User login
Does DTC heart drug advertising discourage lifestyle changes?
A 5-minute bout of direct-to-consumer advertising (DTCA) for prescription heart drugs was associated with favorable perceptions of both medication use and pharmaceutical companies, but did not seem to negate intentions to use lifestyle interventions, a survey study shows.
Participants who watched ads for various prescription heart drugs, with or without price disclosure, were more likely to report positive perceptions of drug companies and intentions to take actions such as switching medications.
The ads did not seem to affect intentions to eat healthfully and exercise.
The study was published online in JAMA Health Forum.
DTCA ‘unlikely to have an adverse effect’
“Increasing prevalence of DTCA may promote an overreliance on medication over healthy lifestyle choices to manage chronic conditions,” coauthor Yashaswini Singh, MPA, a PhD candidate at the Johns Hopkins University, Baltimore, told this news organization. “Thus, we hypothesized that DTCA exposure would reduce the likelihood of individuals engaging in preventive health behaviors.”
“However,” she said, “our results did not support this hypothesis, suggesting that exposure to DTCA for heart disease medication is unlikely to have an adverse effect on individuals’ intentions to engage in diet and exercise.”
That said, she added, “DTCA of prescription drugs can contribute to rising drug costs due to overprescribing of both inappropriate and brand-name drugs over cheaper generic alternatives. While we do not examine this mechanism in our paper, this remains an important question for future research.”
For the study, the team recruited 2,874 individuals (mean age, 53.8 years; 54% men; 83% White) from a U.S. nationally representative sample of people at high risk of cardiovascular disease, the Ipsos Public Affairs KnowledgePanel.
Participants were randomly assigned to one of three interventions: DTCA for heart disease medications, DTCA for heart disease medications with price disclosure, or nonpharmaceutical advertising (control). Each group watched five 1-minute videos for a total of 5 minutes of advertising exposure.
One group viewed ads for four heart disease medications – two ads for sacubitril/valsartan (Entresto, Novartis) and one each for rivaroxaban (Xarelto, Bayer), evolocumab (Repatha, Amgen), and ticagrelor (Brilinta, AstraZeneca); the second group saw the same ads, but with prices spliced in; and controls watched videos for nondrug products, such as consumer electronics.
Participants then completed a questionnaire to measure medication- and lifestyle-related intentions, as well as health-related beliefs and perceptions. Using a scale of 1 (highly unlikely) to 5 (highly likely), they rated the likelihood of their switching medication, asking a physician or insurer about a medication, searching for the drug online, or taking it as directed. The same scale was used to rate the likelihood of their being more physically active or eating more healthfully.
On a scale of 1 (always disagree) to 5 (always agree), they also related their perceptions of pharmaceutical manufacturers as being competent, innovative, and trustworthy.
To measure the magnitude of DTCA associations, the researchers calculated marginal effects (MEs) of treatment – that is, the difference in probability of an outcome between the treatment and control arms.
They found a positive association between DTCA and medication-related behavioral intentions, including intention to switch medication (ME, 0.004; P = .002) and engage in information-seeking behaviors (ME, 0.02; P = .01).
There was no evidence suggesting that pharmaceutical DTCA discouraged use of nonpharmacologic lifestyle interventions to help manage heart disease. DTCA also was positively associated with consumers’ favorable perceptions of pharmaceutical manufacturers (competence: ME, 0.03; P = .01; innovative: ME, 0.03; P = .008).
No differential associations were seen for price disclosures in DTCA.
Questions remain
The authors acknowledged that the study focused on short-term behavioral intentions and that “future research should focus on the long-term effects of advertising in a real-world randomized setting.”
Ms. Singh said additional questions, some of which her team is investigating, include “understanding the interaction between government policies [such as] drug pricing reforms and firms’ advertising decisions; understanding whether observed changes in individuals’ health beliefs translate into actual changes to information-seeking behavior and health care utilization; and whether the demographic, political, and social characteristics of individuals shape their behavioral responses to advertising.”
Johanna Contreras, MD, an advanced heart failure and transplantation cardiologist at Mount Sinai Hospital, New York, said in an interview that the findings don’t surprise her. “The caveat is that this study was an online survey, so it only captured the beliefs and intentions, but not patient demand for the product and use of the product.”
“I do believe DTCA can create positive intentions towards the product ... and could make people more receptive to interventions,” she said. However, the information must be presented in a balanced way.
In addition, she noted, “price is still important. I think people take pricing into account when deciding to proceed with an intervention. If the price is ‘right’ or a little lower than expected, then they will likely consider the product. But if the price is significantly lower, then they may not trust that it is a good product. Generic drugs are an example. Even though they are approved and far cheaper than brand names, patients are often skeptical to take them.”
The study was funded with a grant from the Blue Cross Blue Shield of Illinois Affordability Cures Consortium. Ms. Singh and coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A 5-minute bout of direct-to-consumer advertising (DTCA) for prescription heart drugs was associated with favorable perceptions of both medication use and pharmaceutical companies, but did not seem to negate intentions to use lifestyle interventions, a survey study shows.
Participants who watched ads for various prescription heart drugs, with or without price disclosure, were more likely to report positive perceptions of drug companies and intentions to take actions such as switching medications.
The ads did not seem to affect intentions to eat healthfully and exercise.
The study was published online in JAMA Health Forum.
DTCA ‘unlikely to have an adverse effect’
“Increasing prevalence of DTCA may promote an overreliance on medication over healthy lifestyle choices to manage chronic conditions,” coauthor Yashaswini Singh, MPA, a PhD candidate at the Johns Hopkins University, Baltimore, told this news organization. “Thus, we hypothesized that DTCA exposure would reduce the likelihood of individuals engaging in preventive health behaviors.”
“However,” she said, “our results did not support this hypothesis, suggesting that exposure to DTCA for heart disease medication is unlikely to have an adverse effect on individuals’ intentions to engage in diet and exercise.”
That said, she added, “DTCA of prescription drugs can contribute to rising drug costs due to overprescribing of both inappropriate and brand-name drugs over cheaper generic alternatives. While we do not examine this mechanism in our paper, this remains an important question for future research.”
For the study, the team recruited 2,874 individuals (mean age, 53.8 years; 54% men; 83% White) from a U.S. nationally representative sample of people at high risk of cardiovascular disease, the Ipsos Public Affairs KnowledgePanel.
Participants were randomly assigned to one of three interventions: DTCA for heart disease medications, DTCA for heart disease medications with price disclosure, or nonpharmaceutical advertising (control). Each group watched five 1-minute videos for a total of 5 minutes of advertising exposure.
One group viewed ads for four heart disease medications – two ads for sacubitril/valsartan (Entresto, Novartis) and one each for rivaroxaban (Xarelto, Bayer), evolocumab (Repatha, Amgen), and ticagrelor (Brilinta, AstraZeneca); the second group saw the same ads, but with prices spliced in; and controls watched videos for nondrug products, such as consumer electronics.
Participants then completed a questionnaire to measure medication- and lifestyle-related intentions, as well as health-related beliefs and perceptions. Using a scale of 1 (highly unlikely) to 5 (highly likely), they rated the likelihood of their switching medication, asking a physician or insurer about a medication, searching for the drug online, or taking it as directed. The same scale was used to rate the likelihood of their being more physically active or eating more healthfully.
On a scale of 1 (always disagree) to 5 (always agree), they also related their perceptions of pharmaceutical manufacturers as being competent, innovative, and trustworthy.
To measure the magnitude of DTCA associations, the researchers calculated marginal effects (MEs) of treatment – that is, the difference in probability of an outcome between the treatment and control arms.
They found a positive association between DTCA and medication-related behavioral intentions, including intention to switch medication (ME, 0.004; P = .002) and engage in information-seeking behaviors (ME, 0.02; P = .01).
There was no evidence suggesting that pharmaceutical DTCA discouraged use of nonpharmacologic lifestyle interventions to help manage heart disease. DTCA also was positively associated with consumers’ favorable perceptions of pharmaceutical manufacturers (competence: ME, 0.03; P = .01; innovative: ME, 0.03; P = .008).
No differential associations were seen for price disclosures in DTCA.
Questions remain
The authors acknowledged that the study focused on short-term behavioral intentions and that “future research should focus on the long-term effects of advertising in a real-world randomized setting.”
Ms. Singh said additional questions, some of which her team is investigating, include “understanding the interaction between government policies [such as] drug pricing reforms and firms’ advertising decisions; understanding whether observed changes in individuals’ health beliefs translate into actual changes to information-seeking behavior and health care utilization; and whether the demographic, political, and social characteristics of individuals shape their behavioral responses to advertising.”
Johanna Contreras, MD, an advanced heart failure and transplantation cardiologist at Mount Sinai Hospital, New York, said in an interview that the findings don’t surprise her. “The caveat is that this study was an online survey, so it only captured the beliefs and intentions, but not patient demand for the product and use of the product.”
“I do believe DTCA can create positive intentions towards the product ... and could make people more receptive to interventions,” she said. However, the information must be presented in a balanced way.
In addition, she noted, “price is still important. I think people take pricing into account when deciding to proceed with an intervention. If the price is ‘right’ or a little lower than expected, then they will likely consider the product. But if the price is significantly lower, then they may not trust that it is a good product. Generic drugs are an example. Even though they are approved and far cheaper than brand names, patients are often skeptical to take them.”
The study was funded with a grant from the Blue Cross Blue Shield of Illinois Affordability Cures Consortium. Ms. Singh and coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A 5-minute bout of direct-to-consumer advertising (DTCA) for prescription heart drugs was associated with favorable perceptions of both medication use and pharmaceutical companies, but did not seem to negate intentions to use lifestyle interventions, a survey study shows.
Participants who watched ads for various prescription heart drugs, with or without price disclosure, were more likely to report positive perceptions of drug companies and intentions to take actions such as switching medications.
The ads did not seem to affect intentions to eat healthfully and exercise.
The study was published online in JAMA Health Forum.
DTCA ‘unlikely to have an adverse effect’
“Increasing prevalence of DTCA may promote an overreliance on medication over healthy lifestyle choices to manage chronic conditions,” coauthor Yashaswini Singh, MPA, a PhD candidate at the Johns Hopkins University, Baltimore, told this news organization. “Thus, we hypothesized that DTCA exposure would reduce the likelihood of individuals engaging in preventive health behaviors.”
“However,” she said, “our results did not support this hypothesis, suggesting that exposure to DTCA for heart disease medication is unlikely to have an adverse effect on individuals’ intentions to engage in diet and exercise.”
That said, she added, “DTCA of prescription drugs can contribute to rising drug costs due to overprescribing of both inappropriate and brand-name drugs over cheaper generic alternatives. While we do not examine this mechanism in our paper, this remains an important question for future research.”
For the study, the team recruited 2,874 individuals (mean age, 53.8 years; 54% men; 83% White) from a U.S. nationally representative sample of people at high risk of cardiovascular disease, the Ipsos Public Affairs KnowledgePanel.
Participants were randomly assigned to one of three interventions: DTCA for heart disease medications, DTCA for heart disease medications with price disclosure, or nonpharmaceutical advertising (control). Each group watched five 1-minute videos for a total of 5 minutes of advertising exposure.
One group viewed ads for four heart disease medications – two ads for sacubitril/valsartan (Entresto, Novartis) and one each for rivaroxaban (Xarelto, Bayer), evolocumab (Repatha, Amgen), and ticagrelor (Brilinta, AstraZeneca); the second group saw the same ads, but with prices spliced in; and controls watched videos for nondrug products, such as consumer electronics.
Participants then completed a questionnaire to measure medication- and lifestyle-related intentions, as well as health-related beliefs and perceptions. Using a scale of 1 (highly unlikely) to 5 (highly likely), they rated the likelihood of their switching medication, asking a physician or insurer about a medication, searching for the drug online, or taking it as directed. The same scale was used to rate the likelihood of their being more physically active or eating more healthfully.
On a scale of 1 (always disagree) to 5 (always agree), they also related their perceptions of pharmaceutical manufacturers as being competent, innovative, and trustworthy.
To measure the magnitude of DTCA associations, the researchers calculated marginal effects (MEs) of treatment – that is, the difference in probability of an outcome between the treatment and control arms.
They found a positive association between DTCA and medication-related behavioral intentions, including intention to switch medication (ME, 0.004; P = .002) and engage in information-seeking behaviors (ME, 0.02; P = .01).
There was no evidence suggesting that pharmaceutical DTCA discouraged use of nonpharmacologic lifestyle interventions to help manage heart disease. DTCA also was positively associated with consumers’ favorable perceptions of pharmaceutical manufacturers (competence: ME, 0.03; P = .01; innovative: ME, 0.03; P = .008).
No differential associations were seen for price disclosures in DTCA.
Questions remain
The authors acknowledged that the study focused on short-term behavioral intentions and that “future research should focus on the long-term effects of advertising in a real-world randomized setting.”
Ms. Singh said additional questions, some of which her team is investigating, include “understanding the interaction between government policies [such as] drug pricing reforms and firms’ advertising decisions; understanding whether observed changes in individuals’ health beliefs translate into actual changes to information-seeking behavior and health care utilization; and whether the demographic, political, and social characteristics of individuals shape their behavioral responses to advertising.”
Johanna Contreras, MD, an advanced heart failure and transplantation cardiologist at Mount Sinai Hospital, New York, said in an interview that the findings don’t surprise her. “The caveat is that this study was an online survey, so it only captured the beliefs and intentions, but not patient demand for the product and use of the product.”
“I do believe DTCA can create positive intentions towards the product ... and could make people more receptive to interventions,” she said. However, the information must be presented in a balanced way.
In addition, she noted, “price is still important. I think people take pricing into account when deciding to proceed with an intervention. If the price is ‘right’ or a little lower than expected, then they will likely consider the product. But if the price is significantly lower, then they may not trust that it is a good product. Generic drugs are an example. Even though they are approved and far cheaper than brand names, patients are often skeptical to take them.”
The study was funded with a grant from the Blue Cross Blue Shield of Illinois Affordability Cures Consortium. Ms. Singh and coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA HEALTH FORUM
AHA statement outlines symptoms of common heart diseases
Symptoms of six common cardiovascular diseases (CVD) – acute coronary syndromes, heart failure, valvular disorders, stroke, rhythm disorders, and peripheral vascular disease – often overlap and may vary over time and by sex, the American Heart Association noted in a new scientific statement.
“Symptoms of these cardiovascular diseases can profoundly affect quality of life, and a clear understanding of them is critical for effective diagnosis and treatment decisions,” Corrine Y. Jurgens, PhD, chair of the writing committee, said in a news release.
This scientific statement is a “compendium detailing the symptoms associated with CVD, similarities or differences in symptoms among the conditions, and sex differences in symptom presentation and reporting,” said Dr. Jurgens, associate professor at Connell School of Nursing, Boston College.
The new statement was published online in Circulation.
The writing group noted that measuring CVD symptoms can be challenging because of their subjective nature. Symptoms may go unrecognized or unreported if people don’t think they are important or are related to an existing health condition.
“Some people may not consider symptoms like fatigue, sleep disturbance, weight gain, and depression as important or related to cardiovascular disease. However, research indicates that subtle symptoms such as these may predict acute events and the need for hospitalization,” Dr. Jurgens said.
ACS – chest pain and associated symptoms
The writing group noted that chest pain is the most frequently reported symptom of ACS and has often been described as substernal pressure or discomfort and may radiate to the jaw, shoulder, arm, or upper back.
The most common co-occurring symptoms are dyspnea, diaphoresis, unusual fatigue, nausea, and lightheadedness. Women are more likely than men to report additional symptoms outside of chest pain.
As a result, they have often been labeled “atypical.” However, a recent AHA advisory notes that this label may have been caused by the lack of women included in the clinical trials from which the symptom lists were derived.
The writing group said there is a need to “harmonize” ACS symptom measurement in research. The current lack of harmonization of ACS symptom measurement in research hampers growth in cumulative evidence.
“Therefore, little can be done to synthesize salient findings about symptoms across ischemic heart disease/ACS studies and to incorporate evidence-based information about symptoms into treatment guidelines and patient education materials,” they cautioned.
Heart failure
Turning to heart failure (HF), the writing group noted that dyspnea is the classic symptom and a common reason adults seek medical care.
However, early, more subtle symptoms should be recognized. These include gastrointestinal symptoms such as upset stomach, nausea, vomiting, and loss of appetite; fatigue; exercise intolerance; insomnia; pain (chest and otherwise); mood disturbances (primarily depression and anxiety); and cognitive dysfunction (brain fog, memory problems).
Women with HF report a wider variety of symptoms, are more likely to have depression and anxiety, and report a lower quality of life, compared with men with HF.
“It is important to account for dyspnea heterogeneity in both clinical practice and research by using nuanced measures and probing questions to capture this common and multifaceted symptom,” the writing group said.
“Monitoring symptoms on a spectrum, versus present or not present, with reliable and valid measures may enhance clinical care by identifying more quickly those who may be at risk for poor outcomes, such as lower quality of life, hospitalization, or death,” Dr. Jurgens added.
“Ultimately, we have work to do in terms of determining who needs more frequent monitoring or intervention to avert poor HF outcomes,” she said.
Valvular heart disease
Valvular heart disease is a frequent cause of HF, with symptoms generally indistinguishable from other HF causes. Rheumatic heart disease is still prevalent in low- and middle-income countries but has largely disappeared in high-income countries, with population aging and cardiomyopathies now key drivers of valve disease.
In the absence of acute severe valve dysfunction, patients generally have a prolonged asymptomatic period, followed by a period of progressive symptoms, resulting from the valve lesion itself or secondary myocardial remodeling and dysfunction, the writing group said.
Symptoms of aortic valve disease often differ between men and women. Aortic stenosis is typically silent for years. As stenosis progresses, women report dyspnea and exercise intolerance more often than men. Women are also more likely to be physically frail and to have a higher New York Heart Association class (III/IV) than men. Men are more likely to have chest pain.
“Given the importance of symptom assessment, more work is needed to determine the incremental value of quantitative symptom measurement as an aid to clinical management,” the writing group said.
Stroke
For clinicians, classic stroke symptoms (face drooping, arm weakness, speech difficulty), in addition to nonclassic symptoms, such as partial sensory deficit, dysarthria, vertigo, and diplopia, should be considered for activating a stroke response team, the group says.
A systematic review and meta-analysis revealed that women with stroke were more likely to present with nonfocal symptoms (for example, headache, altered mentality, and coma/stupor) than men, they noted.
To enhance public education about stroke symptoms and to facilitate the diagnosis and treatment of stroke, they say research is needed to better understand the presentation of stroke symptoms by other select demographic characteristics including race and ethnicity, age, and stroke subtype.
Poststroke screening should include assessment for anxiety, depression, fatigue, and pain, the writing group said.
Rhythm disorders
Turning to rhythm disorders, the writing group wrote that cardiac arrhythmias, including atrial fibrillation (AFib), atrial flutter, supraventricular tachycardia, bradyarrhythmia, and ventricular tachycardia, present with common symptoms.
Palpitations are a characteristic symptom of many cardiac arrhythmias. The most common cardiac arrhythmia, AFib, may present with palpitations or less specific symptoms (fatigue, dyspnea, dizziness) that occur with a broad range of rhythm disorders. Chest pain, dizziness, presyncope/syncope, and anxiety occur less frequently in AFib, the group said.
Palpitations are considered the typical symptom presentation for AFib, yet patients with new-onset AFib often present with nonspecific symptoms or no symptoms, they pointed out.
Women and younger individuals with AFib typically present with palpitations, whereas men are more commonly asymptomatic. Older age also increases the likelihood of a nonclassic or asymptomatic presentation of AFib.
Despite non-Hispanic Black individuals being at lower risk for development of AFib, research suggests that Black patients are burdened more with palpitations, dyspnea on exertion, exercise intolerance, dizziness, dyspnea at rest, and chest discomfort, compared with White or Hispanic patients.
Peripheral vascular disease
Classic claudication occurs in roughly one-third of patients with peripheral arterial disease (PAD) and is defined as calf pain that occurs in one or both legs with exertion (walking), does not begin at rest, and resolves within 10 minutes of standing still or rest.
However, non–calf exercise pain is reported more frequently than classic claudication symptoms. Women with PAD are more likely to have nonclassic symptoms or an absence of symptoms.
Assessing symptoms at rest, during exercise, and during recovery can assist with classifying symptoms as ischemic or not, the writing group said.
PAD with symptoms is associated with an increased risk for myocardial infarction and stroke, with men at higher risk than women.
Similar to PAD, peripheral venous disease (PVD) can be symptomatic or asymptomatic. Clinical classification of PVD includes symptoms such as leg pain, aching, fatigue, heaviness, cramping, tightness, restless legs syndrome, and skin irritation.
“Measuring vascular symptoms includes assessing quality of life and activity limitations, as well as the psychological impact of the disease. However, existing measures are often based on the clinician’s appraisal rather than the individual’s self-reported symptoms and severity of symptoms,” Dr. Jurgens commented.
Watch for depression
Finally, the writing group highlighted the importance of depression in cardiac patients, which occurs at about twice the rate, compared with people without any medical condition (10% vs. 5%).
In a prior statement, the AHA said depression should be considered a risk factor for worse outcomes in patients with ACS or CVD diagnosis.
The new statement highlights that people with persistent chest pain, people with HF, as well as stroke survivors and people with PAD commonly have depression and/or anxiety. In addition, cognitive changes after a stroke may affect how and whether symptoms are experienced or noticed.
While symptom relief is an important part of managing CVD, it’s also important to recognize that “factors such as depression and cognitive function may affect symptom detection and reporting,” Dr. Jurgens said.
“Monitoring and measuring symptoms with tools that appropriately account for depression and cognitive function may help to improve patient care by identifying more quickly people who may be at higher risk,” she added.
The scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Cardiovascular and Stroke Nursing; the Council on Hypertension; and the Stroke Council. The research had no commercial funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Symptoms of six common cardiovascular diseases (CVD) – acute coronary syndromes, heart failure, valvular disorders, stroke, rhythm disorders, and peripheral vascular disease – often overlap and may vary over time and by sex, the American Heart Association noted in a new scientific statement.
“Symptoms of these cardiovascular diseases can profoundly affect quality of life, and a clear understanding of them is critical for effective diagnosis and treatment decisions,” Corrine Y. Jurgens, PhD, chair of the writing committee, said in a news release.
This scientific statement is a “compendium detailing the symptoms associated with CVD, similarities or differences in symptoms among the conditions, and sex differences in symptom presentation and reporting,” said Dr. Jurgens, associate professor at Connell School of Nursing, Boston College.
The new statement was published online in Circulation.
The writing group noted that measuring CVD symptoms can be challenging because of their subjective nature. Symptoms may go unrecognized or unreported if people don’t think they are important or are related to an existing health condition.
“Some people may not consider symptoms like fatigue, sleep disturbance, weight gain, and depression as important or related to cardiovascular disease. However, research indicates that subtle symptoms such as these may predict acute events and the need for hospitalization,” Dr. Jurgens said.
ACS – chest pain and associated symptoms
The writing group noted that chest pain is the most frequently reported symptom of ACS and has often been described as substernal pressure or discomfort and may radiate to the jaw, shoulder, arm, or upper back.
The most common co-occurring symptoms are dyspnea, diaphoresis, unusual fatigue, nausea, and lightheadedness. Women are more likely than men to report additional symptoms outside of chest pain.
As a result, they have often been labeled “atypical.” However, a recent AHA advisory notes that this label may have been caused by the lack of women included in the clinical trials from which the symptom lists were derived.
The writing group said there is a need to “harmonize” ACS symptom measurement in research. The current lack of harmonization of ACS symptom measurement in research hampers growth in cumulative evidence.
“Therefore, little can be done to synthesize salient findings about symptoms across ischemic heart disease/ACS studies and to incorporate evidence-based information about symptoms into treatment guidelines and patient education materials,” they cautioned.
Heart failure
Turning to heart failure (HF), the writing group noted that dyspnea is the classic symptom and a common reason adults seek medical care.
However, early, more subtle symptoms should be recognized. These include gastrointestinal symptoms such as upset stomach, nausea, vomiting, and loss of appetite; fatigue; exercise intolerance; insomnia; pain (chest and otherwise); mood disturbances (primarily depression and anxiety); and cognitive dysfunction (brain fog, memory problems).
Women with HF report a wider variety of symptoms, are more likely to have depression and anxiety, and report a lower quality of life, compared with men with HF.
“It is important to account for dyspnea heterogeneity in both clinical practice and research by using nuanced measures and probing questions to capture this common and multifaceted symptom,” the writing group said.
“Monitoring symptoms on a spectrum, versus present or not present, with reliable and valid measures may enhance clinical care by identifying more quickly those who may be at risk for poor outcomes, such as lower quality of life, hospitalization, or death,” Dr. Jurgens added.
“Ultimately, we have work to do in terms of determining who needs more frequent monitoring or intervention to avert poor HF outcomes,” she said.
Valvular heart disease
Valvular heart disease is a frequent cause of HF, with symptoms generally indistinguishable from other HF causes. Rheumatic heart disease is still prevalent in low- and middle-income countries but has largely disappeared in high-income countries, with population aging and cardiomyopathies now key drivers of valve disease.
In the absence of acute severe valve dysfunction, patients generally have a prolonged asymptomatic period, followed by a period of progressive symptoms, resulting from the valve lesion itself or secondary myocardial remodeling and dysfunction, the writing group said.
Symptoms of aortic valve disease often differ between men and women. Aortic stenosis is typically silent for years. As stenosis progresses, women report dyspnea and exercise intolerance more often than men. Women are also more likely to be physically frail and to have a higher New York Heart Association class (III/IV) than men. Men are more likely to have chest pain.
“Given the importance of symptom assessment, more work is needed to determine the incremental value of quantitative symptom measurement as an aid to clinical management,” the writing group said.
Stroke
For clinicians, classic stroke symptoms (face drooping, arm weakness, speech difficulty), in addition to nonclassic symptoms, such as partial sensory deficit, dysarthria, vertigo, and diplopia, should be considered for activating a stroke response team, the group says.
A systematic review and meta-analysis revealed that women with stroke were more likely to present with nonfocal symptoms (for example, headache, altered mentality, and coma/stupor) than men, they noted.
To enhance public education about stroke symptoms and to facilitate the diagnosis and treatment of stroke, they say research is needed to better understand the presentation of stroke symptoms by other select demographic characteristics including race and ethnicity, age, and stroke subtype.
Poststroke screening should include assessment for anxiety, depression, fatigue, and pain, the writing group said.
Rhythm disorders
Turning to rhythm disorders, the writing group wrote that cardiac arrhythmias, including atrial fibrillation (AFib), atrial flutter, supraventricular tachycardia, bradyarrhythmia, and ventricular tachycardia, present with common symptoms.
Palpitations are a characteristic symptom of many cardiac arrhythmias. The most common cardiac arrhythmia, AFib, may present with palpitations or less specific symptoms (fatigue, dyspnea, dizziness) that occur with a broad range of rhythm disorders. Chest pain, dizziness, presyncope/syncope, and anxiety occur less frequently in AFib, the group said.
Palpitations are considered the typical symptom presentation for AFib, yet patients with new-onset AFib often present with nonspecific symptoms or no symptoms, they pointed out.
Women and younger individuals with AFib typically present with palpitations, whereas men are more commonly asymptomatic. Older age also increases the likelihood of a nonclassic or asymptomatic presentation of AFib.
Despite non-Hispanic Black individuals being at lower risk for development of AFib, research suggests that Black patients are burdened more with palpitations, dyspnea on exertion, exercise intolerance, dizziness, dyspnea at rest, and chest discomfort, compared with White or Hispanic patients.
Peripheral vascular disease
Classic claudication occurs in roughly one-third of patients with peripheral arterial disease (PAD) and is defined as calf pain that occurs in one or both legs with exertion (walking), does not begin at rest, and resolves within 10 minutes of standing still or rest.
However, non–calf exercise pain is reported more frequently than classic claudication symptoms. Women with PAD are more likely to have nonclassic symptoms or an absence of symptoms.
Assessing symptoms at rest, during exercise, and during recovery can assist with classifying symptoms as ischemic or not, the writing group said.
PAD with symptoms is associated with an increased risk for myocardial infarction and stroke, with men at higher risk than women.
Similar to PAD, peripheral venous disease (PVD) can be symptomatic or asymptomatic. Clinical classification of PVD includes symptoms such as leg pain, aching, fatigue, heaviness, cramping, tightness, restless legs syndrome, and skin irritation.
“Measuring vascular symptoms includes assessing quality of life and activity limitations, as well as the psychological impact of the disease. However, existing measures are often based on the clinician’s appraisal rather than the individual’s self-reported symptoms and severity of symptoms,” Dr. Jurgens commented.
Watch for depression
Finally, the writing group highlighted the importance of depression in cardiac patients, which occurs at about twice the rate, compared with people without any medical condition (10% vs. 5%).
In a prior statement, the AHA said depression should be considered a risk factor for worse outcomes in patients with ACS or CVD diagnosis.
The new statement highlights that people with persistent chest pain, people with HF, as well as stroke survivors and people with PAD commonly have depression and/or anxiety. In addition, cognitive changes after a stroke may affect how and whether symptoms are experienced or noticed.
While symptom relief is an important part of managing CVD, it’s also important to recognize that “factors such as depression and cognitive function may affect symptom detection and reporting,” Dr. Jurgens said.
“Monitoring and measuring symptoms with tools that appropriately account for depression and cognitive function may help to improve patient care by identifying more quickly people who may be at higher risk,” she added.
The scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Cardiovascular and Stroke Nursing; the Council on Hypertension; and the Stroke Council. The research had no commercial funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Symptoms of six common cardiovascular diseases (CVD) – acute coronary syndromes, heart failure, valvular disorders, stroke, rhythm disorders, and peripheral vascular disease – often overlap and may vary over time and by sex, the American Heart Association noted in a new scientific statement.
“Symptoms of these cardiovascular diseases can profoundly affect quality of life, and a clear understanding of them is critical for effective diagnosis and treatment decisions,” Corrine Y. Jurgens, PhD, chair of the writing committee, said in a news release.
This scientific statement is a “compendium detailing the symptoms associated with CVD, similarities or differences in symptoms among the conditions, and sex differences in symptom presentation and reporting,” said Dr. Jurgens, associate professor at Connell School of Nursing, Boston College.
The new statement was published online in Circulation.
The writing group noted that measuring CVD symptoms can be challenging because of their subjective nature. Symptoms may go unrecognized or unreported if people don’t think they are important or are related to an existing health condition.
“Some people may not consider symptoms like fatigue, sleep disturbance, weight gain, and depression as important or related to cardiovascular disease. However, research indicates that subtle symptoms such as these may predict acute events and the need for hospitalization,” Dr. Jurgens said.
ACS – chest pain and associated symptoms
The writing group noted that chest pain is the most frequently reported symptom of ACS and has often been described as substernal pressure or discomfort and may radiate to the jaw, shoulder, arm, or upper back.
The most common co-occurring symptoms are dyspnea, diaphoresis, unusual fatigue, nausea, and lightheadedness. Women are more likely than men to report additional symptoms outside of chest pain.
As a result, they have often been labeled “atypical.” However, a recent AHA advisory notes that this label may have been caused by the lack of women included in the clinical trials from which the symptom lists were derived.
The writing group said there is a need to “harmonize” ACS symptom measurement in research. The current lack of harmonization of ACS symptom measurement in research hampers growth in cumulative evidence.
“Therefore, little can be done to synthesize salient findings about symptoms across ischemic heart disease/ACS studies and to incorporate evidence-based information about symptoms into treatment guidelines and patient education materials,” they cautioned.
Heart failure
Turning to heart failure (HF), the writing group noted that dyspnea is the classic symptom and a common reason adults seek medical care.
However, early, more subtle symptoms should be recognized. These include gastrointestinal symptoms such as upset stomach, nausea, vomiting, and loss of appetite; fatigue; exercise intolerance; insomnia; pain (chest and otherwise); mood disturbances (primarily depression and anxiety); and cognitive dysfunction (brain fog, memory problems).
Women with HF report a wider variety of symptoms, are more likely to have depression and anxiety, and report a lower quality of life, compared with men with HF.
“It is important to account for dyspnea heterogeneity in both clinical practice and research by using nuanced measures and probing questions to capture this common and multifaceted symptom,” the writing group said.
“Monitoring symptoms on a spectrum, versus present or not present, with reliable and valid measures may enhance clinical care by identifying more quickly those who may be at risk for poor outcomes, such as lower quality of life, hospitalization, or death,” Dr. Jurgens added.
“Ultimately, we have work to do in terms of determining who needs more frequent monitoring or intervention to avert poor HF outcomes,” she said.
Valvular heart disease
Valvular heart disease is a frequent cause of HF, with symptoms generally indistinguishable from other HF causes. Rheumatic heart disease is still prevalent in low- and middle-income countries but has largely disappeared in high-income countries, with population aging and cardiomyopathies now key drivers of valve disease.
In the absence of acute severe valve dysfunction, patients generally have a prolonged asymptomatic period, followed by a period of progressive symptoms, resulting from the valve lesion itself or secondary myocardial remodeling and dysfunction, the writing group said.
Symptoms of aortic valve disease often differ between men and women. Aortic stenosis is typically silent for years. As stenosis progresses, women report dyspnea and exercise intolerance more often than men. Women are also more likely to be physically frail and to have a higher New York Heart Association class (III/IV) than men. Men are more likely to have chest pain.
“Given the importance of symptom assessment, more work is needed to determine the incremental value of quantitative symptom measurement as an aid to clinical management,” the writing group said.
Stroke
For clinicians, classic stroke symptoms (face drooping, arm weakness, speech difficulty), in addition to nonclassic symptoms, such as partial sensory deficit, dysarthria, vertigo, and diplopia, should be considered for activating a stroke response team, the group says.
A systematic review and meta-analysis revealed that women with stroke were more likely to present with nonfocal symptoms (for example, headache, altered mentality, and coma/stupor) than men, they noted.
To enhance public education about stroke symptoms and to facilitate the diagnosis and treatment of stroke, they say research is needed to better understand the presentation of stroke symptoms by other select demographic characteristics including race and ethnicity, age, and stroke subtype.
Poststroke screening should include assessment for anxiety, depression, fatigue, and pain, the writing group said.
Rhythm disorders
Turning to rhythm disorders, the writing group wrote that cardiac arrhythmias, including atrial fibrillation (AFib), atrial flutter, supraventricular tachycardia, bradyarrhythmia, and ventricular tachycardia, present with common symptoms.
Palpitations are a characteristic symptom of many cardiac arrhythmias. The most common cardiac arrhythmia, AFib, may present with palpitations or less specific symptoms (fatigue, dyspnea, dizziness) that occur with a broad range of rhythm disorders. Chest pain, dizziness, presyncope/syncope, and anxiety occur less frequently in AFib, the group said.
Palpitations are considered the typical symptom presentation for AFib, yet patients with new-onset AFib often present with nonspecific symptoms or no symptoms, they pointed out.
Women and younger individuals with AFib typically present with palpitations, whereas men are more commonly asymptomatic. Older age also increases the likelihood of a nonclassic or asymptomatic presentation of AFib.
Despite non-Hispanic Black individuals being at lower risk for development of AFib, research suggests that Black patients are burdened more with palpitations, dyspnea on exertion, exercise intolerance, dizziness, dyspnea at rest, and chest discomfort, compared with White or Hispanic patients.
Peripheral vascular disease
Classic claudication occurs in roughly one-third of patients with peripheral arterial disease (PAD) and is defined as calf pain that occurs in one or both legs with exertion (walking), does not begin at rest, and resolves within 10 minutes of standing still or rest.
However, non–calf exercise pain is reported more frequently than classic claudication symptoms. Women with PAD are more likely to have nonclassic symptoms or an absence of symptoms.
Assessing symptoms at rest, during exercise, and during recovery can assist with classifying symptoms as ischemic or not, the writing group said.
PAD with symptoms is associated with an increased risk for myocardial infarction and stroke, with men at higher risk than women.
Similar to PAD, peripheral venous disease (PVD) can be symptomatic or asymptomatic. Clinical classification of PVD includes symptoms such as leg pain, aching, fatigue, heaviness, cramping, tightness, restless legs syndrome, and skin irritation.
“Measuring vascular symptoms includes assessing quality of life and activity limitations, as well as the psychological impact of the disease. However, existing measures are often based on the clinician’s appraisal rather than the individual’s self-reported symptoms and severity of symptoms,” Dr. Jurgens commented.
Watch for depression
Finally, the writing group highlighted the importance of depression in cardiac patients, which occurs at about twice the rate, compared with people without any medical condition (10% vs. 5%).
In a prior statement, the AHA said depression should be considered a risk factor for worse outcomes in patients with ACS or CVD diagnosis.
The new statement highlights that people with persistent chest pain, people with HF, as well as stroke survivors and people with PAD commonly have depression and/or anxiety. In addition, cognitive changes after a stroke may affect how and whether symptoms are experienced or noticed.
While symptom relief is an important part of managing CVD, it’s also important to recognize that “factors such as depression and cognitive function may affect symptom detection and reporting,” Dr. Jurgens said.
“Monitoring and measuring symptoms with tools that appropriately account for depression and cognitive function may help to improve patient care by identifying more quickly people who may be at higher risk,” she added.
The scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Cardiovascular and Stroke Nursing; the Council on Hypertension; and the Stroke Council. The research had no commercial funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
‘Obesity paradox’ in AFib challenged as mortality climbs with BMI
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.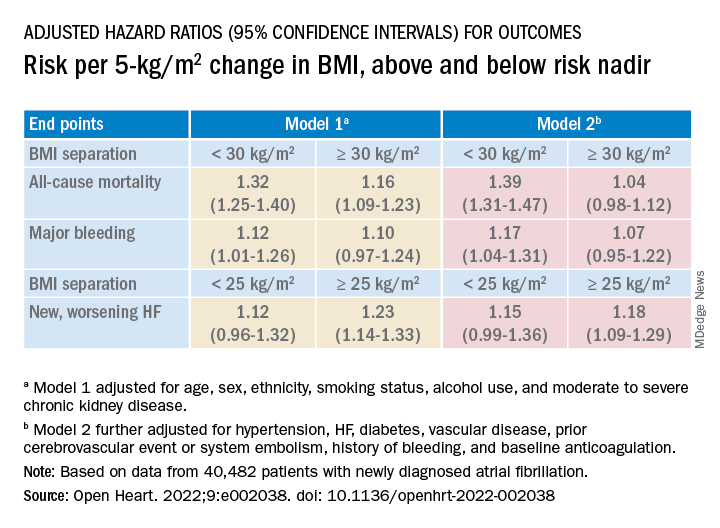
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
FROM OPEN HEART
Strength training overcomes bone effects of vegan diet
People who maintain a vegan diet show significant deficits in bone microarchitecture, compared with omnivores; however, resistance training not only appears to improve those deficits but may have a stronger effect in vegans, suggesting an important strategy in maintaining bone health with a vegan diet.
“We expected better bone structure in both vegans and omnivores who reported resistance training,” first author Robert Wakolbinger-Habel, MD, PhD, of St. Vincent Hospital Vienna and the Medical University of Vienna, said in an interview.
“However, we expected [there would still be] differences in structure between vegans and omnivores [who practiced resistance training], as previous literature reported higher fracture rates in vegans,” he said. “Still, the positive message is that ‘pumping iron’ could counterbalance these differences between vegans and omnivores.”
The research was published online in The Endocrine Society’s Journal of Clinical Endocrinology & Metabolism.
Exercise significantly impacts bone health in vegans
The potential effects of the plant-based vegan diet on bone health have been reported in studies linking the diet to an increased risk of fractures and lower bone mineral density (BMD), with common theories including lower bone- and muscle-building protein in vegan diets.
However, most previous studies have not considered other key factors, such as the effects of exercise, the authors noted.
“While previous studies on bone health in vegans only took BMD, biochemical and nutritional parameters into account, they did not consider the significant effects of physical activity,” they wrote.
“By ignoring these effects, important factors influencing bone health are neglected.”
For the study, 88 participants were enrolled in Vienna, with vegan participants recruited with the help of the Austrian Vegan Society.
Importantly, the study documented participants’ bone microarchitecture, a key measure of bone strength that has also not been previously investigated in vegans, using high-resolution peripheral quantitative CT.
Inclusion criteria included maintaining an omnivore diet of meat and plant-based foods or a vegan diet for at least 5 years, not being underweight or obese (body mass index [BMI], 18.5-30 kg/m2), being age 30-50 years, and being premenopausal.
Of the participants, 43 were vegan and 45 were omnivores, with generally equal ratios of men and women.
Vegan bone deficits disappear with strength training
Overall, compared with omnivores, the vegan group showed significant deficits in 7 of 14 measures of BMI-adjusted trabecular and cortical structure (all P < .05).
Among participants who reported no resistance training, vegans still showed significant decreases in bone microarchitecture, compared with omnivores, including radius trabecular BMD, radius trabecular bone volume fraction, and other tibial and cortical bone microarchitecture measures.
However, among those who did report progressive resistant training (20 vegans and 25 omnivores), defined as using machines, free weights, or bodyweight resistance exercises at least once a week, those differences disappeared and there were no significant differences in BMI-adjusted bone microarchitecture between vegans and omnivores after the 5 years.
Of note, no significant differences in bone microarchitecture were observed between those who performed exclusively aerobic activities and those who reported no sports activities in the vegan or omnivore group.
Based on the findings, “other types of exercise such as aerobics, cycling, etc, would not be sufficient for a similar positive effect on bone [as resistance training],” Dr. Wakolbinger-Habel said.
Although the findings suggest that resistance training seemed to allow vegans to “catch up” with omnivores in terms of bone microarchitecture, Dr. Wakolbinger-Habel cautioned that a study limitation is the relatively low number of participants.
“The absolute numbers suggest that in vegans the differences, and the relative effect, respectively of resistance training might be larger,” he said. “However, the number of participants in the subgroups is small and it is still an observational study, so we need to be careful in drawing causal conclusions.”
Serum bone markers were within normal ranges across all subgroups. And although there were some correlations between nutrient intake and bone microarchitecture among vegans who did and did not practice resistance training, no conclusions could be drawn from that data, the authors noted.
“Based on our data, the structural [differences between vegans and omnivores] cannot solely be explained by deficits in certain nutrients according to lifestyle,” the authors concluded.
Mechanisms
The mechanisms by which progressive resistance training could result in the benefits include that mechanical loads trigger stimulation of key pathways involved in bone formation, or mechanotransduction, the authors explained.
The unique effects have been observed in other studies, including one study showing that, among young adult runners, the addition of resistance training once a week was associated with significantly greater BMD.
“Veganism is a global trend with strongly increasing numbers of people worldwide adhering to a purely plant-based diet,” first author Christian Muschitz, MD, also of St. Vincent Hospital Vienna and the Medical University of Vienna, said in a press statement.
“Our study showed resistance training offsets diminished bone structure in vegan people when compared to omnivores,” he said.
Dr. Wakolbinger-Habel recommended that, based on the findings, “exercise, including resistance training, should be strongly advocated [for vegans], I would say, at least two times per week.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People who maintain a vegan diet show significant deficits in bone microarchitecture, compared with omnivores; however, resistance training not only appears to improve those deficits but may have a stronger effect in vegans, suggesting an important strategy in maintaining bone health with a vegan diet.
“We expected better bone structure in both vegans and omnivores who reported resistance training,” first author Robert Wakolbinger-Habel, MD, PhD, of St. Vincent Hospital Vienna and the Medical University of Vienna, said in an interview.
“However, we expected [there would still be] differences in structure between vegans and omnivores [who practiced resistance training], as previous literature reported higher fracture rates in vegans,” he said. “Still, the positive message is that ‘pumping iron’ could counterbalance these differences between vegans and omnivores.”
The research was published online in The Endocrine Society’s Journal of Clinical Endocrinology & Metabolism.
Exercise significantly impacts bone health in vegans
The potential effects of the plant-based vegan diet on bone health have been reported in studies linking the diet to an increased risk of fractures and lower bone mineral density (BMD), with common theories including lower bone- and muscle-building protein in vegan diets.
However, most previous studies have not considered other key factors, such as the effects of exercise, the authors noted.
“While previous studies on bone health in vegans only took BMD, biochemical and nutritional parameters into account, they did not consider the significant effects of physical activity,” they wrote.
“By ignoring these effects, important factors influencing bone health are neglected.”
For the study, 88 participants were enrolled in Vienna, with vegan participants recruited with the help of the Austrian Vegan Society.
Importantly, the study documented participants’ bone microarchitecture, a key measure of bone strength that has also not been previously investigated in vegans, using high-resolution peripheral quantitative CT.
Inclusion criteria included maintaining an omnivore diet of meat and plant-based foods or a vegan diet for at least 5 years, not being underweight or obese (body mass index [BMI], 18.5-30 kg/m2), being age 30-50 years, and being premenopausal.
Of the participants, 43 were vegan and 45 were omnivores, with generally equal ratios of men and women.
Vegan bone deficits disappear with strength training
Overall, compared with omnivores, the vegan group showed significant deficits in 7 of 14 measures of BMI-adjusted trabecular and cortical structure (all P < .05).
Among participants who reported no resistance training, vegans still showed significant decreases in bone microarchitecture, compared with omnivores, including radius trabecular BMD, radius trabecular bone volume fraction, and other tibial and cortical bone microarchitecture measures.
However, among those who did report progressive resistant training (20 vegans and 25 omnivores), defined as using machines, free weights, or bodyweight resistance exercises at least once a week, those differences disappeared and there were no significant differences in BMI-adjusted bone microarchitecture between vegans and omnivores after the 5 years.
Of note, no significant differences in bone microarchitecture were observed between those who performed exclusively aerobic activities and those who reported no sports activities in the vegan or omnivore group.
Based on the findings, “other types of exercise such as aerobics, cycling, etc, would not be sufficient for a similar positive effect on bone [as resistance training],” Dr. Wakolbinger-Habel said.
Although the findings suggest that resistance training seemed to allow vegans to “catch up” with omnivores in terms of bone microarchitecture, Dr. Wakolbinger-Habel cautioned that a study limitation is the relatively low number of participants.
“The absolute numbers suggest that in vegans the differences, and the relative effect, respectively of resistance training might be larger,” he said. “However, the number of participants in the subgroups is small and it is still an observational study, so we need to be careful in drawing causal conclusions.”
Serum bone markers were within normal ranges across all subgroups. And although there were some correlations between nutrient intake and bone microarchitecture among vegans who did and did not practice resistance training, no conclusions could be drawn from that data, the authors noted.
“Based on our data, the structural [differences between vegans and omnivores] cannot solely be explained by deficits in certain nutrients according to lifestyle,” the authors concluded.
Mechanisms
The mechanisms by which progressive resistance training could result in the benefits include that mechanical loads trigger stimulation of key pathways involved in bone formation, or mechanotransduction, the authors explained.
The unique effects have been observed in other studies, including one study showing that, among young adult runners, the addition of resistance training once a week was associated with significantly greater BMD.
“Veganism is a global trend with strongly increasing numbers of people worldwide adhering to a purely plant-based diet,” first author Christian Muschitz, MD, also of St. Vincent Hospital Vienna and the Medical University of Vienna, said in a press statement.
“Our study showed resistance training offsets diminished bone structure in vegan people when compared to omnivores,” he said.
Dr. Wakolbinger-Habel recommended that, based on the findings, “exercise, including resistance training, should be strongly advocated [for vegans], I would say, at least two times per week.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People who maintain a vegan diet show significant deficits in bone microarchitecture, compared with omnivores; however, resistance training not only appears to improve those deficits but may have a stronger effect in vegans, suggesting an important strategy in maintaining bone health with a vegan diet.
“We expected better bone structure in both vegans and omnivores who reported resistance training,” first author Robert Wakolbinger-Habel, MD, PhD, of St. Vincent Hospital Vienna and the Medical University of Vienna, said in an interview.
“However, we expected [there would still be] differences in structure between vegans and omnivores [who practiced resistance training], as previous literature reported higher fracture rates in vegans,” he said. “Still, the positive message is that ‘pumping iron’ could counterbalance these differences between vegans and omnivores.”
The research was published online in The Endocrine Society’s Journal of Clinical Endocrinology & Metabolism.
Exercise significantly impacts bone health in vegans
The potential effects of the plant-based vegan diet on bone health have been reported in studies linking the diet to an increased risk of fractures and lower bone mineral density (BMD), with common theories including lower bone- and muscle-building protein in vegan diets.
However, most previous studies have not considered other key factors, such as the effects of exercise, the authors noted.
“While previous studies on bone health in vegans only took BMD, biochemical and nutritional parameters into account, they did not consider the significant effects of physical activity,” they wrote.
“By ignoring these effects, important factors influencing bone health are neglected.”
For the study, 88 participants were enrolled in Vienna, with vegan participants recruited with the help of the Austrian Vegan Society.
Importantly, the study documented participants’ bone microarchitecture, a key measure of bone strength that has also not been previously investigated in vegans, using high-resolution peripheral quantitative CT.
Inclusion criteria included maintaining an omnivore diet of meat and plant-based foods or a vegan diet for at least 5 years, not being underweight or obese (body mass index [BMI], 18.5-30 kg/m2), being age 30-50 years, and being premenopausal.
Of the participants, 43 were vegan and 45 were omnivores, with generally equal ratios of men and women.
Vegan bone deficits disappear with strength training
Overall, compared with omnivores, the vegan group showed significant deficits in 7 of 14 measures of BMI-adjusted trabecular and cortical structure (all P < .05).
Among participants who reported no resistance training, vegans still showed significant decreases in bone microarchitecture, compared with omnivores, including radius trabecular BMD, radius trabecular bone volume fraction, and other tibial and cortical bone microarchitecture measures.
However, among those who did report progressive resistant training (20 vegans and 25 omnivores), defined as using machines, free weights, or bodyweight resistance exercises at least once a week, those differences disappeared and there were no significant differences in BMI-adjusted bone microarchitecture between vegans and omnivores after the 5 years.
Of note, no significant differences in bone microarchitecture were observed between those who performed exclusively aerobic activities and those who reported no sports activities in the vegan or omnivore group.
Based on the findings, “other types of exercise such as aerobics, cycling, etc, would not be sufficient for a similar positive effect on bone [as resistance training],” Dr. Wakolbinger-Habel said.
Although the findings suggest that resistance training seemed to allow vegans to “catch up” with omnivores in terms of bone microarchitecture, Dr. Wakolbinger-Habel cautioned that a study limitation is the relatively low number of participants.
“The absolute numbers suggest that in vegans the differences, and the relative effect, respectively of resistance training might be larger,” he said. “However, the number of participants in the subgroups is small and it is still an observational study, so we need to be careful in drawing causal conclusions.”
Serum bone markers were within normal ranges across all subgroups. And although there were some correlations between nutrient intake and bone microarchitecture among vegans who did and did not practice resistance training, no conclusions could be drawn from that data, the authors noted.
“Based on our data, the structural [differences between vegans and omnivores] cannot solely be explained by deficits in certain nutrients according to lifestyle,” the authors concluded.
Mechanisms
The mechanisms by which progressive resistance training could result in the benefits include that mechanical loads trigger stimulation of key pathways involved in bone formation, or mechanotransduction, the authors explained.
The unique effects have been observed in other studies, including one study showing that, among young adult runners, the addition of resistance training once a week was associated with significantly greater BMD.
“Veganism is a global trend with strongly increasing numbers of people worldwide adhering to a purely plant-based diet,” first author Christian Muschitz, MD, also of St. Vincent Hospital Vienna and the Medical University of Vienna, said in a press statement.
“Our study showed resistance training offsets diminished bone structure in vegan people when compared to omnivores,” he said.
Dr. Wakolbinger-Habel recommended that, based on the findings, “exercise, including resistance training, should be strongly advocated [for vegans], I would say, at least two times per week.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Poor sleep raises risk for fatty liver disease
Sleep behaviors, both individually and combined, are associated with an increased risk of developing metabolic dysfunction–associated fatty liver disease (MAFLD), according to a Chinese analysis that suggests the effect may be independent of obesity.
Yan Liu, PhD, from the School of Public Health at Sun Yat-sen University in Guangzhou, China, and colleagues studied data on over 5,000 individuals who self-reported sleep behaviors and underwent liver ultrasound.
, increasing the risk by 37%, 59%, and 17%, respectively, whereas people with both poor nighttime sleep and prolonged daytime napping had the “highest risk for developing fatty liver disease,” said Dr. Liu in a press release.
In contrast, having any of six healthy sleep behaviors decreased the risk by 16% each, and even a “moderate improvement in sleep quality was related to a 29% reduction in the risk for fatty liver disease,” he added.
The research, published online in the Journal of Clinical Endocrinology & Metabolism, also indicated that obesity accounted for only one fifth of the effect of sleep quality on MAFLD risk.
Rise in unhealthy lifestyles leads to increase in MALFD
The authors write that MAFLD is the “leading chronic liver disease worldwide,” affecting around a quarter of the adult population, and may lead to end-stage liver diseases and extrahepatic complications, thus “posing a major health and economic burden.”
Moreover, the disease prevalence is “soaring at an unanticipated rate,” increasing from 18% to 29% in China over the past decade, because of a “rapid rise in unhealthy lifestyles,” the authors note.
Sleep disturbance is increasingly prevalent, “and an emerging contributor to multiple metabolic disorders,” with insomnia and habitual snoring, for example, positively correlated with hypertension, impaired glucose metabolism, and dyslipidemia, report the authors.
However, whether sleep quality, which includes “several metabolic-related sleep behaviors,” constitutes an independent risk for MAFLD “over and above” the effect of obesity remains unclear.
To investigate further, the researchers examined data from the baseline survey of the community-based, prospective South China Cohort study, which was conducted in four regions of Southern China and involved 5,430 individuals aged 30-79 years.
Between March 2018 and October 2019, the participants self-reported sleep behaviors on the Pittsburgh Sleep Quality Index questionnaire and underwent ultrasound examination of the liver.
MAFLD was diagnosed in those with hepatic steatosis and one of the following:
- Overweight/obesity, defined by this study as a body mass index greater than or equal to 23 kg/m2.
- Presence of diabetes.
- Evidence of metabolic dysregulation.
After excluding patients with insufficient data, and those with a history of liver cirrhosis, hepatectomy, or liver cancer, among others, the team included 5,011 individuals with an average age of 64 years and a mean body mass index of 24.31 kg/m2. Forty percent were male.
Obesity was present in 13% of participants, whereas 15% had diabetes, 58% hypertension, and 35% metabolic syndrome.
MAFLD was diagnosed in 28% of the study population. They were older, more likely to be female with a higher education, and had a higher prevalence of preexisting metabolic disorders and worse metabolic profiles, than those without the disease.
Turning to the associations between sleep and the risk of MAFLD, the researchers say that “in contrast to previous reports, neither shorter nor longer sleep duration was found to be associated with the risk for MAFLD.”
However, after adjusting for demographics, lifestyles, medication, and preexisting metabolic comorbidities including hypertension, diabetes, and obesity, they found that the risk of MAFLD was significantly associated with late bedtime (defined as after 10 p.m.), at an odds ratio of 1.37 (P < .05).
MAFLD was also linked to snoring, at an odds ratio of 1.59, and to daytime napping for longer than 30 minutes, at an odds ratio of 1.17 (P < .05 for both).
When the team compared low-risk and high-risk sleep factors, they found that participants who had an early bedtime, slept 7-8 hours per night, never or rarely had insomnia or snoring, had infrequent daytime sleepiness, and daytime napping of half-hour or less had an odds ratio for MAFLD vs. other participants of 0.64 (P < .05).
Combining those factors into a healthy sleep score, the team found that each additional increase of healthy sleep score was associated with a fully adjusted odds ratio for MAFLD of 0.84 (P < .05).
In contrast, individuals with poor nocturnal sleep patterns and prolonged daytime napping had a higher risk for developing MAFLD, compared with those with a healthy nocturnal sleep pattern and daytime napping of half-hour or less, at an odds ratio of 2.38 (P < .05).
Further analysis indicated that individuals with a sedentary lifestyle and central obesity had a higher risk of MAFLD, but that the presence of obesity accounted for only 20.8% of the total effect of sleep quality on the risk of MAFLD.
“Taken together, our results suggests that obesity only partially mediates the effect of overall sleep quality on MAFLD,” the authors write.
“Given that large proportions of subjects suffering from poor sleep quality are underdiagnosed and undertreated, our study calls for more research into this field and strategies to improve sleep quality,” Dr. Liu said.
The study was supported by the “National Key R&D Program” of China, the Fundamental Research Funds for the Central Universities (Sun Yat-sen University), Natural Science Foundation of Guangdong Province, the Key Project of Medicine Discipline of Guangzhou, and Basic Research Project of Key Laboratory of Guangzhou.
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sleep behaviors, both individually and combined, are associated with an increased risk of developing metabolic dysfunction–associated fatty liver disease (MAFLD), according to a Chinese analysis that suggests the effect may be independent of obesity.
Yan Liu, PhD, from the School of Public Health at Sun Yat-sen University in Guangzhou, China, and colleagues studied data on over 5,000 individuals who self-reported sleep behaviors and underwent liver ultrasound.
, increasing the risk by 37%, 59%, and 17%, respectively, whereas people with both poor nighttime sleep and prolonged daytime napping had the “highest risk for developing fatty liver disease,” said Dr. Liu in a press release.
In contrast, having any of six healthy sleep behaviors decreased the risk by 16% each, and even a “moderate improvement in sleep quality was related to a 29% reduction in the risk for fatty liver disease,” he added.
The research, published online in the Journal of Clinical Endocrinology & Metabolism, also indicated that obesity accounted for only one fifth of the effect of sleep quality on MAFLD risk.
Rise in unhealthy lifestyles leads to increase in MALFD
The authors write that MAFLD is the “leading chronic liver disease worldwide,” affecting around a quarter of the adult population, and may lead to end-stage liver diseases and extrahepatic complications, thus “posing a major health and economic burden.”
Moreover, the disease prevalence is “soaring at an unanticipated rate,” increasing from 18% to 29% in China over the past decade, because of a “rapid rise in unhealthy lifestyles,” the authors note.
Sleep disturbance is increasingly prevalent, “and an emerging contributor to multiple metabolic disorders,” with insomnia and habitual snoring, for example, positively correlated with hypertension, impaired glucose metabolism, and dyslipidemia, report the authors.
However, whether sleep quality, which includes “several metabolic-related sleep behaviors,” constitutes an independent risk for MAFLD “over and above” the effect of obesity remains unclear.
To investigate further, the researchers examined data from the baseline survey of the community-based, prospective South China Cohort study, which was conducted in four regions of Southern China and involved 5,430 individuals aged 30-79 years.
Between March 2018 and October 2019, the participants self-reported sleep behaviors on the Pittsburgh Sleep Quality Index questionnaire and underwent ultrasound examination of the liver.
MAFLD was diagnosed in those with hepatic steatosis and one of the following:
- Overweight/obesity, defined by this study as a body mass index greater than or equal to 23 kg/m2.
- Presence of diabetes.
- Evidence of metabolic dysregulation.
After excluding patients with insufficient data, and those with a history of liver cirrhosis, hepatectomy, or liver cancer, among others, the team included 5,011 individuals with an average age of 64 years and a mean body mass index of 24.31 kg/m2. Forty percent were male.
Obesity was present in 13% of participants, whereas 15% had diabetes, 58% hypertension, and 35% metabolic syndrome.
MAFLD was diagnosed in 28% of the study population. They were older, more likely to be female with a higher education, and had a higher prevalence of preexisting metabolic disorders and worse metabolic profiles, than those without the disease.
Turning to the associations between sleep and the risk of MAFLD, the researchers say that “in contrast to previous reports, neither shorter nor longer sleep duration was found to be associated with the risk for MAFLD.”
However, after adjusting for demographics, lifestyles, medication, and preexisting metabolic comorbidities including hypertension, diabetes, and obesity, they found that the risk of MAFLD was significantly associated with late bedtime (defined as after 10 p.m.), at an odds ratio of 1.37 (P < .05).
MAFLD was also linked to snoring, at an odds ratio of 1.59, and to daytime napping for longer than 30 minutes, at an odds ratio of 1.17 (P < .05 for both).
When the team compared low-risk and high-risk sleep factors, they found that participants who had an early bedtime, slept 7-8 hours per night, never or rarely had insomnia or snoring, had infrequent daytime sleepiness, and daytime napping of half-hour or less had an odds ratio for MAFLD vs. other participants of 0.64 (P < .05).
Combining those factors into a healthy sleep score, the team found that each additional increase of healthy sleep score was associated with a fully adjusted odds ratio for MAFLD of 0.84 (P < .05).
In contrast, individuals with poor nocturnal sleep patterns and prolonged daytime napping had a higher risk for developing MAFLD, compared with those with a healthy nocturnal sleep pattern and daytime napping of half-hour or less, at an odds ratio of 2.38 (P < .05).
Further analysis indicated that individuals with a sedentary lifestyle and central obesity had a higher risk of MAFLD, but that the presence of obesity accounted for only 20.8% of the total effect of sleep quality on the risk of MAFLD.
“Taken together, our results suggests that obesity only partially mediates the effect of overall sleep quality on MAFLD,” the authors write.
“Given that large proportions of subjects suffering from poor sleep quality are underdiagnosed and undertreated, our study calls for more research into this field and strategies to improve sleep quality,” Dr. Liu said.
The study was supported by the “National Key R&D Program” of China, the Fundamental Research Funds for the Central Universities (Sun Yat-sen University), Natural Science Foundation of Guangdong Province, the Key Project of Medicine Discipline of Guangzhou, and Basic Research Project of Key Laboratory of Guangzhou.
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sleep behaviors, both individually and combined, are associated with an increased risk of developing metabolic dysfunction–associated fatty liver disease (MAFLD), according to a Chinese analysis that suggests the effect may be independent of obesity.
Yan Liu, PhD, from the School of Public Health at Sun Yat-sen University in Guangzhou, China, and colleagues studied data on over 5,000 individuals who self-reported sleep behaviors and underwent liver ultrasound.
, increasing the risk by 37%, 59%, and 17%, respectively, whereas people with both poor nighttime sleep and prolonged daytime napping had the “highest risk for developing fatty liver disease,” said Dr. Liu in a press release.
In contrast, having any of six healthy sleep behaviors decreased the risk by 16% each, and even a “moderate improvement in sleep quality was related to a 29% reduction in the risk for fatty liver disease,” he added.
The research, published online in the Journal of Clinical Endocrinology & Metabolism, also indicated that obesity accounted for only one fifth of the effect of sleep quality on MAFLD risk.
Rise in unhealthy lifestyles leads to increase in MALFD
The authors write that MAFLD is the “leading chronic liver disease worldwide,” affecting around a quarter of the adult population, and may lead to end-stage liver diseases and extrahepatic complications, thus “posing a major health and economic burden.”
Moreover, the disease prevalence is “soaring at an unanticipated rate,” increasing from 18% to 29% in China over the past decade, because of a “rapid rise in unhealthy lifestyles,” the authors note.
Sleep disturbance is increasingly prevalent, “and an emerging contributor to multiple metabolic disorders,” with insomnia and habitual snoring, for example, positively correlated with hypertension, impaired glucose metabolism, and dyslipidemia, report the authors.
However, whether sleep quality, which includes “several metabolic-related sleep behaviors,” constitutes an independent risk for MAFLD “over and above” the effect of obesity remains unclear.
To investigate further, the researchers examined data from the baseline survey of the community-based, prospective South China Cohort study, which was conducted in four regions of Southern China and involved 5,430 individuals aged 30-79 years.
Between March 2018 and October 2019, the participants self-reported sleep behaviors on the Pittsburgh Sleep Quality Index questionnaire and underwent ultrasound examination of the liver.
MAFLD was diagnosed in those with hepatic steatosis and one of the following:
- Overweight/obesity, defined by this study as a body mass index greater than or equal to 23 kg/m2.
- Presence of diabetes.
- Evidence of metabolic dysregulation.
After excluding patients with insufficient data, and those with a history of liver cirrhosis, hepatectomy, or liver cancer, among others, the team included 5,011 individuals with an average age of 64 years and a mean body mass index of 24.31 kg/m2. Forty percent were male.
Obesity was present in 13% of participants, whereas 15% had diabetes, 58% hypertension, and 35% metabolic syndrome.
MAFLD was diagnosed in 28% of the study population. They were older, more likely to be female with a higher education, and had a higher prevalence of preexisting metabolic disorders and worse metabolic profiles, than those without the disease.
Turning to the associations between sleep and the risk of MAFLD, the researchers say that “in contrast to previous reports, neither shorter nor longer sleep duration was found to be associated with the risk for MAFLD.”
However, after adjusting for demographics, lifestyles, medication, and preexisting metabolic comorbidities including hypertension, diabetes, and obesity, they found that the risk of MAFLD was significantly associated with late bedtime (defined as after 10 p.m.), at an odds ratio of 1.37 (P < .05).
MAFLD was also linked to snoring, at an odds ratio of 1.59, and to daytime napping for longer than 30 minutes, at an odds ratio of 1.17 (P < .05 for both).
When the team compared low-risk and high-risk sleep factors, they found that participants who had an early bedtime, slept 7-8 hours per night, never or rarely had insomnia or snoring, had infrequent daytime sleepiness, and daytime napping of half-hour or less had an odds ratio for MAFLD vs. other participants of 0.64 (P < .05).
Combining those factors into a healthy sleep score, the team found that each additional increase of healthy sleep score was associated with a fully adjusted odds ratio for MAFLD of 0.84 (P < .05).
In contrast, individuals with poor nocturnal sleep patterns and prolonged daytime napping had a higher risk for developing MAFLD, compared with those with a healthy nocturnal sleep pattern and daytime napping of half-hour or less, at an odds ratio of 2.38 (P < .05).
Further analysis indicated that individuals with a sedentary lifestyle and central obesity had a higher risk of MAFLD, but that the presence of obesity accounted for only 20.8% of the total effect of sleep quality on the risk of MAFLD.
“Taken together, our results suggests that obesity only partially mediates the effect of overall sleep quality on MAFLD,” the authors write.
“Given that large proportions of subjects suffering from poor sleep quality are underdiagnosed and undertreated, our study calls for more research into this field and strategies to improve sleep quality,” Dr. Liu said.
The study was supported by the “National Key R&D Program” of China, the Fundamental Research Funds for the Central Universities (Sun Yat-sen University), Natural Science Foundation of Guangdong Province, the Key Project of Medicine Discipline of Guangzhou, and Basic Research Project of Key Laboratory of Guangzhou.
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Risk factors in children linked to stroke as soon as 30s, 40s
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
How nonadherence complicates cardiology, in two trials
Each study adds new twist
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
Each study adds new twist
Each study adds new twist
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Gut metabolites may explain red meat–ASCVD link
The connection between red meat and atherosclerotic cardiovascular disease has been well established, but newly reported findings indicate that metabolites in the gut microbiome may explain that relationship more than cholesterol and blood pressure.
“Eating more meat, especially red meat and processed meats, is associated with a higher risk of cardiovascular disease, even later in life,” co–lead study author Meng Wang, PhD, said in an interview.
The study, from a large community-based cohort of older people, included 3,931 U.S. participants aged 65 and older in the Cardiovascular Health Study (CHS). It found that gut microbiota–generated metabolites of dietary L-carnitine, including trimethylamine N-oxide (TMAO), have a role in the association between unprocessed red meat intake and incident ASCVD.
“TMAO-related metabolites produced by our gut microbes as well as blood-glucose and insulin homeostasis and systematic inflammation appeared to explain much of the association, more so than blood cholesterol or blood pressure,” added Dr. Wang, of the Friedman School of Nutrition Science and Policy at Tufts University, Boston.
Dr. Wang said this study was unique because it focused specifically on older adults; the average participant age was 72.9 years. “Older adults are at the highest risk of CVD, and for them adequate intake of protein may help to offset aging-related loss of muscle mass and strength,” she said. However, the study population was largely white (88%), so, she said, the results may not be generalizable to populations that are younger or of different nationalities and races.
The researchers performed a multivariable analysis that showed that participants who had higher intakes of unprocessed red meat, total meat, and total animal source foods (ASF) had higher hazard ratios of ASCVD risk. The study had a median follow-up of 12.5 years. It divided the study population into five quintiles based on how much unprocessed red met they consumed at baseline and analyzed dietary exposure in the differences between the midpoints of the first and fifth quintiles.
Earlier studies of meat intake and CVD risk focused mostly on saturated fat and blood cholesterol, Dr. Wang added. “But our findings suggest that other components in red meat, such as L-carnitine and heme iron, might play a more important role than saturated fat,” she said.
Higher intake of unprocessed red meat was linked to a 15% higher incidence of ASCVD per interquintile range (hazard ratio, 1.15; 95% confidence interval, 1.01-1.30; P = .031). Total meat intake, defined as unprocessed plus processed red meat, was tied to a 22% higher incidence of ASCVD (HR, 1.22; CI, 1.07-1.39; P = .004).
The study found no significant association between fish, poultry, or egg intake and incident ASCVD, but found total ASF intake had an 18% higher risk (HR, 1.18; CI, 1.03–1.34; P = .016).
Explaining the red meat–CVD connection
“The more novel part of our study is about the mediation analysis,” Dr. Wang said. “It helps explain why meat intake was associated with a higher risk of CVD. We identified several biological pathways, including the novel one through TMAO-related metabolites produced by the gut microbiome.”
Three gut microbiota–generated metabolites of L-carnitine – TMAO, gamma-butyrobetaine, and crotonobetaine – seem to partly explain the association between unprocessed red meat intake and incident ASCVD, the study reported.
The study found 3.92 excess ASCVD events per 1,000 person years associated with each interquintile range of higher unprocessed red meat intake; 10.6% of them were attributed to plasma levels of the three L-carnitine metabolites (95% CI, 1.0-114.5).
In this study, neither blood cholesterol nor blood pressure levels seemed to explain the elevated risk of ASCVD associated with meat intake, but blood glucose and insulin did, with mediation proportions of 26.1% and 11.8%, respectively.
Study strengths are its size and its general population cohort with well-measured CVD risk factors, Dr. Wang pointed out. All participants were free of clinically diagnosed CVD at enrollment, which minimized selection bias and reverse causation, she said. However, she acknowledged that the use of self-reported diet intake data, along with the largely white population, constitute limitations.
“Our study findings need to be confirmed in different populations and more research efforts are needed to better understand the health effects of some of the components in red meat, such as L-carnitine and heme iron,” Dr. Wang said.
“This study is interesting in that it doesn’t just ask the question, ‘Is eating red meat associated with coronary disease and atherosclerotic disease?’ but it tells what the mechanism is,” Robert Vogel, MD, professor at University of Colorado at Denver, Aurora, said in an interview.
The association between red meat and ASCVD is “an established science,” he said. “Where this study adds to the literature is that it suggests that elevated LDL cholesterol or blood pressure, things – especially the former – that are thought to be associated with coronary disease, may or may not be the mechanism.” He cautioned, however, “this is all associative data.”
The study “produces incremental knowledge for the association between eating red met and atherosclerosis, but it does not establish causality,” Dr. Vogel added.
Dr. Wang has no relevant disclosures. Dr. Vogel is a consultant to the Pritikin Longevity Center in Miami.
The connection between red meat and atherosclerotic cardiovascular disease has been well established, but newly reported findings indicate that metabolites in the gut microbiome may explain that relationship more than cholesterol and blood pressure.
“Eating more meat, especially red meat and processed meats, is associated with a higher risk of cardiovascular disease, even later in life,” co–lead study author Meng Wang, PhD, said in an interview.
The study, from a large community-based cohort of older people, included 3,931 U.S. participants aged 65 and older in the Cardiovascular Health Study (CHS). It found that gut microbiota–generated metabolites of dietary L-carnitine, including trimethylamine N-oxide (TMAO), have a role in the association between unprocessed red meat intake and incident ASCVD.
“TMAO-related metabolites produced by our gut microbes as well as blood-glucose and insulin homeostasis and systematic inflammation appeared to explain much of the association, more so than blood cholesterol or blood pressure,” added Dr. Wang, of the Friedman School of Nutrition Science and Policy at Tufts University, Boston.
Dr. Wang said this study was unique because it focused specifically on older adults; the average participant age was 72.9 years. “Older adults are at the highest risk of CVD, and for them adequate intake of protein may help to offset aging-related loss of muscle mass and strength,” she said. However, the study population was largely white (88%), so, she said, the results may not be generalizable to populations that are younger or of different nationalities and races.
The researchers performed a multivariable analysis that showed that participants who had higher intakes of unprocessed red meat, total meat, and total animal source foods (ASF) had higher hazard ratios of ASCVD risk. The study had a median follow-up of 12.5 years. It divided the study population into five quintiles based on how much unprocessed red met they consumed at baseline and analyzed dietary exposure in the differences between the midpoints of the first and fifth quintiles.
Earlier studies of meat intake and CVD risk focused mostly on saturated fat and blood cholesterol, Dr. Wang added. “But our findings suggest that other components in red meat, such as L-carnitine and heme iron, might play a more important role than saturated fat,” she said.
Higher intake of unprocessed red meat was linked to a 15% higher incidence of ASCVD per interquintile range (hazard ratio, 1.15; 95% confidence interval, 1.01-1.30; P = .031). Total meat intake, defined as unprocessed plus processed red meat, was tied to a 22% higher incidence of ASCVD (HR, 1.22; CI, 1.07-1.39; P = .004).
The study found no significant association between fish, poultry, or egg intake and incident ASCVD, but found total ASF intake had an 18% higher risk (HR, 1.18; CI, 1.03–1.34; P = .016).
Explaining the red meat–CVD connection
“The more novel part of our study is about the mediation analysis,” Dr. Wang said. “It helps explain why meat intake was associated with a higher risk of CVD. We identified several biological pathways, including the novel one through TMAO-related metabolites produced by the gut microbiome.”
Three gut microbiota–generated metabolites of L-carnitine – TMAO, gamma-butyrobetaine, and crotonobetaine – seem to partly explain the association between unprocessed red meat intake and incident ASCVD, the study reported.
The study found 3.92 excess ASCVD events per 1,000 person years associated with each interquintile range of higher unprocessed red meat intake; 10.6% of them were attributed to plasma levels of the three L-carnitine metabolites (95% CI, 1.0-114.5).
In this study, neither blood cholesterol nor blood pressure levels seemed to explain the elevated risk of ASCVD associated with meat intake, but blood glucose and insulin did, with mediation proportions of 26.1% and 11.8%, respectively.
Study strengths are its size and its general population cohort with well-measured CVD risk factors, Dr. Wang pointed out. All participants were free of clinically diagnosed CVD at enrollment, which minimized selection bias and reverse causation, she said. However, she acknowledged that the use of self-reported diet intake data, along with the largely white population, constitute limitations.
“Our study findings need to be confirmed in different populations and more research efforts are needed to better understand the health effects of some of the components in red meat, such as L-carnitine and heme iron,” Dr. Wang said.
“This study is interesting in that it doesn’t just ask the question, ‘Is eating red meat associated with coronary disease and atherosclerotic disease?’ but it tells what the mechanism is,” Robert Vogel, MD, professor at University of Colorado at Denver, Aurora, said in an interview.
The association between red meat and ASCVD is “an established science,” he said. “Where this study adds to the literature is that it suggests that elevated LDL cholesterol or blood pressure, things – especially the former – that are thought to be associated with coronary disease, may or may not be the mechanism.” He cautioned, however, “this is all associative data.”
The study “produces incremental knowledge for the association between eating red met and atherosclerosis, but it does not establish causality,” Dr. Vogel added.
Dr. Wang has no relevant disclosures. Dr. Vogel is a consultant to the Pritikin Longevity Center in Miami.
The connection between red meat and atherosclerotic cardiovascular disease has been well established, but newly reported findings indicate that metabolites in the gut microbiome may explain that relationship more than cholesterol and blood pressure.
“Eating more meat, especially red meat and processed meats, is associated with a higher risk of cardiovascular disease, even later in life,” co–lead study author Meng Wang, PhD, said in an interview.
The study, from a large community-based cohort of older people, included 3,931 U.S. participants aged 65 and older in the Cardiovascular Health Study (CHS). It found that gut microbiota–generated metabolites of dietary L-carnitine, including trimethylamine N-oxide (TMAO), have a role in the association between unprocessed red meat intake and incident ASCVD.
“TMAO-related metabolites produced by our gut microbes as well as blood-glucose and insulin homeostasis and systematic inflammation appeared to explain much of the association, more so than blood cholesterol or blood pressure,” added Dr. Wang, of the Friedman School of Nutrition Science and Policy at Tufts University, Boston.
Dr. Wang said this study was unique because it focused specifically on older adults; the average participant age was 72.9 years. “Older adults are at the highest risk of CVD, and for them adequate intake of protein may help to offset aging-related loss of muscle mass and strength,” she said. However, the study population was largely white (88%), so, she said, the results may not be generalizable to populations that are younger or of different nationalities and races.
The researchers performed a multivariable analysis that showed that participants who had higher intakes of unprocessed red meat, total meat, and total animal source foods (ASF) had higher hazard ratios of ASCVD risk. The study had a median follow-up of 12.5 years. It divided the study population into five quintiles based on how much unprocessed red met they consumed at baseline and analyzed dietary exposure in the differences between the midpoints of the first and fifth quintiles.
Earlier studies of meat intake and CVD risk focused mostly on saturated fat and blood cholesterol, Dr. Wang added. “But our findings suggest that other components in red meat, such as L-carnitine and heme iron, might play a more important role than saturated fat,” she said.
Higher intake of unprocessed red meat was linked to a 15% higher incidence of ASCVD per interquintile range (hazard ratio, 1.15; 95% confidence interval, 1.01-1.30; P = .031). Total meat intake, defined as unprocessed plus processed red meat, was tied to a 22% higher incidence of ASCVD (HR, 1.22; CI, 1.07-1.39; P = .004).
The study found no significant association between fish, poultry, or egg intake and incident ASCVD, but found total ASF intake had an 18% higher risk (HR, 1.18; CI, 1.03–1.34; P = .016).
Explaining the red meat–CVD connection
“The more novel part of our study is about the mediation analysis,” Dr. Wang said. “It helps explain why meat intake was associated with a higher risk of CVD. We identified several biological pathways, including the novel one through TMAO-related metabolites produced by the gut microbiome.”
Three gut microbiota–generated metabolites of L-carnitine – TMAO, gamma-butyrobetaine, and crotonobetaine – seem to partly explain the association between unprocessed red meat intake and incident ASCVD, the study reported.
The study found 3.92 excess ASCVD events per 1,000 person years associated with each interquintile range of higher unprocessed red meat intake; 10.6% of them were attributed to plasma levels of the three L-carnitine metabolites (95% CI, 1.0-114.5).
In this study, neither blood cholesterol nor blood pressure levels seemed to explain the elevated risk of ASCVD associated with meat intake, but blood glucose and insulin did, with mediation proportions of 26.1% and 11.8%, respectively.
Study strengths are its size and its general population cohort with well-measured CVD risk factors, Dr. Wang pointed out. All participants were free of clinically diagnosed CVD at enrollment, which minimized selection bias and reverse causation, she said. However, she acknowledged that the use of self-reported diet intake data, along with the largely white population, constitute limitations.
“Our study findings need to be confirmed in different populations and more research efforts are needed to better understand the health effects of some of the components in red meat, such as L-carnitine and heme iron,” Dr. Wang said.
“This study is interesting in that it doesn’t just ask the question, ‘Is eating red meat associated with coronary disease and atherosclerotic disease?’ but it tells what the mechanism is,” Robert Vogel, MD, professor at University of Colorado at Denver, Aurora, said in an interview.
The association between red meat and ASCVD is “an established science,” he said. “Where this study adds to the literature is that it suggests that elevated LDL cholesterol or blood pressure, things – especially the former – that are thought to be associated with coronary disease, may or may not be the mechanism.” He cautioned, however, “this is all associative data.”
The study “produces incremental knowledge for the association between eating red met and atherosclerosis, but it does not establish causality,” Dr. Vogel added.
Dr. Wang has no relevant disclosures. Dr. Vogel is a consultant to the Pritikin Longevity Center in Miami.
FROM ATHEROSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Obesity drug shortage triggers frustrations, workarounds
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
Cardiorespiratory fitness key to longevity for all?
Cardiorespiratory fitness emerged as a stronger predictor of all-cause mortality than did any traditional risk factor across the spectrum of age, sex, and race in a modeling study that included more than 750,000 U.S. veterans.
In addition, mortality risk was cut in half if individuals achieved a moderate cardiorespiratory fitness (CRF) level – that is, by meeting the current U.S. physical activity recommendations of 150 minutes per week, the authors note.
Furthermore, contrary to some previous research, “extremely high” fitness was not associated with an increased risk for mortality in the study, published online in the Journal of the American College of Cardiology.
“This study has been 15 years in the making,” lead author Peter Kokkinos, PhD, Rutgers University, New Brunswick, N.J., and the VA Medical Center, Washington, told this news organization. “We waited until we had the computer power and the right people to really assess this. We wanted to be very liberal in excluding patients we thought might contaminate the results, such as those with cardiovascular disease in the 6 months prior to a stress test.”
Figuring the time was right, the team analyzed data from the VA’s Exercise Testing and Health Outcomes Study (ETHOS) on individuals aged 30-95 years who underwent exercise treadmill tests between 1999 and 2020.
After exclusions, 750,302 individuals (from among 822,995) were included: 6.5% were women; 73.7% were White individuals; 19% were African American individuals; 4.7% were Hispanic individuals; and 2.1% were Native American, Asian, or Hawaiian individuals. Septuagenarians made up 14.7% of the cohort, and octogenarians made up 3.6%.
CRF categories for age and sex were determined by the peak metabolic equivalent of task (MET) achieved during the treadmill test. One MET is the energy spent at rest – that is the basal metabolic rate.
Although some physicians may resist putting patients through a stress test, “the amount of information we get from it is incredible,” Dr. Kokkinos noted. “We get blood pressure, we get heart rate, we get a response if you’re not doing exercise. This tells us a lot more than having you sit around so we can measure resting heart rate and blood pressure.”
Lowest mortality at 14.0 METs
During a median follow-up of 10.2 years (7,803,861 person-years), 23% of participants died, for an average of 22.4 events per 1,000 person-years.
Higher exercise capacity was inversely related to mortality risk across the cohort and within each age category. Specifically, every 1 MET increase in exercise capacity yielded an adjusted hazard ratio for mortality of 0.86 (95% confidence interval, 0.85-0.87; P < .001) for the entire cohort and similar HRs by sex and race.
The mortality risk for the least-fit individuals (20th percentile) was fourfold higher than for extremely fit individuals (HR, 4.09; 95% CI, 3.90-4.20), with the lowest mortality risk at about 14.0 METs for both men (HR, 0.24; 95% CI, 0.23-0.25) and women (HR, 0.23; 95% CI, 0.17-0.29). Extremely high CRF did not increase the risk.
In addition, at 20 years of follow-up, about 80% of men and 95% of women in the highest CRF category (98th percentile) were alive vs. less than 40% of men and approximately 75% of women in the least fit CRF category.
“We know CRF declines by 1% per year after age 30,” Dr. Kokkinos said. “But the age-related decline is cut in half if you are fit, meaning that an expected 10% decline over a decade will be only a 5% decline if you stay active. We cannot stop or reverse the decline, but we can kind of put the brakes on, and that’s a reason for clinicians to continue to encourage fitness.”
Indeed, “improving CRF should be considered a target in CVD prevention, similar to improving lipids, blood sugar, blood pressure, and weight,” Carl J. Lavie, MD, Ochsner Health, New Orleans, and colleagues affirm in a related editorial.
‘A difficult battle’
But that may not happen any time soon. “Unfortunately, despite having been recognized in an American Heart Association scientific statement as a clinical vital sign, aerobic fitness is undervalued and underutilized,” Claudio Gil Araújo, MD, PhD, research director of the Exercise Medicine Clinic-CLINIMEX, Rio de Janeiro, told this news organization.
Dr. Araújo led a recent study showing that the ability to stand on one leg for at least 10 seconds is strongly linked to the risk for death over the next 7 years.
Although physicians should be encouraging fitness, he said that “a substantial part of health professionals are physically unfit and feel uncomfortable talking about and prescribing exercise for their patients. Also, physicians tend to be better trained in treating diseases (using medications and/or prescribing procedures) than in preventing diseases by stimulating adoption of healthy habits. So, this a long road and a difficult battle.”
Nonetheless, he added, “Darwin said a long time ago that only the fittest will survive. If Darwin could read this study, he would surely smile.”
No commercial funding or conflicts of interest related to the study were reported. Dr. Lavie previously served as a speaker and consultant for PAI Health on their PAI (Personalized Activity Intelligence) applications.
A version of this article first appeared on Medscape.com.
Cardiorespiratory fitness emerged as a stronger predictor of all-cause mortality than did any traditional risk factor across the spectrum of age, sex, and race in a modeling study that included more than 750,000 U.S. veterans.
In addition, mortality risk was cut in half if individuals achieved a moderate cardiorespiratory fitness (CRF) level – that is, by meeting the current U.S. physical activity recommendations of 150 minutes per week, the authors note.
Furthermore, contrary to some previous research, “extremely high” fitness was not associated with an increased risk for mortality in the study, published online in the Journal of the American College of Cardiology.
“This study has been 15 years in the making,” lead author Peter Kokkinos, PhD, Rutgers University, New Brunswick, N.J., and the VA Medical Center, Washington, told this news organization. “We waited until we had the computer power and the right people to really assess this. We wanted to be very liberal in excluding patients we thought might contaminate the results, such as those with cardiovascular disease in the 6 months prior to a stress test.”
Figuring the time was right, the team analyzed data from the VA’s Exercise Testing and Health Outcomes Study (ETHOS) on individuals aged 30-95 years who underwent exercise treadmill tests between 1999 and 2020.
After exclusions, 750,302 individuals (from among 822,995) were included: 6.5% were women; 73.7% were White individuals; 19% were African American individuals; 4.7% were Hispanic individuals; and 2.1% were Native American, Asian, or Hawaiian individuals. Septuagenarians made up 14.7% of the cohort, and octogenarians made up 3.6%.
CRF categories for age and sex were determined by the peak metabolic equivalent of task (MET) achieved during the treadmill test. One MET is the energy spent at rest – that is the basal metabolic rate.
Although some physicians may resist putting patients through a stress test, “the amount of information we get from it is incredible,” Dr. Kokkinos noted. “We get blood pressure, we get heart rate, we get a response if you’re not doing exercise. This tells us a lot more than having you sit around so we can measure resting heart rate and blood pressure.”
Lowest mortality at 14.0 METs
During a median follow-up of 10.2 years (7,803,861 person-years), 23% of participants died, for an average of 22.4 events per 1,000 person-years.
Higher exercise capacity was inversely related to mortality risk across the cohort and within each age category. Specifically, every 1 MET increase in exercise capacity yielded an adjusted hazard ratio for mortality of 0.86 (95% confidence interval, 0.85-0.87; P < .001) for the entire cohort and similar HRs by sex and race.
The mortality risk for the least-fit individuals (20th percentile) was fourfold higher than for extremely fit individuals (HR, 4.09; 95% CI, 3.90-4.20), with the lowest mortality risk at about 14.0 METs for both men (HR, 0.24; 95% CI, 0.23-0.25) and women (HR, 0.23; 95% CI, 0.17-0.29). Extremely high CRF did not increase the risk.
In addition, at 20 years of follow-up, about 80% of men and 95% of women in the highest CRF category (98th percentile) were alive vs. less than 40% of men and approximately 75% of women in the least fit CRF category.
“We know CRF declines by 1% per year after age 30,” Dr. Kokkinos said. “But the age-related decline is cut in half if you are fit, meaning that an expected 10% decline over a decade will be only a 5% decline if you stay active. We cannot stop or reverse the decline, but we can kind of put the brakes on, and that’s a reason for clinicians to continue to encourage fitness.”
Indeed, “improving CRF should be considered a target in CVD prevention, similar to improving lipids, blood sugar, blood pressure, and weight,” Carl J. Lavie, MD, Ochsner Health, New Orleans, and colleagues affirm in a related editorial.
‘A difficult battle’
But that may not happen any time soon. “Unfortunately, despite having been recognized in an American Heart Association scientific statement as a clinical vital sign, aerobic fitness is undervalued and underutilized,” Claudio Gil Araújo, MD, PhD, research director of the Exercise Medicine Clinic-CLINIMEX, Rio de Janeiro, told this news organization.
Dr. Araújo led a recent study showing that the ability to stand on one leg for at least 10 seconds is strongly linked to the risk for death over the next 7 years.
Although physicians should be encouraging fitness, he said that “a substantial part of health professionals are physically unfit and feel uncomfortable talking about and prescribing exercise for their patients. Also, physicians tend to be better trained in treating diseases (using medications and/or prescribing procedures) than in preventing diseases by stimulating adoption of healthy habits. So, this a long road and a difficult battle.”
Nonetheless, he added, “Darwin said a long time ago that only the fittest will survive. If Darwin could read this study, he would surely smile.”
No commercial funding or conflicts of interest related to the study were reported. Dr. Lavie previously served as a speaker and consultant for PAI Health on their PAI (Personalized Activity Intelligence) applications.
A version of this article first appeared on Medscape.com.
Cardiorespiratory fitness emerged as a stronger predictor of all-cause mortality than did any traditional risk factor across the spectrum of age, sex, and race in a modeling study that included more than 750,000 U.S. veterans.
In addition, mortality risk was cut in half if individuals achieved a moderate cardiorespiratory fitness (CRF) level – that is, by meeting the current U.S. physical activity recommendations of 150 minutes per week, the authors note.
Furthermore, contrary to some previous research, “extremely high” fitness was not associated with an increased risk for mortality in the study, published online in the Journal of the American College of Cardiology.
“This study has been 15 years in the making,” lead author Peter Kokkinos, PhD, Rutgers University, New Brunswick, N.J., and the VA Medical Center, Washington, told this news organization. “We waited until we had the computer power and the right people to really assess this. We wanted to be very liberal in excluding patients we thought might contaminate the results, such as those with cardiovascular disease in the 6 months prior to a stress test.”
Figuring the time was right, the team analyzed data from the VA’s Exercise Testing and Health Outcomes Study (ETHOS) on individuals aged 30-95 years who underwent exercise treadmill tests between 1999 and 2020.
After exclusions, 750,302 individuals (from among 822,995) were included: 6.5% were women; 73.7% were White individuals; 19% were African American individuals; 4.7% were Hispanic individuals; and 2.1% were Native American, Asian, or Hawaiian individuals. Septuagenarians made up 14.7% of the cohort, and octogenarians made up 3.6%.
CRF categories for age and sex were determined by the peak metabolic equivalent of task (MET) achieved during the treadmill test. One MET is the energy spent at rest – that is the basal metabolic rate.
Although some physicians may resist putting patients through a stress test, “the amount of information we get from it is incredible,” Dr. Kokkinos noted. “We get blood pressure, we get heart rate, we get a response if you’re not doing exercise. This tells us a lot more than having you sit around so we can measure resting heart rate and blood pressure.”
Lowest mortality at 14.0 METs
During a median follow-up of 10.2 years (7,803,861 person-years), 23% of participants died, for an average of 22.4 events per 1,000 person-years.
Higher exercise capacity was inversely related to mortality risk across the cohort and within each age category. Specifically, every 1 MET increase in exercise capacity yielded an adjusted hazard ratio for mortality of 0.86 (95% confidence interval, 0.85-0.87; P < .001) for the entire cohort and similar HRs by sex and race.
The mortality risk for the least-fit individuals (20th percentile) was fourfold higher than for extremely fit individuals (HR, 4.09; 95% CI, 3.90-4.20), with the lowest mortality risk at about 14.0 METs for both men (HR, 0.24; 95% CI, 0.23-0.25) and women (HR, 0.23; 95% CI, 0.17-0.29). Extremely high CRF did not increase the risk.
In addition, at 20 years of follow-up, about 80% of men and 95% of women in the highest CRF category (98th percentile) were alive vs. less than 40% of men and approximately 75% of women in the least fit CRF category.
“We know CRF declines by 1% per year after age 30,” Dr. Kokkinos said. “But the age-related decline is cut in half if you are fit, meaning that an expected 10% decline over a decade will be only a 5% decline if you stay active. We cannot stop or reverse the decline, but we can kind of put the brakes on, and that’s a reason for clinicians to continue to encourage fitness.”
Indeed, “improving CRF should be considered a target in CVD prevention, similar to improving lipids, blood sugar, blood pressure, and weight,” Carl J. Lavie, MD, Ochsner Health, New Orleans, and colleagues affirm in a related editorial.
‘A difficult battle’
But that may not happen any time soon. “Unfortunately, despite having been recognized in an American Heart Association scientific statement as a clinical vital sign, aerobic fitness is undervalued and underutilized,” Claudio Gil Araújo, MD, PhD, research director of the Exercise Medicine Clinic-CLINIMEX, Rio de Janeiro, told this news organization.
Dr. Araújo led a recent study showing that the ability to stand on one leg for at least 10 seconds is strongly linked to the risk for death over the next 7 years.
Although physicians should be encouraging fitness, he said that “a substantial part of health professionals are physically unfit and feel uncomfortable talking about and prescribing exercise for their patients. Also, physicians tend to be better trained in treating diseases (using medications and/or prescribing procedures) than in preventing diseases by stimulating adoption of healthy habits. So, this a long road and a difficult battle.”
Nonetheless, he added, “Darwin said a long time ago that only the fittest will survive. If Darwin could read this study, he would surely smile.”
No commercial funding or conflicts of interest related to the study were reported. Dr. Lavie previously served as a speaker and consultant for PAI Health on their PAI (Personalized Activity Intelligence) applications.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY













