User login
Bone risk: Is time since menopause a better predictor than age?
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Preterm delivery raises lifetime hypertension risk
Women who had a preterm delivery were at least 1.6 times as likely to develop hypertension over the next decade as those who had full-term deliveries, based on data from a national cohort study of more than 2 million women.
Pregnancy complications such as preeclampsia and other hypertensive disorders of pregnancy have been associated with chronic hypertension as well as with preterm delivery, but the independent role of preterm delivery in chronic hypertension risk remains unclear, Casey Crump, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues wrote. “A better understanding of the long-term hypertension risks associated with preterm delivery is needed to improve risk stratification, clinical monitoring, and CVD [cardiovascular disease] prevention in women.”
In a study published in JAMA Cardiology, the researchers reviewed data from 2,195,989 women with 4,308,286 singleton deliveries in Sweden from Jan. 1, 1973, to Dec. 31, 2015. Women with preexisting hypertension before their first pregnancy were excluded. Pregnancy duration was based on maternal reports of the last menstrual period for patients in the 1970s, and based on ultrasound estimates in the 1980s and beyond. Pregnancy duration was divided into six groups in terms of completed weeks of gestation: extremely preterm (22-27 weeks), moderately preterm (28-33 weeks), late preterm (34-36 weeks), early term (37-38 weeks), full term (39-41 weeks), and post term (≥42 weeks). Full-term delivery was used as the reference, and the three preterm groups were combined for summaries of preterm delivery (less than 37 weeks).
Overall, women who delivered at less than 37 weeks’ gestation had a 1.6-fold increased risk of hypertension (adjusted hazard ratio, 1.67) within the next 10 years, compared with women who delivered full term after controlling for preeclampsia, other hypertensive disorders of pregnancy, and maternal factors.
When further stratified by pregnancy duration, the aHRs for extremely preterm, moderately preterm, late preterm, and early term, compared with full-term deliveries were 2.23, 1.85, 1.55, and 1.26, respectively, in the first decade after delivery. Each additional week of pregnancy was associated with a mean 7% reduction in hypertension risk (a HR, 0.93).
The increased hypertension risk following preterm delivery (less than 37 weeks) persisted at 10-19 years, 20-29 years, and 30-43 years, with aHRs of 1.40, 1.20, and 1.12, respectively. Early-term delivery at 37-38 weeks also carried an increased risk of long-term hypertension compared with full-term delivery, with aHRs of 1.12 and 1.06 at 20-29 years and 30-43 years, respectively.
“Cosibling analyses suggested that these findings were only partially explained by familial (genetic and/or early-life environmental) factors that are shared determinants of both preterm delivery and hypertension,” the researchers noted. The findings suggest that preterm delivery itself may contribute to or affect the pathophysiology that leads to cardiovascular disease, they added, hypothesizing that endothelial dysfunction caused by preterm delivery may cause functional impairments in the microvasculature.
The study findings were limited by several factors including the lack of detailed records to verify hypertension and the use of data from a single country, the researchers noted. However, the results were strengthened by the large study population, the use of highly complete prenatal and birth records to minimize selection bias, and the long-term follow-up.
The results are consistent with those from previous studies, and support the recognition of preterm delivery as a lifetime risk factor for hypertension, but future studies should focus on racial and ethnic subgroups already at increased risk for both preterm delivery and hypertension, they added.
“Additional follow-up will be needed to examine these associations in older adulthood when hypertension increasingly and disproportionately affects women,” they concluded.
Data highlight the need for patient and provider education
“This study furthers our knowledge regarding long-term complications associated with the frequent pregnancy complication of preterm delivery,” Stephen S. Crane, MD, an ob.gyn. and maternal-fetal medicine specialist in private practice in Orlando, said in an interview. “Cardiovascular disease is the leading cause of death and often goes unrecognized in women. There are shared risk factors among women and men for developing CVD, the most common being hypertension. However, women have the unique risk factor of pregnancy and its attendant complications including preeclampsia, glucose intolerance, and preterm delivery. Hypertensive disorders in pregnancy often lead to indicated premature delivery, and are associated with development of chronic hypertension and subsequent CVD. However, prior data suggest that preterm delivery itself is a risk factor for developing chronic hypertension later in life.
“The current study, which evaluates one of the most complete population data sets with up to 43 years of follow-up, is the first to assess for familial determinants by cosibling analysis, and supports preterm delivery as an independent risk factor for the development of hypertension,” he said. The study results illustrate that this risk is longstanding, and that recurrent preterm birth further increases the risk of developing hypertension.
Dr. Crane said he was not surprised by the study findings, given that inflammatory processes have been linked to the development of hypertension and CVD. “Similarly, inflammatory processes have been implicated in the pathophysiology of preterm labor and inflammatory cytokines may also play a role in normal term labor. Therefore, it is not surprising that preterm delivery would be a marker for the risk of development of hypertension, as both may be responses to underlying inflammatory processes. Identification of these underlying inflammatory processes and methods for prevention will be critical if we are to decrease both the incidence of preterm birth and CVD.
“As prenatal care may be the only medical care women obtain, it is important to take this opportunity to educate patients regarding their long-term risks of developing hypertension and the need for long-term follow up. Interventions that may help reduce the risk for recurrent preterm birth and long-term risks for developing hypertension and CVD include weight loss, increased activity, and smoking cessation; the resources to achieve these goals need to be shared with patients,” he said.
“Knowledge deficits both on the part of the provider and patient may be a significant barrier to intervention that may be overcome with improved education,” said Dr. Crane. “Care providers need education regarding the long-term risks associated with a history of preterm delivery in order to better educate their patients regarding both prevention of recurrent preterm birth and the development of hypertension and CVD.” However, socioeconomic status, education level, and the inability to obtain further health care remain common barriers to intervention for many women.
“Additional research is needed to identify the causes of inflammatory processes leading to preterm delivery and risks for hypertension and CVD,” said Dr. Crane. “Only after the causes are identified can treatments be sought to successfully treat these conditions.”
The study was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health; the Swedish Research Council; the Swedish Heart-Lung Foundation; and an Avtal om Läkarutbildning och Forskning (Agreement on Medical Training and Research) (ALF) project grant from Region Skåne/Lund University. Neither the researchers nor Dr. Crane had any financial conflicts to disclose.
Women who had a preterm delivery were at least 1.6 times as likely to develop hypertension over the next decade as those who had full-term deliveries, based on data from a national cohort study of more than 2 million women.
Pregnancy complications such as preeclampsia and other hypertensive disorders of pregnancy have been associated with chronic hypertension as well as with preterm delivery, but the independent role of preterm delivery in chronic hypertension risk remains unclear, Casey Crump, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues wrote. “A better understanding of the long-term hypertension risks associated with preterm delivery is needed to improve risk stratification, clinical monitoring, and CVD [cardiovascular disease] prevention in women.”
In a study published in JAMA Cardiology, the researchers reviewed data from 2,195,989 women with 4,308,286 singleton deliveries in Sweden from Jan. 1, 1973, to Dec. 31, 2015. Women with preexisting hypertension before their first pregnancy were excluded. Pregnancy duration was based on maternal reports of the last menstrual period for patients in the 1970s, and based on ultrasound estimates in the 1980s and beyond. Pregnancy duration was divided into six groups in terms of completed weeks of gestation: extremely preterm (22-27 weeks), moderately preterm (28-33 weeks), late preterm (34-36 weeks), early term (37-38 weeks), full term (39-41 weeks), and post term (≥42 weeks). Full-term delivery was used as the reference, and the three preterm groups were combined for summaries of preterm delivery (less than 37 weeks).
Overall, women who delivered at less than 37 weeks’ gestation had a 1.6-fold increased risk of hypertension (adjusted hazard ratio, 1.67) within the next 10 years, compared with women who delivered full term after controlling for preeclampsia, other hypertensive disorders of pregnancy, and maternal factors.
When further stratified by pregnancy duration, the aHRs for extremely preterm, moderately preterm, late preterm, and early term, compared with full-term deliveries were 2.23, 1.85, 1.55, and 1.26, respectively, in the first decade after delivery. Each additional week of pregnancy was associated with a mean 7% reduction in hypertension risk (a HR, 0.93).
The increased hypertension risk following preterm delivery (less than 37 weeks) persisted at 10-19 years, 20-29 years, and 30-43 years, with aHRs of 1.40, 1.20, and 1.12, respectively. Early-term delivery at 37-38 weeks also carried an increased risk of long-term hypertension compared with full-term delivery, with aHRs of 1.12 and 1.06 at 20-29 years and 30-43 years, respectively.
“Cosibling analyses suggested that these findings were only partially explained by familial (genetic and/or early-life environmental) factors that are shared determinants of both preterm delivery and hypertension,” the researchers noted. The findings suggest that preterm delivery itself may contribute to or affect the pathophysiology that leads to cardiovascular disease, they added, hypothesizing that endothelial dysfunction caused by preterm delivery may cause functional impairments in the microvasculature.
The study findings were limited by several factors including the lack of detailed records to verify hypertension and the use of data from a single country, the researchers noted. However, the results were strengthened by the large study population, the use of highly complete prenatal and birth records to minimize selection bias, and the long-term follow-up.
The results are consistent with those from previous studies, and support the recognition of preterm delivery as a lifetime risk factor for hypertension, but future studies should focus on racial and ethnic subgroups already at increased risk for both preterm delivery and hypertension, they added.
“Additional follow-up will be needed to examine these associations in older adulthood when hypertension increasingly and disproportionately affects women,” they concluded.
Data highlight the need for patient and provider education
“This study furthers our knowledge regarding long-term complications associated with the frequent pregnancy complication of preterm delivery,” Stephen S. Crane, MD, an ob.gyn. and maternal-fetal medicine specialist in private practice in Orlando, said in an interview. “Cardiovascular disease is the leading cause of death and often goes unrecognized in women. There are shared risk factors among women and men for developing CVD, the most common being hypertension. However, women have the unique risk factor of pregnancy and its attendant complications including preeclampsia, glucose intolerance, and preterm delivery. Hypertensive disorders in pregnancy often lead to indicated premature delivery, and are associated with development of chronic hypertension and subsequent CVD. However, prior data suggest that preterm delivery itself is a risk factor for developing chronic hypertension later in life.
“The current study, which evaluates one of the most complete population data sets with up to 43 years of follow-up, is the first to assess for familial determinants by cosibling analysis, and supports preterm delivery as an independent risk factor for the development of hypertension,” he said. The study results illustrate that this risk is longstanding, and that recurrent preterm birth further increases the risk of developing hypertension.
Dr. Crane said he was not surprised by the study findings, given that inflammatory processes have been linked to the development of hypertension and CVD. “Similarly, inflammatory processes have been implicated in the pathophysiology of preterm labor and inflammatory cytokines may also play a role in normal term labor. Therefore, it is not surprising that preterm delivery would be a marker for the risk of development of hypertension, as both may be responses to underlying inflammatory processes. Identification of these underlying inflammatory processes and methods for prevention will be critical if we are to decrease both the incidence of preterm birth and CVD.
“As prenatal care may be the only medical care women obtain, it is important to take this opportunity to educate patients regarding their long-term risks of developing hypertension and the need for long-term follow up. Interventions that may help reduce the risk for recurrent preterm birth and long-term risks for developing hypertension and CVD include weight loss, increased activity, and smoking cessation; the resources to achieve these goals need to be shared with patients,” he said.
“Knowledge deficits both on the part of the provider and patient may be a significant barrier to intervention that may be overcome with improved education,” said Dr. Crane. “Care providers need education regarding the long-term risks associated with a history of preterm delivery in order to better educate their patients regarding both prevention of recurrent preterm birth and the development of hypertension and CVD.” However, socioeconomic status, education level, and the inability to obtain further health care remain common barriers to intervention for many women.
“Additional research is needed to identify the causes of inflammatory processes leading to preterm delivery and risks for hypertension and CVD,” said Dr. Crane. “Only after the causes are identified can treatments be sought to successfully treat these conditions.”
The study was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health; the Swedish Research Council; the Swedish Heart-Lung Foundation; and an Avtal om Läkarutbildning och Forskning (Agreement on Medical Training and Research) (ALF) project grant from Region Skåne/Lund University. Neither the researchers nor Dr. Crane had any financial conflicts to disclose.
Women who had a preterm delivery were at least 1.6 times as likely to develop hypertension over the next decade as those who had full-term deliveries, based on data from a national cohort study of more than 2 million women.
Pregnancy complications such as preeclampsia and other hypertensive disorders of pregnancy have been associated with chronic hypertension as well as with preterm delivery, but the independent role of preterm delivery in chronic hypertension risk remains unclear, Casey Crump, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues wrote. “A better understanding of the long-term hypertension risks associated with preterm delivery is needed to improve risk stratification, clinical monitoring, and CVD [cardiovascular disease] prevention in women.”
In a study published in JAMA Cardiology, the researchers reviewed data from 2,195,989 women with 4,308,286 singleton deliveries in Sweden from Jan. 1, 1973, to Dec. 31, 2015. Women with preexisting hypertension before their first pregnancy were excluded. Pregnancy duration was based on maternal reports of the last menstrual period for patients in the 1970s, and based on ultrasound estimates in the 1980s and beyond. Pregnancy duration was divided into six groups in terms of completed weeks of gestation: extremely preterm (22-27 weeks), moderately preterm (28-33 weeks), late preterm (34-36 weeks), early term (37-38 weeks), full term (39-41 weeks), and post term (≥42 weeks). Full-term delivery was used as the reference, and the three preterm groups were combined for summaries of preterm delivery (less than 37 weeks).
Overall, women who delivered at less than 37 weeks’ gestation had a 1.6-fold increased risk of hypertension (adjusted hazard ratio, 1.67) within the next 10 years, compared with women who delivered full term after controlling for preeclampsia, other hypertensive disorders of pregnancy, and maternal factors.
When further stratified by pregnancy duration, the aHRs for extremely preterm, moderately preterm, late preterm, and early term, compared with full-term deliveries were 2.23, 1.85, 1.55, and 1.26, respectively, in the first decade after delivery. Each additional week of pregnancy was associated with a mean 7% reduction in hypertension risk (a HR, 0.93).
The increased hypertension risk following preterm delivery (less than 37 weeks) persisted at 10-19 years, 20-29 years, and 30-43 years, with aHRs of 1.40, 1.20, and 1.12, respectively. Early-term delivery at 37-38 weeks also carried an increased risk of long-term hypertension compared with full-term delivery, with aHRs of 1.12 and 1.06 at 20-29 years and 30-43 years, respectively.
“Cosibling analyses suggested that these findings were only partially explained by familial (genetic and/or early-life environmental) factors that are shared determinants of both preterm delivery and hypertension,” the researchers noted. The findings suggest that preterm delivery itself may contribute to or affect the pathophysiology that leads to cardiovascular disease, they added, hypothesizing that endothelial dysfunction caused by preterm delivery may cause functional impairments in the microvasculature.
The study findings were limited by several factors including the lack of detailed records to verify hypertension and the use of data from a single country, the researchers noted. However, the results were strengthened by the large study population, the use of highly complete prenatal and birth records to minimize selection bias, and the long-term follow-up.
The results are consistent with those from previous studies, and support the recognition of preterm delivery as a lifetime risk factor for hypertension, but future studies should focus on racial and ethnic subgroups already at increased risk for both preterm delivery and hypertension, they added.
“Additional follow-up will be needed to examine these associations in older adulthood when hypertension increasingly and disproportionately affects women,” they concluded.
Data highlight the need for patient and provider education
“This study furthers our knowledge regarding long-term complications associated with the frequent pregnancy complication of preterm delivery,” Stephen S. Crane, MD, an ob.gyn. and maternal-fetal medicine specialist in private practice in Orlando, said in an interview. “Cardiovascular disease is the leading cause of death and often goes unrecognized in women. There are shared risk factors among women and men for developing CVD, the most common being hypertension. However, women have the unique risk factor of pregnancy and its attendant complications including preeclampsia, glucose intolerance, and preterm delivery. Hypertensive disorders in pregnancy often lead to indicated premature delivery, and are associated with development of chronic hypertension and subsequent CVD. However, prior data suggest that preterm delivery itself is a risk factor for developing chronic hypertension later in life.
“The current study, which evaluates one of the most complete population data sets with up to 43 years of follow-up, is the first to assess for familial determinants by cosibling analysis, and supports preterm delivery as an independent risk factor for the development of hypertension,” he said. The study results illustrate that this risk is longstanding, and that recurrent preterm birth further increases the risk of developing hypertension.
Dr. Crane said he was not surprised by the study findings, given that inflammatory processes have been linked to the development of hypertension and CVD. “Similarly, inflammatory processes have been implicated in the pathophysiology of preterm labor and inflammatory cytokines may also play a role in normal term labor. Therefore, it is not surprising that preterm delivery would be a marker for the risk of development of hypertension, as both may be responses to underlying inflammatory processes. Identification of these underlying inflammatory processes and methods for prevention will be critical if we are to decrease both the incidence of preterm birth and CVD.
“As prenatal care may be the only medical care women obtain, it is important to take this opportunity to educate patients regarding their long-term risks of developing hypertension and the need for long-term follow up. Interventions that may help reduce the risk for recurrent preterm birth and long-term risks for developing hypertension and CVD include weight loss, increased activity, and smoking cessation; the resources to achieve these goals need to be shared with patients,” he said.
“Knowledge deficits both on the part of the provider and patient may be a significant barrier to intervention that may be overcome with improved education,” said Dr. Crane. “Care providers need education regarding the long-term risks associated with a history of preterm delivery in order to better educate their patients regarding both prevention of recurrent preterm birth and the development of hypertension and CVD.” However, socioeconomic status, education level, and the inability to obtain further health care remain common barriers to intervention for many women.
“Additional research is needed to identify the causes of inflammatory processes leading to preterm delivery and risks for hypertension and CVD,” said Dr. Crane. “Only after the causes are identified can treatments be sought to successfully treat these conditions.”
The study was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health; the Swedish Research Council; the Swedish Heart-Lung Foundation; and an Avtal om Läkarutbildning och Forskning (Agreement on Medical Training and Research) (ALF) project grant from Region Skåne/Lund University. Neither the researchers nor Dr. Crane had any financial conflicts to disclose.
FROM JAMA CARDIOLOGY
Oral PTH shows promise for osteoporosis in early phase 2 study
An investigational oral form of parathyroid hormone (PTH 1-34), EB 613 (Entera Bio) met its primary efficacy outcome in a phase 2 dosing study involving postmenopausal women with low bone mineral density (BMD).
The adverse effect profile of the drug was similar to that of the injectable PTH 1-34 teriparatide (Forteo), which is approved for osteoporosis.
Arthur C. Santora, MD, chief medical officer, Entera Bio, presented 6-month findings from the study during an oral session at the annual meeting of the American Society of Bone and Mineral Research. The 3-month findings from the study were reported as a poster.
If the drug demonstrates efficacy and safety in larger phase 3 trials, it could be the first oral bone-building (anabolic) therapy for osteoporosis.
Clifford J. Rosen, MD, PhD, who was not involved with the research, told this news organization: “I think this is an intriguing study.” The most likely patients for oral PTH, he added, “are those that have osteoporosis, previous fracture, or very low BMD, particularly those unlikely or unwilling to take bisphosphonates.”
However, “this is very early in the process before this drug could come to market,” cautioned Dr. Rosen, who is director of the Center for Clinical and Translational Research, Maine Medical Research Institute, Scarborough.
“Much more data on efficacy are required at 12 and 24 months for phase 2, and then a full phase 3 [clinical trial] with high-risk fracture patients,” he said.
The company is seeking input from the Food and Drug Administration to develop the protocol for a phase 3 trial. They expect to start this trial in 2022 at sites in the United States, Europe, and Israel, Dr. Santora said.
Primary outcome met
The study randomly assigned 161 postmenopausal women with osteoporosis or low BMD to receive placebo or the investigational oral PTH for 6 months.
Compared with women who received placebo, those who received the study drug experienced a significantly greater increase in the bone formation marker procollagen type I N-terminal propeptide (P1NP) from baseline to 3 months, thereby meeting the study’s primary outcome.
In secondary outcomes, women who received the 2.5-mg/d dose experienced a similar 6-month increase in BMD at the spine and greater increases in BMD at the total hip and femoral neck than those who received injectable teriparatide, Dr. Santora reported.
“The study’s key takeaway is that a once-daily oral PTH [tablet] has the potential to produce the same BMD effects as subcutaneous injections of PTH,” he said in an interview.
Additionally, “the drug was well tolerated when the dose was titrated by adding additional tablets, which suggests that the dose can be tailored to each patient,” he said.
Other study findings
Injectable teriparatide reduces the risk for vertebral fractures by up to 80%, Dr. Santora noted, but the fact that the drug must be administered by injection may deter some older patients from using it.
The company developed an oral form of biosynthetic human PTH with a proprietary drug delivery.
The researchers conducted the phase 2 study at four sites in Israel between June 2019 and May 2021. They enrolled women aged 50 years and older who had entered menopause at least 3 years earlier and who had osteoporosis or low BMD.
Forty-three women received placebo, and the others received oral PTH at doses of 0.5 mg/d (n = 25), 1.0 mg/d (n = 29), 1.5 mg/d (n = 28), 2.5 mg/d (n = 19), or at a dose that was titrated up to 2.5 mg/d starting at 1.5 mg/d for month 1, then 2 mg/d for month 2, and then 2.5 mg/d for months 3 to 6 (n = 17).
The mean age of the patients was 61 years, the mean body mass index was 25-27 kg/m2, and the mean T score at the spine of –2.2 to –2.45.
Among the women who received 2.5 mg/d of oral PTH for the full 6 months, serum levels of the bone resorption marker C-terminal telopeptide of type I collagen (CTX) decreased 21% from baseline to 6 months, and serum levels of P1NP increased at month 1 and then decreased to baseline by month 6.
The women who received 2.5 mg/d of oral PTH for the full 6 months also demonstrated significantly greater increases in BMD at the lumbar spine (3.8%), total hip (1.4%), and femoral neck (2.4%), compared with women who received placebo.
The safety profile of oral PTH was consistent with that of subcutaneous PTH. Patients experienced headache, nausea, presyncope, and dizziness; there were no treatment-emergent hypercalcemia adverse events.
A few ‘unexpected findings’
Suzanne M. Jan De Beur, MD, outgoing ASBMR president, said, “Oral PTH appeared to increase BMD by [dual-energy x-ray absorptiometry] at the lumbar spine effectively and to a similar degree as teriparatide in previous studies.”
She identified two unexpected findings.
“There were increases in BMD by DXA at the femoral neck and total hip at 6 months that were [greater than those] seen in previous trials of teriparatide. Second, markers of bone resorption (CTX) decreased at 6 months, and this is in stark contrast to the increases observed with teriparatide treatment,” she noted in an interview.
Dr. Rosen also noted that “the decrease in CTX is very unusual for PTH and difficult to explain.” He added: “P1NP, a marker of bone formation, was not increased.”
Dr. Jan de Beur continued: “Teriparatide (PTH1-34) and abaloparatide are effective anabolic agents that we use to treat patients with high risk of osteoporotic fracture. Although effective, the burden of daily subcutaneous injection can be a barrier for older individuals, those with poor dexterity, and those that are averse to self-injection.
“Taken together, these results appear promising, that oral PTH may prove to be an effective anabolic agent for osteoporosis treatment,” she summarized.
She stressed that a larger phase 3 study is needed to demonstrate safety and efficacy.
The study was funded by Entera Bio. Dr. Santora is chief medical officer of Entera Bio.
A version of this article first appeared on Medscape.com .
An investigational oral form of parathyroid hormone (PTH 1-34), EB 613 (Entera Bio) met its primary efficacy outcome in a phase 2 dosing study involving postmenopausal women with low bone mineral density (BMD).
The adverse effect profile of the drug was similar to that of the injectable PTH 1-34 teriparatide (Forteo), which is approved for osteoporosis.
Arthur C. Santora, MD, chief medical officer, Entera Bio, presented 6-month findings from the study during an oral session at the annual meeting of the American Society of Bone and Mineral Research. The 3-month findings from the study were reported as a poster.
If the drug demonstrates efficacy and safety in larger phase 3 trials, it could be the first oral bone-building (anabolic) therapy for osteoporosis.
Clifford J. Rosen, MD, PhD, who was not involved with the research, told this news organization: “I think this is an intriguing study.” The most likely patients for oral PTH, he added, “are those that have osteoporosis, previous fracture, or very low BMD, particularly those unlikely or unwilling to take bisphosphonates.”
However, “this is very early in the process before this drug could come to market,” cautioned Dr. Rosen, who is director of the Center for Clinical and Translational Research, Maine Medical Research Institute, Scarborough.
“Much more data on efficacy are required at 12 and 24 months for phase 2, and then a full phase 3 [clinical trial] with high-risk fracture patients,” he said.
The company is seeking input from the Food and Drug Administration to develop the protocol for a phase 3 trial. They expect to start this trial in 2022 at sites in the United States, Europe, and Israel, Dr. Santora said.
Primary outcome met
The study randomly assigned 161 postmenopausal women with osteoporosis or low BMD to receive placebo or the investigational oral PTH for 6 months.
Compared with women who received placebo, those who received the study drug experienced a significantly greater increase in the bone formation marker procollagen type I N-terminal propeptide (P1NP) from baseline to 3 months, thereby meeting the study’s primary outcome.
In secondary outcomes, women who received the 2.5-mg/d dose experienced a similar 6-month increase in BMD at the spine and greater increases in BMD at the total hip and femoral neck than those who received injectable teriparatide, Dr. Santora reported.
“The study’s key takeaway is that a once-daily oral PTH [tablet] has the potential to produce the same BMD effects as subcutaneous injections of PTH,” he said in an interview.
Additionally, “the drug was well tolerated when the dose was titrated by adding additional tablets, which suggests that the dose can be tailored to each patient,” he said.
Other study findings
Injectable teriparatide reduces the risk for vertebral fractures by up to 80%, Dr. Santora noted, but the fact that the drug must be administered by injection may deter some older patients from using it.
The company developed an oral form of biosynthetic human PTH with a proprietary drug delivery.
The researchers conducted the phase 2 study at four sites in Israel between June 2019 and May 2021. They enrolled women aged 50 years and older who had entered menopause at least 3 years earlier and who had osteoporosis or low BMD.
Forty-three women received placebo, and the others received oral PTH at doses of 0.5 mg/d (n = 25), 1.0 mg/d (n = 29), 1.5 mg/d (n = 28), 2.5 mg/d (n = 19), or at a dose that was titrated up to 2.5 mg/d starting at 1.5 mg/d for month 1, then 2 mg/d for month 2, and then 2.5 mg/d for months 3 to 6 (n = 17).
The mean age of the patients was 61 years, the mean body mass index was 25-27 kg/m2, and the mean T score at the spine of –2.2 to –2.45.
Among the women who received 2.5 mg/d of oral PTH for the full 6 months, serum levels of the bone resorption marker C-terminal telopeptide of type I collagen (CTX) decreased 21% from baseline to 6 months, and serum levels of P1NP increased at month 1 and then decreased to baseline by month 6.
The women who received 2.5 mg/d of oral PTH for the full 6 months also demonstrated significantly greater increases in BMD at the lumbar spine (3.8%), total hip (1.4%), and femoral neck (2.4%), compared with women who received placebo.
The safety profile of oral PTH was consistent with that of subcutaneous PTH. Patients experienced headache, nausea, presyncope, and dizziness; there were no treatment-emergent hypercalcemia adverse events.
A few ‘unexpected findings’
Suzanne M. Jan De Beur, MD, outgoing ASBMR president, said, “Oral PTH appeared to increase BMD by [dual-energy x-ray absorptiometry] at the lumbar spine effectively and to a similar degree as teriparatide in previous studies.”
She identified two unexpected findings.
“There were increases in BMD by DXA at the femoral neck and total hip at 6 months that were [greater than those] seen in previous trials of teriparatide. Second, markers of bone resorption (CTX) decreased at 6 months, and this is in stark contrast to the increases observed with teriparatide treatment,” she noted in an interview.
Dr. Rosen also noted that “the decrease in CTX is very unusual for PTH and difficult to explain.” He added: “P1NP, a marker of bone formation, was not increased.”
Dr. Jan de Beur continued: “Teriparatide (PTH1-34) and abaloparatide are effective anabolic agents that we use to treat patients with high risk of osteoporotic fracture. Although effective, the burden of daily subcutaneous injection can be a barrier for older individuals, those with poor dexterity, and those that are averse to self-injection.
“Taken together, these results appear promising, that oral PTH may prove to be an effective anabolic agent for osteoporosis treatment,” she summarized.
She stressed that a larger phase 3 study is needed to demonstrate safety and efficacy.
The study was funded by Entera Bio. Dr. Santora is chief medical officer of Entera Bio.
A version of this article first appeared on Medscape.com .
An investigational oral form of parathyroid hormone (PTH 1-34), EB 613 (Entera Bio) met its primary efficacy outcome in a phase 2 dosing study involving postmenopausal women with low bone mineral density (BMD).
The adverse effect profile of the drug was similar to that of the injectable PTH 1-34 teriparatide (Forteo), which is approved for osteoporosis.
Arthur C. Santora, MD, chief medical officer, Entera Bio, presented 6-month findings from the study during an oral session at the annual meeting of the American Society of Bone and Mineral Research. The 3-month findings from the study were reported as a poster.
If the drug demonstrates efficacy and safety in larger phase 3 trials, it could be the first oral bone-building (anabolic) therapy for osteoporosis.
Clifford J. Rosen, MD, PhD, who was not involved with the research, told this news organization: “I think this is an intriguing study.” The most likely patients for oral PTH, he added, “are those that have osteoporosis, previous fracture, or very low BMD, particularly those unlikely or unwilling to take bisphosphonates.”
However, “this is very early in the process before this drug could come to market,” cautioned Dr. Rosen, who is director of the Center for Clinical and Translational Research, Maine Medical Research Institute, Scarborough.
“Much more data on efficacy are required at 12 and 24 months for phase 2, and then a full phase 3 [clinical trial] with high-risk fracture patients,” he said.
The company is seeking input from the Food and Drug Administration to develop the protocol for a phase 3 trial. They expect to start this trial in 2022 at sites in the United States, Europe, and Israel, Dr. Santora said.
Primary outcome met
The study randomly assigned 161 postmenopausal women with osteoporosis or low BMD to receive placebo or the investigational oral PTH for 6 months.
Compared with women who received placebo, those who received the study drug experienced a significantly greater increase in the bone formation marker procollagen type I N-terminal propeptide (P1NP) from baseline to 3 months, thereby meeting the study’s primary outcome.
In secondary outcomes, women who received the 2.5-mg/d dose experienced a similar 6-month increase in BMD at the spine and greater increases in BMD at the total hip and femoral neck than those who received injectable teriparatide, Dr. Santora reported.
“The study’s key takeaway is that a once-daily oral PTH [tablet] has the potential to produce the same BMD effects as subcutaneous injections of PTH,” he said in an interview.
Additionally, “the drug was well tolerated when the dose was titrated by adding additional tablets, which suggests that the dose can be tailored to each patient,” he said.
Other study findings
Injectable teriparatide reduces the risk for vertebral fractures by up to 80%, Dr. Santora noted, but the fact that the drug must be administered by injection may deter some older patients from using it.
The company developed an oral form of biosynthetic human PTH with a proprietary drug delivery.
The researchers conducted the phase 2 study at four sites in Israel between June 2019 and May 2021. They enrolled women aged 50 years and older who had entered menopause at least 3 years earlier and who had osteoporosis or low BMD.
Forty-three women received placebo, and the others received oral PTH at doses of 0.5 mg/d (n = 25), 1.0 mg/d (n = 29), 1.5 mg/d (n = 28), 2.5 mg/d (n = 19), or at a dose that was titrated up to 2.5 mg/d starting at 1.5 mg/d for month 1, then 2 mg/d for month 2, and then 2.5 mg/d for months 3 to 6 (n = 17).
The mean age of the patients was 61 years, the mean body mass index was 25-27 kg/m2, and the mean T score at the spine of –2.2 to –2.45.
Among the women who received 2.5 mg/d of oral PTH for the full 6 months, serum levels of the bone resorption marker C-terminal telopeptide of type I collagen (CTX) decreased 21% from baseline to 6 months, and serum levels of P1NP increased at month 1 and then decreased to baseline by month 6.
The women who received 2.5 mg/d of oral PTH for the full 6 months also demonstrated significantly greater increases in BMD at the lumbar spine (3.8%), total hip (1.4%), and femoral neck (2.4%), compared with women who received placebo.
The safety profile of oral PTH was consistent with that of subcutaneous PTH. Patients experienced headache, nausea, presyncope, and dizziness; there were no treatment-emergent hypercalcemia adverse events.
A few ‘unexpected findings’
Suzanne M. Jan De Beur, MD, outgoing ASBMR president, said, “Oral PTH appeared to increase BMD by [dual-energy x-ray absorptiometry] at the lumbar spine effectively and to a similar degree as teriparatide in previous studies.”
She identified two unexpected findings.
“There were increases in BMD by DXA at the femoral neck and total hip at 6 months that were [greater than those] seen in previous trials of teriparatide. Second, markers of bone resorption (CTX) decreased at 6 months, and this is in stark contrast to the increases observed with teriparatide treatment,” she noted in an interview.
Dr. Rosen also noted that “the decrease in CTX is very unusual for PTH and difficult to explain.” He added: “P1NP, a marker of bone formation, was not increased.”
Dr. Jan de Beur continued: “Teriparatide (PTH1-34) and abaloparatide are effective anabolic agents that we use to treat patients with high risk of osteoporotic fracture. Although effective, the burden of daily subcutaneous injection can be a barrier for older individuals, those with poor dexterity, and those that are averse to self-injection.
“Taken together, these results appear promising, that oral PTH may prove to be an effective anabolic agent for osteoporosis treatment,” she summarized.
She stressed that a larger phase 3 study is needed to demonstrate safety and efficacy.
The study was funded by Entera Bio. Dr. Santora is chief medical officer of Entera Bio.
A version of this article first appeared on Medscape.com .
Abaloparatide significantly reduced fractures, increased BMD in women at high fracture risk
Postmenopausal women at high or very high risk of fracture gained significantly more bone mineral density and were significantly less likely to experience a fracture when taking abaloparatide for 18 months, according to new research presented at the hybrid annual meeting of the North American Menopause Society.
“The findings showed that abaloparatide was better than teriparatide in a number of parameters important in osteoporosis treatment, and similar in others, in high-risk and very-high-risk postmenopausal women with osteoporosis,” Bart Clarke, MD, a professor of medicine at Mayo Clinic in Rochester, Minn., said in an interview. “Abaloparatide is safe and effective for use in high-risk or very-high-risk postmenopausal women,” as defined by the new American Association of Clinical Endocrinology/American College of Endocrinology osteoporosis guidelines.
Ricardo R. Correa, MD, of the department of endocrinology and director of diversity for graduate medical education at the University of Arizona, Phoenix, said that the study demonstrates that abaloparatide and teriparatide have a very similar effect with abaloparatide providing a slightly better absolute risk reduction in fracture. Dr. Correa was not involved in the research.
“What will drive my decision in what to prescribe will be the cost and insurance coverage,” Dr. Correa said. “At the Veterans Administration hospital, the option that we have is abaloparatide, so this is the option that we use.”
Among women at least 65 years old who have already had one fracture, 1 in 10 will experience another fracture within the next year, and 30% will have another fracture within the next 5 years, the authors noted in their background material. Since phase 3 ACTIVE study data in 2016 showed that abaloparatide reduces fracture risk while increasing bone mineral density, compared with placebo, the researchers reanalyzed that data to assess the drug’s efficacy in patients at high or very high risk for fracture.
The study involved 2,463 postmenopausal women with osteoporosis who received one of three interventions: 80 mcg abaloparatide daily, placebo, or 20 mcg subcutaneous teriparatide daily. Only the abaloparatide and placebo groups were double blinded.
“Teriparatide was used as the comparator drug because teriparatide was previously approved as the first anabolic drug for osteoporosis,” Dr. Clarke said in an interview. “The hope was to show that abaloparatide was a better anabolic drug.”
Women were considered at high or very high risk of fracture if they met at least one of the following four criteria from the 2020 American Association of Clinical Endocrinology guidelines:
- Fracture within the past 12 months or prevalent vertebral fracture.
- Very low T-score (less than –3.0) at baseline at any site.
- Multiple fractures at baseline since age 45.
- Very high fracture risk based on the Fracture Risk Assessment Tool (FRAX) (at least 30% for major osteoporotic fracture or at least 4.5% for hip fracture).
Among the 2,026 patients who met at least one of these criteria, 664 received abaloparatide, 685 received teriparatide, and 677 received placebo. Both the abaloparatide and teriparatide significantly reduced new vertebral fracture risk, compared with placebo. In the abaloparatide group, 0.72% of women had a new vertebral fracture, compared with 0.99% in the teriparatide group and 4.77% in the placebo group (P < .0001).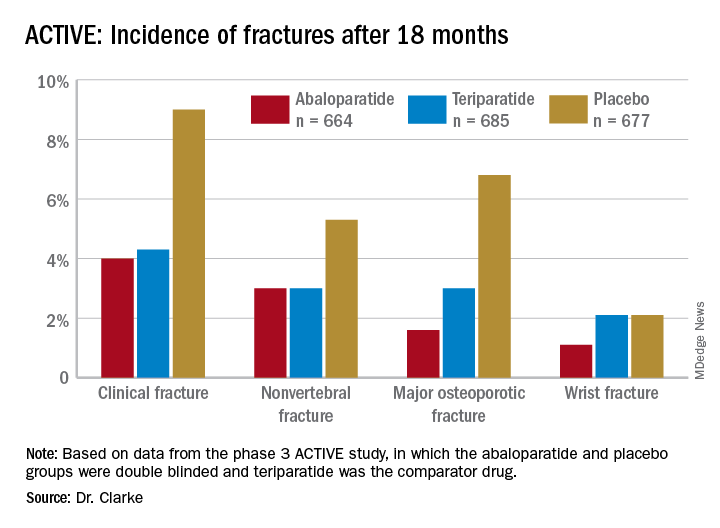
Abaloparatide and teriparatide also led to significant increases in lumbar spine, total hip, and femoral neck bone mineral density, compared with placebo (P < .0001).
The study was limited by its duration of 18 months and the Food and Drug Administration’s restriction on using abaloparatide for more than 2 years because of the theoretical risk of increasing osteosarcoma, although that risk has never been demonstrated in humans, Dr. Correa said. ”We need more data with abaloparitide in more than 2 years,” he added.
In determining which medication clinicians should first prescribe to manage osteoporosis, Dr. Correa said practitioners should consider the type of osteoporosis women have, their preferences, and their labs on kidney function.
With mild to moderate osteoporosis, bisphosphonates will be the first option while denosumab will be preferred for moderate to severe osteoporosis. Teriparatide and abaloparitide are the first-line options for severe osteoporosis, he said.
“If the glomerular filtration rate is low, we cannot use bisphosphonate and we will have to limit our use to denosumab,” he said. Route and frequency of delivery plays a role in patient preferences.
“If the patient prefers an infusion once a year or a pill, then bisphosphonate,” he said, but “if the patient is fine with an injection every 6 months, then denosumab.” Patients who need and can do an injection every day can take abaloparitide or teriparatide.
Failure of previous treatments also guide clinical decisions, he added. ”If the patient has been on one medication and has a fracture or the bone mineral density decreases, then we need to switch to another medication, usually teriparatide or abaloparitide, to build new bone.”
Contraindications for abaloparatide include a high serum calcium before therapy or prior allergic reactions to components in abaloparatide, Dr. Clarke said. No new safety signals showed up in the data analysis.
The research was funded by Radius Health. Dr. Clarke is an advisory board member of Amgen, and another author consults and speaks for Amgen and is a Radius Health Advisory Board member. Two other authors are Radius Health employees who own stock in the company. Dr Correa has no disclosures.
Postmenopausal women at high or very high risk of fracture gained significantly more bone mineral density and were significantly less likely to experience a fracture when taking abaloparatide for 18 months, according to new research presented at the hybrid annual meeting of the North American Menopause Society.
“The findings showed that abaloparatide was better than teriparatide in a number of parameters important in osteoporosis treatment, and similar in others, in high-risk and very-high-risk postmenopausal women with osteoporosis,” Bart Clarke, MD, a professor of medicine at Mayo Clinic in Rochester, Minn., said in an interview. “Abaloparatide is safe and effective for use in high-risk or very-high-risk postmenopausal women,” as defined by the new American Association of Clinical Endocrinology/American College of Endocrinology osteoporosis guidelines.
Ricardo R. Correa, MD, of the department of endocrinology and director of diversity for graduate medical education at the University of Arizona, Phoenix, said that the study demonstrates that abaloparatide and teriparatide have a very similar effect with abaloparatide providing a slightly better absolute risk reduction in fracture. Dr. Correa was not involved in the research.
“What will drive my decision in what to prescribe will be the cost and insurance coverage,” Dr. Correa said. “At the Veterans Administration hospital, the option that we have is abaloparatide, so this is the option that we use.”
Among women at least 65 years old who have already had one fracture, 1 in 10 will experience another fracture within the next year, and 30% will have another fracture within the next 5 years, the authors noted in their background material. Since phase 3 ACTIVE study data in 2016 showed that abaloparatide reduces fracture risk while increasing bone mineral density, compared with placebo, the researchers reanalyzed that data to assess the drug’s efficacy in patients at high or very high risk for fracture.
The study involved 2,463 postmenopausal women with osteoporosis who received one of three interventions: 80 mcg abaloparatide daily, placebo, or 20 mcg subcutaneous teriparatide daily. Only the abaloparatide and placebo groups were double blinded.
“Teriparatide was used as the comparator drug because teriparatide was previously approved as the first anabolic drug for osteoporosis,” Dr. Clarke said in an interview. “The hope was to show that abaloparatide was a better anabolic drug.”
Women were considered at high or very high risk of fracture if they met at least one of the following four criteria from the 2020 American Association of Clinical Endocrinology guidelines:
- Fracture within the past 12 months or prevalent vertebral fracture.
- Very low T-score (less than –3.0) at baseline at any site.
- Multiple fractures at baseline since age 45.
- Very high fracture risk based on the Fracture Risk Assessment Tool (FRAX) (at least 30% for major osteoporotic fracture or at least 4.5% for hip fracture).
Among the 2,026 patients who met at least one of these criteria, 664 received abaloparatide, 685 received teriparatide, and 677 received placebo. Both the abaloparatide and teriparatide significantly reduced new vertebral fracture risk, compared with placebo. In the abaloparatide group, 0.72% of women had a new vertebral fracture, compared with 0.99% in the teriparatide group and 4.77% in the placebo group (P < .0001).
Abaloparatide and teriparatide also led to significant increases in lumbar spine, total hip, and femoral neck bone mineral density, compared with placebo (P < .0001).
The study was limited by its duration of 18 months and the Food and Drug Administration’s restriction on using abaloparatide for more than 2 years because of the theoretical risk of increasing osteosarcoma, although that risk has never been demonstrated in humans, Dr. Correa said. ”We need more data with abaloparitide in more than 2 years,” he added.
In determining which medication clinicians should first prescribe to manage osteoporosis, Dr. Correa said practitioners should consider the type of osteoporosis women have, their preferences, and their labs on kidney function.
With mild to moderate osteoporosis, bisphosphonates will be the first option while denosumab will be preferred for moderate to severe osteoporosis. Teriparatide and abaloparitide are the first-line options for severe osteoporosis, he said.
“If the glomerular filtration rate is low, we cannot use bisphosphonate and we will have to limit our use to denosumab,” he said. Route and frequency of delivery plays a role in patient preferences.
“If the patient prefers an infusion once a year or a pill, then bisphosphonate,” he said, but “if the patient is fine with an injection every 6 months, then denosumab.” Patients who need and can do an injection every day can take abaloparitide or teriparatide.
Failure of previous treatments also guide clinical decisions, he added. ”If the patient has been on one medication and has a fracture or the bone mineral density decreases, then we need to switch to another medication, usually teriparatide or abaloparitide, to build new bone.”
Contraindications for abaloparatide include a high serum calcium before therapy or prior allergic reactions to components in abaloparatide, Dr. Clarke said. No new safety signals showed up in the data analysis.
The research was funded by Radius Health. Dr. Clarke is an advisory board member of Amgen, and another author consults and speaks for Amgen and is a Radius Health Advisory Board member. Two other authors are Radius Health employees who own stock in the company. Dr Correa has no disclosures.
Postmenopausal women at high or very high risk of fracture gained significantly more bone mineral density and were significantly less likely to experience a fracture when taking abaloparatide for 18 months, according to new research presented at the hybrid annual meeting of the North American Menopause Society.
“The findings showed that abaloparatide was better than teriparatide in a number of parameters important in osteoporosis treatment, and similar in others, in high-risk and very-high-risk postmenopausal women with osteoporosis,” Bart Clarke, MD, a professor of medicine at Mayo Clinic in Rochester, Minn., said in an interview. “Abaloparatide is safe and effective for use in high-risk or very-high-risk postmenopausal women,” as defined by the new American Association of Clinical Endocrinology/American College of Endocrinology osteoporosis guidelines.
Ricardo R. Correa, MD, of the department of endocrinology and director of diversity for graduate medical education at the University of Arizona, Phoenix, said that the study demonstrates that abaloparatide and teriparatide have a very similar effect with abaloparatide providing a slightly better absolute risk reduction in fracture. Dr. Correa was not involved in the research.
“What will drive my decision in what to prescribe will be the cost and insurance coverage,” Dr. Correa said. “At the Veterans Administration hospital, the option that we have is abaloparatide, so this is the option that we use.”
Among women at least 65 years old who have already had one fracture, 1 in 10 will experience another fracture within the next year, and 30% will have another fracture within the next 5 years, the authors noted in their background material. Since phase 3 ACTIVE study data in 2016 showed that abaloparatide reduces fracture risk while increasing bone mineral density, compared with placebo, the researchers reanalyzed that data to assess the drug’s efficacy in patients at high or very high risk for fracture.
The study involved 2,463 postmenopausal women with osteoporosis who received one of three interventions: 80 mcg abaloparatide daily, placebo, or 20 mcg subcutaneous teriparatide daily. Only the abaloparatide and placebo groups were double blinded.
“Teriparatide was used as the comparator drug because teriparatide was previously approved as the first anabolic drug for osteoporosis,” Dr. Clarke said in an interview. “The hope was to show that abaloparatide was a better anabolic drug.”
Women were considered at high or very high risk of fracture if they met at least one of the following four criteria from the 2020 American Association of Clinical Endocrinology guidelines:
- Fracture within the past 12 months or prevalent vertebral fracture.
- Very low T-score (less than –3.0) at baseline at any site.
- Multiple fractures at baseline since age 45.
- Very high fracture risk based on the Fracture Risk Assessment Tool (FRAX) (at least 30% for major osteoporotic fracture or at least 4.5% for hip fracture).
Among the 2,026 patients who met at least one of these criteria, 664 received abaloparatide, 685 received teriparatide, and 677 received placebo. Both the abaloparatide and teriparatide significantly reduced new vertebral fracture risk, compared with placebo. In the abaloparatide group, 0.72% of women had a new vertebral fracture, compared with 0.99% in the teriparatide group and 4.77% in the placebo group (P < .0001).
Abaloparatide and teriparatide also led to significant increases in lumbar spine, total hip, and femoral neck bone mineral density, compared with placebo (P < .0001).
The study was limited by its duration of 18 months and the Food and Drug Administration’s restriction on using abaloparatide for more than 2 years because of the theoretical risk of increasing osteosarcoma, although that risk has never been demonstrated in humans, Dr. Correa said. ”We need more data with abaloparitide in more than 2 years,” he added.
In determining which medication clinicians should first prescribe to manage osteoporosis, Dr. Correa said practitioners should consider the type of osteoporosis women have, their preferences, and their labs on kidney function.
With mild to moderate osteoporosis, bisphosphonates will be the first option while denosumab will be preferred for moderate to severe osteoporosis. Teriparatide and abaloparitide are the first-line options for severe osteoporosis, he said.
“If the glomerular filtration rate is low, we cannot use bisphosphonate and we will have to limit our use to denosumab,” he said. Route and frequency of delivery plays a role in patient preferences.
“If the patient prefers an infusion once a year or a pill, then bisphosphonate,” he said, but “if the patient is fine with an injection every 6 months, then denosumab.” Patients who need and can do an injection every day can take abaloparitide or teriparatide.
Failure of previous treatments also guide clinical decisions, he added. ”If the patient has been on one medication and has a fracture or the bone mineral density decreases, then we need to switch to another medication, usually teriparatide or abaloparitide, to build new bone.”
Contraindications for abaloparatide include a high serum calcium before therapy or prior allergic reactions to components in abaloparatide, Dr. Clarke said. No new safety signals showed up in the data analysis.
The research was funded by Radius Health. Dr. Clarke is an advisory board member of Amgen, and another author consults and speaks for Amgen and is a Radius Health Advisory Board member. Two other authors are Radius Health employees who own stock in the company. Dr Correa has no disclosures.
FROM NAMS 2021
New nonhormonal therapies for hot flashes on the horizon
Hot flashes affect three out of four women and can last 7-10 years, but the current standard of care treatment isn’t necessarily appropriate for all women who experience vasomotor symptoms, according to Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health Clinic in Jacksonville, Fla.
For the majority of women under age 60 who are within 10 years of menopause, hormone therapy currently remains the most effective management option for hot flashes where the benefits outweigh the risks, Dr. Faubion told attendees Sept. 25 during a plenary at the annual meeting of the North American Menopause Society. “But really, individualizing treatment is the goal, and there are some women who are going to need some other options.”
Contraindications for hormone therapy include having a history of breast cancer, coronary heart disease, active liver disease, unexplained vaginal bleeding, high-risk endometrial cancer, transient ischemic attack, and a previous venous thromboembolic event or stroke.
“Fortunately, we have things in development,” Dr. Faubion said. She reviewed a wide range of therapies that are not currently Food and Drug Administration approved for vasomotor symptoms but are either available off label or are in clinical trials.
One of these is oxybutynin, an antimuscarinic, anticholinergic agent currently used to treat overactive bladder and overactive sweating. In a 2016 trial, 73% of women taking 15 mg extended-release oxybutynin once daily rated their symptoms as “much better,” compared with 26% who received placebo. The women experienced reduced frequency and severity of hot flashes and better sleep.
Subsequent research found a 60% reduction in hot flash frequency with 2.5 mg twice a day and a 77% reduction with 5 mg twice a day, compared with a 27% reduction with placebo. The only reported side effect that occurred more often with oxybutynin was dry mouth, but there were no significant differences in reasons for discontinuation between the treatment and placebo groups.
There are, however, some potential long-term cognitive effects from oxybutynin, Dr. Faubion said. Some research has shown an increased risk of dementia from oxybutynin and from an overall higher cumulative use of anticholinergics.
“There’s some concern about that for long-term use,” she said, but it’s effective, it’s “probably not harmful [when] used short term in women with significant, bothersome hot flashes who are unwilling or unable to use hormone therapy, and the adverse effects are tolerable for most women.” Women with bladder symptoms would be especially ideal candidates since the drug already treats those.
Dr. Faubion then discussed a new estrogen called estetrol (E4), a naturally occurring estrogen with selection action in tissues that is produced by the fetal liver and crosses the placenta. It has a long half-life of 28-32 hours, and its potential mechanism may give it a different safety profile than estradiol (E2). “There may be a lower risk of drug-drug interactions; lower breast stimulation, pain or carcinogenic impact; lower impact on triglycerides; and a neutral impact on markers of coagulation,” she said.
Though estetrol was recently approved as an oral contraceptive under the name Estelle, it’s also under investigation as a postmenopausal regimen. Preliminary findings suggest it reduces vasomotor symptom severity by 44%, compared with 30% with placebo, at 15 mg, the apparent minimum effective dose. The safety profile showed no endometrial hyperplasia and no unexpected adverse events. In those taking 15 mg of estetrol, mean endometrial thickness increased from 2 to 6 mm but returned to baseline after progestin therapy.
“The 15-mg dose also positively influenced markers of bone turnover, increased HDL [cholesterol], improved glucose tolerance,” and had no effects on coagulation parameters or triglycerides, Dr. Faubion added.
Another group of potential agents being studied for hot flashes are NK3 antagonists, which aim to exploit the recent discovery that kisspeptin, neurokinin B, and dynorphin (KNDy) neurons may play an important role in the etiology of vasomotor symptoms. Development of one of these, MLE 4901, was halted despite a 45% reduction in hot flashes because 3 of 28 women developed transiently elevated liver function tests, about four to six times the upper limit of normal.
Two others, fezolinetant and NT-814, are in phase 2 trials and have shown a significant reduction in symptoms, compared with placebo. The most commonly reported adverse effect in the phase 2a trial was gastrointestinal effects, but none of the participants stopped the drug because of these, and no elevated liver tests occurred. In the larger phase 2b trial, the most commonly reported treatment-emergent adverse events included nausea, diarrhea, fatigue, urinary tract infection, sinusitis, upper respiratory infection, headache, and cough. Five women discontinued the drug because of elevated liver enzymes.
“Overall, NK3 inhibitors appear to be generally well tolerated,” Dr. Faubion said. “There does seem to be mild transaminase elevation,” though it’s not yet known if this is an effect from this class of drugs as a whole. She noted that follicle-stimulating hormone does not significantly increase, which is important because elevated FSH is associated with poor bone health, nor does estradiol significantly increase, which is clinically relevant for women at high risk of breast cancer.
“We don’t know the effects on the heart, the brain, the bone, mood, weight, or sexual health, so there’s a lot that is still not known,” Dr. Faubion said. “We still don’t know about long-term safety and efficacy with these chemical compounds,” but clinical trials of them are ongoing.
They “would be a welcome alternative to hormone therapy for those who can’t or prefer not to use a hormonal option,” Dr. Faubion said. “However, we may need broad education of clinicians to caution against widespread abandonment of hormone therapy, particularly in women with premature or early menopause.”
Donna Klassen, LCSW, the cofounder of Let’s Talk Menopause, asked whether any of these new therapies were being tested in women with breast cancer and whether anything was known about taking oxybutynin at the same time as letrozole.
“I suspect that most women with chronic diseases would have been excluded from these initial studies, but I can’t speak to that,” Dr. Faubion said, and she wasn’t aware of any data related to taking oxybutynin and letrozole concurrently.
James Simon, MD, medical director and founder of IntimMedicine and one of those who led the research on oxybutynin, responded that his trials excluded breast cancer survivors and anyone taking aromatase inhibitors.
“It will be unlikely that, in the very near future, that data will be available because all the clinical developments on these NK3s or KNDy neuron-modulating drugs exclude cancer patients,” Dr. Simon said.
However, another attendee, Lisa Larkin, MD, of Cincinnati, introduced herself as a breast cancer survivor who takes tamoxifen and said she feels “completely comfortable” prescribing oxybutynin to breast cancer survivors.
“In terms of side effects and effectiveness in patients on tamoxifen and aromatase inhibitors, I’ve had incredibly good luck with it, and I think it’s underutilized,” Dr. Larkin said. “The clinical pearl I would tell you is you can start really low, and the dry mouth really seems to improve with time.” She added that patients should be informed that it takes 2 weeks before it begins working, but the side effects eventually go away. “It becomes very tolerable, so I just encourage all of you to consider it as another great option.”
Dr. Faubion had no disclosures. Disclosure information was unavailable for Dr. Simon, Dr. Larkin, and Ms. Klassen.
Hot flashes affect three out of four women and can last 7-10 years, but the current standard of care treatment isn’t necessarily appropriate for all women who experience vasomotor symptoms, according to Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health Clinic in Jacksonville, Fla.
For the majority of women under age 60 who are within 10 years of menopause, hormone therapy currently remains the most effective management option for hot flashes where the benefits outweigh the risks, Dr. Faubion told attendees Sept. 25 during a plenary at the annual meeting of the North American Menopause Society. “But really, individualizing treatment is the goal, and there are some women who are going to need some other options.”
Contraindications for hormone therapy include having a history of breast cancer, coronary heart disease, active liver disease, unexplained vaginal bleeding, high-risk endometrial cancer, transient ischemic attack, and a previous venous thromboembolic event or stroke.
“Fortunately, we have things in development,” Dr. Faubion said. She reviewed a wide range of therapies that are not currently Food and Drug Administration approved for vasomotor symptoms but are either available off label or are in clinical trials.
One of these is oxybutynin, an antimuscarinic, anticholinergic agent currently used to treat overactive bladder and overactive sweating. In a 2016 trial, 73% of women taking 15 mg extended-release oxybutynin once daily rated their symptoms as “much better,” compared with 26% who received placebo. The women experienced reduced frequency and severity of hot flashes and better sleep.
Subsequent research found a 60% reduction in hot flash frequency with 2.5 mg twice a day and a 77% reduction with 5 mg twice a day, compared with a 27% reduction with placebo. The only reported side effect that occurred more often with oxybutynin was dry mouth, but there were no significant differences in reasons for discontinuation between the treatment and placebo groups.
There are, however, some potential long-term cognitive effects from oxybutynin, Dr. Faubion said. Some research has shown an increased risk of dementia from oxybutynin and from an overall higher cumulative use of anticholinergics.
“There’s some concern about that for long-term use,” she said, but it’s effective, it’s “probably not harmful [when] used short term in women with significant, bothersome hot flashes who are unwilling or unable to use hormone therapy, and the adverse effects are tolerable for most women.” Women with bladder symptoms would be especially ideal candidates since the drug already treats those.
Dr. Faubion then discussed a new estrogen called estetrol (E4), a naturally occurring estrogen with selection action in tissues that is produced by the fetal liver and crosses the placenta. It has a long half-life of 28-32 hours, and its potential mechanism may give it a different safety profile than estradiol (E2). “There may be a lower risk of drug-drug interactions; lower breast stimulation, pain or carcinogenic impact; lower impact on triglycerides; and a neutral impact on markers of coagulation,” she said.
Though estetrol was recently approved as an oral contraceptive under the name Estelle, it’s also under investigation as a postmenopausal regimen. Preliminary findings suggest it reduces vasomotor symptom severity by 44%, compared with 30% with placebo, at 15 mg, the apparent minimum effective dose. The safety profile showed no endometrial hyperplasia and no unexpected adverse events. In those taking 15 mg of estetrol, mean endometrial thickness increased from 2 to 6 mm but returned to baseline after progestin therapy.
“The 15-mg dose also positively influenced markers of bone turnover, increased HDL [cholesterol], improved glucose tolerance,” and had no effects on coagulation parameters or triglycerides, Dr. Faubion added.
Another group of potential agents being studied for hot flashes are NK3 antagonists, which aim to exploit the recent discovery that kisspeptin, neurokinin B, and dynorphin (KNDy) neurons may play an important role in the etiology of vasomotor symptoms. Development of one of these, MLE 4901, was halted despite a 45% reduction in hot flashes because 3 of 28 women developed transiently elevated liver function tests, about four to six times the upper limit of normal.
Two others, fezolinetant and NT-814, are in phase 2 trials and have shown a significant reduction in symptoms, compared with placebo. The most commonly reported adverse effect in the phase 2a trial was gastrointestinal effects, but none of the participants stopped the drug because of these, and no elevated liver tests occurred. In the larger phase 2b trial, the most commonly reported treatment-emergent adverse events included nausea, diarrhea, fatigue, urinary tract infection, sinusitis, upper respiratory infection, headache, and cough. Five women discontinued the drug because of elevated liver enzymes.
“Overall, NK3 inhibitors appear to be generally well tolerated,” Dr. Faubion said. “There does seem to be mild transaminase elevation,” though it’s not yet known if this is an effect from this class of drugs as a whole. She noted that follicle-stimulating hormone does not significantly increase, which is important because elevated FSH is associated with poor bone health, nor does estradiol significantly increase, which is clinically relevant for women at high risk of breast cancer.
“We don’t know the effects on the heart, the brain, the bone, mood, weight, or sexual health, so there’s a lot that is still not known,” Dr. Faubion said. “We still don’t know about long-term safety and efficacy with these chemical compounds,” but clinical trials of them are ongoing.
They “would be a welcome alternative to hormone therapy for those who can’t or prefer not to use a hormonal option,” Dr. Faubion said. “However, we may need broad education of clinicians to caution against widespread abandonment of hormone therapy, particularly in women with premature or early menopause.”
Donna Klassen, LCSW, the cofounder of Let’s Talk Menopause, asked whether any of these new therapies were being tested in women with breast cancer and whether anything was known about taking oxybutynin at the same time as letrozole.
“I suspect that most women with chronic diseases would have been excluded from these initial studies, but I can’t speak to that,” Dr. Faubion said, and she wasn’t aware of any data related to taking oxybutynin and letrozole concurrently.
James Simon, MD, medical director and founder of IntimMedicine and one of those who led the research on oxybutynin, responded that his trials excluded breast cancer survivors and anyone taking aromatase inhibitors.
“It will be unlikely that, in the very near future, that data will be available because all the clinical developments on these NK3s or KNDy neuron-modulating drugs exclude cancer patients,” Dr. Simon said.
However, another attendee, Lisa Larkin, MD, of Cincinnati, introduced herself as a breast cancer survivor who takes tamoxifen and said she feels “completely comfortable” prescribing oxybutynin to breast cancer survivors.
“In terms of side effects and effectiveness in patients on tamoxifen and aromatase inhibitors, I’ve had incredibly good luck with it, and I think it’s underutilized,” Dr. Larkin said. “The clinical pearl I would tell you is you can start really low, and the dry mouth really seems to improve with time.” She added that patients should be informed that it takes 2 weeks before it begins working, but the side effects eventually go away. “It becomes very tolerable, so I just encourage all of you to consider it as another great option.”
Dr. Faubion had no disclosures. Disclosure information was unavailable for Dr. Simon, Dr. Larkin, and Ms. Klassen.
Hot flashes affect three out of four women and can last 7-10 years, but the current standard of care treatment isn’t necessarily appropriate for all women who experience vasomotor symptoms, according to Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health Clinic in Jacksonville, Fla.
For the majority of women under age 60 who are within 10 years of menopause, hormone therapy currently remains the most effective management option for hot flashes where the benefits outweigh the risks, Dr. Faubion told attendees Sept. 25 during a plenary at the annual meeting of the North American Menopause Society. “But really, individualizing treatment is the goal, and there are some women who are going to need some other options.”
Contraindications for hormone therapy include having a history of breast cancer, coronary heart disease, active liver disease, unexplained vaginal bleeding, high-risk endometrial cancer, transient ischemic attack, and a previous venous thromboembolic event or stroke.
“Fortunately, we have things in development,” Dr. Faubion said. She reviewed a wide range of therapies that are not currently Food and Drug Administration approved for vasomotor symptoms but are either available off label or are in clinical trials.
One of these is oxybutynin, an antimuscarinic, anticholinergic agent currently used to treat overactive bladder and overactive sweating. In a 2016 trial, 73% of women taking 15 mg extended-release oxybutynin once daily rated their symptoms as “much better,” compared with 26% who received placebo. The women experienced reduced frequency and severity of hot flashes and better sleep.
Subsequent research found a 60% reduction in hot flash frequency with 2.5 mg twice a day and a 77% reduction with 5 mg twice a day, compared with a 27% reduction with placebo. The only reported side effect that occurred more often with oxybutynin was dry mouth, but there were no significant differences in reasons for discontinuation between the treatment and placebo groups.
There are, however, some potential long-term cognitive effects from oxybutynin, Dr. Faubion said. Some research has shown an increased risk of dementia from oxybutynin and from an overall higher cumulative use of anticholinergics.
“There’s some concern about that for long-term use,” she said, but it’s effective, it’s “probably not harmful [when] used short term in women with significant, bothersome hot flashes who are unwilling or unable to use hormone therapy, and the adverse effects are tolerable for most women.” Women with bladder symptoms would be especially ideal candidates since the drug already treats those.
Dr. Faubion then discussed a new estrogen called estetrol (E4), a naturally occurring estrogen with selection action in tissues that is produced by the fetal liver and crosses the placenta. It has a long half-life of 28-32 hours, and its potential mechanism may give it a different safety profile than estradiol (E2). “There may be a lower risk of drug-drug interactions; lower breast stimulation, pain or carcinogenic impact; lower impact on triglycerides; and a neutral impact on markers of coagulation,” she said.
Though estetrol was recently approved as an oral contraceptive under the name Estelle, it’s also under investigation as a postmenopausal regimen. Preliminary findings suggest it reduces vasomotor symptom severity by 44%, compared with 30% with placebo, at 15 mg, the apparent minimum effective dose. The safety profile showed no endometrial hyperplasia and no unexpected adverse events. In those taking 15 mg of estetrol, mean endometrial thickness increased from 2 to 6 mm but returned to baseline after progestin therapy.
“The 15-mg dose also positively influenced markers of bone turnover, increased HDL [cholesterol], improved glucose tolerance,” and had no effects on coagulation parameters or triglycerides, Dr. Faubion added.
Another group of potential agents being studied for hot flashes are NK3 antagonists, which aim to exploit the recent discovery that kisspeptin, neurokinin B, and dynorphin (KNDy) neurons may play an important role in the etiology of vasomotor symptoms. Development of one of these, MLE 4901, was halted despite a 45% reduction in hot flashes because 3 of 28 women developed transiently elevated liver function tests, about four to six times the upper limit of normal.
Two others, fezolinetant and NT-814, are in phase 2 trials and have shown a significant reduction in symptoms, compared with placebo. The most commonly reported adverse effect in the phase 2a trial was gastrointestinal effects, but none of the participants stopped the drug because of these, and no elevated liver tests occurred. In the larger phase 2b trial, the most commonly reported treatment-emergent adverse events included nausea, diarrhea, fatigue, urinary tract infection, sinusitis, upper respiratory infection, headache, and cough. Five women discontinued the drug because of elevated liver enzymes.
“Overall, NK3 inhibitors appear to be generally well tolerated,” Dr. Faubion said. “There does seem to be mild transaminase elevation,” though it’s not yet known if this is an effect from this class of drugs as a whole. She noted that follicle-stimulating hormone does not significantly increase, which is important because elevated FSH is associated with poor bone health, nor does estradiol significantly increase, which is clinically relevant for women at high risk of breast cancer.
“We don’t know the effects on the heart, the brain, the bone, mood, weight, or sexual health, so there’s a lot that is still not known,” Dr. Faubion said. “We still don’t know about long-term safety and efficacy with these chemical compounds,” but clinical trials of them are ongoing.
They “would be a welcome alternative to hormone therapy for those who can’t or prefer not to use a hormonal option,” Dr. Faubion said. “However, we may need broad education of clinicians to caution against widespread abandonment of hormone therapy, particularly in women with premature or early menopause.”
Donna Klassen, LCSW, the cofounder of Let’s Talk Menopause, asked whether any of these new therapies were being tested in women with breast cancer and whether anything was known about taking oxybutynin at the same time as letrozole.
“I suspect that most women with chronic diseases would have been excluded from these initial studies, but I can’t speak to that,” Dr. Faubion said, and she wasn’t aware of any data related to taking oxybutynin and letrozole concurrently.
James Simon, MD, medical director and founder of IntimMedicine and one of those who led the research on oxybutynin, responded that his trials excluded breast cancer survivors and anyone taking aromatase inhibitors.
“It will be unlikely that, in the very near future, that data will be available because all the clinical developments on these NK3s or KNDy neuron-modulating drugs exclude cancer patients,” Dr. Simon said.
However, another attendee, Lisa Larkin, MD, of Cincinnati, introduced herself as a breast cancer survivor who takes tamoxifen and said she feels “completely comfortable” prescribing oxybutynin to breast cancer survivors.
“In terms of side effects and effectiveness in patients on tamoxifen and aromatase inhibitors, I’ve had incredibly good luck with it, and I think it’s underutilized,” Dr. Larkin said. “The clinical pearl I would tell you is you can start really low, and the dry mouth really seems to improve with time.” She added that patients should be informed that it takes 2 weeks before it begins working, but the side effects eventually go away. “It becomes very tolerable, so I just encourage all of you to consider it as another great option.”
Dr. Faubion had no disclosures. Disclosure information was unavailable for Dr. Simon, Dr. Larkin, and Ms. Klassen.
FROM NAMS 2021
No increase in dementia with menopausal HRT
Women who have taken hormone replacement therapy for menopausal symptoms will be relieved by findings from a large British case-control study reporting no overall increased risk of dementia as long as exposure is not long term.
Publishing results online Sept. 29 in BMJ, ( Yana Vinogradova, PhD, a senior research fellow at the University of Nottingham (England), and colleagues made this observation after conducting nested case-control studies involving more than 700,000 women in two U.K. primary care databases. The investigators undertook the study to clarify disparate findings over the past 2 decades on dementia risk associated with menopausal hormone replacement,
“The findings show that menopausal hormone therapy, or MHT, is generally safe for women who require it,” Dr. Vinogradova said in an interview. “A small risk association was found for future development of Alzheimer’s disease increasing with the length of menopausal hormone treatment.” This finding applied only to combined treatments of estrogen plus progestin and became measurable only after long-term use of 5 years or more. “These risk associations, only for long-term use of MHT, are in line with findings related to breast cancer risk,” she said.
The findings also align with previous biological speculations that estrogen combined with progestin may have a harmful effect on the aging brain, she added, “but we also cannot completely rule out other possible factors from our study. For example, some women who were in fact suffering from early signs of Alzheimer’s disease similar to menopausal symptoms may have continued with their menopausal therapy for longer than other women.”
Concerns about the risk of dementia with MHT date back to 2003 when data from the Women’s Health Initiative Memory Study showed that incidence of all-cause dementia doubled in women age 65 years and older after treatment with conjugated equine estrogens and medroxyprogesterone acetate for an average of 4 years. More recently, Finnish research has yielded conflicting data about risks.
The study
The investigators used two U.K. primary care databases (QResearch and CPRD) to analyze MHT prescriptions for 118,501 women age 55 and older diagnosed with dementia between 1998 and 2020 and 497,416 female controls matched by age and general practice, but with no record of dementia.
The cohort was older: mean age of cases was 83.5 years and mean duration of treatment was 16 years for an average age of 67.7 at first captured prescription, considerably later than when most women begin MHT. Relevant factors such as family history, smoking, alcohol consumption, preexisting conditions, and other prescribed drugs were taken into account.
Overall, 16,291 (14%) dementia cases and 68,726 (14%) controls had been exposed to MHT in the period up to 3 years before diagnosis.
After adjusting for potentially confounding factors, the researchers found no overall associations between hormone therapy and risk of dementia, regardless of hormone type, application, dose, or duration of treatment. Within the subgroup of women younger than 80 years who had been taking estrogen-only therapy for 10 years or more, a slightly decreased risk of dementia emerged: odds ratio, 0.85; 95% confidence interval, 0.76-0.94.
However, an analysis of dementia cases with a diagnosis specifically of Alzheimer’s disease showed a slight increase in risk associated with estrogen-progestin therapy. Increased risks of developing specifically Alzheimer’s disease emerged in those who had used combination therapy for 5-9 years (OR, 1.11; 95% CI, 1.04-1.20) and also for 10 years or more (OR, 1.19; 95% CI, 1.06-1.33). This risk rose gradually with each year of exposure, reaching an average 11% increased risk for use from 5-9 years and an average 19% for use 10 years or more – equivalent to, respectively, five and seven extra cases per 10,000 woman-years.
According to Jill M. Rabin, MD, a professor at the Feinstein Institutes for Medical Research and an ob.gyn. with Northwell Health in Manhasset, N.Y., the findings make sense for two reasons. “First, there are other health issues noted in women taking long-term combination hormonal therapy such as an increased risk of breast cancer,” she said in an interview. “Second, progesterone is recommended for women who have retained their uterus in order to counteract the potential effects of estrogen on the uterine lining causing possible overgrowth. There are systemic effects however of progesterone, as it counteracts estrogen, potentially decreasing its benefit on the neurological system.”
She added that this analysis is synchronous with other biological studies demonstrating possible neuroprotective effects of estrogen on the brain, especially among younger women. “The vascular system in the newly menopausal female is noted to have less endothelial and intimal thickening, better blood flow and oxygenation, and in general less vascular damage. Estrogen in these relatively younger, newly menopausal women may help to stabilize the vasculature as well as the neurologic system. On the other hand, estrogen therapy over the age of 80 may be delivered to a neurovasculature damaged with age and time, may be somewhat less beneficial.” Older women also have fewer estrogen receptors and, in general, other medical comorbidities.
According to the authors, the findings will be helpful to policy-makers, doctors, and patients when making choices about hormone therapy.
In an accompanying editorial, two U.S. researchers called the findings reassuring. Pauline M. Maki, PhD, of the University of Illinois at Chicago, and JoAnn E. Manson, MD, DrPH, of Brigham and Women’s Hospital, Harvard Medical School, and Harvard T.H. Chan School of Public Health in Boston, however, pointed out that the current study with its older cohort and older age at MHT initiation could not address the important issue of the “timing hypothesis” – namely, that earlier initiation of hormone therapy might confer greater protection against Alzheimer’s disease, compared with later use.
And while the current observations do not change the recommendation that MHT should not be used to prevent dementia, they are helpful for providers to put dementia findings in context for patients. “The primary indication for hormone therapy continues to be the treatment of vasomotor symptoms, and the current study should provide reassurance for women and their providers when treatment is prescribed for that reason,” they wrote.
This study was funded by the U.K. National Institute for Health Research School for Primary Care Research.
Coauthor Dr. Julia Hippisley-Cox is a director of QResearch, EMIS Health, which supplies the QResearch database used for this work, and is a founder and shareholder of ClinRisk., which produces software to implement clinical risk algorithms.
Women who have taken hormone replacement therapy for menopausal symptoms will be relieved by findings from a large British case-control study reporting no overall increased risk of dementia as long as exposure is not long term.
Publishing results online Sept. 29 in BMJ, ( Yana Vinogradova, PhD, a senior research fellow at the University of Nottingham (England), and colleagues made this observation after conducting nested case-control studies involving more than 700,000 women in two U.K. primary care databases. The investigators undertook the study to clarify disparate findings over the past 2 decades on dementia risk associated with menopausal hormone replacement,
“The findings show that menopausal hormone therapy, or MHT, is generally safe for women who require it,” Dr. Vinogradova said in an interview. “A small risk association was found for future development of Alzheimer’s disease increasing with the length of menopausal hormone treatment.” This finding applied only to combined treatments of estrogen plus progestin and became measurable only after long-term use of 5 years or more. “These risk associations, only for long-term use of MHT, are in line with findings related to breast cancer risk,” she said.
The findings also align with previous biological speculations that estrogen combined with progestin may have a harmful effect on the aging brain, she added, “but we also cannot completely rule out other possible factors from our study. For example, some women who were in fact suffering from early signs of Alzheimer’s disease similar to menopausal symptoms may have continued with their menopausal therapy for longer than other women.”
Concerns about the risk of dementia with MHT date back to 2003 when data from the Women’s Health Initiative Memory Study showed that incidence of all-cause dementia doubled in women age 65 years and older after treatment with conjugated equine estrogens and medroxyprogesterone acetate for an average of 4 years. More recently, Finnish research has yielded conflicting data about risks.
The study
The investigators used two U.K. primary care databases (QResearch and CPRD) to analyze MHT prescriptions for 118,501 women age 55 and older diagnosed with dementia between 1998 and 2020 and 497,416 female controls matched by age and general practice, but with no record of dementia.
The cohort was older: mean age of cases was 83.5 years and mean duration of treatment was 16 years for an average age of 67.7 at first captured prescription, considerably later than when most women begin MHT. Relevant factors such as family history, smoking, alcohol consumption, preexisting conditions, and other prescribed drugs were taken into account.
Overall, 16,291 (14%) dementia cases and 68,726 (14%) controls had been exposed to MHT in the period up to 3 years before diagnosis.
After adjusting for potentially confounding factors, the researchers found no overall associations between hormone therapy and risk of dementia, regardless of hormone type, application, dose, or duration of treatment. Within the subgroup of women younger than 80 years who had been taking estrogen-only therapy for 10 years or more, a slightly decreased risk of dementia emerged: odds ratio, 0.85; 95% confidence interval, 0.76-0.94.
However, an analysis of dementia cases with a diagnosis specifically of Alzheimer’s disease showed a slight increase in risk associated with estrogen-progestin therapy. Increased risks of developing specifically Alzheimer’s disease emerged in those who had used combination therapy for 5-9 years (OR, 1.11; 95% CI, 1.04-1.20) and also for 10 years or more (OR, 1.19; 95% CI, 1.06-1.33). This risk rose gradually with each year of exposure, reaching an average 11% increased risk for use from 5-9 years and an average 19% for use 10 years or more – equivalent to, respectively, five and seven extra cases per 10,000 woman-years.
According to Jill M. Rabin, MD, a professor at the Feinstein Institutes for Medical Research and an ob.gyn. with Northwell Health in Manhasset, N.Y., the findings make sense for two reasons. “First, there are other health issues noted in women taking long-term combination hormonal therapy such as an increased risk of breast cancer,” she said in an interview. “Second, progesterone is recommended for women who have retained their uterus in order to counteract the potential effects of estrogen on the uterine lining causing possible overgrowth. There are systemic effects however of progesterone, as it counteracts estrogen, potentially decreasing its benefit on the neurological system.”
She added that this analysis is synchronous with other biological studies demonstrating possible neuroprotective effects of estrogen on the brain, especially among younger women. “The vascular system in the newly menopausal female is noted to have less endothelial and intimal thickening, better blood flow and oxygenation, and in general less vascular damage. Estrogen in these relatively younger, newly menopausal women may help to stabilize the vasculature as well as the neurologic system. On the other hand, estrogen therapy over the age of 80 may be delivered to a neurovasculature damaged with age and time, may be somewhat less beneficial.” Older women also have fewer estrogen receptors and, in general, other medical comorbidities.
According to the authors, the findings will be helpful to policy-makers, doctors, and patients when making choices about hormone therapy.
In an accompanying editorial, two U.S. researchers called the findings reassuring. Pauline M. Maki, PhD, of the University of Illinois at Chicago, and JoAnn E. Manson, MD, DrPH, of Brigham and Women’s Hospital, Harvard Medical School, and Harvard T.H. Chan School of Public Health in Boston, however, pointed out that the current study with its older cohort and older age at MHT initiation could not address the important issue of the “timing hypothesis” – namely, that earlier initiation of hormone therapy might confer greater protection against Alzheimer’s disease, compared with later use.
And while the current observations do not change the recommendation that MHT should not be used to prevent dementia, they are helpful for providers to put dementia findings in context for patients. “The primary indication for hormone therapy continues to be the treatment of vasomotor symptoms, and the current study should provide reassurance for women and their providers when treatment is prescribed for that reason,” they wrote.
This study was funded by the U.K. National Institute for Health Research School for Primary Care Research.
Coauthor Dr. Julia Hippisley-Cox is a director of QResearch, EMIS Health, which supplies the QResearch database used for this work, and is a founder and shareholder of ClinRisk., which produces software to implement clinical risk algorithms.
Women who have taken hormone replacement therapy for menopausal symptoms will be relieved by findings from a large British case-control study reporting no overall increased risk of dementia as long as exposure is not long term.
Publishing results online Sept. 29 in BMJ, ( Yana Vinogradova, PhD, a senior research fellow at the University of Nottingham (England), and colleagues made this observation after conducting nested case-control studies involving more than 700,000 women in two U.K. primary care databases. The investigators undertook the study to clarify disparate findings over the past 2 decades on dementia risk associated with menopausal hormone replacement,
“The findings show that menopausal hormone therapy, or MHT, is generally safe for women who require it,” Dr. Vinogradova said in an interview. “A small risk association was found for future development of Alzheimer’s disease increasing with the length of menopausal hormone treatment.” This finding applied only to combined treatments of estrogen plus progestin and became measurable only after long-term use of 5 years or more. “These risk associations, only for long-term use of MHT, are in line with findings related to breast cancer risk,” she said.
The findings also align with previous biological speculations that estrogen combined with progestin may have a harmful effect on the aging brain, she added, “but we also cannot completely rule out other possible factors from our study. For example, some women who were in fact suffering from early signs of Alzheimer’s disease similar to menopausal symptoms may have continued with their menopausal therapy for longer than other women.”
Concerns about the risk of dementia with MHT date back to 2003 when data from the Women’s Health Initiative Memory Study showed that incidence of all-cause dementia doubled in women age 65 years and older after treatment with conjugated equine estrogens and medroxyprogesterone acetate for an average of 4 years. More recently, Finnish research has yielded conflicting data about risks.
The study
The investigators used two U.K. primary care databases (QResearch and CPRD) to analyze MHT prescriptions for 118,501 women age 55 and older diagnosed with dementia between 1998 and 2020 and 497,416 female controls matched by age and general practice, but with no record of dementia.
The cohort was older: mean age of cases was 83.5 years and mean duration of treatment was 16 years for an average age of 67.7 at first captured prescription, considerably later than when most women begin MHT. Relevant factors such as family history, smoking, alcohol consumption, preexisting conditions, and other prescribed drugs were taken into account.
Overall, 16,291 (14%) dementia cases and 68,726 (14%) controls had been exposed to MHT in the period up to 3 years before diagnosis.
After adjusting for potentially confounding factors, the researchers found no overall associations between hormone therapy and risk of dementia, regardless of hormone type, application, dose, or duration of treatment. Within the subgroup of women younger than 80 years who had been taking estrogen-only therapy for 10 years or more, a slightly decreased risk of dementia emerged: odds ratio, 0.85; 95% confidence interval, 0.76-0.94.
However, an analysis of dementia cases with a diagnosis specifically of Alzheimer’s disease showed a slight increase in risk associated with estrogen-progestin therapy. Increased risks of developing specifically Alzheimer’s disease emerged in those who had used combination therapy for 5-9 years (OR, 1.11; 95% CI, 1.04-1.20) and also for 10 years or more (OR, 1.19; 95% CI, 1.06-1.33). This risk rose gradually with each year of exposure, reaching an average 11% increased risk for use from 5-9 years and an average 19% for use 10 years or more – equivalent to, respectively, five and seven extra cases per 10,000 woman-years.
According to Jill M. Rabin, MD, a professor at the Feinstein Institutes for Medical Research and an ob.gyn. with Northwell Health in Manhasset, N.Y., the findings make sense for two reasons. “First, there are other health issues noted in women taking long-term combination hormonal therapy such as an increased risk of breast cancer,” she said in an interview. “Second, progesterone is recommended for women who have retained their uterus in order to counteract the potential effects of estrogen on the uterine lining causing possible overgrowth. There are systemic effects however of progesterone, as it counteracts estrogen, potentially decreasing its benefit on the neurological system.”
She added that this analysis is synchronous with other biological studies demonstrating possible neuroprotective effects of estrogen on the brain, especially among younger women. “The vascular system in the newly menopausal female is noted to have less endothelial and intimal thickening, better blood flow and oxygenation, and in general less vascular damage. Estrogen in these relatively younger, newly menopausal women may help to stabilize the vasculature as well as the neurologic system. On the other hand, estrogen therapy over the age of 80 may be delivered to a neurovasculature damaged with age and time, may be somewhat less beneficial.” Older women also have fewer estrogen receptors and, in general, other medical comorbidities.
According to the authors, the findings will be helpful to policy-makers, doctors, and patients when making choices about hormone therapy.
In an accompanying editorial, two U.S. researchers called the findings reassuring. Pauline M. Maki, PhD, of the University of Illinois at Chicago, and JoAnn E. Manson, MD, DrPH, of Brigham and Women’s Hospital, Harvard Medical School, and Harvard T.H. Chan School of Public Health in Boston, however, pointed out that the current study with its older cohort and older age at MHT initiation could not address the important issue of the “timing hypothesis” – namely, that earlier initiation of hormone therapy might confer greater protection against Alzheimer’s disease, compared with later use.
And while the current observations do not change the recommendation that MHT should not be used to prevent dementia, they are helpful for providers to put dementia findings in context for patients. “The primary indication for hormone therapy continues to be the treatment of vasomotor symptoms, and the current study should provide reassurance for women and their providers when treatment is prescribed for that reason,” they wrote.
This study was funded by the U.K. National Institute for Health Research School for Primary Care Research.
Coauthor Dr. Julia Hippisley-Cox is a director of QResearch, EMIS Health, which supplies the QResearch database used for this work, and is a founder and shareholder of ClinRisk., which produces software to implement clinical risk algorithms.
FROM BMJ
Migraine history linked to more severe hot flashes in postmenopausal women
Women with a history of migraine are more likely to experience severe or very severe hot flashes than women without migraines, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society. An estimated one in five women experience migraine, and women tend to have greater migraine symptoms and disability, the authors note in their background information. Since migraines are also linked to a higher risk of cardiovascular disease, the authors sought to learn whether migraines were associated with vasomotor symptoms, another cardiovascular risk factor.
“The question in my mind is, can we do better at predicting cardiovascular risk in women because the risk prediction models that we have really don’t work all that well in women because they were designed for use in men,” Stephanie S. Faubion, MD, MBA, Penny and Bill George Director for Mayo Clinic’s Center for Women’s Health said in an interview. “My ultimate goal is to see if we can somehow use big data, artificial intelligence to figure out how to weight some of these female-specific or female-predominant factors to come up with a better model for cardiovascular risk prediction.”
The researchers analyzed cross-sectional data from 3,308 women who participated in the Data Registry on the Experiences of Aging, Menopause and Sexuality (DREAMS) study through Mayo Clinic sites in Rochester, Minn.; Scottsdale, Ariz.; and Jacksonville, Fla.. The women ranged in age from 45 to 60 years old, with an average age of 53, and the vast majority of them were white (95%) and had at least some college (93%). Most were also in a long-term relationship (85%), and a majority were employed (69%) and postmenopausal (67%).
The data, collected between May 2015 and December 2019, included a self-reported history of migraine and questionnaires that included the Menopause Rating Scale of menopause-related symptoms.
The researchers adjusted their findings to account for body mass index (BMI), menopause status, smoking status, depression, anxiety, current use of hormone therapy, and presence of low back pain within the past year. ”The diagnosis of low back pain, another pain disorder, was used to test the specificity of the association of migraine and vasomotor symptoms,” the authors write.
Just over a quarter of the women (27%) reported a history of migraine, and these women’s Menopause Rating Scale scores were an average 1.36 points greater than women without a history of migraines (P < .001). Women with self-reported migraine were also 40% more likely than women without migraines to report severe or very severe flashes versus reporting no hot flashes at all (odds ratio, 1.4; P = .02).
“The odds of reporting more severe hot flashes increased monotonically in women with a history of migraine,” the authors report. “In addition, women with low back pain had higher Menopause Rating Scale scores, but were no more likely to have severe/very severe hot flashes than those without back pain, confirming the specificity of the link between vasomotor symptoms and migraine.”
It’s not clear if migraine or hot flashes are risk factors that add to a woman’s existing cardiovascular risk profile or whether they are simply biomarkers of a shared pathway, Dr Faubion said in an interview. She speculates that the common link between migraine and vasomotor symptoms could be neurovascular dysregulation.
Rachael B. Smith, DO, of the department of ob.gyn. at the University of Arizona, Phoenix, was not involved in the research but found that hypothesis plausible as well.
“Our neurologic and vascular systems are coordinated physiologic processes working together for basic brain and body function,” Dr. Smith said in an interview. Some of the symptoms of migraines and menopause are similar and both are often explained by the dysfunction of these systems. The association between history of migraines and severity of vasomotor symptoms is very likely to be explained by this dysregulation between the neurologic and vascular systems.”
Dr. Smith also pointed out, however, that the largely homogeneous study population, all from the same national clinic system, makes it difficult to know how generalizable these findings are.
The primary clinical implications of these findings are that women’s providers need to be sure they’re asking their patients about migraine history and symptoms.
“The counseling we provide on menopausal symptoms should be better tailored to our patients’ medical history, specifically inquiring about history of migraines and how this may impact their symptoms,” Dr. Smith said.
The research was funded by the National Institutes of Health. Dr. Faubion and Dr. Smith had no disclosures.
Women with a history of migraine are more likely to experience severe or very severe hot flashes than women without migraines, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society. An estimated one in five women experience migraine, and women tend to have greater migraine symptoms and disability, the authors note in their background information. Since migraines are also linked to a higher risk of cardiovascular disease, the authors sought to learn whether migraines were associated with vasomotor symptoms, another cardiovascular risk factor.
“The question in my mind is, can we do better at predicting cardiovascular risk in women because the risk prediction models that we have really don’t work all that well in women because they were designed for use in men,” Stephanie S. Faubion, MD, MBA, Penny and Bill George Director for Mayo Clinic’s Center for Women’s Health said in an interview. “My ultimate goal is to see if we can somehow use big data, artificial intelligence to figure out how to weight some of these female-specific or female-predominant factors to come up with a better model for cardiovascular risk prediction.”
The researchers analyzed cross-sectional data from 3,308 women who participated in the Data Registry on the Experiences of Aging, Menopause and Sexuality (DREAMS) study through Mayo Clinic sites in Rochester, Minn.; Scottsdale, Ariz.; and Jacksonville, Fla.. The women ranged in age from 45 to 60 years old, with an average age of 53, and the vast majority of them were white (95%) and had at least some college (93%). Most were also in a long-term relationship (85%), and a majority were employed (69%) and postmenopausal (67%).
The data, collected between May 2015 and December 2019, included a self-reported history of migraine and questionnaires that included the Menopause Rating Scale of menopause-related symptoms.
The researchers adjusted their findings to account for body mass index (BMI), menopause status, smoking status, depression, anxiety, current use of hormone therapy, and presence of low back pain within the past year. ”The diagnosis of low back pain, another pain disorder, was used to test the specificity of the association of migraine and vasomotor symptoms,” the authors write.
Just over a quarter of the women (27%) reported a history of migraine, and these women’s Menopause Rating Scale scores were an average 1.36 points greater than women without a history of migraines (P < .001). Women with self-reported migraine were also 40% more likely than women without migraines to report severe or very severe flashes versus reporting no hot flashes at all (odds ratio, 1.4; P = .02).
“The odds of reporting more severe hot flashes increased monotonically in women with a history of migraine,” the authors report. “In addition, women with low back pain had higher Menopause Rating Scale scores, but were no more likely to have severe/very severe hot flashes than those without back pain, confirming the specificity of the link between vasomotor symptoms and migraine.”
It’s not clear if migraine or hot flashes are risk factors that add to a woman’s existing cardiovascular risk profile or whether they are simply biomarkers of a shared pathway, Dr Faubion said in an interview. She speculates that the common link between migraine and vasomotor symptoms could be neurovascular dysregulation.
Rachael B. Smith, DO, of the department of ob.gyn. at the University of Arizona, Phoenix, was not involved in the research but found that hypothesis plausible as well.
“Our neurologic and vascular systems are coordinated physiologic processes working together for basic brain and body function,” Dr. Smith said in an interview. Some of the symptoms of migraines and menopause are similar and both are often explained by the dysfunction of these systems. The association between history of migraines and severity of vasomotor symptoms is very likely to be explained by this dysregulation between the neurologic and vascular systems.”
Dr. Smith also pointed out, however, that the largely homogeneous study population, all from the same national clinic system, makes it difficult to know how generalizable these findings are.
The primary clinical implications of these findings are that women’s providers need to be sure they’re asking their patients about migraine history and symptoms.
“The counseling we provide on menopausal symptoms should be better tailored to our patients’ medical history, specifically inquiring about history of migraines and how this may impact their symptoms,” Dr. Smith said.
The research was funded by the National Institutes of Health. Dr. Faubion and Dr. Smith had no disclosures.
Women with a history of migraine are more likely to experience severe or very severe hot flashes than women without migraines, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society. An estimated one in five women experience migraine, and women tend to have greater migraine symptoms and disability, the authors note in their background information. Since migraines are also linked to a higher risk of cardiovascular disease, the authors sought to learn whether migraines were associated with vasomotor symptoms, another cardiovascular risk factor.
“The question in my mind is, can we do better at predicting cardiovascular risk in women because the risk prediction models that we have really don’t work all that well in women because they were designed for use in men,” Stephanie S. Faubion, MD, MBA, Penny and Bill George Director for Mayo Clinic’s Center for Women’s Health said in an interview. “My ultimate goal is to see if we can somehow use big data, artificial intelligence to figure out how to weight some of these female-specific or female-predominant factors to come up with a better model for cardiovascular risk prediction.”
The researchers analyzed cross-sectional data from 3,308 women who participated in the Data Registry on the Experiences of Aging, Menopause and Sexuality (DREAMS) study through Mayo Clinic sites in Rochester, Minn.; Scottsdale, Ariz.; and Jacksonville, Fla.. The women ranged in age from 45 to 60 years old, with an average age of 53, and the vast majority of them were white (95%) and had at least some college (93%). Most were also in a long-term relationship (85%), and a majority were employed (69%) and postmenopausal (67%).
The data, collected between May 2015 and December 2019, included a self-reported history of migraine and questionnaires that included the Menopause Rating Scale of menopause-related symptoms.
The researchers adjusted their findings to account for body mass index (BMI), menopause status, smoking status, depression, anxiety, current use of hormone therapy, and presence of low back pain within the past year. ”The diagnosis of low back pain, another pain disorder, was used to test the specificity of the association of migraine and vasomotor symptoms,” the authors write.
Just over a quarter of the women (27%) reported a history of migraine, and these women’s Menopause Rating Scale scores were an average 1.36 points greater than women without a history of migraines (P < .001). Women with self-reported migraine were also 40% more likely than women without migraines to report severe or very severe flashes versus reporting no hot flashes at all (odds ratio, 1.4; P = .02).
“The odds of reporting more severe hot flashes increased monotonically in women with a history of migraine,” the authors report. “In addition, women with low back pain had higher Menopause Rating Scale scores, but were no more likely to have severe/very severe hot flashes than those without back pain, confirming the specificity of the link between vasomotor symptoms and migraine.”
It’s not clear if migraine or hot flashes are risk factors that add to a woman’s existing cardiovascular risk profile or whether they are simply biomarkers of a shared pathway, Dr Faubion said in an interview. She speculates that the common link between migraine and vasomotor symptoms could be neurovascular dysregulation.
Rachael B. Smith, DO, of the department of ob.gyn. at the University of Arizona, Phoenix, was not involved in the research but found that hypothesis plausible as well.
“Our neurologic and vascular systems are coordinated physiologic processes working together for basic brain and body function,” Dr. Smith said in an interview. Some of the symptoms of migraines and menopause are similar and both are often explained by the dysfunction of these systems. The association between history of migraines and severity of vasomotor symptoms is very likely to be explained by this dysregulation between the neurologic and vascular systems.”
Dr. Smith also pointed out, however, that the largely homogeneous study population, all from the same national clinic system, makes it difficult to know how generalizable these findings are.
The primary clinical implications of these findings are that women’s providers need to be sure they’re asking their patients about migraine history and symptoms.
“The counseling we provide on menopausal symptoms should be better tailored to our patients’ medical history, specifically inquiring about history of migraines and how this may impact their symptoms,” Dr. Smith said.
The research was funded by the National Institutes of Health. Dr. Faubion and Dr. Smith had no disclosures.
FROM NAMS 2021
PCOS linked to menopausal urogenital symptoms but not hot flashes
Women with a history of polycystic ovary syndrome (PCOS) are more likely to experience somatic and urogenital symptoms post menopause, but they were no more likely to experience severe hot flashes than were other women with similar characteristics, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society.
PCOS and vasomotor symptoms are each risk factors for cardiovascular disease, so researchers wanted to find out whether they were linked to one another, which might indicate that they are markers for the same underlying mechanisms that increase heart disease risk. The lack of an association, however, raises questions about how much each of these conditions might independently increase cardiovascular risk.
“Should we take a little more time to truly risk-assess these patients not just with their ASCVD risk score, but take into account that they have PCOS and they’re going through menopause, and how severe their hot flashes are?” asked Angie S. Lobo, MD, an internal medicine specialist at Mayo Clinic in Rochester, Minn., when she discussed her findings in an interview.
The association between PCOS and urogenital symptoms was surprising, Dr. Lobo said, but she said she suspects the reason for the finding may be the self-reported nature of the study.
“If you ask the question, you get the answer,” Dr. Lobo said. ”Are we just not asking the right questions to our patients? And should we be doing this more often? This is an exciting finding because there’s so much room to improve the clinical care of our patients.”
The researchers analyzed data from 3,308 women, ages 45-60, in a cross-sectional study from the Data Registry on the Experiences of Aging, Menopause, and Sexuality (DREAMS). The study occurred at Mayo Clinic locations between May 2015 and December 2019 in Rochester, Minn., in Scottsdale, Ariz., and in Jacksonville, Fla.
The women were an average 53 years old and were primarily White, educated, and postmenopausal. Among the 4.6% of women with a self-reported history of PCOS, 56% of them reported depression symptoms, compared to 42% of women without PCOS. Those with PCOS also had nearly twice the prevalence of obesity – 42% versus 22.5% among women without PCOS – and had a higher average overall score on the Menopause Rating Scale (17.7 vs. 14.7; P < .001).
Although women with PCOS initially had a greater burden of psychological symptoms on the same scale, that association disappeared after adjustment for menopause status, body mass index, depression, anxiety, and current use of hormone therapy. Even after adjustment, however, women with PCOS had higher average scores for somatic symptoms (6.7 vs. 5.6) and urogenital symptoms (5.2 vs. 4.3) than those of women without PCOS (P < .001).
Severe or very severe hot flashes were no more likely in women with a history of PCOS than in the other women in the study.
”The mechanisms underlying the correlation between PCOS and menopause symptoms in the psychological and urogenital symptom domains requires further study, although the well-known association between PCOS and mood disorders may explain the high psychological symptom burden in these women during the menopause transition,” the authors concluded.
Rachael B. Smith, DO, clinical assistant professor of ob.gyn. at the University of Arizona in Phoenix, said she was not surprised to see an association between PCOS and menopause symptoms overall, but she was surprised that PCOS did not correlate with severity of vasomotor symptoms. But Dr. Smith pointed out that the sample size of women with PCOS is fairly small (n = 151).
“Given that PCOS prevalence is about 6%-10%, I feel this association should be further studied to improve our counseling and treatment for this PCOS population,” Dr. Smith, who was not involved in the research, said in an interview. “The take-home message for physicians is improved patient-tailored counseling that takes into account patients’ prior medical history of PCOS.”
Although it will require more research to find out, Dr. Smith said she suspects that PCOS and vasomotor symptoms are additive risk factors for cardiovascular disease. She also noted that the study is limited by the homogeneity of the study population.
The research was funded by the National Institutes of Health. Dr. Lobo and Dr. Smith had no disclosures.
Women with a history of polycystic ovary syndrome (PCOS) are more likely to experience somatic and urogenital symptoms post menopause, but they were no more likely to experience severe hot flashes than were other women with similar characteristics, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society.
PCOS and vasomotor symptoms are each risk factors for cardiovascular disease, so researchers wanted to find out whether they were linked to one another, which might indicate that they are markers for the same underlying mechanisms that increase heart disease risk. The lack of an association, however, raises questions about how much each of these conditions might independently increase cardiovascular risk.
“Should we take a little more time to truly risk-assess these patients not just with their ASCVD risk score, but take into account that they have PCOS and they’re going through menopause, and how severe their hot flashes are?” asked Angie S. Lobo, MD, an internal medicine specialist at Mayo Clinic in Rochester, Minn., when she discussed her findings in an interview.
The association between PCOS and urogenital symptoms was surprising, Dr. Lobo said, but she said she suspects the reason for the finding may be the self-reported nature of the study.
“If you ask the question, you get the answer,” Dr. Lobo said. ”Are we just not asking the right questions to our patients? And should we be doing this more often? This is an exciting finding because there’s so much room to improve the clinical care of our patients.”
The researchers analyzed data from 3,308 women, ages 45-60, in a cross-sectional study from the Data Registry on the Experiences of Aging, Menopause, and Sexuality (DREAMS). The study occurred at Mayo Clinic locations between May 2015 and December 2019 in Rochester, Minn., in Scottsdale, Ariz., and in Jacksonville, Fla.
The women were an average 53 years old and were primarily White, educated, and postmenopausal. Among the 4.6% of women with a self-reported history of PCOS, 56% of them reported depression symptoms, compared to 42% of women without PCOS. Those with PCOS also had nearly twice the prevalence of obesity – 42% versus 22.5% among women without PCOS – and had a higher average overall score on the Menopause Rating Scale (17.7 vs. 14.7; P < .001).
Although women with PCOS initially had a greater burden of psychological symptoms on the same scale, that association disappeared after adjustment for menopause status, body mass index, depression, anxiety, and current use of hormone therapy. Even after adjustment, however, women with PCOS had higher average scores for somatic symptoms (6.7 vs. 5.6) and urogenital symptoms (5.2 vs. 4.3) than those of women without PCOS (P < .001).
Severe or very severe hot flashes were no more likely in women with a history of PCOS than in the other women in the study.
”The mechanisms underlying the correlation between PCOS and menopause symptoms in the psychological and urogenital symptom domains requires further study, although the well-known association between PCOS and mood disorders may explain the high psychological symptom burden in these women during the menopause transition,” the authors concluded.
Rachael B. Smith, DO, clinical assistant professor of ob.gyn. at the University of Arizona in Phoenix, said she was not surprised to see an association between PCOS and menopause symptoms overall, but she was surprised that PCOS did not correlate with severity of vasomotor symptoms. But Dr. Smith pointed out that the sample size of women with PCOS is fairly small (n = 151).
“Given that PCOS prevalence is about 6%-10%, I feel this association should be further studied to improve our counseling and treatment for this PCOS population,” Dr. Smith, who was not involved in the research, said in an interview. “The take-home message for physicians is improved patient-tailored counseling that takes into account patients’ prior medical history of PCOS.”
Although it will require more research to find out, Dr. Smith said she suspects that PCOS and vasomotor symptoms are additive risk factors for cardiovascular disease. She also noted that the study is limited by the homogeneity of the study population.
The research was funded by the National Institutes of Health. Dr. Lobo and Dr. Smith had no disclosures.
Women with a history of polycystic ovary syndrome (PCOS) are more likely to experience somatic and urogenital symptoms post menopause, but they were no more likely to experience severe hot flashes than were other women with similar characteristics, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society.
PCOS and vasomotor symptoms are each risk factors for cardiovascular disease, so researchers wanted to find out whether they were linked to one another, which might indicate that they are markers for the same underlying mechanisms that increase heart disease risk. The lack of an association, however, raises questions about how much each of these conditions might independently increase cardiovascular risk.
“Should we take a little more time to truly risk-assess these patients not just with their ASCVD risk score, but take into account that they have PCOS and they’re going through menopause, and how severe their hot flashes are?” asked Angie S. Lobo, MD, an internal medicine specialist at Mayo Clinic in Rochester, Minn., when she discussed her findings in an interview.
The association between PCOS and urogenital symptoms was surprising, Dr. Lobo said, but she said she suspects the reason for the finding may be the self-reported nature of the study.
“If you ask the question, you get the answer,” Dr. Lobo said. ”Are we just not asking the right questions to our patients? And should we be doing this more often? This is an exciting finding because there’s so much room to improve the clinical care of our patients.”
The researchers analyzed data from 3,308 women, ages 45-60, in a cross-sectional study from the Data Registry on the Experiences of Aging, Menopause, and Sexuality (DREAMS). The study occurred at Mayo Clinic locations between May 2015 and December 2019 in Rochester, Minn., in Scottsdale, Ariz., and in Jacksonville, Fla.
The women were an average 53 years old and were primarily White, educated, and postmenopausal. Among the 4.6% of women with a self-reported history of PCOS, 56% of them reported depression symptoms, compared to 42% of women without PCOS. Those with PCOS also had nearly twice the prevalence of obesity – 42% versus 22.5% among women without PCOS – and had a higher average overall score on the Menopause Rating Scale (17.7 vs. 14.7; P < .001).
Although women with PCOS initially had a greater burden of psychological symptoms on the same scale, that association disappeared after adjustment for menopause status, body mass index, depression, anxiety, and current use of hormone therapy. Even after adjustment, however, women with PCOS had higher average scores for somatic symptoms (6.7 vs. 5.6) and urogenital symptoms (5.2 vs. 4.3) than those of women without PCOS (P < .001).
Severe or very severe hot flashes were no more likely in women with a history of PCOS than in the other women in the study.
”The mechanisms underlying the correlation between PCOS and menopause symptoms in the psychological and urogenital symptom domains requires further study, although the well-known association between PCOS and mood disorders may explain the high psychological symptom burden in these women during the menopause transition,” the authors concluded.
Rachael B. Smith, DO, clinical assistant professor of ob.gyn. at the University of Arizona in Phoenix, said she was not surprised to see an association between PCOS and menopause symptoms overall, but she was surprised that PCOS did not correlate with severity of vasomotor symptoms. But Dr. Smith pointed out that the sample size of women with PCOS is fairly small (n = 151).
“Given that PCOS prevalence is about 6%-10%, I feel this association should be further studied to improve our counseling and treatment for this PCOS population,” Dr. Smith, who was not involved in the research, said in an interview. “The take-home message for physicians is improved patient-tailored counseling that takes into account patients’ prior medical history of PCOS.”
Although it will require more research to find out, Dr. Smith said she suspects that PCOS and vasomotor symptoms are additive risk factors for cardiovascular disease. She also noted that the study is limited by the homogeneity of the study population.
The research was funded by the National Institutes of Health. Dr. Lobo and Dr. Smith had no disclosures.
FROM NAMS 2021
Updates to CDC’s STI guidelines relevant to midlife women too
Sexually transmitted infection rates have not increased as dramatically in older women as they have in women in their teens and 20s, but rates of chlamydia and gonorrhea in women over age 35 have seen a steady incline over the past decade, and syphilis rates have climbed steeply, according to data from the Centers for Disease Control and Prevention.
That makes the STI treatment guidelines released by the CDC in July even timelier for practitioners of menopause medicine, according to Michael S. Policar, MD, MPH, a professor emeritus of ob.gyn. and reproductive sciences at the University of California, San Francisco.
Dr. Policar discussed what clinicians need to know about STIs in midlife women at the hybrid annual meeting of the North American Menopause Society. Even the nomenclature change in the guidelines from “sexually transmitted diseases” to “sexually transmitted infections” is important “because they want to acknowledge the fact that a lot of the sexually transmitted infections that we’re treating are asymptomatic, are colonizations, and are not yet diseases,” Dr. Policar said. “We’re trying to be much more expansive in thinking about finding these infections before they actually start causing morbidity in the form of a disease.”
Sexual history
The primary guidelines update for taking sexual history is the recommendation to ask patients about their intentions regarding pregnancy. The “5 Ps” of sexual history are now Partners, Practices, Protection from STIs, Past history of STIs, and Pregnancy intention.
“There should be a sixth P that has to do with pleasure questions,” Policar added. “We ask all the time for patients that we see in the context of perimenopausal and menopausal services, ‘Are you satisfied with your sexual relationship with your partner?’ Hopefully that will make it into the CDC guidelines as the sixth P at some point, but for now, that’s aspirational.”
In asking about partners, instead of asking patients whether they have sex with men, women, or both, clinicians should ask first if the patient is having sex of any kind – oral, vaginal, or anal – with anyone. From there, providers should ask how many sex partners the patient has had, the gender(s) of the partners, and whether they or their partners have other sex partners, using more gender-inclusive language.
When asking about practices, in addition to asking about the type of sexual contact patients have had, additional questions include whether the patient met their partners online or through apps, whether they or any of their partners use drugs, and whether the patient has exchanged sex for any needs, such as money, housing, or drugs. The additional questions can identify those at higher risk for STIs.
After reviewing the CDC’s list of risk factors for gonorrhea and chlamydia screening, Dr. Policar shared the screening list from the California Department of Public Health, which he finds more helpful:
- History of gonorrhea, chlamydia, or pelvic inflammatory disease (PID) in the past 2 years.
- More than 1 sexual partner in the past year.
- New sexual partner within 90 days.
- Reason to believe that a sex partner has had other partners in the past year.
- Exchanging sex for drugs or money within the past year.
- Other factors identified locally, including prevalence of infection in the community.
STI screening guidelines
For those with a positive gonorrhea/chlamydia (GC/CT) screen, a nucleic acid amplification test (NAAT) vaginal swab is the preferred specimen source, and self-collection is fine for women of any age, Dr. Policar said. In addition, cis-women who received anal intercourse in the preceding year should consider undergoing a rectal GC/CT NAAT, and those who performed oral sex should consider a pharyngeal GC/CT NAAT, based on shared clinical decision-making. A rectal swab requires an insertion of 3-4 cm and a 360-degree twirl of the wrist, not the swab, to ensure you get a sample from the entire circumference. Pharyngeal samples require swabbing both tonsillar pillars while taking care for those who may gag.
For contact testing – asymptomatic people who have had a high-risk sexual exposure – providers should test for gonorrhea, chlamydia, HIV, and syphilis but not for herpes, high-risk HPV, hepatitis B, hepatitis C, or bacterial vaginosis. “Maybe we’ll do a screen for trichomoniasis, and maybe we’ll offer herpes type 2 serology or antibody screening,” Dr. Policar said. Providers should also ask patients requesting contact testing if they have been vaccinated for hepatitis B. If not, “the conversation should be how can we get you vaccinated for hepatitis B,” Dr. Policar said.
HIV screening only needs to occur once between the ages of 15 and 65 for low-risk people and then once annually (or more often if necessary) for those who have a sex partner with HIV, use injectable drugs, engage in commercial sex work, have a new sex partner with unknown HIV status, received care at an STD or TB clinic, or were in a correctional facility or homeless shelter.
Those at increased risk for syphilis include men who have sex with men, men under age 29, and anyone living with HIV or who has a history of incarceration or a history of commercial sex work. In addition, African Americans have the greatest risk for syphilis of racial/ethnic groups, followed by Hispanics. Most adults only require hepatitis C screening with anti-hep C antibody testing once in their lifetime. Periodic hepatitis C screening should occur for people who inject drugs. If the screening is positive, providers should conduct an RNA polymerase chain reaction (PCR) test to determine whether a chronic infection is present.
Trichomoniasis screening should occur annually in women living with HIV or in correctional facilities. Others to consider screening include people with new or multiple sex partners, a history of STIs, inconsistent condom use, a history of sex work, and intravenous drug use. Dr. Policar also noted that several new assays, including NAAT, PCR, and a rapid test, are available for trichomoniasis.
STI treatment guidelines
For women with mucoprurulent cervicitis, the cause could be chlamydia, gonorrhea, herpes, trichomonas, mycoplasma, or even progesterone from pregnancy or contraception, Dr. Policar said. The new preferred treatment is 100 mg of doxycycline. The alternative, albeit less preferred, treatment is 1 g azithromycin.
The preferred treatment for chlamydia is now 100 mg oral doxycycline twice daily, or doxycycline 200 mg delayed-release once daily, for 7 days. Alternative regimens include 1 g oral azithromycin in a single dose or 500 mg oral levofloxacin once daily for 7 days. The switch to recommending doxycycline over azithromycin is based on recent evidence showing that doxycycline has a slightly higher efficacy for urogenital chlamydia and a substantially higher efficacy for rectal chlamydia. In addition, an increasing proportion of gonorrheal infections have shown resistance to azithromycin, particularly beginning in 2014.
Preferred treatment of new, uncomplicated gonorrhea infections of the cervix, urethra, rectum, and pharynx is one 500-mg dose of ceftriaxone for those weighing under 150 kg and 1 g for those weighing 150 kg or more. If ceftriaxone is unavailable, the new alternative recommended treatment for gonorrhea is 800 mg cefixime. For pharyngeal gonorrhea only, the CDC recommends a test-of-cure 7-14 days after treatment.
For gonorrheal infections, the CDC also recommends treatment with doxycycline if chlamydia has not been excluded, but the agency no longer recommends dual therapy with azithromycin unless it’s used in place of doxycycline for those who are pregnant, have an allergy, or may not be compliant with a 7-day doxycycline regimen.
The preferred treatment for bacterial vaginosis has not changed. The new recommended regimen for trichomoniasis is 500 mg oral metronidazole for 7 days, with the alternative being a single 2-g dose of tinidazole. Male partners should receive 2 g oral metronidazole. The CDC also notes that patients taking metronidazole no longer need to abstain from alcohol during treatment.
”Another area where the guidelines changed is in their description of expedited partner therapy, which means that, when we find an index case who has gonorrhea or chlamydia, we always have a discussion with her about getting her partners treated,” Dr. Policar said. “The CDC was quite clear that the responsibility for discussing partner treatment rests with us as the diagnosing provider” since city and county health departments don’t have the time or resources for contact tracing these STIs.
The two main ways to treat partners are to have the patient bring their partner(s) to the appointment with them or to do patient-delivered partner therapy. Ideally, clinicians who dispense their own medications can give the patient enough drugs to give her partner(s) a complete dose as well. Otherwise, providers can prescribe extra doses in the index patients’ name or write prescriptions in the partner’s name.
“In every state of the union now, it is legal for you to to prescribe antibiotics for partners sight unseen, Dr. Policar said.
Margaret Sullivan, MD, an ob.gyn. from rural western North Carolina, noted during the Q&A that an obstacle to partner therapy at her practice has been cost, particularly since many of the men don’t have insurance.
“I have not heard before of prescribing the extra doses for partners under the patient’s name,” Dr. Sullivan said. “I’ve thought about doing it, but [was worried about] it potentially being fraudulent if that patient has Medicaid and we’re prescribing extra doses under her name, so how do you work around that?”
Dr. Policar acknowledged that barrier and recommended that patients use the website/app Goodrx.com to find discounts for out-of-pocket generic medications. He also noted the occasional obstacle of pharmacists balking at filling a double or triple dose.
“What we’ve been suggesting in that circumstance is to literally copy that part of the CDC guidelines, which explains expedited partner therapy or patient-delivered partner therapy and send that off to the pharmacist so they can see that it’s a national recommendation of the CDC,” Dr. Policar said.
Claudia Rodriguez, MD, an ob.gyn. who works at Sherman Hospital in Elgin, Ill., asked about the CDC recommendations for HPV vaccination in older women. Although the CDC permits women over age 26 to receive the HPV vaccine, the agency does not “make a solid recommendation to have this done, which oftentimes makes a big difference in whether or not health insurance will actually pay for vaccination in that circumstance,” Dr. Policar said.
Patients are welcome to request the vaccine after shared decision-making, but “we should never present this as something which is routine,” he said. For women in their 50s, for example, “there’s virtually no data about any additional degree of protection that you would get” from HPV vaccination, Dr. Policar said in response to a similar question from Tara Allmen, MD, an ob.gyn. in New York City. “If you ask me for my personal clinical opinion about it, I would say it’s not going to be worth it,” he said.
Dr Policar had no disclosures. Disclosures were unavailable for attendees who spoke.
Sexually transmitted infection rates have not increased as dramatically in older women as they have in women in their teens and 20s, but rates of chlamydia and gonorrhea in women over age 35 have seen a steady incline over the past decade, and syphilis rates have climbed steeply, according to data from the Centers for Disease Control and Prevention.
That makes the STI treatment guidelines released by the CDC in July even timelier for practitioners of menopause medicine, according to Michael S. Policar, MD, MPH, a professor emeritus of ob.gyn. and reproductive sciences at the University of California, San Francisco.
Dr. Policar discussed what clinicians need to know about STIs in midlife women at the hybrid annual meeting of the North American Menopause Society. Even the nomenclature change in the guidelines from “sexually transmitted diseases” to “sexually transmitted infections” is important “because they want to acknowledge the fact that a lot of the sexually transmitted infections that we’re treating are asymptomatic, are colonizations, and are not yet diseases,” Dr. Policar said. “We’re trying to be much more expansive in thinking about finding these infections before they actually start causing morbidity in the form of a disease.”
Sexual history
The primary guidelines update for taking sexual history is the recommendation to ask patients about their intentions regarding pregnancy. The “5 Ps” of sexual history are now Partners, Practices, Protection from STIs, Past history of STIs, and Pregnancy intention.
“There should be a sixth P that has to do with pleasure questions,” Policar added. “We ask all the time for patients that we see in the context of perimenopausal and menopausal services, ‘Are you satisfied with your sexual relationship with your partner?’ Hopefully that will make it into the CDC guidelines as the sixth P at some point, but for now, that’s aspirational.”
In asking about partners, instead of asking patients whether they have sex with men, women, or both, clinicians should ask first if the patient is having sex of any kind – oral, vaginal, or anal – with anyone. From there, providers should ask how many sex partners the patient has had, the gender(s) of the partners, and whether they or their partners have other sex partners, using more gender-inclusive language.
When asking about practices, in addition to asking about the type of sexual contact patients have had, additional questions include whether the patient met their partners online or through apps, whether they or any of their partners use drugs, and whether the patient has exchanged sex for any needs, such as money, housing, or drugs. The additional questions can identify those at higher risk for STIs.
After reviewing the CDC’s list of risk factors for gonorrhea and chlamydia screening, Dr. Policar shared the screening list from the California Department of Public Health, which he finds more helpful:
- History of gonorrhea, chlamydia, or pelvic inflammatory disease (PID) in the past 2 years.
- More than 1 sexual partner in the past year.
- New sexual partner within 90 days.
- Reason to believe that a sex partner has had other partners in the past year.
- Exchanging sex for drugs or money within the past year.
- Other factors identified locally, including prevalence of infection in the community.
STI screening guidelines
For those with a positive gonorrhea/chlamydia (GC/CT) screen, a nucleic acid amplification test (NAAT) vaginal swab is the preferred specimen source, and self-collection is fine for women of any age, Dr. Policar said. In addition, cis-women who received anal intercourse in the preceding year should consider undergoing a rectal GC/CT NAAT, and those who performed oral sex should consider a pharyngeal GC/CT NAAT, based on shared clinical decision-making. A rectal swab requires an insertion of 3-4 cm and a 360-degree twirl of the wrist, not the swab, to ensure you get a sample from the entire circumference. Pharyngeal samples require swabbing both tonsillar pillars while taking care for those who may gag.
For contact testing – asymptomatic people who have had a high-risk sexual exposure – providers should test for gonorrhea, chlamydia, HIV, and syphilis but not for herpes, high-risk HPV, hepatitis B, hepatitis C, or bacterial vaginosis. “Maybe we’ll do a screen for trichomoniasis, and maybe we’ll offer herpes type 2 serology or antibody screening,” Dr. Policar said. Providers should also ask patients requesting contact testing if they have been vaccinated for hepatitis B. If not, “the conversation should be how can we get you vaccinated for hepatitis B,” Dr. Policar said.
HIV screening only needs to occur once between the ages of 15 and 65 for low-risk people and then once annually (or more often if necessary) for those who have a sex partner with HIV, use injectable drugs, engage in commercial sex work, have a new sex partner with unknown HIV status, received care at an STD or TB clinic, or were in a correctional facility or homeless shelter.
Those at increased risk for syphilis include men who have sex with men, men under age 29, and anyone living with HIV or who has a history of incarceration or a history of commercial sex work. In addition, African Americans have the greatest risk for syphilis of racial/ethnic groups, followed by Hispanics. Most adults only require hepatitis C screening with anti-hep C antibody testing once in their lifetime. Periodic hepatitis C screening should occur for people who inject drugs. If the screening is positive, providers should conduct an RNA polymerase chain reaction (PCR) test to determine whether a chronic infection is present.
Trichomoniasis screening should occur annually in women living with HIV or in correctional facilities. Others to consider screening include people with new or multiple sex partners, a history of STIs, inconsistent condom use, a history of sex work, and intravenous drug use. Dr. Policar also noted that several new assays, including NAAT, PCR, and a rapid test, are available for trichomoniasis.
STI treatment guidelines
For women with mucoprurulent cervicitis, the cause could be chlamydia, gonorrhea, herpes, trichomonas, mycoplasma, or even progesterone from pregnancy or contraception, Dr. Policar said. The new preferred treatment is 100 mg of doxycycline. The alternative, albeit less preferred, treatment is 1 g azithromycin.
The preferred treatment for chlamydia is now 100 mg oral doxycycline twice daily, or doxycycline 200 mg delayed-release once daily, for 7 days. Alternative regimens include 1 g oral azithromycin in a single dose or 500 mg oral levofloxacin once daily for 7 days. The switch to recommending doxycycline over azithromycin is based on recent evidence showing that doxycycline has a slightly higher efficacy for urogenital chlamydia and a substantially higher efficacy for rectal chlamydia. In addition, an increasing proportion of gonorrheal infections have shown resistance to azithromycin, particularly beginning in 2014.
Preferred treatment of new, uncomplicated gonorrhea infections of the cervix, urethra, rectum, and pharynx is one 500-mg dose of ceftriaxone for those weighing under 150 kg and 1 g for those weighing 150 kg or more. If ceftriaxone is unavailable, the new alternative recommended treatment for gonorrhea is 800 mg cefixime. For pharyngeal gonorrhea only, the CDC recommends a test-of-cure 7-14 days after treatment.
For gonorrheal infections, the CDC also recommends treatment with doxycycline if chlamydia has not been excluded, but the agency no longer recommends dual therapy with azithromycin unless it’s used in place of doxycycline for those who are pregnant, have an allergy, or may not be compliant with a 7-day doxycycline regimen.
The preferred treatment for bacterial vaginosis has not changed. The new recommended regimen for trichomoniasis is 500 mg oral metronidazole for 7 days, with the alternative being a single 2-g dose of tinidazole. Male partners should receive 2 g oral metronidazole. The CDC also notes that patients taking metronidazole no longer need to abstain from alcohol during treatment.
”Another area where the guidelines changed is in their description of expedited partner therapy, which means that, when we find an index case who has gonorrhea or chlamydia, we always have a discussion with her about getting her partners treated,” Dr. Policar said. “The CDC was quite clear that the responsibility for discussing partner treatment rests with us as the diagnosing provider” since city and county health departments don’t have the time or resources for contact tracing these STIs.
The two main ways to treat partners are to have the patient bring their partner(s) to the appointment with them or to do patient-delivered partner therapy. Ideally, clinicians who dispense their own medications can give the patient enough drugs to give her partner(s) a complete dose as well. Otherwise, providers can prescribe extra doses in the index patients’ name or write prescriptions in the partner’s name.
“In every state of the union now, it is legal for you to to prescribe antibiotics for partners sight unseen, Dr. Policar said.
Margaret Sullivan, MD, an ob.gyn. from rural western North Carolina, noted during the Q&A that an obstacle to partner therapy at her practice has been cost, particularly since many of the men don’t have insurance.
“I have not heard before of prescribing the extra doses for partners under the patient’s name,” Dr. Sullivan said. “I’ve thought about doing it, but [was worried about] it potentially being fraudulent if that patient has Medicaid and we’re prescribing extra doses under her name, so how do you work around that?”
Dr. Policar acknowledged that barrier and recommended that patients use the website/app Goodrx.com to find discounts for out-of-pocket generic medications. He also noted the occasional obstacle of pharmacists balking at filling a double or triple dose.
“What we’ve been suggesting in that circumstance is to literally copy that part of the CDC guidelines, which explains expedited partner therapy or patient-delivered partner therapy and send that off to the pharmacist so they can see that it’s a national recommendation of the CDC,” Dr. Policar said.
Claudia Rodriguez, MD, an ob.gyn. who works at Sherman Hospital in Elgin, Ill., asked about the CDC recommendations for HPV vaccination in older women. Although the CDC permits women over age 26 to receive the HPV vaccine, the agency does not “make a solid recommendation to have this done, which oftentimes makes a big difference in whether or not health insurance will actually pay for vaccination in that circumstance,” Dr. Policar said.
Patients are welcome to request the vaccine after shared decision-making, but “we should never present this as something which is routine,” he said. For women in their 50s, for example, “there’s virtually no data about any additional degree of protection that you would get” from HPV vaccination, Dr. Policar said in response to a similar question from Tara Allmen, MD, an ob.gyn. in New York City. “If you ask me for my personal clinical opinion about it, I would say it’s not going to be worth it,” he said.
Dr Policar had no disclosures. Disclosures were unavailable for attendees who spoke.
Sexually transmitted infection rates have not increased as dramatically in older women as they have in women in their teens and 20s, but rates of chlamydia and gonorrhea in women over age 35 have seen a steady incline over the past decade, and syphilis rates have climbed steeply, according to data from the Centers for Disease Control and Prevention.
That makes the STI treatment guidelines released by the CDC in July even timelier for practitioners of menopause medicine, according to Michael S. Policar, MD, MPH, a professor emeritus of ob.gyn. and reproductive sciences at the University of California, San Francisco.
Dr. Policar discussed what clinicians need to know about STIs in midlife women at the hybrid annual meeting of the North American Menopause Society. Even the nomenclature change in the guidelines from “sexually transmitted diseases” to “sexually transmitted infections” is important “because they want to acknowledge the fact that a lot of the sexually transmitted infections that we’re treating are asymptomatic, are colonizations, and are not yet diseases,” Dr. Policar said. “We’re trying to be much more expansive in thinking about finding these infections before they actually start causing morbidity in the form of a disease.”
Sexual history
The primary guidelines update for taking sexual history is the recommendation to ask patients about their intentions regarding pregnancy. The “5 Ps” of sexual history are now Partners, Practices, Protection from STIs, Past history of STIs, and Pregnancy intention.
“There should be a sixth P that has to do with pleasure questions,” Policar added. “We ask all the time for patients that we see in the context of perimenopausal and menopausal services, ‘Are you satisfied with your sexual relationship with your partner?’ Hopefully that will make it into the CDC guidelines as the sixth P at some point, but for now, that’s aspirational.”
In asking about partners, instead of asking patients whether they have sex with men, women, or both, clinicians should ask first if the patient is having sex of any kind – oral, vaginal, or anal – with anyone. From there, providers should ask how many sex partners the patient has had, the gender(s) of the partners, and whether they or their partners have other sex partners, using more gender-inclusive language.
When asking about practices, in addition to asking about the type of sexual contact patients have had, additional questions include whether the patient met their partners online or through apps, whether they or any of their partners use drugs, and whether the patient has exchanged sex for any needs, such as money, housing, or drugs. The additional questions can identify those at higher risk for STIs.
After reviewing the CDC’s list of risk factors for gonorrhea and chlamydia screening, Dr. Policar shared the screening list from the California Department of Public Health, which he finds more helpful:
- History of gonorrhea, chlamydia, or pelvic inflammatory disease (PID) in the past 2 years.
- More than 1 sexual partner in the past year.
- New sexual partner within 90 days.
- Reason to believe that a sex partner has had other partners in the past year.
- Exchanging sex for drugs or money within the past year.
- Other factors identified locally, including prevalence of infection in the community.
STI screening guidelines
For those with a positive gonorrhea/chlamydia (GC/CT) screen, a nucleic acid amplification test (NAAT) vaginal swab is the preferred specimen source, and self-collection is fine for women of any age, Dr. Policar said. In addition, cis-women who received anal intercourse in the preceding year should consider undergoing a rectal GC/CT NAAT, and those who performed oral sex should consider a pharyngeal GC/CT NAAT, based on shared clinical decision-making. A rectal swab requires an insertion of 3-4 cm and a 360-degree twirl of the wrist, not the swab, to ensure you get a sample from the entire circumference. Pharyngeal samples require swabbing both tonsillar pillars while taking care for those who may gag.
For contact testing – asymptomatic people who have had a high-risk sexual exposure – providers should test for gonorrhea, chlamydia, HIV, and syphilis but not for herpes, high-risk HPV, hepatitis B, hepatitis C, or bacterial vaginosis. “Maybe we’ll do a screen for trichomoniasis, and maybe we’ll offer herpes type 2 serology or antibody screening,” Dr. Policar said. Providers should also ask patients requesting contact testing if they have been vaccinated for hepatitis B. If not, “the conversation should be how can we get you vaccinated for hepatitis B,” Dr. Policar said.
HIV screening only needs to occur once between the ages of 15 and 65 for low-risk people and then once annually (or more often if necessary) for those who have a sex partner with HIV, use injectable drugs, engage in commercial sex work, have a new sex partner with unknown HIV status, received care at an STD or TB clinic, or were in a correctional facility or homeless shelter.
Those at increased risk for syphilis include men who have sex with men, men under age 29, and anyone living with HIV or who has a history of incarceration or a history of commercial sex work. In addition, African Americans have the greatest risk for syphilis of racial/ethnic groups, followed by Hispanics. Most adults only require hepatitis C screening with anti-hep C antibody testing once in their lifetime. Periodic hepatitis C screening should occur for people who inject drugs. If the screening is positive, providers should conduct an RNA polymerase chain reaction (PCR) test to determine whether a chronic infection is present.
Trichomoniasis screening should occur annually in women living with HIV or in correctional facilities. Others to consider screening include people with new or multiple sex partners, a history of STIs, inconsistent condom use, a history of sex work, and intravenous drug use. Dr. Policar also noted that several new assays, including NAAT, PCR, and a rapid test, are available for trichomoniasis.
STI treatment guidelines
For women with mucoprurulent cervicitis, the cause could be chlamydia, gonorrhea, herpes, trichomonas, mycoplasma, or even progesterone from pregnancy or contraception, Dr. Policar said. The new preferred treatment is 100 mg of doxycycline. The alternative, albeit less preferred, treatment is 1 g azithromycin.
The preferred treatment for chlamydia is now 100 mg oral doxycycline twice daily, or doxycycline 200 mg delayed-release once daily, for 7 days. Alternative regimens include 1 g oral azithromycin in a single dose or 500 mg oral levofloxacin once daily for 7 days. The switch to recommending doxycycline over azithromycin is based on recent evidence showing that doxycycline has a slightly higher efficacy for urogenital chlamydia and a substantially higher efficacy for rectal chlamydia. In addition, an increasing proportion of gonorrheal infections have shown resistance to azithromycin, particularly beginning in 2014.
Preferred treatment of new, uncomplicated gonorrhea infections of the cervix, urethra, rectum, and pharynx is one 500-mg dose of ceftriaxone for those weighing under 150 kg and 1 g for those weighing 150 kg or more. If ceftriaxone is unavailable, the new alternative recommended treatment for gonorrhea is 800 mg cefixime. For pharyngeal gonorrhea only, the CDC recommends a test-of-cure 7-14 days after treatment.
For gonorrheal infections, the CDC also recommends treatment with doxycycline if chlamydia has not been excluded, but the agency no longer recommends dual therapy with azithromycin unless it’s used in place of doxycycline for those who are pregnant, have an allergy, or may not be compliant with a 7-day doxycycline regimen.
The preferred treatment for bacterial vaginosis has not changed. The new recommended regimen for trichomoniasis is 500 mg oral metronidazole for 7 days, with the alternative being a single 2-g dose of tinidazole. Male partners should receive 2 g oral metronidazole. The CDC also notes that patients taking metronidazole no longer need to abstain from alcohol during treatment.
”Another area where the guidelines changed is in their description of expedited partner therapy, which means that, when we find an index case who has gonorrhea or chlamydia, we always have a discussion with her about getting her partners treated,” Dr. Policar said. “The CDC was quite clear that the responsibility for discussing partner treatment rests with us as the diagnosing provider” since city and county health departments don’t have the time or resources for contact tracing these STIs.
The two main ways to treat partners are to have the patient bring their partner(s) to the appointment with them or to do patient-delivered partner therapy. Ideally, clinicians who dispense their own medications can give the patient enough drugs to give her partner(s) a complete dose as well. Otherwise, providers can prescribe extra doses in the index patients’ name or write prescriptions in the partner’s name.
“In every state of the union now, it is legal for you to to prescribe antibiotics for partners sight unseen, Dr. Policar said.
Margaret Sullivan, MD, an ob.gyn. from rural western North Carolina, noted during the Q&A that an obstacle to partner therapy at her practice has been cost, particularly since many of the men don’t have insurance.
“I have not heard before of prescribing the extra doses for partners under the patient’s name,” Dr. Sullivan said. “I’ve thought about doing it, but [was worried about] it potentially being fraudulent if that patient has Medicaid and we’re prescribing extra doses under her name, so how do you work around that?”
Dr. Policar acknowledged that barrier and recommended that patients use the website/app Goodrx.com to find discounts for out-of-pocket generic medications. He also noted the occasional obstacle of pharmacists balking at filling a double or triple dose.
“What we’ve been suggesting in that circumstance is to literally copy that part of the CDC guidelines, which explains expedited partner therapy or patient-delivered partner therapy and send that off to the pharmacist so they can see that it’s a national recommendation of the CDC,” Dr. Policar said.
Claudia Rodriguez, MD, an ob.gyn. who works at Sherman Hospital in Elgin, Ill., asked about the CDC recommendations for HPV vaccination in older women. Although the CDC permits women over age 26 to receive the HPV vaccine, the agency does not “make a solid recommendation to have this done, which oftentimes makes a big difference in whether or not health insurance will actually pay for vaccination in that circumstance,” Dr. Policar said.
Patients are welcome to request the vaccine after shared decision-making, but “we should never present this as something which is routine,” he said. For women in their 50s, for example, “there’s virtually no data about any additional degree of protection that you would get” from HPV vaccination, Dr. Policar said in response to a similar question from Tara Allmen, MD, an ob.gyn. in New York City. “If you ask me for my personal clinical opinion about it, I would say it’s not going to be worth it,” he said.
Dr Policar had no disclosures. Disclosures were unavailable for attendees who spoke.
FROM NAMS 2021
Moderate alcohol intake may curb subsequent diabetes after gestational diabetes
Among women with a history of gestational diabetes, alcohol intake of half a drink to one drink daily was associated with a 55% lower risk for subsequent type 2 diabetes, based on data from approximately 4,700 women in the Nurses’ Health Study II cohort.
However, the findings must be considered in the context of other risks and benefits of alcohol consumption before making statements or clinical recommendations, wrote Stefanie N. Hinkle, PhD, of the National Institutes of Health, Bethesda, Md., and colleagues.
Women with a history of gestational diabetes remain at increased risk for developing type 2 diabetes, so modifiable diet and lifestyle factors deserve further study, the researchers noted. Previous research has shown an association between light to moderate alcohol consumption and reduced risk of type 2 diabetes among women in the general population, but data on a similar risk reduction for women with a history of gestational diabetes are lacking, they added.
In a study published in JAMA Network Open, the researchers reviewed data from 4,740 women enrolled in the Nurses’ Health Study II who reported a history of gestational diabetes. These women were followed from Jan. 1, 1991, to Dec. 31, 2017, as part of the Diabetes & Women’s Health Study; dietary intake, including alcohol intake, was assessed every 4 years via validated food frequency questionnaires.
The average age at baseline was 38 years, and the median follow-up time was 24 years, yielding a total of 78,328 person-years of follow-up. Alcohol consumption was divided into four categories: none; 0.1 g/day to 4.9 g/day; 5.0 to 14.9 g/day, and 15.0 g/day or higher.
A total of 897 incident cases of type 2 diabetes were reported during the study period. After adjustment for multiple dietary and lifestyle variables, including diet and physical activity, only alcohol consumption of 5.0-14.9 g/day (approximately half a drink to one drink) was associated with a significantly decreased risk for incident type 2 diabetes (hazard ratio, 0.45) compared with women who reported no alcohol consumption.
On further adjustment for body mass index, women who reported alcohol consumption in the 5.0-14.9 g/day range had a 41% lower risk for developing incident type 2 diabetes (HR, 0.59); alcohol consumption in the other ranges remained unassociated with type 2 diabetes risk, although the researchers noted that these estimates were attenuated.
The median daily intake for women who consumed alcohol was 2.3 g/day, approximately one drink per week. Beer was the most frequently consumed type of alcohol.
When the researchers analyzed the data by alcohol type, notably, “only beer consumption of 1 or more servings a week was associated with a lower risk for type 2 diabetes,” although previous studies have suggested a stronger association in diabetes risk reduction with wine consumption vs. beer, the researchers noted.
The study findings were the potential for confounding factors not included in the adjustment, potential underreporting of alcohol intake, and potential screening bias toward women who were more health conscious, the researchers noted. Other limitations were lack of generalizability given that most of the study participants were white women, and a lack of data on binge drinking and whether alcohol was consumed with meals, they added. The study strengths included the prospective design, large size, long-term follow-up, and use of validated questionnaires, they said.
The researchers cautioned that the results should not be interpreted without considering other health outcomes. “Consistent with the 2020 Dietary Guidelines for Americans, which recommend that adults who do not consume alcohol do not initiate drinking, it may not be prudent for those with a history of gestational diabetes who do not consume alcohol to initiate drinking alcohol solely to reduce their risk for type 2 diabetes,” they emphasized.
Risk/benefit ratio for alcohol includes many factors
“There is a relative paucity of data regarding women’s long-term health as it may relate to pregnancy and pregnancy outcomes,” Angela Bianco, MD, of Mount Sinai Hospital, New York, said in an interview.
Dr. Bianco said she was surprised by some of the study findings.
“Generally speaking, I consider alcohol to be of little to no nutritional value, and to have a high sugar content/glycemic index,” she said. “However, a reduced incidence of adult-onset diabetes has been observed among moderate drinkers in other large prospective studies as well,” she noted. “In contrast, some studies have shown an increased risk of diabetes among a proportion of subjects in the top alcohol consumption category, while other studies have found no association. Possible inconsistencies may be due to differences in drinking patterns and the types of beverages consumed,” Dr. Bianco explained.
A key point for clinicians to keep in mind is that “the study may be flawed based on the different criteria used to make a diagnosis of history of gestational diabetes, the fact that they excluded patients that did not return the questionnaires, and the fact that respondents may not have answered correctly due to recall bias” or other reasons, Dr. Bianco said. “Additionally, those who responded obviously had access to health care, which in and of itself is a confounder,” she noted.
Another key point is that “the effect of alcohol being consumed with or without a meal was not examined,” said Dr. Bianco. “Alcohol concentration is reduced if consumed with meals. Alcohol can lead to hypoglycemia (from reduced gluconeogenesis) during fasting states, but after meals (postprandial states) it can result in lower glucose disposal and higher blood glucose levels,” she said. “The available literature suggests that alcohol may improve insulin sensitivity and reduce resistance, but there is likely a U-shaped association between alcohol consumption and the risk of diabetes,” Dr. Bianco noted. “There is likely a delicate balance between benefits and risks of alcohol intake. The inherent benefit/risk ratio must take into account with other potential comorbidities including BMI, activity level, stress, and preexisting conditions,” she said.
“Additional long-term studies engaging patients with diverse ethnic and socioeconomic backgrounds with detailed information regarding the role of nutrition, alcohol intake, tobacco and drug use, environmental exposures, and medical comorbidities need to be performed,” Dr. Bianco concluded.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of General Medical Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases; the Nurses’ Health Study II was supported by the National Institutes of Health. Lead author Dr. Hinkle and coauthor Cuilin Zhang, MD, are employees of the U.S. federal government. The researchers and Dr. Bianco had no financial conflicts to disclose.
Among women with a history of gestational diabetes, alcohol intake of half a drink to one drink daily was associated with a 55% lower risk for subsequent type 2 diabetes, based on data from approximately 4,700 women in the Nurses’ Health Study II cohort.
However, the findings must be considered in the context of other risks and benefits of alcohol consumption before making statements or clinical recommendations, wrote Stefanie N. Hinkle, PhD, of the National Institutes of Health, Bethesda, Md., and colleagues.
Women with a history of gestational diabetes remain at increased risk for developing type 2 diabetes, so modifiable diet and lifestyle factors deserve further study, the researchers noted. Previous research has shown an association between light to moderate alcohol consumption and reduced risk of type 2 diabetes among women in the general population, but data on a similar risk reduction for women with a history of gestational diabetes are lacking, they added.
In a study published in JAMA Network Open, the researchers reviewed data from 4,740 women enrolled in the Nurses’ Health Study II who reported a history of gestational diabetes. These women were followed from Jan. 1, 1991, to Dec. 31, 2017, as part of the Diabetes & Women’s Health Study; dietary intake, including alcohol intake, was assessed every 4 years via validated food frequency questionnaires.
The average age at baseline was 38 years, and the median follow-up time was 24 years, yielding a total of 78,328 person-years of follow-up. Alcohol consumption was divided into four categories: none; 0.1 g/day to 4.9 g/day; 5.0 to 14.9 g/day, and 15.0 g/day or higher.
A total of 897 incident cases of type 2 diabetes were reported during the study period. After adjustment for multiple dietary and lifestyle variables, including diet and physical activity, only alcohol consumption of 5.0-14.9 g/day (approximately half a drink to one drink) was associated with a significantly decreased risk for incident type 2 diabetes (hazard ratio, 0.45) compared with women who reported no alcohol consumption.
On further adjustment for body mass index, women who reported alcohol consumption in the 5.0-14.9 g/day range had a 41% lower risk for developing incident type 2 diabetes (HR, 0.59); alcohol consumption in the other ranges remained unassociated with type 2 diabetes risk, although the researchers noted that these estimates were attenuated.
The median daily intake for women who consumed alcohol was 2.3 g/day, approximately one drink per week. Beer was the most frequently consumed type of alcohol.
When the researchers analyzed the data by alcohol type, notably, “only beer consumption of 1 or more servings a week was associated with a lower risk for type 2 diabetes,” although previous studies have suggested a stronger association in diabetes risk reduction with wine consumption vs. beer, the researchers noted.
The study findings were the potential for confounding factors not included in the adjustment, potential underreporting of alcohol intake, and potential screening bias toward women who were more health conscious, the researchers noted. Other limitations were lack of generalizability given that most of the study participants were white women, and a lack of data on binge drinking and whether alcohol was consumed with meals, they added. The study strengths included the prospective design, large size, long-term follow-up, and use of validated questionnaires, they said.
The researchers cautioned that the results should not be interpreted without considering other health outcomes. “Consistent with the 2020 Dietary Guidelines for Americans, which recommend that adults who do not consume alcohol do not initiate drinking, it may not be prudent for those with a history of gestational diabetes who do not consume alcohol to initiate drinking alcohol solely to reduce their risk for type 2 diabetes,” they emphasized.
Risk/benefit ratio for alcohol includes many factors
“There is a relative paucity of data regarding women’s long-term health as it may relate to pregnancy and pregnancy outcomes,” Angela Bianco, MD, of Mount Sinai Hospital, New York, said in an interview.
Dr. Bianco said she was surprised by some of the study findings.
“Generally speaking, I consider alcohol to be of little to no nutritional value, and to have a high sugar content/glycemic index,” she said. “However, a reduced incidence of adult-onset diabetes has been observed among moderate drinkers in other large prospective studies as well,” she noted. “In contrast, some studies have shown an increased risk of diabetes among a proportion of subjects in the top alcohol consumption category, while other studies have found no association. Possible inconsistencies may be due to differences in drinking patterns and the types of beverages consumed,” Dr. Bianco explained.
A key point for clinicians to keep in mind is that “the study may be flawed based on the different criteria used to make a diagnosis of history of gestational diabetes, the fact that they excluded patients that did not return the questionnaires, and the fact that respondents may not have answered correctly due to recall bias” or other reasons, Dr. Bianco said. “Additionally, those who responded obviously had access to health care, which in and of itself is a confounder,” she noted.
Another key point is that “the effect of alcohol being consumed with or without a meal was not examined,” said Dr. Bianco. “Alcohol concentration is reduced if consumed with meals. Alcohol can lead to hypoglycemia (from reduced gluconeogenesis) during fasting states, but after meals (postprandial states) it can result in lower glucose disposal and higher blood glucose levels,” she said. “The available literature suggests that alcohol may improve insulin sensitivity and reduce resistance, but there is likely a U-shaped association between alcohol consumption and the risk of diabetes,” Dr. Bianco noted. “There is likely a delicate balance between benefits and risks of alcohol intake. The inherent benefit/risk ratio must take into account with other potential comorbidities including BMI, activity level, stress, and preexisting conditions,” she said.
“Additional long-term studies engaging patients with diverse ethnic and socioeconomic backgrounds with detailed information regarding the role of nutrition, alcohol intake, tobacco and drug use, environmental exposures, and medical comorbidities need to be performed,” Dr. Bianco concluded.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of General Medical Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases; the Nurses’ Health Study II was supported by the National Institutes of Health. Lead author Dr. Hinkle and coauthor Cuilin Zhang, MD, are employees of the U.S. federal government. The researchers and Dr. Bianco had no financial conflicts to disclose.
Among women with a history of gestational diabetes, alcohol intake of half a drink to one drink daily was associated with a 55% lower risk for subsequent type 2 diabetes, based on data from approximately 4,700 women in the Nurses’ Health Study II cohort.
However, the findings must be considered in the context of other risks and benefits of alcohol consumption before making statements or clinical recommendations, wrote Stefanie N. Hinkle, PhD, of the National Institutes of Health, Bethesda, Md., and colleagues.
Women with a history of gestational diabetes remain at increased risk for developing type 2 diabetes, so modifiable diet and lifestyle factors deserve further study, the researchers noted. Previous research has shown an association between light to moderate alcohol consumption and reduced risk of type 2 diabetes among women in the general population, but data on a similar risk reduction for women with a history of gestational diabetes are lacking, they added.
In a study published in JAMA Network Open, the researchers reviewed data from 4,740 women enrolled in the Nurses’ Health Study II who reported a history of gestational diabetes. These women were followed from Jan. 1, 1991, to Dec. 31, 2017, as part of the Diabetes & Women’s Health Study; dietary intake, including alcohol intake, was assessed every 4 years via validated food frequency questionnaires.
The average age at baseline was 38 years, and the median follow-up time was 24 years, yielding a total of 78,328 person-years of follow-up. Alcohol consumption was divided into four categories: none; 0.1 g/day to 4.9 g/day; 5.0 to 14.9 g/day, and 15.0 g/day or higher.
A total of 897 incident cases of type 2 diabetes were reported during the study period. After adjustment for multiple dietary and lifestyle variables, including diet and physical activity, only alcohol consumption of 5.0-14.9 g/day (approximately half a drink to one drink) was associated with a significantly decreased risk for incident type 2 diabetes (hazard ratio, 0.45) compared with women who reported no alcohol consumption.
On further adjustment for body mass index, women who reported alcohol consumption in the 5.0-14.9 g/day range had a 41% lower risk for developing incident type 2 diabetes (HR, 0.59); alcohol consumption in the other ranges remained unassociated with type 2 diabetes risk, although the researchers noted that these estimates were attenuated.
The median daily intake for women who consumed alcohol was 2.3 g/day, approximately one drink per week. Beer was the most frequently consumed type of alcohol.
When the researchers analyzed the data by alcohol type, notably, “only beer consumption of 1 or more servings a week was associated with a lower risk for type 2 diabetes,” although previous studies have suggested a stronger association in diabetes risk reduction with wine consumption vs. beer, the researchers noted.
The study findings were the potential for confounding factors not included in the adjustment, potential underreporting of alcohol intake, and potential screening bias toward women who were more health conscious, the researchers noted. Other limitations were lack of generalizability given that most of the study participants were white women, and a lack of data on binge drinking and whether alcohol was consumed with meals, they added. The study strengths included the prospective design, large size, long-term follow-up, and use of validated questionnaires, they said.
The researchers cautioned that the results should not be interpreted without considering other health outcomes. “Consistent with the 2020 Dietary Guidelines for Americans, which recommend that adults who do not consume alcohol do not initiate drinking, it may not be prudent for those with a history of gestational diabetes who do not consume alcohol to initiate drinking alcohol solely to reduce their risk for type 2 diabetes,” they emphasized.
Risk/benefit ratio for alcohol includes many factors
“There is a relative paucity of data regarding women’s long-term health as it may relate to pregnancy and pregnancy outcomes,” Angela Bianco, MD, of Mount Sinai Hospital, New York, said in an interview.
Dr. Bianco said she was surprised by some of the study findings.
“Generally speaking, I consider alcohol to be of little to no nutritional value, and to have a high sugar content/glycemic index,” she said. “However, a reduced incidence of adult-onset diabetes has been observed among moderate drinkers in other large prospective studies as well,” she noted. “In contrast, some studies have shown an increased risk of diabetes among a proportion of subjects in the top alcohol consumption category, while other studies have found no association. Possible inconsistencies may be due to differences in drinking patterns and the types of beverages consumed,” Dr. Bianco explained.
A key point for clinicians to keep in mind is that “the study may be flawed based on the different criteria used to make a diagnosis of history of gestational diabetes, the fact that they excluded patients that did not return the questionnaires, and the fact that respondents may not have answered correctly due to recall bias” or other reasons, Dr. Bianco said. “Additionally, those who responded obviously had access to health care, which in and of itself is a confounder,” she noted.
Another key point is that “the effect of alcohol being consumed with or without a meal was not examined,” said Dr. Bianco. “Alcohol concentration is reduced if consumed with meals. Alcohol can lead to hypoglycemia (from reduced gluconeogenesis) during fasting states, but after meals (postprandial states) it can result in lower glucose disposal and higher blood glucose levels,” she said. “The available literature suggests that alcohol may improve insulin sensitivity and reduce resistance, but there is likely a U-shaped association between alcohol consumption and the risk of diabetes,” Dr. Bianco noted. “There is likely a delicate balance between benefits and risks of alcohol intake. The inherent benefit/risk ratio must take into account with other potential comorbidities including BMI, activity level, stress, and preexisting conditions,” she said.
“Additional long-term studies engaging patients with diverse ethnic and socioeconomic backgrounds with detailed information regarding the role of nutrition, alcohol intake, tobacco and drug use, environmental exposures, and medical comorbidities need to be performed,” Dr. Bianco concluded.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of General Medical Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases; the Nurses’ Health Study II was supported by the National Institutes of Health. Lead author Dr. Hinkle and coauthor Cuilin Zhang, MD, are employees of the U.S. federal government. The researchers and Dr. Bianco had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN






