User login
Low serum AMH predicted miscarriage after IVF
SALT LAKE CITY – Low serum levels of anti-Müllerian hormone predicted miscarriage after in vitro fertilization and embryo transfer, according to a single-center prospective study of more than 2,000 women.
This relationship remained statistically significant after the study controlled for age and response to controlled ovarian hyperstimulation, which suggests that AMH is a marker of the reproductive potential of oocytes, not just of oocyte quantity, Bruno Tarasconi, MD, said at the annual meeting of the American Society for Reproductive Medicine.
“Low” serum AMH was defined as less than 1.61 ng per mL, but specific levels vary by test type, so “this is not a definite cutoff,” Dr. Tarasconi emphasized. “What we need to know is that patients with low AMH are probably experiencing some problems in the ovary that affect oocyte quality.”
The study comprised 2,365 women undergoing 2,688 IVF cycles with fresh oocytes. Levels of AMH were measured by ELISA (AMH Gen II, Beckman Coulter) within 12 months before IVF on cycle days 2 and 4, said Dr. Tarasconi of University of Paris Ouest in France.
Women younger than 34 years old, women 34-36 years old, and women 37 years and older had the highest rates of miscarriage when they had low serum AMH levels. For the two older age groups, low AMH was associated with about a 33% miscarriage rate – about twice the rate of miscarriage among women whose AMH measured at least 5.6 ng per mL. This difference reached statistical significance for both age groups (P less than .05).
Among women younger than 34 years, low serum AMH was associated with a 22% rate of miscarriage, compared with about 13% among women with higher AMH levels. This difference did not reach significance because of a lack of power, Dr. Tarasconi said. “However, binary logistic regression of the whole population confirmed the association between low serum AMH and miscarriage, independent of patient’s age and oocyte yield, with the P value less than .001.”
Low circulating AMH can reflect an above-average prevalence of oocyte abnormalities, which are linked to miscarriage, Dr. Tarasconi noted. “The fact that oocyte yield failed to alter the relationship between AMH and miscarriage suggests that altered per-follicle AMH production, not oocyte or embryo availability, fosters pregnancy loss,” he added. However, the researchers did not test either preimplantation embryos or the expelled gestational sacs to directly link miscarriages to abnormalities such as aneuploidy, he noted.
Low AMH levels also were associated with lower rates of pregnancy after IVF, regardless of age, as has been previously reported. Patients in the study were of normal karyotype, with no history of recurrent pregnancy loss, uterine abnormalities, or family history of genetic or congenital disorders, Dr. Tarasconi noted.
He reported having no financial disclosures.
SALT LAKE CITY – Low serum levels of anti-Müllerian hormone predicted miscarriage after in vitro fertilization and embryo transfer, according to a single-center prospective study of more than 2,000 women.
This relationship remained statistically significant after the study controlled for age and response to controlled ovarian hyperstimulation, which suggests that AMH is a marker of the reproductive potential of oocytes, not just of oocyte quantity, Bruno Tarasconi, MD, said at the annual meeting of the American Society for Reproductive Medicine.
“Low” serum AMH was defined as less than 1.61 ng per mL, but specific levels vary by test type, so “this is not a definite cutoff,” Dr. Tarasconi emphasized. “What we need to know is that patients with low AMH are probably experiencing some problems in the ovary that affect oocyte quality.”
The study comprised 2,365 women undergoing 2,688 IVF cycles with fresh oocytes. Levels of AMH were measured by ELISA (AMH Gen II, Beckman Coulter) within 12 months before IVF on cycle days 2 and 4, said Dr. Tarasconi of University of Paris Ouest in France.
Women younger than 34 years old, women 34-36 years old, and women 37 years and older had the highest rates of miscarriage when they had low serum AMH levels. For the two older age groups, low AMH was associated with about a 33% miscarriage rate – about twice the rate of miscarriage among women whose AMH measured at least 5.6 ng per mL. This difference reached statistical significance for both age groups (P less than .05).
Among women younger than 34 years, low serum AMH was associated with a 22% rate of miscarriage, compared with about 13% among women with higher AMH levels. This difference did not reach significance because of a lack of power, Dr. Tarasconi said. “However, binary logistic regression of the whole population confirmed the association between low serum AMH and miscarriage, independent of patient’s age and oocyte yield, with the P value less than .001.”
Low circulating AMH can reflect an above-average prevalence of oocyte abnormalities, which are linked to miscarriage, Dr. Tarasconi noted. “The fact that oocyte yield failed to alter the relationship between AMH and miscarriage suggests that altered per-follicle AMH production, not oocyte or embryo availability, fosters pregnancy loss,” he added. However, the researchers did not test either preimplantation embryos or the expelled gestational sacs to directly link miscarriages to abnormalities such as aneuploidy, he noted.
Low AMH levels also were associated with lower rates of pregnancy after IVF, regardless of age, as has been previously reported. Patients in the study were of normal karyotype, with no history of recurrent pregnancy loss, uterine abnormalities, or family history of genetic or congenital disorders, Dr. Tarasconi noted.
He reported having no financial disclosures.
SALT LAKE CITY – Low serum levels of anti-Müllerian hormone predicted miscarriage after in vitro fertilization and embryo transfer, according to a single-center prospective study of more than 2,000 women.
This relationship remained statistically significant after the study controlled for age and response to controlled ovarian hyperstimulation, which suggests that AMH is a marker of the reproductive potential of oocytes, not just of oocyte quantity, Bruno Tarasconi, MD, said at the annual meeting of the American Society for Reproductive Medicine.
“Low” serum AMH was defined as less than 1.61 ng per mL, but specific levels vary by test type, so “this is not a definite cutoff,” Dr. Tarasconi emphasized. “What we need to know is that patients with low AMH are probably experiencing some problems in the ovary that affect oocyte quality.”
The study comprised 2,365 women undergoing 2,688 IVF cycles with fresh oocytes. Levels of AMH were measured by ELISA (AMH Gen II, Beckman Coulter) within 12 months before IVF on cycle days 2 and 4, said Dr. Tarasconi of University of Paris Ouest in France.
Women younger than 34 years old, women 34-36 years old, and women 37 years and older had the highest rates of miscarriage when they had low serum AMH levels. For the two older age groups, low AMH was associated with about a 33% miscarriage rate – about twice the rate of miscarriage among women whose AMH measured at least 5.6 ng per mL. This difference reached statistical significance for both age groups (P less than .05).
Among women younger than 34 years, low serum AMH was associated with a 22% rate of miscarriage, compared with about 13% among women with higher AMH levels. This difference did not reach significance because of a lack of power, Dr. Tarasconi said. “However, binary logistic regression of the whole population confirmed the association between low serum AMH and miscarriage, independent of patient’s age and oocyte yield, with the P value less than .001.”
Low circulating AMH can reflect an above-average prevalence of oocyte abnormalities, which are linked to miscarriage, Dr. Tarasconi noted. “The fact that oocyte yield failed to alter the relationship between AMH and miscarriage suggests that altered per-follicle AMH production, not oocyte or embryo availability, fosters pregnancy loss,” he added. However, the researchers did not test either preimplantation embryos or the expelled gestational sacs to directly link miscarriages to abnormalities such as aneuploidy, he noted.
Low AMH levels also were associated with lower rates of pregnancy after IVF, regardless of age, as has been previously reported. Patients in the study were of normal karyotype, with no history of recurrent pregnancy loss, uterine abnormalities, or family history of genetic or congenital disorders, Dr. Tarasconi noted.
He reported having no financial disclosures.
Key clinical point:
Major finding: The miscarriage rate was about 33% in women with low serum AMH levels after IVF, about twice that of women with high AMH levels.
Data source: A single-center prospective study of 2,365 women undergoing 2,688 IVF cycles with fresh oocytes.
Disclosures: Dr. Tarasconi reported having no financial disclosures.
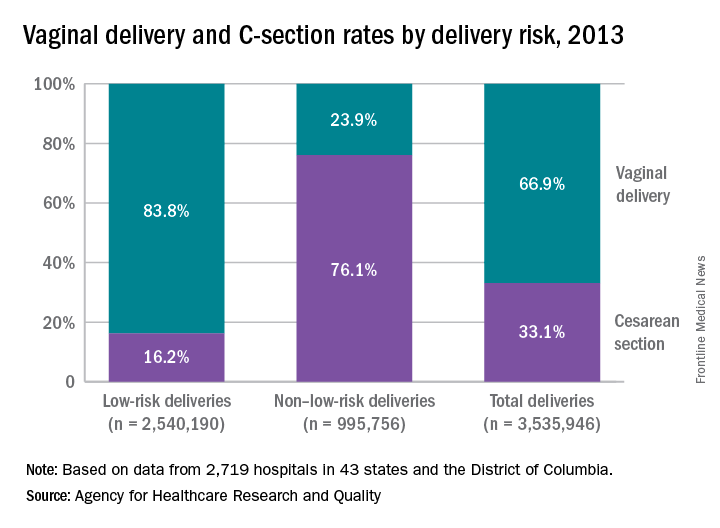
High-risk deliveries much more likely to be C-sections
Cesarean section rates for low-risk deliveries are 4.7 times lower than for deliveries that had a medical indication for the procedure listed in the record, according to the Agency for Healthcare Research and Quality.
The C-section rate for non–low-risk deliveries (deliveries with a medical indication that excluded them from the low-risk category) was 76.1% in 2013, compared with 16.2% for low-risk deliveries. Conversely, 23.9% of non–low-risk deliveries that year were performed vaginally, compared with 83.8% of low-risk deliveries, the AHRQ reported.
The AHRQ analysis combined a new definition of low-risk delivery developed by the Society for Maternal-Fetal Medicine (Am J Obstet Gynecol. 2016;214[2]:153-63) with data from the State Inpatient Databases of 43 states and the District of Columbia. This approach allowed AHRQ researchers to apply the new definition to actual counts of deliveries from 2,719 hospitals – representing 95% of the population – instead of national estimates based on a much smaller sample.
Cesarean section rates for low-risk deliveries are 4.7 times lower than for deliveries that had a medical indication for the procedure listed in the record, according to the Agency for Healthcare Research and Quality.
The C-section rate for non–low-risk deliveries (deliveries with a medical indication that excluded them from the low-risk category) was 76.1% in 2013, compared with 16.2% for low-risk deliveries. Conversely, 23.9% of non–low-risk deliveries that year were performed vaginally, compared with 83.8% of low-risk deliveries, the AHRQ reported.
The AHRQ analysis combined a new definition of low-risk delivery developed by the Society for Maternal-Fetal Medicine (Am J Obstet Gynecol. 2016;214[2]:153-63) with data from the State Inpatient Databases of 43 states and the District of Columbia. This approach allowed AHRQ researchers to apply the new definition to actual counts of deliveries from 2,719 hospitals – representing 95% of the population – instead of national estimates based on a much smaller sample.
Cesarean section rates for low-risk deliveries are 4.7 times lower than for deliveries that had a medical indication for the procedure listed in the record, according to the Agency for Healthcare Research and Quality.
The C-section rate for non–low-risk deliveries (deliveries with a medical indication that excluded them from the low-risk category) was 76.1% in 2013, compared with 16.2% for low-risk deliveries. Conversely, 23.9% of non–low-risk deliveries that year were performed vaginally, compared with 83.8% of low-risk deliveries, the AHRQ reported.
The AHRQ analysis combined a new definition of low-risk delivery developed by the Society for Maternal-Fetal Medicine (Am J Obstet Gynecol. 2016;214[2]:153-63) with data from the State Inpatient Databases of 43 states and the District of Columbia. This approach allowed AHRQ researchers to apply the new definition to actual counts of deliveries from 2,719 hospitals – representing 95% of the population – instead of national estimates based on a much smaller sample.
Claimed missteps lead to brain damage: $53M award
Claimed missteps lead to brain damage: $53M award
At 2:00 AM, a woman at 40 weeks’ gestation went to a hospital because she felt a decrease in fetal movement. At birth, the baby was not breathing. He was rushed to the neonatal intensive care unit where he was resuscitated and placed on life support. He remained in critical care for 4 weeks. The child has cerebral palsy and cannot walk, talk, or care for himself. He will need 24-hour care for the rest of his life.
PARENT’S CLAIM:
The lawsuit cited 20 alleged missteps by physicians and nurses, including failure to: react to abnormal fetal heart-rate patterns that indicated fetal distress, perform a timely cesarean delivery, and follow a chain of command. During the 12 hours that the mother was in labor at the hospital, nurses and physicians allegedly ignored her. Although the fetal heart-rate monitor showed fetal distress, the mother continued to lie unattended. At 12:40 PM, physicians called for cesarean delivery due to fetal distress, but it took an hour for the child to be born.
The negligence of the hospital staff and delay in delivery caused hypoxia, resulting in cerebral palsy. All medical records from the hospital’s neonatal clinic show that he suffered hypoxia at birth.
HOSPITAL’S DEFENSE:
The mother and child were treated for an infection, which is a recognized cause of cerebral palsy. The child was born with normal blood oxygen levels. His injury occurred before the mother came to the hospital.
VERDICT:
A $53 million Illinois verdict was returned. The hospital applied for a mistrial based on allegedly inflammatory comments by the prosecuting attorney, but that was dismissed.
Birth trauma: $2.75M settlement
A woman had her first prenatal visit at 21 weeks’ gestation. Her advanced maternal age (39 years) and poor health history, including prior delivery of a baby with intrauterine growth restriction (IUGR), put her at risk; her prenatal care was transferred to a high-risk clinic.
At her next prenatal visit, records noted that the mother’s job required excessive standing, that she tested positive for marijuana, and that she was at risk for IUGR. Notes did not say that the mother was informed of her IUGR risk nor was a plan created for additional testing to monitor IUGR.
Ultrasonography (US) performed at 25 weeks’ gestation estimated that the baby’s weight was in the 11th- to 12th-week percentile. Amniotic fluid volume was noted as normal.
The mother missed her next appointment but returned at 28 weeks’ gestation, when she reported a headache and was found to have high blood pressure. US revealed normal fetal heart anatomy but amniotic fluid volume was noted to have decreased since the first US. The mother missed the next several appointments.
When she presented at 33 weeks’ gestation, her blood pressure was 160/97 mm Hg, fetal heart-rate tones were normal, and there was positive fetal movement. Fundal height measurement revealed a 3-cm discrepancy in date and size, suggesting a small baby, decreased amniotic fluid, or both. The ObGyn ordered testing for the next day. When a nonstress test performed from 8:44 AM to 9:10 AM was nonreassuring, the mother was ordered to immediately go to the hospital. She did not arrive at the hospital until 11:13 AM, when she was placed on fetal heart-rate monitor; test results were nonreassuring. At 11:30 AM, US revealed IUGR and oligohydramnios. An urgent cesarean delivery was performed and the baby was born at 11:56 AM.
The child’s Apgar scores were 4 and 9 at 1 and 5 minutes, respectively. The baby developed white matter brain damage and grade III and IV intraventricular hemorrhages due to hypoxia, ischemia, and metabolic acidosis. A maternal drug screen was positive for marijuana. Placental pathology revealed multiple abnormalities including placental infarcts involving approximately 50% of placental tissue, abnormal vascular changes, intervillous fibrin deposition, and chronic villitis.
At trial, the child had developmental delays, cognitive defects, learning disabilities, and breakthrough seizures.
PARENT’S CLAIM:
The mother claimed that the high-risk clinic was negligent. A plan should have been put into place at her first visit to monitor for IUGR based on her history. She was not advised of her risk of having another IUGR baby. Fundal height measurement, US to test for IUGR, or assessments of fetal heart-rate tones and fetal movement were not performed regularly. If a nonstress test had been performed earlier than 33 weeks’ gestation, she might have been admitted to the hospital for monitoring and earlier delivery, resulting in a healthier baby.
DEFENDANTS’ DEFENSE:
The mother was noncompliant and missed most of her prenatal appointments. She also continued to smoke marijuana throughout her pregnancy although she was told to stop. When the mother arrived at the prenatal clinic at 33 weeks’ gestation, tests were ordered and delivery occurred in a timely fashion. Any problems suffered by the child were a result of prematurity and damage that occurred during the 5 weeks of missed prenatal appointments.
VERDICT:
A $2.75M Missouri settlement was reached.
Who should have delivered the baby?
A mother’s prenatal care was managed by her family practitioner (FP). The mother went to the FP for induction of labor, but it was unsuccessful. Three days later, the baby was delivered by the FP and began having seizures a few minutes after birth. The child is quadriplegic and has severe cerebral palsy.
PARENTS’ CLAIM:
The FP was negligent in managing labor and delivery. She should have called an ObGyn to manage the labor. She failed to monitor fetal heart-rate tracings and failed to order an emergency cesarean delivery. The FP mismanaged the baby’s condition upon delivery and seizures started.
PHYSICIAN’S DEFENSE:
The FP properly managed the delivery and postdelivery complications. The brain injury had nothing to do with the birth; it was instead caused by a stroke disorder that occurred 3 to 7 days before delivery.
VERDICT:
A Minnesota defense verdict was returned.
Undiagnosed H1N1 influenza (swine flu) during pregnancy; mother and child die: $16.7M verdict
At 7 months’ gestation, a 27-year-old woman presented to a clinic on June 26 with a runny nose, congestion, cough, wheezing, chills, and sweats. The physician noted concern about proteinuria and that the patient reported chills and sweats, but that he was uncertain as to the symptoms’ cause. He recommended that she see her ObGyn immediately and report to the emergency department (ED) if symptoms worsened. The patient called her ObGyn to report having a temperature between 94˚F and 103˚F and taking acetaminophen. The next day, she saw a nurse practitioner in the ObGyn’s office who documented that the patient was not given antiviral medication.
On June 29, the patient was still feeling ill and went to the ED. Although a physician planned to discharge her, an ObGyn nurse recognized that the patient was too ill to leave and had her admitted.
The patient’s condition worsened overnight and she was transferred to the intensive care unit (ICU), where she was intubated and put on a ventilator. Medical notes read “as whether influenza was present was unclear…Tamiflu will be started, but the efficacy of it this late into a possible influenza episode is extremely questionable.” ICU physicians believed that the best option was to deliver the child. After the patient’s husband gave permission, the child was born by cesarean delivery. The mother never regained full consciousness and remained in a medically induced coma. She died on August 11 after the family decided to remove life support.
After being given the diagnosis of intrauterine hypoxia, the child remained in intensive care for several weeks and then was discharged home. Seven months later, the father found his daughter in bed, not breathing, which physicians believed was an episode of sudden infant death syndrome. The father performed CPR and rushed her to the hospital. Physicians tried twice to take the child off the ventilator, but she could not breathe without assistance. On February 21, life support was removed and the child died.
ESTATES’ CLAIM:
The clinic and its physician were negligent for failing to recognize that the mother had influenza; she presented with classic flu symptoms during a worldwide pandemic. In the several months before the patient’s visit, the clinic had received notices from health authorities alerting medical professionals to the dangers of H1N1 influenza, or Swine Flu. The clinic also had received, before the patient’s visit, information warning of an elevated risk of H1N1 to pregnant women, with instructions to administer oseltamivir phosphate (Tamiflu) to any pregnant woman suspected of having influenza.
DEFENDANTS’ DEFENSE:
The clinic and physician denied negligence, claiming that treatment of the mother was appropriate.
VERDICT:
A $16.7 million Washington verdict was returned.
Mother claims to being uninformed of antiepileptics’ risks
A woman with epilepsy gave birth to a child with physical and cognitive birth defects.
PARENT’S CLAIM:
The mother claimed that, although she was of child-bearing age, she had never been informed of the risk of birth defects associated with taking an antiepileptic medication. Had she known of the risk, she would not have chosen to conceive. The physicians should have prescribed a different antiepileptic drug.
DEFENDANTS’ DEFENSE:
The clinic’s physicians met the standard of care in prescribing the drug. They properly informed the mother of the risks of taking the antiepileptic drug during pregnancy. The patient was allergic to all other antiepileptic drugs available at the time, so an alternative was not available.
VERDICT:
An Illinois defense verdict was returned for the clinic.
Mother has stroke during delivery: $3M settlement
A 42-year-old woman had a hemorrhagic stroke during the delivery of her first child. She remained hospitalized for observation with a medical plan to insert a drain if her condition worsened. Initially she did well, but she then began to have episodes of decreased consciousness and loss of function and later became unresponsive. Her physicians then undertook an emergency procedure to attempt to drain blood from her brain, but the surgical measure did not prevent her from incurring significant cognitive and physical injuries.
PATIENT’S CLAIM:
The agreed-upon medical plan of treatment was not followed, resulting in severe brain damage to the patient.
DEFENDANTS’ DEFENSE:
The case was settled during the trial.
VERDICT:
A $3 million Massachusetts settlement was reached.
Did delayed cesarean cause cognitive defects?
When fetal distress was detected, the nurse called the patient’s ObGyn at 12:30 AM. The ObGyn arrived at the hospital at 12:48 AM, ordered a cesarean delivery at 12:56 AM, and the baby was born at 1:20 AM.
PARENTS’ CLAIM:
The ObGyn was negligent for not calling for the hospital’s on-duty resident physician to become involved in the case when the nurse phoned at 12:30 AM. If the ObGyn had done so, cesarean delivery would have been ordered and completed earlier, which would have averted the child’s injuries, including cognitive and physical impairments.
PHYSICIAN’S DEFENSE:
The ObGyn asserted that, based on the information provided to her, there was no reason to request the resident’s involvement. An earlier cesarean delivery was not necessary based on fetal heart-rate monitoring strip results. The ObGyn acted in a timely manner when calling for cesarean delivery. There was no concrete evidence that any alleged delay caused the child’s injuries.
VERDICT:
An Illinois defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Claimed missteps lead to brain damage: $53M award
At 2:00 AM, a woman at 40 weeks’ gestation went to a hospital because she felt a decrease in fetal movement. At birth, the baby was not breathing. He was rushed to the neonatal intensive care unit where he was resuscitated and placed on life support. He remained in critical care for 4 weeks. The child has cerebral palsy and cannot walk, talk, or care for himself. He will need 24-hour care for the rest of his life.
PARENT’S CLAIM:
The lawsuit cited 20 alleged missteps by physicians and nurses, including failure to: react to abnormal fetal heart-rate patterns that indicated fetal distress, perform a timely cesarean delivery, and follow a chain of command. During the 12 hours that the mother was in labor at the hospital, nurses and physicians allegedly ignored her. Although the fetal heart-rate monitor showed fetal distress, the mother continued to lie unattended. At 12:40 PM, physicians called for cesarean delivery due to fetal distress, but it took an hour for the child to be born.
The negligence of the hospital staff and delay in delivery caused hypoxia, resulting in cerebral palsy. All medical records from the hospital’s neonatal clinic show that he suffered hypoxia at birth.
HOSPITAL’S DEFENSE:
The mother and child were treated for an infection, which is a recognized cause of cerebral palsy. The child was born with normal blood oxygen levels. His injury occurred before the mother came to the hospital.
VERDICT:
A $53 million Illinois verdict was returned. The hospital applied for a mistrial based on allegedly inflammatory comments by the prosecuting attorney, but that was dismissed.
Birth trauma: $2.75M settlement
A woman had her first prenatal visit at 21 weeks’ gestation. Her advanced maternal age (39 years) and poor health history, including prior delivery of a baby with intrauterine growth restriction (IUGR), put her at risk; her prenatal care was transferred to a high-risk clinic.
At her next prenatal visit, records noted that the mother’s job required excessive standing, that she tested positive for marijuana, and that she was at risk for IUGR. Notes did not say that the mother was informed of her IUGR risk nor was a plan created for additional testing to monitor IUGR.
Ultrasonography (US) performed at 25 weeks’ gestation estimated that the baby’s weight was in the 11th- to 12th-week percentile. Amniotic fluid volume was noted as normal.
The mother missed her next appointment but returned at 28 weeks’ gestation, when she reported a headache and was found to have high blood pressure. US revealed normal fetal heart anatomy but amniotic fluid volume was noted to have decreased since the first US. The mother missed the next several appointments.
When she presented at 33 weeks’ gestation, her blood pressure was 160/97 mm Hg, fetal heart-rate tones were normal, and there was positive fetal movement. Fundal height measurement revealed a 3-cm discrepancy in date and size, suggesting a small baby, decreased amniotic fluid, or both. The ObGyn ordered testing for the next day. When a nonstress test performed from 8:44 AM to 9:10 AM was nonreassuring, the mother was ordered to immediately go to the hospital. She did not arrive at the hospital until 11:13 AM, when she was placed on fetal heart-rate monitor; test results were nonreassuring. At 11:30 AM, US revealed IUGR and oligohydramnios. An urgent cesarean delivery was performed and the baby was born at 11:56 AM.
The child’s Apgar scores were 4 and 9 at 1 and 5 minutes, respectively. The baby developed white matter brain damage and grade III and IV intraventricular hemorrhages due to hypoxia, ischemia, and metabolic acidosis. A maternal drug screen was positive for marijuana. Placental pathology revealed multiple abnormalities including placental infarcts involving approximately 50% of placental tissue, abnormal vascular changes, intervillous fibrin deposition, and chronic villitis.
At trial, the child had developmental delays, cognitive defects, learning disabilities, and breakthrough seizures.
PARENT’S CLAIM:
The mother claimed that the high-risk clinic was negligent. A plan should have been put into place at her first visit to monitor for IUGR based on her history. She was not advised of her risk of having another IUGR baby. Fundal height measurement, US to test for IUGR, or assessments of fetal heart-rate tones and fetal movement were not performed regularly. If a nonstress test had been performed earlier than 33 weeks’ gestation, she might have been admitted to the hospital for monitoring and earlier delivery, resulting in a healthier baby.
DEFENDANTS’ DEFENSE:
The mother was noncompliant and missed most of her prenatal appointments. She also continued to smoke marijuana throughout her pregnancy although she was told to stop. When the mother arrived at the prenatal clinic at 33 weeks’ gestation, tests were ordered and delivery occurred in a timely fashion. Any problems suffered by the child were a result of prematurity and damage that occurred during the 5 weeks of missed prenatal appointments.
VERDICT:
A $2.75M Missouri settlement was reached.
Who should have delivered the baby?
A mother’s prenatal care was managed by her family practitioner (FP). The mother went to the FP for induction of labor, but it was unsuccessful. Three days later, the baby was delivered by the FP and began having seizures a few minutes after birth. The child is quadriplegic and has severe cerebral palsy.
PARENTS’ CLAIM:
The FP was negligent in managing labor and delivery. She should have called an ObGyn to manage the labor. She failed to monitor fetal heart-rate tracings and failed to order an emergency cesarean delivery. The FP mismanaged the baby’s condition upon delivery and seizures started.
PHYSICIAN’S DEFENSE:
The FP properly managed the delivery and postdelivery complications. The brain injury had nothing to do with the birth; it was instead caused by a stroke disorder that occurred 3 to 7 days before delivery.
VERDICT:
A Minnesota defense verdict was returned.
Undiagnosed H1N1 influenza (swine flu) during pregnancy; mother and child die: $16.7M verdict
At 7 months’ gestation, a 27-year-old woman presented to a clinic on June 26 with a runny nose, congestion, cough, wheezing, chills, and sweats. The physician noted concern about proteinuria and that the patient reported chills and sweats, but that he was uncertain as to the symptoms’ cause. He recommended that she see her ObGyn immediately and report to the emergency department (ED) if symptoms worsened. The patient called her ObGyn to report having a temperature between 94˚F and 103˚F and taking acetaminophen. The next day, she saw a nurse practitioner in the ObGyn’s office who documented that the patient was not given antiviral medication.
On June 29, the patient was still feeling ill and went to the ED. Although a physician planned to discharge her, an ObGyn nurse recognized that the patient was too ill to leave and had her admitted.
The patient’s condition worsened overnight and she was transferred to the intensive care unit (ICU), where she was intubated and put on a ventilator. Medical notes read “as whether influenza was present was unclear…Tamiflu will be started, but the efficacy of it this late into a possible influenza episode is extremely questionable.” ICU physicians believed that the best option was to deliver the child. After the patient’s husband gave permission, the child was born by cesarean delivery. The mother never regained full consciousness and remained in a medically induced coma. She died on August 11 after the family decided to remove life support.
After being given the diagnosis of intrauterine hypoxia, the child remained in intensive care for several weeks and then was discharged home. Seven months later, the father found his daughter in bed, not breathing, which physicians believed was an episode of sudden infant death syndrome. The father performed CPR and rushed her to the hospital. Physicians tried twice to take the child off the ventilator, but she could not breathe without assistance. On February 21, life support was removed and the child died.
ESTATES’ CLAIM:
The clinic and its physician were negligent for failing to recognize that the mother had influenza; she presented with classic flu symptoms during a worldwide pandemic. In the several months before the patient’s visit, the clinic had received notices from health authorities alerting medical professionals to the dangers of H1N1 influenza, or Swine Flu. The clinic also had received, before the patient’s visit, information warning of an elevated risk of H1N1 to pregnant women, with instructions to administer oseltamivir phosphate (Tamiflu) to any pregnant woman suspected of having influenza.
DEFENDANTS’ DEFENSE:
The clinic and physician denied negligence, claiming that treatment of the mother was appropriate.
VERDICT:
A $16.7 million Washington verdict was returned.
Mother claims to being uninformed of antiepileptics’ risks
A woman with epilepsy gave birth to a child with physical and cognitive birth defects.
PARENT’S CLAIM:
The mother claimed that, although she was of child-bearing age, she had never been informed of the risk of birth defects associated with taking an antiepileptic medication. Had she known of the risk, she would not have chosen to conceive. The physicians should have prescribed a different antiepileptic drug.
DEFENDANTS’ DEFENSE:
The clinic’s physicians met the standard of care in prescribing the drug. They properly informed the mother of the risks of taking the antiepileptic drug during pregnancy. The patient was allergic to all other antiepileptic drugs available at the time, so an alternative was not available.
VERDICT:
An Illinois defense verdict was returned for the clinic.
Mother has stroke during delivery: $3M settlement
A 42-year-old woman had a hemorrhagic stroke during the delivery of her first child. She remained hospitalized for observation with a medical plan to insert a drain if her condition worsened. Initially she did well, but she then began to have episodes of decreased consciousness and loss of function and later became unresponsive. Her physicians then undertook an emergency procedure to attempt to drain blood from her brain, but the surgical measure did not prevent her from incurring significant cognitive and physical injuries.
PATIENT’S CLAIM:
The agreed-upon medical plan of treatment was not followed, resulting in severe brain damage to the patient.
DEFENDANTS’ DEFENSE:
The case was settled during the trial.
VERDICT:
A $3 million Massachusetts settlement was reached.
Did delayed cesarean cause cognitive defects?
When fetal distress was detected, the nurse called the patient’s ObGyn at 12:30 AM. The ObGyn arrived at the hospital at 12:48 AM, ordered a cesarean delivery at 12:56 AM, and the baby was born at 1:20 AM.
PARENTS’ CLAIM:
The ObGyn was negligent for not calling for the hospital’s on-duty resident physician to become involved in the case when the nurse phoned at 12:30 AM. If the ObGyn had done so, cesarean delivery would have been ordered and completed earlier, which would have averted the child’s injuries, including cognitive and physical impairments.
PHYSICIAN’S DEFENSE:
The ObGyn asserted that, based on the information provided to her, there was no reason to request the resident’s involvement. An earlier cesarean delivery was not necessary based on fetal heart-rate monitoring strip results. The ObGyn acted in a timely manner when calling for cesarean delivery. There was no concrete evidence that any alleged delay caused the child’s injuries.
VERDICT:
An Illinois defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Claimed missteps lead to brain damage: $53M award
At 2:00 AM, a woman at 40 weeks’ gestation went to a hospital because she felt a decrease in fetal movement. At birth, the baby was not breathing. He was rushed to the neonatal intensive care unit where he was resuscitated and placed on life support. He remained in critical care for 4 weeks. The child has cerebral palsy and cannot walk, talk, or care for himself. He will need 24-hour care for the rest of his life.
PARENT’S CLAIM:
The lawsuit cited 20 alleged missteps by physicians and nurses, including failure to: react to abnormal fetal heart-rate patterns that indicated fetal distress, perform a timely cesarean delivery, and follow a chain of command. During the 12 hours that the mother was in labor at the hospital, nurses and physicians allegedly ignored her. Although the fetal heart-rate monitor showed fetal distress, the mother continued to lie unattended. At 12:40 PM, physicians called for cesarean delivery due to fetal distress, but it took an hour for the child to be born.
The negligence of the hospital staff and delay in delivery caused hypoxia, resulting in cerebral palsy. All medical records from the hospital’s neonatal clinic show that he suffered hypoxia at birth.
HOSPITAL’S DEFENSE:
The mother and child were treated for an infection, which is a recognized cause of cerebral palsy. The child was born with normal blood oxygen levels. His injury occurred before the mother came to the hospital.
VERDICT:
A $53 million Illinois verdict was returned. The hospital applied for a mistrial based on allegedly inflammatory comments by the prosecuting attorney, but that was dismissed.
Birth trauma: $2.75M settlement
A woman had her first prenatal visit at 21 weeks’ gestation. Her advanced maternal age (39 years) and poor health history, including prior delivery of a baby with intrauterine growth restriction (IUGR), put her at risk; her prenatal care was transferred to a high-risk clinic.
At her next prenatal visit, records noted that the mother’s job required excessive standing, that she tested positive for marijuana, and that she was at risk for IUGR. Notes did not say that the mother was informed of her IUGR risk nor was a plan created for additional testing to monitor IUGR.
Ultrasonography (US) performed at 25 weeks’ gestation estimated that the baby’s weight was in the 11th- to 12th-week percentile. Amniotic fluid volume was noted as normal.
The mother missed her next appointment but returned at 28 weeks’ gestation, when she reported a headache and was found to have high blood pressure. US revealed normal fetal heart anatomy but amniotic fluid volume was noted to have decreased since the first US. The mother missed the next several appointments.
When she presented at 33 weeks’ gestation, her blood pressure was 160/97 mm Hg, fetal heart-rate tones were normal, and there was positive fetal movement. Fundal height measurement revealed a 3-cm discrepancy in date and size, suggesting a small baby, decreased amniotic fluid, or both. The ObGyn ordered testing for the next day. When a nonstress test performed from 8:44 AM to 9:10 AM was nonreassuring, the mother was ordered to immediately go to the hospital. She did not arrive at the hospital until 11:13 AM, when she was placed on fetal heart-rate monitor; test results were nonreassuring. At 11:30 AM, US revealed IUGR and oligohydramnios. An urgent cesarean delivery was performed and the baby was born at 11:56 AM.
The child’s Apgar scores were 4 and 9 at 1 and 5 minutes, respectively. The baby developed white matter brain damage and grade III and IV intraventricular hemorrhages due to hypoxia, ischemia, and metabolic acidosis. A maternal drug screen was positive for marijuana. Placental pathology revealed multiple abnormalities including placental infarcts involving approximately 50% of placental tissue, abnormal vascular changes, intervillous fibrin deposition, and chronic villitis.
At trial, the child had developmental delays, cognitive defects, learning disabilities, and breakthrough seizures.
PARENT’S CLAIM:
The mother claimed that the high-risk clinic was negligent. A plan should have been put into place at her first visit to monitor for IUGR based on her history. She was not advised of her risk of having another IUGR baby. Fundal height measurement, US to test for IUGR, or assessments of fetal heart-rate tones and fetal movement were not performed regularly. If a nonstress test had been performed earlier than 33 weeks’ gestation, she might have been admitted to the hospital for monitoring and earlier delivery, resulting in a healthier baby.
DEFENDANTS’ DEFENSE:
The mother was noncompliant and missed most of her prenatal appointments. She also continued to smoke marijuana throughout her pregnancy although she was told to stop. When the mother arrived at the prenatal clinic at 33 weeks’ gestation, tests were ordered and delivery occurred in a timely fashion. Any problems suffered by the child were a result of prematurity and damage that occurred during the 5 weeks of missed prenatal appointments.
VERDICT:
A $2.75M Missouri settlement was reached.
Who should have delivered the baby?
A mother’s prenatal care was managed by her family practitioner (FP). The mother went to the FP for induction of labor, but it was unsuccessful. Three days later, the baby was delivered by the FP and began having seizures a few minutes after birth. The child is quadriplegic and has severe cerebral palsy.
PARENTS’ CLAIM:
The FP was negligent in managing labor and delivery. She should have called an ObGyn to manage the labor. She failed to monitor fetal heart-rate tracings and failed to order an emergency cesarean delivery. The FP mismanaged the baby’s condition upon delivery and seizures started.
PHYSICIAN’S DEFENSE:
The FP properly managed the delivery and postdelivery complications. The brain injury had nothing to do with the birth; it was instead caused by a stroke disorder that occurred 3 to 7 days before delivery.
VERDICT:
A Minnesota defense verdict was returned.
Undiagnosed H1N1 influenza (swine flu) during pregnancy; mother and child die: $16.7M verdict
At 7 months’ gestation, a 27-year-old woman presented to a clinic on June 26 with a runny nose, congestion, cough, wheezing, chills, and sweats. The physician noted concern about proteinuria and that the patient reported chills and sweats, but that he was uncertain as to the symptoms’ cause. He recommended that she see her ObGyn immediately and report to the emergency department (ED) if symptoms worsened. The patient called her ObGyn to report having a temperature between 94˚F and 103˚F and taking acetaminophen. The next day, she saw a nurse practitioner in the ObGyn’s office who documented that the patient was not given antiviral medication.
On June 29, the patient was still feeling ill and went to the ED. Although a physician planned to discharge her, an ObGyn nurse recognized that the patient was too ill to leave and had her admitted.
The patient’s condition worsened overnight and she was transferred to the intensive care unit (ICU), where she was intubated and put on a ventilator. Medical notes read “as whether influenza was present was unclear…Tamiflu will be started, but the efficacy of it this late into a possible influenza episode is extremely questionable.” ICU physicians believed that the best option was to deliver the child. After the patient’s husband gave permission, the child was born by cesarean delivery. The mother never regained full consciousness and remained in a medically induced coma. She died on August 11 after the family decided to remove life support.
After being given the diagnosis of intrauterine hypoxia, the child remained in intensive care for several weeks and then was discharged home. Seven months later, the father found his daughter in bed, not breathing, which physicians believed was an episode of sudden infant death syndrome. The father performed CPR and rushed her to the hospital. Physicians tried twice to take the child off the ventilator, but she could not breathe without assistance. On February 21, life support was removed and the child died.
ESTATES’ CLAIM:
The clinic and its physician were negligent for failing to recognize that the mother had influenza; she presented with classic flu symptoms during a worldwide pandemic. In the several months before the patient’s visit, the clinic had received notices from health authorities alerting medical professionals to the dangers of H1N1 influenza, or Swine Flu. The clinic also had received, before the patient’s visit, information warning of an elevated risk of H1N1 to pregnant women, with instructions to administer oseltamivir phosphate (Tamiflu) to any pregnant woman suspected of having influenza.
DEFENDANTS’ DEFENSE:
The clinic and physician denied negligence, claiming that treatment of the mother was appropriate.
VERDICT:
A $16.7 million Washington verdict was returned.
Mother claims to being uninformed of antiepileptics’ risks
A woman with epilepsy gave birth to a child with physical and cognitive birth defects.
PARENT’S CLAIM:
The mother claimed that, although she was of child-bearing age, she had never been informed of the risk of birth defects associated with taking an antiepileptic medication. Had she known of the risk, she would not have chosen to conceive. The physicians should have prescribed a different antiepileptic drug.
DEFENDANTS’ DEFENSE:
The clinic’s physicians met the standard of care in prescribing the drug. They properly informed the mother of the risks of taking the antiepileptic drug during pregnancy. The patient was allergic to all other antiepileptic drugs available at the time, so an alternative was not available.
VERDICT:
An Illinois defense verdict was returned for the clinic.
Mother has stroke during delivery: $3M settlement
A 42-year-old woman had a hemorrhagic stroke during the delivery of her first child. She remained hospitalized for observation with a medical plan to insert a drain if her condition worsened. Initially she did well, but she then began to have episodes of decreased consciousness and loss of function and later became unresponsive. Her physicians then undertook an emergency procedure to attempt to drain blood from her brain, but the surgical measure did not prevent her from incurring significant cognitive and physical injuries.
PATIENT’S CLAIM:
The agreed-upon medical plan of treatment was not followed, resulting in severe brain damage to the patient.
DEFENDANTS’ DEFENSE:
The case was settled during the trial.
VERDICT:
A $3 million Massachusetts settlement was reached.
Did delayed cesarean cause cognitive defects?
When fetal distress was detected, the nurse called the patient’s ObGyn at 12:30 AM. The ObGyn arrived at the hospital at 12:48 AM, ordered a cesarean delivery at 12:56 AM, and the baby was born at 1:20 AM.
PARENTS’ CLAIM:
The ObGyn was negligent for not calling for the hospital’s on-duty resident physician to become involved in the case when the nurse phoned at 12:30 AM. If the ObGyn had done so, cesarean delivery would have been ordered and completed earlier, which would have averted the child’s injuries, including cognitive and physical impairments.
PHYSICIAN’S DEFENSE:
The ObGyn asserted that, based on the information provided to her, there was no reason to request the resident’s involvement. An earlier cesarean delivery was not necessary based on fetal heart-rate monitoring strip results. The ObGyn acted in a timely manner when calling for cesarean delivery. There was no concrete evidence that any alleged delay caused the child’s injuries.
VERDICT:
An Illinois defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
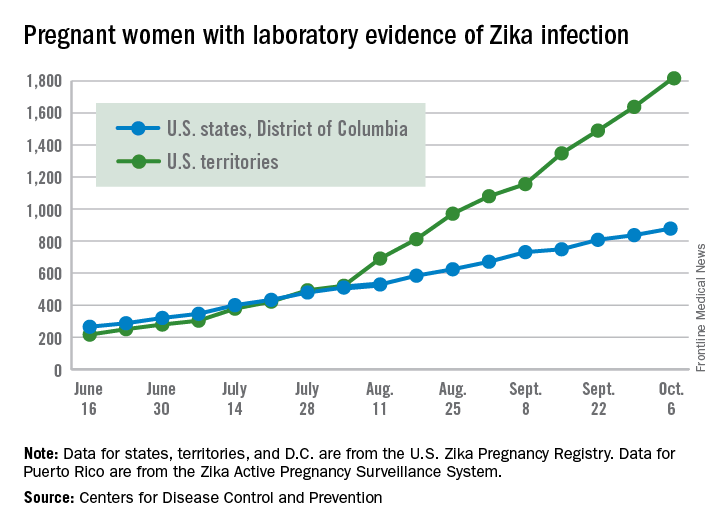
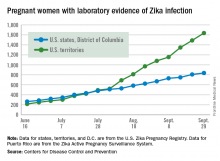
Number of Zika-infected pregnant women continues to climb
The number of pregnant women with laboratory evidence of Zika infection increased by 209 for the week ending Oct. 6, the second-largest weekly increase so far in the United States in 2016, according to the Centers for Disease Control and Prevention.
Another liveborn infant with Zika-related birth defects also was reported for the week, bringing the total for the year to 23 for the 50 states and the District of Columbia. There were no new cases of Zika-related pregnancy losses reported, so the 50-state/DC total remains at five.
Of the 209 new cases reported for the week ending Oct. 6, 41 were in the states/D.C. and 168 were in the territories. The total number of U.S. Zika cases in pregnant women for the year is 2,684: 878 in the states/D.C. and 1,806 in the territories, the CDC said.
Among all Americans, there were 29,891 cases reported as of Oct. 12: 3,936 in the states/D.C. and 25,955 in the territories. Of the territorial cases, 98% have been in Puerto Rico, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The number of pregnant women with laboratory evidence of Zika infection increased by 209 for the week ending Oct. 6, the second-largest weekly increase so far in the United States in 2016, according to the Centers for Disease Control and Prevention.
Another liveborn infant with Zika-related birth defects also was reported for the week, bringing the total for the year to 23 for the 50 states and the District of Columbia. There were no new cases of Zika-related pregnancy losses reported, so the 50-state/DC total remains at five.
Of the 209 new cases reported for the week ending Oct. 6, 41 were in the states/D.C. and 168 were in the territories. The total number of U.S. Zika cases in pregnant women for the year is 2,684: 878 in the states/D.C. and 1,806 in the territories, the CDC said.
Among all Americans, there were 29,891 cases reported as of Oct. 12: 3,936 in the states/D.C. and 25,955 in the territories. Of the territorial cases, 98% have been in Puerto Rico, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The number of pregnant women with laboratory evidence of Zika infection increased by 209 for the week ending Oct. 6, the second-largest weekly increase so far in the United States in 2016, according to the Centers for Disease Control and Prevention.
Another liveborn infant with Zika-related birth defects also was reported for the week, bringing the total for the year to 23 for the 50 states and the District of Columbia. There were no new cases of Zika-related pregnancy losses reported, so the 50-state/DC total remains at five.
Of the 209 new cases reported for the week ending Oct. 6, 41 were in the states/D.C. and 168 were in the territories. The total number of U.S. Zika cases in pregnant women for the year is 2,684: 878 in the states/D.C. and 1,806 in the territories, the CDC said.
Among all Americans, there were 29,891 cases reported as of Oct. 12: 3,936 in the states/D.C. and 25,955 in the territories. Of the territorial cases, 98% have been in Puerto Rico, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Prenatal SSRI exposure linked to speech, language disorders
Selective serotonin reuptake inhibitors taken during pregnancy are linked to a 37% increased risk of speech and language disorders in the children prenatally exposed to them, according to a prospective birth cohort study.
That risk occurs when children exposed prenatally are compared with children whose mothers did not take SSRIs but who had a diagnosis for which the antidepressants are indicated.
“The finding was observed only in offspring of mothers who purchased at least two SSRI prescriptions during pregnancy,” reported Alan S. Brown, MD, of New York State Psychiatric Institute in New York City, and his associates (JAMA Psychiatry. 2016 Oct 12. doi: 10.1001/jamapsychiatry.2016.2594). “This finding is particularly noteworthy because these women were more likely to have taken these medications and were exposed for a longer period and to larger amounts of SSRIs during pregnancy, compared with women who filled only one prescription.”
From among an initial cohort of 845,345 pregnant women in Finland, the researchers followed a final cohort of 56,340 offspring. Most of the children (86.6%) were 9 years old or younger at the end of study follow-up, running from 1996 to 2010, and the oldest children were aged 14 years. The researchers used Finland’s national registries to determine the children’s and mothers’ diagnoses and the mothers’ history of prescriptions from 30 days before pregnancy through delivery.
Among all children, 15,596 children were born to women who took SSRIs for depression or another SSRI-indicated psychiatric condition, and 31,207 children were born to mothers who had neither a psychiatric diagnosis nor a history of taking SSRIs during pregnancy. The remaining 9,537 children had mothers with a psychiatric diagnosis but who did not take SSRIs during pregnancy.
The average ages of the children at diagnosis were 4.4 years for speech or language disorders, 3.6 years for scholastic problems, and 7.7 years for motor disorders. When the researchers compared the children prenatally exposed to SSRIs to the children of mothers with a depression-related diagnosis but not taking SSRIs during pregnancy, the rates of scholastic or motor disorders did not differ.
For language and speech disorders, however, children whose mothers purchased two SSRI prescriptions had a 37% increased risk of a disorder, compared with children whose mothers had a diagnosis but did not purchase SSRIs. Without the requirement of at least two prescriptions, the statistical difference did not exist.
That increased risk occurred after adjustment for sex, mother’s parity, marital status, socioeconomic status, place of residence, both parents’ ages, the children’s gestational age at birth, prenatal exposure to antiepileptic drugs, exposure to anxiolytics/sedatives, history of chronic diseases, death of the child’s parent, mother’s country of birth, maternal smoking or substance abuse, and the psychiatric history of both parents. Data on maternal alcohol consumption were unavailable. Both the children exposed to SSRIs and the children of unmedicated mothers with a psychiatric condition had a higher risk of speech and language disorders.
The research was funded by the National Institutes of Health; the Sackler Foundation of Columbia University, New York; and the University of Turku (Finland). One of the researchers, David Gyllenberg, MD, reported receiving research funding from the Sigrid Juselius Foundation, the Foundation for Pediatric Research in Finland, and the Finnish Medical Foundation. No other conflicts of interest were disclosed.
Alan S. Brown, MD, and his associates examined a great deal of data, but the clinical implications of their findings are fuzzy, wrote Lee S. Cohen, MD, and Ruta Nonacs, MD, in an accompanying editorial (JAMA Psychiatry. 2016 Oct 12. doi: 10.1001/jamapsychiatry.2016.2705).
“The frequency of speech/language problems following referral to specialized health care services are relatively small; disorders occurred in 1.6% of patients from the SSRI-exposed group, 1.9% from the unmedicated group, and 1.0% from the nonexposed group,” Dr. Cohen and Dr. Nonacs wrote. “Are the data presented a signal of concern requiring further study or just background noise?”
Dr. Cohen has received support from several companies, including Cephalon, Takeda/Lundbeck Pharmaceuticals, GlaxoSmithKline, and JayMac Pharmaceuticals. Dr. Nonacs reported no disclosures. Dr. Cohen and Dr. Nonacs are affiliated with the department of psychiatry at Massachusetts General Hospital and Harvard Medical School, both in Boston.
Alan S. Brown, MD, and his associates examined a great deal of data, but the clinical implications of their findings are fuzzy, wrote Lee S. Cohen, MD, and Ruta Nonacs, MD, in an accompanying editorial (JAMA Psychiatry. 2016 Oct 12. doi: 10.1001/jamapsychiatry.2016.2705).
“The frequency of speech/language problems following referral to specialized health care services are relatively small; disorders occurred in 1.6% of patients from the SSRI-exposed group, 1.9% from the unmedicated group, and 1.0% from the nonexposed group,” Dr. Cohen and Dr. Nonacs wrote. “Are the data presented a signal of concern requiring further study or just background noise?”
Dr. Cohen has received support from several companies, including Cephalon, Takeda/Lundbeck Pharmaceuticals, GlaxoSmithKline, and JayMac Pharmaceuticals. Dr. Nonacs reported no disclosures. Dr. Cohen and Dr. Nonacs are affiliated with the department of psychiatry at Massachusetts General Hospital and Harvard Medical School, both in Boston.
Alan S. Brown, MD, and his associates examined a great deal of data, but the clinical implications of their findings are fuzzy, wrote Lee S. Cohen, MD, and Ruta Nonacs, MD, in an accompanying editorial (JAMA Psychiatry. 2016 Oct 12. doi: 10.1001/jamapsychiatry.2016.2705).
“The frequency of speech/language problems following referral to specialized health care services are relatively small; disorders occurred in 1.6% of patients from the SSRI-exposed group, 1.9% from the unmedicated group, and 1.0% from the nonexposed group,” Dr. Cohen and Dr. Nonacs wrote. “Are the data presented a signal of concern requiring further study or just background noise?”
Dr. Cohen has received support from several companies, including Cephalon, Takeda/Lundbeck Pharmaceuticals, GlaxoSmithKline, and JayMac Pharmaceuticals. Dr. Nonacs reported no disclosures. Dr. Cohen and Dr. Nonacs are affiliated with the department of psychiatry at Massachusetts General Hospital and Harvard Medical School, both in Boston.
Selective serotonin reuptake inhibitors taken during pregnancy are linked to a 37% increased risk of speech and language disorders in the children prenatally exposed to them, according to a prospective birth cohort study.
That risk occurs when children exposed prenatally are compared with children whose mothers did not take SSRIs but who had a diagnosis for which the antidepressants are indicated.
“The finding was observed only in offspring of mothers who purchased at least two SSRI prescriptions during pregnancy,” reported Alan S. Brown, MD, of New York State Psychiatric Institute in New York City, and his associates (JAMA Psychiatry. 2016 Oct 12. doi: 10.1001/jamapsychiatry.2016.2594). “This finding is particularly noteworthy because these women were more likely to have taken these medications and were exposed for a longer period and to larger amounts of SSRIs during pregnancy, compared with women who filled only one prescription.”
From among an initial cohort of 845,345 pregnant women in Finland, the researchers followed a final cohort of 56,340 offspring. Most of the children (86.6%) were 9 years old or younger at the end of study follow-up, running from 1996 to 2010, and the oldest children were aged 14 years. The researchers used Finland’s national registries to determine the children’s and mothers’ diagnoses and the mothers’ history of prescriptions from 30 days before pregnancy through delivery.
Among all children, 15,596 children were born to women who took SSRIs for depression or another SSRI-indicated psychiatric condition, and 31,207 children were born to mothers who had neither a psychiatric diagnosis nor a history of taking SSRIs during pregnancy. The remaining 9,537 children had mothers with a psychiatric diagnosis but who did not take SSRIs during pregnancy.
The average ages of the children at diagnosis were 4.4 years for speech or language disorders, 3.6 years for scholastic problems, and 7.7 years for motor disorders. When the researchers compared the children prenatally exposed to SSRIs to the children of mothers with a depression-related diagnosis but not taking SSRIs during pregnancy, the rates of scholastic or motor disorders did not differ.
For language and speech disorders, however, children whose mothers purchased two SSRI prescriptions had a 37% increased risk of a disorder, compared with children whose mothers had a diagnosis but did not purchase SSRIs. Without the requirement of at least two prescriptions, the statistical difference did not exist.
That increased risk occurred after adjustment for sex, mother’s parity, marital status, socioeconomic status, place of residence, both parents’ ages, the children’s gestational age at birth, prenatal exposure to antiepileptic drugs, exposure to anxiolytics/sedatives, history of chronic diseases, death of the child’s parent, mother’s country of birth, maternal smoking or substance abuse, and the psychiatric history of both parents. Data on maternal alcohol consumption were unavailable. Both the children exposed to SSRIs and the children of unmedicated mothers with a psychiatric condition had a higher risk of speech and language disorders.
The research was funded by the National Institutes of Health; the Sackler Foundation of Columbia University, New York; and the University of Turku (Finland). One of the researchers, David Gyllenberg, MD, reported receiving research funding from the Sigrid Juselius Foundation, the Foundation for Pediatric Research in Finland, and the Finnish Medical Foundation. No other conflicts of interest were disclosed.
Selective serotonin reuptake inhibitors taken during pregnancy are linked to a 37% increased risk of speech and language disorders in the children prenatally exposed to them, according to a prospective birth cohort study.
That risk occurs when children exposed prenatally are compared with children whose mothers did not take SSRIs but who had a diagnosis for which the antidepressants are indicated.
“The finding was observed only in offspring of mothers who purchased at least two SSRI prescriptions during pregnancy,” reported Alan S. Brown, MD, of New York State Psychiatric Institute in New York City, and his associates (JAMA Psychiatry. 2016 Oct 12. doi: 10.1001/jamapsychiatry.2016.2594). “This finding is particularly noteworthy because these women were more likely to have taken these medications and were exposed for a longer period and to larger amounts of SSRIs during pregnancy, compared with women who filled only one prescription.”
From among an initial cohort of 845,345 pregnant women in Finland, the researchers followed a final cohort of 56,340 offspring. Most of the children (86.6%) were 9 years old or younger at the end of study follow-up, running from 1996 to 2010, and the oldest children were aged 14 years. The researchers used Finland’s national registries to determine the children’s and mothers’ diagnoses and the mothers’ history of prescriptions from 30 days before pregnancy through delivery.
Among all children, 15,596 children were born to women who took SSRIs for depression or another SSRI-indicated psychiatric condition, and 31,207 children were born to mothers who had neither a psychiatric diagnosis nor a history of taking SSRIs during pregnancy. The remaining 9,537 children had mothers with a psychiatric diagnosis but who did not take SSRIs during pregnancy.
The average ages of the children at diagnosis were 4.4 years for speech or language disorders, 3.6 years for scholastic problems, and 7.7 years for motor disorders. When the researchers compared the children prenatally exposed to SSRIs to the children of mothers with a depression-related diagnosis but not taking SSRIs during pregnancy, the rates of scholastic or motor disorders did not differ.
For language and speech disorders, however, children whose mothers purchased two SSRI prescriptions had a 37% increased risk of a disorder, compared with children whose mothers had a diagnosis but did not purchase SSRIs. Without the requirement of at least two prescriptions, the statistical difference did not exist.
That increased risk occurred after adjustment for sex, mother’s parity, marital status, socioeconomic status, place of residence, both parents’ ages, the children’s gestational age at birth, prenatal exposure to antiepileptic drugs, exposure to anxiolytics/sedatives, history of chronic diseases, death of the child’s parent, mother’s country of birth, maternal smoking or substance abuse, and the psychiatric history of both parents. Data on maternal alcohol consumption were unavailable. Both the children exposed to SSRIs and the children of unmedicated mothers with a psychiatric condition had a higher risk of speech and language disorders.
The research was funded by the National Institutes of Health; the Sackler Foundation of Columbia University, New York; and the University of Turku (Finland). One of the researchers, David Gyllenberg, MD, reported receiving research funding from the Sigrid Juselius Foundation, the Foundation for Pediatric Research in Finland, and the Finnish Medical Foundation. No other conflicts of interest were disclosed.
FROM JAMA PSYCHIATRY
Key clinical point: Selective serotonin reuptake inhibitors taken during pregnancy may increase the risk of speech/language disorders in the offspring.
Major finding: Children of mothers taking SSRIs during pregnancy had a 37% increased risk of speech/language disorders, compared with children of unmedicated mothers with a diagnosis of depression.
Data source: The findings are based on a prospective birth cohort involving 56,340 children tracked in Finland from 1996 to 2010.
Disclosures: The research was funded by the National Institutes of Health; the Sackler Foundation of Columbia University, New York; and the University of Turku (Finland). One of the researchers, David Gyllenberg, MD, reported receiving research funding from the Sigrid Juselius Foundation, the Foundation for Pediatric Research in Finland, and the Finnish Medical Foundation. No other conflicts of interest were disclosed.
Birth outcomes unaffected by paternal immunosuppressive therapy
VIENNA – The use of classic systemic immunosuppressive agents by men in the months shortly before conception was not associated with increased risk of low birthweight, preterm birth, or congenital anomalies in their offspring in a large Danish national registry.
“We didn’t see any real safety signals,” Dr. Alexander Egeberg reported at the annual congress of the European Academy of Dermatology and Venereology.
He and his coinvestigators at the University of Copenhagen decided to examine this issue for a simple reason: “We know quite a lot from registry studies about the safety of these drugs when used by women during pregnancy, but very little about the safety of paternal use,” Dr. Egeberg explained.
Methotrexate, azathioprine, and cyclosporine are often prescribed for patients with moderate to severe psoriasis and psoriatic arthritis as well as other chronic inflammatory disorders. Female patients are typically told to stop using these medications if they’re trying to become pregnant, or as soon as they think they might be pregnant, but nearly half of all pregnancies are unintended.
Using linked comprehensive national Danish databases, the investigators scrutinized the medical records of all children born in Denmark during 2004-2010, as well as those of their parents. They identified 2,235 children whose fathers had been on immunosuppressive therapy for a medical condition at any time prior to conception. There were 1,246 fathers who had been on azathioprine, 848 on methotrexate, and 141 on cyclosporine.
Rates of preterm birth, congenital anomalies, and low birthweight were compared in children born to fathers using immunosuppression and in 415,589 children born to fathers with no history of exposure to the medications. These comparisons entailed multivariate regression analyses adjusted for maternal age, parity, smoking status, and the child’s gender. Dr. Egeberg and his colleagues also compared rates of these reproductive complications in the subgroup of children whose fathers had been on the medications within 3 months prior to the estimated time of conception and in children whose fathers had stopped taking the drugs by that point.
None of the adverse neonatal outcomes were significantly increased in ever or recent paternal users of the medications under study, with one exception. Paternal use of cyclosporine within the last 3 months prior to conception was associated with an adjusted 3.7-fold increased likelihood of having a baby with a congenital anomaly. Dr. Egeberg, however, was quick to state that this finding was based on small numbers of exposures: 18 paternal exposures and four affected offspring.
“The cyclosporine finding should be interpreted quite cautiously,” he emphasized.
The reproductive outcomes study was supported by Danish governmental research funds. Dr. Egeberg reported having received research funding from and serving as a consultant to Pfizer and Eli Lilly.
VIENNA – The use of classic systemic immunosuppressive agents by men in the months shortly before conception was not associated with increased risk of low birthweight, preterm birth, or congenital anomalies in their offspring in a large Danish national registry.
“We didn’t see any real safety signals,” Dr. Alexander Egeberg reported at the annual congress of the European Academy of Dermatology and Venereology.
He and his coinvestigators at the University of Copenhagen decided to examine this issue for a simple reason: “We know quite a lot from registry studies about the safety of these drugs when used by women during pregnancy, but very little about the safety of paternal use,” Dr. Egeberg explained.
Methotrexate, azathioprine, and cyclosporine are often prescribed for patients with moderate to severe psoriasis and psoriatic arthritis as well as other chronic inflammatory disorders. Female patients are typically told to stop using these medications if they’re trying to become pregnant, or as soon as they think they might be pregnant, but nearly half of all pregnancies are unintended.
Using linked comprehensive national Danish databases, the investigators scrutinized the medical records of all children born in Denmark during 2004-2010, as well as those of their parents. They identified 2,235 children whose fathers had been on immunosuppressive therapy for a medical condition at any time prior to conception. There were 1,246 fathers who had been on azathioprine, 848 on methotrexate, and 141 on cyclosporine.
Rates of preterm birth, congenital anomalies, and low birthweight were compared in children born to fathers using immunosuppression and in 415,589 children born to fathers with no history of exposure to the medications. These comparisons entailed multivariate regression analyses adjusted for maternal age, parity, smoking status, and the child’s gender. Dr. Egeberg and his colleagues also compared rates of these reproductive complications in the subgroup of children whose fathers had been on the medications within 3 months prior to the estimated time of conception and in children whose fathers had stopped taking the drugs by that point.
None of the adverse neonatal outcomes were significantly increased in ever or recent paternal users of the medications under study, with one exception. Paternal use of cyclosporine within the last 3 months prior to conception was associated with an adjusted 3.7-fold increased likelihood of having a baby with a congenital anomaly. Dr. Egeberg, however, was quick to state that this finding was based on small numbers of exposures: 18 paternal exposures and four affected offspring.
“The cyclosporine finding should be interpreted quite cautiously,” he emphasized.
The reproductive outcomes study was supported by Danish governmental research funds. Dr. Egeberg reported having received research funding from and serving as a consultant to Pfizer and Eli Lilly.
VIENNA – The use of classic systemic immunosuppressive agents by men in the months shortly before conception was not associated with increased risk of low birthweight, preterm birth, or congenital anomalies in their offspring in a large Danish national registry.
“We didn’t see any real safety signals,” Dr. Alexander Egeberg reported at the annual congress of the European Academy of Dermatology and Venereology.
He and his coinvestigators at the University of Copenhagen decided to examine this issue for a simple reason: “We know quite a lot from registry studies about the safety of these drugs when used by women during pregnancy, but very little about the safety of paternal use,” Dr. Egeberg explained.
Methotrexate, azathioprine, and cyclosporine are often prescribed for patients with moderate to severe psoriasis and psoriatic arthritis as well as other chronic inflammatory disorders. Female patients are typically told to stop using these medications if they’re trying to become pregnant, or as soon as they think they might be pregnant, but nearly half of all pregnancies are unintended.
Using linked comprehensive national Danish databases, the investigators scrutinized the medical records of all children born in Denmark during 2004-2010, as well as those of their parents. They identified 2,235 children whose fathers had been on immunosuppressive therapy for a medical condition at any time prior to conception. There were 1,246 fathers who had been on azathioprine, 848 on methotrexate, and 141 on cyclosporine.
Rates of preterm birth, congenital anomalies, and low birthweight were compared in children born to fathers using immunosuppression and in 415,589 children born to fathers with no history of exposure to the medications. These comparisons entailed multivariate regression analyses adjusted for maternal age, parity, smoking status, and the child’s gender. Dr. Egeberg and his colleagues also compared rates of these reproductive complications in the subgroup of children whose fathers had been on the medications within 3 months prior to the estimated time of conception and in children whose fathers had stopped taking the drugs by that point.
None of the adverse neonatal outcomes were significantly increased in ever or recent paternal users of the medications under study, with one exception. Paternal use of cyclosporine within the last 3 months prior to conception was associated with an adjusted 3.7-fold increased likelihood of having a baby with a congenital anomaly. Dr. Egeberg, however, was quick to state that this finding was based on small numbers of exposures: 18 paternal exposures and four affected offspring.
“The cyclosporine finding should be interpreted quite cautiously,” he emphasized.
The reproductive outcomes study was supported by Danish governmental research funds. Dr. Egeberg reported having received research funding from and serving as a consultant to Pfizer and Eli Lilly.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Adjusted rates of congenital anomalies, preterm birth, and low birthweight are not increased in children with paternal use of azathioprine, methotrexate, or cyclosporine prior to conception.
Data source: This retrospective study utilized linked Danish national registries to compare rates of low birthweight, congenital anomalies, and preterm birth in all Danish children born in 2004-2010 depending upon whether or not the father had been on methotrexate, azathioprine, or cyclosporine prior to the pregnancy.
Disclosures: The study was supported by Danish governmental research funds. Dr. Egeberg reported having received research funding from, and serving as a consultant to, Pfizer and Eli Lilly.
VIDEO: DNA-derived Zika vaccine in humans holds promise
BALTIMORE – Hope is high right now for a genetically engineered Zika virus vaccine currently being tested in humans.
“If we saw something, even early on, that we were concerned about, we would stop the trial and re-evaluate it. And that hasn’t happened, so that’s good news,” said Kathleen M. Neuzil, MD, lead investigator of the trial and a professor at the University of Maryland in Baltimore, where she is also director of the Center for Vaccine Development.
Testing of the DNA-based Zika vaccine began in early August. Now that Congress has approved a $1.1 billion funding package, Dr. Neuzil said investigators can rest assured that there is enough funding for the duration of this trial, which ultimately could take 2 years or longer.
Sponsored by the National Institutes of Health, phase I of the trial is being conducted at three U.S. sites, including the University of Maryland’s Vaccine Development Center, the NIH Clinical Center in Bethesda, Md., and Emory University in Atlanta.
Safety and tolerability results from all three sites are expected by year’s end and will inform how to construct phase II, currently set to begin in early 2017. That phase is expected to include a much larger population than the 80 current participants, although the exact numbers are still to be determined, Dr. Neuzil said. Also still unknown is whether phase II will be conducted internationally.
If the vaccine proves effective, it will be a high point in the annals of maternal-fetal medicine, said Christopher Harman, MD, chair of obstetrics and gynecology and director of the division of maternal-fetal medicine at the University of Maryland. “It’s going to become a story where the power of modern medicine has defeated an epidemic that had enormous potential for harm.”
Dr. Harman also predicted that a viable vaccine, combined with infectious disease prevention services globally, will mitigate Zika’s threat to the population, particularly developing fetuses.
In the meantime, there needs to be continued public education about preventing the spread of Zika, as well as rigorous attention to family planning, Dr. Harman said.
“Planned pregnancy is essential,” he said. “This puts the burden on the couple or the woman. Don’t get pregnant if you’re in an endemic area.”
For more about the vaccine trial and how clinicians can counsel patients about the threat of Zika, watch this video recorded at the University of Maryland.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @whitneymcknight
BALTIMORE – Hope is high right now for a genetically engineered Zika virus vaccine currently being tested in humans.
“If we saw something, even early on, that we were concerned about, we would stop the trial and re-evaluate it. And that hasn’t happened, so that’s good news,” said Kathleen M. Neuzil, MD, lead investigator of the trial and a professor at the University of Maryland in Baltimore, where she is also director of the Center for Vaccine Development.
Testing of the DNA-based Zika vaccine began in early August. Now that Congress has approved a $1.1 billion funding package, Dr. Neuzil said investigators can rest assured that there is enough funding for the duration of this trial, which ultimately could take 2 years or longer.
Sponsored by the National Institutes of Health, phase I of the trial is being conducted at three U.S. sites, including the University of Maryland’s Vaccine Development Center, the NIH Clinical Center in Bethesda, Md., and Emory University in Atlanta.
Safety and tolerability results from all three sites are expected by year’s end and will inform how to construct phase II, currently set to begin in early 2017. That phase is expected to include a much larger population than the 80 current participants, although the exact numbers are still to be determined, Dr. Neuzil said. Also still unknown is whether phase II will be conducted internationally.
If the vaccine proves effective, it will be a high point in the annals of maternal-fetal medicine, said Christopher Harman, MD, chair of obstetrics and gynecology and director of the division of maternal-fetal medicine at the University of Maryland. “It’s going to become a story where the power of modern medicine has defeated an epidemic that had enormous potential for harm.”
Dr. Harman also predicted that a viable vaccine, combined with infectious disease prevention services globally, will mitigate Zika’s threat to the population, particularly developing fetuses.
In the meantime, there needs to be continued public education about preventing the spread of Zika, as well as rigorous attention to family planning, Dr. Harman said.
“Planned pregnancy is essential,” he said. “This puts the burden on the couple or the woman. Don’t get pregnant if you’re in an endemic area.”
For more about the vaccine trial and how clinicians can counsel patients about the threat of Zika, watch this video recorded at the University of Maryland.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @whitneymcknight
BALTIMORE – Hope is high right now for a genetically engineered Zika virus vaccine currently being tested in humans.
“If we saw something, even early on, that we were concerned about, we would stop the trial and re-evaluate it. And that hasn’t happened, so that’s good news,” said Kathleen M. Neuzil, MD, lead investigator of the trial and a professor at the University of Maryland in Baltimore, where she is also director of the Center for Vaccine Development.
Testing of the DNA-based Zika vaccine began in early August. Now that Congress has approved a $1.1 billion funding package, Dr. Neuzil said investigators can rest assured that there is enough funding for the duration of this trial, which ultimately could take 2 years or longer.
Sponsored by the National Institutes of Health, phase I of the trial is being conducted at three U.S. sites, including the University of Maryland’s Vaccine Development Center, the NIH Clinical Center in Bethesda, Md., and Emory University in Atlanta.
Safety and tolerability results from all three sites are expected by year’s end and will inform how to construct phase II, currently set to begin in early 2017. That phase is expected to include a much larger population than the 80 current participants, although the exact numbers are still to be determined, Dr. Neuzil said. Also still unknown is whether phase II will be conducted internationally.
If the vaccine proves effective, it will be a high point in the annals of maternal-fetal medicine, said Christopher Harman, MD, chair of obstetrics and gynecology and director of the division of maternal-fetal medicine at the University of Maryland. “It’s going to become a story where the power of modern medicine has defeated an epidemic that had enormous potential for harm.”
Dr. Harman also predicted that a viable vaccine, combined with infectious disease prevention services globally, will mitigate Zika’s threat to the population, particularly developing fetuses.
In the meantime, there needs to be continued public education about preventing the spread of Zika, as well as rigorous attention to family planning, Dr. Harman said.
“Planned pregnancy is essential,” he said. “This puts the burden on the couple or the woman. Don’t get pregnant if you’re in an endemic area.”
For more about the vaccine trial and how clinicians can counsel patients about the threat of Zika, watch this video recorded at the University of Maryland.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @whitneymcknight
United States nears 2,500 Zika cases in pregnant women
The week also included a report of another live-born infant with Zika-related birth defects, bringing the U.S. total to 23 for the year: 22 in the 50 states and District of Columbia and 1 in the territories, the CDC reported Oct. 6. There also have been six Zika-related pregnancy losses reported so far in the states and territories, a number that has not changed since early August.
There have been 28,019 cases of Zika reported among all Americans as of Oct. 5, with the majority (86%) coming from the territories and the majority of those cases (98%) coming from Puerto Rico, according to an update from the CDC’s Arboviral Disease Branch.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The week also included a report of another live-born infant with Zika-related birth defects, bringing the U.S. total to 23 for the year: 22 in the 50 states and District of Columbia and 1 in the territories, the CDC reported Oct. 6. There also have been six Zika-related pregnancy losses reported so far in the states and territories, a number that has not changed since early August.
There have been 28,019 cases of Zika reported among all Americans as of Oct. 5, with the majority (86%) coming from the territories and the majority of those cases (98%) coming from Puerto Rico, according to an update from the CDC’s Arboviral Disease Branch.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The week also included a report of another live-born infant with Zika-related birth defects, bringing the U.S. total to 23 for the year: 22 in the 50 states and District of Columbia and 1 in the territories, the CDC reported Oct. 6. There also have been six Zika-related pregnancy losses reported so far in the states and territories, a number that has not changed since early August.
There have been 28,019 cases of Zika reported among all Americans as of Oct. 5, with the majority (86%) coming from the territories and the majority of those cases (98%) coming from Puerto Rico, according to an update from the CDC’s Arboviral Disease Branch.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Reassuring findings on neurodevelopmental outcomes in HIV-exposed children
DURBAN, SOUTH AFRICA – Children exposed to HIV in utero but uninfected at birth have neurodevelopmental test scores at age 24 months that are comparable with those of unexposed children, based on a study conducted in Botswana and presented by Jean Leidner at the 21st International AIDS Conference.
“These results provide reassurance regarding the potential effects of in-utero HIV and antiretroviral exposure,” declared Ms. Leidner, CEO of Goodtables Data Consulting in Norman, Okla., and the Botswana Harvard AIDS Institute Partnership.
The two groups of children had virtually identical scores on the cognitive, gross motor, fine motor, expressive language, and receptive language domains measured in the Bayley-III. The same was true for scores on the fine motor, locomotor, language, and personal-social elements of the Developmental Milestone Checklist.
The two groups of children differed in other ways; 17% of the uninfected children exposed to HIV in utero and 8% of the controls were low birth weight. The HIV-exposed children are being raised in a more challenging environment: just 49% have electricity in the home, compared with 64% of control families. Moreover, 53% of the HIV-exposed children and 33% of the controls live under conditions of moderate-to-severe food uncertainty.
Only 8% of the HIV-infected mothers breastfed, whereas breastfeeding was universal among the control group.
More than 99% of the HIV-infected mothers took antiretroviral medication antenatally. Roughly two-thirds were on zidovudine (Retrovir) monotherapy, the rest on a three-drug regimen of nevirapine (Viramune) plus lamivudine/zidovudine (Combivir). These are older antiretrovirals. Additional neurodevelopmental studies are warranted in children with in-utero exposure to newer agents, as well as in older children, Ms. Leidner said.
She reported having no financial conflicts regarding this study, which was funded by the National Institute of Mental Health.
DURBAN, SOUTH AFRICA – Children exposed to HIV in utero but uninfected at birth have neurodevelopmental test scores at age 24 months that are comparable with those of unexposed children, based on a study conducted in Botswana and presented by Jean Leidner at the 21st International AIDS Conference.
“These results provide reassurance regarding the potential effects of in-utero HIV and antiretroviral exposure,” declared Ms. Leidner, CEO of Goodtables Data Consulting in Norman, Okla., and the Botswana Harvard AIDS Institute Partnership.
The two groups of children had virtually identical scores on the cognitive, gross motor, fine motor, expressive language, and receptive language domains measured in the Bayley-III. The same was true for scores on the fine motor, locomotor, language, and personal-social elements of the Developmental Milestone Checklist.
The two groups of children differed in other ways; 17% of the uninfected children exposed to HIV in utero and 8% of the controls were low birth weight. The HIV-exposed children are being raised in a more challenging environment: just 49% have electricity in the home, compared with 64% of control families. Moreover, 53% of the HIV-exposed children and 33% of the controls live under conditions of moderate-to-severe food uncertainty.
Only 8% of the HIV-infected mothers breastfed, whereas breastfeeding was universal among the control group.
More than 99% of the HIV-infected mothers took antiretroviral medication antenatally. Roughly two-thirds were on zidovudine (Retrovir) monotherapy, the rest on a three-drug regimen of nevirapine (Viramune) plus lamivudine/zidovudine (Combivir). These are older antiretrovirals. Additional neurodevelopmental studies are warranted in children with in-utero exposure to newer agents, as well as in older children, Ms. Leidner said.
She reported having no financial conflicts regarding this study, which was funded by the National Institute of Mental Health.
DURBAN, SOUTH AFRICA – Children exposed to HIV in utero but uninfected at birth have neurodevelopmental test scores at age 24 months that are comparable with those of unexposed children, based on a study conducted in Botswana and presented by Jean Leidner at the 21st International AIDS Conference.
“These results provide reassurance regarding the potential effects of in-utero HIV and antiretroviral exposure,” declared Ms. Leidner, CEO of Goodtables Data Consulting in Norman, Okla., and the Botswana Harvard AIDS Institute Partnership.
The two groups of children had virtually identical scores on the cognitive, gross motor, fine motor, expressive language, and receptive language domains measured in the Bayley-III. The same was true for scores on the fine motor, locomotor, language, and personal-social elements of the Developmental Milestone Checklist.
The two groups of children differed in other ways; 17% of the uninfected children exposed to HIV in utero and 8% of the controls were low birth weight. The HIV-exposed children are being raised in a more challenging environment: just 49% have electricity in the home, compared with 64% of control families. Moreover, 53% of the HIV-exposed children and 33% of the controls live under conditions of moderate-to-severe food uncertainty.
Only 8% of the HIV-infected mothers breastfed, whereas breastfeeding was universal among the control group.
More than 99% of the HIV-infected mothers took antiretroviral medication antenatally. Roughly two-thirds were on zidovudine (Retrovir) monotherapy, the rest on a three-drug regimen of nevirapine (Viramune) plus lamivudine/zidovudine (Combivir). These are older antiretrovirals. Additional neurodevelopmental studies are warranted in children with in-utero exposure to newer agents, as well as in older children, Ms. Leidner said.
She reported having no financial conflicts regarding this study, which was funded by the National Institute of Mental Health.
Key clinical point:
Major finding: In-utero exposure to maternal HIV and antiretroviral drugs had no measurable adverse neurodevelopmental effects at age 24 months in uninfected children.
Data source: 337 uninfected children exposed to HIV in-utero and 387 children unexposed to HIV in utero.
Disclosures: The National Institute of Mental Health funded the study. The presenter reported having no financial conflicts of interest.
Teen birth rates continue to decline in the United States
The teen birth rate of 22.3/1,000 females aged 15-19 years for 2015 was down by almost 8% from the year before and marks the seventh consecutive year of historic lows. Since 1991, when 61.8/1,000 teens aged 15-19 gave birth, the rate has fallen 64%, the NCHS reported.
For teens aged 15-19 years, the birth rate declined for each race/ethnicity: dropping 8% for non-Hispanic whites and Hispanics, 9% for non-Hispanic blacks, 10% for Asians or Pacific Islanders, and 6% for American Indians or Alaska Natives. All rates for 2015 were historically low.
The teen birth rate of 22.3/1,000 females aged 15-19 years for 2015 was down by almost 8% from the year before and marks the seventh consecutive year of historic lows. Since 1991, when 61.8/1,000 teens aged 15-19 gave birth, the rate has fallen 64%, the NCHS reported.
For teens aged 15-19 years, the birth rate declined for each race/ethnicity: dropping 8% for non-Hispanic whites and Hispanics, 9% for non-Hispanic blacks, 10% for Asians or Pacific Islanders, and 6% for American Indians or Alaska Natives. All rates for 2015 were historically low.
The teen birth rate of 22.3/1,000 females aged 15-19 years for 2015 was down by almost 8% from the year before and marks the seventh consecutive year of historic lows. Since 1991, when 61.8/1,000 teens aged 15-19 gave birth, the rate has fallen 64%, the NCHS reported.
For teens aged 15-19 years, the birth rate declined for each race/ethnicity: dropping 8% for non-Hispanic whites and Hispanics, 9% for non-Hispanic blacks, 10% for Asians or Pacific Islanders, and 6% for American Indians or Alaska Natives. All rates for 2015 were historically low.