User login
AUA: Long-term use of Botox may decrease urinary incontinence
NEW ORLEANS – Long-term treatment with onabotulinumtoxinA significantly decreased daily urinary incontinence episodes in patients with overactive bladder syndrome, with no increase in adverse effects tied to repeated treatment, according to Dr. Victor W. Nitti.
Dr. Nitti and his colleagues conducted a multicenter extension study evaluating the long-term efficacy and safety of repeated treatments with onabotulinumtoxinA (onabotA) in patients with overactive bladder (OAB).
After completion of either of two 24-week, randomized phase III trials, patients were eligible to enter a 3-year extension study in which they could receive multiple onabotA treatments at 100 units per dose, Dr Nitti reported at the annual meeting of the American Urological Association.
Patients were treated “as needed” based on their request, and their fulfillment of the prespecified qualification criteria. “Patients requesting treatment had to have at least two urgency incontinence episodes in a 3-day diary, at least 12 weeks since their last treatment, and their postvoid residual had to be less than 200 cc,” said Dr. Nitti, professor of urology at New York University, New York. Therefore, the total number of treatments delivered during the study differed among patients depending on need.
Coprimary endpoints included change from baseline in urinary incontinence episodes per day at week 12 and the proportion of patients reporting improvement or great improvement in their urinary incontinence (UI) at 12 weeks. Data were assessed for six subpopulations of patients based on the number of onabotA treatments (one to six) needed during the study; duration of effect (time to request for retreatment) in all six cycles also was evaluated in order to assess the consistency of response to repeated treatments.
Local anesthesia was administered to patients, and onabotA was delivered via injection into the muscle of the bladder. “Once the botulinum toxin gets into the terminal nerve, it will prevent the release of neurotransmitters, particularly acetylcholine; when acetylcholine is not released, there is less of a trigger for the bladder to contract,” he explained.
Of the 829 patients enrolled, 51.7% completed the 3-year study. About 5% did not complete the study because of an adverse event, and only 5.7% dropped out because of a lack of efficacy. “Over the 31/2-year period, patients were lost to follow-up, had protocol violations, and sites closed. So most of the reasons for discontinuation weren’t due to lack of efficacy or adverse events,” Dr. Nitti said.
The baseline mean UI episodes per day was a little over 5.5 for all treatment cycles; consistent reductions in UI episodes were observed in the overall population results regardless of the number of treatments received, with overall reduction between 3.1 and 3.8 episodes per day.
“Also consistent was the number of patients who reported being greatly improved or improved on the treatment benefit scale, which remained at right around 80% regardless of treatment cycle,” he added.
Patients who received fewer treatments had a longer duration of effect than those who received more treatments. The overall median duration of effect was 7.6 months, and 34.2% of the patients reported control of their urinary incontinence symptoms for at least 6 months. The median time to request retreatment was >6 to ≤12 months for 37.2%, and >12 months for 28.5% of patients. Urinary tract infection was the most common adverse event observed.
Based on these data, Dr. Nitti and his colleagues concluded that long-term treatment of OAB with onabotA resulted in decreased daily urinary incontinence episodes, with no increase in adverse events tied to recurrent treatment.
Dr. Nitti disclosed financial relationships with Allergan, and numerous other pharmaceutical and device companies.
NEW ORLEANS – Long-term treatment with onabotulinumtoxinA significantly decreased daily urinary incontinence episodes in patients with overactive bladder syndrome, with no increase in adverse effects tied to repeated treatment, according to Dr. Victor W. Nitti.
Dr. Nitti and his colleagues conducted a multicenter extension study evaluating the long-term efficacy and safety of repeated treatments with onabotulinumtoxinA (onabotA) in patients with overactive bladder (OAB).
After completion of either of two 24-week, randomized phase III trials, patients were eligible to enter a 3-year extension study in which they could receive multiple onabotA treatments at 100 units per dose, Dr Nitti reported at the annual meeting of the American Urological Association.
Patients were treated “as needed” based on their request, and their fulfillment of the prespecified qualification criteria. “Patients requesting treatment had to have at least two urgency incontinence episodes in a 3-day diary, at least 12 weeks since their last treatment, and their postvoid residual had to be less than 200 cc,” said Dr. Nitti, professor of urology at New York University, New York. Therefore, the total number of treatments delivered during the study differed among patients depending on need.
Coprimary endpoints included change from baseline in urinary incontinence episodes per day at week 12 and the proportion of patients reporting improvement or great improvement in their urinary incontinence (UI) at 12 weeks. Data were assessed for six subpopulations of patients based on the number of onabotA treatments (one to six) needed during the study; duration of effect (time to request for retreatment) in all six cycles also was evaluated in order to assess the consistency of response to repeated treatments.
Local anesthesia was administered to patients, and onabotA was delivered via injection into the muscle of the bladder. “Once the botulinum toxin gets into the terminal nerve, it will prevent the release of neurotransmitters, particularly acetylcholine; when acetylcholine is not released, there is less of a trigger for the bladder to contract,” he explained.
Of the 829 patients enrolled, 51.7% completed the 3-year study. About 5% did not complete the study because of an adverse event, and only 5.7% dropped out because of a lack of efficacy. “Over the 31/2-year period, patients were lost to follow-up, had protocol violations, and sites closed. So most of the reasons for discontinuation weren’t due to lack of efficacy or adverse events,” Dr. Nitti said.
The baseline mean UI episodes per day was a little over 5.5 for all treatment cycles; consistent reductions in UI episodes were observed in the overall population results regardless of the number of treatments received, with overall reduction between 3.1 and 3.8 episodes per day.
“Also consistent was the number of patients who reported being greatly improved or improved on the treatment benefit scale, which remained at right around 80% regardless of treatment cycle,” he added.
Patients who received fewer treatments had a longer duration of effect than those who received more treatments. The overall median duration of effect was 7.6 months, and 34.2% of the patients reported control of their urinary incontinence symptoms for at least 6 months. The median time to request retreatment was >6 to ≤12 months for 37.2%, and >12 months for 28.5% of patients. Urinary tract infection was the most common adverse event observed.
Based on these data, Dr. Nitti and his colleagues concluded that long-term treatment of OAB with onabotA resulted in decreased daily urinary incontinence episodes, with no increase in adverse events tied to recurrent treatment.
Dr. Nitti disclosed financial relationships with Allergan, and numerous other pharmaceutical and device companies.
NEW ORLEANS – Long-term treatment with onabotulinumtoxinA significantly decreased daily urinary incontinence episodes in patients with overactive bladder syndrome, with no increase in adverse effects tied to repeated treatment, according to Dr. Victor W. Nitti.
Dr. Nitti and his colleagues conducted a multicenter extension study evaluating the long-term efficacy and safety of repeated treatments with onabotulinumtoxinA (onabotA) in patients with overactive bladder (OAB).
After completion of either of two 24-week, randomized phase III trials, patients were eligible to enter a 3-year extension study in which they could receive multiple onabotA treatments at 100 units per dose, Dr Nitti reported at the annual meeting of the American Urological Association.
Patients were treated “as needed” based on their request, and their fulfillment of the prespecified qualification criteria. “Patients requesting treatment had to have at least two urgency incontinence episodes in a 3-day diary, at least 12 weeks since their last treatment, and their postvoid residual had to be less than 200 cc,” said Dr. Nitti, professor of urology at New York University, New York. Therefore, the total number of treatments delivered during the study differed among patients depending on need.
Coprimary endpoints included change from baseline in urinary incontinence episodes per day at week 12 and the proportion of patients reporting improvement or great improvement in their urinary incontinence (UI) at 12 weeks. Data were assessed for six subpopulations of patients based on the number of onabotA treatments (one to six) needed during the study; duration of effect (time to request for retreatment) in all six cycles also was evaluated in order to assess the consistency of response to repeated treatments.
Local anesthesia was administered to patients, and onabotA was delivered via injection into the muscle of the bladder. “Once the botulinum toxin gets into the terminal nerve, it will prevent the release of neurotransmitters, particularly acetylcholine; when acetylcholine is not released, there is less of a trigger for the bladder to contract,” he explained.
Of the 829 patients enrolled, 51.7% completed the 3-year study. About 5% did not complete the study because of an adverse event, and only 5.7% dropped out because of a lack of efficacy. “Over the 31/2-year period, patients were lost to follow-up, had protocol violations, and sites closed. So most of the reasons for discontinuation weren’t due to lack of efficacy or adverse events,” Dr. Nitti said.
The baseline mean UI episodes per day was a little over 5.5 for all treatment cycles; consistent reductions in UI episodes were observed in the overall population results regardless of the number of treatments received, with overall reduction between 3.1 and 3.8 episodes per day.
“Also consistent was the number of patients who reported being greatly improved or improved on the treatment benefit scale, which remained at right around 80% regardless of treatment cycle,” he added.
Patients who received fewer treatments had a longer duration of effect than those who received more treatments. The overall median duration of effect was 7.6 months, and 34.2% of the patients reported control of their urinary incontinence symptoms for at least 6 months. The median time to request retreatment was >6 to ≤12 months for 37.2%, and >12 months for 28.5% of patients. Urinary tract infection was the most common adverse event observed.
Based on these data, Dr. Nitti and his colleagues concluded that long-term treatment of OAB with onabotA resulted in decreased daily urinary incontinence episodes, with no increase in adverse events tied to recurrent treatment.
Dr. Nitti disclosed financial relationships with Allergan, and numerous other pharmaceutical and device companies.
AT THE AUA ANNUAL MEETING
Key clinical point: OnabotulinumtoxinA delivered via injection into the muscle of the bladder appears to be a good option for patients with overactive bladder syndrome (OAB) experiencing daily urinary incontinence (UI) episodes.
Major finding: Consistent reductions in UI episodes were observed in the overall population results, regardless of the number of treatments received. Overall, reductions were between 3.1 and 3.8 episodes per day.
Data source: A multicenter extension study of more than 400 patients with OAB experiencing daily UI episodes.
Disclosures: Dr. Nitti disclosed financial relationships with Allergan, and numerous other pharmaceutical and device companies.
Perfect is the enemy of good
Urban legends never seem to die. They haunt those who chase the truth because most legends have a kernel of truth. Reflux nephropathy is one of those legends.
In the 1970s, neurosurgeons began treating children with spina bifida rather than allowing them to die shortly after birth. As these children entered their second and third decade of life, episodes of renal failure were noted. The reflux and recurring urinary tract infections (UTIs) from neurogenic bladders damaged kidneys. Self-catheter programs were invented and were effective. Surgical correction of anatomic urinary obstructions and severe reflux yielded similar benefits. By the 1990s, this paradigm had been extrapolated to all children with vesicoureteral reflux (VUR) and codified in the 1999 practice parameter. The unproven hope was that aggressive antibiotic prophylaxis to protect young, growing kidneys from infections would reduce the incidence of renal failure and hypertension in adults.
This is a common methodology for quality improvement at a Mortality and Morbidity conference. A problem is identified. A solution is developed to prevent the bad outcome. The solution is implemented without fully proving that the obvious, customized intervention truly works. No one ever assesses whether the remedy causes more mischief than benefit.
VUR has a pyramid shaped spectrum. Few children have the severe grade V reflux which responds to surgical intervention. At the base of the pyramid are a much larger group of children with mild reflux that usually resolves spontaneously by age 5 years. This pyramid is a setup for overdiagnosis and overtreatment of mild disease. Pediatricians soon recognized that the small portion of the 1999 practice parameter addressing reflux nephropathy was overly aggressive and based on unsound science. However, that same lack of clear evidence delayed creating a new consensus until 2011.
The recent efforts to prove the benefit of prophylaxis used exemplary evidence-based medicine. The RIVUR study over 4 years assessed 10,000 children in a multicenter study involving 19 locations. It enrolled 600 children in a prospective, double-blind, randomized, controlled trial with a placebo control. It followed the children for 2 years. Even by modern standards, this was a huge, prolonged and well-designed trial. It did demonstrate a benefit. About 20% of children on placebo had a recurrent UTI in that 2-year time frame. There was a 50% reduction in the number of UTIs in the children treated with antibiotic prophylaxis. Phrased that way, it was a success. But the numbers can be spun differently. The article duly noted a number needed to treat of eight. Eight children treated for 2 years at 365 days per year and one dose per day, means that 6,000 doses of antibiotics were necessary to prevent one UTI. There was no demonstrated benefit in renal scarring, renal failure, or other long-term outcomes. There was a downside. The rate of antibiotic-resistant organisms in the breakthrough UTIs tripled from 19% of the placebo group to 63% of the prophylaxis group. As large as this study was, it wasn’t able to measure the rate of other known adverse outcomes, such as Stevens-Johnson syndrome from the use of sulfa medications or the impact on resistant infections elsewhere in the body.
With the 2011 practice parameter, pediatricians became less aggressive at working up first UTIs. Urologists disagreed. The May 2015 issue of AAP News had a full page article by Dr. Saul Greenfield, who is the chairperson-elect for the Executive Committee of the American Academy of Pediatrics Section on Urology, a urologist in Buffalo, N.Y., and one of the RIVUR trial’s investigators (AAP News 2015;36:13). He rehashed the RIVUR study results emphasizing the reduction in UTIs, but offered no quantitative assessment of the risks, costs, and harms of prophylaxis.
A June 2015 article in Pediatrics gives the results of the CUTIE study, which ran in parallel with the RIVUR study (Pediatrics 2015 [doi:10.1542/peds.2015-0409]). The conclusions: “VUR and BBD [bladder and bowel dysfunction] are risk factors for recurrent UTI, especially when they appear in combination. Strategies for preventing recurrent UTI include antimicrobial prophylaxis and treatment of BBD.”
The article concludes with, “Therefore, clinicians must help families decide whether the benefits of prophylaxis outweigh the risks and inconvenience. … Additional research is needed to validate the risk factors and profiles that we identified.”
But six pages of discussing renal scarring (which is only a proxy for a small risk of future renal failure or hypertension), followed by a couple paragraphs, without numbers, about the risks of prophylaxis, does not provide the balanced presentation clinicians need to help families make wise decisions. In the new era of Choosing Wisely, scientific articles making clinical recommendations should not be published without an accompanying risk-benefit analysis, either in the article or in an editorial. The maxim in surgery, channeling Voltaire, is that “perfect is the enemy of good.”
There is mounting evidence that giving any antibiotics to young infants is harmful. Even 2 days of antibiotics before 1 month of age leads to measurable changes in the gut microbiota 6 months later. Antibiotics in infancy are associated with obesity at 24 months and at 48 months of age. All medical treatments introduce a substantial risk of harm. As Shakespeare wrote 400 years ago, “Striving to better, oft we mar what’s well.” I don’t doubt the conclusion that prophylaxis reduces UTIs, but giving 6,000 doses to prevent one UTI?! Even Kaley Cuoco can’t sell that.
Ultimately, this choice is not up to the hospitalist, the emergency department doctor, or the urologist. The decision belongs to the parents guided by a primary care doctor they trust. Our professional duty, ethically and legally, is to communicate the risks and benefits to the parents in a manner which they can understand and to provide them the support and counseling necessary to make a wise choice for their child. By focusing on the child and that duty, medical professionals can defuse any clashes of egos, departmental power struggles, or autocratic hierarchy that might interfere. Doctors educate and recommend, but the parent decides what is best for his or her child.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Dr. Powell said he had no relevant financial disclosures or conflicts of interest. E-mail him at [email protected].
Urban legends never seem to die. They haunt those who chase the truth because most legends have a kernel of truth. Reflux nephropathy is one of those legends.
In the 1970s, neurosurgeons began treating children with spina bifida rather than allowing them to die shortly after birth. As these children entered their second and third decade of life, episodes of renal failure were noted. The reflux and recurring urinary tract infections (UTIs) from neurogenic bladders damaged kidneys. Self-catheter programs were invented and were effective. Surgical correction of anatomic urinary obstructions and severe reflux yielded similar benefits. By the 1990s, this paradigm had been extrapolated to all children with vesicoureteral reflux (VUR) and codified in the 1999 practice parameter. The unproven hope was that aggressive antibiotic prophylaxis to protect young, growing kidneys from infections would reduce the incidence of renal failure and hypertension in adults.
This is a common methodology for quality improvement at a Mortality and Morbidity conference. A problem is identified. A solution is developed to prevent the bad outcome. The solution is implemented without fully proving that the obvious, customized intervention truly works. No one ever assesses whether the remedy causes more mischief than benefit.
VUR has a pyramid shaped spectrum. Few children have the severe grade V reflux which responds to surgical intervention. At the base of the pyramid are a much larger group of children with mild reflux that usually resolves spontaneously by age 5 years. This pyramid is a setup for overdiagnosis and overtreatment of mild disease. Pediatricians soon recognized that the small portion of the 1999 practice parameter addressing reflux nephropathy was overly aggressive and based on unsound science. However, that same lack of clear evidence delayed creating a new consensus until 2011.
The recent efforts to prove the benefit of prophylaxis used exemplary evidence-based medicine. The RIVUR study over 4 years assessed 10,000 children in a multicenter study involving 19 locations. It enrolled 600 children in a prospective, double-blind, randomized, controlled trial with a placebo control. It followed the children for 2 years. Even by modern standards, this was a huge, prolonged and well-designed trial. It did demonstrate a benefit. About 20% of children on placebo had a recurrent UTI in that 2-year time frame. There was a 50% reduction in the number of UTIs in the children treated with antibiotic prophylaxis. Phrased that way, it was a success. But the numbers can be spun differently. The article duly noted a number needed to treat of eight. Eight children treated for 2 years at 365 days per year and one dose per day, means that 6,000 doses of antibiotics were necessary to prevent one UTI. There was no demonstrated benefit in renal scarring, renal failure, or other long-term outcomes. There was a downside. The rate of antibiotic-resistant organisms in the breakthrough UTIs tripled from 19% of the placebo group to 63% of the prophylaxis group. As large as this study was, it wasn’t able to measure the rate of other known adverse outcomes, such as Stevens-Johnson syndrome from the use of sulfa medications or the impact on resistant infections elsewhere in the body.
With the 2011 practice parameter, pediatricians became less aggressive at working up first UTIs. Urologists disagreed. The May 2015 issue of AAP News had a full page article by Dr. Saul Greenfield, who is the chairperson-elect for the Executive Committee of the American Academy of Pediatrics Section on Urology, a urologist in Buffalo, N.Y., and one of the RIVUR trial’s investigators (AAP News 2015;36:13). He rehashed the RIVUR study results emphasizing the reduction in UTIs, but offered no quantitative assessment of the risks, costs, and harms of prophylaxis.
A June 2015 article in Pediatrics gives the results of the CUTIE study, which ran in parallel with the RIVUR study (Pediatrics 2015 [doi:10.1542/peds.2015-0409]). The conclusions: “VUR and BBD [bladder and bowel dysfunction] are risk factors for recurrent UTI, especially when they appear in combination. Strategies for preventing recurrent UTI include antimicrobial prophylaxis and treatment of BBD.”
The article concludes with, “Therefore, clinicians must help families decide whether the benefits of prophylaxis outweigh the risks and inconvenience. … Additional research is needed to validate the risk factors and profiles that we identified.”
But six pages of discussing renal scarring (which is only a proxy for a small risk of future renal failure or hypertension), followed by a couple paragraphs, without numbers, about the risks of prophylaxis, does not provide the balanced presentation clinicians need to help families make wise decisions. In the new era of Choosing Wisely, scientific articles making clinical recommendations should not be published without an accompanying risk-benefit analysis, either in the article or in an editorial. The maxim in surgery, channeling Voltaire, is that “perfect is the enemy of good.”
There is mounting evidence that giving any antibiotics to young infants is harmful. Even 2 days of antibiotics before 1 month of age leads to measurable changes in the gut microbiota 6 months later. Antibiotics in infancy are associated with obesity at 24 months and at 48 months of age. All medical treatments introduce a substantial risk of harm. As Shakespeare wrote 400 years ago, “Striving to better, oft we mar what’s well.” I don’t doubt the conclusion that prophylaxis reduces UTIs, but giving 6,000 doses to prevent one UTI?! Even Kaley Cuoco can’t sell that.
Ultimately, this choice is not up to the hospitalist, the emergency department doctor, or the urologist. The decision belongs to the parents guided by a primary care doctor they trust. Our professional duty, ethically and legally, is to communicate the risks and benefits to the parents in a manner which they can understand and to provide them the support and counseling necessary to make a wise choice for their child. By focusing on the child and that duty, medical professionals can defuse any clashes of egos, departmental power struggles, or autocratic hierarchy that might interfere. Doctors educate and recommend, but the parent decides what is best for his or her child.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Dr. Powell said he had no relevant financial disclosures or conflicts of interest. E-mail him at [email protected].
Urban legends never seem to die. They haunt those who chase the truth because most legends have a kernel of truth. Reflux nephropathy is one of those legends.
In the 1970s, neurosurgeons began treating children with spina bifida rather than allowing them to die shortly after birth. As these children entered their second and third decade of life, episodes of renal failure were noted. The reflux and recurring urinary tract infections (UTIs) from neurogenic bladders damaged kidneys. Self-catheter programs were invented and were effective. Surgical correction of anatomic urinary obstructions and severe reflux yielded similar benefits. By the 1990s, this paradigm had been extrapolated to all children with vesicoureteral reflux (VUR) and codified in the 1999 practice parameter. The unproven hope was that aggressive antibiotic prophylaxis to protect young, growing kidneys from infections would reduce the incidence of renal failure and hypertension in adults.
This is a common methodology for quality improvement at a Mortality and Morbidity conference. A problem is identified. A solution is developed to prevent the bad outcome. The solution is implemented without fully proving that the obvious, customized intervention truly works. No one ever assesses whether the remedy causes more mischief than benefit.
VUR has a pyramid shaped spectrum. Few children have the severe grade V reflux which responds to surgical intervention. At the base of the pyramid are a much larger group of children with mild reflux that usually resolves spontaneously by age 5 years. This pyramid is a setup for overdiagnosis and overtreatment of mild disease. Pediatricians soon recognized that the small portion of the 1999 practice parameter addressing reflux nephropathy was overly aggressive and based on unsound science. However, that same lack of clear evidence delayed creating a new consensus until 2011.
The recent efforts to prove the benefit of prophylaxis used exemplary evidence-based medicine. The RIVUR study over 4 years assessed 10,000 children in a multicenter study involving 19 locations. It enrolled 600 children in a prospective, double-blind, randomized, controlled trial with a placebo control. It followed the children for 2 years. Even by modern standards, this was a huge, prolonged and well-designed trial. It did demonstrate a benefit. About 20% of children on placebo had a recurrent UTI in that 2-year time frame. There was a 50% reduction in the number of UTIs in the children treated with antibiotic prophylaxis. Phrased that way, it was a success. But the numbers can be spun differently. The article duly noted a number needed to treat of eight. Eight children treated for 2 years at 365 days per year and one dose per day, means that 6,000 doses of antibiotics were necessary to prevent one UTI. There was no demonstrated benefit in renal scarring, renal failure, or other long-term outcomes. There was a downside. The rate of antibiotic-resistant organisms in the breakthrough UTIs tripled from 19% of the placebo group to 63% of the prophylaxis group. As large as this study was, it wasn’t able to measure the rate of other known adverse outcomes, such as Stevens-Johnson syndrome from the use of sulfa medications or the impact on resistant infections elsewhere in the body.
With the 2011 practice parameter, pediatricians became less aggressive at working up first UTIs. Urologists disagreed. The May 2015 issue of AAP News had a full page article by Dr. Saul Greenfield, who is the chairperson-elect for the Executive Committee of the American Academy of Pediatrics Section on Urology, a urologist in Buffalo, N.Y., and one of the RIVUR trial’s investigators (AAP News 2015;36:13). He rehashed the RIVUR study results emphasizing the reduction in UTIs, but offered no quantitative assessment of the risks, costs, and harms of prophylaxis.
A June 2015 article in Pediatrics gives the results of the CUTIE study, which ran in parallel with the RIVUR study (Pediatrics 2015 [doi:10.1542/peds.2015-0409]). The conclusions: “VUR and BBD [bladder and bowel dysfunction] are risk factors for recurrent UTI, especially when they appear in combination. Strategies for preventing recurrent UTI include antimicrobial prophylaxis and treatment of BBD.”
The article concludes with, “Therefore, clinicians must help families decide whether the benefits of prophylaxis outweigh the risks and inconvenience. … Additional research is needed to validate the risk factors and profiles that we identified.”
But six pages of discussing renal scarring (which is only a proxy for a small risk of future renal failure or hypertension), followed by a couple paragraphs, without numbers, about the risks of prophylaxis, does not provide the balanced presentation clinicians need to help families make wise decisions. In the new era of Choosing Wisely, scientific articles making clinical recommendations should not be published without an accompanying risk-benefit analysis, either in the article or in an editorial. The maxim in surgery, channeling Voltaire, is that “perfect is the enemy of good.”
There is mounting evidence that giving any antibiotics to young infants is harmful. Even 2 days of antibiotics before 1 month of age leads to measurable changes in the gut microbiota 6 months later. Antibiotics in infancy are associated with obesity at 24 months and at 48 months of age. All medical treatments introduce a substantial risk of harm. As Shakespeare wrote 400 years ago, “Striving to better, oft we mar what’s well.” I don’t doubt the conclusion that prophylaxis reduces UTIs, but giving 6,000 doses to prevent one UTI?! Even Kaley Cuoco can’t sell that.
Ultimately, this choice is not up to the hospitalist, the emergency department doctor, or the urologist. The decision belongs to the parents guided by a primary care doctor they trust. Our professional duty, ethically and legally, is to communicate the risks and benefits to the parents in a manner which they can understand and to provide them the support and counseling necessary to make a wise choice for their child. By focusing on the child and that duty, medical professionals can defuse any clashes of egos, departmental power struggles, or autocratic hierarchy that might interfere. Doctors educate and recommend, but the parent decides what is best for his or her child.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Dr. Powell said he had no relevant financial disclosures or conflicts of interest. E-mail him at [email protected].
ADA: Staying fit helps prevent advanced kidney disease in type 2 diabetes
BOSTON – Maintaining or improving cardiorespiratory fitness during the year following an intensive lifestyle intervention contributed to the prevention of advanced chronic kidney disease over 4 years among participants in Look AHEAD, according to findings from a substudy of that randomized clinical trial.
During the first 4 years of follow-up in 4,906 of the 5,145 original study subjects, the incidence of advanced chronic kidney disease – after adjustment for age, race, ethnicity, and disease-related baseline covariates – was reduced by nearly 60% in those who received the intervention in the original study, compared with those who received diabetes support and education (hazard ratio, 0.59), Dr. Margareta I. Hellgren reported at the annual scientific sessions of the American Diabetes Association.
At 1-year follow-up, fitness level was unchanged in 77% of the lifestyle intervention group patients, compared with 58% of those in the diabetes support and education group, and in a model that used the same covariates, plus 1-year fitness change, and which included annually updated weight, blood pressure, and hemoglobin A1c, both intervention group participation and unchanged or improved fitness predicted lower incidence of advanced CKD over 4 years (HR, 0.69, 0.49, respectively), said Dr. Hellgren of the University of Gothenburg, Sweden.
The multicenter Look AHEAD trial included overweight adults with type 2 diabetes who were followed for about 10 years. The findings, which were published in 2013, showed no difference between intensive lifestyle intervention – including physical activity and reduced calorie intake – and diabetes support and education for reducing the incidence of cardiovascular events (the primary outcome measure), but did demonstrate a benefit with intensive lifestyle intervention for a number of secondary outcomes, including nephropathy.
A secondary analysis published in The Lancet in 2014 showed that the incidence of advanced kidney disease was reduced by 31% over 8 years in those in the intervention group, compared with those in the diabetes care and intervention group – an effect that was partly attributable to reductions in body weight, HbA1c, and systolic blood pressure, she noted.
The outcome of the current substudy was very high risk kidney disease (classified according to the Kidney Disease Improving Global Outcomes Guidelines), which is an important cause of disability and high costs and is associated with high mortality, Dr. Hellgren said.
Study subjects had a mean age of 58.6 years at baseline, 59% were women, and 66% were non-Hispanic whites. Known diabetes duration was 6.7 years.
Fitness assessment was based on estimated metabolic equivalents (METs) during a graded treadmill exercise test at baseline and at 1 year. A significant association was seen between baseline fitness and class of chronic kidney disease, Dr. Hellgren said, noting that 31% of those with the lowest fitness level had abnormal kidney function, compared with 14% of those with the highest level.
“Physical fitness at baseline was positively associated with kidney function. Maintaining or improving fitness during the first year was associated with lower incidence of very high risk kidney disease in both intervention groups, with a 51% reduction overall,” she said, adding that the lifestyle intervention effect was only partially attenuated after accounting for fitness change and annually updated wight, blood pressure, and HbA1c, which suggests that an additional unknown mechanism is responsible for the benefits of intervention.
The National Institute of Diabetes and Digestive and Kidney Diseases sponsored Look AHEAD. Dr. Hellgren reported having no disclosures.
BOSTON – Maintaining or improving cardiorespiratory fitness during the year following an intensive lifestyle intervention contributed to the prevention of advanced chronic kidney disease over 4 years among participants in Look AHEAD, according to findings from a substudy of that randomized clinical trial.
During the first 4 years of follow-up in 4,906 of the 5,145 original study subjects, the incidence of advanced chronic kidney disease – after adjustment for age, race, ethnicity, and disease-related baseline covariates – was reduced by nearly 60% in those who received the intervention in the original study, compared with those who received diabetes support and education (hazard ratio, 0.59), Dr. Margareta I. Hellgren reported at the annual scientific sessions of the American Diabetes Association.
At 1-year follow-up, fitness level was unchanged in 77% of the lifestyle intervention group patients, compared with 58% of those in the diabetes support and education group, and in a model that used the same covariates, plus 1-year fitness change, and which included annually updated weight, blood pressure, and hemoglobin A1c, both intervention group participation and unchanged or improved fitness predicted lower incidence of advanced CKD over 4 years (HR, 0.69, 0.49, respectively), said Dr. Hellgren of the University of Gothenburg, Sweden.
The multicenter Look AHEAD trial included overweight adults with type 2 diabetes who were followed for about 10 years. The findings, which were published in 2013, showed no difference between intensive lifestyle intervention – including physical activity and reduced calorie intake – and diabetes support and education for reducing the incidence of cardiovascular events (the primary outcome measure), but did demonstrate a benefit with intensive lifestyle intervention for a number of secondary outcomes, including nephropathy.
A secondary analysis published in The Lancet in 2014 showed that the incidence of advanced kidney disease was reduced by 31% over 8 years in those in the intervention group, compared with those in the diabetes care and intervention group – an effect that was partly attributable to reductions in body weight, HbA1c, and systolic blood pressure, she noted.
The outcome of the current substudy was very high risk kidney disease (classified according to the Kidney Disease Improving Global Outcomes Guidelines), which is an important cause of disability and high costs and is associated with high mortality, Dr. Hellgren said.
Study subjects had a mean age of 58.6 years at baseline, 59% were women, and 66% were non-Hispanic whites. Known diabetes duration was 6.7 years.
Fitness assessment was based on estimated metabolic equivalents (METs) during a graded treadmill exercise test at baseline and at 1 year. A significant association was seen between baseline fitness and class of chronic kidney disease, Dr. Hellgren said, noting that 31% of those with the lowest fitness level had abnormal kidney function, compared with 14% of those with the highest level.
“Physical fitness at baseline was positively associated with kidney function. Maintaining or improving fitness during the first year was associated with lower incidence of very high risk kidney disease in both intervention groups, with a 51% reduction overall,” she said, adding that the lifestyle intervention effect was only partially attenuated after accounting for fitness change and annually updated wight, blood pressure, and HbA1c, which suggests that an additional unknown mechanism is responsible for the benefits of intervention.
The National Institute of Diabetes and Digestive and Kidney Diseases sponsored Look AHEAD. Dr. Hellgren reported having no disclosures.
BOSTON – Maintaining or improving cardiorespiratory fitness during the year following an intensive lifestyle intervention contributed to the prevention of advanced chronic kidney disease over 4 years among participants in Look AHEAD, according to findings from a substudy of that randomized clinical trial.
During the first 4 years of follow-up in 4,906 of the 5,145 original study subjects, the incidence of advanced chronic kidney disease – after adjustment for age, race, ethnicity, and disease-related baseline covariates – was reduced by nearly 60% in those who received the intervention in the original study, compared with those who received diabetes support and education (hazard ratio, 0.59), Dr. Margareta I. Hellgren reported at the annual scientific sessions of the American Diabetes Association.
At 1-year follow-up, fitness level was unchanged in 77% of the lifestyle intervention group patients, compared with 58% of those in the diabetes support and education group, and in a model that used the same covariates, plus 1-year fitness change, and which included annually updated weight, blood pressure, and hemoglobin A1c, both intervention group participation and unchanged or improved fitness predicted lower incidence of advanced CKD over 4 years (HR, 0.69, 0.49, respectively), said Dr. Hellgren of the University of Gothenburg, Sweden.
The multicenter Look AHEAD trial included overweight adults with type 2 diabetes who were followed for about 10 years. The findings, which were published in 2013, showed no difference between intensive lifestyle intervention – including physical activity and reduced calorie intake – and diabetes support and education for reducing the incidence of cardiovascular events (the primary outcome measure), but did demonstrate a benefit with intensive lifestyle intervention for a number of secondary outcomes, including nephropathy.
A secondary analysis published in The Lancet in 2014 showed that the incidence of advanced kidney disease was reduced by 31% over 8 years in those in the intervention group, compared with those in the diabetes care and intervention group – an effect that was partly attributable to reductions in body weight, HbA1c, and systolic blood pressure, she noted.
The outcome of the current substudy was very high risk kidney disease (classified according to the Kidney Disease Improving Global Outcomes Guidelines), which is an important cause of disability and high costs and is associated with high mortality, Dr. Hellgren said.
Study subjects had a mean age of 58.6 years at baseline, 59% were women, and 66% were non-Hispanic whites. Known diabetes duration was 6.7 years.
Fitness assessment was based on estimated metabolic equivalents (METs) during a graded treadmill exercise test at baseline and at 1 year. A significant association was seen between baseline fitness and class of chronic kidney disease, Dr. Hellgren said, noting that 31% of those with the lowest fitness level had abnormal kidney function, compared with 14% of those with the highest level.
“Physical fitness at baseline was positively associated with kidney function. Maintaining or improving fitness during the first year was associated with lower incidence of very high risk kidney disease in both intervention groups, with a 51% reduction overall,” she said, adding that the lifestyle intervention effect was only partially attenuated after accounting for fitness change and annually updated wight, blood pressure, and HbA1c, which suggests that an additional unknown mechanism is responsible for the benefits of intervention.
The National Institute of Diabetes and Digestive and Kidney Diseases sponsored Look AHEAD. Dr. Hellgren reported having no disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Maintaining or improving cardiorespiratory fitness during the year following an intensive lifestyle intervention contributed to the prevention of advanced chronic kidney disease over 4 years among participants in the Look AHEAD trial.
Major finding: Intervention group participation and unchanged or improved fitness predicted lower incidence of advanced CKD over 4 years (hazard ratios, 0.69 and 0.49, respectively).
Data source: A substudy of 4,906 subjects from the randomized Look AHEAD trial.
Disclosures: The National Institute of Diabetes and Digestive and Kidney Diseases sponsored Look AHEAD. Dr. Hellgren reported having no disclosures.
AUA: Renal mass biopsy trend tied to nonsurgical RCC treatment
NEW ORLEANS – Renal mass biopsy has traditionally played a restricted diagnostic role, but with its improved diagnostic accuracy, it is becoming a viable clinical tool in the modern era, according to Dr. Matthew Maurice.
“We were seeking to understand the current role of biopsy in the management of renal masses, said Dr. Maurice, a urology resident at University Hospitals Case Medical Center in Cleveland. “We used the National Cancer Database and looked at data from 2003 to 2011; what we saw was a rise in renal mass biopsy in the final 3 years of the study. It’s a very small increase, but a statistically significant increase, with people in 2011 having 1.3 times higher odds of being biopsied than they would have had in 2003.”
Dr. Maurice and his colleagues at Case Medical conducted a study examining renal mass biopsy use in the modern era, and presented their findings in a poster at the annual meeting of the American Urological Association.
Using the National Cancer Database (NCDB), Dr. Maurice and his colleagues identified all patients diagnosed with renal cell carcinoma (RCC) between 2003 and 2011. Patients within the RCC cohort were then classified as having undergone renal biopsy or not. Renal biopsy utilization rates were plotted over time, and patient, disease, provider, and treatment variables were evaluated via univariate and multivariate logistic regression models to determine the predictors of renal biopsy.
Out of 304,583 patients with kidney cancer, 35,942 patients (11.8%) underwent renal mass biopsy. From 2009 to 2011, Dr. Maurice and his coinvestigators observed a significant increase in biopsy use; patients diagnosed with a renal mass in 2011 had 1.3 times higher odds of being biopsied compared with those diagnosed in 2003 (odds radio, 1.3, confidence interval, 1.3-1.4, P < .01).
Eventual treatment was the strongest predictor of biopsy utilization. “Patients receiving observation or thermal ablative therapy (either cryoablation or radiofrequency ablation) were much more likely to receive biopsy than were those who received surgical therapy such as radical or partial nephrectomy,” Dr. Maurice explained. “So it seems like those treatments are driving the use of renal biopsy utilization in contemporary patients.”
Compared to patients treated with partial nephrectomy, patients managed with observation, cryoablation, or radiofrequency ablation had 4.2, 8.0, and 19.1 times the odds of being biopsied, respectively (OR, 4.2, CI, 4.0-4.5, P < .01; OR, 8.0, CI, 8.0-8.1, P < .01; OR, 19.1, CI, 18.4-19.7, P < .01). Patients with other known cancers, bulky lymph node involvement, or small masses ranging from 2 to 4 cm in size were also more likely to be biopsied (P < .01).
“Nonacademic hospitals were more likely to biopsy,” he added. “It could be that these hospitals are using observation and thermal ablative therapies more frequently.” Conversely, wealthier patients, patients treated at academic hospitals, and patients treated in the Northeast were significantly less likely to be biopsied. (P < .01).
On the basis of the data analyzed in this study, Dr. Maurice and his colleagues concluded that there is a trend in use of renal mass biopsy in nonacademic centers in recent years, particularly among patients with small renal masses and in those who eventually undergo observation or focal ablative therapies. Lesser indications predicting the usage of renal mass biopsy include the existence of other primary cancers and bulky lymph nodes.
Dr. Maurice reported no relevant financial relationships.
NEW ORLEANS – Renal mass biopsy has traditionally played a restricted diagnostic role, but with its improved diagnostic accuracy, it is becoming a viable clinical tool in the modern era, according to Dr. Matthew Maurice.
“We were seeking to understand the current role of biopsy in the management of renal masses, said Dr. Maurice, a urology resident at University Hospitals Case Medical Center in Cleveland. “We used the National Cancer Database and looked at data from 2003 to 2011; what we saw was a rise in renal mass biopsy in the final 3 years of the study. It’s a very small increase, but a statistically significant increase, with people in 2011 having 1.3 times higher odds of being biopsied than they would have had in 2003.”
Dr. Maurice and his colleagues at Case Medical conducted a study examining renal mass biopsy use in the modern era, and presented their findings in a poster at the annual meeting of the American Urological Association.
Using the National Cancer Database (NCDB), Dr. Maurice and his colleagues identified all patients diagnosed with renal cell carcinoma (RCC) between 2003 and 2011. Patients within the RCC cohort were then classified as having undergone renal biopsy or not. Renal biopsy utilization rates were plotted over time, and patient, disease, provider, and treatment variables were evaluated via univariate and multivariate logistic regression models to determine the predictors of renal biopsy.
Out of 304,583 patients with kidney cancer, 35,942 patients (11.8%) underwent renal mass biopsy. From 2009 to 2011, Dr. Maurice and his coinvestigators observed a significant increase in biopsy use; patients diagnosed with a renal mass in 2011 had 1.3 times higher odds of being biopsied compared with those diagnosed in 2003 (odds radio, 1.3, confidence interval, 1.3-1.4, P < .01).
Eventual treatment was the strongest predictor of biopsy utilization. “Patients receiving observation or thermal ablative therapy (either cryoablation or radiofrequency ablation) were much more likely to receive biopsy than were those who received surgical therapy such as radical or partial nephrectomy,” Dr. Maurice explained. “So it seems like those treatments are driving the use of renal biopsy utilization in contemporary patients.”
Compared to patients treated with partial nephrectomy, patients managed with observation, cryoablation, or radiofrequency ablation had 4.2, 8.0, and 19.1 times the odds of being biopsied, respectively (OR, 4.2, CI, 4.0-4.5, P < .01; OR, 8.0, CI, 8.0-8.1, P < .01; OR, 19.1, CI, 18.4-19.7, P < .01). Patients with other known cancers, bulky lymph node involvement, or small masses ranging from 2 to 4 cm in size were also more likely to be biopsied (P < .01).
“Nonacademic hospitals were more likely to biopsy,” he added. “It could be that these hospitals are using observation and thermal ablative therapies more frequently.” Conversely, wealthier patients, patients treated at academic hospitals, and patients treated in the Northeast were significantly less likely to be biopsied. (P < .01).
On the basis of the data analyzed in this study, Dr. Maurice and his colleagues concluded that there is a trend in use of renal mass biopsy in nonacademic centers in recent years, particularly among patients with small renal masses and in those who eventually undergo observation or focal ablative therapies. Lesser indications predicting the usage of renal mass biopsy include the existence of other primary cancers and bulky lymph nodes.
Dr. Maurice reported no relevant financial relationships.
NEW ORLEANS – Renal mass biopsy has traditionally played a restricted diagnostic role, but with its improved diagnostic accuracy, it is becoming a viable clinical tool in the modern era, according to Dr. Matthew Maurice.
“We were seeking to understand the current role of biopsy in the management of renal masses, said Dr. Maurice, a urology resident at University Hospitals Case Medical Center in Cleveland. “We used the National Cancer Database and looked at data from 2003 to 2011; what we saw was a rise in renal mass biopsy in the final 3 years of the study. It’s a very small increase, but a statistically significant increase, with people in 2011 having 1.3 times higher odds of being biopsied than they would have had in 2003.”
Dr. Maurice and his colleagues at Case Medical conducted a study examining renal mass biopsy use in the modern era, and presented their findings in a poster at the annual meeting of the American Urological Association.
Using the National Cancer Database (NCDB), Dr. Maurice and his colleagues identified all patients diagnosed with renal cell carcinoma (RCC) between 2003 and 2011. Patients within the RCC cohort were then classified as having undergone renal biopsy or not. Renal biopsy utilization rates were plotted over time, and patient, disease, provider, and treatment variables were evaluated via univariate and multivariate logistic regression models to determine the predictors of renal biopsy.
Out of 304,583 patients with kidney cancer, 35,942 patients (11.8%) underwent renal mass biopsy. From 2009 to 2011, Dr. Maurice and his coinvestigators observed a significant increase in biopsy use; patients diagnosed with a renal mass in 2011 had 1.3 times higher odds of being biopsied compared with those diagnosed in 2003 (odds radio, 1.3, confidence interval, 1.3-1.4, P < .01).
Eventual treatment was the strongest predictor of biopsy utilization. “Patients receiving observation or thermal ablative therapy (either cryoablation or radiofrequency ablation) were much more likely to receive biopsy than were those who received surgical therapy such as radical or partial nephrectomy,” Dr. Maurice explained. “So it seems like those treatments are driving the use of renal biopsy utilization in contemporary patients.”
Compared to patients treated with partial nephrectomy, patients managed with observation, cryoablation, or radiofrequency ablation had 4.2, 8.0, and 19.1 times the odds of being biopsied, respectively (OR, 4.2, CI, 4.0-4.5, P < .01; OR, 8.0, CI, 8.0-8.1, P < .01; OR, 19.1, CI, 18.4-19.7, P < .01). Patients with other known cancers, bulky lymph node involvement, or small masses ranging from 2 to 4 cm in size were also more likely to be biopsied (P < .01).
“Nonacademic hospitals were more likely to biopsy,” he added. “It could be that these hospitals are using observation and thermal ablative therapies more frequently.” Conversely, wealthier patients, patients treated at academic hospitals, and patients treated in the Northeast were significantly less likely to be biopsied. (P < .01).
On the basis of the data analyzed in this study, Dr. Maurice and his colleagues concluded that there is a trend in use of renal mass biopsy in nonacademic centers in recent years, particularly among patients with small renal masses and in those who eventually undergo observation or focal ablative therapies. Lesser indications predicting the usage of renal mass biopsy include the existence of other primary cancers and bulky lymph nodes.
Dr. Maurice reported no relevant financial relationships.
AT THE AUA ANNUAL MEETING
Key clinical point: Use of renal mass biopsy is increasing and the increase is likely linked to choice of treatment (observation or thermal ablative therapy).
Major finding: Patients diagnosed with renal mass had 1.3 times higher odds of being biopsied in 2011 than they would have had in 2003.
Data source: Sample from 2003-2011 National Cancer Database of 304,583 patients with kidney cancer, 35,942 of whom underwent renal mass biopsy.
Disclosures: Dr. Maurice reported no relevant financial relationships.
Corneal opacities in a man with chronic kidney disease
A 40-year-old man with end-stage renal disease on intermittent hemodialysis presented to the emergency department with a 1-week history of pain affecting his left lower back, left flank, and left lower abdomen, diagnosed as zoster prodrome.
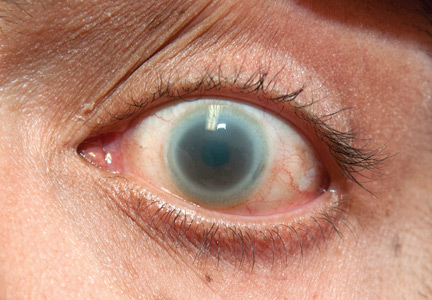
Of note, both corneas were cloudy, most severely in the limbus (Figure 1). His visual acuity and findings on funduscopic examination were normal.
CORNEAL OPACITY
The finding of corneal opacity should prompt an immediate ophthalmologic evaluation by the internist as well as an ophthalmologist. The initial examination should include visual acuity testing; gross examination with the naked eye; penlight examination of the pupil, conjunctiva, and anterior chamber; funduscopic examination to at least confirm a red reflex; and fluorescein examination of the cornea. Fluorescein testing is done last, as the dye may interfere with the other initial tests.1
A number of causes of opacity
A number of conditions can cause corneal opacity. Several genetic conditions can cause developmental anomalies of the cornea, leading to corneal defects present at birth.2 Causes of secondary corneal opacity in early infancy include infections such as herpes, iatrogenic injury during amniocentesis or forceps delivery, and infantile congenital glaucoma.2
Later in life, causes of corneal opacity include cataract, glaucoma, chemical exposure, foreign body injury, irradiation, infection (eg, syphilis, herpes, chlamydia), endophthalmitis, and metabolic genetic disorders such as Fabry disease, trisomy 18 syndrome, and lecithin-cholesterol acyltransferase (LCAT) deficiency.3
LCAT DEFICIENCY
LCAT is a key protein in reverse transport of cholesterol from the systemic circulation to the liver for excretion into the bile. Its deficiency results in low serum concentrations of high-density lipoprotein cholesterol (HDL-C).4 About 80 different mutations in the LCAT gene have been linked to LCAT deficiency.5
LCAT deficiency varies in severity. Patients with complete deficiency can have nearly undetectable levels of HDL-C, eruptive xanthoma, hepatosplenomegaly, and premature coronary artery disease (ie, by age 40).5,6 Features of coronary atherosclerosis can be lacking in patients with partial deficiency.
Regardless of the degree of LCAT deficiency, most patients have corneal opacification that is most severe near the limbus (thus, the term “fish eye syndrome”) and anemia.7 Although corneal opacification presents early in life and persists, it does not seem to affect vision.5 The anemia is associated with enhanced fractional clearance of red blood cells secondary to hypersplenism.8
LCAT deficiency and the kidneys
LCAT deficiency has its most devastating effect on the kidney. Renal disease begins early in life with mild proteinuria and microscopic hematuria. With increasing age, renal function deteriorates and proteinuria and hematuria worsen.9
Renal biopsy study may reveal foam cells in the glomerular tufts, arterioles with thickened intima and narrowed lumens, and subendothelial deposits of lipids in the renal arteries and arterioles.10 Some studies have suggested that kidney disease is most likely initiated by lipid deposition or cellular uptake of lipoproteins in the glomerular basement membrane, mesangium, and capillary subendothelium.
Treatment
There are few treatment options for patients with LCAT deficiency. Control of hypertension, if present, may halt or slow renal deterioration.5 Many patients eventually require dialysis, and some undergo renal transplantation, but the renal disease can recur.
OUR PATIENT
Our patient had a known diagnosis of LCAT deficiency. Five years before this presentation at our emergency department, he developed malignant hypertension, followed shortly by renal disease. Over the next 4 years, his kidney function deteriorated, culminating in the need for dialysis; his corneal opacities manifested and gradually worsened; and after extensive studies including kidney biopsies, he was finally diagnosed with LCAT deficiency.
He also exhibited a chronically low level of HDL-C (2 to 5 mg/dL) and significant coronary artery disease. Although unrelated, his zoster pain was treated with renally dosed acyclovir and gabapentin. He never demonstrated the characteristic rash, and his pain improved significantly within 5 days of treatment.
- Knox KA, McIntee J. Nurse management of corneal abrasion. Br J Nurs 1995; 4:440–460.
- Nischal KK. Congenital corneal opacities—a surgical approach to nomenclature and classification. Eye (Lond) 2007; 21:1326–1337.
- Chiapella AP, Rosenthal AR. One year in an eye casualty clinic. Br J Ophthalmol 1985; 69:865–870.
- Rosenson RS, Brewer HB Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012; 125:1905–1919.
- Roshan B, Ganda OP, Desilva R, et al. Homozygous lecithin:cholesterol acyltransferase (LCAT) deficiency due to a new loss of function mutation and review of the literature. J Clin Lipidol 2011; 5:493–499.
- Kuivenhoven JA, van Voorst tot Voorst EJ, Wiebusch H, et al. A unique genetic and biochemical presentation of fish-eye disease. J Clin Invest 1995; 96:2783–2791.
- Palmiero PM, Sbeity Z, Liebmann J, Ritch R. In vivo imaging of the cornea in a patient with lecithin-cholesterol acyltransferase deficiency. Cornea 2009; 28:1061–1064.
- Norum KR, Gjone E. Familial serum-cholesterol esterification failure. A new inborn error of metabolism. Biochim Biophys Acta 1967; 144:698–700.
- Gjone E, Norum KR. Familial serum cholesterol ester deficiency. Clinical study of a patient with a new syndrome. Acta Med Scand 1968; 183:107–112.
- Lager DJ, Rosenberg BF, Shapiro H, Bernstein J. Lecithin cholesterol acyltransferase deficiency: ultrastructural examination of sequential renal biopsies. Mod Pathol 1991; 4:331–335.
A 40-year-old man with end-stage renal disease on intermittent hemodialysis presented to the emergency department with a 1-week history of pain affecting his left lower back, left flank, and left lower abdomen, diagnosed as zoster prodrome.
Of note, both corneas were cloudy, most severely in the limbus (Figure 1). His visual acuity and findings on funduscopic examination were normal.
CORNEAL OPACITY
The finding of corneal opacity should prompt an immediate ophthalmologic evaluation by the internist as well as an ophthalmologist. The initial examination should include visual acuity testing; gross examination with the naked eye; penlight examination of the pupil, conjunctiva, and anterior chamber; funduscopic examination to at least confirm a red reflex; and fluorescein examination of the cornea. Fluorescein testing is done last, as the dye may interfere with the other initial tests.1
A number of causes of opacity
A number of conditions can cause corneal opacity. Several genetic conditions can cause developmental anomalies of the cornea, leading to corneal defects present at birth.2 Causes of secondary corneal opacity in early infancy include infections such as herpes, iatrogenic injury during amniocentesis or forceps delivery, and infantile congenital glaucoma.2
Later in life, causes of corneal opacity include cataract, glaucoma, chemical exposure, foreign body injury, irradiation, infection (eg, syphilis, herpes, chlamydia), endophthalmitis, and metabolic genetic disorders such as Fabry disease, trisomy 18 syndrome, and lecithin-cholesterol acyltransferase (LCAT) deficiency.3
LCAT DEFICIENCY
LCAT is a key protein in reverse transport of cholesterol from the systemic circulation to the liver for excretion into the bile. Its deficiency results in low serum concentrations of high-density lipoprotein cholesterol (HDL-C).4 About 80 different mutations in the LCAT gene have been linked to LCAT deficiency.5
LCAT deficiency varies in severity. Patients with complete deficiency can have nearly undetectable levels of HDL-C, eruptive xanthoma, hepatosplenomegaly, and premature coronary artery disease (ie, by age 40).5,6 Features of coronary atherosclerosis can be lacking in patients with partial deficiency.
Regardless of the degree of LCAT deficiency, most patients have corneal opacification that is most severe near the limbus (thus, the term “fish eye syndrome”) and anemia.7 Although corneal opacification presents early in life and persists, it does not seem to affect vision.5 The anemia is associated with enhanced fractional clearance of red blood cells secondary to hypersplenism.8
LCAT deficiency and the kidneys
LCAT deficiency has its most devastating effect on the kidney. Renal disease begins early in life with mild proteinuria and microscopic hematuria. With increasing age, renal function deteriorates and proteinuria and hematuria worsen.9
Renal biopsy study may reveal foam cells in the glomerular tufts, arterioles with thickened intima and narrowed lumens, and subendothelial deposits of lipids in the renal arteries and arterioles.10 Some studies have suggested that kidney disease is most likely initiated by lipid deposition or cellular uptake of lipoproteins in the glomerular basement membrane, mesangium, and capillary subendothelium.
Treatment
There are few treatment options for patients with LCAT deficiency. Control of hypertension, if present, may halt or slow renal deterioration.5 Many patients eventually require dialysis, and some undergo renal transplantation, but the renal disease can recur.
OUR PATIENT
Our patient had a known diagnosis of LCAT deficiency. Five years before this presentation at our emergency department, he developed malignant hypertension, followed shortly by renal disease. Over the next 4 years, his kidney function deteriorated, culminating in the need for dialysis; his corneal opacities manifested and gradually worsened; and after extensive studies including kidney biopsies, he was finally diagnosed with LCAT deficiency.
He also exhibited a chronically low level of HDL-C (2 to 5 mg/dL) and significant coronary artery disease. Although unrelated, his zoster pain was treated with renally dosed acyclovir and gabapentin. He never demonstrated the characteristic rash, and his pain improved significantly within 5 days of treatment.
A 40-year-old man with end-stage renal disease on intermittent hemodialysis presented to the emergency department with a 1-week history of pain affecting his left lower back, left flank, and left lower abdomen, diagnosed as zoster prodrome.
Of note, both corneas were cloudy, most severely in the limbus (Figure 1). His visual acuity and findings on funduscopic examination were normal.
CORNEAL OPACITY
The finding of corneal opacity should prompt an immediate ophthalmologic evaluation by the internist as well as an ophthalmologist. The initial examination should include visual acuity testing; gross examination with the naked eye; penlight examination of the pupil, conjunctiva, and anterior chamber; funduscopic examination to at least confirm a red reflex; and fluorescein examination of the cornea. Fluorescein testing is done last, as the dye may interfere with the other initial tests.1
A number of causes of opacity
A number of conditions can cause corneal opacity. Several genetic conditions can cause developmental anomalies of the cornea, leading to corneal defects present at birth.2 Causes of secondary corneal opacity in early infancy include infections such as herpes, iatrogenic injury during amniocentesis or forceps delivery, and infantile congenital glaucoma.2
Later in life, causes of corneal opacity include cataract, glaucoma, chemical exposure, foreign body injury, irradiation, infection (eg, syphilis, herpes, chlamydia), endophthalmitis, and metabolic genetic disorders such as Fabry disease, trisomy 18 syndrome, and lecithin-cholesterol acyltransferase (LCAT) deficiency.3
LCAT DEFICIENCY
LCAT is a key protein in reverse transport of cholesterol from the systemic circulation to the liver for excretion into the bile. Its deficiency results in low serum concentrations of high-density lipoprotein cholesterol (HDL-C).4 About 80 different mutations in the LCAT gene have been linked to LCAT deficiency.5
LCAT deficiency varies in severity. Patients with complete deficiency can have nearly undetectable levels of HDL-C, eruptive xanthoma, hepatosplenomegaly, and premature coronary artery disease (ie, by age 40).5,6 Features of coronary atherosclerosis can be lacking in patients with partial deficiency.
Regardless of the degree of LCAT deficiency, most patients have corneal opacification that is most severe near the limbus (thus, the term “fish eye syndrome”) and anemia.7 Although corneal opacification presents early in life and persists, it does not seem to affect vision.5 The anemia is associated with enhanced fractional clearance of red blood cells secondary to hypersplenism.8
LCAT deficiency and the kidneys
LCAT deficiency has its most devastating effect on the kidney. Renal disease begins early in life with mild proteinuria and microscopic hematuria. With increasing age, renal function deteriorates and proteinuria and hematuria worsen.9
Renal biopsy study may reveal foam cells in the glomerular tufts, arterioles with thickened intima and narrowed lumens, and subendothelial deposits of lipids in the renal arteries and arterioles.10 Some studies have suggested that kidney disease is most likely initiated by lipid deposition or cellular uptake of lipoproteins in the glomerular basement membrane, mesangium, and capillary subendothelium.
Treatment
There are few treatment options for patients with LCAT deficiency. Control of hypertension, if present, may halt or slow renal deterioration.5 Many patients eventually require dialysis, and some undergo renal transplantation, but the renal disease can recur.
OUR PATIENT
Our patient had a known diagnosis of LCAT deficiency. Five years before this presentation at our emergency department, he developed malignant hypertension, followed shortly by renal disease. Over the next 4 years, his kidney function deteriorated, culminating in the need for dialysis; his corneal opacities manifested and gradually worsened; and after extensive studies including kidney biopsies, he was finally diagnosed with LCAT deficiency.
He also exhibited a chronically low level of HDL-C (2 to 5 mg/dL) and significant coronary artery disease. Although unrelated, his zoster pain was treated with renally dosed acyclovir and gabapentin. He never demonstrated the characteristic rash, and his pain improved significantly within 5 days of treatment.
- Knox KA, McIntee J. Nurse management of corneal abrasion. Br J Nurs 1995; 4:440–460.
- Nischal KK. Congenital corneal opacities—a surgical approach to nomenclature and classification. Eye (Lond) 2007; 21:1326–1337.
- Chiapella AP, Rosenthal AR. One year in an eye casualty clinic. Br J Ophthalmol 1985; 69:865–870.
- Rosenson RS, Brewer HB Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012; 125:1905–1919.
- Roshan B, Ganda OP, Desilva R, et al. Homozygous lecithin:cholesterol acyltransferase (LCAT) deficiency due to a new loss of function mutation and review of the literature. J Clin Lipidol 2011; 5:493–499.
- Kuivenhoven JA, van Voorst tot Voorst EJ, Wiebusch H, et al. A unique genetic and biochemical presentation of fish-eye disease. J Clin Invest 1995; 96:2783–2791.
- Palmiero PM, Sbeity Z, Liebmann J, Ritch R. In vivo imaging of the cornea in a patient with lecithin-cholesterol acyltransferase deficiency. Cornea 2009; 28:1061–1064.
- Norum KR, Gjone E. Familial serum-cholesterol esterification failure. A new inborn error of metabolism. Biochim Biophys Acta 1967; 144:698–700.
- Gjone E, Norum KR. Familial serum cholesterol ester deficiency. Clinical study of a patient with a new syndrome. Acta Med Scand 1968; 183:107–112.
- Lager DJ, Rosenberg BF, Shapiro H, Bernstein J. Lecithin cholesterol acyltransferase deficiency: ultrastructural examination of sequential renal biopsies. Mod Pathol 1991; 4:331–335.
- Knox KA, McIntee J. Nurse management of corneal abrasion. Br J Nurs 1995; 4:440–460.
- Nischal KK. Congenital corneal opacities—a surgical approach to nomenclature and classification. Eye (Lond) 2007; 21:1326–1337.
- Chiapella AP, Rosenthal AR. One year in an eye casualty clinic. Br J Ophthalmol 1985; 69:865–870.
- Rosenson RS, Brewer HB Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012; 125:1905–1919.
- Roshan B, Ganda OP, Desilva R, et al. Homozygous lecithin:cholesterol acyltransferase (LCAT) deficiency due to a new loss of function mutation and review of the literature. J Clin Lipidol 2011; 5:493–499.
- Kuivenhoven JA, van Voorst tot Voorst EJ, Wiebusch H, et al. A unique genetic and biochemical presentation of fish-eye disease. J Clin Invest 1995; 96:2783–2791.
- Palmiero PM, Sbeity Z, Liebmann J, Ritch R. In vivo imaging of the cornea in a patient with lecithin-cholesterol acyltransferase deficiency. Cornea 2009; 28:1061–1064.
- Norum KR, Gjone E. Familial serum-cholesterol esterification failure. A new inborn error of metabolism. Biochim Biophys Acta 1967; 144:698–700.
- Gjone E, Norum KR. Familial serum cholesterol ester deficiency. Clinical study of a patient with a new syndrome. Acta Med Scand 1968; 183:107–112.
- Lager DJ, Rosenberg BF, Shapiro H, Bernstein J. Lecithin cholesterol acyltransferase deficiency: ultrastructural examination of sequential renal biopsies. Mod Pathol 1991; 4:331–335.
Vaccination: Special populations are not all the same
Vaccination is the standard of care as part of health maintenance for healthy people and for patients with myriad medical conditions. In an article in this issue of Cleveland Clinic Journal of Medicine, Drs. Faria Farhat and Glenn Wortmann1 make recommendations about vaccinations in special populations, including people with a disordered immune system or who are otherwise at heightened risk of infection (eg, because of older age, international travel, comorbidities, and medications).
But special populations are not all the same in their responses to vaccination. For example, patients with systemic autoimmune diseases are a heterogeneous group with disease-specific immunologic perturbations and immunosuppression that vary by medication and dose, all affecting the response to vaccination. Also, two or more “special” situations may coexist in the same patient.
AREAS OF UNCERTAINTY FOR CLINICAL PRACTICE
Several groups have issued guidelines and recommendations about vaccination in immunocompromised patients, but many areas of uncertainty exist in clinical practice. Most of these arise from a lack of data on immunogenicity and outcomes.
For example, although two pneumococcal vaccines are used in adults, no studies have compared the immunogenicity of the 13-valent pneumococcal conjugate vaccine (PCV13, which is T–cell-dependent) with that of the 23-valent pneumococcal polysaccharide vaccine (PPSV23, which is T–cell-independent) in immunocompromised adults.
Also, whether to use zoster vaccine in immunosuppressed patients ages 50 through 59 is debated. The vaccine is approved by the US Food and Drug Administration (FDA) for this age group, but the Advisory Committee on Immunization Practices (ACIP) does not recommend it, and the Infectious Diseases Society of America (IDSA) suggests that it be “considered” in patients on low-intensity immunosuppressive treatment.2
Testing to ensure optimal response to vaccination has been recommended in several articles and guidelines. However, antibody titers do not necessarily correlate with protection, and at this time no consensus exists about the timing of or need for testing for the response to immunization, the methods to use, how to interpret the results in terms of an adequate or inadequate response, or the role of booster immunization in routine clinical practice.
IS VACCINATION COST-EFFECTIVE?
Also relevant is whether vaccination is cost-effective.
Although vaccination with the PCV13 vaccine is possibly more cost-effective than PPSV23 in US adults based on a model that included immunosuppressed patients, the results of this study were sensitive to several assumptions, and the authors did not extrapolate their conclusion to immunocompromised individuals.3
In another cost-effectiveness analysis, immunocompromised patients were vaccinated with PCV13 at diagnosis and, starting a year later, were followed according to current PPSV23 vaccination guidelines. The PCV13 vaccine’s efficacy against invasive pneumococcal disease and pneumonia based on the modeled program led to cost savings, added quality-adjusted life-years, and prevented invasive pneumococcal disease, mostly in patients with human immunodeficiency virus (HIV) infection and those on dialysis (unpublished data cited in a 2012 US Centers for Disease Control and Prevention [CDC] report).4
No cost-effectiveness studies of influenza vaccination in immunocompromised adults have been conducted in the United States.
The various recommendations by the FDA, the ACIP, and the IDSA regarding the appropriate age at which to give zoster vaccine may also have been influenced by cost-effectiveness studies. These have reported mixed results and have not specifically focused on special populations.5
IMPROVING VACCINATION RATES
Rates of vaccination in special populations are suboptimal, and remedial measures to improve coverage have been proposed. One-page vaccine questionnaires or handouts for patients as well as “pop-up” reminders for vaccination in the electronic health record for physicians have resulted in higher rates of indicated vaccinations. Both the CDC and the American College of Physicians (ACP) offer downloadable tools—the CDC Vaccine Schedules App6 and the ACP Immunization Advisor,7 respectively—that are based on the 2012 ACIP guidelines and are extremely useful for busy practitioners. The CDC also offers patients a vaccine questionnaire8 that allows them to determine which vaccinations they may need and also to learn about those vaccines.
MOVING AHEAD
The development of vaccines is ongoing and will be driven by identification of new molecular targets. Advances in therapies for immunocompromised patients such as those with HIV infection will, we hope, decrease the risk of opportunistic infections. The list of vaccine-preventable diseases may continue to grow, as may the list of special populations. Optimal vaccination and outcomes may emerge from expected improved vaccine coverage as a result of the increased health insurance coverage resulting from the much-maligned Patient Protection and Affordable Care Act and ongoing studies regarding efficacy, safety, and cost-effectiveness, especially pertaining to specific patient populations.
- Farhat F, Wortmann G. Vaccinations in adults who are pregnant, older, diabetic, or immunocompromised, or have chronic kidney disease. Cleve Clin J Med 2015; 82:341–347.
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
- Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA 2012; 307:804–812.
- Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine 2014; 32:1645–1653.
- Centers for Disease Control and Prevention. CDC vaccine schedules app for clinicians and other immunization providers. Available at www.cdc.gov/vaccines/schedules/hcp/schedule-app.html. Accessed April 29, 2015.
- American College of Physicians. Immunization portal. http://immunization.acponline.org/app. Accessed April 29, 2015.
- Centers for Disease Control and Prevention. What vaccines do you need? www2.cdc.gov/nip/adultimmsched. Accessed April 29, 2015.
Vaccination is the standard of care as part of health maintenance for healthy people and for patients with myriad medical conditions. In an article in this issue of Cleveland Clinic Journal of Medicine, Drs. Faria Farhat and Glenn Wortmann1 make recommendations about vaccinations in special populations, including people with a disordered immune system or who are otherwise at heightened risk of infection (eg, because of older age, international travel, comorbidities, and medications).
But special populations are not all the same in their responses to vaccination. For example, patients with systemic autoimmune diseases are a heterogeneous group with disease-specific immunologic perturbations and immunosuppression that vary by medication and dose, all affecting the response to vaccination. Also, two or more “special” situations may coexist in the same patient.
AREAS OF UNCERTAINTY FOR CLINICAL PRACTICE
Several groups have issued guidelines and recommendations about vaccination in immunocompromised patients, but many areas of uncertainty exist in clinical practice. Most of these arise from a lack of data on immunogenicity and outcomes.
For example, although two pneumococcal vaccines are used in adults, no studies have compared the immunogenicity of the 13-valent pneumococcal conjugate vaccine (PCV13, which is T–cell-dependent) with that of the 23-valent pneumococcal polysaccharide vaccine (PPSV23, which is T–cell-independent) in immunocompromised adults.
Also, whether to use zoster vaccine in immunosuppressed patients ages 50 through 59 is debated. The vaccine is approved by the US Food and Drug Administration (FDA) for this age group, but the Advisory Committee on Immunization Practices (ACIP) does not recommend it, and the Infectious Diseases Society of America (IDSA) suggests that it be “considered” in patients on low-intensity immunosuppressive treatment.2
Testing to ensure optimal response to vaccination has been recommended in several articles and guidelines. However, antibody titers do not necessarily correlate with protection, and at this time no consensus exists about the timing of or need for testing for the response to immunization, the methods to use, how to interpret the results in terms of an adequate or inadequate response, or the role of booster immunization in routine clinical practice.
IS VACCINATION COST-EFFECTIVE?
Also relevant is whether vaccination is cost-effective.
Although vaccination with the PCV13 vaccine is possibly more cost-effective than PPSV23 in US adults based on a model that included immunosuppressed patients, the results of this study were sensitive to several assumptions, and the authors did not extrapolate their conclusion to immunocompromised individuals.3
In another cost-effectiveness analysis, immunocompromised patients were vaccinated with PCV13 at diagnosis and, starting a year later, were followed according to current PPSV23 vaccination guidelines. The PCV13 vaccine’s efficacy against invasive pneumococcal disease and pneumonia based on the modeled program led to cost savings, added quality-adjusted life-years, and prevented invasive pneumococcal disease, mostly in patients with human immunodeficiency virus (HIV) infection and those on dialysis (unpublished data cited in a 2012 US Centers for Disease Control and Prevention [CDC] report).4
No cost-effectiveness studies of influenza vaccination in immunocompromised adults have been conducted in the United States.
The various recommendations by the FDA, the ACIP, and the IDSA regarding the appropriate age at which to give zoster vaccine may also have been influenced by cost-effectiveness studies. These have reported mixed results and have not specifically focused on special populations.5
IMPROVING VACCINATION RATES
Rates of vaccination in special populations are suboptimal, and remedial measures to improve coverage have been proposed. One-page vaccine questionnaires or handouts for patients as well as “pop-up” reminders for vaccination in the electronic health record for physicians have resulted in higher rates of indicated vaccinations. Both the CDC and the American College of Physicians (ACP) offer downloadable tools—the CDC Vaccine Schedules App6 and the ACP Immunization Advisor,7 respectively—that are based on the 2012 ACIP guidelines and are extremely useful for busy practitioners. The CDC also offers patients a vaccine questionnaire8 that allows them to determine which vaccinations they may need and also to learn about those vaccines.
MOVING AHEAD
The development of vaccines is ongoing and will be driven by identification of new molecular targets. Advances in therapies for immunocompromised patients such as those with HIV infection will, we hope, decrease the risk of opportunistic infections. The list of vaccine-preventable diseases may continue to grow, as may the list of special populations. Optimal vaccination and outcomes may emerge from expected improved vaccine coverage as a result of the increased health insurance coverage resulting from the much-maligned Patient Protection and Affordable Care Act and ongoing studies regarding efficacy, safety, and cost-effectiveness, especially pertaining to specific patient populations.
Vaccination is the standard of care as part of health maintenance for healthy people and for patients with myriad medical conditions. In an article in this issue of Cleveland Clinic Journal of Medicine, Drs. Faria Farhat and Glenn Wortmann1 make recommendations about vaccinations in special populations, including people with a disordered immune system or who are otherwise at heightened risk of infection (eg, because of older age, international travel, comorbidities, and medications).
But special populations are not all the same in their responses to vaccination. For example, patients with systemic autoimmune diseases are a heterogeneous group with disease-specific immunologic perturbations and immunosuppression that vary by medication and dose, all affecting the response to vaccination. Also, two or more “special” situations may coexist in the same patient.
AREAS OF UNCERTAINTY FOR CLINICAL PRACTICE
Several groups have issued guidelines and recommendations about vaccination in immunocompromised patients, but many areas of uncertainty exist in clinical practice. Most of these arise from a lack of data on immunogenicity and outcomes.
For example, although two pneumococcal vaccines are used in adults, no studies have compared the immunogenicity of the 13-valent pneumococcal conjugate vaccine (PCV13, which is T–cell-dependent) with that of the 23-valent pneumococcal polysaccharide vaccine (PPSV23, which is T–cell-independent) in immunocompromised adults.
Also, whether to use zoster vaccine in immunosuppressed patients ages 50 through 59 is debated. The vaccine is approved by the US Food and Drug Administration (FDA) for this age group, but the Advisory Committee on Immunization Practices (ACIP) does not recommend it, and the Infectious Diseases Society of America (IDSA) suggests that it be “considered” in patients on low-intensity immunosuppressive treatment.2
Testing to ensure optimal response to vaccination has been recommended in several articles and guidelines. However, antibody titers do not necessarily correlate with protection, and at this time no consensus exists about the timing of or need for testing for the response to immunization, the methods to use, how to interpret the results in terms of an adequate or inadequate response, or the role of booster immunization in routine clinical practice.
IS VACCINATION COST-EFFECTIVE?
Also relevant is whether vaccination is cost-effective.
Although vaccination with the PCV13 vaccine is possibly more cost-effective than PPSV23 in US adults based on a model that included immunosuppressed patients, the results of this study were sensitive to several assumptions, and the authors did not extrapolate their conclusion to immunocompromised individuals.3
In another cost-effectiveness analysis, immunocompromised patients were vaccinated with PCV13 at diagnosis and, starting a year later, were followed according to current PPSV23 vaccination guidelines. The PCV13 vaccine’s efficacy against invasive pneumococcal disease and pneumonia based on the modeled program led to cost savings, added quality-adjusted life-years, and prevented invasive pneumococcal disease, mostly in patients with human immunodeficiency virus (HIV) infection and those on dialysis (unpublished data cited in a 2012 US Centers for Disease Control and Prevention [CDC] report).4
No cost-effectiveness studies of influenza vaccination in immunocompromised adults have been conducted in the United States.
The various recommendations by the FDA, the ACIP, and the IDSA regarding the appropriate age at which to give zoster vaccine may also have been influenced by cost-effectiveness studies. These have reported mixed results and have not specifically focused on special populations.5
IMPROVING VACCINATION RATES
Rates of vaccination in special populations are suboptimal, and remedial measures to improve coverage have been proposed. One-page vaccine questionnaires or handouts for patients as well as “pop-up” reminders for vaccination in the electronic health record for physicians have resulted in higher rates of indicated vaccinations. Both the CDC and the American College of Physicians (ACP) offer downloadable tools—the CDC Vaccine Schedules App6 and the ACP Immunization Advisor,7 respectively—that are based on the 2012 ACIP guidelines and are extremely useful for busy practitioners. The CDC also offers patients a vaccine questionnaire8 that allows them to determine which vaccinations they may need and also to learn about those vaccines.
MOVING AHEAD
The development of vaccines is ongoing and will be driven by identification of new molecular targets. Advances in therapies for immunocompromised patients such as those with HIV infection will, we hope, decrease the risk of opportunistic infections. The list of vaccine-preventable diseases may continue to grow, as may the list of special populations. Optimal vaccination and outcomes may emerge from expected improved vaccine coverage as a result of the increased health insurance coverage resulting from the much-maligned Patient Protection and Affordable Care Act and ongoing studies regarding efficacy, safety, and cost-effectiveness, especially pertaining to specific patient populations.
- Farhat F, Wortmann G. Vaccinations in adults who are pregnant, older, diabetic, or immunocompromised, or have chronic kidney disease. Cleve Clin J Med 2015; 82:341–347.
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
- Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA 2012; 307:804–812.
- Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine 2014; 32:1645–1653.
- Centers for Disease Control and Prevention. CDC vaccine schedules app for clinicians and other immunization providers. Available at www.cdc.gov/vaccines/schedules/hcp/schedule-app.html. Accessed April 29, 2015.
- American College of Physicians. Immunization portal. http://immunization.acponline.org/app. Accessed April 29, 2015.
- Centers for Disease Control and Prevention. What vaccines do you need? www2.cdc.gov/nip/adultimmsched. Accessed April 29, 2015.
- Farhat F, Wortmann G. Vaccinations in adults who are pregnant, older, diabetic, or immunocompromised, or have chronic kidney disease. Cleve Clin J Med 2015; 82:341–347.
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
- Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA 2012; 307:804–812.
- Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine 2014; 32:1645–1653.
- Centers for Disease Control and Prevention. CDC vaccine schedules app for clinicians and other immunization providers. Available at www.cdc.gov/vaccines/schedules/hcp/schedule-app.html. Accessed April 29, 2015.
- American College of Physicians. Immunization portal. http://immunization.acponline.org/app. Accessed April 29, 2015.
- Centers for Disease Control and Prevention. What vaccines do you need? www2.cdc.gov/nip/adultimmsched. Accessed April 29, 2015.
Vaccinating adults who are pregnant, older, or immunocompromised, or have chronic kidney disease
Most vaccinations are given during childhood, but some require boosting during adulthood or are indicated for specific patient populations such as international travelers or those with certain medical conditions. Although generally safe, some vaccines contain live, attenuated organisms that can cause disease in immunocompromised patients. Thus, knowledge of the indications for and contraindications to specific vaccinations is critical to protect adults in special circumstances who are at risk.
Vaccines have helped eliminate or significantly reduce the burden of more than a dozen illnesses.1–3 The Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) makes recommendations about vaccinations for normal adults and children as well as for certain groups at high risk of vaccine-preventable infections.4 Tables 1 and 2 summarize the recommendations for vaccination by medical condition.4 In addition, several applications are available online, including downloadable apps from the (www.cdc.gov/vaccines/schedules/Schedulers/adult-scheduler.html) and the American College of Physicians (http://immunization.acponline.org/app/).
HUMANITY’S GREATEST ADVANCES IN PREVENTING INFECTIOUS DISEASE
Immunization and improved sanitation are humanity’s greatest advances in preventing sickness and death from infectious diseases. Since Jenner’s discovery in 1796 that milkmaids who had contracted cowpox (vaccinia) were immune to smallpox, vaccination has eliminated smallpox, markedly decreased the incidence of many infectious diseases, and, most recently, shown efficacy in preventing cervical cancer (with the human papillomavirus vaccine) and hepatocellular cancer (with the hepatitis B vaccine).1–3
Unfortunately, vaccination rates remain low for most routine vaccinations indicated for adults. For example, about 60% of adults over age 65 receive pneumococcal vaccination, and fewer than 10% of black patients over age 60 receive zoster vaccination.5 Various factors may account for these low rates, including financial disincentives.6
Nevertheless, vaccination remains one of medicine’s most effective defenses against infectious diseases and is especially important in the special populations discussed below. By being steadfast proponents of vaccination, especially for the most vulnerable patients, physicians can help ensure the optimum protection for their patients.
VACCINATING PREGNANT PATIENTS
When considering vaccination during pregnancy, one must consider the risk and benefit of the vaccine and the risk of the disease in both the mother and the child.
In general, if a pregnant woman is at high risk of exposure to a particular infection, the benefits of vaccinating her against it outweigh the risks. Vaccinating the mother can also protect against certain infections in early infancy through transfer of vaccine-induced immunoglobin G (IgG) across the placenta.7 In general, inactivated vaccines are considered safe in pregnancy, while live-attenuated vaccines are contraindicated.4 Special considerations for pregnant women include:
Tetanus, diphtheria, and acellular pertussis (Tdap). One dose of Tdap vaccine should be given during each pregnancy, preferably at 27 to 36 weeks of gestation, regardless of when the patient received a previous dose.8
Inactivated influenza vaccine should be given as early as possible during the influenza season (October to March) to all pregnant women, regardless of trimester.
Inactivated polio vaccine may be considered for pregnant women with known exposure to polio or travel to endemic areas.
Hepatitis A, hepatitis B, pneumococcal polysaccharide, meningococcal conjugate, and meningococcal polysaccharide vaccines can be given to women at risk of these infections. If a pregnant patient requires pneumococcal polysaccharide vaccine, it should be given during the second or third trimester, as the safety of this vaccine during the first trimester has not been established.9
Smallpox, measles-mumps-rubella, and varicella-containing vaccines are contraindicated in pregnancy. Household contacts of a pregnant woman should not receive smallpox vaccine, as it is the only vaccine known to cause harm to the fetus.10
Human papillomavirus vaccination is not recommended during pregnancy.
Yellow fever live-attenuated vaccine. The safety of this vaccine during pregnancy has not been established, and it is in the US Food and Drug Administration (FDA) pregnancy category C. However, this vaccine is required for entry into certain countries, and it may be offered if the patient is truly at risk of contracting yellow fever. Because pregnancy may affect immunologic response, serologic testing is recommended to document an immune response. If the patient’s itinerary puts her at low risk of yellow fever, then writing her a vaccine waiver letter can be considered.11
VACCINATING IMMUNOCOMPROMISED PATIENTS (NON-HIV)
People who do not have human immunodeficiency virus (HIV) but have a condition such as functional asplenia (sickle cell disease), anatomic asplenia, or complement component deficiency are at higher risk of infection with the encapsulated bacteria Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b.
Corticosteroids, chemotherapy, radiation for hematologic or solid-organ malignancies, and immune modulators can alter the immune system and pose a risk with the use of live-attenuated vaccines. A corticosteroid dosage equivalent to 2 mg/kg of body weight per day or higher or 20 mg/day of prednisone or higher is generally considered immunosuppressive.
Candidates for organ transplantation should receive vaccinations as early as possible during the disease course leading to transplantation. Vaccinations should be given as soon as the decision is made that the patient is a candidate for transplantation, which could be years or months before the patient actually receives the transplant. In addition to reviewing previously administered vaccinations, pretransplant serologic testing for hepatitis B, varicella, measles, mumps, and rubella antibodies helps to evaluate the need for vaccination.12
Recipients of hematopoietic stem cell transplantation are at risk of infections with encapsulated bacteria and certain other vaccine-preventable infections. Antibody titers are significantly reduced after stem cell transplantation because of ablation of bone marrow, and thus certain vaccines should be readministered 3 to 6 months after transplantation (eg, influenza, pneumococcal, and H influenzae vaccines). If the recipient is presumed to be immunocompetent, then varicella or measles-mumps-rubella vaccine can be given 24 months after transplantation.13
Apart from adhering to the routine vaccination schedule and avoiding live-attenuated vaccines, specific recommendations apply to persons with immunocompromising conditions14:
Quadrivalent meningococcal conjugate vaccine should be given to adults of all ages with asplenia or complement component deficiency. The schedule includes two doses at least 2 months apart initially and then revaccination every 5 years.
H influenzae type b vaccine should be given to people with asplenia and recipients of hematopoietic stem cells. One dose is recommended for those with asplenia (functional, anatomic, or elective splenectomy) or sickle cell disease if they have not already received it. A three-dose schedule is considered for hematopoietic stem cell transplant recipients 6 to 12 months after successful transplantation.
Pneumococcal conjugate (PCV13) and pneumococcal polysaccharide (PPSV23) vaccinations are recommended for people who have immunocompromising conditions. PCV13, the newer pneumococcal vaccine, was approved by the FDA in 2010 for use in children and was recommended by the ACIP in 2012 for adults age 19 and older with immunocompromising conditions.
People who have not previously received either of these vaccines and are age 19 or older with immunocompromising conditions including asplenia, chronic renal failure, nephrotic syndrome, cerebrospinal fluid leakage, or cochlear implant should receive a single dose of PCV13 followed by a dose of PPSV23 at least 8 weeks later. One-time revaccination 5 years after the first dose of PPSV23 is recommended for patients with immunocompromising conditions.
For those who have previously been vaccinated with PPSV23, a dose of PCV13 can be given 1 or more years after the last dose of PPSV23. These dosing intervals are important, as lower opsonophagocytic antibody responses have been noted if repeat doses of either pneumococcal vaccine are given sooner than the recommended interval.15
Inactivated influenza vaccine is recommended annually, except for patients who are unlikely to respond or those who have received anti-B-cell antibodies within 6 months. Live-attenuated influenza vaccine should not be given to immunocompromised patients.
VACCINATING PATIENTS WHO HAVE HIV
People with HIV should be routinely screened for immunity against certain infections and should be offered vaccination if not immune. The response to vaccines may vary depending on the CD4 count, with a good response in patients whose infection is well controlled with antiretroviral agents and with a preserved CD4 count.16 Special considerations for HIV patients include the following:
Hepatitis A vaccine may be offered to all HIV patients who have no evidence of immunity against hepatitis A, with negative antihepatitis A total and IgG antibodies.
Human papillomavirus vaccine is recommended for men and women with HIV through age 26.
Varicella and measles-mumps-rubella are live-attenuated vaccines and may be considered in patients who are nonimmune and with CD4 counts of 200 cells/µL or higher. However, the ACIP does not make a recommendation regarding the zoster vaccine in HIV patients with CD4 cell counts of 200 cells/µL or higher. In general, live-attenuated vaccines should be avoided in patients with CD4 counts less than 200 or with severe immunocompromised status because of risk of acquiring severe, life-threatening infections.
Pneumococcal vaccine should be given to HIV patients if they have not received it before. The schedule is one dose of PCV13, followed by a dose of PPSV23 at least 8 weeks later. If a patient has been previously vaccinated with PPSV23, then PCV13 is recommended at least 1 year after PPSV23.
Inactivated influenza vaccine is recommended annually. Live-attenuated influenza vaccine should not be given.
Hepatitis B vaccine should be given to nonimmune patients without past or present hepatitis B infection. These patients require higher doses of hepatitis B vaccine (40 μg/mL) than immunocompetent patients, who receive 20 μg/mL. The options include Recombivax HB 40 μg/mL given on a three-dose schedule at 0, 1, and 6 months, and Engerix B, two 20-μg/mL injections given simultaneously on a four-dose schedule at 0, 1, 2, and 6 months.
Meningococcal vaccine. HIV infection is not an indication for meningococcal vaccine unless the patient has other risk factors, such as anatomic or functional asplenia, persistent complement component deficiency, occupational exposure, and travel to endemic areas.
VACCINATING PATIENTS WHO ARE OLDER THAN 60
The immune system deteriorates with age, as does immunity gained from previous vaccinations. Vaccination in this age group reduces the risk of illness and death.17
Zoster vaccine should be offered to people age 60 and older regardless of previous episodes of herpes zoster unless there is a contraindication such as severe immunodeficiency. The zoster vaccine can reduce the incidence of postherpetic neuralgia by 66.5% and herpes zoster by 51% in patients over age 60.18
Pneumococcal conjugate vaccine. PCV13 should be offered to all adults age 65 or older. If a person age 65 or older has not received any pneumococcal vaccine before then, PCV13 should be given first, followed by a dose of PPSV23 at least 6 to 12 months after PCV13.
Pneumococcal polysaccharide vaccine. If PPSV23 was given before age 65 for another indication, a dose of PCV 13 should be given at age 65 or later, as long as 6 to 12 months have passed since the previous dose of PPSV 23. The patient should receive the last dose of PPSV23 vaccine 5 years after the first dose of PPSV23.4
Influenza vaccine. People 65 or older are at higher risk of complications from influenza, and vaccine should be offered annually. High-dose inactivated influenza vaccine can be used in this age group.4
Tdap. If never given before, Tdap is recommended regardless of the interval since the most recent Td vaccination, followed by a Td booster every 10 years.
VACCINATING PATIENTS WHO HAVE CHRONIC KIDNEY DISEASE
Patients with chronic kidney disease are at risk of certain infections, so vaccination is an important preventive measure.19 Immunizations should be offered to all patients with chronic kidney disease regardless of the disease stage, but they are recommended during the early stages of progressive renal disease to increase the likelihood of vaccine-induced immunity.20
Pneumococcal conjugate vaccine. PCV13 is recommended for adults 19 or older with chronic renal disease or nephrotic syndrome. One dose of PCV13 should be given, followed by a dose of PPSV23 at least 8 weeks later. If the patient has been previously vaccinated with PPSV23, then PCV13 at least 1 year after PPSV23 is recommended.
Hepatitis B vaccine should be given to nonimmune patients without past or present hepatitis B infection. Adult patients on hemodialysis require higher doses of hepatitis B vaccine. The options include Recombivax HB 40 μg/mL given on a three-dose schedule at 0, 1, and 6 months, and Engerix B, two 20-μg/mL injections given simultaneously on a four-dose schedule at 0, 1, 2, and 6 months.
Influenza vaccine should be offered annually to patients with chronic kidney disease.
VACCINATING IMMUNOCOMPROMISED INTERNATIONAL TRAVELERS
International travel for business or pleasure is increasingly common, and immunocompromised patients require specific attention as they may face unanticipated pathogens or have special requirements. Transplant recipients should ideally receive routine and travel-related vaccines as early as possible before transplantation. Vaccination is generally avoided in the first 6 months after organ transplantation to avoid confusion with early graft dysfunction or rejection.21 However, it should be considered as soon as a patient develops an illness that might lead to transplantation.
Evaluation of patients for vaccination should include an assessment of the travel-specific epidemiologic risk, the nature of the vaccine (live-attenuated or other), and the immune status. As discussed above, live-attenuated vaccines should be avoided in immunocompromised patients, and thus the injectable typhoid vaccine should be given in lieu of the attenuated oral vaccine.
Yellow fever vaccine is required before entrance to certain countries but should not be given to immunocompromised patients, although it can probably be given to asymptomatic HIV-infected adults with a CD4 count higher than 200 cells/μL who are exposed to substantial risk.22 For patients who cannot receive the vaccine, some governments will accept a physician’s letter stating the patient has a contraindication to vaccination.
VACCINATING HOUSEHOLD MEMBERS OF IMMUNOCOMPROMISED PATIENTS
Protecting immunocompromised patients from infectious diseases involves vaccinating not only the patient but also household members so that they do not acquire infections and then bring them into the household. Immunocompetent members of a household can receive inactivated vaccines based on the recommended ACIP schedule.
Annual inactivated influenza vaccination is recommended, although the live-attenuated influenza virus vaccine can be substituted if the immunocompromised patient is not within 2 months of hematopoietic stem cell transplantation, does not have graft-vs-host disease, and does not have severe combined immune deficiency.
Other live-attenuated vaccines can usually be given if indicated, including measles-mumps-rubella vaccine, rotavirus vaccine in infants, varicella vaccine, and zoster vaccine.14
- Crosignani P, De Stefani A, Fara GM, et al. Towards the eradication of HPV infection through universal specific vaccination. BMC Public Health 2013;13:642.
- Plotkin SL, Plotkin SA. A short history of vaccination. In: Plotkin, SA, Orenstein W, Offit PA, editors. Vaccines, 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2008:1–16.
- Wong VW, Chan HL. Prevention of hepatocellular carcinoma: a concise review of contemporary issues. Ann Hepatol 2012; 11:284–293.
- Kim DK, Bridges CB, Harriman K; Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older: United States, 2015. Ann Intern Med 2015; 162:214–223.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults—United States, 2012. MMWR Morb Mortal Wkly Rep 2014; 63:95-102.
- Hurley LP, Bridges CB, Harpaz R, et al. US physicians’ perspective of adult vaccine delivery. Ann Intern Med 2014; 160:161.
- Lindsey B, Kampmann B, Jones C. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr Opin Infect Dis 2013; 26:248–253.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013; 62:131–135.
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:1–24.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003; 52:1–16.
- Staples JE, Gershman M, Fischer M; Centers for Disease Control and Prevention (CDC). Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010; 59:1–27.
- Danziger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice. Vaccination in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):311–317.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–64.
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA, Infectious Diseases Society of America. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:1–10.
- Eilers R, Krabbe PF, van Essen TG, Suijkerbuijk A, van Lier A, de Melker HE. Assessment of vaccine candidates for persons aged 50 and older: a review. BMC Geriatr 2013; 13:32.
- Oxman MN, Levin MJ; Shingles Prevention Study Group. Vaccination against Herpes Zoster and Postherpetic Neuralgia. J Infect Dis 2008; 197(suppl 2):S228–S236.
- Soni R, Horowitz B, Unruh M. Immunization in end-stage renal disease: opportunity to improve outcomes. Semin Dial 2013; 26:416–426.
- Chi C, Patel P, Pilishvili T, Moore M, Murphy T, Strikas R. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. http://www.cdc.gov/vaccines/pubs/downloads/dialysis-guide-2012.pdf. Accessed March 31, 2015.
- Kotton CN, Ryan ET, Fishman JA. Prevention of infection in adult travelers after solid organ transplantation. Am J Transplant 2005; 5:8–14.
- Castelli F, Patroni A. The human immunodeficiency virus-infected traveler. Clin Infect Dis 2000; 31:1403–1408.
Most vaccinations are given during childhood, but some require boosting during adulthood or are indicated for specific patient populations such as international travelers or those with certain medical conditions. Although generally safe, some vaccines contain live, attenuated organisms that can cause disease in immunocompromised patients. Thus, knowledge of the indications for and contraindications to specific vaccinations is critical to protect adults in special circumstances who are at risk.
Vaccines have helped eliminate or significantly reduce the burden of more than a dozen illnesses.1–3 The Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) makes recommendations about vaccinations for normal adults and children as well as for certain groups at high risk of vaccine-preventable infections.4 Tables 1 and 2 summarize the recommendations for vaccination by medical condition.4 In addition, several applications are available online, including downloadable apps from the (www.cdc.gov/vaccines/schedules/Schedulers/adult-scheduler.html) and the American College of Physicians (http://immunization.acponline.org/app/).
HUMANITY’S GREATEST ADVANCES IN PREVENTING INFECTIOUS DISEASE
Immunization and improved sanitation are humanity’s greatest advances in preventing sickness and death from infectious diseases. Since Jenner’s discovery in 1796 that milkmaids who had contracted cowpox (vaccinia) were immune to smallpox, vaccination has eliminated smallpox, markedly decreased the incidence of many infectious diseases, and, most recently, shown efficacy in preventing cervical cancer (with the human papillomavirus vaccine) and hepatocellular cancer (with the hepatitis B vaccine).1–3
Unfortunately, vaccination rates remain low for most routine vaccinations indicated for adults. For example, about 60% of adults over age 65 receive pneumococcal vaccination, and fewer than 10% of black patients over age 60 receive zoster vaccination.5 Various factors may account for these low rates, including financial disincentives.6
Nevertheless, vaccination remains one of medicine’s most effective defenses against infectious diseases and is especially important in the special populations discussed below. By being steadfast proponents of vaccination, especially for the most vulnerable patients, physicians can help ensure the optimum protection for their patients.
VACCINATING PREGNANT PATIENTS
When considering vaccination during pregnancy, one must consider the risk and benefit of the vaccine and the risk of the disease in both the mother and the child.
In general, if a pregnant woman is at high risk of exposure to a particular infection, the benefits of vaccinating her against it outweigh the risks. Vaccinating the mother can also protect against certain infections in early infancy through transfer of vaccine-induced immunoglobin G (IgG) across the placenta.7 In general, inactivated vaccines are considered safe in pregnancy, while live-attenuated vaccines are contraindicated.4 Special considerations for pregnant women include:
Tetanus, diphtheria, and acellular pertussis (Tdap). One dose of Tdap vaccine should be given during each pregnancy, preferably at 27 to 36 weeks of gestation, regardless of when the patient received a previous dose.8
Inactivated influenza vaccine should be given as early as possible during the influenza season (October to March) to all pregnant women, regardless of trimester.
Inactivated polio vaccine may be considered for pregnant women with known exposure to polio or travel to endemic areas.
Hepatitis A, hepatitis B, pneumococcal polysaccharide, meningococcal conjugate, and meningococcal polysaccharide vaccines can be given to women at risk of these infections. If a pregnant patient requires pneumococcal polysaccharide vaccine, it should be given during the second or third trimester, as the safety of this vaccine during the first trimester has not been established.9
Smallpox, measles-mumps-rubella, and varicella-containing vaccines are contraindicated in pregnancy. Household contacts of a pregnant woman should not receive smallpox vaccine, as it is the only vaccine known to cause harm to the fetus.10
Human papillomavirus vaccination is not recommended during pregnancy.
Yellow fever live-attenuated vaccine. The safety of this vaccine during pregnancy has not been established, and it is in the US Food and Drug Administration (FDA) pregnancy category C. However, this vaccine is required for entry into certain countries, and it may be offered if the patient is truly at risk of contracting yellow fever. Because pregnancy may affect immunologic response, serologic testing is recommended to document an immune response. If the patient’s itinerary puts her at low risk of yellow fever, then writing her a vaccine waiver letter can be considered.11
VACCINATING IMMUNOCOMPROMISED PATIENTS (NON-HIV)
People who do not have human immunodeficiency virus (HIV) but have a condition such as functional asplenia (sickle cell disease), anatomic asplenia, or complement component deficiency are at higher risk of infection with the encapsulated bacteria Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b.
Corticosteroids, chemotherapy, radiation for hematologic or solid-organ malignancies, and immune modulators can alter the immune system and pose a risk with the use of live-attenuated vaccines. A corticosteroid dosage equivalent to 2 mg/kg of body weight per day or higher or 20 mg/day of prednisone or higher is generally considered immunosuppressive.
Candidates for organ transplantation should receive vaccinations as early as possible during the disease course leading to transplantation. Vaccinations should be given as soon as the decision is made that the patient is a candidate for transplantation, which could be years or months before the patient actually receives the transplant. In addition to reviewing previously administered vaccinations, pretransplant serologic testing for hepatitis B, varicella, measles, mumps, and rubella antibodies helps to evaluate the need for vaccination.12
Recipients of hematopoietic stem cell transplantation are at risk of infections with encapsulated bacteria and certain other vaccine-preventable infections. Antibody titers are significantly reduced after stem cell transplantation because of ablation of bone marrow, and thus certain vaccines should be readministered 3 to 6 months after transplantation (eg, influenza, pneumococcal, and H influenzae vaccines). If the recipient is presumed to be immunocompetent, then varicella or measles-mumps-rubella vaccine can be given 24 months after transplantation.13
Apart from adhering to the routine vaccination schedule and avoiding live-attenuated vaccines, specific recommendations apply to persons with immunocompromising conditions14:
Quadrivalent meningococcal conjugate vaccine should be given to adults of all ages with asplenia or complement component deficiency. The schedule includes two doses at least 2 months apart initially and then revaccination every 5 years.
H influenzae type b vaccine should be given to people with asplenia and recipients of hematopoietic stem cells. One dose is recommended for those with asplenia (functional, anatomic, or elective splenectomy) or sickle cell disease if they have not already received it. A three-dose schedule is considered for hematopoietic stem cell transplant recipients 6 to 12 months after successful transplantation.
Pneumococcal conjugate (PCV13) and pneumococcal polysaccharide (PPSV23) vaccinations are recommended for people who have immunocompromising conditions. PCV13, the newer pneumococcal vaccine, was approved by the FDA in 2010 for use in children and was recommended by the ACIP in 2012 for adults age 19 and older with immunocompromising conditions.
People who have not previously received either of these vaccines and are age 19 or older with immunocompromising conditions including asplenia, chronic renal failure, nephrotic syndrome, cerebrospinal fluid leakage, or cochlear implant should receive a single dose of PCV13 followed by a dose of PPSV23 at least 8 weeks later. One-time revaccination 5 years after the first dose of PPSV23 is recommended for patients with immunocompromising conditions.
For those who have previously been vaccinated with PPSV23, a dose of PCV13 can be given 1 or more years after the last dose of PPSV23. These dosing intervals are important, as lower opsonophagocytic antibody responses have been noted if repeat doses of either pneumococcal vaccine are given sooner than the recommended interval.15
Inactivated influenza vaccine is recommended annually, except for patients who are unlikely to respond or those who have received anti-B-cell antibodies within 6 months. Live-attenuated influenza vaccine should not be given to immunocompromised patients.
VACCINATING PATIENTS WHO HAVE HIV
People with HIV should be routinely screened for immunity against certain infections and should be offered vaccination if not immune. The response to vaccines may vary depending on the CD4 count, with a good response in patients whose infection is well controlled with antiretroviral agents and with a preserved CD4 count.16 Special considerations for HIV patients include the following:
Hepatitis A vaccine may be offered to all HIV patients who have no evidence of immunity against hepatitis A, with negative antihepatitis A total and IgG antibodies.
Human papillomavirus vaccine is recommended for men and women with HIV through age 26.
Varicella and measles-mumps-rubella are live-attenuated vaccines and may be considered in patients who are nonimmune and with CD4 counts of 200 cells/µL or higher. However, the ACIP does not make a recommendation regarding the zoster vaccine in HIV patients with CD4 cell counts of 200 cells/µL or higher. In general, live-attenuated vaccines should be avoided in patients with CD4 counts less than 200 or with severe immunocompromised status because of risk of acquiring severe, life-threatening infections.
Pneumococcal vaccine should be given to HIV patients if they have not received it before. The schedule is one dose of PCV13, followed by a dose of PPSV23 at least 8 weeks later. If a patient has been previously vaccinated with PPSV23, then PCV13 is recommended at least 1 year after PPSV23.
Inactivated influenza vaccine is recommended annually. Live-attenuated influenza vaccine should not be given.
Hepatitis B vaccine should be given to nonimmune patients without past or present hepatitis B infection. These patients require higher doses of hepatitis B vaccine (40 μg/mL) than immunocompetent patients, who receive 20 μg/mL. The options include Recombivax HB 40 μg/mL given on a three-dose schedule at 0, 1, and 6 months, and Engerix B, two 20-μg/mL injections given simultaneously on a four-dose schedule at 0, 1, 2, and 6 months.
Meningococcal vaccine. HIV infection is not an indication for meningococcal vaccine unless the patient has other risk factors, such as anatomic or functional asplenia, persistent complement component deficiency, occupational exposure, and travel to endemic areas.
VACCINATING PATIENTS WHO ARE OLDER THAN 60
The immune system deteriorates with age, as does immunity gained from previous vaccinations. Vaccination in this age group reduces the risk of illness and death.17
Zoster vaccine should be offered to people age 60 and older regardless of previous episodes of herpes zoster unless there is a contraindication such as severe immunodeficiency. The zoster vaccine can reduce the incidence of postherpetic neuralgia by 66.5% and herpes zoster by 51% in patients over age 60.18
Pneumococcal conjugate vaccine. PCV13 should be offered to all adults age 65 or older. If a person age 65 or older has not received any pneumococcal vaccine before then, PCV13 should be given first, followed by a dose of PPSV23 at least 6 to 12 months after PCV13.
Pneumococcal polysaccharide vaccine. If PPSV23 was given before age 65 for another indication, a dose of PCV 13 should be given at age 65 or later, as long as 6 to 12 months have passed since the previous dose of PPSV 23. The patient should receive the last dose of PPSV23 vaccine 5 years after the first dose of PPSV23.4
Influenza vaccine. People 65 or older are at higher risk of complications from influenza, and vaccine should be offered annually. High-dose inactivated influenza vaccine can be used in this age group.4
Tdap. If never given before, Tdap is recommended regardless of the interval since the most recent Td vaccination, followed by a Td booster every 10 years.
VACCINATING PATIENTS WHO HAVE CHRONIC KIDNEY DISEASE
Patients with chronic kidney disease are at risk of certain infections, so vaccination is an important preventive measure.19 Immunizations should be offered to all patients with chronic kidney disease regardless of the disease stage, but they are recommended during the early stages of progressive renal disease to increase the likelihood of vaccine-induced immunity.20
Pneumococcal conjugate vaccine. PCV13 is recommended for adults 19 or older with chronic renal disease or nephrotic syndrome. One dose of PCV13 should be given, followed by a dose of PPSV23 at least 8 weeks later. If the patient has been previously vaccinated with PPSV23, then PCV13 at least 1 year after PPSV23 is recommended.
Hepatitis B vaccine should be given to nonimmune patients without past or present hepatitis B infection. Adult patients on hemodialysis require higher doses of hepatitis B vaccine. The options include Recombivax HB 40 μg/mL given on a three-dose schedule at 0, 1, and 6 months, and Engerix B, two 20-μg/mL injections given simultaneously on a four-dose schedule at 0, 1, 2, and 6 months.
Influenza vaccine should be offered annually to patients with chronic kidney disease.
VACCINATING IMMUNOCOMPROMISED INTERNATIONAL TRAVELERS
International travel for business or pleasure is increasingly common, and immunocompromised patients require specific attention as they may face unanticipated pathogens or have special requirements. Transplant recipients should ideally receive routine and travel-related vaccines as early as possible before transplantation. Vaccination is generally avoided in the first 6 months after organ transplantation to avoid confusion with early graft dysfunction or rejection.21 However, it should be considered as soon as a patient develops an illness that might lead to transplantation.
Evaluation of patients for vaccination should include an assessment of the travel-specific epidemiologic risk, the nature of the vaccine (live-attenuated or other), and the immune status. As discussed above, live-attenuated vaccines should be avoided in immunocompromised patients, and thus the injectable typhoid vaccine should be given in lieu of the attenuated oral vaccine.
Yellow fever vaccine is required before entrance to certain countries but should not be given to immunocompromised patients, although it can probably be given to asymptomatic HIV-infected adults with a CD4 count higher than 200 cells/μL who are exposed to substantial risk.22 For patients who cannot receive the vaccine, some governments will accept a physician’s letter stating the patient has a contraindication to vaccination.
VACCINATING HOUSEHOLD MEMBERS OF IMMUNOCOMPROMISED PATIENTS
Protecting immunocompromised patients from infectious diseases involves vaccinating not only the patient but also household members so that they do not acquire infections and then bring them into the household. Immunocompetent members of a household can receive inactivated vaccines based on the recommended ACIP schedule.
Annual inactivated influenza vaccination is recommended, although the live-attenuated influenza virus vaccine can be substituted if the immunocompromised patient is not within 2 months of hematopoietic stem cell transplantation, does not have graft-vs-host disease, and does not have severe combined immune deficiency.
Other live-attenuated vaccines can usually be given if indicated, including measles-mumps-rubella vaccine, rotavirus vaccine in infants, varicella vaccine, and zoster vaccine.14
Most vaccinations are given during childhood, but some require boosting during adulthood or are indicated for specific patient populations such as international travelers or those with certain medical conditions. Although generally safe, some vaccines contain live, attenuated organisms that can cause disease in immunocompromised patients. Thus, knowledge of the indications for and contraindications to specific vaccinations is critical to protect adults in special circumstances who are at risk.
Vaccines have helped eliminate or significantly reduce the burden of more than a dozen illnesses.1–3 The Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) makes recommendations about vaccinations for normal adults and children as well as for certain groups at high risk of vaccine-preventable infections.4 Tables 1 and 2 summarize the recommendations for vaccination by medical condition.4 In addition, several applications are available online, including downloadable apps from the (www.cdc.gov/vaccines/schedules/Schedulers/adult-scheduler.html) and the American College of Physicians (http://immunization.acponline.org/app/).
HUMANITY’S GREATEST ADVANCES IN PREVENTING INFECTIOUS DISEASE
Immunization and improved sanitation are humanity’s greatest advances in preventing sickness and death from infectious diseases. Since Jenner’s discovery in 1796 that milkmaids who had contracted cowpox (vaccinia) were immune to smallpox, vaccination has eliminated smallpox, markedly decreased the incidence of many infectious diseases, and, most recently, shown efficacy in preventing cervical cancer (with the human papillomavirus vaccine) and hepatocellular cancer (with the hepatitis B vaccine).1–3
Unfortunately, vaccination rates remain low for most routine vaccinations indicated for adults. For example, about 60% of adults over age 65 receive pneumococcal vaccination, and fewer than 10% of black patients over age 60 receive zoster vaccination.5 Various factors may account for these low rates, including financial disincentives.6
Nevertheless, vaccination remains one of medicine’s most effective defenses against infectious diseases and is especially important in the special populations discussed below. By being steadfast proponents of vaccination, especially for the most vulnerable patients, physicians can help ensure the optimum protection for their patients.
VACCINATING PREGNANT PATIENTS
When considering vaccination during pregnancy, one must consider the risk and benefit of the vaccine and the risk of the disease in both the mother and the child.
In general, if a pregnant woman is at high risk of exposure to a particular infection, the benefits of vaccinating her against it outweigh the risks. Vaccinating the mother can also protect against certain infections in early infancy through transfer of vaccine-induced immunoglobin G (IgG) across the placenta.7 In general, inactivated vaccines are considered safe in pregnancy, while live-attenuated vaccines are contraindicated.4 Special considerations for pregnant women include:
Tetanus, diphtheria, and acellular pertussis (Tdap). One dose of Tdap vaccine should be given during each pregnancy, preferably at 27 to 36 weeks of gestation, regardless of when the patient received a previous dose.8
Inactivated influenza vaccine should be given as early as possible during the influenza season (October to March) to all pregnant women, regardless of trimester.
Inactivated polio vaccine may be considered for pregnant women with known exposure to polio or travel to endemic areas.
Hepatitis A, hepatitis B, pneumococcal polysaccharide, meningococcal conjugate, and meningococcal polysaccharide vaccines can be given to women at risk of these infections. If a pregnant patient requires pneumococcal polysaccharide vaccine, it should be given during the second or third trimester, as the safety of this vaccine during the first trimester has not been established.9
Smallpox, measles-mumps-rubella, and varicella-containing vaccines are contraindicated in pregnancy. Household contacts of a pregnant woman should not receive smallpox vaccine, as it is the only vaccine known to cause harm to the fetus.10
Human papillomavirus vaccination is not recommended during pregnancy.
Yellow fever live-attenuated vaccine. The safety of this vaccine during pregnancy has not been established, and it is in the US Food and Drug Administration (FDA) pregnancy category C. However, this vaccine is required for entry into certain countries, and it may be offered if the patient is truly at risk of contracting yellow fever. Because pregnancy may affect immunologic response, serologic testing is recommended to document an immune response. If the patient’s itinerary puts her at low risk of yellow fever, then writing her a vaccine waiver letter can be considered.11
VACCINATING IMMUNOCOMPROMISED PATIENTS (NON-HIV)
People who do not have human immunodeficiency virus (HIV) but have a condition such as functional asplenia (sickle cell disease), anatomic asplenia, or complement component deficiency are at higher risk of infection with the encapsulated bacteria Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b.
Corticosteroids, chemotherapy, radiation for hematologic or solid-organ malignancies, and immune modulators can alter the immune system and pose a risk with the use of live-attenuated vaccines. A corticosteroid dosage equivalent to 2 mg/kg of body weight per day or higher or 20 mg/day of prednisone or higher is generally considered immunosuppressive.
Candidates for organ transplantation should receive vaccinations as early as possible during the disease course leading to transplantation. Vaccinations should be given as soon as the decision is made that the patient is a candidate for transplantation, which could be years or months before the patient actually receives the transplant. In addition to reviewing previously administered vaccinations, pretransplant serologic testing for hepatitis B, varicella, measles, mumps, and rubella antibodies helps to evaluate the need for vaccination.12
Recipients of hematopoietic stem cell transplantation are at risk of infections with encapsulated bacteria and certain other vaccine-preventable infections. Antibody titers are significantly reduced after stem cell transplantation because of ablation of bone marrow, and thus certain vaccines should be readministered 3 to 6 months after transplantation (eg, influenza, pneumococcal, and H influenzae vaccines). If the recipient is presumed to be immunocompetent, then varicella or measles-mumps-rubella vaccine can be given 24 months after transplantation.13
Apart from adhering to the routine vaccination schedule and avoiding live-attenuated vaccines, specific recommendations apply to persons with immunocompromising conditions14:
Quadrivalent meningococcal conjugate vaccine should be given to adults of all ages with asplenia or complement component deficiency. The schedule includes two doses at least 2 months apart initially and then revaccination every 5 years.
H influenzae type b vaccine should be given to people with asplenia and recipients of hematopoietic stem cells. One dose is recommended for those with asplenia (functional, anatomic, or elective splenectomy) or sickle cell disease if they have not already received it. A three-dose schedule is considered for hematopoietic stem cell transplant recipients 6 to 12 months after successful transplantation.
Pneumococcal conjugate (PCV13) and pneumococcal polysaccharide (PPSV23) vaccinations are recommended for people who have immunocompromising conditions. PCV13, the newer pneumococcal vaccine, was approved by the FDA in 2010 for use in children and was recommended by the ACIP in 2012 for adults age 19 and older with immunocompromising conditions.
People who have not previously received either of these vaccines and are age 19 or older with immunocompromising conditions including asplenia, chronic renal failure, nephrotic syndrome, cerebrospinal fluid leakage, or cochlear implant should receive a single dose of PCV13 followed by a dose of PPSV23 at least 8 weeks later. One-time revaccination 5 years after the first dose of PPSV23 is recommended for patients with immunocompromising conditions.
For those who have previously been vaccinated with PPSV23, a dose of PCV13 can be given 1 or more years after the last dose of PPSV23. These dosing intervals are important, as lower opsonophagocytic antibody responses have been noted if repeat doses of either pneumococcal vaccine are given sooner than the recommended interval.15
Inactivated influenza vaccine is recommended annually, except for patients who are unlikely to respond or those who have received anti-B-cell antibodies within 6 months. Live-attenuated influenza vaccine should not be given to immunocompromised patients.
VACCINATING PATIENTS WHO HAVE HIV
People with HIV should be routinely screened for immunity against certain infections and should be offered vaccination if not immune. The response to vaccines may vary depending on the CD4 count, with a good response in patients whose infection is well controlled with antiretroviral agents and with a preserved CD4 count.16 Special considerations for HIV patients include the following:
Hepatitis A vaccine may be offered to all HIV patients who have no evidence of immunity against hepatitis A, with negative antihepatitis A total and IgG antibodies.
Human papillomavirus vaccine is recommended for men and women with HIV through age 26.
Varicella and measles-mumps-rubella are live-attenuated vaccines and may be considered in patients who are nonimmune and with CD4 counts of 200 cells/µL or higher. However, the ACIP does not make a recommendation regarding the zoster vaccine in HIV patients with CD4 cell counts of 200 cells/µL or higher. In general, live-attenuated vaccines should be avoided in patients with CD4 counts less than 200 or with severe immunocompromised status because of risk of acquiring severe, life-threatening infections.
Pneumococcal vaccine should be given to HIV patients if they have not received it before. The schedule is one dose of PCV13, followed by a dose of PPSV23 at least 8 weeks later. If a patient has been previously vaccinated with PPSV23, then PCV13 is recommended at least 1 year after PPSV23.
Inactivated influenza vaccine is recommended annually. Live-attenuated influenza vaccine should not be given.
Hepatitis B vaccine should be given to nonimmune patients without past or present hepatitis B infection. These patients require higher doses of hepatitis B vaccine (40 μg/mL) than immunocompetent patients, who receive 20 μg/mL. The options include Recombivax HB 40 μg/mL given on a three-dose schedule at 0, 1, and 6 months, and Engerix B, two 20-μg/mL injections given simultaneously on a four-dose schedule at 0, 1, 2, and 6 months.
Meningococcal vaccine. HIV infection is not an indication for meningococcal vaccine unless the patient has other risk factors, such as anatomic or functional asplenia, persistent complement component deficiency, occupational exposure, and travel to endemic areas.
VACCINATING PATIENTS WHO ARE OLDER THAN 60
The immune system deteriorates with age, as does immunity gained from previous vaccinations. Vaccination in this age group reduces the risk of illness and death.17
Zoster vaccine should be offered to people age 60 and older regardless of previous episodes of herpes zoster unless there is a contraindication such as severe immunodeficiency. The zoster vaccine can reduce the incidence of postherpetic neuralgia by 66.5% and herpes zoster by 51% in patients over age 60.18
Pneumococcal conjugate vaccine. PCV13 should be offered to all adults age 65 or older. If a person age 65 or older has not received any pneumococcal vaccine before then, PCV13 should be given first, followed by a dose of PPSV23 at least 6 to 12 months after PCV13.
Pneumococcal polysaccharide vaccine. If PPSV23 was given before age 65 for another indication, a dose of PCV 13 should be given at age 65 or later, as long as 6 to 12 months have passed since the previous dose of PPSV 23. The patient should receive the last dose of PPSV23 vaccine 5 years after the first dose of PPSV23.4
Influenza vaccine. People 65 or older are at higher risk of complications from influenza, and vaccine should be offered annually. High-dose inactivated influenza vaccine can be used in this age group.4
Tdap. If never given before, Tdap is recommended regardless of the interval since the most recent Td vaccination, followed by a Td booster every 10 years.
VACCINATING PATIENTS WHO HAVE CHRONIC KIDNEY DISEASE
Patients with chronic kidney disease are at risk of certain infections, so vaccination is an important preventive measure.19 Immunizations should be offered to all patients with chronic kidney disease regardless of the disease stage, but they are recommended during the early stages of progressive renal disease to increase the likelihood of vaccine-induced immunity.20
Pneumococcal conjugate vaccine. PCV13 is recommended for adults 19 or older with chronic renal disease or nephrotic syndrome. One dose of PCV13 should be given, followed by a dose of PPSV23 at least 8 weeks later. If the patient has been previously vaccinated with PPSV23, then PCV13 at least 1 year after PPSV23 is recommended.
Hepatitis B vaccine should be given to nonimmune patients without past or present hepatitis B infection. Adult patients on hemodialysis require higher doses of hepatitis B vaccine. The options include Recombivax HB 40 μg/mL given on a three-dose schedule at 0, 1, and 6 months, and Engerix B, two 20-μg/mL injections given simultaneously on a four-dose schedule at 0, 1, 2, and 6 months.
Influenza vaccine should be offered annually to patients with chronic kidney disease.
VACCINATING IMMUNOCOMPROMISED INTERNATIONAL TRAVELERS
International travel for business or pleasure is increasingly common, and immunocompromised patients require specific attention as they may face unanticipated pathogens or have special requirements. Transplant recipients should ideally receive routine and travel-related vaccines as early as possible before transplantation. Vaccination is generally avoided in the first 6 months after organ transplantation to avoid confusion with early graft dysfunction or rejection.21 However, it should be considered as soon as a patient develops an illness that might lead to transplantation.
Evaluation of patients for vaccination should include an assessment of the travel-specific epidemiologic risk, the nature of the vaccine (live-attenuated or other), and the immune status. As discussed above, live-attenuated vaccines should be avoided in immunocompromised patients, and thus the injectable typhoid vaccine should be given in lieu of the attenuated oral vaccine.
Yellow fever vaccine is required before entrance to certain countries but should not be given to immunocompromised patients, although it can probably be given to asymptomatic HIV-infected adults with a CD4 count higher than 200 cells/μL who are exposed to substantial risk.22 For patients who cannot receive the vaccine, some governments will accept a physician’s letter stating the patient has a contraindication to vaccination.
VACCINATING HOUSEHOLD MEMBERS OF IMMUNOCOMPROMISED PATIENTS
Protecting immunocompromised patients from infectious diseases involves vaccinating not only the patient but also household members so that they do not acquire infections and then bring them into the household. Immunocompetent members of a household can receive inactivated vaccines based on the recommended ACIP schedule.
Annual inactivated influenza vaccination is recommended, although the live-attenuated influenza virus vaccine can be substituted if the immunocompromised patient is not within 2 months of hematopoietic stem cell transplantation, does not have graft-vs-host disease, and does not have severe combined immune deficiency.
Other live-attenuated vaccines can usually be given if indicated, including measles-mumps-rubella vaccine, rotavirus vaccine in infants, varicella vaccine, and zoster vaccine.14
- Crosignani P, De Stefani A, Fara GM, et al. Towards the eradication of HPV infection through universal specific vaccination. BMC Public Health 2013;13:642.
- Plotkin SL, Plotkin SA. A short history of vaccination. In: Plotkin, SA, Orenstein W, Offit PA, editors. Vaccines, 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2008:1–16.
- Wong VW, Chan HL. Prevention of hepatocellular carcinoma: a concise review of contemporary issues. Ann Hepatol 2012; 11:284–293.
- Kim DK, Bridges CB, Harriman K; Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older: United States, 2015. Ann Intern Med 2015; 162:214–223.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults—United States, 2012. MMWR Morb Mortal Wkly Rep 2014; 63:95-102.
- Hurley LP, Bridges CB, Harpaz R, et al. US physicians’ perspective of adult vaccine delivery. Ann Intern Med 2014; 160:161.
- Lindsey B, Kampmann B, Jones C. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr Opin Infect Dis 2013; 26:248–253.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013; 62:131–135.
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:1–24.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003; 52:1–16.
- Staples JE, Gershman M, Fischer M; Centers for Disease Control and Prevention (CDC). Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010; 59:1–27.
- Danziger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice. Vaccination in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):311–317.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–64.
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA, Infectious Diseases Society of America. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:1–10.
- Eilers R, Krabbe PF, van Essen TG, Suijkerbuijk A, van Lier A, de Melker HE. Assessment of vaccine candidates for persons aged 50 and older: a review. BMC Geriatr 2013; 13:32.
- Oxman MN, Levin MJ; Shingles Prevention Study Group. Vaccination against Herpes Zoster and Postherpetic Neuralgia. J Infect Dis 2008; 197(suppl 2):S228–S236.
- Soni R, Horowitz B, Unruh M. Immunization in end-stage renal disease: opportunity to improve outcomes. Semin Dial 2013; 26:416–426.
- Chi C, Patel P, Pilishvili T, Moore M, Murphy T, Strikas R. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. http://www.cdc.gov/vaccines/pubs/downloads/dialysis-guide-2012.pdf. Accessed March 31, 2015.
- Kotton CN, Ryan ET, Fishman JA. Prevention of infection in adult travelers after solid organ transplantation. Am J Transplant 2005; 5:8–14.
- Castelli F, Patroni A. The human immunodeficiency virus-infected traveler. Clin Infect Dis 2000; 31:1403–1408.
- Crosignani P, De Stefani A, Fara GM, et al. Towards the eradication of HPV infection through universal specific vaccination. BMC Public Health 2013;13:642.
- Plotkin SL, Plotkin SA. A short history of vaccination. In: Plotkin, SA, Orenstein W, Offit PA, editors. Vaccines, 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2008:1–16.
- Wong VW, Chan HL. Prevention of hepatocellular carcinoma: a concise review of contemporary issues. Ann Hepatol 2012; 11:284–293.
- Kim DK, Bridges CB, Harriman K; Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older: United States, 2015. Ann Intern Med 2015; 162:214–223.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults—United States, 2012. MMWR Morb Mortal Wkly Rep 2014; 63:95-102.
- Hurley LP, Bridges CB, Harpaz R, et al. US physicians’ perspective of adult vaccine delivery. Ann Intern Med 2014; 160:161.
- Lindsey B, Kampmann B, Jones C. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr Opin Infect Dis 2013; 26:248–253.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013; 62:131–135.
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:1–24.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003; 52:1–16.
- Staples JE, Gershman M, Fischer M; Centers for Disease Control and Prevention (CDC). Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010; 59:1–27.
- Danziger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice. Vaccination in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):311–317.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–64.
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA, Infectious Diseases Society of America. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:1–10.
- Eilers R, Krabbe PF, van Essen TG, Suijkerbuijk A, van Lier A, de Melker HE. Assessment of vaccine candidates for persons aged 50 and older: a review. BMC Geriatr 2013; 13:32.
- Oxman MN, Levin MJ; Shingles Prevention Study Group. Vaccination against Herpes Zoster and Postherpetic Neuralgia. J Infect Dis 2008; 197(suppl 2):S228–S236.
- Soni R, Horowitz B, Unruh M. Immunization in end-stage renal disease: opportunity to improve outcomes. Semin Dial 2013; 26:416–426.
- Chi C, Patel P, Pilishvili T, Moore M, Murphy T, Strikas R. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. http://www.cdc.gov/vaccines/pubs/downloads/dialysis-guide-2012.pdf. Accessed March 31, 2015.
- Kotton CN, Ryan ET, Fishman JA. Prevention of infection in adult travelers after solid organ transplantation. Am J Transplant 2005; 5:8–14.
- Castelli F, Patroni A. The human immunodeficiency virus-infected traveler. Clin Infect Dis 2000; 31:1403–1408.
KEY POINTS
- Avoid live-attenuated vaccines (influenza, varicella, zoster, measles-mumps-rubella, and yellow fever) in immunocompromised patients.
- Tetanus, diphtheria, and acellular pertussis (Tdap) vaccine is now recommended for pregnant women during each pregnancy, preferably at 27 to 36 weeks of gestation.
- Zoster vaccine is recommended for patients age 60 and older, regardless of earlier episodes of herpes zoster.
AUA: Testosterone may not deserve its reputation as a cardiovascular culprit
NEW ORLEANS – Evidence seems to be mounting that the link between testosterone replacement therapy and increased hematocrit doesn’t lead to more cardiac or thrombotic events in men.
The association between testosterone and secondary erythrocytosis has been known for some time, Dr. Wayne J. G. Hellstrom said at the annual meeting of the American Urological Association. An increase in hematocrit almost invariably follows testosterone supplementation. “The question is, is there a causal relation between testosterone replacement therapy–induced erythrocytosis and venous thromboembolism or major cardiac events?” said Dr. Hellstrom of Tulane Medical Center, New Orleans. “The available evidence doesn’t support this claim.”
Erythrocytosis is defined as a packed red blood cell volume exceeding 125% of the age-predicted mass. This may be primary – an intrinsic alteration of the hematopoietic stem cells – or secondary. “And it may actually be a physiologically appropriate response to something, as in anemia,” Dr. Hellstrom said. “In fact, some anemias are primarily treated with testosterone.”
In the presence of exogenous testosterone, the condition may be due to a couple of things, he noted, such as:
• An overall increase in the erythropoietin set point.
• Increased availability of iron in the liver.
• The conversion of testosterone to estradiol, which tends to stimulate the bone marrow.
Erythrocytosis, obviously then, increases blood viscosity – and this is the primary concern for cardiovascular events.
Intramuscular testosterone is the only form that significantly increases hematocrit above normal levels. However, it does so strongly, with up to a 6% change from baseline. The runner-up is testosterone gel, with an average increase of 2.5% over baseline levels.
But despite concerns – which in March prompted the FDA to require on labeling a warning about the risk of cardiovascular events – the relationship has never been thoroughly investigated, Dr. Hellstrom said.
“We only have retrospective data, primarily extrapolating from the nephrology literature. When we look at the renal literature, we see that 10%-20% of kidney transplant patients develop polycythemia – an increase of both red and white cells, with hematocrit values of more than 51% or 52%.”
This has led to a recommendation by the American Society of Nephrology for frequent complete blood cell counts in the year after transplant and annual measurements thereafter.
The highest-quality mortality data for kidney transplant patients come from a 2013 study of 365 patients; the investigators found that those with polycythemia were 2.7 times more likely to die over 4 years. “But this is a true primary polycythemia,” which is often accompanied by procoagulative changes. It is not the secondary condition induced by testosterone, Dr. Hellstrom said.
Older studies suggested a significant link between increased hematocrit and cardiovascular or thrombotic events, especially after surgery. But prospective data from the Atherosclerosis Risk in Communities and Cardiovascular Health Studies have found no increased risk of cardiovascular death by increasing tertiles of either hematocrit or hemoglobin, with respective cut points of 43% and 14.5 g/dL.
In fact, a recent transgenic mouse model with hematopoietic overexpression, reaching an 85% hematocrit, found no evidence of either lung or cardiovascular thromboses. “This seems to be related to a reduction in clot strength and increased osmotic fragility in the presence of increasing hematocrit. It seems to mechanically deter the interaction of platelets and fibrin in the extravascular space and endothelium.”
He referred to an in-press mouse study showing that a short course of high-dose testosterone did raise whole blood viscosity and hematocrit. “But over time, this returned to normal, even with supraphysiolgic testosterone levels, so it seems likely that there is an adaptive mechanism that occurs in these animals.”
Additionally, he said, men who live at high altitudes develop naturally high hematocrits as a response to decreased oxygen in the atmosphere. “We routinely see men from these locations with hematocrits of 57% and 59% who have no problems at all.”
Extrapolating all these data to the testosterone/thrombosis link is confusing. The most recent study, however, provided some measure of reassurance. The large meta-analysis comprised 75 randomized, placebo-controlled trials involving about 5,500 men; they all examined cardiovascular risk and testosterone therapy.
“Our analyses, performed on the largest number of studies collected so far, indicate that testosterone supplementation is not related to any increase in cardiovascular risk, even when composite or single adverse events were considered,” wrote Dr. Giovanni Corona of the Maggiore-Bellaria Hospital, Bologna, Italy. “In randomized trials performed in subjects with metabolic derangements, a protective effect … was observed. … Our results are in agreement with a large body of literature from the last 20 years supporting testosterone supplementation of hypogonadal men as a valuable strategy in improving a patient’s metabolic profile, reducing body fat, and increasing lean muscle mass, which would ultimately reduce the risk of heart disease
“There is a definite need for large multicenter, randomized trials to determine the true risk,” Dr. Hellstrom said. However, in light of the current evidence, he recommends what he called a “conservative” approach to testosterone prescribing:
• Before prescribing, get a baseline complete blood count.
• If the baseline hematocrit is more than 47%, consider alternative treatments, but proceed if testosterone replacement therapy seems to be the best clinical option. Repeat testing at 3 and 12 months after therapy initiation and then annually.
• If hematocrit increases above 54%, discontinue treatment until there is a further clinical assessment, as detailed by the Endocrine Society.
• Closely monitor any new diagnoses of hypertension.
• If hematocrit does rise precipitously, phlebotomy rapidly resolved the problem.
Dr Hellstrom made the following financial disclosures: consultant, advisor, or leadership position for Abbvie, Allergan, American Medical Systems, Antares, Astellas, Auxilim, Allergan, Coloplast, Endo, Lilly, New England Research Institutes Inc. Pfizer, Promescent, Reros Therapeutics, and Theralogix.
NEW ORLEANS – Evidence seems to be mounting that the link between testosterone replacement therapy and increased hematocrit doesn’t lead to more cardiac or thrombotic events in men.
The association between testosterone and secondary erythrocytosis has been known for some time, Dr. Wayne J. G. Hellstrom said at the annual meeting of the American Urological Association. An increase in hematocrit almost invariably follows testosterone supplementation. “The question is, is there a causal relation between testosterone replacement therapy–induced erythrocytosis and venous thromboembolism or major cardiac events?” said Dr. Hellstrom of Tulane Medical Center, New Orleans. “The available evidence doesn’t support this claim.”
Erythrocytosis is defined as a packed red blood cell volume exceeding 125% of the age-predicted mass. This may be primary – an intrinsic alteration of the hematopoietic stem cells – or secondary. “And it may actually be a physiologically appropriate response to something, as in anemia,” Dr. Hellstrom said. “In fact, some anemias are primarily treated with testosterone.”
In the presence of exogenous testosterone, the condition may be due to a couple of things, he noted, such as:
• An overall increase in the erythropoietin set point.
• Increased availability of iron in the liver.
• The conversion of testosterone to estradiol, which tends to stimulate the bone marrow.
Erythrocytosis, obviously then, increases blood viscosity – and this is the primary concern for cardiovascular events.
Intramuscular testosterone is the only form that significantly increases hematocrit above normal levels. However, it does so strongly, with up to a 6% change from baseline. The runner-up is testosterone gel, with an average increase of 2.5% over baseline levels.
But despite concerns – which in March prompted the FDA to require on labeling a warning about the risk of cardiovascular events – the relationship has never been thoroughly investigated, Dr. Hellstrom said.
“We only have retrospective data, primarily extrapolating from the nephrology literature. When we look at the renal literature, we see that 10%-20% of kidney transplant patients develop polycythemia – an increase of both red and white cells, with hematocrit values of more than 51% or 52%.”
This has led to a recommendation by the American Society of Nephrology for frequent complete blood cell counts in the year after transplant and annual measurements thereafter.
The highest-quality mortality data for kidney transplant patients come from a 2013 study of 365 patients; the investigators found that those with polycythemia were 2.7 times more likely to die over 4 years. “But this is a true primary polycythemia,” which is often accompanied by procoagulative changes. It is not the secondary condition induced by testosterone, Dr. Hellstrom said.
Older studies suggested a significant link between increased hematocrit and cardiovascular or thrombotic events, especially after surgery. But prospective data from the Atherosclerosis Risk in Communities and Cardiovascular Health Studies have found no increased risk of cardiovascular death by increasing tertiles of either hematocrit or hemoglobin, with respective cut points of 43% and 14.5 g/dL.
In fact, a recent transgenic mouse model with hematopoietic overexpression, reaching an 85% hematocrit, found no evidence of either lung or cardiovascular thromboses. “This seems to be related to a reduction in clot strength and increased osmotic fragility in the presence of increasing hematocrit. It seems to mechanically deter the interaction of platelets and fibrin in the extravascular space and endothelium.”
He referred to an in-press mouse study showing that a short course of high-dose testosterone did raise whole blood viscosity and hematocrit. “But over time, this returned to normal, even with supraphysiolgic testosterone levels, so it seems likely that there is an adaptive mechanism that occurs in these animals.”
Additionally, he said, men who live at high altitudes develop naturally high hematocrits as a response to decreased oxygen in the atmosphere. “We routinely see men from these locations with hematocrits of 57% and 59% who have no problems at all.”
Extrapolating all these data to the testosterone/thrombosis link is confusing. The most recent study, however, provided some measure of reassurance. The large meta-analysis comprised 75 randomized, placebo-controlled trials involving about 5,500 men; they all examined cardiovascular risk and testosterone therapy.
“Our analyses, performed on the largest number of studies collected so far, indicate that testosterone supplementation is not related to any increase in cardiovascular risk, even when composite or single adverse events were considered,” wrote Dr. Giovanni Corona of the Maggiore-Bellaria Hospital, Bologna, Italy. “In randomized trials performed in subjects with metabolic derangements, a protective effect … was observed. … Our results are in agreement with a large body of literature from the last 20 years supporting testosterone supplementation of hypogonadal men as a valuable strategy in improving a patient’s metabolic profile, reducing body fat, and increasing lean muscle mass, which would ultimately reduce the risk of heart disease
“There is a definite need for large multicenter, randomized trials to determine the true risk,” Dr. Hellstrom said. However, in light of the current evidence, he recommends what he called a “conservative” approach to testosterone prescribing:
• Before prescribing, get a baseline complete blood count.
• If the baseline hematocrit is more than 47%, consider alternative treatments, but proceed if testosterone replacement therapy seems to be the best clinical option. Repeat testing at 3 and 12 months after therapy initiation and then annually.
• If hematocrit increases above 54%, discontinue treatment until there is a further clinical assessment, as detailed by the Endocrine Society.
• Closely monitor any new diagnoses of hypertension.
• If hematocrit does rise precipitously, phlebotomy rapidly resolved the problem.
Dr Hellstrom made the following financial disclosures: consultant, advisor, or leadership position for Abbvie, Allergan, American Medical Systems, Antares, Astellas, Auxilim, Allergan, Coloplast, Endo, Lilly, New England Research Institutes Inc. Pfizer, Promescent, Reros Therapeutics, and Theralogix.
NEW ORLEANS – Evidence seems to be mounting that the link between testosterone replacement therapy and increased hematocrit doesn’t lead to more cardiac or thrombotic events in men.
The association between testosterone and secondary erythrocytosis has been known for some time, Dr. Wayne J. G. Hellstrom said at the annual meeting of the American Urological Association. An increase in hematocrit almost invariably follows testosterone supplementation. “The question is, is there a causal relation between testosterone replacement therapy–induced erythrocytosis and venous thromboembolism or major cardiac events?” said Dr. Hellstrom of Tulane Medical Center, New Orleans. “The available evidence doesn’t support this claim.”
Erythrocytosis is defined as a packed red blood cell volume exceeding 125% of the age-predicted mass. This may be primary – an intrinsic alteration of the hematopoietic stem cells – or secondary. “And it may actually be a physiologically appropriate response to something, as in anemia,” Dr. Hellstrom said. “In fact, some anemias are primarily treated with testosterone.”
In the presence of exogenous testosterone, the condition may be due to a couple of things, he noted, such as:
• An overall increase in the erythropoietin set point.
• Increased availability of iron in the liver.
• The conversion of testosterone to estradiol, which tends to stimulate the bone marrow.
Erythrocytosis, obviously then, increases blood viscosity – and this is the primary concern for cardiovascular events.
Intramuscular testosterone is the only form that significantly increases hematocrit above normal levels. However, it does so strongly, with up to a 6% change from baseline. The runner-up is testosterone gel, with an average increase of 2.5% over baseline levels.
But despite concerns – which in March prompted the FDA to require on labeling a warning about the risk of cardiovascular events – the relationship has never been thoroughly investigated, Dr. Hellstrom said.
“We only have retrospective data, primarily extrapolating from the nephrology literature. When we look at the renal literature, we see that 10%-20% of kidney transplant patients develop polycythemia – an increase of both red and white cells, with hematocrit values of more than 51% or 52%.”
This has led to a recommendation by the American Society of Nephrology for frequent complete blood cell counts in the year after transplant and annual measurements thereafter.
The highest-quality mortality data for kidney transplant patients come from a 2013 study of 365 patients; the investigators found that those with polycythemia were 2.7 times more likely to die over 4 years. “But this is a true primary polycythemia,” which is often accompanied by procoagulative changes. It is not the secondary condition induced by testosterone, Dr. Hellstrom said.
Older studies suggested a significant link between increased hematocrit and cardiovascular or thrombotic events, especially after surgery. But prospective data from the Atherosclerosis Risk in Communities and Cardiovascular Health Studies have found no increased risk of cardiovascular death by increasing tertiles of either hematocrit or hemoglobin, with respective cut points of 43% and 14.5 g/dL.
In fact, a recent transgenic mouse model with hematopoietic overexpression, reaching an 85% hematocrit, found no evidence of either lung or cardiovascular thromboses. “This seems to be related to a reduction in clot strength and increased osmotic fragility in the presence of increasing hematocrit. It seems to mechanically deter the interaction of platelets and fibrin in the extravascular space and endothelium.”
He referred to an in-press mouse study showing that a short course of high-dose testosterone did raise whole blood viscosity and hematocrit. “But over time, this returned to normal, even with supraphysiolgic testosterone levels, so it seems likely that there is an adaptive mechanism that occurs in these animals.”
Additionally, he said, men who live at high altitudes develop naturally high hematocrits as a response to decreased oxygen in the atmosphere. “We routinely see men from these locations with hematocrits of 57% and 59% who have no problems at all.”
Extrapolating all these data to the testosterone/thrombosis link is confusing. The most recent study, however, provided some measure of reassurance. The large meta-analysis comprised 75 randomized, placebo-controlled trials involving about 5,500 men; they all examined cardiovascular risk and testosterone therapy.
“Our analyses, performed on the largest number of studies collected so far, indicate that testosterone supplementation is not related to any increase in cardiovascular risk, even when composite or single adverse events were considered,” wrote Dr. Giovanni Corona of the Maggiore-Bellaria Hospital, Bologna, Italy. “In randomized trials performed in subjects with metabolic derangements, a protective effect … was observed. … Our results are in agreement with a large body of literature from the last 20 years supporting testosterone supplementation of hypogonadal men as a valuable strategy in improving a patient’s metabolic profile, reducing body fat, and increasing lean muscle mass, which would ultimately reduce the risk of heart disease
“There is a definite need for large multicenter, randomized trials to determine the true risk,” Dr. Hellstrom said. However, in light of the current evidence, he recommends what he called a “conservative” approach to testosterone prescribing:
• Before prescribing, get a baseline complete blood count.
• If the baseline hematocrit is more than 47%, consider alternative treatments, but proceed if testosterone replacement therapy seems to be the best clinical option. Repeat testing at 3 and 12 months after therapy initiation and then annually.
• If hematocrit increases above 54%, discontinue treatment until there is a further clinical assessment, as detailed by the Endocrine Society.
• Closely monitor any new diagnoses of hypertension.
• If hematocrit does rise precipitously, phlebotomy rapidly resolved the problem.
Dr Hellstrom made the following financial disclosures: consultant, advisor, or leadership position for Abbvie, Allergan, American Medical Systems, Antares, Astellas, Auxilim, Allergan, Coloplast, Endo, Lilly, New England Research Institutes Inc. Pfizer, Promescent, Reros Therapeutics, and Theralogix.
EXPERT ANALYSIS FROM THE AUA ANNUAL MEETING
Rate ratio of comorbidity high in SLE patients under 40
MANCHESTER, U.K. – When systemic lupus erythematosus (SLE) occurs before age 40, patients run a high relative risk of end-stage renal disease, data from a retrospective U.K.-based cohort study have shown.
While the risk for cardiovascular disease and stroke has been reported previously, particularly in younger SLE patients, the risks for comorbidities such as end-stage renal failure (ESRF), osteoporosis, and infection were not as clear. “We know that comorbidities are increased in patients with lupus, but we didn’t know by how much,” Dr. Frances Rees of Nottingham University Hospitals NHS Trust, Nottingham, England, explained at the British Society for Rheumatology annual conference.The adjusted incidence rate ratio (IRR) for ESRF was greater than 60 for lupus patients under age 40 and about 10 for those aged 40-69 years.“Although the absolute risk increased with age, the relative risk difference between cases and controls was highest in those at younger ages, so don’t forget primary prevention and screening in younger patients,” Dr. Rees said.
The risk was based on data obtained from the Clinical Practice Research Datalink, an anonymized database of primary care records for approximately 12 million people, on all prevalent cases of SLE occurring between 1999 and 2012 in the United Kingdom. Each of the 7,732 cases was matched to up to four patients who did not have lupus and were seen at the same practice. The control population exceeded 28,000 individuals.
Around 55% of lupus patients had a Charlson Comorbidity Index (CCI) of zero while around 75% of patients without lupus had no comorbidities. About 33% of lupus patients had a CCI of 1-2 as did about 20% of controls; less than 10% of patients had a CCI of 3-5 or more than 5.
“The highest difference between the two groups was for end-stage renal failure even after adjusting for confounders,” she added. IRRs for the other comorbidities were around or just under 2.“When we compared men and women, men had higher risks of cardiovascular disease, stroke, and cancer, but women had higher rates of infection and osteoporosis, which would fit with the underlying population,” Dr. Rees observed.
“What was interesting, however, was the difference in the incidence rates for osteoporosis between cases and controls in men, which was of a bigger relative risk than it was in women,” Dr. Rees noted. “So don’t forget to consider osteoporosis in men,” she advised. For cardiovascular disease, the IRR was much higher in patients under age 40 years than for the older patients (IRR <5).In an interview, Dr. Rees explained that while these data partly confirm what was already known, the research is the first to look at comorbidity in SLE from a community perspective. “Also, some of the previous studies done in hospitals have only really shown that the risk of cardiovascular disease and stroke was in younger people, but we have found that the risk was increased across all age groups.”
The work was supported by a research grant from Lupus UK. Dr. Rees had no conflicts of interest.
MANCHESTER, U.K. – When systemic lupus erythematosus (SLE) occurs before age 40, patients run a high relative risk of end-stage renal disease, data from a retrospective U.K.-based cohort study have shown.
While the risk for cardiovascular disease and stroke has been reported previously, particularly in younger SLE patients, the risks for comorbidities such as end-stage renal failure (ESRF), osteoporosis, and infection were not as clear. “We know that comorbidities are increased in patients with lupus, but we didn’t know by how much,” Dr. Frances Rees of Nottingham University Hospitals NHS Trust, Nottingham, England, explained at the British Society for Rheumatology annual conference.The adjusted incidence rate ratio (IRR) for ESRF was greater than 60 for lupus patients under age 40 and about 10 for those aged 40-69 years.“Although the absolute risk increased with age, the relative risk difference between cases and controls was highest in those at younger ages, so don’t forget primary prevention and screening in younger patients,” Dr. Rees said.
The risk was based on data obtained from the Clinical Practice Research Datalink, an anonymized database of primary care records for approximately 12 million people, on all prevalent cases of SLE occurring between 1999 and 2012 in the United Kingdom. Each of the 7,732 cases was matched to up to four patients who did not have lupus and were seen at the same practice. The control population exceeded 28,000 individuals.
Around 55% of lupus patients had a Charlson Comorbidity Index (CCI) of zero while around 75% of patients without lupus had no comorbidities. About 33% of lupus patients had a CCI of 1-2 as did about 20% of controls; less than 10% of patients had a CCI of 3-5 or more than 5.
“The highest difference between the two groups was for end-stage renal failure even after adjusting for confounders,” she added. IRRs for the other comorbidities were around or just under 2.“When we compared men and women, men had higher risks of cardiovascular disease, stroke, and cancer, but women had higher rates of infection and osteoporosis, which would fit with the underlying population,” Dr. Rees observed.
“What was interesting, however, was the difference in the incidence rates for osteoporosis between cases and controls in men, which was of a bigger relative risk than it was in women,” Dr. Rees noted. “So don’t forget to consider osteoporosis in men,” she advised. For cardiovascular disease, the IRR was much higher in patients under age 40 years than for the older patients (IRR <5).In an interview, Dr. Rees explained that while these data partly confirm what was already known, the research is the first to look at comorbidity in SLE from a community perspective. “Also, some of the previous studies done in hospitals have only really shown that the risk of cardiovascular disease and stroke was in younger people, but we have found that the risk was increased across all age groups.”
The work was supported by a research grant from Lupus UK. Dr. Rees had no conflicts of interest.
MANCHESTER, U.K. – When systemic lupus erythematosus (SLE) occurs before age 40, patients run a high relative risk of end-stage renal disease, data from a retrospective U.K.-based cohort study have shown.
While the risk for cardiovascular disease and stroke has been reported previously, particularly in younger SLE patients, the risks for comorbidities such as end-stage renal failure (ESRF), osteoporosis, and infection were not as clear. “We know that comorbidities are increased in patients with lupus, but we didn’t know by how much,” Dr. Frances Rees of Nottingham University Hospitals NHS Trust, Nottingham, England, explained at the British Society for Rheumatology annual conference.The adjusted incidence rate ratio (IRR) for ESRF was greater than 60 for lupus patients under age 40 and about 10 for those aged 40-69 years.“Although the absolute risk increased with age, the relative risk difference between cases and controls was highest in those at younger ages, so don’t forget primary prevention and screening in younger patients,” Dr. Rees said.
The risk was based on data obtained from the Clinical Practice Research Datalink, an anonymized database of primary care records for approximately 12 million people, on all prevalent cases of SLE occurring between 1999 and 2012 in the United Kingdom. Each of the 7,732 cases was matched to up to four patients who did not have lupus and were seen at the same practice. The control population exceeded 28,000 individuals.
Around 55% of lupus patients had a Charlson Comorbidity Index (CCI) of zero while around 75% of patients without lupus had no comorbidities. About 33% of lupus patients had a CCI of 1-2 as did about 20% of controls; less than 10% of patients had a CCI of 3-5 or more than 5.
“The highest difference between the two groups was for end-stage renal failure even after adjusting for confounders,” she added. IRRs for the other comorbidities were around or just under 2.“When we compared men and women, men had higher risks of cardiovascular disease, stroke, and cancer, but women had higher rates of infection and osteoporosis, which would fit with the underlying population,” Dr. Rees observed.
“What was interesting, however, was the difference in the incidence rates for osteoporosis between cases and controls in men, which was of a bigger relative risk than it was in women,” Dr. Rees noted. “So don’t forget to consider osteoporosis in men,” she advised. For cardiovascular disease, the IRR was much higher in patients under age 40 years than for the older patients (IRR <5).In an interview, Dr. Rees explained that while these data partly confirm what was already known, the research is the first to look at comorbidity in SLE from a community perspective. “Also, some of the previous studies done in hospitals have only really shown that the risk of cardiovascular disease and stroke was in younger people, but we have found that the risk was increased across all age groups.”
The work was supported by a research grant from Lupus UK. Dr. Rees had no conflicts of interest.
Key clinical point: The relative comorbidity burden is highest in SLE patients under age 40.
Major finding: The adjusted incidence rate ratio (IRR) for end-stage renal failure was 60-fold higher in patients under age 40 years and 10-fold higher in patients aged 40-69 years, compared to controls.
Data source: Retrospective cohort study of 7,732 patients with systemic lupus erythematosus and 29,079 lupus-free individuals.
Disclosures: The work was supported by a research grant from Lupus UK. Dr. Rees had no conflicts of interest.
DDW: VIDEO: What we don’t know in the management of liver disease and coagulopathy
WASHINGTON – Your patient has cirrhosis, platelets 60,000 mm3, an INR of 2.0, serum creatinine of 1.2 mg/dL, and requires an endoscopic retrograde cholangiopancreatography with sphincterotomy.
What do you do next?
Management of a patient such as this is challenging, but not just because of the long-perceived risk for bleeding, Dr. Patrick S. Kamath of the Mayo Clinic in Rochester, Minn., said during a clinical symposium at the annual Digestive Disease Week.
Several other factors must be considered, including the clotting risk in patients with liver disease and the fact that procedure-related bleeding risk cannot be adequately determined preprocedure. Transfusions also carry their own dangers in this patient population and should be approached with caution, he said.
To hear more from this world-renowned liver expert, check out our interview as we sat down with Dr. Kamath at this year’s DDW.
Dr. Kamath reported no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
WASHINGTON – Your patient has cirrhosis, platelets 60,000 mm3, an INR of 2.0, serum creatinine of 1.2 mg/dL, and requires an endoscopic retrograde cholangiopancreatography with sphincterotomy.
What do you do next?
Management of a patient such as this is challenging, but not just because of the long-perceived risk for bleeding, Dr. Patrick S. Kamath of the Mayo Clinic in Rochester, Minn., said during a clinical symposium at the annual Digestive Disease Week.
Several other factors must be considered, including the clotting risk in patients with liver disease and the fact that procedure-related bleeding risk cannot be adequately determined preprocedure. Transfusions also carry their own dangers in this patient population and should be approached with caution, he said.
To hear more from this world-renowned liver expert, check out our interview as we sat down with Dr. Kamath at this year’s DDW.
Dr. Kamath reported no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
WASHINGTON – Your patient has cirrhosis, platelets 60,000 mm3, an INR of 2.0, serum creatinine of 1.2 mg/dL, and requires an endoscopic retrograde cholangiopancreatography with sphincterotomy.
What do you do next?
Management of a patient such as this is challenging, but not just because of the long-perceived risk for bleeding, Dr. Patrick S. Kamath of the Mayo Clinic in Rochester, Minn., said during a clinical symposium at the annual Digestive Disease Week.
Several other factors must be considered, including the clotting risk in patients with liver disease and the fact that procedure-related bleeding risk cannot be adequately determined preprocedure. Transfusions also carry their own dangers in this patient population and should be approached with caution, he said.
To hear more from this world-renowned liver expert, check out our interview as we sat down with Dr. Kamath at this year’s DDW.
Dr. Kamath reported no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
AT DDW 2015