User login
A gentler approach to gastroschisis improves outcomes
BALTIMORE – a condition in which infants are born with their intestines and sometimes other organs protruding through a hole beside the umbilicus.
Neonatologists, maternal-fetal health experts, and pediatric surgeons standardized a literature-based approach that was gentler and less invasive than usual management, emphasizing sutureless closure, sometimes at bedside on the first day of life, and early feeding. Often, it turned out, that’s all that children require.
It’s made a big difference. “We reduced the number of trips to the operating room and exposure to general anesthesia. We reduced the number of babies intubated and days on the ventilator. We reduced opioid days and antibiotic days” without increasing bacteremia, and “there are probably long-term benefits beyond the NICU,” said Kara Calkins, MD, at the Pediatric Academic Societies annual meeting.
I think this is definitely ahead of the curve for NICUs. My hope is that the vast majority of universities adopt a similar approach,” said Dr. Calkins, who is an assistant professor of neonatology at the University of California, Los Angeles.
“When I was a fellow,” she explained, “we took all of these babies and intubated them right away and put them on a drip to paralyze and sedate them. We put their bowels into a silo,” essentially a plastic bag suspended by a string, in the hopes that gravity would pull the bowels back into the abdomen. More often than not, however, “the surgeon would come by every day and slowly push them” back in over a week or so. “The fear was if you did it too quickly, you’d invoke an abdominal compartment syndrome, or respiratory decompensation. You had a baby intubated for a week, sedated and paralyzed.”
Infants were kept on total parenteral nutrition for weeks, sometimes through a Broviac central catheter.
It was overkill, Dr. Calkins said, when only the intestines are out and the abdominal wall defect isn’t too large or too small, which is the case for many infants.
For those children, sutureless closure over 1-3 days is the new goal. The bowel is worked back into the abdomen and the umbilical cord is pulled to the side to approximate the edges of the wound, and tacked down; the defect then heals itself. Antibiotics are discontinued 48 hours after closure. Gastric and rectal decompression helps with reduction.
Also, “we give drops of breast milk in their cheek right away, every couple of hours starting on the first day of life. Once the output from the gastric tube is clear, we start feeds. We still give total parenteral nutrition, but through a [peripherally inserted central catheter] in the arm,” Dr. Calkins said. “Use of breast milk for this population is important” to help establish a healthy microbiome, among other reasons.
Another improvement that had been made, according to Dr. Calkins, is that if only the intestines are out, women carry their baby to term and deliver vaginally. The old practice was to deliver babies preterm by Cesarean section, she explained.
To see how it’s worked out, Dr. Calkins and her colleagues reviewed 70 gastroschisis cases managed under the new guidelines. They were uncomplicated cases, with no intestinal atresia, stricture, or ischemia.
Paralysis was avoided for silo placement in 53 infants (76%) and 32 (46%) avoided intubation. Antibiotics were discontinued in 56 (80%) within 48 hours of abdominal wall closure, and routine narcotics were discontinued in 53 infants (76%). Feeds were initiated in almost all children within 48 hours of non-bilious gastric tube output.
Compared with 168 infants treated before the changes were made, silo placement dropped from 71% to 58% of infants, and total ventilator days from a median of 5 to 2.
There was no difference in length of stay, perhaps because the “intestinal dysmotility intrinsic to gastroschisis remains a rate limiting factor for discharge,” the team concluded.
There was no industry funding, and Dr. Calkins didn’t have any disclosures.
SOURCE: Rottkamp CA et al., PAS 2019. Abstract 51.
BALTIMORE – a condition in which infants are born with their intestines and sometimes other organs protruding through a hole beside the umbilicus.
Neonatologists, maternal-fetal health experts, and pediatric surgeons standardized a literature-based approach that was gentler and less invasive than usual management, emphasizing sutureless closure, sometimes at bedside on the first day of life, and early feeding. Often, it turned out, that’s all that children require.
It’s made a big difference. “We reduced the number of trips to the operating room and exposure to general anesthesia. We reduced the number of babies intubated and days on the ventilator. We reduced opioid days and antibiotic days” without increasing bacteremia, and “there are probably long-term benefits beyond the NICU,” said Kara Calkins, MD, at the Pediatric Academic Societies annual meeting.
I think this is definitely ahead of the curve for NICUs. My hope is that the vast majority of universities adopt a similar approach,” said Dr. Calkins, who is an assistant professor of neonatology at the University of California, Los Angeles.
“When I was a fellow,” she explained, “we took all of these babies and intubated them right away and put them on a drip to paralyze and sedate them. We put their bowels into a silo,” essentially a plastic bag suspended by a string, in the hopes that gravity would pull the bowels back into the abdomen. More often than not, however, “the surgeon would come by every day and slowly push them” back in over a week or so. “The fear was if you did it too quickly, you’d invoke an abdominal compartment syndrome, or respiratory decompensation. You had a baby intubated for a week, sedated and paralyzed.”
Infants were kept on total parenteral nutrition for weeks, sometimes through a Broviac central catheter.
It was overkill, Dr. Calkins said, when only the intestines are out and the abdominal wall defect isn’t too large or too small, which is the case for many infants.
For those children, sutureless closure over 1-3 days is the new goal. The bowel is worked back into the abdomen and the umbilical cord is pulled to the side to approximate the edges of the wound, and tacked down; the defect then heals itself. Antibiotics are discontinued 48 hours after closure. Gastric and rectal decompression helps with reduction.
Also, “we give drops of breast milk in their cheek right away, every couple of hours starting on the first day of life. Once the output from the gastric tube is clear, we start feeds. We still give total parenteral nutrition, but through a [peripherally inserted central catheter] in the arm,” Dr. Calkins said. “Use of breast milk for this population is important” to help establish a healthy microbiome, among other reasons.
Another improvement that had been made, according to Dr. Calkins, is that if only the intestines are out, women carry their baby to term and deliver vaginally. The old practice was to deliver babies preterm by Cesarean section, she explained.
To see how it’s worked out, Dr. Calkins and her colleagues reviewed 70 gastroschisis cases managed under the new guidelines. They were uncomplicated cases, with no intestinal atresia, stricture, or ischemia.
Paralysis was avoided for silo placement in 53 infants (76%) and 32 (46%) avoided intubation. Antibiotics were discontinued in 56 (80%) within 48 hours of abdominal wall closure, and routine narcotics were discontinued in 53 infants (76%). Feeds were initiated in almost all children within 48 hours of non-bilious gastric tube output.
Compared with 168 infants treated before the changes were made, silo placement dropped from 71% to 58% of infants, and total ventilator days from a median of 5 to 2.
There was no difference in length of stay, perhaps because the “intestinal dysmotility intrinsic to gastroschisis remains a rate limiting factor for discharge,” the team concluded.
There was no industry funding, and Dr. Calkins didn’t have any disclosures.
SOURCE: Rottkamp CA et al., PAS 2019. Abstract 51.
BALTIMORE – a condition in which infants are born with their intestines and sometimes other organs protruding through a hole beside the umbilicus.
Neonatologists, maternal-fetal health experts, and pediatric surgeons standardized a literature-based approach that was gentler and less invasive than usual management, emphasizing sutureless closure, sometimes at bedside on the first day of life, and early feeding. Often, it turned out, that’s all that children require.
It’s made a big difference. “We reduced the number of trips to the operating room and exposure to general anesthesia. We reduced the number of babies intubated and days on the ventilator. We reduced opioid days and antibiotic days” without increasing bacteremia, and “there are probably long-term benefits beyond the NICU,” said Kara Calkins, MD, at the Pediatric Academic Societies annual meeting.
I think this is definitely ahead of the curve for NICUs. My hope is that the vast majority of universities adopt a similar approach,” said Dr. Calkins, who is an assistant professor of neonatology at the University of California, Los Angeles.
“When I was a fellow,” she explained, “we took all of these babies and intubated them right away and put them on a drip to paralyze and sedate them. We put their bowels into a silo,” essentially a plastic bag suspended by a string, in the hopes that gravity would pull the bowels back into the abdomen. More often than not, however, “the surgeon would come by every day and slowly push them” back in over a week or so. “The fear was if you did it too quickly, you’d invoke an abdominal compartment syndrome, or respiratory decompensation. You had a baby intubated for a week, sedated and paralyzed.”
Infants were kept on total parenteral nutrition for weeks, sometimes through a Broviac central catheter.
It was overkill, Dr. Calkins said, when only the intestines are out and the abdominal wall defect isn’t too large or too small, which is the case for many infants.
For those children, sutureless closure over 1-3 days is the new goal. The bowel is worked back into the abdomen and the umbilical cord is pulled to the side to approximate the edges of the wound, and tacked down; the defect then heals itself. Antibiotics are discontinued 48 hours after closure. Gastric and rectal decompression helps with reduction.
Also, “we give drops of breast milk in their cheek right away, every couple of hours starting on the first day of life. Once the output from the gastric tube is clear, we start feeds. We still give total parenteral nutrition, but through a [peripherally inserted central catheter] in the arm,” Dr. Calkins said. “Use of breast milk for this population is important” to help establish a healthy microbiome, among other reasons.
Another improvement that had been made, according to Dr. Calkins, is that if only the intestines are out, women carry their baby to term and deliver vaginally. The old practice was to deliver babies preterm by Cesarean section, she explained.
To see how it’s worked out, Dr. Calkins and her colleagues reviewed 70 gastroschisis cases managed under the new guidelines. They were uncomplicated cases, with no intestinal atresia, stricture, or ischemia.
Paralysis was avoided for silo placement in 53 infants (76%) and 32 (46%) avoided intubation. Antibiotics were discontinued in 56 (80%) within 48 hours of abdominal wall closure, and routine narcotics were discontinued in 53 infants (76%). Feeds were initiated in almost all children within 48 hours of non-bilious gastric tube output.
Compared with 168 infants treated before the changes were made, silo placement dropped from 71% to 58% of infants, and total ventilator days from a median of 5 to 2.
There was no difference in length of stay, perhaps because the “intestinal dysmotility intrinsic to gastroschisis remains a rate limiting factor for discharge,” the team concluded.
There was no industry funding, and Dr. Calkins didn’t have any disclosures.
SOURCE: Rottkamp CA et al., PAS 2019. Abstract 51.
REPORTING FROM PAS 2019
Multiple sclerosis may not flare up after pregnancy
PHILADELPHIA – according to a study to be presented at the annual meeting of the American Academy of Neurology.
“We did not observe any rebound disease activity,” said Annette Langer-Gould, MD, PhD, and her research colleagues in their report.
The findings contrast with those of 20-year-old studies that first identified a lower risk of relapse during pregnancy but signficant rebound disease activity in the early postpartum period. The initial studies were conducted before disease-modifying treatments (DMTs) were available and before neurologists used MRI to help diagnose MS after one attack, noted Dr. Langer-Gould in a statement.
In the large, contemporary cohort of patients with MS, the annualized relapse rate was 0.39 pre-pregnancy, 0.07-0.14 during pregnancy, 0.27 in the first 3 months postpartum, and 0.37 at 4-6 months postpartum. Exclusive breastfeeding significantly reduced the risk of postpartum relapses by 42% (adjusted hazard ratio = 0.58). Women who supplemented breast milk with formula within 2 months of delivery had the same risk of relapse as women who did not breastfeed, however.
“These results are exciting, as MS is more common among women of childbearing age than in any other group,” said Dr. Langer-Gould, who is regional lead for clinical and translational neuroscience at Kaiser Permanente Southern California in Pasadena, in the statement. “This shows us that women with MS today can have children, breastfeed, and resume their treatment without experiencing an increased risk of relapses during the postpartum period.”
To describe the risk of postpartum relapses and identify potential risk factors for relapse the investigators analyzed prospectively collected data from 466 pregnancies among 375 women with MS from the complete electronic health record at Kaiser Permanente Southern and Northern California between 2008 and 2016. The researchers also used surveys to collect information about treatment history, breastfeeding, and relapses. They used multivariable models to account for intraclass clustering and disease severity.
In 38% of the pregnancies, the mother had not received treatment in the year before conception. In 14.6%, the mother had a clinically isolated syndrome; in 8.4%, the mother had a relapse during pregnancy.
Resuming modestly effective DMTs such as interferon-betas and glatiramer acetate did not affect relapse risk.
In the postpartum year, 26.4% of mothers relapsed, 87% breastfed, 35% breastfed exclusively, and 41.2% resumed using DMT.
The lack of rebound disease activity in this cohort could be related to the high rate of exclusive breastfeeding, as well as the inclusion of women from a population-based setting and the inclusion of women who had incorrectly been diagnosed with MS after a single relapse. Few patients in this cohort had been treated with natalizumab or fingolimod prior to pregnancy, so the study does not address the potential harms of stopping these drugs or the potential benefits of breastfeeding among patients treated with these drugs.
The study was supported by the National Multiple Sclerosis Society. The researchers had no disclosures.
SOURCE: Langer-Gould A et al. AAN 2019, Abstract S6.007.
PHILADELPHIA – according to a study to be presented at the annual meeting of the American Academy of Neurology.
“We did not observe any rebound disease activity,” said Annette Langer-Gould, MD, PhD, and her research colleagues in their report.
The findings contrast with those of 20-year-old studies that first identified a lower risk of relapse during pregnancy but signficant rebound disease activity in the early postpartum period. The initial studies were conducted before disease-modifying treatments (DMTs) were available and before neurologists used MRI to help diagnose MS after one attack, noted Dr. Langer-Gould in a statement.
In the large, contemporary cohort of patients with MS, the annualized relapse rate was 0.39 pre-pregnancy, 0.07-0.14 during pregnancy, 0.27 in the first 3 months postpartum, and 0.37 at 4-6 months postpartum. Exclusive breastfeeding significantly reduced the risk of postpartum relapses by 42% (adjusted hazard ratio = 0.58). Women who supplemented breast milk with formula within 2 months of delivery had the same risk of relapse as women who did not breastfeed, however.
“These results are exciting, as MS is more common among women of childbearing age than in any other group,” said Dr. Langer-Gould, who is regional lead for clinical and translational neuroscience at Kaiser Permanente Southern California in Pasadena, in the statement. “This shows us that women with MS today can have children, breastfeed, and resume their treatment without experiencing an increased risk of relapses during the postpartum period.”
To describe the risk of postpartum relapses and identify potential risk factors for relapse the investigators analyzed prospectively collected data from 466 pregnancies among 375 women with MS from the complete electronic health record at Kaiser Permanente Southern and Northern California between 2008 and 2016. The researchers also used surveys to collect information about treatment history, breastfeeding, and relapses. They used multivariable models to account for intraclass clustering and disease severity.
In 38% of the pregnancies, the mother had not received treatment in the year before conception. In 14.6%, the mother had a clinically isolated syndrome; in 8.4%, the mother had a relapse during pregnancy.
Resuming modestly effective DMTs such as interferon-betas and glatiramer acetate did not affect relapse risk.
In the postpartum year, 26.4% of mothers relapsed, 87% breastfed, 35% breastfed exclusively, and 41.2% resumed using DMT.
The lack of rebound disease activity in this cohort could be related to the high rate of exclusive breastfeeding, as well as the inclusion of women from a population-based setting and the inclusion of women who had incorrectly been diagnosed with MS after a single relapse. Few patients in this cohort had been treated with natalizumab or fingolimod prior to pregnancy, so the study does not address the potential harms of stopping these drugs or the potential benefits of breastfeeding among patients treated with these drugs.
The study was supported by the National Multiple Sclerosis Society. The researchers had no disclosures.
SOURCE: Langer-Gould A et al. AAN 2019, Abstract S6.007.
PHILADELPHIA – according to a study to be presented at the annual meeting of the American Academy of Neurology.
“We did not observe any rebound disease activity,” said Annette Langer-Gould, MD, PhD, and her research colleagues in their report.
The findings contrast with those of 20-year-old studies that first identified a lower risk of relapse during pregnancy but signficant rebound disease activity in the early postpartum period. The initial studies were conducted before disease-modifying treatments (DMTs) were available and before neurologists used MRI to help diagnose MS after one attack, noted Dr. Langer-Gould in a statement.
In the large, contemporary cohort of patients with MS, the annualized relapse rate was 0.39 pre-pregnancy, 0.07-0.14 during pregnancy, 0.27 in the first 3 months postpartum, and 0.37 at 4-6 months postpartum. Exclusive breastfeeding significantly reduced the risk of postpartum relapses by 42% (adjusted hazard ratio = 0.58). Women who supplemented breast milk with formula within 2 months of delivery had the same risk of relapse as women who did not breastfeed, however.
“These results are exciting, as MS is more common among women of childbearing age than in any other group,” said Dr. Langer-Gould, who is regional lead for clinical and translational neuroscience at Kaiser Permanente Southern California in Pasadena, in the statement. “This shows us that women with MS today can have children, breastfeed, and resume their treatment without experiencing an increased risk of relapses during the postpartum period.”
To describe the risk of postpartum relapses and identify potential risk factors for relapse the investigators analyzed prospectively collected data from 466 pregnancies among 375 women with MS from the complete electronic health record at Kaiser Permanente Southern and Northern California between 2008 and 2016. The researchers also used surveys to collect information about treatment history, breastfeeding, and relapses. They used multivariable models to account for intraclass clustering and disease severity.
In 38% of the pregnancies, the mother had not received treatment in the year before conception. In 14.6%, the mother had a clinically isolated syndrome; in 8.4%, the mother had a relapse during pregnancy.
Resuming modestly effective DMTs such as interferon-betas and glatiramer acetate did not affect relapse risk.
In the postpartum year, 26.4% of mothers relapsed, 87% breastfed, 35% breastfed exclusively, and 41.2% resumed using DMT.
The lack of rebound disease activity in this cohort could be related to the high rate of exclusive breastfeeding, as well as the inclusion of women from a population-based setting and the inclusion of women who had incorrectly been diagnosed with MS after a single relapse. Few patients in this cohort had been treated with natalizumab or fingolimod prior to pregnancy, so the study does not address the potential harms of stopping these drugs or the potential benefits of breastfeeding among patients treated with these drugs.
The study was supported by the National Multiple Sclerosis Society. The researchers had no disclosures.
SOURCE: Langer-Gould A et al. AAN 2019, Abstract S6.007.
FROM AAN 2019
ACOG guidance addresses cardiac contributors to maternal mortality
NASHVILLE, TENN. – according to new comprehensive guidance from the American College of Obstetricians and Gynecologists.
The toolkit algorithm is called the California Improving Health Care Response to Cardiovascular Disease in Pregnancy and Postpartum Toolkit. It was developed by the Cardiovascular Disease in Pregnancy Postpartum Task Force to serve as a resource for obstetrics, primary care and emergency medicine providers who provide prenatal care or interact with women during the postpartum period. It incldues an overview of clinical assessment and comprehensive management strategies for cardiovascular disease based on risk factors and presenting symptoms.
The guidance also calls for all pregnant and postpartum women with known or suspected CVD to undergo further evaluation by a “Pregnancy Heart Team that includes a cardiologist and maternal–fetal medicine subspecialist, or both, and other subspecialists as necessary.” The guidance was issued in Practice Bulletin 212, Pregnancy and Heart Disease, which is published in the May edition of Obstetrics & Gynecology (Obstet Gynecol. 2019 May;133[5]:e320-e356).
In all, 27 specific recommendations and conclusions relating to screening, diagnosis, and management of CVD for women during the prepregnancy period through the postpartum period are included in the guidance.
ACOG president Lisa Hollier, MD, convened the task force that developed this guidance to address cardiac contributors to maternal mortality, she said during a press briefing at the ACOG annual clinical and scientific meeting.
“When I began my presidency a year ago, my goal was to bring together a multidisciplinary group of clinicians ... to create clinical guidance that would make a difference in the lives of women," said Dr. Hollier, who is also a professor of obstetrics and gynecology at Baylor College of Medicine, Houston.
Part of her presidential initiative was centered on eliminating preventable maternal death, and this guidance has the potential to make strides toward that goal, she said. When it comes to CVD in pregnancy, “there is so much we can do to prevent negative outcomes and ensure that moms go home with their babies and are around to see them grow up,” she noted.
CVD is the leading cause of death in pregnant women and women in the postpartum period, accounting for 26.5% of U.S. pregnancy-related deaths.
“It’s critical that we as physicians and health care professionals develop expertise in recognizing the signs and symptoms so that we can save women’s lives,” she said in the press breifing. Dr. Hollier also implored her colleagues to “start using this guidance immediately and prevent more women from dying from cardiovascular complications of pregnancy.”
In this video interview, Dr. Hollier further explains the need for the guidance and its potential for improving maternal mortality rates.
Dr. Hollier reported having no relevant disclosures.
SOURCE: Hollier L et al., Obstet Gynecol. 2019 May;133[5]:e320-56.
NASHVILLE, TENN. – according to new comprehensive guidance from the American College of Obstetricians and Gynecologists.
The toolkit algorithm is called the California Improving Health Care Response to Cardiovascular Disease in Pregnancy and Postpartum Toolkit. It was developed by the Cardiovascular Disease in Pregnancy Postpartum Task Force to serve as a resource for obstetrics, primary care and emergency medicine providers who provide prenatal care or interact with women during the postpartum period. It incldues an overview of clinical assessment and comprehensive management strategies for cardiovascular disease based on risk factors and presenting symptoms.
The guidance also calls for all pregnant and postpartum women with known or suspected CVD to undergo further evaluation by a “Pregnancy Heart Team that includes a cardiologist and maternal–fetal medicine subspecialist, or both, and other subspecialists as necessary.” The guidance was issued in Practice Bulletin 212, Pregnancy and Heart Disease, which is published in the May edition of Obstetrics & Gynecology (Obstet Gynecol. 2019 May;133[5]:e320-e356).
In all, 27 specific recommendations and conclusions relating to screening, diagnosis, and management of CVD for women during the prepregnancy period through the postpartum period are included in the guidance.
ACOG president Lisa Hollier, MD, convened the task force that developed this guidance to address cardiac contributors to maternal mortality, she said during a press briefing at the ACOG annual clinical and scientific meeting.
“When I began my presidency a year ago, my goal was to bring together a multidisciplinary group of clinicians ... to create clinical guidance that would make a difference in the lives of women," said Dr. Hollier, who is also a professor of obstetrics and gynecology at Baylor College of Medicine, Houston.
Part of her presidential initiative was centered on eliminating preventable maternal death, and this guidance has the potential to make strides toward that goal, she said. When it comes to CVD in pregnancy, “there is so much we can do to prevent negative outcomes and ensure that moms go home with their babies and are around to see them grow up,” she noted.
CVD is the leading cause of death in pregnant women and women in the postpartum period, accounting for 26.5% of U.S. pregnancy-related deaths.
“It’s critical that we as physicians and health care professionals develop expertise in recognizing the signs and symptoms so that we can save women’s lives,” she said in the press breifing. Dr. Hollier also implored her colleagues to “start using this guidance immediately and prevent more women from dying from cardiovascular complications of pregnancy.”
In this video interview, Dr. Hollier further explains the need for the guidance and its potential for improving maternal mortality rates.
Dr. Hollier reported having no relevant disclosures.
SOURCE: Hollier L et al., Obstet Gynecol. 2019 May;133[5]:e320-56.
NASHVILLE, TENN. – according to new comprehensive guidance from the American College of Obstetricians and Gynecologists.
The toolkit algorithm is called the California Improving Health Care Response to Cardiovascular Disease in Pregnancy and Postpartum Toolkit. It was developed by the Cardiovascular Disease in Pregnancy Postpartum Task Force to serve as a resource for obstetrics, primary care and emergency medicine providers who provide prenatal care or interact with women during the postpartum period. It incldues an overview of clinical assessment and comprehensive management strategies for cardiovascular disease based on risk factors and presenting symptoms.
The guidance also calls for all pregnant and postpartum women with known or suspected CVD to undergo further evaluation by a “Pregnancy Heart Team that includes a cardiologist and maternal–fetal medicine subspecialist, or both, and other subspecialists as necessary.” The guidance was issued in Practice Bulletin 212, Pregnancy and Heart Disease, which is published in the May edition of Obstetrics & Gynecology (Obstet Gynecol. 2019 May;133[5]:e320-e356).
In all, 27 specific recommendations and conclusions relating to screening, diagnosis, and management of CVD for women during the prepregnancy period through the postpartum period are included in the guidance.
ACOG president Lisa Hollier, MD, convened the task force that developed this guidance to address cardiac contributors to maternal mortality, she said during a press briefing at the ACOG annual clinical and scientific meeting.
“When I began my presidency a year ago, my goal was to bring together a multidisciplinary group of clinicians ... to create clinical guidance that would make a difference in the lives of women," said Dr. Hollier, who is also a professor of obstetrics and gynecology at Baylor College of Medicine, Houston.
Part of her presidential initiative was centered on eliminating preventable maternal death, and this guidance has the potential to make strides toward that goal, she said. When it comes to CVD in pregnancy, “there is so much we can do to prevent negative outcomes and ensure that moms go home with their babies and are around to see them grow up,” she noted.
CVD is the leading cause of death in pregnant women and women in the postpartum period, accounting for 26.5% of U.S. pregnancy-related deaths.
“It’s critical that we as physicians and health care professionals develop expertise in recognizing the signs and symptoms so that we can save women’s lives,” she said in the press breifing. Dr. Hollier also implored her colleagues to “start using this guidance immediately and prevent more women from dying from cardiovascular complications of pregnancy.”
In this video interview, Dr. Hollier further explains the need for the guidance and its potential for improving maternal mortality rates.
Dr. Hollier reported having no relevant disclosures.
SOURCE: Hollier L et al., Obstet Gynecol. 2019 May;133[5]:e320-56.
REPORTING FROM ACOG 2019
Good news for ObGyns: Medical liability claims resulting in payment are decreasing!
Medical professional liability claims (claims) are a major cause of worry and agony for physicians who are dedicated to optimizing the health of all their patients. Among physicians, those who practice neurosurgery, thoracic surgery, plastic surgery, and obstetrics and gynecology have the greatest rate of making a payment on a claim per year of practice.1 Physicians who practice psychiatry, pediatrics, pathology, and internal medicine have the lowest rate of making a payment on a claim. Among the physicians in high-risk specialties, greater than 90% will have a claim filed against them during their career.2 Although professional liability exposure reached a crisis during the 1980s and 1990s, recent data have shown a decrease in overall professional liability risk.
The good news: Paid claims per 1,000 ObGyns have decreased greatly
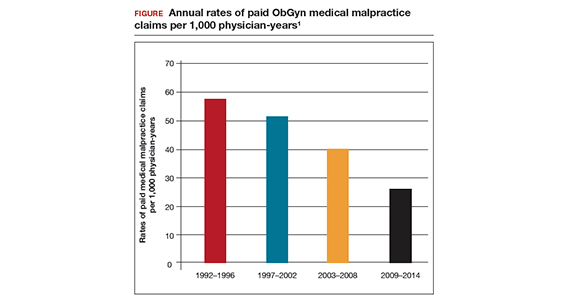
In a review of all paid claims reported to the National Practitioner Data Bank from 1992 to 2014, the annual rate of paid claims per 1,000 ObGyn physician-years was determined.1 For the time periods 1992–1996, 1997–2002, 2003–2008,and 2009–2014, the annual rate of paid claims per 1,000 ObGyn physician-years was 57.6, 51.5, 40.0, and 25.9, representing an astounding 55% decrease in paid claims from 1992 to 2014 (FIGURE).1
The majority of claims result in no payment
In a review of the experience of a nationwide professional liability insurer from 1991 to 2005, only 22% of claims resulted in a payment.2 In this study, for obstetrics and gynecology and gynecologic surgery, only 11% and 8% of claims, respectively, resulted in a payment.2 However, being named in a malpractice claim results in significant stress for a physician and requires a great deal of work and time to defend.
In another study using data from the Physician Insurer’s Association of America, among 10,915 claims closed from 2005 to 2014, 59.5% were dropped, withdrawn, or dismissed; 27.7% were settled; 2.5% were resolved using an alternative dispute resolution process; 1.8% were uncategorized; and 8.6% went to trial.3 Of the cases that went to trial, 87% resulted in a verdict for the physician and 13% resulted in a verdict for the plaintiff.3
Not as good news: Payments per claim and claims settling for a payment > $1 million are increasing
In the period 1992–1996, the average payment per paid claim in the field of obstetrics and gynecology was $387,186, rising to $447,034 in 2009–2014—a 16% increase.1 From 2004 to 2010, million dollar payments occurred in about 8% of cases of paid claims, but they represent 36% of the total of all paid claims.4 In the time periods 1992–1996 and 2009–2014, payments greater than $1 million occurred in 6% and 8% of paid claims, respectively.1
Claims settled for much more than $1 million are of great concern to physicians because the payment may exceed their policy limit, creating a complex legal problem that may take time to resolve. In some cases, where the award is greater than the insurance policy limit, aggressive plaintiff attorneys have obtained a lien on the defendant physician’s home pending settlement of the case. When a multimillion dollar payment is made to settle a professional liability claim, it can greatly influence physician practice and change hospital policies. Frequently, following a multimillion dollar payment a physician may decide to limit their practice to low-risk cases or retire from the practice of medicine.
Liability premiums are stable or decreasing
From 2014 to 2019, my ObGyn professional liability insurance premiums decreased by 18%. During the same time period, my colleagues who practice surgical gynecology (no obstetrics) had a premium decrease of 22%. Insurers use a complex algorithm to determine annual liability insurance premiums, and premiums for ObGyns may not have stabilized or decreased in all regions. Take this Instant Poll:
Create your own user feedback survey
Reform of the liability tort system
Litigation policies and practices that reduce liability risk reduce total medical liability losses. Policies that have helped to constrain medical liability risk include state constitutional amendments limiting payments for pain and suffering, caps on compensation to plaintiff attorneys, increased early resolution programs that compensate patients who experience an adverse event and no-fault conflict resolution programs.5 In 2003, Texas implemented a comprehensive package of tort reform laws. Experts believe the reforms decreased the financial burden of professional liability insurance6 and led to less defensive medical practices, reducing excessive use of imaging and laboratory tests.
Medical factors contributing to a decrease in claims
In 1999, the Institute of Medicine released the report, “To Err is Human,” which galvanized health care systems to deploy systems of care that reduce the rate of adverse patient outcomes.7 Over the past 20 years, health systems have implemented quality improvement programs in obstetrics and gynecology that have contributed to a reduction in the rate of adverse patient outcomes. This may have contributed to the decrease in the rate of paid claims.
In a quasi-experimental study performed in 13 health systems, 7 interventions were implemented with the goal of improving outcomes and reducing medical liability. The 7 interventions included8:
- an elective induction bundle focused on the safe use of oxytocin
- an augmentation bundle focused on early intervention for possible fetal metabolic acidosis
- an operative vaginal delivery bundle
- TeamSTEPPS teamwork training to improve the quality of communication
- best practices education with a focus on electronic fetal monitoring
- regular performance feedback to hospitals and clinicians
- implementation of a quality improvement collaboration to support implementation of the interventions.
During the two-year baseline period prior to the intervention there were 185,373 deliveries with 6.7 perinatal claims made per 10,000 deliveries and 1.3 claims paid per 10,000 deliveries. Following the intervention, the rate of claims made and claims paid per 10,000 deliveries decreased by 22% and 37%, respectively. In addition there was a marked decrease in claims over $1 million paid, greatly limiting total financial liability losses.
Experts with vast experience in obstetrics and obstetric liability litigation have identified 4 priority interventions that may improve outcomes and mitigate liability risk, including: 1) 24-hour in-house physician coverage of an obstetrics service, 2) a conservative approach to trial of labor after a prior cesarean delivery, 3) utilization of a comprehensive, standardized event note in cases of a shoulder dystocia, and 4) judicious use of oxytocin, misoprostol, and magnesium sulfate.9
Other health system interventions that may contribute to a reduction in claims include:
- systematic improvement in the quality of communication among physicians and nurses through the use of team training, preprocedure huddles, and time-out processes10
- rapid response systems to rescue hospital patients with worrisome vital signs11
- standardized responses to a worrisome category 2 or 3 fetal heart-rate tracing12
- rapid recognition, evaluation, and treatment of women with hemorrhage, severe hypertension, sepsis, and venous thromboembolism13
- identification and referral of high-risk patients to tertiary centers14
- closed loop communication of critical imaging and laboratory results15
- universal insurance coverage for health care including contraception, obstetrics, and pediatric care.
Medical liability risk is an important practice issue because it causes excessive use of imaging and laboratory tests and often traumatizes clinicians, which can result in burnout. In the 1980s and 1990s, medical liability litigation reached a crescendo and was a prominent concern among obstetrician-gynecologists. The good news is that, for ObGyns, liability risk has stabilized. Hopefully our resolute efforts to continuously improve the quality of care will result in a long-term reduction in medical liability risk.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992–2014. JAMA Intern Med. 2017;177:710-718.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-e6.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Cardoso R, Zarin W, Nincic V, et al. Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev. 2017;6:181.
- Stewart RM, Geoghegan K, Myers JG, et al. Malpractice risk and costs are significantly reduced after tort reform. J Am Coll Surg. 2011;212:463-467.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- Riley W, Meredith LW, Price R, et al. Decreasing malpractice claims by reducing preventable perinatal harm. Health Serv Res. 2016;51(suppl 3):2453-2471.
- Clark SL, Belfort MA, Dildy GA, et al. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112:1279-1283.
- Haynes AB, Weiser TG, Berry WR, et al; Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499.
- Patel S, Gillon SA, Jones DA. Rapid response systems: recognition and rescue of the deteriorating hospital patient. Br J Hosp Med (Lond). 2017;78:143-148.
- Clark SL, Hamilton EF, Garite TJ, et al. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216:163.e1-163.e6.
- The Council on Patient Safety in Women’s Healthcare website. www.safehealthcareforeverywoman.org. Accessed April 12, 2019.
- Zahn CM, Remick A, Catalano A, et al. Levels of maternal care verification pilot: translating guidance into practice. Obstet Gynecol. 2018;132:1401-1406.
- Zuccotti G, Maloney FL, Feblowitz J, et al. Reducing risk with clinical decision support: a study of closed malpractice claims. Appl Clin Inform. 2014;5:746-756.
Medical professional liability claims (claims) are a major cause of worry and agony for physicians who are dedicated to optimizing the health of all their patients. Among physicians, those who practice neurosurgery, thoracic surgery, plastic surgery, and obstetrics and gynecology have the greatest rate of making a payment on a claim per year of practice.1 Physicians who practice psychiatry, pediatrics, pathology, and internal medicine have the lowest rate of making a payment on a claim. Among the physicians in high-risk specialties, greater than 90% will have a claim filed against them during their career.2 Although professional liability exposure reached a crisis during the 1980s and 1990s, recent data have shown a decrease in overall professional liability risk.
The good news: Paid claims per 1,000 ObGyns have decreased greatly
In a review of all paid claims reported to the National Practitioner Data Bank from 1992 to 2014, the annual rate of paid claims per 1,000 ObGyn physician-years was determined.1 For the time periods 1992–1996, 1997–2002, 2003–2008,and 2009–2014, the annual rate of paid claims per 1,000 ObGyn physician-years was 57.6, 51.5, 40.0, and 25.9, representing an astounding 55% decrease in paid claims from 1992 to 2014 (FIGURE).1
The majority of claims result in no payment
In a review of the experience of a nationwide professional liability insurer from 1991 to 2005, only 22% of claims resulted in a payment.2 In this study, for obstetrics and gynecology and gynecologic surgery, only 11% and 8% of claims, respectively, resulted in a payment.2 However, being named in a malpractice claim results in significant stress for a physician and requires a great deal of work and time to defend.
In another study using data from the Physician Insurer’s Association of America, among 10,915 claims closed from 2005 to 2014, 59.5% were dropped, withdrawn, or dismissed; 27.7% were settled; 2.5% were resolved using an alternative dispute resolution process; 1.8% were uncategorized; and 8.6% went to trial.3 Of the cases that went to trial, 87% resulted in a verdict for the physician and 13% resulted in a verdict for the plaintiff.3
Not as good news: Payments per claim and claims settling for a payment > $1 million are increasing
In the period 1992–1996, the average payment per paid claim in the field of obstetrics and gynecology was $387,186, rising to $447,034 in 2009–2014—a 16% increase.1 From 2004 to 2010, million dollar payments occurred in about 8% of cases of paid claims, but they represent 36% of the total of all paid claims.4 In the time periods 1992–1996 and 2009–2014, payments greater than $1 million occurred in 6% and 8% of paid claims, respectively.1
Claims settled for much more than $1 million are of great concern to physicians because the payment may exceed their policy limit, creating a complex legal problem that may take time to resolve. In some cases, where the award is greater than the insurance policy limit, aggressive plaintiff attorneys have obtained a lien on the defendant physician’s home pending settlement of the case. When a multimillion dollar payment is made to settle a professional liability claim, it can greatly influence physician practice and change hospital policies. Frequently, following a multimillion dollar payment a physician may decide to limit their practice to low-risk cases or retire from the practice of medicine.
Liability premiums are stable or decreasing
From 2014 to 2019, my ObGyn professional liability insurance premiums decreased by 18%. During the same time period, my colleagues who practice surgical gynecology (no obstetrics) had a premium decrease of 22%. Insurers use a complex algorithm to determine annual liability insurance premiums, and premiums for ObGyns may not have stabilized or decreased in all regions. Take this Instant Poll:
Create your own user feedback survey
Reform of the liability tort system
Litigation policies and practices that reduce liability risk reduce total medical liability losses. Policies that have helped to constrain medical liability risk include state constitutional amendments limiting payments for pain and suffering, caps on compensation to plaintiff attorneys, increased early resolution programs that compensate patients who experience an adverse event and no-fault conflict resolution programs.5 In 2003, Texas implemented a comprehensive package of tort reform laws. Experts believe the reforms decreased the financial burden of professional liability insurance6 and led to less defensive medical practices, reducing excessive use of imaging and laboratory tests.
Medical factors contributing to a decrease in claims
In 1999, the Institute of Medicine released the report, “To Err is Human,” which galvanized health care systems to deploy systems of care that reduce the rate of adverse patient outcomes.7 Over the past 20 years, health systems have implemented quality improvement programs in obstetrics and gynecology that have contributed to a reduction in the rate of adverse patient outcomes. This may have contributed to the decrease in the rate of paid claims.
In a quasi-experimental study performed in 13 health systems, 7 interventions were implemented with the goal of improving outcomes and reducing medical liability. The 7 interventions included8:
- an elective induction bundle focused on the safe use of oxytocin
- an augmentation bundle focused on early intervention for possible fetal metabolic acidosis
- an operative vaginal delivery bundle
- TeamSTEPPS teamwork training to improve the quality of communication
- best practices education with a focus on electronic fetal monitoring
- regular performance feedback to hospitals and clinicians
- implementation of a quality improvement collaboration to support implementation of the interventions.
During the two-year baseline period prior to the intervention there were 185,373 deliveries with 6.7 perinatal claims made per 10,000 deliveries and 1.3 claims paid per 10,000 deliveries. Following the intervention, the rate of claims made and claims paid per 10,000 deliveries decreased by 22% and 37%, respectively. In addition there was a marked decrease in claims over $1 million paid, greatly limiting total financial liability losses.
Experts with vast experience in obstetrics and obstetric liability litigation have identified 4 priority interventions that may improve outcomes and mitigate liability risk, including: 1) 24-hour in-house physician coverage of an obstetrics service, 2) a conservative approach to trial of labor after a prior cesarean delivery, 3) utilization of a comprehensive, standardized event note in cases of a shoulder dystocia, and 4) judicious use of oxytocin, misoprostol, and magnesium sulfate.9
Other health system interventions that may contribute to a reduction in claims include:
- systematic improvement in the quality of communication among physicians and nurses through the use of team training, preprocedure huddles, and time-out processes10
- rapid response systems to rescue hospital patients with worrisome vital signs11
- standardized responses to a worrisome category 2 or 3 fetal heart-rate tracing12
- rapid recognition, evaluation, and treatment of women with hemorrhage, severe hypertension, sepsis, and venous thromboembolism13
- identification and referral of high-risk patients to tertiary centers14
- closed loop communication of critical imaging and laboratory results15
- universal insurance coverage for health care including contraception, obstetrics, and pediatric care.
Medical liability risk is an important practice issue because it causes excessive use of imaging and laboratory tests and often traumatizes clinicians, which can result in burnout. In the 1980s and 1990s, medical liability litigation reached a crescendo and was a prominent concern among obstetrician-gynecologists. The good news is that, for ObGyns, liability risk has stabilized. Hopefully our resolute efforts to continuously improve the quality of care will result in a long-term reduction in medical liability risk.
Medical professional liability claims (claims) are a major cause of worry and agony for physicians who are dedicated to optimizing the health of all their patients. Among physicians, those who practice neurosurgery, thoracic surgery, plastic surgery, and obstetrics and gynecology have the greatest rate of making a payment on a claim per year of practice.1 Physicians who practice psychiatry, pediatrics, pathology, and internal medicine have the lowest rate of making a payment on a claim. Among the physicians in high-risk specialties, greater than 90% will have a claim filed against them during their career.2 Although professional liability exposure reached a crisis during the 1980s and 1990s, recent data have shown a decrease in overall professional liability risk.
The good news: Paid claims per 1,000 ObGyns have decreased greatly
In a review of all paid claims reported to the National Practitioner Data Bank from 1992 to 2014, the annual rate of paid claims per 1,000 ObGyn physician-years was determined.1 For the time periods 1992–1996, 1997–2002, 2003–2008,and 2009–2014, the annual rate of paid claims per 1,000 ObGyn physician-years was 57.6, 51.5, 40.0, and 25.9, representing an astounding 55% decrease in paid claims from 1992 to 2014 (FIGURE).1
The majority of claims result in no payment
In a review of the experience of a nationwide professional liability insurer from 1991 to 2005, only 22% of claims resulted in a payment.2 In this study, for obstetrics and gynecology and gynecologic surgery, only 11% and 8% of claims, respectively, resulted in a payment.2 However, being named in a malpractice claim results in significant stress for a physician and requires a great deal of work and time to defend.
In another study using data from the Physician Insurer’s Association of America, among 10,915 claims closed from 2005 to 2014, 59.5% were dropped, withdrawn, or dismissed; 27.7% were settled; 2.5% were resolved using an alternative dispute resolution process; 1.8% were uncategorized; and 8.6% went to trial.3 Of the cases that went to trial, 87% resulted in a verdict for the physician and 13% resulted in a verdict for the plaintiff.3
Not as good news: Payments per claim and claims settling for a payment > $1 million are increasing
In the period 1992–1996, the average payment per paid claim in the field of obstetrics and gynecology was $387,186, rising to $447,034 in 2009–2014—a 16% increase.1 From 2004 to 2010, million dollar payments occurred in about 8% of cases of paid claims, but they represent 36% of the total of all paid claims.4 In the time periods 1992–1996 and 2009–2014, payments greater than $1 million occurred in 6% and 8% of paid claims, respectively.1
Claims settled for much more than $1 million are of great concern to physicians because the payment may exceed their policy limit, creating a complex legal problem that may take time to resolve. In some cases, where the award is greater than the insurance policy limit, aggressive plaintiff attorneys have obtained a lien on the defendant physician’s home pending settlement of the case. When a multimillion dollar payment is made to settle a professional liability claim, it can greatly influence physician practice and change hospital policies. Frequently, following a multimillion dollar payment a physician may decide to limit their practice to low-risk cases or retire from the practice of medicine.
Liability premiums are stable or decreasing
From 2014 to 2019, my ObGyn professional liability insurance premiums decreased by 18%. During the same time period, my colleagues who practice surgical gynecology (no obstetrics) had a premium decrease of 22%. Insurers use a complex algorithm to determine annual liability insurance premiums, and premiums for ObGyns may not have stabilized or decreased in all regions. Take this Instant Poll:
Create your own user feedback survey
Reform of the liability tort system
Litigation policies and practices that reduce liability risk reduce total medical liability losses. Policies that have helped to constrain medical liability risk include state constitutional amendments limiting payments for pain and suffering, caps on compensation to plaintiff attorneys, increased early resolution programs that compensate patients who experience an adverse event and no-fault conflict resolution programs.5 In 2003, Texas implemented a comprehensive package of tort reform laws. Experts believe the reforms decreased the financial burden of professional liability insurance6 and led to less defensive medical practices, reducing excessive use of imaging and laboratory tests.
Medical factors contributing to a decrease in claims
In 1999, the Institute of Medicine released the report, “To Err is Human,” which galvanized health care systems to deploy systems of care that reduce the rate of adverse patient outcomes.7 Over the past 20 years, health systems have implemented quality improvement programs in obstetrics and gynecology that have contributed to a reduction in the rate of adverse patient outcomes. This may have contributed to the decrease in the rate of paid claims.
In a quasi-experimental study performed in 13 health systems, 7 interventions were implemented with the goal of improving outcomes and reducing medical liability. The 7 interventions included8:
- an elective induction bundle focused on the safe use of oxytocin
- an augmentation bundle focused on early intervention for possible fetal metabolic acidosis
- an operative vaginal delivery bundle
- TeamSTEPPS teamwork training to improve the quality of communication
- best practices education with a focus on electronic fetal monitoring
- regular performance feedback to hospitals and clinicians
- implementation of a quality improvement collaboration to support implementation of the interventions.
During the two-year baseline period prior to the intervention there were 185,373 deliveries with 6.7 perinatal claims made per 10,000 deliveries and 1.3 claims paid per 10,000 deliveries. Following the intervention, the rate of claims made and claims paid per 10,000 deliveries decreased by 22% and 37%, respectively. In addition there was a marked decrease in claims over $1 million paid, greatly limiting total financial liability losses.
Experts with vast experience in obstetrics and obstetric liability litigation have identified 4 priority interventions that may improve outcomes and mitigate liability risk, including: 1) 24-hour in-house physician coverage of an obstetrics service, 2) a conservative approach to trial of labor after a prior cesarean delivery, 3) utilization of a comprehensive, standardized event note in cases of a shoulder dystocia, and 4) judicious use of oxytocin, misoprostol, and magnesium sulfate.9
Other health system interventions that may contribute to a reduction in claims include:
- systematic improvement in the quality of communication among physicians and nurses through the use of team training, preprocedure huddles, and time-out processes10
- rapid response systems to rescue hospital patients with worrisome vital signs11
- standardized responses to a worrisome category 2 or 3 fetal heart-rate tracing12
- rapid recognition, evaluation, and treatment of women with hemorrhage, severe hypertension, sepsis, and venous thromboembolism13
- identification and referral of high-risk patients to tertiary centers14
- closed loop communication of critical imaging and laboratory results15
- universal insurance coverage for health care including contraception, obstetrics, and pediatric care.
Medical liability risk is an important practice issue because it causes excessive use of imaging and laboratory tests and often traumatizes clinicians, which can result in burnout. In the 1980s and 1990s, medical liability litigation reached a crescendo and was a prominent concern among obstetrician-gynecologists. The good news is that, for ObGyns, liability risk has stabilized. Hopefully our resolute efforts to continuously improve the quality of care will result in a long-term reduction in medical liability risk.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992–2014. JAMA Intern Med. 2017;177:710-718.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-e6.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Cardoso R, Zarin W, Nincic V, et al. Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev. 2017;6:181.
- Stewart RM, Geoghegan K, Myers JG, et al. Malpractice risk and costs are significantly reduced after tort reform. J Am Coll Surg. 2011;212:463-467.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- Riley W, Meredith LW, Price R, et al. Decreasing malpractice claims by reducing preventable perinatal harm. Health Serv Res. 2016;51(suppl 3):2453-2471.
- Clark SL, Belfort MA, Dildy GA, et al. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112:1279-1283.
- Haynes AB, Weiser TG, Berry WR, et al; Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499.
- Patel S, Gillon SA, Jones DA. Rapid response systems: recognition and rescue of the deteriorating hospital patient. Br J Hosp Med (Lond). 2017;78:143-148.
- Clark SL, Hamilton EF, Garite TJ, et al. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216:163.e1-163.e6.
- The Council on Patient Safety in Women’s Healthcare website. www.safehealthcareforeverywoman.org. Accessed April 12, 2019.
- Zahn CM, Remick A, Catalano A, et al. Levels of maternal care verification pilot: translating guidance into practice. Obstet Gynecol. 2018;132:1401-1406.
- Zuccotti G, Maloney FL, Feblowitz J, et al. Reducing risk with clinical decision support: a study of closed malpractice claims. Appl Clin Inform. 2014;5:746-756.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992–2014. JAMA Intern Med. 2017;177:710-718.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-e6.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Cardoso R, Zarin W, Nincic V, et al. Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev. 2017;6:181.
- Stewart RM, Geoghegan K, Myers JG, et al. Malpractice risk and costs are significantly reduced after tort reform. J Am Coll Surg. 2011;212:463-467.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- Riley W, Meredith LW, Price R, et al. Decreasing malpractice claims by reducing preventable perinatal harm. Health Serv Res. 2016;51(suppl 3):2453-2471.
- Clark SL, Belfort MA, Dildy GA, et al. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112:1279-1283.
- Haynes AB, Weiser TG, Berry WR, et al; Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499.
- Patel S, Gillon SA, Jones DA. Rapid response systems: recognition and rescue of the deteriorating hospital patient. Br J Hosp Med (Lond). 2017;78:143-148.
- Clark SL, Hamilton EF, Garite TJ, et al. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216:163.e1-163.e6.
- The Council on Patient Safety in Women’s Healthcare website. www.safehealthcareforeverywoman.org. Accessed April 12, 2019.
- Zahn CM, Remick A, Catalano A, et al. Levels of maternal care verification pilot: translating guidance into practice. Obstet Gynecol. 2018;132:1401-1406.
- Zuccotti G, Maloney FL, Feblowitz J, et al. Reducing risk with clinical decision support: a study of closed malpractice claims. Appl Clin Inform. 2014;5:746-756.
Are sweeping efforts to reduce primary CD rates associated with an increase in maternal or neonatal AEs?
EXPERT COMMENTARY
Main EK, Chang SC, Cape V, et al. Safety assessment of a large-scale improvement collaborative to reduce nulliparous cesarean delivery rates. Obstet Gynecol. 2019;133:613-623.
Cesarean delivery can be lifesaving for both mother and infant. When compared with successful vaginal delivery, however, CD is associated with higher maternal complication rates (including excessive blood loss requiring blood product transfusion, infectious morbidity, and venous thromboembolic events), longer hospital length of stay, and higher cost. While the optimal CD rate is not well defined, it is generally accepted that the CD rate in the United States is excessively high. As such, efforts to reduce the CD rate should be encouraged, but not at the expense of patient safety.
Details about the study
In keeping with the dictum that the most important CD to prevent is the first one, the California Maternal Quality Care Collaborative (CMQCC) in 2016 introduced a large-scale quality improvement project designed to reduce nulliparous, term, singleton, vertex (NTSV) CDs across the state. This bundle included education around joint guidelines issued by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine on reducing primary CDs,1 introduction of a CMQCC toolkit, increased nursing labor support, and monthly meetings to share best practices across all collaborating sites. The NTSV CD rate in these hospitals did decrease from 29.3% in 2015 to 25.0% in 2017 (adjusted odds ratio, 0.76; 95% confidence interval, 0.73–0.78).
Whether or not implementation of the bundle resulted in an inappropriate delay in indicated CDs and, as such, in an increase in maternal or neonatal morbidity is not known. To address this issue, Main and colleagues collected cross-sectional data from more than 50 hospitals with more than 119,000 deliveries throughout California and measured rates of chorioamnionitis, blood transfusions, third- or fourth-degree perineal lacerations, operative vaginal delivery, severe unexpected newborn complications, and 5-minute Apgar scores of less than 5. None of the 6 safety measures showed any difference when comparing 2017 (after implementation of the CMQCC bundle) to 2015 (before implementation), suggesting that patient safety was not compromised significantly.
Study strengths and weaknesses
Strengths of this study include its large sample size and multicenter design with inclusion of a variety of collaborating hospitals. Earlier studies examining the effect of standardized protocols to reduce CD rates have been largely underpowered and conducted at single institutions.2-6 Moreover, results have been mixed, with some studies reporting an increase in maternal/neonatal adverse events,2-4 while others suggesting an improvement in select newborn quality outcome metrics.5 The current study provides reassurance to providers and institutions employing strategies to reduce NTSV CD rates that such efforts are safe.
Continue to: This study has several limitations...
This study has several limitations. Data collection relied on birth certificate and discharge diagnoses without a robust quality audit. As such, ascertainment bias, random error, and undercounting cannot be excluded. Although the population was heterogeneous, most women had more than a high school education and private insurance, and only 1 in 5 were obese. Whether these findings are generalizable to other areas within the United States is not known.
All reasonable efforts to decrease the CD rate in the United States should be encouraged, with particular attention paid to avoiding the first CD. However, this should not be done at the expense of patient safety. Large-scale quality improvement initiatives, similar to CMQCC efforts in California in 2016, appear to be one such strategy. Other successful strategies may include, for example, routine induction of labor for all low-risk nulliparous women at 39 weeks' gestation.7 The current report suggests that implementing a large-scale quality improvement initiative to reduce the primary CD rate can likely be done safely, without a significant increase in maternal or neonatal morbidity.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. ACOG Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123:693-711.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8.
- Vadnais MA, Hacker MR, Shah NT, et al. Quality improvement initiatives lead to reduction in nulliparous term singleton vertex cesarean delivery rate. Jt Comm J Qual Patient Saf. 2017;43:53-61.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019; 220:191.e1-191.e7.
- Thuillier C, Roy S, Peyronnet V, et al. Impact of recommended changes in labor management for prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2018;218:341.e1-341.e9.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-361.e6.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
EXPERT COMMENTARY
Main EK, Chang SC, Cape V, et al. Safety assessment of a large-scale improvement collaborative to reduce nulliparous cesarean delivery rates. Obstet Gynecol. 2019;133:613-623.
Cesarean delivery can be lifesaving for both mother and infant. When compared with successful vaginal delivery, however, CD is associated with higher maternal complication rates (including excessive blood loss requiring blood product transfusion, infectious morbidity, and venous thromboembolic events), longer hospital length of stay, and higher cost. While the optimal CD rate is not well defined, it is generally accepted that the CD rate in the United States is excessively high. As such, efforts to reduce the CD rate should be encouraged, but not at the expense of patient safety.
Details about the study
In keeping with the dictum that the most important CD to prevent is the first one, the California Maternal Quality Care Collaborative (CMQCC) in 2016 introduced a large-scale quality improvement project designed to reduce nulliparous, term, singleton, vertex (NTSV) CDs across the state. This bundle included education around joint guidelines issued by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine on reducing primary CDs,1 introduction of a CMQCC toolkit, increased nursing labor support, and monthly meetings to share best practices across all collaborating sites. The NTSV CD rate in these hospitals did decrease from 29.3% in 2015 to 25.0% in 2017 (adjusted odds ratio, 0.76; 95% confidence interval, 0.73–0.78).
Whether or not implementation of the bundle resulted in an inappropriate delay in indicated CDs and, as such, in an increase in maternal or neonatal morbidity is not known. To address this issue, Main and colleagues collected cross-sectional data from more than 50 hospitals with more than 119,000 deliveries throughout California and measured rates of chorioamnionitis, blood transfusions, third- or fourth-degree perineal lacerations, operative vaginal delivery, severe unexpected newborn complications, and 5-minute Apgar scores of less than 5. None of the 6 safety measures showed any difference when comparing 2017 (after implementation of the CMQCC bundle) to 2015 (before implementation), suggesting that patient safety was not compromised significantly.
Study strengths and weaknesses
Strengths of this study include its large sample size and multicenter design with inclusion of a variety of collaborating hospitals. Earlier studies examining the effect of standardized protocols to reduce CD rates have been largely underpowered and conducted at single institutions.2-6 Moreover, results have been mixed, with some studies reporting an increase in maternal/neonatal adverse events,2-4 while others suggesting an improvement in select newborn quality outcome metrics.5 The current study provides reassurance to providers and institutions employing strategies to reduce NTSV CD rates that such efforts are safe.
Continue to: This study has several limitations...
This study has several limitations. Data collection relied on birth certificate and discharge diagnoses without a robust quality audit. As such, ascertainment bias, random error, and undercounting cannot be excluded. Although the population was heterogeneous, most women had more than a high school education and private insurance, and only 1 in 5 were obese. Whether these findings are generalizable to other areas within the United States is not known.
All reasonable efforts to decrease the CD rate in the United States should be encouraged, with particular attention paid to avoiding the first CD. However, this should not be done at the expense of patient safety. Large-scale quality improvement initiatives, similar to CMQCC efforts in California in 2016, appear to be one such strategy. Other successful strategies may include, for example, routine induction of labor for all low-risk nulliparous women at 39 weeks' gestation.7 The current report suggests that implementing a large-scale quality improvement initiative to reduce the primary CD rate can likely be done safely, without a significant increase in maternal or neonatal morbidity.
EXPERT COMMENTARY
Main EK, Chang SC, Cape V, et al. Safety assessment of a large-scale improvement collaborative to reduce nulliparous cesarean delivery rates. Obstet Gynecol. 2019;133:613-623.
Cesarean delivery can be lifesaving for both mother and infant. When compared with successful vaginal delivery, however, CD is associated with higher maternal complication rates (including excessive blood loss requiring blood product transfusion, infectious morbidity, and venous thromboembolic events), longer hospital length of stay, and higher cost. While the optimal CD rate is not well defined, it is generally accepted that the CD rate in the United States is excessively high. As such, efforts to reduce the CD rate should be encouraged, but not at the expense of patient safety.
Details about the study
In keeping with the dictum that the most important CD to prevent is the first one, the California Maternal Quality Care Collaborative (CMQCC) in 2016 introduced a large-scale quality improvement project designed to reduce nulliparous, term, singleton, vertex (NTSV) CDs across the state. This bundle included education around joint guidelines issued by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine on reducing primary CDs,1 introduction of a CMQCC toolkit, increased nursing labor support, and monthly meetings to share best practices across all collaborating sites. The NTSV CD rate in these hospitals did decrease from 29.3% in 2015 to 25.0% in 2017 (adjusted odds ratio, 0.76; 95% confidence interval, 0.73–0.78).
Whether or not implementation of the bundle resulted in an inappropriate delay in indicated CDs and, as such, in an increase in maternal or neonatal morbidity is not known. To address this issue, Main and colleagues collected cross-sectional data from more than 50 hospitals with more than 119,000 deliveries throughout California and measured rates of chorioamnionitis, blood transfusions, third- or fourth-degree perineal lacerations, operative vaginal delivery, severe unexpected newborn complications, and 5-minute Apgar scores of less than 5. None of the 6 safety measures showed any difference when comparing 2017 (after implementation of the CMQCC bundle) to 2015 (before implementation), suggesting that patient safety was not compromised significantly.
Study strengths and weaknesses
Strengths of this study include its large sample size and multicenter design with inclusion of a variety of collaborating hospitals. Earlier studies examining the effect of standardized protocols to reduce CD rates have been largely underpowered and conducted at single institutions.2-6 Moreover, results have been mixed, with some studies reporting an increase in maternal/neonatal adverse events,2-4 while others suggesting an improvement in select newborn quality outcome metrics.5 The current study provides reassurance to providers and institutions employing strategies to reduce NTSV CD rates that such efforts are safe.
Continue to: This study has several limitations...
This study has several limitations. Data collection relied on birth certificate and discharge diagnoses without a robust quality audit. As such, ascertainment bias, random error, and undercounting cannot be excluded. Although the population was heterogeneous, most women had more than a high school education and private insurance, and only 1 in 5 were obese. Whether these findings are generalizable to other areas within the United States is not known.
All reasonable efforts to decrease the CD rate in the United States should be encouraged, with particular attention paid to avoiding the first CD. However, this should not be done at the expense of patient safety. Large-scale quality improvement initiatives, similar to CMQCC efforts in California in 2016, appear to be one such strategy. Other successful strategies may include, for example, routine induction of labor for all low-risk nulliparous women at 39 weeks' gestation.7 The current report suggests that implementing a large-scale quality improvement initiative to reduce the primary CD rate can likely be done safely, without a significant increase in maternal or neonatal morbidity.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. ACOG Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123:693-711.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8.
- Vadnais MA, Hacker MR, Shah NT, et al. Quality improvement initiatives lead to reduction in nulliparous term singleton vertex cesarean delivery rate. Jt Comm J Qual Patient Saf. 2017;43:53-61.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019; 220:191.e1-191.e7.
- Thuillier C, Roy S, Peyronnet V, et al. Impact of recommended changes in labor management for prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2018;218:341.e1-341.e9.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-361.e6.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. ACOG Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123:693-711.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8.
- Vadnais MA, Hacker MR, Shah NT, et al. Quality improvement initiatives lead to reduction in nulliparous term singleton vertex cesarean delivery rate. Jt Comm J Qual Patient Saf. 2017;43:53-61.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019; 220:191.e1-191.e7.
- Thuillier C, Roy S, Peyronnet V, et al. Impact of recommended changes in labor management for prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2018;218:341.e1-341.e9.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-361.e6.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
Version success more likely in lower BMI, multiparous breech pregnancies
according to results of a single-center retrospective study.
Writing in Obstetrics & Gynecology, Ofer Isakov, MD, PhD, and colleagues from the Sourasky Medical Center, Tel Aviv, reported the results of a study of 250 women with singleton pregnancies and breech presentation who underwent external cephalic version (ECV) to turn the baby at 36-41 weeks’ gestation.
The overall success rate of the procedure was 65%. However, women with no forebag – the pocket of amniotic fluid in front of the fetal presenting part – had a 3%-10% chance of successful version, while those with a forebag size greater than 1 cm had a 96%-97% probability of success.
Women with a BMI greater than 29 had a very low chance of success, which the authors suggested was likely attributable to a thicker abdominal wall that made manipulation more difficult. However, among women with a BMI of 29 or below, success was significantly associated with forebag size.
Among women with a forebag of 1 cm in size, multiparous women had a significantly higher chance of success than nulliparous women (81%-91% vs. 0%-24%, respectively).
Dr. Isakov and colleagues suggested that the impact of multiparity could relate to late engagement or the relative laxity of the abdomen in women who had experienced previous births.
The authors then developed a decision tree predictive model of success for ECV, which had a prediction accuracy of 92%.
“External version is a simple and effective procedure that can reduce the cesarean delivery rate, but counseling patients on the risks and success rates of version is challenging owing to the lack of validated models to predict success,” Dr. Isakov and colleagues wrote. “The ability to predict the outcome of an ECV attempt may improve the rates of patient consent and prevent the performance of many unpleasant procedures with low chance for success.”
They noted that their success rate was higher than that seen in other studies of ECV and suggested this may be because all the procedures were performed by a single experienced practitioner, and the mean BMI of the cohort was lower than that in earlier studies.
None of the authors declared any relevant financial disclosures, and there was no external funding.
SOURCE: Isakov O et al. Obstet Gynecol. 2019;133:869-78.
With cesarean delivery rates rising, there is a need for vigilance to prevent them from returning to the 2009 peak of 33% of deliveries, and ECV is one strategy to help reduce cesarean rates. While there are some risks associated with ECV, which could contribute to negative attitudes, the lack of acceptance of this procedure may be improved if clinicians can provide an individualized estimate for the chance of success. This study proposes creating a predictive model that discriminates between poor and good changes of ECV success.
The fact that this study is a single-center study with a single physician performing all the procedures does limit its generalizability. However the authors’ use of ultrasound measurements of the forebag is a novel contribution that provides an objective measure of this factor, as well as an objective estimate of the engagement of the breech, which has been lacking.
Dr. Gayle Olson Koutrouvelis is a professor of obstetrics, gynecology, and maternal-fetal medicine at the University of Texas Medical Branch in Galveston. These comments are adapted from an editorial accompanying the article by Isakov et al. (Obstet Gynecol. 2019; 133:855-6.). She declared no conflicts of interest.
With cesarean delivery rates rising, there is a need for vigilance to prevent them from returning to the 2009 peak of 33% of deliveries, and ECV is one strategy to help reduce cesarean rates. While there are some risks associated with ECV, which could contribute to negative attitudes, the lack of acceptance of this procedure may be improved if clinicians can provide an individualized estimate for the chance of success. This study proposes creating a predictive model that discriminates between poor and good changes of ECV success.
The fact that this study is a single-center study with a single physician performing all the procedures does limit its generalizability. However the authors’ use of ultrasound measurements of the forebag is a novel contribution that provides an objective measure of this factor, as well as an objective estimate of the engagement of the breech, which has been lacking.
Dr. Gayle Olson Koutrouvelis is a professor of obstetrics, gynecology, and maternal-fetal medicine at the University of Texas Medical Branch in Galveston. These comments are adapted from an editorial accompanying the article by Isakov et al. (Obstet Gynecol. 2019; 133:855-6.). She declared no conflicts of interest.
With cesarean delivery rates rising, there is a need for vigilance to prevent them from returning to the 2009 peak of 33% of deliveries, and ECV is one strategy to help reduce cesarean rates. While there are some risks associated with ECV, which could contribute to negative attitudes, the lack of acceptance of this procedure may be improved if clinicians can provide an individualized estimate for the chance of success. This study proposes creating a predictive model that discriminates between poor and good changes of ECV success.
The fact that this study is a single-center study with a single physician performing all the procedures does limit its generalizability. However the authors’ use of ultrasound measurements of the forebag is a novel contribution that provides an objective measure of this factor, as well as an objective estimate of the engagement of the breech, which has been lacking.
Dr. Gayle Olson Koutrouvelis is a professor of obstetrics, gynecology, and maternal-fetal medicine at the University of Texas Medical Branch in Galveston. These comments are adapted from an editorial accompanying the article by Isakov et al. (Obstet Gynecol. 2019; 133:855-6.). She declared no conflicts of interest.
according to results of a single-center retrospective study.
Writing in Obstetrics & Gynecology, Ofer Isakov, MD, PhD, and colleagues from the Sourasky Medical Center, Tel Aviv, reported the results of a study of 250 women with singleton pregnancies and breech presentation who underwent external cephalic version (ECV) to turn the baby at 36-41 weeks’ gestation.
The overall success rate of the procedure was 65%. However, women with no forebag – the pocket of amniotic fluid in front of the fetal presenting part – had a 3%-10% chance of successful version, while those with a forebag size greater than 1 cm had a 96%-97% probability of success.
Women with a BMI greater than 29 had a very low chance of success, which the authors suggested was likely attributable to a thicker abdominal wall that made manipulation more difficult. However, among women with a BMI of 29 or below, success was significantly associated with forebag size.
Among women with a forebag of 1 cm in size, multiparous women had a significantly higher chance of success than nulliparous women (81%-91% vs. 0%-24%, respectively).
Dr. Isakov and colleagues suggested that the impact of multiparity could relate to late engagement or the relative laxity of the abdomen in women who had experienced previous births.
The authors then developed a decision tree predictive model of success for ECV, which had a prediction accuracy of 92%.
“External version is a simple and effective procedure that can reduce the cesarean delivery rate, but counseling patients on the risks and success rates of version is challenging owing to the lack of validated models to predict success,” Dr. Isakov and colleagues wrote. “The ability to predict the outcome of an ECV attempt may improve the rates of patient consent and prevent the performance of many unpleasant procedures with low chance for success.”
They noted that their success rate was higher than that seen in other studies of ECV and suggested this may be because all the procedures were performed by a single experienced practitioner, and the mean BMI of the cohort was lower than that in earlier studies.
None of the authors declared any relevant financial disclosures, and there was no external funding.
SOURCE: Isakov O et al. Obstet Gynecol. 2019;133:869-78.
according to results of a single-center retrospective study.
Writing in Obstetrics & Gynecology, Ofer Isakov, MD, PhD, and colleagues from the Sourasky Medical Center, Tel Aviv, reported the results of a study of 250 women with singleton pregnancies and breech presentation who underwent external cephalic version (ECV) to turn the baby at 36-41 weeks’ gestation.
The overall success rate of the procedure was 65%. However, women with no forebag – the pocket of amniotic fluid in front of the fetal presenting part – had a 3%-10% chance of successful version, while those with a forebag size greater than 1 cm had a 96%-97% probability of success.
Women with a BMI greater than 29 had a very low chance of success, which the authors suggested was likely attributable to a thicker abdominal wall that made manipulation more difficult. However, among women with a BMI of 29 or below, success was significantly associated with forebag size.
Among women with a forebag of 1 cm in size, multiparous women had a significantly higher chance of success than nulliparous women (81%-91% vs. 0%-24%, respectively).
Dr. Isakov and colleagues suggested that the impact of multiparity could relate to late engagement or the relative laxity of the abdomen in women who had experienced previous births.
The authors then developed a decision tree predictive model of success for ECV, which had a prediction accuracy of 92%.
“External version is a simple and effective procedure that can reduce the cesarean delivery rate, but counseling patients on the risks and success rates of version is challenging owing to the lack of validated models to predict success,” Dr. Isakov and colleagues wrote. “The ability to predict the outcome of an ECV attempt may improve the rates of patient consent and prevent the performance of many unpleasant procedures with low chance for success.”
They noted that their success rate was higher than that seen in other studies of ECV and suggested this may be because all the procedures were performed by a single experienced practitioner, and the mean BMI of the cohort was lower than that in earlier studies.
None of the authors declared any relevant financial disclosures, and there was no external funding.
SOURCE: Isakov O et al. Obstet Gynecol. 2019;133:869-78.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Multiparity, larger forebag size, and lower BMI are predictors of external cephalic version success.
Major finding: Model of external cephalic version success shows prediction accuracy of 92%.
Study details: A single-center retrospective cohort study in 250 women with breech presentation.
Disclosures: None of the authors declared any relevant financial disclosures, and there was no external funding.
Source: Isakov O et al. Obstet Gynecol. 2019;133:869-78.
Bipolar disorder during pregnancy: Lessons learned
Careful management of bipolar disorder during pregnancy is critical because for so many patients with this illness, the road to emotional well-being has been a long one, requiring a combination of careful pharmacologic and nonpharmacologic strategies.
Half of referrals to our Center for Women’s Mental Health – where we evaluate and treat women before, during, and after pregnancy – are for women who have histories of bipolar disorder. My colleagues and I are asked at continuing medical education programs what we “always do” and “never do” with respect to the treatment of these patients.
What about discontinuation of mood stabilizers during pregnancy and risk of relapse?
We never abruptly stop mood stabilizers if a patient has an unplanned pregnancy – a common scenario, with 50% of pregnancies across the country being unplanned across sociodemographic lines – save for sodium valproate, which is a clearly a documented teratogen; it increases risk for organ malformation and behavioral difficulties in exposed offspring. In our center, we typically view the use of sodium valproate in reproductive age women as contraindicated.
One may then question the circumstances under which lithium might be used during pregnancy, because many clinicians are faced with patients who have been exquisite responders to lithium. Such a patient may present with a history of mania, but there are obvious concerns given the historical literature, and even some more recent reports, that describe an increased risk of teratogenicity with fetal exposure to lithium.
not only to decrease the risk of relapse following discontinuation of mood stabilizers, but because recurrence of illness during pregnancy for these patients is a very strong predictor of risk for postpartum depression. Women with bipolar disorder already are at a fivefold increased risk for postpartum depression, so discussion of sustaining euthymia during pregnancy for bipolar women is particularly timely given the focus nationally on treatment and prevention of postpartum depression.
In patients with history of mania, what about stopping treatment with lithium and other effective treatments during pregnancy?
Historically, we sometimes divided patients with bipolar disorder into those with “more severe recurrent disease” compared with those with more distant, circumscribed disease. In patients with more remote histories of mood dysregulation, we tended to discontinue treatment with mood stabilizers such as lithium or even newer second-generation atypical antipsychotics to see if patients could at least get through earlier stages of pregnancy before going back on anti-manic treatment.
Our experience now over several decades has revealed that this can be a risky clinical move. What we see is that even in patients with histories of mania years in the past (i.e., a circumscribed episode of mania during college in a woman now 35 years old with intervening sustained well-being), discontinuation of treatment that got patients well can lead to recurrence. Hence, we should not confuse an exquisite response to treatment with long periods of well-being as suggesting that the patient has a less severe form of bipolar disorder and hence the capacity to sustain that well-being when treatment is removed.
What about increasing/decreasing lithium dose during pregnancy and around time of delivery?
Select patients may be sensitive to changes in plasma levels of lithium, but the literature suggests that the clinical utility of arbitrarily sustaining plasma levels at the upper limit of the accepted range may be of only modest advantage, if any. With this as a backdrop and even while knowing that increased plasma volume of pregnancy is associated with a fall in plasma level of most medications, we do not arbitrarily increase the dose of lithium across pregnancy merely to sustain a level in the absence of a change in clinical symptoms. Indeed, to my knowledge, currently available data supporting a clear correlation of decline in plasma levels and frank change in symptoms during pregnancy are very sparse, if existent.
Earlier work had suggested that lithium dosage should be reduced proximate to delivery, a period characterized by rapid shifts in plasma volume during the acute peripartum period. Because physicians in our center do not alter lithium dose across pregnancy, we never reduce the dose of lithium proximate to delivery because of a theoretical concern for increased risk of either neonatal toxicity or maternal lithium toxicity, which is essentially nonexistent in terms of systematic reports in the literature.
Obvious concerns about lithium during pregnancy have focused on increased risk of teratogenesis, with the earliest reports supporting an increased risk of Epstein’s anomaly (0.05%-0.1%). More recent reports suggest an increased risk of cardiovascular malformations, which according to some investigators may be dose dependent.
For those patients who are exquisitely responsive to lithium, we typically leave them on the medicine and avail ourselves of current fetal echocardiographic evaluation at 16 weeks to 18 weeks to document the integrity of the fetal cardiac anatomy. Although the risk for cardiac malformations associated with lithium exposure during the first trimester is still exceedingly small, it is still extremely reassuring to patients to know that they are safely on the other side of a teratogenic window.
What about lamotrigine levels across pregnancy?
The last decade has seen a dramatic decrease in the administration of lithium to women with bipolar disorder, and growing use of both lamotrigine and second-generation atypical antipsychotics (frequently in combination) as an alternative. The changes in plasma level of lamotrigine across pregnancy are being increasingly well documented based on rigorous studies (Obstet Gynecol Clin North Am. 2018 Sep;45[3]:403-17).
These are welcome data, but the correlation between plasma concentration of lamotrigine and clinical response is a poor one. To date, there are sparse data to suggest that maintaining plasma levels of lithium or lamotrigine at a certain level during pregnancy changes clinical outcome. Following lamotrigine plasma levels during pregnancy seems more like an academic exercise than a procedure associated with particular clinical value.
As in the case of lithium, we never change lamotrigine doses proximate to pregnancy because of the absence of reports of neonatal toxicity associated with using lamotrigine during the peripartum period. The rationale for removing or minimizing the use of an effective medicine proximate to delivery, a period of risk for bipolar women, is lacking.
In 2019, we clearly are seeing a growing use of atypical antipsychotics for the treatment of bipolar disorder during pregnancy frequently coadministered with medicines such as lamotrigine as opposed to lithium. The accumulated data to date on second-generation atypical antipsychotics are not definitive, but increasingly are reassuring in terms of absence of a clear signal for teratogenicity; hence, our comfort in using this class of medicines is only growing, which is important given the prevalence of use of these agents in reproductive-age women.
If there is a single critical guiding principle for the clinician when it comes to managing bipolar women during pregnancy and the postpartum period, it is sustaining euthymia. With the recent focus of the U.S. Preventive Services Task Force on prevention of postpartum depression, nothing is more helpful perhaps than keeping women with bipolar disorder well, both proximate to pregnancy and during an actual pregnancy. Keeping those patients well maximizes the likelihood that they will proceed across the peripartum and into the postpartum period with a level of emotional well-being that optimizes and maximizes positive long-term outcomes for both patients and families.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Careful management of bipolar disorder during pregnancy is critical because for so many patients with this illness, the road to emotional well-being has been a long one, requiring a combination of careful pharmacologic and nonpharmacologic strategies.
Half of referrals to our Center for Women’s Mental Health – where we evaluate and treat women before, during, and after pregnancy – are for women who have histories of bipolar disorder. My colleagues and I are asked at continuing medical education programs what we “always do” and “never do” with respect to the treatment of these patients.
What about discontinuation of mood stabilizers during pregnancy and risk of relapse?
We never abruptly stop mood stabilizers if a patient has an unplanned pregnancy – a common scenario, with 50% of pregnancies across the country being unplanned across sociodemographic lines – save for sodium valproate, which is a clearly a documented teratogen; it increases risk for organ malformation and behavioral difficulties in exposed offspring. In our center, we typically view the use of sodium valproate in reproductive age women as contraindicated.
One may then question the circumstances under which lithium might be used during pregnancy, because many clinicians are faced with patients who have been exquisite responders to lithium. Such a patient may present with a history of mania, but there are obvious concerns given the historical literature, and even some more recent reports, that describe an increased risk of teratogenicity with fetal exposure to lithium.
not only to decrease the risk of relapse following discontinuation of mood stabilizers, but because recurrence of illness during pregnancy for these patients is a very strong predictor of risk for postpartum depression. Women with bipolar disorder already are at a fivefold increased risk for postpartum depression, so discussion of sustaining euthymia during pregnancy for bipolar women is particularly timely given the focus nationally on treatment and prevention of postpartum depression.
In patients with history of mania, what about stopping treatment with lithium and other effective treatments during pregnancy?
Historically, we sometimes divided patients with bipolar disorder into those with “more severe recurrent disease” compared with those with more distant, circumscribed disease. In patients with more remote histories of mood dysregulation, we tended to discontinue treatment with mood stabilizers such as lithium or even newer second-generation atypical antipsychotics to see if patients could at least get through earlier stages of pregnancy before going back on anti-manic treatment.
Our experience now over several decades has revealed that this can be a risky clinical move. What we see is that even in patients with histories of mania years in the past (i.e., a circumscribed episode of mania during college in a woman now 35 years old with intervening sustained well-being), discontinuation of treatment that got patients well can lead to recurrence. Hence, we should not confuse an exquisite response to treatment with long periods of well-being as suggesting that the patient has a less severe form of bipolar disorder and hence the capacity to sustain that well-being when treatment is removed.
What about increasing/decreasing lithium dose during pregnancy and around time of delivery?
Select patients may be sensitive to changes in plasma levels of lithium, but the literature suggests that the clinical utility of arbitrarily sustaining plasma levels at the upper limit of the accepted range may be of only modest advantage, if any. With this as a backdrop and even while knowing that increased plasma volume of pregnancy is associated with a fall in plasma level of most medications, we do not arbitrarily increase the dose of lithium across pregnancy merely to sustain a level in the absence of a change in clinical symptoms. Indeed, to my knowledge, currently available data supporting a clear correlation of decline in plasma levels and frank change in symptoms during pregnancy are very sparse, if existent.
Earlier work had suggested that lithium dosage should be reduced proximate to delivery, a period characterized by rapid shifts in plasma volume during the acute peripartum period. Because physicians in our center do not alter lithium dose across pregnancy, we never reduce the dose of lithium proximate to delivery because of a theoretical concern for increased risk of either neonatal toxicity or maternal lithium toxicity, which is essentially nonexistent in terms of systematic reports in the literature.
Obvious concerns about lithium during pregnancy have focused on increased risk of teratogenesis, with the earliest reports supporting an increased risk of Epstein’s anomaly (0.05%-0.1%). More recent reports suggest an increased risk of cardiovascular malformations, which according to some investigators may be dose dependent.
For those patients who are exquisitely responsive to lithium, we typically leave them on the medicine and avail ourselves of current fetal echocardiographic evaluation at 16 weeks to 18 weeks to document the integrity of the fetal cardiac anatomy. Although the risk for cardiac malformations associated with lithium exposure during the first trimester is still exceedingly small, it is still extremely reassuring to patients to know that they are safely on the other side of a teratogenic window.
What about lamotrigine levels across pregnancy?
The last decade has seen a dramatic decrease in the administration of lithium to women with bipolar disorder, and growing use of both lamotrigine and second-generation atypical antipsychotics (frequently in combination) as an alternative. The changes in plasma level of lamotrigine across pregnancy are being increasingly well documented based on rigorous studies (Obstet Gynecol Clin North Am. 2018 Sep;45[3]:403-17).
These are welcome data, but the correlation between plasma concentration of lamotrigine and clinical response is a poor one. To date, there are sparse data to suggest that maintaining plasma levels of lithium or lamotrigine at a certain level during pregnancy changes clinical outcome. Following lamotrigine plasma levels during pregnancy seems more like an academic exercise than a procedure associated with particular clinical value.
As in the case of lithium, we never change lamotrigine doses proximate to pregnancy because of the absence of reports of neonatal toxicity associated with using lamotrigine during the peripartum period. The rationale for removing or minimizing the use of an effective medicine proximate to delivery, a period of risk for bipolar women, is lacking.
In 2019, we clearly are seeing a growing use of atypical antipsychotics for the treatment of bipolar disorder during pregnancy frequently coadministered with medicines such as lamotrigine as opposed to lithium. The accumulated data to date on second-generation atypical antipsychotics are not definitive, but increasingly are reassuring in terms of absence of a clear signal for teratogenicity; hence, our comfort in using this class of medicines is only growing, which is important given the prevalence of use of these agents in reproductive-age women.
If there is a single critical guiding principle for the clinician when it comes to managing bipolar women during pregnancy and the postpartum period, it is sustaining euthymia. With the recent focus of the U.S. Preventive Services Task Force on prevention of postpartum depression, nothing is more helpful perhaps than keeping women with bipolar disorder well, both proximate to pregnancy and during an actual pregnancy. Keeping those patients well maximizes the likelihood that they will proceed across the peripartum and into the postpartum period with a level of emotional well-being that optimizes and maximizes positive long-term outcomes for both patients and families.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Careful management of bipolar disorder during pregnancy is critical because for so many patients with this illness, the road to emotional well-being has been a long one, requiring a combination of careful pharmacologic and nonpharmacologic strategies.
Half of referrals to our Center for Women’s Mental Health – where we evaluate and treat women before, during, and after pregnancy – are for women who have histories of bipolar disorder. My colleagues and I are asked at continuing medical education programs what we “always do” and “never do” with respect to the treatment of these patients.
What about discontinuation of mood stabilizers during pregnancy and risk of relapse?
We never abruptly stop mood stabilizers if a patient has an unplanned pregnancy – a common scenario, with 50% of pregnancies across the country being unplanned across sociodemographic lines – save for sodium valproate, which is a clearly a documented teratogen; it increases risk for organ malformation and behavioral difficulties in exposed offspring. In our center, we typically view the use of sodium valproate in reproductive age women as contraindicated.
One may then question the circumstances under which lithium might be used during pregnancy, because many clinicians are faced with patients who have been exquisite responders to lithium. Such a patient may present with a history of mania, but there are obvious concerns given the historical literature, and even some more recent reports, that describe an increased risk of teratogenicity with fetal exposure to lithium.
not only to decrease the risk of relapse following discontinuation of mood stabilizers, but because recurrence of illness during pregnancy for these patients is a very strong predictor of risk for postpartum depression. Women with bipolar disorder already are at a fivefold increased risk for postpartum depression, so discussion of sustaining euthymia during pregnancy for bipolar women is particularly timely given the focus nationally on treatment and prevention of postpartum depression.
In patients with history of mania, what about stopping treatment with lithium and other effective treatments during pregnancy?
Historically, we sometimes divided patients with bipolar disorder into those with “more severe recurrent disease” compared with those with more distant, circumscribed disease. In patients with more remote histories of mood dysregulation, we tended to discontinue treatment with mood stabilizers such as lithium or even newer second-generation atypical antipsychotics to see if patients could at least get through earlier stages of pregnancy before going back on anti-manic treatment.
Our experience now over several decades has revealed that this can be a risky clinical move. What we see is that even in patients with histories of mania years in the past (i.e., a circumscribed episode of mania during college in a woman now 35 years old with intervening sustained well-being), discontinuation of treatment that got patients well can lead to recurrence. Hence, we should not confuse an exquisite response to treatment with long periods of well-being as suggesting that the patient has a less severe form of bipolar disorder and hence the capacity to sustain that well-being when treatment is removed.
What about increasing/decreasing lithium dose during pregnancy and around time of delivery?
Select patients may be sensitive to changes in plasma levels of lithium, but the literature suggests that the clinical utility of arbitrarily sustaining plasma levels at the upper limit of the accepted range may be of only modest advantage, if any. With this as a backdrop and even while knowing that increased plasma volume of pregnancy is associated with a fall in plasma level of most medications, we do not arbitrarily increase the dose of lithium across pregnancy merely to sustain a level in the absence of a change in clinical symptoms. Indeed, to my knowledge, currently available data supporting a clear correlation of decline in plasma levels and frank change in symptoms during pregnancy are very sparse, if existent.
Earlier work had suggested that lithium dosage should be reduced proximate to delivery, a period characterized by rapid shifts in plasma volume during the acute peripartum period. Because physicians in our center do not alter lithium dose across pregnancy, we never reduce the dose of lithium proximate to delivery because of a theoretical concern for increased risk of either neonatal toxicity or maternal lithium toxicity, which is essentially nonexistent in terms of systematic reports in the literature.
Obvious concerns about lithium during pregnancy have focused on increased risk of teratogenesis, with the earliest reports supporting an increased risk of Epstein’s anomaly (0.05%-0.1%). More recent reports suggest an increased risk of cardiovascular malformations, which according to some investigators may be dose dependent.
For those patients who are exquisitely responsive to lithium, we typically leave them on the medicine and avail ourselves of current fetal echocardiographic evaluation at 16 weeks to 18 weeks to document the integrity of the fetal cardiac anatomy. Although the risk for cardiac malformations associated with lithium exposure during the first trimester is still exceedingly small, it is still extremely reassuring to patients to know that they are safely on the other side of a teratogenic window.
What about lamotrigine levels across pregnancy?
The last decade has seen a dramatic decrease in the administration of lithium to women with bipolar disorder, and growing use of both lamotrigine and second-generation atypical antipsychotics (frequently in combination) as an alternative. The changes in plasma level of lamotrigine across pregnancy are being increasingly well documented based on rigorous studies (Obstet Gynecol Clin North Am. 2018 Sep;45[3]:403-17).
These are welcome data, but the correlation between plasma concentration of lamotrigine and clinical response is a poor one. To date, there are sparse data to suggest that maintaining plasma levels of lithium or lamotrigine at a certain level during pregnancy changes clinical outcome. Following lamotrigine plasma levels during pregnancy seems more like an academic exercise than a procedure associated with particular clinical value.
As in the case of lithium, we never change lamotrigine doses proximate to pregnancy because of the absence of reports of neonatal toxicity associated with using lamotrigine during the peripartum period. The rationale for removing or minimizing the use of an effective medicine proximate to delivery, a period of risk for bipolar women, is lacking.
In 2019, we clearly are seeing a growing use of atypical antipsychotics for the treatment of bipolar disorder during pregnancy frequently coadministered with medicines such as lamotrigine as opposed to lithium. The accumulated data to date on second-generation atypical antipsychotics are not definitive, but increasingly are reassuring in terms of absence of a clear signal for teratogenicity; hence, our comfort in using this class of medicines is only growing, which is important given the prevalence of use of these agents in reproductive-age women.
If there is a single critical guiding principle for the clinician when it comes to managing bipolar women during pregnancy and the postpartum period, it is sustaining euthymia. With the recent focus of the U.S. Preventive Services Task Force on prevention of postpartum depression, nothing is more helpful perhaps than keeping women with bipolar disorder well, both proximate to pregnancy and during an actual pregnancy. Keeping those patients well maximizes the likelihood that they will proceed across the peripartum and into the postpartum period with a level of emotional well-being that optimizes and maximizes positive long-term outcomes for both patients and families.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Click for Credit: Migraine & stroke risk; Aspirin for CV events; more
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
The ARRIVE trial: Women’s desideratum versus logistical concerns
Of the 1.5 million nulliparous women who deliver annually in the United States, more than 50% are low-risk pregnancies. Among clinicians, there is a hesitancy to offer elective induction of labor to low-risk nulliparous women, mainly due to early observational studies that noted an association between elective induction of labor and higher rates of cesarean delivery (CD) and other adverse maternal and perinatal outcomes. 1-3 This reluctance over time has permeated throughout the ObGyn specialty and is culturally embedded in contemporary practice. The early observational studies lacked proper comparison groups because outcomes of women undergoing induction (elective and medically indicated) were compared to those in spontaneous labor. Since women who are being induced do not have the option to be in spontaneous labor, the appropriate comparator group for women undergoing elective induction is women who are being managed expectantly.
ARRIVE addresses appropriate comparator groups
Challenging this pervaded practice, in August 2018, Grobman and colleagues published the findings of the ARRIVE trial (A Randomized Trial of Induction Versus Expectant Management).4 This trial, conducted by Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network, recruited participants from 41 geographically dispersed centers in the United States. Nulliparous women with low-risk pregnancies between 34 0/7 and 38 6/7 weeks were randomly assigned to either induction of labor at 39 0/7 to 39 4/7 weeks or to expectant management, which was defined as delaying induction until 40 5/7 to 42 2/7 weeks. The objective of the ARRIVE trial was to determine if, among low-risk nulliparous women, elective induction of labor at 39 weeks, compared with expectant management, would reduce the rate of adverse outcomes.
The primary outcome was a composite: perinatal death or severe neonatal complications (need for respiratory support within 72 hours of birth, Apgar score of ≤ 3 at 5 minutes, hypoxic-ischemic encephalopathy, seizures, infection [confirmed sepsis or pneumonia], meconium aspiration syndrome, birth trauma [bone fracture, neurologic injury, or retinal damage], intracranial or subgaleal hemorrhage, or hypotension requiring vasopressor support). The secondary outcomes included CD, hypertensive disorders of pregnancy, number of hours in the labor and delivery (L&D) unit, length of postpartum hospital stay, and assessment of satisfaction with labor process.
Mothers induced at 39 weeks fared better, while neonatal outcomes were similar. Of 22,533 eligible women, 6,106 (27%) were randomized: 3,062 were assigned to the induction group, and, 3,044 to the expectant management group. The primary composite outcome—perinatal death or severe neonatal complications—was similar in both groups (4.3% in the induction group vs 5.4% in the expectant management group).
However, women who were induced had significantly lower rates of:
- CD (18.6% with induction vs 22.2% for expectant management; relative risk [RR], 0.84; 95% confidence interval [CI], 0.76–0.93)
- hypertensive disorders of pregnancy (9.1% vs 14.1%; RR, 0.64; 95% CI, 0.56–0.74)
- neonatal respiratory support (3.0% vs. 4.2%; RR, 0.71; 95% CI, 0.55–0.93).
In addition, although women in the induction group had a longer stay in the L&D unit (an expected outcome), the overall postpartum length of stay was shorter. Finally, women in the induction group had higher patient satisfaction scores, with less pain and more control reported during labor.
Continue to: What about uncommon adverse outcomes compared at 39 vs 41 weeks?
What about uncommon adverse outcomes compared at 39 vs 41 weeks?
Due to the study’s sample size, ARRIVE investigators could not ascertain if uncommon adverse outcomes (maternal admission to intensive care unit or neonatal seizure) are significantly more common at 40 and 41 weeks, than at 39 weeks.
To address the issue of uncommon adverse outcomes, Chen and colleagues analyzed the US Vital Statistics datasets to compare composite maternal and neonatal morbidity among low-risk nulliparous women with nonanomalous singleton gestations who labored at 39 to 41 weeks.5 The primary outcome was composite neonatal morbidity that included Apgar score < 5 at 5 minutes, assisted ventilation longer than 6 hours, seizure, or neonatal mortality. The secondary outcome was composite maternal morbidity that included intensive care unit admission, blood transfusion, uterine rupture, or unplanned hysterectomy.
The investigators found that from 2011–2015, among 19.8 million live births in the United States, there were 3.3 million live births among low-risk nulliparous women. Among these women, 43% delivered at 39 weeks’ gestation, 41% at 40 weeks, and 15% at 41 weeks. The overall rate of composite neonatal morbidity was 8.8 per 1,000 live births; compared with those who delivered at 39 weeks, composite neonatal morbidity was significantly higher for those delivered at 40 (adjusted RR [aRR], 1.22; 95% CI, 1.19–1.25) and 41 weeks (aRR, 1.53; 95% CI, 1.49–1.58).
The secondary outcome, the overall rate of composite maternal morbidity, was 2.8 per 1,000 live births. As with composite neonatal morbidity, the risk of composite maternal morbidity was also significantly higher for those delivered at 40 (aRR, 1.19; 95% CI, 1.14–1.25) and 41 weeks’ gestation (aRR, 1.56; 95% CI, 1.47–1.65) than at 39 weeks.
Thus, among low-risk nulliparous pregnancies, there is an incremental increase in the rates of composite neonatal and maternal morbidity from 39 to 41 weeks.
Is induction of labor at 39 weeks feasible?
As the evidence demonstrating multiple benefits of 39-week inductions increases, concerns regarding the feasibility and cost of implementation in the current US health care system mount. A planned secondary analysis of the ARRIVE trial evaluated medical resource utilization among low-risk nulliparous women randomly assigned to elective induction at 39 weeks or expectant management.6 Resource utilization was compared between the 2 groups during the antepartum period, delivery admission, and from discharge to 8 weeks postpartum.
For the antepartum period, women in the induction group were significantly less likely than women undergoing expectant management to have at least 1: office visit for routine prenatal care (32.4% vs 68.4%), unanticipated office visit (0.5% vs 2.6%), urgent care/emergency department/triage visit (16.2% vs 44.3%), or hospital admission (0.8% vs 2.2%). When admitted for delivery, as expected, women in the induction group spent significantly more time on the L&D unit (14 hours vs 20 hours) and were more likely to receive interventions for induction (cervical ripening, oxytocin, intrauterine pressure catheter placement). However, they required magnesium sulfate and antibiotics significantly less frequently. For the postpartum group comparison, women in the induction group and their neonates had a significantly shorter duration of hospital stay.
In summary, the investigators found that, compared to women undergoing expectant management, women undergoing elective induction spent longer duration in L&D units and utilized more resources, but they required significantly fewer antepartum clinic and hospital visits, treatments for hypertensive disorders or chorioamnionitis, and had shorter duration of postpartum length of stay.
Continue to: Is induction of labor at 39 weeks cost-effective?
Is induction of labor at 39 weeks cost-effective?
Hersh and colleagues performed a cost-effectiveness analysis for induction of labor at 39 weeks versus expectant management for low-risk nulliparous women.7 Based on 2016 National Vital Statistics Data, there were 3.5 million term births in the United States. Following the exclusion of high-risk pregnancies and term parous low-risk pregnancies, a theoretical cohort of 1.6 million low-risk nulliparous women was included in the analysis. A decision-tree analytic model was created, in which the initial node stratified low-risk nulliparous women into 2 categories: elective induction at 39 weeks and expectant management. Probabilities of maternal and neonatal outcomes were derived from the literature.
Maternal outcomes included hypertensive disorders of pregnancy and delivery mode. Neonatal outcomes included macrosomia, shoulder dystocia, brachial plexus injury, stillbirth, and neonatal death. Costs of clinic and triage visits, induction of labor, modes of delivery, and maternal and neonatal outcomes were derived from previous studies and adjusted for inflation to 2018 dollars. Finally, quality-adjusted life years (QALYs) were calculated for mothers and neonates and were then used to estimate the incremental cost-effectiveness ratio (ICER) of elective induction of labor at 39 weeks. Following accepted standards, the threshold for cost-effectiveness was set at $100,000/QALYs or less.
Induction at 39 weeks comes in lower cost-wise than the standard threshold for QALY. In their analysis, the investigators found that if all 1.6 million women in their theoretical cohort underwent an elective induction of labor at 39 weeks (rather than expectant management), there would be 54,498 fewer CDs, 79,152 fewer cases of hypertensive disorders, 795 fewer cases of stillbirth, and 11 fewer neonatal deaths. Due to the decreased CD rates, the investigators did project an estimated 86 additional cases of neonatal brachial plexus injury. Using these estimates, costs, and utilities, the authors demonstrated that, compared with expectant management, elective induction of labor at 39 weeks was marginally cost-effective with an ICER of $87,692 per QALY, which was lower than the cost-effectiveness threshold of $100,000 per QALY.
Based on additional sensitivity analyses, the authors concluded that cost-effectiveness of elective induction of labor varied based on variations in model inputs. Specifically, the authors demonstrated that cost-effectiveness of induction of labor varied based on labor induction techniques, modes of delivery, and fluctuations in the rates of CD in induction versus expectant management groups.
Despite these theoretically imputed findings, the authors acknowledged the limitations of their study. Their cost-effectiveness model did not account for costs associated with long-term health impact of CD and hypertensive disease of pregnancy. Additionally, their model did not account for an increase in cost and resource utilization associated with increased time on L&D units to accommodate women undergoing induction. Furthermore, the analysis did not take into account the bundled payments for vaginal versus CDs, which are increasing in prevalence. Lastly, the analysis did not consider the incremental increase in severe neonatal and maternal morbidity from 39 to 41 weeks that Chen et al found in their study.5
Will ARRIVE finally arrive?
Cognizant of the medical and economic benefits of 39-week inductions, the Society for Maternal-Fetal Medicine and the American College of Obstetrics and Gynecology published a joint practice advisory recommending “shared decision-making” when counseling low-risk women about induction.8 While more research is needed to validate the aforementioned findings, particularly in regard to resource utilization, the ARRIVE trial and its associated analyses suggest that a reconsideration to deliver term low-risk nulliparous women at 39 weeks is warranted.
In summary, the overwhelming evidence suggests that, among low-risk nulliparous women there are maternal and neonatal benefits with delivery at 39 weeks, as compared with expectant management. Logistical concerns should not interfere with women’s desideratum for optimal outcomes.
- Vardo JH, Thornburg LL, Glantz JC. Maternal and neonatal morbidity among nulliparous women undergoing elective induction of labor. J Reprod Med. 2011;56:25-30.
- Dunne C, Da Silva O, Schmidt G, Natale R. Outcomes of elective labour induction and elective caesarean section in low-risk pregnancies between 37 and 41 weeks’ gestation. J Obstet Gynaecol Can . 2009;31:1124-1130.
- Guerra GV, Cecatti JG, Souza JP, et al; WHO Global Survey on Maternal Perinatal Health in Latin America Study Group. Elective induction versus spontaneous labour in Latin America. Bull World Health Organ. 2011;89:657-665.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Chen HY, Grobman WA, Blackwell SC, et al. Women at 39-41 weeks of gestation among low-risk nulliparous women, several adverse outcomes—including neonatal mortality—are significantly more frequent with delivery at 40 or 41 weeks of gestation than at 39 weeks. Obstet Gynecol. 2019;133:729-737.
- Grobman WA, et al. Resource utilization among low-risk nulliparas randomized to elective induction at 39 weeks or expectant management. Oral presentation at: Society for Maternal-Fetal Medicine 39th Annual Pregnancy Meeting; February 11-16, 2019; Las Vegas, NV.
- Hersh AR, Skeith AE, Sargent JA, et al. Induction of labor at 39 weeks of gestation vs. expectant management for low-risk nulliparous women: a cost-effectiveness analysis. Am J Obstet Gynecol . February 12, 2019. doi: https://doi.org/10.1016/ j.ajog.2019.02.017.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. August 9, 2018. doi: 10.1016/j.ajog.2018.08.009.
Of the 1.5 million nulliparous women who deliver annually in the United States, more than 50% are low-risk pregnancies. Among clinicians, there is a hesitancy to offer elective induction of labor to low-risk nulliparous women, mainly due to early observational studies that noted an association between elective induction of labor and higher rates of cesarean delivery (CD) and other adverse maternal and perinatal outcomes. 1-3 This reluctance over time has permeated throughout the ObGyn specialty and is culturally embedded in contemporary practice. The early observational studies lacked proper comparison groups because outcomes of women undergoing induction (elective and medically indicated) were compared to those in spontaneous labor. Since women who are being induced do not have the option to be in spontaneous labor, the appropriate comparator group for women undergoing elective induction is women who are being managed expectantly.
ARRIVE addresses appropriate comparator groups
Challenging this pervaded practice, in August 2018, Grobman and colleagues published the findings of the ARRIVE trial (A Randomized Trial of Induction Versus Expectant Management).4 This trial, conducted by Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network, recruited participants from 41 geographically dispersed centers in the United States. Nulliparous women with low-risk pregnancies between 34 0/7 and 38 6/7 weeks were randomly assigned to either induction of labor at 39 0/7 to 39 4/7 weeks or to expectant management, which was defined as delaying induction until 40 5/7 to 42 2/7 weeks. The objective of the ARRIVE trial was to determine if, among low-risk nulliparous women, elective induction of labor at 39 weeks, compared with expectant management, would reduce the rate of adverse outcomes.
The primary outcome was a composite: perinatal death or severe neonatal complications (need for respiratory support within 72 hours of birth, Apgar score of ≤ 3 at 5 minutes, hypoxic-ischemic encephalopathy, seizures, infection [confirmed sepsis or pneumonia], meconium aspiration syndrome, birth trauma [bone fracture, neurologic injury, or retinal damage], intracranial or subgaleal hemorrhage, or hypotension requiring vasopressor support). The secondary outcomes included CD, hypertensive disorders of pregnancy, number of hours in the labor and delivery (L&D) unit, length of postpartum hospital stay, and assessment of satisfaction with labor process.
Mothers induced at 39 weeks fared better, while neonatal outcomes were similar. Of 22,533 eligible women, 6,106 (27%) were randomized: 3,062 were assigned to the induction group, and, 3,044 to the expectant management group. The primary composite outcome—perinatal death or severe neonatal complications—was similar in both groups (4.3% in the induction group vs 5.4% in the expectant management group).
However, women who were induced had significantly lower rates of:
- CD (18.6% with induction vs 22.2% for expectant management; relative risk [RR], 0.84; 95% confidence interval [CI], 0.76–0.93)
- hypertensive disorders of pregnancy (9.1% vs 14.1%; RR, 0.64; 95% CI, 0.56–0.74)
- neonatal respiratory support (3.0% vs. 4.2%; RR, 0.71; 95% CI, 0.55–0.93).
In addition, although women in the induction group had a longer stay in the L&D unit (an expected outcome), the overall postpartum length of stay was shorter. Finally, women in the induction group had higher patient satisfaction scores, with less pain and more control reported during labor.
Continue to: What about uncommon adverse outcomes compared at 39 vs 41 weeks?
What about uncommon adverse outcomes compared at 39 vs 41 weeks?
Due to the study’s sample size, ARRIVE investigators could not ascertain if uncommon adverse outcomes (maternal admission to intensive care unit or neonatal seizure) are significantly more common at 40 and 41 weeks, than at 39 weeks.
To address the issue of uncommon adverse outcomes, Chen and colleagues analyzed the US Vital Statistics datasets to compare composite maternal and neonatal morbidity among low-risk nulliparous women with nonanomalous singleton gestations who labored at 39 to 41 weeks.5 The primary outcome was composite neonatal morbidity that included Apgar score < 5 at 5 minutes, assisted ventilation longer than 6 hours, seizure, or neonatal mortality. The secondary outcome was composite maternal morbidity that included intensive care unit admission, blood transfusion, uterine rupture, or unplanned hysterectomy.
The investigators found that from 2011–2015, among 19.8 million live births in the United States, there were 3.3 million live births among low-risk nulliparous women. Among these women, 43% delivered at 39 weeks’ gestation, 41% at 40 weeks, and 15% at 41 weeks. The overall rate of composite neonatal morbidity was 8.8 per 1,000 live births; compared with those who delivered at 39 weeks, composite neonatal morbidity was significantly higher for those delivered at 40 (adjusted RR [aRR], 1.22; 95% CI, 1.19–1.25) and 41 weeks (aRR, 1.53; 95% CI, 1.49–1.58).
The secondary outcome, the overall rate of composite maternal morbidity, was 2.8 per 1,000 live births. As with composite neonatal morbidity, the risk of composite maternal morbidity was also significantly higher for those delivered at 40 (aRR, 1.19; 95% CI, 1.14–1.25) and 41 weeks’ gestation (aRR, 1.56; 95% CI, 1.47–1.65) than at 39 weeks.
Thus, among low-risk nulliparous pregnancies, there is an incremental increase in the rates of composite neonatal and maternal morbidity from 39 to 41 weeks.
Is induction of labor at 39 weeks feasible?
As the evidence demonstrating multiple benefits of 39-week inductions increases, concerns regarding the feasibility and cost of implementation in the current US health care system mount. A planned secondary analysis of the ARRIVE trial evaluated medical resource utilization among low-risk nulliparous women randomly assigned to elective induction at 39 weeks or expectant management.6 Resource utilization was compared between the 2 groups during the antepartum period, delivery admission, and from discharge to 8 weeks postpartum.
For the antepartum period, women in the induction group were significantly less likely than women undergoing expectant management to have at least 1: office visit for routine prenatal care (32.4% vs 68.4%), unanticipated office visit (0.5% vs 2.6%), urgent care/emergency department/triage visit (16.2% vs 44.3%), or hospital admission (0.8% vs 2.2%). When admitted for delivery, as expected, women in the induction group spent significantly more time on the L&D unit (14 hours vs 20 hours) and were more likely to receive interventions for induction (cervical ripening, oxytocin, intrauterine pressure catheter placement). However, they required magnesium sulfate and antibiotics significantly less frequently. For the postpartum group comparison, women in the induction group and their neonates had a significantly shorter duration of hospital stay.
In summary, the investigators found that, compared to women undergoing expectant management, women undergoing elective induction spent longer duration in L&D units and utilized more resources, but they required significantly fewer antepartum clinic and hospital visits, treatments for hypertensive disorders or chorioamnionitis, and had shorter duration of postpartum length of stay.
Continue to: Is induction of labor at 39 weeks cost-effective?
Is induction of labor at 39 weeks cost-effective?
Hersh and colleagues performed a cost-effectiveness analysis for induction of labor at 39 weeks versus expectant management for low-risk nulliparous women.7 Based on 2016 National Vital Statistics Data, there were 3.5 million term births in the United States. Following the exclusion of high-risk pregnancies and term parous low-risk pregnancies, a theoretical cohort of 1.6 million low-risk nulliparous women was included in the analysis. A decision-tree analytic model was created, in which the initial node stratified low-risk nulliparous women into 2 categories: elective induction at 39 weeks and expectant management. Probabilities of maternal and neonatal outcomes were derived from the literature.
Maternal outcomes included hypertensive disorders of pregnancy and delivery mode. Neonatal outcomes included macrosomia, shoulder dystocia, brachial plexus injury, stillbirth, and neonatal death. Costs of clinic and triage visits, induction of labor, modes of delivery, and maternal and neonatal outcomes were derived from previous studies and adjusted for inflation to 2018 dollars. Finally, quality-adjusted life years (QALYs) were calculated for mothers and neonates and were then used to estimate the incremental cost-effectiveness ratio (ICER) of elective induction of labor at 39 weeks. Following accepted standards, the threshold for cost-effectiveness was set at $100,000/QALYs or less.
Induction at 39 weeks comes in lower cost-wise than the standard threshold for QALY. In their analysis, the investigators found that if all 1.6 million women in their theoretical cohort underwent an elective induction of labor at 39 weeks (rather than expectant management), there would be 54,498 fewer CDs, 79,152 fewer cases of hypertensive disorders, 795 fewer cases of stillbirth, and 11 fewer neonatal deaths. Due to the decreased CD rates, the investigators did project an estimated 86 additional cases of neonatal brachial plexus injury. Using these estimates, costs, and utilities, the authors demonstrated that, compared with expectant management, elective induction of labor at 39 weeks was marginally cost-effective with an ICER of $87,692 per QALY, which was lower than the cost-effectiveness threshold of $100,000 per QALY.
Based on additional sensitivity analyses, the authors concluded that cost-effectiveness of elective induction of labor varied based on variations in model inputs. Specifically, the authors demonstrated that cost-effectiveness of induction of labor varied based on labor induction techniques, modes of delivery, and fluctuations in the rates of CD in induction versus expectant management groups.
Despite these theoretically imputed findings, the authors acknowledged the limitations of their study. Their cost-effectiveness model did not account for costs associated with long-term health impact of CD and hypertensive disease of pregnancy. Additionally, their model did not account for an increase in cost and resource utilization associated with increased time on L&D units to accommodate women undergoing induction. Furthermore, the analysis did not take into account the bundled payments for vaginal versus CDs, which are increasing in prevalence. Lastly, the analysis did not consider the incremental increase in severe neonatal and maternal morbidity from 39 to 41 weeks that Chen et al found in their study.5
Will ARRIVE finally arrive?
Cognizant of the medical and economic benefits of 39-week inductions, the Society for Maternal-Fetal Medicine and the American College of Obstetrics and Gynecology published a joint practice advisory recommending “shared decision-making” when counseling low-risk women about induction.8 While more research is needed to validate the aforementioned findings, particularly in regard to resource utilization, the ARRIVE trial and its associated analyses suggest that a reconsideration to deliver term low-risk nulliparous women at 39 weeks is warranted.
In summary, the overwhelming evidence suggests that, among low-risk nulliparous women there are maternal and neonatal benefits with delivery at 39 weeks, as compared with expectant management. Logistical concerns should not interfere with women’s desideratum for optimal outcomes.
Of the 1.5 million nulliparous women who deliver annually in the United States, more than 50% are low-risk pregnancies. Among clinicians, there is a hesitancy to offer elective induction of labor to low-risk nulliparous women, mainly due to early observational studies that noted an association between elective induction of labor and higher rates of cesarean delivery (CD) and other adverse maternal and perinatal outcomes. 1-3 This reluctance over time has permeated throughout the ObGyn specialty and is culturally embedded in contemporary practice. The early observational studies lacked proper comparison groups because outcomes of women undergoing induction (elective and medically indicated) were compared to those in spontaneous labor. Since women who are being induced do not have the option to be in spontaneous labor, the appropriate comparator group for women undergoing elective induction is women who are being managed expectantly.
ARRIVE addresses appropriate comparator groups
Challenging this pervaded practice, in August 2018, Grobman and colleagues published the findings of the ARRIVE trial (A Randomized Trial of Induction Versus Expectant Management).4 This trial, conducted by Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network, recruited participants from 41 geographically dispersed centers in the United States. Nulliparous women with low-risk pregnancies between 34 0/7 and 38 6/7 weeks were randomly assigned to either induction of labor at 39 0/7 to 39 4/7 weeks or to expectant management, which was defined as delaying induction until 40 5/7 to 42 2/7 weeks. The objective of the ARRIVE trial was to determine if, among low-risk nulliparous women, elective induction of labor at 39 weeks, compared with expectant management, would reduce the rate of adverse outcomes.
The primary outcome was a composite: perinatal death or severe neonatal complications (need for respiratory support within 72 hours of birth, Apgar score of ≤ 3 at 5 minutes, hypoxic-ischemic encephalopathy, seizures, infection [confirmed sepsis or pneumonia], meconium aspiration syndrome, birth trauma [bone fracture, neurologic injury, or retinal damage], intracranial or subgaleal hemorrhage, or hypotension requiring vasopressor support). The secondary outcomes included CD, hypertensive disorders of pregnancy, number of hours in the labor and delivery (L&D) unit, length of postpartum hospital stay, and assessment of satisfaction with labor process.
Mothers induced at 39 weeks fared better, while neonatal outcomes were similar. Of 22,533 eligible women, 6,106 (27%) were randomized: 3,062 were assigned to the induction group, and, 3,044 to the expectant management group. The primary composite outcome—perinatal death or severe neonatal complications—was similar in both groups (4.3% in the induction group vs 5.4% in the expectant management group).
However, women who were induced had significantly lower rates of:
- CD (18.6% with induction vs 22.2% for expectant management; relative risk [RR], 0.84; 95% confidence interval [CI], 0.76–0.93)
- hypertensive disorders of pregnancy (9.1% vs 14.1%; RR, 0.64; 95% CI, 0.56–0.74)
- neonatal respiratory support (3.0% vs. 4.2%; RR, 0.71; 95% CI, 0.55–0.93).
In addition, although women in the induction group had a longer stay in the L&D unit (an expected outcome), the overall postpartum length of stay was shorter. Finally, women in the induction group had higher patient satisfaction scores, with less pain and more control reported during labor.
Continue to: What about uncommon adverse outcomes compared at 39 vs 41 weeks?
What about uncommon adverse outcomes compared at 39 vs 41 weeks?
Due to the study’s sample size, ARRIVE investigators could not ascertain if uncommon adverse outcomes (maternal admission to intensive care unit or neonatal seizure) are significantly more common at 40 and 41 weeks, than at 39 weeks.
To address the issue of uncommon adverse outcomes, Chen and colleagues analyzed the US Vital Statistics datasets to compare composite maternal and neonatal morbidity among low-risk nulliparous women with nonanomalous singleton gestations who labored at 39 to 41 weeks.5 The primary outcome was composite neonatal morbidity that included Apgar score < 5 at 5 minutes, assisted ventilation longer than 6 hours, seizure, or neonatal mortality. The secondary outcome was composite maternal morbidity that included intensive care unit admission, blood transfusion, uterine rupture, or unplanned hysterectomy.
The investigators found that from 2011–2015, among 19.8 million live births in the United States, there were 3.3 million live births among low-risk nulliparous women. Among these women, 43% delivered at 39 weeks’ gestation, 41% at 40 weeks, and 15% at 41 weeks. The overall rate of composite neonatal morbidity was 8.8 per 1,000 live births; compared with those who delivered at 39 weeks, composite neonatal morbidity was significantly higher for those delivered at 40 (adjusted RR [aRR], 1.22; 95% CI, 1.19–1.25) and 41 weeks (aRR, 1.53; 95% CI, 1.49–1.58).
The secondary outcome, the overall rate of composite maternal morbidity, was 2.8 per 1,000 live births. As with composite neonatal morbidity, the risk of composite maternal morbidity was also significantly higher for those delivered at 40 (aRR, 1.19; 95% CI, 1.14–1.25) and 41 weeks’ gestation (aRR, 1.56; 95% CI, 1.47–1.65) than at 39 weeks.
Thus, among low-risk nulliparous pregnancies, there is an incremental increase in the rates of composite neonatal and maternal morbidity from 39 to 41 weeks.
Is induction of labor at 39 weeks feasible?
As the evidence demonstrating multiple benefits of 39-week inductions increases, concerns regarding the feasibility and cost of implementation in the current US health care system mount. A planned secondary analysis of the ARRIVE trial evaluated medical resource utilization among low-risk nulliparous women randomly assigned to elective induction at 39 weeks or expectant management.6 Resource utilization was compared between the 2 groups during the antepartum period, delivery admission, and from discharge to 8 weeks postpartum.
For the antepartum period, women in the induction group were significantly less likely than women undergoing expectant management to have at least 1: office visit for routine prenatal care (32.4% vs 68.4%), unanticipated office visit (0.5% vs 2.6%), urgent care/emergency department/triage visit (16.2% vs 44.3%), or hospital admission (0.8% vs 2.2%). When admitted for delivery, as expected, women in the induction group spent significantly more time on the L&D unit (14 hours vs 20 hours) and were more likely to receive interventions for induction (cervical ripening, oxytocin, intrauterine pressure catheter placement). However, they required magnesium sulfate and antibiotics significantly less frequently. For the postpartum group comparison, women in the induction group and their neonates had a significantly shorter duration of hospital stay.
In summary, the investigators found that, compared to women undergoing expectant management, women undergoing elective induction spent longer duration in L&D units and utilized more resources, but they required significantly fewer antepartum clinic and hospital visits, treatments for hypertensive disorders or chorioamnionitis, and had shorter duration of postpartum length of stay.
Continue to: Is induction of labor at 39 weeks cost-effective?
Is induction of labor at 39 weeks cost-effective?
Hersh and colleagues performed a cost-effectiveness analysis for induction of labor at 39 weeks versus expectant management for low-risk nulliparous women.7 Based on 2016 National Vital Statistics Data, there were 3.5 million term births in the United States. Following the exclusion of high-risk pregnancies and term parous low-risk pregnancies, a theoretical cohort of 1.6 million low-risk nulliparous women was included in the analysis. A decision-tree analytic model was created, in which the initial node stratified low-risk nulliparous women into 2 categories: elective induction at 39 weeks and expectant management. Probabilities of maternal and neonatal outcomes were derived from the literature.
Maternal outcomes included hypertensive disorders of pregnancy and delivery mode. Neonatal outcomes included macrosomia, shoulder dystocia, brachial plexus injury, stillbirth, and neonatal death. Costs of clinic and triage visits, induction of labor, modes of delivery, and maternal and neonatal outcomes were derived from previous studies and adjusted for inflation to 2018 dollars. Finally, quality-adjusted life years (QALYs) were calculated for mothers and neonates and were then used to estimate the incremental cost-effectiveness ratio (ICER) of elective induction of labor at 39 weeks. Following accepted standards, the threshold for cost-effectiveness was set at $100,000/QALYs or less.
Induction at 39 weeks comes in lower cost-wise than the standard threshold for QALY. In their analysis, the investigators found that if all 1.6 million women in their theoretical cohort underwent an elective induction of labor at 39 weeks (rather than expectant management), there would be 54,498 fewer CDs, 79,152 fewer cases of hypertensive disorders, 795 fewer cases of stillbirth, and 11 fewer neonatal deaths. Due to the decreased CD rates, the investigators did project an estimated 86 additional cases of neonatal brachial plexus injury. Using these estimates, costs, and utilities, the authors demonstrated that, compared with expectant management, elective induction of labor at 39 weeks was marginally cost-effective with an ICER of $87,692 per QALY, which was lower than the cost-effectiveness threshold of $100,000 per QALY.
Based on additional sensitivity analyses, the authors concluded that cost-effectiveness of elective induction of labor varied based on variations in model inputs. Specifically, the authors demonstrated that cost-effectiveness of induction of labor varied based on labor induction techniques, modes of delivery, and fluctuations in the rates of CD in induction versus expectant management groups.
Despite these theoretically imputed findings, the authors acknowledged the limitations of their study. Their cost-effectiveness model did not account for costs associated with long-term health impact of CD and hypertensive disease of pregnancy. Additionally, their model did not account for an increase in cost and resource utilization associated with increased time on L&D units to accommodate women undergoing induction. Furthermore, the analysis did not take into account the bundled payments for vaginal versus CDs, which are increasing in prevalence. Lastly, the analysis did not consider the incremental increase in severe neonatal and maternal morbidity from 39 to 41 weeks that Chen et al found in their study.5
Will ARRIVE finally arrive?
Cognizant of the medical and economic benefits of 39-week inductions, the Society for Maternal-Fetal Medicine and the American College of Obstetrics and Gynecology published a joint practice advisory recommending “shared decision-making” when counseling low-risk women about induction.8 While more research is needed to validate the aforementioned findings, particularly in regard to resource utilization, the ARRIVE trial and its associated analyses suggest that a reconsideration to deliver term low-risk nulliparous women at 39 weeks is warranted.
In summary, the overwhelming evidence suggests that, among low-risk nulliparous women there are maternal and neonatal benefits with delivery at 39 weeks, as compared with expectant management. Logistical concerns should not interfere with women’s desideratum for optimal outcomes.
- Vardo JH, Thornburg LL, Glantz JC. Maternal and neonatal morbidity among nulliparous women undergoing elective induction of labor. J Reprod Med. 2011;56:25-30.
- Dunne C, Da Silva O, Schmidt G, Natale R. Outcomes of elective labour induction and elective caesarean section in low-risk pregnancies between 37 and 41 weeks’ gestation. J Obstet Gynaecol Can . 2009;31:1124-1130.
- Guerra GV, Cecatti JG, Souza JP, et al; WHO Global Survey on Maternal Perinatal Health in Latin America Study Group. Elective induction versus spontaneous labour in Latin America. Bull World Health Organ. 2011;89:657-665.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Chen HY, Grobman WA, Blackwell SC, et al. Women at 39-41 weeks of gestation among low-risk nulliparous women, several adverse outcomes—including neonatal mortality—are significantly more frequent with delivery at 40 or 41 weeks of gestation than at 39 weeks. Obstet Gynecol. 2019;133:729-737.
- Grobman WA, et al. Resource utilization among low-risk nulliparas randomized to elective induction at 39 weeks or expectant management. Oral presentation at: Society for Maternal-Fetal Medicine 39th Annual Pregnancy Meeting; February 11-16, 2019; Las Vegas, NV.
- Hersh AR, Skeith AE, Sargent JA, et al. Induction of labor at 39 weeks of gestation vs. expectant management for low-risk nulliparous women: a cost-effectiveness analysis. Am J Obstet Gynecol . February 12, 2019. doi: https://doi.org/10.1016/ j.ajog.2019.02.017.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. August 9, 2018. doi: 10.1016/j.ajog.2018.08.009.
- Vardo JH, Thornburg LL, Glantz JC. Maternal and neonatal morbidity among nulliparous women undergoing elective induction of labor. J Reprod Med. 2011;56:25-30.
- Dunne C, Da Silva O, Schmidt G, Natale R. Outcomes of elective labour induction and elective caesarean section in low-risk pregnancies between 37 and 41 weeks’ gestation. J Obstet Gynaecol Can . 2009;31:1124-1130.
- Guerra GV, Cecatti JG, Souza JP, et al; WHO Global Survey on Maternal Perinatal Health in Latin America Study Group. Elective induction versus spontaneous labour in Latin America. Bull World Health Organ. 2011;89:657-665.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Chen HY, Grobman WA, Blackwell SC, et al. Women at 39-41 weeks of gestation among low-risk nulliparous women, several adverse outcomes—including neonatal mortality—are significantly more frequent with delivery at 40 or 41 weeks of gestation than at 39 weeks. Obstet Gynecol. 2019;133:729-737.
- Grobman WA, et al. Resource utilization among low-risk nulliparas randomized to elective induction at 39 weeks or expectant management. Oral presentation at: Society for Maternal-Fetal Medicine 39th Annual Pregnancy Meeting; February 11-16, 2019; Las Vegas, NV.
- Hersh AR, Skeith AE, Sargent JA, et al. Induction of labor at 39 weeks of gestation vs. expectant management for low-risk nulliparous women: a cost-effectiveness analysis. Am J Obstet Gynecol . February 12, 2019. doi: https://doi.org/10.1016/ j.ajog.2019.02.017.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. August 9, 2018. doi: 10.1016/j.ajog.2018.08.009.
Treating the pregnant patient with opioid addiction
OBG Management : How has the opioid crisis affected women in general?
Mishka Terplan, MD: Everyone is aware that we are experiencing a massive opioid crisis in the United States, and from a historical perspective, this is at least the third or fourth significant opioid epidemic in our nation’s history.1 It is similar in some ways to the very first one, which also featured a large proportion of women and also was driven initially by physician prescribing practices. However, the magnitude of this crisis is unparalleled compared with prior opioid epidemics.
There are lots of reasons why women are overrepresented in this crisis. There are gender-based differences in pain—chronic pain syndromes are more common in women. In addition, we have a gender bias in prescribing opioids and prescribe more opioids to women (especially older women) than to men. Cultural differences also contribute. As providers, we tend not to think of women as people who use drugs or people who develop addictions the same way as we think of these risks and behaviors for men. Therefore, compared with men, we are less likely to screen, assess, or refer women for substance use, misuse, and addiction. All of this adds up to creating a crisis in which women are increasingly the face of the epidemic.
OBG Management : What are the concerns about opioid addiction and pregnant women specifically?
Dr. Terplan: Addiction is a chronic condition, just like diabetes or depression, and the same principles that we think of in terms of optimizing maternal and newborn health apply to addiction. Ideally, we want, for women with chronic diseases to have stable disease at the time of conception and through pregnancy. We know this maximizes birth outcomes.
Unfortunately, there is a massive treatment gap in the United States. Most people with addiction receive no treatment. Only 11% of people with a substance use disorder report receipt of treatment. By contrast, more than 70% of people with depression, hypertension, or diabetes receive care. This treatment gap is also present in pregnancy. Among use disorders, treatment receipt is highest for opioid use disorder; however, nationally, at best, 25% of pregnant women with opioid addiction receive any care.
In other words, when we encounter addiction clinically, it is often untreated addiction. Therefore, many times providers will have women presenting to care who are both pregnant and have untreated addiction. From both a public health and a clinical practice perspective, the salient distinction is not between people with addiction and those without but between people with treated disease and people with untreated disease.
Untreated addiction is a serious medical condition. It is associated with preterm delivery and low birth weight infants. It is associated with acquisition and transmission of HIV and hepatitis C. It is associated with overdose and overdose death. By contrast, treated addiction is associated with term delivery and normal weight infants. Pharmacotherapies for opioid use disorder stabilize the intrauterine environment and allow for normal fetal growth. Pharmacotherapies for opioid use disorder help to structure and stabilize the mom’s social circumstance, providing a platform to deliver prenatal care and essential social services. And pharmacotherapies for opioid use disorder protect women and their fetuses from overdose and from overdose deaths. The goal of management of addiction in pregnancy is treatment of the underlying condition, treating the addiction.
Continue to: OBG Management...
OBG Management : What should the ObGyn do when faced with a patient who might have an addiction?
Dr. Terplan: The good news is that there are lots of recently published guidance documents from the World Health Organization,2 the American College of Obstetricians and Gynecologists (ACOG),3 and the Substance Abuse and Mental Health Services Administration (SAMHSA),4 and there have been a whole series of trainings throughout the United States organized by both ACOG and SAMHSA.
There is also a collaboration between ACOG and the American Society of Addiction Medicine (ASAM) to provide buprenorphine waiver trainings specifically designed for ObGyns. Check both the ACOG and ASAM pages for details. I encourage every provider to get a waiver to prescribe buprenorphine. There are about 30 ObGyns who are also board certified in addiction medicine in the United States, and all of us are more than happy to help our colleagues in the clinical care of this population, a population that all of us really enjoy taking care of.
Although care in pregnancy is important, we must not forget about the postpartum period. Generally speaking, women do quite well during pregnancy in terms of treatment. Postpartum, however, is a vulnerable period, where relapse happens, where gaps in care happen, where child welfare involvement and sometimes child removal happens, which can be very stressful for anyone much less somebody with a substance use disorder. Recent data demonstrate that one of the leading causes of maternal mortality in the US in from overdose, and most of these deaths occur in the postpartum period.5 Regardless of what happens during pregnancy, it is essential that we be able to link and continue care for women with opioid use disorder throughout the postpartum period.
OBG Management : How do you treat opioid use disorder in pregnancy?
Dr. Terplan: The standard of care for treatment of opioid use disorder in pregnancy is pharmacotherapy with either methadone or buprenorphine (TABLE) plus behavioral counseling—ideally, co-located with prenatal care. The evidence base for pharmacotherapy for opioid use disorder in pregnancy is supported by every single professional society that has ever issued guidance on this, from the World Health Organization to ACOG, to ASAM, to the Royal College in the UK as well as Canadian and Australian obstetrics and gynecology societies; literally every single professional society supports medication.
The core principle of maternal fetal medicine rests upon the fact that chronic conditions need to be treated and that treated illness improves birth outcomes. For both maternal and fetal health, treated addiction is way better than untreated addiction. One concern people have regarding methadone and buprenorphine is the development of dependence. Dependence is a physiologic effect of medication and occurs with opioids, as well as with many other medications, such as antidepressants and most hypertensive agents. For the fetus, dependence means that at the time of delivery, the infant may go into withdrawal, which is also called neonatal abstinence syndrome. Neonatal abstinence syndrome is an expected outcome of in-utero opioid exposure. It is a time-limited and treatable condition. Prospective data do not demonstrate any long-term harms among infants whose mothers received pharmacotherapy for opioid use disorder during pregnancy.6
The treatment for neonatal abstinence syndrome is costly, especially when in a neonatal intensive care unit. It can be quite concerning to a new mother to have an infant that has to spend extra time in the hospital and sometimes be medicated for management of withdrawal.
There has been a renewed interest amongst ObGyns in investigating medically-supervised withdrawal during pregnancy. Although there are remaining questions, overall, the literature does not support withdrawal during pregnancy—mostly because withdrawal is associated with relapse, and relapse is associated with cessation of care (both prenatal care and addiction treatment), acquisition and transmission of HIV and Hepatitis C, and overdose and overdose death. The pertinent clinical and public health goal is the treatment of the chronic condition of addiction during pregnancy. The standard of care remains pharmacotherapy plus behavioral counseling for the treatment of opioid use disorder in pregnancy.
Clinical care, however, is both evidence-based and person-centered. All of us who have worked in this field, long before there was attention to the opioid crisis, all of us have provided medically-supervised withdrawal of a pregnant person, and that is because we understand the principles of care. When evidence-based care conflicts with person-centered care, the ethical course is the provision of person-centered care. Patients have the right of refusal. If someone wants to discontinue medication, I have tapered the medication during pregnancy, but continued to provide (and often increase) behavioral counseling and prenatal care.
Treated addiction is better for the fetus than untreated addiction. Untreated opioid addiction is associated with preterm birth and low birth weight. These obstetric risks are not because of the opioid per se, but because of the repeated cycles of withdrawal that an individual with untreated addiction experiences. People with untreated addiction are not getting “high” when they use, they are just becoming a little bit less sick. It is this repeated cycle of withdrawal that stresses the fetus, which leads to preterm delivery and low birth weight.
Medications for opioid use disorder are long-acting and dosed daily. In contrast to the repeated cycles of fetal withdrawal in untreated addiction, pharmacotherapy stabilizes the intrauterine environment. There is no cyclic, repeated, stressful withdrawal, and consequentially, the fetus grows normally and delivers at term. Obstetric risk is from repeated cyclic withdrawal more than from opioid exposure itself.
Continue to: OBG Management...
OBG Management : Research reports that women are not using all of the opioids that are prescribed to them after a cesarean delivery. What are the risks for addiction in this setting?
Dr. Terplan: I mark a distinction between use (ie, using something as prescribed) and misuse, which means using a prescribed medication not in the manner in which it was prescribed, or using somebody else’s medications, or using an illicit substance. And I differentiate use and misuse from addiction, which is a behavioral condition, a disease. There has been a lot of attention paid to opioid prescribing in general and in particular postdelivery and post–cesarean delivery, which is one of the most common operative procedures in the United States.
It seems clear from the literature that we have overprescribed opioids postdelivery, and a small number of women, about 1 in 300 will continue an opioid script.7 This means that 1 in 300 women who received an opioid prescription following delivery present for care and get another opioid prescription filled. Now, that is a small number at the level of the individual, but because we do so many cesarean deliveries, this is a large number of women at the level of the population. This does not mean, however, that 1 in 300 women who received opioids after cesarean delivery are going to become addicted to them. It just means that 1 in 300 will continue the prescription. Prescription continuation is a risk factor for opioid misuse, and opioid misuse is on the pathway toward addiction.
Most people who use substances do not develop an addiction to that substance. We know from the opioid literature that at most only 10% of people who receive chronic opioid therapy will meet criteria for opioid use disorder.8 Now 10% is not 100%, nor is it 0%, but because we prescribed so many opioids to so many people for so long, the absolute number of people with opioid use disorder from physician opioid prescribing is large, even though the risk at the level of the individual is not as large as people think.
OBG Management : From your experience in treating addiction during pregnancy, are there clinical pearls you would like to share with ObGyns?
Dr. Terplan: There are a couple of takeaways. One is that all women are motivated to maximize their health and that of their baby to be, and every pregnant woman engages in behavioral change; in fact most women quit or cutback substance use during pregnancy. But some can’t. Those that can’t likely have a substance use disorder. We think of addiction as a chronic condition, centered in the brain, but the primary symptoms of addiction are behaviors. The salient feature of addiction is continued use despite adverse consequences; using something that you know is harming yourself and others but you can’t stop using it. In other words, continuing substance use during pregnancy. When we see clinically a pregnant woman who is using a substance, 99% of the time we are seeing a pregnant woman who has the condition of addiction, and what she needs is treatment. She does not need to be told that injecting heroin is unsafe for her and her fetus, she knows that. What she needs is treatment.
The second point is that pregnant women who use drugs and pregnant women with addiction experience a real specific and strong form of discrimination by providers, by other people with addiction, by the legal system, and by their friends and families. Caring for people who have substance use disorder is grounded in human rights, which means treating people with dignity and respect. It is important for providers to have empathy, especially for pregnant people who use drugs, to counter the discrimination they experience from society and from other health care providers.
Continue to: OBG Management...
OBG Management : Are there specific ways in which ObGyns can show empathy when speaking with a pregnant woman who likely has addiction?
Dr. Terplan: In general when we talk to people about drug use, it is important to ask their permission to talk about it. For example, “Is it okay if I ask you some questions about smoking, drinking, and other drugs?” If someone says, “No, I don’t want you to ask those questions,” we have to respect that. Assessment of substance use should be a universal part of all medical care, as substance use, misuse, and addiction are essential domains of wellness, but I think we should ask permission before screening.
One of the really good things about prenatal care is that people come back; we have multiple visits across the gestational period. The behavioral work of addiction treatment rests upon a strong therapeutic alliance. If you do not respect your patient, then there is no way you can achieve a therapeutic alliance. Asking permission, and then respecting somebody’s answers, I think goes a really long way to establishing a strong therapeutic alliance, which is the basis of any medical care.
- Terplan M. Women and the opioid crisis: historical context and public health solutions. Fertil Steril. 2017;108:195-199.
- Management of substance abuse. World Health Organization website. https://www.who.int/substance_abuse/activities/treatment_opioid_dependence/en/. Accessed March 20, 2019.
- Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Obstet Gynecol. 2017;130(2):e81-e94.
- Substance Abuse and Mental Health Services Administration. Clinical guidance for treating pregnant and parenting women with opioid use disorder and their infants. HHS Publication No. (SMA) 18-5054. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
- Metz TD, Royner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Kaltenbach K, O’Grady E, Heil SH, et al. Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend. 2018;185:40-49.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naive women. Am J Obstet Gynecol. 2016;215:353.e1-353.e18.
OBG Management : How has the opioid crisis affected women in general?
Mishka Terplan, MD: Everyone is aware that we are experiencing a massive opioid crisis in the United States, and from a historical perspective, this is at least the third or fourth significant opioid epidemic in our nation’s history.1 It is similar in some ways to the very first one, which also featured a large proportion of women and also was driven initially by physician prescribing practices. However, the magnitude of this crisis is unparalleled compared with prior opioid epidemics.
There are lots of reasons why women are overrepresented in this crisis. There are gender-based differences in pain—chronic pain syndromes are more common in women. In addition, we have a gender bias in prescribing opioids and prescribe more opioids to women (especially older women) than to men. Cultural differences also contribute. As providers, we tend not to think of women as people who use drugs or people who develop addictions the same way as we think of these risks and behaviors for men. Therefore, compared with men, we are less likely to screen, assess, or refer women for substance use, misuse, and addiction. All of this adds up to creating a crisis in which women are increasingly the face of the epidemic.
OBG Management : What are the concerns about opioid addiction and pregnant women specifically?
Dr. Terplan: Addiction is a chronic condition, just like diabetes or depression, and the same principles that we think of in terms of optimizing maternal and newborn health apply to addiction. Ideally, we want, for women with chronic diseases to have stable disease at the time of conception and through pregnancy. We know this maximizes birth outcomes.
Unfortunately, there is a massive treatment gap in the United States. Most people with addiction receive no treatment. Only 11% of people with a substance use disorder report receipt of treatment. By contrast, more than 70% of people with depression, hypertension, or diabetes receive care. This treatment gap is also present in pregnancy. Among use disorders, treatment receipt is highest for opioid use disorder; however, nationally, at best, 25% of pregnant women with opioid addiction receive any care.
In other words, when we encounter addiction clinically, it is often untreated addiction. Therefore, many times providers will have women presenting to care who are both pregnant and have untreated addiction. From both a public health and a clinical practice perspective, the salient distinction is not between people with addiction and those without but between people with treated disease and people with untreated disease.
Untreated addiction is a serious medical condition. It is associated with preterm delivery and low birth weight infants. It is associated with acquisition and transmission of HIV and hepatitis C. It is associated with overdose and overdose death. By contrast, treated addiction is associated with term delivery and normal weight infants. Pharmacotherapies for opioid use disorder stabilize the intrauterine environment and allow for normal fetal growth. Pharmacotherapies for opioid use disorder help to structure and stabilize the mom’s social circumstance, providing a platform to deliver prenatal care and essential social services. And pharmacotherapies for opioid use disorder protect women and their fetuses from overdose and from overdose deaths. The goal of management of addiction in pregnancy is treatment of the underlying condition, treating the addiction.
Continue to: OBG Management...
OBG Management : What should the ObGyn do when faced with a patient who might have an addiction?
Dr. Terplan: The good news is that there are lots of recently published guidance documents from the World Health Organization,2 the American College of Obstetricians and Gynecologists (ACOG),3 and the Substance Abuse and Mental Health Services Administration (SAMHSA),4 and there have been a whole series of trainings throughout the United States organized by both ACOG and SAMHSA.
There is also a collaboration between ACOG and the American Society of Addiction Medicine (ASAM) to provide buprenorphine waiver trainings specifically designed for ObGyns. Check both the ACOG and ASAM pages for details. I encourage every provider to get a waiver to prescribe buprenorphine. There are about 30 ObGyns who are also board certified in addiction medicine in the United States, and all of us are more than happy to help our colleagues in the clinical care of this population, a population that all of us really enjoy taking care of.
Although care in pregnancy is important, we must not forget about the postpartum period. Generally speaking, women do quite well during pregnancy in terms of treatment. Postpartum, however, is a vulnerable period, where relapse happens, where gaps in care happen, where child welfare involvement and sometimes child removal happens, which can be very stressful for anyone much less somebody with a substance use disorder. Recent data demonstrate that one of the leading causes of maternal mortality in the US in from overdose, and most of these deaths occur in the postpartum period.5 Regardless of what happens during pregnancy, it is essential that we be able to link and continue care for women with opioid use disorder throughout the postpartum period.
OBG Management : How do you treat opioid use disorder in pregnancy?
Dr. Terplan: The standard of care for treatment of opioid use disorder in pregnancy is pharmacotherapy with either methadone or buprenorphine (TABLE) plus behavioral counseling—ideally, co-located with prenatal care. The evidence base for pharmacotherapy for opioid use disorder in pregnancy is supported by every single professional society that has ever issued guidance on this, from the World Health Organization to ACOG, to ASAM, to the Royal College in the UK as well as Canadian and Australian obstetrics and gynecology societies; literally every single professional society supports medication.
The core principle of maternal fetal medicine rests upon the fact that chronic conditions need to be treated and that treated illness improves birth outcomes. For both maternal and fetal health, treated addiction is way better than untreated addiction. One concern people have regarding methadone and buprenorphine is the development of dependence. Dependence is a physiologic effect of medication and occurs with opioids, as well as with many other medications, such as antidepressants and most hypertensive agents. For the fetus, dependence means that at the time of delivery, the infant may go into withdrawal, which is also called neonatal abstinence syndrome. Neonatal abstinence syndrome is an expected outcome of in-utero opioid exposure. It is a time-limited and treatable condition. Prospective data do not demonstrate any long-term harms among infants whose mothers received pharmacotherapy for opioid use disorder during pregnancy.6
The treatment for neonatal abstinence syndrome is costly, especially when in a neonatal intensive care unit. It can be quite concerning to a new mother to have an infant that has to spend extra time in the hospital and sometimes be medicated for management of withdrawal.
There has been a renewed interest amongst ObGyns in investigating medically-supervised withdrawal during pregnancy. Although there are remaining questions, overall, the literature does not support withdrawal during pregnancy—mostly because withdrawal is associated with relapse, and relapse is associated with cessation of care (both prenatal care and addiction treatment), acquisition and transmission of HIV and Hepatitis C, and overdose and overdose death. The pertinent clinical and public health goal is the treatment of the chronic condition of addiction during pregnancy. The standard of care remains pharmacotherapy plus behavioral counseling for the treatment of opioid use disorder in pregnancy.
Clinical care, however, is both evidence-based and person-centered. All of us who have worked in this field, long before there was attention to the opioid crisis, all of us have provided medically-supervised withdrawal of a pregnant person, and that is because we understand the principles of care. When evidence-based care conflicts with person-centered care, the ethical course is the provision of person-centered care. Patients have the right of refusal. If someone wants to discontinue medication, I have tapered the medication during pregnancy, but continued to provide (and often increase) behavioral counseling and prenatal care.
Treated addiction is better for the fetus than untreated addiction. Untreated opioid addiction is associated with preterm birth and low birth weight. These obstetric risks are not because of the opioid per se, but because of the repeated cycles of withdrawal that an individual with untreated addiction experiences. People with untreated addiction are not getting “high” when they use, they are just becoming a little bit less sick. It is this repeated cycle of withdrawal that stresses the fetus, which leads to preterm delivery and low birth weight.
Medications for opioid use disorder are long-acting and dosed daily. In contrast to the repeated cycles of fetal withdrawal in untreated addiction, pharmacotherapy stabilizes the intrauterine environment. There is no cyclic, repeated, stressful withdrawal, and consequentially, the fetus grows normally and delivers at term. Obstetric risk is from repeated cyclic withdrawal more than from opioid exposure itself.
Continue to: OBG Management...
OBG Management : Research reports that women are not using all of the opioids that are prescribed to them after a cesarean delivery. What are the risks for addiction in this setting?
Dr. Terplan: I mark a distinction between use (ie, using something as prescribed) and misuse, which means using a prescribed medication not in the manner in which it was prescribed, or using somebody else’s medications, or using an illicit substance. And I differentiate use and misuse from addiction, which is a behavioral condition, a disease. There has been a lot of attention paid to opioid prescribing in general and in particular postdelivery and post–cesarean delivery, which is one of the most common operative procedures in the United States.
It seems clear from the literature that we have overprescribed opioids postdelivery, and a small number of women, about 1 in 300 will continue an opioid script.7 This means that 1 in 300 women who received an opioid prescription following delivery present for care and get another opioid prescription filled. Now, that is a small number at the level of the individual, but because we do so many cesarean deliveries, this is a large number of women at the level of the population. This does not mean, however, that 1 in 300 women who received opioids after cesarean delivery are going to become addicted to them. It just means that 1 in 300 will continue the prescription. Prescription continuation is a risk factor for opioid misuse, and opioid misuse is on the pathway toward addiction.
Most people who use substances do not develop an addiction to that substance. We know from the opioid literature that at most only 10% of people who receive chronic opioid therapy will meet criteria for opioid use disorder.8 Now 10% is not 100%, nor is it 0%, but because we prescribed so many opioids to so many people for so long, the absolute number of people with opioid use disorder from physician opioid prescribing is large, even though the risk at the level of the individual is not as large as people think.
OBG Management : From your experience in treating addiction during pregnancy, are there clinical pearls you would like to share with ObGyns?
Dr. Terplan: There are a couple of takeaways. One is that all women are motivated to maximize their health and that of their baby to be, and every pregnant woman engages in behavioral change; in fact most women quit or cutback substance use during pregnancy. But some can’t. Those that can’t likely have a substance use disorder. We think of addiction as a chronic condition, centered in the brain, but the primary symptoms of addiction are behaviors. The salient feature of addiction is continued use despite adverse consequences; using something that you know is harming yourself and others but you can’t stop using it. In other words, continuing substance use during pregnancy. When we see clinically a pregnant woman who is using a substance, 99% of the time we are seeing a pregnant woman who has the condition of addiction, and what she needs is treatment. She does not need to be told that injecting heroin is unsafe for her and her fetus, she knows that. What she needs is treatment.
The second point is that pregnant women who use drugs and pregnant women with addiction experience a real specific and strong form of discrimination by providers, by other people with addiction, by the legal system, and by their friends and families. Caring for people who have substance use disorder is grounded in human rights, which means treating people with dignity and respect. It is important for providers to have empathy, especially for pregnant people who use drugs, to counter the discrimination they experience from society and from other health care providers.
Continue to: OBG Management...
OBG Management : Are there specific ways in which ObGyns can show empathy when speaking with a pregnant woman who likely has addiction?
Dr. Terplan: In general when we talk to people about drug use, it is important to ask their permission to talk about it. For example, “Is it okay if I ask you some questions about smoking, drinking, and other drugs?” If someone says, “No, I don’t want you to ask those questions,” we have to respect that. Assessment of substance use should be a universal part of all medical care, as substance use, misuse, and addiction are essential domains of wellness, but I think we should ask permission before screening.
One of the really good things about prenatal care is that people come back; we have multiple visits across the gestational period. The behavioral work of addiction treatment rests upon a strong therapeutic alliance. If you do not respect your patient, then there is no way you can achieve a therapeutic alliance. Asking permission, and then respecting somebody’s answers, I think goes a really long way to establishing a strong therapeutic alliance, which is the basis of any medical care.
OBG Management : How has the opioid crisis affected women in general?
Mishka Terplan, MD: Everyone is aware that we are experiencing a massive opioid crisis in the United States, and from a historical perspective, this is at least the third or fourth significant opioid epidemic in our nation’s history.1 It is similar in some ways to the very first one, which also featured a large proportion of women and also was driven initially by physician prescribing practices. However, the magnitude of this crisis is unparalleled compared with prior opioid epidemics.
There are lots of reasons why women are overrepresented in this crisis. There are gender-based differences in pain—chronic pain syndromes are more common in women. In addition, we have a gender bias in prescribing opioids and prescribe more opioids to women (especially older women) than to men. Cultural differences also contribute. As providers, we tend not to think of women as people who use drugs or people who develop addictions the same way as we think of these risks and behaviors for men. Therefore, compared with men, we are less likely to screen, assess, or refer women for substance use, misuse, and addiction. All of this adds up to creating a crisis in which women are increasingly the face of the epidemic.
OBG Management : What are the concerns about opioid addiction and pregnant women specifically?
Dr. Terplan: Addiction is a chronic condition, just like diabetes or depression, and the same principles that we think of in terms of optimizing maternal and newborn health apply to addiction. Ideally, we want, for women with chronic diseases to have stable disease at the time of conception and through pregnancy. We know this maximizes birth outcomes.
Unfortunately, there is a massive treatment gap in the United States. Most people with addiction receive no treatment. Only 11% of people with a substance use disorder report receipt of treatment. By contrast, more than 70% of people with depression, hypertension, or diabetes receive care. This treatment gap is also present in pregnancy. Among use disorders, treatment receipt is highest for opioid use disorder; however, nationally, at best, 25% of pregnant women with opioid addiction receive any care.
In other words, when we encounter addiction clinically, it is often untreated addiction. Therefore, many times providers will have women presenting to care who are both pregnant and have untreated addiction. From both a public health and a clinical practice perspective, the salient distinction is not between people with addiction and those without but between people with treated disease and people with untreated disease.
Untreated addiction is a serious medical condition. It is associated with preterm delivery and low birth weight infants. It is associated with acquisition and transmission of HIV and hepatitis C. It is associated with overdose and overdose death. By contrast, treated addiction is associated with term delivery and normal weight infants. Pharmacotherapies for opioid use disorder stabilize the intrauterine environment and allow for normal fetal growth. Pharmacotherapies for opioid use disorder help to structure and stabilize the mom’s social circumstance, providing a platform to deliver prenatal care and essential social services. And pharmacotherapies for opioid use disorder protect women and their fetuses from overdose and from overdose deaths. The goal of management of addiction in pregnancy is treatment of the underlying condition, treating the addiction.
Continue to: OBG Management...
OBG Management : What should the ObGyn do when faced with a patient who might have an addiction?
Dr. Terplan: The good news is that there are lots of recently published guidance documents from the World Health Organization,2 the American College of Obstetricians and Gynecologists (ACOG),3 and the Substance Abuse and Mental Health Services Administration (SAMHSA),4 and there have been a whole series of trainings throughout the United States organized by both ACOG and SAMHSA.
There is also a collaboration between ACOG and the American Society of Addiction Medicine (ASAM) to provide buprenorphine waiver trainings specifically designed for ObGyns. Check both the ACOG and ASAM pages for details. I encourage every provider to get a waiver to prescribe buprenorphine. There are about 30 ObGyns who are also board certified in addiction medicine in the United States, and all of us are more than happy to help our colleagues in the clinical care of this population, a population that all of us really enjoy taking care of.
Although care in pregnancy is important, we must not forget about the postpartum period. Generally speaking, women do quite well during pregnancy in terms of treatment. Postpartum, however, is a vulnerable period, where relapse happens, where gaps in care happen, where child welfare involvement and sometimes child removal happens, which can be very stressful for anyone much less somebody with a substance use disorder. Recent data demonstrate that one of the leading causes of maternal mortality in the US in from overdose, and most of these deaths occur in the postpartum period.5 Regardless of what happens during pregnancy, it is essential that we be able to link and continue care for women with opioid use disorder throughout the postpartum period.
OBG Management : How do you treat opioid use disorder in pregnancy?
Dr. Terplan: The standard of care for treatment of opioid use disorder in pregnancy is pharmacotherapy with either methadone or buprenorphine (TABLE) plus behavioral counseling—ideally, co-located with prenatal care. The evidence base for pharmacotherapy for opioid use disorder in pregnancy is supported by every single professional society that has ever issued guidance on this, from the World Health Organization to ACOG, to ASAM, to the Royal College in the UK as well as Canadian and Australian obstetrics and gynecology societies; literally every single professional society supports medication.
The core principle of maternal fetal medicine rests upon the fact that chronic conditions need to be treated and that treated illness improves birth outcomes. For both maternal and fetal health, treated addiction is way better than untreated addiction. One concern people have regarding methadone and buprenorphine is the development of dependence. Dependence is a physiologic effect of medication and occurs with opioids, as well as with many other medications, such as antidepressants and most hypertensive agents. For the fetus, dependence means that at the time of delivery, the infant may go into withdrawal, which is also called neonatal abstinence syndrome. Neonatal abstinence syndrome is an expected outcome of in-utero opioid exposure. It is a time-limited and treatable condition. Prospective data do not demonstrate any long-term harms among infants whose mothers received pharmacotherapy for opioid use disorder during pregnancy.6
The treatment for neonatal abstinence syndrome is costly, especially when in a neonatal intensive care unit. It can be quite concerning to a new mother to have an infant that has to spend extra time in the hospital and sometimes be medicated for management of withdrawal.
There has been a renewed interest amongst ObGyns in investigating medically-supervised withdrawal during pregnancy. Although there are remaining questions, overall, the literature does not support withdrawal during pregnancy—mostly because withdrawal is associated with relapse, and relapse is associated with cessation of care (both prenatal care and addiction treatment), acquisition and transmission of HIV and Hepatitis C, and overdose and overdose death. The pertinent clinical and public health goal is the treatment of the chronic condition of addiction during pregnancy. The standard of care remains pharmacotherapy plus behavioral counseling for the treatment of opioid use disorder in pregnancy.
Clinical care, however, is both evidence-based and person-centered. All of us who have worked in this field, long before there was attention to the opioid crisis, all of us have provided medically-supervised withdrawal of a pregnant person, and that is because we understand the principles of care. When evidence-based care conflicts with person-centered care, the ethical course is the provision of person-centered care. Patients have the right of refusal. If someone wants to discontinue medication, I have tapered the medication during pregnancy, but continued to provide (and often increase) behavioral counseling and prenatal care.
Treated addiction is better for the fetus than untreated addiction. Untreated opioid addiction is associated with preterm birth and low birth weight. These obstetric risks are not because of the opioid per se, but because of the repeated cycles of withdrawal that an individual with untreated addiction experiences. People with untreated addiction are not getting “high” when they use, they are just becoming a little bit less sick. It is this repeated cycle of withdrawal that stresses the fetus, which leads to preterm delivery and low birth weight.
Medications for opioid use disorder are long-acting and dosed daily. In contrast to the repeated cycles of fetal withdrawal in untreated addiction, pharmacotherapy stabilizes the intrauterine environment. There is no cyclic, repeated, stressful withdrawal, and consequentially, the fetus grows normally and delivers at term. Obstetric risk is from repeated cyclic withdrawal more than from opioid exposure itself.
Continue to: OBG Management...
OBG Management : Research reports that women are not using all of the opioids that are prescribed to them after a cesarean delivery. What are the risks for addiction in this setting?
Dr. Terplan: I mark a distinction between use (ie, using something as prescribed) and misuse, which means using a prescribed medication not in the manner in which it was prescribed, or using somebody else’s medications, or using an illicit substance. And I differentiate use and misuse from addiction, which is a behavioral condition, a disease. There has been a lot of attention paid to opioid prescribing in general and in particular postdelivery and post–cesarean delivery, which is one of the most common operative procedures in the United States.
It seems clear from the literature that we have overprescribed opioids postdelivery, and a small number of women, about 1 in 300 will continue an opioid script.7 This means that 1 in 300 women who received an opioid prescription following delivery present for care and get another opioid prescription filled. Now, that is a small number at the level of the individual, but because we do so many cesarean deliveries, this is a large number of women at the level of the population. This does not mean, however, that 1 in 300 women who received opioids after cesarean delivery are going to become addicted to them. It just means that 1 in 300 will continue the prescription. Prescription continuation is a risk factor for opioid misuse, and opioid misuse is on the pathway toward addiction.
Most people who use substances do not develop an addiction to that substance. We know from the opioid literature that at most only 10% of people who receive chronic opioid therapy will meet criteria for opioid use disorder.8 Now 10% is not 100%, nor is it 0%, but because we prescribed so many opioids to so many people for so long, the absolute number of people with opioid use disorder from physician opioid prescribing is large, even though the risk at the level of the individual is not as large as people think.
OBG Management : From your experience in treating addiction during pregnancy, are there clinical pearls you would like to share with ObGyns?
Dr. Terplan: There are a couple of takeaways. One is that all women are motivated to maximize their health and that of their baby to be, and every pregnant woman engages in behavioral change; in fact most women quit or cutback substance use during pregnancy. But some can’t. Those that can’t likely have a substance use disorder. We think of addiction as a chronic condition, centered in the brain, but the primary symptoms of addiction are behaviors. The salient feature of addiction is continued use despite adverse consequences; using something that you know is harming yourself and others but you can’t stop using it. In other words, continuing substance use during pregnancy. When we see clinically a pregnant woman who is using a substance, 99% of the time we are seeing a pregnant woman who has the condition of addiction, and what she needs is treatment. She does not need to be told that injecting heroin is unsafe for her and her fetus, she knows that. What she needs is treatment.
The second point is that pregnant women who use drugs and pregnant women with addiction experience a real specific and strong form of discrimination by providers, by other people with addiction, by the legal system, and by their friends and families. Caring for people who have substance use disorder is grounded in human rights, which means treating people with dignity and respect. It is important for providers to have empathy, especially for pregnant people who use drugs, to counter the discrimination they experience from society and from other health care providers.
Continue to: OBG Management...
OBG Management : Are there specific ways in which ObGyns can show empathy when speaking with a pregnant woman who likely has addiction?
Dr. Terplan: In general when we talk to people about drug use, it is important to ask their permission to talk about it. For example, “Is it okay if I ask you some questions about smoking, drinking, and other drugs?” If someone says, “No, I don’t want you to ask those questions,” we have to respect that. Assessment of substance use should be a universal part of all medical care, as substance use, misuse, and addiction are essential domains of wellness, but I think we should ask permission before screening.
One of the really good things about prenatal care is that people come back; we have multiple visits across the gestational period. The behavioral work of addiction treatment rests upon a strong therapeutic alliance. If you do not respect your patient, then there is no way you can achieve a therapeutic alliance. Asking permission, and then respecting somebody’s answers, I think goes a really long way to establishing a strong therapeutic alliance, which is the basis of any medical care.
- Terplan M. Women and the opioid crisis: historical context and public health solutions. Fertil Steril. 2017;108:195-199.
- Management of substance abuse. World Health Organization website. https://www.who.int/substance_abuse/activities/treatment_opioid_dependence/en/. Accessed March 20, 2019.
- Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Obstet Gynecol. 2017;130(2):e81-e94.
- Substance Abuse and Mental Health Services Administration. Clinical guidance for treating pregnant and parenting women with opioid use disorder and their infants. HHS Publication No. (SMA) 18-5054. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
- Metz TD, Royner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Kaltenbach K, O’Grady E, Heil SH, et al. Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend. 2018;185:40-49.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naive women. Am J Obstet Gynecol. 2016;215:353.e1-353.e18.
- Terplan M. Women and the opioid crisis: historical context and public health solutions. Fertil Steril. 2017;108:195-199.
- Management of substance abuse. World Health Organization website. https://www.who.int/substance_abuse/activities/treatment_opioid_dependence/en/. Accessed March 20, 2019.
- Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Obstet Gynecol. 2017;130(2):e81-e94.
- Substance Abuse and Mental Health Services Administration. Clinical guidance for treating pregnant and parenting women with opioid use disorder and their infants. HHS Publication No. (SMA) 18-5054. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
- Metz TD, Royner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Kaltenbach K, O’Grady E, Heil SH, et al. Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend. 2018;185:40-49.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naive women. Am J Obstet Gynecol. 2016;215:353.e1-353.e18.