User login
Male patients with breast cancer: Special considerations and gender-specific concerns
This transcript has been edited for clarity.
Fatima Cardoso, MD: Today we will be discussing breast cancer in male patients. To join me in this discussion, I have Sharon Giordano and Oliver Bogler. I will ask, to start, that we briefly introduce ourselves.
I’m Fatima Cardoso. I’m a medical oncologist based in Lisbon, Portugal. I have had a special interest in this topic for a couple of years. Sharon?
Sharon H. Giordano, MD, MPH, FASCO: I’m Sharon Giordano. I practice at the University of Texas MD Anderson Cancer Center. I’m also a medical oncologist and treat most of the male breast cancer patients that are seen here.
Oliver Bogler, PhD: I’m Oliver Bogler. I’m a cancer biologist by background and an 11-year survivor of breast cancer. Dr. Giordano was my oncologist during the active phase of my treatment. It’s great to be here with you.
Special considerations surrounding male patients
- Dr. Cardoso: Sharon, when you are treating breast cancer in a male patient, what specific considerations do you have?
- Dr. Giordano: As we all know, breast cancer in men is a rare disease. It makes up about 1 in 1,000 cases of breast cancer.
Often, what we need to do and what we end up doing is extrapolating as much as possible from clinical trials that were conducted in female patients with breast cancer. I think that’s one of the major challenges we face in treating the disease. There have been international efforts to try to put together standardized treatment approaches.
For example, ASCO has created guidelines for the management of male breast cancer. NCCN also has a special page on considerations for treatment of men with breast cancer. I would encourage people to look at those resources if questions do come up on the topic.
Dr. Cardoso: Perhaps we can also mention that the latest clinical trials fortunately have been allowing for male patients to be included, which is very important so that we can start having some data on the new drugs. I think that’s also relevant.
Dr. Giordano: That’s a great point because, historically, most of the trials explicitly excluded men. I don’t know if it was intentional or they just wrote the trials saying “women with breast cancer,” because that’s what most people thought of. I think it’s a great effort by the FDA and by investigators to make sure now that men are included in the trials. That will help build our evidence base.
Dr. Cardoso: Oliver, 11 years ago, you faced the diagnosis and you went through this. Can you speak a little bit about this challenge of going through what is considered a rare disease, but also a disease that is very much associated with the female gender traditionally?
Dr. Bogler: Gladly. For me, it was particularly odd because my wife, at the time that I was diagnosed, was a 5-year survivor of breast cancer. It took me some time to even think that the lump I felt might be the same disease. That seemed very unlikely, statistically, and also odd.
I have to say that I was protected from much of the fish-out-of-water experience that many men have because I both worked and was treated at MD Anderson, where Dr Giordano has a large practice, so my colleagues and my friends were not surprised that a man could get this disease.
Many of the patients I met had that experience, difficulty convincing their primary care physician or even their first-line oncologist that this could be the case. I just want to connect to what you both said, which is that 10 years ago, inclusion of men in clinical trials was not standard. It is a fantastic development to see that because unless we include men, we won’t learn about that type of breast cancer.
Dr. Cardoso: Even if only a few are entering each trial, at least it allows us to see if the drug behaves the same way or if there is any strange behavior of the drug in a male patient. It’s already one step forward. You were going to mention something, Sharon?
Dr. Giordano: I was going to say that, anecdotally, I’ve heard the experience that Oliver referred to, of many men feeling not so much uncomfortable with the diagnosis – although that does happen – but not having an obvious fit within the health care system.
For example, going to get their mammogram as part of their diagnostic workup and whoever might be taking them back saying, “Oh, no, this is Mrs. Jones, not Mr.,” and trying to argue with them that it’s not really meant for them. I had a patient – and this guy had a great sense of humor – who had a biopsy done and the instructions were to place this pink, floral ice pack inside your bra.
Even the materials that we have are gender specific. I think those things all together can certainly contribute to a man feeling like a fish out of water.
Dr. Cardoso: Actually, I fought in my institution because they wanted to call the Breast and Gynecology Unit the Women’s Unit. I said that there is no way you can call it the Women’s Unit because we have male patients. There are small things that we can do in our institutions to try to decrease the stigma and to make it less awkward for a man to be in a waiting room that says Women’s Clinic or something similar to that.
The importance of a support system
Dr. Cardoso: I wanted Oliver, perhaps, to mention experiences that you may have heard from other men. Some men do not feel that comfortable speaking about the disease. Also, some of them do not feel comfortable after treatment to go to the beach, to show the scar, and to show what happens after you have radiation.
Some men actually take it quite heavily, psychologically speaking. Have you encountered some of these men?
Dr. Bogler: Definitely. I think it leads to men not accessing the support opportunities – their family, their friends, or the support groups – and staying away from those because of this feeling of not wanting to share about it. That can be damaging. Cancer treatment is usually a tough road for most people, and the long-term consequences of hormone therapy – most men have hormone-driven disease – can be significant. I agree with you.
I participated in the male breast cancer SCAR Project by David Jay, a famous photographer. One of the high points of my life has been appearing in The New York Times topless, right after my radiation treatment, showing my scar. There are quite a few of us out there who’ve done that.
I’ll just mention in passing the Male Breast Cancer Global Alliance, which is a patient support supergroup, if you will. We’ve got a symposium coming up in November. That’s a great place for men who are early in the stage of their disease, or at any stage, to connect to others who are facing this issue.
Dr. Cardoso: They can also find specific information. This is a really good website where you can find information. One of the most important topics that I’ve heard from my patients is, “I never thought that I could have this disease. I never heard that men could have breast cancer as well.” Information is very crucial.
I believe that if you are well informed, you will also be less scared of the disease. Sources of reliable information are really crucial for patients. Since you mentioned the SCAR Project, we have a similar project here in Portugal that really called attention to the disease. It was very visual and really interesting.
Discussions during and after treatment
Dr. Cardoso: I wanted to say something, and I don’t know if both of you would agree. I think only recently surgeons have started to pay attention to the way they operate on men with breast cancer, and even in considering techniques of breast conservation and oncoplastic surgery. I had the feeling, looking at those photos, that some years ago, it wouldn’t have mattered how they do with the mastectomy scar just because it was a man. This was biased, right?
Just because it was a man, there was no need to pay attention to the aesthetic outcome. That is wrong, in my perspective. I’m very happy to see that now there are surgeons considering other types of breast surgery to conserve as much as possible the aesthetic outcome.
Dr. Bogler: I have to say that I was offered reconstruction at MD Anderson. I declined it. It wasn’t that big a part of my body image. When I raised this issue at home, my kids, who were quite young at the time, just suggested, “Well, Dad, why don’t you just wear a swim shirt?” They came up with a very practical solution for this issue.
I agree with you that it should be an option. I was also offered a nipple tattoo. I have yet to take that up, but maybe one day.
Dr. Cardoso: I’m not sure that we need to go into reconstruction. It also depends on whether a man has gynecomastia, if it’s going to be very asymmetric. There are other techniques to do, and depending on the size of the tumor, we can also do breast conservation, which we have done here in a couple of patients.
It’s quite an interesting approach where, for example, a skin-sparing mastectomy would be less aggressive, let’s say. Sharon?
Dr. Giordano: I completely agree. I’ve noticed increasing attention to the issue over the years that I’ve been in practice. I do think that it’s more front-and-center when the surgeons are having discussions with the patients now.
Also, although it’s still a minority, some do choose to have reconstructive surgery; some have more extensive surgeries, and some maybe have nipple reconstruction or a nipple tattoo. In a few men, like you mentioned, who are somewhat asymmetric, it actually can make a difference even when they’re dressed.
For many men, it’s more that they want to take off their shirt to play basketball or go swimming, and to decrease the feeling of awkwardness or like they have to make an explanation for why they have a nipple missing and a scar across their chest.
Biological aspects of male patients
Dr. Cardoso: Let’s switch gears now to the management, and before that, the biology. Oliver, with your other hat of biology, speak a little bit on what we know so far – whether it is exactly the same disease or there are biological specific characteristics of breast cancer in men.
Dr. Bogler: I should preface this by saying that I spent my career studying brain tumors. That was clearly a mistake.
Dr. Cardoso: It starts with a B. ...
Dr. Bogler: It starts with a B, but it’s the wrong part of the body. The reality is that we don’t really know that much fundamental biology yet, though the picture is changing and it has changed in recent years. Part of the reason is we don’t have many of the tools that we’ve had for the female disease for many years, particularly laboratory models.
On the genetic and transcriptomics front, there has been some really good activity. There was a comprehensive systematic review by Professor Val Speirs from the University of Aberdeen earlier this year that summarized much of the recent data. It showed that there are a handful of molecular hallmarks of the male disease, compared with the female disease, that are worth exploring.
Interestingly enough, one of them is the androgen receptor. It does beg the question of whether hormone-driven disease might not show up quite differently in males and females, where the hormone picture is a little different. I think there’s increasing evidence that there’s information out there to go after.
I will say that I was treated by Dr. Giordano and her colleagues very much like a woman would have been with my disease, and actually, very similarly to my wife. I’ve done well with it, so I would say, in most cases, the current standard of care is very effective but it falls a little short of personalized medicine, particularly when it comes to the hormone component.
Dr. Cardoso: Sharon?
Dr. Giordano: I would add that when I think about it as a clinician, although there’s a large amount of overlap and many similarities, when we’re treating men with breast cancer, almost all of the men have hormone receptor–positive disease, which I think Oliver mentioned earlier. We’re really thinking about endocrine therapy as one of the mainstays of treatment.
Obviously, as he also mentioned, it’s a different biologic background of hormones in a male vs. a female patient. There’s reason to think that some of those treatments could differ. In general, the subtypes are a little bit different. We see very, very few cases of triple-negative breast cancer in men. I think I’ve seen only one or two in my career. The ones I remember were probably radiation induced. They were cancer survivors who’d had chest-wall radiation for previous diseases. Those patients are very uncommon.
We also tend to see that the histology patterns are a little bit different. We tend to see more ductal cancers in men than we do in women as a relative proportion.
One thing that I always try to remember is that the risk for BRCA mutations or underlying germline genetic mutations is higher in men than in women. Just having a diagnosis of male breast cancer is an indication to consider genetic testing or meet with a genetic counselor to look for a BRCA1 or BRCA2 mutation.
Now, most men will not have that. Only roughly 10% of male patients, or maybe a little less, will have a BRCA2 mutation; for BRCA1, it’s more like only 1% or 2%. They’re not that common. Certainly, male breast cancer is recognized as being associated with the BRCA mutations.
Dr. Cardoso: If I have to give a take-home message in terms of biology, it would be that if there is a diagnosis of hormone receptor–negative or HER2-positive disease in a male patient, I would ask for a confirmation of the diagnosis. It’s not that it cannot exist, but it’s so rare that it’s worthwhile to confirm.
You mentioned that triple-negative disease is less than 1%, at about 0.5%, and HER2-positive disease is about 9%-10%. I think it will be very important to keep this in mind and confirm the biology if you have a different diagnosis than ER-positive, HER2-negative. Unfortunately, I received some cases where this was not done, and in fact, it ended up being a technical problem. People can receive the wrong treatment based on that.
Dr. Giordano: I’ve also seen that happen when it’s a metastasis to the breast rather than a primary breast cancer. I completely agree. That’s an excellent point.
Management approaches
Dr. Cardoso: Let’s go now to management and focus on early breast cancer first. Sharon, what are your main take-home messages for a professional who doesn’t see this very often? What does someone need to remember when they manage a male patient who has early breast cancer?
Dr. Giordano: In general, in terms of chemotherapy, we essentially use the same guidelines as we do for women. Most of the male patients will have tumors that are hormone receptor positive. For endocrine therapy, we typically rely on tamoxifen as the standard of care for adjuvant endocrine treatment for breast cancer.
There are some data suggesting that there can be some efficacy of aromatase inhibitors as single agents. In general, and extrapolated from some population-based registry data, the outcomes for men treated with single-agent aromatase inhibitors don’t tend to be as good as for those treated with tamoxifen.
I know that these are not randomized data so there are all the caveats of that, but the best information we have suggests that tamoxifen appears to likely be more effective. Typically, we stay with tamoxifen. If, for some reason, a man cannot tolerate tamoxifen or has a contraindication, then we could use a GnRH agonist along with an aromatase inhibitor.
Dr. Cardoso: I would like to mention that, because it’s ER-positive, HER2-negative disease most of the time, there will be the question as to whether we can use genomic tests. I think it is important that people know that we have much less data regarding the use of Oncotype DX, MammaPrint, or any of the genomic tests in male patients.
We have some data on the distribution of, for example, Oncotype DX or MammaPrint scores. Whether we can use these tests for the decision of chemotherapy, we don’t have much data on that. I’ve seen many people making exactly the same decisions as with female patients, but that’s not really based on very strong evidence.
Dr. Giordano: It’s hard to know what to do with that. There are prognostic data on Oncotype, so the higher-risk tumors do seem to have a worse outcome than the lower-score tumors. You’re right, though; I don’t think we have any predictive information to really show that the Oncotype DX score predicts benefit to chemotherapy.
Having said that, I will sometimes order the test in my practice. If somebody comes back with a score of 5 or a very low-risk score, I will use that in my decision-making.
Dr. Cardoso: There is something we didn’t exactly mention in the diagnosis that may be important. We discussed most men not knowing that they can have breast cancer, and Oliver, you mentioned that sometimes the first-line physicians can think that very often. Usually, we have late diagnosis and that means a higher tumor burden.
Sometimes we have to go to chemotherapy because of locally advanced or very positive axillas and not really because of the biology. That’s one of the reasons to go for chemotherapy in this setting, right?
Dr. Bogler: Yes. I remember that conversation with you, Dr. Giordano. I asked you whether I should do one of these tests. You said, “Don’t worry about it. At stage III, you’re going to have chemo anyway.”
Dr. Cardoso: The problem of these rare diagnoses is the not thinking about it, even from the health professional side, and then having the diagnosis quite late that will demand chemotherapy use.
To clarify to everybody, in terms of distinguishing luminal A–like, luminal B–like, and what that implies in a male patient, we really don’t know if it’s the same as in a female. There have been some very interesting studies from our Nordic country colleagues showing that maybe the subtyping is different. There is likely a male-specific subtype that does not exist in female breast cancer and that probably behaves differently. We still have a large amount of research to do to understand that.
Is there anything else you would like to mention about early breast cancer management?
Dr. Bogler: One of the things that’s probably underexplored is adherence to tamoxifen therapy in men. I do know anecdotally that this is the discussion among men because of the impact on quality of life. I do worry that sometimes men perhaps make the wrong choice, and I think that’s an opportunity for more research. Again, if there were alternative therapies that were perhaps a little less impactful on things like libido, that might be an advance in the field.
Dr. Cardoso: We have been seeing more studies on the issue of quality of life. Noncompliance is also an issue in female patients. We have to acknowledge that. Not everybody is able to keep taking the treatments. Interestingly, when there is a relapse and people had stopped taking the tamoxifen, most of them say, “I stopped because I had not understood exactly how important it is.”
We come back to the importance of explaining that it is the most crucial treatment for this subtype of breast cancer. Again, information is really key.
Sometimes I also use the argument with my patients that the alternative is even worse because if you use an aromatase inhibitor, and you have to use an LHRH agonist, then the implications for your sexual life are even worse. That’s how I try to convince them to stay on tamoxifen.
Let’s finalize with a couple of words on metastatic breast cancer in male patients. Sharon, I’ll start with you again. Is there any difference in the management if you have a patient with metastatic, ER-positive, HER2-negative disease? How do you treat? How do you sequence the available therapies? Is it different from the female patient?
Dr. Giordano: I’d say that, big picture, it’s quite similar. Again, most of the men have hormone receptor–positive disease, so really, the mainstay of treatment and the first treatments are going to be endocrine therapies. We’ll sequence through the endocrine therapies like we do in women. When using aromatase inhibitors, I typically would add a GnRH agonist to that, and I have had that be a very successful therapy, along now with the CDK inhibitors that are also approved.
I don’t think the studies of CDK inhibitors included male patients, but at least palbociclib actually was approved in the United States, based on some real-world evidence of its efficacy. Anecdotally, again, in my clinical practice, that tends to be a really powerful combination of leuprolide, an aromatase inhibitor, and a CDK inhibitor.
I think there’s less information about drugs like fulvestrant, whether that would benefit from combination with a GnRH agonist or whether those should be given as single agents. We just don’t really know. We have a few case series out there.
Similar to the early breast cancer setting, I think it’s really important to remember to check for BRCA1 and BRCA2 mutations. PARP inhibitors could be a part of the treatment plan if those underlying germline mutations are found. Generally, we’re following a similar sequence of endocrine therapies and then, eventually, chemotherapy.
Dr. Cardoso: Maybe, Oliver, you’re also seeing that one consistent finding in the biology study is the importance of the AKT/PI3K/mTOR pathway in male patients with breast cancer, because we now have at least two classes of agents to tackle this pathway. Again, anecdotally – we’re not talking about trials – I’ve been seeing quite interesting responses, for example, to everolimus combined with endocrine therapy.
We have a little less experience with the PI3K inhibitor, but that’s just because of accessibility to the drug. I think this combination is also something to keep in mind that can be quite effective in these patients.
Dr. Bogler: I agree. Those findings are exciting in the context of dealing with something as difficult as metastatic breast cancer. It’s good to know that there’s some information coming and opportunities and options, hopefully, down the road for men facing that problem.
Dr. Cardoso: Sharon, although small numbers, in these cases where there is HER2-positive disease, you would also use the new anti-HER2 agents and more or less the same sequence, right?
Dr. Giordano: Absolutely. It’s not particularly data driven, but yes, I would. If it’s a HER2-positive tumor, I would use the same HER2-targeted therapies that are used for women with breast cancer.
Working toward a balance in patient care
Dr. Cardoso: I would like to add something for all of us to be united in the fight. I don’t know if it happens in the U.S., but in many countries, access to these new agents for male patients is very difficult because of the approval and the labeling. This is why I’m always fighting with those who are proposing that the labeling, again, says “women with breast cancer.”
It is really important that we keep on lobbying and pushing for the labeling to say “patients with breast cancer” so that nobody can withhold access to these new therapies because of gender. In the U.S., maybe you don’t have this problem. There are many European countries where men cannot access, for example, fulvestrant because it has been approved for women with breast cancer.
Dr. Giordano: Thankfully, I have not faced that issue very often. I’ve had occasional issues with getting GnRH agonists approved. Generally, in the U.S., if I provide, for example, the NCCN guideline recommendations, most insurers will cover it. I think it’s often just lack of knowledge.
Dr. Cardoso: It’s something to keep working hard on because for the old drugs that were approved with the wording that still said “women,” we have to keep fighting for accessibility.
I think we had a really nice discussion. I’m going to give you an opportunity for any last words that you want to say on this topic. Perhaps we’ll start with you, Sharon, and we’ll leave the very last word to Oliver.
Dr. Giordano: I would just emphasize the importance of doing research in this area. Hopefully, we will be able to get clinical trials. There are reasons to think that endocrine therapies may behave differently in men and women. We need to continue to work together as a community to collect the data so that we can ultimately improve the outcomes for our patients.
Dr. Bogler: I would echo what you just said, Dr. Giordano. I would like to express my gratitude to both of you. Dr. Giordano, you have a huge practice of men at MD Anderson. You took care of me and many other people I know.
Dr. Cardoso, you are a pioneer of a big registry trial that I am privileged to be working on, trying to gather data on men. You’re both pioneers in this field of working on behalf of people like me. I’m just very grateful for what you do.
Dr. Giordano: Thank you.
Dr. Cardoso: Thank you both for accepting this invitation. We hope that everybody takes more interest in this field. Who knows? Maybe we can find enough funds to run a specific trial for male patients with breast cancer.
Dr. Cardoso is director of the breast unit at Champalimaud Clinical Centre/Champalimaud Foundation, Lisbon. Dr. Giordano is professor of breast medical oncology and chair of health services research at the University of Texas MD Anderson Cancer Center, Houston. Dr. Bogler is a cancer biologist at the Randolph (Vt.) Center. Dr. Cardoso reported conflicts of interest with numerous pharmaceutical companies; Dr. Giordano and Dr. Bogler reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Fatima Cardoso, MD: Today we will be discussing breast cancer in male patients. To join me in this discussion, I have Sharon Giordano and Oliver Bogler. I will ask, to start, that we briefly introduce ourselves.
I’m Fatima Cardoso. I’m a medical oncologist based in Lisbon, Portugal. I have had a special interest in this topic for a couple of years. Sharon?
Sharon H. Giordano, MD, MPH, FASCO: I’m Sharon Giordano. I practice at the University of Texas MD Anderson Cancer Center. I’m also a medical oncologist and treat most of the male breast cancer patients that are seen here.
Oliver Bogler, PhD: I’m Oliver Bogler. I’m a cancer biologist by background and an 11-year survivor of breast cancer. Dr. Giordano was my oncologist during the active phase of my treatment. It’s great to be here with you.
Special considerations surrounding male patients
- Dr. Cardoso: Sharon, when you are treating breast cancer in a male patient, what specific considerations do you have?
- Dr. Giordano: As we all know, breast cancer in men is a rare disease. It makes up about 1 in 1,000 cases of breast cancer.
Often, what we need to do and what we end up doing is extrapolating as much as possible from clinical trials that were conducted in female patients with breast cancer. I think that’s one of the major challenges we face in treating the disease. There have been international efforts to try to put together standardized treatment approaches.
For example, ASCO has created guidelines for the management of male breast cancer. NCCN also has a special page on considerations for treatment of men with breast cancer. I would encourage people to look at those resources if questions do come up on the topic.
Dr. Cardoso: Perhaps we can also mention that the latest clinical trials fortunately have been allowing for male patients to be included, which is very important so that we can start having some data on the new drugs. I think that’s also relevant.
Dr. Giordano: That’s a great point because, historically, most of the trials explicitly excluded men. I don’t know if it was intentional or they just wrote the trials saying “women with breast cancer,” because that’s what most people thought of. I think it’s a great effort by the FDA and by investigators to make sure now that men are included in the trials. That will help build our evidence base.
Dr. Cardoso: Oliver, 11 years ago, you faced the diagnosis and you went through this. Can you speak a little bit about this challenge of going through what is considered a rare disease, but also a disease that is very much associated with the female gender traditionally?
Dr. Bogler: Gladly. For me, it was particularly odd because my wife, at the time that I was diagnosed, was a 5-year survivor of breast cancer. It took me some time to even think that the lump I felt might be the same disease. That seemed very unlikely, statistically, and also odd.
I have to say that I was protected from much of the fish-out-of-water experience that many men have because I both worked and was treated at MD Anderson, where Dr Giordano has a large practice, so my colleagues and my friends were not surprised that a man could get this disease.
Many of the patients I met had that experience, difficulty convincing their primary care physician or even their first-line oncologist that this could be the case. I just want to connect to what you both said, which is that 10 years ago, inclusion of men in clinical trials was not standard. It is a fantastic development to see that because unless we include men, we won’t learn about that type of breast cancer.
Dr. Cardoso: Even if only a few are entering each trial, at least it allows us to see if the drug behaves the same way or if there is any strange behavior of the drug in a male patient. It’s already one step forward. You were going to mention something, Sharon?
Dr. Giordano: I was going to say that, anecdotally, I’ve heard the experience that Oliver referred to, of many men feeling not so much uncomfortable with the diagnosis – although that does happen – but not having an obvious fit within the health care system.
For example, going to get their mammogram as part of their diagnostic workup and whoever might be taking them back saying, “Oh, no, this is Mrs. Jones, not Mr.,” and trying to argue with them that it’s not really meant for them. I had a patient – and this guy had a great sense of humor – who had a biopsy done and the instructions were to place this pink, floral ice pack inside your bra.
Even the materials that we have are gender specific. I think those things all together can certainly contribute to a man feeling like a fish out of water.
Dr. Cardoso: Actually, I fought in my institution because they wanted to call the Breast and Gynecology Unit the Women’s Unit. I said that there is no way you can call it the Women’s Unit because we have male patients. There are small things that we can do in our institutions to try to decrease the stigma and to make it less awkward for a man to be in a waiting room that says Women’s Clinic or something similar to that.
The importance of a support system
Dr. Cardoso: I wanted Oliver, perhaps, to mention experiences that you may have heard from other men. Some men do not feel that comfortable speaking about the disease. Also, some of them do not feel comfortable after treatment to go to the beach, to show the scar, and to show what happens after you have radiation.
Some men actually take it quite heavily, psychologically speaking. Have you encountered some of these men?
Dr. Bogler: Definitely. I think it leads to men not accessing the support opportunities – their family, their friends, or the support groups – and staying away from those because of this feeling of not wanting to share about it. That can be damaging. Cancer treatment is usually a tough road for most people, and the long-term consequences of hormone therapy – most men have hormone-driven disease – can be significant. I agree with you.
I participated in the male breast cancer SCAR Project by David Jay, a famous photographer. One of the high points of my life has been appearing in The New York Times topless, right after my radiation treatment, showing my scar. There are quite a few of us out there who’ve done that.
I’ll just mention in passing the Male Breast Cancer Global Alliance, which is a patient support supergroup, if you will. We’ve got a symposium coming up in November. That’s a great place for men who are early in the stage of their disease, or at any stage, to connect to others who are facing this issue.
Dr. Cardoso: They can also find specific information. This is a really good website where you can find information. One of the most important topics that I’ve heard from my patients is, “I never thought that I could have this disease. I never heard that men could have breast cancer as well.” Information is very crucial.
I believe that if you are well informed, you will also be less scared of the disease. Sources of reliable information are really crucial for patients. Since you mentioned the SCAR Project, we have a similar project here in Portugal that really called attention to the disease. It was very visual and really interesting.
Discussions during and after treatment
Dr. Cardoso: I wanted to say something, and I don’t know if both of you would agree. I think only recently surgeons have started to pay attention to the way they operate on men with breast cancer, and even in considering techniques of breast conservation and oncoplastic surgery. I had the feeling, looking at those photos, that some years ago, it wouldn’t have mattered how they do with the mastectomy scar just because it was a man. This was biased, right?
Just because it was a man, there was no need to pay attention to the aesthetic outcome. That is wrong, in my perspective. I’m very happy to see that now there are surgeons considering other types of breast surgery to conserve as much as possible the aesthetic outcome.
Dr. Bogler: I have to say that I was offered reconstruction at MD Anderson. I declined it. It wasn’t that big a part of my body image. When I raised this issue at home, my kids, who were quite young at the time, just suggested, “Well, Dad, why don’t you just wear a swim shirt?” They came up with a very practical solution for this issue.
I agree with you that it should be an option. I was also offered a nipple tattoo. I have yet to take that up, but maybe one day.
Dr. Cardoso: I’m not sure that we need to go into reconstruction. It also depends on whether a man has gynecomastia, if it’s going to be very asymmetric. There are other techniques to do, and depending on the size of the tumor, we can also do breast conservation, which we have done here in a couple of patients.
It’s quite an interesting approach where, for example, a skin-sparing mastectomy would be less aggressive, let’s say. Sharon?
Dr. Giordano: I completely agree. I’ve noticed increasing attention to the issue over the years that I’ve been in practice. I do think that it’s more front-and-center when the surgeons are having discussions with the patients now.
Also, although it’s still a minority, some do choose to have reconstructive surgery; some have more extensive surgeries, and some maybe have nipple reconstruction or a nipple tattoo. In a few men, like you mentioned, who are somewhat asymmetric, it actually can make a difference even when they’re dressed.
For many men, it’s more that they want to take off their shirt to play basketball or go swimming, and to decrease the feeling of awkwardness or like they have to make an explanation for why they have a nipple missing and a scar across their chest.
Biological aspects of male patients
Dr. Cardoso: Let’s switch gears now to the management, and before that, the biology. Oliver, with your other hat of biology, speak a little bit on what we know so far – whether it is exactly the same disease or there are biological specific characteristics of breast cancer in men.
Dr. Bogler: I should preface this by saying that I spent my career studying brain tumors. That was clearly a mistake.
Dr. Cardoso: It starts with a B. ...
Dr. Bogler: It starts with a B, but it’s the wrong part of the body. The reality is that we don’t really know that much fundamental biology yet, though the picture is changing and it has changed in recent years. Part of the reason is we don’t have many of the tools that we’ve had for the female disease for many years, particularly laboratory models.
On the genetic and transcriptomics front, there has been some really good activity. There was a comprehensive systematic review by Professor Val Speirs from the University of Aberdeen earlier this year that summarized much of the recent data. It showed that there are a handful of molecular hallmarks of the male disease, compared with the female disease, that are worth exploring.
Interestingly enough, one of them is the androgen receptor. It does beg the question of whether hormone-driven disease might not show up quite differently in males and females, where the hormone picture is a little different. I think there’s increasing evidence that there’s information out there to go after.
I will say that I was treated by Dr. Giordano and her colleagues very much like a woman would have been with my disease, and actually, very similarly to my wife. I’ve done well with it, so I would say, in most cases, the current standard of care is very effective but it falls a little short of personalized medicine, particularly when it comes to the hormone component.
Dr. Cardoso: Sharon?
Dr. Giordano: I would add that when I think about it as a clinician, although there’s a large amount of overlap and many similarities, when we’re treating men with breast cancer, almost all of the men have hormone receptor–positive disease, which I think Oliver mentioned earlier. We’re really thinking about endocrine therapy as one of the mainstays of treatment.
Obviously, as he also mentioned, it’s a different biologic background of hormones in a male vs. a female patient. There’s reason to think that some of those treatments could differ. In general, the subtypes are a little bit different. We see very, very few cases of triple-negative breast cancer in men. I think I’ve seen only one or two in my career. The ones I remember were probably radiation induced. They were cancer survivors who’d had chest-wall radiation for previous diseases. Those patients are very uncommon.
We also tend to see that the histology patterns are a little bit different. We tend to see more ductal cancers in men than we do in women as a relative proportion.
One thing that I always try to remember is that the risk for BRCA mutations or underlying germline genetic mutations is higher in men than in women. Just having a diagnosis of male breast cancer is an indication to consider genetic testing or meet with a genetic counselor to look for a BRCA1 or BRCA2 mutation.
Now, most men will not have that. Only roughly 10% of male patients, or maybe a little less, will have a BRCA2 mutation; for BRCA1, it’s more like only 1% or 2%. They’re not that common. Certainly, male breast cancer is recognized as being associated with the BRCA mutations.
Dr. Cardoso: If I have to give a take-home message in terms of biology, it would be that if there is a diagnosis of hormone receptor–negative or HER2-positive disease in a male patient, I would ask for a confirmation of the diagnosis. It’s not that it cannot exist, but it’s so rare that it’s worthwhile to confirm.
You mentioned that triple-negative disease is less than 1%, at about 0.5%, and HER2-positive disease is about 9%-10%. I think it will be very important to keep this in mind and confirm the biology if you have a different diagnosis than ER-positive, HER2-negative. Unfortunately, I received some cases where this was not done, and in fact, it ended up being a technical problem. People can receive the wrong treatment based on that.
Dr. Giordano: I’ve also seen that happen when it’s a metastasis to the breast rather than a primary breast cancer. I completely agree. That’s an excellent point.
Management approaches
Dr. Cardoso: Let’s go now to management and focus on early breast cancer first. Sharon, what are your main take-home messages for a professional who doesn’t see this very often? What does someone need to remember when they manage a male patient who has early breast cancer?
Dr. Giordano: In general, in terms of chemotherapy, we essentially use the same guidelines as we do for women. Most of the male patients will have tumors that are hormone receptor positive. For endocrine therapy, we typically rely on tamoxifen as the standard of care for adjuvant endocrine treatment for breast cancer.
There are some data suggesting that there can be some efficacy of aromatase inhibitors as single agents. In general, and extrapolated from some population-based registry data, the outcomes for men treated with single-agent aromatase inhibitors don’t tend to be as good as for those treated with tamoxifen.
I know that these are not randomized data so there are all the caveats of that, but the best information we have suggests that tamoxifen appears to likely be more effective. Typically, we stay with tamoxifen. If, for some reason, a man cannot tolerate tamoxifen or has a contraindication, then we could use a GnRH agonist along with an aromatase inhibitor.
Dr. Cardoso: I would like to mention that, because it’s ER-positive, HER2-negative disease most of the time, there will be the question as to whether we can use genomic tests. I think it is important that people know that we have much less data regarding the use of Oncotype DX, MammaPrint, or any of the genomic tests in male patients.
We have some data on the distribution of, for example, Oncotype DX or MammaPrint scores. Whether we can use these tests for the decision of chemotherapy, we don’t have much data on that. I’ve seen many people making exactly the same decisions as with female patients, but that’s not really based on very strong evidence.
Dr. Giordano: It’s hard to know what to do with that. There are prognostic data on Oncotype, so the higher-risk tumors do seem to have a worse outcome than the lower-score tumors. You’re right, though; I don’t think we have any predictive information to really show that the Oncotype DX score predicts benefit to chemotherapy.
Having said that, I will sometimes order the test in my practice. If somebody comes back with a score of 5 or a very low-risk score, I will use that in my decision-making.
Dr. Cardoso: There is something we didn’t exactly mention in the diagnosis that may be important. We discussed most men not knowing that they can have breast cancer, and Oliver, you mentioned that sometimes the first-line physicians can think that very often. Usually, we have late diagnosis and that means a higher tumor burden.
Sometimes we have to go to chemotherapy because of locally advanced or very positive axillas and not really because of the biology. That’s one of the reasons to go for chemotherapy in this setting, right?
Dr. Bogler: Yes. I remember that conversation with you, Dr. Giordano. I asked you whether I should do one of these tests. You said, “Don’t worry about it. At stage III, you’re going to have chemo anyway.”
Dr. Cardoso: The problem of these rare diagnoses is the not thinking about it, even from the health professional side, and then having the diagnosis quite late that will demand chemotherapy use.
To clarify to everybody, in terms of distinguishing luminal A–like, luminal B–like, and what that implies in a male patient, we really don’t know if it’s the same as in a female. There have been some very interesting studies from our Nordic country colleagues showing that maybe the subtyping is different. There is likely a male-specific subtype that does not exist in female breast cancer and that probably behaves differently. We still have a large amount of research to do to understand that.
Is there anything else you would like to mention about early breast cancer management?
Dr. Bogler: One of the things that’s probably underexplored is adherence to tamoxifen therapy in men. I do know anecdotally that this is the discussion among men because of the impact on quality of life. I do worry that sometimes men perhaps make the wrong choice, and I think that’s an opportunity for more research. Again, if there were alternative therapies that were perhaps a little less impactful on things like libido, that might be an advance in the field.
Dr. Cardoso: We have been seeing more studies on the issue of quality of life. Noncompliance is also an issue in female patients. We have to acknowledge that. Not everybody is able to keep taking the treatments. Interestingly, when there is a relapse and people had stopped taking the tamoxifen, most of them say, “I stopped because I had not understood exactly how important it is.”
We come back to the importance of explaining that it is the most crucial treatment for this subtype of breast cancer. Again, information is really key.
Sometimes I also use the argument with my patients that the alternative is even worse because if you use an aromatase inhibitor, and you have to use an LHRH agonist, then the implications for your sexual life are even worse. That’s how I try to convince them to stay on tamoxifen.
Let’s finalize with a couple of words on metastatic breast cancer in male patients. Sharon, I’ll start with you again. Is there any difference in the management if you have a patient with metastatic, ER-positive, HER2-negative disease? How do you treat? How do you sequence the available therapies? Is it different from the female patient?
Dr. Giordano: I’d say that, big picture, it’s quite similar. Again, most of the men have hormone receptor–positive disease, so really, the mainstay of treatment and the first treatments are going to be endocrine therapies. We’ll sequence through the endocrine therapies like we do in women. When using aromatase inhibitors, I typically would add a GnRH agonist to that, and I have had that be a very successful therapy, along now with the CDK inhibitors that are also approved.
I don’t think the studies of CDK inhibitors included male patients, but at least palbociclib actually was approved in the United States, based on some real-world evidence of its efficacy. Anecdotally, again, in my clinical practice, that tends to be a really powerful combination of leuprolide, an aromatase inhibitor, and a CDK inhibitor.
I think there’s less information about drugs like fulvestrant, whether that would benefit from combination with a GnRH agonist or whether those should be given as single agents. We just don’t really know. We have a few case series out there.
Similar to the early breast cancer setting, I think it’s really important to remember to check for BRCA1 and BRCA2 mutations. PARP inhibitors could be a part of the treatment plan if those underlying germline mutations are found. Generally, we’re following a similar sequence of endocrine therapies and then, eventually, chemotherapy.
Dr. Cardoso: Maybe, Oliver, you’re also seeing that one consistent finding in the biology study is the importance of the AKT/PI3K/mTOR pathway in male patients with breast cancer, because we now have at least two classes of agents to tackle this pathway. Again, anecdotally – we’re not talking about trials – I’ve been seeing quite interesting responses, for example, to everolimus combined with endocrine therapy.
We have a little less experience with the PI3K inhibitor, but that’s just because of accessibility to the drug. I think this combination is also something to keep in mind that can be quite effective in these patients.
Dr. Bogler: I agree. Those findings are exciting in the context of dealing with something as difficult as metastatic breast cancer. It’s good to know that there’s some information coming and opportunities and options, hopefully, down the road for men facing that problem.
Dr. Cardoso: Sharon, although small numbers, in these cases where there is HER2-positive disease, you would also use the new anti-HER2 agents and more or less the same sequence, right?
Dr. Giordano: Absolutely. It’s not particularly data driven, but yes, I would. If it’s a HER2-positive tumor, I would use the same HER2-targeted therapies that are used for women with breast cancer.
Working toward a balance in patient care
Dr. Cardoso: I would like to add something for all of us to be united in the fight. I don’t know if it happens in the U.S., but in many countries, access to these new agents for male patients is very difficult because of the approval and the labeling. This is why I’m always fighting with those who are proposing that the labeling, again, says “women with breast cancer.”
It is really important that we keep on lobbying and pushing for the labeling to say “patients with breast cancer” so that nobody can withhold access to these new therapies because of gender. In the U.S., maybe you don’t have this problem. There are many European countries where men cannot access, for example, fulvestrant because it has been approved for women with breast cancer.
Dr. Giordano: Thankfully, I have not faced that issue very often. I’ve had occasional issues with getting GnRH agonists approved. Generally, in the U.S., if I provide, for example, the NCCN guideline recommendations, most insurers will cover it. I think it’s often just lack of knowledge.
Dr. Cardoso: It’s something to keep working hard on because for the old drugs that were approved with the wording that still said “women,” we have to keep fighting for accessibility.
I think we had a really nice discussion. I’m going to give you an opportunity for any last words that you want to say on this topic. Perhaps we’ll start with you, Sharon, and we’ll leave the very last word to Oliver.
Dr. Giordano: I would just emphasize the importance of doing research in this area. Hopefully, we will be able to get clinical trials. There are reasons to think that endocrine therapies may behave differently in men and women. We need to continue to work together as a community to collect the data so that we can ultimately improve the outcomes for our patients.
Dr. Bogler: I would echo what you just said, Dr. Giordano. I would like to express my gratitude to both of you. Dr. Giordano, you have a huge practice of men at MD Anderson. You took care of me and many other people I know.
Dr. Cardoso, you are a pioneer of a big registry trial that I am privileged to be working on, trying to gather data on men. You’re both pioneers in this field of working on behalf of people like me. I’m just very grateful for what you do.
Dr. Giordano: Thank you.
Dr. Cardoso: Thank you both for accepting this invitation. We hope that everybody takes more interest in this field. Who knows? Maybe we can find enough funds to run a specific trial for male patients with breast cancer.
Dr. Cardoso is director of the breast unit at Champalimaud Clinical Centre/Champalimaud Foundation, Lisbon. Dr. Giordano is professor of breast medical oncology and chair of health services research at the University of Texas MD Anderson Cancer Center, Houston. Dr. Bogler is a cancer biologist at the Randolph (Vt.) Center. Dr. Cardoso reported conflicts of interest with numerous pharmaceutical companies; Dr. Giordano and Dr. Bogler reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Fatima Cardoso, MD: Today we will be discussing breast cancer in male patients. To join me in this discussion, I have Sharon Giordano and Oliver Bogler. I will ask, to start, that we briefly introduce ourselves.
I’m Fatima Cardoso. I’m a medical oncologist based in Lisbon, Portugal. I have had a special interest in this topic for a couple of years. Sharon?
Sharon H. Giordano, MD, MPH, FASCO: I’m Sharon Giordano. I practice at the University of Texas MD Anderson Cancer Center. I’m also a medical oncologist and treat most of the male breast cancer patients that are seen here.
Oliver Bogler, PhD: I’m Oliver Bogler. I’m a cancer biologist by background and an 11-year survivor of breast cancer. Dr. Giordano was my oncologist during the active phase of my treatment. It’s great to be here with you.
Special considerations surrounding male patients
- Dr. Cardoso: Sharon, when you are treating breast cancer in a male patient, what specific considerations do you have?
- Dr. Giordano: As we all know, breast cancer in men is a rare disease. It makes up about 1 in 1,000 cases of breast cancer.
Often, what we need to do and what we end up doing is extrapolating as much as possible from clinical trials that were conducted in female patients with breast cancer. I think that’s one of the major challenges we face in treating the disease. There have been international efforts to try to put together standardized treatment approaches.
For example, ASCO has created guidelines for the management of male breast cancer. NCCN also has a special page on considerations for treatment of men with breast cancer. I would encourage people to look at those resources if questions do come up on the topic.
Dr. Cardoso: Perhaps we can also mention that the latest clinical trials fortunately have been allowing for male patients to be included, which is very important so that we can start having some data on the new drugs. I think that’s also relevant.
Dr. Giordano: That’s a great point because, historically, most of the trials explicitly excluded men. I don’t know if it was intentional or they just wrote the trials saying “women with breast cancer,” because that’s what most people thought of. I think it’s a great effort by the FDA and by investigators to make sure now that men are included in the trials. That will help build our evidence base.
Dr. Cardoso: Oliver, 11 years ago, you faced the diagnosis and you went through this. Can you speak a little bit about this challenge of going through what is considered a rare disease, but also a disease that is very much associated with the female gender traditionally?
Dr. Bogler: Gladly. For me, it was particularly odd because my wife, at the time that I was diagnosed, was a 5-year survivor of breast cancer. It took me some time to even think that the lump I felt might be the same disease. That seemed very unlikely, statistically, and also odd.
I have to say that I was protected from much of the fish-out-of-water experience that many men have because I both worked and was treated at MD Anderson, where Dr Giordano has a large practice, so my colleagues and my friends were not surprised that a man could get this disease.
Many of the patients I met had that experience, difficulty convincing their primary care physician or even their first-line oncologist that this could be the case. I just want to connect to what you both said, which is that 10 years ago, inclusion of men in clinical trials was not standard. It is a fantastic development to see that because unless we include men, we won’t learn about that type of breast cancer.
Dr. Cardoso: Even if only a few are entering each trial, at least it allows us to see if the drug behaves the same way or if there is any strange behavior of the drug in a male patient. It’s already one step forward. You were going to mention something, Sharon?
Dr. Giordano: I was going to say that, anecdotally, I’ve heard the experience that Oliver referred to, of many men feeling not so much uncomfortable with the diagnosis – although that does happen – but not having an obvious fit within the health care system.
For example, going to get their mammogram as part of their diagnostic workup and whoever might be taking them back saying, “Oh, no, this is Mrs. Jones, not Mr.,” and trying to argue with them that it’s not really meant for them. I had a patient – and this guy had a great sense of humor – who had a biopsy done and the instructions were to place this pink, floral ice pack inside your bra.
Even the materials that we have are gender specific. I think those things all together can certainly contribute to a man feeling like a fish out of water.
Dr. Cardoso: Actually, I fought in my institution because they wanted to call the Breast and Gynecology Unit the Women’s Unit. I said that there is no way you can call it the Women’s Unit because we have male patients. There are small things that we can do in our institutions to try to decrease the stigma and to make it less awkward for a man to be in a waiting room that says Women’s Clinic or something similar to that.
The importance of a support system
Dr. Cardoso: I wanted Oliver, perhaps, to mention experiences that you may have heard from other men. Some men do not feel that comfortable speaking about the disease. Also, some of them do not feel comfortable after treatment to go to the beach, to show the scar, and to show what happens after you have radiation.
Some men actually take it quite heavily, psychologically speaking. Have you encountered some of these men?
Dr. Bogler: Definitely. I think it leads to men not accessing the support opportunities – their family, their friends, or the support groups – and staying away from those because of this feeling of not wanting to share about it. That can be damaging. Cancer treatment is usually a tough road for most people, and the long-term consequences of hormone therapy – most men have hormone-driven disease – can be significant. I agree with you.
I participated in the male breast cancer SCAR Project by David Jay, a famous photographer. One of the high points of my life has been appearing in The New York Times topless, right after my radiation treatment, showing my scar. There are quite a few of us out there who’ve done that.
I’ll just mention in passing the Male Breast Cancer Global Alliance, which is a patient support supergroup, if you will. We’ve got a symposium coming up in November. That’s a great place for men who are early in the stage of their disease, or at any stage, to connect to others who are facing this issue.
Dr. Cardoso: They can also find specific information. This is a really good website where you can find information. One of the most important topics that I’ve heard from my patients is, “I never thought that I could have this disease. I never heard that men could have breast cancer as well.” Information is very crucial.
I believe that if you are well informed, you will also be less scared of the disease. Sources of reliable information are really crucial for patients. Since you mentioned the SCAR Project, we have a similar project here in Portugal that really called attention to the disease. It was very visual and really interesting.
Discussions during and after treatment
Dr. Cardoso: I wanted to say something, and I don’t know if both of you would agree. I think only recently surgeons have started to pay attention to the way they operate on men with breast cancer, and even in considering techniques of breast conservation and oncoplastic surgery. I had the feeling, looking at those photos, that some years ago, it wouldn’t have mattered how they do with the mastectomy scar just because it was a man. This was biased, right?
Just because it was a man, there was no need to pay attention to the aesthetic outcome. That is wrong, in my perspective. I’m very happy to see that now there are surgeons considering other types of breast surgery to conserve as much as possible the aesthetic outcome.
Dr. Bogler: I have to say that I was offered reconstruction at MD Anderson. I declined it. It wasn’t that big a part of my body image. When I raised this issue at home, my kids, who were quite young at the time, just suggested, “Well, Dad, why don’t you just wear a swim shirt?” They came up with a very practical solution for this issue.
I agree with you that it should be an option. I was also offered a nipple tattoo. I have yet to take that up, but maybe one day.
Dr. Cardoso: I’m not sure that we need to go into reconstruction. It also depends on whether a man has gynecomastia, if it’s going to be very asymmetric. There are other techniques to do, and depending on the size of the tumor, we can also do breast conservation, which we have done here in a couple of patients.
It’s quite an interesting approach where, for example, a skin-sparing mastectomy would be less aggressive, let’s say. Sharon?
Dr. Giordano: I completely agree. I’ve noticed increasing attention to the issue over the years that I’ve been in practice. I do think that it’s more front-and-center when the surgeons are having discussions with the patients now.
Also, although it’s still a minority, some do choose to have reconstructive surgery; some have more extensive surgeries, and some maybe have nipple reconstruction or a nipple tattoo. In a few men, like you mentioned, who are somewhat asymmetric, it actually can make a difference even when they’re dressed.
For many men, it’s more that they want to take off their shirt to play basketball or go swimming, and to decrease the feeling of awkwardness or like they have to make an explanation for why they have a nipple missing and a scar across their chest.
Biological aspects of male patients
Dr. Cardoso: Let’s switch gears now to the management, and before that, the biology. Oliver, with your other hat of biology, speak a little bit on what we know so far – whether it is exactly the same disease or there are biological specific characteristics of breast cancer in men.
Dr. Bogler: I should preface this by saying that I spent my career studying brain tumors. That was clearly a mistake.
Dr. Cardoso: It starts with a B. ...
Dr. Bogler: It starts with a B, but it’s the wrong part of the body. The reality is that we don’t really know that much fundamental biology yet, though the picture is changing and it has changed in recent years. Part of the reason is we don’t have many of the tools that we’ve had for the female disease for many years, particularly laboratory models.
On the genetic and transcriptomics front, there has been some really good activity. There was a comprehensive systematic review by Professor Val Speirs from the University of Aberdeen earlier this year that summarized much of the recent data. It showed that there are a handful of molecular hallmarks of the male disease, compared with the female disease, that are worth exploring.
Interestingly enough, one of them is the androgen receptor. It does beg the question of whether hormone-driven disease might not show up quite differently in males and females, where the hormone picture is a little different. I think there’s increasing evidence that there’s information out there to go after.
I will say that I was treated by Dr. Giordano and her colleagues very much like a woman would have been with my disease, and actually, very similarly to my wife. I’ve done well with it, so I would say, in most cases, the current standard of care is very effective but it falls a little short of personalized medicine, particularly when it comes to the hormone component.
Dr. Cardoso: Sharon?
Dr. Giordano: I would add that when I think about it as a clinician, although there’s a large amount of overlap and many similarities, when we’re treating men with breast cancer, almost all of the men have hormone receptor–positive disease, which I think Oliver mentioned earlier. We’re really thinking about endocrine therapy as one of the mainstays of treatment.
Obviously, as he also mentioned, it’s a different biologic background of hormones in a male vs. a female patient. There’s reason to think that some of those treatments could differ. In general, the subtypes are a little bit different. We see very, very few cases of triple-negative breast cancer in men. I think I’ve seen only one or two in my career. The ones I remember were probably radiation induced. They were cancer survivors who’d had chest-wall radiation for previous diseases. Those patients are very uncommon.
We also tend to see that the histology patterns are a little bit different. We tend to see more ductal cancers in men than we do in women as a relative proportion.
One thing that I always try to remember is that the risk for BRCA mutations or underlying germline genetic mutations is higher in men than in women. Just having a diagnosis of male breast cancer is an indication to consider genetic testing or meet with a genetic counselor to look for a BRCA1 or BRCA2 mutation.
Now, most men will not have that. Only roughly 10% of male patients, or maybe a little less, will have a BRCA2 mutation; for BRCA1, it’s more like only 1% or 2%. They’re not that common. Certainly, male breast cancer is recognized as being associated with the BRCA mutations.
Dr. Cardoso: If I have to give a take-home message in terms of biology, it would be that if there is a diagnosis of hormone receptor–negative or HER2-positive disease in a male patient, I would ask for a confirmation of the diagnosis. It’s not that it cannot exist, but it’s so rare that it’s worthwhile to confirm.
You mentioned that triple-negative disease is less than 1%, at about 0.5%, and HER2-positive disease is about 9%-10%. I think it will be very important to keep this in mind and confirm the biology if you have a different diagnosis than ER-positive, HER2-negative. Unfortunately, I received some cases where this was not done, and in fact, it ended up being a technical problem. People can receive the wrong treatment based on that.
Dr. Giordano: I’ve also seen that happen when it’s a metastasis to the breast rather than a primary breast cancer. I completely agree. That’s an excellent point.
Management approaches
Dr. Cardoso: Let’s go now to management and focus on early breast cancer first. Sharon, what are your main take-home messages for a professional who doesn’t see this very often? What does someone need to remember when they manage a male patient who has early breast cancer?
Dr. Giordano: In general, in terms of chemotherapy, we essentially use the same guidelines as we do for women. Most of the male patients will have tumors that are hormone receptor positive. For endocrine therapy, we typically rely on tamoxifen as the standard of care for adjuvant endocrine treatment for breast cancer.
There are some data suggesting that there can be some efficacy of aromatase inhibitors as single agents. In general, and extrapolated from some population-based registry data, the outcomes for men treated with single-agent aromatase inhibitors don’t tend to be as good as for those treated with tamoxifen.
I know that these are not randomized data so there are all the caveats of that, but the best information we have suggests that tamoxifen appears to likely be more effective. Typically, we stay with tamoxifen. If, for some reason, a man cannot tolerate tamoxifen or has a contraindication, then we could use a GnRH agonist along with an aromatase inhibitor.
Dr. Cardoso: I would like to mention that, because it’s ER-positive, HER2-negative disease most of the time, there will be the question as to whether we can use genomic tests. I think it is important that people know that we have much less data regarding the use of Oncotype DX, MammaPrint, or any of the genomic tests in male patients.
We have some data on the distribution of, for example, Oncotype DX or MammaPrint scores. Whether we can use these tests for the decision of chemotherapy, we don’t have much data on that. I’ve seen many people making exactly the same decisions as with female patients, but that’s not really based on very strong evidence.
Dr. Giordano: It’s hard to know what to do with that. There are prognostic data on Oncotype, so the higher-risk tumors do seem to have a worse outcome than the lower-score tumors. You’re right, though; I don’t think we have any predictive information to really show that the Oncotype DX score predicts benefit to chemotherapy.
Having said that, I will sometimes order the test in my practice. If somebody comes back with a score of 5 or a very low-risk score, I will use that in my decision-making.
Dr. Cardoso: There is something we didn’t exactly mention in the diagnosis that may be important. We discussed most men not knowing that they can have breast cancer, and Oliver, you mentioned that sometimes the first-line physicians can think that very often. Usually, we have late diagnosis and that means a higher tumor burden.
Sometimes we have to go to chemotherapy because of locally advanced or very positive axillas and not really because of the biology. That’s one of the reasons to go for chemotherapy in this setting, right?
Dr. Bogler: Yes. I remember that conversation with you, Dr. Giordano. I asked you whether I should do one of these tests. You said, “Don’t worry about it. At stage III, you’re going to have chemo anyway.”
Dr. Cardoso: The problem of these rare diagnoses is the not thinking about it, even from the health professional side, and then having the diagnosis quite late that will demand chemotherapy use.
To clarify to everybody, in terms of distinguishing luminal A–like, luminal B–like, and what that implies in a male patient, we really don’t know if it’s the same as in a female. There have been some very interesting studies from our Nordic country colleagues showing that maybe the subtyping is different. There is likely a male-specific subtype that does not exist in female breast cancer and that probably behaves differently. We still have a large amount of research to do to understand that.
Is there anything else you would like to mention about early breast cancer management?
Dr. Bogler: One of the things that’s probably underexplored is adherence to tamoxifen therapy in men. I do know anecdotally that this is the discussion among men because of the impact on quality of life. I do worry that sometimes men perhaps make the wrong choice, and I think that’s an opportunity for more research. Again, if there were alternative therapies that were perhaps a little less impactful on things like libido, that might be an advance in the field.
Dr. Cardoso: We have been seeing more studies on the issue of quality of life. Noncompliance is also an issue in female patients. We have to acknowledge that. Not everybody is able to keep taking the treatments. Interestingly, when there is a relapse and people had stopped taking the tamoxifen, most of them say, “I stopped because I had not understood exactly how important it is.”
We come back to the importance of explaining that it is the most crucial treatment for this subtype of breast cancer. Again, information is really key.
Sometimes I also use the argument with my patients that the alternative is even worse because if you use an aromatase inhibitor, and you have to use an LHRH agonist, then the implications for your sexual life are even worse. That’s how I try to convince them to stay on tamoxifen.
Let’s finalize with a couple of words on metastatic breast cancer in male patients. Sharon, I’ll start with you again. Is there any difference in the management if you have a patient with metastatic, ER-positive, HER2-negative disease? How do you treat? How do you sequence the available therapies? Is it different from the female patient?
Dr. Giordano: I’d say that, big picture, it’s quite similar. Again, most of the men have hormone receptor–positive disease, so really, the mainstay of treatment and the first treatments are going to be endocrine therapies. We’ll sequence through the endocrine therapies like we do in women. When using aromatase inhibitors, I typically would add a GnRH agonist to that, and I have had that be a very successful therapy, along now with the CDK inhibitors that are also approved.
I don’t think the studies of CDK inhibitors included male patients, but at least palbociclib actually was approved in the United States, based on some real-world evidence of its efficacy. Anecdotally, again, in my clinical practice, that tends to be a really powerful combination of leuprolide, an aromatase inhibitor, and a CDK inhibitor.
I think there’s less information about drugs like fulvestrant, whether that would benefit from combination with a GnRH agonist or whether those should be given as single agents. We just don’t really know. We have a few case series out there.
Similar to the early breast cancer setting, I think it’s really important to remember to check for BRCA1 and BRCA2 mutations. PARP inhibitors could be a part of the treatment plan if those underlying germline mutations are found. Generally, we’re following a similar sequence of endocrine therapies and then, eventually, chemotherapy.
Dr. Cardoso: Maybe, Oliver, you’re also seeing that one consistent finding in the biology study is the importance of the AKT/PI3K/mTOR pathway in male patients with breast cancer, because we now have at least two classes of agents to tackle this pathway. Again, anecdotally – we’re not talking about trials – I’ve been seeing quite interesting responses, for example, to everolimus combined with endocrine therapy.
We have a little less experience with the PI3K inhibitor, but that’s just because of accessibility to the drug. I think this combination is also something to keep in mind that can be quite effective in these patients.
Dr. Bogler: I agree. Those findings are exciting in the context of dealing with something as difficult as metastatic breast cancer. It’s good to know that there’s some information coming and opportunities and options, hopefully, down the road for men facing that problem.
Dr. Cardoso: Sharon, although small numbers, in these cases where there is HER2-positive disease, you would also use the new anti-HER2 agents and more or less the same sequence, right?
Dr. Giordano: Absolutely. It’s not particularly data driven, but yes, I would. If it’s a HER2-positive tumor, I would use the same HER2-targeted therapies that are used for women with breast cancer.
Working toward a balance in patient care
Dr. Cardoso: I would like to add something for all of us to be united in the fight. I don’t know if it happens in the U.S., but in many countries, access to these new agents for male patients is very difficult because of the approval and the labeling. This is why I’m always fighting with those who are proposing that the labeling, again, says “women with breast cancer.”
It is really important that we keep on lobbying and pushing for the labeling to say “patients with breast cancer” so that nobody can withhold access to these new therapies because of gender. In the U.S., maybe you don’t have this problem. There are many European countries where men cannot access, for example, fulvestrant because it has been approved for women with breast cancer.
Dr. Giordano: Thankfully, I have not faced that issue very often. I’ve had occasional issues with getting GnRH agonists approved. Generally, in the U.S., if I provide, for example, the NCCN guideline recommendations, most insurers will cover it. I think it’s often just lack of knowledge.
Dr. Cardoso: It’s something to keep working hard on because for the old drugs that were approved with the wording that still said “women,” we have to keep fighting for accessibility.
I think we had a really nice discussion. I’m going to give you an opportunity for any last words that you want to say on this topic. Perhaps we’ll start with you, Sharon, and we’ll leave the very last word to Oliver.
Dr. Giordano: I would just emphasize the importance of doing research in this area. Hopefully, we will be able to get clinical trials. There are reasons to think that endocrine therapies may behave differently in men and women. We need to continue to work together as a community to collect the data so that we can ultimately improve the outcomes for our patients.
Dr. Bogler: I would echo what you just said, Dr. Giordano. I would like to express my gratitude to both of you. Dr. Giordano, you have a huge practice of men at MD Anderson. You took care of me and many other people I know.
Dr. Cardoso, you are a pioneer of a big registry trial that I am privileged to be working on, trying to gather data on men. You’re both pioneers in this field of working on behalf of people like me. I’m just very grateful for what you do.
Dr. Giordano: Thank you.
Dr. Cardoso: Thank you both for accepting this invitation. We hope that everybody takes more interest in this field. Who knows? Maybe we can find enough funds to run a specific trial for male patients with breast cancer.
Dr. Cardoso is director of the breast unit at Champalimaud Clinical Centre/Champalimaud Foundation, Lisbon. Dr. Giordano is professor of breast medical oncology and chair of health services research at the University of Texas MD Anderson Cancer Center, Houston. Dr. Bogler is a cancer biologist at the Randolph (Vt.) Center. Dr. Cardoso reported conflicts of interest with numerous pharmaceutical companies; Dr. Giordano and Dr. Bogler reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Illicit steroids: If MDs don’t ask, patients won’t tell
Before he attended medical school, Thomas O’Connor, MD, had a not-very-well-kept secret: As a competitive powerlifter, he had used steroids to build strength.
Now an internist and clinical instructor of medicine at the University of Connecticut, Farmington, Dr. O’Connor’s practice focuses on the needs of men taking testosterone and other anabolic steroids – a group he feels is poorly understood and largely neglected by conventional medical care, perceptions borne out by a 2020 study of steroid users he helped conduct.
“They felt discriminated against, they did not feel comfortable working with their physicians, and they felt that the doctors did not know what they were doing,” Dr. O’Connor said in an interview. His patients often express anger and frustration with doctors they had seen previously.
Patients turning to home tests
Clients can order a panel of labs designed to screen for health conditions commonly associated with use of AAS, such as dyslipidemia, renal and hepatic dysfunction, polycythemia, thrombosis, and insulin resistance. The panels also include tests for levels of different hormones.
Sales of direct-to-consumer tests topped $3.6 billion in the United States in 2022 and are predicted to grow. Some of that spending is coming from people, mostly men, using illegally obtained steroids to build muscle. Although published data on the size of the bodybuilder market are unavailable, the Internet is a ready source of relatively inexpensive tests aimed at helping individuals monitor their health.
While clinicians may have their doubts about allowing patients to pick and choose tests and interpret their results, proponents claim they empower consumers to take control of their health – and save themselves money in the process.
But a test panel designed to help the user monitor the effects of banned substances is a bit unnerving to many clinicians, including Dr. O’Connor.
“People using anabolic steroids should be aware of the health risks associated with such use and that laboratory analysis is an important step toward improving health outcomes,” he said, “I’m all about open education, but not self-diagnosis and treatment.”
Testosterone and other AAS such as nandrolone, trenbolone, and boldenone are Schedule III controlled substances that have been banned by numerous athletic governing bodies. Yet recreational users can easily obtain them from online international pharmacies without a prescription. Should they be monitoring themselves for side effects?
A basic problem is that few primary care clinicians routinely ask their patients about the use of AAS or feel competent to manage the complications or withdrawal symptoms associated with the agents. And they may have no idea what the average AAS user looks like.
The American College of Sports Medicine updated its statement on the use of AAS in 2021. The statement warned of a growing new segment of users – up to 70% of people who take the drugs do so recreationally in pursuit of a more muscular appearance, rather than competitive athletes seeking enhanced performance.
The ACSM highlighted the syndrome of muscle dysmorphia, also known as “megarexia” or “bigorexia” (think of it as “reverse anorexia”), as a major risk factor for illicit use of AAS.
Stuart Phillips, PhD, professor and director of the department of kinesiology at McMaster University, Hamilton, Ont., coauthored the ACSM guidelines.
“The prince in Snow White circa 1950s was a guy with nice hair,” said Dr. Phillips, pointing to a change in cultural expectations for the male body. “But then fast forward to the prince or hero in any other Disney movie recently – and the guy is jacked.”
Since the last guidelines were published in 1987, Dr. Phillips has seen some cultural shifts. Testosterone has gone from a banned substance no one talked about to a mainstream medical therapy for men with low androgen levels, as any television viewer of primetime sports can attest. “But the other thing that’s changed,” he added, “is that we’ve seen the proliferation of illicit anabolic steroid use solely for the purpose of aesthetics.”
As an adolescent medicine physician who specializes in eating disorders, Jason Nagata, MD, MSc, sees many young men in his practice who engage in different behaviors to increase their muscle mass – from exercising, consuming high protein diets or taking protein supplements, even injecting AAS.
“A third of teenage boys across the U.S. report they’re trying to gain weight to bulk up and gain muscle,” said Dr. Nagata, an associate professor of pediatrics at the University of California, San Francisco.
In a 2020 study published in JAMA Pediatrics, Dr. Nagata and colleagues found that use of legal performance-enhancing substances in young men aged 18-26 years was associated with a higher odds of using AAS 7 years later (adjusted odds ratio, 3.18; 95% confidence interval, 1.90-5.32). “Some of the legal performance-enhancing substances like the protein powders or creatine may serve as a gateway to use of AAS,” Dr. Nagata said.
Another important factor is exposure to AAS use on social media, where muscular influencers gain huge followings. Dr. Nagata said most of the research on eating disorders and social media has examined the role of media on weight loss in girls.
“Although there’s less research on the social media impact on boys and men, a few studies have shown links between more Instagram use and muscle dissatisfaction, as well as thinking about using steroids,” he said.
The number of people using AAS is not trivial. In a longitudinal study (led by Nagata) of young U.S. adults surveyed multiple times between 1994 and 2002, a total of 2.7% of 18- to 26-year-old men and 0.4% of women reported using AAS. In a more recent cohort of adolescents in Minnesota aged 14-22 years followed between 2010 and 2018, a total of 2.2% of males and 1% of females initiated AAS use.
The Endocrine Society has estimated that between 2.9 and 4 million Americans have used an AAS at some point in their lives. Given that use is illegal without a prescription, a limitation of any survey is that participants may not be willing to disclose their AAS habit, leading to an underestimate of the actual number.
Nor are the complications of AAS use negligible. The drugs can have wide-ranging effects on the body, potentially affecting the brain, heart, liver, kidneys, musculoskeletal system, immune system, and reproductive systems. And individuals might unknowingly expose themselves to AAS: A recent literature review found that over a quarter of dietary supplements tested were found to contain undeclared substances that are on the World Anti-Doping Agency’s list of banned agents.
Alarming mistrust of MDs
Dr. O’Connor’s study shed some light on why AAS users might resort to surfing the Internet looking for a way to diagnose their own complications from steroid use. The web-based survey of nearly 2,400 men who said they took the drugs found that participants considered physicians to be the worst source of information, ranking them below coaches, online bodybuilding forums and sites, other AAS users, and bodybuilding books or magazines. The majority (56%) did not reveal AAS use to their clinicians. Of those who did, 55% reported feeling discriminated against for the admission.
Dr. O’Connor said physicians receive scant education on the many different drugs and regimens used by bodybuilders and have no idea how to a manage withdrawal syndrome for people trying to get off steroids. He urged the medical community to develop an educational campaign for clinicians, similar to those from public health officials aimed at combating the opioid epidemic: “Let’s educate med students and the residents. Let’s put [steroid use] on our agenda.”
Consumer testing evangelist ... or physician nemesis?
Nelson Vergel, BSChE, MBA, is on a mission to make medical lab testing affordable and accessible to everyone. The chemical engineer founded Discounted Labs 8 years ago, offering commonly ordered tests, such as complete blood counts, liver function tests, and cholesterol levels.
Mr. Vergel has advocated for the use of hormones to treat HIV-wasting disease for nearly 40 years, after his own diagnosis of the infection in 1986. After losing 40 pounds, steroids saved his life, he said.
Mr. Vergel said he was shocked to learn about the lack of continuing medical education on AAS for physicians and agreed with Dr. O’Connor that more training is needed for the medical profession. He also recognized that stigma on the part of clinicians is a huge barrier for many AAS users.
“We have to accept the fact that people are using them instead of demonizing them,” Mr. Vergel said. “What I was seeing is that there was so much stigma – and patients would not even talk to their doctors about their use.”
After reviewing Google analytics for his lab’s website and seeing how often “bodybuilder” came up as a search term, he added a panel of labs a year ago that allows AAS users to monitor themselves for adverse events.
Although he doesn’t condone the use of AAS without a medical indication and advises customers to discuss their results with a doctor, “we have to make sure people are reducing their harm or risk,” he said. “That’s really my goal.”
Many health care professionals would disagree with that statement. Dr. Nagata said he was concerned that management of side effects is too complicated. “There are a lot of nuances in the interpretation of these tests.” Arriving at the correct interpretation of the results requires a clinician’s thorough review of each patient’s health history, family history, and mental health history along with lab results.
‘I’m concerned’
In a second article outlining harm-reduction strategies designed to improve care for patients using AAS, Dr. O’Connor and colleagues outlined an approach for talking with patients who are concerned about their health and are seeking guidance from a clinician.
The first step is to work on developing a rapport, and not to demand that patients stop their use of AAS. His recommended opening line is: “I want to be honest with you – I’m concerned.”
The initial interaction is an opportunity to find out why the person uses AAS, what health concerns they have at present, and why they are seeking care. Open-ended questions may reveal concerns that the patient has about fertility or side effects.
Consistent with harm-reduction approaches used for other public health epidemics – such as opioid abuse and blood-borne pathogens among people who inject drugs – follow-up visits can include nonjudgmental discussions about decreasing or stopping their use.
Ultimately, minimizing the harms of AAS use can serve as a bridge to their cessation, but the medical community needs to build up trust with a community of users who currently rely more on each other and the Internet for guidance than their primary care physicians. “We need more education. We’re going to need resources to do it,” Dr. O’Connell said. “And we’re going to have to do it.”
Dr. Phillips and Dr. Nagata have no financial disclosures. Mr. Vergel is the owner and founder of Discounted Labs but reported no other financial conflicts. Dr. O’Connor owns Anabolic Doc but has no additional financial disclosures.
A version of this article first appeared on Medscape.com.
Before he attended medical school, Thomas O’Connor, MD, had a not-very-well-kept secret: As a competitive powerlifter, he had used steroids to build strength.
Now an internist and clinical instructor of medicine at the University of Connecticut, Farmington, Dr. O’Connor’s practice focuses on the needs of men taking testosterone and other anabolic steroids – a group he feels is poorly understood and largely neglected by conventional medical care, perceptions borne out by a 2020 study of steroid users he helped conduct.
“They felt discriminated against, they did not feel comfortable working with their physicians, and they felt that the doctors did not know what they were doing,” Dr. O’Connor said in an interview. His patients often express anger and frustration with doctors they had seen previously.
Patients turning to home tests
Clients can order a panel of labs designed to screen for health conditions commonly associated with use of AAS, such as dyslipidemia, renal and hepatic dysfunction, polycythemia, thrombosis, and insulin resistance. The panels also include tests for levels of different hormones.
Sales of direct-to-consumer tests topped $3.6 billion in the United States in 2022 and are predicted to grow. Some of that spending is coming from people, mostly men, using illegally obtained steroids to build muscle. Although published data on the size of the bodybuilder market are unavailable, the Internet is a ready source of relatively inexpensive tests aimed at helping individuals monitor their health.
While clinicians may have their doubts about allowing patients to pick and choose tests and interpret their results, proponents claim they empower consumers to take control of their health – and save themselves money in the process.
But a test panel designed to help the user monitor the effects of banned substances is a bit unnerving to many clinicians, including Dr. O’Connor.
“People using anabolic steroids should be aware of the health risks associated with such use and that laboratory analysis is an important step toward improving health outcomes,” he said, “I’m all about open education, but not self-diagnosis and treatment.”
Testosterone and other AAS such as nandrolone, trenbolone, and boldenone are Schedule III controlled substances that have been banned by numerous athletic governing bodies. Yet recreational users can easily obtain them from online international pharmacies without a prescription. Should they be monitoring themselves for side effects?
A basic problem is that few primary care clinicians routinely ask their patients about the use of AAS or feel competent to manage the complications or withdrawal symptoms associated with the agents. And they may have no idea what the average AAS user looks like.
The American College of Sports Medicine updated its statement on the use of AAS in 2021. The statement warned of a growing new segment of users – up to 70% of people who take the drugs do so recreationally in pursuit of a more muscular appearance, rather than competitive athletes seeking enhanced performance.
The ACSM highlighted the syndrome of muscle dysmorphia, also known as “megarexia” or “bigorexia” (think of it as “reverse anorexia”), as a major risk factor for illicit use of AAS.
Stuart Phillips, PhD, professor and director of the department of kinesiology at McMaster University, Hamilton, Ont., coauthored the ACSM guidelines.
“The prince in Snow White circa 1950s was a guy with nice hair,” said Dr. Phillips, pointing to a change in cultural expectations for the male body. “But then fast forward to the prince or hero in any other Disney movie recently – and the guy is jacked.”
Since the last guidelines were published in 1987, Dr. Phillips has seen some cultural shifts. Testosterone has gone from a banned substance no one talked about to a mainstream medical therapy for men with low androgen levels, as any television viewer of primetime sports can attest. “But the other thing that’s changed,” he added, “is that we’ve seen the proliferation of illicit anabolic steroid use solely for the purpose of aesthetics.”
As an adolescent medicine physician who specializes in eating disorders, Jason Nagata, MD, MSc, sees many young men in his practice who engage in different behaviors to increase their muscle mass – from exercising, consuming high protein diets or taking protein supplements, even injecting AAS.
“A third of teenage boys across the U.S. report they’re trying to gain weight to bulk up and gain muscle,” said Dr. Nagata, an associate professor of pediatrics at the University of California, San Francisco.
In a 2020 study published in JAMA Pediatrics, Dr. Nagata and colleagues found that use of legal performance-enhancing substances in young men aged 18-26 years was associated with a higher odds of using AAS 7 years later (adjusted odds ratio, 3.18; 95% confidence interval, 1.90-5.32). “Some of the legal performance-enhancing substances like the protein powders or creatine may serve as a gateway to use of AAS,” Dr. Nagata said.
Another important factor is exposure to AAS use on social media, where muscular influencers gain huge followings. Dr. Nagata said most of the research on eating disorders and social media has examined the role of media on weight loss in girls.
“Although there’s less research on the social media impact on boys and men, a few studies have shown links between more Instagram use and muscle dissatisfaction, as well as thinking about using steroids,” he said.
The number of people using AAS is not trivial. In a longitudinal study (led by Nagata) of young U.S. adults surveyed multiple times between 1994 and 2002, a total of 2.7% of 18- to 26-year-old men and 0.4% of women reported using AAS. In a more recent cohort of adolescents in Minnesota aged 14-22 years followed between 2010 and 2018, a total of 2.2% of males and 1% of females initiated AAS use.
The Endocrine Society has estimated that between 2.9 and 4 million Americans have used an AAS at some point in their lives. Given that use is illegal without a prescription, a limitation of any survey is that participants may not be willing to disclose their AAS habit, leading to an underestimate of the actual number.
Nor are the complications of AAS use negligible. The drugs can have wide-ranging effects on the body, potentially affecting the brain, heart, liver, kidneys, musculoskeletal system, immune system, and reproductive systems. And individuals might unknowingly expose themselves to AAS: A recent literature review found that over a quarter of dietary supplements tested were found to contain undeclared substances that are on the World Anti-Doping Agency’s list of banned agents.
Alarming mistrust of MDs
Dr. O’Connor’s study shed some light on why AAS users might resort to surfing the Internet looking for a way to diagnose their own complications from steroid use. The web-based survey of nearly 2,400 men who said they took the drugs found that participants considered physicians to be the worst source of information, ranking them below coaches, online bodybuilding forums and sites, other AAS users, and bodybuilding books or magazines. The majority (56%) did not reveal AAS use to their clinicians. Of those who did, 55% reported feeling discriminated against for the admission.
Dr. O’Connor said physicians receive scant education on the many different drugs and regimens used by bodybuilders and have no idea how to a manage withdrawal syndrome for people trying to get off steroids. He urged the medical community to develop an educational campaign for clinicians, similar to those from public health officials aimed at combating the opioid epidemic: “Let’s educate med students and the residents. Let’s put [steroid use] on our agenda.”
Consumer testing evangelist ... or physician nemesis?
Nelson Vergel, BSChE, MBA, is on a mission to make medical lab testing affordable and accessible to everyone. The chemical engineer founded Discounted Labs 8 years ago, offering commonly ordered tests, such as complete blood counts, liver function tests, and cholesterol levels.
Mr. Vergel has advocated for the use of hormones to treat HIV-wasting disease for nearly 40 years, after his own diagnosis of the infection in 1986. After losing 40 pounds, steroids saved his life, he said.
Mr. Vergel said he was shocked to learn about the lack of continuing medical education on AAS for physicians and agreed with Dr. O’Connor that more training is needed for the medical profession. He also recognized that stigma on the part of clinicians is a huge barrier for many AAS users.
“We have to accept the fact that people are using them instead of demonizing them,” Mr. Vergel said. “What I was seeing is that there was so much stigma – and patients would not even talk to their doctors about their use.”
After reviewing Google analytics for his lab’s website and seeing how often “bodybuilder” came up as a search term, he added a panel of labs a year ago that allows AAS users to monitor themselves for adverse events.
Although he doesn’t condone the use of AAS without a medical indication and advises customers to discuss their results with a doctor, “we have to make sure people are reducing their harm or risk,” he said. “That’s really my goal.”
Many health care professionals would disagree with that statement. Dr. Nagata said he was concerned that management of side effects is too complicated. “There are a lot of nuances in the interpretation of these tests.” Arriving at the correct interpretation of the results requires a clinician’s thorough review of each patient’s health history, family history, and mental health history along with lab results.
‘I’m concerned’
In a second article outlining harm-reduction strategies designed to improve care for patients using AAS, Dr. O’Connor and colleagues outlined an approach for talking with patients who are concerned about their health and are seeking guidance from a clinician.
The first step is to work on developing a rapport, and not to demand that patients stop their use of AAS. His recommended opening line is: “I want to be honest with you – I’m concerned.”
The initial interaction is an opportunity to find out why the person uses AAS, what health concerns they have at present, and why they are seeking care. Open-ended questions may reveal concerns that the patient has about fertility or side effects.
Consistent with harm-reduction approaches used for other public health epidemics – such as opioid abuse and blood-borne pathogens among people who inject drugs – follow-up visits can include nonjudgmental discussions about decreasing or stopping their use.
Ultimately, minimizing the harms of AAS use can serve as a bridge to their cessation, but the medical community needs to build up trust with a community of users who currently rely more on each other and the Internet for guidance than their primary care physicians. “We need more education. We’re going to need resources to do it,” Dr. O’Connell said. “And we’re going to have to do it.”
Dr. Phillips and Dr. Nagata have no financial disclosures. Mr. Vergel is the owner and founder of Discounted Labs but reported no other financial conflicts. Dr. O’Connor owns Anabolic Doc but has no additional financial disclosures.
A version of this article first appeared on Medscape.com.
Before he attended medical school, Thomas O’Connor, MD, had a not-very-well-kept secret: As a competitive powerlifter, he had used steroids to build strength.
Now an internist and clinical instructor of medicine at the University of Connecticut, Farmington, Dr. O’Connor’s practice focuses on the needs of men taking testosterone and other anabolic steroids – a group he feels is poorly understood and largely neglected by conventional medical care, perceptions borne out by a 2020 study of steroid users he helped conduct.
“They felt discriminated against, they did not feel comfortable working with their physicians, and they felt that the doctors did not know what they were doing,” Dr. O’Connor said in an interview. His patients often express anger and frustration with doctors they had seen previously.
Patients turning to home tests
Clients can order a panel of labs designed to screen for health conditions commonly associated with use of AAS, such as dyslipidemia, renal and hepatic dysfunction, polycythemia, thrombosis, and insulin resistance. The panels also include tests for levels of different hormones.
Sales of direct-to-consumer tests topped $3.6 billion in the United States in 2022 and are predicted to grow. Some of that spending is coming from people, mostly men, using illegally obtained steroids to build muscle. Although published data on the size of the bodybuilder market are unavailable, the Internet is a ready source of relatively inexpensive tests aimed at helping individuals monitor their health.
While clinicians may have their doubts about allowing patients to pick and choose tests and interpret their results, proponents claim they empower consumers to take control of their health – and save themselves money in the process.
But a test panel designed to help the user monitor the effects of banned substances is a bit unnerving to many clinicians, including Dr. O’Connor.
“People using anabolic steroids should be aware of the health risks associated with such use and that laboratory analysis is an important step toward improving health outcomes,” he said, “I’m all about open education, but not self-diagnosis and treatment.”
Testosterone and other AAS such as nandrolone, trenbolone, and boldenone are Schedule III controlled substances that have been banned by numerous athletic governing bodies. Yet recreational users can easily obtain them from online international pharmacies without a prescription. Should they be monitoring themselves for side effects?
A basic problem is that few primary care clinicians routinely ask their patients about the use of AAS or feel competent to manage the complications or withdrawal symptoms associated with the agents. And they may have no idea what the average AAS user looks like.
The American College of Sports Medicine updated its statement on the use of AAS in 2021. The statement warned of a growing new segment of users – up to 70% of people who take the drugs do so recreationally in pursuit of a more muscular appearance, rather than competitive athletes seeking enhanced performance.
The ACSM highlighted the syndrome of muscle dysmorphia, also known as “megarexia” or “bigorexia” (think of it as “reverse anorexia”), as a major risk factor for illicit use of AAS.
Stuart Phillips, PhD, professor and director of the department of kinesiology at McMaster University, Hamilton, Ont., coauthored the ACSM guidelines.
“The prince in Snow White circa 1950s was a guy with nice hair,” said Dr. Phillips, pointing to a change in cultural expectations for the male body. “But then fast forward to the prince or hero in any other Disney movie recently – and the guy is jacked.”
Since the last guidelines were published in 1987, Dr. Phillips has seen some cultural shifts. Testosterone has gone from a banned substance no one talked about to a mainstream medical therapy for men with low androgen levels, as any television viewer of primetime sports can attest. “But the other thing that’s changed,” he added, “is that we’ve seen the proliferation of illicit anabolic steroid use solely for the purpose of aesthetics.”
As an adolescent medicine physician who specializes in eating disorders, Jason Nagata, MD, MSc, sees many young men in his practice who engage in different behaviors to increase their muscle mass – from exercising, consuming high protein diets or taking protein supplements, even injecting AAS.
“A third of teenage boys across the U.S. report they’re trying to gain weight to bulk up and gain muscle,” said Dr. Nagata, an associate professor of pediatrics at the University of California, San Francisco.
In a 2020 study published in JAMA Pediatrics, Dr. Nagata and colleagues found that use of legal performance-enhancing substances in young men aged 18-26 years was associated with a higher odds of using AAS 7 years later (adjusted odds ratio, 3.18; 95% confidence interval, 1.90-5.32). “Some of the legal performance-enhancing substances like the protein powders or creatine may serve as a gateway to use of AAS,” Dr. Nagata said.
Another important factor is exposure to AAS use on social media, where muscular influencers gain huge followings. Dr. Nagata said most of the research on eating disorders and social media has examined the role of media on weight loss in girls.
“Although there’s less research on the social media impact on boys and men, a few studies have shown links between more Instagram use and muscle dissatisfaction, as well as thinking about using steroids,” he said.
The number of people using AAS is not trivial. In a longitudinal study (led by Nagata) of young U.S. adults surveyed multiple times between 1994 and 2002, a total of 2.7% of 18- to 26-year-old men and 0.4% of women reported using AAS. In a more recent cohort of adolescents in Minnesota aged 14-22 years followed between 2010 and 2018, a total of 2.2% of males and 1% of females initiated AAS use.
The Endocrine Society has estimated that between 2.9 and 4 million Americans have used an AAS at some point in their lives. Given that use is illegal without a prescription, a limitation of any survey is that participants may not be willing to disclose their AAS habit, leading to an underestimate of the actual number.
Nor are the complications of AAS use negligible. The drugs can have wide-ranging effects on the body, potentially affecting the brain, heart, liver, kidneys, musculoskeletal system, immune system, and reproductive systems. And individuals might unknowingly expose themselves to AAS: A recent literature review found that over a quarter of dietary supplements tested were found to contain undeclared substances that are on the World Anti-Doping Agency’s list of banned agents.
Alarming mistrust of MDs
Dr. O’Connor’s study shed some light on why AAS users might resort to surfing the Internet looking for a way to diagnose their own complications from steroid use. The web-based survey of nearly 2,400 men who said they took the drugs found that participants considered physicians to be the worst source of information, ranking them below coaches, online bodybuilding forums and sites, other AAS users, and bodybuilding books or magazines. The majority (56%) did not reveal AAS use to their clinicians. Of those who did, 55% reported feeling discriminated against for the admission.
Dr. O’Connor said physicians receive scant education on the many different drugs and regimens used by bodybuilders and have no idea how to a manage withdrawal syndrome for people trying to get off steroids. He urged the medical community to develop an educational campaign for clinicians, similar to those from public health officials aimed at combating the opioid epidemic: “Let’s educate med students and the residents. Let’s put [steroid use] on our agenda.”
Consumer testing evangelist ... or physician nemesis?
Nelson Vergel, BSChE, MBA, is on a mission to make medical lab testing affordable and accessible to everyone. The chemical engineer founded Discounted Labs 8 years ago, offering commonly ordered tests, such as complete blood counts, liver function tests, and cholesterol levels.
Mr. Vergel has advocated for the use of hormones to treat HIV-wasting disease for nearly 40 years, after his own diagnosis of the infection in 1986. After losing 40 pounds, steroids saved his life, he said.
Mr. Vergel said he was shocked to learn about the lack of continuing medical education on AAS for physicians and agreed with Dr. O’Connor that more training is needed for the medical profession. He also recognized that stigma on the part of clinicians is a huge barrier for many AAS users.
“We have to accept the fact that people are using them instead of demonizing them,” Mr. Vergel said. “What I was seeing is that there was so much stigma – and patients would not even talk to their doctors about their use.”
After reviewing Google analytics for his lab’s website and seeing how often “bodybuilder” came up as a search term, he added a panel of labs a year ago that allows AAS users to monitor themselves for adverse events.
Although he doesn’t condone the use of AAS without a medical indication and advises customers to discuss their results with a doctor, “we have to make sure people are reducing their harm or risk,” he said. “That’s really my goal.”
Many health care professionals would disagree with that statement. Dr. Nagata said he was concerned that management of side effects is too complicated. “There are a lot of nuances in the interpretation of these tests.” Arriving at the correct interpretation of the results requires a clinician’s thorough review of each patient’s health history, family history, and mental health history along with lab results.
‘I’m concerned’
In a second article outlining harm-reduction strategies designed to improve care for patients using AAS, Dr. O’Connor and colleagues outlined an approach for talking with patients who are concerned about their health and are seeking guidance from a clinician.
The first step is to work on developing a rapport, and not to demand that patients stop their use of AAS. His recommended opening line is: “I want to be honest with you – I’m concerned.”
The initial interaction is an opportunity to find out why the person uses AAS, what health concerns they have at present, and why they are seeking care. Open-ended questions may reveal concerns that the patient has about fertility or side effects.
Consistent with harm-reduction approaches used for other public health epidemics – such as opioid abuse and blood-borne pathogens among people who inject drugs – follow-up visits can include nonjudgmental discussions about decreasing or stopping their use.
Ultimately, minimizing the harms of AAS use can serve as a bridge to their cessation, but the medical community needs to build up trust with a community of users who currently rely more on each other and the Internet for guidance than their primary care physicians. “We need more education. We’re going to need resources to do it,” Dr. O’Connell said. “And we’re going to have to do it.”
Dr. Phillips and Dr. Nagata have no financial disclosures. Mr. Vergel is the owner and founder of Discounted Labs but reported no other financial conflicts. Dr. O’Connor owns Anabolic Doc but has no additional financial disclosures.
A version of this article first appeared on Medscape.com.
The case for ‘pleasure hygiene’: Sexual health in patients with chronic illness
A recent study found a significant association between lower sexual frequency and greater all-cause mortality in young and middle-aged people with hypertension. Should primary care physicians be offering a pleasure prescription to the 6 in 10 Americans living with chronic illness?
Ask, don’t tell
First, we need to ask routinely about sexual well-being and pleasure. Without asking patients their views, we do not know the relevance of sex for their quality of life. Unless we ask, we do not know what specific kinds of sexual play are important for a person’s pleasure, nor can we assume how they prioritize their sexual functioning in the context of their medical care. When I began asking my primary care patients about sexual well-being, many more than I expected were quietly holding on to distressing issues. Now, as a sexual medicine specialist, in each sexual function evaluation, I ask three key questions: What are your goals? What does sex mean to you? What kinds of sexual play are important for your (and your partner’s) pleasure?
Chronic disease – with physical symptoms as well as psychological, relational, and cultural components – affects both general and genital physiology. Any disease process that alters vascular, neuroendocrine, or musculoskeletal function is likely to influence sexual function, either directly through the disease process or indirectly through complications or the effect on identity and well-being. In addition, a host of iatrogenic changes to sexual function may accompany effects of treatments.
Managing the effects of chronic illness on sexuality requires resilience and flexibility. A serious injury may require a massive adjustment to sexuality, but progressive disease may require continuous accommodations to sexual changes. The life stage at which the disease occurs also matters. People facing disease early in life encounter challenges (finding willing sexual partners and limited medical guidance regarding their sexual functioning) as well as benefits (they may integrate their disease as part of their sexual life). Those who experience sexual changes related to their illness later in life may face a loss of “normal” sexual function and well-being.
Meanwhile, the partner who is not ill may have their own sexual needs, fears, and worries. Both patients and partners may experience disenfranchised grief – a sense of loss about something one is not culturally permitted to mourn (“I/my partner is alive in the face of this terrible illness; who am I to worry about our/my sexual pleasure?”).
Positive marital relationships influence health through improved survival, improved medical adherence, better quality of life for the patient, and improved life satisfaction. Sexual satisfaction is an important factor in relational satisfaction. Helping our patients with these changes therefore may improve not only sexual health but overall health.
How, then, should we address sexual pleasure in chronic illness care? Here are a few tips:
Focus on pleasure. “Performance” is foul language when it comes to sex. Full attention to sensation and enjoyment, the only sexual “skill” anyone needs, is impossible while trying to perform.
Encourage flexibility and recognize that sex encompasses a wide and varied menu of experiences that change over a lifetime. Sex is everything from kissing and cuddling to the wildest things a mind can imagine. We can help both patients and partners think about the wide variety of ways to meet sexual needs. Balancing acceptance of sexual changes with motivation for improvement also is part of our role.
Address the effects of illness on the patient’s relationship with their body. Illness may alter not only bodily function but also self-esteem and body image. A reorganization of self-concept may occur (“I am no longer a sexual person; I’m a sexually dysfunctional asthmatic/diabetic/etc. and should avoid sexual intimacy”). Examining these self-constructs allows shifts in thoughts and behaviors, leading to improved psychological and sexual well-being. Encourage patients to explore what feels good in this body now. When possible, we can help with referral for corrective surgeries or direction to resources like stoma covers, wigs, scarves, and tattoos.
We offer suggestions for “sleep hygiene”; how about pleasure hygiene?
- Encourage open communication with partner(s) and offer resources to develop communication skills.
- Consider needs for physical and emotional preparation for sexual play: adequate rest, preparing the environment for body fluids, pillows for comfort or aides for positioning, and plenty of lubricant at hand.
- Allow adequate time for sexual play and encourage the ability to adjust or stop and start over – with humor and self-compassion.
- Use sexual aides to enhance pleasure.
- Seek out sexual medicine and sex therapy colleagues when things become tricky.
All bodies, no matter their health or illness state, are capable of pleasure. Hey, pleasure might even save lives!
Dr. Kranz is an clinical assistant professor of obstetrics/gynecology and family medicine, University of Rochester (N.Y.) Medical Center. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
A recent study found a significant association between lower sexual frequency and greater all-cause mortality in young and middle-aged people with hypertension. Should primary care physicians be offering a pleasure prescription to the 6 in 10 Americans living with chronic illness?
Ask, don’t tell
First, we need to ask routinely about sexual well-being and pleasure. Without asking patients their views, we do not know the relevance of sex for their quality of life. Unless we ask, we do not know what specific kinds of sexual play are important for a person’s pleasure, nor can we assume how they prioritize their sexual functioning in the context of their medical care. When I began asking my primary care patients about sexual well-being, many more than I expected were quietly holding on to distressing issues. Now, as a sexual medicine specialist, in each sexual function evaluation, I ask three key questions: What are your goals? What does sex mean to you? What kinds of sexual play are important for your (and your partner’s) pleasure?
Chronic disease – with physical symptoms as well as psychological, relational, and cultural components – affects both general and genital physiology. Any disease process that alters vascular, neuroendocrine, or musculoskeletal function is likely to influence sexual function, either directly through the disease process or indirectly through complications or the effect on identity and well-being. In addition, a host of iatrogenic changes to sexual function may accompany effects of treatments.
Managing the effects of chronic illness on sexuality requires resilience and flexibility. A serious injury may require a massive adjustment to sexuality, but progressive disease may require continuous accommodations to sexual changes. The life stage at which the disease occurs also matters. People facing disease early in life encounter challenges (finding willing sexual partners and limited medical guidance regarding their sexual functioning) as well as benefits (they may integrate their disease as part of their sexual life). Those who experience sexual changes related to their illness later in life may face a loss of “normal” sexual function and well-being.
Meanwhile, the partner who is not ill may have their own sexual needs, fears, and worries. Both patients and partners may experience disenfranchised grief – a sense of loss about something one is not culturally permitted to mourn (“I/my partner is alive in the face of this terrible illness; who am I to worry about our/my sexual pleasure?”).
Positive marital relationships influence health through improved survival, improved medical adherence, better quality of life for the patient, and improved life satisfaction. Sexual satisfaction is an important factor in relational satisfaction. Helping our patients with these changes therefore may improve not only sexual health but overall health.
How, then, should we address sexual pleasure in chronic illness care? Here are a few tips:
Focus on pleasure. “Performance” is foul language when it comes to sex. Full attention to sensation and enjoyment, the only sexual “skill” anyone needs, is impossible while trying to perform.
Encourage flexibility and recognize that sex encompasses a wide and varied menu of experiences that change over a lifetime. Sex is everything from kissing and cuddling to the wildest things a mind can imagine. We can help both patients and partners think about the wide variety of ways to meet sexual needs. Balancing acceptance of sexual changes with motivation for improvement also is part of our role.
Address the effects of illness on the patient’s relationship with their body. Illness may alter not only bodily function but also self-esteem and body image. A reorganization of self-concept may occur (“I am no longer a sexual person; I’m a sexually dysfunctional asthmatic/diabetic/etc. and should avoid sexual intimacy”). Examining these self-constructs allows shifts in thoughts and behaviors, leading to improved psychological and sexual well-being. Encourage patients to explore what feels good in this body now. When possible, we can help with referral for corrective surgeries or direction to resources like stoma covers, wigs, scarves, and tattoos.
We offer suggestions for “sleep hygiene”; how about pleasure hygiene?
- Encourage open communication with partner(s) and offer resources to develop communication skills.
- Consider needs for physical and emotional preparation for sexual play: adequate rest, preparing the environment for body fluids, pillows for comfort or aides for positioning, and plenty of lubricant at hand.
- Allow adequate time for sexual play and encourage the ability to adjust or stop and start over – with humor and self-compassion.
- Use sexual aides to enhance pleasure.
- Seek out sexual medicine and sex therapy colleagues when things become tricky.
All bodies, no matter their health or illness state, are capable of pleasure. Hey, pleasure might even save lives!
Dr. Kranz is an clinical assistant professor of obstetrics/gynecology and family medicine, University of Rochester (N.Y.) Medical Center. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
A recent study found a significant association between lower sexual frequency and greater all-cause mortality in young and middle-aged people with hypertension. Should primary care physicians be offering a pleasure prescription to the 6 in 10 Americans living with chronic illness?
Ask, don’t tell
First, we need to ask routinely about sexual well-being and pleasure. Without asking patients their views, we do not know the relevance of sex for their quality of life. Unless we ask, we do not know what specific kinds of sexual play are important for a person’s pleasure, nor can we assume how they prioritize their sexual functioning in the context of their medical care. When I began asking my primary care patients about sexual well-being, many more than I expected were quietly holding on to distressing issues. Now, as a sexual medicine specialist, in each sexual function evaluation, I ask three key questions: What are your goals? What does sex mean to you? What kinds of sexual play are important for your (and your partner’s) pleasure?
Chronic disease – with physical symptoms as well as psychological, relational, and cultural components – affects both general and genital physiology. Any disease process that alters vascular, neuroendocrine, or musculoskeletal function is likely to influence sexual function, either directly through the disease process or indirectly through complications or the effect on identity and well-being. In addition, a host of iatrogenic changes to sexual function may accompany effects of treatments.
Managing the effects of chronic illness on sexuality requires resilience and flexibility. A serious injury may require a massive adjustment to sexuality, but progressive disease may require continuous accommodations to sexual changes. The life stage at which the disease occurs also matters. People facing disease early in life encounter challenges (finding willing sexual partners and limited medical guidance regarding their sexual functioning) as well as benefits (they may integrate their disease as part of their sexual life). Those who experience sexual changes related to their illness later in life may face a loss of “normal” sexual function and well-being.
Meanwhile, the partner who is not ill may have their own sexual needs, fears, and worries. Both patients and partners may experience disenfranchised grief – a sense of loss about something one is not culturally permitted to mourn (“I/my partner is alive in the face of this terrible illness; who am I to worry about our/my sexual pleasure?”).
Positive marital relationships influence health through improved survival, improved medical adherence, better quality of life for the patient, and improved life satisfaction. Sexual satisfaction is an important factor in relational satisfaction. Helping our patients with these changes therefore may improve not only sexual health but overall health.
How, then, should we address sexual pleasure in chronic illness care? Here are a few tips:
Focus on pleasure. “Performance” is foul language when it comes to sex. Full attention to sensation and enjoyment, the only sexual “skill” anyone needs, is impossible while trying to perform.
Encourage flexibility and recognize that sex encompasses a wide and varied menu of experiences that change over a lifetime. Sex is everything from kissing and cuddling to the wildest things a mind can imagine. We can help both patients and partners think about the wide variety of ways to meet sexual needs. Balancing acceptance of sexual changes with motivation for improvement also is part of our role.
Address the effects of illness on the patient’s relationship with their body. Illness may alter not only bodily function but also self-esteem and body image. A reorganization of self-concept may occur (“I am no longer a sexual person; I’m a sexually dysfunctional asthmatic/diabetic/etc. and should avoid sexual intimacy”). Examining these self-constructs allows shifts in thoughts and behaviors, leading to improved psychological and sexual well-being. Encourage patients to explore what feels good in this body now. When possible, we can help with referral for corrective surgeries or direction to resources like stoma covers, wigs, scarves, and tattoos.
We offer suggestions for “sleep hygiene”; how about pleasure hygiene?
- Encourage open communication with partner(s) and offer resources to develop communication skills.
- Consider needs for physical and emotional preparation for sexual play: adequate rest, preparing the environment for body fluids, pillows for comfort or aides for positioning, and plenty of lubricant at hand.
- Allow adequate time for sexual play and encourage the ability to adjust or stop and start over – with humor and self-compassion.
- Use sexual aides to enhance pleasure.
- Seek out sexual medicine and sex therapy colleagues when things become tricky.
All bodies, no matter their health or illness state, are capable of pleasure. Hey, pleasure might even save lives!
Dr. Kranz is an clinical assistant professor of obstetrics/gynecology and family medicine, University of Rochester (N.Y.) Medical Center. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Prostate cancer screening guidelines: To PSA or not to PSA
In the United States this year, approximately 288,300 men will be newly diagnosed with prostate cancer and about 34,700 men will die from this disease. It is the second leading cause of cancer in men, and one out of every eight men will be diagnosed with this cancer at some point in their lives.
As primary care physicians, a large part of our role is to prevent or detect cancers early. Patients look to us for this guidance. However, prostate cancer screening has long been a controversial issue. Earlier this year, the American Urological Association along with the Society of Urologic Oncology published updated guidelines.
Clear recommendations that come from this set of guidelines that are relevant to primary care physicians include:
- using PSA as the screening test of choice.
- repeating PSA in patients with newly elevated results before moving on to other test.
- offering PSA screening every 2-4 years in patients aged 50-69 years.
- offering baseline screening in those between 45-50 years of age.
In high-risk patients, screening can be initiated at 40-45 years of age. All of these recommendations come with the caveat that we give the patient all the pros and cons and leave it up to their “values and preferences.”
The guidelines make recommendations regarding PSA screening and biopsy standards. These guidelines are very specific in their recommendations; however, the question about whether to do PSA screening in the first place is left open to debate. While shared decision-making is important with any testing, it is more difficult with prostate cancer screening. Patients need to understand that there are possible adverse events that can result because of an elevated PSA, such as unneeded biopsies that may come with complications.
The authors of this set of guidelines suggest that physicians talk to patients more often about the benefits of the screening than they do about the negative consequences. This assumes that a negative biopsy result is an unnecessary test, which is not a fair assessment. Negative test results can provide useful clinical information. While a PSA result may lead to a biopsy that could have possibly been avoided, we don’t have any better screening tests available. Missing a prostate cancer that could have been detected by PSA screening is also very harmful. Deciding whether to do PSA screening for any given patient then becomes a difficult question.
More research into biomarkers to detect prostate cancer is needed, as suggested by the guideline authors. As primary care doctors, we’re the first ones to order these tests and make decisions regarding the results. While we may not be the ones to do the biopsies, we do need to know when to refer the patients to specialists or when we can just repeat the test.
Population health is often the benchmark used when looking at screening guidelines. But in the primary care setting, we are responsible for individual patients. Applying guidelines that take whole populations into consideration often doesn’t translate well to single patients. We do need to make them responsible for their own health care decisions but, at the same time, we need to offer them some guidance. If the guidelines are clear, this is easy. When they suggest giving patients all the pros and cons and letting them make their own decision, this is hard. Some of them want us to tell them what to do.
Additionally, patients in the primary care setting develop close relationships with their physicians. They are not an elevated PSA test or a negative biopsy result. They have concerns and fears. When they are high risk, the advice is easy. Keeping in mind that prostate cancer is the second leading cause of cancer in men in the United States, we should have clear screening guidelines, such as we do with mammograms in women. Yes, shared decision-making is important, but we also need to know the answer when our patients ask us whether or not they should have a PSA test done.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
In the United States this year, approximately 288,300 men will be newly diagnosed with prostate cancer and about 34,700 men will die from this disease. It is the second leading cause of cancer in men, and one out of every eight men will be diagnosed with this cancer at some point in their lives.
As primary care physicians, a large part of our role is to prevent or detect cancers early. Patients look to us for this guidance. However, prostate cancer screening has long been a controversial issue. Earlier this year, the American Urological Association along with the Society of Urologic Oncology published updated guidelines.
Clear recommendations that come from this set of guidelines that are relevant to primary care physicians include:
- using PSA as the screening test of choice.
- repeating PSA in patients with newly elevated results before moving on to other test.
- offering PSA screening every 2-4 years in patients aged 50-69 years.
- offering baseline screening in those between 45-50 years of age.
In high-risk patients, screening can be initiated at 40-45 years of age. All of these recommendations come with the caveat that we give the patient all the pros and cons and leave it up to their “values and preferences.”
The guidelines make recommendations regarding PSA screening and biopsy standards. These guidelines are very specific in their recommendations; however, the question about whether to do PSA screening in the first place is left open to debate. While shared decision-making is important with any testing, it is more difficult with prostate cancer screening. Patients need to understand that there are possible adverse events that can result because of an elevated PSA, such as unneeded biopsies that may come with complications.
The authors of this set of guidelines suggest that physicians talk to patients more often about the benefits of the screening than they do about the negative consequences. This assumes that a negative biopsy result is an unnecessary test, which is not a fair assessment. Negative test results can provide useful clinical information. While a PSA result may lead to a biopsy that could have possibly been avoided, we don’t have any better screening tests available. Missing a prostate cancer that could have been detected by PSA screening is also very harmful. Deciding whether to do PSA screening for any given patient then becomes a difficult question.
More research into biomarkers to detect prostate cancer is needed, as suggested by the guideline authors. As primary care doctors, we’re the first ones to order these tests and make decisions regarding the results. While we may not be the ones to do the biopsies, we do need to know when to refer the patients to specialists or when we can just repeat the test.
Population health is often the benchmark used when looking at screening guidelines. But in the primary care setting, we are responsible for individual patients. Applying guidelines that take whole populations into consideration often doesn’t translate well to single patients. We do need to make them responsible for their own health care decisions but, at the same time, we need to offer them some guidance. If the guidelines are clear, this is easy. When they suggest giving patients all the pros and cons and letting them make their own decision, this is hard. Some of them want us to tell them what to do.
Additionally, patients in the primary care setting develop close relationships with their physicians. They are not an elevated PSA test or a negative biopsy result. They have concerns and fears. When they are high risk, the advice is easy. Keeping in mind that prostate cancer is the second leading cause of cancer in men in the United States, we should have clear screening guidelines, such as we do with mammograms in women. Yes, shared decision-making is important, but we also need to know the answer when our patients ask us whether or not they should have a PSA test done.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
In the United States this year, approximately 288,300 men will be newly diagnosed with prostate cancer and about 34,700 men will die from this disease. It is the second leading cause of cancer in men, and one out of every eight men will be diagnosed with this cancer at some point in their lives.
As primary care physicians, a large part of our role is to prevent or detect cancers early. Patients look to us for this guidance. However, prostate cancer screening has long been a controversial issue. Earlier this year, the American Urological Association along with the Society of Urologic Oncology published updated guidelines.
Clear recommendations that come from this set of guidelines that are relevant to primary care physicians include:
- using PSA as the screening test of choice.
- repeating PSA in patients with newly elevated results before moving on to other test.
- offering PSA screening every 2-4 years in patients aged 50-69 years.
- offering baseline screening in those between 45-50 years of age.
In high-risk patients, screening can be initiated at 40-45 years of age. All of these recommendations come with the caveat that we give the patient all the pros and cons and leave it up to their “values and preferences.”
The guidelines make recommendations regarding PSA screening and biopsy standards. These guidelines are very specific in their recommendations; however, the question about whether to do PSA screening in the first place is left open to debate. While shared decision-making is important with any testing, it is more difficult with prostate cancer screening. Patients need to understand that there are possible adverse events that can result because of an elevated PSA, such as unneeded biopsies that may come with complications.
The authors of this set of guidelines suggest that physicians talk to patients more often about the benefits of the screening than they do about the negative consequences. This assumes that a negative biopsy result is an unnecessary test, which is not a fair assessment. Negative test results can provide useful clinical information. While a PSA result may lead to a biopsy that could have possibly been avoided, we don’t have any better screening tests available. Missing a prostate cancer that could have been detected by PSA screening is also very harmful. Deciding whether to do PSA screening for any given patient then becomes a difficult question.
More research into biomarkers to detect prostate cancer is needed, as suggested by the guideline authors. As primary care doctors, we’re the first ones to order these tests and make decisions regarding the results. While we may not be the ones to do the biopsies, we do need to know when to refer the patients to specialists or when we can just repeat the test.
Population health is often the benchmark used when looking at screening guidelines. But in the primary care setting, we are responsible for individual patients. Applying guidelines that take whole populations into consideration often doesn’t translate well to single patients. We do need to make them responsible for their own health care decisions but, at the same time, we need to offer them some guidance. If the guidelines are clear, this is easy. When they suggest giving patients all the pros and cons and letting them make their own decision, this is hard. Some of them want us to tell them what to do.
Additionally, patients in the primary care setting develop close relationships with their physicians. They are not an elevated PSA test or a negative biopsy result. They have concerns and fears. When they are high risk, the advice is easy. Keeping in mind that prostate cancer is the second leading cause of cancer in men in the United States, we should have clear screening guidelines, such as we do with mammograms in women. Yes, shared decision-making is important, but we also need to know the answer when our patients ask us whether or not they should have a PSA test done.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
Don’t call them ‘private parts’
This transcript has been edited for clarity.
Today, I’d like to talk about private parts. You know: the genitals, down there.
I hate all of that. I really wish that we can get to a place where we can talk about genitals and sexual health the same way we do about high blood pressure and diabetes. In fact, when a new patient comes in and they get a new diagnosis of diabetes, you spend time explaining to them how their pancreas works. I don’t remember all the details because I’m a urologist. But you explain the details of diabetes, how it works, why therapy is important, and how it’s very important for quality of life.
I say to patients, “You have to know what parts you have in order to figure out how they drive, right?” We want them to drive better.
Let me give you an example. Many men come to see me with complaints of erectile dysfunction. They refuse to take sildenafil and tadalafil (Viagra and Cialis), saying, “Oh my gosh, those are magic pills. I won’t be a man if take them.” We all know that doesn’t make any sense. I explain to them how their penis works: “Your penis is a muscle. The muscle does two things. It contracts and it relaxes, just like your bicep. It’s just that your penis muscle is smooth muscle, which means it responds to fight or flight. It’s on the autonomic nervous system.”
I explain that if the muscle of the penis is relaxed, it fills with blood and expands. It gets big and hard, and it traps the blood. But when the muscles of the penis are contracted, when they are tight, it squeezes out all the blood, like squeezing out a sponge. So the important thing to do if you want to have good erections is to get the muscles to relax. Relaxed muscle increases erections. I get them to understand that sildenafil and tadalafil are phosphodiesterase 5 inhibitors: smooth-muscle relaxants. Instead of saying, “I need to take Viagra or Cialis because I’m broken,” it’s, “Oh hey honey, I need to take my muscle relaxants because my muscles aren’t working the way that they used to.”
In the future, I’ll go into what happens in erectile dysfunction. We’ll go into what can happen with erectile dysfunction and the many reasons why it happens. It’s getting them to understand that if we get the muscles to relax, you will have better erections. This is how the penis works. It’s why the medicine works. The patients will actually try the therapy and they’ll feel so much better about it. They’ll say, “Oh my gosh, this makes so much sense.” They work on their mental muscles to get the muscles of the penis to relax. Understanding anatomy and physiology helps them understand the treatments, which leads to better outcomes.
How about the female side? If a woman comes to see me reporting that she can’t have an orgasm, part of it is education and understanding the anatomy and physiology. The clitoris and the penis are exactly the same thing. The head of the clitoris and the head of the penis are the same. The clitoris has legs that go all the way down to the butt bone. So everyone is sitting on their genitals right now. The butt bones connect to the bottom of the clitoris or the bottom of the penis. They each have legs called crura. When you get patients to understand where their anatomy is and how it functions, they will then understand how to maximize their quality of life.
The clitoris has smooth muscle just like the penis. When that smooth muscle relaxes, it gorges with blood. When you stimulate it, it can lead to orgasm for most people. But, wait a minute. The clitoris is not inside the vagina. It’s outside. It’s behind the labia majora. If you follow the labia minora up, you get to the head of the clitoris. If patients understand that, they then will understand that penetration is not the way the majority of people orgasm.
I love pictures. I show everyone pictures in my office. They help patients to understand why vibration or outside stimulation on the vulva will allow orgasm to happen. And so instead of patients coming in saying, “I’m broken, I can’t orgasm from penetration,” or, “Dr. Rubin, I’m broken because I can’t get erections,” getting them to understand the anatomy and physiology helps them understand the treatment.
As we go forward, I’ll talk more about anatomy and physiology and how to increase the sexual health of our patients. For now though, please stop calling them private parts. Please use your understanding of anatomy and physiology to educate your patients to have better sexual health and higher quality of life. You may be the only clinician to ever do so, and it will make their life so much better.
Dr. Rubin is an assistant clinical professor, department of urology, at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Today, I’d like to talk about private parts. You know: the genitals, down there.
I hate all of that. I really wish that we can get to a place where we can talk about genitals and sexual health the same way we do about high blood pressure and diabetes. In fact, when a new patient comes in and they get a new diagnosis of diabetes, you spend time explaining to them how their pancreas works. I don’t remember all the details because I’m a urologist. But you explain the details of diabetes, how it works, why therapy is important, and how it’s very important for quality of life.
I say to patients, “You have to know what parts you have in order to figure out how they drive, right?” We want them to drive better.
Let me give you an example. Many men come to see me with complaints of erectile dysfunction. They refuse to take sildenafil and tadalafil (Viagra and Cialis), saying, “Oh my gosh, those are magic pills. I won’t be a man if take them.” We all know that doesn’t make any sense. I explain to them how their penis works: “Your penis is a muscle. The muscle does two things. It contracts and it relaxes, just like your bicep. It’s just that your penis muscle is smooth muscle, which means it responds to fight or flight. It’s on the autonomic nervous system.”
I explain that if the muscle of the penis is relaxed, it fills with blood and expands. It gets big and hard, and it traps the blood. But when the muscles of the penis are contracted, when they are tight, it squeezes out all the blood, like squeezing out a sponge. So the important thing to do if you want to have good erections is to get the muscles to relax. Relaxed muscle increases erections. I get them to understand that sildenafil and tadalafil are phosphodiesterase 5 inhibitors: smooth-muscle relaxants. Instead of saying, “I need to take Viagra or Cialis because I’m broken,” it’s, “Oh hey honey, I need to take my muscle relaxants because my muscles aren’t working the way that they used to.”
In the future, I’ll go into what happens in erectile dysfunction. We’ll go into what can happen with erectile dysfunction and the many reasons why it happens. It’s getting them to understand that if we get the muscles to relax, you will have better erections. This is how the penis works. It’s why the medicine works. The patients will actually try the therapy and they’ll feel so much better about it. They’ll say, “Oh my gosh, this makes so much sense.” They work on their mental muscles to get the muscles of the penis to relax. Understanding anatomy and physiology helps them understand the treatments, which leads to better outcomes.
How about the female side? If a woman comes to see me reporting that she can’t have an orgasm, part of it is education and understanding the anatomy and physiology. The clitoris and the penis are exactly the same thing. The head of the clitoris and the head of the penis are the same. The clitoris has legs that go all the way down to the butt bone. So everyone is sitting on their genitals right now. The butt bones connect to the bottom of the clitoris or the bottom of the penis. They each have legs called crura. When you get patients to understand where their anatomy is and how it functions, they will then understand how to maximize their quality of life.
The clitoris has smooth muscle just like the penis. When that smooth muscle relaxes, it gorges with blood. When you stimulate it, it can lead to orgasm for most people. But, wait a minute. The clitoris is not inside the vagina. It’s outside. It’s behind the labia majora. If you follow the labia minora up, you get to the head of the clitoris. If patients understand that, they then will understand that penetration is not the way the majority of people orgasm.
I love pictures. I show everyone pictures in my office. They help patients to understand why vibration or outside stimulation on the vulva will allow orgasm to happen. And so instead of patients coming in saying, “I’m broken, I can’t orgasm from penetration,” or, “Dr. Rubin, I’m broken because I can’t get erections,” getting them to understand the anatomy and physiology helps them understand the treatment.
As we go forward, I’ll talk more about anatomy and physiology and how to increase the sexual health of our patients. For now though, please stop calling them private parts. Please use your understanding of anatomy and physiology to educate your patients to have better sexual health and higher quality of life. You may be the only clinician to ever do so, and it will make their life so much better.
Dr. Rubin is an assistant clinical professor, department of urology, at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Today, I’d like to talk about private parts. You know: the genitals, down there.
I hate all of that. I really wish that we can get to a place where we can talk about genitals and sexual health the same way we do about high blood pressure and diabetes. In fact, when a new patient comes in and they get a new diagnosis of diabetes, you spend time explaining to them how their pancreas works. I don’t remember all the details because I’m a urologist. But you explain the details of diabetes, how it works, why therapy is important, and how it’s very important for quality of life.
I say to patients, “You have to know what parts you have in order to figure out how they drive, right?” We want them to drive better.
Let me give you an example. Many men come to see me with complaints of erectile dysfunction. They refuse to take sildenafil and tadalafil (Viagra and Cialis), saying, “Oh my gosh, those are magic pills. I won’t be a man if take them.” We all know that doesn’t make any sense. I explain to them how their penis works: “Your penis is a muscle. The muscle does two things. It contracts and it relaxes, just like your bicep. It’s just that your penis muscle is smooth muscle, which means it responds to fight or flight. It’s on the autonomic nervous system.”
I explain that if the muscle of the penis is relaxed, it fills with blood and expands. It gets big and hard, and it traps the blood. But when the muscles of the penis are contracted, when they are tight, it squeezes out all the blood, like squeezing out a sponge. So the important thing to do if you want to have good erections is to get the muscles to relax. Relaxed muscle increases erections. I get them to understand that sildenafil and tadalafil are phosphodiesterase 5 inhibitors: smooth-muscle relaxants. Instead of saying, “I need to take Viagra or Cialis because I’m broken,” it’s, “Oh hey honey, I need to take my muscle relaxants because my muscles aren’t working the way that they used to.”
In the future, I’ll go into what happens in erectile dysfunction. We’ll go into what can happen with erectile dysfunction and the many reasons why it happens. It’s getting them to understand that if we get the muscles to relax, you will have better erections. This is how the penis works. It’s why the medicine works. The patients will actually try the therapy and they’ll feel so much better about it. They’ll say, “Oh my gosh, this makes so much sense.” They work on their mental muscles to get the muscles of the penis to relax. Understanding anatomy and physiology helps them understand the treatments, which leads to better outcomes.
How about the female side? If a woman comes to see me reporting that she can’t have an orgasm, part of it is education and understanding the anatomy and physiology. The clitoris and the penis are exactly the same thing. The head of the clitoris and the head of the penis are the same. The clitoris has legs that go all the way down to the butt bone. So everyone is sitting on their genitals right now. The butt bones connect to the bottom of the clitoris or the bottom of the penis. They each have legs called crura. When you get patients to understand where their anatomy is and how it functions, they will then understand how to maximize their quality of life.
The clitoris has smooth muscle just like the penis. When that smooth muscle relaxes, it gorges with blood. When you stimulate it, it can lead to orgasm for most people. But, wait a minute. The clitoris is not inside the vagina. It’s outside. It’s behind the labia majora. If you follow the labia minora up, you get to the head of the clitoris. If patients understand that, they then will understand that penetration is not the way the majority of people orgasm.
I love pictures. I show everyone pictures in my office. They help patients to understand why vibration or outside stimulation on the vulva will allow orgasm to happen. And so instead of patients coming in saying, “I’m broken, I can’t orgasm from penetration,” or, “Dr. Rubin, I’m broken because I can’t get erections,” getting them to understand the anatomy and physiology helps them understand the treatment.
As we go forward, I’ll talk more about anatomy and physiology and how to increase the sexual health of our patients. For now though, please stop calling them private parts. Please use your understanding of anatomy and physiology to educate your patients to have better sexual health and higher quality of life. You may be the only clinician to ever do so, and it will make their life so much better.
Dr. Rubin is an assistant clinical professor, department of urology, at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
Can this device take on enlarged prostates?
Inflating a drug-coated balloon in the prostate is the latest approach to treating a common cause of frequent or difficult urination in older men.
As the prostate naturally grows with age, the gland can obstruct the flow of urine – leading to frequent trips to the bathroom and disrupted nights. An estimated 50% of men aged 60 years and older have benign prostatic hyperplasia (BPH). That figure rises to more than 80% by age 70 and to 90% by age 80.
Transurethral resection of the prostate was the main surgical treatment for symptomatic BPH for much of the 20th century.
More recently, researchers have developed various minimally invasive surgical therapy (MIST) devices to treat the obstruction while limiting effects on sexual function. Some newer devices use lasers or water vapor to remove prostate tissue. Another approach uses implants to move and hold prostate tissue out of the way.
Now drug-coated balloons have entered the picture.
, paclitaxel – best known as a chemotherapy medication – to limit further growth and keep the lobes apart.
The Food and Drug Administration approved Optilume BPH in June. The results from a randomized controlled trial of the device were published in The Journal of Urology.
Uptake of MIST devices for BPH “has been variable due to a host of factors including mixed results, complexity of equipment, and costs,” the journal’s editor, D. Robert Siemens, MD, noted in the issue.
The developer of the device, Urotronic, said it expects that the newest treatment will be commercially available in the near future. Discussions about cost, insurance coverage, and how to train urologists to use it are ongoing, said Ian Schorn, the company’s vice president of clinical affairs.
Raevti Bole, MD, a urologic surgeon at Cleveland Clinic’s Glickman Urological and Kidney Institute, said BPH treatments ideally benefit patients for years, so she is eager to see how patients are doing 5 and 10 years after the Optilume BPH procedure. Studies should also examine its effects on fertility.
But given the safety and efficacy results reported 1 year after treatment, “I think this is something that a lot of people are going to be able to use in their practice and that their patients are going to benefit from,” Dr. Bole told this news organization.
She said she expects most urologists will be able to master the technology. The procedure’s minimal effect on sexual issues and the relatively short time needed to perform it are other advantages.
“All of those things are very positive in terms of whether patients are going to want to consider it and also whether surgeons are going to be able to realistically learn it and offer it at their centers,” Dr. Bole said.
In choosing a particular treatment, Dr. Bole discusses options with patients and takes into account factors such as trial data, the nature and severity of symptoms, treatment goals, comorbidities, and the size of the prostate.
Available MIST devices can vary by institution, and urologists can have different levels of experience with each device. If a patient is interested in an approach a surgeon does not offer, the surgeon can refer the patient to a colleague who does.
Active vs. sham treatment
Urologists may be familiar with another Optilume device, the Optilume urethral drug-coated balloon, that is used for urethral strictures.
The devices have similar names, and the underlying technology is similar, but there are major differences, Mr. Schorn said.
The BPH device expands between the lobes of the prostate, creating an anterior commissurotomy. A double-lobe balloon locks the device in place during inflation.
For the PINNACLE trial of the BPH device, which was conducted at 18 sites in the United States and Canada, Steven A. Kaplan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues enrolled 148 men with symptomatic BPH who were experiencing urinary flow obstruction.
The average age of the patients was 65 years; 100 of them were assigned to undergo active treatment with Optilume BPH. The rest received a sham procedure that mimicked active treatment.
At 3 months, men who received active treatment had an average improvement in the International Prostate Symptom Score of about 11 points. This improvement was maintained at 1 year. Those who received sham treatment experienced an 8-point improvement at 3 months that dissipated over time.
The rate of urine flow increased dramatically with Optilume BPH, the researchers reported.
Five serious adverse events were considered to be possibly related to the device. There were four cases of postprocedural hematuria that required cystoscopic management or extended observation, and one case of urethral false passage that required extended catheterization.
Nonserious adverse events in the men who underwent the Optilume procedure typically resolved in about a month and included hematuria (40%), urinary tract infection (14%), dysuria (9.2%), urge or mixed incontinence (8.2%), mild stress incontinence (7.1%), bladder spasms (6.1%), elevated prostate-specific antigen levels (6.1%), and urinary urgency (6.1%), according to the researchers.
In a subset of participants for whom pharmacokinetic data were available, systemic exposure to paclitaxel was minimal.
Four participants in the Optilume BPH arm (4.1%) reported ejaculatory dysfunction, compared with one man in the sham treatment arm (2.1%). There were no cases of treatment-related erectile dysfunction.
Most patients were treated under deep sedation or general anesthesia, and the average procedure time was 26 minutes.
After the procedure, patients received a Foley catheter, which remained in place for about 2 days, “which is not significantly different from water vapor thermal therapy, holmium laser enucleation of the prostate, or laser photovaporization in similar gland sizes,” Dr. Bole and Petar Bajic, MD, also with Cleveland Clinic, noted in a commentary accompanying the article in The Journal of Urology.
MIST devices can be ideal for patients who prioritize sexual function, but the need for a temporary catheter after the procedure can be a “major postoperative source of patient dissatisfaction,” they acknowledged.
“Consistent with other minimally invasive technologies, the Optilume BPH procedure is a straightforward procedure that can be conducted in an ambulatory or office outpatient setting with pain management at physician and patient discretion,” Dr. Kaplan and his coauthors wrote.
The study was featured on the cover of the journal, which the research team saw as an unusual but welcome spotlight for a treatment for BPH.
“We were thrilled that we got on the cover of The Journal of Urology, which is not a common thing for BPH technology,” Mr. Schorn said.
Urotronic funded the PINNACLE study. Dr. Bole has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Inflating a drug-coated balloon in the prostate is the latest approach to treating a common cause of frequent or difficult urination in older men.
As the prostate naturally grows with age, the gland can obstruct the flow of urine – leading to frequent trips to the bathroom and disrupted nights. An estimated 50% of men aged 60 years and older have benign prostatic hyperplasia (BPH). That figure rises to more than 80% by age 70 and to 90% by age 80.
Transurethral resection of the prostate was the main surgical treatment for symptomatic BPH for much of the 20th century.
More recently, researchers have developed various minimally invasive surgical therapy (MIST) devices to treat the obstruction while limiting effects on sexual function. Some newer devices use lasers or water vapor to remove prostate tissue. Another approach uses implants to move and hold prostate tissue out of the way.
Now drug-coated balloons have entered the picture.
, paclitaxel – best known as a chemotherapy medication – to limit further growth and keep the lobes apart.
The Food and Drug Administration approved Optilume BPH in June. The results from a randomized controlled trial of the device were published in The Journal of Urology.
Uptake of MIST devices for BPH “has been variable due to a host of factors including mixed results, complexity of equipment, and costs,” the journal’s editor, D. Robert Siemens, MD, noted in the issue.
The developer of the device, Urotronic, said it expects that the newest treatment will be commercially available in the near future. Discussions about cost, insurance coverage, and how to train urologists to use it are ongoing, said Ian Schorn, the company’s vice president of clinical affairs.
Raevti Bole, MD, a urologic surgeon at Cleveland Clinic’s Glickman Urological and Kidney Institute, said BPH treatments ideally benefit patients for years, so she is eager to see how patients are doing 5 and 10 years after the Optilume BPH procedure. Studies should also examine its effects on fertility.
But given the safety and efficacy results reported 1 year after treatment, “I think this is something that a lot of people are going to be able to use in their practice and that their patients are going to benefit from,” Dr. Bole told this news organization.
She said she expects most urologists will be able to master the technology. The procedure’s minimal effect on sexual issues and the relatively short time needed to perform it are other advantages.
“All of those things are very positive in terms of whether patients are going to want to consider it and also whether surgeons are going to be able to realistically learn it and offer it at their centers,” Dr. Bole said.
In choosing a particular treatment, Dr. Bole discusses options with patients and takes into account factors such as trial data, the nature and severity of symptoms, treatment goals, comorbidities, and the size of the prostate.
Available MIST devices can vary by institution, and urologists can have different levels of experience with each device. If a patient is interested in an approach a surgeon does not offer, the surgeon can refer the patient to a colleague who does.
Active vs. sham treatment
Urologists may be familiar with another Optilume device, the Optilume urethral drug-coated balloon, that is used for urethral strictures.
The devices have similar names, and the underlying technology is similar, but there are major differences, Mr. Schorn said.
The BPH device expands between the lobes of the prostate, creating an anterior commissurotomy. A double-lobe balloon locks the device in place during inflation.
For the PINNACLE trial of the BPH device, which was conducted at 18 sites in the United States and Canada, Steven A. Kaplan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues enrolled 148 men with symptomatic BPH who were experiencing urinary flow obstruction.
The average age of the patients was 65 years; 100 of them were assigned to undergo active treatment with Optilume BPH. The rest received a sham procedure that mimicked active treatment.
At 3 months, men who received active treatment had an average improvement in the International Prostate Symptom Score of about 11 points. This improvement was maintained at 1 year. Those who received sham treatment experienced an 8-point improvement at 3 months that dissipated over time.
The rate of urine flow increased dramatically with Optilume BPH, the researchers reported.
Five serious adverse events were considered to be possibly related to the device. There were four cases of postprocedural hematuria that required cystoscopic management or extended observation, and one case of urethral false passage that required extended catheterization.
Nonserious adverse events in the men who underwent the Optilume procedure typically resolved in about a month and included hematuria (40%), urinary tract infection (14%), dysuria (9.2%), urge or mixed incontinence (8.2%), mild stress incontinence (7.1%), bladder spasms (6.1%), elevated prostate-specific antigen levels (6.1%), and urinary urgency (6.1%), according to the researchers.
In a subset of participants for whom pharmacokinetic data were available, systemic exposure to paclitaxel was minimal.
Four participants in the Optilume BPH arm (4.1%) reported ejaculatory dysfunction, compared with one man in the sham treatment arm (2.1%). There were no cases of treatment-related erectile dysfunction.
Most patients were treated under deep sedation or general anesthesia, and the average procedure time was 26 minutes.
After the procedure, patients received a Foley catheter, which remained in place for about 2 days, “which is not significantly different from water vapor thermal therapy, holmium laser enucleation of the prostate, or laser photovaporization in similar gland sizes,” Dr. Bole and Petar Bajic, MD, also with Cleveland Clinic, noted in a commentary accompanying the article in The Journal of Urology.
MIST devices can be ideal for patients who prioritize sexual function, but the need for a temporary catheter after the procedure can be a “major postoperative source of patient dissatisfaction,” they acknowledged.
“Consistent with other minimally invasive technologies, the Optilume BPH procedure is a straightforward procedure that can be conducted in an ambulatory or office outpatient setting with pain management at physician and patient discretion,” Dr. Kaplan and his coauthors wrote.
The study was featured on the cover of the journal, which the research team saw as an unusual but welcome spotlight for a treatment for BPH.
“We were thrilled that we got on the cover of The Journal of Urology, which is not a common thing for BPH technology,” Mr. Schorn said.
Urotronic funded the PINNACLE study. Dr. Bole has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Inflating a drug-coated balloon in the prostate is the latest approach to treating a common cause of frequent or difficult urination in older men.
As the prostate naturally grows with age, the gland can obstruct the flow of urine – leading to frequent trips to the bathroom and disrupted nights. An estimated 50% of men aged 60 years and older have benign prostatic hyperplasia (BPH). That figure rises to more than 80% by age 70 and to 90% by age 80.
Transurethral resection of the prostate was the main surgical treatment for symptomatic BPH for much of the 20th century.
More recently, researchers have developed various minimally invasive surgical therapy (MIST) devices to treat the obstruction while limiting effects on sexual function. Some newer devices use lasers or water vapor to remove prostate tissue. Another approach uses implants to move and hold prostate tissue out of the way.
Now drug-coated balloons have entered the picture.
, paclitaxel – best known as a chemotherapy medication – to limit further growth and keep the lobes apart.
The Food and Drug Administration approved Optilume BPH in June. The results from a randomized controlled trial of the device were published in The Journal of Urology.
Uptake of MIST devices for BPH “has been variable due to a host of factors including mixed results, complexity of equipment, and costs,” the journal’s editor, D. Robert Siemens, MD, noted in the issue.
The developer of the device, Urotronic, said it expects that the newest treatment will be commercially available in the near future. Discussions about cost, insurance coverage, and how to train urologists to use it are ongoing, said Ian Schorn, the company’s vice president of clinical affairs.
Raevti Bole, MD, a urologic surgeon at Cleveland Clinic’s Glickman Urological and Kidney Institute, said BPH treatments ideally benefit patients for years, so she is eager to see how patients are doing 5 and 10 years after the Optilume BPH procedure. Studies should also examine its effects on fertility.
But given the safety and efficacy results reported 1 year after treatment, “I think this is something that a lot of people are going to be able to use in their practice and that their patients are going to benefit from,” Dr. Bole told this news organization.
She said she expects most urologists will be able to master the technology. The procedure’s minimal effect on sexual issues and the relatively short time needed to perform it are other advantages.
“All of those things are very positive in terms of whether patients are going to want to consider it and also whether surgeons are going to be able to realistically learn it and offer it at their centers,” Dr. Bole said.
In choosing a particular treatment, Dr. Bole discusses options with patients and takes into account factors such as trial data, the nature and severity of symptoms, treatment goals, comorbidities, and the size of the prostate.
Available MIST devices can vary by institution, and urologists can have different levels of experience with each device. If a patient is interested in an approach a surgeon does not offer, the surgeon can refer the patient to a colleague who does.
Active vs. sham treatment
Urologists may be familiar with another Optilume device, the Optilume urethral drug-coated balloon, that is used for urethral strictures.
The devices have similar names, and the underlying technology is similar, but there are major differences, Mr. Schorn said.
The BPH device expands between the lobes of the prostate, creating an anterior commissurotomy. A double-lobe balloon locks the device in place during inflation.
For the PINNACLE trial of the BPH device, which was conducted at 18 sites in the United States and Canada, Steven A. Kaplan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues enrolled 148 men with symptomatic BPH who were experiencing urinary flow obstruction.
The average age of the patients was 65 years; 100 of them were assigned to undergo active treatment with Optilume BPH. The rest received a sham procedure that mimicked active treatment.
At 3 months, men who received active treatment had an average improvement in the International Prostate Symptom Score of about 11 points. This improvement was maintained at 1 year. Those who received sham treatment experienced an 8-point improvement at 3 months that dissipated over time.
The rate of urine flow increased dramatically with Optilume BPH, the researchers reported.
Five serious adverse events were considered to be possibly related to the device. There were four cases of postprocedural hematuria that required cystoscopic management or extended observation, and one case of urethral false passage that required extended catheterization.
Nonserious adverse events in the men who underwent the Optilume procedure typically resolved in about a month and included hematuria (40%), urinary tract infection (14%), dysuria (9.2%), urge or mixed incontinence (8.2%), mild stress incontinence (7.1%), bladder spasms (6.1%), elevated prostate-specific antigen levels (6.1%), and urinary urgency (6.1%), according to the researchers.
In a subset of participants for whom pharmacokinetic data were available, systemic exposure to paclitaxel was minimal.
Four participants in the Optilume BPH arm (4.1%) reported ejaculatory dysfunction, compared with one man in the sham treatment arm (2.1%). There were no cases of treatment-related erectile dysfunction.
Most patients were treated under deep sedation or general anesthesia, and the average procedure time was 26 minutes.
After the procedure, patients received a Foley catheter, which remained in place for about 2 days, “which is not significantly different from water vapor thermal therapy, holmium laser enucleation of the prostate, or laser photovaporization in similar gland sizes,” Dr. Bole and Petar Bajic, MD, also with Cleveland Clinic, noted in a commentary accompanying the article in The Journal of Urology.
MIST devices can be ideal for patients who prioritize sexual function, but the need for a temporary catheter after the procedure can be a “major postoperative source of patient dissatisfaction,” they acknowledged.
“Consistent with other minimally invasive technologies, the Optilume BPH procedure is a straightforward procedure that can be conducted in an ambulatory or office outpatient setting with pain management at physician and patient discretion,” Dr. Kaplan and his coauthors wrote.
The study was featured on the cover of the journal, which the research team saw as an unusual but welcome spotlight for a treatment for BPH.
“We were thrilled that we got on the cover of The Journal of Urology, which is not a common thing for BPH technology,” Mr. Schorn said.
Urotronic funded the PINNACLE study. Dr. Bole has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Is this the best screening test for prostate cancer?
In the ReIMAGINE study, a group of researchers from the United Kingdom found that half of men with apparently “safe” levels of prostate-specific antigen (PSA) below 3 ng/mL had clinically significant prostate cancers when multiparametric MRI was added to screening. The researchers, whose paper appeared in BMJ Oncology, also found that one in six screened men had a prostate lesion on MRI.
Meanwhile, a large Swedish population-based study, published in JAMA Network Open, showed that pre-biopsy MRIs combined with PSA testing after adoption of guidelines recommending MRIs led to a decrease in the proportion of men with negative biopsies (28% to 7%) and the number of Gleason score 6 cancers (24% to 6%), while the proportion of Gleason score 7-10 cancers rose from 49% to 86%.
Researchers compared prostate MRI uptake rates in the Jönköping Region in southern Sweden over 9 years – 2011 through 2018 before prostate MRIs were recommended nationally, and 2018-2020 when MRIs became commonly used.
David Robinson, MD, PhD, associate professor at Linköping University and leader of the Swedish study, told this news organization: “MRI is now standard for men before biopsy” in that country. In Sweden, which has a high rate of mortality from prostate cancer – about 50 deaths per 100,000 men vs. 12 and 8 per 100,000 in the United Kingdom and United States, respectively – PSA testing is not routine. “Most men that are diagnosed with prostate cancer have no symptoms. They have asked for a PSA when they have visited their general practitioner,” Dr. Robinson said. “To take a PSA test is not encouraged but it is not discouraged either. It is up to each man to decide.”
PSA screening is not common in the United Kingdom. Caroline Moore, MD, chair of urology at University College London and principal investigator on ReIMAGINE, said only 20% of UK men older than age 50 undergo PSA tests because doctors in the United Kingdom are concerned about the sort of overdiagnosis and overtreatment of prostate cancer that has occurred in the United States since the mid-1990s, when PSA screening was adopted here.
The rate of PSA screening in the United States has declined with controversies over recommendations for screening, though they remain above European rates: 37% in 2019, down from 47% in 2005, according to a 2022 Veterans Administration study published in JAMA Oncology.
In the UK study, Dr. Moore’s hospital-based group asked general practitioners to send letters to 2,096 men aged 50-75 years who had not been diagnosed with prostate cancer, inviting them to undergo prostate health checks combining screening with PSA and 10-minute prostate MRIs.
Of the 457 men who responded to the letters, 303 completed both screening tests. Older White men were more likely to respond, and Black men responded 20% less often.
Of the men who completed screening, 29 (9.6%) were diagnosed with clinically significant cancer and 3 were diagnosed with clinically insignificant cancer, the researchers reported.
Dr. Moore said the PSA and MRI-first approach spared men from biopsies as well as the downsides of active surveillance, which include close monitoring with urology visits and occasional MRIs or biopsies over many years. Biopsies are considered undesirable because of pain and the risk for sepsis and other infections associated with transrectal biopsies.
But urologists in America were less convinced by the international data. William J. Catalona, MD, a urologist at Northwestern University in Chicago, who developed the PSA screening test in the 1990s, said he wasn’t surprised so many men in ReIMAGINE with low PSAs had advanced cancers. “Some of the most aggressive prostate cancers occur in men with a low PSA level – not new news,” he said.
Dr. Catalona also disagreed with the UK researchers’ emphasis on MRIs because the readings often are incorrect. A 2021 study in Prostate Cancer and Prostatic Diseases reported that multiparametric MRI had a false-negative rate of between 10% and 20%.
“MRI alone should not be considered more reliable than PSA. Rather, it should be considered complementary,” he said.
Michael S. Leapman, MD, MHS, associate professor of urology at the Yale Cancer Center, New Haven, Conn., said the UK findings point to a role for MRI as a “triage tool” to help identify men with elevated PSAs who should have a prostate biopsy.
But he said the research to date doesn’t support the use of MRI as a stand-alone test for prostate cancer. “In my opinion, it would have to demonstrate some tangible benefit to patients other than finding a greater number of cancers, such as improvement in cancer control, lower burden from the disease overall, or cancer-specific survival,” he said.
Major U.S. guidelines recommend including MRIs before biopsies. Dr. Leapman also pointed out that 2023 recommendations from the National Comprehensive Cancer Network state that MRI is “strongly recommended if available.” Yet fewer than half of U.S. urologists use MRIs as a screening tool, he said.
“My sense is that MRI is not available everywhere. We have also seen that wait times are too long in some centers, leading physicians and patients to opt for biopsy – particularly in cases with higher suspicion,” he said.
The studies from Sweden and the United Kingdom “demonstrate the strides being made in reducing overdetection of low-grade prostate cancer will increase detection of clinically significant Gleason 3+4 or higher” tumors, Dr. Leapman said. “It is unclear whether such patients in whom their otherwise low-risk disease is recast as ‘intermediate risk’ meaningfully stand to benefit in the long term from this detection.”
Dr. Robinson reported no relevant financial conflicts of interest. The Swedish Cancer Society, the Swedish Research Council, Region Jönköping, Futurum, and Clinical Cancer Research Foundation in Jönköping supported the Swedish study. Members of the ReIMAGINE study team disclosed research support from the United Kingdom’s National Institute of Health Research and various industry/other sources. The Medical Research Council and Cancer Research UK funded the ReIMAGINE study.
A version of this article appeared on Medscape.com.
In the ReIMAGINE study, a group of researchers from the United Kingdom found that half of men with apparently “safe” levels of prostate-specific antigen (PSA) below 3 ng/mL had clinically significant prostate cancers when multiparametric MRI was added to screening. The researchers, whose paper appeared in BMJ Oncology, also found that one in six screened men had a prostate lesion on MRI.
Meanwhile, a large Swedish population-based study, published in JAMA Network Open, showed that pre-biopsy MRIs combined with PSA testing after adoption of guidelines recommending MRIs led to a decrease in the proportion of men with negative biopsies (28% to 7%) and the number of Gleason score 6 cancers (24% to 6%), while the proportion of Gleason score 7-10 cancers rose from 49% to 86%.
Researchers compared prostate MRI uptake rates in the Jönköping Region in southern Sweden over 9 years – 2011 through 2018 before prostate MRIs were recommended nationally, and 2018-2020 when MRIs became commonly used.
David Robinson, MD, PhD, associate professor at Linköping University and leader of the Swedish study, told this news organization: “MRI is now standard for men before biopsy” in that country. In Sweden, which has a high rate of mortality from prostate cancer – about 50 deaths per 100,000 men vs. 12 and 8 per 100,000 in the United Kingdom and United States, respectively – PSA testing is not routine. “Most men that are diagnosed with prostate cancer have no symptoms. They have asked for a PSA when they have visited their general practitioner,” Dr. Robinson said. “To take a PSA test is not encouraged but it is not discouraged either. It is up to each man to decide.”
PSA screening is not common in the United Kingdom. Caroline Moore, MD, chair of urology at University College London and principal investigator on ReIMAGINE, said only 20% of UK men older than age 50 undergo PSA tests because doctors in the United Kingdom are concerned about the sort of overdiagnosis and overtreatment of prostate cancer that has occurred in the United States since the mid-1990s, when PSA screening was adopted here.
The rate of PSA screening in the United States has declined with controversies over recommendations for screening, though they remain above European rates: 37% in 2019, down from 47% in 2005, according to a 2022 Veterans Administration study published in JAMA Oncology.
In the UK study, Dr. Moore’s hospital-based group asked general practitioners to send letters to 2,096 men aged 50-75 years who had not been diagnosed with prostate cancer, inviting them to undergo prostate health checks combining screening with PSA and 10-minute prostate MRIs.
Of the 457 men who responded to the letters, 303 completed both screening tests. Older White men were more likely to respond, and Black men responded 20% less often.
Of the men who completed screening, 29 (9.6%) were diagnosed with clinically significant cancer and 3 were diagnosed with clinically insignificant cancer, the researchers reported.
Dr. Moore said the PSA and MRI-first approach spared men from biopsies as well as the downsides of active surveillance, which include close monitoring with urology visits and occasional MRIs or biopsies over many years. Biopsies are considered undesirable because of pain and the risk for sepsis and other infections associated with transrectal biopsies.
But urologists in America were less convinced by the international data. William J. Catalona, MD, a urologist at Northwestern University in Chicago, who developed the PSA screening test in the 1990s, said he wasn’t surprised so many men in ReIMAGINE with low PSAs had advanced cancers. “Some of the most aggressive prostate cancers occur in men with a low PSA level – not new news,” he said.
Dr. Catalona also disagreed with the UK researchers’ emphasis on MRIs because the readings often are incorrect. A 2021 study in Prostate Cancer and Prostatic Diseases reported that multiparametric MRI had a false-negative rate of between 10% and 20%.
“MRI alone should not be considered more reliable than PSA. Rather, it should be considered complementary,” he said.
Michael S. Leapman, MD, MHS, associate professor of urology at the Yale Cancer Center, New Haven, Conn., said the UK findings point to a role for MRI as a “triage tool” to help identify men with elevated PSAs who should have a prostate biopsy.
But he said the research to date doesn’t support the use of MRI as a stand-alone test for prostate cancer. “In my opinion, it would have to demonstrate some tangible benefit to patients other than finding a greater number of cancers, such as improvement in cancer control, lower burden from the disease overall, or cancer-specific survival,” he said.
Major U.S. guidelines recommend including MRIs before biopsies. Dr. Leapman also pointed out that 2023 recommendations from the National Comprehensive Cancer Network state that MRI is “strongly recommended if available.” Yet fewer than half of U.S. urologists use MRIs as a screening tool, he said.
“My sense is that MRI is not available everywhere. We have also seen that wait times are too long in some centers, leading physicians and patients to opt for biopsy – particularly in cases with higher suspicion,” he said.
The studies from Sweden and the United Kingdom “demonstrate the strides being made in reducing overdetection of low-grade prostate cancer will increase detection of clinically significant Gleason 3+4 or higher” tumors, Dr. Leapman said. “It is unclear whether such patients in whom their otherwise low-risk disease is recast as ‘intermediate risk’ meaningfully stand to benefit in the long term from this detection.”
Dr. Robinson reported no relevant financial conflicts of interest. The Swedish Cancer Society, the Swedish Research Council, Region Jönköping, Futurum, and Clinical Cancer Research Foundation in Jönköping supported the Swedish study. Members of the ReIMAGINE study team disclosed research support from the United Kingdom’s National Institute of Health Research and various industry/other sources. The Medical Research Council and Cancer Research UK funded the ReIMAGINE study.
A version of this article appeared on Medscape.com.
In the ReIMAGINE study, a group of researchers from the United Kingdom found that half of men with apparently “safe” levels of prostate-specific antigen (PSA) below 3 ng/mL had clinically significant prostate cancers when multiparametric MRI was added to screening. The researchers, whose paper appeared in BMJ Oncology, also found that one in six screened men had a prostate lesion on MRI.
Meanwhile, a large Swedish population-based study, published in JAMA Network Open, showed that pre-biopsy MRIs combined with PSA testing after adoption of guidelines recommending MRIs led to a decrease in the proportion of men with negative biopsies (28% to 7%) and the number of Gleason score 6 cancers (24% to 6%), while the proportion of Gleason score 7-10 cancers rose from 49% to 86%.
Researchers compared prostate MRI uptake rates in the Jönköping Region in southern Sweden over 9 years – 2011 through 2018 before prostate MRIs were recommended nationally, and 2018-2020 when MRIs became commonly used.
David Robinson, MD, PhD, associate professor at Linköping University and leader of the Swedish study, told this news organization: “MRI is now standard for men before biopsy” in that country. In Sweden, which has a high rate of mortality from prostate cancer – about 50 deaths per 100,000 men vs. 12 and 8 per 100,000 in the United Kingdom and United States, respectively – PSA testing is not routine. “Most men that are diagnosed with prostate cancer have no symptoms. They have asked for a PSA when they have visited their general practitioner,” Dr. Robinson said. “To take a PSA test is not encouraged but it is not discouraged either. It is up to each man to decide.”
PSA screening is not common in the United Kingdom. Caroline Moore, MD, chair of urology at University College London and principal investigator on ReIMAGINE, said only 20% of UK men older than age 50 undergo PSA tests because doctors in the United Kingdom are concerned about the sort of overdiagnosis and overtreatment of prostate cancer that has occurred in the United States since the mid-1990s, when PSA screening was adopted here.
The rate of PSA screening in the United States has declined with controversies over recommendations for screening, though they remain above European rates: 37% in 2019, down from 47% in 2005, according to a 2022 Veterans Administration study published in JAMA Oncology.
In the UK study, Dr. Moore’s hospital-based group asked general practitioners to send letters to 2,096 men aged 50-75 years who had not been diagnosed with prostate cancer, inviting them to undergo prostate health checks combining screening with PSA and 10-minute prostate MRIs.
Of the 457 men who responded to the letters, 303 completed both screening tests. Older White men were more likely to respond, and Black men responded 20% less often.
Of the men who completed screening, 29 (9.6%) were diagnosed with clinically significant cancer and 3 were diagnosed with clinically insignificant cancer, the researchers reported.
Dr. Moore said the PSA and MRI-first approach spared men from biopsies as well as the downsides of active surveillance, which include close monitoring with urology visits and occasional MRIs or biopsies over many years. Biopsies are considered undesirable because of pain and the risk for sepsis and other infections associated with transrectal biopsies.
But urologists in America were less convinced by the international data. William J. Catalona, MD, a urologist at Northwestern University in Chicago, who developed the PSA screening test in the 1990s, said he wasn’t surprised so many men in ReIMAGINE with low PSAs had advanced cancers. “Some of the most aggressive prostate cancers occur in men with a low PSA level – not new news,” he said.
Dr. Catalona also disagreed with the UK researchers’ emphasis on MRIs because the readings often are incorrect. A 2021 study in Prostate Cancer and Prostatic Diseases reported that multiparametric MRI had a false-negative rate of between 10% and 20%.
“MRI alone should not be considered more reliable than PSA. Rather, it should be considered complementary,” he said.
Michael S. Leapman, MD, MHS, associate professor of urology at the Yale Cancer Center, New Haven, Conn., said the UK findings point to a role for MRI as a “triage tool” to help identify men with elevated PSAs who should have a prostate biopsy.
But he said the research to date doesn’t support the use of MRI as a stand-alone test for prostate cancer. “In my opinion, it would have to demonstrate some tangible benefit to patients other than finding a greater number of cancers, such as improvement in cancer control, lower burden from the disease overall, or cancer-specific survival,” he said.
Major U.S. guidelines recommend including MRIs before biopsies. Dr. Leapman also pointed out that 2023 recommendations from the National Comprehensive Cancer Network state that MRI is “strongly recommended if available.” Yet fewer than half of U.S. urologists use MRIs as a screening tool, he said.
“My sense is that MRI is not available everywhere. We have also seen that wait times are too long in some centers, leading physicians and patients to opt for biopsy – particularly in cases with higher suspicion,” he said.
The studies from Sweden and the United Kingdom “demonstrate the strides being made in reducing overdetection of low-grade prostate cancer will increase detection of clinically significant Gleason 3+4 or higher” tumors, Dr. Leapman said. “It is unclear whether such patients in whom their otherwise low-risk disease is recast as ‘intermediate risk’ meaningfully stand to benefit in the long term from this detection.”
Dr. Robinson reported no relevant financial conflicts of interest. The Swedish Cancer Society, the Swedish Research Council, Region Jönköping, Futurum, and Clinical Cancer Research Foundation in Jönköping supported the Swedish study. Members of the ReIMAGINE study team disclosed research support from the United Kingdom’s National Institute of Health Research and various industry/other sources. The Medical Research Council and Cancer Research UK funded the ReIMAGINE study.
A version of this article appeared on Medscape.com.
One in five men carries high-risk HPV in international study
Findings from a meta-analysis of 65 studies conducted in 35 countries indicate that These estimates provide further weight to arguments in favor of vaccinating boys against HPV to prevent certain types of cancer.
“Our results support that sexually active men, regardless of age, are an important reservoir of HPV genital infection,” wrote the authors in The Lancet Global Health . “These estimates emphasize the importance of incorporating men into comprehensive HPV prevention strategies to reduce HPV-related morbidity and mortality in men and ultimately achieve elimination of cervical cancer and other HPV-related diseases.”
Literature review
HPV infection is the most common sexually transmitted viral infection worldwide. More than 200 HPV types can be transmitted sexually, and at least 12 types are oncogenic. Previous studies have shown that most sexually active men and women acquire at least one genital HPV infection during their lifetime.
Although most HPV infections are asymptomatic, they can lead to cancer. Indeed, HPV is involved in the development of cervical, vulval, and vaginal cancers, as well as oropharyngeal and anal cancers, which also affect the male population. More than 25% of cancers caused by HPV occur in men.
Despite these observations, fewer epidemiologic studies have assessed HPV infection in men than in women. To determine the prevalence of HPV infection in the male population, Laia Bruni, MD, MPH, PhD, an epidemiologist at the Catalan Institute of Oncology in Barcelona, and her colleagues collated data from 65 studies conducted in 35 countries pertaining to males older than 15 years.
In this literature review, the researchers selected studies that reported infection rates in males without HPV-related symptoms. Studies conducted exclusively in populations that were considered at increased risk for sexually transmitted infections (STIs) were excluded. Overall, the analysis included close to 45,000 men.
Prevalent HPV genotype
Testing for HPV was conducted on samples collected from the anus and genitals. The results show a global pooled prevalence of HPV infection in males older than 15 years of 31% for any HPV and 21% for HR-HPV. One of these viruses, HPV-16, was the most prevalent HPV genotype (5% prevalence).
HPV prevalence was highest among young adults. It stabilized and decreased from age 50 years. Between ages 25 and 29 years, 35% of men are infected with HPV. It should be noted that prevalence is already high in the youngest group, reaching 28% in males between the ages of 15 and 19 years. The variations are similar for HR-HPV infections.
This age-related change is different from rates in women. Among the female population, HPV prevalence peaks soon after first sexual activity and declines with age, with a slight rebound after ages 50–55 years (i.e., often after or around the time of menopause), wrote the researchers.
The results also show country- and region-based disparities. The pooled prevalence for any HPV was highest in Sub-Saharan Africa (37%), followed by Europe and Northern America (36%). The lowest prevalence was in East and Southeast Asia (15%). Here again, the trends are similar with high-risk HPV.
Preventive measures
“Our study draws attention to the high prevalence, ranging from 20% to 30% for HR-HPV in men across most regions, and the need for strengthening HPV prevention within overall STI control efforts,” wrote the authors.
“Future epidemiological studies are needed to monitor trends in prevalence in men, especially considering the roll-out of HPV vaccination in girls and young women and that many countries are beginning to vaccinate boys.”
In France, the HPV vaccination program was extended in 2021 to include all boys between the ages of 11 and 14 years (two-dose schedule), with a catch-up course in males up to age 19 years (three-dose schedule). This is the same vaccine program as for girls. It is also recommended for men up to age 26 years who have sex with other men.
The 2023 return to school will see the launch of a general vaccination campaign aimed at seventh-grade students, both boys and girls, with parental consent, to increase vaccine coverage. In 2021, vaccine uptake was 43.6% in girls between the ages of 15 and 18 years and scarcely 6% in boys, according to Public Health France.
Two vaccines are in use: the bivalent Cervarix vaccine, which is effective against HPV-16 and HPV-18, and the nonavalent Gardasil 9, which is effective against types 16, 18, 31, 33, 45, 52, and 58. Both provide protection against HPV-16, the type most common in men, which is responsible for more than half of cases of cervical cancer.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Findings from a meta-analysis of 65 studies conducted in 35 countries indicate that These estimates provide further weight to arguments in favor of vaccinating boys against HPV to prevent certain types of cancer.
“Our results support that sexually active men, regardless of age, are an important reservoir of HPV genital infection,” wrote the authors in The Lancet Global Health . “These estimates emphasize the importance of incorporating men into comprehensive HPV prevention strategies to reduce HPV-related morbidity and mortality in men and ultimately achieve elimination of cervical cancer and other HPV-related diseases.”
Literature review
HPV infection is the most common sexually transmitted viral infection worldwide. More than 200 HPV types can be transmitted sexually, and at least 12 types are oncogenic. Previous studies have shown that most sexually active men and women acquire at least one genital HPV infection during their lifetime.
Although most HPV infections are asymptomatic, they can lead to cancer. Indeed, HPV is involved in the development of cervical, vulval, and vaginal cancers, as well as oropharyngeal and anal cancers, which also affect the male population. More than 25% of cancers caused by HPV occur in men.
Despite these observations, fewer epidemiologic studies have assessed HPV infection in men than in women. To determine the prevalence of HPV infection in the male population, Laia Bruni, MD, MPH, PhD, an epidemiologist at the Catalan Institute of Oncology in Barcelona, and her colleagues collated data from 65 studies conducted in 35 countries pertaining to males older than 15 years.
In this literature review, the researchers selected studies that reported infection rates in males without HPV-related symptoms. Studies conducted exclusively in populations that were considered at increased risk for sexually transmitted infections (STIs) were excluded. Overall, the analysis included close to 45,000 men.
Prevalent HPV genotype
Testing for HPV was conducted on samples collected from the anus and genitals. The results show a global pooled prevalence of HPV infection in males older than 15 years of 31% for any HPV and 21% for HR-HPV. One of these viruses, HPV-16, was the most prevalent HPV genotype (5% prevalence).
HPV prevalence was highest among young adults. It stabilized and decreased from age 50 years. Between ages 25 and 29 years, 35% of men are infected with HPV. It should be noted that prevalence is already high in the youngest group, reaching 28% in males between the ages of 15 and 19 years. The variations are similar for HR-HPV infections.
This age-related change is different from rates in women. Among the female population, HPV prevalence peaks soon after first sexual activity and declines with age, with a slight rebound after ages 50–55 years (i.e., often after or around the time of menopause), wrote the researchers.
The results also show country- and region-based disparities. The pooled prevalence for any HPV was highest in Sub-Saharan Africa (37%), followed by Europe and Northern America (36%). The lowest prevalence was in East and Southeast Asia (15%). Here again, the trends are similar with high-risk HPV.
Preventive measures
“Our study draws attention to the high prevalence, ranging from 20% to 30% for HR-HPV in men across most regions, and the need for strengthening HPV prevention within overall STI control efforts,” wrote the authors.
“Future epidemiological studies are needed to monitor trends in prevalence in men, especially considering the roll-out of HPV vaccination in girls and young women and that many countries are beginning to vaccinate boys.”
In France, the HPV vaccination program was extended in 2021 to include all boys between the ages of 11 and 14 years (two-dose schedule), with a catch-up course in males up to age 19 years (three-dose schedule). This is the same vaccine program as for girls. It is also recommended for men up to age 26 years who have sex with other men.
The 2023 return to school will see the launch of a general vaccination campaign aimed at seventh-grade students, both boys and girls, with parental consent, to increase vaccine coverage. In 2021, vaccine uptake was 43.6% in girls between the ages of 15 and 18 years and scarcely 6% in boys, according to Public Health France.
Two vaccines are in use: the bivalent Cervarix vaccine, which is effective against HPV-16 and HPV-18, and the nonavalent Gardasil 9, which is effective against types 16, 18, 31, 33, 45, 52, and 58. Both provide protection against HPV-16, the type most common in men, which is responsible for more than half of cases of cervical cancer.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Findings from a meta-analysis of 65 studies conducted in 35 countries indicate that These estimates provide further weight to arguments in favor of vaccinating boys against HPV to prevent certain types of cancer.
“Our results support that sexually active men, regardless of age, are an important reservoir of HPV genital infection,” wrote the authors in The Lancet Global Health . “These estimates emphasize the importance of incorporating men into comprehensive HPV prevention strategies to reduce HPV-related morbidity and mortality in men and ultimately achieve elimination of cervical cancer and other HPV-related diseases.”
Literature review
HPV infection is the most common sexually transmitted viral infection worldwide. More than 200 HPV types can be transmitted sexually, and at least 12 types are oncogenic. Previous studies have shown that most sexually active men and women acquire at least one genital HPV infection during their lifetime.
Although most HPV infections are asymptomatic, they can lead to cancer. Indeed, HPV is involved in the development of cervical, vulval, and vaginal cancers, as well as oropharyngeal and anal cancers, which also affect the male population. More than 25% of cancers caused by HPV occur in men.
Despite these observations, fewer epidemiologic studies have assessed HPV infection in men than in women. To determine the prevalence of HPV infection in the male population, Laia Bruni, MD, MPH, PhD, an epidemiologist at the Catalan Institute of Oncology in Barcelona, and her colleagues collated data from 65 studies conducted in 35 countries pertaining to males older than 15 years.
In this literature review, the researchers selected studies that reported infection rates in males without HPV-related symptoms. Studies conducted exclusively in populations that were considered at increased risk for sexually transmitted infections (STIs) were excluded. Overall, the analysis included close to 45,000 men.
Prevalent HPV genotype
Testing for HPV was conducted on samples collected from the anus and genitals. The results show a global pooled prevalence of HPV infection in males older than 15 years of 31% for any HPV and 21% for HR-HPV. One of these viruses, HPV-16, was the most prevalent HPV genotype (5% prevalence).
HPV prevalence was highest among young adults. It stabilized and decreased from age 50 years. Between ages 25 and 29 years, 35% of men are infected with HPV. It should be noted that prevalence is already high in the youngest group, reaching 28% in males between the ages of 15 and 19 years. The variations are similar for HR-HPV infections.
This age-related change is different from rates in women. Among the female population, HPV prevalence peaks soon after first sexual activity and declines with age, with a slight rebound after ages 50–55 years (i.e., often after or around the time of menopause), wrote the researchers.
The results also show country- and region-based disparities. The pooled prevalence for any HPV was highest in Sub-Saharan Africa (37%), followed by Europe and Northern America (36%). The lowest prevalence was in East and Southeast Asia (15%). Here again, the trends are similar with high-risk HPV.
Preventive measures
“Our study draws attention to the high prevalence, ranging from 20% to 30% for HR-HPV in men across most regions, and the need for strengthening HPV prevention within overall STI control efforts,” wrote the authors.
“Future epidemiological studies are needed to monitor trends in prevalence in men, especially considering the roll-out of HPV vaccination in girls and young women and that many countries are beginning to vaccinate boys.”
In France, the HPV vaccination program was extended in 2021 to include all boys between the ages of 11 and 14 years (two-dose schedule), with a catch-up course in males up to age 19 years (three-dose schedule). This is the same vaccine program as for girls. It is also recommended for men up to age 26 years who have sex with other men.
The 2023 return to school will see the launch of a general vaccination campaign aimed at seventh-grade students, both boys and girls, with parental consent, to increase vaccine coverage. In 2021, vaccine uptake was 43.6% in girls between the ages of 15 and 18 years and scarcely 6% in boys, according to Public Health France.
Two vaccines are in use: the bivalent Cervarix vaccine, which is effective against HPV-16 and HPV-18, and the nonavalent Gardasil 9, which is effective against types 16, 18, 31, 33, 45, 52, and 58. Both provide protection against HPV-16, the type most common in men, which is responsible for more than half of cases of cervical cancer.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
FROM THE LANCET GLOBAL HEALTH
The four questions you should ask about sexual health
This transcript has been edited for clarity.
When I went to med school, we were taught to take a sexual history. Do you smoke? Do you drink? Do you do drugs? Do you have sex? Men, women, or both? And that was it. We’re telling patients that sex is a vice, something that is dangerous and that you should feel bad about. But sex is how we’re all here and how we even continue as a species. We must get comfortable as doctors talking to our patients about sexual medicine.
What if we move away from sex being in the vice category – the part of the social history that’s the bad stuff you shouldn’t be doing? Maybe we should bring it into the review of systems.
As a very basic first step, I like to ask patients four things. As a sexual medicine doctor, I deal with these four things: libido, arousal, orgasm, and pain.
Why are these important? These are the things our patients really care about; 2.3 of every 1,000 people got divorced in 2021.
Libido. Women who have distressing low sexual desire have sex on average two and a half times per month. We call this mercy sex or duty sex. I don’t know what the half time per month looks like, but people genuinely care about desire and their doctors don’t really know that.
We have a biopsychosocial toolbox to help our patients. Let me give you an example: Antidepressants can have sexual side effects. Could there be medications in our toolbox that can help our patients? Of course there can, and there are. What about education or talk therapy? We should be asking our patients what they care about and why they care about it so we can help them achieve their quality-of-life goals.
Arousal. What about arousal? Did you know that erections are a marker of cardiovascular disease in men? We know this to be true for men, and I’m certain the research would be no different for women. We know that there are many biological causes for decrease in arousal, including sleep apnea, diabetes, hypertension, and smoking. I can convince a lot of men to quit smoking because I tell them it’s bad for their penis. We have to understand what our patients care about and then advise them on why we think we can help improve these issues.
Orgasm. How about orgasm? Have you ever been asked whether you can orgasm? Have you ever been asked whether you have questions about orgasm? About 15%-20% of women report having an orgasm disorder, and we rarely talk about this in an exam room. I’ve certainly never been asked, and everybody knows what I do for a living. Not to mention all the men that I and my colleagues see who have really distressing premature ejaculation or delayed orgasm. This is pathophysiology at its finest and most complex. It is so interesting, and we have so much to learn and understand about orgasm in general.
Pain. Finally, ask about pain. It seems obvious that we should be asking our patients about their pain, which includes pelvic pain, but oftentimes we avoid talking about private parts. Pain affects not just our patients, but also their partners and their families, when our patients can’t sit without discomfort, if they can’t go and perform the daily activities that bring them joy and belonging. We have to really work with our toolbox in a biopsychosocial manner to help our patients. I often use the incredible rehabilitation specialists called pelvic floor physical therapists.
Remember, we’re talking about libido, arousal, orgasm, and pain. Sex is important to us as a species. It’s important to our patients. Ask nonjudgmental and open-ended questions. You actually may be the only doctor to ever do so.
Dr. Rubin is an assistant clinical professor, department of urology, Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I went to med school, we were taught to take a sexual history. Do you smoke? Do you drink? Do you do drugs? Do you have sex? Men, women, or both? And that was it. We’re telling patients that sex is a vice, something that is dangerous and that you should feel bad about. But sex is how we’re all here and how we even continue as a species. We must get comfortable as doctors talking to our patients about sexual medicine.
What if we move away from sex being in the vice category – the part of the social history that’s the bad stuff you shouldn’t be doing? Maybe we should bring it into the review of systems.
As a very basic first step, I like to ask patients four things. As a sexual medicine doctor, I deal with these four things: libido, arousal, orgasm, and pain.
Why are these important? These are the things our patients really care about; 2.3 of every 1,000 people got divorced in 2021.
Libido. Women who have distressing low sexual desire have sex on average two and a half times per month. We call this mercy sex or duty sex. I don’t know what the half time per month looks like, but people genuinely care about desire and their doctors don’t really know that.
We have a biopsychosocial toolbox to help our patients. Let me give you an example: Antidepressants can have sexual side effects. Could there be medications in our toolbox that can help our patients? Of course there can, and there are. What about education or talk therapy? We should be asking our patients what they care about and why they care about it so we can help them achieve their quality-of-life goals.
Arousal. What about arousal? Did you know that erections are a marker of cardiovascular disease in men? We know this to be true for men, and I’m certain the research would be no different for women. We know that there are many biological causes for decrease in arousal, including sleep apnea, diabetes, hypertension, and smoking. I can convince a lot of men to quit smoking because I tell them it’s bad for their penis. We have to understand what our patients care about and then advise them on why we think we can help improve these issues.
Orgasm. How about orgasm? Have you ever been asked whether you can orgasm? Have you ever been asked whether you have questions about orgasm? About 15%-20% of women report having an orgasm disorder, and we rarely talk about this in an exam room. I’ve certainly never been asked, and everybody knows what I do for a living. Not to mention all the men that I and my colleagues see who have really distressing premature ejaculation or delayed orgasm. This is pathophysiology at its finest and most complex. It is so interesting, and we have so much to learn and understand about orgasm in general.
Pain. Finally, ask about pain. It seems obvious that we should be asking our patients about their pain, which includes pelvic pain, but oftentimes we avoid talking about private parts. Pain affects not just our patients, but also their partners and their families, when our patients can’t sit without discomfort, if they can’t go and perform the daily activities that bring them joy and belonging. We have to really work with our toolbox in a biopsychosocial manner to help our patients. I often use the incredible rehabilitation specialists called pelvic floor physical therapists.
Remember, we’re talking about libido, arousal, orgasm, and pain. Sex is important to us as a species. It’s important to our patients. Ask nonjudgmental and open-ended questions. You actually may be the only doctor to ever do so.
Dr. Rubin is an assistant clinical professor, department of urology, Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I went to med school, we were taught to take a sexual history. Do you smoke? Do you drink? Do you do drugs? Do you have sex? Men, women, or both? And that was it. We’re telling patients that sex is a vice, something that is dangerous and that you should feel bad about. But sex is how we’re all here and how we even continue as a species. We must get comfortable as doctors talking to our patients about sexual medicine.
What if we move away from sex being in the vice category – the part of the social history that’s the bad stuff you shouldn’t be doing? Maybe we should bring it into the review of systems.
As a very basic first step, I like to ask patients four things. As a sexual medicine doctor, I deal with these four things: libido, arousal, orgasm, and pain.
Why are these important? These are the things our patients really care about; 2.3 of every 1,000 people got divorced in 2021.
Libido. Women who have distressing low sexual desire have sex on average two and a half times per month. We call this mercy sex or duty sex. I don’t know what the half time per month looks like, but people genuinely care about desire and their doctors don’t really know that.
We have a biopsychosocial toolbox to help our patients. Let me give you an example: Antidepressants can have sexual side effects. Could there be medications in our toolbox that can help our patients? Of course there can, and there are. What about education or talk therapy? We should be asking our patients what they care about and why they care about it so we can help them achieve their quality-of-life goals.
Arousal. What about arousal? Did you know that erections are a marker of cardiovascular disease in men? We know this to be true for men, and I’m certain the research would be no different for women. We know that there are many biological causes for decrease in arousal, including sleep apnea, diabetes, hypertension, and smoking. I can convince a lot of men to quit smoking because I tell them it’s bad for their penis. We have to understand what our patients care about and then advise them on why we think we can help improve these issues.
Orgasm. How about orgasm? Have you ever been asked whether you can orgasm? Have you ever been asked whether you have questions about orgasm? About 15%-20% of women report having an orgasm disorder, and we rarely talk about this in an exam room. I’ve certainly never been asked, and everybody knows what I do for a living. Not to mention all the men that I and my colleagues see who have really distressing premature ejaculation or delayed orgasm. This is pathophysiology at its finest and most complex. It is so interesting, and we have so much to learn and understand about orgasm in general.
Pain. Finally, ask about pain. It seems obvious that we should be asking our patients about their pain, which includes pelvic pain, but oftentimes we avoid talking about private parts. Pain affects not just our patients, but also their partners and their families, when our patients can’t sit without discomfort, if they can’t go and perform the daily activities that bring them joy and belonging. We have to really work with our toolbox in a biopsychosocial manner to help our patients. I often use the incredible rehabilitation specialists called pelvic floor physical therapists.
Remember, we’re talking about libido, arousal, orgasm, and pain. Sex is important to us as a species. It’s important to our patients. Ask nonjudgmental and open-ended questions. You actually may be the only doctor to ever do so.
Dr. Rubin is an assistant clinical professor, department of urology, Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
An STI upsurge requires a nimble approach to care
Except for a drop in the number of sexually transmitted infections (STIs) early in the COVID-19 pandemic (March and April 2020), the incidence of STIs has been rising throughout this century.1 In 2018, 1 in 5 people in the United States had an STI; 26 million new cases were reported that year, resulting in direct costs of $16 billion—85% of which was for the care of HIV infection.2 Also that year, infection with Chlamydia trachomatis (chlamydia), Trichomonas vaginalis (trichomoniasis), herpesvirus type 2 (genital herpes), and/or human papillomavirus (condylomata acuminata) constituted 97.6% of all prevalent and 93.1% of all incident STIs.3 Almost half (45.5%) of new cases of STIs occur in people between the ages of 15 and 24 years.3
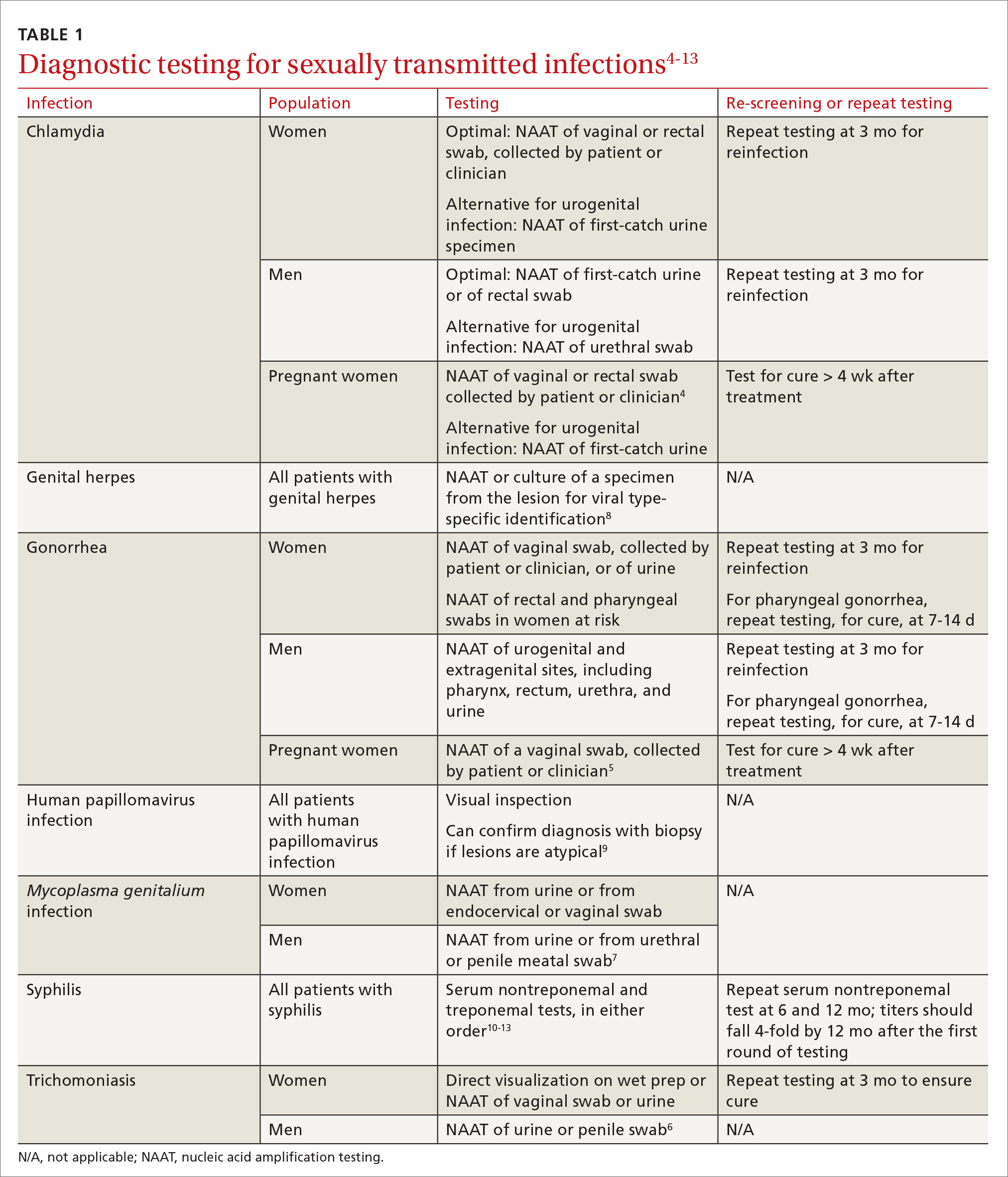
Three factors—changing social patterns, including the increase of social networking; the ability of antiviral therapy to decrease the spread of HIV, leading to a reduction in condom use; and increasing antibiotic resistance—have converged to force changes in screening and treatment recommendations. In this article, we summarize updated guidance for primary care clinicians from several sources—including the Centers for Disease Control and Prevention (CDC), the US Preventive Services Task Force (USPSTF), and the American Society for Colposcopy and Cervical Pathology (ASCCP)—on diagnosing STIs (TABLE 14-13) and providing guideline-based treatment (Table 214). Because of the breadth and complexity of HIV disease, it is not addressed here.
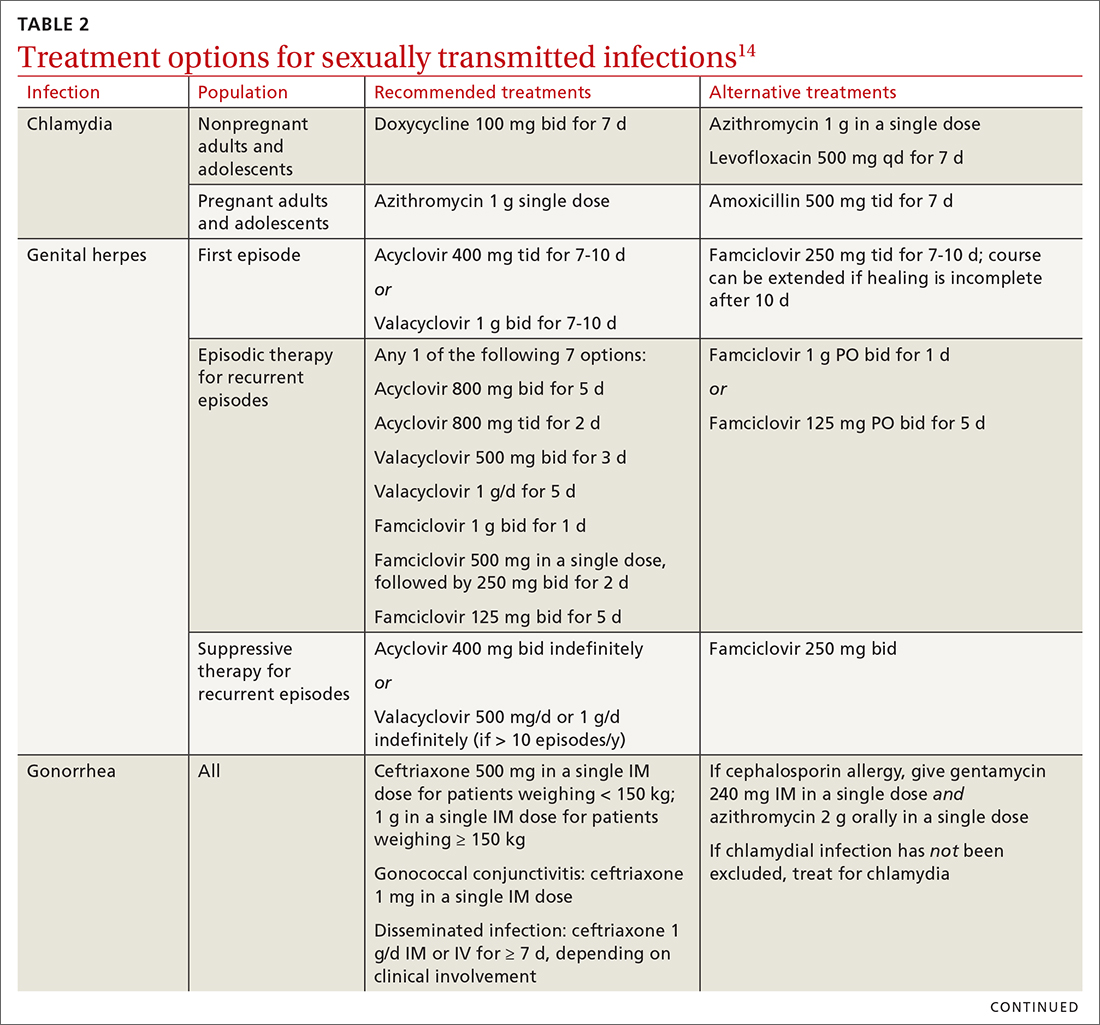
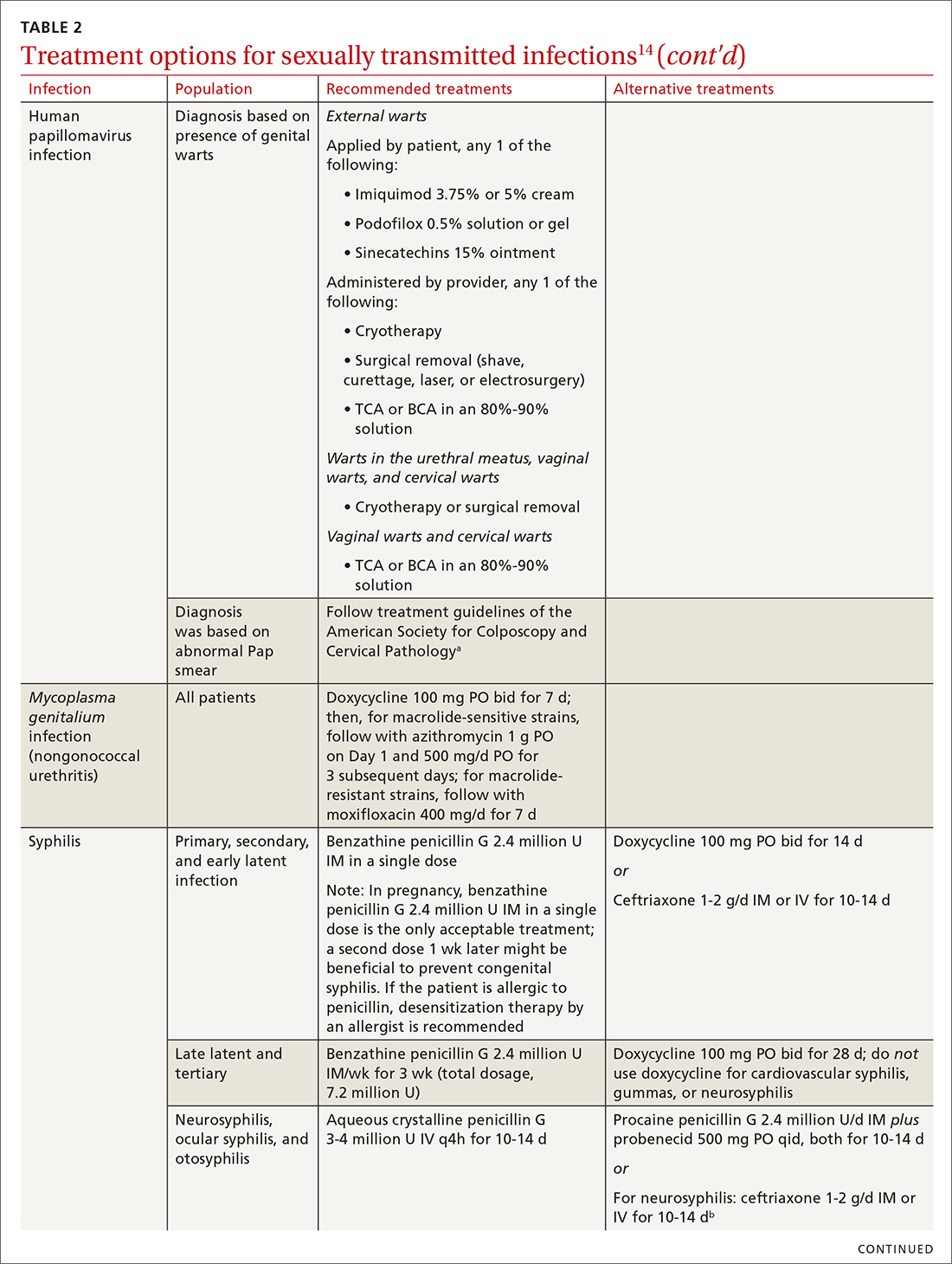
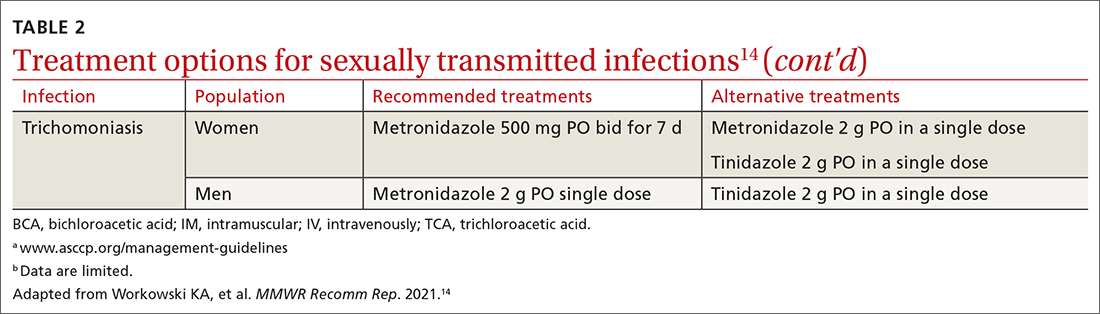
Chlamydia
Infection with Chlamydia trachomatis—the most commonly reported bacterial STI in the United States—primarily causes cervicitis in women and proctitis in men, and can cause urethritis and pharyngitis in men and women. Prevalence is highest in sexually active people younger than 24 years.15
Because most infected people are asymptomatic and show no signs of illness on physical exam, screening is recommended for all sexually active women younger than 25 years and all men who have sex with men (MSM).4 No studies have established proper screening intervals; a reasonable approach, therefore, is to repeat screening for patients who have a sexual history that confers a new or persistent risk for infection since their last negative result.
Depending on the location of the infection, symptoms of chlamydia can include vaginal or penile irritation or discharge, dysuria, pelvic or rectal pain, and sore throat. Breakthrough bleeding in a patient who is taking an oral contraceptive should raise suspicion for chlamydia.
Untreated chlamydia can lead to pelvic inflammatory disease (PID), tubo-ovarian abscess, tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. Infection can be transmitted vertically (mother to baby) antenatally, which can cause ophthalmia neonatorum and pneumonia in these newborns.
Diagnosis. The diagnosis of chlamydia is made using nucleic acid amplification testing (NAAT). Specimens can be collected by the clinician or the patient (self collected) using a vaginal, rectal, or oropharyngeal swab, or a combination of these, and can be obtained from urine or liquid-based cytology material.16
Continue to: Treatment
Treatment. Recommendations for treating chlamydia were updated by the CDC in its 2021 treatment guidelines (Table 214). Doxycycline 100 mg bid for 7 days is the preferred regimen; alternative regiments are (1) azithromycin 1 g in a single dose and (2) levofloxacin 500 mg daily for 7 days.4 A meta-analysis17 and a Cochrane review18 showed that the rate of treatment failure was higher among men when they were treated with azithromycin instead of doxycycline; furthermore, a randomized controlled trial demonstrated that doxycycline is more effective than azithromycin (cure rate, 100%, compared to 74%) at treating rectal chlamydia in MSM.19
Azithromycin is efficacious for urogenital infection in women; however, there is concern that the 33% to 83% of women who have concomitant rectal infection (despite reporting no receptive anorectal sexual activity) would be insufficiently treated. Outside pregnancy, the CDC does not recommend a test of cure but does recommend follow-up testing for reinfection in 3 months. Patients should abstain from sexual activity until 7 days after all sexual partners have been treated.
Expedited partner therapy (EPT) is the practice of treating sexual partners of patients with known chlamydia (and patients with gonococcal infection). Unless prohibited by law in your state, offer EPT to patients with chlamydia if they cannot ensure that their sexual partners from the past 60 days will seek timely treatment.a
Evidence to support EPT comes from 3 US clinical trials, whose subjects comprised heterosexual men and women with chlamydia or gonorrhea.21-23 The role of EPT for MSM is unclear; data are limited. Shared decision-making is recommended to determine whether EPT should be provided, to ensure that co-infection with other bacterial STIs (eg, syphilis) or HIV is not missed.24-26
a Visit www.cdc.gov/std/ept to read updated information about laws and regulations regarding EPT in your state.20
Gonorrhea
Gonorrhea is the second most-reported bacterial communicable disease.5 Infection with Neisseria gonorrhoeae causes urethral discharge in men, leading them to seek treatment; infected women, however, are often asymptomatic. Infected men and women might not recognize symptoms until they have transmitted the disease. Women have a slower natural clearance of gonococcal infection, which might explain their higher prevalence.27 Delayed recognition of symptoms can result in complications, including PID.5
Diagnosis. Specimens for NAAT can be obtained from urine, endocervical, vaginal, rectal, pharyngeal, and male urethral specimens. Reported sexual behaviors and exposures of women and transgender or gender-diverse people should be taken into consideration to determine whether rectal or pharyngeal testing, or both, should be performed.28 MSM should be screened annually at sites of contact, including the urethra, rectum, and pharynx.28 All patients with urogenital or rectal gonorrhea should be asked about oral sexual exposure; if reported, pharyngeal testing should be performed.5
NAAT of urine is at least as sensitive as testing of an endocervical specimen; the same specimen can be used to test for chlamydia and gonorrhea. Patient-collected specimens are a reasonable alternative to clinician-collected swab specimens.29
Continue to: Treatment
Treatment is complicated by the ability of gonorrhea to develop resistance. Intramuscular ceftriaxone 500 mg in a single dose cures 98% to 99% of infections in the United States; however, monitoring local resistance patterns in the community is an important component of treatment.28 (See Table 214 for an alternative regimen for cephalosporin-allergic patients and for treating gonococcal conjunctivitis and disseminated infection.)
In 2007, the CDC identified widespread quinolone-resistant gonococcal strains; therefore, fluoroquinolones no longer are recommended for treating gonorrhea.30 Cefixime has demonstrated only limited success in treating pharyngeal gonorrhea and does not attain a bactericidal level as high as ceftriaxone does; cefixime therefore is recommended only if ceftriaxone is unavailable.28 The national Gonococcal Isolate Surveillance Project is finding emerging evidence of the reduced susceptibility of N gonorrhoeae to azithromycin—making dual therapy for gonococcal infection no longer a recommendation.28
Patients should abstain from sex until 7 days after all sex partners have been treated for gonorrhea. As with chlamydia, the CDC does not recommend a test of cure for uncomplicated urogenital or rectal gonorrhea unless the patient is pregnant, but does recommend testing for reinfection 3 months after treatment.14 For patients with pharyngeal gonorrhea, a test of cure is recommended 7 to 14 days after initial treatment, due to challenges in treatment and because this site of infection is a potential source of antibiotic resistance.28
Trichomoniasis
T vaginalis, the most common nonviral STI worldwide,31 can manifest as a yellow-green vaginal discharge with or without vaginal discomfort, dysuria, epididymitis, and prostatitis; most cases, however, are asymptomatic. On examination, the cervix might be erythematous with punctate lesions (known as strawberry cervix).
Unlike most STIs, trichomoniasis is as common in women older than 24 years as it is in younger women. Infection is associated with a lower educational level, lower socioeconomic status, and having ≥ 2 sexual partners in the past year.32 Prevalence is approximately 10 times as high in Black women as it is in White women.
T vaginalis infection is associated with an increase in the risk for preterm birth, premature rupture of membranes, cervical cancer, and HIV infection. With a lack of high-quality clinical trials on the efficacy of screening, women with HIV are the only group for whom routine screening is recommended.6
Diagnosis. NAAT for trichomoniasis is now available in conjunction with gonorrhea and chlamydia testing of specimens on vaginal or urethral swabs and of urine specimens and liquid Pap smears.
Continue to: Treatment
Treatment. Because of greater efficacy, the treatment recommendation for women has changed from a single 2-g dose of oral metronidazole to 500 mg twice daily for 7 days. The 2-g single oral dose is still recommended for men7 (Table 214 lists alternative regimens).
Mycoplasma genitalium
Infection with M genitalium is common and often asymptomatic. The disease causes approximately 20% of all cases of nongonococcal and nonchlamydial urethritis in men and about 40% of persistent or recurrent infections. M genitalium is present in approximately 20% of women with cervicitis and has been associated with PID, preterm delivery, spontaneous abortion, and infertility.
There are limited and conflicting data regarding outcomes in infected patients other than those with persistent or recurrent infection; furthermore, resistance to azithromycin is increasing rapidly, resulting in an increase in treatment failures. Screening therefore is not recommended, and testing is recommended only in men with nongonococcal urethritis.33,34
Diagnosis. NAAT can be performed on urine or on a urethral, penile meatal, endocervical, or vaginal swab; men with recurrent urethritis or women with recurrent cervicitis should be tested. NAAT also can be considered in women with PID. Testing the specimen for the microorganism’s resistance to macrolide antibiotics is recommended (if such testing is available).
Treatment is initiated with doxycycline 100 mg twice daily for 7 days. If the organism is macrolide sensitive, follow with azithromycin 1 g orally on Day 1, then 500 mg/d for 3 more days. If the organism is macrolide resistant or testing is unavailable, follow doxycycline with oral moxifloxacin 400 mg/d for 7 days.33
Genital herpes (mostly herpesvirus type 2)
Genital herpes, characterized by painful, recurrent outbreaks of genital and anal lesions,35 is a lifelong infection that increases in prevalence with age.8 Because many infected people have disease that is undiagnosed or mild or have unrecognizable symptoms during viral shedding, most genital herpes infections are transmitted by people who are unaware that they are contagious.36 Herpesvirus type 2 (HSV-2) causes most cases of genital herpes, although an increasing percentage of cases are attributed to HSV type 1 (HSV-1) through receptive oral sex from a person who has an oral HSV-1 lesion.
Importantly, HSV-2–infected people are 2 to 3 times more likely to become infected with HIV than people who are not HSV-2 infected.37 This is because CD4+ T cells concentrate at the site of HSV lesions and express a higher level of cell-surface receptors that HIV uses to enter cells. HIV replicates 3 to 5 times more quickly in HSV-infected tissue.38
Continue to: HSV can become disseminated...
HSV can become disseminated, particularly in immunosuppressed people, and can manifest as encephalitis, hepatitis, and pneumonitis. Beyond its significant burden on health, HSV carries significant psychosocial consequences.9
Diagnosis. Clinical diagnosis can be challenging if classic lesions are absent at evaluation. If genital lesions are present, HSV can be identified by NAAT or culture of a specimen of those lesions. False-negative antibody results might be more frequent in early stages of infection; repeating antibody testing 12 weeks after presumed time of acquisition might therefore be indicated, based on clinical judgment. HSV-2 antibody positivity implies anogenital infection because almost all HSV-2 infections are sexually acquired.
HSV-1 antibody positivity alone is more difficult to interpret because this finding does not distinguish between oral and genital lesions, and most HSV-1 seropositivity is acquired during childhood.36 HSV polymerase chain reaction (PCR) testing of blood should not be performed to diagnose genital herpes infection, except in settings in which there is concern about disseminated infection.
Treatment. Management should address the acute episode and the chronic nature of genital herpes. Antivirals will not eradicate latent
- attenuate current infection
- prevent recurrence
- improve quality of life
- suppress the virus to prevent transmission to sexual partners.
All patients experiencing an initial episode of genital herpes should be treated, regardless of symptoms, due to the potential for prolonged or severe symptoms during recurrent episodes.9 Three drugs—acyclovir, valacyclovir, and famciclovir—are approved by the US Food and Drug Administration (FDA) to treat genital herpes and appear equally effective (TABLE 214).
Antiviral therapy for recurrent genital HSV infection can be administered either as suppressive therapy to reduce the frequency of recurrences or episodically to shorten the duration of lesions:
- Suppressive therapy reduces the frequency of recurrence by 70% to 80% among patients with frequent outbreaks. Long-term safety and efficacy are well established.
- Episodic therapy is most effective if started within 1 day after onset of lesions or during the prodrome.36
There is no specific recommendation for when to choose suppressive over episodic therapy; most patients prefer suppressive therapy because it improves quality of life. Use shared clinical decision-making to determine the best option for an individual patient.
Continue to: Human papillomavirus
Human papillomavirus
Condylomata acuminata (genital warts) are caused by human papillomavirus (HPV), most commonly types 6 and 11, which manifest as soft papules or plaques on the external genitalia, perineum, perianal skin, and groin. The warts are usually asymptomatic but can be painful or pruritic, depending on size and location.
Diagnosis is made by visual inspection and can be confirmed by biopsy if lesions are atypical. Lesions can resolve spontaneously, remain unchanged, or grow in size or number.
Treatment. The aim of treatment is relief of symptoms and removal of warts. Treatment does not eradicate HPV infection. Multiple treatments are available that can be applied by the patient as a cream, gel, or ointment or administered by the provider, including cryotherapy, surgical removal, and solutions. The decision on how to treat should be based on the number, size, and
HPV-associated cancers and precancers. This is a broad (and separate) topic. HPV types 16 and 18 cause most cases of cervical, penile, vulvar, vaginal, anal, and oropharyngeal cancer and precancer.39 The USPSTF, the American Cancer Society, and the American College of Obstetricians and Gynecologists all have recommendations for cervical cancer screening in the United States.40 Refer to guidelines of the ASCCP for recommendations on abnormal screening tests.41
Prevention of genital warts. The 9-valent HPV vaccine available in the United States is safe and effective and helps protect against viral types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Types 6 and 11 are the principal causes of genital warts. Types 16 and 18 cause 66% of cervical cancer. The vaccination series can be started at age 9 years and is recommended for everyone through age 26 years. Only 2 doses are needed if the first dose is given prior to age 15 years; given after that age, a 3-dose series is utilized. Refer to CDC vaccine guidelines42 for details on the exact timing of vaccination.
Vaccination for women ages 27 to 45 years is not universally recommended because most people have been exposed to HPV by that age. However, the vaccine can still be administered, depending on clinical circumstances and the risk for new infection.42
Syphilis
Caused by the spirochete Treponema pallidum, syphilis manifests across a spectrum—from congenital to tertiary. The inability of medical science to develop a method for culturing the spirochete has confounded diagnosis and treatment.
Continue to: Since reaching a historic...
Since reaching a historic nadir of incidence in 2000 (5979 cases in the United States), there has been an increasingly rapid rise in that number: to 130,000 in 2020. More than 50% of cases are in MSM; however, the number of cases in heterosexual women is rapidly increasing.43
Routine screening for syphilis should be performed in any person who is at risk: all pregnant women in the first trimester (and in the third trimester and at delivery if they are at risk or live in a community where prevalence is high) and annually in sexually active MSM or anyone with HIV infection.10
Diagnosis. Examination by dark-field microscopy, testing by PCR, and direct fluorescent antibody assay for T pallidum from lesion tissue or exudate provide definitive diagnosis for early and congenital syphilis, but are often unavailable.
Presumptive diagnosis requires 2 serologic tests:
- Nontreponemal tests (the VDRL and rapid plasma reagin tests) identify anticardiolipin antibodies released during syphilis infection, although results also can be elevated in autoimmune disease or after certain immunizations, including the COVID-19 vaccine.
- Treponemal tests (the fluorescent treponemal antibody absorbed assay, T pallidum particulate agglutination assay, enzyme immunoassay, and chemiluminescence immunoassay) are specific antibody tests.
Historically, reactive nontreponemal tests, which are less expensive and easier to perform, were followed by a treponemal test to confirm the presumptive diagnosis. This method continues to be reasonable when screening patients in a low-prevalence population.11 The reverse sequence screening algorithm (ie, begin with a treponemal test) is now frequently used. With this method, a positive treponemal test must be confirmed with a nontreponemal test. If the treponemal test is positive and the nontreponemal test is negative, another treponemal test must be positive to confirm the diagnosis. This algorithm is useful in high-risk populations because it provides earlier detection of recently acquired syphilis and enhanced detection of late latent syphilis.12,13,44 The CDC has not stated a diagnostic preference.
Once the diagnosis is made, a complete history (including a sexual history and a history of syphilis testing and treatment) and a physical exam are necessary to confirm stage of disease.45
Special circumstances. Neurosyphilis, ocular syphilis, and otosyphilis refer to the site of infection and can occur at any stage of disease. The nervous system usually is infected within hours of initial infection, but symptoms might take weeks or years to develop—or might never manifest. Any time a patient develops neurologic, ophthalmologic, or audiologic symptoms, careful neurologic and ophthalmologic evaluation should be performed and the patient should be tested for HIV.
Continue to: Lumbar puncture is warranted...
Lumbar puncture is warranted for evaluation of cerebrospinal fluid if neurologic symptoms are present but is not necessary for isolated ocular syphilis or otosyphilis without neurologic findings. Treatment should not be delayed for test results if ocular syphilis is suspected because permanent blindness can develop. Any patient at high risk for an STI who presents with neurologic or ophthalmologic symptoms should be tested for syphilis and HIV.45
Pregnant women who have a diagnosis of syphilis should be treated with penicillin immediately because treatment ≥ 30 days prior to delivery is likely to prevent most cases of congenital syphilis. However, a course of penicillin might not prevent stillbirth or congenital syphilis in a gravely infected fetus, evidenced by fetal syphilis on a sonogram at the time of treatment. Additional doses of penicillin in pregnant women with early syphilis might be indicated if there is evidence of fetal syphilis on ultrasonography. All women who deliver a stillborn infant (≥ 20 weeks’ gestation) should be tested for syphilis at delivery.46
All patients in whom primary or secondary syphilis has been diagnosed should be tested for HIV at the time of diagnosis and treatment; if the result is negative, they should be offered preexposure prophylaxis (PrEP; discussed shortly). If the incidence of HIV in your community is high, repeat testing for HIV in 3 months. Clinical and serologic evaluation should be performed 6 and 12 months after treatment.47
Treatment. Penicillin remains the standard treatment for syphilis. Primary, secondary, and early tertiary stages (including in pregnancy) are treated with benzathine penicillin G 2.4 million units intramuscular (IM) in a single dose. For pregnant patients, repeating that dose in 1 week generally is recommended. Patients in the late latent (> 1 year) or tertiary stage receive the same dose of penicillin, which is then repeated weekly, for a total of 3 doses. Doxycycline and ceftriaxone are alternatives, except in pregnancy.
Warn patients of the Jarisch-Herxheimer reaction: fever, headache, and myalgias associated with initiation of treatment in the presence of the high bacterial load seen in early syphilis. Treatment is symptomatic, but the Jarisch-Herxheimer reaction can cause fetal distress in pregnancy.
Otosyphilis, ocular syphilis, and neurosyphilis require intravenous (IV) aqueous crystalline penicillin G 3 to 4 million U every 4 hours for 10 to 14 days.45 Alternatively, procaine penicillin G 2.4 million U/d IM can be given daily with oral probenecid 500 mg qid, both for 10 to 14 days (TABLE 214).
Screening andprevention of STIs
Screening recommendations
Follow USPSTF screening guidelines for STIs.10,48-54 Screen annually for:
- gonorrhea and chlamydia in women ages 15 to 24 years and in women older than 25 years if they are at increased risk
- gonorrhea, chlamydia, syphilis, and HIV in MSM, and hepatitis C if they are HIV positive
- trichomoniasis in women who are HIV positive.
Continue to: Consider the community in which...
Consider the community in which you practice when determining risk; you might want to consult local public health authorities for information about local epidemiology and guidance on determining which of your patients are at increased risk.
Preexposure prophylaxis
According to the CDC, all sexually active adults and adolescents should be informed about the availability of PrEP to prevent HIV infection. PrEP should be (1) available to anyone who requests it and (2) recommended for anyone who is sexually active and who practices sexual behaviors that place them at substantial risk for exposure to or acquisition of HIV, or both.
The recommended treatment protocol for men and women who have either an HIV-positive partner or inconsistent condom use or who have had a bacterial STI in the previous 6 months is oral emtricitabine 200 mg plus tenofovir disoproxil fumarate 300 mg/d (sold as Truvada-F/TDF). Men and transgender women (ie, assigned male at birth) with at-risk behaviors also can use emtricitabine plus tenofovir alafenamide 25 mg/d (sold as Descovy-F/TAF).
In addition, cabotegravir plus rilpirivine (sold as Cabenuva), IM every 2 months, was approved by the FDA for PrEP in 2021.
Creatinine clearance should be assessed at baseline and yearly (every 6 months for those older than 50 years) in patients taking PrEP. All patients must be tested for HIV at initiation of treatment and every 3 months thereafter (every 4 months for cabotegravir plus rilpirivine). Patients should be screened for bacterial STIs every 6 months (every 3 months for MSM and transgender women); screening for chlamydia should be done yearly. For patients being treated with emtricitabine plus tenofovir alafenamide, weight and a lipid profile (cholesterol and triglycerides) should be assessed annually.55
Postexposure prophylaxis
The sharp rise in the incidence of STIs in the past few years has brought renewed interest in postexposure prophylaxis (PEP) for STIs. Although PEP should be standard in cases of sexual assault, this protocol also can be considered in other instances of high-risk exposure.
CDC recommendations for PEP in cases of assault are56:
- ceftriaxone 500 mg IM in a single dose (1 g if weight is ≥ 150 kg) plus
- doxycycline 100 mg bid for 7 days plus
- metronidazole 2 g bid for 7 days (for vaginal exposure)
- pregnancy evaluation and emergency contraception
- hepatitis B risk evaluation and vaccination, with or without hepatitis B immune globulin
- HIV risk evaluation, based on CDC guidelines, and possible HIV prophylaxis (PrEP)
- HPV vaccination for patients ages 9 to 26 years if they are not already fully vaccinated.
CORRESPONDENCE
Belinda Vail, MD, 3901 Rainbow Boulevard, Mail Stop 4010, Kansas City, KS 66160; [email protected]
1. Pagaoa M, Grey J, Torrone E, et al. Trends in nationally notifiable sexually transmitted disease case reports during the US COVID-19 pandemic, January to December 2020. Sex Transm Dis. 2021;48:798-804. doi: 10.1097/OLQ.0000000000001506
2. Chesson HW, Spicknall IH; Bingham A, et al. The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Sex Transm Dis. 2021;48:215-221. doi: 10.1097/OLQ.0000000000001380
3. Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis. 2021;48:208-214. doi: 10.1097/OLQ.0000000000001355
4. CDC. Sexually transmitted infections treatment guidelines, 2021: Chlamydial infections among adolescents and adults. US Department of Health and Human Services. July 21, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/chlamydia.htm
5. CDC. Sexually transmitted infections treatment guidelines, 2021: Gonococcal infections among adolescents and adults. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm
6. Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;
7. CDC. Sexually transmitted infections treatment guidelines, 2021. Trichomoniasis. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. December 27, 2021. www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
8. Spicknall IH, Flagg EW, Torrone EA. Estimates of the prevalence and incidence of genital herpes, United States, 2018. Sex Transm Dis. 2021;48:260-265. doi: 10.1097/OLQ.0000000000001375
9. Mark H, Gilbert L, Nanda J. Psychosocial well-being and quality of life among women newly diagnosed with genital herpes. J Obstet Gynecol Neonatal Nurs.
10. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2321-2327. doi: 10.1001/jama.2016.5824
11. Ricco J, Westby A. Syphilis: far from ancient history. Am Fam Physician. 2020;102:91-98.
12. Goza M, Kulwicki B, Akers JM, et al. Syphilis screening: a review of the Syphilis Health Check rapid immunochromatographic test. J Pharm Technol. 2017;33:53-59. doi:10.1177/8755122517691308
13. Henao- AF, Johnson SC. Diagnostic tests for syphilis: new tests and new algorithms. Neurol Clin Pract. 2014;4:114-122. doi: 10.1212/01.CPJ.0000435752.17621.48
14. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
15. CDC. Sexually Transmitted Disease Surveillance 2021. National overview of STDs. US Department of Health and Human Services. April 2023. Accessed May 9, 2023. www.cdc.gov/std/statistics/2021/overview.htm#Chlamydia
16. CDC. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014;63:1-19.
17. Kong FYS, Tabrizi SN, Law M, et al. Azithromycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;59:193-205. doi: 10.1093/cid/ciu220
18. Páez-Canro C, Alzate JP, González LM, et al. Antibiotics for treating urogenital Chlamydia trachomatis infection in men and non-pregnant women. Cochrane Database Syst Rev. 2019;1:CD010871. doi: 10.1002/14651858.CD010871.pub2
19. Dombrowski JC, Wierzbicki MR, Newman LM, et al. Doxycycline versus azithromycin for the treatment of rectal chlamydia in men who have sex with men: a randomized controlled trial. Clin Infect Dis. 2021;73:824-831. doi: 10.1093/cid/ciab153
20. CDC. Sexually transmitted infections treatment guidelines, 2021: Expedited partner therapy. US Department of Health and Human Services. July 22, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/clinical-EPT.htm
21. Golden MR, Whittington WLH, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676-685. doi: 10.1056/NEJMoa041681
22. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
23. Cameron ST, Glasier A, Scott G, et al. Novel interventions to reduce re-infection in women with chlamydia: a randomized controlled trial. Hum Reprod. 2009;24:888-895. doi: 10.1093/humrep/den475
24. McNulty A, Teh MF, Freedman E. Patient delivered partner therapy for chlamydial infection—what would be missed? Sex Transm Dis. 2008;35:834-836. doi: 10.1097/OLQ.0b013e3181761993
25. Stekler J, Bachmann L, Brotman RM, et al. Concurrent sexually transmitted infections (STIs) in sex partners of patients with selected STIs: implications for patient-delivered partner therapy. Clin Infect Dis. 2005;40:787-793. doi: 10.1086/428043
26. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
27. Stupiansky NW, Van der Pol B, Williams JA, et al. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis. 2011;38:750-754. doi: 10.1097/OLQ.0b013e31820ff9a4
28. CDC. Sexually transmitted infections treatment guidelines, 2021: Screening recommendations and considerations referenced in treatment guidelines and original sources. US Department of Health and Human Services. June 6, 2022. Accessed May 9, 2023. www.cdc.gov/std/treatment-guidelines/screening-recommen dations.htm
29. Cantor A, Dana T, Griffen JC, et al. Screening for chlamydial and gonococcal infections: a systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 206. AHRQ Report No. 21-05275-EF-1. Agency for Healthcare Research and Quality. September 2021. www.ncbi.nlm.nih.gov/books/NBK574045
30. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
31. Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019:97:548-562P. doi: 10.2471/BLT.18.228486
32. Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217. doi: 10.1093/cid/ciy079
33. CDC. Sexually transmitted infections treatment guidelines, 2021. Mycoplasma genitalium. US Department of Health and Human Services. July 22, 2021. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm
34. Manhart LE, Broad JM, Bolden MR. Mycoplasma genitalium: should we treat and how? Clin Infect Dis. 2011;53(suppl 3):S129-S142. doi:10.1093/cid/cir702.
35. Corey L, Wald A. Genital herpes. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. McGraw-Hill; 2008:399-437.
36. CDC. Sexually transmitted infections treatment guidelines, 2021: Genital herpes. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/herpes.htm
37. Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17:1303-1316. doi: 10.1016/S1473-3099(17)30405-X
38. Rollenhagen C, Lathrop M, Macura SL, et al. Herpes simplex virus type-2 stimulates HIV-1 replication in cervical tissues: implications for HIV-1 transmission and efficacy of anti-HIV-1 microbicides. Mucosal Immunol. 2014;7:1165-1174. doi: 10.1038/mi.2014.3
39. Cogliano V, Baan R, Straif K, et al; WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol.
40. Simon MA, Tseng CW, Wong JB. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897
41. Perkins RB, Guido RS, Castle PE, et al; . 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
42. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
43. Schmidt R, Carson PJ, Jansen RJ. Resurgence of syphilis in the United States: an assessment of contributing factors. Infect Dis (Auckl). 2019;12:1178633719883282. doi: 10.1177/1178633719883282
44. Boog GHP, Lopes JVZ, Mahler JV, et al. Diagnostic tools for neurosyphilis: a systematic review. BMC Infect Dis. 2021;21:568. doi: 10.1186/s12879-021-06264-8
45. CDC. Sexually transmitted infections treatment guidelines, 2021. Syphilis. US Department of Health and Human Services. April 20, 2023. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/syphilis.htm
46. Matthias JM, Rahman MM, Newman DR, et al. Effectiveness of prenatal screening and treatment to prevent congenital syphilis, Louisiana and Florida, 2013-2014. Sex Transm Dis. 2017;44:498-502. doi: 10.1097/OLQ.0000000000000638
47. Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA. 2014;312:1905-1917. doi: 10.1001/jama.2014.13259
48. Davidson KW, Barry MJ, Mangione CM, et al; US Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:949-956. doi: 10.1001/jama.2021.14081
49. Krist AH, Davidson KW, Mangione CM, et al; US Preventive Services Task Force. Screening for hepatitis B virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2415-2422. doi: 10.1001/jama.2020.22980
50. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:970-975. doi: 10.1001/jama.2020.1123
51. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530. doi: 10.1001/jama.2016.16776
52. Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi: 10.1001/jama.2018.10897
53. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336. doi: 10.1001/jama.2019.6587
54. Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36:502-509. doi: 10.1016/s0091-7435(02)00058-0
55. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. A clinical practice guideline. Centers for Disease Control and Prevention. Accessed April 24, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
56. CDC. Sexually transmitted infections treatment guidelines, 2021: Sexual assault and abuse and STIs—adolescents and adults, 2021. US Department of Health and Human Services. July 22, 2021. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/sexual-assault-adults.htm
Except for a drop in the number of sexually transmitted infections (STIs) early in the COVID-19 pandemic (March and April 2020), the incidence of STIs has been rising throughout this century.1 In 2018, 1 in 5 people in the United States had an STI; 26 million new cases were reported that year, resulting in direct costs of $16 billion—85% of which was for the care of HIV infection.2 Also that year, infection with Chlamydia trachomatis (chlamydia), Trichomonas vaginalis (trichomoniasis), herpesvirus type 2 (genital herpes), and/or human papillomavirus (condylomata acuminata) constituted 97.6% of all prevalent and 93.1% of all incident STIs.3 Almost half (45.5%) of new cases of STIs occur in people between the ages of 15 and 24 years.3

Three factors—changing social patterns, including the increase of social networking; the ability of antiviral therapy to decrease the spread of HIV, leading to a reduction in condom use; and increasing antibiotic resistance—have converged to force changes in screening and treatment recommendations. In this article, we summarize updated guidance for primary care clinicians from several sources—including the Centers for Disease Control and Prevention (CDC), the US Preventive Services Task Force (USPSTF), and the American Society for Colposcopy and Cervical Pathology (ASCCP)—on diagnosing STIs (TABLE 14-13) and providing guideline-based treatment (Table 214). Because of the breadth and complexity of HIV disease, it is not addressed here.



Chlamydia
Infection with Chlamydia trachomatis—the most commonly reported bacterial STI in the United States—primarily causes cervicitis in women and proctitis in men, and can cause urethritis and pharyngitis in men and women. Prevalence is highest in sexually active people younger than 24 years.15
Because most infected people are asymptomatic and show no signs of illness on physical exam, screening is recommended for all sexually active women younger than 25 years and all men who have sex with men (MSM).4 No studies have established proper screening intervals; a reasonable approach, therefore, is to repeat screening for patients who have a sexual history that confers a new or persistent risk for infection since their last negative result.
Depending on the location of the infection, symptoms of chlamydia can include vaginal or penile irritation or discharge, dysuria, pelvic or rectal pain, and sore throat. Breakthrough bleeding in a patient who is taking an oral contraceptive should raise suspicion for chlamydia.
Untreated chlamydia can lead to pelvic inflammatory disease (PID), tubo-ovarian abscess, tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. Infection can be transmitted vertically (mother to baby) antenatally, which can cause ophthalmia neonatorum and pneumonia in these newborns.
Diagnosis. The diagnosis of chlamydia is made using nucleic acid amplification testing (NAAT). Specimens can be collected by the clinician or the patient (self collected) using a vaginal, rectal, or oropharyngeal swab, or a combination of these, and can be obtained from urine or liquid-based cytology material.16
Continue to: Treatment
Treatment. Recommendations for treating chlamydia were updated by the CDC in its 2021 treatment guidelines (Table 214). Doxycycline 100 mg bid for 7 days is the preferred regimen; alternative regiments are (1) azithromycin 1 g in a single dose and (2) levofloxacin 500 mg daily for 7 days.4 A meta-analysis17 and a Cochrane review18 showed that the rate of treatment failure was higher among men when they were treated with azithromycin instead of doxycycline; furthermore, a randomized controlled trial demonstrated that doxycycline is more effective than azithromycin (cure rate, 100%, compared to 74%) at treating rectal chlamydia in MSM.19
Azithromycin is efficacious for urogenital infection in women; however, there is concern that the 33% to 83% of women who have concomitant rectal infection (despite reporting no receptive anorectal sexual activity) would be insufficiently treated. Outside pregnancy, the CDC does not recommend a test of cure but does recommend follow-up testing for reinfection in 3 months. Patients should abstain from sexual activity until 7 days after all sexual partners have been treated.
Expedited partner therapy (EPT) is the practice of treating sexual partners of patients with known chlamydia (and patients with gonococcal infection). Unless prohibited by law in your state, offer EPT to patients with chlamydia if they cannot ensure that their sexual partners from the past 60 days will seek timely treatment.a
Evidence to support EPT comes from 3 US clinical trials, whose subjects comprised heterosexual men and women with chlamydia or gonorrhea.21-23 The role of EPT for MSM is unclear; data are limited. Shared decision-making is recommended to determine whether EPT should be provided, to ensure that co-infection with other bacterial STIs (eg, syphilis) or HIV is not missed.24-26
a Visit www.cdc.gov/std/ept to read updated information about laws and regulations regarding EPT in your state.20
Gonorrhea
Gonorrhea is the second most-reported bacterial communicable disease.5 Infection with Neisseria gonorrhoeae causes urethral discharge in men, leading them to seek treatment; infected women, however, are often asymptomatic. Infected men and women might not recognize symptoms until they have transmitted the disease. Women have a slower natural clearance of gonococcal infection, which might explain their higher prevalence.27 Delayed recognition of symptoms can result in complications, including PID.5
Diagnosis. Specimens for NAAT can be obtained from urine, endocervical, vaginal, rectal, pharyngeal, and male urethral specimens. Reported sexual behaviors and exposures of women and transgender or gender-diverse people should be taken into consideration to determine whether rectal or pharyngeal testing, or both, should be performed.28 MSM should be screened annually at sites of contact, including the urethra, rectum, and pharynx.28 All patients with urogenital or rectal gonorrhea should be asked about oral sexual exposure; if reported, pharyngeal testing should be performed.5
NAAT of urine is at least as sensitive as testing of an endocervical specimen; the same specimen can be used to test for chlamydia and gonorrhea. Patient-collected specimens are a reasonable alternative to clinician-collected swab specimens.29
Continue to: Treatment
Treatment is complicated by the ability of gonorrhea to develop resistance. Intramuscular ceftriaxone 500 mg in a single dose cures 98% to 99% of infections in the United States; however, monitoring local resistance patterns in the community is an important component of treatment.28 (See Table 214 for an alternative regimen for cephalosporin-allergic patients and for treating gonococcal conjunctivitis and disseminated infection.)
In 2007, the CDC identified widespread quinolone-resistant gonococcal strains; therefore, fluoroquinolones no longer are recommended for treating gonorrhea.30 Cefixime has demonstrated only limited success in treating pharyngeal gonorrhea and does not attain a bactericidal level as high as ceftriaxone does; cefixime therefore is recommended only if ceftriaxone is unavailable.28 The national Gonococcal Isolate Surveillance Project is finding emerging evidence of the reduced susceptibility of N gonorrhoeae to azithromycin—making dual therapy for gonococcal infection no longer a recommendation.28
Patients should abstain from sex until 7 days after all sex partners have been treated for gonorrhea. As with chlamydia, the CDC does not recommend a test of cure for uncomplicated urogenital or rectal gonorrhea unless the patient is pregnant, but does recommend testing for reinfection 3 months after treatment.14 For patients with pharyngeal gonorrhea, a test of cure is recommended 7 to 14 days after initial treatment, due to challenges in treatment and because this site of infection is a potential source of antibiotic resistance.28
Trichomoniasis
T vaginalis, the most common nonviral STI worldwide,31 can manifest as a yellow-green vaginal discharge with or without vaginal discomfort, dysuria, epididymitis, and prostatitis; most cases, however, are asymptomatic. On examination, the cervix might be erythematous with punctate lesions (known as strawberry cervix).
Unlike most STIs, trichomoniasis is as common in women older than 24 years as it is in younger women. Infection is associated with a lower educational level, lower socioeconomic status, and having ≥ 2 sexual partners in the past year.32 Prevalence is approximately 10 times as high in Black women as it is in White women.
T vaginalis infection is associated with an increase in the risk for preterm birth, premature rupture of membranes, cervical cancer, and HIV infection. With a lack of high-quality clinical trials on the efficacy of screening, women with HIV are the only group for whom routine screening is recommended.6
Diagnosis. NAAT for trichomoniasis is now available in conjunction with gonorrhea and chlamydia testing of specimens on vaginal or urethral swabs and of urine specimens and liquid Pap smears.
Continue to: Treatment
Treatment. Because of greater efficacy, the treatment recommendation for women has changed from a single 2-g dose of oral metronidazole to 500 mg twice daily for 7 days. The 2-g single oral dose is still recommended for men7 (Table 214 lists alternative regimens).
Mycoplasma genitalium
Infection with M genitalium is common and often asymptomatic. The disease causes approximately 20% of all cases of nongonococcal and nonchlamydial urethritis in men and about 40% of persistent or recurrent infections. M genitalium is present in approximately 20% of women with cervicitis and has been associated with PID, preterm delivery, spontaneous abortion, and infertility.
There are limited and conflicting data regarding outcomes in infected patients other than those with persistent or recurrent infection; furthermore, resistance to azithromycin is increasing rapidly, resulting in an increase in treatment failures. Screening therefore is not recommended, and testing is recommended only in men with nongonococcal urethritis.33,34
Diagnosis. NAAT can be performed on urine or on a urethral, penile meatal, endocervical, or vaginal swab; men with recurrent urethritis or women with recurrent cervicitis should be tested. NAAT also can be considered in women with PID. Testing the specimen for the microorganism’s resistance to macrolide antibiotics is recommended (if such testing is available).
Treatment is initiated with doxycycline 100 mg twice daily for 7 days. If the organism is macrolide sensitive, follow with azithromycin 1 g orally on Day 1, then 500 mg/d for 3 more days. If the organism is macrolide resistant or testing is unavailable, follow doxycycline with oral moxifloxacin 400 mg/d for 7 days.33
Genital herpes (mostly herpesvirus type 2)
Genital herpes, characterized by painful, recurrent outbreaks of genital and anal lesions,35 is a lifelong infection that increases in prevalence with age.8 Because many infected people have disease that is undiagnosed or mild or have unrecognizable symptoms during viral shedding, most genital herpes infections are transmitted by people who are unaware that they are contagious.36 Herpesvirus type 2 (HSV-2) causes most cases of genital herpes, although an increasing percentage of cases are attributed to HSV type 1 (HSV-1) through receptive oral sex from a person who has an oral HSV-1 lesion.
Importantly, HSV-2–infected people are 2 to 3 times more likely to become infected with HIV than people who are not HSV-2 infected.37 This is because CD4+ T cells concentrate at the site of HSV lesions and express a higher level of cell-surface receptors that HIV uses to enter cells. HIV replicates 3 to 5 times more quickly in HSV-infected tissue.38
Continue to: HSV can become disseminated...
HSV can become disseminated, particularly in immunosuppressed people, and can manifest as encephalitis, hepatitis, and pneumonitis. Beyond its significant burden on health, HSV carries significant psychosocial consequences.9
Diagnosis. Clinical diagnosis can be challenging if classic lesions are absent at evaluation. If genital lesions are present, HSV can be identified by NAAT or culture of a specimen of those lesions. False-negative antibody results might be more frequent in early stages of infection; repeating antibody testing 12 weeks after presumed time of acquisition might therefore be indicated, based on clinical judgment. HSV-2 antibody positivity implies anogenital infection because almost all HSV-2 infections are sexually acquired.
HSV-1 antibody positivity alone is more difficult to interpret because this finding does not distinguish between oral and genital lesions, and most HSV-1 seropositivity is acquired during childhood.36 HSV polymerase chain reaction (PCR) testing of blood should not be performed to diagnose genital herpes infection, except in settings in which there is concern about disseminated infection.
Treatment. Management should address the acute episode and the chronic nature of genital herpes. Antivirals will not eradicate latent
- attenuate current infection
- prevent recurrence
- improve quality of life
- suppress the virus to prevent transmission to sexual partners.
All patients experiencing an initial episode of genital herpes should be treated, regardless of symptoms, due to the potential for prolonged or severe symptoms during recurrent episodes.9 Three drugs—acyclovir, valacyclovir, and famciclovir—are approved by the US Food and Drug Administration (FDA) to treat genital herpes and appear equally effective (TABLE 214).
Antiviral therapy for recurrent genital HSV infection can be administered either as suppressive therapy to reduce the frequency of recurrences or episodically to shorten the duration of lesions:
- Suppressive therapy reduces the frequency of recurrence by 70% to 80% among patients with frequent outbreaks. Long-term safety and efficacy are well established.
- Episodic therapy is most effective if started within 1 day after onset of lesions or during the prodrome.36
There is no specific recommendation for when to choose suppressive over episodic therapy; most patients prefer suppressive therapy because it improves quality of life. Use shared clinical decision-making to determine the best option for an individual patient.
Continue to: Human papillomavirus
Human papillomavirus
Condylomata acuminata (genital warts) are caused by human papillomavirus (HPV), most commonly types 6 and 11, which manifest as soft papules or plaques on the external genitalia, perineum, perianal skin, and groin. The warts are usually asymptomatic but can be painful or pruritic, depending on size and location.
Diagnosis is made by visual inspection and can be confirmed by biopsy if lesions are atypical. Lesions can resolve spontaneously, remain unchanged, or grow in size or number.
Treatment. The aim of treatment is relief of symptoms and removal of warts. Treatment does not eradicate HPV infection. Multiple treatments are available that can be applied by the patient as a cream, gel, or ointment or administered by the provider, including cryotherapy, surgical removal, and solutions. The decision on how to treat should be based on the number, size, and
HPV-associated cancers and precancers. This is a broad (and separate) topic. HPV types 16 and 18 cause most cases of cervical, penile, vulvar, vaginal, anal, and oropharyngeal cancer and precancer.39 The USPSTF, the American Cancer Society, and the American College of Obstetricians and Gynecologists all have recommendations for cervical cancer screening in the United States.40 Refer to guidelines of the ASCCP for recommendations on abnormal screening tests.41
Prevention of genital warts. The 9-valent HPV vaccine available in the United States is safe and effective and helps protect against viral types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Types 6 and 11 are the principal causes of genital warts. Types 16 and 18 cause 66% of cervical cancer. The vaccination series can be started at age 9 years and is recommended for everyone through age 26 years. Only 2 doses are needed if the first dose is given prior to age 15 years; given after that age, a 3-dose series is utilized. Refer to CDC vaccine guidelines42 for details on the exact timing of vaccination.
Vaccination for women ages 27 to 45 years is not universally recommended because most people have been exposed to HPV by that age. However, the vaccine can still be administered, depending on clinical circumstances and the risk for new infection.42
Syphilis
Caused by the spirochete Treponema pallidum, syphilis manifests across a spectrum—from congenital to tertiary. The inability of medical science to develop a method for culturing the spirochete has confounded diagnosis and treatment.
Continue to: Since reaching a historic...
Since reaching a historic nadir of incidence in 2000 (5979 cases in the United States), there has been an increasingly rapid rise in that number: to 130,000 in 2020. More than 50% of cases are in MSM; however, the number of cases in heterosexual women is rapidly increasing.43
Routine screening for syphilis should be performed in any person who is at risk: all pregnant women in the first trimester (and in the third trimester and at delivery if they are at risk or live in a community where prevalence is high) and annually in sexually active MSM or anyone with HIV infection.10
Diagnosis. Examination by dark-field microscopy, testing by PCR, and direct fluorescent antibody assay for T pallidum from lesion tissue or exudate provide definitive diagnosis for early and congenital syphilis, but are often unavailable.
Presumptive diagnosis requires 2 serologic tests:
- Nontreponemal tests (the VDRL and rapid plasma reagin tests) identify anticardiolipin antibodies released during syphilis infection, although results also can be elevated in autoimmune disease or after certain immunizations, including the COVID-19 vaccine.
- Treponemal tests (the fluorescent treponemal antibody absorbed assay, T pallidum particulate agglutination assay, enzyme immunoassay, and chemiluminescence immunoassay) are specific antibody tests.
Historically, reactive nontreponemal tests, which are less expensive and easier to perform, were followed by a treponemal test to confirm the presumptive diagnosis. This method continues to be reasonable when screening patients in a low-prevalence population.11 The reverse sequence screening algorithm (ie, begin with a treponemal test) is now frequently used. With this method, a positive treponemal test must be confirmed with a nontreponemal test. If the treponemal test is positive and the nontreponemal test is negative, another treponemal test must be positive to confirm the diagnosis. This algorithm is useful in high-risk populations because it provides earlier detection of recently acquired syphilis and enhanced detection of late latent syphilis.12,13,44 The CDC has not stated a diagnostic preference.
Once the diagnosis is made, a complete history (including a sexual history and a history of syphilis testing and treatment) and a physical exam are necessary to confirm stage of disease.45
Special circumstances. Neurosyphilis, ocular syphilis, and otosyphilis refer to the site of infection and can occur at any stage of disease. The nervous system usually is infected within hours of initial infection, but symptoms might take weeks or years to develop—or might never manifest. Any time a patient develops neurologic, ophthalmologic, or audiologic symptoms, careful neurologic and ophthalmologic evaluation should be performed and the patient should be tested for HIV.
Continue to: Lumbar puncture is warranted...
Lumbar puncture is warranted for evaluation of cerebrospinal fluid if neurologic symptoms are present but is not necessary for isolated ocular syphilis or otosyphilis without neurologic findings. Treatment should not be delayed for test results if ocular syphilis is suspected because permanent blindness can develop. Any patient at high risk for an STI who presents with neurologic or ophthalmologic symptoms should be tested for syphilis and HIV.45
Pregnant women who have a diagnosis of syphilis should be treated with penicillin immediately because treatment ≥ 30 days prior to delivery is likely to prevent most cases of congenital syphilis. However, a course of penicillin might not prevent stillbirth or congenital syphilis in a gravely infected fetus, evidenced by fetal syphilis on a sonogram at the time of treatment. Additional doses of penicillin in pregnant women with early syphilis might be indicated if there is evidence of fetal syphilis on ultrasonography. All women who deliver a stillborn infant (≥ 20 weeks’ gestation) should be tested for syphilis at delivery.46
All patients in whom primary or secondary syphilis has been diagnosed should be tested for HIV at the time of diagnosis and treatment; if the result is negative, they should be offered preexposure prophylaxis (PrEP; discussed shortly). If the incidence of HIV in your community is high, repeat testing for HIV in 3 months. Clinical and serologic evaluation should be performed 6 and 12 months after treatment.47
Treatment. Penicillin remains the standard treatment for syphilis. Primary, secondary, and early tertiary stages (including in pregnancy) are treated with benzathine penicillin G 2.4 million units intramuscular (IM) in a single dose. For pregnant patients, repeating that dose in 1 week generally is recommended. Patients in the late latent (> 1 year) or tertiary stage receive the same dose of penicillin, which is then repeated weekly, for a total of 3 doses. Doxycycline and ceftriaxone are alternatives, except in pregnancy.
Warn patients of the Jarisch-Herxheimer reaction: fever, headache, and myalgias associated with initiation of treatment in the presence of the high bacterial load seen in early syphilis. Treatment is symptomatic, but the Jarisch-Herxheimer reaction can cause fetal distress in pregnancy.
Otosyphilis, ocular syphilis, and neurosyphilis require intravenous (IV) aqueous crystalline penicillin G 3 to 4 million U every 4 hours for 10 to 14 days.45 Alternatively, procaine penicillin G 2.4 million U/d IM can be given daily with oral probenecid 500 mg qid, both for 10 to 14 days (TABLE 214).
Screening andprevention of STIs
Screening recommendations
Follow USPSTF screening guidelines for STIs.10,48-54 Screen annually for:
- gonorrhea and chlamydia in women ages 15 to 24 years and in women older than 25 years if they are at increased risk
- gonorrhea, chlamydia, syphilis, and HIV in MSM, and hepatitis C if they are HIV positive
- trichomoniasis in women who are HIV positive.
Continue to: Consider the community in which...
Consider the community in which you practice when determining risk; you might want to consult local public health authorities for information about local epidemiology and guidance on determining which of your patients are at increased risk.
Preexposure prophylaxis
According to the CDC, all sexually active adults and adolescents should be informed about the availability of PrEP to prevent HIV infection. PrEP should be (1) available to anyone who requests it and (2) recommended for anyone who is sexually active and who practices sexual behaviors that place them at substantial risk for exposure to or acquisition of HIV, or both.
The recommended treatment protocol for men and women who have either an HIV-positive partner or inconsistent condom use or who have had a bacterial STI in the previous 6 months is oral emtricitabine 200 mg plus tenofovir disoproxil fumarate 300 mg/d (sold as Truvada-F/TDF). Men and transgender women (ie, assigned male at birth) with at-risk behaviors also can use emtricitabine plus tenofovir alafenamide 25 mg/d (sold as Descovy-F/TAF).
In addition, cabotegravir plus rilpirivine (sold as Cabenuva), IM every 2 months, was approved by the FDA for PrEP in 2021.
Creatinine clearance should be assessed at baseline and yearly (every 6 months for those older than 50 years) in patients taking PrEP. All patients must be tested for HIV at initiation of treatment and every 3 months thereafter (every 4 months for cabotegravir plus rilpirivine). Patients should be screened for bacterial STIs every 6 months (every 3 months for MSM and transgender women); screening for chlamydia should be done yearly. For patients being treated with emtricitabine plus tenofovir alafenamide, weight and a lipid profile (cholesterol and triglycerides) should be assessed annually.55
Postexposure prophylaxis
The sharp rise in the incidence of STIs in the past few years has brought renewed interest in postexposure prophylaxis (PEP) for STIs. Although PEP should be standard in cases of sexual assault, this protocol also can be considered in other instances of high-risk exposure.
CDC recommendations for PEP in cases of assault are56:
- ceftriaxone 500 mg IM in a single dose (1 g if weight is ≥ 150 kg) plus
- doxycycline 100 mg bid for 7 days plus
- metronidazole 2 g bid for 7 days (for vaginal exposure)
- pregnancy evaluation and emergency contraception
- hepatitis B risk evaluation and vaccination, with or without hepatitis B immune globulin
- HIV risk evaluation, based on CDC guidelines, and possible HIV prophylaxis (PrEP)
- HPV vaccination for patients ages 9 to 26 years if they are not already fully vaccinated.
CORRESPONDENCE
Belinda Vail, MD, 3901 Rainbow Boulevard, Mail Stop 4010, Kansas City, KS 66160; [email protected]
Except for a drop in the number of sexually transmitted infections (STIs) early in the COVID-19 pandemic (March and April 2020), the incidence of STIs has been rising throughout this century.1 In 2018, 1 in 5 people in the United States had an STI; 26 million new cases were reported that year, resulting in direct costs of $16 billion—85% of which was for the care of HIV infection.2 Also that year, infection with Chlamydia trachomatis (chlamydia), Trichomonas vaginalis (trichomoniasis), herpesvirus type 2 (genital herpes), and/or human papillomavirus (condylomata acuminata) constituted 97.6% of all prevalent and 93.1% of all incident STIs.3 Almost half (45.5%) of new cases of STIs occur in people between the ages of 15 and 24 years.3

Three factors—changing social patterns, including the increase of social networking; the ability of antiviral therapy to decrease the spread of HIV, leading to a reduction in condom use; and increasing antibiotic resistance—have converged to force changes in screening and treatment recommendations. In this article, we summarize updated guidance for primary care clinicians from several sources—including the Centers for Disease Control and Prevention (CDC), the US Preventive Services Task Force (USPSTF), and the American Society for Colposcopy and Cervical Pathology (ASCCP)—on diagnosing STIs (TABLE 14-13) and providing guideline-based treatment (Table 214). Because of the breadth and complexity of HIV disease, it is not addressed here.



Chlamydia
Infection with Chlamydia trachomatis—the most commonly reported bacterial STI in the United States—primarily causes cervicitis in women and proctitis in men, and can cause urethritis and pharyngitis in men and women. Prevalence is highest in sexually active people younger than 24 years.15
Because most infected people are asymptomatic and show no signs of illness on physical exam, screening is recommended for all sexually active women younger than 25 years and all men who have sex with men (MSM).4 No studies have established proper screening intervals; a reasonable approach, therefore, is to repeat screening for patients who have a sexual history that confers a new or persistent risk for infection since their last negative result.
Depending on the location of the infection, symptoms of chlamydia can include vaginal or penile irritation or discharge, dysuria, pelvic or rectal pain, and sore throat. Breakthrough bleeding in a patient who is taking an oral contraceptive should raise suspicion for chlamydia.
Untreated chlamydia can lead to pelvic inflammatory disease (PID), tubo-ovarian abscess, tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. Infection can be transmitted vertically (mother to baby) antenatally, which can cause ophthalmia neonatorum and pneumonia in these newborns.
Diagnosis. The diagnosis of chlamydia is made using nucleic acid amplification testing (NAAT). Specimens can be collected by the clinician or the patient (self collected) using a vaginal, rectal, or oropharyngeal swab, or a combination of these, and can be obtained from urine or liquid-based cytology material.16
Continue to: Treatment
Treatment. Recommendations for treating chlamydia were updated by the CDC in its 2021 treatment guidelines (Table 214). Doxycycline 100 mg bid for 7 days is the preferred regimen; alternative regiments are (1) azithromycin 1 g in a single dose and (2) levofloxacin 500 mg daily for 7 days.4 A meta-analysis17 and a Cochrane review18 showed that the rate of treatment failure was higher among men when they were treated with azithromycin instead of doxycycline; furthermore, a randomized controlled trial demonstrated that doxycycline is more effective than azithromycin (cure rate, 100%, compared to 74%) at treating rectal chlamydia in MSM.19
Azithromycin is efficacious for urogenital infection in women; however, there is concern that the 33% to 83% of women who have concomitant rectal infection (despite reporting no receptive anorectal sexual activity) would be insufficiently treated. Outside pregnancy, the CDC does not recommend a test of cure but does recommend follow-up testing for reinfection in 3 months. Patients should abstain from sexual activity until 7 days after all sexual partners have been treated.
Expedited partner therapy (EPT) is the practice of treating sexual partners of patients with known chlamydia (and patients with gonococcal infection). Unless prohibited by law in your state, offer EPT to patients with chlamydia if they cannot ensure that their sexual partners from the past 60 days will seek timely treatment.a
Evidence to support EPT comes from 3 US clinical trials, whose subjects comprised heterosexual men and women with chlamydia or gonorrhea.21-23 The role of EPT for MSM is unclear; data are limited. Shared decision-making is recommended to determine whether EPT should be provided, to ensure that co-infection with other bacterial STIs (eg, syphilis) or HIV is not missed.24-26
a Visit www.cdc.gov/std/ept to read updated information about laws and regulations regarding EPT in your state.20
Gonorrhea
Gonorrhea is the second most-reported bacterial communicable disease.5 Infection with Neisseria gonorrhoeae causes urethral discharge in men, leading them to seek treatment; infected women, however, are often asymptomatic. Infected men and women might not recognize symptoms until they have transmitted the disease. Women have a slower natural clearance of gonococcal infection, which might explain their higher prevalence.27 Delayed recognition of symptoms can result in complications, including PID.5
Diagnosis. Specimens for NAAT can be obtained from urine, endocervical, vaginal, rectal, pharyngeal, and male urethral specimens. Reported sexual behaviors and exposures of women and transgender or gender-diverse people should be taken into consideration to determine whether rectal or pharyngeal testing, or both, should be performed.28 MSM should be screened annually at sites of contact, including the urethra, rectum, and pharynx.28 All patients with urogenital or rectal gonorrhea should be asked about oral sexual exposure; if reported, pharyngeal testing should be performed.5
NAAT of urine is at least as sensitive as testing of an endocervical specimen; the same specimen can be used to test for chlamydia and gonorrhea. Patient-collected specimens are a reasonable alternative to clinician-collected swab specimens.29
Continue to: Treatment
Treatment is complicated by the ability of gonorrhea to develop resistance. Intramuscular ceftriaxone 500 mg in a single dose cures 98% to 99% of infections in the United States; however, monitoring local resistance patterns in the community is an important component of treatment.28 (See Table 214 for an alternative regimen for cephalosporin-allergic patients and for treating gonococcal conjunctivitis and disseminated infection.)
In 2007, the CDC identified widespread quinolone-resistant gonococcal strains; therefore, fluoroquinolones no longer are recommended for treating gonorrhea.30 Cefixime has demonstrated only limited success in treating pharyngeal gonorrhea and does not attain a bactericidal level as high as ceftriaxone does; cefixime therefore is recommended only if ceftriaxone is unavailable.28 The national Gonococcal Isolate Surveillance Project is finding emerging evidence of the reduced susceptibility of N gonorrhoeae to azithromycin—making dual therapy for gonococcal infection no longer a recommendation.28
Patients should abstain from sex until 7 days after all sex partners have been treated for gonorrhea. As with chlamydia, the CDC does not recommend a test of cure for uncomplicated urogenital or rectal gonorrhea unless the patient is pregnant, but does recommend testing for reinfection 3 months after treatment.14 For patients with pharyngeal gonorrhea, a test of cure is recommended 7 to 14 days after initial treatment, due to challenges in treatment and because this site of infection is a potential source of antibiotic resistance.28
Trichomoniasis
T vaginalis, the most common nonviral STI worldwide,31 can manifest as a yellow-green vaginal discharge with or without vaginal discomfort, dysuria, epididymitis, and prostatitis; most cases, however, are asymptomatic. On examination, the cervix might be erythematous with punctate lesions (known as strawberry cervix).
Unlike most STIs, trichomoniasis is as common in women older than 24 years as it is in younger women. Infection is associated with a lower educational level, lower socioeconomic status, and having ≥ 2 sexual partners in the past year.32 Prevalence is approximately 10 times as high in Black women as it is in White women.
T vaginalis infection is associated with an increase in the risk for preterm birth, premature rupture of membranes, cervical cancer, and HIV infection. With a lack of high-quality clinical trials on the efficacy of screening, women with HIV are the only group for whom routine screening is recommended.6
Diagnosis. NAAT for trichomoniasis is now available in conjunction with gonorrhea and chlamydia testing of specimens on vaginal or urethral swabs and of urine specimens and liquid Pap smears.
Continue to: Treatment
Treatment. Because of greater efficacy, the treatment recommendation for women has changed from a single 2-g dose of oral metronidazole to 500 mg twice daily for 7 days. The 2-g single oral dose is still recommended for men7 (Table 214 lists alternative regimens).
Mycoplasma genitalium
Infection with M genitalium is common and often asymptomatic. The disease causes approximately 20% of all cases of nongonococcal and nonchlamydial urethritis in men and about 40% of persistent or recurrent infections. M genitalium is present in approximately 20% of women with cervicitis and has been associated with PID, preterm delivery, spontaneous abortion, and infertility.
There are limited and conflicting data regarding outcomes in infected patients other than those with persistent or recurrent infection; furthermore, resistance to azithromycin is increasing rapidly, resulting in an increase in treatment failures. Screening therefore is not recommended, and testing is recommended only in men with nongonococcal urethritis.33,34
Diagnosis. NAAT can be performed on urine or on a urethral, penile meatal, endocervical, or vaginal swab; men with recurrent urethritis or women with recurrent cervicitis should be tested. NAAT also can be considered in women with PID. Testing the specimen for the microorganism’s resistance to macrolide antibiotics is recommended (if such testing is available).
Treatment is initiated with doxycycline 100 mg twice daily for 7 days. If the organism is macrolide sensitive, follow with azithromycin 1 g orally on Day 1, then 500 mg/d for 3 more days. If the organism is macrolide resistant or testing is unavailable, follow doxycycline with oral moxifloxacin 400 mg/d for 7 days.33
Genital herpes (mostly herpesvirus type 2)
Genital herpes, characterized by painful, recurrent outbreaks of genital and anal lesions,35 is a lifelong infection that increases in prevalence with age.8 Because many infected people have disease that is undiagnosed or mild or have unrecognizable symptoms during viral shedding, most genital herpes infections are transmitted by people who are unaware that they are contagious.36 Herpesvirus type 2 (HSV-2) causes most cases of genital herpes, although an increasing percentage of cases are attributed to HSV type 1 (HSV-1) through receptive oral sex from a person who has an oral HSV-1 lesion.
Importantly, HSV-2–infected people are 2 to 3 times more likely to become infected with HIV than people who are not HSV-2 infected.37 This is because CD4+ T cells concentrate at the site of HSV lesions and express a higher level of cell-surface receptors that HIV uses to enter cells. HIV replicates 3 to 5 times more quickly in HSV-infected tissue.38
Continue to: HSV can become disseminated...
HSV can become disseminated, particularly in immunosuppressed people, and can manifest as encephalitis, hepatitis, and pneumonitis. Beyond its significant burden on health, HSV carries significant psychosocial consequences.9
Diagnosis. Clinical diagnosis can be challenging if classic lesions are absent at evaluation. If genital lesions are present, HSV can be identified by NAAT or culture of a specimen of those lesions. False-negative antibody results might be more frequent in early stages of infection; repeating antibody testing 12 weeks after presumed time of acquisition might therefore be indicated, based on clinical judgment. HSV-2 antibody positivity implies anogenital infection because almost all HSV-2 infections are sexually acquired.
HSV-1 antibody positivity alone is more difficult to interpret because this finding does not distinguish between oral and genital lesions, and most HSV-1 seropositivity is acquired during childhood.36 HSV polymerase chain reaction (PCR) testing of blood should not be performed to diagnose genital herpes infection, except in settings in which there is concern about disseminated infection.
Treatment. Management should address the acute episode and the chronic nature of genital herpes. Antivirals will not eradicate latent
- attenuate current infection
- prevent recurrence
- improve quality of life
- suppress the virus to prevent transmission to sexual partners.
All patients experiencing an initial episode of genital herpes should be treated, regardless of symptoms, due to the potential for prolonged or severe symptoms during recurrent episodes.9 Three drugs—acyclovir, valacyclovir, and famciclovir—are approved by the US Food and Drug Administration (FDA) to treat genital herpes and appear equally effective (TABLE 214).
Antiviral therapy for recurrent genital HSV infection can be administered either as suppressive therapy to reduce the frequency of recurrences or episodically to shorten the duration of lesions:
- Suppressive therapy reduces the frequency of recurrence by 70% to 80% among patients with frequent outbreaks. Long-term safety and efficacy are well established.
- Episodic therapy is most effective if started within 1 day after onset of lesions or during the prodrome.36
There is no specific recommendation for when to choose suppressive over episodic therapy; most patients prefer suppressive therapy because it improves quality of life. Use shared clinical decision-making to determine the best option for an individual patient.
Continue to: Human papillomavirus
Human papillomavirus
Condylomata acuminata (genital warts) are caused by human papillomavirus (HPV), most commonly types 6 and 11, which manifest as soft papules or plaques on the external genitalia, perineum, perianal skin, and groin. The warts are usually asymptomatic but can be painful or pruritic, depending on size and location.
Diagnosis is made by visual inspection and can be confirmed by biopsy if lesions are atypical. Lesions can resolve spontaneously, remain unchanged, or grow in size or number.
Treatment. The aim of treatment is relief of symptoms and removal of warts. Treatment does not eradicate HPV infection. Multiple treatments are available that can be applied by the patient as a cream, gel, or ointment or administered by the provider, including cryotherapy, surgical removal, and solutions. The decision on how to treat should be based on the number, size, and
HPV-associated cancers and precancers. This is a broad (and separate) topic. HPV types 16 and 18 cause most cases of cervical, penile, vulvar, vaginal, anal, and oropharyngeal cancer and precancer.39 The USPSTF, the American Cancer Society, and the American College of Obstetricians and Gynecologists all have recommendations for cervical cancer screening in the United States.40 Refer to guidelines of the ASCCP for recommendations on abnormal screening tests.41
Prevention of genital warts. The 9-valent HPV vaccine available in the United States is safe and effective and helps protect against viral types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Types 6 and 11 are the principal causes of genital warts. Types 16 and 18 cause 66% of cervical cancer. The vaccination series can be started at age 9 years and is recommended for everyone through age 26 years. Only 2 doses are needed if the first dose is given prior to age 15 years; given after that age, a 3-dose series is utilized. Refer to CDC vaccine guidelines42 for details on the exact timing of vaccination.
Vaccination for women ages 27 to 45 years is not universally recommended because most people have been exposed to HPV by that age. However, the vaccine can still be administered, depending on clinical circumstances and the risk for new infection.42
Syphilis
Caused by the spirochete Treponema pallidum, syphilis manifests across a spectrum—from congenital to tertiary. The inability of medical science to develop a method for culturing the spirochete has confounded diagnosis and treatment.
Continue to: Since reaching a historic...
Since reaching a historic nadir of incidence in 2000 (5979 cases in the United States), there has been an increasingly rapid rise in that number: to 130,000 in 2020. More than 50% of cases are in MSM; however, the number of cases in heterosexual women is rapidly increasing.43
Routine screening for syphilis should be performed in any person who is at risk: all pregnant women in the first trimester (and in the third trimester and at delivery if they are at risk or live in a community where prevalence is high) and annually in sexually active MSM or anyone with HIV infection.10
Diagnosis. Examination by dark-field microscopy, testing by PCR, and direct fluorescent antibody assay for T pallidum from lesion tissue or exudate provide definitive diagnosis for early and congenital syphilis, but are often unavailable.
Presumptive diagnosis requires 2 serologic tests:
- Nontreponemal tests (the VDRL and rapid plasma reagin tests) identify anticardiolipin antibodies released during syphilis infection, although results also can be elevated in autoimmune disease or after certain immunizations, including the COVID-19 vaccine.
- Treponemal tests (the fluorescent treponemal antibody absorbed assay, T pallidum particulate agglutination assay, enzyme immunoassay, and chemiluminescence immunoassay) are specific antibody tests.
Historically, reactive nontreponemal tests, which are less expensive and easier to perform, were followed by a treponemal test to confirm the presumptive diagnosis. This method continues to be reasonable when screening patients in a low-prevalence population.11 The reverse sequence screening algorithm (ie, begin with a treponemal test) is now frequently used. With this method, a positive treponemal test must be confirmed with a nontreponemal test. If the treponemal test is positive and the nontreponemal test is negative, another treponemal test must be positive to confirm the diagnosis. This algorithm is useful in high-risk populations because it provides earlier detection of recently acquired syphilis and enhanced detection of late latent syphilis.12,13,44 The CDC has not stated a diagnostic preference.
Once the diagnosis is made, a complete history (including a sexual history and a history of syphilis testing and treatment) and a physical exam are necessary to confirm stage of disease.45
Special circumstances. Neurosyphilis, ocular syphilis, and otosyphilis refer to the site of infection and can occur at any stage of disease. The nervous system usually is infected within hours of initial infection, but symptoms might take weeks or years to develop—or might never manifest. Any time a patient develops neurologic, ophthalmologic, or audiologic symptoms, careful neurologic and ophthalmologic evaluation should be performed and the patient should be tested for HIV.
Continue to: Lumbar puncture is warranted...
Lumbar puncture is warranted for evaluation of cerebrospinal fluid if neurologic symptoms are present but is not necessary for isolated ocular syphilis or otosyphilis without neurologic findings. Treatment should not be delayed for test results if ocular syphilis is suspected because permanent blindness can develop. Any patient at high risk for an STI who presents with neurologic or ophthalmologic symptoms should be tested for syphilis and HIV.45
Pregnant women who have a diagnosis of syphilis should be treated with penicillin immediately because treatment ≥ 30 days prior to delivery is likely to prevent most cases of congenital syphilis. However, a course of penicillin might not prevent stillbirth or congenital syphilis in a gravely infected fetus, evidenced by fetal syphilis on a sonogram at the time of treatment. Additional doses of penicillin in pregnant women with early syphilis might be indicated if there is evidence of fetal syphilis on ultrasonography. All women who deliver a stillborn infant (≥ 20 weeks’ gestation) should be tested for syphilis at delivery.46
All patients in whom primary or secondary syphilis has been diagnosed should be tested for HIV at the time of diagnosis and treatment; if the result is negative, they should be offered preexposure prophylaxis (PrEP; discussed shortly). If the incidence of HIV in your community is high, repeat testing for HIV in 3 months. Clinical and serologic evaluation should be performed 6 and 12 months after treatment.47
Treatment. Penicillin remains the standard treatment for syphilis. Primary, secondary, and early tertiary stages (including in pregnancy) are treated with benzathine penicillin G 2.4 million units intramuscular (IM) in a single dose. For pregnant patients, repeating that dose in 1 week generally is recommended. Patients in the late latent (> 1 year) or tertiary stage receive the same dose of penicillin, which is then repeated weekly, for a total of 3 doses. Doxycycline and ceftriaxone are alternatives, except in pregnancy.
Warn patients of the Jarisch-Herxheimer reaction: fever, headache, and myalgias associated with initiation of treatment in the presence of the high bacterial load seen in early syphilis. Treatment is symptomatic, but the Jarisch-Herxheimer reaction can cause fetal distress in pregnancy.
Otosyphilis, ocular syphilis, and neurosyphilis require intravenous (IV) aqueous crystalline penicillin G 3 to 4 million U every 4 hours for 10 to 14 days.45 Alternatively, procaine penicillin G 2.4 million U/d IM can be given daily with oral probenecid 500 mg qid, both for 10 to 14 days (TABLE 214).
Screening andprevention of STIs
Screening recommendations
Follow USPSTF screening guidelines for STIs.10,48-54 Screen annually for:
- gonorrhea and chlamydia in women ages 15 to 24 years and in women older than 25 years if they are at increased risk
- gonorrhea, chlamydia, syphilis, and HIV in MSM, and hepatitis C if they are HIV positive
- trichomoniasis in women who are HIV positive.
Continue to: Consider the community in which...
Consider the community in which you practice when determining risk; you might want to consult local public health authorities for information about local epidemiology and guidance on determining which of your patients are at increased risk.
Preexposure prophylaxis
According to the CDC, all sexually active adults and adolescents should be informed about the availability of PrEP to prevent HIV infection. PrEP should be (1) available to anyone who requests it and (2) recommended for anyone who is sexually active and who practices sexual behaviors that place them at substantial risk for exposure to or acquisition of HIV, or both.
The recommended treatment protocol for men and women who have either an HIV-positive partner or inconsistent condom use or who have had a bacterial STI in the previous 6 months is oral emtricitabine 200 mg plus tenofovir disoproxil fumarate 300 mg/d (sold as Truvada-F/TDF). Men and transgender women (ie, assigned male at birth) with at-risk behaviors also can use emtricitabine plus tenofovir alafenamide 25 mg/d (sold as Descovy-F/TAF).
In addition, cabotegravir plus rilpirivine (sold as Cabenuva), IM every 2 months, was approved by the FDA for PrEP in 2021.
Creatinine clearance should be assessed at baseline and yearly (every 6 months for those older than 50 years) in patients taking PrEP. All patients must be tested for HIV at initiation of treatment and every 3 months thereafter (every 4 months for cabotegravir plus rilpirivine). Patients should be screened for bacterial STIs every 6 months (every 3 months for MSM and transgender women); screening for chlamydia should be done yearly. For patients being treated with emtricitabine plus tenofovir alafenamide, weight and a lipid profile (cholesterol and triglycerides) should be assessed annually.55
Postexposure prophylaxis
The sharp rise in the incidence of STIs in the past few years has brought renewed interest in postexposure prophylaxis (PEP) for STIs. Although PEP should be standard in cases of sexual assault, this protocol also can be considered in other instances of high-risk exposure.
CDC recommendations for PEP in cases of assault are56:
- ceftriaxone 500 mg IM in a single dose (1 g if weight is ≥ 150 kg) plus
- doxycycline 100 mg bid for 7 days plus
- metronidazole 2 g bid for 7 days (for vaginal exposure)
- pregnancy evaluation and emergency contraception
- hepatitis B risk evaluation and vaccination, with or without hepatitis B immune globulin
- HIV risk evaluation, based on CDC guidelines, and possible HIV prophylaxis (PrEP)
- HPV vaccination for patients ages 9 to 26 years if they are not already fully vaccinated.
CORRESPONDENCE
Belinda Vail, MD, 3901 Rainbow Boulevard, Mail Stop 4010, Kansas City, KS 66160; [email protected]
1. Pagaoa M, Grey J, Torrone E, et al. Trends in nationally notifiable sexually transmitted disease case reports during the US COVID-19 pandemic, January to December 2020. Sex Transm Dis. 2021;48:798-804. doi: 10.1097/OLQ.0000000000001506
2. Chesson HW, Spicknall IH; Bingham A, et al. The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Sex Transm Dis. 2021;48:215-221. doi: 10.1097/OLQ.0000000000001380
3. Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis. 2021;48:208-214. doi: 10.1097/OLQ.0000000000001355
4. CDC. Sexually transmitted infections treatment guidelines, 2021: Chlamydial infections among adolescents and adults. US Department of Health and Human Services. July 21, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/chlamydia.htm
5. CDC. Sexually transmitted infections treatment guidelines, 2021: Gonococcal infections among adolescents and adults. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm
6. Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;
7. CDC. Sexually transmitted infections treatment guidelines, 2021. Trichomoniasis. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. December 27, 2021. www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
8. Spicknall IH, Flagg EW, Torrone EA. Estimates of the prevalence and incidence of genital herpes, United States, 2018. Sex Transm Dis. 2021;48:260-265. doi: 10.1097/OLQ.0000000000001375
9. Mark H, Gilbert L, Nanda J. Psychosocial well-being and quality of life among women newly diagnosed with genital herpes. J Obstet Gynecol Neonatal Nurs.
10. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2321-2327. doi: 10.1001/jama.2016.5824
11. Ricco J, Westby A. Syphilis: far from ancient history. Am Fam Physician. 2020;102:91-98.
12. Goza M, Kulwicki B, Akers JM, et al. Syphilis screening: a review of the Syphilis Health Check rapid immunochromatographic test. J Pharm Technol. 2017;33:53-59. doi:10.1177/8755122517691308
13. Henao- AF, Johnson SC. Diagnostic tests for syphilis: new tests and new algorithms. Neurol Clin Pract. 2014;4:114-122. doi: 10.1212/01.CPJ.0000435752.17621.48
14. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
15. CDC. Sexually Transmitted Disease Surveillance 2021. National overview of STDs. US Department of Health and Human Services. April 2023. Accessed May 9, 2023. www.cdc.gov/std/statistics/2021/overview.htm#Chlamydia
16. CDC. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014;63:1-19.
17. Kong FYS, Tabrizi SN, Law M, et al. Azithromycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;59:193-205. doi: 10.1093/cid/ciu220
18. Páez-Canro C, Alzate JP, González LM, et al. Antibiotics for treating urogenital Chlamydia trachomatis infection in men and non-pregnant women. Cochrane Database Syst Rev. 2019;1:CD010871. doi: 10.1002/14651858.CD010871.pub2
19. Dombrowski JC, Wierzbicki MR, Newman LM, et al. Doxycycline versus azithromycin for the treatment of rectal chlamydia in men who have sex with men: a randomized controlled trial. Clin Infect Dis. 2021;73:824-831. doi: 10.1093/cid/ciab153
20. CDC. Sexually transmitted infections treatment guidelines, 2021: Expedited partner therapy. US Department of Health and Human Services. July 22, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/clinical-EPT.htm
21. Golden MR, Whittington WLH, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676-685. doi: 10.1056/NEJMoa041681
22. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
23. Cameron ST, Glasier A, Scott G, et al. Novel interventions to reduce re-infection in women with chlamydia: a randomized controlled trial. Hum Reprod. 2009;24:888-895. doi: 10.1093/humrep/den475
24. McNulty A, Teh MF, Freedman E. Patient delivered partner therapy for chlamydial infection—what would be missed? Sex Transm Dis. 2008;35:834-836. doi: 10.1097/OLQ.0b013e3181761993
25. Stekler J, Bachmann L, Brotman RM, et al. Concurrent sexually transmitted infections (STIs) in sex partners of patients with selected STIs: implications for patient-delivered partner therapy. Clin Infect Dis. 2005;40:787-793. doi: 10.1086/428043
26. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
27. Stupiansky NW, Van der Pol B, Williams JA, et al. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis. 2011;38:750-754. doi: 10.1097/OLQ.0b013e31820ff9a4
28. CDC. Sexually transmitted infections treatment guidelines, 2021: Screening recommendations and considerations referenced in treatment guidelines and original sources. US Department of Health and Human Services. June 6, 2022. Accessed May 9, 2023. www.cdc.gov/std/treatment-guidelines/screening-recommen dations.htm
29. Cantor A, Dana T, Griffen JC, et al. Screening for chlamydial and gonococcal infections: a systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 206. AHRQ Report No. 21-05275-EF-1. Agency for Healthcare Research and Quality. September 2021. www.ncbi.nlm.nih.gov/books/NBK574045
30. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
31. Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019:97:548-562P. doi: 10.2471/BLT.18.228486
32. Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217. doi: 10.1093/cid/ciy079
33. CDC. Sexually transmitted infections treatment guidelines, 2021. Mycoplasma genitalium. US Department of Health and Human Services. July 22, 2021. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm
34. Manhart LE, Broad JM, Bolden MR. Mycoplasma genitalium: should we treat and how? Clin Infect Dis. 2011;53(suppl 3):S129-S142. doi:10.1093/cid/cir702.
35. Corey L, Wald A. Genital herpes. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. McGraw-Hill; 2008:399-437.
36. CDC. Sexually transmitted infections treatment guidelines, 2021: Genital herpes. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/herpes.htm
37. Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17:1303-1316. doi: 10.1016/S1473-3099(17)30405-X
38. Rollenhagen C, Lathrop M, Macura SL, et al. Herpes simplex virus type-2 stimulates HIV-1 replication in cervical tissues: implications for HIV-1 transmission and efficacy of anti-HIV-1 microbicides. Mucosal Immunol. 2014;7:1165-1174. doi: 10.1038/mi.2014.3
39. Cogliano V, Baan R, Straif K, et al; WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol.
40. Simon MA, Tseng CW, Wong JB. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897
41. Perkins RB, Guido RS, Castle PE, et al; . 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
42. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
43. Schmidt R, Carson PJ, Jansen RJ. Resurgence of syphilis in the United States: an assessment of contributing factors. Infect Dis (Auckl). 2019;12:1178633719883282. doi: 10.1177/1178633719883282
44. Boog GHP, Lopes JVZ, Mahler JV, et al. Diagnostic tools for neurosyphilis: a systematic review. BMC Infect Dis. 2021;21:568. doi: 10.1186/s12879-021-06264-8
45. CDC. Sexually transmitted infections treatment guidelines, 2021. Syphilis. US Department of Health and Human Services. April 20, 2023. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/syphilis.htm
46. Matthias JM, Rahman MM, Newman DR, et al. Effectiveness of prenatal screening and treatment to prevent congenital syphilis, Louisiana and Florida, 2013-2014. Sex Transm Dis. 2017;44:498-502. doi: 10.1097/OLQ.0000000000000638
47. Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA. 2014;312:1905-1917. doi: 10.1001/jama.2014.13259
48. Davidson KW, Barry MJ, Mangione CM, et al; US Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:949-956. doi: 10.1001/jama.2021.14081
49. Krist AH, Davidson KW, Mangione CM, et al; US Preventive Services Task Force. Screening for hepatitis B virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2415-2422. doi: 10.1001/jama.2020.22980
50. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:970-975. doi: 10.1001/jama.2020.1123
51. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530. doi: 10.1001/jama.2016.16776
52. Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi: 10.1001/jama.2018.10897
53. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336. doi: 10.1001/jama.2019.6587
54. Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36:502-509. doi: 10.1016/s0091-7435(02)00058-0
55. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. A clinical practice guideline. Centers for Disease Control and Prevention. Accessed April 24, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
56. CDC. Sexually transmitted infections treatment guidelines, 2021: Sexual assault and abuse and STIs—adolescents and adults, 2021. US Department of Health and Human Services. July 22, 2021. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/sexual-assault-adults.htm
1. Pagaoa M, Grey J, Torrone E, et al. Trends in nationally notifiable sexually transmitted disease case reports during the US COVID-19 pandemic, January to December 2020. Sex Transm Dis. 2021;48:798-804. doi: 10.1097/OLQ.0000000000001506
2. Chesson HW, Spicknall IH; Bingham A, et al. The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Sex Transm Dis. 2021;48:215-221. doi: 10.1097/OLQ.0000000000001380
3. Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis. 2021;48:208-214. doi: 10.1097/OLQ.0000000000001355
4. CDC. Sexually transmitted infections treatment guidelines, 2021: Chlamydial infections among adolescents and adults. US Department of Health and Human Services. July 21, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/chlamydia.htm
5. CDC. Sexually transmitted infections treatment guidelines, 2021: Gonococcal infections among adolescents and adults. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm
6. Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;
7. CDC. Sexually transmitted infections treatment guidelines, 2021. Trichomoniasis. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. December 27, 2021. www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
8. Spicknall IH, Flagg EW, Torrone EA. Estimates of the prevalence and incidence of genital herpes, United States, 2018. Sex Transm Dis. 2021;48:260-265. doi: 10.1097/OLQ.0000000000001375
9. Mark H, Gilbert L, Nanda J. Psychosocial well-being and quality of life among women newly diagnosed with genital herpes. J Obstet Gynecol Neonatal Nurs.
10. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2321-2327. doi: 10.1001/jama.2016.5824
11. Ricco J, Westby A. Syphilis: far from ancient history. Am Fam Physician. 2020;102:91-98.
12. Goza M, Kulwicki B, Akers JM, et al. Syphilis screening: a review of the Syphilis Health Check rapid immunochromatographic test. J Pharm Technol. 2017;33:53-59. doi:10.1177/8755122517691308
13. Henao- AF, Johnson SC. Diagnostic tests for syphilis: new tests and new algorithms. Neurol Clin Pract. 2014;4:114-122. doi: 10.1212/01.CPJ.0000435752.17621.48
14. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
15. CDC. Sexually Transmitted Disease Surveillance 2021. National overview of STDs. US Department of Health and Human Services. April 2023. Accessed May 9, 2023. www.cdc.gov/std/statistics/2021/overview.htm#Chlamydia
16. CDC. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014;63:1-19.
17. Kong FYS, Tabrizi SN, Law M, et al. Azithromycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;59:193-205. doi: 10.1093/cid/ciu220
18. Páez-Canro C, Alzate JP, González LM, et al. Antibiotics for treating urogenital Chlamydia trachomatis infection in men and non-pregnant women. Cochrane Database Syst Rev. 2019;1:CD010871. doi: 10.1002/14651858.CD010871.pub2
19. Dombrowski JC, Wierzbicki MR, Newman LM, et al. Doxycycline versus azithromycin for the treatment of rectal chlamydia in men who have sex with men: a randomized controlled trial. Clin Infect Dis. 2021;73:824-831. doi: 10.1093/cid/ciab153
20. CDC. Sexually transmitted infections treatment guidelines, 2021: Expedited partner therapy. US Department of Health and Human Services. July 22, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/clinical-EPT.htm
21. Golden MR, Whittington WLH, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676-685. doi: 10.1056/NEJMoa041681
22. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
23. Cameron ST, Glasier A, Scott G, et al. Novel interventions to reduce re-infection in women with chlamydia: a randomized controlled trial. Hum Reprod. 2009;24:888-895. doi: 10.1093/humrep/den475
24. McNulty A, Teh MF, Freedman E. Patient delivered partner therapy for chlamydial infection—what would be missed? Sex Transm Dis. 2008;35:834-836. doi: 10.1097/OLQ.0b013e3181761993
25. Stekler J, Bachmann L, Brotman RM, et al. Concurrent sexually transmitted infections (STIs) in sex partners of patients with selected STIs: implications for patient-delivered partner therapy. Clin Infect Dis. 2005;40:787-793. doi: 10.1086/428043
26. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
27. Stupiansky NW, Van der Pol B, Williams JA, et al. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis. 2011;38:750-754. doi: 10.1097/OLQ.0b013e31820ff9a4
28. CDC. Sexually transmitted infections treatment guidelines, 2021: Screening recommendations and considerations referenced in treatment guidelines and original sources. US Department of Health and Human Services. June 6, 2022. Accessed May 9, 2023. www.cdc.gov/std/treatment-guidelines/screening-recommen dations.htm
29. Cantor A, Dana T, Griffen JC, et al. Screening for chlamydial and gonococcal infections: a systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 206. AHRQ Report No. 21-05275-EF-1. Agency for Healthcare Research and Quality. September 2021. www.ncbi.nlm.nih.gov/books/NBK574045
30. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
31. Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019:97:548-562P. doi: 10.2471/BLT.18.228486
32. Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217. doi: 10.1093/cid/ciy079
33. CDC. Sexually transmitted infections treatment guidelines, 2021. Mycoplasma genitalium. US Department of Health and Human Services. July 22, 2021. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm
34. Manhart LE, Broad JM, Bolden MR. Mycoplasma genitalium: should we treat and how? Clin Infect Dis. 2011;53(suppl 3):S129-S142. doi:10.1093/cid/cir702.
35. Corey L, Wald A. Genital herpes. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. McGraw-Hill; 2008:399-437.
36. CDC. Sexually transmitted infections treatment guidelines, 2021: Genital herpes. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/herpes.htm
37. Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17:1303-1316. doi: 10.1016/S1473-3099(17)30405-X
38. Rollenhagen C, Lathrop M, Macura SL, et al. Herpes simplex virus type-2 stimulates HIV-1 replication in cervical tissues: implications for HIV-1 transmission and efficacy of anti-HIV-1 microbicides. Mucosal Immunol. 2014;7:1165-1174. doi: 10.1038/mi.2014.3
39. Cogliano V, Baan R, Straif K, et al; WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol.
40. Simon MA, Tseng CW, Wong JB. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897
41. Perkins RB, Guido RS, Castle PE, et al; . 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
42. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
43. Schmidt R, Carson PJ, Jansen RJ. Resurgence of syphilis in the United States: an assessment of contributing factors. Infect Dis (Auckl). 2019;12:1178633719883282. doi: 10.1177/1178633719883282
44. Boog GHP, Lopes JVZ, Mahler JV, et al. Diagnostic tools for neurosyphilis: a systematic review. BMC Infect Dis. 2021;21:568. doi: 10.1186/s12879-021-06264-8
45. CDC. Sexually transmitted infections treatment guidelines, 2021. Syphilis. US Department of Health and Human Services. April 20, 2023. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/syphilis.htm
46. Matthias JM, Rahman MM, Newman DR, et al. Effectiveness of prenatal screening and treatment to prevent congenital syphilis, Louisiana and Florida, 2013-2014. Sex Transm Dis. 2017;44:498-502. doi: 10.1097/OLQ.0000000000000638
47. Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA. 2014;312:1905-1917. doi: 10.1001/jama.2014.13259
48. Davidson KW, Barry MJ, Mangione CM, et al; US Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:949-956. doi: 10.1001/jama.2021.14081
49. Krist AH, Davidson KW, Mangione CM, et al; US Preventive Services Task Force. Screening for hepatitis B virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2415-2422. doi: 10.1001/jama.2020.22980
50. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:970-975. doi: 10.1001/jama.2020.1123
51. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530. doi: 10.1001/jama.2016.16776
52. Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi: 10.1001/jama.2018.10897
53. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336. doi: 10.1001/jama.2019.6587
54. Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36:502-509. doi: 10.1016/s0091-7435(02)00058-0
55. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. A clinical practice guideline. Centers for Disease Control and Prevention. Accessed April 24, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
56. CDC. Sexually transmitted infections treatment guidelines, 2021: Sexual assault and abuse and STIs—adolescents and adults, 2021. US Department of Health and Human Services. July 22, 2021. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/sexual-assault-adults.htm
PRACTICE RECOMMENDATIONS
› Focus efforts to prevent sexually transmitted infections (STIs) on patients ages 15 to 24 years—because half of new STIs in the United States occur in this age group. A
› Screen for other STIs, including HIV infection, if a person tests positive for a single STI. A
› Treat STIs by following updated (2021) guidelines developed by the Centers for Disease Control and Prevention. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series