User login
Early menopause, early dementia risk, study suggests
Earlier menopause appears to be associated with a higher risk of dementia, and earlier onset of dementia, compared with menopause at normal age or later, according to a large study.
“Being aware of this increased risk can help women practice strategies to prevent dementia and to work with their physicians to closely monitor their cognitive status as they age,” study investigator Wenting Hao, MD, with Shandong University, Jinan, China, says in a news release.
The findings were presented in an e-poster March 1 at the Epidemiology, Prevention, Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
UK Biobank data
Dr. Hao and colleagues examined health data for 153,291 women who were 60 years old on average when they became participants in the UK Biobank.
Age at menopause was categorized as premature (younger than age 40), early (40 to 44 years), reference (45 to 51), 52 to 55 years, and 55+ years.
Compared with women who entered menopause around age 50 years (reference), women who experienced premature menopause were 35% more likely to develop some type of dementia later in life (hazard ratio, 1.35; 95% confidence interval, 1.22 to 1.91).
Women with early menopause were also more likely to develop early-onset dementia, that is, before age 65 (HR, 1.31; 95% confidence interval, 1.07 to 1.72).
Women who entered menopause later (at age 52+) had dementia risk similar to women who entered menopause at the average age of 50 to 51 years.
The results were adjusted for relevant cofactors, including age at last exam, race, educational level, cigarette and alcohol use, body mass index, cardiovascular disease, diabetes, income, and leisure and physical activities.
Blame it on estrogen?
Reduced estrogen levels may be a factor in the possible connection between early menopause and dementia, Dr. Hao and her colleagues say.
Estradiol plays a key role in a range of neurological functions, so the reduction of endogenous estrogen at menopause may aggravate brain changes related to neurodegenerative disease and speed up progression of dementia, they explain.
“We know that the lack of estrogen over the long term enhances oxidative stress, which may increase brain aging and lead to cognitive impairment,” Dr. Hao adds.
Limitations of the study include reliance on self-reported information about age at menopause onset.
Also, the researchers did not evaluate dementia rates in women who had a naturally occurring early menopause separate from the women with surgery-induced menopause, which may affect the results.
Finally, the data used for this study included mostly White women living in the U.K. and may not generalize to other populations.
Supportive evidence, critical area of research
The U.K. study supports results of a previously reported Kaiser Permanente study, which showed women who entered menopause at age 45 or younger were at 28% greater dementia risk, compared with women who experienced menopause after age 45.
Reached for comment, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, noted that nearly two-thirds of Americans with Alzheimer’s are women.
“We know Alzheimer’s and other dementias impact a greater number of women than men, but we don’t know why,” she told this news organization.
“Lifelong differences in women may affect their risk or affect what is contributing to their underlying biology of the disease, and we need more research to better understand what may be these contributing factors,” said Dr. Snyder.
“Reproductive history is one critical area being studied. The physical and hormonal changes that occur during menopause – as well as other hormonal changes throughout life – are considerable, and it’s important to understand what impact, if any, these changes may have on the brain,” Dr. Snyder added.
“The potential link between reproduction history and brain health is intriguing, but much more research in this area is needed to understand these links,” she said.
The study was funded by the Start-up Foundation for Scientific Research at Shandong University. Dr. Hao and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Earlier menopause appears to be associated with a higher risk of dementia, and earlier onset of dementia, compared with menopause at normal age or later, according to a large study.
“Being aware of this increased risk can help women practice strategies to prevent dementia and to work with their physicians to closely monitor their cognitive status as they age,” study investigator Wenting Hao, MD, with Shandong University, Jinan, China, says in a news release.
The findings were presented in an e-poster March 1 at the Epidemiology, Prevention, Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
UK Biobank data
Dr. Hao and colleagues examined health data for 153,291 women who were 60 years old on average when they became participants in the UK Biobank.
Age at menopause was categorized as premature (younger than age 40), early (40 to 44 years), reference (45 to 51), 52 to 55 years, and 55+ years.
Compared with women who entered menopause around age 50 years (reference), women who experienced premature menopause were 35% more likely to develop some type of dementia later in life (hazard ratio, 1.35; 95% confidence interval, 1.22 to 1.91).
Women with early menopause were also more likely to develop early-onset dementia, that is, before age 65 (HR, 1.31; 95% confidence interval, 1.07 to 1.72).
Women who entered menopause later (at age 52+) had dementia risk similar to women who entered menopause at the average age of 50 to 51 years.
The results were adjusted for relevant cofactors, including age at last exam, race, educational level, cigarette and alcohol use, body mass index, cardiovascular disease, diabetes, income, and leisure and physical activities.
Blame it on estrogen?
Reduced estrogen levels may be a factor in the possible connection between early menopause and dementia, Dr. Hao and her colleagues say.
Estradiol plays a key role in a range of neurological functions, so the reduction of endogenous estrogen at menopause may aggravate brain changes related to neurodegenerative disease and speed up progression of dementia, they explain.
“We know that the lack of estrogen over the long term enhances oxidative stress, which may increase brain aging and lead to cognitive impairment,” Dr. Hao adds.
Limitations of the study include reliance on self-reported information about age at menopause onset.
Also, the researchers did not evaluate dementia rates in women who had a naturally occurring early menopause separate from the women with surgery-induced menopause, which may affect the results.
Finally, the data used for this study included mostly White women living in the U.K. and may not generalize to other populations.
Supportive evidence, critical area of research
The U.K. study supports results of a previously reported Kaiser Permanente study, which showed women who entered menopause at age 45 or younger were at 28% greater dementia risk, compared with women who experienced menopause after age 45.
Reached for comment, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, noted that nearly two-thirds of Americans with Alzheimer’s are women.
“We know Alzheimer’s and other dementias impact a greater number of women than men, but we don’t know why,” she told this news organization.
“Lifelong differences in women may affect their risk or affect what is contributing to their underlying biology of the disease, and we need more research to better understand what may be these contributing factors,” said Dr. Snyder.
“Reproductive history is one critical area being studied. The physical and hormonal changes that occur during menopause – as well as other hormonal changes throughout life – are considerable, and it’s important to understand what impact, if any, these changes may have on the brain,” Dr. Snyder added.
“The potential link between reproduction history and brain health is intriguing, but much more research in this area is needed to understand these links,” she said.
The study was funded by the Start-up Foundation for Scientific Research at Shandong University. Dr. Hao and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Earlier menopause appears to be associated with a higher risk of dementia, and earlier onset of dementia, compared with menopause at normal age or later, according to a large study.
“Being aware of this increased risk can help women practice strategies to prevent dementia and to work with their physicians to closely monitor their cognitive status as they age,” study investigator Wenting Hao, MD, with Shandong University, Jinan, China, says in a news release.
The findings were presented in an e-poster March 1 at the Epidemiology, Prevention, Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
UK Biobank data
Dr. Hao and colleagues examined health data for 153,291 women who were 60 years old on average when they became participants in the UK Biobank.
Age at menopause was categorized as premature (younger than age 40), early (40 to 44 years), reference (45 to 51), 52 to 55 years, and 55+ years.
Compared with women who entered menopause around age 50 years (reference), women who experienced premature menopause were 35% more likely to develop some type of dementia later in life (hazard ratio, 1.35; 95% confidence interval, 1.22 to 1.91).
Women with early menopause were also more likely to develop early-onset dementia, that is, before age 65 (HR, 1.31; 95% confidence interval, 1.07 to 1.72).
Women who entered menopause later (at age 52+) had dementia risk similar to women who entered menopause at the average age of 50 to 51 years.
The results were adjusted for relevant cofactors, including age at last exam, race, educational level, cigarette and alcohol use, body mass index, cardiovascular disease, diabetes, income, and leisure and physical activities.
Blame it on estrogen?
Reduced estrogen levels may be a factor in the possible connection between early menopause and dementia, Dr. Hao and her colleagues say.
Estradiol plays a key role in a range of neurological functions, so the reduction of endogenous estrogen at menopause may aggravate brain changes related to neurodegenerative disease and speed up progression of dementia, they explain.
“We know that the lack of estrogen over the long term enhances oxidative stress, which may increase brain aging and lead to cognitive impairment,” Dr. Hao adds.
Limitations of the study include reliance on self-reported information about age at menopause onset.
Also, the researchers did not evaluate dementia rates in women who had a naturally occurring early menopause separate from the women with surgery-induced menopause, which may affect the results.
Finally, the data used for this study included mostly White women living in the U.K. and may not generalize to other populations.
Supportive evidence, critical area of research
The U.K. study supports results of a previously reported Kaiser Permanente study, which showed women who entered menopause at age 45 or younger were at 28% greater dementia risk, compared with women who experienced menopause after age 45.
Reached for comment, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, noted that nearly two-thirds of Americans with Alzheimer’s are women.
“We know Alzheimer’s and other dementias impact a greater number of women than men, but we don’t know why,” she told this news organization.
“Lifelong differences in women may affect their risk or affect what is contributing to their underlying biology of the disease, and we need more research to better understand what may be these contributing factors,” said Dr. Snyder.
“Reproductive history is one critical area being studied. The physical and hormonal changes that occur during menopause – as well as other hormonal changes throughout life – are considerable, and it’s important to understand what impact, if any, these changes may have on the brain,” Dr. Snyder added.
“The potential link between reproduction history and brain health is intriguing, but much more research in this area is needed to understand these links,” she said.
The study was funded by the Start-up Foundation for Scientific Research at Shandong University. Dr. Hao and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Older age for menopause raises risk for lung cancer
This study was published on Medrxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- in analyses of more than 100,000 women that used Mendelian randomization (MR) as a tool to reduce residual confounding.
- The MR analyses showed no significant association between ANM and breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
- The clear lack of a causal effect of ANM on the outcomes of coronary heart disease and ischemic stroke in the MR analyses despite a strong inverse association seen in the observational data of this study (without MR) suggests residual confounding plays a substantial role in driving the observed outcomes.
Why this matters
- The authors said that, to their knowledge, this is the first study that has shown a causal association between older ANM and higher risk of postmenopausal lung cancer.
- This finding was directionally opposite to the significant protective effect of increased ANM documented in an observational analysis of roughly the same data as well as prior reports that did not use MR. This “notable inconsistency” suggests very substantial residual confounding without MR that could be driven by factors such as smoking, diet, and exercise.
- If these results are replicated in additional datasets, it would highlight a need for randomized, controlled trials of antiestrogen therapies in postmenopausal women for the prevention or treatment of lung cancer.
Study design
- The study included data from 106,853 postmenopausal women enrolled in the Women’s Health Initiative (WHI) and 95,464 women who were 37-73 years old included in the UK Biobank (UKB). Analyses for each outcome also included data from smaller numbers of women obtained from several additional datasets.
- The MR analysis used up to 55 single-nucleotide polymorphisms previously discovered through a genome-wide association study of about 70,000 women of European ancestry and independent of all datasets analyzed in the current study. The authors included all single-nucleotide polymorphisms with a consistent direction of effect on ANM.
- The MR analysis for lung cancer included 113,371 women from the two primary datasets and an additional 3012 women from six additional datasets.
- The MR analysis for bone fracture involved 113,239 women from the WHI and UKB only. The MR analysis for osteoporosis involved 137,080 women from the WHI, UKB, and one additional external dataset.
Key results
- Results from a meta-analysis of the MR results using data from the WHI, UKB, and the additional datasets showed ANM was causally associated with an increased risk of lung cancer by an odds ratio of 1.35 for each 5-year increase in ANM. In contrast, the adjusted observational analysis of data just from the WHI and UKB showed a significant 11% relative risk reduction in the incidence of lung cancer for each 5-year increase in ANM.
- The MR results also showed causally protective effects for fracture, with a 24% relative risk reduction, and for osteoporosis, with a 19% relative risk reduction for each 5-year increase in ANM.
- The MR analyses showed no significant association between AMN and outcome for breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
Limitations
The main limitation of the MR study was the potential for inadequate power for assessing some outcomes despite the large overall size of the study cohort. Lack of adequate power may be responsible for some of the nonsignificant associations seen in the study, such as for breast and endometrial cancers, where substantial prior evidence has implicated increased risk through the effects of prolonged exposure to endogenous or exogenous estrogens.
The healthy cohort effect in the UKB is a known weakness of this dataset that may have limited the number of cases and generalizability of findings.
Osteoporosis and Alzheimer’s disease were self-reported.
The study only included participants of European ancestry because most subjects in most of the cohorts examined were White women and the applied MR instruments were found by genome-wide association studies run predominantly in White women. The authors said the causal effects of ANM need study in more diverse populations.
Disclosures
- The study received no commercial funding.
- None of the authors had disclosures.
This is a summary of a preprint research study, “Genetic evidence for causal relationships between age at natural menopause and the risk of aging-associated adverse health outcomes,” written by authors primarily based at Stanford University School of Medicine i
A version of this article first appeared on Medscape.com.
This study was published on Medrxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- in analyses of more than 100,000 women that used Mendelian randomization (MR) as a tool to reduce residual confounding.
- The MR analyses showed no significant association between ANM and breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
- The clear lack of a causal effect of ANM on the outcomes of coronary heart disease and ischemic stroke in the MR analyses despite a strong inverse association seen in the observational data of this study (without MR) suggests residual confounding plays a substantial role in driving the observed outcomes.
Why this matters
- The authors said that, to their knowledge, this is the first study that has shown a causal association between older ANM and higher risk of postmenopausal lung cancer.
- This finding was directionally opposite to the significant protective effect of increased ANM documented in an observational analysis of roughly the same data as well as prior reports that did not use MR. This “notable inconsistency” suggests very substantial residual confounding without MR that could be driven by factors such as smoking, diet, and exercise.
- If these results are replicated in additional datasets, it would highlight a need for randomized, controlled trials of antiestrogen therapies in postmenopausal women for the prevention or treatment of lung cancer.
Study design
- The study included data from 106,853 postmenopausal women enrolled in the Women’s Health Initiative (WHI) and 95,464 women who were 37-73 years old included in the UK Biobank (UKB). Analyses for each outcome also included data from smaller numbers of women obtained from several additional datasets.
- The MR analysis used up to 55 single-nucleotide polymorphisms previously discovered through a genome-wide association study of about 70,000 women of European ancestry and independent of all datasets analyzed in the current study. The authors included all single-nucleotide polymorphisms with a consistent direction of effect on ANM.
- The MR analysis for lung cancer included 113,371 women from the two primary datasets and an additional 3012 women from six additional datasets.
- The MR analysis for bone fracture involved 113,239 women from the WHI and UKB only. The MR analysis for osteoporosis involved 137,080 women from the WHI, UKB, and one additional external dataset.
Key results
- Results from a meta-analysis of the MR results using data from the WHI, UKB, and the additional datasets showed ANM was causally associated with an increased risk of lung cancer by an odds ratio of 1.35 for each 5-year increase in ANM. In contrast, the adjusted observational analysis of data just from the WHI and UKB showed a significant 11% relative risk reduction in the incidence of lung cancer for each 5-year increase in ANM.
- The MR results also showed causally protective effects for fracture, with a 24% relative risk reduction, and for osteoporosis, with a 19% relative risk reduction for each 5-year increase in ANM.
- The MR analyses showed no significant association between AMN and outcome for breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
Limitations
The main limitation of the MR study was the potential for inadequate power for assessing some outcomes despite the large overall size of the study cohort. Lack of adequate power may be responsible for some of the nonsignificant associations seen in the study, such as for breast and endometrial cancers, where substantial prior evidence has implicated increased risk through the effects of prolonged exposure to endogenous or exogenous estrogens.
The healthy cohort effect in the UKB is a known weakness of this dataset that may have limited the number of cases and generalizability of findings.
Osteoporosis and Alzheimer’s disease were self-reported.
The study only included participants of European ancestry because most subjects in most of the cohorts examined were White women and the applied MR instruments were found by genome-wide association studies run predominantly in White women. The authors said the causal effects of ANM need study in more diverse populations.
Disclosures
- The study received no commercial funding.
- None of the authors had disclosures.
This is a summary of a preprint research study, “Genetic evidence for causal relationships between age at natural menopause and the risk of aging-associated adverse health outcomes,” written by authors primarily based at Stanford University School of Medicine i
A version of this article first appeared on Medscape.com.
This study was published on Medrxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- in analyses of more than 100,000 women that used Mendelian randomization (MR) as a tool to reduce residual confounding.
- The MR analyses showed no significant association between ANM and breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
- The clear lack of a causal effect of ANM on the outcomes of coronary heart disease and ischemic stroke in the MR analyses despite a strong inverse association seen in the observational data of this study (without MR) suggests residual confounding plays a substantial role in driving the observed outcomes.
Why this matters
- The authors said that, to their knowledge, this is the first study that has shown a causal association between older ANM and higher risk of postmenopausal lung cancer.
- This finding was directionally opposite to the significant protective effect of increased ANM documented in an observational analysis of roughly the same data as well as prior reports that did not use MR. This “notable inconsistency” suggests very substantial residual confounding without MR that could be driven by factors such as smoking, diet, and exercise.
- If these results are replicated in additional datasets, it would highlight a need for randomized, controlled trials of antiestrogen therapies in postmenopausal women for the prevention or treatment of lung cancer.
Study design
- The study included data from 106,853 postmenopausal women enrolled in the Women’s Health Initiative (WHI) and 95,464 women who were 37-73 years old included in the UK Biobank (UKB). Analyses for each outcome also included data from smaller numbers of women obtained from several additional datasets.
- The MR analysis used up to 55 single-nucleotide polymorphisms previously discovered through a genome-wide association study of about 70,000 women of European ancestry and independent of all datasets analyzed in the current study. The authors included all single-nucleotide polymorphisms with a consistent direction of effect on ANM.
- The MR analysis for lung cancer included 113,371 women from the two primary datasets and an additional 3012 women from six additional datasets.
- The MR analysis for bone fracture involved 113,239 women from the WHI and UKB only. The MR analysis for osteoporosis involved 137,080 women from the WHI, UKB, and one additional external dataset.
Key results
- Results from a meta-analysis of the MR results using data from the WHI, UKB, and the additional datasets showed ANM was causally associated with an increased risk of lung cancer by an odds ratio of 1.35 for each 5-year increase in ANM. In contrast, the adjusted observational analysis of data just from the WHI and UKB showed a significant 11% relative risk reduction in the incidence of lung cancer for each 5-year increase in ANM.
- The MR results also showed causally protective effects for fracture, with a 24% relative risk reduction, and for osteoporosis, with a 19% relative risk reduction for each 5-year increase in ANM.
- The MR analyses showed no significant association between AMN and outcome for breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
Limitations
The main limitation of the MR study was the potential for inadequate power for assessing some outcomes despite the large overall size of the study cohort. Lack of adequate power may be responsible for some of the nonsignificant associations seen in the study, such as for breast and endometrial cancers, where substantial prior evidence has implicated increased risk through the effects of prolonged exposure to endogenous or exogenous estrogens.
The healthy cohort effect in the UKB is a known weakness of this dataset that may have limited the number of cases and generalizability of findings.
Osteoporosis and Alzheimer’s disease were self-reported.
The study only included participants of European ancestry because most subjects in most of the cohorts examined were White women and the applied MR instruments were found by genome-wide association studies run predominantly in White women. The authors said the causal effects of ANM need study in more diverse populations.
Disclosures
- The study received no commercial funding.
- None of the authors had disclosures.
This is a summary of a preprint research study, “Genetic evidence for causal relationships between age at natural menopause and the risk of aging-associated adverse health outcomes,” written by authors primarily based at Stanford University School of Medicine i
A version of this article first appeared on Medscape.com.
HT for women who have had BSO before the age of natural menopause: Discerning the nuances
Women who undergo bilateral salpingo-oophorectomy (BSO) for various indications prior to menopause experience a rapid decline in ovarian hormone levels and consequent vasomotor and other menopausal symptoms. In addition, the resulting estrogen deprivation is associated with such long-term adverse outcomes as osteoporosis and cardiovascular morbidity.
OBG M
Surgical vs natural menopause
Stephanie Faubion, MD, MBA, NCMP: Since the Women’s Health Initiative study was published in 2002,2 many clinicians have been fearful of using systemic HT in menopausal women, and HT use has declined dramatically such that only about 4% to 6% of menopausal women are now receiving systemic HT. Importantly, however, a group of younger menopausal women also are not receiving HT, and that is women who undergo BSO before they reach the average age of menopause, which in the United States is about age 52; this is sometimes referred to as surgical menopause or early surgical menopause. Early surgical menopause has different connotations for long-term health risks than natural menopause at the average age, and we are here to discuss these health effects and their management.
My name is Stephanie Faubion, and I am a women’s health internist and the Chair of the Department of Medicine at Mayo Clinic in Jacksonville, Florida, and Director of Mayo Clinic Women’s Health. I am here with 2 of my esteemed colleagues, Dr. Andrew Kaunitz and Dr. Ekta Kapoor.
Andrew M. Kaunitz, MD, NCMP: Hello, I am an ObGyn with the University of Florida College of Medicine in Jacksonville, with particular interests in contraception, menopause, and gynecologic ultrasonography.
Ekta Kapoor, MBBS, NCMP: And I am an endocrinologist at Mayo Clinic in Rochester with a specific interest in menopause and hormone therapy. I am also the Assistant Director for Mayo Clinic Women’s Health.
Higher-than-standard estrogen doses needed in younger menopausal women
Dr. Faubion: Let’s consider a couple of cases so that we can illustrate some important points regarding hormone management in women who have undergone BSO before the age of natural menopause.
Our first case patient is a woman who is 41 years of age and, because of adenomyosis, she will undergo a hysterectomy. She tells her clinician that she is very concerned about ovarian cancer risk because one of her good friends recently was diagnosed with ovarian cancer, and together they decide to remove her ovaries at the time of hysterectomy. Notably, her ovaries were healthy.
The patient is now menopausal postsurgery, and she is having significant hot flashes and night sweats. She visits her local internist, who is concerned about initiating HT. She is otherwise a healthy woman and does not have any contraindications to HT. Dr. Kaunitz, what would you tell her internist?
Dr. Kaunitz: We are dealing with 2 different issues in terms of decision making about systemic HT for this 41-year-old who has undergone BSO. First, as you mentioned, Dr. Faubion, she has bothersome hot flashes, or vasomotor symptoms. Unless there are contraindications, systemic HT would be appropriate. Although I might start treatment at standard doses, and the accompanying TABLE depicts standard doses for the 2 most common oral estrogen formulations as well as transdermal estradiol, it’s important to recognize that younger menopausal women often will need to use higher-than-standard doses.
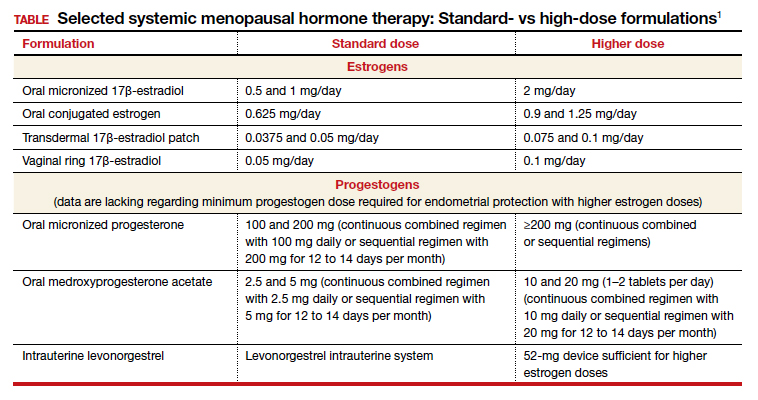
For example, for a 53-year-old woman who has been menopausal for a year or 2 and now has bothersome symptoms, I might start her on estradiol 1 mg tablets with progestin if a uterus is present. However, in this 41-year-old case patient, while I might start treatment at a standard dose, I would anticipate increasing to higher doses, such as 1.5 or 2 mg of daily estradiol until she feels her menopausal symptoms are adequately addressed.
Dr. Faubion: It is important to note that sometimes women with early BSO tend to have more severe vasomotor symptoms. Do you find that sometimes a higher dose is required just to manage symptoms, Dr. Kaunitz?
Dr. Kaunitz: Absolutely, yes. The decision whether or not to use systemic HT might be considered discretionary or elective in the classic 53-year-old woman recently menopausal with hot flashes, a so-called spontaneously or naturally menopausal woman. But my perspective is that unless there are clear contraindications, the decision to start systemic HT in the 41-year-old BSO case patient is actually not discretionary. Unless contraindications are present, it is important not only to treat symptoms but also to prevent an array of chronic major health concerns that are more likely if we don’t prescribe systemic HT.
Continue to: Health effects of not using HT...
Health effects of not using HT
Dr. Faubion: Dr. Kapoor, can you describe the potential long-term adverse health consequences of not using estrogen therapy? Say the same 41-year-old woman does not have many bothersome symptoms. What would you do?
Dr. Kapoor: Thank you for that important question. Building on what Dr. Kaunitz said, in these patients there are really 2 issues that can seem to be independent but are not: The first relates to the immediate consequences of lack of estrogen, ie, the menopause-related symptoms, but the second and perhaps the bigger issue is the long-term risk associated with estrogen deprivation.
The symptoms in these women are often obvious as they can be quite severe and abrupt; one day these women have normal hormone levels and the next day, after BSO, suddenly their hormones are very low. So if symptoms occur, they are usually hard to miss, simply because they are very drastic and very severe.
Historically, patients and their clinicians have targeted these symptoms. Patients experience menopausal symptoms, they seek treatment, and then the clinicians basically titrate the treatment to manage these symptoms. That misses the bigger issue, however, which is that premature estrogen deprivation leads to a host of chronic health conditions, as Dr. Kaunitz mentioned. These mainly include increased risk for cardiovascular disease, diabetes, hypertension, dyslipidemia, increased risk of mortality, dementia, and osteoporosis.
Fairly strong observational evidence suggests that use of estrogen therapy given in replacement doses—doses higher than those typically used in women after natural menopause, therefore considered replacement doses—helps mitigate the risk of some of these adverse health conditions.
In these women, the bigger goal really is to reinstate the hormonal milieu that exists prior to menopause. To your point, Dr. Faubion, if I have a patient who is younger than 46 years, who has her ovaries taken out, and even if she has zero symptoms (and sometimes that does happen), I would still make a case for this patient to utilize hormone therapy unless there is a contraindication such as breast cancer or other estrogen-sensitive cancers.
Dr. Faubion: Again, would you aim for those higher doses rather than treat with the “lowest dose”?
Dr. Kapoor: Absolutely. My punchline to the patients and clinicians in these discussions is that the rules of the game are different for these women. We cannot extrapolate the risks and benefits of HT use in women after natural menopause to younger women who have surgical menopause. Those rules just do not apply with respect to both benefits and risks.
Dr. Faubion: I think it’s important to say that these same “rules” would apply if the women were to go through premature menopause for any other reason, too, such as chemotherapy, radiation therapy, or premature ovarian insufficiency for any number of reasons, including toxic, metabolic, or genetic causes and so on. Would that be true?
Dr. Kapoor: Yes, absolutely so.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: In terms of practical or clinical issues regarding systemic HT management, for the woman in her early 50s who has experienced normal or natural spontaneous menopause, a starting dose of transdermal estradiol would be, for instance, a 0.05-mg patch, which is a patch that over 24 hours releases 0.05 mg of estradiol daily; or standard oral estrogen, including conjugated equine estrogen, a 0.625-mg tablet daily, or estradiol, a 1-mg tablet daily.
But in younger patients, we want to use higher doses. For a patch, for instance, I would aim for a 0.075- or 0.1-mg estradiol patch, which releases a higher daily dose of estradiol than the standard dose. For oral estrogen, the dose would be 0.9- or even 1.25-mg tablets of conjugated equine estrogen or 1.5 mg, which is a 1-mg plus a 0.5-mg estradiol tablet, or a 2-mg estradiol tablet. Estradiol does come in a 2-mg strength.
For oral estrogen, I prefer estradiol because it’s available as a generic medication and often available at a very low cost, sometimes as low as $4 a month from chain pharmacies.
Continue to: Usefulness of monitoring estradiol levels for dosage adjustment...
Usefulness of monitoring estradiol levels for dosage adjustment
Dr. Faubion: That’s a great point, and again it is important to emphasize that we are aiming to recreate the premenopausal hormonal milieu. If you were to check estradiol levels, that would be aiming for a premenopausal range of approximately 80 to 120 pg per mL. Dr. Kapoor, is there utility in monitoring estrogen levels?
Dr. Kapoor: Great question, Dr. Faubion, and as you know it’s a loaded one. We base this on empiric evidence. We know that if the hormonal milieu in a young patient is changed to a postmenopausal one, her risk for many chronic conditions is increased. So if we were to reinstate a premenopausal hormonal milieu, that risk would probably be reduced. It makes good sense to target an empiric goal of 80 to 120 pg per mL of estradiol, which is the average estradiol level in a premenopausal woman. If you were to ask me, however, are there randomized, controlled trial data to support this practice—that is, if you target that level, can you make sure that the risk of diabetes is lower or that the risk of heart disease is lower—that study has yet to be done, and it may not ever be done on a large scale. However, it intuitively makes good sense to target premenopausal estradiol levels.
Dr. Faubion: When might you check an estradiol level in this population? For example, if you are treating a patient with a 0.1-mg estradiol patch and she still has significant hot flashes, would it be useful to check the level?
Dr. Kapoor: It would. In my practice, I check estradiol levels on these patients on an annual basis, regardless of symptoms, but definitely in the patient who has symptoms. It makes good sense, because sometimes these patients don’t absorb the estrogen well, particularly if administered by the transdermal route.
A general rule of thumb is that in the average population, if a patient is on the 0.1-mg patch, for example, you would expect her level to be around 100. If it is much lower than that, which sometimes happens, that speaks for poor absorption. Options at that point would be to treat her with a higher dose patch, depending on what the level is, or switch to a different formulation, such as oral.
In instances in which I have treated patients with a 0.1-mg patch for example, and their estradiol levels are undetectable, that speaks for very poor absorption. For such patients I make a case for switching them to oral therapy. Most definitely that makes sense in a patient who is symptomatic despite treatment. But even for patients who don’t have symptoms, I like to target that level, acknowledging that there is no evidence as such to support this practice.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: Yes, a few practical points. Although patches are available in a wider array of doses than oral estrogen formulations, the highest dose available is 0.1 mg. It’s important for clinicians to recognize that while checking serum levels when indicated can be performed in women using transdermal estradiol or patches, in women who are using oral estrogen, checking blood levels is not going to work well because serum estrogen levels have a daily peak and valley in women who use oral versus transdermal estradiol.
I also wanted to talk about progestins. Although many patients who have had a BSO prior to spontaneous menopause also have had a hysterectomy, others have an intact uterus associated with their BSO, so progestins must be used along with estrogen. And if we are using higher-than-standard doses of estrogen, we also need to use higher-than-standard doses of progestin.
In that classic 53-year-old woman I referred to who had spontaneous normal menopause, if she is taking 1 mg of estradiol daily, or a 0.05-mg patch, or 0.625 mg of conjugated equine estrogen, 2.5 mg of medroxyprogesterone is fine. In fact, that showed excellent progestational protection of the endometrium in the Women’s Health Initiative and in other studies.
However, if we are going to use double the estrogen dose, we should increase the progestin dose too. In some of my patients on higher estrogen doses who have an intact uterus, I’ll use 5 or even 10 mg of daily medroxyprogesterone acetate to ensure adequate progestational suppression.
Dr. Faubion: Another practical tip is that if one is using conjugated equine estrogens, measuring the serum estradiol levels is not useful either.
Dr. Kaunitz: I agree.
Continue to: Oral contraceptives as replacement HT...
Oral contraceptives as replacement HT
Dr. Faubion: Would you comment on use of a birth control pill in this circumstance? Would it be optimal to use a postmenopausal HT regimen as opposed to a birth control pill or combined hormonal contraception?
Dr. Kapoor: In this younger population, sometimes it seems like a more socially acceptable decision to be on a birth control option than on menopausal HT. But there are some issues with being on a contraceptive regimen. One is that we end up using estrogen doses much higher than what is really needed for replacement purposes. It is also a nonphysiologic way of replacement in another sense—as opposed to estradiol, which is the main hormone made by the ovaries, the hormonal contraceptive regimens contain the synthetic estrogen ethinyl estradiol for the most part.
The other issue that is based on some weak evidence is that it appears that the bone health outcomes are probably inferior with combined hormonal contraception. For these reasons, regimens that are based on replacement doses of estradiol are preferred.
Dr. Faubion: Right, although the data are somewhat weak, I agree that thus far it seems optimal to utilize a postmenopausal regimen for various reasons. Dr. Kaunitz, anything to add?
Dr. Kaunitz: Yes, to underscore Dr. Kapoor’s point, a common oral contraceptive that contains 20 µg of ethinyl estradiol is substantially more estrogenic than 1.0 or 2.0 mg of micronized oral estradiol.
Also consider that a 20-µg ethinyl estradiol oral contraceptive may increase the risk of venous thromboembolism more than menopausal doses of oral estradiol, whether it be a micronized estradiol or conjugated equine estrogen.
Dr. Faubion: So the risk may be greater with oral combined hormonal contraception as well?
Dr. Kaunitz: One thing we can do is explain to our patients that their ovaries, prior to surgery or prior to induced menopause, were making substantial quantities of estradiol. Whether we prescribe a patch or oral micronized estradiol, this estrogen is identical to the hormone that their ovaries were making prior to surgery or induced menopause.
Breast cancer concerns
Dr. Faubion: Let’s consider a more complicated case. A 35-year-old woman has an identified BRCA1 mutation; she has not had any cancers but has undergone risk-reducing BSO and her uterus remains. Is this woman a candidate for HT? At what dose, and for how long? Dr. Kaunitz, why don’t you start.
Dr. Kaunitz: That is a challenging case but one that I think our readers will find interesting and maybe even provocative.
We know that women with BRCA1 mutations, the more common of the 2 BRCA mutations, have a very high risk of developing epithelial ovarian cancer at a young age. For this reason, our colleagues in medical oncology who specialize in hereditary ovarian/breast cancer syndromes recommend prophylactic risk-reducing—and I would also say lifesaving—BSO with or without hysterectomy for women with BRCA1 mutations.
However, over the years there has been tremendous reluctance among physicians caring for BRCA patients and the women themselves—I use the term “previvors” to describe BRCA carriers who have not been diagnosed with breast or ovarian cancer—to use HT after BSO because of concerns that HT might increase breast cancer risk in women who are already at high risk for breast cancer.
I assume, Dr. Faubion, that in this case the woman had gynecologic surgery but continues to have intact breasts. Is that correct?
Dr. Faubion: That is correct.
Dr. Kaunitz: Although the assumption has been that it is not safe to prescribe HT in this setting, in fact, the reported cohort studies that have looked at this issue have not found an elevated risk of breast cancer when replacement estrogen, with or without progestin, is prescribed to BRCA1 previvors with intact breasts.
Given what Dr. Kapoor said regarding the morbidity that is associated with BSO without replacement of physiologic estrogen, and also given the severe symptoms that so many of these young menopausal women experience, in my practice I do prescribe estrogen or estrogen-progestin therapy and focus on the higher target doses that we discussed for the earlier case patient who had a hysterectomy for abnormal uterine bleeding with adenomyosis.
Dr. Faubion: Dr. Kapoor, do you agree with this approach? How long would you continue therapy?
Dr. Kapoor: First, in this BRCA1 case we need to appreciate that the indication for the BSO is a legitimate one, in contrast to the first case in which the ovaries were removed in a patient whose average risk of ovarian cancer was low. It is important to recognize that surgery performed in this context is the right thing to do because it does significantly reduce the risk of ovarian cancer.
The second thing to appreciate is that while we reduce the risk of ovarian cancer significantly and make sure that these patients survive longer, it’s striking a fine balance in that you want to make sure that their morbidity is not increased as a result of premature estrogen deprivation.
As Dr. Kaunitz told us, the evidence that we have so far, which granted is not very robust but is fairly strong observational evidence, suggests that the risk of breast cancer is not elevated when these patients are treated with replacement doses of HT.
Having said that, I do have very strong discussions with my patients in this category about having the risk-reducing bilateral mastectomy also, because if they were to get breast cancer because of their increased genetic predisposition, the cancer is likely to grow faster if the patient is on HT. So one of my counseling points to patients is that they strongly consider bilateral mastectomy, which reduces their breast cancer risk by more than 90%. At the same time, I also strongly endorse using HT in replacement doses for the reasons that we have already stated.
Dr. Faubion: Continue HT until age 50 or 52?
Dr. Kapoor: Definitely until that age, and possibly longer, depending on their symptoms. The indications for treating beyond the age of natural menopause are much the same as for women who experience natural menopause.
Dr. Faubion: That is assuming they had a bilateral mastectomy?
Dr. Kapoor: Yes.
Continue to: Continuing HT until the age of natural menopause...
Continuing HT until the age of natural menopause
Dr. Kaunitz: Dr. Kapoor brings up the important point of duration of systemic HT. I agree that similar considerations apply both to the healthy 41-year-old who had a hysterectomy for abnormal uterine bleeding and to the 35-year-old who had risk-reducing surgery because of her BRCA1 mutation.
In the 2 cases, both to treat symptoms and to prevent chronic diseases, it makes sense to continue HT at least until the age of natural menopause. That is consistent with 2017 guidance from The North American Menopause Society (NAMS) position statement on the use of systemic HT, that is, continuing systemic HT at least until the age of natural menopause.3 Then at that point, continuing or discontinuing systemic HT becomes discretionary, and that would be true for both cases. If the patient is slender or has a strong family history of osteoporosis, that tends to push the patient more in terms of continuing systemic HT. Those are just some examples, and Dr. Kapoor may want to detail other relevant considerations.
Dr. Kapoor: I completely agree. The decision is driven by symptoms that are not otherwise well managed, for example, with nonhormone strategies. If we have any concerns utilizing HT beyond the age of natural menopause, then nonhormonal options can be considered; but sometimes those are not as effective. And bone health is very important. You want to avoid using bisphosphonates in younger women and reserve them for older patients in their late 60s and 70s. Hormone therapy use is a very reasonable strategy to prevent bone loss.
Dr. Kaunitz: It is also worth mentioning that sometimes the woman involved in shared decision making with her clinician decides to stop systemic HT. In that setting, should the patient start developing new-onset dyspareunia, vaginal dryness, or other genital or sexuality-related concerns, it takes very little for me to advise that she start low-dose local vaginal estrogen therapy.
Dr. Faubion: In either scenario, if a woman were to develop symptoms consistent with genitourinary syndrome of menopause (GSM), would you use vaginal estrogen in addition to the systemic estrogen or alone after the woman elected to discontinue systemic therapy?
Dr. Kapoor: Yes to both, I would say.
Dr. Kaunitz: As my patients using systemic HT age, often I will lower the dose. For instance, the dose I use in a 53-year-old will be higher than when she is 59 or 62. At the same time, as we lower the dose of systemic estrogen therapy, symptoms of vaginal atrophy or GSM often will appear, and these can be effectively treated by adding low-dose vaginal estrogen therapy. A number of my patients, particularly those who are on lower-than-standard doses of systemic HT, are also using low-dose vaginal estrogen therapy.
There is a “hybrid” product available: the 90-day estradiol vaginal ring. Estring is a low-dose, 2-mg, 90-day estradiol ring that is very useful, but it is effective only for treating GSM or vaginal atrophy. A second menopausal vaginal estradiol ring, Femring, is available in 2 doses: 0.05 mg/day and 0.1 mg/day. These are very effective in treating both systemic issues, such as vasomotor symptoms or prevention of osteoporosis, and very effective in treating GSM or vaginal atrophy. One problem is that Femring, depending on insurance coverage, can be very expensive. It’s not available as a generic, so for insurance or financial reasons I don’t often prescribe it. If I could remove those financial barriers, I would prescribe Femring more often because it is very useful.
Dr. Faubion: You raise an important point, and that is, for women who have been on HT for some time, clinicians often feel the need to slowly reduce the dose. Would you do that same thing, Dr. Kapoor, for a 40-year-old woman? Would you reduce the dose as she approaches age 50? Is there pressure that “she shouldn’t be on that much estrogen”?
Dr. Kapoor: No, I would not feel pressured until the patient turns at least 46. I bring up age 46 because the average age range for menopause is 46 to 55. After that, if there is any concern, we can decrease the dose to half and keep the patient on that until she turns 50 or 51. But most of my patients are on replacement doses until the average age of menopause, which is around 51 years, and that’s when you reduce the dose to that of the typical HT regimens used after natural menopause.
Sometimes patients are told something by a friend or they have read something and they worry about the risk of 2 things. One is breast cancer and the other is venous thromboembolism (VTE), and that may be why they want to be on a lower dose. I counsel patients that while the risk of VTE is real with HT, it is the women after natural menopause who are at risk—because age itself is a risk for VTE—and it also has to do with the kind of HT regimen that a patient is on. High doses of oral estrogens and certain progestogens increase the risk. But again, for estradiol used in replacement doses and the more common progestogens that we now use in practice, such as micronized progesterone, the risk is not the same. The same goes for breast cancer. My biggest message to patients and clinicians who take care of these patients is that the rules that apply to women after natural menopause just do not apply to this very different patient population.
Dr. Faubion: Thank you, Dr. Kaunitz and Dr. Kapoor, for sharing your knowledge and experience. ●
Systemic HT past the age of 65
Dr. Kaunitz: Another practical issue relates to long-term or extended use of systemic HT. It’s not infrequent in my practice to receive mail and faxes from insurance carriers of systemic HT users who are age 65 and older in which the company refers to the American Geriatrics Society’s Beers criteria for potentially inappropriate medication use in older adults,1 suggesting that systemic HT is inappropriate for all women over age 65. In this age group, I use lower doses if I am continuing systemic HT. But the good news is that both NAMS and the American College Obstetricians and Gynecologists indicate that arbitrarily stopping systemic HT at age 65 or for any other arbitrary reason is inappropriate, and that decisions about continuing or discontinuing therapy should be made on an individualized basis using shared decision making. That’s an important message for our readers.
Counseling regarding elective BSO
Dr. Faubion: One final note about elective BSO in the absence of a genetic mutation that predisposes to increased ovarian or breast cancer risk. Fortunately, we have seen rates of oophorectomy before the age of natural menopause decline, but what would your advice be to women or clinicians of these women who say they are “just afraid of ovarian cancer and would like to have their ovaries removed before the age of natural menopause”?
Dr. Kaunitz: If patients have increased anxiety about ovarian cancer and yet they themselves are not known to be at elevated risk, I emphasize that, fortunately, ovarian cancer is uncommon. It is much less common than other cancers the patient might be familiar with, such as breast or colon or lung cancer. I also emphasize that women who have given birth, particularly multiple times; women who nursed their infants; and women who have used combination hormonal contraceptives, particularly if long term, are at markedly lower risk for ovarian cancer as they get older. We are talking about an uncommon cancer that is even less common if women have given birth, nursed their infants, or used combination contraceptives long term.
Dr. Faubion: Dr. Kapoor, what would you say regarding the increased risk they might incur if they do have their ovaries out?
Dr. Kapoor: As Dr. Kaunitz said, this is an uncommon cancer, and pursuing something to reduce the risk of an uncommon cancer does not benefit the community. That is also my counseling point to patients.
I also talk to them extensively about the risk associated with the ovaries being removed, and I tell them that although we have the option of giving them HT, it is hard to replicate the magic of nature. No matter what concoction or regimen we use, we cannot ensure reinstating health to what it was in the premenopausal state, because estrogen has such myriad effects on the body in so many different organ systems.
Reference
1. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
- North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. J North Am Menopause Soc. 2017;24: 728-753.

Women who undergo bilateral salpingo-oophorectomy (BSO) for various indications prior to menopause experience a rapid decline in ovarian hormone levels and consequent vasomotor and other menopausal symptoms. In addition, the resulting estrogen deprivation is associated with such long-term adverse outcomes as osteoporosis and cardiovascular morbidity.
OBG M
Surgical vs natural menopause
Stephanie Faubion, MD, MBA, NCMP: Since the Women’s Health Initiative study was published in 2002,2 many clinicians have been fearful of using systemic HT in menopausal women, and HT use has declined dramatically such that only about 4% to 6% of menopausal women are now receiving systemic HT. Importantly, however, a group of younger menopausal women also are not receiving HT, and that is women who undergo BSO before they reach the average age of menopause, which in the United States is about age 52; this is sometimes referred to as surgical menopause or early surgical menopause. Early surgical menopause has different connotations for long-term health risks than natural menopause at the average age, and we are here to discuss these health effects and their management.
My name is Stephanie Faubion, and I am a women’s health internist and the Chair of the Department of Medicine at Mayo Clinic in Jacksonville, Florida, and Director of Mayo Clinic Women’s Health. I am here with 2 of my esteemed colleagues, Dr. Andrew Kaunitz and Dr. Ekta Kapoor.
Andrew M. Kaunitz, MD, NCMP: Hello, I am an ObGyn with the University of Florida College of Medicine in Jacksonville, with particular interests in contraception, menopause, and gynecologic ultrasonography.
Ekta Kapoor, MBBS, NCMP: And I am an endocrinologist at Mayo Clinic in Rochester with a specific interest in menopause and hormone therapy. I am also the Assistant Director for Mayo Clinic Women’s Health.
Higher-than-standard estrogen doses needed in younger menopausal women
Dr. Faubion: Let’s consider a couple of cases so that we can illustrate some important points regarding hormone management in women who have undergone BSO before the age of natural menopause.
Our first case patient is a woman who is 41 years of age and, because of adenomyosis, she will undergo a hysterectomy. She tells her clinician that she is very concerned about ovarian cancer risk because one of her good friends recently was diagnosed with ovarian cancer, and together they decide to remove her ovaries at the time of hysterectomy. Notably, her ovaries were healthy.
The patient is now menopausal postsurgery, and she is having significant hot flashes and night sweats. She visits her local internist, who is concerned about initiating HT. She is otherwise a healthy woman and does not have any contraindications to HT. Dr. Kaunitz, what would you tell her internist?
Dr. Kaunitz: We are dealing with 2 different issues in terms of decision making about systemic HT for this 41-year-old who has undergone BSO. First, as you mentioned, Dr. Faubion, she has bothersome hot flashes, or vasomotor symptoms. Unless there are contraindications, systemic HT would be appropriate. Although I might start treatment at standard doses, and the accompanying TABLE depicts standard doses for the 2 most common oral estrogen formulations as well as transdermal estradiol, it’s important to recognize that younger menopausal women often will need to use higher-than-standard doses.

For example, for a 53-year-old woman who has been menopausal for a year or 2 and now has bothersome symptoms, I might start her on estradiol 1 mg tablets with progestin if a uterus is present. However, in this 41-year-old case patient, while I might start treatment at a standard dose, I would anticipate increasing to higher doses, such as 1.5 or 2 mg of daily estradiol until she feels her menopausal symptoms are adequately addressed.
Dr. Faubion: It is important to note that sometimes women with early BSO tend to have more severe vasomotor symptoms. Do you find that sometimes a higher dose is required just to manage symptoms, Dr. Kaunitz?
Dr. Kaunitz: Absolutely, yes. The decision whether or not to use systemic HT might be considered discretionary or elective in the classic 53-year-old woman recently menopausal with hot flashes, a so-called spontaneously or naturally menopausal woman. But my perspective is that unless there are clear contraindications, the decision to start systemic HT in the 41-year-old BSO case patient is actually not discretionary. Unless contraindications are present, it is important not only to treat symptoms but also to prevent an array of chronic major health concerns that are more likely if we don’t prescribe systemic HT.
Continue to: Health effects of not using HT...
Health effects of not using HT
Dr. Faubion: Dr. Kapoor, can you describe the potential long-term adverse health consequences of not using estrogen therapy? Say the same 41-year-old woman does not have many bothersome symptoms. What would you do?
Dr. Kapoor: Thank you for that important question. Building on what Dr. Kaunitz said, in these patients there are really 2 issues that can seem to be independent but are not: The first relates to the immediate consequences of lack of estrogen, ie, the menopause-related symptoms, but the second and perhaps the bigger issue is the long-term risk associated with estrogen deprivation.
The symptoms in these women are often obvious as they can be quite severe and abrupt; one day these women have normal hormone levels and the next day, after BSO, suddenly their hormones are very low. So if symptoms occur, they are usually hard to miss, simply because they are very drastic and very severe.
Historically, patients and their clinicians have targeted these symptoms. Patients experience menopausal symptoms, they seek treatment, and then the clinicians basically titrate the treatment to manage these symptoms. That misses the bigger issue, however, which is that premature estrogen deprivation leads to a host of chronic health conditions, as Dr. Kaunitz mentioned. These mainly include increased risk for cardiovascular disease, diabetes, hypertension, dyslipidemia, increased risk of mortality, dementia, and osteoporosis.
Fairly strong observational evidence suggests that use of estrogen therapy given in replacement doses—doses higher than those typically used in women after natural menopause, therefore considered replacement doses—helps mitigate the risk of some of these adverse health conditions.
In these women, the bigger goal really is to reinstate the hormonal milieu that exists prior to menopause. To your point, Dr. Faubion, if I have a patient who is younger than 46 years, who has her ovaries taken out, and even if she has zero symptoms (and sometimes that does happen), I would still make a case for this patient to utilize hormone therapy unless there is a contraindication such as breast cancer or other estrogen-sensitive cancers.
Dr. Faubion: Again, would you aim for those higher doses rather than treat with the “lowest dose”?
Dr. Kapoor: Absolutely. My punchline to the patients and clinicians in these discussions is that the rules of the game are different for these women. We cannot extrapolate the risks and benefits of HT use in women after natural menopause to younger women who have surgical menopause. Those rules just do not apply with respect to both benefits and risks.
Dr. Faubion: I think it’s important to say that these same “rules” would apply if the women were to go through premature menopause for any other reason, too, such as chemotherapy, radiation therapy, or premature ovarian insufficiency for any number of reasons, including toxic, metabolic, or genetic causes and so on. Would that be true?
Dr. Kapoor: Yes, absolutely so.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: In terms of practical or clinical issues regarding systemic HT management, for the woman in her early 50s who has experienced normal or natural spontaneous menopause, a starting dose of transdermal estradiol would be, for instance, a 0.05-mg patch, which is a patch that over 24 hours releases 0.05 mg of estradiol daily; or standard oral estrogen, including conjugated equine estrogen, a 0.625-mg tablet daily, or estradiol, a 1-mg tablet daily.
But in younger patients, we want to use higher doses. For a patch, for instance, I would aim for a 0.075- or 0.1-mg estradiol patch, which releases a higher daily dose of estradiol than the standard dose. For oral estrogen, the dose would be 0.9- or even 1.25-mg tablets of conjugated equine estrogen or 1.5 mg, which is a 1-mg plus a 0.5-mg estradiol tablet, or a 2-mg estradiol tablet. Estradiol does come in a 2-mg strength.
For oral estrogen, I prefer estradiol because it’s available as a generic medication and often available at a very low cost, sometimes as low as $4 a month from chain pharmacies.
Continue to: Usefulness of monitoring estradiol levels for dosage adjustment...
Usefulness of monitoring estradiol levels for dosage adjustment
Dr. Faubion: That’s a great point, and again it is important to emphasize that we are aiming to recreate the premenopausal hormonal milieu. If you were to check estradiol levels, that would be aiming for a premenopausal range of approximately 80 to 120 pg per mL. Dr. Kapoor, is there utility in monitoring estrogen levels?
Dr. Kapoor: Great question, Dr. Faubion, and as you know it’s a loaded one. We base this on empiric evidence. We know that if the hormonal milieu in a young patient is changed to a postmenopausal one, her risk for many chronic conditions is increased. So if we were to reinstate a premenopausal hormonal milieu, that risk would probably be reduced. It makes good sense to target an empiric goal of 80 to 120 pg per mL of estradiol, which is the average estradiol level in a premenopausal woman. If you were to ask me, however, are there randomized, controlled trial data to support this practice—that is, if you target that level, can you make sure that the risk of diabetes is lower or that the risk of heart disease is lower—that study has yet to be done, and it may not ever be done on a large scale. However, it intuitively makes good sense to target premenopausal estradiol levels.
Dr. Faubion: When might you check an estradiol level in this population? For example, if you are treating a patient with a 0.1-mg estradiol patch and she still has significant hot flashes, would it be useful to check the level?
Dr. Kapoor: It would. In my practice, I check estradiol levels on these patients on an annual basis, regardless of symptoms, but definitely in the patient who has symptoms. It makes good sense, because sometimes these patients don’t absorb the estrogen well, particularly if administered by the transdermal route.
A general rule of thumb is that in the average population, if a patient is on the 0.1-mg patch, for example, you would expect her level to be around 100. If it is much lower than that, which sometimes happens, that speaks for poor absorption. Options at that point would be to treat her with a higher dose patch, depending on what the level is, or switch to a different formulation, such as oral.
In instances in which I have treated patients with a 0.1-mg patch for example, and their estradiol levels are undetectable, that speaks for very poor absorption. For such patients I make a case for switching them to oral therapy. Most definitely that makes sense in a patient who is symptomatic despite treatment. But even for patients who don’t have symptoms, I like to target that level, acknowledging that there is no evidence as such to support this practice.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: Yes, a few practical points. Although patches are available in a wider array of doses than oral estrogen formulations, the highest dose available is 0.1 mg. It’s important for clinicians to recognize that while checking serum levels when indicated can be performed in women using transdermal estradiol or patches, in women who are using oral estrogen, checking blood levels is not going to work well because serum estrogen levels have a daily peak and valley in women who use oral versus transdermal estradiol.
I also wanted to talk about progestins. Although many patients who have had a BSO prior to spontaneous menopause also have had a hysterectomy, others have an intact uterus associated with their BSO, so progestins must be used along with estrogen. And if we are using higher-than-standard doses of estrogen, we also need to use higher-than-standard doses of progestin.
In that classic 53-year-old woman I referred to who had spontaneous normal menopause, if she is taking 1 mg of estradiol daily, or a 0.05-mg patch, or 0.625 mg of conjugated equine estrogen, 2.5 mg of medroxyprogesterone is fine. In fact, that showed excellent progestational protection of the endometrium in the Women’s Health Initiative and in other studies.
However, if we are going to use double the estrogen dose, we should increase the progestin dose too. In some of my patients on higher estrogen doses who have an intact uterus, I’ll use 5 or even 10 mg of daily medroxyprogesterone acetate to ensure adequate progestational suppression.
Dr. Faubion: Another practical tip is that if one is using conjugated equine estrogens, measuring the serum estradiol levels is not useful either.
Dr. Kaunitz: I agree.
Continue to: Oral contraceptives as replacement HT...
Oral contraceptives as replacement HT
Dr. Faubion: Would you comment on use of a birth control pill in this circumstance? Would it be optimal to use a postmenopausal HT regimen as opposed to a birth control pill or combined hormonal contraception?
Dr. Kapoor: In this younger population, sometimes it seems like a more socially acceptable decision to be on a birth control option than on menopausal HT. But there are some issues with being on a contraceptive regimen. One is that we end up using estrogen doses much higher than what is really needed for replacement purposes. It is also a nonphysiologic way of replacement in another sense—as opposed to estradiol, which is the main hormone made by the ovaries, the hormonal contraceptive regimens contain the synthetic estrogen ethinyl estradiol for the most part.
The other issue that is based on some weak evidence is that it appears that the bone health outcomes are probably inferior with combined hormonal contraception. For these reasons, regimens that are based on replacement doses of estradiol are preferred.
Dr. Faubion: Right, although the data are somewhat weak, I agree that thus far it seems optimal to utilize a postmenopausal regimen for various reasons. Dr. Kaunitz, anything to add?
Dr. Kaunitz: Yes, to underscore Dr. Kapoor’s point, a common oral contraceptive that contains 20 µg of ethinyl estradiol is substantially more estrogenic than 1.0 or 2.0 mg of micronized oral estradiol.
Also consider that a 20-µg ethinyl estradiol oral contraceptive may increase the risk of venous thromboembolism more than menopausal doses of oral estradiol, whether it be a micronized estradiol or conjugated equine estrogen.
Dr. Faubion: So the risk may be greater with oral combined hormonal contraception as well?
Dr. Kaunitz: One thing we can do is explain to our patients that their ovaries, prior to surgery or prior to induced menopause, were making substantial quantities of estradiol. Whether we prescribe a patch or oral micronized estradiol, this estrogen is identical to the hormone that their ovaries were making prior to surgery or induced menopause.
Breast cancer concerns
Dr. Faubion: Let’s consider a more complicated case. A 35-year-old woman has an identified BRCA1 mutation; she has not had any cancers but has undergone risk-reducing BSO and her uterus remains. Is this woman a candidate for HT? At what dose, and for how long? Dr. Kaunitz, why don’t you start.
Dr. Kaunitz: That is a challenging case but one that I think our readers will find interesting and maybe even provocative.
We know that women with BRCA1 mutations, the more common of the 2 BRCA mutations, have a very high risk of developing epithelial ovarian cancer at a young age. For this reason, our colleagues in medical oncology who specialize in hereditary ovarian/breast cancer syndromes recommend prophylactic risk-reducing—and I would also say lifesaving—BSO with or without hysterectomy for women with BRCA1 mutations.
However, over the years there has been tremendous reluctance among physicians caring for BRCA patients and the women themselves—I use the term “previvors” to describe BRCA carriers who have not been diagnosed with breast or ovarian cancer—to use HT after BSO because of concerns that HT might increase breast cancer risk in women who are already at high risk for breast cancer.
I assume, Dr. Faubion, that in this case the woman had gynecologic surgery but continues to have intact breasts. Is that correct?
Dr. Faubion: That is correct.
Dr. Kaunitz: Although the assumption has been that it is not safe to prescribe HT in this setting, in fact, the reported cohort studies that have looked at this issue have not found an elevated risk of breast cancer when replacement estrogen, with or without progestin, is prescribed to BRCA1 previvors with intact breasts.
Given what Dr. Kapoor said regarding the morbidity that is associated with BSO without replacement of physiologic estrogen, and also given the severe symptoms that so many of these young menopausal women experience, in my practice I do prescribe estrogen or estrogen-progestin therapy and focus on the higher target doses that we discussed for the earlier case patient who had a hysterectomy for abnormal uterine bleeding with adenomyosis.
Dr. Faubion: Dr. Kapoor, do you agree with this approach? How long would you continue therapy?
Dr. Kapoor: First, in this BRCA1 case we need to appreciate that the indication for the BSO is a legitimate one, in contrast to the first case in which the ovaries were removed in a patient whose average risk of ovarian cancer was low. It is important to recognize that surgery performed in this context is the right thing to do because it does significantly reduce the risk of ovarian cancer.
The second thing to appreciate is that while we reduce the risk of ovarian cancer significantly and make sure that these patients survive longer, it’s striking a fine balance in that you want to make sure that their morbidity is not increased as a result of premature estrogen deprivation.
As Dr. Kaunitz told us, the evidence that we have so far, which granted is not very robust but is fairly strong observational evidence, suggests that the risk of breast cancer is not elevated when these patients are treated with replacement doses of HT.
Having said that, I do have very strong discussions with my patients in this category about having the risk-reducing bilateral mastectomy also, because if they were to get breast cancer because of their increased genetic predisposition, the cancer is likely to grow faster if the patient is on HT. So one of my counseling points to patients is that they strongly consider bilateral mastectomy, which reduces their breast cancer risk by more than 90%. At the same time, I also strongly endorse using HT in replacement doses for the reasons that we have already stated.
Dr. Faubion: Continue HT until age 50 or 52?
Dr. Kapoor: Definitely until that age, and possibly longer, depending on their symptoms. The indications for treating beyond the age of natural menopause are much the same as for women who experience natural menopause.
Dr. Faubion: That is assuming they had a bilateral mastectomy?
Dr. Kapoor: Yes.
Continue to: Continuing HT until the age of natural menopause...
Continuing HT until the age of natural menopause
Dr. Kaunitz: Dr. Kapoor brings up the important point of duration of systemic HT. I agree that similar considerations apply both to the healthy 41-year-old who had a hysterectomy for abnormal uterine bleeding and to the 35-year-old who had risk-reducing surgery because of her BRCA1 mutation.
In the 2 cases, both to treat symptoms and to prevent chronic diseases, it makes sense to continue HT at least until the age of natural menopause. That is consistent with 2017 guidance from The North American Menopause Society (NAMS) position statement on the use of systemic HT, that is, continuing systemic HT at least until the age of natural menopause.3 Then at that point, continuing or discontinuing systemic HT becomes discretionary, and that would be true for both cases. If the patient is slender or has a strong family history of osteoporosis, that tends to push the patient more in terms of continuing systemic HT. Those are just some examples, and Dr. Kapoor may want to detail other relevant considerations.
Dr. Kapoor: I completely agree. The decision is driven by symptoms that are not otherwise well managed, for example, with nonhormone strategies. If we have any concerns utilizing HT beyond the age of natural menopause, then nonhormonal options can be considered; but sometimes those are not as effective. And bone health is very important. You want to avoid using bisphosphonates in younger women and reserve them for older patients in their late 60s and 70s. Hormone therapy use is a very reasonable strategy to prevent bone loss.
Dr. Kaunitz: It is also worth mentioning that sometimes the woman involved in shared decision making with her clinician decides to stop systemic HT. In that setting, should the patient start developing new-onset dyspareunia, vaginal dryness, or other genital or sexuality-related concerns, it takes very little for me to advise that she start low-dose local vaginal estrogen therapy.
Dr. Faubion: In either scenario, if a woman were to develop symptoms consistent with genitourinary syndrome of menopause (GSM), would you use vaginal estrogen in addition to the systemic estrogen or alone after the woman elected to discontinue systemic therapy?
Dr. Kapoor: Yes to both, I would say.
Dr. Kaunitz: As my patients using systemic HT age, often I will lower the dose. For instance, the dose I use in a 53-year-old will be higher than when she is 59 or 62. At the same time, as we lower the dose of systemic estrogen therapy, symptoms of vaginal atrophy or GSM often will appear, and these can be effectively treated by adding low-dose vaginal estrogen therapy. A number of my patients, particularly those who are on lower-than-standard doses of systemic HT, are also using low-dose vaginal estrogen therapy.
There is a “hybrid” product available: the 90-day estradiol vaginal ring. Estring is a low-dose, 2-mg, 90-day estradiol ring that is very useful, but it is effective only for treating GSM or vaginal atrophy. A second menopausal vaginal estradiol ring, Femring, is available in 2 doses: 0.05 mg/day and 0.1 mg/day. These are very effective in treating both systemic issues, such as vasomotor symptoms or prevention of osteoporosis, and very effective in treating GSM or vaginal atrophy. One problem is that Femring, depending on insurance coverage, can be very expensive. It’s not available as a generic, so for insurance or financial reasons I don’t often prescribe it. If I could remove those financial barriers, I would prescribe Femring more often because it is very useful.
Dr. Faubion: You raise an important point, and that is, for women who have been on HT for some time, clinicians often feel the need to slowly reduce the dose. Would you do that same thing, Dr. Kapoor, for a 40-year-old woman? Would you reduce the dose as she approaches age 50? Is there pressure that “she shouldn’t be on that much estrogen”?
Dr. Kapoor: No, I would not feel pressured until the patient turns at least 46. I bring up age 46 because the average age range for menopause is 46 to 55. After that, if there is any concern, we can decrease the dose to half and keep the patient on that until she turns 50 or 51. But most of my patients are on replacement doses until the average age of menopause, which is around 51 years, and that’s when you reduce the dose to that of the typical HT regimens used after natural menopause.
Sometimes patients are told something by a friend or they have read something and they worry about the risk of 2 things. One is breast cancer and the other is venous thromboembolism (VTE), and that may be why they want to be on a lower dose. I counsel patients that while the risk of VTE is real with HT, it is the women after natural menopause who are at risk—because age itself is a risk for VTE—and it also has to do with the kind of HT regimen that a patient is on. High doses of oral estrogens and certain progestogens increase the risk. But again, for estradiol used in replacement doses and the more common progestogens that we now use in practice, such as micronized progesterone, the risk is not the same. The same goes for breast cancer. My biggest message to patients and clinicians who take care of these patients is that the rules that apply to women after natural menopause just do not apply to this very different patient population.
Dr. Faubion: Thank you, Dr. Kaunitz and Dr. Kapoor, for sharing your knowledge and experience. ●
Systemic HT past the age of 65
Dr. Kaunitz: Another practical issue relates to long-term or extended use of systemic HT. It’s not infrequent in my practice to receive mail and faxes from insurance carriers of systemic HT users who are age 65 and older in which the company refers to the American Geriatrics Society’s Beers criteria for potentially inappropriate medication use in older adults,1 suggesting that systemic HT is inappropriate for all women over age 65. In this age group, I use lower doses if I am continuing systemic HT. But the good news is that both NAMS and the American College Obstetricians and Gynecologists indicate that arbitrarily stopping systemic HT at age 65 or for any other arbitrary reason is inappropriate, and that decisions about continuing or discontinuing therapy should be made on an individualized basis using shared decision making. That’s an important message for our readers.
Counseling regarding elective BSO
Dr. Faubion: One final note about elective BSO in the absence of a genetic mutation that predisposes to increased ovarian or breast cancer risk. Fortunately, we have seen rates of oophorectomy before the age of natural menopause decline, but what would your advice be to women or clinicians of these women who say they are “just afraid of ovarian cancer and would like to have their ovaries removed before the age of natural menopause”?
Dr. Kaunitz: If patients have increased anxiety about ovarian cancer and yet they themselves are not known to be at elevated risk, I emphasize that, fortunately, ovarian cancer is uncommon. It is much less common than other cancers the patient might be familiar with, such as breast or colon or lung cancer. I also emphasize that women who have given birth, particularly multiple times; women who nursed their infants; and women who have used combination hormonal contraceptives, particularly if long term, are at markedly lower risk for ovarian cancer as they get older. We are talking about an uncommon cancer that is even less common if women have given birth, nursed their infants, or used combination contraceptives long term.
Dr. Faubion: Dr. Kapoor, what would you say regarding the increased risk they might incur if they do have their ovaries out?
Dr. Kapoor: As Dr. Kaunitz said, this is an uncommon cancer, and pursuing something to reduce the risk of an uncommon cancer does not benefit the community. That is also my counseling point to patients.
I also talk to them extensively about the risk associated with the ovaries being removed, and I tell them that although we have the option of giving them HT, it is hard to replicate the magic of nature. No matter what concoction or regimen we use, we cannot ensure reinstating health to what it was in the premenopausal state, because estrogen has such myriad effects on the body in so many different organ systems.
Reference
1. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.

Women who undergo bilateral salpingo-oophorectomy (BSO) for various indications prior to menopause experience a rapid decline in ovarian hormone levels and consequent vasomotor and other menopausal symptoms. In addition, the resulting estrogen deprivation is associated with such long-term adverse outcomes as osteoporosis and cardiovascular morbidity.
OBG M
Surgical vs natural menopause
Stephanie Faubion, MD, MBA, NCMP: Since the Women’s Health Initiative study was published in 2002,2 many clinicians have been fearful of using systemic HT in menopausal women, and HT use has declined dramatically such that only about 4% to 6% of menopausal women are now receiving systemic HT. Importantly, however, a group of younger menopausal women also are not receiving HT, and that is women who undergo BSO before they reach the average age of menopause, which in the United States is about age 52; this is sometimes referred to as surgical menopause or early surgical menopause. Early surgical menopause has different connotations for long-term health risks than natural menopause at the average age, and we are here to discuss these health effects and their management.
My name is Stephanie Faubion, and I am a women’s health internist and the Chair of the Department of Medicine at Mayo Clinic in Jacksonville, Florida, and Director of Mayo Clinic Women’s Health. I am here with 2 of my esteemed colleagues, Dr. Andrew Kaunitz and Dr. Ekta Kapoor.
Andrew M. Kaunitz, MD, NCMP: Hello, I am an ObGyn with the University of Florida College of Medicine in Jacksonville, with particular interests in contraception, menopause, and gynecologic ultrasonography.
Ekta Kapoor, MBBS, NCMP: And I am an endocrinologist at Mayo Clinic in Rochester with a specific interest in menopause and hormone therapy. I am also the Assistant Director for Mayo Clinic Women’s Health.
Higher-than-standard estrogen doses needed in younger menopausal women
Dr. Faubion: Let’s consider a couple of cases so that we can illustrate some important points regarding hormone management in women who have undergone BSO before the age of natural menopause.
Our first case patient is a woman who is 41 years of age and, because of adenomyosis, she will undergo a hysterectomy. She tells her clinician that she is very concerned about ovarian cancer risk because one of her good friends recently was diagnosed with ovarian cancer, and together they decide to remove her ovaries at the time of hysterectomy. Notably, her ovaries were healthy.
The patient is now menopausal postsurgery, and she is having significant hot flashes and night sweats. She visits her local internist, who is concerned about initiating HT. She is otherwise a healthy woman and does not have any contraindications to HT. Dr. Kaunitz, what would you tell her internist?
Dr. Kaunitz: We are dealing with 2 different issues in terms of decision making about systemic HT for this 41-year-old who has undergone BSO. First, as you mentioned, Dr. Faubion, she has bothersome hot flashes, or vasomotor symptoms. Unless there are contraindications, systemic HT would be appropriate. Although I might start treatment at standard doses, and the accompanying TABLE depicts standard doses for the 2 most common oral estrogen formulations as well as transdermal estradiol, it’s important to recognize that younger menopausal women often will need to use higher-than-standard doses.

For example, for a 53-year-old woman who has been menopausal for a year or 2 and now has bothersome symptoms, I might start her on estradiol 1 mg tablets with progestin if a uterus is present. However, in this 41-year-old case patient, while I might start treatment at a standard dose, I would anticipate increasing to higher doses, such as 1.5 or 2 mg of daily estradiol until she feels her menopausal symptoms are adequately addressed.
Dr. Faubion: It is important to note that sometimes women with early BSO tend to have more severe vasomotor symptoms. Do you find that sometimes a higher dose is required just to manage symptoms, Dr. Kaunitz?
Dr. Kaunitz: Absolutely, yes. The decision whether or not to use systemic HT might be considered discretionary or elective in the classic 53-year-old woman recently menopausal with hot flashes, a so-called spontaneously or naturally menopausal woman. But my perspective is that unless there are clear contraindications, the decision to start systemic HT in the 41-year-old BSO case patient is actually not discretionary. Unless contraindications are present, it is important not only to treat symptoms but also to prevent an array of chronic major health concerns that are more likely if we don’t prescribe systemic HT.
Continue to: Health effects of not using HT...
Health effects of not using HT
Dr. Faubion: Dr. Kapoor, can you describe the potential long-term adverse health consequences of not using estrogen therapy? Say the same 41-year-old woman does not have many bothersome symptoms. What would you do?
Dr. Kapoor: Thank you for that important question. Building on what Dr. Kaunitz said, in these patients there are really 2 issues that can seem to be independent but are not: The first relates to the immediate consequences of lack of estrogen, ie, the menopause-related symptoms, but the second and perhaps the bigger issue is the long-term risk associated with estrogen deprivation.
The symptoms in these women are often obvious as they can be quite severe and abrupt; one day these women have normal hormone levels and the next day, after BSO, suddenly their hormones are very low. So if symptoms occur, they are usually hard to miss, simply because they are very drastic and very severe.
Historically, patients and their clinicians have targeted these symptoms. Patients experience menopausal symptoms, they seek treatment, and then the clinicians basically titrate the treatment to manage these symptoms. That misses the bigger issue, however, which is that premature estrogen deprivation leads to a host of chronic health conditions, as Dr. Kaunitz mentioned. These mainly include increased risk for cardiovascular disease, diabetes, hypertension, dyslipidemia, increased risk of mortality, dementia, and osteoporosis.
Fairly strong observational evidence suggests that use of estrogen therapy given in replacement doses—doses higher than those typically used in women after natural menopause, therefore considered replacement doses—helps mitigate the risk of some of these adverse health conditions.
In these women, the bigger goal really is to reinstate the hormonal milieu that exists prior to menopause. To your point, Dr. Faubion, if I have a patient who is younger than 46 years, who has her ovaries taken out, and even if she has zero symptoms (and sometimes that does happen), I would still make a case for this patient to utilize hormone therapy unless there is a contraindication such as breast cancer or other estrogen-sensitive cancers.
Dr. Faubion: Again, would you aim for those higher doses rather than treat with the “lowest dose”?
Dr. Kapoor: Absolutely. My punchline to the patients and clinicians in these discussions is that the rules of the game are different for these women. We cannot extrapolate the risks and benefits of HT use in women after natural menopause to younger women who have surgical menopause. Those rules just do not apply with respect to both benefits and risks.
Dr. Faubion: I think it’s important to say that these same “rules” would apply if the women were to go through premature menopause for any other reason, too, such as chemotherapy, radiation therapy, or premature ovarian insufficiency for any number of reasons, including toxic, metabolic, or genetic causes and so on. Would that be true?
Dr. Kapoor: Yes, absolutely so.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: In terms of practical or clinical issues regarding systemic HT management, for the woman in her early 50s who has experienced normal or natural spontaneous menopause, a starting dose of transdermal estradiol would be, for instance, a 0.05-mg patch, which is a patch that over 24 hours releases 0.05 mg of estradiol daily; or standard oral estrogen, including conjugated equine estrogen, a 0.625-mg tablet daily, or estradiol, a 1-mg tablet daily.
But in younger patients, we want to use higher doses. For a patch, for instance, I would aim for a 0.075- or 0.1-mg estradiol patch, which releases a higher daily dose of estradiol than the standard dose. For oral estrogen, the dose would be 0.9- or even 1.25-mg tablets of conjugated equine estrogen or 1.5 mg, which is a 1-mg plus a 0.5-mg estradiol tablet, or a 2-mg estradiol tablet. Estradiol does come in a 2-mg strength.
For oral estrogen, I prefer estradiol because it’s available as a generic medication and often available at a very low cost, sometimes as low as $4 a month from chain pharmacies.
Continue to: Usefulness of monitoring estradiol levels for dosage adjustment...
Usefulness of monitoring estradiol levels for dosage adjustment
Dr. Faubion: That’s a great point, and again it is important to emphasize that we are aiming to recreate the premenopausal hormonal milieu. If you were to check estradiol levels, that would be aiming for a premenopausal range of approximately 80 to 120 pg per mL. Dr. Kapoor, is there utility in monitoring estrogen levels?
Dr. Kapoor: Great question, Dr. Faubion, and as you know it’s a loaded one. We base this on empiric evidence. We know that if the hormonal milieu in a young patient is changed to a postmenopausal one, her risk for many chronic conditions is increased. So if we were to reinstate a premenopausal hormonal milieu, that risk would probably be reduced. It makes good sense to target an empiric goal of 80 to 120 pg per mL of estradiol, which is the average estradiol level in a premenopausal woman. If you were to ask me, however, are there randomized, controlled trial data to support this practice—that is, if you target that level, can you make sure that the risk of diabetes is lower or that the risk of heart disease is lower—that study has yet to be done, and it may not ever be done on a large scale. However, it intuitively makes good sense to target premenopausal estradiol levels.
Dr. Faubion: When might you check an estradiol level in this population? For example, if you are treating a patient with a 0.1-mg estradiol patch and she still has significant hot flashes, would it be useful to check the level?
Dr. Kapoor: It would. In my practice, I check estradiol levels on these patients on an annual basis, regardless of symptoms, but definitely in the patient who has symptoms. It makes good sense, because sometimes these patients don’t absorb the estrogen well, particularly if administered by the transdermal route.
A general rule of thumb is that in the average population, if a patient is on the 0.1-mg patch, for example, you would expect her level to be around 100. If it is much lower than that, which sometimes happens, that speaks for poor absorption. Options at that point would be to treat her with a higher dose patch, depending on what the level is, or switch to a different formulation, such as oral.
In instances in which I have treated patients with a 0.1-mg patch for example, and their estradiol levels are undetectable, that speaks for very poor absorption. For such patients I make a case for switching them to oral therapy. Most definitely that makes sense in a patient who is symptomatic despite treatment. But even for patients who don’t have symptoms, I like to target that level, acknowledging that there is no evidence as such to support this practice.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: Yes, a few practical points. Although patches are available in a wider array of doses than oral estrogen formulations, the highest dose available is 0.1 mg. It’s important for clinicians to recognize that while checking serum levels when indicated can be performed in women using transdermal estradiol or patches, in women who are using oral estrogen, checking blood levels is not going to work well because serum estrogen levels have a daily peak and valley in women who use oral versus transdermal estradiol.
I also wanted to talk about progestins. Although many patients who have had a BSO prior to spontaneous menopause also have had a hysterectomy, others have an intact uterus associated with their BSO, so progestins must be used along with estrogen. And if we are using higher-than-standard doses of estrogen, we also need to use higher-than-standard doses of progestin.
In that classic 53-year-old woman I referred to who had spontaneous normal menopause, if she is taking 1 mg of estradiol daily, or a 0.05-mg patch, or 0.625 mg of conjugated equine estrogen, 2.5 mg of medroxyprogesterone is fine. In fact, that showed excellent progestational protection of the endometrium in the Women’s Health Initiative and in other studies.
However, if we are going to use double the estrogen dose, we should increase the progestin dose too. In some of my patients on higher estrogen doses who have an intact uterus, I’ll use 5 or even 10 mg of daily medroxyprogesterone acetate to ensure adequate progestational suppression.
Dr. Faubion: Another practical tip is that if one is using conjugated equine estrogens, measuring the serum estradiol levels is not useful either.
Dr. Kaunitz: I agree.
Continue to: Oral contraceptives as replacement HT...
Oral contraceptives as replacement HT
Dr. Faubion: Would you comment on use of a birth control pill in this circumstance? Would it be optimal to use a postmenopausal HT regimen as opposed to a birth control pill or combined hormonal contraception?
Dr. Kapoor: In this younger population, sometimes it seems like a more socially acceptable decision to be on a birth control option than on menopausal HT. But there are some issues with being on a contraceptive regimen. One is that we end up using estrogen doses much higher than what is really needed for replacement purposes. It is also a nonphysiologic way of replacement in another sense—as opposed to estradiol, which is the main hormone made by the ovaries, the hormonal contraceptive regimens contain the synthetic estrogen ethinyl estradiol for the most part.
The other issue that is based on some weak evidence is that it appears that the bone health outcomes are probably inferior with combined hormonal contraception. For these reasons, regimens that are based on replacement doses of estradiol are preferred.
Dr. Faubion: Right, although the data are somewhat weak, I agree that thus far it seems optimal to utilize a postmenopausal regimen for various reasons. Dr. Kaunitz, anything to add?
Dr. Kaunitz: Yes, to underscore Dr. Kapoor’s point, a common oral contraceptive that contains 20 µg of ethinyl estradiol is substantially more estrogenic than 1.0 or 2.0 mg of micronized oral estradiol.
Also consider that a 20-µg ethinyl estradiol oral contraceptive may increase the risk of venous thromboembolism more than menopausal doses of oral estradiol, whether it be a micronized estradiol or conjugated equine estrogen.
Dr. Faubion: So the risk may be greater with oral combined hormonal contraception as well?
Dr. Kaunitz: One thing we can do is explain to our patients that their ovaries, prior to surgery or prior to induced menopause, were making substantial quantities of estradiol. Whether we prescribe a patch or oral micronized estradiol, this estrogen is identical to the hormone that their ovaries were making prior to surgery or induced menopause.
Breast cancer concerns
Dr. Faubion: Let’s consider a more complicated case. A 35-year-old woman has an identified BRCA1 mutation; she has not had any cancers but has undergone risk-reducing BSO and her uterus remains. Is this woman a candidate for HT? At what dose, and for how long? Dr. Kaunitz, why don’t you start.
Dr. Kaunitz: That is a challenging case but one that I think our readers will find interesting and maybe even provocative.
We know that women with BRCA1 mutations, the more common of the 2 BRCA mutations, have a very high risk of developing epithelial ovarian cancer at a young age. For this reason, our colleagues in medical oncology who specialize in hereditary ovarian/breast cancer syndromes recommend prophylactic risk-reducing—and I would also say lifesaving—BSO with or without hysterectomy for women with BRCA1 mutations.
However, over the years there has been tremendous reluctance among physicians caring for BRCA patients and the women themselves—I use the term “previvors” to describe BRCA carriers who have not been diagnosed with breast or ovarian cancer—to use HT after BSO because of concerns that HT might increase breast cancer risk in women who are already at high risk for breast cancer.
I assume, Dr. Faubion, that in this case the woman had gynecologic surgery but continues to have intact breasts. Is that correct?
Dr. Faubion: That is correct.
Dr. Kaunitz: Although the assumption has been that it is not safe to prescribe HT in this setting, in fact, the reported cohort studies that have looked at this issue have not found an elevated risk of breast cancer when replacement estrogen, with or without progestin, is prescribed to BRCA1 previvors with intact breasts.
Given what Dr. Kapoor said regarding the morbidity that is associated with BSO without replacement of physiologic estrogen, and also given the severe symptoms that so many of these young menopausal women experience, in my practice I do prescribe estrogen or estrogen-progestin therapy and focus on the higher target doses that we discussed for the earlier case patient who had a hysterectomy for abnormal uterine bleeding with adenomyosis.
Dr. Faubion: Dr. Kapoor, do you agree with this approach? How long would you continue therapy?
Dr. Kapoor: First, in this BRCA1 case we need to appreciate that the indication for the BSO is a legitimate one, in contrast to the first case in which the ovaries were removed in a patient whose average risk of ovarian cancer was low. It is important to recognize that surgery performed in this context is the right thing to do because it does significantly reduce the risk of ovarian cancer.
The second thing to appreciate is that while we reduce the risk of ovarian cancer significantly and make sure that these patients survive longer, it’s striking a fine balance in that you want to make sure that their morbidity is not increased as a result of premature estrogen deprivation.
As Dr. Kaunitz told us, the evidence that we have so far, which granted is not very robust but is fairly strong observational evidence, suggests that the risk of breast cancer is not elevated when these patients are treated with replacement doses of HT.
Having said that, I do have very strong discussions with my patients in this category about having the risk-reducing bilateral mastectomy also, because if they were to get breast cancer because of their increased genetic predisposition, the cancer is likely to grow faster if the patient is on HT. So one of my counseling points to patients is that they strongly consider bilateral mastectomy, which reduces their breast cancer risk by more than 90%. At the same time, I also strongly endorse using HT in replacement doses for the reasons that we have already stated.
Dr. Faubion: Continue HT until age 50 or 52?
Dr. Kapoor: Definitely until that age, and possibly longer, depending on their symptoms. The indications for treating beyond the age of natural menopause are much the same as for women who experience natural menopause.
Dr. Faubion: That is assuming they had a bilateral mastectomy?
Dr. Kapoor: Yes.
Continue to: Continuing HT until the age of natural menopause...
Continuing HT until the age of natural menopause
Dr. Kaunitz: Dr. Kapoor brings up the important point of duration of systemic HT. I agree that similar considerations apply both to the healthy 41-year-old who had a hysterectomy for abnormal uterine bleeding and to the 35-year-old who had risk-reducing surgery because of her BRCA1 mutation.
In the 2 cases, both to treat symptoms and to prevent chronic diseases, it makes sense to continue HT at least until the age of natural menopause. That is consistent with 2017 guidance from The North American Menopause Society (NAMS) position statement on the use of systemic HT, that is, continuing systemic HT at least until the age of natural menopause.3 Then at that point, continuing or discontinuing systemic HT becomes discretionary, and that would be true for both cases. If the patient is slender or has a strong family history of osteoporosis, that tends to push the patient more in terms of continuing systemic HT. Those are just some examples, and Dr. Kapoor may want to detail other relevant considerations.
Dr. Kapoor: I completely agree. The decision is driven by symptoms that are not otherwise well managed, for example, with nonhormone strategies. If we have any concerns utilizing HT beyond the age of natural menopause, then nonhormonal options can be considered; but sometimes those are not as effective. And bone health is very important. You want to avoid using bisphosphonates in younger women and reserve them for older patients in their late 60s and 70s. Hormone therapy use is a very reasonable strategy to prevent bone loss.
Dr. Kaunitz: It is also worth mentioning that sometimes the woman involved in shared decision making with her clinician decides to stop systemic HT. In that setting, should the patient start developing new-onset dyspareunia, vaginal dryness, or other genital or sexuality-related concerns, it takes very little for me to advise that she start low-dose local vaginal estrogen therapy.
Dr. Faubion: In either scenario, if a woman were to develop symptoms consistent with genitourinary syndrome of menopause (GSM), would you use vaginal estrogen in addition to the systemic estrogen or alone after the woman elected to discontinue systemic therapy?
Dr. Kapoor: Yes to both, I would say.
Dr. Kaunitz: As my patients using systemic HT age, often I will lower the dose. For instance, the dose I use in a 53-year-old will be higher than when she is 59 or 62. At the same time, as we lower the dose of systemic estrogen therapy, symptoms of vaginal atrophy or GSM often will appear, and these can be effectively treated by adding low-dose vaginal estrogen therapy. A number of my patients, particularly those who are on lower-than-standard doses of systemic HT, are also using low-dose vaginal estrogen therapy.
There is a “hybrid” product available: the 90-day estradiol vaginal ring. Estring is a low-dose, 2-mg, 90-day estradiol ring that is very useful, but it is effective only for treating GSM or vaginal atrophy. A second menopausal vaginal estradiol ring, Femring, is available in 2 doses: 0.05 mg/day and 0.1 mg/day. These are very effective in treating both systemic issues, such as vasomotor symptoms or prevention of osteoporosis, and very effective in treating GSM or vaginal atrophy. One problem is that Femring, depending on insurance coverage, can be very expensive. It’s not available as a generic, so for insurance or financial reasons I don’t often prescribe it. If I could remove those financial barriers, I would prescribe Femring more often because it is very useful.
Dr. Faubion: You raise an important point, and that is, for women who have been on HT for some time, clinicians often feel the need to slowly reduce the dose. Would you do that same thing, Dr. Kapoor, for a 40-year-old woman? Would you reduce the dose as she approaches age 50? Is there pressure that “she shouldn’t be on that much estrogen”?
Dr. Kapoor: No, I would not feel pressured until the patient turns at least 46. I bring up age 46 because the average age range for menopause is 46 to 55. After that, if there is any concern, we can decrease the dose to half and keep the patient on that until she turns 50 or 51. But most of my patients are on replacement doses until the average age of menopause, which is around 51 years, and that’s when you reduce the dose to that of the typical HT regimens used after natural menopause.
Sometimes patients are told something by a friend or they have read something and they worry about the risk of 2 things. One is breast cancer and the other is venous thromboembolism (VTE), and that may be why they want to be on a lower dose. I counsel patients that while the risk of VTE is real with HT, it is the women after natural menopause who are at risk—because age itself is a risk for VTE—and it also has to do with the kind of HT regimen that a patient is on. High doses of oral estrogens and certain progestogens increase the risk. But again, for estradiol used in replacement doses and the more common progestogens that we now use in practice, such as micronized progesterone, the risk is not the same. The same goes for breast cancer. My biggest message to patients and clinicians who take care of these patients is that the rules that apply to women after natural menopause just do not apply to this very different patient population.
Dr. Faubion: Thank you, Dr. Kaunitz and Dr. Kapoor, for sharing your knowledge and experience. ●
Systemic HT past the age of 65
Dr. Kaunitz: Another practical issue relates to long-term or extended use of systemic HT. It’s not infrequent in my practice to receive mail and faxes from insurance carriers of systemic HT users who are age 65 and older in which the company refers to the American Geriatrics Society’s Beers criteria for potentially inappropriate medication use in older adults,1 suggesting that systemic HT is inappropriate for all women over age 65. In this age group, I use lower doses if I am continuing systemic HT. But the good news is that both NAMS and the American College Obstetricians and Gynecologists indicate that arbitrarily stopping systemic HT at age 65 or for any other arbitrary reason is inappropriate, and that decisions about continuing or discontinuing therapy should be made on an individualized basis using shared decision making. That’s an important message for our readers.
Counseling regarding elective BSO
Dr. Faubion: One final note about elective BSO in the absence of a genetic mutation that predisposes to increased ovarian or breast cancer risk. Fortunately, we have seen rates of oophorectomy before the age of natural menopause decline, but what would your advice be to women or clinicians of these women who say they are “just afraid of ovarian cancer and would like to have their ovaries removed before the age of natural menopause”?
Dr. Kaunitz: If patients have increased anxiety about ovarian cancer and yet they themselves are not known to be at elevated risk, I emphasize that, fortunately, ovarian cancer is uncommon. It is much less common than other cancers the patient might be familiar with, such as breast or colon or lung cancer. I also emphasize that women who have given birth, particularly multiple times; women who nursed their infants; and women who have used combination hormonal contraceptives, particularly if long term, are at markedly lower risk for ovarian cancer as they get older. We are talking about an uncommon cancer that is even less common if women have given birth, nursed their infants, or used combination contraceptives long term.
Dr. Faubion: Dr. Kapoor, what would you say regarding the increased risk they might incur if they do have their ovaries out?
Dr. Kapoor: As Dr. Kaunitz said, this is an uncommon cancer, and pursuing something to reduce the risk of an uncommon cancer does not benefit the community. That is also my counseling point to patients.
I also talk to them extensively about the risk associated with the ovaries being removed, and I tell them that although we have the option of giving them HT, it is hard to replicate the magic of nature. No matter what concoction or regimen we use, we cannot ensure reinstating health to what it was in the premenopausal state, because estrogen has such myriad effects on the body in so many different organ systems.
Reference
1. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
- North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. J North Am Menopause Soc. 2017;24: 728-753.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
- North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. J North Am Menopause Soc. 2017;24: 728-753.
Do recent data on use of menopausal HT and subsequent risk of dementia indicate an association?
Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
Much interest has surrounded whether the use of menopausal HT impacts future risk of cognitive decline. Recently, Vinogradova and colleagues conducted an observational study using data from 2 large primary care databases, QResearch and the Clinical Practice Research Datalink (CPRD), in the United Kingdom.1 The investigators conducted case-control studies that included women aged 55 and older diagnosed with dementia and up to 5 controls without dementia. Only cases and controls with at least 10 years of medical records prior to the index date (that is, the time of dementia diagnosis in cases) were included. Since early symptoms of dementia prior to diagnosis may cause sleep problems and dysphoria (which also may be symptoms of menopause), HT prescriptions during the 3 years prior to the index date were excluded.
Details of the study
Among 16,291 cases and 68,726 controls, the women’s mean age was approximately 83 years. Cases were identified by using codes for dementia from patients’ clinical records or records of prescriptions for drugs used to treat dementia.
More than half the women were being treated for hypertension, and 14% of women in both groups had used HT. Women were considered users of estrogen-only therapy if they had no prescriptions containing a progestogen after their first prescription for systemic estrogen as the start of exposure to HT. Those with any subsequent prescription containing a progestogen were classified as combined HT users.
Results. In an analysis adjusted for all available potential confounders—including lifestyle factors, ethnicity, family history of dementia, early menopause, oophorectomy/ hysterectomy, comorbidities, and use of other relevant drugs—the use of HT was not associated with risk of dementia.
A reduced risk of dementia was noted among women who had been taking estrogen-only HT for 10 years or more (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.76–0.94). An elevated risk of Alzheimer disease was noted among women who had used estrogen-progestin HT for 5 to 9 years (OR, 1.19; CI, 1.06–1.33).1
Study strengths and limitations
The authors pointed out that this study’s main strengths were that it had a very large sample size representative of the general population and that its design permitted capture of all known cases as well as precision recording for prescribed drugs. On the other hand, the study is limited by the possible lack of data for some older women before the index date; that is, menopause in this latter group started before their registration or before these data were gathered electronically by their practice. ●
The authors of this British large observational study took pains to minimize potential bias. The finding that long-term use of estrogen-only HT may be neuroprotective is consistent with results of recent studies in the United States and Finland,2-4 as well as with the Women’s Health Initiative randomized trial, which found that with 18 years of follow-up, treatment with conjugated estrogen alone was associated with a 26% reduced risk of death from Alzheimer disease.5 Overall, however, the main message we should glean from this important study by Vinogradova and colleagues is that women with bothersome vasomotor symptoms considering use of menopausal HT can be reassured that such therapy has little if any impact on future risk of cognitive decline.
ANDREW M. KAUNITZ, MD, NCMP
- Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
- Matyi JM, Rattinger GB, Schwartz S, et al. Lifetime estrogen exposure and cognition in late life: the Cache County study. Menopause. 2019;26:1366-1374. doi: 10.1097 /GME.0000000000001405.
- Liu JH. Does estrogen provide “neuroprotection” for postmenopausal women? Menopause. 2019;26:1361-1362. doi: 10.1097/GME.0000000000001459.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88:1062-1068. doi: 10.1212 /WNL.0000000000003696.
- Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001 /jama.2017.11217.
Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
Much interest has surrounded whether the use of menopausal HT impacts future risk of cognitive decline. Recently, Vinogradova and colleagues conducted an observational study using data from 2 large primary care databases, QResearch and the Clinical Practice Research Datalink (CPRD), in the United Kingdom.1 The investigators conducted case-control studies that included women aged 55 and older diagnosed with dementia and up to 5 controls without dementia. Only cases and controls with at least 10 years of medical records prior to the index date (that is, the time of dementia diagnosis in cases) were included. Since early symptoms of dementia prior to diagnosis may cause sleep problems and dysphoria (which also may be symptoms of menopause), HT prescriptions during the 3 years prior to the index date were excluded.
Details of the study
Among 16,291 cases and 68,726 controls, the women’s mean age was approximately 83 years. Cases were identified by using codes for dementia from patients’ clinical records or records of prescriptions for drugs used to treat dementia.
More than half the women were being treated for hypertension, and 14% of women in both groups had used HT. Women were considered users of estrogen-only therapy if they had no prescriptions containing a progestogen after their first prescription for systemic estrogen as the start of exposure to HT. Those with any subsequent prescription containing a progestogen were classified as combined HT users.
Results. In an analysis adjusted for all available potential confounders—including lifestyle factors, ethnicity, family history of dementia, early menopause, oophorectomy/ hysterectomy, comorbidities, and use of other relevant drugs—the use of HT was not associated with risk of dementia.
A reduced risk of dementia was noted among women who had been taking estrogen-only HT for 10 years or more (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.76–0.94). An elevated risk of Alzheimer disease was noted among women who had used estrogen-progestin HT for 5 to 9 years (OR, 1.19; CI, 1.06–1.33).1
Study strengths and limitations
The authors pointed out that this study’s main strengths were that it had a very large sample size representative of the general population and that its design permitted capture of all known cases as well as precision recording for prescribed drugs. On the other hand, the study is limited by the possible lack of data for some older women before the index date; that is, menopause in this latter group started before their registration or before these data were gathered electronically by their practice. ●
The authors of this British large observational study took pains to minimize potential bias. The finding that long-term use of estrogen-only HT may be neuroprotective is consistent with results of recent studies in the United States and Finland,2-4 as well as with the Women’s Health Initiative randomized trial, which found that with 18 years of follow-up, treatment with conjugated estrogen alone was associated with a 26% reduced risk of death from Alzheimer disease.5 Overall, however, the main message we should glean from this important study by Vinogradova and colleagues is that women with bothersome vasomotor symptoms considering use of menopausal HT can be reassured that such therapy has little if any impact on future risk of cognitive decline.
ANDREW M. KAUNITZ, MD, NCMP
Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
Much interest has surrounded whether the use of menopausal HT impacts future risk of cognitive decline. Recently, Vinogradova and colleagues conducted an observational study using data from 2 large primary care databases, QResearch and the Clinical Practice Research Datalink (CPRD), in the United Kingdom.1 The investigators conducted case-control studies that included women aged 55 and older diagnosed with dementia and up to 5 controls without dementia. Only cases and controls with at least 10 years of medical records prior to the index date (that is, the time of dementia diagnosis in cases) were included. Since early symptoms of dementia prior to diagnosis may cause sleep problems and dysphoria (which also may be symptoms of menopause), HT prescriptions during the 3 years prior to the index date were excluded.
Details of the study
Among 16,291 cases and 68,726 controls, the women’s mean age was approximately 83 years. Cases were identified by using codes for dementia from patients’ clinical records or records of prescriptions for drugs used to treat dementia.
More than half the women were being treated for hypertension, and 14% of women in both groups had used HT. Women were considered users of estrogen-only therapy if they had no prescriptions containing a progestogen after their first prescription for systemic estrogen as the start of exposure to HT. Those with any subsequent prescription containing a progestogen were classified as combined HT users.
Results. In an analysis adjusted for all available potential confounders—including lifestyle factors, ethnicity, family history of dementia, early menopause, oophorectomy/ hysterectomy, comorbidities, and use of other relevant drugs—the use of HT was not associated with risk of dementia.
A reduced risk of dementia was noted among women who had been taking estrogen-only HT for 10 years or more (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.76–0.94). An elevated risk of Alzheimer disease was noted among women who had used estrogen-progestin HT for 5 to 9 years (OR, 1.19; CI, 1.06–1.33).1
Study strengths and limitations
The authors pointed out that this study’s main strengths were that it had a very large sample size representative of the general population and that its design permitted capture of all known cases as well as precision recording for prescribed drugs. On the other hand, the study is limited by the possible lack of data for some older women before the index date; that is, menopause in this latter group started before their registration or before these data were gathered electronically by their practice. ●
The authors of this British large observational study took pains to minimize potential bias. The finding that long-term use of estrogen-only HT may be neuroprotective is consistent with results of recent studies in the United States and Finland,2-4 as well as with the Women’s Health Initiative randomized trial, which found that with 18 years of follow-up, treatment with conjugated estrogen alone was associated with a 26% reduced risk of death from Alzheimer disease.5 Overall, however, the main message we should glean from this important study by Vinogradova and colleagues is that women with bothersome vasomotor symptoms considering use of menopausal HT can be reassured that such therapy has little if any impact on future risk of cognitive decline.
ANDREW M. KAUNITZ, MD, NCMP
- Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
- Matyi JM, Rattinger GB, Schwartz S, et al. Lifetime estrogen exposure and cognition in late life: the Cache County study. Menopause. 2019;26:1366-1374. doi: 10.1097 /GME.0000000000001405.
- Liu JH. Does estrogen provide “neuroprotection” for postmenopausal women? Menopause. 2019;26:1361-1362. doi: 10.1097/GME.0000000000001459.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88:1062-1068. doi: 10.1212 /WNL.0000000000003696.
- Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001 /jama.2017.11217.
- Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
- Matyi JM, Rattinger GB, Schwartz S, et al. Lifetime estrogen exposure and cognition in late life: the Cache County study. Menopause. 2019;26:1366-1374. doi: 10.1097 /GME.0000000000001405.
- Liu JH. Does estrogen provide “neuroprotection” for postmenopausal women? Menopause. 2019;26:1361-1362. doi: 10.1097/GME.0000000000001459.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88:1062-1068. doi: 10.1212 /WNL.0000000000003696.
- Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001 /jama.2017.11217.
How safe is a drug holiday from bisphosphonates for osteoporosis?
Researchers found a small but greater risk of a hip fracture after 2 years of taking a “drug holiday” – stopping therapy – after long-term (≥3-year) use of one bisphosphonate, risedronate, versus another, alendronate.
The risk of a hip fracture after stopping either of these oral bisphosphonate osteoporosis drugs was similar until 2 years, suggesting that patients who take a drug holiday from risedronate should be revaluated before 2 years.
These top-line findings from a propensity-matched cohort study of older patients in Ontario, Canada, were reported at the annual American Society of Bone and Mineral Research (ASBMR) last fall.
The full study, led by Kaleen N. Hayes, PharmD, PhD, Brown University School of Public Health, Providence, R.I., was published online in the Annals of Internal Medicine.
“We emphasize that our results do not indicate that alendronate therapy should be preferred over risedronate therapy,” the researchers stress, as several real-world studies found a similar risk of fractures while patients were receiving either drug.
“The decision to initiate alendronate or risedronate therapy [the two most commonly prescribed bisphosphonates] is driven by the prescriber,” they note, adding that some patients may prefer risedronate because it is available as a monthly dose or a weekly delayed-release formula that does not require fasting.
“We found little difference in the association between risedronate versus alendronate drug holidays and hip fractures until approximately 2 years of not receiving therapy,” Dr. Hayes and colleagues summarize.
Over 3 years, risedronate drug holidays were associated with an 18% relative and 0.6% absolute increased risk for hip fracture compared with alendronate drug holidays.
“To further inform clinical decision-making on drug holidays,” they conclude, “future research should examine when to start and restart osteoporosis therapy on the basis of initial length and type of treatment, patient characteristics, and relative risk for hip fractures versus [atypical femoral fracture].”
Hip fracture risk with risedronate vs. alendronate drug holiday
Long-term bisphosphonate use is associated with a rare risk of osteonecrosis of the jaw or atypical femoral fractures. At the same time, bisphosphonates continue to have a therapeutic effect after therapy is discontinued.
Guidelines recommend that patients at low risk of fracture should therefore have a “drug holiday” after 3 to 5 years of bisphosphonate use and be reassessed 2 to 3 years later, largely based on the Fracture Intervention Trial Long-Term Extension (FLEX) study of alendronate. But risedronate has a shorter half-life, so it may provide shorter residual fracture protection.
Using Ontario administrative data, Dr. Hayes and associates identified more than 60,000 patients who were over aged 65, had received at least 3 years of continuous alendronate or risedronate, and had a subsequent 3-year drug holiday between 2000 and 2020.
They excluded patients who had a fracture or entered a nursing home within 120 days of starting a drug holiday who may have stopped the bisphosphonate due to declining health rather than a drug holiday.
Roughly half (55%) had been taking risedronate and 45% had been taking alendronate.
Using propensity scores, the researchers matched 25,077 patients who had been taking risedronate with an equal number who had been taking alendronate.
Most of the patients were women (82%) and were White.
They started the drug holiday when they were on average 81 years old, after taking the bisphosphonate for 5.9 years on average.
During the 3-year drug holiday, 915 of the 50,154 patients had hip fractures.
This was equivalent to 12.4 hip fractures per 1,000 patients per year during a risedronate holiday and 10.6 hip fractures per 1,000 patients per year during an alendronate holiday (hazard ratio, 1.18).
The risk of hip fracture was not significantly higher at 1 year (HR, 1.03) or at 2 years of a risedronate holiday versus an alendronate holiday (HR, 1.14).
However, the risk of a hip fracture was significantly higher at 2 to 3 years of a risedronate holiday than after an alendronate holiday (HR, 1.34).
There was no significant difference in the risk of any osteoporotic fracture overall (including hip, vertebrae, pelvis, ribs, forearm), however, during a 3-year risedronate versus alendronate drug holiday (HR, 1.07).
The research was supported by the Canadian Institutes of Health Research and Institute for Clinical Evaluative Sciences. Dr. Hayes was supported by a CIHR doctoral research award. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found a small but greater risk of a hip fracture after 2 years of taking a “drug holiday” – stopping therapy – after long-term (≥3-year) use of one bisphosphonate, risedronate, versus another, alendronate.
The risk of a hip fracture after stopping either of these oral bisphosphonate osteoporosis drugs was similar until 2 years, suggesting that patients who take a drug holiday from risedronate should be revaluated before 2 years.
These top-line findings from a propensity-matched cohort study of older patients in Ontario, Canada, were reported at the annual American Society of Bone and Mineral Research (ASBMR) last fall.
The full study, led by Kaleen N. Hayes, PharmD, PhD, Brown University School of Public Health, Providence, R.I., was published online in the Annals of Internal Medicine.
“We emphasize that our results do not indicate that alendronate therapy should be preferred over risedronate therapy,” the researchers stress, as several real-world studies found a similar risk of fractures while patients were receiving either drug.
“The decision to initiate alendronate or risedronate therapy [the two most commonly prescribed bisphosphonates] is driven by the prescriber,” they note, adding that some patients may prefer risedronate because it is available as a monthly dose or a weekly delayed-release formula that does not require fasting.
“We found little difference in the association between risedronate versus alendronate drug holidays and hip fractures until approximately 2 years of not receiving therapy,” Dr. Hayes and colleagues summarize.
Over 3 years, risedronate drug holidays were associated with an 18% relative and 0.6% absolute increased risk for hip fracture compared with alendronate drug holidays.
“To further inform clinical decision-making on drug holidays,” they conclude, “future research should examine when to start and restart osteoporosis therapy on the basis of initial length and type of treatment, patient characteristics, and relative risk for hip fractures versus [atypical femoral fracture].”
Hip fracture risk with risedronate vs. alendronate drug holiday
Long-term bisphosphonate use is associated with a rare risk of osteonecrosis of the jaw or atypical femoral fractures. At the same time, bisphosphonates continue to have a therapeutic effect after therapy is discontinued.
Guidelines recommend that patients at low risk of fracture should therefore have a “drug holiday” after 3 to 5 years of bisphosphonate use and be reassessed 2 to 3 years later, largely based on the Fracture Intervention Trial Long-Term Extension (FLEX) study of alendronate. But risedronate has a shorter half-life, so it may provide shorter residual fracture protection.
Using Ontario administrative data, Dr. Hayes and associates identified more than 60,000 patients who were over aged 65, had received at least 3 years of continuous alendronate or risedronate, and had a subsequent 3-year drug holiday between 2000 and 2020.
They excluded patients who had a fracture or entered a nursing home within 120 days of starting a drug holiday who may have stopped the bisphosphonate due to declining health rather than a drug holiday.
Roughly half (55%) had been taking risedronate and 45% had been taking alendronate.
Using propensity scores, the researchers matched 25,077 patients who had been taking risedronate with an equal number who had been taking alendronate.
Most of the patients were women (82%) and were White.
They started the drug holiday when they were on average 81 years old, after taking the bisphosphonate for 5.9 years on average.
During the 3-year drug holiday, 915 of the 50,154 patients had hip fractures.
This was equivalent to 12.4 hip fractures per 1,000 patients per year during a risedronate holiday and 10.6 hip fractures per 1,000 patients per year during an alendronate holiday (hazard ratio, 1.18).
The risk of hip fracture was not significantly higher at 1 year (HR, 1.03) or at 2 years of a risedronate holiday versus an alendronate holiday (HR, 1.14).
However, the risk of a hip fracture was significantly higher at 2 to 3 years of a risedronate holiday than after an alendronate holiday (HR, 1.34).
There was no significant difference in the risk of any osteoporotic fracture overall (including hip, vertebrae, pelvis, ribs, forearm), however, during a 3-year risedronate versus alendronate drug holiday (HR, 1.07).
The research was supported by the Canadian Institutes of Health Research and Institute for Clinical Evaluative Sciences. Dr. Hayes was supported by a CIHR doctoral research award. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found a small but greater risk of a hip fracture after 2 years of taking a “drug holiday” – stopping therapy – after long-term (≥3-year) use of one bisphosphonate, risedronate, versus another, alendronate.
The risk of a hip fracture after stopping either of these oral bisphosphonate osteoporosis drugs was similar until 2 years, suggesting that patients who take a drug holiday from risedronate should be revaluated before 2 years.
These top-line findings from a propensity-matched cohort study of older patients in Ontario, Canada, were reported at the annual American Society of Bone and Mineral Research (ASBMR) last fall.
The full study, led by Kaleen N. Hayes, PharmD, PhD, Brown University School of Public Health, Providence, R.I., was published online in the Annals of Internal Medicine.
“We emphasize that our results do not indicate that alendronate therapy should be preferred over risedronate therapy,” the researchers stress, as several real-world studies found a similar risk of fractures while patients were receiving either drug.
“The decision to initiate alendronate or risedronate therapy [the two most commonly prescribed bisphosphonates] is driven by the prescriber,” they note, adding that some patients may prefer risedronate because it is available as a monthly dose or a weekly delayed-release formula that does not require fasting.
“We found little difference in the association between risedronate versus alendronate drug holidays and hip fractures until approximately 2 years of not receiving therapy,” Dr. Hayes and colleagues summarize.
Over 3 years, risedronate drug holidays were associated with an 18% relative and 0.6% absolute increased risk for hip fracture compared with alendronate drug holidays.
“To further inform clinical decision-making on drug holidays,” they conclude, “future research should examine when to start and restart osteoporosis therapy on the basis of initial length and type of treatment, patient characteristics, and relative risk for hip fractures versus [atypical femoral fracture].”
Hip fracture risk with risedronate vs. alendronate drug holiday
Long-term bisphosphonate use is associated with a rare risk of osteonecrosis of the jaw or atypical femoral fractures. At the same time, bisphosphonates continue to have a therapeutic effect after therapy is discontinued.
Guidelines recommend that patients at low risk of fracture should therefore have a “drug holiday” after 3 to 5 years of bisphosphonate use and be reassessed 2 to 3 years later, largely based on the Fracture Intervention Trial Long-Term Extension (FLEX) study of alendronate. But risedronate has a shorter half-life, so it may provide shorter residual fracture protection.
Using Ontario administrative data, Dr. Hayes and associates identified more than 60,000 patients who were over aged 65, had received at least 3 years of continuous alendronate or risedronate, and had a subsequent 3-year drug holiday between 2000 and 2020.
They excluded patients who had a fracture or entered a nursing home within 120 days of starting a drug holiday who may have stopped the bisphosphonate due to declining health rather than a drug holiday.
Roughly half (55%) had been taking risedronate and 45% had been taking alendronate.
Using propensity scores, the researchers matched 25,077 patients who had been taking risedronate with an equal number who had been taking alendronate.
Most of the patients were women (82%) and were White.
They started the drug holiday when they were on average 81 years old, after taking the bisphosphonate for 5.9 years on average.
During the 3-year drug holiday, 915 of the 50,154 patients had hip fractures.
This was equivalent to 12.4 hip fractures per 1,000 patients per year during a risedronate holiday and 10.6 hip fractures per 1,000 patients per year during an alendronate holiday (hazard ratio, 1.18).
The risk of hip fracture was not significantly higher at 1 year (HR, 1.03) or at 2 years of a risedronate holiday versus an alendronate holiday (HR, 1.14).
However, the risk of a hip fracture was significantly higher at 2 to 3 years of a risedronate holiday than after an alendronate holiday (HR, 1.34).
There was no significant difference in the risk of any osteoporotic fracture overall (including hip, vertebrae, pelvis, ribs, forearm), however, during a 3-year risedronate versus alendronate drug holiday (HR, 1.07).
The research was supported by the Canadian Institutes of Health Research and Institute for Clinical Evaluative Sciences. Dr. Hayes was supported by a CIHR doctoral research award. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Midlife cardiovascular conditions tied to greater cognitive decline in women
Even though men in midlife have more cardiovascular (CV) conditions and risk factors than women of the same age, women are more affected by these conditions in terms of cognitive decline, new research suggests.
Analyses of almost 1,400 participants in the population-based Mayo Clinic Study of Aging showed that diabetes, dyslipidemia, and coronary heart disease (CHD) all had stronger associations with global cognitive decline in women than in men.
“All men and women should be treated for cardiovascular risk factors and conditions, but this study really highlights the importance of very early and perhaps more aggressive treatment in women with these conditions,” co-investigator Michelle M. Mielke, PhD, professor of epidemiology and neurology, Mayo Clinic, Rochester, Minn., told this news organization.
The findings were published online Jan. 5 in Neurology.
Assessing sex differences
Most previous studies in this area have focused on CV risk factors in midlife in relation to late-life dementia (after age 75) or on late-life vascular risk factors and late-life dementia, Dr. Mielke noted.
However, a few recent studies have suggested vascular risk factors can affect cognition even in midlife. The current investigators sought to determine whether there are sex differences in these associations.
They assessed 1,857 nondemented participants aged 50 to 69 years from the Mayo Clinic Study on Aging. The mean education level was 14.9 years, and the mean body mass index (BMI) was 29.7.
Among the participants, 78.9% had at least one CV condition or risk factor, and the proportion was higher in men than women (83.4% vs. 74.5%; P < .0001).
Frequency of each individual CV condition or risk factor was also higher in men than women, and they had more years of education and higher BMI but took fewer medications.
Every 15 months, participants had an in-person interview and physical examination that included a neurologic assessment and short test of memory.
The neuropsychological battery included nine tests across four domains: memory, language, executive function, and visuospatial skills. Researchers calculated z-scores for these domains and for global cognition.
Multiple cognitive domains
Whereas this study evaluated multiple cognitive domains, most previous research has focused on global cognitive decline and/or decline in only one or two cognitive domains, the investigators note.
They collected information from medical records on CV conditions such as CHD, arrhythmias, congestive heart failure, peripheral vascular disease (PVD), and stroke; and CV risk factors such as hypertension, diabetes, dyslipidemia, smoking status, and BMI.
Because of the small number of patients with stroke and PVD, these were classified as “other cardiovascular conditions” in the statistical analysis.
Researchers adjusted for sex, age, years of education, depressive symptoms, comorbidities, medications, and apolipoprotein E (APOE) genotyping. The mean follow-up was 3 years and did not differ by sex.
As some participants didn’t have a follow-up visit, the current analysis included 1,394 individuals. Those without follow-up visits were younger, had less education and more comorbidities, and took more medications compared with those with a follow-up.
Results showed most CV conditions were more strongly associated with cognitive function among women than men. For example, CHD was associated with global decline only in women (P < .05).
CHD, diabetes, and dyslipidemia were associated with language decline in women only (all, P < .05), but congestive heart failure was significantly associated with language decline in men only.
Dr. Mielke cautioned about reading too much into the language results for women.
“It’s an intriguing finding and definitely we need to follow up on it,” she said. However, “more studies are needed to examine sex differences before we start saying it only has an effect on language.”
‘Treat aggressively and right away’
The researchers were somewhat surprised by the study findings. Because there is a higher prevalence of CV conditions and risk factors in men, they presumed men would be more affected by these conditions, said Dr. Mielke.
“But that’s not what we saw; we saw the reverse. It was actually the women who were affected more by these cardiovascular risk factors and conditions,” she said.
As midlife is when women enter menopause, fluctuating estrogen levels may help explain the differential impact on cognition among women. But Dr. Mielke said she wants to “move beyond” just looking at hormones.
She pointed out there are a variety of psychosocial factors that may also contribute to an imbalance in the cognitive impact of CV conditions on women.
“Midlife is when many women are still taking care of their children at home, are also taking care of their adult parents, and may be undergoing more stress while continuing to do a job,” Dr. Miekle said.
Structural brain development and genetics may also contribute to the greater effect on cognition in women, the investigators note.
Dr. Mielke stressed that the current study only identifies associations. “The next steps are to understand what some of the underlying mechanisms for this are,” she said.
In the meantime, these new results suggest middle-aged women with high blood pressure, cholesterol, or glucose measures “should be treated aggressively and right away” said Dr. Mielke.
“For example, for women who are just starting to become hypertensive, clinicians should treat them right away and not watch and wait.”
Study limitations cited include that its sample was limited to Olmsted County, Minnesota – so results may not be generalized to other populations. Also, as researchers combined PVD and stroke into one group, larger sample sizes are needed, especially for stroke. Another limitation was the study did not have information on duration of all CV conditions or risk factors.
Helpful for tailoring interventions?
Commenting on the study, Glen R. Finney, MD, director, Memory and Cognition Program, Geisinger Health Clinic, Wilkes-Barre, Pennsylvania, said the results are important.
“The more we understand about risk factors for the development of Alzheimer’s disease and related dementias, the better we understand how we can reduce the risks,” said Dr. Finney, who was not involved with the research.
Awareness that CV conditions are major risk factors in midlife has been “definitely rising,” said Dr. Finney. “Many studies originally were looking at late life and are now looking more at earlier in the disease process, and I think that’s important.”
Understanding how sex, ethnicity, and other demographic variables affect risks can help to “tailor interventions” for individual patients, he said.
The study was supported by the National Institutes of Health, the GHR Foundation, and the Rochester Epidemiology Project. Dr. Mielke is a consultant for Biogen and Brain Protection Company and is on the editorial boards of Neurology and Alzheimer’s and Dementia. Dr. Finney has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even though men in midlife have more cardiovascular (CV) conditions and risk factors than women of the same age, women are more affected by these conditions in terms of cognitive decline, new research suggests.
Analyses of almost 1,400 participants in the population-based Mayo Clinic Study of Aging showed that diabetes, dyslipidemia, and coronary heart disease (CHD) all had stronger associations with global cognitive decline in women than in men.
“All men and women should be treated for cardiovascular risk factors and conditions, but this study really highlights the importance of very early and perhaps more aggressive treatment in women with these conditions,” co-investigator Michelle M. Mielke, PhD, professor of epidemiology and neurology, Mayo Clinic, Rochester, Minn., told this news organization.
The findings were published online Jan. 5 in Neurology.
Assessing sex differences
Most previous studies in this area have focused on CV risk factors in midlife in relation to late-life dementia (after age 75) or on late-life vascular risk factors and late-life dementia, Dr. Mielke noted.
However, a few recent studies have suggested vascular risk factors can affect cognition even in midlife. The current investigators sought to determine whether there are sex differences in these associations.
They assessed 1,857 nondemented participants aged 50 to 69 years from the Mayo Clinic Study on Aging. The mean education level was 14.9 years, and the mean body mass index (BMI) was 29.7.
Among the participants, 78.9% had at least one CV condition or risk factor, and the proportion was higher in men than women (83.4% vs. 74.5%; P < .0001).
Frequency of each individual CV condition or risk factor was also higher in men than women, and they had more years of education and higher BMI but took fewer medications.
Every 15 months, participants had an in-person interview and physical examination that included a neurologic assessment and short test of memory.
The neuropsychological battery included nine tests across four domains: memory, language, executive function, and visuospatial skills. Researchers calculated z-scores for these domains and for global cognition.
Multiple cognitive domains
Whereas this study evaluated multiple cognitive domains, most previous research has focused on global cognitive decline and/or decline in only one or two cognitive domains, the investigators note.
They collected information from medical records on CV conditions such as CHD, arrhythmias, congestive heart failure, peripheral vascular disease (PVD), and stroke; and CV risk factors such as hypertension, diabetes, dyslipidemia, smoking status, and BMI.
Because of the small number of patients with stroke and PVD, these were classified as “other cardiovascular conditions” in the statistical analysis.
Researchers adjusted for sex, age, years of education, depressive symptoms, comorbidities, medications, and apolipoprotein E (APOE) genotyping. The mean follow-up was 3 years and did not differ by sex.
As some participants didn’t have a follow-up visit, the current analysis included 1,394 individuals. Those without follow-up visits were younger, had less education and more comorbidities, and took more medications compared with those with a follow-up.
Results showed most CV conditions were more strongly associated with cognitive function among women than men. For example, CHD was associated with global decline only in women (P < .05).
CHD, diabetes, and dyslipidemia were associated with language decline in women only (all, P < .05), but congestive heart failure was significantly associated with language decline in men only.
Dr. Mielke cautioned about reading too much into the language results for women.
“It’s an intriguing finding and definitely we need to follow up on it,” she said. However, “more studies are needed to examine sex differences before we start saying it only has an effect on language.”
‘Treat aggressively and right away’
The researchers were somewhat surprised by the study findings. Because there is a higher prevalence of CV conditions and risk factors in men, they presumed men would be more affected by these conditions, said Dr. Mielke.
“But that’s not what we saw; we saw the reverse. It was actually the women who were affected more by these cardiovascular risk factors and conditions,” she said.
As midlife is when women enter menopause, fluctuating estrogen levels may help explain the differential impact on cognition among women. But Dr. Mielke said she wants to “move beyond” just looking at hormones.
She pointed out there are a variety of psychosocial factors that may also contribute to an imbalance in the cognitive impact of CV conditions on women.
“Midlife is when many women are still taking care of their children at home, are also taking care of their adult parents, and may be undergoing more stress while continuing to do a job,” Dr. Miekle said.
Structural brain development and genetics may also contribute to the greater effect on cognition in women, the investigators note.
Dr. Mielke stressed that the current study only identifies associations. “The next steps are to understand what some of the underlying mechanisms for this are,” she said.
In the meantime, these new results suggest middle-aged women with high blood pressure, cholesterol, or glucose measures “should be treated aggressively and right away” said Dr. Mielke.
“For example, for women who are just starting to become hypertensive, clinicians should treat them right away and not watch and wait.”
Study limitations cited include that its sample was limited to Olmsted County, Minnesota – so results may not be generalized to other populations. Also, as researchers combined PVD and stroke into one group, larger sample sizes are needed, especially for stroke. Another limitation was the study did not have information on duration of all CV conditions or risk factors.
Helpful for tailoring interventions?
Commenting on the study, Glen R. Finney, MD, director, Memory and Cognition Program, Geisinger Health Clinic, Wilkes-Barre, Pennsylvania, said the results are important.
“The more we understand about risk factors for the development of Alzheimer’s disease and related dementias, the better we understand how we can reduce the risks,” said Dr. Finney, who was not involved with the research.
Awareness that CV conditions are major risk factors in midlife has been “definitely rising,” said Dr. Finney. “Many studies originally were looking at late life and are now looking more at earlier in the disease process, and I think that’s important.”
Understanding how sex, ethnicity, and other demographic variables affect risks can help to “tailor interventions” for individual patients, he said.
The study was supported by the National Institutes of Health, the GHR Foundation, and the Rochester Epidemiology Project. Dr. Mielke is a consultant for Biogen and Brain Protection Company and is on the editorial boards of Neurology and Alzheimer’s and Dementia. Dr. Finney has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even though men in midlife have more cardiovascular (CV) conditions and risk factors than women of the same age, women are more affected by these conditions in terms of cognitive decline, new research suggests.
Analyses of almost 1,400 participants in the population-based Mayo Clinic Study of Aging showed that diabetes, dyslipidemia, and coronary heart disease (CHD) all had stronger associations with global cognitive decline in women than in men.
“All men and women should be treated for cardiovascular risk factors and conditions, but this study really highlights the importance of very early and perhaps more aggressive treatment in women with these conditions,” co-investigator Michelle M. Mielke, PhD, professor of epidemiology and neurology, Mayo Clinic, Rochester, Minn., told this news organization.
The findings were published online Jan. 5 in Neurology.
Assessing sex differences
Most previous studies in this area have focused on CV risk factors in midlife in relation to late-life dementia (after age 75) or on late-life vascular risk factors and late-life dementia, Dr. Mielke noted.
However, a few recent studies have suggested vascular risk factors can affect cognition even in midlife. The current investigators sought to determine whether there are sex differences in these associations.
They assessed 1,857 nondemented participants aged 50 to 69 years from the Mayo Clinic Study on Aging. The mean education level was 14.9 years, and the mean body mass index (BMI) was 29.7.
Among the participants, 78.9% had at least one CV condition or risk factor, and the proportion was higher in men than women (83.4% vs. 74.5%; P < .0001).
Frequency of each individual CV condition or risk factor was also higher in men than women, and they had more years of education and higher BMI but took fewer medications.
Every 15 months, participants had an in-person interview and physical examination that included a neurologic assessment and short test of memory.
The neuropsychological battery included nine tests across four domains: memory, language, executive function, and visuospatial skills. Researchers calculated z-scores for these domains and for global cognition.
Multiple cognitive domains
Whereas this study evaluated multiple cognitive domains, most previous research has focused on global cognitive decline and/or decline in only one or two cognitive domains, the investigators note.
They collected information from medical records on CV conditions such as CHD, arrhythmias, congestive heart failure, peripheral vascular disease (PVD), and stroke; and CV risk factors such as hypertension, diabetes, dyslipidemia, smoking status, and BMI.
Because of the small number of patients with stroke and PVD, these were classified as “other cardiovascular conditions” in the statistical analysis.
Researchers adjusted for sex, age, years of education, depressive symptoms, comorbidities, medications, and apolipoprotein E (APOE) genotyping. The mean follow-up was 3 years and did not differ by sex.
As some participants didn’t have a follow-up visit, the current analysis included 1,394 individuals. Those without follow-up visits were younger, had less education and more comorbidities, and took more medications compared with those with a follow-up.
Results showed most CV conditions were more strongly associated with cognitive function among women than men. For example, CHD was associated with global decline only in women (P < .05).
CHD, diabetes, and dyslipidemia were associated with language decline in women only (all, P < .05), but congestive heart failure was significantly associated with language decline in men only.
Dr. Mielke cautioned about reading too much into the language results for women.
“It’s an intriguing finding and definitely we need to follow up on it,” she said. However, “more studies are needed to examine sex differences before we start saying it only has an effect on language.”
‘Treat aggressively and right away’
The researchers were somewhat surprised by the study findings. Because there is a higher prevalence of CV conditions and risk factors in men, they presumed men would be more affected by these conditions, said Dr. Mielke.
“But that’s not what we saw; we saw the reverse. It was actually the women who were affected more by these cardiovascular risk factors and conditions,” she said.
As midlife is when women enter menopause, fluctuating estrogen levels may help explain the differential impact on cognition among women. But Dr. Mielke said she wants to “move beyond” just looking at hormones.
She pointed out there are a variety of psychosocial factors that may also contribute to an imbalance in the cognitive impact of CV conditions on women.
“Midlife is when many women are still taking care of their children at home, are also taking care of their adult parents, and may be undergoing more stress while continuing to do a job,” Dr. Miekle said.
Structural brain development and genetics may also contribute to the greater effect on cognition in women, the investigators note.
Dr. Mielke stressed that the current study only identifies associations. “The next steps are to understand what some of the underlying mechanisms for this are,” she said.
In the meantime, these new results suggest middle-aged women with high blood pressure, cholesterol, or glucose measures “should be treated aggressively and right away” said Dr. Mielke.
“For example, for women who are just starting to become hypertensive, clinicians should treat them right away and not watch and wait.”
Study limitations cited include that its sample was limited to Olmsted County, Minnesota – so results may not be generalized to other populations. Also, as researchers combined PVD and stroke into one group, larger sample sizes are needed, especially for stroke. Another limitation was the study did not have information on duration of all CV conditions or risk factors.
Helpful for tailoring interventions?
Commenting on the study, Glen R. Finney, MD, director, Memory and Cognition Program, Geisinger Health Clinic, Wilkes-Barre, Pennsylvania, said the results are important.
“The more we understand about risk factors for the development of Alzheimer’s disease and related dementias, the better we understand how we can reduce the risks,” said Dr. Finney, who was not involved with the research.
Awareness that CV conditions are major risk factors in midlife has been “definitely rising,” said Dr. Finney. “Many studies originally were looking at late life and are now looking more at earlier in the disease process, and I think that’s important.”
Understanding how sex, ethnicity, and other demographic variables affect risks can help to “tailor interventions” for individual patients, he said.
The study was supported by the National Institutes of Health, the GHR Foundation, and the Rochester Epidemiology Project. Dr. Mielke is a consultant for Biogen and Brain Protection Company and is on the editorial boards of Neurology and Alzheimer’s and Dementia. Dr. Finney has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular effects of breast cancer treatment vary based on weight, menopausal status
For example, certain chemotherapy drugs may confer higher risk in breast cancer survivors of normal weight, whereas they may lower stroke risk in those who are obese, according to Heather Greenlee, ND, PhD, a public health researcher and naturopathic physician with the Fred Hutchinson Cancer Research Center in Seattle.
In postmenopausal women with breast cancer, aromatase inhibitors may increase cardiovascular risk, while tamoxifen appears to reduce the risk of incident dyslipidemia, she said.
The findings are from separate analyses of data from studies presented during a poster discussion session at the symposium.
Breast cancer treatment and cardiovascular effects: The role of weight
In one analysis, Dr. Greenlee and colleagues examined outcomes in 13,582 breast cancer survivors with a median age of 60 years and median follow-up of 7 years to assess whether cardiovascular disease (CVD) risk associated with specific breast cancer therapies varies by body mass index (BMI) category at diagnosis.
Many routinely used breast cancer therapies are cardiotoxic, and being overweight or obese are known risk factors for CVD, but few studies have assessed whether BMI modifies the effect of these treatment on cardiovascular risk, Dr. Greenlee explained.
After adjusting for baseline demographic and health-related factors, and other breast cancer treatment, they found that:
- Ischemic heart disease risk was higher among normal-weight women who received anthracyclines, compared with those who did not (hazard ratio, 4.2). No other risk associations were observed for other breast cancer therapies and BMI groups.
- Heart failure/cardiomyopathy risk was higher among women with normal weight who received anthracyclines, cyclophosphamides, or left-sided radiation, compared with those who did not (HRs, 5.24, 3.27, and 2.05, respectively), and among overweight women who received anthracyclines, compared with those who did not (HR, 2.18). No risk associations were observed for women who received trastuzumab, taxanes, endocrine therapy, or radiation on any side, and no risk associations were observed for women who were obese.
- Stroke risk was higher in normal-weight women who received taxanes, cyclophosphamides, or left-sided radiation versus those who did not (HRs, 2.14, 2.35, and 1.31, respectively), and stroke risk was lower in obese women who received anthracyclines, taxanes, or cyclophosphamide, compared with those who did not (HRs, 0.32, 0.41, and 0.29, respectively). No risk associations were observed for trastuzumab, endocrine therapy, or radiation on any side, and no risk associations were observed for women who were overweight.
The lack of associations noted between treatments and heart failure risk among obese patients could be caused by the “obesity paradox” observed in prior obese populations, the investigators noted, adding that additional analyses are planned to “examine whether different dosage and duration of breast cancer therapy exposures across the BMI groups contributed to these risk associations.”
Breast cancer treatment and cardiometabolic effects: The role of menopausal status
In a separate analysis, Dr. Greenlee and colleagues looked at the association between endocrine therapies and cardiometabolic risk based on menopausal status.
Endocrine therapy is associated with CVD in breast cancer survivors and may be associated with developing cardiometabolic risk factors like diabetes, dyslipidemia, and hypertension, they noted, explaining that tamoxifen has mixed estrogenic and antiestrogenic activity, while aromatase inhibitors deplete endogenous estrogen.
Since most studies have compared tamoxifen with aromatase inhibitor use, it has been a challenge challenging to discern the effects of each, Dr. Greenlee said.
She and her colleagues reviewed records for 14,942 breast cancer survivors who were diagnosed between 2005 and 2013. The patients had a mean age of 61 years at baseline, and 24.9% were premenopausal at the time of diagnosis. Of the premenopausal women, 27.3% used tamoxifen, 19.2% used aromatase inhibitors, and 53.5% did not use endocrine therapy, and of the postmenopausal women, 6.6% used tamoxifen, 47.7% used aromatase inhibitors, and 45.7% did not use endocrine therapy.
After adjusting for baseline demographics and health factors, the investigators found that:
- The use of tamoxifen or aromatase inhibitors was not associated with a risk of developing diabetes, dyslipidemia, or hypertension in premenopausal women, or with a risk of developing diabetes or hypertension in postmenopausal women.
- The risk of dyslipidemia was higher in postmenopausal aromatase inhibitor users, and lower in postmenopausal tamoxifen users, compared with postmenopausal non-users of endocrine therapy (HRs, 1.15 and 0.75, respectively).
The lack of associations between endocrine therapy and CVD risk in premenopausal women may be from low power, Dr. Greenlee said, noting that analyses in larger sample sizes are needed.
She and her colleagues plan to conduct further analyses looking at treatment dosage and duration, and comparing steroidal versus nonsteroidal aromatase inhibitors.
Future studies should examine the implications of these associations on long-term CVD and how best to manage lipid profiles in postmenopausal breast cancer survivors who have a history of endocrine therapy treatment, they concluded.
This research was funded by grants from the National Cancer Institute.
For example, certain chemotherapy drugs may confer higher risk in breast cancer survivors of normal weight, whereas they may lower stroke risk in those who are obese, according to Heather Greenlee, ND, PhD, a public health researcher and naturopathic physician with the Fred Hutchinson Cancer Research Center in Seattle.
In postmenopausal women with breast cancer, aromatase inhibitors may increase cardiovascular risk, while tamoxifen appears to reduce the risk of incident dyslipidemia, she said.
The findings are from separate analyses of data from studies presented during a poster discussion session at the symposium.
Breast cancer treatment and cardiovascular effects: The role of weight
In one analysis, Dr. Greenlee and colleagues examined outcomes in 13,582 breast cancer survivors with a median age of 60 years and median follow-up of 7 years to assess whether cardiovascular disease (CVD) risk associated with specific breast cancer therapies varies by body mass index (BMI) category at diagnosis.
Many routinely used breast cancer therapies are cardiotoxic, and being overweight or obese are known risk factors for CVD, but few studies have assessed whether BMI modifies the effect of these treatment on cardiovascular risk, Dr. Greenlee explained.
After adjusting for baseline demographic and health-related factors, and other breast cancer treatment, they found that:
- Ischemic heart disease risk was higher among normal-weight women who received anthracyclines, compared with those who did not (hazard ratio, 4.2). No other risk associations were observed for other breast cancer therapies and BMI groups.
- Heart failure/cardiomyopathy risk was higher among women with normal weight who received anthracyclines, cyclophosphamides, or left-sided radiation, compared with those who did not (HRs, 5.24, 3.27, and 2.05, respectively), and among overweight women who received anthracyclines, compared with those who did not (HR, 2.18). No risk associations were observed for women who received trastuzumab, taxanes, endocrine therapy, or radiation on any side, and no risk associations were observed for women who were obese.
- Stroke risk was higher in normal-weight women who received taxanes, cyclophosphamides, or left-sided radiation versus those who did not (HRs, 2.14, 2.35, and 1.31, respectively), and stroke risk was lower in obese women who received anthracyclines, taxanes, or cyclophosphamide, compared with those who did not (HRs, 0.32, 0.41, and 0.29, respectively). No risk associations were observed for trastuzumab, endocrine therapy, or radiation on any side, and no risk associations were observed for women who were overweight.
The lack of associations noted between treatments and heart failure risk among obese patients could be caused by the “obesity paradox” observed in prior obese populations, the investigators noted, adding that additional analyses are planned to “examine whether different dosage and duration of breast cancer therapy exposures across the BMI groups contributed to these risk associations.”
Breast cancer treatment and cardiometabolic effects: The role of menopausal status
In a separate analysis, Dr. Greenlee and colleagues looked at the association between endocrine therapies and cardiometabolic risk based on menopausal status.
Endocrine therapy is associated with CVD in breast cancer survivors and may be associated with developing cardiometabolic risk factors like diabetes, dyslipidemia, and hypertension, they noted, explaining that tamoxifen has mixed estrogenic and antiestrogenic activity, while aromatase inhibitors deplete endogenous estrogen.
Since most studies have compared tamoxifen with aromatase inhibitor use, it has been a challenge challenging to discern the effects of each, Dr. Greenlee said.
She and her colleagues reviewed records for 14,942 breast cancer survivors who were diagnosed between 2005 and 2013. The patients had a mean age of 61 years at baseline, and 24.9% were premenopausal at the time of diagnosis. Of the premenopausal women, 27.3% used tamoxifen, 19.2% used aromatase inhibitors, and 53.5% did not use endocrine therapy, and of the postmenopausal women, 6.6% used tamoxifen, 47.7% used aromatase inhibitors, and 45.7% did not use endocrine therapy.
After adjusting for baseline demographics and health factors, the investigators found that:
- The use of tamoxifen or aromatase inhibitors was not associated with a risk of developing diabetes, dyslipidemia, or hypertension in premenopausal women, or with a risk of developing diabetes or hypertension in postmenopausal women.
- The risk of dyslipidemia was higher in postmenopausal aromatase inhibitor users, and lower in postmenopausal tamoxifen users, compared with postmenopausal non-users of endocrine therapy (HRs, 1.15 and 0.75, respectively).
The lack of associations between endocrine therapy and CVD risk in premenopausal women may be from low power, Dr. Greenlee said, noting that analyses in larger sample sizes are needed.
She and her colleagues plan to conduct further analyses looking at treatment dosage and duration, and comparing steroidal versus nonsteroidal aromatase inhibitors.
Future studies should examine the implications of these associations on long-term CVD and how best to manage lipid profiles in postmenopausal breast cancer survivors who have a history of endocrine therapy treatment, they concluded.
This research was funded by grants from the National Cancer Institute.
For example, certain chemotherapy drugs may confer higher risk in breast cancer survivors of normal weight, whereas they may lower stroke risk in those who are obese, according to Heather Greenlee, ND, PhD, a public health researcher and naturopathic physician with the Fred Hutchinson Cancer Research Center in Seattle.
In postmenopausal women with breast cancer, aromatase inhibitors may increase cardiovascular risk, while tamoxifen appears to reduce the risk of incident dyslipidemia, she said.
The findings are from separate analyses of data from studies presented during a poster discussion session at the symposium.
Breast cancer treatment and cardiovascular effects: The role of weight
In one analysis, Dr. Greenlee and colleagues examined outcomes in 13,582 breast cancer survivors with a median age of 60 years and median follow-up of 7 years to assess whether cardiovascular disease (CVD) risk associated with specific breast cancer therapies varies by body mass index (BMI) category at diagnosis.
Many routinely used breast cancer therapies are cardiotoxic, and being overweight or obese are known risk factors for CVD, but few studies have assessed whether BMI modifies the effect of these treatment on cardiovascular risk, Dr. Greenlee explained.
After adjusting for baseline demographic and health-related factors, and other breast cancer treatment, they found that:
- Ischemic heart disease risk was higher among normal-weight women who received anthracyclines, compared with those who did not (hazard ratio, 4.2). No other risk associations were observed for other breast cancer therapies and BMI groups.
- Heart failure/cardiomyopathy risk was higher among women with normal weight who received anthracyclines, cyclophosphamides, or left-sided radiation, compared with those who did not (HRs, 5.24, 3.27, and 2.05, respectively), and among overweight women who received anthracyclines, compared with those who did not (HR, 2.18). No risk associations were observed for women who received trastuzumab, taxanes, endocrine therapy, or radiation on any side, and no risk associations were observed for women who were obese.
- Stroke risk was higher in normal-weight women who received taxanes, cyclophosphamides, or left-sided radiation versus those who did not (HRs, 2.14, 2.35, and 1.31, respectively), and stroke risk was lower in obese women who received anthracyclines, taxanes, or cyclophosphamide, compared with those who did not (HRs, 0.32, 0.41, and 0.29, respectively). No risk associations were observed for trastuzumab, endocrine therapy, or radiation on any side, and no risk associations were observed for women who were overweight.
The lack of associations noted between treatments and heart failure risk among obese patients could be caused by the “obesity paradox” observed in prior obese populations, the investigators noted, adding that additional analyses are planned to “examine whether different dosage and duration of breast cancer therapy exposures across the BMI groups contributed to these risk associations.”
Breast cancer treatment and cardiometabolic effects: The role of menopausal status
In a separate analysis, Dr. Greenlee and colleagues looked at the association between endocrine therapies and cardiometabolic risk based on menopausal status.
Endocrine therapy is associated with CVD in breast cancer survivors and may be associated with developing cardiometabolic risk factors like diabetes, dyslipidemia, and hypertension, they noted, explaining that tamoxifen has mixed estrogenic and antiestrogenic activity, while aromatase inhibitors deplete endogenous estrogen.
Since most studies have compared tamoxifen with aromatase inhibitor use, it has been a challenge challenging to discern the effects of each, Dr. Greenlee said.
She and her colleagues reviewed records for 14,942 breast cancer survivors who were diagnosed between 2005 and 2013. The patients had a mean age of 61 years at baseline, and 24.9% were premenopausal at the time of diagnosis. Of the premenopausal women, 27.3% used tamoxifen, 19.2% used aromatase inhibitors, and 53.5% did not use endocrine therapy, and of the postmenopausal women, 6.6% used tamoxifen, 47.7% used aromatase inhibitors, and 45.7% did not use endocrine therapy.
After adjusting for baseline demographics and health factors, the investigators found that:
- The use of tamoxifen or aromatase inhibitors was not associated with a risk of developing diabetes, dyslipidemia, or hypertension in premenopausal women, or with a risk of developing diabetes or hypertension in postmenopausal women.
- The risk of dyslipidemia was higher in postmenopausal aromatase inhibitor users, and lower in postmenopausal tamoxifen users, compared with postmenopausal non-users of endocrine therapy (HRs, 1.15 and 0.75, respectively).
The lack of associations between endocrine therapy and CVD risk in premenopausal women may be from low power, Dr. Greenlee said, noting that analyses in larger sample sizes are needed.
She and her colleagues plan to conduct further analyses looking at treatment dosage and duration, and comparing steroidal versus nonsteroidal aromatase inhibitors.
Future studies should examine the implications of these associations on long-term CVD and how best to manage lipid profiles in postmenopausal breast cancer survivors who have a history of endocrine therapy treatment, they concluded.
This research was funded by grants from the National Cancer Institute.
FROM SABCS 2021
Premenopausal bilateral oophorectomy linked to later cognitive impairment
Women whose ovaries were surgically removed before the age of 46 had a higher risk of mild cognitive impairment (MCI) around 30 years later, compared with those who did not undergo bilateral oophorectomy, according to a population-based linkage study published in JAMA Network Open.
The findings suggest that “physicians treating women with premenopausal bilateral oophorectomy need to be aware of their patients’ risk of cognitive impairment or MCI and should consider implementing treatment-monitoring plans,” noted lead author Walter A. Rocca, MD, MPH, from the division of epidemiology, department of quantitative health sciences, at the Mayo Clinic, Rochester, Minn. and colleagues.
The results may particularly “help women at mean risk levels of ovarian cancer to better evaluate the risk-to-benefit ratio of undergoing bilateral oophorectomy prior to spontaneous menopause for the prevention of ovarian cancer,” they emphasized.
While the link between premenopausal bilateral oophorectomy and higher risk of cognitive impairment has been previously suggested, this new study “contributes valuable new data to a major public health importance issue and addresses a number of important shortcomings of existing literature,” Marios K. Georgakis, MD, PhD, and Eleni T. Petridou, MD, PhD, noted in an accompanying commentary.
“As bilateral oophorectomy is still a common procedure at least in well-resourced countries, the results of these studies should alert clinicians about its potential public health consequences. Given that the abrupt cessation of ovarian hormones might be accompanied by previously underestimated long-term adverse effects, treating physicians proposing the operation should weigh its benefits against potential long-term harmful effects, especially among women without an absolute indication,” noted Dr. Georgakis and Dr. Petridou, respectively from the Center for Genomic Medicine at Massachusetts General Hospital in Boston and the National and Kapodistrian University of Athens.
The case-control cross-sectional study used data from the Mayo Clinic Study of Aging (MCSA), a prospective, population-based study examining risk factors for, as well as prevalence and incidence of cognitive decline and MCI among a representative sample of women in Olmsted County, Minn. It included 2,732 women aged 50-89 years who participated in the MCSA study from 2004 to 2019 and underwent a clinical evaluation and comprehensive cognitive testing including nine tests covering four cognitive domains. Almost all of the subjects (98.4%) were White. The mean age of cognitive evaluation was 74 years – at which time 283 women (10.4%) were diagnosed with MCI (197 with amnestic and 86 with nonamnestic MCI). Data from the Rochester Epidemiology Project medical record–linkage system showed a total of 625 women (22.9%) had a history of bilateral oophorectomy. Among this group, 161 women underwent the procedure both before age 46, and before menopause, with 46 (28.6%) receiving oral conjugated equine estrogen (unopposed) and the remaining 95 (59.0%) receiving no estrogen therapy.
The study found that, compared with women who did not undergo bilateral oophorectomy, those who did so before age 46, but not after this age, had statistically significantly increased odds of MCI (adjusted odds ratio, 2.21; P < .001). When type of MCI was examined, the risk was statistically significant for nonamnestic MCI (aOR, 2.96; P < .001), and amnestic (aOR, 1.87; P =.03). The study also found no evidence that estrogen therapy was associated with decreased risk of MCI among women aged less than 46 years, with an aOR of 2.56 in those who received estrogen therapy and 2.05 in those who did not (P = .01 for both).
Finally, in women who had bilateral oophorectomy before menopause and before age 50, surgical indication for the procedure affected the association with MCI. Indications of either cancer or “no ovarian condition” (i.e., performed at the time of hysterectomy) were associated with no increased risk, whereas there was a statistically significantly increased risk associated with benign indications such as an adnexal mass, cyst or endometriosis (aOR, 2.43; P = .003). “This is important,” noted the commentators, “because in many of those cases removal of both ovaries could be avoided.”
The study also found that, compared with women who had not undergone bilateral oophorectomy, those who had also had increased frequency of cardiovascular risk factors, heart disease, and stroke at the time of their cognitive evaluation. “Additional research is needed to clarify the biological explanation of the association,” the investigators said.
The prevailing hypothesis for why premenopausal bilateral oophorectomy is associated with cognitive decline “is that the abrupt endocrine cessation of exposure to ovarian hormones accelerates the aging process,” the commentators noted. “Most important from a clinical perspective is whether these women would benefit from specific hormone replacement therapy schemes. Observational studies cannot reliably answer this question, and possibly it is time to rethink designing trials in specific groups of women who underwent bilateral oophorectomy before 46 years of age starting treatment immediately thereafter.”
In an interview Dr. Georgakis elaborated on this point, saying that, while the Women’s Health Study clearly showed no benefit of hormone replacement therapy for preventing dementia, it recruited women who were aged 65 years or older and had therefore undergone menopause more than 10-15 years earlier. “A hypothesis suggests that a critical vulnerability window exists shortly after menopause during which hormone replacement therapy might be needed to ameliorate any elevated risk,” he said. “Thus, it might make sense to reconsider a trial focused on this group of premenopausal women, who need to undergo oophorectomy at a young age (<46 years). Early initiation would be important. Unfortunately, such a trial would be difficult to conduct, because these women would need to be followed up for very long periods, as cognitive decline usually does not occur before the age of 65.”
Asked to comment on the study, Meadow Good, DO, an ob.gyn., female pelvic medicine and reconstructive surgeon, and physician adviser for Winnie Palmer Hospital for Women & Babies in Orlando, said this study adds credibility to previous studies showing the cognitive risk associated with premenopausal bilateral oophorectomy. “The literature is now pointing to a need to refrain from elective bilateral oophorectomy in women less than 60,” she said in an interview. “It should not be common that a women receives a bilateral oophorectomy before 60 for benign reasons.”
She added that cognition is not the only think at stake. “Bilateral oophorectomy before the age of 60 has a higher risk of incident heart disease, stroke, lung cancer and total cancers,” she said, citing a prospective cohort study within the Nurses’ Health Study.
Dr. Rocca reported financial support from the Mayo Clinic Research Committee during the conduct of the study. One coauthor reported unrestricted grants from Biogen and consulting fees from Brain Protection outside the submitted work. No other disclosures were reported from the authors. Dr. Georgakis, Dr. Petridou, and Dr. Good reported no conflicts of interest. The study was funded by the National Institute on Aging. It also used resources of the Rochester Epidemiology Project medical record–linkage system, which is supported by the NIA, the Mayo Clinic Research Committee, and user fees. Dr. Rocca was partly funded by the Ralph S. and Beverley E. Caulkins Professorship of Neurodegenerative Diseases Research of the Mayo Clinic.
Women whose ovaries were surgically removed before the age of 46 had a higher risk of mild cognitive impairment (MCI) around 30 years later, compared with those who did not undergo bilateral oophorectomy, according to a population-based linkage study published in JAMA Network Open.
The findings suggest that “physicians treating women with premenopausal bilateral oophorectomy need to be aware of their patients’ risk of cognitive impairment or MCI and should consider implementing treatment-monitoring plans,” noted lead author Walter A. Rocca, MD, MPH, from the division of epidemiology, department of quantitative health sciences, at the Mayo Clinic, Rochester, Minn. and colleagues.
The results may particularly “help women at mean risk levels of ovarian cancer to better evaluate the risk-to-benefit ratio of undergoing bilateral oophorectomy prior to spontaneous menopause for the prevention of ovarian cancer,” they emphasized.
While the link between premenopausal bilateral oophorectomy and higher risk of cognitive impairment has been previously suggested, this new study “contributes valuable new data to a major public health importance issue and addresses a number of important shortcomings of existing literature,” Marios K. Georgakis, MD, PhD, and Eleni T. Petridou, MD, PhD, noted in an accompanying commentary.
“As bilateral oophorectomy is still a common procedure at least in well-resourced countries, the results of these studies should alert clinicians about its potential public health consequences. Given that the abrupt cessation of ovarian hormones might be accompanied by previously underestimated long-term adverse effects, treating physicians proposing the operation should weigh its benefits against potential long-term harmful effects, especially among women without an absolute indication,” noted Dr. Georgakis and Dr. Petridou, respectively from the Center for Genomic Medicine at Massachusetts General Hospital in Boston and the National and Kapodistrian University of Athens.
The case-control cross-sectional study used data from the Mayo Clinic Study of Aging (MCSA), a prospective, population-based study examining risk factors for, as well as prevalence and incidence of cognitive decline and MCI among a representative sample of women in Olmsted County, Minn. It included 2,732 women aged 50-89 years who participated in the MCSA study from 2004 to 2019 and underwent a clinical evaluation and comprehensive cognitive testing including nine tests covering four cognitive domains. Almost all of the subjects (98.4%) were White. The mean age of cognitive evaluation was 74 years – at which time 283 women (10.4%) were diagnosed with MCI (197 with amnestic and 86 with nonamnestic MCI). Data from the Rochester Epidemiology Project medical record–linkage system showed a total of 625 women (22.9%) had a history of bilateral oophorectomy. Among this group, 161 women underwent the procedure both before age 46, and before menopause, with 46 (28.6%) receiving oral conjugated equine estrogen (unopposed) and the remaining 95 (59.0%) receiving no estrogen therapy.
The study found that, compared with women who did not undergo bilateral oophorectomy, those who did so before age 46, but not after this age, had statistically significantly increased odds of MCI (adjusted odds ratio, 2.21; P < .001). When type of MCI was examined, the risk was statistically significant for nonamnestic MCI (aOR, 2.96; P < .001), and amnestic (aOR, 1.87; P =.03). The study also found no evidence that estrogen therapy was associated with decreased risk of MCI among women aged less than 46 years, with an aOR of 2.56 in those who received estrogen therapy and 2.05 in those who did not (P = .01 for both).
Finally, in women who had bilateral oophorectomy before menopause and before age 50, surgical indication for the procedure affected the association with MCI. Indications of either cancer or “no ovarian condition” (i.e., performed at the time of hysterectomy) were associated with no increased risk, whereas there was a statistically significantly increased risk associated with benign indications such as an adnexal mass, cyst or endometriosis (aOR, 2.43; P = .003). “This is important,” noted the commentators, “because in many of those cases removal of both ovaries could be avoided.”
The study also found that, compared with women who had not undergone bilateral oophorectomy, those who had also had increased frequency of cardiovascular risk factors, heart disease, and stroke at the time of their cognitive evaluation. “Additional research is needed to clarify the biological explanation of the association,” the investigators said.
The prevailing hypothesis for why premenopausal bilateral oophorectomy is associated with cognitive decline “is that the abrupt endocrine cessation of exposure to ovarian hormones accelerates the aging process,” the commentators noted. “Most important from a clinical perspective is whether these women would benefit from specific hormone replacement therapy schemes. Observational studies cannot reliably answer this question, and possibly it is time to rethink designing trials in specific groups of women who underwent bilateral oophorectomy before 46 years of age starting treatment immediately thereafter.”
In an interview Dr. Georgakis elaborated on this point, saying that, while the Women’s Health Study clearly showed no benefit of hormone replacement therapy for preventing dementia, it recruited women who were aged 65 years or older and had therefore undergone menopause more than 10-15 years earlier. “A hypothesis suggests that a critical vulnerability window exists shortly after menopause during which hormone replacement therapy might be needed to ameliorate any elevated risk,” he said. “Thus, it might make sense to reconsider a trial focused on this group of premenopausal women, who need to undergo oophorectomy at a young age (<46 years). Early initiation would be important. Unfortunately, such a trial would be difficult to conduct, because these women would need to be followed up for very long periods, as cognitive decline usually does not occur before the age of 65.”
Asked to comment on the study, Meadow Good, DO, an ob.gyn., female pelvic medicine and reconstructive surgeon, and physician adviser for Winnie Palmer Hospital for Women & Babies in Orlando, said this study adds credibility to previous studies showing the cognitive risk associated with premenopausal bilateral oophorectomy. “The literature is now pointing to a need to refrain from elective bilateral oophorectomy in women less than 60,” she said in an interview. “It should not be common that a women receives a bilateral oophorectomy before 60 for benign reasons.”
She added that cognition is not the only think at stake. “Bilateral oophorectomy before the age of 60 has a higher risk of incident heart disease, stroke, lung cancer and total cancers,” she said, citing a prospective cohort study within the Nurses’ Health Study.
Dr. Rocca reported financial support from the Mayo Clinic Research Committee during the conduct of the study. One coauthor reported unrestricted grants from Biogen and consulting fees from Brain Protection outside the submitted work. No other disclosures were reported from the authors. Dr. Georgakis, Dr. Petridou, and Dr. Good reported no conflicts of interest. The study was funded by the National Institute on Aging. It also used resources of the Rochester Epidemiology Project medical record–linkage system, which is supported by the NIA, the Mayo Clinic Research Committee, and user fees. Dr. Rocca was partly funded by the Ralph S. and Beverley E. Caulkins Professorship of Neurodegenerative Diseases Research of the Mayo Clinic.
Women whose ovaries were surgically removed before the age of 46 had a higher risk of mild cognitive impairment (MCI) around 30 years later, compared with those who did not undergo bilateral oophorectomy, according to a population-based linkage study published in JAMA Network Open.
The findings suggest that “physicians treating women with premenopausal bilateral oophorectomy need to be aware of their patients’ risk of cognitive impairment or MCI and should consider implementing treatment-monitoring plans,” noted lead author Walter A. Rocca, MD, MPH, from the division of epidemiology, department of quantitative health sciences, at the Mayo Clinic, Rochester, Minn. and colleagues.
The results may particularly “help women at mean risk levels of ovarian cancer to better evaluate the risk-to-benefit ratio of undergoing bilateral oophorectomy prior to spontaneous menopause for the prevention of ovarian cancer,” they emphasized.
While the link between premenopausal bilateral oophorectomy and higher risk of cognitive impairment has been previously suggested, this new study “contributes valuable new data to a major public health importance issue and addresses a number of important shortcomings of existing literature,” Marios K. Georgakis, MD, PhD, and Eleni T. Petridou, MD, PhD, noted in an accompanying commentary.
“As bilateral oophorectomy is still a common procedure at least in well-resourced countries, the results of these studies should alert clinicians about its potential public health consequences. Given that the abrupt cessation of ovarian hormones might be accompanied by previously underestimated long-term adverse effects, treating physicians proposing the operation should weigh its benefits against potential long-term harmful effects, especially among women without an absolute indication,” noted Dr. Georgakis and Dr. Petridou, respectively from the Center for Genomic Medicine at Massachusetts General Hospital in Boston and the National and Kapodistrian University of Athens.
The case-control cross-sectional study used data from the Mayo Clinic Study of Aging (MCSA), a prospective, population-based study examining risk factors for, as well as prevalence and incidence of cognitive decline and MCI among a representative sample of women in Olmsted County, Minn. It included 2,732 women aged 50-89 years who participated in the MCSA study from 2004 to 2019 and underwent a clinical evaluation and comprehensive cognitive testing including nine tests covering four cognitive domains. Almost all of the subjects (98.4%) were White. The mean age of cognitive evaluation was 74 years – at which time 283 women (10.4%) were diagnosed with MCI (197 with amnestic and 86 with nonamnestic MCI). Data from the Rochester Epidemiology Project medical record–linkage system showed a total of 625 women (22.9%) had a history of bilateral oophorectomy. Among this group, 161 women underwent the procedure both before age 46, and before menopause, with 46 (28.6%) receiving oral conjugated equine estrogen (unopposed) and the remaining 95 (59.0%) receiving no estrogen therapy.
The study found that, compared with women who did not undergo bilateral oophorectomy, those who did so before age 46, but not after this age, had statistically significantly increased odds of MCI (adjusted odds ratio, 2.21; P < .001). When type of MCI was examined, the risk was statistically significant for nonamnestic MCI (aOR, 2.96; P < .001), and amnestic (aOR, 1.87; P =.03). The study also found no evidence that estrogen therapy was associated with decreased risk of MCI among women aged less than 46 years, with an aOR of 2.56 in those who received estrogen therapy and 2.05 in those who did not (P = .01 for both).
Finally, in women who had bilateral oophorectomy before menopause and before age 50, surgical indication for the procedure affected the association with MCI. Indications of either cancer or “no ovarian condition” (i.e., performed at the time of hysterectomy) were associated with no increased risk, whereas there was a statistically significantly increased risk associated with benign indications such as an adnexal mass, cyst or endometriosis (aOR, 2.43; P = .003). “This is important,” noted the commentators, “because in many of those cases removal of both ovaries could be avoided.”
The study also found that, compared with women who had not undergone bilateral oophorectomy, those who had also had increased frequency of cardiovascular risk factors, heart disease, and stroke at the time of their cognitive evaluation. “Additional research is needed to clarify the biological explanation of the association,” the investigators said.
The prevailing hypothesis for why premenopausal bilateral oophorectomy is associated with cognitive decline “is that the abrupt endocrine cessation of exposure to ovarian hormones accelerates the aging process,” the commentators noted. “Most important from a clinical perspective is whether these women would benefit from specific hormone replacement therapy schemes. Observational studies cannot reliably answer this question, and possibly it is time to rethink designing trials in specific groups of women who underwent bilateral oophorectomy before 46 years of age starting treatment immediately thereafter.”
In an interview Dr. Georgakis elaborated on this point, saying that, while the Women’s Health Study clearly showed no benefit of hormone replacement therapy for preventing dementia, it recruited women who were aged 65 years or older and had therefore undergone menopause more than 10-15 years earlier. “A hypothesis suggests that a critical vulnerability window exists shortly after menopause during which hormone replacement therapy might be needed to ameliorate any elevated risk,” he said. “Thus, it might make sense to reconsider a trial focused on this group of premenopausal women, who need to undergo oophorectomy at a young age (<46 years). Early initiation would be important. Unfortunately, such a trial would be difficult to conduct, because these women would need to be followed up for very long periods, as cognitive decline usually does not occur before the age of 65.”
Asked to comment on the study, Meadow Good, DO, an ob.gyn., female pelvic medicine and reconstructive surgeon, and physician adviser for Winnie Palmer Hospital for Women & Babies in Orlando, said this study adds credibility to previous studies showing the cognitive risk associated with premenopausal bilateral oophorectomy. “The literature is now pointing to a need to refrain from elective bilateral oophorectomy in women less than 60,” she said in an interview. “It should not be common that a women receives a bilateral oophorectomy before 60 for benign reasons.”
She added that cognition is not the only think at stake. “Bilateral oophorectomy before the age of 60 has a higher risk of incident heart disease, stroke, lung cancer and total cancers,” she said, citing a prospective cohort study within the Nurses’ Health Study.
Dr. Rocca reported financial support from the Mayo Clinic Research Committee during the conduct of the study. One coauthor reported unrestricted grants from Biogen and consulting fees from Brain Protection outside the submitted work. No other disclosures were reported from the authors. Dr. Georgakis, Dr. Petridou, and Dr. Good reported no conflicts of interest. The study was funded by the National Institute on Aging. It also used resources of the Rochester Epidemiology Project medical record–linkage system, which is supported by the NIA, the Mayo Clinic Research Committee, and user fees. Dr. Rocca was partly funded by the Ralph S. and Beverley E. Caulkins Professorship of Neurodegenerative Diseases Research of the Mayo Clinic.
FROM JAMA NETWORK OPEN
Is vaginal laser therapy more efficacious in improving vaginal menopausal symptoms compared with sham therapy?
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
New study ‘changes understanding of bone loss after menopause’
In the longest study of bone loss in postmenopausal women to date, on average, bone mineral density (BMD) at the femoral neck (the most common location for a hip fracture) had dropped by 10% in 25 years – less than expected based on shorter studies.
Specifically, average BMD loss at the femoral neck was 0.4% per year during 25 years in this new study from Finland, compared with a drop of 1.6% per year over 15 years reported in other cohorts.
Five-year BMD change appeared to predict long-term bone loss. However, certain women had faster bone loss, indicating that they should be followed more closely.
“Although the average bone loss was 10.1% ... there is a significant variation in the bone loss rate” among women in the study, senior author Joonas Sirola, MD, PhD, associate professor, University of Eastern Finland, and coauthor Heikki Kröger, MD, PhD, a professor at the same university, explained to this news organization in an email, so “women with fast bone loss should receive special attention.
The findings from the Kuopio Osteoporosis Risk Factor and Prevention study by Anna Moilanen and colleagues were published online October 19 in the Journal of Bone and Mineral Research.
Several factors might explain the lower than expected drop in femoral neck BMD (the site that is used to diagnose osteoporosis), Dr. Sirola and Dr. Kröger said. BMD depends on a person’s age, race, sex, and genes. And compared with other countries, people in Finland consume more dairy products, and more postmenopausal women there take hormone replacement therapy (HRT).
“If otherwise indicated, HRT seemed to effectively protect from bone loss,” the researchers noted.
Also, the number of women who smoked or used corticosteroids was low, so bone loss in other populations may be higher. Moreover, the women who completed the study may have been healthier to start with, so the results should be interpreted with caution, they urge.
Nevertheless, the study sheds light on long-term changes in BMD in postmenopausal women and “stresses the importance of high peak bone mass before menopause and keeping a healthy weight” during aging to protect bone health, they say.
Indeed the work “changes our understanding of bone loss in older women,” said Dr. Kröger in a press release from the university.
Check BMD every 5 years after menopause
Invited to comment, American Society of Bone and Mineral Research President Peter R. Ebeling, MD, who was not involved with the research, noted key findings are that the rate of femoral neck bone loss after perimenopause was far less than previously expected, and 5-year BMD change appeared to predict long-term bone loss in postmenopausal women.
“We know bone loss begins 1 year before menopause and accelerates over the next 5 years,” Dr. Ebeling, from Monash University, Melbourne, added in an email. “This study indicates some stabilization of bone loss thereafter with lesser effects of low estrogen levels on bone.”
“It probably means bone density does not need to be measured as frequently following the menopause transition and could be every 5 years, rather than every 2 years, if there was concern about continuing bone loss.”
Baseline risk factors and long-term changes in BMD
For the study, researchers examined the association between risk factors for bone loss and long-term changes in femoral neck BMD in 2,695 women living in Kuopio who were 47 to 56 years old in 1989. The women were a mean age of 53 years, and 62% were postmenopausal.
They answered questionnaires and had femoral neck BMD measured by DEXA every 5 years.
A total of 2,695, 2,583, 2,482, 2,135, 1,305, and 686 women were assessed at baseline and 5-, 10-, 15-, 20- and 25-year follow-ups, respectively, indicating significant study drop-out by 25 years.
By then, 17% of patients had died, 9% needed long-term care, some were unwilling to continue in the study, and others had factors that would have resulted in DEXA measurement errors (for example, hip implants, spine degeneration).
Researchers divided participants into quartiles of mean initial femoral neck BMD: 1.09 g/cm2, 0.97 g/cm2, 0.89 g/cm2, and 0.79 g/cm2, corresponding with quartiles 1 to 4 respectively (where quartile 1 had the highest initial femoral BMD and quartile 4 the lowest).
At 25 years, the mean femoral BMD had dropped to 0.97 g/cm2, 0.87 g/cm2, 0.80 g/cm2, and 0.73 g/cm2 in these respective quartiles.
Women lost 0.9%, 0.5%, 3.0%, and 1.0% of their initial BMD each year in quartiles 1 to 4, respectively.
And at 25 years, the women had lost 22.5%, 12.5%, 7.5%, and 2.5% of their initial BMD in the four quartiles, respectively.
Women in quartile 1 had the greatest drop in femoral BMD at 25 years, although their mean BMD at 25 years was higher than the mean initial BMD of the other women.
The prevalence of bone-affecting diseases, smoking, and use of vitamin D/calcium supplementation, corticosteroids, or alcohol was similar in the four quartiles and was not associated with significant differences in annual bone loss.
The most important protective factor was HRT
However, body mass index (BMI) and HRT were significantly different in the four quartiles.
On average, women in quartile 1 had a mean BMI of 26.7 kg/m2 at baseline and 27.8 kg/m2 at 25 years. Women in quartile 4 (lowest initial BMD and lowest drop in BMD) had a mean BMI of 24.9 kg/m2 at baseline and 28.4 kg/m2 at 25 years.
Women in quartile 4 (lowest initial BMD and lowest drop in BMD) were more likely to take HRT than women in quartile 1 (highest initial BMD and highest drop in BMD), at 41% versus 26%, respectively.
“The average decrease in bone mineral density was lower than has been assumed on the basis of earlier, shorter follow-ups where the bone loss rate at the femoral neck has been estimated to be even more than 20%,” Dr. Sirola commented in the press release.
“There were also surprisingly few risk factors affecting bone mineral density. The most significant factor protecting against bone loss was hormone replacement therapy. Weight gain during the follow-up also protected against bone loss,” Dr. Sirola added.
The study was funded by the Academy of Finland, Finnish Ministry of Education and Culture, and the Päivikki and Sakari Sohlberg Foundation. The authors and Dr. Ebeling have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In the longest study of bone loss in postmenopausal women to date, on average, bone mineral density (BMD) at the femoral neck (the most common location for a hip fracture) had dropped by 10% in 25 years – less than expected based on shorter studies.
Specifically, average BMD loss at the femoral neck was 0.4% per year during 25 years in this new study from Finland, compared with a drop of 1.6% per year over 15 years reported in other cohorts.
Five-year BMD change appeared to predict long-term bone loss. However, certain women had faster bone loss, indicating that they should be followed more closely.
“Although the average bone loss was 10.1% ... there is a significant variation in the bone loss rate” among women in the study, senior author Joonas Sirola, MD, PhD, associate professor, University of Eastern Finland, and coauthor Heikki Kröger, MD, PhD, a professor at the same university, explained to this news organization in an email, so “women with fast bone loss should receive special attention.
The findings from the Kuopio Osteoporosis Risk Factor and Prevention study by Anna Moilanen and colleagues were published online October 19 in the Journal of Bone and Mineral Research.
Several factors might explain the lower than expected drop in femoral neck BMD (the site that is used to diagnose osteoporosis), Dr. Sirola and Dr. Kröger said. BMD depends on a person’s age, race, sex, and genes. And compared with other countries, people in Finland consume more dairy products, and more postmenopausal women there take hormone replacement therapy (HRT).
“If otherwise indicated, HRT seemed to effectively protect from bone loss,” the researchers noted.
Also, the number of women who smoked or used corticosteroids was low, so bone loss in other populations may be higher. Moreover, the women who completed the study may have been healthier to start with, so the results should be interpreted with caution, they urge.
Nevertheless, the study sheds light on long-term changes in BMD in postmenopausal women and “stresses the importance of high peak bone mass before menopause and keeping a healthy weight” during aging to protect bone health, they say.
Indeed the work “changes our understanding of bone loss in older women,” said Dr. Kröger in a press release from the university.
Check BMD every 5 years after menopause
Invited to comment, American Society of Bone and Mineral Research President Peter R. Ebeling, MD, who was not involved with the research, noted key findings are that the rate of femoral neck bone loss after perimenopause was far less than previously expected, and 5-year BMD change appeared to predict long-term bone loss in postmenopausal women.
“We know bone loss begins 1 year before menopause and accelerates over the next 5 years,” Dr. Ebeling, from Monash University, Melbourne, added in an email. “This study indicates some stabilization of bone loss thereafter with lesser effects of low estrogen levels on bone.”
“It probably means bone density does not need to be measured as frequently following the menopause transition and could be every 5 years, rather than every 2 years, if there was concern about continuing bone loss.”
Baseline risk factors and long-term changes in BMD
For the study, researchers examined the association between risk factors for bone loss and long-term changes in femoral neck BMD in 2,695 women living in Kuopio who were 47 to 56 years old in 1989. The women were a mean age of 53 years, and 62% were postmenopausal.
They answered questionnaires and had femoral neck BMD measured by DEXA every 5 years.
A total of 2,695, 2,583, 2,482, 2,135, 1,305, and 686 women were assessed at baseline and 5-, 10-, 15-, 20- and 25-year follow-ups, respectively, indicating significant study drop-out by 25 years.
By then, 17% of patients had died, 9% needed long-term care, some were unwilling to continue in the study, and others had factors that would have resulted in DEXA measurement errors (for example, hip implants, spine degeneration).
Researchers divided participants into quartiles of mean initial femoral neck BMD: 1.09 g/cm2, 0.97 g/cm2, 0.89 g/cm2, and 0.79 g/cm2, corresponding with quartiles 1 to 4 respectively (where quartile 1 had the highest initial femoral BMD and quartile 4 the lowest).
At 25 years, the mean femoral BMD had dropped to 0.97 g/cm2, 0.87 g/cm2, 0.80 g/cm2, and 0.73 g/cm2 in these respective quartiles.
Women lost 0.9%, 0.5%, 3.0%, and 1.0% of their initial BMD each year in quartiles 1 to 4, respectively.
And at 25 years, the women had lost 22.5%, 12.5%, 7.5%, and 2.5% of their initial BMD in the four quartiles, respectively.
Women in quartile 1 had the greatest drop in femoral BMD at 25 years, although their mean BMD at 25 years was higher than the mean initial BMD of the other women.
The prevalence of bone-affecting diseases, smoking, and use of vitamin D/calcium supplementation, corticosteroids, or alcohol was similar in the four quartiles and was not associated with significant differences in annual bone loss.
The most important protective factor was HRT
However, body mass index (BMI) and HRT were significantly different in the four quartiles.
On average, women in quartile 1 had a mean BMI of 26.7 kg/m2 at baseline and 27.8 kg/m2 at 25 years. Women in quartile 4 (lowest initial BMD and lowest drop in BMD) had a mean BMI of 24.9 kg/m2 at baseline and 28.4 kg/m2 at 25 years.
Women in quartile 4 (lowest initial BMD and lowest drop in BMD) were more likely to take HRT than women in quartile 1 (highest initial BMD and highest drop in BMD), at 41% versus 26%, respectively.
“The average decrease in bone mineral density was lower than has been assumed on the basis of earlier, shorter follow-ups where the bone loss rate at the femoral neck has been estimated to be even more than 20%,” Dr. Sirola commented in the press release.
“There were also surprisingly few risk factors affecting bone mineral density. The most significant factor protecting against bone loss was hormone replacement therapy. Weight gain during the follow-up also protected against bone loss,” Dr. Sirola added.
The study was funded by the Academy of Finland, Finnish Ministry of Education and Culture, and the Päivikki and Sakari Sohlberg Foundation. The authors and Dr. Ebeling have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In the longest study of bone loss in postmenopausal women to date, on average, bone mineral density (BMD) at the femoral neck (the most common location for a hip fracture) had dropped by 10% in 25 years – less than expected based on shorter studies.
Specifically, average BMD loss at the femoral neck was 0.4% per year during 25 years in this new study from Finland, compared with a drop of 1.6% per year over 15 years reported in other cohorts.
Five-year BMD change appeared to predict long-term bone loss. However, certain women had faster bone loss, indicating that they should be followed more closely.
“Although the average bone loss was 10.1% ... there is a significant variation in the bone loss rate” among women in the study, senior author Joonas Sirola, MD, PhD, associate professor, University of Eastern Finland, and coauthor Heikki Kröger, MD, PhD, a professor at the same university, explained to this news organization in an email, so “women with fast bone loss should receive special attention.
The findings from the Kuopio Osteoporosis Risk Factor and Prevention study by Anna Moilanen and colleagues were published online October 19 in the Journal of Bone and Mineral Research.
Several factors might explain the lower than expected drop in femoral neck BMD (the site that is used to diagnose osteoporosis), Dr. Sirola and Dr. Kröger said. BMD depends on a person’s age, race, sex, and genes. And compared with other countries, people in Finland consume more dairy products, and more postmenopausal women there take hormone replacement therapy (HRT).
“If otherwise indicated, HRT seemed to effectively protect from bone loss,” the researchers noted.
Also, the number of women who smoked or used corticosteroids was low, so bone loss in other populations may be higher. Moreover, the women who completed the study may have been healthier to start with, so the results should be interpreted with caution, they urge.
Nevertheless, the study sheds light on long-term changes in BMD in postmenopausal women and “stresses the importance of high peak bone mass before menopause and keeping a healthy weight” during aging to protect bone health, they say.
Indeed the work “changes our understanding of bone loss in older women,” said Dr. Kröger in a press release from the university.
Check BMD every 5 years after menopause
Invited to comment, American Society of Bone and Mineral Research President Peter R. Ebeling, MD, who was not involved with the research, noted key findings are that the rate of femoral neck bone loss after perimenopause was far less than previously expected, and 5-year BMD change appeared to predict long-term bone loss in postmenopausal women.
“We know bone loss begins 1 year before menopause and accelerates over the next 5 years,” Dr. Ebeling, from Monash University, Melbourne, added in an email. “This study indicates some stabilization of bone loss thereafter with lesser effects of low estrogen levels on bone.”
“It probably means bone density does not need to be measured as frequently following the menopause transition and could be every 5 years, rather than every 2 years, if there was concern about continuing bone loss.”
Baseline risk factors and long-term changes in BMD
For the study, researchers examined the association between risk factors for bone loss and long-term changes in femoral neck BMD in 2,695 women living in Kuopio who were 47 to 56 years old in 1989. The women were a mean age of 53 years, and 62% were postmenopausal.
They answered questionnaires and had femoral neck BMD measured by DEXA every 5 years.
A total of 2,695, 2,583, 2,482, 2,135, 1,305, and 686 women were assessed at baseline and 5-, 10-, 15-, 20- and 25-year follow-ups, respectively, indicating significant study drop-out by 25 years.
By then, 17% of patients had died, 9% needed long-term care, some were unwilling to continue in the study, and others had factors that would have resulted in DEXA measurement errors (for example, hip implants, spine degeneration).
Researchers divided participants into quartiles of mean initial femoral neck BMD: 1.09 g/cm2, 0.97 g/cm2, 0.89 g/cm2, and 0.79 g/cm2, corresponding with quartiles 1 to 4 respectively (where quartile 1 had the highest initial femoral BMD and quartile 4 the lowest).
At 25 years, the mean femoral BMD had dropped to 0.97 g/cm2, 0.87 g/cm2, 0.80 g/cm2, and 0.73 g/cm2 in these respective quartiles.
Women lost 0.9%, 0.5%, 3.0%, and 1.0% of their initial BMD each year in quartiles 1 to 4, respectively.
And at 25 years, the women had lost 22.5%, 12.5%, 7.5%, and 2.5% of their initial BMD in the four quartiles, respectively.
Women in quartile 1 had the greatest drop in femoral BMD at 25 years, although their mean BMD at 25 years was higher than the mean initial BMD of the other women.
The prevalence of bone-affecting diseases, smoking, and use of vitamin D/calcium supplementation, corticosteroids, or alcohol was similar in the four quartiles and was not associated with significant differences in annual bone loss.
The most important protective factor was HRT
However, body mass index (BMI) and HRT were significantly different in the four quartiles.
On average, women in quartile 1 had a mean BMI of 26.7 kg/m2 at baseline and 27.8 kg/m2 at 25 years. Women in quartile 4 (lowest initial BMD and lowest drop in BMD) had a mean BMI of 24.9 kg/m2 at baseline and 28.4 kg/m2 at 25 years.
Women in quartile 4 (lowest initial BMD and lowest drop in BMD) were more likely to take HRT than women in quartile 1 (highest initial BMD and highest drop in BMD), at 41% versus 26%, respectively.
“The average decrease in bone mineral density was lower than has been assumed on the basis of earlier, shorter follow-ups where the bone loss rate at the femoral neck has been estimated to be even more than 20%,” Dr. Sirola commented in the press release.
“There were also surprisingly few risk factors affecting bone mineral density. The most significant factor protecting against bone loss was hormone replacement therapy. Weight gain during the follow-up also protected against bone loss,” Dr. Sirola added.
The study was funded by the Academy of Finland, Finnish Ministry of Education and Culture, and the Päivikki and Sakari Sohlberg Foundation. The authors and Dr. Ebeling have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.