User login
Watch for abnormal movements in hospitalized COVID-19 patients
Myoclonus was diagnosed in about half of hospitalized COVID-19 patients who were evaluated for movement disorders, data from 50 cases show.
Abnormal movements often occur as complications from critical illness, and neurologic consultation can determine whether patients have experienced a seizure or stroke. However, restriction of bedside assessment in the wake of the COVID-19 pandemic increases the risk that abnormal movements will be missed, Jeffrey R. Clark and Eric M. Liotta, MD, of Northwestern University, Chicago, and colleagues wrote.
“Given the limited reports of abnormal movements in hospitalized COVID-19 patients and increased recognition of neurologic manifestations of COVID-19, we sought to examine the frequency and etiology of this finding as an indication of neurologic consultation,” they said.
In a study published in the Journal of the Neurological Sciences, the researchers reviewed data from the first 50 consecutive patients with COVID-19 symptoms who were hospitalized at a single center and underwent neurologic consultation between March 17, 2020, and May 18, 2020.
Overall, 11 patients (22.0%) of patients experienced abnormal movement, and all were admitted to the ICU within 7 days of meeting criteria for severe COVID-19. These patients included nine men and two women with an age range of 36-78 years. The most common comorbidities were obesity, hypertension, diabetes, chronic kidney disease, and coronary artery disease.
Myoclonus (generalized and focal) was the most common abnormal movement, and present in 6 of the 11 patients. Three cases were attributed to high-intensity sedation, and three to toxic-metabolic disturbances. In two patients, abnormal movements were attributed to focal seizures in the setting of encephalopathy, with focal facial twitching. An additional two patients experienced tremors; one showed an acute subdural hemorrhage on CT imaging. The second patient showed no sign of stroke or other abnormality on MRI and the tremor improved during the hospital stay. One patient who experienced abnormal high-amplitude nonrhythmic movements of the lower extremities was diagnosed with serotonin syndrome that resolved after discontinuing high-dose fentanyl.
The study findings were limited by several factors, including the small study population and limited availability of MRI, the researchers noted. Assessing severe COVID-19 cases in the ICU setting presents a challenge because of limited patient participation and the potentially confounding effects of sedation and mechanical ventilation.
However, the researchers said.
“A heightened awareness of abnormal eye movements, or subtle facial tremoring, may be the first steps in recognizing potentially dangerous neurologic manifestations,” and clinicians caring for patients with severe COVID-19 should be able to recognize abnormal movements and seek neurologic consultation when indicated, they emphasized.
The study was supported in part by grants to coauthors Nicholas J. Reish, MD, and Dr. Liotta from the National Institutes of Health. The researchers had no financial conflicts to disclose.
Myoclonus was diagnosed in about half of hospitalized COVID-19 patients who were evaluated for movement disorders, data from 50 cases show.
Abnormal movements often occur as complications from critical illness, and neurologic consultation can determine whether patients have experienced a seizure or stroke. However, restriction of bedside assessment in the wake of the COVID-19 pandemic increases the risk that abnormal movements will be missed, Jeffrey R. Clark and Eric M. Liotta, MD, of Northwestern University, Chicago, and colleagues wrote.
“Given the limited reports of abnormal movements in hospitalized COVID-19 patients and increased recognition of neurologic manifestations of COVID-19, we sought to examine the frequency and etiology of this finding as an indication of neurologic consultation,” they said.
In a study published in the Journal of the Neurological Sciences, the researchers reviewed data from the first 50 consecutive patients with COVID-19 symptoms who were hospitalized at a single center and underwent neurologic consultation between March 17, 2020, and May 18, 2020.
Overall, 11 patients (22.0%) of patients experienced abnormal movement, and all were admitted to the ICU within 7 days of meeting criteria for severe COVID-19. These patients included nine men and two women with an age range of 36-78 years. The most common comorbidities were obesity, hypertension, diabetes, chronic kidney disease, and coronary artery disease.
Myoclonus (generalized and focal) was the most common abnormal movement, and present in 6 of the 11 patients. Three cases were attributed to high-intensity sedation, and three to toxic-metabolic disturbances. In two patients, abnormal movements were attributed to focal seizures in the setting of encephalopathy, with focal facial twitching. An additional two patients experienced tremors; one showed an acute subdural hemorrhage on CT imaging. The second patient showed no sign of stroke or other abnormality on MRI and the tremor improved during the hospital stay. One patient who experienced abnormal high-amplitude nonrhythmic movements of the lower extremities was diagnosed with serotonin syndrome that resolved after discontinuing high-dose fentanyl.
The study findings were limited by several factors, including the small study population and limited availability of MRI, the researchers noted. Assessing severe COVID-19 cases in the ICU setting presents a challenge because of limited patient participation and the potentially confounding effects of sedation and mechanical ventilation.
However, the researchers said.
“A heightened awareness of abnormal eye movements, or subtle facial tremoring, may be the first steps in recognizing potentially dangerous neurologic manifestations,” and clinicians caring for patients with severe COVID-19 should be able to recognize abnormal movements and seek neurologic consultation when indicated, they emphasized.
The study was supported in part by grants to coauthors Nicholas J. Reish, MD, and Dr. Liotta from the National Institutes of Health. The researchers had no financial conflicts to disclose.
Myoclonus was diagnosed in about half of hospitalized COVID-19 patients who were evaluated for movement disorders, data from 50 cases show.
Abnormal movements often occur as complications from critical illness, and neurologic consultation can determine whether patients have experienced a seizure or stroke. However, restriction of bedside assessment in the wake of the COVID-19 pandemic increases the risk that abnormal movements will be missed, Jeffrey R. Clark and Eric M. Liotta, MD, of Northwestern University, Chicago, and colleagues wrote.
“Given the limited reports of abnormal movements in hospitalized COVID-19 patients and increased recognition of neurologic manifestations of COVID-19, we sought to examine the frequency and etiology of this finding as an indication of neurologic consultation,” they said.
In a study published in the Journal of the Neurological Sciences, the researchers reviewed data from the first 50 consecutive patients with COVID-19 symptoms who were hospitalized at a single center and underwent neurologic consultation between March 17, 2020, and May 18, 2020.
Overall, 11 patients (22.0%) of patients experienced abnormal movement, and all were admitted to the ICU within 7 days of meeting criteria for severe COVID-19. These patients included nine men and two women with an age range of 36-78 years. The most common comorbidities were obesity, hypertension, diabetes, chronic kidney disease, and coronary artery disease.
Myoclonus (generalized and focal) was the most common abnormal movement, and present in 6 of the 11 patients. Three cases were attributed to high-intensity sedation, and three to toxic-metabolic disturbances. In two patients, abnormal movements were attributed to focal seizures in the setting of encephalopathy, with focal facial twitching. An additional two patients experienced tremors; one showed an acute subdural hemorrhage on CT imaging. The second patient showed no sign of stroke or other abnormality on MRI and the tremor improved during the hospital stay. One patient who experienced abnormal high-amplitude nonrhythmic movements of the lower extremities was diagnosed with serotonin syndrome that resolved after discontinuing high-dose fentanyl.
The study findings were limited by several factors, including the small study population and limited availability of MRI, the researchers noted. Assessing severe COVID-19 cases in the ICU setting presents a challenge because of limited patient participation and the potentially confounding effects of sedation and mechanical ventilation.
However, the researchers said.
“A heightened awareness of abnormal eye movements, or subtle facial tremoring, may be the first steps in recognizing potentially dangerous neurologic manifestations,” and clinicians caring for patients with severe COVID-19 should be able to recognize abnormal movements and seek neurologic consultation when indicated, they emphasized.
The study was supported in part by grants to coauthors Nicholas J. Reish, MD, and Dr. Liotta from the National Institutes of Health. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF THE NEUROLOGICAL SCIENCES
Cardiologists can perform stroke thrombectomy to fill ‘unmet need’
Cardiologists experienced in cardiac interventions can competently perform stroke thrombectomy after a short period of training, with outcomes comparable to those achieved by neuroradiology centers, a new study suggests.
“Using interventional cardiologists in this way will help address the huge unmet need for stroke thrombectomy that currently exists,” senior author Petr Widimsky, MD, said in an interview.
Although this may be a feasible way forward in Europe, there is strong opposition to such a proposal from U.S. neurointerventionalists.
The study, published in the April 12 issue of JACC: Cardiovascular Interventions, describes the establishment of a stroke thrombectomy program in University Hospital Kralovske Vinohrady, a large tertiary hospital in Prague, Czech Republic.
The hospital did not have a neurointerventional program until 2012 when a joint program was started involving an experienced team of cardiologists, angiologists, and one interventional radiologist who trained the cardiologists on the thrombectomy procedure.
The current paper reports on the outcomes of the 333 patients with large vessel occlusion stroke treated under this program between October 2012 and December 2019.
The decision to perform catheter-based thrombectomy was made by a neurologist and was based on acute stroke clinical symptoms and CT angiographic findings.
Results show that functional clinical outcomes, assessed via the Modified Rankin Scale (mRS) score at 3 months, did not vary significantly across years 2012 to 2019, with a favorable outcome (mRS 0 to 2) achieved in 47.9% of patients.
Symptomatic intracerebral hemorrhage occurred in 19 patients (5.7%) and embolization in a new vascular territory occurred in 6 patients (1.8%), outcomes similar to those of neuroradiology centers.
The desired clinical results were achieved from the onset of the program, without any signs of a learning curve effect, they reported.
“These findings support the potential role of interventional cardiac cath labs in the treatment of acute stroke in regions where this therapy is not readily available due to the lack of neurointerventionalists,” the authors concluded.
“Our main message is that our results were excellent from the beginning,” Dr. Widimsky said. “When centers prepare properly, they can achieve excellent results from the beginning with cardiologists who are experienced in interventional procedures and who have spent sufficient time learning about the brain.”
The authors noted that despite thrombectomy being an extremely beneficial treatment for severe stroke, many eligible patients remain untreated, largely because of a lack of neurointerventionalists in many regions worldwide. They estimate that about 15% of all stroke patients are eligible for thrombectomy but only around 2% of stroke patients in Europe actually receive such treatment.
Dr. Widimsky, an interventional cardiologist, first thought of the idea of using cardiologists to perform stroke thrombectomies after a good friend and colleague suffered a severe stroke in 2010.
“This made us realize that our hospital needed to be more active in the stroke field,” he said. “We decided that we needed to start doing stroke interventions.”
But the major problem was the lack of neurointerventionalists.
“There are not enough neurointerventionalists in Europe. Interventional cardiologists can perform thousands of procedures every year whereas a neurointerventionalist will at best perform hundreds a year. It is quicker and simpler to train the cardiologist to do it,” Dr. Widimsky said.
They hired one neurointerventionalist to lead the program. “He was our tutor, he taught us his skills,” Dr. Widimsky said. “The cath lab is open 24/7, but if we only have one neurointerventionalist we cannot offer a 24/7 service for stroke thrombectomy. But if we merge with cardiology then we can,” he added.
Their hospital is a very busy center for myocardial infarction, percutaneous coronary intervention, and carotid stenting, he noted. “It is not difficult to make the step from that to stroke thrombectomy. Interventional cardiologists are used to performing carotid and coronary artery stenting. Stroke thrombectomy is a similar technique. The thrombectomy procedure is different from coronary angioplasty but it is not more difficult. Actually, I think coronary angioplasty can be more difficult.”
Dr. Widimsky explained that cardiologists need to learn about the brain anatomy and physiology and learn the stroke imaging techniques. “I spent 1 month in the U.S. learning stroke interventions working with simulators,” he said. “I think interventional cardiologists can learn what they need to know in about 6 months. I would recommend they should watch about 50 procedures and perform at least 25 under supervision.”
He said this model is the way forward and hopes it will become routine. Thrombectomy is “tremendously effective” in improving outcomes in severe strokes, with a number needed to treat (NNT) of just 2.6 to prevent long-term disability in one patient, he said, while other procedures can have NNTs of 50 or more.
“But millions of patients with acute severe stroke are not getting this life-changing treatment,” he added. “We must do everything we can to make this service available to as many patients as possible.”
Dr. Widimsky acknowledges that there has been opposition to this idea from the neurointerventionalist professional bodies but this has lessened recently, at least in Europe. And a program that allows interventionalists with experience in extracranial carotid and vertebral endovascular procedures to “fast-track” technical training has now been proposed.
“There is an enormous unmet need for stroke thrombectomy in Europe, with some countries needing to increase the number of procedures done by 10 or 20 times. These include the U.K., Sweden, Italy, Spain, and Portugal. This cannot be done without cardiology,” Dr. Widimsky said.
Editorial strongly supportive
An accompanying editorial strongly endorses the idea of using interdisciplinary teams to deliver high standard stroke care.
Marius Hornung, MD, and Horst Sievert, MD, from CardioVascular Center Frankfurt (Germany), point out that many experienced cardiologists are trained in performing carotid artery interventions and are therefore experienced in accessing the supra-aortic arteries.
“To be able to guarantee optimized stroke therapy as soon as possible, disputes over competence among the individual medical societies involved must be ended,” they wrote.
They advocate for the creation of interdisciplinary teams, with diagnostics, patient selection, and follow-up care remaining the core competencies and tasks of neurology; in addition, they call for appropriately trained and experienced physicians – regardless of their specialties – performing acute stroke interventions and endovascular thrombectomy.
“Such a network must be installed as soon as possible to fulfill the mantra ‘time is brain’ ... and not losing unnecessary time to patient transfer, or continuing to offer only the second-best therapy,” they concluded.
Opposition in the United States
Dr. Widimsky explained that this proposal may not be so applicable to the United States, where the need for more clinicians capable of performing stroke thrombectomies does not appear to be as critical, possibly because vascular neurosurgeons as well as neuroradiologists are qualified to undertake these procedures.
In an interview, J. Mocco, MD, director of the cerebrovascular center, department of neurological surgery, at Mount Sinai Health System, New York, confirmed that this was the case.
“There is no legitimate data to support the claim that there is a lack of an adequate workforce to provide stroke thrombectomy, at least in the U.S.,” he said, adding that, rather, the primary limitation to patient access is a lack of adequate systems of care. “We should learn from the trauma model, which is strongly evidence based, and provide emergency stroke care in a similarly regionalized manner.”
Dr. Mocco, vice president of the Society of NeuroInterventional Surgery, was not impressed with the current study.
“This paper is a retrospective, single-center, unadjudicated, nonindependent assessor case series and therefore, as the authors acknowledge in the limitations section of their paper, it is invalid to compare these data to the results from high-quality, prospective, core-lab, and independent assessor adjudicated randomized trials,” he said. “The supposition that this trial provides evidence that the reported model should be widely considered lacks scientific rigor.”
Furthermore, “the interventional cardiology literature is replete with data regarding the importance of technical expertise and content knowledge,” he added. “Why would that community now propose that such expertise and knowledge is not necessary for the brain?”
Dr. Mocco argues that the concept that interventional cardiologists should be fast-tracked to perform stroke interventions because they use similar tools, navigate blood vessels, and are comfortable working in critical situations, does not hold up.
“Liver surgeons and brain surgeons are both familiar with tissue manipulation, are used to operating in critical situations, and use cautery, scissors, and scalpels; but no one would argue that a brain surgeon should be fast-tracked to perform liver surgery, or vice versa.”
He added: “Stroke patients do not have the luxury of choosing the physician who provides their thrombectomy. We should do everything reasonable to ensure that our systems of care are organized so that these vulnerable patients are treated by physicians who have appropriate knowledge and expertise.”
This study was supported by the Charles University Research program. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiologists experienced in cardiac interventions can competently perform stroke thrombectomy after a short period of training, with outcomes comparable to those achieved by neuroradiology centers, a new study suggests.
“Using interventional cardiologists in this way will help address the huge unmet need for stroke thrombectomy that currently exists,” senior author Petr Widimsky, MD, said in an interview.
Although this may be a feasible way forward in Europe, there is strong opposition to such a proposal from U.S. neurointerventionalists.
The study, published in the April 12 issue of JACC: Cardiovascular Interventions, describes the establishment of a stroke thrombectomy program in University Hospital Kralovske Vinohrady, a large tertiary hospital in Prague, Czech Republic.
The hospital did not have a neurointerventional program until 2012 when a joint program was started involving an experienced team of cardiologists, angiologists, and one interventional radiologist who trained the cardiologists on the thrombectomy procedure.
The current paper reports on the outcomes of the 333 patients with large vessel occlusion stroke treated under this program between October 2012 and December 2019.
The decision to perform catheter-based thrombectomy was made by a neurologist and was based on acute stroke clinical symptoms and CT angiographic findings.
Results show that functional clinical outcomes, assessed via the Modified Rankin Scale (mRS) score at 3 months, did not vary significantly across years 2012 to 2019, with a favorable outcome (mRS 0 to 2) achieved in 47.9% of patients.
Symptomatic intracerebral hemorrhage occurred in 19 patients (5.7%) and embolization in a new vascular territory occurred in 6 patients (1.8%), outcomes similar to those of neuroradiology centers.
The desired clinical results were achieved from the onset of the program, without any signs of a learning curve effect, they reported.
“These findings support the potential role of interventional cardiac cath labs in the treatment of acute stroke in regions where this therapy is not readily available due to the lack of neurointerventionalists,” the authors concluded.
“Our main message is that our results were excellent from the beginning,” Dr. Widimsky said. “When centers prepare properly, they can achieve excellent results from the beginning with cardiologists who are experienced in interventional procedures and who have spent sufficient time learning about the brain.”
The authors noted that despite thrombectomy being an extremely beneficial treatment for severe stroke, many eligible patients remain untreated, largely because of a lack of neurointerventionalists in many regions worldwide. They estimate that about 15% of all stroke patients are eligible for thrombectomy but only around 2% of stroke patients in Europe actually receive such treatment.
Dr. Widimsky, an interventional cardiologist, first thought of the idea of using cardiologists to perform stroke thrombectomies after a good friend and colleague suffered a severe stroke in 2010.
“This made us realize that our hospital needed to be more active in the stroke field,” he said. “We decided that we needed to start doing stroke interventions.”
But the major problem was the lack of neurointerventionalists.
“There are not enough neurointerventionalists in Europe. Interventional cardiologists can perform thousands of procedures every year whereas a neurointerventionalist will at best perform hundreds a year. It is quicker and simpler to train the cardiologist to do it,” Dr. Widimsky said.
They hired one neurointerventionalist to lead the program. “He was our tutor, he taught us his skills,” Dr. Widimsky said. “The cath lab is open 24/7, but if we only have one neurointerventionalist we cannot offer a 24/7 service for stroke thrombectomy. But if we merge with cardiology then we can,” he added.
Their hospital is a very busy center for myocardial infarction, percutaneous coronary intervention, and carotid stenting, he noted. “It is not difficult to make the step from that to stroke thrombectomy. Interventional cardiologists are used to performing carotid and coronary artery stenting. Stroke thrombectomy is a similar technique. The thrombectomy procedure is different from coronary angioplasty but it is not more difficult. Actually, I think coronary angioplasty can be more difficult.”
Dr. Widimsky explained that cardiologists need to learn about the brain anatomy and physiology and learn the stroke imaging techniques. “I spent 1 month in the U.S. learning stroke interventions working with simulators,” he said. “I think interventional cardiologists can learn what they need to know in about 6 months. I would recommend they should watch about 50 procedures and perform at least 25 under supervision.”
He said this model is the way forward and hopes it will become routine. Thrombectomy is “tremendously effective” in improving outcomes in severe strokes, with a number needed to treat (NNT) of just 2.6 to prevent long-term disability in one patient, he said, while other procedures can have NNTs of 50 or more.
“But millions of patients with acute severe stroke are not getting this life-changing treatment,” he added. “We must do everything we can to make this service available to as many patients as possible.”
Dr. Widimsky acknowledges that there has been opposition to this idea from the neurointerventionalist professional bodies but this has lessened recently, at least in Europe. And a program that allows interventionalists with experience in extracranial carotid and vertebral endovascular procedures to “fast-track” technical training has now been proposed.
“There is an enormous unmet need for stroke thrombectomy in Europe, with some countries needing to increase the number of procedures done by 10 or 20 times. These include the U.K., Sweden, Italy, Spain, and Portugal. This cannot be done without cardiology,” Dr. Widimsky said.
Editorial strongly supportive
An accompanying editorial strongly endorses the idea of using interdisciplinary teams to deliver high standard stroke care.
Marius Hornung, MD, and Horst Sievert, MD, from CardioVascular Center Frankfurt (Germany), point out that many experienced cardiologists are trained in performing carotid artery interventions and are therefore experienced in accessing the supra-aortic arteries.
“To be able to guarantee optimized stroke therapy as soon as possible, disputes over competence among the individual medical societies involved must be ended,” they wrote.
They advocate for the creation of interdisciplinary teams, with diagnostics, patient selection, and follow-up care remaining the core competencies and tasks of neurology; in addition, they call for appropriately trained and experienced physicians – regardless of their specialties – performing acute stroke interventions and endovascular thrombectomy.
“Such a network must be installed as soon as possible to fulfill the mantra ‘time is brain’ ... and not losing unnecessary time to patient transfer, or continuing to offer only the second-best therapy,” they concluded.
Opposition in the United States
Dr. Widimsky explained that this proposal may not be so applicable to the United States, where the need for more clinicians capable of performing stroke thrombectomies does not appear to be as critical, possibly because vascular neurosurgeons as well as neuroradiologists are qualified to undertake these procedures.
In an interview, J. Mocco, MD, director of the cerebrovascular center, department of neurological surgery, at Mount Sinai Health System, New York, confirmed that this was the case.
“There is no legitimate data to support the claim that there is a lack of an adequate workforce to provide stroke thrombectomy, at least in the U.S.,” he said, adding that, rather, the primary limitation to patient access is a lack of adequate systems of care. “We should learn from the trauma model, which is strongly evidence based, and provide emergency stroke care in a similarly regionalized manner.”
Dr. Mocco, vice president of the Society of NeuroInterventional Surgery, was not impressed with the current study.
“This paper is a retrospective, single-center, unadjudicated, nonindependent assessor case series and therefore, as the authors acknowledge in the limitations section of their paper, it is invalid to compare these data to the results from high-quality, prospective, core-lab, and independent assessor adjudicated randomized trials,” he said. “The supposition that this trial provides evidence that the reported model should be widely considered lacks scientific rigor.”
Furthermore, “the interventional cardiology literature is replete with data regarding the importance of technical expertise and content knowledge,” he added. “Why would that community now propose that such expertise and knowledge is not necessary for the brain?”
Dr. Mocco argues that the concept that interventional cardiologists should be fast-tracked to perform stroke interventions because they use similar tools, navigate blood vessels, and are comfortable working in critical situations, does not hold up.
“Liver surgeons and brain surgeons are both familiar with tissue manipulation, are used to operating in critical situations, and use cautery, scissors, and scalpels; but no one would argue that a brain surgeon should be fast-tracked to perform liver surgery, or vice versa.”
He added: “Stroke patients do not have the luxury of choosing the physician who provides their thrombectomy. We should do everything reasonable to ensure that our systems of care are organized so that these vulnerable patients are treated by physicians who have appropriate knowledge and expertise.”
This study was supported by the Charles University Research program. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiologists experienced in cardiac interventions can competently perform stroke thrombectomy after a short period of training, with outcomes comparable to those achieved by neuroradiology centers, a new study suggests.
“Using interventional cardiologists in this way will help address the huge unmet need for stroke thrombectomy that currently exists,” senior author Petr Widimsky, MD, said in an interview.
Although this may be a feasible way forward in Europe, there is strong opposition to such a proposal from U.S. neurointerventionalists.
The study, published in the April 12 issue of JACC: Cardiovascular Interventions, describes the establishment of a stroke thrombectomy program in University Hospital Kralovske Vinohrady, a large tertiary hospital in Prague, Czech Republic.
The hospital did not have a neurointerventional program until 2012 when a joint program was started involving an experienced team of cardiologists, angiologists, and one interventional radiologist who trained the cardiologists on the thrombectomy procedure.
The current paper reports on the outcomes of the 333 patients with large vessel occlusion stroke treated under this program between October 2012 and December 2019.
The decision to perform catheter-based thrombectomy was made by a neurologist and was based on acute stroke clinical symptoms and CT angiographic findings.
Results show that functional clinical outcomes, assessed via the Modified Rankin Scale (mRS) score at 3 months, did not vary significantly across years 2012 to 2019, with a favorable outcome (mRS 0 to 2) achieved in 47.9% of patients.
Symptomatic intracerebral hemorrhage occurred in 19 patients (5.7%) and embolization in a new vascular territory occurred in 6 patients (1.8%), outcomes similar to those of neuroradiology centers.
The desired clinical results were achieved from the onset of the program, without any signs of a learning curve effect, they reported.
“These findings support the potential role of interventional cardiac cath labs in the treatment of acute stroke in regions where this therapy is not readily available due to the lack of neurointerventionalists,” the authors concluded.
“Our main message is that our results were excellent from the beginning,” Dr. Widimsky said. “When centers prepare properly, they can achieve excellent results from the beginning with cardiologists who are experienced in interventional procedures and who have spent sufficient time learning about the brain.”
The authors noted that despite thrombectomy being an extremely beneficial treatment for severe stroke, many eligible patients remain untreated, largely because of a lack of neurointerventionalists in many regions worldwide. They estimate that about 15% of all stroke patients are eligible for thrombectomy but only around 2% of stroke patients in Europe actually receive such treatment.
Dr. Widimsky, an interventional cardiologist, first thought of the idea of using cardiologists to perform stroke thrombectomies after a good friend and colleague suffered a severe stroke in 2010.
“This made us realize that our hospital needed to be more active in the stroke field,” he said. “We decided that we needed to start doing stroke interventions.”
But the major problem was the lack of neurointerventionalists.
“There are not enough neurointerventionalists in Europe. Interventional cardiologists can perform thousands of procedures every year whereas a neurointerventionalist will at best perform hundreds a year. It is quicker and simpler to train the cardiologist to do it,” Dr. Widimsky said.
They hired one neurointerventionalist to lead the program. “He was our tutor, he taught us his skills,” Dr. Widimsky said. “The cath lab is open 24/7, but if we only have one neurointerventionalist we cannot offer a 24/7 service for stroke thrombectomy. But if we merge with cardiology then we can,” he added.
Their hospital is a very busy center for myocardial infarction, percutaneous coronary intervention, and carotid stenting, he noted. “It is not difficult to make the step from that to stroke thrombectomy. Interventional cardiologists are used to performing carotid and coronary artery stenting. Stroke thrombectomy is a similar technique. The thrombectomy procedure is different from coronary angioplasty but it is not more difficult. Actually, I think coronary angioplasty can be more difficult.”
Dr. Widimsky explained that cardiologists need to learn about the brain anatomy and physiology and learn the stroke imaging techniques. “I spent 1 month in the U.S. learning stroke interventions working with simulators,” he said. “I think interventional cardiologists can learn what they need to know in about 6 months. I would recommend they should watch about 50 procedures and perform at least 25 under supervision.”
He said this model is the way forward and hopes it will become routine. Thrombectomy is “tremendously effective” in improving outcomes in severe strokes, with a number needed to treat (NNT) of just 2.6 to prevent long-term disability in one patient, he said, while other procedures can have NNTs of 50 or more.
“But millions of patients with acute severe stroke are not getting this life-changing treatment,” he added. “We must do everything we can to make this service available to as many patients as possible.”
Dr. Widimsky acknowledges that there has been opposition to this idea from the neurointerventionalist professional bodies but this has lessened recently, at least in Europe. And a program that allows interventionalists with experience in extracranial carotid and vertebral endovascular procedures to “fast-track” technical training has now been proposed.
“There is an enormous unmet need for stroke thrombectomy in Europe, with some countries needing to increase the number of procedures done by 10 or 20 times. These include the U.K., Sweden, Italy, Spain, and Portugal. This cannot be done without cardiology,” Dr. Widimsky said.
Editorial strongly supportive
An accompanying editorial strongly endorses the idea of using interdisciplinary teams to deliver high standard stroke care.
Marius Hornung, MD, and Horst Sievert, MD, from CardioVascular Center Frankfurt (Germany), point out that many experienced cardiologists are trained in performing carotid artery interventions and are therefore experienced in accessing the supra-aortic arteries.
“To be able to guarantee optimized stroke therapy as soon as possible, disputes over competence among the individual medical societies involved must be ended,” they wrote.
They advocate for the creation of interdisciplinary teams, with diagnostics, patient selection, and follow-up care remaining the core competencies and tasks of neurology; in addition, they call for appropriately trained and experienced physicians – regardless of their specialties – performing acute stroke interventions and endovascular thrombectomy.
“Such a network must be installed as soon as possible to fulfill the mantra ‘time is brain’ ... and not losing unnecessary time to patient transfer, or continuing to offer only the second-best therapy,” they concluded.
Opposition in the United States
Dr. Widimsky explained that this proposal may not be so applicable to the United States, where the need for more clinicians capable of performing stroke thrombectomies does not appear to be as critical, possibly because vascular neurosurgeons as well as neuroradiologists are qualified to undertake these procedures.
In an interview, J. Mocco, MD, director of the cerebrovascular center, department of neurological surgery, at Mount Sinai Health System, New York, confirmed that this was the case.
“There is no legitimate data to support the claim that there is a lack of an adequate workforce to provide stroke thrombectomy, at least in the U.S.,” he said, adding that, rather, the primary limitation to patient access is a lack of adequate systems of care. “We should learn from the trauma model, which is strongly evidence based, and provide emergency stroke care in a similarly regionalized manner.”
Dr. Mocco, vice president of the Society of NeuroInterventional Surgery, was not impressed with the current study.
“This paper is a retrospective, single-center, unadjudicated, nonindependent assessor case series and therefore, as the authors acknowledge in the limitations section of their paper, it is invalid to compare these data to the results from high-quality, prospective, core-lab, and independent assessor adjudicated randomized trials,” he said. “The supposition that this trial provides evidence that the reported model should be widely considered lacks scientific rigor.”
Furthermore, “the interventional cardiology literature is replete with data regarding the importance of technical expertise and content knowledge,” he added. “Why would that community now propose that such expertise and knowledge is not necessary for the brain?”
Dr. Mocco argues that the concept that interventional cardiologists should be fast-tracked to perform stroke interventions because they use similar tools, navigate blood vessels, and are comfortable working in critical situations, does not hold up.
“Liver surgeons and brain surgeons are both familiar with tissue manipulation, are used to operating in critical situations, and use cautery, scissors, and scalpels; but no one would argue that a brain surgeon should be fast-tracked to perform liver surgery, or vice versa.”
He added: “Stroke patients do not have the luxury of choosing the physician who provides their thrombectomy. We should do everything reasonable to ensure that our systems of care are organized so that these vulnerable patients are treated by physicians who have appropriate knowledge and expertise.”
This study was supported by the Charles University Research program. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ADVANCES IN NEUROLOGY
New Supplement to Federal Practitioner: Advances in Neurology
Read more about:
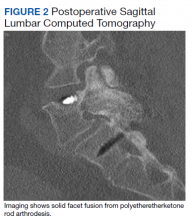
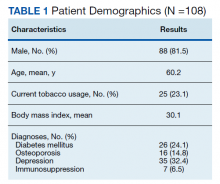
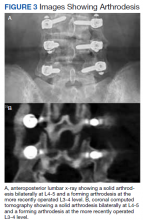
- Lumbar Fusion With PEEK Rods Use for Patients With Degenerative Disease
- Systemic Literature Review of the Use of Virtual Reality for Rehabilitation in Parkinson Disease
- COVID-19 Vaccine in Veterans With Multiple Sclerosis: Protect the Vulnerable
Click here to read the supplement or click on the image
New Supplement to Federal Practitioner: Advances in Neurology
Read more about:
- Lumbar Fusion With PEEK Rods Use for Patients With Degenerative Disease
- Systemic Literature Review of the Use of Virtual Reality for Rehabilitation in Parkinson Disease
- COVID-19 Vaccine in Veterans With Multiple Sclerosis: Protect the Vulnerable
Click here to read the supplement or click on the image
New Supplement to Federal Practitioner: Advances in Neurology
Read more about:
- Lumbar Fusion With PEEK Rods Use for Patients With Degenerative Disease
- Systemic Literature Review of the Use of Virtual Reality for Rehabilitation in Parkinson Disease
- COVID-19 Vaccine in Veterans With Multiple Sclerosis: Protect the Vulnerable
Click here to read the supplement or click on the image
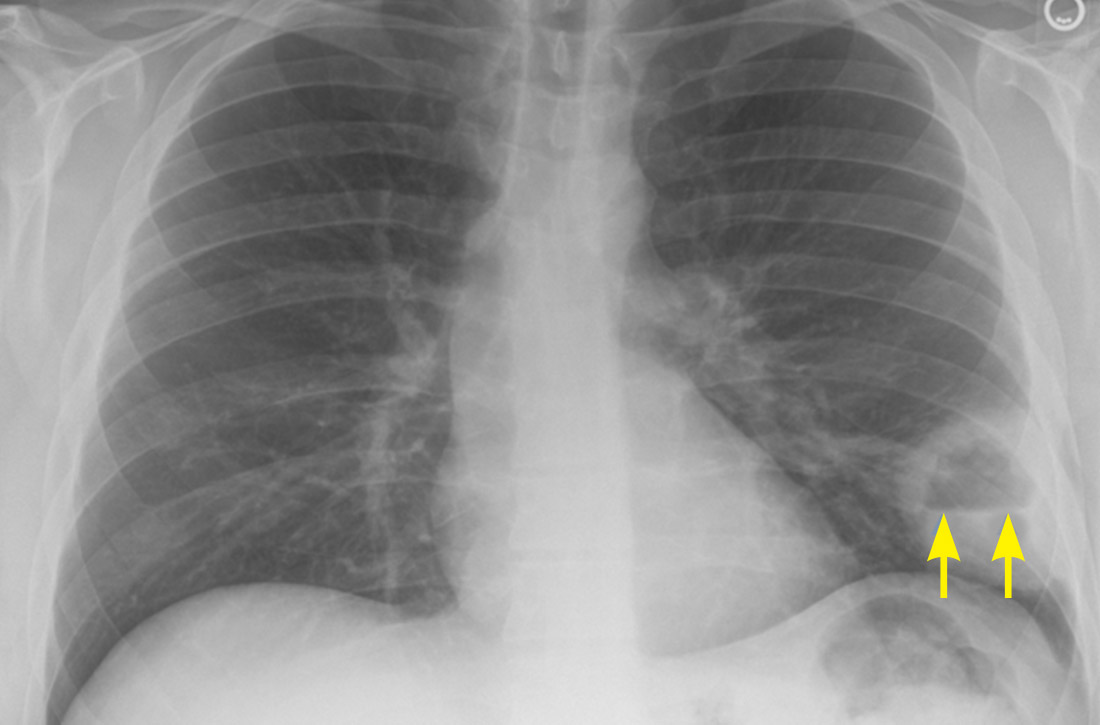
How some COVID-19 vaccines could cause rare blood clots
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
Let’s avoid accepting this headache paradigm as gospel
Dr. Crain’s excellent review, “Breaking the cycle of medication overuse headache” (J Fam Pract. 2021;70:20-28) provides an approach to the diagnosis and treatment of this common disorder that is consistent with most expert opinion and published guidelines. However, like most articles on this subject, it is missing a critical review of the evidence that supports the existence of this condition and the recommended treatments.
The strong association between intractable headaches and quantity of medication used makes the diagnosis of medication overuse headache (MOH) attractive with plausible (if unproven) pathophysiological mechanisms. However, reversing the direction of causation (intractable headaches lead to more medication) seems just as likely. While MOH is taken as an article of faith by most headache experts, high-quality studies in support of this theory have not yet been performed.1
On the other hand, fear of MOH often leads to rigid, arbitrary limitations of abortive medications, blaming of the patient for their symptoms, and the substitution of a host of pharmacologic and nonpharmacologic interventions that similarly lack evidence of efficacy. Patients with chronic migraine are told to take abortive medications early in the headache but not to take them more than twice per week. They hoard their medications while trying to decide if each daily headache is the “big one” that merits depleting their limited supply of medication.
Avoiding medication “overuse” and prescribing from our growing armamentarium of effective preventive medications remain important strategies. However, until we have better evidence, we need to be a little more flexible in prescribing abortive medications and avoid accepting the MOH paradigm as gospel.
David A. Silverstein, MD
Buffalo, NY
1. Vandenbussche N, Laterza D, Lisicki M, et al. Medication-overuse headache: a widely recognized entity amidst ongoing debate. J Headache Pain. 2018;19:50. https://doi.org/10.1186/s10194-018-0875-x
Dr. Crain’s excellent review, “Breaking the cycle of medication overuse headache” (J Fam Pract. 2021;70:20-28) provides an approach to the diagnosis and treatment of this common disorder that is consistent with most expert opinion and published guidelines. However, like most articles on this subject, it is missing a critical review of the evidence that supports the existence of this condition and the recommended treatments.
The strong association between intractable headaches and quantity of medication used makes the diagnosis of medication overuse headache (MOH) attractive with plausible (if unproven) pathophysiological mechanisms. However, reversing the direction of causation (intractable headaches lead to more medication) seems just as likely. While MOH is taken as an article of faith by most headache experts, high-quality studies in support of this theory have not yet been performed.1
On the other hand, fear of MOH often leads to rigid, arbitrary limitations of abortive medications, blaming of the patient for their symptoms, and the substitution of a host of pharmacologic and nonpharmacologic interventions that similarly lack evidence of efficacy. Patients with chronic migraine are told to take abortive medications early in the headache but not to take them more than twice per week. They hoard their medications while trying to decide if each daily headache is the “big one” that merits depleting their limited supply of medication.
Avoiding medication “overuse” and prescribing from our growing armamentarium of effective preventive medications remain important strategies. However, until we have better evidence, we need to be a little more flexible in prescribing abortive medications and avoid accepting the MOH paradigm as gospel.
David A. Silverstein, MD
Buffalo, NY
Dr. Crain’s excellent review, “Breaking the cycle of medication overuse headache” (J Fam Pract. 2021;70:20-28) provides an approach to the diagnosis and treatment of this common disorder that is consistent with most expert opinion and published guidelines. However, like most articles on this subject, it is missing a critical review of the evidence that supports the existence of this condition and the recommended treatments.
The strong association between intractable headaches and quantity of medication used makes the diagnosis of medication overuse headache (MOH) attractive with plausible (if unproven) pathophysiological mechanisms. However, reversing the direction of causation (intractable headaches lead to more medication) seems just as likely. While MOH is taken as an article of faith by most headache experts, high-quality studies in support of this theory have not yet been performed.1
On the other hand, fear of MOH often leads to rigid, arbitrary limitations of abortive medications, blaming of the patient for their symptoms, and the substitution of a host of pharmacologic and nonpharmacologic interventions that similarly lack evidence of efficacy. Patients with chronic migraine are told to take abortive medications early in the headache but not to take them more than twice per week. They hoard their medications while trying to decide if each daily headache is the “big one” that merits depleting their limited supply of medication.
Avoiding medication “overuse” and prescribing from our growing armamentarium of effective preventive medications remain important strategies. However, until we have better evidence, we need to be a little more flexible in prescribing abortive medications and avoid accepting the MOH paradigm as gospel.
David A. Silverstein, MD
Buffalo, NY
1. Vandenbussche N, Laterza D, Lisicki M, et al. Medication-overuse headache: a widely recognized entity amidst ongoing debate. J Headache Pain. 2018;19:50. https://doi.org/10.1186/s10194-018-0875-x
1. Vandenbussche N, Laterza D, Lisicki M, et al. Medication-overuse headache: a widely recognized entity amidst ongoing debate. J Headache Pain. 2018;19:50. https://doi.org/10.1186/s10194-018-0875-x
23-year-old woman • syncopal episode • sinus bradycardia • history of bipolar disorder • Dx?
THE CASE
A 23-year-old woman with past medical history of bipolar II disorder and a REM-specific seizure disorder that resolved at age 9 presented after a syncopal episode. The patient reported an initial sensation of lightheadedness while at work, which was followed by a syncopal episode with brief (1-2 min) loss of consciousness and a minor head injury.
She denied other prodromal symptoms including chest pain, shortness of breath, palpitations, and nausea. She also did not experience convulsions, urinary/bowel incontinence, or confusion upon regaining consciousness.
She denied previous syncopal episodes. However, she reported that, 2 weeks prior, there had been an event similar to that of her presenting complaint. During that episode, she experienced lightheadedness and a fall without loss of consciousness.
The patient had been prescribed a regimen of sertraline 100 mg/d and aripiprazole 10 mg/d to maintain mood stability. She had self-discontinued these medications about 8 months prior to presentation. A recent return of her depressive features had prompted a restart of this regimen 1 week before her first fall, without an initial taper upward.
While in the emergency department, she became bradycardic (heart rate, 38 beats/min) and hypotensive (blood pressure, 70/40 mm Hg). She subsequently became increasingly somnolent and had 1 episode of emesis. An electrocardiogram (EKG) revealed sinus bradycardia without other acute abnormalities (FIGURE).
Blood work including a basic metabolic panel, complete blood count, and cardiac enzymes were all within normal limits. Computed tomography of the head revealed no intracranial pathology. Her vitals were initially unresponsive to a fluid bolus but improved and stabilized after administration of intravenous atropine 0.5 mg.
Aripiprazole was held and sertraline was decreased to 75 mg on hospital Day 1, with close monitoring of her mood. Cardiology was consulted and followed the patient during her stay. The patient was monitored on telemetry for 3 days, exhibiting only sinus bradycardia with a stable heart rate of 45-55 beats/min. Systolic blood pressures were stable within 120 to 130 mm Hg. Transthoracic echocardiogram performed on hospital Day 2 was unremarkable, revealing a normal left ventricular ejection fraction of 65% and no wall motion abnormalities. She had no recurrence of the syncope or emesis.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
Given her benign cardiac work-up and symptom onset coinciding with the abrupt resumption of high doses of aripiprazole after an 8-month abstinence, the patient’s presentation was attributed to a rather uncommon adverse drug reaction to aripiprazole. This has only been described in a few case reports.
DISCUSSION
Aripiprazole (Abilify) is an atypical antipsychotic frequently used in the treatment of psychiatric conditions, including bipolar disorder and schizophrenia. While the specific therapeutic mechanism is unknown, it is believed that drug efficacy is related to partial agonism at dopamine D2, serotonin 5-HT1A, and serotonin 5-HT2A.1 As aripiprazole works on a variety of receptors involved in other physiologic processes, clinical adverse effects have been reported, most of which are associated with the adrenergic alpha1 receptors.1 These include cognitive impairment and seizures. Cardiovascular adverse effects of aripiprazole include orthostatic hypotension, cardiac arrhythmia, prolonged QT interval, and syncope.1-5
Selective serotonin reuptake inhibitors (SSRIs) such as sertraline (Zoloft) have also been shown to cause cardiac arrhythmia and syncope.6 Although sertraline may have contributed to the patient’s cardiac symptoms, it is more likely that the aripiprazole was the direct cause, as she remained asymptomatic while on a therapeutic dose of sertraline. Furthermore, aripiprazole is primarily metabolized though hepatic CYP2D6, which sertraline has been shown to inhibit.1,7 Therefore, the concomitant use of sertraline with no initial taper of either medication likely led to an increased effective dose of aripiprazole in our patient and subsequently to her presentation.
Few prior cases have identified aripiprazole as a cause of antipsychotic-associated bradycardic response.8 Based on the Adverse Drug Reaction Probability Scale, often referred to as the Naranjo Scale, we believe this to be a probable adverse response in our patient.9 Bradycardia followed a reasonable temporal sequence after aripiprazole use with a response previously described in the literature. Symptoms also improved after discontinuation of the drug and other etiologies of the bradycardia were ruled out.
Our patient was discharged with a 30-day cardiac event monitor and a scheduled appointment with Cardiology.
Continue to: THE TAKEAWAY
THE TAKEAWAY
As this case suggests, there may be an association between aripiprazole and symptomatic bradycardia. Therefore, family physicians should inquire about aripiprazole use in patients who present with cardiac symptoms and consider tapering this medication if other causes cannot be identified. Additionally, given the potential cardiac adverse effects of atypical antipsychotics, physicians may consider ordering baseline and follow-up EKGs to monitor for arrhythmias in patients prescribed aripiprazole. This may be especially prudent when an atypical antipsychotic is combined with an SSRI, as potential cardiac adverse effects may occur more frequently.
CORRESPONDENCE
Kyle Fletke, MD, Department of Family and Community Medicine, University of Maryland School of Medicine, 29 South Paca Street, Baltimore, MD 21201; [email protected]
1. Abilify [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2014.
2. Belemonte C, Ochoa D, Román M, et al. Evaluation of the relationship between pharmacokinetics and the safety of aripiprazole and its cardiovascular side effects in health volunteers. J Clin Psychopharmacol. 2016;36:608-614.
3. Torgovnic J, Sethi NK, Arsura E. Aripiprazole-induced orthostatic hypotension and cardiac arrhythmia. Psychiatry Clin Neurosci. 2008:62:485.
4. Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des. 2004;10:2463-2475.
5. Russo L, Rizzo A, Di Vincenzo A, et al. Aripiprazole overdose and transient 2:1 second degree atrioventricular block: only a coincidence? Curr Drug Saf. 2019;14:155-157.
6. Pacher P, Ungvari Z, Kecskemeti V, et al. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Curr Med Chem. 1998;5:381-90.
7. Hemeryck A, Belpaire FM. Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug-drug interactions: an update. Curr Drub Metab. 2002;3:13-37.
8. Snarr BS, Phan SV, Garner A, et al. Symptomatic bradycardia with oral aripiprazole and oral ziprasidone. Ann Pharmacother. 2010;44:760-763.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
THE CASE
A 23-year-old woman with past medical history of bipolar II disorder and a REM-specific seizure disorder that resolved at age 9 presented after a syncopal episode. The patient reported an initial sensation of lightheadedness while at work, which was followed by a syncopal episode with brief (1-2 min) loss of consciousness and a minor head injury.
She denied other prodromal symptoms including chest pain, shortness of breath, palpitations, and nausea. She also did not experience convulsions, urinary/bowel incontinence, or confusion upon regaining consciousness.
She denied previous syncopal episodes. However, she reported that, 2 weeks prior, there had been an event similar to that of her presenting complaint. During that episode, she experienced lightheadedness and a fall without loss of consciousness.
The patient had been prescribed a regimen of sertraline 100 mg/d and aripiprazole 10 mg/d to maintain mood stability. She had self-discontinued these medications about 8 months prior to presentation. A recent return of her depressive features had prompted a restart of this regimen 1 week before her first fall, without an initial taper upward.
While in the emergency department, she became bradycardic (heart rate, 38 beats/min) and hypotensive (blood pressure, 70/40 mm Hg). She subsequently became increasingly somnolent and had 1 episode of emesis. An electrocardiogram (EKG) revealed sinus bradycardia without other acute abnormalities (FIGURE).

Blood work including a basic metabolic panel, complete blood count, and cardiac enzymes were all within normal limits. Computed tomography of the head revealed no intracranial pathology. Her vitals were initially unresponsive to a fluid bolus but improved and stabilized after administration of intravenous atropine 0.5 mg.
Aripiprazole was held and sertraline was decreased to 75 mg on hospital Day 1, with close monitoring of her mood. Cardiology was consulted and followed the patient during her stay. The patient was monitored on telemetry for 3 days, exhibiting only sinus bradycardia with a stable heart rate of 45-55 beats/min. Systolic blood pressures were stable within 120 to 130 mm Hg. Transthoracic echocardiogram performed on hospital Day 2 was unremarkable, revealing a normal left ventricular ejection fraction of 65% and no wall motion abnormalities. She had no recurrence of the syncope or emesis.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
Given her benign cardiac work-up and symptom onset coinciding with the abrupt resumption of high doses of aripiprazole after an 8-month abstinence, the patient’s presentation was attributed to a rather uncommon adverse drug reaction to aripiprazole. This has only been described in a few case reports.
DISCUSSION
Aripiprazole (Abilify) is an atypical antipsychotic frequently used in the treatment of psychiatric conditions, including bipolar disorder and schizophrenia. While the specific therapeutic mechanism is unknown, it is believed that drug efficacy is related to partial agonism at dopamine D2, serotonin 5-HT1A, and serotonin 5-HT2A.1 As aripiprazole works on a variety of receptors involved in other physiologic processes, clinical adverse effects have been reported, most of which are associated with the adrenergic alpha1 receptors.1 These include cognitive impairment and seizures. Cardiovascular adverse effects of aripiprazole include orthostatic hypotension, cardiac arrhythmia, prolonged QT interval, and syncope.1-5
Selective serotonin reuptake inhibitors (SSRIs) such as sertraline (Zoloft) have also been shown to cause cardiac arrhythmia and syncope.6 Although sertraline may have contributed to the patient’s cardiac symptoms, it is more likely that the aripiprazole was the direct cause, as she remained asymptomatic while on a therapeutic dose of sertraline. Furthermore, aripiprazole is primarily metabolized though hepatic CYP2D6, which sertraline has been shown to inhibit.1,7 Therefore, the concomitant use of sertraline with no initial taper of either medication likely led to an increased effective dose of aripiprazole in our patient and subsequently to her presentation.
Few prior cases have identified aripiprazole as a cause of antipsychotic-associated bradycardic response.8 Based on the Adverse Drug Reaction Probability Scale, often referred to as the Naranjo Scale, we believe this to be a probable adverse response in our patient.9 Bradycardia followed a reasonable temporal sequence after aripiprazole use with a response previously described in the literature. Symptoms also improved after discontinuation of the drug and other etiologies of the bradycardia were ruled out.
Our patient was discharged with a 30-day cardiac event monitor and a scheduled appointment with Cardiology.
Continue to: THE TAKEAWAY
THE TAKEAWAY
As this case suggests, there may be an association between aripiprazole and symptomatic bradycardia. Therefore, family physicians should inquire about aripiprazole use in patients who present with cardiac symptoms and consider tapering this medication if other causes cannot be identified. Additionally, given the potential cardiac adverse effects of atypical antipsychotics, physicians may consider ordering baseline and follow-up EKGs to monitor for arrhythmias in patients prescribed aripiprazole. This may be especially prudent when an atypical antipsychotic is combined with an SSRI, as potential cardiac adverse effects may occur more frequently.
CORRESPONDENCE
Kyle Fletke, MD, Department of Family and Community Medicine, University of Maryland School of Medicine, 29 South Paca Street, Baltimore, MD 21201; [email protected]
THE CASE
A 23-year-old woman with past medical history of bipolar II disorder and a REM-specific seizure disorder that resolved at age 9 presented after a syncopal episode. The patient reported an initial sensation of lightheadedness while at work, which was followed by a syncopal episode with brief (1-2 min) loss of consciousness and a minor head injury.
She denied other prodromal symptoms including chest pain, shortness of breath, palpitations, and nausea. She also did not experience convulsions, urinary/bowel incontinence, or confusion upon regaining consciousness.
She denied previous syncopal episodes. However, she reported that, 2 weeks prior, there had been an event similar to that of her presenting complaint. During that episode, she experienced lightheadedness and a fall without loss of consciousness.
The patient had been prescribed a regimen of sertraline 100 mg/d and aripiprazole 10 mg/d to maintain mood stability. She had self-discontinued these medications about 8 months prior to presentation. A recent return of her depressive features had prompted a restart of this regimen 1 week before her first fall, without an initial taper upward.
While in the emergency department, she became bradycardic (heart rate, 38 beats/min) and hypotensive (blood pressure, 70/40 mm Hg). She subsequently became increasingly somnolent and had 1 episode of emesis. An electrocardiogram (EKG) revealed sinus bradycardia without other acute abnormalities (FIGURE).

Blood work including a basic metabolic panel, complete blood count, and cardiac enzymes were all within normal limits. Computed tomography of the head revealed no intracranial pathology. Her vitals were initially unresponsive to a fluid bolus but improved and stabilized after administration of intravenous atropine 0.5 mg.
Aripiprazole was held and sertraline was decreased to 75 mg on hospital Day 1, with close monitoring of her mood. Cardiology was consulted and followed the patient during her stay. The patient was monitored on telemetry for 3 days, exhibiting only sinus bradycardia with a stable heart rate of 45-55 beats/min. Systolic blood pressures were stable within 120 to 130 mm Hg. Transthoracic echocardiogram performed on hospital Day 2 was unremarkable, revealing a normal left ventricular ejection fraction of 65% and no wall motion abnormalities. She had no recurrence of the syncope or emesis.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
Given her benign cardiac work-up and symptom onset coinciding with the abrupt resumption of high doses of aripiprazole after an 8-month abstinence, the patient’s presentation was attributed to a rather uncommon adverse drug reaction to aripiprazole. This has only been described in a few case reports.
DISCUSSION
Aripiprazole (Abilify) is an atypical antipsychotic frequently used in the treatment of psychiatric conditions, including bipolar disorder and schizophrenia. While the specific therapeutic mechanism is unknown, it is believed that drug efficacy is related to partial agonism at dopamine D2, serotonin 5-HT1A, and serotonin 5-HT2A.1 As aripiprazole works on a variety of receptors involved in other physiologic processes, clinical adverse effects have been reported, most of which are associated with the adrenergic alpha1 receptors.1 These include cognitive impairment and seizures. Cardiovascular adverse effects of aripiprazole include orthostatic hypotension, cardiac arrhythmia, prolonged QT interval, and syncope.1-5
Selective serotonin reuptake inhibitors (SSRIs) such as sertraline (Zoloft) have also been shown to cause cardiac arrhythmia and syncope.6 Although sertraline may have contributed to the patient’s cardiac symptoms, it is more likely that the aripiprazole was the direct cause, as she remained asymptomatic while on a therapeutic dose of sertraline. Furthermore, aripiprazole is primarily metabolized though hepatic CYP2D6, which sertraline has been shown to inhibit.1,7 Therefore, the concomitant use of sertraline with no initial taper of either medication likely led to an increased effective dose of aripiprazole in our patient and subsequently to her presentation.
Few prior cases have identified aripiprazole as a cause of antipsychotic-associated bradycardic response.8 Based on the Adverse Drug Reaction Probability Scale, often referred to as the Naranjo Scale, we believe this to be a probable adverse response in our patient.9 Bradycardia followed a reasonable temporal sequence after aripiprazole use with a response previously described in the literature. Symptoms also improved after discontinuation of the drug and other etiologies of the bradycardia were ruled out.
Our patient was discharged with a 30-day cardiac event monitor and a scheduled appointment with Cardiology.
Continue to: THE TAKEAWAY
THE TAKEAWAY
As this case suggests, there may be an association between aripiprazole and symptomatic bradycardia. Therefore, family physicians should inquire about aripiprazole use in patients who present with cardiac symptoms and consider tapering this medication if other causes cannot be identified. Additionally, given the potential cardiac adverse effects of atypical antipsychotics, physicians may consider ordering baseline and follow-up EKGs to monitor for arrhythmias in patients prescribed aripiprazole. This may be especially prudent when an atypical antipsychotic is combined with an SSRI, as potential cardiac adverse effects may occur more frequently.
CORRESPONDENCE
Kyle Fletke, MD, Department of Family and Community Medicine, University of Maryland School of Medicine, 29 South Paca Street, Baltimore, MD 21201; [email protected]
1. Abilify [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2014.
2. Belemonte C, Ochoa D, Román M, et al. Evaluation of the relationship between pharmacokinetics and the safety of aripiprazole and its cardiovascular side effects in health volunteers. J Clin Psychopharmacol. 2016;36:608-614.
3. Torgovnic J, Sethi NK, Arsura E. Aripiprazole-induced orthostatic hypotension and cardiac arrhythmia. Psychiatry Clin Neurosci. 2008:62:485.
4. Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des. 2004;10:2463-2475.
5. Russo L, Rizzo A, Di Vincenzo A, et al. Aripiprazole overdose and transient 2:1 second degree atrioventricular block: only a coincidence? Curr Drug Saf. 2019;14:155-157.
6. Pacher P, Ungvari Z, Kecskemeti V, et al. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Curr Med Chem. 1998;5:381-90.
7. Hemeryck A, Belpaire FM. Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug-drug interactions: an update. Curr Drub Metab. 2002;3:13-37.
8. Snarr BS, Phan SV, Garner A, et al. Symptomatic bradycardia with oral aripiprazole and oral ziprasidone. Ann Pharmacother. 2010;44:760-763.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
1. Abilify [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2014.
2. Belemonte C, Ochoa D, Román M, et al. Evaluation of the relationship between pharmacokinetics and the safety of aripiprazole and its cardiovascular side effects in health volunteers. J Clin Psychopharmacol. 2016;36:608-614.
3. Torgovnic J, Sethi NK, Arsura E. Aripiprazole-induced orthostatic hypotension and cardiac arrhythmia. Psychiatry Clin Neurosci. 2008:62:485.
4. Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des. 2004;10:2463-2475.
5. Russo L, Rizzo A, Di Vincenzo A, et al. Aripiprazole overdose and transient 2:1 second degree atrioventricular block: only a coincidence? Curr Drug Saf. 2019;14:155-157.
6. Pacher P, Ungvari Z, Kecskemeti V, et al. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Curr Med Chem. 1998;5:381-90.
7. Hemeryck A, Belpaire FM. Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug-drug interactions: an update. Curr Drub Metab. 2002;3:13-37.
8. Snarr BS, Phan SV, Garner A, et al. Symptomatic bradycardia with oral aripiprazole and oral ziprasidone. Ann Pharmacother. 2010;44:760-763.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
Systemic Literature Review of the Use of Virtual Reality for Rehabilitation in Parkinson Disease
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease.1 Age-standardized incidence rates of PD in population-based studies in Europe and the United States range from 8.6 to 19.0 per 100,000 individuals, using a strict diagnostic criterion for PD.2 The negative impact of PD on health-related quality of life imposes a heavy burden on veterans. According to the US Department of Veterans Affairs (VA) National Parkinson’s Disease Consortium, the VA has as many as 50,000 patients with PD under its care. Because of this demand, the VA has strived to revolutionize available services for veterans with PD and related movement disorders.3
The classic motor symptoms of resting tremors, bradykinesia, postural instability, and rigidity of this progressive neurodegenerative disorder is a significant cause of functional limitations that lead to increased falls and inability to perform activities of daily living that challenges the individual and caregiver. 4 Rehabilitation has been considered as an adjuvant to surgical and medical treatments for PD to maximize function and minimize complications. High-intensity multimodal exercise boot camps and therapy that focuses on intensely exercising high-amplitude movements, have been shown to improve motor performance in PD.5,6 Available evidence has shown that exercise-dependent plasticity is the main mechanism underlying the effects of physiotherapy because it increases synaptic strength and affects neurotransmission.7 Although there is no consensus on the optimal approach for rehabilitation, innovative techniques have been proposed and studied. One such approach involves virtual reality (VR), which has begun to attract attention for its potential use during rehabilitation.8
VR is a simulated experience created by computer-based technology that grants users access to a virtual environment. There are 2 categories of VR: immersive and nonimmersive. Immersive VR is the most direct experience of virtual environments and usually is implemented through a head-mounted display. These displays have monitors in front of each eye, which can provide monocular or biocular imaging with the most common display being small liquid crystal display (LCD) panels.
Nonimmersive VR typically allows a participant to view a virtual environment by using standard high-resolution monitors rather than a headset or an immersive screen room. Many systems are readily available to the general public as electronic interactive entertainment (ie, video games). Interaction with the virtual world happens through interfaces such as keyboards and controllers while viewing a television or computer monitor. These systems often are more accessible and affordable when compared with immersive VR, although this is changing rapidly.
VR therapy is a noninvasive therapeutic alternative modality for PD. This review aims to study the use of VR to treat PD from a rehabilitative standpoint. Although not the only review on the topic, this systematic review is the first to examine the differences between immersive and nonimmersive VR rehabilitation for PD. VR technology is evolving rapidly and the research behind its clinical applications is steadily growing, especially as accessibility improves. This review also is an updated summary of the current literature on the effectiveness of VR therapy during PD rehabilitation.
Methods
Starting in July 2019, the authors searched several databases (PubMed, Google Scholar, Cochrane, and the Physiotherapy Evidence Database [PEDro]) for articles by using the keyword “Parkinson’s disease” combined with either “virtual reality” or “video games.” To find studies specific to rehabilitation, searches included the additional keyword: “rehabilitation.” After compiling an initial set of 89 articles, titles were reviewed to eliminate duplicates. The authors then read the abstracts to exclude study protocols, systematic reviews, and studies that used VR but did not focus on PD or any therapeutic outcome.
Articles were sorted into immersive or nonimmersive virtual reality categories. To be included as immersive VR, studies had to use any type of VR headset or full-scale VR room. Anything less immersive or similar to a traditional video game was included in the nonimmersive VR category. Articles that met inclusion criteria were selected for the systematic review. Criteria for inclusion in this review were: (1) English language; (2) included a study population focused on PD; (3) used some form of VR therapy; and (4) assessed potential rehabilitation by quantitative outcome measures. Only articles published in peer-reviewed journals were included.
Data were extracted into 2 tables specifically modified for this review: immersive and nonimmersive VR. Extracted data included study author name and publication date, study design, methodologic quality, sample size and group allocation, symptom progression via the Hoehn and Yahr Scale (1 to 5), VR modality, presence of control groups, primary outcomes, and primary findings.
Two of the authors (AS, BC) assessed the quality of each study by using the 11-point PEDro scale for randomized controlled trials (RCTs) (Table 1). Most criterion relate to the design and conduct of the study, but 3 focus on eligibility criteria (item 1), between-group statistical comparisons (item 10), and measures of variability (item 11). The total possible score was 10 because only 2 out of the 3 items on reporting quality contributed points to the total score (eligibility criteria specified did not).9
Results
This review is reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA).10 After screening and assessment, 28 articles met inclusion criteria for this review: 7 using immersive VR and 21 using nonimmersive VR (Figure). The immersive studies included 2 RCTs (both with PEDro scores of 5), 1 controlled study with a PEDro score of 5, 1 pre-post pilot study, and 3 cohort studies (Table 2). The nonimmersive studies included 13 RCTs with an average PEDro score of 5.8; 2 pre-post pilot studies, 1 repeated measures study with a historic control, 1 non-RCT, 2 pre-post prospective studies, and 2 cohort studies (1 retrospective and 1 prospective) (Table 3).
Several outcome and assessment tools were used; the most common measures were related to gait, balance, kinematics, and VR feasibility. Studies varied in VR modalities and protocol, ranging from 21 sessions of Nintendo Wii Fit gaming for 7 weeks to 1 session of VR headset use.
Immersive VR
There were fewer immersive VR studies and these studies had lower mean PEDro scores when compared with nonimmersive VR studies. The VR modalities in the immersive studies used a VR headset or a multisensory immersive system that included polarized glasses. All the studies showed positive improvement in primary outcomes with the exception of Ma and colleagues,which showed no difference in success rates or kinematics with moving balls, and only showed improvement in reaching for stationary balls.11 The mean number of participants in the studies was 18.4.
All 7 studies had each participant complete tasks without VR then with the VR therapy. None of the studies compared immersive VR therapy with more conventional therapies. Robles-Garcia and colleagues compared 2 VR groups where the experimental group imitated an avatar’s finger tapping in the VR system while the control group lacked this imitation.12 The authors found that adding that imitation to the VR group lead to an increase in movement amplitude.
Among the immersive VR studies, only Janeh and colleagues commented on possible adverse effects (AEs) and found that VR was a safe method without AEs of discomfort or simulator sickness.13 The other 6 studies did not make any mention or discussion of AEs related to the training.
Nonimmersive VR
VR modalities used in nonimmersive studies included consumer video gaming systems. Nintendo Wii and Microsoft Xbox Kinect were most commonly used. Among the 21 studies, 14 compared VR therapy with a type of traditional exercise (eg, treadmill training, stretching exercises, balance training). The mean number of participants of the studies was 28.3.
Five studies showed a difference between the VR and traditional training groups.14-18 However, 9 studies showed positive improvement in both groups and found no between-group differences.19-25 Among the remaining 7 studies, all showed improvement in primary outcomes after adding VR interventional therapy. In 1 RCT, 3 groups were compared (no intervention, Nintendo Wii, and Xbox Kinect) for gait tests, anxiety levels, memory, and attention.26 The authors found that only the Nintendo Wii group showed improvement in outcomes. A prospective cohort study was the only one to compare different doses of VR therapy (10 sessions vs 15 sessions of Nintendo Wii Fit).27 The authors found that both groups demonstrated the same amount of improvement on balance performances with no group effect.
Ten studiesreported no AEs during the training, but also did not define what was considered an AE.15,16,19,22-25,27-29 Eight studies did not make any mention of AEs.14,17,21,26,27,30-32 Yen and colleagues reported no AEs during training except for the patients’ tendency to fall.20 However, therapists supervised the patients to avoid falls and no falls occurred. Nuic and colleaguesreported 3 serious AEs, unrelated to the training: severe pneumonia (n = 1) and deep-brain stimulation generator replacement (n = 2).33 During the video game training sessions no specific AEs occurred. Only Pompeu and colleagues defined an AE as any untoward medical occurrence such as convulsion, syncope, dizziness, vertigo, falls, or any medical condition that required hospitalization or disability.34 One researcher registered the occurrence of any AE; however, none occurred during the study period.
Discussion
This systematic review demonstrates that VR therapy is a promising addition to rehabilitation for PD. Evidence supporting VR therapy is limited, but is continually expanding, and current evidence has shown improvement in assessments and rehabilitative outcomes involving PD. Most nonimmersive studies have shown that VR therapy does not lead to better outcomes when compared with traditional therapy but also is not harmful and does provide similar improvement. Immersive VR studies, on the other hand, have not compared therapy with conventional training extensively, and tend to focus more on time for task completion or movement.
There were fewer immersive VR studies than nonimmersive VR studies. This could be because of the increased technological difficulty and demand to correctly execute immersive VR modalities, as well as the—until recently—substantial expense. This might be another reason why the mean PEDro scores for immersive VR RCTs were lower than the mean scores found in nonimmersive RCTs.
Limitations
This review was limited by several factors related to the included studies. A variety of rating scales were used in the immersive and nonimmersive VR studies. Although there was some general overlap with common measurements such as gait, balance, kinematics, and VR feasibility, no studies had the same primary and secondary outcomes. Such heterogeneity in protocols and outcomes limited our ability to draw conclusions from these differing studies. Additionally, the average number of participants of both immersive and nonimmersive studies were small and the statistical significance of findings should be interpreted with caution. Finally, VR devices and systems differed between studies, further limiting comparisons. Although these factors limit this systematic review, we can still identify treatment and research implications. Adequately powered future studies with standardized protocols would further improve the available evidence and support for VR as an intervention.
Conclusions
VR therapy is a promising rehabilitation modality for PD. Additional investigations of VR therapy and PD should include direct comparisons between immersive and nonimmersive VR therapies. It could be hypothesized that the greater immersion and engagement potential of immersive VR would demonstrate greater functional improvement compared with nonimmersive VR, but there is no data to support this for PD. VR therapy for PD appears to be a relatively safe alternative or adjunct to traditional therapy with a potentially positive impact on a variety of symptoms and is growing as an innovative therapeutic approach for PD patients.
1. de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525-535. doi:10.1016/S1474-4422(06)70471-9
2. Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP. Epidemiology of Parkinson’s disease. J Neurol. 2008;255 Suppl 5:18-32. doi:10.1007/s00415-008-5004-3
3. US Department of Veterans Affairs. Parkinson’s Disease Research, Education and Clinical Centers. Updated March 4, 2021. Accessed March 5, 2021. https://www.parkinsons.va.gov/index.asp.
4. Raza C, Anjum R, Shakeel NUA. Parkinson’s disease: mechanisms, translational models and management strategies. Life Sci. 2019;226:77-90. doi:10.1016/j.lfs.2019.03.057
5. Landers MR, Navalta JW, Murtishaw AS, Kinney JW, Pirio Richardson S. A high-intensity exercise boot camp for persons with Parkinson disease: a phase ii, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms. J Neurol Phys Ther. 2019;43(1):12-25. doi:10.1097/NPT.0000000000000249
6. Ebersbach G, Ebersbach A, Edler D, et al. Comparing exercise in Parkinson’s disease--the Berlin LSVT®BIG study [published correction appears in Mov Disord. 2010 Oct 30;25(14):2478]. Mov Disord. 2010;25(12):1902-1908. doi:10.1002/mds.23212
7. Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson’s disease: current outlook and future challenges. Parkinsonism Relat Disord. 2016;22(suppl 1):S60-S64. doi:10.1016/j.parkreldis.2015.09.005
8. Weiss PL, Katz N. The potential of virtual reality for rehabilitation. J Rehabil Res Dev. 2004;41(5):vii-x.
9. da Costa BR, Hilfiker R, Egger M. PEDro’s bias: summary quality scores should not be used in meta-analysis. J Clin Epidemiol. 2013;66(1):75-77.doi:10.1016/j.jclinepi.2012.08.003
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
11. Ma HI, Hwang WJ, Fang JJ, et al. Effects of virtual reality training on functional reaching movements in people with Parkinson’s disease: a randomized controlled pilot trial. Clin Rehabil. 2011;25(10):892-902. doi:10.1177/0269215511406757
12. Robles-García V, Corral-Bergantiños Y, Espinosa N, et al. Effects of movement imitation training in Parkinson’s disease: a virtual reality pilot study. Parkinsonism Relat Disord. 2016;26:17-23. doi:10.1016/j.parkreldis.2016.02.022
13. Janeh O, Fründt O, Schönwald B, et al. Gait Training in virtual reality: short-term effects of different virtual manipulation techniques in Parkinson’s Disease. Cells. 2019;8(5):419. Published 2019 May 6.doi:10.3390/cells8050419
14. Pelosin E, Cerulli C, Ogliastro C, et al. A multimodal training modulates short afferent inhibition and improves complex walking in a cohort of faller older adults with an increased prevalence of Parkinson’s disease. J Gerontol A Biol Sci Med Sci. 2020;75(4):722-728.doi:10.1093/gerona/glz072
15. Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2015;29(7):658-667. doi:10.1177/1545968314562111
16. Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease?. J Gerontol A Biol Sci Med Sci. 2011;66(2):234-240.doi:10.1093/gerona/glq201
17. Lee NY, Lee DK, Song HS. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J Phys Ther Sci. 2015;27(1):145-147. doi:10.1589/jpts.27.145
18. Feng H, Li C, Liu J, et al. Virtual reality rehabilitation versus conventional physical therapy for improving balance and gait in Parkinson’s disease patients: a randomized controlled trial. Med Sci Monit. 2019;25:4186-4192. Published 2019 Jun 5. doi:10.12659/MSM.916455
19. Gandolfi M, Geroin C, Dimitrova E, et al. Virtual reality telerehabilitation for postural instability in Parkinson’s disease: a multicenter, single-blind, randomized, controlled trial. Biomed Res Int. 2017;2017:7962826. doi:10.1155/2017/7962826
20. Yen CY, Lin KH, Hu MH, Wu RM, Lu TW, Lin CH. Effects of virtual reality-augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson disease: a randomized controlled trial. Phys Ther. 2011;91(6):862-874. doi:10.2522/ptj.20100050
21. Yang WC, Wang HK, Wu RM, Lo CS, Lin KH. Home-based virtual reality balance training and conventional balance training in Parkinson’s disease: a randomized controlled trial. J Formos Med Assoc. 2016;115(9):734-743. doi:10.1016/j.jfma.2015.07.012
22. Pompeu JE, Mendes FA, Silva KG, et al. Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: a randomised clinical trial. Physiotherapy. 2012;98(3):196-204. doi:10.1016/j.physio.2012.06.004
23. van den Heuvel MR, Kwakkel G, Beek PJ, Berendse HW, Daffertshofer A, van Wegen EE. Effects of augmented visual feedback during balance training in Parkinson’s disease: a pilot randomized clinical trial. Parkinsonism Relat Disord. 2014;20(12):1352-1358. doi:10.1016/j.parkreldis.2014.09.022
24. Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2015;29(7):658-667. doi:10.1177/1545968314562111
25. Fundarò C, Maestri R, Ferriero G, Chimento P, Taveggia G, Casale R. Self-selected speed gait training in Parkinson’s disease: robot-assisted gait training with virtual reality versus gait training on the ground. Eur J Phys Rehabil Med. 2019;55(4):456-462. doi:10.23736/S1973-9087.18.05368-6
26. Alves MLM, Mesquita BS, Morais WS, Leal JC, Satler CE, Dos Santos Mendes FA. Nintendo Wii™ versus Xbox Kinect™ for assisting people with Parkinson’s disease. Percept Mot Skills. 2018;125(3):546-565. doi:10.1177/0031512518769204
27. Negrini S, Bissolotti L, Ferraris A, Noro F, Bishop MD, Villafañe JH. Nintendo Wii Fit for balance rehabilitation in patients with Parkinson’s disease: A comparative study. J Bodyw Mov Ther. 2017;21(1):117-123. doi:10.1016/j.jbmt.2016.06.001
28. van Beek JJW, van Wegen EEH, Bohlhalter S, Vanbellingen T. Exergaming-based dexterity training in persons with Parkinson disease: a pilot feasibility study. J Neurol Phys Ther. 2019;43(3):168-174. doi:10.1097/NPT.0000000000000278
29. Palacios-Navarro G, García-Magariño I, Ramos-Lorente P. A kinect-based system for lower limb rehabilitation in Parkinson’s disease patients: a pilot study. J Med Syst. 2015;39(9):103. doi:10.1007/s10916-015-0289-0
30. dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, et al. Motor learning, retention and transfer after virtual-reality-based training in Parkinson’s disease--effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy. 2012;98(3):217-223. doi:10.1016/j.physio.2012.06.001
31. de Melo GEL, Kleiner AFR, Lopes JBP, et al. Effect of virtual reality training on walking distance and physical fitness in individuals with Parkinson’s disease. Neuro Rehabilitation. 2018;42(4):473-480. doi:10.3233/NRE-172355
32. Maidan I, Nieuwhof F, Bernad-Elazari H, et al. Evidence for differential effects of 2 forms of exercise on prefrontal plasticity during walking in Parkinson’s disease. Neurorehabil Neural Repair. 2018;32(3):200-208. doi:10.1177/1545968318763750
33. Nuic D, Vinti M, Karachi C, Foulon P, Van Hamme A, Welter ML. The feasibility and positive effects of a customised videogame rehabilitation programme for freezing of gait and falls in Parkinson’s disease patients: a pilot study. J Neuroeng Rehabil. 2018;15(1):31. Published 2018 Apr 10. doi:10.1186/s12984-018-0375-x
34. Pompeu JE, Arduini LA, Botelho AR, et al. Feasibility, safety and outcomes of playing Kinect Adventures!™ for people with Parkinson’s disease: a pilot study. Physiotherapy. 2014;100(2):162-168. doi:10.1016/j.physio.2013.10.003
35. Ma HI, Hwang WJ, Wang CY, Fang JJ, Leong IF, Wang TY. Trunk-arm coordination in reaching for moving targets in people with Parkinson’s disease: comparison between virtual and physical reality. Hum Mov Sci. 2012;31(5):1340-1352. doi:10.1016/j.humov.2011.11.004
36. Griffin HJ, Greenlaw R, Limousin P, Bhatia K, Quinn NP, Jahanshahi M. The effect of real and virtual visual cues on walking in Parkinson’s disease. J Neurol. 2011;258(6):991-1000. doi:10.1007/s00415-010-5866-z
37. Espay AJ, Baram Y, Dwivedi AK, et al. At-home training with closed-loop augmented-reality cueing device for improving gait in patients with Parkinson disease. J Rehabil Res Dev. 2010;47(6):573-581. doi:10.1682/jrrd.2009.10.0165
38. Espay AJ, Gaines L, Gupta R. Sensory feedback in Parkinson’s disease patients with “on”-predominant freezing of gait. Front Neurol. 2013;4:14. Published 2013 Feb 25. doi:10.3389/fneur.2013.00014
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease.1 Age-standardized incidence rates of PD in population-based studies in Europe and the United States range from 8.6 to 19.0 per 100,000 individuals, using a strict diagnostic criterion for PD.2 The negative impact of PD on health-related quality of life imposes a heavy burden on veterans. According to the US Department of Veterans Affairs (VA) National Parkinson’s Disease Consortium, the VA has as many as 50,000 patients with PD under its care. Because of this demand, the VA has strived to revolutionize available services for veterans with PD and related movement disorders.3
The classic motor symptoms of resting tremors, bradykinesia, postural instability, and rigidity of this progressive neurodegenerative disorder is a significant cause of functional limitations that lead to increased falls and inability to perform activities of daily living that challenges the individual and caregiver. 4 Rehabilitation has been considered as an adjuvant to surgical and medical treatments for PD to maximize function and minimize complications. High-intensity multimodal exercise boot camps and therapy that focuses on intensely exercising high-amplitude movements, have been shown to improve motor performance in PD.5,6 Available evidence has shown that exercise-dependent plasticity is the main mechanism underlying the effects of physiotherapy because it increases synaptic strength and affects neurotransmission.7 Although there is no consensus on the optimal approach for rehabilitation, innovative techniques have been proposed and studied. One such approach involves virtual reality (VR), which has begun to attract attention for its potential use during rehabilitation.8
VR is a simulated experience created by computer-based technology that grants users access to a virtual environment. There are 2 categories of VR: immersive and nonimmersive. Immersive VR is the most direct experience of virtual environments and usually is implemented through a head-mounted display. These displays have monitors in front of each eye, which can provide monocular or biocular imaging with the most common display being small liquid crystal display (LCD) panels.
Nonimmersive VR typically allows a participant to view a virtual environment by using standard high-resolution monitors rather than a headset or an immersive screen room. Many systems are readily available to the general public as electronic interactive entertainment (ie, video games). Interaction with the virtual world happens through interfaces such as keyboards and controllers while viewing a television or computer monitor. These systems often are more accessible and affordable when compared with immersive VR, although this is changing rapidly.
VR therapy is a noninvasive therapeutic alternative modality for PD. This review aims to study the use of VR to treat PD from a rehabilitative standpoint. Although not the only review on the topic, this systematic review is the first to examine the differences between immersive and nonimmersive VR rehabilitation for PD. VR technology is evolving rapidly and the research behind its clinical applications is steadily growing, especially as accessibility improves. This review also is an updated summary of the current literature on the effectiveness of VR therapy during PD rehabilitation.
Methods
Starting in July 2019, the authors searched several databases (PubMed, Google Scholar, Cochrane, and the Physiotherapy Evidence Database [PEDro]) for articles by using the keyword “Parkinson’s disease” combined with either “virtual reality” or “video games.” To find studies specific to rehabilitation, searches included the additional keyword: “rehabilitation.” After compiling an initial set of 89 articles, titles were reviewed to eliminate duplicates. The authors then read the abstracts to exclude study protocols, systematic reviews, and studies that used VR but did not focus on PD or any therapeutic outcome.
Articles were sorted into immersive or nonimmersive virtual reality categories. To be included as immersive VR, studies had to use any type of VR headset or full-scale VR room. Anything less immersive or similar to a traditional video game was included in the nonimmersive VR category. Articles that met inclusion criteria were selected for the systematic review. Criteria for inclusion in this review were: (1) English language; (2) included a study population focused on PD; (3) used some form of VR therapy; and (4) assessed potential rehabilitation by quantitative outcome measures. Only articles published in peer-reviewed journals were included.
Data were extracted into 2 tables specifically modified for this review: immersive and nonimmersive VR. Extracted data included study author name and publication date, study design, methodologic quality, sample size and group allocation, symptom progression via the Hoehn and Yahr Scale (1 to 5), VR modality, presence of control groups, primary outcomes, and primary findings.
Two of the authors (AS, BC) assessed the quality of each study by using the 11-point PEDro scale for randomized controlled trials (RCTs) (Table 1). Most criterion relate to the design and conduct of the study, but 3 focus on eligibility criteria (item 1), between-group statistical comparisons (item 10), and measures of variability (item 11). The total possible score was 10 because only 2 out of the 3 items on reporting quality contributed points to the total score (eligibility criteria specified did not).9
Results
This review is reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA).10 After screening and assessment, 28 articles met inclusion criteria for this review: 7 using immersive VR and 21 using nonimmersive VR (Figure). The immersive studies included 2 RCTs (both with PEDro scores of 5), 1 controlled study with a PEDro score of 5, 1 pre-post pilot study, and 3 cohort studies (Table 2). The nonimmersive studies included 13 RCTs with an average PEDro score of 5.8; 2 pre-post pilot studies, 1 repeated measures study with a historic control, 1 non-RCT, 2 pre-post prospective studies, and 2 cohort studies (1 retrospective and 1 prospective) (Table 3).
Several outcome and assessment tools were used; the most common measures were related to gait, balance, kinematics, and VR feasibility. Studies varied in VR modalities and protocol, ranging from 21 sessions of Nintendo Wii Fit gaming for 7 weeks to 1 session of VR headset use.
Immersive VR
There were fewer immersive VR studies and these studies had lower mean PEDro scores when compared with nonimmersive VR studies. The VR modalities in the immersive studies used a VR headset or a multisensory immersive system that included polarized glasses. All the studies showed positive improvement in primary outcomes with the exception of Ma and colleagues,which showed no difference in success rates or kinematics with moving balls, and only showed improvement in reaching for stationary balls.11 The mean number of participants in the studies was 18.4.
All 7 studies had each participant complete tasks without VR then with the VR therapy. None of the studies compared immersive VR therapy with more conventional therapies. Robles-Garcia and colleagues compared 2 VR groups where the experimental group imitated an avatar’s finger tapping in the VR system while the control group lacked this imitation.12 The authors found that adding that imitation to the VR group lead to an increase in movement amplitude.
Among the immersive VR studies, only Janeh and colleagues commented on possible adverse effects (AEs) and found that VR was a safe method without AEs of discomfort or simulator sickness.13 The other 6 studies did not make any mention or discussion of AEs related to the training.
Nonimmersive VR
VR modalities used in nonimmersive studies included consumer video gaming systems. Nintendo Wii and Microsoft Xbox Kinect were most commonly used. Among the 21 studies, 14 compared VR therapy with a type of traditional exercise (eg, treadmill training, stretching exercises, balance training). The mean number of participants of the studies was 28.3.
Five studies showed a difference between the VR and traditional training groups.14-18 However, 9 studies showed positive improvement in both groups and found no between-group differences.19-25 Among the remaining 7 studies, all showed improvement in primary outcomes after adding VR interventional therapy. In 1 RCT, 3 groups were compared (no intervention, Nintendo Wii, and Xbox Kinect) for gait tests, anxiety levels, memory, and attention.26 The authors found that only the Nintendo Wii group showed improvement in outcomes. A prospective cohort study was the only one to compare different doses of VR therapy (10 sessions vs 15 sessions of Nintendo Wii Fit).27 The authors found that both groups demonstrated the same amount of improvement on balance performances with no group effect.
Ten studiesreported no AEs during the training, but also did not define what was considered an AE.15,16,19,22-25,27-29 Eight studies did not make any mention of AEs.14,17,21,26,27,30-32 Yen and colleagues reported no AEs during training except for the patients’ tendency to fall.20 However, therapists supervised the patients to avoid falls and no falls occurred. Nuic and colleaguesreported 3 serious AEs, unrelated to the training: severe pneumonia (n = 1) and deep-brain stimulation generator replacement (n = 2).33 During the video game training sessions no specific AEs occurred. Only Pompeu and colleagues defined an AE as any untoward medical occurrence such as convulsion, syncope, dizziness, vertigo, falls, or any medical condition that required hospitalization or disability.34 One researcher registered the occurrence of any AE; however, none occurred during the study period.
Discussion
This systematic review demonstrates that VR therapy is a promising addition to rehabilitation for PD. Evidence supporting VR therapy is limited, but is continually expanding, and current evidence has shown improvement in assessments and rehabilitative outcomes involving PD. Most nonimmersive studies have shown that VR therapy does not lead to better outcomes when compared with traditional therapy but also is not harmful and does provide similar improvement. Immersive VR studies, on the other hand, have not compared therapy with conventional training extensively, and tend to focus more on time for task completion or movement.
There were fewer immersive VR studies than nonimmersive VR studies. This could be because of the increased technological difficulty and demand to correctly execute immersive VR modalities, as well as the—until recently—substantial expense. This might be another reason why the mean PEDro scores for immersive VR RCTs were lower than the mean scores found in nonimmersive RCTs.
Limitations
This review was limited by several factors related to the included studies. A variety of rating scales were used in the immersive and nonimmersive VR studies. Although there was some general overlap with common measurements such as gait, balance, kinematics, and VR feasibility, no studies had the same primary and secondary outcomes. Such heterogeneity in protocols and outcomes limited our ability to draw conclusions from these differing studies. Additionally, the average number of participants of both immersive and nonimmersive studies were small and the statistical significance of findings should be interpreted with caution. Finally, VR devices and systems differed between studies, further limiting comparisons. Although these factors limit this systematic review, we can still identify treatment and research implications. Adequately powered future studies with standardized protocols would further improve the available evidence and support for VR as an intervention.
Conclusions
VR therapy is a promising rehabilitation modality for PD. Additional investigations of VR therapy and PD should include direct comparisons between immersive and nonimmersive VR therapies. It could be hypothesized that the greater immersion and engagement potential of immersive VR would demonstrate greater functional improvement compared with nonimmersive VR, but there is no data to support this for PD. VR therapy for PD appears to be a relatively safe alternative or adjunct to traditional therapy with a potentially positive impact on a variety of symptoms and is growing as an innovative therapeutic approach for PD patients.
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease.1 Age-standardized incidence rates of PD in population-based studies in Europe and the United States range from 8.6 to 19.0 per 100,000 individuals, using a strict diagnostic criterion for PD.2 The negative impact of PD on health-related quality of life imposes a heavy burden on veterans. According to the US Department of Veterans Affairs (VA) National Parkinson’s Disease Consortium, the VA has as many as 50,000 patients with PD under its care. Because of this demand, the VA has strived to revolutionize available services for veterans with PD and related movement disorders.3
The classic motor symptoms of resting tremors, bradykinesia, postural instability, and rigidity of this progressive neurodegenerative disorder is a significant cause of functional limitations that lead to increased falls and inability to perform activities of daily living that challenges the individual and caregiver. 4 Rehabilitation has been considered as an adjuvant to surgical and medical treatments for PD to maximize function and minimize complications. High-intensity multimodal exercise boot camps and therapy that focuses on intensely exercising high-amplitude movements, have been shown to improve motor performance in PD.5,6 Available evidence has shown that exercise-dependent plasticity is the main mechanism underlying the effects of physiotherapy because it increases synaptic strength and affects neurotransmission.7 Although there is no consensus on the optimal approach for rehabilitation, innovative techniques have been proposed and studied. One such approach involves virtual reality (VR), which has begun to attract attention for its potential use during rehabilitation.8
VR is a simulated experience created by computer-based technology that grants users access to a virtual environment. There are 2 categories of VR: immersive and nonimmersive. Immersive VR is the most direct experience of virtual environments and usually is implemented through a head-mounted display. These displays have monitors in front of each eye, which can provide monocular or biocular imaging with the most common display being small liquid crystal display (LCD) panels.
Nonimmersive VR typically allows a participant to view a virtual environment by using standard high-resolution monitors rather than a headset or an immersive screen room. Many systems are readily available to the general public as electronic interactive entertainment (ie, video games). Interaction with the virtual world happens through interfaces such as keyboards and controllers while viewing a television or computer monitor. These systems often are more accessible and affordable when compared with immersive VR, although this is changing rapidly.
VR therapy is a noninvasive therapeutic alternative modality for PD. This review aims to study the use of VR to treat PD from a rehabilitative standpoint. Although not the only review on the topic, this systematic review is the first to examine the differences between immersive and nonimmersive VR rehabilitation for PD. VR technology is evolving rapidly and the research behind its clinical applications is steadily growing, especially as accessibility improves. This review also is an updated summary of the current literature on the effectiveness of VR therapy during PD rehabilitation.
Methods
Starting in July 2019, the authors searched several databases (PubMed, Google Scholar, Cochrane, and the Physiotherapy Evidence Database [PEDro]) for articles by using the keyword “Parkinson’s disease” combined with either “virtual reality” or “video games.” To find studies specific to rehabilitation, searches included the additional keyword: “rehabilitation.” After compiling an initial set of 89 articles, titles were reviewed to eliminate duplicates. The authors then read the abstracts to exclude study protocols, systematic reviews, and studies that used VR but did not focus on PD or any therapeutic outcome.
Articles were sorted into immersive or nonimmersive virtual reality categories. To be included as immersive VR, studies had to use any type of VR headset or full-scale VR room. Anything less immersive or similar to a traditional video game was included in the nonimmersive VR category. Articles that met inclusion criteria were selected for the systematic review. Criteria for inclusion in this review were: (1) English language; (2) included a study population focused on PD; (3) used some form of VR therapy; and (4) assessed potential rehabilitation by quantitative outcome measures. Only articles published in peer-reviewed journals were included.
Data were extracted into 2 tables specifically modified for this review: immersive and nonimmersive VR. Extracted data included study author name and publication date, study design, methodologic quality, sample size and group allocation, symptom progression via the Hoehn and Yahr Scale (1 to 5), VR modality, presence of control groups, primary outcomes, and primary findings.
Two of the authors (AS, BC) assessed the quality of each study by using the 11-point PEDro scale for randomized controlled trials (RCTs) (Table 1). Most criterion relate to the design and conduct of the study, but 3 focus on eligibility criteria (item 1), between-group statistical comparisons (item 10), and measures of variability (item 11). The total possible score was 10 because only 2 out of the 3 items on reporting quality contributed points to the total score (eligibility criteria specified did not).9
Results
This review is reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA).10 After screening and assessment, 28 articles met inclusion criteria for this review: 7 using immersive VR and 21 using nonimmersive VR (Figure). The immersive studies included 2 RCTs (both with PEDro scores of 5), 1 controlled study with a PEDro score of 5, 1 pre-post pilot study, and 3 cohort studies (Table 2). The nonimmersive studies included 13 RCTs with an average PEDro score of 5.8; 2 pre-post pilot studies, 1 repeated measures study with a historic control, 1 non-RCT, 2 pre-post prospective studies, and 2 cohort studies (1 retrospective and 1 prospective) (Table 3).
Several outcome and assessment tools were used; the most common measures were related to gait, balance, kinematics, and VR feasibility. Studies varied in VR modalities and protocol, ranging from 21 sessions of Nintendo Wii Fit gaming for 7 weeks to 1 session of VR headset use.
Immersive VR
There were fewer immersive VR studies and these studies had lower mean PEDro scores when compared with nonimmersive VR studies. The VR modalities in the immersive studies used a VR headset or a multisensory immersive system that included polarized glasses. All the studies showed positive improvement in primary outcomes with the exception of Ma and colleagues,which showed no difference in success rates or kinematics with moving balls, and only showed improvement in reaching for stationary balls.11 The mean number of participants in the studies was 18.4.
All 7 studies had each participant complete tasks without VR then with the VR therapy. None of the studies compared immersive VR therapy with more conventional therapies. Robles-Garcia and colleagues compared 2 VR groups where the experimental group imitated an avatar’s finger tapping in the VR system while the control group lacked this imitation.12 The authors found that adding that imitation to the VR group lead to an increase in movement amplitude.
Among the immersive VR studies, only Janeh and colleagues commented on possible adverse effects (AEs) and found that VR was a safe method without AEs of discomfort or simulator sickness.13 The other 6 studies did not make any mention or discussion of AEs related to the training.
Nonimmersive VR
VR modalities used in nonimmersive studies included consumer video gaming systems. Nintendo Wii and Microsoft Xbox Kinect were most commonly used. Among the 21 studies, 14 compared VR therapy with a type of traditional exercise (eg, treadmill training, stretching exercises, balance training). The mean number of participants of the studies was 28.3.
Five studies showed a difference between the VR and traditional training groups.14-18 However, 9 studies showed positive improvement in both groups and found no between-group differences.19-25 Among the remaining 7 studies, all showed improvement in primary outcomes after adding VR interventional therapy. In 1 RCT, 3 groups were compared (no intervention, Nintendo Wii, and Xbox Kinect) for gait tests, anxiety levels, memory, and attention.26 The authors found that only the Nintendo Wii group showed improvement in outcomes. A prospective cohort study was the only one to compare different doses of VR therapy (10 sessions vs 15 sessions of Nintendo Wii Fit).27 The authors found that both groups demonstrated the same amount of improvement on balance performances with no group effect.
Ten studiesreported no AEs during the training, but also did not define what was considered an AE.15,16,19,22-25,27-29 Eight studies did not make any mention of AEs.14,17,21,26,27,30-32 Yen and colleagues reported no AEs during training except for the patients’ tendency to fall.20 However, therapists supervised the patients to avoid falls and no falls occurred. Nuic and colleaguesreported 3 serious AEs, unrelated to the training: severe pneumonia (n = 1) and deep-brain stimulation generator replacement (n = 2).33 During the video game training sessions no specific AEs occurred. Only Pompeu and colleagues defined an AE as any untoward medical occurrence such as convulsion, syncope, dizziness, vertigo, falls, or any medical condition that required hospitalization or disability.34 One researcher registered the occurrence of any AE; however, none occurred during the study period.
Discussion
This systematic review demonstrates that VR therapy is a promising addition to rehabilitation for PD. Evidence supporting VR therapy is limited, but is continually expanding, and current evidence has shown improvement in assessments and rehabilitative outcomes involving PD. Most nonimmersive studies have shown that VR therapy does not lead to better outcomes when compared with traditional therapy but also is not harmful and does provide similar improvement. Immersive VR studies, on the other hand, have not compared therapy with conventional training extensively, and tend to focus more on time for task completion or movement.
There were fewer immersive VR studies than nonimmersive VR studies. This could be because of the increased technological difficulty and demand to correctly execute immersive VR modalities, as well as the—until recently—substantial expense. This might be another reason why the mean PEDro scores for immersive VR RCTs were lower than the mean scores found in nonimmersive RCTs.
Limitations
This review was limited by several factors related to the included studies. A variety of rating scales were used in the immersive and nonimmersive VR studies. Although there was some general overlap with common measurements such as gait, balance, kinematics, and VR feasibility, no studies had the same primary and secondary outcomes. Such heterogeneity in protocols and outcomes limited our ability to draw conclusions from these differing studies. Additionally, the average number of participants of both immersive and nonimmersive studies were small and the statistical significance of findings should be interpreted with caution. Finally, VR devices and systems differed between studies, further limiting comparisons. Although these factors limit this systematic review, we can still identify treatment and research implications. Adequately powered future studies with standardized protocols would further improve the available evidence and support for VR as an intervention.
Conclusions
VR therapy is a promising rehabilitation modality for PD. Additional investigations of VR therapy and PD should include direct comparisons between immersive and nonimmersive VR therapies. It could be hypothesized that the greater immersion and engagement potential of immersive VR would demonstrate greater functional improvement compared with nonimmersive VR, but there is no data to support this for PD. VR therapy for PD appears to be a relatively safe alternative or adjunct to traditional therapy with a potentially positive impact on a variety of symptoms and is growing as an innovative therapeutic approach for PD patients.
1. de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525-535. doi:10.1016/S1474-4422(06)70471-9
2. Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP. Epidemiology of Parkinson’s disease. J Neurol. 2008;255 Suppl 5:18-32. doi:10.1007/s00415-008-5004-3
3. US Department of Veterans Affairs. Parkinson’s Disease Research, Education and Clinical Centers. Updated March 4, 2021. Accessed March 5, 2021. https://www.parkinsons.va.gov/index.asp.
4. Raza C, Anjum R, Shakeel NUA. Parkinson’s disease: mechanisms, translational models and management strategies. Life Sci. 2019;226:77-90. doi:10.1016/j.lfs.2019.03.057
5. Landers MR, Navalta JW, Murtishaw AS, Kinney JW, Pirio Richardson S. A high-intensity exercise boot camp for persons with Parkinson disease: a phase ii, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms. J Neurol Phys Ther. 2019;43(1):12-25. doi:10.1097/NPT.0000000000000249
6. Ebersbach G, Ebersbach A, Edler D, et al. Comparing exercise in Parkinson’s disease--the Berlin LSVT®BIG study [published correction appears in Mov Disord. 2010 Oct 30;25(14):2478]. Mov Disord. 2010;25(12):1902-1908. doi:10.1002/mds.23212
7. Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson’s disease: current outlook and future challenges. Parkinsonism Relat Disord. 2016;22(suppl 1):S60-S64. doi:10.1016/j.parkreldis.2015.09.005
8. Weiss PL, Katz N. The potential of virtual reality for rehabilitation. J Rehabil Res Dev. 2004;41(5):vii-x.
9. da Costa BR, Hilfiker R, Egger M. PEDro’s bias: summary quality scores should not be used in meta-analysis. J Clin Epidemiol. 2013;66(1):75-77.doi:10.1016/j.jclinepi.2012.08.003
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
11. Ma HI, Hwang WJ, Fang JJ, et al. Effects of virtual reality training on functional reaching movements in people with Parkinson’s disease: a randomized controlled pilot trial. Clin Rehabil. 2011;25(10):892-902. doi:10.1177/0269215511406757
12. Robles-García V, Corral-Bergantiños Y, Espinosa N, et al. Effects of movement imitation training in Parkinson’s disease: a virtual reality pilot study. Parkinsonism Relat Disord. 2016;26:17-23. doi:10.1016/j.parkreldis.2016.02.022
13. Janeh O, Fründt O, Schönwald B, et al. Gait Training in virtual reality: short-term effects of different virtual manipulation techniques in Parkinson’s Disease. Cells. 2019;8(5):419. Published 2019 May 6.doi:10.3390/cells8050419
14. Pelosin E, Cerulli C, Ogliastro C, et al. A multimodal training modulates short afferent inhibition and improves complex walking in a cohort of faller older adults with an increased prevalence of Parkinson’s disease. J Gerontol A Biol Sci Med Sci. 2020;75(4):722-728.doi:10.1093/gerona/glz072
15. Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2015;29(7):658-667. doi:10.1177/1545968314562111
16. Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease?. J Gerontol A Biol Sci Med Sci. 2011;66(2):234-240.doi:10.1093/gerona/glq201
17. Lee NY, Lee DK, Song HS. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J Phys Ther Sci. 2015;27(1):145-147. doi:10.1589/jpts.27.145
18. Feng H, Li C, Liu J, et al. Virtual reality rehabilitation versus conventional physical therapy for improving balance and gait in Parkinson’s disease patients: a randomized controlled trial. Med Sci Monit. 2019;25:4186-4192. Published 2019 Jun 5. doi:10.12659/MSM.916455
19. Gandolfi M, Geroin C, Dimitrova E, et al. Virtual reality telerehabilitation for postural instability in Parkinson’s disease: a multicenter, single-blind, randomized, controlled trial. Biomed Res Int. 2017;2017:7962826. doi:10.1155/2017/7962826
20. Yen CY, Lin KH, Hu MH, Wu RM, Lu TW, Lin CH. Effects of virtual reality-augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson disease: a randomized controlled trial. Phys Ther. 2011;91(6):862-874. doi:10.2522/ptj.20100050
21. Yang WC, Wang HK, Wu RM, Lo CS, Lin KH. Home-based virtual reality balance training and conventional balance training in Parkinson’s disease: a randomized controlled trial. J Formos Med Assoc. 2016;115(9):734-743. doi:10.1016/j.jfma.2015.07.012
22. Pompeu JE, Mendes FA, Silva KG, et al. Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: a randomised clinical trial. Physiotherapy. 2012;98(3):196-204. doi:10.1016/j.physio.2012.06.004
23. van den Heuvel MR, Kwakkel G, Beek PJ, Berendse HW, Daffertshofer A, van Wegen EE. Effects of augmented visual feedback during balance training in Parkinson’s disease: a pilot randomized clinical trial. Parkinsonism Relat Disord. 2014;20(12):1352-1358. doi:10.1016/j.parkreldis.2014.09.022
24. Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2015;29(7):658-667. doi:10.1177/1545968314562111
25. Fundarò C, Maestri R, Ferriero G, Chimento P, Taveggia G, Casale R. Self-selected speed gait training in Parkinson’s disease: robot-assisted gait training with virtual reality versus gait training on the ground. Eur J Phys Rehabil Med. 2019;55(4):456-462. doi:10.23736/S1973-9087.18.05368-6
26. Alves MLM, Mesquita BS, Morais WS, Leal JC, Satler CE, Dos Santos Mendes FA. Nintendo Wii™ versus Xbox Kinect™ for assisting people with Parkinson’s disease. Percept Mot Skills. 2018;125(3):546-565. doi:10.1177/0031512518769204
27. Negrini S, Bissolotti L, Ferraris A, Noro F, Bishop MD, Villafañe JH. Nintendo Wii Fit for balance rehabilitation in patients with Parkinson’s disease: A comparative study. J Bodyw Mov Ther. 2017;21(1):117-123. doi:10.1016/j.jbmt.2016.06.001
28. van Beek JJW, van Wegen EEH, Bohlhalter S, Vanbellingen T. Exergaming-based dexterity training in persons with Parkinson disease: a pilot feasibility study. J Neurol Phys Ther. 2019;43(3):168-174. doi:10.1097/NPT.0000000000000278
29. Palacios-Navarro G, García-Magariño I, Ramos-Lorente P. A kinect-based system for lower limb rehabilitation in Parkinson’s disease patients: a pilot study. J Med Syst. 2015;39(9):103. doi:10.1007/s10916-015-0289-0
30. dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, et al. Motor learning, retention and transfer after virtual-reality-based training in Parkinson’s disease--effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy. 2012;98(3):217-223. doi:10.1016/j.physio.2012.06.001
31. de Melo GEL, Kleiner AFR, Lopes JBP, et al. Effect of virtual reality training on walking distance and physical fitness in individuals with Parkinson’s disease. Neuro Rehabilitation. 2018;42(4):473-480. doi:10.3233/NRE-172355
32. Maidan I, Nieuwhof F, Bernad-Elazari H, et al. Evidence for differential effects of 2 forms of exercise on prefrontal plasticity during walking in Parkinson’s disease. Neurorehabil Neural Repair. 2018;32(3):200-208. doi:10.1177/1545968318763750
33. Nuic D, Vinti M, Karachi C, Foulon P, Van Hamme A, Welter ML. The feasibility and positive effects of a customised videogame rehabilitation programme for freezing of gait and falls in Parkinson’s disease patients: a pilot study. J Neuroeng Rehabil. 2018;15(1):31. Published 2018 Apr 10. doi:10.1186/s12984-018-0375-x
34. Pompeu JE, Arduini LA, Botelho AR, et al. Feasibility, safety and outcomes of playing Kinect Adventures!™ for people with Parkinson’s disease: a pilot study. Physiotherapy. 2014;100(2):162-168. doi:10.1016/j.physio.2013.10.003
35. Ma HI, Hwang WJ, Wang CY, Fang JJ, Leong IF, Wang TY. Trunk-arm coordination in reaching for moving targets in people with Parkinson’s disease: comparison between virtual and physical reality. Hum Mov Sci. 2012;31(5):1340-1352. doi:10.1016/j.humov.2011.11.004
36. Griffin HJ, Greenlaw R, Limousin P, Bhatia K, Quinn NP, Jahanshahi M. The effect of real and virtual visual cues on walking in Parkinson’s disease. J Neurol. 2011;258(6):991-1000. doi:10.1007/s00415-010-5866-z
37. Espay AJ, Baram Y, Dwivedi AK, et al. At-home training with closed-loop augmented-reality cueing device for improving gait in patients with Parkinson disease. J Rehabil Res Dev. 2010;47(6):573-581. doi:10.1682/jrrd.2009.10.0165
38. Espay AJ, Gaines L, Gupta R. Sensory feedback in Parkinson’s disease patients with “on”-predominant freezing of gait. Front Neurol. 2013;4:14. Published 2013 Feb 25. doi:10.3389/fneur.2013.00014
1. de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525-535. doi:10.1016/S1474-4422(06)70471-9
2. Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP. Epidemiology of Parkinson’s disease. J Neurol. 2008;255 Suppl 5:18-32. doi:10.1007/s00415-008-5004-3
3. US Department of Veterans Affairs. Parkinson’s Disease Research, Education and Clinical Centers. Updated March 4, 2021. Accessed March 5, 2021. https://www.parkinsons.va.gov/index.asp.
4. Raza C, Anjum R, Shakeel NUA. Parkinson’s disease: mechanisms, translational models and management strategies. Life Sci. 2019;226:77-90. doi:10.1016/j.lfs.2019.03.057
5. Landers MR, Navalta JW, Murtishaw AS, Kinney JW, Pirio Richardson S. A high-intensity exercise boot camp for persons with Parkinson disease: a phase ii, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms. J Neurol Phys Ther. 2019;43(1):12-25. doi:10.1097/NPT.0000000000000249
6. Ebersbach G, Ebersbach A, Edler D, et al. Comparing exercise in Parkinson’s disease--the Berlin LSVT®BIG study [published correction appears in Mov Disord. 2010 Oct 30;25(14):2478]. Mov Disord. 2010;25(12):1902-1908. doi:10.1002/mds.23212
7. Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson’s disease: current outlook and future challenges. Parkinsonism Relat Disord. 2016;22(suppl 1):S60-S64. doi:10.1016/j.parkreldis.2015.09.005
8. Weiss PL, Katz N. The potential of virtual reality for rehabilitation. J Rehabil Res Dev. 2004;41(5):vii-x.
9. da Costa BR, Hilfiker R, Egger M. PEDro’s bias: summary quality scores should not be used in meta-analysis. J Clin Epidemiol. 2013;66(1):75-77.doi:10.1016/j.jclinepi.2012.08.003
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
11. Ma HI, Hwang WJ, Fang JJ, et al. Effects of virtual reality training on functional reaching movements in people with Parkinson’s disease: a randomized controlled pilot trial. Clin Rehabil. 2011;25(10):892-902. doi:10.1177/0269215511406757
12. Robles-García V, Corral-Bergantiños Y, Espinosa N, et al. Effects of movement imitation training in Parkinson’s disease: a virtual reality pilot study. Parkinsonism Relat Disord. 2016;26:17-23. doi:10.1016/j.parkreldis.2016.02.022
13. Janeh O, Fründt O, Schönwald B, et al. Gait Training in virtual reality: short-term effects of different virtual manipulation techniques in Parkinson’s Disease. Cells. 2019;8(5):419. Published 2019 May 6.doi:10.3390/cells8050419
14. Pelosin E, Cerulli C, Ogliastro C, et al. A multimodal training modulates short afferent inhibition and improves complex walking in a cohort of faller older adults with an increased prevalence of Parkinson’s disease. J Gerontol A Biol Sci Med Sci. 2020;75(4):722-728.doi:10.1093/gerona/glz072
15. Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2015;29(7):658-667. doi:10.1177/1545968314562111
16. Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease?. J Gerontol A Biol Sci Med Sci. 2011;66(2):234-240.doi:10.1093/gerona/glq201
17. Lee NY, Lee DK, Song HS. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J Phys Ther Sci. 2015;27(1):145-147. doi:10.1589/jpts.27.145
18. Feng H, Li C, Liu J, et al. Virtual reality rehabilitation versus conventional physical therapy for improving balance and gait in Parkinson’s disease patients: a randomized controlled trial. Med Sci Monit. 2019;25:4186-4192. Published 2019 Jun 5. doi:10.12659/MSM.916455
19. Gandolfi M, Geroin C, Dimitrova E, et al. Virtual reality telerehabilitation for postural instability in Parkinson’s disease: a multicenter, single-blind, randomized, controlled trial. Biomed Res Int. 2017;2017:7962826. doi:10.1155/2017/7962826
20. Yen CY, Lin KH, Hu MH, Wu RM, Lu TW, Lin CH. Effects of virtual reality-augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson disease: a randomized controlled trial. Phys Ther. 2011;91(6):862-874. doi:10.2522/ptj.20100050
21. Yang WC, Wang HK, Wu RM, Lo CS, Lin KH. Home-based virtual reality balance training and conventional balance training in Parkinson’s disease: a randomized controlled trial. J Formos Med Assoc. 2016;115(9):734-743. doi:10.1016/j.jfma.2015.07.012
22. Pompeu JE, Mendes FA, Silva KG, et al. Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: a randomised clinical trial. Physiotherapy. 2012;98(3):196-204. doi:10.1016/j.physio.2012.06.004
23. van den Heuvel MR, Kwakkel G, Beek PJ, Berendse HW, Daffertshofer A, van Wegen EE. Effects of augmented visual feedback during balance training in Parkinson’s disease: a pilot randomized clinical trial. Parkinsonism Relat Disord. 2014;20(12):1352-1358. doi:10.1016/j.parkreldis.2014.09.022
24. Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2015;29(7):658-667. doi:10.1177/1545968314562111
25. Fundarò C, Maestri R, Ferriero G, Chimento P, Taveggia G, Casale R. Self-selected speed gait training in Parkinson’s disease: robot-assisted gait training with virtual reality versus gait training on the ground. Eur J Phys Rehabil Med. 2019;55(4):456-462. doi:10.23736/S1973-9087.18.05368-6
26. Alves MLM, Mesquita BS, Morais WS, Leal JC, Satler CE, Dos Santos Mendes FA. Nintendo Wii™ versus Xbox Kinect™ for assisting people with Parkinson’s disease. Percept Mot Skills. 2018;125(3):546-565. doi:10.1177/0031512518769204
27. Negrini S, Bissolotti L, Ferraris A, Noro F, Bishop MD, Villafañe JH. Nintendo Wii Fit for balance rehabilitation in patients with Parkinson’s disease: A comparative study. J Bodyw Mov Ther. 2017;21(1):117-123. doi:10.1016/j.jbmt.2016.06.001
28. van Beek JJW, van Wegen EEH, Bohlhalter S, Vanbellingen T. Exergaming-based dexterity training in persons with Parkinson disease: a pilot feasibility study. J Neurol Phys Ther. 2019;43(3):168-174. doi:10.1097/NPT.0000000000000278
29. Palacios-Navarro G, García-Magariño I, Ramos-Lorente P. A kinect-based system for lower limb rehabilitation in Parkinson’s disease patients: a pilot study. J Med Syst. 2015;39(9):103. doi:10.1007/s10916-015-0289-0
30. dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, et al. Motor learning, retention and transfer after virtual-reality-based training in Parkinson’s disease--effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy. 2012;98(3):217-223. doi:10.1016/j.physio.2012.06.001
31. de Melo GEL, Kleiner AFR, Lopes JBP, et al. Effect of virtual reality training on walking distance and physical fitness in individuals with Parkinson’s disease. Neuro Rehabilitation. 2018;42(4):473-480. doi:10.3233/NRE-172355
32. Maidan I, Nieuwhof F, Bernad-Elazari H, et al. Evidence for differential effects of 2 forms of exercise on prefrontal plasticity during walking in Parkinson’s disease. Neurorehabil Neural Repair. 2018;32(3):200-208. doi:10.1177/1545968318763750
33. Nuic D, Vinti M, Karachi C, Foulon P, Van Hamme A, Welter ML. The feasibility and positive effects of a customised videogame rehabilitation programme for freezing of gait and falls in Parkinson’s disease patients: a pilot study. J Neuroeng Rehabil. 2018;15(1):31. Published 2018 Apr 10. doi:10.1186/s12984-018-0375-x
34. Pompeu JE, Arduini LA, Botelho AR, et al. Feasibility, safety and outcomes of playing Kinect Adventures!™ for people with Parkinson’s disease: a pilot study. Physiotherapy. 2014;100(2):162-168. doi:10.1016/j.physio.2013.10.003
35. Ma HI, Hwang WJ, Wang CY, Fang JJ, Leong IF, Wang TY. Trunk-arm coordination in reaching for moving targets in people with Parkinson’s disease: comparison between virtual and physical reality. Hum Mov Sci. 2012;31(5):1340-1352. doi:10.1016/j.humov.2011.11.004
36. Griffin HJ, Greenlaw R, Limousin P, Bhatia K, Quinn NP, Jahanshahi M. The effect of real and virtual visual cues on walking in Parkinson’s disease. J Neurol. 2011;258(6):991-1000. doi:10.1007/s00415-010-5866-z
37. Espay AJ, Baram Y, Dwivedi AK, et al. At-home training with closed-loop augmented-reality cueing device for improving gait in patients with Parkinson disease. J Rehabil Res Dev. 2010;47(6):573-581. doi:10.1682/jrrd.2009.10.0165
38. Espay AJ, Gaines L, Gupta R. Sensory feedback in Parkinson’s disease patients with “on”-predominant freezing of gait. Front Neurol. 2013;4:14. Published 2013 Feb 25. doi:10.3389/fneur.2013.00014
Lumbar Fusion With Polyetheretherketone Rods Use for Patients With Degenerative Disease
Surgical treatment of degenerative lumbar spine disease has been rising steadily in the United States, and an increasing fraction of surgery involves lumbar fusion.1,2 Various techniques are used to accomplish a lumbar fusion, including noninstrumented fusion, anterior lumbar interbody fusion (ALIF), lateral lumbar interbody fusion (XLIF, OLIF), posterior pedicle screw fusion, posterior cortical screw fusion, posterior interbody fusion (TLIF, PLIF), and interspinous process fusion. Rigid, metallic fusion hardware provides high stability and fusion rates, but it likely leads to stress shielding and adjacent segment disease.3 There is interest in less rigid and dynamic stabilization techniques to reduce the risk of adjacent segment disease, such as polyetheretherketone (PEEK) rods, which have been available since 2007. However, literature regarding PEEK rod utility is sparse and of mixed outcomes.3,4 Additional patient reported outcome (PRO) information would be useful to both surgeons and patients. Using institutional data, this review was designed to examine our experience with PEEK rod lumbar fusion and to document PROs.
Methods
The study was approved by the institutional review board at the US Department of Veterans Affairs (VA) Portland Health Care System (VAPHCS) in Oregon with a waiver of authorization. In this retrospective, single center study, data were queried from the senior author’s (DAR) case logs from VA Computerized Patient Record System (CPRS). Electronic medical records, imaging, and PROs of all consecutive patients undergoing lumbar fusion at 1 or 2 levels with PEEK rods for degenerative disease were retrospectively reviewed. Cases of trauma, malignancy, or infection were excluded. From March 2011 through October 2019, 108 patients underwent lumbar fusion with PEEK rods.
Surgeries were conducted on a Mizuho OSI Jackson Table via bilateral 3 to 4 cm Wiltse incisions using the Medtronic Quadrant retractor system. Medtronic O-Arm images were acquired and delivered to a Medtronic Stealth Station for navigation of the screws. Monopolar coagulation was not used. PEEK pedicle screws were placed and verified with a second O-Arm spin before placing lordotic PEEK rods in the screw heads. No attempt was made to reduce any spondylolisthesis, but distraction was used to open the foramina and indirectly decompress the canal. An interbody device was placed only in treatment of multiply recurrent disc protrusion. After decortication of the transverse processes and facets, intertransverse fusion constructs consisting of calcium hydroxyapatite soaked in autologous bone marrow blood and wrapped in 6-mg bone morphogenetic protein-soaked sponges were placed on the bone. If canal decompression was indicated, a Medtronic Metrx retractor tube was then placed through one of the incisions and decompression carried out. Wounds were closed with absorbable suture. No bracing was used postoperatively. Figure 1 shows a typical single level PEEK rod fusion construct.
Patient pre- and postoperative Short Form-36 (SF-36) physical function (PF) scores and Oswestry Disability Index (ODI) scores had been obtained at routine clinic visits.
Static radiographs were used to assess the fusion. Dynamic films and/or computed tomography (CT) scans were obtained only when symptomatic pseudarthrosis was suspected. Some patients had abdominal or lumbar CT scans for other indications, and these were reviewed when available. Particular care was taken to assess facet fusion as an indicator of arthrodesis (Figure 2).5
Statistical Analysis
Pre- and postoperative pairwise t tests were completed for patients with a complete data, using SAS 9.2 statistical package. Data are presented as standard deviation (SD) of the mean.
Results
Following application of the inclusion/exclusion criteria, 108 patients had undergone lumbar fusion with PEEK rods. Mean (SD) patient age was 60.2 (10.3) years and 88 patients were male (Table 1). Most surgeries were at L5-S1 and L4-5. There were 97 single-level fusions and 11 bilevel fusions. Seventy-four procedures were for spondylolisthesis, 23 for foraminal stenosis, 5 for degenerative disc disease, 3 for coronal imbalance with foraminal stenosis, 2 for pseudarthrosis after surgery elsewhere, and 1 for multiple recurrent disc herniation (Table 2). Twenty-five patients (23.1%) were current tobacco users and 28 (25.9%) were former smokers, 26 (24.1%) had diabetes mellitus (DM), 16 (14.8%) had low bone density by dual energy X-ray absorptiometry (DEXA) imaging, 35 (32.4%) had depression, and 7 (6.5%) were taking an immunosuppressive agent (chronic steroids, biological response modifiers, or methotrexate). Mean body mass index was 30.1.
Surgical Procedure
Of the 108 patients, the first 18 underwent a procedure with fluoroscopic guidance and the Medtronic FluoroNav and Stealth Systems. The next 90 patients underwent a procedure with O-Arm intraoperative CT scanning and Stealth frameless stereotactic navigation. The mean (SD) length of stay was 1.7 (1.3) days. There were no wound infections and no new neurologic deficits. Mean (SD) follow up time was 30.3 (21.8) months.
Imaging
Final imaging was by radiograph in 73 patients, CT in 31, and magnetic resonance imaging (MRI) in 3 (1 patient had no imaging). Sixty-seven patients (62.0%) had a bilateral arthrodesis, and 15 (13.9%) had at least a unilateral arthrodesis. MRI was not used to assess arthrodesis. Eight patients (7.4%) had no definite arthrodesis. Seventeen patients had inadequate or early imaging from which a fusion determination could not be made. Of 81 patients with > 11 months of follow up, 58 (71.6%) had a bilateral arthrodesis, 12 (14.8%) had a unilateral arthrodesis, 8 (9.9%) had no arthrodesis, and 3 (3.7%) were indeterminate.
No patient had any revision fusion surgery at the index level during follow up. Two patients had adjacent level fusions at 27 and 60 months after the index procedure. One patient had a laminectomy at an adjacent segment at 18 months postfusion, and 1 had a foraminotomy at an adjacent segment 89 months post fusion (Figure 3). Overall, there were 4 (3.7%) adjacent segment surgeries at a mean of 48.5 months after surgery. One patient had a sacro-iliac joint fusion below an L5-S1 fusion 17 months prior for persisting pain after the fusion procedure.
Patient Reported Outcomes
Preoperative SF-36 PF and ODI scores were available for 81 patients (Table 3). Postoperative SF-36 PF scores were obtained at 3 months for 65 of these patients, and at 1 year for 63 patients. Postoperative ODI scores were obtained at 3 months for 65 patients, and at 1 year for 55 patients. Among the 65 patients with completed SF-36 scores at 3 months, a mean increase of 22.4 (95% CI, 17-27; P < .001) was noted, and for the 63 patients at 1 year a mean increase of 30.3 (95% CI, 25-35; P < .001) was noted. Among the 65 patients with completed ODI scores at 3 months, a mean decrease of 6.8 (95% CI, 4.9-8.6; P < .001) was noted, and for the 55 patients with completed ODI scores at 1 year a mean decrease of 10.3 (n = 55; 95% CI, 8.4-12.2; P < .001) was noted.
Cost
We compared the hardware cost of a single level construct consisting of 4 pedicle screws, 4 locking caps, and 2 rods using a PEEK system with that of 2 other titanium construct systems. At VAPHCS, the PEEK system cost was about 71% of the cost of 2 other titanium construct systems and 62% of the cost when compared with Medtronic titanium rods.
Discussion
PEEK is useful for spine and cranial implants. It is inert and fully biocompatible with a modulus of elasticity between that of cortical and cancellous bone, and much lower than that of titanium, and is therefore considered to be semirigid.3,4,6 PEEK rods are intermediate in stiffness between titanium rods (110 Gigapascals) and dynamic devices such as the Zimmer Biomet DYNESYS dynamic stabilization system or the Premia Spine TOPS system.3 Carbon fiber rods and carbon fiber reinforced PEEK implants are other semirigid rod alternatives.7,8 PEEK rods for posterior lumbar fusion surgery were introduced in 2007. Li and colleagues provide a thorough review of the biomechanical properties of PEEK rods.3
PEEK is thought to have several advantages when compared with titanium. These advantages include more physiologic load sharing and reduction in stress shielding, improved durability, reduced risk of failure in osteoporotic bone, less wear debris, no change in bone forming environment, and imaging radiolucency.4,9 Spinal PEEK cages have been reported to allow more uniform radiation dose distribution compared with metal constructs, an advantage that also may pertain to PEEK rods.10 Disadvantages of PEEK rods include an inability to detect rod breakage easily, lack of data on the use in more than minimally unstable clinical situations, and greater expense, although this was not the authors’ observation.3,4,11
Importantly, it has been reported that PEEK rods permit a greater range of motion in all planes when compared with titanium rods.9 Polyetheretherketone rods unload the bone screw interface and increased the anterior column load to a more physiologic 75% when compared with titanium rods.6,9 However, in another biomechanical study that compared titanium rods, PEEK rods, and a dynamic stabilization device, it was reported that anterior load sharing was 55%, 59%, and 75%, respectively.12 This indicated that PEEK rods are closer to metal rods than truly dynamic devices for anterior load sharing. The endurance limit of a PEEK rod construct was similar to that of clinically useful metal systems.9 PEEK rods resulted in no increase in postfatigue motion compared with titanium rods in a biomechanical model.13 Intradiscal pressures at PEEK instrumented segments were similar to uninstrumented segments and greater than those with titanium rod constructs.14 Intradiscal pressures at adjacent segments were highest with dynamic devices, intermediate with semirigid rods, and lowest with rigid constructs; however, stress values at adjacent segments were lower in PEEK than titanium constructs in any direction of motion.15,16
Fusion Rates
The use of PEEK rods in lumbar fusion has been reported previously.3,4,17,18 However, these studies featured small sample sizes, short follow up times, and contradictory results.4 Of 8 outcome reports found in a systematic review, 2 studies reported on procedures designed to create nonfusion outcomes (a third similar trial from 2013 was not included in the systematic review), and 1 study reported only on the condition of PEEK rods removed at subsequent surgery.3,19-21 Reported fusion rates varied from 86 to 100%.
In 42 patients with PEEK rod fusions who were followed for a mean of 31.4 months, 5 patients required adjacent segment surgery and 3 patients were treated for interbody cage migration and nonunion.17 Radiographic fusion rate was 86%. These authors concluded that PEEK rod fusion results were similar to those of other constructs, but not better, or perhaps worse than, metal rods.
Other studies have reported better results with PEEK.11,18,19,22-24 Highsmith and colleagues reported on 3 successful example cases of the use of PEEK rods.11 De Iure and colleagues reported on 30 cases up to 5 levels (mean, 2.9) using autograft bone, with a mean follow up of 18 months.23 Results were reported as satisfactory. Three patients had radiographic nonunions, 1 of which required revision for asymptomatic screw loosening at the cranial end of the construct. Qi and colleagues, reported on 20 patients with PEEK rods compared to 21 patients with titanium alloy rods.24 Both groups had similar clinical outcomes, structural parameters, and 100% fusion rates. Athanasakopoulos and colleagues reported on 52 patients with up to 3 level fusions followed for a mean of 3 years.22 There were significant improvements in PROs: at 1 year 96% had radiographic union. Two patients had screw breakage, 1 of whom required revision to a metal rod construct. Colangeli and colleagues reported on 12 patients treated with PEEK rods compared with 12 who were treated with a dynamic system.18 They reported significant improvements, no complications, and 100% fusion at 6 months. Huang and colleagues reported on 38 patients intended to undergo a nonfusion procedure with 2 years of follow up.19 They reported good outcomes and 1 case of screw loosening. As no fusion was intended, no fusion outcomes were reported. All these studies suggested that longer follow up and more patients would be needed to assess the role of PEEK rods in lumbar fusion.3
Our results show a radiographic fusion rate of 86.4% and a radiographic nonunion rate of 9.9% in patients followed for at least 12 months. There was no clinical need for revision fusion at the index level. In our retrospective review, patients had high levels of smoking, DM, depression, immunosuppression, and obesity, which may negatively influence radiographic fusion rates when compared with other studies with 100% reported fusion rates. There was no instance of construct breakage or screw breakout, indicating that PEEK rods may allow enough flexibility to avoid construct failure under stress as in a fall.
Patient Reported Outcomes
Recent large studies were reviewed to assess the pre- and postoperative patient PROs reported in comparison with our study population (Table 4). In the Swedish Spine Registry analysis of 765 patients with 3 different types of lumbar fusion, the mean preoperative ODI score was 37 and mean SF-36 physical component score (PCS) was 35 for the most similar approach (posterolateral fusion with instrumentation).25 At 1 year postoperation, the mean ODI was 26 and mean SF-36 PCS was 43. In the Spine Patient Outcomes Research Trial (SPORT) spondylolisthesis trial of 3 fusion types, the mean preoperative ODI was 41.2 and mean SF-36 PF score was 31.2 for the most similar approach (posterolateral instrumented fusion with pedicle screws).26 Postoperative ODI scores at 1 year decreased by a mean 20.9 points and mean SF-36 PF scores increased by 29.9.
We report a mean preoperative SF-36 PF score of 28.9, which is lower than the SPORT study score for posterolateral fusion with instrumentation and the Swedish Study score for posterolateral instrumented fusion with pedicle screws. Similarly, our mean ODI score of 24.8 was better than the scores reported in the Swedish and SPORT studies. Our mean SF-36 PF score at 1 year postoperation was 59.3, compared with 58.5 for the SPORT study group and 46.0 in the Swedish study group. Mean ODI score at 1 year postoperatively was 14.5, which is better than the scores reported in the Swedish and SPORT studies.
Minimally clinically important difference (MCID) is a parameter used to gauge the efficacy of spine surgery. The utility of the MCID based upon PROs has been questioned in lumbar fusion surgery, as it has been thought to measure if the patient is “feeling” rather than “doing” better, the latter of which can be better measured by functional performance measures and objective, external socioeconomic anchors such as return to work and health care costs.27 Nevertheless, validated PROs are reported widely in the spine surgery literature. The MCID in the SF-36 is not well established and can depend upon whether the scores are at the extremes or more in the central range and whether there is large variability in the scores.28 Rheumatoid arthritis was estimated to be 7.1 points on the PF scale and 7.2 on the physical component summary (PCS).29 For total knee replacement, it has been estimated to be 10 points on the SF-36 PCS.30 Lumbar surgery was estimated to be 4.9 points for the SF-36 PCS and 12.8 points for the ODI.31 And the SPORT trial it has been estimated that a 30% change in the possible gain (or loss) may be an appropriate criterion.28
With a preoperative mean SF-36 PF of 28.9, a 30% improvement in the available range (70.1) would be 21 points, making our data mean improvement of 30 points above the MCID. With a mean preoperative ODI of 24.6, a 30% improvement in the available range (25.4) would be 7.6 points, making our data mean improvement of 10.3 points better than the MCID. Therefore, our outcome results are comparable with other lumbar fusion outcome studies in terms of degree of disability prior to surgery and amount of improvement from surgery.
Adjacent Segment Disease
The precise factors resulting in adjacent segment disease are not fully defined.3,32 In reviews of lumbar adjacent segment disease, reported rates ranged from 2.5% at 1 year up to 80 to 100% at 10 years, with lower rates with noninstrumented fusions.4,32-34 Annual incidence of symptomatic adjacent segment disease following lumbar fusion ranges from 0.6 to 3.9% per year.32,35,36 Mismatch between lumbar lordosis and pelvic incidence after fusion is thought to lead to higher rates of adjacent segment disease, as can a laminectomy at an adjacent segment.32,36 Percutaneous fusion techniques or use of the Wiltse approach may lower the risk of adjacent segment disease due to avoidance of facet capsule disruption.37,38
Dynamic stabilization techniques do not appear be clearly protective against adjacent segment disease, although biomechanical models suggest that they may do so.33,39,40 A review by Wang and colleagues pooled studies to assess the risk of lumbar adjacent segment disease in spinal fusion to compare to disc arthroplasty and concluded that fusion carried a higher risk of adjacent segment disease.41 Definitive data on other types of motion preservation devices is lacking.3We show 3 adjacent segment fusions and 1 laminectomy have been needed in 108 patients and at a mean of 46 months after the index procedure and over 2.5 years of mean overall follow up. This is a low adjacent segment surgery rate compared to the historical data cited above, and may suggest some advantage for PEEK rods over more rigid constructs.
Strengths and Limitations
Strengths of this study include larger numbers than prior series of PEEK rod use and use in a population with high comorbidities linked to poor results without reduction in good outcomes. PEEK rods as used at the VAPHCS do not result in higher instrumentation costs than all metal constructs.
Study limitations include the retrospective nature with loss of follow up on some patients and incomplete radiographic and PROs in some patients. The use of 100% stereotactic guidance, the avoidance of interbody devices, and the off-label use of bone morphogenetic protein as part of the fusion construct introduce additional variables that may influence comparison to other studies. To avoid unnecessary radiation exposure, flexion extension films or CT scans were not routinely obtained if patients were doing well.42 Additionally, the degree of motion on dynamic views that would differentiate pseudarthrosis from arthrodesis has not been defined.5
Conclusions
The results presented show that lumbar fusion with PEEK rods can be undertaken with short hospitalization times and low complication rates, produce satisfactory clinical improvements, and result in radiographic fusion rates similar to metal constructs. Low rates of hardware failure or need for revision surgery were found. Preliminarily results of low rates of adjacent segment surgery are comparable with previously published metal construct rates. Longer follow up is needed to confirm these findings and to investigate whether semirigid constructs truly offer some protection from adjacent segment disease when compared to all metal constructs.
Acknowledgments
The authors thank Shirley McCartney, PhD, for editorial assistance.
1. Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259-1265. doi:10.1001/jama.2010.338
2. Machado GC, Maher CG, Ferreira PH, et al. Trends, complications, and costs for hospital admission and surgery for lumbar spinal stenosis. Spine (Phila Pa 1976). 2017;42(22):1737-1743. doi:10.1097/BRS.0000000000002207
3. Li C, Liu L, Shi JY, Yan KZ, Shen WZ, Yang ZR. Clinical and biomechanical researches of polyetheretherketone (PEEK) rods for semi-rigid lumbar fusion: a systematic review. Neurosurg Rev. 2018;41(2):375-389. doi:10.1007/s10143-016-0763-2
4. Mavrogenis AF, Vottis C, Triantafyllopoulos G, Papagelopoulos PJ, Pneumaticos SG. PEEK rod systems for the spine. Eur J Orthop Surg Traumatol. 2014;24 Suppl 1:S111-S116. doi:10.1007/s00590-014-1421-4
5. Choudhri TF, Mummaneni PV, Dhall SS, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion status. J Neurosurg Spine. 2014;21(1):23-30. doi:10.3171/2014.4.SPINE14267
6. Ahn YH, Chen WM, Lee KY, Park KW, Lee SJ. Comparison of the load-sharing characteristics between pedicle-based dynamic and rigid rod devices. Biomed Mater. 2008;3(4):044101. doi:10.1088/1748-6041/3/4/044101
7. Ozer AF, Cevik OM, Erbulut DU, et al. A novel modular dynamic stabilization system for the treatment of degenerative spinal pathologies. Turk Neurosurg. 2019;29(1):115-120. doi:10.5137/1019-5149.JTN.23227-18.1
8. Hak DJ, Mauffrey C, Seligson D, Lindeque B. Use of carbon-fiber-reinforced composite implants in orthopedic surgery. Orthopedics. 2014;37(12):825-830. doi:10.3928/01477447-20141124-05
9. Gornet MF, Chan FW, Coleman JC, et al. Biomechanical assessment of a PEEK rod system for semi-rigid fixation of lumbar fusion constructs. J Biomech Eng. 2011;133(8):081009. doi:10.1115/1.4004862
10. Jackson JB 3rd, Crimaldi AJ, Peindl R, Norton HJ, Anderson WE, Patt JC. Effect of polyether ether ketone on therapeutic radiation to the spine: a pilot study. Spine (Phila Pa 1976). 2017;42(1):E1-E7. doi:10.1097/BRS.0000000000001695
11. Highsmith JM, Tumialán LM, Rodts GE Jr. Flexible rods and the case for dynamic stabilization. Neurosurg Focus. 2007;22(1):E11. Published 2007 Jan 15. doi:10.3171/foc.2007.22.1.11
12. Sengupta DK, Bucklen B, McAfee PC, Nichols J, Angara R, Khalil S. The comprehensive biomechanics and load-sharing of semirigid PEEK and semirigid posterior dynamic stabilization systems. Adv Orthop. 2013;2013:745610. doi:10.1155/2013/745610
13. Agarwal A, Ingels M, Kodigudla M, Momeni N, Goel V, Agarwal AK. Adjacent-level hypermobility and instrumented-level fatigue loosening with titanium and PEEK rods for a pedicle screw system: an in vitro study. J Biomech Eng. 2016;138(5):051004. doi:10.1115/1.4032965
14. Chou WK, Chien A, Wang JL. Biomechanical analysis between PEEK and titanium screw-rods spinal construct subjected to fatigue loading. J Spinal Disord Tech. 2015;28(3):E121-E125. doi:10.1097/BSD.0000000000000176
15. Shih KS Hsu CC, Zhou SY, Hou SM. Biomechanical investigation of pedicle screw-based posterior stabilization systems for the treatment of lumbar degenerative disc disease using finite element analyses. Biomed Eng: Appl Basis Commun. 2015;27(06):1550060. doi: 10.4015/S101623721550060X

16. Chang TK, Huang CH, Liu YC, et al. Biomechanical evaluation and comparison of polyetheretherketone rod system to traditional titanium rod fixation on adjacent levels. Formosan J Musculoskeletal Disord. 2013;4(2):42-47. doi: 10.1016/j.fjmd.2013.04.003
17. Ormond DR, Albert L Jr, Das K. Polyetheretherketone (PEEK) rods in lumbar spine degenerative disease: a case series. Clin Spine Surg. 2016;29(7):E371-E375. doi:10.1097/BSD.0b013e318277cb9b
18. Colangeli S, Barbanti Brodàno G, Gasbarrini A, et al. Polyetheretherketone (PEEK) rods: short-term results in lumbar spine degenerative disease. J Neurosurg Sci. 2015;59(2):91-96.
19. Huang W, Chang Z, Song R, Zhou K, Yu X. Non-fusion procedure using PEEK rod systems for lumbar degenerative diseases: clinical experience with a 2-year follow-up. BMC Musculoskelet Disord. 2016;17:53. Published 2016 Feb 1. doi:10.1186/s12891-016-0913-2
20. Wang C-J, Graf H, Wei H-W. Clinical outcomes of the dynamic lumbar pedicle screw-rod stabilization. Neurosurg Q. 2016;26(3):214-218. doi:10.1097/WNQ.0000000000000169
21. Kurtz SM, Lanman TH, Higgs G, et al. Retrieval analysis of PEEK rods for posterior fusion and motion preservation. Eur Spine J. 2013;22(12):2752-2759. doi:10.1007/s00586-013-2920-4
22. Athanasakopoulos M, Mavrogenis AF, Triantafyllopoulos G, Koufos S, Pneumaticos SG. Posterior spinal fusion using pedicle screws. Orthopedics. 2013;36(7):e951-e957. doi:10.3928/01477447-20130624-28
23. De Iure F, Bosco G, Cappuccio M, Paderni S, Amendola L. Posterior lumbar fusion by peek rods in degenerative spine: preliminary report on 30 cases. Eur Spine J. 2012;21 Suppl 1(Suppl 1):S50-S54. doi:10.1007/s00586-012-2219-x
24. Qi L, Li M, Zhang S, Xue J, Si H. Comparative effectiveness of PEEK rods versus titanium alloy rods in lumbar fusion: a preliminary report. Acta Neurochir (Wien). 2013;155(7):1187-1193. doi:10.1007/s00701-013-1772-3
25. Endler P, Ekman P, Möller H, Gerdhem P. Outcomes of posterolateral fusion with and without instrumentation and of interbody fusion for isthmic spondylolisthesis: a prospective study. J Bone Joint Surg Am. 2017;99(9):743-752. doi:10.2106/JBJS.16.00679
26. Abdu WA, Lurie JD, Spratt KF, et al. Degenerative spondylolisthesis: does fusion method influence outcome? Four-year results of the spine patient outcomes research trial. Spine (Phila Pa 1976). 2009;34(21):2351-2360. doi:10.1097/BRS.0b013e3181b8a829
27. Gatchel RJ, Mayer TG, Chou R. What does/should the minimum clinically important difference measure? A reconsideration of its clinical value in evaluating efficacy of lumbar fusion surgery. Clin J Pain. 2012;28(5):387-397. doi:10.1097/AJP.0b013e3182327f20
28. Spratt KF. Patient-level minimal clinically important difference based on clinical judgment and minimally detectable measurement difference: a rationale for the SF-36 physical function scale in the SPORT intervertebral disc herniation cohort. Spine (Phila Pa 1976). 2009;34(16):1722-1731. doi:10.1097/BRS.0b013e3181a8faf2
29. Ward MM, Guthrie LC, Alba MI. Clinically important changes in short form 36 health survey scales for use in rheumatoid arthritis clinical trials: the impact of low responsiveness. Arthritis Care Res (Hoboken). 2014;66(12):1783-1789. doi:10.1002/acr.22392
30. Escobar A, Quintana JM, Bilbao A, Aróstegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthritis Cartilage. 2007;15(3):273-280. doi:10.1016/j.joca.2006.09.001
31. Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008;8(6):968-974. doi:10.1016/j.spinee.2007.11.006
32. Radcliff KE, Kepler CK, Jakoi A, et al. Adjacent segment disease in the lumbar spine following different treatment interventions. Spine J. 2013;13(10):1339-1349. doi:10.1016/j.spinee.2013.03.020
33. Epstein NE. Adjacent level disease following lumbar spine surgery: a review. Surg Neurol Int. 2015;6(Suppl 24):S591-S599. Published 2015 Nov 25. doi:10.4103/2152-7806.170432
34. Epstein NE. A review: reduced reoperation rate for multilevel lumbar laminectomies with noninstrumented versus instrumented fusions. Surg Neurol Int. 2016;7(Suppl 13):S337-S346. Published 2016 May 17. doi:10.4103/2152-7806.182546
35. Scemama C, Magrino B, Gillet P, Guigui P. Risk of adjacent-segment disease requiring surgery after short lumbar fusion: results of the French Spine Surgery Society Series. J Neurosurg Spine. 2016;25(1):46-51. doi:10.3171/2015.11.SPINE15700
36. Tempel ZJ, Gandhoke GS, Bolinger BD, et al. The influence of pelvic incidence and lumbar lordosis mismatch on development of symptomatic adjacent level disease following single-level transforaminal lumbar interbody fusion. Neurosurgery. 2017;80(6):880-886. doi:10.1093/neuros/nyw073

37. Cheng YW, Chang PY, Wu JC, et al. Letter to the editor: Pedicle screw-based dynamic stabilization and adjacent-segment disease. J Neurosurg Spine. 2017;26(3):405-406. doi:10.3171/2016.7.SPINE16816
38. Street JT, Andrew Glennie R, Dea N, et al. A comparison of the Wiltse versus midline approaches in degenerative conditions of the lumbar spine. J Neurosurg Spine. 2016;25(3):332-338. doi:10.3171/2016.2.SPINE151018
39. Kuo CH, Huang WC, Wu JC, et al. Radiological adjacent-segment degeneration in L4-5 spondylolisthesis: comparison between dynamic stabilization and minimally invasive transforaminal lumbar interbody fusion. J Neurosurg Spine. 2018;29(3):250-258. doi:10.3171/2018.1.SPINE17993
40. Lee CH, Kim YE, Lee HJ, Kim DG, Kim CH. Biomechanical effects of hybrid stabilization on the risk of proximal adjacent-segment degeneration following lumbar spinal fusion using an interspinous device or a pedicle screw-based dynamic fixator. J Neurosurg Spine. 2017;27(6):643-649. doi:10.3171/2017.3.SPINE161169
41. Wang JC, Arnold PM, Hermsmeyer JT, Norvell DC. Do lumbar motion preserving devices reduce the risk of adjacent segment pathology compared with fusion surgery? A systematic review. Spine (Phila Pa 1976). 2012;37(22 Suppl):S133-S143. doi:10.1097/BRS.0b013e31826cadf2
42. Ross DA. Letter to the editor: steroid use in anterior cervical discectomy and fusion. J Neurosurg Spine. 2016;24(6):998-1000. doi:10.3171/2015.9.SPINE151052
Surgical treatment of degenerative lumbar spine disease has been rising steadily in the United States, and an increasing fraction of surgery involves lumbar fusion.1,2 Various techniques are used to accomplish a lumbar fusion, including noninstrumented fusion, anterior lumbar interbody fusion (ALIF), lateral lumbar interbody fusion (XLIF, OLIF), posterior pedicle screw fusion, posterior cortical screw fusion, posterior interbody fusion (TLIF, PLIF), and interspinous process fusion. Rigid, metallic fusion hardware provides high stability and fusion rates, but it likely leads to stress shielding and adjacent segment disease.3 There is interest in less rigid and dynamic stabilization techniques to reduce the risk of adjacent segment disease, such as polyetheretherketone (PEEK) rods, which have been available since 2007. However, literature regarding PEEK rod utility is sparse and of mixed outcomes.3,4 Additional patient reported outcome (PRO) information would be useful to both surgeons and patients. Using institutional data, this review was designed to examine our experience with PEEK rod lumbar fusion and to document PROs.
Methods
The study was approved by the institutional review board at the US Department of Veterans Affairs (VA) Portland Health Care System (VAPHCS) in Oregon with a waiver of authorization. In this retrospective, single center study, data were queried from the senior author’s (DAR) case logs from VA Computerized Patient Record System (CPRS). Electronic medical records, imaging, and PROs of all consecutive patients undergoing lumbar fusion at 1 or 2 levels with PEEK rods for degenerative disease were retrospectively reviewed. Cases of trauma, malignancy, or infection were excluded. From March 2011 through October 2019, 108 patients underwent lumbar fusion with PEEK rods.
Surgeries were conducted on a Mizuho OSI Jackson Table via bilateral 3 to 4 cm Wiltse incisions using the Medtronic Quadrant retractor system. Medtronic O-Arm images were acquired and delivered to a Medtronic Stealth Station for navigation of the screws. Monopolar coagulation was not used. PEEK pedicle screws were placed and verified with a second O-Arm spin before placing lordotic PEEK rods in the screw heads. No attempt was made to reduce any spondylolisthesis, but distraction was used to open the foramina and indirectly decompress the canal. An interbody device was placed only in treatment of multiply recurrent disc protrusion. After decortication of the transverse processes and facets, intertransverse fusion constructs consisting of calcium hydroxyapatite soaked in autologous bone marrow blood and wrapped in 6-mg bone morphogenetic protein-soaked sponges were placed on the bone. If canal decompression was indicated, a Medtronic Metrx retractor tube was then placed through one of the incisions and decompression carried out. Wounds were closed with absorbable suture. No bracing was used postoperatively. Figure 1 shows a typical single level PEEK rod fusion construct.
Patient pre- and postoperative Short Form-36 (SF-36) physical function (PF) scores and Oswestry Disability Index (ODI) scores had been obtained at routine clinic visits.
Static radiographs were used to assess the fusion. Dynamic films and/or computed tomography (CT) scans were obtained only when symptomatic pseudarthrosis was suspected. Some patients had abdominal or lumbar CT scans for other indications, and these were reviewed when available. Particular care was taken to assess facet fusion as an indicator of arthrodesis (Figure 2).5
Statistical Analysis
Pre- and postoperative pairwise t tests were completed for patients with a complete data, using SAS 9.2 statistical package. Data are presented as standard deviation (SD) of the mean.
Results
Following application of the inclusion/exclusion criteria, 108 patients had undergone lumbar fusion with PEEK rods. Mean (SD) patient age was 60.2 (10.3) years and 88 patients were male (Table 1). Most surgeries were at L5-S1 and L4-5. There were 97 single-level fusions and 11 bilevel fusions. Seventy-four procedures were for spondylolisthesis, 23 for foraminal stenosis, 5 for degenerative disc disease, 3 for coronal imbalance with foraminal stenosis, 2 for pseudarthrosis after surgery elsewhere, and 1 for multiple recurrent disc herniation (Table 2). Twenty-five patients (23.1%) were current tobacco users and 28 (25.9%) were former smokers, 26 (24.1%) had diabetes mellitus (DM), 16 (14.8%) had low bone density by dual energy X-ray absorptiometry (DEXA) imaging, 35 (32.4%) had depression, and 7 (6.5%) were taking an immunosuppressive agent (chronic steroids, biological response modifiers, or methotrexate). Mean body mass index was 30.1.
Surgical Procedure
Of the 108 patients, the first 18 underwent a procedure with fluoroscopic guidance and the Medtronic FluoroNav and Stealth Systems. The next 90 patients underwent a procedure with O-Arm intraoperative CT scanning and Stealth frameless stereotactic navigation. The mean (SD) length of stay was 1.7 (1.3) days. There were no wound infections and no new neurologic deficits. Mean (SD) follow up time was 30.3 (21.8) months.
Imaging
Final imaging was by radiograph in 73 patients, CT in 31, and magnetic resonance imaging (MRI) in 3 (1 patient had no imaging). Sixty-seven patients (62.0%) had a bilateral arthrodesis, and 15 (13.9%) had at least a unilateral arthrodesis. MRI was not used to assess arthrodesis. Eight patients (7.4%) had no definite arthrodesis. Seventeen patients had inadequate or early imaging from which a fusion determination could not be made. Of 81 patients with > 11 months of follow up, 58 (71.6%) had a bilateral arthrodesis, 12 (14.8%) had a unilateral arthrodesis, 8 (9.9%) had no arthrodesis, and 3 (3.7%) were indeterminate.
No patient had any revision fusion surgery at the index level during follow up. Two patients had adjacent level fusions at 27 and 60 months after the index procedure. One patient had a laminectomy at an adjacent segment at 18 months postfusion, and 1 had a foraminotomy at an adjacent segment 89 months post fusion (Figure 3). Overall, there were 4 (3.7%) adjacent segment surgeries at a mean of 48.5 months after surgery. One patient had a sacro-iliac joint fusion below an L5-S1 fusion 17 months prior for persisting pain after the fusion procedure.
Patient Reported Outcomes
Preoperative SF-36 PF and ODI scores were available for 81 patients (Table 3). Postoperative SF-36 PF scores were obtained at 3 months for 65 of these patients, and at 1 year for 63 patients. Postoperative ODI scores were obtained at 3 months for 65 patients, and at 1 year for 55 patients. Among the 65 patients with completed SF-36 scores at 3 months, a mean increase of 22.4 (95% CI, 17-27; P < .001) was noted, and for the 63 patients at 1 year a mean increase of 30.3 (95% CI, 25-35; P < .001) was noted. Among the 65 patients with completed ODI scores at 3 months, a mean decrease of 6.8 (95% CI, 4.9-8.6; P < .001) was noted, and for the 55 patients with completed ODI scores at 1 year a mean decrease of 10.3 (n = 55; 95% CI, 8.4-12.2; P < .001) was noted.
Cost
We compared the hardware cost of a single level construct consisting of 4 pedicle screws, 4 locking caps, and 2 rods using a PEEK system with that of 2 other titanium construct systems. At VAPHCS, the PEEK system cost was about 71% of the cost of 2 other titanium construct systems and 62% of the cost when compared with Medtronic titanium rods.
Discussion
PEEK is useful for spine and cranial implants. It is inert and fully biocompatible with a modulus of elasticity between that of cortical and cancellous bone, and much lower than that of titanium, and is therefore considered to be semirigid.3,4,6 PEEK rods are intermediate in stiffness between titanium rods (110 Gigapascals) and dynamic devices such as the Zimmer Biomet DYNESYS dynamic stabilization system or the Premia Spine TOPS system.3 Carbon fiber rods and carbon fiber reinforced PEEK implants are other semirigid rod alternatives.7,8 PEEK rods for posterior lumbar fusion surgery were introduced in 2007. Li and colleagues provide a thorough review of the biomechanical properties of PEEK rods.3
PEEK is thought to have several advantages when compared with titanium. These advantages include more physiologic load sharing and reduction in stress shielding, improved durability, reduced risk of failure in osteoporotic bone, less wear debris, no change in bone forming environment, and imaging radiolucency.4,9 Spinal PEEK cages have been reported to allow more uniform radiation dose distribution compared with metal constructs, an advantage that also may pertain to PEEK rods.10 Disadvantages of PEEK rods include an inability to detect rod breakage easily, lack of data on the use in more than minimally unstable clinical situations, and greater expense, although this was not the authors’ observation.3,4,11
Importantly, it has been reported that PEEK rods permit a greater range of motion in all planes when compared with titanium rods.9 Polyetheretherketone rods unload the bone screw interface and increased the anterior column load to a more physiologic 75% when compared with titanium rods.6,9 However, in another biomechanical study that compared titanium rods, PEEK rods, and a dynamic stabilization device, it was reported that anterior load sharing was 55%, 59%, and 75%, respectively.12 This indicated that PEEK rods are closer to metal rods than truly dynamic devices for anterior load sharing. The endurance limit of a PEEK rod construct was similar to that of clinically useful metal systems.9 PEEK rods resulted in no increase in postfatigue motion compared with titanium rods in a biomechanical model.13 Intradiscal pressures at PEEK instrumented segments were similar to uninstrumented segments and greater than those with titanium rod constructs.14 Intradiscal pressures at adjacent segments were highest with dynamic devices, intermediate with semirigid rods, and lowest with rigid constructs; however, stress values at adjacent segments were lower in PEEK than titanium constructs in any direction of motion.15,16
Fusion Rates
The use of PEEK rods in lumbar fusion has been reported previously.3,4,17,18 However, these studies featured small sample sizes, short follow up times, and contradictory results.4 Of 8 outcome reports found in a systematic review, 2 studies reported on procedures designed to create nonfusion outcomes (a third similar trial from 2013 was not included in the systematic review), and 1 study reported only on the condition of PEEK rods removed at subsequent surgery.3,19-21 Reported fusion rates varied from 86 to 100%.
In 42 patients with PEEK rod fusions who were followed for a mean of 31.4 months, 5 patients required adjacent segment surgery and 3 patients were treated for interbody cage migration and nonunion.17 Radiographic fusion rate was 86%. These authors concluded that PEEK rod fusion results were similar to those of other constructs, but not better, or perhaps worse than, metal rods.
Other studies have reported better results with PEEK.11,18,19,22-24 Highsmith and colleagues reported on 3 successful example cases of the use of PEEK rods.11 De Iure and colleagues reported on 30 cases up to 5 levels (mean, 2.9) using autograft bone, with a mean follow up of 18 months.23 Results were reported as satisfactory. Three patients had radiographic nonunions, 1 of which required revision for asymptomatic screw loosening at the cranial end of the construct. Qi and colleagues, reported on 20 patients with PEEK rods compared to 21 patients with titanium alloy rods.24 Both groups had similar clinical outcomes, structural parameters, and 100% fusion rates. Athanasakopoulos and colleagues reported on 52 patients with up to 3 level fusions followed for a mean of 3 years.22 There were significant improvements in PROs: at 1 year 96% had radiographic union. Two patients had screw breakage, 1 of whom required revision to a metal rod construct. Colangeli and colleagues reported on 12 patients treated with PEEK rods compared with 12 who were treated with a dynamic system.18 They reported significant improvements, no complications, and 100% fusion at 6 months. Huang and colleagues reported on 38 patients intended to undergo a nonfusion procedure with 2 years of follow up.19 They reported good outcomes and 1 case of screw loosening. As no fusion was intended, no fusion outcomes were reported. All these studies suggested that longer follow up and more patients would be needed to assess the role of PEEK rods in lumbar fusion.3
Our results show a radiographic fusion rate of 86.4% and a radiographic nonunion rate of 9.9% in patients followed for at least 12 months. There was no clinical need for revision fusion at the index level. In our retrospective review, patients had high levels of smoking, DM, depression, immunosuppression, and obesity, which may negatively influence radiographic fusion rates when compared with other studies with 100% reported fusion rates. There was no instance of construct breakage or screw breakout, indicating that PEEK rods may allow enough flexibility to avoid construct failure under stress as in a fall.
Patient Reported Outcomes
Recent large studies were reviewed to assess the pre- and postoperative patient PROs reported in comparison with our study population (Table 4). In the Swedish Spine Registry analysis of 765 patients with 3 different types of lumbar fusion, the mean preoperative ODI score was 37 and mean SF-36 physical component score (PCS) was 35 for the most similar approach (posterolateral fusion with instrumentation).25 At 1 year postoperation, the mean ODI was 26 and mean SF-36 PCS was 43. In the Spine Patient Outcomes Research Trial (SPORT) spondylolisthesis trial of 3 fusion types, the mean preoperative ODI was 41.2 and mean SF-36 PF score was 31.2 for the most similar approach (posterolateral instrumented fusion with pedicle screws).26 Postoperative ODI scores at 1 year decreased by a mean 20.9 points and mean SF-36 PF scores increased by 29.9.
We report a mean preoperative SF-36 PF score of 28.9, which is lower than the SPORT study score for posterolateral fusion with instrumentation and the Swedish Study score for posterolateral instrumented fusion with pedicle screws. Similarly, our mean ODI score of 24.8 was better than the scores reported in the Swedish and SPORT studies. Our mean SF-36 PF score at 1 year postoperation was 59.3, compared with 58.5 for the SPORT study group and 46.0 in the Swedish study group. Mean ODI score at 1 year postoperatively was 14.5, which is better than the scores reported in the Swedish and SPORT studies.
Minimally clinically important difference (MCID) is a parameter used to gauge the efficacy of spine surgery. The utility of the MCID based upon PROs has been questioned in lumbar fusion surgery, as it has been thought to measure if the patient is “feeling” rather than “doing” better, the latter of which can be better measured by functional performance measures and objective, external socioeconomic anchors such as return to work and health care costs.27 Nevertheless, validated PROs are reported widely in the spine surgery literature. The MCID in the SF-36 is not well established and can depend upon whether the scores are at the extremes or more in the central range and whether there is large variability in the scores.28 Rheumatoid arthritis was estimated to be 7.1 points on the PF scale and 7.2 on the physical component summary (PCS).29 For total knee replacement, it has been estimated to be 10 points on the SF-36 PCS.30 Lumbar surgery was estimated to be 4.9 points for the SF-36 PCS and 12.8 points for the ODI.31 And the SPORT trial it has been estimated that a 30% change in the possible gain (or loss) may be an appropriate criterion.28
With a preoperative mean SF-36 PF of 28.9, a 30% improvement in the available range (70.1) would be 21 points, making our data mean improvement of 30 points above the MCID. With a mean preoperative ODI of 24.6, a 30% improvement in the available range (25.4) would be 7.6 points, making our data mean improvement of 10.3 points better than the MCID. Therefore, our outcome results are comparable with other lumbar fusion outcome studies in terms of degree of disability prior to surgery and amount of improvement from surgery.
Adjacent Segment Disease
The precise factors resulting in adjacent segment disease are not fully defined.3,32 In reviews of lumbar adjacent segment disease, reported rates ranged from 2.5% at 1 year up to 80 to 100% at 10 years, with lower rates with noninstrumented fusions.4,32-34 Annual incidence of symptomatic adjacent segment disease following lumbar fusion ranges from 0.6 to 3.9% per year.32,35,36 Mismatch between lumbar lordosis and pelvic incidence after fusion is thought to lead to higher rates of adjacent segment disease, as can a laminectomy at an adjacent segment.32,36 Percutaneous fusion techniques or use of the Wiltse approach may lower the risk of adjacent segment disease due to avoidance of facet capsule disruption.37,38
Dynamic stabilization techniques do not appear be clearly protective against adjacent segment disease, although biomechanical models suggest that they may do so.33,39,40 A review by Wang and colleagues pooled studies to assess the risk of lumbar adjacent segment disease in spinal fusion to compare to disc arthroplasty and concluded that fusion carried a higher risk of adjacent segment disease.41 Definitive data on other types of motion preservation devices is lacking.3We show 3 adjacent segment fusions and 1 laminectomy have been needed in 108 patients and at a mean of 46 months after the index procedure and over 2.5 years of mean overall follow up. This is a low adjacent segment surgery rate compared to the historical data cited above, and may suggest some advantage for PEEK rods over more rigid constructs.
Strengths and Limitations
Strengths of this study include larger numbers than prior series of PEEK rod use and use in a population with high comorbidities linked to poor results without reduction in good outcomes. PEEK rods as used at the VAPHCS do not result in higher instrumentation costs than all metal constructs.
Study limitations include the retrospective nature with loss of follow up on some patients and incomplete radiographic and PROs in some patients. The use of 100% stereotactic guidance, the avoidance of interbody devices, and the off-label use of bone morphogenetic protein as part of the fusion construct introduce additional variables that may influence comparison to other studies. To avoid unnecessary radiation exposure, flexion extension films or CT scans were not routinely obtained if patients were doing well.42 Additionally, the degree of motion on dynamic views that would differentiate pseudarthrosis from arthrodesis has not been defined.5
Conclusions
The results presented show that lumbar fusion with PEEK rods can be undertaken with short hospitalization times and low complication rates, produce satisfactory clinical improvements, and result in radiographic fusion rates similar to metal constructs. Low rates of hardware failure or need for revision surgery were found. Preliminarily results of low rates of adjacent segment surgery are comparable with previously published metal construct rates. Longer follow up is needed to confirm these findings and to investigate whether semirigid constructs truly offer some protection from adjacent segment disease when compared to all metal constructs.
Acknowledgments
The authors thank Shirley McCartney, PhD, for editorial assistance.
Surgical treatment of degenerative lumbar spine disease has been rising steadily in the United States, and an increasing fraction of surgery involves lumbar fusion.1,2 Various techniques are used to accomplish a lumbar fusion, including noninstrumented fusion, anterior lumbar interbody fusion (ALIF), lateral lumbar interbody fusion (XLIF, OLIF), posterior pedicle screw fusion, posterior cortical screw fusion, posterior interbody fusion (TLIF, PLIF), and interspinous process fusion. Rigid, metallic fusion hardware provides high stability and fusion rates, but it likely leads to stress shielding and adjacent segment disease.3 There is interest in less rigid and dynamic stabilization techniques to reduce the risk of adjacent segment disease, such as polyetheretherketone (PEEK) rods, which have been available since 2007. However, literature regarding PEEK rod utility is sparse and of mixed outcomes.3,4 Additional patient reported outcome (PRO) information would be useful to both surgeons and patients. Using institutional data, this review was designed to examine our experience with PEEK rod lumbar fusion and to document PROs.
Methods
The study was approved by the institutional review board at the US Department of Veterans Affairs (VA) Portland Health Care System (VAPHCS) in Oregon with a waiver of authorization. In this retrospective, single center study, data were queried from the senior author’s (DAR) case logs from VA Computerized Patient Record System (CPRS). Electronic medical records, imaging, and PROs of all consecutive patients undergoing lumbar fusion at 1 or 2 levels with PEEK rods for degenerative disease were retrospectively reviewed. Cases of trauma, malignancy, or infection were excluded. From March 2011 through October 2019, 108 patients underwent lumbar fusion with PEEK rods.
Surgeries were conducted on a Mizuho OSI Jackson Table via bilateral 3 to 4 cm Wiltse incisions using the Medtronic Quadrant retractor system. Medtronic O-Arm images were acquired and delivered to a Medtronic Stealth Station for navigation of the screws. Monopolar coagulation was not used. PEEK pedicle screws were placed and verified with a second O-Arm spin before placing lordotic PEEK rods in the screw heads. No attempt was made to reduce any spondylolisthesis, but distraction was used to open the foramina and indirectly decompress the canal. An interbody device was placed only in treatment of multiply recurrent disc protrusion. After decortication of the transverse processes and facets, intertransverse fusion constructs consisting of calcium hydroxyapatite soaked in autologous bone marrow blood and wrapped in 6-mg bone morphogenetic protein-soaked sponges were placed on the bone. If canal decompression was indicated, a Medtronic Metrx retractor tube was then placed through one of the incisions and decompression carried out. Wounds were closed with absorbable suture. No bracing was used postoperatively. Figure 1 shows a typical single level PEEK rod fusion construct.
Patient pre- and postoperative Short Form-36 (SF-36) physical function (PF) scores and Oswestry Disability Index (ODI) scores had been obtained at routine clinic visits.
Static radiographs were used to assess the fusion. Dynamic films and/or computed tomography (CT) scans were obtained only when symptomatic pseudarthrosis was suspected. Some patients had abdominal or lumbar CT scans for other indications, and these were reviewed when available. Particular care was taken to assess facet fusion as an indicator of arthrodesis (Figure 2).5
Statistical Analysis
Pre- and postoperative pairwise t tests were completed for patients with a complete data, using SAS 9.2 statistical package. Data are presented as standard deviation (SD) of the mean.
Results
Following application of the inclusion/exclusion criteria, 108 patients had undergone lumbar fusion with PEEK rods. Mean (SD) patient age was 60.2 (10.3) years and 88 patients were male (Table 1). Most surgeries were at L5-S1 and L4-5. There were 97 single-level fusions and 11 bilevel fusions. Seventy-four procedures were for spondylolisthesis, 23 for foraminal stenosis, 5 for degenerative disc disease, 3 for coronal imbalance with foraminal stenosis, 2 for pseudarthrosis after surgery elsewhere, and 1 for multiple recurrent disc herniation (Table 2). Twenty-five patients (23.1%) were current tobacco users and 28 (25.9%) were former smokers, 26 (24.1%) had diabetes mellitus (DM), 16 (14.8%) had low bone density by dual energy X-ray absorptiometry (DEXA) imaging, 35 (32.4%) had depression, and 7 (6.5%) were taking an immunosuppressive agent (chronic steroids, biological response modifiers, or methotrexate). Mean body mass index was 30.1.
Surgical Procedure
Of the 108 patients, the first 18 underwent a procedure with fluoroscopic guidance and the Medtronic FluoroNav and Stealth Systems. The next 90 patients underwent a procedure with O-Arm intraoperative CT scanning and Stealth frameless stereotactic navigation. The mean (SD) length of stay was 1.7 (1.3) days. There were no wound infections and no new neurologic deficits. Mean (SD) follow up time was 30.3 (21.8) months.
Imaging
Final imaging was by radiograph in 73 patients, CT in 31, and magnetic resonance imaging (MRI) in 3 (1 patient had no imaging). Sixty-seven patients (62.0%) had a bilateral arthrodesis, and 15 (13.9%) had at least a unilateral arthrodesis. MRI was not used to assess arthrodesis. Eight patients (7.4%) had no definite arthrodesis. Seventeen patients had inadequate or early imaging from which a fusion determination could not be made. Of 81 patients with > 11 months of follow up, 58 (71.6%) had a bilateral arthrodesis, 12 (14.8%) had a unilateral arthrodesis, 8 (9.9%) had no arthrodesis, and 3 (3.7%) were indeterminate.
No patient had any revision fusion surgery at the index level during follow up. Two patients had adjacent level fusions at 27 and 60 months after the index procedure. One patient had a laminectomy at an adjacent segment at 18 months postfusion, and 1 had a foraminotomy at an adjacent segment 89 months post fusion (Figure 3). Overall, there were 4 (3.7%) adjacent segment surgeries at a mean of 48.5 months after surgery. One patient had a sacro-iliac joint fusion below an L5-S1 fusion 17 months prior for persisting pain after the fusion procedure.
Patient Reported Outcomes
Preoperative SF-36 PF and ODI scores were available for 81 patients (Table 3). Postoperative SF-36 PF scores were obtained at 3 months for 65 of these patients, and at 1 year for 63 patients. Postoperative ODI scores were obtained at 3 months for 65 patients, and at 1 year for 55 patients. Among the 65 patients with completed SF-36 scores at 3 months, a mean increase of 22.4 (95% CI, 17-27; P < .001) was noted, and for the 63 patients at 1 year a mean increase of 30.3 (95% CI, 25-35; P < .001) was noted. Among the 65 patients with completed ODI scores at 3 months, a mean decrease of 6.8 (95% CI, 4.9-8.6; P < .001) was noted, and for the 55 patients with completed ODI scores at 1 year a mean decrease of 10.3 (n = 55; 95% CI, 8.4-12.2; P < .001) was noted.
Cost
We compared the hardware cost of a single level construct consisting of 4 pedicle screws, 4 locking caps, and 2 rods using a PEEK system with that of 2 other titanium construct systems. At VAPHCS, the PEEK system cost was about 71% of the cost of 2 other titanium construct systems and 62% of the cost when compared with Medtronic titanium rods.
Discussion
PEEK is useful for spine and cranial implants. It is inert and fully biocompatible with a modulus of elasticity between that of cortical and cancellous bone, and much lower than that of titanium, and is therefore considered to be semirigid.3,4,6 PEEK rods are intermediate in stiffness between titanium rods (110 Gigapascals) and dynamic devices such as the Zimmer Biomet DYNESYS dynamic stabilization system or the Premia Spine TOPS system.3 Carbon fiber rods and carbon fiber reinforced PEEK implants are other semirigid rod alternatives.7,8 PEEK rods for posterior lumbar fusion surgery were introduced in 2007. Li and colleagues provide a thorough review of the biomechanical properties of PEEK rods.3
PEEK is thought to have several advantages when compared with titanium. These advantages include more physiologic load sharing and reduction in stress shielding, improved durability, reduced risk of failure in osteoporotic bone, less wear debris, no change in bone forming environment, and imaging radiolucency.4,9 Spinal PEEK cages have been reported to allow more uniform radiation dose distribution compared with metal constructs, an advantage that also may pertain to PEEK rods.10 Disadvantages of PEEK rods include an inability to detect rod breakage easily, lack of data on the use in more than minimally unstable clinical situations, and greater expense, although this was not the authors’ observation.3,4,11
Importantly, it has been reported that PEEK rods permit a greater range of motion in all planes when compared with titanium rods.9 Polyetheretherketone rods unload the bone screw interface and increased the anterior column load to a more physiologic 75% when compared with titanium rods.6,9 However, in another biomechanical study that compared titanium rods, PEEK rods, and a dynamic stabilization device, it was reported that anterior load sharing was 55%, 59%, and 75%, respectively.12 This indicated that PEEK rods are closer to metal rods than truly dynamic devices for anterior load sharing. The endurance limit of a PEEK rod construct was similar to that of clinically useful metal systems.9 PEEK rods resulted in no increase in postfatigue motion compared with titanium rods in a biomechanical model.13 Intradiscal pressures at PEEK instrumented segments were similar to uninstrumented segments and greater than those with titanium rod constructs.14 Intradiscal pressures at adjacent segments were highest with dynamic devices, intermediate with semirigid rods, and lowest with rigid constructs; however, stress values at adjacent segments were lower in PEEK than titanium constructs in any direction of motion.15,16
Fusion Rates
The use of PEEK rods in lumbar fusion has been reported previously.3,4,17,18 However, these studies featured small sample sizes, short follow up times, and contradictory results.4 Of 8 outcome reports found in a systematic review, 2 studies reported on procedures designed to create nonfusion outcomes (a third similar trial from 2013 was not included in the systematic review), and 1 study reported only on the condition of PEEK rods removed at subsequent surgery.3,19-21 Reported fusion rates varied from 86 to 100%.
In 42 patients with PEEK rod fusions who were followed for a mean of 31.4 months, 5 patients required adjacent segment surgery and 3 patients were treated for interbody cage migration and nonunion.17 Radiographic fusion rate was 86%. These authors concluded that PEEK rod fusion results were similar to those of other constructs, but not better, or perhaps worse than, metal rods.
Other studies have reported better results with PEEK.11,18,19,22-24 Highsmith and colleagues reported on 3 successful example cases of the use of PEEK rods.11 De Iure and colleagues reported on 30 cases up to 5 levels (mean, 2.9) using autograft bone, with a mean follow up of 18 months.23 Results were reported as satisfactory. Three patients had radiographic nonunions, 1 of which required revision for asymptomatic screw loosening at the cranial end of the construct. Qi and colleagues, reported on 20 patients with PEEK rods compared to 21 patients with titanium alloy rods.24 Both groups had similar clinical outcomes, structural parameters, and 100% fusion rates. Athanasakopoulos and colleagues reported on 52 patients with up to 3 level fusions followed for a mean of 3 years.22 There were significant improvements in PROs: at 1 year 96% had radiographic union. Two patients had screw breakage, 1 of whom required revision to a metal rod construct. Colangeli and colleagues reported on 12 patients treated with PEEK rods compared with 12 who were treated with a dynamic system.18 They reported significant improvements, no complications, and 100% fusion at 6 months. Huang and colleagues reported on 38 patients intended to undergo a nonfusion procedure with 2 years of follow up.19 They reported good outcomes and 1 case of screw loosening. As no fusion was intended, no fusion outcomes were reported. All these studies suggested that longer follow up and more patients would be needed to assess the role of PEEK rods in lumbar fusion.3
Our results show a radiographic fusion rate of 86.4% and a radiographic nonunion rate of 9.9% in patients followed for at least 12 months. There was no clinical need for revision fusion at the index level. In our retrospective review, patients had high levels of smoking, DM, depression, immunosuppression, and obesity, which may negatively influence radiographic fusion rates when compared with other studies with 100% reported fusion rates. There was no instance of construct breakage or screw breakout, indicating that PEEK rods may allow enough flexibility to avoid construct failure under stress as in a fall.
Patient Reported Outcomes
Recent large studies were reviewed to assess the pre- and postoperative patient PROs reported in comparison with our study population (Table 4). In the Swedish Spine Registry analysis of 765 patients with 3 different types of lumbar fusion, the mean preoperative ODI score was 37 and mean SF-36 physical component score (PCS) was 35 for the most similar approach (posterolateral fusion with instrumentation).25 At 1 year postoperation, the mean ODI was 26 and mean SF-36 PCS was 43. In the Spine Patient Outcomes Research Trial (SPORT) spondylolisthesis trial of 3 fusion types, the mean preoperative ODI was 41.2 and mean SF-36 PF score was 31.2 for the most similar approach (posterolateral instrumented fusion with pedicle screws).26 Postoperative ODI scores at 1 year decreased by a mean 20.9 points and mean SF-36 PF scores increased by 29.9.
We report a mean preoperative SF-36 PF score of 28.9, which is lower than the SPORT study score for posterolateral fusion with instrumentation and the Swedish Study score for posterolateral instrumented fusion with pedicle screws. Similarly, our mean ODI score of 24.8 was better than the scores reported in the Swedish and SPORT studies. Our mean SF-36 PF score at 1 year postoperation was 59.3, compared with 58.5 for the SPORT study group and 46.0 in the Swedish study group. Mean ODI score at 1 year postoperatively was 14.5, which is better than the scores reported in the Swedish and SPORT studies.
Minimally clinically important difference (MCID) is a parameter used to gauge the efficacy of spine surgery. The utility of the MCID based upon PROs has been questioned in lumbar fusion surgery, as it has been thought to measure if the patient is “feeling” rather than “doing” better, the latter of which can be better measured by functional performance measures and objective, external socioeconomic anchors such as return to work and health care costs.27 Nevertheless, validated PROs are reported widely in the spine surgery literature. The MCID in the SF-36 is not well established and can depend upon whether the scores are at the extremes or more in the central range and whether there is large variability in the scores.28 Rheumatoid arthritis was estimated to be 7.1 points on the PF scale and 7.2 on the physical component summary (PCS).29 For total knee replacement, it has been estimated to be 10 points on the SF-36 PCS.30 Lumbar surgery was estimated to be 4.9 points for the SF-36 PCS and 12.8 points for the ODI.31 And the SPORT trial it has been estimated that a 30% change in the possible gain (or loss) may be an appropriate criterion.28
With a preoperative mean SF-36 PF of 28.9, a 30% improvement in the available range (70.1) would be 21 points, making our data mean improvement of 30 points above the MCID. With a mean preoperative ODI of 24.6, a 30% improvement in the available range (25.4) would be 7.6 points, making our data mean improvement of 10.3 points better than the MCID. Therefore, our outcome results are comparable with other lumbar fusion outcome studies in terms of degree of disability prior to surgery and amount of improvement from surgery.
Adjacent Segment Disease
The precise factors resulting in adjacent segment disease are not fully defined.3,32 In reviews of lumbar adjacent segment disease, reported rates ranged from 2.5% at 1 year up to 80 to 100% at 10 years, with lower rates with noninstrumented fusions.4,32-34 Annual incidence of symptomatic adjacent segment disease following lumbar fusion ranges from 0.6 to 3.9% per year.32,35,36 Mismatch between lumbar lordosis and pelvic incidence after fusion is thought to lead to higher rates of adjacent segment disease, as can a laminectomy at an adjacent segment.32,36 Percutaneous fusion techniques or use of the Wiltse approach may lower the risk of adjacent segment disease due to avoidance of facet capsule disruption.37,38
Dynamic stabilization techniques do not appear be clearly protective against adjacent segment disease, although biomechanical models suggest that they may do so.33,39,40 A review by Wang and colleagues pooled studies to assess the risk of lumbar adjacent segment disease in spinal fusion to compare to disc arthroplasty and concluded that fusion carried a higher risk of adjacent segment disease.41 Definitive data on other types of motion preservation devices is lacking.3We show 3 adjacent segment fusions and 1 laminectomy have been needed in 108 patients and at a mean of 46 months after the index procedure and over 2.5 years of mean overall follow up. This is a low adjacent segment surgery rate compared to the historical data cited above, and may suggest some advantage for PEEK rods over more rigid constructs.
Strengths and Limitations
Strengths of this study include larger numbers than prior series of PEEK rod use and use in a population with high comorbidities linked to poor results without reduction in good outcomes. PEEK rods as used at the VAPHCS do not result in higher instrumentation costs than all metal constructs.
Study limitations include the retrospective nature with loss of follow up on some patients and incomplete radiographic and PROs in some patients. The use of 100% stereotactic guidance, the avoidance of interbody devices, and the off-label use of bone morphogenetic protein as part of the fusion construct introduce additional variables that may influence comparison to other studies. To avoid unnecessary radiation exposure, flexion extension films or CT scans were not routinely obtained if patients were doing well.42 Additionally, the degree of motion on dynamic views that would differentiate pseudarthrosis from arthrodesis has not been defined.5
Conclusions
The results presented show that lumbar fusion with PEEK rods can be undertaken with short hospitalization times and low complication rates, produce satisfactory clinical improvements, and result in radiographic fusion rates similar to metal constructs. Low rates of hardware failure or need for revision surgery were found. Preliminarily results of low rates of adjacent segment surgery are comparable with previously published metal construct rates. Longer follow up is needed to confirm these findings and to investigate whether semirigid constructs truly offer some protection from adjacent segment disease when compared to all metal constructs.
Acknowledgments
The authors thank Shirley McCartney, PhD, for editorial assistance.
1. Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259-1265. doi:10.1001/jama.2010.338
2. Machado GC, Maher CG, Ferreira PH, et al. Trends, complications, and costs for hospital admission and surgery for lumbar spinal stenosis. Spine (Phila Pa 1976). 2017;42(22):1737-1743. doi:10.1097/BRS.0000000000002207
3. Li C, Liu L, Shi JY, Yan KZ, Shen WZ, Yang ZR. Clinical and biomechanical researches of polyetheretherketone (PEEK) rods for semi-rigid lumbar fusion: a systematic review. Neurosurg Rev. 2018;41(2):375-389. doi:10.1007/s10143-016-0763-2
4. Mavrogenis AF, Vottis C, Triantafyllopoulos G, Papagelopoulos PJ, Pneumaticos SG. PEEK rod systems for the spine. Eur J Orthop Surg Traumatol. 2014;24 Suppl 1:S111-S116. doi:10.1007/s00590-014-1421-4
5. Choudhri TF, Mummaneni PV, Dhall SS, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion status. J Neurosurg Spine. 2014;21(1):23-30. doi:10.3171/2014.4.SPINE14267
6. Ahn YH, Chen WM, Lee KY, Park KW, Lee SJ. Comparison of the load-sharing characteristics between pedicle-based dynamic and rigid rod devices. Biomed Mater. 2008;3(4):044101. doi:10.1088/1748-6041/3/4/044101
7. Ozer AF, Cevik OM, Erbulut DU, et al. A novel modular dynamic stabilization system for the treatment of degenerative spinal pathologies. Turk Neurosurg. 2019;29(1):115-120. doi:10.5137/1019-5149.JTN.23227-18.1
8. Hak DJ, Mauffrey C, Seligson D, Lindeque B. Use of carbon-fiber-reinforced composite implants in orthopedic surgery. Orthopedics. 2014;37(12):825-830. doi:10.3928/01477447-20141124-05
9. Gornet MF, Chan FW, Coleman JC, et al. Biomechanical assessment of a PEEK rod system for semi-rigid fixation of lumbar fusion constructs. J Biomech Eng. 2011;133(8):081009. doi:10.1115/1.4004862
10. Jackson JB 3rd, Crimaldi AJ, Peindl R, Norton HJ, Anderson WE, Patt JC. Effect of polyether ether ketone on therapeutic radiation to the spine: a pilot study. Spine (Phila Pa 1976). 2017;42(1):E1-E7. doi:10.1097/BRS.0000000000001695
11. Highsmith JM, Tumialán LM, Rodts GE Jr. Flexible rods and the case for dynamic stabilization. Neurosurg Focus. 2007;22(1):E11. Published 2007 Jan 15. doi:10.3171/foc.2007.22.1.11
12. Sengupta DK, Bucklen B, McAfee PC, Nichols J, Angara R, Khalil S. The comprehensive biomechanics and load-sharing of semirigid PEEK and semirigid posterior dynamic stabilization systems. Adv Orthop. 2013;2013:745610. doi:10.1155/2013/745610
13. Agarwal A, Ingels M, Kodigudla M, Momeni N, Goel V, Agarwal AK. Adjacent-level hypermobility and instrumented-level fatigue loosening with titanium and PEEK rods for a pedicle screw system: an in vitro study. J Biomech Eng. 2016;138(5):051004. doi:10.1115/1.4032965
14. Chou WK, Chien A, Wang JL. Biomechanical analysis between PEEK and titanium screw-rods spinal construct subjected to fatigue loading. J Spinal Disord Tech. 2015;28(3):E121-E125. doi:10.1097/BSD.0000000000000176
15. Shih KS Hsu CC, Zhou SY, Hou SM. Biomechanical investigation of pedicle screw-based posterior stabilization systems for the treatment of lumbar degenerative disc disease using finite element analyses. Biomed Eng: Appl Basis Commun. 2015;27(06):1550060. doi: 10.4015/S101623721550060X

16. Chang TK, Huang CH, Liu YC, et al. Biomechanical evaluation and comparison of polyetheretherketone rod system to traditional titanium rod fixation on adjacent levels. Formosan J Musculoskeletal Disord. 2013;4(2):42-47. doi: 10.1016/j.fjmd.2013.04.003
17. Ormond DR, Albert L Jr, Das K. Polyetheretherketone (PEEK) rods in lumbar spine degenerative disease: a case series. Clin Spine Surg. 2016;29(7):E371-E375. doi:10.1097/BSD.0b013e318277cb9b
18. Colangeli S, Barbanti Brodàno G, Gasbarrini A, et al. Polyetheretherketone (PEEK) rods: short-term results in lumbar spine degenerative disease. J Neurosurg Sci. 2015;59(2):91-96.
19. Huang W, Chang Z, Song R, Zhou K, Yu X. Non-fusion procedure using PEEK rod systems for lumbar degenerative diseases: clinical experience with a 2-year follow-up. BMC Musculoskelet Disord. 2016;17:53. Published 2016 Feb 1. doi:10.1186/s12891-016-0913-2
20. Wang C-J, Graf H, Wei H-W. Clinical outcomes of the dynamic lumbar pedicle screw-rod stabilization. Neurosurg Q. 2016;26(3):214-218. doi:10.1097/WNQ.0000000000000169
21. Kurtz SM, Lanman TH, Higgs G, et al. Retrieval analysis of PEEK rods for posterior fusion and motion preservation. Eur Spine J. 2013;22(12):2752-2759. doi:10.1007/s00586-013-2920-4
22. Athanasakopoulos M, Mavrogenis AF, Triantafyllopoulos G, Koufos S, Pneumaticos SG. Posterior spinal fusion using pedicle screws. Orthopedics. 2013;36(7):e951-e957. doi:10.3928/01477447-20130624-28
23. De Iure F, Bosco G, Cappuccio M, Paderni S, Amendola L. Posterior lumbar fusion by peek rods in degenerative spine: preliminary report on 30 cases. Eur Spine J. 2012;21 Suppl 1(Suppl 1):S50-S54. doi:10.1007/s00586-012-2219-x
24. Qi L, Li M, Zhang S, Xue J, Si H. Comparative effectiveness of PEEK rods versus titanium alloy rods in lumbar fusion: a preliminary report. Acta Neurochir (Wien). 2013;155(7):1187-1193. doi:10.1007/s00701-013-1772-3
25. Endler P, Ekman P, Möller H, Gerdhem P. Outcomes of posterolateral fusion with and without instrumentation and of interbody fusion for isthmic spondylolisthesis: a prospective study. J Bone Joint Surg Am. 2017;99(9):743-752. doi:10.2106/JBJS.16.00679
26. Abdu WA, Lurie JD, Spratt KF, et al. Degenerative spondylolisthesis: does fusion method influence outcome? Four-year results of the spine patient outcomes research trial. Spine (Phila Pa 1976). 2009;34(21):2351-2360. doi:10.1097/BRS.0b013e3181b8a829
27. Gatchel RJ, Mayer TG, Chou R. What does/should the minimum clinically important difference measure? A reconsideration of its clinical value in evaluating efficacy of lumbar fusion surgery. Clin J Pain. 2012;28(5):387-397. doi:10.1097/AJP.0b013e3182327f20
28. Spratt KF. Patient-level minimal clinically important difference based on clinical judgment and minimally detectable measurement difference: a rationale for the SF-36 physical function scale in the SPORT intervertebral disc herniation cohort. Spine (Phila Pa 1976). 2009;34(16):1722-1731. doi:10.1097/BRS.0b013e3181a8faf2
29. Ward MM, Guthrie LC, Alba MI. Clinically important changes in short form 36 health survey scales for use in rheumatoid arthritis clinical trials: the impact of low responsiveness. Arthritis Care Res (Hoboken). 2014;66(12):1783-1789. doi:10.1002/acr.22392
30. Escobar A, Quintana JM, Bilbao A, Aróstegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthritis Cartilage. 2007;15(3):273-280. doi:10.1016/j.joca.2006.09.001
31. Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008;8(6):968-974. doi:10.1016/j.spinee.2007.11.006
32. Radcliff KE, Kepler CK, Jakoi A, et al. Adjacent segment disease in the lumbar spine following different treatment interventions. Spine J. 2013;13(10):1339-1349. doi:10.1016/j.spinee.2013.03.020
33. Epstein NE. Adjacent level disease following lumbar spine surgery: a review. Surg Neurol Int. 2015;6(Suppl 24):S591-S599. Published 2015 Nov 25. doi:10.4103/2152-7806.170432
34. Epstein NE. A review: reduced reoperation rate for multilevel lumbar laminectomies with noninstrumented versus instrumented fusions. Surg Neurol Int. 2016;7(Suppl 13):S337-S346. Published 2016 May 17. doi:10.4103/2152-7806.182546
35. Scemama C, Magrino B, Gillet P, Guigui P. Risk of adjacent-segment disease requiring surgery after short lumbar fusion: results of the French Spine Surgery Society Series. J Neurosurg Spine. 2016;25(1):46-51. doi:10.3171/2015.11.SPINE15700
36. Tempel ZJ, Gandhoke GS, Bolinger BD, et al. The influence of pelvic incidence and lumbar lordosis mismatch on development of symptomatic adjacent level disease following single-level transforaminal lumbar interbody fusion. Neurosurgery. 2017;80(6):880-886. doi:10.1093/neuros/nyw073

37. Cheng YW, Chang PY, Wu JC, et al. Letter to the editor: Pedicle screw-based dynamic stabilization and adjacent-segment disease. J Neurosurg Spine. 2017;26(3):405-406. doi:10.3171/2016.7.SPINE16816
38. Street JT, Andrew Glennie R, Dea N, et al. A comparison of the Wiltse versus midline approaches in degenerative conditions of the lumbar spine. J Neurosurg Spine. 2016;25(3):332-338. doi:10.3171/2016.2.SPINE151018
39. Kuo CH, Huang WC, Wu JC, et al. Radiological adjacent-segment degeneration in L4-5 spondylolisthesis: comparison between dynamic stabilization and minimally invasive transforaminal lumbar interbody fusion. J Neurosurg Spine. 2018;29(3):250-258. doi:10.3171/2018.1.SPINE17993
40. Lee CH, Kim YE, Lee HJ, Kim DG, Kim CH. Biomechanical effects of hybrid stabilization on the risk of proximal adjacent-segment degeneration following lumbar spinal fusion using an interspinous device or a pedicle screw-based dynamic fixator. J Neurosurg Spine. 2017;27(6):643-649. doi:10.3171/2017.3.SPINE161169
41. Wang JC, Arnold PM, Hermsmeyer JT, Norvell DC. Do lumbar motion preserving devices reduce the risk of adjacent segment pathology compared with fusion surgery? A systematic review. Spine (Phila Pa 1976). 2012;37(22 Suppl):S133-S143. doi:10.1097/BRS.0b013e31826cadf2
42. Ross DA. Letter to the editor: steroid use in anterior cervical discectomy and fusion. J Neurosurg Spine. 2016;24(6):998-1000. doi:10.3171/2015.9.SPINE151052
1. Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259-1265. doi:10.1001/jama.2010.338
2. Machado GC, Maher CG, Ferreira PH, et al. Trends, complications, and costs for hospital admission and surgery for lumbar spinal stenosis. Spine (Phila Pa 1976). 2017;42(22):1737-1743. doi:10.1097/BRS.0000000000002207
3. Li C, Liu L, Shi JY, Yan KZ, Shen WZ, Yang ZR. Clinical and biomechanical researches of polyetheretherketone (PEEK) rods for semi-rigid lumbar fusion: a systematic review. Neurosurg Rev. 2018;41(2):375-389. doi:10.1007/s10143-016-0763-2
4. Mavrogenis AF, Vottis C, Triantafyllopoulos G, Papagelopoulos PJ, Pneumaticos SG. PEEK rod systems for the spine. Eur J Orthop Surg Traumatol. 2014;24 Suppl 1:S111-S116. doi:10.1007/s00590-014-1421-4
5. Choudhri TF, Mummaneni PV, Dhall SS, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion status. J Neurosurg Spine. 2014;21(1):23-30. doi:10.3171/2014.4.SPINE14267
6. Ahn YH, Chen WM, Lee KY, Park KW, Lee SJ. Comparison of the load-sharing characteristics between pedicle-based dynamic and rigid rod devices. Biomed Mater. 2008;3(4):044101. doi:10.1088/1748-6041/3/4/044101
7. Ozer AF, Cevik OM, Erbulut DU, et al. A novel modular dynamic stabilization system for the treatment of degenerative spinal pathologies. Turk Neurosurg. 2019;29(1):115-120. doi:10.5137/1019-5149.JTN.23227-18.1
8. Hak DJ, Mauffrey C, Seligson D, Lindeque B. Use of carbon-fiber-reinforced composite implants in orthopedic surgery. Orthopedics. 2014;37(12):825-830. doi:10.3928/01477447-20141124-05
9. Gornet MF, Chan FW, Coleman JC, et al. Biomechanical assessment of a PEEK rod system for semi-rigid fixation of lumbar fusion constructs. J Biomech Eng. 2011;133(8):081009. doi:10.1115/1.4004862
10. Jackson JB 3rd, Crimaldi AJ, Peindl R, Norton HJ, Anderson WE, Patt JC. Effect of polyether ether ketone on therapeutic radiation to the spine: a pilot study. Spine (Phila Pa 1976). 2017;42(1):E1-E7. doi:10.1097/BRS.0000000000001695
11. Highsmith JM, Tumialán LM, Rodts GE Jr. Flexible rods and the case for dynamic stabilization. Neurosurg Focus. 2007;22(1):E11. Published 2007 Jan 15. doi:10.3171/foc.2007.22.1.11
12. Sengupta DK, Bucklen B, McAfee PC, Nichols J, Angara R, Khalil S. The comprehensive biomechanics and load-sharing of semirigid PEEK and semirigid posterior dynamic stabilization systems. Adv Orthop. 2013;2013:745610. doi:10.1155/2013/745610
13. Agarwal A, Ingels M, Kodigudla M, Momeni N, Goel V, Agarwal AK. Adjacent-level hypermobility and instrumented-level fatigue loosening with titanium and PEEK rods for a pedicle screw system: an in vitro study. J Biomech Eng. 2016;138(5):051004. doi:10.1115/1.4032965
14. Chou WK, Chien A, Wang JL. Biomechanical analysis between PEEK and titanium screw-rods spinal construct subjected to fatigue loading. J Spinal Disord Tech. 2015;28(3):E121-E125. doi:10.1097/BSD.0000000000000176
15. Shih KS Hsu CC, Zhou SY, Hou SM. Biomechanical investigation of pedicle screw-based posterior stabilization systems for the treatment of lumbar degenerative disc disease using finite element analyses. Biomed Eng: Appl Basis Commun. 2015;27(06):1550060. doi: 10.4015/S101623721550060X

16. Chang TK, Huang CH, Liu YC, et al. Biomechanical evaluation and comparison of polyetheretherketone rod system to traditional titanium rod fixation on adjacent levels. Formosan J Musculoskeletal Disord. 2013;4(2):42-47. doi: 10.1016/j.fjmd.2013.04.003
17. Ormond DR, Albert L Jr, Das K. Polyetheretherketone (PEEK) rods in lumbar spine degenerative disease: a case series. Clin Spine Surg. 2016;29(7):E371-E375. doi:10.1097/BSD.0b013e318277cb9b
18. Colangeli S, Barbanti Brodàno G, Gasbarrini A, et al. Polyetheretherketone (PEEK) rods: short-term results in lumbar spine degenerative disease. J Neurosurg Sci. 2015;59(2):91-96.
19. Huang W, Chang Z, Song R, Zhou K, Yu X. Non-fusion procedure using PEEK rod systems for lumbar degenerative diseases: clinical experience with a 2-year follow-up. BMC Musculoskelet Disord. 2016;17:53. Published 2016 Feb 1. doi:10.1186/s12891-016-0913-2
20. Wang C-J, Graf H, Wei H-W. Clinical outcomes of the dynamic lumbar pedicle screw-rod stabilization. Neurosurg Q. 2016;26(3):214-218. doi:10.1097/WNQ.0000000000000169
21. Kurtz SM, Lanman TH, Higgs G, et al. Retrieval analysis of PEEK rods for posterior fusion and motion preservation. Eur Spine J. 2013;22(12):2752-2759. doi:10.1007/s00586-013-2920-4
22. Athanasakopoulos M, Mavrogenis AF, Triantafyllopoulos G, Koufos S, Pneumaticos SG. Posterior spinal fusion using pedicle screws. Orthopedics. 2013;36(7):e951-e957. doi:10.3928/01477447-20130624-28
23. De Iure F, Bosco G, Cappuccio M, Paderni S, Amendola L. Posterior lumbar fusion by peek rods in degenerative spine: preliminary report on 30 cases. Eur Spine J. 2012;21 Suppl 1(Suppl 1):S50-S54. doi:10.1007/s00586-012-2219-x
24. Qi L, Li M, Zhang S, Xue J, Si H. Comparative effectiveness of PEEK rods versus titanium alloy rods in lumbar fusion: a preliminary report. Acta Neurochir (Wien). 2013;155(7):1187-1193. doi:10.1007/s00701-013-1772-3
25. Endler P, Ekman P, Möller H, Gerdhem P. Outcomes of posterolateral fusion with and without instrumentation and of interbody fusion for isthmic spondylolisthesis: a prospective study. J Bone Joint Surg Am. 2017;99(9):743-752. doi:10.2106/JBJS.16.00679
26. Abdu WA, Lurie JD, Spratt KF, et al. Degenerative spondylolisthesis: does fusion method influence outcome? Four-year results of the spine patient outcomes research trial. Spine (Phila Pa 1976). 2009;34(21):2351-2360. doi:10.1097/BRS.0b013e3181b8a829
27. Gatchel RJ, Mayer TG, Chou R. What does/should the minimum clinically important difference measure? A reconsideration of its clinical value in evaluating efficacy of lumbar fusion surgery. Clin J Pain. 2012;28(5):387-397. doi:10.1097/AJP.0b013e3182327f20
28. Spratt KF. Patient-level minimal clinically important difference based on clinical judgment and minimally detectable measurement difference: a rationale for the SF-36 physical function scale in the SPORT intervertebral disc herniation cohort. Spine (Phila Pa 1976). 2009;34(16):1722-1731. doi:10.1097/BRS.0b013e3181a8faf2
29. Ward MM, Guthrie LC, Alba MI. Clinically important changes in short form 36 health survey scales for use in rheumatoid arthritis clinical trials: the impact of low responsiveness. Arthritis Care Res (Hoboken). 2014;66(12):1783-1789. doi:10.1002/acr.22392
30. Escobar A, Quintana JM, Bilbao A, Aróstegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthritis Cartilage. 2007;15(3):273-280. doi:10.1016/j.joca.2006.09.001
31. Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008;8(6):968-974. doi:10.1016/j.spinee.2007.11.006
32. Radcliff KE, Kepler CK, Jakoi A, et al. Adjacent segment disease in the lumbar spine following different treatment interventions. Spine J. 2013;13(10):1339-1349. doi:10.1016/j.spinee.2013.03.020
33. Epstein NE. Adjacent level disease following lumbar spine surgery: a review. Surg Neurol Int. 2015;6(Suppl 24):S591-S599. Published 2015 Nov 25. doi:10.4103/2152-7806.170432
34. Epstein NE. A review: reduced reoperation rate for multilevel lumbar laminectomies with noninstrumented versus instrumented fusions. Surg Neurol Int. 2016;7(Suppl 13):S337-S346. Published 2016 May 17. doi:10.4103/2152-7806.182546
35. Scemama C, Magrino B, Gillet P, Guigui P. Risk of adjacent-segment disease requiring surgery after short lumbar fusion: results of the French Spine Surgery Society Series. J Neurosurg Spine. 2016;25(1):46-51. doi:10.3171/2015.11.SPINE15700
36. Tempel ZJ, Gandhoke GS, Bolinger BD, et al. The influence of pelvic incidence and lumbar lordosis mismatch on development of symptomatic adjacent level disease following single-level transforaminal lumbar interbody fusion. Neurosurgery. 2017;80(6):880-886. doi:10.1093/neuros/nyw073

37. Cheng YW, Chang PY, Wu JC, et al. Letter to the editor: Pedicle screw-based dynamic stabilization and adjacent-segment disease. J Neurosurg Spine. 2017;26(3):405-406. doi:10.3171/2016.7.SPINE16816
38. Street JT, Andrew Glennie R, Dea N, et al. A comparison of the Wiltse versus midline approaches in degenerative conditions of the lumbar spine. J Neurosurg Spine. 2016;25(3):332-338. doi:10.3171/2016.2.SPINE151018
39. Kuo CH, Huang WC, Wu JC, et al. Radiological adjacent-segment degeneration in L4-5 spondylolisthesis: comparison between dynamic stabilization and minimally invasive transforaminal lumbar interbody fusion. J Neurosurg Spine. 2018;29(3):250-258. doi:10.3171/2018.1.SPINE17993
40. Lee CH, Kim YE, Lee HJ, Kim DG, Kim CH. Biomechanical effects of hybrid stabilization on the risk of proximal adjacent-segment degeneration following lumbar spinal fusion using an interspinous device or a pedicle screw-based dynamic fixator. J Neurosurg Spine. 2017;27(6):643-649. doi:10.3171/2017.3.SPINE161169
41. Wang JC, Arnold PM, Hermsmeyer JT, Norvell DC. Do lumbar motion preserving devices reduce the risk of adjacent segment pathology compared with fusion surgery? A systematic review. Spine (Phila Pa 1976). 2012;37(22 Suppl):S133-S143. doi:10.1097/BRS.0b013e31826cadf2
42. Ross DA. Letter to the editor: steroid use in anterior cervical discectomy and fusion. J Neurosurg Spine. 2016;24(6):998-1000. doi:10.3171/2015.9.SPINE151052
37-year-old man • cough • increasing shortness of breath • pleuritic chest pain • Dx?
THE CASE
A 37-year-old man with a history of asthma, schizoaffective disorder, and tobacco use (36 packs per year) presented to the clinic after 5 days of worsening cough, reproducible left-sided chest pain, and increasing shortness of breath. He also experienced chills, fatigue, nausea, and vomiting but was afebrile. The patient had not travelled recently nor had direct contact with anyone sick. He also denied intravenous (IV) drug use, alcohol use, and bloody sputum. Recently, he had intentionally lost weight, as recommended by his psychiatrist.
Medication review revealed that he was taking many central-acting agents for schizoaffective disorder, including alprazolam, aripiprazole, desvenlafaxine, and quetiapine. Due to his intermittent asthma since childhood, he used an albuterol inhaler as needed, which currently offered only minimal relief. He denied any history of hospitalization or intubation for asthma.
During the clinic visit, his blood pressure was 90/60 mm Hg and his heart rate was normal. His pulse oximetry was 92% on room air. On physical examination, he had normal-appearing dentition. Auscultation revealed bilateral expiratory wheezes with decreased breath sounds at the left lower lobe.
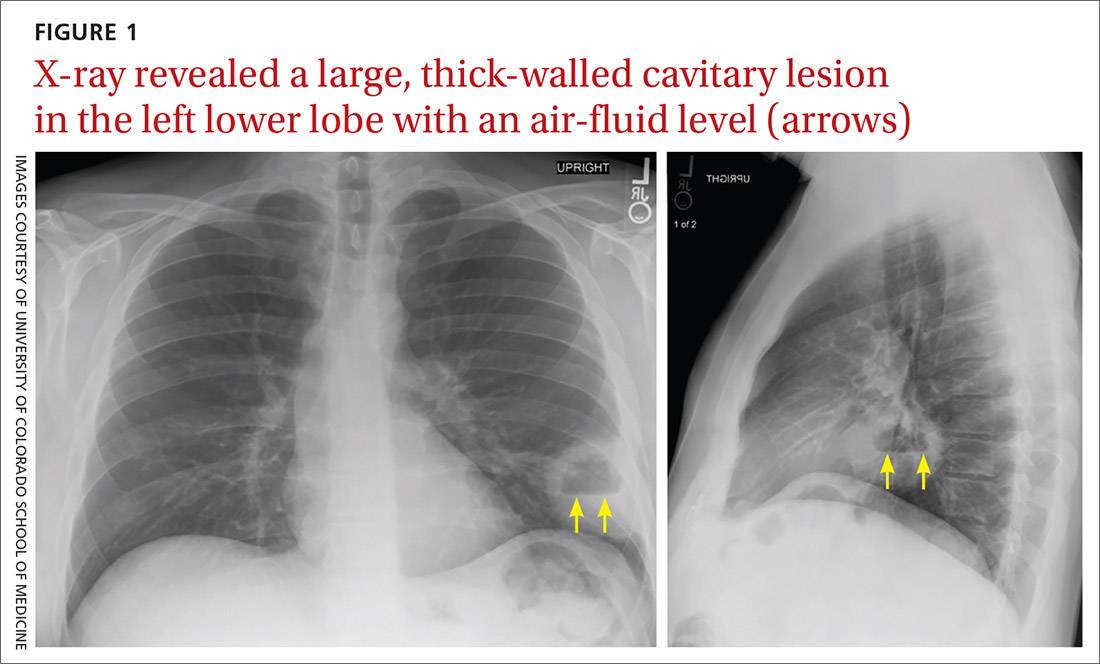
A plain chest radiograph (CXR) performed in the clinic (FIGURE 1) showed a large, thick-walled cavitary lesion with an air-fluid level in the left lower lobe. The patient was directly admitted to the Family Medicine Inpatient Service. Computed tomography (CT) of the chest with contrast was ordered to rule out empyema or malignancy. The chest CT confirmed the previous findings while also revealing a surrounding satellite nodularity in the left lower lobe (FIGURE 2). QuantiFERON-TB Gold and HIV tests were both negative.
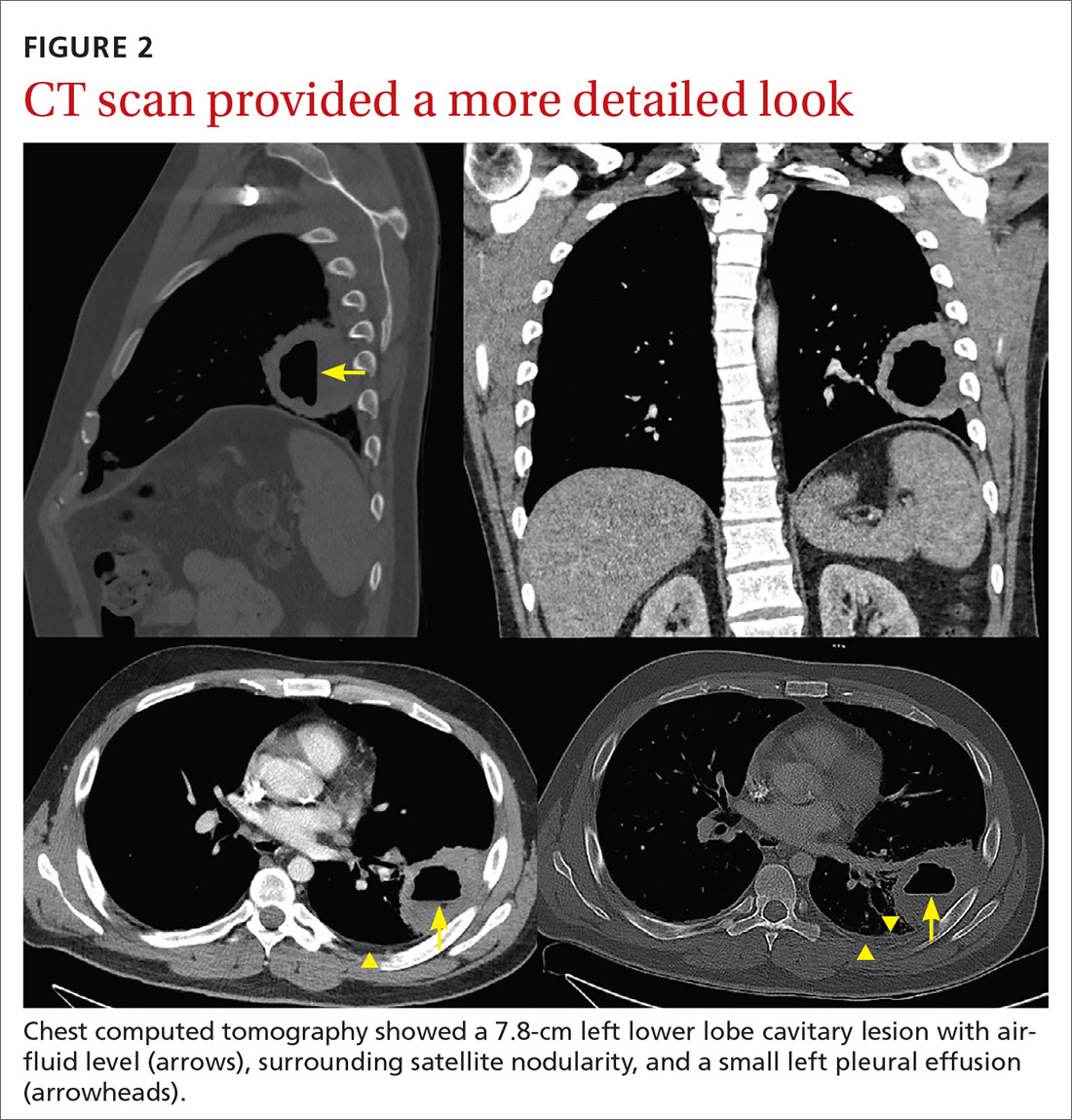
THE DIAGNOSIS
The patient was given a diagnosis of a lung abscess based on symptoms and imaging. An extensive smoking history, as well as multiple sedating medications, increased his likelihood of aspiration.
DISCUSSION
Lung abscess is the probable diagnosis in a patient with indolent infectious symptoms (cough, fever, night sweats) developing over days to weeks and a CXR finding of pulmonary opacity, often with an air-fluid level.1-4 A lung abscess is a circumscribed collection of pus in the lung parenchyma that develops as a result of microbial infection.4
Primary vs secondary abscess. Lung abscesses can be divided into 2 groups: primary and secondary abscesses. Primary abscesses (60%) occur without any other medical condition or in patients prone to aspiration.5 Secondary abscesses occur in the setting of a comorbid medical condition, such as lung disease, heart disease, bronchogenic neoplasm, or immunocompromised status.5
Continue to: With a primary lung abscess...
With a primary lung abscess, oropharyngeal contents are aspirated (generally while the patient is unconscious) and contain mixed flora.2 The aspirate typically migrates to the posterior segments of the upper lobes and to the superior segments of the lower lobes. These abscesses are usually singular and have an air-fluid level.1,2
Secondary lung abscesses occur in bronchial obstruction (by tumor, foreign body, or enlarged lymph nodes), with coexisting lung diseases (bronchiectasis, cystic fibrosis, infected pulmonary infarcts, lung contusion) or by direct spread (broncho-esophageal fistula, subphrenic abscess).6 Secondary abscesses are associated with a poorer prognosis, dependent on the patient’s general condition and underlying disease.7
What to rule out
The differential diagnosis of cavitary lung lesion includes tuberculosis, necrotizing pneumonia, bronchial carcinoma, pulmonary embolism, vasculitis (eg, Churg-Strauss syndrome), and localized pleural empyema.1,4 A CT scan is helpful to differentiate between a parenchymal lesion and pleural collection, which may not be as clear on CXR.1,4
Tuberculosis manifests with fatigue, weight loss, and night sweats; a chest CT will reveal a cavitating lesion (usually upper lobe) with a characteristic “rim sign” that includes caseous necrosis surrounded by a peripheral enhancing rim.8
Necrotizing pneumonia manifests as acute, fulminant infection. The most common causative organisms on sputum culture are Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas species. Plain radiography will reveal multiple cavities and often associated pleural effusion and empyema.9
Continue to: Excavating bronchogenic carcinomas
Excavating bronchogenic carcinomas differ from a lung abscess in that a patient with the latter is typically, but not always, febrile and has purulent sputum. On imaging, a bronchogenic carcinoma has a thicker and more irregular wall than a lung abscess.10
Treatment
When antibiotics first became available, penicillin was used to treat lung abscess.11 Then IV clindamycin became the drug of choice after 2 trials demonstrated its superiority to IV penicillin.12,13 More recently, clindamycin alone has fallen out of favor due to growing anaerobic resistance.14
Current therapy includes beta-lactam with beta-lactamase inhibitors.14 Lung abscesses are typically polymicrobial and thus carry different degrees of antibiotic resistance.15,16 If culture data are available, targeted therapy is preferred, especially for secondary abscesses.7 Antibiotic therapy is usually continued until a CXR reveals a small lesion or is clear, which may require several months of outpatient oral antibiotic therapy.4
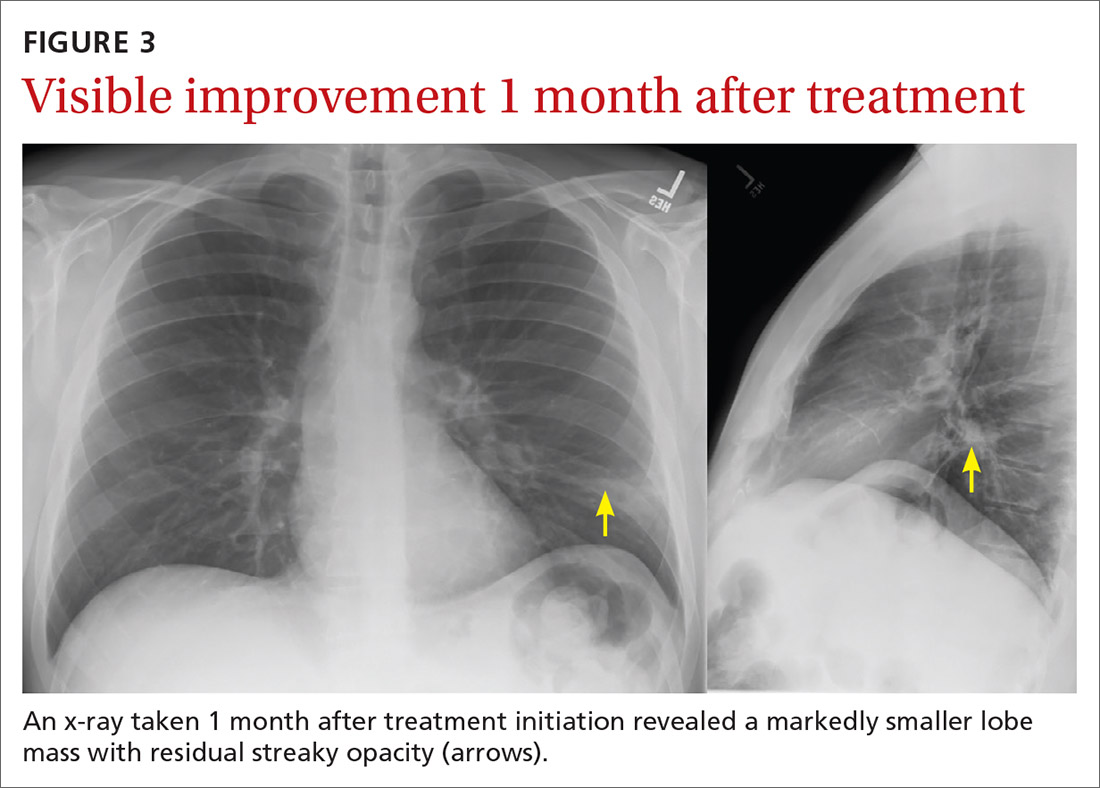
Our patient was treated with IV clindamycin for 3 days in the hospital. Clindamycin was chosen due to his penicillin allergy and started empirically without any culture data. He was transitioned to oral clindamycin and completed a total 3-week course as his CXR continued to show improvement (FIGURE 3). He did not undergo bronchoscopy. A follow-up CXR showed resolution of lung abscess at 9 months. (FIGURE 4).
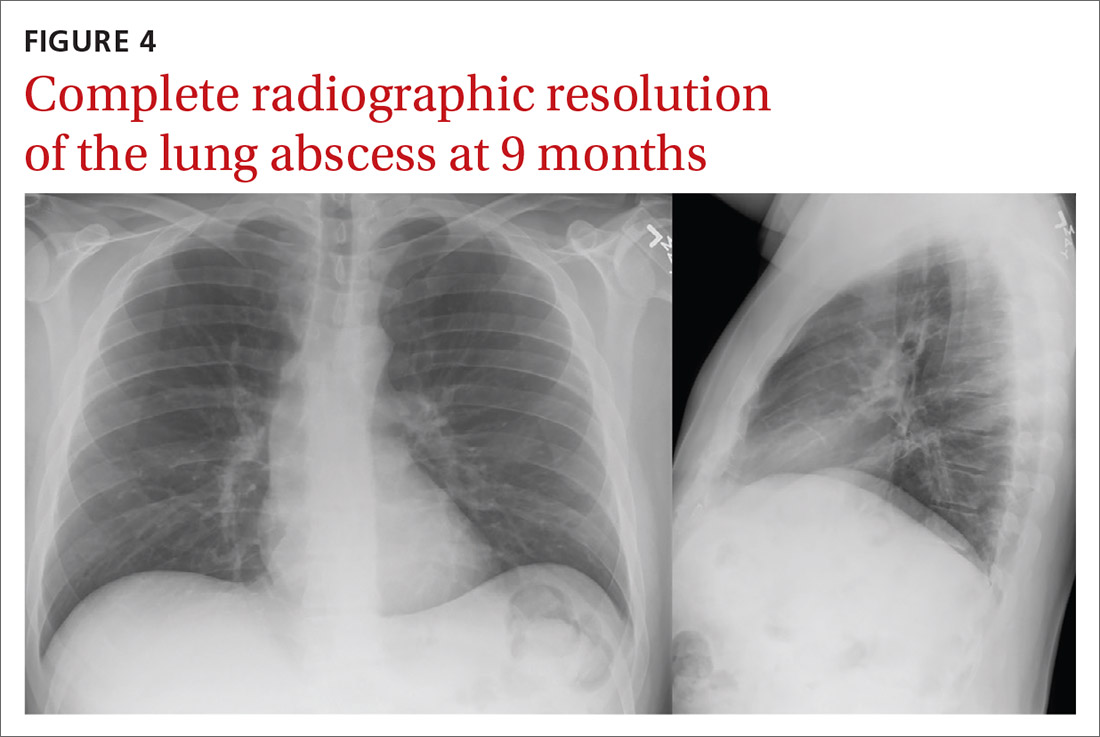
THE TAKEAWAY
All patients with lung abscesses should have sputum culture with gram stain done—ideally prior to starting antibiotics.3,4 Bronchoscopy should be considered for patients with atypical presentations or those who fail standard therapy, but may be used in other cases, as well.3
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
1. Hassan M, Asciak R, Rizk R, et al. Lung abscess or empyema? Taking a closer look. Thorax. 2018;73:887-889. https://doi. org/10.1136/thoraxjnl-2018-211604
2. Moreira J da SM, Camargo J de JP, Felicetti JC, et al. Lung abscess: analysis of 252 consecutive cases diagnosed between 1968 and 2004. J Bras Pneumol. 2006;32:136-43. https://doi.org/10.1590/ s1806-37132006000200009
3. Schiza S, Siafakas NM. Clinical presentation and management of empyema, lung abscess and pleural effusion. Curr Opin Pulm Med. 2006;12:205-211. https://doi.org/10.1097/01. mcp.0000219270.73180.8b
4. Yazbeck MF, Dahdel M, Kalra A, et al. Lung abscess: update on microbiology and management. Am J Ther. 2014;21:217-221. https://doi.org/10.1097/MJT.0b013e3182383c9b
5. Nicolini A, Cilloniz C, Senarega R, et al. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82:276-285. https://doi. org/10.5603/PiAP.2014.0033
6. Puligandla PS, Laberge J-M. Respiratory infections: pneumonia, lung abscess, and empyema. Semin Pediatr Surg. 2008;17:42-52. https://doi.org/10.1053/j.sempedsurg.2007.10.007
7. Marra A, Hillejan L, Ukena D. [Management of Lung Abscess]. Zentralbl Chir. 2015;140 (suppl 1):S47-S53. https://doi. org/10.1055/s-0035-1557883
THE CASE
A 37-year-old man with a history of asthma, schizoaffective disorder, and tobacco use (36 packs per year) presented to the clinic after 5 days of worsening cough, reproducible left-sided chest pain, and increasing shortness of breath. He also experienced chills, fatigue, nausea, and vomiting but was afebrile. The patient had not travelled recently nor had direct contact with anyone sick. He also denied intravenous (IV) drug use, alcohol use, and bloody sputum. Recently, he had intentionally lost weight, as recommended by his psychiatrist.
Medication review revealed that he was taking many central-acting agents for schizoaffective disorder, including alprazolam, aripiprazole, desvenlafaxine, and quetiapine. Due to his intermittent asthma since childhood, he used an albuterol inhaler as needed, which currently offered only minimal relief. He denied any history of hospitalization or intubation for asthma.
During the clinic visit, his blood pressure was 90/60 mm Hg and his heart rate was normal. His pulse oximetry was 92% on room air. On physical examination, he had normal-appearing dentition. Auscultation revealed bilateral expiratory wheezes with decreased breath sounds at the left lower lobe.

A plain chest radiograph (CXR) performed in the clinic (FIGURE 1) showed a large, thick-walled cavitary lesion with an air-fluid level in the left lower lobe. The patient was directly admitted to the Family Medicine Inpatient Service. Computed tomography (CT) of the chest with contrast was ordered to rule out empyema or malignancy. The chest CT confirmed the previous findings while also revealing a surrounding satellite nodularity in the left lower lobe (FIGURE 2). QuantiFERON-TB Gold and HIV tests were both negative.

THE DIAGNOSIS
The patient was given a diagnosis of a lung abscess based on symptoms and imaging. An extensive smoking history, as well as multiple sedating medications, increased his likelihood of aspiration.
DISCUSSION
Lung abscess is the probable diagnosis in a patient with indolent infectious symptoms (cough, fever, night sweats) developing over days to weeks and a CXR finding of pulmonary opacity, often with an air-fluid level.1-4 A lung abscess is a circumscribed collection of pus in the lung parenchyma that develops as a result of microbial infection.4
Primary vs secondary abscess. Lung abscesses can be divided into 2 groups: primary and secondary abscesses. Primary abscesses (60%) occur without any other medical condition or in patients prone to aspiration.5 Secondary abscesses occur in the setting of a comorbid medical condition, such as lung disease, heart disease, bronchogenic neoplasm, or immunocompromised status.5
Continue to: With a primary lung abscess...
With a primary lung abscess, oropharyngeal contents are aspirated (generally while the patient is unconscious) and contain mixed flora.2 The aspirate typically migrates to the posterior segments of the upper lobes and to the superior segments of the lower lobes. These abscesses are usually singular and have an air-fluid level.1,2
Secondary lung abscesses occur in bronchial obstruction (by tumor, foreign body, or enlarged lymph nodes), with coexisting lung diseases (bronchiectasis, cystic fibrosis, infected pulmonary infarcts, lung contusion) or by direct spread (broncho-esophageal fistula, subphrenic abscess).6 Secondary abscesses are associated with a poorer prognosis, dependent on the patient’s general condition and underlying disease.7
What to rule out
The differential diagnosis of cavitary lung lesion includes tuberculosis, necrotizing pneumonia, bronchial carcinoma, pulmonary embolism, vasculitis (eg, Churg-Strauss syndrome), and localized pleural empyema.1,4 A CT scan is helpful to differentiate between a parenchymal lesion and pleural collection, which may not be as clear on CXR.1,4
Tuberculosis manifests with fatigue, weight loss, and night sweats; a chest CT will reveal a cavitating lesion (usually upper lobe) with a characteristic “rim sign” that includes caseous necrosis surrounded by a peripheral enhancing rim.8
Necrotizing pneumonia manifests as acute, fulminant infection. The most common causative organisms on sputum culture are Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas species. Plain radiography will reveal multiple cavities and often associated pleural effusion and empyema.9
Continue to: Excavating bronchogenic carcinomas
Excavating bronchogenic carcinomas differ from a lung abscess in that a patient with the latter is typically, but not always, febrile and has purulent sputum. On imaging, a bronchogenic carcinoma has a thicker and more irregular wall than a lung abscess.10
Treatment
When antibiotics first became available, penicillin was used to treat lung abscess.11 Then IV clindamycin became the drug of choice after 2 trials demonstrated its superiority to IV penicillin.12,13 More recently, clindamycin alone has fallen out of favor due to growing anaerobic resistance.14
Current therapy includes beta-lactam with beta-lactamase inhibitors.14 Lung abscesses are typically polymicrobial and thus carry different degrees of antibiotic resistance.15,16 If culture data are available, targeted therapy is preferred, especially for secondary abscesses.7 Antibiotic therapy is usually continued until a CXR reveals a small lesion or is clear, which may require several months of outpatient oral antibiotic therapy.4

Our patient was treated with IV clindamycin for 3 days in the hospital. Clindamycin was chosen due to his penicillin allergy and started empirically without any culture data. He was transitioned to oral clindamycin and completed a total 3-week course as his CXR continued to show improvement (FIGURE 3). He did not undergo bronchoscopy. A follow-up CXR showed resolution of lung abscess at 9 months. (FIGURE 4).

THE TAKEAWAY
All patients with lung abscesses should have sputum culture with gram stain done—ideally prior to starting antibiotics.3,4 Bronchoscopy should be considered for patients with atypical presentations or those who fail standard therapy, but may be used in other cases, as well.3
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
THE CASE
A 37-year-old man with a history of asthma, schizoaffective disorder, and tobacco use (36 packs per year) presented to the clinic after 5 days of worsening cough, reproducible left-sided chest pain, and increasing shortness of breath. He also experienced chills, fatigue, nausea, and vomiting but was afebrile. The patient had not travelled recently nor had direct contact with anyone sick. He also denied intravenous (IV) drug use, alcohol use, and bloody sputum. Recently, he had intentionally lost weight, as recommended by his psychiatrist.
Medication review revealed that he was taking many central-acting agents for schizoaffective disorder, including alprazolam, aripiprazole, desvenlafaxine, and quetiapine. Due to his intermittent asthma since childhood, he used an albuterol inhaler as needed, which currently offered only minimal relief. He denied any history of hospitalization or intubation for asthma.
During the clinic visit, his blood pressure was 90/60 mm Hg and his heart rate was normal. His pulse oximetry was 92% on room air. On physical examination, he had normal-appearing dentition. Auscultation revealed bilateral expiratory wheezes with decreased breath sounds at the left lower lobe.

A plain chest radiograph (CXR) performed in the clinic (FIGURE 1) showed a large, thick-walled cavitary lesion with an air-fluid level in the left lower lobe. The patient was directly admitted to the Family Medicine Inpatient Service. Computed tomography (CT) of the chest with contrast was ordered to rule out empyema or malignancy. The chest CT confirmed the previous findings while also revealing a surrounding satellite nodularity in the left lower lobe (FIGURE 2). QuantiFERON-TB Gold and HIV tests were both negative.

THE DIAGNOSIS
The patient was given a diagnosis of a lung abscess based on symptoms and imaging. An extensive smoking history, as well as multiple sedating medications, increased his likelihood of aspiration.
DISCUSSION
Lung abscess is the probable diagnosis in a patient with indolent infectious symptoms (cough, fever, night sweats) developing over days to weeks and a CXR finding of pulmonary opacity, often with an air-fluid level.1-4 A lung abscess is a circumscribed collection of pus in the lung parenchyma that develops as a result of microbial infection.4
Primary vs secondary abscess. Lung abscesses can be divided into 2 groups: primary and secondary abscesses. Primary abscesses (60%) occur without any other medical condition or in patients prone to aspiration.5 Secondary abscesses occur in the setting of a comorbid medical condition, such as lung disease, heart disease, bronchogenic neoplasm, or immunocompromised status.5
Continue to: With a primary lung abscess...
With a primary lung abscess, oropharyngeal contents are aspirated (generally while the patient is unconscious) and contain mixed flora.2 The aspirate typically migrates to the posterior segments of the upper lobes and to the superior segments of the lower lobes. These abscesses are usually singular and have an air-fluid level.1,2
Secondary lung abscesses occur in bronchial obstruction (by tumor, foreign body, or enlarged lymph nodes), with coexisting lung diseases (bronchiectasis, cystic fibrosis, infected pulmonary infarcts, lung contusion) or by direct spread (broncho-esophageal fistula, subphrenic abscess).6 Secondary abscesses are associated with a poorer prognosis, dependent on the patient’s general condition and underlying disease.7
What to rule out
The differential diagnosis of cavitary lung lesion includes tuberculosis, necrotizing pneumonia, bronchial carcinoma, pulmonary embolism, vasculitis (eg, Churg-Strauss syndrome), and localized pleural empyema.1,4 A CT scan is helpful to differentiate between a parenchymal lesion and pleural collection, which may not be as clear on CXR.1,4
Tuberculosis manifests with fatigue, weight loss, and night sweats; a chest CT will reveal a cavitating lesion (usually upper lobe) with a characteristic “rim sign” that includes caseous necrosis surrounded by a peripheral enhancing rim.8
Necrotizing pneumonia manifests as acute, fulminant infection. The most common causative organisms on sputum culture are Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas species. Plain radiography will reveal multiple cavities and often associated pleural effusion and empyema.9
Continue to: Excavating bronchogenic carcinomas
Excavating bronchogenic carcinomas differ from a lung abscess in that a patient with the latter is typically, but not always, febrile and has purulent sputum. On imaging, a bronchogenic carcinoma has a thicker and more irregular wall than a lung abscess.10
Treatment
When antibiotics first became available, penicillin was used to treat lung abscess.11 Then IV clindamycin became the drug of choice after 2 trials demonstrated its superiority to IV penicillin.12,13 More recently, clindamycin alone has fallen out of favor due to growing anaerobic resistance.14
Current therapy includes beta-lactam with beta-lactamase inhibitors.14 Lung abscesses are typically polymicrobial and thus carry different degrees of antibiotic resistance.15,16 If culture data are available, targeted therapy is preferred, especially for secondary abscesses.7 Antibiotic therapy is usually continued until a CXR reveals a small lesion or is clear, which may require several months of outpatient oral antibiotic therapy.4

Our patient was treated with IV clindamycin for 3 days in the hospital. Clindamycin was chosen due to his penicillin allergy and started empirically without any culture data. He was transitioned to oral clindamycin and completed a total 3-week course as his CXR continued to show improvement (FIGURE 3). He did not undergo bronchoscopy. A follow-up CXR showed resolution of lung abscess at 9 months. (FIGURE 4).

THE TAKEAWAY
All patients with lung abscesses should have sputum culture with gram stain done—ideally prior to starting antibiotics.3,4 Bronchoscopy should be considered for patients with atypical presentations or those who fail standard therapy, but may be used in other cases, as well.3
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
1. Hassan M, Asciak R, Rizk R, et al. Lung abscess or empyema? Taking a closer look. Thorax. 2018;73:887-889. https://doi. org/10.1136/thoraxjnl-2018-211604
2. Moreira J da SM, Camargo J de JP, Felicetti JC, et al. Lung abscess: analysis of 252 consecutive cases diagnosed between 1968 and 2004. J Bras Pneumol. 2006;32:136-43. https://doi.org/10.1590/ s1806-37132006000200009
3. Schiza S, Siafakas NM. Clinical presentation and management of empyema, lung abscess and pleural effusion. Curr Opin Pulm Med. 2006;12:205-211. https://doi.org/10.1097/01. mcp.0000219270.73180.8b
4. Yazbeck MF, Dahdel M, Kalra A, et al. Lung abscess: update on microbiology and management. Am J Ther. 2014;21:217-221. https://doi.org/10.1097/MJT.0b013e3182383c9b
5. Nicolini A, Cilloniz C, Senarega R, et al. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82:276-285. https://doi. org/10.5603/PiAP.2014.0033
6. Puligandla PS, Laberge J-M. Respiratory infections: pneumonia, lung abscess, and empyema. Semin Pediatr Surg. 2008;17:42-52. https://doi.org/10.1053/j.sempedsurg.2007.10.007
7. Marra A, Hillejan L, Ukena D. [Management of Lung Abscess]. Zentralbl Chir. 2015;140 (suppl 1):S47-S53. https://doi. org/10.1055/s-0035-1557883
1. Hassan M, Asciak R, Rizk R, et al. Lung abscess or empyema? Taking a closer look. Thorax. 2018;73:887-889. https://doi. org/10.1136/thoraxjnl-2018-211604
2. Moreira J da SM, Camargo J de JP, Felicetti JC, et al. Lung abscess: analysis of 252 consecutive cases diagnosed between 1968 and 2004. J Bras Pneumol. 2006;32:136-43. https://doi.org/10.1590/ s1806-37132006000200009
3. Schiza S, Siafakas NM. Clinical presentation and management of empyema, lung abscess and pleural effusion. Curr Opin Pulm Med. 2006;12:205-211. https://doi.org/10.1097/01. mcp.0000219270.73180.8b
4. Yazbeck MF, Dahdel M, Kalra A, et al. Lung abscess: update on microbiology and management. Am J Ther. 2014;21:217-221. https://doi.org/10.1097/MJT.0b013e3182383c9b
5. Nicolini A, Cilloniz C, Senarega R, et al. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82:276-285. https://doi. org/10.5603/PiAP.2014.0033
6. Puligandla PS, Laberge J-M. Respiratory infections: pneumonia, lung abscess, and empyema. Semin Pediatr Surg. 2008;17:42-52. https://doi.org/10.1053/j.sempedsurg.2007.10.007
7. Marra A, Hillejan L, Ukena D. [Management of Lung Abscess]. Zentralbl Chir. 2015;140 (suppl 1):S47-S53. https://doi. org/10.1055/s-0035-1557883
COVID-19 Vaccine in Veterans with Multiple Sclerosis: Protect the Vulnerable
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.