User login
Functional Knee Outcomes in Infrapatellar and Suprapatellar Tibial Nailing: Does Approach Matter?
With an incidence of 75,000 per year in the United States alone, fractures of the tibial shaft are among the most common long-bone fractures.1 Diaphyseal tibial fractures present a unique treatment challenge because of complications, including nonunion, malunion, and the potential for an open injury. Intramedullary fixation of these fractures has long been the standard of care, allowing for early mobilization, shorter time to weight-bearing, and high union rates.2-4
The classic infrapatellar approach to intramedullary nailing involves placing the knee in hyperflexion over a bump or radiolucent triangle and inserting the nail through a longitudinal incision in line with the fibers of the patellar tendon. Deforming muscle forces often cause proximal-third tibial fractures and segmental fractures to fall into valgus and procurvatum. To counter these deforming forces, orthopedic surgeons have used some novel surgical approaches, including use of blocking screws5 and a parapatellar approach that could be used with the knee in semi-extended position.6 Anterior knee pain has been reported as a common complication of tibial nailing (reported incidence, 56%).7 In a prospective randomized controlled study, Toivanen and colleagues8 found no difference in incidence of knee pain between patellar tendon splitting and parapatellar approaches.
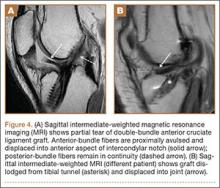
Techniques have been developed to insert the nail through a semi-extended suprapatellar approach to facilitate intraoperative imaging, allow easier access to starting-site position, and counter deforming forces. Although outcomes of traditional infrapatellar nailing have been well documented, there is a paucity of literature on outcomes of using a suprapatellar approach. Splitting the quadriceps tendon causes scar tissue to form superior to the patella versus the anterior knee, which may reduce flexion-related pain or kneeling pain.9 The infrapatellar nerve is also well protected with this approach.
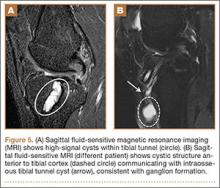
We conducted a study to determine differences in functional knee pain in patients who underwent either traditional infrapatellar nailing or suprapatellar nailing. We hypothesized that there would be no difference in functional knee scores between these approaches and that, when compared with the infrapatellar approach, the suprapatellar approach would result in improved postoperative reduction and reduced intraoperative fluoroscopy time.
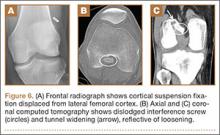
Materials and Methods
This study was approved by our institutional review board. We searched our level I trauma center’s database for Current Procedural Terminology (CPT) code 27759 to identify all patients who had a tibial shaft fracture fixed with an intramedullary implant between January 2009 and February 2013. Radiographs, operative reports, and inpatient records were reviewed. Patients older than 18 years at time of injury and patients with an isolated tibial shaft fracture (Orthopaedic Trauma Association type 42 A-C) surgically fixed with an intramedullary nail through either a traditional infrapatellar approach or a suprapatellar approach were included in the study. Exclusion criteria were required fasciotomy, Gustilo type 3B or 3C open fracture, prior knee surgery, additional orthopedic injury, and preexisting radiographic evidence of degenerative joint disease.
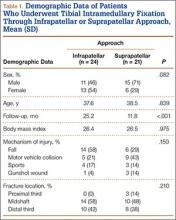
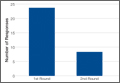
In addition to surgical approach, demographic data, including body mass index (BMI), age, sex, and mechanism of injury, were documented from the medical record. Each patient was contacted by telephone by an investigator blinded to surgical exposure, and the 12-item Oxford Knee Score (OKS) questionnaire was administered (Figure). Operative time, quality of reduction on postoperative radiographs, and intraoperative fluoroscopy time were compared between the 2 approaches. We determined quality of reduction by measuring the angle between the line perpendicular to the tibial plateau and plafond on both the anteroposterior and lateral postoperative radiographs. Rotation was determined by measuring displacement of the fracture by cortical widths. The infrapatellar and suprapatellar groups were statistically analyzed with an unpaired, 2-tailed Student t test. Categorical variables between groups were analyzed with the χ2 test or, when expected values in a cell were less than 5, the Fisher exact test.
We then conducted an a priori power analysis to determine the appropriate sample size. To detect the reported minimally clinically important difference in the OKS of 5.2,10 estimating an approximate 20% larger patient population in the infrapatellar group, we would need to enroll 24 infrapatellar patients and 20 suprapatellar patients to achieve a power of 0.80 with a type I error rate of 0.05.11 This analysis is also based on an estimated OKS standard deviation of 6, which has been reported in several studies.12,13
Results
We identified 176 patients who had the CPT code for intramedullary fixation of a tibial shaft fracture between January 2009 and February 2013. After analysis of radiographs and medical records, 82 patients met the inclusion criteria. Thirty-six (45%) of the original 82 patients were lost to follow-up after attempts to contact them by telephone. One patient refused to participate in the study. Twenty-four patients underwent traditional infrapatellar nailing, and 21 patients had a suprapatellar nail placed with approach-specific instrumentation. Nine patients had an open fracture. There was no significant difference between the groups in terms of sex, age, BMI, mechanism of injury, or operative time (Table 1). There was also no difference (P = .210) in fracture location between groups (0 proximal-third, 14 midshaft, 10 distal-third vs 3 proximal-third, 10 midshaft, 8 distal-third). Mean age was 37.6 years (range, 20-65 years) for the infrapatellar group and 38.5 years (range, 18-68 years) for the suprapatellar group (P = .839). Mean follow-up was significantly (P < .001) shorter for the suprapatellar group (12 mo; range, 3-33 mo) than for the infrapatellar group (25 mo; range, 4-43 mo).
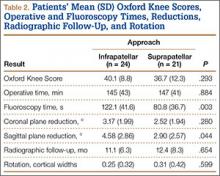
Mean OKS (maximum, 48 points) was 40.1 (range, 11-48) for the infrapatellar group and 36.7 (range, 2-48) for the suprapatellar group (P = .293). Table 2 summarizes the data. Radiographic reduction in the sagittal plane was improved (P = .044) in the suprapatellar group (2.90°) compared with the infrapatellar group (4.58°). There was no difference in rotational malreduction (0.31 vs 0.25 cortical width; P = .599) or in reduction in the coronal plane (2.52° vs 3.17°; P = .280). All patients in both groups maintained radiographic reduction within 5° in any plane throughout follow-up. There was no difference (P = .654) in radiographic follow-up between the infrapatellar group (11 mo) and the suprapatellar group (12 mo). The 1 nonunion in the suprapatellar group required return to the operating room for exchange intramedullary nailing. The suprapatellar approach required less (P = .003) operative fluoroscopy time (80.8 s; range, 46-180 s) than the standard infrapatellar approach (122.1 s; range, 71-240 s). Two patients in the suprapatellar group and 8 in the infrapatellar group did not have their fluoroscopy time recorded in the operative report.
Discussion
We have described the first retrospective cohort-comparison study of functional knee scores associated with traditional infrapatellar nailing and suprapatellar nailing. Although much has been written about the incidence of anterior knee pain with use of a patellar splitting or parapatellar approach, the clinical effects of knee pain after use of suprapatellar nails are yet to be addressed. In a cadaveric study, Gelbke and colleagues14 found higher mean patellofemoral pressures and higher peak contact pressures with a suprapatellar approach. These numbers, however, were still far below the threshold for chondrocyte damage, and that study is yet to be clinically validated. Our data showed no difference in OKS between the 2 groups. Despite being intra-articular, approach-specific instrumentation may protect the trochlea and patellar cartilage.
Although the OKS questionnaire was originally developed and widely validated to describe clinical outcomes of total knee arthroplasty,15,16 it has also been evaluated for other interventions, including viscosupplementation injections17 and high tibial osteotomy.18 We used the OKS questionnaire in our study because it is simple to administer by telephone and is not as cumbersome as the Knee Society Score or the Western Ontario and McMaster Universities Osteoarthritis Index. It is also more specific to the knee than generalized outcome measures used in trauma, such as the Short Form 36 (SF-36). Sanders and colleagues19 reported excellent tibial alignment, radiographic union, and knee range of motion using semi-extended tibial nailing with a suprapatellar approach. For outcome measures, they used the Lysholm Knee Score and the SF-36. Our clinical and radiographic results confirmed their finding—that the semi-extended suprapatellar approach is an option for tibial nailing.
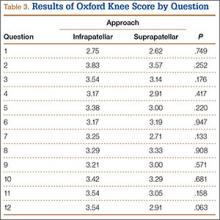
OKS results by question (Table 3) showed that the infrapatellar group had less pain walking down stairs. This result approached statistical significance (P = .063). As surgeons at our institution began using the suprapatellar approach only during the final 2 years of the study period, mean follow-up was significantly (P < .001) less than for the infrapatellar group (12 vs 25 mo). Although there was no statistically significant difference in reduction quality on anteroposterior radiographs, the suprapatellar approach had improved (P = .044) reduction on lateral radiographs (2.90° vs 4.58°).
Although operative time did not differ between our 2 groups, significantly (P = .003) less fluoroscopy time was required for suprapatellar nails (80.8 s) than for infrapatellar nails (122.1 s). Positioning the knee in the semi-extended position offers easier access for fluoroscopy and less radiation exposure for the patient. Placing the nail in extension also helps eliminate the deforming forces that cause malreduction of proximal tibial shaft or segmental fractures. However, our study was limited in that only 2 surgeons at our institution used the suprapatellar approach, and both were fellowship-trained in orthopedic traumatology. This situation could have introduced bias into the interpretation of fluoroscopy data, as these surgeons may have been more comfortable with the procedure and less likely to use fluoroscopy. Both surgeons also performed infrapatellar nailing during the study period, and there was no statistical difference in fracture patterns between the groups, thus minimizing bias.
This study was retrospective but had several strengths. Sample size met the prestudy power analysis to determine a minimally clinically important difference in OKS results. The investigator who administered the telephone survey was blinded to surgical approach. This study was also the first clinical study to compare outcomes of infrapatellar and suprapatellar nailing. However, the study’s follow-up rate was a weakness. The patient population at our academic, urban, level I trauma center is transient. We lost 36 patients (45%) to follow-up; their telephone numbers in the hospital records likely changed since surgery, and we could not contact these patients.
Conclusion
Our retrospective cohort study found no difference in OKS between traditional infrapatellar nailing and suprapatellar nailing for diaphyseal tibia fractures. Suprapatellar nails require less fluoroscopy time and may show improved radiographic reduction in the sagittal plane. Although further study is needed, the suprapatellar entry portal appears to be a safe alternative for tibial nailing with use of appropriate instrumentation.
1. Praemer A, Furner S, Rice DP. Musculoskeletal Conditions in the United States. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1992.
2. Bone LB, Sucato D, Stegemann PM, Rohrbacher BJ. Displaced isolated fractures of the tibial shaft treated with either a cast or intramedullary nailing. An outcome analysis of matched pairs of patients. J Bone Joint Surg Am. 1997;79(9):1336-1341.
3. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
4. Alho A, Benterud JG, Høgevold HE, Ekeland A, Strømsøe K. Comparison of functional bracing and locked intramedullary nailing in the treatment of displaced tibial shaft fractures. Clin Orthop Relat Res. 1992;(277):243-250.
5. Ricci WM, O’Boyle M, Borrelli J, Bellabarba C, Sanders R. Fractures of the proximal third of the tibial shaft treated with intramedullary nails and blocking screws. J Orthop Trauma. 2001;15(4):264-270.
6. Tornetta P 3rd, Collins E. Semiextended position of intramedullary nailing of the proximal tibia. Clin Orthop Relat Res. 1996;(328):185-189.
7. Court-Brown CM, Gustilo T, Shaw AD. Knee pain after intramedullary tibial nailing: its incidence, etiology, and outcome. J Orthop Trauma. 1997;11(2):103-105.
8. Toivanen JA, Väistö O, Kannus P, Latvala K, Honkonen SE, Järvinen MJ. Anterior knee pain after intramedullary nailing of fractures of the tibial shaft. A prospective, randomized study comparing two different nail-insertion techniques. J Bone Joint Surg Am. 2002;84(4):580-585.
9. Morandi M, Banka T, Gairarsa GP, et al. Intramedullary nailing of tibial fractures: review of surgical techniques and description of a percutaneous lateral suprapatellar approach. Orthopaedics. 2010;33(3):172-179.
10. Bohm ER, Loucks L, Tan QE, et al. Determining minimum clinically important difference and targeted clinical improvement values for the Oxford 12. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; 2012; San Francisco, CA.
11. Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11(2):116-128.
12. Streit MR, Walker T, Bruckner T, et al. Mobile-bearing lateral unicompartmental knee replacement with the Oxford domed tibial component: an independent series. J Bone Joint Surg Br. 2012;94(10):1356-1361.
13. Jenny JY, Diesinger Y. The Oxford Knee Score: compared performance before and after knee replacement. Orthop Traumatol Surg Res. 2012;98(4):409-412.
14. Gelbke MK, Coombs D, Powell S, et al. Suprapatellar versus infra-patellar intramedullary nail insertion of the tibia: a cadaveric model for comparison of patellofemoral contact pressures and forces. J Orthop Trauma. 2010;24(11):665-671.
15. Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br. 1998;80(1):63-69.
16. Dunbar MJ, Robertsson O, Ryd L, Lidgren L. Translation and validation of the Oxford-12 item knee score for use in Sweden. Acta Orthop Scand. 2000;71(3):268-274.
17. Clarke S, Lock V, Duddy J, Sharif M, Newman JH, Kirwan JR. Intra-articular hylan G-F 20 (Synvisc) in the management of patellofemoral osteoarthritis of the knee (POAK). Knee. 2005;12(1):57-62.
18. Weale AE, Lee AS, MacEachern AG. High tibial osteotomy using a dynamic axial external fixator. Clin Orthop Relat Res. 2001;(382):154-167.
19. Sanders RW, DiPasquale TG, Jordan CJ, Arrington JA, Sagi HC. Semiextended intramedullary nailing of the tibia using a suprapatellar approach: radiographic results and clinical outcomes at a minimum of 12 months follow-up. J Orthop Trauma. 2014;28(suppl 8):S29-S39.
With an incidence of 75,000 per year in the United States alone, fractures of the tibial shaft are among the most common long-bone fractures.1 Diaphyseal tibial fractures present a unique treatment challenge because of complications, including nonunion, malunion, and the potential for an open injury. Intramedullary fixation of these fractures has long been the standard of care, allowing for early mobilization, shorter time to weight-bearing, and high union rates.2-4
The classic infrapatellar approach to intramedullary nailing involves placing the knee in hyperflexion over a bump or radiolucent triangle and inserting the nail through a longitudinal incision in line with the fibers of the patellar tendon. Deforming muscle forces often cause proximal-third tibial fractures and segmental fractures to fall into valgus and procurvatum. To counter these deforming forces, orthopedic surgeons have used some novel surgical approaches, including use of blocking screws5 and a parapatellar approach that could be used with the knee in semi-extended position.6 Anterior knee pain has been reported as a common complication of tibial nailing (reported incidence, 56%).7 In a prospective randomized controlled study, Toivanen and colleagues8 found no difference in incidence of knee pain between patellar tendon splitting and parapatellar approaches.
Techniques have been developed to insert the nail through a semi-extended suprapatellar approach to facilitate intraoperative imaging, allow easier access to starting-site position, and counter deforming forces. Although outcomes of traditional infrapatellar nailing have been well documented, there is a paucity of literature on outcomes of using a suprapatellar approach. Splitting the quadriceps tendon causes scar tissue to form superior to the patella versus the anterior knee, which may reduce flexion-related pain or kneeling pain.9 The infrapatellar nerve is also well protected with this approach.
We conducted a study to determine differences in functional knee pain in patients who underwent either traditional infrapatellar nailing or suprapatellar nailing. We hypothesized that there would be no difference in functional knee scores between these approaches and that, when compared with the infrapatellar approach, the suprapatellar approach would result in improved postoperative reduction and reduced intraoperative fluoroscopy time.
Materials and Methods
This study was approved by our institutional review board. We searched our level I trauma center’s database for Current Procedural Terminology (CPT) code 27759 to identify all patients who had a tibial shaft fracture fixed with an intramedullary implant between January 2009 and February 2013. Radiographs, operative reports, and inpatient records were reviewed. Patients older than 18 years at time of injury and patients with an isolated tibial shaft fracture (Orthopaedic Trauma Association type 42 A-C) surgically fixed with an intramedullary nail through either a traditional infrapatellar approach or a suprapatellar approach were included in the study. Exclusion criteria were required fasciotomy, Gustilo type 3B or 3C open fracture, prior knee surgery, additional orthopedic injury, and preexisting radiographic evidence of degenerative joint disease.
In addition to surgical approach, demographic data, including body mass index (BMI), age, sex, and mechanism of injury, were documented from the medical record. Each patient was contacted by telephone by an investigator blinded to surgical exposure, and the 12-item Oxford Knee Score (OKS) questionnaire was administered (Figure). Operative time, quality of reduction on postoperative radiographs, and intraoperative fluoroscopy time were compared between the 2 approaches. We determined quality of reduction by measuring the angle between the line perpendicular to the tibial plateau and plafond on both the anteroposterior and lateral postoperative radiographs. Rotation was determined by measuring displacement of the fracture by cortical widths. The infrapatellar and suprapatellar groups were statistically analyzed with an unpaired, 2-tailed Student t test. Categorical variables between groups were analyzed with the χ2 test or, when expected values in a cell were less than 5, the Fisher exact test.
We then conducted an a priori power analysis to determine the appropriate sample size. To detect the reported minimally clinically important difference in the OKS of 5.2,10 estimating an approximate 20% larger patient population in the infrapatellar group, we would need to enroll 24 infrapatellar patients and 20 suprapatellar patients to achieve a power of 0.80 with a type I error rate of 0.05.11 This analysis is also based on an estimated OKS standard deviation of 6, which has been reported in several studies.12,13
Results
We identified 176 patients who had the CPT code for intramedullary fixation of a tibial shaft fracture between January 2009 and February 2013. After analysis of radiographs and medical records, 82 patients met the inclusion criteria. Thirty-six (45%) of the original 82 patients were lost to follow-up after attempts to contact them by telephone. One patient refused to participate in the study. Twenty-four patients underwent traditional infrapatellar nailing, and 21 patients had a suprapatellar nail placed with approach-specific instrumentation. Nine patients had an open fracture. There was no significant difference between the groups in terms of sex, age, BMI, mechanism of injury, or operative time (Table 1). There was also no difference (P = .210) in fracture location between groups (0 proximal-third, 14 midshaft, 10 distal-third vs 3 proximal-third, 10 midshaft, 8 distal-third). Mean age was 37.6 years (range, 20-65 years) for the infrapatellar group and 38.5 years (range, 18-68 years) for the suprapatellar group (P = .839). Mean follow-up was significantly (P < .001) shorter for the suprapatellar group (12 mo; range, 3-33 mo) than for the infrapatellar group (25 mo; range, 4-43 mo).
Mean OKS (maximum, 48 points) was 40.1 (range, 11-48) for the infrapatellar group and 36.7 (range, 2-48) for the suprapatellar group (P = .293). Table 2 summarizes the data. Radiographic reduction in the sagittal plane was improved (P = .044) in the suprapatellar group (2.90°) compared with the infrapatellar group (4.58°). There was no difference in rotational malreduction (0.31 vs 0.25 cortical width; P = .599) or in reduction in the coronal plane (2.52° vs 3.17°; P = .280). All patients in both groups maintained radiographic reduction within 5° in any plane throughout follow-up. There was no difference (P = .654) in radiographic follow-up between the infrapatellar group (11 mo) and the suprapatellar group (12 mo). The 1 nonunion in the suprapatellar group required return to the operating room for exchange intramedullary nailing. The suprapatellar approach required less (P = .003) operative fluoroscopy time (80.8 s; range, 46-180 s) than the standard infrapatellar approach (122.1 s; range, 71-240 s). Two patients in the suprapatellar group and 8 in the infrapatellar group did not have their fluoroscopy time recorded in the operative report.
Discussion
We have described the first retrospective cohort-comparison study of functional knee scores associated with traditional infrapatellar nailing and suprapatellar nailing. Although much has been written about the incidence of anterior knee pain with use of a patellar splitting or parapatellar approach, the clinical effects of knee pain after use of suprapatellar nails are yet to be addressed. In a cadaveric study, Gelbke and colleagues14 found higher mean patellofemoral pressures and higher peak contact pressures with a suprapatellar approach. These numbers, however, were still far below the threshold for chondrocyte damage, and that study is yet to be clinically validated. Our data showed no difference in OKS between the 2 groups. Despite being intra-articular, approach-specific instrumentation may protect the trochlea and patellar cartilage.
Although the OKS questionnaire was originally developed and widely validated to describe clinical outcomes of total knee arthroplasty,15,16 it has also been evaluated for other interventions, including viscosupplementation injections17 and high tibial osteotomy.18 We used the OKS questionnaire in our study because it is simple to administer by telephone and is not as cumbersome as the Knee Society Score or the Western Ontario and McMaster Universities Osteoarthritis Index. It is also more specific to the knee than generalized outcome measures used in trauma, such as the Short Form 36 (SF-36). Sanders and colleagues19 reported excellent tibial alignment, radiographic union, and knee range of motion using semi-extended tibial nailing with a suprapatellar approach. For outcome measures, they used the Lysholm Knee Score and the SF-36. Our clinical and radiographic results confirmed their finding—that the semi-extended suprapatellar approach is an option for tibial nailing.
OKS results by question (Table 3) showed that the infrapatellar group had less pain walking down stairs. This result approached statistical significance (P = .063). As surgeons at our institution began using the suprapatellar approach only during the final 2 years of the study period, mean follow-up was significantly (P < .001) less than for the infrapatellar group (12 vs 25 mo). Although there was no statistically significant difference in reduction quality on anteroposterior radiographs, the suprapatellar approach had improved (P = .044) reduction on lateral radiographs (2.90° vs 4.58°).
Although operative time did not differ between our 2 groups, significantly (P = .003) less fluoroscopy time was required for suprapatellar nails (80.8 s) than for infrapatellar nails (122.1 s). Positioning the knee in the semi-extended position offers easier access for fluoroscopy and less radiation exposure for the patient. Placing the nail in extension also helps eliminate the deforming forces that cause malreduction of proximal tibial shaft or segmental fractures. However, our study was limited in that only 2 surgeons at our institution used the suprapatellar approach, and both were fellowship-trained in orthopedic traumatology. This situation could have introduced bias into the interpretation of fluoroscopy data, as these surgeons may have been more comfortable with the procedure and less likely to use fluoroscopy. Both surgeons also performed infrapatellar nailing during the study period, and there was no statistical difference in fracture patterns between the groups, thus minimizing bias.
This study was retrospective but had several strengths. Sample size met the prestudy power analysis to determine a minimally clinically important difference in OKS results. The investigator who administered the telephone survey was blinded to surgical approach. This study was also the first clinical study to compare outcomes of infrapatellar and suprapatellar nailing. However, the study’s follow-up rate was a weakness. The patient population at our academic, urban, level I trauma center is transient. We lost 36 patients (45%) to follow-up; their telephone numbers in the hospital records likely changed since surgery, and we could not contact these patients.
Conclusion
Our retrospective cohort study found no difference in OKS between traditional infrapatellar nailing and suprapatellar nailing for diaphyseal tibia fractures. Suprapatellar nails require less fluoroscopy time and may show improved radiographic reduction in the sagittal plane. Although further study is needed, the suprapatellar entry portal appears to be a safe alternative for tibial nailing with use of appropriate instrumentation.
With an incidence of 75,000 per year in the United States alone, fractures of the tibial shaft are among the most common long-bone fractures.1 Diaphyseal tibial fractures present a unique treatment challenge because of complications, including nonunion, malunion, and the potential for an open injury. Intramedullary fixation of these fractures has long been the standard of care, allowing for early mobilization, shorter time to weight-bearing, and high union rates.2-4
The classic infrapatellar approach to intramedullary nailing involves placing the knee in hyperflexion over a bump or radiolucent triangle and inserting the nail through a longitudinal incision in line with the fibers of the patellar tendon. Deforming muscle forces often cause proximal-third tibial fractures and segmental fractures to fall into valgus and procurvatum. To counter these deforming forces, orthopedic surgeons have used some novel surgical approaches, including use of blocking screws5 and a parapatellar approach that could be used with the knee in semi-extended position.6 Anterior knee pain has been reported as a common complication of tibial nailing (reported incidence, 56%).7 In a prospective randomized controlled study, Toivanen and colleagues8 found no difference in incidence of knee pain between patellar tendon splitting and parapatellar approaches.
Techniques have been developed to insert the nail through a semi-extended suprapatellar approach to facilitate intraoperative imaging, allow easier access to starting-site position, and counter deforming forces. Although outcomes of traditional infrapatellar nailing have been well documented, there is a paucity of literature on outcomes of using a suprapatellar approach. Splitting the quadriceps tendon causes scar tissue to form superior to the patella versus the anterior knee, which may reduce flexion-related pain or kneeling pain.9 The infrapatellar nerve is also well protected with this approach.
We conducted a study to determine differences in functional knee pain in patients who underwent either traditional infrapatellar nailing or suprapatellar nailing. We hypothesized that there would be no difference in functional knee scores between these approaches and that, when compared with the infrapatellar approach, the suprapatellar approach would result in improved postoperative reduction and reduced intraoperative fluoroscopy time.
Materials and Methods
This study was approved by our institutional review board. We searched our level I trauma center’s database for Current Procedural Terminology (CPT) code 27759 to identify all patients who had a tibial shaft fracture fixed with an intramedullary implant between January 2009 and February 2013. Radiographs, operative reports, and inpatient records were reviewed. Patients older than 18 years at time of injury and patients with an isolated tibial shaft fracture (Orthopaedic Trauma Association type 42 A-C) surgically fixed with an intramedullary nail through either a traditional infrapatellar approach or a suprapatellar approach were included in the study. Exclusion criteria were required fasciotomy, Gustilo type 3B or 3C open fracture, prior knee surgery, additional orthopedic injury, and preexisting radiographic evidence of degenerative joint disease.
In addition to surgical approach, demographic data, including body mass index (BMI), age, sex, and mechanism of injury, were documented from the medical record. Each patient was contacted by telephone by an investigator blinded to surgical exposure, and the 12-item Oxford Knee Score (OKS) questionnaire was administered (Figure). Operative time, quality of reduction on postoperative radiographs, and intraoperative fluoroscopy time were compared between the 2 approaches. We determined quality of reduction by measuring the angle between the line perpendicular to the tibial plateau and plafond on both the anteroposterior and lateral postoperative radiographs. Rotation was determined by measuring displacement of the fracture by cortical widths. The infrapatellar and suprapatellar groups were statistically analyzed with an unpaired, 2-tailed Student t test. Categorical variables between groups were analyzed with the χ2 test or, when expected values in a cell were less than 5, the Fisher exact test.
We then conducted an a priori power analysis to determine the appropriate sample size. To detect the reported minimally clinically important difference in the OKS of 5.2,10 estimating an approximate 20% larger patient population in the infrapatellar group, we would need to enroll 24 infrapatellar patients and 20 suprapatellar patients to achieve a power of 0.80 with a type I error rate of 0.05.11 This analysis is also based on an estimated OKS standard deviation of 6, which has been reported in several studies.12,13
Results
We identified 176 patients who had the CPT code for intramedullary fixation of a tibial shaft fracture between January 2009 and February 2013. After analysis of radiographs and medical records, 82 patients met the inclusion criteria. Thirty-six (45%) of the original 82 patients were lost to follow-up after attempts to contact them by telephone. One patient refused to participate in the study. Twenty-four patients underwent traditional infrapatellar nailing, and 21 patients had a suprapatellar nail placed with approach-specific instrumentation. Nine patients had an open fracture. There was no significant difference between the groups in terms of sex, age, BMI, mechanism of injury, or operative time (Table 1). There was also no difference (P = .210) in fracture location between groups (0 proximal-third, 14 midshaft, 10 distal-third vs 3 proximal-third, 10 midshaft, 8 distal-third). Mean age was 37.6 years (range, 20-65 years) for the infrapatellar group and 38.5 years (range, 18-68 years) for the suprapatellar group (P = .839). Mean follow-up was significantly (P < .001) shorter for the suprapatellar group (12 mo; range, 3-33 mo) than for the infrapatellar group (25 mo; range, 4-43 mo).
Mean OKS (maximum, 48 points) was 40.1 (range, 11-48) for the infrapatellar group and 36.7 (range, 2-48) for the suprapatellar group (P = .293). Table 2 summarizes the data. Radiographic reduction in the sagittal plane was improved (P = .044) in the suprapatellar group (2.90°) compared with the infrapatellar group (4.58°). There was no difference in rotational malreduction (0.31 vs 0.25 cortical width; P = .599) or in reduction in the coronal plane (2.52° vs 3.17°; P = .280). All patients in both groups maintained radiographic reduction within 5° in any plane throughout follow-up. There was no difference (P = .654) in radiographic follow-up between the infrapatellar group (11 mo) and the suprapatellar group (12 mo). The 1 nonunion in the suprapatellar group required return to the operating room for exchange intramedullary nailing. The suprapatellar approach required less (P = .003) operative fluoroscopy time (80.8 s; range, 46-180 s) than the standard infrapatellar approach (122.1 s; range, 71-240 s). Two patients in the suprapatellar group and 8 in the infrapatellar group did not have their fluoroscopy time recorded in the operative report.
Discussion
We have described the first retrospective cohort-comparison study of functional knee scores associated with traditional infrapatellar nailing and suprapatellar nailing. Although much has been written about the incidence of anterior knee pain with use of a patellar splitting or parapatellar approach, the clinical effects of knee pain after use of suprapatellar nails are yet to be addressed. In a cadaveric study, Gelbke and colleagues14 found higher mean patellofemoral pressures and higher peak contact pressures with a suprapatellar approach. These numbers, however, were still far below the threshold for chondrocyte damage, and that study is yet to be clinically validated. Our data showed no difference in OKS between the 2 groups. Despite being intra-articular, approach-specific instrumentation may protect the trochlea and patellar cartilage.
Although the OKS questionnaire was originally developed and widely validated to describe clinical outcomes of total knee arthroplasty,15,16 it has also been evaluated for other interventions, including viscosupplementation injections17 and high tibial osteotomy.18 We used the OKS questionnaire in our study because it is simple to administer by telephone and is not as cumbersome as the Knee Society Score or the Western Ontario and McMaster Universities Osteoarthritis Index. It is also more specific to the knee than generalized outcome measures used in trauma, such as the Short Form 36 (SF-36). Sanders and colleagues19 reported excellent tibial alignment, radiographic union, and knee range of motion using semi-extended tibial nailing with a suprapatellar approach. For outcome measures, they used the Lysholm Knee Score and the SF-36. Our clinical and radiographic results confirmed their finding—that the semi-extended suprapatellar approach is an option for tibial nailing.
OKS results by question (Table 3) showed that the infrapatellar group had less pain walking down stairs. This result approached statistical significance (P = .063). As surgeons at our institution began using the suprapatellar approach only during the final 2 years of the study period, mean follow-up was significantly (P < .001) less than for the infrapatellar group (12 vs 25 mo). Although there was no statistically significant difference in reduction quality on anteroposterior radiographs, the suprapatellar approach had improved (P = .044) reduction on lateral radiographs (2.90° vs 4.58°).
Although operative time did not differ between our 2 groups, significantly (P = .003) less fluoroscopy time was required for suprapatellar nails (80.8 s) than for infrapatellar nails (122.1 s). Positioning the knee in the semi-extended position offers easier access for fluoroscopy and less radiation exposure for the patient. Placing the nail in extension also helps eliminate the deforming forces that cause malreduction of proximal tibial shaft or segmental fractures. However, our study was limited in that only 2 surgeons at our institution used the suprapatellar approach, and both were fellowship-trained in orthopedic traumatology. This situation could have introduced bias into the interpretation of fluoroscopy data, as these surgeons may have been more comfortable with the procedure and less likely to use fluoroscopy. Both surgeons also performed infrapatellar nailing during the study period, and there was no statistical difference in fracture patterns between the groups, thus minimizing bias.
This study was retrospective but had several strengths. Sample size met the prestudy power analysis to determine a minimally clinically important difference in OKS results. The investigator who administered the telephone survey was blinded to surgical approach. This study was also the first clinical study to compare outcomes of infrapatellar and suprapatellar nailing. However, the study’s follow-up rate was a weakness. The patient population at our academic, urban, level I trauma center is transient. We lost 36 patients (45%) to follow-up; their telephone numbers in the hospital records likely changed since surgery, and we could not contact these patients.
Conclusion
Our retrospective cohort study found no difference in OKS between traditional infrapatellar nailing and suprapatellar nailing for diaphyseal tibia fractures. Suprapatellar nails require less fluoroscopy time and may show improved radiographic reduction in the sagittal plane. Although further study is needed, the suprapatellar entry portal appears to be a safe alternative for tibial nailing with use of appropriate instrumentation.
1. Praemer A, Furner S, Rice DP. Musculoskeletal Conditions in the United States. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1992.
2. Bone LB, Sucato D, Stegemann PM, Rohrbacher BJ. Displaced isolated fractures of the tibial shaft treated with either a cast or intramedullary nailing. An outcome analysis of matched pairs of patients. J Bone Joint Surg Am. 1997;79(9):1336-1341.
3. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
4. Alho A, Benterud JG, Høgevold HE, Ekeland A, Strømsøe K. Comparison of functional bracing and locked intramedullary nailing in the treatment of displaced tibial shaft fractures. Clin Orthop Relat Res. 1992;(277):243-250.
5. Ricci WM, O’Boyle M, Borrelli J, Bellabarba C, Sanders R. Fractures of the proximal third of the tibial shaft treated with intramedullary nails and blocking screws. J Orthop Trauma. 2001;15(4):264-270.
6. Tornetta P 3rd, Collins E. Semiextended position of intramedullary nailing of the proximal tibia. Clin Orthop Relat Res. 1996;(328):185-189.
7. Court-Brown CM, Gustilo T, Shaw AD. Knee pain after intramedullary tibial nailing: its incidence, etiology, and outcome. J Orthop Trauma. 1997;11(2):103-105.
8. Toivanen JA, Väistö O, Kannus P, Latvala K, Honkonen SE, Järvinen MJ. Anterior knee pain after intramedullary nailing of fractures of the tibial shaft. A prospective, randomized study comparing two different nail-insertion techniques. J Bone Joint Surg Am. 2002;84(4):580-585.
9. Morandi M, Banka T, Gairarsa GP, et al. Intramedullary nailing of tibial fractures: review of surgical techniques and description of a percutaneous lateral suprapatellar approach. Orthopaedics. 2010;33(3):172-179.
10. Bohm ER, Loucks L, Tan QE, et al. Determining minimum clinically important difference and targeted clinical improvement values for the Oxford 12. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; 2012; San Francisco, CA.
11. Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11(2):116-128.
12. Streit MR, Walker T, Bruckner T, et al. Mobile-bearing lateral unicompartmental knee replacement with the Oxford domed tibial component: an independent series. J Bone Joint Surg Br. 2012;94(10):1356-1361.
13. Jenny JY, Diesinger Y. The Oxford Knee Score: compared performance before and after knee replacement. Orthop Traumatol Surg Res. 2012;98(4):409-412.
14. Gelbke MK, Coombs D, Powell S, et al. Suprapatellar versus infra-patellar intramedullary nail insertion of the tibia: a cadaveric model for comparison of patellofemoral contact pressures and forces. J Orthop Trauma. 2010;24(11):665-671.
15. Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br. 1998;80(1):63-69.
16. Dunbar MJ, Robertsson O, Ryd L, Lidgren L. Translation and validation of the Oxford-12 item knee score for use in Sweden. Acta Orthop Scand. 2000;71(3):268-274.
17. Clarke S, Lock V, Duddy J, Sharif M, Newman JH, Kirwan JR. Intra-articular hylan G-F 20 (Synvisc) in the management of patellofemoral osteoarthritis of the knee (POAK). Knee. 2005;12(1):57-62.
18. Weale AE, Lee AS, MacEachern AG. High tibial osteotomy using a dynamic axial external fixator. Clin Orthop Relat Res. 2001;(382):154-167.
19. Sanders RW, DiPasquale TG, Jordan CJ, Arrington JA, Sagi HC. Semiextended intramedullary nailing of the tibia using a suprapatellar approach: radiographic results and clinical outcomes at a minimum of 12 months follow-up. J Orthop Trauma. 2014;28(suppl 8):S29-S39.
1. Praemer A, Furner S, Rice DP. Musculoskeletal Conditions in the United States. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1992.
2. Bone LB, Sucato D, Stegemann PM, Rohrbacher BJ. Displaced isolated fractures of the tibial shaft treated with either a cast or intramedullary nailing. An outcome analysis of matched pairs of patients. J Bone Joint Surg Am. 1997;79(9):1336-1341.
3. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
4. Alho A, Benterud JG, Høgevold HE, Ekeland A, Strømsøe K. Comparison of functional bracing and locked intramedullary nailing in the treatment of displaced tibial shaft fractures. Clin Orthop Relat Res. 1992;(277):243-250.
5. Ricci WM, O’Boyle M, Borrelli J, Bellabarba C, Sanders R. Fractures of the proximal third of the tibial shaft treated with intramedullary nails and blocking screws. J Orthop Trauma. 2001;15(4):264-270.
6. Tornetta P 3rd, Collins E. Semiextended position of intramedullary nailing of the proximal tibia. Clin Orthop Relat Res. 1996;(328):185-189.
7. Court-Brown CM, Gustilo T, Shaw AD. Knee pain after intramedullary tibial nailing: its incidence, etiology, and outcome. J Orthop Trauma. 1997;11(2):103-105.
8. Toivanen JA, Väistö O, Kannus P, Latvala K, Honkonen SE, Järvinen MJ. Anterior knee pain after intramedullary nailing of fractures of the tibial shaft. A prospective, randomized study comparing two different nail-insertion techniques. J Bone Joint Surg Am. 2002;84(4):580-585.
9. Morandi M, Banka T, Gairarsa GP, et al. Intramedullary nailing of tibial fractures: review of surgical techniques and description of a percutaneous lateral suprapatellar approach. Orthopaedics. 2010;33(3):172-179.
10. Bohm ER, Loucks L, Tan QE, et al. Determining minimum clinically important difference and targeted clinical improvement values for the Oxford 12. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; 2012; San Francisco, CA.
11. Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11(2):116-128.
12. Streit MR, Walker T, Bruckner T, et al. Mobile-bearing lateral unicompartmental knee replacement with the Oxford domed tibial component: an independent series. J Bone Joint Surg Br. 2012;94(10):1356-1361.
13. Jenny JY, Diesinger Y. The Oxford Knee Score: compared performance before and after knee replacement. Orthop Traumatol Surg Res. 2012;98(4):409-412.
14. Gelbke MK, Coombs D, Powell S, et al. Suprapatellar versus infra-patellar intramedullary nail insertion of the tibia: a cadaveric model for comparison of patellofemoral contact pressures and forces. J Orthop Trauma. 2010;24(11):665-671.
15. Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br. 1998;80(1):63-69.
16. Dunbar MJ, Robertsson O, Ryd L, Lidgren L. Translation and validation of the Oxford-12 item knee score for use in Sweden. Acta Orthop Scand. 2000;71(3):268-274.
17. Clarke S, Lock V, Duddy J, Sharif M, Newman JH, Kirwan JR. Intra-articular hylan G-F 20 (Synvisc) in the management of patellofemoral osteoarthritis of the knee (POAK). Knee. 2005;12(1):57-62.
18. Weale AE, Lee AS, MacEachern AG. High tibial osteotomy using a dynamic axial external fixator. Clin Orthop Relat Res. 2001;(382):154-167.
19. Sanders RW, DiPasquale TG, Jordan CJ, Arrington JA, Sagi HC. Semiextended intramedullary nailing of the tibia using a suprapatellar approach: radiographic results and clinical outcomes at a minimum of 12 months follow-up. J Orthop Trauma. 2014;28(suppl 8):S29-S39.
Total Knee Arthroplasty in Hemophilic Arthropathy
Chronic hemophilic arthropathy, a well-known complication of hemophilia, develops as a long-term consequence of recurrent joint bleeds resulting in synovial hypertrophy (chronic proliferative synovitis) and joint cartilage destruction. Hemophilic arthropathy mostly affects the knees, ankles, and elbows and causes chronic joint pain and functional impairment in relatively young patients who have not received adequate primary prophylactic replacement therapy with factor concentrates from early childhood.1-3
In the late stages of hemophilic arthropathy of the knee, total knee arthroplasty (TKA) provides dramatic joint pain relief, improves knee functional status, and reduces rebleeding into the joint.4-8 TKA performed on a patient with hemophilia was first reported in the mid-1970s.9,10 In these cases, the surgical procedure itself is often complicated by severe fibrosis developing in the joint soft tissues, flexion joint contracture, and poor quality of the joint bone structures. Even though TKA significantly reduces joint pain in patients with chronic hemophilic arthropathy, some authors have achieved only modest functional outcomes and experienced a high rate of complications (infection, prosthetic loosening).11-13 Data on TKA outcomes are still scarce, and most studies have enrolled a limited number of patients.
We retrospectively evaluated the outcomes of 88 primary TKAs performed on patients with severe hemophilia at a single institution. Clinical outcomes and complications were assessed with a special focus on prosthetic survival and infection.
Patients and Methods
Ninety-one primary TKAs were performed in 77 patients with severe hemophilia A and B (factor VIII [FVIII] and factor IX plasma concentration, <1% each) between January 1, 1999, and December 31, 2011, and the medical records of all these patients were thoroughly reviewed in 2013. The cases of 3 patients who died shortly after surgery were excluded from analysis. Thus, 88 TKAs and 74 patients (74 males) were finally available for evaluation. Fourteen patients underwent bilateral TKAs but none concurrently. The patients provided written informed consent for print and electronic publication of their outcomes.
We recorded demographic data, type and severity of hemophilia, human immunodeficiency virus (HIV) status, hepatitis C virus (HCV) status, and Knee Society Scale (KSS) scores.14 KSS scores include Knee score (pain, range of motion [ROM], stability) and Function score (walking, stairs), both of which range from 0 (normal knee) to 100 (most affected knee). Prosthetic infection was classified (Segawa and colleagues15) as early or late, depending on timing of symptom onset (4 weeks after replacement surgery was the threshold used).
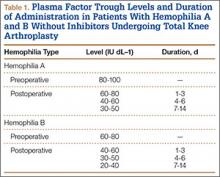
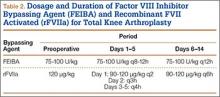
Patients received an intravenous bolus infusion of the deficient factor concentrate followed by continuous infusion to reach a plasma factor level of 100% just before surgery and during the first 7 postoperative days and 50% over the next 7 days (Table 1). Patients with a circulating inhibitor (3 overall) received bypassing agents FEIBA (FVIII inhibitor bypassing agent) or rFVIIa (recombinant factor VII activated) (Table 2). Patients were not given any antifibrinolytic treatment or thromboprophylaxis.
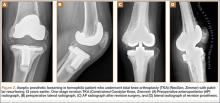
Surgery was performed in a standard surgical room. Patients were placed on the operating table in decubitus supinus position. A parapatellar medial incision was made on a bloodless surgical field (achieved with tourniquet ischemia). The prosthesis model used was always the cemented (gentamicin bone cement) NexGen (Zimmer). Patellar resurfacing was done in all cases (Figures 1A–1D). All TKAs were performed by Dr. Rodríguez-Merchán. Intravenous antibiotic prophylaxis was administered at anesthetic induction and during the first 48 hours after surgery (3 further doses). Active exercises were started on postoperative day 1. Joint load aided with 2 crutches was allowed starting on postoperative day 2.
Mean patient age was 38.2 years (range, 24-73 years). Of the 74 patients, 55 had a diagnosis of severe hemophilia A, and 19 had a diagnosis of severe hemophilia B. During the follow-up period, 23 patients died (mean time, 6.4 years; range, 4-9 years). Causes of death were acquired immune deficiency syndrome (AIDS), liver cirrhosis, and intracranial bleeding. Mean follow-up for the full series of patients was 8 years (range, 1-13 years).
Descriptive statistical analysis was performed with SPSS Windows Version 18.0. Prosthetic failure was regarded as implant removal for any reason. Student t test was used to compare continuous variables, and either χ2 test or Fisher exact test was used to compare categorical variables. P < .05 (2-sided) was considered significant.
Results
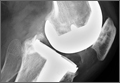
Prosthetic survival rates with implant removal for any reason regarded as final endpoint was 92%. Causes of failure were prosthetic infection (6 cases, 6.8%) and loosening (2 cases, 2.2%). Of the 6 prosthetic infections, 5 were regarded as late and 1 as early. Late infections were successfully sorted by performing 2-stage revision TKA with the Constrained Condylar Knee (Zimmer). Acute infections were managed by open joint débridement and polyethylene exchange. Both cases of aseptic loosening of the TKA were successfully managed with 1-stage revision TKA using the same implant model (Figures 2A–2D).
Mean KSS Knee score improved from 79 before surgery to 36 after surgery, and mean KSS Function score improved from 63 to 33. KSS Pain score, which is included in the Knee score, 0 (no pain) to 50 (most severe pain), improved from 47 to 8. Patients receiving inhibitors and patients who were HIV- or HCV-positive did not have poorer outcomes relative to those of patients not receiving inhibitors and patients who were HIV- or HCV-negative. Patients with liver cirrhosis had a lower prosthetic survival rate and lower Knee scores.
Discussion
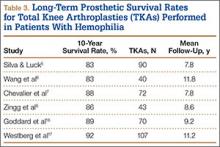
The prosthetic survival rate found in this study compares well with other reported rates for patients with hemophilia and other bleeding disorders. However, evidence regarding long-term prosthesis survival in TKAs performed for patients with hemophilia is limited. Table 3 summarizes the main reported series of patients with hemophilia with 10-year prosthetic survival rates, number of TKAs performed, and mean follow-up period; in all these series, implant removal for any reason was regarded as the final endpoint.5-8,16,17 Mean follow-up in our study was 8 years. Clinical outcomes of TKA in patients with severe hemophilia and related disorders are expected to be inferior to those achieved in patients without a bleeding condition. The overall 10-year prosthetic survival rate for cemented TKA implants, as reported by the Norwegian Arthroplasty Register, was on the order of 93%.18 Mean age of our patients at time of surgery was only 38.2 years. TKAs performed in younger patients without a bleeding disorder have been associated with shorter implant survival times relative to those of elderly patients.19 Thus, Diduch and colleagues20 reported a prosthetic survival rate of 87% at 18 years in 108 TKAs performed on patients under age 55 years. Lonner and colleagues21 reported a better implant survival rate (90% at 8 years) in a series of patients under age 40 years (32 TKAs). In a study by Duffy and colleagues,22 the implant survival rate was 85% at 15 years in patients under age 55 years (74 TKAs). The results from our retrospective case assessment are quite similar to the overall prosthetic survival rates reported for TKAs performed on patients without hemophilia.
Rates of periprosthetic infection after primary TKA in patients with hemophilia and other bleeding conditions are much higher (up to 11%), with a mean infection rate of 6.2% (range, 1% to 11%), consistent with the rate found in our series of patients (6.8%)7,16,17,23,24 (Table 4). This rate is much higher than that reported after primary TKA in patients without hemophilia but is similar to some rates reported for patients with hemophilia. In our experience, most periprosthetic infections (5/6) were sorted as late.
Late infection is a major concern after TKA in patients with hemophilia, and various factors have been hypothesized as contributing to the high prevalence. An important factor is the high rate of HIV-positive patients among patients with hemophilia—which acts as a strong predisposing factor because of the often low CD4 counts and associated immune deficiency,25 but different reports have provided conflicting results in this respect.5,6,12 We found no relationship between HIV status and risk for periprosthetic infection, but conclusions are limited by the low number of HIV-positive patients in our series (14/74, 18.9%). Our patients’ late periprosthetic infections were diagnosed several years after TKA, suggesting hematogenous spread of infection. Most of these patients either were on regular prophylactic factor infusions or were being treated on demand, which might entail a risk for contamination of infusions by skin bacteria from the puncture site. Therefore, having an aseptic technique for administering coagulation factor concentrates is of paramount importance for patients with hemophilia and a knee implant.
Another important complication of TKA surgery is aseptic loosening of the prosthesis. Aseptic loosening occurred in 2.2% of our patients, but higher rates have been reported elsewhere.11,26 Rates of this complication increase over follow-up, and some authors have linked this complication to TKA polyethylene wear.27 Development of a reactive and destructive bone–cement interface and microhemorrhages into such interface might be implicated in the higher rate of loosening observed among patients with hemophilia.28
In the present study, preoperative and postoperative functional outcomes differed significantly. A modest postoperative total ROM of 69º to 79º has been reported by several authors.5,6 Postoperative ROM may vary—may be slightly increased, remain unchanged, or may even be reduced.4,23,26 Even though little improvement in total ROM is achieved after TKA, many authors have reported reduced flexion contracture and hence an easier gait. However, along with functional improvement, dramatic pain relief after TKA is perhaps the most remarkable aspect, and it has a strong effect on patient satisfaction after surgery.5,7,8,18,23
Our study had 2 main limitations. First, it was a retrospective case series evaluation with the usual issues of potential inaccuracy of medical records and information bias. Second, the study did not include a control group.
Conclusion
The primary TKAs performed in our patients with hemophilia have had a good prosthetic survival rate. Even though such a result is slightly inferior to results in patients without hemophilia, our prosthetic survival rate is not significantly different from the rates reported in other, younger patient subsets. Late periprosthetic infections are a major concern, and taking precautions to avoid hematogenous spread of infections during factor concentrate infusions is strongly encouraged.
1. Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977;59(3):287-305.
2. Rodriguez-Merchan EC. Common orthopaedic problems in haemophilia. Haemophilia. 1999;5(suppl 1):53-60.
3. Steen Carlsson K, Höjgård S, Glomstein A, et al. On-demand vs. prophylactic treatment for severe haemophilia in Norway and Sweden: differences in treatment characteristics and outcome. Haemophilia. 2003;9(5):555-566.
4. Teigland JC, Tjønnfjord GE, Evensen SA, Charania B. Knee arthroplasty in hemophilia. 5-12 year follow-up of 15 patients. Acta Orthop Scand. 1993;64(2):153-156.
5. Silva M, Luck JV Jr. Long-term results of primary total knee replacement in patients with hemophilia. J Bone Joint Surg Am. 2005;87(1):85-91.
6. Wang K, Street A, Dowrick A, Liew S. Clinical outcomes and patient satisfaction following total joint replacement in haemophilia—23-year experience in knees, hips and elbows. Haemophilia. 2012;18(1):86-93.
7. Chevalier Y, Dargaud Y, Lienhart A, Chamouard V, Negrier C. Seventy-two total knee arthroplasties performed in patients with haemophilia using continuous infusion. Vox Sang. 2013;104(2):135-143.
8. Zingg PO, Fucentese SF, Lutz W, Brand B, Mamisch N, Koch PP. Haemophilic knee arthropathy: long-term outcome after total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2465-2470.
9. Kjaersgaard-Andersen P, Christiansen SE, Ingerslev J, Sneppen O. Total knee arthroplasty in classic hemophilia. Clin Orthop Relat Res. 1990;(256):137-146.
10. Cohen I, Heim M, Martinowitz U, Chechick A. Orthopaedic outcome of total knee replacement in haemophilia A. Haemophilia. 2000;6(2):104-109.
11. Fehily M, Fleming P, O’Shea E, Smith O, Smyth H. Total knee arthroplasty in patients with severe haemophilia. Int Orthop. 2002;26(2):89-91.
12. Legroux-Gérot I, Strouk G, Parquet A, Goodemand J, Gougeon F, Duquesnoy B. Total knee arthroplasty in hemophilic arthropathy. Joint Bone Spine. 2003;70(1):22-32.
13. Sheth DS, Oldfield D, Ambrose C, Clyburn T. Total knee arthroplasty in hemophilic arthropathy. J Arthroplasty. 2004;19(1):56-60.
14. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
15. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
16. Goddard NJ, Mann HA, Lee CA. Total knee replacement in patients with end-stage haemophilic arthropathy. 25-year results. J Bone Joint Surg Br. 2010;92(8):1085-1089.
17. Westberg M, Paus AC, Holme PA, Tjønnfjord GE. Haemophilic arthropathy: long-term outcomes in 107 primary total knee arthroplasties. Knee. 2014;21(1):147-150.
18. Lygre SH, Espehaug B, Havelin LI, Vollset SE, Furnes O. Failure of total knee arthroplasty with or without patella resurfacing. A study from the Norwegian Arthroplasty Register with 0-15 years of follow-up. Acta Orthop. 2011;82(3):282-292.
19. Post M, Telfer MC. Surgery in hemophilic patients. J Bone Joint Surg Am. 1975;57(8):1136-1145.
20. Diduch DR, Insall JN, Scott WN, Scuderi GR, Font-Rodriguez D. Total knee replacement in young, active patients. Long-term follow-up and functional outcome. J Bone Joint Surg Am. 1997;79(4):575-582.
21. Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000;(380):85-90.
22. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 suppl 2):67-70.
23. Chiang CC, Chen PQ, Shen MC, Tsai W. Total knee arthroplasty for severe haemophilic arthropathy: long-term experience in Taiwan. Haemophilia. 2008;14(4):828-834.
24. Solimeno LP, Mancuso ME, Pasta G, Santagostino E, Perfetto S, Mannucci PM. Factors influencing the long-term outcome of primary total knee replacement in haemophiliacs: a review of 116 procedures at a single institution. Br J Haematol. 2009;145(2):227-234.
25. Jämsen E, Varonen M, Huhtala H, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty. 2010;25(1):87-92.
26. Ragni MV, Crossett LS, Herndon JH. Postoperative infection following orthopaedic surgery in human immunodeficiency virus–infected hemophiliacs with CD4 counts < or = 200/mm3. J Arthroplasty. 1995;10(6):716-721.
27. Hicks JL, Ribbans WJ, Buzzard B, et al. Infected joint replacements in HIV-positive patients with haemophilia. J Bone Joint Surg Br. 2001;83(7):1050-1054.
28. Figgie MP, Goldberg VM, Figgie HE 3rd, Heiple KG, Sobel M. Total knee arthroplasty for the treatment of chronic hemophilic arthropathy. Clin Orthop Relat Res. 1989;(248):98-107.
Chronic hemophilic arthropathy, a well-known complication of hemophilia, develops as a long-term consequence of recurrent joint bleeds resulting in synovial hypertrophy (chronic proliferative synovitis) and joint cartilage destruction. Hemophilic arthropathy mostly affects the knees, ankles, and elbows and causes chronic joint pain and functional impairment in relatively young patients who have not received adequate primary prophylactic replacement therapy with factor concentrates from early childhood.1-3
In the late stages of hemophilic arthropathy of the knee, total knee arthroplasty (TKA) provides dramatic joint pain relief, improves knee functional status, and reduces rebleeding into the joint.4-8 TKA performed on a patient with hemophilia was first reported in the mid-1970s.9,10 In these cases, the surgical procedure itself is often complicated by severe fibrosis developing in the joint soft tissues, flexion joint contracture, and poor quality of the joint bone structures. Even though TKA significantly reduces joint pain in patients with chronic hemophilic arthropathy, some authors have achieved only modest functional outcomes and experienced a high rate of complications (infection, prosthetic loosening).11-13 Data on TKA outcomes are still scarce, and most studies have enrolled a limited number of patients.
We retrospectively evaluated the outcomes of 88 primary TKAs performed on patients with severe hemophilia at a single institution. Clinical outcomes and complications were assessed with a special focus on prosthetic survival and infection.
Patients and Methods
Ninety-one primary TKAs were performed in 77 patients with severe hemophilia A and B (factor VIII [FVIII] and factor IX plasma concentration, <1% each) between January 1, 1999, and December 31, 2011, and the medical records of all these patients were thoroughly reviewed in 2013. The cases of 3 patients who died shortly after surgery were excluded from analysis. Thus, 88 TKAs and 74 patients (74 males) were finally available for evaluation. Fourteen patients underwent bilateral TKAs but none concurrently. The patients provided written informed consent for print and electronic publication of their outcomes.
We recorded demographic data, type and severity of hemophilia, human immunodeficiency virus (HIV) status, hepatitis C virus (HCV) status, and Knee Society Scale (KSS) scores.14 KSS scores include Knee score (pain, range of motion [ROM], stability) and Function score (walking, stairs), both of which range from 0 (normal knee) to 100 (most affected knee). Prosthetic infection was classified (Segawa and colleagues15) as early or late, depending on timing of symptom onset (4 weeks after replacement surgery was the threshold used).
Patients received an intravenous bolus infusion of the deficient factor concentrate followed by continuous infusion to reach a plasma factor level of 100% just before surgery and during the first 7 postoperative days and 50% over the next 7 days (Table 1). Patients with a circulating inhibitor (3 overall) received bypassing agents FEIBA (FVIII inhibitor bypassing agent) or rFVIIa (recombinant factor VII activated) (Table 2). Patients were not given any antifibrinolytic treatment or thromboprophylaxis.
Surgery was performed in a standard surgical room. Patients were placed on the operating table in decubitus supinus position. A parapatellar medial incision was made on a bloodless surgical field (achieved with tourniquet ischemia). The prosthesis model used was always the cemented (gentamicin bone cement) NexGen (Zimmer). Patellar resurfacing was done in all cases (Figures 1A–1D). All TKAs were performed by Dr. Rodríguez-Merchán. Intravenous antibiotic prophylaxis was administered at anesthetic induction and during the first 48 hours after surgery (3 further doses). Active exercises were started on postoperative day 1. Joint load aided with 2 crutches was allowed starting on postoperative day 2.
Mean patient age was 38.2 years (range, 24-73 years). Of the 74 patients, 55 had a diagnosis of severe hemophilia A, and 19 had a diagnosis of severe hemophilia B. During the follow-up period, 23 patients died (mean time, 6.4 years; range, 4-9 years). Causes of death were acquired immune deficiency syndrome (AIDS), liver cirrhosis, and intracranial bleeding. Mean follow-up for the full series of patients was 8 years (range, 1-13 years).
Descriptive statistical analysis was performed with SPSS Windows Version 18.0. Prosthetic failure was regarded as implant removal for any reason. Student t test was used to compare continuous variables, and either χ2 test or Fisher exact test was used to compare categorical variables. P < .05 (2-sided) was considered significant.
Results
Prosthetic survival rates with implant removal for any reason regarded as final endpoint was 92%. Causes of failure were prosthetic infection (6 cases, 6.8%) and loosening (2 cases, 2.2%). Of the 6 prosthetic infections, 5 were regarded as late and 1 as early. Late infections were successfully sorted by performing 2-stage revision TKA with the Constrained Condylar Knee (Zimmer). Acute infections were managed by open joint débridement and polyethylene exchange. Both cases of aseptic loosening of the TKA were successfully managed with 1-stage revision TKA using the same implant model (Figures 2A–2D).
Mean KSS Knee score improved from 79 before surgery to 36 after surgery, and mean KSS Function score improved from 63 to 33. KSS Pain score, which is included in the Knee score, 0 (no pain) to 50 (most severe pain), improved from 47 to 8. Patients receiving inhibitors and patients who were HIV- or HCV-positive did not have poorer outcomes relative to those of patients not receiving inhibitors and patients who were HIV- or HCV-negative. Patients with liver cirrhosis had a lower prosthetic survival rate and lower Knee scores.
Discussion
The prosthetic survival rate found in this study compares well with other reported rates for patients with hemophilia and other bleeding disorders. However, evidence regarding long-term prosthesis survival in TKAs performed for patients with hemophilia is limited. Table 3 summarizes the main reported series of patients with hemophilia with 10-year prosthetic survival rates, number of TKAs performed, and mean follow-up period; in all these series, implant removal for any reason was regarded as the final endpoint.5-8,16,17 Mean follow-up in our study was 8 years. Clinical outcomes of TKA in patients with severe hemophilia and related disorders are expected to be inferior to those achieved in patients without a bleeding condition. The overall 10-year prosthetic survival rate for cemented TKA implants, as reported by the Norwegian Arthroplasty Register, was on the order of 93%.18 Mean age of our patients at time of surgery was only 38.2 years. TKAs performed in younger patients without a bleeding disorder have been associated with shorter implant survival times relative to those of elderly patients.19 Thus, Diduch and colleagues20 reported a prosthetic survival rate of 87% at 18 years in 108 TKAs performed on patients under age 55 years. Lonner and colleagues21 reported a better implant survival rate (90% at 8 years) in a series of patients under age 40 years (32 TKAs). In a study by Duffy and colleagues,22 the implant survival rate was 85% at 15 years in patients under age 55 years (74 TKAs). The results from our retrospective case assessment are quite similar to the overall prosthetic survival rates reported for TKAs performed on patients without hemophilia.
Rates of periprosthetic infection after primary TKA in patients with hemophilia and other bleeding conditions are much higher (up to 11%), with a mean infection rate of 6.2% (range, 1% to 11%), consistent with the rate found in our series of patients (6.8%)7,16,17,23,24 (Table 4). This rate is much higher than that reported after primary TKA in patients without hemophilia but is similar to some rates reported for patients with hemophilia. In our experience, most periprosthetic infections (5/6) were sorted as late.
Late infection is a major concern after TKA in patients with hemophilia, and various factors have been hypothesized as contributing to the high prevalence. An important factor is the high rate of HIV-positive patients among patients with hemophilia—which acts as a strong predisposing factor because of the often low CD4 counts and associated immune deficiency,25 but different reports have provided conflicting results in this respect.5,6,12 We found no relationship between HIV status and risk for periprosthetic infection, but conclusions are limited by the low number of HIV-positive patients in our series (14/74, 18.9%). Our patients’ late periprosthetic infections were diagnosed several years after TKA, suggesting hematogenous spread of infection. Most of these patients either were on regular prophylactic factor infusions or were being treated on demand, which might entail a risk for contamination of infusions by skin bacteria from the puncture site. Therefore, having an aseptic technique for administering coagulation factor concentrates is of paramount importance for patients with hemophilia and a knee implant.
Another important complication of TKA surgery is aseptic loosening of the prosthesis. Aseptic loosening occurred in 2.2% of our patients, but higher rates have been reported elsewhere.11,26 Rates of this complication increase over follow-up, and some authors have linked this complication to TKA polyethylene wear.27 Development of a reactive and destructive bone–cement interface and microhemorrhages into such interface might be implicated in the higher rate of loosening observed among patients with hemophilia.28
In the present study, preoperative and postoperative functional outcomes differed significantly. A modest postoperative total ROM of 69º to 79º has been reported by several authors.5,6 Postoperative ROM may vary—may be slightly increased, remain unchanged, or may even be reduced.4,23,26 Even though little improvement in total ROM is achieved after TKA, many authors have reported reduced flexion contracture and hence an easier gait. However, along with functional improvement, dramatic pain relief after TKA is perhaps the most remarkable aspect, and it has a strong effect on patient satisfaction after surgery.5,7,8,18,23
Our study had 2 main limitations. First, it was a retrospective case series evaluation with the usual issues of potential inaccuracy of medical records and information bias. Second, the study did not include a control group.
Conclusion
The primary TKAs performed in our patients with hemophilia have had a good prosthetic survival rate. Even though such a result is slightly inferior to results in patients without hemophilia, our prosthetic survival rate is not significantly different from the rates reported in other, younger patient subsets. Late periprosthetic infections are a major concern, and taking precautions to avoid hematogenous spread of infections during factor concentrate infusions is strongly encouraged.
Chronic hemophilic arthropathy, a well-known complication of hemophilia, develops as a long-term consequence of recurrent joint bleeds resulting in synovial hypertrophy (chronic proliferative synovitis) and joint cartilage destruction. Hemophilic arthropathy mostly affects the knees, ankles, and elbows and causes chronic joint pain and functional impairment in relatively young patients who have not received adequate primary prophylactic replacement therapy with factor concentrates from early childhood.1-3
In the late stages of hemophilic arthropathy of the knee, total knee arthroplasty (TKA) provides dramatic joint pain relief, improves knee functional status, and reduces rebleeding into the joint.4-8 TKA performed on a patient with hemophilia was first reported in the mid-1970s.9,10 In these cases, the surgical procedure itself is often complicated by severe fibrosis developing in the joint soft tissues, flexion joint contracture, and poor quality of the joint bone structures. Even though TKA significantly reduces joint pain in patients with chronic hemophilic arthropathy, some authors have achieved only modest functional outcomes and experienced a high rate of complications (infection, prosthetic loosening).11-13 Data on TKA outcomes are still scarce, and most studies have enrolled a limited number of patients.
We retrospectively evaluated the outcomes of 88 primary TKAs performed on patients with severe hemophilia at a single institution. Clinical outcomes and complications were assessed with a special focus on prosthetic survival and infection.
Patients and Methods
Ninety-one primary TKAs were performed in 77 patients with severe hemophilia A and B (factor VIII [FVIII] and factor IX plasma concentration, <1% each) between January 1, 1999, and December 31, 2011, and the medical records of all these patients were thoroughly reviewed in 2013. The cases of 3 patients who died shortly after surgery were excluded from analysis. Thus, 88 TKAs and 74 patients (74 males) were finally available for evaluation. Fourteen patients underwent bilateral TKAs but none concurrently. The patients provided written informed consent for print and electronic publication of their outcomes.
We recorded demographic data, type and severity of hemophilia, human immunodeficiency virus (HIV) status, hepatitis C virus (HCV) status, and Knee Society Scale (KSS) scores.14 KSS scores include Knee score (pain, range of motion [ROM], stability) and Function score (walking, stairs), both of which range from 0 (normal knee) to 100 (most affected knee). Prosthetic infection was classified (Segawa and colleagues15) as early or late, depending on timing of symptom onset (4 weeks after replacement surgery was the threshold used).
Patients received an intravenous bolus infusion of the deficient factor concentrate followed by continuous infusion to reach a plasma factor level of 100% just before surgery and during the first 7 postoperative days and 50% over the next 7 days (Table 1). Patients with a circulating inhibitor (3 overall) received bypassing agents FEIBA (FVIII inhibitor bypassing agent) or rFVIIa (recombinant factor VII activated) (Table 2). Patients were not given any antifibrinolytic treatment or thromboprophylaxis.
Surgery was performed in a standard surgical room. Patients were placed on the operating table in decubitus supinus position. A parapatellar medial incision was made on a bloodless surgical field (achieved with tourniquet ischemia). The prosthesis model used was always the cemented (gentamicin bone cement) NexGen (Zimmer). Patellar resurfacing was done in all cases (Figures 1A–1D). All TKAs were performed by Dr. Rodríguez-Merchán. Intravenous antibiotic prophylaxis was administered at anesthetic induction and during the first 48 hours after surgery (3 further doses). Active exercises were started on postoperative day 1. Joint load aided with 2 crutches was allowed starting on postoperative day 2.
Mean patient age was 38.2 years (range, 24-73 years). Of the 74 patients, 55 had a diagnosis of severe hemophilia A, and 19 had a diagnosis of severe hemophilia B. During the follow-up period, 23 patients died (mean time, 6.4 years; range, 4-9 years). Causes of death were acquired immune deficiency syndrome (AIDS), liver cirrhosis, and intracranial bleeding. Mean follow-up for the full series of patients was 8 years (range, 1-13 years).
Descriptive statistical analysis was performed with SPSS Windows Version 18.0. Prosthetic failure was regarded as implant removal for any reason. Student t test was used to compare continuous variables, and either χ2 test or Fisher exact test was used to compare categorical variables. P < .05 (2-sided) was considered significant.
Results
Prosthetic survival rates with implant removal for any reason regarded as final endpoint was 92%. Causes of failure were prosthetic infection (6 cases, 6.8%) and loosening (2 cases, 2.2%). Of the 6 prosthetic infections, 5 were regarded as late and 1 as early. Late infections were successfully sorted by performing 2-stage revision TKA with the Constrained Condylar Knee (Zimmer). Acute infections were managed by open joint débridement and polyethylene exchange. Both cases of aseptic loosening of the TKA were successfully managed with 1-stage revision TKA using the same implant model (Figures 2A–2D).
Mean KSS Knee score improved from 79 before surgery to 36 after surgery, and mean KSS Function score improved from 63 to 33. KSS Pain score, which is included in the Knee score, 0 (no pain) to 50 (most severe pain), improved from 47 to 8. Patients receiving inhibitors and patients who were HIV- or HCV-positive did not have poorer outcomes relative to those of patients not receiving inhibitors and patients who were HIV- or HCV-negative. Patients with liver cirrhosis had a lower prosthetic survival rate and lower Knee scores.
Discussion
The prosthetic survival rate found in this study compares well with other reported rates for patients with hemophilia and other bleeding disorders. However, evidence regarding long-term prosthesis survival in TKAs performed for patients with hemophilia is limited. Table 3 summarizes the main reported series of patients with hemophilia with 10-year prosthetic survival rates, number of TKAs performed, and mean follow-up period; in all these series, implant removal for any reason was regarded as the final endpoint.5-8,16,17 Mean follow-up in our study was 8 years. Clinical outcomes of TKA in patients with severe hemophilia and related disorders are expected to be inferior to those achieved in patients without a bleeding condition. The overall 10-year prosthetic survival rate for cemented TKA implants, as reported by the Norwegian Arthroplasty Register, was on the order of 93%.18 Mean age of our patients at time of surgery was only 38.2 years. TKAs performed in younger patients without a bleeding disorder have been associated with shorter implant survival times relative to those of elderly patients.19 Thus, Diduch and colleagues20 reported a prosthetic survival rate of 87% at 18 years in 108 TKAs performed on patients under age 55 years. Lonner and colleagues21 reported a better implant survival rate (90% at 8 years) in a series of patients under age 40 years (32 TKAs). In a study by Duffy and colleagues,22 the implant survival rate was 85% at 15 years in patients under age 55 years (74 TKAs). The results from our retrospective case assessment are quite similar to the overall prosthetic survival rates reported for TKAs performed on patients without hemophilia.
Rates of periprosthetic infection after primary TKA in patients with hemophilia and other bleeding conditions are much higher (up to 11%), with a mean infection rate of 6.2% (range, 1% to 11%), consistent with the rate found in our series of patients (6.8%)7,16,17,23,24 (Table 4). This rate is much higher than that reported after primary TKA in patients without hemophilia but is similar to some rates reported for patients with hemophilia. In our experience, most periprosthetic infections (5/6) were sorted as late.
Late infection is a major concern after TKA in patients with hemophilia, and various factors have been hypothesized as contributing to the high prevalence. An important factor is the high rate of HIV-positive patients among patients with hemophilia—which acts as a strong predisposing factor because of the often low CD4 counts and associated immune deficiency,25 but different reports have provided conflicting results in this respect.5,6,12 We found no relationship between HIV status and risk for periprosthetic infection, but conclusions are limited by the low number of HIV-positive patients in our series (14/74, 18.9%). Our patients’ late periprosthetic infections were diagnosed several years after TKA, suggesting hematogenous spread of infection. Most of these patients either were on regular prophylactic factor infusions or were being treated on demand, which might entail a risk for contamination of infusions by skin bacteria from the puncture site. Therefore, having an aseptic technique for administering coagulation factor concentrates is of paramount importance for patients with hemophilia and a knee implant.
Another important complication of TKA surgery is aseptic loosening of the prosthesis. Aseptic loosening occurred in 2.2% of our patients, but higher rates have been reported elsewhere.11,26 Rates of this complication increase over follow-up, and some authors have linked this complication to TKA polyethylene wear.27 Development of a reactive and destructive bone–cement interface and microhemorrhages into such interface might be implicated in the higher rate of loosening observed among patients with hemophilia.28
In the present study, preoperative and postoperative functional outcomes differed significantly. A modest postoperative total ROM of 69º to 79º has been reported by several authors.5,6 Postoperative ROM may vary—may be slightly increased, remain unchanged, or may even be reduced.4,23,26 Even though little improvement in total ROM is achieved after TKA, many authors have reported reduced flexion contracture and hence an easier gait. However, along with functional improvement, dramatic pain relief after TKA is perhaps the most remarkable aspect, and it has a strong effect on patient satisfaction after surgery.5,7,8,18,23
Our study had 2 main limitations. First, it was a retrospective case series evaluation with the usual issues of potential inaccuracy of medical records and information bias. Second, the study did not include a control group.
Conclusion
The primary TKAs performed in our patients with hemophilia have had a good prosthetic survival rate. Even though such a result is slightly inferior to results in patients without hemophilia, our prosthetic survival rate is not significantly different from the rates reported in other, younger patient subsets. Late periprosthetic infections are a major concern, and taking precautions to avoid hematogenous spread of infections during factor concentrate infusions is strongly encouraged.
1. Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977;59(3):287-305.
2. Rodriguez-Merchan EC. Common orthopaedic problems in haemophilia. Haemophilia. 1999;5(suppl 1):53-60.
3. Steen Carlsson K, Höjgård S, Glomstein A, et al. On-demand vs. prophylactic treatment for severe haemophilia in Norway and Sweden: differences in treatment characteristics and outcome. Haemophilia. 2003;9(5):555-566.
4. Teigland JC, Tjønnfjord GE, Evensen SA, Charania B. Knee arthroplasty in hemophilia. 5-12 year follow-up of 15 patients. Acta Orthop Scand. 1993;64(2):153-156.
5. Silva M, Luck JV Jr. Long-term results of primary total knee replacement in patients with hemophilia. J Bone Joint Surg Am. 2005;87(1):85-91.
6. Wang K, Street A, Dowrick A, Liew S. Clinical outcomes and patient satisfaction following total joint replacement in haemophilia—23-year experience in knees, hips and elbows. Haemophilia. 2012;18(1):86-93.
7. Chevalier Y, Dargaud Y, Lienhart A, Chamouard V, Negrier C. Seventy-two total knee arthroplasties performed in patients with haemophilia using continuous infusion. Vox Sang. 2013;104(2):135-143.
8. Zingg PO, Fucentese SF, Lutz W, Brand B, Mamisch N, Koch PP. Haemophilic knee arthropathy: long-term outcome after total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2465-2470.
9. Kjaersgaard-Andersen P, Christiansen SE, Ingerslev J, Sneppen O. Total knee arthroplasty in classic hemophilia. Clin Orthop Relat Res. 1990;(256):137-146.
10. Cohen I, Heim M, Martinowitz U, Chechick A. Orthopaedic outcome of total knee replacement in haemophilia A. Haemophilia. 2000;6(2):104-109.
11. Fehily M, Fleming P, O’Shea E, Smith O, Smyth H. Total knee arthroplasty in patients with severe haemophilia. Int Orthop. 2002;26(2):89-91.
12. Legroux-Gérot I, Strouk G, Parquet A, Goodemand J, Gougeon F, Duquesnoy B. Total knee arthroplasty in hemophilic arthropathy. Joint Bone Spine. 2003;70(1):22-32.
13. Sheth DS, Oldfield D, Ambrose C, Clyburn T. Total knee arthroplasty in hemophilic arthropathy. J Arthroplasty. 2004;19(1):56-60.
14. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
15. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
16. Goddard NJ, Mann HA, Lee CA. Total knee replacement in patients with end-stage haemophilic arthropathy. 25-year results. J Bone Joint Surg Br. 2010;92(8):1085-1089.
17. Westberg M, Paus AC, Holme PA, Tjønnfjord GE. Haemophilic arthropathy: long-term outcomes in 107 primary total knee arthroplasties. Knee. 2014;21(1):147-150.
18. Lygre SH, Espehaug B, Havelin LI, Vollset SE, Furnes O. Failure of total knee arthroplasty with or without patella resurfacing. A study from the Norwegian Arthroplasty Register with 0-15 years of follow-up. Acta Orthop. 2011;82(3):282-292.
19. Post M, Telfer MC. Surgery in hemophilic patients. J Bone Joint Surg Am. 1975;57(8):1136-1145.
20. Diduch DR, Insall JN, Scott WN, Scuderi GR, Font-Rodriguez D. Total knee replacement in young, active patients. Long-term follow-up and functional outcome. J Bone Joint Surg Am. 1997;79(4):575-582.
21. Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000;(380):85-90.
22. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 suppl 2):67-70.
23. Chiang CC, Chen PQ, Shen MC, Tsai W. Total knee arthroplasty for severe haemophilic arthropathy: long-term experience in Taiwan. Haemophilia. 2008;14(4):828-834.
24. Solimeno LP, Mancuso ME, Pasta G, Santagostino E, Perfetto S, Mannucci PM. Factors influencing the long-term outcome of primary total knee replacement in haemophiliacs: a review of 116 procedures at a single institution. Br J Haematol. 2009;145(2):227-234.
25. Jämsen E, Varonen M, Huhtala H, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty. 2010;25(1):87-92.
26. Ragni MV, Crossett LS, Herndon JH. Postoperative infection following orthopaedic surgery in human immunodeficiency virus–infected hemophiliacs with CD4 counts < or = 200/mm3. J Arthroplasty. 1995;10(6):716-721.
27. Hicks JL, Ribbans WJ, Buzzard B, et al. Infected joint replacements in HIV-positive patients with haemophilia. J Bone Joint Surg Br. 2001;83(7):1050-1054.
28. Figgie MP, Goldberg VM, Figgie HE 3rd, Heiple KG, Sobel M. Total knee arthroplasty for the treatment of chronic hemophilic arthropathy. Clin Orthop Relat Res. 1989;(248):98-107.
1. Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977;59(3):287-305.
2. Rodriguez-Merchan EC. Common orthopaedic problems in haemophilia. Haemophilia. 1999;5(suppl 1):53-60.
3. Steen Carlsson K, Höjgård S, Glomstein A, et al. On-demand vs. prophylactic treatment for severe haemophilia in Norway and Sweden: differences in treatment characteristics and outcome. Haemophilia. 2003;9(5):555-566.
4. Teigland JC, Tjønnfjord GE, Evensen SA, Charania B. Knee arthroplasty in hemophilia. 5-12 year follow-up of 15 patients. Acta Orthop Scand. 1993;64(2):153-156.
5. Silva M, Luck JV Jr. Long-term results of primary total knee replacement in patients with hemophilia. J Bone Joint Surg Am. 2005;87(1):85-91.
6. Wang K, Street A, Dowrick A, Liew S. Clinical outcomes and patient satisfaction following total joint replacement in haemophilia—23-year experience in knees, hips and elbows. Haemophilia. 2012;18(1):86-93.
7. Chevalier Y, Dargaud Y, Lienhart A, Chamouard V, Negrier C. Seventy-two total knee arthroplasties performed in patients with haemophilia using continuous infusion. Vox Sang. 2013;104(2):135-143.
8. Zingg PO, Fucentese SF, Lutz W, Brand B, Mamisch N, Koch PP. Haemophilic knee arthropathy: long-term outcome after total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2465-2470.
9. Kjaersgaard-Andersen P, Christiansen SE, Ingerslev J, Sneppen O. Total knee arthroplasty in classic hemophilia. Clin Orthop Relat Res. 1990;(256):137-146.
10. Cohen I, Heim M, Martinowitz U, Chechick A. Orthopaedic outcome of total knee replacement in haemophilia A. Haemophilia. 2000;6(2):104-109.
11. Fehily M, Fleming P, O’Shea E, Smith O, Smyth H. Total knee arthroplasty in patients with severe haemophilia. Int Orthop. 2002;26(2):89-91.
12. Legroux-Gérot I, Strouk G, Parquet A, Goodemand J, Gougeon F, Duquesnoy B. Total knee arthroplasty in hemophilic arthropathy. Joint Bone Spine. 2003;70(1):22-32.
13. Sheth DS, Oldfield D, Ambrose C, Clyburn T. Total knee arthroplasty in hemophilic arthropathy. J Arthroplasty. 2004;19(1):56-60.
14. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
15. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
16. Goddard NJ, Mann HA, Lee CA. Total knee replacement in patients with end-stage haemophilic arthropathy. 25-year results. J Bone Joint Surg Br. 2010;92(8):1085-1089.
17. Westberg M, Paus AC, Holme PA, Tjønnfjord GE. Haemophilic arthropathy: long-term outcomes in 107 primary total knee arthroplasties. Knee. 2014;21(1):147-150.
18. Lygre SH, Espehaug B, Havelin LI, Vollset SE, Furnes O. Failure of total knee arthroplasty with or without patella resurfacing. A study from the Norwegian Arthroplasty Register with 0-15 years of follow-up. Acta Orthop. 2011;82(3):282-292.
19. Post M, Telfer MC. Surgery in hemophilic patients. J Bone Joint Surg Am. 1975;57(8):1136-1145.
20. Diduch DR, Insall JN, Scott WN, Scuderi GR, Font-Rodriguez D. Total knee replacement in young, active patients. Long-term follow-up and functional outcome. J Bone Joint Surg Am. 1997;79(4):575-582.
21. Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000;(380):85-90.
22. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 suppl 2):67-70.
23. Chiang CC, Chen PQ, Shen MC, Tsai W. Total knee arthroplasty for severe haemophilic arthropathy: long-term experience in Taiwan. Haemophilia. 2008;14(4):828-834.
24. Solimeno LP, Mancuso ME, Pasta G, Santagostino E, Perfetto S, Mannucci PM. Factors influencing the long-term outcome of primary total knee replacement in haemophiliacs: a review of 116 procedures at a single institution. Br J Haematol. 2009;145(2):227-234.
25. Jämsen E, Varonen M, Huhtala H, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty. 2010;25(1):87-92.
26. Ragni MV, Crossett LS, Herndon JH. Postoperative infection following orthopaedic surgery in human immunodeficiency virus–infected hemophiliacs with CD4 counts < or = 200/mm3. J Arthroplasty. 1995;10(6):716-721.
27. Hicks JL, Ribbans WJ, Buzzard B, et al. Infected joint replacements in HIV-positive patients with haemophilia. J Bone Joint Surg Br. 2001;83(7):1050-1054.
28. Figgie MP, Goldberg VM, Figgie HE 3rd, Heiple KG, Sobel M. Total knee arthroplasty for the treatment of chronic hemophilic arthropathy. Clin Orthop Relat Res. 1989;(248):98-107.
Orthopedic Practice Patterns Relating to Anterior Cruciate Ligament Reconstruction in Elite Athletes
National Hockey League (NHL), Major League Soccer (MLS), and US Olympic/World Cup Ski/Snowboard (Olympic) athletes receive orthopedic care from a select group of surgeons. There are 30 NHL teams, 19 MLS teams, 1 Olympic ski team, and 1 Olympic snowboard team, for a total of 51 teams and a rough total of 2229 athletes (1500 NHL, 570 MLS, 159 Olympic).1
Studies have shown that MLS athletes and X-Game skiers and snowboarders have performed well on return to sport (RTS) after anterior cruciate ligament (ACL) reconstruction.2,3 However, the techniques, graft choices, and rehabilitation protocols used to return these elite athletes to their preinjury level of performance have not been elucidated. It is unclear if the treatment given to these elite athletes differs from that given to recreational athletes and nonathletes. Bradley and colleagues4 examined how 32 NFL team orthopedists treated ACL tears, and Erickson and colleagues5 recently surveyed NFL and National Collegiate Athletic Association (NCAA) team physicians to determine practice patterns (eg, surgical techniques, graft choices, postoperative protocols) in treating ACL tears. Until now, however, no one has examined NHL, MLS, or Olympic team orthopedic surgeons’ practice patterns as they relate to ACL reconstruction.
We conducted an online survey of NHL, MLS, and Olympic team orthopedic surgeons to determine practice patterns relating to ACL reconstruction in elite athletes. Given the practice patterns of surgeons in our practice, we hypothesized that the surveyed surgeons treating these elite athletes would most commonly use bone–patellar tendon–bone (BPTB) autograft with a single-bundle technique. We also hypothesized that they would permit RTS without a brace at a minimum of 6 months after surgery, with a normal physical examination, and after successful completion of a structured battery of RTS tests.
Materials and Methods
On the SurveyMonkey website (http://www.surveymonkey.com), we created a 7-question base survey, with other questions added for the NHL and MLS surveys (Figure 1). We sent this survey to 94 team orthopedic surgeons (41 NHL, 26 MLS, 27 Olympic) identified through Internet searches and direct contact with team public relations departments. The survey was approved by MLS and NHL research committees. In 2013, each survey was sent out 5 times. The response rates for each round are shown in Figure 2. All responses remained confidential; we did not learn surgeons’ identities. Data were collected and analyzed through the SurveyMonkey website. Each surgeon was instructed to respond to all relevant questions in the survey. The survey was designed such that the participant could not submit the survey without answering all the questions. Descriptive statistics were calculated for each study and parameter analyzed. Continuous variable data are reported as means and standard deviations (weighted means where applicable). Categorical data are reported as frequencies with percentages.
Results
Of the 94 team orthopedic surgeons surveyed, 47 (50%) responded (NHL, 49%; MLS, 50%; Olympic, 52%). Mean (SD) experience as a team physician was 7.73 (5.33) years (range, 2-20 years) for NHL, 6.77 (6.64) years (range, 2-20 years) for MLS, and 1.14 (0.36) years (range, 1-10 years) for Olympic. Mean (SD) number of ACL reconstructions performed in 2012 was 101 (51) for NHL (range, 50-200), 78 (38) for MLS (range, 20-150), and 110 (105) for Olympic (range, 25-175) (Table 1). Of the 47 surgeons, 42 (89.4%) used autograft in the treatment of elite athletes, and 5 (10.6%) used allograft. Autograft choices were BPTB (n = 33; 70.2%), 4-strand semitendinosus (n = 7; 14.9%), and quadriceps (n = 2; 4.3%); allograft choices were 4-strand semitendinosus (n = 4; 8.5%) and BPTB (n = 1; 2.1%) (Table 2).
Of the 40 surgeons (85.1%) who indicated they would use autograft in 25-year-old recreational athletes, 25 (53.2%) would use BPTB, 13 (27.7%) would use 4-strand semitendinosus, and 2 (4.3%) would use quadriceps; of the 7 who indicated they would use allograft, 4 (8.5%) would use 4-strand semitendinosus, and 3 (6.4%) would use BPTB. In the NHL and MLS surveys, 19 surgeons (57.6%) indicated they would use autograft (6 would use BPTB, 13 would use 4-strand semitendinosus), and 14 (42.4%) would use allograft (7 would use BPTB, 5 would use Achilles, and 2 would use tibialis anterior) in 35-year-old recreational athletes.
Twenty-one surgeons (44.7%) were drilling the femoral tunnel through a transtibial portal, 36.2% through an anteromedial portal, and 12.8% using a 2-incision technique. All surgeons indicated they were using a single-bundle technique in ACL reconstruction. Thirty-three surgeons (70.2%) did not recommend a brace for their elite athletes on RTS. Olympic team surgeons had the highest rate of brace wear in RTS (50%, both skiers and snowboarders); NHL and MLS surgeons had significantly lower rates (25% and 15.4%, respectively) (Table 3).
Twenty (60.6%) of the NHL and MLS surgeons recommended waiting at least 6 months before RTS; 2 (6.1%) recommended waiting at least 9 months; no surgeon recommended waiting at least 12 months; and the others did not have a specific time frame for RTS. Twenty-seven surgeons (81.8%) recommended RTS after an athlete passed a series of RTS tests (eg, Vail, single-leg hop). Nineteen surgeons (57.6%) recommended waiting until the athlete had full range of motion, no pain, full strength, and subjective stability in the knee. Physicians could choose more than one answer for the previous question, allowing for a total percentage higher than 100%.
Discussion
The goal of this study was to determine how NHL, MLS, and Olympic team orthopedic surgeons manage ACL tears in elite and recreational athletes. Our study hypotheses were confirmed, as 70.2% of those surveyed used BPTB autograft for elite athletes, 100% used the single-bundle technique, 70.2% did not require a brace on RTS, 81.8% recommended RTS after the athlete passed a series of RTS tests (eg, Vail, single-leg hop), and 60.6% waited at least 6 months after surgery.
As soccer and skiing are the top 2 sports in which participants sustain ACL tears, it is necessary to report how surgeons obtain successful results in these patient populations.6 Using the US and Norwegian ACL reconstruction registries, Granan and colleagues6 found that, over a 7-year period, 5760 ACL tears occurred during soccer, and 2030 occurred during skiing. The scope of ACL injuries is broad, and treatment patterns must be elucidated. Although most surgeons do not treat elite athletes, many high school and college athletes compete at very high levels. Therefore, replicating the methods of the surgeons who treat elite athletes may be warranted.
In our survey, autograft (89.4%), particularly BPTB autograft (70.2%), was the most common graft choice for elite athletes. The rate of allograft use (42.4%) was higher for 35-year-old recreational athletes. As BPTB autograft produces reliable long-term results, this graft type is a reasonable choice.7 However, only 18% of our surveyed orthopedic surgeons indicated they would use BPTB autograft in older, recreational athletes. This stark difference is likely related to the more than 40% long-term side effects of anterior knee pain and graft harvest site morbidity with BPTB autograft as opposed to allograft and other types of autograft.8,9 Younger patients may be more willing to accept some anterior knee pain to ensure bone-to-bone healing with BPTB autograft. This shift in graft choice may also reflect the desire to minimize skin incisions and their resulting scars, especially in female recreational athletes.
In a meta-analysis of more than 5000 patients, Kraeutler and colleagues7 found that BPTB autograft outperformed allograft according to several knee scores, including Lysholm and Tegner, and had a lower re-rupture rate (4.3% vs 12.7%). However, despite the superior performance of BPTB autograft, graft choice cannot overcome surgeon error in graft placement.10 BPTB autograft appears to remain the gold standard for ACL reconstruction for many reasons, including low failure rates and decreased costs.11 Recently, investigators have tried to challenge the superiority of BPTB autograft. In a retrospective case–control study, Mascarenhas and colleagues12 found that hamstring autograft afforded patients better extension and higher subjective outcome scores. Bourke and colleagues13 found a higher rate of contralateral ACL rupture in patients treated with BPTB autograft compared with hamstring autograft.
According to this survey, 44.7% of surgeons indicated they drilled the femoral tunnel through a transtibial portal, 36.2% used an anteromedial portal, and 12.8% used the 2-incision technique. These methods were recently evaluated to determine if any is superior to the others, but the study results were not definitive.14 Franceschi and colleagues15 found improved rotational and anterior stability of the knee with use of an anteromedial approach, but their findings were not clinically or functionally significant. Wang and colleagues16 found an extension loss in the late-stance phase of gait with the anteromedial approach; the transtibial approach was correlated with inferior anterior-posterior stability during the stance phase of gait. Therefore, our results parallel those in the current literature in that the surveyed population is split on which technique to use and likely bases its practice on comfort level and residency/fellowship training.
Limitations
This study had several limitations. First, it provided level V evidence of team physicians in 3 major sports. Although some of these physicians were also treating athletes in other sports, our survey targeted NHL, MLS, and Olympic athletes. It did not address all ages and both sexes—which is significant, given the higher rate of ACL tears in females. All NHL and MLS players are male, and there was a high rate of BPTB graft use in these sports. However, recreational athletes include both males and females, and the fact that some surgeons would choose a hamstring graft for a female for cosmetic reasons must not be overlooked. Conversely, that there was no difference in the number of BPTB autografts chosen between NHL and MLS surgeons versus Olympic surgeons, where females are included (all chose about 60% BPTB autografts for their elite athletes), disputes this limitation. Our survey response rate was 50%. Other studies have had similar rates in relation to ACL practices,17 especially elite team physicians’ practices,5 and recent literature has confirmed that lower response rates in surveys did not alter results and may in fact have improved results.18,19 This percentage could be falsely low if some of our email addresses were incorrect. This rate also raises the possibility of selection bias, as surgeons who routinely used allograft in their athlete population may not have wanted to admit this. It is possible that some NHL, MLS, and Olympic athletes were treated by surgeons not included in this survey (in some cases, a non–team surgeon may have performed the athlete’s surgery). This survey did not address concomitant knee pathology or cover all possible technique variables.
Conclusion
Most of the NHL, MLS, and Olympic team orthopedic surgeons who were surveyed perform their ACL reconstructions using BPTB autograft, using a single-bundle technique, through a transtibial portal, and do not require bracing for their athletes returning to sport. Most required their athletes to complete a series of RTS tests before resuming competitive play.
1. Team USA. 2013. US Olympic Committee website. http://www.teamusa.org/athletes?pg=1&seasonId=%7BCF2DC66A-C2B3-44A8-ABB8-A486F3FBFDDF%7D&ngbId=%7BB36167A0-2AC8-4B0F-876F-93D0A44DF60A%7D. Accessed October 23, 2015.
2. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):1-8.
3. Erickson BJ, Harris JD, Fillingham YA, et al. Performance and return to sport after anterior cruciate ligament reconstruction in X-Games skiers and snowboarders. Orthop J Sports Med. 2013;1(6):1-5.
4. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
5. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
6. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
7. Kraeutler MJ, Bravman JT, McCarty EC. Bone–patellar tendon–bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
8. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
9. Kartus J, Magnusson L, Stener S, Brandsson S, Eriksson BI, Karlsson J. Complications following arthroscopic anterior cruciate ligament reconstruction. A 2-5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(1):2-8.
10. Boszotta H. Arthroscopic anterior cruciate ligament reconstruction using a patellar tendon graft in press-fit technique: surgical technique and follow-up. Arthroscopy. 1997;13(3):332-339.
11. Hospodar SJ, Miller MD. Controversies in ACL reconstruction: bone–patellar tendon–bone anterior cruciate ligament reconstruction remains the gold standard. Sports Med Arthrosc Rev. 2009;17(4):242-246.
12. Mascarenhas R, Tranovich MJ, Kropf EJ, Fu FH, Harner CD. Bone–patellar tendon–bone autograft versus hamstring autograft anterior cruciate ligament reconstruction in the young athlete: a retrospective matched analysis with 2-10 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1520-1527.
13. Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985-1992.
14. Chalmers PN, Mall NA, Cole BJ, Verma NN, Bush-Joseph CA, Bach BR Jr. Anteromedial versus transtibial tunnel drilling in anterior cruciate ligament reconstructions: a systematic review. Arthroscopy. 2013;29(7):1235-1242.
15. Franceschi F, Papalia R, Rizzello G, Del Buono A, Maffulli N, Denaro V. Anteromedial portal versus transtibial drilling techniques in anterior cruciate ligament reconstruction: any clinical relevance? A retrospective comparative study. Arthroscopy. 2013;29(8):1330-1337.
16. Wang H, Fleischli JE, Zheng NN. Transtibial versus anteromedial portal technique in single-bundle anterior cruciate ligament reconstruction: outcomes of knee joint kinematics during walking. Am J Sports Med. 2013;41(8):1847-1856.
17. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
18. Keeter S, Miller C, Kohut A, Groves RM, Presser S. Consequences of reducing nonresponse in a national telephone survey. Public Opin Q. 2000;64(2):125-148.
19. Curtin R, Presser S, Singer E. The effects of response rate changes on the index of consumer sentiment. Public Opin Q. 2000;64(4):413-428.
National Hockey League (NHL), Major League Soccer (MLS), and US Olympic/World Cup Ski/Snowboard (Olympic) athletes receive orthopedic care from a select group of surgeons. There are 30 NHL teams, 19 MLS teams, 1 Olympic ski team, and 1 Olympic snowboard team, for a total of 51 teams and a rough total of 2229 athletes (1500 NHL, 570 MLS, 159 Olympic).1
Studies have shown that MLS athletes and X-Game skiers and snowboarders have performed well on return to sport (RTS) after anterior cruciate ligament (ACL) reconstruction.2,3 However, the techniques, graft choices, and rehabilitation protocols used to return these elite athletes to their preinjury level of performance have not been elucidated. It is unclear if the treatment given to these elite athletes differs from that given to recreational athletes and nonathletes. Bradley and colleagues4 examined how 32 NFL team orthopedists treated ACL tears, and Erickson and colleagues5 recently surveyed NFL and National Collegiate Athletic Association (NCAA) team physicians to determine practice patterns (eg, surgical techniques, graft choices, postoperative protocols) in treating ACL tears. Until now, however, no one has examined NHL, MLS, or Olympic team orthopedic surgeons’ practice patterns as they relate to ACL reconstruction.
We conducted an online survey of NHL, MLS, and Olympic team orthopedic surgeons to determine practice patterns relating to ACL reconstruction in elite athletes. Given the practice patterns of surgeons in our practice, we hypothesized that the surveyed surgeons treating these elite athletes would most commonly use bone–patellar tendon–bone (BPTB) autograft with a single-bundle technique. We also hypothesized that they would permit RTS without a brace at a minimum of 6 months after surgery, with a normal physical examination, and after successful completion of a structured battery of RTS tests.
Materials and Methods
On the SurveyMonkey website (http://www.surveymonkey.com), we created a 7-question base survey, with other questions added for the NHL and MLS surveys (Figure 1). We sent this survey to 94 team orthopedic surgeons (41 NHL, 26 MLS, 27 Olympic) identified through Internet searches and direct contact with team public relations departments. The survey was approved by MLS and NHL research committees. In 2013, each survey was sent out 5 times. The response rates for each round are shown in Figure 2. All responses remained confidential; we did not learn surgeons’ identities. Data were collected and analyzed through the SurveyMonkey website. Each surgeon was instructed to respond to all relevant questions in the survey. The survey was designed such that the participant could not submit the survey without answering all the questions. Descriptive statistics were calculated for each study and parameter analyzed. Continuous variable data are reported as means and standard deviations (weighted means where applicable). Categorical data are reported as frequencies with percentages.
Results
Of the 94 team orthopedic surgeons surveyed, 47 (50%) responded (NHL, 49%; MLS, 50%; Olympic, 52%). Mean (SD) experience as a team physician was 7.73 (5.33) years (range, 2-20 years) for NHL, 6.77 (6.64) years (range, 2-20 years) for MLS, and 1.14 (0.36) years (range, 1-10 years) for Olympic. Mean (SD) number of ACL reconstructions performed in 2012 was 101 (51) for NHL (range, 50-200), 78 (38) for MLS (range, 20-150), and 110 (105) for Olympic (range, 25-175) (Table 1). Of the 47 surgeons, 42 (89.4%) used autograft in the treatment of elite athletes, and 5 (10.6%) used allograft. Autograft choices were BPTB (n = 33; 70.2%), 4-strand semitendinosus (n = 7; 14.9%), and quadriceps (n = 2; 4.3%); allograft choices were 4-strand semitendinosus (n = 4; 8.5%) and BPTB (n = 1; 2.1%) (Table 2).
Of the 40 surgeons (85.1%) who indicated they would use autograft in 25-year-old recreational athletes, 25 (53.2%) would use BPTB, 13 (27.7%) would use 4-strand semitendinosus, and 2 (4.3%) would use quadriceps; of the 7 who indicated they would use allograft, 4 (8.5%) would use 4-strand semitendinosus, and 3 (6.4%) would use BPTB. In the NHL and MLS surveys, 19 surgeons (57.6%) indicated they would use autograft (6 would use BPTB, 13 would use 4-strand semitendinosus), and 14 (42.4%) would use allograft (7 would use BPTB, 5 would use Achilles, and 2 would use tibialis anterior) in 35-year-old recreational athletes.
Twenty-one surgeons (44.7%) were drilling the femoral tunnel through a transtibial portal, 36.2% through an anteromedial portal, and 12.8% using a 2-incision technique. All surgeons indicated they were using a single-bundle technique in ACL reconstruction. Thirty-three surgeons (70.2%) did not recommend a brace for their elite athletes on RTS. Olympic team surgeons had the highest rate of brace wear in RTS (50%, both skiers and snowboarders); NHL and MLS surgeons had significantly lower rates (25% and 15.4%, respectively) (Table 3).
Twenty (60.6%) of the NHL and MLS surgeons recommended waiting at least 6 months before RTS; 2 (6.1%) recommended waiting at least 9 months; no surgeon recommended waiting at least 12 months; and the others did not have a specific time frame for RTS. Twenty-seven surgeons (81.8%) recommended RTS after an athlete passed a series of RTS tests (eg, Vail, single-leg hop). Nineteen surgeons (57.6%) recommended waiting until the athlete had full range of motion, no pain, full strength, and subjective stability in the knee. Physicians could choose more than one answer for the previous question, allowing for a total percentage higher than 100%.
Discussion
The goal of this study was to determine how NHL, MLS, and Olympic team orthopedic surgeons manage ACL tears in elite and recreational athletes. Our study hypotheses were confirmed, as 70.2% of those surveyed used BPTB autograft for elite athletes, 100% used the single-bundle technique, 70.2% did not require a brace on RTS, 81.8% recommended RTS after the athlete passed a series of RTS tests (eg, Vail, single-leg hop), and 60.6% waited at least 6 months after surgery.
As soccer and skiing are the top 2 sports in which participants sustain ACL tears, it is necessary to report how surgeons obtain successful results in these patient populations.6 Using the US and Norwegian ACL reconstruction registries, Granan and colleagues6 found that, over a 7-year period, 5760 ACL tears occurred during soccer, and 2030 occurred during skiing. The scope of ACL injuries is broad, and treatment patterns must be elucidated. Although most surgeons do not treat elite athletes, many high school and college athletes compete at very high levels. Therefore, replicating the methods of the surgeons who treat elite athletes may be warranted.
In our survey, autograft (89.4%), particularly BPTB autograft (70.2%), was the most common graft choice for elite athletes. The rate of allograft use (42.4%) was higher for 35-year-old recreational athletes. As BPTB autograft produces reliable long-term results, this graft type is a reasonable choice.7 However, only 18% of our surveyed orthopedic surgeons indicated they would use BPTB autograft in older, recreational athletes. This stark difference is likely related to the more than 40% long-term side effects of anterior knee pain and graft harvest site morbidity with BPTB autograft as opposed to allograft and other types of autograft.8,9 Younger patients may be more willing to accept some anterior knee pain to ensure bone-to-bone healing with BPTB autograft. This shift in graft choice may also reflect the desire to minimize skin incisions and their resulting scars, especially in female recreational athletes.
In a meta-analysis of more than 5000 patients, Kraeutler and colleagues7 found that BPTB autograft outperformed allograft according to several knee scores, including Lysholm and Tegner, and had a lower re-rupture rate (4.3% vs 12.7%). However, despite the superior performance of BPTB autograft, graft choice cannot overcome surgeon error in graft placement.10 BPTB autograft appears to remain the gold standard for ACL reconstruction for many reasons, including low failure rates and decreased costs.11 Recently, investigators have tried to challenge the superiority of BPTB autograft. In a retrospective case–control study, Mascarenhas and colleagues12 found that hamstring autograft afforded patients better extension and higher subjective outcome scores. Bourke and colleagues13 found a higher rate of contralateral ACL rupture in patients treated with BPTB autograft compared with hamstring autograft.
According to this survey, 44.7% of surgeons indicated they drilled the femoral tunnel through a transtibial portal, 36.2% used an anteromedial portal, and 12.8% used the 2-incision technique. These methods were recently evaluated to determine if any is superior to the others, but the study results were not definitive.14 Franceschi and colleagues15 found improved rotational and anterior stability of the knee with use of an anteromedial approach, but their findings were not clinically or functionally significant. Wang and colleagues16 found an extension loss in the late-stance phase of gait with the anteromedial approach; the transtibial approach was correlated with inferior anterior-posterior stability during the stance phase of gait. Therefore, our results parallel those in the current literature in that the surveyed population is split on which technique to use and likely bases its practice on comfort level and residency/fellowship training.
Limitations
This study had several limitations. First, it provided level V evidence of team physicians in 3 major sports. Although some of these physicians were also treating athletes in other sports, our survey targeted NHL, MLS, and Olympic athletes. It did not address all ages and both sexes—which is significant, given the higher rate of ACL tears in females. All NHL and MLS players are male, and there was a high rate of BPTB graft use in these sports. However, recreational athletes include both males and females, and the fact that some surgeons would choose a hamstring graft for a female for cosmetic reasons must not be overlooked. Conversely, that there was no difference in the number of BPTB autografts chosen between NHL and MLS surgeons versus Olympic surgeons, where females are included (all chose about 60% BPTB autografts for their elite athletes), disputes this limitation. Our survey response rate was 50%. Other studies have had similar rates in relation to ACL practices,17 especially elite team physicians’ practices,5 and recent literature has confirmed that lower response rates in surveys did not alter results and may in fact have improved results.18,19 This percentage could be falsely low if some of our email addresses were incorrect. This rate also raises the possibility of selection bias, as surgeons who routinely used allograft in their athlete population may not have wanted to admit this. It is possible that some NHL, MLS, and Olympic athletes were treated by surgeons not included in this survey (in some cases, a non–team surgeon may have performed the athlete’s surgery). This survey did not address concomitant knee pathology or cover all possible technique variables.
Conclusion
Most of the NHL, MLS, and Olympic team orthopedic surgeons who were surveyed perform their ACL reconstructions using BPTB autograft, using a single-bundle technique, through a transtibial portal, and do not require bracing for their athletes returning to sport. Most required their athletes to complete a series of RTS tests before resuming competitive play.
National Hockey League (NHL), Major League Soccer (MLS), and US Olympic/World Cup Ski/Snowboard (Olympic) athletes receive orthopedic care from a select group of surgeons. There are 30 NHL teams, 19 MLS teams, 1 Olympic ski team, and 1 Olympic snowboard team, for a total of 51 teams and a rough total of 2229 athletes (1500 NHL, 570 MLS, 159 Olympic).1
Studies have shown that MLS athletes and X-Game skiers and snowboarders have performed well on return to sport (RTS) after anterior cruciate ligament (ACL) reconstruction.2,3 However, the techniques, graft choices, and rehabilitation protocols used to return these elite athletes to their preinjury level of performance have not been elucidated. It is unclear if the treatment given to these elite athletes differs from that given to recreational athletes and nonathletes. Bradley and colleagues4 examined how 32 NFL team orthopedists treated ACL tears, and Erickson and colleagues5 recently surveyed NFL and National Collegiate Athletic Association (NCAA) team physicians to determine practice patterns (eg, surgical techniques, graft choices, postoperative protocols) in treating ACL tears. Until now, however, no one has examined NHL, MLS, or Olympic team orthopedic surgeons’ practice patterns as they relate to ACL reconstruction.
We conducted an online survey of NHL, MLS, and Olympic team orthopedic surgeons to determine practice patterns relating to ACL reconstruction in elite athletes. Given the practice patterns of surgeons in our practice, we hypothesized that the surveyed surgeons treating these elite athletes would most commonly use bone–patellar tendon–bone (BPTB) autograft with a single-bundle technique. We also hypothesized that they would permit RTS without a brace at a minimum of 6 months after surgery, with a normal physical examination, and after successful completion of a structured battery of RTS tests.
Materials and Methods
On the SurveyMonkey website (http://www.surveymonkey.com), we created a 7-question base survey, with other questions added for the NHL and MLS surveys (Figure 1). We sent this survey to 94 team orthopedic surgeons (41 NHL, 26 MLS, 27 Olympic) identified through Internet searches and direct contact with team public relations departments. The survey was approved by MLS and NHL research committees. In 2013, each survey was sent out 5 times. The response rates for each round are shown in Figure 2. All responses remained confidential; we did not learn surgeons’ identities. Data were collected and analyzed through the SurveyMonkey website. Each surgeon was instructed to respond to all relevant questions in the survey. The survey was designed such that the participant could not submit the survey without answering all the questions. Descriptive statistics were calculated for each study and parameter analyzed. Continuous variable data are reported as means and standard deviations (weighted means where applicable). Categorical data are reported as frequencies with percentages.
Results
Of the 94 team orthopedic surgeons surveyed, 47 (50%) responded (NHL, 49%; MLS, 50%; Olympic, 52%). Mean (SD) experience as a team physician was 7.73 (5.33) years (range, 2-20 years) for NHL, 6.77 (6.64) years (range, 2-20 years) for MLS, and 1.14 (0.36) years (range, 1-10 years) for Olympic. Mean (SD) number of ACL reconstructions performed in 2012 was 101 (51) for NHL (range, 50-200), 78 (38) for MLS (range, 20-150), and 110 (105) for Olympic (range, 25-175) (Table 1). Of the 47 surgeons, 42 (89.4%) used autograft in the treatment of elite athletes, and 5 (10.6%) used allograft. Autograft choices were BPTB (n = 33; 70.2%), 4-strand semitendinosus (n = 7; 14.9%), and quadriceps (n = 2; 4.3%); allograft choices were 4-strand semitendinosus (n = 4; 8.5%) and BPTB (n = 1; 2.1%) (Table 2).
Of the 40 surgeons (85.1%) who indicated they would use autograft in 25-year-old recreational athletes, 25 (53.2%) would use BPTB, 13 (27.7%) would use 4-strand semitendinosus, and 2 (4.3%) would use quadriceps; of the 7 who indicated they would use allograft, 4 (8.5%) would use 4-strand semitendinosus, and 3 (6.4%) would use BPTB. In the NHL and MLS surveys, 19 surgeons (57.6%) indicated they would use autograft (6 would use BPTB, 13 would use 4-strand semitendinosus), and 14 (42.4%) would use allograft (7 would use BPTB, 5 would use Achilles, and 2 would use tibialis anterior) in 35-year-old recreational athletes.
Twenty-one surgeons (44.7%) were drilling the femoral tunnel through a transtibial portal, 36.2% through an anteromedial portal, and 12.8% using a 2-incision technique. All surgeons indicated they were using a single-bundle technique in ACL reconstruction. Thirty-three surgeons (70.2%) did not recommend a brace for their elite athletes on RTS. Olympic team surgeons had the highest rate of brace wear in RTS (50%, both skiers and snowboarders); NHL and MLS surgeons had significantly lower rates (25% and 15.4%, respectively) (Table 3).
Twenty (60.6%) of the NHL and MLS surgeons recommended waiting at least 6 months before RTS; 2 (6.1%) recommended waiting at least 9 months; no surgeon recommended waiting at least 12 months; and the others did not have a specific time frame for RTS. Twenty-seven surgeons (81.8%) recommended RTS after an athlete passed a series of RTS tests (eg, Vail, single-leg hop). Nineteen surgeons (57.6%) recommended waiting until the athlete had full range of motion, no pain, full strength, and subjective stability in the knee. Physicians could choose more than one answer for the previous question, allowing for a total percentage higher than 100%.
Discussion
The goal of this study was to determine how NHL, MLS, and Olympic team orthopedic surgeons manage ACL tears in elite and recreational athletes. Our study hypotheses were confirmed, as 70.2% of those surveyed used BPTB autograft for elite athletes, 100% used the single-bundle technique, 70.2% did not require a brace on RTS, 81.8% recommended RTS after the athlete passed a series of RTS tests (eg, Vail, single-leg hop), and 60.6% waited at least 6 months after surgery.
As soccer and skiing are the top 2 sports in which participants sustain ACL tears, it is necessary to report how surgeons obtain successful results in these patient populations.6 Using the US and Norwegian ACL reconstruction registries, Granan and colleagues6 found that, over a 7-year period, 5760 ACL tears occurred during soccer, and 2030 occurred during skiing. The scope of ACL injuries is broad, and treatment patterns must be elucidated. Although most surgeons do not treat elite athletes, many high school and college athletes compete at very high levels. Therefore, replicating the methods of the surgeons who treat elite athletes may be warranted.
In our survey, autograft (89.4%), particularly BPTB autograft (70.2%), was the most common graft choice for elite athletes. The rate of allograft use (42.4%) was higher for 35-year-old recreational athletes. As BPTB autograft produces reliable long-term results, this graft type is a reasonable choice.7 However, only 18% of our surveyed orthopedic surgeons indicated they would use BPTB autograft in older, recreational athletes. This stark difference is likely related to the more than 40% long-term side effects of anterior knee pain and graft harvest site morbidity with BPTB autograft as opposed to allograft and other types of autograft.8,9 Younger patients may be more willing to accept some anterior knee pain to ensure bone-to-bone healing with BPTB autograft. This shift in graft choice may also reflect the desire to minimize skin incisions and their resulting scars, especially in female recreational athletes.
In a meta-analysis of more than 5000 patients, Kraeutler and colleagues7 found that BPTB autograft outperformed allograft according to several knee scores, including Lysholm and Tegner, and had a lower re-rupture rate (4.3% vs 12.7%). However, despite the superior performance of BPTB autograft, graft choice cannot overcome surgeon error in graft placement.10 BPTB autograft appears to remain the gold standard for ACL reconstruction for many reasons, including low failure rates and decreased costs.11 Recently, investigators have tried to challenge the superiority of BPTB autograft. In a retrospective case–control study, Mascarenhas and colleagues12 found that hamstring autograft afforded patients better extension and higher subjective outcome scores. Bourke and colleagues13 found a higher rate of contralateral ACL rupture in patients treated with BPTB autograft compared with hamstring autograft.
According to this survey, 44.7% of surgeons indicated they drilled the femoral tunnel through a transtibial portal, 36.2% used an anteromedial portal, and 12.8% used the 2-incision technique. These methods were recently evaluated to determine if any is superior to the others, but the study results were not definitive.14 Franceschi and colleagues15 found improved rotational and anterior stability of the knee with use of an anteromedial approach, but their findings were not clinically or functionally significant. Wang and colleagues16 found an extension loss in the late-stance phase of gait with the anteromedial approach; the transtibial approach was correlated with inferior anterior-posterior stability during the stance phase of gait. Therefore, our results parallel those in the current literature in that the surveyed population is split on which technique to use and likely bases its practice on comfort level and residency/fellowship training.
Limitations
This study had several limitations. First, it provided level V evidence of team physicians in 3 major sports. Although some of these physicians were also treating athletes in other sports, our survey targeted NHL, MLS, and Olympic athletes. It did not address all ages and both sexes—which is significant, given the higher rate of ACL tears in females. All NHL and MLS players are male, and there was a high rate of BPTB graft use in these sports. However, recreational athletes include both males and females, and the fact that some surgeons would choose a hamstring graft for a female for cosmetic reasons must not be overlooked. Conversely, that there was no difference in the number of BPTB autografts chosen between NHL and MLS surgeons versus Olympic surgeons, where females are included (all chose about 60% BPTB autografts for their elite athletes), disputes this limitation. Our survey response rate was 50%. Other studies have had similar rates in relation to ACL practices,17 especially elite team physicians’ practices,5 and recent literature has confirmed that lower response rates in surveys did not alter results and may in fact have improved results.18,19 This percentage could be falsely low if some of our email addresses were incorrect. This rate also raises the possibility of selection bias, as surgeons who routinely used allograft in their athlete population may not have wanted to admit this. It is possible that some NHL, MLS, and Olympic athletes were treated by surgeons not included in this survey (in some cases, a non–team surgeon may have performed the athlete’s surgery). This survey did not address concomitant knee pathology or cover all possible technique variables.
Conclusion
Most of the NHL, MLS, and Olympic team orthopedic surgeons who were surveyed perform their ACL reconstructions using BPTB autograft, using a single-bundle technique, through a transtibial portal, and do not require bracing for their athletes returning to sport. Most required their athletes to complete a series of RTS tests before resuming competitive play.
1. Team USA. 2013. US Olympic Committee website. http://www.teamusa.org/athletes?pg=1&seasonId=%7BCF2DC66A-C2B3-44A8-ABB8-A486F3FBFDDF%7D&ngbId=%7BB36167A0-2AC8-4B0F-876F-93D0A44DF60A%7D. Accessed October 23, 2015.
2. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):1-8.
3. Erickson BJ, Harris JD, Fillingham YA, et al. Performance and return to sport after anterior cruciate ligament reconstruction in X-Games skiers and snowboarders. Orthop J Sports Med. 2013;1(6):1-5.
4. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
5. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
6. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
7. Kraeutler MJ, Bravman JT, McCarty EC. Bone–patellar tendon–bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
8. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
9. Kartus J, Magnusson L, Stener S, Brandsson S, Eriksson BI, Karlsson J. Complications following arthroscopic anterior cruciate ligament reconstruction. A 2-5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(1):2-8.
10. Boszotta H. Arthroscopic anterior cruciate ligament reconstruction using a patellar tendon graft in press-fit technique: surgical technique and follow-up. Arthroscopy. 1997;13(3):332-339.
11. Hospodar SJ, Miller MD. Controversies in ACL reconstruction: bone–patellar tendon–bone anterior cruciate ligament reconstruction remains the gold standard. Sports Med Arthrosc Rev. 2009;17(4):242-246.
12. Mascarenhas R, Tranovich MJ, Kropf EJ, Fu FH, Harner CD. Bone–patellar tendon–bone autograft versus hamstring autograft anterior cruciate ligament reconstruction in the young athlete: a retrospective matched analysis with 2-10 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1520-1527.
13. Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985-1992.
14. Chalmers PN, Mall NA, Cole BJ, Verma NN, Bush-Joseph CA, Bach BR Jr. Anteromedial versus transtibial tunnel drilling in anterior cruciate ligament reconstructions: a systematic review. Arthroscopy. 2013;29(7):1235-1242.
15. Franceschi F, Papalia R, Rizzello G, Del Buono A, Maffulli N, Denaro V. Anteromedial portal versus transtibial drilling techniques in anterior cruciate ligament reconstruction: any clinical relevance? A retrospective comparative study. Arthroscopy. 2013;29(8):1330-1337.
16. Wang H, Fleischli JE, Zheng NN. Transtibial versus anteromedial portal technique in single-bundle anterior cruciate ligament reconstruction: outcomes of knee joint kinematics during walking. Am J Sports Med. 2013;41(8):1847-1856.
17. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
18. Keeter S, Miller C, Kohut A, Groves RM, Presser S. Consequences of reducing nonresponse in a national telephone survey. Public Opin Q. 2000;64(2):125-148.
19. Curtin R, Presser S, Singer E. The effects of response rate changes on the index of consumer sentiment. Public Opin Q. 2000;64(4):413-428.
1. Team USA. 2013. US Olympic Committee website. http://www.teamusa.org/athletes?pg=1&seasonId=%7BCF2DC66A-C2B3-44A8-ABB8-A486F3FBFDDF%7D&ngbId=%7BB36167A0-2AC8-4B0F-876F-93D0A44DF60A%7D. Accessed October 23, 2015.
2. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):1-8.
3. Erickson BJ, Harris JD, Fillingham YA, et al. Performance and return to sport after anterior cruciate ligament reconstruction in X-Games skiers and snowboarders. Orthop J Sports Med. 2013;1(6):1-5.
4. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
5. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
6. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
7. Kraeutler MJ, Bravman JT, McCarty EC. Bone–patellar tendon–bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
8. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
9. Kartus J, Magnusson L, Stener S, Brandsson S, Eriksson BI, Karlsson J. Complications following arthroscopic anterior cruciate ligament reconstruction. A 2-5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(1):2-8.
10. Boszotta H. Arthroscopic anterior cruciate ligament reconstruction using a patellar tendon graft in press-fit technique: surgical technique and follow-up. Arthroscopy. 1997;13(3):332-339.
11. Hospodar SJ, Miller MD. Controversies in ACL reconstruction: bone–patellar tendon–bone anterior cruciate ligament reconstruction remains the gold standard. Sports Med Arthrosc Rev. 2009;17(4):242-246.
12. Mascarenhas R, Tranovich MJ, Kropf EJ, Fu FH, Harner CD. Bone–patellar tendon–bone autograft versus hamstring autograft anterior cruciate ligament reconstruction in the young athlete: a retrospective matched analysis with 2-10 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1520-1527.
13. Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985-1992.
14. Chalmers PN, Mall NA, Cole BJ, Verma NN, Bush-Joseph CA, Bach BR Jr. Anteromedial versus transtibial tunnel drilling in anterior cruciate ligament reconstructions: a systematic review. Arthroscopy. 2013;29(7):1235-1242.
15. Franceschi F, Papalia R, Rizzello G, Del Buono A, Maffulli N, Denaro V. Anteromedial portal versus transtibial drilling techniques in anterior cruciate ligament reconstruction: any clinical relevance? A retrospective comparative study. Arthroscopy. 2013;29(8):1330-1337.
16. Wang H, Fleischli JE, Zheng NN. Transtibial versus anteromedial portal technique in single-bundle anterior cruciate ligament reconstruction: outcomes of knee joint kinematics during walking. Am J Sports Med. 2013;41(8):1847-1856.
17. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
18. Keeter S, Miller C, Kohut A, Groves RM, Presser S. Consequences of reducing nonresponse in a national telephone survey. Public Opin Q. 2000;64(2):125-148.
19. Curtin R, Presser S, Singer E. The effects of response rate changes on the index of consumer sentiment. Public Opin Q. 2000;64(4):413-428.
Magnetic Resonance Imaging of Complications of Anterior Cruciate Ligament Reconstruction
Magnetic resonance imaging (MRI) is the preferred modality in the evaluation of complications of anterior cruciate ligament reconstruction (ACL-R).1-3 ACL-R complications may be broadly characterized as those resulting in decreased range of motion (ROM), eg, arthrofibrosis and impingement, and those resulting in increased laxity, ie, graft disruption.4 Short tau inversion recovery (STIR) sequences best minimize artifact related to field inhomogeneity in the presence of metal-containing fixation devices. Patients with contraindications to MRI may undergo high-resolution computed tomographic arthrography of the knee for evaluation of postoperative graft abnormalities.1
Arthrofibrosis refers to focal or diffuse synovial scar tissue, which may limit ROM. Preoperative irritation, preoperative limited ROM, and reconstruction within 4 weeks of trauma may all play a role in the development of arthrofibrosis.5,6 The focal form, cyclops lesion, named for its arthroscopic appearance, has been reported in 1% to 10% of patients with ACL-R.1 On MRI, focal arthrofibrosis may be seen as a focal or diffuse intermediate signal lesion in the anterior intercondylar notch extending linearly along the intercondylar roof1 (Figure 1).
MRI can be used to accurately determine the position of the femoral and tibial tunnels. Correct femoral tunnel position results in isometry of the graft during full ROM of the knee. Graft impingement can occur when the tibial tunnel is placed too far anteriorly such that the graft contacts the roof of the intercondylar notch before the knee is able to fully extend.7 A tibial tunnel placed anterior to the intersection of the Blumensaat line and the tibia is at higher risk for impingement.1,4 Impingement may be accompanied by signal change in the graft on intermediate-weighted and fluid-sensitive sequences. The signal abnormality is usually focal and persists longer than the expected signal changes related to revascularization of immature grafts within the first year (Figure 2). If left untreated, impingement may progress to graft rupture.4
Complete graft rupture is diagnosed on the basis of discontinuity of the graft fibers. MRI findings include fluid-filled defect or absence of intact graft fibers. Other reliable signs include large joint effusion, anterior tibial translation, pivot-shift–type marrow edema pattern, and horizontal orientation, laxity, or resorption of the graft fibers.1,8,9 The diagnosis of partial graft rupture may be challenging, as there are several other causes of increased graft signal, including revascularization (within 12 months after procedure), signal heterogeneity between individual bundles of hamstring grafts, and focal signal changes related to impingment (Figures 3, 4).
Fluid within the tunnels is a normal finding after surgery and typically resolves within the first 18 months.1 Cyst formation within the tibial tunnel is an uncommon complication of ACL-R and may be incidental to or present with clinical symptoms caused by extension into the pretibial soft tissues or expansion of the tunnel (Figure 5). Communication of cyst with joint space is important, as a noncommunicating cyst requires simple excision without need for bone grafting.7
Hardware-related complications (eg, loosening of fixation devices) are uncommon but may require revision surgery (Figure 6). Septic arthritis after ACL-R has a cumulative incidence of 0.1% to 0.9% and may be difficult to diagnose clinically because of the lack of classic symptoms of a septic joint.1 Diagnosis requires joint aspiration.
MRI is reliably and accurately used to assess ACL-R complications. The clinical history helps in stratifying complications that result in decreased ROM or increased laxity.
1. Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009;29(7):2115-2126.
2. Recht MP, Kramer J. MR imaging of the postoperative knee: a pictorial essay. Radiographics. 2002;22(4):765-774.
3. Papakonstantinou O, Chung CB, Chanchairujira K, Resnick DL. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13(5):1106-1117.
4. Meyers AB, Haims AH, Menn K, Moukaddam H. Imaging of anterior cruciate ligament repair and its complications. AJR Am J Roentgenol. 2010;194(2):476-484.
5. Kwok CS, Harrison T, Servant C. The optimal timing for anterior cruciate ligament reconstruction with respect to the risk of postoperative stiffness. Arthroscopy. 2013;29(3):556-565.
6. Mayr HO, Weig TG, Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124(8):518-522.
7. Ghazikhanian V, Beltran J, Nikac V, Feldman M, Bencardino JT. Tibial tunnel and pretibial cysts following ACL graft reconstruction: MR imaging diagnosis. Skeletal Radiol. 2012;41(11):1375-1379.
8. Collins MS, Unruh KP, Bond JR, Mandrekar JN. Magnetic resonance imaging of surgically confirmed anterior cruciate ligament graft disruption. Skeletal Radiol. 2008;37(3):233-243.
9. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249(2):581-590.
Magnetic resonance imaging (MRI) is the preferred modality in the evaluation of complications of anterior cruciate ligament reconstruction (ACL-R).1-3 ACL-R complications may be broadly characterized as those resulting in decreased range of motion (ROM), eg, arthrofibrosis and impingement, and those resulting in increased laxity, ie, graft disruption.4 Short tau inversion recovery (STIR) sequences best minimize artifact related to field inhomogeneity in the presence of metal-containing fixation devices. Patients with contraindications to MRI may undergo high-resolution computed tomographic arthrography of the knee for evaluation of postoperative graft abnormalities.1
Arthrofibrosis refers to focal or diffuse synovial scar tissue, which may limit ROM. Preoperative irritation, preoperative limited ROM, and reconstruction within 4 weeks of trauma may all play a role in the development of arthrofibrosis.5,6 The focal form, cyclops lesion, named for its arthroscopic appearance, has been reported in 1% to 10% of patients with ACL-R.1 On MRI, focal arthrofibrosis may be seen as a focal or diffuse intermediate signal lesion in the anterior intercondylar notch extending linearly along the intercondylar roof1 (Figure 1).
MRI can be used to accurately determine the position of the femoral and tibial tunnels. Correct femoral tunnel position results in isometry of the graft during full ROM of the knee. Graft impingement can occur when the tibial tunnel is placed too far anteriorly such that the graft contacts the roof of the intercondylar notch before the knee is able to fully extend.7 A tibial tunnel placed anterior to the intersection of the Blumensaat line and the tibia is at higher risk for impingement.1,4 Impingement may be accompanied by signal change in the graft on intermediate-weighted and fluid-sensitive sequences. The signal abnormality is usually focal and persists longer than the expected signal changes related to revascularization of immature grafts within the first year (Figure 2). If left untreated, impingement may progress to graft rupture.4
Complete graft rupture is diagnosed on the basis of discontinuity of the graft fibers. MRI findings include fluid-filled defect or absence of intact graft fibers. Other reliable signs include large joint effusion, anterior tibial translation, pivot-shift–type marrow edema pattern, and horizontal orientation, laxity, or resorption of the graft fibers.1,8,9 The diagnosis of partial graft rupture may be challenging, as there are several other causes of increased graft signal, including revascularization (within 12 months after procedure), signal heterogeneity between individual bundles of hamstring grafts, and focal signal changes related to impingment (Figures 3, 4).
Fluid within the tunnels is a normal finding after surgery and typically resolves within the first 18 months.1 Cyst formation within the tibial tunnel is an uncommon complication of ACL-R and may be incidental to or present with clinical symptoms caused by extension into the pretibial soft tissues or expansion of the tunnel (Figure 5). Communication of cyst with joint space is important, as a noncommunicating cyst requires simple excision without need for bone grafting.7
Hardware-related complications (eg, loosening of fixation devices) are uncommon but may require revision surgery (Figure 6). Septic arthritis after ACL-R has a cumulative incidence of 0.1% to 0.9% and may be difficult to diagnose clinically because of the lack of classic symptoms of a septic joint.1 Diagnosis requires joint aspiration.
MRI is reliably and accurately used to assess ACL-R complications. The clinical history helps in stratifying complications that result in decreased ROM or increased laxity.
Magnetic resonance imaging (MRI) is the preferred modality in the evaluation of complications of anterior cruciate ligament reconstruction (ACL-R).1-3 ACL-R complications may be broadly characterized as those resulting in decreased range of motion (ROM), eg, arthrofibrosis and impingement, and those resulting in increased laxity, ie, graft disruption.4 Short tau inversion recovery (STIR) sequences best minimize artifact related to field inhomogeneity in the presence of metal-containing fixation devices. Patients with contraindications to MRI may undergo high-resolution computed tomographic arthrography of the knee for evaluation of postoperative graft abnormalities.1
Arthrofibrosis refers to focal or diffuse synovial scar tissue, which may limit ROM. Preoperative irritation, preoperative limited ROM, and reconstruction within 4 weeks of trauma may all play a role in the development of arthrofibrosis.5,6 The focal form, cyclops lesion, named for its arthroscopic appearance, has been reported in 1% to 10% of patients with ACL-R.1 On MRI, focal arthrofibrosis may be seen as a focal or diffuse intermediate signal lesion in the anterior intercondylar notch extending linearly along the intercondylar roof1 (Figure 1).
MRI can be used to accurately determine the position of the femoral and tibial tunnels. Correct femoral tunnel position results in isometry of the graft during full ROM of the knee. Graft impingement can occur when the tibial tunnel is placed too far anteriorly such that the graft contacts the roof of the intercondylar notch before the knee is able to fully extend.7 A tibial tunnel placed anterior to the intersection of the Blumensaat line and the tibia is at higher risk for impingement.1,4 Impingement may be accompanied by signal change in the graft on intermediate-weighted and fluid-sensitive sequences. The signal abnormality is usually focal and persists longer than the expected signal changes related to revascularization of immature grafts within the first year (Figure 2). If left untreated, impingement may progress to graft rupture.4
Complete graft rupture is diagnosed on the basis of discontinuity of the graft fibers. MRI findings include fluid-filled defect or absence of intact graft fibers. Other reliable signs include large joint effusion, anterior tibial translation, pivot-shift–type marrow edema pattern, and horizontal orientation, laxity, or resorption of the graft fibers.1,8,9 The diagnosis of partial graft rupture may be challenging, as there are several other causes of increased graft signal, including revascularization (within 12 months after procedure), signal heterogeneity between individual bundles of hamstring grafts, and focal signal changes related to impingment (Figures 3, 4).
Fluid within the tunnels is a normal finding after surgery and typically resolves within the first 18 months.1 Cyst formation within the tibial tunnel is an uncommon complication of ACL-R and may be incidental to or present with clinical symptoms caused by extension into the pretibial soft tissues or expansion of the tunnel (Figure 5). Communication of cyst with joint space is important, as a noncommunicating cyst requires simple excision without need for bone grafting.7
Hardware-related complications (eg, loosening of fixation devices) are uncommon but may require revision surgery (Figure 6). Septic arthritis after ACL-R has a cumulative incidence of 0.1% to 0.9% and may be difficult to diagnose clinically because of the lack of classic symptoms of a septic joint.1 Diagnosis requires joint aspiration.
MRI is reliably and accurately used to assess ACL-R complications. The clinical history helps in stratifying complications that result in decreased ROM or increased laxity.
1. Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009;29(7):2115-2126.
2. Recht MP, Kramer J. MR imaging of the postoperative knee: a pictorial essay. Radiographics. 2002;22(4):765-774.
3. Papakonstantinou O, Chung CB, Chanchairujira K, Resnick DL. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13(5):1106-1117.
4. Meyers AB, Haims AH, Menn K, Moukaddam H. Imaging of anterior cruciate ligament repair and its complications. AJR Am J Roentgenol. 2010;194(2):476-484.
5. Kwok CS, Harrison T, Servant C. The optimal timing for anterior cruciate ligament reconstruction with respect to the risk of postoperative stiffness. Arthroscopy. 2013;29(3):556-565.
6. Mayr HO, Weig TG, Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124(8):518-522.
7. Ghazikhanian V, Beltran J, Nikac V, Feldman M, Bencardino JT. Tibial tunnel and pretibial cysts following ACL graft reconstruction: MR imaging diagnosis. Skeletal Radiol. 2012;41(11):1375-1379.
8. Collins MS, Unruh KP, Bond JR, Mandrekar JN. Magnetic resonance imaging of surgically confirmed anterior cruciate ligament graft disruption. Skeletal Radiol. 2008;37(3):233-243.
9. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249(2):581-590.
1. Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009;29(7):2115-2126.
2. Recht MP, Kramer J. MR imaging of the postoperative knee: a pictorial essay. Radiographics. 2002;22(4):765-774.
3. Papakonstantinou O, Chung CB, Chanchairujira K, Resnick DL. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13(5):1106-1117.
4. Meyers AB, Haims AH, Menn K, Moukaddam H. Imaging of anterior cruciate ligament repair and its complications. AJR Am J Roentgenol. 2010;194(2):476-484.
5. Kwok CS, Harrison T, Servant C. The optimal timing for anterior cruciate ligament reconstruction with respect to the risk of postoperative stiffness. Arthroscopy. 2013;29(3):556-565.
6. Mayr HO, Weig TG, Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124(8):518-522.
7. Ghazikhanian V, Beltran J, Nikac V, Feldman M, Bencardino JT. Tibial tunnel and pretibial cysts following ACL graft reconstruction: MR imaging diagnosis. Skeletal Radiol. 2012;41(11):1375-1379.
8. Collins MS, Unruh KP, Bond JR, Mandrekar JN. Magnetic resonance imaging of surgically confirmed anterior cruciate ligament graft disruption. Skeletal Radiol. 2008;37(3):233-243.
9. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249(2):581-590.
Multifocal Langerhans Cell Histiocytosis in an Adult
Eosinophilic granuloma (EG) is the most common benign form of Langerhans cell histiocytosis (LCH). Initially described by Lichtenstein in 1953, LCH encompasses a triad of proliferative granulomatous disorders primarily affecting children: EG, Hand-Schüller-Christian disease, and Letterer-Siwe disease.1 Lichtenstein first termed the disease histiocytosis X, after recognizing that the 3 syndromes had the same histology.1 The term was updated after the clonal proliferation of Langerhans cells in the pathogenesis of the disease was discovered.
As LCH is generally considered a pediatric disease, there is little in the literature regarding adult-onset LCH. The incidence of LCH in adults is reported as 1 to 2 cases per million, significantly lower than that in children.2,3 Two studies have reported the mean age at diagnosis in adults as the fourth decade of life, and have suggested a male predominance.4,5 The vast majority of adult LCH cases described are simple EG, with very few cases of multisystem disseminated disease reported.5
Adult patients with LCH typically present with solitary lesions in bone. Approximately 10% of cases have extraosseous involvement, with the lung being the most common site.6 Lesions tend to be unifocal, with fewer than 10 reports describing multifocal EG.1,7-13 The axial skeleton is most frequently involved, with the majority of lesions occurring in the skull, ribs, vertebrae, or mandible.14 While less common, the femur, humerus, and clavicle are most often involved when the appendicular skeleton is affected.5
In a literature review, a few case reports describe adult-onset EG of the skull. Only 5 case reports since the 1970s describe adult patients with EG of the femur. We present a rare case of multifocal EG in a 48-year-old woman with lesions of the femur and skull, as well as a review of the literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 48-year-old woman presented with progressive right knee pain that was exacerbated by weight-bearing. She denied trauma, fevers, fatigue, or weight change. Her history was significant for an EG of the skull, excised at an outside institution 2 years prior to presentation. The patient also admitted to recent onset of right-sided skull pain, near the region of her previous surgery.
Physical examination demonstrated tenderness to palpation and fullness over the right medial distal femur and a normal neurovascular examination of the right lower extremity. Radiographs of the knee showed a cortically based, lytic, destructive lesion involving the medial femoral condyle, with soft-tissue extension (Figures 1A, 1B). Magnetic resonance imaging (MRI) of the right knee showed the lesion, with extraosseous soft-tissue extension (Figures 2A, 2B). The mass was isointense to muscle on T1-weighted images and hyperintense on T2-weighted images. Technetium bone scanning showed increased uptake in the right femur and the right skull (Figures 3A, 3B). MRI of the brain confirmed a new lesion in the right diploic space, distinct from the previous EG lesion site (Figures 4A-4D). An ultrasound-guided biopsy of the femur was performed and was consistent with EG.
After reevaluation and clearance by her neurosurgeon, the patient underwent curettage and allografting of the femoral lesion, with prophylactic internal fixation using a titanium distal femoral locking plate (Figure 5). Intraoperative frozen section was consistent with EG, which was confirmed with additional immunohistochemical workup (Figures 6A-6D).
The patient recovered uneventfully and follow-up radiographs showed restoration of the bony cortex of the medial femoral condyle (Figure 7). The second skull lesion, which was also consistent with EG, was excised by her neurosurgeon.
The patient remained asymptomatic until 2 years later, when she began experiencing mild pain in her right distal thigh and knee. Radiographs showed a new lytic focus in the right distal metadiaphysis (Figure 8) which was not present on her last radiograph 6 months prior. A computed tomography (CT) scan showed a lytic lesion involving the right distal femur medullary canal with cortical thinning and destruction, most pronounced posteriorly (Figures 9A, 9B). There was also an extraosseous soft-tissue component to the lesion. Bone scan showed increased uptake in the area of the new lesion. There was no increased uptake elsewhere, including the medial distal femur at the site of the old lesion, to suggest other lesions, and no increased uptake in the skull.
Given that the location of the lesion was distinct from the prior site of curettage and bone grafting, it was thought to be consistent with a new EG lesion. The patient underwent CT-guided biopsy, with simultaneous intralesional corticosteroid injection to treat the lesion when on-site pathology confirmed the etiology. Further surgical management was deemed unnecessary because internal fixation was present and spanned the new lesion. Final analysis of the fine-needle aspirate of the new lesion was positive for numerous eosinophils and histiocytes, consistent with EG.
At 6-week follow-up after the intralesional steroid injection, the patient’s pain continued to abate, and she was ambulating with crutches. Repeat CT scan of the right distal femur showed improvement of the extraosseous soft-tissue component, while the lucency in the femur itself remained unchanged. The decision was made to proceed with a second intralesional corticosteroid injection under CT guidance. The patient’s symptoms continued to improve, and repeat imaging 1 year after her steroid injections showed substantial bony healing with reconstitution of her cortical bone (Figures 10A-10E).
The patient had had 4 distinct tumors consistent with EG and was referred to a medical oncologist for further workup. The patient began treatment with zoledronic acid to prevent development of further lesions. At most recent follow-up, the patient was 18 months out from her second intralesional corticosteroid injection and was doing very well. She reported being pain-free and was walking 3 to 4 miles per week without gait aids. There was no evidence of new disease. The medial distal femur lesion was completely healed, and the distal metaphyseal lesion was nearly healed, with very little residual evidence of lesions.
Discussion
Adult-onset multifocal EG is a rare entity. Most affected patients develop lesions in the axial skeleton, with the skull, mandible, and vertebrae most commonly involved.14 Only 5 cases of femoral EG have been reported, one of which was multifocal.11,14-17
Of these patients, 3 were between the ages of 33 and 53 years and had insidious onset of hip pain that failed conservative management.14,15,17 Further imaging and biopsy revealed unifocal EG in the proximal femur in each case. Each patient received a different form of treatment, including curettage and radiation, radiofrequency ablation, and/or physical therapy. At the time of publication, all patients had reported improvement in their clinical symptoms.14,15,17 The fourth patient was a man with human immunodeficiency virus (HIV) with 3 months of progressive thigh pain. Further evaluation found an isolated EG of the femoral diaphysis that progressed to pathologic fracture. He was treated with curettage and intramedullary nailing, and had improved symptoms and radiographic signs of healing at 30-month follow-up.16
An interesting case by Kerzl and colleagues11 reported a 63-year-old woman with a 24-year history of multiple symmetric lesions of the femora, leading to multiple pathologic fractures. Like our patient, her initial lesion was in the skull. Initial pathology specimens led to the diagnosis of EG. However, as the patient aged, she developed symptoms of diabetes insipidus and xanthelasma, which led to reevaluation of histology from 3 bony lesions. The patient was determined to have multifocal EG of the skull and femur, with simultaneous occurrence of Erdheim-Chester disease, which also causes bone lesions in addition to diabetes insipidus and xanthelasma.11
Though LCH was initially described more than 50 years ago, many aspects of LCH remain an enigma, especially in adults. The etiology of the disease is poorly understood. Controversy exists regarding whether LCH is primarily an immunoregulatory, neoplastic, or reactive disorder. The vast majority of adult cases described in the literature are EG, with very few cases of multisystem disseminated disease reported.5
The spectrum of disorders constituting LCH is heterogenous. Eosinophilic granuloma is the most common form, reportedly accounting for 60% to 70% of all cases, usually presenting as solitary bone lesions.6 Eosinophilic granuloma refers to the localized form of LCH, in which the disease is limited to bone or lung.18 This is the least aggressive form of the disease, with the most favorable prognosis. Hand- Schüller-Christian disease is a chronic, recurring form of LCH, with disseminated disease, affecting both bone and extraskeletal sites. Hand-Schüller-Christian disease is known for the classic triad of diabetes insipidus, exophthalmos, and destructive bone lesions. Patients may also present with otitis media or neurologic complaints from pathologic vertebral fractures. Letterer-Siwe disease refers to the acute, disseminated, fulminant form of LCH. This is the least common form of LCH and is predominately described in young children. Patients present with hepatosplenomegaly, lymphadenopathy, skin rash, fever, anemia, and thrombocytopenia.19 It is rapidly progressive, leading to multiorgan dysfunction and death within 1 to 2 years.18
The classification of LCH follows the Histiocyte Society guidelines developed from multicenter randomized trials in children.3 Classification is based on affected organs and is divided into 2 categories: single-system disease or multisystem disease. Single-system disease may be single site (bone, skin, or solitary lymph node) or multisite (multifocal bone disease or multiple lymph nodes). Multisystem disease is further classified into low-risk or risk groups. The low-risk group involves disseminated disease without involvement of risk organs (lungs, liver, spleen, and hematopoietic system). Involvement of 1 or more risk organs places the patient in the risk group, associated with the least favorable prognosis.3
In adults, the most common presenting symptoms are local pain from bony involvement, weight loss, and fever. Bony lesions most often occur in the skull, especially in the jaw. Long bones are less frequently involved, with lesions occurring in the long bones in approximately 17% of patients.3 The rib has also been reported as a common site of involvement in adults.5 Similar to children, diabetes insipidus remains a classic manifestation of LCH because of pituitary gland involvement. Other common symptoms of LCH in adults are cough, dyspnea, and chest pain from pulmonary involvement. Up to 20% to 30% of adult LCH patients have isolated pulmonary lesions, although pulmonary LCH may also occur as part of multisystem disease (risk group).3,4,20
Eosinophilic granuloma bone lesions have a variety of radiographic appearances but most commonly appear as lytic lesions. They often mimic aggressive lesions with permeative bone destruction, periostitis, ill-defined borders, and cortical erosion. Most lesions arise in the medullary space but can present as a destructive, cortically based lesion, as it did in our patient’s first femoral lesion. The differential diagnosis for a lytic medullary bone lesion includes benign entities, such as nonossifying fibromas, bone cysts, or osteomyelitis, but also includes malignant tumors, such as metastases, Ewing sarcoma, and lymphoma. A destructive, cortically based lesion in an adult should raise a very high suspicion for metastatic carcinoma until proven otherwise. Other diagnostic considerations for a cortically based lesion include chondromyxoid fibroma and surface bone lesions, such as surface chondroma and osteoma, or osteosarcoma (parosteal and periosteal). In the skull, lesions commonly erode the outer table more than the inner table (the typical “beveled-edge” appearance). Skull lesions also may have a small, central, dense focus within the lytic lesion (“button sequestrum”).
Bone scanning is often not as sensitive in detecting EG lesions compared with other bone tumors, although in our patient the bone scan was positive. In patients with a negative bone scan but a high index of suspicion, a radiographic skeletal survey should be obtained to rule out other lesions. MRI typically shows T2-hyperintense, T1-hypointense lesions with surrounding bone marrow edema and variable contrast enhancement, which is relatively nonspecific. The high sensitivity of MRI allows accurate delineation of the extent of the lesions and evaluates for the presence of an extraosseous soft-tissue component. Biopsy is generally necessary to establish a definitive histologic diagnosis. In our patient, despite her history of biopsy-proven EG, the aggressive appearance of a destructive, cortically based lesion made obtaining a biopsy critical to establish a definitive diagnosis in this case.
The histopathologic examination of the tissue from our patient was typical of that seen in patients with EG. It revealed tissue fragments with diffuse sheets of histiocytes displaying nuclear grooves, admixed numerous eosinophils with eosinophilic microabscesses, and scattered lymphocytes (Figures 6A, 6B). There were areas of necrosis, raising the possibility of osteomyelitis. However, the presence of classic histomorphologic features of LCH in the majority of the tissue fragments, along with CD1a- and S100-positivity in the histiocytes, confirmed the diagnosis of LCH (Figures 6C, 6D). Although not highly specific, a positive CD1a immunostain with the described histomorphologic findings in the proper clinical setting is often considered sufficient for LCH diagnosis. S100 is an important adjunct immunostain in the evaluation of histiocytic disorders. A positive S100 immunostain helps identify histiocytes, which are also CD1a-positive, because the latter immunostain can also be positive in some lymphomas and thymomas.21
After diagnosis of LCH has been confirmed, staging includes radiographs of any suspicious bone lesions, chest radiograph, bone scan, abdominal ultrasound, routine laboratory studies, and chest CT if pulmonary LCH is suspected.
The optimal treatment strategy for adult patients has not been clearly defined, and current strategies for LCH vary depending on organ involvement and extent of disease. Therapeutic options include observation, local treatment with steroids, local excision with curettage with or without bone grafting, chemotherapy, immunomodulation, irradiation, and stem cell transplantation in advanced disease. In general, patients who benefit from systemic therapy, such as chemotherapy or immunomodulation, include those with multisystem disease, refractory or recurrent lesions, and multifocal skeletal involvement.22
Patients with more limited disease, such as EG of bone, may undergo observation or local intralesional treatment. Eosinophilic granuloma of bone may resolve spontaneously and commonly does so when it is located in the pediatric spine. However, the therapeutic approach in adults with EG is controversial, given that spontaneous resolution is less likely to occur in the skeletally mature. Plasschaert and colleagues23 reported a recurrence rate of 26% in skeletally mature patients with EG of bone treated with biopsy followed by curettage with or without grafting. In the skeletally immature group, there were no clinical or radiographic signs of recurrence in the 2-year follow-up period.23 Thus, treatment in the adult population must be considered separate from the skeletally immature and in the appropriate clinical context. Depending on the location of the lesion, patients may become symptomatic or be at risk for pathologic fracture. In such circumstances, curettage with or without bone grafting and prophylactic internal fixation may be indicated. Other treatments, such as intralesional infiltration with corticosteroids, have been reported, but the role of such treatment in adults is undetermined.24,25 Radiation is typically not recommended in single-system disease unless a vital organ is threatened.26 Overall, patients with single-system disease have an excellent prognosis, and treatment should be determined on an individual basis.3
Eosinophilic granuloma represents less than 1% of all bone tumors, and adult presentation is very rare. The differential diagnosis of lytic bone lesions is broad and includes metastatic carcinoma, lymphoma/myeloma, osteomyelitis, osteoblastoma, aneurysmal bone cyst, and Ewing sarcoma. While EG is more common and easily diagnosed in children, it should be considered in the differential diagnosis in adults, so that the appropriate diagnostic workup and treatment can be performed.
1. Lahiani D, Hammami BK, Maâloul I, et al. Multifocal Langerhans cell histiocytosis of bone: late revelation in a 76-year-old woman. Rev Med Interne. 2008;29(3):249-251.
2. Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28(1):9-14.
3. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76(5):363-368.
4. Aricò M, Girschikofsky M, Généreau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39(16):2341-2348.
5. Islinger RB, Kuklo TR, Owens BD, et al. Langerhans’ cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res. 2000;379:231-235.
6. Key SJ, O’Brien CJ, Silvester KC, Crean SJ. Eosinophilic granuloma: resolution of maxillofacial bony lesions following minimal intervention. Report of three cases and a review of the literature. J Craniomaxillofac Surg. 2004;32(3):170-175.
7. Bodner G, Kreczy A, Rachbauer F, Baechter O, Peer S. Eosinophilic granuloma of the bone: ultrasonographic imaging. Australas Radiol. 2002;46(4):418-421.
8. Boutsen Y, Esselinckx W, Delos M, Nisolle JF. Adult onset of multifocal eosinophilic granuloma of bone: a long-term follow-up with evaluation of various treatment options and spontaneous healing. Clin Rheumatol. 1999;18(1):69-73.
9. Corti F, Valicenti A, Bertolucci D, Bruno J, Gustinucci R. Multifocal Langerhans cell granulomatosis. Report of a clinical case. Minerva Med. 1994;85(7-8):413-416.
10. Demirci I. Adult eosinophilic granuloma of the lumbar spine with atypical dissemination. Case report: a long-term follow-up. Zentralbl Neurochir. 2004;65(2):84-87.
11. Kerzl R, Eyerich K, Eberlein B, et al. Parallel occurrence of Erdheim-Chester disease and eosinophilic granuloma in the same patient. J Eur Acad Dermatol Venereol. 2009;23(2):224-226.
12. Nguyen BD, Roarke MC, Chivers SF. Multifocal Langerhans cell histiocytosis with infiltrative pelvic lesions: PET/CT imaging. Clin Nucl Med. 2010;35(10): 824-826.
13. Scolozzi P, Lombardi T, Monnier P, Jaques B. Multisystem Langerhans’ cell histiocytosis (Hand-Schuller-Christian disease) in an adult: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2004;261(6):326-330.
14. King JJ, Melvin JS, Iwenofu OH, Fox EJ. Thigh pain in a 53-year-old woman. Clin Orthop Relat Res. 2009;467(6):1652-1657.
15. Hair LC, Deyle GD. Eosinophilic granuloma in a patient with hip pain. J Orthop Sports Phys Ther. 2011;41(2):119.
16. Panayiotakopoulos GD, Sipsas NV, Kontos A, et al. Eosinophilic granuloma of the femur in an HIV-1 positive patient. AIDS Patient Care STDS. 2002;16(3):103-106.
17. Rodrigues RJ, Lewis HH. Eosinophilic granuloma of bone. Review of literature and case presentation. Clin Orthop Relat Res. 1971;77:183-192.
18. Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics. 1992;12(4):801-823.
19. Lichtenstein L. Histiocytosis X (eosinophilic granuloma of bone, Letterer-Siwe disease, and Schueller-Christian disease). Further observations of pathological and clinical importance. J Bone Joint Surg Am. 1964;46:76-90.
20. Götz G, Fichter J. Langerhans’-cell histiocytosis in 58 adults. Eur J Med Res. 2004;9(11):510-514.
21. Cheng KL, Glu PG, Weiss LM. Hematopoeitic tumors. In: Peiguo C, Weiss L, eds. Modern Immunohistochemistry. New York, NY: Cambridge University Press; 2009:503.
22. Broadbent V, Gadner H. Current therapy for Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12(2):327-338.
23. Plasschaert F, Craig C, Bell R, Cole WG, Wunder JS, Alman BA. Eosinophilic granuloma. A different behaviour in children than in adults. J Bone Joint Surg Br. 2002;84(6):870-872.
24. Capanna R, Springfield DS, Ruggieri P, et al. Direct cortisone injection in osinophilic granuloma of bone: a preliminary report on 11 patients. J Pediatr Orthop. 1985;5(3):339-342.
25. Egeler RM, Thompson RC Jr, Voûte PA, Nesbit ME Jr. Intralesional infiltration of corticosteroids in localized Langerhans’ cell histiocytosis. J Pediatr Orthop. 1992;12(6):811-814.
26. Ladisch S, Gadner H. Treatment of Langerhans cell histiocytosis–evolution and current approaches. Br J Cancer Suppl. 1994;23:S41-S46.
Eosinophilic granuloma (EG) is the most common benign form of Langerhans cell histiocytosis (LCH). Initially described by Lichtenstein in 1953, LCH encompasses a triad of proliferative granulomatous disorders primarily affecting children: EG, Hand-Schüller-Christian disease, and Letterer-Siwe disease.1 Lichtenstein first termed the disease histiocytosis X, after recognizing that the 3 syndromes had the same histology.1 The term was updated after the clonal proliferation of Langerhans cells in the pathogenesis of the disease was discovered.
As LCH is generally considered a pediatric disease, there is little in the literature regarding adult-onset LCH. The incidence of LCH in adults is reported as 1 to 2 cases per million, significantly lower than that in children.2,3 Two studies have reported the mean age at diagnosis in adults as the fourth decade of life, and have suggested a male predominance.4,5 The vast majority of adult LCH cases described are simple EG, with very few cases of multisystem disseminated disease reported.5
Adult patients with LCH typically present with solitary lesions in bone. Approximately 10% of cases have extraosseous involvement, with the lung being the most common site.6 Lesions tend to be unifocal, with fewer than 10 reports describing multifocal EG.1,7-13 The axial skeleton is most frequently involved, with the majority of lesions occurring in the skull, ribs, vertebrae, or mandible.14 While less common, the femur, humerus, and clavicle are most often involved when the appendicular skeleton is affected.5
In a literature review, a few case reports describe adult-onset EG of the skull. Only 5 case reports since the 1970s describe adult patients with EG of the femur. We present a rare case of multifocal EG in a 48-year-old woman with lesions of the femur and skull, as well as a review of the literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 48-year-old woman presented with progressive right knee pain that was exacerbated by weight-bearing. She denied trauma, fevers, fatigue, or weight change. Her history was significant for an EG of the skull, excised at an outside institution 2 years prior to presentation. The patient also admitted to recent onset of right-sided skull pain, near the region of her previous surgery.
Physical examination demonstrated tenderness to palpation and fullness over the right medial distal femur and a normal neurovascular examination of the right lower extremity. Radiographs of the knee showed a cortically based, lytic, destructive lesion involving the medial femoral condyle, with soft-tissue extension (Figures 1A, 1B). Magnetic resonance imaging (MRI) of the right knee showed the lesion, with extraosseous soft-tissue extension (Figures 2A, 2B). The mass was isointense to muscle on T1-weighted images and hyperintense on T2-weighted images. Technetium bone scanning showed increased uptake in the right femur and the right skull (Figures 3A, 3B). MRI of the brain confirmed a new lesion in the right diploic space, distinct from the previous EG lesion site (Figures 4A-4D). An ultrasound-guided biopsy of the femur was performed and was consistent with EG.
After reevaluation and clearance by her neurosurgeon, the patient underwent curettage and allografting of the femoral lesion, with prophylactic internal fixation using a titanium distal femoral locking plate (Figure 5). Intraoperative frozen section was consistent with EG, which was confirmed with additional immunohistochemical workup (Figures 6A-6D).
The patient recovered uneventfully and follow-up radiographs showed restoration of the bony cortex of the medial femoral condyle (Figure 7). The second skull lesion, which was also consistent with EG, was excised by her neurosurgeon.
The patient remained asymptomatic until 2 years later, when she began experiencing mild pain in her right distal thigh and knee. Radiographs showed a new lytic focus in the right distal metadiaphysis (Figure 8) which was not present on her last radiograph 6 months prior. A computed tomography (CT) scan showed a lytic lesion involving the right distal femur medullary canal with cortical thinning and destruction, most pronounced posteriorly (Figures 9A, 9B). There was also an extraosseous soft-tissue component to the lesion. Bone scan showed increased uptake in the area of the new lesion. There was no increased uptake elsewhere, including the medial distal femur at the site of the old lesion, to suggest other lesions, and no increased uptake in the skull.
Given that the location of the lesion was distinct from the prior site of curettage and bone grafting, it was thought to be consistent with a new EG lesion. The patient underwent CT-guided biopsy, with simultaneous intralesional corticosteroid injection to treat the lesion when on-site pathology confirmed the etiology. Further surgical management was deemed unnecessary because internal fixation was present and spanned the new lesion. Final analysis of the fine-needle aspirate of the new lesion was positive for numerous eosinophils and histiocytes, consistent with EG.
At 6-week follow-up after the intralesional steroid injection, the patient’s pain continued to abate, and she was ambulating with crutches. Repeat CT scan of the right distal femur showed improvement of the extraosseous soft-tissue component, while the lucency in the femur itself remained unchanged. The decision was made to proceed with a second intralesional corticosteroid injection under CT guidance. The patient’s symptoms continued to improve, and repeat imaging 1 year after her steroid injections showed substantial bony healing with reconstitution of her cortical bone (Figures 10A-10E).
The patient had had 4 distinct tumors consistent with EG and was referred to a medical oncologist for further workup. The patient began treatment with zoledronic acid to prevent development of further lesions. At most recent follow-up, the patient was 18 months out from her second intralesional corticosteroid injection and was doing very well. She reported being pain-free and was walking 3 to 4 miles per week without gait aids. There was no evidence of new disease. The medial distal femur lesion was completely healed, and the distal metaphyseal lesion was nearly healed, with very little residual evidence of lesions.
Discussion
Adult-onset multifocal EG is a rare entity. Most affected patients develop lesions in the axial skeleton, with the skull, mandible, and vertebrae most commonly involved.14 Only 5 cases of femoral EG have been reported, one of which was multifocal.11,14-17
Of these patients, 3 were between the ages of 33 and 53 years and had insidious onset of hip pain that failed conservative management.14,15,17 Further imaging and biopsy revealed unifocal EG in the proximal femur in each case. Each patient received a different form of treatment, including curettage and radiation, radiofrequency ablation, and/or physical therapy. At the time of publication, all patients had reported improvement in their clinical symptoms.14,15,17 The fourth patient was a man with human immunodeficiency virus (HIV) with 3 months of progressive thigh pain. Further evaluation found an isolated EG of the femoral diaphysis that progressed to pathologic fracture. He was treated with curettage and intramedullary nailing, and had improved symptoms and radiographic signs of healing at 30-month follow-up.16
An interesting case by Kerzl and colleagues11 reported a 63-year-old woman with a 24-year history of multiple symmetric lesions of the femora, leading to multiple pathologic fractures. Like our patient, her initial lesion was in the skull. Initial pathology specimens led to the diagnosis of EG. However, as the patient aged, she developed symptoms of diabetes insipidus and xanthelasma, which led to reevaluation of histology from 3 bony lesions. The patient was determined to have multifocal EG of the skull and femur, with simultaneous occurrence of Erdheim-Chester disease, which also causes bone lesions in addition to diabetes insipidus and xanthelasma.11
Though LCH was initially described more than 50 years ago, many aspects of LCH remain an enigma, especially in adults. The etiology of the disease is poorly understood. Controversy exists regarding whether LCH is primarily an immunoregulatory, neoplastic, or reactive disorder. The vast majority of adult cases described in the literature are EG, with very few cases of multisystem disseminated disease reported.5
The spectrum of disorders constituting LCH is heterogenous. Eosinophilic granuloma is the most common form, reportedly accounting for 60% to 70% of all cases, usually presenting as solitary bone lesions.6 Eosinophilic granuloma refers to the localized form of LCH, in which the disease is limited to bone or lung.18 This is the least aggressive form of the disease, with the most favorable prognosis. Hand- Schüller-Christian disease is a chronic, recurring form of LCH, with disseminated disease, affecting both bone and extraskeletal sites. Hand-Schüller-Christian disease is known for the classic triad of diabetes insipidus, exophthalmos, and destructive bone lesions. Patients may also present with otitis media or neurologic complaints from pathologic vertebral fractures. Letterer-Siwe disease refers to the acute, disseminated, fulminant form of LCH. This is the least common form of LCH and is predominately described in young children. Patients present with hepatosplenomegaly, lymphadenopathy, skin rash, fever, anemia, and thrombocytopenia.19 It is rapidly progressive, leading to multiorgan dysfunction and death within 1 to 2 years.18
The classification of LCH follows the Histiocyte Society guidelines developed from multicenter randomized trials in children.3 Classification is based on affected organs and is divided into 2 categories: single-system disease or multisystem disease. Single-system disease may be single site (bone, skin, or solitary lymph node) or multisite (multifocal bone disease or multiple lymph nodes). Multisystem disease is further classified into low-risk or risk groups. The low-risk group involves disseminated disease without involvement of risk organs (lungs, liver, spleen, and hematopoietic system). Involvement of 1 or more risk organs places the patient in the risk group, associated with the least favorable prognosis.3
In adults, the most common presenting symptoms are local pain from bony involvement, weight loss, and fever. Bony lesions most often occur in the skull, especially in the jaw. Long bones are less frequently involved, with lesions occurring in the long bones in approximately 17% of patients.3 The rib has also been reported as a common site of involvement in adults.5 Similar to children, diabetes insipidus remains a classic manifestation of LCH because of pituitary gland involvement. Other common symptoms of LCH in adults are cough, dyspnea, and chest pain from pulmonary involvement. Up to 20% to 30% of adult LCH patients have isolated pulmonary lesions, although pulmonary LCH may also occur as part of multisystem disease (risk group).3,4,20
Eosinophilic granuloma bone lesions have a variety of radiographic appearances but most commonly appear as lytic lesions. They often mimic aggressive lesions with permeative bone destruction, periostitis, ill-defined borders, and cortical erosion. Most lesions arise in the medullary space but can present as a destructive, cortically based lesion, as it did in our patient’s first femoral lesion. The differential diagnosis for a lytic medullary bone lesion includes benign entities, such as nonossifying fibromas, bone cysts, or osteomyelitis, but also includes malignant tumors, such as metastases, Ewing sarcoma, and lymphoma. A destructive, cortically based lesion in an adult should raise a very high suspicion for metastatic carcinoma until proven otherwise. Other diagnostic considerations for a cortically based lesion include chondromyxoid fibroma and surface bone lesions, such as surface chondroma and osteoma, or osteosarcoma (parosteal and periosteal). In the skull, lesions commonly erode the outer table more than the inner table (the typical “beveled-edge” appearance). Skull lesions also may have a small, central, dense focus within the lytic lesion (“button sequestrum”).
Bone scanning is often not as sensitive in detecting EG lesions compared with other bone tumors, although in our patient the bone scan was positive. In patients with a negative bone scan but a high index of suspicion, a radiographic skeletal survey should be obtained to rule out other lesions. MRI typically shows T2-hyperintense, T1-hypointense lesions with surrounding bone marrow edema and variable contrast enhancement, which is relatively nonspecific. The high sensitivity of MRI allows accurate delineation of the extent of the lesions and evaluates for the presence of an extraosseous soft-tissue component. Biopsy is generally necessary to establish a definitive histologic diagnosis. In our patient, despite her history of biopsy-proven EG, the aggressive appearance of a destructive, cortically based lesion made obtaining a biopsy critical to establish a definitive diagnosis in this case.
The histopathologic examination of the tissue from our patient was typical of that seen in patients with EG. It revealed tissue fragments with diffuse sheets of histiocytes displaying nuclear grooves, admixed numerous eosinophils with eosinophilic microabscesses, and scattered lymphocytes (Figures 6A, 6B). There were areas of necrosis, raising the possibility of osteomyelitis. However, the presence of classic histomorphologic features of LCH in the majority of the tissue fragments, along with CD1a- and S100-positivity in the histiocytes, confirmed the diagnosis of LCH (Figures 6C, 6D). Although not highly specific, a positive CD1a immunostain with the described histomorphologic findings in the proper clinical setting is often considered sufficient for LCH diagnosis. S100 is an important adjunct immunostain in the evaluation of histiocytic disorders. A positive S100 immunostain helps identify histiocytes, which are also CD1a-positive, because the latter immunostain can also be positive in some lymphomas and thymomas.21
After diagnosis of LCH has been confirmed, staging includes radiographs of any suspicious bone lesions, chest radiograph, bone scan, abdominal ultrasound, routine laboratory studies, and chest CT if pulmonary LCH is suspected.
The optimal treatment strategy for adult patients has not been clearly defined, and current strategies for LCH vary depending on organ involvement and extent of disease. Therapeutic options include observation, local treatment with steroids, local excision with curettage with or without bone grafting, chemotherapy, immunomodulation, irradiation, and stem cell transplantation in advanced disease. In general, patients who benefit from systemic therapy, such as chemotherapy or immunomodulation, include those with multisystem disease, refractory or recurrent lesions, and multifocal skeletal involvement.22
Patients with more limited disease, such as EG of bone, may undergo observation or local intralesional treatment. Eosinophilic granuloma of bone may resolve spontaneously and commonly does so when it is located in the pediatric spine. However, the therapeutic approach in adults with EG is controversial, given that spontaneous resolution is less likely to occur in the skeletally mature. Plasschaert and colleagues23 reported a recurrence rate of 26% in skeletally mature patients with EG of bone treated with biopsy followed by curettage with or without grafting. In the skeletally immature group, there were no clinical or radiographic signs of recurrence in the 2-year follow-up period.23 Thus, treatment in the adult population must be considered separate from the skeletally immature and in the appropriate clinical context. Depending on the location of the lesion, patients may become symptomatic or be at risk for pathologic fracture. In such circumstances, curettage with or without bone grafting and prophylactic internal fixation may be indicated. Other treatments, such as intralesional infiltration with corticosteroids, have been reported, but the role of such treatment in adults is undetermined.24,25 Radiation is typically not recommended in single-system disease unless a vital organ is threatened.26 Overall, patients with single-system disease have an excellent prognosis, and treatment should be determined on an individual basis.3
Eosinophilic granuloma represents less than 1% of all bone tumors, and adult presentation is very rare. The differential diagnosis of lytic bone lesions is broad and includes metastatic carcinoma, lymphoma/myeloma, osteomyelitis, osteoblastoma, aneurysmal bone cyst, and Ewing sarcoma. While EG is more common and easily diagnosed in children, it should be considered in the differential diagnosis in adults, so that the appropriate diagnostic workup and treatment can be performed.
Eosinophilic granuloma (EG) is the most common benign form of Langerhans cell histiocytosis (LCH). Initially described by Lichtenstein in 1953, LCH encompasses a triad of proliferative granulomatous disorders primarily affecting children: EG, Hand-Schüller-Christian disease, and Letterer-Siwe disease.1 Lichtenstein first termed the disease histiocytosis X, after recognizing that the 3 syndromes had the same histology.1 The term was updated after the clonal proliferation of Langerhans cells in the pathogenesis of the disease was discovered.
As LCH is generally considered a pediatric disease, there is little in the literature regarding adult-onset LCH. The incidence of LCH in adults is reported as 1 to 2 cases per million, significantly lower than that in children.2,3 Two studies have reported the mean age at diagnosis in adults as the fourth decade of life, and have suggested a male predominance.4,5 The vast majority of adult LCH cases described are simple EG, with very few cases of multisystem disseminated disease reported.5
Adult patients with LCH typically present with solitary lesions in bone. Approximately 10% of cases have extraosseous involvement, with the lung being the most common site.6 Lesions tend to be unifocal, with fewer than 10 reports describing multifocal EG.1,7-13 The axial skeleton is most frequently involved, with the majority of lesions occurring in the skull, ribs, vertebrae, or mandible.14 While less common, the femur, humerus, and clavicle are most often involved when the appendicular skeleton is affected.5
In a literature review, a few case reports describe adult-onset EG of the skull. Only 5 case reports since the 1970s describe adult patients with EG of the femur. We present a rare case of multifocal EG in a 48-year-old woman with lesions of the femur and skull, as well as a review of the literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 48-year-old woman presented with progressive right knee pain that was exacerbated by weight-bearing. She denied trauma, fevers, fatigue, or weight change. Her history was significant for an EG of the skull, excised at an outside institution 2 years prior to presentation. The patient also admitted to recent onset of right-sided skull pain, near the region of her previous surgery.
Physical examination demonstrated tenderness to palpation and fullness over the right medial distal femur and a normal neurovascular examination of the right lower extremity. Radiographs of the knee showed a cortically based, lytic, destructive lesion involving the medial femoral condyle, with soft-tissue extension (Figures 1A, 1B). Magnetic resonance imaging (MRI) of the right knee showed the lesion, with extraosseous soft-tissue extension (Figures 2A, 2B). The mass was isointense to muscle on T1-weighted images and hyperintense on T2-weighted images. Technetium bone scanning showed increased uptake in the right femur and the right skull (Figures 3A, 3B). MRI of the brain confirmed a new lesion in the right diploic space, distinct from the previous EG lesion site (Figures 4A-4D). An ultrasound-guided biopsy of the femur was performed and was consistent with EG.
After reevaluation and clearance by her neurosurgeon, the patient underwent curettage and allografting of the femoral lesion, with prophylactic internal fixation using a titanium distal femoral locking plate (Figure 5). Intraoperative frozen section was consistent with EG, which was confirmed with additional immunohistochemical workup (Figures 6A-6D).
The patient recovered uneventfully and follow-up radiographs showed restoration of the bony cortex of the medial femoral condyle (Figure 7). The second skull lesion, which was also consistent with EG, was excised by her neurosurgeon.
The patient remained asymptomatic until 2 years later, when she began experiencing mild pain in her right distal thigh and knee. Radiographs showed a new lytic focus in the right distal metadiaphysis (Figure 8) which was not present on her last radiograph 6 months prior. A computed tomography (CT) scan showed a lytic lesion involving the right distal femur medullary canal with cortical thinning and destruction, most pronounced posteriorly (Figures 9A, 9B). There was also an extraosseous soft-tissue component to the lesion. Bone scan showed increased uptake in the area of the new lesion. There was no increased uptake elsewhere, including the medial distal femur at the site of the old lesion, to suggest other lesions, and no increased uptake in the skull.
Given that the location of the lesion was distinct from the prior site of curettage and bone grafting, it was thought to be consistent with a new EG lesion. The patient underwent CT-guided biopsy, with simultaneous intralesional corticosteroid injection to treat the lesion when on-site pathology confirmed the etiology. Further surgical management was deemed unnecessary because internal fixation was present and spanned the new lesion. Final analysis of the fine-needle aspirate of the new lesion was positive for numerous eosinophils and histiocytes, consistent with EG.
At 6-week follow-up after the intralesional steroid injection, the patient’s pain continued to abate, and she was ambulating with crutches. Repeat CT scan of the right distal femur showed improvement of the extraosseous soft-tissue component, while the lucency in the femur itself remained unchanged. The decision was made to proceed with a second intralesional corticosteroid injection under CT guidance. The patient’s symptoms continued to improve, and repeat imaging 1 year after her steroid injections showed substantial bony healing with reconstitution of her cortical bone (Figures 10A-10E).
The patient had had 4 distinct tumors consistent with EG and was referred to a medical oncologist for further workup. The patient began treatment with zoledronic acid to prevent development of further lesions. At most recent follow-up, the patient was 18 months out from her second intralesional corticosteroid injection and was doing very well. She reported being pain-free and was walking 3 to 4 miles per week without gait aids. There was no evidence of new disease. The medial distal femur lesion was completely healed, and the distal metaphyseal lesion was nearly healed, with very little residual evidence of lesions.
Discussion
Adult-onset multifocal EG is a rare entity. Most affected patients develop lesions in the axial skeleton, with the skull, mandible, and vertebrae most commonly involved.14 Only 5 cases of femoral EG have been reported, one of which was multifocal.11,14-17
Of these patients, 3 were between the ages of 33 and 53 years and had insidious onset of hip pain that failed conservative management.14,15,17 Further imaging and biopsy revealed unifocal EG in the proximal femur in each case. Each patient received a different form of treatment, including curettage and radiation, radiofrequency ablation, and/or physical therapy. At the time of publication, all patients had reported improvement in their clinical symptoms.14,15,17 The fourth patient was a man with human immunodeficiency virus (HIV) with 3 months of progressive thigh pain. Further evaluation found an isolated EG of the femoral diaphysis that progressed to pathologic fracture. He was treated with curettage and intramedullary nailing, and had improved symptoms and radiographic signs of healing at 30-month follow-up.16
An interesting case by Kerzl and colleagues11 reported a 63-year-old woman with a 24-year history of multiple symmetric lesions of the femora, leading to multiple pathologic fractures. Like our patient, her initial lesion was in the skull. Initial pathology specimens led to the diagnosis of EG. However, as the patient aged, she developed symptoms of diabetes insipidus and xanthelasma, which led to reevaluation of histology from 3 bony lesions. The patient was determined to have multifocal EG of the skull and femur, with simultaneous occurrence of Erdheim-Chester disease, which also causes bone lesions in addition to diabetes insipidus and xanthelasma.11
Though LCH was initially described more than 50 years ago, many aspects of LCH remain an enigma, especially in adults. The etiology of the disease is poorly understood. Controversy exists regarding whether LCH is primarily an immunoregulatory, neoplastic, or reactive disorder. The vast majority of adult cases described in the literature are EG, with very few cases of multisystem disseminated disease reported.5
The spectrum of disorders constituting LCH is heterogenous. Eosinophilic granuloma is the most common form, reportedly accounting for 60% to 70% of all cases, usually presenting as solitary bone lesions.6 Eosinophilic granuloma refers to the localized form of LCH, in which the disease is limited to bone or lung.18 This is the least aggressive form of the disease, with the most favorable prognosis. Hand- Schüller-Christian disease is a chronic, recurring form of LCH, with disseminated disease, affecting both bone and extraskeletal sites. Hand-Schüller-Christian disease is known for the classic triad of diabetes insipidus, exophthalmos, and destructive bone lesions. Patients may also present with otitis media or neurologic complaints from pathologic vertebral fractures. Letterer-Siwe disease refers to the acute, disseminated, fulminant form of LCH. This is the least common form of LCH and is predominately described in young children. Patients present with hepatosplenomegaly, lymphadenopathy, skin rash, fever, anemia, and thrombocytopenia.19 It is rapidly progressive, leading to multiorgan dysfunction and death within 1 to 2 years.18
The classification of LCH follows the Histiocyte Society guidelines developed from multicenter randomized trials in children.3 Classification is based on affected organs and is divided into 2 categories: single-system disease or multisystem disease. Single-system disease may be single site (bone, skin, or solitary lymph node) or multisite (multifocal bone disease or multiple lymph nodes). Multisystem disease is further classified into low-risk or risk groups. The low-risk group involves disseminated disease without involvement of risk organs (lungs, liver, spleen, and hematopoietic system). Involvement of 1 or more risk organs places the patient in the risk group, associated with the least favorable prognosis.3
In adults, the most common presenting symptoms are local pain from bony involvement, weight loss, and fever. Bony lesions most often occur in the skull, especially in the jaw. Long bones are less frequently involved, with lesions occurring in the long bones in approximately 17% of patients.3 The rib has also been reported as a common site of involvement in adults.5 Similar to children, diabetes insipidus remains a classic manifestation of LCH because of pituitary gland involvement. Other common symptoms of LCH in adults are cough, dyspnea, and chest pain from pulmonary involvement. Up to 20% to 30% of adult LCH patients have isolated pulmonary lesions, although pulmonary LCH may also occur as part of multisystem disease (risk group).3,4,20
Eosinophilic granuloma bone lesions have a variety of radiographic appearances but most commonly appear as lytic lesions. They often mimic aggressive lesions with permeative bone destruction, periostitis, ill-defined borders, and cortical erosion. Most lesions arise in the medullary space but can present as a destructive, cortically based lesion, as it did in our patient’s first femoral lesion. The differential diagnosis for a lytic medullary bone lesion includes benign entities, such as nonossifying fibromas, bone cysts, or osteomyelitis, but also includes malignant tumors, such as metastases, Ewing sarcoma, and lymphoma. A destructive, cortically based lesion in an adult should raise a very high suspicion for metastatic carcinoma until proven otherwise. Other diagnostic considerations for a cortically based lesion include chondromyxoid fibroma and surface bone lesions, such as surface chondroma and osteoma, or osteosarcoma (parosteal and periosteal). In the skull, lesions commonly erode the outer table more than the inner table (the typical “beveled-edge” appearance). Skull lesions also may have a small, central, dense focus within the lytic lesion (“button sequestrum”).
Bone scanning is often not as sensitive in detecting EG lesions compared with other bone tumors, although in our patient the bone scan was positive. In patients with a negative bone scan but a high index of suspicion, a radiographic skeletal survey should be obtained to rule out other lesions. MRI typically shows T2-hyperintense, T1-hypointense lesions with surrounding bone marrow edema and variable contrast enhancement, which is relatively nonspecific. The high sensitivity of MRI allows accurate delineation of the extent of the lesions and evaluates for the presence of an extraosseous soft-tissue component. Biopsy is generally necessary to establish a definitive histologic diagnosis. In our patient, despite her history of biopsy-proven EG, the aggressive appearance of a destructive, cortically based lesion made obtaining a biopsy critical to establish a definitive diagnosis in this case.
The histopathologic examination of the tissue from our patient was typical of that seen in patients with EG. It revealed tissue fragments with diffuse sheets of histiocytes displaying nuclear grooves, admixed numerous eosinophils with eosinophilic microabscesses, and scattered lymphocytes (Figures 6A, 6B). There were areas of necrosis, raising the possibility of osteomyelitis. However, the presence of classic histomorphologic features of LCH in the majority of the tissue fragments, along with CD1a- and S100-positivity in the histiocytes, confirmed the diagnosis of LCH (Figures 6C, 6D). Although not highly specific, a positive CD1a immunostain with the described histomorphologic findings in the proper clinical setting is often considered sufficient for LCH diagnosis. S100 is an important adjunct immunostain in the evaluation of histiocytic disorders. A positive S100 immunostain helps identify histiocytes, which are also CD1a-positive, because the latter immunostain can also be positive in some lymphomas and thymomas.21
After diagnosis of LCH has been confirmed, staging includes radiographs of any suspicious bone lesions, chest radiograph, bone scan, abdominal ultrasound, routine laboratory studies, and chest CT if pulmonary LCH is suspected.
The optimal treatment strategy for adult patients has not been clearly defined, and current strategies for LCH vary depending on organ involvement and extent of disease. Therapeutic options include observation, local treatment with steroids, local excision with curettage with or without bone grafting, chemotherapy, immunomodulation, irradiation, and stem cell transplantation in advanced disease. In general, patients who benefit from systemic therapy, such as chemotherapy or immunomodulation, include those with multisystem disease, refractory or recurrent lesions, and multifocal skeletal involvement.22
Patients with more limited disease, such as EG of bone, may undergo observation or local intralesional treatment. Eosinophilic granuloma of bone may resolve spontaneously and commonly does so when it is located in the pediatric spine. However, the therapeutic approach in adults with EG is controversial, given that spontaneous resolution is less likely to occur in the skeletally mature. Plasschaert and colleagues23 reported a recurrence rate of 26% in skeletally mature patients with EG of bone treated with biopsy followed by curettage with or without grafting. In the skeletally immature group, there were no clinical or radiographic signs of recurrence in the 2-year follow-up period.23 Thus, treatment in the adult population must be considered separate from the skeletally immature and in the appropriate clinical context. Depending on the location of the lesion, patients may become symptomatic or be at risk for pathologic fracture. In such circumstances, curettage with or without bone grafting and prophylactic internal fixation may be indicated. Other treatments, such as intralesional infiltration with corticosteroids, have been reported, but the role of such treatment in adults is undetermined.24,25 Radiation is typically not recommended in single-system disease unless a vital organ is threatened.26 Overall, patients with single-system disease have an excellent prognosis, and treatment should be determined on an individual basis.3
Eosinophilic granuloma represents less than 1% of all bone tumors, and adult presentation is very rare. The differential diagnosis of lytic bone lesions is broad and includes metastatic carcinoma, lymphoma/myeloma, osteomyelitis, osteoblastoma, aneurysmal bone cyst, and Ewing sarcoma. While EG is more common and easily diagnosed in children, it should be considered in the differential diagnosis in adults, so that the appropriate diagnostic workup and treatment can be performed.
1. Lahiani D, Hammami BK, Maâloul I, et al. Multifocal Langerhans cell histiocytosis of bone: late revelation in a 76-year-old woman. Rev Med Interne. 2008;29(3):249-251.
2. Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28(1):9-14.
3. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76(5):363-368.
4. Aricò M, Girschikofsky M, Généreau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39(16):2341-2348.
5. Islinger RB, Kuklo TR, Owens BD, et al. Langerhans’ cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res. 2000;379:231-235.
6. Key SJ, O’Brien CJ, Silvester KC, Crean SJ. Eosinophilic granuloma: resolution of maxillofacial bony lesions following minimal intervention. Report of three cases and a review of the literature. J Craniomaxillofac Surg. 2004;32(3):170-175.
7. Bodner G, Kreczy A, Rachbauer F, Baechter O, Peer S. Eosinophilic granuloma of the bone: ultrasonographic imaging. Australas Radiol. 2002;46(4):418-421.
8. Boutsen Y, Esselinckx W, Delos M, Nisolle JF. Adult onset of multifocal eosinophilic granuloma of bone: a long-term follow-up with evaluation of various treatment options and spontaneous healing. Clin Rheumatol. 1999;18(1):69-73.
9. Corti F, Valicenti A, Bertolucci D, Bruno J, Gustinucci R. Multifocal Langerhans cell granulomatosis. Report of a clinical case. Minerva Med. 1994;85(7-8):413-416.
10. Demirci I. Adult eosinophilic granuloma of the lumbar spine with atypical dissemination. Case report: a long-term follow-up. Zentralbl Neurochir. 2004;65(2):84-87.
11. Kerzl R, Eyerich K, Eberlein B, et al. Parallel occurrence of Erdheim-Chester disease and eosinophilic granuloma in the same patient. J Eur Acad Dermatol Venereol. 2009;23(2):224-226.
12. Nguyen BD, Roarke MC, Chivers SF. Multifocal Langerhans cell histiocytosis with infiltrative pelvic lesions: PET/CT imaging. Clin Nucl Med. 2010;35(10): 824-826.
13. Scolozzi P, Lombardi T, Monnier P, Jaques B. Multisystem Langerhans’ cell histiocytosis (Hand-Schuller-Christian disease) in an adult: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2004;261(6):326-330.
14. King JJ, Melvin JS, Iwenofu OH, Fox EJ. Thigh pain in a 53-year-old woman. Clin Orthop Relat Res. 2009;467(6):1652-1657.
15. Hair LC, Deyle GD. Eosinophilic granuloma in a patient with hip pain. J Orthop Sports Phys Ther. 2011;41(2):119.
16. Panayiotakopoulos GD, Sipsas NV, Kontos A, et al. Eosinophilic granuloma of the femur in an HIV-1 positive patient. AIDS Patient Care STDS. 2002;16(3):103-106.
17. Rodrigues RJ, Lewis HH. Eosinophilic granuloma of bone. Review of literature and case presentation. Clin Orthop Relat Res. 1971;77:183-192.
18. Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics. 1992;12(4):801-823.
19. Lichtenstein L. Histiocytosis X (eosinophilic granuloma of bone, Letterer-Siwe disease, and Schueller-Christian disease). Further observations of pathological and clinical importance. J Bone Joint Surg Am. 1964;46:76-90.
20. Götz G, Fichter J. Langerhans’-cell histiocytosis in 58 adults. Eur J Med Res. 2004;9(11):510-514.
21. Cheng KL, Glu PG, Weiss LM. Hematopoeitic tumors. In: Peiguo C, Weiss L, eds. Modern Immunohistochemistry. New York, NY: Cambridge University Press; 2009:503.
22. Broadbent V, Gadner H. Current therapy for Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12(2):327-338.
23. Plasschaert F, Craig C, Bell R, Cole WG, Wunder JS, Alman BA. Eosinophilic granuloma. A different behaviour in children than in adults. J Bone Joint Surg Br. 2002;84(6):870-872.
24. Capanna R, Springfield DS, Ruggieri P, et al. Direct cortisone injection in osinophilic granuloma of bone: a preliminary report on 11 patients. J Pediatr Orthop. 1985;5(3):339-342.
25. Egeler RM, Thompson RC Jr, Voûte PA, Nesbit ME Jr. Intralesional infiltration of corticosteroids in localized Langerhans’ cell histiocytosis. J Pediatr Orthop. 1992;12(6):811-814.
26. Ladisch S, Gadner H. Treatment of Langerhans cell histiocytosis–evolution and current approaches. Br J Cancer Suppl. 1994;23:S41-S46.
1. Lahiani D, Hammami BK, Maâloul I, et al. Multifocal Langerhans cell histiocytosis of bone: late revelation in a 76-year-old woman. Rev Med Interne. 2008;29(3):249-251.
2. Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28(1):9-14.
3. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76(5):363-368.
4. Aricò M, Girschikofsky M, Généreau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39(16):2341-2348.
5. Islinger RB, Kuklo TR, Owens BD, et al. Langerhans’ cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res. 2000;379:231-235.
6. Key SJ, O’Brien CJ, Silvester KC, Crean SJ. Eosinophilic granuloma: resolution of maxillofacial bony lesions following minimal intervention. Report of three cases and a review of the literature. J Craniomaxillofac Surg. 2004;32(3):170-175.
7. Bodner G, Kreczy A, Rachbauer F, Baechter O, Peer S. Eosinophilic granuloma of the bone: ultrasonographic imaging. Australas Radiol. 2002;46(4):418-421.
8. Boutsen Y, Esselinckx W, Delos M, Nisolle JF. Adult onset of multifocal eosinophilic granuloma of bone: a long-term follow-up with evaluation of various treatment options and spontaneous healing. Clin Rheumatol. 1999;18(1):69-73.
9. Corti F, Valicenti A, Bertolucci D, Bruno J, Gustinucci R. Multifocal Langerhans cell granulomatosis. Report of a clinical case. Minerva Med. 1994;85(7-8):413-416.
10. Demirci I. Adult eosinophilic granuloma of the lumbar spine with atypical dissemination. Case report: a long-term follow-up. Zentralbl Neurochir. 2004;65(2):84-87.
11. Kerzl R, Eyerich K, Eberlein B, et al. Parallel occurrence of Erdheim-Chester disease and eosinophilic granuloma in the same patient. J Eur Acad Dermatol Venereol. 2009;23(2):224-226.
12. Nguyen BD, Roarke MC, Chivers SF. Multifocal Langerhans cell histiocytosis with infiltrative pelvic lesions: PET/CT imaging. Clin Nucl Med. 2010;35(10): 824-826.
13. Scolozzi P, Lombardi T, Monnier P, Jaques B. Multisystem Langerhans’ cell histiocytosis (Hand-Schuller-Christian disease) in an adult: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2004;261(6):326-330.
14. King JJ, Melvin JS, Iwenofu OH, Fox EJ. Thigh pain in a 53-year-old woman. Clin Orthop Relat Res. 2009;467(6):1652-1657.
15. Hair LC, Deyle GD. Eosinophilic granuloma in a patient with hip pain. J Orthop Sports Phys Ther. 2011;41(2):119.
16. Panayiotakopoulos GD, Sipsas NV, Kontos A, et al. Eosinophilic granuloma of the femur in an HIV-1 positive patient. AIDS Patient Care STDS. 2002;16(3):103-106.
17. Rodrigues RJ, Lewis HH. Eosinophilic granuloma of bone. Review of literature and case presentation. Clin Orthop Relat Res. 1971;77:183-192.
18. Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics. 1992;12(4):801-823.
19. Lichtenstein L. Histiocytosis X (eosinophilic granuloma of bone, Letterer-Siwe disease, and Schueller-Christian disease). Further observations of pathological and clinical importance. J Bone Joint Surg Am. 1964;46:76-90.
20. Götz G, Fichter J. Langerhans’-cell histiocytosis in 58 adults. Eur J Med Res. 2004;9(11):510-514.
21. Cheng KL, Glu PG, Weiss LM. Hematopoeitic tumors. In: Peiguo C, Weiss L, eds. Modern Immunohistochemistry. New York, NY: Cambridge University Press; 2009:503.
22. Broadbent V, Gadner H. Current therapy for Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12(2):327-338.
23. Plasschaert F, Craig C, Bell R, Cole WG, Wunder JS, Alman BA. Eosinophilic granuloma. A different behaviour in children than in adults. J Bone Joint Surg Br. 2002;84(6):870-872.
24. Capanna R, Springfield DS, Ruggieri P, et al. Direct cortisone injection in osinophilic granuloma of bone: a preliminary report on 11 patients. J Pediatr Orthop. 1985;5(3):339-342.
25. Egeler RM, Thompson RC Jr, Voûte PA, Nesbit ME Jr. Intralesional infiltration of corticosteroids in localized Langerhans’ cell histiocytosis. J Pediatr Orthop. 1992;12(6):811-814.
26. Ladisch S, Gadner H. Treatment of Langerhans cell histiocytosis–evolution and current approaches. Br J Cancer Suppl. 1994;23:S41-S46.
Postsurgical Analgesic Found to Decrease Opioid Use, Hospital Stay, and Readmission Rates After Knee Replacement Surgery
DALLAS—Positive data about the use of Exparel (bupivacaine liposome injectable suspension) as a postsurgical analgesic following total knee replacement surgery was presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
The study, which compared the use of bupivacaine liposome injectable suspension infiltration to the standard of care in 1,110 patients, found that bupivacaine liposome injectable suspension was associated with significant improvements in a variety of patient and health economic outcomes, including opioid use, hospital stay, and readmission rate.
Patients who underwent total knee arthroplasty (TKA) received identical pre-, intra-, and postoperative pain management protocols, with the exception of 527 patients who received bupivacaine liposome injectable suspension infiltration in place of a femoral nerve block.
The study authors compared several patient and cost-related outcomes. Opioid use during hospitalization was statistically significantly reduced in the bupivacaine liposome injectable suspension group. Other key findings included:
• Shorter hospital length of stay (2.93 days for the bupivacaine liposome injectable suspension group vs 3.19 days for the femoral nerve block group, P<0.001)
• Increased rate of discharge to home (77.8% for the bupivacaine liposome injectable suspension group vs 72.21% for the femoral nerve block group, P=0.032)
• Reduced inpatient fall rate (0.56% for the bupivacaine liposome injectable suspension group vs 2.11% for the femoral nerve block group, P=0.03)
• Lower 30-day all-cause readmission rate (0.95% for the bupivacaine liposome injectable suspension group vs 2.57% for the femoral nerve block group, P=0.041)
“Based on our analysis, incorporating liposomal bupivacaine into the postsurgical analgesic protocol following total knee arthroplasty has significant and quantifiable benefits to both the patient and the institution,” said Richard Iorio, MD, Professor of Orthopaedic Surgery at NYU School of Medicine in New York. “The measurable opioid-sparing effect of this new regimen has enabled us to virtually eliminate intravenous patient-controlled analgesia, or PCA, devices from the standard of care in total joint arthroplasty patients, without compromising patient comfort. In addition, we found that the incremental cost of adding this new modality was offset by meaningful savings from shorter anesthesia induction time in the operating room, shorter hospital stays and lower rates of 30-day readmission.”
DALLAS—Positive data about the use of Exparel (bupivacaine liposome injectable suspension) as a postsurgical analgesic following total knee replacement surgery was presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
The study, which compared the use of bupivacaine liposome injectable suspension infiltration to the standard of care in 1,110 patients, found that bupivacaine liposome injectable suspension was associated with significant improvements in a variety of patient and health economic outcomes, including opioid use, hospital stay, and readmission rate.
Patients who underwent total knee arthroplasty (TKA) received identical pre-, intra-, and postoperative pain management protocols, with the exception of 527 patients who received bupivacaine liposome injectable suspension infiltration in place of a femoral nerve block.
The study authors compared several patient and cost-related outcomes. Opioid use during hospitalization was statistically significantly reduced in the bupivacaine liposome injectable suspension group. Other key findings included:
• Shorter hospital length of stay (2.93 days for the bupivacaine liposome injectable suspension group vs 3.19 days for the femoral nerve block group, P<0.001)
• Increased rate of discharge to home (77.8% for the bupivacaine liposome injectable suspension group vs 72.21% for the femoral nerve block group, P=0.032)
• Reduced inpatient fall rate (0.56% for the bupivacaine liposome injectable suspension group vs 2.11% for the femoral nerve block group, P=0.03)
• Lower 30-day all-cause readmission rate (0.95% for the bupivacaine liposome injectable suspension group vs 2.57% for the femoral nerve block group, P=0.041)
“Based on our analysis, incorporating liposomal bupivacaine into the postsurgical analgesic protocol following total knee arthroplasty has significant and quantifiable benefits to both the patient and the institution,” said Richard Iorio, MD, Professor of Orthopaedic Surgery at NYU School of Medicine in New York. “The measurable opioid-sparing effect of this new regimen has enabled us to virtually eliminate intravenous patient-controlled analgesia, or PCA, devices from the standard of care in total joint arthroplasty patients, without compromising patient comfort. In addition, we found that the incremental cost of adding this new modality was offset by meaningful savings from shorter anesthesia induction time in the operating room, shorter hospital stays and lower rates of 30-day readmission.”
DALLAS—Positive data about the use of Exparel (bupivacaine liposome injectable suspension) as a postsurgical analgesic following total knee replacement surgery was presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
The study, which compared the use of bupivacaine liposome injectable suspension infiltration to the standard of care in 1,110 patients, found that bupivacaine liposome injectable suspension was associated with significant improvements in a variety of patient and health economic outcomes, including opioid use, hospital stay, and readmission rate.
Patients who underwent total knee arthroplasty (TKA) received identical pre-, intra-, and postoperative pain management protocols, with the exception of 527 patients who received bupivacaine liposome injectable suspension infiltration in place of a femoral nerve block.
The study authors compared several patient and cost-related outcomes. Opioid use during hospitalization was statistically significantly reduced in the bupivacaine liposome injectable suspension group. Other key findings included:
• Shorter hospital length of stay (2.93 days for the bupivacaine liposome injectable suspension group vs 3.19 days for the femoral nerve block group, P<0.001)
• Increased rate of discharge to home (77.8% for the bupivacaine liposome injectable suspension group vs 72.21% for the femoral nerve block group, P=0.032)
• Reduced inpatient fall rate (0.56% for the bupivacaine liposome injectable suspension group vs 2.11% for the femoral nerve block group, P=0.03)
• Lower 30-day all-cause readmission rate (0.95% for the bupivacaine liposome injectable suspension group vs 2.57% for the femoral nerve block group, P=0.041)
“Based on our analysis, incorporating liposomal bupivacaine into the postsurgical analgesic protocol following total knee arthroplasty has significant and quantifiable benefits to both the patient and the institution,” said Richard Iorio, MD, Professor of Orthopaedic Surgery at NYU School of Medicine in New York. “The measurable opioid-sparing effect of this new regimen has enabled us to virtually eliminate intravenous patient-controlled analgesia, or PCA, devices from the standard of care in total joint arthroplasty patients, without compromising patient comfort. In addition, we found that the incremental cost of adding this new modality was offset by meaningful savings from shorter anesthesia induction time in the operating room, shorter hospital stays and lower rates of 30-day readmission.”
Medial Patellar Subluxation: Diagnosis and Treatment
Medial patellar subluxation (MPS) is a disabling condition caused by an imbalance in the medial and lateral forces in the normal knee, allowing the patella to displace medially. Normally, the patella glides appropriately in the femoral trochlea, but alteration in this medial–lateral equilibrium can lead to pain and instability.1 MPS was first described in 1987 by Betz and colleagues2 as a complication of lateral retinacular release. Since then, multiple cases of iatrogenic, traumatic, and isolated medial subluxation have been reported.3–15 However, MPS after lateral release is the most common cause, accounting for the majority of published cases, whereas only 8 cases of isolated MPS have been reported to date.
Optimal treatment for MPS is not well understood. To better comprehend and manage MPS, we must fully appreciate the pathoanatomy, biomechanics, and current research. In this review, we focus on the anatomy of the lateral retinaculum, diagnosis and treatment of MPS, and outcomes of current treatment techniques.
Anatomy
In 1980, Fulkerson and Gossling16 delineated the anatomy of the knee joint lateral retinaculum. They described a 2-layered system with separate distinct anatomical structures. The lateral retinaculum is oriented longitudinally with the knee extended but exerts a posterolateral force on the lateral aspect of the patella as the knee is flexed. The superficial layer is composed of oblique fibers of the lateral retinaculum originating from the iliotibial band and the vastus lateralis fascia and inserting into the lateral margin of the patella and the patella tendon. The deep layer of the retinaculum consists of several structures, including the deep transverse retinaculum, lateral patellofemoral ligament (LPFL), and the patellotibial band.
Over the years, several studies have described the importance of the lateral retinaculum and, in particular, the LPFL. Examining the functional anatomy of the knee in 1962, Kaplan17 first described the lateral epicondylopatellar ligament as a palpable thickening of the joint capsule. Reider and colleagues18 later named this structure the lateral patellofemoral ligament in their anatomical study of 21 fresh cadaver knees. They described its width as ranging from 3 to 10 mm. In a comprehensive cadaveric study of the LPFL, Navarro and colleagues19,20 found it to be a distinct structure present in all 20 of their dissected specimens. They found its femoral insertion at the lateral epicondyle with a fanlike expansion of the fibers predominantly in the posterior region proximal to the lateral epicondyle. The patellar insertion was found in the posterior half and upper lateral aspect, also with expanded fibers. Mean length of the LPFL is 42.1 mm, and mean width is 16.1 mm.
Medial and lateral forces are balanced in a normal knee, and the patella glides appropriately in the femoral trochlea. Alteration in this medial–lateral equilibrium can lead to pain and instability.1 Normally, the patella lies laterally with the knee extended, but in early flexion the patella moves medially as it engages in the trochlea. As the knee continues to flex, the patella flexes and translates distally.21 By 45°, the patella is fully engaged in the trochlear groove throughout the remainder of the knee’s range of motion (ROM).
Lateral release procedures, as described in the literature, result in sectioning of both layers of the lateral retinaculum. In a biomechanical study, Merican and colleagues22 found that staged release of the lateral retinaculum reduced the medial stability of the patellofemoral joint progressively, making it easier to push the patella medially. At 30° of flexion, the transverse fibers of the midsection of the lateral retinaculum were found to be the main contributor to the lateral restraint of the patella. When the release extends too far proximally, the transverse fibers that anchor the lateral patella and the vastus lateralis oblique tendon to the iliotibial band are disrupted. Subsequent loss of a dynamic muscular pull in the orientation of the lateral stabilizing structures results in medial subluxation in a range from full knee extension to about 30° of flexion.
Furthermore, the attachments of the LPFL and the orientation of its fibers suggest that the LPFL may have a significant role in limiting medial excursion of the patella. Vieira and colleagues23 resected the LPFL in 10 fresh cadaver knees. They noticed that, after resection, the patella spontaneously traveled medially, demonstrating the importance of this ligament in patellar stability. In cases of isolated MPS, there have been no reports of associated pathology, such as muscular imbalance or coronal/rotational malalignment of the lower extremity. With an intact lateral retinaculum, medial subluxation is likely caused by pathology in the normal histologic structure of the LPFL and lateral retinaculum. However, the histologic structure of the LPFL and its contribution to the understanding of the pathoetiology of MPS have not been documented.
Diagnosis
MPS diagnosis can be challenging. Often, clinical examination findings are subtle, and radiographs may not show significant pathology. The most accurate diagnosis is obtained by combining patient history, physical examination findings, imaging studies, and diagnostic arthroscopy.
Patient History
Patients with MPS report chronic pain localized to the inferior medial patella and anterior-medial joint line. Occasionally, they complain of crepitus and intermittent swelling. Other symptoms include pain with knee flexion activity, such as squatting and climbing or descending stairs. Some patients describe episodes of giving way and feelings of instability. Often, they are aware the direction of instability is medial. The pain typically is not relieved by medication, physical therapy, or bracing.
Physical Examination
MPS must be identified by clinical examination. Peripatellar tenderness is typically noted. There is often no effusion or crepitus, but the patella is unstable in early flexion. Active and passive ROM is painful through the first 30° of knee flexion. The patient may have a positive medial apprehension test7 in which he or she experiences apprehension of the patella being subluxated with a medially directed force on the lateral border of the patella.
The gravity subluxation test described by Nonweiler and DeLee6 is useful in detecting MPS after lateral release and indicates that the vastus lateralis muscle has been detached from the patella and that the lateral retinaculum is lax. In this test, the patient is positioned in the lateral decubitus position with the involved knee farthest from the table. In this position, gravity causes the patella to subluxate out of the trochlea. The test is positive for MPS when a voluntary contraction of the quadriceps does not center the patella into the trochlear groove. Patients with MPS without previous lateral release can have the patella subluxate medially in the lateral decubitus position, but it is pulled back into the trochlea with active quadriceps contraction (Figure 1).
Patients with MPS often have lateral patellar laxity (LPL), which allows the patella to rotate upward on the lateral side and skid across the medial facet of the femoral trochlea. A physical examination sign combining lateral patellar glide and tilt was described by Shneider24 to identify LPL. This “lateral patellar float” sign is present when the patella translates laterally and rotates or tilts upward with medial pressure on the patella (Figure 2). Another maneuver to test for subtle MPS involves manually centering the patella in the trochlea during active knee flexion and extension. The involved knee is examined in the seated position. The examiner attempts to center the patella in the trochlea with a laterally directed force from the examiner’s thumb on the medial border of the patella. This will usually provide immediate relief as the patient actively ranges the knee.
Imaging Studies
Diagnostic imaging is a crucial component of the evaluation and treatment decision process. Plain radiographs often are not helpful in diagnosing MPS but may provide additional information.5 A variety of radiographic measurements have been described as indicators of structural disease, but there is a lack of comprehensive information recommending radiographic evaluation and interpretation of patients with patellofemoral dysfunction. It is crucial that orthopedic surgeons have common and consistent radiographic views for plain radiographic assessment that can serve as a basis for accurate diagnosis and surgical decision-making.
Standard knee radiographs should include a standing anteroposterior view of bilateral knees, a standing lateral view of the symptomatic knee in 30° of flexion, a patellar axial view, and a tunnel view. These views, occasionally combined with magnetic resonance imaging (MRI), can yield information vital to surgical decision-making. Image quality is highly technique-dependent, and variability in patient positioning can substantially affect the ability to properly diagnose structural abnormalities. For improved diagnostic accuracy and disease classification, radiographs must be obtained with use of the same standardized imaging protocol.
Kinetic MRI was shown by Shellock and colleagues25 to provide diagnostic information related to patellar malalignment. As kinetic MRI can image the patellofemoral joint within the initial 20° to 30° of flexion, it is useful in detecting some of the more subtle patellar tracking problems. In their study of 43 knees (40 patients) with symptoms after lateral release, Shellock and colleagues25 found that 27 knees (63%) had medial subluxation of the patella as the knee moved from extension to flexion. Furthermore, MPS was noted on the contralateral, unoperated knee in 17 (43%) of the 40 patients.
Diagnostic Arthroscopy
Once MPS is suspected after a thorough history and physical examination, examination under anesthesia accompanied by diagnostic arthroscopy confirms the diagnosis. Lateral forces are applied to the patella in full knee extension and 30° of flexion (Figure 3). During arthroscopy, the patellofemoral compartment is viewed from the anterolateral portal. With the knee at full extension, the lateral laxity and medial tilt of the patella can be identified (Figure 4). As the knee is flexed to 30°, the patella moves medially and can subluxate over the edge of the medial facet of the trochlea (Figure 5).
Treatment
Nonsurgical Management
Treatment of MPS depends entirely on making an accurate diagnosis and determining the degree of impairment. Patients with symptomatic MPS should initially undergo supervised rehabilitation focusing on balancing the medial and lateral forces that influence patellar tracking. Patients should be evaluated for specific muscle tightness, weakness, and biomechanical abnormalities. Each problem should be addressed with an individualized rehabilitation prescription. Emphasis is placed on balance, proprioception, and strengthening of the quadriceps, hip abductors/external rotators, and abdominal core muscle groups.
In some patients, symptomatic MPS may be reduced with a patella-stabilizing brace with a medial buttress.3,5,26 Although bracing should be regarded as an adjuvant to a structured physical therapy program, it can also be helpful in confirming the diagnosis of MPS. Shannon and Keene3 reported that all patients in their study experienced significant pain relief and decreased medial patellar subluxations when they wore a medial patella–stabilizing brace. Shellock and colleagues25 used kinematic MRI to investigate the effect of a patella-realignment brace and found that bracing counteracted patellar subluxation in the majority of knees studied.
Surgical Management
When conservative management fails and patients continue to experience pain and instability, surgical intervention is often required. Although various surgical techniques have been used (Table),3–6,8–10,14,15,27,28 the optimal surgical treatment for MPS has not been identified.
Lateral Retinaculum Imbrication. Lateral retinaculum imbrication has been used to centralize patella tracking and stabilize the patella. Richman and Scheller5 reported on a 17-year-old patient who had isolated medial subluxation of the patella without having undergone a previous lateral release. At 3-month follow-up, there was no recurrent instability; there was only intermittent medial knee soreness with weight-bearing activity.
Lateral Retinaculum Repair/Reconstruction. Hughston and colleagues8 treated 65 knees for MPS. Most had undergone lateral release. Of the 65 knees, 39 were treated with direct repair of the lateral retinaculum, and 26 with reconstruction of the lateral patellotibial ligament using locally available tissue, such as strips of iliotibial band or patellar tendon. Results were good to excellent in 80% of patients at a mean follow-up of 53.7 months. Nonweiler and DeLee6 reconstructed the lateral retinaculum in 5 patients with MPS that developed after isolated lateral retinacular release. Four (80%) of the 5 patients had no symptoms or physical signs of instability at a mean follow-up of 3.3 years. Results were excellent (3 knees) and good (2 knees) according to the Merchant and Mercer rating scale. Akşahin and colleagues28 reported on a single case of spontaneous medial patellar instability. At surgery, imbrication of the lateral structures failed to prevent the medial subluxation. Lateral patellotibial ligament augmentation was performed using an iliotibial band flap that effectively corrected the instability. At 1 year, the patient was characterized as engaging in vigorous recreational activity, according to the clinical score defined by Hughston and colleagues.8 He had mild pain with competitive sports but no pain with daily activity. Abhaykumar and Craig9 reported on 4 surgically treated knees with medial instability. They reconstructed the lateral retinaculum using a strip of fascia lata. By follow-up (5-7 years), each knee had its instability resolved and full ROM restored. Johnson and Wakeley26 reported on a case of iatrogenic MPS after lateral release. Treatment consisted of mobilization and direct repair of the lateral retinaculum. At 12-month follow-up, there was no instability. Although symptom-free with light activity, the patient had patellofemoral pain with strenuous activity. Sanchis-Alfonso and colleagues14 reported the results of isolated lateral retinacular reconstruction for iatrogenic MPS in 17 patients. At mean follow-up of 56 months, results were good or excellent in 65% of patients, and the Lysholm score improved from 36.4 preoperatively to 86.1 postoperatively.
Medial Retinaculum Release. Medial retinaculum release has been used as an alternative to open reconstruction. Shannon and Keene3 reported the results of medial retinacular release procedures on 9 knees. Four (44%) of the 9 patients had either spontaneous or traumatic onset of instability. All cases were treated with arthroscopic medial retinacular release, extending 2 cm medial to the superior pole of the patella down to the anteromedial portal. This avoided releasing the attachment of the vastus medialis oblique muscle to the patella and removing its dynamic medial stabilizing force. At a mean follow-up of 2.7 years, both medial subluxation and knee pain were relieved in all 9 knees without complications or further realignment surgery. Results were excellent in 6 knees (66.7%) and good in 3 knees (33.3%). Shannon and Keene3 emphasized that the procedure should not be used in patients with hypermobile patellae or in cases of failed lateral retinacular releases in which MPS is not clearly and carefully documented.
LPFL Reconstruction. Before coming to our practice, most patients have tried several months of formal physical rehabilitation, medications, and bracing. Many have already had surgical procedures, including arthroscopy, lateral release, and tibial tubercle transfer. When the diagnosis of MPS is suspected after a thorough history and physical examination, LPFL reconstruction is offered. Management of MPS with LPFL reconstruction has yielded excellent and reliable clinical results. Teitge and Torga Spak10 described an LPFL reconstruction technique that is used as a salvage procedure in managing medial iatrogenic patellar instability (the patient’s own quadriceps tendon is used). In their experience, direct repair or imbrication of the lateral retinaculum failed to provide long-term stability because medial excursion usually appeared after 1 year. The 60 patients’ outcomes were excellent with respect to patellar stability, and there were no cases of recurrent subluxation. Borbas and colleagues15 reported a case of LPFL reconstruction in a symptomatic medial subluxated patella resulting from TKA and extended lateral release. Using a free gracilis autograft through patellar bone tunnels to reconstruct the LPFL, the patient was free of pain and very satisfied with the result at 1 year postoperatively. Our current strategy is anatomical reconstruction of the LPFL using a quadriceps tendon graft and no bone tunnels, screws, or anchors in the patella.27 We previously reported a single case of isolated medial instability.4 At 2-year follow-up, there was no recurrent instability, and the functional outcome was excellent. This LPFL reconstruction method has been used in 10 patients with isolated MPS. There has been no residual medial subluxation on follow-up ranging from 3 months to 2 years. Outcome studies are in progress.
Rehabilitation. The initial goal of rehabilitation after surgical reconstruction of the lateral retinaculum or LPFL is to protect the healing soft tissues, restore normal knee ROM, and normalize gait. The knee is immobilized in a brace for weight-bearing activity for 4 to 6 weeks, until limb control is sufficient to prevent rotational stress on the knee. Gradual increase to full weight-bearing without bracing is permitted as quadriceps strength is restored. As motion is regained, strength, balance, and proprioception are emphasized for the entire lower extremity and core.
Functional limb training, including rotational activity, begins at 12 weeks. As strength and neuromuscular control progress, single-leg activity may be started with particular attention to proper alignment of the pelvis and the entire lower extremity. For competitive or recreational athletes, the final stages of rehabilitation focus on dynamic lower extremity control during sport-specific movements. Patients return to unrestricted activity by 6 months to 1 year after surgery.
Summary
MPS is a disabling condition that can limit daily functional activity because of apprehension and pain. Initially described as a complication of lateral retinacular release, isolated MPS can occur in the absence of a previous lateral release. Thorough physical examination and identification during arthroscopy are crucial for proper MPS diagnosis and management. When nonsurgical measures fail, LPFL reconstruction can provide patellofemoral stability and excellent functional outcomes.
1. Marumoto JM, Jordan C, Akins R. A biomechanical comparison of lateral retinacular releases. Am J Sports Med. 1995;23(2):151-155.
2. Betz RR, Magill JT, Lonergan RP. The percutaneous lateral retinacular release. Am J Sports Med. 1987;15(5):477-482.
3. Shannon BD, Keene JS. Results of arthroscopic medial retinacular release for treatment of medial subluxation of the patella. Am J Sports Med. 2007;35(7):1180-1187.
4. Saper MG, Shneider DA. Medial patellar subluxation without previous lateral release: a case report. J Pediatr Orthop B. 2014;23(4):350-353.
5. Richman NM, Scheller AD Jr. Medial subluxation of the patella without previous lateral retinacular release. Orthopedics. 1998;21(7):810-813.
6. Nonweiler DE, DeLee JC. The diagnosis and treatment of medial subluxation of the patella after lateral retinacular release. Am J Sports Med. 1994;22(5):680-686.
7. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
8. Hughston JC, Flandry F, Brinker MR, Terry GC, Mills JC 3rd. Surgical correction of medial subluxation of the patella. Am J Sports Med. 1996;24(4):486-491.
9. Abhaykumar S, Craig DM. Fascia lata sling reconstruction for recurrent medial dislocation of the patella. The Knee. 1999;6(1):55-57.
10. Teitge RA, Torga Spak R. Lateral patellofemoral ligament reconstruction. Arthroscopy. 2004;20(9):998-1002.
11. Kusano M, Horibe S, Tanaka Y, et al. Simultaneous MPFL and LPFL reconstruction for recurrent lateral patellar dislocation with medial patellofemoral instability. Asia-Pac J Sports Med Arthrosc Rehabil Technol. 2014;1:42-46.
12. Saper MG, Shneider DA. Simultaneous medial and lateral patellofemoral ligament reconstruction for combined medial and lateral patellar subluxation. Arthrosc Tech. 2014,3(2):e227-e231.
13. Udagawa K, Niki Y, Matsumoto H, et al. Lateral patellar retinaculum reconstruction for medial patellar instability following lateral retinacular release: a case report. Knee. 2014;21(1):336-339.
14. Sanchis-Alfonso V, Montesinos-Berry E, Monllau JC, Merchant AC. Results of isolated lateral retinacular reconstruction for iatrogenic medial patellar instability. Arthroscopy. 2015;31(3):422-427.
15. Borbas P, Koch PP, Fucentese SF. Lateral patellofemoral ligament reconstruction using a free gracilis autograft. Orthopedics. 2014;37(7):e665-e668.
16. Fulkerson JP, Gossling H. Anatomy of the knee joint lateral retinaculum. Clin Orthop Relat Res. 1980;153:183-188.
17. Kaplan E. Some aspects of functional anatomy of the human knee joint. Clin Orthop Relat Res. 1962;23:18-29.
18. Reider B, Marshall J, Koslin B, Ring B, Girgis F. The anterior aspect of the knee joint. J Bone Joint Surg Am. 1981;63(3):351-356.
19. Navarro MS, Navarro RD, Akita Junior J, Cohen M. Anatomical study of the lateral patellofemoral ligament in cadaver knees. Rev Bras Ortop. 2008;43(7):300-307.
20. Navarro MS, Beltrani Filho CA, Akita Junior J, Navarro RD, Cohen M. Relationship between the lateral patellofemoral ligament and the width of the lateral patellar facet. Acta Ortop Bras. 2010;18(1):19-22.
21. Salsich GB, Ward SR, Terk MR, Powers CM. In vivo assessment of patellofemoral joint contact area in individuals who are pain free. Clin Orthop Relat Res. 2003;417:277-284.
22. Merican AM, Kondo E, Amis AA. The effect on patellofemoral joint stability of selective cutting of lateral retinacular and capsular structures. J Biomech. 2009;42(3):291-296.
23. Vieira EL, Vieira EÁ, da Silva RT, Berlfein PA, Abdalla RJ, Cohen M. An anatomic study of the iliotibial tract. Arthroscopy. 2007;23(3):269-274.
24. Shneider DA. Lateral patellar laxity—identification, significance, treatment. Poster session presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 25-28, 2009; Las Vegas, NV.
25. Shellock FG, Mink JH, Deutsch A, Fox JM, Ferkel RD. Evaluation of patients with persistent symptoms after lateral retinacular release by kinematic magnetic resonance imaging of the patellofemoral joint. Arthroscopy. 1990;6(3):226-234.
26. Johnson DP, Wakeley C. Reconstruction of the lateral patellar retinaculum following lateral release: a case report. Knee Surg Sports Traumatol Arthrosc. 2002;10(6):361-363.
27. Saper MG, Shneider DA. Lateral patellofemoral ligament reconstruction using a quadriceps tendon graft. Arthrosc Tech. 2014;3(4):e445-e448.
28. Akşahin E, Yumrukçal F, Yüksel HY, Doğruyol D, Celebi L. Role of pathophysiology of patellofemoral instability in the treatment of spontaneous medial patellofemoral subluxation: a case report. J Med Case Rep. 2010;4:148.
Medial patellar subluxation (MPS) is a disabling condition caused by an imbalance in the medial and lateral forces in the normal knee, allowing the patella to displace medially. Normally, the patella glides appropriately in the femoral trochlea, but alteration in this medial–lateral equilibrium can lead to pain and instability.1 MPS was first described in 1987 by Betz and colleagues2 as a complication of lateral retinacular release. Since then, multiple cases of iatrogenic, traumatic, and isolated medial subluxation have been reported.3–15 However, MPS after lateral release is the most common cause, accounting for the majority of published cases, whereas only 8 cases of isolated MPS have been reported to date.
Optimal treatment for MPS is not well understood. To better comprehend and manage MPS, we must fully appreciate the pathoanatomy, biomechanics, and current research. In this review, we focus on the anatomy of the lateral retinaculum, diagnosis and treatment of MPS, and outcomes of current treatment techniques.
Anatomy
In 1980, Fulkerson and Gossling16 delineated the anatomy of the knee joint lateral retinaculum. They described a 2-layered system with separate distinct anatomical structures. The lateral retinaculum is oriented longitudinally with the knee extended but exerts a posterolateral force on the lateral aspect of the patella as the knee is flexed. The superficial layer is composed of oblique fibers of the lateral retinaculum originating from the iliotibial band and the vastus lateralis fascia and inserting into the lateral margin of the patella and the patella tendon. The deep layer of the retinaculum consists of several structures, including the deep transverse retinaculum, lateral patellofemoral ligament (LPFL), and the patellotibial band.
Over the years, several studies have described the importance of the lateral retinaculum and, in particular, the LPFL. Examining the functional anatomy of the knee in 1962, Kaplan17 first described the lateral epicondylopatellar ligament as a palpable thickening of the joint capsule. Reider and colleagues18 later named this structure the lateral patellofemoral ligament in their anatomical study of 21 fresh cadaver knees. They described its width as ranging from 3 to 10 mm. In a comprehensive cadaveric study of the LPFL, Navarro and colleagues19,20 found it to be a distinct structure present in all 20 of their dissected specimens. They found its femoral insertion at the lateral epicondyle with a fanlike expansion of the fibers predominantly in the posterior region proximal to the lateral epicondyle. The patellar insertion was found in the posterior half and upper lateral aspect, also with expanded fibers. Mean length of the LPFL is 42.1 mm, and mean width is 16.1 mm.
Medial and lateral forces are balanced in a normal knee, and the patella glides appropriately in the femoral trochlea. Alteration in this medial–lateral equilibrium can lead to pain and instability.1 Normally, the patella lies laterally with the knee extended, but in early flexion the patella moves medially as it engages in the trochlea. As the knee continues to flex, the patella flexes and translates distally.21 By 45°, the patella is fully engaged in the trochlear groove throughout the remainder of the knee’s range of motion (ROM).
Lateral release procedures, as described in the literature, result in sectioning of both layers of the lateral retinaculum. In a biomechanical study, Merican and colleagues22 found that staged release of the lateral retinaculum reduced the medial stability of the patellofemoral joint progressively, making it easier to push the patella medially. At 30° of flexion, the transverse fibers of the midsection of the lateral retinaculum were found to be the main contributor to the lateral restraint of the patella. When the release extends too far proximally, the transverse fibers that anchor the lateral patella and the vastus lateralis oblique tendon to the iliotibial band are disrupted. Subsequent loss of a dynamic muscular pull in the orientation of the lateral stabilizing structures results in medial subluxation in a range from full knee extension to about 30° of flexion.
Furthermore, the attachments of the LPFL and the orientation of its fibers suggest that the LPFL may have a significant role in limiting medial excursion of the patella. Vieira and colleagues23 resected the LPFL in 10 fresh cadaver knees. They noticed that, after resection, the patella spontaneously traveled medially, demonstrating the importance of this ligament in patellar stability. In cases of isolated MPS, there have been no reports of associated pathology, such as muscular imbalance or coronal/rotational malalignment of the lower extremity. With an intact lateral retinaculum, medial subluxation is likely caused by pathology in the normal histologic structure of the LPFL and lateral retinaculum. However, the histologic structure of the LPFL and its contribution to the understanding of the pathoetiology of MPS have not been documented.
Diagnosis
MPS diagnosis can be challenging. Often, clinical examination findings are subtle, and radiographs may not show significant pathology. The most accurate diagnosis is obtained by combining patient history, physical examination findings, imaging studies, and diagnostic arthroscopy.
Patient History
Patients with MPS report chronic pain localized to the inferior medial patella and anterior-medial joint line. Occasionally, they complain of crepitus and intermittent swelling. Other symptoms include pain with knee flexion activity, such as squatting and climbing or descending stairs. Some patients describe episodes of giving way and feelings of instability. Often, they are aware the direction of instability is medial. The pain typically is not relieved by medication, physical therapy, or bracing.
Physical Examination
MPS must be identified by clinical examination. Peripatellar tenderness is typically noted. There is often no effusion or crepitus, but the patella is unstable in early flexion. Active and passive ROM is painful through the first 30° of knee flexion. The patient may have a positive medial apprehension test7 in which he or she experiences apprehension of the patella being subluxated with a medially directed force on the lateral border of the patella.
The gravity subluxation test described by Nonweiler and DeLee6 is useful in detecting MPS after lateral release and indicates that the vastus lateralis muscle has been detached from the patella and that the lateral retinaculum is lax. In this test, the patient is positioned in the lateral decubitus position with the involved knee farthest from the table. In this position, gravity causes the patella to subluxate out of the trochlea. The test is positive for MPS when a voluntary contraction of the quadriceps does not center the patella into the trochlear groove. Patients with MPS without previous lateral release can have the patella subluxate medially in the lateral decubitus position, but it is pulled back into the trochlea with active quadriceps contraction (Figure 1).
Patients with MPS often have lateral patellar laxity (LPL), which allows the patella to rotate upward on the lateral side and skid across the medial facet of the femoral trochlea. A physical examination sign combining lateral patellar glide and tilt was described by Shneider24 to identify LPL. This “lateral patellar float” sign is present when the patella translates laterally and rotates or tilts upward with medial pressure on the patella (Figure 2). Another maneuver to test for subtle MPS involves manually centering the patella in the trochlea during active knee flexion and extension. The involved knee is examined in the seated position. The examiner attempts to center the patella in the trochlea with a laterally directed force from the examiner’s thumb on the medial border of the patella. This will usually provide immediate relief as the patient actively ranges the knee.
Imaging Studies
Diagnostic imaging is a crucial component of the evaluation and treatment decision process. Plain radiographs often are not helpful in diagnosing MPS but may provide additional information.5 A variety of radiographic measurements have been described as indicators of structural disease, but there is a lack of comprehensive information recommending radiographic evaluation and interpretation of patients with patellofemoral dysfunction. It is crucial that orthopedic surgeons have common and consistent radiographic views for plain radiographic assessment that can serve as a basis for accurate diagnosis and surgical decision-making.
Standard knee radiographs should include a standing anteroposterior view of bilateral knees, a standing lateral view of the symptomatic knee in 30° of flexion, a patellar axial view, and a tunnel view. These views, occasionally combined with magnetic resonance imaging (MRI), can yield information vital to surgical decision-making. Image quality is highly technique-dependent, and variability in patient positioning can substantially affect the ability to properly diagnose structural abnormalities. For improved diagnostic accuracy and disease classification, radiographs must be obtained with use of the same standardized imaging protocol.
Kinetic MRI was shown by Shellock and colleagues25 to provide diagnostic information related to patellar malalignment. As kinetic MRI can image the patellofemoral joint within the initial 20° to 30° of flexion, it is useful in detecting some of the more subtle patellar tracking problems. In their study of 43 knees (40 patients) with symptoms after lateral release, Shellock and colleagues25 found that 27 knees (63%) had medial subluxation of the patella as the knee moved from extension to flexion. Furthermore, MPS was noted on the contralateral, unoperated knee in 17 (43%) of the 40 patients.
Diagnostic Arthroscopy
Once MPS is suspected after a thorough history and physical examination, examination under anesthesia accompanied by diagnostic arthroscopy confirms the diagnosis. Lateral forces are applied to the patella in full knee extension and 30° of flexion (Figure 3). During arthroscopy, the patellofemoral compartment is viewed from the anterolateral portal. With the knee at full extension, the lateral laxity and medial tilt of the patella can be identified (Figure 4). As the knee is flexed to 30°, the patella moves medially and can subluxate over the edge of the medial facet of the trochlea (Figure 5).
Treatment
Nonsurgical Management
Treatment of MPS depends entirely on making an accurate diagnosis and determining the degree of impairment. Patients with symptomatic MPS should initially undergo supervised rehabilitation focusing on balancing the medial and lateral forces that influence patellar tracking. Patients should be evaluated for specific muscle tightness, weakness, and biomechanical abnormalities. Each problem should be addressed with an individualized rehabilitation prescription. Emphasis is placed on balance, proprioception, and strengthening of the quadriceps, hip abductors/external rotators, and abdominal core muscle groups.
In some patients, symptomatic MPS may be reduced with a patella-stabilizing brace with a medial buttress.3,5,26 Although bracing should be regarded as an adjuvant to a structured physical therapy program, it can also be helpful in confirming the diagnosis of MPS. Shannon and Keene3 reported that all patients in their study experienced significant pain relief and decreased medial patellar subluxations when they wore a medial patella–stabilizing brace. Shellock and colleagues25 used kinematic MRI to investigate the effect of a patella-realignment brace and found that bracing counteracted patellar subluxation in the majority of knees studied.
Surgical Management
When conservative management fails and patients continue to experience pain and instability, surgical intervention is often required. Although various surgical techniques have been used (Table),3–6,8–10,14,15,27,28 the optimal surgical treatment for MPS has not been identified.
Lateral Retinaculum Imbrication. Lateral retinaculum imbrication has been used to centralize patella tracking and stabilize the patella. Richman and Scheller5 reported on a 17-year-old patient who had isolated medial subluxation of the patella without having undergone a previous lateral release. At 3-month follow-up, there was no recurrent instability; there was only intermittent medial knee soreness with weight-bearing activity.
Lateral Retinaculum Repair/Reconstruction. Hughston and colleagues8 treated 65 knees for MPS. Most had undergone lateral release. Of the 65 knees, 39 were treated with direct repair of the lateral retinaculum, and 26 with reconstruction of the lateral patellotibial ligament using locally available tissue, such as strips of iliotibial band or patellar tendon. Results were good to excellent in 80% of patients at a mean follow-up of 53.7 months. Nonweiler and DeLee6 reconstructed the lateral retinaculum in 5 patients with MPS that developed after isolated lateral retinacular release. Four (80%) of the 5 patients had no symptoms or physical signs of instability at a mean follow-up of 3.3 years. Results were excellent (3 knees) and good (2 knees) according to the Merchant and Mercer rating scale. Akşahin and colleagues28 reported on a single case of spontaneous medial patellar instability. At surgery, imbrication of the lateral structures failed to prevent the medial subluxation. Lateral patellotibial ligament augmentation was performed using an iliotibial band flap that effectively corrected the instability. At 1 year, the patient was characterized as engaging in vigorous recreational activity, according to the clinical score defined by Hughston and colleagues.8 He had mild pain with competitive sports but no pain with daily activity. Abhaykumar and Craig9 reported on 4 surgically treated knees with medial instability. They reconstructed the lateral retinaculum using a strip of fascia lata. By follow-up (5-7 years), each knee had its instability resolved and full ROM restored. Johnson and Wakeley26 reported on a case of iatrogenic MPS after lateral release. Treatment consisted of mobilization and direct repair of the lateral retinaculum. At 12-month follow-up, there was no instability. Although symptom-free with light activity, the patient had patellofemoral pain with strenuous activity. Sanchis-Alfonso and colleagues14 reported the results of isolated lateral retinacular reconstruction for iatrogenic MPS in 17 patients. At mean follow-up of 56 months, results were good or excellent in 65% of patients, and the Lysholm score improved from 36.4 preoperatively to 86.1 postoperatively.
Medial Retinaculum Release. Medial retinaculum release has been used as an alternative to open reconstruction. Shannon and Keene3 reported the results of medial retinacular release procedures on 9 knees. Four (44%) of the 9 patients had either spontaneous or traumatic onset of instability. All cases were treated with arthroscopic medial retinacular release, extending 2 cm medial to the superior pole of the patella down to the anteromedial portal. This avoided releasing the attachment of the vastus medialis oblique muscle to the patella and removing its dynamic medial stabilizing force. At a mean follow-up of 2.7 years, both medial subluxation and knee pain were relieved in all 9 knees without complications or further realignment surgery. Results were excellent in 6 knees (66.7%) and good in 3 knees (33.3%). Shannon and Keene3 emphasized that the procedure should not be used in patients with hypermobile patellae or in cases of failed lateral retinacular releases in which MPS is not clearly and carefully documented.
LPFL Reconstruction. Before coming to our practice, most patients have tried several months of formal physical rehabilitation, medications, and bracing. Many have already had surgical procedures, including arthroscopy, lateral release, and tibial tubercle transfer. When the diagnosis of MPS is suspected after a thorough history and physical examination, LPFL reconstruction is offered. Management of MPS with LPFL reconstruction has yielded excellent and reliable clinical results. Teitge and Torga Spak10 described an LPFL reconstruction technique that is used as a salvage procedure in managing medial iatrogenic patellar instability (the patient’s own quadriceps tendon is used). In their experience, direct repair or imbrication of the lateral retinaculum failed to provide long-term stability because medial excursion usually appeared after 1 year. The 60 patients’ outcomes were excellent with respect to patellar stability, and there were no cases of recurrent subluxation. Borbas and colleagues15 reported a case of LPFL reconstruction in a symptomatic medial subluxated patella resulting from TKA and extended lateral release. Using a free gracilis autograft through patellar bone tunnels to reconstruct the LPFL, the patient was free of pain and very satisfied with the result at 1 year postoperatively. Our current strategy is anatomical reconstruction of the LPFL using a quadriceps tendon graft and no bone tunnels, screws, or anchors in the patella.27 We previously reported a single case of isolated medial instability.4 At 2-year follow-up, there was no recurrent instability, and the functional outcome was excellent. This LPFL reconstruction method has been used in 10 patients with isolated MPS. There has been no residual medial subluxation on follow-up ranging from 3 months to 2 years. Outcome studies are in progress.
Rehabilitation. The initial goal of rehabilitation after surgical reconstruction of the lateral retinaculum or LPFL is to protect the healing soft tissues, restore normal knee ROM, and normalize gait. The knee is immobilized in a brace for weight-bearing activity for 4 to 6 weeks, until limb control is sufficient to prevent rotational stress on the knee. Gradual increase to full weight-bearing without bracing is permitted as quadriceps strength is restored. As motion is regained, strength, balance, and proprioception are emphasized for the entire lower extremity and core.
Functional limb training, including rotational activity, begins at 12 weeks. As strength and neuromuscular control progress, single-leg activity may be started with particular attention to proper alignment of the pelvis and the entire lower extremity. For competitive or recreational athletes, the final stages of rehabilitation focus on dynamic lower extremity control during sport-specific movements. Patients return to unrestricted activity by 6 months to 1 year after surgery.
Summary
MPS is a disabling condition that can limit daily functional activity because of apprehension and pain. Initially described as a complication of lateral retinacular release, isolated MPS can occur in the absence of a previous lateral release. Thorough physical examination and identification during arthroscopy are crucial for proper MPS diagnosis and management. When nonsurgical measures fail, LPFL reconstruction can provide patellofemoral stability and excellent functional outcomes.
Medial patellar subluxation (MPS) is a disabling condition caused by an imbalance in the medial and lateral forces in the normal knee, allowing the patella to displace medially. Normally, the patella glides appropriately in the femoral trochlea, but alteration in this medial–lateral equilibrium can lead to pain and instability.1 MPS was first described in 1987 by Betz and colleagues2 as a complication of lateral retinacular release. Since then, multiple cases of iatrogenic, traumatic, and isolated medial subluxation have been reported.3–15 However, MPS after lateral release is the most common cause, accounting for the majority of published cases, whereas only 8 cases of isolated MPS have been reported to date.
Optimal treatment for MPS is not well understood. To better comprehend and manage MPS, we must fully appreciate the pathoanatomy, biomechanics, and current research. In this review, we focus on the anatomy of the lateral retinaculum, diagnosis and treatment of MPS, and outcomes of current treatment techniques.
Anatomy
In 1980, Fulkerson and Gossling16 delineated the anatomy of the knee joint lateral retinaculum. They described a 2-layered system with separate distinct anatomical structures. The lateral retinaculum is oriented longitudinally with the knee extended but exerts a posterolateral force on the lateral aspect of the patella as the knee is flexed. The superficial layer is composed of oblique fibers of the lateral retinaculum originating from the iliotibial band and the vastus lateralis fascia and inserting into the lateral margin of the patella and the patella tendon. The deep layer of the retinaculum consists of several structures, including the deep transverse retinaculum, lateral patellofemoral ligament (LPFL), and the patellotibial band.
Over the years, several studies have described the importance of the lateral retinaculum and, in particular, the LPFL. Examining the functional anatomy of the knee in 1962, Kaplan17 first described the lateral epicondylopatellar ligament as a palpable thickening of the joint capsule. Reider and colleagues18 later named this structure the lateral patellofemoral ligament in their anatomical study of 21 fresh cadaver knees. They described its width as ranging from 3 to 10 mm. In a comprehensive cadaveric study of the LPFL, Navarro and colleagues19,20 found it to be a distinct structure present in all 20 of their dissected specimens. They found its femoral insertion at the lateral epicondyle with a fanlike expansion of the fibers predominantly in the posterior region proximal to the lateral epicondyle. The patellar insertion was found in the posterior half and upper lateral aspect, also with expanded fibers. Mean length of the LPFL is 42.1 mm, and mean width is 16.1 mm.
Medial and lateral forces are balanced in a normal knee, and the patella glides appropriately in the femoral trochlea. Alteration in this medial–lateral equilibrium can lead to pain and instability.1 Normally, the patella lies laterally with the knee extended, but in early flexion the patella moves medially as it engages in the trochlea. As the knee continues to flex, the patella flexes and translates distally.21 By 45°, the patella is fully engaged in the trochlear groove throughout the remainder of the knee’s range of motion (ROM).
Lateral release procedures, as described in the literature, result in sectioning of both layers of the lateral retinaculum. In a biomechanical study, Merican and colleagues22 found that staged release of the lateral retinaculum reduced the medial stability of the patellofemoral joint progressively, making it easier to push the patella medially. At 30° of flexion, the transverse fibers of the midsection of the lateral retinaculum were found to be the main contributor to the lateral restraint of the patella. When the release extends too far proximally, the transverse fibers that anchor the lateral patella and the vastus lateralis oblique tendon to the iliotibial band are disrupted. Subsequent loss of a dynamic muscular pull in the orientation of the lateral stabilizing structures results in medial subluxation in a range from full knee extension to about 30° of flexion.
Furthermore, the attachments of the LPFL and the orientation of its fibers suggest that the LPFL may have a significant role in limiting medial excursion of the patella. Vieira and colleagues23 resected the LPFL in 10 fresh cadaver knees. They noticed that, after resection, the patella spontaneously traveled medially, demonstrating the importance of this ligament in patellar stability. In cases of isolated MPS, there have been no reports of associated pathology, such as muscular imbalance or coronal/rotational malalignment of the lower extremity. With an intact lateral retinaculum, medial subluxation is likely caused by pathology in the normal histologic structure of the LPFL and lateral retinaculum. However, the histologic structure of the LPFL and its contribution to the understanding of the pathoetiology of MPS have not been documented.
Diagnosis
MPS diagnosis can be challenging. Often, clinical examination findings are subtle, and radiographs may not show significant pathology. The most accurate diagnosis is obtained by combining patient history, physical examination findings, imaging studies, and diagnostic arthroscopy.
Patient History
Patients with MPS report chronic pain localized to the inferior medial patella and anterior-medial joint line. Occasionally, they complain of crepitus and intermittent swelling. Other symptoms include pain with knee flexion activity, such as squatting and climbing or descending stairs. Some patients describe episodes of giving way and feelings of instability. Often, they are aware the direction of instability is medial. The pain typically is not relieved by medication, physical therapy, or bracing.
Physical Examination
MPS must be identified by clinical examination. Peripatellar tenderness is typically noted. There is often no effusion or crepitus, but the patella is unstable in early flexion. Active and passive ROM is painful through the first 30° of knee flexion. The patient may have a positive medial apprehension test7 in which he or she experiences apprehension of the patella being subluxated with a medially directed force on the lateral border of the patella.
The gravity subluxation test described by Nonweiler and DeLee6 is useful in detecting MPS after lateral release and indicates that the vastus lateralis muscle has been detached from the patella and that the lateral retinaculum is lax. In this test, the patient is positioned in the lateral decubitus position with the involved knee farthest from the table. In this position, gravity causes the patella to subluxate out of the trochlea. The test is positive for MPS when a voluntary contraction of the quadriceps does not center the patella into the trochlear groove. Patients with MPS without previous lateral release can have the patella subluxate medially in the lateral decubitus position, but it is pulled back into the trochlea with active quadriceps contraction (Figure 1).
Patients with MPS often have lateral patellar laxity (LPL), which allows the patella to rotate upward on the lateral side and skid across the medial facet of the femoral trochlea. A physical examination sign combining lateral patellar glide and tilt was described by Shneider24 to identify LPL. This “lateral patellar float” sign is present when the patella translates laterally and rotates or tilts upward with medial pressure on the patella (Figure 2). Another maneuver to test for subtle MPS involves manually centering the patella in the trochlea during active knee flexion and extension. The involved knee is examined in the seated position. The examiner attempts to center the patella in the trochlea with a laterally directed force from the examiner’s thumb on the medial border of the patella. This will usually provide immediate relief as the patient actively ranges the knee.
Imaging Studies
Diagnostic imaging is a crucial component of the evaluation and treatment decision process. Plain radiographs often are not helpful in diagnosing MPS but may provide additional information.5 A variety of radiographic measurements have been described as indicators of structural disease, but there is a lack of comprehensive information recommending radiographic evaluation and interpretation of patients with patellofemoral dysfunction. It is crucial that orthopedic surgeons have common and consistent radiographic views for plain radiographic assessment that can serve as a basis for accurate diagnosis and surgical decision-making.
Standard knee radiographs should include a standing anteroposterior view of bilateral knees, a standing lateral view of the symptomatic knee in 30° of flexion, a patellar axial view, and a tunnel view. These views, occasionally combined with magnetic resonance imaging (MRI), can yield information vital to surgical decision-making. Image quality is highly technique-dependent, and variability in patient positioning can substantially affect the ability to properly diagnose structural abnormalities. For improved diagnostic accuracy and disease classification, radiographs must be obtained with use of the same standardized imaging protocol.
Kinetic MRI was shown by Shellock and colleagues25 to provide diagnostic information related to patellar malalignment. As kinetic MRI can image the patellofemoral joint within the initial 20° to 30° of flexion, it is useful in detecting some of the more subtle patellar tracking problems. In their study of 43 knees (40 patients) with symptoms after lateral release, Shellock and colleagues25 found that 27 knees (63%) had medial subluxation of the patella as the knee moved from extension to flexion. Furthermore, MPS was noted on the contralateral, unoperated knee in 17 (43%) of the 40 patients.
Diagnostic Arthroscopy
Once MPS is suspected after a thorough history and physical examination, examination under anesthesia accompanied by diagnostic arthroscopy confirms the diagnosis. Lateral forces are applied to the patella in full knee extension and 30° of flexion (Figure 3). During arthroscopy, the patellofemoral compartment is viewed from the anterolateral portal. With the knee at full extension, the lateral laxity and medial tilt of the patella can be identified (Figure 4). As the knee is flexed to 30°, the patella moves medially and can subluxate over the edge of the medial facet of the trochlea (Figure 5).
Treatment
Nonsurgical Management
Treatment of MPS depends entirely on making an accurate diagnosis and determining the degree of impairment. Patients with symptomatic MPS should initially undergo supervised rehabilitation focusing on balancing the medial and lateral forces that influence patellar tracking. Patients should be evaluated for specific muscle tightness, weakness, and biomechanical abnormalities. Each problem should be addressed with an individualized rehabilitation prescription. Emphasis is placed on balance, proprioception, and strengthening of the quadriceps, hip abductors/external rotators, and abdominal core muscle groups.
In some patients, symptomatic MPS may be reduced with a patella-stabilizing brace with a medial buttress.3,5,26 Although bracing should be regarded as an adjuvant to a structured physical therapy program, it can also be helpful in confirming the diagnosis of MPS. Shannon and Keene3 reported that all patients in their study experienced significant pain relief and decreased medial patellar subluxations when they wore a medial patella–stabilizing brace. Shellock and colleagues25 used kinematic MRI to investigate the effect of a patella-realignment brace and found that bracing counteracted patellar subluxation in the majority of knees studied.
Surgical Management
When conservative management fails and patients continue to experience pain and instability, surgical intervention is often required. Although various surgical techniques have been used (Table),3–6,8–10,14,15,27,28 the optimal surgical treatment for MPS has not been identified.
Lateral Retinaculum Imbrication. Lateral retinaculum imbrication has been used to centralize patella tracking and stabilize the patella. Richman and Scheller5 reported on a 17-year-old patient who had isolated medial subluxation of the patella without having undergone a previous lateral release. At 3-month follow-up, there was no recurrent instability; there was only intermittent medial knee soreness with weight-bearing activity.
Lateral Retinaculum Repair/Reconstruction. Hughston and colleagues8 treated 65 knees for MPS. Most had undergone lateral release. Of the 65 knees, 39 were treated with direct repair of the lateral retinaculum, and 26 with reconstruction of the lateral patellotibial ligament using locally available tissue, such as strips of iliotibial band or patellar tendon. Results were good to excellent in 80% of patients at a mean follow-up of 53.7 months. Nonweiler and DeLee6 reconstructed the lateral retinaculum in 5 patients with MPS that developed after isolated lateral retinacular release. Four (80%) of the 5 patients had no symptoms or physical signs of instability at a mean follow-up of 3.3 years. Results were excellent (3 knees) and good (2 knees) according to the Merchant and Mercer rating scale. Akşahin and colleagues28 reported on a single case of spontaneous medial patellar instability. At surgery, imbrication of the lateral structures failed to prevent the medial subluxation. Lateral patellotibial ligament augmentation was performed using an iliotibial band flap that effectively corrected the instability. At 1 year, the patient was characterized as engaging in vigorous recreational activity, according to the clinical score defined by Hughston and colleagues.8 He had mild pain with competitive sports but no pain with daily activity. Abhaykumar and Craig9 reported on 4 surgically treated knees with medial instability. They reconstructed the lateral retinaculum using a strip of fascia lata. By follow-up (5-7 years), each knee had its instability resolved and full ROM restored. Johnson and Wakeley26 reported on a case of iatrogenic MPS after lateral release. Treatment consisted of mobilization and direct repair of the lateral retinaculum. At 12-month follow-up, there was no instability. Although symptom-free with light activity, the patient had patellofemoral pain with strenuous activity. Sanchis-Alfonso and colleagues14 reported the results of isolated lateral retinacular reconstruction for iatrogenic MPS in 17 patients. At mean follow-up of 56 months, results were good or excellent in 65% of patients, and the Lysholm score improved from 36.4 preoperatively to 86.1 postoperatively.
Medial Retinaculum Release. Medial retinaculum release has been used as an alternative to open reconstruction. Shannon and Keene3 reported the results of medial retinacular release procedures on 9 knees. Four (44%) of the 9 patients had either spontaneous or traumatic onset of instability. All cases were treated with arthroscopic medial retinacular release, extending 2 cm medial to the superior pole of the patella down to the anteromedial portal. This avoided releasing the attachment of the vastus medialis oblique muscle to the patella and removing its dynamic medial stabilizing force. At a mean follow-up of 2.7 years, both medial subluxation and knee pain were relieved in all 9 knees without complications or further realignment surgery. Results were excellent in 6 knees (66.7%) and good in 3 knees (33.3%). Shannon and Keene3 emphasized that the procedure should not be used in patients with hypermobile patellae or in cases of failed lateral retinacular releases in which MPS is not clearly and carefully documented.
LPFL Reconstruction. Before coming to our practice, most patients have tried several months of formal physical rehabilitation, medications, and bracing. Many have already had surgical procedures, including arthroscopy, lateral release, and tibial tubercle transfer. When the diagnosis of MPS is suspected after a thorough history and physical examination, LPFL reconstruction is offered. Management of MPS with LPFL reconstruction has yielded excellent and reliable clinical results. Teitge and Torga Spak10 described an LPFL reconstruction technique that is used as a salvage procedure in managing medial iatrogenic patellar instability (the patient’s own quadriceps tendon is used). In their experience, direct repair or imbrication of the lateral retinaculum failed to provide long-term stability because medial excursion usually appeared after 1 year. The 60 patients’ outcomes were excellent with respect to patellar stability, and there were no cases of recurrent subluxation. Borbas and colleagues15 reported a case of LPFL reconstruction in a symptomatic medial subluxated patella resulting from TKA and extended lateral release. Using a free gracilis autograft through patellar bone tunnels to reconstruct the LPFL, the patient was free of pain and very satisfied with the result at 1 year postoperatively. Our current strategy is anatomical reconstruction of the LPFL using a quadriceps tendon graft and no bone tunnels, screws, or anchors in the patella.27 We previously reported a single case of isolated medial instability.4 At 2-year follow-up, there was no recurrent instability, and the functional outcome was excellent. This LPFL reconstruction method has been used in 10 patients with isolated MPS. There has been no residual medial subluxation on follow-up ranging from 3 months to 2 years. Outcome studies are in progress.
Rehabilitation. The initial goal of rehabilitation after surgical reconstruction of the lateral retinaculum or LPFL is to protect the healing soft tissues, restore normal knee ROM, and normalize gait. The knee is immobilized in a brace for weight-bearing activity for 4 to 6 weeks, until limb control is sufficient to prevent rotational stress on the knee. Gradual increase to full weight-bearing without bracing is permitted as quadriceps strength is restored. As motion is regained, strength, balance, and proprioception are emphasized for the entire lower extremity and core.
Functional limb training, including rotational activity, begins at 12 weeks. As strength and neuromuscular control progress, single-leg activity may be started with particular attention to proper alignment of the pelvis and the entire lower extremity. For competitive or recreational athletes, the final stages of rehabilitation focus on dynamic lower extremity control during sport-specific movements. Patients return to unrestricted activity by 6 months to 1 year after surgery.
Summary
MPS is a disabling condition that can limit daily functional activity because of apprehension and pain. Initially described as a complication of lateral retinacular release, isolated MPS can occur in the absence of a previous lateral release. Thorough physical examination and identification during arthroscopy are crucial for proper MPS diagnosis and management. When nonsurgical measures fail, LPFL reconstruction can provide patellofemoral stability and excellent functional outcomes.
1. Marumoto JM, Jordan C, Akins R. A biomechanical comparison of lateral retinacular releases. Am J Sports Med. 1995;23(2):151-155.
2. Betz RR, Magill JT, Lonergan RP. The percutaneous lateral retinacular release. Am J Sports Med. 1987;15(5):477-482.
3. Shannon BD, Keene JS. Results of arthroscopic medial retinacular release for treatment of medial subluxation of the patella. Am J Sports Med. 2007;35(7):1180-1187.
4. Saper MG, Shneider DA. Medial patellar subluxation without previous lateral release: a case report. J Pediatr Orthop B. 2014;23(4):350-353.
5. Richman NM, Scheller AD Jr. Medial subluxation of the patella without previous lateral retinacular release. Orthopedics. 1998;21(7):810-813.
6. Nonweiler DE, DeLee JC. The diagnosis and treatment of medial subluxation of the patella after lateral retinacular release. Am J Sports Med. 1994;22(5):680-686.
7. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
8. Hughston JC, Flandry F, Brinker MR, Terry GC, Mills JC 3rd. Surgical correction of medial subluxation of the patella. Am J Sports Med. 1996;24(4):486-491.
9. Abhaykumar S, Craig DM. Fascia lata sling reconstruction for recurrent medial dislocation of the patella. The Knee. 1999;6(1):55-57.
10. Teitge RA, Torga Spak R. Lateral patellofemoral ligament reconstruction. Arthroscopy. 2004;20(9):998-1002.
11. Kusano M, Horibe S, Tanaka Y, et al. Simultaneous MPFL and LPFL reconstruction for recurrent lateral patellar dislocation with medial patellofemoral instability. Asia-Pac J Sports Med Arthrosc Rehabil Technol. 2014;1:42-46.
12. Saper MG, Shneider DA. Simultaneous medial and lateral patellofemoral ligament reconstruction for combined medial and lateral patellar subluxation. Arthrosc Tech. 2014,3(2):e227-e231.
13. Udagawa K, Niki Y, Matsumoto H, et al. Lateral patellar retinaculum reconstruction for medial patellar instability following lateral retinacular release: a case report. Knee. 2014;21(1):336-339.
14. Sanchis-Alfonso V, Montesinos-Berry E, Monllau JC, Merchant AC. Results of isolated lateral retinacular reconstruction for iatrogenic medial patellar instability. Arthroscopy. 2015;31(3):422-427.
15. Borbas P, Koch PP, Fucentese SF. Lateral patellofemoral ligament reconstruction using a free gracilis autograft. Orthopedics. 2014;37(7):e665-e668.
16. Fulkerson JP, Gossling H. Anatomy of the knee joint lateral retinaculum. Clin Orthop Relat Res. 1980;153:183-188.
17. Kaplan E. Some aspects of functional anatomy of the human knee joint. Clin Orthop Relat Res. 1962;23:18-29.
18. Reider B, Marshall J, Koslin B, Ring B, Girgis F. The anterior aspect of the knee joint. J Bone Joint Surg Am. 1981;63(3):351-356.
19. Navarro MS, Navarro RD, Akita Junior J, Cohen M. Anatomical study of the lateral patellofemoral ligament in cadaver knees. Rev Bras Ortop. 2008;43(7):300-307.
20. Navarro MS, Beltrani Filho CA, Akita Junior J, Navarro RD, Cohen M. Relationship between the lateral patellofemoral ligament and the width of the lateral patellar facet. Acta Ortop Bras. 2010;18(1):19-22.
21. Salsich GB, Ward SR, Terk MR, Powers CM. In vivo assessment of patellofemoral joint contact area in individuals who are pain free. Clin Orthop Relat Res. 2003;417:277-284.
22. Merican AM, Kondo E, Amis AA. The effect on patellofemoral joint stability of selective cutting of lateral retinacular and capsular structures. J Biomech. 2009;42(3):291-296.
23. Vieira EL, Vieira EÁ, da Silva RT, Berlfein PA, Abdalla RJ, Cohen M. An anatomic study of the iliotibial tract. Arthroscopy. 2007;23(3):269-274.
24. Shneider DA. Lateral patellar laxity—identification, significance, treatment. Poster session presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 25-28, 2009; Las Vegas, NV.
25. Shellock FG, Mink JH, Deutsch A, Fox JM, Ferkel RD. Evaluation of patients with persistent symptoms after lateral retinacular release by kinematic magnetic resonance imaging of the patellofemoral joint. Arthroscopy. 1990;6(3):226-234.
26. Johnson DP, Wakeley C. Reconstruction of the lateral patellar retinaculum following lateral release: a case report. Knee Surg Sports Traumatol Arthrosc. 2002;10(6):361-363.
27. Saper MG, Shneider DA. Lateral patellofemoral ligament reconstruction using a quadriceps tendon graft. Arthrosc Tech. 2014;3(4):e445-e448.
28. Akşahin E, Yumrukçal F, Yüksel HY, Doğruyol D, Celebi L. Role of pathophysiology of patellofemoral instability in the treatment of spontaneous medial patellofemoral subluxation: a case report. J Med Case Rep. 2010;4:148.
1. Marumoto JM, Jordan C, Akins R. A biomechanical comparison of lateral retinacular releases. Am J Sports Med. 1995;23(2):151-155.
2. Betz RR, Magill JT, Lonergan RP. The percutaneous lateral retinacular release. Am J Sports Med. 1987;15(5):477-482.
3. Shannon BD, Keene JS. Results of arthroscopic medial retinacular release for treatment of medial subluxation of the patella. Am J Sports Med. 2007;35(7):1180-1187.
4. Saper MG, Shneider DA. Medial patellar subluxation without previous lateral release: a case report. J Pediatr Orthop B. 2014;23(4):350-353.
5. Richman NM, Scheller AD Jr. Medial subluxation of the patella without previous lateral retinacular release. Orthopedics. 1998;21(7):810-813.
6. Nonweiler DE, DeLee JC. The diagnosis and treatment of medial subluxation of the patella after lateral retinacular release. Am J Sports Med. 1994;22(5):680-686.
7. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
8. Hughston JC, Flandry F, Brinker MR, Terry GC, Mills JC 3rd. Surgical correction of medial subluxation of the patella. Am J Sports Med. 1996;24(4):486-491.
9. Abhaykumar S, Craig DM. Fascia lata sling reconstruction for recurrent medial dislocation of the patella. The Knee. 1999;6(1):55-57.
10. Teitge RA, Torga Spak R. Lateral patellofemoral ligament reconstruction. Arthroscopy. 2004;20(9):998-1002.
11. Kusano M, Horibe S, Tanaka Y, et al. Simultaneous MPFL and LPFL reconstruction for recurrent lateral patellar dislocation with medial patellofemoral instability. Asia-Pac J Sports Med Arthrosc Rehabil Technol. 2014;1:42-46.
12. Saper MG, Shneider DA. Simultaneous medial and lateral patellofemoral ligament reconstruction for combined medial and lateral patellar subluxation. Arthrosc Tech. 2014,3(2):e227-e231.
13. Udagawa K, Niki Y, Matsumoto H, et al. Lateral patellar retinaculum reconstruction for medial patellar instability following lateral retinacular release: a case report. Knee. 2014;21(1):336-339.
14. Sanchis-Alfonso V, Montesinos-Berry E, Monllau JC, Merchant AC. Results of isolated lateral retinacular reconstruction for iatrogenic medial patellar instability. Arthroscopy. 2015;31(3):422-427.
15. Borbas P, Koch PP, Fucentese SF. Lateral patellofemoral ligament reconstruction using a free gracilis autograft. Orthopedics. 2014;37(7):e665-e668.
16. Fulkerson JP, Gossling H. Anatomy of the knee joint lateral retinaculum. Clin Orthop Relat Res. 1980;153:183-188.
17. Kaplan E. Some aspects of functional anatomy of the human knee joint. Clin Orthop Relat Res. 1962;23:18-29.
18. Reider B, Marshall J, Koslin B, Ring B, Girgis F. The anterior aspect of the knee joint. J Bone Joint Surg Am. 1981;63(3):351-356.
19. Navarro MS, Navarro RD, Akita Junior J, Cohen M. Anatomical study of the lateral patellofemoral ligament in cadaver knees. Rev Bras Ortop. 2008;43(7):300-307.
20. Navarro MS, Beltrani Filho CA, Akita Junior J, Navarro RD, Cohen M. Relationship between the lateral patellofemoral ligament and the width of the lateral patellar facet. Acta Ortop Bras. 2010;18(1):19-22.
21. Salsich GB, Ward SR, Terk MR, Powers CM. In vivo assessment of patellofemoral joint contact area in individuals who are pain free. Clin Orthop Relat Res. 2003;417:277-284.
22. Merican AM, Kondo E, Amis AA. The effect on patellofemoral joint stability of selective cutting of lateral retinacular and capsular structures. J Biomech. 2009;42(3):291-296.
23. Vieira EL, Vieira EÁ, da Silva RT, Berlfein PA, Abdalla RJ, Cohen M. An anatomic study of the iliotibial tract. Arthroscopy. 2007;23(3):269-274.
24. Shneider DA. Lateral patellar laxity—identification, significance, treatment. Poster session presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 25-28, 2009; Las Vegas, NV.
25. Shellock FG, Mink JH, Deutsch A, Fox JM, Ferkel RD. Evaluation of patients with persistent symptoms after lateral retinacular release by kinematic magnetic resonance imaging of the patellofemoral joint. Arthroscopy. 1990;6(3):226-234.
26. Johnson DP, Wakeley C. Reconstruction of the lateral patellar retinaculum following lateral release: a case report. Knee Surg Sports Traumatol Arthrosc. 2002;10(6):361-363.
27. Saper MG, Shneider DA. Lateral patellofemoral ligament reconstruction using a quadriceps tendon graft. Arthrosc Tech. 2014;3(4):e445-e448.
28. Akşahin E, Yumrukçal F, Yüksel HY, Doğruyol D, Celebi L. Role of pathophysiology of patellofemoral instability in the treatment of spontaneous medial patellofemoral subluxation: a case report. J Med Case Rep. 2010;4:148.
Do Women With Knee Osteoarthritis Experience Greater Pain Sensitivity Than Men?
Among patients with osteoarthritis of the knee, women experienced greater sensitivity to various pain modalities, such as lower tolerance to heat, cold, and pressure, and greater widespread pain than men, according to a study published online ahead of print October 5 in Arthritis Care & Research.
“Many questions still remain as to why women with knee osteoarthritis are more sensitive to painful stimuli than are men. While therapeutic approaches to control pain are only beginning to take these sex differences into account, there is still quite a bit of research yet to be done to help reduce this gender gap and improve clinical therapies for men and women alike,” said lead author Emily J. Bartley, PhD, a Research Assistant Professor at the University of Florida College of Dentistry in Gainsville.
For this study, 288 participants between the ages of 45 and 85 completed a battery of quantitative sensory pain procedures assessing sensitivity to contact heat, cold pressor, mechanical pressure, and punctate stimuli. Differences in temporal summation were examined, along with measures of clinical pain and functional performance.
When compared to men, women exhibited greater sensitivity to multiple pain modalities (eg, lower heat, cold, pressure thresholds/tolerances, greater temporal summation of pain). There were no sex differences in clinical pain with the exception of greater widespread pain observed in women. Although there were select age-related differences in pain sensitivity, sex differences in pain varied minimally across age cohort.
“Overall, these findings provide evidence for greater overall sensitivity to experimental pain in women with symptomatic knee osteoarthritis compared with men, suggesting that enhanced central sensitivity may be an important contributor to pain in this group,” wrote Dr. Bartley and colleagues.
Suggested Reading
Bartley EJ, King CD, Sibille KT, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res (Hoboken). 2015 Oct 5. [Epub ahead of print].
Among patients with osteoarthritis of the knee, women experienced greater sensitivity to various pain modalities, such as lower tolerance to heat, cold, and pressure, and greater widespread pain than men, according to a study published online ahead of print October 5 in Arthritis Care & Research.
“Many questions still remain as to why women with knee osteoarthritis are more sensitive to painful stimuli than are men. While therapeutic approaches to control pain are only beginning to take these sex differences into account, there is still quite a bit of research yet to be done to help reduce this gender gap and improve clinical therapies for men and women alike,” said lead author Emily J. Bartley, PhD, a Research Assistant Professor at the University of Florida College of Dentistry in Gainsville.
For this study, 288 participants between the ages of 45 and 85 completed a battery of quantitative sensory pain procedures assessing sensitivity to contact heat, cold pressor, mechanical pressure, and punctate stimuli. Differences in temporal summation were examined, along with measures of clinical pain and functional performance.
When compared to men, women exhibited greater sensitivity to multiple pain modalities (eg, lower heat, cold, pressure thresholds/tolerances, greater temporal summation of pain). There were no sex differences in clinical pain with the exception of greater widespread pain observed in women. Although there were select age-related differences in pain sensitivity, sex differences in pain varied minimally across age cohort.
“Overall, these findings provide evidence for greater overall sensitivity to experimental pain in women with symptomatic knee osteoarthritis compared with men, suggesting that enhanced central sensitivity may be an important contributor to pain in this group,” wrote Dr. Bartley and colleagues.
Among patients with osteoarthritis of the knee, women experienced greater sensitivity to various pain modalities, such as lower tolerance to heat, cold, and pressure, and greater widespread pain than men, according to a study published online ahead of print October 5 in Arthritis Care & Research.
“Many questions still remain as to why women with knee osteoarthritis are more sensitive to painful stimuli than are men. While therapeutic approaches to control pain are only beginning to take these sex differences into account, there is still quite a bit of research yet to be done to help reduce this gender gap and improve clinical therapies for men and women alike,” said lead author Emily J. Bartley, PhD, a Research Assistant Professor at the University of Florida College of Dentistry in Gainsville.
For this study, 288 participants between the ages of 45 and 85 completed a battery of quantitative sensory pain procedures assessing sensitivity to contact heat, cold pressor, mechanical pressure, and punctate stimuli. Differences in temporal summation were examined, along with measures of clinical pain and functional performance.
When compared to men, women exhibited greater sensitivity to multiple pain modalities (eg, lower heat, cold, pressure thresholds/tolerances, greater temporal summation of pain). There were no sex differences in clinical pain with the exception of greater widespread pain observed in women. Although there were select age-related differences in pain sensitivity, sex differences in pain varied minimally across age cohort.
“Overall, these findings provide evidence for greater overall sensitivity to experimental pain in women with symptomatic knee osteoarthritis compared with men, suggesting that enhanced central sensitivity may be an important contributor to pain in this group,” wrote Dr. Bartley and colleagues.
Suggested Reading
Bartley EJ, King CD, Sibille KT, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res (Hoboken). 2015 Oct 5. [Epub ahead of print].
Suggested Reading
Bartley EJ, King CD, Sibille KT, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res (Hoboken). 2015 Oct 5. [Epub ahead of print].
Benefits, risks of total knee replacement for OA illuminated in trial
Total knee replacement was superior to nonsurgical treatment in relieving pain, restoring function, and improving quality of life for patients with moderate to severe knee osteoarthritis, according to a report published online Oct. 22 in the New England Journal of Medicine.
Even though the number of total knee replacements performed each year is large and steadily increasing – with more than 670,000 done in 2012 in the United States alone – no high-quality randomized, controlled trials have ever compared the effectiveness of the procedure against nonsurgical treatment, said Søren T. Skou, Ph.D., of the Research Unit for Musculoskeletal Function and Physiotherapy, Institute of Sports Science and Clinical Biomechanics, University of Southern Denmark, Odense, and his associates.
Dr. Skou and his colleagues remedied that situation by randomly assigning 100 adults (mean age, 66 years) who were eligible for unilateral total knee replacement to either undergo the procedure and then receive a comprehensive nonsurgical intervention (50 patients) or receive the comprehensive nonsurgical intervention alone (50 patients) at two specialized university clinics in Denmark. The 12-week nonsurgical intervention comprised a twice-weekly group exercise program to restore neutral, functional realignment of the legs; two 1-hour education sessions regarding osteoarthritis characteristics, treatments, and self-help strategies; a dietary (weight-loss) program; provision of individually fitted insoles with medial arch support and a lateral wedge if patients had knee-lateral-to-foot positioning; and as-needed pain medication for pain – acetaminophen and ibuprofen – and pantoprazole, a proton-pump inhibitor.
The primary outcome measure in the trial was the between-group difference at 1 year in improvement on four subscales of the Knee Injury and Osteoarthritis Outcome Scores (KOOS) for pain, symptoms, activities of daily living, and quality of life. The surgical group showed a significantly greater improvement (32.5 out of a possible 100 points) than the nonsurgical group (16.0 points) in this outcome. The surgical group also showed significantly greater improvements in all five individual subscales and in a timed chair-rising test, a timed 20-meter walk test, and on a quality-of-life index, the investigators said (N Engl J Med. 2015 373;17:1597-606).
However, it is important to note that patients who had only the nonsurgical intervention showed clinically relevant improvements, and only 26% of them chose to have the surgery after the conclusion of the study. As expected, the surgical group had more serious adverse events than did the nonsurgical group (24 vs. 6), including three cases of deep venous thrombosis and three cases of knee stiffness requiring brisement forcé while the patient was anesthetized, Dr. Skou and his associates said.
This study was supported by the Obel Family Foundation, the Danish Rheumatism Association, the Health Science Foundation of the North Denmark Region, Foot Science International, Spar Nord Foundation, the Bevica Foundation, the Association of Danish Physiotherapists Research Fund, the Medical Specialist Heinrich Kopp’s Grant, and the Danish Medical Association Research Fund. Dr. Skou and his associates reported having no relevant financial disclosures.

|
Dr. Jeffrey N. Katz |
This study provides the first rigorously controlled data to inform discussions about whether patients should undergo total knee replacement or opt for comprehensive nonsurgical treatment. Surgery proved markedly superior in this trial, with 85% of surgical patients reporting a clinically important improvement in pain and function at 1 year, compared with 68% of nonsurgical patients.
But surgery was associated with several severe adverse events, including deep venous thrombosis, deep wound infection, supracondylar fracture, and stiffness requiring treatment under general anesthesia. Each patient must weigh these considerations; each physician should present the relevant data to their patients and then listen carefully to their preferences.
Dr. Jeffrey N. Katz is in the departments of medicine and orthopedic surgery at Brigham and Women’s Hospital and Harvard University, Boston. He reported having no relevant financial disclosures. Dr. Katz made these remarks in an editorial accompanying Dr. Skou’s report (N Engl J Med. 2015 373;17:1668-9).

|
Dr. Jeffrey N. Katz |
This study provides the first rigorously controlled data to inform discussions about whether patients should undergo total knee replacement or opt for comprehensive nonsurgical treatment. Surgery proved markedly superior in this trial, with 85% of surgical patients reporting a clinically important improvement in pain and function at 1 year, compared with 68% of nonsurgical patients.
But surgery was associated with several severe adverse events, including deep venous thrombosis, deep wound infection, supracondylar fracture, and stiffness requiring treatment under general anesthesia. Each patient must weigh these considerations; each physician should present the relevant data to their patients and then listen carefully to their preferences.
Dr. Jeffrey N. Katz is in the departments of medicine and orthopedic surgery at Brigham and Women’s Hospital and Harvard University, Boston. He reported having no relevant financial disclosures. Dr. Katz made these remarks in an editorial accompanying Dr. Skou’s report (N Engl J Med. 2015 373;17:1668-9).

|
Dr. Jeffrey N. Katz |
This study provides the first rigorously controlled data to inform discussions about whether patients should undergo total knee replacement or opt for comprehensive nonsurgical treatment. Surgery proved markedly superior in this trial, with 85% of surgical patients reporting a clinically important improvement in pain and function at 1 year, compared with 68% of nonsurgical patients.
But surgery was associated with several severe adverse events, including deep venous thrombosis, deep wound infection, supracondylar fracture, and stiffness requiring treatment under general anesthesia. Each patient must weigh these considerations; each physician should present the relevant data to their patients and then listen carefully to their preferences.
Dr. Jeffrey N. Katz is in the departments of medicine and orthopedic surgery at Brigham and Women’s Hospital and Harvard University, Boston. He reported having no relevant financial disclosures. Dr. Katz made these remarks in an editorial accompanying Dr. Skou’s report (N Engl J Med. 2015 373;17:1668-9).
Total knee replacement was superior to nonsurgical treatment in relieving pain, restoring function, and improving quality of life for patients with moderate to severe knee osteoarthritis, according to a report published online Oct. 22 in the New England Journal of Medicine.
Even though the number of total knee replacements performed each year is large and steadily increasing – with more than 670,000 done in 2012 in the United States alone – no high-quality randomized, controlled trials have ever compared the effectiveness of the procedure against nonsurgical treatment, said Søren T. Skou, Ph.D., of the Research Unit for Musculoskeletal Function and Physiotherapy, Institute of Sports Science and Clinical Biomechanics, University of Southern Denmark, Odense, and his associates.
Dr. Skou and his colleagues remedied that situation by randomly assigning 100 adults (mean age, 66 years) who were eligible for unilateral total knee replacement to either undergo the procedure and then receive a comprehensive nonsurgical intervention (50 patients) or receive the comprehensive nonsurgical intervention alone (50 patients) at two specialized university clinics in Denmark. The 12-week nonsurgical intervention comprised a twice-weekly group exercise program to restore neutral, functional realignment of the legs; two 1-hour education sessions regarding osteoarthritis characteristics, treatments, and self-help strategies; a dietary (weight-loss) program; provision of individually fitted insoles with medial arch support and a lateral wedge if patients had knee-lateral-to-foot positioning; and as-needed pain medication for pain – acetaminophen and ibuprofen – and pantoprazole, a proton-pump inhibitor.
The primary outcome measure in the trial was the between-group difference at 1 year in improvement on four subscales of the Knee Injury and Osteoarthritis Outcome Scores (KOOS) for pain, symptoms, activities of daily living, and quality of life. The surgical group showed a significantly greater improvement (32.5 out of a possible 100 points) than the nonsurgical group (16.0 points) in this outcome. The surgical group also showed significantly greater improvements in all five individual subscales and in a timed chair-rising test, a timed 20-meter walk test, and on a quality-of-life index, the investigators said (N Engl J Med. 2015 373;17:1597-606).
However, it is important to note that patients who had only the nonsurgical intervention showed clinically relevant improvements, and only 26% of them chose to have the surgery after the conclusion of the study. As expected, the surgical group had more serious adverse events than did the nonsurgical group (24 vs. 6), including three cases of deep venous thrombosis and three cases of knee stiffness requiring brisement forcé while the patient was anesthetized, Dr. Skou and his associates said.
This study was supported by the Obel Family Foundation, the Danish Rheumatism Association, the Health Science Foundation of the North Denmark Region, Foot Science International, Spar Nord Foundation, the Bevica Foundation, the Association of Danish Physiotherapists Research Fund, the Medical Specialist Heinrich Kopp’s Grant, and the Danish Medical Association Research Fund. Dr. Skou and his associates reported having no relevant financial disclosures.
Total knee replacement was superior to nonsurgical treatment in relieving pain, restoring function, and improving quality of life for patients with moderate to severe knee osteoarthritis, according to a report published online Oct. 22 in the New England Journal of Medicine.
Even though the number of total knee replacements performed each year is large and steadily increasing – with more than 670,000 done in 2012 in the United States alone – no high-quality randomized, controlled trials have ever compared the effectiveness of the procedure against nonsurgical treatment, said Søren T. Skou, Ph.D., of the Research Unit for Musculoskeletal Function and Physiotherapy, Institute of Sports Science and Clinical Biomechanics, University of Southern Denmark, Odense, and his associates.
Dr. Skou and his colleagues remedied that situation by randomly assigning 100 adults (mean age, 66 years) who were eligible for unilateral total knee replacement to either undergo the procedure and then receive a comprehensive nonsurgical intervention (50 patients) or receive the comprehensive nonsurgical intervention alone (50 patients) at two specialized university clinics in Denmark. The 12-week nonsurgical intervention comprised a twice-weekly group exercise program to restore neutral, functional realignment of the legs; two 1-hour education sessions regarding osteoarthritis characteristics, treatments, and self-help strategies; a dietary (weight-loss) program; provision of individually fitted insoles with medial arch support and a lateral wedge if patients had knee-lateral-to-foot positioning; and as-needed pain medication for pain – acetaminophen and ibuprofen – and pantoprazole, a proton-pump inhibitor.
The primary outcome measure in the trial was the between-group difference at 1 year in improvement on four subscales of the Knee Injury and Osteoarthritis Outcome Scores (KOOS) for pain, symptoms, activities of daily living, and quality of life. The surgical group showed a significantly greater improvement (32.5 out of a possible 100 points) than the nonsurgical group (16.0 points) in this outcome. The surgical group also showed significantly greater improvements in all five individual subscales and in a timed chair-rising test, a timed 20-meter walk test, and on a quality-of-life index, the investigators said (N Engl J Med. 2015 373;17:1597-606).
However, it is important to note that patients who had only the nonsurgical intervention showed clinically relevant improvements, and only 26% of them chose to have the surgery after the conclusion of the study. As expected, the surgical group had more serious adverse events than did the nonsurgical group (24 vs. 6), including three cases of deep venous thrombosis and three cases of knee stiffness requiring brisement forcé while the patient was anesthetized, Dr. Skou and his associates said.
This study was supported by the Obel Family Foundation, the Danish Rheumatism Association, the Health Science Foundation of the North Denmark Region, Foot Science International, Spar Nord Foundation, the Bevica Foundation, the Association of Danish Physiotherapists Research Fund, the Medical Specialist Heinrich Kopp’s Grant, and the Danish Medical Association Research Fund. Dr. Skou and his associates reported having no relevant financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Total knee replacement is superior to nonsurgical treatment in decreasing pain and improving function and quality of life.
Major finding: The surgical group showed a significantly greater improvement 1 year from baseline (32.5 out of a possible 100 points) than did the nonsurgical group (16.0 points) in mean Knee Injury and Osteoarthritis Outcome Scores (KOOS) for pain, symptoms, activities of daily living, and quality of life.
Data source: A randomized, controlled trial comparing 1-year outcomes after total knee replacement (50 patients) vs. nonsurgical treatment (50 patients) for osteoarthritis.
Disclosures: This study was supported by the Obel Family Foundation, the Danish Rheumatism Association, the Health Science Foundation of the North Denmark Region, Foot Science International, Spar Nord Foundation, the Bevica Foundation, the Association of Danish Physiotherapists Research Fund, the Medical Specialist Heinrich Kopp’s Grant, and the Danish Medical Association Research Fund. Dr. Skou and his associates reported having no relevant financial disclosures.
Osteofibrous Dysplasia–like Adamantinoma of the Tibia in a 15-Year-Old Girl
Adamantinomas are rare primary malignant bone tumors (less than 1% of all bone tumors) that arise most commonly in the tibia.1 There is a predilection for adult men aged 20 to 50 years, with rare occurrences in children. These tumors are malignant, highly invasive, and have significant metastatic potential.2 A rarely seen, benign variant, known as osteofibrous dysplasia–like adamantinoma, is described in the literature, with fewer than 135 cases reported.3-5 This variant predominantly has benign characteristics of an osteofibrous dysplasia lesion but has the potential to transform into an adamantinoma.6 Osteofibrous dysplasia–like adamantinoma has been observed to regress with age and is also referred to as a regressing adamantinoma or differentiated adamantinoma.7
We report an uncommon case of an osteofibrous dysplasia–like adamantinoma of the tibia in a 15-year-old girl. We decided to observe the tumor with regular 3- to 6-month follow-ups. Osteofibrous dysplasia–like adamantinoma in our patient has remained stable for 2 years and has an excellent prognosis.8 We report this case for its rarity, its short-term stability, and significant treatment implications due to its potential to regress or develop into a malignant form. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 15-year-old girl was referred to our institution for evaluation of anterior left knee pain. She had sustained a fall while playing basketball 3 months earlier and had been having left knee pain since that time. She did not have any swelling, catching, or locking in her left knee. She denied any recent fever, chills, night sweats, weight loss, nausea, vomiting, or diarrhea. On physical examination, her gait was normal and no swelling, erythema, or tenderness was noticed around the left knee.
Plain radiographs revealed a heterogeneous lesion with sclerosis and thickening of the anteromedial cortex of the proximal left tibia (Figures 1A, 1B). A computed tomography (CT) scan of the abdomen, pelvis, and chest showed no osseous abnormalities. A whole-body bone scan showed activity in the anterior aspect of the left proximal tibia. No other areas of activity were noted. Magnetic resonance imaging of the left leg showed an elongated, multiloculated, enhancing mass arising from the anterolateral cortex and extending from the tibial tuberosity to the mid-diaphysis of the left tibia. Histologic examination of the CT-guided core needle biopsy specimen showed that the lesion was composed of dense fibrocollagenous tissue separating irregular bony trabeculae with osteoblastic and osteoclastic activity. There was no evidence of any atypical cells, necrosis, or significant mitotic activity. No epithelial cells were identified on hematoxylin-eosin (H&E) stain (Figure 2). However, immunohistochemical staining was positive for focal cytokeratin-positive epithelial cells (Figure 3). The lesion was diagnosed as an osteofibrous dysplasia–like adamantinoma on the basis of the radiographic and histologic findings. We elected nonoperative intervention given the benign nature of the lesion and its potential to regress. Given the possibility of sampling error and potential for progression, the patient was followed regularly at 3- to 6-month intervals over a 2-year period without disease progression.
Discussion
Osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma are rare fibro-osseous lesions that largely involve the midshaft of the tibia. Osteofibrous dysplasia accounts for 0.2% of primary bone tumors, whereas adamantinoma accounts for 0.1% to 0.5% of malignant bone tumors.9 Osteofibrous dysplasia is a benign lesion composed primarily of fibro-osseous tissue. Adamantinoma, however, is a slow-growing, low-grade, malignant biphasic tumor with intermingled epithelial and fibro-osseous components. It is an aggressive tumor that is locally invasive and can metastasize.2 Osteofibrous dysplasia–like adamantinoma (also known as differentiated or regressing adamantinoma) is a benign lesion like osteofibrous dysplasia but has features of both osteofibrous dysplasia and adamantinoma. Osteofibrous dysplasia–like adamantinoma may progress and become a malignant adamantinoma.6,10
The radiologic features of the 3 lesions are quite similar. It is not possible to distinguish between osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma based on imaging alone.9 Adamantinoma, being highly invasive, can be distinguished from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma according to the extent of involvement of the medullary cavity seen on magnetic resonance imaging.9 Complete involvement of the medullary cavity is almost always seen in an adamantinoma. Involvement of the medullary cavity is minimal or absent in osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma lesions.
Tissue confirmation through biopsy is crucial for accurate diagnosis. A biopsy should always be performed on any suspicious lesion,3,6 and the fibro-osseous lesion should be treated as an adamantinoma if findings are equivocal. A biopsy also distinctly distinguishes these lesions from benign fibrous cortical defects, which have a similar radiographic appearance. While open biopsy is the gold standard, minimally invasive techniques such as core needle biopsy and fine needle biopsy are increasingly used.6 Because of the higher risk of misdiagnosis with minimally invasive techniques, radiographic confirmation is highly recommended.5
Histologically, both osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma do not stain for cytokeratin on H&E stain. However, they can be differentiated based on immunohistochemical staining for cytokeratin. Osteofibrous dysplasia lesions exhibit diffuse staining whereas osteofibrous dysplasia–like adamantinoma lesions show focal staining of small nests of epithelial cells. Adamantinoma, in comparison, exhibits a biphasic pattern on H&E stain, representing areas of epithelial and osteofibrous cells. Immunohistochemical staining for cytokeratin of an adamantinoma reveals large nests of epithelial cells.
The association between osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma is not clearly established. However, it is widely believed that these 3 lesions represent a spectrum of the same disease and are linearly related in disease progression, with osteofibrous dysplasia at the benign end of the spectrum, osteofibrous dysplasia–like adamantinoma the intermediate form, and adamantinoma at the malignant end of the spectrum.11
Hazelbag and colleagues6 and Springfield and colleagues10 point out that osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma could be precursor lesions of adamantinoma. We found several studies in the literature that support and document progression from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma to an adamantinoma.4,6,10,12 Other studies, however, showed no progression from either a benign osteofibrous dysplasia or an osteofibrous dysplasia–like adamantinoma lesion to a malignant adamantinoma. Park and colleagues13 described 41 cases of osteofibrous dysplasia that did not progress to adamantinoma. Kuruvilla and Steiner8 described 5 cases of osteofibrous dysplasia–like adamantinoma that showed no progression to adamantinoma. Additionally, our case has not progressed and has remained radiographically stable over a 2-year follow-up. Czerniak and colleagues7 and Ueda and colleagues14 postulated, based on histologic and immunohistochemical studies, that osteofibrous dysplasia–like adamantinoma might be a regressing form of an adamantinoma that is undergoing reparative processes that could result in complete elimination of all tumor cells.
In general, any lesion with absent to low malignant potential could be managed nonoperatively with periodic observation and without the need for surgical intervention. Thus, identification of a stable or nonprogressing osteofibrous dysplasia–like adamantinoma lesion has significant treatment implications. Campanacci and Laus15 at the Rizzoli Institute in Milan, through long term follow-up of their patients with osteofibrous dysplasia, found that most lesions had a tendency to regress spontaneously by puberty. They recommended that nonextensive osteofibrous dysplasia lesions should be observed, and surgery should be delayed until puberty. Gleason and colleagues16 also recommended nonoperative management of osteofibrous dysplasia lesions, with surgery used only for large, deforming, and highly invasive lesions. We recommend a similar treatment approach for osteofibrous dysplasia–like adamantinoma lesions.
Adamantinomas, however, are usually symptomatic, are highly invasive, have a high recurrence rate, and can metastasize.9 In these patients, a wide en bloc resection or amputation should be performed as soon as possible.11 Our case highlights that osteofibrous dysplasia–like adamantinoma lesions can occur in children and can remain stable, especially over the short term. Such lesions can be observed without surgical intervention.
1. Kanakaraddi SV, Nagaraj G, Ravinath TM. Adamantinoma of the tibia with late skeletal metastasis: an unusual presentation. J Bone Joint Surg Br. 2007;89(3):388-389.
2. Van Geel AN, Hazelbag HM, Slingerland R, Vermeulen MI. Disseminating adamantinoma of the tibia. Sarcoma. 1997;1(2):109-111.
3. Povysil C, Kohout A, Urban K, Horak M. Differentiated adamantinoma of the fibula: a rhabdoid variant. Skeletal Radiol. 2004;33(8):488-492.
4. Hatori M, Watanabe M, Hosaka M, Sasano H, Narita M, Kokubun S. A classic adamantinoma arising from osteofibrous dysplasia-like adamantinoma in the lower leg: a case report and review of the literature. Tohoku J Exp Med. 2006;209(1):53-59.
5. Khanna M, Delaney D, Tirabosco R, Saifuddin A. Osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma and adamantinoma: correlation of radiological imaging features with surgical histology and assessment of the use of radiology in contributing to needle biopsy diagnosis. Skeletal Radiol. 2008;37(12):1077-1084.
6. Hazelbag HM, Taminiau AH, Fleuren GJ, Hogendoorn PC. Adamantinoma of the long bones. A clinicopathological study of thirty-two patients with emphasis on histological subtype, precursor lesion, and biological behavior. J Bone Joint Surg Am. 1994;76(10):1482-1499.
7. Czerniak B, Rojas-Corona RR, Dorfman HD. Morphologic diversity of long bone adamantinoma. The concept of differentiated (regressing) adamantinoma and its relationship to osteofibrous dysplasia. Cancer. 1989;64(11):2319-2334.
8. Kuruvilla G, Steiner GC. Osteofibrous dysplasia-like adamantinoma of bone: a report of five cases with immunohistochemical and ultrastructural studies. Hum Pathol. 1998;29(8):809-814.
9. Bethapudi S, Ritchie DA, Macduff E, Straiton J. Imaging in osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma, and classic adamantinoma. Clin Radiol. 2014;69(2):200-208.
10. Springfield DS, Rosenberg AE, Mankin HJ, Mindell ER. Relationship between osteofibrous dysplasia and adamantinoma. Clin Orthop Relat Res. 1994;(309):234-244.
11. Most MJ, Sim FH, Inwards CY. Osteofibrous dysplasia and adamantinoma. J Am Acad Orthop Surg. 2010;18(6):358-366.
12. Lee RS, Weitzel S, Eastwood DM, et al. Osteofibrous dysplasia of the tibia. Is there a need for a radical surgical approach? J Bone Joint Surg Br. 2006;88(5):658-664.
13. Park YK, Unni KK, McLeod RA, Pritchard DJ. Osteofibrous dysplasia: clinicopathologic study of 80 cases. Hum Pathol. 1993;24(12):1339-1347.
14. Ueda Y, Roessner A, Bosse A, Edel G, Bocker W, Wuisman P. Juvenile intracortical adamantinoma of the tibia with predominant osteofibrous dysplasia-like features. Pathol Res Pract. 1991;187(8):1039-1043; discussion 1043-1034.
15. Campanacci M, Laus M. Osteofibrous dysplasia of the tibia and fibula. J Bone Joint Surg Am. 1981;63(3):367-375.
16. Gleason BC, Liegl-Atzwanger B, Kozakewich HP, et al. Osteofibrous dysplasia and adamantinoma in children and adolescents: a clinicopathologic reappraisal. Am J Surg Pathol. 2008;32(3):363-376.
Adamantinomas are rare primary malignant bone tumors (less than 1% of all bone tumors) that arise most commonly in the tibia.1 There is a predilection for adult men aged 20 to 50 years, with rare occurrences in children. These tumors are malignant, highly invasive, and have significant metastatic potential.2 A rarely seen, benign variant, known as osteofibrous dysplasia–like adamantinoma, is described in the literature, with fewer than 135 cases reported.3-5 This variant predominantly has benign characteristics of an osteofibrous dysplasia lesion but has the potential to transform into an adamantinoma.6 Osteofibrous dysplasia–like adamantinoma has been observed to regress with age and is also referred to as a regressing adamantinoma or differentiated adamantinoma.7
We report an uncommon case of an osteofibrous dysplasia–like adamantinoma of the tibia in a 15-year-old girl. We decided to observe the tumor with regular 3- to 6-month follow-ups. Osteofibrous dysplasia–like adamantinoma in our patient has remained stable for 2 years and has an excellent prognosis.8 We report this case for its rarity, its short-term stability, and significant treatment implications due to its potential to regress or develop into a malignant form. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 15-year-old girl was referred to our institution for evaluation of anterior left knee pain. She had sustained a fall while playing basketball 3 months earlier and had been having left knee pain since that time. She did not have any swelling, catching, or locking in her left knee. She denied any recent fever, chills, night sweats, weight loss, nausea, vomiting, or diarrhea. On physical examination, her gait was normal and no swelling, erythema, or tenderness was noticed around the left knee.
Plain radiographs revealed a heterogeneous lesion with sclerosis and thickening of the anteromedial cortex of the proximal left tibia (Figures 1A, 1B). A computed tomography (CT) scan of the abdomen, pelvis, and chest showed no osseous abnormalities. A whole-body bone scan showed activity in the anterior aspect of the left proximal tibia. No other areas of activity were noted. Magnetic resonance imaging of the left leg showed an elongated, multiloculated, enhancing mass arising from the anterolateral cortex and extending from the tibial tuberosity to the mid-diaphysis of the left tibia. Histologic examination of the CT-guided core needle biopsy specimen showed that the lesion was composed of dense fibrocollagenous tissue separating irregular bony trabeculae with osteoblastic and osteoclastic activity. There was no evidence of any atypical cells, necrosis, or significant mitotic activity. No epithelial cells were identified on hematoxylin-eosin (H&E) stain (Figure 2). However, immunohistochemical staining was positive for focal cytokeratin-positive epithelial cells (Figure 3). The lesion was diagnosed as an osteofibrous dysplasia–like adamantinoma on the basis of the radiographic and histologic findings. We elected nonoperative intervention given the benign nature of the lesion and its potential to regress. Given the possibility of sampling error and potential for progression, the patient was followed regularly at 3- to 6-month intervals over a 2-year period without disease progression.
Discussion
Osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma are rare fibro-osseous lesions that largely involve the midshaft of the tibia. Osteofibrous dysplasia accounts for 0.2% of primary bone tumors, whereas adamantinoma accounts for 0.1% to 0.5% of malignant bone tumors.9 Osteofibrous dysplasia is a benign lesion composed primarily of fibro-osseous tissue. Adamantinoma, however, is a slow-growing, low-grade, malignant biphasic tumor with intermingled epithelial and fibro-osseous components. It is an aggressive tumor that is locally invasive and can metastasize.2 Osteofibrous dysplasia–like adamantinoma (also known as differentiated or regressing adamantinoma) is a benign lesion like osteofibrous dysplasia but has features of both osteofibrous dysplasia and adamantinoma. Osteofibrous dysplasia–like adamantinoma may progress and become a malignant adamantinoma.6,10
The radiologic features of the 3 lesions are quite similar. It is not possible to distinguish between osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma based on imaging alone.9 Adamantinoma, being highly invasive, can be distinguished from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma according to the extent of involvement of the medullary cavity seen on magnetic resonance imaging.9 Complete involvement of the medullary cavity is almost always seen in an adamantinoma. Involvement of the medullary cavity is minimal or absent in osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma lesions.
Tissue confirmation through biopsy is crucial for accurate diagnosis. A biopsy should always be performed on any suspicious lesion,3,6 and the fibro-osseous lesion should be treated as an adamantinoma if findings are equivocal. A biopsy also distinctly distinguishes these lesions from benign fibrous cortical defects, which have a similar radiographic appearance. While open biopsy is the gold standard, minimally invasive techniques such as core needle biopsy and fine needle biopsy are increasingly used.6 Because of the higher risk of misdiagnosis with minimally invasive techniques, radiographic confirmation is highly recommended.5
Histologically, both osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma do not stain for cytokeratin on H&E stain. However, they can be differentiated based on immunohistochemical staining for cytokeratin. Osteofibrous dysplasia lesions exhibit diffuse staining whereas osteofibrous dysplasia–like adamantinoma lesions show focal staining of small nests of epithelial cells. Adamantinoma, in comparison, exhibits a biphasic pattern on H&E stain, representing areas of epithelial and osteofibrous cells. Immunohistochemical staining for cytokeratin of an adamantinoma reveals large nests of epithelial cells.
The association between osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma is not clearly established. However, it is widely believed that these 3 lesions represent a spectrum of the same disease and are linearly related in disease progression, with osteofibrous dysplasia at the benign end of the spectrum, osteofibrous dysplasia–like adamantinoma the intermediate form, and adamantinoma at the malignant end of the spectrum.11
Hazelbag and colleagues6 and Springfield and colleagues10 point out that osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma could be precursor lesions of adamantinoma. We found several studies in the literature that support and document progression from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma to an adamantinoma.4,6,10,12 Other studies, however, showed no progression from either a benign osteofibrous dysplasia or an osteofibrous dysplasia–like adamantinoma lesion to a malignant adamantinoma. Park and colleagues13 described 41 cases of osteofibrous dysplasia that did not progress to adamantinoma. Kuruvilla and Steiner8 described 5 cases of osteofibrous dysplasia–like adamantinoma that showed no progression to adamantinoma. Additionally, our case has not progressed and has remained radiographically stable over a 2-year follow-up. Czerniak and colleagues7 and Ueda and colleagues14 postulated, based on histologic and immunohistochemical studies, that osteofibrous dysplasia–like adamantinoma might be a regressing form of an adamantinoma that is undergoing reparative processes that could result in complete elimination of all tumor cells.
In general, any lesion with absent to low malignant potential could be managed nonoperatively with periodic observation and without the need for surgical intervention. Thus, identification of a stable or nonprogressing osteofibrous dysplasia–like adamantinoma lesion has significant treatment implications. Campanacci and Laus15 at the Rizzoli Institute in Milan, through long term follow-up of their patients with osteofibrous dysplasia, found that most lesions had a tendency to regress spontaneously by puberty. They recommended that nonextensive osteofibrous dysplasia lesions should be observed, and surgery should be delayed until puberty. Gleason and colleagues16 also recommended nonoperative management of osteofibrous dysplasia lesions, with surgery used only for large, deforming, and highly invasive lesions. We recommend a similar treatment approach for osteofibrous dysplasia–like adamantinoma lesions.
Adamantinomas, however, are usually symptomatic, are highly invasive, have a high recurrence rate, and can metastasize.9 In these patients, a wide en bloc resection or amputation should be performed as soon as possible.11 Our case highlights that osteofibrous dysplasia–like adamantinoma lesions can occur in children and can remain stable, especially over the short term. Such lesions can be observed without surgical intervention.
Adamantinomas are rare primary malignant bone tumors (less than 1% of all bone tumors) that arise most commonly in the tibia.1 There is a predilection for adult men aged 20 to 50 years, with rare occurrences in children. These tumors are malignant, highly invasive, and have significant metastatic potential.2 A rarely seen, benign variant, known as osteofibrous dysplasia–like adamantinoma, is described in the literature, with fewer than 135 cases reported.3-5 This variant predominantly has benign characteristics of an osteofibrous dysplasia lesion but has the potential to transform into an adamantinoma.6 Osteofibrous dysplasia–like adamantinoma has been observed to regress with age and is also referred to as a regressing adamantinoma or differentiated adamantinoma.7
We report an uncommon case of an osteofibrous dysplasia–like adamantinoma of the tibia in a 15-year-old girl. We decided to observe the tumor with regular 3- to 6-month follow-ups. Osteofibrous dysplasia–like adamantinoma in our patient has remained stable for 2 years and has an excellent prognosis.8 We report this case for its rarity, its short-term stability, and significant treatment implications due to its potential to regress or develop into a malignant form. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 15-year-old girl was referred to our institution for evaluation of anterior left knee pain. She had sustained a fall while playing basketball 3 months earlier and had been having left knee pain since that time. She did not have any swelling, catching, or locking in her left knee. She denied any recent fever, chills, night sweats, weight loss, nausea, vomiting, or diarrhea. On physical examination, her gait was normal and no swelling, erythema, or tenderness was noticed around the left knee.
Plain radiographs revealed a heterogeneous lesion with sclerosis and thickening of the anteromedial cortex of the proximal left tibia (Figures 1A, 1B). A computed tomography (CT) scan of the abdomen, pelvis, and chest showed no osseous abnormalities. A whole-body bone scan showed activity in the anterior aspect of the left proximal tibia. No other areas of activity were noted. Magnetic resonance imaging of the left leg showed an elongated, multiloculated, enhancing mass arising from the anterolateral cortex and extending from the tibial tuberosity to the mid-diaphysis of the left tibia. Histologic examination of the CT-guided core needle biopsy specimen showed that the lesion was composed of dense fibrocollagenous tissue separating irregular bony trabeculae with osteoblastic and osteoclastic activity. There was no evidence of any atypical cells, necrosis, or significant mitotic activity. No epithelial cells were identified on hematoxylin-eosin (H&E) stain (Figure 2). However, immunohistochemical staining was positive for focal cytokeratin-positive epithelial cells (Figure 3). The lesion was diagnosed as an osteofibrous dysplasia–like adamantinoma on the basis of the radiographic and histologic findings. We elected nonoperative intervention given the benign nature of the lesion and its potential to regress. Given the possibility of sampling error and potential for progression, the patient was followed regularly at 3- to 6-month intervals over a 2-year period without disease progression.
Discussion
Osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma are rare fibro-osseous lesions that largely involve the midshaft of the tibia. Osteofibrous dysplasia accounts for 0.2% of primary bone tumors, whereas adamantinoma accounts for 0.1% to 0.5% of malignant bone tumors.9 Osteofibrous dysplasia is a benign lesion composed primarily of fibro-osseous tissue. Adamantinoma, however, is a slow-growing, low-grade, malignant biphasic tumor with intermingled epithelial and fibro-osseous components. It is an aggressive tumor that is locally invasive and can metastasize.2 Osteofibrous dysplasia–like adamantinoma (also known as differentiated or regressing adamantinoma) is a benign lesion like osteofibrous dysplasia but has features of both osteofibrous dysplasia and adamantinoma. Osteofibrous dysplasia–like adamantinoma may progress and become a malignant adamantinoma.6,10
The radiologic features of the 3 lesions are quite similar. It is not possible to distinguish between osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma based on imaging alone.9 Adamantinoma, being highly invasive, can be distinguished from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma according to the extent of involvement of the medullary cavity seen on magnetic resonance imaging.9 Complete involvement of the medullary cavity is almost always seen in an adamantinoma. Involvement of the medullary cavity is minimal or absent in osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma lesions.
Tissue confirmation through biopsy is crucial for accurate diagnosis. A biopsy should always be performed on any suspicious lesion,3,6 and the fibro-osseous lesion should be treated as an adamantinoma if findings are equivocal. A biopsy also distinctly distinguishes these lesions from benign fibrous cortical defects, which have a similar radiographic appearance. While open biopsy is the gold standard, minimally invasive techniques such as core needle biopsy and fine needle biopsy are increasingly used.6 Because of the higher risk of misdiagnosis with minimally invasive techniques, radiographic confirmation is highly recommended.5
Histologically, both osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma do not stain for cytokeratin on H&E stain. However, they can be differentiated based on immunohistochemical staining for cytokeratin. Osteofibrous dysplasia lesions exhibit diffuse staining whereas osteofibrous dysplasia–like adamantinoma lesions show focal staining of small nests of epithelial cells. Adamantinoma, in comparison, exhibits a biphasic pattern on H&E stain, representing areas of epithelial and osteofibrous cells. Immunohistochemical staining for cytokeratin of an adamantinoma reveals large nests of epithelial cells.
The association between osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma is not clearly established. However, it is widely believed that these 3 lesions represent a spectrum of the same disease and are linearly related in disease progression, with osteofibrous dysplasia at the benign end of the spectrum, osteofibrous dysplasia–like adamantinoma the intermediate form, and adamantinoma at the malignant end of the spectrum.11
Hazelbag and colleagues6 and Springfield and colleagues10 point out that osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma could be precursor lesions of adamantinoma. We found several studies in the literature that support and document progression from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma to an adamantinoma.4,6,10,12 Other studies, however, showed no progression from either a benign osteofibrous dysplasia or an osteofibrous dysplasia–like adamantinoma lesion to a malignant adamantinoma. Park and colleagues13 described 41 cases of osteofibrous dysplasia that did not progress to adamantinoma. Kuruvilla and Steiner8 described 5 cases of osteofibrous dysplasia–like adamantinoma that showed no progression to adamantinoma. Additionally, our case has not progressed and has remained radiographically stable over a 2-year follow-up. Czerniak and colleagues7 and Ueda and colleagues14 postulated, based on histologic and immunohistochemical studies, that osteofibrous dysplasia–like adamantinoma might be a regressing form of an adamantinoma that is undergoing reparative processes that could result in complete elimination of all tumor cells.
In general, any lesion with absent to low malignant potential could be managed nonoperatively with periodic observation and without the need for surgical intervention. Thus, identification of a stable or nonprogressing osteofibrous dysplasia–like adamantinoma lesion has significant treatment implications. Campanacci and Laus15 at the Rizzoli Institute in Milan, through long term follow-up of their patients with osteofibrous dysplasia, found that most lesions had a tendency to regress spontaneously by puberty. They recommended that nonextensive osteofibrous dysplasia lesions should be observed, and surgery should be delayed until puberty. Gleason and colleagues16 also recommended nonoperative management of osteofibrous dysplasia lesions, with surgery used only for large, deforming, and highly invasive lesions. We recommend a similar treatment approach for osteofibrous dysplasia–like adamantinoma lesions.
Adamantinomas, however, are usually symptomatic, are highly invasive, have a high recurrence rate, and can metastasize.9 In these patients, a wide en bloc resection or amputation should be performed as soon as possible.11 Our case highlights that osteofibrous dysplasia–like adamantinoma lesions can occur in children and can remain stable, especially over the short term. Such lesions can be observed without surgical intervention.
1. Kanakaraddi SV, Nagaraj G, Ravinath TM. Adamantinoma of the tibia with late skeletal metastasis: an unusual presentation. J Bone Joint Surg Br. 2007;89(3):388-389.
2. Van Geel AN, Hazelbag HM, Slingerland R, Vermeulen MI. Disseminating adamantinoma of the tibia. Sarcoma. 1997;1(2):109-111.
3. Povysil C, Kohout A, Urban K, Horak M. Differentiated adamantinoma of the fibula: a rhabdoid variant. Skeletal Radiol. 2004;33(8):488-492.
4. Hatori M, Watanabe M, Hosaka M, Sasano H, Narita M, Kokubun S. A classic adamantinoma arising from osteofibrous dysplasia-like adamantinoma in the lower leg: a case report and review of the literature. Tohoku J Exp Med. 2006;209(1):53-59.
5. Khanna M, Delaney D, Tirabosco R, Saifuddin A. Osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma and adamantinoma: correlation of radiological imaging features with surgical histology and assessment of the use of radiology in contributing to needle biopsy diagnosis. Skeletal Radiol. 2008;37(12):1077-1084.
6. Hazelbag HM, Taminiau AH, Fleuren GJ, Hogendoorn PC. Adamantinoma of the long bones. A clinicopathological study of thirty-two patients with emphasis on histological subtype, precursor lesion, and biological behavior. J Bone Joint Surg Am. 1994;76(10):1482-1499.
7. Czerniak B, Rojas-Corona RR, Dorfman HD. Morphologic diversity of long bone adamantinoma. The concept of differentiated (regressing) adamantinoma and its relationship to osteofibrous dysplasia. Cancer. 1989;64(11):2319-2334.
8. Kuruvilla G, Steiner GC. Osteofibrous dysplasia-like adamantinoma of bone: a report of five cases with immunohistochemical and ultrastructural studies. Hum Pathol. 1998;29(8):809-814.
9. Bethapudi S, Ritchie DA, Macduff E, Straiton J. Imaging in osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma, and classic adamantinoma. Clin Radiol. 2014;69(2):200-208.
10. Springfield DS, Rosenberg AE, Mankin HJ, Mindell ER. Relationship between osteofibrous dysplasia and adamantinoma. Clin Orthop Relat Res. 1994;(309):234-244.
11. Most MJ, Sim FH, Inwards CY. Osteofibrous dysplasia and adamantinoma. J Am Acad Orthop Surg. 2010;18(6):358-366.
12. Lee RS, Weitzel S, Eastwood DM, et al. Osteofibrous dysplasia of the tibia. Is there a need for a radical surgical approach? J Bone Joint Surg Br. 2006;88(5):658-664.
13. Park YK, Unni KK, McLeod RA, Pritchard DJ. Osteofibrous dysplasia: clinicopathologic study of 80 cases. Hum Pathol. 1993;24(12):1339-1347.
14. Ueda Y, Roessner A, Bosse A, Edel G, Bocker W, Wuisman P. Juvenile intracortical adamantinoma of the tibia with predominant osteofibrous dysplasia-like features. Pathol Res Pract. 1991;187(8):1039-1043; discussion 1043-1034.
15. Campanacci M, Laus M. Osteofibrous dysplasia of the tibia and fibula. J Bone Joint Surg Am. 1981;63(3):367-375.
16. Gleason BC, Liegl-Atzwanger B, Kozakewich HP, et al. Osteofibrous dysplasia and adamantinoma in children and adolescents: a clinicopathologic reappraisal. Am J Surg Pathol. 2008;32(3):363-376.
1. Kanakaraddi SV, Nagaraj G, Ravinath TM. Adamantinoma of the tibia with late skeletal metastasis: an unusual presentation. J Bone Joint Surg Br. 2007;89(3):388-389.
2. Van Geel AN, Hazelbag HM, Slingerland R, Vermeulen MI. Disseminating adamantinoma of the tibia. Sarcoma. 1997;1(2):109-111.
3. Povysil C, Kohout A, Urban K, Horak M. Differentiated adamantinoma of the fibula: a rhabdoid variant. Skeletal Radiol. 2004;33(8):488-492.
4. Hatori M, Watanabe M, Hosaka M, Sasano H, Narita M, Kokubun S. A classic adamantinoma arising from osteofibrous dysplasia-like adamantinoma in the lower leg: a case report and review of the literature. Tohoku J Exp Med. 2006;209(1):53-59.
5. Khanna M, Delaney D, Tirabosco R, Saifuddin A. Osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma and adamantinoma: correlation of radiological imaging features with surgical histology and assessment of the use of radiology in contributing to needle biopsy diagnosis. Skeletal Radiol. 2008;37(12):1077-1084.
6. Hazelbag HM, Taminiau AH, Fleuren GJ, Hogendoorn PC. Adamantinoma of the long bones. A clinicopathological study of thirty-two patients with emphasis on histological subtype, precursor lesion, and biological behavior. J Bone Joint Surg Am. 1994;76(10):1482-1499.
7. Czerniak B, Rojas-Corona RR, Dorfman HD. Morphologic diversity of long bone adamantinoma. The concept of differentiated (regressing) adamantinoma and its relationship to osteofibrous dysplasia. Cancer. 1989;64(11):2319-2334.
8. Kuruvilla G, Steiner GC. Osteofibrous dysplasia-like adamantinoma of bone: a report of five cases with immunohistochemical and ultrastructural studies. Hum Pathol. 1998;29(8):809-814.
9. Bethapudi S, Ritchie DA, Macduff E, Straiton J. Imaging in osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma, and classic adamantinoma. Clin Radiol. 2014;69(2):200-208.
10. Springfield DS, Rosenberg AE, Mankin HJ, Mindell ER. Relationship between osteofibrous dysplasia and adamantinoma. Clin Orthop Relat Res. 1994;(309):234-244.
11. Most MJ, Sim FH, Inwards CY. Osteofibrous dysplasia and adamantinoma. J Am Acad Orthop Surg. 2010;18(6):358-366.
12. Lee RS, Weitzel S, Eastwood DM, et al. Osteofibrous dysplasia of the tibia. Is there a need for a radical surgical approach? J Bone Joint Surg Br. 2006;88(5):658-664.
13. Park YK, Unni KK, McLeod RA, Pritchard DJ. Osteofibrous dysplasia: clinicopathologic study of 80 cases. Hum Pathol. 1993;24(12):1339-1347.
14. Ueda Y, Roessner A, Bosse A, Edel G, Bocker W, Wuisman P. Juvenile intracortical adamantinoma of the tibia with predominant osteofibrous dysplasia-like features. Pathol Res Pract. 1991;187(8):1039-1043; discussion 1043-1034.
15. Campanacci M, Laus M. Osteofibrous dysplasia of the tibia and fibula. J Bone Joint Surg Am. 1981;63(3):367-375.
16. Gleason BC, Liegl-Atzwanger B, Kozakewich HP, et al. Osteofibrous dysplasia and adamantinoma in children and adolescents: a clinicopathologic reappraisal. Am J Surg Pathol. 2008;32(3):363-376.