User login
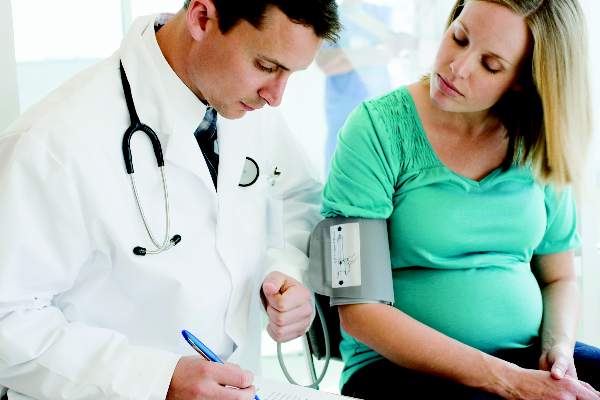
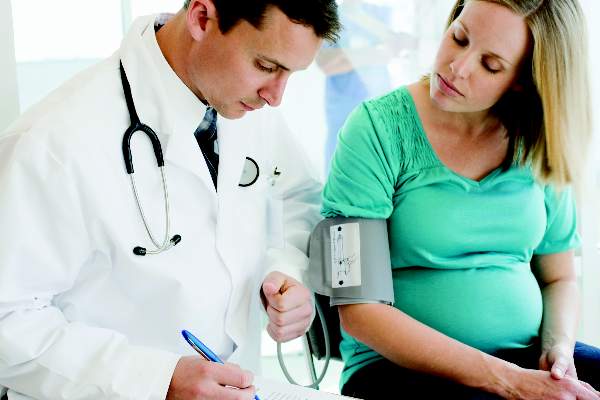
Breastfeeding protects against postpartum MS relapse
New mothers with MS are less likely to have a relapse within 6 months of delivery if they breastfeed their babies exclusively for at least 2 months, according to the findings of a prospective study of 201 women published online Aug. 31 in JAMA Neurology.
“Our findings indicate that women with MS should be supported if they choose to breastfeed exclusively since it clearly does not increase the risk of postpartum relapse. Relapse in the first 6 months post partum may be diminished by exclusive breastfeeding, but once regular feedings are introduced, disease activity is likely to return,” wrote Dr. Kerstin Hellwig of Ruhr-University Bochum (Germany) and her colleagues (JAMA Neurol. 2015 Aug 31. doi: 10.1001/jamaneurol.2015.1806).
The effect of breastfeeding on postpartum MS relapse has been controversial. Some studies have found that exclusive breastfeeding for at least the first 2 months might be beneficial, while others – studies that “defined breastfeeding crudely and/or measured breastfeeding retrospectively,” the authors said – found no protective effect.
The 201 women in the study had relapsing-remitting MS for a median of about 4.5 years and had voluntarily enrolled while pregnant in the German MS and pregnancy registry. They completed a series of questionnaires during pregnancy and the first post partum year.
Overall, 120 women (59.7%) intended to breastfeed exclusively for at least 2 months; 42 (20.9%) combined breastfeeding with supplemental feedings within the first 2 months; and 39 (19.4%) did not breastfeed; 178 (88.6%) reported using disease-modifying therapies before pregnancy, most often glatiramer acetate or interferon beta.
Among the 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first 6 post partum months, compared with 29 women (24.2%) among the 120 who breastfed their children exclusively (adjusted hazard ratio, 1.70; 95% confidence interval, 1.02-2.85; P = 0.04).
The researchers described exclusively breastfeeding as acting like a “modestly effective treatment with a natural end date.”
“During exclusive breastfeeding, the pulsatile release of gonadotropin-releasing hormone and luteinizing hormone is suppressed with a corresponding suppression of the growth of ovarian follicles resulting in lactational amenorrhea and anovulation. Shortly after the breastfeeding frequency is reduced (1 or 2 regularly replaced breastfeeding meals are sufficient to interrupt this cycle), the ovarian activity resumes with the return of menses,” Dr. Hellwig and her associates wrote.
They also speculated that the “hormonal changes leading to anovulation might play a key role since women with MS are less likely to receive the diagnosis during their anovulatory years (childhood or after menopause) and women with MS were found to be more likely to experience relapse shortly before menstruation.”
The mean age in the study was about 31 years, but women who breastfed exclusively tended to be older than their peers and less likely to have received disease-modifying therapies before or at the time of conception. The first postpartum menses among exclusive breastfeeders came at a median of 185 days vs. 64 days for other women.
“In the present study, we observed that an earlier return of menses was associated with a higher risk of relapse in the first 6 months post-partum,” the authors wrote.
The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer Healthcare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
New mothers with MS are less likely to have a relapse within 6 months of delivery if they breastfeed their babies exclusively for at least 2 months, according to the findings of a prospective study of 201 women published online Aug. 31 in JAMA Neurology.
“Our findings indicate that women with MS should be supported if they choose to breastfeed exclusively since it clearly does not increase the risk of postpartum relapse. Relapse in the first 6 months post partum may be diminished by exclusive breastfeeding, but once regular feedings are introduced, disease activity is likely to return,” wrote Dr. Kerstin Hellwig of Ruhr-University Bochum (Germany) and her colleagues (JAMA Neurol. 2015 Aug 31. doi: 10.1001/jamaneurol.2015.1806).
The effect of breastfeeding on postpartum MS relapse has been controversial. Some studies have found that exclusive breastfeeding for at least the first 2 months might be beneficial, while others – studies that “defined breastfeeding crudely and/or measured breastfeeding retrospectively,” the authors said – found no protective effect.
The 201 women in the study had relapsing-remitting MS for a median of about 4.5 years and had voluntarily enrolled while pregnant in the German MS and pregnancy registry. They completed a series of questionnaires during pregnancy and the first post partum year.
Overall, 120 women (59.7%) intended to breastfeed exclusively for at least 2 months; 42 (20.9%) combined breastfeeding with supplemental feedings within the first 2 months; and 39 (19.4%) did not breastfeed; 178 (88.6%) reported using disease-modifying therapies before pregnancy, most often glatiramer acetate or interferon beta.
Among the 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first 6 post partum months, compared with 29 women (24.2%) among the 120 who breastfed their children exclusively (adjusted hazard ratio, 1.70; 95% confidence interval, 1.02-2.85; P = 0.04).
The researchers described exclusively breastfeeding as acting like a “modestly effective treatment with a natural end date.”
“During exclusive breastfeeding, the pulsatile release of gonadotropin-releasing hormone and luteinizing hormone is suppressed with a corresponding suppression of the growth of ovarian follicles resulting in lactational amenorrhea and anovulation. Shortly after the breastfeeding frequency is reduced (1 or 2 regularly replaced breastfeeding meals are sufficient to interrupt this cycle), the ovarian activity resumes with the return of menses,” Dr. Hellwig and her associates wrote.
They also speculated that the “hormonal changes leading to anovulation might play a key role since women with MS are less likely to receive the diagnosis during their anovulatory years (childhood or after menopause) and women with MS were found to be more likely to experience relapse shortly before menstruation.”
The mean age in the study was about 31 years, but women who breastfed exclusively tended to be older than their peers and less likely to have received disease-modifying therapies before or at the time of conception. The first postpartum menses among exclusive breastfeeders came at a median of 185 days vs. 64 days for other women.
“In the present study, we observed that an earlier return of menses was associated with a higher risk of relapse in the first 6 months post-partum,” the authors wrote.
The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer Healthcare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
New mothers with MS are less likely to have a relapse within 6 months of delivery if they breastfeed their babies exclusively for at least 2 months, according to the findings of a prospective study of 201 women published online Aug. 31 in JAMA Neurology.
“Our findings indicate that women with MS should be supported if they choose to breastfeed exclusively since it clearly does not increase the risk of postpartum relapse. Relapse in the first 6 months post partum may be diminished by exclusive breastfeeding, but once regular feedings are introduced, disease activity is likely to return,” wrote Dr. Kerstin Hellwig of Ruhr-University Bochum (Germany) and her colleagues (JAMA Neurol. 2015 Aug 31. doi: 10.1001/jamaneurol.2015.1806).
The effect of breastfeeding on postpartum MS relapse has been controversial. Some studies have found that exclusive breastfeeding for at least the first 2 months might be beneficial, while others – studies that “defined breastfeeding crudely and/or measured breastfeeding retrospectively,” the authors said – found no protective effect.
The 201 women in the study had relapsing-remitting MS for a median of about 4.5 years and had voluntarily enrolled while pregnant in the German MS and pregnancy registry. They completed a series of questionnaires during pregnancy and the first post partum year.
Overall, 120 women (59.7%) intended to breastfeed exclusively for at least 2 months; 42 (20.9%) combined breastfeeding with supplemental feedings within the first 2 months; and 39 (19.4%) did not breastfeed; 178 (88.6%) reported using disease-modifying therapies before pregnancy, most often glatiramer acetate or interferon beta.
Among the 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first 6 post partum months, compared with 29 women (24.2%) among the 120 who breastfed their children exclusively (adjusted hazard ratio, 1.70; 95% confidence interval, 1.02-2.85; P = 0.04).
The researchers described exclusively breastfeeding as acting like a “modestly effective treatment with a natural end date.”
“During exclusive breastfeeding, the pulsatile release of gonadotropin-releasing hormone and luteinizing hormone is suppressed with a corresponding suppression of the growth of ovarian follicles resulting in lactational amenorrhea and anovulation. Shortly after the breastfeeding frequency is reduced (1 or 2 regularly replaced breastfeeding meals are sufficient to interrupt this cycle), the ovarian activity resumes with the return of menses,” Dr. Hellwig and her associates wrote.
They also speculated that the “hormonal changes leading to anovulation might play a key role since women with MS are less likely to receive the diagnosis during their anovulatory years (childhood or after menopause) and women with MS were found to be more likely to experience relapse shortly before menstruation.”
The mean age in the study was about 31 years, but women who breastfed exclusively tended to be older than their peers and less likely to have received disease-modifying therapies before or at the time of conception. The first postpartum menses among exclusive breastfeeders came at a median of 185 days vs. 64 days for other women.
“In the present study, we observed that an earlier return of menses was associated with a higher risk of relapse in the first 6 months post-partum,” the authors wrote.
The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer Healthcare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
FROM JAMA NEUROLOGY
Key clinical point: Don’t discourage new mothers with multiple sclerosis from breastfeeding.
Major finding: Among 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first 6 postpartum months, compared with 29 women (24.2%) among the 120 who intended to breastfeed their children exclusively for at least 2 months (adjusted HR, 1.70).
Data source: A prospective study of 201 pregnant women with relapsing-remitting MS who were followed for 1 year post partum.
Disclosures: The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer Healthcare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
ICD-10-CM documentation and coding for obstetric procedures
The countdown is on for the big coding switch. Last month I wrote about changes in International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes that will occur in relation to gynecologic services, but now it’s time to tackle obstetric services. For obstetricians, the changes will be all about definitions. And documentation of obstetric conditions will be more complicated due to several factors, including the need to report trimester information and gestational age, use of a placeholder code, more complex guidelines for certain conditions, chorionicity for multiple gestations, and use of a 7th digit to identify the fetus with a problem.
No one is expecting clinicians to instantly be fluent in code-speak, but in order for the most specific diagnoses to be reported, the clinical documentation must be spot on. Think of it this way: ICD-10-CM is not requiring you to document more, it’s requiring you to document more precisely.
How to get started
Figuring out where you are now goes a long way toward knowing where you need to be when the calendar changes to October 1—and the best way to do it is to perform a gap analysis. This analysis can be carried out by the clinician or a qualified practice staff person.
To begin, run a report of the distinct obstetric codes you have billed in 2015 by frequency. Then sort them in numeric order so that each individual code category is captured for all of the 5th digits (and the code then will be counted as a single code). Finally, review 5 medical records for each of the top 10 reported diagnosis categories and determine whether you could have reported a more specific ICD-10-CM code.
The information you gain will go a long way toward identifying potential weaknesses in the documentation, or, if you are currently using an electronic health record (EHR) to look up a code, it will point up any weak points in searching for the right code, based on your specific documentation at the encounter. Remember, practice makes perfect…eventually.
Well-trained staff can help
Not only must you, the clinician, learn about the part your clinical documentation will play in providing the most specific information that will lead to a very specific code, but your coding and billing staff will need training as well. They are the ones who should be checking your claims for accuracy from October 1 forward, as they will know the basic rules about which codes can be billed together, code order, place codes, and so on. In other words, while you as a clinician should be responsible for picking the more specific code in ICD-10-CM, your staff is your backup when you don’t.
Feedback from your staff on how the claims are being processed and, perhaps, the overuse of unspecified codes will keep you moving toward the goal of complete and precise clinical documentation and the reporting of diagnoses at the highest level possible given the documentation.
Highlights of ICD-10-CM obstetric coding
Given the complexity of obstetric coding, this article deals only with the most important changes. It will be up to each clinician to learn the rules that surround the diagnostic codes that you report most frequently. Here again, a trained staff can help by preparing specific coding tools for the most frequently used diagnoses, including notes about what must be in the record to report the most specific code.
Trimester, gestational age, and timing definitions
The majority of obstetric complication codes (these are the codes that start with the letter “O”) and the “Z” codes for supervision of a normal pregnancy require trimester information to be valid. In the outpatient setting, the trimester will be based on the gestational age at the date of the encounter. For inpatient admissions, the trimester will be based on the age at the time of admission; if the patient is hospitalized over more than one trimester, it is the admission trimester that continues to be recorded, not the discharge trimester.
Although there are codes that indicate an unspecified trimester, they should be reported rarely if this information is, in fact, available. Trimesters are defined as:
- first: less than 14 weeks, 0 days
- second: 14 weeks, 0 days to less than 28 weeks, 0 days
- third: 28 weeks, 0 days until delivery.
Examples of trimester codes include:
- O25.11Malnutrition in pregnancy, first trimester
- O14.02 Mild to moderate preeclampsia, second trimester
- O24.013 Preexisting diabetes mellitus, type 1, in pregnancy, third trimester.
However, definitions in ICD-10-CM go beyond this, and these definitions will have to be taken into account to provide sufficient documentation to report the condition. In ICD-9-CM, a missed abortion and early hemorrhage in pregnancy occurred prior to 22 completed weeks, but in ICD-10-CM that definition changes to prior to 20 completed weeks.
Additional definitions that may impact coding:
- preterm labor or delivery: 20 completed weeks to less than 37 completed weeks
- full-term labor or delivery: 37 completed weeks to 40 completed weeks
- postterm pregnancy: more than 40 completed weeks to 42 completed weeks
- prolonged pregnancy: more than 42 completed weeks.
You also will be required to include a code for gestational age any time you report an obstetric complication. This and the trimester information will change as the pregnancy advances, so always be sure that the code selected matches the gestational age on the flow sheet at the time of the encounter. The gestational age code is Z3A.__, with the final 2 digits representing the weeks of gestation (for instance, from 27 weeks, 0 days to 27 weeks, 6 days, the final 2 digits will be “27”).
ICD-10-CM also has different conventions when it comes to timing as it relates to conditions that are present during the episode in which the patient delivers. When this is the case, an “in childbirth” code must be selected instead of assigning the diagnosis by trimester, if one is available. There also are codes that are specific to “in the puer-perium,” and these generally will be reported after the patient has been discharged after delivery but also would be reported if there is no “in childbirth” code available at the time of delivery. The code categories to which this concept will apply are:
- preexisting hypertension
- diabetes mellitus
- malnutrition
- liver and biliary tract disorders
- subluxation of symphysis (pubis)
- obstetric embolism
- maternal infectious and parasitic diseases classifiable elsewhere
- other maternal diseases classifiable elsewhere
- maternal malignant neoplasms, traumatic injuries, and abuse classifiable elsewhere.
Taking time to read a code description from a search program or drop-down menu also will be important because some codes refer to “of the puerperium” versus “complicating the puerperium” or “in the puerperium.” The first reference means that the condition develops after delivery, while the second and third terms mean that it developed prior to delivery. For example, code O90.81, Anemia of the puerperium, refers to anemia that develops following delivery, while code O99.03 Anemia complicating the puerperium, denotes preexisting anemia that is still present in the postpartum period.
Multiple gestation coding and the 7th digit
The first thing you will notice here is that several code categories require a 7th numeric character of 0 or 1 through 9. This rule will apply to the following categories:
- complications specific to multiple gestation
- maternal care for malpresentation of fetus
- maternal care for disproportion
- maternal care for known or suspected fetal abnormality and damage
- maternal care for other fetal problems
- polyhydramnios
- other disorders of amniotic fluid and membranes
- preterm labor with preterm delivery
- term delivery with preterm labor
- obstructed labor due to malposition and malpresentation of fetus
- labor and delivery with umbilical cord complications.
A 7th character of 0 will be reported if this is a singleton pregnancy, and the numbers 1 through 5 and 9 refer to which fetus of the multiple gestation has the problem. The number 9 would indicate any fetus that was not labeled as 1 to 5.
The trick in documentation will be identifying the fetus with the problem consistently while still recognizing that, in some cases, such as fetal position, twins may switch places. On the other hand, if one fetus is small for dates, chances are good that this fetus will remain so during pregnancy when twins are present.
A code will be denied as invalid without this 7th digit, so it will be good practice for the clinician to document this information at each visit.
Additional information in regard to multiple gestations will be the chorionicity of the pregnancy, if known, but there will also be an “unable to determine” and an “unspecified” code available if that better fits the documentation for the visit. Note, however, that there is no code for a trichorionic/ triamniotic pregnancy; therefore, only the unspecified code would be reported in that case. In addition, if there is a continuing pregnancy after fetal loss, the cause must be identified within the code (that is, fetal reduction, fetal demise [and retained], or spontaneous abortion).
Documentation requirements for certain conditions
If you plan on reporting any complication of pregnancy at the time of the encounter, information about that condition needs to be part of the antepartum flow sheet comments. If, at the time of the encounter, a condition the patient has is not addressed and the entire visit involves only routine care, you would report the code for routine supervision of preg- nancy rather than the complication code. If the complication is again addressed at a later visit, the complication code would be reported again for that visit. The routine supervision code and the complication code cannot be reported on the record for the same encounter under ICD-10-CM rules.
Hypertension. Documentation needs to state whether the hypertension is preexisting or gestational. If it is preexisting, it needs to be identified as essential or secondary. If the patient also has hypertensive heart disease or chronic kidney disease, this information should be included, as different codes must be selected.
Diabetes. The documentation needs to state whether it is preexisting or gestational. If preexisting, you must document whether it is type 1 or type 2. If it is type 2, you must report an additional code for long-term insulin use, if applicable. The assumption for a woman with type 1 diabetes is that she is always insulin-dependent, so long-term use is not reported separately. Note, however, that neither metformin nor glyburide is considered insulin and there is no mechanism for reporting control with these medications.
If diabetes is gestational, you must indicate whether the patient’s blood glucose level is controlled by diet or insulin. If both, report only the insulin. There is no code for the use of other medications for the control of gestational diabetes, so you would have to report an unspecified code in that case.
Also note that ICD-10-CM differentiates between an abnormal 1-hour glucose tolerance test (GTT) and gestational diabetes. Unless a 3-specimen or 4-specimen GTT has been performed and results are abnormal, a diagnosis of gestational diabetes should not be reported.
An additional code outside of the obstetric complication chapter is required to denote any manifestations of diabetes. If there are none, then a diabetes uncomplicated manifestation code must be reported.
Preterm labor and delivery. Your documentation must clearly indicate whether the patient has preterm labor with preterm delivery or whether the delivery is term in addition to the trimester. For instance, if you document that Mary presents with preterm labor at 27 weeks, 2 days and delivers a girl at 28 weeks, 6 days, your code will describe Preterm labor second trimester with preterm delivery third trimester. However, if Susan presents with preterm labor at 30 weeks, 2 days and is managed until 37 weeks, 1 day, when she delivers a baby boy, your code would describe Term delivery with preterm labor, third trimester.
New coding options
Among the new coding options under ICD-10-CM:
- Abnormal findings on antenatal screening. These would be reported when the antenatal test is abnormal but you have not yet determined a definitive diagnosis.
- Alcohol, drug, and tobacco use during pregnancy. If you report any of these codes, you must also report a manifestation code for the patient’s condition. If the use is uncomplicated, you would report that code instead.
- Abuse of the pregnant patient. You can report sexual, physical, or psychological abuse, but you also must report a code for any applicable injury to the patient and identify the abuser, if known.
- Pruritic urticarial papules and plaques of pregnancy
- Retained intrauterine contraceptive device in pregnancy
- Maternal care due to uterine scar from other previous surgery. This would mean a surgery other than a previous cesarean delivery.
- Maternal care for (suspected) damage to the fetus by other medical procedures
- Maternal care for hydrops fetalis
- Maternal care for viable fetus in abdominal pregnancy
- Malignant neoplasm complicating pregnancy
- Failed attempt at vaginal birth after previous cesarean delivery
- Supervision of high-risk pregnancy due to social problems (for instance, a homeless patient)
- Rh incompatibility status (when you lack confirmation of serum antibodies and are giving prophylactic Rho[D] immune globulin).
CMS takes steps to ease transition to ICD-10-CM
To help health care providers get “up to speed” on the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM), which takes effect October 1, 2015, the Centers for Medicare and Medicaid Services (CMS) has launched a new series for specialists. A guide tailored to ObGyns is available at http://roadto10.org/example-practice-obgyn. The guide includes:
Parting words
ICD-10-CM may seem like the end of the world, but its difficulty is exaggerated. If you fail to prepare, you will fail, and money coming in the door may be affected. If you prepare with training and practice, you will have a short learning curve. I wish you all the best. If you have specific questions about your practice, don’t hesitate to let us know so they can be addressed early.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The countdown is on for the big coding switch. Last month I wrote about changes in International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes that will occur in relation to gynecologic services, but now it’s time to tackle obstetric services. For obstetricians, the changes will be all about definitions. And documentation of obstetric conditions will be more complicated due to several factors, including the need to report trimester information and gestational age, use of a placeholder code, more complex guidelines for certain conditions, chorionicity for multiple gestations, and use of a 7th digit to identify the fetus with a problem.
No one is expecting clinicians to instantly be fluent in code-speak, but in order for the most specific diagnoses to be reported, the clinical documentation must be spot on. Think of it this way: ICD-10-CM is not requiring you to document more, it’s requiring you to document more precisely.
How to get started
Figuring out where you are now goes a long way toward knowing where you need to be when the calendar changes to October 1—and the best way to do it is to perform a gap analysis. This analysis can be carried out by the clinician or a qualified practice staff person.
To begin, run a report of the distinct obstetric codes you have billed in 2015 by frequency. Then sort them in numeric order so that each individual code category is captured for all of the 5th digits (and the code then will be counted as a single code). Finally, review 5 medical records for each of the top 10 reported diagnosis categories and determine whether you could have reported a more specific ICD-10-CM code.
The information you gain will go a long way toward identifying potential weaknesses in the documentation, or, if you are currently using an electronic health record (EHR) to look up a code, it will point up any weak points in searching for the right code, based on your specific documentation at the encounter. Remember, practice makes perfect…eventually.
Well-trained staff can help
Not only must you, the clinician, learn about the part your clinical documentation will play in providing the most specific information that will lead to a very specific code, but your coding and billing staff will need training as well. They are the ones who should be checking your claims for accuracy from October 1 forward, as they will know the basic rules about which codes can be billed together, code order, place codes, and so on. In other words, while you as a clinician should be responsible for picking the more specific code in ICD-10-CM, your staff is your backup when you don’t.
Feedback from your staff on how the claims are being processed and, perhaps, the overuse of unspecified codes will keep you moving toward the goal of complete and precise clinical documentation and the reporting of diagnoses at the highest level possible given the documentation.
Highlights of ICD-10-CM obstetric coding
Given the complexity of obstetric coding, this article deals only with the most important changes. It will be up to each clinician to learn the rules that surround the diagnostic codes that you report most frequently. Here again, a trained staff can help by preparing specific coding tools for the most frequently used diagnoses, including notes about what must be in the record to report the most specific code.
Trimester, gestational age, and timing definitions
The majority of obstetric complication codes (these are the codes that start with the letter “O”) and the “Z” codes for supervision of a normal pregnancy require trimester information to be valid. In the outpatient setting, the trimester will be based on the gestational age at the date of the encounter. For inpatient admissions, the trimester will be based on the age at the time of admission; if the patient is hospitalized over more than one trimester, it is the admission trimester that continues to be recorded, not the discharge trimester.
Although there are codes that indicate an unspecified trimester, they should be reported rarely if this information is, in fact, available. Trimesters are defined as:
- first: less than 14 weeks, 0 days
- second: 14 weeks, 0 days to less than 28 weeks, 0 days
- third: 28 weeks, 0 days until delivery.
Examples of trimester codes include:
- O25.11Malnutrition in pregnancy, first trimester
- O14.02 Mild to moderate preeclampsia, second trimester
- O24.013 Preexisting diabetes mellitus, type 1, in pregnancy, third trimester.
However, definitions in ICD-10-CM go beyond this, and these definitions will have to be taken into account to provide sufficient documentation to report the condition. In ICD-9-CM, a missed abortion and early hemorrhage in pregnancy occurred prior to 22 completed weeks, but in ICD-10-CM that definition changes to prior to 20 completed weeks.
Additional definitions that may impact coding:
- preterm labor or delivery: 20 completed weeks to less than 37 completed weeks
- full-term labor or delivery: 37 completed weeks to 40 completed weeks
- postterm pregnancy: more than 40 completed weeks to 42 completed weeks
- prolonged pregnancy: more than 42 completed weeks.
You also will be required to include a code for gestational age any time you report an obstetric complication. This and the trimester information will change as the pregnancy advances, so always be sure that the code selected matches the gestational age on the flow sheet at the time of the encounter. The gestational age code is Z3A.__, with the final 2 digits representing the weeks of gestation (for instance, from 27 weeks, 0 days to 27 weeks, 6 days, the final 2 digits will be “27”).
ICD-10-CM also has different conventions when it comes to timing as it relates to conditions that are present during the episode in which the patient delivers. When this is the case, an “in childbirth” code must be selected instead of assigning the diagnosis by trimester, if one is available. There also are codes that are specific to “in the puer-perium,” and these generally will be reported after the patient has been discharged after delivery but also would be reported if there is no “in childbirth” code available at the time of delivery. The code categories to which this concept will apply are:
- preexisting hypertension
- diabetes mellitus
- malnutrition
- liver and biliary tract disorders
- subluxation of symphysis (pubis)
- obstetric embolism
- maternal infectious and parasitic diseases classifiable elsewhere
- other maternal diseases classifiable elsewhere
- maternal malignant neoplasms, traumatic injuries, and abuse classifiable elsewhere.
Taking time to read a code description from a search program or drop-down menu also will be important because some codes refer to “of the puerperium” versus “complicating the puerperium” or “in the puerperium.” The first reference means that the condition develops after delivery, while the second and third terms mean that it developed prior to delivery. For example, code O90.81, Anemia of the puerperium, refers to anemia that develops following delivery, while code O99.03 Anemia complicating the puerperium, denotes preexisting anemia that is still present in the postpartum period.
Multiple gestation coding and the 7th digit
The first thing you will notice here is that several code categories require a 7th numeric character of 0 or 1 through 9. This rule will apply to the following categories:
- complications specific to multiple gestation
- maternal care for malpresentation of fetus
- maternal care for disproportion
- maternal care for known or suspected fetal abnormality and damage
- maternal care for other fetal problems
- polyhydramnios
- other disorders of amniotic fluid and membranes
- preterm labor with preterm delivery
- term delivery with preterm labor
- obstructed labor due to malposition and malpresentation of fetus
- labor and delivery with umbilical cord complications.
A 7th character of 0 will be reported if this is a singleton pregnancy, and the numbers 1 through 5 and 9 refer to which fetus of the multiple gestation has the problem. The number 9 would indicate any fetus that was not labeled as 1 to 5.
The trick in documentation will be identifying the fetus with the problem consistently while still recognizing that, in some cases, such as fetal position, twins may switch places. On the other hand, if one fetus is small for dates, chances are good that this fetus will remain so during pregnancy when twins are present.
A code will be denied as invalid without this 7th digit, so it will be good practice for the clinician to document this information at each visit.
Additional information in regard to multiple gestations will be the chorionicity of the pregnancy, if known, but there will also be an “unable to determine” and an “unspecified” code available if that better fits the documentation for the visit. Note, however, that there is no code for a trichorionic/ triamniotic pregnancy; therefore, only the unspecified code would be reported in that case. In addition, if there is a continuing pregnancy after fetal loss, the cause must be identified within the code (that is, fetal reduction, fetal demise [and retained], or spontaneous abortion).
Documentation requirements for certain conditions
If you plan on reporting any complication of pregnancy at the time of the encounter, information about that condition needs to be part of the antepartum flow sheet comments. If, at the time of the encounter, a condition the patient has is not addressed and the entire visit involves only routine care, you would report the code for routine supervision of preg- nancy rather than the complication code. If the complication is again addressed at a later visit, the complication code would be reported again for that visit. The routine supervision code and the complication code cannot be reported on the record for the same encounter under ICD-10-CM rules.
Hypertension. Documentation needs to state whether the hypertension is preexisting or gestational. If it is preexisting, it needs to be identified as essential or secondary. If the patient also has hypertensive heart disease or chronic kidney disease, this information should be included, as different codes must be selected.
Diabetes. The documentation needs to state whether it is preexisting or gestational. If preexisting, you must document whether it is type 1 or type 2. If it is type 2, you must report an additional code for long-term insulin use, if applicable. The assumption for a woman with type 1 diabetes is that she is always insulin-dependent, so long-term use is not reported separately. Note, however, that neither metformin nor glyburide is considered insulin and there is no mechanism for reporting control with these medications.
If diabetes is gestational, you must indicate whether the patient’s blood glucose level is controlled by diet or insulin. If both, report only the insulin. There is no code for the use of other medications for the control of gestational diabetes, so you would have to report an unspecified code in that case.
Also note that ICD-10-CM differentiates between an abnormal 1-hour glucose tolerance test (GTT) and gestational diabetes. Unless a 3-specimen or 4-specimen GTT has been performed and results are abnormal, a diagnosis of gestational diabetes should not be reported.
An additional code outside of the obstetric complication chapter is required to denote any manifestations of diabetes. If there are none, then a diabetes uncomplicated manifestation code must be reported.
Preterm labor and delivery. Your documentation must clearly indicate whether the patient has preterm labor with preterm delivery or whether the delivery is term in addition to the trimester. For instance, if you document that Mary presents with preterm labor at 27 weeks, 2 days and delivers a girl at 28 weeks, 6 days, your code will describe Preterm labor second trimester with preterm delivery third trimester. However, if Susan presents with preterm labor at 30 weeks, 2 days and is managed until 37 weeks, 1 day, when she delivers a baby boy, your code would describe Term delivery with preterm labor, third trimester.
New coding options
Among the new coding options under ICD-10-CM:
- Abnormal findings on antenatal screening. These would be reported when the antenatal test is abnormal but you have not yet determined a definitive diagnosis.
- Alcohol, drug, and tobacco use during pregnancy. If you report any of these codes, you must also report a manifestation code for the patient’s condition. If the use is uncomplicated, you would report that code instead.
- Abuse of the pregnant patient. You can report sexual, physical, or psychological abuse, but you also must report a code for any applicable injury to the patient and identify the abuser, if known.
- Pruritic urticarial papules and plaques of pregnancy
- Retained intrauterine contraceptive device in pregnancy
- Maternal care due to uterine scar from other previous surgery. This would mean a surgery other than a previous cesarean delivery.
- Maternal care for (suspected) damage to the fetus by other medical procedures
- Maternal care for hydrops fetalis
- Maternal care for viable fetus in abdominal pregnancy
- Malignant neoplasm complicating pregnancy
- Failed attempt at vaginal birth after previous cesarean delivery
- Supervision of high-risk pregnancy due to social problems (for instance, a homeless patient)
- Rh incompatibility status (when you lack confirmation of serum antibodies and are giving prophylactic Rho[D] immune globulin).
CMS takes steps to ease transition to ICD-10-CM
To help health care providers get “up to speed” on the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM), which takes effect October 1, 2015, the Centers for Medicare and Medicaid Services (CMS) has launched a new series for specialists. A guide tailored to ObGyns is available at http://roadto10.org/example-practice-obgyn. The guide includes:
Parting words
ICD-10-CM may seem like the end of the world, but its difficulty is exaggerated. If you fail to prepare, you will fail, and money coming in the door may be affected. If you prepare with training and practice, you will have a short learning curve. I wish you all the best. If you have specific questions about your practice, don’t hesitate to let us know so they can be addressed early.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The countdown is on for the big coding switch. Last month I wrote about changes in International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes that will occur in relation to gynecologic services, but now it’s time to tackle obstetric services. For obstetricians, the changes will be all about definitions. And documentation of obstetric conditions will be more complicated due to several factors, including the need to report trimester information and gestational age, use of a placeholder code, more complex guidelines for certain conditions, chorionicity for multiple gestations, and use of a 7th digit to identify the fetus with a problem.
No one is expecting clinicians to instantly be fluent in code-speak, but in order for the most specific diagnoses to be reported, the clinical documentation must be spot on. Think of it this way: ICD-10-CM is not requiring you to document more, it’s requiring you to document more precisely.
How to get started
Figuring out where you are now goes a long way toward knowing where you need to be when the calendar changes to October 1—and the best way to do it is to perform a gap analysis. This analysis can be carried out by the clinician or a qualified practice staff person.
To begin, run a report of the distinct obstetric codes you have billed in 2015 by frequency. Then sort them in numeric order so that each individual code category is captured for all of the 5th digits (and the code then will be counted as a single code). Finally, review 5 medical records for each of the top 10 reported diagnosis categories and determine whether you could have reported a more specific ICD-10-CM code.
The information you gain will go a long way toward identifying potential weaknesses in the documentation, or, if you are currently using an electronic health record (EHR) to look up a code, it will point up any weak points in searching for the right code, based on your specific documentation at the encounter. Remember, practice makes perfect…eventually.
Well-trained staff can help
Not only must you, the clinician, learn about the part your clinical documentation will play in providing the most specific information that will lead to a very specific code, but your coding and billing staff will need training as well. They are the ones who should be checking your claims for accuracy from October 1 forward, as they will know the basic rules about which codes can be billed together, code order, place codes, and so on. In other words, while you as a clinician should be responsible for picking the more specific code in ICD-10-CM, your staff is your backup when you don’t.
Feedback from your staff on how the claims are being processed and, perhaps, the overuse of unspecified codes will keep you moving toward the goal of complete and precise clinical documentation and the reporting of diagnoses at the highest level possible given the documentation.
Highlights of ICD-10-CM obstetric coding
Given the complexity of obstetric coding, this article deals only with the most important changes. It will be up to each clinician to learn the rules that surround the diagnostic codes that you report most frequently. Here again, a trained staff can help by preparing specific coding tools for the most frequently used diagnoses, including notes about what must be in the record to report the most specific code.
Trimester, gestational age, and timing definitions
The majority of obstetric complication codes (these are the codes that start with the letter “O”) and the “Z” codes for supervision of a normal pregnancy require trimester information to be valid. In the outpatient setting, the trimester will be based on the gestational age at the date of the encounter. For inpatient admissions, the trimester will be based on the age at the time of admission; if the patient is hospitalized over more than one trimester, it is the admission trimester that continues to be recorded, not the discharge trimester.
Although there are codes that indicate an unspecified trimester, they should be reported rarely if this information is, in fact, available. Trimesters are defined as:
- first: less than 14 weeks, 0 days
- second: 14 weeks, 0 days to less than 28 weeks, 0 days
- third: 28 weeks, 0 days until delivery.
Examples of trimester codes include:
- O25.11Malnutrition in pregnancy, first trimester
- O14.02 Mild to moderate preeclampsia, second trimester
- O24.013 Preexisting diabetes mellitus, type 1, in pregnancy, third trimester.
However, definitions in ICD-10-CM go beyond this, and these definitions will have to be taken into account to provide sufficient documentation to report the condition. In ICD-9-CM, a missed abortion and early hemorrhage in pregnancy occurred prior to 22 completed weeks, but in ICD-10-CM that definition changes to prior to 20 completed weeks.
Additional definitions that may impact coding:
- preterm labor or delivery: 20 completed weeks to less than 37 completed weeks
- full-term labor or delivery: 37 completed weeks to 40 completed weeks
- postterm pregnancy: more than 40 completed weeks to 42 completed weeks
- prolonged pregnancy: more than 42 completed weeks.
You also will be required to include a code for gestational age any time you report an obstetric complication. This and the trimester information will change as the pregnancy advances, so always be sure that the code selected matches the gestational age on the flow sheet at the time of the encounter. The gestational age code is Z3A.__, with the final 2 digits representing the weeks of gestation (for instance, from 27 weeks, 0 days to 27 weeks, 6 days, the final 2 digits will be “27”).
ICD-10-CM also has different conventions when it comes to timing as it relates to conditions that are present during the episode in which the patient delivers. When this is the case, an “in childbirth” code must be selected instead of assigning the diagnosis by trimester, if one is available. There also are codes that are specific to “in the puer-perium,” and these generally will be reported after the patient has been discharged after delivery but also would be reported if there is no “in childbirth” code available at the time of delivery. The code categories to which this concept will apply are:
- preexisting hypertension
- diabetes mellitus
- malnutrition
- liver and biliary tract disorders
- subluxation of symphysis (pubis)
- obstetric embolism
- maternal infectious and parasitic diseases classifiable elsewhere
- other maternal diseases classifiable elsewhere
- maternal malignant neoplasms, traumatic injuries, and abuse classifiable elsewhere.
Taking time to read a code description from a search program or drop-down menu also will be important because some codes refer to “of the puerperium” versus “complicating the puerperium” or “in the puerperium.” The first reference means that the condition develops after delivery, while the second and third terms mean that it developed prior to delivery. For example, code O90.81, Anemia of the puerperium, refers to anemia that develops following delivery, while code O99.03 Anemia complicating the puerperium, denotes preexisting anemia that is still present in the postpartum period.
Multiple gestation coding and the 7th digit
The first thing you will notice here is that several code categories require a 7th numeric character of 0 or 1 through 9. This rule will apply to the following categories:
- complications specific to multiple gestation
- maternal care for malpresentation of fetus
- maternal care for disproportion
- maternal care for known or suspected fetal abnormality and damage
- maternal care for other fetal problems
- polyhydramnios
- other disorders of amniotic fluid and membranes
- preterm labor with preterm delivery
- term delivery with preterm labor
- obstructed labor due to malposition and malpresentation of fetus
- labor and delivery with umbilical cord complications.
A 7th character of 0 will be reported if this is a singleton pregnancy, and the numbers 1 through 5 and 9 refer to which fetus of the multiple gestation has the problem. The number 9 would indicate any fetus that was not labeled as 1 to 5.
The trick in documentation will be identifying the fetus with the problem consistently while still recognizing that, in some cases, such as fetal position, twins may switch places. On the other hand, if one fetus is small for dates, chances are good that this fetus will remain so during pregnancy when twins are present.
A code will be denied as invalid without this 7th digit, so it will be good practice for the clinician to document this information at each visit.
Additional information in regard to multiple gestations will be the chorionicity of the pregnancy, if known, but there will also be an “unable to determine” and an “unspecified” code available if that better fits the documentation for the visit. Note, however, that there is no code for a trichorionic/ triamniotic pregnancy; therefore, only the unspecified code would be reported in that case. In addition, if there is a continuing pregnancy after fetal loss, the cause must be identified within the code (that is, fetal reduction, fetal demise [and retained], or spontaneous abortion).
Documentation requirements for certain conditions
If you plan on reporting any complication of pregnancy at the time of the encounter, information about that condition needs to be part of the antepartum flow sheet comments. If, at the time of the encounter, a condition the patient has is not addressed and the entire visit involves only routine care, you would report the code for routine supervision of preg- nancy rather than the complication code. If the complication is again addressed at a later visit, the complication code would be reported again for that visit. The routine supervision code and the complication code cannot be reported on the record for the same encounter under ICD-10-CM rules.
Hypertension. Documentation needs to state whether the hypertension is preexisting or gestational. If it is preexisting, it needs to be identified as essential or secondary. If the patient also has hypertensive heart disease or chronic kidney disease, this information should be included, as different codes must be selected.
Diabetes. The documentation needs to state whether it is preexisting or gestational. If preexisting, you must document whether it is type 1 or type 2. If it is type 2, you must report an additional code for long-term insulin use, if applicable. The assumption for a woman with type 1 diabetes is that she is always insulin-dependent, so long-term use is not reported separately. Note, however, that neither metformin nor glyburide is considered insulin and there is no mechanism for reporting control with these medications.
If diabetes is gestational, you must indicate whether the patient’s blood glucose level is controlled by diet or insulin. If both, report only the insulin. There is no code for the use of other medications for the control of gestational diabetes, so you would have to report an unspecified code in that case.
Also note that ICD-10-CM differentiates between an abnormal 1-hour glucose tolerance test (GTT) and gestational diabetes. Unless a 3-specimen or 4-specimen GTT has been performed and results are abnormal, a diagnosis of gestational diabetes should not be reported.
An additional code outside of the obstetric complication chapter is required to denote any manifestations of diabetes. If there are none, then a diabetes uncomplicated manifestation code must be reported.
Preterm labor and delivery. Your documentation must clearly indicate whether the patient has preterm labor with preterm delivery or whether the delivery is term in addition to the trimester. For instance, if you document that Mary presents with preterm labor at 27 weeks, 2 days and delivers a girl at 28 weeks, 6 days, your code will describe Preterm labor second trimester with preterm delivery third trimester. However, if Susan presents with preterm labor at 30 weeks, 2 days and is managed until 37 weeks, 1 day, when she delivers a baby boy, your code would describe Term delivery with preterm labor, third trimester.
New coding options
Among the new coding options under ICD-10-CM:
- Abnormal findings on antenatal screening. These would be reported when the antenatal test is abnormal but you have not yet determined a definitive diagnosis.
- Alcohol, drug, and tobacco use during pregnancy. If you report any of these codes, you must also report a manifestation code for the patient’s condition. If the use is uncomplicated, you would report that code instead.
- Abuse of the pregnant patient. You can report sexual, physical, or psychological abuse, but you also must report a code for any applicable injury to the patient and identify the abuser, if known.
- Pruritic urticarial papules and plaques of pregnancy
- Retained intrauterine contraceptive device in pregnancy
- Maternal care due to uterine scar from other previous surgery. This would mean a surgery other than a previous cesarean delivery.
- Maternal care for (suspected) damage to the fetus by other medical procedures
- Maternal care for hydrops fetalis
- Maternal care for viable fetus in abdominal pregnancy
- Malignant neoplasm complicating pregnancy
- Failed attempt at vaginal birth after previous cesarean delivery
- Supervision of high-risk pregnancy due to social problems (for instance, a homeless patient)
- Rh incompatibility status (when you lack confirmation of serum antibodies and are giving prophylactic Rho[D] immune globulin).
CMS takes steps to ease transition to ICD-10-CM
To help health care providers get “up to speed” on the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM), which takes effect October 1, 2015, the Centers for Medicare and Medicaid Services (CMS) has launched a new series for specialists. A guide tailored to ObGyns is available at http://roadto10.org/example-practice-obgyn. The guide includes:
Parting words
ICD-10-CM may seem like the end of the world, but its difficulty is exaggerated. If you fail to prepare, you will fail, and money coming in the door may be affected. If you prepare with training and practice, you will have a short learning curve. I wish you all the best. If you have specific questions about your practice, don’t hesitate to let us know so they can be addressed early.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Article
- How to do a gap analysis
- Highlights of OB coding
- Condition-specific coding notations
ACOG Recommends Against First-trimester Preeclampsia Tests
Commercial screening tests that purport to predict early-onset preeclampsia may do more harm than good and are not recommended, according to the American College of Obstetricians and Gynecologists.
Early-onset preeclampsia does signal great risk for both mother and infant, and early identification of pregnancies at high risk “would theoretically allow referral for more intensive surveillance or application of preventive therapies to reduce the risk of severe disease.” But the effectiveness of this approach is undercut by the low positive predictive value of currently available tests, according to a new policy statement from ACOG’s Committee on Obstetric Practice published Aug. 19 (Obstet Gynecol 2015;126:e25-7.).
“These tests require a large number of women to be identified as high risk and to potentially undergo intensive surveillance in order to detect one case of early-onset preeclampsia,” the committee members wrote.
Moreover, there are no data showing that using these screening tests improve clinical outcomes.
Taking a detailed medical history to assess risk factors remains “the best and only recommended screening approach for preeclampsia,” according to ACOG. The medical history should remain the screening method of choice until high-quality evidence demonstrates that aspirin and other interventions reduce the incidence of preeclampsia for women at high risk based on first-trimester predictive tests, ACOG recommended.
“For a first-trimester risk assessment for preeclampsia to be useful in clinical practice, future screening tests will need to have sensitivities and [positive predictive values] high enough to accurately identify women who will develop preeclampsia, and interventions will need to be available that improve clinical outcome in women who test positive,” the committee members wrote.
The new committee opinion has been endorsed by the Society for Maternal-Fetal Medicine.
Commercial screening tests that purport to predict early-onset preeclampsia may do more harm than good and are not recommended, according to the American College of Obstetricians and Gynecologists.
Early-onset preeclampsia does signal great risk for both mother and infant, and early identification of pregnancies at high risk “would theoretically allow referral for more intensive surveillance or application of preventive therapies to reduce the risk of severe disease.” But the effectiveness of this approach is undercut by the low positive predictive value of currently available tests, according to a new policy statement from ACOG’s Committee on Obstetric Practice published Aug. 19 (Obstet Gynecol 2015;126:e25-7.).
“These tests require a large number of women to be identified as high risk and to potentially undergo intensive surveillance in order to detect one case of early-onset preeclampsia,” the committee members wrote.
Moreover, there are no data showing that using these screening tests improve clinical outcomes.
Taking a detailed medical history to assess risk factors remains “the best and only recommended screening approach for preeclampsia,” according to ACOG. The medical history should remain the screening method of choice until high-quality evidence demonstrates that aspirin and other interventions reduce the incidence of preeclampsia for women at high risk based on first-trimester predictive tests, ACOG recommended.
“For a first-trimester risk assessment for preeclampsia to be useful in clinical practice, future screening tests will need to have sensitivities and [positive predictive values] high enough to accurately identify women who will develop preeclampsia, and interventions will need to be available that improve clinical outcome in women who test positive,” the committee members wrote.
The new committee opinion has been endorsed by the Society for Maternal-Fetal Medicine.
Commercial screening tests that purport to predict early-onset preeclampsia may do more harm than good and are not recommended, according to the American College of Obstetricians and Gynecologists.
Early-onset preeclampsia does signal great risk for both mother and infant, and early identification of pregnancies at high risk “would theoretically allow referral for more intensive surveillance or application of preventive therapies to reduce the risk of severe disease.” But the effectiveness of this approach is undercut by the low positive predictive value of currently available tests, according to a new policy statement from ACOG’s Committee on Obstetric Practice published Aug. 19 (Obstet Gynecol 2015;126:e25-7.).
“These tests require a large number of women to be identified as high risk and to potentially undergo intensive surveillance in order to detect one case of early-onset preeclampsia,” the committee members wrote.
Moreover, there are no data showing that using these screening tests improve clinical outcomes.
Taking a detailed medical history to assess risk factors remains “the best and only recommended screening approach for preeclampsia,” according to ACOG. The medical history should remain the screening method of choice until high-quality evidence demonstrates that aspirin and other interventions reduce the incidence of preeclampsia for women at high risk based on first-trimester predictive tests, ACOG recommended.
“For a first-trimester risk assessment for preeclampsia to be useful in clinical practice, future screening tests will need to have sensitivities and [positive predictive values] high enough to accurately identify women who will develop preeclampsia, and interventions will need to be available that improve clinical outcome in women who test positive,” the committee members wrote.
The new committee opinion has been endorsed by the Society for Maternal-Fetal Medicine.
FROM OBSTETRICS & GYNECOLOGY
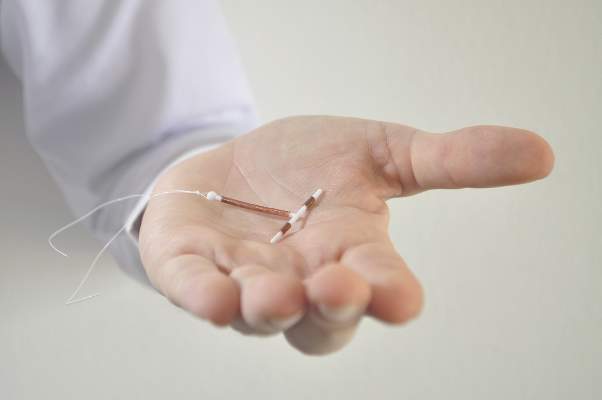
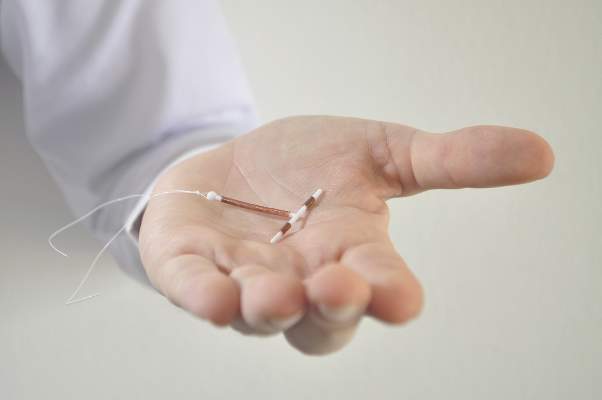
ACOG: Copper IUD Best for Emergency Contraception in Obese Patients
The copper intrauterine device is the most effective method of emergency contraception and may a good option for obese patients who have higher failure rates on oral emergency contraception, according to an updated practice bulletin from the American College of Obstetricians and Gynecologists.
Levonorgestrel emergency contraception may be less effective among overweight (BMI 25-29.9 kg/m2) and obese (BMI 30 kg/m2 or greater) women, and ulipristal acetate may be less effective among obese women. The copper IUD, however, is not affected by body weight.
“Consideration should be given to use of a copper IUD as an alternative to oral emergency contraception in obese women. However, oral emergency contraception should not be withheld from women who are overweight or obese because no research to date has been powered adequately to evaluate a threshold weight at which it would be ineffective,” ACOG officials wrote in a practice bulletin published on Aug. 19 (Obstet Gynecol. 2015;126:e1-11).
Among oral emergency contraception methods, ulipristal acetate is more effective than the levonorgestrel-only regimen and is effective for up to 5 days. But the levonorgestrel-only method is more effective than the combined hormonal regimen, with less nausea and vomiting.
The most common adverse effects for the pills are headache and nausea, as well as irregular menstrual bleeding. Less often reported effects include breast tenderness, abdominal pain, dizziness, and fatigue. Uterine perforation can occur in 1 of every 1,000 insertions of the copper IUD, which can also cause cramping and increased or painful menstrual flow.
The ACOG bulletin does not recommend a clinical exam or pregnancy testing before a patient uses emergency contraception. However, patients should be evaluated if their menses is delayed by a week or more after taking emergency contraception, or if they experience lower abdominal pain or persistent irregular bleeding, according to the bulletin.
The copper intrauterine device is the most effective method of emergency contraception and may a good option for obese patients who have higher failure rates on oral emergency contraception, according to an updated practice bulletin from the American College of Obstetricians and Gynecologists.
Levonorgestrel emergency contraception may be less effective among overweight (BMI 25-29.9 kg/m2) and obese (BMI 30 kg/m2 or greater) women, and ulipristal acetate may be less effective among obese women. The copper IUD, however, is not affected by body weight.
“Consideration should be given to use of a copper IUD as an alternative to oral emergency contraception in obese women. However, oral emergency contraception should not be withheld from women who are overweight or obese because no research to date has been powered adequately to evaluate a threshold weight at which it would be ineffective,” ACOG officials wrote in a practice bulletin published on Aug. 19 (Obstet Gynecol. 2015;126:e1-11).
Among oral emergency contraception methods, ulipristal acetate is more effective than the levonorgestrel-only regimen and is effective for up to 5 days. But the levonorgestrel-only method is more effective than the combined hormonal regimen, with less nausea and vomiting.
The most common adverse effects for the pills are headache and nausea, as well as irregular menstrual bleeding. Less often reported effects include breast tenderness, abdominal pain, dizziness, and fatigue. Uterine perforation can occur in 1 of every 1,000 insertions of the copper IUD, which can also cause cramping and increased or painful menstrual flow.
The ACOG bulletin does not recommend a clinical exam or pregnancy testing before a patient uses emergency contraception. However, patients should be evaluated if their menses is delayed by a week or more after taking emergency contraception, or if they experience lower abdominal pain or persistent irregular bleeding, according to the bulletin.
The copper intrauterine device is the most effective method of emergency contraception and may a good option for obese patients who have higher failure rates on oral emergency contraception, according to an updated practice bulletin from the American College of Obstetricians and Gynecologists.
Levonorgestrel emergency contraception may be less effective among overweight (BMI 25-29.9 kg/m2) and obese (BMI 30 kg/m2 or greater) women, and ulipristal acetate may be less effective among obese women. The copper IUD, however, is not affected by body weight.
“Consideration should be given to use of a copper IUD as an alternative to oral emergency contraception in obese women. However, oral emergency contraception should not be withheld from women who are overweight or obese because no research to date has been powered adequately to evaluate a threshold weight at which it would be ineffective,” ACOG officials wrote in a practice bulletin published on Aug. 19 (Obstet Gynecol. 2015;126:e1-11).
Among oral emergency contraception methods, ulipristal acetate is more effective than the levonorgestrel-only regimen and is effective for up to 5 days. But the levonorgestrel-only method is more effective than the combined hormonal regimen, with less nausea and vomiting.
The most common adverse effects for the pills are headache and nausea, as well as irregular menstrual bleeding. Less often reported effects include breast tenderness, abdominal pain, dizziness, and fatigue. Uterine perforation can occur in 1 of every 1,000 insertions of the copper IUD, which can also cause cramping and increased or painful menstrual flow.
The ACOG bulletin does not recommend a clinical exam or pregnancy testing before a patient uses emergency contraception. However, patients should be evaluated if their menses is delayed by a week or more after taking emergency contraception, or if they experience lower abdominal pain or persistent irregular bleeding, according to the bulletin.
FROM AN ACOG PRACTICE BULLETIN
ACOG: Copper IUD best for emergency contraception in obese patients
The copper intrauterine device is the most effective method of emergency contraception and may a good option for obese patients who have higher failure rates on oral emergency contraception, according to an updated practice bulletin from the American College of Obstetricians and Gynecologists.
Levonorgestrel emergency contraception may be less effective among overweight (BMI 25-29.9 kg/m2) and obese (BMI 30 kg/m2 or greater) women, and ulipristal acetate may be less effective among obese women. The copper IUD, however, is not affected by body weight.
“Consideration should be given to use of a copper IUD as an alternative to oral emergency contraception in obese women. However, oral emergency contraception should not be withheld from women who are overweight or obese because no research to date has been powered adequately to evaluate a threshold weight at which it would be ineffective,” ACOG officials wrote in a practice bulletin published on Aug. 19 (Obstet Gynecol. 2015;126:e1-11).
Among oral emergency contraception methods, ulipristal acetate is more effective than the levonorgestrel-only regimen and is effective for up to 5 days. But the levonorgestrel-only method is more effective than the combined hormonal regimen, with less nausea and vomiting.
The most common adverse effects for the pills are headache and nausea, as well as irregular menstrual bleeding. Less often reported effects include breast tenderness, abdominal pain, dizziness, and fatigue. Uterine perforation can occur in 1 of every 1,000 insertions of the copper IUD, which can also cause cramping and increased or painful menstrual flow.
The ACOG bulletin does not recommend a clinical exam or pregnancy testing before a patient uses emergency contraception. However, patients should be evaluated if their menses is delayed by a week or more after taking emergency contraception, or if they experience lower abdominal pain or persistent irregular bleeding, according to the bulletin.
The copper intrauterine device is the most effective method of emergency contraception and may a good option for obese patients who have higher failure rates on oral emergency contraception, according to an updated practice bulletin from the American College of Obstetricians and Gynecologists.
Levonorgestrel emergency contraception may be less effective among overweight (BMI 25-29.9 kg/m2) and obese (BMI 30 kg/m2 or greater) women, and ulipristal acetate may be less effective among obese women. The copper IUD, however, is not affected by body weight.
“Consideration should be given to use of a copper IUD as an alternative to oral emergency contraception in obese women. However, oral emergency contraception should not be withheld from women who are overweight or obese because no research to date has been powered adequately to evaluate a threshold weight at which it would be ineffective,” ACOG officials wrote in a practice bulletin published on Aug. 19 (Obstet Gynecol. 2015;126:e1-11).
Among oral emergency contraception methods, ulipristal acetate is more effective than the levonorgestrel-only regimen and is effective for up to 5 days. But the levonorgestrel-only method is more effective than the combined hormonal regimen, with less nausea and vomiting.
The most common adverse effects for the pills are headache and nausea, as well as irregular menstrual bleeding. Less often reported effects include breast tenderness, abdominal pain, dizziness, and fatigue. Uterine perforation can occur in 1 of every 1,000 insertions of the copper IUD, which can also cause cramping and increased or painful menstrual flow.
The ACOG bulletin does not recommend a clinical exam or pregnancy testing before a patient uses emergency contraception. However, patients should be evaluated if their menses is delayed by a week or more after taking emergency contraception, or if they experience lower abdominal pain or persistent irregular bleeding, according to the bulletin.
The copper intrauterine device is the most effective method of emergency contraception and may a good option for obese patients who have higher failure rates on oral emergency contraception, according to an updated practice bulletin from the American College of Obstetricians and Gynecologists.
Levonorgestrel emergency contraception may be less effective among overweight (BMI 25-29.9 kg/m2) and obese (BMI 30 kg/m2 or greater) women, and ulipristal acetate may be less effective among obese women. The copper IUD, however, is not affected by body weight.
“Consideration should be given to use of a copper IUD as an alternative to oral emergency contraception in obese women. However, oral emergency contraception should not be withheld from women who are overweight or obese because no research to date has been powered adequately to evaluate a threshold weight at which it would be ineffective,” ACOG officials wrote in a practice bulletin published on Aug. 19 (Obstet Gynecol. 2015;126:e1-11).
Among oral emergency contraception methods, ulipristal acetate is more effective than the levonorgestrel-only regimen and is effective for up to 5 days. But the levonorgestrel-only method is more effective than the combined hormonal regimen, with less nausea and vomiting.
The most common adverse effects for the pills are headache and nausea, as well as irregular menstrual bleeding. Less often reported effects include breast tenderness, abdominal pain, dizziness, and fatigue. Uterine perforation can occur in 1 of every 1,000 insertions of the copper IUD, which can also cause cramping and increased or painful menstrual flow.
The ACOG bulletin does not recommend a clinical exam or pregnancy testing before a patient uses emergency contraception. However, patients should be evaluated if their menses is delayed by a week or more after taking emergency contraception, or if they experience lower abdominal pain or persistent irregular bleeding, according to the bulletin.
FROM AN ACOG PRACTICE BULLETIN
ACOG offers advice on early treatment of morning sickness
Physicians should consider early treatment of nausea and vomiting in pregnancy to prevent progression to hyperemesis gravidarum, according to an updated practice bulletin from the American College of Obstetricians and Gynecologists.
Recommendations with the strongest evidence for prevention and management of nausea and vomiting in pregnancy include taking prenatal vitamins for 3 months prior to conception, and treating nausea and vomiting with vitamin B6 or vitamin B6 plus doxylamine.
Patients with hyperemesis gravidarum (HG) and suppressed thyroid-stimulating hormone levels should not receive hyperthyroidism treatment until evidence of thyroid disease has been clearly identified, according to the bulletin published on Aug. 19 (Obstet Gynecol. 2015;126:e12–24).
Though the evidence is weaker, women can also use ginger to ease nausea symptoms. Women experiencing particularly unmanageable nausea and vomiting or HG may find methylprednisolone, 48 mg taken daily orally or intravenously for 3 days, effective in reducing symptoms. But its efficacy is unclear, and it may increase the risk of oral clefts by one or two cases per 1,000 treated women. For this reason, ACOG urged physicians to use caution in prescribing the drug for HG and to avoid it as a first-line agent before 10 weeks gestation.
“Treatment of severe nausea and vomiting of pregnancy or hyperemesis gravidarum with methylprednisolone may be efficacious in refractory cases; however, the risk profile of methylprednisolone suggests it should be a last-resort treatment,” ACOG wrote.
Consensus and expert opinion support the recommendation to use IV hydration for women with HG who are dehydrated or cannot tolerate oral liquids.
“Dextrose and vitamins should be included in the therapy when prolonged vomiting is present, and thiamine should be administered before dextrose infusion to prevent Wernicke encephalopathy,” ACOG recommended.
Women with HG who cannot maintain their weight or do not respond to medical therapy may require enteral tube feeding for nutrition. These women should not receive peripherally inserted central catheters unless absolutely necessary because of the risk of complications.
About half of all pregnant women experience vomiting, and up to 80% experience nausea while pregnant, though only about 0.3%-3% of pregnancies progress to HG, according to ACOG. Although HG, typically diagnosed once other causes have been excluded, lacks clear criteria, it commonly includes at least 5% loss of prepregnancy weight, persistent vomiting not related to other causes, and some measure of acute starvation, such as large ketonuria. There may also be electrolyte, thyroid, and liver abnormalities.
While one study has categorized the severity of nausea and vomiting in pregnancy along a continuum, the most clinically relevant factor is how the patient is affected by her symptoms.
“The woman’s perception of the severity of her symptoms, her desire for treatment, and the potential effect of treatment on her fetus are more likely to influence clinical decision making,” the bulletin states.
The recommendations in the bulletin are based on a literature search between January 1985 and April 2015 of English-language articles in the MEDLINE database, Cochrane Library, and the American College of Obstetricians and Gynecologists’ internal resources and documents.
Physicians should consider early treatment of nausea and vomiting in pregnancy to prevent progression to hyperemesis gravidarum, according to an updated practice bulletin from the American College of Obstetricians and Gynecologists.
Recommendations with the strongest evidence for prevention and management of nausea and vomiting in pregnancy include taking prenatal vitamins for 3 months prior to conception, and treating nausea and vomiting with vitamin B6 or vitamin B6 plus doxylamine.
Patients with hyperemesis gravidarum (HG) and suppressed thyroid-stimulating hormone levels should not receive hyperthyroidism treatment until evidence of thyroid disease has been clearly identified, according to the bulletin published on Aug. 19 (Obstet Gynecol. 2015;126:e12–24).
Though the evidence is weaker, women can also use ginger to ease nausea symptoms. Women experiencing particularly unmanageable nausea and vomiting or HG may find methylprednisolone, 48 mg taken daily orally or intravenously for 3 days, effective in reducing symptoms. But its efficacy is unclear, and it may increase the risk of oral clefts by one or two cases per 1,000 treated women. For this reason, ACOG urged physicians to use caution in prescribing the drug for HG and to avoid it as a first-line agent before 10 weeks gestation.
“Treatment of severe nausea and vomiting of pregnancy or hyperemesis gravidarum with methylprednisolone may be efficacious in refractory cases; however, the risk profile of methylprednisolone suggests it should be a last-resort treatment,” ACOG wrote.
Consensus and expert opinion support the recommendation to use IV hydration for women with HG who are dehydrated or cannot tolerate oral liquids.
“Dextrose and vitamins should be included in the therapy when prolonged vomiting is present, and thiamine should be administered before dextrose infusion to prevent Wernicke encephalopathy,” ACOG recommended.
Women with HG who cannot maintain their weight or do not respond to medical therapy may require enteral tube feeding for nutrition. These women should not receive peripherally inserted central catheters unless absolutely necessary because of the risk of complications.
About half of all pregnant women experience vomiting, and up to 80% experience nausea while pregnant, though only about 0.3%-3% of pregnancies progress to HG, according to ACOG. Although HG, typically diagnosed once other causes have been excluded, lacks clear criteria, it commonly includes at least 5% loss of prepregnancy weight, persistent vomiting not related to other causes, and some measure of acute starvation, such as large ketonuria. There may also be electrolyte, thyroid, and liver abnormalities.
While one study has categorized the severity of nausea and vomiting in pregnancy along a continuum, the most clinically relevant factor is how the patient is affected by her symptoms.
“The woman’s perception of the severity of her symptoms, her desire for treatment, and the potential effect of treatment on her fetus are more likely to influence clinical decision making,” the bulletin states.
The recommendations in the bulletin are based on a literature search between January 1985 and April 2015 of English-language articles in the MEDLINE database, Cochrane Library, and the American College of Obstetricians and Gynecologists’ internal resources and documents.
Physicians should consider early treatment of nausea and vomiting in pregnancy to prevent progression to hyperemesis gravidarum, according to an updated practice bulletin from the American College of Obstetricians and Gynecologists.
Recommendations with the strongest evidence for prevention and management of nausea and vomiting in pregnancy include taking prenatal vitamins for 3 months prior to conception, and treating nausea and vomiting with vitamin B6 or vitamin B6 plus doxylamine.
Patients with hyperemesis gravidarum (HG) and suppressed thyroid-stimulating hormone levels should not receive hyperthyroidism treatment until evidence of thyroid disease has been clearly identified, according to the bulletin published on Aug. 19 (Obstet Gynecol. 2015;126:e12–24).
Though the evidence is weaker, women can also use ginger to ease nausea symptoms. Women experiencing particularly unmanageable nausea and vomiting or HG may find methylprednisolone, 48 mg taken daily orally or intravenously for 3 days, effective in reducing symptoms. But its efficacy is unclear, and it may increase the risk of oral clefts by one or two cases per 1,000 treated women. For this reason, ACOG urged physicians to use caution in prescribing the drug for HG and to avoid it as a first-line agent before 10 weeks gestation.
“Treatment of severe nausea and vomiting of pregnancy or hyperemesis gravidarum with methylprednisolone may be efficacious in refractory cases; however, the risk profile of methylprednisolone suggests it should be a last-resort treatment,” ACOG wrote.
Consensus and expert opinion support the recommendation to use IV hydration for women with HG who are dehydrated or cannot tolerate oral liquids.
“Dextrose and vitamins should be included in the therapy when prolonged vomiting is present, and thiamine should be administered before dextrose infusion to prevent Wernicke encephalopathy,” ACOG recommended.
Women with HG who cannot maintain their weight or do not respond to medical therapy may require enteral tube feeding for nutrition. These women should not receive peripherally inserted central catheters unless absolutely necessary because of the risk of complications.
About half of all pregnant women experience vomiting, and up to 80% experience nausea while pregnant, though only about 0.3%-3% of pregnancies progress to HG, according to ACOG. Although HG, typically diagnosed once other causes have been excluded, lacks clear criteria, it commonly includes at least 5% loss of prepregnancy weight, persistent vomiting not related to other causes, and some measure of acute starvation, such as large ketonuria. There may also be electrolyte, thyroid, and liver abnormalities.
While one study has categorized the severity of nausea and vomiting in pregnancy along a continuum, the most clinically relevant factor is how the patient is affected by her symptoms.
“The woman’s perception of the severity of her symptoms, her desire for treatment, and the potential effect of treatment on her fetus are more likely to influence clinical decision making,” the bulletin states.
The recommendations in the bulletin are based on a literature search between January 1985 and April 2015 of English-language articles in the MEDLINE database, Cochrane Library, and the American College of Obstetricians and Gynecologists’ internal resources and documents.
FROM AN ACOG PRACTICE BULLETIN
ACOG recommends against first-trimester preeclampsia tests
Commercial screening tests that purport to predict early-onset preeclampsia may do more harm than good and are not recommended, according to the American College of Obstetricians and Gynecologists.
Early-onset preeclampsia does signal great risk for both mother and infant, and early identification of pregnancies at high risk “would theoretically allow referral for more intensive surveillance or application of preventive therapies to reduce the risk of severe disease.” But the effectiveness of this approach is undercut by the low positive predictive value of currently available tests, according to a new policy statement from ACOG’s Committee on Obstetric Practice published Aug. 19 (Obstet Gynecol 2015;126:e25-7.).
“These tests require a large number of women to be identified as high risk and to potentially undergo intensive surveillance in order to detect one case of early-onset preeclampsia,” the committee members wrote.
Moreover, there are no data showing that using these screening tests improve clinical outcomes.
Taking a detailed medical history to assess risk factors remains “the best and only recommended screening approach for preeclampsia,” according to ACOG. The medical history should remain the screening method of choice until high-quality evidence demonstrates that aspirin and other interventions reduce the incidence of preeclampsia for women at high risk based on first-trimester predictive tests, ACOG recommended.
“For a first-trimester risk assessment for preeclampsia to be useful in clinical practice, future screening tests will need to have sensitivities and [positive predictive values] high enough to accurately identify women who will develop preeclampsia, and interventions will need to be available that improve clinical outcome in women who test positive,” the committee members wrote.
The new committee opinion has been endorsed by the Society for Maternal-Fetal Medicine.
Commercial screening tests that purport to predict early-onset preeclampsia may do more harm than good and are not recommended, according to the American College of Obstetricians and Gynecologists.
Early-onset preeclampsia does signal great risk for both mother and infant, and early identification of pregnancies at high risk “would theoretically allow referral for more intensive surveillance or application of preventive therapies to reduce the risk of severe disease.” But the effectiveness of this approach is undercut by the low positive predictive value of currently available tests, according to a new policy statement from ACOG’s Committee on Obstetric Practice published Aug. 19 (Obstet Gynecol 2015;126:e25-7.).
“These tests require a large number of women to be identified as high risk and to potentially undergo intensive surveillance in order to detect one case of early-onset preeclampsia,” the committee members wrote.
Moreover, there are no data showing that using these screening tests improve clinical outcomes.
Taking a detailed medical history to assess risk factors remains “the best and only recommended screening approach for preeclampsia,” according to ACOG. The medical history should remain the screening method of choice until high-quality evidence demonstrates that aspirin and other interventions reduce the incidence of preeclampsia for women at high risk based on first-trimester predictive tests, ACOG recommended.
“For a first-trimester risk assessment for preeclampsia to be useful in clinical practice, future screening tests will need to have sensitivities and [positive predictive values] high enough to accurately identify women who will develop preeclampsia, and interventions will need to be available that improve clinical outcome in women who test positive,” the committee members wrote.
The new committee opinion has been endorsed by the Society for Maternal-Fetal Medicine.
Commercial screening tests that purport to predict early-onset preeclampsia may do more harm than good and are not recommended, according to the American College of Obstetricians and Gynecologists.
Early-onset preeclampsia does signal great risk for both mother and infant, and early identification of pregnancies at high risk “would theoretically allow referral for more intensive surveillance or application of preventive therapies to reduce the risk of severe disease.” But the effectiveness of this approach is undercut by the low positive predictive value of currently available tests, according to a new policy statement from ACOG’s Committee on Obstetric Practice published Aug. 19 (Obstet Gynecol 2015;126:e25-7.).
“These tests require a large number of women to be identified as high risk and to potentially undergo intensive surveillance in order to detect one case of early-onset preeclampsia,” the committee members wrote.
Moreover, there are no data showing that using these screening tests improve clinical outcomes.
Taking a detailed medical history to assess risk factors remains “the best and only recommended screening approach for preeclampsia,” according to ACOG. The medical history should remain the screening method of choice until high-quality evidence demonstrates that aspirin and other interventions reduce the incidence of preeclampsia for women at high risk based on first-trimester predictive tests, ACOG recommended.
“For a first-trimester risk assessment for preeclampsia to be useful in clinical practice, future screening tests will need to have sensitivities and [positive predictive values] high enough to accurately identify women who will develop preeclampsia, and interventions will need to be available that improve clinical outcome in women who test positive,” the committee members wrote.
The new committee opinion has been endorsed by the Society for Maternal-Fetal Medicine.
FROM OBSTETRICS & GYNECOLOGY
Experts call for greater scrutiny of egg donation practices
Fertility clinics are quick to tout their success rates to attract patients, but are they doing enough to disclose potential conflicts of interest and risks to oocyte donors?
A new report calls for professional societies to develop guidelines specifically addressing conflicts of interest in oocyte donation and to adopt tougher reporting and advertising standards (J Law Med Ethics. 2015 Jun;43[2]:410-24.).
While at least some measure of professional guidance addresses the potential conflicts that can arise at all four phases of oocyte donation – recruitment, screening and informed consent, ovarian stimulation, and follow-up – there is “a fracture between this guidance and actual practice,” the authors argued.
Some clinics and agencies may be minimizing risks in recruitment ads and websites, incentivizing repeated donations, or overstimulating donors for the benefit of recipients and clinic success rates.
One low-cost step to better inform a woman’s choice to donate would be to make complication rates publicly available along with the federally regulated annual success rates reported by the Centers for Disease Control and Prevention, said Aaron Levine, Ph.D., one of the report authors and an associate professor of public policy at the Georgia Institute of Technology in Atlanta.
“Sure, the clinics can complain it will require a little more paperwork and so on, but they should be tracking this already and if they’re not, a little nudge to track it better would be beneficial, in my view,” Dr. Levine said.
In 2010,Dr. Levine helped spark national debate after reporting that egg-donor payments rose $2,350 for every 100-point increase in SAT scores. The practice is not illegal and reflects, in part, the shortage of donors of certain ethnicities; however, the American Society for Reproductive Medicine (ASRM) egg donor compensation guidelines recommend payment not vary based on donor traits.
A separate systematic analysis of agency and clinic websites found that 34% of sites explicitly mentioned paying more for particular traits, 41% accepted donors under the ASRM recommended age minimum of 21, and many failed to present short-term (56%), long-term (92%), or psychological/emotional (77%) risks (Fertil Steril. 2012 Oct;98[4]: 995-1000).
A simple, cost-free solution may be to publish the names of programs that fail to follow ASRM/Society for Assisted Reproductive Technology (SART) guidelines, said study author Dr. Robert Klitzman, professor of psychiatry at Columbia University, New York, and director of the university’s master program in bioethics.
“There are carrots and sticks,” he said. “You can name-and-shame or you can praise those who are doing it.”
While fertility clinics tended to observe the guidelines, the analysis found that egg donor agencies or brokers were far more likely to advertise high payments, observed Dr. James Toner of the Atlanta Center for Reproductive Medicine.
“The profession cannot control what agencies offer their donors, nor prohibit private citizens from trying to find their own donors and offering exorbitant amounts,” said Dr. Toner, who is president of SART. “The true source of these guideline violations sits outside the profession.”
Dr. Klitzman argued that longitudinal follow-up is costly, but necessary to better understand the emotional and medical risks associated with egg donation. There is little interest from industry and the egg donors themselves may not want to think about creating a child for money. But, they may also look back in regret if they are childless at age 40 and have biological children they’re not in touch with, he said.
“Of course, if you go after 18- to 20-year-olds, their eggs are going to be better quality, but they’re probably going to think of these issues less,” he added.
Several studies have also shown that sperm donor–conceived offspring often want to know who their biological parent is, partly, for genetic and medical reasons to understand disease risk, Dr. Klitzman noted. In September 2014, the ASRM Ethics Committee updated its report on the interests, obligations, and rights in gamete donation and recommended that, “at minimum, gamete donors and recipients should be encouraged to provide appropriate medical updates.”
Dr. Levine acknowledged that ethical issues aren’t new to the infertility field, but said renewed attention is called for because of the increasingly high stakes involved in maintaining an adequate egg supply, increasing evidence of limited compliance with self-regulatory guidelines, and legal challenges to professional self-regulation.
In February 2015, the U.S. District Court for the Northern District of California granted in part the plaintiff’s motion for class certification in Kamakahi v. ASRM, a case filed in 2010 alleging that ASRM and SART engaged in price fixing by capping “appropriate” compensation in guidelines at $5,000, or $10,000 with justification.
The suit also names all SART-member fertility clinics and all egg agencies that agreed to abide by the ASRM-SART guidelines maximum price from April 12, 2007 to the present.
Also, in January 2015, the U.S. Tax Court ruled in favor of the Internal Revenue Service that $20,000 paid to a California woman for donating her eggs was compensation, not damages, and therefore taxable income.
The Kamakahi v. ASRM case is ongoing, but is already prompting some agencies to drop out of the ASRM, according to Andrew Vorzimer, a Woodland Hills, Calif., fertility lawyer. Mr. Vorzimer used to own Egg Donation Inc., but said he sold the Encino, Calif., infertility center in part because he could no longer compete with agencies who ignored the ASRM guidelines and were willing to offer six-figure payments for highly sought after donors. He continues to provide legal counsel to Egg Donation Inc.
“Most agencies ignore these guidelines anyway, so I think the likely result is, No. 1, that ASRM may simply withdraw all their guidelines and stay out of this entirely because they just don’t need the headache or liability, and you’re going to see the cost of egg donation skyrocket because the $10,000 dollar cap is going to go out the window,” Mr. Vorzimer said.
The IRS tax decision will also add upward pressure on egg prices because women are “going to want a larger amount to offset their tax liability,” he said.
Dr. Toner said that if the antitrust argument prevails in the courts, “...then I think the profession would stop providing guidance about reimbursement.”
Compensation, however, would be unlikely to take off. Even with the $10,000 cap in place, the going rate for eggs in Atlanta is about $6,000 per cycle. Few recipients, who ultimately pay the bill, would seek fertility treatment if the cost of eggs suddenly jumped from $6,000 to $30,000, he said. This downward pressure on the market is already being seen in states without insurance treatment mandates, whereas the “utilization of fertilization services is double or triple in states with coverage mandates,” Dr. Toner said.
Online ads may not include all the necessary information needed by donors, but “where the rubber hits the road” is in the clinic, he said, adding: “The profession has done a laudable job when it comes to egg donors. They are fully informed, treated as patients and fairly compensated for their time and effort.”
The new report from Dr. Levine and his colleagues discusses various possible oversight models, but ultimately balks at recommending federal or state laws or regulations. The authors conclude that improved self-regulation is the “most appropriate first step,” given the absence of compelling evidence of harm to donors associated with potential conflicts and feasibility concerns associated with other oversight models.
“Everyone is clamoring for more regulation,” said Dr. Mark V. Sauer, a member of ASRM’s Ethics Committee and director of Columbia’s Center for Women’s Reproductive Care. But he contended that the practice of reproductive medicine is probably already the most regulated form of medicine in the country. Moreover, the ASRM, not unlike the American Medical Association, is “not a policing professional society. They’re not a regulatory society,” he said.
Dr. Sauer declined to comment on the Kamakahi case, but said compensation rates in the highly competitive market of New York City have settled over the last 5 years at about $8,000 – the amount Columbia pays per cycle.
Fertility clinics are quick to tout their success rates to attract patients, but are they doing enough to disclose potential conflicts of interest and risks to oocyte donors?
A new report calls for professional societies to develop guidelines specifically addressing conflicts of interest in oocyte donation and to adopt tougher reporting and advertising standards (J Law Med Ethics. 2015 Jun;43[2]:410-24.).
While at least some measure of professional guidance addresses the potential conflicts that can arise at all four phases of oocyte donation – recruitment, screening and informed consent, ovarian stimulation, and follow-up – there is “a fracture between this guidance and actual practice,” the authors argued.
Some clinics and agencies may be minimizing risks in recruitment ads and websites, incentivizing repeated donations, or overstimulating donors for the benefit of recipients and clinic success rates.
One low-cost step to better inform a woman’s choice to donate would be to make complication rates publicly available along with the federally regulated annual success rates reported by the Centers for Disease Control and Prevention, said Aaron Levine, Ph.D., one of the report authors and an associate professor of public policy at the Georgia Institute of Technology in Atlanta.
“Sure, the clinics can complain it will require a little more paperwork and so on, but they should be tracking this already and if they’re not, a little nudge to track it better would be beneficial, in my view,” Dr. Levine said.
In 2010,Dr. Levine helped spark national debate after reporting that egg-donor payments rose $2,350 for every 100-point increase in SAT scores. The practice is not illegal and reflects, in part, the shortage of donors of certain ethnicities; however, the American Society for Reproductive Medicine (ASRM) egg donor compensation guidelines recommend payment not vary based on donor traits.
A separate systematic analysis of agency and clinic websites found that 34% of sites explicitly mentioned paying more for particular traits, 41% accepted donors under the ASRM recommended age minimum of 21, and many failed to present short-term (56%), long-term (92%), or psychological/emotional (77%) risks (Fertil Steril. 2012 Oct;98[4]: 995-1000).
A simple, cost-free solution may be to publish the names of programs that fail to follow ASRM/Society for Assisted Reproductive Technology (SART) guidelines, said study author Dr. Robert Klitzman, professor of psychiatry at Columbia University, New York, and director of the university’s master program in bioethics.
“There are carrots and sticks,” he said. “You can name-and-shame or you can praise those who are doing it.”
While fertility clinics tended to observe the guidelines, the analysis found that egg donor agencies or brokers were far more likely to advertise high payments, observed Dr. James Toner of the Atlanta Center for Reproductive Medicine.
“The profession cannot control what agencies offer their donors, nor prohibit private citizens from trying to find their own donors and offering exorbitant amounts,” said Dr. Toner, who is president of SART. “The true source of these guideline violations sits outside the profession.”
Dr. Klitzman argued that longitudinal follow-up is costly, but necessary to better understand the emotional and medical risks associated with egg donation. There is little interest from industry and the egg donors themselves may not want to think about creating a child for money. But, they may also look back in regret if they are childless at age 40 and have biological children they’re not in touch with, he said.
“Of course, if you go after 18- to 20-year-olds, their eggs are going to be better quality, but they’re probably going to think of these issues less,” he added.
Several studies have also shown that sperm donor–conceived offspring often want to know who their biological parent is, partly, for genetic and medical reasons to understand disease risk, Dr. Klitzman noted. In September 2014, the ASRM Ethics Committee updated its report on the interests, obligations, and rights in gamete donation and recommended that, “at minimum, gamete donors and recipients should be encouraged to provide appropriate medical updates.”
Dr. Levine acknowledged that ethical issues aren’t new to the infertility field, but said renewed attention is called for because of the increasingly high stakes involved in maintaining an adequate egg supply, increasing evidence of limited compliance with self-regulatory guidelines, and legal challenges to professional self-regulation.
In February 2015, the U.S. District Court for the Northern District of California granted in part the plaintiff’s motion for class certification in Kamakahi v. ASRM, a case filed in 2010 alleging that ASRM and SART engaged in price fixing by capping “appropriate” compensation in guidelines at $5,000, or $10,000 with justification.
The suit also names all SART-member fertility clinics and all egg agencies that agreed to abide by the ASRM-SART guidelines maximum price from April 12, 2007 to the present.
Also, in January 2015, the U.S. Tax Court ruled in favor of the Internal Revenue Service that $20,000 paid to a California woman for donating her eggs was compensation, not damages, and therefore taxable income.
The Kamakahi v. ASRM case is ongoing, but is already prompting some agencies to drop out of the ASRM, according to Andrew Vorzimer, a Woodland Hills, Calif., fertility lawyer. Mr. Vorzimer used to own Egg Donation Inc., but said he sold the Encino, Calif., infertility center in part because he could no longer compete with agencies who ignored the ASRM guidelines and were willing to offer six-figure payments for highly sought after donors. He continues to provide legal counsel to Egg Donation Inc.
“Most agencies ignore these guidelines anyway, so I think the likely result is, No. 1, that ASRM may simply withdraw all their guidelines and stay out of this entirely because they just don’t need the headache or liability, and you’re going to see the cost of egg donation skyrocket because the $10,000 dollar cap is going to go out the window,” Mr. Vorzimer said.
The IRS tax decision will also add upward pressure on egg prices because women are “going to want a larger amount to offset their tax liability,” he said.
Dr. Toner said that if the antitrust argument prevails in the courts, “...then I think the profession would stop providing guidance about reimbursement.”
Compensation, however, would be unlikely to take off. Even with the $10,000 cap in place, the going rate for eggs in Atlanta is about $6,000 per cycle. Few recipients, who ultimately pay the bill, would seek fertility treatment if the cost of eggs suddenly jumped from $6,000 to $30,000, he said. This downward pressure on the market is already being seen in states without insurance treatment mandates, whereas the “utilization of fertilization services is double or triple in states with coverage mandates,” Dr. Toner said.
Online ads may not include all the necessary information needed by donors, but “where the rubber hits the road” is in the clinic, he said, adding: “The profession has done a laudable job when it comes to egg donors. They are fully informed, treated as patients and fairly compensated for their time and effort.”
The new report from Dr. Levine and his colleagues discusses various possible oversight models, but ultimately balks at recommending federal or state laws or regulations. The authors conclude that improved self-regulation is the “most appropriate first step,” given the absence of compelling evidence of harm to donors associated with potential conflicts and feasibility concerns associated with other oversight models.
“Everyone is clamoring for more regulation,” said Dr. Mark V. Sauer, a member of ASRM’s Ethics Committee and director of Columbia’s Center for Women’s Reproductive Care. But he contended that the practice of reproductive medicine is probably already the most regulated form of medicine in the country. Moreover, the ASRM, not unlike the American Medical Association, is “not a policing professional society. They’re not a regulatory society,” he said.
Dr. Sauer declined to comment on the Kamakahi case, but said compensation rates in the highly competitive market of New York City have settled over the last 5 years at about $8,000 – the amount Columbia pays per cycle.
Fertility clinics are quick to tout their success rates to attract patients, but are they doing enough to disclose potential conflicts of interest and risks to oocyte donors?
A new report calls for professional societies to develop guidelines specifically addressing conflicts of interest in oocyte donation and to adopt tougher reporting and advertising standards (J Law Med Ethics. 2015 Jun;43[2]:410-24.).
While at least some measure of professional guidance addresses the potential conflicts that can arise at all four phases of oocyte donation – recruitment, screening and informed consent, ovarian stimulation, and follow-up – there is “a fracture between this guidance and actual practice,” the authors argued.
Some clinics and agencies may be minimizing risks in recruitment ads and websites, incentivizing repeated donations, or overstimulating donors for the benefit of recipients and clinic success rates.
One low-cost step to better inform a woman’s choice to donate would be to make complication rates publicly available along with the federally regulated annual success rates reported by the Centers for Disease Control and Prevention, said Aaron Levine, Ph.D., one of the report authors and an associate professor of public policy at the Georgia Institute of Technology in Atlanta.
“Sure, the clinics can complain it will require a little more paperwork and so on, but they should be tracking this already and if they’re not, a little nudge to track it better would be beneficial, in my view,” Dr. Levine said.
In 2010,Dr. Levine helped spark national debate after reporting that egg-donor payments rose $2,350 for every 100-point increase in SAT scores. The practice is not illegal and reflects, in part, the shortage of donors of certain ethnicities; however, the American Society for Reproductive Medicine (ASRM) egg donor compensation guidelines recommend payment not vary based on donor traits.
A separate systematic analysis of agency and clinic websites found that 34% of sites explicitly mentioned paying more for particular traits, 41% accepted donors under the ASRM recommended age minimum of 21, and many failed to present short-term (56%), long-term (92%), or psychological/emotional (77%) risks (Fertil Steril. 2012 Oct;98[4]: 995-1000).
A simple, cost-free solution may be to publish the names of programs that fail to follow ASRM/Society for Assisted Reproductive Technology (SART) guidelines, said study author Dr. Robert Klitzman, professor of psychiatry at Columbia University, New York, and director of the university’s master program in bioethics.
“There are carrots and sticks,” he said. “You can name-and-shame or you can praise those who are doing it.”
While fertility clinics tended to observe the guidelines, the analysis found that egg donor agencies or brokers were far more likely to advertise high payments, observed Dr. James Toner of the Atlanta Center for Reproductive Medicine.
“The profession cannot control what agencies offer their donors, nor prohibit private citizens from trying to find their own donors and offering exorbitant amounts,” said Dr. Toner, who is president of SART. “The true source of these guideline violations sits outside the profession.”
Dr. Klitzman argued that longitudinal follow-up is costly, but necessary to better understand the emotional and medical risks associated with egg donation. There is little interest from industry and the egg donors themselves may not want to think about creating a child for money. But, they may also look back in regret if they are childless at age 40 and have biological children they’re not in touch with, he said.
“Of course, if you go after 18- to 20-year-olds, their eggs are going to be better quality, but they’re probably going to think of these issues less,” he added.
Several studies have also shown that sperm donor–conceived offspring often want to know who their biological parent is, partly, for genetic and medical reasons to understand disease risk, Dr. Klitzman noted. In September 2014, the ASRM Ethics Committee updated its report on the interests, obligations, and rights in gamete donation and recommended that, “at minimum, gamete donors and recipients should be encouraged to provide appropriate medical updates.”
Dr. Levine acknowledged that ethical issues aren’t new to the infertility field, but said renewed attention is called for because of the increasingly high stakes involved in maintaining an adequate egg supply, increasing evidence of limited compliance with self-regulatory guidelines, and legal challenges to professional self-regulation.
In February 2015, the U.S. District Court for the Northern District of California granted in part the plaintiff’s motion for class certification in Kamakahi v. ASRM, a case filed in 2010 alleging that ASRM and SART engaged in price fixing by capping “appropriate” compensation in guidelines at $5,000, or $10,000 with justification.
The suit also names all SART-member fertility clinics and all egg agencies that agreed to abide by the ASRM-SART guidelines maximum price from April 12, 2007 to the present.
Also, in January 2015, the U.S. Tax Court ruled in favor of the Internal Revenue Service that $20,000 paid to a California woman for donating her eggs was compensation, not damages, and therefore taxable income.
The Kamakahi v. ASRM case is ongoing, but is already prompting some agencies to drop out of the ASRM, according to Andrew Vorzimer, a Woodland Hills, Calif., fertility lawyer. Mr. Vorzimer used to own Egg Donation Inc., but said he sold the Encino, Calif., infertility center in part because he could no longer compete with agencies who ignored the ASRM guidelines and were willing to offer six-figure payments for highly sought after donors. He continues to provide legal counsel to Egg Donation Inc.
“Most agencies ignore these guidelines anyway, so I think the likely result is, No. 1, that ASRM may simply withdraw all their guidelines and stay out of this entirely because they just don’t need the headache or liability, and you’re going to see the cost of egg donation skyrocket because the $10,000 dollar cap is going to go out the window,” Mr. Vorzimer said.
The IRS tax decision will also add upward pressure on egg prices because women are “going to want a larger amount to offset their tax liability,” he said.
Dr. Toner said that if the antitrust argument prevails in the courts, “...then I think the profession would stop providing guidance about reimbursement.”
Compensation, however, would be unlikely to take off. Even with the $10,000 cap in place, the going rate for eggs in Atlanta is about $6,000 per cycle. Few recipients, who ultimately pay the bill, would seek fertility treatment if the cost of eggs suddenly jumped from $6,000 to $30,000, he said. This downward pressure on the market is already being seen in states without insurance treatment mandates, whereas the “utilization of fertilization services is double or triple in states with coverage mandates,” Dr. Toner said.
Online ads may not include all the necessary information needed by donors, but “where the rubber hits the road” is in the clinic, he said, adding: “The profession has done a laudable job when it comes to egg donors. They are fully informed, treated as patients and fairly compensated for their time and effort.”
The new report from Dr. Levine and his colleagues discusses various possible oversight models, but ultimately balks at recommending federal or state laws or regulations. The authors conclude that improved self-regulation is the “most appropriate first step,” given the absence of compelling evidence of harm to donors associated with potential conflicts and feasibility concerns associated with other oversight models.
“Everyone is clamoring for more regulation,” said Dr. Mark V. Sauer, a member of ASRM’s Ethics Committee and director of Columbia’s Center for Women’s Reproductive Care. But he contended that the practice of reproductive medicine is probably already the most regulated form of medicine in the country. Moreover, the ASRM, not unlike the American Medical Association, is “not a policing professional society. They’re not a regulatory society,” he said.
Dr. Sauer declined to comment on the Kamakahi case, but said compensation rates in the highly competitive market of New York City have settled over the last 5 years at about $8,000 – the amount Columbia pays per cycle.
Lifestyle intervention can reduce gestational diabetes incidence
The incidence of gestational diabetes can be reduced in high-risk women with individualized lifestyle intervention focused on diet and physical activity, new research suggests.
A randomized, controlled trial of 269 women with a history of gestational diabetes or a prepregnancy body mass index of at least 30 kg/m2 showed the intervention was associated with a 36% reduction in the incidence of gestational diabetes, compared with usual care (13.9% vs. 21.6%, P = .044).
This was after adjustment for age, prepregnancy BMI, previous gestational diabetes status, and number of weeks’ gestation at the time of the oral glucose tolerance test, according to a paper published online July 29 in Diabetes Care.
From baseline to the second trimester oral glucose tolerance test – when the gestational diabetes diagnosis was made – women in the intervention group gained slightly less weight than did those in the control group (2.5 kg vs. 3.1 kg).
The intervention was associated with a significantly greater reduction in fasting plasma glucose concentration from baseline to third trimester, but there were no impacts on other pregnancy or birth outcomes such as preeclampsia, birth weight, or gestational age at birth.
“This is, to our knowledge, the first randomized controlled lifestyle intervention trial that has succeeded in reducing the overall incidence of GDM [gestational diabetes mellitus] among high-risk pregnant women,” wrote Dr. Saila B. Koivusalo of University of Helsinki and Helsinki University Hospital.
Women in the intervention arm were counseled by study nurses and dietitians three times during their pregnancy. Counseling was tailored to their risk and stage of pregnancy. The women continued their normal antenatal clinic visits (Diabetes Care 2015, Jul 29 [doi:10.2337/dc15-0511]).
Women with a prepregnancy BMI of 30 kg/m2 were advised not to gain any weight during the first two trimesters, and were given dietary counseling to optimize their consumption of healthy foods and reduce their intake of sugar-rich foods.
They also were counseled to aim for a minimum of 150 minutes of moderate-intensity physical activity per week, as part of an active lifestyle.
Participants in the control group received leaflets on diet and physical activity, which were usually provide by the local antenatal clinics.
Women who undertook the intervention showed significantly greater improvements in their dietary index scores than did those in the control arm, and they increased their median weekly physical activity by 15 minutes while the control group showed no increases.
However, there was no significant difference between the two groups in the number of women who met the physical activity goal of 150 minutes/week in their second trimester (26% of intervention group vs. 23% of the control group).
“Despite the fact that only a small proportion of the women in the intervention group reached the physical activity goals, and the difference in weight gain was modest between the groups, it is obvious that the individual changes in lifestyle do not need to be large but together they have a beneficial effect on the reduction of the incidence of GDM,” the authors wrote.
They pointed out that as the women participating in the trial were all known to be at high risk for gestational diabetes, even the women in the control group would have received advice on weight control as part of their antenatal care and were therefore more of a “mini-intervention” group than pure control.
“We believe that in an unselected high-risk population the impact of this kind of lifestyle intervention could be even more pronounced.”
The Ahokas Foundation, Finnish Foundation for Cardiovascular Disease, Special State Subsidy for Health Science Research of Helsinki University Central Hospital, Samfundet Folkhälsan, Finnish Diabetes Research Foundation, State Provincial Office of Southern Finland, and Social Insurance Institution of Finland funded the study. There were no conflicts of interest declared.
The incidence of gestational diabetes can be reduced in high-risk women with individualized lifestyle intervention focused on diet and physical activity, new research suggests.
A randomized, controlled trial of 269 women with a history of gestational diabetes or a prepregnancy body mass index of at least 30 kg/m2 showed the intervention was associated with a 36% reduction in the incidence of gestational diabetes, compared with usual care (13.9% vs. 21.6%, P = .044).
This was after adjustment for age, prepregnancy BMI, previous gestational diabetes status, and number of weeks’ gestation at the time of the oral glucose tolerance test, according to a paper published online July 29 in Diabetes Care.
From baseline to the second trimester oral glucose tolerance test – when the gestational diabetes diagnosis was made – women in the intervention group gained slightly less weight than did those in the control group (2.5 kg vs. 3.1 kg).
The intervention was associated with a significantly greater reduction in fasting plasma glucose concentration from baseline to third trimester, but there were no impacts on other pregnancy or birth outcomes such as preeclampsia, birth weight, or gestational age at birth.
“This is, to our knowledge, the first randomized controlled lifestyle intervention trial that has succeeded in reducing the overall incidence of GDM [gestational diabetes mellitus] among high-risk pregnant women,” wrote Dr. Saila B. Koivusalo of University of Helsinki and Helsinki University Hospital.
Women in the intervention arm were counseled by study nurses and dietitians three times during their pregnancy. Counseling was tailored to their risk and stage of pregnancy. The women continued their normal antenatal clinic visits (Diabetes Care 2015, Jul 29 [doi:10.2337/dc15-0511]).
Women with a prepregnancy BMI of 30 kg/m2 were advised not to gain any weight during the first two trimesters, and were given dietary counseling to optimize their consumption of healthy foods and reduce their intake of sugar-rich foods.
They also were counseled to aim for a minimum of 150 minutes of moderate-intensity physical activity per week, as part of an active lifestyle.
Participants in the control group received leaflets on diet and physical activity, which were usually provide by the local antenatal clinics.
Women who undertook the intervention showed significantly greater improvements in their dietary index scores than did those in the control arm, and they increased their median weekly physical activity by 15 minutes while the control group showed no increases.
However, there was no significant difference between the two groups in the number of women who met the physical activity goal of 150 minutes/week in their second trimester (26% of intervention group vs. 23% of the control group).
“Despite the fact that only a small proportion of the women in the intervention group reached the physical activity goals, and the difference in weight gain was modest between the groups, it is obvious that the individual changes in lifestyle do not need to be large but together they have a beneficial effect on the reduction of the incidence of GDM,” the authors wrote.
They pointed out that as the women participating in the trial were all known to be at high risk for gestational diabetes, even the women in the control group would have received advice on weight control as part of their antenatal care and were therefore more of a “mini-intervention” group than pure control.
“We believe that in an unselected high-risk population the impact of this kind of lifestyle intervention could be even more pronounced.”
The Ahokas Foundation, Finnish Foundation for Cardiovascular Disease, Special State Subsidy for Health Science Research of Helsinki University Central Hospital, Samfundet Folkhälsan, Finnish Diabetes Research Foundation, State Provincial Office of Southern Finland, and Social Insurance Institution of Finland funded the study. There were no conflicts of interest declared.
The incidence of gestational diabetes can be reduced in high-risk women with individualized lifestyle intervention focused on diet and physical activity, new research suggests.
A randomized, controlled trial of 269 women with a history of gestational diabetes or a prepregnancy body mass index of at least 30 kg/m2 showed the intervention was associated with a 36% reduction in the incidence of gestational diabetes, compared with usual care (13.9% vs. 21.6%, P = .044).
This was after adjustment for age, prepregnancy BMI, previous gestational diabetes status, and number of weeks’ gestation at the time of the oral glucose tolerance test, according to a paper published online July 29 in Diabetes Care.
From baseline to the second trimester oral glucose tolerance test – when the gestational diabetes diagnosis was made – women in the intervention group gained slightly less weight than did those in the control group (2.5 kg vs. 3.1 kg).
The intervention was associated with a significantly greater reduction in fasting plasma glucose concentration from baseline to third trimester, but there were no impacts on other pregnancy or birth outcomes such as preeclampsia, birth weight, or gestational age at birth.
“This is, to our knowledge, the first randomized controlled lifestyle intervention trial that has succeeded in reducing the overall incidence of GDM [gestational diabetes mellitus] among high-risk pregnant women,” wrote Dr. Saila B. Koivusalo of University of Helsinki and Helsinki University Hospital.
Women in the intervention arm were counseled by study nurses and dietitians three times during their pregnancy. Counseling was tailored to their risk and stage of pregnancy. The women continued their normal antenatal clinic visits (Diabetes Care 2015, Jul 29 [doi:10.2337/dc15-0511]).
Women with a prepregnancy BMI of 30 kg/m2 were advised not to gain any weight during the first two trimesters, and were given dietary counseling to optimize their consumption of healthy foods and reduce their intake of sugar-rich foods.
They also were counseled to aim for a minimum of 150 minutes of moderate-intensity physical activity per week, as part of an active lifestyle.
Participants in the control group received leaflets on diet and physical activity, which were usually provide by the local antenatal clinics.
Women who undertook the intervention showed significantly greater improvements in their dietary index scores than did those in the control arm, and they increased their median weekly physical activity by 15 minutes while the control group showed no increases.
However, there was no significant difference between the two groups in the number of women who met the physical activity goal of 150 minutes/week in their second trimester (26% of intervention group vs. 23% of the control group).
“Despite the fact that only a small proportion of the women in the intervention group reached the physical activity goals, and the difference in weight gain was modest between the groups, it is obvious that the individual changes in lifestyle do not need to be large but together they have a beneficial effect on the reduction of the incidence of GDM,” the authors wrote.
They pointed out that as the women participating in the trial were all known to be at high risk for gestational diabetes, even the women in the control group would have received advice on weight control as part of their antenatal care and were therefore more of a “mini-intervention” group than pure control.
“We believe that in an unselected high-risk population the impact of this kind of lifestyle intervention could be even more pronounced.”
The Ahokas Foundation, Finnish Foundation for Cardiovascular Disease, Special State Subsidy for Health Science Research of Helsinki University Central Hospital, Samfundet Folkhälsan, Finnish Diabetes Research Foundation, State Provincial Office of Southern Finland, and Social Insurance Institution of Finland funded the study. There were no conflicts of interest declared.
FROM DIABETES CARE
Key clinical point: Individualized lifestyle intervention focusing on diet and physical activity can reduce the risk of gestational diabetes in high-risk women.
Major finding: An individualized lifestyle intervention was associated with a 36% reduction in the incidence of gestational diabetes, compared with usual care.
Data source: A randomized, controlled trial of 269 women with a history of gestational diabetes or with a prepregnancy BMI of at least 30 kg/m2.
Disclosures: The Ahokas Foundation, Finnish Foundation for Cardiovascular Disease, Special State Subsidy for Health Science Research of Helsinki University Central Hospital, Samfundet Folkhälsan, Finnish Diabetes Research Foundation, State Provincial Office of Southern Finland, and Social Insurance Institution of Finland funded the study. There were no conflicts of interest declared.
Unvetted drug indications mean safety uncertainties
The coincidence of two recent news items highlighted the tension between a more unfettered approach to pharmaceutical drug marketing and the need for caution when promoting and prescribing drugs.
A federal court judge issued a decision August 7 that ruled the drug company Amarin was not subject to Food and Drug Administration penalty for truthful but off-label promotion of the FDA-approved drug Vascepa, a triglyceride-lowering agent. Also on August 7, Dr. Frances Oldham Kelsey died at 101 years old. She was the FDA staffer singled out for recognition in 1962 as the primary firewall who prevented the agency from approving thalidomide for U.S. marketing as a treatment for morning sickness in pregnant woman, thereby sparing America from the epidemic of thalidomide-induced birth defects seen in Europe.
Despite the interesting juxtaposition of these two events, talking about them in the same breath requires a couple of important caveats: Vascepa has received FDA acceptance as safe for its approved indication, and circumstances would need to be unusual and severe for physicians to consider prescribing it to pregnant women. Society has dramatically changed the way it thinks about dosing pregnant women with drugs, compared with 60 years ago. It’s an attitudinal change driven at least in part by the thalidomide tragedy.
But the careful line that regulatory agencies and physicians must navigate that can mean denying patients potentially useful or even life-saving drugs because of insufficient evidence of safety and the need for caution against unsuspected consequences remains an enduring fact of medical practice.
Underappreciated threats exist even from drugs widely perceived as commonplace and benign. Consider NSAIDs. In July, the FDA strengthened and broadened its label warning for drugs in this class to say that treatment with NSAIDs of all types, including those sold OTC, can increase a person’s risk for MI, stroke, and heart failure, and that the elevated risk occurs even after just a few weeks of NSAID use.
Also in July, I covered a report at the Alzheimer’s Association International Conference 2015 on the off-label use for treating agitation in Alzheimer’s disease patients with the combination of dextromethorphan and quinidine, a formulation branded as Nuedexta with FDA approval for treating pseudobulbar affect. Although this combination is already on the U.S. market, and the main active agent dextromethorphan is also widely marketed in several OTC cough-medicine products, the principal investigator of the Alzheimer’s disease study, Dr. Jeffrey L. Cummings, told me that he cautions physicians against prescribing the dextromethorphan plus quinidine combination to patients with Alzheimer’s disease agitation, even though controlling Alzheimer’s agitation is a major unmet need for patients and their families. Dr. Cummings stressed that the 93 patients he studied during 10 weeks of treatment were too few people followed for too short a period to draw any conclusions on safety in this new patient population.
Once a drug receives FDA approval, and so presumably has an adequate evidence base proving clinical safety, extrapolation of its safety to different patient types can be tricky. As clinical experience with a drug or a drug class accumulates and as use expands to different sorts of patients, appreciation often grows for subtle or uncommon adverse effects not signaled during initial testing. How many physicians or patients suspected a potential risk from all prescription NSAIDs before the FDA issued its first warning about the class 10 year ago, and how many remained oblivious to the danger from OTC NSAIDs until the FDA strengthened its warning a few weeks ago? And nearly 60 years ago, few physicians aside from Dr. Kelsey and her associates at the FDA focused on the unanticipated danger posed by thalidomide treatment during pregnancy.
Truthful free speech about possible benefits of FDA-approved drugs for additional indications is a interesting concept, but physicians and patients must remain mindful of the need for caution and the danger of extrapolating too much and being too aggressive with drug use when an agent’s safety is uncertain.
On Twitter @mitchelzoler
The coincidence of two recent news items highlighted the tension between a more unfettered approach to pharmaceutical drug marketing and the need for caution when promoting and prescribing drugs.
A federal court judge issued a decision August 7 that ruled the drug company Amarin was not subject to Food and Drug Administration penalty for truthful but off-label promotion of the FDA-approved drug Vascepa, a triglyceride-lowering agent. Also on August 7, Dr. Frances Oldham Kelsey died at 101 years old. She was the FDA staffer singled out for recognition in 1962 as the primary firewall who prevented the agency from approving thalidomide for U.S. marketing as a treatment for morning sickness in pregnant woman, thereby sparing America from the epidemic of thalidomide-induced birth defects seen in Europe.
Despite the interesting juxtaposition of these two events, talking about them in the same breath requires a couple of important caveats: Vascepa has received FDA acceptance as safe for its approved indication, and circumstances would need to be unusual and severe for physicians to consider prescribing it to pregnant women. Society has dramatically changed the way it thinks about dosing pregnant women with drugs, compared with 60 years ago. It’s an attitudinal change driven at least in part by the thalidomide tragedy.
But the careful line that regulatory agencies and physicians must navigate that can mean denying patients potentially useful or even life-saving drugs because of insufficient evidence of safety and the need for caution against unsuspected consequences remains an enduring fact of medical practice.
Underappreciated threats exist even from drugs widely perceived as commonplace and benign. Consider NSAIDs. In July, the FDA strengthened and broadened its label warning for drugs in this class to say that treatment with NSAIDs of all types, including those sold OTC, can increase a person’s risk for MI, stroke, and heart failure, and that the elevated risk occurs even after just a few weeks of NSAID use.
Also in July, I covered a report at the Alzheimer’s Association International Conference 2015 on the off-label use for treating agitation in Alzheimer’s disease patients with the combination of dextromethorphan and quinidine, a formulation branded as Nuedexta with FDA approval for treating pseudobulbar affect. Although this combination is already on the U.S. market, and the main active agent dextromethorphan is also widely marketed in several OTC cough-medicine products, the principal investigator of the Alzheimer’s disease study, Dr. Jeffrey L. Cummings, told me that he cautions physicians against prescribing the dextromethorphan plus quinidine combination to patients with Alzheimer’s disease agitation, even though controlling Alzheimer’s agitation is a major unmet need for patients and their families. Dr. Cummings stressed that the 93 patients he studied during 10 weeks of treatment were too few people followed for too short a period to draw any conclusions on safety in this new patient population.
Once a drug receives FDA approval, and so presumably has an adequate evidence base proving clinical safety, extrapolation of its safety to different patient types can be tricky. As clinical experience with a drug or a drug class accumulates and as use expands to different sorts of patients, appreciation often grows for subtle or uncommon adverse effects not signaled during initial testing. How many physicians or patients suspected a potential risk from all prescription NSAIDs before the FDA issued its first warning about the class 10 year ago, and how many remained oblivious to the danger from OTC NSAIDs until the FDA strengthened its warning a few weeks ago? And nearly 60 years ago, few physicians aside from Dr. Kelsey and her associates at the FDA focused on the unanticipated danger posed by thalidomide treatment during pregnancy.
Truthful free speech about possible benefits of FDA-approved drugs for additional indications is a interesting concept, but physicians and patients must remain mindful of the need for caution and the danger of extrapolating too much and being too aggressive with drug use when an agent’s safety is uncertain.
On Twitter @mitchelzoler
The coincidence of two recent news items highlighted the tension between a more unfettered approach to pharmaceutical drug marketing and the need for caution when promoting and prescribing drugs.
A federal court judge issued a decision August 7 that ruled the drug company Amarin was not subject to Food and Drug Administration penalty for truthful but off-label promotion of the FDA-approved drug Vascepa, a triglyceride-lowering agent. Also on August 7, Dr. Frances Oldham Kelsey died at 101 years old. She was the FDA staffer singled out for recognition in 1962 as the primary firewall who prevented the agency from approving thalidomide for U.S. marketing as a treatment for morning sickness in pregnant woman, thereby sparing America from the epidemic of thalidomide-induced birth defects seen in Europe.
Despite the interesting juxtaposition of these two events, talking about them in the same breath requires a couple of important caveats: Vascepa has received FDA acceptance as safe for its approved indication, and circumstances would need to be unusual and severe for physicians to consider prescribing it to pregnant women. Society has dramatically changed the way it thinks about dosing pregnant women with drugs, compared with 60 years ago. It’s an attitudinal change driven at least in part by the thalidomide tragedy.
But the careful line that regulatory agencies and physicians must navigate that can mean denying patients potentially useful or even life-saving drugs because of insufficient evidence of safety and the need for caution against unsuspected consequences remains an enduring fact of medical practice.
Underappreciated threats exist even from drugs widely perceived as commonplace and benign. Consider NSAIDs. In July, the FDA strengthened and broadened its label warning for drugs in this class to say that treatment with NSAIDs of all types, including those sold OTC, can increase a person’s risk for MI, stroke, and heart failure, and that the elevated risk occurs even after just a few weeks of NSAID use.
Also in July, I covered a report at the Alzheimer’s Association International Conference 2015 on the off-label use for treating agitation in Alzheimer’s disease patients with the combination of dextromethorphan and quinidine, a formulation branded as Nuedexta with FDA approval for treating pseudobulbar affect. Although this combination is already on the U.S. market, and the main active agent dextromethorphan is also widely marketed in several OTC cough-medicine products, the principal investigator of the Alzheimer’s disease study, Dr. Jeffrey L. Cummings, told me that he cautions physicians against prescribing the dextromethorphan plus quinidine combination to patients with Alzheimer’s disease agitation, even though controlling Alzheimer’s agitation is a major unmet need for patients and their families. Dr. Cummings stressed that the 93 patients he studied during 10 weeks of treatment were too few people followed for too short a period to draw any conclusions on safety in this new patient population.
Once a drug receives FDA approval, and so presumably has an adequate evidence base proving clinical safety, extrapolation of its safety to different patient types can be tricky. As clinical experience with a drug or a drug class accumulates and as use expands to different sorts of patients, appreciation often grows for subtle or uncommon adverse effects not signaled during initial testing. How many physicians or patients suspected a potential risk from all prescription NSAIDs before the FDA issued its first warning about the class 10 year ago, and how many remained oblivious to the danger from OTC NSAIDs until the FDA strengthened its warning a few weeks ago? And nearly 60 years ago, few physicians aside from Dr. Kelsey and her associates at the FDA focused on the unanticipated danger posed by thalidomide treatment during pregnancy.
Truthful free speech about possible benefits of FDA-approved drugs for additional indications is a interesting concept, but physicians and patients must remain mindful of the need for caution and the danger of extrapolating too much and being too aggressive with drug use when an agent’s safety is uncertain.
On Twitter @mitchelzoler