User login
Study documents obesity-related defecation disorders
, as well as clinically significant rectocele and increased anal resting and rectal pressures.
The study, which was published in the American Journal of Gastroenterology and led by Pam Chaichanavichkij, MBChB, MRCS, of Queen Mary University, London, included 1,155 patients (84% female, median age 52) who were obese (31.7%), overweight (34.8%), or of normal weight 33.5%).
“These results support the notion that rectal evacuation disorder/incomplete evacuation may be an important underlying mechanism for fecal incontinence in obese patients,” the authors wrote.
Obese patients had higher odds of fecal incontinence to liquid stools (69.9 vs. 47.8%; odds ratio, 1.96 [confidence interval, 1.43-2.70]), use of containment products (54.6% vs. 32.6%; OR, 1.81 [CI, 1.31-2.51]), fecal urgency (74.6% vs. 60.7%; OR, 1.54 [CI, 1.11-2.14]), urge fecal incontinence (63.4% vs. 47.3%, OR, 1.68 [CI, 1.23-2.29]), and vaginal digitation (18.0% vs. 9.7%; OR, 2.18 [CI, 1.26-3.86]).
Obese patients were also more likely to have functional constipation (50.3%), compared with overweight (44.8%) and normal weight patients (41.1%).
There was a positive linear association between body mass index (BMI) and anal resting pressure (beta 0.45; R2, 0.25, P = 0.0003), though the odds of anal hypertension were not significantly higher after Benjamini-Hochberg correction. Obese patients more often had a large clinically significant rectocele (34.4% vs. 20.6%; OR, 2.62 [CI, 1.51-4.55]), compared with normal BMI patients.
The data showed higher rates of gynecological surgery, cholecystectomy, diabetes, and self-reported use of opioids, antidepressants, and anticholinergic medications in the obese group, compared with the others.
In morphological differences measured by x-ray defecography, obese patients had more than two-fold higher odds of having a rectocele and even greater odds of the rectocele being large and clinically significant. Anal and rectal resting pressures were linearly related to increasing BMI, the authors report.
Because most patients in the study were female, the findings may not be generalizable to the general population or male patients. Also, diet and exercise, two factors that may affect defecation disorders, were not accounted for in this study.
Dr. Chaichanavichkij reported no disclosures. Two other authors reported financial relationships with Medtronic Inc. and MMS/Laborie.
, as well as clinically significant rectocele and increased anal resting and rectal pressures.
The study, which was published in the American Journal of Gastroenterology and led by Pam Chaichanavichkij, MBChB, MRCS, of Queen Mary University, London, included 1,155 patients (84% female, median age 52) who were obese (31.7%), overweight (34.8%), or of normal weight 33.5%).
“These results support the notion that rectal evacuation disorder/incomplete evacuation may be an important underlying mechanism for fecal incontinence in obese patients,” the authors wrote.
Obese patients had higher odds of fecal incontinence to liquid stools (69.9 vs. 47.8%; odds ratio, 1.96 [confidence interval, 1.43-2.70]), use of containment products (54.6% vs. 32.6%; OR, 1.81 [CI, 1.31-2.51]), fecal urgency (74.6% vs. 60.7%; OR, 1.54 [CI, 1.11-2.14]), urge fecal incontinence (63.4% vs. 47.3%, OR, 1.68 [CI, 1.23-2.29]), and vaginal digitation (18.0% vs. 9.7%; OR, 2.18 [CI, 1.26-3.86]).
Obese patients were also more likely to have functional constipation (50.3%), compared with overweight (44.8%) and normal weight patients (41.1%).
There was a positive linear association between body mass index (BMI) and anal resting pressure (beta 0.45; R2, 0.25, P = 0.0003), though the odds of anal hypertension were not significantly higher after Benjamini-Hochberg correction. Obese patients more often had a large clinically significant rectocele (34.4% vs. 20.6%; OR, 2.62 [CI, 1.51-4.55]), compared with normal BMI patients.
The data showed higher rates of gynecological surgery, cholecystectomy, diabetes, and self-reported use of opioids, antidepressants, and anticholinergic medications in the obese group, compared with the others.
In morphological differences measured by x-ray defecography, obese patients had more than two-fold higher odds of having a rectocele and even greater odds of the rectocele being large and clinically significant. Anal and rectal resting pressures were linearly related to increasing BMI, the authors report.
Because most patients in the study were female, the findings may not be generalizable to the general population or male patients. Also, diet and exercise, two factors that may affect defecation disorders, were not accounted for in this study.
Dr. Chaichanavichkij reported no disclosures. Two other authors reported financial relationships with Medtronic Inc. and MMS/Laborie.
, as well as clinically significant rectocele and increased anal resting and rectal pressures.
The study, which was published in the American Journal of Gastroenterology and led by Pam Chaichanavichkij, MBChB, MRCS, of Queen Mary University, London, included 1,155 patients (84% female, median age 52) who were obese (31.7%), overweight (34.8%), or of normal weight 33.5%).
“These results support the notion that rectal evacuation disorder/incomplete evacuation may be an important underlying mechanism for fecal incontinence in obese patients,” the authors wrote.
Obese patients had higher odds of fecal incontinence to liquid stools (69.9 vs. 47.8%; odds ratio, 1.96 [confidence interval, 1.43-2.70]), use of containment products (54.6% vs. 32.6%; OR, 1.81 [CI, 1.31-2.51]), fecal urgency (74.6% vs. 60.7%; OR, 1.54 [CI, 1.11-2.14]), urge fecal incontinence (63.4% vs. 47.3%, OR, 1.68 [CI, 1.23-2.29]), and vaginal digitation (18.0% vs. 9.7%; OR, 2.18 [CI, 1.26-3.86]).
Obese patients were also more likely to have functional constipation (50.3%), compared with overweight (44.8%) and normal weight patients (41.1%).
There was a positive linear association between body mass index (BMI) and anal resting pressure (beta 0.45; R2, 0.25, P = 0.0003), though the odds of anal hypertension were not significantly higher after Benjamini-Hochberg correction. Obese patients more often had a large clinically significant rectocele (34.4% vs. 20.6%; OR, 2.62 [CI, 1.51-4.55]), compared with normal BMI patients.
The data showed higher rates of gynecological surgery, cholecystectomy, diabetes, and self-reported use of opioids, antidepressants, and anticholinergic medications in the obese group, compared with the others.
In morphological differences measured by x-ray defecography, obese patients had more than two-fold higher odds of having a rectocele and even greater odds of the rectocele being large and clinically significant. Anal and rectal resting pressures were linearly related to increasing BMI, the authors report.
Because most patients in the study were female, the findings may not be generalizable to the general population or male patients. Also, diet and exercise, two factors that may affect defecation disorders, were not accounted for in this study.
Dr. Chaichanavichkij reported no disclosures. Two other authors reported financial relationships with Medtronic Inc. and MMS/Laborie.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Revised presentation of obesity may reduce internalized bias
a new study suggests.
In an online study, patients with obesity reported significantly less internalized weight bias and significantly enhanced perceptions of positive communication with their medical providers after watching a video of a doctor who framed obesity as a treatable medical condition, compared with a video of a doctor who emphasized willpower.
“Recent research has identified the dominant role that biology (both genetics as well as homeostatic, hedonic, and executive brain systems) and environment, rather than willpower, play in the development of obesity and the resistance to weight loss,” wrote study authors Sara English, a medical student, and Michael Vallis, MD, associate professor of family medicine, both at Dalhousie University, Halifax, N.S. “Yet the false narrative that ideal or goal weight can be achieved by eating less and moving more using willpower continues to dominate the public narrative.”
The findings were published in Clinical Obesity.
Medical complexity
The public discussion generally places all responsibility for the health outcomes of obesity on the patient. As a result, patients with obesity face bias and stigma from the public and the health care system, wrote the authors.
This stigmatization contributes to increased mortality and morbidity by promoting maladaptive eating behaviors and stress. It also causes mistrust of health care professionals, which, in turn, leads to worse health outcomes and increased health care costs.
The 2020 Canadian clinical practice guidelines for obesity management in adults emphasize that obesity is complex and that nonbehavioral factors strongly influence it. They recommend that treatment focus on improving patient-centered health outcomes and address the root causes of obesity, instead of focusing on weight loss alone.
In the present study, Ms. English and Dr. Vallis evaluated how presenting obesity as a treatable medical condition affected participants’ internalized weight bias and their perceived relationship with their health care provider. They asked 61 patients with obesity (average age, 49 years; average body mass index, 41 kg/m2) to watch two videos, the first showing a doctor endorsing the traditional “eat less, move more approach,” and the second showing a doctor describing obesity as a chronic, treatable medical condition.
Nearly half (49.5%) of participants reported that their health care provider rarely or never discusses weight loss, and almost two-thirds of participants (64%) reported feeling stigmatized by their health care provider because of their weight at least some of the time.
After having watched each video, participants were asked to imagine that they were being treated by the corresponding doctor and to complete two measures: the Weight Bias Internalization Scale (WBIS), which measures the degree to which a respondent believes the negative stereotypes about obese people, and the Patient-Health Care Provider Communication Scale (PHCPCS), which assesses the quality of patient–health care provider communication.
Virtually all participants preferred the care provider in the video with the revised presentation of obesity. Only one preferred the traditional video. The video with the revised presentation was associated with significant reductions in internalized weight bias. Participants’ WBIS total score decreased from 4.49 to 3.36 (P < .001). The revised narrative video also had a positive effect on patients’ perception of their health care providers. The PHCPCS total score increased from 2.65 to 4.20 (P < .001).
A chronic disease
In a comment, Yoni Freedhoff, MD, associate professor of family medicine at the University of Ottawa, said: “If you’re asking me if it is a good idea to treat obesity like a chronic disease, the answer would be yes, we absolutely should. It is a chronic disease, and it shouldn’t have a treatment paradigm different from the other chronic diseases.” Dr. Freedhoff did not participate in the study.
“We certainly don’t blame patients for having other chronic conditions,” Dr. Freedhoff added. “We don’t have a narrative that, in order for them to qualify for medication or other treatment options, they have to audition for them by failing lifestyle approaches first. And yet, I’d say at least 85% of chronic noncommunicable diseases have lifestyle factors, but obesity is the only one where we consider that there is a necessity for these lifestyle changes, as if there have been studies demonstrating durable and reproducible outcomes for lifestyle in obesity. There have not.”
Telling patients and doctors that obesity is a chronic disease driven by biology, not a failure of willpower, is going to reduce stigma, “which is what this study was able to demonstrate to some degree,” Dr. Freedhoff said.
“What is more stigmatizing? Being told that if you just try hard enough, you’ll succeed, and if you don’t succeed, the corollary, of course, is that you did not try hard enough? Versus, you’ve got a medical condition where you’ve got biological drivers beyond your locus of control, affecting behaviors that, in turn, contribute to your adiposity? I’m pretty sure the second statement will have far less impact on a person’s internalized weight bias than what we’ve unfortunately been doing up until now with the focus on willpower,” Dr. Freedhoff said.
No funding for the study was reported. Ms. English and Dr. Vallis reported no relevant financial relationships. Dr. Freedhoff reported receiving clinical grants from Novo Nordisk.
A version of this article first appeared on Medscape.com.
a new study suggests.
In an online study, patients with obesity reported significantly less internalized weight bias and significantly enhanced perceptions of positive communication with their medical providers after watching a video of a doctor who framed obesity as a treatable medical condition, compared with a video of a doctor who emphasized willpower.
“Recent research has identified the dominant role that biology (both genetics as well as homeostatic, hedonic, and executive brain systems) and environment, rather than willpower, play in the development of obesity and the resistance to weight loss,” wrote study authors Sara English, a medical student, and Michael Vallis, MD, associate professor of family medicine, both at Dalhousie University, Halifax, N.S. “Yet the false narrative that ideal or goal weight can be achieved by eating less and moving more using willpower continues to dominate the public narrative.”
The findings were published in Clinical Obesity.
Medical complexity
The public discussion generally places all responsibility for the health outcomes of obesity on the patient. As a result, patients with obesity face bias and stigma from the public and the health care system, wrote the authors.
This stigmatization contributes to increased mortality and morbidity by promoting maladaptive eating behaviors and stress. It also causes mistrust of health care professionals, which, in turn, leads to worse health outcomes and increased health care costs.
The 2020 Canadian clinical practice guidelines for obesity management in adults emphasize that obesity is complex and that nonbehavioral factors strongly influence it. They recommend that treatment focus on improving patient-centered health outcomes and address the root causes of obesity, instead of focusing on weight loss alone.
In the present study, Ms. English and Dr. Vallis evaluated how presenting obesity as a treatable medical condition affected participants’ internalized weight bias and their perceived relationship with their health care provider. They asked 61 patients with obesity (average age, 49 years; average body mass index, 41 kg/m2) to watch two videos, the first showing a doctor endorsing the traditional “eat less, move more approach,” and the second showing a doctor describing obesity as a chronic, treatable medical condition.
Nearly half (49.5%) of participants reported that their health care provider rarely or never discusses weight loss, and almost two-thirds of participants (64%) reported feeling stigmatized by their health care provider because of their weight at least some of the time.
After having watched each video, participants were asked to imagine that they were being treated by the corresponding doctor and to complete two measures: the Weight Bias Internalization Scale (WBIS), which measures the degree to which a respondent believes the negative stereotypes about obese people, and the Patient-Health Care Provider Communication Scale (PHCPCS), which assesses the quality of patient–health care provider communication.
Virtually all participants preferred the care provider in the video with the revised presentation of obesity. Only one preferred the traditional video. The video with the revised presentation was associated with significant reductions in internalized weight bias. Participants’ WBIS total score decreased from 4.49 to 3.36 (P < .001). The revised narrative video also had a positive effect on patients’ perception of their health care providers. The PHCPCS total score increased from 2.65 to 4.20 (P < .001).
A chronic disease
In a comment, Yoni Freedhoff, MD, associate professor of family medicine at the University of Ottawa, said: “If you’re asking me if it is a good idea to treat obesity like a chronic disease, the answer would be yes, we absolutely should. It is a chronic disease, and it shouldn’t have a treatment paradigm different from the other chronic diseases.” Dr. Freedhoff did not participate in the study.
“We certainly don’t blame patients for having other chronic conditions,” Dr. Freedhoff added. “We don’t have a narrative that, in order for them to qualify for medication or other treatment options, they have to audition for them by failing lifestyle approaches first. And yet, I’d say at least 85% of chronic noncommunicable diseases have lifestyle factors, but obesity is the only one where we consider that there is a necessity for these lifestyle changes, as if there have been studies demonstrating durable and reproducible outcomes for lifestyle in obesity. There have not.”
Telling patients and doctors that obesity is a chronic disease driven by biology, not a failure of willpower, is going to reduce stigma, “which is what this study was able to demonstrate to some degree,” Dr. Freedhoff said.
“What is more stigmatizing? Being told that if you just try hard enough, you’ll succeed, and if you don’t succeed, the corollary, of course, is that you did not try hard enough? Versus, you’ve got a medical condition where you’ve got biological drivers beyond your locus of control, affecting behaviors that, in turn, contribute to your adiposity? I’m pretty sure the second statement will have far less impact on a person’s internalized weight bias than what we’ve unfortunately been doing up until now with the focus on willpower,” Dr. Freedhoff said.
No funding for the study was reported. Ms. English and Dr. Vallis reported no relevant financial relationships. Dr. Freedhoff reported receiving clinical grants from Novo Nordisk.
A version of this article first appeared on Medscape.com.
a new study suggests.
In an online study, patients with obesity reported significantly less internalized weight bias and significantly enhanced perceptions of positive communication with their medical providers after watching a video of a doctor who framed obesity as a treatable medical condition, compared with a video of a doctor who emphasized willpower.
“Recent research has identified the dominant role that biology (both genetics as well as homeostatic, hedonic, and executive brain systems) and environment, rather than willpower, play in the development of obesity and the resistance to weight loss,” wrote study authors Sara English, a medical student, and Michael Vallis, MD, associate professor of family medicine, both at Dalhousie University, Halifax, N.S. “Yet the false narrative that ideal or goal weight can be achieved by eating less and moving more using willpower continues to dominate the public narrative.”
The findings were published in Clinical Obesity.
Medical complexity
The public discussion generally places all responsibility for the health outcomes of obesity on the patient. As a result, patients with obesity face bias and stigma from the public and the health care system, wrote the authors.
This stigmatization contributes to increased mortality and morbidity by promoting maladaptive eating behaviors and stress. It also causes mistrust of health care professionals, which, in turn, leads to worse health outcomes and increased health care costs.
The 2020 Canadian clinical practice guidelines for obesity management in adults emphasize that obesity is complex and that nonbehavioral factors strongly influence it. They recommend that treatment focus on improving patient-centered health outcomes and address the root causes of obesity, instead of focusing on weight loss alone.
In the present study, Ms. English and Dr. Vallis evaluated how presenting obesity as a treatable medical condition affected participants’ internalized weight bias and their perceived relationship with their health care provider. They asked 61 patients with obesity (average age, 49 years; average body mass index, 41 kg/m2) to watch two videos, the first showing a doctor endorsing the traditional “eat less, move more approach,” and the second showing a doctor describing obesity as a chronic, treatable medical condition.
Nearly half (49.5%) of participants reported that their health care provider rarely or never discusses weight loss, and almost two-thirds of participants (64%) reported feeling stigmatized by their health care provider because of their weight at least some of the time.
After having watched each video, participants were asked to imagine that they were being treated by the corresponding doctor and to complete two measures: the Weight Bias Internalization Scale (WBIS), which measures the degree to which a respondent believes the negative stereotypes about obese people, and the Patient-Health Care Provider Communication Scale (PHCPCS), which assesses the quality of patient–health care provider communication.
Virtually all participants preferred the care provider in the video with the revised presentation of obesity. Only one preferred the traditional video. The video with the revised presentation was associated with significant reductions in internalized weight bias. Participants’ WBIS total score decreased from 4.49 to 3.36 (P < .001). The revised narrative video also had a positive effect on patients’ perception of their health care providers. The PHCPCS total score increased from 2.65 to 4.20 (P < .001).
A chronic disease
In a comment, Yoni Freedhoff, MD, associate professor of family medicine at the University of Ottawa, said: “If you’re asking me if it is a good idea to treat obesity like a chronic disease, the answer would be yes, we absolutely should. It is a chronic disease, and it shouldn’t have a treatment paradigm different from the other chronic diseases.” Dr. Freedhoff did not participate in the study.
“We certainly don’t blame patients for having other chronic conditions,” Dr. Freedhoff added. “We don’t have a narrative that, in order for them to qualify for medication or other treatment options, they have to audition for them by failing lifestyle approaches first. And yet, I’d say at least 85% of chronic noncommunicable diseases have lifestyle factors, but obesity is the only one where we consider that there is a necessity for these lifestyle changes, as if there have been studies demonstrating durable and reproducible outcomes for lifestyle in obesity. There have not.”
Telling patients and doctors that obesity is a chronic disease driven by biology, not a failure of willpower, is going to reduce stigma, “which is what this study was able to demonstrate to some degree,” Dr. Freedhoff said.
“What is more stigmatizing? Being told that if you just try hard enough, you’ll succeed, and if you don’t succeed, the corollary, of course, is that you did not try hard enough? Versus, you’ve got a medical condition where you’ve got biological drivers beyond your locus of control, affecting behaviors that, in turn, contribute to your adiposity? I’m pretty sure the second statement will have far less impact on a person’s internalized weight bias than what we’ve unfortunately been doing up until now with the focus on willpower,” Dr. Freedhoff said.
No funding for the study was reported. Ms. English and Dr. Vallis reported no relevant financial relationships. Dr. Freedhoff reported receiving clinical grants from Novo Nordisk.
A version of this article first appeared on Medscape.com.
FROM CLINICAL OBESITY
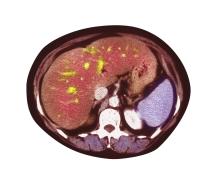
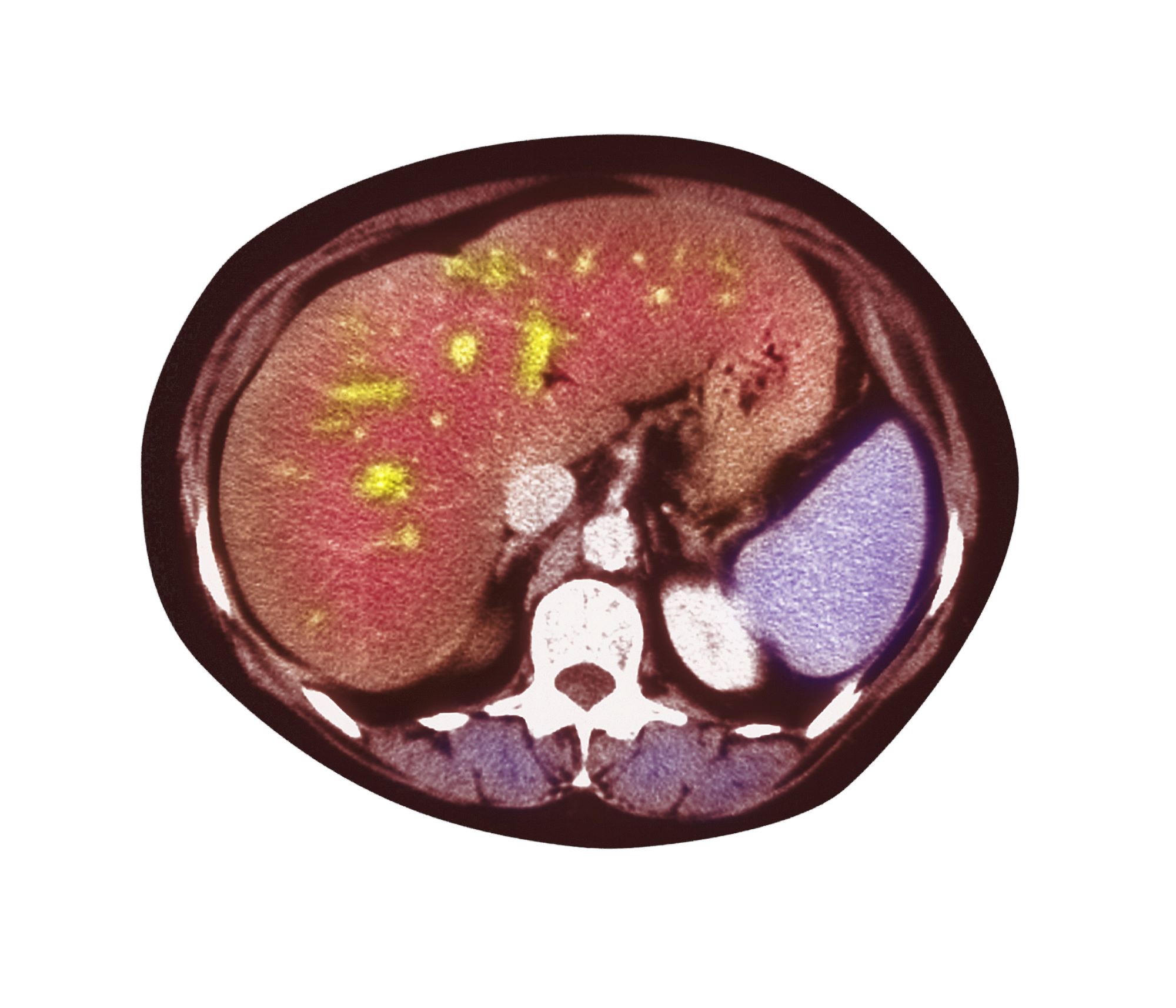
Pain in upper right abdomen
The patient's history, symptomatology, and assessments suggest a diagnosis of nonalcoholic fatty liver disease (NAFLD). The primary care physician recommends referral to a hepatologist for evaluation and possible liver biopsy.
NAFLD involves an accumulation of triglycerides and other fats in the liver (unrelated to alcohol consumption and other liver disease), with the presence of hepatic steatosis in more than 5% of hepatocytes. NAFLD affects 25% to 35% of the general population, making it the most common cause of chronic liver disease. The rate increases among patients with obesity, 80% of whom are affected by NAFLD.
NAFLD should be considered in patients with unexplained elevations in serum aminotransferases (without positive viral markers or autoantibodies and no history of alcohol use) and a high risk for steatohepatitis, including obesity. The standard NAFLD assessment for biopsy specimens is the Brunt system, and disease stage is determined using the NAFLD activity score and the amount of fibrosis present.
A study of the natural history of NAFLD in patients who were followed for 3 years showed that without pharmacologic intervention, one third experienced disease progression, one third remained stable, and one third improved. An independent risk factor for progression of nonalcoholic steatohepatitis was abnormal glucose tolerance testing. In another natural history study, a 10% higher rate of mortality over 10 years was demonstrated among those with NAFLD vs controls, with the top three causes of death being cancer, heart disease, and liver-related disease. Prevalence of chronic liver disease and cirrhosis has been shown to be elevated in Latino and Japanese American populations.
Patients with NAFLD should be seen regularly to assess for disease progression and receive guidance on weight management interventions and exercise. A weight loss of more than 5% has been shown to reduce liver fat and provide cardiometabolic benefits; a weight reduction of more than 10% can help reverse steatohepatitis or liver fibrosis. In addition to weight loss management strategies, physicians should discuss the importance of controlling hyperlipidemia, insulin resistance, and T2D with their patients and share the importance of avoiding alcohol and other hepatotoxic substances.
According to the American Association of Clinical Endocrinology Clinical Practice Guideline: "There are no U.S. Food and Drug Administration-approved medications for the treatment of NAFLD; however, some diabetes and anti-obesity medications can be beneficial. Bariatric surgery is also effective for weight loss and reducing liver fat in persons with severe obesity."
Courtney Whittle, MD, MSW, Diplomate of ABOM, Pediatric Lead, Obesity Champion, TSPMG, Weight A Minute Clinic, Atlanta, Georgia.
Courtney Whittle, MD, MSW, Diplomate of ABOM, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The patient's history, symptomatology, and assessments suggest a diagnosis of nonalcoholic fatty liver disease (NAFLD). The primary care physician recommends referral to a hepatologist for evaluation and possible liver biopsy.
NAFLD involves an accumulation of triglycerides and other fats in the liver (unrelated to alcohol consumption and other liver disease), with the presence of hepatic steatosis in more than 5% of hepatocytes. NAFLD affects 25% to 35% of the general population, making it the most common cause of chronic liver disease. The rate increases among patients with obesity, 80% of whom are affected by NAFLD.
NAFLD should be considered in patients with unexplained elevations in serum aminotransferases (without positive viral markers or autoantibodies and no history of alcohol use) and a high risk for steatohepatitis, including obesity. The standard NAFLD assessment for biopsy specimens is the Brunt system, and disease stage is determined using the NAFLD activity score and the amount of fibrosis present.
A study of the natural history of NAFLD in patients who were followed for 3 years showed that without pharmacologic intervention, one third experienced disease progression, one third remained stable, and one third improved. An independent risk factor for progression of nonalcoholic steatohepatitis was abnormal glucose tolerance testing. In another natural history study, a 10% higher rate of mortality over 10 years was demonstrated among those with NAFLD vs controls, with the top three causes of death being cancer, heart disease, and liver-related disease. Prevalence of chronic liver disease and cirrhosis has been shown to be elevated in Latino and Japanese American populations.
Patients with NAFLD should be seen regularly to assess for disease progression and receive guidance on weight management interventions and exercise. A weight loss of more than 5% has been shown to reduce liver fat and provide cardiometabolic benefits; a weight reduction of more than 10% can help reverse steatohepatitis or liver fibrosis. In addition to weight loss management strategies, physicians should discuss the importance of controlling hyperlipidemia, insulin resistance, and T2D with their patients and share the importance of avoiding alcohol and other hepatotoxic substances.
According to the American Association of Clinical Endocrinology Clinical Practice Guideline: "There are no U.S. Food and Drug Administration-approved medications for the treatment of NAFLD; however, some diabetes and anti-obesity medications can be beneficial. Bariatric surgery is also effective for weight loss and reducing liver fat in persons with severe obesity."
Courtney Whittle, MD, MSW, Diplomate of ABOM, Pediatric Lead, Obesity Champion, TSPMG, Weight A Minute Clinic, Atlanta, Georgia.
Courtney Whittle, MD, MSW, Diplomate of ABOM, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The patient's history, symptomatology, and assessments suggest a diagnosis of nonalcoholic fatty liver disease (NAFLD). The primary care physician recommends referral to a hepatologist for evaluation and possible liver biopsy.
NAFLD involves an accumulation of triglycerides and other fats in the liver (unrelated to alcohol consumption and other liver disease), with the presence of hepatic steatosis in more than 5% of hepatocytes. NAFLD affects 25% to 35% of the general population, making it the most common cause of chronic liver disease. The rate increases among patients with obesity, 80% of whom are affected by NAFLD.
NAFLD should be considered in patients with unexplained elevations in serum aminotransferases (without positive viral markers or autoantibodies and no history of alcohol use) and a high risk for steatohepatitis, including obesity. The standard NAFLD assessment for biopsy specimens is the Brunt system, and disease stage is determined using the NAFLD activity score and the amount of fibrosis present.
A study of the natural history of NAFLD in patients who were followed for 3 years showed that without pharmacologic intervention, one third experienced disease progression, one third remained stable, and one third improved. An independent risk factor for progression of nonalcoholic steatohepatitis was abnormal glucose tolerance testing. In another natural history study, a 10% higher rate of mortality over 10 years was demonstrated among those with NAFLD vs controls, with the top three causes of death being cancer, heart disease, and liver-related disease. Prevalence of chronic liver disease and cirrhosis has been shown to be elevated in Latino and Japanese American populations.
Patients with NAFLD should be seen regularly to assess for disease progression and receive guidance on weight management interventions and exercise. A weight loss of more than 5% has been shown to reduce liver fat and provide cardiometabolic benefits; a weight reduction of more than 10% can help reverse steatohepatitis or liver fibrosis. In addition to weight loss management strategies, physicians should discuss the importance of controlling hyperlipidemia, insulin resistance, and T2D with their patients and share the importance of avoiding alcohol and other hepatotoxic substances.
According to the American Association of Clinical Endocrinology Clinical Practice Guideline: "There are no U.S. Food and Drug Administration-approved medications for the treatment of NAFLD; however, some diabetes and anti-obesity medications can be beneficial. Bariatric surgery is also effective for weight loss and reducing liver fat in persons with severe obesity."
Courtney Whittle, MD, MSW, Diplomate of ABOM, Pediatric Lead, Obesity Champion, TSPMG, Weight A Minute Clinic, Atlanta, Georgia.
Courtney Whittle, MD, MSW, Diplomate of ABOM, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 51-year-old Hispanic man presents to his primary care physician with fatigue and pain in the upper right abdomen. Physical exam reveals ascites and splenomegaly. His height is 5 ft 8 in and weight is 274 lb; his BMI is 41.7. For the past 5 years, the patient has seen his physician for routine annual exams, during which time he has consistently met the criteria for World Health Organization Class 3 overweight (BMI ≥ 40) and has taken metformin, with varying degrees of adherence, for type 2 diabetes (T2D). Now, given the patient's symptoms and the potential for uncontrolled diabetes, the physician orders laboratory studies and viral serologies for hepatitis. Results of these assessments exclude viral infection but demonstrate abnormal levels of fasting insulin and glucose, hypertriglyceridemia, and elevated transaminase levels that are sixfold above normal levels, with an aspartate aminotransferase-to-alanine transaminase ratio < 1:1.
‘Triple G’ agonist hits new weight loss heights
A novel triple agonist to receptors for three nutrient-stimulated hormones led to weight loss as high as 24% among people with overweight or obesity but who did not have type 2 diabetes when used at the highest tested dose for 48 weeks. The results are from a phase 2 study of retatrutide that was published in The New England Journal of Medicine (2023 Aug 10. doi: 10.1056/NEJMoa2301972).
This level of weight loss is “unprecedented” for a medication administered for 48 weeks, Mary-Elizabeth Patti, MD, said in an editorial that accompanied the report.
The findings “offer further optimism ... that effective pharmacologic management of obesity and related disorders is possible,” wrote Dr. Patti, a principal investigator at the Joslin Diabetes Center in Boston.
The study randomly assigned 338 adults with obesity or overweight – a body mass index (BMI) of ≥ 27 kg/m2 – and at least one weight-related complication to receive either weekly subcutaneous injections of retatrutide in any of six dose regimens or placebo over 48 weeks. The primary outcome was weight change from baseline after 24 weeks.
The highest dose of retatrutide safely produced an average 17.5% drop from baseline weight, compared with an average 1.6% reduction in the placebo group, after 24 weeks, a significant difference.
After 48 weeks, the highest retatrutide dose safely cut baseline weight by an average of 24.2%, compared with an average 2.1% drop among placebo control patients, Ania M. Jastreboff, MD, PhD, and her coauthors wrote in their report. Weight loss levels after 24 and 48 weeks of retatrutide treatment followed a clear dose-response pattern.
Weight losses never before seen
“I have never seen weight loss at this level” after nearly 1 year of treatment, Dr. Jastreboff said when she discussed these findings in a press conference at the annual scientific sessions of the American Diabetes Association in San Diego in late June.
A separate presentation at the ADA meeting documented unprecedented weight loss levels in a study of 281 people with obesity or overweight and type 2 diabetes.
“No other medication has shown an average 17% reduction from baseline bodyweight after 36 weeks in people with type 2 diabetes,” said Julio Rosenstock, MD, director of the Dallas Diabetes Research Center at Medical City, Texas, who formally presented the results from the study of retatrutide in people with type 2 diabetes at the ADA meeting.
The mechanism behind retatrutide’s potent weight-loss effect seems likely tied to its action on three human receptors that naturally respond to three nutrient-stimulated hormones that control appetite, metabolism, fat mobilization, and related functions.
The three hormones that the retatrutide molecule simultaneously mimics are glucagon-like peptide-1 (GLP-1), such as agents in the class of GLP-1 agonists that includes liraglutide (Victoza/Saxenda) and semaglutide (Ozempic/Wegovy); the glucose-dependent insulinotropic polypeptide (GIP), the receptor that is also activated by tirzepatide (Mounjaro), a dual-incretin receptor agonist that mimics both GLP-1 and GIP; and glucagon. Survodutide is a dual GLP-1 and glucagon receptor agonist in phase 2 development.
Retatrutide is currently unique among agents with reported clinical results by having agonist effects on the receptors for all three of these hormones, a property that led Dr. Patti to call retatrutide a “triple G” hormone-receptor agonist in her editorial.
Triple agonist has added effect on liver fat clearance
The glucagon-receptor agonism appears to give retatrutide added effects beyond those of the GLP-1 agonists or GLP-1/GIP dual agonists that are increasingly used in U.S. practice.
A prespecified subgroup analysis of the no diabetes/Jastreboff study (but that was not included in the NEJM report) showed that at both 8-mg and 12-mg weekly doses, 24 weeks of retatrutide produced complete resolution of excess liver fat (hepatic steatosis) in about 80% of the people eligible for the analysis (those whose liver volume was at least 10% fat at study entry).
That percentage increased to about 90% of people receiving these doses after 48 weeks, Lee M. Kaplan, MD, reported during a separate presentation at the ADA meeting.
“When you add glucagon activity, liver-fat clearance goes up tremendously,” observed Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
The average age of the participants in the new study of the use of retatrutide for those with obesity/overweight but not diabetes was 48 years. By design, 52% were men. (The study sought to enroll roughly equal numbers of men and women.) Average BMI at study entry was 37 kg/m2.
Treatment with retatrutide was also significantly associated with improvements in several cardiometabolic measures in exploratory analyses, including systolic and diastolic blood pressure, A1c, fasting glucose, insulin, and some (but not all) lipids, Dr. Jastreboff, director of the Yale Obesity Research Center of Yale University in New Haven, Conn., and her coauthors reported in the NEJM article.
The safety profile of retatrutide was consistent with reported phase 1 findings for the agent among people with type 2 diabetes and resembled the safety profiles of other agents based on GLP-1 or GIP–GLP-1 mimicry for the treatment of type 2 diabetes or obesity.
The most frequently reported adverse events from retatrutide were transient, mostly mild to moderate gastrointestinal events. They occurred primarily during dose escalation. Discontinuation of retatrutide or placebo because of adverse events occurred in 6% to 16% of the participants who received retatrutide and in none of the participants who received placebo.
Lilly, the company developing retatrutide, previously announced the launch of four phase 3 trials to gather further data on retatrutide for use in a marketing-approval application to the Food and Drug Administration.
The four trials – TRIUMPH-1, TRIUMPH-2, TRIUMPH-3, and TRIUMPH-4 – are evaluating the safety and efficacy of retatrutide for chronic weight management for those with obesity or overweight, including those who also have obstructive sleep apnea, knee osteoarthritis, type 2 diabetes, or cardiovascular disease.
The study was sponsored by Lilly, the company developing retatrutide. Dr. Patti has been a consultant to AstraZeneca, Dexcom, Hanmi, and MBX. She has received funding from Dexcom and has been a monitor for a trial funded by Fractyl. Dr. Jastreboff, Dr. Kaplan, and Dr. Rosenstock have reported financial relationships with Lilly as well as other companies.
A version of this article first appeared on Medscape.com.
A novel triple agonist to receptors for three nutrient-stimulated hormones led to weight loss as high as 24% among people with overweight or obesity but who did not have type 2 diabetes when used at the highest tested dose for 48 weeks. The results are from a phase 2 study of retatrutide that was published in The New England Journal of Medicine (2023 Aug 10. doi: 10.1056/NEJMoa2301972).
This level of weight loss is “unprecedented” for a medication administered for 48 weeks, Mary-Elizabeth Patti, MD, said in an editorial that accompanied the report.
The findings “offer further optimism ... that effective pharmacologic management of obesity and related disorders is possible,” wrote Dr. Patti, a principal investigator at the Joslin Diabetes Center in Boston.
The study randomly assigned 338 adults with obesity or overweight – a body mass index (BMI) of ≥ 27 kg/m2 – and at least one weight-related complication to receive either weekly subcutaneous injections of retatrutide in any of six dose regimens or placebo over 48 weeks. The primary outcome was weight change from baseline after 24 weeks.
The highest dose of retatrutide safely produced an average 17.5% drop from baseline weight, compared with an average 1.6% reduction in the placebo group, after 24 weeks, a significant difference.
After 48 weeks, the highest retatrutide dose safely cut baseline weight by an average of 24.2%, compared with an average 2.1% drop among placebo control patients, Ania M. Jastreboff, MD, PhD, and her coauthors wrote in their report. Weight loss levels after 24 and 48 weeks of retatrutide treatment followed a clear dose-response pattern.
Weight losses never before seen
“I have never seen weight loss at this level” after nearly 1 year of treatment, Dr. Jastreboff said when she discussed these findings in a press conference at the annual scientific sessions of the American Diabetes Association in San Diego in late June.
A separate presentation at the ADA meeting documented unprecedented weight loss levels in a study of 281 people with obesity or overweight and type 2 diabetes.
“No other medication has shown an average 17% reduction from baseline bodyweight after 36 weeks in people with type 2 diabetes,” said Julio Rosenstock, MD, director of the Dallas Diabetes Research Center at Medical City, Texas, who formally presented the results from the study of retatrutide in people with type 2 diabetes at the ADA meeting.
The mechanism behind retatrutide’s potent weight-loss effect seems likely tied to its action on three human receptors that naturally respond to three nutrient-stimulated hormones that control appetite, metabolism, fat mobilization, and related functions.
The three hormones that the retatrutide molecule simultaneously mimics are glucagon-like peptide-1 (GLP-1), such as agents in the class of GLP-1 agonists that includes liraglutide (Victoza/Saxenda) and semaglutide (Ozempic/Wegovy); the glucose-dependent insulinotropic polypeptide (GIP), the receptor that is also activated by tirzepatide (Mounjaro), a dual-incretin receptor agonist that mimics both GLP-1 and GIP; and glucagon. Survodutide is a dual GLP-1 and glucagon receptor agonist in phase 2 development.
Retatrutide is currently unique among agents with reported clinical results by having agonist effects on the receptors for all three of these hormones, a property that led Dr. Patti to call retatrutide a “triple G” hormone-receptor agonist in her editorial.
Triple agonist has added effect on liver fat clearance
The glucagon-receptor agonism appears to give retatrutide added effects beyond those of the GLP-1 agonists or GLP-1/GIP dual agonists that are increasingly used in U.S. practice.
A prespecified subgroup analysis of the no diabetes/Jastreboff study (but that was not included in the NEJM report) showed that at both 8-mg and 12-mg weekly doses, 24 weeks of retatrutide produced complete resolution of excess liver fat (hepatic steatosis) in about 80% of the people eligible for the analysis (those whose liver volume was at least 10% fat at study entry).
That percentage increased to about 90% of people receiving these doses after 48 weeks, Lee M. Kaplan, MD, reported during a separate presentation at the ADA meeting.
“When you add glucagon activity, liver-fat clearance goes up tremendously,” observed Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
The average age of the participants in the new study of the use of retatrutide for those with obesity/overweight but not diabetes was 48 years. By design, 52% were men. (The study sought to enroll roughly equal numbers of men and women.) Average BMI at study entry was 37 kg/m2.
Treatment with retatrutide was also significantly associated with improvements in several cardiometabolic measures in exploratory analyses, including systolic and diastolic blood pressure, A1c, fasting glucose, insulin, and some (but not all) lipids, Dr. Jastreboff, director of the Yale Obesity Research Center of Yale University in New Haven, Conn., and her coauthors reported in the NEJM article.
The safety profile of retatrutide was consistent with reported phase 1 findings for the agent among people with type 2 diabetes and resembled the safety profiles of other agents based on GLP-1 or GIP–GLP-1 mimicry for the treatment of type 2 diabetes or obesity.
The most frequently reported adverse events from retatrutide were transient, mostly mild to moderate gastrointestinal events. They occurred primarily during dose escalation. Discontinuation of retatrutide or placebo because of adverse events occurred in 6% to 16% of the participants who received retatrutide and in none of the participants who received placebo.
Lilly, the company developing retatrutide, previously announced the launch of four phase 3 trials to gather further data on retatrutide for use in a marketing-approval application to the Food and Drug Administration.
The four trials – TRIUMPH-1, TRIUMPH-2, TRIUMPH-3, and TRIUMPH-4 – are evaluating the safety and efficacy of retatrutide for chronic weight management for those with obesity or overweight, including those who also have obstructive sleep apnea, knee osteoarthritis, type 2 diabetes, or cardiovascular disease.
The study was sponsored by Lilly, the company developing retatrutide. Dr. Patti has been a consultant to AstraZeneca, Dexcom, Hanmi, and MBX. She has received funding from Dexcom and has been a monitor for a trial funded by Fractyl. Dr. Jastreboff, Dr. Kaplan, and Dr. Rosenstock have reported financial relationships with Lilly as well as other companies.
A version of this article first appeared on Medscape.com.
A novel triple agonist to receptors for three nutrient-stimulated hormones led to weight loss as high as 24% among people with overweight or obesity but who did not have type 2 diabetes when used at the highest tested dose for 48 weeks. The results are from a phase 2 study of retatrutide that was published in The New England Journal of Medicine (2023 Aug 10. doi: 10.1056/NEJMoa2301972).
This level of weight loss is “unprecedented” for a medication administered for 48 weeks, Mary-Elizabeth Patti, MD, said in an editorial that accompanied the report.
The findings “offer further optimism ... that effective pharmacologic management of obesity and related disorders is possible,” wrote Dr. Patti, a principal investigator at the Joslin Diabetes Center in Boston.
The study randomly assigned 338 adults with obesity or overweight – a body mass index (BMI) of ≥ 27 kg/m2 – and at least one weight-related complication to receive either weekly subcutaneous injections of retatrutide in any of six dose regimens or placebo over 48 weeks. The primary outcome was weight change from baseline after 24 weeks.
The highest dose of retatrutide safely produced an average 17.5% drop from baseline weight, compared with an average 1.6% reduction in the placebo group, after 24 weeks, a significant difference.
After 48 weeks, the highest retatrutide dose safely cut baseline weight by an average of 24.2%, compared with an average 2.1% drop among placebo control patients, Ania M. Jastreboff, MD, PhD, and her coauthors wrote in their report. Weight loss levels after 24 and 48 weeks of retatrutide treatment followed a clear dose-response pattern.
Weight losses never before seen
“I have never seen weight loss at this level” after nearly 1 year of treatment, Dr. Jastreboff said when she discussed these findings in a press conference at the annual scientific sessions of the American Diabetes Association in San Diego in late June.
A separate presentation at the ADA meeting documented unprecedented weight loss levels in a study of 281 people with obesity or overweight and type 2 diabetes.
“No other medication has shown an average 17% reduction from baseline bodyweight after 36 weeks in people with type 2 diabetes,” said Julio Rosenstock, MD, director of the Dallas Diabetes Research Center at Medical City, Texas, who formally presented the results from the study of retatrutide in people with type 2 diabetes at the ADA meeting.
The mechanism behind retatrutide’s potent weight-loss effect seems likely tied to its action on three human receptors that naturally respond to three nutrient-stimulated hormones that control appetite, metabolism, fat mobilization, and related functions.
The three hormones that the retatrutide molecule simultaneously mimics are glucagon-like peptide-1 (GLP-1), such as agents in the class of GLP-1 agonists that includes liraglutide (Victoza/Saxenda) and semaglutide (Ozempic/Wegovy); the glucose-dependent insulinotropic polypeptide (GIP), the receptor that is also activated by tirzepatide (Mounjaro), a dual-incretin receptor agonist that mimics both GLP-1 and GIP; and glucagon. Survodutide is a dual GLP-1 and glucagon receptor agonist in phase 2 development.
Retatrutide is currently unique among agents with reported clinical results by having agonist effects on the receptors for all three of these hormones, a property that led Dr. Patti to call retatrutide a “triple G” hormone-receptor agonist in her editorial.
Triple agonist has added effect on liver fat clearance
The glucagon-receptor agonism appears to give retatrutide added effects beyond those of the GLP-1 agonists or GLP-1/GIP dual agonists that are increasingly used in U.S. practice.
A prespecified subgroup analysis of the no diabetes/Jastreboff study (but that was not included in the NEJM report) showed that at both 8-mg and 12-mg weekly doses, 24 weeks of retatrutide produced complete resolution of excess liver fat (hepatic steatosis) in about 80% of the people eligible for the analysis (those whose liver volume was at least 10% fat at study entry).
That percentage increased to about 90% of people receiving these doses after 48 weeks, Lee M. Kaplan, MD, reported during a separate presentation at the ADA meeting.
“When you add glucagon activity, liver-fat clearance goes up tremendously,” observed Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
The average age of the participants in the new study of the use of retatrutide for those with obesity/overweight but not diabetes was 48 years. By design, 52% were men. (The study sought to enroll roughly equal numbers of men and women.) Average BMI at study entry was 37 kg/m2.
Treatment with retatrutide was also significantly associated with improvements in several cardiometabolic measures in exploratory analyses, including systolic and diastolic blood pressure, A1c, fasting glucose, insulin, and some (but not all) lipids, Dr. Jastreboff, director of the Yale Obesity Research Center of Yale University in New Haven, Conn., and her coauthors reported in the NEJM article.
The safety profile of retatrutide was consistent with reported phase 1 findings for the agent among people with type 2 diabetes and resembled the safety profiles of other agents based on GLP-1 or GIP–GLP-1 mimicry for the treatment of type 2 diabetes or obesity.
The most frequently reported adverse events from retatrutide were transient, mostly mild to moderate gastrointestinal events. They occurred primarily during dose escalation. Discontinuation of retatrutide or placebo because of adverse events occurred in 6% to 16% of the participants who received retatrutide and in none of the participants who received placebo.
Lilly, the company developing retatrutide, previously announced the launch of four phase 3 trials to gather further data on retatrutide for use in a marketing-approval application to the Food and Drug Administration.
The four trials – TRIUMPH-1, TRIUMPH-2, TRIUMPH-3, and TRIUMPH-4 – are evaluating the safety and efficacy of retatrutide for chronic weight management for those with obesity or overweight, including those who also have obstructive sleep apnea, knee osteoarthritis, type 2 diabetes, or cardiovascular disease.
The study was sponsored by Lilly, the company developing retatrutide. Dr. Patti has been a consultant to AstraZeneca, Dexcom, Hanmi, and MBX. She has received funding from Dexcom and has been a monitor for a trial funded by Fractyl. Dr. Jastreboff, Dr. Kaplan, and Dr. Rosenstock have reported financial relationships with Lilly as well as other companies.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
How newly discovered genes might fit into obesity
Identifying specific genes adds to growing evidence that biology, in part, drives obesity. Researchers hope the findings will lead to effective treatments, and in the meantime add to the understanding that there are many types of obesity that come from a mix of genes and environmental factors.
Although the study is not the first to point to specific genes, “we were quite surprised by the proposed function of some of the genes we identified,” Lena R. Kaisinger, lead study investigator and a PhD student in the MRC Epidemiology Unit at the University of Cambridge (England), wrote in an email. For example, the genes also manage cell death and influence how cells respond to DNA damage.
The investigators are not sure why genes involved in body size perform this kind of double duty, which opens avenues for future research.
The gene sequencing study was published online in the journal Cell Genomics.
Differences between women and men
The researchers found five new genes in females and two new genes in males linked to greater body mass index (BMI): DIDO1, KIAA1109, MC4R, PTPRG and SLC12A5 in women and MC4R and SLTM in men. People who recall having obesity as a child were more likely to have rare genetic changes in two other genes, OBSCN and MADD.
“The key thing is that when you see real genes with real gene names, it really makes real the notion that genetics underlie obesity,” said Lee Kaplan, MD, PhD, director of the Obesity and Metabolism Institute in Boston, who was not affiliated with the research.
Ms. Kaisinger and colleagues found these significant genetic differences by studying genomes of about 420,000 people stored in the UK Biobank, a huge biomedical database. The researchers decided to look at genes by sex and age because these are “two areas that we still know very little about,” Ms. Kaisinger said.
“We know that different types of obesity connect to different ages,” said Dr. Kaplan, who is also past president of the Obesity Society. “But what they’ve done now is find genes that are associated with particular subtypes of obesity ... some more common in one sex and some more common in different phases of life, including early onset obesity.”
The future is already here
Treatment for obesity based on a person’s genes already exists. For example, in June 2022, the Food and Drug Administration approved setmelanotide (Imcivree) for weight management in adults and children aged over 6 years with specific genetic markers.
Even as encouraging as setmelanotide is to Ms. Kaisinger and colleagues, these are still early days for translating the current research findings into clinical obesity tests and potential treatment, she said.
The “holy grail,” Dr. Kaplan said, is a future where people get screened for a particular genetic profile and their provider can say something like, “You’re probably most susceptible to this type, so we’ll treat you with this particular drug that’s been developed for people with this phenotype.”
Dr. Kaplan added: “That’s exactly what we are trying to do.”
Moving forward, Ms. Kaisinger and colleagues plan to repeat the study in larger and more diverse populations. They also plan to reverse the usual road map for studies, which typically start in animals and then progress to humans.
“We plan to take the most promising gene candidates forward into mouse models to learn more about their function and how exactly their dysfunction results in obesity,” Ms. Kaisinger said.
Three study coauthors are employees and shareholders of Adrestia Therapeutics. No other conflicts of interest were reported.
A version of this article appeared on WebMD.com.
Identifying specific genes adds to growing evidence that biology, in part, drives obesity. Researchers hope the findings will lead to effective treatments, and in the meantime add to the understanding that there are many types of obesity that come from a mix of genes and environmental factors.
Although the study is not the first to point to specific genes, “we were quite surprised by the proposed function of some of the genes we identified,” Lena R. Kaisinger, lead study investigator and a PhD student in the MRC Epidemiology Unit at the University of Cambridge (England), wrote in an email. For example, the genes also manage cell death and influence how cells respond to DNA damage.
The investigators are not sure why genes involved in body size perform this kind of double duty, which opens avenues for future research.
The gene sequencing study was published online in the journal Cell Genomics.
Differences between women and men
The researchers found five new genes in females and two new genes in males linked to greater body mass index (BMI): DIDO1, KIAA1109, MC4R, PTPRG and SLC12A5 in women and MC4R and SLTM in men. People who recall having obesity as a child were more likely to have rare genetic changes in two other genes, OBSCN and MADD.
“The key thing is that when you see real genes with real gene names, it really makes real the notion that genetics underlie obesity,” said Lee Kaplan, MD, PhD, director of the Obesity and Metabolism Institute in Boston, who was not affiliated with the research.
Ms. Kaisinger and colleagues found these significant genetic differences by studying genomes of about 420,000 people stored in the UK Biobank, a huge biomedical database. The researchers decided to look at genes by sex and age because these are “two areas that we still know very little about,” Ms. Kaisinger said.
“We know that different types of obesity connect to different ages,” said Dr. Kaplan, who is also past president of the Obesity Society. “But what they’ve done now is find genes that are associated with particular subtypes of obesity ... some more common in one sex and some more common in different phases of life, including early onset obesity.”
The future is already here
Treatment for obesity based on a person’s genes already exists. For example, in June 2022, the Food and Drug Administration approved setmelanotide (Imcivree) for weight management in adults and children aged over 6 years with specific genetic markers.
Even as encouraging as setmelanotide is to Ms. Kaisinger and colleagues, these are still early days for translating the current research findings into clinical obesity tests and potential treatment, she said.
The “holy grail,” Dr. Kaplan said, is a future where people get screened for a particular genetic profile and their provider can say something like, “You’re probably most susceptible to this type, so we’ll treat you with this particular drug that’s been developed for people with this phenotype.”
Dr. Kaplan added: “That’s exactly what we are trying to do.”
Moving forward, Ms. Kaisinger and colleagues plan to repeat the study in larger and more diverse populations. They also plan to reverse the usual road map for studies, which typically start in animals and then progress to humans.
“We plan to take the most promising gene candidates forward into mouse models to learn more about their function and how exactly their dysfunction results in obesity,” Ms. Kaisinger said.
Three study coauthors are employees and shareholders of Adrestia Therapeutics. No other conflicts of interest were reported.
A version of this article appeared on WebMD.com.
Identifying specific genes adds to growing evidence that biology, in part, drives obesity. Researchers hope the findings will lead to effective treatments, and in the meantime add to the understanding that there are many types of obesity that come from a mix of genes and environmental factors.
Although the study is not the first to point to specific genes, “we were quite surprised by the proposed function of some of the genes we identified,” Lena R. Kaisinger, lead study investigator and a PhD student in the MRC Epidemiology Unit at the University of Cambridge (England), wrote in an email. For example, the genes also manage cell death and influence how cells respond to DNA damage.
The investigators are not sure why genes involved in body size perform this kind of double duty, which opens avenues for future research.
The gene sequencing study was published online in the journal Cell Genomics.
Differences between women and men
The researchers found five new genes in females and two new genes in males linked to greater body mass index (BMI): DIDO1, KIAA1109, MC4R, PTPRG and SLC12A5 in women and MC4R and SLTM in men. People who recall having obesity as a child were more likely to have rare genetic changes in two other genes, OBSCN and MADD.
“The key thing is that when you see real genes with real gene names, it really makes real the notion that genetics underlie obesity,” said Lee Kaplan, MD, PhD, director of the Obesity and Metabolism Institute in Boston, who was not affiliated with the research.
Ms. Kaisinger and colleagues found these significant genetic differences by studying genomes of about 420,000 people stored in the UK Biobank, a huge biomedical database. The researchers decided to look at genes by sex and age because these are “two areas that we still know very little about,” Ms. Kaisinger said.
“We know that different types of obesity connect to different ages,” said Dr. Kaplan, who is also past president of the Obesity Society. “But what they’ve done now is find genes that are associated with particular subtypes of obesity ... some more common in one sex and some more common in different phases of life, including early onset obesity.”
The future is already here
Treatment for obesity based on a person’s genes already exists. For example, in June 2022, the Food and Drug Administration approved setmelanotide (Imcivree) for weight management in adults and children aged over 6 years with specific genetic markers.
Even as encouraging as setmelanotide is to Ms. Kaisinger and colleagues, these are still early days for translating the current research findings into clinical obesity tests and potential treatment, she said.
The “holy grail,” Dr. Kaplan said, is a future where people get screened for a particular genetic profile and their provider can say something like, “You’re probably most susceptible to this type, so we’ll treat you with this particular drug that’s been developed for people with this phenotype.”
Dr. Kaplan added: “That’s exactly what we are trying to do.”
Moving forward, Ms. Kaisinger and colleagues plan to repeat the study in larger and more diverse populations. They also plan to reverse the usual road map for studies, which typically start in animals and then progress to humans.
“We plan to take the most promising gene candidates forward into mouse models to learn more about their function and how exactly their dysfunction results in obesity,” Ms. Kaisinger said.
Three study coauthors are employees and shareholders of Adrestia Therapeutics. No other conflicts of interest were reported.
A version of this article appeared on WebMD.com.
FROM CELL GENOMICS
Semaglutide cuts cardiovascular events in landmark trial
, in the pivotal SELECT trial, with more than 17,000 enrolled people with overweight or obesity and established cardiovascular disease (CVD), but no diabetes.
The finding should fuel improved patient access to this glucagon-like peptide-1 (GLP-1) agonist weight-loss agent that has historically been hindered by skepticism among U.S. payers, many of whom have criticized the health benefits and cost effectiveness of this drug in people whose only indication for treatment is overweight or obesity.
According to top-line results from SELECT released by Novo Nordisk on Aug. 8, the people randomly assigned to receive weekly 2.4-mg subcutaneous injections of semaglutide showed a significant 20% reduction in their incidence of the combined endpoint of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The announcement added that semaglutide treatment also significantly linked with a drop in the incidence of each of these individual three endpoints; the magnitude of these reductions, however, wasn’t specified, nor was the duration of treatment and follow-up.
The results also showed a level of safety and patient tolerance for weekly 2.4-mg injections of semaglutide that were consistent with prior reports on the agent. Semaglutide as Wegovy received marketing approval from the U.S. Food and Drug Administration in 2021 for weight loss, and in 2017 for glucose control in people with type 2 diabetes, at a weekly maximum dose of 2.0 mg (for which it’s marketed as Ozempic).
SELECT began in 2018 and randomly assigned 17,604 adults aged 45 years and older at more than 800 sites in 41 countries. The company’s announcement noted that the trial had accrued a total of 1,270 study participants with a first MACE event but did not break this total down based on treatment received.
‘A good result for patients’
“The topline results from SELECT are exciting, as preventing heart attacks and stroke with a drug that also lowers weight is very important for many patients, especially if the data also show – as I suspect they will – a meaningful improvement of quality of life for patients due to associated weight loss,” commented Naveed Sattar, PhD, a professor of metabolic medicine at the University of Glasgow who was not involved with the study.
Despite this lack of current clarity over the role that weight loss by itself played in driving the observed result, the SELECT findings seem poised to reset a long-standing prejudice against the medical necessity and safety of weight-loss agents when used for the sole indication of helping people lose weight.
Changing how obesity is regarded
“To date, there are no approved weight management medications proven to deliver effective weight management while also reducing the risk of heart attack, stroke, or cardiovascular death,” said Martin Holst Lange, executive vice president for development at Novo Nordisk, in the company’s press release.
“SELECT is a landmark trial and has demonstrated that semaglutide 2.4 mg has the potential to change how obesity is regarded and treated.”
Several of the early medical options for aiding weight loss had substantial adverse effects, including increased MACE rates, a history that led to pervasive wariness among physicians over the safety of antiobesity agents and the wisdom of using medically aided weight loss to produce health benefits.
This attitude also helped dampen health insurance coverage of weight-loss treatments. For example, Medicare has a long-standing policy against reimbursing the cost for medications that are used for the indication of weight loss, and a 2003 U.S. law prohibited part D plans from providing this coverage.
Semaglutide belongs to the class of agents that mimic the action of the incretin GLP-1. The introduction of this class of GLP-1 agonists for weight loss began in 2014 with the FDA’s approval of liraglutide (Saxenda), a daily subcutaneous injection that marked the first step toward establishing the class as safe and effective for weight loss and launching a new era in weight-loss treatment.
According to the Novo Nordisk announcement, a full report on results from SELECT will occur “at a scientific meeting later in 2023.”
SELECT is sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Sattar is a consultant to several companies that market GLP-1 receptor agonists, including Novo Nordisk and Lilly, but has had no involvement in SELECT.
A version of this article first appeared on Medscape.com.
, in the pivotal SELECT trial, with more than 17,000 enrolled people with overweight or obesity and established cardiovascular disease (CVD), but no diabetes.
The finding should fuel improved patient access to this glucagon-like peptide-1 (GLP-1) agonist weight-loss agent that has historically been hindered by skepticism among U.S. payers, many of whom have criticized the health benefits and cost effectiveness of this drug in people whose only indication for treatment is overweight or obesity.
According to top-line results from SELECT released by Novo Nordisk on Aug. 8, the people randomly assigned to receive weekly 2.4-mg subcutaneous injections of semaglutide showed a significant 20% reduction in their incidence of the combined endpoint of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The announcement added that semaglutide treatment also significantly linked with a drop in the incidence of each of these individual three endpoints; the magnitude of these reductions, however, wasn’t specified, nor was the duration of treatment and follow-up.
The results also showed a level of safety and patient tolerance for weekly 2.4-mg injections of semaglutide that were consistent with prior reports on the agent. Semaglutide as Wegovy received marketing approval from the U.S. Food and Drug Administration in 2021 for weight loss, and in 2017 for glucose control in people with type 2 diabetes, at a weekly maximum dose of 2.0 mg (for which it’s marketed as Ozempic).
SELECT began in 2018 and randomly assigned 17,604 adults aged 45 years and older at more than 800 sites in 41 countries. The company’s announcement noted that the trial had accrued a total of 1,270 study participants with a first MACE event but did not break this total down based on treatment received.
‘A good result for patients’
“The topline results from SELECT are exciting, as preventing heart attacks and stroke with a drug that also lowers weight is very important for many patients, especially if the data also show – as I suspect they will – a meaningful improvement of quality of life for patients due to associated weight loss,” commented Naveed Sattar, PhD, a professor of metabolic medicine at the University of Glasgow who was not involved with the study.
Despite this lack of current clarity over the role that weight loss by itself played in driving the observed result, the SELECT findings seem poised to reset a long-standing prejudice against the medical necessity and safety of weight-loss agents when used for the sole indication of helping people lose weight.
Changing how obesity is regarded
“To date, there are no approved weight management medications proven to deliver effective weight management while also reducing the risk of heart attack, stroke, or cardiovascular death,” said Martin Holst Lange, executive vice president for development at Novo Nordisk, in the company’s press release.
“SELECT is a landmark trial and has demonstrated that semaglutide 2.4 mg has the potential to change how obesity is regarded and treated.”
Several of the early medical options for aiding weight loss had substantial adverse effects, including increased MACE rates, a history that led to pervasive wariness among physicians over the safety of antiobesity agents and the wisdom of using medically aided weight loss to produce health benefits.
This attitude also helped dampen health insurance coverage of weight-loss treatments. For example, Medicare has a long-standing policy against reimbursing the cost for medications that are used for the indication of weight loss, and a 2003 U.S. law prohibited part D plans from providing this coverage.
Semaglutide belongs to the class of agents that mimic the action of the incretin GLP-1. The introduction of this class of GLP-1 agonists for weight loss began in 2014 with the FDA’s approval of liraglutide (Saxenda), a daily subcutaneous injection that marked the first step toward establishing the class as safe and effective for weight loss and launching a new era in weight-loss treatment.
According to the Novo Nordisk announcement, a full report on results from SELECT will occur “at a scientific meeting later in 2023.”
SELECT is sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Sattar is a consultant to several companies that market GLP-1 receptor agonists, including Novo Nordisk and Lilly, but has had no involvement in SELECT.
A version of this article first appeared on Medscape.com.
, in the pivotal SELECT trial, with more than 17,000 enrolled people with overweight or obesity and established cardiovascular disease (CVD), but no diabetes.
The finding should fuel improved patient access to this glucagon-like peptide-1 (GLP-1) agonist weight-loss agent that has historically been hindered by skepticism among U.S. payers, many of whom have criticized the health benefits and cost effectiveness of this drug in people whose only indication for treatment is overweight or obesity.
According to top-line results from SELECT released by Novo Nordisk on Aug. 8, the people randomly assigned to receive weekly 2.4-mg subcutaneous injections of semaglutide showed a significant 20% reduction in their incidence of the combined endpoint of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The announcement added that semaglutide treatment also significantly linked with a drop in the incidence of each of these individual three endpoints; the magnitude of these reductions, however, wasn’t specified, nor was the duration of treatment and follow-up.
The results also showed a level of safety and patient tolerance for weekly 2.4-mg injections of semaglutide that were consistent with prior reports on the agent. Semaglutide as Wegovy received marketing approval from the U.S. Food and Drug Administration in 2021 for weight loss, and in 2017 for glucose control in people with type 2 diabetes, at a weekly maximum dose of 2.0 mg (for which it’s marketed as Ozempic).
SELECT began in 2018 and randomly assigned 17,604 adults aged 45 years and older at more than 800 sites in 41 countries. The company’s announcement noted that the trial had accrued a total of 1,270 study participants with a first MACE event but did not break this total down based on treatment received.
‘A good result for patients’
“The topline results from SELECT are exciting, as preventing heart attacks and stroke with a drug that also lowers weight is very important for many patients, especially if the data also show – as I suspect they will – a meaningful improvement of quality of life for patients due to associated weight loss,” commented Naveed Sattar, PhD, a professor of metabolic medicine at the University of Glasgow who was not involved with the study.
Despite this lack of current clarity over the role that weight loss by itself played in driving the observed result, the SELECT findings seem poised to reset a long-standing prejudice against the medical necessity and safety of weight-loss agents when used for the sole indication of helping people lose weight.
Changing how obesity is regarded
“To date, there are no approved weight management medications proven to deliver effective weight management while also reducing the risk of heart attack, stroke, or cardiovascular death,” said Martin Holst Lange, executive vice president for development at Novo Nordisk, in the company’s press release.
“SELECT is a landmark trial and has demonstrated that semaglutide 2.4 mg has the potential to change how obesity is regarded and treated.”
Several of the early medical options for aiding weight loss had substantial adverse effects, including increased MACE rates, a history that led to pervasive wariness among physicians over the safety of antiobesity agents and the wisdom of using medically aided weight loss to produce health benefits.
This attitude also helped dampen health insurance coverage of weight-loss treatments. For example, Medicare has a long-standing policy against reimbursing the cost for medications that are used for the indication of weight loss, and a 2003 U.S. law prohibited part D plans from providing this coverage.
Semaglutide belongs to the class of agents that mimic the action of the incretin GLP-1. The introduction of this class of GLP-1 agonists for weight loss began in 2014 with the FDA’s approval of liraglutide (Saxenda), a daily subcutaneous injection that marked the first step toward establishing the class as safe and effective for weight loss and launching a new era in weight-loss treatment.
According to the Novo Nordisk announcement, a full report on results from SELECT will occur “at a scientific meeting later in 2023.”
SELECT is sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Sattar is a consultant to several companies that market GLP-1 receptor agonists, including Novo Nordisk and Lilly, but has had no involvement in SELECT.
A version of this article first appeared on Medscape.com.
Should we rename obesity?
Public perception of disease is everything. “Diabetics” are now referred to as “people living with diabetes,” and an “obese person” is now an “individual living with obesity.”
And is the term so tainted in negativity, blame, and bias that the only solution is to scrap it and completely rename it? Society (and medicine) have changed significantly since the Latin word obesitas was adopted back in the 1600s.
Despite so much hinging on the word “obesity,” it’s remarkable that the label persists while the concepts underpinning it have evolved significantly. So perhaps it is more about finding the least-worst option rather than pursuing the impossibility of a solution that suits all?
This is precisely the challenge faced by a Lancet Diabetes & Endocrinology Commission on the Definition and Diagnosis of Clinical Obesity, which is due to publish its initial findings this coming fall. The global task force has 60 leaders in the clinical management of obesity, including representatives with lived experiences of obesity. Leading the project is Francesco Rubino, MD, chair of metabolic and bariatric surgery at King’s College London.
“Renaming ‘obesity’ is very important,” states Dr. Rubino. “The word is so stigmatized, with so much misunderstanding and misperception, some might say the only solution is to change the name.”
One possibility for a new name, introduced by the American Association of Clinical Endocrinologists (now –Endocrinology) and the American College of Endocrinology back in 2016, was based on framing the disease on the central characteristic of adiposity and was termed ABCD, for adiposity-based chronic disease.
Dr. Rubino welcomes “ABCD” but has some reservations. “It is good from a physiological point of view, but the problem is it speaks to scientists and medical professionals. I don’t know how much it would appeal to the general public. ‘ABCD’ still falls short of telling us what the illness is.”
He adds that the Lancet Commission’s approach is rather to call it “clinical obesity.” “ ‘Obesity’ itself doesn’t necessarily convey the message that you have a disease or an illness,” he observes. “It is similar to the difference in meaning between depression and clinical depression, which communicate two different things.”
But underpinning any renaming is greater clarification of the definition and diagnosis of obesity. In 1997, the World Health Organization recognized obesity as a chronic disease; in 2013, the American Medical Association did likewise, adding that it warranted medical attention; while it took until 2021 for the European Commission to define obesity as a “chronic relapsing disease, which in turn acts as a gateway to a range of other non-communicable diseases.”
Yet, 25 years after the initial recognition of obesity as a disease, the concept is still riddled with negativity, whether openly or unconsciously. Such stigma denigrates overweight people and those with obesity as “lazy, sloppy, unintelligent, and unattractive.”
Dr. Rubino explains that first, it’s important to establish and define the essential components and characteristics of the disease of obesity. This is key to improving access to clinical care, reducing personal blame, and nurturing a more supportive research environment to help inform both clinical and policy decision-making.
“This is the question that is at the core of our commission. We have a problem with the current definition of obesity, and the way we measure it does not allow us to accurately define a state of illness with obesity,” he explains.
Labels shape public perceptions of disease; ‘obesity’ epitomizes this
Another expert championing the need for a name that better reflects the definition – whatever that turns out to be – is Margaret Steele, PhD, School of Public Health, University College Cork (Ireland), who, according to her university webpage, has a special interest in “ ‘fatness’ as a cultural, social and political phenomenon.”
She believes that labels, including “obesity,” have a pivotal role in shaping public perceptions. In our digital, information-rich age, the boundaries of medicine and society overlap, with public perception shaping decisions of a medical nature as never before. But with this comes controversy and division – obesity management being a case in point.
Specifically, the word “obesity” is too widely associated with negative connotations, she says, and therefore she welcomes the dialogue around redefining and renaming it. Despite wide general support for a name and definition that reflects adiposity, due to its central physiologic role in the complications of obesity, Dr. Steele believes that the “effects on adipose tissue are downstream of brain issues and the food environment,” and she wants to see more attention brought to this.
Referring to most Westernized societies, she describes how people who grew up in times of food scarcity, before processed foods became widely available, have a different taste profile from those who grew up afterwards. “Growing up in 1940s and ‘50s Ireland, people recall how they remember getting an orange as a treat at Christmas, because the idea that you could have food all year-round – any fruit and veg that you want, when you want it – just wasn’t there.”
By comparison, societal changes leading to more financial and time pressure in later decades meant that fast, high-fat, high-sugar, and processed foods became more desirable, she points out. “Most young children now recognize the company name, and even the specific fast-food brand [they like], before they know their alphabet.”
The current environment has cultivated “a very different physical reaction to foods, maybe a different kind of emotional response,” she believes, highlighting the tightly woven relationship between obesity, society, mental health, and food options.
Dr. Steele wants to stimulate a conversation about the term used to describe individuals, conventionally described as ‘”obese” or using the word “obesity.” “We’re thinking in terms of maybe chronic appetite, chronic food intake, or dietary intake dysregulation.”
Changing medical terminology when it has become useless or harmful is not new, she argues, with co-author, Francis Finucane, MD, consultant endocrinologist at Galway University Hospitals, Ireland, in a recent paper on the subject.
“In the 20th century, the terms ‘feeble-minded’ and ‘moron’ had become used in a pejorative way in the wider culture and were dropped from medical usage,” Dr. Steele points out. She adds that changing the term “obesity” can facilitate pursuit of the strategic goals of clinical medicine “without causing needless controversy with those who, given their own goals and contexts, understand body mass index or body weight in a radically different way.”
Obesity: Disease, risk factor, or both?
Dr. Rubino stresses that prior to any renaming, there is a need to establish and define the essential components and characteristics of the disease of obesity. “This question is at the core of our Commission, and it is not an easy conversation to have.” He further explains that the struggle with the current definition of obesity, and the way it is conceived, is largely centered on it still being considered a risk factor for something else.
Disease is characterized by three things, says Dr. Rubino. These comprise the phenomenon of having a pathogenic cause, leading to pathophysiologic alterations (of the organs), causing clinical manifestations.
He adds that obesity is currently described by what it can cause – for example, type 2 diabetes, cancer, or hypertension. “Each of these things have their own clinical manifestations but obesity doesn’t. [As a disease], we don’t have a definition of the clinical manifestations of obesity other than excess adiposity.”
“If we use BMI, this does not predict excess adiposity, nor does it determine a disease here and now. There is no disease without an illness, which is the clinical manifestation, and the perception by the patient of it being an illness,” explains Dr. Rubino, pointing out that the Lancet Commission is filling this gap in knowledge by asking, “If obesity is an illness, then what does it look like?”
He adds that waist circumference probably provides a better measure than BMI in directly indicating the abnormal distribution of adiposity, known to be associated with poor cardiometabolic outcomes, “but it doesn’t tell you if you have an illness here and now – only that someone is at risk of developing cardiovascular disease in the future. Most people with some excess fat around the waist are perfectly functional and don’t feel ill.”
He also explains that confusion persists around whether obesity – or excess adiposity – is a risk factor for or a symptom of another disease. “The picture is blurred, and we do not know how to discriminate between these. We only have one name, and it applies to all those things, and we have one criterion – BMI – to diagnose it!”
Dr. Rubino adds, “So, what defines it? Is it diabetes? No, because that is another disease. You don’t define a disease by another. It has to stand on its own.”
Recently, the American Medical Association advised that BMI now be used in conjunction with other valid measures of risk such as, but not limited to, measurements of visceral fat, body adiposity index, body composition, relative fat mass, waist circumference, and genetic/metabolic factors.
Aayush Visaria, MD, an internal medicine resident at Rutgers University, New Brunswick, New Jersey, agrees that a new name might help change public perception of obesity for the better. A study he presented at the 2023 Endocrine Society Meeting found that BMI “vastly underestimates” obesity.
He agrees with Dr. Rubino that the challenge lies in the lack of precise understanding of the mechanisms driving obesity: “It’s multifactorial, so not just appetite or food intake. Putting this into one phrase is difficult.”
However, if a new term can incorporate the many facets of the disease, “overall, it’ll reduce stigma because we’ll start to think about obesity as a disease process, not a personal thing with blame attached,” says Dr. Visaria.
But simultaneously, he expresses caution around possible negative connotations associated with the classification of obesity as a disease. Dr. Steele also reflects on this risk, highlighting that medicalizing body size can be counterproductive in feeding into weight stigma and fatphobia.
“Medicalizing obesity can be discouraging rather than empowering, but by specifying more clearly that we’re talking about a specific set of interrelated metabolic conditions, it would make it much clearer, and that ... this isn’t about making people skinny, it isn’t about an aesthetic thing,” Dr. Steele observes.
The word ‘obesity’ hinders disease explanations
Dr. Steele explains that her goal is to overcome the ambiguity around the word “obesity” that hinders explanations of the disease of obesity to the wider public.
“Much confusion and controversy might be avoided if we were to clarify that when doctors say that obesity is a disease, they do not mean that being ‘fat’ is a disease.”
Nevertheless, adipose tissue is an active endocrine organ, producing hormones that function less well in people with obesity, she notes. “This new knowledge has led to better treatments, including drugs like semaglutide and tirzepatide. These drugs, like bariatric surgery, typically lead to significant weight loss and to improvements in overall metabolic health.”
Dr. Rubino also expresses concerns around medicalization, as determined by definition and diagnosis and the availability of drug treatment that could potentially lead to overtreatment. “Currently, when everyone with a BMI of greater than 30 gets access to every obesity treatment out there, we see drugs are running out of stock. We should prioritize that treatment.”
Ultimately, the diagnosis of obesity as a disease needs an anthropometric biomarker that provides, on an individual level, the confidence that a person has a disease today, or at least close to a 100% likelihood of developing this disease and illness, asserts Dr. Rubino.
“If we use BMI, or even waist circumference, these might diagnose the disease; but if the person lives to 90 years, what’s the point of labeling somebody as having an illness?” he points out.
“As doctors, we have to be cautious. We say this is a disease, but you must think about the implications for the person on the receiving end of that diagnosis of a chronic disease that is substantially incurable. When we say it, we need to be certain.”
Dr. Steele and Dr. Visaria have disclosed no relevant financial relationships. Dr. Rubino disclosed that he has received research grants from Novo Nordisk, Medtronic, and Johnson & Johnson. He has undertaken paid consultancy work for GI Dynamics and received honoraria for lectures from Medtronic, Novo Nordisk, and Johnson & Johnson. He is a member of the data safety monitoring board for GT Metabolic Solutions and has provided scientific advice to Keyron, Metadeq, GHP Scientific, and ViBo Health for no remuneration.
A version of this article first appeared on Medscape.com.
Public perception of disease is everything. “Diabetics” are now referred to as “people living with diabetes,” and an “obese person” is now an “individual living with obesity.”
And is the term so tainted in negativity, blame, and bias that the only solution is to scrap it and completely rename it? Society (and medicine) have changed significantly since the Latin word obesitas was adopted back in the 1600s.
Despite so much hinging on the word “obesity,” it’s remarkable that the label persists while the concepts underpinning it have evolved significantly. So perhaps it is more about finding the least-worst option rather than pursuing the impossibility of a solution that suits all?
This is precisely the challenge faced by a Lancet Diabetes & Endocrinology Commission on the Definition and Diagnosis of Clinical Obesity, which is due to publish its initial findings this coming fall. The global task force has 60 leaders in the clinical management of obesity, including representatives with lived experiences of obesity. Leading the project is Francesco Rubino, MD, chair of metabolic and bariatric surgery at King’s College London.
“Renaming ‘obesity’ is very important,” states Dr. Rubino. “The word is so stigmatized, with so much misunderstanding and misperception, some might say the only solution is to change the name.”
One possibility for a new name, introduced by the American Association of Clinical Endocrinologists (now –Endocrinology) and the American College of Endocrinology back in 2016, was based on framing the disease on the central characteristic of adiposity and was termed ABCD, for adiposity-based chronic disease.
Dr. Rubino welcomes “ABCD” but has some reservations. “It is good from a physiological point of view, but the problem is it speaks to scientists and medical professionals. I don’t know how much it would appeal to the general public. ‘ABCD’ still falls short of telling us what the illness is.”
He adds that the Lancet Commission’s approach is rather to call it “clinical obesity.” “ ‘Obesity’ itself doesn’t necessarily convey the message that you have a disease or an illness,” he observes. “It is similar to the difference in meaning between depression and clinical depression, which communicate two different things.”
But underpinning any renaming is greater clarification of the definition and diagnosis of obesity. In 1997, the World Health Organization recognized obesity as a chronic disease; in 2013, the American Medical Association did likewise, adding that it warranted medical attention; while it took until 2021 for the European Commission to define obesity as a “chronic relapsing disease, which in turn acts as a gateway to a range of other non-communicable diseases.”
Yet, 25 years after the initial recognition of obesity as a disease, the concept is still riddled with negativity, whether openly or unconsciously. Such stigma denigrates overweight people and those with obesity as “lazy, sloppy, unintelligent, and unattractive.”
Dr. Rubino explains that first, it’s important to establish and define the essential components and characteristics of the disease of obesity. This is key to improving access to clinical care, reducing personal blame, and nurturing a more supportive research environment to help inform both clinical and policy decision-making.
“This is the question that is at the core of our commission. We have a problem with the current definition of obesity, and the way we measure it does not allow us to accurately define a state of illness with obesity,” he explains.
Labels shape public perceptions of disease; ‘obesity’ epitomizes this
Another expert championing the need for a name that better reflects the definition – whatever that turns out to be – is Margaret Steele, PhD, School of Public Health, University College Cork (Ireland), who, according to her university webpage, has a special interest in “ ‘fatness’ as a cultural, social and political phenomenon.”
She believes that labels, including “obesity,” have a pivotal role in shaping public perceptions. In our digital, information-rich age, the boundaries of medicine and society overlap, with public perception shaping decisions of a medical nature as never before. But with this comes controversy and division – obesity management being a case in point.
Specifically, the word “obesity” is too widely associated with negative connotations, she says, and therefore she welcomes the dialogue around redefining and renaming it. Despite wide general support for a name and definition that reflects adiposity, due to its central physiologic role in the complications of obesity, Dr. Steele believes that the “effects on adipose tissue are downstream of brain issues and the food environment,” and she wants to see more attention brought to this.
Referring to most Westernized societies, she describes how people who grew up in times of food scarcity, before processed foods became widely available, have a different taste profile from those who grew up afterwards. “Growing up in 1940s and ‘50s Ireland, people recall how they remember getting an orange as a treat at Christmas, because the idea that you could have food all year-round – any fruit and veg that you want, when you want it – just wasn’t there.”
By comparison, societal changes leading to more financial and time pressure in later decades meant that fast, high-fat, high-sugar, and processed foods became more desirable, she points out. “Most young children now recognize the company name, and even the specific fast-food brand [they like], before they know their alphabet.”
The current environment has cultivated “a very different physical reaction to foods, maybe a different kind of emotional response,” she believes, highlighting the tightly woven relationship between obesity, society, mental health, and food options.
Dr. Steele wants to stimulate a conversation about the term used to describe individuals, conventionally described as ‘”obese” or using the word “obesity.” “We’re thinking in terms of maybe chronic appetite, chronic food intake, or dietary intake dysregulation.”
Changing medical terminology when it has become useless or harmful is not new, she argues, with co-author, Francis Finucane, MD, consultant endocrinologist at Galway University Hospitals, Ireland, in a recent paper on the subject.
“In the 20th century, the terms ‘feeble-minded’ and ‘moron’ had become used in a pejorative way in the wider culture and were dropped from medical usage,” Dr. Steele points out. She adds that changing the term “obesity” can facilitate pursuit of the strategic goals of clinical medicine “without causing needless controversy with those who, given their own goals and contexts, understand body mass index or body weight in a radically different way.”
Obesity: Disease, risk factor, or both?
Dr. Rubino stresses that prior to any renaming, there is a need to establish and define the essential components and characteristics of the disease of obesity. “This question is at the core of our Commission, and it is not an easy conversation to have.” He further explains that the struggle with the current definition of obesity, and the way it is conceived, is largely centered on it still being considered a risk factor for something else.
Disease is characterized by three things, says Dr. Rubino. These comprise the phenomenon of having a pathogenic cause, leading to pathophysiologic alterations (of the organs), causing clinical manifestations.
He adds that obesity is currently described by what it can cause – for example, type 2 diabetes, cancer, or hypertension. “Each of these things have their own clinical manifestations but obesity doesn’t. [As a disease], we don’t have a definition of the clinical manifestations of obesity other than excess adiposity.”
“If we use BMI, this does not predict excess adiposity, nor does it determine a disease here and now. There is no disease without an illness, which is the clinical manifestation, and the perception by the patient of it being an illness,” explains Dr. Rubino, pointing out that the Lancet Commission is filling this gap in knowledge by asking, “If obesity is an illness, then what does it look like?”
He adds that waist circumference probably provides a better measure than BMI in directly indicating the abnormal distribution of adiposity, known to be associated with poor cardiometabolic outcomes, “but it doesn’t tell you if you have an illness here and now – only that someone is at risk of developing cardiovascular disease in the future. Most people with some excess fat around the waist are perfectly functional and don’t feel ill.”
He also explains that confusion persists around whether obesity – or excess adiposity – is a risk factor for or a symptom of another disease. “The picture is blurred, and we do not know how to discriminate between these. We only have one name, and it applies to all those things, and we have one criterion – BMI – to diagnose it!”
Dr. Rubino adds, “So, what defines it? Is it diabetes? No, because that is another disease. You don’t define a disease by another. It has to stand on its own.”
Recently, the American Medical Association advised that BMI now be used in conjunction with other valid measures of risk such as, but not limited to, measurements of visceral fat, body adiposity index, body composition, relative fat mass, waist circumference, and genetic/metabolic factors.
Aayush Visaria, MD, an internal medicine resident at Rutgers University, New Brunswick, New Jersey, agrees that a new name might help change public perception of obesity for the better. A study he presented at the 2023 Endocrine Society Meeting found that BMI “vastly underestimates” obesity.
He agrees with Dr. Rubino that the challenge lies in the lack of precise understanding of the mechanisms driving obesity: “It’s multifactorial, so not just appetite or food intake. Putting this into one phrase is difficult.”
However, if a new term can incorporate the many facets of the disease, “overall, it’ll reduce stigma because we’ll start to think about obesity as a disease process, not a personal thing with blame attached,” says Dr. Visaria.
But simultaneously, he expresses caution around possible negative connotations associated with the classification of obesity as a disease. Dr. Steele also reflects on this risk, highlighting that medicalizing body size can be counterproductive in feeding into weight stigma and fatphobia.
“Medicalizing obesity can be discouraging rather than empowering, but by specifying more clearly that we’re talking about a specific set of interrelated metabolic conditions, it would make it much clearer, and that ... this isn’t about making people skinny, it isn’t about an aesthetic thing,” Dr. Steele observes.
The word ‘obesity’ hinders disease explanations
Dr. Steele explains that her goal is to overcome the ambiguity around the word “obesity” that hinders explanations of the disease of obesity to the wider public.
“Much confusion and controversy might be avoided if we were to clarify that when doctors say that obesity is a disease, they do not mean that being ‘fat’ is a disease.”
Nevertheless, adipose tissue is an active endocrine organ, producing hormones that function less well in people with obesity, she notes. “This new knowledge has led to better treatments, including drugs like semaglutide and tirzepatide. These drugs, like bariatric surgery, typically lead to significant weight loss and to improvements in overall metabolic health.”
Dr. Rubino also expresses concerns around medicalization, as determined by definition and diagnosis and the availability of drug treatment that could potentially lead to overtreatment. “Currently, when everyone with a BMI of greater than 30 gets access to every obesity treatment out there, we see drugs are running out of stock. We should prioritize that treatment.”
Ultimately, the diagnosis of obesity as a disease needs an anthropometric biomarker that provides, on an individual level, the confidence that a person has a disease today, or at least close to a 100% likelihood of developing this disease and illness, asserts Dr. Rubino.
“If we use BMI, or even waist circumference, these might diagnose the disease; but if the person lives to 90 years, what’s the point of labeling somebody as having an illness?” he points out.
“As doctors, we have to be cautious. We say this is a disease, but you must think about the implications for the person on the receiving end of that diagnosis of a chronic disease that is substantially incurable. When we say it, we need to be certain.”
Dr. Steele and Dr. Visaria have disclosed no relevant financial relationships. Dr. Rubino disclosed that he has received research grants from Novo Nordisk, Medtronic, and Johnson & Johnson. He has undertaken paid consultancy work for GI Dynamics and received honoraria for lectures from Medtronic, Novo Nordisk, and Johnson & Johnson. He is a member of the data safety monitoring board for GT Metabolic Solutions and has provided scientific advice to Keyron, Metadeq, GHP Scientific, and ViBo Health for no remuneration.
A version of this article first appeared on Medscape.com.
Public perception of disease is everything. “Diabetics” are now referred to as “people living with diabetes,” and an “obese person” is now an “individual living with obesity.”
And is the term so tainted in negativity, blame, and bias that the only solution is to scrap it and completely rename it? Society (and medicine) have changed significantly since the Latin word obesitas was adopted back in the 1600s.
Despite so much hinging on the word “obesity,” it’s remarkable that the label persists while the concepts underpinning it have evolved significantly. So perhaps it is more about finding the least-worst option rather than pursuing the impossibility of a solution that suits all?
This is precisely the challenge faced by a Lancet Diabetes & Endocrinology Commission on the Definition and Diagnosis of Clinical Obesity, which is due to publish its initial findings this coming fall. The global task force has 60 leaders in the clinical management of obesity, including representatives with lived experiences of obesity. Leading the project is Francesco Rubino, MD, chair of metabolic and bariatric surgery at King’s College London.
“Renaming ‘obesity’ is very important,” states Dr. Rubino. “The word is so stigmatized, with so much misunderstanding and misperception, some might say the only solution is to change the name.”
One possibility for a new name, introduced by the American Association of Clinical Endocrinologists (now –Endocrinology) and the American College of Endocrinology back in 2016, was based on framing the disease on the central characteristic of adiposity and was termed ABCD, for adiposity-based chronic disease.
Dr. Rubino welcomes “ABCD” but has some reservations. “It is good from a physiological point of view, but the problem is it speaks to scientists and medical professionals. I don’t know how much it would appeal to the general public. ‘ABCD’ still falls short of telling us what the illness is.”
He adds that the Lancet Commission’s approach is rather to call it “clinical obesity.” “ ‘Obesity’ itself doesn’t necessarily convey the message that you have a disease or an illness,” he observes. “It is similar to the difference in meaning between depression and clinical depression, which communicate two different things.”
But underpinning any renaming is greater clarification of the definition and diagnosis of obesity. In 1997, the World Health Organization recognized obesity as a chronic disease; in 2013, the American Medical Association did likewise, adding that it warranted medical attention; while it took until 2021 for the European Commission to define obesity as a “chronic relapsing disease, which in turn acts as a gateway to a range of other non-communicable diseases.”
Yet, 25 years after the initial recognition of obesity as a disease, the concept is still riddled with negativity, whether openly or unconsciously. Such stigma denigrates overweight people and those with obesity as “lazy, sloppy, unintelligent, and unattractive.”
Dr. Rubino explains that first, it’s important to establish and define the essential components and characteristics of the disease of obesity. This is key to improving access to clinical care, reducing personal blame, and nurturing a more supportive research environment to help inform both clinical and policy decision-making.
“This is the question that is at the core of our commission. We have a problem with the current definition of obesity, and the way we measure it does not allow us to accurately define a state of illness with obesity,” he explains.
Labels shape public perceptions of disease; ‘obesity’ epitomizes this
Another expert championing the need for a name that better reflects the definition – whatever that turns out to be – is Margaret Steele, PhD, School of Public Health, University College Cork (Ireland), who, according to her university webpage, has a special interest in “ ‘fatness’ as a cultural, social and political phenomenon.”
She believes that labels, including “obesity,” have a pivotal role in shaping public perceptions. In our digital, information-rich age, the boundaries of medicine and society overlap, with public perception shaping decisions of a medical nature as never before. But with this comes controversy and division – obesity management being a case in point.
Specifically, the word “obesity” is too widely associated with negative connotations, she says, and therefore she welcomes the dialogue around redefining and renaming it. Despite wide general support for a name and definition that reflects adiposity, due to its central physiologic role in the complications of obesity, Dr. Steele believes that the “effects on adipose tissue are downstream of brain issues and the food environment,” and she wants to see more attention brought to this.
Referring to most Westernized societies, she describes how people who grew up in times of food scarcity, before processed foods became widely available, have a different taste profile from those who grew up afterwards. “Growing up in 1940s and ‘50s Ireland, people recall how they remember getting an orange as a treat at Christmas, because the idea that you could have food all year-round – any fruit and veg that you want, when you want it – just wasn’t there.”
By comparison, societal changes leading to more financial and time pressure in later decades meant that fast, high-fat, high-sugar, and processed foods became more desirable, she points out. “Most young children now recognize the company name, and even the specific fast-food brand [they like], before they know their alphabet.”
The current environment has cultivated “a very different physical reaction to foods, maybe a different kind of emotional response,” she believes, highlighting the tightly woven relationship between obesity, society, mental health, and food options.
Dr. Steele wants to stimulate a conversation about the term used to describe individuals, conventionally described as ‘”obese” or using the word “obesity.” “We’re thinking in terms of maybe chronic appetite, chronic food intake, or dietary intake dysregulation.”
Changing medical terminology when it has become useless or harmful is not new, she argues, with co-author, Francis Finucane, MD, consultant endocrinologist at Galway University Hospitals, Ireland, in a recent paper on the subject.
“In the 20th century, the terms ‘feeble-minded’ and ‘moron’ had become used in a pejorative way in the wider culture and were dropped from medical usage,” Dr. Steele points out. She adds that changing the term “obesity” can facilitate pursuit of the strategic goals of clinical medicine “without causing needless controversy with those who, given their own goals and contexts, understand body mass index or body weight in a radically different way.”
Obesity: Disease, risk factor, or both?
Dr. Rubino stresses that prior to any renaming, there is a need to establish and define the essential components and characteristics of the disease of obesity. “This question is at the core of our Commission, and it is not an easy conversation to have.” He further explains that the struggle with the current definition of obesity, and the way it is conceived, is largely centered on it still being considered a risk factor for something else.
Disease is characterized by three things, says Dr. Rubino. These comprise the phenomenon of having a pathogenic cause, leading to pathophysiologic alterations (of the organs), causing clinical manifestations.
He adds that obesity is currently described by what it can cause – for example, type 2 diabetes, cancer, or hypertension. “Each of these things have their own clinical manifestations but obesity doesn’t. [As a disease], we don’t have a definition of the clinical manifestations of obesity other than excess adiposity.”
“If we use BMI, this does not predict excess adiposity, nor does it determine a disease here and now. There is no disease without an illness, which is the clinical manifestation, and the perception by the patient of it being an illness,” explains Dr. Rubino, pointing out that the Lancet Commission is filling this gap in knowledge by asking, “If obesity is an illness, then what does it look like?”
He adds that waist circumference probably provides a better measure than BMI in directly indicating the abnormal distribution of adiposity, known to be associated with poor cardiometabolic outcomes, “but it doesn’t tell you if you have an illness here and now – only that someone is at risk of developing cardiovascular disease in the future. Most people with some excess fat around the waist are perfectly functional and don’t feel ill.”
He also explains that confusion persists around whether obesity – or excess adiposity – is a risk factor for or a symptom of another disease. “The picture is blurred, and we do not know how to discriminate between these. We only have one name, and it applies to all those things, and we have one criterion – BMI – to diagnose it!”
Dr. Rubino adds, “So, what defines it? Is it diabetes? No, because that is another disease. You don’t define a disease by another. It has to stand on its own.”
Recently, the American Medical Association advised that BMI now be used in conjunction with other valid measures of risk such as, but not limited to, measurements of visceral fat, body adiposity index, body composition, relative fat mass, waist circumference, and genetic/metabolic factors.
Aayush Visaria, MD, an internal medicine resident at Rutgers University, New Brunswick, New Jersey, agrees that a new name might help change public perception of obesity for the better. A study he presented at the 2023 Endocrine Society Meeting found that BMI “vastly underestimates” obesity.
He agrees with Dr. Rubino that the challenge lies in the lack of precise understanding of the mechanisms driving obesity: “It’s multifactorial, so not just appetite or food intake. Putting this into one phrase is difficult.”
However, if a new term can incorporate the many facets of the disease, “overall, it’ll reduce stigma because we’ll start to think about obesity as a disease process, not a personal thing with blame attached,” says Dr. Visaria.
But simultaneously, he expresses caution around possible negative connotations associated with the classification of obesity as a disease. Dr. Steele also reflects on this risk, highlighting that medicalizing body size can be counterproductive in feeding into weight stigma and fatphobia.
“Medicalizing obesity can be discouraging rather than empowering, but by specifying more clearly that we’re talking about a specific set of interrelated metabolic conditions, it would make it much clearer, and that ... this isn’t about making people skinny, it isn’t about an aesthetic thing,” Dr. Steele observes.
The word ‘obesity’ hinders disease explanations
Dr. Steele explains that her goal is to overcome the ambiguity around the word “obesity” that hinders explanations of the disease of obesity to the wider public.
“Much confusion and controversy might be avoided if we were to clarify that when doctors say that obesity is a disease, they do not mean that being ‘fat’ is a disease.”
Nevertheless, adipose tissue is an active endocrine organ, producing hormones that function less well in people with obesity, she notes. “This new knowledge has led to better treatments, including drugs like semaglutide and tirzepatide. These drugs, like bariatric surgery, typically lead to significant weight loss and to improvements in overall metabolic health.”
Dr. Rubino also expresses concerns around medicalization, as determined by definition and diagnosis and the availability of drug treatment that could potentially lead to overtreatment. “Currently, when everyone with a BMI of greater than 30 gets access to every obesity treatment out there, we see drugs are running out of stock. We should prioritize that treatment.”
Ultimately, the diagnosis of obesity as a disease needs an anthropometric biomarker that provides, on an individual level, the confidence that a person has a disease today, or at least close to a 100% likelihood of developing this disease and illness, asserts Dr. Rubino.
“If we use BMI, or even waist circumference, these might diagnose the disease; but if the person lives to 90 years, what’s the point of labeling somebody as having an illness?” he points out.
“As doctors, we have to be cautious. We say this is a disease, but you must think about the implications for the person on the receiving end of that diagnosis of a chronic disease that is substantially incurable. When we say it, we need to be certain.”
Dr. Steele and Dr. Visaria have disclosed no relevant financial relationships. Dr. Rubino disclosed that he has received research grants from Novo Nordisk, Medtronic, and Johnson & Johnson. He has undertaken paid consultancy work for GI Dynamics and received honoraria for lectures from Medtronic, Novo Nordisk, and Johnson & Johnson. He is a member of the data safety monitoring board for GT Metabolic Solutions and has provided scientific advice to Keyron, Metadeq, GHP Scientific, and ViBo Health for no remuneration.
A version of this article first appeared on Medscape.com.
Just 1 in 10 with overweight/obesity lose 5% of body weight
On the brighter side, those with higher body mass index (BMI) had greater odds of losing at least 5% of body weight than those with lower BMI, and women were more likely to do so than men. The chances of achieving a healthy weight category – defined as BMI of 18.5-24.9 kg/m2 – was less likely than losing 5% in all groups, however.
Even a modest 5% weight loss at any BMI has been associated with improved health measures, including lower systolic and diastolic blood pressure, lower fasting glucose level, lower hemoglobin A1c level, and higher HDL cholesterol level, write Lyudmyla Kompaniyets, PhD, of the National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and colleagues.
The data from more than 18 million U.S. adults from a nationwide ambulatory electronic medical record database, called IQVIA, suggest that “clinicians and public health efforts can focus on messaging and referrals to interventions that support individuals with excess weight in achieving and sustaining meaningful weight loss, i.e., ≥ 5% for adults at any level of excess weight,” the authors say.
The study population was health care–seeking but not necessarily for weight loss, and their intent to lose weight was unknown. “Several studies suggest that persons who are trying to lose weight may experience greater reductions in weight,” the researchers point out in their article, which was published in JAMA Network Open.
At the initial visit, 72.5% of the participants were categorized as having either overweight (BMI, 25.0-29.9kg/m2) or obesity (BMI, ≥ 30.0 kg/m2). The median age of the patients was 54 years. A little over half (56.7%) were women, 72.3% were White, and 7.7% were Black.
During a maximum follow-up period of 14 years, the proportion with 5% or greater weight loss was 33.4% of those with initial overweight and 41.8% with initial obesity. The proportion achieving healthy weight (BMI, 18.5-24.9 kg/m2) was just 23.2% and 2.0%, respectively.
For the combined overweight/obesity groups, the adjusted annual probability of 5% or greater weight loss was 1 in 10, increasing with BMI category from 1 in 12 for those with initial overweight to 1 in 6 for those with initial BMI of 45 kg/m2 or higher. The annual probability was slightly lower among Black than White women (1 in 9 vs. 1 in 8, respectively).
In contrast, the adjusted annual probability of reducing BMI to the healthy category ranged from 1 in 19 with initial overweight to 1 in 1,667 with initial BMI of 45 kg/m2 or higher. This probability was higher among women than men and was highest among White women.
“These findings could, in part, be explained by barriers in availability of and access to obesity management options, including lifestyle interventions and pharmacotherapy. There is a continual need for policies and strategies that ensure community access to nutrition and physical activity opportunities,” Dr. Kompaniyets and colleague write.
Moreover, they say, “understanding patterns of weight loss could help support populations, including Hispanic or Latino and non-Hispanic Black individuals, who are disproportionately affected by obesity due to factors such as structural racism and race and ethnicity-based social and economic disadvantages.”
The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
On the brighter side, those with higher body mass index (BMI) had greater odds of losing at least 5% of body weight than those with lower BMI, and women were more likely to do so than men. The chances of achieving a healthy weight category – defined as BMI of 18.5-24.9 kg/m2 – was less likely than losing 5% in all groups, however.
Even a modest 5% weight loss at any BMI has been associated with improved health measures, including lower systolic and diastolic blood pressure, lower fasting glucose level, lower hemoglobin A1c level, and higher HDL cholesterol level, write Lyudmyla Kompaniyets, PhD, of the National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and colleagues.
The data from more than 18 million U.S. adults from a nationwide ambulatory electronic medical record database, called IQVIA, suggest that “clinicians and public health efforts can focus on messaging and referrals to interventions that support individuals with excess weight in achieving and sustaining meaningful weight loss, i.e., ≥ 5% for adults at any level of excess weight,” the authors say.
The study population was health care–seeking but not necessarily for weight loss, and their intent to lose weight was unknown. “Several studies suggest that persons who are trying to lose weight may experience greater reductions in weight,” the researchers point out in their article, which was published in JAMA Network Open.
At the initial visit, 72.5% of the participants were categorized as having either overweight (BMI, 25.0-29.9kg/m2) or obesity (BMI, ≥ 30.0 kg/m2). The median age of the patients was 54 years. A little over half (56.7%) were women, 72.3% were White, and 7.7% were Black.
During a maximum follow-up period of 14 years, the proportion with 5% or greater weight loss was 33.4% of those with initial overweight and 41.8% with initial obesity. The proportion achieving healthy weight (BMI, 18.5-24.9 kg/m2) was just 23.2% and 2.0%, respectively.
For the combined overweight/obesity groups, the adjusted annual probability of 5% or greater weight loss was 1 in 10, increasing with BMI category from 1 in 12 for those with initial overweight to 1 in 6 for those with initial BMI of 45 kg/m2 or higher. The annual probability was slightly lower among Black than White women (1 in 9 vs. 1 in 8, respectively).
In contrast, the adjusted annual probability of reducing BMI to the healthy category ranged from 1 in 19 with initial overweight to 1 in 1,667 with initial BMI of 45 kg/m2 or higher. This probability was higher among women than men and was highest among White women.
“These findings could, in part, be explained by barriers in availability of and access to obesity management options, including lifestyle interventions and pharmacotherapy. There is a continual need for policies and strategies that ensure community access to nutrition and physical activity opportunities,” Dr. Kompaniyets and colleague write.
Moreover, they say, “understanding patterns of weight loss could help support populations, including Hispanic or Latino and non-Hispanic Black individuals, who are disproportionately affected by obesity due to factors such as structural racism and race and ethnicity-based social and economic disadvantages.”
The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
On the brighter side, those with higher body mass index (BMI) had greater odds of losing at least 5% of body weight than those with lower BMI, and women were more likely to do so than men. The chances of achieving a healthy weight category – defined as BMI of 18.5-24.9 kg/m2 – was less likely than losing 5% in all groups, however.
Even a modest 5% weight loss at any BMI has been associated with improved health measures, including lower systolic and diastolic blood pressure, lower fasting glucose level, lower hemoglobin A1c level, and higher HDL cholesterol level, write Lyudmyla Kompaniyets, PhD, of the National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and colleagues.
The data from more than 18 million U.S. adults from a nationwide ambulatory electronic medical record database, called IQVIA, suggest that “clinicians and public health efforts can focus on messaging and referrals to interventions that support individuals with excess weight in achieving and sustaining meaningful weight loss, i.e., ≥ 5% for adults at any level of excess weight,” the authors say.
The study population was health care–seeking but not necessarily for weight loss, and their intent to lose weight was unknown. “Several studies suggest that persons who are trying to lose weight may experience greater reductions in weight,” the researchers point out in their article, which was published in JAMA Network Open.
At the initial visit, 72.5% of the participants were categorized as having either overweight (BMI, 25.0-29.9kg/m2) or obesity (BMI, ≥ 30.0 kg/m2). The median age of the patients was 54 years. A little over half (56.7%) were women, 72.3% were White, and 7.7% were Black.
During a maximum follow-up period of 14 years, the proportion with 5% or greater weight loss was 33.4% of those with initial overweight and 41.8% with initial obesity. The proportion achieving healthy weight (BMI, 18.5-24.9 kg/m2) was just 23.2% and 2.0%, respectively.
For the combined overweight/obesity groups, the adjusted annual probability of 5% or greater weight loss was 1 in 10, increasing with BMI category from 1 in 12 for those with initial overweight to 1 in 6 for those with initial BMI of 45 kg/m2 or higher. The annual probability was slightly lower among Black than White women (1 in 9 vs. 1 in 8, respectively).
In contrast, the adjusted annual probability of reducing BMI to the healthy category ranged from 1 in 19 with initial overweight to 1 in 1,667 with initial BMI of 45 kg/m2 or higher. This probability was higher among women than men and was highest among White women.
“These findings could, in part, be explained by barriers in availability of and access to obesity management options, including lifestyle interventions and pharmacotherapy. There is a continual need for policies and strategies that ensure community access to nutrition and physical activity opportunities,” Dr. Kompaniyets and colleague write.
Moreover, they say, “understanding patterns of weight loss could help support populations, including Hispanic or Latino and non-Hispanic Black individuals, who are disproportionately affected by obesity due to factors such as structural racism and race and ethnicity-based social and economic disadvantages.”
The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Obesity cardiomyopathy tied to sudden cardiac death
a new case-control study suggests.
Researchers who analyzed hearts taken at autopsy from people who had died from sudden cardiac death found that a number of the hearts obtained from obese decedents were heavier than those from normal-weight decedents and that the hazard ratio of unexplained cardiomegaly in this cohort was 5.3, compared with normal-weight individuals.
“Even when we ruled out any conditions that could potentially cause enlargement of the heart, including hypertension, heart valve problems, diabetes, and other cardiovascular risk factors, the association with obesity cardiomyopathy, or OCM, and sudden cardiac death remained,” lead author Joseph Westaby, PhD, from the Cardiac Risk in the Young (CRY) Cardiovascular Pathology Laboratories at St George’s University of London, said in an interview.
The study was published online in JACC: Advances.
Intrigued by this finding, Dr. Westaby and associates sought to characterize the clinical and pathological features of OCM associated with sudden cardiac death by comparing this population to two control groups: sudden cardiac death patients who were either obese or of normal weight, and had morphologically normal hearts.
Their group is uniquely positioned to do such research, Dr. Westaby explained.
“Here at St George’s University of London, we have a specialized cardiovascular pathology service. ... All hearts obtained at autopsy from individuals who have died from sudden cardiac death, or who were suspected to have had a cardiovascular cause of death, anywhere in the U.K., are referred to the CRY Centre for further analysis,” he said.
Patients were divided into two groups according to body mass index: an obesity group (BMI > 30 kg/m2) and a normal-weight group (BMI, 18.5-24.9).
An increased heart weight above 550 g in men and 450 g in women in the absence of coronary artery disease, hypertension, diabetes, or valvular disease was classified as unexplained cardiomegaly, and individuals with obesity and cardiomegaly were defined as obesity cardiomyopathy.
Age- and sex-matched controls with obesity (n = 106) were selected based on a BMI greater than 30, with a morphologically normal heart weighing less than 550 g in men and than 450 g in women.
Age- and sex-matched normal weight controls (n = 106) were selected based on a BMI of 18.5-24.9 and a morphologically normal heart weighing less than 550 g in men and less than 450 g in women.
The researchers identified 53 OCM cases from a cohort of more than 4,500 sudden cardiac death cases that had BMI measurements. In normal-weight patients, there were 14 cases of unexplained cardiomegaly.
The mean age at death of individuals with OCM was 42 years (range, 30-54 years). Most of the deaths occurred in men (n = 34; 64%), who also died younger than women (40 ± 13 years vs. 45 ± 10 years; P = .036).
The average heart weight in OCM patients was 598 ± 93 g. Risk of sudden cardiac death increased when BMI reached 35.
Compared with matched controls, there were increases in right and left ventricular wall thickness (all P < .05) in OCM cases. Right ventricular epicardial fat was increased in OCM cases, compared with normal-weight controls only.
Left ventricular fibrosis was identified in seven (13%) OCM cases.
Role of genetics to be explored
“This study highlights the need for further investigation into these individuals because, at the moment, we can’t be sure that the only contributing factor to this is the obesity,” said Dr. Westaby.
In the works are plans to see if there may be an underlying genetic predisposition in obese individuals that may have contributed to the development of an enlarged heart. The group also plans to study the families of the deceased individuals to determine if they are at risk of developing cardiomegaly, he said.
“This paper makes an important contribution to the literature that raises many important questions for future research,” Timothy P. Fitzgibbons, MD, PhD, from the University of Massachusetts, Worcester, wrote in an accompanying editorial.
Being able to access so many autopsy samples gives the current study considerable heft, Dr. Fitzgibbons said in an interview.
“A lot has been made of the obesity paradox and the perhaps benign nature of obesity but this paper suggests the opposite, that it is a very serious problem and can, in fact, in and of itself, cause heart abnormalities that could cause sudden death,” he noted.
The fact that only 13% of OCM cases had fibrosis on histology suggests that fibrosis was not the main cause of sudden cardiac death, he said.
“Often we will do MRIs to look for areas of fibrosis within the heart because those areas make patients prone to re-entry arrhythmias, in particular, ventricular tachycardia. But the authors suggest that the enlarged myocytes may themselves be predisposing to arrhythmias, rather than fibrosis,” Dr. Fitzgibbons said.
The study was supported by Cardiac Risk in the Young. Dr. Westaby and Dr. Fitzgibbons have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
a new case-control study suggests.
Researchers who analyzed hearts taken at autopsy from people who had died from sudden cardiac death found that a number of the hearts obtained from obese decedents were heavier than those from normal-weight decedents and that the hazard ratio of unexplained cardiomegaly in this cohort was 5.3, compared with normal-weight individuals.
“Even when we ruled out any conditions that could potentially cause enlargement of the heart, including hypertension, heart valve problems, diabetes, and other cardiovascular risk factors, the association with obesity cardiomyopathy, or OCM, and sudden cardiac death remained,” lead author Joseph Westaby, PhD, from the Cardiac Risk in the Young (CRY) Cardiovascular Pathology Laboratories at St George’s University of London, said in an interview.
The study was published online in JACC: Advances.
Intrigued by this finding, Dr. Westaby and associates sought to characterize the clinical and pathological features of OCM associated with sudden cardiac death by comparing this population to two control groups: sudden cardiac death patients who were either obese or of normal weight, and had morphologically normal hearts.
Their group is uniquely positioned to do such research, Dr. Westaby explained.
“Here at St George’s University of London, we have a specialized cardiovascular pathology service. ... All hearts obtained at autopsy from individuals who have died from sudden cardiac death, or who were suspected to have had a cardiovascular cause of death, anywhere in the U.K., are referred to the CRY Centre for further analysis,” he said.
Patients were divided into two groups according to body mass index: an obesity group (BMI > 30 kg/m2) and a normal-weight group (BMI, 18.5-24.9).
An increased heart weight above 550 g in men and 450 g in women in the absence of coronary artery disease, hypertension, diabetes, or valvular disease was classified as unexplained cardiomegaly, and individuals with obesity and cardiomegaly were defined as obesity cardiomyopathy.
Age- and sex-matched controls with obesity (n = 106) were selected based on a BMI greater than 30, with a morphologically normal heart weighing less than 550 g in men and than 450 g in women.
Age- and sex-matched normal weight controls (n = 106) were selected based on a BMI of 18.5-24.9 and a morphologically normal heart weighing less than 550 g in men and less than 450 g in women.
The researchers identified 53 OCM cases from a cohort of more than 4,500 sudden cardiac death cases that had BMI measurements. In normal-weight patients, there were 14 cases of unexplained cardiomegaly.
The mean age at death of individuals with OCM was 42 years (range, 30-54 years). Most of the deaths occurred in men (n = 34; 64%), who also died younger than women (40 ± 13 years vs. 45 ± 10 years; P = .036).
The average heart weight in OCM patients was 598 ± 93 g. Risk of sudden cardiac death increased when BMI reached 35.
Compared with matched controls, there were increases in right and left ventricular wall thickness (all P < .05) in OCM cases. Right ventricular epicardial fat was increased in OCM cases, compared with normal-weight controls only.
Left ventricular fibrosis was identified in seven (13%) OCM cases.
Role of genetics to be explored
“This study highlights the need for further investigation into these individuals because, at the moment, we can’t be sure that the only contributing factor to this is the obesity,” said Dr. Westaby.
In the works are plans to see if there may be an underlying genetic predisposition in obese individuals that may have contributed to the development of an enlarged heart. The group also plans to study the families of the deceased individuals to determine if they are at risk of developing cardiomegaly, he said.
“This paper makes an important contribution to the literature that raises many important questions for future research,” Timothy P. Fitzgibbons, MD, PhD, from the University of Massachusetts, Worcester, wrote in an accompanying editorial.
Being able to access so many autopsy samples gives the current study considerable heft, Dr. Fitzgibbons said in an interview.
“A lot has been made of the obesity paradox and the perhaps benign nature of obesity but this paper suggests the opposite, that it is a very serious problem and can, in fact, in and of itself, cause heart abnormalities that could cause sudden death,” he noted.
The fact that only 13% of OCM cases had fibrosis on histology suggests that fibrosis was not the main cause of sudden cardiac death, he said.
“Often we will do MRIs to look for areas of fibrosis within the heart because those areas make patients prone to re-entry arrhythmias, in particular, ventricular tachycardia. But the authors suggest that the enlarged myocytes may themselves be predisposing to arrhythmias, rather than fibrosis,” Dr. Fitzgibbons said.
The study was supported by Cardiac Risk in the Young. Dr. Westaby and Dr. Fitzgibbons have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
a new case-control study suggests.
Researchers who analyzed hearts taken at autopsy from people who had died from sudden cardiac death found that a number of the hearts obtained from obese decedents were heavier than those from normal-weight decedents and that the hazard ratio of unexplained cardiomegaly in this cohort was 5.3, compared with normal-weight individuals.
“Even when we ruled out any conditions that could potentially cause enlargement of the heart, including hypertension, heart valve problems, diabetes, and other cardiovascular risk factors, the association with obesity cardiomyopathy, or OCM, and sudden cardiac death remained,” lead author Joseph Westaby, PhD, from the Cardiac Risk in the Young (CRY) Cardiovascular Pathology Laboratories at St George’s University of London, said in an interview.
The study was published online in JACC: Advances.
Intrigued by this finding, Dr. Westaby and associates sought to characterize the clinical and pathological features of OCM associated with sudden cardiac death by comparing this population to two control groups: sudden cardiac death patients who were either obese or of normal weight, and had morphologically normal hearts.
Their group is uniquely positioned to do such research, Dr. Westaby explained.
“Here at St George’s University of London, we have a specialized cardiovascular pathology service. ... All hearts obtained at autopsy from individuals who have died from sudden cardiac death, or who were suspected to have had a cardiovascular cause of death, anywhere in the U.K., are referred to the CRY Centre for further analysis,” he said.
Patients were divided into two groups according to body mass index: an obesity group (BMI > 30 kg/m2) and a normal-weight group (BMI, 18.5-24.9).
An increased heart weight above 550 g in men and 450 g in women in the absence of coronary artery disease, hypertension, diabetes, or valvular disease was classified as unexplained cardiomegaly, and individuals with obesity and cardiomegaly were defined as obesity cardiomyopathy.
Age- and sex-matched controls with obesity (n = 106) were selected based on a BMI greater than 30, with a morphologically normal heart weighing less than 550 g in men and than 450 g in women.
Age- and sex-matched normal weight controls (n = 106) were selected based on a BMI of 18.5-24.9 and a morphologically normal heart weighing less than 550 g in men and less than 450 g in women.
The researchers identified 53 OCM cases from a cohort of more than 4,500 sudden cardiac death cases that had BMI measurements. In normal-weight patients, there were 14 cases of unexplained cardiomegaly.
The mean age at death of individuals with OCM was 42 years (range, 30-54 years). Most of the deaths occurred in men (n = 34; 64%), who also died younger than women (40 ± 13 years vs. 45 ± 10 years; P = .036).
The average heart weight in OCM patients was 598 ± 93 g. Risk of sudden cardiac death increased when BMI reached 35.
Compared with matched controls, there were increases in right and left ventricular wall thickness (all P < .05) in OCM cases. Right ventricular epicardial fat was increased in OCM cases, compared with normal-weight controls only.
Left ventricular fibrosis was identified in seven (13%) OCM cases.
Role of genetics to be explored
“This study highlights the need for further investigation into these individuals because, at the moment, we can’t be sure that the only contributing factor to this is the obesity,” said Dr. Westaby.
In the works are plans to see if there may be an underlying genetic predisposition in obese individuals that may have contributed to the development of an enlarged heart. The group also plans to study the families of the deceased individuals to determine if they are at risk of developing cardiomegaly, he said.
“This paper makes an important contribution to the literature that raises many important questions for future research,” Timothy P. Fitzgibbons, MD, PhD, from the University of Massachusetts, Worcester, wrote in an accompanying editorial.
Being able to access so many autopsy samples gives the current study considerable heft, Dr. Fitzgibbons said in an interview.
“A lot has been made of the obesity paradox and the perhaps benign nature of obesity but this paper suggests the opposite, that it is a very serious problem and can, in fact, in and of itself, cause heart abnormalities that could cause sudden death,” he noted.
The fact that only 13% of OCM cases had fibrosis on histology suggests that fibrosis was not the main cause of sudden cardiac death, he said.
“Often we will do MRIs to look for areas of fibrosis within the heart because those areas make patients prone to re-entry arrhythmias, in particular, ventricular tachycardia. But the authors suggest that the enlarged myocytes may themselves be predisposing to arrhythmias, rather than fibrosis,” Dr. Fitzgibbons said.
The study was supported by Cardiac Risk in the Young. Dr. Westaby and Dr. Fitzgibbons have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM JACC: ADVANCES
Higher occurrence of kidney stones with more added sugar
Consuming a higher percentage of calories from added sugars is linked with a higher prevalence of kidney stones, new research suggests.
Though added sugars have been linked with multiple poor health outcomes, their link with kidney stones has been unclear.
Added sugars are sugars or caloric sweeteners added to foods or drinks during processing or preparation to add flavor or shelf life. They do not include natural sugars such as lactose in milk and fructose in fruits.
Researchers, led by Shan Yin, a urologist at Affiliated Hospital of North Sichuan Medical College, in Nanchong, China, compared the added-sugar intake by quartiles in the U.S. National Health and Nutrition Examination Survey 2007-2018.
A total of 28,303 adults were included in this study, with an average age of 48. Women who consumed less than 600 or more than 3,500 kcal or men who consumed less than 800 or more than 4,200 kcal were excluded.
Researchers adjusted for factors including age, race, education, income, physical activity, and marital, employment, and smoking status.
Compared with the first quartile of percentage added-sugar calorie intake, the population in the fourth quartile, with the highest added sugar intake, had a higher prevalence of kidney stones (odds ratio, 1.39; 95% confidence interval, 1.17-1.65).
Compared with the group with fewer than 5% of calories from added sugar, the group that consumed at least 25% of calories from added sugar had nearly twice the prevalence of kidney stones (OR, 1.88; 95% CI, 1.52-2.32).
Findings were published online in Frontiers in Nutrition.
“By identifying this association, policymakers and health professionals can emphasize the need for public health initiatives to reduce added sugar consumption and promote healthy dietary habits,” the authors write.
Added sugar in the U.S. diet
Sugar-sweetened beverages such as soft drinks and energy and sports drinks account for 34.4% of added sugars in the American diet. Previous studies have shown the relationship between consuming sugar-sweetened beverages and a higher risk of obesity, diabetes, and cardiovascular disease, diseases that often co-occur with kidney stones.
Researchers note that even though most added sugars in the United States come from sugar-sweetened beverages, it’s unclear whether the association between added sugars and kidney stones is caused by the beverages or other sources. For instance, fructose intake has been found to be independently associated with kidney stones.
How much is too much?
The recommended upper limit on added sugar is controversial and varies widely by health organization. The American Heart Association says daily average intake from added sugars should be no more than 150 kcal for adult males (about 9 teaspoons) and no more than 100 kcal for women (about 6 teaspoons). The Institute of Medicine allows up to 25% of calories to be consumed from added sugars. The 2020 Dietary Guidelines for Americans and World Health Organization set 10% of calories as the recommended upper limit.
Further investigating what causes kidney stones is critical as kidney stones are common worldwide, affecting about 1 in 10 people in the United States alone, and occurrence is increasing. Kidney stones have a high recurrence rate – about half of people who get them have a second episode within 10 years, the authors note.
The researchers acknowledge that because participants self-reported food intake, there is the potential for recall bias. Additionally, because of the cross-sectional design, the researchers were not able to determine whether sugar intake or kidney stone occurrence came first.
This work was supported by the Doctoral Fund Project of North Sichuan Medical College. The authors declare no relevant financial relationships.
Consuming a higher percentage of calories from added sugars is linked with a higher prevalence of kidney stones, new research suggests.
Though added sugars have been linked with multiple poor health outcomes, their link with kidney stones has been unclear.
Added sugars are sugars or caloric sweeteners added to foods or drinks during processing or preparation to add flavor or shelf life. They do not include natural sugars such as lactose in milk and fructose in fruits.
Researchers, led by Shan Yin, a urologist at Affiliated Hospital of North Sichuan Medical College, in Nanchong, China, compared the added-sugar intake by quartiles in the U.S. National Health and Nutrition Examination Survey 2007-2018.
A total of 28,303 adults were included in this study, with an average age of 48. Women who consumed less than 600 or more than 3,500 kcal or men who consumed less than 800 or more than 4,200 kcal were excluded.
Researchers adjusted for factors including age, race, education, income, physical activity, and marital, employment, and smoking status.
Compared with the first quartile of percentage added-sugar calorie intake, the population in the fourth quartile, with the highest added sugar intake, had a higher prevalence of kidney stones (odds ratio, 1.39; 95% confidence interval, 1.17-1.65).
Compared with the group with fewer than 5% of calories from added sugar, the group that consumed at least 25% of calories from added sugar had nearly twice the prevalence of kidney stones (OR, 1.88; 95% CI, 1.52-2.32).
Findings were published online in Frontiers in Nutrition.
“By identifying this association, policymakers and health professionals can emphasize the need for public health initiatives to reduce added sugar consumption and promote healthy dietary habits,” the authors write.
Added sugar in the U.S. diet
Sugar-sweetened beverages such as soft drinks and energy and sports drinks account for 34.4% of added sugars in the American diet. Previous studies have shown the relationship between consuming sugar-sweetened beverages and a higher risk of obesity, diabetes, and cardiovascular disease, diseases that often co-occur with kidney stones.
Researchers note that even though most added sugars in the United States come from sugar-sweetened beverages, it’s unclear whether the association between added sugars and kidney stones is caused by the beverages or other sources. For instance, fructose intake has been found to be independently associated with kidney stones.
How much is too much?
The recommended upper limit on added sugar is controversial and varies widely by health organization. The American Heart Association says daily average intake from added sugars should be no more than 150 kcal for adult males (about 9 teaspoons) and no more than 100 kcal for women (about 6 teaspoons). The Institute of Medicine allows up to 25% of calories to be consumed from added sugars. The 2020 Dietary Guidelines for Americans and World Health Organization set 10% of calories as the recommended upper limit.
Further investigating what causes kidney stones is critical as kidney stones are common worldwide, affecting about 1 in 10 people in the United States alone, and occurrence is increasing. Kidney stones have a high recurrence rate – about half of people who get them have a second episode within 10 years, the authors note.
The researchers acknowledge that because participants self-reported food intake, there is the potential for recall bias. Additionally, because of the cross-sectional design, the researchers were not able to determine whether sugar intake or kidney stone occurrence came first.
This work was supported by the Doctoral Fund Project of North Sichuan Medical College. The authors declare no relevant financial relationships.
Consuming a higher percentage of calories from added sugars is linked with a higher prevalence of kidney stones, new research suggests.
Though added sugars have been linked with multiple poor health outcomes, their link with kidney stones has been unclear.
Added sugars are sugars or caloric sweeteners added to foods or drinks during processing or preparation to add flavor or shelf life. They do not include natural sugars such as lactose in milk and fructose in fruits.
Researchers, led by Shan Yin, a urologist at Affiliated Hospital of North Sichuan Medical College, in Nanchong, China, compared the added-sugar intake by quartiles in the U.S. National Health and Nutrition Examination Survey 2007-2018.
A total of 28,303 adults were included in this study, with an average age of 48. Women who consumed less than 600 or more than 3,500 kcal or men who consumed less than 800 or more than 4,200 kcal were excluded.
Researchers adjusted for factors including age, race, education, income, physical activity, and marital, employment, and smoking status.
Compared with the first quartile of percentage added-sugar calorie intake, the population in the fourth quartile, with the highest added sugar intake, had a higher prevalence of kidney stones (odds ratio, 1.39; 95% confidence interval, 1.17-1.65).
Compared with the group with fewer than 5% of calories from added sugar, the group that consumed at least 25% of calories from added sugar had nearly twice the prevalence of kidney stones (OR, 1.88; 95% CI, 1.52-2.32).
Findings were published online in Frontiers in Nutrition.
“By identifying this association, policymakers and health professionals can emphasize the need for public health initiatives to reduce added sugar consumption and promote healthy dietary habits,” the authors write.
Added sugar in the U.S. diet
Sugar-sweetened beverages such as soft drinks and energy and sports drinks account for 34.4% of added sugars in the American diet. Previous studies have shown the relationship between consuming sugar-sweetened beverages and a higher risk of obesity, diabetes, and cardiovascular disease, diseases that often co-occur with kidney stones.
Researchers note that even though most added sugars in the United States come from sugar-sweetened beverages, it’s unclear whether the association between added sugars and kidney stones is caused by the beverages or other sources. For instance, fructose intake has been found to be independently associated with kidney stones.
How much is too much?
The recommended upper limit on added sugar is controversial and varies widely by health organization. The American Heart Association says daily average intake from added sugars should be no more than 150 kcal for adult males (about 9 teaspoons) and no more than 100 kcal for women (about 6 teaspoons). The Institute of Medicine allows up to 25% of calories to be consumed from added sugars. The 2020 Dietary Guidelines for Americans and World Health Organization set 10% of calories as the recommended upper limit.
Further investigating what causes kidney stones is critical as kidney stones are common worldwide, affecting about 1 in 10 people in the United States alone, and occurrence is increasing. Kidney stones have a high recurrence rate – about half of people who get them have a second episode within 10 years, the authors note.
The researchers acknowledge that because participants self-reported food intake, there is the potential for recall bias. Additionally, because of the cross-sectional design, the researchers were not able to determine whether sugar intake or kidney stone occurrence came first.
This work was supported by the Doctoral Fund Project of North Sichuan Medical College. The authors declare no relevant financial relationships.
FROM FRONTIERS IN NUTRITION