User login
Age-related cognitive decline not inevitable?
Investigators found that despite the presence of neuropathologies associated with Alzheimer’s disease (AD), many centenarians maintained high levels of cognitive performance.
“Cognitive decline is not inevitable,” senior author Henne Holstege, PhD, assistant professor, Amsterdam Alzheimer Center and Clinical Genetics, Amsterdam University Medical Center, said in an interview.
“At 100 years or older, high levels of cognitive performance can be maintained for several years, even when individuals are exposed to risk factors associated with cognitive decline,” she said.
The study was published online Jan. 15 in JAMA Network Open.
Escaping cognitive decline
Dr. Holstege said her interest in researching aging and cognitive health was inspired by the “fascinating” story of Hendrikje van Andel-Schipper, who died at age 115 in 2015 “completely cognitively healthy.” Her mother, who died at age 100, also was cognitively intact at the end of her life.
“I wanted to know how it is possible that some people can completely escape all aspects of cognitive decline while reaching extreme ages,” Dr. Holstege said.
To discover the secret to cognitive health in the oldest old, Dr. Holstege initiated the 100-Plus Study, which involved a cohort of healthy centenarians.
The investigators conducted extensive neuropsychological testing and collected blood and fecal samples to examine “the myriad factors that influence physical health, including genetics, neuropathology, blood markers, and the gut microbiome, to explore the molecular and neuropsychologic constellations associated with the escape from cognitive decline.”
The goal of the research was to investigate “to what extent centenarians were able to maintain their cognitive health after study inclusion, and to what extent this was associated with genetic, physical, or neuropathological features,” she said.
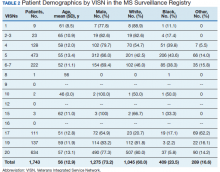
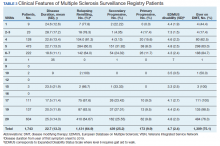
The study included 330 centenarians who completed one or more neuropsychological assessments. Neuropathologic studies were available for 44 participants.
To assess baseline cognitive performance, the researchers administered a wide array of neurocognitive tests, as well as the Mini–Mental State Examination, from which mean z scores for cognitive domains were calculated.
Additional factors in the analysis included sex, age, APOE status, cognitive reserve, physical health, and whether participants lived independently.
At autopsy, amyloid-beta (A-beta) level, the level of intracellular accumulation of phosphorylated tau protein in neurofibrillary tangles (NFTs), and the neuritic plaque (NP) load were assessed.
Resilience and cognitive reserve
At baseline, the median age of the centenarians (n = 330, 72.4% women) was 100.5 years (interquartile range, 100.2-101.7). A little over half (56.7%) lived independently, and the majority had good vision (65%) and hearing (56.4%). Most (78.8%) were able to walk independently, and 37.9% had achieved the highest International Standard Classification of Education level of postsecondary education.
The researchers found “varying degrees of neuropathology” in the brains of the 44 donors, including A-beta, NFT, and NPs.
The duration of follow-up in analyzing cognitive trajectories ranged from 0 to 4 years (median, 1.6 years).
Assessments of all cognitive domains showed no decline, with the exception of a “slight” decrement in memory function (beta −.10 SD per year; 95% confidence interval, –.14 to –.05 SD; P < .001).
Cognitive performance was associated with factors of physical health or cognitive reserve, for example, greater independence in performing activities of daily living, as assessed by the Barthel index (beta .37 SD per year; 95% CI, .24-.49; P < .001), or higher educational level (beta .41 SD per year; 95% CI, .29-.53; P < .001).
Despite findings of neuropathologic “hallmarks” of AD post mortem in the brains of the centenarians, these were not associated with cognitive performance or rate of decline.
APOE epsilon-4 or an APOE epsilon-3 alleles also were not significantly associated with cognitive performance or decline, suggesting that the “effects of APOE alleles are exerted before the age of 100 years,” the authors noted.
“Our findings suggest that after reaching age 100 years, cognitive performance remains relatively stable during ensuing years. Therefore, these centenarians might be resilient or resistant against different risk factors of cognitive decline,” the authors wrote. They also speculate that resilience may be attributable to greater cognitive reserve.
“Our preliminary data indicate that approximately 60% of the chance to reach 100 years old is heritable. Therefore, to get a better understanding of which genetic factors associate with the prolonged maintenance of cognitive health, we are looking into which genetic variants occur more commonly in centenarians compared to younger individuals,” said Dr. Holstege.
“Of course, more research needs to be performed to get a better understanding of how such genetic elements might sustain brain health,” she added.
A ‘landmark study’
Commenting on the study in an interview, Thomas Perls, MD, MPH, professor of medicine, Boston University, called it a “landmark” study in research on exceptional longevity in humans.
Dr. Perls, the author of an accompanying editorial, noted that “one cannot absolutely assume a certain level or disability or risk for disease just because a person has achieved extreme age – in fact, if anything, their ability to achieve much older ages likely indicates that they have resistance or resilience to aging-related problems.”
Understanding the mechanism of the resilience could lead to treatment or prevention of AD, said Dr. Perls, who was not involved in the research.
“People have to be careful about ageist myths and attitudes and not have the ageist idea that the older you get, the sicker you get, because many individuals disprove that,” he cautioned.
The study was supported by Stichting Alzheimer Nederland and Stichting Vumc Fonds. Research from the Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Dr. Holstege and Dr. Perls reported having no relevant financial relationships. The other authors’ disclosures are listed on the original article.
A version of this article first appeared on Medscape.com.
Investigators found that despite the presence of neuropathologies associated with Alzheimer’s disease (AD), many centenarians maintained high levels of cognitive performance.
“Cognitive decline is not inevitable,” senior author Henne Holstege, PhD, assistant professor, Amsterdam Alzheimer Center and Clinical Genetics, Amsterdam University Medical Center, said in an interview.
“At 100 years or older, high levels of cognitive performance can be maintained for several years, even when individuals are exposed to risk factors associated with cognitive decline,” she said.
The study was published online Jan. 15 in JAMA Network Open.
Escaping cognitive decline
Dr. Holstege said her interest in researching aging and cognitive health was inspired by the “fascinating” story of Hendrikje van Andel-Schipper, who died at age 115 in 2015 “completely cognitively healthy.” Her mother, who died at age 100, also was cognitively intact at the end of her life.
“I wanted to know how it is possible that some people can completely escape all aspects of cognitive decline while reaching extreme ages,” Dr. Holstege said.
To discover the secret to cognitive health in the oldest old, Dr. Holstege initiated the 100-Plus Study, which involved a cohort of healthy centenarians.
The investigators conducted extensive neuropsychological testing and collected blood and fecal samples to examine “the myriad factors that influence physical health, including genetics, neuropathology, blood markers, and the gut microbiome, to explore the molecular and neuropsychologic constellations associated with the escape from cognitive decline.”
The goal of the research was to investigate “to what extent centenarians were able to maintain their cognitive health after study inclusion, and to what extent this was associated with genetic, physical, or neuropathological features,” she said.
The study included 330 centenarians who completed one or more neuropsychological assessments. Neuropathologic studies were available for 44 participants.
To assess baseline cognitive performance, the researchers administered a wide array of neurocognitive tests, as well as the Mini–Mental State Examination, from which mean z scores for cognitive domains were calculated.
Additional factors in the analysis included sex, age, APOE status, cognitive reserve, physical health, and whether participants lived independently.
At autopsy, amyloid-beta (A-beta) level, the level of intracellular accumulation of phosphorylated tau protein in neurofibrillary tangles (NFTs), and the neuritic plaque (NP) load were assessed.
Resilience and cognitive reserve
At baseline, the median age of the centenarians (n = 330, 72.4% women) was 100.5 years (interquartile range, 100.2-101.7). A little over half (56.7%) lived independently, and the majority had good vision (65%) and hearing (56.4%). Most (78.8%) were able to walk independently, and 37.9% had achieved the highest International Standard Classification of Education level of postsecondary education.
The researchers found “varying degrees of neuropathology” in the brains of the 44 donors, including A-beta, NFT, and NPs.
The duration of follow-up in analyzing cognitive trajectories ranged from 0 to 4 years (median, 1.6 years).
Assessments of all cognitive domains showed no decline, with the exception of a “slight” decrement in memory function (beta −.10 SD per year; 95% confidence interval, –.14 to –.05 SD; P < .001).
Cognitive performance was associated with factors of physical health or cognitive reserve, for example, greater independence in performing activities of daily living, as assessed by the Barthel index (beta .37 SD per year; 95% CI, .24-.49; P < .001), or higher educational level (beta .41 SD per year; 95% CI, .29-.53; P < .001).
Despite findings of neuropathologic “hallmarks” of AD post mortem in the brains of the centenarians, these were not associated with cognitive performance or rate of decline.
APOE epsilon-4 or an APOE epsilon-3 alleles also were not significantly associated with cognitive performance or decline, suggesting that the “effects of APOE alleles are exerted before the age of 100 years,” the authors noted.
“Our findings suggest that after reaching age 100 years, cognitive performance remains relatively stable during ensuing years. Therefore, these centenarians might be resilient or resistant against different risk factors of cognitive decline,” the authors wrote. They also speculate that resilience may be attributable to greater cognitive reserve.
“Our preliminary data indicate that approximately 60% of the chance to reach 100 years old is heritable. Therefore, to get a better understanding of which genetic factors associate with the prolonged maintenance of cognitive health, we are looking into which genetic variants occur more commonly in centenarians compared to younger individuals,” said Dr. Holstege.
“Of course, more research needs to be performed to get a better understanding of how such genetic elements might sustain brain health,” she added.
A ‘landmark study’
Commenting on the study in an interview, Thomas Perls, MD, MPH, professor of medicine, Boston University, called it a “landmark” study in research on exceptional longevity in humans.
Dr. Perls, the author of an accompanying editorial, noted that “one cannot absolutely assume a certain level or disability or risk for disease just because a person has achieved extreme age – in fact, if anything, their ability to achieve much older ages likely indicates that they have resistance or resilience to aging-related problems.”
Understanding the mechanism of the resilience could lead to treatment or prevention of AD, said Dr. Perls, who was not involved in the research.
“People have to be careful about ageist myths and attitudes and not have the ageist idea that the older you get, the sicker you get, because many individuals disprove that,” he cautioned.
The study was supported by Stichting Alzheimer Nederland and Stichting Vumc Fonds. Research from the Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Dr. Holstege and Dr. Perls reported having no relevant financial relationships. The other authors’ disclosures are listed on the original article.
A version of this article first appeared on Medscape.com.
Investigators found that despite the presence of neuropathologies associated with Alzheimer’s disease (AD), many centenarians maintained high levels of cognitive performance.
“Cognitive decline is not inevitable,” senior author Henne Holstege, PhD, assistant professor, Amsterdam Alzheimer Center and Clinical Genetics, Amsterdam University Medical Center, said in an interview.
“At 100 years or older, high levels of cognitive performance can be maintained for several years, even when individuals are exposed to risk factors associated with cognitive decline,” she said.
The study was published online Jan. 15 in JAMA Network Open.
Escaping cognitive decline
Dr. Holstege said her interest in researching aging and cognitive health was inspired by the “fascinating” story of Hendrikje van Andel-Schipper, who died at age 115 in 2015 “completely cognitively healthy.” Her mother, who died at age 100, also was cognitively intact at the end of her life.
“I wanted to know how it is possible that some people can completely escape all aspects of cognitive decline while reaching extreme ages,” Dr. Holstege said.
To discover the secret to cognitive health in the oldest old, Dr. Holstege initiated the 100-Plus Study, which involved a cohort of healthy centenarians.
The investigators conducted extensive neuropsychological testing and collected blood and fecal samples to examine “the myriad factors that influence physical health, including genetics, neuropathology, blood markers, and the gut microbiome, to explore the molecular and neuropsychologic constellations associated with the escape from cognitive decline.”
The goal of the research was to investigate “to what extent centenarians were able to maintain their cognitive health after study inclusion, and to what extent this was associated with genetic, physical, or neuropathological features,” she said.
The study included 330 centenarians who completed one or more neuropsychological assessments. Neuropathologic studies were available for 44 participants.
To assess baseline cognitive performance, the researchers administered a wide array of neurocognitive tests, as well as the Mini–Mental State Examination, from which mean z scores for cognitive domains were calculated.
Additional factors in the analysis included sex, age, APOE status, cognitive reserve, physical health, and whether participants lived independently.
At autopsy, amyloid-beta (A-beta) level, the level of intracellular accumulation of phosphorylated tau protein in neurofibrillary tangles (NFTs), and the neuritic plaque (NP) load were assessed.
Resilience and cognitive reserve
At baseline, the median age of the centenarians (n = 330, 72.4% women) was 100.5 years (interquartile range, 100.2-101.7). A little over half (56.7%) lived independently, and the majority had good vision (65%) and hearing (56.4%). Most (78.8%) were able to walk independently, and 37.9% had achieved the highest International Standard Classification of Education level of postsecondary education.
The researchers found “varying degrees of neuropathology” in the brains of the 44 donors, including A-beta, NFT, and NPs.
The duration of follow-up in analyzing cognitive trajectories ranged from 0 to 4 years (median, 1.6 years).
Assessments of all cognitive domains showed no decline, with the exception of a “slight” decrement in memory function (beta −.10 SD per year; 95% confidence interval, –.14 to –.05 SD; P < .001).
Cognitive performance was associated with factors of physical health or cognitive reserve, for example, greater independence in performing activities of daily living, as assessed by the Barthel index (beta .37 SD per year; 95% CI, .24-.49; P < .001), or higher educational level (beta .41 SD per year; 95% CI, .29-.53; P < .001).
Despite findings of neuropathologic “hallmarks” of AD post mortem in the brains of the centenarians, these were not associated with cognitive performance or rate of decline.
APOE epsilon-4 or an APOE epsilon-3 alleles also were not significantly associated with cognitive performance or decline, suggesting that the “effects of APOE alleles are exerted before the age of 100 years,” the authors noted.
“Our findings suggest that after reaching age 100 years, cognitive performance remains relatively stable during ensuing years. Therefore, these centenarians might be resilient or resistant against different risk factors of cognitive decline,” the authors wrote. They also speculate that resilience may be attributable to greater cognitive reserve.
“Our preliminary data indicate that approximately 60% of the chance to reach 100 years old is heritable. Therefore, to get a better understanding of which genetic factors associate with the prolonged maintenance of cognitive health, we are looking into which genetic variants occur more commonly in centenarians compared to younger individuals,” said Dr. Holstege.
“Of course, more research needs to be performed to get a better understanding of how such genetic elements might sustain brain health,” she added.
A ‘landmark study’
Commenting on the study in an interview, Thomas Perls, MD, MPH, professor of medicine, Boston University, called it a “landmark” study in research on exceptional longevity in humans.
Dr. Perls, the author of an accompanying editorial, noted that “one cannot absolutely assume a certain level or disability or risk for disease just because a person has achieved extreme age – in fact, if anything, their ability to achieve much older ages likely indicates that they have resistance or resilience to aging-related problems.”
Understanding the mechanism of the resilience could lead to treatment or prevention of AD, said Dr. Perls, who was not involved in the research.
“People have to be careful about ageist myths and attitudes and not have the ageist idea that the older you get, the sicker you get, because many individuals disprove that,” he cautioned.
The study was supported by Stichting Alzheimer Nederland and Stichting Vumc Fonds. Research from the Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Dr. Holstege and Dr. Perls reported having no relevant financial relationships. The other authors’ disclosures are listed on the original article.
A version of this article first appeared on Medscape.com.
Green light puts the stop on migraine
, according to results of a small study from the University of Arizona, Tucson.
“This is the first clinical study to evaluate green light exposure as a potential preventive therapy for patients with migraine, “ senior author Mohab M. Ibrahim, MD, PhD, said in a press release. “Now I have another tool in my toolbox to treat one of the most difficult neurologic conditions – migraine.”
“Given the safety, affordability, and efficacy of green light exposure, there is merit to conduct a larger study,” he and coauthors from the university wrote in their paper.
The study included 29 adult patients (average age 52.2 years), 22 with chronic migraine and the rest with episodic migraine who were recruited from the University of Arizona/Banner Medical Center chronic pain clinic. To be included, patients had to meet the International Headache Society diagnostic criteria for chronic or episodic migraine, have an average headache pain intensity of 5 out of 10 or greater on the numeric pain scale (NPS) over the 10 weeks prior to enrolling in the study, and be dissatisfied with their current migraine therapy.
The patients were free to start, continue, or discontinue any other migraine treatments as recommended by their physicians as long as this was reported to the study team.
White versus green
The one-way crossover design involved exposure to 10 weeks of white light emitting diodes, for 1-2 hours per day, followed by a 2-week washout period and then 10 weeks’ exposure to green light emitting diodes (GLED) for the same daily duration. The protocol involved use of a light strip emitting an intensity of between 4 and 100 lux measured at approximately 2 m and 1 m from a lux meter.
Patients were instructed to use the light in a dark room, without falling asleep, and to participate in activities that did not require external light sources, such as listening to music, reading books, doing exercises, or engaging in similar activities. The daily minimum exposure of 1 hour, up to a maximum of 2 hours, was to be completed in one sitting.
The primary outcome measure was the number of headache days per month, defined as days with moderate to severe headache pain for at least 4 hours. Secondary outcomes included perceived reduction in duration and intensity of the headache phase of the migraine episodes assessed every 2 weeks with the NPS, improved ability to fall and stay asleep, improved ability to perform work and daily activity, improved quality of life, and reduction of pain medications.
The researchers found that when the patients with chronic migraine and episodic migraine were examined as separate groups, white light exposure did not significantly reduce the number of headache days per month, but when the chronic migraine and episodic migraine groups were combined there was a significant reduction from 18.2 to 16.5 headache days per month.
On the other hand, green light did result in significantly reduced headache days both in the separate (from 7.9 to 2.4 days in the episodic migraine group and 22.3 to 9.4 days in the chronic migraine group) and combined groups (from 18.4 to 7.4 days).
“While some improvement in secondary outcomes was observed with white light emitting diodes, more secondary outcomes with significantly greater magnitude including assessments of quality of life, Short-Form McGill Pain Questionnaire, Headache Impact Test-6, and Five-level version of the EuroQol five-dimensional survey without reported side effects were observed with green light emitting diodes,” the authors reported.
“The use of a nonpharmacological therapy such as green light can be of tremendous help to a variety of patients that either do not want to be on medications or do not respond to them,” coauthor Amol M. Patwardhan, MD, PhD, said in the press release. “The beauty of this approach is the lack of associated side effects. If at all, it appears to improve sleep and other quality of life measures,” said Dr. Patwardhan, associate professor and vice chair of research in the University of Arizona’s department of anesthesiology.
Better than white light
Asked to comment on the findings, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said research has shown for some time that exposure to green light has beneficial effects in migraine patients. This study, although small, does indicate that green light is more beneficial than is white light and reduces headache days and intensity. “I believe patients would be willing to spend 1-2 hours a day in green light to reduce and improve their migraine with few side effects. A larger randomized trial should be done,” he said.
The study was funded by support from the National Center for Complementary and Integrative Health (to Dr. Ibrahim), the Comprehensive Chronic Pain and Addiction Center–University of Arizona, and the University of Arizona CHiLLI initiative. Dr. Ibrahim and one coauthor have a patent pending through the University of Arizona for use of green light therapy for the management of chronic pain. Dr. Rapoport is a former president of the International Headache Society. He is an editor of Headache and CNS Drugs, and Editor-in-Chief of Neurology Reviews. He reviews for many peer-reviewed journals such as Cephalalgia, Neurology, New England Journal of Medicine, and Headache.
, according to results of a small study from the University of Arizona, Tucson.
“This is the first clinical study to evaluate green light exposure as a potential preventive therapy for patients with migraine, “ senior author Mohab M. Ibrahim, MD, PhD, said in a press release. “Now I have another tool in my toolbox to treat one of the most difficult neurologic conditions – migraine.”
“Given the safety, affordability, and efficacy of green light exposure, there is merit to conduct a larger study,” he and coauthors from the university wrote in their paper.
The study included 29 adult patients (average age 52.2 years), 22 with chronic migraine and the rest with episodic migraine who were recruited from the University of Arizona/Banner Medical Center chronic pain clinic. To be included, patients had to meet the International Headache Society diagnostic criteria for chronic or episodic migraine, have an average headache pain intensity of 5 out of 10 or greater on the numeric pain scale (NPS) over the 10 weeks prior to enrolling in the study, and be dissatisfied with their current migraine therapy.
The patients were free to start, continue, or discontinue any other migraine treatments as recommended by their physicians as long as this was reported to the study team.
White versus green
The one-way crossover design involved exposure to 10 weeks of white light emitting diodes, for 1-2 hours per day, followed by a 2-week washout period and then 10 weeks’ exposure to green light emitting diodes (GLED) for the same daily duration. The protocol involved use of a light strip emitting an intensity of between 4 and 100 lux measured at approximately 2 m and 1 m from a lux meter.
Patients were instructed to use the light in a dark room, without falling asleep, and to participate in activities that did not require external light sources, such as listening to music, reading books, doing exercises, or engaging in similar activities. The daily minimum exposure of 1 hour, up to a maximum of 2 hours, was to be completed in one sitting.
The primary outcome measure was the number of headache days per month, defined as days with moderate to severe headache pain for at least 4 hours. Secondary outcomes included perceived reduction in duration and intensity of the headache phase of the migraine episodes assessed every 2 weeks with the NPS, improved ability to fall and stay asleep, improved ability to perform work and daily activity, improved quality of life, and reduction of pain medications.
The researchers found that when the patients with chronic migraine and episodic migraine were examined as separate groups, white light exposure did not significantly reduce the number of headache days per month, but when the chronic migraine and episodic migraine groups were combined there was a significant reduction from 18.2 to 16.5 headache days per month.
On the other hand, green light did result in significantly reduced headache days both in the separate (from 7.9 to 2.4 days in the episodic migraine group and 22.3 to 9.4 days in the chronic migraine group) and combined groups (from 18.4 to 7.4 days).
“While some improvement in secondary outcomes was observed with white light emitting diodes, more secondary outcomes with significantly greater magnitude including assessments of quality of life, Short-Form McGill Pain Questionnaire, Headache Impact Test-6, and Five-level version of the EuroQol five-dimensional survey without reported side effects were observed with green light emitting diodes,” the authors reported.
“The use of a nonpharmacological therapy such as green light can be of tremendous help to a variety of patients that either do not want to be on medications or do not respond to them,” coauthor Amol M. Patwardhan, MD, PhD, said in the press release. “The beauty of this approach is the lack of associated side effects. If at all, it appears to improve sleep and other quality of life measures,” said Dr. Patwardhan, associate professor and vice chair of research in the University of Arizona’s department of anesthesiology.
Better than white light
Asked to comment on the findings, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said research has shown for some time that exposure to green light has beneficial effects in migraine patients. This study, although small, does indicate that green light is more beneficial than is white light and reduces headache days and intensity. “I believe patients would be willing to spend 1-2 hours a day in green light to reduce and improve their migraine with few side effects. A larger randomized trial should be done,” he said.
The study was funded by support from the National Center for Complementary and Integrative Health (to Dr. Ibrahim), the Comprehensive Chronic Pain and Addiction Center–University of Arizona, and the University of Arizona CHiLLI initiative. Dr. Ibrahim and one coauthor have a patent pending through the University of Arizona for use of green light therapy for the management of chronic pain. Dr. Rapoport is a former president of the International Headache Society. He is an editor of Headache and CNS Drugs, and Editor-in-Chief of Neurology Reviews. He reviews for many peer-reviewed journals such as Cephalalgia, Neurology, New England Journal of Medicine, and Headache.
, according to results of a small study from the University of Arizona, Tucson.
“This is the first clinical study to evaluate green light exposure as a potential preventive therapy for patients with migraine, “ senior author Mohab M. Ibrahim, MD, PhD, said in a press release. “Now I have another tool in my toolbox to treat one of the most difficult neurologic conditions – migraine.”
“Given the safety, affordability, and efficacy of green light exposure, there is merit to conduct a larger study,” he and coauthors from the university wrote in their paper.
The study included 29 adult patients (average age 52.2 years), 22 with chronic migraine and the rest with episodic migraine who were recruited from the University of Arizona/Banner Medical Center chronic pain clinic. To be included, patients had to meet the International Headache Society diagnostic criteria for chronic or episodic migraine, have an average headache pain intensity of 5 out of 10 or greater on the numeric pain scale (NPS) over the 10 weeks prior to enrolling in the study, and be dissatisfied with their current migraine therapy.
The patients were free to start, continue, or discontinue any other migraine treatments as recommended by their physicians as long as this was reported to the study team.
White versus green
The one-way crossover design involved exposure to 10 weeks of white light emitting diodes, for 1-2 hours per day, followed by a 2-week washout period and then 10 weeks’ exposure to green light emitting diodes (GLED) for the same daily duration. The protocol involved use of a light strip emitting an intensity of between 4 and 100 lux measured at approximately 2 m and 1 m from a lux meter.
Patients were instructed to use the light in a dark room, without falling asleep, and to participate in activities that did not require external light sources, such as listening to music, reading books, doing exercises, or engaging in similar activities. The daily minimum exposure of 1 hour, up to a maximum of 2 hours, was to be completed in one sitting.
The primary outcome measure was the number of headache days per month, defined as days with moderate to severe headache pain for at least 4 hours. Secondary outcomes included perceived reduction in duration and intensity of the headache phase of the migraine episodes assessed every 2 weeks with the NPS, improved ability to fall and stay asleep, improved ability to perform work and daily activity, improved quality of life, and reduction of pain medications.
The researchers found that when the patients with chronic migraine and episodic migraine were examined as separate groups, white light exposure did not significantly reduce the number of headache days per month, but when the chronic migraine and episodic migraine groups were combined there was a significant reduction from 18.2 to 16.5 headache days per month.
On the other hand, green light did result in significantly reduced headache days both in the separate (from 7.9 to 2.4 days in the episodic migraine group and 22.3 to 9.4 days in the chronic migraine group) and combined groups (from 18.4 to 7.4 days).
“While some improvement in secondary outcomes was observed with white light emitting diodes, more secondary outcomes with significantly greater magnitude including assessments of quality of life, Short-Form McGill Pain Questionnaire, Headache Impact Test-6, and Five-level version of the EuroQol five-dimensional survey without reported side effects were observed with green light emitting diodes,” the authors reported.
“The use of a nonpharmacological therapy such as green light can be of tremendous help to a variety of patients that either do not want to be on medications or do not respond to them,” coauthor Amol M. Patwardhan, MD, PhD, said in the press release. “The beauty of this approach is the lack of associated side effects. If at all, it appears to improve sleep and other quality of life measures,” said Dr. Patwardhan, associate professor and vice chair of research in the University of Arizona’s department of anesthesiology.
Better than white light
Asked to comment on the findings, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said research has shown for some time that exposure to green light has beneficial effects in migraine patients. This study, although small, does indicate that green light is more beneficial than is white light and reduces headache days and intensity. “I believe patients would be willing to spend 1-2 hours a day in green light to reduce and improve their migraine with few side effects. A larger randomized trial should be done,” he said.
The study was funded by support from the National Center for Complementary and Integrative Health (to Dr. Ibrahim), the Comprehensive Chronic Pain and Addiction Center–University of Arizona, and the University of Arizona CHiLLI initiative. Dr. Ibrahim and one coauthor have a patent pending through the University of Arizona for use of green light therapy for the management of chronic pain. Dr. Rapoport is a former president of the International Headache Society. He is an editor of Headache and CNS Drugs, and Editor-in-Chief of Neurology Reviews. He reviews for many peer-reviewed journals such as Cephalalgia, Neurology, New England Journal of Medicine, and Headache.
FROM CEPHALALGIA
The Future of Progressive Multiple Sclerosis Therapies (FULL)
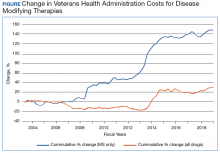
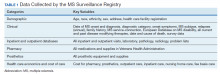
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system, with recent estimates of around 1 million people living with MS in the US.1 In many countries, MS is a leading cause of disability among young adults, second only to trauma.2 Clinically, neurologic worsening (ie, disability) in MS can occur in the relapsing-remitting (RRMS) phase of disease due to incomplete recovery from neuroinflammatory relapses. However, in the 15% of patients with a progressive course from onset (PPMS), and in those with RRMS who transition to a secondary progressive phenotype (SPMS), neurologic worsening follows a slowly progressive pattern.3 A progressive disease course—either PPMS at onset or transitioning to SPMS—is the dominant factor affecting MS-related neurologic disability accumulation. In particular, epidemiologic studies have shown that, in the absence of transitioning to a progressive disease course, < 5% of individuals with MS will accumulate sufficient disability to necessitate use of a cane for ambulation.4-6 Therefore, developing disease modifying therapies (DMTs) that are highly effective at slowing or stopping the gradual accumulation of neurologic disability in progressive MS represent a critical unmet need.
Research into the development of DMTs for progressive MS has been hindered by a number of factors. In particular, the clinical definition and diagnosis of progressive MS has been an evolving concept. Diagnostic criteria for MS, which help facilitate the enrollment of appropriate subjects into clinical trials, have only recently clarified the current consensus definition for progressive MS—steadily increasing neurologic disability independent of clinical relapses. Looking back to the Schumacher criteria in 1965 and Poser criteria in 1983, it was acknowledged that neurologic symptoms in MS may follow a relapsing-remitting or progressive pattern, but little attempt was made to define progressive MS.7,8 The original McDonald criteria in 2001 defined diagnostic criteria for progressive MS.9 These criteria continued to evolve through subsequent revisions (eg, cerebrospinal fluid [CSF] specific oligoclonal bands no longer are an absolute requirement), and only in the 2017 revision was it emphasized that disability progression must occur independent of clinical relapses, concordant with similar emphasis in the 2013 revision of MS clinical course definitions.3,10
The interpretation of prior clinical trials of DMT for progressive MS must consider this evolving clinical definition. The US Food and Drug Administration (FDA) approved mitoxantrone in 2000—making it the first DMT to carry an approved label for SPMS. While achieving significant clinical efficacy, it is clear from the details of the trial that the enrolled subjects had a high degree of inflammatory disease activity, which suggests that mitoxantrone treats neuroinflammation and not relapse-independent worsening. More recently, disparate results were seen in the anti-CD20 (rituximab, ocrelizumab) and S1P receptor modulator (fingolimod, siponimod) trials targeted at patients with primary and secondary progressive MS.11-14 Although there are differences between these therapies, they are more similar than not within the same therapeutic class. Taken together, these trials illustrate the critical impact the narrower inclusion/exclusion criteria (namely age and extent of inflammatory activity) had on attaining positive outcomes. Other considerations, such as confounding illness, also may impact trial recruitment and generalizability of findings.
The lack of known biological targets in progressive MS, which is a complex disease with an incompletely understood and heterogeneous pathology, also hinders DMT development. Decades of research has characterized multifocal central nervous system (CNS) lesions that exhibit features of demyelination, inflammation, reactive gliosis, axonal loss, and neuronal damage. Until recently, however, much of this research focused on the relapsing phase of disease, and so the understanding of the pathologic underpinnings of progressive disease has remained limited. Current areas of investigation encompass a broad range of pathological processes, such as microglial activation, meningeal lymphoid follicles, remyelination failure, vulnerability of chronically demyelinated axons, oxidative injury, iron accumulation, mitochondrial damage, and others. There is the added complication that the pathologic processes underlying progressive MS are superimposed on the CNS aging process. In particular, the transition to progressive MS and the rate of disability accumulation during progressive MS show strong correlation with age.6,15-17
Finally, DMT development for progressive MS also is hindered by the lack of specific surrogate and clinical outcome measures. Trials for relapsing MS have benefited greatly from the relatively straightforward assessment of clinical relapses and inflammatory disease activity on magnetic resonance imaging (MRI). With the goal of developing DMTs that are highly effective at slowing or stopping the gradual accumulation of neurologic disability in progressive MS, which by definition occurs independent of clinical relapses, these measures are not directly relevant. The longitudinal clinical disability outcome measures change at a slower rate than in early, relapsing disease. The use of standardized scales (eg, the Expanded Disability Status Scale [EDSS]), lower limb function, upper limb function, cognition, or a combination is a subject of ongoing debate. For example, the ASCEND and IMPACT trials (placebo-controlled trials for SPMS with natalizumab and interferon β-1a, respectively) showed no significant impact in EDSS progression—but in both of these trials, the 9-hole peg test (9-HPT), a performance measure for upper limb function, showed significant improvement.10,18 Particularly in those with an EDSS of > 6.5, who are unlikely to have measurable EDSS progression, functional tests such as the 9-HPT or timed 25-foot walk may be more sensitive as measures for disability progression.11 MRI measures of brain atrophy is the current gold standard surrogate outcome for clinical trials in progressive MS, but others that may warrant consideration include optical coherence tomography (OCT) or CSF markers of axonal degeneration.
DMT for Progressive MS
Current diagnostic nomenclature separates patients with active (superimposed clinical and/or radiographic relapses) from those with inactive disease.3,12 Relapsing forms of MS include all RRMS and those with SPMS with superimposed relapses (ie, active SPMS). Following this paradigm shift, the FDA changed the indication for already approved DMT from RRMS to relapsing forms of MS. Below is a discussion of DMT that specifically use the term SPMS and PPMS in the indication, where phase 3 trial data for progressive MS is available.
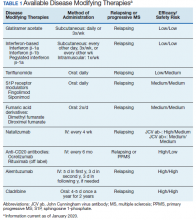
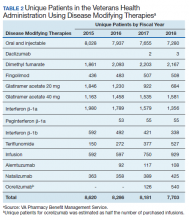
In 2019, the FDA approved the first oral medication (siponimod) for active SPMS. Subsequently, updates to the labels of the older DMT expanded to include active SPMS. Until 2019, the only FDA approved medication for SPMS was mitoxantrone, and use of this medication was limited due to unfavorable adverse effects (AEs). No medications had obtained FDA approval for inactive SPMS to this point, which represented an unmet need for a considerable number of patients.
Mitoxantrone became the first DMT approved for use in SPMS in 2000 following early trials that showed reductions in EDSS worsening, change in ambulation index, reduced number of treated relapses, and prolonged time to first treated relapse. However, as with some of the other positive trials in progressive MS, it is difficult to discern the impact of suppression of relapses as opposed to direct impact on progressive pathophysiology. Within the placebo arm, for example, there were 129 relapses among the 64 subjects, which suggests that these cases had particularly active disease or were in the early stages of SPMS.13 Furthermore, the use of this medication was limited due to concerns of cardiotoxicity and hematologic malignancy as serious AEs.
The trials of interferon β-1b illustrate the same difficulty of isolating possible benefits in disease progression from disability accumulated from relapses. The first interferon β-1b trial for SPMS, was conducted in Europe using fingolimod and showed a delay in confirmed disability progression compared to placebo as measured with the EDSS.14 However, a North American trial that followed in 2004 was unable to replicate this finding.15 The patients in the European trial appeared to be in an earlier phase of SPMS with more active disease, and a post-hoc pooled analysis suggested that patients with active disease and those with more pronounced disability progression were more likely to benefit from treatment.16 Overall, interferons do not appear to appreciably alter disability in the long-term for patients with SPMS, though they may modify short-term, relapse-related disability.
Perhaps the most encouraging data for SPMS was found in the EXPAND trial, which investigated siponimod, an S1P receptor modulator that is more selective than fingolimod. The trial identified a 21% reduction in 3-month confirmed disability progression for SPMS patients taking siponimod compared with those taking a placebo.17 Although the patients in EXPAND did seem to have relatively less disease activity at baseline than those who participated in other SPMS trials, those who benefitted from siponimod were primarily patients who had clinical and/or radiographic relapses over the prior 2 years. Based on this, the FDA approved siponimod for active SPMS. The extent to which siponimod exerts a true neuroprotective effect beyond reducing inflammation has not been clearly established.
B-cell depleting therapies rituximab and ocrelizumab have been evaluated in both primary and secondary progressive MS populations. Early investigations of the chimeric rituximab in PPMS did not show benefits on disability (EDSS) progression; however, benefits were seen in analysis of some subgroups.18 With this in mind, the ORATORIO trial for the humanized version, ocreluzimab, included PPMS patients that were younger (aged < 55 years) and had cutoffs for disease duration (< 15 years for those with EDSS more than 5 years, < 10 years for those with EDSS less than 5 years). The study showed statistically significant changes on disability progression, which led to ocrelizumab receiving the first FDA indication for PPMS.11 There are substantial pathophysiologic similarities between PPMS and SPMS in the progressive phase.19 While these medications may have similar effects across these disease processes, these benefits have not yet been demonstrated in a prospective trial for the SPMS population. Regardless, B-cell depleting therapy is a reasonable consideration for select patients with active SPMS, consistent with a relapsing form of MS.
Therapies in Development
DMT development for progressive MS is a high priority area. Current immunomodulatory therapies for RRMS have consistently been ineffective in the inactive forms of MS, with the possible exceptions of ocrelizumab and siponimod. Therefore, instead of immunosuppression, many agents currently in phase 2 and 3 clinical trials target alternative pathophysiological processes in order to provide neuroprotection, and/or promote remyelination and neurogenesis. These targets include oxidative stress (OS), non-T cell mediated inflammation, and mitochondrial/energy failure.20 Below we review a selection of clinical trials testing agents following these approaches. Many agents have more than one potential mechanism of action (MOA) that could benefit progressive MS.
Lipoic acid (LA), also known as α-lipoic acid and thiotic acid, is one such agent targeting alternative pathophysiology in SPMS. LA is an endogenous agent synthesized de novo from fatty acids and cysteine as well as obtained in small amounts from foods.21 It has antioxidant (AO) properties including direct radical scavenging, regeneration of glutathione, and upregulation of AO enzymes via the NrF2 pathway.22 It supports mitochondria as a key cofactor for pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, and it also aids mitochondrial DNA synthesis.21,22 Studies in experimental autoimmune encephalomyelitis, a widely used experimental mouse model of inflammatory demyelinating disease, also indicate a reduction in excessive microglial activation.23 A phase 2 pilot randomized controlled trial (RCT) of 1200 mg LA in SPMS (n = 51) resulted in significantly less whole brain atrophy by SIENA (Structural Image Evaluation, Using Normalization, of Atrophy) at 2 years.24 A follow-up multicenter trial is ongoing.
Simvastatin also targets alternative pathophysiology in SPMS. It has anti-inflammatory effects, improves vascular function, and promotes neuroprotection by reducing excitotoxicity. A phase 2 RCT demonstrated a reduction in whole brain atrophy in SPMS (n = 140), and a phase 3 trial is underway.25 Ibudilast is another repurposed drug that targets alternative inflammation by inhibiting several cyclic nucleotide phosphodiesterases, macrophage migration inhibitory factor and toll-like receptor 4. A phase 2 trial (n = 225) in both SPMS and PPMS also demonstrated a reduction in brain atrophy, but participants had high rates of AEs.26
Lithium and riluzole promote neuroprotection by reducing excitotoxicity. Lithium is a pharmacologic active cation used as a mood stabilizer and causes inhibition of glycogen synthase kinase-3β. Animal models also indicate that lithium may decrease inflammation and positively impact neurogenesis.27 A crossover pilot trial demonstrated tolerability with trends toward stabilization of EDSS and reductions in brain atrophy.28 Three neuroprotective agents, riluzole (reduces glutamate excitotoxicity), fluoxetine (stimulates glycogenolysis and improves mitochondrial energy production), and amiloride (an acid-sensing ion channel blocker that opens in response to inflammation) were tested in a phase 2b multi-arm, multi-site parallel group RCT in SPMS (n = 445). The study failed to yield differences from placebo for any agent in reduction of brain volume loss.29 A prior study of lamotrigine, a sodium channel blocker, also failed to find changes in brain volume loss.30 These studies highlight the large sample sizes and/or long study durations needed to test agents using brain atrophy as primary outcome. In the future, precise surrogate markers of neuroprotection will be a great need for earlier phase trials. These results also suggest that targeting > 1 MOA may be necessary to treat SPMS effectively.
Efforts to promote remyelination target one hallmark of MS damage. High dose biotin (about 10,000× usual dose) may promote myelin repair as a cofactor for fatty acid synthesis and support mitochondrial oxidative phosphorylation. While a RCT yielded a greater proportion of participants with either PPMS or SPMS with improvement in disability than placebo at 12 months, an open label trial suggested otherwise indicating a need for a more definitive trial.31,32
Anti-LINGO-1 (opicinumab) is a monoclonal antibody that targets LINGO, a potent negative regulator of oligodendrocyte differentiation and myelination.33 Although this agent failed in a phase 2 trial in relapsing MS, and is thus unlikely to be tested in progressive forms, the innovative approach to stimulating oligodendrocytes is ongoing. One such effort is to use thyroid hormone, crucial to myelin formation during development, as a repair agent in MS.34 A dose-finding study of thyroid hormone was completed and efforts to develop a thyromimetic agent are ongoing.
Finally, efforts to promote neurogenesis remain a goal for many neurodegenerative diseases. Exercise appears to prevent age-related atrophy of the hippocampus in animals and humans and help maintain neuronal health.35 In patients with RRMS, cortical thickness is impacted positively by resistance training, which suggests a neuroprotective effect.36 A multi-center trial of exercise in patients with progressive MS investigating cognitive outcomes is ongoing.
Discontinuing DMT
In the early 1990s, the successful development of immune modulating therapies that reliably reduced disease activity in RRMS led to widespread initiation in patients with relapsing disease. However, guidance on when or if to discontinue DMT, even in those who have transitioned to SPMS, remains largely absent at this time. Requests to discontinue DMT may come from patients weary of taking medication (especially injections), bothered by AEs, or those who no longer perceive efficacy from their treatments. Clinicians also may question the benefit of immune modulation in patients with longstanding freedom from relapses or changes in MRI lesion burden.
To inform discussion centered on treatment discontinuation, a clinical trial is currently underway to better answer the question of when and how to withdraw MS therapy. Discontinuation of Disease Modifying Therapies in Multiple Sclerosis (DISCO-MS) is a prospective, placebo-controlled RCT and its primary endpoint is recurrence of disease activity over a 2 year follow-up period.37 Eligibility requirements for the trial include age > 55 years, 5-year freedom from relapses, and 3-year freedom from new MRI lesions (criteria informed by progressive MS cohort studies).31 In addition to demonstrating the active disease recurrence rates in this patient population, the trial also aims to identify risk factors for recurrent disease activity among treated MS patients.37 DISCO-MS builds upon a series of retrospective and observational studies that partially answered these questions, albeit in the context of biases inherent in retrospective or observational studies.
A Minneapolis MS Treatment and Research Center single-center study identified 77 SPMS patients with no acute CNS inflammatory events over 2 to 20 years and advised these patients to stop taking DMT.32 In this group, 11.7% of subjects experienced recurrent active disease. Age was the primary discriminating factor. The mean age of those who experienced disease activity was 56 years vs 61 years those who did not. A second observational study from France found that among 100 SPMS patients treated either with interferon β or glatiramer acetate for at least 6 months, 35% experienced some form of inflammatory disease upon discontinuation.38 Sixteen patients relapsed and 19 developed gadolinium-enhancing MR lesions after DMT discontinuation. However, the age of the cohort was younger than the Minneapolis study (47.2 years vs 61 years), and reasons for discontinuation (eg, AEs or lack of disease activity) were not considered in the analysis.
Other studies examining the safety of DMT discontinuation have not considered MS subtype or excluded patients with progressive subtypes of MS. The largest studies to date on DMT discontinuation utilized the international MSBase global patient registry, which identified nearly 5,000 patients who discontinued interferons (73%), glatiramer acetate (18%), natalizumab (6%), or fingolimod (3%), without specifying the reasons for discontinuation.39 Despite these shortcomings, data reveal trends that are helpful in predicting how MS tends to behave in patients who have discontinued therapy. Not surprisingly, disability progression was most likely among patients already characterized as having a progressive phenotype, while relapses were less likely to occur among older, progressive patients.
Although clinicians may be increasingly willing to discuss DMT discontinuation with their patients, at least 1 study exploring patient perspectives on stopping treatment suggests widespread reluctance to stop treatment. A survey conducted with participants in the North American Research Committee on Multiple Sclerosis patient-report registry found that among survey respondents, only 11.9% would discontinue their MS medication if deemed stable, while 66.3% stated they were unlikely to stop treatment.40
These results suggest that before clinicians incorporate DMT discontinuation into the normal course of discussion with patients, they should be prepared to provide both education (on the wisdom of stopping under the right circumstances) and evidence to support when and how to make the recommendation. Based on existing evidence, criteria for recommending treatment discontinuation might include prolonged freedom from disease activity (≥ 5 years), age > 55 years or 60 years, and a progressive disease course. Thus far, no combination of factors has been shown to completely predict an event-free transition off of medicine. Since no fixed algorithm yet exists to guide DMT stoppage in MS, reasonable suggestions for monitoring patients might include surveillance MRIs, more frequent clinic visits, and possible transitional treatment for patients coming off of natalizumab or fingolimod, since these drugs have been associated with rebound disease activity when discontinued.41,42
Clinicians wishing to maximize function and quality of life for their patients at any age or stage of disease should look to nonpharmacologic interventions to lessen disability and maximize quality of life. While beyond the scope of this discussion, preliminary evidence suggests multimodal (aerobic, resistance, balance) exercise may enhance endurance and cognitive processing speed, and that treatment of comorbid diseases affecting vascular health benefits MS. 43
Conclusions
The development of numerous treatments for RRMS has established an entirely new landscape and disease course for those with MS. While this benefit has not entirely extended to those with progressive MS, those with active disease with superimposed relapses may receive limited benefit from these medications. New insights into the pathophysiology of progressive MS may lead us to new treatments through multiple alternative pathophysiologic pathways. Some early studies using this strategy show promise in slowing the progressive phase. Medication development for progressive MS faces multiple challenges due to lack of a single animal model demonstrating both pathology and clinical effects, absence of phase 1 surrogate biomarkers, and later phase trial endpoints that require large sample sizes and extended study durations. Nevertheless, the increase in number of trials and diversity of therapeutic approaches for progressive MS provides hope for effective therapy. Currently, the heterogeneity of the population with progressive MS requires an individualized treatment approach, and in some of these patients, stopping therapy may be a reasonable consideration. Symptomatic management remains critical for all patients with progressive MS as well as non-pharmacologic approaches that maximize quality of life.
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data [published correction appears in Neurology. 2019;93(15):688]. Neurology. 2019;92(10):e1029-e1040.
2. Browne P, Chandraratna D, Angood C, et al. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83(11):1022-1024.
3. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
4. Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112(Pt 1):133-146. 5. Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129(Pt 3):595-605.
6. Tutuncu M, Tang J, Zeid NA, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler. 2013;19(2):188-198.
7. Schumacher GA, Beebe G, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci. 1965;122:552-568.
8. Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
9. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
10. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
11. Montalban X, Hauser SL, Kappos L, et al; ORATORIO Clinical Investigators. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209-220.
12. Hawker K, O’Connor P, Freedman MS, et al; OLYMPUS trial group. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471.
13. Kappos L, Bar-Or A, Cree BAC, et al; EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study [published correction appears in Lancet. 2018;392(10160):2170]. Lancet. 2018;391(10127):1263-1273.
14. Lublin F, Miller DH, Freedman MS, et al; INFORMS study investigators. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial [published correction appears in Lancet. 2017;389(10066):254]. Lancet. 2016;387(10023):1075-1084.
15. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430-1438.
16. Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain. 2006;129(Pt 3):584-594.
17. Leray E, Yaouanq J, Le Page E, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain. 2010;133(Pt 7):1900–1913.
18. Kapoor R, Ho PR, Campbell N, et al; ASCEND investigators. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405-415.
19. Koch MW, Mostert J, Uitdehaag B, Cutter G. Clinical outcome measures in SPMS trials: an analysis of the IMPACT and ASCEND original trial data sets [published online ahead of print, 2019 Sep 13]. Mult Scler. 2019;1352458519876701.
20. Hartung HP, Gonsette R, König N, et al; Mitoxantrone in Multiple Sclerosis Study Group (MIMS). Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018-2025.
21. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet. 1998;352(9139):1491-1497.
22. Gorąca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid - biological activity and therapeutic potential. Pharmacol Rep. 2011;63:849-858.
23. Chaudhary P, Marracci G, Pocius E, Galipeau D, Morris B, Bourdette D. Effects of lipoic acid on primary murine microglial cells. J Neuroimmunol. 2019;334:576972.
24. Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4:e374.
25. Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383:2213-2221.
26. Fox RJ, Coffey CS, Conwit R, et al. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N Engl J Med. 2018;379:846-855.
27. Rinker JR, 2nd, Cossey TC, Cutter GR, Culpepper WJ. A retrospective review of lithium usage in veterans with multiple sclerosis. Mult Scler Relat Disord. 2013;2:327-333.
28. Rinker JR, W Meador, V Sung, A Nicholas, G Cutter. Results of a pilot trial of lithium in progressive multiple sclerosis. ECTRIMS Online Library. 09/16/16; 145965; P12822016.
29. Chataway J, De Angelis F, Connick P, et al; MS-SMART Investigators. Efficacy of three neuroprotective drugs in secondary progressive multiple sclerosis (MS-SMART): a phase 2b, multiarm, double-blind, randomised placebo-controlled trial. Lancet Neurol. 2020;19(3):214-225.
30. Kapoor R, Furby J, Hayton T, et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:681-688.
31. Paz Soldan MM, Novotna M, Abou Zeid N, et al. Relapses and disability accumulation in progressive multiple sclerosis. Neurology. 2015;84:81-88.
32. Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19:11-14.
33. Ruggieri S, Tortorella C, Gasperini C. Anti lingo 1 (opicinumab) a new monoclonal antibody tested in relapsing remitting multiple sclerosis. Expert Rev Neurother 2017;17:1081-1089.
34. Hartley MD, Banerji T, Tagge IJ, et al. Myelin repair stimulated by CNS-selective thyroid hormone action. JCI Insight. 2019;4(8):e126329.
35. Firth J, Stubbs B, Vancampfort D, et al. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage. 2018;166:230-238.
36. Kjolhede T, Siemonsen S, Wenzel D, et al. Can resistance training impact MRI outcomes in relapsing-remitting multiple sclerosis? Mult Scler. 2018;24:1356-1365.
37. US National Library of Medicine, Clinicaltrials.gov. Discontinuation of Disease Modifying Therapies (DMTs) in Multiple Sclerosis (MS) (DISCOMS). https://clinicaltrials.gov/ct2/show/NCT03073603. Updated February 10, 2020. Accessed March 26, 2020.
38. Bonenfant J, Bajeux E, Deburghgraeve V, Le Page E, Edan G, Kerbrat A. Can we stop immunomodulatory treatments in secondary progressive multiple sclerosis? Eur J Neurol. 2017;24:237-244.
39. Kister I, Spelman T, Patti F, et al. Predictors of relapse and disability progression in MS patients who discontinue disease-modifying therapy. J Neurol Sci. 2018;391:72-76.
40. McGinley MP, Cola PA, Fox RJ, Cohen JA, Corboy JJ, Miller D. Perspectives of individuals with multiple sclerosis on discontinuation of disease-modifying therapies. Mult Scler. 2019:1352458519867314.
41. Hatcher SE, Waubant E, Graves JS. Rebound Syndrome in Multiple Sclerosis After Fingolimod Cessation-Reply. JAMA Neurol. 2016;73:1376.
42. Vellinga MM, Castelijns JA, Barkhof F, Uitdehaag BM, Polman CH. Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology. 2008;70:1150-1151.
43. Sandroff BM, Bollaert RE, Pilutti LA, et al. Multimodal exercise training in multiple sclerosis: A randomized controlled trial in persons with substantial mobility disability. Contemp Clin Trials 2017;61:39-47.
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system, with recent estimates of around 1 million people living with MS in the US.1 In many countries, MS is a leading cause of disability among young adults, second only to trauma.2 Clinically, neurologic worsening (ie, disability) in MS can occur in the relapsing-remitting (RRMS) phase of disease due to incomplete recovery from neuroinflammatory relapses. However, in the 15% of patients with a progressive course from onset (PPMS), and in those with RRMS who transition to a secondary progressive phenotype (SPMS), neurologic worsening follows a slowly progressive pattern.3 A progressive disease course—either PPMS at onset or transitioning to SPMS—is the dominant factor affecting MS-related neurologic disability accumulation. In particular, epidemiologic studies have shown that, in the absence of transitioning to a progressive disease course, < 5% of individuals with MS will accumulate sufficient disability to necessitate use of a cane for ambulation.4-6 Therefore, developing disease modifying therapies (DMTs) that are highly effective at slowing or stopping the gradual accumulation of neurologic disability in progressive MS represent a critical unmet need.
Research into the development of DMTs for progressive MS has been hindered by a number of factors. In particular, the clinical definition and diagnosis of progressive MS has been an evolving concept. Diagnostic criteria for MS, which help facilitate the enrollment of appropriate subjects into clinical trials, have only recently clarified the current consensus definition for progressive MS—steadily increasing neurologic disability independent of clinical relapses. Looking back to the Schumacher criteria in 1965 and Poser criteria in 1983, it was acknowledged that neurologic symptoms in MS may follow a relapsing-remitting or progressive pattern, but little attempt was made to define progressive MS.7,8 The original McDonald criteria in 2001 defined diagnostic criteria for progressive MS.9 These criteria continued to evolve through subsequent revisions (eg, cerebrospinal fluid [CSF] specific oligoclonal bands no longer are an absolute requirement), and only in the 2017 revision was it emphasized that disability progression must occur independent of clinical relapses, concordant with similar emphasis in the 2013 revision of MS clinical course definitions.3,10
The interpretation of prior clinical trials of DMT for progressive MS must consider this evolving clinical definition. The US Food and Drug Administration (FDA) approved mitoxantrone in 2000—making it the first DMT to carry an approved label for SPMS. While achieving significant clinical efficacy, it is clear from the details of the trial that the enrolled subjects had a high degree of inflammatory disease activity, which suggests that mitoxantrone treats neuroinflammation and not relapse-independent worsening. More recently, disparate results were seen in the anti-CD20 (rituximab, ocrelizumab) and S1P receptor modulator (fingolimod, siponimod) trials targeted at patients with primary and secondary progressive MS.11-14 Although there are differences between these therapies, they are more similar than not within the same therapeutic class. Taken together, these trials illustrate the critical impact the narrower inclusion/exclusion criteria (namely age and extent of inflammatory activity) had on attaining positive outcomes. Other considerations, such as confounding illness, also may impact trial recruitment and generalizability of findings.
The lack of known biological targets in progressive MS, which is a complex disease with an incompletely understood and heterogeneous pathology, also hinders DMT development. Decades of research has characterized multifocal central nervous system (CNS) lesions that exhibit features of demyelination, inflammation, reactive gliosis, axonal loss, and neuronal damage. Until recently, however, much of this research focused on the relapsing phase of disease, and so the understanding of the pathologic underpinnings of progressive disease has remained limited. Current areas of investigation encompass a broad range of pathological processes, such as microglial activation, meningeal lymphoid follicles, remyelination failure, vulnerability of chronically demyelinated axons, oxidative injury, iron accumulation, mitochondrial damage, and others. There is the added complication that the pathologic processes underlying progressive MS are superimposed on the CNS aging process. In particular, the transition to progressive MS and the rate of disability accumulation during progressive MS show strong correlation with age.6,15-17
Finally, DMT development for progressive MS also is hindered by the lack of specific surrogate and clinical outcome measures. Trials for relapsing MS have benefited greatly from the relatively straightforward assessment of clinical relapses and inflammatory disease activity on magnetic resonance imaging (MRI). With the goal of developing DMTs that are highly effective at slowing or stopping the gradual accumulation of neurologic disability in progressive MS, which by definition occurs independent of clinical relapses, these measures are not directly relevant. The longitudinal clinical disability outcome measures change at a slower rate than in early, relapsing disease. The use of standardized scales (eg, the Expanded Disability Status Scale [EDSS]), lower limb function, upper limb function, cognition, or a combination is a subject of ongoing debate. For example, the ASCEND and IMPACT trials (placebo-controlled trials for SPMS with natalizumab and interferon β-1a, respectively) showed no significant impact in EDSS progression—but in both of these trials, the 9-hole peg test (9-HPT), a performance measure for upper limb function, showed significant improvement.10,18 Particularly in those with an EDSS of > 6.5, who are unlikely to have measurable EDSS progression, functional tests such as the 9-HPT or timed 25-foot walk may be more sensitive as measures for disability progression.11 MRI measures of brain atrophy is the current gold standard surrogate outcome for clinical trials in progressive MS, but others that may warrant consideration include optical coherence tomography (OCT) or CSF markers of axonal degeneration.
DMT for Progressive MS
Current diagnostic nomenclature separates patients with active (superimposed clinical and/or radiographic relapses) from those with inactive disease.3,12 Relapsing forms of MS include all RRMS and those with SPMS with superimposed relapses (ie, active SPMS). Following this paradigm shift, the FDA changed the indication for already approved DMT from RRMS to relapsing forms of MS. Below is a discussion of DMT that specifically use the term SPMS and PPMS in the indication, where phase 3 trial data for progressive MS is available.
In 2019, the FDA approved the first oral medication (siponimod) for active SPMS. Subsequently, updates to the labels of the older DMT expanded to include active SPMS. Until 2019, the only FDA approved medication for SPMS was mitoxantrone, and use of this medication was limited due to unfavorable adverse effects (AEs). No medications had obtained FDA approval for inactive SPMS to this point, which represented an unmet need for a considerable number of patients.
Mitoxantrone became the first DMT approved for use in SPMS in 2000 following early trials that showed reductions in EDSS worsening, change in ambulation index, reduced number of treated relapses, and prolonged time to first treated relapse. However, as with some of the other positive trials in progressive MS, it is difficult to discern the impact of suppression of relapses as opposed to direct impact on progressive pathophysiology. Within the placebo arm, for example, there were 129 relapses among the 64 subjects, which suggests that these cases had particularly active disease or were in the early stages of SPMS.13 Furthermore, the use of this medication was limited due to concerns of cardiotoxicity and hematologic malignancy as serious AEs.
The trials of interferon β-1b illustrate the same difficulty of isolating possible benefits in disease progression from disability accumulated from relapses. The first interferon β-1b trial for SPMS, was conducted in Europe using fingolimod and showed a delay in confirmed disability progression compared to placebo as measured with the EDSS.14 However, a North American trial that followed in 2004 was unable to replicate this finding.15 The patients in the European trial appeared to be in an earlier phase of SPMS with more active disease, and a post-hoc pooled analysis suggested that patients with active disease and those with more pronounced disability progression were more likely to benefit from treatment.16 Overall, interferons do not appear to appreciably alter disability in the long-term for patients with SPMS, though they may modify short-term, relapse-related disability.
Perhaps the most encouraging data for SPMS was found in the EXPAND trial, which investigated siponimod, an S1P receptor modulator that is more selective than fingolimod. The trial identified a 21% reduction in 3-month confirmed disability progression for SPMS patients taking siponimod compared with those taking a placebo.17 Although the patients in EXPAND did seem to have relatively less disease activity at baseline than those who participated in other SPMS trials, those who benefitted from siponimod were primarily patients who had clinical and/or radiographic relapses over the prior 2 years. Based on this, the FDA approved siponimod for active SPMS. The extent to which siponimod exerts a true neuroprotective effect beyond reducing inflammation has not been clearly established.
B-cell depleting therapies rituximab and ocrelizumab have been evaluated in both primary and secondary progressive MS populations. Early investigations of the chimeric rituximab in PPMS did not show benefits on disability (EDSS) progression; however, benefits were seen in analysis of some subgroups.18 With this in mind, the ORATORIO trial for the humanized version, ocreluzimab, included PPMS patients that were younger (aged < 55 years) and had cutoffs for disease duration (< 15 years for those with EDSS more than 5 years, < 10 years for those with EDSS less than 5 years). The study showed statistically significant changes on disability progression, which led to ocrelizumab receiving the first FDA indication for PPMS.11 There are substantial pathophysiologic similarities between PPMS and SPMS in the progressive phase.19 While these medications may have similar effects across these disease processes, these benefits have not yet been demonstrated in a prospective trial for the SPMS population. Regardless, B-cell depleting therapy is a reasonable consideration for select patients with active SPMS, consistent with a relapsing form of MS.
Therapies in Development
DMT development for progressive MS is a high priority area. Current immunomodulatory therapies for RRMS have consistently been ineffective in the inactive forms of MS, with the possible exceptions of ocrelizumab and siponimod. Therefore, instead of immunosuppression, many agents currently in phase 2 and 3 clinical trials target alternative pathophysiological processes in order to provide neuroprotection, and/or promote remyelination and neurogenesis. These targets include oxidative stress (OS), non-T cell mediated inflammation, and mitochondrial/energy failure.20 Below we review a selection of clinical trials testing agents following these approaches. Many agents have more than one potential mechanism of action (MOA) that could benefit progressive MS.
Lipoic acid (LA), also known as α-lipoic acid and thiotic acid, is one such agent targeting alternative pathophysiology in SPMS. LA is an endogenous agent synthesized de novo from fatty acids and cysteine as well as obtained in small amounts from foods.21 It has antioxidant (AO) properties including direct radical scavenging, regeneration of glutathione, and upregulation of AO enzymes via the NrF2 pathway.22 It supports mitochondria as a key cofactor for pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, and it also aids mitochondrial DNA synthesis.21,22 Studies in experimental autoimmune encephalomyelitis, a widely used experimental mouse model of inflammatory demyelinating disease, also indicate a reduction in excessive microglial activation.23 A phase 2 pilot randomized controlled trial (RCT) of 1200 mg LA in SPMS (n = 51) resulted in significantly less whole brain atrophy by SIENA (Structural Image Evaluation, Using Normalization, of Atrophy) at 2 years.24 A follow-up multicenter trial is ongoing.
Simvastatin also targets alternative pathophysiology in SPMS. It has anti-inflammatory effects, improves vascular function, and promotes neuroprotection by reducing excitotoxicity. A phase 2 RCT demonstrated a reduction in whole brain atrophy in SPMS (n = 140), and a phase 3 trial is underway.25 Ibudilast is another repurposed drug that targets alternative inflammation by inhibiting several cyclic nucleotide phosphodiesterases, macrophage migration inhibitory factor and toll-like receptor 4. A phase 2 trial (n = 225) in both SPMS and PPMS also demonstrated a reduction in brain atrophy, but participants had high rates of AEs.26
Lithium and riluzole promote neuroprotection by reducing excitotoxicity. Lithium is a pharmacologic active cation used as a mood stabilizer and causes inhibition of glycogen synthase kinase-3β. Animal models also indicate that lithium may decrease inflammation and positively impact neurogenesis.27 A crossover pilot trial demonstrated tolerability with trends toward stabilization of EDSS and reductions in brain atrophy.28 Three neuroprotective agents, riluzole (reduces glutamate excitotoxicity), fluoxetine (stimulates glycogenolysis and improves mitochondrial energy production), and amiloride (an acid-sensing ion channel blocker that opens in response to inflammation) were tested in a phase 2b multi-arm, multi-site parallel group RCT in SPMS (n = 445). The study failed to yield differences from placebo for any agent in reduction of brain volume loss.29 A prior study of lamotrigine, a sodium channel blocker, also failed to find changes in brain volume loss.30 These studies highlight the large sample sizes and/or long study durations needed to test agents using brain atrophy as primary outcome. In the future, precise surrogate markers of neuroprotection will be a great need for earlier phase trials. These results also suggest that targeting > 1 MOA may be necessary to treat SPMS effectively.
Efforts to promote remyelination target one hallmark of MS damage. High dose biotin (about 10,000× usual dose) may promote myelin repair as a cofactor for fatty acid synthesis and support mitochondrial oxidative phosphorylation. While a RCT yielded a greater proportion of participants with either PPMS or SPMS with improvement in disability than placebo at 12 months, an open label trial suggested otherwise indicating a need for a more definitive trial.31,32
Anti-LINGO-1 (opicinumab) is a monoclonal antibody that targets LINGO, a potent negative regulator of oligodendrocyte differentiation and myelination.33 Although this agent failed in a phase 2 trial in relapsing MS, and is thus unlikely to be tested in progressive forms, the innovative approach to stimulating oligodendrocytes is ongoing. One such effort is to use thyroid hormone, crucial to myelin formation during development, as a repair agent in MS.34 A dose-finding study of thyroid hormone was completed and efforts to develop a thyromimetic agent are ongoing.
Finally, efforts to promote neurogenesis remain a goal for many neurodegenerative diseases. Exercise appears to prevent age-related atrophy of the hippocampus in animals and humans and help maintain neuronal health.35 In patients with RRMS, cortical thickness is impacted positively by resistance training, which suggests a neuroprotective effect.36 A multi-center trial of exercise in patients with progressive MS investigating cognitive outcomes is ongoing.
Discontinuing DMT
In the early 1990s, the successful development of immune modulating therapies that reliably reduced disease activity in RRMS led to widespread initiation in patients with relapsing disease. However, guidance on when or if to discontinue DMT, even in those who have transitioned to SPMS, remains largely absent at this time. Requests to discontinue DMT may come from patients weary of taking medication (especially injections), bothered by AEs, or those who no longer perceive efficacy from their treatments. Clinicians also may question the benefit of immune modulation in patients with longstanding freedom from relapses or changes in MRI lesion burden.
To inform discussion centered on treatment discontinuation, a clinical trial is currently underway to better answer the question of when and how to withdraw MS therapy. Discontinuation of Disease Modifying Therapies in Multiple Sclerosis (DISCO-MS) is a prospective, placebo-controlled RCT and its primary endpoint is recurrence of disease activity over a 2 year follow-up period.37 Eligibility requirements for the trial include age > 55 years, 5-year freedom from relapses, and 3-year freedom from new MRI lesions (criteria informed by progressive MS cohort studies).31 In addition to demonstrating the active disease recurrence rates in this patient population, the trial also aims to identify risk factors for recurrent disease activity among treated MS patients.37 DISCO-MS builds upon a series of retrospective and observational studies that partially answered these questions, albeit in the context of biases inherent in retrospective or observational studies.
A Minneapolis MS Treatment and Research Center single-center study identified 77 SPMS patients with no acute CNS inflammatory events over 2 to 20 years and advised these patients to stop taking DMT.32 In this group, 11.7% of subjects experienced recurrent active disease. Age was the primary discriminating factor. The mean age of those who experienced disease activity was 56 years vs 61 years those who did not. A second observational study from France found that among 100 SPMS patients treated either with interferon β or glatiramer acetate for at least 6 months, 35% experienced some form of inflammatory disease upon discontinuation.38 Sixteen patients relapsed and 19 developed gadolinium-enhancing MR lesions after DMT discontinuation. However, the age of the cohort was younger than the Minneapolis study (47.2 years vs 61 years), and reasons for discontinuation (eg, AEs or lack of disease activity) were not considered in the analysis.
Other studies examining the safety of DMT discontinuation have not considered MS subtype or excluded patients with progressive subtypes of MS. The largest studies to date on DMT discontinuation utilized the international MSBase global patient registry, which identified nearly 5,000 patients who discontinued interferons (73%), glatiramer acetate (18%), natalizumab (6%), or fingolimod (3%), without specifying the reasons for discontinuation.39 Despite these shortcomings, data reveal trends that are helpful in predicting how MS tends to behave in patients who have discontinued therapy. Not surprisingly, disability progression was most likely among patients already characterized as having a progressive phenotype, while relapses were less likely to occur among older, progressive patients.
Although clinicians may be increasingly willing to discuss DMT discontinuation with their patients, at least 1 study exploring patient perspectives on stopping treatment suggests widespread reluctance to stop treatment. A survey conducted with participants in the North American Research Committee on Multiple Sclerosis patient-report registry found that among survey respondents, only 11.9% would discontinue their MS medication if deemed stable, while 66.3% stated they were unlikely to stop treatment.40
These results suggest that before clinicians incorporate DMT discontinuation into the normal course of discussion with patients, they should be prepared to provide both education (on the wisdom of stopping under the right circumstances) and evidence to support when and how to make the recommendation. Based on existing evidence, criteria for recommending treatment discontinuation might include prolonged freedom from disease activity (≥ 5 years), age > 55 years or 60 years, and a progressive disease course. Thus far, no combination of factors has been shown to completely predict an event-free transition off of medicine. Since no fixed algorithm yet exists to guide DMT stoppage in MS, reasonable suggestions for monitoring patients might include surveillance MRIs, more frequent clinic visits, and possible transitional treatment for patients coming off of natalizumab or fingolimod, since these drugs have been associated with rebound disease activity when discontinued.41,42
Clinicians wishing to maximize function and quality of life for their patients at any age or stage of disease should look to nonpharmacologic interventions to lessen disability and maximize quality of life. While beyond the scope of this discussion, preliminary evidence suggests multimodal (aerobic, resistance, balance) exercise may enhance endurance and cognitive processing speed, and that treatment of comorbid diseases affecting vascular health benefits MS. 43
Conclusions
The development of numerous treatments for RRMS has established an entirely new landscape and disease course for those with MS. While this benefit has not entirely extended to those with progressive MS, those with active disease with superimposed relapses may receive limited benefit from these medications. New insights into the pathophysiology of progressive MS may lead us to new treatments through multiple alternative pathophysiologic pathways. Some early studies using this strategy show promise in slowing the progressive phase. Medication development for progressive MS faces multiple challenges due to lack of a single animal model demonstrating both pathology and clinical effects, absence of phase 1 surrogate biomarkers, and later phase trial endpoints that require large sample sizes and extended study durations. Nevertheless, the increase in number of trials and diversity of therapeutic approaches for progressive MS provides hope for effective therapy. Currently, the heterogeneity of the population with progressive MS requires an individualized treatment approach, and in some of these patients, stopping therapy may be a reasonable consideration. Symptomatic management remains critical for all patients with progressive MS as well as non-pharmacologic approaches that maximize quality of life.
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system, with recent estimates of around 1 million people living with MS in the US.1 In many countries, MS is a leading cause of disability among young adults, second only to trauma.2 Clinically, neurologic worsening (ie, disability) in MS can occur in the relapsing-remitting (RRMS) phase of disease due to incomplete recovery from neuroinflammatory relapses. However, in the 15% of patients with a progressive course from onset (PPMS), and in those with RRMS who transition to a secondary progressive phenotype (SPMS), neurologic worsening follows a slowly progressive pattern.3 A progressive disease course—either PPMS at onset or transitioning to SPMS—is the dominant factor affecting MS-related neurologic disability accumulation. In particular, epidemiologic studies have shown that, in the absence of transitioning to a progressive disease course, < 5% of individuals with MS will accumulate sufficient disability to necessitate use of a cane for ambulation.4-6 Therefore, developing disease modifying therapies (DMTs) that are highly effective at slowing or stopping the gradual accumulation of neurologic disability in progressive MS represent a critical unmet need.
Research into the development of DMTs for progressive MS has been hindered by a number of factors. In particular, the clinical definition and diagnosis of progressive MS has been an evolving concept. Diagnostic criteria for MS, which help facilitate the enrollment of appropriate subjects into clinical trials, have only recently clarified the current consensus definition for progressive MS—steadily increasing neurologic disability independent of clinical relapses. Looking back to the Schumacher criteria in 1965 and Poser criteria in 1983, it was acknowledged that neurologic symptoms in MS may follow a relapsing-remitting or progressive pattern, but little attempt was made to define progressive MS.7,8 The original McDonald criteria in 2001 defined diagnostic criteria for progressive MS.9 These criteria continued to evolve through subsequent revisions (eg, cerebrospinal fluid [CSF] specific oligoclonal bands no longer are an absolute requirement), and only in the 2017 revision was it emphasized that disability progression must occur independent of clinical relapses, concordant with similar emphasis in the 2013 revision of MS clinical course definitions.3,10
The interpretation of prior clinical trials of DMT for progressive MS must consider this evolving clinical definition. The US Food and Drug Administration (FDA) approved mitoxantrone in 2000—making it the first DMT to carry an approved label for SPMS. While achieving significant clinical efficacy, it is clear from the details of the trial that the enrolled subjects had a high degree of inflammatory disease activity, which suggests that mitoxantrone treats neuroinflammation and not relapse-independent worsening. More recently, disparate results were seen in the anti-CD20 (rituximab, ocrelizumab) and S1P receptor modulator (fingolimod, siponimod) trials targeted at patients with primary and secondary progressive MS.11-14 Although there are differences between these therapies, they are more similar than not within the same therapeutic class. Taken together, these trials illustrate the critical impact the narrower inclusion/exclusion criteria (namely age and extent of inflammatory activity) had on attaining positive outcomes. Other considerations, such as confounding illness, also may impact trial recruitment and generalizability of findings.
The lack of known biological targets in progressive MS, which is a complex disease with an incompletely understood and heterogeneous pathology, also hinders DMT development. Decades of research has characterized multifocal central nervous system (CNS) lesions that exhibit features of demyelination, inflammation, reactive gliosis, axonal loss, and neuronal damage. Until recently, however, much of this research focused on the relapsing phase of disease, and so the understanding of the pathologic underpinnings of progressive disease has remained limited. Current areas of investigation encompass a broad range of pathological processes, such as microglial activation, meningeal lymphoid follicles, remyelination failure, vulnerability of chronically demyelinated axons, oxidative injury, iron accumulation, mitochondrial damage, and others. There is the added complication that the pathologic processes underlying progressive MS are superimposed on the CNS aging process. In particular, the transition to progressive MS and the rate of disability accumulation during progressive MS show strong correlation with age.6,15-17
Finally, DMT development for progressive MS also is hindered by the lack of specific surrogate and clinical outcome measures. Trials for relapsing MS have benefited greatly from the relatively straightforward assessment of clinical relapses and inflammatory disease activity on magnetic resonance imaging (MRI). With the goal of developing DMTs that are highly effective at slowing or stopping the gradual accumulation of neurologic disability in progressive MS, which by definition occurs independent of clinical relapses, these measures are not directly relevant. The longitudinal clinical disability outcome measures change at a slower rate than in early, relapsing disease. The use of standardized scales (eg, the Expanded Disability Status Scale [EDSS]), lower limb function, upper limb function, cognition, or a combination is a subject of ongoing debate. For example, the ASCEND and IMPACT trials (placebo-controlled trials for SPMS with natalizumab and interferon β-1a, respectively) showed no significant impact in EDSS progression—but in both of these trials, the 9-hole peg test (9-HPT), a performance measure for upper limb function, showed significant improvement.10,18 Particularly in those with an EDSS of > 6.5, who are unlikely to have measurable EDSS progression, functional tests such as the 9-HPT or timed 25-foot walk may be more sensitive as measures for disability progression.11 MRI measures of brain atrophy is the current gold standard surrogate outcome for clinical trials in progressive MS, but others that may warrant consideration include optical coherence tomography (OCT) or CSF markers of axonal degeneration.
DMT for Progressive MS
Current diagnostic nomenclature separates patients with active (superimposed clinical and/or radiographic relapses) from those with inactive disease.3,12 Relapsing forms of MS include all RRMS and those with SPMS with superimposed relapses (ie, active SPMS). Following this paradigm shift, the FDA changed the indication for already approved DMT from RRMS to relapsing forms of MS. Below is a discussion of DMT that specifically use the term SPMS and PPMS in the indication, where phase 3 trial data for progressive MS is available.
In 2019, the FDA approved the first oral medication (siponimod) for active SPMS. Subsequently, updates to the labels of the older DMT expanded to include active SPMS. Until 2019, the only FDA approved medication for SPMS was mitoxantrone, and use of this medication was limited due to unfavorable adverse effects (AEs). No medications had obtained FDA approval for inactive SPMS to this point, which represented an unmet need for a considerable number of patients.
Mitoxantrone became the first DMT approved for use in SPMS in 2000 following early trials that showed reductions in EDSS worsening, change in ambulation index, reduced number of treated relapses, and prolonged time to first treated relapse. However, as with some of the other positive trials in progressive MS, it is difficult to discern the impact of suppression of relapses as opposed to direct impact on progressive pathophysiology. Within the placebo arm, for example, there were 129 relapses among the 64 subjects, which suggests that these cases had particularly active disease or were in the early stages of SPMS.13 Furthermore, the use of this medication was limited due to concerns of cardiotoxicity and hematologic malignancy as serious AEs.
The trials of interferon β-1b illustrate the same difficulty of isolating possible benefits in disease progression from disability accumulated from relapses. The first interferon β-1b trial for SPMS, was conducted in Europe using fingolimod and showed a delay in confirmed disability progression compared to placebo as measured with the EDSS.14 However, a North American trial that followed in 2004 was unable to replicate this finding.15 The patients in the European trial appeared to be in an earlier phase of SPMS with more active disease, and a post-hoc pooled analysis suggested that patients with active disease and those with more pronounced disability progression were more likely to benefit from treatment.16 Overall, interferons do not appear to appreciably alter disability in the long-term for patients with SPMS, though they may modify short-term, relapse-related disability.
Perhaps the most encouraging data for SPMS was found in the EXPAND trial, which investigated siponimod, an S1P receptor modulator that is more selective than fingolimod. The trial identified a 21% reduction in 3-month confirmed disability progression for SPMS patients taking siponimod compared with those taking a placebo.17 Although the patients in EXPAND did seem to have relatively less disease activity at baseline than those who participated in other SPMS trials, those who benefitted from siponimod were primarily patients who had clinical and/or radiographic relapses over the prior 2 years. Based on this, the FDA approved siponimod for active SPMS. The extent to which siponimod exerts a true neuroprotective effect beyond reducing inflammation has not been clearly established.
B-cell depleting therapies rituximab and ocrelizumab have been evaluated in both primary and secondary progressive MS populations. Early investigations of the chimeric rituximab in PPMS did not show benefits on disability (EDSS) progression; however, benefits were seen in analysis of some subgroups.18 With this in mind, the ORATORIO trial for the humanized version, ocreluzimab, included PPMS patients that were younger (aged < 55 years) and had cutoffs for disease duration (< 15 years for those with EDSS more than 5 years, < 10 years for those with EDSS less than 5 years). The study showed statistically significant changes on disability progression, which led to ocrelizumab receiving the first FDA indication for PPMS.11 There are substantial pathophysiologic similarities between PPMS and SPMS in the progressive phase.19 While these medications may have similar effects across these disease processes, these benefits have not yet been demonstrated in a prospective trial for the SPMS population. Regardless, B-cell depleting therapy is a reasonable consideration for select patients with active SPMS, consistent with a relapsing form of MS.
Therapies in Development
DMT development for progressive MS is a high priority area. Current immunomodulatory therapies for RRMS have consistently been ineffective in the inactive forms of MS, with the possible exceptions of ocrelizumab and siponimod. Therefore, instead of immunosuppression, many agents currently in phase 2 and 3 clinical trials target alternative pathophysiological processes in order to provide neuroprotection, and/or promote remyelination and neurogenesis. These targets include oxidative stress (OS), non-T cell mediated inflammation, and mitochondrial/energy failure.20 Below we review a selection of clinical trials testing agents following these approaches. Many agents have more than one potential mechanism of action (MOA) that could benefit progressive MS.
Lipoic acid (LA), also known as α-lipoic acid and thiotic acid, is one such agent targeting alternative pathophysiology in SPMS. LA is an endogenous agent synthesized de novo from fatty acids and cysteine as well as obtained in small amounts from foods.21 It has antioxidant (AO) properties including direct radical scavenging, regeneration of glutathione, and upregulation of AO enzymes via the NrF2 pathway.22 It supports mitochondria as a key cofactor for pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, and it also aids mitochondrial DNA synthesis.21,22 Studies in experimental autoimmune encephalomyelitis, a widely used experimental mouse model of inflammatory demyelinating disease, also indicate a reduction in excessive microglial activation.23 A phase 2 pilot randomized controlled trial (RCT) of 1200 mg LA in SPMS (n = 51) resulted in significantly less whole brain atrophy by SIENA (Structural Image Evaluation, Using Normalization, of Atrophy) at 2 years.24 A follow-up multicenter trial is ongoing.
Simvastatin also targets alternative pathophysiology in SPMS. It has anti-inflammatory effects, improves vascular function, and promotes neuroprotection by reducing excitotoxicity. A phase 2 RCT demonstrated a reduction in whole brain atrophy in SPMS (n = 140), and a phase 3 trial is underway.25 Ibudilast is another repurposed drug that targets alternative inflammation by inhibiting several cyclic nucleotide phosphodiesterases, macrophage migration inhibitory factor and toll-like receptor 4. A phase 2 trial (n = 225) in both SPMS and PPMS also demonstrated a reduction in brain atrophy, but participants had high rates of AEs.26
Lithium and riluzole promote neuroprotection by reducing excitotoxicity. Lithium is a pharmacologic active cation used as a mood stabilizer and causes inhibition of glycogen synthase kinase-3β. Animal models also indicate that lithium may decrease inflammation and positively impact neurogenesis.27 A crossover pilot trial demonstrated tolerability with trends toward stabilization of EDSS and reductions in brain atrophy.28 Three neuroprotective agents, riluzole (reduces glutamate excitotoxicity), fluoxetine (stimulates glycogenolysis and improves mitochondrial energy production), and amiloride (an acid-sensing ion channel blocker that opens in response to inflammation) were tested in a phase 2b multi-arm, multi-site parallel group RCT in SPMS (n = 445). The study failed to yield differences from placebo for any agent in reduction of brain volume loss.29 A prior study of lamotrigine, a sodium channel blocker, also failed to find changes in brain volume loss.30 These studies highlight the large sample sizes and/or long study durations needed to test agents using brain atrophy as primary outcome. In the future, precise surrogate markers of neuroprotection will be a great need for earlier phase trials. These results also suggest that targeting > 1 MOA may be necessary to treat SPMS effectively.
Efforts to promote remyelination target one hallmark of MS damage. High dose biotin (about 10,000× usual dose) may promote myelin repair as a cofactor for fatty acid synthesis and support mitochondrial oxidative phosphorylation. While a RCT yielded a greater proportion of participants with either PPMS or SPMS with improvement in disability than placebo at 12 months, an open label trial suggested otherwise indicating a need for a more definitive trial.31,32
Anti-LINGO-1 (opicinumab) is a monoclonal antibody that targets LINGO, a potent negative regulator of oligodendrocyte differentiation and myelination.33 Although this agent failed in a phase 2 trial in relapsing MS, and is thus unlikely to be tested in progressive forms, the innovative approach to stimulating oligodendrocytes is ongoing. One such effort is to use thyroid hormone, crucial to myelin formation during development, as a repair agent in MS.34 A dose-finding study of thyroid hormone was completed and efforts to develop a thyromimetic agent are ongoing.
Finally, efforts to promote neurogenesis remain a goal for many neurodegenerative diseases. Exercise appears to prevent age-related atrophy of the hippocampus in animals and humans and help maintain neuronal health.35 In patients with RRMS, cortical thickness is impacted positively by resistance training, which suggests a neuroprotective effect.36 A multi-center trial of exercise in patients with progressive MS investigating cognitive outcomes is ongoing.
Discontinuing DMT
In the early 1990s, the successful development of immune modulating therapies that reliably reduced disease activity in RRMS led to widespread initiation in patients with relapsing disease. However, guidance on when or if to discontinue DMT, even in those who have transitioned to SPMS, remains largely absent at this time. Requests to discontinue DMT may come from patients weary of taking medication (especially injections), bothered by AEs, or those who no longer perceive efficacy from their treatments. Clinicians also may question the benefit of immune modulation in patients with longstanding freedom from relapses or changes in MRI lesion burden.
To inform discussion centered on treatment discontinuation, a clinical trial is currently underway to better answer the question of when and how to withdraw MS therapy. Discontinuation of Disease Modifying Therapies in Multiple Sclerosis (DISCO-MS) is a prospective, placebo-controlled RCT and its primary endpoint is recurrence of disease activity over a 2 year follow-up period.37 Eligibility requirements for the trial include age > 55 years, 5-year freedom from relapses, and 3-year freedom from new MRI lesions (criteria informed by progressive MS cohort studies).31 In addition to demonstrating the active disease recurrence rates in this patient population, the trial also aims to identify risk factors for recurrent disease activity among treated MS patients.37 DISCO-MS builds upon a series of retrospective and observational studies that partially answered these questions, albeit in the context of biases inherent in retrospective or observational studies.
A Minneapolis MS Treatment and Research Center single-center study identified 77 SPMS patients with no acute CNS inflammatory events over 2 to 20 years and advised these patients to stop taking DMT.32 In this group, 11.7% of subjects experienced recurrent active disease. Age was the primary discriminating factor. The mean age of those who experienced disease activity was 56 years vs 61 years those who did not. A second observational study from France found that among 100 SPMS patients treated either with interferon β or glatiramer acetate for at least 6 months, 35% experienced some form of inflammatory disease upon discontinuation.38 Sixteen patients relapsed and 19 developed gadolinium-enhancing MR lesions after DMT discontinuation. However, the age of the cohort was younger than the Minneapolis study (47.2 years vs 61 years), and reasons for discontinuation (eg, AEs or lack of disease activity) were not considered in the analysis.
Other studies examining the safety of DMT discontinuation have not considered MS subtype or excluded patients with progressive subtypes of MS. The largest studies to date on DMT discontinuation utilized the international MSBase global patient registry, which identified nearly 5,000 patients who discontinued interferons (73%), glatiramer acetate (18%), natalizumab (6%), or fingolimod (3%), without specifying the reasons for discontinuation.39 Despite these shortcomings, data reveal trends that are helpful in predicting how MS tends to behave in patients who have discontinued therapy. Not surprisingly, disability progression was most likely among patients already characterized as having a progressive phenotype, while relapses were less likely to occur among older, progressive patients.
Although clinicians may be increasingly willing to discuss DMT discontinuation with their patients, at least 1 study exploring patient perspectives on stopping treatment suggests widespread reluctance to stop treatment. A survey conducted with participants in the North American Research Committee on Multiple Sclerosis patient-report registry found that among survey respondents, only 11.9% would discontinue their MS medication if deemed stable, while 66.3% stated they were unlikely to stop treatment.40
These results suggest that before clinicians incorporate DMT discontinuation into the normal course of discussion with patients, they should be prepared to provide both education (on the wisdom of stopping under the right circumstances) and evidence to support when and how to make the recommendation. Based on existing evidence, criteria for recommending treatment discontinuation might include prolonged freedom from disease activity (≥ 5 years), age > 55 years or 60 years, and a progressive disease course. Thus far, no combination of factors has been shown to completely predict an event-free transition off of medicine. Since no fixed algorithm yet exists to guide DMT stoppage in MS, reasonable suggestions for monitoring patients might include surveillance MRIs, more frequent clinic visits, and possible transitional treatment for patients coming off of natalizumab or fingolimod, since these drugs have been associated with rebound disease activity when discontinued.41,42
Clinicians wishing to maximize function and quality of life for their patients at any age or stage of disease should look to nonpharmacologic interventions to lessen disability and maximize quality of life. While beyond the scope of this discussion, preliminary evidence suggests multimodal (aerobic, resistance, balance) exercise may enhance endurance and cognitive processing speed, and that treatment of comorbid diseases affecting vascular health benefits MS. 43
Conclusions
The development of numerous treatments for RRMS has established an entirely new landscape and disease course for those with MS. While this benefit has not entirely extended to those with progressive MS, those with active disease with superimposed relapses may receive limited benefit from these medications. New insights into the pathophysiology of progressive MS may lead us to new treatments through multiple alternative pathophysiologic pathways. Some early studies using this strategy show promise in slowing the progressive phase. Medication development for progressive MS faces multiple challenges due to lack of a single animal model demonstrating both pathology and clinical effects, absence of phase 1 surrogate biomarkers, and later phase trial endpoints that require large sample sizes and extended study durations. Nevertheless, the increase in number of trials and diversity of therapeutic approaches for progressive MS provides hope for effective therapy. Currently, the heterogeneity of the population with progressive MS requires an individualized treatment approach, and in some of these patients, stopping therapy may be a reasonable consideration. Symptomatic management remains critical for all patients with progressive MS as well as non-pharmacologic approaches that maximize quality of life.
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data [published correction appears in Neurology. 2019;93(15):688]. Neurology. 2019;92(10):e1029-e1040.
2. Browne P, Chandraratna D, Angood C, et al. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83(11):1022-1024.
3. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
4. Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112(Pt 1):133-146. 5. Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129(Pt 3):595-605.
6. Tutuncu M, Tang J, Zeid NA, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler. 2013;19(2):188-198.
7. Schumacher GA, Beebe G, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci. 1965;122:552-568.
8. Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
9. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
10. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
11. Montalban X, Hauser SL, Kappos L, et al; ORATORIO Clinical Investigators. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209-220.
12. Hawker K, O’Connor P, Freedman MS, et al; OLYMPUS trial group. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471.
13. Kappos L, Bar-Or A, Cree BAC, et al; EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study [published correction appears in Lancet. 2018;392(10160):2170]. Lancet. 2018;391(10127):1263-1273.
14. Lublin F, Miller DH, Freedman MS, et al; INFORMS study investigators. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial [published correction appears in Lancet. 2017;389(10066):254]. Lancet. 2016;387(10023):1075-1084.
15. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430-1438.
16. Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain. 2006;129(Pt 3):584-594.
17. Leray E, Yaouanq J, Le Page E, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain. 2010;133(Pt 7):1900–1913.
18. Kapoor R, Ho PR, Campbell N, et al; ASCEND investigators. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405-415.
19. Koch MW, Mostert J, Uitdehaag B, Cutter G. Clinical outcome measures in SPMS trials: an analysis of the IMPACT and ASCEND original trial data sets [published online ahead of print, 2019 Sep 13]. Mult Scler. 2019;1352458519876701.
20. Hartung HP, Gonsette R, König N, et al; Mitoxantrone in Multiple Sclerosis Study Group (MIMS). Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018-2025.
21. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet. 1998;352(9139):1491-1497.
22. Gorąca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid - biological activity and therapeutic potential. Pharmacol Rep. 2011;63:849-858.
23. Chaudhary P, Marracci G, Pocius E, Galipeau D, Morris B, Bourdette D. Effects of lipoic acid on primary murine microglial cells. J Neuroimmunol. 2019;334:576972.
24. Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4:e374.
25. Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383:2213-2221.
26. Fox RJ, Coffey CS, Conwit R, et al. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N Engl J Med. 2018;379:846-855.
27. Rinker JR, 2nd, Cossey TC, Cutter GR, Culpepper WJ. A retrospective review of lithium usage in veterans with multiple sclerosis. Mult Scler Relat Disord. 2013;2:327-333.
28. Rinker JR, W Meador, V Sung, A Nicholas, G Cutter. Results of a pilot trial of lithium in progressive multiple sclerosis. ECTRIMS Online Library. 09/16/16; 145965; P12822016.
29. Chataway J, De Angelis F, Connick P, et al; MS-SMART Investigators. Efficacy of three neuroprotective drugs in secondary progressive multiple sclerosis (MS-SMART): a phase 2b, multiarm, double-blind, randomised placebo-controlled trial. Lancet Neurol. 2020;19(3):214-225.
30. Kapoor R, Furby J, Hayton T, et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:681-688.
31. Paz Soldan MM, Novotna M, Abou Zeid N, et al. Relapses and disability accumulation in progressive multiple sclerosis. Neurology. 2015;84:81-88.
32. Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19:11-14.
33. Ruggieri S, Tortorella C, Gasperini C. Anti lingo 1 (opicinumab) a new monoclonal antibody tested in relapsing remitting multiple sclerosis. Expert Rev Neurother 2017;17:1081-1089.
34. Hartley MD, Banerji T, Tagge IJ, et al. Myelin repair stimulated by CNS-selective thyroid hormone action. JCI Insight. 2019;4(8):e126329.
35. Firth J, Stubbs B, Vancampfort D, et al. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage. 2018;166:230-238.
36. Kjolhede T, Siemonsen S, Wenzel D, et al. Can resistance training impact MRI outcomes in relapsing-remitting multiple sclerosis? Mult Scler. 2018;24:1356-1365.
37. US National Library of Medicine, Clinicaltrials.gov. Discontinuation of Disease Modifying Therapies (DMTs) in Multiple Sclerosis (MS) (DISCOMS). https://clinicaltrials.gov/ct2/show/NCT03073603. Updated February 10, 2020. Accessed March 26, 2020.
38. Bonenfant J, Bajeux E, Deburghgraeve V, Le Page E, Edan G, Kerbrat A. Can we stop immunomodulatory treatments in secondary progressive multiple sclerosis? Eur J Neurol. 2017;24:237-244.
39. Kister I, Spelman T, Patti F, et al. Predictors of relapse and disability progression in MS patients who discontinue disease-modifying therapy. J Neurol Sci. 2018;391:72-76.
40. McGinley MP, Cola PA, Fox RJ, Cohen JA, Corboy JJ, Miller D. Perspectives of individuals with multiple sclerosis on discontinuation of disease-modifying therapies. Mult Scler. 2019:1352458519867314.
41. Hatcher SE, Waubant E, Graves JS. Rebound Syndrome in Multiple Sclerosis After Fingolimod Cessation-Reply. JAMA Neurol. 2016;73:1376.
42. Vellinga MM, Castelijns JA, Barkhof F, Uitdehaag BM, Polman CH. Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology. 2008;70:1150-1151.
43. Sandroff BM, Bollaert RE, Pilutti LA, et al. Multimodal exercise training in multiple sclerosis: A randomized controlled trial in persons with substantial mobility disability. Contemp Clin Trials 2017;61:39-47.
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data [published correction appears in Neurology. 2019;93(15):688]. Neurology. 2019;92(10):e1029-e1040.
2. Browne P, Chandraratna D, Angood C, et al. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83(11):1022-1024.
3. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
4. Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112(Pt 1):133-146. 5. Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129(Pt 3):595-605.
6. Tutuncu M, Tang J, Zeid NA, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler. 2013;19(2):188-198.
7. Schumacher GA, Beebe G, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci. 1965;122:552-568.
8. Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
9. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
10. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
11. Montalban X, Hauser SL, Kappos L, et al; ORATORIO Clinical Investigators. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209-220.
12. Hawker K, O’Connor P, Freedman MS, et al; OLYMPUS trial group. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471.
13. Kappos L, Bar-Or A, Cree BAC, et al; EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study [published correction appears in Lancet. 2018;392(10160):2170]. Lancet. 2018;391(10127):1263-1273.
14. Lublin F, Miller DH, Freedman MS, et al; INFORMS study investigators. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial [published correction appears in Lancet. 2017;389(10066):254]. Lancet. 2016;387(10023):1075-1084.
15. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430-1438.
16. Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain. 2006;129(Pt 3):584-594.
17. Leray E, Yaouanq J, Le Page E, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain. 2010;133(Pt 7):1900–1913.
18. Kapoor R, Ho PR, Campbell N, et al; ASCEND investigators. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405-415.
19. Koch MW, Mostert J, Uitdehaag B, Cutter G. Clinical outcome measures in SPMS trials: an analysis of the IMPACT and ASCEND original trial data sets [published online ahead of print, 2019 Sep 13]. Mult Scler. 2019;1352458519876701.
20. Hartung HP, Gonsette R, König N, et al; Mitoxantrone in Multiple Sclerosis Study Group (MIMS). Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018-2025.
21. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet. 1998;352(9139):1491-1497.
22. Gorąca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid - biological activity and therapeutic potential. Pharmacol Rep. 2011;63:849-858.
23. Chaudhary P, Marracci G, Pocius E, Galipeau D, Morris B, Bourdette D. Effects of lipoic acid on primary murine microglial cells. J Neuroimmunol. 2019;334:576972.
24. Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4:e374.
25. Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383:2213-2221.
26. Fox RJ, Coffey CS, Conwit R, et al. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N Engl J Med. 2018;379:846-855.
27. Rinker JR, 2nd, Cossey TC, Cutter GR, Culpepper WJ. A retrospective review of lithium usage in veterans with multiple sclerosis. Mult Scler Relat Disord. 2013;2:327-333.
28. Rinker JR, W Meador, V Sung, A Nicholas, G Cutter. Results of a pilot trial of lithium in progressive multiple sclerosis. ECTRIMS Online Library. 09/16/16; 145965; P12822016.
29. Chataway J, De Angelis F, Connick P, et al; MS-SMART Investigators. Efficacy of three neuroprotective drugs in secondary progressive multiple sclerosis (MS-SMART): a phase 2b, multiarm, double-blind, randomised placebo-controlled trial. Lancet Neurol. 2020;19(3):214-225.
30. Kapoor R, Furby J, Hayton T, et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:681-688.
31. Paz Soldan MM, Novotna M, Abou Zeid N, et al. Relapses and disability accumulation in progressive multiple sclerosis. Neurology. 2015;84:81-88.
32. Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19:11-14.
33. Ruggieri S, Tortorella C, Gasperini C. Anti lingo 1 (opicinumab) a new monoclonal antibody tested in relapsing remitting multiple sclerosis. Expert Rev Neurother 2017;17:1081-1089.
34. Hartley MD, Banerji T, Tagge IJ, et al. Myelin repair stimulated by CNS-selective thyroid hormone action. JCI Insight. 2019;4(8):e126329.
35. Firth J, Stubbs B, Vancampfort D, et al. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage. 2018;166:230-238.
36. Kjolhede T, Siemonsen S, Wenzel D, et al. Can resistance training impact MRI outcomes in relapsing-remitting multiple sclerosis? Mult Scler. 2018;24:1356-1365.
37. US National Library of Medicine, Clinicaltrials.gov. Discontinuation of Disease Modifying Therapies (DMTs) in Multiple Sclerosis (MS) (DISCOMS). https://clinicaltrials.gov/ct2/show/NCT03073603. Updated February 10, 2020. Accessed March 26, 2020.
38. Bonenfant J, Bajeux E, Deburghgraeve V, Le Page E, Edan G, Kerbrat A. Can we stop immunomodulatory treatments in secondary progressive multiple sclerosis? Eur J Neurol. 2017;24:237-244.
39. Kister I, Spelman T, Patti F, et al. Predictors of relapse and disability progression in MS patients who discontinue disease-modifying therapy. J Neurol Sci. 2018;391:72-76.
40. McGinley MP, Cola PA, Fox RJ, Cohen JA, Corboy JJ, Miller D. Perspectives of individuals with multiple sclerosis on discontinuation of disease-modifying therapies. Mult Scler. 2019:1352458519867314.
41. Hatcher SE, Waubant E, Graves JS. Rebound Syndrome in Multiple Sclerosis After Fingolimod Cessation-Reply. JAMA Neurol. 2016;73:1376.
42. Vellinga MM, Castelijns JA, Barkhof F, Uitdehaag BM, Polman CH. Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology. 2008;70:1150-1151.
43. Sandroff BM, Bollaert RE, Pilutti LA, et al. Multimodal exercise training in multiple sclerosis: A randomized controlled trial in persons with substantial mobility disability. Contemp Clin Trials 2017;61:39-47.
Multiple Sclerosis Medications in the VHA: Delivering Specialty, High-Cost, Pharmacy Care in a National System (FULL)
Prior to the first approved disease modifying therapy (DMT) in the 1990s, treatment approaches for multiple sclerosis (MS) were not well understood. The discovery that MS was an immune mediated inflammatory disease paved the way for the treatments we know today. In 1993, interferon β‐1b became the first DMT for MS approved by the US Food and Drug Administration (FDA). Approvals for interferon β‐1a as well as glatiramer acetate (GA) soon followed. Today, we consider these the mildest immunosuppressant DMTs; however, their success verified that suppressing the immune system had a positive effect on the MS disease process.
Following these approvals, the disease process in MS is now better understood. Recently approved therapies include monoclonal antibodies, which affect other immune pathways. Today, there are 14 approved DMTs (Table 1). Although the advent of these newer DMTs has revolutionized care for patients with MS, it has been accompanied by increasing costs for the agents. Direct medical costs associated with MS management, coupled with indirect costs from lost productivity, have been estimated to be $24.2 billion annually in the US.1 These increases have been seen across many levels of insurance coverage—private payer, Medicare, and the Veterans Health Administration (VHA).2,3
The Figure demonstrates the cost increase that have been seen across VHA between 2004 and 2019 for the DMTs identified in Table 1. Indeed, this compound annual growth rate may be an underestimate because infusion therapies (eg, natalizumab, ocrelizumab, and alemtuzumab) are difficult to track as they may be dispensed directly via a Risk Evaluation Medication Strategy (REMS) program. According to the VHA Pharmacy Benefit Management Service (PBM), in September 2019, dimethyl fumarate (DMF) had the 13th highest total outpatient drug cost for the US Department of Veterans Affairs (VA), interferon β‐1a ranked 62nd and 83rd (prefilled pen and syringe, respectively), and GA 40 mg ranked 89th.
The DMT landscape has demonstrated significant price fluctuations and given rise to a class of medications that requires extensive oversight in terms of efficacy, safety, and cost minimization. The purpose of this article is to show how delivery of this specialty group of medications can be optimized with safety, efficacy, and cost value within a large health care system.
Factors Impacting DMT Use
Recent changes to MS typing have impacted utilization of DMTs. Traditionally, there were 4 subtypes of MS: relapsing remitting (RRMS), secondary progressive (SPMS), progressive relapsing (PRMS), and primary progressive (PPMS). These subtypes are now viewed more broadly and grouped as either relapsing or progressive. The traditional subtypes fall under these broader definitions. Additionally, SPMS has been broken into active SPMS, characterized by continued worsening of disability unrelated to acute relapses, superimposed with activity that can be seen on magnetic resonance images (MRIs), and nonactive SPMS, which has the same disability progression as active SPMS but without MRI-visible activity.4-6 In 2019, these supplementary designations to SPMS made their first appearance in FDA-approved indications. All existing DMTs now include this terminology in their labelling and are indicated in active SPMS. There remain no DMTs that treat nonactive SPMS.
The current landscape of DMTs is highly varied in method of administration, risks, and benefits. As efficacy of these medications often is marked by how well they can prevent the immune system from attacking myelin, an inverse relationship between safety and efficacy results. The standard treatment outcomes in MS have evolved over time. The following are the commonly used primary outcomes in clinical trials: relapse reduction; increased time between relapses; decreased severity of relapses; prevention or extend time to disability milestones as measured by the Expanded Disability Status Scale (EDSS) and other disability measures; prevention or extension of time to onset of secondary progressive disease; prevention or reduction of the number and size of new and enhancing lesions on MRI; and limitation of overall MRI lesion burden in the central nervous system (CNS).
Newer treatment outcomes employed in more recent trials include: measures of axonal damage, CNS atrophy, evidence of microscopic disease via conventional MRI and advanced imaging modalities, biomarkers associated with inflammatory disease activity and neurodegeneration in MS, and the use of no evidence of disease activity (NEDA). These outcomes also must be evaluated by the safety concerns of each agent. Short- and long-term safety are critical factors in the selection of DMTs for MS. The injectable therapies for MS (interferon β‐1a, interferon β‐1b, and GA) have established long-term safety profiles from > 20 years of continuous use. The long-term safety profiles of oral immunomodulatory agents and monoclonal antibodies for these drugs in MS have yet to be determined. Safety concerns associated with some therapies and added requirements for safety monitoring may increase the complexity of a therapeutic selection.
Current cost minimization strategies for DMT include limiting DMT agents on formularies, tier systems that incentivize patients/prescribers to select the lowest priced agents on the formulary, negotiating arrangements with manufacturers to freeze prices or provide discounts in exchange for a priority position in the formulary, and requiring prior authorization to initiate or switch therapy. The use of generic medications and interchange to these agents from a brand name formulation can help reduce expense. Several of these strategies have been implemented in VHA.
Disease-Modifying Therapies
In 2019, 18,645 veterans with MS had either a MS-specific DMT or ≥ 1 annual encounters with a primary diagnosis of MS. Of this population, 4,720 were female and 13,357 were service connected according to VA data. About 50% of veterans with MS take a DMT. This percentage has remained stable over the past decade (Table 2). Although it appears the number of unique veterans prescribed an outpatient DMT is decreasing, this does not include the growing use of infused DMTs or DMTs obtained through the Veterans Choice Program (VCP)/Community Care (CC).
The overall outpatient pharmacy costs for veterans have remained constant despite the reduction in outpatient pharmacy prescription numbers. This may be due to increases in DMT cost to the VHA and the use of more expensive oral agents over the previously used platform injection DMTs.
Generic Conversion
GA is available in 20 mg daily and 40 mg3 times weekly subcutaneous injection dosing. The first evidence of clinical efficacy for a generic formulation for GA was evaluated by the GATE trial.7 This trial was a multicenter, randomized, double-blind, active- and placebo-controlled phase 3 trial. Eligible participants were randomized to receive daily SC injection for 9 months of 20 mg generic GA (n = 5,353), 20 mg brand GA (n = 5,357), or placebo (n = 584). The primary endpoint was the mean number of gadolinium (Gd1) lesions visible on MRIs during months 7, 8, and 9, which were significantly reduced in the combined GA-treated group and in each GA group individually when compared with the placebo group, confirming the study sensitivity (ie, GA was effective under the conditions of the study). Tolerability (including injection site reactions) and safety (incidence, spectrum, and severity of adverse events [AEs]) were similar in the generic and brand GA groups. These results demonstrated that generic and brand GA had equivalent efficacy, tolerability, and safety over a 9-month period.7
Results of a 15-month extension of the study were presented in 2015 and showed similar efficacy, safety, and tolerability in participants treated with generic GA for 2 years and patients switched from brand to generic GA.8 Multiple shifts for GA occurred, most notably the conversion from branded Copaxone (Teva Pharmaceutical Industries) to generic Glatopa (Sandoz). Subsequently, Sandoz released a generic 40 mg 3 times weekly formulation. Additionally, Mylan entered the generic GA market. With 3 competing manufacturers, internal data from the VHA indicated that it was able to negotiate a single source contract for this medication that provided a savings of $32,088,904.69 between September 2016 and May 2019.
The impact of generic conversions is just being realized. Soon, patents will begin to expire for oral DMTs, leading to an expected growth of generic alternatives. Already the FDA has approved 4 generic alternatives for teriflunomide, 3 for fingolimod (with 13 tentative approvals), and 15 generic alternatives for dimethyl fumarate (DMF). Implementation of therapeutic interchanges will be pursued by VHA as clinically supported by evidence.
Criteria for Use
PBM supports utilizing criteria to help guide providers on DMT options and promote safe, effective, and value-based selection of a DMT. The PBM creates monographs and criteria for use (CFU) for new medications. The monograph contains a literature evaluation of all studies available to date that concern both safety and efficacy of the new medication. Therapeutic alternatives also are presented and assessed for key elements that may determine the most safe and effective use. Additional safety areas for the new medications such as look-alike, sound-alike potential, special populations use (ie, those who are pregnant, the elderly, and those with liver or renal dysfunction), and drug-drug interactions are presented. Lastly, and possibly most importantly in an ever-growing growing world of DMTs, the monograph describes a reasonable place in therapy for the new DMT.
CFU are additional guidance for some DMTs. The development of CFU are based on several questions that arise during the monograph development for a DMT. These include, but are not limited to:
- Are there safety concerns that require the drug to receive a review to ensure safe prescribing (eg, agents with REMS programs, or safety concerns in specific populations)?
- Does the drug require a specialty provider type with knowledge and experience in those disease states to ensure appropriate and safe prescribing (eg restricted to infectious diseases)?
- Do VHA or non-VHA guidelines suggest alternative therapy be used prior to the agent?
- Is a review deemed necessary to ensure the preferred agent is used first (eg, second-line therapy)?
The CFU defines parameters of drug use consistent with high quality and evidence-based patient care. CFUs also serve as a basis for monitoring local, regional, and national patterns of pharmacologic care and help guide health care providers (HCPs) on appropriate use of medication.
CFUs are designed to ensure the HCP is safely starting a medication that has evidence for efficacy for their patient. For example, alemtuzumab is a high-risk, high-efficacy DMT. The alemtuzumab CFU acknowledges this by having exclusion criteria that prevent a veteran at high risk (ie, on another immunosuppressant) from being exposed to severe AEs (ie, severe leukopenia) that are associated with the medication. On the other hand, the inclusion criteria recognize the benefits of alemtuzumab and allows those with highly active MS who have failed other DMTs to receive the medication.
The drug monograph and CFU process is an important part of VHA efforts to optimize patient care. After a draft version is developed, HCPs can provide feedback on the exclusion/inclusion criteria and describe how they anticipate using the medication in their practice. This insight can be beneficial for MS treatment as diverse HCPs may have distinct viewpoints on how DMTs should be started. Pharmacists and physicians on a national level then discuss and decide together what to include in the final drafts of the drug monograph and CFU. Final documents are disseminated to all sites, which encourages consistent practices across the VHA.9 These documents are reviewed on a regular basis and updated as needed based on available literature evidence.
It is well accepted that early use of DMT correlates with lower accumulated long-term disability.10 However, discontinuation of DMT should be treated with equal importance. This benefits the patient by reducing their risk of AEs from DMTs and provides cost savings. Age and disease stability are factors to consider for DMT discontinuation. In a study with patients aged > 45 years and another with patients aged > 60 years, discontinuing DMT rarely had a negative impact and improved quality of life.11,12 A retrospective meta-analysis of age-dependent efficacy of current DMTs predicted that DMT loses efficacy at age 53 years. In addition, higher efficacy DMT only outperforms lower efficacy DMT in patients aged < 40.5 years.13 Stability of disease and lack of relapses for ≥ 2 years also may be a positive predictor to safely discontinue DMT.14,15 The growing literature to support safe discontinuation of DMT makes this a more convincing strategy to avoid unnecessary costs associated with current DMTs. With an average age of 59 years for veterans with MS, this may be one of the largest areas of cost avoidance to consider.
Off-Label Use
Other potential ways to reduce DMT costs is to consider off-label treatments. The OLYMPUS trial studied off-label use of rituximab, an anti-CD20 antibody like ocrelizumab. It did not meet statistical significance for its primary endpoint; however, in a subgroup analysis, off-label use was found to be more effective in a population aged < 51 years.16 Other case reports and smaller scale studies also describe rituximab’s efficacy in MS.17,18 In 2018, the FDA approved the first rituximab biosimilar.19 Further competition from biosimilars likely will make rituximab an even more cost-effective choice when compared with ocrelizumab.
Alternate Dosing Regimens
Extended interval dosing of natalizumab has been studied, extending the standard infusion interval from every 4 weeks to 5- to 8-week intervals. One recent article compared these interval extensions and found that all extended intervals of up to 56 days did not increase new or enhancing lesions on MRI when compared with standard interval dosing.20 Another larger randomized trial is underway to evaluate efficacy and safety of extended interval dosing of natalizumab (NCT03689972). Utilization of this dosing may reduce natalizumab annual costs by up to 50%.
Safety Monitoring
DMF is an oral DMT on the VHA formulary with CFU. Since leukopenia is a known AE, baseline and quarterly monitoring of the complete blood count (CBC) is recommended for patients taking DMF. Additionally, DMF should be held if white blood cell count (WBC) falls below 2,000/mm3.21 There have been recent reports of death secondary to progressive multifocal leukoencephalopathy (PML) among European patients taking DMF.22-24 This has raised concerns about adherence to recommended CBC monitoring in veterans taking DMF. The association of DMF and leukopenia has been evident since early clinical trials.25 Leukopenia in immunocompromised patients increases the risk of PML.
In the long-term extension study ENDORSE, 6% to 7% of patients continuing DMF had WBC counts of 3.0×109/L compared with 7% to 10% in the new to DMF group.26 In addition 6% to 8% of patients continuing DMF had lymphocyte counts of 0.5×109/L, compared with 5% to 9% in the new to DMF group. The cases of PML occurred in patients who had low lymphocyte counts over an extended period with no adjustment to DMF therapy, such as holding the drug until WBC counts returned to normal levels or stopping the drug. Discussion and review within VHA resulted in the recommendation for quarterly WBC monitoring criteria.
PBM and VA Center for Medication Safety (MedSafe) conducted a medication usage evaluation (MUE) on adherence to the WBC monitoring set forth in the CFU. Data collection began in fourth quarter of fiscal year (FY) 2015 with the most recent reporting period of fourth quarter of FY 2017. The Medication Utilization Evaluation Tool tracks patients with no reported WBC in 90 days and WBC < 2,000/mm3. Over the reporting period, 20% to 23% of patients have not received appropriate quarterly monitoring. Additionally, there have been 4 cases where the WBC decreased below the threshold limit. To ensure safe and effective use of DMF, it is important to adhere to the monitoring requirements set forth in the CFU.
Impact of REMS and Special Distribution
As DMTs increase in efficacy, there are often more risks associated with them. Some of these high-risk medications, including natalizumab and alemtuzumab, have REMS programs and/or have special distribution procedures. Although REMS are imperative for patient safety, the complexity of these programs can be difficult to navigate, which can create a barrier to access. The PBM helps to assist all sites with navigating and adhering to required actions to dispense and administer these medications through a national Special Handling Drugs Microsoft SharePoint site, which provides access to REMS forms and procurement information when drugs are dispensed from specialty pharmacies. Easing this process nationwide empowers more sites to be confident they can dispense specialty medications appropriately.
Clinical Pharmacists
The VHA is unique in its utilization of pharmacists in outpatient clinic settings. Utilization of an interdisciplinary team for medication management has been highly used in VHA for areas like primary care; however, pharmacist involvement in specialty areas is on the rise and MS is no exception. Pharmacists stationed in clinics, such as neurology or spinal cord injury, can impact care for veterans with MS. Interdisciplinary teams that include a pharmacist have been shown to increase patient adherence to DMTs.27 However, pharmacists often assist with medication education and monitoring, which adds an additional layer of safety to DMT treatment. At the VHA, pharmacists also can obtain a scope of practice that allows them to prescribe medications and increase access to care for veterans with MS.
Education
The VHA demonstrates how education on a disease state like MS can be distributed on a large, national scale through drug monographs, CFU, and Microsoft SharePoint sites. In addition, VHA has created the MS Centers of Excellence (MSCoE) that serve as a hub of specialized health care providers in all aspects of MS care.
A core function of the MSCoE is to provide education to both HCPs and patients. The MSCoE and its regional hubs support sites that may not have an HCP who specializes in MS by providing advice on DMT selection, how to obtain specialty medications, and monitoring that needs to be completed to ensure veterans’ safety. The MSCoE also has partnered with the National MS Society to hold a lecture series on topics in MS. This free series is available online to all HCPs who interact with patients who have MS and is a way that VA is extending its best practices and expertise beyond its own health care system. There also is a quarterly newsletter for veterans with MS that highlights new information on DMTs that can affect their care.
Conclusion
It is an exciting and challenging period in MS treatment. New DMTs are being approved and entering clinical trials at a rapid pace. These new DMT agents may offer increased efficacy, improvements in AE profiles, and the possibility of increased medication adherence—but often at a higher cost. The utilization of CFU and formulary management provides the ability to ensure the safe and appropriate use of medications by veterans, with a secondary outcome of controlling pharmacy expenditures.
The VHA had expenditures of $142,135,938 for DMT use in FY 2018. As the VHA sees the new contract prices for DMT in January 2020, we are reminded that costs will continue to rise with some pharmaceutical manufacturers implementing prices 8% to 11% higher than 2019 prices, when the consumer price index defines an increase of 1.0% for 2020 and 1.4% in 2021.28 It is imperative that the VHA formulary be managed judiciously and the necessary measures be in place for VHA practitioners to enable effective, safe and value-based care to the veteran population.
1. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81(4):479-484.
2. Hartung DM, Bourdette DN, Ahmed SM, Whitham RH. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? [published correction appears in Neurology. 2015;85(19):1728]. Neurology. 2015;84(21):2185–2192.
3. San-Juan-Rodriguez A, Good CB, Heyman RA, Parekh N, Shrank WH, Hernandez I. Trends in prices, market share, and spending on self-administered disease-modifying therapies for multiple sclerosis in Medicare Part D. JAMA Neurol. 2019;76(11):1386-1390.
4. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
5. Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis [published correction appears in Mult Scler. 2003;9(6):641]. Mult Scler. 2003;9(3):260-274.
6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
7. Cohen J, Belova A, Selmaj K, et al. Equivalence of generic glatiramer acetate in multiple sclerosis: a randomized clinical trial. JAMA Neurol. 2015;72(12):1433-1441.
8. Selmaj K, Barkhof F, Belova AN, et al; GATE study group. Switching from branded to generic glatiramer acetate: 15-month GATE trial extension results. Mult Scler. 2017;23(14):1909-1917.
9. Aspinall SL, Sales MM, Good CB, et al. Pharmacy benefits management in the Veterans Health Administration revisited: a decade of advancements, 2004-2014. J Manag Care Spec Pharm. 2016;22(9):1058-1063.
10. Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321(2):175-187.
11. Hua LH, Harris H, Conway D, Thompson NR. Changes in patient-reported outcomes between continuers and discontinuers of disease modifying therapy in patients with multiple sclerosis over age 60 [published correction appears in Mult Scler Relat Disord. 2019;30:293]. Mult Scler Relat Disord. 2019;30:252-256.
12. Bsteh G, Feige J, Ehling R, et al. Discontinuation of disease-modifying therapies in multiple sclerosis - Clinical outcome and prognostic factors. Mult Scler. 2017;23(9):1241-1248.
13. Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.
14. Kister I, Spelman T, Alroughani R, et al; MSBase Study Group. Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study [published correction appears in J Neurol Neurosurg Psychiatry. 2019;90(4):e2]. J Neurol Neurosurg Psychiatry. 2016;87(10):1133-1137.
15. Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19(1):11-14.
16. Hawker K, O’Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471.
17. Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688.
18. Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79(6):950–958.
19. Rituximab-abbs [package insert]. North Wales, PA: Teva Pharmaceuticals; 2018.
20. Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(8):885-889.
21. Dimethyl fumarate [package insert]. Cambridge, MA: Biogen Inc; 2015.
22. van Kester MS, Bouwes Bavinck JN, Quint KD. PML in Patients treated with dimethyl fumarate. N Engl J Med. 2015;373(6):583-584.
23. Nieuwkamp DJ, Murk JL, van Oosten BW. PML in patients treated with dimethyl fumarate. N Engl J Med. 2015;373(6):584.
24. Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372(15):1476-1478.
25. Longbrake EE, Cross AH. Dimethyl fumarate associated lymphopenia in clinical practice. Mult Scler. 2015;21(6):796-797.
26. Gold R, Arnold DL, Bar-Or A, et al. Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: Interim analysis of ENDORSE, a randomized extension study. Mult Scler. 2017;23(2):253–265.
27. Hanson RL, Habibi M, Khamo N, Abdou S, Stubbings J. Integrated clinical and specialty pharmacy practice model for management of patients with multiple sclerosis. Am J Health Syst Pharm. 2014;71(6):463-469.
28. Federal Planning Bureau. Consumer Price Index - Inflation forecasts. https://www.plan.be/databases/17-en-consumer+price+index+inflation+forecasts. Updated March 3, 2020. Accessed March 9, 2020.
Prior to the first approved disease modifying therapy (DMT) in the 1990s, treatment approaches for multiple sclerosis (MS) were not well understood. The discovery that MS was an immune mediated inflammatory disease paved the way for the treatments we know today. In 1993, interferon β‐1b became the first DMT for MS approved by the US Food and Drug Administration (FDA). Approvals for interferon β‐1a as well as glatiramer acetate (GA) soon followed. Today, we consider these the mildest immunosuppressant DMTs; however, their success verified that suppressing the immune system had a positive effect on the MS disease process.
Following these approvals, the disease process in MS is now better understood. Recently approved therapies include monoclonal antibodies, which affect other immune pathways. Today, there are 14 approved DMTs (Table 1). Although the advent of these newer DMTs has revolutionized care for patients with MS, it has been accompanied by increasing costs for the agents. Direct medical costs associated with MS management, coupled with indirect costs from lost productivity, have been estimated to be $24.2 billion annually in the US.1 These increases have been seen across many levels of insurance coverage—private payer, Medicare, and the Veterans Health Administration (VHA).2,3
The Figure demonstrates the cost increase that have been seen across VHA between 2004 and 2019 for the DMTs identified in Table 1. Indeed, this compound annual growth rate may be an underestimate because infusion therapies (eg, natalizumab, ocrelizumab, and alemtuzumab) are difficult to track as they may be dispensed directly via a Risk Evaluation Medication Strategy (REMS) program. According to the VHA Pharmacy Benefit Management Service (PBM), in September 2019, dimethyl fumarate (DMF) had the 13th highest total outpatient drug cost for the US Department of Veterans Affairs (VA), interferon β‐1a ranked 62nd and 83rd (prefilled pen and syringe, respectively), and GA 40 mg ranked 89th.
The DMT landscape has demonstrated significant price fluctuations and given rise to a class of medications that requires extensive oversight in terms of efficacy, safety, and cost minimization. The purpose of this article is to show how delivery of this specialty group of medications can be optimized with safety, efficacy, and cost value within a large health care system.
Factors Impacting DMT Use
Recent changes to MS typing have impacted utilization of DMTs. Traditionally, there were 4 subtypes of MS: relapsing remitting (RRMS), secondary progressive (SPMS), progressive relapsing (PRMS), and primary progressive (PPMS). These subtypes are now viewed more broadly and grouped as either relapsing or progressive. The traditional subtypes fall under these broader definitions. Additionally, SPMS has been broken into active SPMS, characterized by continued worsening of disability unrelated to acute relapses, superimposed with activity that can be seen on magnetic resonance images (MRIs), and nonactive SPMS, which has the same disability progression as active SPMS but without MRI-visible activity.4-6 In 2019, these supplementary designations to SPMS made their first appearance in FDA-approved indications. All existing DMTs now include this terminology in their labelling and are indicated in active SPMS. There remain no DMTs that treat nonactive SPMS.
The current landscape of DMTs is highly varied in method of administration, risks, and benefits. As efficacy of these medications often is marked by how well they can prevent the immune system from attacking myelin, an inverse relationship between safety and efficacy results. The standard treatment outcomes in MS have evolved over time. The following are the commonly used primary outcomes in clinical trials: relapse reduction; increased time between relapses; decreased severity of relapses; prevention or extend time to disability milestones as measured by the Expanded Disability Status Scale (EDSS) and other disability measures; prevention or extension of time to onset of secondary progressive disease; prevention or reduction of the number and size of new and enhancing lesions on MRI; and limitation of overall MRI lesion burden in the central nervous system (CNS).
Newer treatment outcomes employed in more recent trials include: measures of axonal damage, CNS atrophy, evidence of microscopic disease via conventional MRI and advanced imaging modalities, biomarkers associated with inflammatory disease activity and neurodegeneration in MS, and the use of no evidence of disease activity (NEDA). These outcomes also must be evaluated by the safety concerns of each agent. Short- and long-term safety are critical factors in the selection of DMTs for MS. The injectable therapies for MS (interferon β‐1a, interferon β‐1b, and GA) have established long-term safety profiles from > 20 years of continuous use. The long-term safety profiles of oral immunomodulatory agents and monoclonal antibodies for these drugs in MS have yet to be determined. Safety concerns associated with some therapies and added requirements for safety monitoring may increase the complexity of a therapeutic selection.
Current cost minimization strategies for DMT include limiting DMT agents on formularies, tier systems that incentivize patients/prescribers to select the lowest priced agents on the formulary, negotiating arrangements with manufacturers to freeze prices or provide discounts in exchange for a priority position in the formulary, and requiring prior authorization to initiate or switch therapy. The use of generic medications and interchange to these agents from a brand name formulation can help reduce expense. Several of these strategies have been implemented in VHA.
Disease-Modifying Therapies
In 2019, 18,645 veterans with MS had either a MS-specific DMT or ≥ 1 annual encounters with a primary diagnosis of MS. Of this population, 4,720 were female and 13,357 were service connected according to VA data. About 50% of veterans with MS take a DMT. This percentage has remained stable over the past decade (Table 2). Although it appears the number of unique veterans prescribed an outpatient DMT is decreasing, this does not include the growing use of infused DMTs or DMTs obtained through the Veterans Choice Program (VCP)/Community Care (CC).
The overall outpatient pharmacy costs for veterans have remained constant despite the reduction in outpatient pharmacy prescription numbers. This may be due to increases in DMT cost to the VHA and the use of more expensive oral agents over the previously used platform injection DMTs.
Generic Conversion
GA is available in 20 mg daily and 40 mg3 times weekly subcutaneous injection dosing. The first evidence of clinical efficacy for a generic formulation for GA was evaluated by the GATE trial.7 This trial was a multicenter, randomized, double-blind, active- and placebo-controlled phase 3 trial. Eligible participants were randomized to receive daily SC injection for 9 months of 20 mg generic GA (n = 5,353), 20 mg brand GA (n = 5,357), or placebo (n = 584). The primary endpoint was the mean number of gadolinium (Gd1) lesions visible on MRIs during months 7, 8, and 9, which were significantly reduced in the combined GA-treated group and in each GA group individually when compared with the placebo group, confirming the study sensitivity (ie, GA was effective under the conditions of the study). Tolerability (including injection site reactions) and safety (incidence, spectrum, and severity of adverse events [AEs]) were similar in the generic and brand GA groups. These results demonstrated that generic and brand GA had equivalent efficacy, tolerability, and safety over a 9-month period.7
Results of a 15-month extension of the study were presented in 2015 and showed similar efficacy, safety, and tolerability in participants treated with generic GA for 2 years and patients switched from brand to generic GA.8 Multiple shifts for GA occurred, most notably the conversion from branded Copaxone (Teva Pharmaceutical Industries) to generic Glatopa (Sandoz). Subsequently, Sandoz released a generic 40 mg 3 times weekly formulation. Additionally, Mylan entered the generic GA market. With 3 competing manufacturers, internal data from the VHA indicated that it was able to negotiate a single source contract for this medication that provided a savings of $32,088,904.69 between September 2016 and May 2019.
The impact of generic conversions is just being realized. Soon, patents will begin to expire for oral DMTs, leading to an expected growth of generic alternatives. Already the FDA has approved 4 generic alternatives for teriflunomide, 3 for fingolimod (with 13 tentative approvals), and 15 generic alternatives for dimethyl fumarate (DMF). Implementation of therapeutic interchanges will be pursued by VHA as clinically supported by evidence.
Criteria for Use
PBM supports utilizing criteria to help guide providers on DMT options and promote safe, effective, and value-based selection of a DMT. The PBM creates monographs and criteria for use (CFU) for new medications. The monograph contains a literature evaluation of all studies available to date that concern both safety and efficacy of the new medication. Therapeutic alternatives also are presented and assessed for key elements that may determine the most safe and effective use. Additional safety areas for the new medications such as look-alike, sound-alike potential, special populations use (ie, those who are pregnant, the elderly, and those with liver or renal dysfunction), and drug-drug interactions are presented. Lastly, and possibly most importantly in an ever-growing growing world of DMTs, the monograph describes a reasonable place in therapy for the new DMT.
CFU are additional guidance for some DMTs. The development of CFU are based on several questions that arise during the monograph development for a DMT. These include, but are not limited to:
- Are there safety concerns that require the drug to receive a review to ensure safe prescribing (eg, agents with REMS programs, or safety concerns in specific populations)?
- Does the drug require a specialty provider type with knowledge and experience in those disease states to ensure appropriate and safe prescribing (eg restricted to infectious diseases)?
- Do VHA or non-VHA guidelines suggest alternative therapy be used prior to the agent?
- Is a review deemed necessary to ensure the preferred agent is used first (eg, second-line therapy)?
The CFU defines parameters of drug use consistent with high quality and evidence-based patient care. CFUs also serve as a basis for monitoring local, regional, and national patterns of pharmacologic care and help guide health care providers (HCPs) on appropriate use of medication.
CFUs are designed to ensure the HCP is safely starting a medication that has evidence for efficacy for their patient. For example, alemtuzumab is a high-risk, high-efficacy DMT. The alemtuzumab CFU acknowledges this by having exclusion criteria that prevent a veteran at high risk (ie, on another immunosuppressant) from being exposed to severe AEs (ie, severe leukopenia) that are associated with the medication. On the other hand, the inclusion criteria recognize the benefits of alemtuzumab and allows those with highly active MS who have failed other DMTs to receive the medication.
The drug monograph and CFU process is an important part of VHA efforts to optimize patient care. After a draft version is developed, HCPs can provide feedback on the exclusion/inclusion criteria and describe how they anticipate using the medication in their practice. This insight can be beneficial for MS treatment as diverse HCPs may have distinct viewpoints on how DMTs should be started. Pharmacists and physicians on a national level then discuss and decide together what to include in the final drafts of the drug monograph and CFU. Final documents are disseminated to all sites, which encourages consistent practices across the VHA.9 These documents are reviewed on a regular basis and updated as needed based on available literature evidence.
It is well accepted that early use of DMT correlates with lower accumulated long-term disability.10 However, discontinuation of DMT should be treated with equal importance. This benefits the patient by reducing their risk of AEs from DMTs and provides cost savings. Age and disease stability are factors to consider for DMT discontinuation. In a study with patients aged > 45 years and another with patients aged > 60 years, discontinuing DMT rarely had a negative impact and improved quality of life.11,12 A retrospective meta-analysis of age-dependent efficacy of current DMTs predicted that DMT loses efficacy at age 53 years. In addition, higher efficacy DMT only outperforms lower efficacy DMT in patients aged < 40.5 years.13 Stability of disease and lack of relapses for ≥ 2 years also may be a positive predictor to safely discontinue DMT.14,15 The growing literature to support safe discontinuation of DMT makes this a more convincing strategy to avoid unnecessary costs associated with current DMTs. With an average age of 59 years for veterans with MS, this may be one of the largest areas of cost avoidance to consider.
Off-Label Use
Other potential ways to reduce DMT costs is to consider off-label treatments. The OLYMPUS trial studied off-label use of rituximab, an anti-CD20 antibody like ocrelizumab. It did not meet statistical significance for its primary endpoint; however, in a subgroup analysis, off-label use was found to be more effective in a population aged < 51 years.16 Other case reports and smaller scale studies also describe rituximab’s efficacy in MS.17,18 In 2018, the FDA approved the first rituximab biosimilar.19 Further competition from biosimilars likely will make rituximab an even more cost-effective choice when compared with ocrelizumab.
Alternate Dosing Regimens
Extended interval dosing of natalizumab has been studied, extending the standard infusion interval from every 4 weeks to 5- to 8-week intervals. One recent article compared these interval extensions and found that all extended intervals of up to 56 days did not increase new or enhancing lesions on MRI when compared with standard interval dosing.20 Another larger randomized trial is underway to evaluate efficacy and safety of extended interval dosing of natalizumab (NCT03689972). Utilization of this dosing may reduce natalizumab annual costs by up to 50%.
Safety Monitoring
DMF is an oral DMT on the VHA formulary with CFU. Since leukopenia is a known AE, baseline and quarterly monitoring of the complete blood count (CBC) is recommended for patients taking DMF. Additionally, DMF should be held if white blood cell count (WBC) falls below 2,000/mm3.21 There have been recent reports of death secondary to progressive multifocal leukoencephalopathy (PML) among European patients taking DMF.22-24 This has raised concerns about adherence to recommended CBC monitoring in veterans taking DMF. The association of DMF and leukopenia has been evident since early clinical trials.25 Leukopenia in immunocompromised patients increases the risk of PML.
In the long-term extension study ENDORSE, 6% to 7% of patients continuing DMF had WBC counts of 3.0×109/L compared with 7% to 10% in the new to DMF group.26 In addition 6% to 8% of patients continuing DMF had lymphocyte counts of 0.5×109/L, compared with 5% to 9% in the new to DMF group. The cases of PML occurred in patients who had low lymphocyte counts over an extended period with no adjustment to DMF therapy, such as holding the drug until WBC counts returned to normal levels or stopping the drug. Discussion and review within VHA resulted in the recommendation for quarterly WBC monitoring criteria.
PBM and VA Center for Medication Safety (MedSafe) conducted a medication usage evaluation (MUE) on adherence to the WBC monitoring set forth in the CFU. Data collection began in fourth quarter of fiscal year (FY) 2015 with the most recent reporting period of fourth quarter of FY 2017. The Medication Utilization Evaluation Tool tracks patients with no reported WBC in 90 days and WBC < 2,000/mm3. Over the reporting period, 20% to 23% of patients have not received appropriate quarterly monitoring. Additionally, there have been 4 cases where the WBC decreased below the threshold limit. To ensure safe and effective use of DMF, it is important to adhere to the monitoring requirements set forth in the CFU.
Impact of REMS and Special Distribution
As DMTs increase in efficacy, there are often more risks associated with them. Some of these high-risk medications, including natalizumab and alemtuzumab, have REMS programs and/or have special distribution procedures. Although REMS are imperative for patient safety, the complexity of these programs can be difficult to navigate, which can create a barrier to access. The PBM helps to assist all sites with navigating and adhering to required actions to dispense and administer these medications through a national Special Handling Drugs Microsoft SharePoint site, which provides access to REMS forms and procurement information when drugs are dispensed from specialty pharmacies. Easing this process nationwide empowers more sites to be confident they can dispense specialty medications appropriately.
Clinical Pharmacists
The VHA is unique in its utilization of pharmacists in outpatient clinic settings. Utilization of an interdisciplinary team for medication management has been highly used in VHA for areas like primary care; however, pharmacist involvement in specialty areas is on the rise and MS is no exception. Pharmacists stationed in clinics, such as neurology or spinal cord injury, can impact care for veterans with MS. Interdisciplinary teams that include a pharmacist have been shown to increase patient adherence to DMTs.27 However, pharmacists often assist with medication education and monitoring, which adds an additional layer of safety to DMT treatment. At the VHA, pharmacists also can obtain a scope of practice that allows them to prescribe medications and increase access to care for veterans with MS.
Education
The VHA demonstrates how education on a disease state like MS can be distributed on a large, national scale through drug monographs, CFU, and Microsoft SharePoint sites. In addition, VHA has created the MS Centers of Excellence (MSCoE) that serve as a hub of specialized health care providers in all aspects of MS care.
A core function of the MSCoE is to provide education to both HCPs and patients. The MSCoE and its regional hubs support sites that may not have an HCP who specializes in MS by providing advice on DMT selection, how to obtain specialty medications, and monitoring that needs to be completed to ensure veterans’ safety. The MSCoE also has partnered with the National MS Society to hold a lecture series on topics in MS. This free series is available online to all HCPs who interact with patients who have MS and is a way that VA is extending its best practices and expertise beyond its own health care system. There also is a quarterly newsletter for veterans with MS that highlights new information on DMTs that can affect their care.
Conclusion
It is an exciting and challenging period in MS treatment. New DMTs are being approved and entering clinical trials at a rapid pace. These new DMT agents may offer increased efficacy, improvements in AE profiles, and the possibility of increased medication adherence—but often at a higher cost. The utilization of CFU and formulary management provides the ability to ensure the safe and appropriate use of medications by veterans, with a secondary outcome of controlling pharmacy expenditures.
The VHA had expenditures of $142,135,938 for DMT use in FY 2018. As the VHA sees the new contract prices for DMT in January 2020, we are reminded that costs will continue to rise with some pharmaceutical manufacturers implementing prices 8% to 11% higher than 2019 prices, when the consumer price index defines an increase of 1.0% for 2020 and 1.4% in 2021.28 It is imperative that the VHA formulary be managed judiciously and the necessary measures be in place for VHA practitioners to enable effective, safe and value-based care to the veteran population.
Prior to the first approved disease modifying therapy (DMT) in the 1990s, treatment approaches for multiple sclerosis (MS) were not well understood. The discovery that MS was an immune mediated inflammatory disease paved the way for the treatments we know today. In 1993, interferon β‐1b became the first DMT for MS approved by the US Food and Drug Administration (FDA). Approvals for interferon β‐1a as well as glatiramer acetate (GA) soon followed. Today, we consider these the mildest immunosuppressant DMTs; however, their success verified that suppressing the immune system had a positive effect on the MS disease process.
Following these approvals, the disease process in MS is now better understood. Recently approved therapies include monoclonal antibodies, which affect other immune pathways. Today, there are 14 approved DMTs (Table 1). Although the advent of these newer DMTs has revolutionized care for patients with MS, it has been accompanied by increasing costs for the agents. Direct medical costs associated with MS management, coupled with indirect costs from lost productivity, have been estimated to be $24.2 billion annually in the US.1 These increases have been seen across many levels of insurance coverage—private payer, Medicare, and the Veterans Health Administration (VHA).2,3
The Figure demonstrates the cost increase that have been seen across VHA between 2004 and 2019 for the DMTs identified in Table 1. Indeed, this compound annual growth rate may be an underestimate because infusion therapies (eg, natalizumab, ocrelizumab, and alemtuzumab) are difficult to track as they may be dispensed directly via a Risk Evaluation Medication Strategy (REMS) program. According to the VHA Pharmacy Benefit Management Service (PBM), in September 2019, dimethyl fumarate (DMF) had the 13th highest total outpatient drug cost for the US Department of Veterans Affairs (VA), interferon β‐1a ranked 62nd and 83rd (prefilled pen and syringe, respectively), and GA 40 mg ranked 89th.
The DMT landscape has demonstrated significant price fluctuations and given rise to a class of medications that requires extensive oversight in terms of efficacy, safety, and cost minimization. The purpose of this article is to show how delivery of this specialty group of medications can be optimized with safety, efficacy, and cost value within a large health care system.
Factors Impacting DMT Use
Recent changes to MS typing have impacted utilization of DMTs. Traditionally, there were 4 subtypes of MS: relapsing remitting (RRMS), secondary progressive (SPMS), progressive relapsing (PRMS), and primary progressive (PPMS). These subtypes are now viewed more broadly and grouped as either relapsing or progressive. The traditional subtypes fall under these broader definitions. Additionally, SPMS has been broken into active SPMS, characterized by continued worsening of disability unrelated to acute relapses, superimposed with activity that can be seen on magnetic resonance images (MRIs), and nonactive SPMS, which has the same disability progression as active SPMS but without MRI-visible activity.4-6 In 2019, these supplementary designations to SPMS made their first appearance in FDA-approved indications. All existing DMTs now include this terminology in their labelling and are indicated in active SPMS. There remain no DMTs that treat nonactive SPMS.
The current landscape of DMTs is highly varied in method of administration, risks, and benefits. As efficacy of these medications often is marked by how well they can prevent the immune system from attacking myelin, an inverse relationship between safety and efficacy results. The standard treatment outcomes in MS have evolved over time. The following are the commonly used primary outcomes in clinical trials: relapse reduction; increased time between relapses; decreased severity of relapses; prevention or extend time to disability milestones as measured by the Expanded Disability Status Scale (EDSS) and other disability measures; prevention or extension of time to onset of secondary progressive disease; prevention or reduction of the number and size of new and enhancing lesions on MRI; and limitation of overall MRI lesion burden in the central nervous system (CNS).
Newer treatment outcomes employed in more recent trials include: measures of axonal damage, CNS atrophy, evidence of microscopic disease via conventional MRI and advanced imaging modalities, biomarkers associated with inflammatory disease activity and neurodegeneration in MS, and the use of no evidence of disease activity (NEDA). These outcomes also must be evaluated by the safety concerns of each agent. Short- and long-term safety are critical factors in the selection of DMTs for MS. The injectable therapies for MS (interferon β‐1a, interferon β‐1b, and GA) have established long-term safety profiles from > 20 years of continuous use. The long-term safety profiles of oral immunomodulatory agents and monoclonal antibodies for these drugs in MS have yet to be determined. Safety concerns associated with some therapies and added requirements for safety monitoring may increase the complexity of a therapeutic selection.
Current cost minimization strategies for DMT include limiting DMT agents on formularies, tier systems that incentivize patients/prescribers to select the lowest priced agents on the formulary, negotiating arrangements with manufacturers to freeze prices or provide discounts in exchange for a priority position in the formulary, and requiring prior authorization to initiate or switch therapy. The use of generic medications and interchange to these agents from a brand name formulation can help reduce expense. Several of these strategies have been implemented in VHA.
Disease-Modifying Therapies
In 2019, 18,645 veterans with MS had either a MS-specific DMT or ≥ 1 annual encounters with a primary diagnosis of MS. Of this population, 4,720 were female and 13,357 were service connected according to VA data. About 50% of veterans with MS take a DMT. This percentage has remained stable over the past decade (Table 2). Although it appears the number of unique veterans prescribed an outpatient DMT is decreasing, this does not include the growing use of infused DMTs or DMTs obtained through the Veterans Choice Program (VCP)/Community Care (CC).
The overall outpatient pharmacy costs for veterans have remained constant despite the reduction in outpatient pharmacy prescription numbers. This may be due to increases in DMT cost to the VHA and the use of more expensive oral agents over the previously used platform injection DMTs.
Generic Conversion
GA is available in 20 mg daily and 40 mg3 times weekly subcutaneous injection dosing. The first evidence of clinical efficacy for a generic formulation for GA was evaluated by the GATE trial.7 This trial was a multicenter, randomized, double-blind, active- and placebo-controlled phase 3 trial. Eligible participants were randomized to receive daily SC injection for 9 months of 20 mg generic GA (n = 5,353), 20 mg brand GA (n = 5,357), or placebo (n = 584). The primary endpoint was the mean number of gadolinium (Gd1) lesions visible on MRIs during months 7, 8, and 9, which were significantly reduced in the combined GA-treated group and in each GA group individually when compared with the placebo group, confirming the study sensitivity (ie, GA was effective under the conditions of the study). Tolerability (including injection site reactions) and safety (incidence, spectrum, and severity of adverse events [AEs]) were similar in the generic and brand GA groups. These results demonstrated that generic and brand GA had equivalent efficacy, tolerability, and safety over a 9-month period.7
Results of a 15-month extension of the study were presented in 2015 and showed similar efficacy, safety, and tolerability in participants treated with generic GA for 2 years and patients switched from brand to generic GA.8 Multiple shifts for GA occurred, most notably the conversion from branded Copaxone (Teva Pharmaceutical Industries) to generic Glatopa (Sandoz). Subsequently, Sandoz released a generic 40 mg 3 times weekly formulation. Additionally, Mylan entered the generic GA market. With 3 competing manufacturers, internal data from the VHA indicated that it was able to negotiate a single source contract for this medication that provided a savings of $32,088,904.69 between September 2016 and May 2019.
The impact of generic conversions is just being realized. Soon, patents will begin to expire for oral DMTs, leading to an expected growth of generic alternatives. Already the FDA has approved 4 generic alternatives for teriflunomide, 3 for fingolimod (with 13 tentative approvals), and 15 generic alternatives for dimethyl fumarate (DMF). Implementation of therapeutic interchanges will be pursued by VHA as clinically supported by evidence.
Criteria for Use
PBM supports utilizing criteria to help guide providers on DMT options and promote safe, effective, and value-based selection of a DMT. The PBM creates monographs and criteria for use (CFU) for new medications. The monograph contains a literature evaluation of all studies available to date that concern both safety and efficacy of the new medication. Therapeutic alternatives also are presented and assessed for key elements that may determine the most safe and effective use. Additional safety areas for the new medications such as look-alike, sound-alike potential, special populations use (ie, those who are pregnant, the elderly, and those with liver or renal dysfunction), and drug-drug interactions are presented. Lastly, and possibly most importantly in an ever-growing growing world of DMTs, the monograph describes a reasonable place in therapy for the new DMT.
CFU are additional guidance for some DMTs. The development of CFU are based on several questions that arise during the monograph development for a DMT. These include, but are not limited to:
- Are there safety concerns that require the drug to receive a review to ensure safe prescribing (eg, agents with REMS programs, or safety concerns in specific populations)?
- Does the drug require a specialty provider type with knowledge and experience in those disease states to ensure appropriate and safe prescribing (eg restricted to infectious diseases)?
- Do VHA or non-VHA guidelines suggest alternative therapy be used prior to the agent?
- Is a review deemed necessary to ensure the preferred agent is used first (eg, second-line therapy)?
The CFU defines parameters of drug use consistent with high quality and evidence-based patient care. CFUs also serve as a basis for monitoring local, regional, and national patterns of pharmacologic care and help guide health care providers (HCPs) on appropriate use of medication.
CFUs are designed to ensure the HCP is safely starting a medication that has evidence for efficacy for their patient. For example, alemtuzumab is a high-risk, high-efficacy DMT. The alemtuzumab CFU acknowledges this by having exclusion criteria that prevent a veteran at high risk (ie, on another immunosuppressant) from being exposed to severe AEs (ie, severe leukopenia) that are associated with the medication. On the other hand, the inclusion criteria recognize the benefits of alemtuzumab and allows those with highly active MS who have failed other DMTs to receive the medication.
The drug monograph and CFU process is an important part of VHA efforts to optimize patient care. After a draft version is developed, HCPs can provide feedback on the exclusion/inclusion criteria and describe how they anticipate using the medication in their practice. This insight can be beneficial for MS treatment as diverse HCPs may have distinct viewpoints on how DMTs should be started. Pharmacists and physicians on a national level then discuss and decide together what to include in the final drafts of the drug monograph and CFU. Final documents are disseminated to all sites, which encourages consistent practices across the VHA.9 These documents are reviewed on a regular basis and updated as needed based on available literature evidence.
It is well accepted that early use of DMT correlates with lower accumulated long-term disability.10 However, discontinuation of DMT should be treated with equal importance. This benefits the patient by reducing their risk of AEs from DMTs and provides cost savings. Age and disease stability are factors to consider for DMT discontinuation. In a study with patients aged > 45 years and another with patients aged > 60 years, discontinuing DMT rarely had a negative impact and improved quality of life.11,12 A retrospective meta-analysis of age-dependent efficacy of current DMTs predicted that DMT loses efficacy at age 53 years. In addition, higher efficacy DMT only outperforms lower efficacy DMT in patients aged < 40.5 years.13 Stability of disease and lack of relapses for ≥ 2 years also may be a positive predictor to safely discontinue DMT.14,15 The growing literature to support safe discontinuation of DMT makes this a more convincing strategy to avoid unnecessary costs associated with current DMTs. With an average age of 59 years for veterans with MS, this may be one of the largest areas of cost avoidance to consider.
Off-Label Use
Other potential ways to reduce DMT costs is to consider off-label treatments. The OLYMPUS trial studied off-label use of rituximab, an anti-CD20 antibody like ocrelizumab. It did not meet statistical significance for its primary endpoint; however, in a subgroup analysis, off-label use was found to be more effective in a population aged < 51 years.16 Other case reports and smaller scale studies also describe rituximab’s efficacy in MS.17,18 In 2018, the FDA approved the first rituximab biosimilar.19 Further competition from biosimilars likely will make rituximab an even more cost-effective choice when compared with ocrelizumab.
Alternate Dosing Regimens
Extended interval dosing of natalizumab has been studied, extending the standard infusion interval from every 4 weeks to 5- to 8-week intervals. One recent article compared these interval extensions and found that all extended intervals of up to 56 days did not increase new or enhancing lesions on MRI when compared with standard interval dosing.20 Another larger randomized trial is underway to evaluate efficacy and safety of extended interval dosing of natalizumab (NCT03689972). Utilization of this dosing may reduce natalizumab annual costs by up to 50%.
Safety Monitoring
DMF is an oral DMT on the VHA formulary with CFU. Since leukopenia is a known AE, baseline and quarterly monitoring of the complete blood count (CBC) is recommended for patients taking DMF. Additionally, DMF should be held if white blood cell count (WBC) falls below 2,000/mm3.21 There have been recent reports of death secondary to progressive multifocal leukoencephalopathy (PML) among European patients taking DMF.22-24 This has raised concerns about adherence to recommended CBC monitoring in veterans taking DMF. The association of DMF and leukopenia has been evident since early clinical trials.25 Leukopenia in immunocompromised patients increases the risk of PML.
In the long-term extension study ENDORSE, 6% to 7% of patients continuing DMF had WBC counts of 3.0×109/L compared with 7% to 10% in the new to DMF group.26 In addition 6% to 8% of patients continuing DMF had lymphocyte counts of 0.5×109/L, compared with 5% to 9% in the new to DMF group. The cases of PML occurred in patients who had low lymphocyte counts over an extended period with no adjustment to DMF therapy, such as holding the drug until WBC counts returned to normal levels or stopping the drug. Discussion and review within VHA resulted in the recommendation for quarterly WBC monitoring criteria.
PBM and VA Center for Medication Safety (MedSafe) conducted a medication usage evaluation (MUE) on adherence to the WBC monitoring set forth in the CFU. Data collection began in fourth quarter of fiscal year (FY) 2015 with the most recent reporting period of fourth quarter of FY 2017. The Medication Utilization Evaluation Tool tracks patients with no reported WBC in 90 days and WBC < 2,000/mm3. Over the reporting period, 20% to 23% of patients have not received appropriate quarterly monitoring. Additionally, there have been 4 cases where the WBC decreased below the threshold limit. To ensure safe and effective use of DMF, it is important to adhere to the monitoring requirements set forth in the CFU.
Impact of REMS and Special Distribution
As DMTs increase in efficacy, there are often more risks associated with them. Some of these high-risk medications, including natalizumab and alemtuzumab, have REMS programs and/or have special distribution procedures. Although REMS are imperative for patient safety, the complexity of these programs can be difficult to navigate, which can create a barrier to access. The PBM helps to assist all sites with navigating and adhering to required actions to dispense and administer these medications through a national Special Handling Drugs Microsoft SharePoint site, which provides access to REMS forms and procurement information when drugs are dispensed from specialty pharmacies. Easing this process nationwide empowers more sites to be confident they can dispense specialty medications appropriately.
Clinical Pharmacists
The VHA is unique in its utilization of pharmacists in outpatient clinic settings. Utilization of an interdisciplinary team for medication management has been highly used in VHA for areas like primary care; however, pharmacist involvement in specialty areas is on the rise and MS is no exception. Pharmacists stationed in clinics, such as neurology or spinal cord injury, can impact care for veterans with MS. Interdisciplinary teams that include a pharmacist have been shown to increase patient adherence to DMTs.27 However, pharmacists often assist with medication education and monitoring, which adds an additional layer of safety to DMT treatment. At the VHA, pharmacists also can obtain a scope of practice that allows them to prescribe medications and increase access to care for veterans with MS.
Education
The VHA demonstrates how education on a disease state like MS can be distributed on a large, national scale through drug monographs, CFU, and Microsoft SharePoint sites. In addition, VHA has created the MS Centers of Excellence (MSCoE) that serve as a hub of specialized health care providers in all aspects of MS care.
A core function of the MSCoE is to provide education to both HCPs and patients. The MSCoE and its regional hubs support sites that may not have an HCP who specializes in MS by providing advice on DMT selection, how to obtain specialty medications, and monitoring that needs to be completed to ensure veterans’ safety. The MSCoE also has partnered with the National MS Society to hold a lecture series on topics in MS. This free series is available online to all HCPs who interact with patients who have MS and is a way that VA is extending its best practices and expertise beyond its own health care system. There also is a quarterly newsletter for veterans with MS that highlights new information on DMTs that can affect their care.
Conclusion
It is an exciting and challenging period in MS treatment. New DMTs are being approved and entering clinical trials at a rapid pace. These new DMT agents may offer increased efficacy, improvements in AE profiles, and the possibility of increased medication adherence—but often at a higher cost. The utilization of CFU and formulary management provides the ability to ensure the safe and appropriate use of medications by veterans, with a secondary outcome of controlling pharmacy expenditures.
The VHA had expenditures of $142,135,938 for DMT use in FY 2018. As the VHA sees the new contract prices for DMT in January 2020, we are reminded that costs will continue to rise with some pharmaceutical manufacturers implementing prices 8% to 11% higher than 2019 prices, when the consumer price index defines an increase of 1.0% for 2020 and 1.4% in 2021.28 It is imperative that the VHA formulary be managed judiciously and the necessary measures be in place for VHA practitioners to enable effective, safe and value-based care to the veteran population.
1. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81(4):479-484.
2. Hartung DM, Bourdette DN, Ahmed SM, Whitham RH. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? [published correction appears in Neurology. 2015;85(19):1728]. Neurology. 2015;84(21):2185–2192.
3. San-Juan-Rodriguez A, Good CB, Heyman RA, Parekh N, Shrank WH, Hernandez I. Trends in prices, market share, and spending on self-administered disease-modifying therapies for multiple sclerosis in Medicare Part D. JAMA Neurol. 2019;76(11):1386-1390.
4. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
5. Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis [published correction appears in Mult Scler. 2003;9(6):641]. Mult Scler. 2003;9(3):260-274.
6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
7. Cohen J, Belova A, Selmaj K, et al. Equivalence of generic glatiramer acetate in multiple sclerosis: a randomized clinical trial. JAMA Neurol. 2015;72(12):1433-1441.
8. Selmaj K, Barkhof F, Belova AN, et al; GATE study group. Switching from branded to generic glatiramer acetate: 15-month GATE trial extension results. Mult Scler. 2017;23(14):1909-1917.
9. Aspinall SL, Sales MM, Good CB, et al. Pharmacy benefits management in the Veterans Health Administration revisited: a decade of advancements, 2004-2014. J Manag Care Spec Pharm. 2016;22(9):1058-1063.
10. Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321(2):175-187.
11. Hua LH, Harris H, Conway D, Thompson NR. Changes in patient-reported outcomes between continuers and discontinuers of disease modifying therapy in patients with multiple sclerosis over age 60 [published correction appears in Mult Scler Relat Disord. 2019;30:293]. Mult Scler Relat Disord. 2019;30:252-256.
12. Bsteh G, Feige J, Ehling R, et al. Discontinuation of disease-modifying therapies in multiple sclerosis - Clinical outcome and prognostic factors. Mult Scler. 2017;23(9):1241-1248.
13. Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.
14. Kister I, Spelman T, Alroughani R, et al; MSBase Study Group. Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study [published correction appears in J Neurol Neurosurg Psychiatry. 2019;90(4):e2]. J Neurol Neurosurg Psychiatry. 2016;87(10):1133-1137.
15. Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19(1):11-14.
16. Hawker K, O’Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471.
17. Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688.
18. Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79(6):950–958.
19. Rituximab-abbs [package insert]. North Wales, PA: Teva Pharmaceuticals; 2018.
20. Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(8):885-889.
21. Dimethyl fumarate [package insert]. Cambridge, MA: Biogen Inc; 2015.
22. van Kester MS, Bouwes Bavinck JN, Quint KD. PML in Patients treated with dimethyl fumarate. N Engl J Med. 2015;373(6):583-584.
23. Nieuwkamp DJ, Murk JL, van Oosten BW. PML in patients treated with dimethyl fumarate. N Engl J Med. 2015;373(6):584.
24. Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372(15):1476-1478.
25. Longbrake EE, Cross AH. Dimethyl fumarate associated lymphopenia in clinical practice. Mult Scler. 2015;21(6):796-797.
26. Gold R, Arnold DL, Bar-Or A, et al. Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: Interim analysis of ENDORSE, a randomized extension study. Mult Scler. 2017;23(2):253–265.
27. Hanson RL, Habibi M, Khamo N, Abdou S, Stubbings J. Integrated clinical and specialty pharmacy practice model for management of patients with multiple sclerosis. Am J Health Syst Pharm. 2014;71(6):463-469.
28. Federal Planning Bureau. Consumer Price Index - Inflation forecasts. https://www.plan.be/databases/17-en-consumer+price+index+inflation+forecasts. Updated March 3, 2020. Accessed March 9, 2020.
1. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81(4):479-484.
2. Hartung DM, Bourdette DN, Ahmed SM, Whitham RH. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? [published correction appears in Neurology. 2015;85(19):1728]. Neurology. 2015;84(21):2185–2192.
3. San-Juan-Rodriguez A, Good CB, Heyman RA, Parekh N, Shrank WH, Hernandez I. Trends in prices, market share, and spending on self-administered disease-modifying therapies for multiple sclerosis in Medicare Part D. JAMA Neurol. 2019;76(11):1386-1390.
4. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
5. Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis [published correction appears in Mult Scler. 2003;9(6):641]. Mult Scler. 2003;9(3):260-274.
6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
7. Cohen J, Belova A, Selmaj K, et al. Equivalence of generic glatiramer acetate in multiple sclerosis: a randomized clinical trial. JAMA Neurol. 2015;72(12):1433-1441.
8. Selmaj K, Barkhof F, Belova AN, et al; GATE study group. Switching from branded to generic glatiramer acetate: 15-month GATE trial extension results. Mult Scler. 2017;23(14):1909-1917.
9. Aspinall SL, Sales MM, Good CB, et al. Pharmacy benefits management in the Veterans Health Administration revisited: a decade of advancements, 2004-2014. J Manag Care Spec Pharm. 2016;22(9):1058-1063.
10. Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321(2):175-187.
11. Hua LH, Harris H, Conway D, Thompson NR. Changes in patient-reported outcomes between continuers and discontinuers of disease modifying therapy in patients with multiple sclerosis over age 60 [published correction appears in Mult Scler Relat Disord. 2019;30:293]. Mult Scler Relat Disord. 2019;30:252-256.
12. Bsteh G, Feige J, Ehling R, et al. Discontinuation of disease-modifying therapies in multiple sclerosis - Clinical outcome and prognostic factors. Mult Scler. 2017;23(9):1241-1248.
13. Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.
14. Kister I, Spelman T, Alroughani R, et al; MSBase Study Group. Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study [published correction appears in J Neurol Neurosurg Psychiatry. 2019;90(4):e2]. J Neurol Neurosurg Psychiatry. 2016;87(10):1133-1137.
15. Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19(1):11-14.
16. Hawker K, O’Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471.
17. Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688.
18. Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79(6):950–958.
19. Rituximab-abbs [package insert]. North Wales, PA: Teva Pharmaceuticals; 2018.
20. Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(8):885-889.
21. Dimethyl fumarate [package insert]. Cambridge, MA: Biogen Inc; 2015.
22. van Kester MS, Bouwes Bavinck JN, Quint KD. PML in Patients treated with dimethyl fumarate. N Engl J Med. 2015;373(6):583-584.
23. Nieuwkamp DJ, Murk JL, van Oosten BW. PML in patients treated with dimethyl fumarate. N Engl J Med. 2015;373(6):584.
24. Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372(15):1476-1478.
25. Longbrake EE, Cross AH. Dimethyl fumarate associated lymphopenia in clinical practice. Mult Scler. 2015;21(6):796-797.
26. Gold R, Arnold DL, Bar-Or A, et al. Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: Interim analysis of ENDORSE, a randomized extension study. Mult Scler. 2017;23(2):253–265.
27. Hanson RL, Habibi M, Khamo N, Abdou S, Stubbings J. Integrated clinical and specialty pharmacy practice model for management of patients with multiple sclerosis. Am J Health Syst Pharm. 2014;71(6):463-469.
28. Federal Planning Bureau. Consumer Price Index - Inflation forecasts. https://www.plan.be/databases/17-en-consumer+price+index+inflation+forecasts. Updated March 3, 2020. Accessed March 9, 2020.
Behavioral Interventions in Multiple Sclerosis
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system that affects nearly 1 million people in the US.1 In addition to the accumulation of functional limitations, patients with MS commonly experience mental health and physical symptoms such as depression, anxiety, stress, fatigue, and pain. Day-to-day life with MS requires adaptation to challenges and active maintenance of health and well-being over time. Behavioral intervention and treatment, whether in the form of psychotherapy, health behavior coaching, or the promotion of active self-management, is an integral component of interprofessional care and key aspect of living well with MS.
Behavioral Comorbidities
Depression
Depression is a common concern among individuals with MS. Population-based studies suggest that individuals with MS have a roughly 1 in 4 chance of developing major depressive disorder over their lifetime.2 However, at any given time, between 40% and 60% of individuals with MS report clinically meaningful levels of depressive symptoms.3 Although the relationship between MS disease characteristics and depression is unclear, some evidence suggests that depressive symptoms are more common at certain points in illness, such as early in the disease process as individuals grapple with the onset of new symptoms, late in the disease process as they accumulate greater disability, and during active clinical relapses.3-5
Depression often is comorbid with, and adds to the symptom burden of, other common conditions in MS such as fatigue and cognitive dysfunction.6-8 Thus, it is not surprising that it associated with poorer overall quality of life (QOL).9 Depression also is a risk factor for suicidal ideation and suicide for patients with MS.10,11
Fortunately, several behavioral interventions show promise in treating depression in patients with MS. Both individual and group formats of cognitive behavioral therapy (CBT), a treatment focused on challenging maladaptive patterns of thought and behavior, have been shown to improve depressive symptoms for people with MS.12,13 Several brief and efficient group-based programs grounded in CBT and focused on the development of specific skills, including problem solving, goal setting, relationship management, and managing emotions, have been shown to reduce depressive symptoms.13,14 CBT for depression in MS has been shown to be effective when delivered via telephone.15,16
Anxiety
Anxiety is common among individuals with MS. Existing data suggest more than one-third of individuals with MS will qualify for a diagnosis of anxiety disorder during their lifetime.17 The characteristics of anxiety disorders are broad and heterogenous, including generalized anxiety disorder, panic disorder, obsessive compulsive disorders, and health-specific phobias such as needle/injection anxiety. Some estimates suggest a point prevalence of 34% for the presence of clinically meaningful symptoms.18 Similar to depression, anxiety symptoms can be more common during periods of stress, threat, and transition including early in the disease course while adapting to new diagnosis, late in the disease course with increasing disability, and during clinical relapses.19-21
The efficacy of behavioral interventions for anxiety in MS is less well established than it is for depression, but some preliminary evidence suggests that individual CBT may be effective for reducing general symptoms of anxiety as well as health-related anxiety.22,23 Brief, targeted CBT also has been shown to improve injection anxiety, removing a barrier to self-care including the administration of MS disease modifying therapies (DMTs).24
Stress
Stress is commonly conceptualized as a person’s perception that efforts to manage internal and external demands exceed available coping resources.25 Such demands involve both psychological and physiological processes and come in many forms for people with MS and can include daily hassles, major life events, traumatic stress, and perceptions of global nonspecific stress. The relationship between stress and MS remains complex and poorly understood. Nonetheless, individuals with MS frequently report that stress exacerbates their symptoms.26
Some evidence also suggests stress may exacerbate the MS disease process, resulting in more frequent relapses and increased lesion activity visible on MRI.27,28 In addition to mindfulness (described below), stress inoculation training (CBT and relaxation training), and stress-focused group-based self-management have been shown to be beneficial.29,30 In an intriguing and rigorous trial, a 24-week stress management therapy based on CBT was associated with the development of fewer new MS lesions visible on MRI.31
Adaptation to Illness
MS presents challenges that vary between patients and over time. Individuals may confront new physical and cognitive limitations that inhibit the completion of daily tasks, reduce independence, and limit participation in valued and meaningful activities. In addition, the unpredictability of the disease contributes to perceptions of uncertainty and uncontrollability, which in turn result in higher illness impact and poorer psychological outcomes.32 Building cognitive and behavioral skills to address these challenges can promote adaptation to illness and reduce overall distress associated with chronic illness.33 Psychosocial intervention also can address the uncertainty commonly experienced by individuals with MS.34
Self-Management
As with any chronic illness, living well with MS requires ongoing commitment and active engagement with health and personal care over time. The process of building knowledge and skills to manage the day-to-day physical, emotional, and social aspects of living with illness often is referred to as self-management.35 For individuals with MS, this may take the form of participation in programs that address adaptation and psychological distress like those described above, but it also may include improving health behavior (eg, physical activity, DMT adherence, modification of maladaptive habits like smoking or hazardous alcohol use) and symptom management (eg, fatigue, pain). Self-management programs typically include education, the practice of identifying, problem solving, and following through with specific and realistic health and wellness goals, as well as the bolstering of self-efficacy.
Physical Activity
Once discouraged for patients with MS, physical activity is now considered a cornerstone of health and wellness. Physical activity and interventions that target various forms of exercise have been shown to improve strength and endurance, reduce functional decline, enhance QOL, and likely reduce mortality.35-39 A variety of brief behavioral interventions have been shown to improve physical activity in MS. Structured group-based exercise classes focusing on various activities such as aerobic training (eg, cycling) or resistance training (eg, lower extremity strengthening) have demonstrated improvements in various measures of fitness and mood states such as depression and QOL. Brief home-based telephone counseling interventions based in social cognitive theory (eg, goal setting, navigating obstacles) and motivational interviewing strategies (eg, open-ended questions, affirmation, reflective listening, summarizing) also have been shown to be effective not only at increasing physical activity and improving depression and fatigue.40,41
Adherence to Treatment
One primary focus of adherence to treatment is medication management. For individuals with MS, DMTs represent a primary means of reducing disease burden and delaying functional decline. Many DMTs require consistent self-administration over time. Some evidence suggests that poorer adherence is associated with a greater risk of relapse and more rapid disease progression.42,43 Brief telephone counseling, again based on social cognitive theory, and principles of motivational interviewing combined with home telehealth monitoring by a care coordinator has been shown to improve adherence to DMTs.44
Mindfulness
In recent years, mindfulness training has emerged as a popular and common behavioral intervention among individuals with MS. Programs like Mindfulness-Based Stress Reduction (MBSR) provide training in meditation techniques designed to promote mindfulness, which is defined as paying attention to present moment experience, including sensations, thoughts, and emotions, without judgment or attachment.45 Cultivating mindfulness helps people with MS cope with and adapt to symptoms and stressors.46 Mindfulness interventions typically are delivered in a group format. For example, MBSR consists of 8 in-person group sessions with daily meditation practice homework. Mindfulness interventions also have been delivered effectively with smartphone apps.47 Mindfulness programs have been shown to improve depression, anxiety, fatigue, stress, and QOL for patients with MS.48-50
Fatigue
More than 90% of individuals with MS report fatigue, and many identify it as their most disabling symptom.51 Often defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities,” fatigue has been shown to be associated with longer disease duration, greater physical disability, progressive subtype, and depressive symptoms, although the relative and possibly overlapping impact of these issues is only partially understood.52,53 Fatigue is associated with poorer overall mental health and negatively impacts work and social roles.54
Several behavioral interventions have been developed to address fatigue in MS. Using both individual and group based formats and across several modalities (eg, in-person, telephone, online modules, or a combination), behavioral fatigue interventions most commonly combine traditional general CBT skills (eg, addressing maladaptive thoughts and behaviors) with a variety of fatigue-specific skill building exercises that may include fatigue education, energy conservation strategies, improving sleep, enlisting social support, and self-management goal setting strategies.35,55-57
Pain
Chronic pain is common and disabling in people with MS.58,59 Nearly 50% report experiencing moderate to severe chronic pain.59,60 Individuals with MS reporting pain often are older, more disabled (higher Expanded Disability Status Scale score), and have longer disease duration that those who are not experiencing chronic pain.61 Patients report various types of pain in the following order of frequency: dysesthetic pain (18.1%), back pain (16.4%), painful tonic spasms (11.0%), Lhermitte sign (9.0%), visceral pain (2.9%), and trigeminal neuralgia (2.0%).61 Chronic pain has a negative impact on QOL in the areas of sleep, work, maintaining relationships, recreational activities, and overall life enjoyment.59 Additionally, research has shown that greater pain intensity and pain-related interference with activities of daily living are both associated with greater depression severity.62,63
The literature supports the use of behavioral interventions for pain in people with MS.61 Behavioral interventions include in-person exercise interventions (eg, water aerobics, cycling, rowing ergometer, treadmill walking, and resistance training), self-hypnosis, and telephone-based self-management programs based on CBT.35,64,65 As described above, CBT-based self-management programs combine learning CBT skills (eg, modifying maladaptive thoughts) with pain-specific skill building such as pain education, pacing activities, and improving sleep. Of note, MS education including, but not limited to, pain was as effective as a CBT-based self-management program in reducing pain intensity and interference.35 In addition, there is evidence to support acceptance- and mindfulness-based interventions for chronic pain, and online mindfulness-based cognitive therapy for MS related pain is currently being tested in a randomized controlled trial.35,66
Conclusion
People with MS face significant challenges in coping with and adapting to a chronic and unpredictable disease. However, there is considerable evidence that behavioral interventions can improve many of the most common and disabling symptoms in MS including depression, anxiety, stress, fatigue, and pain as well as health behavior and self-care. Research also suggests that improvements in one of these problems (eg, physical inactivity) can influence improvement in other symptoms (eg, depression and fatigue). Unlike other treatment options, behavioral interventions can be delivered in various formats (eg, in-person and electronic health), are time-limited, and cause few (if any) undesirable systemic adverse effects. Behavioral interventions are therefore, an essential part of interprofessional care and rehabilitation for patients with MS.
1. Wallin MT, Culpepper WJ, Campbell JD, et al; US Multiple Sclerosis Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
3. Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862-1868.
4. Williams RM, Turner AP, Hatzakis M Jr, Bowen JD, Rodriquez AA, Haselkorn JK. Prevalence and correlates of depression among veterans with multiple sclerosis. Neurology. 2005;64(1):75-80.
5. Moore P, Hirst C, Harding KE, Clarkson H, Pickersgill TP, Robertson NP. Multiple sclerosis relapses and depression. J Psychosom Res. 2012;73(4):272-276.
6. Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19(2):217-224.
7. Arnett PA, Higginson CI, Voss WD, et al. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology. 1999;13(3):434-446.
8. Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23(2):189-199.
9. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231(1-2):29-34.
10. Turner AP, Williams RM, Bowen JD, Kivlahan DR, Haselkorn JK. Suicidal ideation in multiple sclerosis. Arch Phys Med Rehabil. 2006;87(8):1073-1078.
11. Stenager EN, Koch-Henriksen N, Stenager E. Risk factors for suicide in multiple sclerosis. Psychother Psychosom. 1996;65(2):86-90.
12. Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69(6):942-949.
13. Larcombe NA, Wilson PH. An evaluation of cognitive-behaviour therapy for depression in patients with multiple sclerosis. Br J Psychiatry. 1984;145:366-371.
14. Lincoln NB, Yuill F, Holmes J, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler. 2011;17(10):1250-1257.
15. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol. 2000;68(2):356-361.
16. Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62(9):1007-1014.
17. Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13(1):67-72.
18. Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331-341.
19. Dahl OP, Stordal E, Lydersen S, Midgard R. Anxiety and depression in multiple sclerosis. A comparative population-based study in Nord-Trøndelag County, Norway. Mult Scler. 2009;15(12):1495-1501.
20. Burns MN, Nawacki E, Siddique J, Pelletier D, Mohr DC. Prospective examination of anxiety and depression before and during confirmed and pseudoexacerbations in patients with multiple sclerosis. Psychosom Med. 2013;75(1):76-82.
21. Uguz F, Akpinar Z, Ozkan I, Tokgoz S. Mood and anxiety disorders in patients with multiple sclerosis. Int J Psychiatry Clin Pract. 2008;12(1):19-24.
22. Askey-Jones S, David AS, Silber E, Shaw P, Chalder T. Cognitive behaviour therapy for common mental disorders in people with multiple sclerosis: a bench marking study. Behav Res Ther. 2013;51(10):648-655.
23. Carrigan N, Dysch L, Salkovskis PM. The impact of health anxiety in multiple sclerosis: a replication and treatment case series. Behav Cogn Psychother. 2018;46(2):148-167.
24. Mohr DC, Cox D, Merluzzi N. Self-injection anxiety training: a treatment for patients unable to self-inject injectable medications. Mult Scler. 2005;11(2):182-185.
25. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984.
26. Ackerman KD, Heyman R, Rabin BS, et al. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64(6):916-920.
27. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
28. Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61.
29. Foley FW, Bedell JR, LaRocca NG, Scheinberg LC, Reznikoff M. Efficacy of stress-inoculation training in coping with multiple sclerosis. J Consult Clin Psychol. 1987;55(6):919-922.
30. Hughes RB, Robinson-Whelen S, Taylor HB, Hall JW. Stress self-management: an intervention for women with physical disabilities. Womens Health Issues. 2006;16(6):389-399.
31. Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419.
32. Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009;29(2):141-153.
33. Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work? J Consult Clin Psychol. 2013;81(2):251-262.
34. Molton IR, Koelmel E, Curran M, von Geldern G, Ordway A, Alschuler KN. Pilot intervention to promote tolerance for uncertainty in early multiple sclerosis. Rehabil Psychol. 2019;64(3):339-350.
35. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945-1958.e2.
36. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85(2):290-297.
37. Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(7):935-943.
38. Turner AP, Hartoonian N, Maynard C, Leipertz SL, Haselkorn JK. Smoking and physical activity: examining health behaviors and 15-year mortality among individuals with multiple sclerosis. Arch Phys Med Rehabil. 2015;96(3):402-409.
39. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
40. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
41. Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81(1):89-99.
42. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251-260.
43. Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;7 Suppl 1:S1-S9.
44. Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136-146.
45. Kabat-Zinn J. Full Catastrophe Living. London, UK: Piatkus; 2013.
46. Bishop SR. What do we really know about mindfulness-based stress reduction? [published correction appears in Psychosom Med. 2002;64(3):449]. Psychosom Med. 2002;64(1):71-83.
47. Lindsay EK, Young S, Smyth JM, Brown KW, Creswell JD. Acceptance lowers stress reactivity: dismantling mindfulness training in a randomized controlled trial. Psychoneuroendocrinology. 2018;87:63-73.
48. Simpson R, Mair FS, Mercer SW. Mindfulness-based stress reduction for people with multiple sclerosis - a feasibility randomised controlled trial. BMC Neurol. 2017;17(1):94.
49. Cavalera C, Rovaris M, Mendozzi L, et al. Online meditation training for people with multiple sclerosis: a randomized controlled trial. Mult Scler. 2019;25(4):610-617.
50. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141-1149.
51. Shah A. Fatigue in multiple sclerosis. Phys Med Rehabil Clin N Am. 2009;20(2):363-372.
52. Guidelines MSCfCP. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
53. Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225-234.
54. Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1996;77(2):165-170.
55. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50(6):415-421.
56. Thomas PW, Thomas S, Kersten P, et al. Multi-centre parallel arm randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based cognitive behavioural approach to managing fatigue in people with multiple sclerosis. BMC Neurol. 2010;10:43.
57. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70(2):205-213.
58. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154(5):632-642.
59. O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
60. Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12(5):629-638.
61. Aboud T, Schuster NM. Pain management in multiple sclerosis: a review of available treatment options. Curr Treat Options Neurol. 2019;21(12):62.
62. Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015;60(1):81-90.
63. Arewasikporn A, Turner AP, Alschuler KN, Hughes AJ, Ehde DM. Cognitive and affective mechanisms of pain and fatigue in multiple sclerosis. Health Psychol. 2018;37(6):544-552.
64. Demaneuf T, Aitken Z, Karahalios A, et al. Effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2019;100(1):128-139.
65. Jensen MP, Barber J, Romano JM, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2009;57(2):198-221.
66. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533-542.
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system that affects nearly 1 million people in the US.1 In addition to the accumulation of functional limitations, patients with MS commonly experience mental health and physical symptoms such as depression, anxiety, stress, fatigue, and pain. Day-to-day life with MS requires adaptation to challenges and active maintenance of health and well-being over time. Behavioral intervention and treatment, whether in the form of psychotherapy, health behavior coaching, or the promotion of active self-management, is an integral component of interprofessional care and key aspect of living well with MS.
Behavioral Comorbidities
Depression
Depression is a common concern among individuals with MS. Population-based studies suggest that individuals with MS have a roughly 1 in 4 chance of developing major depressive disorder over their lifetime.2 However, at any given time, between 40% and 60% of individuals with MS report clinically meaningful levels of depressive symptoms.3 Although the relationship between MS disease characteristics and depression is unclear, some evidence suggests that depressive symptoms are more common at certain points in illness, such as early in the disease process as individuals grapple with the onset of new symptoms, late in the disease process as they accumulate greater disability, and during active clinical relapses.3-5
Depression often is comorbid with, and adds to the symptom burden of, other common conditions in MS such as fatigue and cognitive dysfunction.6-8 Thus, it is not surprising that it associated with poorer overall quality of life (QOL).9 Depression also is a risk factor for suicidal ideation and suicide for patients with MS.10,11
Fortunately, several behavioral interventions show promise in treating depression in patients with MS. Both individual and group formats of cognitive behavioral therapy (CBT), a treatment focused on challenging maladaptive patterns of thought and behavior, have been shown to improve depressive symptoms for people with MS.12,13 Several brief and efficient group-based programs grounded in CBT and focused on the development of specific skills, including problem solving, goal setting, relationship management, and managing emotions, have been shown to reduce depressive symptoms.13,14 CBT for depression in MS has been shown to be effective when delivered via telephone.15,16
Anxiety
Anxiety is common among individuals with MS. Existing data suggest more than one-third of individuals with MS will qualify for a diagnosis of anxiety disorder during their lifetime.17 The characteristics of anxiety disorders are broad and heterogenous, including generalized anxiety disorder, panic disorder, obsessive compulsive disorders, and health-specific phobias such as needle/injection anxiety. Some estimates suggest a point prevalence of 34% for the presence of clinically meaningful symptoms.18 Similar to depression, anxiety symptoms can be more common during periods of stress, threat, and transition including early in the disease course while adapting to new diagnosis, late in the disease course with increasing disability, and during clinical relapses.19-21
The efficacy of behavioral interventions for anxiety in MS is less well established than it is for depression, but some preliminary evidence suggests that individual CBT may be effective for reducing general symptoms of anxiety as well as health-related anxiety.22,23 Brief, targeted CBT also has been shown to improve injection anxiety, removing a barrier to self-care including the administration of MS disease modifying therapies (DMTs).24
Stress
Stress is commonly conceptualized as a person’s perception that efforts to manage internal and external demands exceed available coping resources.25 Such demands involve both psychological and physiological processes and come in many forms for people with MS and can include daily hassles, major life events, traumatic stress, and perceptions of global nonspecific stress. The relationship between stress and MS remains complex and poorly understood. Nonetheless, individuals with MS frequently report that stress exacerbates their symptoms.26
Some evidence also suggests stress may exacerbate the MS disease process, resulting in more frequent relapses and increased lesion activity visible on MRI.27,28 In addition to mindfulness (described below), stress inoculation training (CBT and relaxation training), and stress-focused group-based self-management have been shown to be beneficial.29,30 In an intriguing and rigorous trial, a 24-week stress management therapy based on CBT was associated with the development of fewer new MS lesions visible on MRI.31
Adaptation to Illness
MS presents challenges that vary between patients and over time. Individuals may confront new physical and cognitive limitations that inhibit the completion of daily tasks, reduce independence, and limit participation in valued and meaningful activities. In addition, the unpredictability of the disease contributes to perceptions of uncertainty and uncontrollability, which in turn result in higher illness impact and poorer psychological outcomes.32 Building cognitive and behavioral skills to address these challenges can promote adaptation to illness and reduce overall distress associated with chronic illness.33 Psychosocial intervention also can address the uncertainty commonly experienced by individuals with MS.34
Self-Management
As with any chronic illness, living well with MS requires ongoing commitment and active engagement with health and personal care over time. The process of building knowledge and skills to manage the day-to-day physical, emotional, and social aspects of living with illness often is referred to as self-management.35 For individuals with MS, this may take the form of participation in programs that address adaptation and psychological distress like those described above, but it also may include improving health behavior (eg, physical activity, DMT adherence, modification of maladaptive habits like smoking or hazardous alcohol use) and symptom management (eg, fatigue, pain). Self-management programs typically include education, the practice of identifying, problem solving, and following through with specific and realistic health and wellness goals, as well as the bolstering of self-efficacy.
Physical Activity
Once discouraged for patients with MS, physical activity is now considered a cornerstone of health and wellness. Physical activity and interventions that target various forms of exercise have been shown to improve strength and endurance, reduce functional decline, enhance QOL, and likely reduce mortality.35-39 A variety of brief behavioral interventions have been shown to improve physical activity in MS. Structured group-based exercise classes focusing on various activities such as aerobic training (eg, cycling) or resistance training (eg, lower extremity strengthening) have demonstrated improvements in various measures of fitness and mood states such as depression and QOL. Brief home-based telephone counseling interventions based in social cognitive theory (eg, goal setting, navigating obstacles) and motivational interviewing strategies (eg, open-ended questions, affirmation, reflective listening, summarizing) also have been shown to be effective not only at increasing physical activity and improving depression and fatigue.40,41
Adherence to Treatment
One primary focus of adherence to treatment is medication management. For individuals with MS, DMTs represent a primary means of reducing disease burden and delaying functional decline. Many DMTs require consistent self-administration over time. Some evidence suggests that poorer adherence is associated with a greater risk of relapse and more rapid disease progression.42,43 Brief telephone counseling, again based on social cognitive theory, and principles of motivational interviewing combined with home telehealth monitoring by a care coordinator has been shown to improve adherence to DMTs.44
Mindfulness
In recent years, mindfulness training has emerged as a popular and common behavioral intervention among individuals with MS. Programs like Mindfulness-Based Stress Reduction (MBSR) provide training in meditation techniques designed to promote mindfulness, which is defined as paying attention to present moment experience, including sensations, thoughts, and emotions, without judgment or attachment.45 Cultivating mindfulness helps people with MS cope with and adapt to symptoms and stressors.46 Mindfulness interventions typically are delivered in a group format. For example, MBSR consists of 8 in-person group sessions with daily meditation practice homework. Mindfulness interventions also have been delivered effectively with smartphone apps.47 Mindfulness programs have been shown to improve depression, anxiety, fatigue, stress, and QOL for patients with MS.48-50
Fatigue
More than 90% of individuals with MS report fatigue, and many identify it as their most disabling symptom.51 Often defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities,” fatigue has been shown to be associated with longer disease duration, greater physical disability, progressive subtype, and depressive symptoms, although the relative and possibly overlapping impact of these issues is only partially understood.52,53 Fatigue is associated with poorer overall mental health and negatively impacts work and social roles.54
Several behavioral interventions have been developed to address fatigue in MS. Using both individual and group based formats and across several modalities (eg, in-person, telephone, online modules, or a combination), behavioral fatigue interventions most commonly combine traditional general CBT skills (eg, addressing maladaptive thoughts and behaviors) with a variety of fatigue-specific skill building exercises that may include fatigue education, energy conservation strategies, improving sleep, enlisting social support, and self-management goal setting strategies.35,55-57
Pain
Chronic pain is common and disabling in people with MS.58,59 Nearly 50% report experiencing moderate to severe chronic pain.59,60 Individuals with MS reporting pain often are older, more disabled (higher Expanded Disability Status Scale score), and have longer disease duration that those who are not experiencing chronic pain.61 Patients report various types of pain in the following order of frequency: dysesthetic pain (18.1%), back pain (16.4%), painful tonic spasms (11.0%), Lhermitte sign (9.0%), visceral pain (2.9%), and trigeminal neuralgia (2.0%).61 Chronic pain has a negative impact on QOL in the areas of sleep, work, maintaining relationships, recreational activities, and overall life enjoyment.59 Additionally, research has shown that greater pain intensity and pain-related interference with activities of daily living are both associated with greater depression severity.62,63
The literature supports the use of behavioral interventions for pain in people with MS.61 Behavioral interventions include in-person exercise interventions (eg, water aerobics, cycling, rowing ergometer, treadmill walking, and resistance training), self-hypnosis, and telephone-based self-management programs based on CBT.35,64,65 As described above, CBT-based self-management programs combine learning CBT skills (eg, modifying maladaptive thoughts) with pain-specific skill building such as pain education, pacing activities, and improving sleep. Of note, MS education including, but not limited to, pain was as effective as a CBT-based self-management program in reducing pain intensity and interference.35 In addition, there is evidence to support acceptance- and mindfulness-based interventions for chronic pain, and online mindfulness-based cognitive therapy for MS related pain is currently being tested in a randomized controlled trial.35,66
Conclusion
People with MS face significant challenges in coping with and adapting to a chronic and unpredictable disease. However, there is considerable evidence that behavioral interventions can improve many of the most common and disabling symptoms in MS including depression, anxiety, stress, fatigue, and pain as well as health behavior and self-care. Research also suggests that improvements in one of these problems (eg, physical inactivity) can influence improvement in other symptoms (eg, depression and fatigue). Unlike other treatment options, behavioral interventions can be delivered in various formats (eg, in-person and electronic health), are time-limited, and cause few (if any) undesirable systemic adverse effects. Behavioral interventions are therefore, an essential part of interprofessional care and rehabilitation for patients with MS.
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system that affects nearly 1 million people in the US.1 In addition to the accumulation of functional limitations, patients with MS commonly experience mental health and physical symptoms such as depression, anxiety, stress, fatigue, and pain. Day-to-day life with MS requires adaptation to challenges and active maintenance of health and well-being over time. Behavioral intervention and treatment, whether in the form of psychotherapy, health behavior coaching, or the promotion of active self-management, is an integral component of interprofessional care and key aspect of living well with MS.
Behavioral Comorbidities
Depression
Depression is a common concern among individuals with MS. Population-based studies suggest that individuals with MS have a roughly 1 in 4 chance of developing major depressive disorder over their lifetime.2 However, at any given time, between 40% and 60% of individuals with MS report clinically meaningful levels of depressive symptoms.3 Although the relationship between MS disease characteristics and depression is unclear, some evidence suggests that depressive symptoms are more common at certain points in illness, such as early in the disease process as individuals grapple with the onset of new symptoms, late in the disease process as they accumulate greater disability, and during active clinical relapses.3-5
Depression often is comorbid with, and adds to the symptom burden of, other common conditions in MS such as fatigue and cognitive dysfunction.6-8 Thus, it is not surprising that it associated with poorer overall quality of life (QOL).9 Depression also is a risk factor for suicidal ideation and suicide for patients with MS.10,11
Fortunately, several behavioral interventions show promise in treating depression in patients with MS. Both individual and group formats of cognitive behavioral therapy (CBT), a treatment focused on challenging maladaptive patterns of thought and behavior, have been shown to improve depressive symptoms for people with MS.12,13 Several brief and efficient group-based programs grounded in CBT and focused on the development of specific skills, including problem solving, goal setting, relationship management, and managing emotions, have been shown to reduce depressive symptoms.13,14 CBT for depression in MS has been shown to be effective when delivered via telephone.15,16
Anxiety
Anxiety is common among individuals with MS. Existing data suggest more than one-third of individuals with MS will qualify for a diagnosis of anxiety disorder during their lifetime.17 The characteristics of anxiety disorders are broad and heterogenous, including generalized anxiety disorder, panic disorder, obsessive compulsive disorders, and health-specific phobias such as needle/injection anxiety. Some estimates suggest a point prevalence of 34% for the presence of clinically meaningful symptoms.18 Similar to depression, anxiety symptoms can be more common during periods of stress, threat, and transition including early in the disease course while adapting to new diagnosis, late in the disease course with increasing disability, and during clinical relapses.19-21
The efficacy of behavioral interventions for anxiety in MS is less well established than it is for depression, but some preliminary evidence suggests that individual CBT may be effective for reducing general symptoms of anxiety as well as health-related anxiety.22,23 Brief, targeted CBT also has been shown to improve injection anxiety, removing a barrier to self-care including the administration of MS disease modifying therapies (DMTs).24
Stress
Stress is commonly conceptualized as a person’s perception that efforts to manage internal and external demands exceed available coping resources.25 Such demands involve both psychological and physiological processes and come in many forms for people with MS and can include daily hassles, major life events, traumatic stress, and perceptions of global nonspecific stress. The relationship between stress and MS remains complex and poorly understood. Nonetheless, individuals with MS frequently report that stress exacerbates their symptoms.26
Some evidence also suggests stress may exacerbate the MS disease process, resulting in more frequent relapses and increased lesion activity visible on MRI.27,28 In addition to mindfulness (described below), stress inoculation training (CBT and relaxation training), and stress-focused group-based self-management have been shown to be beneficial.29,30 In an intriguing and rigorous trial, a 24-week stress management therapy based on CBT was associated with the development of fewer new MS lesions visible on MRI.31
Adaptation to Illness
MS presents challenges that vary between patients and over time. Individuals may confront new physical and cognitive limitations that inhibit the completion of daily tasks, reduce independence, and limit participation in valued and meaningful activities. In addition, the unpredictability of the disease contributes to perceptions of uncertainty and uncontrollability, which in turn result in higher illness impact and poorer psychological outcomes.32 Building cognitive and behavioral skills to address these challenges can promote adaptation to illness and reduce overall distress associated with chronic illness.33 Psychosocial intervention also can address the uncertainty commonly experienced by individuals with MS.34
Self-Management
As with any chronic illness, living well with MS requires ongoing commitment and active engagement with health and personal care over time. The process of building knowledge and skills to manage the day-to-day physical, emotional, and social aspects of living with illness often is referred to as self-management.35 For individuals with MS, this may take the form of participation in programs that address adaptation and psychological distress like those described above, but it also may include improving health behavior (eg, physical activity, DMT adherence, modification of maladaptive habits like smoking or hazardous alcohol use) and symptom management (eg, fatigue, pain). Self-management programs typically include education, the practice of identifying, problem solving, and following through with specific and realistic health and wellness goals, as well as the bolstering of self-efficacy.
Physical Activity
Once discouraged for patients with MS, physical activity is now considered a cornerstone of health and wellness. Physical activity and interventions that target various forms of exercise have been shown to improve strength and endurance, reduce functional decline, enhance QOL, and likely reduce mortality.35-39 A variety of brief behavioral interventions have been shown to improve physical activity in MS. Structured group-based exercise classes focusing on various activities such as aerobic training (eg, cycling) or resistance training (eg, lower extremity strengthening) have demonstrated improvements in various measures of fitness and mood states such as depression and QOL. Brief home-based telephone counseling interventions based in social cognitive theory (eg, goal setting, navigating obstacles) and motivational interviewing strategies (eg, open-ended questions, affirmation, reflective listening, summarizing) also have been shown to be effective not only at increasing physical activity and improving depression and fatigue.40,41
Adherence to Treatment
One primary focus of adherence to treatment is medication management. For individuals with MS, DMTs represent a primary means of reducing disease burden and delaying functional decline. Many DMTs require consistent self-administration over time. Some evidence suggests that poorer adherence is associated with a greater risk of relapse and more rapid disease progression.42,43 Brief telephone counseling, again based on social cognitive theory, and principles of motivational interviewing combined with home telehealth monitoring by a care coordinator has been shown to improve adherence to DMTs.44
Mindfulness
In recent years, mindfulness training has emerged as a popular and common behavioral intervention among individuals with MS. Programs like Mindfulness-Based Stress Reduction (MBSR) provide training in meditation techniques designed to promote mindfulness, which is defined as paying attention to present moment experience, including sensations, thoughts, and emotions, without judgment or attachment.45 Cultivating mindfulness helps people with MS cope with and adapt to symptoms and stressors.46 Mindfulness interventions typically are delivered in a group format. For example, MBSR consists of 8 in-person group sessions with daily meditation practice homework. Mindfulness interventions also have been delivered effectively with smartphone apps.47 Mindfulness programs have been shown to improve depression, anxiety, fatigue, stress, and QOL for patients with MS.48-50
Fatigue
More than 90% of individuals with MS report fatigue, and many identify it as their most disabling symptom.51 Often defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities,” fatigue has been shown to be associated with longer disease duration, greater physical disability, progressive subtype, and depressive symptoms, although the relative and possibly overlapping impact of these issues is only partially understood.52,53 Fatigue is associated with poorer overall mental health and negatively impacts work and social roles.54
Several behavioral interventions have been developed to address fatigue in MS. Using both individual and group based formats and across several modalities (eg, in-person, telephone, online modules, or a combination), behavioral fatigue interventions most commonly combine traditional general CBT skills (eg, addressing maladaptive thoughts and behaviors) with a variety of fatigue-specific skill building exercises that may include fatigue education, energy conservation strategies, improving sleep, enlisting social support, and self-management goal setting strategies.35,55-57
Pain
Chronic pain is common and disabling in people with MS.58,59 Nearly 50% report experiencing moderate to severe chronic pain.59,60 Individuals with MS reporting pain often are older, more disabled (higher Expanded Disability Status Scale score), and have longer disease duration that those who are not experiencing chronic pain.61 Patients report various types of pain in the following order of frequency: dysesthetic pain (18.1%), back pain (16.4%), painful tonic spasms (11.0%), Lhermitte sign (9.0%), visceral pain (2.9%), and trigeminal neuralgia (2.0%).61 Chronic pain has a negative impact on QOL in the areas of sleep, work, maintaining relationships, recreational activities, and overall life enjoyment.59 Additionally, research has shown that greater pain intensity and pain-related interference with activities of daily living are both associated with greater depression severity.62,63
The literature supports the use of behavioral interventions for pain in people with MS.61 Behavioral interventions include in-person exercise interventions (eg, water aerobics, cycling, rowing ergometer, treadmill walking, and resistance training), self-hypnosis, and telephone-based self-management programs based on CBT.35,64,65 As described above, CBT-based self-management programs combine learning CBT skills (eg, modifying maladaptive thoughts) with pain-specific skill building such as pain education, pacing activities, and improving sleep. Of note, MS education including, but not limited to, pain was as effective as a CBT-based self-management program in reducing pain intensity and interference.35 In addition, there is evidence to support acceptance- and mindfulness-based interventions for chronic pain, and online mindfulness-based cognitive therapy for MS related pain is currently being tested in a randomized controlled trial.35,66
Conclusion
People with MS face significant challenges in coping with and adapting to a chronic and unpredictable disease. However, there is considerable evidence that behavioral interventions can improve many of the most common and disabling symptoms in MS including depression, anxiety, stress, fatigue, and pain as well as health behavior and self-care. Research also suggests that improvements in one of these problems (eg, physical inactivity) can influence improvement in other symptoms (eg, depression and fatigue). Unlike other treatment options, behavioral interventions can be delivered in various formats (eg, in-person and electronic health), are time-limited, and cause few (if any) undesirable systemic adverse effects. Behavioral interventions are therefore, an essential part of interprofessional care and rehabilitation for patients with MS.
1. Wallin MT, Culpepper WJ, Campbell JD, et al; US Multiple Sclerosis Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
3. Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862-1868.
4. Williams RM, Turner AP, Hatzakis M Jr, Bowen JD, Rodriquez AA, Haselkorn JK. Prevalence and correlates of depression among veterans with multiple sclerosis. Neurology. 2005;64(1):75-80.
5. Moore P, Hirst C, Harding KE, Clarkson H, Pickersgill TP, Robertson NP. Multiple sclerosis relapses and depression. J Psychosom Res. 2012;73(4):272-276.
6. Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19(2):217-224.
7. Arnett PA, Higginson CI, Voss WD, et al. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology. 1999;13(3):434-446.
8. Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23(2):189-199.
9. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231(1-2):29-34.
10. Turner AP, Williams RM, Bowen JD, Kivlahan DR, Haselkorn JK. Suicidal ideation in multiple sclerosis. Arch Phys Med Rehabil. 2006;87(8):1073-1078.
11. Stenager EN, Koch-Henriksen N, Stenager E. Risk factors for suicide in multiple sclerosis. Psychother Psychosom. 1996;65(2):86-90.
12. Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69(6):942-949.
13. Larcombe NA, Wilson PH. An evaluation of cognitive-behaviour therapy for depression in patients with multiple sclerosis. Br J Psychiatry. 1984;145:366-371.
14. Lincoln NB, Yuill F, Holmes J, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler. 2011;17(10):1250-1257.
15. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol. 2000;68(2):356-361.
16. Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62(9):1007-1014.
17. Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13(1):67-72.
18. Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331-341.
19. Dahl OP, Stordal E, Lydersen S, Midgard R. Anxiety and depression in multiple sclerosis. A comparative population-based study in Nord-Trøndelag County, Norway. Mult Scler. 2009;15(12):1495-1501.
20. Burns MN, Nawacki E, Siddique J, Pelletier D, Mohr DC. Prospective examination of anxiety and depression before and during confirmed and pseudoexacerbations in patients with multiple sclerosis. Psychosom Med. 2013;75(1):76-82.
21. Uguz F, Akpinar Z, Ozkan I, Tokgoz S. Mood and anxiety disorders in patients with multiple sclerosis. Int J Psychiatry Clin Pract. 2008;12(1):19-24.
22. Askey-Jones S, David AS, Silber E, Shaw P, Chalder T. Cognitive behaviour therapy for common mental disorders in people with multiple sclerosis: a bench marking study. Behav Res Ther. 2013;51(10):648-655.
23. Carrigan N, Dysch L, Salkovskis PM. The impact of health anxiety in multiple sclerosis: a replication and treatment case series. Behav Cogn Psychother. 2018;46(2):148-167.
24. Mohr DC, Cox D, Merluzzi N. Self-injection anxiety training: a treatment for patients unable to self-inject injectable medications. Mult Scler. 2005;11(2):182-185.
25. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984.
26. Ackerman KD, Heyman R, Rabin BS, et al. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64(6):916-920.
27. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
28. Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61.
29. Foley FW, Bedell JR, LaRocca NG, Scheinberg LC, Reznikoff M. Efficacy of stress-inoculation training in coping with multiple sclerosis. J Consult Clin Psychol. 1987;55(6):919-922.
30. Hughes RB, Robinson-Whelen S, Taylor HB, Hall JW. Stress self-management: an intervention for women with physical disabilities. Womens Health Issues. 2006;16(6):389-399.
31. Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419.
32. Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009;29(2):141-153.
33. Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work? J Consult Clin Psychol. 2013;81(2):251-262.
34. Molton IR, Koelmel E, Curran M, von Geldern G, Ordway A, Alschuler KN. Pilot intervention to promote tolerance for uncertainty in early multiple sclerosis. Rehabil Psychol. 2019;64(3):339-350.
35. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945-1958.e2.
36. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85(2):290-297.
37. Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(7):935-943.
38. Turner AP, Hartoonian N, Maynard C, Leipertz SL, Haselkorn JK. Smoking and physical activity: examining health behaviors and 15-year mortality among individuals with multiple sclerosis. Arch Phys Med Rehabil. 2015;96(3):402-409.
39. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
40. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
41. Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81(1):89-99.
42. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251-260.
43. Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;7 Suppl 1:S1-S9.
44. Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136-146.
45. Kabat-Zinn J. Full Catastrophe Living. London, UK: Piatkus; 2013.
46. Bishop SR. What do we really know about mindfulness-based stress reduction? [published correction appears in Psychosom Med. 2002;64(3):449]. Psychosom Med. 2002;64(1):71-83.
47. Lindsay EK, Young S, Smyth JM, Brown KW, Creswell JD. Acceptance lowers stress reactivity: dismantling mindfulness training in a randomized controlled trial. Psychoneuroendocrinology. 2018;87:63-73.
48. Simpson R, Mair FS, Mercer SW. Mindfulness-based stress reduction for people with multiple sclerosis - a feasibility randomised controlled trial. BMC Neurol. 2017;17(1):94.
49. Cavalera C, Rovaris M, Mendozzi L, et al. Online meditation training for people with multiple sclerosis: a randomized controlled trial. Mult Scler. 2019;25(4):610-617.
50. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141-1149.
51. Shah A. Fatigue in multiple sclerosis. Phys Med Rehabil Clin N Am. 2009;20(2):363-372.
52. Guidelines MSCfCP. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
53. Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225-234.
54. Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1996;77(2):165-170.
55. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50(6):415-421.
56. Thomas PW, Thomas S, Kersten P, et al. Multi-centre parallel arm randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based cognitive behavioural approach to managing fatigue in people with multiple sclerosis. BMC Neurol. 2010;10:43.
57. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70(2):205-213.
58. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154(5):632-642.
59. O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
60. Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12(5):629-638.
61. Aboud T, Schuster NM. Pain management in multiple sclerosis: a review of available treatment options. Curr Treat Options Neurol. 2019;21(12):62.
62. Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015;60(1):81-90.
63. Arewasikporn A, Turner AP, Alschuler KN, Hughes AJ, Ehde DM. Cognitive and affective mechanisms of pain and fatigue in multiple sclerosis. Health Psychol. 2018;37(6):544-552.
64. Demaneuf T, Aitken Z, Karahalios A, et al. Effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2019;100(1):128-139.
65. Jensen MP, Barber J, Romano JM, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2009;57(2):198-221.
66. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533-542.
1. Wallin MT, Culpepper WJ, Campbell JD, et al; US Multiple Sclerosis Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
3. Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862-1868.
4. Williams RM, Turner AP, Hatzakis M Jr, Bowen JD, Rodriquez AA, Haselkorn JK. Prevalence and correlates of depression among veterans with multiple sclerosis. Neurology. 2005;64(1):75-80.
5. Moore P, Hirst C, Harding KE, Clarkson H, Pickersgill TP, Robertson NP. Multiple sclerosis relapses and depression. J Psychosom Res. 2012;73(4):272-276.
6. Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19(2):217-224.
7. Arnett PA, Higginson CI, Voss WD, et al. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology. 1999;13(3):434-446.
8. Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23(2):189-199.
9. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231(1-2):29-34.
10. Turner AP, Williams RM, Bowen JD, Kivlahan DR, Haselkorn JK. Suicidal ideation in multiple sclerosis. Arch Phys Med Rehabil. 2006;87(8):1073-1078.
11. Stenager EN, Koch-Henriksen N, Stenager E. Risk factors for suicide in multiple sclerosis. Psychother Psychosom. 1996;65(2):86-90.
12. Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69(6):942-949.
13. Larcombe NA, Wilson PH. An evaluation of cognitive-behaviour therapy for depression in patients with multiple sclerosis. Br J Psychiatry. 1984;145:366-371.
14. Lincoln NB, Yuill F, Holmes J, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler. 2011;17(10):1250-1257.
15. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol. 2000;68(2):356-361.
16. Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62(9):1007-1014.
17. Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13(1):67-72.
18. Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331-341.
19. Dahl OP, Stordal E, Lydersen S, Midgard R. Anxiety and depression in multiple sclerosis. A comparative population-based study in Nord-Trøndelag County, Norway. Mult Scler. 2009;15(12):1495-1501.
20. Burns MN, Nawacki E, Siddique J, Pelletier D, Mohr DC. Prospective examination of anxiety and depression before and during confirmed and pseudoexacerbations in patients with multiple sclerosis. Psychosom Med. 2013;75(1):76-82.
21. Uguz F, Akpinar Z, Ozkan I, Tokgoz S. Mood and anxiety disorders in patients with multiple sclerosis. Int J Psychiatry Clin Pract. 2008;12(1):19-24.
22. Askey-Jones S, David AS, Silber E, Shaw P, Chalder T. Cognitive behaviour therapy for common mental disorders in people with multiple sclerosis: a bench marking study. Behav Res Ther. 2013;51(10):648-655.
23. Carrigan N, Dysch L, Salkovskis PM. The impact of health anxiety in multiple sclerosis: a replication and treatment case series. Behav Cogn Psychother. 2018;46(2):148-167.
24. Mohr DC, Cox D, Merluzzi N. Self-injection anxiety training: a treatment for patients unable to self-inject injectable medications. Mult Scler. 2005;11(2):182-185.
25. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984.
26. Ackerman KD, Heyman R, Rabin BS, et al. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64(6):916-920.
27. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
28. Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61.
29. Foley FW, Bedell JR, LaRocca NG, Scheinberg LC, Reznikoff M. Efficacy of stress-inoculation training in coping with multiple sclerosis. J Consult Clin Psychol. 1987;55(6):919-922.
30. Hughes RB, Robinson-Whelen S, Taylor HB, Hall JW. Stress self-management: an intervention for women with physical disabilities. Womens Health Issues. 2006;16(6):389-399.
31. Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419.
32. Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009;29(2):141-153.
33. Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work? J Consult Clin Psychol. 2013;81(2):251-262.
34. Molton IR, Koelmel E, Curran M, von Geldern G, Ordway A, Alschuler KN. Pilot intervention to promote tolerance for uncertainty in early multiple sclerosis. Rehabil Psychol. 2019;64(3):339-350.
35. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945-1958.e2.
36. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85(2):290-297.
37. Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(7):935-943.
38. Turner AP, Hartoonian N, Maynard C, Leipertz SL, Haselkorn JK. Smoking and physical activity: examining health behaviors and 15-year mortality among individuals with multiple sclerosis. Arch Phys Med Rehabil. 2015;96(3):402-409.
39. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
40. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
41. Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81(1):89-99.
42. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251-260.
43. Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;7 Suppl 1:S1-S9.
44. Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136-146.
45. Kabat-Zinn J. Full Catastrophe Living. London, UK: Piatkus; 2013.
46. Bishop SR. What do we really know about mindfulness-based stress reduction? [published correction appears in Psychosom Med. 2002;64(3):449]. Psychosom Med. 2002;64(1):71-83.
47. Lindsay EK, Young S, Smyth JM, Brown KW, Creswell JD. Acceptance lowers stress reactivity: dismantling mindfulness training in a randomized controlled trial. Psychoneuroendocrinology. 2018;87:63-73.
48. Simpson R, Mair FS, Mercer SW. Mindfulness-based stress reduction for people with multiple sclerosis - a feasibility randomised controlled trial. BMC Neurol. 2017;17(1):94.
49. Cavalera C, Rovaris M, Mendozzi L, et al. Online meditation training for people with multiple sclerosis: a randomized controlled trial. Mult Scler. 2019;25(4):610-617.
50. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141-1149.
51. Shah A. Fatigue in multiple sclerosis. Phys Med Rehabil Clin N Am. 2009;20(2):363-372.
52. Guidelines MSCfCP. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
53. Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225-234.
54. Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1996;77(2):165-170.
55. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50(6):415-421.
56. Thomas PW, Thomas S, Kersten P, et al. Multi-centre parallel arm randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based cognitive behavioural approach to managing fatigue in people with multiple sclerosis. BMC Neurol. 2010;10:43.
57. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70(2):205-213.
58. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154(5):632-642.
59. O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
60. Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12(5):629-638.
61. Aboud T, Schuster NM. Pain management in multiple sclerosis: a review of available treatment options. Curr Treat Options Neurol. 2019;21(12):62.
62. Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015;60(1):81-90.
63. Arewasikporn A, Turner AP, Alschuler KN, Hughes AJ, Ehde DM. Cognitive and affective mechanisms of pain and fatigue in multiple sclerosis. Health Psychol. 2018;37(6):544-552.
64. Demaneuf T, Aitken Z, Karahalios A, et al. Effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2019;100(1):128-139.
65. Jensen MP, Barber J, Romano JM, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2009;57(2):198-221.
66. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533-542.
The Multiple Sclerosis Surveillance Registry: A Novel Interactive Database Within the Veterans Health Administration (FULL)
The VA MS Surveillance Registry combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US.
A number of large registries exist for multiple sclerosis (MS) in North America and Europe. The Scandinavian countries have some of the longest running and integrated MS registries to date. The Danish MS Registry was initiated in 1948 and has been consistently maintained to track MS epidemiologic trends.2 Similar databases exist in Swedenand Norway that were created in the later 20th century.3,4 The Rochester Epidemiology Project, launched by Len Kurland at the Mayo Clinic, has tracked the morbidity of MS and many other conditions in Olmsted county Minnesota for > 60 years.5
The Canadian provinces of British Columbia, Ontario, and Manitoba also have long standing MS registries.6-8 Other North American MS registries have gathered state-wide cases, such as the New York State MS Consortium.9 Some registries have gathered a population-based sample throughout the US, such as the Sonya Slifka MS Study.10 The North American Research Consortium on MS (NARCOMS) registry is a patient-driven registry within the US that has enrolled > 30,000 cases.11 The MSBase is the largest online registry to date utilizing data from several countries.12 The MS Bioscreen, based at the University of California San Francisco, is a recent effort to create a longitudinal clinical dataset.13 This electronic registry integrates clinical disease morbidity scales, neuroimaging, genetics and laboratory data for individual patients with the goal of providing predictive tools.
The US military provides a unique population to study MS and has the oldest and largest nation-wide MS cohort in existence starting with World War I service members and continuing through the recent Gulf War Era.14 With the advent of EHRs in the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) in the mid-1990s and large clinical databases, the possibility of an integrated registry for chronic conditions was created. In this report, we describe the creation of the VA MS Surveillance Registry (MSSR) and the initial roll out to several VA medical centers within the MS Center of Excellence (MSCoE). The MSSR is a unique platform with potential for improving MS patient care and clinical research.
Methods
The MSSR was designed by MSCoE health care providers in conjunction with IT specialists from the VA Northwest Innovation Center. Between 2012 and 2013, the team developed and tested a core template for data entry and refined an efficient data dashboard display to optimize clinical decisions. IT programmers created data entry templates that were tested by 4 to 5 clinicians who provided feedback in biweekly meetings. Technical problems were addressed and enhancements added and the trial process was repeated.
After creation of the prototype MS Assessment Tool (MSAT) data entry template that fed into the prototype MSSR, our team received a grant in 2013 for national development and sustainment. The MSSR was established on the VA Converged Registries Solution (CRS) platform, which is a hardware and software architecture designed to host individual clinical registries and eliminate duplicative development effort while maximizing the ability to create new patient registries. The common platform includes a relational database, Health Level 7 messaging, software classes, security modules, extraction services, and other components. The CR obtains data from the VA Corporate Data Warehouse (CDW), directly from the Veterans Health Information Systems and Technology Architecture (VISTA) and via direct user input using MSAT.
From 2016 to 2019, data from patients with MS followed in several VA MS regional programs were inputted into MSSR. A roll-out process to start patient data entry at VA medical centers began in 2017 that included an orientation, technical support, and quality assurance review. Twelve sites from Veteran Integrated Service Network (VISN) 5 (mid-Atlantic) and VISN 20 (Pacific Northwest) were included in the initial roll-out.
Results
After a live or remote telehealth or telephone visit, a clinician can access MSAT from the Computerized Patient Record System (CPRS) or directly from the MSSR online portal (Figure 1). The tool uses radio buttons and pull-down menus and takes about 5 to 15 minutes to complete with a list of required variables. Data is auto-saved for efficiency, and the key variables that are collected in MSAT are noted in Table 1. The MSAT subsequently creates a text integration utility progress note with health factors that is processed through an integration engine and eventually transmitted to VISTA and becomes part of the EHR and available to all health care providers involved in that patient’s care. Additionally, data from VA outpatient and inpatient utilization files, pharmacy, prosthetics, laboratory, and radiology databases are included in the CDW and are included in MSSR. With data from 1998 to the present, the MSAT and CDW databases can provide longitudinal data analysis.
Between 18,000 and 20,000 patients with MS are evaluated in the VHA annually, and 56,000 unique patients have been assessed since 1998. From 2016 to 2019, 1,743 patients with MS or related disorders were enrolled in MSSR (Table 2 and Figure 2). The mean (SD) age of patients was 56.0 (12.9) years and the male:female ratio was 2.7. Racial minorities make up 40% of the cohort. Among those with definite and possible MS, the mean disease duration was 22.7 years and the mean (SD) European Database for MS disability score was 4.7 (2.4) (Table 3). Three-quarters of the MSSR cohort have used ≥ 1 MS disease modifying therapy and 65% were classified as relapsing-remitting MS. An electronic dashboard was developed for health care providers to easily access demographic and clinical data for individuals and groups of patients (Figure 3). Standard and ad hoc reports can be generated from the MSSR. Larger longitudinal analyses can be performed with MSAT and clinical data from CDW. Data on comorbid conditions, pharmacy, radiology and prosthetics utilization, outpatient clinic and inpatient admission can be accessed for each patient or a group of patients.
In 2015, MSCoE published a larger national survey of the VA MS population.15 This study revealed that the majority of clinical features and demographics of the MSSR were not significantly different from other major US MS registries including the North American Research Committee on MS, the New York State MS Consortium, and the Sonya Slifka Study.16-18
Discussion
The MSSR is novel in that it combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US. This new registry leverages the existing databases related to cost of care, utilization, and pharmacy services to provide surveillance tools for longitudinal follow-up of the MS population within the VHA. Because the structure of the MSAT and MSSR were developed in a partnership between IT developers and clinicians, there has been mutual buy-in for those who use it and maintain it. This registry can be a test bed for standardized patient outcomes including the recently released MS Quality measures from the American Academy of Neurology.19
To achieve greater numbers across populations, there has been efforts in Europe to combine registries into a common European Register for MS. A recent survey found that although many European registries were heterogeneous, it would be possible to have a minimum common data set for limited epidemiologic studies.20 Still many registries do not have environmental or genetic data to evaluate etiologic questions.21 Additionally, most registries are not set up to evaluate cost or quality of care within a health care system.
Recommendations for maximizing the impact of existing MS registries were recently released by a panel of MS clinicians and researchers.22 The first recommendation was to create a broad network of registries that would communicate and collaborate. This group of MS registries would have strategic oversight and direction that would greatly streamline and leverage existing and future efforts. Second, registries should standardize data collection and management thereby enhancing the ability to share data and perform meta-analyses with aggregated data. Third, the collection of physician- and patient-reported outcomes should be encouraged to provide a more complete picture of MS. Finally, registries should prioritize research questions and utilize new technologies for data collection. These recommendations would help to coordinate existing registries and accelerate knowledge discovery.
The MSSR will contribute to the growing registry network of data. The MSSR can address questions about clinical outcomes, cost, quality with a growing data repository and linked biobank. Based on the CR platform, the MSSR allows for integration with other VA clinical registries, including registries for traumatic brain injuries, oncology, HIV, hepatitis C virus, and eye injuries. Identifying case outcomes related to other registries is optimized with the CR common structure.
Conclusion
The MSSR has been a useful tool for clinicians managing individual patients and their regional referral populations with real-time access to clinical and utilization data. It will also be a useful research tool in tracking epidemiological trends for the military population. The MSSR has enhanced clinical management of MS and serves as a national source for clinical outcomes.
1. Flachenecker P. Multiple sclerosis databases: present and future. Eur Neurol. 2014;72(suppl 1):29-31.
2. Koch-Henriksen N, Magyari M, Laursen B. Registers of multiple sclerosis in Denmark. Acta Neurol Scand. 2015;132(199):4-10.
3. Alping P, Piehl F, Langer-Gould A, Frisell T; COMBAT-MS Study Group. Validation of the Swedish Multiple Sclerosis Register: further improving a resource for pharmacoepidemiologic evaluations. Epidemiology. 2019;30(2):230-233.
4. Benjaminsen E, Myhr KM, Grytten N, Alstadhaug KB. Validation of the multiple sclerosis diagnosis in the Norwegian Patient Registry. Brain Behav. 2019;9(11):e01422.
5. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202-1213.
6. Kingwell E, Zhu F, Marrie RA, et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991-2010). J Neurol. 2015;262(10):2352-2363.
7. Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914-1929.
8. Mahmud SM, Bozat-Emre S, Mostaço-Guidolin LC, Marrie RA. Registry cohort study to determine risk for multiple sclerosis after vaccination for pandemic influenza A(H1N1) with Arepanrix, Manitoba, Canada. Emerg Infect Dis. 2018;24(7):1267-1274.
9. Kister I, Chamot E, Bacon JH, Cutter G, Herbert J; New York State Multiple Sclerosis Consortium. Trend for decreasing Multiple Sclerosis Severity Scores (MSSS) with increasing calendar year of enrollment into the New York State Multiple Sclerosis Consortium. Mult Scler. 2011;17(6):725-733.
10. Minden SL, Frankel D, Hadden L, Perloffp J, Srinath KP, Hoaglin DC. The Sonya Slifka Longitudinal Multiple Sclerosis Study: methods and sample characteristics. Mult Scler. 2006;12(1):24-38.
11. Fox RJ, Salter A, Alster JM, et al. Risk tolerance to MS therapies: survey results from the NARCOMS registry. Mult Scler Relat Disord. 2015;4(3):241-249.
12. Kalincik T, Butzkueven H. The MSBase registry: Informing clinical practice. Mult Scler. 2019;25(14):1828-1834.
13. Gourraud PA, Henry RG, Cree BA, et al. Precision medicine in chronic disease management: the multiple sclerosis BioScreen. Ann Neurol. 2014;76(5):633-642.
14. Wallin MT, Culpepper WJ, Coffman P, et al. The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain. 2012;135(Pt 6):1778-1785.
15. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272.
16. Salter A, Stahmann A, Ellenberger D, et al. Data harmonization for collaborative research among MS registries: a case study in employment [published online ahead of print, 2020 Mar 12]. Mult Scler. 2020;1352458520910499.
17. Vaughn CB, Kavak KS, Dwyer MG, et al. Fatigue at enrollment predicts EDSS worsening in the New York State Multiple Sclerosis Consortium. Mult Scler. 2020;26(1):99-108.
18. Minden SL, Kinkel RP, Machado HT, et al. Use and cost of disease-modifying therapies by Sonya Slifka Study participants: has anything really changed since 2000 and 2009? Mult Scler J Exp Transl Clin. 2019;5(1):2055217318820888.
19. Rae-Grant A, Bennett A, Sanders AE, Phipps M, Cheng E, Bever C. Quality improvement in neurology: multiple sclerosis quality measures: Executive summary [published correction appears in Neurology. 2016;86(15):1465]. Neurology. 2015;85(21):1904-1908.
20. Flachenecker P, Buckow K, Pugliatti M, et al; EUReMS Consortium. Multiple sclerosis registries in Europe - results of a systematic survey. Mult Scler. 2014;20(11):1523-1532.
21. Traboulsee A, McMullen K. How useful are MS registries?. Mult Scler. 2014;20(11):1423-1424.
22. Bebo BF Jr, Fox RJ, Lee K, Utz U, Thompson AJ. Landscape of MS patient cohorts and registries: Recommendations for maximizing impact. Mult Scler. 2018;24(5):579-586.
The VA MS Surveillance Registry combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US.
The VA MS Surveillance Registry combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US.
A number of large registries exist for multiple sclerosis (MS) in North America and Europe. The Scandinavian countries have some of the longest running and integrated MS registries to date. The Danish MS Registry was initiated in 1948 and has been consistently maintained to track MS epidemiologic trends.2 Similar databases exist in Swedenand Norway that were created in the later 20th century.3,4 The Rochester Epidemiology Project, launched by Len Kurland at the Mayo Clinic, has tracked the morbidity of MS and many other conditions in Olmsted county Minnesota for > 60 years.5
The Canadian provinces of British Columbia, Ontario, and Manitoba also have long standing MS registries.6-8 Other North American MS registries have gathered state-wide cases, such as the New York State MS Consortium.9 Some registries have gathered a population-based sample throughout the US, such as the Sonya Slifka MS Study.10 The North American Research Consortium on MS (NARCOMS) registry is a patient-driven registry within the US that has enrolled > 30,000 cases.11 The MSBase is the largest online registry to date utilizing data from several countries.12 The MS Bioscreen, based at the University of California San Francisco, is a recent effort to create a longitudinal clinical dataset.13 This electronic registry integrates clinical disease morbidity scales, neuroimaging, genetics and laboratory data for individual patients with the goal of providing predictive tools.
The US military provides a unique population to study MS and has the oldest and largest nation-wide MS cohort in existence starting with World War I service members and continuing through the recent Gulf War Era.14 With the advent of EHRs in the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) in the mid-1990s and large clinical databases, the possibility of an integrated registry for chronic conditions was created. In this report, we describe the creation of the VA MS Surveillance Registry (MSSR) and the initial roll out to several VA medical centers within the MS Center of Excellence (MSCoE). The MSSR is a unique platform with potential for improving MS patient care and clinical research.
Methods
The MSSR was designed by MSCoE health care providers in conjunction with IT specialists from the VA Northwest Innovation Center. Between 2012 and 2013, the team developed and tested a core template for data entry and refined an efficient data dashboard display to optimize clinical decisions. IT programmers created data entry templates that were tested by 4 to 5 clinicians who provided feedback in biweekly meetings. Technical problems were addressed and enhancements added and the trial process was repeated.
After creation of the prototype MS Assessment Tool (MSAT) data entry template that fed into the prototype MSSR, our team received a grant in 2013 for national development and sustainment. The MSSR was established on the VA Converged Registries Solution (CRS) platform, which is a hardware and software architecture designed to host individual clinical registries and eliminate duplicative development effort while maximizing the ability to create new patient registries. The common platform includes a relational database, Health Level 7 messaging, software classes, security modules, extraction services, and other components. The CR obtains data from the VA Corporate Data Warehouse (CDW), directly from the Veterans Health Information Systems and Technology Architecture (VISTA) and via direct user input using MSAT.
From 2016 to 2019, data from patients with MS followed in several VA MS regional programs were inputted into MSSR. A roll-out process to start patient data entry at VA medical centers began in 2017 that included an orientation, technical support, and quality assurance review. Twelve sites from Veteran Integrated Service Network (VISN) 5 (mid-Atlantic) and VISN 20 (Pacific Northwest) were included in the initial roll-out.
Results
After a live or remote telehealth or telephone visit, a clinician can access MSAT from the Computerized Patient Record System (CPRS) or directly from the MSSR online portal (Figure 1). The tool uses radio buttons and pull-down menus and takes about 5 to 15 minutes to complete with a list of required variables. Data is auto-saved for efficiency, and the key variables that are collected in MSAT are noted in Table 1. The MSAT subsequently creates a text integration utility progress note with health factors that is processed through an integration engine and eventually transmitted to VISTA and becomes part of the EHR and available to all health care providers involved in that patient’s care. Additionally, data from VA outpatient and inpatient utilization files, pharmacy, prosthetics, laboratory, and radiology databases are included in the CDW and are included in MSSR. With data from 1998 to the present, the MSAT and CDW databases can provide longitudinal data analysis.
Between 18,000 and 20,000 patients with MS are evaluated in the VHA annually, and 56,000 unique patients have been assessed since 1998. From 2016 to 2019, 1,743 patients with MS or related disorders were enrolled in MSSR (Table 2 and Figure 2). The mean (SD) age of patients was 56.0 (12.9) years and the male:female ratio was 2.7. Racial minorities make up 40% of the cohort. Among those with definite and possible MS, the mean disease duration was 22.7 years and the mean (SD) European Database for MS disability score was 4.7 (2.4) (Table 3). Three-quarters of the MSSR cohort have used ≥ 1 MS disease modifying therapy and 65% were classified as relapsing-remitting MS. An electronic dashboard was developed for health care providers to easily access demographic and clinical data for individuals and groups of patients (Figure 3). Standard and ad hoc reports can be generated from the MSSR. Larger longitudinal analyses can be performed with MSAT and clinical data from CDW. Data on comorbid conditions, pharmacy, radiology and prosthetics utilization, outpatient clinic and inpatient admission can be accessed for each patient or a group of patients.
In 2015, MSCoE published a larger national survey of the VA MS population.15 This study revealed that the majority of clinical features and demographics of the MSSR were not significantly different from other major US MS registries including the North American Research Committee on MS, the New York State MS Consortium, and the Sonya Slifka Study.16-18
Discussion
The MSSR is novel in that it combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US. This new registry leverages the existing databases related to cost of care, utilization, and pharmacy services to provide surveillance tools for longitudinal follow-up of the MS population within the VHA. Because the structure of the MSAT and MSSR were developed in a partnership between IT developers and clinicians, there has been mutual buy-in for those who use it and maintain it. This registry can be a test bed for standardized patient outcomes including the recently released MS Quality measures from the American Academy of Neurology.19
To achieve greater numbers across populations, there has been efforts in Europe to combine registries into a common European Register for MS. A recent survey found that although many European registries were heterogeneous, it would be possible to have a minimum common data set for limited epidemiologic studies.20 Still many registries do not have environmental or genetic data to evaluate etiologic questions.21 Additionally, most registries are not set up to evaluate cost or quality of care within a health care system.
Recommendations for maximizing the impact of existing MS registries were recently released by a panel of MS clinicians and researchers.22 The first recommendation was to create a broad network of registries that would communicate and collaborate. This group of MS registries would have strategic oversight and direction that would greatly streamline and leverage existing and future efforts. Second, registries should standardize data collection and management thereby enhancing the ability to share data and perform meta-analyses with aggregated data. Third, the collection of physician- and patient-reported outcomes should be encouraged to provide a more complete picture of MS. Finally, registries should prioritize research questions and utilize new technologies for data collection. These recommendations would help to coordinate existing registries and accelerate knowledge discovery.
The MSSR will contribute to the growing registry network of data. The MSSR can address questions about clinical outcomes, cost, quality with a growing data repository and linked biobank. Based on the CR platform, the MSSR allows for integration with other VA clinical registries, including registries for traumatic brain injuries, oncology, HIV, hepatitis C virus, and eye injuries. Identifying case outcomes related to other registries is optimized with the CR common structure.
Conclusion
The MSSR has been a useful tool for clinicians managing individual patients and their regional referral populations with real-time access to clinical and utilization data. It will also be a useful research tool in tracking epidemiological trends for the military population. The MSSR has enhanced clinical management of MS and serves as a national source for clinical outcomes.
A number of large registries exist for multiple sclerosis (MS) in North America and Europe. The Scandinavian countries have some of the longest running and integrated MS registries to date. The Danish MS Registry was initiated in 1948 and has been consistently maintained to track MS epidemiologic trends.2 Similar databases exist in Swedenand Norway that were created in the later 20th century.3,4 The Rochester Epidemiology Project, launched by Len Kurland at the Mayo Clinic, has tracked the morbidity of MS and many other conditions in Olmsted county Minnesota for > 60 years.5
The Canadian provinces of British Columbia, Ontario, and Manitoba also have long standing MS registries.6-8 Other North American MS registries have gathered state-wide cases, such as the New York State MS Consortium.9 Some registries have gathered a population-based sample throughout the US, such as the Sonya Slifka MS Study.10 The North American Research Consortium on MS (NARCOMS) registry is a patient-driven registry within the US that has enrolled > 30,000 cases.11 The MSBase is the largest online registry to date utilizing data from several countries.12 The MS Bioscreen, based at the University of California San Francisco, is a recent effort to create a longitudinal clinical dataset.13 This electronic registry integrates clinical disease morbidity scales, neuroimaging, genetics and laboratory data for individual patients with the goal of providing predictive tools.
The US military provides a unique population to study MS and has the oldest and largest nation-wide MS cohort in existence starting with World War I service members and continuing through the recent Gulf War Era.14 With the advent of EHRs in the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) in the mid-1990s and large clinical databases, the possibility of an integrated registry for chronic conditions was created. In this report, we describe the creation of the VA MS Surveillance Registry (MSSR) and the initial roll out to several VA medical centers within the MS Center of Excellence (MSCoE). The MSSR is a unique platform with potential for improving MS patient care and clinical research.
Methods
The MSSR was designed by MSCoE health care providers in conjunction with IT specialists from the VA Northwest Innovation Center. Between 2012 and 2013, the team developed and tested a core template for data entry and refined an efficient data dashboard display to optimize clinical decisions. IT programmers created data entry templates that were tested by 4 to 5 clinicians who provided feedback in biweekly meetings. Technical problems were addressed and enhancements added and the trial process was repeated.
After creation of the prototype MS Assessment Tool (MSAT) data entry template that fed into the prototype MSSR, our team received a grant in 2013 for national development and sustainment. The MSSR was established on the VA Converged Registries Solution (CRS) platform, which is a hardware and software architecture designed to host individual clinical registries and eliminate duplicative development effort while maximizing the ability to create new patient registries. The common platform includes a relational database, Health Level 7 messaging, software classes, security modules, extraction services, and other components. The CR obtains data from the VA Corporate Data Warehouse (CDW), directly from the Veterans Health Information Systems and Technology Architecture (VISTA) and via direct user input using MSAT.
From 2016 to 2019, data from patients with MS followed in several VA MS regional programs were inputted into MSSR. A roll-out process to start patient data entry at VA medical centers began in 2017 that included an orientation, technical support, and quality assurance review. Twelve sites from Veteran Integrated Service Network (VISN) 5 (mid-Atlantic) and VISN 20 (Pacific Northwest) were included in the initial roll-out.
Results
After a live or remote telehealth or telephone visit, a clinician can access MSAT from the Computerized Patient Record System (CPRS) or directly from the MSSR online portal (Figure 1). The tool uses radio buttons and pull-down menus and takes about 5 to 15 minutes to complete with a list of required variables. Data is auto-saved for efficiency, and the key variables that are collected in MSAT are noted in Table 1. The MSAT subsequently creates a text integration utility progress note with health factors that is processed through an integration engine and eventually transmitted to VISTA and becomes part of the EHR and available to all health care providers involved in that patient’s care. Additionally, data from VA outpatient and inpatient utilization files, pharmacy, prosthetics, laboratory, and radiology databases are included in the CDW and are included in MSSR. With data from 1998 to the present, the MSAT and CDW databases can provide longitudinal data analysis.
Between 18,000 and 20,000 patients with MS are evaluated in the VHA annually, and 56,000 unique patients have been assessed since 1998. From 2016 to 2019, 1,743 patients with MS or related disorders were enrolled in MSSR (Table 2 and Figure 2). The mean (SD) age of patients was 56.0 (12.9) years and the male:female ratio was 2.7. Racial minorities make up 40% of the cohort. Among those with definite and possible MS, the mean disease duration was 22.7 years and the mean (SD) European Database for MS disability score was 4.7 (2.4) (Table 3). Three-quarters of the MSSR cohort have used ≥ 1 MS disease modifying therapy and 65% were classified as relapsing-remitting MS. An electronic dashboard was developed for health care providers to easily access demographic and clinical data for individuals and groups of patients (Figure 3). Standard and ad hoc reports can be generated from the MSSR. Larger longitudinal analyses can be performed with MSAT and clinical data from CDW. Data on comorbid conditions, pharmacy, radiology and prosthetics utilization, outpatient clinic and inpatient admission can be accessed for each patient or a group of patients.
In 2015, MSCoE published a larger national survey of the VA MS population.15 This study revealed that the majority of clinical features and demographics of the MSSR were not significantly different from other major US MS registries including the North American Research Committee on MS, the New York State MS Consortium, and the Sonya Slifka Study.16-18
Discussion
The MSSR is novel in that it combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US. This new registry leverages the existing databases related to cost of care, utilization, and pharmacy services to provide surveillance tools for longitudinal follow-up of the MS population within the VHA. Because the structure of the MSAT and MSSR were developed in a partnership between IT developers and clinicians, there has been mutual buy-in for those who use it and maintain it. This registry can be a test bed for standardized patient outcomes including the recently released MS Quality measures from the American Academy of Neurology.19
To achieve greater numbers across populations, there has been efforts in Europe to combine registries into a common European Register for MS. A recent survey found that although many European registries were heterogeneous, it would be possible to have a minimum common data set for limited epidemiologic studies.20 Still many registries do not have environmental or genetic data to evaluate etiologic questions.21 Additionally, most registries are not set up to evaluate cost or quality of care within a health care system.
Recommendations for maximizing the impact of existing MS registries were recently released by a panel of MS clinicians and researchers.22 The first recommendation was to create a broad network of registries that would communicate and collaborate. This group of MS registries would have strategic oversight and direction that would greatly streamline and leverage existing and future efforts. Second, registries should standardize data collection and management thereby enhancing the ability to share data and perform meta-analyses with aggregated data. Third, the collection of physician- and patient-reported outcomes should be encouraged to provide a more complete picture of MS. Finally, registries should prioritize research questions and utilize new technologies for data collection. These recommendations would help to coordinate existing registries and accelerate knowledge discovery.
The MSSR will contribute to the growing registry network of data. The MSSR can address questions about clinical outcomes, cost, quality with a growing data repository and linked biobank. Based on the CR platform, the MSSR allows for integration with other VA clinical registries, including registries for traumatic brain injuries, oncology, HIV, hepatitis C virus, and eye injuries. Identifying case outcomes related to other registries is optimized with the CR common structure.
Conclusion
The MSSR has been a useful tool for clinicians managing individual patients and their regional referral populations with real-time access to clinical and utilization data. It will also be a useful research tool in tracking epidemiological trends for the military population. The MSSR has enhanced clinical management of MS and serves as a national source for clinical outcomes.
1. Flachenecker P. Multiple sclerosis databases: present and future. Eur Neurol. 2014;72(suppl 1):29-31.
2. Koch-Henriksen N, Magyari M, Laursen B. Registers of multiple sclerosis in Denmark. Acta Neurol Scand. 2015;132(199):4-10.
3. Alping P, Piehl F, Langer-Gould A, Frisell T; COMBAT-MS Study Group. Validation of the Swedish Multiple Sclerosis Register: further improving a resource for pharmacoepidemiologic evaluations. Epidemiology. 2019;30(2):230-233.
4. Benjaminsen E, Myhr KM, Grytten N, Alstadhaug KB. Validation of the multiple sclerosis diagnosis in the Norwegian Patient Registry. Brain Behav. 2019;9(11):e01422.
5. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202-1213.
6. Kingwell E, Zhu F, Marrie RA, et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991-2010). J Neurol. 2015;262(10):2352-2363.
7. Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914-1929.
8. Mahmud SM, Bozat-Emre S, Mostaço-Guidolin LC, Marrie RA. Registry cohort study to determine risk for multiple sclerosis after vaccination for pandemic influenza A(H1N1) with Arepanrix, Manitoba, Canada. Emerg Infect Dis. 2018;24(7):1267-1274.
9. Kister I, Chamot E, Bacon JH, Cutter G, Herbert J; New York State Multiple Sclerosis Consortium. Trend for decreasing Multiple Sclerosis Severity Scores (MSSS) with increasing calendar year of enrollment into the New York State Multiple Sclerosis Consortium. Mult Scler. 2011;17(6):725-733.
10. Minden SL, Frankel D, Hadden L, Perloffp J, Srinath KP, Hoaglin DC. The Sonya Slifka Longitudinal Multiple Sclerosis Study: methods and sample characteristics. Mult Scler. 2006;12(1):24-38.
11. Fox RJ, Salter A, Alster JM, et al. Risk tolerance to MS therapies: survey results from the NARCOMS registry. Mult Scler Relat Disord. 2015;4(3):241-249.
12. Kalincik T, Butzkueven H. The MSBase registry: Informing clinical practice. Mult Scler. 2019;25(14):1828-1834.
13. Gourraud PA, Henry RG, Cree BA, et al. Precision medicine in chronic disease management: the multiple sclerosis BioScreen. Ann Neurol. 2014;76(5):633-642.
14. Wallin MT, Culpepper WJ, Coffman P, et al. The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain. 2012;135(Pt 6):1778-1785.
15. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272.
16. Salter A, Stahmann A, Ellenberger D, et al. Data harmonization for collaborative research among MS registries: a case study in employment [published online ahead of print, 2020 Mar 12]. Mult Scler. 2020;1352458520910499.
17. Vaughn CB, Kavak KS, Dwyer MG, et al. Fatigue at enrollment predicts EDSS worsening in the New York State Multiple Sclerosis Consortium. Mult Scler. 2020;26(1):99-108.
18. Minden SL, Kinkel RP, Machado HT, et al. Use and cost of disease-modifying therapies by Sonya Slifka Study participants: has anything really changed since 2000 and 2009? Mult Scler J Exp Transl Clin. 2019;5(1):2055217318820888.
19. Rae-Grant A, Bennett A, Sanders AE, Phipps M, Cheng E, Bever C. Quality improvement in neurology: multiple sclerosis quality measures: Executive summary [published correction appears in Neurology. 2016;86(15):1465]. Neurology. 2015;85(21):1904-1908.
20. Flachenecker P, Buckow K, Pugliatti M, et al; EUReMS Consortium. Multiple sclerosis registries in Europe - results of a systematic survey. Mult Scler. 2014;20(11):1523-1532.
21. Traboulsee A, McMullen K. How useful are MS registries?. Mult Scler. 2014;20(11):1423-1424.
22. Bebo BF Jr, Fox RJ, Lee K, Utz U, Thompson AJ. Landscape of MS patient cohorts and registries: Recommendations for maximizing impact. Mult Scler. 2018;24(5):579-586.
1. Flachenecker P. Multiple sclerosis databases: present and future. Eur Neurol. 2014;72(suppl 1):29-31.
2. Koch-Henriksen N, Magyari M, Laursen B. Registers of multiple sclerosis in Denmark. Acta Neurol Scand. 2015;132(199):4-10.
3. Alping P, Piehl F, Langer-Gould A, Frisell T; COMBAT-MS Study Group. Validation of the Swedish Multiple Sclerosis Register: further improving a resource for pharmacoepidemiologic evaluations. Epidemiology. 2019;30(2):230-233.
4. Benjaminsen E, Myhr KM, Grytten N, Alstadhaug KB. Validation of the multiple sclerosis diagnosis in the Norwegian Patient Registry. Brain Behav. 2019;9(11):e01422.
5. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202-1213.
6. Kingwell E, Zhu F, Marrie RA, et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991-2010). J Neurol. 2015;262(10):2352-2363.
7. Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914-1929.
8. Mahmud SM, Bozat-Emre S, Mostaço-Guidolin LC, Marrie RA. Registry cohort study to determine risk for multiple sclerosis after vaccination for pandemic influenza A(H1N1) with Arepanrix, Manitoba, Canada. Emerg Infect Dis. 2018;24(7):1267-1274.
9. Kister I, Chamot E, Bacon JH, Cutter G, Herbert J; New York State Multiple Sclerosis Consortium. Trend for decreasing Multiple Sclerosis Severity Scores (MSSS) with increasing calendar year of enrollment into the New York State Multiple Sclerosis Consortium. Mult Scler. 2011;17(6):725-733.
10. Minden SL, Frankel D, Hadden L, Perloffp J, Srinath KP, Hoaglin DC. The Sonya Slifka Longitudinal Multiple Sclerosis Study: methods and sample characteristics. Mult Scler. 2006;12(1):24-38.
11. Fox RJ, Salter A, Alster JM, et al. Risk tolerance to MS therapies: survey results from the NARCOMS registry. Mult Scler Relat Disord. 2015;4(3):241-249.
12. Kalincik T, Butzkueven H. The MSBase registry: Informing clinical practice. Mult Scler. 2019;25(14):1828-1834.
13. Gourraud PA, Henry RG, Cree BA, et al. Precision medicine in chronic disease management: the multiple sclerosis BioScreen. Ann Neurol. 2014;76(5):633-642.
14. Wallin MT, Culpepper WJ, Coffman P, et al. The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain. 2012;135(Pt 6):1778-1785.
15. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272.
16. Salter A, Stahmann A, Ellenberger D, et al. Data harmonization for collaborative research among MS registries: a case study in employment [published online ahead of print, 2020 Mar 12]. Mult Scler. 2020;1352458520910499.
17. Vaughn CB, Kavak KS, Dwyer MG, et al. Fatigue at enrollment predicts EDSS worsening in the New York State Multiple Sclerosis Consortium. Mult Scler. 2020;26(1):99-108.
18. Minden SL, Kinkel RP, Machado HT, et al. Use and cost of disease-modifying therapies by Sonya Slifka Study participants: has anything really changed since 2000 and 2009? Mult Scler J Exp Transl Clin. 2019;5(1):2055217318820888.
19. Rae-Grant A, Bennett A, Sanders AE, Phipps M, Cheng E, Bever C. Quality improvement in neurology: multiple sclerosis quality measures: Executive summary [published correction appears in Neurology. 2016;86(15):1465]. Neurology. 2015;85(21):1904-1908.
20. Flachenecker P, Buckow K, Pugliatti M, et al; EUReMS Consortium. Multiple sclerosis registries in Europe - results of a systematic survey. Mult Scler. 2014;20(11):1523-1532.
21. Traboulsee A, McMullen K. How useful are MS registries?. Mult Scler. 2014;20(11):1423-1424.
22. Bebo BF Jr, Fox RJ, Lee K, Utz U, Thompson AJ. Landscape of MS patient cohorts and registries: Recommendations for maximizing impact. Mult Scler. 2018;24(5):579-586.
The Multiple Sclerosis Centers of Excellence: A Model of Excellence in the VA (FULL)
The Veterans Health Administration (VHA) has established a number of centers of excellence (CoEs), including centers focused on posttraumatic stress disorder, suicide prevention, epilepsy, and, most recently, the Senator Elizabeth Dole CoE for Veteran and Caregiver Research. Some VA CoE serve as centralized locations for specialty care. For example, the VA Epilepsy CoE is a network of 16 facilities that provide comprehensive epilepsy care for veterans with seizure disorders, including expert and presurgical evaluations and inpatient monitoring.
In contrast, other CoEs, including the multiple sclerosis (MS) CoE, achieve their missions by serving as a resource center to a network of regional and supporting various programs to optimize the care of veterans across the nation within their home US Department of Veterans Affairs (VA) medical center (VAMC). The MSCoE are charged, through VHA Directive 1011.06, with establishing at least 1 VA MS Regional Program in each of the 21 Veteran Integrated Service Networks (VISNs) across the country and integrating these and affiliated MS Support Programs into the MS National Network. Currently, there are 29 MS regional programs and 49 MS support programs across the US.1
Established in 2003, the MSCoE is dedicated to furthering the understanding of MS, its impact on veterans, and effective treatments to help manage the disease and its symptoms. In 2002, 2 coordinating centers were selected based on a competitive review process. The MSCoE-East is located at the Baltimore, Maryland and Washington, DC VAMC and serves VISNs 1 to 10. The MSCoE-West serves VISNs 11 to 23 and is jointly-based at VA Puget Sound Health Care System in Seattle, Washington and VA Portland Health Care System in Portland, Oregon. The MSCoEs were made permanent by The Veteran’ Benefits, Healthcare and Information Technology Act of 2006 (38 USC §7330). By partnering with veterans, caregivers, health care professionals, and other affiliates, the MSCoE endeavor to optimize health, activities, participation and quality of life for veterans with MS.
Core Functions
The MSCoE has a 3-part mission. First, the MSCoE seeks to expand care coordination between VAMCs by developing a national network of VA MSCoE Regional and Support Programs. Second, the MSCoE provides resources to VA health care providers (HCPs) through a collaborative approach to clinical care, education, research, and informatics. Third, the MSCoE improves the quality and consistency of health care services delivered to veterans diagnosed with MS nationwide. To meet its objectives, the MSCoE activities are organized around 4 functional cores: clinical care, research, education and training, and informatics and telemedicine.
Clinical Care
The MSCoE delivers high-quality clinical care by identifying veterans with MS who use VA services, understanding their needs, and facilitating appropriate interventions. Veterans with MS are a special cohort for many reasons including that about 70% are male. Men and women veterans not only have different genetics, but also may have different environmental exposures and other risk factors for MS. Since 1998, the VHA has evaluated > 50,000 veterans with MS. Over the past decade, between 18,000 and 20,000 veterans with MS have accessed care within the VHA annually.
The MSCoE advocates for appropriate and safe use of currently available MS disease modifying therapies through collaborations with the VA Pharmacy Benefits Management Service (PBM). The MSCoE partners with PBM to develop and disseminate Criteria For Use, safety, and economic monitoring of the impacts of the MS therapies. The MSCoE also provide national consultation services for complex MS cases, clinical education to VA HCPs, and mentors fellows, residents, and medical students.
The VA provides numerous resources that are not readily available in other health care systems and facilitate the care for patients with chronic diseases, including providing low or no co-pays to patients for MS disease modifying agents and other MS related medications, access to medically necessary adaptive equipment at no charge, the Home Improvement and Structural Alteration (HISA) grant for assistance with safe home ingress and egress, respite care, access to a homemaker/home health aide, and caregiver support programs. Eligible veterans also can access additional resources such as adaptive housing and an automobile grant. The VA also provides substantial hands-on assistance to veterans who are homeless. The clinical team and a veteran with MS can leverage VA resources through the National MS Society (NMSS) Navigator Program as well as other community resources.2
The VHA encourages physical activity and wellness through sports and leisure. Veterans with MS can participate in sports programs and special events, including the National Veterans Wheelchair Games, the National Disabled Veterans Winter Sports Clinic, the National Disabled Veterans TEE (Training, Exposure and Experience) golf tournament, the National Veterans Summer Sports Clinic, the National Veterans Golden Age Games, and the National Veterans Creative Sports Festival. HCPs or veterans who are not sure how to access any of these programs can contact the MSCoE or their local VA social workers.
Research
The primary goal of the MSCoE research core is to conduct clinical, health services, epidemiologic, and basic science research relevant to veterans with MS. The MSCoE serves to enhance collaboration among VAMCs, increase the participation of veterans in research, and provide research mentorship for the next generation of VA MS scientists. MSCoE research is carried out by investigators at the MSCoE and the MS Regional Programs, often in collaboration with investigators at academic institutions. This research is supported by competitive grant awards from a variety of funding agencies including the VA Research and Development Service (R&D) and the NMSS. Results from about 40 research grants in Fiscal Year 2019 were disseminated through 34 peer-reviewed publications, 30 posters, presentations, abstracts, and clinical practice guidelines.
There are many examples of recent high impact MS research performed by MSCoE investigators. For example, MSCoE researchers noted an increase in the estimated prevalence of MS to 1 million individuals in the US, about twice the previously estimated prevalence.3-5 In addition, a multicenter study highlighted the prevalence of MS misdiagnosis and common confounders for MS.6 Other research includes pilot clinical trials evaluating lipoic acid as a potential disease modifying therapy in people with secondary progressive MS and the impact of a multicomponent walking aid selection, fitting, and training program for preventing falls in people with MS.7,8 Clinical trial also are investigating telehealth counseling to improve physical activity in MS and a systematic review of rehabilitation interventions in MS.9,10
Education and Training
A unified program of education is essential to effective management of MS nationally. The primary goal of the education and training core is to provide a national program of MS education for HCPs, veterans, and caregivers to improve knowledge, enhance access to resources, and promote effective management strategies. The MSCoE collaborate with the Paralyzed Veterans of America (PVA), the Consortium of MS Centers (CMSC), the NMSS, and other national service organizations to increase educational opportunities, share knowledge, and expand participation.
The MSCoE education and training core produces a range of products both veterans, HCPs, and others affected by MS. The MSCoE sends a biannual patient newsletter to > 20,000 veterans and a monthly email to > 1,000 VA HCPs. Specific opportunities for HCP education include accredited multidisciplinary MS webinars, sponsored symposia and workshops at the CMSC and PVA Summit annual meetings, and presentations at other university and professional conferences. Enduring educational opportunities for veterans, caregivers, and HCPs can also be found by visiting www.va.gov/ms.
The MSCoE coordinate postdoctoral fellowship training programs to develop expertise in MS health care for the future. It offers VA physician fellowships for neurologists in Baltimore and Portland and for physiatrists in Seattle as well as NMSS fellowships for education and research. In 2019, MSCoE had 6 MD Fellows and 1 PhD Fellow.
Clinical Informatics and Telehealth
The primary goal of the informatics and telemedicine core is to employ state-of-the-art informatics, telemedicine technology, and the MSCoE website, to improve MS health care delivery. The VA has a integrated electronic health record and various data repositories are stored in the VHA Corporate Data Warehouse (CDW). MSCoE utilizes the CDW to maintain a national MS administrative data repository to understand the VHA care provided to veterans with MS. Data from the CDW have also served as an important resource to facilitate a wide range of veteran-focused MS research. This research has addressed clinical conditions like pain and obesity; health behaviors like smoking, alcohol use, and exercise as well as issues related to care delivery such as specialty care access, medication adherence, and appointment attendance.11-19
Monitoring the health of veterans with MS in the VA requires additional data not available in the CDW. To this end, we have developed the MS Surveillance Registry (MSSR), funded and maintained by the VA Office of Information Technology as part of their Veteran Integrated Registry Platform (VIRP). The purpose of the MSSR is to understand the unique characteristics and treatment patterns of veterans with MS in order to optimize their VHA care. HCPs input MS-specific clinical data on their patients into the MSSR, either through the MS Assessment Tool (MSAT) in the Computerized Patient Record System (CPRS) or through a secure online portal. Other data from existing databases from the CDW is also automatically fed into the MSSR. The MSSR continues to be developed and populated to serve as a resource for the future.
Neurologists, physiatrists, psychologists, and rehabilitation specialists can use telehealth to evaluate and treat veterans who have difficulty accessing outpatient clinics, either because of mobility limitations, or distance. Between 2012 and 2015, the VA MSCoE, together with the Epilepsy CoE and the Parkinson’s Disease Research and Clinical Centers in VISNs 5, 6 (mid-Atlantic) and 20 (Pacific Northwest) initiated an integrated teleneurology project. The goal of this project was to improve patient access to care at 4 tertiary and 12 regional VAMCs. A study team, with administrators and key clinical stakeholders, followed a traditional project management approach to design, plan, implement and evaluate an optimal model for communication and referrals with both live visits and telehealth (Table). Major outcomes of the project included: delivering subspecialty teleneurology to 47 patient sites, increasing interfacility consultation by 133% while reducing wait times by roughly 40%, and increasing telemedicine workload at these centers from 95 annual encounters in 2012 to 1,245 annual encounters in 2015 (Figure).
Today, telehealth for veterans with MS can be delivered to nearby VA facilities closer to their home, within their home, or anywhere else the veteran can use a cellphone or tablet. Telehealth visits can save travel time and expenses and optimize VA productivity and clinic use. The MSCoE and many of the MS regional programs are using telehealth for MS physician follow-up and therapies. The VA Office of Rural Health is also currently working with the MS network to use telehealth to increase access to physical therapy to those who have difficulty coming into clinic.
MSCoE Resources
The MSCoE is funded by VA Central Office through the Office of Specialty Care by Special Purpose funds. The directive specifies that funding for the regional and support programs is through Veterans Equitable Resource Allocation based on VISN and facility workload and complexity. Any research is funded separately through grants, some from VA R&D and others from other sources including the National Institutes of Health, the Patient Centered Outcome Research Institute, affiliated universities, the NMSS, the MS Society of Canada, the Consortium of MS Centers, foundations, and industry.
In 2019, MSCoE investigators received grants totaling > $18 million in funding. In-kind support also is provided by the PVA, the CMSC, the NMSS, and others. The first 3 foundations have been supporters since the inception of the MSCoE and have provided opportunities for the dissemination of education and research for HCPs, fellows, residents and medical students; travel; meeting rooms for MSCoE national meetings; exhibit space for HCP outreach; competitive research and educational grant support; programming and resources for veterans and significant others; organizational expertise; and opportunities for VA HCPs, veterans, and caregivers to learn how to navigate MS with others in the private sector.
Conclusion
The MSCoE had a tremendous impact on improving the consistency and quality of care for veterans with MS through clinical care, research, education and informatics and telehealth. Since opening in 2003, there has been an increase in the number of MS specialty clinics, served veterans with MS, and veterans receiving specialty neurologic and rehabilitation services in VA. Research programs in MS have been initiated to address key questions relevant to veterans with MS, including immunology, epidemiology, clinical care, and rehabilitation. Educational programs and products have evolved with technology and had a greater impact through partnerships with veteran and MS nonprofit organizations.
MSCoE strives to minimize impairment and maximize quality of life for veterans with MS by leveraging integrated electronic health records, data repositories, and telehealth services. These efforts have all improved veteran health, access and safety. We look forward to continuing into the next decade by bringing fresh ideas to the care of veterans with MS, their families and caregivers.
1. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Multiple Sclerosis System of Care-VHA Directive 1101.06 and Multiple Sclerosis Centers of Excellence network facilities. https://www.va.gov/MS/veterans/find_a_clinic/index_clinics.asp. Updated February 26, 2020. Accessed March 6, 2020.
2. National MS Society. MS navigator program. https://www.nationalmssociety.org/For-Professionals/Clinical-Care/MS-Navigator-Program. Accessed March 6, 2020.
3. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92:e1029-e1040.
4. GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269-285.
5. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480.
6. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87(13):1393-1399.
7. Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e374.
8. Martini DN, Zeeboer E, Hildebrand A, Fling BW, Hugos CL, Cameron MH. ADSTEP: preliminary investigation of a multicomponent walking aid program in people with multiple sclerosis. Arch Phys Med Rehabil. 2018;99(10):2050-2058.
9. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
10. Haselkorn JK, Hughes C, Rae-Grant A, et al. Summary of comprehensive systematic review: rehabilitation in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2015;85(21):1896-1903.
11. Hirsh AT, Turner AP, Ehde DM, Haselkorn JK. Prevalence and impact of pain in multiple sclerosis: physical and psychologic contributors. Arch Phys Med Rehabil. 2009;90(4):646-651.
12. Khurana SR, Bamer AM, Turner AP, et al. The prevalence of overweight and obesity in veterans with multiple sclerosis. Am J Phys Med Rehabil. 2009;88(2):83-91.
13. Turner AP, Kivlahan DR, Kazis LE, Haselkorn JK. Smoking among veterans with multiple sclerosis: prevalence correlates, quit attempts, and unmet need for services. Arch Phys Med Rehabil. 2007;88(11):1394-1399.
14. Turner AP, Hawkins EJ, Haselkorn JK, Kivlahan DR. Alcohol misuse and multiple sclerosis. Arch Phys Med Rehabil. 2009;90(5):842-848.
15. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
16. Turner AP, Chapko MK, Yanez D, et al. Access to multiple sclerosis specialty care. PM R. 2013;5(12):1044-1050.
17. Gromisch ES, Turner AP, Leipertz SL, Beauvais J, Haselkorn JK. Risk factors for suboptimal medication adherence in persons with multiple sclerosis: development of an electronic health record-based explanatory model for disease-modifying therapy use [published online ahead of print, 2019 Dec 3]. Arch Phys Med Rehabil. 2019;S0003-9993(19)31430-3143.
18. Settle JR, Maloni H, Bedra M, Finkelstein J, Zhan M, Wallin M. Monitoring medication adherence in multiple sclerosis using a novel web-based tool. J Telemed Telecare. 2016;22:225-233.
19. Gromisch ES, Turner AP, Leipertz SL, Beauvais J, Haselkorn JK. Who is not coming to clinic? A predictive model of excessive missed appointments in persons with multiple sclerosis. Mult Scler Rel Dis. In Press.
The Veterans Health Administration (VHA) has established a number of centers of excellence (CoEs), including centers focused on posttraumatic stress disorder, suicide prevention, epilepsy, and, most recently, the Senator Elizabeth Dole CoE for Veteran and Caregiver Research. Some VA CoE serve as centralized locations for specialty care. For example, the VA Epilepsy CoE is a network of 16 facilities that provide comprehensive epilepsy care for veterans with seizure disorders, including expert and presurgical evaluations and inpatient monitoring.
In contrast, other CoEs, including the multiple sclerosis (MS) CoE, achieve their missions by serving as a resource center to a network of regional and supporting various programs to optimize the care of veterans across the nation within their home US Department of Veterans Affairs (VA) medical center (VAMC). The MSCoE are charged, through VHA Directive 1011.06, with establishing at least 1 VA MS Regional Program in each of the 21 Veteran Integrated Service Networks (VISNs) across the country and integrating these and affiliated MS Support Programs into the MS National Network. Currently, there are 29 MS regional programs and 49 MS support programs across the US.1
Established in 2003, the MSCoE is dedicated to furthering the understanding of MS, its impact on veterans, and effective treatments to help manage the disease and its symptoms. In 2002, 2 coordinating centers were selected based on a competitive review process. The MSCoE-East is located at the Baltimore, Maryland and Washington, DC VAMC and serves VISNs 1 to 10. The MSCoE-West serves VISNs 11 to 23 and is jointly-based at VA Puget Sound Health Care System in Seattle, Washington and VA Portland Health Care System in Portland, Oregon. The MSCoEs were made permanent by The Veteran’ Benefits, Healthcare and Information Technology Act of 2006 (38 USC §7330). By partnering with veterans, caregivers, health care professionals, and other affiliates, the MSCoE endeavor to optimize health, activities, participation and quality of life for veterans with MS.
Core Functions
The MSCoE has a 3-part mission. First, the MSCoE seeks to expand care coordination between VAMCs by developing a national network of VA MSCoE Regional and Support Programs. Second, the MSCoE provides resources to VA health care providers (HCPs) through a collaborative approach to clinical care, education, research, and informatics. Third, the MSCoE improves the quality and consistency of health care services delivered to veterans diagnosed with MS nationwide. To meet its objectives, the MSCoE activities are organized around 4 functional cores: clinical care, research, education and training, and informatics and telemedicine.
Clinical Care
The MSCoE delivers high-quality clinical care by identifying veterans with MS who use VA services, understanding their needs, and facilitating appropriate interventions. Veterans with MS are a special cohort for many reasons including that about 70% are male. Men and women veterans not only have different genetics, but also may have different environmental exposures and other risk factors for MS. Since 1998, the VHA has evaluated > 50,000 veterans with MS. Over the past decade, between 18,000 and 20,000 veterans with MS have accessed care within the VHA annually.
The MSCoE advocates for appropriate and safe use of currently available MS disease modifying therapies through collaborations with the VA Pharmacy Benefits Management Service (PBM). The MSCoE partners with PBM to develop and disseminate Criteria For Use, safety, and economic monitoring of the impacts of the MS therapies. The MSCoE also provide national consultation services for complex MS cases, clinical education to VA HCPs, and mentors fellows, residents, and medical students.
The VA provides numerous resources that are not readily available in other health care systems and facilitate the care for patients with chronic diseases, including providing low or no co-pays to patients for MS disease modifying agents and other MS related medications, access to medically necessary adaptive equipment at no charge, the Home Improvement and Structural Alteration (HISA) grant for assistance with safe home ingress and egress, respite care, access to a homemaker/home health aide, and caregiver support programs. Eligible veterans also can access additional resources such as adaptive housing and an automobile grant. The VA also provides substantial hands-on assistance to veterans who are homeless. The clinical team and a veteran with MS can leverage VA resources through the National MS Society (NMSS) Navigator Program as well as other community resources.2
The VHA encourages physical activity and wellness through sports and leisure. Veterans with MS can participate in sports programs and special events, including the National Veterans Wheelchair Games, the National Disabled Veterans Winter Sports Clinic, the National Disabled Veterans TEE (Training, Exposure and Experience) golf tournament, the National Veterans Summer Sports Clinic, the National Veterans Golden Age Games, and the National Veterans Creative Sports Festival. HCPs or veterans who are not sure how to access any of these programs can contact the MSCoE or their local VA social workers.
Research
The primary goal of the MSCoE research core is to conduct clinical, health services, epidemiologic, and basic science research relevant to veterans with MS. The MSCoE serves to enhance collaboration among VAMCs, increase the participation of veterans in research, and provide research mentorship for the next generation of VA MS scientists. MSCoE research is carried out by investigators at the MSCoE and the MS Regional Programs, often in collaboration with investigators at academic institutions. This research is supported by competitive grant awards from a variety of funding agencies including the VA Research and Development Service (R&D) and the NMSS. Results from about 40 research grants in Fiscal Year 2019 were disseminated through 34 peer-reviewed publications, 30 posters, presentations, abstracts, and clinical practice guidelines.
There are many examples of recent high impact MS research performed by MSCoE investigators. For example, MSCoE researchers noted an increase in the estimated prevalence of MS to 1 million individuals in the US, about twice the previously estimated prevalence.3-5 In addition, a multicenter study highlighted the prevalence of MS misdiagnosis and common confounders for MS.6 Other research includes pilot clinical trials evaluating lipoic acid as a potential disease modifying therapy in people with secondary progressive MS and the impact of a multicomponent walking aid selection, fitting, and training program for preventing falls in people with MS.7,8 Clinical trial also are investigating telehealth counseling to improve physical activity in MS and a systematic review of rehabilitation interventions in MS.9,10
Education and Training
A unified program of education is essential to effective management of MS nationally. The primary goal of the education and training core is to provide a national program of MS education for HCPs, veterans, and caregivers to improve knowledge, enhance access to resources, and promote effective management strategies. The MSCoE collaborate with the Paralyzed Veterans of America (PVA), the Consortium of MS Centers (CMSC), the NMSS, and other national service organizations to increase educational opportunities, share knowledge, and expand participation.
The MSCoE education and training core produces a range of products both veterans, HCPs, and others affected by MS. The MSCoE sends a biannual patient newsletter to > 20,000 veterans and a monthly email to > 1,000 VA HCPs. Specific opportunities for HCP education include accredited multidisciplinary MS webinars, sponsored symposia and workshops at the CMSC and PVA Summit annual meetings, and presentations at other university and professional conferences. Enduring educational opportunities for veterans, caregivers, and HCPs can also be found by visiting www.va.gov/ms.
The MSCoE coordinate postdoctoral fellowship training programs to develop expertise in MS health care for the future. It offers VA physician fellowships for neurologists in Baltimore and Portland and for physiatrists in Seattle as well as NMSS fellowships for education and research. In 2019, MSCoE had 6 MD Fellows and 1 PhD Fellow.
Clinical Informatics and Telehealth
The primary goal of the informatics and telemedicine core is to employ state-of-the-art informatics, telemedicine technology, and the MSCoE website, to improve MS health care delivery. The VA has a integrated electronic health record and various data repositories are stored in the VHA Corporate Data Warehouse (CDW). MSCoE utilizes the CDW to maintain a national MS administrative data repository to understand the VHA care provided to veterans with MS. Data from the CDW have also served as an important resource to facilitate a wide range of veteran-focused MS research. This research has addressed clinical conditions like pain and obesity; health behaviors like smoking, alcohol use, and exercise as well as issues related to care delivery such as specialty care access, medication adherence, and appointment attendance.11-19
Monitoring the health of veterans with MS in the VA requires additional data not available in the CDW. To this end, we have developed the MS Surveillance Registry (MSSR), funded and maintained by the VA Office of Information Technology as part of their Veteran Integrated Registry Platform (VIRP). The purpose of the MSSR is to understand the unique characteristics and treatment patterns of veterans with MS in order to optimize their VHA care. HCPs input MS-specific clinical data on their patients into the MSSR, either through the MS Assessment Tool (MSAT) in the Computerized Patient Record System (CPRS) or through a secure online portal. Other data from existing databases from the CDW is also automatically fed into the MSSR. The MSSR continues to be developed and populated to serve as a resource for the future.
Neurologists, physiatrists, psychologists, and rehabilitation specialists can use telehealth to evaluate and treat veterans who have difficulty accessing outpatient clinics, either because of mobility limitations, or distance. Between 2012 and 2015, the VA MSCoE, together with the Epilepsy CoE and the Parkinson’s Disease Research and Clinical Centers in VISNs 5, 6 (mid-Atlantic) and 20 (Pacific Northwest) initiated an integrated teleneurology project. The goal of this project was to improve patient access to care at 4 tertiary and 12 regional VAMCs. A study team, with administrators and key clinical stakeholders, followed a traditional project management approach to design, plan, implement and evaluate an optimal model for communication and referrals with both live visits and telehealth (Table). Major outcomes of the project included: delivering subspecialty teleneurology to 47 patient sites, increasing interfacility consultation by 133% while reducing wait times by roughly 40%, and increasing telemedicine workload at these centers from 95 annual encounters in 2012 to 1,245 annual encounters in 2015 (Figure).
Today, telehealth for veterans with MS can be delivered to nearby VA facilities closer to their home, within their home, or anywhere else the veteran can use a cellphone or tablet. Telehealth visits can save travel time and expenses and optimize VA productivity and clinic use. The MSCoE and many of the MS regional programs are using telehealth for MS physician follow-up and therapies. The VA Office of Rural Health is also currently working with the MS network to use telehealth to increase access to physical therapy to those who have difficulty coming into clinic.
MSCoE Resources
The MSCoE is funded by VA Central Office through the Office of Specialty Care by Special Purpose funds. The directive specifies that funding for the regional and support programs is through Veterans Equitable Resource Allocation based on VISN and facility workload and complexity. Any research is funded separately through grants, some from VA R&D and others from other sources including the National Institutes of Health, the Patient Centered Outcome Research Institute, affiliated universities, the NMSS, the MS Society of Canada, the Consortium of MS Centers, foundations, and industry.
In 2019, MSCoE investigators received grants totaling > $18 million in funding. In-kind support also is provided by the PVA, the CMSC, the NMSS, and others. The first 3 foundations have been supporters since the inception of the MSCoE and have provided opportunities for the dissemination of education and research for HCPs, fellows, residents and medical students; travel; meeting rooms for MSCoE national meetings; exhibit space for HCP outreach; competitive research and educational grant support; programming and resources for veterans and significant others; organizational expertise; and opportunities for VA HCPs, veterans, and caregivers to learn how to navigate MS with others in the private sector.
Conclusion
The MSCoE had a tremendous impact on improving the consistency and quality of care for veterans with MS through clinical care, research, education and informatics and telehealth. Since opening in 2003, there has been an increase in the number of MS specialty clinics, served veterans with MS, and veterans receiving specialty neurologic and rehabilitation services in VA. Research programs in MS have been initiated to address key questions relevant to veterans with MS, including immunology, epidemiology, clinical care, and rehabilitation. Educational programs and products have evolved with technology and had a greater impact through partnerships with veteran and MS nonprofit organizations.
MSCoE strives to minimize impairment and maximize quality of life for veterans with MS by leveraging integrated electronic health records, data repositories, and telehealth services. These efforts have all improved veteran health, access and safety. We look forward to continuing into the next decade by bringing fresh ideas to the care of veterans with MS, their families and caregivers.
The Veterans Health Administration (VHA) has established a number of centers of excellence (CoEs), including centers focused on posttraumatic stress disorder, suicide prevention, epilepsy, and, most recently, the Senator Elizabeth Dole CoE for Veteran and Caregiver Research. Some VA CoE serve as centralized locations for specialty care. For example, the VA Epilepsy CoE is a network of 16 facilities that provide comprehensive epilepsy care for veterans with seizure disorders, including expert and presurgical evaluations and inpatient monitoring.
In contrast, other CoEs, including the multiple sclerosis (MS) CoE, achieve their missions by serving as a resource center to a network of regional and supporting various programs to optimize the care of veterans across the nation within their home US Department of Veterans Affairs (VA) medical center (VAMC). The MSCoE are charged, through VHA Directive 1011.06, with establishing at least 1 VA MS Regional Program in each of the 21 Veteran Integrated Service Networks (VISNs) across the country and integrating these and affiliated MS Support Programs into the MS National Network. Currently, there are 29 MS regional programs and 49 MS support programs across the US.1
Established in 2003, the MSCoE is dedicated to furthering the understanding of MS, its impact on veterans, and effective treatments to help manage the disease and its symptoms. In 2002, 2 coordinating centers were selected based on a competitive review process. The MSCoE-East is located at the Baltimore, Maryland and Washington, DC VAMC and serves VISNs 1 to 10. The MSCoE-West serves VISNs 11 to 23 and is jointly-based at VA Puget Sound Health Care System in Seattle, Washington and VA Portland Health Care System in Portland, Oregon. The MSCoEs were made permanent by The Veteran’ Benefits, Healthcare and Information Technology Act of 2006 (38 USC §7330). By partnering with veterans, caregivers, health care professionals, and other affiliates, the MSCoE endeavor to optimize health, activities, participation and quality of life for veterans with MS.
Core Functions
The MSCoE has a 3-part mission. First, the MSCoE seeks to expand care coordination between VAMCs by developing a national network of VA MSCoE Regional and Support Programs. Second, the MSCoE provides resources to VA health care providers (HCPs) through a collaborative approach to clinical care, education, research, and informatics. Third, the MSCoE improves the quality and consistency of health care services delivered to veterans diagnosed with MS nationwide. To meet its objectives, the MSCoE activities are organized around 4 functional cores: clinical care, research, education and training, and informatics and telemedicine.
Clinical Care
The MSCoE delivers high-quality clinical care by identifying veterans with MS who use VA services, understanding their needs, and facilitating appropriate interventions. Veterans with MS are a special cohort for many reasons including that about 70% are male. Men and women veterans not only have different genetics, but also may have different environmental exposures and other risk factors for MS. Since 1998, the VHA has evaluated > 50,000 veterans with MS. Over the past decade, between 18,000 and 20,000 veterans with MS have accessed care within the VHA annually.
The MSCoE advocates for appropriate and safe use of currently available MS disease modifying therapies through collaborations with the VA Pharmacy Benefits Management Service (PBM). The MSCoE partners with PBM to develop and disseminate Criteria For Use, safety, and economic monitoring of the impacts of the MS therapies. The MSCoE also provide national consultation services for complex MS cases, clinical education to VA HCPs, and mentors fellows, residents, and medical students.
The VA provides numerous resources that are not readily available in other health care systems and facilitate the care for patients with chronic diseases, including providing low or no co-pays to patients for MS disease modifying agents and other MS related medications, access to medically necessary adaptive equipment at no charge, the Home Improvement and Structural Alteration (HISA) grant for assistance with safe home ingress and egress, respite care, access to a homemaker/home health aide, and caregiver support programs. Eligible veterans also can access additional resources such as adaptive housing and an automobile grant. The VA also provides substantial hands-on assistance to veterans who are homeless. The clinical team and a veteran with MS can leverage VA resources through the National MS Society (NMSS) Navigator Program as well as other community resources.2
The VHA encourages physical activity and wellness through sports and leisure. Veterans with MS can participate in sports programs and special events, including the National Veterans Wheelchair Games, the National Disabled Veterans Winter Sports Clinic, the National Disabled Veterans TEE (Training, Exposure and Experience) golf tournament, the National Veterans Summer Sports Clinic, the National Veterans Golden Age Games, and the National Veterans Creative Sports Festival. HCPs or veterans who are not sure how to access any of these programs can contact the MSCoE or their local VA social workers.
Research
The primary goal of the MSCoE research core is to conduct clinical, health services, epidemiologic, and basic science research relevant to veterans with MS. The MSCoE serves to enhance collaboration among VAMCs, increase the participation of veterans in research, and provide research mentorship for the next generation of VA MS scientists. MSCoE research is carried out by investigators at the MSCoE and the MS Regional Programs, often in collaboration with investigators at academic institutions. This research is supported by competitive grant awards from a variety of funding agencies including the VA Research and Development Service (R&D) and the NMSS. Results from about 40 research grants in Fiscal Year 2019 were disseminated through 34 peer-reviewed publications, 30 posters, presentations, abstracts, and clinical practice guidelines.
There are many examples of recent high impact MS research performed by MSCoE investigators. For example, MSCoE researchers noted an increase in the estimated prevalence of MS to 1 million individuals in the US, about twice the previously estimated prevalence.3-5 In addition, a multicenter study highlighted the prevalence of MS misdiagnosis and common confounders for MS.6 Other research includes pilot clinical trials evaluating lipoic acid as a potential disease modifying therapy in people with secondary progressive MS and the impact of a multicomponent walking aid selection, fitting, and training program for preventing falls in people with MS.7,8 Clinical trial also are investigating telehealth counseling to improve physical activity in MS and a systematic review of rehabilitation interventions in MS.9,10
Education and Training
A unified program of education is essential to effective management of MS nationally. The primary goal of the education and training core is to provide a national program of MS education for HCPs, veterans, and caregivers to improve knowledge, enhance access to resources, and promote effective management strategies. The MSCoE collaborate with the Paralyzed Veterans of America (PVA), the Consortium of MS Centers (CMSC), the NMSS, and other national service organizations to increase educational opportunities, share knowledge, and expand participation.
The MSCoE education and training core produces a range of products both veterans, HCPs, and others affected by MS. The MSCoE sends a biannual patient newsletter to > 20,000 veterans and a monthly email to > 1,000 VA HCPs. Specific opportunities for HCP education include accredited multidisciplinary MS webinars, sponsored symposia and workshops at the CMSC and PVA Summit annual meetings, and presentations at other university and professional conferences. Enduring educational opportunities for veterans, caregivers, and HCPs can also be found by visiting www.va.gov/ms.
The MSCoE coordinate postdoctoral fellowship training programs to develop expertise in MS health care for the future. It offers VA physician fellowships for neurologists in Baltimore and Portland and for physiatrists in Seattle as well as NMSS fellowships for education and research. In 2019, MSCoE had 6 MD Fellows and 1 PhD Fellow.
Clinical Informatics and Telehealth
The primary goal of the informatics and telemedicine core is to employ state-of-the-art informatics, telemedicine technology, and the MSCoE website, to improve MS health care delivery. The VA has a integrated electronic health record and various data repositories are stored in the VHA Corporate Data Warehouse (CDW). MSCoE utilizes the CDW to maintain a national MS administrative data repository to understand the VHA care provided to veterans with MS. Data from the CDW have also served as an important resource to facilitate a wide range of veteran-focused MS research. This research has addressed clinical conditions like pain and obesity; health behaviors like smoking, alcohol use, and exercise as well as issues related to care delivery such as specialty care access, medication adherence, and appointment attendance.11-19
Monitoring the health of veterans with MS in the VA requires additional data not available in the CDW. To this end, we have developed the MS Surveillance Registry (MSSR), funded and maintained by the VA Office of Information Technology as part of their Veteran Integrated Registry Platform (VIRP). The purpose of the MSSR is to understand the unique characteristics and treatment patterns of veterans with MS in order to optimize their VHA care. HCPs input MS-specific clinical data on their patients into the MSSR, either through the MS Assessment Tool (MSAT) in the Computerized Patient Record System (CPRS) or through a secure online portal. Other data from existing databases from the CDW is also automatically fed into the MSSR. The MSSR continues to be developed and populated to serve as a resource for the future.
Neurologists, physiatrists, psychologists, and rehabilitation specialists can use telehealth to evaluate and treat veterans who have difficulty accessing outpatient clinics, either because of mobility limitations, or distance. Between 2012 and 2015, the VA MSCoE, together with the Epilepsy CoE and the Parkinson’s Disease Research and Clinical Centers in VISNs 5, 6 (mid-Atlantic) and 20 (Pacific Northwest) initiated an integrated teleneurology project. The goal of this project was to improve patient access to care at 4 tertiary and 12 regional VAMCs. A study team, with administrators and key clinical stakeholders, followed a traditional project management approach to design, plan, implement and evaluate an optimal model for communication and referrals with both live visits and telehealth (Table). Major outcomes of the project included: delivering subspecialty teleneurology to 47 patient sites, increasing interfacility consultation by 133% while reducing wait times by roughly 40%, and increasing telemedicine workload at these centers from 95 annual encounters in 2012 to 1,245 annual encounters in 2015 (Figure).
Today, telehealth for veterans with MS can be delivered to nearby VA facilities closer to their home, within their home, or anywhere else the veteran can use a cellphone or tablet. Telehealth visits can save travel time and expenses and optimize VA productivity and clinic use. The MSCoE and many of the MS regional programs are using telehealth for MS physician follow-up and therapies. The VA Office of Rural Health is also currently working with the MS network to use telehealth to increase access to physical therapy to those who have difficulty coming into clinic.
MSCoE Resources
The MSCoE is funded by VA Central Office through the Office of Specialty Care by Special Purpose funds. The directive specifies that funding for the regional and support programs is through Veterans Equitable Resource Allocation based on VISN and facility workload and complexity. Any research is funded separately through grants, some from VA R&D and others from other sources including the National Institutes of Health, the Patient Centered Outcome Research Institute, affiliated universities, the NMSS, the MS Society of Canada, the Consortium of MS Centers, foundations, and industry.
In 2019, MSCoE investigators received grants totaling > $18 million in funding. In-kind support also is provided by the PVA, the CMSC, the NMSS, and others. The first 3 foundations have been supporters since the inception of the MSCoE and have provided opportunities for the dissemination of education and research for HCPs, fellows, residents and medical students; travel; meeting rooms for MSCoE national meetings; exhibit space for HCP outreach; competitive research and educational grant support; programming and resources for veterans and significant others; organizational expertise; and opportunities for VA HCPs, veterans, and caregivers to learn how to navigate MS with others in the private sector.
Conclusion
The MSCoE had a tremendous impact on improving the consistency and quality of care for veterans with MS through clinical care, research, education and informatics and telehealth. Since opening in 2003, there has been an increase in the number of MS specialty clinics, served veterans with MS, and veterans receiving specialty neurologic and rehabilitation services in VA. Research programs in MS have been initiated to address key questions relevant to veterans with MS, including immunology, epidemiology, clinical care, and rehabilitation. Educational programs and products have evolved with technology and had a greater impact through partnerships with veteran and MS nonprofit organizations.
MSCoE strives to minimize impairment and maximize quality of life for veterans with MS by leveraging integrated electronic health records, data repositories, and telehealth services. These efforts have all improved veteran health, access and safety. We look forward to continuing into the next decade by bringing fresh ideas to the care of veterans with MS, their families and caregivers.
1. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Multiple Sclerosis System of Care-VHA Directive 1101.06 and Multiple Sclerosis Centers of Excellence network facilities. https://www.va.gov/MS/veterans/find_a_clinic/index_clinics.asp. Updated February 26, 2020. Accessed March 6, 2020.
2. National MS Society. MS navigator program. https://www.nationalmssociety.org/For-Professionals/Clinical-Care/MS-Navigator-Program. Accessed March 6, 2020.
3. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92:e1029-e1040.
4. GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269-285.
5. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480.
6. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87(13):1393-1399.
7. Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e374.
8. Martini DN, Zeeboer E, Hildebrand A, Fling BW, Hugos CL, Cameron MH. ADSTEP: preliminary investigation of a multicomponent walking aid program in people with multiple sclerosis. Arch Phys Med Rehabil. 2018;99(10):2050-2058.
9. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
10. Haselkorn JK, Hughes C, Rae-Grant A, et al. Summary of comprehensive systematic review: rehabilitation in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2015;85(21):1896-1903.
11. Hirsh AT, Turner AP, Ehde DM, Haselkorn JK. Prevalence and impact of pain in multiple sclerosis: physical and psychologic contributors. Arch Phys Med Rehabil. 2009;90(4):646-651.
12. Khurana SR, Bamer AM, Turner AP, et al. The prevalence of overweight and obesity in veterans with multiple sclerosis. Am J Phys Med Rehabil. 2009;88(2):83-91.
13. Turner AP, Kivlahan DR, Kazis LE, Haselkorn JK. Smoking among veterans with multiple sclerosis: prevalence correlates, quit attempts, and unmet need for services. Arch Phys Med Rehabil. 2007;88(11):1394-1399.
14. Turner AP, Hawkins EJ, Haselkorn JK, Kivlahan DR. Alcohol misuse and multiple sclerosis. Arch Phys Med Rehabil. 2009;90(5):842-848.
15. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
16. Turner AP, Chapko MK, Yanez D, et al. Access to multiple sclerosis specialty care. PM R. 2013;5(12):1044-1050.
17. Gromisch ES, Turner AP, Leipertz SL, Beauvais J, Haselkorn JK. Risk factors for suboptimal medication adherence in persons with multiple sclerosis: development of an electronic health record-based explanatory model for disease-modifying therapy use [published online ahead of print, 2019 Dec 3]. Arch Phys Med Rehabil. 2019;S0003-9993(19)31430-3143.
18. Settle JR, Maloni H, Bedra M, Finkelstein J, Zhan M, Wallin M. Monitoring medication adherence in multiple sclerosis using a novel web-based tool. J Telemed Telecare. 2016;22:225-233.
19. Gromisch ES, Turner AP, Leipertz SL, Beauvais J, Haselkorn JK. Who is not coming to clinic? A predictive model of excessive missed appointments in persons with multiple sclerosis. Mult Scler Rel Dis. In Press.
1. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Multiple Sclerosis System of Care-VHA Directive 1101.06 and Multiple Sclerosis Centers of Excellence network facilities. https://www.va.gov/MS/veterans/find_a_clinic/index_clinics.asp. Updated February 26, 2020. Accessed March 6, 2020.
2. National MS Society. MS navigator program. https://www.nationalmssociety.org/For-Professionals/Clinical-Care/MS-Navigator-Program. Accessed March 6, 2020.
3. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92:e1029-e1040.
4. GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269-285.
5. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480.
6. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87(13):1393-1399.
7. Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e374.
8. Martini DN, Zeeboer E, Hildebrand A, Fling BW, Hugos CL, Cameron MH. ADSTEP: preliminary investigation of a multicomponent walking aid program in people with multiple sclerosis. Arch Phys Med Rehabil. 2018;99(10):2050-2058.
9. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
10. Haselkorn JK, Hughes C, Rae-Grant A, et al. Summary of comprehensive systematic review: rehabilitation in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2015;85(21):1896-1903.
11. Hirsh AT, Turner AP, Ehde DM, Haselkorn JK. Prevalence and impact of pain in multiple sclerosis: physical and psychologic contributors. Arch Phys Med Rehabil. 2009;90(4):646-651.
12. Khurana SR, Bamer AM, Turner AP, et al. The prevalence of overweight and obesity in veterans with multiple sclerosis. Am J Phys Med Rehabil. 2009;88(2):83-91.
13. Turner AP, Kivlahan DR, Kazis LE, Haselkorn JK. Smoking among veterans with multiple sclerosis: prevalence correlates, quit attempts, and unmet need for services. Arch Phys Med Rehabil. 2007;88(11):1394-1399.
14. Turner AP, Hawkins EJ, Haselkorn JK, Kivlahan DR. Alcohol misuse and multiple sclerosis. Arch Phys Med Rehabil. 2009;90(5):842-848.
15. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
16. Turner AP, Chapko MK, Yanez D, et al. Access to multiple sclerosis specialty care. PM R. 2013;5(12):1044-1050.
17. Gromisch ES, Turner AP, Leipertz SL, Beauvais J, Haselkorn JK. Risk factors for suboptimal medication adherence in persons with multiple sclerosis: development of an electronic health record-based explanatory model for disease-modifying therapy use [published online ahead of print, 2019 Dec 3]. Arch Phys Med Rehabil. 2019;S0003-9993(19)31430-3143.
18. Settle JR, Maloni H, Bedra M, Finkelstein J, Zhan M, Wallin M. Monitoring medication adherence in multiple sclerosis using a novel web-based tool. J Telemed Telecare. 2016;22:225-233.
19. Gromisch ES, Turner AP, Leipertz SL, Beauvais J, Haselkorn JK. Who is not coming to clinic? A predictive model of excessive missed appointments in persons with multiple sclerosis. Mult Scler Rel Dis. In Press.
Encephalopathy common, often lethal in hospitalized patients with COVID-19
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.
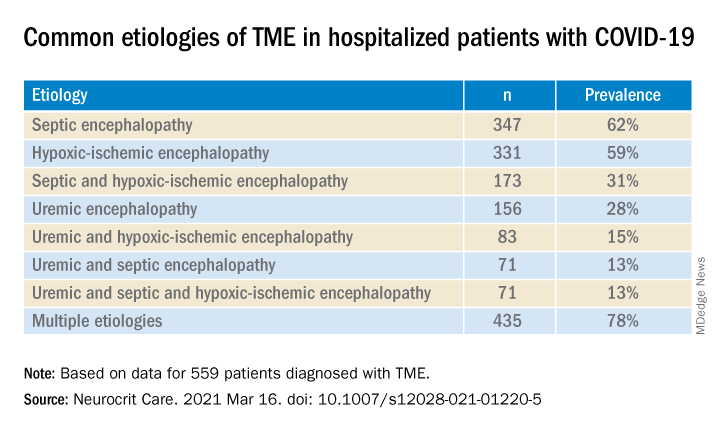
Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROCRITICAL CARE
Time is of the essence: DST up for debate again
Seasonal time change is now up for consideration in the U.S. Congress, prompting sleep medicine specialists to weigh in on the health impact of a major policy change.
As lawmakers in Washington propose an end to seasonal time changes by permanently establishing daylight saving time (DST), the American Academy of Sleep Medicine (AASM) is pushing for a Congressional hearing so scientists can present evidence in favor of converse legislation – to make standard time the new norm.
According to the AASM, ; however, the switch from standard time to DST incurs more risk.
“Current evidence best supports the adoption of year-round standard time, which aligns best with human circadian biology and provides distinct benefits for public health and safety,” the AASM noted in a 2020 position statement on DST.
The statement cites a number of studies that have reported associations between the switch to DST and acute, negative health outcomes, including higher rates of hospital admission, cardiovascular morbidity, atrial fibrillation, and stroke. The time shift has been associated with a spectrum of cellular, metabolic, and circadian derangements, from increased production of inflammatory markers, to higher blood pressure, and loss of sleep. These biological effects may have far-reaching consequences, including increased rates of fatal motor accidents in the days following the time change, and even increased volatility in the stock market, which may stem from cognitive deficits.
U.S. Senator Marco Rubio (R-Fla.) and others in the U.S. Congress have reintroduced the 2019 Sunshine Protection Act, legislation that would make DST permanent across the country. According to a statement on Sen. Rubio’s website, “The bill reflects the Florida legislature’s 2018 enactment of year-round DST; however, for Florida’s change to apply, a change in the federal statute is required. Fifteen other states – Arkansas, Alabama, California, Delaware, Georgia, Idaho, Louisiana, Maine, Ohio, Oregon, South Carolina, Tennessee, Utah, Washington, and Wyoming – have passed similar laws, resolutions, or voter initiatives, and dozens more are looking. The legislation, if enacted, would apply to those states [that] currently participate in DST, which most states observe for eight months out of the year.”
A stitch in time
“The sudden change in clock time disrupts sleep/wake patterns, decreasing total sleep time and sleep quality, leading to decrements in daytime cognition,” said Kannan Ramar, MBBS, MD, president of the AASM and a sleep medicine specialist at Mayo Clinic, Rochester, Minn.
Emphasizing this point, Dr. Ramar noted a recent study that reported an 18% increase in “patient safety-related incidents associated with human error” among health care workers within a week of the spring time change.
“Irregular bedtimes and wake times disrupt the timing of our circadian rhythms, which can lead to symptoms of insomnia or long-term, excessive daytime sleepiness. Lack of sleep can lead to numerous adverse effects on our minds, including decreased cognitive function, trouble concentrating, and general moodiness,” Dr. Ramar said.
He noted that these impacts may be more significant among certain individuals.
“The daylight saving time changes can be especially problematic for any populations that already experience chronic insufficient sleep or other sleep difficulties,” Dr. Ramar said. “Populations at greatest risk include teenagers, who tend to experience chronic sleep restriction during the school week, and night shift workers, who often struggle to sleep well during daytime hours.”
While fewer studies have evaluated the long-term effects of seasonal time changes, the AASM position statement cited evidence that “the body clock does not adjust to daylight saving time after several months,” possibly because “daylight saving time is less well-aligned with intrinsic human circadian physiology, and it disrupts the natural seasonal adjustment of the human clock due to the effect of late-evening light on the circadian rhythm.”
According to the AASM, permanent DST, as proposed by Sen. Rubio and colleagues, could “result in permanent phase delay, a condition that can also lead to a perpetual discrepancy between the innate biological clock and the extrinsic environmental clock, as well as chronic sleep loss due to early morning social demands that truncate the opportunity to sleep.” This mismatch between sleep/wake cycles and social demands, known as “social jet lag,” has been associated with chronic health risks, including metabolic syndrome, obesity, depression, and cardiovascular disease.
Cardiac impacts of seasonal time change
Muhammad Adeel Rishi, MD, a sleep specialist at Mayo Clinic, Eau Claire, Wis., and lead author of the AASM position statement, highlighted cardiovascular risks in a written statement for this article, noting increased rates of heart attack following the spring time change, and a higher risk of atrial fibrillation.
“Mayo Clinic has not taken a position on this issue,” Dr. Rishi noted. Still, he advocated for permanent standard time as the author of the AASM position statement and vice chair of the AASM public safety committee.
Jay Chudow, MD, and Andrew K. Krumerman, MD, of Montefiore Medical Center, New York, lead author and principal author, respectively, of a recent study that reported increased rates of atrial fibrillation admissions after DST transitions, had the same stance.
“We support elimination of seasonal time changes from a health perspective,” they wrote in a joint comment. “There is mounting evidence of a negative health impact with these seasonal time changes related to effects on sleep and circadian rhythm. Our work found the spring change was associated with more admissions for atrial fibrillation. This added to prior evidence of increased cardiovascular events related to these time changes. If physicians counsel patients on reducing risk factors for disease, shouldn’t we do the same as a society?”
Pros and cons
Not all sleep experts are convinced. Mary Jo Farmer, MD, PhD, FCCP, a sleep specialist and director of pulmonary hypertension services at Baystate Medical Center, and assistant professor of medicine at the University of Massachusetts, Springfield, considers perspectives from both sides of the issue.
“Daylight saving time promotes active lifestyles as people engage in more outdoor activities after work and school, [and] daylight saving time produces economic and safety benefits to society as retail revenues are higher and crimes are lower,” Dr. Farmer said. “Alternatively, moving the clocks forward is a cost burden to the U.S. economy when health issues, decreased productivity, and workplace injuries are considered.”
If one time system is permanently established, Dr. Farmer anticipates divided opinions from patients with sleep issues, regardless of which system is chosen.
“I can tell you, I have a cohort of sleep patients who prefer more evening light and look forward to the spring time change to daylight saving time,” she said. “However, they would not want the sun coming up at 9:00 a.m. in the winter months if we stayed on daylight saving time year-round. Similarly, patients would not want the sun coming up at 4:00 a.m. on the longest day of the year if we stayed on standard time all year round.”
Dr. Farmer called for more research before a decision is made.
“I suggest we need more information about the dangers of staying on daylight saving or standard time year-round because perhaps the current strategy of keeping morning light consistent is not so bad,” she said.
Time for a Congressional hearing?
According to Dr. Ramar, the time is now for a Congressional hearing, as lawmakers and the public need to be adequately informed when considering new legislation.
“There are public misconceptions about daylight saving time and standard time,” Dr. Ramar said. “People often like the idea of daylight saving time because they think it provides more light, and they dislike the concept of standard time because they think it provides more darkness. The reality is that neither time system provides more light or darkness than the other; it is only the timing that changes.”
Until new legislation is introduced, Dr. Ramar offered some practical advice for navigating seasonal time shifts.
“Beginning 2-3 days before the time change, it can be helpful to gradually adjust sleep and wake times, as well as other daily routines such as meal times,” he said. “After the time change, going outside for some morning light can help adjust the timing of your internal body clock.”
The investigators reported no conflicts of interest.
Seasonal time change is now up for consideration in the U.S. Congress, prompting sleep medicine specialists to weigh in on the health impact of a major policy change.
As lawmakers in Washington propose an end to seasonal time changes by permanently establishing daylight saving time (DST), the American Academy of Sleep Medicine (AASM) is pushing for a Congressional hearing so scientists can present evidence in favor of converse legislation – to make standard time the new norm.
According to the AASM, ; however, the switch from standard time to DST incurs more risk.
“Current evidence best supports the adoption of year-round standard time, which aligns best with human circadian biology and provides distinct benefits for public health and safety,” the AASM noted in a 2020 position statement on DST.
The statement cites a number of studies that have reported associations between the switch to DST and acute, negative health outcomes, including higher rates of hospital admission, cardiovascular morbidity, atrial fibrillation, and stroke. The time shift has been associated with a spectrum of cellular, metabolic, and circadian derangements, from increased production of inflammatory markers, to higher blood pressure, and loss of sleep. These biological effects may have far-reaching consequences, including increased rates of fatal motor accidents in the days following the time change, and even increased volatility in the stock market, which may stem from cognitive deficits.
U.S. Senator Marco Rubio (R-Fla.) and others in the U.S. Congress have reintroduced the 2019 Sunshine Protection Act, legislation that would make DST permanent across the country. According to a statement on Sen. Rubio’s website, “The bill reflects the Florida legislature’s 2018 enactment of year-round DST; however, for Florida’s change to apply, a change in the federal statute is required. Fifteen other states – Arkansas, Alabama, California, Delaware, Georgia, Idaho, Louisiana, Maine, Ohio, Oregon, South Carolina, Tennessee, Utah, Washington, and Wyoming – have passed similar laws, resolutions, or voter initiatives, and dozens more are looking. The legislation, if enacted, would apply to those states [that] currently participate in DST, which most states observe for eight months out of the year.”
A stitch in time
“The sudden change in clock time disrupts sleep/wake patterns, decreasing total sleep time and sleep quality, leading to decrements in daytime cognition,” said Kannan Ramar, MBBS, MD, president of the AASM and a sleep medicine specialist at Mayo Clinic, Rochester, Minn.
Emphasizing this point, Dr. Ramar noted a recent study that reported an 18% increase in “patient safety-related incidents associated with human error” among health care workers within a week of the spring time change.
“Irregular bedtimes and wake times disrupt the timing of our circadian rhythms, which can lead to symptoms of insomnia or long-term, excessive daytime sleepiness. Lack of sleep can lead to numerous adverse effects on our minds, including decreased cognitive function, trouble concentrating, and general moodiness,” Dr. Ramar said.
He noted that these impacts may be more significant among certain individuals.
“The daylight saving time changes can be especially problematic for any populations that already experience chronic insufficient sleep or other sleep difficulties,” Dr. Ramar said. “Populations at greatest risk include teenagers, who tend to experience chronic sleep restriction during the school week, and night shift workers, who often struggle to sleep well during daytime hours.”
While fewer studies have evaluated the long-term effects of seasonal time changes, the AASM position statement cited evidence that “the body clock does not adjust to daylight saving time after several months,” possibly because “daylight saving time is less well-aligned with intrinsic human circadian physiology, and it disrupts the natural seasonal adjustment of the human clock due to the effect of late-evening light on the circadian rhythm.”
According to the AASM, permanent DST, as proposed by Sen. Rubio and colleagues, could “result in permanent phase delay, a condition that can also lead to a perpetual discrepancy between the innate biological clock and the extrinsic environmental clock, as well as chronic sleep loss due to early morning social demands that truncate the opportunity to sleep.” This mismatch between sleep/wake cycles and social demands, known as “social jet lag,” has been associated with chronic health risks, including metabolic syndrome, obesity, depression, and cardiovascular disease.
Cardiac impacts of seasonal time change
Muhammad Adeel Rishi, MD, a sleep specialist at Mayo Clinic, Eau Claire, Wis., and lead author of the AASM position statement, highlighted cardiovascular risks in a written statement for this article, noting increased rates of heart attack following the spring time change, and a higher risk of atrial fibrillation.
“Mayo Clinic has not taken a position on this issue,” Dr. Rishi noted. Still, he advocated for permanent standard time as the author of the AASM position statement and vice chair of the AASM public safety committee.
Jay Chudow, MD, and Andrew K. Krumerman, MD, of Montefiore Medical Center, New York, lead author and principal author, respectively, of a recent study that reported increased rates of atrial fibrillation admissions after DST transitions, had the same stance.
“We support elimination of seasonal time changes from a health perspective,” they wrote in a joint comment. “There is mounting evidence of a negative health impact with these seasonal time changes related to effects on sleep and circadian rhythm. Our work found the spring change was associated with more admissions for atrial fibrillation. This added to prior evidence of increased cardiovascular events related to these time changes. If physicians counsel patients on reducing risk factors for disease, shouldn’t we do the same as a society?”
Pros and cons
Not all sleep experts are convinced. Mary Jo Farmer, MD, PhD, FCCP, a sleep specialist and director of pulmonary hypertension services at Baystate Medical Center, and assistant professor of medicine at the University of Massachusetts, Springfield, considers perspectives from both sides of the issue.
“Daylight saving time promotes active lifestyles as people engage in more outdoor activities after work and school, [and] daylight saving time produces economic and safety benefits to society as retail revenues are higher and crimes are lower,” Dr. Farmer said. “Alternatively, moving the clocks forward is a cost burden to the U.S. economy when health issues, decreased productivity, and workplace injuries are considered.”
If one time system is permanently established, Dr. Farmer anticipates divided opinions from patients with sleep issues, regardless of which system is chosen.
“I can tell you, I have a cohort of sleep patients who prefer more evening light and look forward to the spring time change to daylight saving time,” she said. “However, they would not want the sun coming up at 9:00 a.m. in the winter months if we stayed on daylight saving time year-round. Similarly, patients would not want the sun coming up at 4:00 a.m. on the longest day of the year if we stayed on standard time all year round.”
Dr. Farmer called for more research before a decision is made.
“I suggest we need more information about the dangers of staying on daylight saving or standard time year-round because perhaps the current strategy of keeping morning light consistent is not so bad,” she said.
Time for a Congressional hearing?
According to Dr. Ramar, the time is now for a Congressional hearing, as lawmakers and the public need to be adequately informed when considering new legislation.
“There are public misconceptions about daylight saving time and standard time,” Dr. Ramar said. “People often like the idea of daylight saving time because they think it provides more light, and they dislike the concept of standard time because they think it provides more darkness. The reality is that neither time system provides more light or darkness than the other; it is only the timing that changes.”
Until new legislation is introduced, Dr. Ramar offered some practical advice for navigating seasonal time shifts.
“Beginning 2-3 days before the time change, it can be helpful to gradually adjust sleep and wake times, as well as other daily routines such as meal times,” he said. “After the time change, going outside for some morning light can help adjust the timing of your internal body clock.”
The investigators reported no conflicts of interest.
Seasonal time change is now up for consideration in the U.S. Congress, prompting sleep medicine specialists to weigh in on the health impact of a major policy change.
As lawmakers in Washington propose an end to seasonal time changes by permanently establishing daylight saving time (DST), the American Academy of Sleep Medicine (AASM) is pushing for a Congressional hearing so scientists can present evidence in favor of converse legislation – to make standard time the new norm.
According to the AASM, ; however, the switch from standard time to DST incurs more risk.
“Current evidence best supports the adoption of year-round standard time, which aligns best with human circadian biology and provides distinct benefits for public health and safety,” the AASM noted in a 2020 position statement on DST.
The statement cites a number of studies that have reported associations between the switch to DST and acute, negative health outcomes, including higher rates of hospital admission, cardiovascular morbidity, atrial fibrillation, and stroke. The time shift has been associated with a spectrum of cellular, metabolic, and circadian derangements, from increased production of inflammatory markers, to higher blood pressure, and loss of sleep. These biological effects may have far-reaching consequences, including increased rates of fatal motor accidents in the days following the time change, and even increased volatility in the stock market, which may stem from cognitive deficits.
U.S. Senator Marco Rubio (R-Fla.) and others in the U.S. Congress have reintroduced the 2019 Sunshine Protection Act, legislation that would make DST permanent across the country. According to a statement on Sen. Rubio’s website, “The bill reflects the Florida legislature’s 2018 enactment of year-round DST; however, for Florida’s change to apply, a change in the federal statute is required. Fifteen other states – Arkansas, Alabama, California, Delaware, Georgia, Idaho, Louisiana, Maine, Ohio, Oregon, South Carolina, Tennessee, Utah, Washington, and Wyoming – have passed similar laws, resolutions, or voter initiatives, and dozens more are looking. The legislation, if enacted, would apply to those states [that] currently participate in DST, which most states observe for eight months out of the year.”
A stitch in time
“The sudden change in clock time disrupts sleep/wake patterns, decreasing total sleep time and sleep quality, leading to decrements in daytime cognition,” said Kannan Ramar, MBBS, MD, president of the AASM and a sleep medicine specialist at Mayo Clinic, Rochester, Minn.
Emphasizing this point, Dr. Ramar noted a recent study that reported an 18% increase in “patient safety-related incidents associated with human error” among health care workers within a week of the spring time change.
“Irregular bedtimes and wake times disrupt the timing of our circadian rhythms, which can lead to symptoms of insomnia or long-term, excessive daytime sleepiness. Lack of sleep can lead to numerous adverse effects on our minds, including decreased cognitive function, trouble concentrating, and general moodiness,” Dr. Ramar said.
He noted that these impacts may be more significant among certain individuals.
“The daylight saving time changes can be especially problematic for any populations that already experience chronic insufficient sleep or other sleep difficulties,” Dr. Ramar said. “Populations at greatest risk include teenagers, who tend to experience chronic sleep restriction during the school week, and night shift workers, who often struggle to sleep well during daytime hours.”
While fewer studies have evaluated the long-term effects of seasonal time changes, the AASM position statement cited evidence that “the body clock does not adjust to daylight saving time after several months,” possibly because “daylight saving time is less well-aligned with intrinsic human circadian physiology, and it disrupts the natural seasonal adjustment of the human clock due to the effect of late-evening light on the circadian rhythm.”
According to the AASM, permanent DST, as proposed by Sen. Rubio and colleagues, could “result in permanent phase delay, a condition that can also lead to a perpetual discrepancy between the innate biological clock and the extrinsic environmental clock, as well as chronic sleep loss due to early morning social demands that truncate the opportunity to sleep.” This mismatch between sleep/wake cycles and social demands, known as “social jet lag,” has been associated with chronic health risks, including metabolic syndrome, obesity, depression, and cardiovascular disease.
Cardiac impacts of seasonal time change
Muhammad Adeel Rishi, MD, a sleep specialist at Mayo Clinic, Eau Claire, Wis., and lead author of the AASM position statement, highlighted cardiovascular risks in a written statement for this article, noting increased rates of heart attack following the spring time change, and a higher risk of atrial fibrillation.
“Mayo Clinic has not taken a position on this issue,” Dr. Rishi noted. Still, he advocated for permanent standard time as the author of the AASM position statement and vice chair of the AASM public safety committee.
Jay Chudow, MD, and Andrew K. Krumerman, MD, of Montefiore Medical Center, New York, lead author and principal author, respectively, of a recent study that reported increased rates of atrial fibrillation admissions after DST transitions, had the same stance.
“We support elimination of seasonal time changes from a health perspective,” they wrote in a joint comment. “There is mounting evidence of a negative health impact with these seasonal time changes related to effects on sleep and circadian rhythm. Our work found the spring change was associated with more admissions for atrial fibrillation. This added to prior evidence of increased cardiovascular events related to these time changes. If physicians counsel patients on reducing risk factors for disease, shouldn’t we do the same as a society?”
Pros and cons
Not all sleep experts are convinced. Mary Jo Farmer, MD, PhD, FCCP, a sleep specialist and director of pulmonary hypertension services at Baystate Medical Center, and assistant professor of medicine at the University of Massachusetts, Springfield, considers perspectives from both sides of the issue.
“Daylight saving time promotes active lifestyles as people engage in more outdoor activities after work and school, [and] daylight saving time produces economic and safety benefits to society as retail revenues are higher and crimes are lower,” Dr. Farmer said. “Alternatively, moving the clocks forward is a cost burden to the U.S. economy when health issues, decreased productivity, and workplace injuries are considered.”
If one time system is permanently established, Dr. Farmer anticipates divided opinions from patients with sleep issues, regardless of which system is chosen.
“I can tell you, I have a cohort of sleep patients who prefer more evening light and look forward to the spring time change to daylight saving time,” she said. “However, they would not want the sun coming up at 9:00 a.m. in the winter months if we stayed on daylight saving time year-round. Similarly, patients would not want the sun coming up at 4:00 a.m. on the longest day of the year if we stayed on standard time all year round.”
Dr. Farmer called for more research before a decision is made.
“I suggest we need more information about the dangers of staying on daylight saving or standard time year-round because perhaps the current strategy of keeping morning light consistent is not so bad,” she said.
Time for a Congressional hearing?
According to Dr. Ramar, the time is now for a Congressional hearing, as lawmakers and the public need to be adequately informed when considering new legislation.
“There are public misconceptions about daylight saving time and standard time,” Dr. Ramar said. “People often like the idea of daylight saving time because they think it provides more light, and they dislike the concept of standard time because they think it provides more darkness. The reality is that neither time system provides more light or darkness than the other; it is only the timing that changes.”
Until new legislation is introduced, Dr. Ramar offered some practical advice for navigating seasonal time shifts.
“Beginning 2-3 days before the time change, it can be helpful to gradually adjust sleep and wake times, as well as other daily routines such as meal times,” he said. “After the time change, going outside for some morning light can help adjust the timing of your internal body clock.”
The investigators reported no conflicts of interest.
Prenatal dietary folate not enough to offset AEDs’ effect on kids’ cognition
New research underscores the importance of folic acid supplementation for pregnant women with epilepsy who are taking antiepileptic drugs (AEDs).
Dietary folate alone, even in the United States, where food is fortified with folic acid, is “not sufficient” to improve cognitive outcomes for children of women who take AEDs during pregnancy, the researchers report.
“We found that dietary folate was not related to outcomes,” study investigator Kimford Meador, MD, professor of neurology and neurologic sciences, Stanford (Calif.) University, told this news organization.
“Only when the mother was taking extra folate did we see an improvement in child outcomes,” he added.
The findings were published online Feb. 23 in Epilepsy and Behavior.
Cognitive boost
“Daily folate is recommended to women in the general populations to reduce congenital malformations,” Dr. Meador said. In addition, periconceptional use of folate has been shown in previous research to improve neurodevelopmental outcomes for children of mothers with epilepsy who are taking AEDs.
Whether folate-fortified food alone, without supplements, has any effect on cognitive outcomes in this population of children has not been examined previously.
To investigate, the researchers assessed 117 children from the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, a prospective, observational study of women with epilepsy who were taking one of four AEDs: carbamazepine, lamotrigine, phenytoin, or valproate.
Results showed that dietary folate from fortified food alone, without supplements, had no significant impact on IQ at age 6 years among children with prenatal exposure to AEDs.
In contrast, use of periconceptual folate supplements was significantly associated with a 10-point higher IQ at age 6 in the adjusted analyses (95% confidence interval, 5.2-15.0; P < .001).
These six other nutrients from food and supplements had no significant association with IQ at age 6 years: vitamins C, D, and E, omega-3, gamma tocopherol, and vitamin B12.
Optimal dose unclear
The findings indicate that folates, including natural folate and folic acid, in food do not have positive cognitive effects for children of women with epilepsy who take AEDs, the researchers write.
Dr. Meador noted that the optimal dose of folic acid supplementation to provide a cognitive benefit remains unclear.
The U.S. Centers for Disease Control recommends 0.4 mg/d for the general population of women of childbearing age. In Europe, the recommendation is 1 mg/d.
“Higher doses are recommended if there is a personal or family history of spina bifida in prior pregnancies, but there is some concern that very high doses of folate may be detrimental,” Dr. Meador said.
For women with epilepsy, he would recommend “at least 1 mg/d and not more than 4 mg/d.”
Proves a point?
Commenting on the study for this news organization, Derek Chong, MD, vice chair of neurology and director of epilepsy at Lenox Hill Hospital, New York, said the finding that folate fortification of food alone is not adequate for women with epilepsy is “not groundbreaking” but does prove something previously thought.
“Folic acid is important for all women, but it does seem like folic acid may be even more important in the epilepsy population,” said Dr. Chong, who was not involved with the research.
He cautioned that the current analysis included only four medications, three of which are not used very often anymore.
“Lamotrigine is probably the most commonly used one now. It’s unfortunate that this study did not include Keppra [levetiracetam], which probably is the number one medication that we use now,” Dr. Chong said.
The research was supported by the National Institutes of Health. Dr. Meador and Dr. Chong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research underscores the importance of folic acid supplementation for pregnant women with epilepsy who are taking antiepileptic drugs (AEDs).
Dietary folate alone, even in the United States, where food is fortified with folic acid, is “not sufficient” to improve cognitive outcomes for children of women who take AEDs during pregnancy, the researchers report.
“We found that dietary folate was not related to outcomes,” study investigator Kimford Meador, MD, professor of neurology and neurologic sciences, Stanford (Calif.) University, told this news organization.
“Only when the mother was taking extra folate did we see an improvement in child outcomes,” he added.
The findings were published online Feb. 23 in Epilepsy and Behavior.
Cognitive boost
“Daily folate is recommended to women in the general populations to reduce congenital malformations,” Dr. Meador said. In addition, periconceptional use of folate has been shown in previous research to improve neurodevelopmental outcomes for children of mothers with epilepsy who are taking AEDs.
Whether folate-fortified food alone, without supplements, has any effect on cognitive outcomes in this population of children has not been examined previously.
To investigate, the researchers assessed 117 children from the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, a prospective, observational study of women with epilepsy who were taking one of four AEDs: carbamazepine, lamotrigine, phenytoin, or valproate.
Results showed that dietary folate from fortified food alone, without supplements, had no significant impact on IQ at age 6 years among children with prenatal exposure to AEDs.
In contrast, use of periconceptual folate supplements was significantly associated with a 10-point higher IQ at age 6 in the adjusted analyses (95% confidence interval, 5.2-15.0; P < .001).
These six other nutrients from food and supplements had no significant association with IQ at age 6 years: vitamins C, D, and E, omega-3, gamma tocopherol, and vitamin B12.
Optimal dose unclear
The findings indicate that folates, including natural folate and folic acid, in food do not have positive cognitive effects for children of women with epilepsy who take AEDs, the researchers write.
Dr. Meador noted that the optimal dose of folic acid supplementation to provide a cognitive benefit remains unclear.
The U.S. Centers for Disease Control recommends 0.4 mg/d for the general population of women of childbearing age. In Europe, the recommendation is 1 mg/d.
“Higher doses are recommended if there is a personal or family history of spina bifida in prior pregnancies, but there is some concern that very high doses of folate may be detrimental,” Dr. Meador said.
For women with epilepsy, he would recommend “at least 1 mg/d and not more than 4 mg/d.”
Proves a point?
Commenting on the study for this news organization, Derek Chong, MD, vice chair of neurology and director of epilepsy at Lenox Hill Hospital, New York, said the finding that folate fortification of food alone is not adequate for women with epilepsy is “not groundbreaking” but does prove something previously thought.
“Folic acid is important for all women, but it does seem like folic acid may be even more important in the epilepsy population,” said Dr. Chong, who was not involved with the research.
He cautioned that the current analysis included only four medications, three of which are not used very often anymore.
“Lamotrigine is probably the most commonly used one now. It’s unfortunate that this study did not include Keppra [levetiracetam], which probably is the number one medication that we use now,” Dr. Chong said.
The research was supported by the National Institutes of Health. Dr. Meador and Dr. Chong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research underscores the importance of folic acid supplementation for pregnant women with epilepsy who are taking antiepileptic drugs (AEDs).
Dietary folate alone, even in the United States, where food is fortified with folic acid, is “not sufficient” to improve cognitive outcomes for children of women who take AEDs during pregnancy, the researchers report.
“We found that dietary folate was not related to outcomes,” study investigator Kimford Meador, MD, professor of neurology and neurologic sciences, Stanford (Calif.) University, told this news organization.
“Only when the mother was taking extra folate did we see an improvement in child outcomes,” he added.
The findings were published online Feb. 23 in Epilepsy and Behavior.
Cognitive boost
“Daily folate is recommended to women in the general populations to reduce congenital malformations,” Dr. Meador said. In addition, periconceptional use of folate has been shown in previous research to improve neurodevelopmental outcomes for children of mothers with epilepsy who are taking AEDs.
Whether folate-fortified food alone, without supplements, has any effect on cognitive outcomes in this population of children has not been examined previously.
To investigate, the researchers assessed 117 children from the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, a prospective, observational study of women with epilepsy who were taking one of four AEDs: carbamazepine, lamotrigine, phenytoin, or valproate.
Results showed that dietary folate from fortified food alone, without supplements, had no significant impact on IQ at age 6 years among children with prenatal exposure to AEDs.
In contrast, use of periconceptual folate supplements was significantly associated with a 10-point higher IQ at age 6 in the adjusted analyses (95% confidence interval, 5.2-15.0; P < .001).
These six other nutrients from food and supplements had no significant association with IQ at age 6 years: vitamins C, D, and E, omega-3, gamma tocopherol, and vitamin B12.
Optimal dose unclear
The findings indicate that folates, including natural folate and folic acid, in food do not have positive cognitive effects for children of women with epilepsy who take AEDs, the researchers write.
Dr. Meador noted that the optimal dose of folic acid supplementation to provide a cognitive benefit remains unclear.
The U.S. Centers for Disease Control recommends 0.4 mg/d for the general population of women of childbearing age. In Europe, the recommendation is 1 mg/d.
“Higher doses are recommended if there is a personal or family history of spina bifida in prior pregnancies, but there is some concern that very high doses of folate may be detrimental,” Dr. Meador said.
For women with epilepsy, he would recommend “at least 1 mg/d and not more than 4 mg/d.”
Proves a point?
Commenting on the study for this news organization, Derek Chong, MD, vice chair of neurology and director of epilepsy at Lenox Hill Hospital, New York, said the finding that folate fortification of food alone is not adequate for women with epilepsy is “not groundbreaking” but does prove something previously thought.
“Folic acid is important for all women, but it does seem like folic acid may be even more important in the epilepsy population,” said Dr. Chong, who was not involved with the research.
He cautioned that the current analysis included only four medications, three of which are not used very often anymore.
“Lamotrigine is probably the most commonly used one now. It’s unfortunate that this study did not include Keppra [levetiracetam], which probably is the number one medication that we use now,” Dr. Chong said.
The research was supported by the National Institutes of Health. Dr. Meador and Dr. Chong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.