User login
FDA approves intravenous immunoglobulin for dermatomyositis
, according to a statement from manufacturer Octapharma USA.
Dermatomyositis is a rare, idiopathic autoimmune disorder that affects approximately 10 out of every million people in the United States, mainly adults in their late 40s to early 60s, according to the company, but children aged 5-15 years can be affected. The disease is characterized by skin rashes, chronic muscle inflammation, progressive muscle weakness, and risk for mortality that is three times higher than for the general population.
There are no previously approved treatments for dermatomyositis prior to Octagam 10%, which also is indicated for chronic immune thrombocytopenic purpura in adults.
The approval for dermatomyositis was based on the results of a phase 3 randomized, double-blind, placebo-controlled clinical trial (the ProDERM trial) that included 95 adult patients at 36 sites worldwide, with 17 sites in the United States. In the trial, 78.7% of patients with dermatomyositis who were randomized to receive 2 g/kg of Octagam 10% every 4 weeks showed response at 16 weeks, compared with 43.8% of patients who received placebo. Response was based on the 2016 American College of Rheumatology/European Alliance of Associations for Rheumatology myositis response criteria. Placebo patients who switched to intravenous immunoglobulin (IVIG) during a trial extension had response rates at week 40 similar to the original patients at week 16.
“The study gives clinicians much more confidence in the efficacy and safety of intravenous immunoglobulin and provides valuable information about what type of patient is best suited for the treatment,” Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh and a member of the ProDERM study Steering Committee, said in the Octapharma statement.
Safety and tolerability were similar to profiles seen with other IVIG medications, according to the statement. The medication does carry a boxed warning from its chronic ITP approval, cautioning about the potential for thrombosis, renal dysfunction, and acute renal failure.
The most common adverse reactions reported by dermatomyositis patients in the ProDERM trial were headache, fever, nausea, vomiting, increased blood pressure, chills, musculoskeletal pain, increased heart rate, dyspnea, and reactions at the infusion sites.
Read the full prescribing information here.
, according to a statement from manufacturer Octapharma USA.
Dermatomyositis is a rare, idiopathic autoimmune disorder that affects approximately 10 out of every million people in the United States, mainly adults in their late 40s to early 60s, according to the company, but children aged 5-15 years can be affected. The disease is characterized by skin rashes, chronic muscle inflammation, progressive muscle weakness, and risk for mortality that is three times higher than for the general population.
There are no previously approved treatments for dermatomyositis prior to Octagam 10%, which also is indicated for chronic immune thrombocytopenic purpura in adults.
The approval for dermatomyositis was based on the results of a phase 3 randomized, double-blind, placebo-controlled clinical trial (the ProDERM trial) that included 95 adult patients at 36 sites worldwide, with 17 sites in the United States. In the trial, 78.7% of patients with dermatomyositis who were randomized to receive 2 g/kg of Octagam 10% every 4 weeks showed response at 16 weeks, compared with 43.8% of patients who received placebo. Response was based on the 2016 American College of Rheumatology/European Alliance of Associations for Rheumatology myositis response criteria. Placebo patients who switched to intravenous immunoglobulin (IVIG) during a trial extension had response rates at week 40 similar to the original patients at week 16.
“The study gives clinicians much more confidence in the efficacy and safety of intravenous immunoglobulin and provides valuable information about what type of patient is best suited for the treatment,” Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh and a member of the ProDERM study Steering Committee, said in the Octapharma statement.
Safety and tolerability were similar to profiles seen with other IVIG medications, according to the statement. The medication does carry a boxed warning from its chronic ITP approval, cautioning about the potential for thrombosis, renal dysfunction, and acute renal failure.
The most common adverse reactions reported by dermatomyositis patients in the ProDERM trial were headache, fever, nausea, vomiting, increased blood pressure, chills, musculoskeletal pain, increased heart rate, dyspnea, and reactions at the infusion sites.
Read the full prescribing information here.
, according to a statement from manufacturer Octapharma USA.
Dermatomyositis is a rare, idiopathic autoimmune disorder that affects approximately 10 out of every million people in the United States, mainly adults in their late 40s to early 60s, according to the company, but children aged 5-15 years can be affected. The disease is characterized by skin rashes, chronic muscle inflammation, progressive muscle weakness, and risk for mortality that is three times higher than for the general population.
There are no previously approved treatments for dermatomyositis prior to Octagam 10%, which also is indicated for chronic immune thrombocytopenic purpura in adults.
The approval for dermatomyositis was based on the results of a phase 3 randomized, double-blind, placebo-controlled clinical trial (the ProDERM trial) that included 95 adult patients at 36 sites worldwide, with 17 sites in the United States. In the trial, 78.7% of patients with dermatomyositis who were randomized to receive 2 g/kg of Octagam 10% every 4 weeks showed response at 16 weeks, compared with 43.8% of patients who received placebo. Response was based on the 2016 American College of Rheumatology/European Alliance of Associations for Rheumatology myositis response criteria. Placebo patients who switched to intravenous immunoglobulin (IVIG) during a trial extension had response rates at week 40 similar to the original patients at week 16.
“The study gives clinicians much more confidence in the efficacy and safety of intravenous immunoglobulin and provides valuable information about what type of patient is best suited for the treatment,” Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh and a member of the ProDERM study Steering Committee, said in the Octapharma statement.
Safety and tolerability were similar to profiles seen with other IVIG medications, according to the statement. The medication does carry a boxed warning from its chronic ITP approval, cautioning about the potential for thrombosis, renal dysfunction, and acute renal failure.
The most common adverse reactions reported by dermatomyositis patients in the ProDERM trial were headache, fever, nausea, vomiting, increased blood pressure, chills, musculoskeletal pain, increased heart rate, dyspnea, and reactions at the infusion sites.
Read the full prescribing information here.
Three new ACR guidelines recommend treatment for six forms of vasculitis
Three new guidelines from the American College of Rheumatology, in partnership with the Vasculitis Foundation, offer evidence-based recommendations for managing and treating six different forms of systemic vasculitis.
“It’s not unusual for many rheumatologists to have fairly limited experience caring for patients with vasculitis,” coauthor Sharon Chung, MD, director of the Vasculitis Clinic at the University of California, San Francisco, said in an interview. “And with limited experience comes anxiety and concerns about whether or not one is treating patients appropriately. First and foremost, these guidelines are to help rheumatologists who may not have experience treating patients with vasculitides, to provide them with a framework they can use.”
The guidelines – the first to be produced and endorsed by both the ACR and the Vasculitis Foundation – were published July 8 in both Arthritis & Rheumatology and Arthritis Care & Research.
To assess the recent expansion in diagnostic and treatment options for various forms of vasculitis, the ACR assembled a literature review team, an 11-person patient panel, and a voting panel – made up of 9 adult rheumatologists, 5 pediatric rheumatologists, and 2 patients – to evaluate evidence, provide feedback, and formulate and vote on recommendations, respectively. The guidelines cover six types of vasculitis: one focusing on giant cell arteritis (GCA) and Takayasu arteritis (TAK); one on polyarteritis nodosa (PAN), and another on three forms of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV): granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA).
As with other ACR guidelines, these three were developed via the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which was used to rate the quality of the gathered evidence. For a recommendation to be published, it required 70% consensus or greater from the voting panel.
GCA and TAK guideline
Regarding the management and treatment of GCA and TAK, the guideline offers 42 recommendations and three ungraded position statements. Due to the low quality of evidence – “reflecting the paucity of randomized clinical trials in these diseases,” the authors noted – only one of the GCA recommendations and one of the TAK recommendations are strong; the rest are conditional.
For patients with GCA, the guideline strongly recommends long-term clinical monitoring over no clinical monitoring for anyone in apparent clinical remission. Other notable recommendations include favoring oral glucocorticoids (GCs) with tocilizumab (Actemra) over oral glucocorticoids alone in newly diagnosed GCA, adding a non-GC immunosuppressive agent to oral GCs for GCA patients with active extracranial large vessel involvement, and preferring temporary artery biopsy as their “diagnostic test of choice at this time.”
“The Europeans generally are more comfortable relying on temporal artery ultrasound,” Robert F. Spiera, MD, director of the vasculitis and scleroderma program at the Hospital for Special Surgery, New York, said in an interview. “In this country, possibly in part due to less uniform expertise in performing these ultrasounds, we have not had as much success in terms of accuracy.
These ACR guidelines therefore recommended biopsy to establish the diagnosis in patients with cranial presentations, whereas in the EULAR guidelines, ultrasound was felt to be preferable to biopsy.”
“While we have temporal artery ultrasound available in the United States, we just don’t have the expertise at this point to perform or interpret that test like the European rheumatologists do,” Dr. Chung agreed. “But I think we’re all hopeful that experience with temporal artery ultrasound will improve in the future, so we can use that test instead of an invasive biopsy.”
Dr. Spiera, who was not a coauthor on any of the guidelines, also highlighted the conditional recommendation of noninvasive vascular imaging of the large vessels in patients with newly diagnosed GCA.
“It is well recognized that a substantial portion of patients with GCA have unrecognized evidence of large vessel involvement, and patients with GCA in general are at higher risk of aneurysms later in the disease course,” he said. “These guidelines suggest screening even patients with purely cranial presentations for large vessel involvement with imaging to possibly identify the patients at higher risk for those later complications.
“What they didn’t offer were recommendations on how to follow up on that imaging,” he added, “which is an important and as-yet-unanswered question.”
For patients with TAK, the guideline again strongly recommends long-term clinical monitoring over no clinical monitoring for anyone in apparent clinical remission. Other conditional recommendations include choosing a non-GC immunosuppressive agent such as methotrexate or a tumor necrosis factor (TNF) inhibitor over tocilizumab as initial therapy because “the efficacy of tocilizumab in TAK is not established at this time.”
AAV guideline
Regarding the management and treatment of GPA, MPA, and EGPA, the guideline offers 41 recommendations and 10 ungraded position statements. All recommendations were conditional, and many address GPA and MPA together because, as the authors noted, “pivotal trials have enrolled both groups and presented results for these diseases together.”
One notable recommendation is their preference for rituximab over cyclophosphamide for remission induction and for rituximab over methotrexate or azathioprine for remission maintenance in patients with severe GPA or MPA. “I don’t think this is a surprise to people, but I think it reaffirms where our current practice is moving,” Dr. Chung said.
“The literature supports that in patients with relapsing disease, rituximab works better than cyclophosphamide for remission induction,” Dr. Spiera said. “But in these guidelines, even in new disease, rituximab is suggested as the agent of choice to induce remission. I would say that that is reasonable, but you could make an argument that it’s maybe beyond what the literature supports, particularly in patients with advanced renal insufficiency attributable to that initial vasculitis flare.”
Other recommendations include being against routinely adding plasma exchange to remission induction therapy in GPA or MPA patients with active glomerulonephritis – although they added that it should be considered in patients at high risk of end-stage kidney disease – as well as preferring cyclophosphamide or rituximab over mepolizumab for remission induction in patients with severe EGPA.
“We, to the surprise of many, were more supportive for the use of rituximab in EGPA than others were expecting, given the limited evidence,” Dr. Chung said. “One of the reasons for that is the wide experience we’ve had with rituximab in GPA and MPA, and our recognition that there is a population of patients with EGPA who are ANCA positive who do seem to benefit from rituximab therapy.”
And although the voting panel strongly favored treatment with methotrexate or azathioprine over trimethoprim/sulfamethoxazole for GPA patients in remission, they ultimately labeled the recommendation as conditional “due to the lack of sufficient high-quality evidence comparing the two treatments.”
“There has been progress in terms of well-done clinical trials to inform our decision-making, particularly for ANCA-associated vasculitis, both in terms of how to induce and maintain remission,” Dr. Spiera said. “Though the recommendations were conditional, I think there’s very strong data to support many of them.”
PAN guideline
Regarding the management and treatment of PAN, the guideline offers 16 recommendations – all but one are conditional – and one ungraded position statement. Their strong recommendation was for treatment with TNF inhibitors over GCs in patients with clinical manifestations of deficiency of adenosine deaminase 2, which they asked doctors to consider “in the setting of a PAN-like syndrome with strokes.” Other conditional recommendations include treating patients with newly diagnosed, severe PAN with cyclophosphamide and GCs, as well as the use of abdominal vascular imaging and/or a deep-skin biopsy to help establish a diagnosis.
According to the authors, a fourth guideline on treating and managing Kawasaki syndrome will be released in the coming weeks.
The guidelines were supported by the ACR and the Vasculitis Foundation. Several authors acknowledged potential conflicts of interest, including receiving speaking and consulting fees, research grants, and honoraria from various pharmaceutical companies. Dr. Spiera has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, Chemocentryx, Corbus, Formation Biologics, InflaRx, Kadmon, AstraZeneca, AbbVie, CSL Behring, Sanofi, and Janssen.
Three new guidelines from the American College of Rheumatology, in partnership with the Vasculitis Foundation, offer evidence-based recommendations for managing and treating six different forms of systemic vasculitis.
“It’s not unusual for many rheumatologists to have fairly limited experience caring for patients with vasculitis,” coauthor Sharon Chung, MD, director of the Vasculitis Clinic at the University of California, San Francisco, said in an interview. “And with limited experience comes anxiety and concerns about whether or not one is treating patients appropriately. First and foremost, these guidelines are to help rheumatologists who may not have experience treating patients with vasculitides, to provide them with a framework they can use.”
The guidelines – the first to be produced and endorsed by both the ACR and the Vasculitis Foundation – were published July 8 in both Arthritis & Rheumatology and Arthritis Care & Research.
To assess the recent expansion in diagnostic and treatment options for various forms of vasculitis, the ACR assembled a literature review team, an 11-person patient panel, and a voting panel – made up of 9 adult rheumatologists, 5 pediatric rheumatologists, and 2 patients – to evaluate evidence, provide feedback, and formulate and vote on recommendations, respectively. The guidelines cover six types of vasculitis: one focusing on giant cell arteritis (GCA) and Takayasu arteritis (TAK); one on polyarteritis nodosa (PAN), and another on three forms of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV): granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA).
As with other ACR guidelines, these three were developed via the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which was used to rate the quality of the gathered evidence. For a recommendation to be published, it required 70% consensus or greater from the voting panel.
GCA and TAK guideline
Regarding the management and treatment of GCA and TAK, the guideline offers 42 recommendations and three ungraded position statements. Due to the low quality of evidence – “reflecting the paucity of randomized clinical trials in these diseases,” the authors noted – only one of the GCA recommendations and one of the TAK recommendations are strong; the rest are conditional.
For patients with GCA, the guideline strongly recommends long-term clinical monitoring over no clinical monitoring for anyone in apparent clinical remission. Other notable recommendations include favoring oral glucocorticoids (GCs) with tocilizumab (Actemra) over oral glucocorticoids alone in newly diagnosed GCA, adding a non-GC immunosuppressive agent to oral GCs for GCA patients with active extracranial large vessel involvement, and preferring temporary artery biopsy as their “diagnostic test of choice at this time.”
“The Europeans generally are more comfortable relying on temporal artery ultrasound,” Robert F. Spiera, MD, director of the vasculitis and scleroderma program at the Hospital for Special Surgery, New York, said in an interview. “In this country, possibly in part due to less uniform expertise in performing these ultrasounds, we have not had as much success in terms of accuracy.
These ACR guidelines therefore recommended biopsy to establish the diagnosis in patients with cranial presentations, whereas in the EULAR guidelines, ultrasound was felt to be preferable to biopsy.”
“While we have temporal artery ultrasound available in the United States, we just don’t have the expertise at this point to perform or interpret that test like the European rheumatologists do,” Dr. Chung agreed. “But I think we’re all hopeful that experience with temporal artery ultrasound will improve in the future, so we can use that test instead of an invasive biopsy.”
Dr. Spiera, who was not a coauthor on any of the guidelines, also highlighted the conditional recommendation of noninvasive vascular imaging of the large vessels in patients with newly diagnosed GCA.
“It is well recognized that a substantial portion of patients with GCA have unrecognized evidence of large vessel involvement, and patients with GCA in general are at higher risk of aneurysms later in the disease course,” he said. “These guidelines suggest screening even patients with purely cranial presentations for large vessel involvement with imaging to possibly identify the patients at higher risk for those later complications.
“What they didn’t offer were recommendations on how to follow up on that imaging,” he added, “which is an important and as-yet-unanswered question.”
For patients with TAK, the guideline again strongly recommends long-term clinical monitoring over no clinical monitoring for anyone in apparent clinical remission. Other conditional recommendations include choosing a non-GC immunosuppressive agent such as methotrexate or a tumor necrosis factor (TNF) inhibitor over tocilizumab as initial therapy because “the efficacy of tocilizumab in TAK is not established at this time.”
AAV guideline
Regarding the management and treatment of GPA, MPA, and EGPA, the guideline offers 41 recommendations and 10 ungraded position statements. All recommendations were conditional, and many address GPA and MPA together because, as the authors noted, “pivotal trials have enrolled both groups and presented results for these diseases together.”
One notable recommendation is their preference for rituximab over cyclophosphamide for remission induction and for rituximab over methotrexate or azathioprine for remission maintenance in patients with severe GPA or MPA. “I don’t think this is a surprise to people, but I think it reaffirms where our current practice is moving,” Dr. Chung said.
“The literature supports that in patients with relapsing disease, rituximab works better than cyclophosphamide for remission induction,” Dr. Spiera said. “But in these guidelines, even in new disease, rituximab is suggested as the agent of choice to induce remission. I would say that that is reasonable, but you could make an argument that it’s maybe beyond what the literature supports, particularly in patients with advanced renal insufficiency attributable to that initial vasculitis flare.”
Other recommendations include being against routinely adding plasma exchange to remission induction therapy in GPA or MPA patients with active glomerulonephritis – although they added that it should be considered in patients at high risk of end-stage kidney disease – as well as preferring cyclophosphamide or rituximab over mepolizumab for remission induction in patients with severe EGPA.
“We, to the surprise of many, were more supportive for the use of rituximab in EGPA than others were expecting, given the limited evidence,” Dr. Chung said. “One of the reasons for that is the wide experience we’ve had with rituximab in GPA and MPA, and our recognition that there is a population of patients with EGPA who are ANCA positive who do seem to benefit from rituximab therapy.”
And although the voting panel strongly favored treatment with methotrexate or azathioprine over trimethoprim/sulfamethoxazole for GPA patients in remission, they ultimately labeled the recommendation as conditional “due to the lack of sufficient high-quality evidence comparing the two treatments.”
“There has been progress in terms of well-done clinical trials to inform our decision-making, particularly for ANCA-associated vasculitis, both in terms of how to induce and maintain remission,” Dr. Spiera said. “Though the recommendations were conditional, I think there’s very strong data to support many of them.”
PAN guideline
Regarding the management and treatment of PAN, the guideline offers 16 recommendations – all but one are conditional – and one ungraded position statement. Their strong recommendation was for treatment with TNF inhibitors over GCs in patients with clinical manifestations of deficiency of adenosine deaminase 2, which they asked doctors to consider “in the setting of a PAN-like syndrome with strokes.” Other conditional recommendations include treating patients with newly diagnosed, severe PAN with cyclophosphamide and GCs, as well as the use of abdominal vascular imaging and/or a deep-skin biopsy to help establish a diagnosis.
According to the authors, a fourth guideline on treating and managing Kawasaki syndrome will be released in the coming weeks.
The guidelines were supported by the ACR and the Vasculitis Foundation. Several authors acknowledged potential conflicts of interest, including receiving speaking and consulting fees, research grants, and honoraria from various pharmaceutical companies. Dr. Spiera has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, Chemocentryx, Corbus, Formation Biologics, InflaRx, Kadmon, AstraZeneca, AbbVie, CSL Behring, Sanofi, and Janssen.
Three new guidelines from the American College of Rheumatology, in partnership with the Vasculitis Foundation, offer evidence-based recommendations for managing and treating six different forms of systemic vasculitis.
“It’s not unusual for many rheumatologists to have fairly limited experience caring for patients with vasculitis,” coauthor Sharon Chung, MD, director of the Vasculitis Clinic at the University of California, San Francisco, said in an interview. “And with limited experience comes anxiety and concerns about whether or not one is treating patients appropriately. First and foremost, these guidelines are to help rheumatologists who may not have experience treating patients with vasculitides, to provide them with a framework they can use.”
The guidelines – the first to be produced and endorsed by both the ACR and the Vasculitis Foundation – were published July 8 in both Arthritis & Rheumatology and Arthritis Care & Research.
To assess the recent expansion in diagnostic and treatment options for various forms of vasculitis, the ACR assembled a literature review team, an 11-person patient panel, and a voting panel – made up of 9 adult rheumatologists, 5 pediatric rheumatologists, and 2 patients – to evaluate evidence, provide feedback, and formulate and vote on recommendations, respectively. The guidelines cover six types of vasculitis: one focusing on giant cell arteritis (GCA) and Takayasu arteritis (TAK); one on polyarteritis nodosa (PAN), and another on three forms of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV): granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA).
As with other ACR guidelines, these three were developed via the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which was used to rate the quality of the gathered evidence. For a recommendation to be published, it required 70% consensus or greater from the voting panel.
GCA and TAK guideline
Regarding the management and treatment of GCA and TAK, the guideline offers 42 recommendations and three ungraded position statements. Due to the low quality of evidence – “reflecting the paucity of randomized clinical trials in these diseases,” the authors noted – only one of the GCA recommendations and one of the TAK recommendations are strong; the rest are conditional.
For patients with GCA, the guideline strongly recommends long-term clinical monitoring over no clinical monitoring for anyone in apparent clinical remission. Other notable recommendations include favoring oral glucocorticoids (GCs) with tocilizumab (Actemra) over oral glucocorticoids alone in newly diagnosed GCA, adding a non-GC immunosuppressive agent to oral GCs for GCA patients with active extracranial large vessel involvement, and preferring temporary artery biopsy as their “diagnostic test of choice at this time.”
“The Europeans generally are more comfortable relying on temporal artery ultrasound,” Robert F. Spiera, MD, director of the vasculitis and scleroderma program at the Hospital for Special Surgery, New York, said in an interview. “In this country, possibly in part due to less uniform expertise in performing these ultrasounds, we have not had as much success in terms of accuracy.
These ACR guidelines therefore recommended biopsy to establish the diagnosis in patients with cranial presentations, whereas in the EULAR guidelines, ultrasound was felt to be preferable to biopsy.”
“While we have temporal artery ultrasound available in the United States, we just don’t have the expertise at this point to perform or interpret that test like the European rheumatologists do,” Dr. Chung agreed. “But I think we’re all hopeful that experience with temporal artery ultrasound will improve in the future, so we can use that test instead of an invasive biopsy.”
Dr. Spiera, who was not a coauthor on any of the guidelines, also highlighted the conditional recommendation of noninvasive vascular imaging of the large vessels in patients with newly diagnosed GCA.
“It is well recognized that a substantial portion of patients with GCA have unrecognized evidence of large vessel involvement, and patients with GCA in general are at higher risk of aneurysms later in the disease course,” he said. “These guidelines suggest screening even patients with purely cranial presentations for large vessel involvement with imaging to possibly identify the patients at higher risk for those later complications.
“What they didn’t offer were recommendations on how to follow up on that imaging,” he added, “which is an important and as-yet-unanswered question.”
For patients with TAK, the guideline again strongly recommends long-term clinical monitoring over no clinical monitoring for anyone in apparent clinical remission. Other conditional recommendations include choosing a non-GC immunosuppressive agent such as methotrexate or a tumor necrosis factor (TNF) inhibitor over tocilizumab as initial therapy because “the efficacy of tocilizumab in TAK is not established at this time.”
AAV guideline
Regarding the management and treatment of GPA, MPA, and EGPA, the guideline offers 41 recommendations and 10 ungraded position statements. All recommendations were conditional, and many address GPA and MPA together because, as the authors noted, “pivotal trials have enrolled both groups and presented results for these diseases together.”
One notable recommendation is their preference for rituximab over cyclophosphamide for remission induction and for rituximab over methotrexate or azathioprine for remission maintenance in patients with severe GPA or MPA. “I don’t think this is a surprise to people, but I think it reaffirms where our current practice is moving,” Dr. Chung said.
“The literature supports that in patients with relapsing disease, rituximab works better than cyclophosphamide for remission induction,” Dr. Spiera said. “But in these guidelines, even in new disease, rituximab is suggested as the agent of choice to induce remission. I would say that that is reasonable, but you could make an argument that it’s maybe beyond what the literature supports, particularly in patients with advanced renal insufficiency attributable to that initial vasculitis flare.”
Other recommendations include being against routinely adding plasma exchange to remission induction therapy in GPA or MPA patients with active glomerulonephritis – although they added that it should be considered in patients at high risk of end-stage kidney disease – as well as preferring cyclophosphamide or rituximab over mepolizumab for remission induction in patients with severe EGPA.
“We, to the surprise of many, were more supportive for the use of rituximab in EGPA than others were expecting, given the limited evidence,” Dr. Chung said. “One of the reasons for that is the wide experience we’ve had with rituximab in GPA and MPA, and our recognition that there is a population of patients with EGPA who are ANCA positive who do seem to benefit from rituximab therapy.”
And although the voting panel strongly favored treatment with methotrexate or azathioprine over trimethoprim/sulfamethoxazole for GPA patients in remission, they ultimately labeled the recommendation as conditional “due to the lack of sufficient high-quality evidence comparing the two treatments.”
“There has been progress in terms of well-done clinical trials to inform our decision-making, particularly for ANCA-associated vasculitis, both in terms of how to induce and maintain remission,” Dr. Spiera said. “Though the recommendations were conditional, I think there’s very strong data to support many of them.”
PAN guideline
Regarding the management and treatment of PAN, the guideline offers 16 recommendations – all but one are conditional – and one ungraded position statement. Their strong recommendation was for treatment with TNF inhibitors over GCs in patients with clinical manifestations of deficiency of adenosine deaminase 2, which they asked doctors to consider “in the setting of a PAN-like syndrome with strokes.” Other conditional recommendations include treating patients with newly diagnosed, severe PAN with cyclophosphamide and GCs, as well as the use of abdominal vascular imaging and/or a deep-skin biopsy to help establish a diagnosis.
According to the authors, a fourth guideline on treating and managing Kawasaki syndrome will be released in the coming weeks.
The guidelines were supported by the ACR and the Vasculitis Foundation. Several authors acknowledged potential conflicts of interest, including receiving speaking and consulting fees, research grants, and honoraria from various pharmaceutical companies. Dr. Spiera has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, Chemocentryx, Corbus, Formation Biologics, InflaRx, Kadmon, AstraZeneca, AbbVie, CSL Behring, Sanofi, and Janssen.
FROM ARTHRITIS & RHEUMATOLOGY
Pharmacovigilance data implicate three new drugs in ANCA-associated vasculitis
The overactive bladder treatment mirabegron (Myrbetriq) is one of three new drugs to be linked to cases of drug-associated antineutrophil cytoplasmic antibody–associated vasculitis (DA-AAV) according to pharmacovigilance data.
The other two drugs are the antiviral agent sofosbuvir (Sovaldi) and the tyrosine kinase inhibitor nintedanib (Ofev), which is indicated for chronic fibrosing interstitial lung diseases (ILDs) with a progressive phenotype.
The study findings also strengthen previously suspected associations, Samuel Deshayes, MD, of Caen (France) University Hospital and coauthors report in Arthritis & Rheumatology. This includes associations between hydralazine, propylthiouracil, thiamazole, minocycline, and carbimazole to name a few more of the 15 drugs where associations were found.
“These data imply specific prescriber attention to these drugs and argue for experimental studies on AAV pathophysiology with [the] recent drugs,” Dr. Deshayes and colleagues wrote.
Identifying whether AAV could be due to drug treatment is important as there have been suggestions that this may confer a better prognosis than for primary AAV. In addition, “withdrawal of the culprit drug” may be enough to treat the patient in milder cases, the researchers suggested.
That said, the researchers acknowledged that it can be difficult to attribute AAV to a specific drug, particularly as data are often from case reports or small series of patients and, until now, research had considered older medications.
To update current knowledge and include newer treatments, Dr. Deshayes and associates took data from the World Health Organization pharmacovigilance database (VigiBase) and performed a retrospective analysis.
They collected data on all adverse drug reactions (ADRs) reported using the Medical Dictionary for Drug Regulatory Activities preferred term ‘anti-neutrophil’ cytoplasmic antibody positive vasculitis’ from its introduction in 2006 up until November 2020.
Drugs used in the management of AAV, such as azathioprine, cyclophosphamide, leflunomide, and methotrexate, among others, were not included in the analysis “due to potential indication bias,” they explained.
Among 483 individual “deduplicated” case safety reports, most cases of DA-AAV were reported in women (71.2% of cases) and the median age at onset was 62 years. The median time to develop AAV after drug initiation was 9 months.
DA-AAV was considered serious in almost all (98%) of the reported cases, meaning that it was life-threatening, had resulted in or had prolonged hospital treatment, caused significant or persistent disability or other anomalies such as birth defects, or resulted in the death of the individual. With regard to the latter, DA-AAV was reported as fatal in 8.9% of cases.
The highest rates of reported DA-AAV were seen in people treated with the antihypertensive drug hydralazine, the antithyroid drugs propylthiouracil, thiamazole, and carbimazole, the antibiotic minocycline, mirabegron, sofosbuvir, and nintedanib.
A further seven drugs were also linked to cases of AAV, bringing the total to list to 15 drugs associated with overreporting of AAV. This included the heavy metal antagonist penicillamine, the influenza vaccine, the gout medication allopurinol, the anti-tuberculosis drug rifampin, the antiviral agent peginterferon alfa-2b, the anti-asthma drug montelukast, and the anti-cholesterol agent rosuvastatin.
Dr. Deshayes and coauthors pointed out that the associations do not imply causality. The data mining approach used allowed for the “automated detection of drug safety signals associated with rare events such as AAV,” they said.
There are limitations, they acknowledged, which are common to studies that use pharmacovigilance data. For one, “notoriety bias” could be at play. This is where there is an “increased number of reports after safety alerts in the media.” Missing data, “including delay between the introduction of the drug and AAV,” could be a factor, as could “bias related to its retrospective and declarative design.”
The study received no commercial funding. All study authors declared no competing interests.
The overactive bladder treatment mirabegron (Myrbetriq) is one of three new drugs to be linked to cases of drug-associated antineutrophil cytoplasmic antibody–associated vasculitis (DA-AAV) according to pharmacovigilance data.
The other two drugs are the antiviral agent sofosbuvir (Sovaldi) and the tyrosine kinase inhibitor nintedanib (Ofev), which is indicated for chronic fibrosing interstitial lung diseases (ILDs) with a progressive phenotype.
The study findings also strengthen previously suspected associations, Samuel Deshayes, MD, of Caen (France) University Hospital and coauthors report in Arthritis & Rheumatology. This includes associations between hydralazine, propylthiouracil, thiamazole, minocycline, and carbimazole to name a few more of the 15 drugs where associations were found.
“These data imply specific prescriber attention to these drugs and argue for experimental studies on AAV pathophysiology with [the] recent drugs,” Dr. Deshayes and colleagues wrote.
Identifying whether AAV could be due to drug treatment is important as there have been suggestions that this may confer a better prognosis than for primary AAV. In addition, “withdrawal of the culprit drug” may be enough to treat the patient in milder cases, the researchers suggested.
That said, the researchers acknowledged that it can be difficult to attribute AAV to a specific drug, particularly as data are often from case reports or small series of patients and, until now, research had considered older medications.
To update current knowledge and include newer treatments, Dr. Deshayes and associates took data from the World Health Organization pharmacovigilance database (VigiBase) and performed a retrospective analysis.
They collected data on all adverse drug reactions (ADRs) reported using the Medical Dictionary for Drug Regulatory Activities preferred term ‘anti-neutrophil’ cytoplasmic antibody positive vasculitis’ from its introduction in 2006 up until November 2020.
Drugs used in the management of AAV, such as azathioprine, cyclophosphamide, leflunomide, and methotrexate, among others, were not included in the analysis “due to potential indication bias,” they explained.
Among 483 individual “deduplicated” case safety reports, most cases of DA-AAV were reported in women (71.2% of cases) and the median age at onset was 62 years. The median time to develop AAV after drug initiation was 9 months.
DA-AAV was considered serious in almost all (98%) of the reported cases, meaning that it was life-threatening, had resulted in or had prolonged hospital treatment, caused significant or persistent disability or other anomalies such as birth defects, or resulted in the death of the individual. With regard to the latter, DA-AAV was reported as fatal in 8.9% of cases.
The highest rates of reported DA-AAV were seen in people treated with the antihypertensive drug hydralazine, the antithyroid drugs propylthiouracil, thiamazole, and carbimazole, the antibiotic minocycline, mirabegron, sofosbuvir, and nintedanib.
A further seven drugs were also linked to cases of AAV, bringing the total to list to 15 drugs associated with overreporting of AAV. This included the heavy metal antagonist penicillamine, the influenza vaccine, the gout medication allopurinol, the anti-tuberculosis drug rifampin, the antiviral agent peginterferon alfa-2b, the anti-asthma drug montelukast, and the anti-cholesterol agent rosuvastatin.
Dr. Deshayes and coauthors pointed out that the associations do not imply causality. The data mining approach used allowed for the “automated detection of drug safety signals associated with rare events such as AAV,” they said.
There are limitations, they acknowledged, which are common to studies that use pharmacovigilance data. For one, “notoriety bias” could be at play. This is where there is an “increased number of reports after safety alerts in the media.” Missing data, “including delay between the introduction of the drug and AAV,” could be a factor, as could “bias related to its retrospective and declarative design.”
The study received no commercial funding. All study authors declared no competing interests.
The overactive bladder treatment mirabegron (Myrbetriq) is one of three new drugs to be linked to cases of drug-associated antineutrophil cytoplasmic antibody–associated vasculitis (DA-AAV) according to pharmacovigilance data.
The other two drugs are the antiviral agent sofosbuvir (Sovaldi) and the tyrosine kinase inhibitor nintedanib (Ofev), which is indicated for chronic fibrosing interstitial lung diseases (ILDs) with a progressive phenotype.
The study findings also strengthen previously suspected associations, Samuel Deshayes, MD, of Caen (France) University Hospital and coauthors report in Arthritis & Rheumatology. This includes associations between hydralazine, propylthiouracil, thiamazole, minocycline, and carbimazole to name a few more of the 15 drugs where associations were found.
“These data imply specific prescriber attention to these drugs and argue for experimental studies on AAV pathophysiology with [the] recent drugs,” Dr. Deshayes and colleagues wrote.
Identifying whether AAV could be due to drug treatment is important as there have been suggestions that this may confer a better prognosis than for primary AAV. In addition, “withdrawal of the culprit drug” may be enough to treat the patient in milder cases, the researchers suggested.
That said, the researchers acknowledged that it can be difficult to attribute AAV to a specific drug, particularly as data are often from case reports or small series of patients and, until now, research had considered older medications.
To update current knowledge and include newer treatments, Dr. Deshayes and associates took data from the World Health Organization pharmacovigilance database (VigiBase) and performed a retrospective analysis.
They collected data on all adverse drug reactions (ADRs) reported using the Medical Dictionary for Drug Regulatory Activities preferred term ‘anti-neutrophil’ cytoplasmic antibody positive vasculitis’ from its introduction in 2006 up until November 2020.
Drugs used in the management of AAV, such as azathioprine, cyclophosphamide, leflunomide, and methotrexate, among others, were not included in the analysis “due to potential indication bias,” they explained.
Among 483 individual “deduplicated” case safety reports, most cases of DA-AAV were reported in women (71.2% of cases) and the median age at onset was 62 years. The median time to develop AAV after drug initiation was 9 months.
DA-AAV was considered serious in almost all (98%) of the reported cases, meaning that it was life-threatening, had resulted in or had prolonged hospital treatment, caused significant or persistent disability or other anomalies such as birth defects, or resulted in the death of the individual. With regard to the latter, DA-AAV was reported as fatal in 8.9% of cases.
The highest rates of reported DA-AAV were seen in people treated with the antihypertensive drug hydralazine, the antithyroid drugs propylthiouracil, thiamazole, and carbimazole, the antibiotic minocycline, mirabegron, sofosbuvir, and nintedanib.
A further seven drugs were also linked to cases of AAV, bringing the total to list to 15 drugs associated with overreporting of AAV. This included the heavy metal antagonist penicillamine, the influenza vaccine, the gout medication allopurinol, the anti-tuberculosis drug rifampin, the antiviral agent peginterferon alfa-2b, the anti-asthma drug montelukast, and the anti-cholesterol agent rosuvastatin.
Dr. Deshayes and coauthors pointed out that the associations do not imply causality. The data mining approach used allowed for the “automated detection of drug safety signals associated with rare events such as AAV,” they said.
There are limitations, they acknowledged, which are common to studies that use pharmacovigilance data. For one, “notoriety bias” could be at play. This is where there is an “increased number of reports after safety alerts in the media.” Missing data, “including delay between the introduction of the drug and AAV,” could be a factor, as could “bias related to its retrospective and declarative design.”
The study received no commercial funding. All study authors declared no competing interests.
FROM ARTHRITIS & RHEUMATOLOGY
Neuropsychiatric event etiology in lupus helps define predictors, outcomes
Different kinds of neuropsychiatric (NP) events in patients with systemic lupus erythematosus (SLE) have substantial variability in their occurrence, resolution, and recurrence over time, as well as in their predictors, according to new research from a large, prospective, international, inception cohort study.
Because “multiple NP events due to different causes may present concurrently in individual patients, the findings emphasize the importance of recognizing attribution of NP events as a determinant of clinical outcome,” John G. Hanly, MD, of Queen Elizabeth II Health Sciences Centre and Dalhousie University, Halifax, N.S., and colleagues wrote in Arthritis & Rheumatology.
In a previous study of the same group of 1,827 patients with SLE, NP events occurred in about half and approximately one-third of these events were deemed disease related. They also “occurred most frequently around the diagnosis of SLE and had a significant negative impact on health-related quality of life,” the researchers wrote.
Researchers involved with the Systemic Lupus International Collaborating Clinics recruited the 1,827 adults with SLE over an 11-year period during 1999-2011 from a total of 31 sites in Europe, Asia, and North America. The average age of the patients at study enrollment was 35 years, 89% were women, and 49% were White. The mean disease duration was 5.6 months, and 70% of patients were taking corticosteroids at enrollment.
Over an average follow-up period of 7.6 years, 955 patients (52.3%) experienced a single neuropsychiatric event, and 493 (27.0%) experienced two or more events; the total number of unique NP events was 1,910. Most of these unique events (92%) involved the central nervous system, and 8.4% involved the peripheral nervous system.
The researchers used multistate models to attribute NP events to SLE based on factors that included the temporal onset of NP events in relation to SLE diagnosis, concurrent non-SLE factors, and NP events that are common in healthy controls. The four states in the multistate models were no NP events, no current NP event but a history of at least one event, new or ongoing NP events, and death. The results included a multivariate analysis of a model involving 492 observed transitions into new or ongoing NP events.
In the multivariate analysis, factors positively associated with SLE-attributed NP events included male sex (hazard ratio, 1.35; P = .028), concurrent non-SLE NP events excluding headache (HR, 1.83; P < .001), active SLE based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (HR, 1.19; P = .012), and corticosteroid use (HR, 1.59; P = .008). The researchers also found that SLE-attributed NP events were negatively associated with Asian race/ethnicity, postsecondary education, and use of immunosuppressive drugs.
Another multivariate analysis found that non-SLE NP events were positively associated with only concurrent SLE-attributed NP events excluding headache (HR, 2.31; P < .001), but negative associations were seen with non-U.S. African race/ethnicity and Asian race/ethnicity.
The researchers found that SLE-attributed NP events had higher rates of resolution, compared with non-SLE NP events, with the exception of headache, which had similar resolution for both event groups.
“Resolution of SLE events was more likely in patients with Asian race/ethnicity and those with Central/Focal nervous system disease with no effect seen for age at diagnosis,” the researchers noted. “For non-SLE NP events, African race/ethnicity at non-U.S. sites and younger age at diagnosis was associated with a better outcome.”
The study findings were limited by several factors including the predominantly White patient population and the clustering of NP events into limited categories, which may have reduced the identification of more specific associations, the researchers noted. Also, the assessment of NP event outcomes did not include patient perceptions, and the relatively short follow-up period does not allow for assessment of later NP events such as cerebrovascular disease. However, “despite these limitations the current study provides valuable data on the presentation, outcome and predictors of NP disease in SLE patients enrolled in a long-term, international, disease inception cohort,” the researchers concluded.
The study received no outside funding. Dr. Hanly was supported by a grant from the Canadian Institutes of Health Research but had no financial conflicts to disclose. Several coauthors received grant support from various institutions, but not from industry, and had no financial conflicts to disclose.
Different kinds of neuropsychiatric (NP) events in patients with systemic lupus erythematosus (SLE) have substantial variability in their occurrence, resolution, and recurrence over time, as well as in their predictors, according to new research from a large, prospective, international, inception cohort study.
Because “multiple NP events due to different causes may present concurrently in individual patients, the findings emphasize the importance of recognizing attribution of NP events as a determinant of clinical outcome,” John G. Hanly, MD, of Queen Elizabeth II Health Sciences Centre and Dalhousie University, Halifax, N.S., and colleagues wrote in Arthritis & Rheumatology.
In a previous study of the same group of 1,827 patients with SLE, NP events occurred in about half and approximately one-third of these events were deemed disease related. They also “occurred most frequently around the diagnosis of SLE and had a significant negative impact on health-related quality of life,” the researchers wrote.
Researchers involved with the Systemic Lupus International Collaborating Clinics recruited the 1,827 adults with SLE over an 11-year period during 1999-2011 from a total of 31 sites in Europe, Asia, and North America. The average age of the patients at study enrollment was 35 years, 89% were women, and 49% were White. The mean disease duration was 5.6 months, and 70% of patients were taking corticosteroids at enrollment.
Over an average follow-up period of 7.6 years, 955 patients (52.3%) experienced a single neuropsychiatric event, and 493 (27.0%) experienced two or more events; the total number of unique NP events was 1,910. Most of these unique events (92%) involved the central nervous system, and 8.4% involved the peripheral nervous system.
The researchers used multistate models to attribute NP events to SLE based on factors that included the temporal onset of NP events in relation to SLE diagnosis, concurrent non-SLE factors, and NP events that are common in healthy controls. The four states in the multistate models were no NP events, no current NP event but a history of at least one event, new or ongoing NP events, and death. The results included a multivariate analysis of a model involving 492 observed transitions into new or ongoing NP events.
In the multivariate analysis, factors positively associated with SLE-attributed NP events included male sex (hazard ratio, 1.35; P = .028), concurrent non-SLE NP events excluding headache (HR, 1.83; P < .001), active SLE based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (HR, 1.19; P = .012), and corticosteroid use (HR, 1.59; P = .008). The researchers also found that SLE-attributed NP events were negatively associated with Asian race/ethnicity, postsecondary education, and use of immunosuppressive drugs.
Another multivariate analysis found that non-SLE NP events were positively associated with only concurrent SLE-attributed NP events excluding headache (HR, 2.31; P < .001), but negative associations were seen with non-U.S. African race/ethnicity and Asian race/ethnicity.
The researchers found that SLE-attributed NP events had higher rates of resolution, compared with non-SLE NP events, with the exception of headache, which had similar resolution for both event groups.
“Resolution of SLE events was more likely in patients with Asian race/ethnicity and those with Central/Focal nervous system disease with no effect seen for age at diagnosis,” the researchers noted. “For non-SLE NP events, African race/ethnicity at non-U.S. sites and younger age at diagnosis was associated with a better outcome.”
The study findings were limited by several factors including the predominantly White patient population and the clustering of NP events into limited categories, which may have reduced the identification of more specific associations, the researchers noted. Also, the assessment of NP event outcomes did not include patient perceptions, and the relatively short follow-up period does not allow for assessment of later NP events such as cerebrovascular disease. However, “despite these limitations the current study provides valuable data on the presentation, outcome and predictors of NP disease in SLE patients enrolled in a long-term, international, disease inception cohort,” the researchers concluded.
The study received no outside funding. Dr. Hanly was supported by a grant from the Canadian Institutes of Health Research but had no financial conflicts to disclose. Several coauthors received grant support from various institutions, but not from industry, and had no financial conflicts to disclose.
Different kinds of neuropsychiatric (NP) events in patients with systemic lupus erythematosus (SLE) have substantial variability in their occurrence, resolution, and recurrence over time, as well as in their predictors, according to new research from a large, prospective, international, inception cohort study.
Because “multiple NP events due to different causes may present concurrently in individual patients, the findings emphasize the importance of recognizing attribution of NP events as a determinant of clinical outcome,” John G. Hanly, MD, of Queen Elizabeth II Health Sciences Centre and Dalhousie University, Halifax, N.S., and colleagues wrote in Arthritis & Rheumatology.
In a previous study of the same group of 1,827 patients with SLE, NP events occurred in about half and approximately one-third of these events were deemed disease related. They also “occurred most frequently around the diagnosis of SLE and had a significant negative impact on health-related quality of life,” the researchers wrote.
Researchers involved with the Systemic Lupus International Collaborating Clinics recruited the 1,827 adults with SLE over an 11-year period during 1999-2011 from a total of 31 sites in Europe, Asia, and North America. The average age of the patients at study enrollment was 35 years, 89% were women, and 49% were White. The mean disease duration was 5.6 months, and 70% of patients were taking corticosteroids at enrollment.
Over an average follow-up period of 7.6 years, 955 patients (52.3%) experienced a single neuropsychiatric event, and 493 (27.0%) experienced two or more events; the total number of unique NP events was 1,910. Most of these unique events (92%) involved the central nervous system, and 8.4% involved the peripheral nervous system.
The researchers used multistate models to attribute NP events to SLE based on factors that included the temporal onset of NP events in relation to SLE diagnosis, concurrent non-SLE factors, and NP events that are common in healthy controls. The four states in the multistate models were no NP events, no current NP event but a history of at least one event, new or ongoing NP events, and death. The results included a multivariate analysis of a model involving 492 observed transitions into new or ongoing NP events.
In the multivariate analysis, factors positively associated with SLE-attributed NP events included male sex (hazard ratio, 1.35; P = .028), concurrent non-SLE NP events excluding headache (HR, 1.83; P < .001), active SLE based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (HR, 1.19; P = .012), and corticosteroid use (HR, 1.59; P = .008). The researchers also found that SLE-attributed NP events were negatively associated with Asian race/ethnicity, postsecondary education, and use of immunosuppressive drugs.
Another multivariate analysis found that non-SLE NP events were positively associated with only concurrent SLE-attributed NP events excluding headache (HR, 2.31; P < .001), but negative associations were seen with non-U.S. African race/ethnicity and Asian race/ethnicity.
The researchers found that SLE-attributed NP events had higher rates of resolution, compared with non-SLE NP events, with the exception of headache, which had similar resolution for both event groups.
“Resolution of SLE events was more likely in patients with Asian race/ethnicity and those with Central/Focal nervous system disease with no effect seen for age at diagnosis,” the researchers noted. “For non-SLE NP events, African race/ethnicity at non-U.S. sites and younger age at diagnosis was associated with a better outcome.”
The study findings were limited by several factors including the predominantly White patient population and the clustering of NP events into limited categories, which may have reduced the identification of more specific associations, the researchers noted. Also, the assessment of NP event outcomes did not include patient perceptions, and the relatively short follow-up period does not allow for assessment of later NP events such as cerebrovascular disease. However, “despite these limitations the current study provides valuable data on the presentation, outcome and predictors of NP disease in SLE patients enrolled in a long-term, international, disease inception cohort,” the researchers concluded.
The study received no outside funding. Dr. Hanly was supported by a grant from the Canadian Institutes of Health Research but had no financial conflicts to disclose. Several coauthors received grant support from various institutions, but not from industry, and had no financial conflicts to disclose.
FROM ARTHRITIS & RHEUMATOLOGY
Lupus images fall short on diverse examples
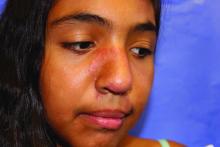
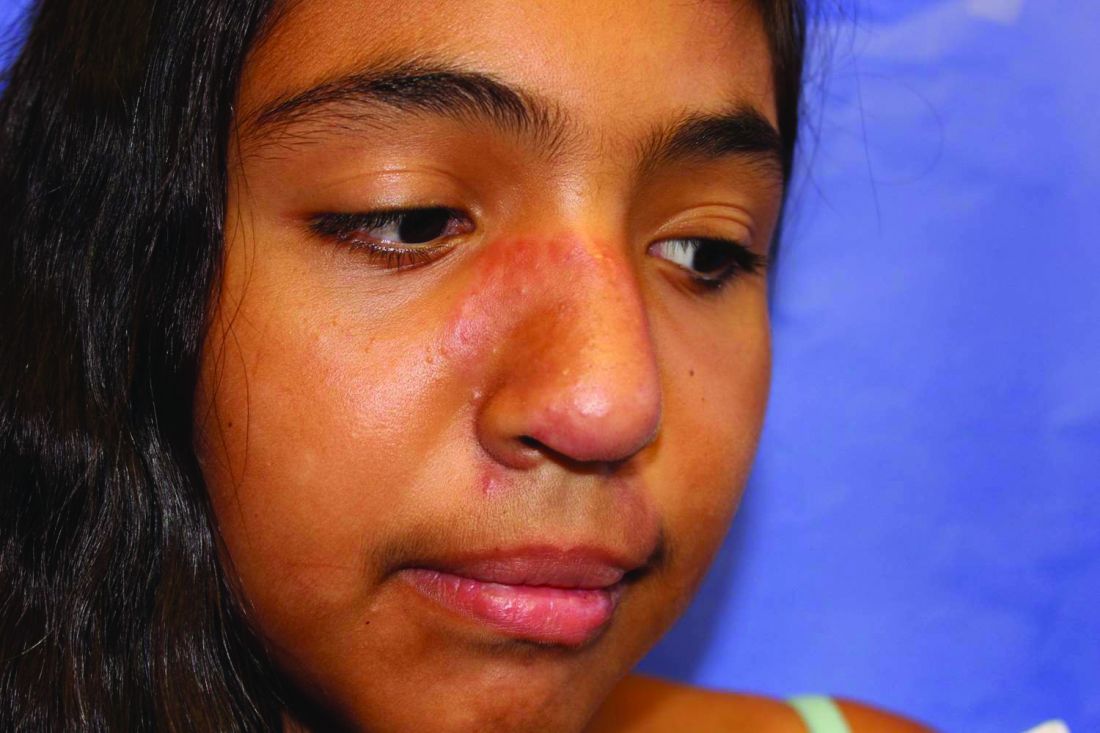
Lupus images in medical resource materials underrepresent patients with skin of color, based on data from a review of more than 1,400 images published between 2014 and 2019 in materials from a university’s online medical library.
Patients with skin of color who develop lupus tend to present earlier and with more severe cases, and often experience worse outcomes, compared with other populations, wrote Amaad Rana, MD, of Washington University, St. Louis, and colleagues. Medical resources in general have historically underrepresented patients of color, and the researchers reviewed lupus materials for a similar publication bias.
In a study published in Arthritis Care & Research, the investigators identified 1,417 images in rheumatology, dermatology, and internal medicine resources, including 119 medical textbooks, 15 medical journals, 2 online image libraries, and the online image collections of Google and UpToDate. An additional 24 images came from skin of color atlases.
Excluding the skin of color atlases, 56.4% of the images represented light skin, 35.1% showed medium skin, and 8.5% showed dark skin. Overall, publishers were more than twice as likely to portray light skin tones and were significantly less likely to portray dark skin tones (odds ratios, 2.59 and 0.19, respectively), compared with an equal representation of skin tones; however, the difference was not significant for portrayal of medium skin tones (OR, 1.08).
By specialty, dermatology was more inclusive of skin of color images than rheumatology or internal medicine, although the internal medicine sample size was too small for comparable analysis, the researchers noted. Dermatology textbooks were 2.42 times more likely and rheumatology textbooks were 4.87 times more likely to depict light skin tones than an equal representation of light, medium, and dark skin tones.
The researchers rated the skin color in the images using the New Immigrant Survey Skin Color Scale and categorized the images as representing light (NISSCS scores, 1-2), medium (NISSCS scores, 3-5), or dark skin (NISSCS scores, 6-10). Medical journals had the most images of dark skin, excluding skin of color atlases. In a comparison of specialties, dermatology materials included the most images of medium and darker skin tones.
The underrepresentation of skin of color patients can contribute to a limited knowledge of lupus presentation that could lead to disparate health outcomes, the researchers noted.
The study findings were limited by several factors, including the review of only the online textbooks and journals available through the medical library of a single university, the researchers noted. In addition, definitions of light, medium, and dark skin tones were variable among studies, and the researchers did not distinguish among lupus pathologies.
“Further research is needed to quantitatively assess the influence these materials have on healthcare providers’ ability to care for patients with lupus and SOC, and new material and strategies will be required to correct this disparity and promote equitable representation,” the researchers emphasized. “Ultimately, this will arm practitioners with the resources to competently treat patients with any skin color and work towards reducing disparities in health outcomes.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
Lupus images in medical resource materials underrepresent patients with skin of color, based on data from a review of more than 1,400 images published between 2014 and 2019 in materials from a university’s online medical library.
Patients with skin of color who develop lupus tend to present earlier and with more severe cases, and often experience worse outcomes, compared with other populations, wrote Amaad Rana, MD, of Washington University, St. Louis, and colleagues. Medical resources in general have historically underrepresented patients of color, and the researchers reviewed lupus materials for a similar publication bias.
In a study published in Arthritis Care & Research, the investigators identified 1,417 images in rheumatology, dermatology, and internal medicine resources, including 119 medical textbooks, 15 medical journals, 2 online image libraries, and the online image collections of Google and UpToDate. An additional 24 images came from skin of color atlases.
Excluding the skin of color atlases, 56.4% of the images represented light skin, 35.1% showed medium skin, and 8.5% showed dark skin. Overall, publishers were more than twice as likely to portray light skin tones and were significantly less likely to portray dark skin tones (odds ratios, 2.59 and 0.19, respectively), compared with an equal representation of skin tones; however, the difference was not significant for portrayal of medium skin tones (OR, 1.08).
By specialty, dermatology was more inclusive of skin of color images than rheumatology or internal medicine, although the internal medicine sample size was too small for comparable analysis, the researchers noted. Dermatology textbooks were 2.42 times more likely and rheumatology textbooks were 4.87 times more likely to depict light skin tones than an equal representation of light, medium, and dark skin tones.
The researchers rated the skin color in the images using the New Immigrant Survey Skin Color Scale and categorized the images as representing light (NISSCS scores, 1-2), medium (NISSCS scores, 3-5), or dark skin (NISSCS scores, 6-10). Medical journals had the most images of dark skin, excluding skin of color atlases. In a comparison of specialties, dermatology materials included the most images of medium and darker skin tones.
The underrepresentation of skin of color patients can contribute to a limited knowledge of lupus presentation that could lead to disparate health outcomes, the researchers noted.
The study findings were limited by several factors, including the review of only the online textbooks and journals available through the medical library of a single university, the researchers noted. In addition, definitions of light, medium, and dark skin tones were variable among studies, and the researchers did not distinguish among lupus pathologies.
“Further research is needed to quantitatively assess the influence these materials have on healthcare providers’ ability to care for patients with lupus and SOC, and new material and strategies will be required to correct this disparity and promote equitable representation,” the researchers emphasized. “Ultimately, this will arm practitioners with the resources to competently treat patients with any skin color and work towards reducing disparities in health outcomes.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
Lupus images in medical resource materials underrepresent patients with skin of color, based on data from a review of more than 1,400 images published between 2014 and 2019 in materials from a university’s online medical library.
Patients with skin of color who develop lupus tend to present earlier and with more severe cases, and often experience worse outcomes, compared with other populations, wrote Amaad Rana, MD, of Washington University, St. Louis, and colleagues. Medical resources in general have historically underrepresented patients of color, and the researchers reviewed lupus materials for a similar publication bias.
In a study published in Arthritis Care & Research, the investigators identified 1,417 images in rheumatology, dermatology, and internal medicine resources, including 119 medical textbooks, 15 medical journals, 2 online image libraries, and the online image collections of Google and UpToDate. An additional 24 images came from skin of color atlases.
Excluding the skin of color atlases, 56.4% of the images represented light skin, 35.1% showed medium skin, and 8.5% showed dark skin. Overall, publishers were more than twice as likely to portray light skin tones and were significantly less likely to portray dark skin tones (odds ratios, 2.59 and 0.19, respectively), compared with an equal representation of skin tones; however, the difference was not significant for portrayal of medium skin tones (OR, 1.08).
By specialty, dermatology was more inclusive of skin of color images than rheumatology or internal medicine, although the internal medicine sample size was too small for comparable analysis, the researchers noted. Dermatology textbooks were 2.42 times more likely and rheumatology textbooks were 4.87 times more likely to depict light skin tones than an equal representation of light, medium, and dark skin tones.
The researchers rated the skin color in the images using the New Immigrant Survey Skin Color Scale and categorized the images as representing light (NISSCS scores, 1-2), medium (NISSCS scores, 3-5), or dark skin (NISSCS scores, 6-10). Medical journals had the most images of dark skin, excluding skin of color atlases. In a comparison of specialties, dermatology materials included the most images of medium and darker skin tones.
The underrepresentation of skin of color patients can contribute to a limited knowledge of lupus presentation that could lead to disparate health outcomes, the researchers noted.
The study findings were limited by several factors, including the review of only the online textbooks and journals available through the medical library of a single university, the researchers noted. In addition, definitions of light, medium, and dark skin tones were variable among studies, and the researchers did not distinguish among lupus pathologies.
“Further research is needed to quantitatively assess the influence these materials have on healthcare providers’ ability to care for patients with lupus and SOC, and new material and strategies will be required to correct this disparity and promote equitable representation,” the researchers emphasized. “Ultimately, this will arm practitioners with the resources to competently treat patients with any skin color and work towards reducing disparities in health outcomes.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM ARTHRITIS CARE & RESEARCH
New biomarkers may predict interstitial lung disease progression in patients with systemic sclerosis
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Lenabasum missed mark for systemic sclerosis but may show promise for adjunctive therapy
Although a phase 3 trial of lenabasum did not meet its primary endpoint for treatment of diffuse cutaneous systemic sclerosis (dcSSc), the drug led to more improvement in participants who were not receiving background immunosuppressant therapy during the trial than that seen in participants who received the placebo. Lenabasum also had a favorable safety profile, according to findings presented at the annual European Congress of Rheumatology.
The double-blind, randomized, placebo-controlled trial involved 363 adults who had had dcSSc for up to 6 years. One third of the participants received 5 mg of oral lenabasum, one third received 20 mg, and one third received a placebo. Patients already receiving immunosuppressant therapy could continue to receive it during the trial if the dose had been stable for at least 8 weeks before screening and corticosteroid therapy did not exceed 10 mg prednisone per day or the equivalent.
“The decision to allow background immunosuppressant therapies was made to reflect real-world clinical practice,” coprincipal investigator Robert Dr. Spiera, MD, director of the Vasculitis and Scleroderma Program at the Hospital for Special Surgery, New York, told attendees.
“It is surprising that we do not see any added efficacy of lenabasum in this trial, given the fact that the previous phase 2 trial in 42 patients did show a clear benefit of lenabasum over placebo in the same population,” Jeska K. de Vries-Bouwstra, MD, PhD, a rheumatologist at Leiden (the Netherlands) University Medical Center told this news organization. “Even more, the clinical response in the phase 2 study was supported by a greater change in gene expression in skin tissue of pathways involved in inflammation and fibrosis with lenabasum as compared to placebo.”
Background immunosuppressants contribute to unprecedented placebo responses
The researchers compared the ACR CRISS (Combined Response Index in Diffuse Cutaneous Systemic Sclerosis) score and several secondary endpoints at 52 weeks between the 123 participants who received the placebo and the 120 participants who received 20 mg of lenabasum. A total of 60% of the lenabasum group and 66% of the placebo group had a disease duration of 3 or fewer years, and the modified Rodnan skin score (mRSS) was 22 in the lenabasum group and 23.3 in the placebo group at baseline.
A large majority of participants in both groups – 89% in the lenabasum group and 84% in the placebo group – were receiving background immunosuppressant therapy during the trial. Specifically, 53% of each group was taking mycophenolate, and 23% of the lenabasum group and 32% of the placebo group were taking corticosteroids. In addition, 22% of the lenabasum group and 12% of the placebo group were on methotrexate, and 27% of the lenabasum group and 22% of the placebo group were on another immunosuppressant therapy.
Half of the placebo group and 58% of the lenabasum group were taking only one immunosuppressive therapy. About one-third of the lenabasum (32%) and placebo (34%) groups were taking two or more immunosuppressive therapies.
The primary endpoint at 52 weeks was not significantly different between the two groups: a CRISS score of 0.888 in the lenabasum group and 0.887 in the placebo group. A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Patients with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
“We had very high CRISS scores in all three groups, and they were comparable in all three groups,” Dr. Spiera reported. Because improvement in placebo group far exceeded expectations, the researchers were unable to discern the treatment effect of lenabasum on top of the placebo effect.
The placebo group had better outcomes than expected because of the background immunosuppressant therapy, particularly the use of mycophenolate. When the researchers looked only at placebo participants, the CRISS score was 0.936 in the 97 patients receiving background immunosuppressant therapy of any kind and 0.935 in the 29 patients taking only mycophenolate with no other immunosuppressant therapy, compared with 0.417 in the 16 patients not receiving any background therapy.
In a prespecified analysis, the researchers investigated background immunosuppressive therapy as a mediator. The CRISS score for the 10 lenabasum participants not receiving background therapy was 0.811, compared with 0.417 seen in the placebo group patients not on background therapy.
Among the 173 participants taking mycophenolate in particular, the mycophenolate “had a statistically significant improvement on CRISS score that increased with each visit,” Dr. Spiera reported. The duration of mycophenolate therapy also affected efficacy results.
Patients who had been taking mycophenolate longer saw less improvement in their CRISS score over time. Those taking it more than 2 years at baseline had a CRISS score of 0.86, compared with 0.96 for those taking it for 1-2 years at baseline and 0.98 for those taking it from 6 months to 1 year at baseline. Those who had only been taking mycophenolate for up to 6 months at baseline had a CRISS score of 0.99. Meanwhile, patients not taking any background immunosuppressant therapies only had a CRISS score of about 0.35.
Changes in secondary endpoints followed same pattern as CRISS
The secondary endpoints similarly showed no statistically significant difference when comparing the lenabasum and placebo groups overall. These endpoints included change in mRSS score, change in forced vital capacity (FVC) percentage and volume, and change in the Health Assessment Questionnaire Disability Index (HAQ-DI) score.
However, the researchers again found that duration of background therapy affected FVC.
“You were more likely to have declined [in FVC] if you were on placebo and more likely to have improved or stayed stable if you were on lenabasum if you were a patient on more than 2 years of immunomodulatory therapy at baseline,” Dr. Spiera reported. “There was evidence for an effect of lenabasum on FVC suggested by post-hoc analyses that considered the effect of background immunosuppressive therapies on outcomes, but those results would require confirmation in additional studies to determine the potential of lenabasum for treating patients with diffuse cutaneous systemic sclerosis,” Dr. Spiera noted in his conclusions.
Serious adverse events occurred in 9.2% of the lenabasum group and 5.8% of the placebo group. Rates of severe adverse events were similar between the lenabasum (14.6%) and placebo (13%) groups.
Is there a subgroup for whom lenabasum would be efficacious?
Although De Vries-Bouwstra of Leiden University Medical Center acknowledged the role of mycophenolate in the trial, she does not think background therapy can totally explain the observation and speculated on other possibilities.
“For example, there were fewer males in the placebo group as compared to the phase 2 study. From previous cohort studies we know that males have higher risk of worsening of skin disease,” she said. “In addition, it could be worthwhile to evaluate antibody profiles of the population under study; some subpopulations defined by autoantibody have higher risk for skin progression, while others can show spontaneous improvement.”
Dr. De Vries-Bouwstra said that, although it’s not currently appropriate to advocate for lenabasum to treat dcSSc, it may eventually become an additional treatment in those who still show active skin or lung disease after 2 years of mycophenolate treatment if future research identifies a benefit from that application. She would also like to see an evaluation of lenabasum’s possible benefits in patients with very early and active inflammatory disease. “Ideally, one could stratify patients based on biomarkers reflecting activation in relevant pathways, for example by using gene expression analysis from skin tissue to stratify,” she said.
Jacob M. van Laar, MD, PhD, professor of rheumatology at University Medical Center Utrecht (the Netherlands), also commented on the potential differences in using the drug in early versus later disease.
“Based on ex vivo analyses of skin samples from systemic sclerosis patients, one would expect such a mechanism of action to be particularly relevant in very early disease, so the observation that it might also be effective at a later disease stage is interesting,” Dr. van Laar told this news organization. “We still have a lot to learn about this complex disease.”
Given that safety does not appear to be a major concern and that there may be a benefit in a subgroup of patients, Dr. van Laar also said he hoped “the company is not deterred by the seemingly negative result of the primary endpoint.”
Dr. Spiera expressed optimism about what this trial’s findings have revealed about management of dcSSc.
“Independent of what lenabasum did or didn’t do in this trial, I think there’s going to be a lot that we’re going to learn from this trial and that we’re already learning and analyzing right now about treating scleroderma,” he said in an interview.
He reiterated the value of allowing background therapy in the trial to ensure it better replicated real-world clinical practice.
“You’re not withholding therapies that we think are probably active from patients with active disease that, once you incur organ damage, is probably not going to be reversible,” Dr. Spiera said. “The downside is that it makes it harder to see an effect of a drug on top of the background therapy if that background therapy is effective. So what we saw in terms of this absence of benefit from lenabasum really may have been a ceiling effect.”
Nevertheless, Dr. Spiera said the findings still strongly suggest that lenabasum is an active compound.
“It’s not an enormously powerful effect, but it probably has a role as an adjunctive therapy in people on stable background therapy who have either plateaued or are getting worse,” he said. “The thing we have to keep in mind also is this was an incredibly safe therapy. It’s not immunosuppressive.”
The trial was funded by Corbus. Dr. Spiera has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, Chemocentryx, Corbus, Formation Biologics, Inflarx, Kadmon, AstraZeneca, AbbVie, CSL Behring, Sanofi, and Janssen. Dr. De Vries-Bouwstra has received consulting fees from AbbVie and Boehringer Ingelheim and research grants from Galapagos and Janssen. Dr. Van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Although a phase 3 trial of lenabasum did not meet its primary endpoint for treatment of diffuse cutaneous systemic sclerosis (dcSSc), the drug led to more improvement in participants who were not receiving background immunosuppressant therapy during the trial than that seen in participants who received the placebo. Lenabasum also had a favorable safety profile, according to findings presented at the annual European Congress of Rheumatology.
The double-blind, randomized, placebo-controlled trial involved 363 adults who had had dcSSc for up to 6 years. One third of the participants received 5 mg of oral lenabasum, one third received 20 mg, and one third received a placebo. Patients already receiving immunosuppressant therapy could continue to receive it during the trial if the dose had been stable for at least 8 weeks before screening and corticosteroid therapy did not exceed 10 mg prednisone per day or the equivalent.
“The decision to allow background immunosuppressant therapies was made to reflect real-world clinical practice,” coprincipal investigator Robert Dr. Spiera, MD, director of the Vasculitis and Scleroderma Program at the Hospital for Special Surgery, New York, told attendees.
“It is surprising that we do not see any added efficacy of lenabasum in this trial, given the fact that the previous phase 2 trial in 42 patients did show a clear benefit of lenabasum over placebo in the same population,” Jeska K. de Vries-Bouwstra, MD, PhD, a rheumatologist at Leiden (the Netherlands) University Medical Center told this news organization. “Even more, the clinical response in the phase 2 study was supported by a greater change in gene expression in skin tissue of pathways involved in inflammation and fibrosis with lenabasum as compared to placebo.”
Background immunosuppressants contribute to unprecedented placebo responses
The researchers compared the ACR CRISS (Combined Response Index in Diffuse Cutaneous Systemic Sclerosis) score and several secondary endpoints at 52 weeks between the 123 participants who received the placebo and the 120 participants who received 20 mg of lenabasum. A total of 60% of the lenabasum group and 66% of the placebo group had a disease duration of 3 or fewer years, and the modified Rodnan skin score (mRSS) was 22 in the lenabasum group and 23.3 in the placebo group at baseline.
A large majority of participants in both groups – 89% in the lenabasum group and 84% in the placebo group – were receiving background immunosuppressant therapy during the trial. Specifically, 53% of each group was taking mycophenolate, and 23% of the lenabasum group and 32% of the placebo group were taking corticosteroids. In addition, 22% of the lenabasum group and 12% of the placebo group were on methotrexate, and 27% of the lenabasum group and 22% of the placebo group were on another immunosuppressant therapy.
Half of the placebo group and 58% of the lenabasum group were taking only one immunosuppressive therapy. About one-third of the lenabasum (32%) and placebo (34%) groups were taking two or more immunosuppressive therapies.
The primary endpoint at 52 weeks was not significantly different between the two groups: a CRISS score of 0.888 in the lenabasum group and 0.887 in the placebo group. A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Patients with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
“We had very high CRISS scores in all three groups, and they were comparable in all three groups,” Dr. Spiera reported. Because improvement in placebo group far exceeded expectations, the researchers were unable to discern the treatment effect of lenabasum on top of the placebo effect.
The placebo group had better outcomes than expected because of the background immunosuppressant therapy, particularly the use of mycophenolate. When the researchers looked only at placebo participants, the CRISS score was 0.936 in the 97 patients receiving background immunosuppressant therapy of any kind and 0.935 in the 29 patients taking only mycophenolate with no other immunosuppressant therapy, compared with 0.417 in the 16 patients not receiving any background therapy.
In a prespecified analysis, the researchers investigated background immunosuppressive therapy as a mediator. The CRISS score for the 10 lenabasum participants not receiving background therapy was 0.811, compared with 0.417 seen in the placebo group patients not on background therapy.
Among the 173 participants taking mycophenolate in particular, the mycophenolate “had a statistically significant improvement on CRISS score that increased with each visit,” Dr. Spiera reported. The duration of mycophenolate therapy also affected efficacy results.
Patients who had been taking mycophenolate longer saw less improvement in their CRISS score over time. Those taking it more than 2 years at baseline had a CRISS score of 0.86, compared with 0.96 for those taking it for 1-2 years at baseline and 0.98 for those taking it from 6 months to 1 year at baseline. Those who had only been taking mycophenolate for up to 6 months at baseline had a CRISS score of 0.99. Meanwhile, patients not taking any background immunosuppressant therapies only had a CRISS score of about 0.35.
Changes in secondary endpoints followed same pattern as CRISS
The secondary endpoints similarly showed no statistically significant difference when comparing the lenabasum and placebo groups overall. These endpoints included change in mRSS score, change in forced vital capacity (FVC) percentage and volume, and change in the Health Assessment Questionnaire Disability Index (HAQ-DI) score.
However, the researchers again found that duration of background therapy affected FVC.
“You were more likely to have declined [in FVC] if you were on placebo and more likely to have improved or stayed stable if you were on lenabasum if you were a patient on more than 2 years of immunomodulatory therapy at baseline,” Dr. Spiera reported. “There was evidence for an effect of lenabasum on FVC suggested by post-hoc analyses that considered the effect of background immunosuppressive therapies on outcomes, but those results would require confirmation in additional studies to determine the potential of lenabasum for treating patients with diffuse cutaneous systemic sclerosis,” Dr. Spiera noted in his conclusions.
Serious adverse events occurred in 9.2% of the lenabasum group and 5.8% of the placebo group. Rates of severe adverse events were similar between the lenabasum (14.6%) and placebo (13%) groups.
Is there a subgroup for whom lenabasum would be efficacious?
Although De Vries-Bouwstra of Leiden University Medical Center acknowledged the role of mycophenolate in the trial, she does not think background therapy can totally explain the observation and speculated on other possibilities.
“For example, there were fewer males in the placebo group as compared to the phase 2 study. From previous cohort studies we know that males have higher risk of worsening of skin disease,” she said. “In addition, it could be worthwhile to evaluate antibody profiles of the population under study; some subpopulations defined by autoantibody have higher risk for skin progression, while others can show spontaneous improvement.”
Dr. De Vries-Bouwstra said that, although it’s not currently appropriate to advocate for lenabasum to treat dcSSc, it may eventually become an additional treatment in those who still show active skin or lung disease after 2 years of mycophenolate treatment if future research identifies a benefit from that application. She would also like to see an evaluation of lenabasum’s possible benefits in patients with very early and active inflammatory disease. “Ideally, one could stratify patients based on biomarkers reflecting activation in relevant pathways, for example by using gene expression analysis from skin tissue to stratify,” she said.
Jacob M. van Laar, MD, PhD, professor of rheumatology at University Medical Center Utrecht (the Netherlands), also commented on the potential differences in using the drug in early versus later disease.
“Based on ex vivo analyses of skin samples from systemic sclerosis patients, one would expect such a mechanism of action to be particularly relevant in very early disease, so the observation that it might also be effective at a later disease stage is interesting,” Dr. van Laar told this news organization. “We still have a lot to learn about this complex disease.”
Given that safety does not appear to be a major concern and that there may be a benefit in a subgroup of patients, Dr. van Laar also said he hoped “the company is not deterred by the seemingly negative result of the primary endpoint.”
Dr. Spiera expressed optimism about what this trial’s findings have revealed about management of dcSSc.
“Independent of what lenabasum did or didn’t do in this trial, I think there’s going to be a lot that we’re going to learn from this trial and that we’re already learning and analyzing right now about treating scleroderma,” he said in an interview.
He reiterated the value of allowing background therapy in the trial to ensure it better replicated real-world clinical practice.
“You’re not withholding therapies that we think are probably active from patients with active disease that, once you incur organ damage, is probably not going to be reversible,” Dr. Spiera said. “The downside is that it makes it harder to see an effect of a drug on top of the background therapy if that background therapy is effective. So what we saw in terms of this absence of benefit from lenabasum really may have been a ceiling effect.”
Nevertheless, Dr. Spiera said the findings still strongly suggest that lenabasum is an active compound.
“It’s not an enormously powerful effect, but it probably has a role as an adjunctive therapy in people on stable background therapy who have either plateaued or are getting worse,” he said. “The thing we have to keep in mind also is this was an incredibly safe therapy. It’s not immunosuppressive.”
The trial was funded by Corbus. Dr. Spiera has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, Chemocentryx, Corbus, Formation Biologics, Inflarx, Kadmon, AstraZeneca, AbbVie, CSL Behring, Sanofi, and Janssen. Dr. De Vries-Bouwstra has received consulting fees from AbbVie and Boehringer Ingelheim and research grants from Galapagos and Janssen. Dr. Van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Although a phase 3 trial of lenabasum did not meet its primary endpoint for treatment of diffuse cutaneous systemic sclerosis (dcSSc), the drug led to more improvement in participants who were not receiving background immunosuppressant therapy during the trial than that seen in participants who received the placebo. Lenabasum also had a favorable safety profile, according to findings presented at the annual European Congress of Rheumatology.
The double-blind, randomized, placebo-controlled trial involved 363 adults who had had dcSSc for up to 6 years. One third of the participants received 5 mg of oral lenabasum, one third received 20 mg, and one third received a placebo. Patients already receiving immunosuppressant therapy could continue to receive it during the trial if the dose had been stable for at least 8 weeks before screening and corticosteroid therapy did not exceed 10 mg prednisone per day or the equivalent.
“The decision to allow background immunosuppressant therapies was made to reflect real-world clinical practice,” coprincipal investigator Robert Dr. Spiera, MD, director of the Vasculitis and Scleroderma Program at the Hospital for Special Surgery, New York, told attendees.
“It is surprising that we do not see any added efficacy of lenabasum in this trial, given the fact that the previous phase 2 trial in 42 patients did show a clear benefit of lenabasum over placebo in the same population,” Jeska K. de Vries-Bouwstra, MD, PhD, a rheumatologist at Leiden (the Netherlands) University Medical Center told this news organization. “Even more, the clinical response in the phase 2 study was supported by a greater change in gene expression in skin tissue of pathways involved in inflammation and fibrosis with lenabasum as compared to placebo.”
Background immunosuppressants contribute to unprecedented placebo responses
The researchers compared the ACR CRISS (Combined Response Index in Diffuse Cutaneous Systemic Sclerosis) score and several secondary endpoints at 52 weeks between the 123 participants who received the placebo and the 120 participants who received 20 mg of lenabasum. A total of 60% of the lenabasum group and 66% of the placebo group had a disease duration of 3 or fewer years, and the modified Rodnan skin score (mRSS) was 22 in the lenabasum group and 23.3 in the placebo group at baseline.
A large majority of participants in both groups – 89% in the lenabasum group and 84% in the placebo group – were receiving background immunosuppressant therapy during the trial. Specifically, 53% of each group was taking mycophenolate, and 23% of the lenabasum group and 32% of the placebo group were taking corticosteroids. In addition, 22% of the lenabasum group and 12% of the placebo group were on methotrexate, and 27% of the lenabasum group and 22% of the placebo group were on another immunosuppressant therapy.
Half of the placebo group and 58% of the lenabasum group were taking only one immunosuppressive therapy. About one-third of the lenabasum (32%) and placebo (34%) groups were taking two or more immunosuppressive therapies.
The primary endpoint at 52 weeks was not significantly different between the two groups: a CRISS score of 0.888 in the lenabasum group and 0.887 in the placebo group. A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Patients with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
“We had very high CRISS scores in all three groups, and they were comparable in all three groups,” Dr. Spiera reported. Because improvement in placebo group far exceeded expectations, the researchers were unable to discern the treatment effect of lenabasum on top of the placebo effect.
The placebo group had better outcomes than expected because of the background immunosuppressant therapy, particularly the use of mycophenolate. When the researchers looked only at placebo participants, the CRISS score was 0.936 in the 97 patients receiving background immunosuppressant therapy of any kind and 0.935 in the 29 patients taking only mycophenolate with no other immunosuppressant therapy, compared with 0.417 in the 16 patients not receiving any background therapy.
In a prespecified analysis, the researchers investigated background immunosuppressive therapy as a mediator. The CRISS score for the 10 lenabasum participants not receiving background therapy was 0.811, compared with 0.417 seen in the placebo group patients not on background therapy.
Among the 173 participants taking mycophenolate in particular, the mycophenolate “had a statistically significant improvement on CRISS score that increased with each visit,” Dr. Spiera reported. The duration of mycophenolate therapy also affected efficacy results.
Patients who had been taking mycophenolate longer saw less improvement in their CRISS score over time. Those taking it more than 2 years at baseline had a CRISS score of 0.86, compared with 0.96 for those taking it for 1-2 years at baseline and 0.98 for those taking it from 6 months to 1 year at baseline. Those who had only been taking mycophenolate for up to 6 months at baseline had a CRISS score of 0.99. Meanwhile, patients not taking any background immunosuppressant therapies only had a CRISS score of about 0.35.
Changes in secondary endpoints followed same pattern as CRISS
The secondary endpoints similarly showed no statistically significant difference when comparing the lenabasum and placebo groups overall. These endpoints included change in mRSS score, change in forced vital capacity (FVC) percentage and volume, and change in the Health Assessment Questionnaire Disability Index (HAQ-DI) score.
However, the researchers again found that duration of background therapy affected FVC.
“You were more likely to have declined [in FVC] if you were on placebo and more likely to have improved or stayed stable if you were on lenabasum if you were a patient on more than 2 years of immunomodulatory therapy at baseline,” Dr. Spiera reported. “There was evidence for an effect of lenabasum on FVC suggested by post-hoc analyses that considered the effect of background immunosuppressive therapies on outcomes, but those results would require confirmation in additional studies to determine the potential of lenabasum for treating patients with diffuse cutaneous systemic sclerosis,” Dr. Spiera noted in his conclusions.
Serious adverse events occurred in 9.2% of the lenabasum group and 5.8% of the placebo group. Rates of severe adverse events were similar between the lenabasum (14.6%) and placebo (13%) groups.
Is there a subgroup for whom lenabasum would be efficacious?
Although De Vries-Bouwstra of Leiden University Medical Center acknowledged the role of mycophenolate in the trial, she does not think background therapy can totally explain the observation and speculated on other possibilities.
“For example, there were fewer males in the placebo group as compared to the phase 2 study. From previous cohort studies we know that males have higher risk of worsening of skin disease,” she said. “In addition, it could be worthwhile to evaluate antibody profiles of the population under study; some subpopulations defined by autoantibody have higher risk for skin progression, while others can show spontaneous improvement.”
Dr. De Vries-Bouwstra said that, although it’s not currently appropriate to advocate for lenabasum to treat dcSSc, it may eventually become an additional treatment in those who still show active skin or lung disease after 2 years of mycophenolate treatment if future research identifies a benefit from that application. She would also like to see an evaluation of lenabasum’s possible benefits in patients with very early and active inflammatory disease. “Ideally, one could stratify patients based on biomarkers reflecting activation in relevant pathways, for example by using gene expression analysis from skin tissue to stratify,” she said.
Jacob M. van Laar, MD, PhD, professor of rheumatology at University Medical Center Utrecht (the Netherlands), also commented on the potential differences in using the drug in early versus later disease.
“Based on ex vivo analyses of skin samples from systemic sclerosis patients, one would expect such a mechanism of action to be particularly relevant in very early disease, so the observation that it might also be effective at a later disease stage is interesting,” Dr. van Laar told this news organization. “We still have a lot to learn about this complex disease.”
Given that safety does not appear to be a major concern and that there may be a benefit in a subgroup of patients, Dr. van Laar also said he hoped “the company is not deterred by the seemingly negative result of the primary endpoint.”
Dr. Spiera expressed optimism about what this trial’s findings have revealed about management of dcSSc.
“Independent of what lenabasum did or didn’t do in this trial, I think there’s going to be a lot that we’re going to learn from this trial and that we’re already learning and analyzing right now about treating scleroderma,” he said in an interview.
He reiterated the value of allowing background therapy in the trial to ensure it better replicated real-world clinical practice.
“You’re not withholding therapies that we think are probably active from patients with active disease that, once you incur organ damage, is probably not going to be reversible,” Dr. Spiera said. “The downside is that it makes it harder to see an effect of a drug on top of the background therapy if that background therapy is effective. So what we saw in terms of this absence of benefit from lenabasum really may have been a ceiling effect.”
Nevertheless, Dr. Spiera said the findings still strongly suggest that lenabasum is an active compound.
“It’s not an enormously powerful effect, but it probably has a role as an adjunctive therapy in people on stable background therapy who have either plateaued or are getting worse,” he said. “The thing we have to keep in mind also is this was an incredibly safe therapy. It’s not immunosuppressive.”
The trial was funded by Corbus. Dr. Spiera has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, Chemocentryx, Corbus, Formation Biologics, Inflarx, Kadmon, AstraZeneca, AbbVie, CSL Behring, Sanofi, and Janssen. Dr. De Vries-Bouwstra has received consulting fees from AbbVie and Boehringer Ingelheim and research grants from Galapagos and Janssen. Dr. Van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
BEAT-LUPUS: Belimumab after rituximab delays severe flares
Using belimumab after rituximab to treat patients with systemic lupus erythematosus (SLE) refractory to conventional therapy not only significantly decreased levels of serum IgG anti-dsDNA antibody levels but also prolonged the time before severe flares of disease occurred in the phase 2b BEAT-LUPUS (Belimumab after B cell depletion in SLE) study.
The trial’s primary outcome of serum IgG anti-dsDNA antibody levels showed a decline from a geometric mean of 162 IU/mL at baseline to 69 IU/mL at 24 weeks and 47 IU/mL at 1 year in patients treated with belimumab (Benlysta) after rituximab (Rituxan and biosimilars). These reductions were significantly lower than the values seen in the placebo after rituximab arm (a respective 121 IU/mL, 99 IU/mL, and 103 IU/mL; P < .001).
Just 3 patients who received belimumab versus 10 who received placebo after rituximab experienced a severe BILAG (British Isles Lupus Assessment Group) index A flare by the end of the study at 52 weeks. The hazard ratio (HR) for the flare reduction was 0.27 (P = .03), indicating a 73% reduction.
The use of belimumab rather than a placebo also led to a small reduction in total serum IgG, and significantly suppressed B-cell repopulation (P = .03).
These results need confirming in a larger, phase 3 trial, the trial’s principal investigator, Michael Ehrenstein, PhD, said at the annual European Congress of Rheumatology. They are “clearly encouraging” and “support the hypothesis that BAFF [B-cell–activating factor] can drive flares after rituximab,” he said.
Although B-cell depletion with rituximab is recommended by national and international guidelines to treat some patients with SLE who are refractory to conventional therapy, its use is not licensed.
“Certainly, rituximab is a controversial drug in lupus,” Dr. Ehrenstein, a consultant rheumatologist based at University College London, said in an interview. Although there is real-world evidence from registries and open-label studies suggesting that it is widely used and effective in some patients, the randomized, controlled trials conducted with rituximab about 10 years ago failed to meet their primary endpoints.
“A lot has been written about why that was, but probably the biggest reason was the high dose of steroids in both groups,” Dr. Ehrenstein said. To try to avoid muddying the waters of the BEAT-LUPUS trial findings, the maximum dose of prednisolone allowed to be used as background therapy was 20 mg/day. The trial’s investigators were also encouraged to reduce the baseline steroid dose to at least 50% by the trial’s 6-month halfway point.
“We tried to reflect what was going on in the U.K.,” Dr. Ehrenstein said, noting that the inspiration for the trial was a patient who had received sequential rituximab treatment. Although she got better with each cycle of rituximab, when her disease flared it would be worse than the time before, with increasingly higher anti-dsDNA levels recorded. The reason for this seemed to be because of increasing BAFF levels, and so the hypothesis was that if rituximab was associated with increased BAFF levels, then co-targeting BAFF with belimumab should be able to prevent those flares from happening.
The BEAT-LUPUS trial has been a huge collaborative effort and was conducted across 16 U.K. centers. From initial funding to the data analysis, it has taken 6 years to complete and was made possible by a unique partnership between Versus Arthritis, University College London Hospitals Biomedical Research Center, the National Institute for Health Research UK Musculoskeletal Translational Research Collaboration, and GlaxoSmithKline (GSK). GSK provided belimumab free of charge, as well as additional funding, but had no role in the design of the study and will not have any role going forward.
From an initial 172 patients assessed for eligibility, 52 patients were finally enrolled into the trial and received rituximab as two infusions given 2 weeks apart. Patients were then randomized in a double-blind manner to receive either belimumab (n = 26) or placebo (n = 26) 4-8 weeks after their first dose of rituximab. The intention-to-treat population consisted of 43 patients.
The use of belimumab after rituximab did not increase the risk for infection – serious or otherwise – or adverse effects, Dr. Ehrenstein reported. Serious adverse events were reported in six (23%) patients in each arm, and serious infections were seen in two (8%) of the belimumab- and four (15%) of the placebo-treated patients.
“I think the take-home message is that it seems that belimumab can reduce the number of severe flares that occur after rituximab therapy,” Dr. Ehrenstein said. “It’s promising, but not definitive,” he added. The next step is of course to publish these data and to perform a phase 3 trial.
In the discussion time following the presentation, session moderator Xavier Mariette, MD, PhD, of Bicêtre Hospital, Paris-Saclay University, asked why not give belimumab first before rituximab if using belimumab afterward works?
“Our strategy was driven by the observation that BAFF levels surged after rituximab, and therefore it’s logical to give the belimumab to block that BAFF surge,” he answered.
“Certainly, there are ideas that belimumab releases mature B cells into the circulation and rituximab can target that,” he added. That strategy is under investigation in the BLISS-BELIEVE trial, which should also report by the end of this year. It’s a much larger, phase 3 trial, involving nearly 300 patients and is sponsored by GSK.
“Clearly, this is a combination treatment [but] whether you give one before the other is uncertain,” Dr. Ehrenstein observed.
Another member of the viewing audience asked whether it would have been a fairer comparison if another dose of rituximab had been given to patients at week 24 instead of no treatment. Dr. Ehrenstein noted that it was a “good point” to make, but the investigators mainly wanted to answer whether giving belimumab after rituximab would target BAFF and thereby drop serum anti-dsDNA antibody levels. He said that a full trial of rituximab for patients with SLE, perhaps adding this extra dose, needs to be conducted.
Dr. Ehrenstein disclosed receiving research funding and educational grants from GSK and participating in advisory panels for the company.
Using belimumab after rituximab to treat patients with systemic lupus erythematosus (SLE) refractory to conventional therapy not only significantly decreased levels of serum IgG anti-dsDNA antibody levels but also prolonged the time before severe flares of disease occurred in the phase 2b BEAT-LUPUS (Belimumab after B cell depletion in SLE) study.
The trial’s primary outcome of serum IgG anti-dsDNA antibody levels showed a decline from a geometric mean of 162 IU/mL at baseline to 69 IU/mL at 24 weeks and 47 IU/mL at 1 year in patients treated with belimumab (Benlysta) after rituximab (Rituxan and biosimilars). These reductions were significantly lower than the values seen in the placebo after rituximab arm (a respective 121 IU/mL, 99 IU/mL, and 103 IU/mL; P < .001).
Just 3 patients who received belimumab versus 10 who received placebo after rituximab experienced a severe BILAG (British Isles Lupus Assessment Group) index A flare by the end of the study at 52 weeks. The hazard ratio (HR) for the flare reduction was 0.27 (P = .03), indicating a 73% reduction.
The use of belimumab rather than a placebo also led to a small reduction in total serum IgG, and significantly suppressed B-cell repopulation (P = .03).
These results need confirming in a larger, phase 3 trial, the trial’s principal investigator, Michael Ehrenstein, PhD, said at the annual European Congress of Rheumatology. They are “clearly encouraging” and “support the hypothesis that BAFF [B-cell–activating factor] can drive flares after rituximab,” he said.
Although B-cell depletion with rituximab is recommended by national and international guidelines to treat some patients with SLE who are refractory to conventional therapy, its use is not licensed.
“Certainly, rituximab is a controversial drug in lupus,” Dr. Ehrenstein, a consultant rheumatologist based at University College London, said in an interview. Although there is real-world evidence from registries and open-label studies suggesting that it is widely used and effective in some patients, the randomized, controlled trials conducted with rituximab about 10 years ago failed to meet their primary endpoints.
“A lot has been written about why that was, but probably the biggest reason was the high dose of steroids in both groups,” Dr. Ehrenstein said. To try to avoid muddying the waters of the BEAT-LUPUS trial findings, the maximum dose of prednisolone allowed to be used as background therapy was 20 mg/day. The trial’s investigators were also encouraged to reduce the baseline steroid dose to at least 50% by the trial’s 6-month halfway point.
“We tried to reflect what was going on in the U.K.,” Dr. Ehrenstein said, noting that the inspiration for the trial was a patient who had received sequential rituximab treatment. Although she got better with each cycle of rituximab, when her disease flared it would be worse than the time before, with increasingly higher anti-dsDNA levels recorded. The reason for this seemed to be because of increasing BAFF levels, and so the hypothesis was that if rituximab was associated with increased BAFF levels, then co-targeting BAFF with belimumab should be able to prevent those flares from happening.
The BEAT-LUPUS trial has been a huge collaborative effort and was conducted across 16 U.K. centers. From initial funding to the data analysis, it has taken 6 years to complete and was made possible by a unique partnership between Versus Arthritis, University College London Hospitals Biomedical Research Center, the National Institute for Health Research UK Musculoskeletal Translational Research Collaboration, and GlaxoSmithKline (GSK). GSK provided belimumab free of charge, as well as additional funding, but had no role in the design of the study and will not have any role going forward.
From an initial 172 patients assessed for eligibility, 52 patients were finally enrolled into the trial and received rituximab as two infusions given 2 weeks apart. Patients were then randomized in a double-blind manner to receive either belimumab (n = 26) or placebo (n = 26) 4-8 weeks after their first dose of rituximab. The intention-to-treat population consisted of 43 patients.
The use of belimumab after rituximab did not increase the risk for infection – serious or otherwise – or adverse effects, Dr. Ehrenstein reported. Serious adverse events were reported in six (23%) patients in each arm, and serious infections were seen in two (8%) of the belimumab- and four (15%) of the placebo-treated patients.
“I think the take-home message is that it seems that belimumab can reduce the number of severe flares that occur after rituximab therapy,” Dr. Ehrenstein said. “It’s promising, but not definitive,” he added. The next step is of course to publish these data and to perform a phase 3 trial.
In the discussion time following the presentation, session moderator Xavier Mariette, MD, PhD, of Bicêtre Hospital, Paris-Saclay University, asked why not give belimumab first before rituximab if using belimumab afterward works?
“Our strategy was driven by the observation that BAFF levels surged after rituximab, and therefore it’s logical to give the belimumab to block that BAFF surge,” he answered.
“Certainly, there are ideas that belimumab releases mature B cells into the circulation and rituximab can target that,” he added. That strategy is under investigation in the BLISS-BELIEVE trial, which should also report by the end of this year. It’s a much larger, phase 3 trial, involving nearly 300 patients and is sponsored by GSK.
“Clearly, this is a combination treatment [but] whether you give one before the other is uncertain,” Dr. Ehrenstein observed.
Another member of the viewing audience asked whether it would have been a fairer comparison if another dose of rituximab had been given to patients at week 24 instead of no treatment. Dr. Ehrenstein noted that it was a “good point” to make, but the investigators mainly wanted to answer whether giving belimumab after rituximab would target BAFF and thereby drop serum anti-dsDNA antibody levels. He said that a full trial of rituximab for patients with SLE, perhaps adding this extra dose, needs to be conducted.
Dr. Ehrenstein disclosed receiving research funding and educational grants from GSK and participating in advisory panels for the company.
Using belimumab after rituximab to treat patients with systemic lupus erythematosus (SLE) refractory to conventional therapy not only significantly decreased levels of serum IgG anti-dsDNA antibody levels but also prolonged the time before severe flares of disease occurred in the phase 2b BEAT-LUPUS (Belimumab after B cell depletion in SLE) study.
The trial’s primary outcome of serum IgG anti-dsDNA antibody levels showed a decline from a geometric mean of 162 IU/mL at baseline to 69 IU/mL at 24 weeks and 47 IU/mL at 1 year in patients treated with belimumab (Benlysta) after rituximab (Rituxan and biosimilars). These reductions were significantly lower than the values seen in the placebo after rituximab arm (a respective 121 IU/mL, 99 IU/mL, and 103 IU/mL; P < .001).
Just 3 patients who received belimumab versus 10 who received placebo after rituximab experienced a severe BILAG (British Isles Lupus Assessment Group) index A flare by the end of the study at 52 weeks. The hazard ratio (HR) for the flare reduction was 0.27 (P = .03), indicating a 73% reduction.
The use of belimumab rather than a placebo also led to a small reduction in total serum IgG, and significantly suppressed B-cell repopulation (P = .03).
These results need confirming in a larger, phase 3 trial, the trial’s principal investigator, Michael Ehrenstein, PhD, said at the annual European Congress of Rheumatology. They are “clearly encouraging” and “support the hypothesis that BAFF [B-cell–activating factor] can drive flares after rituximab,” he said.
Although B-cell depletion with rituximab is recommended by national and international guidelines to treat some patients with SLE who are refractory to conventional therapy, its use is not licensed.
“Certainly, rituximab is a controversial drug in lupus,” Dr. Ehrenstein, a consultant rheumatologist based at University College London, said in an interview. Although there is real-world evidence from registries and open-label studies suggesting that it is widely used and effective in some patients, the randomized, controlled trials conducted with rituximab about 10 years ago failed to meet their primary endpoints.
“A lot has been written about why that was, but probably the biggest reason was the high dose of steroids in both groups,” Dr. Ehrenstein said. To try to avoid muddying the waters of the BEAT-LUPUS trial findings, the maximum dose of prednisolone allowed to be used as background therapy was 20 mg/day. The trial’s investigators were also encouraged to reduce the baseline steroid dose to at least 50% by the trial’s 6-month halfway point.
“We tried to reflect what was going on in the U.K.,” Dr. Ehrenstein said, noting that the inspiration for the trial was a patient who had received sequential rituximab treatment. Although she got better with each cycle of rituximab, when her disease flared it would be worse than the time before, with increasingly higher anti-dsDNA levels recorded. The reason for this seemed to be because of increasing BAFF levels, and so the hypothesis was that if rituximab was associated with increased BAFF levels, then co-targeting BAFF with belimumab should be able to prevent those flares from happening.
The BEAT-LUPUS trial has been a huge collaborative effort and was conducted across 16 U.K. centers. From initial funding to the data analysis, it has taken 6 years to complete and was made possible by a unique partnership between Versus Arthritis, University College London Hospitals Biomedical Research Center, the National Institute for Health Research UK Musculoskeletal Translational Research Collaboration, and GlaxoSmithKline (GSK). GSK provided belimumab free of charge, as well as additional funding, but had no role in the design of the study and will not have any role going forward.
From an initial 172 patients assessed for eligibility, 52 patients were finally enrolled into the trial and received rituximab as two infusions given 2 weeks apart. Patients were then randomized in a double-blind manner to receive either belimumab (n = 26) or placebo (n = 26) 4-8 weeks after their first dose of rituximab. The intention-to-treat population consisted of 43 patients.
The use of belimumab after rituximab did not increase the risk for infection – serious or otherwise – or adverse effects, Dr. Ehrenstein reported. Serious adverse events were reported in six (23%) patients in each arm, and serious infections were seen in two (8%) of the belimumab- and four (15%) of the placebo-treated patients.
“I think the take-home message is that it seems that belimumab can reduce the number of severe flares that occur after rituximab therapy,” Dr. Ehrenstein said. “It’s promising, but not definitive,” he added. The next step is of course to publish these data and to perform a phase 3 trial.
In the discussion time following the presentation, session moderator Xavier Mariette, MD, PhD, of Bicêtre Hospital, Paris-Saclay University, asked why not give belimumab first before rituximab if using belimumab afterward works?
“Our strategy was driven by the observation that BAFF levels surged after rituximab, and therefore it’s logical to give the belimumab to block that BAFF surge,” he answered.
“Certainly, there are ideas that belimumab releases mature B cells into the circulation and rituximab can target that,” he added. That strategy is under investigation in the BLISS-BELIEVE trial, which should also report by the end of this year. It’s a much larger, phase 3 trial, involving nearly 300 patients and is sponsored by GSK.
“Clearly, this is a combination treatment [but] whether you give one before the other is uncertain,” Dr. Ehrenstein observed.
Another member of the viewing audience asked whether it would have been a fairer comparison if another dose of rituximab had been given to patients at week 24 instead of no treatment. Dr. Ehrenstein noted that it was a “good point” to make, but the investigators mainly wanted to answer whether giving belimumab after rituximab would target BAFF and thereby drop serum anti-dsDNA antibody levels. He said that a full trial of rituximab for patients with SLE, perhaps adding this extra dose, needs to be conducted.
Dr. Ehrenstein disclosed receiving research funding and educational grants from GSK and participating in advisory panels for the company.
FROM THE EULAR 2021 CONGRESS
Intravenous immunoglobulin controls dermatomyositis in phase 3 trial
Nearly 50% achieve moderate improvement or better
The first multinational, phase 3, placebo-controlled trial conducted with intravenous immunoglobulin therapy (IVIg) for dermatomyositis has confirmed significant efficacy and acceptable safety, according to data presented at the opening plenary abstract session of the annual European Congress of Rheumatology.
At the week 16 evaluation of the trial, called ProDERM, the response rates were 78.7% and 43.8% (P = .0008) for active therapy and placebo, respectively, reported Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh.
ProDERM is a “much-awaited study,” according to session moderator Hendrik Schulze-Koops, MD, PhD, of the division of rheumatology and clinical immunology at Ludwig Maximilian University of Munich (Germany). He was not involved in the study.
“We all have been doing what we have been doing,” Dr. Schulze-Koops said, referring to the use of IVIg for the control of dermatomyositis, “but we had no evidence for support.”
This statement could apply not only to IVIg, which has long been listed among treatment options by the Myositis Association despite the absence of controlled studies, but also to most immunosuppressive therapies and other options used for this challenging disease.
The proprietary IVIg employed in this study, Octagam 10%, has been approved in the United States for the treatment of chronic immune thrombocytopenic purpura. Its manufacturer, Octagam, plans to file a supplemental new drug application with the Food and Drug Administration for the treatment of dermatomyositis. The agent is already approved for dermatomyositis by the European Medicines Agency, according to Dr. Aggarwal.
Multiple response criteria favor IVIg
In the trial, 95 patients with dermatomyositis were randomized to 2 g/kg of IVIg (Octagam 10%) or placebo administered every 4 weeks. In a subsequent open-label extension study in which patients on placebo were switched to active therapy, the same every-4-week treatment schedule was used. The patients’ mean age was 53; 75% were women, and 92% were White.
The primary endpoint was at least minimal improvement on 2016 ACR/EULAR (American College of Rheumatology/European Alliance of Associations for Rheumatology) myositis response criteria, defined as a 20-point or greater gain in the Total Improvement Score (TIS) and no clinical worsening at two consecutive visits. But IVIg also provided a large relative benefit over placebo using more rigorous definitions of improvement. For moderate improvement, defined as at least a 40-point TIS improvement, there was a 45.2% relative advantage for IVIg over placebo (68.1% vs. 22.9%; P < .0001). For major improvement, defined as at least a 60-point TIS improvement, the relative advantage was 23.6% (31.9% vs. 8.3%; P < .0062).
At 16 weeks, the mean TIS score was more than twice as high in those receiving IVIg than in those randomized to placebo (48.4 vs. 21.6). At that point, an open-label extension was initiated. Those in the IVIg group were permitted to remain on therapy for an additional 24 weeks if they had not worsened in the blinded phase.
The mean TIS score in the IVIg group continued to rise during the extension phase. By 12 weeks in this phase, it reached 54.0. Over the same period, mean TIS scores climbed steeply among the placebo-treated patients who had switched to active therapy, reaching 44.4.
At the end of 24 weeks of the extension trial, when patients initiated on IVIg had been on active therapy for 40 weeks, the mean TIS score advantage of starting on IVIg rather than placebo was relatively modest (55.4 vs. 51.1).
Benefit is significant for skin and muscle
Changes in the two major components of dermatomyositis were tracked individually. For skin symptoms, patients were evaluated with the Cutaneous Dermatomyositis Disease Areas and Severity Index (CDASI). For muscle involvement, symptoms were evaluated with the 8-item Manual Muscle Testing (MMT-8) tool.
“The effects of IVIg on the muscle and the skin were both highly statistically significant,” Dr. Aggarwal reported. He said the CDASI score was reduced by almost half at the end of 16 weeks among those treated with IVIg relative to those treated with placebo. Improvement in MMT-8 scores were also clinically as well as statistically significant.
The IVIg therapy was well tolerated. The most common adverse effects in this study, like those reported with IVIg when used to treat other diseases, were headache, pyrexia, and nausea, but Dr. Aggarwal reported that these were generally mild.
Serious adverse events, particularly thromboembolism, did occur over the course of the study, but the rate of events was only slightly higher in the group receiving active therapy (5.8% vs. 4.2%).
Patients who entered the study were permitted to remain on most immunosuppressive therapies, such as methotrexate, mycophenolate, tacrolimus, and glucocorticoids. Dr. Aggarwal said that the majority of patients were taking a glucocorticoid and at least one nonglucocorticoid immunosuppressant.
Effect on associated conditions is planned
The data from this trial have not yet been analyzed for the impact of IVIg on conditions that occur frequently in association with dermatomyositis, such as interstitial lung disease (ILD) and dysphagia, but Dr. Aggarwal reported that there are plans to do so. Although severe ILD was a trial exclusion, the presence of mild to moderate ILD and dysphagia were evaluated at baseline, so the impact of treatment can be assessed.
There are also plans to evaluate how the presence or absence of myositis-specific antibodies, which were also evaluated at baseline, affected response to IVIg.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Dr. Schulze-Koops reported no relevant potential conflicts of interest.
Nearly 50% achieve moderate improvement or better
Nearly 50% achieve moderate improvement or better
The first multinational, phase 3, placebo-controlled trial conducted with intravenous immunoglobulin therapy (IVIg) for dermatomyositis has confirmed significant efficacy and acceptable safety, according to data presented at the opening plenary abstract session of the annual European Congress of Rheumatology.
At the week 16 evaluation of the trial, called ProDERM, the response rates were 78.7% and 43.8% (P = .0008) for active therapy and placebo, respectively, reported Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh.
ProDERM is a “much-awaited study,” according to session moderator Hendrik Schulze-Koops, MD, PhD, of the division of rheumatology and clinical immunology at Ludwig Maximilian University of Munich (Germany). He was not involved in the study.
“We all have been doing what we have been doing,” Dr. Schulze-Koops said, referring to the use of IVIg for the control of dermatomyositis, “but we had no evidence for support.”
This statement could apply not only to IVIg, which has long been listed among treatment options by the Myositis Association despite the absence of controlled studies, but also to most immunosuppressive therapies and other options used for this challenging disease.
The proprietary IVIg employed in this study, Octagam 10%, has been approved in the United States for the treatment of chronic immune thrombocytopenic purpura. Its manufacturer, Octagam, plans to file a supplemental new drug application with the Food and Drug Administration for the treatment of dermatomyositis. The agent is already approved for dermatomyositis by the European Medicines Agency, according to Dr. Aggarwal.
Multiple response criteria favor IVIg
In the trial, 95 patients with dermatomyositis were randomized to 2 g/kg of IVIg (Octagam 10%) or placebo administered every 4 weeks. In a subsequent open-label extension study in which patients on placebo were switched to active therapy, the same every-4-week treatment schedule was used. The patients’ mean age was 53; 75% were women, and 92% were White.
The primary endpoint was at least minimal improvement on 2016 ACR/EULAR (American College of Rheumatology/European Alliance of Associations for Rheumatology) myositis response criteria, defined as a 20-point or greater gain in the Total Improvement Score (TIS) and no clinical worsening at two consecutive visits. But IVIg also provided a large relative benefit over placebo using more rigorous definitions of improvement. For moderate improvement, defined as at least a 40-point TIS improvement, there was a 45.2% relative advantage for IVIg over placebo (68.1% vs. 22.9%; P < .0001). For major improvement, defined as at least a 60-point TIS improvement, the relative advantage was 23.6% (31.9% vs. 8.3%; P < .0062).
At 16 weeks, the mean TIS score was more than twice as high in those receiving IVIg than in those randomized to placebo (48.4 vs. 21.6). At that point, an open-label extension was initiated. Those in the IVIg group were permitted to remain on therapy for an additional 24 weeks if they had not worsened in the blinded phase.
The mean TIS score in the IVIg group continued to rise during the extension phase. By 12 weeks in this phase, it reached 54.0. Over the same period, mean TIS scores climbed steeply among the placebo-treated patients who had switched to active therapy, reaching 44.4.
At the end of 24 weeks of the extension trial, when patients initiated on IVIg had been on active therapy for 40 weeks, the mean TIS score advantage of starting on IVIg rather than placebo was relatively modest (55.4 vs. 51.1).
Benefit is significant for skin and muscle
Changes in the two major components of dermatomyositis were tracked individually. For skin symptoms, patients were evaluated with the Cutaneous Dermatomyositis Disease Areas and Severity Index (CDASI). For muscle involvement, symptoms were evaluated with the 8-item Manual Muscle Testing (MMT-8) tool.
“The effects of IVIg on the muscle and the skin were both highly statistically significant,” Dr. Aggarwal reported. He said the CDASI score was reduced by almost half at the end of 16 weeks among those treated with IVIg relative to those treated with placebo. Improvement in MMT-8 scores were also clinically as well as statistically significant.
The IVIg therapy was well tolerated. The most common adverse effects in this study, like those reported with IVIg when used to treat other diseases, were headache, pyrexia, and nausea, but Dr. Aggarwal reported that these were generally mild.
Serious adverse events, particularly thromboembolism, did occur over the course of the study, but the rate of events was only slightly higher in the group receiving active therapy (5.8% vs. 4.2%).
Patients who entered the study were permitted to remain on most immunosuppressive therapies, such as methotrexate, mycophenolate, tacrolimus, and glucocorticoids. Dr. Aggarwal said that the majority of patients were taking a glucocorticoid and at least one nonglucocorticoid immunosuppressant.
Effect on associated conditions is planned
The data from this trial have not yet been analyzed for the impact of IVIg on conditions that occur frequently in association with dermatomyositis, such as interstitial lung disease (ILD) and dysphagia, but Dr. Aggarwal reported that there are plans to do so. Although severe ILD was a trial exclusion, the presence of mild to moderate ILD and dysphagia were evaluated at baseline, so the impact of treatment can be assessed.
There are also plans to evaluate how the presence or absence of myositis-specific antibodies, which were also evaluated at baseline, affected response to IVIg.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Dr. Schulze-Koops reported no relevant potential conflicts of interest.
The first multinational, phase 3, placebo-controlled trial conducted with intravenous immunoglobulin therapy (IVIg) for dermatomyositis has confirmed significant efficacy and acceptable safety, according to data presented at the opening plenary abstract session of the annual European Congress of Rheumatology.
At the week 16 evaluation of the trial, called ProDERM, the response rates were 78.7% and 43.8% (P = .0008) for active therapy and placebo, respectively, reported Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh.
ProDERM is a “much-awaited study,” according to session moderator Hendrik Schulze-Koops, MD, PhD, of the division of rheumatology and clinical immunology at Ludwig Maximilian University of Munich (Germany). He was not involved in the study.
“We all have been doing what we have been doing,” Dr. Schulze-Koops said, referring to the use of IVIg for the control of dermatomyositis, “but we had no evidence for support.”
This statement could apply not only to IVIg, which has long been listed among treatment options by the Myositis Association despite the absence of controlled studies, but also to most immunosuppressive therapies and other options used for this challenging disease.
The proprietary IVIg employed in this study, Octagam 10%, has been approved in the United States for the treatment of chronic immune thrombocytopenic purpura. Its manufacturer, Octagam, plans to file a supplemental new drug application with the Food and Drug Administration for the treatment of dermatomyositis. The agent is already approved for dermatomyositis by the European Medicines Agency, according to Dr. Aggarwal.
Multiple response criteria favor IVIg
In the trial, 95 patients with dermatomyositis were randomized to 2 g/kg of IVIg (Octagam 10%) or placebo administered every 4 weeks. In a subsequent open-label extension study in which patients on placebo were switched to active therapy, the same every-4-week treatment schedule was used. The patients’ mean age was 53; 75% were women, and 92% were White.
The primary endpoint was at least minimal improvement on 2016 ACR/EULAR (American College of Rheumatology/European Alliance of Associations for Rheumatology) myositis response criteria, defined as a 20-point or greater gain in the Total Improvement Score (TIS) and no clinical worsening at two consecutive visits. But IVIg also provided a large relative benefit over placebo using more rigorous definitions of improvement. For moderate improvement, defined as at least a 40-point TIS improvement, there was a 45.2% relative advantage for IVIg over placebo (68.1% vs. 22.9%; P < .0001). For major improvement, defined as at least a 60-point TIS improvement, the relative advantage was 23.6% (31.9% vs. 8.3%; P < .0062).
At 16 weeks, the mean TIS score was more than twice as high in those receiving IVIg than in those randomized to placebo (48.4 vs. 21.6). At that point, an open-label extension was initiated. Those in the IVIg group were permitted to remain on therapy for an additional 24 weeks if they had not worsened in the blinded phase.
The mean TIS score in the IVIg group continued to rise during the extension phase. By 12 weeks in this phase, it reached 54.0. Over the same period, mean TIS scores climbed steeply among the placebo-treated patients who had switched to active therapy, reaching 44.4.
At the end of 24 weeks of the extension trial, when patients initiated on IVIg had been on active therapy for 40 weeks, the mean TIS score advantage of starting on IVIg rather than placebo was relatively modest (55.4 vs. 51.1).
Benefit is significant for skin and muscle
Changes in the two major components of dermatomyositis were tracked individually. For skin symptoms, patients were evaluated with the Cutaneous Dermatomyositis Disease Areas and Severity Index (CDASI). For muscle involvement, symptoms were evaluated with the 8-item Manual Muscle Testing (MMT-8) tool.
“The effects of IVIg on the muscle and the skin were both highly statistically significant,” Dr. Aggarwal reported. He said the CDASI score was reduced by almost half at the end of 16 weeks among those treated with IVIg relative to those treated with placebo. Improvement in MMT-8 scores were also clinically as well as statistically significant.
The IVIg therapy was well tolerated. The most common adverse effects in this study, like those reported with IVIg when used to treat other diseases, were headache, pyrexia, and nausea, but Dr. Aggarwal reported that these were generally mild.
Serious adverse events, particularly thromboembolism, did occur over the course of the study, but the rate of events was only slightly higher in the group receiving active therapy (5.8% vs. 4.2%).
Patients who entered the study were permitted to remain on most immunosuppressive therapies, such as methotrexate, mycophenolate, tacrolimus, and glucocorticoids. Dr. Aggarwal said that the majority of patients were taking a glucocorticoid and at least one nonglucocorticoid immunosuppressant.
Effect on associated conditions is planned
The data from this trial have not yet been analyzed for the impact of IVIg on conditions that occur frequently in association with dermatomyositis, such as interstitial lung disease (ILD) and dysphagia, but Dr. Aggarwal reported that there are plans to do so. Although severe ILD was a trial exclusion, the presence of mild to moderate ILD and dysphagia were evaluated at baseline, so the impact of treatment can be assessed.
There are also plans to evaluate how the presence or absence of myositis-specific antibodies, which were also evaluated at baseline, affected response to IVIg.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Dr. Schulze-Koops reported no relevant potential conflicts of interest.
FROM THE EULAR 2021 CONGRESS
Lower glucocorticoid dose proves effective for new-onset ANCA-associated vasculitis
A reduced dose of prednisolone plus rituximab was as effective as a conventionally high dose in treating patients with newly diagnosed antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, according to a new Japanese study, along with significantly fewer adverse events as well.
“To our knowledge, this is the first trial in ANCA-associated vasculitis showing that a lower glucocorticoid dose may reduce serious adverse events,” wrote Shunsuke Furuta, MD, PhD, of the department of allergy and clinical immunology at Chiba (Japan) University Hospital, and colleagues. The study was published June 1, 2021, in JAMA.
To determine the most effective and safe dose of glucocorticoids for treating this specific subset of patients with vasculitis, the researchers launched an open-label noninferiority clinical trial at 21 hospitals in Japan. A group of 140 patients with new-onset ANCA-associated vasculitis (AAV) without severe glomerulonephritis or alveolar hemorrhage were enrolled and split evenly into two treatment subgroups: reduced-dose prednisolone (0.5 mg/kg per day) plus four doses of rituximab (375 mg/m2 per week) or high-dose prednisolone (1 mg/kg per day) plus rituximab. The median age for all enrolled patients was 73, and approximately 58% were women.
Of the 140 original patients, 134 (95.7%) completed the trial. After 6 months, 49 participants in the reduced-dose group (71%) and 45 in the high-dose group (69.2%) achieved remission, as assessed via the Birmingham Vasculitis Activity Score. The difference between the two groups – 1.8 percentage points (one-sided 97.5% confidence interval, –13.7 to infinity) – met the prespecified margin of –20 percentage points for noninferiority (P = .003 for noninferiority). Relapse within 6 months occurred in three participants in the reduced-dose group and zero in the high-dose group, frequencies that the researchers identified as not statistically different (difference, 4.3%; 95% CI, –0.5% to 9.3%; P = .24).
Serious adverse events occurred less frequently in the reduced-dose group (21 events in 13 patients, 18.8%), compared with the high-dose group (41 events in 24 patients, 36.9%), as did serious infections in the reduced-dose group (7 events in 5 patients, 7.2%) versus the high-dose group (20 events in 13 patients, 20%). Two patients died in the reduced-dose group and three died in the high-dose group; those frequencies were noted as not statistically different (difference, –1.7%; 95% CI, –4.7% to 8.2%; P = .67). Causes of death included subarachnoid hemorrhage in a 58-year-old in the reduced-dose group, along with a case of sepsis in an 80-year-old and two gastrointestinal bleedings in a 75-year-old and an 85-year-old in the high-dose group.
End-stage kidney disease (ESKD) occurred in one patient in the high-dose group and none in the reduced-dose group. Cumulative survival rates at 6 months were not significantly different between the reduced-dose (97.1%) and the high-dose (95.3%) groups (95% CI, –4.7% to 8.2%; P = .58).
Less glucocorticoids makes sense for subset of patients with milder vasculitis
“We always worry about how much steroids we’re giving our patients,” said Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago. “And it’s not a shock to find out that we can use less. That’s been the theme of many studies across vasculitities that have been coming out: ‘Maybe we are using too much steroids.’ It’s really important to have actual data supporting that, though, so clinicians can feel more confident and figure out what population it applies to.”
She added that, because the study focused on patients with milder disease, it’s no surprise that remission was achieved with a lesser dose.
“I see a lot of vasculitis patients, and this gives me more confidence in a subset of them – new ANCA vasculitis, MPO positive, not very severe disease – to get away with using less steroids up front,” she said.
To apply these findings more broadly across vasculitis patients, Dr. Dua stressed the need for a follow-up study, preferably a randomized, controlled trial, with an expanded population and a longer duration.
“There were 3 relapses in the low-dose group and zero in the high-dose group in the first 6 months,” she said. “I’d be interested to know when those happened and also, over time, whether the low-dose regiment up front impacts the rate of relapse in the long term.”
The authors acknowledged the study’s limitations, including the necessity of an open-label trial because of the inevitable visible effects of high-dose glucocorticoid on patients. They also addressed the potential subjectivity of the Birmingham Vasculitis Activity Score, though they added that “other endpoints, including death, ESKD, and serious adverse events, were objective.” Finally, they acknowledged that their study was nationwide but not international, with disease phenotypes that were typical of Japanese patients with AAV. That said, “previous studies have shown that treatment responses are similar between Japan and other countries,” they wrote.
The study was funded by an intramural competitive grant from Chiba University Hospital. The authors reported numerous potential conflicts of interest, including receiving grant support, lecture fees, and personal fees from various pharmaceutical companies.
A reduced dose of prednisolone plus rituximab was as effective as a conventionally high dose in treating patients with newly diagnosed antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, according to a new Japanese study, along with significantly fewer adverse events as well.
“To our knowledge, this is the first trial in ANCA-associated vasculitis showing that a lower glucocorticoid dose may reduce serious adverse events,” wrote Shunsuke Furuta, MD, PhD, of the department of allergy and clinical immunology at Chiba (Japan) University Hospital, and colleagues. The study was published June 1, 2021, in JAMA.
To determine the most effective and safe dose of glucocorticoids for treating this specific subset of patients with vasculitis, the researchers launched an open-label noninferiority clinical trial at 21 hospitals in Japan. A group of 140 patients with new-onset ANCA-associated vasculitis (AAV) without severe glomerulonephritis or alveolar hemorrhage were enrolled and split evenly into two treatment subgroups: reduced-dose prednisolone (0.5 mg/kg per day) plus four doses of rituximab (375 mg/m2 per week) or high-dose prednisolone (1 mg/kg per day) plus rituximab. The median age for all enrolled patients was 73, and approximately 58% were women.
Of the 140 original patients, 134 (95.7%) completed the trial. After 6 months, 49 participants in the reduced-dose group (71%) and 45 in the high-dose group (69.2%) achieved remission, as assessed via the Birmingham Vasculitis Activity Score. The difference between the two groups – 1.8 percentage points (one-sided 97.5% confidence interval, –13.7 to infinity) – met the prespecified margin of –20 percentage points for noninferiority (P = .003 for noninferiority). Relapse within 6 months occurred in three participants in the reduced-dose group and zero in the high-dose group, frequencies that the researchers identified as not statistically different (difference, 4.3%; 95% CI, –0.5% to 9.3%; P = .24).
Serious adverse events occurred less frequently in the reduced-dose group (21 events in 13 patients, 18.8%), compared with the high-dose group (41 events in 24 patients, 36.9%), as did serious infections in the reduced-dose group (7 events in 5 patients, 7.2%) versus the high-dose group (20 events in 13 patients, 20%). Two patients died in the reduced-dose group and three died in the high-dose group; those frequencies were noted as not statistically different (difference, –1.7%; 95% CI, –4.7% to 8.2%; P = .67). Causes of death included subarachnoid hemorrhage in a 58-year-old in the reduced-dose group, along with a case of sepsis in an 80-year-old and two gastrointestinal bleedings in a 75-year-old and an 85-year-old in the high-dose group.
End-stage kidney disease (ESKD) occurred in one patient in the high-dose group and none in the reduced-dose group. Cumulative survival rates at 6 months were not significantly different between the reduced-dose (97.1%) and the high-dose (95.3%) groups (95% CI, –4.7% to 8.2%; P = .58).
Less glucocorticoids makes sense for subset of patients with milder vasculitis
“We always worry about how much steroids we’re giving our patients,” said Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago. “And it’s not a shock to find out that we can use less. That’s been the theme of many studies across vasculitities that have been coming out: ‘Maybe we are using too much steroids.’ It’s really important to have actual data supporting that, though, so clinicians can feel more confident and figure out what population it applies to.”
She added that, because the study focused on patients with milder disease, it’s no surprise that remission was achieved with a lesser dose.
“I see a lot of vasculitis patients, and this gives me more confidence in a subset of them – new ANCA vasculitis, MPO positive, not very severe disease – to get away with using less steroids up front,” she said.
To apply these findings more broadly across vasculitis patients, Dr. Dua stressed the need for a follow-up study, preferably a randomized, controlled trial, with an expanded population and a longer duration.
“There were 3 relapses in the low-dose group and zero in the high-dose group in the first 6 months,” she said. “I’d be interested to know when those happened and also, over time, whether the low-dose regiment up front impacts the rate of relapse in the long term.”
The authors acknowledged the study’s limitations, including the necessity of an open-label trial because of the inevitable visible effects of high-dose glucocorticoid on patients. They also addressed the potential subjectivity of the Birmingham Vasculitis Activity Score, though they added that “other endpoints, including death, ESKD, and serious adverse events, were objective.” Finally, they acknowledged that their study was nationwide but not international, with disease phenotypes that were typical of Japanese patients with AAV. That said, “previous studies have shown that treatment responses are similar between Japan and other countries,” they wrote.
The study was funded by an intramural competitive grant from Chiba University Hospital. The authors reported numerous potential conflicts of interest, including receiving grant support, lecture fees, and personal fees from various pharmaceutical companies.
A reduced dose of prednisolone plus rituximab was as effective as a conventionally high dose in treating patients with newly diagnosed antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, according to a new Japanese study, along with significantly fewer adverse events as well.
“To our knowledge, this is the first trial in ANCA-associated vasculitis showing that a lower glucocorticoid dose may reduce serious adverse events,” wrote Shunsuke Furuta, MD, PhD, of the department of allergy and clinical immunology at Chiba (Japan) University Hospital, and colleagues. The study was published June 1, 2021, in JAMA.
To determine the most effective and safe dose of glucocorticoids for treating this specific subset of patients with vasculitis, the researchers launched an open-label noninferiority clinical trial at 21 hospitals in Japan. A group of 140 patients with new-onset ANCA-associated vasculitis (AAV) without severe glomerulonephritis or alveolar hemorrhage were enrolled and split evenly into two treatment subgroups: reduced-dose prednisolone (0.5 mg/kg per day) plus four doses of rituximab (375 mg/m2 per week) or high-dose prednisolone (1 mg/kg per day) plus rituximab. The median age for all enrolled patients was 73, and approximately 58% were women.
Of the 140 original patients, 134 (95.7%) completed the trial. After 6 months, 49 participants in the reduced-dose group (71%) and 45 in the high-dose group (69.2%) achieved remission, as assessed via the Birmingham Vasculitis Activity Score. The difference between the two groups – 1.8 percentage points (one-sided 97.5% confidence interval, –13.7 to infinity) – met the prespecified margin of –20 percentage points for noninferiority (P = .003 for noninferiority). Relapse within 6 months occurred in three participants in the reduced-dose group and zero in the high-dose group, frequencies that the researchers identified as not statistically different (difference, 4.3%; 95% CI, –0.5% to 9.3%; P = .24).
Serious adverse events occurred less frequently in the reduced-dose group (21 events in 13 patients, 18.8%), compared with the high-dose group (41 events in 24 patients, 36.9%), as did serious infections in the reduced-dose group (7 events in 5 patients, 7.2%) versus the high-dose group (20 events in 13 patients, 20%). Two patients died in the reduced-dose group and three died in the high-dose group; those frequencies were noted as not statistically different (difference, –1.7%; 95% CI, –4.7% to 8.2%; P = .67). Causes of death included subarachnoid hemorrhage in a 58-year-old in the reduced-dose group, along with a case of sepsis in an 80-year-old and two gastrointestinal bleedings in a 75-year-old and an 85-year-old in the high-dose group.
End-stage kidney disease (ESKD) occurred in one patient in the high-dose group and none in the reduced-dose group. Cumulative survival rates at 6 months were not significantly different between the reduced-dose (97.1%) and the high-dose (95.3%) groups (95% CI, –4.7% to 8.2%; P = .58).
Less glucocorticoids makes sense for subset of patients with milder vasculitis
“We always worry about how much steroids we’re giving our patients,” said Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago. “And it’s not a shock to find out that we can use less. That’s been the theme of many studies across vasculitities that have been coming out: ‘Maybe we are using too much steroids.’ It’s really important to have actual data supporting that, though, so clinicians can feel more confident and figure out what population it applies to.”
She added that, because the study focused on patients with milder disease, it’s no surprise that remission was achieved with a lesser dose.
“I see a lot of vasculitis patients, and this gives me more confidence in a subset of them – new ANCA vasculitis, MPO positive, not very severe disease – to get away with using less steroids up front,” she said.
To apply these findings more broadly across vasculitis patients, Dr. Dua stressed the need for a follow-up study, preferably a randomized, controlled trial, with an expanded population and a longer duration.
“There were 3 relapses in the low-dose group and zero in the high-dose group in the first 6 months,” she said. “I’d be interested to know when those happened and also, over time, whether the low-dose regiment up front impacts the rate of relapse in the long term.”
The authors acknowledged the study’s limitations, including the necessity of an open-label trial because of the inevitable visible effects of high-dose glucocorticoid on patients. They also addressed the potential subjectivity of the Birmingham Vasculitis Activity Score, though they added that “other endpoints, including death, ESKD, and serious adverse events, were objective.” Finally, they acknowledged that their study was nationwide but not international, with disease phenotypes that were typical of Japanese patients with AAV. That said, “previous studies have shown that treatment responses are similar between Japan and other countries,” they wrote.
The study was funded by an intramural competitive grant from Chiba University Hospital. The authors reported numerous potential conflicts of interest, including receiving grant support, lecture fees, and personal fees from various pharmaceutical companies.
FROM JAMA