User login
Novel drugs approved in 2016
The Food and Drug Administration approved 22 new drug products in 12 pharmacologic classes in 2016. Additionally, daclizumab (Zenapax), which was approved several years ago for prophylaxis of acute organ rejection in patients receiving renal transplants, was approved for multiple sclerosis treatment (Zinbryta) last year, and sofosbuvir (Sovaldi), which was approved in 2013 for the treatment of hepatitis C virus, is now combined with velpatasvir (Epclusa) to treat all six major forms of hepatitis C.
There are 22 drugs that can be considered novel drugs. As defined by the FDA, novel drugs have never been approved for human use. There are no human pregnancy data for any of the newly approved drugs or drug combinations. As such, it is important to consider that high molecular weight drugs that probably do not cross the placenta in the first half of pregnancy may do so in late pregnancy.
Antineoplastics
Atezolizumab (Tecentriq) is a programmed death ligand–blocking antibody that is indicated for locally advanced or metastatic urothelial carcinoma and metastatic nonsmall cell lung cancer following platinum-containing chemotherapy. Animal reproduction studies have not been conducted, but, based on its mechanism of action, fetal exposure may increase the risk of developing immune-mediated disorders or altering the normal immune response. The molecular weight is high (145,000) and the terminal half-life is long (27 days).
Olaratumab (Lartruvo) is a platelet-derived growth factor receptor– alpha-blocking antibody. It is indicated, in combination with doxorubicin, for the treatment of soft tissue sarcoma. Although the estimated elimination half-life is long (about 11 days with a range of 6-24 days), the high molecular weight (about 154,000) should limit fetal exposure, at least in the first half of pregnancy. The drug should be avoided in pregnancy, however, based on the animal data, the mechanism of action, and its combination with doxorubicin.
Rucaparib (Rubraca) is a poly (adenosine diphosphate–ribose) polymerase inhibitor indicated for the treatment of ovarian cancer. The drug could cause human fetal harm based on the animal data, mechanism of action, relatively low molecular weight (about 556), and terminal half-life (17 hours).
Venetoclax (Venclexta) is a B-cell lymphoma 2 inhibitor indicated for the treatment of chronic lymphocytic leukemia. Although the animal data, molecular weight (about 868), and elimination half-life (about 26 hours) suggest embryo-fetal risk, the high plasma protein binding (99.9%) should limit the amount crossing the placenta.
Anti-infectives
There are two new monoclonal antibodies in this class. Bezlotoxumab (Zinplava) is used to reduce recurrence of Clostridium difficile. Animal reproduction studies have not been conducted. As the molecular weight is about 148,000, the drug will not cross the placenta, at least not in the first half of pregnancy. However, the drug has a long elimination half-life (about 19 days), so, depending on when it was given, it could cross in late pregnancy. Obiltoxaximab (Anthim), administered as a single IV dose, is indicated for the treatment of inhaled anthrax due to Bacillus anthracis. No fetal harm was observed in animal reproduction studies. The high molecular weight (about 148,000) suggests that the drug will not cross to the embryo and/or fetus, at least not in the first part of pregnancy.
Elbasvir/Grazoprevir (Zepatier) is indicated for the treatment of chronic hepatitis C virus genotype 1 or 4. Animal reproduction studies found no evidence of adverse developmental outcomes. The molecular weights of the two components are about 882 and 767, respectively. Both are extensively bound to plasma proteins, 99.9% and 98.8%, respectively, and the terminal half-lives are 24 and 31 hours. Thus, the product appears to be low risk if used in human pregnancy. However, it is contraindicated if given with ribavirin.
Central nervous system agents
Brivaracetam (Briviact) is an anticonvulsant used to treat partial-onset seizures. Animal reproduction studies suggest moderate risk. The molecular weight (about 212), low plasma protein binding (less than or equal to 20%), and terminal plasma half-life of about 9 hours suggest that the drug will cross the placenta. The manufacturer recommends that the drug should be used in pregnancy only if the potential benefit justifies the potential risk to the embryo/fetus.
Pimavanserin (Nuplazid) is an atypical antipsychotic indicated for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. Reproduction studies in animals suggest low risk. The molecular weight of the free base (about 428) and the long mean plasma half-lives of the parent drug and active metabolite (57 and 200 hours) suggest that the drug will cross the placenta. However, the high plasma protein binding (about 95%) may limit the exposure. Nevertheless, avoiding the period of organogenesis appears to be best.
Dermatologic agents
Crisaborole (Eucrisa) is indicated for topical treatment of mild to moderate atopic dermatitis. In 33 pediatric subjects (aged 2-17 years) who applied the ointment twice daily for 8 days, low amounts were absorbed systemically with plasma concentrations in the nanogram/milliliter range. Plasma protein binding was 97%. With oral formulations of the drug, animal reproduction studies suggest low risk. Taken in sum, the human pregnancy risk appears to be low.
Ixekizumab (Taltz) is a humanized interleukin-17A antagonist, administered subcutaneously, that is indicated for the treatment of adults with moderate to severe plaque psoriasis. The drug did not cause developmental toxicity in monkeys. The molecular weight for the drug’s protein backbone is 146,158, and the mean elimination half-life was 13 days. The human embryo-fetal risk in the first half of pregnancy appears to be low.
Diagnostic agents
Fluciclovine F 18 (Axumin) is a radioactive diagnostic agent indicated for positron emission tomography imaging in men with suspected prostate cancer. Since the agent is only used in men, there are no human or animal pregnancy data.
Gallium GA 68 dotatate injection, a diagnostic imaging agent to detect rare neuroendocrine tumors, is not yet on the market.
Endocrine/metabolic agents
Lixisenatide (Adlyxin) is a glucagon-like–peptide-1 receptor agonist that is administered subcutaneously. It is indicated as an adjunct to diet and exercise to improve glycemic control in type 2 diabetes. The drug was teratogenic in two animal species. The molecular weight is about 4,859, and the mean terminal half-life was about 3 hours. Since tight control of glucose levels in type 2 diabetes is required during pregnancy, insulin is the treatment of choice. Consequently, lixisenatide should not be used during pregnancy.
Gastrointestinal agents
Obeticholic acid (Ocaliva) is a farnesoid X receptor agonist that is given orally for the treatment of primary biliary cholangitis, in combination with ursodiol, or ursodeoxycholic acid. I have classified ursodiol as compatible in pregnancy in the 10th edition of “Drugs in Pregnancy and Lactation” (2011: Wolters Kluwer Health). The animal reproduction data for both drugs suggest low risk. Based on the molecular weight of obeticholic (about 421), the drug will probably cross to the embryo/fetus, but the high plasma protein binding (greater than 99%) may limit exposure. The elimination half-life is apparently unknown.
Hematologic agents
Defibrotide sodium (Defitelio), given as an intravenous infusion, is an oligonucleotide mixture. It is indicated for the treatment of hepatic veno-occlusive disease, also known as sinusoidal obstruction syndrome, with renal or pulmonary dysfunction following hematopoietic stem-cell transplantation. Animal reproduction studies in two species suggest risk. The mean molecular weight is 13,000-20,000. Plasma protein binding is an average 93%, and the elimination half-life is less than 2 hours. It is doubtful if the drug crosses the placenta, especially in the first half of pregnancy. If possible, avoid the drug in the second half of pregnancy.
Immunologics
Two indications have been approved for daclizumab. The first was in 2005 for the prophylaxis of acute organ rejection of renal transplants (Zenapax), and the second was in 2016 for the treatment of relapsing forms of multiple sclerosis (Zinbryta). Reproduction studies in monkeys with Zinbryta can be classified as low risk. The molecular weight (about 144,000) suggests that the drug will not cross the placenta, at least in the first half of pregnancy. However, depending on when the drug is given, the long elimination half-life of 21 days might allow the drug to cross in late pregnancy. Regardless, if the perceived maternal benefit exceeds the potential embryo-fetal risk, the drug should not be withheld because of pregnancy.
Muscular disorder agents
Eteplirsen (Exondys 51) is an antisense oligonucleotide that is given intravenously. It is indicated for the treatment of Duchenne muscular dystrophy in patients who have a confirmed mutation of the related gene, which is amenable to exon 51 skipping. There are no animal reproduction data. The molecular weight is about 10,306. This suggests that the drug will not cross the placenta, at least in the first half of pregnancy. The elimination half-life is 3-4 hours, and the plasma concentration 24 hours after a dose was 0.07% of the peak plasma concentration. The drug is given once weekly, and waiting for 24 hours or slightly longer after a dose should reduce the exposure, if any, of the embryo-fetus during the first half of pregnancy.
Nusinersen (Spinraza), a survival motor neuron 2 directed–antisense oligonucleotide, is given as an intrathecal dose. It is indicated for the treatment of spinal muscular atrophy. Subcutaneous doses in two animal species caused no developmental toxicity. The molecular weight of 7,501 suggests that the drug will not cross the human placenta, at least not in the first half of pregnancy. The mean terminal elimination half-life in cerebrospinal fluid was 135-177 days and 63-87 days in plasma.
Ophthalmic agents
Lifitegrast (Xiidra) is an ophthalmic solution of a lymphocyte function-associated–antigen-1 antagonist. It is indicated for the treatment of the signs and symptoms of dry eye disease. The animal reproduction data suggest low risk. The molecular weight is about 616, suggesting that the drug would cross the placenta. However, in a Phase III trial conducted before FDA approval, 47 patients with dry eye disease were given 1 drop twice daily for periods up to 360 days. Nine patients (19%) had plasma predose (trough) concentrations above 0.5 ng/mL, the lower limit of quantitation. Trough plasma concentrations in these patients ranged from 0.55 ng/mL to 3.74 ng/mL. These amounts do not appear to represent an embryo-fetal risk.
Respiratory agents
Reslizumab (Cinqair), an interleukin-5 antagonist monoclonal antibody, is given intravenously. It is indicated for add-on maintenance treatment of severe asthma in patients with an eosinophilic phenotype. Animal data in two species suggest low risk. The molecular weight is about 147,000, and the elimination half-life is about 24 days. This suggests that exposure of the embryo and fetus will be minimal, at least in the first half of pregnancy. The maternal benefit appears to outweigh the unknown embryo-fetal risk.
Lactation
None of the above drugs have been studied during breastfeeding. Many drugs, regardless of their molecular weight, will cross into milk in small amounts during the first postpartum week. The effects of this exposure on a nursing infant are unknown. Based on the potential for nursing infant harm, the drugs that probably should not be given during breastfeeding include the four antineoplastics, the atypical antipsychotic pimavanserin, and the diabetes injection lixisenatide.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, and at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation,” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
The Food and Drug Administration approved 22 new drug products in 12 pharmacologic classes in 2016. Additionally, daclizumab (Zenapax), which was approved several years ago for prophylaxis of acute organ rejection in patients receiving renal transplants, was approved for multiple sclerosis treatment (Zinbryta) last year, and sofosbuvir (Sovaldi), which was approved in 2013 for the treatment of hepatitis C virus, is now combined with velpatasvir (Epclusa) to treat all six major forms of hepatitis C.
There are 22 drugs that can be considered novel drugs. As defined by the FDA, novel drugs have never been approved for human use. There are no human pregnancy data for any of the newly approved drugs or drug combinations. As such, it is important to consider that high molecular weight drugs that probably do not cross the placenta in the first half of pregnancy may do so in late pregnancy.
Antineoplastics
Atezolizumab (Tecentriq) is a programmed death ligand–blocking antibody that is indicated for locally advanced or metastatic urothelial carcinoma and metastatic nonsmall cell lung cancer following platinum-containing chemotherapy. Animal reproduction studies have not been conducted, but, based on its mechanism of action, fetal exposure may increase the risk of developing immune-mediated disorders or altering the normal immune response. The molecular weight is high (145,000) and the terminal half-life is long (27 days).
Olaratumab (Lartruvo) is a platelet-derived growth factor receptor– alpha-blocking antibody. It is indicated, in combination with doxorubicin, for the treatment of soft tissue sarcoma. Although the estimated elimination half-life is long (about 11 days with a range of 6-24 days), the high molecular weight (about 154,000) should limit fetal exposure, at least in the first half of pregnancy. The drug should be avoided in pregnancy, however, based on the animal data, the mechanism of action, and its combination with doxorubicin.
Rucaparib (Rubraca) is a poly (adenosine diphosphate–ribose) polymerase inhibitor indicated for the treatment of ovarian cancer. The drug could cause human fetal harm based on the animal data, mechanism of action, relatively low molecular weight (about 556), and terminal half-life (17 hours).
Venetoclax (Venclexta) is a B-cell lymphoma 2 inhibitor indicated for the treatment of chronic lymphocytic leukemia. Although the animal data, molecular weight (about 868), and elimination half-life (about 26 hours) suggest embryo-fetal risk, the high plasma protein binding (99.9%) should limit the amount crossing the placenta.
Anti-infectives
There are two new monoclonal antibodies in this class. Bezlotoxumab (Zinplava) is used to reduce recurrence of Clostridium difficile. Animal reproduction studies have not been conducted. As the molecular weight is about 148,000, the drug will not cross the placenta, at least not in the first half of pregnancy. However, the drug has a long elimination half-life (about 19 days), so, depending on when it was given, it could cross in late pregnancy. Obiltoxaximab (Anthim), administered as a single IV dose, is indicated for the treatment of inhaled anthrax due to Bacillus anthracis. No fetal harm was observed in animal reproduction studies. The high molecular weight (about 148,000) suggests that the drug will not cross to the embryo and/or fetus, at least not in the first part of pregnancy.
Elbasvir/Grazoprevir (Zepatier) is indicated for the treatment of chronic hepatitis C virus genotype 1 or 4. Animal reproduction studies found no evidence of adverse developmental outcomes. The molecular weights of the two components are about 882 and 767, respectively. Both are extensively bound to plasma proteins, 99.9% and 98.8%, respectively, and the terminal half-lives are 24 and 31 hours. Thus, the product appears to be low risk if used in human pregnancy. However, it is contraindicated if given with ribavirin.
Central nervous system agents
Brivaracetam (Briviact) is an anticonvulsant used to treat partial-onset seizures. Animal reproduction studies suggest moderate risk. The molecular weight (about 212), low plasma protein binding (less than or equal to 20%), and terminal plasma half-life of about 9 hours suggest that the drug will cross the placenta. The manufacturer recommends that the drug should be used in pregnancy only if the potential benefit justifies the potential risk to the embryo/fetus.
Pimavanserin (Nuplazid) is an atypical antipsychotic indicated for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. Reproduction studies in animals suggest low risk. The molecular weight of the free base (about 428) and the long mean plasma half-lives of the parent drug and active metabolite (57 and 200 hours) suggest that the drug will cross the placenta. However, the high plasma protein binding (about 95%) may limit the exposure. Nevertheless, avoiding the period of organogenesis appears to be best.
Dermatologic agents
Crisaborole (Eucrisa) is indicated for topical treatment of mild to moderate atopic dermatitis. In 33 pediatric subjects (aged 2-17 years) who applied the ointment twice daily for 8 days, low amounts were absorbed systemically with plasma concentrations in the nanogram/milliliter range. Plasma protein binding was 97%. With oral formulations of the drug, animal reproduction studies suggest low risk. Taken in sum, the human pregnancy risk appears to be low.
Ixekizumab (Taltz) is a humanized interleukin-17A antagonist, administered subcutaneously, that is indicated for the treatment of adults with moderate to severe plaque psoriasis. The drug did not cause developmental toxicity in monkeys. The molecular weight for the drug’s protein backbone is 146,158, and the mean elimination half-life was 13 days. The human embryo-fetal risk in the first half of pregnancy appears to be low.
Diagnostic agents
Fluciclovine F 18 (Axumin) is a radioactive diagnostic agent indicated for positron emission tomography imaging in men with suspected prostate cancer. Since the agent is only used in men, there are no human or animal pregnancy data.
Gallium GA 68 dotatate injection, a diagnostic imaging agent to detect rare neuroendocrine tumors, is not yet on the market.
Endocrine/metabolic agents
Lixisenatide (Adlyxin) is a glucagon-like–peptide-1 receptor agonist that is administered subcutaneously. It is indicated as an adjunct to diet and exercise to improve glycemic control in type 2 diabetes. The drug was teratogenic in two animal species. The molecular weight is about 4,859, and the mean terminal half-life was about 3 hours. Since tight control of glucose levels in type 2 diabetes is required during pregnancy, insulin is the treatment of choice. Consequently, lixisenatide should not be used during pregnancy.
Gastrointestinal agents
Obeticholic acid (Ocaliva) is a farnesoid X receptor agonist that is given orally for the treatment of primary biliary cholangitis, in combination with ursodiol, or ursodeoxycholic acid. I have classified ursodiol as compatible in pregnancy in the 10th edition of “Drugs in Pregnancy and Lactation” (2011: Wolters Kluwer Health). The animal reproduction data for both drugs suggest low risk. Based on the molecular weight of obeticholic (about 421), the drug will probably cross to the embryo/fetus, but the high plasma protein binding (greater than 99%) may limit exposure. The elimination half-life is apparently unknown.
Hematologic agents
Defibrotide sodium (Defitelio), given as an intravenous infusion, is an oligonucleotide mixture. It is indicated for the treatment of hepatic veno-occlusive disease, also known as sinusoidal obstruction syndrome, with renal or pulmonary dysfunction following hematopoietic stem-cell transplantation. Animal reproduction studies in two species suggest risk. The mean molecular weight is 13,000-20,000. Plasma protein binding is an average 93%, and the elimination half-life is less than 2 hours. It is doubtful if the drug crosses the placenta, especially in the first half of pregnancy. If possible, avoid the drug in the second half of pregnancy.
Immunologics
Two indications have been approved for daclizumab. The first was in 2005 for the prophylaxis of acute organ rejection of renal transplants (Zenapax), and the second was in 2016 for the treatment of relapsing forms of multiple sclerosis (Zinbryta). Reproduction studies in monkeys with Zinbryta can be classified as low risk. The molecular weight (about 144,000) suggests that the drug will not cross the placenta, at least in the first half of pregnancy. However, depending on when the drug is given, the long elimination half-life of 21 days might allow the drug to cross in late pregnancy. Regardless, if the perceived maternal benefit exceeds the potential embryo-fetal risk, the drug should not be withheld because of pregnancy.
Muscular disorder agents
Eteplirsen (Exondys 51) is an antisense oligonucleotide that is given intravenously. It is indicated for the treatment of Duchenne muscular dystrophy in patients who have a confirmed mutation of the related gene, which is amenable to exon 51 skipping. There are no animal reproduction data. The molecular weight is about 10,306. This suggests that the drug will not cross the placenta, at least in the first half of pregnancy. The elimination half-life is 3-4 hours, and the plasma concentration 24 hours after a dose was 0.07% of the peak plasma concentration. The drug is given once weekly, and waiting for 24 hours or slightly longer after a dose should reduce the exposure, if any, of the embryo-fetus during the first half of pregnancy.
Nusinersen (Spinraza), a survival motor neuron 2 directed–antisense oligonucleotide, is given as an intrathecal dose. It is indicated for the treatment of spinal muscular atrophy. Subcutaneous doses in two animal species caused no developmental toxicity. The molecular weight of 7,501 suggests that the drug will not cross the human placenta, at least not in the first half of pregnancy. The mean terminal elimination half-life in cerebrospinal fluid was 135-177 days and 63-87 days in plasma.
Ophthalmic agents
Lifitegrast (Xiidra) is an ophthalmic solution of a lymphocyte function-associated–antigen-1 antagonist. It is indicated for the treatment of the signs and symptoms of dry eye disease. The animal reproduction data suggest low risk. The molecular weight is about 616, suggesting that the drug would cross the placenta. However, in a Phase III trial conducted before FDA approval, 47 patients with dry eye disease were given 1 drop twice daily for periods up to 360 days. Nine patients (19%) had plasma predose (trough) concentrations above 0.5 ng/mL, the lower limit of quantitation. Trough plasma concentrations in these patients ranged from 0.55 ng/mL to 3.74 ng/mL. These amounts do not appear to represent an embryo-fetal risk.
Respiratory agents
Reslizumab (Cinqair), an interleukin-5 antagonist monoclonal antibody, is given intravenously. It is indicated for add-on maintenance treatment of severe asthma in patients with an eosinophilic phenotype. Animal data in two species suggest low risk. The molecular weight is about 147,000, and the elimination half-life is about 24 days. This suggests that exposure of the embryo and fetus will be minimal, at least in the first half of pregnancy. The maternal benefit appears to outweigh the unknown embryo-fetal risk.
Lactation
None of the above drugs have been studied during breastfeeding. Many drugs, regardless of their molecular weight, will cross into milk in small amounts during the first postpartum week. The effects of this exposure on a nursing infant are unknown. Based on the potential for nursing infant harm, the drugs that probably should not be given during breastfeeding include the four antineoplastics, the atypical antipsychotic pimavanserin, and the diabetes injection lixisenatide.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, and at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation,” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
The Food and Drug Administration approved 22 new drug products in 12 pharmacologic classes in 2016. Additionally, daclizumab (Zenapax), which was approved several years ago for prophylaxis of acute organ rejection in patients receiving renal transplants, was approved for multiple sclerosis treatment (Zinbryta) last year, and sofosbuvir (Sovaldi), which was approved in 2013 for the treatment of hepatitis C virus, is now combined with velpatasvir (Epclusa) to treat all six major forms of hepatitis C.
There are 22 drugs that can be considered novel drugs. As defined by the FDA, novel drugs have never been approved for human use. There are no human pregnancy data for any of the newly approved drugs or drug combinations. As such, it is important to consider that high molecular weight drugs that probably do not cross the placenta in the first half of pregnancy may do so in late pregnancy.
Antineoplastics
Atezolizumab (Tecentriq) is a programmed death ligand–blocking antibody that is indicated for locally advanced or metastatic urothelial carcinoma and metastatic nonsmall cell lung cancer following platinum-containing chemotherapy. Animal reproduction studies have not been conducted, but, based on its mechanism of action, fetal exposure may increase the risk of developing immune-mediated disorders or altering the normal immune response. The molecular weight is high (145,000) and the terminal half-life is long (27 days).
Olaratumab (Lartruvo) is a platelet-derived growth factor receptor– alpha-blocking antibody. It is indicated, in combination with doxorubicin, for the treatment of soft tissue sarcoma. Although the estimated elimination half-life is long (about 11 days with a range of 6-24 days), the high molecular weight (about 154,000) should limit fetal exposure, at least in the first half of pregnancy. The drug should be avoided in pregnancy, however, based on the animal data, the mechanism of action, and its combination with doxorubicin.
Rucaparib (Rubraca) is a poly (adenosine diphosphate–ribose) polymerase inhibitor indicated for the treatment of ovarian cancer. The drug could cause human fetal harm based on the animal data, mechanism of action, relatively low molecular weight (about 556), and terminal half-life (17 hours).
Venetoclax (Venclexta) is a B-cell lymphoma 2 inhibitor indicated for the treatment of chronic lymphocytic leukemia. Although the animal data, molecular weight (about 868), and elimination half-life (about 26 hours) suggest embryo-fetal risk, the high plasma protein binding (99.9%) should limit the amount crossing the placenta.
Anti-infectives
There are two new monoclonal antibodies in this class. Bezlotoxumab (Zinplava) is used to reduce recurrence of Clostridium difficile. Animal reproduction studies have not been conducted. As the molecular weight is about 148,000, the drug will not cross the placenta, at least not in the first half of pregnancy. However, the drug has a long elimination half-life (about 19 days), so, depending on when it was given, it could cross in late pregnancy. Obiltoxaximab (Anthim), administered as a single IV dose, is indicated for the treatment of inhaled anthrax due to Bacillus anthracis. No fetal harm was observed in animal reproduction studies. The high molecular weight (about 148,000) suggests that the drug will not cross to the embryo and/or fetus, at least not in the first part of pregnancy.
Elbasvir/Grazoprevir (Zepatier) is indicated for the treatment of chronic hepatitis C virus genotype 1 or 4. Animal reproduction studies found no evidence of adverse developmental outcomes. The molecular weights of the two components are about 882 and 767, respectively. Both are extensively bound to plasma proteins, 99.9% and 98.8%, respectively, and the terminal half-lives are 24 and 31 hours. Thus, the product appears to be low risk if used in human pregnancy. However, it is contraindicated if given with ribavirin.
Central nervous system agents
Brivaracetam (Briviact) is an anticonvulsant used to treat partial-onset seizures. Animal reproduction studies suggest moderate risk. The molecular weight (about 212), low plasma protein binding (less than or equal to 20%), and terminal plasma half-life of about 9 hours suggest that the drug will cross the placenta. The manufacturer recommends that the drug should be used in pregnancy only if the potential benefit justifies the potential risk to the embryo/fetus.
Pimavanserin (Nuplazid) is an atypical antipsychotic indicated for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. Reproduction studies in animals suggest low risk. The molecular weight of the free base (about 428) and the long mean plasma half-lives of the parent drug and active metabolite (57 and 200 hours) suggest that the drug will cross the placenta. However, the high plasma protein binding (about 95%) may limit the exposure. Nevertheless, avoiding the period of organogenesis appears to be best.
Dermatologic agents
Crisaborole (Eucrisa) is indicated for topical treatment of mild to moderate atopic dermatitis. In 33 pediatric subjects (aged 2-17 years) who applied the ointment twice daily for 8 days, low amounts were absorbed systemically with plasma concentrations in the nanogram/milliliter range. Plasma protein binding was 97%. With oral formulations of the drug, animal reproduction studies suggest low risk. Taken in sum, the human pregnancy risk appears to be low.
Ixekizumab (Taltz) is a humanized interleukin-17A antagonist, administered subcutaneously, that is indicated for the treatment of adults with moderate to severe plaque psoriasis. The drug did not cause developmental toxicity in monkeys. The molecular weight for the drug’s protein backbone is 146,158, and the mean elimination half-life was 13 days. The human embryo-fetal risk in the first half of pregnancy appears to be low.
Diagnostic agents
Fluciclovine F 18 (Axumin) is a radioactive diagnostic agent indicated for positron emission tomography imaging in men with suspected prostate cancer. Since the agent is only used in men, there are no human or animal pregnancy data.
Gallium GA 68 dotatate injection, a diagnostic imaging agent to detect rare neuroendocrine tumors, is not yet on the market.
Endocrine/metabolic agents
Lixisenatide (Adlyxin) is a glucagon-like–peptide-1 receptor agonist that is administered subcutaneously. It is indicated as an adjunct to diet and exercise to improve glycemic control in type 2 diabetes. The drug was teratogenic in two animal species. The molecular weight is about 4,859, and the mean terminal half-life was about 3 hours. Since tight control of glucose levels in type 2 diabetes is required during pregnancy, insulin is the treatment of choice. Consequently, lixisenatide should not be used during pregnancy.
Gastrointestinal agents
Obeticholic acid (Ocaliva) is a farnesoid X receptor agonist that is given orally for the treatment of primary biliary cholangitis, in combination with ursodiol, or ursodeoxycholic acid. I have classified ursodiol as compatible in pregnancy in the 10th edition of “Drugs in Pregnancy and Lactation” (2011: Wolters Kluwer Health). The animal reproduction data for both drugs suggest low risk. Based on the molecular weight of obeticholic (about 421), the drug will probably cross to the embryo/fetus, but the high plasma protein binding (greater than 99%) may limit exposure. The elimination half-life is apparently unknown.
Hematologic agents
Defibrotide sodium (Defitelio), given as an intravenous infusion, is an oligonucleotide mixture. It is indicated for the treatment of hepatic veno-occlusive disease, also known as sinusoidal obstruction syndrome, with renal or pulmonary dysfunction following hematopoietic stem-cell transplantation. Animal reproduction studies in two species suggest risk. The mean molecular weight is 13,000-20,000. Plasma protein binding is an average 93%, and the elimination half-life is less than 2 hours. It is doubtful if the drug crosses the placenta, especially in the first half of pregnancy. If possible, avoid the drug in the second half of pregnancy.
Immunologics
Two indications have been approved for daclizumab. The first was in 2005 for the prophylaxis of acute organ rejection of renal transplants (Zenapax), and the second was in 2016 for the treatment of relapsing forms of multiple sclerosis (Zinbryta). Reproduction studies in monkeys with Zinbryta can be classified as low risk. The molecular weight (about 144,000) suggests that the drug will not cross the placenta, at least in the first half of pregnancy. However, depending on when the drug is given, the long elimination half-life of 21 days might allow the drug to cross in late pregnancy. Regardless, if the perceived maternal benefit exceeds the potential embryo-fetal risk, the drug should not be withheld because of pregnancy.
Muscular disorder agents
Eteplirsen (Exondys 51) is an antisense oligonucleotide that is given intravenously. It is indicated for the treatment of Duchenne muscular dystrophy in patients who have a confirmed mutation of the related gene, which is amenable to exon 51 skipping. There are no animal reproduction data. The molecular weight is about 10,306. This suggests that the drug will not cross the placenta, at least in the first half of pregnancy. The elimination half-life is 3-4 hours, and the plasma concentration 24 hours after a dose was 0.07% of the peak plasma concentration. The drug is given once weekly, and waiting for 24 hours or slightly longer after a dose should reduce the exposure, if any, of the embryo-fetus during the first half of pregnancy.
Nusinersen (Spinraza), a survival motor neuron 2 directed–antisense oligonucleotide, is given as an intrathecal dose. It is indicated for the treatment of spinal muscular atrophy. Subcutaneous doses in two animal species caused no developmental toxicity. The molecular weight of 7,501 suggests that the drug will not cross the human placenta, at least not in the first half of pregnancy. The mean terminal elimination half-life in cerebrospinal fluid was 135-177 days and 63-87 days in plasma.
Ophthalmic agents
Lifitegrast (Xiidra) is an ophthalmic solution of a lymphocyte function-associated–antigen-1 antagonist. It is indicated for the treatment of the signs and symptoms of dry eye disease. The animal reproduction data suggest low risk. The molecular weight is about 616, suggesting that the drug would cross the placenta. However, in a Phase III trial conducted before FDA approval, 47 patients with dry eye disease were given 1 drop twice daily for periods up to 360 days. Nine patients (19%) had plasma predose (trough) concentrations above 0.5 ng/mL, the lower limit of quantitation. Trough plasma concentrations in these patients ranged from 0.55 ng/mL to 3.74 ng/mL. These amounts do not appear to represent an embryo-fetal risk.
Respiratory agents
Reslizumab (Cinqair), an interleukin-5 antagonist monoclonal antibody, is given intravenously. It is indicated for add-on maintenance treatment of severe asthma in patients with an eosinophilic phenotype. Animal data in two species suggest low risk. The molecular weight is about 147,000, and the elimination half-life is about 24 days. This suggests that exposure of the embryo and fetus will be minimal, at least in the first half of pregnancy. The maternal benefit appears to outweigh the unknown embryo-fetal risk.
Lactation
None of the above drugs have been studied during breastfeeding. Many drugs, regardless of their molecular weight, will cross into milk in small amounts during the first postpartum week. The effects of this exposure on a nursing infant are unknown. Based on the potential for nursing infant harm, the drugs that probably should not be given during breastfeeding include the four antineoplastics, the atypical antipsychotic pimavanserin, and the diabetes injection lixisenatide.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, and at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation,” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Anthony Fauci faces the ‘perpetual challenge’ of emerging infections
SAN DIEGO – Reflecting on his 33-year career as director of the National Institute of Allergy and Infectious Diseases, Anthony S. Fauci, MD, can say one thing for certain: Emerging and re-emerging infectious diseases in the continental United States are here to stay.
In an article that he and his colleagues published in the Lancet in 2008, they used the term “perpetual challenge” to describe emerging infections, a descriptor that resonates with him to this day.
Global examples of emerging and re-emerging infectious diseases he discussed include dengue, West Nile virus, chikungunya, and carbapenem-resistant Enterobacteriaceae, “which is becoming a progressively more serious problem in hospitalized patients,” said Dr. Fauci, who is also chief of the NIAID Laboratory of Immunoregulation.
“We had a serious challenge with that at our own clinical center in Bethesda just a few years ago,” he noted. Numerous cases of antimicrobial resistance in methicillin-resistant Staphylococcus aureus, Clostridium difficile, and Neisseria gonorrhoeae have been reported.
Dr. Fauci described the Ebola outbreak as “a globally important disease that had ripple effects in the United States that were unpredicted,” referring to the case of the infected man who traveled from Monrovia to Dallas on Sept. 19, 2014, and developed Ebola symptoms 5 days later. Between 2014 and 2016, there were 28,616 cases and 11,310 deaths combined in the countries of Guinea, Sierra Leone, and Liberia.
“There is virtually no health care system in those three countries,” he said. “There’s a distrust in authority, and anything we tried to do as a global health [effort] made things worse. What we’re trying to do now is built sustainable health care issues in countries that don’t have it.”
Of particular concern to public health officials worldwide is getting a lid on Zika virus, a mosquito-borne illness that can be passed from a pregnant woman to her fetus and cause an increased risk of microcephaly, particularly during the first trimester.
“Not only is there microcephaly, there’s a whole host of abnormalities that involve hearing loss, visual abnormalities, and a variety of other issues,” Dr. Fauci said. “There are about 50 countries in the Americas and the Caribbean that have Zika virus transmission.”
According to data from the Centers for Disease Control and Prevention, from Jan. 1, 2015, to March 29, 2017, there were 5,182 reported cases of Zika virus disease in the 50 states and the District of Columbia. The majority of those (4,886) were travel associated, 222 were locally acquired mosquito-borne, 45 were sexually transmitted, 27 congenital, 1 was laboratory acquired, and 1 was unknown.
At the same time, there have been 38,303 cases in the U.S. territories. Of those, 38,156 were locally acquired, and 147 were travel associated. “That’s why there’s such an intense effort to develop a Zika vaccine,” he said.
According to the CDC, as of March 15, 2017, there are 265 cases of locally transmitted cases in Florida: 216 by mosquito and the rest by sexual transmission. “Talk about surprises,” Dr. Fauci said. “Zika is the first mosquito-borne infection that can result in a congenital abnormality, the first mosquito-borne infection that can be sexually transmitted, and now we’re learning more about this problem, which is the reason why it’s very important for us to develop a vaccine.”
A phase I trial of a DNA vaccine developed by the NIH Vaccine Research Center has reached its enrollment goal of 80 patients age 18-35 years. Initial results are expected sometime in the first quarter of 2017. A phase II trial in the United States and Puerto Rico is expected to launch soon.
Dr. Fauci closed his presentation by sharing lessons learned from previous pandemics.
The first lesson is that global surveillance is required. “Namely, know what’s going on in real time,” he said. “That has to be linked to transparency and communication. So that if something happens in China, we don’t find out about it months later, but we know about it in real time.”
Infrastructure and capacity building are also important. “The lack of capacity in West Africa can ultimately have an indirect impact on us here in the United States,” he said.
“Finally, we need to coordinate and collaborate; we need adaptable platforms for vaccines,” Dr. Fauci cautioned. “Importantly, we need a stable funding mechanism such as a public health emergency fund so that we do not have to go scrambling before the Congress when we need emergency funds.”
SAN DIEGO – Reflecting on his 33-year career as director of the National Institute of Allergy and Infectious Diseases, Anthony S. Fauci, MD, can say one thing for certain: Emerging and re-emerging infectious diseases in the continental United States are here to stay.
In an article that he and his colleagues published in the Lancet in 2008, they used the term “perpetual challenge” to describe emerging infections, a descriptor that resonates with him to this day.
Global examples of emerging and re-emerging infectious diseases he discussed include dengue, West Nile virus, chikungunya, and carbapenem-resistant Enterobacteriaceae, “which is becoming a progressively more serious problem in hospitalized patients,” said Dr. Fauci, who is also chief of the NIAID Laboratory of Immunoregulation.
“We had a serious challenge with that at our own clinical center in Bethesda just a few years ago,” he noted. Numerous cases of antimicrobial resistance in methicillin-resistant Staphylococcus aureus, Clostridium difficile, and Neisseria gonorrhoeae have been reported.
Dr. Fauci described the Ebola outbreak as “a globally important disease that had ripple effects in the United States that were unpredicted,” referring to the case of the infected man who traveled from Monrovia to Dallas on Sept. 19, 2014, and developed Ebola symptoms 5 days later. Between 2014 and 2016, there were 28,616 cases and 11,310 deaths combined in the countries of Guinea, Sierra Leone, and Liberia.
“There is virtually no health care system in those three countries,” he said. “There’s a distrust in authority, and anything we tried to do as a global health [effort] made things worse. What we’re trying to do now is built sustainable health care issues in countries that don’t have it.”
Of particular concern to public health officials worldwide is getting a lid on Zika virus, a mosquito-borne illness that can be passed from a pregnant woman to her fetus and cause an increased risk of microcephaly, particularly during the first trimester.
“Not only is there microcephaly, there’s a whole host of abnormalities that involve hearing loss, visual abnormalities, and a variety of other issues,” Dr. Fauci said. “There are about 50 countries in the Americas and the Caribbean that have Zika virus transmission.”
According to data from the Centers for Disease Control and Prevention, from Jan. 1, 2015, to March 29, 2017, there were 5,182 reported cases of Zika virus disease in the 50 states and the District of Columbia. The majority of those (4,886) were travel associated, 222 were locally acquired mosquito-borne, 45 were sexually transmitted, 27 congenital, 1 was laboratory acquired, and 1 was unknown.
At the same time, there have been 38,303 cases in the U.S. territories. Of those, 38,156 were locally acquired, and 147 were travel associated. “That’s why there’s such an intense effort to develop a Zika vaccine,” he said.
According to the CDC, as of March 15, 2017, there are 265 cases of locally transmitted cases in Florida: 216 by mosquito and the rest by sexual transmission. “Talk about surprises,” Dr. Fauci said. “Zika is the first mosquito-borne infection that can result in a congenital abnormality, the first mosquito-borne infection that can be sexually transmitted, and now we’re learning more about this problem, which is the reason why it’s very important for us to develop a vaccine.”
A phase I trial of a DNA vaccine developed by the NIH Vaccine Research Center has reached its enrollment goal of 80 patients age 18-35 years. Initial results are expected sometime in the first quarter of 2017. A phase II trial in the United States and Puerto Rico is expected to launch soon.
Dr. Fauci closed his presentation by sharing lessons learned from previous pandemics.
The first lesson is that global surveillance is required. “Namely, know what’s going on in real time,” he said. “That has to be linked to transparency and communication. So that if something happens in China, we don’t find out about it months later, but we know about it in real time.”
Infrastructure and capacity building are also important. “The lack of capacity in West Africa can ultimately have an indirect impact on us here in the United States,” he said.
“Finally, we need to coordinate and collaborate; we need adaptable platforms for vaccines,” Dr. Fauci cautioned. “Importantly, we need a stable funding mechanism such as a public health emergency fund so that we do not have to go scrambling before the Congress when we need emergency funds.”
SAN DIEGO – Reflecting on his 33-year career as director of the National Institute of Allergy and Infectious Diseases, Anthony S. Fauci, MD, can say one thing for certain: Emerging and re-emerging infectious diseases in the continental United States are here to stay.
In an article that he and his colleagues published in the Lancet in 2008, they used the term “perpetual challenge” to describe emerging infections, a descriptor that resonates with him to this day.
Global examples of emerging and re-emerging infectious diseases he discussed include dengue, West Nile virus, chikungunya, and carbapenem-resistant Enterobacteriaceae, “which is becoming a progressively more serious problem in hospitalized patients,” said Dr. Fauci, who is also chief of the NIAID Laboratory of Immunoregulation.
“We had a serious challenge with that at our own clinical center in Bethesda just a few years ago,” he noted. Numerous cases of antimicrobial resistance in methicillin-resistant Staphylococcus aureus, Clostridium difficile, and Neisseria gonorrhoeae have been reported.
Dr. Fauci described the Ebola outbreak as “a globally important disease that had ripple effects in the United States that were unpredicted,” referring to the case of the infected man who traveled from Monrovia to Dallas on Sept. 19, 2014, and developed Ebola symptoms 5 days later. Between 2014 and 2016, there were 28,616 cases and 11,310 deaths combined in the countries of Guinea, Sierra Leone, and Liberia.
“There is virtually no health care system in those three countries,” he said. “There’s a distrust in authority, and anything we tried to do as a global health [effort] made things worse. What we’re trying to do now is built sustainable health care issues in countries that don’t have it.”
Of particular concern to public health officials worldwide is getting a lid on Zika virus, a mosquito-borne illness that can be passed from a pregnant woman to her fetus and cause an increased risk of microcephaly, particularly during the first trimester.
“Not only is there microcephaly, there’s a whole host of abnormalities that involve hearing loss, visual abnormalities, and a variety of other issues,” Dr. Fauci said. “There are about 50 countries in the Americas and the Caribbean that have Zika virus transmission.”
According to data from the Centers for Disease Control and Prevention, from Jan. 1, 2015, to March 29, 2017, there were 5,182 reported cases of Zika virus disease in the 50 states and the District of Columbia. The majority of those (4,886) were travel associated, 222 were locally acquired mosquito-borne, 45 were sexually transmitted, 27 congenital, 1 was laboratory acquired, and 1 was unknown.
At the same time, there have been 38,303 cases in the U.S. territories. Of those, 38,156 were locally acquired, and 147 were travel associated. “That’s why there’s such an intense effort to develop a Zika vaccine,” he said.
According to the CDC, as of March 15, 2017, there are 265 cases of locally transmitted cases in Florida: 216 by mosquito and the rest by sexual transmission. “Talk about surprises,” Dr. Fauci said. “Zika is the first mosquito-borne infection that can result in a congenital abnormality, the first mosquito-borne infection that can be sexually transmitted, and now we’re learning more about this problem, which is the reason why it’s very important for us to develop a vaccine.”
A phase I trial of a DNA vaccine developed by the NIH Vaccine Research Center has reached its enrollment goal of 80 patients age 18-35 years. Initial results are expected sometime in the first quarter of 2017. A phase II trial in the United States and Puerto Rico is expected to launch soon.
Dr. Fauci closed his presentation by sharing lessons learned from previous pandemics.
The first lesson is that global surveillance is required. “Namely, know what’s going on in real time,” he said. “That has to be linked to transparency and communication. So that if something happens in China, we don’t find out about it months later, but we know about it in real time.”
Infrastructure and capacity building are also important. “The lack of capacity in West Africa can ultimately have an indirect impact on us here in the United States,” he said.
“Finally, we need to coordinate and collaborate; we need adaptable platforms for vaccines,” Dr. Fauci cautioned. “Importantly, we need a stable funding mechanism such as a public health emergency fund so that we do not have to go scrambling before the Congress when we need emergency funds.”
EXPERT ANALYSIS FROM ACP INTERNAL MEDICINE
Exposure to HPV vaccine in pregnancy not linked to birth defects
The quadrivalent human papillomavirus (HPV) vaccine was not associated with any adverse pregnancy outcomes when inadvertently given during pregnancy, according to the findings from a nationwide Danish study.
“Our results are consistent with other evidence that does not indicate that the vaccination of pregnant women with inactivated virus, bacterial, or toxoid vaccines generally confers a higher risk of adverse pregnancy outcomes than no such vaccination. Our results also confirm and considerably expand on results from previous studies of the quadrivalent HPV vaccine,” wrote Nikolai M. Scheller, MD, of the Statens Serum Institut, Copenhagen, and his colleagues.
Quadrivalent HPV vaccination was not associated with any increase in risk for major birth defects (prevalence odds ratio, 1.19), spontaneous abortion (hazard ratio, 0.71), preterm birth (prevalence OR, 1.15), small size for gestational age (prevalence OR, 0.86), or low birth weight (prevalence OR, 1.10). It also was not associated with increased risk for stillbirth, but this outcome occurred in only two case patients and four controls, making it “impossible to draw clinically meaningful conclusions” regarding the risk for stillbirth, Dr. Scheller and his colleagues reported (N Engl J Med. 2017;376[13]:1223-33).
“Because many [adverse] pregnancy outcomes are rare, our study did not have the statistical power to assess the risks of stillbirth and specific major birth defects associated with quadrivalent HPV vaccination. Larger studies would be needed to address these outcomes,” they added.
Novo Nordisk and the Danish Medical Research Council funded the study. Dr. Scheller reported having no relevant financial disclosures; two of his coauthors reported receiving research grants from the Novo Nordisk Foundation and the Danish Medical Research Council.
Although a large number of women (1,665) were immunized in the first trimester of pregnancy, when organogenesis occurs, their offspring did not have a significantly higher rate of major birth defects compared with offspring born to unvaccinated women. The numbers of spontaneous abortions, preterm births, infants with low birth weight, infants who were small for gestational age, and stillbirths were not higher in the vaccinated cohorts than in the unvaccinated cohorts, although the number of stillbirths was small.
These data are very encouraging and strongly support the safety of HPV vaccines if they are inadvertently given in pregnancy, a finding that complements previous safety reports of HPV vaccine in nonpregnant women. These data also show that adverse outcomes occur at a baseline rate in pregnancy and that when no control group is included in studies, these outcomes may be inappropriately attributed to the vaccine.
Kathryn M. Edwards, MD, is in the division of infectious diseases and the department of pediatrics at Vanderbilt University, Nashville, Tenn. She reported receiving grants from Novartis for research on group B strep vaccines in pregnancy. These comments are excerpted from an accompanying editorial (N Engl J Med. 2017;376[13]:1280-2).
Although a large number of women (1,665) were immunized in the first trimester of pregnancy, when organogenesis occurs, their offspring did not have a significantly higher rate of major birth defects compared with offspring born to unvaccinated women. The numbers of spontaneous abortions, preterm births, infants with low birth weight, infants who were small for gestational age, and stillbirths were not higher in the vaccinated cohorts than in the unvaccinated cohorts, although the number of stillbirths was small.
These data are very encouraging and strongly support the safety of HPV vaccines if they are inadvertently given in pregnancy, a finding that complements previous safety reports of HPV vaccine in nonpregnant women. These data also show that adverse outcomes occur at a baseline rate in pregnancy and that when no control group is included in studies, these outcomes may be inappropriately attributed to the vaccine.
Kathryn M. Edwards, MD, is in the division of infectious diseases and the department of pediatrics at Vanderbilt University, Nashville, Tenn. She reported receiving grants from Novartis for research on group B strep vaccines in pregnancy. These comments are excerpted from an accompanying editorial (N Engl J Med. 2017;376[13]:1280-2).
Although a large number of women (1,665) were immunized in the first trimester of pregnancy, when organogenesis occurs, their offspring did not have a significantly higher rate of major birth defects compared with offspring born to unvaccinated women. The numbers of spontaneous abortions, preterm births, infants with low birth weight, infants who were small for gestational age, and stillbirths were not higher in the vaccinated cohorts than in the unvaccinated cohorts, although the number of stillbirths was small.
These data are very encouraging and strongly support the safety of HPV vaccines if they are inadvertently given in pregnancy, a finding that complements previous safety reports of HPV vaccine in nonpregnant women. These data also show that adverse outcomes occur at a baseline rate in pregnancy and that when no control group is included in studies, these outcomes may be inappropriately attributed to the vaccine.
Kathryn M. Edwards, MD, is in the division of infectious diseases and the department of pediatrics at Vanderbilt University, Nashville, Tenn. She reported receiving grants from Novartis for research on group B strep vaccines in pregnancy. These comments are excerpted from an accompanying editorial (N Engl J Med. 2017;376[13]:1280-2).
The quadrivalent human papillomavirus (HPV) vaccine was not associated with any adverse pregnancy outcomes when inadvertently given during pregnancy, according to the findings from a nationwide Danish study.
“Our results are consistent with other evidence that does not indicate that the vaccination of pregnant women with inactivated virus, bacterial, or toxoid vaccines generally confers a higher risk of adverse pregnancy outcomes than no such vaccination. Our results also confirm and considerably expand on results from previous studies of the quadrivalent HPV vaccine,” wrote Nikolai M. Scheller, MD, of the Statens Serum Institut, Copenhagen, and his colleagues.
Quadrivalent HPV vaccination was not associated with any increase in risk for major birth defects (prevalence odds ratio, 1.19), spontaneous abortion (hazard ratio, 0.71), preterm birth (prevalence OR, 1.15), small size for gestational age (prevalence OR, 0.86), or low birth weight (prevalence OR, 1.10). It also was not associated with increased risk for stillbirth, but this outcome occurred in only two case patients and four controls, making it “impossible to draw clinically meaningful conclusions” regarding the risk for stillbirth, Dr. Scheller and his colleagues reported (N Engl J Med. 2017;376[13]:1223-33).
“Because many [adverse] pregnancy outcomes are rare, our study did not have the statistical power to assess the risks of stillbirth and specific major birth defects associated with quadrivalent HPV vaccination. Larger studies would be needed to address these outcomes,” they added.
Novo Nordisk and the Danish Medical Research Council funded the study. Dr. Scheller reported having no relevant financial disclosures; two of his coauthors reported receiving research grants from the Novo Nordisk Foundation and the Danish Medical Research Council.
The quadrivalent human papillomavirus (HPV) vaccine was not associated with any adverse pregnancy outcomes when inadvertently given during pregnancy, according to the findings from a nationwide Danish study.
“Our results are consistent with other evidence that does not indicate that the vaccination of pregnant women with inactivated virus, bacterial, or toxoid vaccines generally confers a higher risk of adverse pregnancy outcomes than no such vaccination. Our results also confirm and considerably expand on results from previous studies of the quadrivalent HPV vaccine,” wrote Nikolai M. Scheller, MD, of the Statens Serum Institut, Copenhagen, and his colleagues.
Quadrivalent HPV vaccination was not associated with any increase in risk for major birth defects (prevalence odds ratio, 1.19), spontaneous abortion (hazard ratio, 0.71), preterm birth (prevalence OR, 1.15), small size for gestational age (prevalence OR, 0.86), or low birth weight (prevalence OR, 1.10). It also was not associated with increased risk for stillbirth, but this outcome occurred in only two case patients and four controls, making it “impossible to draw clinically meaningful conclusions” regarding the risk for stillbirth, Dr. Scheller and his colleagues reported (N Engl J Med. 2017;376[13]:1223-33).
“Because many [adverse] pregnancy outcomes are rare, our study did not have the statistical power to assess the risks of stillbirth and specific major birth defects associated with quadrivalent HPV vaccination. Larger studies would be needed to address these outcomes,” they added.
Novo Nordisk and the Danish Medical Research Council funded the study. Dr. Scheller reported having no relevant financial disclosures; two of his coauthors reported receiving research grants from the Novo Nordisk Foundation and the Danish Medical Research Council.
Key clinical point:
Major finding: Quadrivalent HPV vaccination was not associated with any increase in risk for major birth defects (prevalence odds ratio, 1.19).
Data source: A population-based cohort study involving 581,550 pregnancies across Denmark during a 7-year period, including 1,665 in which the mother inadvertently received the HPV vaccine.
Disclosures: Novo Nordisk and the Danish Medical Research Council funded the study. Dr. Scheller reported having no relevant financial disclosures; two of his coauthors reported receiving research grants from the Novo Nordisk Foundation and the Danish Medical Research Council.
Delivering clinician should be seated
“MANAGEMENT OF WOUND COMPLICATIONS FOLLOWING OBSTETRIC ANAL SPHINCTER INJURY (OASIS)”
ROBERT L. BARBIERI, MD, AND JEANNINE M. MIRANNE, MD, MS (EDITORIAL; DECEMBER 2016)
Delivering clinician should be seated
Indeed, obstetric anal sphincter injuries (OASIS),1 with their short- and long-term consequences, merit clinical attention, as spotlighted in Dr. Barbieri and Dr. Miranne’s article. An issue not discussed is the position of the obstetrician.
In our practice, we sit down to perform a vaginal delivery, as taught by Soranus of Ephesus.2 We strive to be at the bedside sooner than when the nurse calls “she is crowning.” This allows communication with the woman, attending nurse, and support person(s), as well as for a brief review of recent estimated fetal weight, length of the second stage, position of the presenting part, degree of flexion, presence of caput, and other last-minute details. Sitting down in front of the outlet permits uninterrupted visual evaluation of the distention of the soft perineal tissues. All traditional maneuvers are performed comfortably from the sitting position: the vertex is controlled by hands-on, and a quick reach with the nonpredominant hand searches for a loop of cord or a small part procidentia to resolve it. The patient is coached either for the next bearing-down effort or to not push to allow for gradual, controlled delivery of the fetal shoulder girdle. We avoid use of the fetal head for traction and move to facilitate “shrugging” with reduction of the bisacromial to facilitate delivery.
In our experience, the sitting position is ideal to observe uninterruptedly the tension of the perineal body during vertex and shoulders delivery, without having to flex and rotate our back and neck in repeatedly nonergonomic positions.
If an obstetrician of above-average height stands for the delivery, the obstetric bed should be elevated to fit her or his reach. Should shoulder dystocia occur, an assistant will stand on a chair and hover over the maternal abdomen to provide suprapubic pressure (indeed, an indelible memory for any parturient and her family). From the sitting position, exploration of the birth canal and repair of any injury, if necessary, can be conducted without technical impediments.
These simple steps have provided our patients and ourselves with clinical and professional satisfaction with minimal OASIS events as shown by others.3 Ironically, if we successfully avoid perineal injuries, our young trainees may require simulation training to learn this tedious repair procedure. In our geographic practice area, a new “collaborative” expects the frequency of episiotomy to be less than 4.6%. Third- and 4th-degree spontaneous or procedure-related perineal injuries still are used to measure quality of care despite demonstrated reasons for this parameter to be a noncredible metric.
Federico G. Mariona, MD
Dearborn, Michigan
Dr. Barbieri responds
I agree with Dr. Mariona that in some cases the fetal head delivers without causing a 3rd- or 4th-degree laceration, but then the delivery of the posterior shoulder causes a severe perineal injury. Dr. Mariona’s clinical pearl is that the delivering clinician should be seated, carefully observe the delivery of the shoulders, and facilitate fetal shrugging by gently reducing the bisacromial diameter as the posterior shoulder transitions over the perineal body.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Verghese TS, Champaneria R, Kapoor DS, Latthe PM. Obstetric anal sphincter injuries after episiotomy: systematic review and meta-analysis. Int Urogynecol J. 2016;27(10):1459–1467.
- Drife J. The start of life: a history of obstetrics. Postgrad Med J. 2002;78(919):311–315.
- Basu M, Smith D, Edwards R; STOMP Project Team. Can the incidence of obstetric anal sphincter injury be reduced? The STOMP experience. Eur J Obstet Gynecol Reprod Biol. 2016;202:55–59.
“MANAGEMENT OF WOUND COMPLICATIONS FOLLOWING OBSTETRIC ANAL SPHINCTER INJURY (OASIS)”
ROBERT L. BARBIERI, MD, AND JEANNINE M. MIRANNE, MD, MS (EDITORIAL; DECEMBER 2016)
Delivering clinician should be seated
Indeed, obstetric anal sphincter injuries (OASIS),1 with their short- and long-term consequences, merit clinical attention, as spotlighted in Dr. Barbieri and Dr. Miranne’s article. An issue not discussed is the position of the obstetrician.
In our practice, we sit down to perform a vaginal delivery, as taught by Soranus of Ephesus.2 We strive to be at the bedside sooner than when the nurse calls “she is crowning.” This allows communication with the woman, attending nurse, and support person(s), as well as for a brief review of recent estimated fetal weight, length of the second stage, position of the presenting part, degree of flexion, presence of caput, and other last-minute details. Sitting down in front of the outlet permits uninterrupted visual evaluation of the distention of the soft perineal tissues. All traditional maneuvers are performed comfortably from the sitting position: the vertex is controlled by hands-on, and a quick reach with the nonpredominant hand searches for a loop of cord or a small part procidentia to resolve it. The patient is coached either for the next bearing-down effort or to not push to allow for gradual, controlled delivery of the fetal shoulder girdle. We avoid use of the fetal head for traction and move to facilitate “shrugging” with reduction of the bisacromial to facilitate delivery.
In our experience, the sitting position is ideal to observe uninterruptedly the tension of the perineal body during vertex and shoulders delivery, without having to flex and rotate our back and neck in repeatedly nonergonomic positions.
If an obstetrician of above-average height stands for the delivery, the obstetric bed should be elevated to fit her or his reach. Should shoulder dystocia occur, an assistant will stand on a chair and hover over the maternal abdomen to provide suprapubic pressure (indeed, an indelible memory for any parturient and her family). From the sitting position, exploration of the birth canal and repair of any injury, if necessary, can be conducted without technical impediments.
These simple steps have provided our patients and ourselves with clinical and professional satisfaction with minimal OASIS events as shown by others.3 Ironically, if we successfully avoid perineal injuries, our young trainees may require simulation training to learn this tedious repair procedure. In our geographic practice area, a new “collaborative” expects the frequency of episiotomy to be less than 4.6%. Third- and 4th-degree spontaneous or procedure-related perineal injuries still are used to measure quality of care despite demonstrated reasons for this parameter to be a noncredible metric.
Federico G. Mariona, MD
Dearborn, Michigan
Dr. Barbieri responds
I agree with Dr. Mariona that in some cases the fetal head delivers without causing a 3rd- or 4th-degree laceration, but then the delivery of the posterior shoulder causes a severe perineal injury. Dr. Mariona’s clinical pearl is that the delivering clinician should be seated, carefully observe the delivery of the shoulders, and facilitate fetal shrugging by gently reducing the bisacromial diameter as the posterior shoulder transitions over the perineal body.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“MANAGEMENT OF WOUND COMPLICATIONS FOLLOWING OBSTETRIC ANAL SPHINCTER INJURY (OASIS)”
ROBERT L. BARBIERI, MD, AND JEANNINE M. MIRANNE, MD, MS (EDITORIAL; DECEMBER 2016)
Delivering clinician should be seated
Indeed, obstetric anal sphincter injuries (OASIS),1 with their short- and long-term consequences, merit clinical attention, as spotlighted in Dr. Barbieri and Dr. Miranne’s article. An issue not discussed is the position of the obstetrician.
In our practice, we sit down to perform a vaginal delivery, as taught by Soranus of Ephesus.2 We strive to be at the bedside sooner than when the nurse calls “she is crowning.” This allows communication with the woman, attending nurse, and support person(s), as well as for a brief review of recent estimated fetal weight, length of the second stage, position of the presenting part, degree of flexion, presence of caput, and other last-minute details. Sitting down in front of the outlet permits uninterrupted visual evaluation of the distention of the soft perineal tissues. All traditional maneuvers are performed comfortably from the sitting position: the vertex is controlled by hands-on, and a quick reach with the nonpredominant hand searches for a loop of cord or a small part procidentia to resolve it. The patient is coached either for the next bearing-down effort or to not push to allow for gradual, controlled delivery of the fetal shoulder girdle. We avoid use of the fetal head for traction and move to facilitate “shrugging” with reduction of the bisacromial to facilitate delivery.
In our experience, the sitting position is ideal to observe uninterruptedly the tension of the perineal body during vertex and shoulders delivery, without having to flex and rotate our back and neck in repeatedly nonergonomic positions.
If an obstetrician of above-average height stands for the delivery, the obstetric bed should be elevated to fit her or his reach. Should shoulder dystocia occur, an assistant will stand on a chair and hover over the maternal abdomen to provide suprapubic pressure (indeed, an indelible memory for any parturient and her family). From the sitting position, exploration of the birth canal and repair of any injury, if necessary, can be conducted without technical impediments.
These simple steps have provided our patients and ourselves with clinical and professional satisfaction with minimal OASIS events as shown by others.3 Ironically, if we successfully avoid perineal injuries, our young trainees may require simulation training to learn this tedious repair procedure. In our geographic practice area, a new “collaborative” expects the frequency of episiotomy to be less than 4.6%. Third- and 4th-degree spontaneous or procedure-related perineal injuries still are used to measure quality of care despite demonstrated reasons for this parameter to be a noncredible metric.
Federico G. Mariona, MD
Dearborn, Michigan
Dr. Barbieri responds
I agree with Dr. Mariona that in some cases the fetal head delivers without causing a 3rd- or 4th-degree laceration, but then the delivery of the posterior shoulder causes a severe perineal injury. Dr. Mariona’s clinical pearl is that the delivering clinician should be seated, carefully observe the delivery of the shoulders, and facilitate fetal shrugging by gently reducing the bisacromial diameter as the posterior shoulder transitions over the perineal body.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Verghese TS, Champaneria R, Kapoor DS, Latthe PM. Obstetric anal sphincter injuries after episiotomy: systematic review and meta-analysis. Int Urogynecol J. 2016;27(10):1459–1467.
- Drife J. The start of life: a history of obstetrics. Postgrad Med J. 2002;78(919):311–315.
- Basu M, Smith D, Edwards R; STOMP Project Team. Can the incidence of obstetric anal sphincter injury be reduced? The STOMP experience. Eur J Obstet Gynecol Reprod Biol. 2016;202:55–59.
- Verghese TS, Champaneria R, Kapoor DS, Latthe PM. Obstetric anal sphincter injuries after episiotomy: systematic review and meta-analysis. Int Urogynecol J. 2016;27(10):1459–1467.
- Drife J. The start of life: a history of obstetrics. Postgrad Med J. 2002;78(919):311–315.
- Basu M, Smith D, Edwards R; STOMP Project Team. Can the incidence of obstetric anal sphincter injury be reduced? The STOMP experience. Eur J Obstet Gynecol Reprod Biol. 2016;202:55–59.
Montgomery maneuver for shoulder dystocia
“SHOULDER DYSTOCIA: TAKING THE FEAR OUT OF MANAGEMENT”
JOHN T. REPKE, MD, AND RONALD T. BURKMAN, MD (WEB EXCLUSIVE; APRIL 2016)
Montgomery maneuver for shoulder dystocia
In managing shoulder dystocia, my maneuver is to use my elbow to maximize mechanical advantage when applying suprapubic pressure to push the trapped shoulder down. It works well and is more efficient than having a nurse standing off to the side.
J.S. Montgomery, MD
Cypress, Texas

Dr. Barbieri responds
I thank Dr. Montgomery for sharing his maneuver for dislodging the trapped anterior shoulder by using his elbow to apply suprapubic pressure. There is vast knowledge and experience in our clinical community, and sharing insights is helpful to all our readers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“SHOULDER DYSTOCIA: TAKING THE FEAR OUT OF MANAGEMENT”
JOHN T. REPKE, MD, AND RONALD T. BURKMAN, MD (WEB EXCLUSIVE; APRIL 2016)
Montgomery maneuver for shoulder dystocia
In managing shoulder dystocia, my maneuver is to use my elbow to maximize mechanical advantage when applying suprapubic pressure to push the trapped shoulder down. It works well and is more efficient than having a nurse standing off to the side.
J.S. Montgomery, MD
Cypress, Texas

Dr. Barbieri responds
I thank Dr. Montgomery for sharing his maneuver for dislodging the trapped anterior shoulder by using his elbow to apply suprapubic pressure. There is vast knowledge and experience in our clinical community, and sharing insights is helpful to all our readers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“SHOULDER DYSTOCIA: TAKING THE FEAR OUT OF MANAGEMENT”
JOHN T. REPKE, MD, AND RONALD T. BURKMAN, MD (WEB EXCLUSIVE; APRIL 2016)
Montgomery maneuver for shoulder dystocia
In managing shoulder dystocia, my maneuver is to use my elbow to maximize mechanical advantage when applying suprapubic pressure to push the trapped shoulder down. It works well and is more efficient than having a nurse standing off to the side.
J.S. Montgomery, MD
Cypress, Texas

Dr. Barbieri responds
I thank Dr. Montgomery for sharing his maneuver for dislodging the trapped anterior shoulder by using his elbow to apply suprapubic pressure. There is vast knowledge and experience in our clinical community, and sharing insights is helpful to all our readers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Forceful use of forceps, infant dies: $10.2M verdict
Forceful use of forceps, infant dies: $10.2M verdict
A woman in her mid-20s went to the hospital in labor. After several hours, fetal heart-rate (FHR) monitor results became nonreassuring. The ObGyn and the nurse in charge disagreed on the interpretation of the FHR monitor strips. The nurse went to her supervisor, who confronted the ObGyn 2 hours later, saying that fetal distress was a serious concern and necessitated the cessation of oxytocin. The ObGyn disagreed and ordered another nurse to increase the oxytocin dose.
Three hours later, when the FHR monitoring strips showed severe distress, the ObGyn decided to undertake an operative vaginal delivery. During a 17-minute period, the ObGyn unsuccessfully used forceps 3 times. On the second attempt, a cracking noise was heard. Then a cesarean delivery was ordered; the baby was born limp, lifeless, and unresponsive. She was found to have hypoxic ischemic encephalopathy, was removed from life support, and died.
PARENTS’ CLAIM:
Oxytocin should not have been continued when the baby was clearly in distress. The supervising nurse should have contacted her supervisor and continued up the chain of command until the ObGyn was forced to stop the oxytocin.
Physicians are prohibited from using their leg muscles when applying forceps; gentle action is critical. During one attempt, the ObGyn had his leg on the bed to increase the force with which he pulled on the forceps. The ObGyn’s reckless use of forceps caused a skull fracture to depress into the brain. The ObGyn also tried to turn the baby using forceps, which is outside the standard of care because of the risk of rotational injury. A mother’s pushing rarely causes such severe damage to the baby.
DEFENDANTS’ DEFENSE:
There was no negligence. The hypoxia was due to a hemorrhage. Natural forces of a long delivery caused the skull injury.
VERDICT:
A $10,200,575 Texas verdict was returned.
After long labor, baby has CP: $8.4M settlement
Early on March 20, a 30-year-old woman who weighed 300 lbs was admitted for delivery at 40 weeks’ gestation. Labor was induced with oxytocin. Within 30 minutes, FHR monitoring showed that the baby’s baseline began to climb, accelerations ceased, and late decelerations commenced. The oxytocin dose was steadily increased throughout the day. A nurse decided that the baby was not tolerating the contractions and discontinued oxytocin. The attending ObGyn ordered oxytocin be restarted after giving the baby a chance to recover. The mother requested a cesarean delivery, but the ObGyn refused, saying that he was concerned with the risk due to her excessive weight and prior heart surgery. When his shift ended, his partner took over.
On March 21, a nurse reported that the FHR had climbed to 160 bpm although labor had not progressed. The ObGyn ordered terbutaline to slow contractions but he did not examine the mother. An hour after terbutaline administration, the FHR showed a deceleration. An emergency cesarean delivery was performed. The baby, born severely depressed, was resuscitated. Magnetic resonance imaging performed at 23 days of life showed that the child had a hypoxic ischemic injury. She has cerebral palsy and is nonambulatory with significant cognitive deficits.
PARENTS’ CLAIM:
The care provided by 2 ObGyns, nursing staff, and hospital was negligent. A cesarean delivery should have been performed on March 20 when the nurse identified fetal distress. The nurses should have been more assertive in recommending cesarean delivery. The injury occurred 30 minutes prior to delivery and could have been prevented by an earlier cesarean delivery.
DEFENDANTS’ DEFENSE:
FHR strips on March 20 were not as nonreassuring as claimed and did not warrant cesarean delivery, which was performed when needed.
VERDICT:
An $8.4 million Wisconsin settlement was reached by mediation.
Eclamptic seizure, twins stillborn: $4.25M
A 29-year-old woman pregnant with twins had an eclamptic seizure at 33 4/7 weeks’ gestation. The babies were stillborn.
PARENTS’ CLAIM:
The ObGyn failed to properly treat the patient’s preeclampsia for more than 11 weeks. The seizure caused hypovolemic shock, tachycardia, and massive hemorrhaging and required an emergency hysterectomy and bilateral salpingo-oophorectomy. The patient has no children and has been rendered unable to conceive. She sought to apportion 60% of the settlement proceeds to her distress claim and 20% each to wrongful-death and survival claims. She also sought to bar the twins’ biological father from sharing in the recovery due to abandonment.
HOSPITAL'S DEFENSE:
The case was settled during the trial.
VERDICT:
The mother agreed to receive 65% of the wrongful-death and survival funds, with 35% going to the father. A Pennsylvania settlement of $4.25 million was reached.
Brachial plexus injury: $4.8M verdict
A woman gave birth with assistance from a midwife. During delivery, shoulder dystocia was encountered. The baby has a permanent brachial plexus injury.
PARENTS’ CLAIM:
The midwife mismanaged shoulder dystocia by applying excessive traction to the baby’s head. The ObGyn in charge of the mother’s care did not provide adequate supervision.
DEFENDANTS’ DEFENSE:
The hospital settled prior to trial. The midwife and ObGyn denied negligence during delivery and contended that the child’s injury occurred as a result of the natural forces of labor.
VERDICT:
The jury found the midwife 60% negligent and the ObGyn 40% negligent. A $4.82 million Florida verdict was returned.
What caused infant's death?
During prenatal care, a woman underwent weekly nonstress tests due to excessive amniotic fluid until the level returned to normal. Near the end of her pregnancy, the patient noticed a decrease in fetal movement and called her ObGyn group. She was told to perform a fetal kick count and go to the emergency department (ED) if the count was abnormal, but she fell asleep. In the morning, she presented to the ObGyns’ office and was sent to the hospital for emergency cesarean delivery, which was performed 2.5 hrs after her arrival. The infant was born in distress and died 8 hours later.
PARENTS’ CLAIM:
The ObGyns should have continued weekly tests even after the amniotic fluid level returned to normal. She should have been sent to the ED when she initially reported decreased fetal movement. Cesarean delivery should have been performed immediately upon her arrival at the hospital.
PHYSICIAN’S DEFENSE:
Further prenatal testing for amniotic fluid levels was unwarranted. Telephone advice to count fetal kicks was appropriate. The delay in performing a cesarean delivery was beyond the ObGyns’ control. The outcome would have been the same regardless of their actions.
VERDICT:
A Michigan defense verdict was returned.
Perineal laceration during vaginal delivery
During vaginal delivery, a 27-year-old woman suffered a 4th-degree perineal laceration. She developed a retrovaginal fistula and has permanent fecal incontinence.
PARENTS’ CLAIM:
The ObGyn’s care was negligent. She failed to perform a rectal examination to assess the severity of the perineal laceration. The laceration was improperly repaired, and, as a result, the patient developed a retrovaginal fistula that persisted for 6 months until it was surgically repaired. A divot in her anal canal causes fecal incontinence.
PHYSICIAN’S DEFENSE:
The ObGyn contended she correctly diagnosed and repaired a 3rd-degree laceration. The wound later broke down for unknown reasons.
VERDICT:
An Arizona defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Forceful use of forceps, infant dies: $10.2M verdict
A woman in her mid-20s went to the hospital in labor. After several hours, fetal heart-rate (FHR) monitor results became nonreassuring. The ObGyn and the nurse in charge disagreed on the interpretation of the FHR monitor strips. The nurse went to her supervisor, who confronted the ObGyn 2 hours later, saying that fetal distress was a serious concern and necessitated the cessation of oxytocin. The ObGyn disagreed and ordered another nurse to increase the oxytocin dose.
Three hours later, when the FHR monitoring strips showed severe distress, the ObGyn decided to undertake an operative vaginal delivery. During a 17-minute period, the ObGyn unsuccessfully used forceps 3 times. On the second attempt, a cracking noise was heard. Then a cesarean delivery was ordered; the baby was born limp, lifeless, and unresponsive. She was found to have hypoxic ischemic encephalopathy, was removed from life support, and died.
PARENTS’ CLAIM:
Oxytocin should not have been continued when the baby was clearly in distress. The supervising nurse should have contacted her supervisor and continued up the chain of command until the ObGyn was forced to stop the oxytocin.
Physicians are prohibited from using their leg muscles when applying forceps; gentle action is critical. During one attempt, the ObGyn had his leg on the bed to increase the force with which he pulled on the forceps. The ObGyn’s reckless use of forceps caused a skull fracture to depress into the brain. The ObGyn also tried to turn the baby using forceps, which is outside the standard of care because of the risk of rotational injury. A mother’s pushing rarely causes such severe damage to the baby.
DEFENDANTS’ DEFENSE:
There was no negligence. The hypoxia was due to a hemorrhage. Natural forces of a long delivery caused the skull injury.
VERDICT:
A $10,200,575 Texas verdict was returned.
After long labor, baby has CP: $8.4M settlement
Early on March 20, a 30-year-old woman who weighed 300 lbs was admitted for delivery at 40 weeks’ gestation. Labor was induced with oxytocin. Within 30 minutes, FHR monitoring showed that the baby’s baseline began to climb, accelerations ceased, and late decelerations commenced. The oxytocin dose was steadily increased throughout the day. A nurse decided that the baby was not tolerating the contractions and discontinued oxytocin. The attending ObGyn ordered oxytocin be restarted after giving the baby a chance to recover. The mother requested a cesarean delivery, but the ObGyn refused, saying that he was concerned with the risk due to her excessive weight and prior heart surgery. When his shift ended, his partner took over.
On March 21, a nurse reported that the FHR had climbed to 160 bpm although labor had not progressed. The ObGyn ordered terbutaline to slow contractions but he did not examine the mother. An hour after terbutaline administration, the FHR showed a deceleration. An emergency cesarean delivery was performed. The baby, born severely depressed, was resuscitated. Magnetic resonance imaging performed at 23 days of life showed that the child had a hypoxic ischemic injury. She has cerebral palsy and is nonambulatory with significant cognitive deficits.
PARENTS’ CLAIM:
The care provided by 2 ObGyns, nursing staff, and hospital was negligent. A cesarean delivery should have been performed on March 20 when the nurse identified fetal distress. The nurses should have been more assertive in recommending cesarean delivery. The injury occurred 30 minutes prior to delivery and could have been prevented by an earlier cesarean delivery.
DEFENDANTS’ DEFENSE:
FHR strips on March 20 were not as nonreassuring as claimed and did not warrant cesarean delivery, which was performed when needed.
VERDICT:
An $8.4 million Wisconsin settlement was reached by mediation.
Eclamptic seizure, twins stillborn: $4.25M
A 29-year-old woman pregnant with twins had an eclamptic seizure at 33 4/7 weeks’ gestation. The babies were stillborn.
PARENTS’ CLAIM:
The ObGyn failed to properly treat the patient’s preeclampsia for more than 11 weeks. The seizure caused hypovolemic shock, tachycardia, and massive hemorrhaging and required an emergency hysterectomy and bilateral salpingo-oophorectomy. The patient has no children and has been rendered unable to conceive. She sought to apportion 60% of the settlement proceeds to her distress claim and 20% each to wrongful-death and survival claims. She also sought to bar the twins’ biological father from sharing in the recovery due to abandonment.
HOSPITAL'S DEFENSE:
The case was settled during the trial.
VERDICT:
The mother agreed to receive 65% of the wrongful-death and survival funds, with 35% going to the father. A Pennsylvania settlement of $4.25 million was reached.
Brachial plexus injury: $4.8M verdict
A woman gave birth with assistance from a midwife. During delivery, shoulder dystocia was encountered. The baby has a permanent brachial plexus injury.
PARENTS’ CLAIM:
The midwife mismanaged shoulder dystocia by applying excessive traction to the baby’s head. The ObGyn in charge of the mother’s care did not provide adequate supervision.
DEFENDANTS’ DEFENSE:
The hospital settled prior to trial. The midwife and ObGyn denied negligence during delivery and contended that the child’s injury occurred as a result of the natural forces of labor.
VERDICT:
The jury found the midwife 60% negligent and the ObGyn 40% negligent. A $4.82 million Florida verdict was returned.
What caused infant's death?
During prenatal care, a woman underwent weekly nonstress tests due to excessive amniotic fluid until the level returned to normal. Near the end of her pregnancy, the patient noticed a decrease in fetal movement and called her ObGyn group. She was told to perform a fetal kick count and go to the emergency department (ED) if the count was abnormal, but she fell asleep. In the morning, she presented to the ObGyns’ office and was sent to the hospital for emergency cesarean delivery, which was performed 2.5 hrs after her arrival. The infant was born in distress and died 8 hours later.
PARENTS’ CLAIM:
The ObGyns should have continued weekly tests even after the amniotic fluid level returned to normal. She should have been sent to the ED when she initially reported decreased fetal movement. Cesarean delivery should have been performed immediately upon her arrival at the hospital.
PHYSICIAN’S DEFENSE:
Further prenatal testing for amniotic fluid levels was unwarranted. Telephone advice to count fetal kicks was appropriate. The delay in performing a cesarean delivery was beyond the ObGyns’ control. The outcome would have been the same regardless of their actions.
VERDICT:
A Michigan defense verdict was returned.
Perineal laceration during vaginal delivery
During vaginal delivery, a 27-year-old woman suffered a 4th-degree perineal laceration. She developed a retrovaginal fistula and has permanent fecal incontinence.
PARENTS’ CLAIM:
The ObGyn’s care was negligent. She failed to perform a rectal examination to assess the severity of the perineal laceration. The laceration was improperly repaired, and, as a result, the patient developed a retrovaginal fistula that persisted for 6 months until it was surgically repaired. A divot in her anal canal causes fecal incontinence.
PHYSICIAN’S DEFENSE:
The ObGyn contended she correctly diagnosed and repaired a 3rd-degree laceration. The wound later broke down for unknown reasons.
VERDICT:
An Arizona defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Forceful use of forceps, infant dies: $10.2M verdict
A woman in her mid-20s went to the hospital in labor. After several hours, fetal heart-rate (FHR) monitor results became nonreassuring. The ObGyn and the nurse in charge disagreed on the interpretation of the FHR monitor strips. The nurse went to her supervisor, who confronted the ObGyn 2 hours later, saying that fetal distress was a serious concern and necessitated the cessation of oxytocin. The ObGyn disagreed and ordered another nurse to increase the oxytocin dose.
Three hours later, when the FHR monitoring strips showed severe distress, the ObGyn decided to undertake an operative vaginal delivery. During a 17-minute period, the ObGyn unsuccessfully used forceps 3 times. On the second attempt, a cracking noise was heard. Then a cesarean delivery was ordered; the baby was born limp, lifeless, and unresponsive. She was found to have hypoxic ischemic encephalopathy, was removed from life support, and died.
PARENTS’ CLAIM:
Oxytocin should not have been continued when the baby was clearly in distress. The supervising nurse should have contacted her supervisor and continued up the chain of command until the ObGyn was forced to stop the oxytocin.
Physicians are prohibited from using their leg muscles when applying forceps; gentle action is critical. During one attempt, the ObGyn had his leg on the bed to increase the force with which he pulled on the forceps. The ObGyn’s reckless use of forceps caused a skull fracture to depress into the brain. The ObGyn also tried to turn the baby using forceps, which is outside the standard of care because of the risk of rotational injury. A mother’s pushing rarely causes such severe damage to the baby.
DEFENDANTS’ DEFENSE:
There was no negligence. The hypoxia was due to a hemorrhage. Natural forces of a long delivery caused the skull injury.
VERDICT:
A $10,200,575 Texas verdict was returned.
After long labor, baby has CP: $8.4M settlement
Early on March 20, a 30-year-old woman who weighed 300 lbs was admitted for delivery at 40 weeks’ gestation. Labor was induced with oxytocin. Within 30 minutes, FHR monitoring showed that the baby’s baseline began to climb, accelerations ceased, and late decelerations commenced. The oxytocin dose was steadily increased throughout the day. A nurse decided that the baby was not tolerating the contractions and discontinued oxytocin. The attending ObGyn ordered oxytocin be restarted after giving the baby a chance to recover. The mother requested a cesarean delivery, but the ObGyn refused, saying that he was concerned with the risk due to her excessive weight and prior heart surgery. When his shift ended, his partner took over.
On March 21, a nurse reported that the FHR had climbed to 160 bpm although labor had not progressed. The ObGyn ordered terbutaline to slow contractions but he did not examine the mother. An hour after terbutaline administration, the FHR showed a deceleration. An emergency cesarean delivery was performed. The baby, born severely depressed, was resuscitated. Magnetic resonance imaging performed at 23 days of life showed that the child had a hypoxic ischemic injury. She has cerebral palsy and is nonambulatory with significant cognitive deficits.
PARENTS’ CLAIM:
The care provided by 2 ObGyns, nursing staff, and hospital was negligent. A cesarean delivery should have been performed on March 20 when the nurse identified fetal distress. The nurses should have been more assertive in recommending cesarean delivery. The injury occurred 30 minutes prior to delivery and could have been prevented by an earlier cesarean delivery.
DEFENDANTS’ DEFENSE:
FHR strips on March 20 were not as nonreassuring as claimed and did not warrant cesarean delivery, which was performed when needed.
VERDICT:
An $8.4 million Wisconsin settlement was reached by mediation.
Eclamptic seizure, twins stillborn: $4.25M
A 29-year-old woman pregnant with twins had an eclamptic seizure at 33 4/7 weeks’ gestation. The babies were stillborn.
PARENTS’ CLAIM:
The ObGyn failed to properly treat the patient’s preeclampsia for more than 11 weeks. The seizure caused hypovolemic shock, tachycardia, and massive hemorrhaging and required an emergency hysterectomy and bilateral salpingo-oophorectomy. The patient has no children and has been rendered unable to conceive. She sought to apportion 60% of the settlement proceeds to her distress claim and 20% each to wrongful-death and survival claims. She also sought to bar the twins’ biological father from sharing in the recovery due to abandonment.
HOSPITAL'S DEFENSE:
The case was settled during the trial.
VERDICT:
The mother agreed to receive 65% of the wrongful-death and survival funds, with 35% going to the father. A Pennsylvania settlement of $4.25 million was reached.
Brachial plexus injury: $4.8M verdict
A woman gave birth with assistance from a midwife. During delivery, shoulder dystocia was encountered. The baby has a permanent brachial plexus injury.
PARENTS’ CLAIM:
The midwife mismanaged shoulder dystocia by applying excessive traction to the baby’s head. The ObGyn in charge of the mother’s care did not provide adequate supervision.
DEFENDANTS’ DEFENSE:
The hospital settled prior to trial. The midwife and ObGyn denied negligence during delivery and contended that the child’s injury occurred as a result of the natural forces of labor.
VERDICT:
The jury found the midwife 60% negligent and the ObGyn 40% negligent. A $4.82 million Florida verdict was returned.
What caused infant's death?
During prenatal care, a woman underwent weekly nonstress tests due to excessive amniotic fluid until the level returned to normal. Near the end of her pregnancy, the patient noticed a decrease in fetal movement and called her ObGyn group. She was told to perform a fetal kick count and go to the emergency department (ED) if the count was abnormal, but she fell asleep. In the morning, she presented to the ObGyns’ office and was sent to the hospital for emergency cesarean delivery, which was performed 2.5 hrs after her arrival. The infant was born in distress and died 8 hours later.
PARENTS’ CLAIM:
The ObGyns should have continued weekly tests even after the amniotic fluid level returned to normal. She should have been sent to the ED when she initially reported decreased fetal movement. Cesarean delivery should have been performed immediately upon her arrival at the hospital.
PHYSICIAN’S DEFENSE:
Further prenatal testing for amniotic fluid levels was unwarranted. Telephone advice to count fetal kicks was appropriate. The delay in performing a cesarean delivery was beyond the ObGyns’ control. The outcome would have been the same regardless of their actions.
VERDICT:
A Michigan defense verdict was returned.
Perineal laceration during vaginal delivery
During vaginal delivery, a 27-year-old woman suffered a 4th-degree perineal laceration. She developed a retrovaginal fistula and has permanent fecal incontinence.
PARENTS’ CLAIM:
The ObGyn’s care was negligent. She failed to perform a rectal examination to assess the severity of the perineal laceration. The laceration was improperly repaired, and, as a result, the patient developed a retrovaginal fistula that persisted for 6 months until it was surgically repaired. A divot in her anal canal causes fecal incontinence.
PHYSICIAN’S DEFENSE:
The ObGyn contended she correctly diagnosed and repaired a 3rd-degree laceration. The wound later broke down for unknown reasons.
VERDICT:
An Arizona defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CDC reports two new Zika-related pregnancy losses
The first pregnancy losses with Zika-related birth defects since last summer were reported March 14 by the Centers for Disease Control and Prevention.
Two new cases were reported to the U.S. Zika Pregnancy Registry between Feb. 21 and March 14 – the time period covered by the most recent release of data from the CDC. That brings the total number of Zika virus–related pregnancy losses to seven since the beginning of 2016 in the 50 states and the District of Columbia, along with 54 liveborn infants with Zika-related birth defects. The CDC stopped reporting adverse pregnancy outcomes in Puerto Rico and the other U.S. territories in early October 2016 because Puerto Rico’s Zika Active Pregnancy Surveillance System is not using the same inclusion criteria as its mainland counterpart.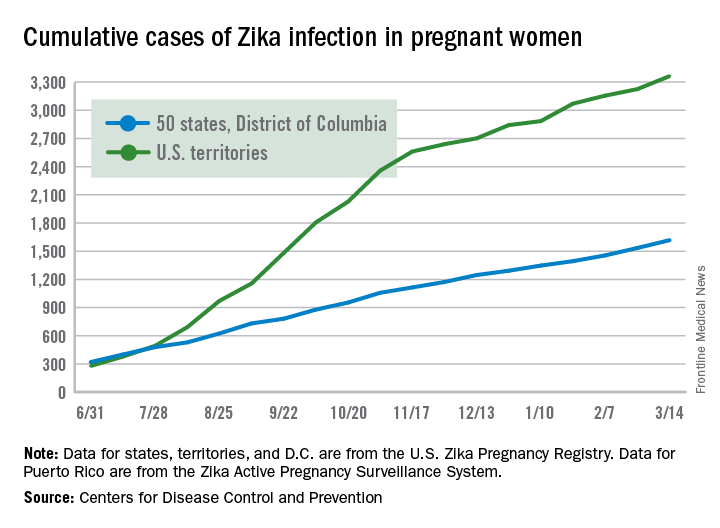
These are not real-time data and reflect only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, although it is not known if Zika virus was the cause of the poor outcomes. Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, or termination with evidence of birth defects.
The first pregnancy losses with Zika-related birth defects since last summer were reported March 14 by the Centers for Disease Control and Prevention.
Two new cases were reported to the U.S. Zika Pregnancy Registry between Feb. 21 and March 14 – the time period covered by the most recent release of data from the CDC. That brings the total number of Zika virus–related pregnancy losses to seven since the beginning of 2016 in the 50 states and the District of Columbia, along with 54 liveborn infants with Zika-related birth defects. The CDC stopped reporting adverse pregnancy outcomes in Puerto Rico and the other U.S. territories in early October 2016 because Puerto Rico’s Zika Active Pregnancy Surveillance System is not using the same inclusion criteria as its mainland counterpart.
These are not real-time data and reflect only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, although it is not known if Zika virus was the cause of the poor outcomes. Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, or termination with evidence of birth defects.
The first pregnancy losses with Zika-related birth defects since last summer were reported March 14 by the Centers for Disease Control and Prevention.
Two new cases were reported to the U.S. Zika Pregnancy Registry between Feb. 21 and March 14 – the time period covered by the most recent release of data from the CDC. That brings the total number of Zika virus–related pregnancy losses to seven since the beginning of 2016 in the 50 states and the District of Columbia, along with 54 liveborn infants with Zika-related birth defects. The CDC stopped reporting adverse pregnancy outcomes in Puerto Rico and the other U.S. territories in early October 2016 because Puerto Rico’s Zika Active Pregnancy Surveillance System is not using the same inclusion criteria as its mainland counterpart.
These are not real-time data and reflect only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, although it is not known if Zika virus was the cause of the poor outcomes. Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, or termination with evidence of birth defects.
American Samoa could be a model for responding to Zika outbreaks
Syndromic and enhanced surveillance employed during a Zika outbreak in American Samoa last year is credited for bringing a quick end to ongoing transmission and could be used to predict when ongoing transmission in similarly small populations will end.
“Establishing a timeline for discontinuing screening of asymptomatic pregnant women allows ASDoH [the American Samoa Department of Health] to allocate resources appropriately toward early interventions for children and families affected by Zika virus,” W. Thane Hancock, MD, of the CDC’s Office of Public Health Preparedness and Response, and his colleagues wrote in the Morbidity and Mortality Weekly Report (2017 Mar 24;66[11]:299-301).
Laboratory-confirmed Zika cases first cropped up in American Samoa in January 2016, prompting the implementation of protocols to reduce the mosquito population, along with syndromic surveillance based on electronic health records to determine which patients may be at higher risk. Routine testing of all asymptomatic pregnant woman, along with individuals with one or more symptoms of Zika virus, also became standard procedure. Real-time, reverse transcription–polymerase chain reaction (rRT-PCR) testing was used to confirm suspected Zika virus cases.
“Early in the response, collection and testing of specimens from pregnant women was prioritized over the collection from symptomatic nonpregnant patients because of limited testing and shipping capacity,” the researchers wrote.
By May 2016, suspected cases had dropped from six per week during January and February, to just one per week, with the last laboratory-confirmed case occurring on June 19, 2016. In August, the ASDoH proposed ending routine screening of asymptomatic pregnant women by Oct. 15, 2016. Transmission data from American Samoa and the CDC were used to determine the Oct. 15 date as an estimate for when active mosquito-borne transmission would end.
To confirm that local transmission was no longer ongoing, enhanced surveillance was implemented from Aug. 31 to Oct. 15, involving free testing at local clinics. Over the course of 45 days, there were a total of 32 patients suspected of having a Zika virus infection.
Two of these subjects’ specimens were not sufficient in sample size to be tested, but the other 30 underwent rRT-PCR testing within 30 days of symptom onset, and all the results were negative. Additionally, 277 asymptomatic pregnant women were tested and 86 (31%) tested anti-Zika IgM–positive or equivocal. All 86 specimens later tested negative by rRT-PCR.
Given the possibility that Zika virus can persist in semen for 6 months, officials recommended permissive testing of asymptomatic pregnant women who conceive through April 15, 2017 “as a conservative approach.”
“The surveillance processes outlined and the timeline established for American Samoa might have implications for jurisdictions where small populations and similar population immunity following widespread Zika virus exposure can facilitate interruption of transmission,” the researchers wrote.
Syndromic and enhanced surveillance employed during a Zika outbreak in American Samoa last year is credited for bringing a quick end to ongoing transmission and could be used to predict when ongoing transmission in similarly small populations will end.
“Establishing a timeline for discontinuing screening of asymptomatic pregnant women allows ASDoH [the American Samoa Department of Health] to allocate resources appropriately toward early interventions for children and families affected by Zika virus,” W. Thane Hancock, MD, of the CDC’s Office of Public Health Preparedness and Response, and his colleagues wrote in the Morbidity and Mortality Weekly Report (2017 Mar 24;66[11]:299-301).
Laboratory-confirmed Zika cases first cropped up in American Samoa in January 2016, prompting the implementation of protocols to reduce the mosquito population, along with syndromic surveillance based on electronic health records to determine which patients may be at higher risk. Routine testing of all asymptomatic pregnant woman, along with individuals with one or more symptoms of Zika virus, also became standard procedure. Real-time, reverse transcription–polymerase chain reaction (rRT-PCR) testing was used to confirm suspected Zika virus cases.
“Early in the response, collection and testing of specimens from pregnant women was prioritized over the collection from symptomatic nonpregnant patients because of limited testing and shipping capacity,” the researchers wrote.
By May 2016, suspected cases had dropped from six per week during January and February, to just one per week, with the last laboratory-confirmed case occurring on June 19, 2016. In August, the ASDoH proposed ending routine screening of asymptomatic pregnant women by Oct. 15, 2016. Transmission data from American Samoa and the CDC were used to determine the Oct. 15 date as an estimate for when active mosquito-borne transmission would end.
To confirm that local transmission was no longer ongoing, enhanced surveillance was implemented from Aug. 31 to Oct. 15, involving free testing at local clinics. Over the course of 45 days, there were a total of 32 patients suspected of having a Zika virus infection.
Two of these subjects’ specimens were not sufficient in sample size to be tested, but the other 30 underwent rRT-PCR testing within 30 days of symptom onset, and all the results were negative. Additionally, 277 asymptomatic pregnant women were tested and 86 (31%) tested anti-Zika IgM–positive or equivocal. All 86 specimens later tested negative by rRT-PCR.
Given the possibility that Zika virus can persist in semen for 6 months, officials recommended permissive testing of asymptomatic pregnant women who conceive through April 15, 2017 “as a conservative approach.”
“The surveillance processes outlined and the timeline established for American Samoa might have implications for jurisdictions where small populations and similar population immunity following widespread Zika virus exposure can facilitate interruption of transmission,” the researchers wrote.
Syndromic and enhanced surveillance employed during a Zika outbreak in American Samoa last year is credited for bringing a quick end to ongoing transmission and could be used to predict when ongoing transmission in similarly small populations will end.
“Establishing a timeline for discontinuing screening of asymptomatic pregnant women allows ASDoH [the American Samoa Department of Health] to allocate resources appropriately toward early interventions for children and families affected by Zika virus,” W. Thane Hancock, MD, of the CDC’s Office of Public Health Preparedness and Response, and his colleagues wrote in the Morbidity and Mortality Weekly Report (2017 Mar 24;66[11]:299-301).
Laboratory-confirmed Zika cases first cropped up in American Samoa in January 2016, prompting the implementation of protocols to reduce the mosquito population, along with syndromic surveillance based on electronic health records to determine which patients may be at higher risk. Routine testing of all asymptomatic pregnant woman, along with individuals with one or more symptoms of Zika virus, also became standard procedure. Real-time, reverse transcription–polymerase chain reaction (rRT-PCR) testing was used to confirm suspected Zika virus cases.
“Early in the response, collection and testing of specimens from pregnant women was prioritized over the collection from symptomatic nonpregnant patients because of limited testing and shipping capacity,” the researchers wrote.
By May 2016, suspected cases had dropped from six per week during January and February, to just one per week, with the last laboratory-confirmed case occurring on June 19, 2016. In August, the ASDoH proposed ending routine screening of asymptomatic pregnant women by Oct. 15, 2016. Transmission data from American Samoa and the CDC were used to determine the Oct. 15 date as an estimate for when active mosquito-borne transmission would end.
To confirm that local transmission was no longer ongoing, enhanced surveillance was implemented from Aug. 31 to Oct. 15, involving free testing at local clinics. Over the course of 45 days, there were a total of 32 patients suspected of having a Zika virus infection.
Two of these subjects’ specimens were not sufficient in sample size to be tested, but the other 30 underwent rRT-PCR testing within 30 days of symptom onset, and all the results were negative. Additionally, 277 asymptomatic pregnant women were tested and 86 (31%) tested anti-Zika IgM–positive or equivocal. All 86 specimens later tested negative by rRT-PCR.
Given the possibility that Zika virus can persist in semen for 6 months, officials recommended permissive testing of asymptomatic pregnant women who conceive through April 15, 2017 “as a conservative approach.”
“The surveillance processes outlined and the timeline established for American Samoa might have implications for jurisdictions where small populations and similar population immunity following widespread Zika virus exposure can facilitate interruption of transmission,” the researchers wrote.
FROM MMWR
Universal preterm birth screening not ready for prime time
Two screens to predict spontaneous preterm birth in low-risk women, which were rapidly adapted into clinical practice despite a lack of supportive evidence, proved to have little predictive value in a large cohort study.
Transvaginal ultrasound examination for short cervical length and quantitative cervicovaginal swabbing for fetal fibronectin are routinely used to predict spontaneous preterm birth. To assess the accuracy of both screens individually and in combination, researchers analyzed data for women participating in the prospective multicenter Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b). They focused on 9,410 nulliparous women with singleton pregnancies who were followed at eight clinical centers across the country. In addition to regular pregnancy visits, these women underwent both screening procedures at approximately 12 weeks, 19 weeks, and 28 weeks.
Both screens had relatively low sensitivity and low positive predictive value, regardless of when they were performed, which threshold values were used, and whether the results were considered individually or in combination (JAMA. 2017;317[10]:1047-56).
Transvaginal cervical length at 22-30 weeks’ gestation was the most accurate predictor of spontaneous preterm birth before 37 weeks, outperforming fetal fibronectin assessment alone. However, with a threshold of 25 mm or less – the most commonly used clinical cutoff – cervical length screening identified just 23.3% of spontaneous preterm births before 37 weeks. Use of that same threshold at 16-22 weeks – the most common time for screening in clinical practice – identified just 8% of subsequent spontaneous preterm births. The addition of fetal fibronectin did not increase the predictive performance of cervical length alone, according to the findings.
The researchers cited the low incidence of short cervix (1% at 16-22 weeks) as a potential reason why the ultrasound screen was not useful. “Using the most conservative threshold of 25 mm or less in the most common time for clinical screening (16-22 weeks’ gestation), 247 women would need to be screened to identify 1 case of spontaneous preterm birth,” the researchers wrote. Using a transvaginal cervical length of 15 mm or less during the same time period, the number needed to screen rose to 680 to identify a single case of spontaneous preterm birth.
These findings do not support the routine use of these two screens among nulliparous women with singleton pregnancies, the researchers wrote. Screening procedures with relatively poor predictive values “may sometimes be useful if they are inexpensive, lack serious adverse effects, and address a serious condition for which an effective intervention exists. Neither of these tests, alone or in combination, meets all of these criteria,” they added.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. Dr. Esplin reported holding a patent for serum markers of preterm birth and ties to Sera Prognostics and Clinical Innovations. One of his coauthors reported ties to Natera, Sequenom, Illumina, March of Dimes, Ariosa Diagnostics/Roche, KellBenx, and LabCorp.
The report by Esplin and colleagues is important for at least two reasons. First, this large and well-executed prospective study provides compelling data that two popular screening modalities, either alone or in combination, do not accurately predict which women will deliver preterm. Second, this report provides provocative insights as to how medical practice determined through consensus, rather than reproducible evidence, contributes to the narrative in the United States regarding the cost and quality of health care.
In 2012, the publications committee of the Society for Maternal-Fetal Medicine provided a consensus clinical guideline for use of progesterone for the prevention of preterm birth. The guideline indicated that vaginal progesterone was beneficial for women at low risk for preterm birth who were discovered to have short cervical length during an ultrasound examination. This recommendation raised the issue of universal testing for short cervix in women such as those screened in the study by Esplin and colleagues. The authors of the clinical guideline, which was reaffirmed in 2016, stated that “cervical length screening in singleton gestations without prior preterm birth cannot yet be universally mandated. Nonetheless, implementation of such a screening strategy can be viewed as reasonable, and can be considered by individual practitioners.” Similarly, one of the recommendations – although based on limited or inconsistent scientific evidence – promulgated by the American College of Obstetricians and Gynecologists in its 2012 Practice Bulletin on Prediction and Prevention of Preterm Birth, and also reaffirmed in 2016, stated that “although this document does not mandate universal cervical length screening in women without a prior preterm birth, this screening strategy may be considered.”
The important study by Esplin and colleagues should serve to temper the use of two controversial screening approaches. While it is gratifying that such research takes place, that research should have preceded the adoption of this screening strategy into practice.
Steven L. Bloom, MD, and Kenneth J. Leveno, MD, are in the department of ob.gyn. at the University of Texas Southwestern Medical Center, Dallas. They reported having no relevant financial disclosures. These comments are excerpted from an editorial ( JAMA. 2017;317[10]:1025-26).
The report by Esplin and colleagues is important for at least two reasons. First, this large and well-executed prospective study provides compelling data that two popular screening modalities, either alone or in combination, do not accurately predict which women will deliver preterm. Second, this report provides provocative insights as to how medical practice determined through consensus, rather than reproducible evidence, contributes to the narrative in the United States regarding the cost and quality of health care.
In 2012, the publications committee of the Society for Maternal-Fetal Medicine provided a consensus clinical guideline for use of progesterone for the prevention of preterm birth. The guideline indicated that vaginal progesterone was beneficial for women at low risk for preterm birth who were discovered to have short cervical length during an ultrasound examination. This recommendation raised the issue of universal testing for short cervix in women such as those screened in the study by Esplin and colleagues. The authors of the clinical guideline, which was reaffirmed in 2016, stated that “cervical length screening in singleton gestations without prior preterm birth cannot yet be universally mandated. Nonetheless, implementation of such a screening strategy can be viewed as reasonable, and can be considered by individual practitioners.” Similarly, one of the recommendations – although based on limited or inconsistent scientific evidence – promulgated by the American College of Obstetricians and Gynecologists in its 2012 Practice Bulletin on Prediction and Prevention of Preterm Birth, and also reaffirmed in 2016, stated that “although this document does not mandate universal cervical length screening in women without a prior preterm birth, this screening strategy may be considered.”
The important study by Esplin and colleagues should serve to temper the use of two controversial screening approaches. While it is gratifying that such research takes place, that research should have preceded the adoption of this screening strategy into practice.
Steven L. Bloom, MD, and Kenneth J. Leveno, MD, are in the department of ob.gyn. at the University of Texas Southwestern Medical Center, Dallas. They reported having no relevant financial disclosures. These comments are excerpted from an editorial ( JAMA. 2017;317[10]:1025-26).
The report by Esplin and colleagues is important for at least two reasons. First, this large and well-executed prospective study provides compelling data that two popular screening modalities, either alone or in combination, do not accurately predict which women will deliver preterm. Second, this report provides provocative insights as to how medical practice determined through consensus, rather than reproducible evidence, contributes to the narrative in the United States regarding the cost and quality of health care.
In 2012, the publications committee of the Society for Maternal-Fetal Medicine provided a consensus clinical guideline for use of progesterone for the prevention of preterm birth. The guideline indicated that vaginal progesterone was beneficial for women at low risk for preterm birth who were discovered to have short cervical length during an ultrasound examination. This recommendation raised the issue of universal testing for short cervix in women such as those screened in the study by Esplin and colleagues. The authors of the clinical guideline, which was reaffirmed in 2016, stated that “cervical length screening in singleton gestations without prior preterm birth cannot yet be universally mandated. Nonetheless, implementation of such a screening strategy can be viewed as reasonable, and can be considered by individual practitioners.” Similarly, one of the recommendations – although based on limited or inconsistent scientific evidence – promulgated by the American College of Obstetricians and Gynecologists in its 2012 Practice Bulletin on Prediction and Prevention of Preterm Birth, and also reaffirmed in 2016, stated that “although this document does not mandate universal cervical length screening in women without a prior preterm birth, this screening strategy may be considered.”
The important study by Esplin and colleagues should serve to temper the use of two controversial screening approaches. While it is gratifying that such research takes place, that research should have preceded the adoption of this screening strategy into practice.
Steven L. Bloom, MD, and Kenneth J. Leveno, MD, are in the department of ob.gyn. at the University of Texas Southwestern Medical Center, Dallas. They reported having no relevant financial disclosures. These comments are excerpted from an editorial ( JAMA. 2017;317[10]:1025-26).
Two screens to predict spontaneous preterm birth in low-risk women, which were rapidly adapted into clinical practice despite a lack of supportive evidence, proved to have little predictive value in a large cohort study.
Transvaginal ultrasound examination for short cervical length and quantitative cervicovaginal swabbing for fetal fibronectin are routinely used to predict spontaneous preterm birth. To assess the accuracy of both screens individually and in combination, researchers analyzed data for women participating in the prospective multicenter Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b). They focused on 9,410 nulliparous women with singleton pregnancies who were followed at eight clinical centers across the country. In addition to regular pregnancy visits, these women underwent both screening procedures at approximately 12 weeks, 19 weeks, and 28 weeks.
Both screens had relatively low sensitivity and low positive predictive value, regardless of when they were performed, which threshold values were used, and whether the results were considered individually or in combination (JAMA. 2017;317[10]:1047-56).
Transvaginal cervical length at 22-30 weeks’ gestation was the most accurate predictor of spontaneous preterm birth before 37 weeks, outperforming fetal fibronectin assessment alone. However, with a threshold of 25 mm or less – the most commonly used clinical cutoff – cervical length screening identified just 23.3% of spontaneous preterm births before 37 weeks. Use of that same threshold at 16-22 weeks – the most common time for screening in clinical practice – identified just 8% of subsequent spontaneous preterm births. The addition of fetal fibronectin did not increase the predictive performance of cervical length alone, according to the findings.
The researchers cited the low incidence of short cervix (1% at 16-22 weeks) as a potential reason why the ultrasound screen was not useful. “Using the most conservative threshold of 25 mm or less in the most common time for clinical screening (16-22 weeks’ gestation), 247 women would need to be screened to identify 1 case of spontaneous preterm birth,” the researchers wrote. Using a transvaginal cervical length of 15 mm or less during the same time period, the number needed to screen rose to 680 to identify a single case of spontaneous preterm birth.
These findings do not support the routine use of these two screens among nulliparous women with singleton pregnancies, the researchers wrote. Screening procedures with relatively poor predictive values “may sometimes be useful if they are inexpensive, lack serious adverse effects, and address a serious condition for which an effective intervention exists. Neither of these tests, alone or in combination, meets all of these criteria,” they added.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. Dr. Esplin reported holding a patent for serum markers of preterm birth and ties to Sera Prognostics and Clinical Innovations. One of his coauthors reported ties to Natera, Sequenom, Illumina, March of Dimes, Ariosa Diagnostics/Roche, KellBenx, and LabCorp.
Two screens to predict spontaneous preterm birth in low-risk women, which were rapidly adapted into clinical practice despite a lack of supportive evidence, proved to have little predictive value in a large cohort study.
Transvaginal ultrasound examination for short cervical length and quantitative cervicovaginal swabbing for fetal fibronectin are routinely used to predict spontaneous preterm birth. To assess the accuracy of both screens individually and in combination, researchers analyzed data for women participating in the prospective multicenter Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b). They focused on 9,410 nulliparous women with singleton pregnancies who were followed at eight clinical centers across the country. In addition to regular pregnancy visits, these women underwent both screening procedures at approximately 12 weeks, 19 weeks, and 28 weeks.
Both screens had relatively low sensitivity and low positive predictive value, regardless of when they were performed, which threshold values were used, and whether the results were considered individually or in combination (JAMA. 2017;317[10]:1047-56).
Transvaginal cervical length at 22-30 weeks’ gestation was the most accurate predictor of spontaneous preterm birth before 37 weeks, outperforming fetal fibronectin assessment alone. However, with a threshold of 25 mm or less – the most commonly used clinical cutoff – cervical length screening identified just 23.3% of spontaneous preterm births before 37 weeks. Use of that same threshold at 16-22 weeks – the most common time for screening in clinical practice – identified just 8% of subsequent spontaneous preterm births. The addition of fetal fibronectin did not increase the predictive performance of cervical length alone, according to the findings.
The researchers cited the low incidence of short cervix (1% at 16-22 weeks) as a potential reason why the ultrasound screen was not useful. “Using the most conservative threshold of 25 mm or less in the most common time for clinical screening (16-22 weeks’ gestation), 247 women would need to be screened to identify 1 case of spontaneous preterm birth,” the researchers wrote. Using a transvaginal cervical length of 15 mm or less during the same time period, the number needed to screen rose to 680 to identify a single case of spontaneous preterm birth.
These findings do not support the routine use of these two screens among nulliparous women with singleton pregnancies, the researchers wrote. Screening procedures with relatively poor predictive values “may sometimes be useful if they are inexpensive, lack serious adverse effects, and address a serious condition for which an effective intervention exists. Neither of these tests, alone or in combination, meets all of these criteria,” they added.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. Dr. Esplin reported holding a patent for serum markers of preterm birth and ties to Sera Prognostics and Clinical Innovations. One of his coauthors reported ties to Natera, Sequenom, Illumina, March of Dimes, Ariosa Diagnostics/Roche, KellBenx, and LabCorp.
FROM JAMA
Key clinical point:
Major finding: With a threshold of transvaginal cervical length of 25 mm or less at 16-22 weeks’ gestation, 247 women would need to be screened to identify one case of spontaneous preterm birth before 37 weeks’ gestation.
Data source: A prospective multicenter observational cohort study involving 9,410 nulliparous women across the United States.
Disclosures: The Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. Dr. Esplin reported holding a patent for serum markers of preterm birth and ties to Sera Prognostics and Clinical Innovations. An associate reported ties to Natera, Sequenom, Illumina, March of Dimes, Ariosa Diagnostics/Roche, KellBenx, and LabCorp.
U.S. chikungunya epidemic would likely put rheumatologists on front line
SNOWMASS, COLO. – The continental United States is vulnerable to an epidemic of chikungunya virus disease, an event which would have profound consequences for rheumatologists, Robert T. Schoen, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“We certainly have all the factors in place where we could have a major epidemic of chikungunya, particularly in Florida, Texas, and neighboring states. As rheumatologists, I think we can own this infection if we want to because it causes an arthritis that is a true arthritis in a significant percentage of patients,” said Dr. Schoen, a rheumatologist at Yale University in New Haven, Conn.
“That’s a major impact when you think that these epidemics affect tens of thousands of individuals. If 25% of them develop long-term arthritis disability, that’s a whole new world for us,” the rheumatologist observed.
Chikungunya is a single-stranded RNA virus in the togaviridae family, which also contains rubella. The infection is transmitted by Aedes aegypti and A. albopictus, also known as the yellow fever and Asian tiger mosquitoes, respectively. Both mosquitoes are established inhabitants of much of the United States.
“This infection is a one-bite deal. These are very aggressive mosquitoes,” Dr. Schoen observed. “If you haven’t seen a case of chikungunya yet, you will soon,” he added.
In 2014, at the height of a massive Caribbean epidemic which included half a million cases in Puerto Rico, roughly 2,800 cases of chikungunya were imported into 46 U.S. states. In 2015, however, as the Caribbean epidemic waned and herd immunity developed, that figure fell to 653 imported cases. But the epidemic in India, which began in 2008, remains ongoing with no end in sight. Several of Dr. Schoen’s patients with chikungunya had recently returned from India when they first became ill.
“India provides an unlimited reservoir of immunologically naive patients to perpetuate the infection,” he observed.
Course of illness
The rate of asymptomatic infection has been variously estimated at 3%-25%. Symptomatic chikungunya is a biphasic illness. After a 2- to 6-day incubation period, patients develop rapid-onset fever with severe joint pain and muscle aches. Indeed, chikungunya means “bent over” in Makonde, an African Bantu language.
Roughly 60% of patients develop a rash during the acute febrile phase of the illness, which lasts about a week. The dermatitis is most often a maculopapular rash on the trunk which is “absolutely indistinguishable” from the rash caused by Zika virus infection, transmitted by the same vectors, Dr. Schoen noted.
During this acute phase, almost all patients develop a severe polyarthritis which can last for weeks or, less commonly, for months or years. This polyarthritis mimics seronegative rheumatoid arthritis. It is usually symmetric and often affects the hands, wrists, and feet.
The acute febrile phase is characterized by high levels of viremia, with up to 1 billion viral particles per milliliter of blood. During this period, definitive diagnosis of chikungunya can be made through reverse transcriptase–polymerase chain reaction testing or viral culture.
Typically, though, patients make their way to a rheumatologist only well after the viremic period is over. In that situation, the diagnostic mainstay is serologic testing. Antichikungunya virus Immunoglobulin M is detectable starting on about day 5 after symptom onset, and it persists for the next 1-3 months. Immunoglobulin G (IgG) antibodies become detectable in the same time frame and remain elevated for years.
Dr. Schoen highlighted a small but intriguing study from Singapore that suggests that the early appearance of chikungunya-specific neutralizing IgG3–antibodies may constitute a marker for favorable long-term prognosis. The observational study involved 30 patients hospitalized for severe acute chikungunya infection. The investigators classified them into two groups: 14 patients had low levels of acute viremia, a less severe acute illness, and late development of IgG3 antibodies; the other 16 had a high initial viral load, a more severe acute phase, and a rapid IgG3 and interleukin-6 response to infection.
The patients with a robust early IgG3 response cleared the virus faster and none of them developed chronic arthritis. In contrast, those without early, chikungunya-specific IgG3 had a high rate of persistent arthralgia (J Infect Dis. 2012 Apr 1;205[7]:1147-54).
Treatment
No evidence-based treatment for chikungunya exists. Treatment in the acute phase is primarily supportive care. Rheumatologists on the Caribbean island of Martinique have reported a favorable experience using methotrexate at doses up to 25 mg/week or with standard doses of anti–tumor necrosis factor agents in chikungunya patients with severe bilateral symmetric chronic inflammatory joint disease arising during the 2013-2015 epidemic. The treatment response and tolerability were comparable with the results typically obtained in rheumatoid arthritis patients, according to the rheumatologists (Arthritis Rheumatol. 2016 Nov;68[11]:2817-24).
Dr. Schoen found the report from Martinique to be reassuring. He too has resorted to familiar rheumatologic medications in his severely affected patients. Anecdotally, his greatest success involved a patient with a 5-year history of disabling chikungunya polyarthritis who showed marked improvement after several months on hydroxychloroquine.
The Martinique investigators also reported on 22 patients who developed chikungunya while on biologic agents for rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, or systemic lupus erythematosus. The biologics weren’t protective against the viral disease in these patients. All 22 of them developed severe acute chikungunya polyarthritis with a mean swollen joint count of 9.6 (Joint Bone Spine. 2016 Mar;83[2]:245-6).
Infection during pregnancy
There is a legitimate concern about chikungunya infection occurring during pregnancy, but, based on the experiences documented on Reunion Island, the risk to the baby is confined to intrapartum exposure in a viremic mother.
Reunion Island is a French region in the Indian Ocean off the eastern coast of Africa. It has first-world medical care. Much of the best documented early work on chikungunya outbreaks grew out of the 2005-2006 epidemic there, which affected more than one-third of the island’s 800,000-plus residents.
In a prospective study of 7,504 deliveries on the island during a 22-month period, mother-to-child transmission of chikungunya occurred only in the context of intrapartum viremia, with 19 cases of vertical transmission occurring among 39 affected mothers who delivered at a median 38 weeks of gestation. Cesarean section had no protective effect. All 19 infected neonates were asymptomatic at birth, with median onset of pain, fever, and thrombocytopenia on day 4. Nine of the 19 infected neonates developed encephalopathy with pathologic MRI findings, including cerebral hemorrhage in two cases (PLoS Med. 2008 Mar 18;5[3]:e60).
The case fatality rate for chikungunya is low at 0.1%. Most deaths occur in young children or elderly individuals with major comorbid conditions. Despite this low mortality, interest in chikungunya vaccine development is being driven by the infection’s impact on tourism, its spread to temperate climates, the economic impact of the considerable time lost from work, and military need. A promising attenuated, virus-like, particle-based vaccine is currently in phase II testing in the Caribbean.
Dr. Schoen reported having no financial conflicts.
SNOWMASS, COLO. – The continental United States is vulnerable to an epidemic of chikungunya virus disease, an event which would have profound consequences for rheumatologists, Robert T. Schoen, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“We certainly have all the factors in place where we could have a major epidemic of chikungunya, particularly in Florida, Texas, and neighboring states. As rheumatologists, I think we can own this infection if we want to because it causes an arthritis that is a true arthritis in a significant percentage of patients,” said Dr. Schoen, a rheumatologist at Yale University in New Haven, Conn.
“That’s a major impact when you think that these epidemics affect tens of thousands of individuals. If 25% of them develop long-term arthritis disability, that’s a whole new world for us,” the rheumatologist observed.
Chikungunya is a single-stranded RNA virus in the togaviridae family, which also contains rubella. The infection is transmitted by Aedes aegypti and A. albopictus, also known as the yellow fever and Asian tiger mosquitoes, respectively. Both mosquitoes are established inhabitants of much of the United States.
“This infection is a one-bite deal. These are very aggressive mosquitoes,” Dr. Schoen observed. “If you haven’t seen a case of chikungunya yet, you will soon,” he added.
In 2014, at the height of a massive Caribbean epidemic which included half a million cases in Puerto Rico, roughly 2,800 cases of chikungunya were imported into 46 U.S. states. In 2015, however, as the Caribbean epidemic waned and herd immunity developed, that figure fell to 653 imported cases. But the epidemic in India, which began in 2008, remains ongoing with no end in sight. Several of Dr. Schoen’s patients with chikungunya had recently returned from India when they first became ill.
“India provides an unlimited reservoir of immunologically naive patients to perpetuate the infection,” he observed.
Course of illness
The rate of asymptomatic infection has been variously estimated at 3%-25%. Symptomatic chikungunya is a biphasic illness. After a 2- to 6-day incubation period, patients develop rapid-onset fever with severe joint pain and muscle aches. Indeed, chikungunya means “bent over” in Makonde, an African Bantu language.
Roughly 60% of patients develop a rash during the acute febrile phase of the illness, which lasts about a week. The dermatitis is most often a maculopapular rash on the trunk which is “absolutely indistinguishable” from the rash caused by Zika virus infection, transmitted by the same vectors, Dr. Schoen noted.
During this acute phase, almost all patients develop a severe polyarthritis which can last for weeks or, less commonly, for months or years. This polyarthritis mimics seronegative rheumatoid arthritis. It is usually symmetric and often affects the hands, wrists, and feet.
The acute febrile phase is characterized by high levels of viremia, with up to 1 billion viral particles per milliliter of blood. During this period, definitive diagnosis of chikungunya can be made through reverse transcriptase–polymerase chain reaction testing or viral culture.
Typically, though, patients make their way to a rheumatologist only well after the viremic period is over. In that situation, the diagnostic mainstay is serologic testing. Antichikungunya virus Immunoglobulin M is detectable starting on about day 5 after symptom onset, and it persists for the next 1-3 months. Immunoglobulin G (IgG) antibodies become detectable in the same time frame and remain elevated for years.
Dr. Schoen highlighted a small but intriguing study from Singapore that suggests that the early appearance of chikungunya-specific neutralizing IgG3–antibodies may constitute a marker for favorable long-term prognosis. The observational study involved 30 patients hospitalized for severe acute chikungunya infection. The investigators classified them into two groups: 14 patients had low levels of acute viremia, a less severe acute illness, and late development of IgG3 antibodies; the other 16 had a high initial viral load, a more severe acute phase, and a rapid IgG3 and interleukin-6 response to infection.
The patients with a robust early IgG3 response cleared the virus faster and none of them developed chronic arthritis. In contrast, those without early, chikungunya-specific IgG3 had a high rate of persistent arthralgia (J Infect Dis. 2012 Apr 1;205[7]:1147-54).
Treatment
No evidence-based treatment for chikungunya exists. Treatment in the acute phase is primarily supportive care. Rheumatologists on the Caribbean island of Martinique have reported a favorable experience using methotrexate at doses up to 25 mg/week or with standard doses of anti–tumor necrosis factor agents in chikungunya patients with severe bilateral symmetric chronic inflammatory joint disease arising during the 2013-2015 epidemic. The treatment response and tolerability were comparable with the results typically obtained in rheumatoid arthritis patients, according to the rheumatologists (Arthritis Rheumatol. 2016 Nov;68[11]:2817-24).
Dr. Schoen found the report from Martinique to be reassuring. He too has resorted to familiar rheumatologic medications in his severely affected patients. Anecdotally, his greatest success involved a patient with a 5-year history of disabling chikungunya polyarthritis who showed marked improvement after several months on hydroxychloroquine.
The Martinique investigators also reported on 22 patients who developed chikungunya while on biologic agents for rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, or systemic lupus erythematosus. The biologics weren’t protective against the viral disease in these patients. All 22 of them developed severe acute chikungunya polyarthritis with a mean swollen joint count of 9.6 (Joint Bone Spine. 2016 Mar;83[2]:245-6).
Infection during pregnancy
There is a legitimate concern about chikungunya infection occurring during pregnancy, but, based on the experiences documented on Reunion Island, the risk to the baby is confined to intrapartum exposure in a viremic mother.
Reunion Island is a French region in the Indian Ocean off the eastern coast of Africa. It has first-world medical care. Much of the best documented early work on chikungunya outbreaks grew out of the 2005-2006 epidemic there, which affected more than one-third of the island’s 800,000-plus residents.
In a prospective study of 7,504 deliveries on the island during a 22-month period, mother-to-child transmission of chikungunya occurred only in the context of intrapartum viremia, with 19 cases of vertical transmission occurring among 39 affected mothers who delivered at a median 38 weeks of gestation. Cesarean section had no protective effect. All 19 infected neonates were asymptomatic at birth, with median onset of pain, fever, and thrombocytopenia on day 4. Nine of the 19 infected neonates developed encephalopathy with pathologic MRI findings, including cerebral hemorrhage in two cases (PLoS Med. 2008 Mar 18;5[3]:e60).
The case fatality rate for chikungunya is low at 0.1%. Most deaths occur in young children or elderly individuals with major comorbid conditions. Despite this low mortality, interest in chikungunya vaccine development is being driven by the infection’s impact on tourism, its spread to temperate climates, the economic impact of the considerable time lost from work, and military need. A promising attenuated, virus-like, particle-based vaccine is currently in phase II testing in the Caribbean.
Dr. Schoen reported having no financial conflicts.
SNOWMASS, COLO. – The continental United States is vulnerable to an epidemic of chikungunya virus disease, an event which would have profound consequences for rheumatologists, Robert T. Schoen, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“We certainly have all the factors in place where we could have a major epidemic of chikungunya, particularly in Florida, Texas, and neighboring states. As rheumatologists, I think we can own this infection if we want to because it causes an arthritis that is a true arthritis in a significant percentage of patients,” said Dr. Schoen, a rheumatologist at Yale University in New Haven, Conn.
“That’s a major impact when you think that these epidemics affect tens of thousands of individuals. If 25% of them develop long-term arthritis disability, that’s a whole new world for us,” the rheumatologist observed.
Chikungunya is a single-stranded RNA virus in the togaviridae family, which also contains rubella. The infection is transmitted by Aedes aegypti and A. albopictus, also known as the yellow fever and Asian tiger mosquitoes, respectively. Both mosquitoes are established inhabitants of much of the United States.
“This infection is a one-bite deal. These are very aggressive mosquitoes,” Dr. Schoen observed. “If you haven’t seen a case of chikungunya yet, you will soon,” he added.
In 2014, at the height of a massive Caribbean epidemic which included half a million cases in Puerto Rico, roughly 2,800 cases of chikungunya were imported into 46 U.S. states. In 2015, however, as the Caribbean epidemic waned and herd immunity developed, that figure fell to 653 imported cases. But the epidemic in India, which began in 2008, remains ongoing with no end in sight. Several of Dr. Schoen’s patients with chikungunya had recently returned from India when they first became ill.
“India provides an unlimited reservoir of immunologically naive patients to perpetuate the infection,” he observed.
Course of illness
The rate of asymptomatic infection has been variously estimated at 3%-25%. Symptomatic chikungunya is a biphasic illness. After a 2- to 6-day incubation period, patients develop rapid-onset fever with severe joint pain and muscle aches. Indeed, chikungunya means “bent over” in Makonde, an African Bantu language.
Roughly 60% of patients develop a rash during the acute febrile phase of the illness, which lasts about a week. The dermatitis is most often a maculopapular rash on the trunk which is “absolutely indistinguishable” from the rash caused by Zika virus infection, transmitted by the same vectors, Dr. Schoen noted.
During this acute phase, almost all patients develop a severe polyarthritis which can last for weeks or, less commonly, for months or years. This polyarthritis mimics seronegative rheumatoid arthritis. It is usually symmetric and often affects the hands, wrists, and feet.
The acute febrile phase is characterized by high levels of viremia, with up to 1 billion viral particles per milliliter of blood. During this period, definitive diagnosis of chikungunya can be made through reverse transcriptase–polymerase chain reaction testing or viral culture.
Typically, though, patients make their way to a rheumatologist only well after the viremic period is over. In that situation, the diagnostic mainstay is serologic testing. Antichikungunya virus Immunoglobulin M is detectable starting on about day 5 after symptom onset, and it persists for the next 1-3 months. Immunoglobulin G (IgG) antibodies become detectable in the same time frame and remain elevated for years.
Dr. Schoen highlighted a small but intriguing study from Singapore that suggests that the early appearance of chikungunya-specific neutralizing IgG3–antibodies may constitute a marker for favorable long-term prognosis. The observational study involved 30 patients hospitalized for severe acute chikungunya infection. The investigators classified them into two groups: 14 patients had low levels of acute viremia, a less severe acute illness, and late development of IgG3 antibodies; the other 16 had a high initial viral load, a more severe acute phase, and a rapid IgG3 and interleukin-6 response to infection.
The patients with a robust early IgG3 response cleared the virus faster and none of them developed chronic arthritis. In contrast, those without early, chikungunya-specific IgG3 had a high rate of persistent arthralgia (J Infect Dis. 2012 Apr 1;205[7]:1147-54).
Treatment
No evidence-based treatment for chikungunya exists. Treatment in the acute phase is primarily supportive care. Rheumatologists on the Caribbean island of Martinique have reported a favorable experience using methotrexate at doses up to 25 mg/week or with standard doses of anti–tumor necrosis factor agents in chikungunya patients with severe bilateral symmetric chronic inflammatory joint disease arising during the 2013-2015 epidemic. The treatment response and tolerability were comparable with the results typically obtained in rheumatoid arthritis patients, according to the rheumatologists (Arthritis Rheumatol. 2016 Nov;68[11]:2817-24).
Dr. Schoen found the report from Martinique to be reassuring. He too has resorted to familiar rheumatologic medications in his severely affected patients. Anecdotally, his greatest success involved a patient with a 5-year history of disabling chikungunya polyarthritis who showed marked improvement after several months on hydroxychloroquine.
The Martinique investigators also reported on 22 patients who developed chikungunya while on biologic agents for rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, or systemic lupus erythematosus. The biologics weren’t protective against the viral disease in these patients. All 22 of them developed severe acute chikungunya polyarthritis with a mean swollen joint count of 9.6 (Joint Bone Spine. 2016 Mar;83[2]:245-6).
Infection during pregnancy
There is a legitimate concern about chikungunya infection occurring during pregnancy, but, based on the experiences documented on Reunion Island, the risk to the baby is confined to intrapartum exposure in a viremic mother.
Reunion Island is a French region in the Indian Ocean off the eastern coast of Africa. It has first-world medical care. Much of the best documented early work on chikungunya outbreaks grew out of the 2005-2006 epidemic there, which affected more than one-third of the island’s 800,000-plus residents.
In a prospective study of 7,504 deliveries on the island during a 22-month period, mother-to-child transmission of chikungunya occurred only in the context of intrapartum viremia, with 19 cases of vertical transmission occurring among 39 affected mothers who delivered at a median 38 weeks of gestation. Cesarean section had no protective effect. All 19 infected neonates were asymptomatic at birth, with median onset of pain, fever, and thrombocytopenia on day 4. Nine of the 19 infected neonates developed encephalopathy with pathologic MRI findings, including cerebral hemorrhage in two cases (PLoS Med. 2008 Mar 18;5[3]:e60).
The case fatality rate for chikungunya is low at 0.1%. Most deaths occur in young children or elderly individuals with major comorbid conditions. Despite this low mortality, interest in chikungunya vaccine development is being driven by the infection’s impact on tourism, its spread to temperate climates, the economic impact of the considerable time lost from work, and military need. A promising attenuated, virus-like, particle-based vaccine is currently in phase II testing in the Caribbean.
Dr. Schoen reported having no financial conflicts.