User login
Mild TBI may predict PTSD, not postconcussion syndrome
Patients with certain symptoms after mild traumatic brain injury should be considered to have a form of posttraumatic stress disorder, rather than postconcussion syndrome, a prospective study of more than 1,300 patients shows.
Several published diagnostic criteria exist for postconcussion syndrome (PCS), but it is defined in the DSM-IV as a head trauma causing concussion followed by cognitive problems and at least three of eight symptoms – among them headache, dizziness, and personality change – lasting 3 months or longer. Several studies have questioned the specificity of the PCS diagnosis, finding overlap of symptoms among patients with head injuries and other types of injuries. And as traumatic brain injury is strongly associated with PTSD, researchers have suggested that certain symptoms thought to be indicative of brain injury might instead reflect stress reactions stemming from trauma.
In a study published online July 16 in JAMA Psychiatry (doi:10.1001/jamapsychiatry.2014.666), Emmanuel Lagarde, Ph.D., of the INSERM Research Center in Bordeaux, France, and colleagues, analyzed data from 1,361 patients recruited from a hospital emergency department, of whom 534 had head injuries and 827 had injuries to other parts of the body. At 3 months’ follow-up, 21.2% of the mild traumatic brain injury (MTBI) patients and 16.3% of controls without head injuries could be diagnosed with PCS based on the DSM-IV criteria.
Moreover, 8.8% of patients with head injuries fulfilled diagnostic criteria for PTSD, compared with only 2.2% of controls. In the study group as a whole, MTBI was seen as a strong predictor of PTSD (odds ratio 4.47; 95% CI 2.38-8.4) but not of PCS (OR, 1.13; 95% CI 0.82-1.55). MTBI predicted PTSD regardless of whether the injury occurred as a result of a road crash, assault, or fall.
Assault was seen as a predictor of PCS as defined by DSM-IV, while severity of injury was not, suggesting that "psychological stress, and not potential brain injury, causes these symptoms, reinforcing the idea that they should be considered part of PTSD and not PCS," the researchers wrote.
They also found that symptoms suggestive of PCS under the DSM-IV criteria clustered in a way that paralleled a group of PTSD symptoms categorized as hyperarousal.
"The rationale to define a PCS that is specific to head-trauma patients is weak," Dr. Lagarde and colleagues wrote in their analysis. "Our results also suggested that the misunderstanding related to the relevance of defining such a syndrome could be explained by the overlapping pattern with symptoms of the PTSD hyperarousal dimension."
Available evidence does not support the use of PCS, they concluded, adding that clinicians should consider PTSD risk and treatment for patients with MTBI.
Dr. Lagarde and colleagues acknowledged as limitations of their study the fact that PTSD diagnoses were not made through formal clinical exams and that researchers focused on the traumatic events that landed subjects in the emergency department without capturing information on past traumatic events.
INSERM, the REUNICA Group, and Bordeaux University Hospital funded the study. None of the authors disclosed conflicts of interest.
Patients with certain symptoms after mild traumatic brain injury should be considered to have a form of posttraumatic stress disorder, rather than postconcussion syndrome, a prospective study of more than 1,300 patients shows.
Several published diagnostic criteria exist for postconcussion syndrome (PCS), but it is defined in the DSM-IV as a head trauma causing concussion followed by cognitive problems and at least three of eight symptoms – among them headache, dizziness, and personality change – lasting 3 months or longer. Several studies have questioned the specificity of the PCS diagnosis, finding overlap of symptoms among patients with head injuries and other types of injuries. And as traumatic brain injury is strongly associated with PTSD, researchers have suggested that certain symptoms thought to be indicative of brain injury might instead reflect stress reactions stemming from trauma.
In a study published online July 16 in JAMA Psychiatry (doi:10.1001/jamapsychiatry.2014.666), Emmanuel Lagarde, Ph.D., of the INSERM Research Center in Bordeaux, France, and colleagues, analyzed data from 1,361 patients recruited from a hospital emergency department, of whom 534 had head injuries and 827 had injuries to other parts of the body. At 3 months’ follow-up, 21.2% of the mild traumatic brain injury (MTBI) patients and 16.3% of controls without head injuries could be diagnosed with PCS based on the DSM-IV criteria.
Moreover, 8.8% of patients with head injuries fulfilled diagnostic criteria for PTSD, compared with only 2.2% of controls. In the study group as a whole, MTBI was seen as a strong predictor of PTSD (odds ratio 4.47; 95% CI 2.38-8.4) but not of PCS (OR, 1.13; 95% CI 0.82-1.55). MTBI predicted PTSD regardless of whether the injury occurred as a result of a road crash, assault, or fall.
Assault was seen as a predictor of PCS as defined by DSM-IV, while severity of injury was not, suggesting that "psychological stress, and not potential brain injury, causes these symptoms, reinforcing the idea that they should be considered part of PTSD and not PCS," the researchers wrote.
They also found that symptoms suggestive of PCS under the DSM-IV criteria clustered in a way that paralleled a group of PTSD symptoms categorized as hyperarousal.
"The rationale to define a PCS that is specific to head-trauma patients is weak," Dr. Lagarde and colleagues wrote in their analysis. "Our results also suggested that the misunderstanding related to the relevance of defining such a syndrome could be explained by the overlapping pattern with symptoms of the PTSD hyperarousal dimension."
Available evidence does not support the use of PCS, they concluded, adding that clinicians should consider PTSD risk and treatment for patients with MTBI.
Dr. Lagarde and colleagues acknowledged as limitations of their study the fact that PTSD diagnoses were not made through formal clinical exams and that researchers focused on the traumatic events that landed subjects in the emergency department without capturing information on past traumatic events.
INSERM, the REUNICA Group, and Bordeaux University Hospital funded the study. None of the authors disclosed conflicts of interest.
Patients with certain symptoms after mild traumatic brain injury should be considered to have a form of posttraumatic stress disorder, rather than postconcussion syndrome, a prospective study of more than 1,300 patients shows.
Several published diagnostic criteria exist for postconcussion syndrome (PCS), but it is defined in the DSM-IV as a head trauma causing concussion followed by cognitive problems and at least three of eight symptoms – among them headache, dizziness, and personality change – lasting 3 months or longer. Several studies have questioned the specificity of the PCS diagnosis, finding overlap of symptoms among patients with head injuries and other types of injuries. And as traumatic brain injury is strongly associated with PTSD, researchers have suggested that certain symptoms thought to be indicative of brain injury might instead reflect stress reactions stemming from trauma.
In a study published online July 16 in JAMA Psychiatry (doi:10.1001/jamapsychiatry.2014.666), Emmanuel Lagarde, Ph.D., of the INSERM Research Center in Bordeaux, France, and colleagues, analyzed data from 1,361 patients recruited from a hospital emergency department, of whom 534 had head injuries and 827 had injuries to other parts of the body. At 3 months’ follow-up, 21.2% of the mild traumatic brain injury (MTBI) patients and 16.3% of controls without head injuries could be diagnosed with PCS based on the DSM-IV criteria.
Moreover, 8.8% of patients with head injuries fulfilled diagnostic criteria for PTSD, compared with only 2.2% of controls. In the study group as a whole, MTBI was seen as a strong predictor of PTSD (odds ratio 4.47; 95% CI 2.38-8.4) but not of PCS (OR, 1.13; 95% CI 0.82-1.55). MTBI predicted PTSD regardless of whether the injury occurred as a result of a road crash, assault, or fall.
Assault was seen as a predictor of PCS as defined by DSM-IV, while severity of injury was not, suggesting that "psychological stress, and not potential brain injury, causes these symptoms, reinforcing the idea that they should be considered part of PTSD and not PCS," the researchers wrote.
They also found that symptoms suggestive of PCS under the DSM-IV criteria clustered in a way that paralleled a group of PTSD symptoms categorized as hyperarousal.
"The rationale to define a PCS that is specific to head-trauma patients is weak," Dr. Lagarde and colleagues wrote in their analysis. "Our results also suggested that the misunderstanding related to the relevance of defining such a syndrome could be explained by the overlapping pattern with symptoms of the PTSD hyperarousal dimension."
Available evidence does not support the use of PCS, they concluded, adding that clinicians should consider PTSD risk and treatment for patients with MTBI.
Dr. Lagarde and colleagues acknowledged as limitations of their study the fact that PTSD diagnoses were not made through formal clinical exams and that researchers focused on the traumatic events that landed subjects in the emergency department without capturing information on past traumatic events.
INSERM, the REUNICA Group, and Bordeaux University Hospital funded the study. None of the authors disclosed conflicts of interest.
FROM JAMA PSYCHIATRY
Key clinical point: Head trauma often occurs within the context of a psychologically distressing event.
Major finding: Injured patients with persistent symptoms 3 months after a traumatic event should be considered as having the hyperarousal dimension of PTSD rather than postconcussion syndrome.
Data source: A prospective study of 1,361 patients with mild traumatic injuries who were recruited from a hospital emergency department from Dec. 4, 2007, to Feb. 25, 2009.
Disclosures: INSERM, the REUNICA Group, and Bordeaux University Hospital funded the study. None of the authors disclosed conflicts of interest.
Position paper on sports-related concussion sets the right tone
The American Academy of Neurology’s position paper on the evaluation and management of sports-related concussion states a belief in the ethical obligation of the treating physician to make sure an athlete is ready to return to play following a concussion and that he or she is educated and protected from what could be a serious neurological injury.
As a practicing sports neurologist for the past 30 years, I agree with the stance that the AAN has taken, and I have found in my own practice that while the injured player and coaches are often anxious for the injured player to get back on the field, it is imperative for the player sit out until he or she has been given the okay by a qualified physician.
In my work with athletes from different sports and of all ages – as an adviser for Pop Warner football up through working on the NFL Players Association Mackey-White Traumatic Brain Injury Committee – the care of the patient has always been and will always be my greatest concern. Contact and collision sports, like football, hockey, and boxing, will never be completely safe, but that doesn’t lessen our responsibility as physicians to shield players from unnecessary injury and to ensure they are well enough to continue the sport.
If a player returns to the game before he is ready, he raises the likelihood of sustaining a second concussion, which is most likely to occur within 10 days of the initial concussion. He also increases his risk of "second-impact syndrome." While this is rare, its effects can be disastrous: traumatic injury and even death has occurred. A concussion is not just a "bump on the head," and everyone involved in a player’s care should take that seriously.
The AAN statement also supports the inclusion of concussion evaluation and training for neurology residency programs. Education on this particular issue is paramount in the fight to keep athletes safe. Making sure that all neurologists have the proper tools and are kept abreast of any changes to concussion management guidelines and testing will continue to ensure players’ safety.
Education for athletes, coaches, athletic trainers, parents, and the community in general also is critical. Once they know and understand the signs and symptoms – and the impact – of a concussion, the easier it will be for everyone to be on board with keeping an athlete out of play until she has been cleared by her doctor. It’s also important to have a better understanding of the scope of the problem and its impact. To that end, the AAN’s idea to create a mandatory national concussion registry is definitely a step in the right direction and could be an integral way to gain a better understanding of the issue at hand.
No matter whether a player has dedicated his life to the sport or if he is in his first Pop Warner season, it will always be tough to tell him that he has to sit out for one or more games. (And, for the younger set, that is often difficult for parents to hear.) But it is the ethical obligation of a physician to make sure a player has no visible signs or symptoms and is cognitively ready to return to play. That, combined with education and knowledge on the subject, as the AAN recommends, will help neurologists and other physicians throughout the country keep players safe and limit the number of concussions and their impact on today’s athletes.
Dr. Jordan is the director of the brain injury program and the memory evaluation treatment service at Burke Rehabilitation Hospital in White Plains, N.Y. He also serves as the assistant medical director there. He currently serves as the chief medical officer of the New York State Athletic Commission, as a team physician for USA Boxing, and as a member of the NFL Players Association Mackey-White Traumatic Brain Injury Committee and the NFL Neuro-Cognitive Disability Committee.
The American Academy of Neurology’s position paper on the evaluation and management of sports-related concussion states a belief in the ethical obligation of the treating physician to make sure an athlete is ready to return to play following a concussion and that he or she is educated and protected from what could be a serious neurological injury.
As a practicing sports neurologist for the past 30 years, I agree with the stance that the AAN has taken, and I have found in my own practice that while the injured player and coaches are often anxious for the injured player to get back on the field, it is imperative for the player sit out until he or she has been given the okay by a qualified physician.
In my work with athletes from different sports and of all ages – as an adviser for Pop Warner football up through working on the NFL Players Association Mackey-White Traumatic Brain Injury Committee – the care of the patient has always been and will always be my greatest concern. Contact and collision sports, like football, hockey, and boxing, will never be completely safe, but that doesn’t lessen our responsibility as physicians to shield players from unnecessary injury and to ensure they are well enough to continue the sport.
If a player returns to the game before he is ready, he raises the likelihood of sustaining a second concussion, which is most likely to occur within 10 days of the initial concussion. He also increases his risk of "second-impact syndrome." While this is rare, its effects can be disastrous: traumatic injury and even death has occurred. A concussion is not just a "bump on the head," and everyone involved in a player’s care should take that seriously.
The AAN statement also supports the inclusion of concussion evaluation and training for neurology residency programs. Education on this particular issue is paramount in the fight to keep athletes safe. Making sure that all neurologists have the proper tools and are kept abreast of any changes to concussion management guidelines and testing will continue to ensure players’ safety.
Education for athletes, coaches, athletic trainers, parents, and the community in general also is critical. Once they know and understand the signs and symptoms – and the impact – of a concussion, the easier it will be for everyone to be on board with keeping an athlete out of play until she has been cleared by her doctor. It’s also important to have a better understanding of the scope of the problem and its impact. To that end, the AAN’s idea to create a mandatory national concussion registry is definitely a step in the right direction and could be an integral way to gain a better understanding of the issue at hand.
No matter whether a player has dedicated his life to the sport or if he is in his first Pop Warner season, it will always be tough to tell him that he has to sit out for one or more games. (And, for the younger set, that is often difficult for parents to hear.) But it is the ethical obligation of a physician to make sure a player has no visible signs or symptoms and is cognitively ready to return to play. That, combined with education and knowledge on the subject, as the AAN recommends, will help neurologists and other physicians throughout the country keep players safe and limit the number of concussions and their impact on today’s athletes.
Dr. Jordan is the director of the brain injury program and the memory evaluation treatment service at Burke Rehabilitation Hospital in White Plains, N.Y. He also serves as the assistant medical director there. He currently serves as the chief medical officer of the New York State Athletic Commission, as a team physician for USA Boxing, and as a member of the NFL Players Association Mackey-White Traumatic Brain Injury Committee and the NFL Neuro-Cognitive Disability Committee.
The American Academy of Neurology’s position paper on the evaluation and management of sports-related concussion states a belief in the ethical obligation of the treating physician to make sure an athlete is ready to return to play following a concussion and that he or she is educated and protected from what could be a serious neurological injury.
As a practicing sports neurologist for the past 30 years, I agree with the stance that the AAN has taken, and I have found in my own practice that while the injured player and coaches are often anxious for the injured player to get back on the field, it is imperative for the player sit out until he or she has been given the okay by a qualified physician.
In my work with athletes from different sports and of all ages – as an adviser for Pop Warner football up through working on the NFL Players Association Mackey-White Traumatic Brain Injury Committee – the care of the patient has always been and will always be my greatest concern. Contact and collision sports, like football, hockey, and boxing, will never be completely safe, but that doesn’t lessen our responsibility as physicians to shield players from unnecessary injury and to ensure they are well enough to continue the sport.
If a player returns to the game before he is ready, he raises the likelihood of sustaining a second concussion, which is most likely to occur within 10 days of the initial concussion. He also increases his risk of "second-impact syndrome." While this is rare, its effects can be disastrous: traumatic injury and even death has occurred. A concussion is not just a "bump on the head," and everyone involved in a player’s care should take that seriously.
The AAN statement also supports the inclusion of concussion evaluation and training for neurology residency programs. Education on this particular issue is paramount in the fight to keep athletes safe. Making sure that all neurologists have the proper tools and are kept abreast of any changes to concussion management guidelines and testing will continue to ensure players’ safety.
Education for athletes, coaches, athletic trainers, parents, and the community in general also is critical. Once they know and understand the signs and symptoms – and the impact – of a concussion, the easier it will be for everyone to be on board with keeping an athlete out of play until she has been cleared by her doctor. It’s also important to have a better understanding of the scope of the problem and its impact. To that end, the AAN’s idea to create a mandatory national concussion registry is definitely a step in the right direction and could be an integral way to gain a better understanding of the issue at hand.
No matter whether a player has dedicated his life to the sport or if he is in his first Pop Warner season, it will always be tough to tell him that he has to sit out for one or more games. (And, for the younger set, that is often difficult for parents to hear.) But it is the ethical obligation of a physician to make sure a player has no visible signs or symptoms and is cognitively ready to return to play. That, combined with education and knowledge on the subject, as the AAN recommends, will help neurologists and other physicians throughout the country keep players safe and limit the number of concussions and their impact on today’s athletes.
Dr. Jordan is the director of the brain injury program and the memory evaluation treatment service at Burke Rehabilitation Hospital in White Plains, N.Y. He also serves as the assistant medical director there. He currently serves as the chief medical officer of the New York State Athletic Commission, as a team physician for USA Boxing, and as a member of the NFL Players Association Mackey-White Traumatic Brain Injury Committee and the NFL Neuro-Cognitive Disability Committee.
Return-to-play decisions a key challenge in management of sports-related concussion
Privacy laws can present a challenge to physicians managing athletes with concussion, particularly if those athletes push to return to play against the physician’s advice, but waivers may avoid this challenge, according to the authors of a position paper on sports-related concussion.
"Evaluating and managing sports-related concussion raises a variety of distinctive ethical and legal issues for physicians, especially relating to return-to-play decisions," Dr. Matthew P. Kirschen of the Children’s Hospital of Philadelphia and his colleagues wrote in Neurology July 9.
Lack of training is also a major issue in sports-related concussion, with a previous survey by the American Academy of Neurology finding that while most neurologists do see patients with sports-related concussion, few have had formal or informal training on managing concussion.
One of the most challenging components of managing sports-related concussion is the decision about when the athlete can return to play, which can be problematic if the athlete-patient wants to return to play prematurely.
Athletes may ignore their physician’s advice or even "doctor shop" for a physician who will approve their return to play, which may bring the physician into conflict with privacy laws restricting the sharing of personal health information without the patient’s consent.
"Thus, the evaluating physician could find himself or herself in the difficult position of being legally restricted from sharing a concussion evaluation with the athlete’s coaches and school personnel, even though making such a disclosure might be in the best interest of the athlete’s health," the authors wrote in the document, which is an official position paper of the Ethics, Law, and Humanities Committee, a joint committee of the American Academy of Neurology, American Neurological Association, and the Child Neurology Society (Neurology 2014 July 9 [doi:10.1212/WNL.0000000000000613]).
In response, some institutions now require athletes to sign waivers, allowing personal health information to be shared between the physician affiliated with the school department and the coaches and other team or school staff.
While all 50 states have adopted youth sports concussion laws addressing the three main components of education, removal from play, and return to play, statutes differ over who is authorized to clear an athlete to return to the field – some specify a physician while others allow athletic trainers, nurse practitioners, and physician assistants to make the decision. "States do not uniformly require that individuals providing clearance be trained in the evaluation and management of concussion," the authors reported.
However, the authors of the report stressed that physicians responsible for the care of athletes, either on or off the sidelines, should ensure they have appropriate training and experience in recognizing, evaluating, and managing concussion and potential brain injury.
Fortunately, state-based youth sports concussion laws generally have a low "removal from play" threshold to protect young athletes from harm, which the authors said should encourage coaches, parents, and athletes to take the risks of concussion more seriously.
In an editorial commentDr. Ellen Deibert of Wellspan Neurology, York, Pa., said discussion of sports-related concussion was timely, with the Centers for Disease Control and Prevention recently estimating around 1.6-3.8 million sports and recreation-related concussions each year, which has skyrocketed from the previous annual estimate of 300,000.
"Overall, the article is a refreshing reminder of the issues surrounding the treatment of sports-related concussion and the need for continued education and research on this topic," Dr. Deibert wrote.
The position paper authors call for the establishment of a concussion registry to improve understanding of the condition. Such a registry "would need to be interdisciplinary and in collaboration with other subspecialists already involved in concussion management. The role the neurologist plays will eventually be defined during that process. However, in 2014, there remains an immediate need for providers to treat concussion patients. The only question you need to answer is what your role will be in supporting this effort," she said.
Several authors of the paper reported authorship honorariums, royalties, and editorial positions. The editorial author declared no relevant conflicts of interest.
Privacy laws can present a challenge to physicians managing athletes with concussion, particularly if those athletes push to return to play against the physician’s advice, but waivers may avoid this challenge, according to the authors of a position paper on sports-related concussion.
"Evaluating and managing sports-related concussion raises a variety of distinctive ethical and legal issues for physicians, especially relating to return-to-play decisions," Dr. Matthew P. Kirschen of the Children’s Hospital of Philadelphia and his colleagues wrote in Neurology July 9.
Lack of training is also a major issue in sports-related concussion, with a previous survey by the American Academy of Neurology finding that while most neurologists do see patients with sports-related concussion, few have had formal or informal training on managing concussion.
One of the most challenging components of managing sports-related concussion is the decision about when the athlete can return to play, which can be problematic if the athlete-patient wants to return to play prematurely.
Athletes may ignore their physician’s advice or even "doctor shop" for a physician who will approve their return to play, which may bring the physician into conflict with privacy laws restricting the sharing of personal health information without the patient’s consent.
"Thus, the evaluating physician could find himself or herself in the difficult position of being legally restricted from sharing a concussion evaluation with the athlete’s coaches and school personnel, even though making such a disclosure might be in the best interest of the athlete’s health," the authors wrote in the document, which is an official position paper of the Ethics, Law, and Humanities Committee, a joint committee of the American Academy of Neurology, American Neurological Association, and the Child Neurology Society (Neurology 2014 July 9 [doi:10.1212/WNL.0000000000000613]).
In response, some institutions now require athletes to sign waivers, allowing personal health information to be shared between the physician affiliated with the school department and the coaches and other team or school staff.
While all 50 states have adopted youth sports concussion laws addressing the three main components of education, removal from play, and return to play, statutes differ over who is authorized to clear an athlete to return to the field – some specify a physician while others allow athletic trainers, nurse practitioners, and physician assistants to make the decision. "States do not uniformly require that individuals providing clearance be trained in the evaluation and management of concussion," the authors reported.
However, the authors of the report stressed that physicians responsible for the care of athletes, either on or off the sidelines, should ensure they have appropriate training and experience in recognizing, evaluating, and managing concussion and potential brain injury.
Fortunately, state-based youth sports concussion laws generally have a low "removal from play" threshold to protect young athletes from harm, which the authors said should encourage coaches, parents, and athletes to take the risks of concussion more seriously.
In an editorial commentDr. Ellen Deibert of Wellspan Neurology, York, Pa., said discussion of sports-related concussion was timely, with the Centers for Disease Control and Prevention recently estimating around 1.6-3.8 million sports and recreation-related concussions each year, which has skyrocketed from the previous annual estimate of 300,000.
"Overall, the article is a refreshing reminder of the issues surrounding the treatment of sports-related concussion and the need for continued education and research on this topic," Dr. Deibert wrote.
The position paper authors call for the establishment of a concussion registry to improve understanding of the condition. Such a registry "would need to be interdisciplinary and in collaboration with other subspecialists already involved in concussion management. The role the neurologist plays will eventually be defined during that process. However, in 2014, there remains an immediate need for providers to treat concussion patients. The only question you need to answer is what your role will be in supporting this effort," she said.
Several authors of the paper reported authorship honorariums, royalties, and editorial positions. The editorial author declared no relevant conflicts of interest.
Privacy laws can present a challenge to physicians managing athletes with concussion, particularly if those athletes push to return to play against the physician’s advice, but waivers may avoid this challenge, according to the authors of a position paper on sports-related concussion.
"Evaluating and managing sports-related concussion raises a variety of distinctive ethical and legal issues for physicians, especially relating to return-to-play decisions," Dr. Matthew P. Kirschen of the Children’s Hospital of Philadelphia and his colleagues wrote in Neurology July 9.
Lack of training is also a major issue in sports-related concussion, with a previous survey by the American Academy of Neurology finding that while most neurologists do see patients with sports-related concussion, few have had formal or informal training on managing concussion.
One of the most challenging components of managing sports-related concussion is the decision about when the athlete can return to play, which can be problematic if the athlete-patient wants to return to play prematurely.
Athletes may ignore their physician’s advice or even "doctor shop" for a physician who will approve their return to play, which may bring the physician into conflict with privacy laws restricting the sharing of personal health information without the patient’s consent.
"Thus, the evaluating physician could find himself or herself in the difficult position of being legally restricted from sharing a concussion evaluation with the athlete’s coaches and school personnel, even though making such a disclosure might be in the best interest of the athlete’s health," the authors wrote in the document, which is an official position paper of the Ethics, Law, and Humanities Committee, a joint committee of the American Academy of Neurology, American Neurological Association, and the Child Neurology Society (Neurology 2014 July 9 [doi:10.1212/WNL.0000000000000613]).
In response, some institutions now require athletes to sign waivers, allowing personal health information to be shared between the physician affiliated with the school department and the coaches and other team or school staff.
While all 50 states have adopted youth sports concussion laws addressing the three main components of education, removal from play, and return to play, statutes differ over who is authorized to clear an athlete to return to the field – some specify a physician while others allow athletic trainers, nurse practitioners, and physician assistants to make the decision. "States do not uniformly require that individuals providing clearance be trained in the evaluation and management of concussion," the authors reported.
However, the authors of the report stressed that physicians responsible for the care of athletes, either on or off the sidelines, should ensure they have appropriate training and experience in recognizing, evaluating, and managing concussion and potential brain injury.
Fortunately, state-based youth sports concussion laws generally have a low "removal from play" threshold to protect young athletes from harm, which the authors said should encourage coaches, parents, and athletes to take the risks of concussion more seriously.
In an editorial commentDr. Ellen Deibert of Wellspan Neurology, York, Pa., said discussion of sports-related concussion was timely, with the Centers for Disease Control and Prevention recently estimating around 1.6-3.8 million sports and recreation-related concussions each year, which has skyrocketed from the previous annual estimate of 300,000.
"Overall, the article is a refreshing reminder of the issues surrounding the treatment of sports-related concussion and the need for continued education and research on this topic," Dr. Deibert wrote.
The position paper authors call for the establishment of a concussion registry to improve understanding of the condition. Such a registry "would need to be interdisciplinary and in collaboration with other subspecialists already involved in concussion management. The role the neurologist plays will eventually be defined during that process. However, in 2014, there remains an immediate need for providers to treat concussion patients. The only question you need to answer is what your role will be in supporting this effort," she said.
Several authors of the paper reported authorship honorariums, royalties, and editorial positions. The editorial author declared no relevant conflicts of interest.
FROM NEUROLOGY
FDA clears marketing of robotic exoskeleton for at-home use
The Food and Drug Administration has cleared the marketing of the ReWalk Personal System, thereby for the first time enabling individuals with lower-body paralysis because of a spinal cord injury to use a wearable motorized exoskeleton at home.
The robotic device consists of a fitted, metal brace worn over the legs and part of the upper body and is controlled by a wireless remote. It accommodates individuals with heights from 5’3" to 6’3" and weighs up to 220 pounds.
ReWalk costs $69,500 and is currently not covered by insurance, although company officials are in talks with insurance companies, according to a spokesperson. The company also says that the potential medical benefits of using the device will offset the cost by reducing hospitalization and some medications.
ReWalk Personal System is not the only motorized exoskeleton available in the United States, but so far, ReWalk, along with similar (but different) exoskeleton systems such as Ekso Bionics, have been mainly used in rehabilitation facilities and Veterans Affairs hospitals.
"Innovative devices such as ReWalk go a long way towards helping individuals with spinal cord injuries gain some mobility," Christy Foreman, director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health, said in a written statement. "Along with physical therapy, training, and assistance from a caregiver, these individuals may be able to use these devices to walk again in their homes and in their communities."
Individuals with paraplegia due to spinal cord injuries at level T7 (seventh thoracic vertebra) to L5 (fifth lumbar vertebra), can use the device when accompanied by a specially trained caregiver, according to the FDA.
People with spinal cord injury at levels T4 (fourth thoracic vertebra) to T6 (sixth thoracic vertebra) can also use the device, but only in rehab facilities, according to the FDA. The device is not for sports or climbing stairs.
Consumers and their caregivers, who could be a spouse or home health aid, must successfully complete a training program designed by ReWalk at a rehabilitation center or a VA hospital. Physicians evaluate the individual’s skill level based on training before writing a prescription for the device.
But, according to the FDA, prior to being trained to use ReWalk:
• Patients should be able to stand using an assistive standing device (e.g., standing frame), and their hands and shoulders should be able to support crutches or a walker.
• Patients should not use the device if they have a history of severe neurologic injuries other than spinal cord injury, or have severe spasticity, significant contractures, unstable spine, unhealed limb fractures, or pelvic fractures.
• Patients should also not use the device if they have severe concurrent medical diseases such as infection, circulatory conditions, heart or lung conditions, or pressure sores.
The ReWalk Personal System consists of motors that help with movement of hips, knees, and ankles, enabling people to sit, stand, turn and walk, with assistance from a trained person. The device has a tilt sensor, and its computer and power supply are contained in a backpack. Patients use crutches, and they control the device with a wireless remote control worn on the wrist. The device weighs 46 pounds, but the user feels mostly the weight of the backpack, which is about 5 pounds, according to the company.
"The person walks the system, the system does not walk them," Dr. Amit Goffer, the Israeli inventor of ReWalk who became quadriplegic after an ATV accident in 1997, said in a company news release. "The users are in control – when they want to sit, they sit, when then want to stand and walk, they do so."
The device, manufactured by Argo Medical Technologies, has been in use in Europe, Israel, and other countries. The rehab version of ReWalk became available internationally in 2011, while sales of the personal system began in 2012.
It is not clear when another brand of exoskeletons will be cleared for marketing by the FDA. "We’re excited that the FDA has opened this gateway and look forward to seeing how the market reacts," said a spokesperson for Ekso Bionics, noting that the company is currently focused on the rehabilitation market, but some individuals use the device at home with a physical therapist and in clinical studies conducted by the company.
The ReWalk personal system was reviewed through FDA’s de novo classification process, used for novel, first-of-its-kind medical devices that are low to moderate risk. The agency cleared the device after reviewing its durability, hardware, software, and battery system, in addition to assessing clinical data from a 30-subject study and a 16-patient observational study.
The company reports that "Study data of the ReWalk system indicate potential improvements in cardiovascular health, loss of fat tissue, building of lean muscle mass, and improved bowel function. Feedback from ReWalk users supports these potential benefits and others, such as better pain management, fewer medications, and potentially reduced hospitalizations."
The device’s manufacturer is required to complete a postmarket clinical study to collect data on adverse events related to device use and to assess the adequacy of its training program, according to the FDA.
On Twitter @naseemmiller
The Food and Drug Administration has cleared the marketing of the ReWalk Personal System, thereby for the first time enabling individuals with lower-body paralysis because of a spinal cord injury to use a wearable motorized exoskeleton at home.
The robotic device consists of a fitted, metal brace worn over the legs and part of the upper body and is controlled by a wireless remote. It accommodates individuals with heights from 5’3" to 6’3" and weighs up to 220 pounds.
ReWalk costs $69,500 and is currently not covered by insurance, although company officials are in talks with insurance companies, according to a spokesperson. The company also says that the potential medical benefits of using the device will offset the cost by reducing hospitalization and some medications.
ReWalk Personal System is not the only motorized exoskeleton available in the United States, but so far, ReWalk, along with similar (but different) exoskeleton systems such as Ekso Bionics, have been mainly used in rehabilitation facilities and Veterans Affairs hospitals.
"Innovative devices such as ReWalk go a long way towards helping individuals with spinal cord injuries gain some mobility," Christy Foreman, director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health, said in a written statement. "Along with physical therapy, training, and assistance from a caregiver, these individuals may be able to use these devices to walk again in their homes and in their communities."
Individuals with paraplegia due to spinal cord injuries at level T7 (seventh thoracic vertebra) to L5 (fifth lumbar vertebra), can use the device when accompanied by a specially trained caregiver, according to the FDA.
People with spinal cord injury at levels T4 (fourth thoracic vertebra) to T6 (sixth thoracic vertebra) can also use the device, but only in rehab facilities, according to the FDA. The device is not for sports or climbing stairs.
Consumers and their caregivers, who could be a spouse or home health aid, must successfully complete a training program designed by ReWalk at a rehabilitation center or a VA hospital. Physicians evaluate the individual’s skill level based on training before writing a prescription for the device.
But, according to the FDA, prior to being trained to use ReWalk:
• Patients should be able to stand using an assistive standing device (e.g., standing frame), and their hands and shoulders should be able to support crutches or a walker.
• Patients should not use the device if they have a history of severe neurologic injuries other than spinal cord injury, or have severe spasticity, significant contractures, unstable spine, unhealed limb fractures, or pelvic fractures.
• Patients should also not use the device if they have severe concurrent medical diseases such as infection, circulatory conditions, heart or lung conditions, or pressure sores.
The ReWalk Personal System consists of motors that help with movement of hips, knees, and ankles, enabling people to sit, stand, turn and walk, with assistance from a trained person. The device has a tilt sensor, and its computer and power supply are contained in a backpack. Patients use crutches, and they control the device with a wireless remote control worn on the wrist. The device weighs 46 pounds, but the user feels mostly the weight of the backpack, which is about 5 pounds, according to the company.
"The person walks the system, the system does not walk them," Dr. Amit Goffer, the Israeli inventor of ReWalk who became quadriplegic after an ATV accident in 1997, said in a company news release. "The users are in control – when they want to sit, they sit, when then want to stand and walk, they do so."
The device, manufactured by Argo Medical Technologies, has been in use in Europe, Israel, and other countries. The rehab version of ReWalk became available internationally in 2011, while sales of the personal system began in 2012.
It is not clear when another brand of exoskeletons will be cleared for marketing by the FDA. "We’re excited that the FDA has opened this gateway and look forward to seeing how the market reacts," said a spokesperson for Ekso Bionics, noting that the company is currently focused on the rehabilitation market, but some individuals use the device at home with a physical therapist and in clinical studies conducted by the company.
The ReWalk personal system was reviewed through FDA’s de novo classification process, used for novel, first-of-its-kind medical devices that are low to moderate risk. The agency cleared the device after reviewing its durability, hardware, software, and battery system, in addition to assessing clinical data from a 30-subject study and a 16-patient observational study.
The company reports that "Study data of the ReWalk system indicate potential improvements in cardiovascular health, loss of fat tissue, building of lean muscle mass, and improved bowel function. Feedback from ReWalk users supports these potential benefits and others, such as better pain management, fewer medications, and potentially reduced hospitalizations."
The device’s manufacturer is required to complete a postmarket clinical study to collect data on adverse events related to device use and to assess the adequacy of its training program, according to the FDA.
On Twitter @naseemmiller
The Food and Drug Administration has cleared the marketing of the ReWalk Personal System, thereby for the first time enabling individuals with lower-body paralysis because of a spinal cord injury to use a wearable motorized exoskeleton at home.
The robotic device consists of a fitted, metal brace worn over the legs and part of the upper body and is controlled by a wireless remote. It accommodates individuals with heights from 5’3" to 6’3" and weighs up to 220 pounds.
ReWalk costs $69,500 and is currently not covered by insurance, although company officials are in talks with insurance companies, according to a spokesperson. The company also says that the potential medical benefits of using the device will offset the cost by reducing hospitalization and some medications.
ReWalk Personal System is not the only motorized exoskeleton available in the United States, but so far, ReWalk, along with similar (but different) exoskeleton systems such as Ekso Bionics, have been mainly used in rehabilitation facilities and Veterans Affairs hospitals.
"Innovative devices such as ReWalk go a long way towards helping individuals with spinal cord injuries gain some mobility," Christy Foreman, director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health, said in a written statement. "Along with physical therapy, training, and assistance from a caregiver, these individuals may be able to use these devices to walk again in their homes and in their communities."
Individuals with paraplegia due to spinal cord injuries at level T7 (seventh thoracic vertebra) to L5 (fifth lumbar vertebra), can use the device when accompanied by a specially trained caregiver, according to the FDA.
People with spinal cord injury at levels T4 (fourth thoracic vertebra) to T6 (sixth thoracic vertebra) can also use the device, but only in rehab facilities, according to the FDA. The device is not for sports or climbing stairs.
Consumers and their caregivers, who could be a spouse or home health aid, must successfully complete a training program designed by ReWalk at a rehabilitation center or a VA hospital. Physicians evaluate the individual’s skill level based on training before writing a prescription for the device.
But, according to the FDA, prior to being trained to use ReWalk:
• Patients should be able to stand using an assistive standing device (e.g., standing frame), and their hands and shoulders should be able to support crutches or a walker.
• Patients should not use the device if they have a history of severe neurologic injuries other than spinal cord injury, or have severe spasticity, significant contractures, unstable spine, unhealed limb fractures, or pelvic fractures.
• Patients should also not use the device if they have severe concurrent medical diseases such as infection, circulatory conditions, heart or lung conditions, or pressure sores.
The ReWalk Personal System consists of motors that help with movement of hips, knees, and ankles, enabling people to sit, stand, turn and walk, with assistance from a trained person. The device has a tilt sensor, and its computer and power supply are contained in a backpack. Patients use crutches, and they control the device with a wireless remote control worn on the wrist. The device weighs 46 pounds, but the user feels mostly the weight of the backpack, which is about 5 pounds, according to the company.
"The person walks the system, the system does not walk them," Dr. Amit Goffer, the Israeli inventor of ReWalk who became quadriplegic after an ATV accident in 1997, said in a company news release. "The users are in control – when they want to sit, they sit, when then want to stand and walk, they do so."
The device, manufactured by Argo Medical Technologies, has been in use in Europe, Israel, and other countries. The rehab version of ReWalk became available internationally in 2011, while sales of the personal system began in 2012.
It is not clear when another brand of exoskeletons will be cleared for marketing by the FDA. "We’re excited that the FDA has opened this gateway and look forward to seeing how the market reacts," said a spokesperson for Ekso Bionics, noting that the company is currently focused on the rehabilitation market, but some individuals use the device at home with a physical therapist and in clinical studies conducted by the company.
The ReWalk personal system was reviewed through FDA’s de novo classification process, used for novel, first-of-its-kind medical devices that are low to moderate risk. The agency cleared the device after reviewing its durability, hardware, software, and battery system, in addition to assessing clinical data from a 30-subject study and a 16-patient observational study.
The company reports that "Study data of the ReWalk system indicate potential improvements in cardiovascular health, loss of fat tissue, building of lean muscle mass, and improved bowel function. Feedback from ReWalk users supports these potential benefits and others, such as better pain management, fewer medications, and potentially reduced hospitalizations."
The device’s manufacturer is required to complete a postmarket clinical study to collect data on adverse events related to device use and to assess the adequacy of its training program, according to the FDA.
On Twitter @naseemmiller
First guidelines on pediatric concussion reflect new knowledge of impacts
Children with concussion may not look injured but may experience attention and memory deficits, become clumsy and accident prone, and experience social withdrawal for weeks or even months after injury, according to the first comprehensive guidelines on the diagnosis and management of pediatric concussion.
Published by the Ontario Neurotrauma Foundation, with involvement of the American Academy of Neurology, the guidelines recommend that clinicians use validated tools such as the Postconcussion Symptom Inventory, and work closely with the child and his or her family and school to implement an individualized plan for both physical and cognitive rest. The guidelines are free and available here and here.
The guidelines recommended the practice of "if in doubt, sit them out" when unsure about whether concussion had occurred, and cited strong evidence in favor of both verbal information and printed handouts for patients and parents.
They also suggested clinicians consider baseline neurocognitive testing but only if the child or adolescent plays high-risk sports.
"Our hope is that these guidelines will lead to significant improvements in the current management, since our survey has demonstrated that the current management is suboptimal," said guidelines chair Dr. Roger Zemek, director of pediatric emergency research at the Children’s Hospital of Eastern Ontario, Ottawa.
"The literature examining pediatric concussion has seen exponential growth over the past decade, and the latest evidence points to different diagnostic and management approaches than 10 years (or even just 5 years) ago."
The guidelines’ 32-member panel involved pediatric neurologists, sports medicine physicians, and neuropsychologists, and doctors of rehabilitation medicine, emergency medicine, family medicine, pediatric neurosurgery, and speech and language pathology. The panelists also included health professionals in physical and occupational therapy, rehabilitation nursing, and child and youth health system planning, as well as a pediatric brain injury outreach program school liaison and a patient advocacy group adviser.
There were no relevant conflicts of interest declared.
Children with concussion may not look injured but may experience attention and memory deficits, become clumsy and accident prone, and experience social withdrawal for weeks or even months after injury, according to the first comprehensive guidelines on the diagnosis and management of pediatric concussion.
Published by the Ontario Neurotrauma Foundation, with involvement of the American Academy of Neurology, the guidelines recommend that clinicians use validated tools such as the Postconcussion Symptom Inventory, and work closely with the child and his or her family and school to implement an individualized plan for both physical and cognitive rest. The guidelines are free and available here and here.
The guidelines recommended the practice of "if in doubt, sit them out" when unsure about whether concussion had occurred, and cited strong evidence in favor of both verbal information and printed handouts for patients and parents.
They also suggested clinicians consider baseline neurocognitive testing but only if the child or adolescent plays high-risk sports.
"Our hope is that these guidelines will lead to significant improvements in the current management, since our survey has demonstrated that the current management is suboptimal," said guidelines chair Dr. Roger Zemek, director of pediatric emergency research at the Children’s Hospital of Eastern Ontario, Ottawa.
"The literature examining pediatric concussion has seen exponential growth over the past decade, and the latest evidence points to different diagnostic and management approaches than 10 years (or even just 5 years) ago."
The guidelines’ 32-member panel involved pediatric neurologists, sports medicine physicians, and neuropsychologists, and doctors of rehabilitation medicine, emergency medicine, family medicine, pediatric neurosurgery, and speech and language pathology. The panelists also included health professionals in physical and occupational therapy, rehabilitation nursing, and child and youth health system planning, as well as a pediatric brain injury outreach program school liaison and a patient advocacy group adviser.
There were no relevant conflicts of interest declared.
Children with concussion may not look injured but may experience attention and memory deficits, become clumsy and accident prone, and experience social withdrawal for weeks or even months after injury, according to the first comprehensive guidelines on the diagnosis and management of pediatric concussion.
Published by the Ontario Neurotrauma Foundation, with involvement of the American Academy of Neurology, the guidelines recommend that clinicians use validated tools such as the Postconcussion Symptom Inventory, and work closely with the child and his or her family and school to implement an individualized plan for both physical and cognitive rest. The guidelines are free and available here and here.
The guidelines recommended the practice of "if in doubt, sit them out" when unsure about whether concussion had occurred, and cited strong evidence in favor of both verbal information and printed handouts for patients and parents.
They also suggested clinicians consider baseline neurocognitive testing but only if the child or adolescent plays high-risk sports.
"Our hope is that these guidelines will lead to significant improvements in the current management, since our survey has demonstrated that the current management is suboptimal," said guidelines chair Dr. Roger Zemek, director of pediatric emergency research at the Children’s Hospital of Eastern Ontario, Ottawa.
"The literature examining pediatric concussion has seen exponential growth over the past decade, and the latest evidence points to different diagnostic and management approaches than 10 years (or even just 5 years) ago."
The guidelines’ 32-member panel involved pediatric neurologists, sports medicine physicians, and neuropsychologists, and doctors of rehabilitation medicine, emergency medicine, family medicine, pediatric neurosurgery, and speech and language pathology. The panelists also included health professionals in physical and occupational therapy, rehabilitation nursing, and child and youth health system planning, as well as a pediatric brain injury outreach program school liaison and a patient advocacy group adviser.
There were no relevant conflicts of interest declared.
Disability rates from blast-related, nonblast TBI similar
The adverse effects of concussive traumatic brain injury are similar whether the injury was sustained in a blast-related incident such as a bomb or improvised explosive device detonation or a nonblast incident such as a fall or car crash, a report published online June 16 in JAMA Neurology shows.
Researchers studied this issue by prospectively assessing the clinical outcomes of the two types of traumatic brain injury (TBI) in 178 patients injured while serving in Iraq and Afghanistan during a 3-year period. Study participants were enrolled immediately after evacuation from combat theaters, when they were medically assessed at Landstuhl Regional Medical Center in Germany, and were followed up at 6 and 12 months afterward, said Christine L. MacDonald, Ph.D., of the department of neurology, Washington University, St. Louis, and her associates.
A total of 53 patients sustained blast-plus-impact TBI; 29 sustained nonblast TBI from falls, crashes, or being struck in the head by a blunt object; and 96 who had no head injuries served as control subjects. In the control group, 27 had been exposed to blasts, and 69 had not been exposed to blasts; most required medical evaluation for gastrointestinal, dermatologic, gynecologic, or orthopedic indications.
Global outcomes as measured by the Glasgow Outcome Scale-Extended were "essentially indistinguishable" between blast-related and nonblast TBI, as were numerous neuropsychological abnormalities; neurobehavioral impairments; focal neurological deficits such as those affecting olfaction, gait, and limb ataxia; headache-related disability; PTSD and its components; depression; alcohol misuse; and sleep disturbances. This suggests that TBI itself, independent of the mechanism of injury and of the intensity of combat exposure, is the primary driver of adverse outcomes, Dr. MacDonald and her colleagues said (JAMA Neurol. 2014 June 16 [doi:10.1001/jamaneurol.2014.1114]).
Another important finding was that control subjects who had been exposed to blasts showed significantly worse outcomes than controls who had not been exposed to blasts on measures of neurobehavioral, psychiatric, and headache-related impairment. It is possible that subconcussive blast exposure might cause direct structural damage to the brain or that other factors associated with blast exposure might play a role, the investigators added.
The relatively modest sample size and potential selection bias were cited by Dr. MacDonald and her associates as possible study limitations.
This study was funded by the Congressionally Directed Medical Research Programs. Dr. MacDonald and her associates reported no financial conflicts of interest.
The adverse effects of concussive traumatic brain injury are similar whether the injury was sustained in a blast-related incident such as a bomb or improvised explosive device detonation or a nonblast incident such as a fall or car crash, a report published online June 16 in JAMA Neurology shows.
Researchers studied this issue by prospectively assessing the clinical outcomes of the two types of traumatic brain injury (TBI) in 178 patients injured while serving in Iraq and Afghanistan during a 3-year period. Study participants were enrolled immediately after evacuation from combat theaters, when they were medically assessed at Landstuhl Regional Medical Center in Germany, and were followed up at 6 and 12 months afterward, said Christine L. MacDonald, Ph.D., of the department of neurology, Washington University, St. Louis, and her associates.
A total of 53 patients sustained blast-plus-impact TBI; 29 sustained nonblast TBI from falls, crashes, or being struck in the head by a blunt object; and 96 who had no head injuries served as control subjects. In the control group, 27 had been exposed to blasts, and 69 had not been exposed to blasts; most required medical evaluation for gastrointestinal, dermatologic, gynecologic, or orthopedic indications.
Global outcomes as measured by the Glasgow Outcome Scale-Extended were "essentially indistinguishable" between blast-related and nonblast TBI, as were numerous neuropsychological abnormalities; neurobehavioral impairments; focal neurological deficits such as those affecting olfaction, gait, and limb ataxia; headache-related disability; PTSD and its components; depression; alcohol misuse; and sleep disturbances. This suggests that TBI itself, independent of the mechanism of injury and of the intensity of combat exposure, is the primary driver of adverse outcomes, Dr. MacDonald and her colleagues said (JAMA Neurol. 2014 June 16 [doi:10.1001/jamaneurol.2014.1114]).
Another important finding was that control subjects who had been exposed to blasts showed significantly worse outcomes than controls who had not been exposed to blasts on measures of neurobehavioral, psychiatric, and headache-related impairment. It is possible that subconcussive blast exposure might cause direct structural damage to the brain or that other factors associated with blast exposure might play a role, the investigators added.
The relatively modest sample size and potential selection bias were cited by Dr. MacDonald and her associates as possible study limitations.
This study was funded by the Congressionally Directed Medical Research Programs. Dr. MacDonald and her associates reported no financial conflicts of interest.
The adverse effects of concussive traumatic brain injury are similar whether the injury was sustained in a blast-related incident such as a bomb or improvised explosive device detonation or a nonblast incident such as a fall or car crash, a report published online June 16 in JAMA Neurology shows.
Researchers studied this issue by prospectively assessing the clinical outcomes of the two types of traumatic brain injury (TBI) in 178 patients injured while serving in Iraq and Afghanistan during a 3-year period. Study participants were enrolled immediately after evacuation from combat theaters, when they were medically assessed at Landstuhl Regional Medical Center in Germany, and were followed up at 6 and 12 months afterward, said Christine L. MacDonald, Ph.D., of the department of neurology, Washington University, St. Louis, and her associates.
A total of 53 patients sustained blast-plus-impact TBI; 29 sustained nonblast TBI from falls, crashes, or being struck in the head by a blunt object; and 96 who had no head injuries served as control subjects. In the control group, 27 had been exposed to blasts, and 69 had not been exposed to blasts; most required medical evaluation for gastrointestinal, dermatologic, gynecologic, or orthopedic indications.
Global outcomes as measured by the Glasgow Outcome Scale-Extended were "essentially indistinguishable" between blast-related and nonblast TBI, as were numerous neuropsychological abnormalities; neurobehavioral impairments; focal neurological deficits such as those affecting olfaction, gait, and limb ataxia; headache-related disability; PTSD and its components; depression; alcohol misuse; and sleep disturbances. This suggests that TBI itself, independent of the mechanism of injury and of the intensity of combat exposure, is the primary driver of adverse outcomes, Dr. MacDonald and her colleagues said (JAMA Neurol. 2014 June 16 [doi:10.1001/jamaneurol.2014.1114]).
Another important finding was that control subjects who had been exposed to blasts showed significantly worse outcomes than controls who had not been exposed to blasts on measures of neurobehavioral, psychiatric, and headache-related impairment. It is possible that subconcussive blast exposure might cause direct structural damage to the brain or that other factors associated with blast exposure might play a role, the investigators added.
The relatively modest sample size and potential selection bias were cited by Dr. MacDonald and her associates as possible study limitations.
This study was funded by the Congressionally Directed Medical Research Programs. Dr. MacDonald and her associates reported no financial conflicts of interest.
FROM JAMA NEUROLOGY
Key clinical point: TBI itself, regardless of injury mechanism or the intensity of combat exposure, appears to be a "primary driver of adverse outcomes."
Major finding: Global outcomes as measured by the Glasgow Outcome Scale-Extended were "essentially indistinguishable" between blast-related and nonblast TBI, as were numerous neuropsychological abnormalities; neurobehavioral impairments; focal neurological deficits such as those affecting olfaction, gait, and limb ataxia; headache-related disability; PTSD and its components; depression; alcohol misuse; and sleep disturbances.
Data source: A prospective cohort study involving 178 military personnel who sustained blast-related TBI, TBI related to other, nonblast mechanisms, or other medical disorders and were assessed for a wide variety of adverse outcomes at 6 months and 12 months.
Disclosures: This study was funded by the Congressionally Directed Medical Research Programs. Dr. MacDonald and her associates reported no financial conflicts of interest.
Ethical quagmires in preventing and managing concussion
Proper medical surveillance is one of the best tools for preventing and managing concussion. As neurologists, we play a key role in this aspect of concussion prevention and recovery, with the ultimate responsibility of protecting the health and safety of athletes.
That said, there are several instances in which neurologists and sports doctors could face ethical quagmires as they strive toward this goal. One of them is determining when athletes can return to play, if at all, as repetitive injuries are more likely to cause serious brain injury. Those players who have had previous concussions are also at a higher risk for sustaining another one, with football players having a four to six times higher risk than do others with prior concussions. Additionally, those who go back to play too soon are vulnerable to sustaining a second concussion, typically within 10 days of the prior one.
Therefore, much care and consideration must be taken into account when assessing a concussed athlete’s condition. And not all neurologists will be qualified to perform the necessary evaluations, treatment recommendations, and postconcussion follow-up. It is the ethical responsibility of the licensed health care professional to ensure that whoever is treating the concussed athlete is experienced and knowledgeable about treating this type of trauma, including sending the athlete to a different neurologist if necessary.
There’s also a 2013 study that began to take a look at two particular genetic polymorphisms, an amino acid switch in exon 6 of the MAPT (microtubule-associated protein tau) gene and in the promoter region of the APOE (apolipoprotein E) gene, and their relation to postconcussion neurocognitive function/reaction time and outcome in a group of college athletes comprising men’s football and men’s and women’s soccer players. The goal of the study is to determine whether an athlete’s genetic makeup determines the severity of postconcussive brain function. The outcome of the study could potentially add another wrinkle to the question of whether to let players go back to the game or not.
A similar ethical question arises when considering whether to let athletes with certain genetic dispositions play at all. Evidence has been found that boxers with the APOE epsilon-4 allele were more likely to suffer from the effects of chronic traumatic brain injury as their careers went on than did those who did not have the genotype (JAMA 1997;278:136-140). This genotype has also been linked with Alzheimer’s disease (Arch. Neurol. 1995;52:1074-9).
Knowledge of an athlete’s genetic vulnerability to brain injury creates an ethical grey area because the misuse of the information could harm his or her career. Like other HIPAA-protected information, this type of information should be kept confidential. However, sports doctor always needs to keep the athlete’s safety in mind, and if confronted with an athlete who has a genetic predisposition to concussion or poor recovery from concussion, they need to advise the athletes to get regular brain scans to ensure there’s no damage but not necessarily to stop them from playing.
There are other factors at play beyond genetic disposition, and more studies need to be done to get a better understanding of this public health risk. Until then, neurologists need to make careful judgments when dealing with concussions.
Dr. Jordan is the director of the brain injury program and the memory evaluation treatment service at Burke Rehabilitation Hospital in White Plains, N.Y. He also serves as the assistant medical director there. He currently serves as the chief medical officer of the New York State Athletic Commission, as a team physician for USA Boxing, and as a member of the NFL Players Association Mackey-White Traumatic Brain Injury Committee and the NFL Neuro-Cognitive Disability Committee.
Proper medical surveillance is one of the best tools for preventing and managing concussion. As neurologists, we play a key role in this aspect of concussion prevention and recovery, with the ultimate responsibility of protecting the health and safety of athletes.
That said, there are several instances in which neurologists and sports doctors could face ethical quagmires as they strive toward this goal. One of them is determining when athletes can return to play, if at all, as repetitive injuries are more likely to cause serious brain injury. Those players who have had previous concussions are also at a higher risk for sustaining another one, with football players having a four to six times higher risk than do others with prior concussions. Additionally, those who go back to play too soon are vulnerable to sustaining a second concussion, typically within 10 days of the prior one.
Therefore, much care and consideration must be taken into account when assessing a concussed athlete’s condition. And not all neurologists will be qualified to perform the necessary evaluations, treatment recommendations, and postconcussion follow-up. It is the ethical responsibility of the licensed health care professional to ensure that whoever is treating the concussed athlete is experienced and knowledgeable about treating this type of trauma, including sending the athlete to a different neurologist if necessary.
There’s also a 2013 study that began to take a look at two particular genetic polymorphisms, an amino acid switch in exon 6 of the MAPT (microtubule-associated protein tau) gene and in the promoter region of the APOE (apolipoprotein E) gene, and their relation to postconcussion neurocognitive function/reaction time and outcome in a group of college athletes comprising men’s football and men’s and women’s soccer players. The goal of the study is to determine whether an athlete’s genetic makeup determines the severity of postconcussive brain function. The outcome of the study could potentially add another wrinkle to the question of whether to let players go back to the game or not.
A similar ethical question arises when considering whether to let athletes with certain genetic dispositions play at all. Evidence has been found that boxers with the APOE epsilon-4 allele were more likely to suffer from the effects of chronic traumatic brain injury as their careers went on than did those who did not have the genotype (JAMA 1997;278:136-140). This genotype has also been linked with Alzheimer’s disease (Arch. Neurol. 1995;52:1074-9).
Knowledge of an athlete’s genetic vulnerability to brain injury creates an ethical grey area because the misuse of the information could harm his or her career. Like other HIPAA-protected information, this type of information should be kept confidential. However, sports doctor always needs to keep the athlete’s safety in mind, and if confronted with an athlete who has a genetic predisposition to concussion or poor recovery from concussion, they need to advise the athletes to get regular brain scans to ensure there’s no damage but not necessarily to stop them from playing.
There are other factors at play beyond genetic disposition, and more studies need to be done to get a better understanding of this public health risk. Until then, neurologists need to make careful judgments when dealing with concussions.
Dr. Jordan is the director of the brain injury program and the memory evaluation treatment service at Burke Rehabilitation Hospital in White Plains, N.Y. He also serves as the assistant medical director there. He currently serves as the chief medical officer of the New York State Athletic Commission, as a team physician for USA Boxing, and as a member of the NFL Players Association Mackey-White Traumatic Brain Injury Committee and the NFL Neuro-Cognitive Disability Committee.
Proper medical surveillance is one of the best tools for preventing and managing concussion. As neurologists, we play a key role in this aspect of concussion prevention and recovery, with the ultimate responsibility of protecting the health and safety of athletes.
That said, there are several instances in which neurologists and sports doctors could face ethical quagmires as they strive toward this goal. One of them is determining when athletes can return to play, if at all, as repetitive injuries are more likely to cause serious brain injury. Those players who have had previous concussions are also at a higher risk for sustaining another one, with football players having a four to six times higher risk than do others with prior concussions. Additionally, those who go back to play too soon are vulnerable to sustaining a second concussion, typically within 10 days of the prior one.
Therefore, much care and consideration must be taken into account when assessing a concussed athlete’s condition. And not all neurologists will be qualified to perform the necessary evaluations, treatment recommendations, and postconcussion follow-up. It is the ethical responsibility of the licensed health care professional to ensure that whoever is treating the concussed athlete is experienced and knowledgeable about treating this type of trauma, including sending the athlete to a different neurologist if necessary.
There’s also a 2013 study that began to take a look at two particular genetic polymorphisms, an amino acid switch in exon 6 of the MAPT (microtubule-associated protein tau) gene and in the promoter region of the APOE (apolipoprotein E) gene, and their relation to postconcussion neurocognitive function/reaction time and outcome in a group of college athletes comprising men’s football and men’s and women’s soccer players. The goal of the study is to determine whether an athlete’s genetic makeup determines the severity of postconcussive brain function. The outcome of the study could potentially add another wrinkle to the question of whether to let players go back to the game or not.
A similar ethical question arises when considering whether to let athletes with certain genetic dispositions play at all. Evidence has been found that boxers with the APOE epsilon-4 allele were more likely to suffer from the effects of chronic traumatic brain injury as their careers went on than did those who did not have the genotype (JAMA 1997;278:136-140). This genotype has also been linked with Alzheimer’s disease (Arch. Neurol. 1995;52:1074-9).
Knowledge of an athlete’s genetic vulnerability to brain injury creates an ethical grey area because the misuse of the information could harm his or her career. Like other HIPAA-protected information, this type of information should be kept confidential. However, sports doctor always needs to keep the athlete’s safety in mind, and if confronted with an athlete who has a genetic predisposition to concussion or poor recovery from concussion, they need to advise the athletes to get regular brain scans to ensure there’s no damage but not necessarily to stop them from playing.
There are other factors at play beyond genetic disposition, and more studies need to be done to get a better understanding of this public health risk. Until then, neurologists need to make careful judgments when dealing with concussions.
Dr. Jordan is the director of the brain injury program and the memory evaluation treatment service at Burke Rehabilitation Hospital in White Plains, N.Y. He also serves as the assistant medical director there. He currently serves as the chief medical officer of the New York State Athletic Commission, as a team physician for USA Boxing, and as a member of the NFL Players Association Mackey-White Traumatic Brain Injury Committee and the NFL Neuro-Cognitive Disability Committee.
Pseudobulbar affect common in vets with mild TBI
Symptoms of pseudobulbar affect were reported by 60% of veterans with mild traumatic brain injury in a survey of patients in the Veterans Affairs system.
Pseudobulbar affect (PBA) – a neurologic condition involving uncontrollable, disruptive outbursts of crying and/or laughing – was more common among 758 veterans with mild TBI than were posttraumatic stress disorder (48%), major depression (31%), and anxiety disorders (18%), according to Dr. Regina McGlinchey of the VA Boston Healthcare System and her associates, who reported their findings at the Tenth World Congress on Brain Injury in San Francisco.
Veterans with PBA symptoms were more likely to also have PTSD, a finding not previously reported, the investigators said. In the survey, 54% of patients with PBA also reported PTSD, compared with 32% of those who did not have PBA.
Veterans with PBA symptoms also were significantly more likely to be using antidepressants (46%) than were those without PBA (31%), and "those with PBA symptoms reported significantly worse health-related quality of life scores," Dr. McGlinchey said in a statement.
The survey was sponsored by Avanir, maker of Nuedexta (dextromethorphan hydrobromide/quinidine sulfate), which is indicated for treatment of pseudobulbar affect.
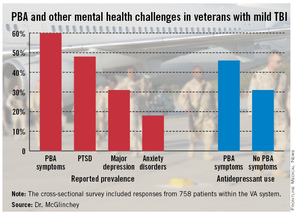
Symptoms of pseudobulbar affect were reported by 60% of veterans with mild traumatic brain injury in a survey of patients in the Veterans Affairs system.
Pseudobulbar affect (PBA) – a neurologic condition involving uncontrollable, disruptive outbursts of crying and/or laughing – was more common among 758 veterans with mild TBI than were posttraumatic stress disorder (48%), major depression (31%), and anxiety disorders (18%), according to Dr. Regina McGlinchey of the VA Boston Healthcare System and her associates, who reported their findings at the Tenth World Congress on Brain Injury in San Francisco.
Veterans with PBA symptoms were more likely to also have PTSD, a finding not previously reported, the investigators said. In the survey, 54% of patients with PBA also reported PTSD, compared with 32% of those who did not have PBA.
Veterans with PBA symptoms also were significantly more likely to be using antidepressants (46%) than were those without PBA (31%), and "those with PBA symptoms reported significantly worse health-related quality of life scores," Dr. McGlinchey said in a statement.
The survey was sponsored by Avanir, maker of Nuedexta (dextromethorphan hydrobromide/quinidine sulfate), which is indicated for treatment of pseudobulbar affect.

Symptoms of pseudobulbar affect were reported by 60% of veterans with mild traumatic brain injury in a survey of patients in the Veterans Affairs system.
Pseudobulbar affect (PBA) – a neurologic condition involving uncontrollable, disruptive outbursts of crying and/or laughing – was more common among 758 veterans with mild TBI than were posttraumatic stress disorder (48%), major depression (31%), and anxiety disorders (18%), according to Dr. Regina McGlinchey of the VA Boston Healthcare System and her associates, who reported their findings at the Tenth World Congress on Brain Injury in San Francisco.
Veterans with PBA symptoms were more likely to also have PTSD, a finding not previously reported, the investigators said. In the survey, 54% of patients with PBA also reported PTSD, compared with 32% of those who did not have PBA.
Veterans with PBA symptoms also were significantly more likely to be using antidepressants (46%) than were those without PBA (31%), and "those with PBA symptoms reported significantly worse health-related quality of life scores," Dr. McGlinchey said in a statement.
The survey was sponsored by Avanir, maker of Nuedexta (dextromethorphan hydrobromide/quinidine sulfate), which is indicated for treatment of pseudobulbar affect.

Pseudobulbar affect: More common than you’d think
ORLANDO – The prevalence of pseudobulbar affect symptoms – that is, uncontrollable, disruptive outbursts of crying and/or laughing – is considerably greater across a range of neurologic disorders than previously appreciated, according to the largest-ever study to screen for this condition.
Pseudobulbar affect (PBA) symptoms were found in the study to be more common among neurology patients under age 65; however, the adverse impact of PBA symptoms upon quality of life was greater in the elderly, Dr. David W. Crumpacker reported at the annual meeting of the American Association for Geriatric Psychiatry.
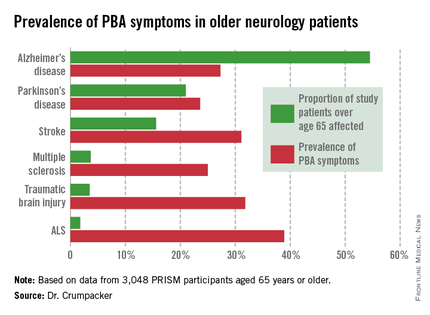
He presented the results of the PRISM (PBA Registry Series) study, which enrolled 5,290 patients on the basis of having any of six neurologic disorders: Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, Parkinson’s disease, stroke, or traumatic brain injury. They were screened for the presence of PBA symptoms using the validated Center for Neurologic Study–Lability Scale (CNS-LS). A score of 13 or more was deemed positive, based upon its demonstrated good predictive value for physician diagnosis of PBA in patients with ALS.
The CNS-LS is a simple test that can be completed quickly by either the patient or caregiver. The test is well-suited for routine use in clinical practice, noted Dr. W. Crumpacker, a psychiatrist at Baylor University Medical Center, Dallas.
The overall prevalence of PBA symptoms among the 3,048 PRISM participants aged 65 years or older was 27.4%, with the highest rate seen in patients having ALS (see chart). In contrast, the prevalence of PBA symptoms among patients under age 65 years was 49.5%, with the highest rate – 56.9% – being seen in traumatic brain injury patients.
Patients or caregivers were asked to rate on a 0-10 scale the impact their primary neurologic disease has had on their quality of life. Patients 65 years and older with PBA symptoms reported a significantly greater negative impact than did those without PBA symptoms, with mean scores on the quality of life impact scale of 6.3 vs. 4.6. The quality of life difference between those with PBA symptoms and those without was significant for patients with each of the neurologic diseases except for ALS.
As another measure of the adverse impact of having PBA symptoms, 56% of affected older patients were on at least one antipsychotic or antidepressant, compared with 35% of older patients without PBA symptoms.
PBA is thought to result from injury to neurologic pathways that regulate emotional expression as a secondary consequence of a variety of neurologic disorders.
In an interview, Dr. Crumpacker said PBA is greatly underdiagnosed and often gets misdiagnosed as depression.
"The symptoms are extremely disturbing to others, and patients are acutely aware of that. I tell my friends in neurology, it’s the psychiatric pathology that causes people problems in their lives. No one gets divorced over neurologic pathology, they get divorced over psychiatric pathology. It’s not, ‘I got a divorce because he had a stroke.’ " "It’s "We got divorced because he had a stroke and it changed his personality; he was a different person and I couldn’t be around him anymore,’ " the psychiatrist said.
PBA became a diagnosable disorder with its own ICD-9 code, albeit a diagnosis that can’t be made in the absence of neurologic pathology, at the behest of the Food and Drug Administration, Dr. Crumpacker explained. The impetus was the discovery of an effective treatment, dextromethorphan HBr and quinidine sulfate (Nuedexta), which received FDA approval for PBA 3 years ago.
Nuedexta’s development as the sole medication indicated for PBA was serendipitous, according to Dr. Crumpacker.
"The drug was being tested in Alzheimer’s disease. The jury is still out on whether it helps. But families of study participants came back saying, ‘You know that stuff dad used to do – the crying, the inappropriate laughter, the anger? He doesn’t do those kinds of things anymore,’ " Dr. Crumpacker recalled.
He reported serving on a scientific advisory board for Avanir Pharmaceuticals, which markets Nuedexta.
ORLANDO – The prevalence of pseudobulbar affect symptoms – that is, uncontrollable, disruptive outbursts of crying and/or laughing – is considerably greater across a range of neurologic disorders than previously appreciated, according to the largest-ever study to screen for this condition.
Pseudobulbar affect (PBA) symptoms were found in the study to be more common among neurology patients under age 65; however, the adverse impact of PBA symptoms upon quality of life was greater in the elderly, Dr. David W. Crumpacker reported at the annual meeting of the American Association for Geriatric Psychiatry.

He presented the results of the PRISM (PBA Registry Series) study, which enrolled 5,290 patients on the basis of having any of six neurologic disorders: Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, Parkinson’s disease, stroke, or traumatic brain injury. They were screened for the presence of PBA symptoms using the validated Center for Neurologic Study–Lability Scale (CNS-LS). A score of 13 or more was deemed positive, based upon its demonstrated good predictive value for physician diagnosis of PBA in patients with ALS.
The CNS-LS is a simple test that can be completed quickly by either the patient or caregiver. The test is well-suited for routine use in clinical practice, noted Dr. W. Crumpacker, a psychiatrist at Baylor University Medical Center, Dallas.
The overall prevalence of PBA symptoms among the 3,048 PRISM participants aged 65 years or older was 27.4%, with the highest rate seen in patients having ALS (see chart). In contrast, the prevalence of PBA symptoms among patients under age 65 years was 49.5%, with the highest rate – 56.9% – being seen in traumatic brain injury patients.
Patients or caregivers were asked to rate on a 0-10 scale the impact their primary neurologic disease has had on their quality of life. Patients 65 years and older with PBA symptoms reported a significantly greater negative impact than did those without PBA symptoms, with mean scores on the quality of life impact scale of 6.3 vs. 4.6. The quality of life difference between those with PBA symptoms and those without was significant for patients with each of the neurologic diseases except for ALS.
As another measure of the adverse impact of having PBA symptoms, 56% of affected older patients were on at least one antipsychotic or antidepressant, compared with 35% of older patients without PBA symptoms.
PBA is thought to result from injury to neurologic pathways that regulate emotional expression as a secondary consequence of a variety of neurologic disorders.
In an interview, Dr. Crumpacker said PBA is greatly underdiagnosed and often gets misdiagnosed as depression.
"The symptoms are extremely disturbing to others, and patients are acutely aware of that. I tell my friends in neurology, it’s the psychiatric pathology that causes people problems in their lives. No one gets divorced over neurologic pathology, they get divorced over psychiatric pathology. It’s not, ‘I got a divorce because he had a stroke.’ " "It’s "We got divorced because he had a stroke and it changed his personality; he was a different person and I couldn’t be around him anymore,’ " the psychiatrist said.
PBA became a diagnosable disorder with its own ICD-9 code, albeit a diagnosis that can’t be made in the absence of neurologic pathology, at the behest of the Food and Drug Administration, Dr. Crumpacker explained. The impetus was the discovery of an effective treatment, dextromethorphan HBr and quinidine sulfate (Nuedexta), which received FDA approval for PBA 3 years ago.
Nuedexta’s development as the sole medication indicated for PBA was serendipitous, according to Dr. Crumpacker.
"The drug was being tested in Alzheimer’s disease. The jury is still out on whether it helps. But families of study participants came back saying, ‘You know that stuff dad used to do – the crying, the inappropriate laughter, the anger? He doesn’t do those kinds of things anymore,’ " Dr. Crumpacker recalled.
He reported serving on a scientific advisory board for Avanir Pharmaceuticals, which markets Nuedexta.
ORLANDO – The prevalence of pseudobulbar affect symptoms – that is, uncontrollable, disruptive outbursts of crying and/or laughing – is considerably greater across a range of neurologic disorders than previously appreciated, according to the largest-ever study to screen for this condition.
Pseudobulbar affect (PBA) symptoms were found in the study to be more common among neurology patients under age 65; however, the adverse impact of PBA symptoms upon quality of life was greater in the elderly, Dr. David W. Crumpacker reported at the annual meeting of the American Association for Geriatric Psychiatry.

He presented the results of the PRISM (PBA Registry Series) study, which enrolled 5,290 patients on the basis of having any of six neurologic disorders: Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, Parkinson’s disease, stroke, or traumatic brain injury. They were screened for the presence of PBA symptoms using the validated Center for Neurologic Study–Lability Scale (CNS-LS). A score of 13 or more was deemed positive, based upon its demonstrated good predictive value for physician diagnosis of PBA in patients with ALS.
The CNS-LS is a simple test that can be completed quickly by either the patient or caregiver. The test is well-suited for routine use in clinical practice, noted Dr. W. Crumpacker, a psychiatrist at Baylor University Medical Center, Dallas.
The overall prevalence of PBA symptoms among the 3,048 PRISM participants aged 65 years or older was 27.4%, with the highest rate seen in patients having ALS (see chart). In contrast, the prevalence of PBA symptoms among patients under age 65 years was 49.5%, with the highest rate – 56.9% – being seen in traumatic brain injury patients.
Patients or caregivers were asked to rate on a 0-10 scale the impact their primary neurologic disease has had on their quality of life. Patients 65 years and older with PBA symptoms reported a significantly greater negative impact than did those without PBA symptoms, with mean scores on the quality of life impact scale of 6.3 vs. 4.6. The quality of life difference between those with PBA symptoms and those without was significant for patients with each of the neurologic diseases except for ALS.
As another measure of the adverse impact of having PBA symptoms, 56% of affected older patients were on at least one antipsychotic or antidepressant, compared with 35% of older patients without PBA symptoms.
PBA is thought to result from injury to neurologic pathways that regulate emotional expression as a secondary consequence of a variety of neurologic disorders.
In an interview, Dr. Crumpacker said PBA is greatly underdiagnosed and often gets misdiagnosed as depression.
"The symptoms are extremely disturbing to others, and patients are acutely aware of that. I tell my friends in neurology, it’s the psychiatric pathology that causes people problems in their lives. No one gets divorced over neurologic pathology, they get divorced over psychiatric pathology. It’s not, ‘I got a divorce because he had a stroke.’ " "It’s "We got divorced because he had a stroke and it changed his personality; he was a different person and I couldn’t be around him anymore,’ " the psychiatrist said.
PBA became a diagnosable disorder with its own ICD-9 code, albeit a diagnosis that can’t be made in the absence of neurologic pathology, at the behest of the Food and Drug Administration, Dr. Crumpacker explained. The impetus was the discovery of an effective treatment, dextromethorphan HBr and quinidine sulfate (Nuedexta), which received FDA approval for PBA 3 years ago.
Nuedexta’s development as the sole medication indicated for PBA was serendipitous, according to Dr. Crumpacker.
"The drug was being tested in Alzheimer’s disease. The jury is still out on whether it helps. But families of study participants came back saying, ‘You know that stuff dad used to do – the crying, the inappropriate laughter, the anger? He doesn’t do those kinds of things anymore,’ " Dr. Crumpacker recalled.
He reported serving on a scientific advisory board for Avanir Pharmaceuticals, which markets Nuedexta.
AT THE AAGP ANNUAL MEETING
Major finding: The prevalence of PBA symptoms among patients over age 65 years with any of six underlying neurologic disorders was 27.4%. That was significantly less than in younger patients with the same disorders, but the adverse effect of having PBA symptoms upon quality of life was markedly greater in the older group.
Data source: The PRISM study included 5,290 patients with Alzheimer’s disease or any of five other less common neurologic disorders, all of whom were screened for the presence of PBA symptoms using a brief validated measure.
Disclosures: The presenter serves on a scientific advisory board for Avanir Pharmaceuticals, which funded the PRISM study.
Strong nerves, teamwork key in managing neurosurgical patients, devices
SCOTTSDALE, ARIZ. – Comanaging neurosurgical patients requires a delicate dance between primary practitioners, surgeons, anesthesiologists, and, in some cases, the makers of implantable neurostimulators, according to perioperative medicine specialists.
Special considerations with patients scheduled for neurosurgical procedures include hypertension, fever, hyponatremia, and risk of deep vein thromboembolism (DVT) and coagulopathies, said Dr. Richard Huh, director of the inpatient medical consultation service at Rush University Medical Center in Chicago, at a meeting on perioperative medicine sponsored by the University of Miami.
For example, hospitalists are typically involved in the management of blood pressure in neurosurgery patients because of the importance of controlling intracranial pressure, Dr. Huh noted.
"The trick is for the blood pressure not to get too high or too low. The Handbook of Neurosurgery suggests a goal of 140 over 90 [mm Hg]," he said.
Patients with acute subarachnoid hemorrhage are at risk for vasospasm, most frequently within 7-10 days of hemorrhage. Hypovolemia is a common cause of vasospasm and should be avoided. Medications such as nimodipine (Nimotop) can help prevent this complication.
Patients undergoing spinal procedures tend to have low blood pressure from acute blood loss or intravenous pain medications, and may require hold parameters on medications to avoid complications from hypotension.
However, on the day following spinal surgery, some patients develop hypertension, and may require additional medications for BP control.
Hyponatremia
The reported prevalence of hyponatremia in hospitalized patients ranges from 1%-7%, and the rate is even higher in neurosurgical patients, possibility because the brain’s response to changes in osmolality. Clinicians managing neurosurgical patients should be aware of the syndrome of inappropriate antidiuretic hormone secretion (SIADH) and cerebral salt wasting, Dr. Huh said.
Cerebral salt wasting may be cause by damaged brain cells that affect sympathetic neural input to the kidneys, decreasing sodium resorption and an increase in atrial natriuretic peptide and brain natriuretic peptide.
The syndrome looks similar to SIADH, with high urinary sodium and osmolality, low serum osmolality, and decreased serum uric acid. But cerebral salt wasting is distinguished from SIADH by the presence of hypovolemia. Cerebral salt wasting is treated with saline and/or salt tablets.
DVT
Neurosurgical patients are at increased risk for DVT and pulmonary embolism compared with the general postoperative population. However, 2012 guidelines on antithrombotic therapy from the American College of Chest Physicians recommend against pharmacologic prophylaxis except for high risk patients, such as patients with intracranial masses. Dr. Huh said.
Implantable neurostimulators
Patients with implanted devices such as deep-brain stimulators for control of Parkinson’s disease, hypoglossal nerve stimulators for severe sleep apnea, or vagal nerve stimulators for epilepsy also require special consideration throughout the perioperative period.
Issues to consider when managing a patient with an implantable device include the device site and its proximity to the planned surgical field, indication for the device, comorbidities, and the patient’s goals for treatment, said Dr. Deborah Richman, section chief of preoperative services at Stony Brook (New York) University Medical Center.
"What do we do with device itself? This is a team approach, and we find that it’s best coordinated by our nurses in the preop holding area, because they know what time the surgery is, they have the device company rep’s phone number, they make sure the patient is there on time, and they put everything together to prevent delays on the day of surgery," she said.
In general, device manufacturers recommend turning devices off, and to turn the amplitude down to zero to prevent accidental activation of magnetic on-off switches.
The distance from the surgery to the pulse generator should be a minimum of 20 cm, and electrocautery, if used, should be bipolar rather than monopolar, Dr. Richman said.
Patients with implanted devices will also require prophylactic antibiotics to prevent potential bacterial seeding of the device or leads, she said,
Coordination of perioperative care "is best done with clinical management pathways, so that when you have one of these patients who present to you, you have a checklist that includes the device company’s phone number, and it’s an easy go-to, so that you don’t have to start from scratch each time," she concluded.
Dr. Huh and Dr. Richman reported having no financial disclosures.
SCOTTSDALE, ARIZ. – Comanaging neurosurgical patients requires a delicate dance between primary practitioners, surgeons, anesthesiologists, and, in some cases, the makers of implantable neurostimulators, according to perioperative medicine specialists.
Special considerations with patients scheduled for neurosurgical procedures include hypertension, fever, hyponatremia, and risk of deep vein thromboembolism (DVT) and coagulopathies, said Dr. Richard Huh, director of the inpatient medical consultation service at Rush University Medical Center in Chicago, at a meeting on perioperative medicine sponsored by the University of Miami.
For example, hospitalists are typically involved in the management of blood pressure in neurosurgery patients because of the importance of controlling intracranial pressure, Dr. Huh noted.
"The trick is for the blood pressure not to get too high or too low. The Handbook of Neurosurgery suggests a goal of 140 over 90 [mm Hg]," he said.
Patients with acute subarachnoid hemorrhage are at risk for vasospasm, most frequently within 7-10 days of hemorrhage. Hypovolemia is a common cause of vasospasm and should be avoided. Medications such as nimodipine (Nimotop) can help prevent this complication.
Patients undergoing spinal procedures tend to have low blood pressure from acute blood loss or intravenous pain medications, and may require hold parameters on medications to avoid complications from hypotension.
However, on the day following spinal surgery, some patients develop hypertension, and may require additional medications for BP control.
Hyponatremia
The reported prevalence of hyponatremia in hospitalized patients ranges from 1%-7%, and the rate is even higher in neurosurgical patients, possibility because the brain’s response to changes in osmolality. Clinicians managing neurosurgical patients should be aware of the syndrome of inappropriate antidiuretic hormone secretion (SIADH) and cerebral salt wasting, Dr. Huh said.
Cerebral salt wasting may be cause by damaged brain cells that affect sympathetic neural input to the kidneys, decreasing sodium resorption and an increase in atrial natriuretic peptide and brain natriuretic peptide.
The syndrome looks similar to SIADH, with high urinary sodium and osmolality, low serum osmolality, and decreased serum uric acid. But cerebral salt wasting is distinguished from SIADH by the presence of hypovolemia. Cerebral salt wasting is treated with saline and/or salt tablets.
DVT
Neurosurgical patients are at increased risk for DVT and pulmonary embolism compared with the general postoperative population. However, 2012 guidelines on antithrombotic therapy from the American College of Chest Physicians recommend against pharmacologic prophylaxis except for high risk patients, such as patients with intracranial masses. Dr. Huh said.
Implantable neurostimulators
Patients with implanted devices such as deep-brain stimulators for control of Parkinson’s disease, hypoglossal nerve stimulators for severe sleep apnea, or vagal nerve stimulators for epilepsy also require special consideration throughout the perioperative period.
Issues to consider when managing a patient with an implantable device include the device site and its proximity to the planned surgical field, indication for the device, comorbidities, and the patient’s goals for treatment, said Dr. Deborah Richman, section chief of preoperative services at Stony Brook (New York) University Medical Center.
"What do we do with device itself? This is a team approach, and we find that it’s best coordinated by our nurses in the preop holding area, because they know what time the surgery is, they have the device company rep’s phone number, they make sure the patient is there on time, and they put everything together to prevent delays on the day of surgery," she said.
In general, device manufacturers recommend turning devices off, and to turn the amplitude down to zero to prevent accidental activation of magnetic on-off switches.
The distance from the surgery to the pulse generator should be a minimum of 20 cm, and electrocautery, if used, should be bipolar rather than monopolar, Dr. Richman said.
Patients with implanted devices will also require prophylactic antibiotics to prevent potential bacterial seeding of the device or leads, she said,
Coordination of perioperative care "is best done with clinical management pathways, so that when you have one of these patients who present to you, you have a checklist that includes the device company’s phone number, and it’s an easy go-to, so that you don’t have to start from scratch each time," she concluded.
Dr. Huh and Dr. Richman reported having no financial disclosures.
SCOTTSDALE, ARIZ. – Comanaging neurosurgical patients requires a delicate dance between primary practitioners, surgeons, anesthesiologists, and, in some cases, the makers of implantable neurostimulators, according to perioperative medicine specialists.
Special considerations with patients scheduled for neurosurgical procedures include hypertension, fever, hyponatremia, and risk of deep vein thromboembolism (DVT) and coagulopathies, said Dr. Richard Huh, director of the inpatient medical consultation service at Rush University Medical Center in Chicago, at a meeting on perioperative medicine sponsored by the University of Miami.
For example, hospitalists are typically involved in the management of blood pressure in neurosurgery patients because of the importance of controlling intracranial pressure, Dr. Huh noted.
"The trick is for the blood pressure not to get too high or too low. The Handbook of Neurosurgery suggests a goal of 140 over 90 [mm Hg]," he said.
Patients with acute subarachnoid hemorrhage are at risk for vasospasm, most frequently within 7-10 days of hemorrhage. Hypovolemia is a common cause of vasospasm and should be avoided. Medications such as nimodipine (Nimotop) can help prevent this complication.
Patients undergoing spinal procedures tend to have low blood pressure from acute blood loss or intravenous pain medications, and may require hold parameters on medications to avoid complications from hypotension.
However, on the day following spinal surgery, some patients develop hypertension, and may require additional medications for BP control.
Hyponatremia
The reported prevalence of hyponatremia in hospitalized patients ranges from 1%-7%, and the rate is even higher in neurosurgical patients, possibility because the brain’s response to changes in osmolality. Clinicians managing neurosurgical patients should be aware of the syndrome of inappropriate antidiuretic hormone secretion (SIADH) and cerebral salt wasting, Dr. Huh said.
Cerebral salt wasting may be cause by damaged brain cells that affect sympathetic neural input to the kidneys, decreasing sodium resorption and an increase in atrial natriuretic peptide and brain natriuretic peptide.
The syndrome looks similar to SIADH, with high urinary sodium and osmolality, low serum osmolality, and decreased serum uric acid. But cerebral salt wasting is distinguished from SIADH by the presence of hypovolemia. Cerebral salt wasting is treated with saline and/or salt tablets.
DVT
Neurosurgical patients are at increased risk for DVT and pulmonary embolism compared with the general postoperative population. However, 2012 guidelines on antithrombotic therapy from the American College of Chest Physicians recommend against pharmacologic prophylaxis except for high risk patients, such as patients with intracranial masses. Dr. Huh said.
Implantable neurostimulators
Patients with implanted devices such as deep-brain stimulators for control of Parkinson’s disease, hypoglossal nerve stimulators for severe sleep apnea, or vagal nerve stimulators for epilepsy also require special consideration throughout the perioperative period.
Issues to consider when managing a patient with an implantable device include the device site and its proximity to the planned surgical field, indication for the device, comorbidities, and the patient’s goals for treatment, said Dr. Deborah Richman, section chief of preoperative services at Stony Brook (New York) University Medical Center.
"What do we do with device itself? This is a team approach, and we find that it’s best coordinated by our nurses in the preop holding area, because they know what time the surgery is, they have the device company rep’s phone number, they make sure the patient is there on time, and they put everything together to prevent delays on the day of surgery," she said.
In general, device manufacturers recommend turning devices off, and to turn the amplitude down to zero to prevent accidental activation of magnetic on-off switches.
The distance from the surgery to the pulse generator should be a minimum of 20 cm, and electrocautery, if used, should be bipolar rather than monopolar, Dr. Richman said.
Patients with implanted devices will also require prophylactic antibiotics to prevent potential bacterial seeding of the device or leads, she said,
Coordination of perioperative care "is best done with clinical management pathways, so that when you have one of these patients who present to you, you have a checklist that includes the device company’s phone number, and it’s an easy go-to, so that you don’t have to start from scratch each time," she concluded.
Dr. Huh and Dr. Richman reported having no financial disclosures.
AT THE PERIOPERATIVE MEDICINE SUMMIT
Major finding: Neurosurgical procedures are associated with increased risk for complications, such as hyponatremia and deep vein thromboembolism, compared with general surgeries.
Data source: Reviews of recommendations on the perioperative management of neurosurgical patients and those with implanted neurostimulating devices.
Disclosures: Dr. Huh and Dr. Richman reported having no financial disclosures.