User login
Factors that change our brains; The APA’s stance on neuroimaging
Factors that change our brains
I greatly enjoyed Dr. Nasrallah’s editorial, “Your patient’s brain is different at every visit” (From the Editor,
In reading this editorial, it is clear that a myriad of factors we consider and address with our patients during each visit underly intricate neurobiologic mechanisms and processes that ever deepen our understanding of the brain. In discussing the changes taking place in our patients, I can’t help but wonder what changes are also occurring in our brains (as Dr. Nasrallah noted). What would be the resulting impact of these changes in our next patient interaction and/or subsequent interaction(s) with the same patient? Looking through the editorial’s bullet points, many (if not all) of the factors contributing to brain changes apply equally and naturally to clinicians as well as patients. In this light, the editorial serves not only as a broad guideline for patient psychoeducation but also as a reminder of wellness and well-being for clinicians.
As a “fresh-out-of-training” psychiatrist, I can definitely work on several of the factors, such as diet and exercise. Trainees and residents can be more susceptible to overlook and befall some of these factors and changes, and may already be basing the clinical advice they give to their patients on these same factors and changes. As a child psychiatrist, I value the importance of modeling healthy behaviors for my patients, and their families and with coworkers or colleagues. In accordance with the impact these factors have on our brains, it’s important to emphasize what we can do to further strengthen rapport and therapeutic value through modeling. I strive to model the desired behaviors, attitudes, and dynamics that are the external, observable manifestation or symptomology of what takes place in my brain. To do so, I understand I need to be mindful in proactively managing the contributing factors, such as those listed in Dr. Nasrallah’s editorial. I imagine patients and their families would easily notice if we are in suboptimal physical and/or mental health that results in us not being prompt, fully engaged, or receptive. I believe that attending to these facets during training falls under the umbrella of professionalism. Being a professional in our field often entails practicing what we preach. So, I’m grateful that what we preach is informed by our field’s exciting research, continued advancements, and expertise that benefits our patients and us professionally and personally.
Philip Yen-Tsun Liu, MD
Child and adolescent psychiatrist
innovaTel Telepsychiatry
San Antonio, Texas
Dr. Nasrallah responds
I would like to thank Dr. Liu for his thoughtful response to my editorial. He seems to be very cognizant of the fact that experiential neuroplasticity and brain tissue remodeling occurs in both the patient and physician. I admire his focus on psychoeducation, wellness, and professionalism. He is right that we as psychiatrists (and nurse practitioners) must be role models for our patients in multiple ways, because it may help enhance clinical outcomes and have a positive impact on their brains.
I would also like to point Dr. Liu to the editorial “The most powerful placebo is not a pill” (From the Editor,
Henry A. Nasrallah, MD
Editor-in-Chief
Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
The APA’s stance on neuroimaging
Can anyone in the modern world argue that the brain is irrelevant to psychiatry? Yet surprisingly, in September 2018, the American Psychiatric Association (APA) officially declared that neuroimaging of the brain has no clinical value in psychiatry.1
Unfortunately, the APA focused almost exclusively on functional magnetic resonance imaging (fMRI) and neglected an extensive library of studies of single-photon emission computed tomography (SPECT) and positron emission tomography (PET). The APA’s position on neuroimaging is as follows1,2:
- A neuroimaging finding must have a sensitivity and specificity (S/sp) of no less than 80%.
- The psychiatric imaging literature does not support using neuroimaging in psychiatric diagnostics or treatment.
- Neuroimaging has not had a significant impact on the diagnosis and treatment of psychiatric disorders.
The APA set unrealistic standards for biomarkers in a field that lacks pathologic markers of specific disease entities.3 Moreover, numerous widely used tests fall below the APA’s unrealistic S/sp cutoff, including the Hamilton Depression Rating Scale,4 Zung Depression Scale,5 the clock drawing test,6 and even the chest X-ray.3 Curiously, numerous replicated SPECT and PET studies were not included in the APA’s analysis.1-3 For example, in a study of 196 veterans, posttraumatic stress disorder was distinguished from traumatic brain injury with an S/sp of 0.92/0.85.7,8 Also, fluorodeoxyglucose (FDG)-PET has an S/sp of 0.84/0.74 in differentiating patients with Alzheimer’s disease from controls, while perfusion SPECT, using multi-detector cameras, has an S/sp of 0.93/0.84.3,9 Moreover, both FDG-PET and SPECT can differentiate other forms of dementia from Alzheimer’s disease, yielding an additional benefit compared to amyloid imaging alone.2,9 As President of the International Society of Applied Neuroimaging, I suggest neuroimaging should not be feared. Neuroimaging does not replace the diagnostician; rather, it aids him/her in a complex case.
Theodore A. Henderson, MD, PhD
President
Neuro-Luminance Brain Health Centers, Inc.
Denver, Colorado
Director
The Synaptic Space
Vice President
The Neuro-Laser Foundation
President
International Society of Applied Neuroimaging
Centennial, Colorado
Disclosure
The author has no ownership in, and receives no remuneration from, any neuroimaging company.
References
1. First MB, Drevets WC, Carter C, et al. Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018:175:
2. First MB, Drevets WC, Carter C, et al. Data supplement for Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018;175(suppl).
3. Henderson TA. Brain SPECT imaging in neuropsychiatric diagnosis and monitoring. EPatient. http://nmpangea.com/2018/10/09/738/. Published 2018. Accessed May 31, 2019.
4. Bagby RM, Ryder AG, Schuller DR, et al. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161(12):2163-2177.
5. Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung Self-rating Depression Scale. Br J Psychiatry. 1978;132:381-385.
6. Seigerschmidt E, Mösch E, Siemen M, et al. The clock drawing test and questionable dementia: reliability and validity. Int J Geriatr Psychiatry. 2002;17(11):1048-1054.
7. Raji CA, Willeumier K, Taylor D, et al. Functional neuroimaging with default mode network regions distinguishes PTSD from TBI in a military veteran population. Brain Imaging Behav. 2015;9(3):527-534.
8. Amen DG, Raji CA, Willeumier K, et al. Functional neuroimaging distinguishes posttraumatic stress disorder from traumatic brain injury in focused and large community datasets. PLoS One. 2015;10(7):e0129659. doi: 10.1371/journal.pone.0129659.
9. Henderson TA. The diagnosis and evaluation of dementia and mild cognitive impairment with emphasis on SPECT perfusion neuroimaging. CNS Spectr. 2012;17(4):176-206.
Factors that change our brains
I greatly enjoyed Dr. Nasrallah’s editorial, “Your patient’s brain is different at every visit” (From the Editor,
In reading this editorial, it is clear that a myriad of factors we consider and address with our patients during each visit underly intricate neurobiologic mechanisms and processes that ever deepen our understanding of the brain. In discussing the changes taking place in our patients, I can’t help but wonder what changes are also occurring in our brains (as Dr. Nasrallah noted). What would be the resulting impact of these changes in our next patient interaction and/or subsequent interaction(s) with the same patient? Looking through the editorial’s bullet points, many (if not all) of the factors contributing to brain changes apply equally and naturally to clinicians as well as patients. In this light, the editorial serves not only as a broad guideline for patient psychoeducation but also as a reminder of wellness and well-being for clinicians.
As a “fresh-out-of-training” psychiatrist, I can definitely work on several of the factors, such as diet and exercise. Trainees and residents can be more susceptible to overlook and befall some of these factors and changes, and may already be basing the clinical advice they give to their patients on these same factors and changes. As a child psychiatrist, I value the importance of modeling healthy behaviors for my patients, and their families and with coworkers or colleagues. In accordance with the impact these factors have on our brains, it’s important to emphasize what we can do to further strengthen rapport and therapeutic value through modeling. I strive to model the desired behaviors, attitudes, and dynamics that are the external, observable manifestation or symptomology of what takes place in my brain. To do so, I understand I need to be mindful in proactively managing the contributing factors, such as those listed in Dr. Nasrallah’s editorial. I imagine patients and their families would easily notice if we are in suboptimal physical and/or mental health that results in us not being prompt, fully engaged, or receptive. I believe that attending to these facets during training falls under the umbrella of professionalism. Being a professional in our field often entails practicing what we preach. So, I’m grateful that what we preach is informed by our field’s exciting research, continued advancements, and expertise that benefits our patients and us professionally and personally.
Philip Yen-Tsun Liu, MD
Child and adolescent psychiatrist
innovaTel Telepsychiatry
San Antonio, Texas
Dr. Nasrallah responds
I would like to thank Dr. Liu for his thoughtful response to my editorial. He seems to be very cognizant of the fact that experiential neuroplasticity and brain tissue remodeling occurs in both the patient and physician. I admire his focus on psychoeducation, wellness, and professionalism. He is right that we as psychiatrists (and nurse practitioners) must be role models for our patients in multiple ways, because it may help enhance clinical outcomes and have a positive impact on their brains.
I would also like to point Dr. Liu to the editorial “The most powerful placebo is not a pill” (From the Editor,
Henry A. Nasrallah, MD
Editor-in-Chief
Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
The APA’s stance on neuroimaging
Can anyone in the modern world argue that the brain is irrelevant to psychiatry? Yet surprisingly, in September 2018, the American Psychiatric Association (APA) officially declared that neuroimaging of the brain has no clinical value in psychiatry.1
Unfortunately, the APA focused almost exclusively on functional magnetic resonance imaging (fMRI) and neglected an extensive library of studies of single-photon emission computed tomography (SPECT) and positron emission tomography (PET). The APA’s position on neuroimaging is as follows1,2:
- A neuroimaging finding must have a sensitivity and specificity (S/sp) of no less than 80%.
- The psychiatric imaging literature does not support using neuroimaging in psychiatric diagnostics or treatment.
- Neuroimaging has not had a significant impact on the diagnosis and treatment of psychiatric disorders.
The APA set unrealistic standards for biomarkers in a field that lacks pathologic markers of specific disease entities.3 Moreover, numerous widely used tests fall below the APA’s unrealistic S/sp cutoff, including the Hamilton Depression Rating Scale,4 Zung Depression Scale,5 the clock drawing test,6 and even the chest X-ray.3 Curiously, numerous replicated SPECT and PET studies were not included in the APA’s analysis.1-3 For example, in a study of 196 veterans, posttraumatic stress disorder was distinguished from traumatic brain injury with an S/sp of 0.92/0.85.7,8 Also, fluorodeoxyglucose (FDG)-PET has an S/sp of 0.84/0.74 in differentiating patients with Alzheimer’s disease from controls, while perfusion SPECT, using multi-detector cameras, has an S/sp of 0.93/0.84.3,9 Moreover, both FDG-PET and SPECT can differentiate other forms of dementia from Alzheimer’s disease, yielding an additional benefit compared to amyloid imaging alone.2,9 As President of the International Society of Applied Neuroimaging, I suggest neuroimaging should not be feared. Neuroimaging does not replace the diagnostician; rather, it aids him/her in a complex case.
Theodore A. Henderson, MD, PhD
President
Neuro-Luminance Brain Health Centers, Inc.
Denver, Colorado
Director
The Synaptic Space
Vice President
The Neuro-Laser Foundation
President
International Society of Applied Neuroimaging
Centennial, Colorado
Disclosure
The author has no ownership in, and receives no remuneration from, any neuroimaging company.
References
1. First MB, Drevets WC, Carter C, et al. Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018:175:
2. First MB, Drevets WC, Carter C, et al. Data supplement for Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018;175(suppl).
3. Henderson TA. Brain SPECT imaging in neuropsychiatric diagnosis and monitoring. EPatient. http://nmpangea.com/2018/10/09/738/. Published 2018. Accessed May 31, 2019.
4. Bagby RM, Ryder AG, Schuller DR, et al. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161(12):2163-2177.
5. Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung Self-rating Depression Scale. Br J Psychiatry. 1978;132:381-385.
6. Seigerschmidt E, Mösch E, Siemen M, et al. The clock drawing test and questionable dementia: reliability and validity. Int J Geriatr Psychiatry. 2002;17(11):1048-1054.
7. Raji CA, Willeumier K, Taylor D, et al. Functional neuroimaging with default mode network regions distinguishes PTSD from TBI in a military veteran population. Brain Imaging Behav. 2015;9(3):527-534.
8. Amen DG, Raji CA, Willeumier K, et al. Functional neuroimaging distinguishes posttraumatic stress disorder from traumatic brain injury in focused and large community datasets. PLoS One. 2015;10(7):e0129659. doi: 10.1371/journal.pone.0129659.
9. Henderson TA. The diagnosis and evaluation of dementia and mild cognitive impairment with emphasis on SPECT perfusion neuroimaging. CNS Spectr. 2012;17(4):176-206.
Factors that change our brains
I greatly enjoyed Dr. Nasrallah’s editorial, “Your patient’s brain is different at every visit” (From the Editor,
In reading this editorial, it is clear that a myriad of factors we consider and address with our patients during each visit underly intricate neurobiologic mechanisms and processes that ever deepen our understanding of the brain. In discussing the changes taking place in our patients, I can’t help but wonder what changes are also occurring in our brains (as Dr. Nasrallah noted). What would be the resulting impact of these changes in our next patient interaction and/or subsequent interaction(s) with the same patient? Looking through the editorial’s bullet points, many (if not all) of the factors contributing to brain changes apply equally and naturally to clinicians as well as patients. In this light, the editorial serves not only as a broad guideline for patient psychoeducation but also as a reminder of wellness and well-being for clinicians.
As a “fresh-out-of-training” psychiatrist, I can definitely work on several of the factors, such as diet and exercise. Trainees and residents can be more susceptible to overlook and befall some of these factors and changes, and may already be basing the clinical advice they give to their patients on these same factors and changes. As a child psychiatrist, I value the importance of modeling healthy behaviors for my patients, and their families and with coworkers or colleagues. In accordance with the impact these factors have on our brains, it’s important to emphasize what we can do to further strengthen rapport and therapeutic value through modeling. I strive to model the desired behaviors, attitudes, and dynamics that are the external, observable manifestation or symptomology of what takes place in my brain. To do so, I understand I need to be mindful in proactively managing the contributing factors, such as those listed in Dr. Nasrallah’s editorial. I imagine patients and their families would easily notice if we are in suboptimal physical and/or mental health that results in us not being prompt, fully engaged, or receptive. I believe that attending to these facets during training falls under the umbrella of professionalism. Being a professional in our field often entails practicing what we preach. So, I’m grateful that what we preach is informed by our field’s exciting research, continued advancements, and expertise that benefits our patients and us professionally and personally.
Philip Yen-Tsun Liu, MD
Child and adolescent psychiatrist
innovaTel Telepsychiatry
San Antonio, Texas
Dr. Nasrallah responds
I would like to thank Dr. Liu for his thoughtful response to my editorial. He seems to be very cognizant of the fact that experiential neuroplasticity and brain tissue remodeling occurs in both the patient and physician. I admire his focus on psychoeducation, wellness, and professionalism. He is right that we as psychiatrists (and nurse practitioners) must be role models for our patients in multiple ways, because it may help enhance clinical outcomes and have a positive impact on their brains.
I would also like to point Dr. Liu to the editorial “The most powerful placebo is not a pill” (From the Editor,
Henry A. Nasrallah, MD
Editor-in-Chief
Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
The APA’s stance on neuroimaging
Can anyone in the modern world argue that the brain is irrelevant to psychiatry? Yet surprisingly, in September 2018, the American Psychiatric Association (APA) officially declared that neuroimaging of the brain has no clinical value in psychiatry.1
Unfortunately, the APA focused almost exclusively on functional magnetic resonance imaging (fMRI) and neglected an extensive library of studies of single-photon emission computed tomography (SPECT) and positron emission tomography (PET). The APA’s position on neuroimaging is as follows1,2:
- A neuroimaging finding must have a sensitivity and specificity (S/sp) of no less than 80%.
- The psychiatric imaging literature does not support using neuroimaging in psychiatric diagnostics or treatment.
- Neuroimaging has not had a significant impact on the diagnosis and treatment of psychiatric disorders.
The APA set unrealistic standards for biomarkers in a field that lacks pathologic markers of specific disease entities.3 Moreover, numerous widely used tests fall below the APA’s unrealistic S/sp cutoff, including the Hamilton Depression Rating Scale,4 Zung Depression Scale,5 the clock drawing test,6 and even the chest X-ray.3 Curiously, numerous replicated SPECT and PET studies were not included in the APA’s analysis.1-3 For example, in a study of 196 veterans, posttraumatic stress disorder was distinguished from traumatic brain injury with an S/sp of 0.92/0.85.7,8 Also, fluorodeoxyglucose (FDG)-PET has an S/sp of 0.84/0.74 in differentiating patients with Alzheimer’s disease from controls, while perfusion SPECT, using multi-detector cameras, has an S/sp of 0.93/0.84.3,9 Moreover, both FDG-PET and SPECT can differentiate other forms of dementia from Alzheimer’s disease, yielding an additional benefit compared to amyloid imaging alone.2,9 As President of the International Society of Applied Neuroimaging, I suggest neuroimaging should not be feared. Neuroimaging does not replace the diagnostician; rather, it aids him/her in a complex case.
Theodore A. Henderson, MD, PhD
President
Neuro-Luminance Brain Health Centers, Inc.
Denver, Colorado
Director
The Synaptic Space
Vice President
The Neuro-Laser Foundation
President
International Society of Applied Neuroimaging
Centennial, Colorado
Disclosure
The author has no ownership in, and receives no remuneration from, any neuroimaging company.
References
1. First MB, Drevets WC, Carter C, et al. Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018:175:
2. First MB, Drevets WC, Carter C, et al. Data supplement for Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018;175(suppl).
3. Henderson TA. Brain SPECT imaging in neuropsychiatric diagnosis and monitoring. EPatient. http://nmpangea.com/2018/10/09/738/. Published 2018. Accessed May 31, 2019.
4. Bagby RM, Ryder AG, Schuller DR, et al. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161(12):2163-2177.
5. Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung Self-rating Depression Scale. Br J Psychiatry. 1978;132:381-385.
6. Seigerschmidt E, Mösch E, Siemen M, et al. The clock drawing test and questionable dementia: reliability and validity. Int J Geriatr Psychiatry. 2002;17(11):1048-1054.
7. Raji CA, Willeumier K, Taylor D, et al. Functional neuroimaging with default mode network regions distinguishes PTSD from TBI in a military veteran population. Brain Imaging Behav. 2015;9(3):527-534.
8. Amen DG, Raji CA, Willeumier K, et al. Functional neuroimaging distinguishes posttraumatic stress disorder from traumatic brain injury in focused and large community datasets. PLoS One. 2015;10(7):e0129659. doi: 10.1371/journal.pone.0129659.
9. Henderson TA. The diagnosis and evaluation of dementia and mild cognitive impairment with emphasis on SPECT perfusion neuroimaging. CNS Spectr. 2012;17(4):176-206.
Stigma in dementia: It’s time to talk about it
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).

Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
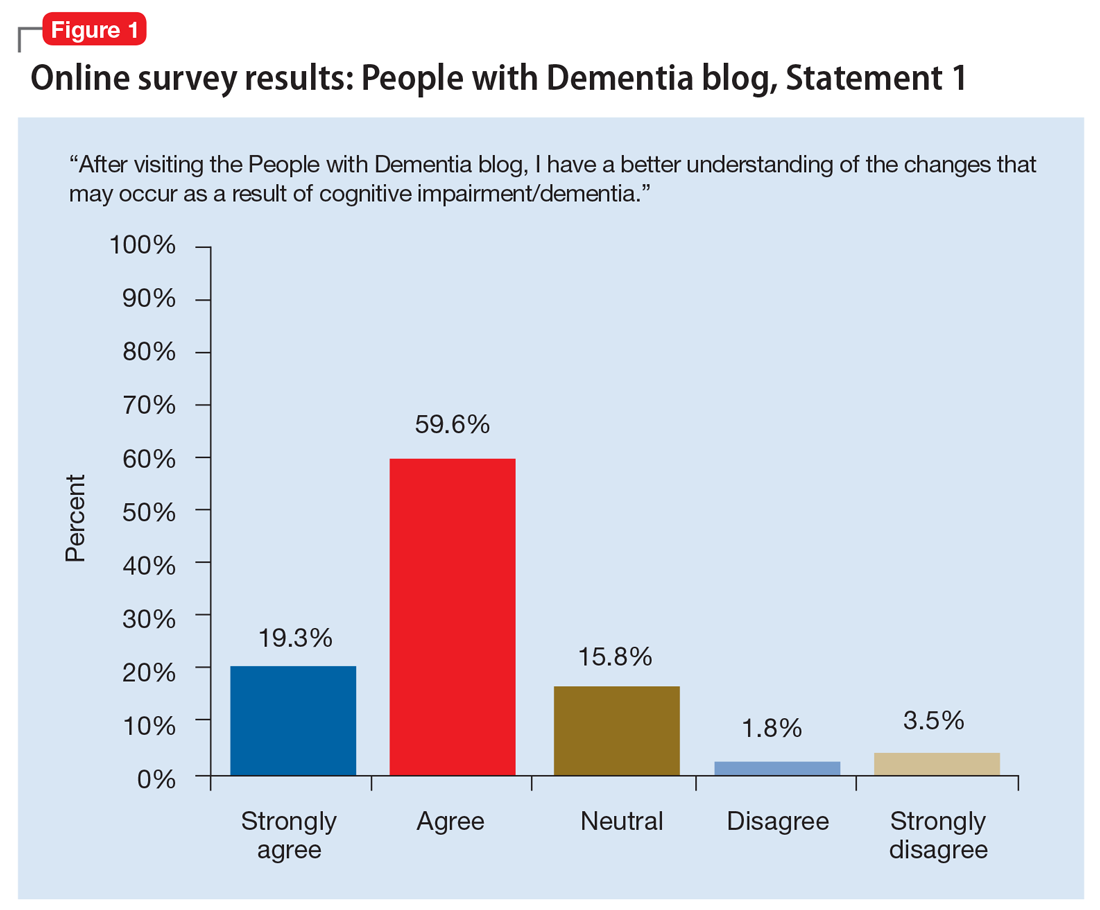
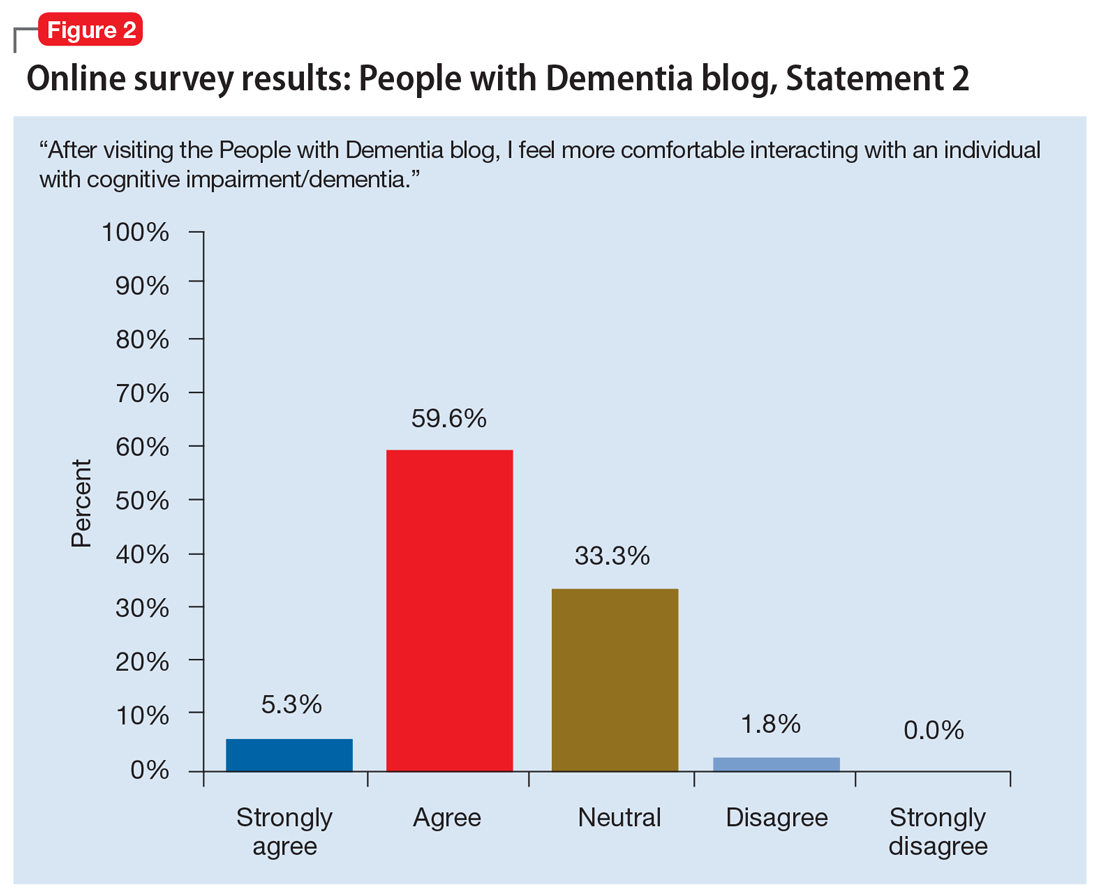
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.
Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.
State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a
Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).

Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.

Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.

State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a

Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).

Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.

Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.

State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a

Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
Study eyes narcolepsy’s impact on patient quality of life
SAN ANTONIO – Narcolepsy adversely impacts one’s health-related quality of life in a variety of ways, from elevated levels of depression to negative social stigma, results from a mixed methods study suggest.
“Despite established pharmacological treatments to reduce narcolepsy symptoms, health-related quality of life remains poor,” the study’s first author, Jason C. Ong, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “The impact these symptoms have on functioning, the disease burden, and psychosocial functioning in particular is very important. Psychosocial functioning is particularly poor.”
Previous research has shown that people with narcolepsy have two- to four times the rate of psychiatric comorbidities and that health-related stigma is a predictor of depression and poor functioning, said Dr. Ong, a psychologist with the Center for Circadian and Sleep Medicine at the Northwestern University Feinberg School of Medicine, Chicago. In an effort to assess current practices for addressing the psychosocial needs of persons with narcolepsy and to identify potential strategies that could be used to develop a psychosocial intervention, he and his associates conducted a mixed methods study to examine how narcolepsy symptoms impact health-related quality of life and the appropriateness of different health-related quality of life measures for the disorder. “Our long-term goal is to see if we can use this information to help inform the feasibility of a psychosocial intervention to improve health-related quality of life,” he said.
For the study, 29 adults with an established diagnosis of narcolepsy completed online versions of the Patient Health Questionnaire-9 (PHQ-9), the Patient Reported Outcomes Measurement Information System (PROMIS), the 36-item Short Form Survey (SF-36), and the Epworth Sleepiness Scale (ESS). They also participated in a focus group, which consisted of questions pertaining to quality of life for persons with narcolepsy, current practices for addressing psychosocial health of affected individuals, and suggestions for developing a psychosocial intervention. The researchers used thematic analysis to reduce the qualitative data to key themes.
Most of the study participants (93%) were female, 90% were white, their mean age was 31, and their mean time since narcolepsy diagnosis was 4.3 years. Clinically significant elevations on the PROMIS scale, defined as a t-score of greater than 60, were reported for depression (t-score of 64.8), anxiety (66.3), fatigue (68.3), and sleep impairment (66.9). Elevations in depressive symptoms were reported on the PHQ-9 (a mean of 15.79), “which corresponds to moderately severe levels,” Dr. Ong said. “The ESS was highly elevated and fit well with the scales for sleep impairment as well as fatigue on the PROMIS. Overall, there was nice congruence across these measures.”
On the SF-36, the researchers observed that there were deficits in physical and emotional aspects of role limitations, and in energy/fatigue. “One thing we did find was a significant difference in general functioning, where patients with type 1 narcolepsy were worse off than those with type 2 narcolepsy (P less than .05).”
Qualitative data from focus groups revealed several key themes, including the perception that narcolepsy is poorly understood by the public and health care providers.
“People have the perception that if you have narcolepsy, you just feel fine and then you fall asleep,” Dr. Ong said. “They don’t understand that it’s a constant thing. Negative social stigma was also common. As a result, we found a lot of negative impact on self-esteem and self-efficacy. People talked about being hesitant to tell other people about their diagnosis, feeling that they’re ashamed of having narcolepsy. They felt less capable. One person said, ‘I get tired trying to explain why I’m tired.’”
Another common theme that emerged was the challenge of optimal treatment for their narcolepsy. Most patients met with sleep doctors or clinics every 3-6 months. “They said that this was generally good for discussing medications and symptom management, but there didn’t seem to be much time to talk about psychosocial aspects,” Dr. Ong said. “That seemed to be one area of need. There was also a strong dissatisfaction with mental health providers. People talked about how their mental health provider really didn’t understand narcolepsy. It did seem to reduce rapport and the ability to trust their therapist. Some talked about the challenges of accessibility. In some cases, people said their narcolepsy symptoms created challenges with appointment attendance.”
In terms of preferences for a psychosocial intervention, respondents generally “preferred some kind of online or Internet delivery,” he said. “They prefer a team approach with a clinician who’s knowledgeable about both sleep and mental health.”
Dr. Ong acknowledged certain limitations of the study, including its small sample size and the fact that it was not adequately powered to detect differences between type 1 and type 2 narcolepsy.
The study was funded by a grant from Wake Up Narcolepsy. Dr. Ong reported having no relevant financial disclosures.
SOURCE: Ong J et al., SLEEP 2019, abstract 0624.
SAN ANTONIO – Narcolepsy adversely impacts one’s health-related quality of life in a variety of ways, from elevated levels of depression to negative social stigma, results from a mixed methods study suggest.
“Despite established pharmacological treatments to reduce narcolepsy symptoms, health-related quality of life remains poor,” the study’s first author, Jason C. Ong, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “The impact these symptoms have on functioning, the disease burden, and psychosocial functioning in particular is very important. Psychosocial functioning is particularly poor.”
Previous research has shown that people with narcolepsy have two- to four times the rate of psychiatric comorbidities and that health-related stigma is a predictor of depression and poor functioning, said Dr. Ong, a psychologist with the Center for Circadian and Sleep Medicine at the Northwestern University Feinberg School of Medicine, Chicago. In an effort to assess current practices for addressing the psychosocial needs of persons with narcolepsy and to identify potential strategies that could be used to develop a psychosocial intervention, he and his associates conducted a mixed methods study to examine how narcolepsy symptoms impact health-related quality of life and the appropriateness of different health-related quality of life measures for the disorder. “Our long-term goal is to see if we can use this information to help inform the feasibility of a psychosocial intervention to improve health-related quality of life,” he said.
For the study, 29 adults with an established diagnosis of narcolepsy completed online versions of the Patient Health Questionnaire-9 (PHQ-9), the Patient Reported Outcomes Measurement Information System (PROMIS), the 36-item Short Form Survey (SF-36), and the Epworth Sleepiness Scale (ESS). They also participated in a focus group, which consisted of questions pertaining to quality of life for persons with narcolepsy, current practices for addressing psychosocial health of affected individuals, and suggestions for developing a psychosocial intervention. The researchers used thematic analysis to reduce the qualitative data to key themes.
Most of the study participants (93%) were female, 90% were white, their mean age was 31, and their mean time since narcolepsy diagnosis was 4.3 years. Clinically significant elevations on the PROMIS scale, defined as a t-score of greater than 60, were reported for depression (t-score of 64.8), anxiety (66.3), fatigue (68.3), and sleep impairment (66.9). Elevations in depressive symptoms were reported on the PHQ-9 (a mean of 15.79), “which corresponds to moderately severe levels,” Dr. Ong said. “The ESS was highly elevated and fit well with the scales for sleep impairment as well as fatigue on the PROMIS. Overall, there was nice congruence across these measures.”
On the SF-36, the researchers observed that there were deficits in physical and emotional aspects of role limitations, and in energy/fatigue. “One thing we did find was a significant difference in general functioning, where patients with type 1 narcolepsy were worse off than those with type 2 narcolepsy (P less than .05).”
Qualitative data from focus groups revealed several key themes, including the perception that narcolepsy is poorly understood by the public and health care providers.
“People have the perception that if you have narcolepsy, you just feel fine and then you fall asleep,” Dr. Ong said. “They don’t understand that it’s a constant thing. Negative social stigma was also common. As a result, we found a lot of negative impact on self-esteem and self-efficacy. People talked about being hesitant to tell other people about their diagnosis, feeling that they’re ashamed of having narcolepsy. They felt less capable. One person said, ‘I get tired trying to explain why I’m tired.’”
Another common theme that emerged was the challenge of optimal treatment for their narcolepsy. Most patients met with sleep doctors or clinics every 3-6 months. “They said that this was generally good for discussing medications and symptom management, but there didn’t seem to be much time to talk about psychosocial aspects,” Dr. Ong said. “That seemed to be one area of need. There was also a strong dissatisfaction with mental health providers. People talked about how their mental health provider really didn’t understand narcolepsy. It did seem to reduce rapport and the ability to trust their therapist. Some talked about the challenges of accessibility. In some cases, people said their narcolepsy symptoms created challenges with appointment attendance.”
In terms of preferences for a psychosocial intervention, respondents generally “preferred some kind of online or Internet delivery,” he said. “They prefer a team approach with a clinician who’s knowledgeable about both sleep and mental health.”
Dr. Ong acknowledged certain limitations of the study, including its small sample size and the fact that it was not adequately powered to detect differences between type 1 and type 2 narcolepsy.
The study was funded by a grant from Wake Up Narcolepsy. Dr. Ong reported having no relevant financial disclosures.
SOURCE: Ong J et al., SLEEP 2019, abstract 0624.
SAN ANTONIO – Narcolepsy adversely impacts one’s health-related quality of life in a variety of ways, from elevated levels of depression to negative social stigma, results from a mixed methods study suggest.
“Despite established pharmacological treatments to reduce narcolepsy symptoms, health-related quality of life remains poor,” the study’s first author, Jason C. Ong, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “The impact these symptoms have on functioning, the disease burden, and psychosocial functioning in particular is very important. Psychosocial functioning is particularly poor.”
Previous research has shown that people with narcolepsy have two- to four times the rate of psychiatric comorbidities and that health-related stigma is a predictor of depression and poor functioning, said Dr. Ong, a psychologist with the Center for Circadian and Sleep Medicine at the Northwestern University Feinberg School of Medicine, Chicago. In an effort to assess current practices for addressing the psychosocial needs of persons with narcolepsy and to identify potential strategies that could be used to develop a psychosocial intervention, he and his associates conducted a mixed methods study to examine how narcolepsy symptoms impact health-related quality of life and the appropriateness of different health-related quality of life measures for the disorder. “Our long-term goal is to see if we can use this information to help inform the feasibility of a psychosocial intervention to improve health-related quality of life,” he said.
For the study, 29 adults with an established diagnosis of narcolepsy completed online versions of the Patient Health Questionnaire-9 (PHQ-9), the Patient Reported Outcomes Measurement Information System (PROMIS), the 36-item Short Form Survey (SF-36), and the Epworth Sleepiness Scale (ESS). They also participated in a focus group, which consisted of questions pertaining to quality of life for persons with narcolepsy, current practices for addressing psychosocial health of affected individuals, and suggestions for developing a psychosocial intervention. The researchers used thematic analysis to reduce the qualitative data to key themes.
Most of the study participants (93%) were female, 90% were white, their mean age was 31, and their mean time since narcolepsy diagnosis was 4.3 years. Clinically significant elevations on the PROMIS scale, defined as a t-score of greater than 60, were reported for depression (t-score of 64.8), anxiety (66.3), fatigue (68.3), and sleep impairment (66.9). Elevations in depressive symptoms were reported on the PHQ-9 (a mean of 15.79), “which corresponds to moderately severe levels,” Dr. Ong said. “The ESS was highly elevated and fit well with the scales for sleep impairment as well as fatigue on the PROMIS. Overall, there was nice congruence across these measures.”
On the SF-36, the researchers observed that there were deficits in physical and emotional aspects of role limitations, and in energy/fatigue. “One thing we did find was a significant difference in general functioning, where patients with type 1 narcolepsy were worse off than those with type 2 narcolepsy (P less than .05).”
Qualitative data from focus groups revealed several key themes, including the perception that narcolepsy is poorly understood by the public and health care providers.
“People have the perception that if you have narcolepsy, you just feel fine and then you fall asleep,” Dr. Ong said. “They don’t understand that it’s a constant thing. Negative social stigma was also common. As a result, we found a lot of negative impact on self-esteem and self-efficacy. People talked about being hesitant to tell other people about their diagnosis, feeling that they’re ashamed of having narcolepsy. They felt less capable. One person said, ‘I get tired trying to explain why I’m tired.’”
Another common theme that emerged was the challenge of optimal treatment for their narcolepsy. Most patients met with sleep doctors or clinics every 3-6 months. “They said that this was generally good for discussing medications and symptom management, but there didn’t seem to be much time to talk about psychosocial aspects,” Dr. Ong said. “That seemed to be one area of need. There was also a strong dissatisfaction with mental health providers. People talked about how their mental health provider really didn’t understand narcolepsy. It did seem to reduce rapport and the ability to trust their therapist. Some talked about the challenges of accessibility. In some cases, people said their narcolepsy symptoms created challenges with appointment attendance.”
In terms of preferences for a psychosocial intervention, respondents generally “preferred some kind of online or Internet delivery,” he said. “They prefer a team approach with a clinician who’s knowledgeable about both sleep and mental health.”
Dr. Ong acknowledged certain limitations of the study, including its small sample size and the fact that it was not adequately powered to detect differences between type 1 and type 2 narcolepsy.
The study was funded by a grant from Wake Up Narcolepsy. Dr. Ong reported having no relevant financial disclosures.
SOURCE: Ong J et al., SLEEP 2019, abstract 0624.
REPORTING FROM SLEEP 2019
Subset of patients benefits from in-hospital sleep apnea screening
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
EXPERT ANALYSIS FROM SLEEP 2019
Question Marks Lead to Dollar Signs
An employee of a sawmill in Kentucky sustained paralyzing injuries when a large piece of milling equipment struck him in the back. His coworkers took him to the hospital in the back of a pickup truck.
At the time, some of the hospital’s nursing staff were on strike and had been replaced by temporary staff provided by US Nursing Corporation. A female nurse helped load the patient into a wheelchair for transfer into the hospital.
The patient was evaluated for a spinal injury; it was determined that he had sustained an L-3 burst fracture that impinged his spine. He was transferred to another hospital. However, due to the nature of his injuries, he is permanently paralyzed from the waist down.
The plaintiff presented a products liability claim against the machinery manufacturers, which was settled for $3.05 million. He later filed a medical malpractice complaint to include the nursing contractor and 3 individual nurses (1 from the contractor and 2 employed by the hospital). The complaint alleged that the nurses “failed to stabilize and immobilize” the patient when moving him from the pickup truck to the emergency department (ED), which worsened his injuries. A nurse employed by the contractor was identified as the nurse who had transferred him to the wheelchair.
The latter case was litigated for several years. On the eve of trial, the hospital settled for $2 million and the nursing contractor for $1.1 million. However, the hospital brought an indemnification claim against the nursing contractor to recover the $2 million settlement.
At the time of trial, there was a question regarding the identity of the nurse who had transferred the plaintiff from the pickup truck to the wheelchair. The US Nursing Corporation contract nurse contended she did not transfer the plaintiff to the wheelchair. Resolving the uncertainty, the jury concluded that the contract nurse was the nurse who had transferred the plaintiff.
VERDICT
At the conclusion of a 7-day trial, the jury awarded the plaintiff $2,823,522.
Continue to: COMMENTARY
COMMENTARY
Who doesn’t love a good mystery, right? Well, not everyone. Years ago, I was given a gift: a “host your own murder mystery party” game. I recently gave it away when I realized I was statistically more likely to be murdered than ever to host a “murder mystery party.” Love them or hate them, I think you will agree: Mysteries belong in novels or movies or board games. They have no place in your clinical practice.
In litigation, lawyers obsess over trivial details. I’ve attended enough malpractice depositions to see physicians, NPs, PAs, and nurses, with puzzled faces, answering seemingly nonsensical questions that appear to have no bearing on clinical matters. The clinicians respond half amused and half annoyed, through a litany of telephone logs, record access logs, chain-of-custody records, transfer center logs, recorded ambulance communications, time-stamped records, and recollections of who brought a specimen to the lab or what time someone was at the nurses’ station—all peripheral to practice. I understand the quizzical looks and sympathize with providers’ annoyance at having to answer seemingly inane questions. Yet these matters, collateral to practice, can take center stage in a legal case.
These issues form part of the puzzle: the who, what, where, when, why, and how of any case. For example
Who carried a specimen from the operating room (OR)? (Because it was sent from the OR, but the lab has no record of receiving it and knowing the identity of the runner is now key.)
What time did the attending call the hospital to alert the surgical team? (Because precise timing from surgeon’s knowledge to first incision is now at issue.)
Continue to: Where...
Where, specifically, was the culture taken from? (Because there were three wounds, and it turns out later two wounds were from a different source than the third.)
When did scrub tech A clock out of a surgery and scrub tech B clock in? (Because one of the surgical counts was wrong, and a surgical item was retained.)
Why did the patient leave against medical advice? (Because in the ED, he said he “needed to feed his cat.” This wasn’t recorded; the chart only states “patient left AMA.” During litigation, plaintiff claims he left because a nurse told him “it would be better to see your regular doctor.”)
How did a patient get a KFC value meal to eat in his hospital bed when strict oral intake was needed? (Because the hospital’s knowledge of the patient’s dietary intake is now at issue.)
I know—such a list of who, what, etc, can appear cutesy and cloying. Further, some of these trivial details are not recorded by clinicians, so why bring them up? I raise it because in your practice setting, you may be in a position to influence decision-making with regard to recording those minor details, which can become critically important later.
Continue to: In a medical malpractice case...
In a medical malpractice case, every tiny detail is potentially part of the puzzle. If a piece of the puzzle is missing, it becomes a mystery, and a mystery can become a problem. A plaintiff’s lawyer who sees question marks also sees dollar signs.
In this case, the presence of a “mystery nurse” likely kicked up enough dust to confuse the jury. Most clinicians are aware a malpractice plaintiff must prove 4 elements: (1) duty, (2) breach of duty, (3) causation, and (4) harm. The plaintiff must prove all elements by a preponderance of the evidence (ie, greater than 50% likely). Duty and damages are not at issue in this case; there was a clear patient relationship, and the plaintiff is clearly paralyzed. The plaintiff has the burden to prove elements (2) and (3): that there was a breach of the standard of care and that breach caused the plaintiff’s harm.
With respect to element (2), the plaintiff had the burden of showing that the act of putting him into the wheelchair was a breach of the standard of care. I think we’d all agree: The standard of care requires a registered nurse to recognize that a patient struck by a heavy object is at risk for spinal injury and spinal immobilization is required. The patient should have been removed from the vehicle with spinal immobilization techniques.
However, with respect to the causation element, the plaintiff would have been required to prove it was more probable than not (ie, 51% or greater) that the act of putting him into the wheelchair caused the paralysis. This is a stretch. The jury would have to believe it was at least 51% likely that the act of car-to-wheelchair transfer caused the injury—not the heavy mill equipment falling on him in the first place, not the efforts of his coworkers to move him from the scene, not the efforts of his coworkers to load him into the truck, not the bouncy ride in the back of a truck over to the hospital. The plaintiff was able to overcome a big causation hurdle because the identity of the nurse was not known.
The plaintiff would also generally have to show that the coworkers did not mislead the transferring nurse—that is, the statements made at the time of transfer would lead a reasonably skilled nurse to suspect spinal injury, halt transfer attempts, and see to it the patient’s spine was immobilized. Although doubtful, it is possible that in the split seconds when the car arrived at the ED, the initial communications were errant and a reasonable nurse would not have just cause to suspect spinal injury. However, we will never know. We don’t have testimony on what was said during transfer.
Continue to: So we don't know who...
So we don’t know who the nurse was. We don’t know what was said. We don’t know exactly how the plaintiff was transferred out of the vehicle. And those mysteries, to a jury, are suspicious.
IN SUMMARY
Any time a lawyer can draw a giant “?” on a whiteboard during summation, rest assured, someone is in trouble. That someone could be you. I’ve seen lots of question marks in my life; none carry a $1 million/$3 million malpractice policy. The presence of a mystery will transform a case that was defensible into one with unanswered questions. Those unanswered questions open the door to the suggestion or outright accusation of a cover-up. Do your best to document details and work within your system to encourage documentation. In short, don’t let the plaintiff host a mystery party at your expense.
An employee of a sawmill in Kentucky sustained paralyzing injuries when a large piece of milling equipment struck him in the back. His coworkers took him to the hospital in the back of a pickup truck.
At the time, some of the hospital’s nursing staff were on strike and had been replaced by temporary staff provided by US Nursing Corporation. A female nurse helped load the patient into a wheelchair for transfer into the hospital.
The patient was evaluated for a spinal injury; it was determined that he had sustained an L-3 burst fracture that impinged his spine. He was transferred to another hospital. However, due to the nature of his injuries, he is permanently paralyzed from the waist down.
The plaintiff presented a products liability claim against the machinery manufacturers, which was settled for $3.05 million. He later filed a medical malpractice complaint to include the nursing contractor and 3 individual nurses (1 from the contractor and 2 employed by the hospital). The complaint alleged that the nurses “failed to stabilize and immobilize” the patient when moving him from the pickup truck to the emergency department (ED), which worsened his injuries. A nurse employed by the contractor was identified as the nurse who had transferred him to the wheelchair.
The latter case was litigated for several years. On the eve of trial, the hospital settled for $2 million and the nursing contractor for $1.1 million. However, the hospital brought an indemnification claim against the nursing contractor to recover the $2 million settlement.
At the time of trial, there was a question regarding the identity of the nurse who had transferred the plaintiff from the pickup truck to the wheelchair. The US Nursing Corporation contract nurse contended she did not transfer the plaintiff to the wheelchair. Resolving the uncertainty, the jury concluded that the contract nurse was the nurse who had transferred the plaintiff.
VERDICT
At the conclusion of a 7-day trial, the jury awarded the plaintiff $2,823,522.
Continue to: COMMENTARY
COMMENTARY
Who doesn’t love a good mystery, right? Well, not everyone. Years ago, I was given a gift: a “host your own murder mystery party” game. I recently gave it away when I realized I was statistically more likely to be murdered than ever to host a “murder mystery party.” Love them or hate them, I think you will agree: Mysteries belong in novels or movies or board games. They have no place in your clinical practice.
In litigation, lawyers obsess over trivial details. I’ve attended enough malpractice depositions to see physicians, NPs, PAs, and nurses, with puzzled faces, answering seemingly nonsensical questions that appear to have no bearing on clinical matters. The clinicians respond half amused and half annoyed, through a litany of telephone logs, record access logs, chain-of-custody records, transfer center logs, recorded ambulance communications, time-stamped records, and recollections of who brought a specimen to the lab or what time someone was at the nurses’ station—all peripheral to practice. I understand the quizzical looks and sympathize with providers’ annoyance at having to answer seemingly inane questions. Yet these matters, collateral to practice, can take center stage in a legal case.
These issues form part of the puzzle: the who, what, where, when, why, and how of any case. For example
Who carried a specimen from the operating room (OR)? (Because it was sent from the OR, but the lab has no record of receiving it and knowing the identity of the runner is now key.)
What time did the attending call the hospital to alert the surgical team? (Because precise timing from surgeon’s knowledge to first incision is now at issue.)
Continue to: Where...
Where, specifically, was the culture taken from? (Because there were three wounds, and it turns out later two wounds were from a different source than the third.)
When did scrub tech A clock out of a surgery and scrub tech B clock in? (Because one of the surgical counts was wrong, and a surgical item was retained.)
Why did the patient leave against medical advice? (Because in the ED, he said he “needed to feed his cat.” This wasn’t recorded; the chart only states “patient left AMA.” During litigation, plaintiff claims he left because a nurse told him “it would be better to see your regular doctor.”)
How did a patient get a KFC value meal to eat in his hospital bed when strict oral intake was needed? (Because the hospital’s knowledge of the patient’s dietary intake is now at issue.)
I know—such a list of who, what, etc, can appear cutesy and cloying. Further, some of these trivial details are not recorded by clinicians, so why bring them up? I raise it because in your practice setting, you may be in a position to influence decision-making with regard to recording those minor details, which can become critically important later.
Continue to: In a medical malpractice case...
In a medical malpractice case, every tiny detail is potentially part of the puzzle. If a piece of the puzzle is missing, it becomes a mystery, and a mystery can become a problem. A plaintiff’s lawyer who sees question marks also sees dollar signs.
In this case, the presence of a “mystery nurse” likely kicked up enough dust to confuse the jury. Most clinicians are aware a malpractice plaintiff must prove 4 elements: (1) duty, (2) breach of duty, (3) causation, and (4) harm. The plaintiff must prove all elements by a preponderance of the evidence (ie, greater than 50% likely). Duty and damages are not at issue in this case; there was a clear patient relationship, and the plaintiff is clearly paralyzed. The plaintiff has the burden to prove elements (2) and (3): that there was a breach of the standard of care and that breach caused the plaintiff’s harm.
With respect to element (2), the plaintiff had the burden of showing that the act of putting him into the wheelchair was a breach of the standard of care. I think we’d all agree: The standard of care requires a registered nurse to recognize that a patient struck by a heavy object is at risk for spinal injury and spinal immobilization is required. The patient should have been removed from the vehicle with spinal immobilization techniques.
However, with respect to the causation element, the plaintiff would have been required to prove it was more probable than not (ie, 51% or greater) that the act of putting him into the wheelchair caused the paralysis. This is a stretch. The jury would have to believe it was at least 51% likely that the act of car-to-wheelchair transfer caused the injury—not the heavy mill equipment falling on him in the first place, not the efforts of his coworkers to move him from the scene, not the efforts of his coworkers to load him into the truck, not the bouncy ride in the back of a truck over to the hospital. The plaintiff was able to overcome a big causation hurdle because the identity of the nurse was not known.
The plaintiff would also generally have to show that the coworkers did not mislead the transferring nurse—that is, the statements made at the time of transfer would lead a reasonably skilled nurse to suspect spinal injury, halt transfer attempts, and see to it the patient’s spine was immobilized. Although doubtful, it is possible that in the split seconds when the car arrived at the ED, the initial communications were errant and a reasonable nurse would not have just cause to suspect spinal injury. However, we will never know. We don’t have testimony on what was said during transfer.
Continue to: So we don't know who...
So we don’t know who the nurse was. We don’t know what was said. We don’t know exactly how the plaintiff was transferred out of the vehicle. And those mysteries, to a jury, are suspicious.
IN SUMMARY
Any time a lawyer can draw a giant “?” on a whiteboard during summation, rest assured, someone is in trouble. That someone could be you. I’ve seen lots of question marks in my life; none carry a $1 million/$3 million malpractice policy. The presence of a mystery will transform a case that was defensible into one with unanswered questions. Those unanswered questions open the door to the suggestion or outright accusation of a cover-up. Do your best to document details and work within your system to encourage documentation. In short, don’t let the plaintiff host a mystery party at your expense.
An employee of a sawmill in Kentucky sustained paralyzing injuries when a large piece of milling equipment struck him in the back. His coworkers took him to the hospital in the back of a pickup truck.
At the time, some of the hospital’s nursing staff were on strike and had been replaced by temporary staff provided by US Nursing Corporation. A female nurse helped load the patient into a wheelchair for transfer into the hospital.
The patient was evaluated for a spinal injury; it was determined that he had sustained an L-3 burst fracture that impinged his spine. He was transferred to another hospital. However, due to the nature of his injuries, he is permanently paralyzed from the waist down.
The plaintiff presented a products liability claim against the machinery manufacturers, which was settled for $3.05 million. He later filed a medical malpractice complaint to include the nursing contractor and 3 individual nurses (1 from the contractor and 2 employed by the hospital). The complaint alleged that the nurses “failed to stabilize and immobilize” the patient when moving him from the pickup truck to the emergency department (ED), which worsened his injuries. A nurse employed by the contractor was identified as the nurse who had transferred him to the wheelchair.
The latter case was litigated for several years. On the eve of trial, the hospital settled for $2 million and the nursing contractor for $1.1 million. However, the hospital brought an indemnification claim against the nursing contractor to recover the $2 million settlement.
At the time of trial, there was a question regarding the identity of the nurse who had transferred the plaintiff from the pickup truck to the wheelchair. The US Nursing Corporation contract nurse contended she did not transfer the plaintiff to the wheelchair. Resolving the uncertainty, the jury concluded that the contract nurse was the nurse who had transferred the plaintiff.
VERDICT
At the conclusion of a 7-day trial, the jury awarded the plaintiff $2,823,522.
Continue to: COMMENTARY
COMMENTARY
Who doesn’t love a good mystery, right? Well, not everyone. Years ago, I was given a gift: a “host your own murder mystery party” game. I recently gave it away when I realized I was statistically more likely to be murdered than ever to host a “murder mystery party.” Love them or hate them, I think you will agree: Mysteries belong in novels or movies or board games. They have no place in your clinical practice.
In litigation, lawyers obsess over trivial details. I’ve attended enough malpractice depositions to see physicians, NPs, PAs, and nurses, with puzzled faces, answering seemingly nonsensical questions that appear to have no bearing on clinical matters. The clinicians respond half amused and half annoyed, through a litany of telephone logs, record access logs, chain-of-custody records, transfer center logs, recorded ambulance communications, time-stamped records, and recollections of who brought a specimen to the lab or what time someone was at the nurses’ station—all peripheral to practice. I understand the quizzical looks and sympathize with providers’ annoyance at having to answer seemingly inane questions. Yet these matters, collateral to practice, can take center stage in a legal case.
These issues form part of the puzzle: the who, what, where, when, why, and how of any case. For example
Who carried a specimen from the operating room (OR)? (Because it was sent from the OR, but the lab has no record of receiving it and knowing the identity of the runner is now key.)
What time did the attending call the hospital to alert the surgical team? (Because precise timing from surgeon’s knowledge to first incision is now at issue.)
Continue to: Where...
Where, specifically, was the culture taken from? (Because there were three wounds, and it turns out later two wounds were from a different source than the third.)
When did scrub tech A clock out of a surgery and scrub tech B clock in? (Because one of the surgical counts was wrong, and a surgical item was retained.)
Why did the patient leave against medical advice? (Because in the ED, he said he “needed to feed his cat.” This wasn’t recorded; the chart only states “patient left AMA.” During litigation, plaintiff claims he left because a nurse told him “it would be better to see your regular doctor.”)
How did a patient get a KFC value meal to eat in his hospital bed when strict oral intake was needed? (Because the hospital’s knowledge of the patient’s dietary intake is now at issue.)
I know—such a list of who, what, etc, can appear cutesy and cloying. Further, some of these trivial details are not recorded by clinicians, so why bring them up? I raise it because in your practice setting, you may be in a position to influence decision-making with regard to recording those minor details, which can become critically important later.
Continue to: In a medical malpractice case...
In a medical malpractice case, every tiny detail is potentially part of the puzzle. If a piece of the puzzle is missing, it becomes a mystery, and a mystery can become a problem. A plaintiff’s lawyer who sees question marks also sees dollar signs.
In this case, the presence of a “mystery nurse” likely kicked up enough dust to confuse the jury. Most clinicians are aware a malpractice plaintiff must prove 4 elements: (1) duty, (2) breach of duty, (3) causation, and (4) harm. The plaintiff must prove all elements by a preponderance of the evidence (ie, greater than 50% likely). Duty and damages are not at issue in this case; there was a clear patient relationship, and the plaintiff is clearly paralyzed. The plaintiff has the burden to prove elements (2) and (3): that there was a breach of the standard of care and that breach caused the plaintiff’s harm.
With respect to element (2), the plaintiff had the burden of showing that the act of putting him into the wheelchair was a breach of the standard of care. I think we’d all agree: The standard of care requires a registered nurse to recognize that a patient struck by a heavy object is at risk for spinal injury and spinal immobilization is required. The patient should have been removed from the vehicle with spinal immobilization techniques.
However, with respect to the causation element, the plaintiff would have been required to prove it was more probable than not (ie, 51% or greater) that the act of putting him into the wheelchair caused the paralysis. This is a stretch. The jury would have to believe it was at least 51% likely that the act of car-to-wheelchair transfer caused the injury—not the heavy mill equipment falling on him in the first place, not the efforts of his coworkers to move him from the scene, not the efforts of his coworkers to load him into the truck, not the bouncy ride in the back of a truck over to the hospital. The plaintiff was able to overcome a big causation hurdle because the identity of the nurse was not known.
The plaintiff would also generally have to show that the coworkers did not mislead the transferring nurse—that is, the statements made at the time of transfer would lead a reasonably skilled nurse to suspect spinal injury, halt transfer attempts, and see to it the patient’s spine was immobilized. Although doubtful, it is possible that in the split seconds when the car arrived at the ED, the initial communications were errant and a reasonable nurse would not have just cause to suspect spinal injury. However, we will never know. We don’t have testimony on what was said during transfer.
Continue to: So we don't know who...
So we don’t know who the nurse was. We don’t know what was said. We don’t know exactly how the plaintiff was transferred out of the vehicle. And those mysteries, to a jury, are suspicious.
IN SUMMARY
Any time a lawyer can draw a giant “?” on a whiteboard during summation, rest assured, someone is in trouble. That someone could be you. I’ve seen lots of question marks in my life; none carry a $1 million/$3 million malpractice policy. The presence of a mystery will transform a case that was defensible into one with unanswered questions. Those unanswered questions open the door to the suggestion or outright accusation of a cover-up. Do your best to document details and work within your system to encourage documentation. In short, don’t let the plaintiff host a mystery party at your expense.
Imaging predicts early postural instability in Parkinson’s disease
PHILADELPHIA – Diffusion-weighted MRI and the presence of at least five of seven clinical features may prove useful for determining which newly diagnosed patients with Parkinson’s disease are likely to have rapidly progressive disease, Frank M. Skidmore, MD, reported at the annual meeting of the American Academy of Neurology.
Patients with gray matter and axonal disease on initial imaging were found to have more aggressive disease associated with early gait dysfunction than were patients with primarily white matter and axonal disease, said Dr. Skidmore, associate professor of neurology at the University of Alabama, Birmingham.
Diffusion-weighted imaging provides a way to assess cellular fluid partitioning and directional information in gray and white matter. Thus, it has the potential to identify brainstem pathology that is associated with disease progression, he said. “Our approach provides a pathway towards using MR to detect early, prognostic, neurodegenerative changes in diseases of the brain.”
Dr. Skidmore and colleagues performed diffusion-weighted imaging on 101 patients with newly diagnosed Parkinson’s disease and 56 healthy controls. They found that Parkinson’s disease was associated with altered radial diffusion in white matter. Changes were observed mainly in the striatonigral tract and the substantia nigra. The investigators also noted atrophy in the cerebellar peduncle among patients with Parkinson’s disease.
At baseline, the patients who went on to have subsequent development of early postural instability and gait dysfunction had decreased intracellular fluid partitioning in the substantia nigra and the mesencephalic locomotor region, which are predominantly gray matter regions. These participants had a lower orientation diffusion index (ODI) and a lower estimate of cellularity, Dr. Skidmore said.
The researchers defined early gait dysfunction as the achievement of a Hoehn and Yahr score of 3 at least once while on medication during the first 5 years after Parkinson’s disease diagnosis. Follow-up was at least 5 years in 79 of the patients.
To identify clinical features associated with early postural instability and gait difficulty, the investigators examined data for 301 patients. In this population, Dr. Skidmore and colleagues identified 218 patients whose Hoehn and Yahr scores never exceeded 2 and 83 patients with at least one Hoehn and Yahr score of 3 or more. Using Bonferroni correction, they examined Unified Parkinson’s Disease Rating Scale (UPDRS) data for all patients to identify significant differences between these two groups. Seven items distinguished patients who developed early postural instability and gait difficulty. They included lightheadedness, fatigue, difficulty walking, ability to rise from a chair, and postural problems. The seven-item scale was superior to the Unified Parkinson’s Disease Rating Scale (UPDRS) at predicting which newly diagnosed patients would develop early postural and gait difficulties
SOURCE: Skidmore F et al. AANN 2019, Abstract S41.004.
PHILADELPHIA – Diffusion-weighted MRI and the presence of at least five of seven clinical features may prove useful for determining which newly diagnosed patients with Parkinson’s disease are likely to have rapidly progressive disease, Frank M. Skidmore, MD, reported at the annual meeting of the American Academy of Neurology.
Patients with gray matter and axonal disease on initial imaging were found to have more aggressive disease associated with early gait dysfunction than were patients with primarily white matter and axonal disease, said Dr. Skidmore, associate professor of neurology at the University of Alabama, Birmingham.
Diffusion-weighted imaging provides a way to assess cellular fluid partitioning and directional information in gray and white matter. Thus, it has the potential to identify brainstem pathology that is associated with disease progression, he said. “Our approach provides a pathway towards using MR to detect early, prognostic, neurodegenerative changes in diseases of the brain.”
Dr. Skidmore and colleagues performed diffusion-weighted imaging on 101 patients with newly diagnosed Parkinson’s disease and 56 healthy controls. They found that Parkinson’s disease was associated with altered radial diffusion in white matter. Changes were observed mainly in the striatonigral tract and the substantia nigra. The investigators also noted atrophy in the cerebellar peduncle among patients with Parkinson’s disease.
At baseline, the patients who went on to have subsequent development of early postural instability and gait dysfunction had decreased intracellular fluid partitioning in the substantia nigra and the mesencephalic locomotor region, which are predominantly gray matter regions. These participants had a lower orientation diffusion index (ODI) and a lower estimate of cellularity, Dr. Skidmore said.
The researchers defined early gait dysfunction as the achievement of a Hoehn and Yahr score of 3 at least once while on medication during the first 5 years after Parkinson’s disease diagnosis. Follow-up was at least 5 years in 79 of the patients.
To identify clinical features associated with early postural instability and gait difficulty, the investigators examined data for 301 patients. In this population, Dr. Skidmore and colleagues identified 218 patients whose Hoehn and Yahr scores never exceeded 2 and 83 patients with at least one Hoehn and Yahr score of 3 or more. Using Bonferroni correction, they examined Unified Parkinson’s Disease Rating Scale (UPDRS) data for all patients to identify significant differences between these two groups. Seven items distinguished patients who developed early postural instability and gait difficulty. They included lightheadedness, fatigue, difficulty walking, ability to rise from a chair, and postural problems. The seven-item scale was superior to the Unified Parkinson’s Disease Rating Scale (UPDRS) at predicting which newly diagnosed patients would develop early postural and gait difficulties
SOURCE: Skidmore F et al. AANN 2019, Abstract S41.004.
PHILADELPHIA – Diffusion-weighted MRI and the presence of at least five of seven clinical features may prove useful for determining which newly diagnosed patients with Parkinson’s disease are likely to have rapidly progressive disease, Frank M. Skidmore, MD, reported at the annual meeting of the American Academy of Neurology.
Patients with gray matter and axonal disease on initial imaging were found to have more aggressive disease associated with early gait dysfunction than were patients with primarily white matter and axonal disease, said Dr. Skidmore, associate professor of neurology at the University of Alabama, Birmingham.
Diffusion-weighted imaging provides a way to assess cellular fluid partitioning and directional information in gray and white matter. Thus, it has the potential to identify brainstem pathology that is associated with disease progression, he said. “Our approach provides a pathway towards using MR to detect early, prognostic, neurodegenerative changes in diseases of the brain.”
Dr. Skidmore and colleagues performed diffusion-weighted imaging on 101 patients with newly diagnosed Parkinson’s disease and 56 healthy controls. They found that Parkinson’s disease was associated with altered radial diffusion in white matter. Changes were observed mainly in the striatonigral tract and the substantia nigra. The investigators also noted atrophy in the cerebellar peduncle among patients with Parkinson’s disease.
At baseline, the patients who went on to have subsequent development of early postural instability and gait dysfunction had decreased intracellular fluid partitioning in the substantia nigra and the mesencephalic locomotor region, which are predominantly gray matter regions. These participants had a lower orientation diffusion index (ODI) and a lower estimate of cellularity, Dr. Skidmore said.
The researchers defined early gait dysfunction as the achievement of a Hoehn and Yahr score of 3 at least once while on medication during the first 5 years after Parkinson’s disease diagnosis. Follow-up was at least 5 years in 79 of the patients.
To identify clinical features associated with early postural instability and gait difficulty, the investigators examined data for 301 patients. In this population, Dr. Skidmore and colleagues identified 218 patients whose Hoehn and Yahr scores never exceeded 2 and 83 patients with at least one Hoehn and Yahr score of 3 or more. Using Bonferroni correction, they examined Unified Parkinson’s Disease Rating Scale (UPDRS) data for all patients to identify significant differences between these two groups. Seven items distinguished patients who developed early postural instability and gait difficulty. They included lightheadedness, fatigue, difficulty walking, ability to rise from a chair, and postural problems. The seven-item scale was superior to the Unified Parkinson’s Disease Rating Scale (UPDRS) at predicting which newly diagnosed patients would develop early postural and gait difficulties
SOURCE: Skidmore F et al. AANN 2019, Abstract S41.004.
REPORTING FROM AAN 2019
Opioid use curbed with patient education and lower prescription quantities
Patients given lower prescription quantities of opioid tablets with and without opioid education used significantly less of the medication compared with those given more tablets and no education, according to data from 264 adults and adolescents who underwent anterior cruciate ligament (ACL) surgery.
Although lower default prescription programs have been shown to reduce the number of tablets prescribed, “the effect of reduced prescription quantities on actual patient opioid consumption remains undetermined,” wrote Kevin X. Farley, BS, of Emory University, Atlanta, and colleagues.
In a study published in JAMA, the researchers examined whether patients took fewer tablets if given fewer, and whether patient education about opioids further reduced the number of tablets taken.
The study population included adults and adolescents who underwent ACL surgery at a single center. The patients were divided into three groups: 109 patients received 50 opioid tablets after surgery, 78 received 30 tablets plus education prior to surgery about appropriate opioid use and alternative pain management, and 77 received 30 tablets but no education on opioid use.
Patients given 50 tablets consumed an average of 25 tablets for an average of 5.8 days. By contrast, patients given 30 tablets but no opioid education consumed an average of 16 tablets for an average of 4.5 days, and those given 30 tablets and preoperative education consumed an average of 12 tablets for an average of 3.5 days.
In addition, patients given 30 tablets reported lower levels of constipation and fatigue compared with patients given 50 tablets. No differences were seen in medication refills among the groups.
The findings were limited by several factors including the use of data from a single center, the lack of randomization, and the potential for recall bias, the researchers noted. However, the results suggest that prescribing fewer tablets may further reduce use, as each group consumed approximately half of the tablets given, the researchers added.
“Further investigation should evaluate whether similar opioid stewardship and education protocols would be successful in other patient populations,” they said.
Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
Patients given lower prescription quantities of opioid tablets with and without opioid education used significantly less of the medication compared with those given more tablets and no education, according to data from 264 adults and adolescents who underwent anterior cruciate ligament (ACL) surgery.
Although lower default prescription programs have been shown to reduce the number of tablets prescribed, “the effect of reduced prescription quantities on actual patient opioid consumption remains undetermined,” wrote Kevin X. Farley, BS, of Emory University, Atlanta, and colleagues.
In a study published in JAMA, the researchers examined whether patients took fewer tablets if given fewer, and whether patient education about opioids further reduced the number of tablets taken.
The study population included adults and adolescents who underwent ACL surgery at a single center. The patients were divided into three groups: 109 patients received 50 opioid tablets after surgery, 78 received 30 tablets plus education prior to surgery about appropriate opioid use and alternative pain management, and 77 received 30 tablets but no education on opioid use.
Patients given 50 tablets consumed an average of 25 tablets for an average of 5.8 days. By contrast, patients given 30 tablets but no opioid education consumed an average of 16 tablets for an average of 4.5 days, and those given 30 tablets and preoperative education consumed an average of 12 tablets for an average of 3.5 days.
In addition, patients given 30 tablets reported lower levels of constipation and fatigue compared with patients given 50 tablets. No differences were seen in medication refills among the groups.
The findings were limited by several factors including the use of data from a single center, the lack of randomization, and the potential for recall bias, the researchers noted. However, the results suggest that prescribing fewer tablets may further reduce use, as each group consumed approximately half of the tablets given, the researchers added.
“Further investigation should evaluate whether similar opioid stewardship and education protocols would be successful in other patient populations,” they said.
Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
Patients given lower prescription quantities of opioid tablets with and without opioid education used significantly less of the medication compared with those given more tablets and no education, according to data from 264 adults and adolescents who underwent anterior cruciate ligament (ACL) surgery.
Although lower default prescription programs have been shown to reduce the number of tablets prescribed, “the effect of reduced prescription quantities on actual patient opioid consumption remains undetermined,” wrote Kevin X. Farley, BS, of Emory University, Atlanta, and colleagues.
In a study published in JAMA, the researchers examined whether patients took fewer tablets if given fewer, and whether patient education about opioids further reduced the number of tablets taken.
The study population included adults and adolescents who underwent ACL surgery at a single center. The patients were divided into three groups: 109 patients received 50 opioid tablets after surgery, 78 received 30 tablets plus education prior to surgery about appropriate opioid use and alternative pain management, and 77 received 30 tablets but no education on opioid use.
Patients given 50 tablets consumed an average of 25 tablets for an average of 5.8 days. By contrast, patients given 30 tablets but no opioid education consumed an average of 16 tablets for an average of 4.5 days, and those given 30 tablets and preoperative education consumed an average of 12 tablets for an average of 3.5 days.
In addition, patients given 30 tablets reported lower levels of constipation and fatigue compared with patients given 50 tablets. No differences were seen in medication refills among the groups.
The findings were limited by several factors including the use of data from a single center, the lack of randomization, and the potential for recall bias, the researchers noted. However, the results suggest that prescribing fewer tablets may further reduce use, as each group consumed approximately half of the tablets given, the researchers added.
“Further investigation should evaluate whether similar opioid stewardship and education protocols would be successful in other patient populations,” they said.
Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
FROM JAMA
Key clinical point: Patient education and fewer tablets prescribed significantly reduced the amount of opioid tablets taken compared with no education and more tablets prescribed.
Major finding: Patients given 50 tablets and no patient education, 30 tablets and no patient education, and 30 tablets plus education consumed an average of 25, 16, and 12 tablets, respectively.
Study details: The data come from 264 adolescents and adults who underwent ACL surgery at a single center.
Disclosures: Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
Source: Farley KX et al. JAMA. 2019 June 25.321(24):2465-7.
Anticholinergic drugs linked to dementia in older populations
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
FROM JAMA INTERNAL MEDICINE
Food insecurity tied to migraine in young adults
, according to Jason M. Nagata, MD, of the University of California, San Francisco, and associates.
Data were collected from a cross-sectional, nationally representative set of 14,786 young adults in the United States aged 24-32 years who participated in the 2008 National Longitudinal Study of Adolescent to Adult Health, the investigators wrote in a research letter published in JAMA Neurology.
Food insecurity was assessed by self-report through the interview question, “In the past 12 months, was there a time when (you/your household were/was) worried whether food would run out before you would get money to buy more?” Migraine was assessed by a positive answer to the interview question, “Has a doctor, nurse, or other health care professional ever told you that you have or had migraine headaches?”
In all, 1,647 study participants (11%) reported food insecurity; the prevalence of migraine in this group was 23.9%, compared with a prevalence of 13.6% in participants who did not report food insecurity. The association between food insecurity and migraine was significant both before (odds ratio, 2.00; 95% confidence interval, 1.68-2.38; P less than .001) and after adjustment (adjusted OR, .58; 95% CI, 1.30-1.95; P less than .001).
“Health care clinicians caring for persons who experience migraine should consider screening for food insecurity as a potential contributor to migraine exacerbations and provide referrals to programs such as the Supplemental Nutrition Assistance Program [formerly the Food Stamp Program] when appropriate,” the investigators concluded (JAMA Neurol. 2019 Jun 24. doi: 10.1001/jamaneurol.2019.1663).
No conflicts of interest were reported. The study was supported by grants from the University of California Global Food Initiative Fellowship, the American Academy of Pediatrics, the American Pediatric Society, and the Norman Schlossberger Research Fund from the University of California.
, according to Jason M. Nagata, MD, of the University of California, San Francisco, and associates.
Data were collected from a cross-sectional, nationally representative set of 14,786 young adults in the United States aged 24-32 years who participated in the 2008 National Longitudinal Study of Adolescent to Adult Health, the investigators wrote in a research letter published in JAMA Neurology.
Food insecurity was assessed by self-report through the interview question, “In the past 12 months, was there a time when (you/your household were/was) worried whether food would run out before you would get money to buy more?” Migraine was assessed by a positive answer to the interview question, “Has a doctor, nurse, or other health care professional ever told you that you have or had migraine headaches?”
In all, 1,647 study participants (11%) reported food insecurity; the prevalence of migraine in this group was 23.9%, compared with a prevalence of 13.6% in participants who did not report food insecurity. The association between food insecurity and migraine was significant both before (odds ratio, 2.00; 95% confidence interval, 1.68-2.38; P less than .001) and after adjustment (adjusted OR, .58; 95% CI, 1.30-1.95; P less than .001).
“Health care clinicians caring for persons who experience migraine should consider screening for food insecurity as a potential contributor to migraine exacerbations and provide referrals to programs such as the Supplemental Nutrition Assistance Program [formerly the Food Stamp Program] when appropriate,” the investigators concluded (JAMA Neurol. 2019 Jun 24. doi: 10.1001/jamaneurol.2019.1663).
No conflicts of interest were reported. The study was supported by grants from the University of California Global Food Initiative Fellowship, the American Academy of Pediatrics, the American Pediatric Society, and the Norman Schlossberger Research Fund from the University of California.
, according to Jason M. Nagata, MD, of the University of California, San Francisco, and associates.
Data were collected from a cross-sectional, nationally representative set of 14,786 young adults in the United States aged 24-32 years who participated in the 2008 National Longitudinal Study of Adolescent to Adult Health, the investigators wrote in a research letter published in JAMA Neurology.
Food insecurity was assessed by self-report through the interview question, “In the past 12 months, was there a time when (you/your household were/was) worried whether food would run out before you would get money to buy more?” Migraine was assessed by a positive answer to the interview question, “Has a doctor, nurse, or other health care professional ever told you that you have or had migraine headaches?”
In all, 1,647 study participants (11%) reported food insecurity; the prevalence of migraine in this group was 23.9%, compared with a prevalence of 13.6% in participants who did not report food insecurity. The association between food insecurity and migraine was significant both before (odds ratio, 2.00; 95% confidence interval, 1.68-2.38; P less than .001) and after adjustment (adjusted OR, .58; 95% CI, 1.30-1.95; P less than .001).
“Health care clinicians caring for persons who experience migraine should consider screening for food insecurity as a potential contributor to migraine exacerbations and provide referrals to programs such as the Supplemental Nutrition Assistance Program [formerly the Food Stamp Program] when appropriate,” the investigators concluded (JAMA Neurol. 2019 Jun 24. doi: 10.1001/jamaneurol.2019.1663).
No conflicts of interest were reported. The study was supported by grants from the University of California Global Food Initiative Fellowship, the American Academy of Pediatrics, the American Pediatric Society, and the Norman Schlossberger Research Fund from the University of California.
FROM JAMA NEUROLOGY
Mindfulness-based stress reduction reduces migraine frequency
SAN ANTONIO – Episodic migraine patients benefit from mindfulness-based stress reduction training, according to new research. The intervention reduced headache frequency, slightly increased whole-brain gray matter volume, and reduced symptoms of anxiety, depression, and stress.
The gray matter findings may indicate opportunities for therapeutic targets, while the psychosocial findings are important in understanding migraine burden, treatment response, and personalized medicine opportunities, Shana Burrowes, PhD, a postdoctoral associate at Boston University, said at the annual meeting of the College on Problems of Drug Dependence.
In a session focused on exploring alternatives to opioids for pain treatment, Dr. Burrowes described interim results of a randomized, controlled trial testing the effectiveness of mindfulness-based stress reduction (MBSR) training for managing migraine.
In discussing the rationale for study endpoints, she explained a three-pronged model for understanding migraine. Those elements include the symptoms themselves – unilateral throbbing pain, nausea, and photophobia – and the psychosocial symptoms and comorbidities, including anxiety, depression, stress, and catastrophizing. Up to 30%* of migraine patients have comorbid depression.
Those two prongs have a bidirectional relationship, since each increases the risk of the other. For example, frequent migraine can leave people feeling anxious about when their next migraine will occur, and that anxiety can increase the risk of it occurring.
Both elements lead to the third prong, which is change in gray matter volume. “If you’re a patient with migraine, an MRI on your brain is going to look different from somebody who does not have migraine,” Dr. Burrowes said. “With all these things going on in a patient, a migraine patient is actually pretty difficult to treat.”
Therefore, the researchers focused on outcomes from each of these three domains: gray matter volume in MRI; headache frequency as a clinical outcome; and the psychosocial comorbidities of anxiety, stress, and depression.
Study participants included 98 patients with episodic migraine, defined as fewer than 15 headache days a month, and 27 controls* matched by demographics to the patients and without any chronic pain conditions. The groups were 92% female and had similar ratios of whites (75% and 77%) and college graduates (95% and 96%).
Only the patients were randomized to the two interventions, one a training on MBSR and the other focusing on stress management for headache (SMH).
The MBSR training involved group sessions, eight 2.5-hour meditation sessions, at-home practice, a half-day retreat, and then an additional four biweekly sessions. The mindfulness training specifically focused on intentionally paying attention in the moment without judgment. The SMH arm focused on education for managing headache symptoms, stress, sleep hygiene, and diet, but it did not involve any specific skills training, such as relaxation training.
All participants, including healthy controls, underwent clinical assessment and baseline MRI and psychosocial questionnaires, followed by MRI and psychosocial questionnaire follow-ups at 3 and 6 months. MRI imaging focused on the whole brain and on the bilateral insula, dorsolateral prefrontal cortex, anterior cingulate cortex, and superior frontal gyrus. Patients also kept headache diaries throughout the trial.
Both intervention groups showed an increase in gray matter volume over 6 months, compared with healthy controls: 1.3% in the whole brain for SMH participants and 1.01% in the MBSR patients, compared with –1.37% in healthy participants. In the right superior frontal gyrus, gray matter volume also increased 2.62% in SMH participants and 1.25% in MBSR patients but decreased 0.19% in healthy participants.
Dr. Burrowes said she could not share specific findings on headache frequency and psychosocial outcomes because her team’s research is currently under review. Overall, however, headache frequency declined more than 50% post intervention, and 39% of migraine patients responded to the therapy.
In addition, anxiety, stress, and depression symptoms all saw improvements from MBSR and slightly but significantly mediated the effect of MBSR on migraine reduction.
Dr. Burrowes reported having no disclosures.
*The story was updated 6/20/2019.
SAN ANTONIO – Episodic migraine patients benefit from mindfulness-based stress reduction training, according to new research. The intervention reduced headache frequency, slightly increased whole-brain gray matter volume, and reduced symptoms of anxiety, depression, and stress.
The gray matter findings may indicate opportunities for therapeutic targets, while the psychosocial findings are important in understanding migraine burden, treatment response, and personalized medicine opportunities, Shana Burrowes, PhD, a postdoctoral associate at Boston University, said at the annual meeting of the College on Problems of Drug Dependence.
In a session focused on exploring alternatives to opioids for pain treatment, Dr. Burrowes described interim results of a randomized, controlled trial testing the effectiveness of mindfulness-based stress reduction (MBSR) training for managing migraine.
In discussing the rationale for study endpoints, she explained a three-pronged model for understanding migraine. Those elements include the symptoms themselves – unilateral throbbing pain, nausea, and photophobia – and the psychosocial symptoms and comorbidities, including anxiety, depression, stress, and catastrophizing. Up to 30%* of migraine patients have comorbid depression.
Those two prongs have a bidirectional relationship, since each increases the risk of the other. For example, frequent migraine can leave people feeling anxious about when their next migraine will occur, and that anxiety can increase the risk of it occurring.
Both elements lead to the third prong, which is change in gray matter volume. “If you’re a patient with migraine, an MRI on your brain is going to look different from somebody who does not have migraine,” Dr. Burrowes said. “With all these things going on in a patient, a migraine patient is actually pretty difficult to treat.”
Therefore, the researchers focused on outcomes from each of these three domains: gray matter volume in MRI; headache frequency as a clinical outcome; and the psychosocial comorbidities of anxiety, stress, and depression.
Study participants included 98 patients with episodic migraine, defined as fewer than 15 headache days a month, and 27 controls* matched by demographics to the patients and without any chronic pain conditions. The groups were 92% female and had similar ratios of whites (75% and 77%) and college graduates (95% and 96%).
Only the patients were randomized to the two interventions, one a training on MBSR and the other focusing on stress management for headache (SMH).
The MBSR training involved group sessions, eight 2.5-hour meditation sessions, at-home practice, a half-day retreat, and then an additional four biweekly sessions. The mindfulness training specifically focused on intentionally paying attention in the moment without judgment. The SMH arm focused on education for managing headache symptoms, stress, sleep hygiene, and diet, but it did not involve any specific skills training, such as relaxation training.
All participants, including healthy controls, underwent clinical assessment and baseline MRI and psychosocial questionnaires, followed by MRI and psychosocial questionnaire follow-ups at 3 and 6 months. MRI imaging focused on the whole brain and on the bilateral insula, dorsolateral prefrontal cortex, anterior cingulate cortex, and superior frontal gyrus. Patients also kept headache diaries throughout the trial.
Both intervention groups showed an increase in gray matter volume over 6 months, compared with healthy controls: 1.3% in the whole brain for SMH participants and 1.01% in the MBSR patients, compared with –1.37% in healthy participants. In the right superior frontal gyrus, gray matter volume also increased 2.62% in SMH participants and 1.25% in MBSR patients but decreased 0.19% in healthy participants.
Dr. Burrowes said she could not share specific findings on headache frequency and psychosocial outcomes because her team’s research is currently under review. Overall, however, headache frequency declined more than 50% post intervention, and 39% of migraine patients responded to the therapy.
In addition, anxiety, stress, and depression symptoms all saw improvements from MBSR and slightly but significantly mediated the effect of MBSR on migraine reduction.
Dr. Burrowes reported having no disclosures.
*The story was updated 6/20/2019.
SAN ANTONIO – Episodic migraine patients benefit from mindfulness-based stress reduction training, according to new research. The intervention reduced headache frequency, slightly increased whole-brain gray matter volume, and reduced symptoms of anxiety, depression, and stress.
The gray matter findings may indicate opportunities for therapeutic targets, while the psychosocial findings are important in understanding migraine burden, treatment response, and personalized medicine opportunities, Shana Burrowes, PhD, a postdoctoral associate at Boston University, said at the annual meeting of the College on Problems of Drug Dependence.
In a session focused on exploring alternatives to opioids for pain treatment, Dr. Burrowes described interim results of a randomized, controlled trial testing the effectiveness of mindfulness-based stress reduction (MBSR) training for managing migraine.
In discussing the rationale for study endpoints, she explained a three-pronged model for understanding migraine. Those elements include the symptoms themselves – unilateral throbbing pain, nausea, and photophobia – and the psychosocial symptoms and comorbidities, including anxiety, depression, stress, and catastrophizing. Up to 30%* of migraine patients have comorbid depression.
Those two prongs have a bidirectional relationship, since each increases the risk of the other. For example, frequent migraine can leave people feeling anxious about when their next migraine will occur, and that anxiety can increase the risk of it occurring.
Both elements lead to the third prong, which is change in gray matter volume. “If you’re a patient with migraine, an MRI on your brain is going to look different from somebody who does not have migraine,” Dr. Burrowes said. “With all these things going on in a patient, a migraine patient is actually pretty difficult to treat.”
Therefore, the researchers focused on outcomes from each of these three domains: gray matter volume in MRI; headache frequency as a clinical outcome; and the psychosocial comorbidities of anxiety, stress, and depression.
Study participants included 98 patients with episodic migraine, defined as fewer than 15 headache days a month, and 27 controls* matched by demographics to the patients and without any chronic pain conditions. The groups were 92% female and had similar ratios of whites (75% and 77%) and college graduates (95% and 96%).
Only the patients were randomized to the two interventions, one a training on MBSR and the other focusing on stress management for headache (SMH).
The MBSR training involved group sessions, eight 2.5-hour meditation sessions, at-home practice, a half-day retreat, and then an additional four biweekly sessions. The mindfulness training specifically focused on intentionally paying attention in the moment without judgment. The SMH arm focused on education for managing headache symptoms, stress, sleep hygiene, and diet, but it did not involve any specific skills training, such as relaxation training.
All participants, including healthy controls, underwent clinical assessment and baseline MRI and psychosocial questionnaires, followed by MRI and psychosocial questionnaire follow-ups at 3 and 6 months. MRI imaging focused on the whole brain and on the bilateral insula, dorsolateral prefrontal cortex, anterior cingulate cortex, and superior frontal gyrus. Patients also kept headache diaries throughout the trial.
Both intervention groups showed an increase in gray matter volume over 6 months, compared with healthy controls: 1.3% in the whole brain for SMH participants and 1.01% in the MBSR patients, compared with –1.37% in healthy participants. In the right superior frontal gyrus, gray matter volume also increased 2.62% in SMH participants and 1.25% in MBSR patients but decreased 0.19% in healthy participants.
Dr. Burrowes said she could not share specific findings on headache frequency and psychosocial outcomes because her team’s research is currently under review. Overall, however, headache frequency declined more than 50% post intervention, and 39% of migraine patients responded to the therapy.
In addition, anxiety, stress, and depression symptoms all saw improvements from MBSR and slightly but significantly mediated the effect of MBSR on migraine reduction.
Dr. Burrowes reported having no disclosures.
*The story was updated 6/20/2019.
REPORTING FROM CPDD 2019