User login
How to counsel women about marijuana in pregnancy
DENVER – , Torri D. Metz, MD, observed at the annual meeting of the Teratology Society.
This is of particular concern because the increasing legalization of recreational marijuana across the United States means growing use, possibly including use by pregnant women. National surveys indicate a high percentage of pregnant women believe there is slight or no harm in using marijuana once or twice per week, said Dr. Metz, an ob.gyn. at the University of Colorado, Denver, who is researching the effects of marijuana in pregnancy.
Here’s how she likes to handle that situation: She starts out by freely admitting that that’s true. The available evidence is limited, mixed, and often flawed.
“I say, ‘I can’t give you data that says absolutely it’s not safe, but I also absolutely cannot give you data saying it is safe.’ I would favor saying, ‘I can’t tell you it’s safe. And if there’s any possible risk, let’s talk about things we know are safe we can use as alternatives for whatever you’re using cannabis for,’ ” she explained.
A Colorado survey of more than 1,700 mothers in the WIC (Women, Infants, and Children) nutrition program shed light on the reasons women use marijuana while pregnant or breastfeeding. Sixty-three percent of current users cited as a perceived benefit that it helped with depression, anxiety, and/or stress. Sixty percent reported it helped with pain. Nearly half used marijuana for nausea and vomiting. Just 39% did so for recreation.
Dr. Metz’s anecdotal experience has been that many health care providers are flubbing the opportunity to counsel women about marijuana use in pregnancy. This impression was bolstered by a recent study by investigators at the University of Pittsburgh who audio-recorded 468 first prenatal visits.
In total, 19% of patients disclosed marijuana use to 47 health care providers. In nearly half of those encounters, the providers didn’t respond to the disclosure at all. And when they did respond, it typically wasn’t by providing thoughtful, informed counseling on the risks or outcomes of using marijuana in pregnancy. Instead, the response was most often punitive: for example, a warning that evidence of use at delivery would result in a call to child protective services (Obstet Gynecol. 2016 Apr;127[4]:681-7).
Because of Colorado’s lengthy experience with legalized marijuana, the state Department of Public Health and Environment has endeavored to create resources of value for health care providers and patients (www.colorado.gov/cdphe/marijuana-clinical-guidelines). The website contains a fact sheet for patients regarding marijuana in pregnancy and breastfeeding. For physicians, there is plain-language guidance on how to talk effectively about marijuana with patients, including suggested responses to selected commonly voiced misconceptions.
The website also includes the results of a 2014 marijuana-in-pregnancy literature review by a state advisory committee composed of Colorado specialists in pediatrics, ob.gyn., family medicine, public health, and addiction medicine.
The committee determined that there is moderate evidence that the use of marijuana in pregnancy is associated with increased risk of reduced fetal growth, lower IQ scores in young children, adverse effects on a child’s cognitive function and academic ability, and an increase in attention problems. There was deemed to be limited evidence of an association with stillbirth and isolated ventricular septal defects. There is also “mixed” evidence for associations with preterm delivery, reduced birth weight, and selected congenital anomalies.
Since that 2014 review, a new signal of potential harm stemming from maternal marijuana use in pregnancy has appeared: a possible increased risk of neonatal ICU admission. In one retrospective study including 361 marijuana users and 6,107 nonusers, the users had a 1.54-fold increased risk for neonatal ICU admission in an analysis adjusted for maternal demographics and tobacco use (J Perinatol. 2015 Dec;35[12]:991-5).
Moreover, investigators at the University of Arizona in Tucson performed a meta-analysis of 24 studies and concluded that infants exposed to cannabis in utero were at 2.02-fold increased likelihood of neonatal ICU admission, a 1.77-fold increased risk of low birth weight, and 1.36-fold increased odds of anemia (BMJ Open. 2016 Apr 5;6[4]:e0009986. doi: 10.1136/bmjopen-2015-009986).
“That obviously would have a big public health impact,” Dr. Metz said.
In marked contrast, however, just a few months later investigators at Washington University in St. Louis reported finding no significantly increased risk of neonatal ICU admission or any other adverse neonatal outcome after adjustment for tobacco use and other potential confounders in a meta-analysis of 31 studies (Obstet Gynecol. 2016 Oct;128[4]:713-23).
These contradictory meta-analyses underscore a key point about the existing literature on the safety of marijuana use in pregnancy: It provides few, if any, definitive answers. The studies conducted in the 1980s and 1990s are of limited generalizability because concentrations of tetrahydrocannabinol were so small, compared with today’s products. Ascertainment of exposure to marijuana in pregnancy is unreliable in the absence of confirmatory biologic sampling. Self-reported use is unreliable and is typically an underestimate. Adjustment for confounders associated with adverse neonatal outcomes is challenging.
“Biologic sampling is critical,” Dr. Metz said. “We actually don’t know who’s using, and we lack information on the timing and quantity of exposure.
“Part of the problem is the data are so mixed that you can really find whatever you want in the literature to support your bias,” she added.
Still, in light of the signals of possible harm, she urged her colleagues to advise patients not to use marijuana in pregnancy. Patients need to understand that there are no known benefits of marijuana use in pregnancy, there are possible risks, and there is no known safe amount of cannabis in pregnancy.
Dr. Metz reported having no financial conflicts related to her presentation.
DENVER – , Torri D. Metz, MD, observed at the annual meeting of the Teratology Society.
This is of particular concern because the increasing legalization of recreational marijuana across the United States means growing use, possibly including use by pregnant women. National surveys indicate a high percentage of pregnant women believe there is slight or no harm in using marijuana once or twice per week, said Dr. Metz, an ob.gyn. at the University of Colorado, Denver, who is researching the effects of marijuana in pregnancy.
Here’s how she likes to handle that situation: She starts out by freely admitting that that’s true. The available evidence is limited, mixed, and often flawed.
“I say, ‘I can’t give you data that says absolutely it’s not safe, but I also absolutely cannot give you data saying it is safe.’ I would favor saying, ‘I can’t tell you it’s safe. And if there’s any possible risk, let’s talk about things we know are safe we can use as alternatives for whatever you’re using cannabis for,’ ” she explained.
A Colorado survey of more than 1,700 mothers in the WIC (Women, Infants, and Children) nutrition program shed light on the reasons women use marijuana while pregnant or breastfeeding. Sixty-three percent of current users cited as a perceived benefit that it helped with depression, anxiety, and/or stress. Sixty percent reported it helped with pain. Nearly half used marijuana for nausea and vomiting. Just 39% did so for recreation.
Dr. Metz’s anecdotal experience has been that many health care providers are flubbing the opportunity to counsel women about marijuana use in pregnancy. This impression was bolstered by a recent study by investigators at the University of Pittsburgh who audio-recorded 468 first prenatal visits.
In total, 19% of patients disclosed marijuana use to 47 health care providers. In nearly half of those encounters, the providers didn’t respond to the disclosure at all. And when they did respond, it typically wasn’t by providing thoughtful, informed counseling on the risks or outcomes of using marijuana in pregnancy. Instead, the response was most often punitive: for example, a warning that evidence of use at delivery would result in a call to child protective services (Obstet Gynecol. 2016 Apr;127[4]:681-7).
Because of Colorado’s lengthy experience with legalized marijuana, the state Department of Public Health and Environment has endeavored to create resources of value for health care providers and patients (www.colorado.gov/cdphe/marijuana-clinical-guidelines). The website contains a fact sheet for patients regarding marijuana in pregnancy and breastfeeding. For physicians, there is plain-language guidance on how to talk effectively about marijuana with patients, including suggested responses to selected commonly voiced misconceptions.
The website also includes the results of a 2014 marijuana-in-pregnancy literature review by a state advisory committee composed of Colorado specialists in pediatrics, ob.gyn., family medicine, public health, and addiction medicine.
The committee determined that there is moderate evidence that the use of marijuana in pregnancy is associated with increased risk of reduced fetal growth, lower IQ scores in young children, adverse effects on a child’s cognitive function and academic ability, and an increase in attention problems. There was deemed to be limited evidence of an association with stillbirth and isolated ventricular septal defects. There is also “mixed” evidence for associations with preterm delivery, reduced birth weight, and selected congenital anomalies.
Since that 2014 review, a new signal of potential harm stemming from maternal marijuana use in pregnancy has appeared: a possible increased risk of neonatal ICU admission. In one retrospective study including 361 marijuana users and 6,107 nonusers, the users had a 1.54-fold increased risk for neonatal ICU admission in an analysis adjusted for maternal demographics and tobacco use (J Perinatol. 2015 Dec;35[12]:991-5).
Moreover, investigators at the University of Arizona in Tucson performed a meta-analysis of 24 studies and concluded that infants exposed to cannabis in utero were at 2.02-fold increased likelihood of neonatal ICU admission, a 1.77-fold increased risk of low birth weight, and 1.36-fold increased odds of anemia (BMJ Open. 2016 Apr 5;6[4]:e0009986. doi: 10.1136/bmjopen-2015-009986).
“That obviously would have a big public health impact,” Dr. Metz said.
In marked contrast, however, just a few months later investigators at Washington University in St. Louis reported finding no significantly increased risk of neonatal ICU admission or any other adverse neonatal outcome after adjustment for tobacco use and other potential confounders in a meta-analysis of 31 studies (Obstet Gynecol. 2016 Oct;128[4]:713-23).
These contradictory meta-analyses underscore a key point about the existing literature on the safety of marijuana use in pregnancy: It provides few, if any, definitive answers. The studies conducted in the 1980s and 1990s are of limited generalizability because concentrations of tetrahydrocannabinol were so small, compared with today’s products. Ascertainment of exposure to marijuana in pregnancy is unreliable in the absence of confirmatory biologic sampling. Self-reported use is unreliable and is typically an underestimate. Adjustment for confounders associated with adverse neonatal outcomes is challenging.
“Biologic sampling is critical,” Dr. Metz said. “We actually don’t know who’s using, and we lack information on the timing and quantity of exposure.
“Part of the problem is the data are so mixed that you can really find whatever you want in the literature to support your bias,” she added.
Still, in light of the signals of possible harm, she urged her colleagues to advise patients not to use marijuana in pregnancy. Patients need to understand that there are no known benefits of marijuana use in pregnancy, there are possible risks, and there is no known safe amount of cannabis in pregnancy.
Dr. Metz reported having no financial conflicts related to her presentation.
DENVER – , Torri D. Metz, MD, observed at the annual meeting of the Teratology Society.
This is of particular concern because the increasing legalization of recreational marijuana across the United States means growing use, possibly including use by pregnant women. National surveys indicate a high percentage of pregnant women believe there is slight or no harm in using marijuana once or twice per week, said Dr. Metz, an ob.gyn. at the University of Colorado, Denver, who is researching the effects of marijuana in pregnancy.
Here’s how she likes to handle that situation: She starts out by freely admitting that that’s true. The available evidence is limited, mixed, and often flawed.
“I say, ‘I can’t give you data that says absolutely it’s not safe, but I also absolutely cannot give you data saying it is safe.’ I would favor saying, ‘I can’t tell you it’s safe. And if there’s any possible risk, let’s talk about things we know are safe we can use as alternatives for whatever you’re using cannabis for,’ ” she explained.
A Colorado survey of more than 1,700 mothers in the WIC (Women, Infants, and Children) nutrition program shed light on the reasons women use marijuana while pregnant or breastfeeding. Sixty-three percent of current users cited as a perceived benefit that it helped with depression, anxiety, and/or stress. Sixty percent reported it helped with pain. Nearly half used marijuana for nausea and vomiting. Just 39% did so for recreation.
Dr. Metz’s anecdotal experience has been that many health care providers are flubbing the opportunity to counsel women about marijuana use in pregnancy. This impression was bolstered by a recent study by investigators at the University of Pittsburgh who audio-recorded 468 first prenatal visits.
In total, 19% of patients disclosed marijuana use to 47 health care providers. In nearly half of those encounters, the providers didn’t respond to the disclosure at all. And when they did respond, it typically wasn’t by providing thoughtful, informed counseling on the risks or outcomes of using marijuana in pregnancy. Instead, the response was most often punitive: for example, a warning that evidence of use at delivery would result in a call to child protective services (Obstet Gynecol. 2016 Apr;127[4]:681-7).
Because of Colorado’s lengthy experience with legalized marijuana, the state Department of Public Health and Environment has endeavored to create resources of value for health care providers and patients (www.colorado.gov/cdphe/marijuana-clinical-guidelines). The website contains a fact sheet for patients regarding marijuana in pregnancy and breastfeeding. For physicians, there is plain-language guidance on how to talk effectively about marijuana with patients, including suggested responses to selected commonly voiced misconceptions.
The website also includes the results of a 2014 marijuana-in-pregnancy literature review by a state advisory committee composed of Colorado specialists in pediatrics, ob.gyn., family medicine, public health, and addiction medicine.
The committee determined that there is moderate evidence that the use of marijuana in pregnancy is associated with increased risk of reduced fetal growth, lower IQ scores in young children, adverse effects on a child’s cognitive function and academic ability, and an increase in attention problems. There was deemed to be limited evidence of an association with stillbirth and isolated ventricular septal defects. There is also “mixed” evidence for associations with preterm delivery, reduced birth weight, and selected congenital anomalies.
Since that 2014 review, a new signal of potential harm stemming from maternal marijuana use in pregnancy has appeared: a possible increased risk of neonatal ICU admission. In one retrospective study including 361 marijuana users and 6,107 nonusers, the users had a 1.54-fold increased risk for neonatal ICU admission in an analysis adjusted for maternal demographics and tobacco use (J Perinatol. 2015 Dec;35[12]:991-5).
Moreover, investigators at the University of Arizona in Tucson performed a meta-analysis of 24 studies and concluded that infants exposed to cannabis in utero were at 2.02-fold increased likelihood of neonatal ICU admission, a 1.77-fold increased risk of low birth weight, and 1.36-fold increased odds of anemia (BMJ Open. 2016 Apr 5;6[4]:e0009986. doi: 10.1136/bmjopen-2015-009986).
“That obviously would have a big public health impact,” Dr. Metz said.
In marked contrast, however, just a few months later investigators at Washington University in St. Louis reported finding no significantly increased risk of neonatal ICU admission or any other adverse neonatal outcome after adjustment for tobacco use and other potential confounders in a meta-analysis of 31 studies (Obstet Gynecol. 2016 Oct;128[4]:713-23).
These contradictory meta-analyses underscore a key point about the existing literature on the safety of marijuana use in pregnancy: It provides few, if any, definitive answers. The studies conducted in the 1980s and 1990s are of limited generalizability because concentrations of tetrahydrocannabinol were so small, compared with today’s products. Ascertainment of exposure to marijuana in pregnancy is unreliable in the absence of confirmatory biologic sampling. Self-reported use is unreliable and is typically an underestimate. Adjustment for confounders associated with adverse neonatal outcomes is challenging.
“Biologic sampling is critical,” Dr. Metz said. “We actually don’t know who’s using, and we lack information on the timing and quantity of exposure.
“Part of the problem is the data are so mixed that you can really find whatever you want in the literature to support your bias,” she added.
Still, in light of the signals of possible harm, she urged her colleagues to advise patients not to use marijuana in pregnancy. Patients need to understand that there are no known benefits of marijuana use in pregnancy, there are possible risks, and there is no known safe amount of cannabis in pregnancy.
Dr. Metz reported having no financial conflicts related to her presentation.
EXPERT ANALYSIS FROM TERATOLOGY SOCIETY 2017
Opening the door to gene editing?
In early August, in Portland, Ore. The scale and success of such experimentation with human embryos is unprecedented in the United States. Given the highly experimental nature of fertility clinics in the United States and abroad, many suggest that these findings open the door to designer babies. A careful read of the report, however, indicates that the door is still quite closed, perhaps cracked open just a little.
The research team used a new method of cutting the genome, called CRISPR-Cas9. CRISPR utilizes two key components that the team combined in a test tube together: a Cas9 protein that can cut the DNA and a synthetic RNA that can guide the protein to cut a 20-letter sequence in the human genome specifically. In these experiments, the Cas9-RNA protein was designed to cut a pathogenic mutation in the MYBPC3 gene, which can cause hypertrophic cardiomyopathy. The research team could not obtain human zygotes with this mutation on both copies of the genome (a rare homozygous genotype). Such zygotes would have the most severe phenotype and be the most compelling test case for CRISPR. Instead, they focused on gene editing heterozygous human zygotes that have one normal maternal copy of the MYBPC3 gene and one pathogenic paternal copy. The heterozygous zygotes were produced by the research team via in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) using sperm donated by males carrying the pathogenic mutation (Nature. 2017 Aug 2. doi: 10.1038/nature23305).
When researchers injected the Cas9-RNA protein targeting the mutation into already fertilized zygotes, they found that 67% of the resulting embryos had two normal copies of the MYBPC3 gene. Without gene editing, approximately 50% of the embryos would have two normal copies, because the male sperm donor would produce equal numbers of sperm with normal and pathogenic genotypes. Thus, editing likely corrected only about 17% of the embryos that would have otherwise had one pathogenic paternal mutation. Thirty-six percent of embryos had additional mutations from imprecise gene editing. Further, some of the gene edits and additional mutations were mosaic, meaning that the resulting embryo harbored many different genotypes.
To overcome these challenges, the research team precisely controlled the timing of CRISPR injection to coincide with fertilization. With controlled timing, gene editing was restricted to only the paternal pathogenic mutation, resulting in 72% of all injected embryos having two normal copies of the gene in all cells without any mosaicism. Whole genome sequencing revealed no additional mutations above the detection limit of the assay. Finally, preimplantation development proceeded normally to the blastocyst stage, suggesting that the edited embryos have no functional deficits from the procedure.
A surprising finding was that new sequences could not be put into the embryo. The research team had coinjected a synthetic DNA template that differed from the normal maternal copy, but never saw this sequence incorporated into any embryo. Instead, the zygote utilized the maternal copy of the gene with the normal sequence as a template for repairing the DNA cut in the paternal copy produced by CRISPR. The biology behind this repair process is poorly understood and has not been previously reported with other human cell types. These observations suggest that we cannot easily “write” our genome. Instead, our vocabulary is limited to what is already within either the maternal or paternal copy of the genome. In other words, designer babies are not around the corner. While preimplantation genetic diagnosis (PGD) is still currently the safest way to avoid passing on autosomal dominant mutations, these new findings could enable correction of such mutations within IVF embryos, resulting in a larger pool of embryos for IVF clinics to work with.
Apart from these technical challenges, the National Academies has not given a green light to implant edited human embryos. Instead, the organization calls for several requirements to be met, including “broad societal consensus” on the need for this type of intervention. While it is not clear whether or how consensus could be achieved, it is clear that scientists, clinicians, and patients will need help from the rest of society for this research to have an impact clinically.
Dr. Saha is assistant professor of biomedical engineering at the Wisconsin Institute for Discovery at the University of Wisconsin, Madison. His lab works on gene editing of human cells. He has patent filings through the Wisconsin Alumni Research Foundation on gene editing inventions.
In early August, in Portland, Ore. The scale and success of such experimentation with human embryos is unprecedented in the United States. Given the highly experimental nature of fertility clinics in the United States and abroad, many suggest that these findings open the door to designer babies. A careful read of the report, however, indicates that the door is still quite closed, perhaps cracked open just a little.
The research team used a new method of cutting the genome, called CRISPR-Cas9. CRISPR utilizes two key components that the team combined in a test tube together: a Cas9 protein that can cut the DNA and a synthetic RNA that can guide the protein to cut a 20-letter sequence in the human genome specifically. In these experiments, the Cas9-RNA protein was designed to cut a pathogenic mutation in the MYBPC3 gene, which can cause hypertrophic cardiomyopathy. The research team could not obtain human zygotes with this mutation on both copies of the genome (a rare homozygous genotype). Such zygotes would have the most severe phenotype and be the most compelling test case for CRISPR. Instead, they focused on gene editing heterozygous human zygotes that have one normal maternal copy of the MYBPC3 gene and one pathogenic paternal copy. The heterozygous zygotes were produced by the research team via in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) using sperm donated by males carrying the pathogenic mutation (Nature. 2017 Aug 2. doi: 10.1038/nature23305).
When researchers injected the Cas9-RNA protein targeting the mutation into already fertilized zygotes, they found that 67% of the resulting embryos had two normal copies of the MYBPC3 gene. Without gene editing, approximately 50% of the embryos would have two normal copies, because the male sperm donor would produce equal numbers of sperm with normal and pathogenic genotypes. Thus, editing likely corrected only about 17% of the embryos that would have otherwise had one pathogenic paternal mutation. Thirty-six percent of embryos had additional mutations from imprecise gene editing. Further, some of the gene edits and additional mutations were mosaic, meaning that the resulting embryo harbored many different genotypes.
To overcome these challenges, the research team precisely controlled the timing of CRISPR injection to coincide with fertilization. With controlled timing, gene editing was restricted to only the paternal pathogenic mutation, resulting in 72% of all injected embryos having two normal copies of the gene in all cells without any mosaicism. Whole genome sequencing revealed no additional mutations above the detection limit of the assay. Finally, preimplantation development proceeded normally to the blastocyst stage, suggesting that the edited embryos have no functional deficits from the procedure.
A surprising finding was that new sequences could not be put into the embryo. The research team had coinjected a synthetic DNA template that differed from the normal maternal copy, but never saw this sequence incorporated into any embryo. Instead, the zygote utilized the maternal copy of the gene with the normal sequence as a template for repairing the DNA cut in the paternal copy produced by CRISPR. The biology behind this repair process is poorly understood and has not been previously reported with other human cell types. These observations suggest that we cannot easily “write” our genome. Instead, our vocabulary is limited to what is already within either the maternal or paternal copy of the genome. In other words, designer babies are not around the corner. While preimplantation genetic diagnosis (PGD) is still currently the safest way to avoid passing on autosomal dominant mutations, these new findings could enable correction of such mutations within IVF embryos, resulting in a larger pool of embryos for IVF clinics to work with.
Apart from these technical challenges, the National Academies has not given a green light to implant edited human embryos. Instead, the organization calls for several requirements to be met, including “broad societal consensus” on the need for this type of intervention. While it is not clear whether or how consensus could be achieved, it is clear that scientists, clinicians, and patients will need help from the rest of society for this research to have an impact clinically.
Dr. Saha is assistant professor of biomedical engineering at the Wisconsin Institute for Discovery at the University of Wisconsin, Madison. His lab works on gene editing of human cells. He has patent filings through the Wisconsin Alumni Research Foundation on gene editing inventions.
In early August, in Portland, Ore. The scale and success of such experimentation with human embryos is unprecedented in the United States. Given the highly experimental nature of fertility clinics in the United States and abroad, many suggest that these findings open the door to designer babies. A careful read of the report, however, indicates that the door is still quite closed, perhaps cracked open just a little.
The research team used a new method of cutting the genome, called CRISPR-Cas9. CRISPR utilizes two key components that the team combined in a test tube together: a Cas9 protein that can cut the DNA and a synthetic RNA that can guide the protein to cut a 20-letter sequence in the human genome specifically. In these experiments, the Cas9-RNA protein was designed to cut a pathogenic mutation in the MYBPC3 gene, which can cause hypertrophic cardiomyopathy. The research team could not obtain human zygotes with this mutation on both copies of the genome (a rare homozygous genotype). Such zygotes would have the most severe phenotype and be the most compelling test case for CRISPR. Instead, they focused on gene editing heterozygous human zygotes that have one normal maternal copy of the MYBPC3 gene and one pathogenic paternal copy. The heterozygous zygotes were produced by the research team via in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) using sperm donated by males carrying the pathogenic mutation (Nature. 2017 Aug 2. doi: 10.1038/nature23305).
When researchers injected the Cas9-RNA protein targeting the mutation into already fertilized zygotes, they found that 67% of the resulting embryos had two normal copies of the MYBPC3 gene. Without gene editing, approximately 50% of the embryos would have two normal copies, because the male sperm donor would produce equal numbers of sperm with normal and pathogenic genotypes. Thus, editing likely corrected only about 17% of the embryos that would have otherwise had one pathogenic paternal mutation. Thirty-six percent of embryos had additional mutations from imprecise gene editing. Further, some of the gene edits and additional mutations were mosaic, meaning that the resulting embryo harbored many different genotypes.
To overcome these challenges, the research team precisely controlled the timing of CRISPR injection to coincide with fertilization. With controlled timing, gene editing was restricted to only the paternal pathogenic mutation, resulting in 72% of all injected embryos having two normal copies of the gene in all cells without any mosaicism. Whole genome sequencing revealed no additional mutations above the detection limit of the assay. Finally, preimplantation development proceeded normally to the blastocyst stage, suggesting that the edited embryos have no functional deficits from the procedure.
A surprising finding was that new sequences could not be put into the embryo. The research team had coinjected a synthetic DNA template that differed from the normal maternal copy, but never saw this sequence incorporated into any embryo. Instead, the zygote utilized the maternal copy of the gene with the normal sequence as a template for repairing the DNA cut in the paternal copy produced by CRISPR. The biology behind this repair process is poorly understood and has not been previously reported with other human cell types. These observations suggest that we cannot easily “write” our genome. Instead, our vocabulary is limited to what is already within either the maternal or paternal copy of the genome. In other words, designer babies are not around the corner. While preimplantation genetic diagnosis (PGD) is still currently the safest way to avoid passing on autosomal dominant mutations, these new findings could enable correction of such mutations within IVF embryos, resulting in a larger pool of embryos for IVF clinics to work with.
Apart from these technical challenges, the National Academies has not given a green light to implant edited human embryos. Instead, the organization calls for several requirements to be met, including “broad societal consensus” on the need for this type of intervention. While it is not clear whether or how consensus could be achieved, it is clear that scientists, clinicians, and patients will need help from the rest of society for this research to have an impact clinically.
Dr. Saha is assistant professor of biomedical engineering at the Wisconsin Institute for Discovery at the University of Wisconsin, Madison. His lab works on gene editing of human cells. He has patent filings through the Wisconsin Alumni Research Foundation on gene editing inventions.
C-section raises hysterectomy complication risk later
Women who have at least one cesarean delivery have a more than 30% risk of a complication requiring reoperation after benign hysterectomy later in life, compared with women who have had vaginal deliveries only, according to a study of more than 7,600 women in a Danish patient registry.
Cesarean delivery is the most common major surgery performed in the world, and the rate is rapidly increasing, with the global average cesarean rate estimated at 18.6%, and rates as high as 52% in some European countries. However, the impact cesarean deliveries have on surgical complications later in life has not been thoroughly studied. The study authors said this might be the first population study of the association of cesarean delivery with hysterectomy complications.
Of the 388 women (5%) who had a hysterectomy and then a reoperation within 30 days, the risk increased with the number of previous cesarean deliveries. Those who had vaginal-only deliveries had reoperation rates of 4.4%, compared with 6.2% for those who had one cesarean delivery and 6.8% for those who had two or more. That represents increased risks of 31% and 35% for women who had one cesarean delivery and two or more cesarean deliveries, respectively, compared with women who had only vaginal deliveries.
Likewise, surgical complications were 16% more frequent in women who had one previous cesarean delivery and 30% more likely in women with two or more cesarean deliveries. Women who had two or more cesarean deliveries were almost twice as likely (odds ratio, 1.93) to receive a blood transfusion.
“Our results imply that information on long-term associations should be made more readily available to women, clinicians, and policymakers and suggest that decisions on cesarean delivery should take into account not only immediate maternal and neonatal influences, but also women’s health in the long term, including an increased risk of reoperation and complications associated with surgery later in life,” the researchers wrote. “The results support policies and clinical efforts to prevent cesarean deliveries that are not medically indicated.”
The study noted some limitations, including the observational design, which did not allow for elimination of all potential confounding factors.
The researchers reported having no relevant financial disclosures.
Women who have at least one cesarean delivery have a more than 30% risk of a complication requiring reoperation after benign hysterectomy later in life, compared with women who have had vaginal deliveries only, according to a study of more than 7,600 women in a Danish patient registry.
Cesarean delivery is the most common major surgery performed in the world, and the rate is rapidly increasing, with the global average cesarean rate estimated at 18.6%, and rates as high as 52% in some European countries. However, the impact cesarean deliveries have on surgical complications later in life has not been thoroughly studied. The study authors said this might be the first population study of the association of cesarean delivery with hysterectomy complications.
Of the 388 women (5%) who had a hysterectomy and then a reoperation within 30 days, the risk increased with the number of previous cesarean deliveries. Those who had vaginal-only deliveries had reoperation rates of 4.4%, compared with 6.2% for those who had one cesarean delivery and 6.8% for those who had two or more. That represents increased risks of 31% and 35% for women who had one cesarean delivery and two or more cesarean deliveries, respectively, compared with women who had only vaginal deliveries.
Likewise, surgical complications were 16% more frequent in women who had one previous cesarean delivery and 30% more likely in women with two or more cesarean deliveries. Women who had two or more cesarean deliveries were almost twice as likely (odds ratio, 1.93) to receive a blood transfusion.
“Our results imply that information on long-term associations should be made more readily available to women, clinicians, and policymakers and suggest that decisions on cesarean delivery should take into account not only immediate maternal and neonatal influences, but also women’s health in the long term, including an increased risk of reoperation and complications associated with surgery later in life,” the researchers wrote. “The results support policies and clinical efforts to prevent cesarean deliveries that are not medically indicated.”
The study noted some limitations, including the observational design, which did not allow for elimination of all potential confounding factors.
The researchers reported having no relevant financial disclosures.
Women who have at least one cesarean delivery have a more than 30% risk of a complication requiring reoperation after benign hysterectomy later in life, compared with women who have had vaginal deliveries only, according to a study of more than 7,600 women in a Danish patient registry.
Cesarean delivery is the most common major surgery performed in the world, and the rate is rapidly increasing, with the global average cesarean rate estimated at 18.6%, and rates as high as 52% in some European countries. However, the impact cesarean deliveries have on surgical complications later in life has not been thoroughly studied. The study authors said this might be the first population study of the association of cesarean delivery with hysterectomy complications.
Of the 388 women (5%) who had a hysterectomy and then a reoperation within 30 days, the risk increased with the number of previous cesarean deliveries. Those who had vaginal-only deliveries had reoperation rates of 4.4%, compared with 6.2% for those who had one cesarean delivery and 6.8% for those who had two or more. That represents increased risks of 31% and 35% for women who had one cesarean delivery and two or more cesarean deliveries, respectively, compared with women who had only vaginal deliveries.
Likewise, surgical complications were 16% more frequent in women who had one previous cesarean delivery and 30% more likely in women with two or more cesarean deliveries. Women who had two or more cesarean deliveries were almost twice as likely (odds ratio, 1.93) to receive a blood transfusion.
“Our results imply that information on long-term associations should be made more readily available to women, clinicians, and policymakers and suggest that decisions on cesarean delivery should take into account not only immediate maternal and neonatal influences, but also women’s health in the long term, including an increased risk of reoperation and complications associated with surgery later in life,” the researchers wrote. “The results support policies and clinical efforts to prevent cesarean deliveries that are not medically indicated.”
The study noted some limitations, including the observational design, which did not allow for elimination of all potential confounding factors.
The researchers reported having no relevant financial disclosures.
FROM JAMA SURGERY
Key clinical point:
Major finding: The rate of complications after hysterectomy was 4.4% for women who had vaginal birth only, 6.2% for those who had one cesarean delivery, and 6.8% for those who had two or more cesarean deliveries.
Data source: Danish National Patient Registry–based cohort study of 7,685 women who gave birth from 1993 to 2012.
Disclosures: The researchers reported having no financial disclosures.
Researchers identify ‘congenital NAD deficiency disorders’
Mutations that disrupt de novo synthesis of nicotinamide adenine dinucleotide (NAD) were associated with multiple congenital malformations in humans and mice, and supplementing niacin during gestation prevented these malformations in mice, new research suggests.
The malformations include vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities (VACTERL), “a nonrandom combination of congenital defects without a known cause,” wrote Hongjun Shi, PhD, of Victor Chang Cardiac Research Institute, New South Wales, Australia, and colleagues (N Engl J Med. 2017;377:544-52).
Numerous genetic and environmental factors can potentially cause NAD deficiency during gestation and the investigators suggested collectively referring to the resulting malformations as “congenital NAD deficiency disorders.”
Congenital defects can occur together in newborns more often than would be expected by chance, but “in many such cases, it has proved difficult to identify a genetic cause,” the investigators noted. Using genomic sequencing, they looked for possible pathogenic gene variants within four unrelated families in which a person was born with multiple congenital malformations. Next, they evaluated the function of the variants by testing in vitro enzyme activity and measuring relevant plasma metabolites. Finally, they used the CRISPR (clustered regularly interspaced short palindromic repeats)–Cas9 system to create mouse models with similar variants.
This approach identified variants in two genes encoding enzymes of the kynurenine pathway: 3-hydroxyanthranilic acid 3,4-dioxygenase (HAAO) and kynureninase (KYNU). Three patients had homozygous variants associated with loss-of-function changes in these proteins. A fourth patient had heterozygous variants in the gene encoding KYNU.
“The mutant enzymes had greatly reduced activity in vitro,” the researchers wrote. Patients had decreased circulating levels of NAD, which tryptophan synthesizes through the kynurenine pathway. Notably, mouse embryos lacking the mouse equivalents of HAAO or KYNU also had congenital defects associated with NAD deficiency. Preventing NAD deficiency during gestation averted these defects in mice.
“The NAD de novo synthesis pathway catabolizes tryptophan,” the researchers added. “Although metabolite levels upstream of the block are elevated, and the metabolites have postnatal functions, we found that it is the deficiency in embryonic NAD, downstream of the block, that is disrupting embryogenesis.”
The study was supported by the Australian and New South Wales governments and foundations. The investigators reported having no other financial disclosures.
Shi et al. report that a deficiency of nicotinamide adenine dinucleotide (NAD) causes congenital malformations, suggesting that interventions to raise NAD levels during fetal and early postnatal development might further reduce the incidence of congenital anomalies.
Regardless of how NAD depletion leads to congenital malformations (whether by compromising the detection of DNA damage by PARP proteins, reducing the supply of nucleotides, or both), dietary supplementation with NAD precursors merits further study. At high doses, niacin can cause flushing and gastrointestinal symptoms, but it has few side effects at lower doses.
Nicotinamide mononucleotide, nicotinamide riboside, and nicotinamide itself are better tolerated than niacin and are generally considered to be safe as dietary supplements, but the doses of NAD precursors required to reduce the risk of congenital malformations in humans are not known. Also unknown is the extent to which raising dietary levels of NAD would limit cognitive impairment in infants with congenital malformations.
Matthew G. Vander Heiden, MD, PhD, is with the Massachusetts Institute of Technology, Cambridge, Mass., and the Dana Farber Cancer Center, Boston. He reported receiving personal fees from Agios Pharmaceuticals and Aeglea Biotherapeutics outside the submitted work. These comments are adapted from an editorial (N Engl J Med. 2007;377:509-11).
Shi et al. report that a deficiency of nicotinamide adenine dinucleotide (NAD) causes congenital malformations, suggesting that interventions to raise NAD levels during fetal and early postnatal development might further reduce the incidence of congenital anomalies.
Regardless of how NAD depletion leads to congenital malformations (whether by compromising the detection of DNA damage by PARP proteins, reducing the supply of nucleotides, or both), dietary supplementation with NAD precursors merits further study. At high doses, niacin can cause flushing and gastrointestinal symptoms, but it has few side effects at lower doses.
Nicotinamide mononucleotide, nicotinamide riboside, and nicotinamide itself are better tolerated than niacin and are generally considered to be safe as dietary supplements, but the doses of NAD precursors required to reduce the risk of congenital malformations in humans are not known. Also unknown is the extent to which raising dietary levels of NAD would limit cognitive impairment in infants with congenital malformations.
Matthew G. Vander Heiden, MD, PhD, is with the Massachusetts Institute of Technology, Cambridge, Mass., and the Dana Farber Cancer Center, Boston. He reported receiving personal fees from Agios Pharmaceuticals and Aeglea Biotherapeutics outside the submitted work. These comments are adapted from an editorial (N Engl J Med. 2007;377:509-11).
Shi et al. report that a deficiency of nicotinamide adenine dinucleotide (NAD) causes congenital malformations, suggesting that interventions to raise NAD levels during fetal and early postnatal development might further reduce the incidence of congenital anomalies.
Regardless of how NAD depletion leads to congenital malformations (whether by compromising the detection of DNA damage by PARP proteins, reducing the supply of nucleotides, or both), dietary supplementation with NAD precursors merits further study. At high doses, niacin can cause flushing and gastrointestinal symptoms, but it has few side effects at lower doses.
Nicotinamide mononucleotide, nicotinamide riboside, and nicotinamide itself are better tolerated than niacin and are generally considered to be safe as dietary supplements, but the doses of NAD precursors required to reduce the risk of congenital malformations in humans are not known. Also unknown is the extent to which raising dietary levels of NAD would limit cognitive impairment in infants with congenital malformations.
Matthew G. Vander Heiden, MD, PhD, is with the Massachusetts Institute of Technology, Cambridge, Mass., and the Dana Farber Cancer Center, Boston. He reported receiving personal fees from Agios Pharmaceuticals and Aeglea Biotherapeutics outside the submitted work. These comments are adapted from an editorial (N Engl J Med. 2007;377:509-11).
Mutations that disrupt de novo synthesis of nicotinamide adenine dinucleotide (NAD) were associated with multiple congenital malformations in humans and mice, and supplementing niacin during gestation prevented these malformations in mice, new research suggests.
The malformations include vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities (VACTERL), “a nonrandom combination of congenital defects without a known cause,” wrote Hongjun Shi, PhD, of Victor Chang Cardiac Research Institute, New South Wales, Australia, and colleagues (N Engl J Med. 2017;377:544-52).
Numerous genetic and environmental factors can potentially cause NAD deficiency during gestation and the investigators suggested collectively referring to the resulting malformations as “congenital NAD deficiency disorders.”
Congenital defects can occur together in newborns more often than would be expected by chance, but “in many such cases, it has proved difficult to identify a genetic cause,” the investigators noted. Using genomic sequencing, they looked for possible pathogenic gene variants within four unrelated families in which a person was born with multiple congenital malformations. Next, they evaluated the function of the variants by testing in vitro enzyme activity and measuring relevant plasma metabolites. Finally, they used the CRISPR (clustered regularly interspaced short palindromic repeats)–Cas9 system to create mouse models with similar variants.
This approach identified variants in two genes encoding enzymes of the kynurenine pathway: 3-hydroxyanthranilic acid 3,4-dioxygenase (HAAO) and kynureninase (KYNU). Three patients had homozygous variants associated with loss-of-function changes in these proteins. A fourth patient had heterozygous variants in the gene encoding KYNU.
“The mutant enzymes had greatly reduced activity in vitro,” the researchers wrote. Patients had decreased circulating levels of NAD, which tryptophan synthesizes through the kynurenine pathway. Notably, mouse embryos lacking the mouse equivalents of HAAO or KYNU also had congenital defects associated with NAD deficiency. Preventing NAD deficiency during gestation averted these defects in mice.
“The NAD de novo synthesis pathway catabolizes tryptophan,” the researchers added. “Although metabolite levels upstream of the block are elevated, and the metabolites have postnatal functions, we found that it is the deficiency in embryonic NAD, downstream of the block, that is disrupting embryogenesis.”
The study was supported by the Australian and New South Wales governments and foundations. The investigators reported having no other financial disclosures.
Mutations that disrupt de novo synthesis of nicotinamide adenine dinucleotide (NAD) were associated with multiple congenital malformations in humans and mice, and supplementing niacin during gestation prevented these malformations in mice, new research suggests.
The malformations include vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities (VACTERL), “a nonrandom combination of congenital defects without a known cause,” wrote Hongjun Shi, PhD, of Victor Chang Cardiac Research Institute, New South Wales, Australia, and colleagues (N Engl J Med. 2017;377:544-52).
Numerous genetic and environmental factors can potentially cause NAD deficiency during gestation and the investigators suggested collectively referring to the resulting malformations as “congenital NAD deficiency disorders.”
Congenital defects can occur together in newborns more often than would be expected by chance, but “in many such cases, it has proved difficult to identify a genetic cause,” the investigators noted. Using genomic sequencing, they looked for possible pathogenic gene variants within four unrelated families in which a person was born with multiple congenital malformations. Next, they evaluated the function of the variants by testing in vitro enzyme activity and measuring relevant plasma metabolites. Finally, they used the CRISPR (clustered regularly interspaced short palindromic repeats)–Cas9 system to create mouse models with similar variants.
This approach identified variants in two genes encoding enzymes of the kynurenine pathway: 3-hydroxyanthranilic acid 3,4-dioxygenase (HAAO) and kynureninase (KYNU). Three patients had homozygous variants associated with loss-of-function changes in these proteins. A fourth patient had heterozygous variants in the gene encoding KYNU.
“The mutant enzymes had greatly reduced activity in vitro,” the researchers wrote. Patients had decreased circulating levels of NAD, which tryptophan synthesizes through the kynurenine pathway. Notably, mouse embryos lacking the mouse equivalents of HAAO or KYNU also had congenital defects associated with NAD deficiency. Preventing NAD deficiency during gestation averted these defects in mice.
“The NAD de novo synthesis pathway catabolizes tryptophan,” the researchers added. “Although metabolite levels upstream of the block are elevated, and the metabolites have postnatal functions, we found that it is the deficiency in embryonic NAD, downstream of the block, that is disrupting embryogenesis.”
The study was supported by the Australian and New South Wales governments and foundations. The investigators reported having no other financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Major congenital defects affecting unrelated families were associated with variants in genes encoding 3-hydroxyanthranilic acid 3,4-dioxygenase (HAAO) and kynureninase (KYNU).
Data source: Genomic sequencing of four unrelated families in which a person was born with multiple congenital malformations, plus in vitro measurements of enzyme activity and plasma metabolites and studies of mouse models created with the CRISPR–Cas9 system.
Disclosures: The study was supported by the Australian and New South Wales governments and foundations. The investigators reported having no other financial disclosures.
Prenatal ART regimen with lowest risk is TDF-FTC-EFV
The antiretroviral therapy (ART) regimen associated with the least risk of adverse birth outcomes among pregnant women with HIV, relative to other regimens, is tenofovir disoproxil fumarate (TDF), emtricitabine (FTC), and efavirenz (EFV), according to a new study.
“Our results provide reassurance for the more than 90% of HIV-infected women who live in countries that follow WHO recommendations to use TDF-FTC-EFV,” wrote Rebecca Zash, MD, of Beth Israel Deaconess Medical Center in Boston and her associates in JAMA Pediatrics.
Using data collected from August 2014 through August 2016, the researchers compared outcomes among 47,027 births by women from Botswana, average age 26 years, who reached at least 24 weeks’ gestation. The study’s data came from eight government hospitals throughout Botswana, where approximately 45% of births had occurred nationwide.
The 11,932 infants exposed to HIV, representing about a quarter of all infants in the study, had a higher risk of adverse birth outcomes: 39.6% of HIV-exposed infants had adverse outcomes, compared with 28.9% of unexposed infants.
Nearly half (48.4%) of the HIV-exposed infants had also been exposed to ART from conception. Among these 5,780 infants, those exposed to the ART regimen comprising tenofovir disoproxil fumarate (TDF), emtricitabine (FTC), and efavirenz (EFV) had the lowest rate of adverse birth outcomes. The following percentages of infants exposed to different ART regimens had adverse outcomes:
- 36.4% of infants exposed to TDF-FTC-EFV.
- 41.7% of infants exposed to TDF-FTC and nevirapine (NVP).
- 44.9% of infants exposed to zidovudine (ZDV), lamivudine (3TC), and lopinavir-ritonavir (LPV-R).
- 47.4% of infants exposed to ZDV-3TC-NVP.
- 48.5% of infants exposed to TDF-FTC-LPV-R.
The risk of adverse birth outcomes, compared with exposure to TDF-FTC-EFV, was 15% higher for TDF-FTC-NVP, 21% higher for ZDV-3TC-LPV-R, 30% higher for ZDV-3TC-NVP, and 31% higher for TDF-FTC-LPV-R after researchers adjusted for age and potential sociodemographic confounders.
The risk of severe adverse outcomes for ART exposure from conception was as follows:
- 12.3% for exposure to TDF-FTC-EFV.
- 17.9% for exposure to TDF-FTC-NVP.
- 19.5% for TDF-FTC–LPV-R.
- 20.7% for ZDV-3TC-NVP.
- 23.4% for ZDV-3TC–LPV-R.
The risk for giving birth to an infant small for gestational age was lowest for TDF-FTC-EFV, compared with the other regimens.
“Differences between TDF-FTC-EFV and other ART regimens were greater for small for gestational age than for preterm birth,” suggesting a “drug-specific mechanism at the placental level because the health of the placenta is directly related to fetal growth,” the researchers wrote. “An ART effect at the level of the placenta may also explain why women receiving ART before conception have more adverse outcomes than [do] those who start ART after conception because endothelial dysfunction during placentation would be expected to have a more detrimental effect on the pregnancy,” they added.
The ZDV-3TC-NVP regimen was linked to greater risk for stillbirth, very preterm birth, and neonatal death; the ZDV-3TC-LPV-R regimen was linked to a greater risk of preterm and very preterm birth, as well as neonatal death.
“Our study findings may be difficult to integrate into settings with ART regimen choices beyond those available in Botswana,” the authors wrote. “Whether the magnitude of the differences we found in Botswana will be similar in higher-resource settings is unclear.”
The research was funded by the National Institutes of Health. The authors reported no conflicts of interest.
The antiretroviral therapy (ART) regimen associated with the least risk of adverse birth outcomes among pregnant women with HIV, relative to other regimens, is tenofovir disoproxil fumarate (TDF), emtricitabine (FTC), and efavirenz (EFV), according to a new study.
“Our results provide reassurance for the more than 90% of HIV-infected women who live in countries that follow WHO recommendations to use TDF-FTC-EFV,” wrote Rebecca Zash, MD, of Beth Israel Deaconess Medical Center in Boston and her associates in JAMA Pediatrics.
Using data collected from August 2014 through August 2016, the researchers compared outcomes among 47,027 births by women from Botswana, average age 26 years, who reached at least 24 weeks’ gestation. The study’s data came from eight government hospitals throughout Botswana, where approximately 45% of births had occurred nationwide.
The 11,932 infants exposed to HIV, representing about a quarter of all infants in the study, had a higher risk of adverse birth outcomes: 39.6% of HIV-exposed infants had adverse outcomes, compared with 28.9% of unexposed infants.
Nearly half (48.4%) of the HIV-exposed infants had also been exposed to ART from conception. Among these 5,780 infants, those exposed to the ART regimen comprising tenofovir disoproxil fumarate (TDF), emtricitabine (FTC), and efavirenz (EFV) had the lowest rate of adverse birth outcomes. The following percentages of infants exposed to different ART regimens had adverse outcomes:
- 36.4% of infants exposed to TDF-FTC-EFV.
- 41.7% of infants exposed to TDF-FTC and nevirapine (NVP).
- 44.9% of infants exposed to zidovudine (ZDV), lamivudine (3TC), and lopinavir-ritonavir (LPV-R).
- 47.4% of infants exposed to ZDV-3TC-NVP.
- 48.5% of infants exposed to TDF-FTC-LPV-R.
The risk of adverse birth outcomes, compared with exposure to TDF-FTC-EFV, was 15% higher for TDF-FTC-NVP, 21% higher for ZDV-3TC-LPV-R, 30% higher for ZDV-3TC-NVP, and 31% higher for TDF-FTC-LPV-R after researchers adjusted for age and potential sociodemographic confounders.
The risk of severe adverse outcomes for ART exposure from conception was as follows:
- 12.3% for exposure to TDF-FTC-EFV.
- 17.9% for exposure to TDF-FTC-NVP.
- 19.5% for TDF-FTC–LPV-R.
- 20.7% for ZDV-3TC-NVP.
- 23.4% for ZDV-3TC–LPV-R.
The risk for giving birth to an infant small for gestational age was lowest for TDF-FTC-EFV, compared with the other regimens.
“Differences between TDF-FTC-EFV and other ART regimens were greater for small for gestational age than for preterm birth,” suggesting a “drug-specific mechanism at the placental level because the health of the placenta is directly related to fetal growth,” the researchers wrote. “An ART effect at the level of the placenta may also explain why women receiving ART before conception have more adverse outcomes than [do] those who start ART after conception because endothelial dysfunction during placentation would be expected to have a more detrimental effect on the pregnancy,” they added.
The ZDV-3TC-NVP regimen was linked to greater risk for stillbirth, very preterm birth, and neonatal death; the ZDV-3TC-LPV-R regimen was linked to a greater risk of preterm and very preterm birth, as well as neonatal death.
“Our study findings may be difficult to integrate into settings with ART regimen choices beyond those available in Botswana,” the authors wrote. “Whether the magnitude of the differences we found in Botswana will be similar in higher-resource settings is unclear.”
The research was funded by the National Institutes of Health. The authors reported no conflicts of interest.
The antiretroviral therapy (ART) regimen associated with the least risk of adverse birth outcomes among pregnant women with HIV, relative to other regimens, is tenofovir disoproxil fumarate (TDF), emtricitabine (FTC), and efavirenz (EFV), according to a new study.
“Our results provide reassurance for the more than 90% of HIV-infected women who live in countries that follow WHO recommendations to use TDF-FTC-EFV,” wrote Rebecca Zash, MD, of Beth Israel Deaconess Medical Center in Boston and her associates in JAMA Pediatrics.
Using data collected from August 2014 through August 2016, the researchers compared outcomes among 47,027 births by women from Botswana, average age 26 years, who reached at least 24 weeks’ gestation. The study’s data came from eight government hospitals throughout Botswana, where approximately 45% of births had occurred nationwide.
The 11,932 infants exposed to HIV, representing about a quarter of all infants in the study, had a higher risk of adverse birth outcomes: 39.6% of HIV-exposed infants had adverse outcomes, compared with 28.9% of unexposed infants.
Nearly half (48.4%) of the HIV-exposed infants had also been exposed to ART from conception. Among these 5,780 infants, those exposed to the ART regimen comprising tenofovir disoproxil fumarate (TDF), emtricitabine (FTC), and efavirenz (EFV) had the lowest rate of adverse birth outcomes. The following percentages of infants exposed to different ART regimens had adverse outcomes:
- 36.4% of infants exposed to TDF-FTC-EFV.
- 41.7% of infants exposed to TDF-FTC and nevirapine (NVP).
- 44.9% of infants exposed to zidovudine (ZDV), lamivudine (3TC), and lopinavir-ritonavir (LPV-R).
- 47.4% of infants exposed to ZDV-3TC-NVP.
- 48.5% of infants exposed to TDF-FTC-LPV-R.
The risk of adverse birth outcomes, compared with exposure to TDF-FTC-EFV, was 15% higher for TDF-FTC-NVP, 21% higher for ZDV-3TC-LPV-R, 30% higher for ZDV-3TC-NVP, and 31% higher for TDF-FTC-LPV-R after researchers adjusted for age and potential sociodemographic confounders.
The risk of severe adverse outcomes for ART exposure from conception was as follows:
- 12.3% for exposure to TDF-FTC-EFV.
- 17.9% for exposure to TDF-FTC-NVP.
- 19.5% for TDF-FTC–LPV-R.
- 20.7% for ZDV-3TC-NVP.
- 23.4% for ZDV-3TC–LPV-R.
The risk for giving birth to an infant small for gestational age was lowest for TDF-FTC-EFV, compared with the other regimens.
“Differences between TDF-FTC-EFV and other ART regimens were greater for small for gestational age than for preterm birth,” suggesting a “drug-specific mechanism at the placental level because the health of the placenta is directly related to fetal growth,” the researchers wrote. “An ART effect at the level of the placenta may also explain why women receiving ART before conception have more adverse outcomes than [do] those who start ART after conception because endothelial dysfunction during placentation would be expected to have a more detrimental effect on the pregnancy,” they added.
The ZDV-3TC-NVP regimen was linked to greater risk for stillbirth, very preterm birth, and neonatal death; the ZDV-3TC-LPV-R regimen was linked to a greater risk of preterm and very preterm birth, as well as neonatal death.
“Our study findings may be difficult to integrate into settings with ART regimen choices beyond those available in Botswana,” the authors wrote. “Whether the magnitude of the differences we found in Botswana will be similar in higher-resource settings is unclear.”
The research was funded by the National Institutes of Health. The authors reported no conflicts of interest.
FROM JAMA PEDIATRICS
Key clinical point: Different antiretroviral regimens pose different adverse birth outcome risks for pregnant women with HIV.
Major finding: The ART regimen with the lowest level of risk was tenofovir, emtricitabine, and efavirenz, with a 36.5% risk for adverse outcomes and 12.3% risk for serious adverse outcomes.
Data source: The findings are based on an observational study of 47,027 births to women at eight government hospitals in Botswana from 2014 to 2016.
Disclosures: The research was funded by the National Institutes of Health. The authors reported no conflicts of interest.
Recurrent UTIs in Women: How to Refine Your Care

For the third time in nine months, Joan, 28, presents with complaints of painful, frequent, and urgent urination. Joan is sexually active; her medical history is otherwise unremarkable. In each of the previous two episodes, her urine culture grew Escherichia coli, and she was treated with a five-day course of nitrofurantoin. Now, she asks about the need for additional workup and treatment, as well as whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women and account for an estimated 5.4 million primary care office visits and 2.3 million emergency department visits annually.1,2 For women, the lifetime risk for a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women younger than 55 and 53% of women older than 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 100,000 colony-forming units (ie, viable bacteria) per milliliter of urine collected midstream on two consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant or who have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI; the term applies when such an infection occurs within six months of the previous UTI or when three or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within two weeks of the original treatment.9 Reinfection is a UTI that occurs more than two weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following seven to 14 days of appropriate antibiotic treatment.9
FACTORS THAT INCREASE RISK FOR RECURRENT UTI
Premenopausal women
Both modifiable and nonmodifiable factors (see Table 1) have been associated with increased risk for recurrent UTI in premenopausal women.10-21 Among those with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other nonmodifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are those whose mothers had no such history.13
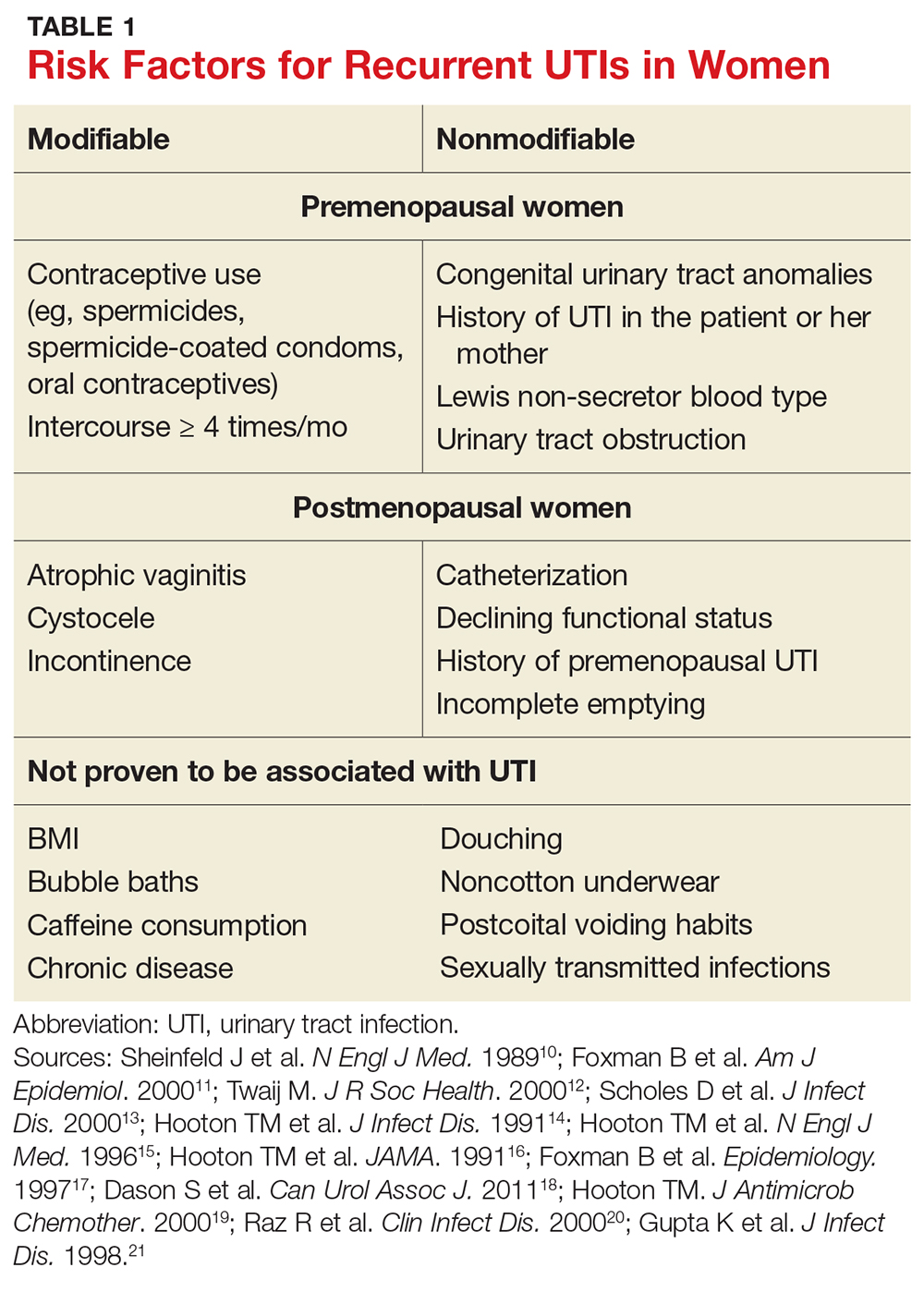
Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥ 4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse, sexually active college women who had engaged in intercourse three times in a week had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk for UTI.15This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women, which is thought to predispose them to UTI.21 These risk factors are summarized in Table 1.10-21
INITIAL EVALUATION OF RECURRENT UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (see Table 2).18 Likewise, perform a pelvic exam to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

TREATMENT OPTIONS AND PRECAUTIONS
As with isolated UTI, E coli is the most common pathogen in recurrent UTI. However, recurrent UTI is more likely than isolated UTI to result from other pathogens (odds ratio [OR], 1.5), such as Klebsiella, Enterococcus, Proteus, and Citrobacter.23 Since a patient’s recurrent UTI most likely arises from the same pathogen that caused the prior infection, start an antibiotic you know is effective against it.8 Additionally, take into account local resistance rates; antibiotic availability, cost, and adverse effects; and a patient’s drug allergies.
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX; 160 mg/800 mg bid for 3 d) has long been the mainstay of treatment for uncomplicated UTI. In recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as firstline treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin (100 mg bid for 5 d) has efficacy similar to that of TMP-SMX but without significant bacterial resistance. While fosfomycin (3 g as a single dose) is still recommended as firstline treatment, it is less effective than either TMP-SMX or nitrofurantoin. Table 3 summarizes these antibiotic choices and their efficacies.24
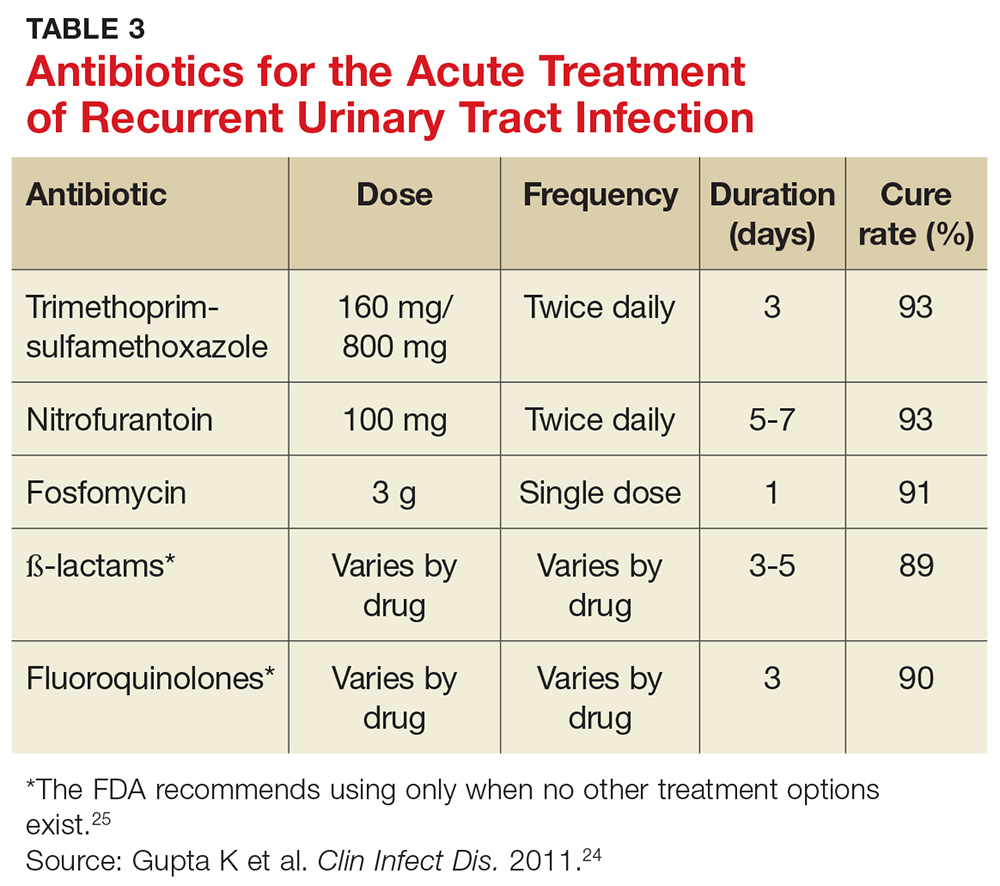
Agents to avoid or use only as a last resort. For patients who are unable to take any of the mentioned drugs, consider ß-lactam antibiotics—although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the FDA issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25 Do not use ampicillin or amoxicillin, which lack effectiveness for this indication and are compromised by high levels of bacterial resistance.
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 d) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 d). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for those who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (see Table 3).24 Patient-initiated treatment yields similar rates of efficacy as clinician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies (see text).28
TIME FOR IMAGING AND REFERRAL?
For patients with a high risk for complicated UTI or a surgically amenable condition, either ultrasound or CT of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in Table 2.18
Two weeks later … and it’s back? Finally, for women who experience recurrent symptoms within two weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
PREVENTION DOS AND DON’TS
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or those who douche, consume caffeinated beverages, or wear noncotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Clinicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had two or more UTIs in the past six months or three or more UTIs in the past year. 29,30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or postcoital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to two weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin (see Table 4), none of which demonstrated superiority in a Cochrane review.31-33 Although the same review showed no optimal duration of treatment, six to 24 months of treatment is usually recommended.29,33
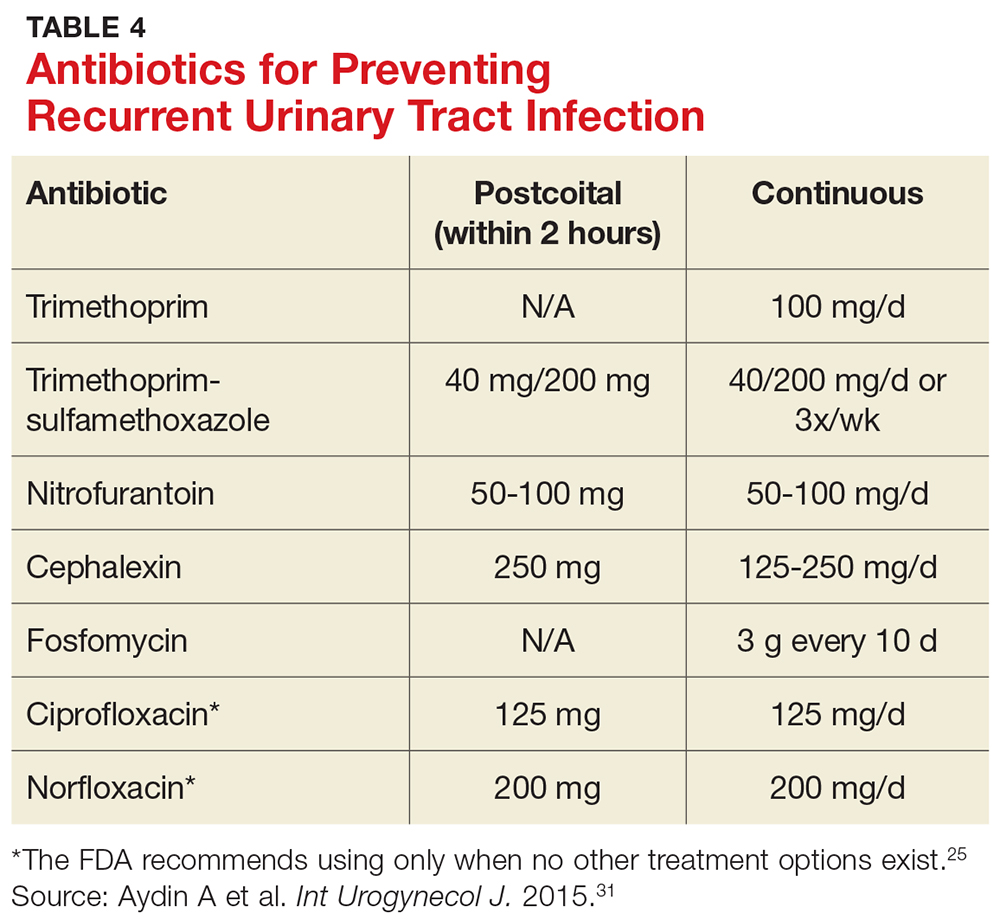
A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about the FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence (see Table 4).31,35,36
Several nonpharmacologic strategies have been suggested for prevention of recurrent UTI. Among them are use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a noninferiority trial, and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolyzed to bactericidal ammonia and formaldehyde), and
As noted, the only behavioral modifications that have been shown to decrease the risk for recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
Joan is started on a three-day course of TMP-SMX. Further questioning reveals that each of her three UTIs followed sexual intercourse. Her clinician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision-making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. CDC. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed June 8, 2017.
3. Griebling TL. Urologic Diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991; 164: 1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000; 30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. FDA. FDA drug safety communication. www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed June 8, 2017.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988; 9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157: 935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trial. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012; (10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Krancˇec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014;32:79-84.

For the third time in nine months, Joan, 28, presents with complaints of painful, frequent, and urgent urination. Joan is sexually active; her medical history is otherwise unremarkable. In each of the previous two episodes, her urine culture grew Escherichia coli, and she was treated with a five-day course of nitrofurantoin. Now, she asks about the need for additional workup and treatment, as well as whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women and account for an estimated 5.4 million primary care office visits and 2.3 million emergency department visits annually.1,2 For women, the lifetime risk for a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women younger than 55 and 53% of women older than 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 100,000 colony-forming units (ie, viable bacteria) per milliliter of urine collected midstream on two consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant or who have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI; the term applies when such an infection occurs within six months of the previous UTI or when three or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within two weeks of the original treatment.9 Reinfection is a UTI that occurs more than two weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following seven to 14 days of appropriate antibiotic treatment.9

FACTORS THAT INCREASE RISK FOR RECURRENT UTI
Premenopausal women
Both modifiable and nonmodifiable factors (see Table 1) have been associated with increased risk for recurrent UTI in premenopausal women.10-21 Among those with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other nonmodifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are those whose mothers had no such history.13

Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥ 4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse, sexually active college women who had engaged in intercourse three times in a week had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk for UTI.15This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women, which is thought to predispose them to UTI.21 These risk factors are summarized in Table 1.10-21
INITIAL EVALUATION OF RECURRENT UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (see Table 2).18 Likewise, perform a pelvic exam to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

TREATMENT OPTIONS AND PRECAUTIONS
As with isolated UTI, E coli is the most common pathogen in recurrent UTI. However, recurrent UTI is more likely than isolated UTI to result from other pathogens (odds ratio [OR], 1.5), such as Klebsiella, Enterococcus, Proteus, and Citrobacter.23 Since a patient’s recurrent UTI most likely arises from the same pathogen that caused the prior infection, start an antibiotic you know is effective against it.8 Additionally, take into account local resistance rates; antibiotic availability, cost, and adverse effects; and a patient’s drug allergies.
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX; 160 mg/800 mg bid for 3 d) has long been the mainstay of treatment for uncomplicated UTI. In recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as firstline treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin (100 mg bid for 5 d) has efficacy similar to that of TMP-SMX but without significant bacterial resistance. While fosfomycin (3 g as a single dose) is still recommended as firstline treatment, it is less effective than either TMP-SMX or nitrofurantoin. Table 3 summarizes these antibiotic choices and their efficacies.24

Agents to avoid or use only as a last resort. For patients who are unable to take any of the mentioned drugs, consider ß-lactam antibiotics—although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the FDA issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25 Do not use ampicillin or amoxicillin, which lack effectiveness for this indication and are compromised by high levels of bacterial resistance.
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 d) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 d). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for those who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (see Table 3).24 Patient-initiated treatment yields similar rates of efficacy as clinician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies (see text).28
TIME FOR IMAGING AND REFERRAL?
For patients with a high risk for complicated UTI or a surgically amenable condition, either ultrasound or CT of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in Table 2.18
Two weeks later … and it’s back? Finally, for women who experience recurrent symptoms within two weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
PREVENTION DOS AND DON’TS
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or those who douche, consume caffeinated beverages, or wear noncotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Clinicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had two or more UTIs in the past six months or three or more UTIs in the past year. 29,30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or postcoital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to two weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin (see Table 4), none of which demonstrated superiority in a Cochrane review.31-33 Although the same review showed no optimal duration of treatment, six to 24 months of treatment is usually recommended.29,33

A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about the FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence (see Table 4).31,35,36
Several nonpharmacologic strategies have been suggested for prevention of recurrent UTI. Among them are use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a noninferiority trial, and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolyzed to bactericidal ammonia and formaldehyde), and
As noted, the only behavioral modifications that have been shown to decrease the risk for recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
Joan is started on a three-day course of TMP-SMX. Further questioning reveals that each of her three UTIs followed sexual intercourse. Her clinician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision-making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.

For the third time in nine months, Joan, 28, presents with complaints of painful, frequent, and urgent urination. Joan is sexually active; her medical history is otherwise unremarkable. In each of the previous two episodes, her urine culture grew Escherichia coli, and she was treated with a five-day course of nitrofurantoin. Now, she asks about the need for additional workup and treatment, as well as whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women and account for an estimated 5.4 million primary care office visits and 2.3 million emergency department visits annually.1,2 For women, the lifetime risk for a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women younger than 55 and 53% of women older than 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 100,000 colony-forming units (ie, viable bacteria) per milliliter of urine collected midstream on two consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant or who have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI; the term applies when such an infection occurs within six months of the previous UTI or when three or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within two weeks of the original treatment.9 Reinfection is a UTI that occurs more than two weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following seven to 14 days of appropriate antibiotic treatment.9

FACTORS THAT INCREASE RISK FOR RECURRENT UTI
Premenopausal women
Both modifiable and nonmodifiable factors (see Table 1) have been associated with increased risk for recurrent UTI in premenopausal women.10-21 Among those with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other nonmodifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are those whose mothers had no such history.13

Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥ 4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse, sexually active college women who had engaged in intercourse three times in a week had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk for UTI.15This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women, which is thought to predispose them to UTI.21 These risk factors are summarized in Table 1.10-21
INITIAL EVALUATION OF RECURRENT UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (see Table 2).18 Likewise, perform a pelvic exam to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

TREATMENT OPTIONS AND PRECAUTIONS
As with isolated UTI, E coli is the most common pathogen in recurrent UTI. However, recurrent UTI is more likely than isolated UTI to result from other pathogens (odds ratio [OR], 1.5), such as Klebsiella, Enterococcus, Proteus, and Citrobacter.23 Since a patient’s recurrent UTI most likely arises from the same pathogen that caused the prior infection, start an antibiotic you know is effective against it.8 Additionally, take into account local resistance rates; antibiotic availability, cost, and adverse effects; and a patient’s drug allergies.
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX; 160 mg/800 mg bid for 3 d) has long been the mainstay of treatment for uncomplicated UTI. In recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as firstline treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin (100 mg bid for 5 d) has efficacy similar to that of TMP-SMX but without significant bacterial resistance. While fosfomycin (3 g as a single dose) is still recommended as firstline treatment, it is less effective than either TMP-SMX or nitrofurantoin. Table 3 summarizes these antibiotic choices and their efficacies.24

Agents to avoid or use only as a last resort. For patients who are unable to take any of the mentioned drugs, consider ß-lactam antibiotics—although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the FDA issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25 Do not use ampicillin or amoxicillin, which lack effectiveness for this indication and are compromised by high levels of bacterial resistance.
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 d) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 d). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for those who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (see Table 3).24 Patient-initiated treatment yields similar rates of efficacy as clinician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies (see text).28
TIME FOR IMAGING AND REFERRAL?
For patients with a high risk for complicated UTI or a surgically amenable condition, either ultrasound or CT of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in Table 2.18
Two weeks later … and it’s back? Finally, for women who experience recurrent symptoms within two weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
PREVENTION DOS AND DON’TS
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or those who douche, consume caffeinated beverages, or wear noncotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Clinicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had two or more UTIs in the past six months or three or more UTIs in the past year. 29,30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or postcoital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to two weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin (see Table 4), none of which demonstrated superiority in a Cochrane review.31-33 Although the same review showed no optimal duration of treatment, six to 24 months of treatment is usually recommended.29,33

A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about the FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence (see Table 4).31,35,36
Several nonpharmacologic strategies have been suggested for prevention of recurrent UTI. Among them are use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a noninferiority trial, and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolyzed to bactericidal ammonia and formaldehyde), and
As noted, the only behavioral modifications that have been shown to decrease the risk for recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
Joan is started on a three-day course of TMP-SMX. Further questioning reveals that each of her three UTIs followed sexual intercourse. Her clinician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision-making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. CDC. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed June 8, 2017.
3. Griebling TL. Urologic Diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991; 164: 1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000; 30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. FDA. FDA drug safety communication. www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed June 8, 2017.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988; 9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157: 935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trial. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012; (10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Krancˇec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014;32:79-84.
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. CDC. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed June 8, 2017.
3. Griebling TL. Urologic Diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991; 164: 1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000; 30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. FDA. FDA drug safety communication. www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed June 8, 2017.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988; 9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157: 935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trial. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012; (10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Krancˇec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014;32:79-84.
E-cigarettes: A health threat or cessation tool?
DENVER –
“So far, the evidence regarding e-cigarettes’ effectiveness for smoking cessation is equivocal at best,” Alison Breland, PhD, said at the annual meeting of the Teratology Society.
But Dr. Breland noted that there is significant controversy around this topic. “I can tell you that, at the conferences I go to, where there are lots of people studying nicotine and tobacco, scientists are fighting with each other over this question,” said Dr. Breland, a psychologist and project director at the Center for the Study of Tobacco Products at Virginia Commonwealth University in Richmond.
That being said, she noted that this meta-analysis has generated unusually harsh printed comments from its critics.
“We could argue about the methodology of the studies all day. If you think all the studies are garbage then you won’t believe the odds ratio, either. But I think right now the evidence shows that e-cigarettes don’t seem to help people quit,” she said. “That may change in the future with testing of different kinds of devices.”
To be useful for smoking cessation, she explained, a device would need to consistently deliver enough nicotine to enable the smoker to fend off withdrawal symptoms but not so much that the wish to quit evaporates. It’s a matter of finding the sweet spot in what is technically termed device nicotine flux.
There is a great deal of misconception about e-cigarettes, Dr. Breland said, some of it promoted through misleading product advertising. She sought to set the record straight.
How e-cigarettes work
What are e-cigarettes? They are basically nicotine delivery devices. They use electricity to power a heating element that aerosolizes a liquid containing varying concentrations of nicotine; solvents, such as propylene glycol and vegetable glycerins; and flavorants. As a class, e-cigarettes are rapidly evolving. A vast array of devices are marketed with wide differences in design, materials, construction, amount of nicotine delivered, and electrical power – which, along with puff duration, is a key factor in how much nicotine gets into a user’s blood.
“Most of the devices have a battery, but it’s important to know that some of them can be plugged directly into a USB port on a computer,” Dr. Breland said.
E-cigarettes don’t generate a vapor, as is widely believed. It’s an aerosol, and it contains toxic byproducts. On the plus side, unlike combustible cigarettes, e-cigarettes don’t deliver carbon monoxide.
A vast array of flavorant mixtures are sold, including some that are clearly designed to be attractive to children, with names like “blue cotton candy” and “Apple Jacks.”
User demographics
Who is using e-cigarettes? Primarily adolescents and young adults in prime reproductive age. National surveys indicate e-cigarettes are now the most widely used tobacco product among U.S. high school students, well ahead of combustible cigarettes.
Of particular concern, data from the Centers for Disease Control and Prevention’s National Health Interview Survey indicate that, among 18- to 24-year-olds who use e-cigarettes, about 40% also currently use conventional cigarettes, about 20% are former cigarette smokers, and about 40% are never smokers – that is, have never smoked combustible cigarettes (MMWR Morb Mortal Wkly Rep. 2016;65:1177. doi: 10.15585/mmwr.mm6542a7).
“We don’t know what’s going to happen to these never smokers who are currently using e-cigarettes. Are they starting on a lifetime of nicotine dependence via e-cigarettes, or perhaps even worse, are they going to transition to combustible cigarettes? There’s more and more evidence showing that’s happening,” Dr. Breland said.
The CDC survey also showed that 59% of adult users of e-cigarettes are what Dr. Breland called “dualies,” individuals who also smoke conventional cigarettes.
“That really diminishes any potential benefit of e-cigarettes,” she said.
Impact on pregnancy
What is known about the impact of e-cigarettes on pregnancy and birth outcomes? Almost nothing at this point. E-cigarettes deliver nicotine to the bloodstream, and nicotine is known to cause unwelcome, long-term changes in fetal brain development and in that of adolescents as well. The other aerosolized toxicants have not been well studied. A few small surveys conducted in obstetric practices indicate some pregnant women perceive e-cigarettes as posing only minor health risks and safer than combustible cigarettes. And some pregnant women are using e-cigarettes.
“I think it’s notable that we’re not finding exclusive e-cigarette users. It’s early in the study, but so far the dual users are smoking the same number of cigarettes per day as cigarette-only users, and they have the same expired carbon monoxide levels. It makes me feel concerned in particular about dual use in pregnancy,” she said.
Regulation
One audience member asked what the point of allowing e-cigarettes is since, under a best-case scenario, their effectiveness as a smoking cessation tool is similar to a nicotine patch, and smokers already have access to the patch as well as nicotine gum.
Dr. Breland replied that the patch and gum deliver nicotine very slowly, so they are not as satisfying as smoking.
“The hope with e-cigarettes is that, since they get nicotine into your blood pretty fast – similar to a cigarette – they can more effectively suppress your withdrawal,” she said. “Whether or not that’s true isn’t known yet.”
The Food and Drug Administration has the authority to regulate e-cigarettes through several different mechanisms but, in late July 2017, announced a delay in issuing new regulations that would likely have removed many of the devices and flavorings from the marketplace.
Dr. Breland’s research is supported by the National Institute on Drug Abuse and the Food and Drug Administration. She reported having no financial conflicts of interest.
DENVER –
“So far, the evidence regarding e-cigarettes’ effectiveness for smoking cessation is equivocal at best,” Alison Breland, PhD, said at the annual meeting of the Teratology Society.
But Dr. Breland noted that there is significant controversy around this topic. “I can tell you that, at the conferences I go to, where there are lots of people studying nicotine and tobacco, scientists are fighting with each other over this question,” said Dr. Breland, a psychologist and project director at the Center for the Study of Tobacco Products at Virginia Commonwealth University in Richmond.
That being said, she noted that this meta-analysis has generated unusually harsh printed comments from its critics.
“We could argue about the methodology of the studies all day. If you think all the studies are garbage then you won’t believe the odds ratio, either. But I think right now the evidence shows that e-cigarettes don’t seem to help people quit,” she said. “That may change in the future with testing of different kinds of devices.”
To be useful for smoking cessation, she explained, a device would need to consistently deliver enough nicotine to enable the smoker to fend off withdrawal symptoms but not so much that the wish to quit evaporates. It’s a matter of finding the sweet spot in what is technically termed device nicotine flux.
There is a great deal of misconception about e-cigarettes, Dr. Breland said, some of it promoted through misleading product advertising. She sought to set the record straight.
How e-cigarettes work
What are e-cigarettes? They are basically nicotine delivery devices. They use electricity to power a heating element that aerosolizes a liquid containing varying concentrations of nicotine; solvents, such as propylene glycol and vegetable glycerins; and flavorants. As a class, e-cigarettes are rapidly evolving. A vast array of devices are marketed with wide differences in design, materials, construction, amount of nicotine delivered, and electrical power – which, along with puff duration, is a key factor in how much nicotine gets into a user’s blood.
“Most of the devices have a battery, but it’s important to know that some of them can be plugged directly into a USB port on a computer,” Dr. Breland said.
E-cigarettes don’t generate a vapor, as is widely believed. It’s an aerosol, and it contains toxic byproducts. On the plus side, unlike combustible cigarettes, e-cigarettes don’t deliver carbon monoxide.
A vast array of flavorant mixtures are sold, including some that are clearly designed to be attractive to children, with names like “blue cotton candy” and “Apple Jacks.”
User demographics
Who is using e-cigarettes? Primarily adolescents and young adults in prime reproductive age. National surveys indicate e-cigarettes are now the most widely used tobacco product among U.S. high school students, well ahead of combustible cigarettes.
Of particular concern, data from the Centers for Disease Control and Prevention’s National Health Interview Survey indicate that, among 18- to 24-year-olds who use e-cigarettes, about 40% also currently use conventional cigarettes, about 20% are former cigarette smokers, and about 40% are never smokers – that is, have never smoked combustible cigarettes (MMWR Morb Mortal Wkly Rep. 2016;65:1177. doi: 10.15585/mmwr.mm6542a7).
“We don’t know what’s going to happen to these never smokers who are currently using e-cigarettes. Are they starting on a lifetime of nicotine dependence via e-cigarettes, or perhaps even worse, are they going to transition to combustible cigarettes? There’s more and more evidence showing that’s happening,” Dr. Breland said.
The CDC survey also showed that 59% of adult users of e-cigarettes are what Dr. Breland called “dualies,” individuals who also smoke conventional cigarettes.
“That really diminishes any potential benefit of e-cigarettes,” she said.
Impact on pregnancy
What is known about the impact of e-cigarettes on pregnancy and birth outcomes? Almost nothing at this point. E-cigarettes deliver nicotine to the bloodstream, and nicotine is known to cause unwelcome, long-term changes in fetal brain development and in that of adolescents as well. The other aerosolized toxicants have not been well studied. A few small surveys conducted in obstetric practices indicate some pregnant women perceive e-cigarettes as posing only minor health risks and safer than combustible cigarettes. And some pregnant women are using e-cigarettes.
“I think it’s notable that we’re not finding exclusive e-cigarette users. It’s early in the study, but so far the dual users are smoking the same number of cigarettes per day as cigarette-only users, and they have the same expired carbon monoxide levels. It makes me feel concerned in particular about dual use in pregnancy,” she said.
Regulation
One audience member asked what the point of allowing e-cigarettes is since, under a best-case scenario, their effectiveness as a smoking cessation tool is similar to a nicotine patch, and smokers already have access to the patch as well as nicotine gum.
Dr. Breland replied that the patch and gum deliver nicotine very slowly, so they are not as satisfying as smoking.
“The hope with e-cigarettes is that, since they get nicotine into your blood pretty fast – similar to a cigarette – they can more effectively suppress your withdrawal,” she said. “Whether or not that’s true isn’t known yet.”
The Food and Drug Administration has the authority to regulate e-cigarettes through several different mechanisms but, in late July 2017, announced a delay in issuing new regulations that would likely have removed many of the devices and flavorings from the marketplace.
Dr. Breland’s research is supported by the National Institute on Drug Abuse and the Food and Drug Administration. She reported having no financial conflicts of interest.
DENVER –
“So far, the evidence regarding e-cigarettes’ effectiveness for smoking cessation is equivocal at best,” Alison Breland, PhD, said at the annual meeting of the Teratology Society.
But Dr. Breland noted that there is significant controversy around this topic. “I can tell you that, at the conferences I go to, where there are lots of people studying nicotine and tobacco, scientists are fighting with each other over this question,” said Dr. Breland, a psychologist and project director at the Center for the Study of Tobacco Products at Virginia Commonwealth University in Richmond.
That being said, she noted that this meta-analysis has generated unusually harsh printed comments from its critics.
“We could argue about the methodology of the studies all day. If you think all the studies are garbage then you won’t believe the odds ratio, either. But I think right now the evidence shows that e-cigarettes don’t seem to help people quit,” she said. “That may change in the future with testing of different kinds of devices.”
To be useful for smoking cessation, she explained, a device would need to consistently deliver enough nicotine to enable the smoker to fend off withdrawal symptoms but not so much that the wish to quit evaporates. It’s a matter of finding the sweet spot in what is technically termed device nicotine flux.
There is a great deal of misconception about e-cigarettes, Dr. Breland said, some of it promoted through misleading product advertising. She sought to set the record straight.
How e-cigarettes work
What are e-cigarettes? They are basically nicotine delivery devices. They use electricity to power a heating element that aerosolizes a liquid containing varying concentrations of nicotine; solvents, such as propylene glycol and vegetable glycerins; and flavorants. As a class, e-cigarettes are rapidly evolving. A vast array of devices are marketed with wide differences in design, materials, construction, amount of nicotine delivered, and electrical power – which, along with puff duration, is a key factor in how much nicotine gets into a user’s blood.
“Most of the devices have a battery, but it’s important to know that some of them can be plugged directly into a USB port on a computer,” Dr. Breland said.
E-cigarettes don’t generate a vapor, as is widely believed. It’s an aerosol, and it contains toxic byproducts. On the plus side, unlike combustible cigarettes, e-cigarettes don’t deliver carbon monoxide.
A vast array of flavorant mixtures are sold, including some that are clearly designed to be attractive to children, with names like “blue cotton candy” and “Apple Jacks.”
User demographics
Who is using e-cigarettes? Primarily adolescents and young adults in prime reproductive age. National surveys indicate e-cigarettes are now the most widely used tobacco product among U.S. high school students, well ahead of combustible cigarettes.
Of particular concern, data from the Centers for Disease Control and Prevention’s National Health Interview Survey indicate that, among 18- to 24-year-olds who use e-cigarettes, about 40% also currently use conventional cigarettes, about 20% are former cigarette smokers, and about 40% are never smokers – that is, have never smoked combustible cigarettes (MMWR Morb Mortal Wkly Rep. 2016;65:1177. doi: 10.15585/mmwr.mm6542a7).
“We don’t know what’s going to happen to these never smokers who are currently using e-cigarettes. Are they starting on a lifetime of nicotine dependence via e-cigarettes, or perhaps even worse, are they going to transition to combustible cigarettes? There’s more and more evidence showing that’s happening,” Dr. Breland said.
The CDC survey also showed that 59% of adult users of e-cigarettes are what Dr. Breland called “dualies,” individuals who also smoke conventional cigarettes.
“That really diminishes any potential benefit of e-cigarettes,” she said.
Impact on pregnancy
What is known about the impact of e-cigarettes on pregnancy and birth outcomes? Almost nothing at this point. E-cigarettes deliver nicotine to the bloodstream, and nicotine is known to cause unwelcome, long-term changes in fetal brain development and in that of adolescents as well. The other aerosolized toxicants have not been well studied. A few small surveys conducted in obstetric practices indicate some pregnant women perceive e-cigarettes as posing only minor health risks and safer than combustible cigarettes. And some pregnant women are using e-cigarettes.
“I think it’s notable that we’re not finding exclusive e-cigarette users. It’s early in the study, but so far the dual users are smoking the same number of cigarettes per day as cigarette-only users, and they have the same expired carbon monoxide levels. It makes me feel concerned in particular about dual use in pregnancy,” she said.
Regulation
One audience member asked what the point of allowing e-cigarettes is since, under a best-case scenario, their effectiveness as a smoking cessation tool is similar to a nicotine patch, and smokers already have access to the patch as well as nicotine gum.
Dr. Breland replied that the patch and gum deliver nicotine very slowly, so they are not as satisfying as smoking.
“The hope with e-cigarettes is that, since they get nicotine into your blood pretty fast – similar to a cigarette – they can more effectively suppress your withdrawal,” she said. “Whether or not that’s true isn’t known yet.”
The Food and Drug Administration has the authority to regulate e-cigarettes through several different mechanisms but, in late July 2017, announced a delay in issuing new regulations that would likely have removed many of the devices and flavorings from the marketplace.
Dr. Breland’s research is supported by the National Institute on Drug Abuse and the Food and Drug Administration. She reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM TERATOLOGY SOCIETY 2017
Umbilical hernia repair during pregnancy safe, but often serious
Umbilical hernia repair during pregnancy is rare and safe, but more than half of surgeries required incarceration or strangulation repair, according to Dr. I.N. Haskins and associates.
A total of 126 pregnant women underwent umbilical hernia repair from 2005 to 2014, according to data collected from the American College of Surgeons National Surgical Quality Improvement Program. All but six women underwent open surgery, and of these 120 patients, 71 had umbilical hernia incarceration or strangulation at the time of surgery.
“Additional studies are needed to determine the long-term recurrence rate of umbilical hernia repairs performed in pregnant patients and the effects of surgical intervention and approach on the fetus,” the investigators concluded.
Find the study in Hernia (doi: 10.1007/s10029-017-1633-8).
Umbilical hernia repair during pregnancy is rare and safe, but more than half of surgeries required incarceration or strangulation repair, according to Dr. I.N. Haskins and associates.
A total of 126 pregnant women underwent umbilical hernia repair from 2005 to 2014, according to data collected from the American College of Surgeons National Surgical Quality Improvement Program. All but six women underwent open surgery, and of these 120 patients, 71 had umbilical hernia incarceration or strangulation at the time of surgery.
“Additional studies are needed to determine the long-term recurrence rate of umbilical hernia repairs performed in pregnant patients and the effects of surgical intervention and approach on the fetus,” the investigators concluded.
Find the study in Hernia (doi: 10.1007/s10029-017-1633-8).
Umbilical hernia repair during pregnancy is rare and safe, but more than half of surgeries required incarceration or strangulation repair, according to Dr. I.N. Haskins and associates.
A total of 126 pregnant women underwent umbilical hernia repair from 2005 to 2014, according to data collected from the American College of Surgeons National Surgical Quality Improvement Program. All but six women underwent open surgery, and of these 120 patients, 71 had umbilical hernia incarceration or strangulation at the time of surgery.
“Additional studies are needed to determine the long-term recurrence rate of umbilical hernia repairs performed in pregnant patients and the effects of surgical intervention and approach on the fetus,” the investigators concluded.
Find the study in Hernia (doi: 10.1007/s10029-017-1633-8).
FROM HERNIA
Prescribe This Combined OC With CV Safety in Mind

A 28-year-old woman presents to your office for a routine health maintenance exam. She is currently using an oral contraceptive containing desogestrel and ethinyl estradiol for contraception and is inquiring about a refill for the coming year. What would you recommend?
When choosing a combined oral contraceptive (COC) for a pa
In general, when compared with nonusers, women who use COCs have a two- to four-fold increase in risk for venous thromboembolism (VTE) and an increased risk for myocardial infarction (MI) and stroke.2,3 More specifically, higher doses of estrogen combined with the progesterones gestodene, desogestrel, and levonorgestrel, are associated with a higher risk for VTE.2-6
In 2012, the European Medicines Agency warned that COCs containing drospirenone were associated with a higher risk for VTE than other preparations, despite similar estrogen content.7 The FDA produced a similar statement that same year, recommending that providers carefully consider the risks and benefits before prescribing contraceptives containing drospirenone.8
The risks for ischemic stroke and MI have not been clearly established for varying doses of estrogen and different progesterones. This large observational study fills that informational gap by providing risk estimates for the various COC options.
STUDY SUMMARY
One COC comes out ahead
The authors used an observational cohort model to determine the effects of different doses of estrogen combined with different progesterones in COCs on the risks for pulmonary embolism (PE), ischemic stroke, and MI.1 Data were collected from the French national health insurance database and the French national hospital discharge database.9,10 The study included nearly 5 million women ages 15 to 49, living in France, who had at least one prescription filled for COCs between July 2010 and September 2012.
The investigators calculated the absolute and relative risks for first PE, ischemic stroke, and MI in women using COC formulations containing either low-dose estrogen (20 µg) or high-dose estrogen (30-40 µg) combined with one of five progesterones (norethisterone, norgestrel, levonorgestrel, desogestrel, gestodene). The relative risk (RR) was adjusted for confounding factors, including age, complimentary universal health insurance, socioeconomic status, hypertension, diabetes, and consultation with a gynecologist in the previous year.
The absolute risk per 100,000 woman-years for all COC use was 33 for PE, 19 for ischemic stroke, and 7 for MI, with a composite risk of 60. The RRs for low-dose estrogen vs high-dose estrogen were 0.75 for PE, 0.82 for ischemic stroke, and 0.56 for MI. The absolute risk reduction (ARR) with low-dose estrogen vs high-dose estrogen was 14/100,000 person-years of use; the number needed to harm (NNH) was 7,143.
Compared with levonorgestrel, desogestrel and gestodene were associated with higher RRs for PE but not arterial events (2.16 for desogestrel and 1.63 for gestodene). For PE, the ARR with levonorgestrel compared to desogestrel and gestodene, respectively, was 19/100,000 and 12/100,000 person-years of use (NNH, 5,263 and 8,333, respectively). The authors concluded that for the same progesterone, using a lower dose of estrogen decreases risk for PE, ischemic stroke, and MI, and that oral contraceptives containing levonorgestrel and low-dose estrogen resulted in the lowest overall risks for PE and arterial thromboembolism.
WHAT’S NEW?
Low-dose estrogen + levonorgestrel confer lowest risk
Prior studies have shown that COCs increase the risk for PE and may also increase the risks for ischemic stroke and MI.3,11 Studies have also suggested that a higher dose of estrogen in COCs is associated with an increased risk for VTE.11,12 This study shows that 20 µg of estrogen combined with levonorgestrel is associated with the lowest risks for PE, MI, and ischemic stroke.
CAVEATS
Cohort study, no start date, incomplete tobacco use data
This is an observational cohort study, so it is subject to confounding factors and biases. It does, however, include a very large population, which improves validity. The study did not account for COC start date, which may be confounding because the risk for VTE is highest in the first three months to one year of COC use.12 Data on tobacco use, a significant independent risk factor for arterial but not venous thromboembolism, was incomplete; however, in other studies, it has only marginally affected outcomes.3,13
CHALLENGES TO IMPLEMENTATION
Increased vaginal spotting
One potential challenge to implementing this practice changer may be the increased rate of vaginal spotting associated with low-dose estrogen. COCs containing 20 µg of estrogen are associated with spotting in approximately two-thirds of menstrual cycles over the course of a year.14 That said, women may prefer to endure the spotting in light of the improved safety profile of a lower-dose estrogen pill.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[7]:454-456).
1. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.
2. Lidegaard Ø, Løkkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.
3. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
4. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298.
5. FDA. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. www.fda.gov/downloads/drugs/drugsafety/ucm277384. Accessed July 5, 2017.
6. Seeger JD, Loughlin J, Eng PM, et al. Risk of thromboembolism in women taking ethinyl estradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007;110:587-593.
7. European Medicines Agency. PhVWP monthly report on safety concerns, guidelines and general matters. 2012. www.ema.europa.eu/docs/en_GB/document_library/Report/2012/01/WC500121387.pdf. Accessed July 5, 2017.
8. FDA. FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone. 2012. www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed July 5, 2017.
9. Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286-290.
10. Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36:411-417.
11. Farmer RD, Lawrenson RA, Thompson CR, et al. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83-88.
12. Lidegaard Ø, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423.
13. Zhang G, Xu X, Su W, et al. Smoking and risk of venous thromboembolism: a systematic review. Southeast Asian J Trop Med Public Health. 2014;45:736-745.
14. Akerlund M, Røde A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 micrograms desogestrel and either 30 micrograms or 20 micrograms ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.

A 28-year-old woman presents to your office for a routine health maintenance exam. She is currently using an oral contraceptive containing desogestrel and ethinyl estradiol for contraception and is inquiring about a refill for the coming year. What would you recommend?
When choosing a combined oral contraceptive (COC) for a pa
In general, when compared with nonusers, women who use COCs have a two- to four-fold increase in risk for venous thromboembolism (VTE) and an increased risk for myocardial infarction (MI) and stroke.2,3 More specifically, higher doses of estrogen combined with the progesterones gestodene, desogestrel, and levonorgestrel, are associated with a higher risk for VTE.2-6
In 2012, the European Medicines Agency warned that COCs containing drospirenone were associated with a higher risk for VTE than other preparations, despite similar estrogen content.7 The FDA produced a similar statement that same year, recommending that providers carefully consider the risks and benefits before prescribing contraceptives containing drospirenone.8
The risks for ischemic stroke and MI have not been clearly established for varying doses of estrogen and different progesterones. This large observational study fills that informational gap by providing risk estimates for the various COC options.
STUDY SUMMARY
One COC comes out ahead
The authors used an observational cohort model to determine the effects of different doses of estrogen combined with different progesterones in COCs on the risks for pulmonary embolism (PE), ischemic stroke, and MI.1 Data were collected from the French national health insurance database and the French national hospital discharge database.9,10 The study included nearly 5 million women ages 15 to 49, living in France, who had at least one prescription filled for COCs between July 2010 and September 2012.
The investigators calculated the absolute and relative risks for first PE, ischemic stroke, and MI in women using COC formulations containing either low-dose estrogen (20 µg) or high-dose estrogen (30-40 µg) combined with one of five progesterones (norethisterone, norgestrel, levonorgestrel, desogestrel, gestodene). The relative risk (RR) was adjusted for confounding factors, including age, complimentary universal health insurance, socioeconomic status, hypertension, diabetes, and consultation with a gynecologist in the previous year.
The absolute risk per 100,000 woman-years for all COC use was 33 for PE, 19 for ischemic stroke, and 7 for MI, with a composite risk of 60. The RRs for low-dose estrogen vs high-dose estrogen were 0.75 for PE, 0.82 for ischemic stroke, and 0.56 for MI. The absolute risk reduction (ARR) with low-dose estrogen vs high-dose estrogen was 14/100,000 person-years of use; the number needed to harm (NNH) was 7,143.
Compared with levonorgestrel, desogestrel and gestodene were associated with higher RRs for PE but not arterial events (2.16 for desogestrel and 1.63 for gestodene). For PE, the ARR with levonorgestrel compared to desogestrel and gestodene, respectively, was 19/100,000 and 12/100,000 person-years of use (NNH, 5,263 and 8,333, respectively). The authors concluded that for the same progesterone, using a lower dose of estrogen decreases risk for PE, ischemic stroke, and MI, and that oral contraceptives containing levonorgestrel and low-dose estrogen resulted in the lowest overall risks for PE and arterial thromboembolism.

WHAT’S NEW?
Low-dose estrogen + levonorgestrel confer lowest risk
Prior studies have shown that COCs increase the risk for PE and may also increase the risks for ischemic stroke and MI.3,11 Studies have also suggested that a higher dose of estrogen in COCs is associated with an increased risk for VTE.11,12 This study shows that 20 µg of estrogen combined with levonorgestrel is associated with the lowest risks for PE, MI, and ischemic stroke.
CAVEATS
Cohort study, no start date, incomplete tobacco use data
This is an observational cohort study, so it is subject to confounding factors and biases. It does, however, include a very large population, which improves validity. The study did not account for COC start date, which may be confounding because the risk for VTE is highest in the first three months to one year of COC use.12 Data on tobacco use, a significant independent risk factor for arterial but not venous thromboembolism, was incomplete; however, in other studies, it has only marginally affected outcomes.3,13
CHALLENGES TO IMPLEMENTATION
Increased vaginal spotting
One potential challenge to implementing this practice changer may be the increased rate of vaginal spotting associated with low-dose estrogen. COCs containing 20 µg of estrogen are associated with spotting in approximately two-thirds of menstrual cycles over the course of a year.14 That said, women may prefer to endure the spotting in light of the improved safety profile of a lower-dose estrogen pill.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[7]:454-456).

A 28-year-old woman presents to your office for a routine health maintenance exam. She is currently using an oral contraceptive containing desogestrel and ethinyl estradiol for contraception and is inquiring about a refill for the coming year. What would you recommend?
When choosing a combined oral contraceptive (COC) for a pa
In general, when compared with nonusers, women who use COCs have a two- to four-fold increase in risk for venous thromboembolism (VTE) and an increased risk for myocardial infarction (MI) and stroke.2,3 More specifically, higher doses of estrogen combined with the progesterones gestodene, desogestrel, and levonorgestrel, are associated with a higher risk for VTE.2-6
In 2012, the European Medicines Agency warned that COCs containing drospirenone were associated with a higher risk for VTE than other preparations, despite similar estrogen content.7 The FDA produced a similar statement that same year, recommending that providers carefully consider the risks and benefits before prescribing contraceptives containing drospirenone.8
The risks for ischemic stroke and MI have not been clearly established for varying doses of estrogen and different progesterones. This large observational study fills that informational gap by providing risk estimates for the various COC options.
STUDY SUMMARY
One COC comes out ahead
The authors used an observational cohort model to determine the effects of different doses of estrogen combined with different progesterones in COCs on the risks for pulmonary embolism (PE), ischemic stroke, and MI.1 Data were collected from the French national health insurance database and the French national hospital discharge database.9,10 The study included nearly 5 million women ages 15 to 49, living in France, who had at least one prescription filled for COCs between July 2010 and September 2012.
The investigators calculated the absolute and relative risks for first PE, ischemic stroke, and MI in women using COC formulations containing either low-dose estrogen (20 µg) or high-dose estrogen (30-40 µg) combined with one of five progesterones (norethisterone, norgestrel, levonorgestrel, desogestrel, gestodene). The relative risk (RR) was adjusted for confounding factors, including age, complimentary universal health insurance, socioeconomic status, hypertension, diabetes, and consultation with a gynecologist in the previous year.
The absolute risk per 100,000 woman-years for all COC use was 33 for PE, 19 for ischemic stroke, and 7 for MI, with a composite risk of 60. The RRs for low-dose estrogen vs high-dose estrogen were 0.75 for PE, 0.82 for ischemic stroke, and 0.56 for MI. The absolute risk reduction (ARR) with low-dose estrogen vs high-dose estrogen was 14/100,000 person-years of use; the number needed to harm (NNH) was 7,143.
Compared with levonorgestrel, desogestrel and gestodene were associated with higher RRs for PE but not arterial events (2.16 for desogestrel and 1.63 for gestodene). For PE, the ARR with levonorgestrel compared to desogestrel and gestodene, respectively, was 19/100,000 and 12/100,000 person-years of use (NNH, 5,263 and 8,333, respectively). The authors concluded that for the same progesterone, using a lower dose of estrogen decreases risk for PE, ischemic stroke, and MI, and that oral contraceptives containing levonorgestrel and low-dose estrogen resulted in the lowest overall risks for PE and arterial thromboembolism.

WHAT’S NEW?
Low-dose estrogen + levonorgestrel confer lowest risk
Prior studies have shown that COCs increase the risk for PE and may also increase the risks for ischemic stroke and MI.3,11 Studies have also suggested that a higher dose of estrogen in COCs is associated with an increased risk for VTE.11,12 This study shows that 20 µg of estrogen combined with levonorgestrel is associated with the lowest risks for PE, MI, and ischemic stroke.
CAVEATS
Cohort study, no start date, incomplete tobacco use data
This is an observational cohort study, so it is subject to confounding factors and biases. It does, however, include a very large population, which improves validity. The study did not account for COC start date, which may be confounding because the risk for VTE is highest in the first three months to one year of COC use.12 Data on tobacco use, a significant independent risk factor for arterial but not venous thromboembolism, was incomplete; however, in other studies, it has only marginally affected outcomes.3,13
CHALLENGES TO IMPLEMENTATION
Increased vaginal spotting
One potential challenge to implementing this practice changer may be the increased rate of vaginal spotting associated with low-dose estrogen. COCs containing 20 µg of estrogen are associated with spotting in approximately two-thirds of menstrual cycles over the course of a year.14 That said, women may prefer to endure the spotting in light of the improved safety profile of a lower-dose estrogen pill.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[7]:454-456).
1. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.
2. Lidegaard Ø, Løkkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.
3. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
4. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298.
5. FDA. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. www.fda.gov/downloads/drugs/drugsafety/ucm277384. Accessed July 5, 2017.
6. Seeger JD, Loughlin J, Eng PM, et al. Risk of thromboembolism in women taking ethinyl estradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007;110:587-593.
7. European Medicines Agency. PhVWP monthly report on safety concerns, guidelines and general matters. 2012. www.ema.europa.eu/docs/en_GB/document_library/Report/2012/01/WC500121387.pdf. Accessed July 5, 2017.
8. FDA. FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone. 2012. www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed July 5, 2017.
9. Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286-290.
10. Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36:411-417.
11. Farmer RD, Lawrenson RA, Thompson CR, et al. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83-88.
12. Lidegaard Ø, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423.
13. Zhang G, Xu X, Su W, et al. Smoking and risk of venous thromboembolism: a systematic review. Southeast Asian J Trop Med Public Health. 2014;45:736-745.
14. Akerlund M, Røde A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 micrograms desogestrel and either 30 micrograms or 20 micrograms ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
1. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.
2. Lidegaard Ø, Løkkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.
3. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
4. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298.
5. FDA. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. www.fda.gov/downloads/drugs/drugsafety/ucm277384. Accessed July 5, 2017.
6. Seeger JD, Loughlin J, Eng PM, et al. Risk of thromboembolism in women taking ethinyl estradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007;110:587-593.
7. European Medicines Agency. PhVWP monthly report on safety concerns, guidelines and general matters. 2012. www.ema.europa.eu/docs/en_GB/document_library/Report/2012/01/WC500121387.pdf. Accessed July 5, 2017.
8. FDA. FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone. 2012. www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed July 5, 2017.
9. Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286-290.
10. Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36:411-417.
11. Farmer RD, Lawrenson RA, Thompson CR, et al. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83-88.
12. Lidegaard Ø, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423.
13. Zhang G, Xu X, Su W, et al. Smoking and risk of venous thromboembolism: a systematic review. Southeast Asian J Trop Med Public Health. 2014;45:736-745.
14. Akerlund M, Røde A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 micrograms desogestrel and either 30 micrograms or 20 micrograms ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
Diabetes’ social determinants: What they mean in our practices
More than many other pregnancy complications, diabetes exemplifies the impact of social determinants of health.
The medical management of diabetes during pregnancy involves major lifestyle changes. Diabetes care is largely a patient-driven social experience involving complex and demanding self-care behaviors and tasks.
The pregnant woman with diabetes is placed on a diet that is often novel to her and may be in conflict with the eating patterns of her family. She is advised to exercise, read nutrition labels, and purchase and cook healthy food. She often has to pick up prescriptions, check finger sticks and log results, accurately draw up insulin, and manage strict schedules.
Management requires a tremendous amount of daily engagement during a period of time that, in and of itself, is cognitively demanding.
Outcomes, in turn, are impacted by social context and social factors – by the patient’s economic stability and the safety and characteristics of her neighborhood, for instance, as well as her work schedule, her social support, and her level of health literacy. Each of these factors can influence behaviors and decision making, and ultimately glycemic control and perinatal outcomes.
The social determinants of diabetes-related health are so individualized and impactful that they must be realized and addressed throughout our care, from the way in which we communicate at the initial prenatal checkup to the support we offer for self-management.
Barriers to diabetes self-care
While the incidence of type 2 diabetes is increasing among all social, ethnic, and racial groups, its prevalence among nonpregnant U.S. adults is greatest among racial/ethnic minorities, as well as in individuals with a low-income status. Women who enter pregnancy with preexisting diabetes are more likely to be racial/ethnic minorities.
In pregnancy, minority women (especially Hispanic, but also Asian and non-Hispanic black women), and women with low-income status are similarly predisposed to developing gestational diabetes mellitus (GDM).
Social determinants of health are interwoven with inequities stemming from race/ethnicity, income, and other factors that affect outcomes. For example, not only do non-Hispanic black women experience a greater incidence of GDM than non-Hispanic white women, but when they have GDM, they also appear to experience worse pregnancy outcomes compared with white women who also have GDM. In addition, they have a greater likelihood of developing type 2 diabetes after a pregnancy with GDM.
I care for a population that consists largely of minority, low-income women with either gestational or pregestational diabetes. Despite their best intentions and efforts – and despite seemingly high motivation levels – these women struggle to achieve the levels of glycemic control necessary for preventing maternal and fetal complications.
Several years ago, I sought to better understand the barriers to diabetes self-care and behavioral change these women face. Through a series of in-depth, semi-structured interviews with 10 English-speaking women (half with pregestational diabetes) over the course of their pregnancies, we found that the barriers to self-care related to the following: disease novelty, social and economic instability, nutrition challenges, psychological stressors, a failure of outcome expectations, and the burden of disease management (J Health Care Poor Underserved. 2015 Aug;26[3]:926-40; J Nutr Educ Behav. 2016 Mar;48[3]:170-80.e1).
Some of these barriers, such as the lack of any prior experience with diabetes (through a family member, for instance) or the inability to believe that behavior change and other treatment could impact her diabetes and her fetus’ health, echoed other limited published data. However, women in our study also appeared to be affected by barriers driven by social instability (e.g., a lack of partner or family support, family conflict, or neighborhood violence), inadequate access to healthy food, and the psychological impact of diabetes.
They often felt isolated and overwhelmed by their diabetes; the condition amplified stresses they were already experiencing and contributed to worsening mental health in those who already had depression or anxiety. In the other direction, women also described how preexisting mental health challenges affected their ability to sustain recommended behavior changes.
However, we also identified factors that empowered women in this community to succeed with their diabetes during pregnancy – these included having prior familiarity and diabetes self-efficacy, being motivated by the health of the fetus or older children, having a supportive social and physical environment, and having the ability to self-regulate or set and achieve goals (J Perinatol. 2016 Jan;36[1]:13-8).
To address these barriers, my group has undertaken a series of projects aimed at improving care for pregnant women with diabetes. We developed a diabetes-specific text message support system, for instance, and are now transitioning this support to an advanced mobile health tool that can help patients beyond our site.
What we can do
Much of what we can do in our practices to identify and address social determinants and alleviate barriers to effective diabetes management is about finding the “sweet spot” – about being able to convey the right information in the right amount, with the right timing and the right delivery.
While we can’t improve a woman’s neighborhood or resolve food instability, I believe that we can still work to improve outcomes for women who experience these problems. Here are some key strategies for optimal support of our patients:
Inquire about social factors
Identify hurdles by asking questions such as: Where do you live? Is it safe to walk in your neighborhood? If not, where’s your closest mall? What kind of job do you have, and does your employer allow breaks to take care of your health? How are things going at home? Who is at home to help you? Are you having any trouble affording food? How can we help you learn to adapt your personal or cultural food preferences to healthier options?
Look for small actions to take. I often write letters to my patients’ employers requesting that they be given short, frequent breaks to accommodate their care regimens. I also work to ensure that diet recommendations and medication/insulin regimens are customized for patients with irregular meal and sleep schedules, such as those working night shifts.
Employ a social worker if possible, especially if your practice cares for large numbers of underserved women.
Serve as a resource center, and engage your team in doing so. Be prepared to refer women for social services support, food banks, intimate partner violence support services, and other local resources.
Take a low-health-literacy approach
Health literacy is the ability to obtain and utilize health information. It has been widely investigated outside of pregnancy (and to some extent during pregnancy), and has been found to be at the root of many disparities in health care and health outcomes. Numeracy, a type of health literacy, is the ability to understand numbers, perform basic calculations, and use simple math skills in a way that helps one’s health.
The barriers created by inadequate health literacy are distinct from language barriers. I’ve had patients who can read the labels on their insulin vials but cannot distinguish the short-acting from the long-acting formulation, or who can read the words on a nutrition label but don’t know how to interpret the amount of carbohydrates and determine if a food fits the diet plan.
Moreover, while health literacy is correlated with cognitive ability, it still is a distinct skill set. Studies have shown that patients educated in a traditional sense – college-educated professionals, for instance – will not necessarily understand health-related words and instructions.
Research similarly suggests that a low-health-literacy approach that uses focused, simple, and straightforward messages benefits everyone. This type of approach involves the following:
Simple language
Teach-back techniques (“tell me you what your understanding is of what I just told you”)
Diagrams, handouts, and brochures written at a sixth-grade level.
Teaching that is limited to five to eight key messages per session, and reinforcement of these messages over time.
Promote self-efficacy
Self-efficacy is the confidence in one’s ability to perform certain health behaviors. It involves motivation as well as knowledge of the disease, the rationale for treatment, and the specific behaviors that are required for effective self-care.
Help patients understand “why it matters” – that diabetes raises the risk of macrosomia, shoulder dystocia, hypertension, long-term diabetes, and other adverse maternal and neonatal outcomes. Explain basic physiologic concepts and provide background information. This builds self-efficacy.
Do not issue recommendations for exercising and eating well without asking: How can I help you do this? What do you need to be able to eat healthy? Do you need an appointment with a nutritionist? Do you need to see a social worker?
Inquire about and help patients identify supportive family members or other “champions.” Look for ways to incorporate these support people into the patient’s care. At a minimum, encourage the patient to ask her support person to eat healthy with her and/or to understand her daily tasks so that this individual can offer reminders and be a source of support when she feels exhausted or overwhelmed.
If possible, facilitate some type of “diabetes buddy” program to offer peer support and help patients stay engaged in their care, or use group education sessions.
Piggyback on your patients’ own motivating factors. Research has shown that women are extraordinarily motivated to stop smoking during pregnancy because of the health of the fetus. This should extend as well to the difficult lifestyle changes required for diabetes self-care.
View pregnancy as a “golden opportunity” to promote healthy life changes that endure because of the often-extraordinary levels of motivation that women feel or can be encouraged to feel.
Facilitate access
The ability to attend frequent appointments and to juggle the logistics of transportation, child care, and time off work (all part of the burden of disease management) is a social determinant of health. It’s something we should ask about, and it is often something we can positively impact by modifying our practice hours and/or using telehealth or mobile health techniques.
Coordinating newborn and pediatric care with the mother’s subsequent primary care is optimal. Women often prioritize their babies’ health over their own health and they rarely miss pediatric appointments. Coordinating care through medical homes or other mechanisms may help women remain engaged and may lessen the gaps between obstetrical and subsequent primary care.
For me, facilitating doctor-to-doctor transitions sometimes entails picking up the phone or sending communication to a primary care doctor to say, for instance, “I’m worried about my patient’s lifetime risk of type 2 diabetes, and I’d like to hand off her care to you.” This is one of many small but meaningful steps we can take.
Dr. Yee is an assistant professor in the division of maternal-fetal medicine at Northwestern University, Chicago. She reported having no relevant financial disclosures.
More than many other pregnancy complications, diabetes exemplifies the impact of social determinants of health.
The medical management of diabetes during pregnancy involves major lifestyle changes. Diabetes care is largely a patient-driven social experience involving complex and demanding self-care behaviors and tasks.
The pregnant woman with diabetes is placed on a diet that is often novel to her and may be in conflict with the eating patterns of her family. She is advised to exercise, read nutrition labels, and purchase and cook healthy food. She often has to pick up prescriptions, check finger sticks and log results, accurately draw up insulin, and manage strict schedules.
Management requires a tremendous amount of daily engagement during a period of time that, in and of itself, is cognitively demanding.
Outcomes, in turn, are impacted by social context and social factors – by the patient’s economic stability and the safety and characteristics of her neighborhood, for instance, as well as her work schedule, her social support, and her level of health literacy. Each of these factors can influence behaviors and decision making, and ultimately glycemic control and perinatal outcomes.
The social determinants of diabetes-related health are so individualized and impactful that they must be realized and addressed throughout our care, from the way in which we communicate at the initial prenatal checkup to the support we offer for self-management.
Barriers to diabetes self-care
While the incidence of type 2 diabetes is increasing among all social, ethnic, and racial groups, its prevalence among nonpregnant U.S. adults is greatest among racial/ethnic minorities, as well as in individuals with a low-income status. Women who enter pregnancy with preexisting diabetes are more likely to be racial/ethnic minorities.

In pregnancy, minority women (especially Hispanic, but also Asian and non-Hispanic black women), and women with low-income status are similarly predisposed to developing gestational diabetes mellitus (GDM).
Social determinants of health are interwoven with inequities stemming from race/ethnicity, income, and other factors that affect outcomes. For example, not only do non-Hispanic black women experience a greater incidence of GDM than non-Hispanic white women, but when they have GDM, they also appear to experience worse pregnancy outcomes compared with white women who also have GDM. In addition, they have a greater likelihood of developing type 2 diabetes after a pregnancy with GDM.
I care for a population that consists largely of minority, low-income women with either gestational or pregestational diabetes. Despite their best intentions and efforts – and despite seemingly high motivation levels – these women struggle to achieve the levels of glycemic control necessary for preventing maternal and fetal complications.
Several years ago, I sought to better understand the barriers to diabetes self-care and behavioral change these women face. Through a series of in-depth, semi-structured interviews with 10 English-speaking women (half with pregestational diabetes) over the course of their pregnancies, we found that the barriers to self-care related to the following: disease novelty, social and economic instability, nutrition challenges, psychological stressors, a failure of outcome expectations, and the burden of disease management (J Health Care Poor Underserved. 2015 Aug;26[3]:926-40; J Nutr Educ Behav. 2016 Mar;48[3]:170-80.e1).
Some of these barriers, such as the lack of any prior experience with diabetes (through a family member, for instance) or the inability to believe that behavior change and other treatment could impact her diabetes and her fetus’ health, echoed other limited published data. However, women in our study also appeared to be affected by barriers driven by social instability (e.g., a lack of partner or family support, family conflict, or neighborhood violence), inadequate access to healthy food, and the psychological impact of diabetes.
They often felt isolated and overwhelmed by their diabetes; the condition amplified stresses they were already experiencing and contributed to worsening mental health in those who already had depression or anxiety. In the other direction, women also described how preexisting mental health challenges affected their ability to sustain recommended behavior changes.
However, we also identified factors that empowered women in this community to succeed with their diabetes during pregnancy – these included having prior familiarity and diabetes self-efficacy, being motivated by the health of the fetus or older children, having a supportive social and physical environment, and having the ability to self-regulate or set and achieve goals (J Perinatol. 2016 Jan;36[1]:13-8).
To address these barriers, my group has undertaken a series of projects aimed at improving care for pregnant women with diabetes. We developed a diabetes-specific text message support system, for instance, and are now transitioning this support to an advanced mobile health tool that can help patients beyond our site.
What we can do
Much of what we can do in our practices to identify and address social determinants and alleviate barriers to effective diabetes management is about finding the “sweet spot” – about being able to convey the right information in the right amount, with the right timing and the right delivery.
While we can’t improve a woman’s neighborhood or resolve food instability, I believe that we can still work to improve outcomes for women who experience these problems. Here are some key strategies for optimal support of our patients:
Inquire about social factors
Identify hurdles by asking questions such as: Where do you live? Is it safe to walk in your neighborhood? If not, where’s your closest mall? What kind of job do you have, and does your employer allow breaks to take care of your health? How are things going at home? Who is at home to help you? Are you having any trouble affording food? How can we help you learn to adapt your personal or cultural food preferences to healthier options?
Look for small actions to take. I often write letters to my patients’ employers requesting that they be given short, frequent breaks to accommodate their care regimens. I also work to ensure that diet recommendations and medication/insulin regimens are customized for patients with irregular meal and sleep schedules, such as those working night shifts.
Employ a social worker if possible, especially if your practice cares for large numbers of underserved women.
Serve as a resource center, and engage your team in doing so. Be prepared to refer women for social services support, food banks, intimate partner violence support services, and other local resources.
Take a low-health-literacy approach
Health literacy is the ability to obtain and utilize health information. It has been widely investigated outside of pregnancy (and to some extent during pregnancy), and has been found to be at the root of many disparities in health care and health outcomes. Numeracy, a type of health literacy, is the ability to understand numbers, perform basic calculations, and use simple math skills in a way that helps one’s health.
The barriers created by inadequate health literacy are distinct from language barriers. I’ve had patients who can read the labels on their insulin vials but cannot distinguish the short-acting from the long-acting formulation, or who can read the words on a nutrition label but don’t know how to interpret the amount of carbohydrates and determine if a food fits the diet plan.
Moreover, while health literacy is correlated with cognitive ability, it still is a distinct skill set. Studies have shown that patients educated in a traditional sense – college-educated professionals, for instance – will not necessarily understand health-related words and instructions.
Research similarly suggests that a low-health-literacy approach that uses focused, simple, and straightforward messages benefits everyone. This type of approach involves the following:
Simple language
Teach-back techniques (“tell me you what your understanding is of what I just told you”)
Diagrams, handouts, and brochures written at a sixth-grade level.
Teaching that is limited to five to eight key messages per session, and reinforcement of these messages over time.
Promote self-efficacy
Self-efficacy is the confidence in one’s ability to perform certain health behaviors. It involves motivation as well as knowledge of the disease, the rationale for treatment, and the specific behaviors that are required for effective self-care.
Help patients understand “why it matters” – that diabetes raises the risk of macrosomia, shoulder dystocia, hypertension, long-term diabetes, and other adverse maternal and neonatal outcomes. Explain basic physiologic concepts and provide background information. This builds self-efficacy.
Do not issue recommendations for exercising and eating well without asking: How can I help you do this? What do you need to be able to eat healthy? Do you need an appointment with a nutritionist? Do you need to see a social worker?
Inquire about and help patients identify supportive family members or other “champions.” Look for ways to incorporate these support people into the patient’s care. At a minimum, encourage the patient to ask her support person to eat healthy with her and/or to understand her daily tasks so that this individual can offer reminders and be a source of support when she feels exhausted or overwhelmed.
If possible, facilitate some type of “diabetes buddy” program to offer peer support and help patients stay engaged in their care, or use group education sessions.
Piggyback on your patients’ own motivating factors. Research has shown that women are extraordinarily motivated to stop smoking during pregnancy because of the health of the fetus. This should extend as well to the difficult lifestyle changes required for diabetes self-care.
View pregnancy as a “golden opportunity” to promote healthy life changes that endure because of the often-extraordinary levels of motivation that women feel or can be encouraged to feel.
Facilitate access
The ability to attend frequent appointments and to juggle the logistics of transportation, child care, and time off work (all part of the burden of disease management) is a social determinant of health. It’s something we should ask about, and it is often something we can positively impact by modifying our practice hours and/or using telehealth or mobile health techniques.
Coordinating newborn and pediatric care with the mother’s subsequent primary care is optimal. Women often prioritize their babies’ health over their own health and they rarely miss pediatric appointments. Coordinating care through medical homes or other mechanisms may help women remain engaged and may lessen the gaps between obstetrical and subsequent primary care.
For me, facilitating doctor-to-doctor transitions sometimes entails picking up the phone or sending communication to a primary care doctor to say, for instance, “I’m worried about my patient’s lifetime risk of type 2 diabetes, and I’d like to hand off her care to you.” This is one of many small but meaningful steps we can take.
Dr. Yee is an assistant professor in the division of maternal-fetal medicine at Northwestern University, Chicago. She reported having no relevant financial disclosures.
More than many other pregnancy complications, diabetes exemplifies the impact of social determinants of health.
The medical management of diabetes during pregnancy involves major lifestyle changes. Diabetes care is largely a patient-driven social experience involving complex and demanding self-care behaviors and tasks.
The pregnant woman with diabetes is placed on a diet that is often novel to her and may be in conflict with the eating patterns of her family. She is advised to exercise, read nutrition labels, and purchase and cook healthy food. She often has to pick up prescriptions, check finger sticks and log results, accurately draw up insulin, and manage strict schedules.
Management requires a tremendous amount of daily engagement during a period of time that, in and of itself, is cognitively demanding.
Outcomes, in turn, are impacted by social context and social factors – by the patient’s economic stability and the safety and characteristics of her neighborhood, for instance, as well as her work schedule, her social support, and her level of health literacy. Each of these factors can influence behaviors and decision making, and ultimately glycemic control and perinatal outcomes.
The social determinants of diabetes-related health are so individualized and impactful that they must be realized and addressed throughout our care, from the way in which we communicate at the initial prenatal checkup to the support we offer for self-management.
Barriers to diabetes self-care
While the incidence of type 2 diabetes is increasing among all social, ethnic, and racial groups, its prevalence among nonpregnant U.S. adults is greatest among racial/ethnic minorities, as well as in individuals with a low-income status. Women who enter pregnancy with preexisting diabetes are more likely to be racial/ethnic minorities.

In pregnancy, minority women (especially Hispanic, but also Asian and non-Hispanic black women), and women with low-income status are similarly predisposed to developing gestational diabetes mellitus (GDM).
Social determinants of health are interwoven with inequities stemming from race/ethnicity, income, and other factors that affect outcomes. For example, not only do non-Hispanic black women experience a greater incidence of GDM than non-Hispanic white women, but when they have GDM, they also appear to experience worse pregnancy outcomes compared with white women who also have GDM. In addition, they have a greater likelihood of developing type 2 diabetes after a pregnancy with GDM.
I care for a population that consists largely of minority, low-income women with either gestational or pregestational diabetes. Despite their best intentions and efforts – and despite seemingly high motivation levels – these women struggle to achieve the levels of glycemic control necessary for preventing maternal and fetal complications.
Several years ago, I sought to better understand the barriers to diabetes self-care and behavioral change these women face. Through a series of in-depth, semi-structured interviews with 10 English-speaking women (half with pregestational diabetes) over the course of their pregnancies, we found that the barriers to self-care related to the following: disease novelty, social and economic instability, nutrition challenges, psychological stressors, a failure of outcome expectations, and the burden of disease management (J Health Care Poor Underserved. 2015 Aug;26[3]:926-40; J Nutr Educ Behav. 2016 Mar;48[3]:170-80.e1).
Some of these barriers, such as the lack of any prior experience with diabetes (through a family member, for instance) or the inability to believe that behavior change and other treatment could impact her diabetes and her fetus’ health, echoed other limited published data. However, women in our study also appeared to be affected by barriers driven by social instability (e.g., a lack of partner or family support, family conflict, or neighborhood violence), inadequate access to healthy food, and the psychological impact of diabetes.
They often felt isolated and overwhelmed by their diabetes; the condition amplified stresses they were already experiencing and contributed to worsening mental health in those who already had depression or anxiety. In the other direction, women also described how preexisting mental health challenges affected their ability to sustain recommended behavior changes.
However, we also identified factors that empowered women in this community to succeed with their diabetes during pregnancy – these included having prior familiarity and diabetes self-efficacy, being motivated by the health of the fetus or older children, having a supportive social and physical environment, and having the ability to self-regulate or set and achieve goals (J Perinatol. 2016 Jan;36[1]:13-8).
To address these barriers, my group has undertaken a series of projects aimed at improving care for pregnant women with diabetes. We developed a diabetes-specific text message support system, for instance, and are now transitioning this support to an advanced mobile health tool that can help patients beyond our site.
What we can do
Much of what we can do in our practices to identify and address social determinants and alleviate barriers to effective diabetes management is about finding the “sweet spot” – about being able to convey the right information in the right amount, with the right timing and the right delivery.
While we can’t improve a woman’s neighborhood or resolve food instability, I believe that we can still work to improve outcomes for women who experience these problems. Here are some key strategies for optimal support of our patients:
Inquire about social factors
Identify hurdles by asking questions such as: Where do you live? Is it safe to walk in your neighborhood? If not, where’s your closest mall? What kind of job do you have, and does your employer allow breaks to take care of your health? How are things going at home? Who is at home to help you? Are you having any trouble affording food? How can we help you learn to adapt your personal or cultural food preferences to healthier options?
Look for small actions to take. I often write letters to my patients’ employers requesting that they be given short, frequent breaks to accommodate their care regimens. I also work to ensure that diet recommendations and medication/insulin regimens are customized for patients with irregular meal and sleep schedules, such as those working night shifts.
Employ a social worker if possible, especially if your practice cares for large numbers of underserved women.
Serve as a resource center, and engage your team in doing so. Be prepared to refer women for social services support, food banks, intimate partner violence support services, and other local resources.
Take a low-health-literacy approach
Health literacy is the ability to obtain and utilize health information. It has been widely investigated outside of pregnancy (and to some extent during pregnancy), and has been found to be at the root of many disparities in health care and health outcomes. Numeracy, a type of health literacy, is the ability to understand numbers, perform basic calculations, and use simple math skills in a way that helps one’s health.
The barriers created by inadequate health literacy are distinct from language barriers. I’ve had patients who can read the labels on their insulin vials but cannot distinguish the short-acting from the long-acting formulation, or who can read the words on a nutrition label but don’t know how to interpret the amount of carbohydrates and determine if a food fits the diet plan.
Moreover, while health literacy is correlated with cognitive ability, it still is a distinct skill set. Studies have shown that patients educated in a traditional sense – college-educated professionals, for instance – will not necessarily understand health-related words and instructions.
Research similarly suggests that a low-health-literacy approach that uses focused, simple, and straightforward messages benefits everyone. This type of approach involves the following:
Simple language
Teach-back techniques (“tell me you what your understanding is of what I just told you”)
Diagrams, handouts, and brochures written at a sixth-grade level.
Teaching that is limited to five to eight key messages per session, and reinforcement of these messages over time.
Promote self-efficacy
Self-efficacy is the confidence in one’s ability to perform certain health behaviors. It involves motivation as well as knowledge of the disease, the rationale for treatment, and the specific behaviors that are required for effective self-care.
Help patients understand “why it matters” – that diabetes raises the risk of macrosomia, shoulder dystocia, hypertension, long-term diabetes, and other adverse maternal and neonatal outcomes. Explain basic physiologic concepts and provide background information. This builds self-efficacy.
Do not issue recommendations for exercising and eating well without asking: How can I help you do this? What do you need to be able to eat healthy? Do you need an appointment with a nutritionist? Do you need to see a social worker?
Inquire about and help patients identify supportive family members or other “champions.” Look for ways to incorporate these support people into the patient’s care. At a minimum, encourage the patient to ask her support person to eat healthy with her and/or to understand her daily tasks so that this individual can offer reminders and be a source of support when she feels exhausted or overwhelmed.
If possible, facilitate some type of “diabetes buddy” program to offer peer support and help patients stay engaged in their care, or use group education sessions.
Piggyback on your patients’ own motivating factors. Research has shown that women are extraordinarily motivated to stop smoking during pregnancy because of the health of the fetus. This should extend as well to the difficult lifestyle changes required for diabetes self-care.
View pregnancy as a “golden opportunity” to promote healthy life changes that endure because of the often-extraordinary levels of motivation that women feel or can be encouraged to feel.
Facilitate access
The ability to attend frequent appointments and to juggle the logistics of transportation, child care, and time off work (all part of the burden of disease management) is a social determinant of health. It’s something we should ask about, and it is often something we can positively impact by modifying our practice hours and/or using telehealth or mobile health techniques.
Coordinating newborn and pediatric care with the mother’s subsequent primary care is optimal. Women often prioritize their babies’ health over their own health and they rarely miss pediatric appointments. Coordinating care through medical homes or other mechanisms may help women remain engaged and may lessen the gaps between obstetrical and subsequent primary care.
For me, facilitating doctor-to-doctor transitions sometimes entails picking up the phone or sending communication to a primary care doctor to say, for instance, “I’m worried about my patient’s lifetime risk of type 2 diabetes, and I’d like to hand off her care to you.” This is one of many small but meaningful steps we can take.
Dr. Yee is an assistant professor in the division of maternal-fetal medicine at Northwestern University, Chicago. She reported having no relevant financial disclosures.