User login
High parathyroid hormone level a marker for NAFLD and possibly NASH
TOPLINE:
METHODOLOGY:
- The researchers conducted a systematic review and meta-analysis of 12 case-control studies of patients with NAFLD/NASH and a comparison group without NAFLD/NASH.
- All studies had data on mean PTH levels in cases and controls.
- Pooled weighted mean difference (WMD) was calculated by combining WMDs of each study using a random-effects model.
TAKEAWAY:
- A meta-analysis of 10 studies with 1,051 patients with NAFLD and 1,510 controls revealed a significant association between high PTH level and NAFLD, with a pooled WMD of 5.479.
- A meta-analysis of four studies with 99 patients with NASH and 143 controls revealed a trend toward an association of high PTH level and NASH, with a pooled WMD of 11.995; statistical significance was not achieved owing to inadequate power.
- Both meta-analyses had high statistical heterogeneity (I2 of 82.4% for NAFLD and 81.0% for NASH).
IN PRACTICE:
“These findings may have clinical implications as they may suggest that high PTH level could be another biochemical marker of presence of NAFLD and possibly NASH,” the researchers wrote.
SOURCE:
This study was led by Aunchalee Jaroenlapnopparat, MD, Mount Auburn Hospital/Beth Israel Lahey Health, Cambridge, Mass. It was published online in Diabetes & Metabolic Syndrome: Research & Reviews. The study had no funding.
LIMITATIONS:
This systematic review and meta-analysis included observational studies, which might not show a causal relationship owing to potential confounding effects. Both meta-analyses demonstrated high statistical heterogeneity, probably because of differences in study design, population, and quality among the included studies. The number of studies and participants in the NASH-related analysis were limited, which may have compromised the statistical power of the analysis.
DISCLOSURES:
The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The researchers conducted a systematic review and meta-analysis of 12 case-control studies of patients with NAFLD/NASH and a comparison group without NAFLD/NASH.
- All studies had data on mean PTH levels in cases and controls.
- Pooled weighted mean difference (WMD) was calculated by combining WMDs of each study using a random-effects model.
TAKEAWAY:
- A meta-analysis of 10 studies with 1,051 patients with NAFLD and 1,510 controls revealed a significant association between high PTH level and NAFLD, with a pooled WMD of 5.479.
- A meta-analysis of four studies with 99 patients with NASH and 143 controls revealed a trend toward an association of high PTH level and NASH, with a pooled WMD of 11.995; statistical significance was not achieved owing to inadequate power.
- Both meta-analyses had high statistical heterogeneity (I2 of 82.4% for NAFLD and 81.0% for NASH).
IN PRACTICE:
“These findings may have clinical implications as they may suggest that high PTH level could be another biochemical marker of presence of NAFLD and possibly NASH,” the researchers wrote.
SOURCE:
This study was led by Aunchalee Jaroenlapnopparat, MD, Mount Auburn Hospital/Beth Israel Lahey Health, Cambridge, Mass. It was published online in Diabetes & Metabolic Syndrome: Research & Reviews. The study had no funding.
LIMITATIONS:
This systematic review and meta-analysis included observational studies, which might not show a causal relationship owing to potential confounding effects. Both meta-analyses demonstrated high statistical heterogeneity, probably because of differences in study design, population, and quality among the included studies. The number of studies and participants in the NASH-related analysis were limited, which may have compromised the statistical power of the analysis.
DISCLOSURES:
The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The researchers conducted a systematic review and meta-analysis of 12 case-control studies of patients with NAFLD/NASH and a comparison group without NAFLD/NASH.
- All studies had data on mean PTH levels in cases and controls.
- Pooled weighted mean difference (WMD) was calculated by combining WMDs of each study using a random-effects model.
TAKEAWAY:
- A meta-analysis of 10 studies with 1,051 patients with NAFLD and 1,510 controls revealed a significant association between high PTH level and NAFLD, with a pooled WMD of 5.479.
- A meta-analysis of four studies with 99 patients with NASH and 143 controls revealed a trend toward an association of high PTH level and NASH, with a pooled WMD of 11.995; statistical significance was not achieved owing to inadequate power.
- Both meta-analyses had high statistical heterogeneity (I2 of 82.4% for NAFLD and 81.0% for NASH).
IN PRACTICE:
“These findings may have clinical implications as they may suggest that high PTH level could be another biochemical marker of presence of NAFLD and possibly NASH,” the researchers wrote.
SOURCE:
This study was led by Aunchalee Jaroenlapnopparat, MD, Mount Auburn Hospital/Beth Israel Lahey Health, Cambridge, Mass. It was published online in Diabetes & Metabolic Syndrome: Research & Reviews. The study had no funding.
LIMITATIONS:
This systematic review and meta-analysis included observational studies, which might not show a causal relationship owing to potential confounding effects. Both meta-analyses demonstrated high statistical heterogeneity, probably because of differences in study design, population, and quality among the included studies. The number of studies and participants in the NASH-related analysis were limited, which may have compromised the statistical power of the analysis.
DISCLOSURES:
The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM DIABETES & METABOLIC SYNDROME: RESEARCH & REVIEWS
Using Ozempic for ‘minor’ weight loss: Fair or foul?
Ashley Raibick is familiar with the weight loss yo-yo. She’s bounced through the big names: Weight Watchers, Jenny Craig, and so on. She drops 10 pounds and then slides off the plan only to see her weight pop back up.
But a day at her local med spa – where she gets facials, Botox, and fillers – changed all that for the 28-year-old hairstylist who just wanted to lose 18 pounds.
During one of her visits, she noticed that the spa’s owner was thinner. When Ms. Raibick asked her how she did it, the owner explained that she was on semaglutide and talked Ms. Raibick through the process. Ms. Raibick was convinced. That same day, she got a prescription from a doctor at the spa and got her first shot.
“Are people going to think I’m crazy for doing this?” she recalls thinking.
At 5 foot 4, her starting weight before the drug was 158, which would put her in the overweight, but not obese, category based on body mass index (BMI). And she really just wanted to get down to 140 and stop there.
Ozempic is part of an ever-growing group of GLP-1 receptor agonists that contain a peptide called semaglutide as its main ingredient. Although first meant to treat type 2 diabetes, the reputation of Ozempic and its siblings picked up when already-thin celebrities were suspected of using the injectable drugs to become even slimmer.
The FDA approved Ozempic’s cousin, Wegovy, for “weight management” in patients with obesity a few years ago, whereas Ozempic is currently approved only for diabetes treatment. Curious patients who don’t fit the criteria can – and do – get off-label prescriptions if they can afford to pay out of pocket, often to the tune of more than $1,400 a month.
For many – mainly those who have been on the drug for a couple of months and have lost weight as a result – taking Ozempic has not only helped them shed stubborn weight, but has also freed them from the constant internal chatter around eating, commonly called “food noise.” But experts do not all agree that semaglutide is the right path for those who aren’t technically obese – especially in the long term.
After her first 9 weeks on semaglutide, Ms. Raibick had already lost 18 pounds. That’s when she decided to post about it on TikTok, and her videos on GLP-1s were viewed hundreds of thousands of times.
For the time being, there is no data on how many semaglutide takers are using the drug for diabetes and/or obesity, and how many are using it off-label for weight loss alone. But the company that makes Ozempic, Novo Nordisk, has reported sharp increases in sales and projects more profits down the road.
Ms. Raibick knows of others like her, who sought out the drug for more minor weight loss but aren’t as candid about their journeys. Some feel a stigma about having to resort to a weight-loss drug intended to treat obesity, rather than achieving their goals with diet and lifestyle change alone.
Another reason for the secrecy is the guilt some who take Ozempic feel about using their financial privilege to get a drug that had serious shortages, which made it harder for some patients who need the drug for diabetes or obesity treatment to get their doses.
That’s what Diana Thiara, MD, the medical director of the University of California, San Francisco’s weight management program, has been seeing on the ground.
“It’s one of the most depressing things I’ve experienced as a physician,” she said. In her practice, she has seen patients who have finally been able to access GLP-1s and have started to lose weight, only for them to regain the weight in the time it takes to find another prescription under their insurance coverage.
“It’s just horrible, there are patients spending all day calling dozens of pharmacies. I’ve never had a situation like this in my career,” said Dr. Thiara.
Ann, 48, a mom who works from home full-time, has been taking Ozempic since the end of January. (Ann is not her real name; she asked that we use a pseudonym in order to feel comfortable speaking publicly about her use of Ozempic). Like Ms. Raibick, she has been paying out of pocket for her shots. At first, she was going to have to pay $1,400 a month, but she found a pharmacy in Canada that offers the medication for $350. It’s sourced globally, she said, so sometimes her Ozempic boxes will be in Czech or another foreign language.
Unlike a lot of women, Ann never had any qualms with her weight or the way her body looked. She was never big on exercise, but it wasn’t until the pandemic that she started to gain weight. She noticed the changes in her body once places started opening back up, and her clothes didn’t fit anymore.
She tried moving more and eating healthier. She tried former Real Housewives of Beverly Hills cast member Teddi Mellencamp’s controversial weight-loss program, infamous for its incredibly restrictive dietary plan and excessive cardio recommendations. Nothing worked until another mom at her daughter’s school mentioned that she was on Ozempic.
Ann also started to get hot flashes and missed periods. The doctor who prescribed her Ozempic confirmed that she was perimenopausal and that, for women in this stage of life, losing weight can be harder than ever.
Ann, who is 5 foot 7, started out at 176 pounds (considered overweight) and now weighs in at 151, which is considered a normal weight by BMI measurements. She’s still on Ozempic but continues to struggle with the shame around the idea she’s potentially taking the drug away from someone else who might desperately need it. And she doesn’t know how long she’ll have to stay on Ozempic to maintain her weight loss.
Ann has reason for concern. A 2022 study found that most people regain the weight they lost within a year of stopping Ozempic.
Once Ms. Raibick hit her initial goal weight, she felt that she could keep going and lose a little more. It wasn’t until she got into the 120-pound range that she decided it was time to wean off the dose of semaglutide she had been taking.
“I got to the point where my mom was like, ‘All right, you’re a little too thin.’ But I’m just so happy where I’m at. I’m not mentally stressed out about fitting into clothes or getting into a bathing suit,” said Ms. Raibick, who has now lost around 30 pounds in total since she started the shots.
At one point, she stopped taking the drug altogether, and all of the hunger cravings and food noise semaglutide had suppressed came back to the surface. She didn’t gain any weight that month, she said, but the internal chatter around food was enough to make her start back on a lower dose, geared toward weight maintenance.
There’s also the issue of side effects. Ms. Raibick says she never had the overwhelming nausea and digestive problems that so many on the drug – including Ann – have reported. But Dr. Thiara said that even beyond these more common side effects, there are a number of other concerns – like the long-lasting effects on thyroid and reproductive health, especially for women – that we still don’t know enough about. And just recently, CNN reported that some Ozempic users have developed stomach paralysis due to the drug’s ability to slow down the passage of food through the digestive tract.
For Ms. Raibick, the out-of-pocket cost for the drug is around $600 a month. It’s an expense she’s willing to keep paying for, even just for the peace of mind the drug provides. She doesn’t have any plans to stop her semaglutide shots soon.
“There is nothing stopping me from – a year from now, when I’ve put a little weight back on – looking back at photos from this time and thinking I was way too skinny.”
Dan Azagury, MD, a bariatric surgeon and associate professor of surgery at Stanford (Calif.) University, tries GLP-1s for patients with obesity before considering bariatric surgery. For his patient population, it’s possible that drugs like Ozempic will be part of their lifelong treatment plans.
“We’re not doing it for the cosmetic part of it, we’re doing it for health,” he said. “What I tell my patients is, if you’re planning to start on this medication, you should be OK with the idea of staying on it forever.”
For doctors like Dr. Thiara who specialize in weight management, using Ozempic long-term for patients in a healthy weight range is the wrong approach.
“It’s not about the way people look, it’s about health. If you’re a normal weight or even in an overweight category, but not showing signs of risk of having elevated cardiometabolic disease ... You don’t need to be taking medications for weight loss,” she said. “This idea of using medications for aesthetic reasons is really more related to societal ills around how we value fitness above anything else. That’s not the goal, and it’s not safe.”
A version of this article first appeared on WebMD.com.
Ashley Raibick is familiar with the weight loss yo-yo. She’s bounced through the big names: Weight Watchers, Jenny Craig, and so on. She drops 10 pounds and then slides off the plan only to see her weight pop back up.
But a day at her local med spa – where she gets facials, Botox, and fillers – changed all that for the 28-year-old hairstylist who just wanted to lose 18 pounds.
During one of her visits, she noticed that the spa’s owner was thinner. When Ms. Raibick asked her how she did it, the owner explained that she was on semaglutide and talked Ms. Raibick through the process. Ms. Raibick was convinced. That same day, she got a prescription from a doctor at the spa and got her first shot.
“Are people going to think I’m crazy for doing this?” she recalls thinking.
At 5 foot 4, her starting weight before the drug was 158, which would put her in the overweight, but not obese, category based on body mass index (BMI). And she really just wanted to get down to 140 and stop there.
Ozempic is part of an ever-growing group of GLP-1 receptor agonists that contain a peptide called semaglutide as its main ingredient. Although first meant to treat type 2 diabetes, the reputation of Ozempic and its siblings picked up when already-thin celebrities were suspected of using the injectable drugs to become even slimmer.
The FDA approved Ozempic’s cousin, Wegovy, for “weight management” in patients with obesity a few years ago, whereas Ozempic is currently approved only for diabetes treatment. Curious patients who don’t fit the criteria can – and do – get off-label prescriptions if they can afford to pay out of pocket, often to the tune of more than $1,400 a month.
For many – mainly those who have been on the drug for a couple of months and have lost weight as a result – taking Ozempic has not only helped them shed stubborn weight, but has also freed them from the constant internal chatter around eating, commonly called “food noise.” But experts do not all agree that semaglutide is the right path for those who aren’t technically obese – especially in the long term.
After her first 9 weeks on semaglutide, Ms. Raibick had already lost 18 pounds. That’s when she decided to post about it on TikTok, and her videos on GLP-1s were viewed hundreds of thousands of times.
For the time being, there is no data on how many semaglutide takers are using the drug for diabetes and/or obesity, and how many are using it off-label for weight loss alone. But the company that makes Ozempic, Novo Nordisk, has reported sharp increases in sales and projects more profits down the road.
Ms. Raibick knows of others like her, who sought out the drug for more minor weight loss but aren’t as candid about their journeys. Some feel a stigma about having to resort to a weight-loss drug intended to treat obesity, rather than achieving their goals with diet and lifestyle change alone.
Another reason for the secrecy is the guilt some who take Ozempic feel about using their financial privilege to get a drug that had serious shortages, which made it harder for some patients who need the drug for diabetes or obesity treatment to get their doses.
That’s what Diana Thiara, MD, the medical director of the University of California, San Francisco’s weight management program, has been seeing on the ground.
“It’s one of the most depressing things I’ve experienced as a physician,” she said. In her practice, she has seen patients who have finally been able to access GLP-1s and have started to lose weight, only for them to regain the weight in the time it takes to find another prescription under their insurance coverage.
“It’s just horrible, there are patients spending all day calling dozens of pharmacies. I’ve never had a situation like this in my career,” said Dr. Thiara.
Ann, 48, a mom who works from home full-time, has been taking Ozempic since the end of January. (Ann is not her real name; she asked that we use a pseudonym in order to feel comfortable speaking publicly about her use of Ozempic). Like Ms. Raibick, she has been paying out of pocket for her shots. At first, she was going to have to pay $1,400 a month, but she found a pharmacy in Canada that offers the medication for $350. It’s sourced globally, she said, so sometimes her Ozempic boxes will be in Czech or another foreign language.
Unlike a lot of women, Ann never had any qualms with her weight or the way her body looked. She was never big on exercise, but it wasn’t until the pandemic that she started to gain weight. She noticed the changes in her body once places started opening back up, and her clothes didn’t fit anymore.
She tried moving more and eating healthier. She tried former Real Housewives of Beverly Hills cast member Teddi Mellencamp’s controversial weight-loss program, infamous for its incredibly restrictive dietary plan and excessive cardio recommendations. Nothing worked until another mom at her daughter’s school mentioned that she was on Ozempic.
Ann also started to get hot flashes and missed periods. The doctor who prescribed her Ozempic confirmed that she was perimenopausal and that, for women in this stage of life, losing weight can be harder than ever.
Ann, who is 5 foot 7, started out at 176 pounds (considered overweight) and now weighs in at 151, which is considered a normal weight by BMI measurements. She’s still on Ozempic but continues to struggle with the shame around the idea she’s potentially taking the drug away from someone else who might desperately need it. And she doesn’t know how long she’ll have to stay on Ozempic to maintain her weight loss.
Ann has reason for concern. A 2022 study found that most people regain the weight they lost within a year of stopping Ozempic.
Once Ms. Raibick hit her initial goal weight, she felt that she could keep going and lose a little more. It wasn’t until she got into the 120-pound range that she decided it was time to wean off the dose of semaglutide she had been taking.
“I got to the point where my mom was like, ‘All right, you’re a little too thin.’ But I’m just so happy where I’m at. I’m not mentally stressed out about fitting into clothes or getting into a bathing suit,” said Ms. Raibick, who has now lost around 30 pounds in total since she started the shots.
At one point, she stopped taking the drug altogether, and all of the hunger cravings and food noise semaglutide had suppressed came back to the surface. She didn’t gain any weight that month, she said, but the internal chatter around food was enough to make her start back on a lower dose, geared toward weight maintenance.
There’s also the issue of side effects. Ms. Raibick says she never had the overwhelming nausea and digestive problems that so many on the drug – including Ann – have reported. But Dr. Thiara said that even beyond these more common side effects, there are a number of other concerns – like the long-lasting effects on thyroid and reproductive health, especially for women – that we still don’t know enough about. And just recently, CNN reported that some Ozempic users have developed stomach paralysis due to the drug’s ability to slow down the passage of food through the digestive tract.
For Ms. Raibick, the out-of-pocket cost for the drug is around $600 a month. It’s an expense she’s willing to keep paying for, even just for the peace of mind the drug provides. She doesn’t have any plans to stop her semaglutide shots soon.
“There is nothing stopping me from – a year from now, when I’ve put a little weight back on – looking back at photos from this time and thinking I was way too skinny.”
Dan Azagury, MD, a bariatric surgeon and associate professor of surgery at Stanford (Calif.) University, tries GLP-1s for patients with obesity before considering bariatric surgery. For his patient population, it’s possible that drugs like Ozempic will be part of their lifelong treatment plans.
“We’re not doing it for the cosmetic part of it, we’re doing it for health,” he said. “What I tell my patients is, if you’re planning to start on this medication, you should be OK with the idea of staying on it forever.”
For doctors like Dr. Thiara who specialize in weight management, using Ozempic long-term for patients in a healthy weight range is the wrong approach.
“It’s not about the way people look, it’s about health. If you’re a normal weight or even in an overweight category, but not showing signs of risk of having elevated cardiometabolic disease ... You don’t need to be taking medications for weight loss,” she said. “This idea of using medications for aesthetic reasons is really more related to societal ills around how we value fitness above anything else. That’s not the goal, and it’s not safe.”
A version of this article first appeared on WebMD.com.
Ashley Raibick is familiar with the weight loss yo-yo. She’s bounced through the big names: Weight Watchers, Jenny Craig, and so on. She drops 10 pounds and then slides off the plan only to see her weight pop back up.
But a day at her local med spa – where she gets facials, Botox, and fillers – changed all that for the 28-year-old hairstylist who just wanted to lose 18 pounds.
During one of her visits, she noticed that the spa’s owner was thinner. When Ms. Raibick asked her how she did it, the owner explained that she was on semaglutide and talked Ms. Raibick through the process. Ms. Raibick was convinced. That same day, she got a prescription from a doctor at the spa and got her first shot.
“Are people going to think I’m crazy for doing this?” she recalls thinking.
At 5 foot 4, her starting weight before the drug was 158, which would put her in the overweight, but not obese, category based on body mass index (BMI). And she really just wanted to get down to 140 and stop there.
Ozempic is part of an ever-growing group of GLP-1 receptor agonists that contain a peptide called semaglutide as its main ingredient. Although first meant to treat type 2 diabetes, the reputation of Ozempic and its siblings picked up when already-thin celebrities were suspected of using the injectable drugs to become even slimmer.
The FDA approved Ozempic’s cousin, Wegovy, for “weight management” in patients with obesity a few years ago, whereas Ozempic is currently approved only for diabetes treatment. Curious patients who don’t fit the criteria can – and do – get off-label prescriptions if they can afford to pay out of pocket, often to the tune of more than $1,400 a month.
For many – mainly those who have been on the drug for a couple of months and have lost weight as a result – taking Ozempic has not only helped them shed stubborn weight, but has also freed them from the constant internal chatter around eating, commonly called “food noise.” But experts do not all agree that semaglutide is the right path for those who aren’t technically obese – especially in the long term.
After her first 9 weeks on semaglutide, Ms. Raibick had already lost 18 pounds. That’s when she decided to post about it on TikTok, and her videos on GLP-1s were viewed hundreds of thousands of times.
For the time being, there is no data on how many semaglutide takers are using the drug for diabetes and/or obesity, and how many are using it off-label for weight loss alone. But the company that makes Ozempic, Novo Nordisk, has reported sharp increases in sales and projects more profits down the road.
Ms. Raibick knows of others like her, who sought out the drug for more minor weight loss but aren’t as candid about their journeys. Some feel a stigma about having to resort to a weight-loss drug intended to treat obesity, rather than achieving their goals with diet and lifestyle change alone.
Another reason for the secrecy is the guilt some who take Ozempic feel about using their financial privilege to get a drug that had serious shortages, which made it harder for some patients who need the drug for diabetes or obesity treatment to get their doses.
That’s what Diana Thiara, MD, the medical director of the University of California, San Francisco’s weight management program, has been seeing on the ground.
“It’s one of the most depressing things I’ve experienced as a physician,” she said. In her practice, she has seen patients who have finally been able to access GLP-1s and have started to lose weight, only for them to regain the weight in the time it takes to find another prescription under their insurance coverage.
“It’s just horrible, there are patients spending all day calling dozens of pharmacies. I’ve never had a situation like this in my career,” said Dr. Thiara.
Ann, 48, a mom who works from home full-time, has been taking Ozempic since the end of January. (Ann is not her real name; she asked that we use a pseudonym in order to feel comfortable speaking publicly about her use of Ozempic). Like Ms. Raibick, she has been paying out of pocket for her shots. At first, she was going to have to pay $1,400 a month, but she found a pharmacy in Canada that offers the medication for $350. It’s sourced globally, she said, so sometimes her Ozempic boxes will be in Czech or another foreign language.
Unlike a lot of women, Ann never had any qualms with her weight or the way her body looked. She was never big on exercise, but it wasn’t until the pandemic that she started to gain weight. She noticed the changes in her body once places started opening back up, and her clothes didn’t fit anymore.
She tried moving more and eating healthier. She tried former Real Housewives of Beverly Hills cast member Teddi Mellencamp’s controversial weight-loss program, infamous for its incredibly restrictive dietary plan and excessive cardio recommendations. Nothing worked until another mom at her daughter’s school mentioned that she was on Ozempic.
Ann also started to get hot flashes and missed periods. The doctor who prescribed her Ozempic confirmed that she was perimenopausal and that, for women in this stage of life, losing weight can be harder than ever.
Ann, who is 5 foot 7, started out at 176 pounds (considered overweight) and now weighs in at 151, which is considered a normal weight by BMI measurements. She’s still on Ozempic but continues to struggle with the shame around the idea she’s potentially taking the drug away from someone else who might desperately need it. And she doesn’t know how long she’ll have to stay on Ozempic to maintain her weight loss.
Ann has reason for concern. A 2022 study found that most people regain the weight they lost within a year of stopping Ozempic.
Once Ms. Raibick hit her initial goal weight, she felt that she could keep going and lose a little more. It wasn’t until she got into the 120-pound range that she decided it was time to wean off the dose of semaglutide she had been taking.
“I got to the point where my mom was like, ‘All right, you’re a little too thin.’ But I’m just so happy where I’m at. I’m not mentally stressed out about fitting into clothes or getting into a bathing suit,” said Ms. Raibick, who has now lost around 30 pounds in total since she started the shots.
At one point, she stopped taking the drug altogether, and all of the hunger cravings and food noise semaglutide had suppressed came back to the surface. She didn’t gain any weight that month, she said, but the internal chatter around food was enough to make her start back on a lower dose, geared toward weight maintenance.
There’s also the issue of side effects. Ms. Raibick says she never had the overwhelming nausea and digestive problems that so many on the drug – including Ann – have reported. But Dr. Thiara said that even beyond these more common side effects, there are a number of other concerns – like the long-lasting effects on thyroid and reproductive health, especially for women – that we still don’t know enough about. And just recently, CNN reported that some Ozempic users have developed stomach paralysis due to the drug’s ability to slow down the passage of food through the digestive tract.
For Ms. Raibick, the out-of-pocket cost for the drug is around $600 a month. It’s an expense she’s willing to keep paying for, even just for the peace of mind the drug provides. She doesn’t have any plans to stop her semaglutide shots soon.
“There is nothing stopping me from – a year from now, when I’ve put a little weight back on – looking back at photos from this time and thinking I was way too skinny.”
Dan Azagury, MD, a bariatric surgeon and associate professor of surgery at Stanford (Calif.) University, tries GLP-1s for patients with obesity before considering bariatric surgery. For his patient population, it’s possible that drugs like Ozempic will be part of their lifelong treatment plans.
“We’re not doing it for the cosmetic part of it, we’re doing it for health,” he said. “What I tell my patients is, if you’re planning to start on this medication, you should be OK with the idea of staying on it forever.”
For doctors like Dr. Thiara who specialize in weight management, using Ozempic long-term for patients in a healthy weight range is the wrong approach.
“It’s not about the way people look, it’s about health. If you’re a normal weight or even in an overweight category, but not showing signs of risk of having elevated cardiometabolic disease ... You don’t need to be taking medications for weight loss,” she said. “This idea of using medications for aesthetic reasons is really more related to societal ills around how we value fitness above anything else. That’s not the goal, and it’s not safe.”
A version of this article first appeared on WebMD.com.
Obesity: Don’t separate mental health from physical health
“The patient is ready,” the medical assistant informs you while handing you the chart. The chart reads: “Chief complaint: Weight gain/Discuss weight loss options.” You note the normal vital signs other than an increased BMI to 34 from 4 months ago. You knock on the exam room door with your plan half-formulated.
“Come in,” the patient says, almost too softly for you to hear. Shock overtakes you as you enter the room and see something you never imagined. The patient is holding their disconnected head in their lap as they say, “Nice to see you, Doc. I want to do something about my weight.”
You’re baffled at how they are speaking with a disconnected head. Of course, this outlandish patient scenario isn’t real. Or is it?
Patients with mental health concerns don’t literally present with their head disconnected from their bodies. Too often, mental health is treated as separate from physical health, especially regarding weight management and obesity. However, studies have shown an association between mental health and obesity. In this pivotal time of pharmacologic innovation in obesity care, we must also ensure that we effectively address the mental health of our patients with obesity.
Screening
Mental health conditions can look different for everyone. It can be hard to diagnose a mental health condition without validated screening. For example, depression is one of the most common mental health disorders. The U.S. Preventive Services Task Force recommends depression screening in all adults.
The Patient Health Questionnaire-2 (PHQ-2) is one screening tool that can alert doctors and clinicians to potential depression. Patients with obesity have higher rates of depression and other mental health conditions. It’s even more critical to screen for depression and other mental health disorders when prescribing these new medications, given recent reports of suicidal ideation with certain antiobesity medications.
Stigma
Mental health–related stigma can trigger shame and prevent patients from seeking psychological help. Furthermore, compounded stigma in patients with larger bodies (weight bias) and from marginalized communities such as the Black community (racial discrimination) add more barriers to seeking mental health care. When patients seek care for mental health conditions, they may feel more comfortable seeing a primary care physician or other clinician than a mental health professional. Therefore, all physicians and clinicians are integral in normalizing mental health care. Instead of treating mental health as separate from physical health, discussing the bidirectional relationship between mental health conditions and physiologic diseases can help patients understand that having a mental health condition isn’t a choice and facilitate openness to multiple treatment options to improve their quality of life.
Support
Addressing mental health effectively often requires multiple layers of patient support. Support can come from loved ones or community groups. But for severe stress and other mental health conditions, treatment with psychotherapy or psychiatric medications is essential. Unfortunately, even if a patient is willing to see a mental health professional, availability or access may be a challenge. Therefore, other clinicians may have to step in and serve as a bridge to mental health care. It’s also essential to ensure that patients are aware of crisis support lines and online resources for mental health care.
Stress
Having a high level of stress can be harmful physically and can also worsen mental health conditions. Additionally, it can contribute to a higher risk for obesity and can trigger emotional eating. Chronic stress has become so common in society that patients often underestimate how much stress they are under. Assessments like the Holmes-Rahe Stress Inventory can help patients identify and quantify potential stressors. While some stressors are uncontrollable, such as social determinants of health (SDOH), addressing controllable stressors and improving coping mechanisms is possible. For instance, mindfulness and breathwork are easy to follow and relatively accessible for most patients.
Social determinants of health
For a treatment plan to be maximally impactful, we must incorporate SDOH in clinical care. SDOH includes financial instability, safe neighborhoods, and more, and can significantly influence an ideal treatment plan. Furthermore, a high SDOH burden can negatively affect mental health and obesity rates. It’s helpful to incorporate patients’ SDOH burden into treatment planning. Learn how to take action on SDOH.
Empowerment
Patients who address their mental health have taken a courageous step toward health and healing. As mentioned, they may experience gaps in care while awaiting connection to the next steps of their journey, such as starting care with a mental health professional or waiting for a medication to take effect. All clinicians can empower patients about their weight by informing them that:
Food may affect their mood. Studies show that certain foods and eating patterns are associated with high levels of depression and anxiety. Limiting processed foods and increasing fruits, vegetables, and foods high in vitamin D, C, and other nutrients is helpful. Everyone is different, so encourage patients to pay attention to how food uniquely affects their mood by keeping a food/feeling log for 1-3 days.
Move more. Increased physical activity can improve mental health.
Get outdoors. Time in nature is associated with better mental health. Spending as little as 10 minutes outside can be beneficial. It’s important to be aware that SDOH factors such as unsafe environments or limited outdoor access may make this difficult for some patients.
Positive stress-relieving activities. Each person has their own way of reducing stress. It is helpful to remind patients of unhealthy stress relievers such as overeating, drinking alcohol, and smoking, and encourage them to replace those with positive stress relievers.
Spiritual well-being. Spirituality is often overlooked in health care. But studies have shown that incorporating a person’s spirituality may have positive health benefits.
It’s time to stop disconnecting mental health from physical health. Each clinician plays a vital role in treating the whole person. Just as you wouldn’t let a patient with a disconnected head leave the office without addressing it, let’s not leave mental health out when addressing our patients’ weight concerns.
Dr. Gonsahn-Bollie is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating. Connect with her at www.embraceyouweightloss.com or on Instagram @embraceyoumd. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness,” (Baltimore: Purposely Created Publishing Group, 2019) was Healthline.com’s Best Overall Weight Loss Book of 2022 and one of Livestrong.com’s 8 Best Weight-Loss Books to Read in 2022.
Dr. Gonsahn-Bollie is CEO and Lead Physician, Embrace You Weight and Wellness, Telehealth & Virtual Counseling. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“The patient is ready,” the medical assistant informs you while handing you the chart. The chart reads: “Chief complaint: Weight gain/Discuss weight loss options.” You note the normal vital signs other than an increased BMI to 34 from 4 months ago. You knock on the exam room door with your plan half-formulated.
“Come in,” the patient says, almost too softly for you to hear. Shock overtakes you as you enter the room and see something you never imagined. The patient is holding their disconnected head in their lap as they say, “Nice to see you, Doc. I want to do something about my weight.”
You’re baffled at how they are speaking with a disconnected head. Of course, this outlandish patient scenario isn’t real. Or is it?
Patients with mental health concerns don’t literally present with their head disconnected from their bodies. Too often, mental health is treated as separate from physical health, especially regarding weight management and obesity. However, studies have shown an association between mental health and obesity. In this pivotal time of pharmacologic innovation in obesity care, we must also ensure that we effectively address the mental health of our patients with obesity.
Screening
Mental health conditions can look different for everyone. It can be hard to diagnose a mental health condition without validated screening. For example, depression is one of the most common mental health disorders. The U.S. Preventive Services Task Force recommends depression screening in all adults.
The Patient Health Questionnaire-2 (PHQ-2) is one screening tool that can alert doctors and clinicians to potential depression. Patients with obesity have higher rates of depression and other mental health conditions. It’s even more critical to screen for depression and other mental health disorders when prescribing these new medications, given recent reports of suicidal ideation with certain antiobesity medications.
Stigma
Mental health–related stigma can trigger shame and prevent patients from seeking psychological help. Furthermore, compounded stigma in patients with larger bodies (weight bias) and from marginalized communities such as the Black community (racial discrimination) add more barriers to seeking mental health care. When patients seek care for mental health conditions, they may feel more comfortable seeing a primary care physician or other clinician than a mental health professional. Therefore, all physicians and clinicians are integral in normalizing mental health care. Instead of treating mental health as separate from physical health, discussing the bidirectional relationship between mental health conditions and physiologic diseases can help patients understand that having a mental health condition isn’t a choice and facilitate openness to multiple treatment options to improve their quality of life.
Support
Addressing mental health effectively often requires multiple layers of patient support. Support can come from loved ones or community groups. But for severe stress and other mental health conditions, treatment with psychotherapy or psychiatric medications is essential. Unfortunately, even if a patient is willing to see a mental health professional, availability or access may be a challenge. Therefore, other clinicians may have to step in and serve as a bridge to mental health care. It’s also essential to ensure that patients are aware of crisis support lines and online resources for mental health care.
Stress
Having a high level of stress can be harmful physically and can also worsen mental health conditions. Additionally, it can contribute to a higher risk for obesity and can trigger emotional eating. Chronic stress has become so common in society that patients often underestimate how much stress they are under. Assessments like the Holmes-Rahe Stress Inventory can help patients identify and quantify potential stressors. While some stressors are uncontrollable, such as social determinants of health (SDOH), addressing controllable stressors and improving coping mechanisms is possible. For instance, mindfulness and breathwork are easy to follow and relatively accessible for most patients.
Social determinants of health
For a treatment plan to be maximally impactful, we must incorporate SDOH in clinical care. SDOH includes financial instability, safe neighborhoods, and more, and can significantly influence an ideal treatment plan. Furthermore, a high SDOH burden can negatively affect mental health and obesity rates. It’s helpful to incorporate patients’ SDOH burden into treatment planning. Learn how to take action on SDOH.
Empowerment
Patients who address their mental health have taken a courageous step toward health and healing. As mentioned, they may experience gaps in care while awaiting connection to the next steps of their journey, such as starting care with a mental health professional or waiting for a medication to take effect. All clinicians can empower patients about their weight by informing them that:
Food may affect their mood. Studies show that certain foods and eating patterns are associated with high levels of depression and anxiety. Limiting processed foods and increasing fruits, vegetables, and foods high in vitamin D, C, and other nutrients is helpful. Everyone is different, so encourage patients to pay attention to how food uniquely affects their mood by keeping a food/feeling log for 1-3 days.
Move more. Increased physical activity can improve mental health.
Get outdoors. Time in nature is associated with better mental health. Spending as little as 10 minutes outside can be beneficial. It’s important to be aware that SDOH factors such as unsafe environments or limited outdoor access may make this difficult for some patients.
Positive stress-relieving activities. Each person has their own way of reducing stress. It is helpful to remind patients of unhealthy stress relievers such as overeating, drinking alcohol, and smoking, and encourage them to replace those with positive stress relievers.
Spiritual well-being. Spirituality is often overlooked in health care. But studies have shown that incorporating a person’s spirituality may have positive health benefits.
It’s time to stop disconnecting mental health from physical health. Each clinician plays a vital role in treating the whole person. Just as you wouldn’t let a patient with a disconnected head leave the office without addressing it, let’s not leave mental health out when addressing our patients’ weight concerns.
Dr. Gonsahn-Bollie is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating. Connect with her at www.embraceyouweightloss.com or on Instagram @embraceyoumd. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness,” (Baltimore: Purposely Created Publishing Group, 2019) was Healthline.com’s Best Overall Weight Loss Book of 2022 and one of Livestrong.com’s 8 Best Weight-Loss Books to Read in 2022.
Dr. Gonsahn-Bollie is CEO and Lead Physician, Embrace You Weight and Wellness, Telehealth & Virtual Counseling. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“The patient is ready,” the medical assistant informs you while handing you the chart. The chart reads: “Chief complaint: Weight gain/Discuss weight loss options.” You note the normal vital signs other than an increased BMI to 34 from 4 months ago. You knock on the exam room door with your plan half-formulated.
“Come in,” the patient says, almost too softly for you to hear. Shock overtakes you as you enter the room and see something you never imagined. The patient is holding their disconnected head in their lap as they say, “Nice to see you, Doc. I want to do something about my weight.”
You’re baffled at how they are speaking with a disconnected head. Of course, this outlandish patient scenario isn’t real. Or is it?
Patients with mental health concerns don’t literally present with their head disconnected from their bodies. Too often, mental health is treated as separate from physical health, especially regarding weight management and obesity. However, studies have shown an association between mental health and obesity. In this pivotal time of pharmacologic innovation in obesity care, we must also ensure that we effectively address the mental health of our patients with obesity.
Screening
Mental health conditions can look different for everyone. It can be hard to diagnose a mental health condition without validated screening. For example, depression is one of the most common mental health disorders. The U.S. Preventive Services Task Force recommends depression screening in all adults.
The Patient Health Questionnaire-2 (PHQ-2) is one screening tool that can alert doctors and clinicians to potential depression. Patients with obesity have higher rates of depression and other mental health conditions. It’s even more critical to screen for depression and other mental health disorders when prescribing these new medications, given recent reports of suicidal ideation with certain antiobesity medications.
Stigma
Mental health–related stigma can trigger shame and prevent patients from seeking psychological help. Furthermore, compounded stigma in patients with larger bodies (weight bias) and from marginalized communities such as the Black community (racial discrimination) add more barriers to seeking mental health care. When patients seek care for mental health conditions, they may feel more comfortable seeing a primary care physician or other clinician than a mental health professional. Therefore, all physicians and clinicians are integral in normalizing mental health care. Instead of treating mental health as separate from physical health, discussing the bidirectional relationship between mental health conditions and physiologic diseases can help patients understand that having a mental health condition isn’t a choice and facilitate openness to multiple treatment options to improve their quality of life.
Support
Addressing mental health effectively often requires multiple layers of patient support. Support can come from loved ones or community groups. But for severe stress and other mental health conditions, treatment with psychotherapy or psychiatric medications is essential. Unfortunately, even if a patient is willing to see a mental health professional, availability or access may be a challenge. Therefore, other clinicians may have to step in and serve as a bridge to mental health care. It’s also essential to ensure that patients are aware of crisis support lines and online resources for mental health care.
Stress
Having a high level of stress can be harmful physically and can also worsen mental health conditions. Additionally, it can contribute to a higher risk for obesity and can trigger emotional eating. Chronic stress has become so common in society that patients often underestimate how much stress they are under. Assessments like the Holmes-Rahe Stress Inventory can help patients identify and quantify potential stressors. While some stressors are uncontrollable, such as social determinants of health (SDOH), addressing controllable stressors and improving coping mechanisms is possible. For instance, mindfulness and breathwork are easy to follow and relatively accessible for most patients.
Social determinants of health
For a treatment plan to be maximally impactful, we must incorporate SDOH in clinical care. SDOH includes financial instability, safe neighborhoods, and more, and can significantly influence an ideal treatment plan. Furthermore, a high SDOH burden can negatively affect mental health and obesity rates. It’s helpful to incorporate patients’ SDOH burden into treatment planning. Learn how to take action on SDOH.
Empowerment
Patients who address their mental health have taken a courageous step toward health and healing. As mentioned, they may experience gaps in care while awaiting connection to the next steps of their journey, such as starting care with a mental health professional or waiting for a medication to take effect. All clinicians can empower patients about their weight by informing them that:
Food may affect their mood. Studies show that certain foods and eating patterns are associated with high levels of depression and anxiety. Limiting processed foods and increasing fruits, vegetables, and foods high in vitamin D, C, and other nutrients is helpful. Everyone is different, so encourage patients to pay attention to how food uniquely affects their mood by keeping a food/feeling log for 1-3 days.
Move more. Increased physical activity can improve mental health.
Get outdoors. Time in nature is associated with better mental health. Spending as little as 10 minutes outside can be beneficial. It’s important to be aware that SDOH factors such as unsafe environments or limited outdoor access may make this difficult for some patients.
Positive stress-relieving activities. Each person has their own way of reducing stress. It is helpful to remind patients of unhealthy stress relievers such as overeating, drinking alcohol, and smoking, and encourage them to replace those with positive stress relievers.
Spiritual well-being. Spirituality is often overlooked in health care. But studies have shown that incorporating a person’s spirituality may have positive health benefits.
It’s time to stop disconnecting mental health from physical health. Each clinician plays a vital role in treating the whole person. Just as you wouldn’t let a patient with a disconnected head leave the office without addressing it, let’s not leave mental health out when addressing our patients’ weight concerns.
Dr. Gonsahn-Bollie is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating. Connect with her at www.embraceyouweightloss.com or on Instagram @embraceyoumd. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness,” (Baltimore: Purposely Created Publishing Group, 2019) was Healthline.com’s Best Overall Weight Loss Book of 2022 and one of Livestrong.com’s 8 Best Weight-Loss Books to Read in 2022.
Dr. Gonsahn-Bollie is CEO and Lead Physician, Embrace You Weight and Wellness, Telehealth & Virtual Counseling. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
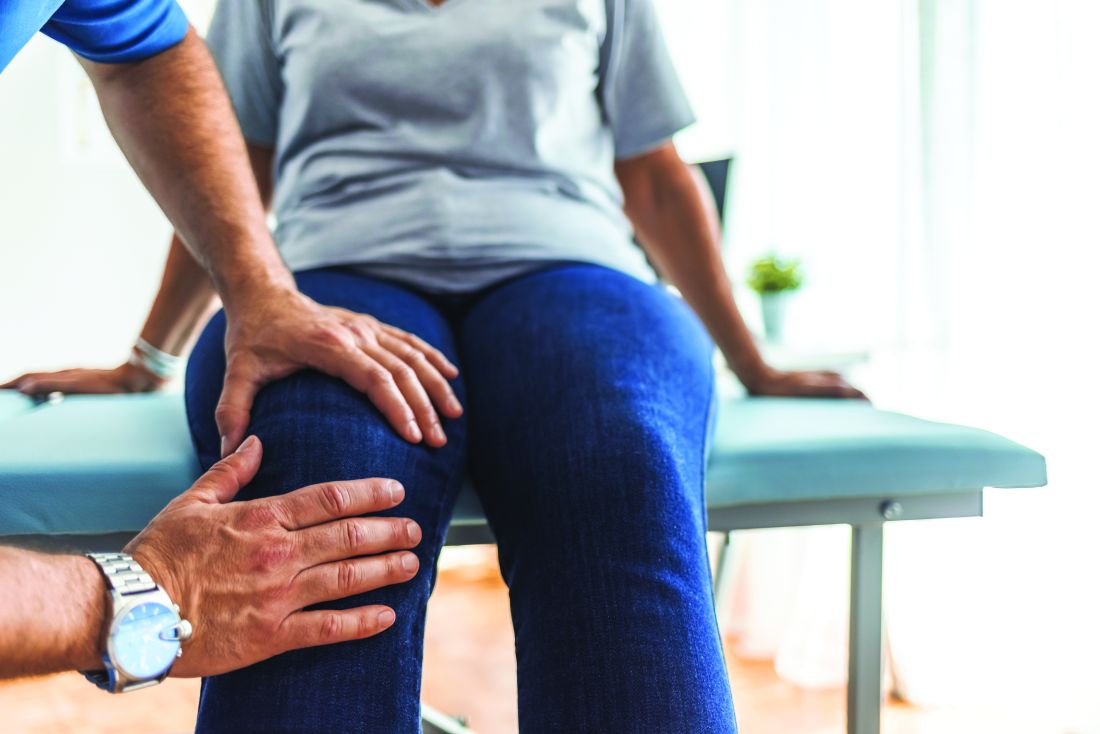
Nonalcohol substance use disorder tied to bariatric surgery
Nonalcohol substance use disorder (SUD) was 2.5 times more common in people who had gastric bypass surgery, compared with a control group who received usual obesity care, a new prospective study has found.
The findings suggest that the risk for nonalcohol SUD should be carefully explained to patients getting a gastric bypass and that the risk should be considered in care before and after the surgery, said the study authors and editorialists.
Though alcohol use disorder is a well-known side effect for some bariatric procedures, little is known about the link between the procedures and other substance abuse, wrote the study authors, led by Per-Arne Svensson, PhD, with the department of molecular and clinical medicine, Institute of Medicine, at the University of Gothenburg (Sweden).
The study was published online in Obesity.
The researchers analyzed data from the SOS study. It was originally designed to compare bariatric surgery with usual obesity care, with overall mortality as the primary outcome. The protocol also called for reporting negative effects of included treatments.
The study was conducted throughout Sweden at 25 public surgical departments and 480 primary health centers. Participants were between ages 37 and 60 years and had a body mass index of at least 34 kg/m2 for men and 38 for women.
After people with previous nonalcoholic SUD were excluded, the study population included 1,990 patients who had undergone bariatric surgery between September 1987 and January 2001, as well as 2,030 matched controls who received usual obesity care. The three types of bariatric surgery were gastric bypass (264 patients), vertical banded gastroplasty (1,353), and gastric banding (373), as chosen by the surgeons.
The follow-up was nearly 24 years.
Link found only with gastric bypass
The researchers identified participants who had nonalcoholic SUDs using the ICD from the Swedish National Patient Register covering hospital treatment (hospital stays or hospital-based outpatient care) but not primary care.
Only gastric bypass was associated with an increased incidence of nonalcoholic SUD (adjusted hazard ratio, 2.54; 95% confidence interval, 1.14-5.65), compared with controls during the follow-up period.
Among those who had gastric bypass surgery, three developed opioid-related disorders; three had sedative-, hypnotic-, or anxiolytic-related disorders; and three had other psychoactive substance–related disorders, the study authors wrote.
The researchers found no statistical difference in the incidence of nonalcoholic SUD when the groups who had undergone different surgical procedures were compared with each other.
“It is important to acknowledge that the number of affected patients was relatively low, in the single digits,” Jihad Kudsi, MD, a bariatric surgeon and chairman of surgery at Duly Health and Care, Oak Brook, Ill., said in a press release.
The findings “highlight the critical role of bariatric behavioral health clinicians in the comprehensive evaluation and care of patients both before and after weight loss surgery,” added Dr. Kudsi, who was not associated with the research.
Bariatric surgery candidates should be warned, monitored
The data indicate that patients who are candidates for bariatric surgery should be “carefully warned” about risks for nonalcoholic SUD and be monitored after the procedure, wrote James E. Mitchell, MD, a psychiatrist with the department of psychiatry and behavioral science, University of North Dakota, Fargo, and Devika Umashanker, MD, with Obesity Medicine, Hartford (Conn.) Health Care, in an accompanying editorial.
They acknowledged, however, that monitoring can be difficult given the typical low rate of follow-up of these patients.
Though the reasons for the rise in nonalcoholic SUD are not clear, Dr. Mitchell and Dr. Umashanker said biologic and psychosocial issues may be contributors to the increase.
The persistence of medical comorbidities and a lack of noted improvement in quality of life or physical mobility after the surgery has been addressed in a paper on suicide risk after bariatric surgery, the study authors also noted.
Dr. Svensson said in an interview that a mechanism for alcohol abuse after gastric bypass surgery is more evident, as measured by “increased blood alcohol levels after the surgery for a given amount of alcohol.” However, for other addictive substances, the mechanism is not obvious and needs further study.
The editorialists reminded clinicians that measuring phosphatidylethanol can be very useful in identifying and quantifying recent alcohol intake, suggesting that all clinicians, not just those in bariatric surgery clinics, should be aware of the connection between the procedures and subsequent alcohol abuse and monitor those patients carefully.
Both the study authors and the editorialists pointed out that the SOS cohort was recruited when vertical banded gastroplasty and banding were commonly used, and both methods are now rarely, if ever, used. Gastric sleeve procedures are now the most common approach, and those patients were not included in the study.
“However, gastric bypass surgery patients were included, albeit in a minority of the sample,” Dr. Mitchell and Dr. Umashanker wrote. In addition, the sample size of patients with SUD was too small to determine the drugs that were being abused.
Dr. Svensson said in an interview the main limitation is that SUD events were identified in the Swedish National Patient Register, which misses nonhospitalized patients.
“This register is very complete for hospitals, but it does not include SUD events detected in the primary health care setting,” he said. “Hence, the absolute number of events is probably a clear underestimation. However, it is unlikely that this limitation would affect the study groups (control group vs. groups with different surgical procedures) in different ways and hence the conclusions from this study are most likely valid.”
The study authors and the editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nonalcohol substance use disorder (SUD) was 2.5 times more common in people who had gastric bypass surgery, compared with a control group who received usual obesity care, a new prospective study has found.
The findings suggest that the risk for nonalcohol SUD should be carefully explained to patients getting a gastric bypass and that the risk should be considered in care before and after the surgery, said the study authors and editorialists.
Though alcohol use disorder is a well-known side effect for some bariatric procedures, little is known about the link between the procedures and other substance abuse, wrote the study authors, led by Per-Arne Svensson, PhD, with the department of molecular and clinical medicine, Institute of Medicine, at the University of Gothenburg (Sweden).
The study was published online in Obesity.
The researchers analyzed data from the SOS study. It was originally designed to compare bariatric surgery with usual obesity care, with overall mortality as the primary outcome. The protocol also called for reporting negative effects of included treatments.
The study was conducted throughout Sweden at 25 public surgical departments and 480 primary health centers. Participants were between ages 37 and 60 years and had a body mass index of at least 34 kg/m2 for men and 38 for women.
After people with previous nonalcoholic SUD were excluded, the study population included 1,990 patients who had undergone bariatric surgery between September 1987 and January 2001, as well as 2,030 matched controls who received usual obesity care. The three types of bariatric surgery were gastric bypass (264 patients), vertical banded gastroplasty (1,353), and gastric banding (373), as chosen by the surgeons.
The follow-up was nearly 24 years.
Link found only with gastric bypass
The researchers identified participants who had nonalcoholic SUDs using the ICD from the Swedish National Patient Register covering hospital treatment (hospital stays or hospital-based outpatient care) but not primary care.
Only gastric bypass was associated with an increased incidence of nonalcoholic SUD (adjusted hazard ratio, 2.54; 95% confidence interval, 1.14-5.65), compared with controls during the follow-up period.
Among those who had gastric bypass surgery, three developed opioid-related disorders; three had sedative-, hypnotic-, or anxiolytic-related disorders; and three had other psychoactive substance–related disorders, the study authors wrote.
The researchers found no statistical difference in the incidence of nonalcoholic SUD when the groups who had undergone different surgical procedures were compared with each other.
“It is important to acknowledge that the number of affected patients was relatively low, in the single digits,” Jihad Kudsi, MD, a bariatric surgeon and chairman of surgery at Duly Health and Care, Oak Brook, Ill., said in a press release.
The findings “highlight the critical role of bariatric behavioral health clinicians in the comprehensive evaluation and care of patients both before and after weight loss surgery,” added Dr. Kudsi, who was not associated with the research.
Bariatric surgery candidates should be warned, monitored
The data indicate that patients who are candidates for bariatric surgery should be “carefully warned” about risks for nonalcoholic SUD and be monitored after the procedure, wrote James E. Mitchell, MD, a psychiatrist with the department of psychiatry and behavioral science, University of North Dakota, Fargo, and Devika Umashanker, MD, with Obesity Medicine, Hartford (Conn.) Health Care, in an accompanying editorial.
They acknowledged, however, that monitoring can be difficult given the typical low rate of follow-up of these patients.
Though the reasons for the rise in nonalcoholic SUD are not clear, Dr. Mitchell and Dr. Umashanker said biologic and psychosocial issues may be contributors to the increase.
The persistence of medical comorbidities and a lack of noted improvement in quality of life or physical mobility after the surgery has been addressed in a paper on suicide risk after bariatric surgery, the study authors also noted.
Dr. Svensson said in an interview that a mechanism for alcohol abuse after gastric bypass surgery is more evident, as measured by “increased blood alcohol levels after the surgery for a given amount of alcohol.” However, for other addictive substances, the mechanism is not obvious and needs further study.
The editorialists reminded clinicians that measuring phosphatidylethanol can be very useful in identifying and quantifying recent alcohol intake, suggesting that all clinicians, not just those in bariatric surgery clinics, should be aware of the connection between the procedures and subsequent alcohol abuse and monitor those patients carefully.
Both the study authors and the editorialists pointed out that the SOS cohort was recruited when vertical banded gastroplasty and banding were commonly used, and both methods are now rarely, if ever, used. Gastric sleeve procedures are now the most common approach, and those patients were not included in the study.
“However, gastric bypass surgery patients were included, albeit in a minority of the sample,” Dr. Mitchell and Dr. Umashanker wrote. In addition, the sample size of patients with SUD was too small to determine the drugs that were being abused.
Dr. Svensson said in an interview the main limitation is that SUD events were identified in the Swedish National Patient Register, which misses nonhospitalized patients.
“This register is very complete for hospitals, but it does not include SUD events detected in the primary health care setting,” he said. “Hence, the absolute number of events is probably a clear underestimation. However, it is unlikely that this limitation would affect the study groups (control group vs. groups with different surgical procedures) in different ways and hence the conclusions from this study are most likely valid.”
The study authors and the editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nonalcohol substance use disorder (SUD) was 2.5 times more common in people who had gastric bypass surgery, compared with a control group who received usual obesity care, a new prospective study has found.
The findings suggest that the risk for nonalcohol SUD should be carefully explained to patients getting a gastric bypass and that the risk should be considered in care before and after the surgery, said the study authors and editorialists.
Though alcohol use disorder is a well-known side effect for some bariatric procedures, little is known about the link between the procedures and other substance abuse, wrote the study authors, led by Per-Arne Svensson, PhD, with the department of molecular and clinical medicine, Institute of Medicine, at the University of Gothenburg (Sweden).
The study was published online in Obesity.
The researchers analyzed data from the SOS study. It was originally designed to compare bariatric surgery with usual obesity care, with overall mortality as the primary outcome. The protocol also called for reporting negative effects of included treatments.
The study was conducted throughout Sweden at 25 public surgical departments and 480 primary health centers. Participants were between ages 37 and 60 years and had a body mass index of at least 34 kg/m2 for men and 38 for women.
After people with previous nonalcoholic SUD were excluded, the study population included 1,990 patients who had undergone bariatric surgery between September 1987 and January 2001, as well as 2,030 matched controls who received usual obesity care. The three types of bariatric surgery were gastric bypass (264 patients), vertical banded gastroplasty (1,353), and gastric banding (373), as chosen by the surgeons.
The follow-up was nearly 24 years.
Link found only with gastric bypass
The researchers identified participants who had nonalcoholic SUDs using the ICD from the Swedish National Patient Register covering hospital treatment (hospital stays or hospital-based outpatient care) but not primary care.
Only gastric bypass was associated with an increased incidence of nonalcoholic SUD (adjusted hazard ratio, 2.54; 95% confidence interval, 1.14-5.65), compared with controls during the follow-up period.
Among those who had gastric bypass surgery, three developed opioid-related disorders; three had sedative-, hypnotic-, or anxiolytic-related disorders; and three had other psychoactive substance–related disorders, the study authors wrote.
The researchers found no statistical difference in the incidence of nonalcoholic SUD when the groups who had undergone different surgical procedures were compared with each other.
“It is important to acknowledge that the number of affected patients was relatively low, in the single digits,” Jihad Kudsi, MD, a bariatric surgeon and chairman of surgery at Duly Health and Care, Oak Brook, Ill., said in a press release.
The findings “highlight the critical role of bariatric behavioral health clinicians in the comprehensive evaluation and care of patients both before and after weight loss surgery,” added Dr. Kudsi, who was not associated with the research.
Bariatric surgery candidates should be warned, monitored
The data indicate that patients who are candidates for bariatric surgery should be “carefully warned” about risks for nonalcoholic SUD and be monitored after the procedure, wrote James E. Mitchell, MD, a psychiatrist with the department of psychiatry and behavioral science, University of North Dakota, Fargo, and Devika Umashanker, MD, with Obesity Medicine, Hartford (Conn.) Health Care, in an accompanying editorial.
They acknowledged, however, that monitoring can be difficult given the typical low rate of follow-up of these patients.
Though the reasons for the rise in nonalcoholic SUD are not clear, Dr. Mitchell and Dr. Umashanker said biologic and psychosocial issues may be contributors to the increase.
The persistence of medical comorbidities and a lack of noted improvement in quality of life or physical mobility after the surgery has been addressed in a paper on suicide risk after bariatric surgery, the study authors also noted.
Dr. Svensson said in an interview that a mechanism for alcohol abuse after gastric bypass surgery is more evident, as measured by “increased blood alcohol levels after the surgery for a given amount of alcohol.” However, for other addictive substances, the mechanism is not obvious and needs further study.
The editorialists reminded clinicians that measuring phosphatidylethanol can be very useful in identifying and quantifying recent alcohol intake, suggesting that all clinicians, not just those in bariatric surgery clinics, should be aware of the connection between the procedures and subsequent alcohol abuse and monitor those patients carefully.
Both the study authors and the editorialists pointed out that the SOS cohort was recruited when vertical banded gastroplasty and banding were commonly used, and both methods are now rarely, if ever, used. Gastric sleeve procedures are now the most common approach, and those patients were not included in the study.
“However, gastric bypass surgery patients were included, albeit in a minority of the sample,” Dr. Mitchell and Dr. Umashanker wrote. In addition, the sample size of patients with SUD was too small to determine the drugs that were being abused.
Dr. Svensson said in an interview the main limitation is that SUD events were identified in the Swedish National Patient Register, which misses nonhospitalized patients.
“This register is very complete for hospitals, but it does not include SUD events detected in the primary health care setting,” he said. “Hence, the absolute number of events is probably a clear underestimation. However, it is unlikely that this limitation would affect the study groups (control group vs. groups with different surgical procedures) in different ways and hence the conclusions from this study are most likely valid.”
The study authors and the editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM OBESITY
Benefits of bariatric surgery persist for 12 years
SAN DIEGO – and a baseline body mass index (BMI) of at least 27 kg/m2, according to new study results.
The findings are from ARMMS-T2D, a prospective, controlled trial with the largest cohort and longest follow-up of bariatric surgery reported to date. The results reinforce the potential role of surgery “as an option to improve diabetes-related outcomes, including people with a BMI of less than 35 kg/m2,” said Anita P. Courcoulas, MD, at the recent annual scientific sessions of the American Diabetes Association.
People who underwent bariatric surgery (gastric band, sleeve gastrectomy, or Roux-en-Y gastric bypass) had an average 1.6–percentage point drop in hemoglobin A1c from baseline 7 years after surgery and an average 1.4–percentage point reduction from baseline after 12 years. Average decreases from baseline were 0.2 and 0.3 percentage points at these time points, respectively, among controls who underwent lifestyle and medical interventions only. Between-group differences were significant at both the 7-year (primary endpoint) and 12-year time points in the intention-to-treat analysis, reported Dr. Courcoulas, a professor of surgery at the University of Pittsburgh.
Average weight loss from baseline to 7 and 12 years was 19.9% and 19.3%, respectively, in the surgery group and 8.3% and 10.8%, respectively, among controls, which was significantly different between groups at both time points (a secondary endpoint).
Dr. Courcoulas highlighted that the 10.8% average weight loss after 12 years among controls included crossovers, with 25% of patients progressing from their initial intervention of lifestyle and medical management to undergoing bariatric surgery during follow-up. Among the controls who never underwent surgery (per-protocol analysis), the 12-year average weight loss from baseline was 7.3%.
High-dose incretin-hormone therapy missing
A major limitation of ARMMS-T2D (Alliance of Randomized Trials of Medicine vs. Metabolic Surgery in Type 2 Diabetes) is that it prospectively followed a combined cohort from four independently run controlled U.S. trials that all began more than a decade ago, before the contemporary era of medical weight loss management that’s been revolutionized by incretin-hormone receptor agonists such as semaglutide (Ozempic/Wegovy, Novo Nordisk) and tirzepatide (Mounjaro, Lilly).
New randomized, controlled trials “are needed” that compare metabolic bariatric surgery with medical and lifestyle management that includes “high-dose incretin-hormone therapy,” commented Robert H. Eckel, MD, designated discussant for ARMMS-T2D at the session.
The results also showed notable rates of two adverse events associated with bariatric surgery: a 14% incidence of bone fractures, compared with a rate of 5% among controls, and a 12% incidence of anemia after surgery, compared with a rate of 3% among controls.
The control group also had a significantly higher 3% incidence of new need for hemodialysis, compared with no incident dialysis cases among the surgery patients.
“The fracture difference [after bariatric surgery] needs more careful follow-up,” commented Dr. Eckel, an endocrinologist and emeritus professor at the University of Colorado at Denver, Aurora.
ARMMS-T2D included data from 262 people with overweight or obesity and type 2 diabetes randomized in any of four U.S. studies that compared the outcomes of 166 patients who underwent bariatric surgery with 96 patients who served as controls and had lifestyle and medical interventions for weight loss and glycemic control. Seven-year follow-up included 82 (85%) of the initial 96 control patients and 136 (82%) of the initial 166 surgery patients. After 12 years, 31 of the controls (32%) and 83 surgery patients (50%) remained for the A1c analysis.
A quartet of studies joined together
The ARMMS-T2D prospective analysis resulted from an early partnership by the organizers of the four independent randomized studies that compared bariatric surgery with lifestyle and medical intervention in people with type 2 diabetes and overweight or obesity: STAMPEDE, which included 150 people at the Cleveland Clinic starting in 2007; SLIMM-T2D, which included 88 people at Brigham and Women’s Hospital and the Joslin Diabetes Center in Boston starting in 2010; TRIABETES, which included 69 people at the University of Pittsburgh starting in 2009; and CROSSROADS, which included 43 people at the University of Washington, Seattle, starting in 2011.
Further secondary findings from the ARMMS-T2D analyses showed that 38% of the surgery patients and 17% of controls had an A1c < 6.5% after 7 years.
At 7 years, type 2 diabetes remission, defined as those with an A1c < 6.5% who were not taking any antidiabetes medications, was reached in 18% of surgery patients and 6% of controls. At 12 years, 13% of the surgery patients and none of the controls met this metric, Dr. Courcoulas said.
The duration of diabetes a person had before undergoing bariatric surgery “may be an important factor” as to whether patients undergo remission, suggested Dr. Eckel. He noted that longer duration type 2 diabetes usually results in increased glucose intolerance and makes remission less likely
Roux-en-Y gastric bypass appeared to have the best rates of patients achieving both lower A1c levels and more weight loss, followed by sleeve gastrectomy and gastric banding, which had the worst performance. But Dr. Courcoulas cautioned that the study was underpowered to reliably compare individual surgical procedures.
In terms of those with an A1c < 7.0%, surgery patients maintained a steady prevalence rate of about 55% during the first 5 years of follow-up, roughly twice the rate of controls, at 28% during all years of follow-up starting at year 5.
About 37% of enrolled patients had a BMI < 35 kg/m2, and the A1c-lowering benefit and weight loss in this subgroup were consistent with the overall findings, which supports consideration of bariatric surgery for people with type 2 diabetes and a BMI < 35 kg/m2, Dr. Courcoulas said.
She also highlighted that bariatric surgery was linked with significant reductions in triglyceride levels and increased high-density lipoprotein cholesterol levels, compared with controls. However, 22% of surgery patients experienced abdominal pain, compared with 10% of controls, and 7% experienced dysphagia, compared with no cases among the controls.
ARMMS-T2D received no commercial funding. Dr. Courcoulas had no disclosures. Dr. Eckel has been a consultant to numerous companies but said he had no relevant disclosures.
A version of this article first appeared on Medscape.com.
SAN DIEGO – and a baseline body mass index (BMI) of at least 27 kg/m2, according to new study results.
The findings are from ARMMS-T2D, a prospective, controlled trial with the largest cohort and longest follow-up of bariatric surgery reported to date. The results reinforce the potential role of surgery “as an option to improve diabetes-related outcomes, including people with a BMI of less than 35 kg/m2,” said Anita P. Courcoulas, MD, at the recent annual scientific sessions of the American Diabetes Association.
People who underwent bariatric surgery (gastric band, sleeve gastrectomy, or Roux-en-Y gastric bypass) had an average 1.6–percentage point drop in hemoglobin A1c from baseline 7 years after surgery and an average 1.4–percentage point reduction from baseline after 12 years. Average decreases from baseline were 0.2 and 0.3 percentage points at these time points, respectively, among controls who underwent lifestyle and medical interventions only. Between-group differences were significant at both the 7-year (primary endpoint) and 12-year time points in the intention-to-treat analysis, reported Dr. Courcoulas, a professor of surgery at the University of Pittsburgh.
Average weight loss from baseline to 7 and 12 years was 19.9% and 19.3%, respectively, in the surgery group and 8.3% and 10.8%, respectively, among controls, which was significantly different between groups at both time points (a secondary endpoint).
Dr. Courcoulas highlighted that the 10.8% average weight loss after 12 years among controls included crossovers, with 25% of patients progressing from their initial intervention of lifestyle and medical management to undergoing bariatric surgery during follow-up. Among the controls who never underwent surgery (per-protocol analysis), the 12-year average weight loss from baseline was 7.3%.
High-dose incretin-hormone therapy missing
A major limitation of ARMMS-T2D (Alliance of Randomized Trials of Medicine vs. Metabolic Surgery in Type 2 Diabetes) is that it prospectively followed a combined cohort from four independently run controlled U.S. trials that all began more than a decade ago, before the contemporary era of medical weight loss management that’s been revolutionized by incretin-hormone receptor agonists such as semaglutide (Ozempic/Wegovy, Novo Nordisk) and tirzepatide (Mounjaro, Lilly).
New randomized, controlled trials “are needed” that compare metabolic bariatric surgery with medical and lifestyle management that includes “high-dose incretin-hormone therapy,” commented Robert H. Eckel, MD, designated discussant for ARMMS-T2D at the session.
The results also showed notable rates of two adverse events associated with bariatric surgery: a 14% incidence of bone fractures, compared with a rate of 5% among controls, and a 12% incidence of anemia after surgery, compared with a rate of 3% among controls.
The control group also had a significantly higher 3% incidence of new need for hemodialysis, compared with no incident dialysis cases among the surgery patients.
“The fracture difference [after bariatric surgery] needs more careful follow-up,” commented Dr. Eckel, an endocrinologist and emeritus professor at the University of Colorado at Denver, Aurora.
ARMMS-T2D included data from 262 people with overweight or obesity and type 2 diabetes randomized in any of four U.S. studies that compared the outcomes of 166 patients who underwent bariatric surgery with 96 patients who served as controls and had lifestyle and medical interventions for weight loss and glycemic control. Seven-year follow-up included 82 (85%) of the initial 96 control patients and 136 (82%) of the initial 166 surgery patients. After 12 years, 31 of the controls (32%) and 83 surgery patients (50%) remained for the A1c analysis.
A quartet of studies joined together
The ARMMS-T2D prospective analysis resulted from an early partnership by the organizers of the four independent randomized studies that compared bariatric surgery with lifestyle and medical intervention in people with type 2 diabetes and overweight or obesity: STAMPEDE, which included 150 people at the Cleveland Clinic starting in 2007; SLIMM-T2D, which included 88 people at Brigham and Women’s Hospital and the Joslin Diabetes Center in Boston starting in 2010; TRIABETES, which included 69 people at the University of Pittsburgh starting in 2009; and CROSSROADS, which included 43 people at the University of Washington, Seattle, starting in 2011.
Further secondary findings from the ARMMS-T2D analyses showed that 38% of the surgery patients and 17% of controls had an A1c < 6.5% after 7 years.
At 7 years, type 2 diabetes remission, defined as those with an A1c < 6.5% who were not taking any antidiabetes medications, was reached in 18% of surgery patients and 6% of controls. At 12 years, 13% of the surgery patients and none of the controls met this metric, Dr. Courcoulas said.
The duration of diabetes a person had before undergoing bariatric surgery “may be an important factor” as to whether patients undergo remission, suggested Dr. Eckel. He noted that longer duration type 2 diabetes usually results in increased glucose intolerance and makes remission less likely
Roux-en-Y gastric bypass appeared to have the best rates of patients achieving both lower A1c levels and more weight loss, followed by sleeve gastrectomy and gastric banding, which had the worst performance. But Dr. Courcoulas cautioned that the study was underpowered to reliably compare individual surgical procedures.
In terms of those with an A1c < 7.0%, surgery patients maintained a steady prevalence rate of about 55% during the first 5 years of follow-up, roughly twice the rate of controls, at 28% during all years of follow-up starting at year 5.
About 37% of enrolled patients had a BMI < 35 kg/m2, and the A1c-lowering benefit and weight loss in this subgroup were consistent with the overall findings, which supports consideration of bariatric surgery for people with type 2 diabetes and a BMI < 35 kg/m2, Dr. Courcoulas said.
She also highlighted that bariatric surgery was linked with significant reductions in triglyceride levels and increased high-density lipoprotein cholesterol levels, compared with controls. However, 22% of surgery patients experienced abdominal pain, compared with 10% of controls, and 7% experienced dysphagia, compared with no cases among the controls.
ARMMS-T2D received no commercial funding. Dr. Courcoulas had no disclosures. Dr. Eckel has been a consultant to numerous companies but said he had no relevant disclosures.
A version of this article first appeared on Medscape.com.
SAN DIEGO – and a baseline body mass index (BMI) of at least 27 kg/m2, according to new study results.
The findings are from ARMMS-T2D, a prospective, controlled trial with the largest cohort and longest follow-up of bariatric surgery reported to date. The results reinforce the potential role of surgery “as an option to improve diabetes-related outcomes, including people with a BMI of less than 35 kg/m2,” said Anita P. Courcoulas, MD, at the recent annual scientific sessions of the American Diabetes Association.
People who underwent bariatric surgery (gastric band, sleeve gastrectomy, or Roux-en-Y gastric bypass) had an average 1.6–percentage point drop in hemoglobin A1c from baseline 7 years after surgery and an average 1.4–percentage point reduction from baseline after 12 years. Average decreases from baseline were 0.2 and 0.3 percentage points at these time points, respectively, among controls who underwent lifestyle and medical interventions only. Between-group differences were significant at both the 7-year (primary endpoint) and 12-year time points in the intention-to-treat analysis, reported Dr. Courcoulas, a professor of surgery at the University of Pittsburgh.
Average weight loss from baseline to 7 and 12 years was 19.9% and 19.3%, respectively, in the surgery group and 8.3% and 10.8%, respectively, among controls, which was significantly different between groups at both time points (a secondary endpoint).
Dr. Courcoulas highlighted that the 10.8% average weight loss after 12 years among controls included crossovers, with 25% of patients progressing from their initial intervention of lifestyle and medical management to undergoing bariatric surgery during follow-up. Among the controls who never underwent surgery (per-protocol analysis), the 12-year average weight loss from baseline was 7.3%.
High-dose incretin-hormone therapy missing
A major limitation of ARMMS-T2D (Alliance of Randomized Trials of Medicine vs. Metabolic Surgery in Type 2 Diabetes) is that it prospectively followed a combined cohort from four independently run controlled U.S. trials that all began more than a decade ago, before the contemporary era of medical weight loss management that’s been revolutionized by incretin-hormone receptor agonists such as semaglutide (Ozempic/Wegovy, Novo Nordisk) and tirzepatide (Mounjaro, Lilly).
New randomized, controlled trials “are needed” that compare metabolic bariatric surgery with medical and lifestyle management that includes “high-dose incretin-hormone therapy,” commented Robert H. Eckel, MD, designated discussant for ARMMS-T2D at the session.
The results also showed notable rates of two adverse events associated with bariatric surgery: a 14% incidence of bone fractures, compared with a rate of 5% among controls, and a 12% incidence of anemia after surgery, compared with a rate of 3% among controls.
The control group also had a significantly higher 3% incidence of new need for hemodialysis, compared with no incident dialysis cases among the surgery patients.
“The fracture difference [after bariatric surgery] needs more careful follow-up,” commented Dr. Eckel, an endocrinologist and emeritus professor at the University of Colorado at Denver, Aurora.
ARMMS-T2D included data from 262 people with overweight or obesity and type 2 diabetes randomized in any of four U.S. studies that compared the outcomes of 166 patients who underwent bariatric surgery with 96 patients who served as controls and had lifestyle and medical interventions for weight loss and glycemic control. Seven-year follow-up included 82 (85%) of the initial 96 control patients and 136 (82%) of the initial 166 surgery patients. After 12 years, 31 of the controls (32%) and 83 surgery patients (50%) remained for the A1c analysis.
A quartet of studies joined together
The ARMMS-T2D prospective analysis resulted from an early partnership by the organizers of the four independent randomized studies that compared bariatric surgery with lifestyle and medical intervention in people with type 2 diabetes and overweight or obesity: STAMPEDE, which included 150 people at the Cleveland Clinic starting in 2007; SLIMM-T2D, which included 88 people at Brigham and Women’s Hospital and the Joslin Diabetes Center in Boston starting in 2010; TRIABETES, which included 69 people at the University of Pittsburgh starting in 2009; and CROSSROADS, which included 43 people at the University of Washington, Seattle, starting in 2011.
Further secondary findings from the ARMMS-T2D analyses showed that 38% of the surgery patients and 17% of controls had an A1c < 6.5% after 7 years.
At 7 years, type 2 diabetes remission, defined as those with an A1c < 6.5% who were not taking any antidiabetes medications, was reached in 18% of surgery patients and 6% of controls. At 12 years, 13% of the surgery patients and none of the controls met this metric, Dr. Courcoulas said.
The duration of diabetes a person had before undergoing bariatric surgery “may be an important factor” as to whether patients undergo remission, suggested Dr. Eckel. He noted that longer duration type 2 diabetes usually results in increased glucose intolerance and makes remission less likely
Roux-en-Y gastric bypass appeared to have the best rates of patients achieving both lower A1c levels and more weight loss, followed by sleeve gastrectomy and gastric banding, which had the worst performance. But Dr. Courcoulas cautioned that the study was underpowered to reliably compare individual surgical procedures.
In terms of those with an A1c < 7.0%, surgery patients maintained a steady prevalence rate of about 55% during the first 5 years of follow-up, roughly twice the rate of controls, at 28% during all years of follow-up starting at year 5.
About 37% of enrolled patients had a BMI < 35 kg/m2, and the A1c-lowering benefit and weight loss in this subgroup were consistent with the overall findings, which supports consideration of bariatric surgery for people with type 2 diabetes and a BMI < 35 kg/m2, Dr. Courcoulas said.
She also highlighted that bariatric surgery was linked with significant reductions in triglyceride levels and increased high-density lipoprotein cholesterol levels, compared with controls. However, 22% of surgery patients experienced abdominal pain, compared with 10% of controls, and 7% experienced dysphagia, compared with no cases among the controls.
ARMMS-T2D received no commercial funding. Dr. Courcoulas had no disclosures. Dr. Eckel has been a consultant to numerous companies but said he had no relevant disclosures.
A version of this article first appeared on Medscape.com.
AT ADA 2023
Continuous glucose monitoring might help in managing postoperative hypoglycemia
Continuous glucose monitors (CGMs) may help curb the severity of hypoglycemia after weight loss operations and even other gastrointestinal procedures, according to recent findings from a small study published in Diabetes, Obesity, and Metabolism.
Hypoglycemia is a chronic and persistent complication common in patients following bariatric surgery, affecting as many as 30% of people who undergo a sleeve gastrectomy or Roux-en-Y gastric bypass.
The symptoms of hypoglycemia, including lightheadedness, heart palpitations, difficulty concentrating, and confusion, can mimic anxiety disorders, arrhythmia, and dumping syndrome.
If a postoperative patient experiences these symptoms within a few hours following a meal or exercising, “primary care doctors should consider the possibility that hypoglycemia may be a contributor,” said Mary-Elizabeth Patti, MD, director of the Hypoglycemia Clinic at the Joslin Diabetes Center in Boston and senior author of the new study.
“In fact, hypoglycemia is a possible diagnosis even among those who underwent [operations other than bariatric, including] fundoplication or other upper gastrointestinal or esophageal surgeries,” she said.
To understand how CGM could benefit patients, Dr. Patti and colleagues recruited 22 participants who had undergone bariatric surgery more than 8 years prior and had postbariatric hypoglycemia. Their mean age was 51 years, 90% were women, 82% were diagnosed with level 3 hypoglycemia, and none had type 1 or 2 diabetes.
All participants experienced neuronal dysfunction with symptoms like fatigue, concentration difficulties, and confusion. More than 90% had received medical nutrition therapy for postbariatric hypoglycemia in the past.
CGM data were collected in the 22 individuals in two sequential phases: masked (no access to sensor glucose or alarms) and unmasked (access to sensor glucose and alarms for low or rapidly declining sensor glucose). Twelve participants wore a CGM (Dexcom G4 device) for a total of 28 days, whereas 10 wore a CGM (the Dexcom G6 device) for a total of 20 days.
The team observed that the percentage of time when the participants’ blood glucose was below 70 mg/dL – the definition of hypoglycemia – was significantly lower during the unmasked phase.
Though CGM devices are not sensitive enough to serve as a diagnostic tool for hypoglycemia, “the alarms on CGM devices can provide some much-needed awareness,” Dr. Patti said. “After a detailed diagnosis, CGM devices can be a helpful tool to assess dietary patterns and make modifications that could reduce the severity of postbariatric hypoglycemia.”
If a patient frequently experiences hypoglycemia, they may not sense when their glucose levels drop, also known as hypoglycemia unawareness, according to Dr. Patti. Studies have found that postbariatric hypoglycemia remains underdiagnosed because most patients are asymptomatic.
the researchers conclude.
Next steps
Patients are more vulnerable to hypoglycemia after a sleeve gastrectomy or gastric bypass surgery because these procedures involve removing the pylorus. This valve plays a crucial role in only allowing small portions of food to enter the intestine and prevents sudden spikes in blood glucose.
Without the pylorus, large amounts of food directly enter the intestine and soon result in large amounts of glucose getting absorbed, according to Sriram Machineni, MD, an associate professor of medicine at Albert Einstein College of Medicine, New York, who was not affiliated with the study.
“The pancreas then goes into overdrive and produces a lot of insulin, which continues reducing sugar levels,” Dr. Machineni said. “That is what causes hypoglycemia.”
Dr. Patti and associates are next working on research using CGM-derived data to investigate how different types of meals, physical activities, and other factors could influence glucose metabolism patterns in patients with hypoglycemia.
The study was funded by Dexcom, a manufacturer of continuous glucose monitoring systems. Dr. Patti reported receiving grant funding from the Diabetes Research Center.
A version of this article appeared on Medscape.com.
Continuous glucose monitors (CGMs) may help curb the severity of hypoglycemia after weight loss operations and even other gastrointestinal procedures, according to recent findings from a small study published in Diabetes, Obesity, and Metabolism.
Hypoglycemia is a chronic and persistent complication common in patients following bariatric surgery, affecting as many as 30% of people who undergo a sleeve gastrectomy or Roux-en-Y gastric bypass.
The symptoms of hypoglycemia, including lightheadedness, heart palpitations, difficulty concentrating, and confusion, can mimic anxiety disorders, arrhythmia, and dumping syndrome.
If a postoperative patient experiences these symptoms within a few hours following a meal or exercising, “primary care doctors should consider the possibility that hypoglycemia may be a contributor,” said Mary-Elizabeth Patti, MD, director of the Hypoglycemia Clinic at the Joslin Diabetes Center in Boston and senior author of the new study.
“In fact, hypoglycemia is a possible diagnosis even among those who underwent [operations other than bariatric, including] fundoplication or other upper gastrointestinal or esophageal surgeries,” she said.
To understand how CGM could benefit patients, Dr. Patti and colleagues recruited 22 participants who had undergone bariatric surgery more than 8 years prior and had postbariatric hypoglycemia. Their mean age was 51 years, 90% were women, 82% were diagnosed with level 3 hypoglycemia, and none had type 1 or 2 diabetes.
All participants experienced neuronal dysfunction with symptoms like fatigue, concentration difficulties, and confusion. More than 90% had received medical nutrition therapy for postbariatric hypoglycemia in the past.
CGM data were collected in the 22 individuals in two sequential phases: masked (no access to sensor glucose or alarms) and unmasked (access to sensor glucose and alarms for low or rapidly declining sensor glucose). Twelve participants wore a CGM (Dexcom G4 device) for a total of 28 days, whereas 10 wore a CGM (the Dexcom G6 device) for a total of 20 days.
The team observed that the percentage of time when the participants’ blood glucose was below 70 mg/dL – the definition of hypoglycemia – was significantly lower during the unmasked phase.
Though CGM devices are not sensitive enough to serve as a diagnostic tool for hypoglycemia, “the alarms on CGM devices can provide some much-needed awareness,” Dr. Patti said. “After a detailed diagnosis, CGM devices can be a helpful tool to assess dietary patterns and make modifications that could reduce the severity of postbariatric hypoglycemia.”
If a patient frequently experiences hypoglycemia, they may not sense when their glucose levels drop, also known as hypoglycemia unawareness, according to Dr. Patti. Studies have found that postbariatric hypoglycemia remains underdiagnosed because most patients are asymptomatic.
the researchers conclude.
Next steps
Patients are more vulnerable to hypoglycemia after a sleeve gastrectomy or gastric bypass surgery because these procedures involve removing the pylorus. This valve plays a crucial role in only allowing small portions of food to enter the intestine and prevents sudden spikes in blood glucose.
Without the pylorus, large amounts of food directly enter the intestine and soon result in large amounts of glucose getting absorbed, according to Sriram Machineni, MD, an associate professor of medicine at Albert Einstein College of Medicine, New York, who was not affiliated with the study.
“The pancreas then goes into overdrive and produces a lot of insulin, which continues reducing sugar levels,” Dr. Machineni said. “That is what causes hypoglycemia.”
Dr. Patti and associates are next working on research using CGM-derived data to investigate how different types of meals, physical activities, and other factors could influence glucose metabolism patterns in patients with hypoglycemia.
The study was funded by Dexcom, a manufacturer of continuous glucose monitoring systems. Dr. Patti reported receiving grant funding from the Diabetes Research Center.
A version of this article appeared on Medscape.com.
Continuous glucose monitors (CGMs) may help curb the severity of hypoglycemia after weight loss operations and even other gastrointestinal procedures, according to recent findings from a small study published in Diabetes, Obesity, and Metabolism.
Hypoglycemia is a chronic and persistent complication common in patients following bariatric surgery, affecting as many as 30% of people who undergo a sleeve gastrectomy or Roux-en-Y gastric bypass.
The symptoms of hypoglycemia, including lightheadedness, heart palpitations, difficulty concentrating, and confusion, can mimic anxiety disorders, arrhythmia, and dumping syndrome.
If a postoperative patient experiences these symptoms within a few hours following a meal or exercising, “primary care doctors should consider the possibility that hypoglycemia may be a contributor,” said Mary-Elizabeth Patti, MD, director of the Hypoglycemia Clinic at the Joslin Diabetes Center in Boston and senior author of the new study.
“In fact, hypoglycemia is a possible diagnosis even among those who underwent [operations other than bariatric, including] fundoplication or other upper gastrointestinal or esophageal surgeries,” she said.
To understand how CGM could benefit patients, Dr. Patti and colleagues recruited 22 participants who had undergone bariatric surgery more than 8 years prior and had postbariatric hypoglycemia. Their mean age was 51 years, 90% were women, 82% were diagnosed with level 3 hypoglycemia, and none had type 1 or 2 diabetes.
All participants experienced neuronal dysfunction with symptoms like fatigue, concentration difficulties, and confusion. More than 90% had received medical nutrition therapy for postbariatric hypoglycemia in the past.
CGM data were collected in the 22 individuals in two sequential phases: masked (no access to sensor glucose or alarms) and unmasked (access to sensor glucose and alarms for low or rapidly declining sensor glucose). Twelve participants wore a CGM (Dexcom G4 device) for a total of 28 days, whereas 10 wore a CGM (the Dexcom G6 device) for a total of 20 days.
The team observed that the percentage of time when the participants’ blood glucose was below 70 mg/dL – the definition of hypoglycemia – was significantly lower during the unmasked phase.
Though CGM devices are not sensitive enough to serve as a diagnostic tool for hypoglycemia, “the alarms on CGM devices can provide some much-needed awareness,” Dr. Patti said. “After a detailed diagnosis, CGM devices can be a helpful tool to assess dietary patterns and make modifications that could reduce the severity of postbariatric hypoglycemia.”
If a patient frequently experiences hypoglycemia, they may not sense when their glucose levels drop, also known as hypoglycemia unawareness, according to Dr. Patti. Studies have found that postbariatric hypoglycemia remains underdiagnosed because most patients are asymptomatic.
the researchers conclude.
Next steps
Patients are more vulnerable to hypoglycemia after a sleeve gastrectomy or gastric bypass surgery because these procedures involve removing the pylorus. This valve plays a crucial role in only allowing small portions of food to enter the intestine and prevents sudden spikes in blood glucose.
Without the pylorus, large amounts of food directly enter the intestine and soon result in large amounts of glucose getting absorbed, according to Sriram Machineni, MD, an associate professor of medicine at Albert Einstein College of Medicine, New York, who was not affiliated with the study.
“The pancreas then goes into overdrive and produces a lot of insulin, which continues reducing sugar levels,” Dr. Machineni said. “That is what causes hypoglycemia.”
Dr. Patti and associates are next working on research using CGM-derived data to investigate how different types of meals, physical activities, and other factors could influence glucose metabolism patterns in patients with hypoglycemia.
The study was funded by Dexcom, a manufacturer of continuous glucose monitoring systems. Dr. Patti reported receiving grant funding from the Diabetes Research Center.
A version of this article appeared on Medscape.com.
FROM DIABETES, OBESITY, AND METABOLISM
Exercise program boosted physical, but not mental, health in young children with overweight
A defined exercise program significantly improved cardiometabolic health and body composition in children with overweight and obesity, but no effect was seen on mental health, based on data from 92 children.
Childhood obesity is associated with negative health outcomes including type 2 diabetes, cardiovascular disease, and mental health disorders, and exercise is considered essential to treatment, wrote Jairo H. Migueles, PhD, of the University of Granada, Spain, and colleagues. However, the effect on children with obesity and overweight of an exercise program on physical and mental health, including within-individual changes, has not been well studied, they said.
In a study published in JAMA Network Open, the researchers reviewed data from 36 girls and 56 boys with overweight or obesity who were randomized to a 20-week exercise program with aerobic and resistance elements, or waitlisted to serve as controls. The participants ranged in age from 8 to 11 years with a mean age of 10 years. The data were collected between Nov. 1, 2014, and June 30, 2016, as part of a parallel-group randomized clinical trial. The exercise program consisted of three to five 90-minute exercise sessions per week for 20 weeks, and the control children continued their usual routines.
The main cardiometabolic outcomes measured in the study were divided into three categories: body composition, physical fitness, and traditional risk factors (waist circumference, blood lipid levels, glucose levels, insulin levels, and blood pressure).
A cardiometabolic risk score was defined by z score. The researchers also added cardiorespiratory fitness (CRF) to the cardiometabolic risk score. Mental health was assessed using composite standardized scores for psychological well-being and poor mental health.
After 20 weeks, cardiometabolic risk scores decreased by approximately 0.38 standard deviations in the exercise group compared with the control group. In addition, specific measures of cardiometabolic health improved significantly from baseline in the exercise group compared with control children for low-density lipoprotein (change of –7.00 mg/dL), body mass index (–5.9 kg/m2), fat mass index (−0.67), and visceral adipose tissue (31.44 g).
Cardiorespiratory fitness improved by 2.75 laps in the exercise group compared with control children. In addition, significantly more children in the exercise group showed meaningful changes (defined as individual changes of at least 0.2 SDs) compared with control children in measures of fat mass index (37 vs. 17, P < .001) and CRF performance (30 vs. 17, P = .03).
However, no significant effects appeared on mental health outcomes in exercisers, the researchers noted.
The reduction in cardiometabolic score was attributable mainly to improvements in cardiovascular fitness, blood lipid levels, and total and visceral adiposity, the researchers wrote in their discussion. The lack of changes in mental health measures may be a result of the healthy mental state of the children at the study outset, they said. “The null effect on mental health outcomes needs to be further investigated, including, among other things, whether the instruments are sensitive enough to detect changes and whether there is a ceiling effect in young children who might be mentally healthy overall,” they wrote.
The findings were limited by several factors, including the relatively small sample size and lack of blinding for some evaluators. However, the results show the potential of exercise programs to affect meaningful change and improve cardiometabolic health in overweight and obese children, although more research is needed to explore the effects of larger-scale and longer-lasting public health interventions combining exercise and other health behaviors such as diet, the researchers concluded.
Bottom line: Exercise works
The increasing rates of overweight and obesity in children in the United States have “significant downstream consequences that include increased risk of metabolic disease, including diabetes and hypertension, as well as increased rates of anxiety and depression,” Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, said in an interview.
Therefore, the effect of interventions such as exercise training on outcomes is important, he said.
The current study findings are “what you would hope for and expect – improvement in cardiometabolic parameters and fitness,” said Dr. Skolnik. “It was encouraging to see the effect of this relatively short duration of intervention has a clear positive effect on weight, BMI, and cardiometabolic parameters,” he said. “The real benefit, of course, comes from sustaining these habits over a long period of time.”
The lack of improvement in mental health is not surprising given the small study population “who did not have a high rate of mental health problems to begin with,” Dr. Skolnik added.
Barriers to promoting exercise programs for obese and overweight children in primary care are many, Dr. Skolnik said, including “having the motivation and funding to create programs like this so they are readily available to youth.”
However, the key message from the current study is simple and straightforward, according to Dr. Skolnik. “Exercise works! It works to improve fitness, cardiometabolic parameters, and weight control,” he said.
“There is always room for more research,” Dr. Skolnik added. The questions now are not about whether exercise benefits health; they are about figuring out how to implement the known benefits of exercise into daily living for all children, athletes and nonathletes alike, he said. “We need to find nonjudgmental ways to encourage exercise as a part of routine daily healthy living, up there with brushing teeth every day,” he emphasized.
The study was supported by grants from the Spanish Ministry of Economy and Competitiveness and El Fondo Europeo de Desarrollo Regional (FEDER) and by the MCIN (Ministerio de Ciencia e Innovación) / AEI (Agencia Estatal de Investigación. The researchers and Dr. Skolnik had no financial conflicts to disclose. Dr. Skolnik serves on the editorial advisory board of Family Practice News.
A defined exercise program significantly improved cardiometabolic health and body composition in children with overweight and obesity, but no effect was seen on mental health, based on data from 92 children.
Childhood obesity is associated with negative health outcomes including type 2 diabetes, cardiovascular disease, and mental health disorders, and exercise is considered essential to treatment, wrote Jairo H. Migueles, PhD, of the University of Granada, Spain, and colleagues. However, the effect on children with obesity and overweight of an exercise program on physical and mental health, including within-individual changes, has not been well studied, they said.
In a study published in JAMA Network Open, the researchers reviewed data from 36 girls and 56 boys with overweight or obesity who were randomized to a 20-week exercise program with aerobic and resistance elements, or waitlisted to serve as controls. The participants ranged in age from 8 to 11 years with a mean age of 10 years. The data were collected between Nov. 1, 2014, and June 30, 2016, as part of a parallel-group randomized clinical trial. The exercise program consisted of three to five 90-minute exercise sessions per week for 20 weeks, and the control children continued their usual routines.
The main cardiometabolic outcomes measured in the study were divided into three categories: body composition, physical fitness, and traditional risk factors (waist circumference, blood lipid levels, glucose levels, insulin levels, and blood pressure).
A cardiometabolic risk score was defined by z score. The researchers also added cardiorespiratory fitness (CRF) to the cardiometabolic risk score. Mental health was assessed using composite standardized scores for psychological well-being and poor mental health.
After 20 weeks, cardiometabolic risk scores decreased by approximately 0.38 standard deviations in the exercise group compared with the control group. In addition, specific measures of cardiometabolic health improved significantly from baseline in the exercise group compared with control children for low-density lipoprotein (change of –7.00 mg/dL), body mass index (–5.9 kg/m2), fat mass index (−0.67), and visceral adipose tissue (31.44 g).
Cardiorespiratory fitness improved by 2.75 laps in the exercise group compared with control children. In addition, significantly more children in the exercise group showed meaningful changes (defined as individual changes of at least 0.2 SDs) compared with control children in measures of fat mass index (37 vs. 17, P < .001) and CRF performance (30 vs. 17, P = .03).
However, no significant effects appeared on mental health outcomes in exercisers, the researchers noted.
The reduction in cardiometabolic score was attributable mainly to improvements in cardiovascular fitness, blood lipid levels, and total and visceral adiposity, the researchers wrote in their discussion. The lack of changes in mental health measures may be a result of the healthy mental state of the children at the study outset, they said. “The null effect on mental health outcomes needs to be further investigated, including, among other things, whether the instruments are sensitive enough to detect changes and whether there is a ceiling effect in young children who might be mentally healthy overall,” they wrote.
The findings were limited by several factors, including the relatively small sample size and lack of blinding for some evaluators. However, the results show the potential of exercise programs to affect meaningful change and improve cardiometabolic health in overweight and obese children, although more research is needed to explore the effects of larger-scale and longer-lasting public health interventions combining exercise and other health behaviors such as diet, the researchers concluded.
Bottom line: Exercise works
The increasing rates of overweight and obesity in children in the United States have “significant downstream consequences that include increased risk of metabolic disease, including diabetes and hypertension, as well as increased rates of anxiety and depression,” Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, said in an interview.
Therefore, the effect of interventions such as exercise training on outcomes is important, he said.
The current study findings are “what you would hope for and expect – improvement in cardiometabolic parameters and fitness,” said Dr. Skolnik. “It was encouraging to see the effect of this relatively short duration of intervention has a clear positive effect on weight, BMI, and cardiometabolic parameters,” he said. “The real benefit, of course, comes from sustaining these habits over a long period of time.”
The lack of improvement in mental health is not surprising given the small study population “who did not have a high rate of mental health problems to begin with,” Dr. Skolnik added.
Barriers to promoting exercise programs for obese and overweight children in primary care are many, Dr. Skolnik said, including “having the motivation and funding to create programs like this so they are readily available to youth.”
However, the key message from the current study is simple and straightforward, according to Dr. Skolnik. “Exercise works! It works to improve fitness, cardiometabolic parameters, and weight control,” he said.
“There is always room for more research,” Dr. Skolnik added. The questions now are not about whether exercise benefits health; they are about figuring out how to implement the known benefits of exercise into daily living for all children, athletes and nonathletes alike, he said. “We need to find nonjudgmental ways to encourage exercise as a part of routine daily healthy living, up there with brushing teeth every day,” he emphasized.
The study was supported by grants from the Spanish Ministry of Economy and Competitiveness and El Fondo Europeo de Desarrollo Regional (FEDER) and by the MCIN (Ministerio de Ciencia e Innovación) / AEI (Agencia Estatal de Investigación. The researchers and Dr. Skolnik had no financial conflicts to disclose. Dr. Skolnik serves on the editorial advisory board of Family Practice News.
A defined exercise program significantly improved cardiometabolic health and body composition in children with overweight and obesity, but no effect was seen on mental health, based on data from 92 children.
Childhood obesity is associated with negative health outcomes including type 2 diabetes, cardiovascular disease, and mental health disorders, and exercise is considered essential to treatment, wrote Jairo H. Migueles, PhD, of the University of Granada, Spain, and colleagues. However, the effect on children with obesity and overweight of an exercise program on physical and mental health, including within-individual changes, has not been well studied, they said.
In a study published in JAMA Network Open, the researchers reviewed data from 36 girls and 56 boys with overweight or obesity who were randomized to a 20-week exercise program with aerobic and resistance elements, or waitlisted to serve as controls. The participants ranged in age from 8 to 11 years with a mean age of 10 years. The data were collected between Nov. 1, 2014, and June 30, 2016, as part of a parallel-group randomized clinical trial. The exercise program consisted of three to five 90-minute exercise sessions per week for 20 weeks, and the control children continued their usual routines.
The main cardiometabolic outcomes measured in the study were divided into three categories: body composition, physical fitness, and traditional risk factors (waist circumference, blood lipid levels, glucose levels, insulin levels, and blood pressure).
A cardiometabolic risk score was defined by z score. The researchers also added cardiorespiratory fitness (CRF) to the cardiometabolic risk score. Mental health was assessed using composite standardized scores for psychological well-being and poor mental health.
After 20 weeks, cardiometabolic risk scores decreased by approximately 0.38 standard deviations in the exercise group compared with the control group. In addition, specific measures of cardiometabolic health improved significantly from baseline in the exercise group compared with control children for low-density lipoprotein (change of –7.00 mg/dL), body mass index (–5.9 kg/m2), fat mass index (−0.67), and visceral adipose tissue (31.44 g).
Cardiorespiratory fitness improved by 2.75 laps in the exercise group compared with control children. In addition, significantly more children in the exercise group showed meaningful changes (defined as individual changes of at least 0.2 SDs) compared with control children in measures of fat mass index (37 vs. 17, P < .001) and CRF performance (30 vs. 17, P = .03).
However, no significant effects appeared on mental health outcomes in exercisers, the researchers noted.
The reduction in cardiometabolic score was attributable mainly to improvements in cardiovascular fitness, blood lipid levels, and total and visceral adiposity, the researchers wrote in their discussion. The lack of changes in mental health measures may be a result of the healthy mental state of the children at the study outset, they said. “The null effect on mental health outcomes needs to be further investigated, including, among other things, whether the instruments are sensitive enough to detect changes and whether there is a ceiling effect in young children who might be mentally healthy overall,” they wrote.
The findings were limited by several factors, including the relatively small sample size and lack of blinding for some evaluators. However, the results show the potential of exercise programs to affect meaningful change and improve cardiometabolic health in overweight and obese children, although more research is needed to explore the effects of larger-scale and longer-lasting public health interventions combining exercise and other health behaviors such as diet, the researchers concluded.
Bottom line: Exercise works
The increasing rates of overweight and obesity in children in the United States have “significant downstream consequences that include increased risk of metabolic disease, including diabetes and hypertension, as well as increased rates of anxiety and depression,” Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, said in an interview.
Therefore, the effect of interventions such as exercise training on outcomes is important, he said.
The current study findings are “what you would hope for and expect – improvement in cardiometabolic parameters and fitness,” said Dr. Skolnik. “It was encouraging to see the effect of this relatively short duration of intervention has a clear positive effect on weight, BMI, and cardiometabolic parameters,” he said. “The real benefit, of course, comes from sustaining these habits over a long period of time.”
The lack of improvement in mental health is not surprising given the small study population “who did not have a high rate of mental health problems to begin with,” Dr. Skolnik added.
Barriers to promoting exercise programs for obese and overweight children in primary care are many, Dr. Skolnik said, including “having the motivation and funding to create programs like this so they are readily available to youth.”
However, the key message from the current study is simple and straightforward, according to Dr. Skolnik. “Exercise works! It works to improve fitness, cardiometabolic parameters, and weight control,” he said.
“There is always room for more research,” Dr. Skolnik added. The questions now are not about whether exercise benefits health; they are about figuring out how to implement the known benefits of exercise into daily living for all children, athletes and nonathletes alike, he said. “We need to find nonjudgmental ways to encourage exercise as a part of routine daily healthy living, up there with brushing teeth every day,” he emphasized.
The study was supported by grants from the Spanish Ministry of Economy and Competitiveness and El Fondo Europeo de Desarrollo Regional (FEDER) and by the MCIN (Ministerio de Ciencia e Innovación) / AEI (Agencia Estatal de Investigación. The researchers and Dr. Skolnik had no financial conflicts to disclose. Dr. Skolnik serves on the editorial advisory board of Family Practice News.
FROM JAMA NETWORK OPEN
Poor weight loss after bariatric surgery? Liraglutide may help
Up to one in four patients who undergo metabolic/bariatric surgery have less than 20% weight loss and patients need additional strategies to help them reach their goals.
In the new BARI-OPTIMISE trial, patients with poor weight loss after such surgery were randomized to the glucagonlike peptide–1 (GLP-1) agonist liraglutide 3.0 mg/day or placebo. , report Jessica Mok, BMBS, MPhil, University College London and colleagues, in their study published online in JAMA Surgery.
Weight loss in BARI-OPTIMISE (–9.2 kg or –20 lb) was greater than the weight loss in the earlier GRAVITAS trial of 80 patients with persistent or recurrent type 2 diabetes randomized to liraglutide 1.8 mg/day or placebo, Dr. Mok and colleagues note. And more patients in BARI-OPTIMISE than in GRAVITAS lost 5% or more of their baseline weight (72% vs. 46%).
“Our findings therefore suggest that liraglutide, 3.0 mg, may have a role in the treatment of people with poor weight loss following metabolic surgery,” they write.
However, newer gut hormone–based therapies with greater efficacy than liraglutide 3.0 mg (for example, semaglutide and tirzepatide) are emerging, they add.
Therefore, “randomized clinical trials investigating the efficacy of novel pharmaceutical agents will be needed to generate the evidence required to deliver individualized precision-medicine approaches to patients with obesity and suboptimal weight loss following metabolic surgery,” the researchers urge.
‘Extremely welcome tools for severe obesity’
“The additional weight loss with associated favorable metabolic changes achieved with liraglutide reported in [the BARI-OPTIMISE and GRAVITAS trials] is extremely welcomed with the new antiobesity medications ... adding another effective tool in the toolbox for the treatment of severe obesity,” Paulina Salminen, MD, PhD, Turku (Finland) University Hospital, and Ali Aminian, MD, Cleveland Clinic, write in an accompanying editorial.
However, they also point to limitations of the current trial.
Almost all patients (65 of 70 [93%]) underwent laparoscopic sleeve gastrectomy in BARI-OPTIMISE. However, “there are safe and more effective surgical options that can be considered in patients with suboptimal initial clinical response or recurrent weight gain” after laparoscopic sleeve gastrectomy, such as “conversion to Roux-en-Y gastric bypass (RYGB), duodenal switch, or single-anastomosis duodeno-ileal bypass,” they note.
The small number of patients and low follow-up rate of 81% (57 of 70 patients) for the short intervention are other limitations.
“In treating a patient with ischemic heart disease, a combination of lifestyle intervention, risk factor modification, pharmacotherapy, coronary stenting, and open-heart surgery may be needed,” note the editorialists. “A very similar concept would be applicable in the management of severe obesity.”
In the past, they add, there was not much progress with combination therapies for obesity because of a lack of effective antiobesity medications.
However, “with the better availability of potent [antiobesity medications] now and in the near future, the practice of combination therapy will grow as [metabolic and bariatric surgery] and [antiobesity medications] work in synergy in both treating severe obesity and hopefully also in enabling increased access to effective obesity treatment,” Dr. Salminen and Dr. Aminian speculate.
“Hopefully, based on findings of future studies and the use of global uniform criteria for evaluating treatment outcomes,” the editorialists conclude, “we can develop practice guidelines to assist and optimize phenotype-tailored multimodal treatment of this heterogeneous chronic disease of severe obesity.”
Most patients had severe obesity, sleeve gastrectomy
Individuals with poor weight loss after surgery have increased appetite coupled with an unfavorable gut hormone profile, including lower circulating GLP-1 levels, Dr. Mok and colleagues note.
In 2018 and 2019, they recruited and randomized 70 adults who had had metabolic surgery at two hospitals in London at least a year earlier and had 20% or less weight loss, compared with the day of surgery, as well as a suboptimal nutrient-stimulated GLP-1 response.
Patients were excluded if they had type 1 diabetes or were taking a GLP-1 agonist, insulin, or other medications that can affect weight, among other criteria.
The mean age of patients was 48 years, and 74% were women; 13% had type 2 diabetes.
Participants had a mean weight of 120 kg, and a mean body mass index of 43 kg/m2 (57% had a BMI ≥ 40 kg/m2). Almost all patients (93%) had had sleeve gastrectomy and 7% had RYGB.
On average, they had surgery 4.3 years earlier and had lost 7% of their initial weight.
Patients were randomized 1:1 to receive liraglutide 3.0 mg or placebo daily for 24 weeks. All patients received dietary counseling and aimed for a 500 kcal/day energy deficit. They were encouraged to do a minimum of 150 minutes of moderate to vigorous exercise each week.
The primary endpoint, percentage change in body weight from baseline to week 24, was –8.8% with liraglutide versus –0.53% with placebo.
Adverse effects were predominantly gastrointestinal in nature and were more frequent with liraglutide (80%) than placebo (57%). There were no serious adverse events.
This study was funded by the Sir Jules Thorn Charitable Trust and the National Institute for Health and Care Research. Novo Nordisk provided the liraglutide and placebo pens. Author disclosures are listed with the article. Dr. Salminen has reported receiving personal fees from Novo Nordisk. Dr. Aminian has reported receiving received grants and personal fees from Medtronic and Ethicon.
A version of this article appeared on Medscape.com.
Up to one in four patients who undergo metabolic/bariatric surgery have less than 20% weight loss and patients need additional strategies to help them reach their goals.
In the new BARI-OPTIMISE trial, patients with poor weight loss after such surgery were randomized to the glucagonlike peptide–1 (GLP-1) agonist liraglutide 3.0 mg/day or placebo. , report Jessica Mok, BMBS, MPhil, University College London and colleagues, in their study published online in JAMA Surgery.
Weight loss in BARI-OPTIMISE (–9.2 kg or –20 lb) was greater than the weight loss in the earlier GRAVITAS trial of 80 patients with persistent or recurrent type 2 diabetes randomized to liraglutide 1.8 mg/day or placebo, Dr. Mok and colleagues note. And more patients in BARI-OPTIMISE than in GRAVITAS lost 5% or more of their baseline weight (72% vs. 46%).
“Our findings therefore suggest that liraglutide, 3.0 mg, may have a role in the treatment of people with poor weight loss following metabolic surgery,” they write.
However, newer gut hormone–based therapies with greater efficacy than liraglutide 3.0 mg (for example, semaglutide and tirzepatide) are emerging, they add.
Therefore, “randomized clinical trials investigating the efficacy of novel pharmaceutical agents will be needed to generate the evidence required to deliver individualized precision-medicine approaches to patients with obesity and suboptimal weight loss following metabolic surgery,” the researchers urge.
‘Extremely welcome tools for severe obesity’
“The additional weight loss with associated favorable metabolic changes achieved with liraglutide reported in [the BARI-OPTIMISE and GRAVITAS trials] is extremely welcomed with the new antiobesity medications ... adding another effective tool in the toolbox for the treatment of severe obesity,” Paulina Salminen, MD, PhD, Turku (Finland) University Hospital, and Ali Aminian, MD, Cleveland Clinic, write in an accompanying editorial.
However, they also point to limitations of the current trial.
Almost all patients (65 of 70 [93%]) underwent laparoscopic sleeve gastrectomy in BARI-OPTIMISE. However, “there are safe and more effective surgical options that can be considered in patients with suboptimal initial clinical response or recurrent weight gain” after laparoscopic sleeve gastrectomy, such as “conversion to Roux-en-Y gastric bypass (RYGB), duodenal switch, or single-anastomosis duodeno-ileal bypass,” they note.
The small number of patients and low follow-up rate of 81% (57 of 70 patients) for the short intervention are other limitations.
“In treating a patient with ischemic heart disease, a combination of lifestyle intervention, risk factor modification, pharmacotherapy, coronary stenting, and open-heart surgery may be needed,” note the editorialists. “A very similar concept would be applicable in the management of severe obesity.”
In the past, they add, there was not much progress with combination therapies for obesity because of a lack of effective antiobesity medications.
However, “with the better availability of potent [antiobesity medications] now and in the near future, the practice of combination therapy will grow as [metabolic and bariatric surgery] and [antiobesity medications] work in synergy in both treating severe obesity and hopefully also in enabling increased access to effective obesity treatment,” Dr. Salminen and Dr. Aminian speculate.
“Hopefully, based on findings of future studies and the use of global uniform criteria for evaluating treatment outcomes,” the editorialists conclude, “we can develop practice guidelines to assist and optimize phenotype-tailored multimodal treatment of this heterogeneous chronic disease of severe obesity.”
Most patients had severe obesity, sleeve gastrectomy
Individuals with poor weight loss after surgery have increased appetite coupled with an unfavorable gut hormone profile, including lower circulating GLP-1 levels, Dr. Mok and colleagues note.
In 2018 and 2019, they recruited and randomized 70 adults who had had metabolic surgery at two hospitals in London at least a year earlier and had 20% or less weight loss, compared with the day of surgery, as well as a suboptimal nutrient-stimulated GLP-1 response.
Patients were excluded if they had type 1 diabetes or were taking a GLP-1 agonist, insulin, or other medications that can affect weight, among other criteria.
The mean age of patients was 48 years, and 74% were women; 13% had type 2 diabetes.
Participants had a mean weight of 120 kg, and a mean body mass index of 43 kg/m2 (57% had a BMI ≥ 40 kg/m2). Almost all patients (93%) had had sleeve gastrectomy and 7% had RYGB.
On average, they had surgery 4.3 years earlier and had lost 7% of their initial weight.
Patients were randomized 1:1 to receive liraglutide 3.0 mg or placebo daily for 24 weeks. All patients received dietary counseling and aimed for a 500 kcal/day energy deficit. They were encouraged to do a minimum of 150 minutes of moderate to vigorous exercise each week.
The primary endpoint, percentage change in body weight from baseline to week 24, was –8.8% with liraglutide versus –0.53% with placebo.
Adverse effects were predominantly gastrointestinal in nature and were more frequent with liraglutide (80%) than placebo (57%). There were no serious adverse events.
This study was funded by the Sir Jules Thorn Charitable Trust and the National Institute for Health and Care Research. Novo Nordisk provided the liraglutide and placebo pens. Author disclosures are listed with the article. Dr. Salminen has reported receiving personal fees from Novo Nordisk. Dr. Aminian has reported receiving received grants and personal fees from Medtronic and Ethicon.
A version of this article appeared on Medscape.com.
Up to one in four patients who undergo metabolic/bariatric surgery have less than 20% weight loss and patients need additional strategies to help them reach their goals.
In the new BARI-OPTIMISE trial, patients with poor weight loss after such surgery were randomized to the glucagonlike peptide–1 (GLP-1) agonist liraglutide 3.0 mg/day or placebo. , report Jessica Mok, BMBS, MPhil, University College London and colleagues, in their study published online in JAMA Surgery.
Weight loss in BARI-OPTIMISE (–9.2 kg or –20 lb) was greater than the weight loss in the earlier GRAVITAS trial of 80 patients with persistent or recurrent type 2 diabetes randomized to liraglutide 1.8 mg/day or placebo, Dr. Mok and colleagues note. And more patients in BARI-OPTIMISE than in GRAVITAS lost 5% or more of their baseline weight (72% vs. 46%).
“Our findings therefore suggest that liraglutide, 3.0 mg, may have a role in the treatment of people with poor weight loss following metabolic surgery,” they write.
However, newer gut hormone–based therapies with greater efficacy than liraglutide 3.0 mg (for example, semaglutide and tirzepatide) are emerging, they add.
Therefore, “randomized clinical trials investigating the efficacy of novel pharmaceutical agents will be needed to generate the evidence required to deliver individualized precision-medicine approaches to patients with obesity and suboptimal weight loss following metabolic surgery,” the researchers urge.
‘Extremely welcome tools for severe obesity’
“The additional weight loss with associated favorable metabolic changes achieved with liraglutide reported in [the BARI-OPTIMISE and GRAVITAS trials] is extremely welcomed with the new antiobesity medications ... adding another effective tool in the toolbox for the treatment of severe obesity,” Paulina Salminen, MD, PhD, Turku (Finland) University Hospital, and Ali Aminian, MD, Cleveland Clinic, write in an accompanying editorial.
However, they also point to limitations of the current trial.
Almost all patients (65 of 70 [93%]) underwent laparoscopic sleeve gastrectomy in BARI-OPTIMISE. However, “there are safe and more effective surgical options that can be considered in patients with suboptimal initial clinical response or recurrent weight gain” after laparoscopic sleeve gastrectomy, such as “conversion to Roux-en-Y gastric bypass (RYGB), duodenal switch, or single-anastomosis duodeno-ileal bypass,” they note.
The small number of patients and low follow-up rate of 81% (57 of 70 patients) for the short intervention are other limitations.
“In treating a patient with ischemic heart disease, a combination of lifestyle intervention, risk factor modification, pharmacotherapy, coronary stenting, and open-heart surgery may be needed,” note the editorialists. “A very similar concept would be applicable in the management of severe obesity.”
In the past, they add, there was not much progress with combination therapies for obesity because of a lack of effective antiobesity medications.
However, “with the better availability of potent [antiobesity medications] now and in the near future, the practice of combination therapy will grow as [metabolic and bariatric surgery] and [antiobesity medications] work in synergy in both treating severe obesity and hopefully also in enabling increased access to effective obesity treatment,” Dr. Salminen and Dr. Aminian speculate.
“Hopefully, based on findings of future studies and the use of global uniform criteria for evaluating treatment outcomes,” the editorialists conclude, “we can develop practice guidelines to assist and optimize phenotype-tailored multimodal treatment of this heterogeneous chronic disease of severe obesity.”
Most patients had severe obesity, sleeve gastrectomy
Individuals with poor weight loss after surgery have increased appetite coupled with an unfavorable gut hormone profile, including lower circulating GLP-1 levels, Dr. Mok and colleagues note.
In 2018 and 2019, they recruited and randomized 70 adults who had had metabolic surgery at two hospitals in London at least a year earlier and had 20% or less weight loss, compared with the day of surgery, as well as a suboptimal nutrient-stimulated GLP-1 response.
Patients were excluded if they had type 1 diabetes or were taking a GLP-1 agonist, insulin, or other medications that can affect weight, among other criteria.
The mean age of patients was 48 years, and 74% were women; 13% had type 2 diabetes.
Participants had a mean weight of 120 kg, and a mean body mass index of 43 kg/m2 (57% had a BMI ≥ 40 kg/m2). Almost all patients (93%) had had sleeve gastrectomy and 7% had RYGB.
On average, they had surgery 4.3 years earlier and had lost 7% of their initial weight.
Patients were randomized 1:1 to receive liraglutide 3.0 mg or placebo daily for 24 weeks. All patients received dietary counseling and aimed for a 500 kcal/day energy deficit. They were encouraged to do a minimum of 150 minutes of moderate to vigorous exercise each week.
The primary endpoint, percentage change in body weight from baseline to week 24, was –8.8% with liraglutide versus –0.53% with placebo.
Adverse effects were predominantly gastrointestinal in nature and were more frequent with liraglutide (80%) than placebo (57%). There were no serious adverse events.
This study was funded by the Sir Jules Thorn Charitable Trust and the National Institute for Health and Care Research. Novo Nordisk provided the liraglutide and placebo pens. Author disclosures are listed with the article. Dr. Salminen has reported receiving personal fees from Novo Nordisk. Dr. Aminian has reported receiving received grants and personal fees from Medtronic and Ethicon.
A version of this article appeared on Medscape.com.
FROM JAMA SURGERY
Tirzepatide powers weight loss in two more pivotal trials
The primary weight-loss results from the SURMOUNT-3 and SURMOUNT-4 studies in a combined total of 1,249 randomized adults add to positive data previously reported from more than 3,400 randomized patients in SURMOUNT-1 and SURMOUNT-2, also in people with overweight or obesity. The results from these four trials collectively create a compelling picture of safety and efficacy as tirzepatide (Mounjaro, Lilly) nears a decision, expected later in 2023, from the U.S. Food and Drug Administration for approval as a weight-loss agent in people with or without type 2 diabetes.
Tirzepatide received FDA approval in May 2022 for the indication of improving glycemic control in people with type 2 diabetes.
SURMOUNT-3 included intensive lifestyle management
SURMOUNT-3 initially enrolled 806 adults with obesity or overweight plus one or more weight-related comorbidities who received a 12-week intensive lifestyle-intervention program. People who lost at least 5% of their baseline weight could continue, and in the second phase, investigators randomized 579 people to 72 weeks of treatment with weekly injections of tirzepatide or placebo while they continued the lifestyle intervention. In the intervention group, tirzepatide was gradually up-titrated to a 10-mg or 15-mg weekly dose, depending on tolerance.
People taking tirzepatide lost an average of 21.1% of body weight after 72 weeks from time of randomization, compared with an average weight gain of 3.3% among controls, an overall incremental loss of 24.5% of body weight with tirzepatide, compared with placebo, one of the trial’s two primary endpoints. The second primary endpoint was the percentage of people achieving at least a 5% weight loss from time of randomization, which occurred in 94.4% of people taking tirzepatide and 10.7% of controls.
SURMOUNT-4 tested tirzepatide discontinuation
SURMOUNT-4 started with a 36-week lead-in period during which 783 adults with obesity or overweight plus comorbidities received weekly injections of tirzepatide, which led to an average weight loss of 21.1% from baseline. Researchers then randomized 670 of these participants to continue weekly tirzepatide for another 52 weeks or continue placebo injections. At the end of the 1-year randomized phase, those who continued tirzepatide had an average additional weight loss of 6.7%, while those who switched to placebo had an average 14.8% weight gain during the 52-week phase, producing a placebo-adjusted weight loss with tirzepatide of 21.4% for this phase.
As a secondary endpoint, those who received tirzepatide continuously for 88 weeks (the 36-week run-in plus the 52-week randomized phase) had an overall average weight loss from baseline of 26.0%. In SURMOUNT-3, participants randomized to receive tirzepatide during the second phase had an overall average weight loss, compared with baseline, before the lifestyle-intervention lead-in of 26.6% during 84 total weeks of treatment. These weight-loss levels, 26.0% and 26.6%, were “the highest level of weight loss observed in the SURMOUNT program to date,” said a Lilly official in a written statement. The findings from this trial also highlighted the importance of ongoing tirzepatide treatment to maintain weight loss.
Safety findings from both trials were consistent with prior studies of tirzepatide, as well as other agents that act by mimicking the action of human incretin hormones, the glucagonlike peptide-1 (GLP-1) receptor agonists. The most common adverse effects with tirzepatide were gastrointestinal and were generally mild to moderate in severity. Tirzepatide is a twincretin that has agonist activity for both the GLP-1 receptor and the glucose-dependent insulinotropic polypeptide receptor.
According to Lilly’s announcement, the SURMOUNT-3 results will be reported at Obesity Week, being held Oct. 14-17 in Dallas, and the SURMOUNT-4 findings will be reported at the European Association for the Study of Diabetes 2023 annual meeting, being held Oct. 2-6 in Hamburg, Germany.
The SURMOUNT trials have been funded by Lilly, the company that markets tirzepatide (Mounjaro).
A version of this article first appeared on Medscape.com.
The primary weight-loss results from the SURMOUNT-3 and SURMOUNT-4 studies in a combined total of 1,249 randomized adults add to positive data previously reported from more than 3,400 randomized patients in SURMOUNT-1 and SURMOUNT-2, also in people with overweight or obesity. The results from these four trials collectively create a compelling picture of safety and efficacy as tirzepatide (Mounjaro, Lilly) nears a decision, expected later in 2023, from the U.S. Food and Drug Administration for approval as a weight-loss agent in people with or without type 2 diabetes.
Tirzepatide received FDA approval in May 2022 for the indication of improving glycemic control in people with type 2 diabetes.
SURMOUNT-3 included intensive lifestyle management
SURMOUNT-3 initially enrolled 806 adults with obesity or overweight plus one or more weight-related comorbidities who received a 12-week intensive lifestyle-intervention program. People who lost at least 5% of their baseline weight could continue, and in the second phase, investigators randomized 579 people to 72 weeks of treatment with weekly injections of tirzepatide or placebo while they continued the lifestyle intervention. In the intervention group, tirzepatide was gradually up-titrated to a 10-mg or 15-mg weekly dose, depending on tolerance.
People taking tirzepatide lost an average of 21.1% of body weight after 72 weeks from time of randomization, compared with an average weight gain of 3.3% among controls, an overall incremental loss of 24.5% of body weight with tirzepatide, compared with placebo, one of the trial’s two primary endpoints. The second primary endpoint was the percentage of people achieving at least a 5% weight loss from time of randomization, which occurred in 94.4% of people taking tirzepatide and 10.7% of controls.
SURMOUNT-4 tested tirzepatide discontinuation
SURMOUNT-4 started with a 36-week lead-in period during which 783 adults with obesity or overweight plus comorbidities received weekly injections of tirzepatide, which led to an average weight loss of 21.1% from baseline. Researchers then randomized 670 of these participants to continue weekly tirzepatide for another 52 weeks or continue placebo injections. At the end of the 1-year randomized phase, those who continued tirzepatide had an average additional weight loss of 6.7%, while those who switched to placebo had an average 14.8% weight gain during the 52-week phase, producing a placebo-adjusted weight loss with tirzepatide of 21.4% for this phase.
As a secondary endpoint, those who received tirzepatide continuously for 88 weeks (the 36-week run-in plus the 52-week randomized phase) had an overall average weight loss from baseline of 26.0%. In SURMOUNT-3, participants randomized to receive tirzepatide during the second phase had an overall average weight loss, compared with baseline, before the lifestyle-intervention lead-in of 26.6% during 84 total weeks of treatment. These weight-loss levels, 26.0% and 26.6%, were “the highest level of weight loss observed in the SURMOUNT program to date,” said a Lilly official in a written statement. The findings from this trial also highlighted the importance of ongoing tirzepatide treatment to maintain weight loss.
Safety findings from both trials were consistent with prior studies of tirzepatide, as well as other agents that act by mimicking the action of human incretin hormones, the glucagonlike peptide-1 (GLP-1) receptor agonists. The most common adverse effects with tirzepatide were gastrointestinal and were generally mild to moderate in severity. Tirzepatide is a twincretin that has agonist activity for both the GLP-1 receptor and the glucose-dependent insulinotropic polypeptide receptor.
According to Lilly’s announcement, the SURMOUNT-3 results will be reported at Obesity Week, being held Oct. 14-17 in Dallas, and the SURMOUNT-4 findings will be reported at the European Association for the Study of Diabetes 2023 annual meeting, being held Oct. 2-6 in Hamburg, Germany.
The SURMOUNT trials have been funded by Lilly, the company that markets tirzepatide (Mounjaro).
A version of this article first appeared on Medscape.com.
The primary weight-loss results from the SURMOUNT-3 and SURMOUNT-4 studies in a combined total of 1,249 randomized adults add to positive data previously reported from more than 3,400 randomized patients in SURMOUNT-1 and SURMOUNT-2, also in people with overweight or obesity. The results from these four trials collectively create a compelling picture of safety and efficacy as tirzepatide (Mounjaro, Lilly) nears a decision, expected later in 2023, from the U.S. Food and Drug Administration for approval as a weight-loss agent in people with or without type 2 diabetes.
Tirzepatide received FDA approval in May 2022 for the indication of improving glycemic control in people with type 2 diabetes.
SURMOUNT-3 included intensive lifestyle management
SURMOUNT-3 initially enrolled 806 adults with obesity or overweight plus one or more weight-related comorbidities who received a 12-week intensive lifestyle-intervention program. People who lost at least 5% of their baseline weight could continue, and in the second phase, investigators randomized 579 people to 72 weeks of treatment with weekly injections of tirzepatide or placebo while they continued the lifestyle intervention. In the intervention group, tirzepatide was gradually up-titrated to a 10-mg or 15-mg weekly dose, depending on tolerance.
People taking tirzepatide lost an average of 21.1% of body weight after 72 weeks from time of randomization, compared with an average weight gain of 3.3% among controls, an overall incremental loss of 24.5% of body weight with tirzepatide, compared with placebo, one of the trial’s two primary endpoints. The second primary endpoint was the percentage of people achieving at least a 5% weight loss from time of randomization, which occurred in 94.4% of people taking tirzepatide and 10.7% of controls.
SURMOUNT-4 tested tirzepatide discontinuation
SURMOUNT-4 started with a 36-week lead-in period during which 783 adults with obesity or overweight plus comorbidities received weekly injections of tirzepatide, which led to an average weight loss of 21.1% from baseline. Researchers then randomized 670 of these participants to continue weekly tirzepatide for another 52 weeks or continue placebo injections. At the end of the 1-year randomized phase, those who continued tirzepatide had an average additional weight loss of 6.7%, while those who switched to placebo had an average 14.8% weight gain during the 52-week phase, producing a placebo-adjusted weight loss with tirzepatide of 21.4% for this phase.
As a secondary endpoint, those who received tirzepatide continuously for 88 weeks (the 36-week run-in plus the 52-week randomized phase) had an overall average weight loss from baseline of 26.0%. In SURMOUNT-3, participants randomized to receive tirzepatide during the second phase had an overall average weight loss, compared with baseline, before the lifestyle-intervention lead-in of 26.6% during 84 total weeks of treatment. These weight-loss levels, 26.0% and 26.6%, were “the highest level of weight loss observed in the SURMOUNT program to date,” said a Lilly official in a written statement. The findings from this trial also highlighted the importance of ongoing tirzepatide treatment to maintain weight loss.
Safety findings from both trials were consistent with prior studies of tirzepatide, as well as other agents that act by mimicking the action of human incretin hormones, the glucagonlike peptide-1 (GLP-1) receptor agonists. The most common adverse effects with tirzepatide were gastrointestinal and were generally mild to moderate in severity. Tirzepatide is a twincretin that has agonist activity for both the GLP-1 receptor and the glucose-dependent insulinotropic polypeptide receptor.
According to Lilly’s announcement, the SURMOUNT-3 results will be reported at Obesity Week, being held Oct. 14-17 in Dallas, and the SURMOUNT-4 findings will be reported at the European Association for the Study of Diabetes 2023 annual meeting, being held Oct. 2-6 in Hamburg, Germany.
The SURMOUNT trials have been funded by Lilly, the company that markets tirzepatide (Mounjaro).
A version of this article first appeared on Medscape.com.
Could GLP-1 receptor agonists ease knee osteoarthritis pain, slow progression?
Could glucagon-like peptide-1 receptor agonists, such as liraglutide and semaglutide, also be potential disease-modifying treatments for knee osteoarthritis (KOA)?
Weight loss is recommended for patients with KOA, and GLP-1 receptor agonists are approved for weight loss. New early research suggests these drugs might have a disease-modifying effect for KOA.
Three recently published studies investigated this:
- The LOSEIT phase 4, randomized controlled trial of liraglutide vs. placebo in patients with obesity/overweight and KOA.
- A large observational study out of China in patients with KOA and type 2 diabetes.
- A preclinical trial of liraglutide in mouse models of KOA.
The preclinical trial and the observational study report promising results, and the lack of KOA pain relief in patients in the phase 4 trial may be explained by the trial design. Three other trials are in the works.
This news organization invited two researchers and two outside experts to discuss these studies and potential future treatment of KOA with GLP-1 receptor agonists.
The big picture, as seen by two experts
The GLP-1 receptor agonists liraglutide (Victoza) and semaglutide (Ozempic) are approved for type 2 diabetes, and, in higher doses, liraglutide (Saxenda) and semaglutide (Wegovy) are approved for weight loss in patients with obesity (or overweight with comorbidities), and given as weekly injections.
Victoza and Saxenda are expected to come off patent in December 2023, and in 2026, respectively.
Lauren King, MD, PhD, a rheumatologist and clinician scientist who was not involved with the recent investigational studies of GLP-1 receptor agonists for KOA, noted that obesity is the most important, guideline-recommended, modifiable risk factor for KOA.
“In people with overweight and obesity, losing weight can improve knee osteoarthritis symptoms, and some evidence supports that it may also slow joint structural changes,” Dr. King, of the department of medicine at the University of Toronto, said in an interview.
Large trials of GLP-1 receptor agonists in people with overweight and obesity, such as the STEP trials of semaglutide, she noted, “provide evidence that these medications are safe and effective, facilitating clinically relevant and sustained weight loss.”
Further research is needed, she said, to better understand disease-modifying effects of GLP-1 receptor agonists in patients with KOA.
Similarly, W. Timothy Garvey, MD, professor in the department of nutrition sciences at the University of Alabama at Birmingham and director of the UAB Diabetes Research Center, who was not involved with this research, noted that weight loss improves KOA symptoms.
Dr. Garvey was lead investigator in the STEP 5 trial of semaglutide and lead author of the American Association of Clinical Endocrinologists 2016 Obesity Management guidelines.
“The question is whether these GLP-1 receptor agonists have anything to offer over and above weight loss per se, and we don’t know for sure,” he said.
They “do have anti-inflammatory actions,” and “there are GLP-1 receptors in locations where you think GLP-1 receptor agonism may help inflammation in the knee, in joints, and in other tissues,” he noted.
He looks forward to results of the phase 3 trial of semaglutide in patients with KOA, expected this fall.
Three published studies
LOSEIT: RCT of liraglutide for pain and weight control in KOA
Henrik Rindel Gudbergsen, MD, PhD, and colleagues published results of the only randomized controlled trial of a GLP-1 receptor agonist (liraglutide, Saxenda) vs. placebo in patients with overweight/obesity and KOA, the LOSEIT trial.
All patients first entered an 8-week, pre-randomization phase where they had strict caloric restriction (and ate meal replacements) and lost at least 5% of their initial weight. They also had less knee pain at the end of this phase.
Then they were randomly assigned to receive 3 mg liraglutide or placebo daily injections for 1 year.
From randomization until week 52, the liraglutide group had greater mean weight loss than the placebo group (but this was < 5% of their weight). They did not have greater reduction in knee pain than patients in the placebo group.
“Our interpretation was that dieting results in weight loss and diminishes knee pain (which we knew), and that the impact of liraglutide following severe calorie restriction and weight loss and improvement of pain was limited,” Dr. Gudbergsen, a physician and associate professor at The Parker Institute, University of Copenhagen, told this news organization.
“That was the surprise for us as investigators,” he said, “and, I assume, why Novo Nordisk is now pursuing the investigation of semaglutide for KOA, as this is expected to create a larger effect on body weight and knee symptoms.”
The weight loss was about 12.5 kg (27.5 pounds) prior to randomization, and the subsequent weight loss with liraglutide was about 2.8 kg (6 pounds; about 4% of their weight). “Thus, it could seem that the participants’ potential for weight loss as well as symptom reduction was fully exploited in the pre–random assignment dietary intervention period,” according to the researchers.
“It seems highly relevant to use liraglutide or semaglutide for patients impacted by obesity and KOA, as it is in line with guidelines suggesting weight loss for this group,” Dr. Gudbergsen said. “However, whether liraglutide and/or semaglutide, acting via an anti-inflammatory effect, for example, has an added positive impact on cartilage quality remains to be clarified,” he said.
Others who were not involved in this study suggest that the lack of pain-reduction benefit with liraglutide vs. placebo can be explained by the short-term use of liraglutide (1 year), small weight loss (< 5%), and systemic rather than intraarticular injection.
The LOSEIT trial design “is problematic and could not provide a confirmative conclusion,” Hongyi Zhu, MD, PhD, Shanghai Sixth People’s Hospital, China, and colleagues wrote in their observational study. The small weight loss of < 5% in the liraglutide group may explain why the pain relief was not better than with placebo. A longer study duration with significant weight loss/maintenance may be needed, they noted.
Francis Berenbaum, MD, PhD, senior author of a preclinical study of liraglutide, said that in the LOSEIT trial, “daily systemic injections of liraglutide did not ameliorate OA-related pain, probably because of poor access and hence poor local concentrations of liraglutide in the knee joint.”
Dr. Berenbaum is professor of rheumatology at Sorbonne University and director of the department of rheumatology at AP-HP Saint-Antoine Hospital in Paris. He is cofounder and CEO of 4Moving Biotech (a subsidiary of 4P Pharma, an innovator accelerator biotech company), which is testing liraglutide for KOA.
In experiments in mice, systemic injections of liraglutide did not lead to high enough concentration in synovial fluid to show efficacy for pain relief, he told this news organization. “In order to get the direct effect of liraglutide, it should be injected intraarticularly,” he said.
Observational study of patients with diabetes and KOA
Dr. Zhu and colleagues recently published results of the first clinical investigation of long-term effects of GLP-1 receptor agonists on KOA in patients with comorbid type 2 diabetes.
They analyzed data from a subset of patients with KOA and type 2 diabetes from the Shanghai Osteoarthritis Cohort, including 233 patients who received a GLP-1 receptor agonist (semaglutide, liraglutide, or dulaglutide [Trulicity]) for at least 2 years and 1,574 patients who did not receive this therapy.
The patients had a mean weight of 66 kg (145 pounds), a mean body mass index of 27 kg/m2, and a mean A1c of 7.3%.
“According to conventional wisdom, a weight change greater than 5% is considered clinically relevant for KOA,” the researchers wrote. They found that patients had substantial weight loss after GLP-1 receptor agonist therapy.
The primary outcome, the incidence of knee surgery, was lower in the patients who received a GLP-1 receptor agonist than in the other patients (1.7% vs. 5.9%; adjusted P = .014).
Patients who received a GLP-1 receptor agonist also had greater improvements in secondary outcomes than did other patients, including pain subscale scores and cartilage-loss velocity of the medial femorotibial joint in patients with predominantly lateral OA.
“The effects of GLP-1 receptor agonists on arthritic knees were largely mediated by weight loss instead of glycemic control,” Dr. Zhu and colleagues reported.
They concluded that with long-enough treatment, “GLP-1 receptor agonist therapies might be disease-modifying for KOA patients with comorbid [type 2 diabetes mellitus].”
They called for further research to elucidate the effects of GLP-1 receptor agonists on the disease process, joint structure, and patient-reported outcomes of OA.
Dr. Garvey noted that “whether your BMI is 30 or 40, if there are complications, that tells you that degree of adiposity is sufficient to impair health.” So, if a patient in southeast China has a BMI of 27 kg/m2 and has osteoarthritis, he or she could still benefit from weight loss, he said.
Liraglutide and pain-related behavior in mouse models of OA
Dr. Berenbaum and colleagues reported that liraglutide alleviated pain-related behavior in sodium monoiodoacetate mouse models of KOA.
In addition, liraglutide had anti-inflammatory and anticatabolic effects in synovial fluid from the knees of six patients with OA of varying severity.
The researchers analyzed generic liraglutide (from Hybio Pharmaceuticals, Shenzhen, China) and nongeneric liraglutide (from Novo Nordisk, Bagsværd, Denmark).
They found that “when injected intra-articularly, liraglutide blunts the inflammatory process that is present in OA synovial tissue, explaining the acute analgesic effect,” Dr. Berenbaum said.
“Liraglutide could be a game-changer,” he said, “by demonstrating not only an effect on joint structures like synovial tissue and cartilage, but also on symptoms in a short-term period.”
Dr. Garvey said the symptom improvements after intrasynovial infusion of liraglutide in this trial were “impressive.” This study “adds credence to the hypothesis that these GLP-1 receptor agonists could have effects above and beyond weight loss,” he said.
Two trials near completion, one is upcoming
Phase 1 and 2 trials of 4P-004
“We are now in a phase 1 clinical trial [of 4P-004/liraglutide] in patients suffering from knee OA and should start a large phase 2 trial next year,” said Dr. Berenbaum.
The phase 1 LASARE trial, sponsored by 4Moving Biotech, planned to enroll 32 patients with KOA.
The primary outcome is safety and tolerability of single IA administration of 4P-004 at escalating doses in patients with KOA. Secondary outcomes include plasma concentration of liraglutide when administered this way.
Phase 3 trial of semaglutide for KOA
Novo Nordisk is performing a phase 3 study, “Effect of Subcutaneous Semaglutide 2.4 mg Once-weekly Compared to Placebo in Subjects With Obesity and Knee Osteoarthritis,” with an expected enrollment of 407 patients with KOA and estimated trial completion in September.
Eligible patients were aged 18 and older, with BMI > 30 kg/m2 and KOA with Kellgren-Lawrence grades 2 or 3. The co-primary outcomes are change in body weight and change in WOMAC pain score, from baseline to 68 weeks.
The LOSEIT trial was supported by Novo Nordisk and the Cambridge Weight Plan. The observational study in China was supported by the Shanghai Shenkang Hospital Development Centre, the Clinical Research Plan of SHDC, and the National Natural Science Foundation of China. The preclinical trial was supported by 4P Pharma/4Moving Biotech.
Dr. Berenbaum is CEO of 4Moving Biotech and chair of the scientific advisory board of 4P Pharma. He has received personal fees from 4P Pharma as well as numerous other pharmaceutical companies. Dr. Garvey has reported being a consultant to Boehringer Ingelheim, Novo Nordisk, Eli Lilly, Merck, Fractyl Health, and Alnylam Pharmaceuticals, and reported being an investigator for studies sponsored by Novo Nordisk, Eli Lilly, Pfizer, and Epitomee. Dr. Gudbergsen, Dr. King, and Dr. Zhu report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Could glucagon-like peptide-1 receptor agonists, such as liraglutide and semaglutide, also be potential disease-modifying treatments for knee osteoarthritis (KOA)?
Weight loss is recommended for patients with KOA, and GLP-1 receptor agonists are approved for weight loss. New early research suggests these drugs might have a disease-modifying effect for KOA.
Three recently published studies investigated this:
- The LOSEIT phase 4, randomized controlled trial of liraglutide vs. placebo in patients with obesity/overweight and KOA.
- A large observational study out of China in patients with KOA and type 2 diabetes.
- A preclinical trial of liraglutide in mouse models of KOA.
The preclinical trial and the observational study report promising results, and the lack of KOA pain relief in patients in the phase 4 trial may be explained by the trial design. Three other trials are in the works.
This news organization invited two researchers and two outside experts to discuss these studies and potential future treatment of KOA with GLP-1 receptor agonists.
The big picture, as seen by two experts
The GLP-1 receptor agonists liraglutide (Victoza) and semaglutide (Ozempic) are approved for type 2 diabetes, and, in higher doses, liraglutide (Saxenda) and semaglutide (Wegovy) are approved for weight loss in patients with obesity (or overweight with comorbidities), and given as weekly injections.
Victoza and Saxenda are expected to come off patent in December 2023, and in 2026, respectively.
Lauren King, MD, PhD, a rheumatologist and clinician scientist who was not involved with the recent investigational studies of GLP-1 receptor agonists for KOA, noted that obesity is the most important, guideline-recommended, modifiable risk factor for KOA.
“In people with overweight and obesity, losing weight can improve knee osteoarthritis symptoms, and some evidence supports that it may also slow joint structural changes,” Dr. King, of the department of medicine at the University of Toronto, said in an interview.
Large trials of GLP-1 receptor agonists in people with overweight and obesity, such as the STEP trials of semaglutide, she noted, “provide evidence that these medications are safe and effective, facilitating clinically relevant and sustained weight loss.”
Further research is needed, she said, to better understand disease-modifying effects of GLP-1 receptor agonists in patients with KOA.
Similarly, W. Timothy Garvey, MD, professor in the department of nutrition sciences at the University of Alabama at Birmingham and director of the UAB Diabetes Research Center, who was not involved with this research, noted that weight loss improves KOA symptoms.
Dr. Garvey was lead investigator in the STEP 5 trial of semaglutide and lead author of the American Association of Clinical Endocrinologists 2016 Obesity Management guidelines.
“The question is whether these GLP-1 receptor agonists have anything to offer over and above weight loss per se, and we don’t know for sure,” he said.
They “do have anti-inflammatory actions,” and “there are GLP-1 receptors in locations where you think GLP-1 receptor agonism may help inflammation in the knee, in joints, and in other tissues,” he noted.
He looks forward to results of the phase 3 trial of semaglutide in patients with KOA, expected this fall.
Three published studies
LOSEIT: RCT of liraglutide for pain and weight control in KOA
Henrik Rindel Gudbergsen, MD, PhD, and colleagues published results of the only randomized controlled trial of a GLP-1 receptor agonist (liraglutide, Saxenda) vs. placebo in patients with overweight/obesity and KOA, the LOSEIT trial.
All patients first entered an 8-week, pre-randomization phase where they had strict caloric restriction (and ate meal replacements) and lost at least 5% of their initial weight. They also had less knee pain at the end of this phase.
Then they were randomly assigned to receive 3 mg liraglutide or placebo daily injections for 1 year.
From randomization until week 52, the liraglutide group had greater mean weight loss than the placebo group (but this was < 5% of their weight). They did not have greater reduction in knee pain than patients in the placebo group.
“Our interpretation was that dieting results in weight loss and diminishes knee pain (which we knew), and that the impact of liraglutide following severe calorie restriction and weight loss and improvement of pain was limited,” Dr. Gudbergsen, a physician and associate professor at The Parker Institute, University of Copenhagen, told this news organization.
“That was the surprise for us as investigators,” he said, “and, I assume, why Novo Nordisk is now pursuing the investigation of semaglutide for KOA, as this is expected to create a larger effect on body weight and knee symptoms.”
The weight loss was about 12.5 kg (27.5 pounds) prior to randomization, and the subsequent weight loss with liraglutide was about 2.8 kg (6 pounds; about 4% of their weight). “Thus, it could seem that the participants’ potential for weight loss as well as symptom reduction was fully exploited in the pre–random assignment dietary intervention period,” according to the researchers.
“It seems highly relevant to use liraglutide or semaglutide for patients impacted by obesity and KOA, as it is in line with guidelines suggesting weight loss for this group,” Dr. Gudbergsen said. “However, whether liraglutide and/or semaglutide, acting via an anti-inflammatory effect, for example, has an added positive impact on cartilage quality remains to be clarified,” he said.
Others who were not involved in this study suggest that the lack of pain-reduction benefit with liraglutide vs. placebo can be explained by the short-term use of liraglutide (1 year), small weight loss (< 5%), and systemic rather than intraarticular injection.
The LOSEIT trial design “is problematic and could not provide a confirmative conclusion,” Hongyi Zhu, MD, PhD, Shanghai Sixth People’s Hospital, China, and colleagues wrote in their observational study. The small weight loss of < 5% in the liraglutide group may explain why the pain relief was not better than with placebo. A longer study duration with significant weight loss/maintenance may be needed, they noted.
Francis Berenbaum, MD, PhD, senior author of a preclinical study of liraglutide, said that in the LOSEIT trial, “daily systemic injections of liraglutide did not ameliorate OA-related pain, probably because of poor access and hence poor local concentrations of liraglutide in the knee joint.”
Dr. Berenbaum is professor of rheumatology at Sorbonne University and director of the department of rheumatology at AP-HP Saint-Antoine Hospital in Paris. He is cofounder and CEO of 4Moving Biotech (a subsidiary of 4P Pharma, an innovator accelerator biotech company), which is testing liraglutide for KOA.
In experiments in mice, systemic injections of liraglutide did not lead to high enough concentration in synovial fluid to show efficacy for pain relief, he told this news organization. “In order to get the direct effect of liraglutide, it should be injected intraarticularly,” he said.
Observational study of patients with diabetes and KOA
Dr. Zhu and colleagues recently published results of the first clinical investigation of long-term effects of GLP-1 receptor agonists on KOA in patients with comorbid type 2 diabetes.
They analyzed data from a subset of patients with KOA and type 2 diabetes from the Shanghai Osteoarthritis Cohort, including 233 patients who received a GLP-1 receptor agonist (semaglutide, liraglutide, or dulaglutide [Trulicity]) for at least 2 years and 1,574 patients who did not receive this therapy.
The patients had a mean weight of 66 kg (145 pounds), a mean body mass index of 27 kg/m2, and a mean A1c of 7.3%.
“According to conventional wisdom, a weight change greater than 5% is considered clinically relevant for KOA,” the researchers wrote. They found that patients had substantial weight loss after GLP-1 receptor agonist therapy.
The primary outcome, the incidence of knee surgery, was lower in the patients who received a GLP-1 receptor agonist than in the other patients (1.7% vs. 5.9%; adjusted P = .014).
Patients who received a GLP-1 receptor agonist also had greater improvements in secondary outcomes than did other patients, including pain subscale scores and cartilage-loss velocity of the medial femorotibial joint in patients with predominantly lateral OA.
“The effects of GLP-1 receptor agonists on arthritic knees were largely mediated by weight loss instead of glycemic control,” Dr. Zhu and colleagues reported.
They concluded that with long-enough treatment, “GLP-1 receptor agonist therapies might be disease-modifying for KOA patients with comorbid [type 2 diabetes mellitus].”
They called for further research to elucidate the effects of GLP-1 receptor agonists on the disease process, joint structure, and patient-reported outcomes of OA.
Dr. Garvey noted that “whether your BMI is 30 or 40, if there are complications, that tells you that degree of adiposity is sufficient to impair health.” So, if a patient in southeast China has a BMI of 27 kg/m2 and has osteoarthritis, he or she could still benefit from weight loss, he said.
Liraglutide and pain-related behavior in mouse models of OA
Dr. Berenbaum and colleagues reported that liraglutide alleviated pain-related behavior in sodium monoiodoacetate mouse models of KOA.
In addition, liraglutide had anti-inflammatory and anticatabolic effects in synovial fluid from the knees of six patients with OA of varying severity.
The researchers analyzed generic liraglutide (from Hybio Pharmaceuticals, Shenzhen, China) and nongeneric liraglutide (from Novo Nordisk, Bagsværd, Denmark).
They found that “when injected intra-articularly, liraglutide blunts the inflammatory process that is present in OA synovial tissue, explaining the acute analgesic effect,” Dr. Berenbaum said.
“Liraglutide could be a game-changer,” he said, “by demonstrating not only an effect on joint structures like synovial tissue and cartilage, but also on symptoms in a short-term period.”
Dr. Garvey said the symptom improvements after intrasynovial infusion of liraglutide in this trial were “impressive.” This study “adds credence to the hypothesis that these GLP-1 receptor agonists could have effects above and beyond weight loss,” he said.
Two trials near completion, one is upcoming
Phase 1 and 2 trials of 4P-004
“We are now in a phase 1 clinical trial [of 4P-004/liraglutide] in patients suffering from knee OA and should start a large phase 2 trial next year,” said Dr. Berenbaum.
The phase 1 LASARE trial, sponsored by 4Moving Biotech, planned to enroll 32 patients with KOA.
The primary outcome is safety and tolerability of single IA administration of 4P-004 at escalating doses in patients with KOA. Secondary outcomes include plasma concentration of liraglutide when administered this way.
Phase 3 trial of semaglutide for KOA
Novo Nordisk is performing a phase 3 study, “Effect of Subcutaneous Semaglutide 2.4 mg Once-weekly Compared to Placebo in Subjects With Obesity and Knee Osteoarthritis,” with an expected enrollment of 407 patients with KOA and estimated trial completion in September.
Eligible patients were aged 18 and older, with BMI > 30 kg/m2 and KOA with Kellgren-Lawrence grades 2 or 3. The co-primary outcomes are change in body weight and change in WOMAC pain score, from baseline to 68 weeks.
The LOSEIT trial was supported by Novo Nordisk and the Cambridge Weight Plan. The observational study in China was supported by the Shanghai Shenkang Hospital Development Centre, the Clinical Research Plan of SHDC, and the National Natural Science Foundation of China. The preclinical trial was supported by 4P Pharma/4Moving Biotech.
Dr. Berenbaum is CEO of 4Moving Biotech and chair of the scientific advisory board of 4P Pharma. He has received personal fees from 4P Pharma as well as numerous other pharmaceutical companies. Dr. Garvey has reported being a consultant to Boehringer Ingelheim, Novo Nordisk, Eli Lilly, Merck, Fractyl Health, and Alnylam Pharmaceuticals, and reported being an investigator for studies sponsored by Novo Nordisk, Eli Lilly, Pfizer, and Epitomee. Dr. Gudbergsen, Dr. King, and Dr. Zhu report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Could glucagon-like peptide-1 receptor agonists, such as liraglutide and semaglutide, also be potential disease-modifying treatments for knee osteoarthritis (KOA)?
Weight loss is recommended for patients with KOA, and GLP-1 receptor agonists are approved for weight loss. New early research suggests these drugs might have a disease-modifying effect for KOA.
Three recently published studies investigated this:
- The LOSEIT phase 4, randomized controlled trial of liraglutide vs. placebo in patients with obesity/overweight and KOA.
- A large observational study out of China in patients with KOA and type 2 diabetes.
- A preclinical trial of liraglutide in mouse models of KOA.
The preclinical trial and the observational study report promising results, and the lack of KOA pain relief in patients in the phase 4 trial may be explained by the trial design. Three other trials are in the works.
This news organization invited two researchers and two outside experts to discuss these studies and potential future treatment of KOA with GLP-1 receptor agonists.
The big picture, as seen by two experts
The GLP-1 receptor agonists liraglutide (Victoza) and semaglutide (Ozempic) are approved for type 2 diabetes, and, in higher doses, liraglutide (Saxenda) and semaglutide (Wegovy) are approved for weight loss in patients with obesity (or overweight with comorbidities), and given as weekly injections.
Victoza and Saxenda are expected to come off patent in December 2023, and in 2026, respectively.
Lauren King, MD, PhD, a rheumatologist and clinician scientist who was not involved with the recent investigational studies of GLP-1 receptor agonists for KOA, noted that obesity is the most important, guideline-recommended, modifiable risk factor for KOA.
“In people with overweight and obesity, losing weight can improve knee osteoarthritis symptoms, and some evidence supports that it may also slow joint structural changes,” Dr. King, of the department of medicine at the University of Toronto, said in an interview.
Large trials of GLP-1 receptor agonists in people with overweight and obesity, such as the STEP trials of semaglutide, she noted, “provide evidence that these medications are safe and effective, facilitating clinically relevant and sustained weight loss.”
Further research is needed, she said, to better understand disease-modifying effects of GLP-1 receptor agonists in patients with KOA.
Similarly, W. Timothy Garvey, MD, professor in the department of nutrition sciences at the University of Alabama at Birmingham and director of the UAB Diabetes Research Center, who was not involved with this research, noted that weight loss improves KOA symptoms.
Dr. Garvey was lead investigator in the STEP 5 trial of semaglutide and lead author of the American Association of Clinical Endocrinologists 2016 Obesity Management guidelines.
“The question is whether these GLP-1 receptor agonists have anything to offer over and above weight loss per se, and we don’t know for sure,” he said.
They “do have anti-inflammatory actions,” and “there are GLP-1 receptors in locations where you think GLP-1 receptor agonism may help inflammation in the knee, in joints, and in other tissues,” he noted.
He looks forward to results of the phase 3 trial of semaglutide in patients with KOA, expected this fall.
Three published studies
LOSEIT: RCT of liraglutide for pain and weight control in KOA
Henrik Rindel Gudbergsen, MD, PhD, and colleagues published results of the only randomized controlled trial of a GLP-1 receptor agonist (liraglutide, Saxenda) vs. placebo in patients with overweight/obesity and KOA, the LOSEIT trial.
All patients first entered an 8-week, pre-randomization phase where they had strict caloric restriction (and ate meal replacements) and lost at least 5% of their initial weight. They also had less knee pain at the end of this phase.
Then they were randomly assigned to receive 3 mg liraglutide or placebo daily injections for 1 year.
From randomization until week 52, the liraglutide group had greater mean weight loss than the placebo group (but this was < 5% of their weight). They did not have greater reduction in knee pain than patients in the placebo group.
“Our interpretation was that dieting results in weight loss and diminishes knee pain (which we knew), and that the impact of liraglutide following severe calorie restriction and weight loss and improvement of pain was limited,” Dr. Gudbergsen, a physician and associate professor at The Parker Institute, University of Copenhagen, told this news organization.
“That was the surprise for us as investigators,” he said, “and, I assume, why Novo Nordisk is now pursuing the investigation of semaglutide for KOA, as this is expected to create a larger effect on body weight and knee symptoms.”
The weight loss was about 12.5 kg (27.5 pounds) prior to randomization, and the subsequent weight loss with liraglutide was about 2.8 kg (6 pounds; about 4% of their weight). “Thus, it could seem that the participants’ potential for weight loss as well as symptom reduction was fully exploited in the pre–random assignment dietary intervention period,” according to the researchers.
“It seems highly relevant to use liraglutide or semaglutide for patients impacted by obesity and KOA, as it is in line with guidelines suggesting weight loss for this group,” Dr. Gudbergsen said. “However, whether liraglutide and/or semaglutide, acting via an anti-inflammatory effect, for example, has an added positive impact on cartilage quality remains to be clarified,” he said.
Others who were not involved in this study suggest that the lack of pain-reduction benefit with liraglutide vs. placebo can be explained by the short-term use of liraglutide (1 year), small weight loss (< 5%), and systemic rather than intraarticular injection.
The LOSEIT trial design “is problematic and could not provide a confirmative conclusion,” Hongyi Zhu, MD, PhD, Shanghai Sixth People’s Hospital, China, and colleagues wrote in their observational study. The small weight loss of < 5% in the liraglutide group may explain why the pain relief was not better than with placebo. A longer study duration with significant weight loss/maintenance may be needed, they noted.
Francis Berenbaum, MD, PhD, senior author of a preclinical study of liraglutide, said that in the LOSEIT trial, “daily systemic injections of liraglutide did not ameliorate OA-related pain, probably because of poor access and hence poor local concentrations of liraglutide in the knee joint.”
Dr. Berenbaum is professor of rheumatology at Sorbonne University and director of the department of rheumatology at AP-HP Saint-Antoine Hospital in Paris. He is cofounder and CEO of 4Moving Biotech (a subsidiary of 4P Pharma, an innovator accelerator biotech company), which is testing liraglutide for KOA.
In experiments in mice, systemic injections of liraglutide did not lead to high enough concentration in synovial fluid to show efficacy for pain relief, he told this news organization. “In order to get the direct effect of liraglutide, it should be injected intraarticularly,” he said.
Observational study of patients with diabetes and KOA
Dr. Zhu and colleagues recently published results of the first clinical investigation of long-term effects of GLP-1 receptor agonists on KOA in patients with comorbid type 2 diabetes.
They analyzed data from a subset of patients with KOA and type 2 diabetes from the Shanghai Osteoarthritis Cohort, including 233 patients who received a GLP-1 receptor agonist (semaglutide, liraglutide, or dulaglutide [Trulicity]) for at least 2 years and 1,574 patients who did not receive this therapy.
The patients had a mean weight of 66 kg (145 pounds), a mean body mass index of 27 kg/m2, and a mean A1c of 7.3%.
“According to conventional wisdom, a weight change greater than 5% is considered clinically relevant for KOA,” the researchers wrote. They found that patients had substantial weight loss after GLP-1 receptor agonist therapy.
The primary outcome, the incidence of knee surgery, was lower in the patients who received a GLP-1 receptor agonist than in the other patients (1.7% vs. 5.9%; adjusted P = .014).
Patients who received a GLP-1 receptor agonist also had greater improvements in secondary outcomes than did other patients, including pain subscale scores and cartilage-loss velocity of the medial femorotibial joint in patients with predominantly lateral OA.
“The effects of GLP-1 receptor agonists on arthritic knees were largely mediated by weight loss instead of glycemic control,” Dr. Zhu and colleagues reported.
They concluded that with long-enough treatment, “GLP-1 receptor agonist therapies might be disease-modifying for KOA patients with comorbid [type 2 diabetes mellitus].”
They called for further research to elucidate the effects of GLP-1 receptor agonists on the disease process, joint structure, and patient-reported outcomes of OA.
Dr. Garvey noted that “whether your BMI is 30 or 40, if there are complications, that tells you that degree of adiposity is sufficient to impair health.” So, if a patient in southeast China has a BMI of 27 kg/m2 and has osteoarthritis, he or she could still benefit from weight loss, he said.
Liraglutide and pain-related behavior in mouse models of OA
Dr. Berenbaum and colleagues reported that liraglutide alleviated pain-related behavior in sodium monoiodoacetate mouse models of KOA.
In addition, liraglutide had anti-inflammatory and anticatabolic effects in synovial fluid from the knees of six patients with OA of varying severity.
The researchers analyzed generic liraglutide (from Hybio Pharmaceuticals, Shenzhen, China) and nongeneric liraglutide (from Novo Nordisk, Bagsværd, Denmark).
They found that “when injected intra-articularly, liraglutide blunts the inflammatory process that is present in OA synovial tissue, explaining the acute analgesic effect,” Dr. Berenbaum said.
“Liraglutide could be a game-changer,” he said, “by demonstrating not only an effect on joint structures like synovial tissue and cartilage, but also on symptoms in a short-term period.”
Dr. Garvey said the symptom improvements after intrasynovial infusion of liraglutide in this trial were “impressive.” This study “adds credence to the hypothesis that these GLP-1 receptor agonists could have effects above and beyond weight loss,” he said.
Two trials near completion, one is upcoming
Phase 1 and 2 trials of 4P-004
“We are now in a phase 1 clinical trial [of 4P-004/liraglutide] in patients suffering from knee OA and should start a large phase 2 trial next year,” said Dr. Berenbaum.
The phase 1 LASARE trial, sponsored by 4Moving Biotech, planned to enroll 32 patients with KOA.
The primary outcome is safety and tolerability of single IA administration of 4P-004 at escalating doses in patients with KOA. Secondary outcomes include plasma concentration of liraglutide when administered this way.
Phase 3 trial of semaglutide for KOA
Novo Nordisk is performing a phase 3 study, “Effect of Subcutaneous Semaglutide 2.4 mg Once-weekly Compared to Placebo in Subjects With Obesity and Knee Osteoarthritis,” with an expected enrollment of 407 patients with KOA and estimated trial completion in September.
Eligible patients were aged 18 and older, with BMI > 30 kg/m2 and KOA with Kellgren-Lawrence grades 2 or 3. The co-primary outcomes are change in body weight and change in WOMAC pain score, from baseline to 68 weeks.
The LOSEIT trial was supported by Novo Nordisk and the Cambridge Weight Plan. The observational study in China was supported by the Shanghai Shenkang Hospital Development Centre, the Clinical Research Plan of SHDC, and the National Natural Science Foundation of China. The preclinical trial was supported by 4P Pharma/4Moving Biotech.
Dr. Berenbaum is CEO of 4Moving Biotech and chair of the scientific advisory board of 4P Pharma. He has received personal fees from 4P Pharma as well as numerous other pharmaceutical companies. Dr. Garvey has reported being a consultant to Boehringer Ingelheim, Novo Nordisk, Eli Lilly, Merck, Fractyl Health, and Alnylam Pharmaceuticals, and reported being an investigator for studies sponsored by Novo Nordisk, Eli Lilly, Pfizer, and Epitomee. Dr. Gudbergsen, Dr. King, and Dr. Zhu report no relevant financial relationships.
A version of this article appeared on Medscape.com.