User login
Rheumatology clinics find success with smoking cessation referral program
A new protocol designed to help patients in rheumatology clinics quit smoking proved both efficient and effective in referring willing participants to free tobacco quit lines.
“Rheumatology visits provide a unique opportunity to address smoking as a chronic modifiable risk factor in populations at high risk for cardiovascular disease, pulmonary disease, and rheumatic disease progression,” wrote Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, and colleagues. The study was published in Arthritis Care & Research.
To assess the effectiveness of implementing a smoking cessation protocol for patients with rheumatic diseases, the researchers launched a quasi-experimental cohort study in which their Quit Connect protocol was tested at three rheumatology clinics. Adapting the Ask, Advise, Connect primary care protocol to a new setting, nurses and medical assistants were trained to use electronic health record (EHR) prompts that would check if patients who smoked were ready to quit within 30 days, advise them to do so, and then use electronic referrals to connect them to state-run tobacco quit lines. An extended baseline period – October 2012 to March 2016 – was compared to a 6-month intervention period from April to October 2016.
Across 54,090 pre- and postimplementation rheumatology clinic visits, 4,601 were with current smokers. Demographics were similar across both periods: The mean age of the patients was 51 years, about two-thirds were female, and 85% were White.
Clinicians’ assessment of tobacco use before and after implementation of the program stayed steady at 96% of patient visits, but the percentage of tobacco users’ visits that included checking for readiness to quit within the next 30 days rose from 3% (135 of 4,078) to 80% (421 of 523).
Before the implementation of the program, 0.6% of eligible visits with current smokers included a quit-line referral offer. After implementation, 93 (18%) of the 523 smokers who visited – 122 of whom said they were ready to quit – were offered referrals, a 26-fold increase. Of the 93 offered referrals, 66 (71%) accepted and 16 set a quit date or reported having quit; 11 accepted counseling services and nicotine replacement.
Although clinic staff reported encountering several obstacles, such as the need to craft nonthreatening language for challenging patients, they also contributed their own talking points that were included in the EHR tools and desktop brochures. On average, the protocol took less than 90 seconds to perform.
Rheumatologists can make headway on patients quitting smoking
“While smoking cessation programs require time and resources to implement, this study suggests a role for evidence-based protocols within rheumatology centers,” Medha Barbhaiya, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. “Given that current smokers are at an increased risk of developing more severe rheumatic disease and cardiovascular disease, and patients often visit their rheumatologist multiple times yearly, rheumatologists may be well-positioned to address smoking cessation with patients.”
In regard to next steps, she noted that “while future large studies in diverse cohorts are needed to confirm these findings, implementing a formal smoking cessation protocol within rheumatology centers may provide a unique opportunity for rheumatologists to directly help patients modify their disease risk, leading to improved health outcomes.”
The authors acknowledged their study’s limitations, including the fact that it was a prepost design and not a randomized trial. They also recognized that many tobacco users require 8-10 attempts before permanently quitting, likely lessening the lasting impact of the short-term study. They did cite expert analysis, however, that says “connecting patients to evidence-based resources makes them more likely to permanently quit.”
The study was supported in part by Pfizer’s office of Independent Grants for Learning and Change and by a grant collaboration from the University of Wisconsin Clinical and Translational Science Award and the University of Wisconsin School of Medicine and Public Health’s Wisconsin Partnership Program, through the NIH National Center for Advancing Translational Sciences.
A new protocol designed to help patients in rheumatology clinics quit smoking proved both efficient and effective in referring willing participants to free tobacco quit lines.
“Rheumatology visits provide a unique opportunity to address smoking as a chronic modifiable risk factor in populations at high risk for cardiovascular disease, pulmonary disease, and rheumatic disease progression,” wrote Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, and colleagues. The study was published in Arthritis Care & Research.
To assess the effectiveness of implementing a smoking cessation protocol for patients with rheumatic diseases, the researchers launched a quasi-experimental cohort study in which their Quit Connect protocol was tested at three rheumatology clinics. Adapting the Ask, Advise, Connect primary care protocol to a new setting, nurses and medical assistants were trained to use electronic health record (EHR) prompts that would check if patients who smoked were ready to quit within 30 days, advise them to do so, and then use electronic referrals to connect them to state-run tobacco quit lines. An extended baseline period – October 2012 to March 2016 – was compared to a 6-month intervention period from April to October 2016.
Across 54,090 pre- and postimplementation rheumatology clinic visits, 4,601 were with current smokers. Demographics were similar across both periods: The mean age of the patients was 51 years, about two-thirds were female, and 85% were White.
Clinicians’ assessment of tobacco use before and after implementation of the program stayed steady at 96% of patient visits, but the percentage of tobacco users’ visits that included checking for readiness to quit within the next 30 days rose from 3% (135 of 4,078) to 80% (421 of 523).
Before the implementation of the program, 0.6% of eligible visits with current smokers included a quit-line referral offer. After implementation, 93 (18%) of the 523 smokers who visited – 122 of whom said they were ready to quit – were offered referrals, a 26-fold increase. Of the 93 offered referrals, 66 (71%) accepted and 16 set a quit date or reported having quit; 11 accepted counseling services and nicotine replacement.
Although clinic staff reported encountering several obstacles, such as the need to craft nonthreatening language for challenging patients, they also contributed their own talking points that were included in the EHR tools and desktop brochures. On average, the protocol took less than 90 seconds to perform.
Rheumatologists can make headway on patients quitting smoking
“While smoking cessation programs require time and resources to implement, this study suggests a role for evidence-based protocols within rheumatology centers,” Medha Barbhaiya, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. “Given that current smokers are at an increased risk of developing more severe rheumatic disease and cardiovascular disease, and patients often visit their rheumatologist multiple times yearly, rheumatologists may be well-positioned to address smoking cessation with patients.”
In regard to next steps, she noted that “while future large studies in diverse cohorts are needed to confirm these findings, implementing a formal smoking cessation protocol within rheumatology centers may provide a unique opportunity for rheumatologists to directly help patients modify their disease risk, leading to improved health outcomes.”
The authors acknowledged their study’s limitations, including the fact that it was a prepost design and not a randomized trial. They also recognized that many tobacco users require 8-10 attempts before permanently quitting, likely lessening the lasting impact of the short-term study. They did cite expert analysis, however, that says “connecting patients to evidence-based resources makes them more likely to permanently quit.”
The study was supported in part by Pfizer’s office of Independent Grants for Learning and Change and by a grant collaboration from the University of Wisconsin Clinical and Translational Science Award and the University of Wisconsin School of Medicine and Public Health’s Wisconsin Partnership Program, through the NIH National Center for Advancing Translational Sciences.
A new protocol designed to help patients in rheumatology clinics quit smoking proved both efficient and effective in referring willing participants to free tobacco quit lines.
“Rheumatology visits provide a unique opportunity to address smoking as a chronic modifiable risk factor in populations at high risk for cardiovascular disease, pulmonary disease, and rheumatic disease progression,” wrote Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, and colleagues. The study was published in Arthritis Care & Research.
To assess the effectiveness of implementing a smoking cessation protocol for patients with rheumatic diseases, the researchers launched a quasi-experimental cohort study in which their Quit Connect protocol was tested at three rheumatology clinics. Adapting the Ask, Advise, Connect primary care protocol to a new setting, nurses and medical assistants were trained to use electronic health record (EHR) prompts that would check if patients who smoked were ready to quit within 30 days, advise them to do so, and then use electronic referrals to connect them to state-run tobacco quit lines. An extended baseline period – October 2012 to March 2016 – was compared to a 6-month intervention period from April to October 2016.
Across 54,090 pre- and postimplementation rheumatology clinic visits, 4,601 were with current smokers. Demographics were similar across both periods: The mean age of the patients was 51 years, about two-thirds were female, and 85% were White.
Clinicians’ assessment of tobacco use before and after implementation of the program stayed steady at 96% of patient visits, but the percentage of tobacco users’ visits that included checking for readiness to quit within the next 30 days rose from 3% (135 of 4,078) to 80% (421 of 523).
Before the implementation of the program, 0.6% of eligible visits with current smokers included a quit-line referral offer. After implementation, 93 (18%) of the 523 smokers who visited – 122 of whom said they were ready to quit – were offered referrals, a 26-fold increase. Of the 93 offered referrals, 66 (71%) accepted and 16 set a quit date or reported having quit; 11 accepted counseling services and nicotine replacement.
Although clinic staff reported encountering several obstacles, such as the need to craft nonthreatening language for challenging patients, they also contributed their own talking points that were included in the EHR tools and desktop brochures. On average, the protocol took less than 90 seconds to perform.
Rheumatologists can make headway on patients quitting smoking
“While smoking cessation programs require time and resources to implement, this study suggests a role for evidence-based protocols within rheumatology centers,” Medha Barbhaiya, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. “Given that current smokers are at an increased risk of developing more severe rheumatic disease and cardiovascular disease, and patients often visit their rheumatologist multiple times yearly, rheumatologists may be well-positioned to address smoking cessation with patients.”
In regard to next steps, she noted that “while future large studies in diverse cohorts are needed to confirm these findings, implementing a formal smoking cessation protocol within rheumatology centers may provide a unique opportunity for rheumatologists to directly help patients modify their disease risk, leading to improved health outcomes.”
The authors acknowledged their study’s limitations, including the fact that it was a prepost design and not a randomized trial. They also recognized that many tobacco users require 8-10 attempts before permanently quitting, likely lessening the lasting impact of the short-term study. They did cite expert analysis, however, that says “connecting patients to evidence-based resources makes them more likely to permanently quit.”
The study was supported in part by Pfizer’s office of Independent Grants for Learning and Change and by a grant collaboration from the University of Wisconsin Clinical and Translational Science Award and the University of Wisconsin School of Medicine and Public Health’s Wisconsin Partnership Program, through the NIH National Center for Advancing Translational Sciences.
FROM ARTHRITIS CARE & RESEARCH
VEXAS: A novel rheumatologic, hematologic syndrome that’s making waves
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
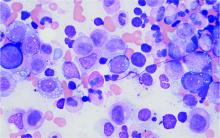
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
COVID-19 vaccination in RMD patients: Safety data “reassuring”
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
FROM ANNALS OF THE RHEUMATIC DISEASES
COVID-19’s impact on lupus inpatients examined in study
Severe COVID-19 infection was more likely in hospitalized patients with systemic lupus erythematosus (SLE) who had comorbidities and risk factors associated with severe infection in the general population, notably older age, male gender, and hypertension, based on data from a nationwide epidemiologic study of inpatients in France.
“Recently, anti-interferon antibodies have been implicated in severe SARS-CoV-2 infection while it has been known for decades that patients with SLE may produce such autoantibodies,” but large-scale data on the risk of severe COVID-19 infection in SLE patients are limited, Arthur Mageau, MD, of Bichat–Claude Bernard Hospital in Paris, and colleagues wrote.
In a research letter published in Annals of the Rheumatic Diseases, the researchers used the French health care database Programme de Médicalisation des Systèmes d’Information to identify 11,055 adult SLE patients who had at least one hospital stay between March 1, 2020, and Oct.31, 2020. Of these, 1,411 (12.8%) also were diagnosed with COVID-19, and these patients had a total of 1,721 hospital stays.
Overall, in-hospital mortality was approximately four times higher among SLE patients with COVID-19 infection, compared with SLE patients without COVID-19 infection (9.5% vs. 2.4%, P < .001), and 293 (17%) of the COVID-19 hospital stays involved an intensive care unit. In the ICU, 78 (26.7%) of the COVID-19 patients required invasive ventilation, and 71 (24.7%) required noninvasive mechanical ventilation.
The SLE patients with COVID-19 who died were significantly more likely than the SLE patients with COVID-19 who recovered to be older and male, and to have conditions including chronic kidney disease, high blood pressure, chronic pulmonary disease, and a history of cardiovascular events or lupus nephritis. The study findings were limited by the focus on hospitalized patients only, so the results cannot be generalized to all lupus patients, the researchers said.
“Interestingly, while the overall mortality rate was lower in SLE/COVID-19–positive inpatients as compared with the total population admitted for SARS-CoV-2 infection in France during the same period (9.5% vs 15.7%, P < .0001), the mortality rate at a younger age tended to be higher in patients with SLE,” the researchers wrote, but the difference for these younger patients was not statistically significant. This disparity may be caused by the reduced need for immunosuppressive drugs in SLE patients as they age, and the observed increased mortality in younger SLE patients, compared with the general population, suggests that SLE may promote poor outcomes from COVID-19 infection.
Dr. Mageau received PhD fellowship support from the Agence Nationale pour la recherche. He and the other researchers had no financial conflicts to disclose. The study received no outside funding.
Severe COVID-19 infection was more likely in hospitalized patients with systemic lupus erythematosus (SLE) who had comorbidities and risk factors associated with severe infection in the general population, notably older age, male gender, and hypertension, based on data from a nationwide epidemiologic study of inpatients in France.
“Recently, anti-interferon antibodies have been implicated in severe SARS-CoV-2 infection while it has been known for decades that patients with SLE may produce such autoantibodies,” but large-scale data on the risk of severe COVID-19 infection in SLE patients are limited, Arthur Mageau, MD, of Bichat–Claude Bernard Hospital in Paris, and colleagues wrote.
In a research letter published in Annals of the Rheumatic Diseases, the researchers used the French health care database Programme de Médicalisation des Systèmes d’Information to identify 11,055 adult SLE patients who had at least one hospital stay between March 1, 2020, and Oct.31, 2020. Of these, 1,411 (12.8%) also were diagnosed with COVID-19, and these patients had a total of 1,721 hospital stays.
Overall, in-hospital mortality was approximately four times higher among SLE patients with COVID-19 infection, compared with SLE patients without COVID-19 infection (9.5% vs. 2.4%, P < .001), and 293 (17%) of the COVID-19 hospital stays involved an intensive care unit. In the ICU, 78 (26.7%) of the COVID-19 patients required invasive ventilation, and 71 (24.7%) required noninvasive mechanical ventilation.
The SLE patients with COVID-19 who died were significantly more likely than the SLE patients with COVID-19 who recovered to be older and male, and to have conditions including chronic kidney disease, high blood pressure, chronic pulmonary disease, and a history of cardiovascular events or lupus nephritis. The study findings were limited by the focus on hospitalized patients only, so the results cannot be generalized to all lupus patients, the researchers said.
“Interestingly, while the overall mortality rate was lower in SLE/COVID-19–positive inpatients as compared with the total population admitted for SARS-CoV-2 infection in France during the same period (9.5% vs 15.7%, P < .0001), the mortality rate at a younger age tended to be higher in patients with SLE,” the researchers wrote, but the difference for these younger patients was not statistically significant. This disparity may be caused by the reduced need for immunosuppressive drugs in SLE patients as they age, and the observed increased mortality in younger SLE patients, compared with the general population, suggests that SLE may promote poor outcomes from COVID-19 infection.
Dr. Mageau received PhD fellowship support from the Agence Nationale pour la recherche. He and the other researchers had no financial conflicts to disclose. The study received no outside funding.
Severe COVID-19 infection was more likely in hospitalized patients with systemic lupus erythematosus (SLE) who had comorbidities and risk factors associated with severe infection in the general population, notably older age, male gender, and hypertension, based on data from a nationwide epidemiologic study of inpatients in France.
“Recently, anti-interferon antibodies have been implicated in severe SARS-CoV-2 infection while it has been known for decades that patients with SLE may produce such autoantibodies,” but large-scale data on the risk of severe COVID-19 infection in SLE patients are limited, Arthur Mageau, MD, of Bichat–Claude Bernard Hospital in Paris, and colleagues wrote.
In a research letter published in Annals of the Rheumatic Diseases, the researchers used the French health care database Programme de Médicalisation des Systèmes d’Information to identify 11,055 adult SLE patients who had at least one hospital stay between March 1, 2020, and Oct.31, 2020. Of these, 1,411 (12.8%) also were diagnosed with COVID-19, and these patients had a total of 1,721 hospital stays.
Overall, in-hospital mortality was approximately four times higher among SLE patients with COVID-19 infection, compared with SLE patients without COVID-19 infection (9.5% vs. 2.4%, P < .001), and 293 (17%) of the COVID-19 hospital stays involved an intensive care unit. In the ICU, 78 (26.7%) of the COVID-19 patients required invasive ventilation, and 71 (24.7%) required noninvasive mechanical ventilation.
The SLE patients with COVID-19 who died were significantly more likely than the SLE patients with COVID-19 who recovered to be older and male, and to have conditions including chronic kidney disease, high blood pressure, chronic pulmonary disease, and a history of cardiovascular events or lupus nephritis. The study findings were limited by the focus on hospitalized patients only, so the results cannot be generalized to all lupus patients, the researchers said.
“Interestingly, while the overall mortality rate was lower in SLE/COVID-19–positive inpatients as compared with the total population admitted for SARS-CoV-2 infection in France during the same period (9.5% vs 15.7%, P < .0001), the mortality rate at a younger age tended to be higher in patients with SLE,” the researchers wrote, but the difference for these younger patients was not statistically significant. This disparity may be caused by the reduced need for immunosuppressive drugs in SLE patients as they age, and the observed increased mortality in younger SLE patients, compared with the general population, suggests that SLE may promote poor outcomes from COVID-19 infection.
Dr. Mageau received PhD fellowship support from the Agence Nationale pour la recherche. He and the other researchers had no financial conflicts to disclose. The study received no outside funding.
FROM ANNALS OF THE RHEUMATIC DISEASES
Checkpoint inhibitor–induced rheumatic complications often arise late
Most checkpoint inhibitor–induced rheumatic complications in cancer patients can be treated successfully with corticosteroids, albeit often at considerably higher doses than rheumatologists typically use in managing rheumatoid arthritis, Eric M. Ruderman, MD, observed at the 2021 Rheumatology Winter Clinical Symposium.
“In RA, we’re all used to the idea that 5 or 10 mg of corticosteroids per day can make a tremendous difference. That’s not always the case here. Patients who develop rheumatic immunotherapy-related adverse events often require 20-30 mg/day to get symptoms under control,” according to Dr. Ruderman, professor of medicine (rheumatology) at Northwestern University, Chicago.
This may be in part because oncologists typically don’t refer affected patients to rheumatologists early on. Guidelines from the National Comprehensive Cancer Network and other oncology groups suggest referral only once a patient develops grade 3 immunotherapy-related rheumatic adverse events, meaning the symptoms significantly impair daily activities, he explained.
Checkpoint inhibitors, which induce T-cell activation to fight the patient’s malignancy, can produce a plethora of off-target effects. These adverse events may involve the skin, heart, lungs, kidneys, eyes, blood, GI tract, and endocrine organs. The drugs also can cause rheumatic or neurologic complications. The most common of these adverse events are colitis and rash. Next most common are arthritis and arthralgia. Rheumatic side effects are most common as a consequence of immunotherapy using a CTLA4 (cytotoxic T-lymphocyte-associated protein 4) inhibitor, but can also occur in association with programmed cell death protein 1 (PD-1) inhibitors and PD-ligand 1 inhibitors. Arthritis and other rheumatic adverse events are more common in patients undergoing combination therapy.
Some form of frank inflammatory arthritis occurs in 5%-10% of cancer patients undergoing checkpoint inhibitor therapy. This can manifest as an RA-like polyarthritis, spondyloarthritis, polymyalgia rheumatica, necrotizing myositis, or vasculitis. Arthralgia occurs in up to 40% of treated patients.
This immunotherapy-related arthritis is typically more inflammatory than RA. It also has a much more abrupt onset. It is usually seronegative and has no gender predisposition, and the limited available evidence to date suggests there is no increased risk of this complication in checkpoint inhibitor–treated patients with a history of prior rheumatic disease, according to Dr. Ruderman.
Delayed onset and resolution of rheumatologic immune-related adverse events
“Onset and resolution of rheumatologic adverse events with immunotherapy may be delayed. This is an important point: While skin rash and colitis often show up pretty early in the course of immunotherapy, some of the arthritic events can happen later. They can actually continue after the immunotherapy is stopped,” the rheumatologist said.
Indeed, a retrospective nationwide Canadian study of 117 patients at nine academic centers who developed 136 rheumatic immune-related adverse events in conjunction with cancer immunotherapy found that the mean time to the first such event was 6.8 months into checkpoint inhibitor therapy. The most common rheumatic complication was symmetric polyarthritis, affecting 45 patients. Other rheumatologic immune-related complications included polymyalgia rheumatica in 17 patients, noninflammatory musculoskeletal symptoms in 18, and myositis in 9.
Seventy-six patients were treated with prednisone for a mean of 8.4 months at a maximum dose of 60 mg/day. Forty-two moved up the treatment ladder to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) to manage their symptoms. Only two patients required escalation to biologic therapy. A reassuring finding in this relatively small study was that treatment of the patients’ rheumatic complications didn’t appear to worsen the tumor response to immunotherapy: Twenty-three patients experienced tumor progression prior to treatment of their rheumatic disorder, and 14 did so following treatment.
Flares of preexisting rheumatic diseases
These tend to occur much earlier in the course of immune checkpoint inhibitor therapy for cancer than de novo immunotherapy-related rheumatic adverse events. In a retrospective Australian study of 12 cancer patients with preexisting rheumatic disease before going on a PD-1 inhibitor and 24 others with no such history, all of whom developed rheumatic adverse events while on the checkpoint inhibitor, the mean time to a flare of preexisting rheumatic disease was 6.2 weeks, compared to 21.5 weeks in patients who experienced a de novo rheumatic adverse event.
Dr. Ruderman supports recommendations from the European Alliance of Associations for Rheumatology (EULAR) for the management of rheumatic immune-related adverse events due to cancer immunotherapy, even though the underlying level of evidence is fairly weak. The recommendations call for the use of csDMARDs when corticosteroids don’t adequately control symptoms. And when the response to csDMARDs is insufficient, the next step is a biologic, preferably a tumor necrosis factor inhibitor or interleukin-6 inhibitor.
“At our institution, the oncologists are a little bit nervous about using biologics in cancer patients, but I think more and more they’re going to have to accept it. And so far there isn’t a ton of evidence that suggests the addition of biologics interferes with the efficacy of the immunotherapy,” the rheumatologist said.
He underscored the critical importance of one of the overarching principles of the EULAR guidelines: the need for interdisciplinary coordination between rheumatologists and oncologists regarding the problem of rheumatologic immune-related adverse events.
“Oncologists aren’t good at managing inflammatory arthritis. I think they really need us,” he said.
Dr. Ruderman reported serving as a consultant to and/or receiving a research grant from nine pharmaceutical companies.
Most checkpoint inhibitor–induced rheumatic complications in cancer patients can be treated successfully with corticosteroids, albeit often at considerably higher doses than rheumatologists typically use in managing rheumatoid arthritis, Eric M. Ruderman, MD, observed at the 2021 Rheumatology Winter Clinical Symposium.
“In RA, we’re all used to the idea that 5 or 10 mg of corticosteroids per day can make a tremendous difference. That’s not always the case here. Patients who develop rheumatic immunotherapy-related adverse events often require 20-30 mg/day to get symptoms under control,” according to Dr. Ruderman, professor of medicine (rheumatology) at Northwestern University, Chicago.
This may be in part because oncologists typically don’t refer affected patients to rheumatologists early on. Guidelines from the National Comprehensive Cancer Network and other oncology groups suggest referral only once a patient develops grade 3 immunotherapy-related rheumatic adverse events, meaning the symptoms significantly impair daily activities, he explained.
Checkpoint inhibitors, which induce T-cell activation to fight the patient’s malignancy, can produce a plethora of off-target effects. These adverse events may involve the skin, heart, lungs, kidneys, eyes, blood, GI tract, and endocrine organs. The drugs also can cause rheumatic or neurologic complications. The most common of these adverse events are colitis and rash. Next most common are arthritis and arthralgia. Rheumatic side effects are most common as a consequence of immunotherapy using a CTLA4 (cytotoxic T-lymphocyte-associated protein 4) inhibitor, but can also occur in association with programmed cell death protein 1 (PD-1) inhibitors and PD-ligand 1 inhibitors. Arthritis and other rheumatic adverse events are more common in patients undergoing combination therapy.
Some form of frank inflammatory arthritis occurs in 5%-10% of cancer patients undergoing checkpoint inhibitor therapy. This can manifest as an RA-like polyarthritis, spondyloarthritis, polymyalgia rheumatica, necrotizing myositis, or vasculitis. Arthralgia occurs in up to 40% of treated patients.
This immunotherapy-related arthritis is typically more inflammatory than RA. It also has a much more abrupt onset. It is usually seronegative and has no gender predisposition, and the limited available evidence to date suggests there is no increased risk of this complication in checkpoint inhibitor–treated patients with a history of prior rheumatic disease, according to Dr. Ruderman.
Delayed onset and resolution of rheumatologic immune-related adverse events
“Onset and resolution of rheumatologic adverse events with immunotherapy may be delayed. This is an important point: While skin rash and colitis often show up pretty early in the course of immunotherapy, some of the arthritic events can happen later. They can actually continue after the immunotherapy is stopped,” the rheumatologist said.
Indeed, a retrospective nationwide Canadian study of 117 patients at nine academic centers who developed 136 rheumatic immune-related adverse events in conjunction with cancer immunotherapy found that the mean time to the first such event was 6.8 months into checkpoint inhibitor therapy. The most common rheumatic complication was symmetric polyarthritis, affecting 45 patients. Other rheumatologic immune-related complications included polymyalgia rheumatica in 17 patients, noninflammatory musculoskeletal symptoms in 18, and myositis in 9.
Seventy-six patients were treated with prednisone for a mean of 8.4 months at a maximum dose of 60 mg/day. Forty-two moved up the treatment ladder to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) to manage their symptoms. Only two patients required escalation to biologic therapy. A reassuring finding in this relatively small study was that treatment of the patients’ rheumatic complications didn’t appear to worsen the tumor response to immunotherapy: Twenty-three patients experienced tumor progression prior to treatment of their rheumatic disorder, and 14 did so following treatment.
Flares of preexisting rheumatic diseases
These tend to occur much earlier in the course of immune checkpoint inhibitor therapy for cancer than de novo immunotherapy-related rheumatic adverse events. In a retrospective Australian study of 12 cancer patients with preexisting rheumatic disease before going on a PD-1 inhibitor and 24 others with no such history, all of whom developed rheumatic adverse events while on the checkpoint inhibitor, the mean time to a flare of preexisting rheumatic disease was 6.2 weeks, compared to 21.5 weeks in patients who experienced a de novo rheumatic adverse event.
Dr. Ruderman supports recommendations from the European Alliance of Associations for Rheumatology (EULAR) for the management of rheumatic immune-related adverse events due to cancer immunotherapy, even though the underlying level of evidence is fairly weak. The recommendations call for the use of csDMARDs when corticosteroids don’t adequately control symptoms. And when the response to csDMARDs is insufficient, the next step is a biologic, preferably a tumor necrosis factor inhibitor or interleukin-6 inhibitor.
“At our institution, the oncologists are a little bit nervous about using biologics in cancer patients, but I think more and more they’re going to have to accept it. And so far there isn’t a ton of evidence that suggests the addition of biologics interferes with the efficacy of the immunotherapy,” the rheumatologist said.
He underscored the critical importance of one of the overarching principles of the EULAR guidelines: the need for interdisciplinary coordination between rheumatologists and oncologists regarding the problem of rheumatologic immune-related adverse events.
“Oncologists aren’t good at managing inflammatory arthritis. I think they really need us,” he said.
Dr. Ruderman reported serving as a consultant to and/or receiving a research grant from nine pharmaceutical companies.
Most checkpoint inhibitor–induced rheumatic complications in cancer patients can be treated successfully with corticosteroids, albeit often at considerably higher doses than rheumatologists typically use in managing rheumatoid arthritis, Eric M. Ruderman, MD, observed at the 2021 Rheumatology Winter Clinical Symposium.
“In RA, we’re all used to the idea that 5 or 10 mg of corticosteroids per day can make a tremendous difference. That’s not always the case here. Patients who develop rheumatic immunotherapy-related adverse events often require 20-30 mg/day to get symptoms under control,” according to Dr. Ruderman, professor of medicine (rheumatology) at Northwestern University, Chicago.
This may be in part because oncologists typically don’t refer affected patients to rheumatologists early on. Guidelines from the National Comprehensive Cancer Network and other oncology groups suggest referral only once a patient develops grade 3 immunotherapy-related rheumatic adverse events, meaning the symptoms significantly impair daily activities, he explained.
Checkpoint inhibitors, which induce T-cell activation to fight the patient’s malignancy, can produce a plethora of off-target effects. These adverse events may involve the skin, heart, lungs, kidneys, eyes, blood, GI tract, and endocrine organs. The drugs also can cause rheumatic or neurologic complications. The most common of these adverse events are colitis and rash. Next most common are arthritis and arthralgia. Rheumatic side effects are most common as a consequence of immunotherapy using a CTLA4 (cytotoxic T-lymphocyte-associated protein 4) inhibitor, but can also occur in association with programmed cell death protein 1 (PD-1) inhibitors and PD-ligand 1 inhibitors. Arthritis and other rheumatic adverse events are more common in patients undergoing combination therapy.
Some form of frank inflammatory arthritis occurs in 5%-10% of cancer patients undergoing checkpoint inhibitor therapy. This can manifest as an RA-like polyarthritis, spondyloarthritis, polymyalgia rheumatica, necrotizing myositis, or vasculitis. Arthralgia occurs in up to 40% of treated patients.
This immunotherapy-related arthritis is typically more inflammatory than RA. It also has a much more abrupt onset. It is usually seronegative and has no gender predisposition, and the limited available evidence to date suggests there is no increased risk of this complication in checkpoint inhibitor–treated patients with a history of prior rheumatic disease, according to Dr. Ruderman.
Delayed onset and resolution of rheumatologic immune-related adverse events
“Onset and resolution of rheumatologic adverse events with immunotherapy may be delayed. This is an important point: While skin rash and colitis often show up pretty early in the course of immunotherapy, some of the arthritic events can happen later. They can actually continue after the immunotherapy is stopped,” the rheumatologist said.
Indeed, a retrospective nationwide Canadian study of 117 patients at nine academic centers who developed 136 rheumatic immune-related adverse events in conjunction with cancer immunotherapy found that the mean time to the first such event was 6.8 months into checkpoint inhibitor therapy. The most common rheumatic complication was symmetric polyarthritis, affecting 45 patients. Other rheumatologic immune-related complications included polymyalgia rheumatica in 17 patients, noninflammatory musculoskeletal symptoms in 18, and myositis in 9.
Seventy-six patients were treated with prednisone for a mean of 8.4 months at a maximum dose of 60 mg/day. Forty-two moved up the treatment ladder to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) to manage their symptoms. Only two patients required escalation to biologic therapy. A reassuring finding in this relatively small study was that treatment of the patients’ rheumatic complications didn’t appear to worsen the tumor response to immunotherapy: Twenty-three patients experienced tumor progression prior to treatment of their rheumatic disorder, and 14 did so following treatment.
Flares of preexisting rheumatic diseases
These tend to occur much earlier in the course of immune checkpoint inhibitor therapy for cancer than de novo immunotherapy-related rheumatic adverse events. In a retrospective Australian study of 12 cancer patients with preexisting rheumatic disease before going on a PD-1 inhibitor and 24 others with no such history, all of whom developed rheumatic adverse events while on the checkpoint inhibitor, the mean time to a flare of preexisting rheumatic disease was 6.2 weeks, compared to 21.5 weeks in patients who experienced a de novo rheumatic adverse event.
Dr. Ruderman supports recommendations from the European Alliance of Associations for Rheumatology (EULAR) for the management of rheumatic immune-related adverse events due to cancer immunotherapy, even though the underlying level of evidence is fairly weak. The recommendations call for the use of csDMARDs when corticosteroids don’t adequately control symptoms. And when the response to csDMARDs is insufficient, the next step is a biologic, preferably a tumor necrosis factor inhibitor or interleukin-6 inhibitor.
“At our institution, the oncologists are a little bit nervous about using biologics in cancer patients, but I think more and more they’re going to have to accept it. And so far there isn’t a ton of evidence that suggests the addition of biologics interferes with the efficacy of the immunotherapy,” the rheumatologist said.
He underscored the critical importance of one of the overarching principles of the EULAR guidelines: the need for interdisciplinary coordination between rheumatologists and oncologists regarding the problem of rheumatologic immune-related adverse events.
“Oncologists aren’t good at managing inflammatory arthritis. I think they really need us,” he said.
Dr. Ruderman reported serving as a consultant to and/or receiving a research grant from nine pharmaceutical companies.
FROM RWCS 2021
Nearly 20% of lupus patients have severe infection in first decade after diagnosis
People with systemic lupus erythematosus (SLE) experienced significantly higher rates of first severe infections, a higher number of severe infections overall, and greater infection-related mortality, compared with controls, based on data from a population-based cohort study of more than 30,000 individuals.
Infections remain a leading cause of morbidity and early mortality in patients with SLE, wrote Kai Zhao, MSc, of Arthritis Research Canada, Richmond, and colleagues. However, “limitations from existing studies including selected samples, small sizes, and prevalent cohorts can negatively affect the accuracy of both the absolute and relative risk estimates of infections in SLE at the population level,” they said.
In a study published in Rheumatology, the researchers identified 5,169 people newly diagnosed with SLE between Jan. 1, 1997, and March 31, 2015, and matched them with 25,845 non-SLE controls using an administrative health database of all health care services funded in British Columbia during the time period. The investigators said the study is the first “to evaluate the risk of severe infections in a large population-based and incident SLE cohort.”
The average age of the patients was 46.9 at the time of their index SLE diagnosis, and 86% were women. The average follow-up period was approximately 10 years.
The primary outcome was the first severe infection after the onset of SLE that required hospitalization or occurred in the hospital setting. A total of 955 (18.5%) first severe infections occurred in the SLE group, compared with 1,988 (7.7%) in the controls, for incidence rates of 19.7 events per 1,000 person-years and 7.6 events per 1,000 person-years, respectively, yielding an 82% increased risk of severe infection for SLE patients after adjustment for confounding baseline factors.
Secondary outcomes of the total number of severe infections and infection-related mortality both showed significant increases in SLE patients, compared with controls. The total number of severe infections in the SLE and control groups was 1,898 and 3,114, respectively, with an adjusted risk ratio of 2.07.
As for mortality, a total of 539 deaths occurred in SLE patients during the study period, and 114 (21%) were related to severe infection. A total of 1,495 deaths occurred in the control group, including 269 (18%) related to severe infection. The adjusted hazard ratio was 1.61 after adjustment for confounding baseline variables.
The risks for first severe infection, total number of severe infections, and infection-related mortality were “independent of traditional risk factors for infection and the results remain robust in the presence of an unmeasured confounder (smoking) and competing risk of death,” the researchers said. Reasons for the increased risk are uncertain, but likely result from intrinsic factors such as immune system dysfunction and extrinsic factors such as the impact of immunosuppressive medications. “Future research can focus on quantifying the relative contributions of these intrinsic and extrinsic factors on the increased infection risk in SLE patients,” they added.
The study findings were limited by several factors linked to the observational design, including possible misdiagnosis of SLE and inaccurate measure of SLE onset, the researchers noted. In addition, no data were available for certain confounders such as smoking and nonhospitalized infections, they said.
However, the results were strengthened by the large size and general population and the use of sensitivity analyses, they noted. For SLE patients, “increased awareness of the risk of infections can identify their early signs and potentially prevent hospitalizations,” and clinicians can promote infection prevention strategies, including vaccinations when appropriate, they added.
Based on their findings, “we recommend a closer surveillance for severe infections in SLE patients and risk assessment for severe infections for SLE patients after diagnosis,” the researchers emphasized. “Further studies are warranted to further identify risk factors for infections in SLE patients to develop personalized treatment regimens and to select treatment in practice by synthesizing patient information,” they concluded.
The study was supported by the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
People with systemic lupus erythematosus (SLE) experienced significantly higher rates of first severe infections, a higher number of severe infections overall, and greater infection-related mortality, compared with controls, based on data from a population-based cohort study of more than 30,000 individuals.
Infections remain a leading cause of morbidity and early mortality in patients with SLE, wrote Kai Zhao, MSc, of Arthritis Research Canada, Richmond, and colleagues. However, “limitations from existing studies including selected samples, small sizes, and prevalent cohorts can negatively affect the accuracy of both the absolute and relative risk estimates of infections in SLE at the population level,” they said.
In a study published in Rheumatology, the researchers identified 5,169 people newly diagnosed with SLE between Jan. 1, 1997, and March 31, 2015, and matched them with 25,845 non-SLE controls using an administrative health database of all health care services funded in British Columbia during the time period. The investigators said the study is the first “to evaluate the risk of severe infections in a large population-based and incident SLE cohort.”
The average age of the patients was 46.9 at the time of their index SLE diagnosis, and 86% were women. The average follow-up period was approximately 10 years.
The primary outcome was the first severe infection after the onset of SLE that required hospitalization or occurred in the hospital setting. A total of 955 (18.5%) first severe infections occurred in the SLE group, compared with 1,988 (7.7%) in the controls, for incidence rates of 19.7 events per 1,000 person-years and 7.6 events per 1,000 person-years, respectively, yielding an 82% increased risk of severe infection for SLE patients after adjustment for confounding baseline factors.
Secondary outcomes of the total number of severe infections and infection-related mortality both showed significant increases in SLE patients, compared with controls. The total number of severe infections in the SLE and control groups was 1,898 and 3,114, respectively, with an adjusted risk ratio of 2.07.
As for mortality, a total of 539 deaths occurred in SLE patients during the study period, and 114 (21%) were related to severe infection. A total of 1,495 deaths occurred in the control group, including 269 (18%) related to severe infection. The adjusted hazard ratio was 1.61 after adjustment for confounding baseline variables.
The risks for first severe infection, total number of severe infections, and infection-related mortality were “independent of traditional risk factors for infection and the results remain robust in the presence of an unmeasured confounder (smoking) and competing risk of death,” the researchers said. Reasons for the increased risk are uncertain, but likely result from intrinsic factors such as immune system dysfunction and extrinsic factors such as the impact of immunosuppressive medications. “Future research can focus on quantifying the relative contributions of these intrinsic and extrinsic factors on the increased infection risk in SLE patients,” they added.
The study findings were limited by several factors linked to the observational design, including possible misdiagnosis of SLE and inaccurate measure of SLE onset, the researchers noted. In addition, no data were available for certain confounders such as smoking and nonhospitalized infections, they said.
However, the results were strengthened by the large size and general population and the use of sensitivity analyses, they noted. For SLE patients, “increased awareness of the risk of infections can identify their early signs and potentially prevent hospitalizations,” and clinicians can promote infection prevention strategies, including vaccinations when appropriate, they added.
Based on their findings, “we recommend a closer surveillance for severe infections in SLE patients and risk assessment for severe infections for SLE patients after diagnosis,” the researchers emphasized. “Further studies are warranted to further identify risk factors for infections in SLE patients to develop personalized treatment regimens and to select treatment in practice by synthesizing patient information,” they concluded.
The study was supported by the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
People with systemic lupus erythematosus (SLE) experienced significantly higher rates of first severe infections, a higher number of severe infections overall, and greater infection-related mortality, compared with controls, based on data from a population-based cohort study of more than 30,000 individuals.
Infections remain a leading cause of morbidity and early mortality in patients with SLE, wrote Kai Zhao, MSc, of Arthritis Research Canada, Richmond, and colleagues. However, “limitations from existing studies including selected samples, small sizes, and prevalent cohorts can negatively affect the accuracy of both the absolute and relative risk estimates of infections in SLE at the population level,” they said.
In a study published in Rheumatology, the researchers identified 5,169 people newly diagnosed with SLE between Jan. 1, 1997, and March 31, 2015, and matched them with 25,845 non-SLE controls using an administrative health database of all health care services funded in British Columbia during the time period. The investigators said the study is the first “to evaluate the risk of severe infections in a large population-based and incident SLE cohort.”
The average age of the patients was 46.9 at the time of their index SLE diagnosis, and 86% were women. The average follow-up period was approximately 10 years.
The primary outcome was the first severe infection after the onset of SLE that required hospitalization or occurred in the hospital setting. A total of 955 (18.5%) first severe infections occurred in the SLE group, compared with 1,988 (7.7%) in the controls, for incidence rates of 19.7 events per 1,000 person-years and 7.6 events per 1,000 person-years, respectively, yielding an 82% increased risk of severe infection for SLE patients after adjustment for confounding baseline factors.
Secondary outcomes of the total number of severe infections and infection-related mortality both showed significant increases in SLE patients, compared with controls. The total number of severe infections in the SLE and control groups was 1,898 and 3,114, respectively, with an adjusted risk ratio of 2.07.
As for mortality, a total of 539 deaths occurred in SLE patients during the study period, and 114 (21%) were related to severe infection. A total of 1,495 deaths occurred in the control group, including 269 (18%) related to severe infection. The adjusted hazard ratio was 1.61 after adjustment for confounding baseline variables.
The risks for first severe infection, total number of severe infections, and infection-related mortality were “independent of traditional risk factors for infection and the results remain robust in the presence of an unmeasured confounder (smoking) and competing risk of death,” the researchers said. Reasons for the increased risk are uncertain, but likely result from intrinsic factors such as immune system dysfunction and extrinsic factors such as the impact of immunosuppressive medications. “Future research can focus on quantifying the relative contributions of these intrinsic and extrinsic factors on the increased infection risk in SLE patients,” they added.
The study findings were limited by several factors linked to the observational design, including possible misdiagnosis of SLE and inaccurate measure of SLE onset, the researchers noted. In addition, no data were available for certain confounders such as smoking and nonhospitalized infections, they said.
However, the results were strengthened by the large size and general population and the use of sensitivity analyses, they noted. For SLE patients, “increased awareness of the risk of infections can identify their early signs and potentially prevent hospitalizations,” and clinicians can promote infection prevention strategies, including vaccinations when appropriate, they added.
Based on their findings, “we recommend a closer surveillance for severe infections in SLE patients and risk assessment for severe infections for SLE patients after diagnosis,” the researchers emphasized. “Further studies are warranted to further identify risk factors for infections in SLE patients to develop personalized treatment regimens and to select treatment in practice by synthesizing patient information,” they concluded.
The study was supported by the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
FROM RHEUMATOLOGY
Novel lupus therapies take center stage
It’s been a banner year for treatment advances in systemic lupus erythematosus (SLE), with two drugs gaining approval for lupus nephritis while other promising molecules with novel mechanisms of action advanced smartly through the developmental pipeline, speakers agreed at the 2021 Rheumatology Winter Clinical Symposium.
“I think the most important thing in rheumatology in the last year is where we are now with lupus. With two drugs being approved for lupus nephritis, I think that’s really huge as we talk about treat-to-target,” said Alvin F. Wells, MD, PhD, a rheumatologist in Franklin, Wisc.
Martin Bergman, MD, concurred.
“Lupus has been blowing up in the past year. We have two new medications for lupus nephritis, we have two or three new mechanisms of action for therapy. I think that was one of the biggest things in rheumatology in the past year,” said Dr. Bergman, a rheumatologist at Drexel University in Philadelphia and in private practice in Ridley Park, Pa.
Together with Roy Fleischmann, MD, Dr. Wells spotlighted promising new molecules for the treatment of SLE, giant cell arteritis, vasculitis, rheumatoid arthritis, and osteoarthritis.
SLE
The two drugs approved in recent months specifically for lupus nephritis are voclosporin (Lupkynis) and belimumab (Benlysta), which has been approved for lupus for a decade. Voclosporin, an oral calcineurin inhibitor, is a modification of cyclosporine offering significant advantages over the older drug: It’s more potent, requires no dose titration, has a better safety profile, and is metabolized more quickly.
“A safer and easier-to-use calcineurin inhibitor is going to be huge,” Dr. Wells predicted.
Up for Food and Drug Administration review in the coming year on the basis of the positive phase 3 TULIP-1 and TULIP-2 trials is anifrolumab, a monoclonal antibody that binds to the type 1 interferon receptor subunit 1d. At 52 weeks in the pooled analysis, one or more SLE flares occurred in 33.6% of patients on anifrolumab and 42.9% of placebo-treated controls.
“This is not a blockbuster, but it’s a worthwhile addition, like belimumab,” according to Dr. Fleischmann, a rheumatologist at the University of Texas, Dallas.
Dr. Wells concurred, with a reservation: In a subgroup analysis of the TULIP trials, anifrolumab wasn’t significantly better than placebo in black patients, who tend to have more severe and tough-to-treat renal disease.
“Anifrolumab doesn’t look as effective as some other agents, and I’d be disinclined to give it to my black patients,” the rheumatologist said.
Dr. Fleischmann was far more enthusiastic about obinutuzumab (Gazyva), a humanized anti-CD20 monoclonal antibody already approved for the treatment of chronic lymphocytic leukemia and follicular lymphoma.
“It’s an anti-CD20, like rituximab. But it’s better than rituximab, it’s much more effective,” he said.
He pointed to the phase 2 NOBILITY trial, in which 125 patients with class III/IV lupus nephritis were randomized to a 1,000-mg infusion of obinutuzumab or placebo at weeks 0, 2, 24, and 26 and followed for 2 years. The complete renal response rate at 104 weeks in the obinutuzumab group was 41% and the partial renal response rate was 13%, compared to 23% and 6% in controls. The obinutuzumab group also did significantly better in terms of improvement in complement levels, double-stranded DNA, and estimated glomerular filtration rate. All this was accomplished even though the reduction in peripheral B cells dropped from 93% at week 24 to just 16% at week 104. This suggests that tissue levels of B cells in the kidney, joints, and skin may be more important than circulating B cell levels.
“This looks like a very promising agent for patients with lupus nephritis,” Dr. Wells said. “The fact that they got this long-term effect for 2 years with just four infusions is really impressive.”
Another promising drug is iberdomide, an oral modulator of the E3 ubiquitin ligase complex which decreases plasmacytoid dendritic cells and B cells while increasing T regulatory cells. In a phase 2b clinical trial in 288 patients with active SLE, all on background standard-of-care therapy, a 4-point or greater reduction in the SLE Responder Index (SRI-4) at week 24 was achieved in 54.3% of the group on iberdomide at 0.45 mg/day, a significantly better result than the 34.9% rate with placebo. This absolute 19.4% difference was even greater in the subgroup of patients with a high baseline level of the transcription factor Aiolos, where the absolute improvement over placebo was 32.9%. Similarly, the benefit of iberdomide was also enhanced in patients with a high baseline level of type 1 interferon, where the absolute difference was 26.8%. This raises the prospect that a bioassay could be developed to predict the likelihood of a favorable clinical response to the drug. Iberdomide was well tolerated, with fewer severe adverse events than in the control group.
A humanized monoclonal antibody known for now as BIIB059 demonstrated efficacy and was well tolerated in the phase 2 LILAC trial. BIIB059 binds to blood dendritic cell antigen 2 (BDCA2), a receptor specific to plasmacytoid dendritic cells, resulting in decreased production of type 1 interferon and other inflammatory cytokines. The LILAC trial included 132 SLE patients with active arthritis and skin disease who received subcutaneous injections of BIIB059 at 450 mg or placebo every 4 weeks, with an extra dose at week 2. The primary endpoint was met, with an absolute 15-joint reduction in the total number of tender or swollen joints from baseline to week 24 in the BIIB059 group, compared to an 11.6-joint reduction with placebo. In addition, the likelihood of an SRI-4 response at week 24 was 3.49-fold greater with BIIB059 than with placebo.
Dr. Wells noted that the BIIB059 group showed continued improvement from week 12 to week 24, unlike the response pattern seen with many biologics for rheumatoid arthritis, where a plateau is reached by 8-12 weeks.
Vasculitis
The positive results for the C5a receptor inhibitor avacopan for treatment of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis in the phase-3 ADVOCATE trial have been hailed by some rheumatologists as a major breakthrough, but Dr. Fleischmann isn’t so sure.
The trial randomized 331 patients to oral avacopan at 30 mg twice daily or oral prednisone, with all patients on either cyclophosphamide or rituximab. Avacopan was noninferior to prednisone in terms of remission at week 26, but superior to prednisone for sustained taper at week 52. The rate of serious adverse events was 45.1% with prednisone and 42.2% in the avacopan arm.
“This is a drug that’s going to be much, much more expensive than prednisone. There were people in our group who were ecstatic that this drug is going to come, but how much it’s going to be used, I don’t know,” Dr. Fleischmann said.
Dr. Wells said cost-benefit analyses will be needed in order to learn if avacopan’s anticipated high sticker price is offset by the cost of serious corticosteroid side effects such as avascular necrosis.
Giant cell arteritis
Mavrilimumab is a human monoclonal antibody that inhibits human granulocyte macrophage colony stimulating factor receptor alpha. It demonstrated impressive efficacy in a phase 2, double-blind, randomized, placebo-controlled trial conducted in 70 patients with biopsy-confirmed giant cell arteritis. Participants were on corticosteroids until they went into remission and were then randomized to mavrilimumab or placebo, with the steroids stopped. By week 26, 19% of patients in the mavrilimumab arm had flared, as compared to 46.4% of controls.
“This is a game changer,” Dr. Wells declared. “I struggle with these patients because I can’t get the IL-6 drugs approved for them. I need something else.”
Dr. Fleischmann has a good idea how he’ll use mavrilimumab, if it wins approval: “I think this is clearly a drug you would use in a patient you can’t get off steroids and you’re having all the steroid toxicity. I don’t know that you’d use it right away.”
Osteoarthritis
Dr. Fleischmann predicted that tanezumab, a monoclonal antibody directed against nerve growth factor, will win FDA approval in 2021 for the treatment of osteoarthritis pain in patients with an inadequate response or intolerance to standard-of-care NSAIDs and opioids. But he cautioned his colleagues not to expect too much from the biologic, which has a long and checkered developmental history.
“It works better than placebo. It does not work better than an NSAID or an opioid. So it should be reasonable in patients who cannot take an NSAID or cannot or will not take an opioid,” he said.
There are safety issues to be aware of with tanezumab, he added: clinically significant increased risks of peripheral neuropathy and joint space narrowing.
Rheumatoid arthritis
Dr. Wells thought one of the most interesting novel therapies for RA in the past year didn’t involve a pharmaceutical, but rather noninvasive auricular branch stimulation of the vagus nerve. He cited an open-label, 12-week, uncontrolled study in 27 patients with active RA who wore an ear clip for vagal nerve stimulation for 12 weeks. The mean Disease Activity Score in 28 joints using C-reactive protein (DAS28-CRP) – the primary study endpoint – improved from 6.30 at baseline to 3.76 at week 12. The number of tender joints dropped from 12.17 to 4.7, while the swollen joint count went from 7.0 to 3.44. Pain scores improved from 75.23 to 43.3. Scores on the Health Assessment Questionnaire Disability Index improved from 1.59 to 1.05. There was no significant change in CRP. All in all, a modest clinical effect achieved noninvasively.
“The thing that did it for me was the effect on MRI from baseline: decreased synovitis, osteitis, and bone erosion scores,” Dr. Wells said. “This is noninvasive, so patients who want to do medical marijuana or CBD can put an earring on their auricular nerve.”
Dr. Fleischmann scoffed. “An open-label study, 27 patients? Let me see the real study,” he quipped.
Dr. Fleischmann reported receiving clinical trial research grants from and serving as a consultant to more than a dozen pharmaceutical companies. Dr. Wells serves as a consultant to MiCare Path.
It’s been a banner year for treatment advances in systemic lupus erythematosus (SLE), with two drugs gaining approval for lupus nephritis while other promising molecules with novel mechanisms of action advanced smartly through the developmental pipeline, speakers agreed at the 2021 Rheumatology Winter Clinical Symposium.
“I think the most important thing in rheumatology in the last year is where we are now with lupus. With two drugs being approved for lupus nephritis, I think that’s really huge as we talk about treat-to-target,” said Alvin F. Wells, MD, PhD, a rheumatologist in Franklin, Wisc.
Martin Bergman, MD, concurred.
“Lupus has been blowing up in the past year. We have two new medications for lupus nephritis, we have two or three new mechanisms of action for therapy. I think that was one of the biggest things in rheumatology in the past year,” said Dr. Bergman, a rheumatologist at Drexel University in Philadelphia and in private practice in Ridley Park, Pa.
Together with Roy Fleischmann, MD, Dr. Wells spotlighted promising new molecules for the treatment of SLE, giant cell arteritis, vasculitis, rheumatoid arthritis, and osteoarthritis.
SLE
The two drugs approved in recent months specifically for lupus nephritis are voclosporin (Lupkynis) and belimumab (Benlysta), which has been approved for lupus for a decade. Voclosporin, an oral calcineurin inhibitor, is a modification of cyclosporine offering significant advantages over the older drug: It’s more potent, requires no dose titration, has a better safety profile, and is metabolized more quickly.
“A safer and easier-to-use calcineurin inhibitor is going to be huge,” Dr. Wells predicted.
Up for Food and Drug Administration review in the coming year on the basis of the positive phase 3 TULIP-1 and TULIP-2 trials is anifrolumab, a monoclonal antibody that binds to the type 1 interferon receptor subunit 1d. At 52 weeks in the pooled analysis, one or more SLE flares occurred in 33.6% of patients on anifrolumab and 42.9% of placebo-treated controls.
“This is not a blockbuster, but it’s a worthwhile addition, like belimumab,” according to Dr. Fleischmann, a rheumatologist at the University of Texas, Dallas.
Dr. Wells concurred, with a reservation: In a subgroup analysis of the TULIP trials, anifrolumab wasn’t significantly better than placebo in black patients, who tend to have more severe and tough-to-treat renal disease.
“Anifrolumab doesn’t look as effective as some other agents, and I’d be disinclined to give it to my black patients,” the rheumatologist said.
Dr. Fleischmann was far more enthusiastic about obinutuzumab (Gazyva), a humanized anti-CD20 monoclonal antibody already approved for the treatment of chronic lymphocytic leukemia and follicular lymphoma.
“It’s an anti-CD20, like rituximab. But it’s better than rituximab, it’s much more effective,” he said.
He pointed to the phase 2 NOBILITY trial, in which 125 patients with class III/IV lupus nephritis were randomized to a 1,000-mg infusion of obinutuzumab or placebo at weeks 0, 2, 24, and 26 and followed for 2 years. The complete renal response rate at 104 weeks in the obinutuzumab group was 41% and the partial renal response rate was 13%, compared to 23% and 6% in controls. The obinutuzumab group also did significantly better in terms of improvement in complement levels, double-stranded DNA, and estimated glomerular filtration rate. All this was accomplished even though the reduction in peripheral B cells dropped from 93% at week 24 to just 16% at week 104. This suggests that tissue levels of B cells in the kidney, joints, and skin may be more important than circulating B cell levels.
“This looks like a very promising agent for patients with lupus nephritis,” Dr. Wells said. “The fact that they got this long-term effect for 2 years with just four infusions is really impressive.”
Another promising drug is iberdomide, an oral modulator of the E3 ubiquitin ligase complex which decreases plasmacytoid dendritic cells and B cells while increasing T regulatory cells. In a phase 2b clinical trial in 288 patients with active SLE, all on background standard-of-care therapy, a 4-point or greater reduction in the SLE Responder Index (SRI-4) at week 24 was achieved in 54.3% of the group on iberdomide at 0.45 mg/day, a significantly better result than the 34.9% rate with placebo. This absolute 19.4% difference was even greater in the subgroup of patients with a high baseline level of the transcription factor Aiolos, where the absolute improvement over placebo was 32.9%. Similarly, the benefit of iberdomide was also enhanced in patients with a high baseline level of type 1 interferon, where the absolute difference was 26.8%. This raises the prospect that a bioassay could be developed to predict the likelihood of a favorable clinical response to the drug. Iberdomide was well tolerated, with fewer severe adverse events than in the control group.
A humanized monoclonal antibody known for now as BIIB059 demonstrated efficacy and was well tolerated in the phase 2 LILAC trial. BIIB059 binds to blood dendritic cell antigen 2 (BDCA2), a receptor specific to plasmacytoid dendritic cells, resulting in decreased production of type 1 interferon and other inflammatory cytokines. The LILAC trial included 132 SLE patients with active arthritis and skin disease who received subcutaneous injections of BIIB059 at 450 mg or placebo every 4 weeks, with an extra dose at week 2. The primary endpoint was met, with an absolute 15-joint reduction in the total number of tender or swollen joints from baseline to week 24 in the BIIB059 group, compared to an 11.6-joint reduction with placebo. In addition, the likelihood of an SRI-4 response at week 24 was 3.49-fold greater with BIIB059 than with placebo.
Dr. Wells noted that the BIIB059 group showed continued improvement from week 12 to week 24, unlike the response pattern seen with many biologics for rheumatoid arthritis, where a plateau is reached by 8-12 weeks.
Vasculitis
The positive results for the C5a receptor inhibitor avacopan for treatment of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis in the phase-3 ADVOCATE trial have been hailed by some rheumatologists as a major breakthrough, but Dr. Fleischmann isn’t so sure.
The trial randomized 331 patients to oral avacopan at 30 mg twice daily or oral prednisone, with all patients on either cyclophosphamide or rituximab. Avacopan was noninferior to prednisone in terms of remission at week 26, but superior to prednisone for sustained taper at week 52. The rate of serious adverse events was 45.1% with prednisone and 42.2% in the avacopan arm.
“This is a drug that’s going to be much, much more expensive than prednisone. There were people in our group who were ecstatic that this drug is going to come, but how much it’s going to be used, I don’t know,” Dr. Fleischmann said.
Dr. Wells said cost-benefit analyses will be needed in order to learn if avacopan’s anticipated high sticker price is offset by the cost of serious corticosteroid side effects such as avascular necrosis.
Giant cell arteritis
Mavrilimumab is a human monoclonal antibody that inhibits human granulocyte macrophage colony stimulating factor receptor alpha. It demonstrated impressive efficacy in a phase 2, double-blind, randomized, placebo-controlled trial conducted in 70 patients with biopsy-confirmed giant cell arteritis. Participants were on corticosteroids until they went into remission and were then randomized to mavrilimumab or placebo, with the steroids stopped. By week 26, 19% of patients in the mavrilimumab arm had flared, as compared to 46.4% of controls.
“This is a game changer,” Dr. Wells declared. “I struggle with these patients because I can’t get the IL-6 drugs approved for them. I need something else.”
Dr. Fleischmann has a good idea how he’ll use mavrilimumab, if it wins approval: “I think this is clearly a drug you would use in a patient you can’t get off steroids and you’re having all the steroid toxicity. I don’t know that you’d use it right away.”
Osteoarthritis
Dr. Fleischmann predicted that tanezumab, a monoclonal antibody directed against nerve growth factor, will win FDA approval in 2021 for the treatment of osteoarthritis pain in patients with an inadequate response or intolerance to standard-of-care NSAIDs and opioids. But he cautioned his colleagues not to expect too much from the biologic, which has a long and checkered developmental history.
“It works better than placebo. It does not work better than an NSAID or an opioid. So it should be reasonable in patients who cannot take an NSAID or cannot or will not take an opioid,” he said.
There are safety issues to be aware of with tanezumab, he added: clinically significant increased risks of peripheral neuropathy and joint space narrowing.
Rheumatoid arthritis
Dr. Wells thought one of the most interesting novel therapies for RA in the past year didn’t involve a pharmaceutical, but rather noninvasive auricular branch stimulation of the vagus nerve. He cited an open-label, 12-week, uncontrolled study in 27 patients with active RA who wore an ear clip for vagal nerve stimulation for 12 weeks. The mean Disease Activity Score in 28 joints using C-reactive protein (DAS28-CRP) – the primary study endpoint – improved from 6.30 at baseline to 3.76 at week 12. The number of tender joints dropped from 12.17 to 4.7, while the swollen joint count went from 7.0 to 3.44. Pain scores improved from 75.23 to 43.3. Scores on the Health Assessment Questionnaire Disability Index improved from 1.59 to 1.05. There was no significant change in CRP. All in all, a modest clinical effect achieved noninvasively.
“The thing that did it for me was the effect on MRI from baseline: decreased synovitis, osteitis, and bone erosion scores,” Dr. Wells said. “This is noninvasive, so patients who want to do medical marijuana or CBD can put an earring on their auricular nerve.”
Dr. Fleischmann scoffed. “An open-label study, 27 patients? Let me see the real study,” he quipped.
Dr. Fleischmann reported receiving clinical trial research grants from and serving as a consultant to more than a dozen pharmaceutical companies. Dr. Wells serves as a consultant to MiCare Path.
It’s been a banner year for treatment advances in systemic lupus erythematosus (SLE), with two drugs gaining approval for lupus nephritis while other promising molecules with novel mechanisms of action advanced smartly through the developmental pipeline, speakers agreed at the 2021 Rheumatology Winter Clinical Symposium.
“I think the most important thing in rheumatology in the last year is where we are now with lupus. With two drugs being approved for lupus nephritis, I think that’s really huge as we talk about treat-to-target,” said Alvin F. Wells, MD, PhD, a rheumatologist in Franklin, Wisc.
Martin Bergman, MD, concurred.
“Lupus has been blowing up in the past year. We have two new medications for lupus nephritis, we have two or three new mechanisms of action for therapy. I think that was one of the biggest things in rheumatology in the past year,” said Dr. Bergman, a rheumatologist at Drexel University in Philadelphia and in private practice in Ridley Park, Pa.
Together with Roy Fleischmann, MD, Dr. Wells spotlighted promising new molecules for the treatment of SLE, giant cell arteritis, vasculitis, rheumatoid arthritis, and osteoarthritis.
SLE
The two drugs approved in recent months specifically for lupus nephritis are voclosporin (Lupkynis) and belimumab (Benlysta), which has been approved for lupus for a decade. Voclosporin, an oral calcineurin inhibitor, is a modification of cyclosporine offering significant advantages over the older drug: It’s more potent, requires no dose titration, has a better safety profile, and is metabolized more quickly.
“A safer and easier-to-use calcineurin inhibitor is going to be huge,” Dr. Wells predicted.
Up for Food and Drug Administration review in the coming year on the basis of the positive phase 3 TULIP-1 and TULIP-2 trials is anifrolumab, a monoclonal antibody that binds to the type 1 interferon receptor subunit 1d. At 52 weeks in the pooled analysis, one or more SLE flares occurred in 33.6% of patients on anifrolumab and 42.9% of placebo-treated controls.
“This is not a blockbuster, but it’s a worthwhile addition, like belimumab,” according to Dr. Fleischmann, a rheumatologist at the University of Texas, Dallas.
Dr. Wells concurred, with a reservation: In a subgroup analysis of the TULIP trials, anifrolumab wasn’t significantly better than placebo in black patients, who tend to have more severe and tough-to-treat renal disease.
“Anifrolumab doesn’t look as effective as some other agents, and I’d be disinclined to give it to my black patients,” the rheumatologist said.
Dr. Fleischmann was far more enthusiastic about obinutuzumab (Gazyva), a humanized anti-CD20 monoclonal antibody already approved for the treatment of chronic lymphocytic leukemia and follicular lymphoma.
“It’s an anti-CD20, like rituximab. But it’s better than rituximab, it’s much more effective,” he said.
He pointed to the phase 2 NOBILITY trial, in which 125 patients with class III/IV lupus nephritis were randomized to a 1,000-mg infusion of obinutuzumab or placebo at weeks 0, 2, 24, and 26 and followed for 2 years. The complete renal response rate at 104 weeks in the obinutuzumab group was 41% and the partial renal response rate was 13%, compared to 23% and 6% in controls. The obinutuzumab group also did significantly better in terms of improvement in complement levels, double-stranded DNA, and estimated glomerular filtration rate. All this was accomplished even though the reduction in peripheral B cells dropped from 93% at week 24 to just 16% at week 104. This suggests that tissue levels of B cells in the kidney, joints, and skin may be more important than circulating B cell levels.
“This looks like a very promising agent for patients with lupus nephritis,” Dr. Wells said. “The fact that they got this long-term effect for 2 years with just four infusions is really impressive.”
Another promising drug is iberdomide, an oral modulator of the E3 ubiquitin ligase complex which decreases plasmacytoid dendritic cells and B cells while increasing T regulatory cells. In a phase 2b clinical trial in 288 patients with active SLE, all on background standard-of-care therapy, a 4-point or greater reduction in the SLE Responder Index (SRI-4) at week 24 was achieved in 54.3% of the group on iberdomide at 0.45 mg/day, a significantly better result than the 34.9% rate with placebo. This absolute 19.4% difference was even greater in the subgroup of patients with a high baseline level of the transcription factor Aiolos, where the absolute improvement over placebo was 32.9%. Similarly, the benefit of iberdomide was also enhanced in patients with a high baseline level of type 1 interferon, where the absolute difference was 26.8%. This raises the prospect that a bioassay could be developed to predict the likelihood of a favorable clinical response to the drug. Iberdomide was well tolerated, with fewer severe adverse events than in the control group.
A humanized monoclonal antibody known for now as BIIB059 demonstrated efficacy and was well tolerated in the phase 2 LILAC trial. BIIB059 binds to blood dendritic cell antigen 2 (BDCA2), a receptor specific to plasmacytoid dendritic cells, resulting in decreased production of type 1 interferon and other inflammatory cytokines. The LILAC trial included 132 SLE patients with active arthritis and skin disease who received subcutaneous injections of BIIB059 at 450 mg or placebo every 4 weeks, with an extra dose at week 2. The primary endpoint was met, with an absolute 15-joint reduction in the total number of tender or swollen joints from baseline to week 24 in the BIIB059 group, compared to an 11.6-joint reduction with placebo. In addition, the likelihood of an SRI-4 response at week 24 was 3.49-fold greater with BIIB059 than with placebo.
Dr. Wells noted that the BIIB059 group showed continued improvement from week 12 to week 24, unlike the response pattern seen with many biologics for rheumatoid arthritis, where a plateau is reached by 8-12 weeks.
Vasculitis
The positive results for the C5a receptor inhibitor avacopan for treatment of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis in the phase-3 ADVOCATE trial have been hailed by some rheumatologists as a major breakthrough, but Dr. Fleischmann isn’t so sure.
The trial randomized 331 patients to oral avacopan at 30 mg twice daily or oral prednisone, with all patients on either cyclophosphamide or rituximab. Avacopan was noninferior to prednisone in terms of remission at week 26, but superior to prednisone for sustained taper at week 52. The rate of serious adverse events was 45.1% with prednisone and 42.2% in the avacopan arm.
“This is a drug that’s going to be much, much more expensive than prednisone. There were people in our group who were ecstatic that this drug is going to come, but how much it’s going to be used, I don’t know,” Dr. Fleischmann said.
Dr. Wells said cost-benefit analyses will be needed in order to learn if avacopan’s anticipated high sticker price is offset by the cost of serious corticosteroid side effects such as avascular necrosis.
Giant cell arteritis
Mavrilimumab is a human monoclonal antibody that inhibits human granulocyte macrophage colony stimulating factor receptor alpha. It demonstrated impressive efficacy in a phase 2, double-blind, randomized, placebo-controlled trial conducted in 70 patients with biopsy-confirmed giant cell arteritis. Participants were on corticosteroids until they went into remission and were then randomized to mavrilimumab or placebo, with the steroids stopped. By week 26, 19% of patients in the mavrilimumab arm had flared, as compared to 46.4% of controls.
“This is a game changer,” Dr. Wells declared. “I struggle with these patients because I can’t get the IL-6 drugs approved for them. I need something else.”
Dr. Fleischmann has a good idea how he’ll use mavrilimumab, if it wins approval: “I think this is clearly a drug you would use in a patient you can’t get off steroids and you’re having all the steroid toxicity. I don’t know that you’d use it right away.”
Osteoarthritis
Dr. Fleischmann predicted that tanezumab, a monoclonal antibody directed against nerve growth factor, will win FDA approval in 2021 for the treatment of osteoarthritis pain in patients with an inadequate response or intolerance to standard-of-care NSAIDs and opioids. But he cautioned his colleagues not to expect too much from the biologic, which has a long and checkered developmental history.
“It works better than placebo. It does not work better than an NSAID or an opioid. So it should be reasonable in patients who cannot take an NSAID or cannot or will not take an opioid,” he said.
There are safety issues to be aware of with tanezumab, he added: clinically significant increased risks of peripheral neuropathy and joint space narrowing.
Rheumatoid arthritis
Dr. Wells thought one of the most interesting novel therapies for RA in the past year didn’t involve a pharmaceutical, but rather noninvasive auricular branch stimulation of the vagus nerve. He cited an open-label, 12-week, uncontrolled study in 27 patients with active RA who wore an ear clip for vagal nerve stimulation for 12 weeks. The mean Disease Activity Score in 28 joints using C-reactive protein (DAS28-CRP) – the primary study endpoint – improved from 6.30 at baseline to 3.76 at week 12. The number of tender joints dropped from 12.17 to 4.7, while the swollen joint count went from 7.0 to 3.44. Pain scores improved from 75.23 to 43.3. Scores on the Health Assessment Questionnaire Disability Index improved from 1.59 to 1.05. There was no significant change in CRP. All in all, a modest clinical effect achieved noninvasively.
“The thing that did it for me was the effect on MRI from baseline: decreased synovitis, osteitis, and bone erosion scores,” Dr. Wells said. “This is noninvasive, so patients who want to do medical marijuana or CBD can put an earring on their auricular nerve.”
Dr. Fleischmann scoffed. “An open-label study, 27 patients? Let me see the real study,” he quipped.
Dr. Fleischmann reported receiving clinical trial research grants from and serving as a consultant to more than a dozen pharmaceutical companies. Dr. Wells serves as a consultant to MiCare Path.
FROM RWCS 2021
Tocilizumab (Actemra) scores FDA approval for systemic sclerosis–associated interstitial lung disease
The Food and Drug Administration has approved subcutaneously-injected tocilizumab (Actemra) to reduce the rate of pulmonary function decline in systemic sclerosis–associated interstitial lung disease (SSc-ILD) patients, according to a press release from manufacturer Genentech.
Tocilizumab is the first biologic to be approved by the agency for adults with SSc-ILD, a rare and potentially life-threatening condition that may affect up to 80% of SSc patients and lead to lung inflammation and scarring.
The approval was based primarily on data from a phase 3 randomized, double-blind, placebo-controlled clinical trial (the focuSSced trial) that included 212 adults with SSc. Although that study failed to meet its primary endpoint of change from baseline to 48 weeks in the modified Rodnan Skin Score, the researchers observed a significantly reduced lung function decline as measured by forced vital capacity (FVC) and percent predicted forced vital capacity (ppFVC) among tocilizumab-treated patients, compared with those who received placebo. A total of 68 patients (65%) in the tocilizumab group and 68 patients (64%) in the placebo group had SSc-ILD at baseline.
In a subgroup analysis, patients taking tocilizumab had a smaller decline in mean ppFVC, compared with placebo patients (0.07% vs. –6.4%; mean difference, 6.47%), and a smaller decline in FVC (mean change –14 mL vs. –255 mL with placebo; mean difference, 241 mL).
The mean change from baseline to week 48 in modified Rodnan Skin Score was –5.88 for patients on tocilizumab and –3.77 with placebo.
Safety data were similar between tocilizumab and placebo groups through 48 weeks, and similar for patients with and without SSc-ILD. In general, tocilizumab side effects include increased susceptibility to infections, and serious side effects may include stomach tears, hepatotoxicity, and increased risk of cancer and hepatitis B, according to the prescribing information. However, the most common side effects are upper respiratory tract infections, headache, hypertension, and injection-site reactions.
Tocilizumab, an interleukin-6 receptor antagonist, is already approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis, as well as for adult patients with giant cell arteritis; patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis or active systemic juvenile idiopathic arthritis; and adults and pediatric patients 2 years of age and older with chimeric antigen receptor T-cell–induced severe or life-threatening cytokine release syndrome.
Prescribing information is available here.
The Food and Drug Administration has approved subcutaneously-injected tocilizumab (Actemra) to reduce the rate of pulmonary function decline in systemic sclerosis–associated interstitial lung disease (SSc-ILD) patients, according to a press release from manufacturer Genentech.
Tocilizumab is the first biologic to be approved by the agency for adults with SSc-ILD, a rare and potentially life-threatening condition that may affect up to 80% of SSc patients and lead to lung inflammation and scarring.
The approval was based primarily on data from a phase 3 randomized, double-blind, placebo-controlled clinical trial (the focuSSced trial) that included 212 adults with SSc. Although that study failed to meet its primary endpoint of change from baseline to 48 weeks in the modified Rodnan Skin Score, the researchers observed a significantly reduced lung function decline as measured by forced vital capacity (FVC) and percent predicted forced vital capacity (ppFVC) among tocilizumab-treated patients, compared with those who received placebo. A total of 68 patients (65%) in the tocilizumab group and 68 patients (64%) in the placebo group had SSc-ILD at baseline.
In a subgroup analysis, patients taking tocilizumab had a smaller decline in mean ppFVC, compared with placebo patients (0.07% vs. –6.4%; mean difference, 6.47%), and a smaller decline in FVC (mean change –14 mL vs. –255 mL with placebo; mean difference, 241 mL).
The mean change from baseline to week 48 in modified Rodnan Skin Score was –5.88 for patients on tocilizumab and –3.77 with placebo.
Safety data were similar between tocilizumab and placebo groups through 48 weeks, and similar for patients with and without SSc-ILD. In general, tocilizumab side effects include increased susceptibility to infections, and serious side effects may include stomach tears, hepatotoxicity, and increased risk of cancer and hepatitis B, according to the prescribing information. However, the most common side effects are upper respiratory tract infections, headache, hypertension, and injection-site reactions.
Tocilizumab, an interleukin-6 receptor antagonist, is already approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis, as well as for adult patients with giant cell arteritis; patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis or active systemic juvenile idiopathic arthritis; and adults and pediatric patients 2 years of age and older with chimeric antigen receptor T-cell–induced severe or life-threatening cytokine release syndrome.
Prescribing information is available here.
The Food and Drug Administration has approved subcutaneously-injected tocilizumab (Actemra) to reduce the rate of pulmonary function decline in systemic sclerosis–associated interstitial lung disease (SSc-ILD) patients, according to a press release from manufacturer Genentech.
Tocilizumab is the first biologic to be approved by the agency for adults with SSc-ILD, a rare and potentially life-threatening condition that may affect up to 80% of SSc patients and lead to lung inflammation and scarring.
The approval was based primarily on data from a phase 3 randomized, double-blind, placebo-controlled clinical trial (the focuSSced trial) that included 212 adults with SSc. Although that study failed to meet its primary endpoint of change from baseline to 48 weeks in the modified Rodnan Skin Score, the researchers observed a significantly reduced lung function decline as measured by forced vital capacity (FVC) and percent predicted forced vital capacity (ppFVC) among tocilizumab-treated patients, compared with those who received placebo. A total of 68 patients (65%) in the tocilizumab group and 68 patients (64%) in the placebo group had SSc-ILD at baseline.
In a subgroup analysis, patients taking tocilizumab had a smaller decline in mean ppFVC, compared with placebo patients (0.07% vs. –6.4%; mean difference, 6.47%), and a smaller decline in FVC (mean change –14 mL vs. –255 mL with placebo; mean difference, 241 mL).
The mean change from baseline to week 48 in modified Rodnan Skin Score was –5.88 for patients on tocilizumab and –3.77 with placebo.
Safety data were similar between tocilizumab and placebo groups through 48 weeks, and similar for patients with and without SSc-ILD. In general, tocilizumab side effects include increased susceptibility to infections, and serious side effects may include stomach tears, hepatotoxicity, and increased risk of cancer and hepatitis B, according to the prescribing information. However, the most common side effects are upper respiratory tract infections, headache, hypertension, and injection-site reactions.
Tocilizumab, an interleukin-6 receptor antagonist, is already approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis, as well as for adult patients with giant cell arteritis; patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis or active systemic juvenile idiopathic arthritis; and adults and pediatric patients 2 years of age and older with chimeric antigen receptor T-cell–induced severe or life-threatening cytokine release syndrome.
Prescribing information is available here.
ACR, AAD, AAO, RDS issue joint statement on safe use of hydroxychloroquine
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
FROM ARTHRITIS & rHEUMATOLOGY
COVID-19 vaccination recommended for rheumatology patients
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.