User login
The Failure to Deliver as Promised
In March 2009, a 63-year-old man was diagnosed with stage IV gastric carcinoma with metastasis to the liver. His treating oncologist gave him a prognosis of about 10 months’ life expectancy with chemotherapy. The patient’s family searched for alternative treatment options and found a natural alternative treatment center claiming the ability to cure the patient.
The patient and his family decided to defer chemotherapy, and he was admitted to the alternative treatment center for three to four weeks of inpatient care. The treatment consisted of “colonic hydrotherapy,” supplements designed to cleanse the body, and a diet restricted to seed milk, vegetable juice, and spinach soup.
After six days, the patient developed severe diarrhea, confusion, and profound weakness. He was taken to a local hospital and admitted with a diagnosis of acute renal failure. Dialysis attempts were unsuccessful, and the man died of respiratory distress secondary to acute renal failure a week later.
The plaintiff claimed that the treatment provided by the defendants was contraindicated and caused acute renal failure, noting that the patient’s kidney function had been relatively normal when he entered the treatment facility. The plaintiff claimed that defendant Dr N., a chiropractor, never reviewed any of the decedent’s medical records, did not discuss the proposed treatment plan with his treating physicians, and failed to properly monitor the patient’s condition, notice his deterioration, and provide timely transfer to a hospital.
The defendant claimed that the treatment given had no adverse effects on the decedent and that the acute renal failure was due to hepatorenal syndrome due to his advanced metastatic liver cancer.
What was the outcome? >>
OUTCOME
A $2.5 million verdict was returned. An appeal was pending.
COMMENT
This is a case against a chiropractor, so why discuss it in a journal dedicated to NP and PA practice? Because it involves scope of practice, alternative medicine, the safety of “natural” treatments, and the ethical and legal problems of making unsupportable promises to patients.
Know your scope of practice, and don’t overextend. Clinicians trained as specialists (eg, in pediatrics, midwifery, or anesthesia) should use caution departing from that area. Those trained as “generalists” need to be careful as well; even if you were trained in a family practice program, if you are a PA who has worked in dermatology for the past 10 years, think twice about giving treatment or advice to your friend with a neurologic complaint. In the event of a lawsuit, the plaintiff will spend a great deal of time building your resume as an expert in your discipline, only to attack you as inexperienced and unqualified in the case in which you extended yourself.
Here a chiropractor, without ever examining the patient, directed the treatment of a very sick man in an area in which he was not qualified. While chiropractors may claim the ability to treat outside their traditional scope, the jury’s verdict in this case proves that they were not persuaded he was right to do so. The chiropractor, Dr N., eventually lost his license, based in part on the false promises he made about his ability to cure patients of “any and all diseases, including cancer, by restoring the body to its natural state ….” This opportunistic preying upon the most ill and vulnerable in our society likely irked the jurors, who returned a substantial award, considering that the patient’s short life expectancy was uncontested.
Handle alternative medicine with particular care, because an alternative treatment may not qualify as “medicine” at all. If we define medicine as the application of scientific principles to health care, an alternative that is unproven, unstudied, and unknown does not qualify. Rather, it is guesswork—with potentially devastating consequences.
In this case, through his company, the chiropractor based his treatment plan on guesswork that colonic hydrotherapy and severe dietary restrictions would help a patient with stage IV metastatic gastric carcinoma. He was wrong, and the jury concluded that these alternatives injured the patient and hastened his death.
Certainly, Western medicine has been rightly and fairly criticized for failing to promote wellness through a healthy lifestyle, including diet, exercise, safety, emotional well-being, and stress management. However, when venturing from generally accepted health promotion strategies to a specific recommendation that an alternative agent “is good for” a specific problem, be careful. You may believe lavender oil is an effective antibiotic—but can you prove it?
If you choose lavender oil over a demonstrably effective antibiotic to treat pneumonia, and the patient deteriorates, you will be held accountable. The plaintiff will demand answers, and the jury will await your explanation. Reliance on vague concepts, not generally accepted in the literature (eg, “energy management,” detoxifying, unblocking “clogged” nervous systems), will be ridiculed by the plaintiff’s experts, and you will be skewered on cross-examination. It is not enough to personally “believe” in the alternative; you must be able to support your treatment decisions through the best evidence possible.
To be fair, this cuts both ways: Some Western medical practices are based on anecdotal evidence with minimal scientific support. There was a time when a corneal abrasion was patched, a fractured clavicle was stabilized with a figure-of-eight dressing, and narcotics were withheld from a suffering patient with acute abdomen because it would “mask signs.” Our “Western” system is not immune from the impact of poor research, group-think, dogmas leading to inappropriate practice, and other sources of logical fallacy.
As NPs and PAs, we will be held to a scientific evidentiary standard. The standard of care will be based upon the care a reasonably prudent clinician would deliver in a similar situation. At trial, you will be confronted with a PA or NP on the stand testifying against you regarding what is reasonably prudent, acceptable care. Make sure your actions are scientifically defensible.
Interestingly, the standard for admitting a scientific opinion as expert testimony has changed. In 1923, Frye v United States1 established that, for an expert opinion to be admissible, the testimony had to be based on what is “generally accepted in the scientific community.” In 1993, the Supreme Court case Daubert v Merrell Dow Pharmaceuticals2 determined that the opinion need not be “generally accepted” but must be based on scientific method and must be relevant to the case; the judge serves as a “gatekeeper” to be sure the opinions flow from “scientific knowledge.”
Medical malpractice cases are based on state law. Some follow Frye, some Daubert. The latter is a more relaxed standard, but even in states following Daubert, an expert witness who purports to testify on an alternative treatment must follow the scientific method. For example, the webpage of the defendant chiropractor’s institute (still in business) currently claims that “Heart/Brain Entrainment Therapy balances frequencies of organs/glands/tissues. Everything in the universe resonates at a particular frequency—light, sound, and every cell, organ, gland, and tissue in you.”3
So, whatever Heart/Brain Entrainment Therapy is, for that theory to be admissible in a Frye jurisdiction it would likely have to be “generally accepted” in the medical community. To be admissible in a Daubert jurisdiction, proponents of the testimony would have to show evidence of a scientific methodology supporting the theory before the jury could hear any testimony about it. In either case, strategically, the defense attorney would likely file a motion to block either certain parts of the testimony or the testimony entirely.
IN SUM
Jurors expect sound scientific methodology supporting medical decisions; use care when selecting treatment for patients. Robustly adopt health promotion and general wellness strategies. However, if you use alternatives directed toward a specific therapy solving a specific problem, use them cautiously and with an awareness that the indication for the therapy should be scientifically defensible. —DML
REFERENCES
1. Frye v United States, 293 F. 1013 (D.C. Cir. 1923).
2. Daubert v Merrell Dow Pharmaceuticals, 509 U.S. 579 (1993).
3. Total Health Institute. Bioelectrical Energy, Quantum Frequency Resonance. www.totalhealthinstitute.com/about. Accessed July 14, 2015.
In March 2009, a 63-year-old man was diagnosed with stage IV gastric carcinoma with metastasis to the liver. His treating oncologist gave him a prognosis of about 10 months’ life expectancy with chemotherapy. The patient’s family searched for alternative treatment options and found a natural alternative treatment center claiming the ability to cure the patient.
The patient and his family decided to defer chemotherapy, and he was admitted to the alternative treatment center for three to four weeks of inpatient care. The treatment consisted of “colonic hydrotherapy,” supplements designed to cleanse the body, and a diet restricted to seed milk, vegetable juice, and spinach soup.
After six days, the patient developed severe diarrhea, confusion, and profound weakness. He was taken to a local hospital and admitted with a diagnosis of acute renal failure. Dialysis attempts were unsuccessful, and the man died of respiratory distress secondary to acute renal failure a week later.
The plaintiff claimed that the treatment provided by the defendants was contraindicated and caused acute renal failure, noting that the patient’s kidney function had been relatively normal when he entered the treatment facility. The plaintiff claimed that defendant Dr N., a chiropractor, never reviewed any of the decedent’s medical records, did not discuss the proposed treatment plan with his treating physicians, and failed to properly monitor the patient’s condition, notice his deterioration, and provide timely transfer to a hospital.
The defendant claimed that the treatment given had no adverse effects on the decedent and that the acute renal failure was due to hepatorenal syndrome due to his advanced metastatic liver cancer.
What was the outcome? >>
OUTCOME
A $2.5 million verdict was returned. An appeal was pending.
COMMENT
This is a case against a chiropractor, so why discuss it in a journal dedicated to NP and PA practice? Because it involves scope of practice, alternative medicine, the safety of “natural” treatments, and the ethical and legal problems of making unsupportable promises to patients.
Know your scope of practice, and don’t overextend. Clinicians trained as specialists (eg, in pediatrics, midwifery, or anesthesia) should use caution departing from that area. Those trained as “generalists” need to be careful as well; even if you were trained in a family practice program, if you are a PA who has worked in dermatology for the past 10 years, think twice about giving treatment or advice to your friend with a neurologic complaint. In the event of a lawsuit, the plaintiff will spend a great deal of time building your resume as an expert in your discipline, only to attack you as inexperienced and unqualified in the case in which you extended yourself.
Here a chiropractor, without ever examining the patient, directed the treatment of a very sick man in an area in which he was not qualified. While chiropractors may claim the ability to treat outside their traditional scope, the jury’s verdict in this case proves that they were not persuaded he was right to do so. The chiropractor, Dr N., eventually lost his license, based in part on the false promises he made about his ability to cure patients of “any and all diseases, including cancer, by restoring the body to its natural state ….” This opportunistic preying upon the most ill and vulnerable in our society likely irked the jurors, who returned a substantial award, considering that the patient’s short life expectancy was uncontested.
Handle alternative medicine with particular care, because an alternative treatment may not qualify as “medicine” at all. If we define medicine as the application of scientific principles to health care, an alternative that is unproven, unstudied, and unknown does not qualify. Rather, it is guesswork—with potentially devastating consequences.
In this case, through his company, the chiropractor based his treatment plan on guesswork that colonic hydrotherapy and severe dietary restrictions would help a patient with stage IV metastatic gastric carcinoma. He was wrong, and the jury concluded that these alternatives injured the patient and hastened his death.
Certainly, Western medicine has been rightly and fairly criticized for failing to promote wellness through a healthy lifestyle, including diet, exercise, safety, emotional well-being, and stress management. However, when venturing from generally accepted health promotion strategies to a specific recommendation that an alternative agent “is good for” a specific problem, be careful. You may believe lavender oil is an effective antibiotic—but can you prove it?
If you choose lavender oil over a demonstrably effective antibiotic to treat pneumonia, and the patient deteriorates, you will be held accountable. The plaintiff will demand answers, and the jury will await your explanation. Reliance on vague concepts, not generally accepted in the literature (eg, “energy management,” detoxifying, unblocking “clogged” nervous systems), will be ridiculed by the plaintiff’s experts, and you will be skewered on cross-examination. It is not enough to personally “believe” in the alternative; you must be able to support your treatment decisions through the best evidence possible.
To be fair, this cuts both ways: Some Western medical practices are based on anecdotal evidence with minimal scientific support. There was a time when a corneal abrasion was patched, a fractured clavicle was stabilized with a figure-of-eight dressing, and narcotics were withheld from a suffering patient with acute abdomen because it would “mask signs.” Our “Western” system is not immune from the impact of poor research, group-think, dogmas leading to inappropriate practice, and other sources of logical fallacy.
As NPs and PAs, we will be held to a scientific evidentiary standard. The standard of care will be based upon the care a reasonably prudent clinician would deliver in a similar situation. At trial, you will be confronted with a PA or NP on the stand testifying against you regarding what is reasonably prudent, acceptable care. Make sure your actions are scientifically defensible.
Interestingly, the standard for admitting a scientific opinion as expert testimony has changed. In 1923, Frye v United States1 established that, for an expert opinion to be admissible, the testimony had to be based on what is “generally accepted in the scientific community.” In 1993, the Supreme Court case Daubert v Merrell Dow Pharmaceuticals2 determined that the opinion need not be “generally accepted” but must be based on scientific method and must be relevant to the case; the judge serves as a “gatekeeper” to be sure the opinions flow from “scientific knowledge.”
Medical malpractice cases are based on state law. Some follow Frye, some Daubert. The latter is a more relaxed standard, but even in states following Daubert, an expert witness who purports to testify on an alternative treatment must follow the scientific method. For example, the webpage of the defendant chiropractor’s institute (still in business) currently claims that “Heart/Brain Entrainment Therapy balances frequencies of organs/glands/tissues. Everything in the universe resonates at a particular frequency—light, sound, and every cell, organ, gland, and tissue in you.”3
So, whatever Heart/Brain Entrainment Therapy is, for that theory to be admissible in a Frye jurisdiction it would likely have to be “generally accepted” in the medical community. To be admissible in a Daubert jurisdiction, proponents of the testimony would have to show evidence of a scientific methodology supporting the theory before the jury could hear any testimony about it. In either case, strategically, the defense attorney would likely file a motion to block either certain parts of the testimony or the testimony entirely.
IN SUM
Jurors expect sound scientific methodology supporting medical decisions; use care when selecting treatment for patients. Robustly adopt health promotion and general wellness strategies. However, if you use alternatives directed toward a specific therapy solving a specific problem, use them cautiously and with an awareness that the indication for the therapy should be scientifically defensible. —DML
REFERENCES
1. Frye v United States, 293 F. 1013 (D.C. Cir. 1923).
2. Daubert v Merrell Dow Pharmaceuticals, 509 U.S. 579 (1993).
3. Total Health Institute. Bioelectrical Energy, Quantum Frequency Resonance. www.totalhealthinstitute.com/about. Accessed July 14, 2015.
In March 2009, a 63-year-old man was diagnosed with stage IV gastric carcinoma with metastasis to the liver. His treating oncologist gave him a prognosis of about 10 months’ life expectancy with chemotherapy. The patient’s family searched for alternative treatment options and found a natural alternative treatment center claiming the ability to cure the patient.
The patient and his family decided to defer chemotherapy, and he was admitted to the alternative treatment center for three to four weeks of inpatient care. The treatment consisted of “colonic hydrotherapy,” supplements designed to cleanse the body, and a diet restricted to seed milk, vegetable juice, and spinach soup.
After six days, the patient developed severe diarrhea, confusion, and profound weakness. He was taken to a local hospital and admitted with a diagnosis of acute renal failure. Dialysis attempts were unsuccessful, and the man died of respiratory distress secondary to acute renal failure a week later.
The plaintiff claimed that the treatment provided by the defendants was contraindicated and caused acute renal failure, noting that the patient’s kidney function had been relatively normal when he entered the treatment facility. The plaintiff claimed that defendant Dr N., a chiropractor, never reviewed any of the decedent’s medical records, did not discuss the proposed treatment plan with his treating physicians, and failed to properly monitor the patient’s condition, notice his deterioration, and provide timely transfer to a hospital.
The defendant claimed that the treatment given had no adverse effects on the decedent and that the acute renal failure was due to hepatorenal syndrome due to his advanced metastatic liver cancer.
What was the outcome? >>
OUTCOME
A $2.5 million verdict was returned. An appeal was pending.
COMMENT
This is a case against a chiropractor, so why discuss it in a journal dedicated to NP and PA practice? Because it involves scope of practice, alternative medicine, the safety of “natural” treatments, and the ethical and legal problems of making unsupportable promises to patients.
Know your scope of practice, and don’t overextend. Clinicians trained as specialists (eg, in pediatrics, midwifery, or anesthesia) should use caution departing from that area. Those trained as “generalists” need to be careful as well; even if you were trained in a family practice program, if you are a PA who has worked in dermatology for the past 10 years, think twice about giving treatment or advice to your friend with a neurologic complaint. In the event of a lawsuit, the plaintiff will spend a great deal of time building your resume as an expert in your discipline, only to attack you as inexperienced and unqualified in the case in which you extended yourself.
Here a chiropractor, without ever examining the patient, directed the treatment of a very sick man in an area in which he was not qualified. While chiropractors may claim the ability to treat outside their traditional scope, the jury’s verdict in this case proves that they were not persuaded he was right to do so. The chiropractor, Dr N., eventually lost his license, based in part on the false promises he made about his ability to cure patients of “any and all diseases, including cancer, by restoring the body to its natural state ….” This opportunistic preying upon the most ill and vulnerable in our society likely irked the jurors, who returned a substantial award, considering that the patient’s short life expectancy was uncontested.
Handle alternative medicine with particular care, because an alternative treatment may not qualify as “medicine” at all. If we define medicine as the application of scientific principles to health care, an alternative that is unproven, unstudied, and unknown does not qualify. Rather, it is guesswork—with potentially devastating consequences.
In this case, through his company, the chiropractor based his treatment plan on guesswork that colonic hydrotherapy and severe dietary restrictions would help a patient with stage IV metastatic gastric carcinoma. He was wrong, and the jury concluded that these alternatives injured the patient and hastened his death.
Certainly, Western medicine has been rightly and fairly criticized for failing to promote wellness through a healthy lifestyle, including diet, exercise, safety, emotional well-being, and stress management. However, when venturing from generally accepted health promotion strategies to a specific recommendation that an alternative agent “is good for” a specific problem, be careful. You may believe lavender oil is an effective antibiotic—but can you prove it?
If you choose lavender oil over a demonstrably effective antibiotic to treat pneumonia, and the patient deteriorates, you will be held accountable. The plaintiff will demand answers, and the jury will await your explanation. Reliance on vague concepts, not generally accepted in the literature (eg, “energy management,” detoxifying, unblocking “clogged” nervous systems), will be ridiculed by the plaintiff’s experts, and you will be skewered on cross-examination. It is not enough to personally “believe” in the alternative; you must be able to support your treatment decisions through the best evidence possible.
To be fair, this cuts both ways: Some Western medical practices are based on anecdotal evidence with minimal scientific support. There was a time when a corneal abrasion was patched, a fractured clavicle was stabilized with a figure-of-eight dressing, and narcotics were withheld from a suffering patient with acute abdomen because it would “mask signs.” Our “Western” system is not immune from the impact of poor research, group-think, dogmas leading to inappropriate practice, and other sources of logical fallacy.
As NPs and PAs, we will be held to a scientific evidentiary standard. The standard of care will be based upon the care a reasonably prudent clinician would deliver in a similar situation. At trial, you will be confronted with a PA or NP on the stand testifying against you regarding what is reasonably prudent, acceptable care. Make sure your actions are scientifically defensible.
Interestingly, the standard for admitting a scientific opinion as expert testimony has changed. In 1923, Frye v United States1 established that, for an expert opinion to be admissible, the testimony had to be based on what is “generally accepted in the scientific community.” In 1993, the Supreme Court case Daubert v Merrell Dow Pharmaceuticals2 determined that the opinion need not be “generally accepted” but must be based on scientific method and must be relevant to the case; the judge serves as a “gatekeeper” to be sure the opinions flow from “scientific knowledge.”
Medical malpractice cases are based on state law. Some follow Frye, some Daubert. The latter is a more relaxed standard, but even in states following Daubert, an expert witness who purports to testify on an alternative treatment must follow the scientific method. For example, the webpage of the defendant chiropractor’s institute (still in business) currently claims that “Heart/Brain Entrainment Therapy balances frequencies of organs/glands/tissues. Everything in the universe resonates at a particular frequency—light, sound, and every cell, organ, gland, and tissue in you.”3
So, whatever Heart/Brain Entrainment Therapy is, for that theory to be admissible in a Frye jurisdiction it would likely have to be “generally accepted” in the medical community. To be admissible in a Daubert jurisdiction, proponents of the testimony would have to show evidence of a scientific methodology supporting the theory before the jury could hear any testimony about it. In either case, strategically, the defense attorney would likely file a motion to block either certain parts of the testimony or the testimony entirely.
IN SUM
Jurors expect sound scientific methodology supporting medical decisions; use care when selecting treatment for patients. Robustly adopt health promotion and general wellness strategies. However, if you use alternatives directed toward a specific therapy solving a specific problem, use them cautiously and with an awareness that the indication for the therapy should be scientifically defensible. —DML
REFERENCES
1. Frye v United States, 293 F. 1013 (D.C. Cir. 1923).
2. Daubert v Merrell Dow Pharmaceuticals, 509 U.S. 579 (1993).
3. Total Health Institute. Bioelectrical Energy, Quantum Frequency Resonance. www.totalhealthinstitute.com/about. Accessed July 14, 2015.
Does stenting of severe renal artery stenosis improve outomes compared with medical therapy alone?
No. In patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting offers no additional benefit when added to comprehensive medical therapy.
In these patients, renal artery stenting in addition to antihypertensive drug therapy can improve blood pressure control modestly but has no significant effect on outcomes such as adverse cardiovascular events and death. And because renal artery stenting carries a risk of complications, medical management should continue to be the first-line therapy.
RENAL ARTERY STENOSIS
Renal artery stenosis is a common form of peripheral artery disease. Atherosclerosis is the most common cause, but it can also be caused by fibromuscular dysplasia or vasculitis (eg, Takayasu arteritis). It is most often unilateral, but bilateral disease has also been reported.
The prevalence of atherosclerotic renal vascular disease in the US Medicare population is 0.5%, and 5.5% in those with chronic kidney disease.1 Furthermore, renal artery stenosis is found in 6.8% of adults over age 65.2 The prevalence increases with age and is higher in patients with hyperlipidemia, peripheral arterial disease, and hypertension. The prevalence of renal artery stenosis in patients with atherosclerotic disease and renal dysfunction is as high as 50%.3
Patients with peripheral artery disease may be five times more likely to develop renal artery stenosis than people without peripheral artery disease.4 Significant stenosis can result in resistant arterial hypertension, renal insufficiency, left ventricular hypertrophy, and congestive heart failure.5
Nephropathy due to renal artery stenosis is complex and is caused by hypoperfusion and chronic microatheroembolism. Renal artery stenosis leads to oxidative stress, inflammation, fibrosis in the stenotic kidney, and, over time, loss of kidney function. Hypoperfusion also leads to activation of the renin-angiotensin-aldosterone system, which plays a role in development of left ventricular hypertrophy.5,6
Adequate blood pressure control, goal-directed lipid-lowering therapy, smoking cessation, and other preventive measures are the foundation of management.
RENAL ARTERY STENOSIS AND HYPERTENSION
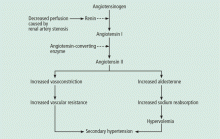
Renal artery stenosis is a cause of secondary hypertension. The stenosis decreases renal perfusion pressure, activating the release of renin and the production of angiotensin II, which in turn raises the blood pressure by two mechanisms (Figure 1): directly, by causing generalized vasoconstriction, and indirectly, by stimulating the release of aldosterone, which in turn increases the reabsorption of sodium and causes hypervolemia. These two mechanisms play a major role in renal vascular hypertension when renal artery stenosis is bilateral. In unilateral renal artery stenosis, pressure diuresis in the unaffected kidney compensates for the reabsorption of sodium in the affected kidney, keeping the blood pressure down. However, with time, the unaffected kidney will develop hypertensive nephropathy, and pressure diuresis will be lost.7,8 In addition, the activation of the renin-angiotensin-aldosterone system results in structural heart disease, such as left ventricular hypertrophy,5 and may shorten survival.
STENTING PLUS ANTIHYPERTENSIVE DRUG THERAPY
Because observational studies showed improvement in blood pressure control after endovascular stenting of atherosclerotic renal artery stenosis,9,10 this approach became a treatment option for uncontrolled hypertension in these patients. The 2005 joint guidelines of the American College of Cardiology and the American Heart Association11 considered percutaneous revascularization a reasonable option (level of evidence B) for patients who meet one of the following criteria:
- Hemodynamically significant stenosis and accelerated, resistant, or malignant hypertension, hypertension with an unexplained unilateral small kidney, or hypertension with intolerance to medication
- Renal artery stenosis and progressive chronic kidney disease with bilateral stenosis or stenosis in a solitary functioning kidney
- Hemodynamically significant stenosis and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema or unstable angina.11
However, no randomized study has shown a direct benefit of renal artery stenting on rates of cardiovascular events or renal function compared with drug therapy alone.
TRIALS OF STENTING VS MEDICAL THERAPY ALONE
Technical improvements have led to more widespread use of diagnostic and interventional endovascular tools for renal artery revascularization. Studies over the past 10 years examined the impact of stenting in patients with uncontrolled hypertension.
The STAR trial
In the Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial,9 patients with creatinine clearance less than 80 mL/min/1.73 m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy or medical therapy alone. The authors concluded that stenting had no effect on the progression of renal dysfunction but led to a small number of significant, procedure-related complications. The study was criticized for including patients with mild stenosis (< 50% stenosis) and for being underpowered for the primary end point.
The ASTRAL study
The Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) study10 was a similar comparison with similar results, showing no benefit from stenting with respect to renal function, systolic blood pressure control, cardiovascular events, or death.
HERCULES
The Herculink Elite Cobalt Chromium Renal Stent Trial to Demonstrate Efficacy and Safety (HERCULES)12 was a prospective multicenter study of the effects of renal artery stenting in 202 patients with significant renal artery stenosis and uncontrolled hypertension. It showed a reduction in systolic blood pressure from baseline (P < .0001). However, follow-up was only 9 months, which was insufficient to show a significant effect on long-term cardiovascular and cerebrovascular outcomes.
The CORAL trial
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial13 used more stringent definitions and longer follow-up. It randomized 947 patients to either stenting plus medical therapy or medical therapy alone. Patients had atherosclerotic renal artery stenosis, defined as stenosis of at least 80% or stenosis of 60% to 80% with a gradient of at least 20 mm Hg in the systolic pressure), and either systolic hypertension while taking two or more antihypertensive drugs or stage 3 or higher chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease formula).
Participants were followed for 43 months to detect the occurrence of adverse cardiovascular and renal events. There was no significant difference in primary outcome between stenting plus drug therapy and drug therapy alone (35.1% and 35.8%, respectively; P = .58). However, stenting plus drug therapy was associated with modestly lower systolic pressures compared with drug therapy alone (−2.3 mm Hg, 95% confidence interval −4.4 to −0.2 mm Hg, P = .03).13 This study provided strong evidence that renal artery stenting offers no significant benefit to patients with moderately severe atherosclerotic renal artery stenosis, and that stenting may actually pose an unnecessary risk.
COMPLICATIONS OF RENAL ARTERY STENTING
Complications of renal artery stenting are a limiting factor compared with drug therapy alone, especially since the procedure offers no significant benefit in outcome. Procedural complication rates of 10% to 15% have been reported.9,10,12 The CORAL trial reported arterial dissection in 2.2%, branch-vessel occlusion in 1.2%, and distal embolization in 1.2% of patients undergoing stenting.13 Other reported complications have included stent misplacement requiring an additional stent, access-vessel damage, stent embolization, renal artery thrombosis or occlusion, and death.10,12
- Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 68:293–301.
- Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Uzu T, Takeji M, Yamada N, et al. Prevalence and outcome of renal artery stenosis in atherosclerotic patients with renal dysfunction. Hypertens Res 2002; 25:537–542.
- Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014; 113:687–690.
- Wu S, Polavarapu N, Stouffer GA. Left ventricular hypertrophy in patients with renal artery stenosis. Am J Med Sci 2006; 332:334–338.
- Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52:196–203.
- Black HR, Glickman MG, Schiff M Jr, Pingoud EG. Renovascular hypertension: pathophysiology, diagnosis, and treatment. Yale J Biol Med 1978; 51:635–654.
- Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. Can J Cardiol 2006; 22:623–628.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of stent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by atherosclerotic ostial stenosis of the renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
No. In patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting offers no additional benefit when added to comprehensive medical therapy.
In these patients, renal artery stenting in addition to antihypertensive drug therapy can improve blood pressure control modestly but has no significant effect on outcomes such as adverse cardiovascular events and death. And because renal artery stenting carries a risk of complications, medical management should continue to be the first-line therapy.
RENAL ARTERY STENOSIS
Renal artery stenosis is a common form of peripheral artery disease. Atherosclerosis is the most common cause, but it can also be caused by fibromuscular dysplasia or vasculitis (eg, Takayasu arteritis). It is most often unilateral, but bilateral disease has also been reported.
The prevalence of atherosclerotic renal vascular disease in the US Medicare population is 0.5%, and 5.5% in those with chronic kidney disease.1 Furthermore, renal artery stenosis is found in 6.8% of adults over age 65.2 The prevalence increases with age and is higher in patients with hyperlipidemia, peripheral arterial disease, and hypertension. The prevalence of renal artery stenosis in patients with atherosclerotic disease and renal dysfunction is as high as 50%.3
Patients with peripheral artery disease may be five times more likely to develop renal artery stenosis than people without peripheral artery disease.4 Significant stenosis can result in resistant arterial hypertension, renal insufficiency, left ventricular hypertrophy, and congestive heart failure.5
Nephropathy due to renal artery stenosis is complex and is caused by hypoperfusion and chronic microatheroembolism. Renal artery stenosis leads to oxidative stress, inflammation, fibrosis in the stenotic kidney, and, over time, loss of kidney function. Hypoperfusion also leads to activation of the renin-angiotensin-aldosterone system, which plays a role in development of left ventricular hypertrophy.5,6
Adequate blood pressure control, goal-directed lipid-lowering therapy, smoking cessation, and other preventive measures are the foundation of management.
RENAL ARTERY STENOSIS AND HYPERTENSION
Renal artery stenosis is a cause of secondary hypertension. The stenosis decreases renal perfusion pressure, activating the release of renin and the production of angiotensin II, which in turn raises the blood pressure by two mechanisms (Figure 1): directly, by causing generalized vasoconstriction, and indirectly, by stimulating the release of aldosterone, which in turn increases the reabsorption of sodium and causes hypervolemia. These two mechanisms play a major role in renal vascular hypertension when renal artery stenosis is bilateral. In unilateral renal artery stenosis, pressure diuresis in the unaffected kidney compensates for the reabsorption of sodium in the affected kidney, keeping the blood pressure down. However, with time, the unaffected kidney will develop hypertensive nephropathy, and pressure diuresis will be lost.7,8 In addition, the activation of the renin-angiotensin-aldosterone system results in structural heart disease, such as left ventricular hypertrophy,5 and may shorten survival.
STENTING PLUS ANTIHYPERTENSIVE DRUG THERAPY
Because observational studies showed improvement in blood pressure control after endovascular stenting of atherosclerotic renal artery stenosis,9,10 this approach became a treatment option for uncontrolled hypertension in these patients. The 2005 joint guidelines of the American College of Cardiology and the American Heart Association11 considered percutaneous revascularization a reasonable option (level of evidence B) for patients who meet one of the following criteria:
- Hemodynamically significant stenosis and accelerated, resistant, or malignant hypertension, hypertension with an unexplained unilateral small kidney, or hypertension with intolerance to medication
- Renal artery stenosis and progressive chronic kidney disease with bilateral stenosis or stenosis in a solitary functioning kidney
- Hemodynamically significant stenosis and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema or unstable angina.11
However, no randomized study has shown a direct benefit of renal artery stenting on rates of cardiovascular events or renal function compared with drug therapy alone.
TRIALS OF STENTING VS MEDICAL THERAPY ALONE
Technical improvements have led to more widespread use of diagnostic and interventional endovascular tools for renal artery revascularization. Studies over the past 10 years examined the impact of stenting in patients with uncontrolled hypertension.
The STAR trial
In the Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial,9 patients with creatinine clearance less than 80 mL/min/1.73 m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy or medical therapy alone. The authors concluded that stenting had no effect on the progression of renal dysfunction but led to a small number of significant, procedure-related complications. The study was criticized for including patients with mild stenosis (< 50% stenosis) and for being underpowered for the primary end point.
The ASTRAL study
The Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) study10 was a similar comparison with similar results, showing no benefit from stenting with respect to renal function, systolic blood pressure control, cardiovascular events, or death.
HERCULES
The Herculink Elite Cobalt Chromium Renal Stent Trial to Demonstrate Efficacy and Safety (HERCULES)12 was a prospective multicenter study of the effects of renal artery stenting in 202 patients with significant renal artery stenosis and uncontrolled hypertension. It showed a reduction in systolic blood pressure from baseline (P < .0001). However, follow-up was only 9 months, which was insufficient to show a significant effect on long-term cardiovascular and cerebrovascular outcomes.
The CORAL trial
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial13 used more stringent definitions and longer follow-up. It randomized 947 patients to either stenting plus medical therapy or medical therapy alone. Patients had atherosclerotic renal artery stenosis, defined as stenosis of at least 80% or stenosis of 60% to 80% with a gradient of at least 20 mm Hg in the systolic pressure), and either systolic hypertension while taking two or more antihypertensive drugs or stage 3 or higher chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease formula).
Participants were followed for 43 months to detect the occurrence of adverse cardiovascular and renal events. There was no significant difference in primary outcome between stenting plus drug therapy and drug therapy alone (35.1% and 35.8%, respectively; P = .58). However, stenting plus drug therapy was associated with modestly lower systolic pressures compared with drug therapy alone (−2.3 mm Hg, 95% confidence interval −4.4 to −0.2 mm Hg, P = .03).13 This study provided strong evidence that renal artery stenting offers no significant benefit to patients with moderately severe atherosclerotic renal artery stenosis, and that stenting may actually pose an unnecessary risk.
COMPLICATIONS OF RENAL ARTERY STENTING
Complications of renal artery stenting are a limiting factor compared with drug therapy alone, especially since the procedure offers no significant benefit in outcome. Procedural complication rates of 10% to 15% have been reported.9,10,12 The CORAL trial reported arterial dissection in 2.2%, branch-vessel occlusion in 1.2%, and distal embolization in 1.2% of patients undergoing stenting.13 Other reported complications have included stent misplacement requiring an additional stent, access-vessel damage, stent embolization, renal artery thrombosis or occlusion, and death.10,12
No. In patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting offers no additional benefit when added to comprehensive medical therapy.
In these patients, renal artery stenting in addition to antihypertensive drug therapy can improve blood pressure control modestly but has no significant effect on outcomes such as adverse cardiovascular events and death. And because renal artery stenting carries a risk of complications, medical management should continue to be the first-line therapy.
RENAL ARTERY STENOSIS
Renal artery stenosis is a common form of peripheral artery disease. Atherosclerosis is the most common cause, but it can also be caused by fibromuscular dysplasia or vasculitis (eg, Takayasu arteritis). It is most often unilateral, but bilateral disease has also been reported.
The prevalence of atherosclerotic renal vascular disease in the US Medicare population is 0.5%, and 5.5% in those with chronic kidney disease.1 Furthermore, renal artery stenosis is found in 6.8% of adults over age 65.2 The prevalence increases with age and is higher in patients with hyperlipidemia, peripheral arterial disease, and hypertension. The prevalence of renal artery stenosis in patients with atherosclerotic disease and renal dysfunction is as high as 50%.3
Patients with peripheral artery disease may be five times more likely to develop renal artery stenosis than people without peripheral artery disease.4 Significant stenosis can result in resistant arterial hypertension, renal insufficiency, left ventricular hypertrophy, and congestive heart failure.5
Nephropathy due to renal artery stenosis is complex and is caused by hypoperfusion and chronic microatheroembolism. Renal artery stenosis leads to oxidative stress, inflammation, fibrosis in the stenotic kidney, and, over time, loss of kidney function. Hypoperfusion also leads to activation of the renin-angiotensin-aldosterone system, which plays a role in development of left ventricular hypertrophy.5,6
Adequate blood pressure control, goal-directed lipid-lowering therapy, smoking cessation, and other preventive measures are the foundation of management.
RENAL ARTERY STENOSIS AND HYPERTENSION
Renal artery stenosis is a cause of secondary hypertension. The stenosis decreases renal perfusion pressure, activating the release of renin and the production of angiotensin II, which in turn raises the blood pressure by two mechanisms (Figure 1): directly, by causing generalized vasoconstriction, and indirectly, by stimulating the release of aldosterone, which in turn increases the reabsorption of sodium and causes hypervolemia. These two mechanisms play a major role in renal vascular hypertension when renal artery stenosis is bilateral. In unilateral renal artery stenosis, pressure diuresis in the unaffected kidney compensates for the reabsorption of sodium in the affected kidney, keeping the blood pressure down. However, with time, the unaffected kidney will develop hypertensive nephropathy, and pressure diuresis will be lost.7,8 In addition, the activation of the renin-angiotensin-aldosterone system results in structural heart disease, such as left ventricular hypertrophy,5 and may shorten survival.
STENTING PLUS ANTIHYPERTENSIVE DRUG THERAPY
Because observational studies showed improvement in blood pressure control after endovascular stenting of atherosclerotic renal artery stenosis,9,10 this approach became a treatment option for uncontrolled hypertension in these patients. The 2005 joint guidelines of the American College of Cardiology and the American Heart Association11 considered percutaneous revascularization a reasonable option (level of evidence B) for patients who meet one of the following criteria:
- Hemodynamically significant stenosis and accelerated, resistant, or malignant hypertension, hypertension with an unexplained unilateral small kidney, or hypertension with intolerance to medication
- Renal artery stenosis and progressive chronic kidney disease with bilateral stenosis or stenosis in a solitary functioning kidney
- Hemodynamically significant stenosis and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema or unstable angina.11
However, no randomized study has shown a direct benefit of renal artery stenting on rates of cardiovascular events or renal function compared with drug therapy alone.
TRIALS OF STENTING VS MEDICAL THERAPY ALONE
Technical improvements have led to more widespread use of diagnostic and interventional endovascular tools for renal artery revascularization. Studies over the past 10 years examined the impact of stenting in patients with uncontrolled hypertension.
The STAR trial
In the Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial,9 patients with creatinine clearance less than 80 mL/min/1.73 m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy or medical therapy alone. The authors concluded that stenting had no effect on the progression of renal dysfunction but led to a small number of significant, procedure-related complications. The study was criticized for including patients with mild stenosis (< 50% stenosis) and for being underpowered for the primary end point.
The ASTRAL study
The Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) study10 was a similar comparison with similar results, showing no benefit from stenting with respect to renal function, systolic blood pressure control, cardiovascular events, or death.
HERCULES
The Herculink Elite Cobalt Chromium Renal Stent Trial to Demonstrate Efficacy and Safety (HERCULES)12 was a prospective multicenter study of the effects of renal artery stenting in 202 patients with significant renal artery stenosis and uncontrolled hypertension. It showed a reduction in systolic blood pressure from baseline (P < .0001). However, follow-up was only 9 months, which was insufficient to show a significant effect on long-term cardiovascular and cerebrovascular outcomes.
The CORAL trial
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial13 used more stringent definitions and longer follow-up. It randomized 947 patients to either stenting plus medical therapy or medical therapy alone. Patients had atherosclerotic renal artery stenosis, defined as stenosis of at least 80% or stenosis of 60% to 80% with a gradient of at least 20 mm Hg in the systolic pressure), and either systolic hypertension while taking two or more antihypertensive drugs or stage 3 or higher chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease formula).
Participants were followed for 43 months to detect the occurrence of adverse cardiovascular and renal events. There was no significant difference in primary outcome between stenting plus drug therapy and drug therapy alone (35.1% and 35.8%, respectively; P = .58). However, stenting plus drug therapy was associated with modestly lower systolic pressures compared with drug therapy alone (−2.3 mm Hg, 95% confidence interval −4.4 to −0.2 mm Hg, P = .03).13 This study provided strong evidence that renal artery stenting offers no significant benefit to patients with moderately severe atherosclerotic renal artery stenosis, and that stenting may actually pose an unnecessary risk.
COMPLICATIONS OF RENAL ARTERY STENTING
Complications of renal artery stenting are a limiting factor compared with drug therapy alone, especially since the procedure offers no significant benefit in outcome. Procedural complication rates of 10% to 15% have been reported.9,10,12 The CORAL trial reported arterial dissection in 2.2%, branch-vessel occlusion in 1.2%, and distal embolization in 1.2% of patients undergoing stenting.13 Other reported complications have included stent misplacement requiring an additional stent, access-vessel damage, stent embolization, renal artery thrombosis or occlusion, and death.10,12
- Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 68:293–301.
- Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Uzu T, Takeji M, Yamada N, et al. Prevalence and outcome of renal artery stenosis in atherosclerotic patients with renal dysfunction. Hypertens Res 2002; 25:537–542.
- Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014; 113:687–690.
- Wu S, Polavarapu N, Stouffer GA. Left ventricular hypertrophy in patients with renal artery stenosis. Am J Med Sci 2006; 332:334–338.
- Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52:196–203.
- Black HR, Glickman MG, Schiff M Jr, Pingoud EG. Renovascular hypertension: pathophysiology, diagnosis, and treatment. Yale J Biol Med 1978; 51:635–654.
- Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. Can J Cardiol 2006; 22:623–628.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of stent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by atherosclerotic ostial stenosis of the renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
- Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 68:293–301.
- Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Uzu T, Takeji M, Yamada N, et al. Prevalence and outcome of renal artery stenosis in atherosclerotic patients with renal dysfunction. Hypertens Res 2002; 25:537–542.
- Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014; 113:687–690.
- Wu S, Polavarapu N, Stouffer GA. Left ventricular hypertrophy in patients with renal artery stenosis. Am J Med Sci 2006; 332:334–338.
- Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52:196–203.
- Black HR, Glickman MG, Schiff M Jr, Pingoud EG. Renovascular hypertension: pathophysiology, diagnosis, and treatment. Yale J Biol Med 1978; 51:635–654.
- Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. Can J Cardiol 2006; 22:623–628.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of stent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by atherosclerotic ostial stenosis of the renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
Stenting may benefit select patients with severe renal artery stenosis
In their article in this issue of the Cleveland Clinic Journal of Medicine, Kabach et al answer no to the question of whether stenting of severe renal artery stenosis improves outcomes compared with medical therapy alone.1 They review the findings of four key studies2–5 published between 2003 and 2014 and conclude that, in patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting with medical therapy can improve blood pressure control but has no significant impact on cardiovascular or mortality outcomes.1
Furthermore, the authors state that in view of the risk of complications associated with stenting, medical management should continue to be the first-line therapy.1 Indeed, the ASTRAL study (Angioplasty and Stenting for Renal Artery Lesions) investigators found substantial risks without evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.3
Nevertheless, I believe that this procedure may benefit certain patients.
MAYO CLINIC COHORT STUDY
In 2008, our group at Mayo Clinic Health system in Eau Claire, Wisconsin, published the results of a prospective cohort study in 26 patients with renal artery stenosis and chronic kidney disease who presented with rapidly worsening renal failure (defined as an increase in serum creatinine of ≥ 25%) while receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB).6,7
These drugs—inhibitors of the renin-angiotensin-aldosterone system—slow the progression of chronic kidney disease but can acutely worsen renal function, especially in patients with renal artery stenosis, and withdrawing them in this situation was the focus of our study.
The patients (10 men and 16 women) ranged in age from 63 to 87 (mean age 75.3).
At enrollment, the ACE inhibitors and ARBs were discontinued, standard nephrologic care was applied, and the glomerular filtration rate (estimated by the Modification of Diet in Renal Disease Study equation) was monitored. After at least 2 weeks, percutaneous renal angioplasty with stent placement was considered if the patient met any of the following criteria:
- Persistence of renal failure
- Flash pulmonary edema
- Uncontrolled hypertension despite the use of at least three antihypertensive medications.
Nine patients underwent percutaneous angioplasty and stenting and 17 did not. The procedure was done on one renal artery in 8 patients and both renal arteries in 1. Indications for the procedure were recent worsening of renal failure in 8 patients and recent worsening renal failure together with symptomatic flash pulmonary edema in 1 patient. (Flash pulmonary edema is the only class I recommendation for percutaneous renal angioplasty in the 2006 joint guidelines of the American College of Cardiology and the American Heart Association.8) As noted above, all the patients were experiencing acute exacerbation of chronic kidney disease at the time.
We found clear evidence of additional improved and sustained renal function in the patients who underwent percutaneous renal angioplasty and stenting compared with the patients who did not (Figures 1 and 2).6,7
In an editorial9 following publication of the ASTRAL study, our group reported a subsequent 82-month analysis of our 26-patient cohort completed in June 2009. In the 7 surviving patients who had undergone percutaneous renal angioplasty and stenting, the estimated glomerular filtration rate had increased from 27.4 mL/min/1.73 m2 (± 12.7, range 11–47) at baseline to 50.3 (± 21.7, range 23–68) (P = .018) after 46.9 months.
PATIENT NUMBER 13
To illustrate how percutaneous renal angioplasty and stenting can reverse recently worsening renal failure in renal artery stenosis, I would like to discuss in greater detail a patient from our two previous reports.6,7
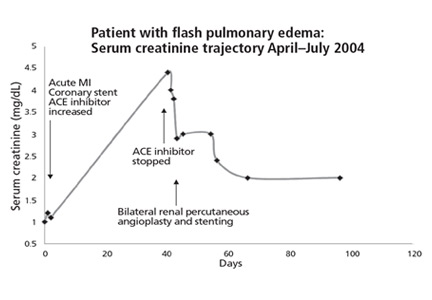
Patient 13, a 67-year-old woman with hypertension, was referred to our nephrology service in September 2004 to consider starting hemodialysis for symptomatic renal failure. Her serum creatinine had increased to 3.4 mg/dL from a previous baseline level of 2.0 mg/dL, and she also had worsening anemia and hyperkalemia. She had been taking lisinopril 10 mg/day for the last 12 months. Magnetic resonance angiography revealed high-grade (> 95%) bilateral renal artery stenosis with an atrophic left kidney.
Lisinopril was promptly discontinued, and within 2 weeks her serum creatinine level had decreased by more than 0.5 mg/dL. In mid-November 2004, she underwent right renal artery angioplasty with stent placement. After that, her serum creatinine decreased further, and 3 months later it had dropped to 1.6 mg/dL. The value continued to improve, with the lowest measurement of 1.1 mg/dL, equivalent to an estimated glomerular filtration rate of 51 mL/min/1.73 m2. This was in May 2006, 19 months after stopping lisinopril and 17 months after angioplasty and stenting. The last available serum creatinine level (August 2006) was 1.2 mg/dL, equivalent to an estimated glomerular filtration rate of 45 mL/min/1.73 m2 (Figure 1). Unfortunately, the patient died of metastatic lung cancer in December of that year.
Also of note, the patient who underwent angioplasty because of recurrent flash pulmonary edema had no recurrences of it afterward.
We concluded that, in patients with hemodynamically significant renal artery stenosis presenting with acutely worsening renal failure, renal angioplasty with stenting has the potential to reverse renal failure, improve blood pressure control, and stop flash pulmonary edema.6–8
Notably, all patients in our study who underwent renal angioplasty with stenting had new-onset acute renal injury as defined by an increase in the baseline serum creatinine of more than 25% during the 3 months before stent placement.6–8 Patients in the STAR,2 HERCULES,4 and CORAL5 studies had renal artery stenosis but otherwise stable chronic kidney disease at the time of enrollment. In the ASTRAL study,3 12% of the patients had acute kidney injury on study enrollment, defined as an increase in the serum creatinine level of more than 20% or of more than 1.13 mg/dL.3
While we strongly agree with aggressive yet monitored medical therapy for patients with hemodynamically significant renal artery stenosis, I posit that selected patients do indeed derive significant clinical benefits from renal angioplasty and stenting. Our group’s prospective individual-patient-level data support the paradigm that angioplasty with stenting is useful in patients with renal artery stenosis who experience “acute-on-chronic” kidney disease.
The pathophysiology of renal artery stenosis and the progression of chronic kidney disease are complex, and many factors affect patient outcomes and response to treatment. Thus, the message is that treatment of severe renal artery stenosis must be individualized.9–11 No one treatment fits all.10,11
- Kabach A, Agha OQ, Baibars M, Alraiyes AH, Alraies MC. Does stenting of severe renal artery stenosis improve outcomes compared with medical therapy alone? Cleve Clin J Med 2015; 82:491–494.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Jaff MR, Bates M, Sullivan T, et al; HERCULES Investigators. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Catheter Cardiovasc Interv 2012; 80:343–350.
- Cooper CJ, Murphy TP, Cutlip DE, et al; CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370:13–22.
- Onuigbo MAC, Onuigbo NTC. Worsening renal failure in older chronic kidney disease patients with renal artery stenosis concurrently on renin angiotensin aldosterone system blockade: a prospective 50-month Mayo Health System clinic analysis. QJM 2008; 101:519–527.
- Onuigbo MA, Onuigbo NT. Renal failure and concurrent RAAS blockade in older CKD patients with renal artery stenosis: an extended Mayo Clinic prospective 63-month experience. Ren Fail 2008; 30:363–371.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
- Onuigbo M, Frenandes R, Nijhawan V. The ASTRAL trial results revisited—to stent or not to stent in renal artery stenosis? QJM 2010; 103:357.
- Singh M, Kovacs DF, Singh A, Dhaliwal P, Khosla S. ACE inhibition and renal artery stenosis: what lessons have we learnt? A 21st century perspective. In: Onuigbo MAC, ed. ACE inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 2. New York, NY: NOVA Publishers; 2013:203–218.
- Onuigbo MA, Onuigbo C, Onuigbo V, et al. The CKD enigma, reengineering CKD care, narrowing asymmetric information and confronting ethicomedicinomics in CKD care: the introduction of the new 'CKD express©' IT software program. In: Onuigbo MAC, ed. ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 1. New York, NY: NOVA Publishers; 2013: 41–56.
In their article in this issue of the Cleveland Clinic Journal of Medicine, Kabach et al answer no to the question of whether stenting of severe renal artery stenosis improves outcomes compared with medical therapy alone.1 They review the findings of four key studies2–5 published between 2003 and 2014 and conclude that, in patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting with medical therapy can improve blood pressure control but has no significant impact on cardiovascular or mortality outcomes.1
Furthermore, the authors state that in view of the risk of complications associated with stenting, medical management should continue to be the first-line therapy.1 Indeed, the ASTRAL study (Angioplasty and Stenting for Renal Artery Lesions) investigators found substantial risks without evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.3
Nevertheless, I believe that this procedure may benefit certain patients.
MAYO CLINIC COHORT STUDY
In 2008, our group at Mayo Clinic Health system in Eau Claire, Wisconsin, published the results of a prospective cohort study in 26 patients with renal artery stenosis and chronic kidney disease who presented with rapidly worsening renal failure (defined as an increase in serum creatinine of ≥ 25%) while receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB).6,7
These drugs—inhibitors of the renin-angiotensin-aldosterone system—slow the progression of chronic kidney disease but can acutely worsen renal function, especially in patients with renal artery stenosis, and withdrawing them in this situation was the focus of our study.
The patients (10 men and 16 women) ranged in age from 63 to 87 (mean age 75.3).
At enrollment, the ACE inhibitors and ARBs were discontinued, standard nephrologic care was applied, and the glomerular filtration rate (estimated by the Modification of Diet in Renal Disease Study equation) was monitored. After at least 2 weeks, percutaneous renal angioplasty with stent placement was considered if the patient met any of the following criteria:
- Persistence of renal failure
- Flash pulmonary edema
- Uncontrolled hypertension despite the use of at least three antihypertensive medications.
Nine patients underwent percutaneous angioplasty and stenting and 17 did not. The procedure was done on one renal artery in 8 patients and both renal arteries in 1. Indications for the procedure were recent worsening of renal failure in 8 patients and recent worsening renal failure together with symptomatic flash pulmonary edema in 1 patient. (Flash pulmonary edema is the only class I recommendation for percutaneous renal angioplasty in the 2006 joint guidelines of the American College of Cardiology and the American Heart Association.8) As noted above, all the patients were experiencing acute exacerbation of chronic kidney disease at the time.
We found clear evidence of additional improved and sustained renal function in the patients who underwent percutaneous renal angioplasty and stenting compared with the patients who did not (Figures 1 and 2).6,7
In an editorial9 following publication of the ASTRAL study, our group reported a subsequent 82-month analysis of our 26-patient cohort completed in June 2009. In the 7 surviving patients who had undergone percutaneous renal angioplasty and stenting, the estimated glomerular filtration rate had increased from 27.4 mL/min/1.73 m2 (± 12.7, range 11–47) at baseline to 50.3 (± 21.7, range 23–68) (P = .018) after 46.9 months.
PATIENT NUMBER 13
To illustrate how percutaneous renal angioplasty and stenting can reverse recently worsening renal failure in renal artery stenosis, I would like to discuss in greater detail a patient from our two previous reports.6,7
Patient 13, a 67-year-old woman with hypertension, was referred to our nephrology service in September 2004 to consider starting hemodialysis for symptomatic renal failure. Her serum creatinine had increased to 3.4 mg/dL from a previous baseline level of 2.0 mg/dL, and she also had worsening anemia and hyperkalemia. She had been taking lisinopril 10 mg/day for the last 12 months. Magnetic resonance angiography revealed high-grade (> 95%) bilateral renal artery stenosis with an atrophic left kidney.
Lisinopril was promptly discontinued, and within 2 weeks her serum creatinine level had decreased by more than 0.5 mg/dL. In mid-November 2004, she underwent right renal artery angioplasty with stent placement. After that, her serum creatinine decreased further, and 3 months later it had dropped to 1.6 mg/dL. The value continued to improve, with the lowest measurement of 1.1 mg/dL, equivalent to an estimated glomerular filtration rate of 51 mL/min/1.73 m2. This was in May 2006, 19 months after stopping lisinopril and 17 months after angioplasty and stenting. The last available serum creatinine level (August 2006) was 1.2 mg/dL, equivalent to an estimated glomerular filtration rate of 45 mL/min/1.73 m2 (Figure 1). Unfortunately, the patient died of metastatic lung cancer in December of that year.
Also of note, the patient who underwent angioplasty because of recurrent flash pulmonary edema had no recurrences of it afterward.
We concluded that, in patients with hemodynamically significant renal artery stenosis presenting with acutely worsening renal failure, renal angioplasty with stenting has the potential to reverse renal failure, improve blood pressure control, and stop flash pulmonary edema.6–8
Notably, all patients in our study who underwent renal angioplasty with stenting had new-onset acute renal injury as defined by an increase in the baseline serum creatinine of more than 25% during the 3 months before stent placement.6–8 Patients in the STAR,2 HERCULES,4 and CORAL5 studies had renal artery stenosis but otherwise stable chronic kidney disease at the time of enrollment. In the ASTRAL study,3 12% of the patients had acute kidney injury on study enrollment, defined as an increase in the serum creatinine level of more than 20% or of more than 1.13 mg/dL.3
While we strongly agree with aggressive yet monitored medical therapy for patients with hemodynamically significant renal artery stenosis, I posit that selected patients do indeed derive significant clinical benefits from renal angioplasty and stenting. Our group’s prospective individual-patient-level data support the paradigm that angioplasty with stenting is useful in patients with renal artery stenosis who experience “acute-on-chronic” kidney disease.
The pathophysiology of renal artery stenosis and the progression of chronic kidney disease are complex, and many factors affect patient outcomes and response to treatment. Thus, the message is that treatment of severe renal artery stenosis must be individualized.9–11 No one treatment fits all.10,11
In their article in this issue of the Cleveland Clinic Journal of Medicine, Kabach et al answer no to the question of whether stenting of severe renal artery stenosis improves outcomes compared with medical therapy alone.1 They review the findings of four key studies2–5 published between 2003 and 2014 and conclude that, in patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting with medical therapy can improve blood pressure control but has no significant impact on cardiovascular or mortality outcomes.1
Furthermore, the authors state that in view of the risk of complications associated with stenting, medical management should continue to be the first-line therapy.1 Indeed, the ASTRAL study (Angioplasty and Stenting for Renal Artery Lesions) investigators found substantial risks without evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.3
Nevertheless, I believe that this procedure may benefit certain patients.
MAYO CLINIC COHORT STUDY
In 2008, our group at Mayo Clinic Health system in Eau Claire, Wisconsin, published the results of a prospective cohort study in 26 patients with renal artery stenosis and chronic kidney disease who presented with rapidly worsening renal failure (defined as an increase in serum creatinine of ≥ 25%) while receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB).6,7
These drugs—inhibitors of the renin-angiotensin-aldosterone system—slow the progression of chronic kidney disease but can acutely worsen renal function, especially in patients with renal artery stenosis, and withdrawing them in this situation was the focus of our study.
The patients (10 men and 16 women) ranged in age from 63 to 87 (mean age 75.3).
At enrollment, the ACE inhibitors and ARBs were discontinued, standard nephrologic care was applied, and the glomerular filtration rate (estimated by the Modification of Diet in Renal Disease Study equation) was monitored. After at least 2 weeks, percutaneous renal angioplasty with stent placement was considered if the patient met any of the following criteria:
- Persistence of renal failure
- Flash pulmonary edema
- Uncontrolled hypertension despite the use of at least three antihypertensive medications.
Nine patients underwent percutaneous angioplasty and stenting and 17 did not. The procedure was done on one renal artery in 8 patients and both renal arteries in 1. Indications for the procedure were recent worsening of renal failure in 8 patients and recent worsening renal failure together with symptomatic flash pulmonary edema in 1 patient. (Flash pulmonary edema is the only class I recommendation for percutaneous renal angioplasty in the 2006 joint guidelines of the American College of Cardiology and the American Heart Association.8) As noted above, all the patients were experiencing acute exacerbation of chronic kidney disease at the time.
We found clear evidence of additional improved and sustained renal function in the patients who underwent percutaneous renal angioplasty and stenting compared with the patients who did not (Figures 1 and 2).6,7
In an editorial9 following publication of the ASTRAL study, our group reported a subsequent 82-month analysis of our 26-patient cohort completed in June 2009. In the 7 surviving patients who had undergone percutaneous renal angioplasty and stenting, the estimated glomerular filtration rate had increased from 27.4 mL/min/1.73 m2 (± 12.7, range 11–47) at baseline to 50.3 (± 21.7, range 23–68) (P = .018) after 46.9 months.
PATIENT NUMBER 13
To illustrate how percutaneous renal angioplasty and stenting can reverse recently worsening renal failure in renal artery stenosis, I would like to discuss in greater detail a patient from our two previous reports.6,7
Patient 13, a 67-year-old woman with hypertension, was referred to our nephrology service in September 2004 to consider starting hemodialysis for symptomatic renal failure. Her serum creatinine had increased to 3.4 mg/dL from a previous baseline level of 2.0 mg/dL, and she also had worsening anemia and hyperkalemia. She had been taking lisinopril 10 mg/day for the last 12 months. Magnetic resonance angiography revealed high-grade (> 95%) bilateral renal artery stenosis with an atrophic left kidney.
Lisinopril was promptly discontinued, and within 2 weeks her serum creatinine level had decreased by more than 0.5 mg/dL. In mid-November 2004, she underwent right renal artery angioplasty with stent placement. After that, her serum creatinine decreased further, and 3 months later it had dropped to 1.6 mg/dL. The value continued to improve, with the lowest measurement of 1.1 mg/dL, equivalent to an estimated glomerular filtration rate of 51 mL/min/1.73 m2. This was in May 2006, 19 months after stopping lisinopril and 17 months after angioplasty and stenting. The last available serum creatinine level (August 2006) was 1.2 mg/dL, equivalent to an estimated glomerular filtration rate of 45 mL/min/1.73 m2 (Figure 1). Unfortunately, the patient died of metastatic lung cancer in December of that year.
Also of note, the patient who underwent angioplasty because of recurrent flash pulmonary edema had no recurrences of it afterward.
We concluded that, in patients with hemodynamically significant renal artery stenosis presenting with acutely worsening renal failure, renal angioplasty with stenting has the potential to reverse renal failure, improve blood pressure control, and stop flash pulmonary edema.6–8
Notably, all patients in our study who underwent renal angioplasty with stenting had new-onset acute renal injury as defined by an increase in the baseline serum creatinine of more than 25% during the 3 months before stent placement.6–8 Patients in the STAR,2 HERCULES,4 and CORAL5 studies had renal artery stenosis but otherwise stable chronic kidney disease at the time of enrollment. In the ASTRAL study,3 12% of the patients had acute kidney injury on study enrollment, defined as an increase in the serum creatinine level of more than 20% or of more than 1.13 mg/dL.3
While we strongly agree with aggressive yet monitored medical therapy for patients with hemodynamically significant renal artery stenosis, I posit that selected patients do indeed derive significant clinical benefits from renal angioplasty and stenting. Our group’s prospective individual-patient-level data support the paradigm that angioplasty with stenting is useful in patients with renal artery stenosis who experience “acute-on-chronic” kidney disease.
The pathophysiology of renal artery stenosis and the progression of chronic kidney disease are complex, and many factors affect patient outcomes and response to treatment. Thus, the message is that treatment of severe renal artery stenosis must be individualized.9–11 No one treatment fits all.10,11
- Kabach A, Agha OQ, Baibars M, Alraiyes AH, Alraies MC. Does stenting of severe renal artery stenosis improve outcomes compared with medical therapy alone? Cleve Clin J Med 2015; 82:491–494.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Jaff MR, Bates M, Sullivan T, et al; HERCULES Investigators. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Catheter Cardiovasc Interv 2012; 80:343–350.
- Cooper CJ, Murphy TP, Cutlip DE, et al; CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370:13–22.
- Onuigbo MAC, Onuigbo NTC. Worsening renal failure in older chronic kidney disease patients with renal artery stenosis concurrently on renin angiotensin aldosterone system blockade: a prospective 50-month Mayo Health System clinic analysis. QJM 2008; 101:519–527.
- Onuigbo MA, Onuigbo NT. Renal failure and concurrent RAAS blockade in older CKD patients with renal artery stenosis: an extended Mayo Clinic prospective 63-month experience. Ren Fail 2008; 30:363–371.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
- Onuigbo M, Frenandes R, Nijhawan V. The ASTRAL trial results revisited—to stent or not to stent in renal artery stenosis? QJM 2010; 103:357.
- Singh M, Kovacs DF, Singh A, Dhaliwal P, Khosla S. ACE inhibition and renal artery stenosis: what lessons have we learnt? A 21st century perspective. In: Onuigbo MAC, ed. ACE inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 2. New York, NY: NOVA Publishers; 2013:203–218.
- Onuigbo MA, Onuigbo C, Onuigbo V, et al. The CKD enigma, reengineering CKD care, narrowing asymmetric information and confronting ethicomedicinomics in CKD care: the introduction of the new 'CKD express©' IT software program. In: Onuigbo MAC, ed. ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 1. New York, NY: NOVA Publishers; 2013: 41–56.
- Kabach A, Agha OQ, Baibars M, Alraiyes AH, Alraies MC. Does stenting of severe renal artery stenosis improve outcomes compared with medical therapy alone? Cleve Clin J Med 2015; 82:491–494.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Jaff MR, Bates M, Sullivan T, et al; HERCULES Investigators. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Catheter Cardiovasc Interv 2012; 80:343–350.
- Cooper CJ, Murphy TP, Cutlip DE, et al; CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370:13–22.
- Onuigbo MAC, Onuigbo NTC. Worsening renal failure in older chronic kidney disease patients with renal artery stenosis concurrently on renin angiotensin aldosterone system blockade: a prospective 50-month Mayo Health System clinic analysis. QJM 2008; 101:519–527.
- Onuigbo MA, Onuigbo NT. Renal failure and concurrent RAAS blockade in older CKD patients with renal artery stenosis: an extended Mayo Clinic prospective 63-month experience. Ren Fail 2008; 30:363–371.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
- Onuigbo M, Frenandes R, Nijhawan V. The ASTRAL trial results revisited—to stent or not to stent in renal artery stenosis? QJM 2010; 103:357.
- Singh M, Kovacs DF, Singh A, Dhaliwal P, Khosla S. ACE inhibition and renal artery stenosis: what lessons have we learnt? A 21st century perspective. In: Onuigbo MAC, ed. ACE inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 2. New York, NY: NOVA Publishers; 2013:203–218.
- Onuigbo MA, Onuigbo C, Onuigbo V, et al. The CKD enigma, reengineering CKD care, narrowing asymmetric information and confronting ethicomedicinomics in CKD care: the introduction of the new 'CKD express©' IT software program. In: Onuigbo MAC, ed. ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 1. New York, NY: NOVA Publishers; 2013: 41–56.
Remembering that old dogs can still do tricks
More and more we are realizing that we need trials that use hard clinical end points to inform our clinical practice. Several things we used to do based on observational studies have fallen from grace after being evaluated in interventional trials. And faced with the US Food and Drug Administration’s mandate to demonstrate clinical impact, pharmaceutical companies can rarely count on using even well-accepted biomarkers instead of clinical outcomes when trying to bring new drugs to market.
This atmosphere often makes us a bit uncomfortable when prescribing older drugs that have passed the test of time and collective anecdotal experience but not rigorous clinical testing. In some cases this is good, and robust evaluation provides greater confidence in our choice of therapy: witness the demise of digoxin for heart failure.
Many older drugs have never been compared with newer drugs in well-designed trials using hard clinical outcomes and likely never will, owing to cost, marketing, and logistic reasons. But sometimes these trials are done, and the results are surprising. For instance, methotrexate in appropriate doses may actually be comparable to newer and far more expensive tumor necrosis factor inhibitors when used to treat rheumatoid arthritis.
Should we be willing to sometimes accept data on surrogate markers (eg, low-density lipoprotein cholesterol levels, blood pressure, hemoglobin A1c ) or even extensive clinical experience in the absence of hard outcome data when using older, tried-and-true drugs? Markers can mislead: consider the higher number of deaths recorded in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial in the group receiving more aggressive control of their glucose levels.
So we should not be totally sanguine when using older drugs instead of newer ones. But some drugs may have slipped out of our mental formularies yet still have real value in niche or even common settings. Methyldopa remains an effective antihypertensive drug and may be especially useful in peripartum patients. Yet relatively few young physicians know the drug.
And so it may be with chlorthalidone. In this issue of the Journal, Cooney et al remind us not only that this drug is still around, but that it has proven efficacy and, compared with its more popular cousin hydrochlorothiazide, favorable pharmacokinetic properties such as longer action. Not to mention that it was a comparator drug in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack (ALLHAT) trial.
In our current cost-saving environment, we should remember that some old dogs can still do good tricks.
More and more we are realizing that we need trials that use hard clinical end points to inform our clinical practice. Several things we used to do based on observational studies have fallen from grace after being evaluated in interventional trials. And faced with the US Food and Drug Administration’s mandate to demonstrate clinical impact, pharmaceutical companies can rarely count on using even well-accepted biomarkers instead of clinical outcomes when trying to bring new drugs to market.
This atmosphere often makes us a bit uncomfortable when prescribing older drugs that have passed the test of time and collective anecdotal experience but not rigorous clinical testing. In some cases this is good, and robust evaluation provides greater confidence in our choice of therapy: witness the demise of digoxin for heart failure.
Many older drugs have never been compared with newer drugs in well-designed trials using hard clinical outcomes and likely never will, owing to cost, marketing, and logistic reasons. But sometimes these trials are done, and the results are surprising. For instance, methotrexate in appropriate doses may actually be comparable to newer and far more expensive tumor necrosis factor inhibitors when used to treat rheumatoid arthritis.
Should we be willing to sometimes accept data on surrogate markers (eg, low-density lipoprotein cholesterol levels, blood pressure, hemoglobin A1c ) or even extensive clinical experience in the absence of hard outcome data when using older, tried-and-true drugs? Markers can mislead: consider the higher number of deaths recorded in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial in the group receiving more aggressive control of their glucose levels.
So we should not be totally sanguine when using older drugs instead of newer ones. But some drugs may have slipped out of our mental formularies yet still have real value in niche or even common settings. Methyldopa remains an effective antihypertensive drug and may be especially useful in peripartum patients. Yet relatively few young physicians know the drug.
And so it may be with chlorthalidone. In this issue of the Journal, Cooney et al remind us not only that this drug is still around, but that it has proven efficacy and, compared with its more popular cousin hydrochlorothiazide, favorable pharmacokinetic properties such as longer action. Not to mention that it was a comparator drug in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack (ALLHAT) trial.
In our current cost-saving environment, we should remember that some old dogs can still do good tricks.
More and more we are realizing that we need trials that use hard clinical end points to inform our clinical practice. Several things we used to do based on observational studies have fallen from grace after being evaluated in interventional trials. And faced with the US Food and Drug Administration’s mandate to demonstrate clinical impact, pharmaceutical companies can rarely count on using even well-accepted biomarkers instead of clinical outcomes when trying to bring new drugs to market.
This atmosphere often makes us a bit uncomfortable when prescribing older drugs that have passed the test of time and collective anecdotal experience but not rigorous clinical testing. In some cases this is good, and robust evaluation provides greater confidence in our choice of therapy: witness the demise of digoxin for heart failure.
Many older drugs have never been compared with newer drugs in well-designed trials using hard clinical outcomes and likely never will, owing to cost, marketing, and logistic reasons. But sometimes these trials are done, and the results are surprising. For instance, methotrexate in appropriate doses may actually be comparable to newer and far more expensive tumor necrosis factor inhibitors when used to treat rheumatoid arthritis.
Should we be willing to sometimes accept data on surrogate markers (eg, low-density lipoprotein cholesterol levels, blood pressure, hemoglobin A1c ) or even extensive clinical experience in the absence of hard outcome data when using older, tried-and-true drugs? Markers can mislead: consider the higher number of deaths recorded in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial in the group receiving more aggressive control of their glucose levels.
So we should not be totally sanguine when using older drugs instead of newer ones. But some drugs may have slipped out of our mental formularies yet still have real value in niche or even common settings. Methyldopa remains an effective antihypertensive drug and may be especially useful in peripartum patients. Yet relatively few young physicians know the drug.
And so it may be with chlorthalidone. In this issue of the Journal, Cooney et al remind us not only that this drug is still around, but that it has proven efficacy and, compared with its more popular cousin hydrochlorothiazide, favorable pharmacokinetic properties such as longer action. Not to mention that it was a comparator drug in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack (ALLHAT) trial.
In our current cost-saving environment, we should remember that some old dogs can still do good tricks.
Diuretics for hypertension: Hydrochlorothiazide or chlorthalidone?
The thiazide diuretic hydrochlorothiazide and the thiazidelike diuretic chlorthalidone are two old drugs that are still useful. Although similar, they differ in important ways still not fully appreciated more than a half century after they were introduced.
Most hypertension guidelines recommend thiazide diuretics as one of the classes of agents that can be used either as initial antihypertensive drug therapy or as part of combination therapy.1–3
In the United States, hydrochlorothiazide is used more often than chlorthalidone, but many clinical trials of antihypertensive therapy have used chlorthalidone.4,5 In recent years, particularly after the publication of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), interest in chlorthalidone has been increasing, and new data are now available comparing these two diuretics.6 While current US guidelines do not recommend one over the other, British guidelines prefer chlorthalidone.7
This review summarizes the data comparing the two drugs’ pharmacology, antihypertensive effect, and impact on clinical outcomes to help guide clinicians in choosing antihypertensive drug therapy.
PHARMACOLOGY AND MECHANISM OF ACTION
Many of the differences in effectiveness and adverse effects of hydrochlorothiazide and chlorthalidone are thought to be due to their different pharmacodynamic and pharmacokinetic effects.
Pharmacodynamic effects
Hydrochlorothiazide and chlorthalidone differ significantly in chemical structure (Figure 1), but both contain a sulfonamide group that inhibits carbonic anhydrase activity, which may be associated with lower vascular contractility. Both drugs are concentrated in the kidney and secreted into the tubular lumen8; therefore, their therapeutic diuretic effects are often achieved with relatively low plasma concentrations.
Both drugs inhibit the sodium-chloride cotransporter in the luminal membrane of the distal convoluted tubule of the ascending loop of Henle, leading to a modest natriuresis and diuresis. The exact mechanism by which they lower blood pressure is not known: while the initial response is from diuresis and volume changes, long-term reduction in blood pressure is through uncertain mechanisms. In addition, chlorthalidone may have beneficial effects on endothelial function and oxidative stress.9,10
Both drugs also increase secretion of potassium and hydrogen ions and promote increased reabsorption of calcium through increased expression of a sodium-calcium exchange channel.8 Chlorthalidone may cause more inhibition of carbonic anhydrase than hydrochlorothiazide, which can lead to lower intracellular pH and cell volume. This effect may in part explain a pleiotropic effect of chlorthalidone, ie, inhibition of platelet function, which in turn may contribute to this drug’s beneficial effect on cardiovascular outcomes.9
Pharmacokinetic differences
Hydrochlorothiazide and chlorthalidone have important differences in their pharmacokinetic properties (Table 1).11
Hydrochlorothiazide has its onset of action in about 2 hours, and it reaches its peak in 4 to 6 hours. Though its duration of action is short—up to 12 hours—its pharmacodynamic response can be much longer than predicted by its kinetics, allowing once-daily dosing.8
Chlorthalidone has a longer duration of action than hydrochlorothiazide. This may be because it has a very high volume of distribution, since it is taken up into red blood cells and is bound to carbonic anhydrase.12 This may result in a “drug reservoir” that keeps drug levels higher for a longer time.13 Its long duration of action makes it a favorable choice for patients who have difficulty adhering to medication instructions. In addition, a missed dose is unlikely to have a “rebound” effect like that seen with some other antihypertensive agents. However, both chlorthalidone and hydrochlorothiazide are effective if taken once daily.
BLOOD PRESSURE-LOWERING
Both hydrochlorothiazide and chlorthalidone are effective antihypertensive agents. Table 2 summarizes findings from studies that evaluated their blood pressure-lowering effect at various doses.14–33 However, relatively few studies have directly compared these two agents’ effects on blood pressure.
Ernst et al,34 in a small study (but probably the best one to address this issue), compared chlorthalidone 12.5 mg/day (force-titrated to 25 mg/day) and hydrochlorothiazide 25 mg/day (force-titrated to 50 mg/day) in untreated hypertensive patients. After 8 weeks, ambulatory blood pressure monitoring indicated a greater reduction from baseline in systolic blood pressure with chlorthalidone 25 mg/day than with hydrochlorothiazide 50 mg/day (24-hour mean –12.4 vs –7.4 mm Hg, P = .05). Interestingly, the change in nighttime blood pressure was greater in the chlorthalidone group (–13.5 mm Hg) than in the hydrochlorothiazide group (–6.4 mm Hg; P = .009). These data suggest that at the doses studied, chlorthalidone is more effective than hydrochlorothiazide in lowering systolic blood pressure.
Bakris et al,35 using a different study design, compared the single-pill combination of azilsartan medoxomil and chlorthalidone vs coadministration of azilsartan medoxomil and hydrochlorothiazide in participants with stage 2 primary hypertension (≥ 160/100 mm Hg). Systolic blood pressure, as measured in the clinic, declined more with the chlorthalidone combination (–35.1 mm Hg) than with the hydrochlorothiazide combination (–29.5 mm Hg, mean difference –5.6 mm Hg, P < .001).
Meta-analyses also support the conclusion that chlorthalidone is more potent than hydrochlorothiazide in lowering blood pressure.35,36 Several studies have shown that chlorthalidone at the same dose is 1.5 to 2 times as potent as hydrochlorothiazide.33,36,37 Therefore, for clinical purposes, it is reasonable to consider chlorthalidone 12.5 mg daily as similar to 25 mg of hydrochlorothiazide daily.
ADVERSE EFFECTS
Electrolyte disturbances are a common adverse effect of thiazide diuretics.
Hypokalemia. All thiazide diuretics cause potassium wasting. The frequency of hypokalemia depends on the dose, frequency of administration, diet, and other pharmacologic agents used.
Two large clinical trials, the Systolic Hypertension in the Elderly Program and ALLHAT, found that chlorthalidone caused hypokalemia requiring therapy in about 6% to 8% of patients.38,39 Chlorthalidone therapy was associated with a lowering of serum potassium levels of 0.2 to 0.5 mmol/L.36 In ALLHAT, chlorthalidone was associated with a reduction in potassium levels of approximately 0.2 mmol/L after 4 years.38
All diuretics require monitoring of electrolytes, especially during the first 2 weeks of therapy. Once a steady state is reached, patients are not usually at risk of hypokalemia unless the dose is increased, extrarenal losses of potassium increase, or dietary potassium is reduced.
Other electrolyte changes. Thiazide and thiazide-like diuretics can cause other metabolic and endocrine abnormalities such as hypochloremic alkalosis, hyponatremia, and hypercalcemia.40,41 They can also cause photosensitivity and can precipitate gout.42
Observational studies have suggested that metabolic adverse effects such as hypokalemia and hyperuricemia are more common with chlorthalidone than with hydrochlorothiazide.43 It is prudent to monitor laboratory values periodically in patients on diuretic therapy.
DRUG INTERACTIONS
The drug interaction profiles of hydrochlorothiazide and chlorthalidone are also similar. The most common interactions are pharmacodynamic interactions resulting from potassium depletion caused by the diuretics.
Antiarrythymic drugs. Hypokalemia is a risk factor for arrhythmias such as torsades de pointes, and the risk is magnified with concomitant therapy with antiarrhythmic agents that prolong the QT interval independently of serum potassium concentration (eg, sotalol, dronedarone, ibutilide, propafenone). Therefore, combinations of drugs that can cause hypokalemia (eg, diuretics) and antiarrhythmic agents require vigilant monitoring of potassium and appropriate replenishment.44
Dofetilide is a class III antiarrhythmic agent and, like other antiarrhythmic drugs, carries a risk of QT prolongation and torsades de pointes, which is magnified by hypokalemia.45 In addition, dofetilide undergoes active tubular secretion in the kidney via the cation transport system, which is inhibited by hydrochlorothiazide.45 The resulting increase in plasma concentrations of dofetilide may magnify the risk of arrhythmias. Chlorthalidone has not been specifically studied in combination with dofetilide, but thiazide diuretics in general are thought to have a similar effect on tubular secretion and, therefore, should be considered similar to hydrochlorothiazide in this regard.
Digoxin. Similarly, digoxin toxicity may be enhanced in hypokalemia. As with antiarrhythmic agents, serum potassium should be carefully monitored and replenished appropriately when diuretics are used in combination with digoxin.
Lithium is reabsorbed in the proximal tubule along with sodium. Diuretics including hydrochlorothiazide and chlorthalidone that alter sodium reabsorption can also alter lithium absorption.46 When sodium reabsorption is decreased, lithium ion reabsorption is increased and may result in lithium toxicity. Although this combination is not contraindicated, monitoring of serum lithium concentrations and clinical signs and symptoms of lithium toxicity is recommended during initiation and dose adjustments of thiazide diuretics.
Nonsteroidal anti-inflammatory drugs can decrease the natriuretic, diuretic, and antihypertensive effects of both hydrochlorothiazide and chlorthalidone.47
Renin-angiotensin-aldosterone system antagonists, ie, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and the renin inhibitor aliskiren, have potentially beneficial interactions with hydrochlorothiazide and chlorthalidone, producing additive decreases in blood pressure when coadministered with these diuretics. These effects may be particularly potent early in concomitant therapy and allow use of lower doses of diuretics, typically 12.5 mg of hydrochlorothiazide in combination therapy.
LONG-TERM EFFECTS ON CARDIOVASCULAR EVENTS
The long-term goal in treating hypertension is to lower the risk of cardiovascular disease. Therefore, the clinician needs to consider the effect of antihypertensive drug therapy on long-term clinical outcomes.
Antihypertensive drug therapy based on thiazide diuretics has been shown to lower cardiovascular risk when compared with placebo.48 In addition, the effect of chlorthalidone-based antihypertensive therapy was similar to that of other antihypertensive drug classes in preventing most cardiovascular outcomes in ALLHAT.4
However, no study has directly compared hydrochlorothiazide and chlorthalidone with the primary outcome of reduction in long-term cardiovascular events. The data to date come from observational studies and meta-analyses. For example, in a retrospective analysis of the Multiple Risk Factor Intervention Trial, cardiovascular events were significantly fewer in those receiving chlorthalidone vs hydrochlorothiazide (P = .0016).43
In a systematic review and meta-analysis, chlorthalidone was associated with a 23% lower risk of heart failure and a 21% lower risk of all cardiovascular events.49
However, a Canadian observational study of 29,873 patients found no difference in the composite outcome of death or hospitalization for heart failure, stroke, or myocardial infarction between chlorthalidone recipients (3.2 events per 100 person-years) and hydrochlorothiazide recipients (3.4 events per 100 person-years; adjusted hazard ratio 0.93, 95% confidence interval 0.81–1.06).50
In summary, it is unclear whether chlorthalidone or hydrochlorothiazide is superior in preventing cardiovascular events.
SUMMARY
Thiazide and thiazidelike diuretics play an important role in managing hypertension in most patients. The eighth Joint National Committee guidelines do not recommend either hydrochlorothiazide or chlorthalidone over the other. The target dose recommendations are hydrochlorothiazide 25 to 50 mg or chlorthalidone 12.5 to 25 mg daily, with lower doses considered for the elderly.
There are important differences between hydrochlorothiazide and chlorthalidone in pharmacology, potency, and frequency of metabolic side effects. Clinicians should consider these factors to tailor the choice of thiazide diuretic therapy in hypertensive patients.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Dasgupta K, Quinn RR, Zarnke KB, et al; Canadian Hypertension Education Program. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol 2014; 30:485–501.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997.
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265:3255–3264.
- Roush GC, Kaur R, Ernst ME. Diuretics: a review and update. J Cardiovasc Pharmacol Ther 2014; 19:5–13.
- McCormack T, Krause T, O’Flynn N. Management of hypertension in adults in primary care: NICE guideline. Br J Gen Pract 2012; 62:163–164.
- Bhattacharaya M, Alper SL. Pharmacology of volume regulation. In: Golan DE, Tashjian AH Jr, Armstrong EJ, Armstrong AW, editors. Principles of Pharmacology: The pathophysiologic Basis of Drug Therapy. 3rd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012:332–352.
- Woodman R, Brown C, Lockette W. Chlorthalidone decreases platelet aggregation and vascular permeability and promotes angiogenesis. Hypertension 2010; 56:463–470.
- Sato K, Dohi Y, Kojima M, Takase H, Suzuki S, Ito S. Antioxidative effects of thiazide diuretics in refractory hypertensive patients. A randomized crossover trial of chlortalidone and trichlormethiazide. Arzneimittelforschung 2010; 60:612–616.
- US National Library of Medicine. Dailymed. dailymed.nlm.nih.gov. Accessed May 14, 2015.
- Collste P, Garle M, Rawlins MD, Sjöqvist F. Interindividual differences in chlorthalidone concentration in plasma and red cells of man after single and multiple doses. Eur J Clin Pharmacol 1976; 9:319–325.
- Roush GC, Buddharaju V, Ernst ME, Holford TR. Chlorthalidone: mechanisms of action and effect on cardiovascular events. Curr Hypertens Rep 2013; 15:514–521.
- Pool JL, Cushman WC, Saini RK, Nwachuku CE, Battikha JP. Use of the factorial design and quadratic response surface models to evaluate the fosinopril and hydrochlorothiazide combination therapy in hypertension. Am J Hypertens 1997; 10:117–123.
- Pool JL, Glazer R, Weinberger M, Alvarado R, Huang J, Graff A. Comparison of valsartan/hydrochlorothiazide combination therapy at doses up to 320/25 mg versus monotherapy: a double-blind, placebo-controlled study followed by long-term combination therapy in hypertensive adults. Clin Ther 2007; 29:61–73.
- Horie Y, Higaki J, Takeuchi M. Design, statistical analysis and sample size calculation of dose response study of telmisartan and hydrochlorothiazide. Contemp Clin Trials 2007; 28:647–653.
- Chrysant SG. Antihypertensive effectiveness of low-dose lisinopril-hydrochlorothiazide combination. A large multicenter study. Lisinopril-Hydrochlorothiazide Group. Arch Intern Med 1994; 154:737–743.
- Lacourcière Y, Arnott W. Placebo-controlled comparison of the effects of nebivolol and low-dose hydrochlorothiazide as monotherapies and in combination on blood pressure and lipid profile in hypertensive patients. J Hum Hypertens 1994; 8:283–288.
- Villamil A, Chrysant SG, Calhoun D, et al. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens 2007; 25:217–226.
- McGill JB, Reilly PA. Telmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. Clin Ther 2001; 23:833–850.
- Weir MR, Weber MA, Punzi HA, Serfer HM, Rosenblatt S, Cady WJ. A dose escalation trial comparing the combination of diltiazem SR and hydrochlorothiazide with the monotherapies in patients with essential hypertension. J Hum Hypertens 1992; 6:133–138.
- Goldberg MR, Rockhold FW, Offen WW, Dornseif BE. Dose-effect and concentration-effect relationships of pinacidil and hydrochlorothiazide in hypertension. Clin Pharmacol Ther 1989; 46:208–218.
- Papademetriou V, Hainer JW, Sugg J, Munzer D; ATTACH Study Group. Factorial antihypertensive study of an extended-release metoprolol and hydrochlorothiazide combination. Am J Hypertens 2006; 19:1217–1225.
- Chrysant SG, Chrysant GS. Antihypertensive efficacy of olmesartan medoxomil alone and in combination with hydrochlorothiazide. Expert Opin Pharmacother 2004; 5:657–667.
- Kochar M, Guthrie R, Triscari J, Kassler-Taub K, Reeves RA. Matrix study of irbesartan with hydrochlorothiazide in mild-to-moderate hypertension. Am J Hypertens 1999; 12:797–805.
- Benz JR, Black HR, Graff A, Reed A, Fitzsimmons S, Shi Y. Valsartan and hydrochlorothiazide in patients with essential hypertension. A multiple dose, double-blind, placebo controlled trial comparing combination therapy with monotherapy. J Hum Hypertens 1998; 12:861–866.
- Jounela AJ, Lilja M, Lumme J, et al. Relation between low dose of hydrochlorothiazide, antihypertensive effect and adverse effects. Blood Press 1994; 3:231–235.
- Scholze J, Breitstadt A, Cairns V, et al. Short report: ramipril and hydrochlorothiazide combination therapy in hypertension: a clinical trial of factorial design. East Germany Collaborative Trial Group. J Hypertens 1993; 11:217–221.
- Canter D, Frank GJ, Knapp LE, Phelps M, Quade M, Texter M. Quinapril and hydrochlorothiazide combination for control of hypertension: assessment by factorial design. Quinapril Investigator Group. J Hum Hypertens 1994; 8:155–162.
- Vardan S, Mehrotra KG, Mookherjee S, Willsey GA, Gens JD, Green DE. Efficacy and reduced metabolic side effects of a 15-mg chlorthalidone formulation in the treatment of mild hypertension. A multicenter study. JAMA 1987; 258:484–488.
- Materson BJ, Oster JR, Michael UF, et al. Dose response to chlorthalidone in patients with mild hypertension. Efficacy of a lower dose. Clin Pharmacol Ther 1978; 24:192–198.
- Morledge JH, Ettinger B, Aranda J, et al. Isolated systolic hypertension in the elderly. A placebo-controlled, dose-response evaluation of chlorthalidone. J Am Geriatr Soc 1986; 34:199–206.
- Peterzan MA, Hardy R, Chaturvedi N, Hughes AD. Meta-analysis of dose-response relationships for hydrochlorothiazide, chlorthalidone, and bendroflumethiazide on blood pressure, serum potassium, and urate. Hypertension 2012; 59:1104–1109.
- Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension 2006; 47:352–358.
- Bakris GL, Sica D, White WB, et al. Antihypertensive efficacy of hydrochlorothiazide vs chlorthalidone combined with azilsartan medoxomil. Am J Med 2012; 25:1229.e1–1229.e10.
- Ernst ME, Carter BL, Zheng S, Grimm RH Jr. Meta-analysis of dose-response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens 2010; 23:440–446.
- Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension 2004; 43:4–9.
- Alderman MH, Piller LB, Ford CE, et al; Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Clinical significance of incident hypokalemia and hyperkalemia in treated hypertensive patients in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Hypertension 2012; 59:926–933.
- Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly Program. Hypertension 2000; 35:1025–1030.
- Egom EE, Chirico D, Clark AL. A review of thiazide-induced hyponatraemia. Clin Med 2011; 11:448–451.
- Palmer BF. Metabolic complications associated with use of diuretics. Semin Nephrol 2011; 31:542–552.
- Hueskes BA, Roovers EA, Mantel-Teeuwisse AK, Janssens HJ, van de Lisdonk EH, Janssen M. Use of diuretics and the risk of gouty arthritis: a systematic review. Semin Arthritis Rheum 2012; 41:879–889.
- Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension 2011; 57:689–694.
- Trinkley KE, Page RL 2nd, Lien H, Yamanouye K, Tisdale JE. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin 2013; 29:1719–1726.
- Crist LW, Dixon DL. Considerations for dofetilide use in the elderly. Consult Pharm 2014; 29:270–274.
- Handler J. Lithium and antihypertensive medication: a potentially dangerous interaction. J Clin Hypertens (Greenwich) 2009; 11:738–742.
- Pavlicević I, Kuzmanić M, Rumboldt M, Rumboldt Z. Interaction between antihypertensives and NSAIDs in primary care: a controlled trial. Can J Clin Pharmacol 2008; 15:e372–e382.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension 2012; 59:1110–1117.
- Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med 2013; 158:447–455.
The thiazide diuretic hydrochlorothiazide and the thiazidelike diuretic chlorthalidone are two old drugs that are still useful. Although similar, they differ in important ways still not fully appreciated more than a half century after they were introduced.
Most hypertension guidelines recommend thiazide diuretics as one of the classes of agents that can be used either as initial antihypertensive drug therapy or as part of combination therapy.1–3
In the United States, hydrochlorothiazide is used more often than chlorthalidone, but many clinical trials of antihypertensive therapy have used chlorthalidone.4,5 In recent years, particularly after the publication of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), interest in chlorthalidone has been increasing, and new data are now available comparing these two diuretics.6 While current US guidelines do not recommend one over the other, British guidelines prefer chlorthalidone.7
This review summarizes the data comparing the two drugs’ pharmacology, antihypertensive effect, and impact on clinical outcomes to help guide clinicians in choosing antihypertensive drug therapy.
PHARMACOLOGY AND MECHANISM OF ACTION
Many of the differences in effectiveness and adverse effects of hydrochlorothiazide and chlorthalidone are thought to be due to their different pharmacodynamic and pharmacokinetic effects.
Pharmacodynamic effects
Hydrochlorothiazide and chlorthalidone differ significantly in chemical structure (Figure 1), but both contain a sulfonamide group that inhibits carbonic anhydrase activity, which may be associated with lower vascular contractility. Both drugs are concentrated in the kidney and secreted into the tubular lumen8; therefore, their therapeutic diuretic effects are often achieved with relatively low plasma concentrations.
Both drugs inhibit the sodium-chloride cotransporter in the luminal membrane of the distal convoluted tubule of the ascending loop of Henle, leading to a modest natriuresis and diuresis. The exact mechanism by which they lower blood pressure is not known: while the initial response is from diuresis and volume changes, long-term reduction in blood pressure is through uncertain mechanisms. In addition, chlorthalidone may have beneficial effects on endothelial function and oxidative stress.9,10
Both drugs also increase secretion of potassium and hydrogen ions and promote increased reabsorption of calcium through increased expression of a sodium-calcium exchange channel.8 Chlorthalidone may cause more inhibition of carbonic anhydrase than hydrochlorothiazide, which can lead to lower intracellular pH and cell volume. This effect may in part explain a pleiotropic effect of chlorthalidone, ie, inhibition of platelet function, which in turn may contribute to this drug’s beneficial effect on cardiovascular outcomes.9
Pharmacokinetic differences
Hydrochlorothiazide and chlorthalidone have important differences in their pharmacokinetic properties (Table 1).11
Hydrochlorothiazide has its onset of action in about 2 hours, and it reaches its peak in 4 to 6 hours. Though its duration of action is short—up to 12 hours—its pharmacodynamic response can be much longer than predicted by its kinetics, allowing once-daily dosing.8
Chlorthalidone has a longer duration of action than hydrochlorothiazide. This may be because it has a very high volume of distribution, since it is taken up into red blood cells and is bound to carbonic anhydrase.12 This may result in a “drug reservoir” that keeps drug levels higher for a longer time.13 Its long duration of action makes it a favorable choice for patients who have difficulty adhering to medication instructions. In addition, a missed dose is unlikely to have a “rebound” effect like that seen with some other antihypertensive agents. However, both chlorthalidone and hydrochlorothiazide are effective if taken once daily.
BLOOD PRESSURE-LOWERING
Both hydrochlorothiazide and chlorthalidone are effective antihypertensive agents. Table 2 summarizes findings from studies that evaluated their blood pressure-lowering effect at various doses.14–33 However, relatively few studies have directly compared these two agents’ effects on blood pressure.
Ernst et al,34 in a small study (but probably the best one to address this issue), compared chlorthalidone 12.5 mg/day (force-titrated to 25 mg/day) and hydrochlorothiazide 25 mg/day (force-titrated to 50 mg/day) in untreated hypertensive patients. After 8 weeks, ambulatory blood pressure monitoring indicated a greater reduction from baseline in systolic blood pressure with chlorthalidone 25 mg/day than with hydrochlorothiazide 50 mg/day (24-hour mean –12.4 vs –7.4 mm Hg, P = .05). Interestingly, the change in nighttime blood pressure was greater in the chlorthalidone group (–13.5 mm Hg) than in the hydrochlorothiazide group (–6.4 mm Hg; P = .009). These data suggest that at the doses studied, chlorthalidone is more effective than hydrochlorothiazide in lowering systolic blood pressure.
Bakris et al,35 using a different study design, compared the single-pill combination of azilsartan medoxomil and chlorthalidone vs coadministration of azilsartan medoxomil and hydrochlorothiazide in participants with stage 2 primary hypertension (≥ 160/100 mm Hg). Systolic blood pressure, as measured in the clinic, declined more with the chlorthalidone combination (–35.1 mm Hg) than with the hydrochlorothiazide combination (–29.5 mm Hg, mean difference –5.6 mm Hg, P < .001).
Meta-analyses also support the conclusion that chlorthalidone is more potent than hydrochlorothiazide in lowering blood pressure.35,36 Several studies have shown that chlorthalidone at the same dose is 1.5 to 2 times as potent as hydrochlorothiazide.33,36,37 Therefore, for clinical purposes, it is reasonable to consider chlorthalidone 12.5 mg daily as similar to 25 mg of hydrochlorothiazide daily.
ADVERSE EFFECTS
Electrolyte disturbances are a common adverse effect of thiazide diuretics.
Hypokalemia. All thiazide diuretics cause potassium wasting. The frequency of hypokalemia depends on the dose, frequency of administration, diet, and other pharmacologic agents used.
Two large clinical trials, the Systolic Hypertension in the Elderly Program and ALLHAT, found that chlorthalidone caused hypokalemia requiring therapy in about 6% to 8% of patients.38,39 Chlorthalidone therapy was associated with a lowering of serum potassium levels of 0.2 to 0.5 mmol/L.36 In ALLHAT, chlorthalidone was associated with a reduction in potassium levels of approximately 0.2 mmol/L after 4 years.38
All diuretics require monitoring of electrolytes, especially during the first 2 weeks of therapy. Once a steady state is reached, patients are not usually at risk of hypokalemia unless the dose is increased, extrarenal losses of potassium increase, or dietary potassium is reduced.
Other electrolyte changes. Thiazide and thiazide-like diuretics can cause other metabolic and endocrine abnormalities such as hypochloremic alkalosis, hyponatremia, and hypercalcemia.40,41 They can also cause photosensitivity and can precipitate gout.42
Observational studies have suggested that metabolic adverse effects such as hypokalemia and hyperuricemia are more common with chlorthalidone than with hydrochlorothiazide.43 It is prudent to monitor laboratory values periodically in patients on diuretic therapy.
DRUG INTERACTIONS
The drug interaction profiles of hydrochlorothiazide and chlorthalidone are also similar. The most common interactions are pharmacodynamic interactions resulting from potassium depletion caused by the diuretics.
Antiarrythymic drugs. Hypokalemia is a risk factor for arrhythmias such as torsades de pointes, and the risk is magnified with concomitant therapy with antiarrhythmic agents that prolong the QT interval independently of serum potassium concentration (eg, sotalol, dronedarone, ibutilide, propafenone). Therefore, combinations of drugs that can cause hypokalemia (eg, diuretics) and antiarrhythmic agents require vigilant monitoring of potassium and appropriate replenishment.44
Dofetilide is a class III antiarrhythmic agent and, like other antiarrhythmic drugs, carries a risk of QT prolongation and torsades de pointes, which is magnified by hypokalemia.45 In addition, dofetilide undergoes active tubular secretion in the kidney via the cation transport system, which is inhibited by hydrochlorothiazide.45 The resulting increase in plasma concentrations of dofetilide may magnify the risk of arrhythmias. Chlorthalidone has not been specifically studied in combination with dofetilide, but thiazide diuretics in general are thought to have a similar effect on tubular secretion and, therefore, should be considered similar to hydrochlorothiazide in this regard.
Digoxin. Similarly, digoxin toxicity may be enhanced in hypokalemia. As with antiarrhythmic agents, serum potassium should be carefully monitored and replenished appropriately when diuretics are used in combination with digoxin.
Lithium is reabsorbed in the proximal tubule along with sodium. Diuretics including hydrochlorothiazide and chlorthalidone that alter sodium reabsorption can also alter lithium absorption.46 When sodium reabsorption is decreased, lithium ion reabsorption is increased and may result in lithium toxicity. Although this combination is not contraindicated, monitoring of serum lithium concentrations and clinical signs and symptoms of lithium toxicity is recommended during initiation and dose adjustments of thiazide diuretics.
Nonsteroidal anti-inflammatory drugs can decrease the natriuretic, diuretic, and antihypertensive effects of both hydrochlorothiazide and chlorthalidone.47
Renin-angiotensin-aldosterone system antagonists, ie, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and the renin inhibitor aliskiren, have potentially beneficial interactions with hydrochlorothiazide and chlorthalidone, producing additive decreases in blood pressure when coadministered with these diuretics. These effects may be particularly potent early in concomitant therapy and allow use of lower doses of diuretics, typically 12.5 mg of hydrochlorothiazide in combination therapy.
LONG-TERM EFFECTS ON CARDIOVASCULAR EVENTS
The long-term goal in treating hypertension is to lower the risk of cardiovascular disease. Therefore, the clinician needs to consider the effect of antihypertensive drug therapy on long-term clinical outcomes.
Antihypertensive drug therapy based on thiazide diuretics has been shown to lower cardiovascular risk when compared with placebo.48 In addition, the effect of chlorthalidone-based antihypertensive therapy was similar to that of other antihypertensive drug classes in preventing most cardiovascular outcomes in ALLHAT.4
However, no study has directly compared hydrochlorothiazide and chlorthalidone with the primary outcome of reduction in long-term cardiovascular events. The data to date come from observational studies and meta-analyses. For example, in a retrospective analysis of the Multiple Risk Factor Intervention Trial, cardiovascular events were significantly fewer in those receiving chlorthalidone vs hydrochlorothiazide (P = .0016).43
In a systematic review and meta-analysis, chlorthalidone was associated with a 23% lower risk of heart failure and a 21% lower risk of all cardiovascular events.49
However, a Canadian observational study of 29,873 patients found no difference in the composite outcome of death or hospitalization for heart failure, stroke, or myocardial infarction between chlorthalidone recipients (3.2 events per 100 person-years) and hydrochlorothiazide recipients (3.4 events per 100 person-years; adjusted hazard ratio 0.93, 95% confidence interval 0.81–1.06).50
In summary, it is unclear whether chlorthalidone or hydrochlorothiazide is superior in preventing cardiovascular events.
SUMMARY
Thiazide and thiazidelike diuretics play an important role in managing hypertension in most patients. The eighth Joint National Committee guidelines do not recommend either hydrochlorothiazide or chlorthalidone over the other. The target dose recommendations are hydrochlorothiazide 25 to 50 mg or chlorthalidone 12.5 to 25 mg daily, with lower doses considered for the elderly.
There are important differences between hydrochlorothiazide and chlorthalidone in pharmacology, potency, and frequency of metabolic side effects. Clinicians should consider these factors to tailor the choice of thiazide diuretic therapy in hypertensive patients.
The thiazide diuretic hydrochlorothiazide and the thiazidelike diuretic chlorthalidone are two old drugs that are still useful. Although similar, they differ in important ways still not fully appreciated more than a half century after they were introduced.
Most hypertension guidelines recommend thiazide diuretics as one of the classes of agents that can be used either as initial antihypertensive drug therapy or as part of combination therapy.1–3
In the United States, hydrochlorothiazide is used more often than chlorthalidone, but many clinical trials of antihypertensive therapy have used chlorthalidone.4,5 In recent years, particularly after the publication of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), interest in chlorthalidone has been increasing, and new data are now available comparing these two diuretics.6 While current US guidelines do not recommend one over the other, British guidelines prefer chlorthalidone.7
This review summarizes the data comparing the two drugs’ pharmacology, antihypertensive effect, and impact on clinical outcomes to help guide clinicians in choosing antihypertensive drug therapy.
PHARMACOLOGY AND MECHANISM OF ACTION
Many of the differences in effectiveness and adverse effects of hydrochlorothiazide and chlorthalidone are thought to be due to their different pharmacodynamic and pharmacokinetic effects.
Pharmacodynamic effects
Hydrochlorothiazide and chlorthalidone differ significantly in chemical structure (Figure 1), but both contain a sulfonamide group that inhibits carbonic anhydrase activity, which may be associated with lower vascular contractility. Both drugs are concentrated in the kidney and secreted into the tubular lumen8; therefore, their therapeutic diuretic effects are often achieved with relatively low plasma concentrations.
Both drugs inhibit the sodium-chloride cotransporter in the luminal membrane of the distal convoluted tubule of the ascending loop of Henle, leading to a modest natriuresis and diuresis. The exact mechanism by which they lower blood pressure is not known: while the initial response is from diuresis and volume changes, long-term reduction in blood pressure is through uncertain mechanisms. In addition, chlorthalidone may have beneficial effects on endothelial function and oxidative stress.9,10
Both drugs also increase secretion of potassium and hydrogen ions and promote increased reabsorption of calcium through increased expression of a sodium-calcium exchange channel.8 Chlorthalidone may cause more inhibition of carbonic anhydrase than hydrochlorothiazide, which can lead to lower intracellular pH and cell volume. This effect may in part explain a pleiotropic effect of chlorthalidone, ie, inhibition of platelet function, which in turn may contribute to this drug’s beneficial effect on cardiovascular outcomes.9
Pharmacokinetic differences
Hydrochlorothiazide and chlorthalidone have important differences in their pharmacokinetic properties (Table 1).11
Hydrochlorothiazide has its onset of action in about 2 hours, and it reaches its peak in 4 to 6 hours. Though its duration of action is short—up to 12 hours—its pharmacodynamic response can be much longer than predicted by its kinetics, allowing once-daily dosing.8
Chlorthalidone has a longer duration of action than hydrochlorothiazide. This may be because it has a very high volume of distribution, since it is taken up into red blood cells and is bound to carbonic anhydrase.12 This may result in a “drug reservoir” that keeps drug levels higher for a longer time.13 Its long duration of action makes it a favorable choice for patients who have difficulty adhering to medication instructions. In addition, a missed dose is unlikely to have a “rebound” effect like that seen with some other antihypertensive agents. However, both chlorthalidone and hydrochlorothiazide are effective if taken once daily.
BLOOD PRESSURE-LOWERING
Both hydrochlorothiazide and chlorthalidone are effective antihypertensive agents. Table 2 summarizes findings from studies that evaluated their blood pressure-lowering effect at various doses.14–33 However, relatively few studies have directly compared these two agents’ effects on blood pressure.
Ernst et al,34 in a small study (but probably the best one to address this issue), compared chlorthalidone 12.5 mg/day (force-titrated to 25 mg/day) and hydrochlorothiazide 25 mg/day (force-titrated to 50 mg/day) in untreated hypertensive patients. After 8 weeks, ambulatory blood pressure monitoring indicated a greater reduction from baseline in systolic blood pressure with chlorthalidone 25 mg/day than with hydrochlorothiazide 50 mg/day (24-hour mean –12.4 vs –7.4 mm Hg, P = .05). Interestingly, the change in nighttime blood pressure was greater in the chlorthalidone group (–13.5 mm Hg) than in the hydrochlorothiazide group (–6.4 mm Hg; P = .009). These data suggest that at the doses studied, chlorthalidone is more effective than hydrochlorothiazide in lowering systolic blood pressure.
Bakris et al,35 using a different study design, compared the single-pill combination of azilsartan medoxomil and chlorthalidone vs coadministration of azilsartan medoxomil and hydrochlorothiazide in participants with stage 2 primary hypertension (≥ 160/100 mm Hg). Systolic blood pressure, as measured in the clinic, declined more with the chlorthalidone combination (–35.1 mm Hg) than with the hydrochlorothiazide combination (–29.5 mm Hg, mean difference –5.6 mm Hg, P < .001).
Meta-analyses also support the conclusion that chlorthalidone is more potent than hydrochlorothiazide in lowering blood pressure.35,36 Several studies have shown that chlorthalidone at the same dose is 1.5 to 2 times as potent as hydrochlorothiazide.33,36,37 Therefore, for clinical purposes, it is reasonable to consider chlorthalidone 12.5 mg daily as similar to 25 mg of hydrochlorothiazide daily.
ADVERSE EFFECTS
Electrolyte disturbances are a common adverse effect of thiazide diuretics.
Hypokalemia. All thiazide diuretics cause potassium wasting. The frequency of hypokalemia depends on the dose, frequency of administration, diet, and other pharmacologic agents used.
Two large clinical trials, the Systolic Hypertension in the Elderly Program and ALLHAT, found that chlorthalidone caused hypokalemia requiring therapy in about 6% to 8% of patients.38,39 Chlorthalidone therapy was associated with a lowering of serum potassium levels of 0.2 to 0.5 mmol/L.36 In ALLHAT, chlorthalidone was associated with a reduction in potassium levels of approximately 0.2 mmol/L after 4 years.38
All diuretics require monitoring of electrolytes, especially during the first 2 weeks of therapy. Once a steady state is reached, patients are not usually at risk of hypokalemia unless the dose is increased, extrarenal losses of potassium increase, or dietary potassium is reduced.
Other electrolyte changes. Thiazide and thiazide-like diuretics can cause other metabolic and endocrine abnormalities such as hypochloremic alkalosis, hyponatremia, and hypercalcemia.40,41 They can also cause photosensitivity and can precipitate gout.42
Observational studies have suggested that metabolic adverse effects such as hypokalemia and hyperuricemia are more common with chlorthalidone than with hydrochlorothiazide.43 It is prudent to monitor laboratory values periodically in patients on diuretic therapy.
DRUG INTERACTIONS
The drug interaction profiles of hydrochlorothiazide and chlorthalidone are also similar. The most common interactions are pharmacodynamic interactions resulting from potassium depletion caused by the diuretics.
Antiarrythymic drugs. Hypokalemia is a risk factor for arrhythmias such as torsades de pointes, and the risk is magnified with concomitant therapy with antiarrhythmic agents that prolong the QT interval independently of serum potassium concentration (eg, sotalol, dronedarone, ibutilide, propafenone). Therefore, combinations of drugs that can cause hypokalemia (eg, diuretics) and antiarrhythmic agents require vigilant monitoring of potassium and appropriate replenishment.44
Dofetilide is a class III antiarrhythmic agent and, like other antiarrhythmic drugs, carries a risk of QT prolongation and torsades de pointes, which is magnified by hypokalemia.45 In addition, dofetilide undergoes active tubular secretion in the kidney via the cation transport system, which is inhibited by hydrochlorothiazide.45 The resulting increase in plasma concentrations of dofetilide may magnify the risk of arrhythmias. Chlorthalidone has not been specifically studied in combination with dofetilide, but thiazide diuretics in general are thought to have a similar effect on tubular secretion and, therefore, should be considered similar to hydrochlorothiazide in this regard.
Digoxin. Similarly, digoxin toxicity may be enhanced in hypokalemia. As with antiarrhythmic agents, serum potassium should be carefully monitored and replenished appropriately when diuretics are used in combination with digoxin.
Lithium is reabsorbed in the proximal tubule along with sodium. Diuretics including hydrochlorothiazide and chlorthalidone that alter sodium reabsorption can also alter lithium absorption.46 When sodium reabsorption is decreased, lithium ion reabsorption is increased and may result in lithium toxicity. Although this combination is not contraindicated, monitoring of serum lithium concentrations and clinical signs and symptoms of lithium toxicity is recommended during initiation and dose adjustments of thiazide diuretics.
Nonsteroidal anti-inflammatory drugs can decrease the natriuretic, diuretic, and antihypertensive effects of both hydrochlorothiazide and chlorthalidone.47
Renin-angiotensin-aldosterone system antagonists, ie, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and the renin inhibitor aliskiren, have potentially beneficial interactions with hydrochlorothiazide and chlorthalidone, producing additive decreases in blood pressure when coadministered with these diuretics. These effects may be particularly potent early in concomitant therapy and allow use of lower doses of diuretics, typically 12.5 mg of hydrochlorothiazide in combination therapy.
LONG-TERM EFFECTS ON CARDIOVASCULAR EVENTS
The long-term goal in treating hypertension is to lower the risk of cardiovascular disease. Therefore, the clinician needs to consider the effect of antihypertensive drug therapy on long-term clinical outcomes.
Antihypertensive drug therapy based on thiazide diuretics has been shown to lower cardiovascular risk when compared with placebo.48 In addition, the effect of chlorthalidone-based antihypertensive therapy was similar to that of other antihypertensive drug classes in preventing most cardiovascular outcomes in ALLHAT.4
However, no study has directly compared hydrochlorothiazide and chlorthalidone with the primary outcome of reduction in long-term cardiovascular events. The data to date come from observational studies and meta-analyses. For example, in a retrospective analysis of the Multiple Risk Factor Intervention Trial, cardiovascular events were significantly fewer in those receiving chlorthalidone vs hydrochlorothiazide (P = .0016).43
In a systematic review and meta-analysis, chlorthalidone was associated with a 23% lower risk of heart failure and a 21% lower risk of all cardiovascular events.49
However, a Canadian observational study of 29,873 patients found no difference in the composite outcome of death or hospitalization for heart failure, stroke, or myocardial infarction between chlorthalidone recipients (3.2 events per 100 person-years) and hydrochlorothiazide recipients (3.4 events per 100 person-years; adjusted hazard ratio 0.93, 95% confidence interval 0.81–1.06).50
In summary, it is unclear whether chlorthalidone or hydrochlorothiazide is superior in preventing cardiovascular events.
SUMMARY
Thiazide and thiazidelike diuretics play an important role in managing hypertension in most patients. The eighth Joint National Committee guidelines do not recommend either hydrochlorothiazide or chlorthalidone over the other. The target dose recommendations are hydrochlorothiazide 25 to 50 mg or chlorthalidone 12.5 to 25 mg daily, with lower doses considered for the elderly.
There are important differences between hydrochlorothiazide and chlorthalidone in pharmacology, potency, and frequency of metabolic side effects. Clinicians should consider these factors to tailor the choice of thiazide diuretic therapy in hypertensive patients.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Dasgupta K, Quinn RR, Zarnke KB, et al; Canadian Hypertension Education Program. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol 2014; 30:485–501.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997.
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265:3255–3264.
- Roush GC, Kaur R, Ernst ME. Diuretics: a review and update. J Cardiovasc Pharmacol Ther 2014; 19:5–13.
- McCormack T, Krause T, O’Flynn N. Management of hypertension in adults in primary care: NICE guideline. Br J Gen Pract 2012; 62:163–164.
- Bhattacharaya M, Alper SL. Pharmacology of volume regulation. In: Golan DE, Tashjian AH Jr, Armstrong EJ, Armstrong AW, editors. Principles of Pharmacology: The pathophysiologic Basis of Drug Therapy. 3rd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012:332–352.
- Woodman R, Brown C, Lockette W. Chlorthalidone decreases platelet aggregation and vascular permeability and promotes angiogenesis. Hypertension 2010; 56:463–470.
- Sato K, Dohi Y, Kojima M, Takase H, Suzuki S, Ito S. Antioxidative effects of thiazide diuretics in refractory hypertensive patients. A randomized crossover trial of chlortalidone and trichlormethiazide. Arzneimittelforschung 2010; 60:612–616.
- US National Library of Medicine. Dailymed. dailymed.nlm.nih.gov. Accessed May 14, 2015.
- Collste P, Garle M, Rawlins MD, Sjöqvist F. Interindividual differences in chlorthalidone concentration in plasma and red cells of man after single and multiple doses. Eur J Clin Pharmacol 1976; 9:319–325.
- Roush GC, Buddharaju V, Ernst ME, Holford TR. Chlorthalidone: mechanisms of action and effect on cardiovascular events. Curr Hypertens Rep 2013; 15:514–521.
- Pool JL, Cushman WC, Saini RK, Nwachuku CE, Battikha JP. Use of the factorial design and quadratic response surface models to evaluate the fosinopril and hydrochlorothiazide combination therapy in hypertension. Am J Hypertens 1997; 10:117–123.
- Pool JL, Glazer R, Weinberger M, Alvarado R, Huang J, Graff A. Comparison of valsartan/hydrochlorothiazide combination therapy at doses up to 320/25 mg versus monotherapy: a double-blind, placebo-controlled study followed by long-term combination therapy in hypertensive adults. Clin Ther 2007; 29:61–73.
- Horie Y, Higaki J, Takeuchi M. Design, statistical analysis and sample size calculation of dose response study of telmisartan and hydrochlorothiazide. Contemp Clin Trials 2007; 28:647–653.
- Chrysant SG. Antihypertensive effectiveness of low-dose lisinopril-hydrochlorothiazide combination. A large multicenter study. Lisinopril-Hydrochlorothiazide Group. Arch Intern Med 1994; 154:737–743.
- Lacourcière Y, Arnott W. Placebo-controlled comparison of the effects of nebivolol and low-dose hydrochlorothiazide as monotherapies and in combination on blood pressure and lipid profile in hypertensive patients. J Hum Hypertens 1994; 8:283–288.
- Villamil A, Chrysant SG, Calhoun D, et al. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens 2007; 25:217–226.
- McGill JB, Reilly PA. Telmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. Clin Ther 2001; 23:833–850.
- Weir MR, Weber MA, Punzi HA, Serfer HM, Rosenblatt S, Cady WJ. A dose escalation trial comparing the combination of diltiazem SR and hydrochlorothiazide with the monotherapies in patients with essential hypertension. J Hum Hypertens 1992; 6:133–138.
- Goldberg MR, Rockhold FW, Offen WW, Dornseif BE. Dose-effect and concentration-effect relationships of pinacidil and hydrochlorothiazide in hypertension. Clin Pharmacol Ther 1989; 46:208–218.
- Papademetriou V, Hainer JW, Sugg J, Munzer D; ATTACH Study Group. Factorial antihypertensive study of an extended-release metoprolol and hydrochlorothiazide combination. Am J Hypertens 2006; 19:1217–1225.
- Chrysant SG, Chrysant GS. Antihypertensive efficacy of olmesartan medoxomil alone and in combination with hydrochlorothiazide. Expert Opin Pharmacother 2004; 5:657–667.
- Kochar M, Guthrie R, Triscari J, Kassler-Taub K, Reeves RA. Matrix study of irbesartan with hydrochlorothiazide in mild-to-moderate hypertension. Am J Hypertens 1999; 12:797–805.
- Benz JR, Black HR, Graff A, Reed A, Fitzsimmons S, Shi Y. Valsartan and hydrochlorothiazide in patients with essential hypertension. A multiple dose, double-blind, placebo controlled trial comparing combination therapy with monotherapy. J Hum Hypertens 1998; 12:861–866.
- Jounela AJ, Lilja M, Lumme J, et al. Relation between low dose of hydrochlorothiazide, antihypertensive effect and adverse effects. Blood Press 1994; 3:231–235.
- Scholze J, Breitstadt A, Cairns V, et al. Short report: ramipril and hydrochlorothiazide combination therapy in hypertension: a clinical trial of factorial design. East Germany Collaborative Trial Group. J Hypertens 1993; 11:217–221.
- Canter D, Frank GJ, Knapp LE, Phelps M, Quade M, Texter M. Quinapril and hydrochlorothiazide combination for control of hypertension: assessment by factorial design. Quinapril Investigator Group. J Hum Hypertens 1994; 8:155–162.
- Vardan S, Mehrotra KG, Mookherjee S, Willsey GA, Gens JD, Green DE. Efficacy and reduced metabolic side effects of a 15-mg chlorthalidone formulation in the treatment of mild hypertension. A multicenter study. JAMA 1987; 258:484–488.
- Materson BJ, Oster JR, Michael UF, et al. Dose response to chlorthalidone in patients with mild hypertension. Efficacy of a lower dose. Clin Pharmacol Ther 1978; 24:192–198.
- Morledge JH, Ettinger B, Aranda J, et al. Isolated systolic hypertension in the elderly. A placebo-controlled, dose-response evaluation of chlorthalidone. J Am Geriatr Soc 1986; 34:199–206.
- Peterzan MA, Hardy R, Chaturvedi N, Hughes AD. Meta-analysis of dose-response relationships for hydrochlorothiazide, chlorthalidone, and bendroflumethiazide on blood pressure, serum potassium, and urate. Hypertension 2012; 59:1104–1109.
- Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension 2006; 47:352–358.
- Bakris GL, Sica D, White WB, et al. Antihypertensive efficacy of hydrochlorothiazide vs chlorthalidone combined with azilsartan medoxomil. Am J Med 2012; 25:1229.e1–1229.e10.
- Ernst ME, Carter BL, Zheng S, Grimm RH Jr. Meta-analysis of dose-response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens 2010; 23:440–446.
- Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension 2004; 43:4–9.
- Alderman MH, Piller LB, Ford CE, et al; Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Clinical significance of incident hypokalemia and hyperkalemia in treated hypertensive patients in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Hypertension 2012; 59:926–933.
- Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly Program. Hypertension 2000; 35:1025–1030.
- Egom EE, Chirico D, Clark AL. A review of thiazide-induced hyponatraemia. Clin Med 2011; 11:448–451.
- Palmer BF. Metabolic complications associated with use of diuretics. Semin Nephrol 2011; 31:542–552.
- Hueskes BA, Roovers EA, Mantel-Teeuwisse AK, Janssens HJ, van de Lisdonk EH, Janssen M. Use of diuretics and the risk of gouty arthritis: a systematic review. Semin Arthritis Rheum 2012; 41:879–889.
- Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension 2011; 57:689–694.
- Trinkley KE, Page RL 2nd, Lien H, Yamanouye K, Tisdale JE. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin 2013; 29:1719–1726.
- Crist LW, Dixon DL. Considerations for dofetilide use in the elderly. Consult Pharm 2014; 29:270–274.
- Handler J. Lithium and antihypertensive medication: a potentially dangerous interaction. J Clin Hypertens (Greenwich) 2009; 11:738–742.
- Pavlicević I, Kuzmanić M, Rumboldt M, Rumboldt Z. Interaction between antihypertensives and NSAIDs in primary care: a controlled trial. Can J Clin Pharmacol 2008; 15:e372–e382.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension 2012; 59:1110–1117.
- Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med 2013; 158:447–455.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Dasgupta K, Quinn RR, Zarnke KB, et al; Canadian Hypertension Education Program. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol 2014; 30:485–501.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997.
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265:3255–3264.
- Roush GC, Kaur R, Ernst ME. Diuretics: a review and update. J Cardiovasc Pharmacol Ther 2014; 19:5–13.
- McCormack T, Krause T, O’Flynn N. Management of hypertension in adults in primary care: NICE guideline. Br J Gen Pract 2012; 62:163–164.
- Bhattacharaya M, Alper SL. Pharmacology of volume regulation. In: Golan DE, Tashjian AH Jr, Armstrong EJ, Armstrong AW, editors. Principles of Pharmacology: The pathophysiologic Basis of Drug Therapy. 3rd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012:332–352.
- Woodman R, Brown C, Lockette W. Chlorthalidone decreases platelet aggregation and vascular permeability and promotes angiogenesis. Hypertension 2010; 56:463–470.
- Sato K, Dohi Y, Kojima M, Takase H, Suzuki S, Ito S. Antioxidative effects of thiazide diuretics in refractory hypertensive patients. A randomized crossover trial of chlortalidone and trichlormethiazide. Arzneimittelforschung 2010; 60:612–616.
- US National Library of Medicine. Dailymed. dailymed.nlm.nih.gov. Accessed May 14, 2015.
- Collste P, Garle M, Rawlins MD, Sjöqvist F. Interindividual differences in chlorthalidone concentration in plasma and red cells of man after single and multiple doses. Eur J Clin Pharmacol 1976; 9:319–325.
- Roush GC, Buddharaju V, Ernst ME, Holford TR. Chlorthalidone: mechanisms of action and effect on cardiovascular events. Curr Hypertens Rep 2013; 15:514–521.
- Pool JL, Cushman WC, Saini RK, Nwachuku CE, Battikha JP. Use of the factorial design and quadratic response surface models to evaluate the fosinopril and hydrochlorothiazide combination therapy in hypertension. Am J Hypertens 1997; 10:117–123.
- Pool JL, Glazer R, Weinberger M, Alvarado R, Huang J, Graff A. Comparison of valsartan/hydrochlorothiazide combination therapy at doses up to 320/25 mg versus monotherapy: a double-blind, placebo-controlled study followed by long-term combination therapy in hypertensive adults. Clin Ther 2007; 29:61–73.
- Horie Y, Higaki J, Takeuchi M. Design, statistical analysis and sample size calculation of dose response study of telmisartan and hydrochlorothiazide. Contemp Clin Trials 2007; 28:647–653.
- Chrysant SG. Antihypertensive effectiveness of low-dose lisinopril-hydrochlorothiazide combination. A large multicenter study. Lisinopril-Hydrochlorothiazide Group. Arch Intern Med 1994; 154:737–743.
- Lacourcière Y, Arnott W. Placebo-controlled comparison of the effects of nebivolol and low-dose hydrochlorothiazide as monotherapies and in combination on blood pressure and lipid profile in hypertensive patients. J Hum Hypertens 1994; 8:283–288.
- Villamil A, Chrysant SG, Calhoun D, et al. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens 2007; 25:217–226.
- McGill JB, Reilly PA. Telmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. Clin Ther 2001; 23:833–850.
- Weir MR, Weber MA, Punzi HA, Serfer HM, Rosenblatt S, Cady WJ. A dose escalation trial comparing the combination of diltiazem SR and hydrochlorothiazide with the monotherapies in patients with essential hypertension. J Hum Hypertens 1992; 6:133–138.
- Goldberg MR, Rockhold FW, Offen WW, Dornseif BE. Dose-effect and concentration-effect relationships of pinacidil and hydrochlorothiazide in hypertension. Clin Pharmacol Ther 1989; 46:208–218.
- Papademetriou V, Hainer JW, Sugg J, Munzer D; ATTACH Study Group. Factorial antihypertensive study of an extended-release metoprolol and hydrochlorothiazide combination. Am J Hypertens 2006; 19:1217–1225.
- Chrysant SG, Chrysant GS. Antihypertensive efficacy of olmesartan medoxomil alone and in combination with hydrochlorothiazide. Expert Opin Pharmacother 2004; 5:657–667.
- Kochar M, Guthrie R, Triscari J, Kassler-Taub K, Reeves RA. Matrix study of irbesartan with hydrochlorothiazide in mild-to-moderate hypertension. Am J Hypertens 1999; 12:797–805.
- Benz JR, Black HR, Graff A, Reed A, Fitzsimmons S, Shi Y. Valsartan and hydrochlorothiazide in patients with essential hypertension. A multiple dose, double-blind, placebo controlled trial comparing combination therapy with monotherapy. J Hum Hypertens 1998; 12:861–866.
- Jounela AJ, Lilja M, Lumme J, et al. Relation between low dose of hydrochlorothiazide, antihypertensive effect and adverse effects. Blood Press 1994; 3:231–235.
- Scholze J, Breitstadt A, Cairns V, et al. Short report: ramipril and hydrochlorothiazide combination therapy in hypertension: a clinical trial of factorial design. East Germany Collaborative Trial Group. J Hypertens 1993; 11:217–221.
- Canter D, Frank GJ, Knapp LE, Phelps M, Quade M, Texter M. Quinapril and hydrochlorothiazide combination for control of hypertension: assessment by factorial design. Quinapril Investigator Group. J Hum Hypertens 1994; 8:155–162.
- Vardan S, Mehrotra KG, Mookherjee S, Willsey GA, Gens JD, Green DE. Efficacy and reduced metabolic side effects of a 15-mg chlorthalidone formulation in the treatment of mild hypertension. A multicenter study. JAMA 1987; 258:484–488.
- Materson BJ, Oster JR, Michael UF, et al. Dose response to chlorthalidone in patients with mild hypertension. Efficacy of a lower dose. Clin Pharmacol Ther 1978; 24:192–198.
- Morledge JH, Ettinger B, Aranda J, et al. Isolated systolic hypertension in the elderly. A placebo-controlled, dose-response evaluation of chlorthalidone. J Am Geriatr Soc 1986; 34:199–206.
- Peterzan MA, Hardy R, Chaturvedi N, Hughes AD. Meta-analysis of dose-response relationships for hydrochlorothiazide, chlorthalidone, and bendroflumethiazide on blood pressure, serum potassium, and urate. Hypertension 2012; 59:1104–1109.
- Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension 2006; 47:352–358.
- Bakris GL, Sica D, White WB, et al. Antihypertensive efficacy of hydrochlorothiazide vs chlorthalidone combined with azilsartan medoxomil. Am J Med 2012; 25:1229.e1–1229.e10.
- Ernst ME, Carter BL, Zheng S, Grimm RH Jr. Meta-analysis of dose-response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens 2010; 23:440–446.
- Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension 2004; 43:4–9.
- Alderman MH, Piller LB, Ford CE, et al; Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Clinical significance of incident hypokalemia and hyperkalemia in treated hypertensive patients in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Hypertension 2012; 59:926–933.
- Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly Program. Hypertension 2000; 35:1025–1030.
- Egom EE, Chirico D, Clark AL. A review of thiazide-induced hyponatraemia. Clin Med 2011; 11:448–451.
- Palmer BF. Metabolic complications associated with use of diuretics. Semin Nephrol 2011; 31:542–552.
- Hueskes BA, Roovers EA, Mantel-Teeuwisse AK, Janssens HJ, van de Lisdonk EH, Janssen M. Use of diuretics and the risk of gouty arthritis: a systematic review. Semin Arthritis Rheum 2012; 41:879–889.
- Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension 2011; 57:689–694.
- Trinkley KE, Page RL 2nd, Lien H, Yamanouye K, Tisdale JE. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin 2013; 29:1719–1726.
- Crist LW, Dixon DL. Considerations for dofetilide use in the elderly. Consult Pharm 2014; 29:270–274.
- Handler J. Lithium and antihypertensive medication: a potentially dangerous interaction. J Clin Hypertens (Greenwich) 2009; 11:738–742.
- Pavlicević I, Kuzmanić M, Rumboldt M, Rumboldt Z. Interaction between antihypertensives and NSAIDs in primary care: a controlled trial. Can J Clin Pharmacol 2008; 15:e372–e382.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension 2012; 59:1110–1117.
- Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med 2013; 158:447–455.
KEY POINTS
- Chlorthalidone has a longer duration of action and a longer half-life than hydrochlorothiazide.
- Chlorthalidone may be more potent than hydrochlorothiazide in lowering blood pressure, but it also may be associated with more metabolic adverse effects, such as hypokalemia.
- No study has conclusively shown either drug to be better in preventing adverse clinical outcomes.
- These differences should be considered when making choices about thiazide diuretic therapy for hypertension.
Patiromer cuts potassium in diabetic CKD with hyperkalemia
Patiromer, an orally administered potassium-binding polymer, significantly decreased serum potassium in adults who had diabetic kidney disease with hyperkalemia in a phase II study funded by and conducted with the manufacturer.
Patiromer consists of tiny, smooth beads in a liquid suspension and works by binding potassium throughout the GI tract, allowing it to be excreted in the feces. After preliminary studies demonstrated the agent’s usefulness in reducing hyperkalemia for up to 12 weeks in high-risk patients, researchers performed this open-label, uncontrolled trial to determine optimal dosing and to assess its longer-term safety. The findings were reported online July 14 in JAMA.
All four doses of patiromer studied significantly reduced serum potassium, beginning within 48 hours of the initial dose and continuing through all 52 weeks of treatment. The proportion of patients whose potassium levels reached target range at all scheduled visits ranged from 83% to 93% in those who had mild hyperkalemia at baseline and from 77% to 95% in those who had moderate hyperkalemia at baseline. Hyperkalemia quickly reappeared when patiromer treatment ended, reverting to baseline levels, wrote Dr. George L. Bakris of the ASH Comprehensive Hypertension Center, division of endocrinology, diabetes, and metabolism, University of Chicago, and his associates.
Study participants were 222 white patients with mild and 84 with moderate hyperkalemia (mean age, 66 years). All had type 2 diabetes and hypertension, and approximately one-third had heart failure; 65% had stage 3 and 22% had stage 4 chronic kidney disease (CKD), All were taking an ACE inhibitor, an angiotensin II receptor blocker, or both, with or without spironolactone. At 48 medical centers in five European countries, they were randomly assigned to receive one of four doses of patiromer that could be titrated up or down as needed for 4 weeks. Patients then entered an 8-week treatment phase, after which they continued maintenance therapy for a further 40 weeks (JAMA 2015 July 14 [doi:10.1001/jama.2015.7446]).
The optimal doses of patiromer were found to be the two lowest doses assessed in this study, 8.4 g daily and 16.8 g daily, which significantly decreased serum potassium without provoking hypokalemia. These doses will now be tested in a phase III study.
The most frequent treatment-related adverse events in the phase II study were nonsevere hypomagnesemia (7.2%), constipation (4.6%), and diarrhea (2.7%). A total of 28 patients (9.2%) developed at least one adverse event prompting them to discontinue patiromer, including worsening of CKD (which was considered unrelated to treatment), hypokalemia (5 patients), and one hypertensive crisis (also unrelated to treatment). No dose-related edema was noted, and there were no clinically relevant changes in serum calcium or phosphate levels.
The findings of Bakris et al. have the potential to fundamentally change the current treatment approach for hyperkalemia.
Relypsa, the manufacturer of patiromer, filed a New Drug Application with the Food and Drug Administration, and the agent will likely be approved for use in the United States by the end of October. The FDA should consider mandating a sizable postmarketing trial and safety surveillance program to clearly establish the agent’s safety and effectiveness for the hard endpoints that patients care about: halting the progression of chronic kidney disease and thus deferring dialysis, and improving heart failure. Otherwise the manufacturer may not be motivated to conduct such crucial trials, especially if it could instead spend those dollars on marketing and company-directed contract research.
Wolfgang C. Winkelmayer, M.D., Sc.D., is in the nephrology section at Baylor University, Houston, and is an associate editor at JAMA. He reported serving as an adviser or consultant to Amgen, AstraZeneca, Bayer, Keryx, Medgenics, Medtronic, Mitsubishi Tanabe, and Rockwell Pharma. Dr. Winkelmayer made these remarks in an editorial accompanying Dr. Bakris’ report (JAMA 2015 July 14;314:129-30).
The findings of Bakris et al. have the potential to fundamentally change the current treatment approach for hyperkalemia.
Relypsa, the manufacturer of patiromer, filed a New Drug Application with the Food and Drug Administration, and the agent will likely be approved for use in the United States by the end of October. The FDA should consider mandating a sizable postmarketing trial and safety surveillance program to clearly establish the agent’s safety and effectiveness for the hard endpoints that patients care about: halting the progression of chronic kidney disease and thus deferring dialysis, and improving heart failure. Otherwise the manufacturer may not be motivated to conduct such crucial trials, especially if it could instead spend those dollars on marketing and company-directed contract research.
Wolfgang C. Winkelmayer, M.D., Sc.D., is in the nephrology section at Baylor University, Houston, and is an associate editor at JAMA. He reported serving as an adviser or consultant to Amgen, AstraZeneca, Bayer, Keryx, Medgenics, Medtronic, Mitsubishi Tanabe, and Rockwell Pharma. Dr. Winkelmayer made these remarks in an editorial accompanying Dr. Bakris’ report (JAMA 2015 July 14;314:129-30).
The findings of Bakris et al. have the potential to fundamentally change the current treatment approach for hyperkalemia.
Relypsa, the manufacturer of patiromer, filed a New Drug Application with the Food and Drug Administration, and the agent will likely be approved for use in the United States by the end of October. The FDA should consider mandating a sizable postmarketing trial and safety surveillance program to clearly establish the agent’s safety and effectiveness for the hard endpoints that patients care about: halting the progression of chronic kidney disease and thus deferring dialysis, and improving heart failure. Otherwise the manufacturer may not be motivated to conduct such crucial trials, especially if it could instead spend those dollars on marketing and company-directed contract research.
Wolfgang C. Winkelmayer, M.D., Sc.D., is in the nephrology section at Baylor University, Houston, and is an associate editor at JAMA. He reported serving as an adviser or consultant to Amgen, AstraZeneca, Bayer, Keryx, Medgenics, Medtronic, Mitsubishi Tanabe, and Rockwell Pharma. Dr. Winkelmayer made these remarks in an editorial accompanying Dr. Bakris’ report (JAMA 2015 July 14;314:129-30).
Patiromer, an orally administered potassium-binding polymer, significantly decreased serum potassium in adults who had diabetic kidney disease with hyperkalemia in a phase II study funded by and conducted with the manufacturer.
Patiromer consists of tiny, smooth beads in a liquid suspension and works by binding potassium throughout the GI tract, allowing it to be excreted in the feces. After preliminary studies demonstrated the agent’s usefulness in reducing hyperkalemia for up to 12 weeks in high-risk patients, researchers performed this open-label, uncontrolled trial to determine optimal dosing and to assess its longer-term safety. The findings were reported online July 14 in JAMA.
All four doses of patiromer studied significantly reduced serum potassium, beginning within 48 hours of the initial dose and continuing through all 52 weeks of treatment. The proportion of patients whose potassium levels reached target range at all scheduled visits ranged from 83% to 93% in those who had mild hyperkalemia at baseline and from 77% to 95% in those who had moderate hyperkalemia at baseline. Hyperkalemia quickly reappeared when patiromer treatment ended, reverting to baseline levels, wrote Dr. George L. Bakris of the ASH Comprehensive Hypertension Center, division of endocrinology, diabetes, and metabolism, University of Chicago, and his associates.
Study participants were 222 white patients with mild and 84 with moderate hyperkalemia (mean age, 66 years). All had type 2 diabetes and hypertension, and approximately one-third had heart failure; 65% had stage 3 and 22% had stage 4 chronic kidney disease (CKD), All were taking an ACE inhibitor, an angiotensin II receptor blocker, or both, with or without spironolactone. At 48 medical centers in five European countries, they were randomly assigned to receive one of four doses of patiromer that could be titrated up or down as needed for 4 weeks. Patients then entered an 8-week treatment phase, after which they continued maintenance therapy for a further 40 weeks (JAMA 2015 July 14 [doi:10.1001/jama.2015.7446]).
The optimal doses of patiromer were found to be the two lowest doses assessed in this study, 8.4 g daily and 16.8 g daily, which significantly decreased serum potassium without provoking hypokalemia. These doses will now be tested in a phase III study.
The most frequent treatment-related adverse events in the phase II study were nonsevere hypomagnesemia (7.2%), constipation (4.6%), and diarrhea (2.7%). A total of 28 patients (9.2%) developed at least one adverse event prompting them to discontinue patiromer, including worsening of CKD (which was considered unrelated to treatment), hypokalemia (5 patients), and one hypertensive crisis (also unrelated to treatment). No dose-related edema was noted, and there were no clinically relevant changes in serum calcium or phosphate levels.
Patiromer, an orally administered potassium-binding polymer, significantly decreased serum potassium in adults who had diabetic kidney disease with hyperkalemia in a phase II study funded by and conducted with the manufacturer.
Patiromer consists of tiny, smooth beads in a liquid suspension and works by binding potassium throughout the GI tract, allowing it to be excreted in the feces. After preliminary studies demonstrated the agent’s usefulness in reducing hyperkalemia for up to 12 weeks in high-risk patients, researchers performed this open-label, uncontrolled trial to determine optimal dosing and to assess its longer-term safety. The findings were reported online July 14 in JAMA.
All four doses of patiromer studied significantly reduced serum potassium, beginning within 48 hours of the initial dose and continuing through all 52 weeks of treatment. The proportion of patients whose potassium levels reached target range at all scheduled visits ranged from 83% to 93% in those who had mild hyperkalemia at baseline and from 77% to 95% in those who had moderate hyperkalemia at baseline. Hyperkalemia quickly reappeared when patiromer treatment ended, reverting to baseline levels, wrote Dr. George L. Bakris of the ASH Comprehensive Hypertension Center, division of endocrinology, diabetes, and metabolism, University of Chicago, and his associates.
Study participants were 222 white patients with mild and 84 with moderate hyperkalemia (mean age, 66 years). All had type 2 diabetes and hypertension, and approximately one-third had heart failure; 65% had stage 3 and 22% had stage 4 chronic kidney disease (CKD), All were taking an ACE inhibitor, an angiotensin II receptor blocker, or both, with or without spironolactone. At 48 medical centers in five European countries, they were randomly assigned to receive one of four doses of patiromer that could be titrated up or down as needed for 4 weeks. Patients then entered an 8-week treatment phase, after which they continued maintenance therapy for a further 40 weeks (JAMA 2015 July 14 [doi:10.1001/jama.2015.7446]).
The optimal doses of patiromer were found to be the two lowest doses assessed in this study, 8.4 g daily and 16.8 g daily, which significantly decreased serum potassium without provoking hypokalemia. These doses will now be tested in a phase III study.
The most frequent treatment-related adverse events in the phase II study were nonsevere hypomagnesemia (7.2%), constipation (4.6%), and diarrhea (2.7%). A total of 28 patients (9.2%) developed at least one adverse event prompting them to discontinue patiromer, including worsening of CKD (which was considered unrelated to treatment), hypokalemia (5 patients), and one hypertensive crisis (also unrelated to treatment). No dose-related edema was noted, and there were no clinically relevant changes in serum calcium or phosphate levels.
FROM JAMA
Key clinical point: The oral potassium-binding polymer patiromer decreased serum potassium in patients who had diabetic kidney disease with hyperkalemia.
Major finding: The proportion of patients whose potassium levels remained within target range throughout 1 year of treatment was 83%-93% in those who had mild hyperkalemia at baseline and 77%-95% in those who had moderate hyperkalemia at baseline.
Data source: A multicenter open-label, noncontrolled phase II, randomized trial involving 306 adults with diabetic kidney disease and mild to moderate hyperkalemia treated for 1 year.
Disclosures: This study was funded by Relypsa, maker of patiromer. Relypsa also was involved in designing and conducting the study; collecting, analyzing, and interpreting the data; and preparing the manuscript. Dr. Bakris reported receiving personal fees from AbbVie, Takeda. Medtronic, Relypsa, Janssen, Daiichi-Sankyo, Novartis, and Bayer, as well as grants from Takeda. His associates reported ties to numerous industry sources.
Abdominal distention • loss of appetite • elevated creatinine • Dx?
THE CASE
A 21-year-old male college student sought care at our urology clinic for a 2-year history of progressive abdominal distention and loss of appetite due to abdominal pressure. On physical examination, his abdomen was distended and tense, but without any tenderness on palpation or any costovertebral angle tenderness. He had no abdominal or flank pain, and wasn’t in acute distress. His blood pressure was normal.
Initial lab test results were significant for elevated creatinine at 2.7 mg/dL (normal: 0.7-1.3 mg/dL) and blood urea nitrogen (BUN) at 31.1 mg/dL (normal: 6-20 mg/dL). Results of a complete blood count (CBC) were within normal ranges, including a white blood cell (WBC) count of 7900, hemoglobin level of 15.1 g/dL, and platelet count of 217,000/mcL. A urinalysis showed only a mild increase in the WBC count.
THE DIAGNOSIS
We performed a computed tomography (CT) scan of the patient’s abdomen, which revealed bilateral hydronephrosis secondary to ureteropelvic junction obstruction (UPJO). The patient’s right kidney was mildly to moderately enlarged, but the left kidney was massive (FIGURE 1A). The hydronephrotic left kidney had extended itself across the midline (FIGURE 1B), pushed the ipsilateral diaphragm upward, and displaced the bladder downward.
The patient underwent right-sided ureteral stent placement for temporary drainage and a complete left-sided nephrectomy. During the surgery, the left kidney was first aspirated, and more than 11,000 cc of clear urine was drained. (Aspiration reduced the kidney size, allowing the surgeon to make a smaller incision.) The removed kidney contained an additional 1200 cc of cloudy residual fluid (FIGURE 2). UPJO was confirmed by the pathological examination of the excised organ.
DISCUSSION
UPJO is the most common etiology for congenital hydronephrosis.1 Because it can cause little to no pain, hydronephrosis secondary to UPJO can be asymptomatic and may not present until later in life. Frequently, an abdominal mass is the initial clinical presentation.
When the hydronephrotic fluid exceeds 1000 cc, the condition is referred to as giant hydronephrosis.2 Although several cases of giant hydronephrosis secondary to UPJO have been reported in the medical literature,3-5 the volume of the hydronephrotic fluid in these cases rarely exceeded 10,000 cc. We believe our patient may be the most severe case of hydronephrosis secondary to bilateral UPJO, with 12,200 cc of fluid. His condition reached this late stage only because his right kidney retained adequate function.
Diagnosis of hydronephrosis is straightforward with an abdominal ultrasound and/or CT scan. Widespread use of abdominal ultrasound as a screening tool has significantly increased the diagnosis of asymptomatic hydronephrosis, and many cases are secondary to UPJO.6 The true incidence of UPJO is unknown, but it is more prevalent in males than in females, and in 10% to 40% of cases, the condition is bilateral.7 Congenital UPJO typically results from intrinsic pathology of the ureter. The diseased segment is often fibrotic, strictured, and aperistaltic.8
Treatment choice depends on whether renal function can be preserved
Treatment of hydronephrosis is straightforward; when there is little or no salvageable renal function (<10%), a simple nephrectomy is indicated, as was the case for our patient. Nephrectomy can be accomplished by either an open or laparoscopic approach.
When there is salvageable renal function, treatment options include pyeloplasty and pyelotomy. Traditionally, open dismembered pyeloplasty has been the gold standard. However, with advances in endoscopic and laparoscopic techniques, there has been a shift toward minimally invasive procedures. Laparoscopic pyeloplasty—with or without robotic assistance—and endoscopic pyelotomy—with either a percutaneous or retrograde approach—are now typically performed. Ureteral stenting should only be used as a temporary measure.
Our patient. Four weeks after the nephrectomy, our patient underwent a right side pyeloplasty, which was successful. He had an uneventful recovery from both procedures. His renal function stabilized and other than routine follow-up, he required no additional treatment.
THE TAKEAWAY
Most cases of hydronephrosis in young people are due to congenital abnormalities, and UPJO is the leading cause. However, the condition can be asymptomatic and may not present until later in life. Whenever a patient presents with an asymptomatic abdominal mass, hydronephrosis should be part of the differential diagnosis. Treatment options include nephrectomy when there is no salvageable kidney function or pyeloplasty and pyelotomy when some kidney function can be preserved.
1. Brown T, Mandell J, Lebowitz RL. Neonatal hydronephrosis in the era ultrasonography. AJR Am J Roentgenol. 1987;148:959-963.
2. Stirling WC. Massive hydronephrosis complicated by hydroureter: Report of 3 cases. J Urol. 1939;42:520.
3. Chiang PH, Chen MT, Chou YH, et al. Giant hydronephrosis: report of 4 cases with review of the literature. J Formos Med Assoc. 1990;89:811-817.
4. Aguiar MFM, Oliveira APS, Silva SC, et al. Giant hydronephrosis secondary to ureteropelvic junction obstruction. Gazzetta Medica Italiana-Archivio per le Scienze Mediche. 2009;168:207.
5. Sepulveda L, Rodriguesa F. Giant hydronephrosis - a late diagnosis of ureteropelvic junction obstruction. World J Nephrol Urol. 2013;2:33.
6. Bernstein GT, Mandell J, Lebowitz RL, et al. Ureteropelvic junction obstruction in neonate. J Urol. 1988;140:1216-1221.
7. Johnston JH, Evans JP, Glassberg KI, et al. Pelvic hydronephrosis in children: a review of 219 personal cases. J Urol. 1977;117:97-101.
8. Gosling JA, Dixon JS. Functional obstruction of the ureter and renal pelvis. A histological and electron microscopic study. Br J Urol. 1978;50:145-152.
THE CASE
A 21-year-old male college student sought care at our urology clinic for a 2-year history of progressive abdominal distention and loss of appetite due to abdominal pressure. On physical examination, his abdomen was distended and tense, but without any tenderness on palpation or any costovertebral angle tenderness. He had no abdominal or flank pain, and wasn’t in acute distress. His blood pressure was normal.
Initial lab test results were significant for elevated creatinine at 2.7 mg/dL (normal: 0.7-1.3 mg/dL) and blood urea nitrogen (BUN) at 31.1 mg/dL (normal: 6-20 mg/dL). Results of a complete blood count (CBC) were within normal ranges, including a white blood cell (WBC) count of 7900, hemoglobin level of 15.1 g/dL, and platelet count of 217,000/mcL. A urinalysis showed only a mild increase in the WBC count.
THE DIAGNOSIS
We performed a computed tomography (CT) scan of the patient’s abdomen, which revealed bilateral hydronephrosis secondary to ureteropelvic junction obstruction (UPJO). The patient’s right kidney was mildly to moderately enlarged, but the left kidney was massive (FIGURE 1A). The hydronephrotic left kidney had extended itself across the midline (FIGURE 1B), pushed the ipsilateral diaphragm upward, and displaced the bladder downward.
The patient underwent right-sided ureteral stent placement for temporary drainage and a complete left-sided nephrectomy. During the surgery, the left kidney was first aspirated, and more than 11,000 cc of clear urine was drained. (Aspiration reduced the kidney size, allowing the surgeon to make a smaller incision.) The removed kidney contained an additional 1200 cc of cloudy residual fluid (FIGURE 2). UPJO was confirmed by the pathological examination of the excised organ.
DISCUSSION
UPJO is the most common etiology for congenital hydronephrosis.1 Because it can cause little to no pain, hydronephrosis secondary to UPJO can be asymptomatic and may not present until later in life. Frequently, an abdominal mass is the initial clinical presentation.
When the hydronephrotic fluid exceeds 1000 cc, the condition is referred to as giant hydronephrosis.2 Although several cases of giant hydronephrosis secondary to UPJO have been reported in the medical literature,3-5 the volume of the hydronephrotic fluid in these cases rarely exceeded 10,000 cc. We believe our patient may be the most severe case of hydronephrosis secondary to bilateral UPJO, with 12,200 cc of fluid. His condition reached this late stage only because his right kidney retained adequate function.
Diagnosis of hydronephrosis is straightforward with an abdominal ultrasound and/or CT scan. Widespread use of abdominal ultrasound as a screening tool has significantly increased the diagnosis of asymptomatic hydronephrosis, and many cases are secondary to UPJO.6 The true incidence of UPJO is unknown, but it is more prevalent in males than in females, and in 10% to 40% of cases, the condition is bilateral.7 Congenital UPJO typically results from intrinsic pathology of the ureter. The diseased segment is often fibrotic, strictured, and aperistaltic.8
Treatment choice depends on whether renal function can be preserved
Treatment of hydronephrosis is straightforward; when there is little or no salvageable renal function (<10%), a simple nephrectomy is indicated, as was the case for our patient. Nephrectomy can be accomplished by either an open or laparoscopic approach.
When there is salvageable renal function, treatment options include pyeloplasty and pyelotomy. Traditionally, open dismembered pyeloplasty has been the gold standard. However, with advances in endoscopic and laparoscopic techniques, there has been a shift toward minimally invasive procedures. Laparoscopic pyeloplasty—with or without robotic assistance—and endoscopic pyelotomy—with either a percutaneous or retrograde approach—are now typically performed. Ureteral stenting should only be used as a temporary measure.
Our patient. Four weeks after the nephrectomy, our patient underwent a right side pyeloplasty, which was successful. He had an uneventful recovery from both procedures. His renal function stabilized and other than routine follow-up, he required no additional treatment.
THE TAKEAWAY
Most cases of hydronephrosis in young people are due to congenital abnormalities, and UPJO is the leading cause. However, the condition can be asymptomatic and may not present until later in life. Whenever a patient presents with an asymptomatic abdominal mass, hydronephrosis should be part of the differential diagnosis. Treatment options include nephrectomy when there is no salvageable kidney function or pyeloplasty and pyelotomy when some kidney function can be preserved.
THE CASE
A 21-year-old male college student sought care at our urology clinic for a 2-year history of progressive abdominal distention and loss of appetite due to abdominal pressure. On physical examination, his abdomen was distended and tense, but without any tenderness on palpation or any costovertebral angle tenderness. He had no abdominal or flank pain, and wasn’t in acute distress. His blood pressure was normal.
Initial lab test results were significant for elevated creatinine at 2.7 mg/dL (normal: 0.7-1.3 mg/dL) and blood urea nitrogen (BUN) at 31.1 mg/dL (normal: 6-20 mg/dL). Results of a complete blood count (CBC) were within normal ranges, including a white blood cell (WBC) count of 7900, hemoglobin level of 15.1 g/dL, and platelet count of 217,000/mcL. A urinalysis showed only a mild increase in the WBC count.
THE DIAGNOSIS
We performed a computed tomography (CT) scan of the patient’s abdomen, which revealed bilateral hydronephrosis secondary to ureteropelvic junction obstruction (UPJO). The patient’s right kidney was mildly to moderately enlarged, but the left kidney was massive (FIGURE 1A). The hydronephrotic left kidney had extended itself across the midline (FIGURE 1B), pushed the ipsilateral diaphragm upward, and displaced the bladder downward.
The patient underwent right-sided ureteral stent placement for temporary drainage and a complete left-sided nephrectomy. During the surgery, the left kidney was first aspirated, and more than 11,000 cc of clear urine was drained. (Aspiration reduced the kidney size, allowing the surgeon to make a smaller incision.) The removed kidney contained an additional 1200 cc of cloudy residual fluid (FIGURE 2). UPJO was confirmed by the pathological examination of the excised organ.
DISCUSSION
UPJO is the most common etiology for congenital hydronephrosis.1 Because it can cause little to no pain, hydronephrosis secondary to UPJO can be asymptomatic and may not present until later in life. Frequently, an abdominal mass is the initial clinical presentation.
When the hydronephrotic fluid exceeds 1000 cc, the condition is referred to as giant hydronephrosis.2 Although several cases of giant hydronephrosis secondary to UPJO have been reported in the medical literature,3-5 the volume of the hydronephrotic fluid in these cases rarely exceeded 10,000 cc. We believe our patient may be the most severe case of hydronephrosis secondary to bilateral UPJO, with 12,200 cc of fluid. His condition reached this late stage only because his right kidney retained adequate function.
Diagnosis of hydronephrosis is straightforward with an abdominal ultrasound and/or CT scan. Widespread use of abdominal ultrasound as a screening tool has significantly increased the diagnosis of asymptomatic hydronephrosis, and many cases are secondary to UPJO.6 The true incidence of UPJO is unknown, but it is more prevalent in males than in females, and in 10% to 40% of cases, the condition is bilateral.7 Congenital UPJO typically results from intrinsic pathology of the ureter. The diseased segment is often fibrotic, strictured, and aperistaltic.8
Treatment choice depends on whether renal function can be preserved
Treatment of hydronephrosis is straightforward; when there is little or no salvageable renal function (<10%), a simple nephrectomy is indicated, as was the case for our patient. Nephrectomy can be accomplished by either an open or laparoscopic approach.
When there is salvageable renal function, treatment options include pyeloplasty and pyelotomy. Traditionally, open dismembered pyeloplasty has been the gold standard. However, with advances in endoscopic and laparoscopic techniques, there has been a shift toward minimally invasive procedures. Laparoscopic pyeloplasty—with or without robotic assistance—and endoscopic pyelotomy—with either a percutaneous or retrograde approach—are now typically performed. Ureteral stenting should only be used as a temporary measure.
Our patient. Four weeks after the nephrectomy, our patient underwent a right side pyeloplasty, which was successful. He had an uneventful recovery from both procedures. His renal function stabilized and other than routine follow-up, he required no additional treatment.
THE TAKEAWAY
Most cases of hydronephrosis in young people are due to congenital abnormalities, and UPJO is the leading cause. However, the condition can be asymptomatic and may not present until later in life. Whenever a patient presents with an asymptomatic abdominal mass, hydronephrosis should be part of the differential diagnosis. Treatment options include nephrectomy when there is no salvageable kidney function or pyeloplasty and pyelotomy when some kidney function can be preserved.
1. Brown T, Mandell J, Lebowitz RL. Neonatal hydronephrosis in the era ultrasonography. AJR Am J Roentgenol. 1987;148:959-963.
2. Stirling WC. Massive hydronephrosis complicated by hydroureter: Report of 3 cases. J Urol. 1939;42:520.
3. Chiang PH, Chen MT, Chou YH, et al. Giant hydronephrosis: report of 4 cases with review of the literature. J Formos Med Assoc. 1990;89:811-817.
4. Aguiar MFM, Oliveira APS, Silva SC, et al. Giant hydronephrosis secondary to ureteropelvic junction obstruction. Gazzetta Medica Italiana-Archivio per le Scienze Mediche. 2009;168:207.
5. Sepulveda L, Rodriguesa F. Giant hydronephrosis - a late diagnosis of ureteropelvic junction obstruction. World J Nephrol Urol. 2013;2:33.
6. Bernstein GT, Mandell J, Lebowitz RL, et al. Ureteropelvic junction obstruction in neonate. J Urol. 1988;140:1216-1221.
7. Johnston JH, Evans JP, Glassberg KI, et al. Pelvic hydronephrosis in children: a review of 219 personal cases. J Urol. 1977;117:97-101.
8. Gosling JA, Dixon JS. Functional obstruction of the ureter and renal pelvis. A histological and electron microscopic study. Br J Urol. 1978;50:145-152.
1. Brown T, Mandell J, Lebowitz RL. Neonatal hydronephrosis in the era ultrasonography. AJR Am J Roentgenol. 1987;148:959-963.
2. Stirling WC. Massive hydronephrosis complicated by hydroureter: Report of 3 cases. J Urol. 1939;42:520.
3. Chiang PH, Chen MT, Chou YH, et al. Giant hydronephrosis: report of 4 cases with review of the literature. J Formos Med Assoc. 1990;89:811-817.
4. Aguiar MFM, Oliveira APS, Silva SC, et al. Giant hydronephrosis secondary to ureteropelvic junction obstruction. Gazzetta Medica Italiana-Archivio per le Scienze Mediche. 2009;168:207.
5. Sepulveda L, Rodriguesa F. Giant hydronephrosis - a late diagnosis of ureteropelvic junction obstruction. World J Nephrol Urol. 2013;2:33.
6. Bernstein GT, Mandell J, Lebowitz RL, et al. Ureteropelvic junction obstruction in neonate. J Urol. 1988;140:1216-1221.
7. Johnston JH, Evans JP, Glassberg KI, et al. Pelvic hydronephrosis in children: a review of 219 personal cases. J Urol. 1977;117:97-101.
8. Gosling JA, Dixon JS. Functional obstruction of the ureter and renal pelvis. A histological and electron microscopic study. Br J Urol. 1978;50:145-152.
Understanding Hematuria: Causes
Q) I have been treating a 60-year-old man with a long history of microscopic hematuria and waxing/waning proteinuria. What could be the cause of his hematuria?
Hematuria is a consequence of erythrocytes, or red blood cells (RBCs), in the urine. This can cause a visible change in color, considered gross or macroscopic hematuria; or the blood may only be visible under microscopy or by urine dipstick (referred to as microscopic hematuria).
Both findings are followed up with urinalysis to quantify erythrocytes, protein, and presence of casts and to review RBC morphology. This information will assist in determining if the hematuria is glomerular or nonglomerular in origin.1
The examination and treatment plan for nonglomerular hematuria will focus on urinary tract diseases. If the patient is found to have glomerular hematuria, the focus will be on diseases of the kidney. A thorough history and physical should be performed in addition to urinalysis.
Glomerular disease is suggested in those with micro- or macroscopic proteinuria, proteinuria > 1 g/24h, or an absence of casts. Our index patient has microscopic hematuria and “waxing/waning” (unquantified) proteinuria, suggesting glomerular origin.
There are a number of renal causes for glomerular bleeding, including primary glomerulonephritis, multisystem autoimmune disease, and hereditary or infective glomerulonephritis.2 Renal biopsy is recommended for patients who have hypertension, proteinuria, and hematuria, to determine the cause and thus determine the appropriate treatment.
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
Q) I have been treating a 60-year-old man with a long history of microscopic hematuria and waxing/waning proteinuria. What could be the cause of his hematuria?
Hematuria is a consequence of erythrocytes, or red blood cells (RBCs), in the urine. This can cause a visible change in color, considered gross or macroscopic hematuria; or the blood may only be visible under microscopy or by urine dipstick (referred to as microscopic hematuria).
Both findings are followed up with urinalysis to quantify erythrocytes, protein, and presence of casts and to review RBC morphology. This information will assist in determining if the hematuria is glomerular or nonglomerular in origin.1
The examination and treatment plan for nonglomerular hematuria will focus on urinary tract diseases. If the patient is found to have glomerular hematuria, the focus will be on diseases of the kidney. A thorough history and physical should be performed in addition to urinalysis.
Glomerular disease is suggested in those with micro- or macroscopic proteinuria, proteinuria > 1 g/24h, or an absence of casts. Our index patient has microscopic hematuria and “waxing/waning” (unquantified) proteinuria, suggesting glomerular origin.
There are a number of renal causes for glomerular bleeding, including primary glomerulonephritis, multisystem autoimmune disease, and hereditary or infective glomerulonephritis.2 Renal biopsy is recommended for patients who have hypertension, proteinuria, and hematuria, to determine the cause and thus determine the appropriate treatment.
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
Q) I have been treating a 60-year-old man with a long history of microscopic hematuria and waxing/waning proteinuria. What could be the cause of his hematuria?
Hematuria is a consequence of erythrocytes, or red blood cells (RBCs), in the urine. This can cause a visible change in color, considered gross or macroscopic hematuria; or the blood may only be visible under microscopy or by urine dipstick (referred to as microscopic hematuria).
Both findings are followed up with urinalysis to quantify erythrocytes, protein, and presence of casts and to review RBC morphology. This information will assist in determining if the hematuria is glomerular or nonglomerular in origin.1
The examination and treatment plan for nonglomerular hematuria will focus on urinary tract diseases. If the patient is found to have glomerular hematuria, the focus will be on diseases of the kidney. A thorough history and physical should be performed in addition to urinalysis.
Glomerular disease is suggested in those with micro- or macroscopic proteinuria, proteinuria > 1 g/24h, or an absence of casts. Our index patient has microscopic hematuria and “waxing/waning” (unquantified) proteinuria, suggesting glomerular origin.
There are a number of renal causes for glomerular bleeding, including primary glomerulonephritis, multisystem autoimmune disease, and hereditary or infective glomerulonephritis.2 Renal biopsy is recommended for patients who have hypertension, proteinuria, and hematuria, to determine the cause and thus determine the appropriate treatment.
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
Understanding Hematuria: IgA Nephropathy
Q) My hematuria patient had more significant proteinuria recently, so the nephrologist sent him for kidney biopsy. It was read as IgA nephropathy: “classic mesangial staining on IF with moderate-advanced chronic injury (15/32 gloms globally sclerosed, 40% IFTA, mild arteriosclerosis).” What exactly does this mean, and what is IgA nephropathy?
Immunoglobulin A (IgA) nephropathy is the most common type of glomerulonephritis; up to 40% of patients with IgA nephropathy develop end-stage renal disease within 20 years of diagnosis. More common in men, IgA nephropathy is usually diagnosed in people in their second or third decades of life.2,3
Considered an autoimmune disease, IgA nephropathy typically presents with macroscopic or gross hematuria that occurs within 24 hours of the onset of an upper respiratory infection (URI). The hematuria typically resolves quickly, in one to three days. An individual bacterial or viral element has not yet been identified.
IgA nephropathy is an immune response to the URI. IgA is secreted from mucosal surfaces at the back of the mouth and then deposited in the glomerular mesangium, a “stalk of cells” associated with the capillaries of the renal glomerulus.1 It is speculated that genetics, environment, and/or hypersensitivity to food antigens may play a part in IgA nephropathy. Results from biopsies of blood relatives of patients with confirmed IgA nephropathy suggest a familial role.1
IgA nephropathy is prevalent in persons who live in the Pacific Rim and Southern Europe. However, this association may be the result of a sampling error due to investigation of all microscopic hematuria in these areas. In all, 90% of IgA is sporadic.4 It is often asymptomatic, aside from occasional back and flank pain secondary to inflammation of the renal capsule. Unfortunately, many patients develop renal impairment and hypertension by the time they are diagnosed.
Renal biopsy is used to confirm/diagnose IgA nephropathy. IgA, deposited in the mesangium of the glomerulus, lights up under immunofluorescence (IF; see Figure 1). In some patients, this mesangial deposition results in sclerosis, scarring, and/or inflammation of the glomerulus (see Figure 2).
An international panel of experts created guidelines (the Oxford classification system) for reporting IgA kidney biopsies. Six adverse pathologic features have been identified:
• Mesangial cellularity score
• Percentage of segmental sclerosis
• Endocapillary hypercellularity
• Cellular and/or fibrocellular crescents
• Percentage of interstitial fibrosis/tubular atrophy (IFTA)
• Arteriosclerosis score5,6
Interstitial fibrosis, crescents, and as little as 25% glomerular sclerosis found on biopsy increases the likelihood of disease progression.5 Clinically, hypertension, a reduced glomerular filtration rate, increasing age, and proteinuria of > 1g/24h have been identified as risk factors for progression of IgA nephropathy. Up to 30% of patients diagnosed will require renal replacement therapy within 20 years.1
The case patient’s findings include the typical IF staining of IgA in the glomerulus. The biopsy report also indicates that 40% of the glomeruli (less than half) have interstitial fibrosis and that the structural integrity of the tubules has been affected secondary to IgA accumulation in the mesangium. These findings are suggestive of progressive disease.
There is no known way to stop IgA deposition in the mesangium. Tonsillectomy to reduce mucosal IgA release has been suggested but is controversial.
Treatment of IgA nephropathy focuses on preserving renal function by reducing proteinuria through the use of ACE inhibitors and/or angiotensin receptor blockers. Aggressive blood pressure management is achieved by blocking the renin-angiotensin-aldosterone system (RAAS).
Other methods for decreasing progression of renal disease are directed at reducing the immune and inflammatory response via immunosuppressant medications.3 The use of immunosuppressive agents, though controversial, is recommended for those who have progressive disease and/or proteinuria despite achieving target blood pressure with full RAAS blockade.1
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
3. Barratt J, Feehally J. Treatment of IgA nephropathy. Kidney Int. 2006;69(11):1934-1938.
4. Johnson R, Feehally J. Comprehensive Clinical Nephrology. 2nd ed. London: Mosby; 2000.
5. Walsh M, Sar A, Lee D, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010; 5(3):425-430.
6. Roberts I. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009; 76(5):546-556.
Q) My hematuria patient had more significant proteinuria recently, so the nephrologist sent him for kidney biopsy. It was read as IgA nephropathy: “classic mesangial staining on IF with moderate-advanced chronic injury (15/32 gloms globally sclerosed, 40% IFTA, mild arteriosclerosis).” What exactly does this mean, and what is IgA nephropathy?
Immunoglobulin A (IgA) nephropathy is the most common type of glomerulonephritis; up to 40% of patients with IgA nephropathy develop end-stage renal disease within 20 years of diagnosis. More common in men, IgA nephropathy is usually diagnosed in people in their second or third decades of life.2,3
Considered an autoimmune disease, IgA nephropathy typically presents with macroscopic or gross hematuria that occurs within 24 hours of the onset of an upper respiratory infection (URI). The hematuria typically resolves quickly, in one to three days. An individual bacterial or viral element has not yet been identified.
IgA nephropathy is an immune response to the URI. IgA is secreted from mucosal surfaces at the back of the mouth and then deposited in the glomerular mesangium, a “stalk of cells” associated with the capillaries of the renal glomerulus.1 It is speculated that genetics, environment, and/or hypersensitivity to food antigens may play a part in IgA nephropathy. Results from biopsies of blood relatives of patients with confirmed IgA nephropathy suggest a familial role.1
IgA nephropathy is prevalent in persons who live in the Pacific Rim and Southern Europe. However, this association may be the result of a sampling error due to investigation of all microscopic hematuria in these areas. In all, 90% of IgA is sporadic.4 It is often asymptomatic, aside from occasional back and flank pain secondary to inflammation of the renal capsule. Unfortunately, many patients develop renal impairment and hypertension by the time they are diagnosed.
Renal biopsy is used to confirm/diagnose IgA nephropathy. IgA, deposited in the mesangium of the glomerulus, lights up under immunofluorescence (IF; see Figure 1). In some patients, this mesangial deposition results in sclerosis, scarring, and/or inflammation of the glomerulus (see Figure 2).
An international panel of experts created guidelines (the Oxford classification system) for reporting IgA kidney biopsies. Six adverse pathologic features have been identified:
• Mesangial cellularity score
• Percentage of segmental sclerosis
• Endocapillary hypercellularity
• Cellular and/or fibrocellular crescents
• Percentage of interstitial fibrosis/tubular atrophy (IFTA)
• Arteriosclerosis score5,6
Interstitial fibrosis, crescents, and as little as 25% glomerular sclerosis found on biopsy increases the likelihood of disease progression.5 Clinically, hypertension, a reduced glomerular filtration rate, increasing age, and proteinuria of > 1g/24h have been identified as risk factors for progression of IgA nephropathy. Up to 30% of patients diagnosed will require renal replacement therapy within 20 years.1
The case patient’s findings include the typical IF staining of IgA in the glomerulus. The biopsy report also indicates that 40% of the glomeruli (less than half) have interstitial fibrosis and that the structural integrity of the tubules has been affected secondary to IgA accumulation in the mesangium. These findings are suggestive of progressive disease.
There is no known way to stop IgA deposition in the mesangium. Tonsillectomy to reduce mucosal IgA release has been suggested but is controversial.
Treatment of IgA nephropathy focuses on preserving renal function by reducing proteinuria through the use of ACE inhibitors and/or angiotensin receptor blockers. Aggressive blood pressure management is achieved by blocking the renin-angiotensin-aldosterone system (RAAS).
Other methods for decreasing progression of renal disease are directed at reducing the immune and inflammatory response via immunosuppressant medications.3 The use of immunosuppressive agents, though controversial, is recommended for those who have progressive disease and/or proteinuria despite achieving target blood pressure with full RAAS blockade.1
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
3. Barratt J, Feehally J. Treatment of IgA nephropathy. Kidney Int. 2006;69(11):1934-1938.
4. Johnson R, Feehally J. Comprehensive Clinical Nephrology. 2nd ed. London: Mosby; 2000.
5. Walsh M, Sar A, Lee D, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010; 5(3):425-430.
6. Roberts I. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009; 76(5):546-556.
Q) My hematuria patient had more significant proteinuria recently, so the nephrologist sent him for kidney biopsy. It was read as IgA nephropathy: “classic mesangial staining on IF with moderate-advanced chronic injury (15/32 gloms globally sclerosed, 40% IFTA, mild arteriosclerosis).” What exactly does this mean, and what is IgA nephropathy?
Immunoglobulin A (IgA) nephropathy is the most common type of glomerulonephritis; up to 40% of patients with IgA nephropathy develop end-stage renal disease within 20 years of diagnosis. More common in men, IgA nephropathy is usually diagnosed in people in their second or third decades of life.2,3
Considered an autoimmune disease, IgA nephropathy typically presents with macroscopic or gross hematuria that occurs within 24 hours of the onset of an upper respiratory infection (URI). The hematuria typically resolves quickly, in one to three days. An individual bacterial or viral element has not yet been identified.
IgA nephropathy is an immune response to the URI. IgA is secreted from mucosal surfaces at the back of the mouth and then deposited in the glomerular mesangium, a “stalk of cells” associated with the capillaries of the renal glomerulus.1 It is speculated that genetics, environment, and/or hypersensitivity to food antigens may play a part in IgA nephropathy. Results from biopsies of blood relatives of patients with confirmed IgA nephropathy suggest a familial role.1
IgA nephropathy is prevalent in persons who live in the Pacific Rim and Southern Europe. However, this association may be the result of a sampling error due to investigation of all microscopic hematuria in these areas. In all, 90% of IgA is sporadic.4 It is often asymptomatic, aside from occasional back and flank pain secondary to inflammation of the renal capsule. Unfortunately, many patients develop renal impairment and hypertension by the time they are diagnosed.
Renal biopsy is used to confirm/diagnose IgA nephropathy. IgA, deposited in the mesangium of the glomerulus, lights up under immunofluorescence (IF; see Figure 1). In some patients, this mesangial deposition results in sclerosis, scarring, and/or inflammation of the glomerulus (see Figure 2).
An international panel of experts created guidelines (the Oxford classification system) for reporting IgA kidney biopsies. Six adverse pathologic features have been identified:
• Mesangial cellularity score
• Percentage of segmental sclerosis
• Endocapillary hypercellularity
• Cellular and/or fibrocellular crescents
• Percentage of interstitial fibrosis/tubular atrophy (IFTA)
• Arteriosclerosis score5,6
Interstitial fibrosis, crescents, and as little as 25% glomerular sclerosis found on biopsy increases the likelihood of disease progression.5 Clinically, hypertension, a reduced glomerular filtration rate, increasing age, and proteinuria of > 1g/24h have been identified as risk factors for progression of IgA nephropathy. Up to 30% of patients diagnosed will require renal replacement therapy within 20 years.1
The case patient’s findings include the typical IF staining of IgA in the glomerulus. The biopsy report also indicates that 40% of the glomeruli (less than half) have interstitial fibrosis and that the structural integrity of the tubules has been affected secondary to IgA accumulation in the mesangium. These findings are suggestive of progressive disease.
There is no known way to stop IgA deposition in the mesangium. Tonsillectomy to reduce mucosal IgA release has been suggested but is controversial.
Treatment of IgA nephropathy focuses on preserving renal function by reducing proteinuria through the use of ACE inhibitors and/or angiotensin receptor blockers. Aggressive blood pressure management is achieved by blocking the renin-angiotensin-aldosterone system (RAAS).
Other methods for decreasing progression of renal disease are directed at reducing the immune and inflammatory response via immunosuppressant medications.3 The use of immunosuppressive agents, though controversial, is recommended for those who have progressive disease and/or proteinuria despite achieving target blood pressure with full RAAS blockade.1
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
3. Barratt J, Feehally J. Treatment of IgA nephropathy. Kidney Int. 2006;69(11):1934-1938.
4. Johnson R, Feehally J. Comprehensive Clinical Nephrology. 2nd ed. London: Mosby; 2000.
5. Walsh M, Sar A, Lee D, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010; 5(3):425-430.
6. Roberts I. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009; 76(5):546-556.
Urologic applications of botulinum toxin
Patients with loss of bladder control experience discomfort, embarrassment, personal care and health issues, and, often, significant pain, all with a decidedly negative impact on quality of life. Although some patients may find lifestyle modifications, drug therapy, and self-catheterization acceptable and effective, there is a clear need for more options.
Botulinum toxin, or onabotulinumtoxinA, is currently approved by the US Food and Drug Administration (FDA) for neurogenic detrusor overactivity and overactive bladder refractory to drug therapy. Studies so far have shown botulinum toxin injection to be safe and effective for these conditions, and these results have led to interest in off-label uses, eg, for detrusor external sphincter dyssynergia (DESD), motor and sensory urgency, and painful bladder syndrome/interstitial cystitis (Table 1).
Although more data from clinical trials are needed, botulinum toxin injection offers patients a much-needed treatment option.
HOW BOTULINUM TOXIN WORKS
Seven serotypes identified
Discovered in 1897, botulinum toxin is a neurotoxin produced by the gram-positive, rod-shaped anaerobic bacterium Clostridium botulinum1 and is the most poisonous naturally occurring toxin known.2 Seven immunologically distinct antigenic serotypes have been identified (A, B, C1, D, E, F, and G),1 but only types A and B are available for clinical use.
Most research into potential therapeutic uses has focused on type A, which has the longest duration of action, a clinical advantage.3 Recently, work has been done to further characterize other serotypes and to isolate additional variants of botulinum toxin. For example, serotype E, the predominant serotype associated with foodborne botulism, is being studied in an effort to prevent future outbreaks.4
Our discussion focuses on clinical uses of the serotype A botulinum toxin preparation, which we will refer to simply as botulinum toxin.
Studies exploring how it works
Botulinum toxin exerts its effects by binding to peripheral cholinergic terminals, inhibiting release of acetylcholine at the neuromuscular junction. Flaccid paralysis ensues as a result.
Results of animal studies have shed additional light on the specific actions of botulinum toxin A:
- It may alter levels of nerve growth factor and transient receptor potential vanilloid 1 in rats, and this may provide an additional mechanism of reducing bladder detrusor overactivity.5
- In addition to blocking acetylcholine release from motor neurons, it inhibits the release of neurotransmitters involved in bladder sensory afferent pathways.6
- It inhibits the release of substance P and glutamate, neuropeptides involved in sensory and nociceptive pathways.6,7
- It promotes apoptosis in prostatic tissue; however, this effect has not been shown in the bladder.3
The time necessary to recover function after botulinum toxin paralysis depends on the subtype of botulinum toxin as well as on the type of nerve terminal. Chemodenervation lasts from 3 to 6 months when the toxin is injected into the neuromuscular junction of skeletal muscle, and considerably longer (up to 1 year) when injected into the autonomic neurons of smooth muscle.2,6
TREATMENT OF NEUROGENIC DETRUSOR OVERACTIVITY
Neurogenic detrusor overactivity involves involuntary contractions of the bladder resulting from spinal cord injury, multiple sclerosis, and other neurologic conditions. An estimated 273,000 people in the United States have a spinal cord injury, and 81% of them have urologic symptoms ranging from areflexia to overactivity.8 From 75% to 100% of patients with multiple sclerosis have urologic symptoms, and detrusor overactivity is the most common.9
Detrusor overactivity can cause urinary urgency, urinary frequency, and urgency incontinence, significantly affecting quality of life and leading to skin breakdown, sacral ulcerations, and challenges with personal care.
Anticholinergic drugs have been the mainstay of therapy. If drug therapy failed, the next option was reconstructive surgery, often augmentation cystoplasty. Thus, botulinum toxin injection is an important advance in treatment options.
Studies that showed effectiveness
Botulinum toxin for neurogenic detrusor overactivity was first studied by Schurch et al.10 In their study, 200 U or 300 U was injected into the trigone of 21 patients with spinal cord injury and urgency incontinence managed with intermittent self-catheterization.10 At 6 weeks after injection, 17 of the 19 patients seen at follow-up visits were completely continent. Urodynamic evaluation revealed significant increases in maximum cystometric capacity and in volume at first involuntary detrusor contraction, and a decrease in detrusor voiding pressure. Of the 11 patients available for follow-up at 16 and 36 weeks, improvements in measures of incontinence and urodynamic function persisted.
In addition, two small randomized controlled trials11,12 showed significant increases in cystometric bladder capacity, significant improvement in quality-of-life measures, and reduction in episodes of urgency incontinence.
In 2011 and 2012, two multicenter double-blind randomized controlled trials reported on patients with multiple sclerosis and spinal cord injury with neurogenic detrusor overactivity inadequately managed with drug therapy. The patients were randomized to botulinum toxin injection (200 U or 300 U) or placebo injection.13,14 The primary end point for both studies was the change from baseline in episodes of urinary incontinence per week at week 6. Secondary end points were maximum cystometric capacity, maximum detrusor pressure during first involuntary detrusor contraction, and score on the Incontinence Quality of Life scale.15
In both studies, the mean number of urinary incontinence episodes per week was 33 at baseline. At week 6, Cruz et al14 found that patients who received botulinum toxin injection had significantly fewer episodes per week (21.8 fewer with 200 U, 19.4 fewer with 300 U) than those in the placebo group, who had 13.2 fewer episodes per week (P < .01). Ginsberg et al13 reported decreases in the mean number of episodes of urinary incontinence of 21, 23, and 9 episodes per week in the 200 U, 300 U, and placebo groups, respectively (P < .001). The patients who received botulinum toxin had statistically significant improvements in maximum cystometric capacity, maximum detrusor pressure during first involuntary detrusor contraction, and Incontinence Quality of Life scores compared with placebo (P < .001). Thirty-eight percent of patients in the treatment group were fully continent.13,14
Safety and adverse effects
The most frequently reported adverse events were urinary tract infection (24% of patients)13,14 and urinary retention requiring initiation of clean intermittent catheterization. In the study by Cruz et al,14 these were reported in 30% with 200 U, 42% with 300 U, and 12% with placebo, while in the study by Ginsberg et al13 they were reported in 35% with 200 U, 42% with 300 U, and 10% with placebo.
In a study of long-term safety and efficacy of botulinum toxin injection in patients with neurogenic detrusor overactivity, Kennelly et al16 found that patients undergoing repeat injections had sustained reductions in episodes of incontinence and increases in the maximum cystometric capacity and quality of life scores, with no increase in adverse events over time.16
But is it cost-effective?
While botulinum toxin injection may be safe and effective for neurogenic detrusor overactivity, is it cost-effective?
Carlson et al17 used a Markov State Transition model to assess the cost of refractory neurogenic detrusor overactivity in patients receiving botulinum toxin vs best supportive care (incontinence pads, medications, intermittent self-catheterization).17 They found that the injections were more expensive than supportive care but were cost-effective when considering the reduction in episodes of incontinence, the reduced need for incontinence products, and improvement in measures of quality of life.
What the evidence indicates
Trials of botulinum toxin injection for neurogenic detrusor overactivity have shown that it improves continence, maximum cystometric capacity, detrusor pressures, and quality of life. The main adverse effects are urinary tract infection and urinary retention requiring intermittent self-catheterization.
Although many patients with this condition are already self-catheterizing, the physician must discuss this before botulinum toxin therapy to ensure that the patient or a family member is able to perform catheterization. Studies have shown that patients have an increase in urinary tract infections after botulinum injections. But in these studies, a urinary tract infection was defined as 100,000 colony-forming units or the presence of leukocytosis with or without symptoms. It is important to remember that patients on intermittent catheterization have bacteriuria and should be treated only for symptomatic, not asymptomatic, bacteriuria.
TREATMENT OF IDIOPATHIC OVERACTIVE BLADDER
Patients with idiopathic overactive bladder have urinary urgency accompanied by urgency incontinence, nocturia, or urinary frequency.18 The prevalence of this condition has been reported to range from 1.7% to 13.3% in men age 30 and older and 7% to 30.3% in women of similar ages. About one-third of women with overactive bladder also have detrusor overactivity.19 Overactive bladder presents a significant economic and medical burden on the healthcare system, as well as having a negative impact on quality of life.
The FDA approved botulinum toxin injection for treatment of idiopathic overactive bladder in January 2013.
Evidence of effectiveness
Two multicenter randomized controlled trials20,21 of botulinum toxin 100 U enrolled patients age 18 and older who had more than three episodes of urinary urgency incontinence in a 3-day period or more than eight micturitions per day inadequately managed by anticholinergic drug therapy. Primary end points were the change from baseline in the number of episodes of urinary incontinence per day and the proportion of patients with a positive response on the Treatment Benefit Scale22 at week 12. Secondary end points included episodes of urinary urgency incontinence, micturition, urgency, and nocturia, and scores on health-related quality of life questionnaires (Incontinence Quality of Life scale, King’s Health Questionnaire).
In both studies, patients receiving botulinum toxin had significantly fewer episodes of incontinence compared with placebo (−2.65 vs −0.87; P < .001 and −2.95 vs −1.03; P < .001).20,21 Reductions from baseline in all other symptoms of overactive bladder, a positive treatment response on the treatment benefit scale, and improvements in quality-of-life scores were also significantly greater with botulinum toxin injection than with placebo (P ≤ .01).
As in the studies of neurogenic detrusor overactivity, the most common adverse effects were urinary tract infection (occurring in 15.5%20 and 24.1%21 of patients) and urinary retention requiring self-catheterization (5.4%20 and 6.9%21).
The largest study to date of anticholinergic therapy vs botulinum toxin injection23 in women with urinary urgency incontinence, published in 2012, studied nearly 250 women who had five or more episodes of idiopathic urgency incontinence in a 3-day period. They were randomized either to daily oral therapy (solifenacin 5 mg with possible escalation to 10 mg and, if necessary, a subsequent switch to extended-release trospium 60 mg) plus one intradetrusor injection of saline, or to a daily oral placebo plus one injection of botulinum toxin 100 U.23
The dropout rate was low in both groups, with 93% of patients in both groups completing the 6-month protocol. Women experienced a mean reduction in urgency incontinence episodes of 3.4 per day (baseline 5) in the anticholinergic group vs 3.3 episodes in the botulinum toxin group (P = .81). However, more patients achieved complete resolution of urinary urgency incontinence in the botulinum toxin group than in the anticholinergic therapy group (27% vs 13%; P = .003). Quality of life improved in both groups without a significant difference between the groups. The botulinum toxin group had higher rates of initiation of self-catheterization (5% vs 0%, P = .01) and urinary tract infection (33% vs 13%, P < .001).23
Botulinum toxin as a third-line therapy
In May 2014, the American Urological Association updated its guidelines on idiopathic overactive bladder24 to include botulinum toxin injection as standard third-line therapy for patients in whom behavioral and medical management (ie, anticholinergics and beta-3-agonists) failed.
Interpreting the evidence to date
Overall, studies in idiopathic overactive bladder have shown a reduction in episodes of urgency incontinence and other symptoms, with some data also demonstrating a corresponding improvement in quality of life.
As in neurogenic detrusor overactivity, the main risks associated with botulinum toxin injection are urinary tract infection and the need to initiate self-catheterization. Although 94% of patients studied did not require self-catheterization after injection, the patient’s ability to perform self-catheterization should be determined before proceeding with botulinum toxin injections.
DETRUSOR EXTERNAL SPHINCTER DYSSYNERGIA
Botulinum toxin has been used not only to improve bladder storage but also to facilitate bladder emptying, as in patients with DESD, a lack of coordination between the bladder and the urinary sphincter. Normal voiding involves relaxation of the urinary sphincter and contraction of the bladder; in DESD the sphincter contracts and works against the bladder’s ability to empty. This leads not only to difficulty emptying the bladder but also to elevated bladder pressure, which can cause renal damage if untreated.
DESD can be seen after injury between the pontine micturition center, which coordinates activity between the bladder and the sphincter, and the caudal spinal cord. This can occur in spinal cord injury, multiple sclerosis, myelomeningocele, and transverse myelitis and can cause significant morbidity for the patient.
Treatment options include drug therapy, injection of botulinum toxin into the sphincter, clean intermittent catheterization, indwelling catheterization, urethral stenting, sphincterotomy, and reconstructive surgery such as urinary diversion.25
The goals of therapy are to avoid the need for clean intermittent catheterization in patients who have difficulty with manual dexterity, and to avoid the need for surgical procedures such as sphincterotomy and urinary diversion. The efficacy of urethral stenting is low, and medical management can be limited.26
In the first published report on botulinum toxin for DESD (in 1988),27 of 11 patients with spinal cord injury and DESD who received botulinum toxin injected into the external urethral sphincter, 10 showed signs of sphincter denervation on electromyography and reductions in urethral pressure profiles and postvoid residual volumes. Schurch et al28 and de Sèze et al29 also reported reductions in postvoid residual volume and maximal urethral pressures in patients with spinal cord injury and DESD.
In 2005, Gallien et al30 reported what is still the largest multicenter randomized controlled trial of botulinum toxin injection in DESD. Eighty-six patients with multiple sclerosis, DESD, and chronic urinary retention were randomized to receive either a single transperineal botulinum toxin injection of 100 U plus the alpha-1-blocker alfuzosin, or a placebo injection plus alfuzosin. Botulinum toxin treatment was associated with significantly increased voided volumes and reduced premicturition and maximal detrusor pressures, but no significant decrease in postvoid residual volume.30
More study needed
Despite these findings, a Cochrane Review concluded that, given the limited experience with intrasphincteric injection of botulinum toxin, data from larger randomized controlled trials are needed before making definitive recommendations.25 In the meantime, the clinician must weigh the low morbidity of the procedure against the limited options in the treatment of these patients.
OFF-LABEL UROLOGIC INDICATIONS
Botulinum toxin injection has been studied off-label for painful bladder syndrome/interstitial cystitis and for chronic prostatic pain. Patients with these conditions often describe pain with filling of the bladder, which leads to urinary frequency in an attempt to relieve the pain.
These pain syndromes can be difficult to treat and can have a devastating impact on quality of life. Treatment options include pain management, stress management, physical therapy, intravesical therapies, cystoscopy with hydrodistention, neuromodulation, cyclosporine, urinary diversion surgery, and botulinum toxin injection (an off-label use).31
In painful bladder syndrome/interstitial cystitis, botulinum toxin is thought to act on sensory afferent pathways, as well as to inhibit the release of substance P and glutamate, neuropeptides involved in sensory and nociceptive pathways.6 In animal studies,32 botulinum toxin was found to inhibit the afferent neural response by inhibiting mechanoreceptor-mediated release of adenosine triphosphate and by causing a decrease in calcitonin gene-related peptide, which helps regulate micturition and mediates painful bladder sensation.
Clinical studies to date in pelvic pain syndromes
Data from clinical studies of botulinum toxin injection for pelvic pain syndromes are limited. Zermann et al33 performed transurethral perisphincteric injection in 11 men with chronic prostatic pain, 9 of whom reported subjective pain relief, with an average decrease from 7.2 to 1.6 on a visual analogue scale. Postinjection urodynamic studies showed a decrease in functional urethral length, urethral closure pressure, and postvoid residual volume, and an increase in the peak and average flow rates.33
Abbott et al34 evaluated the effect of botulinum toxin injection into the levator ani in 12 women with chronic pelvic pain and pelvic floor hypertonicity. Pelvic floor manometry showed significant reduction in resting muscle pressures and improvements in dyspareunia and nonmenstrual pain. There were also improvements in quality of life and dyschezia, but these were not statistically significant.
Smith et al35 injected botulinum toxin into the detrusor of 13 women with refractory painful bladder syndrome and interstitial cystitis,35 and 9 women (69%) noted statistically significant improvements in the Interstitial Cystitis Symptom Index and Interstitial Cystitis Problem Index, daytime frequency, nocturia, pain, and urodynamic parameters (volume at first desire to void, and maximum cystometric capacity).
In a prospective randomized study of patients with refractory painful bladder syndrome and interstitial cystitis, Kuo and Chancellor36 compared suburothelial injection of 200 U or 100 U of botulinum toxin plus hydrodistention against hydrodistention alone.Patients who received botulinum toxin had increased bladder capacity and improved long-term pain relief, but no difference was noted between 200 U and 100 U, and more adverse effects were seen with the higher dose.36
Pinto et al37 treated 16 women with refractory painful bladder syndrome and interstitial cystitis with intratrigonal injections of botulinum toxin and reported improvements in pain scores, symptom scores, urinary frequency, and quality-of-life measures. The effect lasted 9.9 months (± 2.4 months) and persisted with successive injections.37
More study needed
Although these studies show that botulinum toxin injection for pelvic pain syndromes has the potential to improve pain, urinary frequency, bladder sensation, bladder capacity, and quality of life, larger randomized controlled trials are needed.
Again, the treatment options are limited for refractory painful bladder syndrome and interstitial cystitis. Patients may be desperate for relief from their symptoms. Practitioners must manage expectations and properly inform patients of the potential risks of treatments, especially with patients who will easily agree to further treatment with the smallest hope of relief.
INJECTION TECHNIQUES
For general points about the procedure to discuss with patients, see “What to tell patients.”
Cystoscopic detrusor injection
This procedure is usually done on an outpatient basis (eg, office, ambulatory surgery center). With the patient in the lithotomy position, 100 mL of 2% lidocaine is instilled into the bladder and is allowed 15 to 20 minutes to take effect. A flexible or rigid cystoscope can be used. Depending on the indication, the bladder is injected with 100 U to 300 U of botulinum toxin. The ideal depth of injection is 2 mm in the detrusor muscle, with each injection spaced about 1 cm apart. The recommended administration for 100 U is to inject 20 sites with 0.5 U per mL of saline and, for 200 U, to inject 30 sites with about 0.67 U per mL of saline.38 The location of the injections into the detrusor can vary, as long as adequate spacing is assured.
Injection sites vary. Proponents of injecting the trigone argue that as it is an area of greater nerve density, patients will have a better clinical response. Opponents argue that trigonal injection could result in distal ureteral paralysis and subsequent ureteral reflux. However, this theoretical concern has not been observed clinically.
Urethral injection (off-label use)
The urethra can be injected cystoscopically or periurethrally. Cystoscopic injection involves localization of the external sphincter using the rigid cystoscope and collagen needle; a total of 100 U is injected into the sphincter under direct vision, typically at the 3 o’clock and 9 o’clock positions.35 The periurethral technique is an option in women and involves a spinal needle with 100 U to 200 U of botulinum toxin injected into the external sphincter muscle at the 2 o’clock and 10 o’clock positions.
ADVERSE EFFECTS AND CONTRAINDICATIONS
Adverse effects are rare for urologic applications. The injections are localized, with little systemic absorption, and the doses are 1/1,000th of the theorized lethal dose in a 70-kg male.2 The maximum recommended dose for a 3-month period is 360 U.
Generalized muscle weakness has been reported in a paraplegic patient and in a tetraplegic patient after detrusor injections.2 Interestingly, both patients had return of bladder spasticity within 2 months, prompting speculation about diffusion of botulinum toxin through the bladder wall.2
Repeat injections can cause an immune response in up to 5% of patients.6 Patients undergoing repeat injections are at risk of forming neutralizing antibodies that can interfere with the efficacy of botulinum toxin.6 In a study by Schulte-Baukloh et al, all patients with antibodies to botulinum toxin had a history of recurrent urinary tract infection.39
Botulinum toxin injection is contraindicated in patients with preexisting neuromuscular disease, such as myasthenia gravis, Eaton-Lambert syndrome, and amyotrophic lateral sclerosis. It should also be avoided in patients who are breastfeeding, pregnant, or using agents that potentiate neuromuscular weakness, such as aminoglycosides.
Patients should be informed that some formulations of botulinum toxin include a stabilizer such as albumin derived from human blood, as this may be of religious or cultural significance.
- Leippold T, Reitz A, Schurch B. Botulinum toxin as a new therapy option for voiding disorders: current state of the art. Eur Urol 2003; 44:165–174.
- Sahai A, Khan M, Fowler CJ, Dasgupta P. Botulinum toxin for the treatment of lower urinary tract symptoms: a review. Neurourol Urodyn 2005; 24:2–12.
- Cruz F. Targets for botulinum toxin in the lower urinary tract. Neurourol Urodyn 2014; 33:31–38.
- Weedmark KA, Lambert DL, Mabon P, et al. Two novel toxin variants revealed by whole-genome sequencing of 175 Clostridium botulinum type E strains. Appl Environ Microbiol 2014; 80:6334–6345.
- Ha US, Park EY, Kim JC. Effect of botulinum toxin on expression of nerve growth factor and transient receptor potential vanilloid 1 in urothelium and detrusor muscle of rats with bladder outlet obstruction-induced detrusor overactivity. Urology 2011; 78:721.e1–721.e6
- Frenkl TL, Rackley RR. Injectable neuromodulatory agents: botulinum toxin therapy. Urol Clin North Am 2005; 32:89–99.
- Ikeda Y, Zabbarova IV, Birder LA, et al. Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder. Eur Urol 2012; 62:1157–1164.
- Goldmark E, Niver B, Ginsberg DA. Neurogenic bladder: from diagnosis to management. Curr Urol Rep 2014; 15:448.
- Andersson KE. Current and future drugs for treatment of MS-associated bladder dysfunction. Ann Phys Rehabil Med 2014; 57:321–328.
- Schurch B, Stöhrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol 2000; 164:692–697.
- Schurch B, de Sèze M, Denys P, et al; Botox Detrusor Hyperreflexia Study Team. Botulinum toxin type a is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol 2005; 174:196–200.
- Ehren I, Volz D, Farrelly E, et al. Efficacy and impact of botulinum toxin A on quality of life in patients with neurogenic detrusor overactivity: a randomised, placebo-controlled, double-blind study. Scand J Urol Nephrol 2007; 41:335–340.
- Ginsberg D, Gousse A, Keppenne V, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol 2012; 187:2131–2139.
- Cruz F, Herschorn S, Aliotta P, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur Urol 2011; 60:742–750.
- Wagner TH, Patrick DL, Bavendam TG, Martin ML, Buesching DP. Quality of life of persons with urinary incontinence: development of a new measure. Urology 1996: 47:67–71.
- Kennelly M, Dmochowski R, Ethans K, et al. Long-term efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: an interim analysis. Urology 2013; 81:491–497.
- Carlson JJ, Hansen RN, Dmochowski RR, Globe DR, Colayco DC, Sullivan SD. Estimating the cost-effectiveness of onabotulinumtoxinA for neurogenic detrusor overactivity in the United States. Clin Ther 2013; 35:414–424.
- Abrams P, Cardozo L, Fall M, et al; Standardisation Sub-Committee of the International Continence Society. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003; 61:37–49.
- Milsom I, Coyne KS, Nicholson S, Kvasz M, Chen CI, Wein AJ. Global prevalence and economic burden of urgency urinary incontinence: a systematic review. Eur Urol 2014; 65:79–95.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol 2013; 189:2186–2193.
- Chapple C, Sievert KD, MacDiarmid S, et al. OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: a randomised, double-blind, placebo-controlled trial. Eur Urol 2013; 64:249–256.
- Colman S, Chapple C, Nitti V, Haag-Molkenteller C, Hastedt C, Massow U. Validation of Treatment Benefit Scale for assessing subjective outcomes in treatment of overactive bladder. Urology 2008; 72:803–807.
- Visco AG, Brubaker L, Richter HE, et al; Pelvic Floor Disorders Network. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. N Engl J Med 2012; 367:1803–1813.
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. www.auanet.org/education/guidelines/overactive-bladder.cfm. Accessed June 11, 2015.
- Utomo E, Groen J, Blok BF. Surgical management of functional bladder outlet obstruction in adults with neurogenic bladder dysfunction. Cochrane Database Syst Rev 2014; 5:CD004927.
- Mahfouz W, Corcos J. Management of detrusor external sphincter dyssynergia in neurogenic bladder. Eur J Phys Rehabil Med 2011; 47:639–650.
- Dykstra DD, Sidi AA, Scott AB, Pagel JM, Goldish GD. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1988; 139:919–922.
- Schurch B, Hauri D, Rodic B, Curt A, Meyer M, Rossier AB. Botulinum-A toxin as a treatment of detrusor-sphincter dyssynergia: a prospective study in 24 spinal cord injury patients. J Urol 1996; 155:1023–1029.
- de Sèze M, Petit H, Gallien, de Sèze MP, Joseph PA, Mazaux JM, Barat M. Botulinum a toxin and detrusor sphincter dyssynergia: a double-blind lidocaine-controlled study in 13 patients with spinal cord disease. Eur Urol 2002; 42:56–62.
- Gallien P, Reymann JM, Amarenco G, Nicolas B, de Sèze M, Bellissant E. Placebo controlled, randomised, double blind study of the effects of botulinum A toxin on detrusor sphincter dyssynergia in multiple sclerosis patients. J Neurol Neurosurg Psychiatry 2005; 76:1670–1676.
- Hanno PM, Burks DA, Clemens JQ, et al; Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 2011; 185:2162–2170.
- Chuang YC, Yoshimura N, Huang CC, Chiang PH, Chancellor MB. Intravesical botulinum toxin a administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol 2004; 172:1529–1532.
- Zermann DH, Ishigooka M, Schubert J, Schmidt RA. Perisphincteric injection of botulinum toxin type A. A treatment option for patients with chronic prostatic pain? Eur Urol 2000; 38:393–399.
- Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol 2006; 108:915–923.
- Smith CP, Radziszewski P, Borkowski A, Somogyi GT, Boone TB, Chancellor MB. Botulinum toxin A has antinociceptive effects in treating interstitial cystitis. Urology 2004; 64:871–875.
- Kuo HC, Chancellor MB. Comparison of intravesical botulinum toxin type A injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJU Int 2009: 104:657–661.
- Pinto R, Lopes T, Silva J, Silva C, Dinis P, Cruz F. Persistent therapeutic effect of repeated injections of onabotulinum toxin a in refractory bladder pain syndrome/interstitial cystitis. J Urol 2013; 189:548–553.
- Rovner E. Chapter 6: Practical aspects of administration of onabotulinumtoxinA. Neurourol Urodyn 2014; 33(suppl 3):S32–S37.
- Schulte-Baukloh H, Herholz J, Bigalke H, Miller K, Knispel HH. Results of a BoNT/A antibody study in children and adolescents after onabotulinumtoxin A (Botox®) detrusor injection. Urol Int 2011; 87:434–438.
Patients with loss of bladder control experience discomfort, embarrassment, personal care and health issues, and, often, significant pain, all with a decidedly negative impact on quality of life. Although some patients may find lifestyle modifications, drug therapy, and self-catheterization acceptable and effective, there is a clear need for more options.
Botulinum toxin, or onabotulinumtoxinA, is currently approved by the US Food and Drug Administration (FDA) for neurogenic detrusor overactivity and overactive bladder refractory to drug therapy. Studies so far have shown botulinum toxin injection to be safe and effective for these conditions, and these results have led to interest in off-label uses, eg, for detrusor external sphincter dyssynergia (DESD), motor and sensory urgency, and painful bladder syndrome/interstitial cystitis (Table 1).
Although more data from clinical trials are needed, botulinum toxin injection offers patients a much-needed treatment option.
HOW BOTULINUM TOXIN WORKS
Seven serotypes identified
Discovered in 1897, botulinum toxin is a neurotoxin produced by the gram-positive, rod-shaped anaerobic bacterium Clostridium botulinum1 and is the most poisonous naturally occurring toxin known.2 Seven immunologically distinct antigenic serotypes have been identified (A, B, C1, D, E, F, and G),1 but only types A and B are available for clinical use.
Most research into potential therapeutic uses has focused on type A, which has the longest duration of action, a clinical advantage.3 Recently, work has been done to further characterize other serotypes and to isolate additional variants of botulinum toxin. For example, serotype E, the predominant serotype associated with foodborne botulism, is being studied in an effort to prevent future outbreaks.4
Our discussion focuses on clinical uses of the serotype A botulinum toxin preparation, which we will refer to simply as botulinum toxin.
Studies exploring how it works
Botulinum toxin exerts its effects by binding to peripheral cholinergic terminals, inhibiting release of acetylcholine at the neuromuscular junction. Flaccid paralysis ensues as a result.
Results of animal studies have shed additional light on the specific actions of botulinum toxin A:
- It may alter levels of nerve growth factor and transient receptor potential vanilloid 1 in rats, and this may provide an additional mechanism of reducing bladder detrusor overactivity.5
- In addition to blocking acetylcholine release from motor neurons, it inhibits the release of neurotransmitters involved in bladder sensory afferent pathways.6
- It inhibits the release of substance P and glutamate, neuropeptides involved in sensory and nociceptive pathways.6,7
- It promotes apoptosis in prostatic tissue; however, this effect has not been shown in the bladder.3
The time necessary to recover function after botulinum toxin paralysis depends on the subtype of botulinum toxin as well as on the type of nerve terminal. Chemodenervation lasts from 3 to 6 months when the toxin is injected into the neuromuscular junction of skeletal muscle, and considerably longer (up to 1 year) when injected into the autonomic neurons of smooth muscle.2,6
TREATMENT OF NEUROGENIC DETRUSOR OVERACTIVITY
Neurogenic detrusor overactivity involves involuntary contractions of the bladder resulting from spinal cord injury, multiple sclerosis, and other neurologic conditions. An estimated 273,000 people in the United States have a spinal cord injury, and 81% of them have urologic symptoms ranging from areflexia to overactivity.8 From 75% to 100% of patients with multiple sclerosis have urologic symptoms, and detrusor overactivity is the most common.9
Detrusor overactivity can cause urinary urgency, urinary frequency, and urgency incontinence, significantly affecting quality of life and leading to skin breakdown, sacral ulcerations, and challenges with personal care.
Anticholinergic drugs have been the mainstay of therapy. If drug therapy failed, the next option was reconstructive surgery, often augmentation cystoplasty. Thus, botulinum toxin injection is an important advance in treatment options.
Studies that showed effectiveness
Botulinum toxin for neurogenic detrusor overactivity was first studied by Schurch et al.10 In their study, 200 U or 300 U was injected into the trigone of 21 patients with spinal cord injury and urgency incontinence managed with intermittent self-catheterization.10 At 6 weeks after injection, 17 of the 19 patients seen at follow-up visits were completely continent. Urodynamic evaluation revealed significant increases in maximum cystometric capacity and in volume at first involuntary detrusor contraction, and a decrease in detrusor voiding pressure. Of the 11 patients available for follow-up at 16 and 36 weeks, improvements in measures of incontinence and urodynamic function persisted.
In addition, two small randomized controlled trials11,12 showed significant increases in cystometric bladder capacity, significant improvement in quality-of-life measures, and reduction in episodes of urgency incontinence.
In 2011 and 2012, two multicenter double-blind randomized controlled trials reported on patients with multiple sclerosis and spinal cord injury with neurogenic detrusor overactivity inadequately managed with drug therapy. The patients were randomized to botulinum toxin injection (200 U or 300 U) or placebo injection.13,14 The primary end point for both studies was the change from baseline in episodes of urinary incontinence per week at week 6. Secondary end points were maximum cystometric capacity, maximum detrusor pressure during first involuntary detrusor contraction, and score on the Incontinence Quality of Life scale.15
In both studies, the mean number of urinary incontinence episodes per week was 33 at baseline. At week 6, Cruz et al14 found that patients who received botulinum toxin injection had significantly fewer episodes per week (21.8 fewer with 200 U, 19.4 fewer with 300 U) than those in the placebo group, who had 13.2 fewer episodes per week (P < .01). Ginsberg et al13 reported decreases in the mean number of episodes of urinary incontinence of 21, 23, and 9 episodes per week in the 200 U, 300 U, and placebo groups, respectively (P < .001). The patients who received botulinum toxin had statistically significant improvements in maximum cystometric capacity, maximum detrusor pressure during first involuntary detrusor contraction, and Incontinence Quality of Life scores compared with placebo (P < .001). Thirty-eight percent of patients in the treatment group were fully continent.13,14
Safety and adverse effects
The most frequently reported adverse events were urinary tract infection (24% of patients)13,14 and urinary retention requiring initiation of clean intermittent catheterization. In the study by Cruz et al,14 these were reported in 30% with 200 U, 42% with 300 U, and 12% with placebo, while in the study by Ginsberg et al13 they were reported in 35% with 200 U, 42% with 300 U, and 10% with placebo.
In a study of long-term safety and efficacy of botulinum toxin injection in patients with neurogenic detrusor overactivity, Kennelly et al16 found that patients undergoing repeat injections had sustained reductions in episodes of incontinence and increases in the maximum cystometric capacity and quality of life scores, with no increase in adverse events over time.16
But is it cost-effective?
While botulinum toxin injection may be safe and effective for neurogenic detrusor overactivity, is it cost-effective?
Carlson et al17 used a Markov State Transition model to assess the cost of refractory neurogenic detrusor overactivity in patients receiving botulinum toxin vs best supportive care (incontinence pads, medications, intermittent self-catheterization).17 They found that the injections were more expensive than supportive care but were cost-effective when considering the reduction in episodes of incontinence, the reduced need for incontinence products, and improvement in measures of quality of life.
What the evidence indicates
Trials of botulinum toxin injection for neurogenic detrusor overactivity have shown that it improves continence, maximum cystometric capacity, detrusor pressures, and quality of life. The main adverse effects are urinary tract infection and urinary retention requiring intermittent self-catheterization.
Although many patients with this condition are already self-catheterizing, the physician must discuss this before botulinum toxin therapy to ensure that the patient or a family member is able to perform catheterization. Studies have shown that patients have an increase in urinary tract infections after botulinum injections. But in these studies, a urinary tract infection was defined as 100,000 colony-forming units or the presence of leukocytosis with or without symptoms. It is important to remember that patients on intermittent catheterization have bacteriuria and should be treated only for symptomatic, not asymptomatic, bacteriuria.
TREATMENT OF IDIOPATHIC OVERACTIVE BLADDER
Patients with idiopathic overactive bladder have urinary urgency accompanied by urgency incontinence, nocturia, or urinary frequency.18 The prevalence of this condition has been reported to range from 1.7% to 13.3% in men age 30 and older and 7% to 30.3% in women of similar ages. About one-third of women with overactive bladder also have detrusor overactivity.19 Overactive bladder presents a significant economic and medical burden on the healthcare system, as well as having a negative impact on quality of life.
The FDA approved botulinum toxin injection for treatment of idiopathic overactive bladder in January 2013.
Evidence of effectiveness
Two multicenter randomized controlled trials20,21 of botulinum toxin 100 U enrolled patients age 18 and older who had more than three episodes of urinary urgency incontinence in a 3-day period or more than eight micturitions per day inadequately managed by anticholinergic drug therapy. Primary end points were the change from baseline in the number of episodes of urinary incontinence per day and the proportion of patients with a positive response on the Treatment Benefit Scale22 at week 12. Secondary end points included episodes of urinary urgency incontinence, micturition, urgency, and nocturia, and scores on health-related quality of life questionnaires (Incontinence Quality of Life scale, King’s Health Questionnaire).
In both studies, patients receiving botulinum toxin had significantly fewer episodes of incontinence compared with placebo (−2.65 vs −0.87; P < .001 and −2.95 vs −1.03; P < .001).20,21 Reductions from baseline in all other symptoms of overactive bladder, a positive treatment response on the treatment benefit scale, and improvements in quality-of-life scores were also significantly greater with botulinum toxin injection than with placebo (P ≤ .01).
As in the studies of neurogenic detrusor overactivity, the most common adverse effects were urinary tract infection (occurring in 15.5%20 and 24.1%21 of patients) and urinary retention requiring self-catheterization (5.4%20 and 6.9%21).
The largest study to date of anticholinergic therapy vs botulinum toxin injection23 in women with urinary urgency incontinence, published in 2012, studied nearly 250 women who had five or more episodes of idiopathic urgency incontinence in a 3-day period. They were randomized either to daily oral therapy (solifenacin 5 mg with possible escalation to 10 mg and, if necessary, a subsequent switch to extended-release trospium 60 mg) plus one intradetrusor injection of saline, or to a daily oral placebo plus one injection of botulinum toxin 100 U.23
The dropout rate was low in both groups, with 93% of patients in both groups completing the 6-month protocol. Women experienced a mean reduction in urgency incontinence episodes of 3.4 per day (baseline 5) in the anticholinergic group vs 3.3 episodes in the botulinum toxin group (P = .81). However, more patients achieved complete resolution of urinary urgency incontinence in the botulinum toxin group than in the anticholinergic therapy group (27% vs 13%; P = .003). Quality of life improved in both groups without a significant difference between the groups. The botulinum toxin group had higher rates of initiation of self-catheterization (5% vs 0%, P = .01) and urinary tract infection (33% vs 13%, P < .001).23
Botulinum toxin as a third-line therapy
In May 2014, the American Urological Association updated its guidelines on idiopathic overactive bladder24 to include botulinum toxin injection as standard third-line therapy for patients in whom behavioral and medical management (ie, anticholinergics and beta-3-agonists) failed.
Interpreting the evidence to date
Overall, studies in idiopathic overactive bladder have shown a reduction in episodes of urgency incontinence and other symptoms, with some data also demonstrating a corresponding improvement in quality of life.
As in neurogenic detrusor overactivity, the main risks associated with botulinum toxin injection are urinary tract infection and the need to initiate self-catheterization. Although 94% of patients studied did not require self-catheterization after injection, the patient’s ability to perform self-catheterization should be determined before proceeding with botulinum toxin injections.
DETRUSOR EXTERNAL SPHINCTER DYSSYNERGIA
Botulinum toxin has been used not only to improve bladder storage but also to facilitate bladder emptying, as in patients with DESD, a lack of coordination between the bladder and the urinary sphincter. Normal voiding involves relaxation of the urinary sphincter and contraction of the bladder; in DESD the sphincter contracts and works against the bladder’s ability to empty. This leads not only to difficulty emptying the bladder but also to elevated bladder pressure, which can cause renal damage if untreated.
DESD can be seen after injury between the pontine micturition center, which coordinates activity between the bladder and the sphincter, and the caudal spinal cord. This can occur in spinal cord injury, multiple sclerosis, myelomeningocele, and transverse myelitis and can cause significant morbidity for the patient.
Treatment options include drug therapy, injection of botulinum toxin into the sphincter, clean intermittent catheterization, indwelling catheterization, urethral stenting, sphincterotomy, and reconstructive surgery such as urinary diversion.25
The goals of therapy are to avoid the need for clean intermittent catheterization in patients who have difficulty with manual dexterity, and to avoid the need for surgical procedures such as sphincterotomy and urinary diversion. The efficacy of urethral stenting is low, and medical management can be limited.26
In the first published report on botulinum toxin for DESD (in 1988),27 of 11 patients with spinal cord injury and DESD who received botulinum toxin injected into the external urethral sphincter, 10 showed signs of sphincter denervation on electromyography and reductions in urethral pressure profiles and postvoid residual volumes. Schurch et al28 and de Sèze et al29 also reported reductions in postvoid residual volume and maximal urethral pressures in patients with spinal cord injury and DESD.
In 2005, Gallien et al30 reported what is still the largest multicenter randomized controlled trial of botulinum toxin injection in DESD. Eighty-six patients with multiple sclerosis, DESD, and chronic urinary retention were randomized to receive either a single transperineal botulinum toxin injection of 100 U plus the alpha-1-blocker alfuzosin, or a placebo injection plus alfuzosin. Botulinum toxin treatment was associated with significantly increased voided volumes and reduced premicturition and maximal detrusor pressures, but no significant decrease in postvoid residual volume.30
More study needed
Despite these findings, a Cochrane Review concluded that, given the limited experience with intrasphincteric injection of botulinum toxin, data from larger randomized controlled trials are needed before making definitive recommendations.25 In the meantime, the clinician must weigh the low morbidity of the procedure against the limited options in the treatment of these patients.
OFF-LABEL UROLOGIC INDICATIONS
Botulinum toxin injection has been studied off-label for painful bladder syndrome/interstitial cystitis and for chronic prostatic pain. Patients with these conditions often describe pain with filling of the bladder, which leads to urinary frequency in an attempt to relieve the pain.
These pain syndromes can be difficult to treat and can have a devastating impact on quality of life. Treatment options include pain management, stress management, physical therapy, intravesical therapies, cystoscopy with hydrodistention, neuromodulation, cyclosporine, urinary diversion surgery, and botulinum toxin injection (an off-label use).31
In painful bladder syndrome/interstitial cystitis, botulinum toxin is thought to act on sensory afferent pathways, as well as to inhibit the release of substance P and glutamate, neuropeptides involved in sensory and nociceptive pathways.6 In animal studies,32 botulinum toxin was found to inhibit the afferent neural response by inhibiting mechanoreceptor-mediated release of adenosine triphosphate and by causing a decrease in calcitonin gene-related peptide, which helps regulate micturition and mediates painful bladder sensation.
Clinical studies to date in pelvic pain syndromes
Data from clinical studies of botulinum toxin injection for pelvic pain syndromes are limited. Zermann et al33 performed transurethral perisphincteric injection in 11 men with chronic prostatic pain, 9 of whom reported subjective pain relief, with an average decrease from 7.2 to 1.6 on a visual analogue scale. Postinjection urodynamic studies showed a decrease in functional urethral length, urethral closure pressure, and postvoid residual volume, and an increase in the peak and average flow rates.33
Abbott et al34 evaluated the effect of botulinum toxin injection into the levator ani in 12 women with chronic pelvic pain and pelvic floor hypertonicity. Pelvic floor manometry showed significant reduction in resting muscle pressures and improvements in dyspareunia and nonmenstrual pain. There were also improvements in quality of life and dyschezia, but these were not statistically significant.
Smith et al35 injected botulinum toxin into the detrusor of 13 women with refractory painful bladder syndrome and interstitial cystitis,35 and 9 women (69%) noted statistically significant improvements in the Interstitial Cystitis Symptom Index and Interstitial Cystitis Problem Index, daytime frequency, nocturia, pain, and urodynamic parameters (volume at first desire to void, and maximum cystometric capacity).
In a prospective randomized study of patients with refractory painful bladder syndrome and interstitial cystitis, Kuo and Chancellor36 compared suburothelial injection of 200 U or 100 U of botulinum toxin plus hydrodistention against hydrodistention alone.Patients who received botulinum toxin had increased bladder capacity and improved long-term pain relief, but no difference was noted between 200 U and 100 U, and more adverse effects were seen with the higher dose.36
Pinto et al37 treated 16 women with refractory painful bladder syndrome and interstitial cystitis with intratrigonal injections of botulinum toxin and reported improvements in pain scores, symptom scores, urinary frequency, and quality-of-life measures. The effect lasted 9.9 months (± 2.4 months) and persisted with successive injections.37
More study needed
Although these studies show that botulinum toxin injection for pelvic pain syndromes has the potential to improve pain, urinary frequency, bladder sensation, bladder capacity, and quality of life, larger randomized controlled trials are needed.
Again, the treatment options are limited for refractory painful bladder syndrome and interstitial cystitis. Patients may be desperate for relief from their symptoms. Practitioners must manage expectations and properly inform patients of the potential risks of treatments, especially with patients who will easily agree to further treatment with the smallest hope of relief.
INJECTION TECHNIQUES
For general points about the procedure to discuss with patients, see “What to tell patients.”
Cystoscopic detrusor injection
This procedure is usually done on an outpatient basis (eg, office, ambulatory surgery center). With the patient in the lithotomy position, 100 mL of 2% lidocaine is instilled into the bladder and is allowed 15 to 20 minutes to take effect. A flexible or rigid cystoscope can be used. Depending on the indication, the bladder is injected with 100 U to 300 U of botulinum toxin. The ideal depth of injection is 2 mm in the detrusor muscle, with each injection spaced about 1 cm apart. The recommended administration for 100 U is to inject 20 sites with 0.5 U per mL of saline and, for 200 U, to inject 30 sites with about 0.67 U per mL of saline.38 The location of the injections into the detrusor can vary, as long as adequate spacing is assured.
Injection sites vary. Proponents of injecting the trigone argue that as it is an area of greater nerve density, patients will have a better clinical response. Opponents argue that trigonal injection could result in distal ureteral paralysis and subsequent ureteral reflux. However, this theoretical concern has not been observed clinically.
Urethral injection (off-label use)
The urethra can be injected cystoscopically or periurethrally. Cystoscopic injection involves localization of the external sphincter using the rigid cystoscope and collagen needle; a total of 100 U is injected into the sphincter under direct vision, typically at the 3 o’clock and 9 o’clock positions.35 The periurethral technique is an option in women and involves a spinal needle with 100 U to 200 U of botulinum toxin injected into the external sphincter muscle at the 2 o’clock and 10 o’clock positions.
ADVERSE EFFECTS AND CONTRAINDICATIONS
Adverse effects are rare for urologic applications. The injections are localized, with little systemic absorption, and the doses are 1/1,000th of the theorized lethal dose in a 70-kg male.2 The maximum recommended dose for a 3-month period is 360 U.
Generalized muscle weakness has been reported in a paraplegic patient and in a tetraplegic patient after detrusor injections.2 Interestingly, both patients had return of bladder spasticity within 2 months, prompting speculation about diffusion of botulinum toxin through the bladder wall.2
Repeat injections can cause an immune response in up to 5% of patients.6 Patients undergoing repeat injections are at risk of forming neutralizing antibodies that can interfere with the efficacy of botulinum toxin.6 In a study by Schulte-Baukloh et al, all patients with antibodies to botulinum toxin had a history of recurrent urinary tract infection.39
Botulinum toxin injection is contraindicated in patients with preexisting neuromuscular disease, such as myasthenia gravis, Eaton-Lambert syndrome, and amyotrophic lateral sclerosis. It should also be avoided in patients who are breastfeeding, pregnant, or using agents that potentiate neuromuscular weakness, such as aminoglycosides.
Patients should be informed that some formulations of botulinum toxin include a stabilizer such as albumin derived from human blood, as this may be of religious or cultural significance.
Patients with loss of bladder control experience discomfort, embarrassment, personal care and health issues, and, often, significant pain, all with a decidedly negative impact on quality of life. Although some patients may find lifestyle modifications, drug therapy, and self-catheterization acceptable and effective, there is a clear need for more options.
Botulinum toxin, or onabotulinumtoxinA, is currently approved by the US Food and Drug Administration (FDA) for neurogenic detrusor overactivity and overactive bladder refractory to drug therapy. Studies so far have shown botulinum toxin injection to be safe and effective for these conditions, and these results have led to interest in off-label uses, eg, for detrusor external sphincter dyssynergia (DESD), motor and sensory urgency, and painful bladder syndrome/interstitial cystitis (Table 1).
Although more data from clinical trials are needed, botulinum toxin injection offers patients a much-needed treatment option.
HOW BOTULINUM TOXIN WORKS
Seven serotypes identified
Discovered in 1897, botulinum toxin is a neurotoxin produced by the gram-positive, rod-shaped anaerobic bacterium Clostridium botulinum1 and is the most poisonous naturally occurring toxin known.2 Seven immunologically distinct antigenic serotypes have been identified (A, B, C1, D, E, F, and G),1 but only types A and B are available for clinical use.
Most research into potential therapeutic uses has focused on type A, which has the longest duration of action, a clinical advantage.3 Recently, work has been done to further characterize other serotypes and to isolate additional variants of botulinum toxin. For example, serotype E, the predominant serotype associated with foodborne botulism, is being studied in an effort to prevent future outbreaks.4
Our discussion focuses on clinical uses of the serotype A botulinum toxin preparation, which we will refer to simply as botulinum toxin.
Studies exploring how it works
Botulinum toxin exerts its effects by binding to peripheral cholinergic terminals, inhibiting release of acetylcholine at the neuromuscular junction. Flaccid paralysis ensues as a result.
Results of animal studies have shed additional light on the specific actions of botulinum toxin A:
- It may alter levels of nerve growth factor and transient receptor potential vanilloid 1 in rats, and this may provide an additional mechanism of reducing bladder detrusor overactivity.5
- In addition to blocking acetylcholine release from motor neurons, it inhibits the release of neurotransmitters involved in bladder sensory afferent pathways.6
- It inhibits the release of substance P and glutamate, neuropeptides involved in sensory and nociceptive pathways.6,7
- It promotes apoptosis in prostatic tissue; however, this effect has not been shown in the bladder.3
The time necessary to recover function after botulinum toxin paralysis depends on the subtype of botulinum toxin as well as on the type of nerve terminal. Chemodenervation lasts from 3 to 6 months when the toxin is injected into the neuromuscular junction of skeletal muscle, and considerably longer (up to 1 year) when injected into the autonomic neurons of smooth muscle.2,6
TREATMENT OF NEUROGENIC DETRUSOR OVERACTIVITY
Neurogenic detrusor overactivity involves involuntary contractions of the bladder resulting from spinal cord injury, multiple sclerosis, and other neurologic conditions. An estimated 273,000 people in the United States have a spinal cord injury, and 81% of them have urologic symptoms ranging from areflexia to overactivity.8 From 75% to 100% of patients with multiple sclerosis have urologic symptoms, and detrusor overactivity is the most common.9
Detrusor overactivity can cause urinary urgency, urinary frequency, and urgency incontinence, significantly affecting quality of life and leading to skin breakdown, sacral ulcerations, and challenges with personal care.
Anticholinergic drugs have been the mainstay of therapy. If drug therapy failed, the next option was reconstructive surgery, often augmentation cystoplasty. Thus, botulinum toxin injection is an important advance in treatment options.
Studies that showed effectiveness
Botulinum toxin for neurogenic detrusor overactivity was first studied by Schurch et al.10 In their study, 200 U or 300 U was injected into the trigone of 21 patients with spinal cord injury and urgency incontinence managed with intermittent self-catheterization.10 At 6 weeks after injection, 17 of the 19 patients seen at follow-up visits were completely continent. Urodynamic evaluation revealed significant increases in maximum cystometric capacity and in volume at first involuntary detrusor contraction, and a decrease in detrusor voiding pressure. Of the 11 patients available for follow-up at 16 and 36 weeks, improvements in measures of incontinence and urodynamic function persisted.
In addition, two small randomized controlled trials11,12 showed significant increases in cystometric bladder capacity, significant improvement in quality-of-life measures, and reduction in episodes of urgency incontinence.
In 2011 and 2012, two multicenter double-blind randomized controlled trials reported on patients with multiple sclerosis and spinal cord injury with neurogenic detrusor overactivity inadequately managed with drug therapy. The patients were randomized to botulinum toxin injection (200 U or 300 U) or placebo injection.13,14 The primary end point for both studies was the change from baseline in episodes of urinary incontinence per week at week 6. Secondary end points were maximum cystometric capacity, maximum detrusor pressure during first involuntary detrusor contraction, and score on the Incontinence Quality of Life scale.15
In both studies, the mean number of urinary incontinence episodes per week was 33 at baseline. At week 6, Cruz et al14 found that patients who received botulinum toxin injection had significantly fewer episodes per week (21.8 fewer with 200 U, 19.4 fewer with 300 U) than those in the placebo group, who had 13.2 fewer episodes per week (P < .01). Ginsberg et al13 reported decreases in the mean number of episodes of urinary incontinence of 21, 23, and 9 episodes per week in the 200 U, 300 U, and placebo groups, respectively (P < .001). The patients who received botulinum toxin had statistically significant improvements in maximum cystometric capacity, maximum detrusor pressure during first involuntary detrusor contraction, and Incontinence Quality of Life scores compared with placebo (P < .001). Thirty-eight percent of patients in the treatment group were fully continent.13,14
Safety and adverse effects
The most frequently reported adverse events were urinary tract infection (24% of patients)13,14 and urinary retention requiring initiation of clean intermittent catheterization. In the study by Cruz et al,14 these were reported in 30% with 200 U, 42% with 300 U, and 12% with placebo, while in the study by Ginsberg et al13 they were reported in 35% with 200 U, 42% with 300 U, and 10% with placebo.
In a study of long-term safety and efficacy of botulinum toxin injection in patients with neurogenic detrusor overactivity, Kennelly et al16 found that patients undergoing repeat injections had sustained reductions in episodes of incontinence and increases in the maximum cystometric capacity and quality of life scores, with no increase in adverse events over time.16
But is it cost-effective?
While botulinum toxin injection may be safe and effective for neurogenic detrusor overactivity, is it cost-effective?
Carlson et al17 used a Markov State Transition model to assess the cost of refractory neurogenic detrusor overactivity in patients receiving botulinum toxin vs best supportive care (incontinence pads, medications, intermittent self-catheterization).17 They found that the injections were more expensive than supportive care but were cost-effective when considering the reduction in episodes of incontinence, the reduced need for incontinence products, and improvement in measures of quality of life.
What the evidence indicates
Trials of botulinum toxin injection for neurogenic detrusor overactivity have shown that it improves continence, maximum cystometric capacity, detrusor pressures, and quality of life. The main adverse effects are urinary tract infection and urinary retention requiring intermittent self-catheterization.
Although many patients with this condition are already self-catheterizing, the physician must discuss this before botulinum toxin therapy to ensure that the patient or a family member is able to perform catheterization. Studies have shown that patients have an increase in urinary tract infections after botulinum injections. But in these studies, a urinary tract infection was defined as 100,000 colony-forming units or the presence of leukocytosis with or without symptoms. It is important to remember that patients on intermittent catheterization have bacteriuria and should be treated only for symptomatic, not asymptomatic, bacteriuria.
TREATMENT OF IDIOPATHIC OVERACTIVE BLADDER
Patients with idiopathic overactive bladder have urinary urgency accompanied by urgency incontinence, nocturia, or urinary frequency.18 The prevalence of this condition has been reported to range from 1.7% to 13.3% in men age 30 and older and 7% to 30.3% in women of similar ages. About one-third of women with overactive bladder also have detrusor overactivity.19 Overactive bladder presents a significant economic and medical burden on the healthcare system, as well as having a negative impact on quality of life.
The FDA approved botulinum toxin injection for treatment of idiopathic overactive bladder in January 2013.
Evidence of effectiveness
Two multicenter randomized controlled trials20,21 of botulinum toxin 100 U enrolled patients age 18 and older who had more than three episodes of urinary urgency incontinence in a 3-day period or more than eight micturitions per day inadequately managed by anticholinergic drug therapy. Primary end points were the change from baseline in the number of episodes of urinary incontinence per day and the proportion of patients with a positive response on the Treatment Benefit Scale22 at week 12. Secondary end points included episodes of urinary urgency incontinence, micturition, urgency, and nocturia, and scores on health-related quality of life questionnaires (Incontinence Quality of Life scale, King’s Health Questionnaire).
In both studies, patients receiving botulinum toxin had significantly fewer episodes of incontinence compared with placebo (−2.65 vs −0.87; P < .001 and −2.95 vs −1.03; P < .001).20,21 Reductions from baseline in all other symptoms of overactive bladder, a positive treatment response on the treatment benefit scale, and improvements in quality-of-life scores were also significantly greater with botulinum toxin injection than with placebo (P ≤ .01).
As in the studies of neurogenic detrusor overactivity, the most common adverse effects were urinary tract infection (occurring in 15.5%20 and 24.1%21 of patients) and urinary retention requiring self-catheterization (5.4%20 and 6.9%21).
The largest study to date of anticholinergic therapy vs botulinum toxin injection23 in women with urinary urgency incontinence, published in 2012, studied nearly 250 women who had five or more episodes of idiopathic urgency incontinence in a 3-day period. They were randomized either to daily oral therapy (solifenacin 5 mg with possible escalation to 10 mg and, if necessary, a subsequent switch to extended-release trospium 60 mg) plus one intradetrusor injection of saline, or to a daily oral placebo plus one injection of botulinum toxin 100 U.23
The dropout rate was low in both groups, with 93% of patients in both groups completing the 6-month protocol. Women experienced a mean reduction in urgency incontinence episodes of 3.4 per day (baseline 5) in the anticholinergic group vs 3.3 episodes in the botulinum toxin group (P = .81). However, more patients achieved complete resolution of urinary urgency incontinence in the botulinum toxin group than in the anticholinergic therapy group (27% vs 13%; P = .003). Quality of life improved in both groups without a significant difference between the groups. The botulinum toxin group had higher rates of initiation of self-catheterization (5% vs 0%, P = .01) and urinary tract infection (33% vs 13%, P < .001).23
Botulinum toxin as a third-line therapy
In May 2014, the American Urological Association updated its guidelines on idiopathic overactive bladder24 to include botulinum toxin injection as standard third-line therapy for patients in whom behavioral and medical management (ie, anticholinergics and beta-3-agonists) failed.
Interpreting the evidence to date
Overall, studies in idiopathic overactive bladder have shown a reduction in episodes of urgency incontinence and other symptoms, with some data also demonstrating a corresponding improvement in quality of life.
As in neurogenic detrusor overactivity, the main risks associated with botulinum toxin injection are urinary tract infection and the need to initiate self-catheterization. Although 94% of patients studied did not require self-catheterization after injection, the patient’s ability to perform self-catheterization should be determined before proceeding with botulinum toxin injections.
DETRUSOR EXTERNAL SPHINCTER DYSSYNERGIA
Botulinum toxin has been used not only to improve bladder storage but also to facilitate bladder emptying, as in patients with DESD, a lack of coordination between the bladder and the urinary sphincter. Normal voiding involves relaxation of the urinary sphincter and contraction of the bladder; in DESD the sphincter contracts and works against the bladder’s ability to empty. This leads not only to difficulty emptying the bladder but also to elevated bladder pressure, which can cause renal damage if untreated.
DESD can be seen after injury between the pontine micturition center, which coordinates activity between the bladder and the sphincter, and the caudal spinal cord. This can occur in spinal cord injury, multiple sclerosis, myelomeningocele, and transverse myelitis and can cause significant morbidity for the patient.
Treatment options include drug therapy, injection of botulinum toxin into the sphincter, clean intermittent catheterization, indwelling catheterization, urethral stenting, sphincterotomy, and reconstructive surgery such as urinary diversion.25
The goals of therapy are to avoid the need for clean intermittent catheterization in patients who have difficulty with manual dexterity, and to avoid the need for surgical procedures such as sphincterotomy and urinary diversion. The efficacy of urethral stenting is low, and medical management can be limited.26
In the first published report on botulinum toxin for DESD (in 1988),27 of 11 patients with spinal cord injury and DESD who received botulinum toxin injected into the external urethral sphincter, 10 showed signs of sphincter denervation on electromyography and reductions in urethral pressure profiles and postvoid residual volumes. Schurch et al28 and de Sèze et al29 also reported reductions in postvoid residual volume and maximal urethral pressures in patients with spinal cord injury and DESD.
In 2005, Gallien et al30 reported what is still the largest multicenter randomized controlled trial of botulinum toxin injection in DESD. Eighty-six patients with multiple sclerosis, DESD, and chronic urinary retention were randomized to receive either a single transperineal botulinum toxin injection of 100 U plus the alpha-1-blocker alfuzosin, or a placebo injection plus alfuzosin. Botulinum toxin treatment was associated with significantly increased voided volumes and reduced premicturition and maximal detrusor pressures, but no significant decrease in postvoid residual volume.30
More study needed
Despite these findings, a Cochrane Review concluded that, given the limited experience with intrasphincteric injection of botulinum toxin, data from larger randomized controlled trials are needed before making definitive recommendations.25 In the meantime, the clinician must weigh the low morbidity of the procedure against the limited options in the treatment of these patients.
OFF-LABEL UROLOGIC INDICATIONS
Botulinum toxin injection has been studied off-label for painful bladder syndrome/interstitial cystitis and for chronic prostatic pain. Patients with these conditions often describe pain with filling of the bladder, which leads to urinary frequency in an attempt to relieve the pain.
These pain syndromes can be difficult to treat and can have a devastating impact on quality of life. Treatment options include pain management, stress management, physical therapy, intravesical therapies, cystoscopy with hydrodistention, neuromodulation, cyclosporine, urinary diversion surgery, and botulinum toxin injection (an off-label use).31
In painful bladder syndrome/interstitial cystitis, botulinum toxin is thought to act on sensory afferent pathways, as well as to inhibit the release of substance P and glutamate, neuropeptides involved in sensory and nociceptive pathways.6 In animal studies,32 botulinum toxin was found to inhibit the afferent neural response by inhibiting mechanoreceptor-mediated release of adenosine triphosphate and by causing a decrease in calcitonin gene-related peptide, which helps regulate micturition and mediates painful bladder sensation.
Clinical studies to date in pelvic pain syndromes
Data from clinical studies of botulinum toxin injection for pelvic pain syndromes are limited. Zermann et al33 performed transurethral perisphincteric injection in 11 men with chronic prostatic pain, 9 of whom reported subjective pain relief, with an average decrease from 7.2 to 1.6 on a visual analogue scale. Postinjection urodynamic studies showed a decrease in functional urethral length, urethral closure pressure, and postvoid residual volume, and an increase in the peak and average flow rates.33
Abbott et al34 evaluated the effect of botulinum toxin injection into the levator ani in 12 women with chronic pelvic pain and pelvic floor hypertonicity. Pelvic floor manometry showed significant reduction in resting muscle pressures and improvements in dyspareunia and nonmenstrual pain. There were also improvements in quality of life and dyschezia, but these were not statistically significant.
Smith et al35 injected botulinum toxin into the detrusor of 13 women with refractory painful bladder syndrome and interstitial cystitis,35 and 9 women (69%) noted statistically significant improvements in the Interstitial Cystitis Symptom Index and Interstitial Cystitis Problem Index, daytime frequency, nocturia, pain, and urodynamic parameters (volume at first desire to void, and maximum cystometric capacity).
In a prospective randomized study of patients with refractory painful bladder syndrome and interstitial cystitis, Kuo and Chancellor36 compared suburothelial injection of 200 U or 100 U of botulinum toxin plus hydrodistention against hydrodistention alone.Patients who received botulinum toxin had increased bladder capacity and improved long-term pain relief, but no difference was noted between 200 U and 100 U, and more adverse effects were seen with the higher dose.36
Pinto et al37 treated 16 women with refractory painful bladder syndrome and interstitial cystitis with intratrigonal injections of botulinum toxin and reported improvements in pain scores, symptom scores, urinary frequency, and quality-of-life measures. The effect lasted 9.9 months (± 2.4 months) and persisted with successive injections.37
More study needed
Although these studies show that botulinum toxin injection for pelvic pain syndromes has the potential to improve pain, urinary frequency, bladder sensation, bladder capacity, and quality of life, larger randomized controlled trials are needed.
Again, the treatment options are limited for refractory painful bladder syndrome and interstitial cystitis. Patients may be desperate for relief from their symptoms. Practitioners must manage expectations and properly inform patients of the potential risks of treatments, especially with patients who will easily agree to further treatment with the smallest hope of relief.
INJECTION TECHNIQUES
For general points about the procedure to discuss with patients, see “What to tell patients.”
Cystoscopic detrusor injection
This procedure is usually done on an outpatient basis (eg, office, ambulatory surgery center). With the patient in the lithotomy position, 100 mL of 2% lidocaine is instilled into the bladder and is allowed 15 to 20 minutes to take effect. A flexible or rigid cystoscope can be used. Depending on the indication, the bladder is injected with 100 U to 300 U of botulinum toxin. The ideal depth of injection is 2 mm in the detrusor muscle, with each injection spaced about 1 cm apart. The recommended administration for 100 U is to inject 20 sites with 0.5 U per mL of saline and, for 200 U, to inject 30 sites with about 0.67 U per mL of saline.38 The location of the injections into the detrusor can vary, as long as adequate spacing is assured.
Injection sites vary. Proponents of injecting the trigone argue that as it is an area of greater nerve density, patients will have a better clinical response. Opponents argue that trigonal injection could result in distal ureteral paralysis and subsequent ureteral reflux. However, this theoretical concern has not been observed clinically.
Urethral injection (off-label use)
The urethra can be injected cystoscopically or periurethrally. Cystoscopic injection involves localization of the external sphincter using the rigid cystoscope and collagen needle; a total of 100 U is injected into the sphincter under direct vision, typically at the 3 o’clock and 9 o’clock positions.35 The periurethral technique is an option in women and involves a spinal needle with 100 U to 200 U of botulinum toxin injected into the external sphincter muscle at the 2 o’clock and 10 o’clock positions.
ADVERSE EFFECTS AND CONTRAINDICATIONS
Adverse effects are rare for urologic applications. The injections are localized, with little systemic absorption, and the doses are 1/1,000th of the theorized lethal dose in a 70-kg male.2 The maximum recommended dose for a 3-month period is 360 U.
Generalized muscle weakness has been reported in a paraplegic patient and in a tetraplegic patient after detrusor injections.2 Interestingly, both patients had return of bladder spasticity within 2 months, prompting speculation about diffusion of botulinum toxin through the bladder wall.2
Repeat injections can cause an immune response in up to 5% of patients.6 Patients undergoing repeat injections are at risk of forming neutralizing antibodies that can interfere with the efficacy of botulinum toxin.6 In a study by Schulte-Baukloh et al, all patients with antibodies to botulinum toxin had a history of recurrent urinary tract infection.39
Botulinum toxin injection is contraindicated in patients with preexisting neuromuscular disease, such as myasthenia gravis, Eaton-Lambert syndrome, and amyotrophic lateral sclerosis. It should also be avoided in patients who are breastfeeding, pregnant, or using agents that potentiate neuromuscular weakness, such as aminoglycosides.
Patients should be informed that some formulations of botulinum toxin include a stabilizer such as albumin derived from human blood, as this may be of religious or cultural significance.
- Leippold T, Reitz A, Schurch B. Botulinum toxin as a new therapy option for voiding disorders: current state of the art. Eur Urol 2003; 44:165–174.
- Sahai A, Khan M, Fowler CJ, Dasgupta P. Botulinum toxin for the treatment of lower urinary tract symptoms: a review. Neurourol Urodyn 2005; 24:2–12.
- Cruz F. Targets for botulinum toxin in the lower urinary tract. Neurourol Urodyn 2014; 33:31–38.
- Weedmark KA, Lambert DL, Mabon P, et al. Two novel toxin variants revealed by whole-genome sequencing of 175 Clostridium botulinum type E strains. Appl Environ Microbiol 2014; 80:6334–6345.
- Ha US, Park EY, Kim JC. Effect of botulinum toxin on expression of nerve growth factor and transient receptor potential vanilloid 1 in urothelium and detrusor muscle of rats with bladder outlet obstruction-induced detrusor overactivity. Urology 2011; 78:721.e1–721.e6
- Frenkl TL, Rackley RR. Injectable neuromodulatory agents: botulinum toxin therapy. Urol Clin North Am 2005; 32:89–99.
- Ikeda Y, Zabbarova IV, Birder LA, et al. Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder. Eur Urol 2012; 62:1157–1164.
- Goldmark E, Niver B, Ginsberg DA. Neurogenic bladder: from diagnosis to management. Curr Urol Rep 2014; 15:448.
- Andersson KE. Current and future drugs for treatment of MS-associated bladder dysfunction. Ann Phys Rehabil Med 2014; 57:321–328.
- Schurch B, Stöhrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol 2000; 164:692–697.
- Schurch B, de Sèze M, Denys P, et al; Botox Detrusor Hyperreflexia Study Team. Botulinum toxin type a is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol 2005; 174:196–200.
- Ehren I, Volz D, Farrelly E, et al. Efficacy and impact of botulinum toxin A on quality of life in patients with neurogenic detrusor overactivity: a randomised, placebo-controlled, double-blind study. Scand J Urol Nephrol 2007; 41:335–340.
- Ginsberg D, Gousse A, Keppenne V, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol 2012; 187:2131–2139.
- Cruz F, Herschorn S, Aliotta P, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur Urol 2011; 60:742–750.
- Wagner TH, Patrick DL, Bavendam TG, Martin ML, Buesching DP. Quality of life of persons with urinary incontinence: development of a new measure. Urology 1996: 47:67–71.
- Kennelly M, Dmochowski R, Ethans K, et al. Long-term efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: an interim analysis. Urology 2013; 81:491–497.
- Carlson JJ, Hansen RN, Dmochowski RR, Globe DR, Colayco DC, Sullivan SD. Estimating the cost-effectiveness of onabotulinumtoxinA for neurogenic detrusor overactivity in the United States. Clin Ther 2013; 35:414–424.
- Abrams P, Cardozo L, Fall M, et al; Standardisation Sub-Committee of the International Continence Society. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003; 61:37–49.
- Milsom I, Coyne KS, Nicholson S, Kvasz M, Chen CI, Wein AJ. Global prevalence and economic burden of urgency urinary incontinence: a systematic review. Eur Urol 2014; 65:79–95.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol 2013; 189:2186–2193.
- Chapple C, Sievert KD, MacDiarmid S, et al. OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: a randomised, double-blind, placebo-controlled trial. Eur Urol 2013; 64:249–256.
- Colman S, Chapple C, Nitti V, Haag-Molkenteller C, Hastedt C, Massow U. Validation of Treatment Benefit Scale for assessing subjective outcomes in treatment of overactive bladder. Urology 2008; 72:803–807.
- Visco AG, Brubaker L, Richter HE, et al; Pelvic Floor Disorders Network. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. N Engl J Med 2012; 367:1803–1813.
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. www.auanet.org/education/guidelines/overactive-bladder.cfm. Accessed June 11, 2015.
- Utomo E, Groen J, Blok BF. Surgical management of functional bladder outlet obstruction in adults with neurogenic bladder dysfunction. Cochrane Database Syst Rev 2014; 5:CD004927.
- Mahfouz W, Corcos J. Management of detrusor external sphincter dyssynergia in neurogenic bladder. Eur J Phys Rehabil Med 2011; 47:639–650.
- Dykstra DD, Sidi AA, Scott AB, Pagel JM, Goldish GD. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1988; 139:919–922.
- Schurch B, Hauri D, Rodic B, Curt A, Meyer M, Rossier AB. Botulinum-A toxin as a treatment of detrusor-sphincter dyssynergia: a prospective study in 24 spinal cord injury patients. J Urol 1996; 155:1023–1029.
- de Sèze M, Petit H, Gallien, de Sèze MP, Joseph PA, Mazaux JM, Barat M. Botulinum a toxin and detrusor sphincter dyssynergia: a double-blind lidocaine-controlled study in 13 patients with spinal cord disease. Eur Urol 2002; 42:56–62.
- Gallien P, Reymann JM, Amarenco G, Nicolas B, de Sèze M, Bellissant E. Placebo controlled, randomised, double blind study of the effects of botulinum A toxin on detrusor sphincter dyssynergia in multiple sclerosis patients. J Neurol Neurosurg Psychiatry 2005; 76:1670–1676.
- Hanno PM, Burks DA, Clemens JQ, et al; Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 2011; 185:2162–2170.
- Chuang YC, Yoshimura N, Huang CC, Chiang PH, Chancellor MB. Intravesical botulinum toxin a administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol 2004; 172:1529–1532.
- Zermann DH, Ishigooka M, Schubert J, Schmidt RA. Perisphincteric injection of botulinum toxin type A. A treatment option for patients with chronic prostatic pain? Eur Urol 2000; 38:393–399.
- Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol 2006; 108:915–923.
- Smith CP, Radziszewski P, Borkowski A, Somogyi GT, Boone TB, Chancellor MB. Botulinum toxin A has antinociceptive effects in treating interstitial cystitis. Urology 2004; 64:871–875.
- Kuo HC, Chancellor MB. Comparison of intravesical botulinum toxin type A injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJU Int 2009: 104:657–661.
- Pinto R, Lopes T, Silva J, Silva C, Dinis P, Cruz F. Persistent therapeutic effect of repeated injections of onabotulinum toxin a in refractory bladder pain syndrome/interstitial cystitis. J Urol 2013; 189:548–553.
- Rovner E. Chapter 6: Practical aspects of administration of onabotulinumtoxinA. Neurourol Urodyn 2014; 33(suppl 3):S32–S37.
- Schulte-Baukloh H, Herholz J, Bigalke H, Miller K, Knispel HH. Results of a BoNT/A antibody study in children and adolescents after onabotulinumtoxin A (Botox®) detrusor injection. Urol Int 2011; 87:434–438.
- Leippold T, Reitz A, Schurch B. Botulinum toxin as a new therapy option for voiding disorders: current state of the art. Eur Urol 2003; 44:165–174.
- Sahai A, Khan M, Fowler CJ, Dasgupta P. Botulinum toxin for the treatment of lower urinary tract symptoms: a review. Neurourol Urodyn 2005; 24:2–12.
- Cruz F. Targets for botulinum toxin in the lower urinary tract. Neurourol Urodyn 2014; 33:31–38.
- Weedmark KA, Lambert DL, Mabon P, et al. Two novel toxin variants revealed by whole-genome sequencing of 175 Clostridium botulinum type E strains. Appl Environ Microbiol 2014; 80:6334–6345.
- Ha US, Park EY, Kim JC. Effect of botulinum toxin on expression of nerve growth factor and transient receptor potential vanilloid 1 in urothelium and detrusor muscle of rats with bladder outlet obstruction-induced detrusor overactivity. Urology 2011; 78:721.e1–721.e6
- Frenkl TL, Rackley RR. Injectable neuromodulatory agents: botulinum toxin therapy. Urol Clin North Am 2005; 32:89–99.
- Ikeda Y, Zabbarova IV, Birder LA, et al. Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder. Eur Urol 2012; 62:1157–1164.
- Goldmark E, Niver B, Ginsberg DA. Neurogenic bladder: from diagnosis to management. Curr Urol Rep 2014; 15:448.
- Andersson KE. Current and future drugs for treatment of MS-associated bladder dysfunction. Ann Phys Rehabil Med 2014; 57:321–328.
- Schurch B, Stöhrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol 2000; 164:692–697.
- Schurch B, de Sèze M, Denys P, et al; Botox Detrusor Hyperreflexia Study Team. Botulinum toxin type a is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol 2005; 174:196–200.
- Ehren I, Volz D, Farrelly E, et al. Efficacy and impact of botulinum toxin A on quality of life in patients with neurogenic detrusor overactivity: a randomised, placebo-controlled, double-blind study. Scand J Urol Nephrol 2007; 41:335–340.
- Ginsberg D, Gousse A, Keppenne V, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol 2012; 187:2131–2139.
- Cruz F, Herschorn S, Aliotta P, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur Urol 2011; 60:742–750.
- Wagner TH, Patrick DL, Bavendam TG, Martin ML, Buesching DP. Quality of life of persons with urinary incontinence: development of a new measure. Urology 1996: 47:67–71.
- Kennelly M, Dmochowski R, Ethans K, et al. Long-term efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: an interim analysis. Urology 2013; 81:491–497.
- Carlson JJ, Hansen RN, Dmochowski RR, Globe DR, Colayco DC, Sullivan SD. Estimating the cost-effectiveness of onabotulinumtoxinA for neurogenic detrusor overactivity in the United States. Clin Ther 2013; 35:414–424.
- Abrams P, Cardozo L, Fall M, et al; Standardisation Sub-Committee of the International Continence Society. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003; 61:37–49.
- Milsom I, Coyne KS, Nicholson S, Kvasz M, Chen CI, Wein AJ. Global prevalence and economic burden of urgency urinary incontinence: a systematic review. Eur Urol 2014; 65:79–95.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol 2013; 189:2186–2193.
- Chapple C, Sievert KD, MacDiarmid S, et al. OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: a randomised, double-blind, placebo-controlled trial. Eur Urol 2013; 64:249–256.
- Colman S, Chapple C, Nitti V, Haag-Molkenteller C, Hastedt C, Massow U. Validation of Treatment Benefit Scale for assessing subjective outcomes in treatment of overactive bladder. Urology 2008; 72:803–807.
- Visco AG, Brubaker L, Richter HE, et al; Pelvic Floor Disorders Network. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. N Engl J Med 2012; 367:1803–1813.
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. www.auanet.org/education/guidelines/overactive-bladder.cfm. Accessed June 11, 2015.
- Utomo E, Groen J, Blok BF. Surgical management of functional bladder outlet obstruction in adults with neurogenic bladder dysfunction. Cochrane Database Syst Rev 2014; 5:CD004927.
- Mahfouz W, Corcos J. Management of detrusor external sphincter dyssynergia in neurogenic bladder. Eur J Phys Rehabil Med 2011; 47:639–650.
- Dykstra DD, Sidi AA, Scott AB, Pagel JM, Goldish GD. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1988; 139:919–922.
- Schurch B, Hauri D, Rodic B, Curt A, Meyer M, Rossier AB. Botulinum-A toxin as a treatment of detrusor-sphincter dyssynergia: a prospective study in 24 spinal cord injury patients. J Urol 1996; 155:1023–1029.
- de Sèze M, Petit H, Gallien, de Sèze MP, Joseph PA, Mazaux JM, Barat M. Botulinum a toxin and detrusor sphincter dyssynergia: a double-blind lidocaine-controlled study in 13 patients with spinal cord disease. Eur Urol 2002; 42:56–62.
- Gallien P, Reymann JM, Amarenco G, Nicolas B, de Sèze M, Bellissant E. Placebo controlled, randomised, double blind study of the effects of botulinum A toxin on detrusor sphincter dyssynergia in multiple sclerosis patients. J Neurol Neurosurg Psychiatry 2005; 76:1670–1676.
- Hanno PM, Burks DA, Clemens JQ, et al; Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 2011; 185:2162–2170.
- Chuang YC, Yoshimura N, Huang CC, Chiang PH, Chancellor MB. Intravesical botulinum toxin a administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol 2004; 172:1529–1532.
- Zermann DH, Ishigooka M, Schubert J, Schmidt RA. Perisphincteric injection of botulinum toxin type A. A treatment option for patients with chronic prostatic pain? Eur Urol 2000; 38:393–399.
- Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol 2006; 108:915–923.
- Smith CP, Radziszewski P, Borkowski A, Somogyi GT, Boone TB, Chancellor MB. Botulinum toxin A has antinociceptive effects in treating interstitial cystitis. Urology 2004; 64:871–875.
- Kuo HC, Chancellor MB. Comparison of intravesical botulinum toxin type A injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJU Int 2009: 104:657–661.
- Pinto R, Lopes T, Silva J, Silva C, Dinis P, Cruz F. Persistent therapeutic effect of repeated injections of onabotulinum toxin a in refractory bladder pain syndrome/interstitial cystitis. J Urol 2013; 189:548–553.
- Rovner E. Chapter 6: Practical aspects of administration of onabotulinumtoxinA. Neurourol Urodyn 2014; 33(suppl 3):S32–S37.
- Schulte-Baukloh H, Herholz J, Bigalke H, Miller K, Knispel HH. Results of a BoNT/A antibody study in children and adolescents after onabotulinumtoxin A (Botox®) detrusor injection. Urol Int 2011; 87:434–438.
KEY POINTS
- Anticholinergic drugs have been the first-line therapy for neurogenic detrusor overactivity. If drug therapy failed, the next option was reconstructive surgery such as cystoplasty. Botulinum toxin injection may be an option in select patients.
- Urinary tract infection and urinary retention requiring intermittent self-catheterization are the most common adverse events of botulinum toxin injection in trials of patients with neurogenic detrusor overactivity or idiopathic overactive bladder.
- Small studies have shown that botulinum toxin injection for painful bladder syndrome/interstitial cystitis can improve pain, urinary frequency, and quality of life. But larger randomized controlled trials are needed.