User login
2015 Update on contraception
Unintended pregnancy remains a serious problem in the United States, and the rate continues to increase. Currently, the unintended pregnancy rate is at an all-time high, estimated at 51% of all pregnancies.1 Sobering statistics reveal that the United States has a significantly higher rate of unintended pregnancy than any other developed country.2,3
So the question remains: If we have been introducing new contraceptive methods, why do unintended pregnancy rates keep rising in the United States?
The answer: Key barriers prevent women from attaining their desired contraceptive—foremost among them, cost.
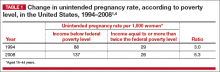
The unintended pregnancy rate is highest among women who are poor, young (aged 18–24 years), minorities, or cohabitating.1 The unintended pregnancy rate among poor women (income below the federal poverty level) of reproductive age has increased over the past few decades, whereas the rate among high-income women (more than twice the federal poverty level) has declined.1 The unintended pregnancy rate discrepancy between poor women and those with means has increased 77%, from a threefold difference in 1995 to a difference of more than fivefold in 2008 (TABLE 1).1,4
These data indicate that we are doing well providing contraception to women of means. However, as a society, we need to improve how we deliver contraceptives—especially highly effective methods—to poor women. As one might expect, given these numbers, low-income women have rates of abortion and unplanned birth that are nearly 6 times higher than their higher-income counterparts.1 The resultant cost to society is substantial, with 68% of unplanned births paid for by public insurance programs such as Medicaid, compared with 38% of planned births.5
As women’s health providers, we must work to improve these numbers and advocate for our patients to help them gain access to the contraceptives they need in accordance with their reproductive life plan. In this article, we hope to put this information in context as we:
- review reports and studies that evaluatethe trend of unintended pregnancy
- describe one state initiative that reduced the rates of unintended pregnancy, birth, and abortion
- introduce a new, highly effective levonorgestrel-releasing intrauterine system (Liletta) being marketed with the goal of reaching women who receive care from private physicians as well as public health clinics.
National and state snapshots reveal shifting proportions of intended, unintended pregnancies
Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(suppl 1):s43–s48.
Kost K. Unintended pregnancy rates at the state level: estimates for 2010 and trends since 2002. Guttmacher Institute. https://www.guttmacher.org/pubs/StateUP10.pdf. Published January 2015. Accessed June 29, 2015.
Recent studies have demonstrated trends in unplanned pregnancy over the past 2 decades on both a national and statewide level. Data from the National Survey of Family Growth have highlighted trends in abortion and miscarriage, and data from the National Center for Health Statistics have shed light on birth trends. Of 6 million births in 2008, 51% were unintended.1 Unintended pregnancy was defined as a gestation that was mistimed or unwanted. Intended pregnancy was defined as one that was desired at the time it occurred or sooner.
2008 data focus on the national level
Although the overall pregnancy rate for US women aged 15 to 44 years is relatively unchanged, there is a small change in whether or not the pregnancy was intended. For 2008, as the rate of intended pregnancy dropped slightly, from 54 to 51 pregnancies per 1,000 women, the unintended pregnancy rate increased by 10%, from 49 to 54 pregnancies per 1,000 women. Proportionally, unintended pregnancies that resulted in abortion declined from 47% to 40%, whereas the rate of unintended pregnancies ending in birth increased to 27 births per 1,000 women.1
2010 data focus on individual states
We now have state-specific data on trends in unintended pregnancy rates from 2002 to 2010 in the United States.6 In 28 of 50 states, more than half of all pregnancies were unintended, with rates ranging from 36% to 62%. The median rate was 47 unintended pregnancies per 1,000 women aged 15 to 44, with the lowest unintended pregnancy rate in New Hampshire at 32 per 1,000 women and highest rates in Delaware, Hawaii, and New York at 61 to 62 unintended pregnancies per 1,000 women.6
Between 2002 and 2010, unintended pregnancy rates fell 5% or more in 18 states and rose 5% or more in 4 states. In the remaining 12 states for which there are data, unintended pregnancy rates remained unchanged.
Interestingly, 16 states had increases in unintended pregnancy rates of 5% or more between 2002 and 2006. The trend reversed between 2006 and 2010, during which 28 of 41 states with available data experienced decreases of 5% or more and only 1 state experienced an increase of 5% or more. These latest numbers suggest we may be making some progress in reducing the overall rate of unintended pregnancy.
What this EVIDENCE means for practice
Although the rate of unintended pregnancy is declining in some states, the national rate is still increasing. This information emphasizes the need for all providers to consider initiating discussions about pregnancy intentions—a step that may be as important as obtaining blood pressure and weight. When women are seen for any health visit, they should be asked about their reproductive plans. The Centers for Disease Control and Prevention (CDC) has issued a helpful set of questions to guide the discussion of timing and planning pregnancy. It also provides useful information on ways to increase utilization of long-acting reversible contraceptives (LARCs) to help realize the goal of fewer unintended pregnancies. (For more on this discussion, see “How to motivate your patient to create a reproductive life plan,” below.)
How to motivate your patient to create a reproductive life plan
The Centers for Disease Control and Prevention (CDC) offers a tool for health care professionals to use to encourage patients to think about their reproductive goals and make a plan to facilitate those goals. It’s available at: http://www.cdc.gov/preconception/documents/rlphealthproviders.pdf.
Questions to ask your patient
- Do you plan to have any (more) children at any time in your future?
IF YES
- How many children would you like to have?
- How long would you like to wait until you or your partner become pregnant?
- What family planning method do you plan to use until you or your partner are ready to become pregnant?
- How sure are you that you will be able to use this method without any problems?
IF NO
- What family planning method will you use to avoid pregnancy?
- How sure are you that you will be able to use this method without any problems?
- People’s plans change. Is it possible that you or your partner could ever decide to become pregnant?
Action plan
Encourage your patient to make a plan and take action. Remind her that the plan doesn’t have to be set in stone.
How Colorado broke down barriers to highly effective contraception—and saved $42.5 million in 1 year
Ricketts S, Klinger G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline of births among young, low-income women. Perspect Sexual Reprod Health. 2014;46(3):125–132.
The Contraceptive CHOICE Project in St. Louis County, Missouri, is an incredible success story. In it, clinicians made highly effective LARCs readily available and free of charge. When a large percentage of women chose a LARC method, the unintended pregnancy and abortion rates declined.7 In Colorado, providers put this study into practice, creating a successful statewide initiative to reduce the unintended pregnancy rate.8
The data behind LARC methods
LARC methods are backed by endorsements from the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the CDC, and the World Health Organization, which recommend them for adolescents because of their superiority to shorter-acting methods. With LARCs, failure rates are lower and compliance is greater, making them ideal for adolescents, who have high unintended pregnancy rates.1
However, based on 2011 data, only 2% of LARC users nationwide are aged 20 or younger. A number of barriers prevent young women from obtaining LARCs, including lack of education, limited access to and availability of the contraceptives, and, importantly, cost. Many state plans have adopted Medicaid expansions to reduce barriers to LARCs. However, this benefit is still not available in many states, Colorado being 1 of them.
How the Colorado initiative worked
In 2005, 40% of Colorado’s births were unintended, and 60% of those unintended births occurred in women aged 15 to 24 years. About three-quarters of women who were using a contraceptive method at the time of unintended pregnancy reported that it was a low-cost, high-failure method such as condoms or withdrawal.
In response, Colorado’s Department of Public Health and Environment and the Colorado Family Planning Initiative (CFPI) used private funds from an anonymous foundation to provide LARC products at no cost to the Title X–funded clinics in the state.
The initiative began in 2009 in clinics that served 95% of the state’s total population. The funding provided the products themselves (intrauterine device [IUD], implant), as well as training for providers and staff.
Before the initiative began, 52,645 clients received services in these clinics annually. In the third year of the initiative, that number had increased to 64,928 annually. About 55% of clients receiving services both preinitiative and postinitiative were younger than 25 years, and most (92% in 2011) had income below the poverty level.
LARC use increased fourfold over the 3 years of the funded program, from less than 4.5% to 19.4% in the third year. Contraceptive implant use increased tenfold, and IUD use increased by almost threefold. At the same time, oral contraceptive use declined 13%. Before the initiative, only 620 young, low-income women used a LARC method; afterward, 8,435 did.
These changes in contraceptive practice triggered a significant decline in pregnancy rates (TABLE 2) and abortion rates (TABLE 3). Abortion rates increased 8% among 20- to 24-year-olds who were not enrolled in the initiative and decreased 18% among those who were. The proportion of high-risk births (births to unmarried, low-income women with less than a high school education) dropped 24% after the initiative began. The proportion of high-risk births in counties not receiving CFPI funds stayed the same at 7%.
Colorado program saved $42.5 million in public funds
This Colorado program demonstrated that the CHOICE Project can be translated to a statewide initiative. Whereas CHOICE enrolled 9,256 women over 4 years, the Colorado initiative included more than 50,000 clients annually over 3 years. Colorado did not use any state funds for this project, which resulted in significant decreases in the unintended birth rate, abortion rate, and rate of high-risk births.
The Colorado governor’s office estimates that the CFPI saved $5.68 in Medicaid costs for Colorado for every dollar spent on contraceptives. In just 1 year (2010), the program saved approximately $42.5 million in public funds.
Ironically, despite the success of this project, the Colorado legislature denied further funding once the initial financial support ceased.
What this EVIDENCE means for practice
The Colorado program demonstrates that we all can provide LARC methods in practice, especially to young women. In this population, use of highly effective contraception resulted in fewer unintended pregnancies, births, and abortions statewide.
We also need to advocate for our patients, particularly those who have less means and rely on public assistance. Public funding of LARC methods clearly improves outcomes at an individual and population level.
A new, more affordable IUD enters the market
Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
Programs such as the Contraceptive CHOICE Project and the Colorado Family Planning Initiative relied on private foundations for financial support, largely because of the high cost of the IUDs and implants currently available in the United States. Even with the Affordable Care Act (ACA) reducing the costs of LARC products and other contraceptives for patients, there are still many women not covered by these programs. For example, “grandfathered” health insurance plans do not need to follow some aspects of the ACA.
Just as important, the high cost of LARC products takes a toll on providers and clinics that must finance the cost per unit to have stock on hand and then wait for months for reimbursement by insurance companies. As a result, some providers do not stock IUDs and implants and only order them as they are needed and approved by insurance for a particular patient. These barriers limit access to LARC methods and reduce the number of women who receive the products.8
Liletta is less expensive than other IUDs
Enter Liletta, a new levonorgestrel-releasing, 52-mg intrauterine system (IUS) that has been in clinical trials since 2009.9 ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an Intrauterine System) was initiated by Medicines360, a unique nonprofit pharmaceutical company committed to ensuring access to reproductive health products for all women (private and public sector).
ACCESS IUS is the largest IUD approval study ever performed exclusively in the United States. The Phase 3, open-label clinical trial was conducted at 29 sites around the United States, enrolling healthy, nonpregnant, sexually active women aged 16 to 45 years with regular menstrual cycles. Both nulliparous and parous women were included, with no weight or body mass index (BMI) restrictions applied. The study is ongoing and will continue for as long as 7 years. Eisenberg and colleagues published the data used for initial approval for 3 years of use in the United States and Europe.9
Details of the trial
A total of 1,600 women aged 16 to 35 years comprised the group in which efficacy was evaluated. An additional 151 women aged 36 to 45 years were evaluated for safety only. Of the enrolled women, 1,011 (58%) were nulliparous, making ACCESS IUS the largest product approval study of nulliparous women. In addition, 438 women (25.1%) were obese, and 5% of these women had a BMI greater than 40 kg/m2.
Liletta was placed successfully in 1,714 women (97.9%). Fifteen women did not have placement attempted due to uterine factors (the uterus could not be sounded, or the sound was <5.5 cm) or factors unrelated to the product or inserter. In women in whom placement was attempted, the success rate was 98.7%.
The first-year Pearl index for Liletta was 0.15. Life-table pregnancy rates were 0.14 through year 1 and 0.55 through year 3. Four of the 6 pregnancies reported through 3 years of use were ectopic. Adverse events and their incidence, occurring in more than 2% of users, were acne (6%), expulsion (3.5%), dyspareunia (2.8%), and mastalgia (2.0%). The most common adverse events leading to discontinuation were expulsion (3.5%), bleeding complaints (1.5%), acne (1.3%), and mood swings (1.3%).
Uterine perforation with Liletta was diagnosed in 2 participants (0.11%). Expulsion occurred in 62 users (3.5%) and was more frequent among parous than nulliparous women (5.6% vs 2.0%, respectively; P<.001). Most (80.6%) of the expulsions were reported in the first year of product use. Pelvic infection was reported in 10 participants (0.6%), and all cases resolved with outpatient antibiotic treatment.9
Keep in mind that this is an ongoing study—not all women have reached a full 3 years of use. Updates on efficacy and adverse events will be published in the future. This current publication demonstrates the high efficacy and safety of the product through 3 years of use, permitting its approval for contraception in the United States.
In Europe, the product is approved for both contraception and the treatment of heavy menstrual bleeding, based on a European study.10
What this EVIDENCE means for practice
Liletta is a branded product (not generic) designed to be similar to Mirena, with the same size, frame, hormone content, and hormone release rate.11 Medicines360 has entered a groundbreaking marketing partnership with Actavis to make Liletta widely available and affordable. For most public sector providers and clinics in the United States, Liletta costs only $50, significantly less than other LARC methods available in the United States. Actavis also has a program that ensures that any woman lacking insurance coverage for an IUD and not receiving care at a public sector clinic will not be charged more than $75 for her IUD. However, the price of the device is only one aspect of its overall cost, as women still need to pay for any office visit or insertion fees.
For society, this unique business partnership has to include providers and patients as well. Sales of Liletta in the private sector will support the very low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we can all help poor women access very affordable highly effective contraception.
For providers, Liletta is a lower-cost alternative to currently available hormonal IUDs and should perform well over the long term. The highly successful use of Liletta in nulliparous women demonstrates its safety in this population. The 3-year approval is the first step, as the Phase 3 study continues. In the future, Liletta is expected to be approved for 5 years or longer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(suppl 1):s43–s48.
2. Guttmacher Institute. Fact Sheet: Unintended Pregnancy in the United States. http://www.guttmacher.org/pubs/FB-Unintended-Pregnancy-US.html. Published February 2015. Accessed June 29, 2015.
3. Singh S, Sedgh G, Hussain R. Unintended pregnancy: worldwide levels, trends and outcomes. Stud Fam Plann. 2010;41(4):241–250.
4. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspectives. 1998;30(1):24–29, 46.
5. Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care. National and States Estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed June 29, 2015.
6. Kost K. Unintended pregnancy rates at the state level: estimates for 2010 and trends since 2002. Guttmacher Institute. http://www.guttmacher.org/pubs/StateUP10.pdf. Published January 2015. Accessed June 30, 2015.
7. Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Preventing unintended pregnancy: the ContraceptiveCHOICE Project in review. J Womens Health. 2015; 24(5):349–353.
8. Ricketts S, Klinger G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline of births among young, low-income women. Perspect Sexual Reprod Health. 2014;46(3):125–132.
9. Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
10. Mawet M, Nollevaux F, Nizet D, et al. Impact of a new levonorgestrel intrauterine system, Levosert, on heavy menstrual bleeding: results of a one-year randomised controlled trial. Eur J Contracept Reprod Health Care. 2014;19(3):169–179.
11. Gopalakrishnan M, Liu T, Gobburu J, Creinin MD. Levonorgestrel release rates with LNG20, a new levonorgestrel intrauterine system. Obstet Gynecol. 2015;125(suppl 1): 62S–63S.
Unintended pregnancy remains a serious problem in the United States, and the rate continues to increase. Currently, the unintended pregnancy rate is at an all-time high, estimated at 51% of all pregnancies.1 Sobering statistics reveal that the United States has a significantly higher rate of unintended pregnancy than any other developed country.2,3
So the question remains: If we have been introducing new contraceptive methods, why do unintended pregnancy rates keep rising in the United States?
The answer: Key barriers prevent women from attaining their desired contraceptive—foremost among them, cost.
The unintended pregnancy rate is highest among women who are poor, young (aged 18–24 years), minorities, or cohabitating.1 The unintended pregnancy rate among poor women (income below the federal poverty level) of reproductive age has increased over the past few decades, whereas the rate among high-income women (more than twice the federal poverty level) has declined.1 The unintended pregnancy rate discrepancy between poor women and those with means has increased 77%, from a threefold difference in 1995 to a difference of more than fivefold in 2008 (TABLE 1).1,4
These data indicate that we are doing well providing contraception to women of means. However, as a society, we need to improve how we deliver contraceptives—especially highly effective methods—to poor women. As one might expect, given these numbers, low-income women have rates of abortion and unplanned birth that are nearly 6 times higher than their higher-income counterparts.1 The resultant cost to society is substantial, with 68% of unplanned births paid for by public insurance programs such as Medicaid, compared with 38% of planned births.5
As women’s health providers, we must work to improve these numbers and advocate for our patients to help them gain access to the contraceptives they need in accordance with their reproductive life plan. In this article, we hope to put this information in context as we:
- review reports and studies that evaluatethe trend of unintended pregnancy
- describe one state initiative that reduced the rates of unintended pregnancy, birth, and abortion
- introduce a new, highly effective levonorgestrel-releasing intrauterine system (Liletta) being marketed with the goal of reaching women who receive care from private physicians as well as public health clinics.
National and state snapshots reveal shifting proportions of intended, unintended pregnancies
Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(suppl 1):s43–s48.
Kost K. Unintended pregnancy rates at the state level: estimates for 2010 and trends since 2002. Guttmacher Institute. https://www.guttmacher.org/pubs/StateUP10.pdf. Published January 2015. Accessed June 29, 2015.
Recent studies have demonstrated trends in unplanned pregnancy over the past 2 decades on both a national and statewide level. Data from the National Survey of Family Growth have highlighted trends in abortion and miscarriage, and data from the National Center for Health Statistics have shed light on birth trends. Of 6 million births in 2008, 51% were unintended.1 Unintended pregnancy was defined as a gestation that was mistimed or unwanted. Intended pregnancy was defined as one that was desired at the time it occurred or sooner.
2008 data focus on the national level
Although the overall pregnancy rate for US women aged 15 to 44 years is relatively unchanged, there is a small change in whether or not the pregnancy was intended. For 2008, as the rate of intended pregnancy dropped slightly, from 54 to 51 pregnancies per 1,000 women, the unintended pregnancy rate increased by 10%, from 49 to 54 pregnancies per 1,000 women. Proportionally, unintended pregnancies that resulted in abortion declined from 47% to 40%, whereas the rate of unintended pregnancies ending in birth increased to 27 births per 1,000 women.1
2010 data focus on individual states
We now have state-specific data on trends in unintended pregnancy rates from 2002 to 2010 in the United States.6 In 28 of 50 states, more than half of all pregnancies were unintended, with rates ranging from 36% to 62%. The median rate was 47 unintended pregnancies per 1,000 women aged 15 to 44, with the lowest unintended pregnancy rate in New Hampshire at 32 per 1,000 women and highest rates in Delaware, Hawaii, and New York at 61 to 62 unintended pregnancies per 1,000 women.6
Between 2002 and 2010, unintended pregnancy rates fell 5% or more in 18 states and rose 5% or more in 4 states. In the remaining 12 states for which there are data, unintended pregnancy rates remained unchanged.
Interestingly, 16 states had increases in unintended pregnancy rates of 5% or more between 2002 and 2006. The trend reversed between 2006 and 2010, during which 28 of 41 states with available data experienced decreases of 5% or more and only 1 state experienced an increase of 5% or more. These latest numbers suggest we may be making some progress in reducing the overall rate of unintended pregnancy.
What this EVIDENCE means for practice
Although the rate of unintended pregnancy is declining in some states, the national rate is still increasing. This information emphasizes the need for all providers to consider initiating discussions about pregnancy intentions—a step that may be as important as obtaining blood pressure and weight. When women are seen for any health visit, they should be asked about their reproductive plans. The Centers for Disease Control and Prevention (CDC) has issued a helpful set of questions to guide the discussion of timing and planning pregnancy. It also provides useful information on ways to increase utilization of long-acting reversible contraceptives (LARCs) to help realize the goal of fewer unintended pregnancies. (For more on this discussion, see “How to motivate your patient to create a reproductive life plan,” below.)
How to motivate your patient to create a reproductive life plan
The Centers for Disease Control and Prevention (CDC) offers a tool for health care professionals to use to encourage patients to think about their reproductive goals and make a plan to facilitate those goals. It’s available at: http://www.cdc.gov/preconception/documents/rlphealthproviders.pdf.
Questions to ask your patient
- Do you plan to have any (more) children at any time in your future?
IF YES
- How many children would you like to have?
- How long would you like to wait until you or your partner become pregnant?
- What family planning method do you plan to use until you or your partner are ready to become pregnant?
- How sure are you that you will be able to use this method without any problems?
IF NO
- What family planning method will you use to avoid pregnancy?
- How sure are you that you will be able to use this method without any problems?
- People’s plans change. Is it possible that you or your partner could ever decide to become pregnant?
Action plan
Encourage your patient to make a plan and take action. Remind her that the plan doesn’t have to be set in stone.
How Colorado broke down barriers to highly effective contraception—and saved $42.5 million in 1 year
Ricketts S, Klinger G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline of births among young, low-income women. Perspect Sexual Reprod Health. 2014;46(3):125–132.
The Contraceptive CHOICE Project in St. Louis County, Missouri, is an incredible success story. In it, clinicians made highly effective LARCs readily available and free of charge. When a large percentage of women chose a LARC method, the unintended pregnancy and abortion rates declined.7 In Colorado, providers put this study into practice, creating a successful statewide initiative to reduce the unintended pregnancy rate.8
The data behind LARC methods
LARC methods are backed by endorsements from the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the CDC, and the World Health Organization, which recommend them for adolescents because of their superiority to shorter-acting methods. With LARCs, failure rates are lower and compliance is greater, making them ideal for adolescents, who have high unintended pregnancy rates.1
However, based on 2011 data, only 2% of LARC users nationwide are aged 20 or younger. A number of barriers prevent young women from obtaining LARCs, including lack of education, limited access to and availability of the contraceptives, and, importantly, cost. Many state plans have adopted Medicaid expansions to reduce barriers to LARCs. However, this benefit is still not available in many states, Colorado being 1 of them.
How the Colorado initiative worked
In 2005, 40% of Colorado’s births were unintended, and 60% of those unintended births occurred in women aged 15 to 24 years. About three-quarters of women who were using a contraceptive method at the time of unintended pregnancy reported that it was a low-cost, high-failure method such as condoms or withdrawal.
In response, Colorado’s Department of Public Health and Environment and the Colorado Family Planning Initiative (CFPI) used private funds from an anonymous foundation to provide LARC products at no cost to the Title X–funded clinics in the state.
The initiative began in 2009 in clinics that served 95% of the state’s total population. The funding provided the products themselves (intrauterine device [IUD], implant), as well as training for providers and staff.
Before the initiative began, 52,645 clients received services in these clinics annually. In the third year of the initiative, that number had increased to 64,928 annually. About 55% of clients receiving services both preinitiative and postinitiative were younger than 25 years, and most (92% in 2011) had income below the poverty level.
LARC use increased fourfold over the 3 years of the funded program, from less than 4.5% to 19.4% in the third year. Contraceptive implant use increased tenfold, and IUD use increased by almost threefold. At the same time, oral contraceptive use declined 13%. Before the initiative, only 620 young, low-income women used a LARC method; afterward, 8,435 did.
These changes in contraceptive practice triggered a significant decline in pregnancy rates (TABLE 2) and abortion rates (TABLE 3). Abortion rates increased 8% among 20- to 24-year-olds who were not enrolled in the initiative and decreased 18% among those who were. The proportion of high-risk births (births to unmarried, low-income women with less than a high school education) dropped 24% after the initiative began. The proportion of high-risk births in counties not receiving CFPI funds stayed the same at 7%.
Colorado program saved $42.5 million in public funds
This Colorado program demonstrated that the CHOICE Project can be translated to a statewide initiative. Whereas CHOICE enrolled 9,256 women over 4 years, the Colorado initiative included more than 50,000 clients annually over 3 years. Colorado did not use any state funds for this project, which resulted in significant decreases in the unintended birth rate, abortion rate, and rate of high-risk births.
The Colorado governor’s office estimates that the CFPI saved $5.68 in Medicaid costs for Colorado for every dollar spent on contraceptives. In just 1 year (2010), the program saved approximately $42.5 million in public funds.
Ironically, despite the success of this project, the Colorado legislature denied further funding once the initial financial support ceased.
What this EVIDENCE means for practice
The Colorado program demonstrates that we all can provide LARC methods in practice, especially to young women. In this population, use of highly effective contraception resulted in fewer unintended pregnancies, births, and abortions statewide.
We also need to advocate for our patients, particularly those who have less means and rely on public assistance. Public funding of LARC methods clearly improves outcomes at an individual and population level.
A new, more affordable IUD enters the market
Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
Programs such as the Contraceptive CHOICE Project and the Colorado Family Planning Initiative relied on private foundations for financial support, largely because of the high cost of the IUDs and implants currently available in the United States. Even with the Affordable Care Act (ACA) reducing the costs of LARC products and other contraceptives for patients, there are still many women not covered by these programs. For example, “grandfathered” health insurance plans do not need to follow some aspects of the ACA.
Just as important, the high cost of LARC products takes a toll on providers and clinics that must finance the cost per unit to have stock on hand and then wait for months for reimbursement by insurance companies. As a result, some providers do not stock IUDs and implants and only order them as they are needed and approved by insurance for a particular patient. These barriers limit access to LARC methods and reduce the number of women who receive the products.8
Liletta is less expensive than other IUDs
Enter Liletta, a new levonorgestrel-releasing, 52-mg intrauterine system (IUS) that has been in clinical trials since 2009.9 ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an Intrauterine System) was initiated by Medicines360, a unique nonprofit pharmaceutical company committed to ensuring access to reproductive health products for all women (private and public sector).
ACCESS IUS is the largest IUD approval study ever performed exclusively in the United States. The Phase 3, open-label clinical trial was conducted at 29 sites around the United States, enrolling healthy, nonpregnant, sexually active women aged 16 to 45 years with regular menstrual cycles. Both nulliparous and parous women were included, with no weight or body mass index (BMI) restrictions applied. The study is ongoing and will continue for as long as 7 years. Eisenberg and colleagues published the data used for initial approval for 3 years of use in the United States and Europe.9
Details of the trial
A total of 1,600 women aged 16 to 35 years comprised the group in which efficacy was evaluated. An additional 151 women aged 36 to 45 years were evaluated for safety only. Of the enrolled women, 1,011 (58%) were nulliparous, making ACCESS IUS the largest product approval study of nulliparous women. In addition, 438 women (25.1%) were obese, and 5% of these women had a BMI greater than 40 kg/m2.
Liletta was placed successfully in 1,714 women (97.9%). Fifteen women did not have placement attempted due to uterine factors (the uterus could not be sounded, or the sound was <5.5 cm) or factors unrelated to the product or inserter. In women in whom placement was attempted, the success rate was 98.7%.
The first-year Pearl index for Liletta was 0.15. Life-table pregnancy rates were 0.14 through year 1 and 0.55 through year 3. Four of the 6 pregnancies reported through 3 years of use were ectopic. Adverse events and their incidence, occurring in more than 2% of users, were acne (6%), expulsion (3.5%), dyspareunia (2.8%), and mastalgia (2.0%). The most common adverse events leading to discontinuation were expulsion (3.5%), bleeding complaints (1.5%), acne (1.3%), and mood swings (1.3%).
Uterine perforation with Liletta was diagnosed in 2 participants (0.11%). Expulsion occurred in 62 users (3.5%) and was more frequent among parous than nulliparous women (5.6% vs 2.0%, respectively; P<.001). Most (80.6%) of the expulsions were reported in the first year of product use. Pelvic infection was reported in 10 participants (0.6%), and all cases resolved with outpatient antibiotic treatment.9
Keep in mind that this is an ongoing study—not all women have reached a full 3 years of use. Updates on efficacy and adverse events will be published in the future. This current publication demonstrates the high efficacy and safety of the product through 3 years of use, permitting its approval for contraception in the United States.
In Europe, the product is approved for both contraception and the treatment of heavy menstrual bleeding, based on a European study.10
What this EVIDENCE means for practice
Liletta is a branded product (not generic) designed to be similar to Mirena, with the same size, frame, hormone content, and hormone release rate.11 Medicines360 has entered a groundbreaking marketing partnership with Actavis to make Liletta widely available and affordable. For most public sector providers and clinics in the United States, Liletta costs only $50, significantly less than other LARC methods available in the United States. Actavis also has a program that ensures that any woman lacking insurance coverage for an IUD and not receiving care at a public sector clinic will not be charged more than $75 for her IUD. However, the price of the device is only one aspect of its overall cost, as women still need to pay for any office visit or insertion fees.
For society, this unique business partnership has to include providers and patients as well. Sales of Liletta in the private sector will support the very low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we can all help poor women access very affordable highly effective contraception.
For providers, Liletta is a lower-cost alternative to currently available hormonal IUDs and should perform well over the long term. The highly successful use of Liletta in nulliparous women demonstrates its safety in this population. The 3-year approval is the first step, as the Phase 3 study continues. In the future, Liletta is expected to be approved for 5 years or longer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Unintended pregnancy remains a serious problem in the United States, and the rate continues to increase. Currently, the unintended pregnancy rate is at an all-time high, estimated at 51% of all pregnancies.1 Sobering statistics reveal that the United States has a significantly higher rate of unintended pregnancy than any other developed country.2,3
So the question remains: If we have been introducing new contraceptive methods, why do unintended pregnancy rates keep rising in the United States?
The answer: Key barriers prevent women from attaining their desired contraceptive—foremost among them, cost.
The unintended pregnancy rate is highest among women who are poor, young (aged 18–24 years), minorities, or cohabitating.1 The unintended pregnancy rate among poor women (income below the federal poverty level) of reproductive age has increased over the past few decades, whereas the rate among high-income women (more than twice the federal poverty level) has declined.1 The unintended pregnancy rate discrepancy between poor women and those with means has increased 77%, from a threefold difference in 1995 to a difference of more than fivefold in 2008 (TABLE 1).1,4
These data indicate that we are doing well providing contraception to women of means. However, as a society, we need to improve how we deliver contraceptives—especially highly effective methods—to poor women. As one might expect, given these numbers, low-income women have rates of abortion and unplanned birth that are nearly 6 times higher than their higher-income counterparts.1 The resultant cost to society is substantial, with 68% of unplanned births paid for by public insurance programs such as Medicaid, compared with 38% of planned births.5
As women’s health providers, we must work to improve these numbers and advocate for our patients to help them gain access to the contraceptives they need in accordance with their reproductive life plan. In this article, we hope to put this information in context as we:
- review reports and studies that evaluatethe trend of unintended pregnancy
- describe one state initiative that reduced the rates of unintended pregnancy, birth, and abortion
- introduce a new, highly effective levonorgestrel-releasing intrauterine system (Liletta) being marketed with the goal of reaching women who receive care from private physicians as well as public health clinics.
National and state snapshots reveal shifting proportions of intended, unintended pregnancies
Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(suppl 1):s43–s48.
Kost K. Unintended pregnancy rates at the state level: estimates for 2010 and trends since 2002. Guttmacher Institute. https://www.guttmacher.org/pubs/StateUP10.pdf. Published January 2015. Accessed June 29, 2015.
Recent studies have demonstrated trends in unplanned pregnancy over the past 2 decades on both a national and statewide level. Data from the National Survey of Family Growth have highlighted trends in abortion and miscarriage, and data from the National Center for Health Statistics have shed light on birth trends. Of 6 million births in 2008, 51% were unintended.1 Unintended pregnancy was defined as a gestation that was mistimed or unwanted. Intended pregnancy was defined as one that was desired at the time it occurred or sooner.
2008 data focus on the national level
Although the overall pregnancy rate for US women aged 15 to 44 years is relatively unchanged, there is a small change in whether or not the pregnancy was intended. For 2008, as the rate of intended pregnancy dropped slightly, from 54 to 51 pregnancies per 1,000 women, the unintended pregnancy rate increased by 10%, from 49 to 54 pregnancies per 1,000 women. Proportionally, unintended pregnancies that resulted in abortion declined from 47% to 40%, whereas the rate of unintended pregnancies ending in birth increased to 27 births per 1,000 women.1
2010 data focus on individual states
We now have state-specific data on trends in unintended pregnancy rates from 2002 to 2010 in the United States.6 In 28 of 50 states, more than half of all pregnancies were unintended, with rates ranging from 36% to 62%. The median rate was 47 unintended pregnancies per 1,000 women aged 15 to 44, with the lowest unintended pregnancy rate in New Hampshire at 32 per 1,000 women and highest rates in Delaware, Hawaii, and New York at 61 to 62 unintended pregnancies per 1,000 women.6
Between 2002 and 2010, unintended pregnancy rates fell 5% or more in 18 states and rose 5% or more in 4 states. In the remaining 12 states for which there are data, unintended pregnancy rates remained unchanged.
Interestingly, 16 states had increases in unintended pregnancy rates of 5% or more between 2002 and 2006. The trend reversed between 2006 and 2010, during which 28 of 41 states with available data experienced decreases of 5% or more and only 1 state experienced an increase of 5% or more. These latest numbers suggest we may be making some progress in reducing the overall rate of unintended pregnancy.
What this EVIDENCE means for practice
Although the rate of unintended pregnancy is declining in some states, the national rate is still increasing. This information emphasizes the need for all providers to consider initiating discussions about pregnancy intentions—a step that may be as important as obtaining blood pressure and weight. When women are seen for any health visit, they should be asked about their reproductive plans. The Centers for Disease Control and Prevention (CDC) has issued a helpful set of questions to guide the discussion of timing and planning pregnancy. It also provides useful information on ways to increase utilization of long-acting reversible contraceptives (LARCs) to help realize the goal of fewer unintended pregnancies. (For more on this discussion, see “How to motivate your patient to create a reproductive life plan,” below.)
How to motivate your patient to create a reproductive life plan
The Centers for Disease Control and Prevention (CDC) offers a tool for health care professionals to use to encourage patients to think about their reproductive goals and make a plan to facilitate those goals. It’s available at: http://www.cdc.gov/preconception/documents/rlphealthproviders.pdf.
Questions to ask your patient
- Do you plan to have any (more) children at any time in your future?
IF YES
- How many children would you like to have?
- How long would you like to wait until you or your partner become pregnant?
- What family planning method do you plan to use until you or your partner are ready to become pregnant?
- How sure are you that you will be able to use this method without any problems?
IF NO
- What family planning method will you use to avoid pregnancy?
- How sure are you that you will be able to use this method without any problems?
- People’s plans change. Is it possible that you or your partner could ever decide to become pregnant?
Action plan
Encourage your patient to make a plan and take action. Remind her that the plan doesn’t have to be set in stone.
How Colorado broke down barriers to highly effective contraception—and saved $42.5 million in 1 year
Ricketts S, Klinger G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline of births among young, low-income women. Perspect Sexual Reprod Health. 2014;46(3):125–132.
The Contraceptive CHOICE Project in St. Louis County, Missouri, is an incredible success story. In it, clinicians made highly effective LARCs readily available and free of charge. When a large percentage of women chose a LARC method, the unintended pregnancy and abortion rates declined.7 In Colorado, providers put this study into practice, creating a successful statewide initiative to reduce the unintended pregnancy rate.8
The data behind LARC methods
LARC methods are backed by endorsements from the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the CDC, and the World Health Organization, which recommend them for adolescents because of their superiority to shorter-acting methods. With LARCs, failure rates are lower and compliance is greater, making them ideal for adolescents, who have high unintended pregnancy rates.1
However, based on 2011 data, only 2% of LARC users nationwide are aged 20 or younger. A number of barriers prevent young women from obtaining LARCs, including lack of education, limited access to and availability of the contraceptives, and, importantly, cost. Many state plans have adopted Medicaid expansions to reduce barriers to LARCs. However, this benefit is still not available in many states, Colorado being 1 of them.
How the Colorado initiative worked
In 2005, 40% of Colorado’s births were unintended, and 60% of those unintended births occurred in women aged 15 to 24 years. About three-quarters of women who were using a contraceptive method at the time of unintended pregnancy reported that it was a low-cost, high-failure method such as condoms or withdrawal.
In response, Colorado’s Department of Public Health and Environment and the Colorado Family Planning Initiative (CFPI) used private funds from an anonymous foundation to provide LARC products at no cost to the Title X–funded clinics in the state.
The initiative began in 2009 in clinics that served 95% of the state’s total population. The funding provided the products themselves (intrauterine device [IUD], implant), as well as training for providers and staff.
Before the initiative began, 52,645 clients received services in these clinics annually. In the third year of the initiative, that number had increased to 64,928 annually. About 55% of clients receiving services both preinitiative and postinitiative were younger than 25 years, and most (92% in 2011) had income below the poverty level.
LARC use increased fourfold over the 3 years of the funded program, from less than 4.5% to 19.4% in the third year. Contraceptive implant use increased tenfold, and IUD use increased by almost threefold. At the same time, oral contraceptive use declined 13%. Before the initiative, only 620 young, low-income women used a LARC method; afterward, 8,435 did.
These changes in contraceptive practice triggered a significant decline in pregnancy rates (TABLE 2) and abortion rates (TABLE 3). Abortion rates increased 8% among 20- to 24-year-olds who were not enrolled in the initiative and decreased 18% among those who were. The proportion of high-risk births (births to unmarried, low-income women with less than a high school education) dropped 24% after the initiative began. The proportion of high-risk births in counties not receiving CFPI funds stayed the same at 7%.
Colorado program saved $42.5 million in public funds
This Colorado program demonstrated that the CHOICE Project can be translated to a statewide initiative. Whereas CHOICE enrolled 9,256 women over 4 years, the Colorado initiative included more than 50,000 clients annually over 3 years. Colorado did not use any state funds for this project, which resulted in significant decreases in the unintended birth rate, abortion rate, and rate of high-risk births.
The Colorado governor’s office estimates that the CFPI saved $5.68 in Medicaid costs for Colorado for every dollar spent on contraceptives. In just 1 year (2010), the program saved approximately $42.5 million in public funds.
Ironically, despite the success of this project, the Colorado legislature denied further funding once the initial financial support ceased.
What this EVIDENCE means for practice
The Colorado program demonstrates that we all can provide LARC methods in practice, especially to young women. In this population, use of highly effective contraception resulted in fewer unintended pregnancies, births, and abortions statewide.
We also need to advocate for our patients, particularly those who have less means and rely on public assistance. Public funding of LARC methods clearly improves outcomes at an individual and population level.
A new, more affordable IUD enters the market
Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
Programs such as the Contraceptive CHOICE Project and the Colorado Family Planning Initiative relied on private foundations for financial support, largely because of the high cost of the IUDs and implants currently available in the United States. Even with the Affordable Care Act (ACA) reducing the costs of LARC products and other contraceptives for patients, there are still many women not covered by these programs. For example, “grandfathered” health insurance plans do not need to follow some aspects of the ACA.
Just as important, the high cost of LARC products takes a toll on providers and clinics that must finance the cost per unit to have stock on hand and then wait for months for reimbursement by insurance companies. As a result, some providers do not stock IUDs and implants and only order them as they are needed and approved by insurance for a particular patient. These barriers limit access to LARC methods and reduce the number of women who receive the products.8
Liletta is less expensive than other IUDs
Enter Liletta, a new levonorgestrel-releasing, 52-mg intrauterine system (IUS) that has been in clinical trials since 2009.9 ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an Intrauterine System) was initiated by Medicines360, a unique nonprofit pharmaceutical company committed to ensuring access to reproductive health products for all women (private and public sector).
ACCESS IUS is the largest IUD approval study ever performed exclusively in the United States. The Phase 3, open-label clinical trial was conducted at 29 sites around the United States, enrolling healthy, nonpregnant, sexually active women aged 16 to 45 years with regular menstrual cycles. Both nulliparous and parous women were included, with no weight or body mass index (BMI) restrictions applied. The study is ongoing and will continue for as long as 7 years. Eisenberg and colleagues published the data used for initial approval for 3 years of use in the United States and Europe.9
Details of the trial
A total of 1,600 women aged 16 to 35 years comprised the group in which efficacy was evaluated. An additional 151 women aged 36 to 45 years were evaluated for safety only. Of the enrolled women, 1,011 (58%) were nulliparous, making ACCESS IUS the largest product approval study of nulliparous women. In addition, 438 women (25.1%) were obese, and 5% of these women had a BMI greater than 40 kg/m2.
Liletta was placed successfully in 1,714 women (97.9%). Fifteen women did not have placement attempted due to uterine factors (the uterus could not be sounded, or the sound was <5.5 cm) or factors unrelated to the product or inserter. In women in whom placement was attempted, the success rate was 98.7%.
The first-year Pearl index for Liletta was 0.15. Life-table pregnancy rates were 0.14 through year 1 and 0.55 through year 3. Four of the 6 pregnancies reported through 3 years of use were ectopic. Adverse events and their incidence, occurring in more than 2% of users, were acne (6%), expulsion (3.5%), dyspareunia (2.8%), and mastalgia (2.0%). The most common adverse events leading to discontinuation were expulsion (3.5%), bleeding complaints (1.5%), acne (1.3%), and mood swings (1.3%).
Uterine perforation with Liletta was diagnosed in 2 participants (0.11%). Expulsion occurred in 62 users (3.5%) and was more frequent among parous than nulliparous women (5.6% vs 2.0%, respectively; P<.001). Most (80.6%) of the expulsions were reported in the first year of product use. Pelvic infection was reported in 10 participants (0.6%), and all cases resolved with outpatient antibiotic treatment.9
Keep in mind that this is an ongoing study—not all women have reached a full 3 years of use. Updates on efficacy and adverse events will be published in the future. This current publication demonstrates the high efficacy and safety of the product through 3 years of use, permitting its approval for contraception in the United States.
In Europe, the product is approved for both contraception and the treatment of heavy menstrual bleeding, based on a European study.10
What this EVIDENCE means for practice
Liletta is a branded product (not generic) designed to be similar to Mirena, with the same size, frame, hormone content, and hormone release rate.11 Medicines360 has entered a groundbreaking marketing partnership with Actavis to make Liletta widely available and affordable. For most public sector providers and clinics in the United States, Liletta costs only $50, significantly less than other LARC methods available in the United States. Actavis also has a program that ensures that any woman lacking insurance coverage for an IUD and not receiving care at a public sector clinic will not be charged more than $75 for her IUD. However, the price of the device is only one aspect of its overall cost, as women still need to pay for any office visit or insertion fees.
For society, this unique business partnership has to include providers and patients as well. Sales of Liletta in the private sector will support the very low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we can all help poor women access very affordable highly effective contraception.
For providers, Liletta is a lower-cost alternative to currently available hormonal IUDs and should perform well over the long term. The highly successful use of Liletta in nulliparous women demonstrates its safety in this population. The 3-year approval is the first step, as the Phase 3 study continues. In the future, Liletta is expected to be approved for 5 years or longer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(suppl 1):s43–s48.
2. Guttmacher Institute. Fact Sheet: Unintended Pregnancy in the United States. http://www.guttmacher.org/pubs/FB-Unintended-Pregnancy-US.html. Published February 2015. Accessed June 29, 2015.
3. Singh S, Sedgh G, Hussain R. Unintended pregnancy: worldwide levels, trends and outcomes. Stud Fam Plann. 2010;41(4):241–250.
4. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspectives. 1998;30(1):24–29, 46.
5. Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care. National and States Estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed June 29, 2015.
6. Kost K. Unintended pregnancy rates at the state level: estimates for 2010 and trends since 2002. Guttmacher Institute. http://www.guttmacher.org/pubs/StateUP10.pdf. Published January 2015. Accessed June 30, 2015.
7. Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Preventing unintended pregnancy: the ContraceptiveCHOICE Project in review. J Womens Health. 2015; 24(5):349–353.
8. Ricketts S, Klinger G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline of births among young, low-income women. Perspect Sexual Reprod Health. 2014;46(3):125–132.
9. Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
10. Mawet M, Nollevaux F, Nizet D, et al. Impact of a new levonorgestrel intrauterine system, Levosert, on heavy menstrual bleeding: results of a one-year randomised controlled trial. Eur J Contracept Reprod Health Care. 2014;19(3):169–179.
11. Gopalakrishnan M, Liu T, Gobburu J, Creinin MD. Levonorgestrel release rates with LNG20, a new levonorgestrel intrauterine system. Obstet Gynecol. 2015;125(suppl 1): 62S–63S.
1. Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(suppl 1):s43–s48.
2. Guttmacher Institute. Fact Sheet: Unintended Pregnancy in the United States. http://www.guttmacher.org/pubs/FB-Unintended-Pregnancy-US.html. Published February 2015. Accessed June 29, 2015.
3. Singh S, Sedgh G, Hussain R. Unintended pregnancy: worldwide levels, trends and outcomes. Stud Fam Plann. 2010;41(4):241–250.
4. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspectives. 1998;30(1):24–29, 46.
5. Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care. National and States Estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed June 29, 2015.
6. Kost K. Unintended pregnancy rates at the state level: estimates for 2010 and trends since 2002. Guttmacher Institute. http://www.guttmacher.org/pubs/StateUP10.pdf. Published January 2015. Accessed June 30, 2015.
7. Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Preventing unintended pregnancy: the ContraceptiveCHOICE Project in review. J Womens Health. 2015; 24(5):349–353.
8. Ricketts S, Klinger G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline of births among young, low-income women. Perspect Sexual Reprod Health. 2014;46(3):125–132.
9. Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
10. Mawet M, Nollevaux F, Nizet D, et al. Impact of a new levonorgestrel intrauterine system, Levosert, on heavy menstrual bleeding: results of a one-year randomised controlled trial. Eur J Contracept Reprod Health Care. 2014;19(3):169–179.
11. Gopalakrishnan M, Liu T, Gobburu J, Creinin MD. Levonorgestrel release rates with LNG20, a new levonorgestrel intrauterine system. Obstet Gynecol. 2015;125(suppl 1): 62S–63S.
In this Article
- How Colorado broke down barriers to highly effective contraception
- How to motivate your patient to create a reproductive life plan
- A more affordable IUD enters the market
Intragestational injection of methotrexate
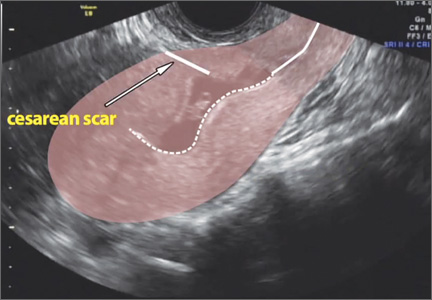
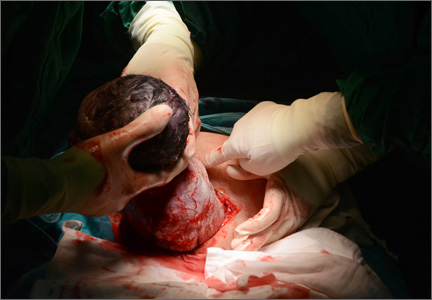
The presentation of a cesarean scar ectopic pregnancy can at times be daunting, especially without familiarity regarding its management. Women with cesarean scar ectopic pregnancy most often have no symptoms, although vaginal bleeding and abdominal pain can present. Upon visual diagnosis with transabdominal or transvaginal ultrasound, the preferred treatment method is direct injection of methotrexate into the gestational sac within the cesarean scar.
In this video, my colleagues review the indications and contraindications for direct injection of methotrexate as well as alternative treatment methods for this type of nonviable pregnancy that is increasing in frequency (given the US cesarean delivery rate). Demonstrated is the technique for methotrexate injection in the case of a 34-year-old woman (G6P0232) with ultrasound and beta−human chorionic gonadotropin confirmation of cesarean scar ectopic pregnancy.
We hope this video serves as a useful reference in your practice.

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The presentation of a cesarean scar ectopic pregnancy can at times be daunting, especially without familiarity regarding its management. Women with cesarean scar ectopic pregnancy most often have no symptoms, although vaginal bleeding and abdominal pain can present. Upon visual diagnosis with transabdominal or transvaginal ultrasound, the preferred treatment method is direct injection of methotrexate into the gestational sac within the cesarean scar.
In this video, my colleagues review the indications and contraindications for direct injection of methotrexate as well as alternative treatment methods for this type of nonviable pregnancy that is increasing in frequency (given the US cesarean delivery rate). Demonstrated is the technique for methotrexate injection in the case of a 34-year-old woman (G6P0232) with ultrasound and beta−human chorionic gonadotropin confirmation of cesarean scar ectopic pregnancy.
We hope this video serves as a useful reference in your practice.

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The presentation of a cesarean scar ectopic pregnancy can at times be daunting, especially without familiarity regarding its management. Women with cesarean scar ectopic pregnancy most often have no symptoms, although vaginal bleeding and abdominal pain can present. Upon visual diagnosis with transabdominal or transvaginal ultrasound, the preferred treatment method is direct injection of methotrexate into the gestational sac within the cesarean scar.
In this video, my colleagues review the indications and contraindications for direct injection of methotrexate as well as alternative treatment methods for this type of nonviable pregnancy that is increasing in frequency (given the US cesarean delivery rate). Demonstrated is the technique for methotrexate injection in the case of a 34-year-old woman (G6P0232) with ultrasound and beta−human chorionic gonadotropin confirmation of cesarean scar ectopic pregnancy.
We hope this video serves as a useful reference in your practice.

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Does labor induction (vs expectant management) increase the risk of failed TOLAC?
Past research into outcomes for induction of labor for women attempting trial of labor after cesarean (TOLAC) has compared labor induction with spontaneous labor. This comparison may be biased against induction, say Lappen and colleagues, who conducted this recent study with the goal of characterizing the likelihood of failed TOLAC with induction and assessing maternal and neonatal outcomes of induction, compared with expectant management, by week of gestation (between 37 and 40 completed weeks).
Details of the study
The researchers analyzed data from the Consortium on Safe Labor,1 excluding women who had:
- no or more than 1 prior cesarean delivery
- multiple gestations
- fetal anomalies
- preterm delivery
- unknown labor type
- repeat cesarean delivery without a trial of labor (including those for whom TOLAC was contraindicated).
Their final, primary cohort included 6,033 women undergoing TOLAC (1,626 underwent induction of labor; 4,407 did not). For this group, induction of labor was defined to include all medically indicated and elective inductions.
They also analyzed a secondary cohort, for which they redefined the induction group to only include those inductions that were nonmedically indicated. This was a “low risk” cohort (n = 500) that excluded women with chronic conditions (hypertension, gestational diabetes, etc) that could result in medically indicated induction.
Induction of labor still associated with failed TOLAC
Comparing induction of labor with expectant management, the frequency of failed TOLAC was higher at each week of gestation, but not at 40 weeks. The adjusted odds ratios were:
- 37 weeks: 1.53 (95% confidence interval [CI], 1.02−2.28)
- 38 weeks: 1.74 (95% CI, 1.29−2.34)
- 39 weeks: 2.16 (95% CI, 1.76−2.67)
- 40 weeks: 1.21 (95% CI, 0.9−1.66).
Induction was associated with an increased risk of composite maternal morbidity at 39 weeks’ gestation. The authors attributed this to a statistically significant increase in the risk of transfusion. Induction was not associated with increased neonatal morbidity.
The authors point out that, since their data set collection, ACOG recommended against nonmedically indicated inductions before 39 weeks’ gestation, but argue that their results remain generalizable and clinically pertinent because medically indicated early-term inductions remain common.
What this evidence means for practice
The authors identified a significant increase in risk of failed TOLAC with induction of labor. These findings are consistent with prior work describing the favorable relationship between TOLAC success and spontaneous labor and thus should not alter current obstetric practice. The study authors used a large, reliable database for the analysis and controlled for maternal age, body mass index, and history of any prior vaginal birth. However, as the authors point out, the study was limited by a lack of data on obstetric factors that have been identified in prior studies to be pertinent to the likelihood of success of TOLAC, such as Bishop score, indication for prior cesarean delivery, and history of any successful vaginal birth after cesarean. Clinicians should consider each patient’s predictors for successful TOLAC individually and provide appropriate counseling. An induction of labor remains appropriate in well-selected patients attempting TOLAC.
— Janine S. Rhoades, MD, and Alison G. Cahill, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
1. Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–1287.
Past research into outcomes for induction of labor for women attempting trial of labor after cesarean (TOLAC) has compared labor induction with spontaneous labor. This comparison may be biased against induction, say Lappen and colleagues, who conducted this recent study with the goal of characterizing the likelihood of failed TOLAC with induction and assessing maternal and neonatal outcomes of induction, compared with expectant management, by week of gestation (between 37 and 40 completed weeks).
Details of the study
The researchers analyzed data from the Consortium on Safe Labor,1 excluding women who had:
- no or more than 1 prior cesarean delivery
- multiple gestations
- fetal anomalies
- preterm delivery
- unknown labor type
- repeat cesarean delivery without a trial of labor (including those for whom TOLAC was contraindicated).
Their final, primary cohort included 6,033 women undergoing TOLAC (1,626 underwent induction of labor; 4,407 did not). For this group, induction of labor was defined to include all medically indicated and elective inductions.
They also analyzed a secondary cohort, for which they redefined the induction group to only include those inductions that were nonmedically indicated. This was a “low risk” cohort (n = 500) that excluded women with chronic conditions (hypertension, gestational diabetes, etc) that could result in medically indicated induction.
Induction of labor still associated with failed TOLAC
Comparing induction of labor with expectant management, the frequency of failed TOLAC was higher at each week of gestation, but not at 40 weeks. The adjusted odds ratios were:
- 37 weeks: 1.53 (95% confidence interval [CI], 1.02−2.28)
- 38 weeks: 1.74 (95% CI, 1.29−2.34)
- 39 weeks: 2.16 (95% CI, 1.76−2.67)
- 40 weeks: 1.21 (95% CI, 0.9−1.66).
Induction was associated with an increased risk of composite maternal morbidity at 39 weeks’ gestation. The authors attributed this to a statistically significant increase in the risk of transfusion. Induction was not associated with increased neonatal morbidity.
The authors point out that, since their data set collection, ACOG recommended against nonmedically indicated inductions before 39 weeks’ gestation, but argue that their results remain generalizable and clinically pertinent because medically indicated early-term inductions remain common.
What this evidence means for practice
The authors identified a significant increase in risk of failed TOLAC with induction of labor. These findings are consistent with prior work describing the favorable relationship between TOLAC success and spontaneous labor and thus should not alter current obstetric practice. The study authors used a large, reliable database for the analysis and controlled for maternal age, body mass index, and history of any prior vaginal birth. However, as the authors point out, the study was limited by a lack of data on obstetric factors that have been identified in prior studies to be pertinent to the likelihood of success of TOLAC, such as Bishop score, indication for prior cesarean delivery, and history of any successful vaginal birth after cesarean. Clinicians should consider each patient’s predictors for successful TOLAC individually and provide appropriate counseling. An induction of labor remains appropriate in well-selected patients attempting TOLAC.
— Janine S. Rhoades, MD, and Alison G. Cahill, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Past research into outcomes for induction of labor for women attempting trial of labor after cesarean (TOLAC) has compared labor induction with spontaneous labor. This comparison may be biased against induction, say Lappen and colleagues, who conducted this recent study with the goal of characterizing the likelihood of failed TOLAC with induction and assessing maternal and neonatal outcomes of induction, compared with expectant management, by week of gestation (between 37 and 40 completed weeks).
Details of the study
The researchers analyzed data from the Consortium on Safe Labor,1 excluding women who had:
- no or more than 1 prior cesarean delivery
- multiple gestations
- fetal anomalies
- preterm delivery
- unknown labor type
- repeat cesarean delivery without a trial of labor (including those for whom TOLAC was contraindicated).
Their final, primary cohort included 6,033 women undergoing TOLAC (1,626 underwent induction of labor; 4,407 did not). For this group, induction of labor was defined to include all medically indicated and elective inductions.
They also analyzed a secondary cohort, for which they redefined the induction group to only include those inductions that were nonmedically indicated. This was a “low risk” cohort (n = 500) that excluded women with chronic conditions (hypertension, gestational diabetes, etc) that could result in medically indicated induction.
Induction of labor still associated with failed TOLAC
Comparing induction of labor with expectant management, the frequency of failed TOLAC was higher at each week of gestation, but not at 40 weeks. The adjusted odds ratios were:
- 37 weeks: 1.53 (95% confidence interval [CI], 1.02−2.28)
- 38 weeks: 1.74 (95% CI, 1.29−2.34)
- 39 weeks: 2.16 (95% CI, 1.76−2.67)
- 40 weeks: 1.21 (95% CI, 0.9−1.66).
Induction was associated with an increased risk of composite maternal morbidity at 39 weeks’ gestation. The authors attributed this to a statistically significant increase in the risk of transfusion. Induction was not associated with increased neonatal morbidity.
The authors point out that, since their data set collection, ACOG recommended against nonmedically indicated inductions before 39 weeks’ gestation, but argue that their results remain generalizable and clinically pertinent because medically indicated early-term inductions remain common.
What this evidence means for practice
The authors identified a significant increase in risk of failed TOLAC with induction of labor. These findings are consistent with prior work describing the favorable relationship between TOLAC success and spontaneous labor and thus should not alter current obstetric practice. The study authors used a large, reliable database for the analysis and controlled for maternal age, body mass index, and history of any prior vaginal birth. However, as the authors point out, the study was limited by a lack of data on obstetric factors that have been identified in prior studies to be pertinent to the likelihood of success of TOLAC, such as Bishop score, indication for prior cesarean delivery, and history of any successful vaginal birth after cesarean. Clinicians should consider each patient’s predictors for successful TOLAC individually and provide appropriate counseling. An induction of labor remains appropriate in well-selected patients attempting TOLAC.
— Janine S. Rhoades, MD, and Alison G. Cahill, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
1. Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–1287.
Reference
1. Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–1287.
Do ACOG guidelines protect us from liability?
“THE SGR IS ABOLISHED! WHAT COMES NEXT?”
LUCIA DIVENERE, MA (PRACTICE MANAGEMENT; JUNE 2015)
Do ACOG guidelines protect us from liability?
I read Ms. DiVenere’s June article with interest, but I found this point she quoted confusing: "The law protects physicians from liability from federal or state standards of care. No health care guideline or other standard developed under federal or state requirements associated with this law may be used as a standard of care or duty of care owed by a health care professional to a patient in a medical liability lawsuit."
I have 2 questions: How do you interpret the use of guidelines by the American College of Obstetricians and Gynecologists (ACOG), since they are developed independently by a specialty society rather than by federal or state “requirements”? Does this only pertain to liability lawsuits concerning billing of fees, or does it pertain to medical malpractice civil lawsuits?
In the Medicare Access and CHIP Reauthorization Act, I find this section that seems to contradict the protection1:
What is the bottom line? No law can protect and provide immunity to a physician for true medical malpractice. This federal law says “no preemption.”
Arnold D. Wharton, MD
Tyler, Texas
Reference
1. Pub L No. 114–10. Medicare Access and CHIP Reauthorization Act of 2015. 114th Congress. Title 1—SGR repeal and Medicare Provider Payment Modernization. §106. Reducing administrative burden and other provisions. 129 STAT.143. http://www.gpo.gov/fdsys/pkg/PLAW-114publ10/pdf/PLAW-114publ10.pdf. Accessed June 10, 2015.
Ms. DiVenere responds
I thank Dr. Wharton for his interesting perspective. To answer the first questions, this section of the law only applies to guidelines and standards created by a federal or state entity, not to ACOG guidelines, and is intended to provide one area of protection from medical malpractice lawsuits. Interestingly, legislation has been introduced in the US House by Congressman Andy Barr (R-KY), with ACOG’s support, to create liability safe harbors for physicians who follow care guidelines developed by their relevant specialty society.
As for the question about preemption, this section of the law allows stronger state laws to stand; this federal law would not preempt state laws.
Statute of limitations still in effect; contact your insurer
While the end result to dismiss the patient was achieved, the statute of limitations for a possible malpractice suit had not fully run. I would suggest that the physician contact his/her insurer so that they can open a file and be alerted for a possible suit. Insurers generally require physicians to notify them of any potential suits.
Lynn Frame, MD, JD
Tulsa, Oklahoma
Dr. Sanfilippo and Mr. Smith respond
Our thanks to Dr. Frame for the good reminder that physicians should always remember the obligation to inform malpractice insurance carriers when a malpractice claim is being, or may be, filed. Insurance contracts vary somewhat regarding when notice must be given.
In the hypothetical case, there was an angry patient but no formal threat of legal action. Some lawyers take the sensible position that “when in doubt, notify.” Others are reluctant to “over notify” carriers. Our view is that this is one of the areas in which it may be beneficial for a physician to have an ongoing professional relationship with an attorney to allow for advice on when to provide insurance carrier notification.
Videos show very useful techniques for malpositioned IUDs
I have placed somewhere in the ballpark of 2,000 intrauterine devices (IUDs) and have had 2 perforations that I am aware of (and probably many more malpositioned IUDs that I am unaware of). Some of those were likely the cause of a patient’s pain and were either removed or hysteroscopically repositioned. Dr. Advincula’s edited video from several cases demonstrates very useful techniques in the surgical management of these problems.
Philip Ivey, MD
Casa Grande, Arizona
The IUD might not stay where I put it
For the past several years I have performed the majority (more than 95%) of IUD insertions with ultrasound guidance and have been very thankful at times for the assistance of my sonographer. Despite my knowledge of accurate placement, there are still patients who return months or years later with a malpositioned IUD. I have come to realize that the uterus is a dynamic organ—not a piece of concrete. Just because I put the IUD in the right place does not ensure that it will stay there. Fortunately, I have not yet had a perforation into the abdominal cavity.
I really enjoyed the videos and advice, as always!
Elizabeth Street, MD
Marietta, Georgia
Improved care for pregnant women during Ebola crisis
The article on Ebola in pregnancy noted how little we actually know about the Ebola virus. The Ebola virus was first documented in 1976 in Sudan and the Democratic Republic of the Congo,1 not in 1967 as the article stated. The Marburg virus outbreak occurred in 1967. Closely related, both viruses are filo viruses that cause hemorrhagic fever. A significant difference between the 2 is that the natural reservoir for the Marburg virus was identified. The outbreak in Marburg, Germany, which the virus is named for, was linked to African green monkeys imported from Uganda, East Africa.2 Bats also have been identified as a reservoir for the Marburg virus.3 However, there is only speculation as to whether the natural reservoir for the Ebola virus is fruit bats. A 3-month research study following the 1995 outbreak of Ebola virus in Kikwit, Democratic Republic of the Congo, tested more than 3,000 vertebrate species and was still unable to identify a natural carrier for the virus.4
The Ebola virus was first documented nearly 40 years ago and yet we know so little about it. This demonstrates the ongoing disparity in funding and research devoted to disease conditions that most often affect only third-world nations.
Also, I’d like to point out that the article’s comment that pregnant patients are triaged “last” during the current Ebola virus outbreak may not be completely accurate. Yes, pregnant women have a significantly higher rate of mortality with Ebola viral infection. I spoke with a nurse (name and location withheld for confidentiality) who is currently the Clinical Lead at an Ebola Holding Unit for pregnant and lactating women in a West African nation. According to her, improved resources were quickly mobilized by nongovernment organizations and other foreign health care volunteers following the initial reports of disease, a factor that significantly increased access to care for pregnant women and improved outcomes. Erin Kiser, DNP, FNP-BC, WHNP-BC
Fayetteville, North Carolina
References
1. World Health Organization. Ebola virus disease. Fact sheet No. 103. http://www.who.int/mediacentre/factsheets/fs103/en/. Updated April 2015. Accessed July 6, 2015.
2. World Health Organization. Marburg haemorrhagic fever. Fact sheet. http://www.who.int/mediacentre/factsheets/fs_marburg/en/. Published November 2012. Accessed July 8, 2015.
3. Towner JS, Pourrut X, Albariño CG, et al. Marburg virus infection detected in a common African bat. PLoS One. 2007;2(8):e764. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0000764.
4. Leirs H, Mills JN, Krebs JW, et al. Search for the Ebola virus reservoir in Kikwit, Democratic Republic of the Congo: Reflections on a vertebrate collection. J Infect Dis. 1999;179(suppl 1):S155–S163.
Had the chance to change my specialty, but didn’t
I trained in Mexico, where I was a board certified ObGyn and a maternal-fetal medicine specialist. When I came to the United States I had the opportunity to change my specialty, and I didn’t. As a “free agent” international medical graduate, I had to go through many hurdles. My gate to enter the American medical world was through a family practice residency. After a year, I realized my love was still obstetrics and gynecology. In 1996, I finished an ObGyn residency at Loma Linda University Medical Center in California, and have been board certified since 1998.
There are many things I like about this specialty. Mainly, it’s the diversity. A well-rounded ObGyn has to know internal medicine, pediatrics, and surgery and apply this knowledge to the pregnant patient—a feat somehow exclusive to ObGyns.
I have enjoyed a wonderful career and many rewards. I never stop thanking all those professors and colleagues who helped me develop the set of skills that I now possess.
Tomas A. Hernandez, MD
Pasco, Washington
Not again!
I would not go into obstetrics and gynecology again because of many reasons:
- It is a very difficult life, with no family time and calls 24 hours per day.
- The specialty is the bread and butter of malpractice attorneys, causing a lot of stress.
- Insurance companies, health maintenance organizations (HMOs), etc, pay ridiculously low reimbursement for obstetric and gynecologic procedures.
- Malpractice insurance premiums are so high that you can be forced to be without malpractice and therefore more exposed.
- Patients are extremely demanding. Because pregnancy is not a disease but a natural process, they expect perfect results every time (as if congenital malformations, chromosomal abnormalities, and pregnancy complications are your fault).
- There is no patient loyalty, or very little. If a patient changes HMOs she changes obstetricians. If a woman has to wait 20 minutes in the waiting room, she changes doctors—to one who doesn’t do obstetrics (too many pregnant women!).
I would like to say that ObGyn is a beautiful specialty, most likely the best of all medical specialties, if it was not for the attorneys’ greed and patients’ lack of understanding that we are not God. We are only doctors, working within a system that contributes to all of the above.
Manuel S. Mendizabal, MD
Miami, Florida
Are men discouraged from entering the ObGyn field?
Dr. Barbieri asks, “Why is obstetrics and gynecology a popular choice for medical students?” The unaddressed question is why is it unpopular for half of medical students? Ninety-three percent of resident graduates in the field are women, while women account for half of medical student graduates. Men rarely go into the specialty today. Perhaps job advertisements touting physician opportunities in “all female groups” discourage males. Perhaps hospitals’ “women’s health centers,” with “women taking care of women,” discourage males. Perhaps receptionists’ asking patients whether they prefer a male or female physician discourages male ObGyns. In the United States, two-thirds of outpatient office visits are made by women, and academic centers and hospitals focus on this demographic in their marketing. The business ends justify the unethical means.
The result of discouraging half your medical students from the field is a lower quality field. If male and female medical students are equally qualified for any field, and I believe this is true, then discouraging half the candidates from a field lowers the quality of the resulting field. This has been the product of all discrimination throughout the ages.
Joe Walsh, MD
Philadelphia, Pennsylvania
Dr. Barbieri responds
Drs. Hernandez and Mendizabal provide 2 divergent perspectives on our field. Dr. Hernandez cherishes the diversity of the clinical work in the field, and Dr. Mendizabal warns that night call and medical malpractice take a toll on a physician. Both perspectives are valid and important, and medical students entering the field should be alerted to these rewards and challenges.
I agree with Dr. Walsh that the majority of residents in obstetrics and gynecology are women. On December 31, 2013, of the 4,942 residents in obstetrics and gynecology in the United States, 82.5% were women.1 In the fields of orthopedic surgery, neurosurgery, and urology, male residents dominate the resident complement, constituting 86.3%, 84.1%, and 77.3% of the residents, respectively.1 It is interesting that the fields of obstetrics and gynecology, orthopedic surgery, neurosurgery, and urology are among the most competitive fields in the resident match. Based on personal observation, medical student clerkship directors and obstetrics and gynecology residency programs encourage both women and men to consider a career in obstetrics and gynecology and warmly welcome male applicants. Medical students select their preferred future specialty based on many factors. It is clear that in the past few years the medical students applying to obstetrics and gynecology are extremely capable, and I am confident that the future of women’s health is in the hands ofexcellent clinicians.
Reference
1. Brotherton SE, Etzel SI. Graduate medical education, 2013–2014. JAMA. 2014;312(22):2427–2445.
Who will teach this dying art to a new generation?
The article on rotational forceps has what I consider one glaring defect—who will teach this dying art to a new generation?
Now retired, I was military-residency trained in the 1970s when you had to do your own regional and conduction anesthesia as well as operative forceps delivery—and that did not mean a silastic cup vacuum extractor, though we had just started using the Malstrom vacuum. Breech forceps, Kielland rotations, occipito-transverse forceps application—you name it and we did it as we had to keep our cesarean delivery rate down. All of us were well skilled in operative vaginal delivery.
When I stopped practicing obstetrics, the fresh-out-of-residency people coming into our practice couldn’t do a low forceps delivery. If there is to be a reteaching of rotational forceps, they’d better catch us old codgers fast before we die off (I am 72) and grant us malpractice relief (I no longer have insurance). This is an art, not a science, and can’t be taught from a book or a computer model. Set up a crash course to teach this dying art, pay us well, and perhaps we will be able to pass this skill along. Otherwise it will be gone forever.
I have always said that forceps are like a shoehorn—used correctly, they make things so much easier.
Robert Frischer, MD
Wichita Falls, Texas
Why I now recommend 3D ultrasonography to my high-risk patients
In 2012, I attended a medical staff meeting where Dr. Ruby Chang spoke about a newly available modality at our hospital: 3D ultrasonography. Her slideshow included some impressive images of cancers that were not seen on mammogram but were unmistakable on sonography.
I decided to have a 3D ultrasound for myself in order to tell my patients what it was like. I also have “heterogeneously dense breasts” on mammogram. For the previous 10 years, my annual screening mammograms had all been negative. The 3D ultrasound showed an 8-mm cancer in my left breast—not palpable to me. A subsequent mammogram was still negative for cancer.
Luckily, the breast cancer was Stage 1 at surgery, and I did not need chemotherapy or radiation, opting for skin- and nipple-sparing double mastectomy. I had a double mastectomy because I believed that I could no longer trust screening mammography for a timely diagnosis.
To this day, I explain breast density to all of my higher-risk patients who have either heterogeneously or extremely dense breasts. I tell them that their mammograms may miss a cancer and that there is another test that might help detect cancer early. It’s a good thing to have another way to evaluate the breast, especially when our patients are being sent letters about their “dense breasts.” (The majority of my patients do not understand what this means.)
I realize that data may show that this modality isn’t the perfect solution and may lead to more testing and procedures, but in my case, it was worth it!
Strangely, to this day, I have not had one patient who had breast cancer diagnosed in this way.
It’s a shame that insurance companies don’t cover even partial cost for eligible patients.
Bettina Zatuchni, MD
Pleasanton, California
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“THE SGR IS ABOLISHED! WHAT COMES NEXT?”
LUCIA DIVENERE, MA (PRACTICE MANAGEMENT; JUNE 2015)
Do ACOG guidelines protect us from liability?
I read Ms. DiVenere’s June article with interest, but I found this point she quoted confusing: "The law protects physicians from liability from federal or state standards of care. No health care guideline or other standard developed under federal or state requirements associated with this law may be used as a standard of care or duty of care owed by a health care professional to a patient in a medical liability lawsuit."
I have 2 questions: How do you interpret the use of guidelines by the American College of Obstetricians and Gynecologists (ACOG), since they are developed independently by a specialty society rather than by federal or state “requirements”? Does this only pertain to liability lawsuits concerning billing of fees, or does it pertain to medical malpractice civil lawsuits?
In the Medicare Access and CHIP Reauthorization Act, I find this section that seems to contradict the protection1:
What is the bottom line? No law can protect and provide immunity to a physician for true medical malpractice. This federal law says “no preemption.”
Arnold D. Wharton, MD
Tyler, Texas
Reference
1. Pub L No. 114–10. Medicare Access and CHIP Reauthorization Act of 2015. 114th Congress. Title 1—SGR repeal and Medicare Provider Payment Modernization. §106. Reducing administrative burden and other provisions. 129 STAT.143. http://www.gpo.gov/fdsys/pkg/PLAW-114publ10/pdf/PLAW-114publ10.pdf. Accessed June 10, 2015.
Ms. DiVenere responds
I thank Dr. Wharton for his interesting perspective. To answer the first questions, this section of the law only applies to guidelines and standards created by a federal or state entity, not to ACOG guidelines, and is intended to provide one area of protection from medical malpractice lawsuits. Interestingly, legislation has been introduced in the US House by Congressman Andy Barr (R-KY), with ACOG’s support, to create liability safe harbors for physicians who follow care guidelines developed by their relevant specialty society.
As for the question about preemption, this section of the law allows stronger state laws to stand; this federal law would not preempt state laws.
Statute of limitations still in effect; contact your insurer
While the end result to dismiss the patient was achieved, the statute of limitations for a possible malpractice suit had not fully run. I would suggest that the physician contact his/her insurer so that they can open a file and be alerted for a possible suit. Insurers generally require physicians to notify them of any potential suits.
Lynn Frame, MD, JD
Tulsa, Oklahoma
Dr. Sanfilippo and Mr. Smith respond
Our thanks to Dr. Frame for the good reminder that physicians should always remember the obligation to inform malpractice insurance carriers when a malpractice claim is being, or may be, filed. Insurance contracts vary somewhat regarding when notice must be given.
In the hypothetical case, there was an angry patient but no formal threat of legal action. Some lawyers take the sensible position that “when in doubt, notify.” Others are reluctant to “over notify” carriers. Our view is that this is one of the areas in which it may be beneficial for a physician to have an ongoing professional relationship with an attorney to allow for advice on when to provide insurance carrier notification.
Videos show very useful techniques for malpositioned IUDs
I have placed somewhere in the ballpark of 2,000 intrauterine devices (IUDs) and have had 2 perforations that I am aware of (and probably many more malpositioned IUDs that I am unaware of). Some of those were likely the cause of a patient’s pain and were either removed or hysteroscopically repositioned. Dr. Advincula’s edited video from several cases demonstrates very useful techniques in the surgical management of these problems.
Philip Ivey, MD
Casa Grande, Arizona
The IUD might not stay where I put it
For the past several years I have performed the majority (more than 95%) of IUD insertions with ultrasound guidance and have been very thankful at times for the assistance of my sonographer. Despite my knowledge of accurate placement, there are still patients who return months or years later with a malpositioned IUD. I have come to realize that the uterus is a dynamic organ—not a piece of concrete. Just because I put the IUD in the right place does not ensure that it will stay there. Fortunately, I have not yet had a perforation into the abdominal cavity.
I really enjoyed the videos and advice, as always!
Elizabeth Street, MD
Marietta, Georgia
Improved care for pregnant women during Ebola crisis
The article on Ebola in pregnancy noted how little we actually know about the Ebola virus. The Ebola virus was first documented in 1976 in Sudan and the Democratic Republic of the Congo,1 not in 1967 as the article stated. The Marburg virus outbreak occurred in 1967. Closely related, both viruses are filo viruses that cause hemorrhagic fever. A significant difference between the 2 is that the natural reservoir for the Marburg virus was identified. The outbreak in Marburg, Germany, which the virus is named for, was linked to African green monkeys imported from Uganda, East Africa.2 Bats also have been identified as a reservoir for the Marburg virus.3 However, there is only speculation as to whether the natural reservoir for the Ebola virus is fruit bats. A 3-month research study following the 1995 outbreak of Ebola virus in Kikwit, Democratic Republic of the Congo, tested more than 3,000 vertebrate species and was still unable to identify a natural carrier for the virus.4
The Ebola virus was first documented nearly 40 years ago and yet we know so little about it. This demonstrates the ongoing disparity in funding and research devoted to disease conditions that most often affect only third-world nations.
Also, I’d like to point out that the article’s comment that pregnant patients are triaged “last” during the current Ebola virus outbreak may not be completely accurate. Yes, pregnant women have a significantly higher rate of mortality with Ebola viral infection. I spoke with a nurse (name and location withheld for confidentiality) who is currently the Clinical Lead at an Ebola Holding Unit for pregnant and lactating women in a West African nation. According to her, improved resources were quickly mobilized by nongovernment organizations and other foreign health care volunteers following the initial reports of disease, a factor that significantly increased access to care for pregnant women and improved outcomes. Erin Kiser, DNP, FNP-BC, WHNP-BC
Fayetteville, North Carolina
References
1. World Health Organization. Ebola virus disease. Fact sheet No. 103. http://www.who.int/mediacentre/factsheets/fs103/en/. Updated April 2015. Accessed July 6, 2015.
2. World Health Organization. Marburg haemorrhagic fever. Fact sheet. http://www.who.int/mediacentre/factsheets/fs_marburg/en/. Published November 2012. Accessed July 8, 2015.
3. Towner JS, Pourrut X, Albariño CG, et al. Marburg virus infection detected in a common African bat. PLoS One. 2007;2(8):e764. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0000764.
4. Leirs H, Mills JN, Krebs JW, et al. Search for the Ebola virus reservoir in Kikwit, Democratic Republic of the Congo: Reflections on a vertebrate collection. J Infect Dis. 1999;179(suppl 1):S155–S163.
Had the chance to change my specialty, but didn’t
I trained in Mexico, where I was a board certified ObGyn and a maternal-fetal medicine specialist. When I came to the United States I had the opportunity to change my specialty, and I didn’t. As a “free agent” international medical graduate, I had to go through many hurdles. My gate to enter the American medical world was through a family practice residency. After a year, I realized my love was still obstetrics and gynecology. In 1996, I finished an ObGyn residency at Loma Linda University Medical Center in California, and have been board certified since 1998.
There are many things I like about this specialty. Mainly, it’s the diversity. A well-rounded ObGyn has to know internal medicine, pediatrics, and surgery and apply this knowledge to the pregnant patient—a feat somehow exclusive to ObGyns.
I have enjoyed a wonderful career and many rewards. I never stop thanking all those professors and colleagues who helped me develop the set of skills that I now possess.
Tomas A. Hernandez, MD
Pasco, Washington
Not again!
I would not go into obstetrics and gynecology again because of many reasons:
- It is a very difficult life, with no family time and calls 24 hours per day.
- The specialty is the bread and butter of malpractice attorneys, causing a lot of stress.
- Insurance companies, health maintenance organizations (HMOs), etc, pay ridiculously low reimbursement for obstetric and gynecologic procedures.
- Malpractice insurance premiums are so high that you can be forced to be without malpractice and therefore more exposed.
- Patients are extremely demanding. Because pregnancy is not a disease but a natural process, they expect perfect results every time (as if congenital malformations, chromosomal abnormalities, and pregnancy complications are your fault).
- There is no patient loyalty, or very little. If a patient changes HMOs she changes obstetricians. If a woman has to wait 20 minutes in the waiting room, she changes doctors—to one who doesn’t do obstetrics (too many pregnant women!).
I would like to say that ObGyn is a beautiful specialty, most likely the best of all medical specialties, if it was not for the attorneys’ greed and patients’ lack of understanding that we are not God. We are only doctors, working within a system that contributes to all of the above.
Manuel S. Mendizabal, MD
Miami, Florida
Are men discouraged from entering the ObGyn field?
Dr. Barbieri asks, “Why is obstetrics and gynecology a popular choice for medical students?” The unaddressed question is why is it unpopular for half of medical students? Ninety-three percent of resident graduates in the field are women, while women account for half of medical student graduates. Men rarely go into the specialty today. Perhaps job advertisements touting physician opportunities in “all female groups” discourage males. Perhaps hospitals’ “women’s health centers,” with “women taking care of women,” discourage males. Perhaps receptionists’ asking patients whether they prefer a male or female physician discourages male ObGyns. In the United States, two-thirds of outpatient office visits are made by women, and academic centers and hospitals focus on this demographic in their marketing. The business ends justify the unethical means.
The result of discouraging half your medical students from the field is a lower quality field. If male and female medical students are equally qualified for any field, and I believe this is true, then discouraging half the candidates from a field lowers the quality of the resulting field. This has been the product of all discrimination throughout the ages.
Joe Walsh, MD
Philadelphia, Pennsylvania
Dr. Barbieri responds
Drs. Hernandez and Mendizabal provide 2 divergent perspectives on our field. Dr. Hernandez cherishes the diversity of the clinical work in the field, and Dr. Mendizabal warns that night call and medical malpractice take a toll on a physician. Both perspectives are valid and important, and medical students entering the field should be alerted to these rewards and challenges.
I agree with Dr. Walsh that the majority of residents in obstetrics and gynecology are women. On December 31, 2013, of the 4,942 residents in obstetrics and gynecology in the United States, 82.5% were women.1 In the fields of orthopedic surgery, neurosurgery, and urology, male residents dominate the resident complement, constituting 86.3%, 84.1%, and 77.3% of the residents, respectively.1 It is interesting that the fields of obstetrics and gynecology, orthopedic surgery, neurosurgery, and urology are among the most competitive fields in the resident match. Based on personal observation, medical student clerkship directors and obstetrics and gynecology residency programs encourage both women and men to consider a career in obstetrics and gynecology and warmly welcome male applicants. Medical students select their preferred future specialty based on many factors. It is clear that in the past few years the medical students applying to obstetrics and gynecology are extremely capable, and I am confident that the future of women’s health is in the hands ofexcellent clinicians.
Reference
1. Brotherton SE, Etzel SI. Graduate medical education, 2013–2014. JAMA. 2014;312(22):2427–2445.
Who will teach this dying art to a new generation?
The article on rotational forceps has what I consider one glaring defect—who will teach this dying art to a new generation?
Now retired, I was military-residency trained in the 1970s when you had to do your own regional and conduction anesthesia as well as operative forceps delivery—and that did not mean a silastic cup vacuum extractor, though we had just started using the Malstrom vacuum. Breech forceps, Kielland rotations, occipito-transverse forceps application—you name it and we did it as we had to keep our cesarean delivery rate down. All of us were well skilled in operative vaginal delivery.
When I stopped practicing obstetrics, the fresh-out-of-residency people coming into our practice couldn’t do a low forceps delivery. If there is to be a reteaching of rotational forceps, they’d better catch us old codgers fast before we die off (I am 72) and grant us malpractice relief (I no longer have insurance). This is an art, not a science, and can’t be taught from a book or a computer model. Set up a crash course to teach this dying art, pay us well, and perhaps we will be able to pass this skill along. Otherwise it will be gone forever.
I have always said that forceps are like a shoehorn—used correctly, they make things so much easier.
Robert Frischer, MD
Wichita Falls, Texas
Why I now recommend 3D ultrasonography to my high-risk patients
In 2012, I attended a medical staff meeting where Dr. Ruby Chang spoke about a newly available modality at our hospital: 3D ultrasonography. Her slideshow included some impressive images of cancers that were not seen on mammogram but were unmistakable on sonography.
I decided to have a 3D ultrasound for myself in order to tell my patients what it was like. I also have “heterogeneously dense breasts” on mammogram. For the previous 10 years, my annual screening mammograms had all been negative. The 3D ultrasound showed an 8-mm cancer in my left breast—not palpable to me. A subsequent mammogram was still negative for cancer.
Luckily, the breast cancer was Stage 1 at surgery, and I did not need chemotherapy or radiation, opting for skin- and nipple-sparing double mastectomy. I had a double mastectomy because I believed that I could no longer trust screening mammography for a timely diagnosis.
To this day, I explain breast density to all of my higher-risk patients who have either heterogeneously or extremely dense breasts. I tell them that their mammograms may miss a cancer and that there is another test that might help detect cancer early. It’s a good thing to have another way to evaluate the breast, especially when our patients are being sent letters about their “dense breasts.” (The majority of my patients do not understand what this means.)
I realize that data may show that this modality isn’t the perfect solution and may lead to more testing and procedures, but in my case, it was worth it!
Strangely, to this day, I have not had one patient who had breast cancer diagnosed in this way.
It’s a shame that insurance companies don’t cover even partial cost for eligible patients.
Bettina Zatuchni, MD
Pleasanton, California
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“THE SGR IS ABOLISHED! WHAT COMES NEXT?”
LUCIA DIVENERE, MA (PRACTICE MANAGEMENT; JUNE 2015)
Do ACOG guidelines protect us from liability?
I read Ms. DiVenere’s June article with interest, but I found this point she quoted confusing: "The law protects physicians from liability from federal or state standards of care. No health care guideline or other standard developed under federal or state requirements associated with this law may be used as a standard of care or duty of care owed by a health care professional to a patient in a medical liability lawsuit."
I have 2 questions: How do you interpret the use of guidelines by the American College of Obstetricians and Gynecologists (ACOG), since they are developed independently by a specialty society rather than by federal or state “requirements”? Does this only pertain to liability lawsuits concerning billing of fees, or does it pertain to medical malpractice civil lawsuits?
In the Medicare Access and CHIP Reauthorization Act, I find this section that seems to contradict the protection1:
What is the bottom line? No law can protect and provide immunity to a physician for true medical malpractice. This federal law says “no preemption.”
Arnold D. Wharton, MD
Tyler, Texas
Reference
1. Pub L No. 114–10. Medicare Access and CHIP Reauthorization Act of 2015. 114th Congress. Title 1—SGR repeal and Medicare Provider Payment Modernization. §106. Reducing administrative burden and other provisions. 129 STAT.143. http://www.gpo.gov/fdsys/pkg/PLAW-114publ10/pdf/PLAW-114publ10.pdf. Accessed June 10, 2015.
Ms. DiVenere responds
I thank Dr. Wharton for his interesting perspective. To answer the first questions, this section of the law only applies to guidelines and standards created by a federal or state entity, not to ACOG guidelines, and is intended to provide one area of protection from medical malpractice lawsuits. Interestingly, legislation has been introduced in the US House by Congressman Andy Barr (R-KY), with ACOG’s support, to create liability safe harbors for physicians who follow care guidelines developed by their relevant specialty society.
As for the question about preemption, this section of the law allows stronger state laws to stand; this federal law would not preempt state laws.
Statute of limitations still in effect; contact your insurer
While the end result to dismiss the patient was achieved, the statute of limitations for a possible malpractice suit had not fully run. I would suggest that the physician contact his/her insurer so that they can open a file and be alerted for a possible suit. Insurers generally require physicians to notify them of any potential suits.
Lynn Frame, MD, JD
Tulsa, Oklahoma
Dr. Sanfilippo and Mr. Smith respond
Our thanks to Dr. Frame for the good reminder that physicians should always remember the obligation to inform malpractice insurance carriers when a malpractice claim is being, or may be, filed. Insurance contracts vary somewhat regarding when notice must be given.
In the hypothetical case, there was an angry patient but no formal threat of legal action. Some lawyers take the sensible position that “when in doubt, notify.” Others are reluctant to “over notify” carriers. Our view is that this is one of the areas in which it may be beneficial for a physician to have an ongoing professional relationship with an attorney to allow for advice on when to provide insurance carrier notification.
Videos show very useful techniques for malpositioned IUDs
I have placed somewhere in the ballpark of 2,000 intrauterine devices (IUDs) and have had 2 perforations that I am aware of (and probably many more malpositioned IUDs that I am unaware of). Some of those were likely the cause of a patient’s pain and were either removed or hysteroscopically repositioned. Dr. Advincula’s edited video from several cases demonstrates very useful techniques in the surgical management of these problems.
Philip Ivey, MD
Casa Grande, Arizona
The IUD might not stay where I put it
For the past several years I have performed the majority (more than 95%) of IUD insertions with ultrasound guidance and have been very thankful at times for the assistance of my sonographer. Despite my knowledge of accurate placement, there are still patients who return months or years later with a malpositioned IUD. I have come to realize that the uterus is a dynamic organ—not a piece of concrete. Just because I put the IUD in the right place does not ensure that it will stay there. Fortunately, I have not yet had a perforation into the abdominal cavity.
I really enjoyed the videos and advice, as always!
Elizabeth Street, MD
Marietta, Georgia
Improved care for pregnant women during Ebola crisis
The article on Ebola in pregnancy noted how little we actually know about the Ebola virus. The Ebola virus was first documented in 1976 in Sudan and the Democratic Republic of the Congo,1 not in 1967 as the article stated. The Marburg virus outbreak occurred in 1967. Closely related, both viruses are filo viruses that cause hemorrhagic fever. A significant difference between the 2 is that the natural reservoir for the Marburg virus was identified. The outbreak in Marburg, Germany, which the virus is named for, was linked to African green monkeys imported from Uganda, East Africa.2 Bats also have been identified as a reservoir for the Marburg virus.3 However, there is only speculation as to whether the natural reservoir for the Ebola virus is fruit bats. A 3-month research study following the 1995 outbreak of Ebola virus in Kikwit, Democratic Republic of the Congo, tested more than 3,000 vertebrate species and was still unable to identify a natural carrier for the virus.4
The Ebola virus was first documented nearly 40 years ago and yet we know so little about it. This demonstrates the ongoing disparity in funding and research devoted to disease conditions that most often affect only third-world nations.
Also, I’d like to point out that the article’s comment that pregnant patients are triaged “last” during the current Ebola virus outbreak may not be completely accurate. Yes, pregnant women have a significantly higher rate of mortality with Ebola viral infection. I spoke with a nurse (name and location withheld for confidentiality) who is currently the Clinical Lead at an Ebola Holding Unit for pregnant and lactating women in a West African nation. According to her, improved resources were quickly mobilized by nongovernment organizations and other foreign health care volunteers following the initial reports of disease, a factor that significantly increased access to care for pregnant women and improved outcomes. Erin Kiser, DNP, FNP-BC, WHNP-BC
Fayetteville, North Carolina
References
1. World Health Organization. Ebola virus disease. Fact sheet No. 103. http://www.who.int/mediacentre/factsheets/fs103/en/. Updated April 2015. Accessed July 6, 2015.
2. World Health Organization. Marburg haemorrhagic fever. Fact sheet. http://www.who.int/mediacentre/factsheets/fs_marburg/en/. Published November 2012. Accessed July 8, 2015.
3. Towner JS, Pourrut X, Albariño CG, et al. Marburg virus infection detected in a common African bat. PLoS One. 2007;2(8):e764. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0000764.
4. Leirs H, Mills JN, Krebs JW, et al. Search for the Ebola virus reservoir in Kikwit, Democratic Republic of the Congo: Reflections on a vertebrate collection. J Infect Dis. 1999;179(suppl 1):S155–S163.
Had the chance to change my specialty, but didn’t
I trained in Mexico, where I was a board certified ObGyn and a maternal-fetal medicine specialist. When I came to the United States I had the opportunity to change my specialty, and I didn’t. As a “free agent” international medical graduate, I had to go through many hurdles. My gate to enter the American medical world was through a family practice residency. After a year, I realized my love was still obstetrics and gynecology. In 1996, I finished an ObGyn residency at Loma Linda University Medical Center in California, and have been board certified since 1998.
There are many things I like about this specialty. Mainly, it’s the diversity. A well-rounded ObGyn has to know internal medicine, pediatrics, and surgery and apply this knowledge to the pregnant patient—a feat somehow exclusive to ObGyns.
I have enjoyed a wonderful career and many rewards. I never stop thanking all those professors and colleagues who helped me develop the set of skills that I now possess.
Tomas A. Hernandez, MD
Pasco, Washington
Not again!
I would not go into obstetrics and gynecology again because of many reasons:
- It is a very difficult life, with no family time and calls 24 hours per day.
- The specialty is the bread and butter of malpractice attorneys, causing a lot of stress.
- Insurance companies, health maintenance organizations (HMOs), etc, pay ridiculously low reimbursement for obstetric and gynecologic procedures.
- Malpractice insurance premiums are so high that you can be forced to be without malpractice and therefore more exposed.
- Patients are extremely demanding. Because pregnancy is not a disease but a natural process, they expect perfect results every time (as if congenital malformations, chromosomal abnormalities, and pregnancy complications are your fault).
- There is no patient loyalty, or very little. If a patient changes HMOs she changes obstetricians. If a woman has to wait 20 minutes in the waiting room, she changes doctors—to one who doesn’t do obstetrics (too many pregnant women!).
I would like to say that ObGyn is a beautiful specialty, most likely the best of all medical specialties, if it was not for the attorneys’ greed and patients’ lack of understanding that we are not God. We are only doctors, working within a system that contributes to all of the above.
Manuel S. Mendizabal, MD
Miami, Florida
Are men discouraged from entering the ObGyn field?
Dr. Barbieri asks, “Why is obstetrics and gynecology a popular choice for medical students?” The unaddressed question is why is it unpopular for half of medical students? Ninety-three percent of resident graduates in the field are women, while women account for half of medical student graduates. Men rarely go into the specialty today. Perhaps job advertisements touting physician opportunities in “all female groups” discourage males. Perhaps hospitals’ “women’s health centers,” with “women taking care of women,” discourage males. Perhaps receptionists’ asking patients whether they prefer a male or female physician discourages male ObGyns. In the United States, two-thirds of outpatient office visits are made by women, and academic centers and hospitals focus on this demographic in their marketing. The business ends justify the unethical means.
The result of discouraging half your medical students from the field is a lower quality field. If male and female medical students are equally qualified for any field, and I believe this is true, then discouraging half the candidates from a field lowers the quality of the resulting field. This has been the product of all discrimination throughout the ages.
Joe Walsh, MD
Philadelphia, Pennsylvania
Dr. Barbieri responds
Drs. Hernandez and Mendizabal provide 2 divergent perspectives on our field. Dr. Hernandez cherishes the diversity of the clinical work in the field, and Dr. Mendizabal warns that night call and medical malpractice take a toll on a physician. Both perspectives are valid and important, and medical students entering the field should be alerted to these rewards and challenges.
I agree with Dr. Walsh that the majority of residents in obstetrics and gynecology are women. On December 31, 2013, of the 4,942 residents in obstetrics and gynecology in the United States, 82.5% were women.1 In the fields of orthopedic surgery, neurosurgery, and urology, male residents dominate the resident complement, constituting 86.3%, 84.1%, and 77.3% of the residents, respectively.1 It is interesting that the fields of obstetrics and gynecology, orthopedic surgery, neurosurgery, and urology are among the most competitive fields in the resident match. Based on personal observation, medical student clerkship directors and obstetrics and gynecology residency programs encourage both women and men to consider a career in obstetrics and gynecology and warmly welcome male applicants. Medical students select their preferred future specialty based on many factors. It is clear that in the past few years the medical students applying to obstetrics and gynecology are extremely capable, and I am confident that the future of women’s health is in the hands ofexcellent clinicians.
Reference
1. Brotherton SE, Etzel SI. Graduate medical education, 2013–2014. JAMA. 2014;312(22):2427–2445.
Who will teach this dying art to a new generation?
The article on rotational forceps has what I consider one glaring defect—who will teach this dying art to a new generation?
Now retired, I was military-residency trained in the 1970s when you had to do your own regional and conduction anesthesia as well as operative forceps delivery—and that did not mean a silastic cup vacuum extractor, though we had just started using the Malstrom vacuum. Breech forceps, Kielland rotations, occipito-transverse forceps application—you name it and we did it as we had to keep our cesarean delivery rate down. All of us were well skilled in operative vaginal delivery.
When I stopped practicing obstetrics, the fresh-out-of-residency people coming into our practice couldn’t do a low forceps delivery. If there is to be a reteaching of rotational forceps, they’d better catch us old codgers fast before we die off (I am 72) and grant us malpractice relief (I no longer have insurance). This is an art, not a science, and can’t be taught from a book or a computer model. Set up a crash course to teach this dying art, pay us well, and perhaps we will be able to pass this skill along. Otherwise it will be gone forever.
I have always said that forceps are like a shoehorn—used correctly, they make things so much easier.
Robert Frischer, MD
Wichita Falls, Texas
Why I now recommend 3D ultrasonography to my high-risk patients
In 2012, I attended a medical staff meeting where Dr. Ruby Chang spoke about a newly available modality at our hospital: 3D ultrasonography. Her slideshow included some impressive images of cancers that were not seen on mammogram but were unmistakable on sonography.
I decided to have a 3D ultrasound for myself in order to tell my patients what it was like. I also have “heterogeneously dense breasts” on mammogram. For the previous 10 years, my annual screening mammograms had all been negative. The 3D ultrasound showed an 8-mm cancer in my left breast—not palpable to me. A subsequent mammogram was still negative for cancer.
Luckily, the breast cancer was Stage 1 at surgery, and I did not need chemotherapy or radiation, opting for skin- and nipple-sparing double mastectomy. I had a double mastectomy because I believed that I could no longer trust screening mammography for a timely diagnosis.
To this day, I explain breast density to all of my higher-risk patients who have either heterogeneously or extremely dense breasts. I tell them that their mammograms may miss a cancer and that there is another test that might help detect cancer early. It’s a good thing to have another way to evaluate the breast, especially when our patients are being sent letters about their “dense breasts.” (The majority of my patients do not understand what this means.)
I realize that data may show that this modality isn’t the perfect solution and may lead to more testing and procedures, but in my case, it was worth it!
Strangely, to this day, I have not had one patient who had breast cancer diagnosed in this way.
It’s a shame that insurance companies don’t cover even partial cost for eligible patients.
Bettina Zatuchni, MD
Pleasanton, California
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
USPSTF recommends depression screening for adults
The U.S. Preventive Services Task Force has issued a draft grade B recommendation endorsing depression screening in the general adult population, the group announced July 28.
The draft is an update to the 2009 USPSTF recommendation, which suggested screening only when staff-assisted depression care supports are in place.
“In recognition that such support is now much more widely available and accepted as part of mental health care, the current recommendation statement has omitted the recommendation regarding selective screening, as it is no longer representative of current clinical practice,” the USPSTF said in a statement.
In addition, the new recommendation supports screening for depression in pregnant and postpartum women, groups that were not reviewed for the 2009 recommendation.
About 7% of the U.S. population met the criteria for a current depressive disorder from 2009 to 2012, according to the National Survey on Drug Use and Health and the National Health and Nutrition Examination Survey, the USPSTF noted.
“Major depressive disorder is a common and significant health care problem,” the statement said. “It is the leading cause of disability among adults in high-income countries and is associated with increased mortality due to suicide and impaired ability to manage other health issues.”
A USPSTF review of clinical trials found that adult patients reported a 46% remission rate with antidepressants and a 48% remission rate with psychotherapy after 10-16 weeks. A separate review concluded that older adults who received antidepressants were twice as likely to achieve remission as older adults who received placebo (odds ratio, 2.03), the task force reported.
In addition, a review of clinical trials that evaluated the effect of screening in pregnant and postpartum women showed 28%-59% reductions in risk of depression at follow-up, compared with usual care. Another trial, which evaluated screening plus provider support, found that 45% of intervention participants reported a 5-point or greater reduction in Patient Health Questionnaire-9 scores, compared with 35% of usual care participants (OR, 1.74), the report said.
Data from the 2004-2005 National Epidemiologic Survey on Alcohol and Related Conditions reported depression prevalence of 9.1% in pregnant women, 10.2% in postpartum women, and 13.1% in women in childbearing age who were not in the postpartum period.
“For pregnant and postpartum women, there is at least moderate certainty that the net benefit of screening for depression is moderate based on the evidence of benefits and harms when [cognitive behavioral therapy] or other evidence-based counseling is available,” the USPSTF reported.
The draft recommendation is open for public comment on the USPSTF website until Aug. 24.
The U.S. Preventive Services Task Force has issued a draft grade B recommendation endorsing depression screening in the general adult population, the group announced July 28.
The draft is an update to the 2009 USPSTF recommendation, which suggested screening only when staff-assisted depression care supports are in place.
“In recognition that such support is now much more widely available and accepted as part of mental health care, the current recommendation statement has omitted the recommendation regarding selective screening, as it is no longer representative of current clinical practice,” the USPSTF said in a statement.
In addition, the new recommendation supports screening for depression in pregnant and postpartum women, groups that were not reviewed for the 2009 recommendation.
About 7% of the U.S. population met the criteria for a current depressive disorder from 2009 to 2012, according to the National Survey on Drug Use and Health and the National Health and Nutrition Examination Survey, the USPSTF noted.
“Major depressive disorder is a common and significant health care problem,” the statement said. “It is the leading cause of disability among adults in high-income countries and is associated with increased mortality due to suicide and impaired ability to manage other health issues.”
A USPSTF review of clinical trials found that adult patients reported a 46% remission rate with antidepressants and a 48% remission rate with psychotherapy after 10-16 weeks. A separate review concluded that older adults who received antidepressants were twice as likely to achieve remission as older adults who received placebo (odds ratio, 2.03), the task force reported.
In addition, a review of clinical trials that evaluated the effect of screening in pregnant and postpartum women showed 28%-59% reductions in risk of depression at follow-up, compared with usual care. Another trial, which evaluated screening plus provider support, found that 45% of intervention participants reported a 5-point or greater reduction in Patient Health Questionnaire-9 scores, compared with 35% of usual care participants (OR, 1.74), the report said.
Data from the 2004-2005 National Epidemiologic Survey on Alcohol and Related Conditions reported depression prevalence of 9.1% in pregnant women, 10.2% in postpartum women, and 13.1% in women in childbearing age who were not in the postpartum period.
“For pregnant and postpartum women, there is at least moderate certainty that the net benefit of screening for depression is moderate based on the evidence of benefits and harms when [cognitive behavioral therapy] or other evidence-based counseling is available,” the USPSTF reported.
The draft recommendation is open for public comment on the USPSTF website until Aug. 24.
The U.S. Preventive Services Task Force has issued a draft grade B recommendation endorsing depression screening in the general adult population, the group announced July 28.
The draft is an update to the 2009 USPSTF recommendation, which suggested screening only when staff-assisted depression care supports are in place.
“In recognition that such support is now much more widely available and accepted as part of mental health care, the current recommendation statement has omitted the recommendation regarding selective screening, as it is no longer representative of current clinical practice,” the USPSTF said in a statement.
In addition, the new recommendation supports screening for depression in pregnant and postpartum women, groups that were not reviewed for the 2009 recommendation.
About 7% of the U.S. population met the criteria for a current depressive disorder from 2009 to 2012, according to the National Survey on Drug Use and Health and the National Health and Nutrition Examination Survey, the USPSTF noted.
“Major depressive disorder is a common and significant health care problem,” the statement said. “It is the leading cause of disability among adults in high-income countries and is associated with increased mortality due to suicide and impaired ability to manage other health issues.”
A USPSTF review of clinical trials found that adult patients reported a 46% remission rate with antidepressants and a 48% remission rate with psychotherapy after 10-16 weeks. A separate review concluded that older adults who received antidepressants were twice as likely to achieve remission as older adults who received placebo (odds ratio, 2.03), the task force reported.
In addition, a review of clinical trials that evaluated the effect of screening in pregnant and postpartum women showed 28%-59% reductions in risk of depression at follow-up, compared with usual care. Another trial, which evaluated screening plus provider support, found that 45% of intervention participants reported a 5-point or greater reduction in Patient Health Questionnaire-9 scores, compared with 35% of usual care participants (OR, 1.74), the report said.
Data from the 2004-2005 National Epidemiologic Survey on Alcohol and Related Conditions reported depression prevalence of 9.1% in pregnant women, 10.2% in postpartum women, and 13.1% in women in childbearing age who were not in the postpartum period.
“For pregnant and postpartum women, there is at least moderate certainty that the net benefit of screening for depression is moderate based on the evidence of benefits and harms when [cognitive behavioral therapy] or other evidence-based counseling is available,” the USPSTF reported.
The draft recommendation is open for public comment on the USPSTF website until Aug. 24.
Was the ObGyn’s dexterity compromised?
Was the ObGyn’s dexterity compromised?
A woman underwent a hysterectomy. During surgery, the patient’s bladder was injured; the ObGyn called in a urologist to make the repair.
Patient’s claim The ObGyn failed to inform the patient about the possible complications from hysterectomy. The patient also claimed fraudulent concealment because the ObGyn had suffered a serious injury 3 years earlier that affected his dexterity. At the time of surgery, the ObGyn had a pending lawsuit against the owner of the premises where he fell in which he claimed that he was unable to continue his surgical practice because of the injury. The ObGyn never informed the patient of the extent of his injury or any associated risks related to his injury.
Defendants’ defense The patient was fully informed that bladder injury is a known risk of the procedure. The ObGyn maintained that his injury only affected his ability to stand for many hours while operating. The hospital settled during trial.
Verdict A $12,000 Louisiana settlement was reached with the hospital. Summary judgment was granted to the ObGyn on the informed consent claim. A $30,000 verdict was returned on the fraud count.
Placental abruption: Was child dead?
At 32 weeks’ gestation, a woman was found to have placental abruption. At the hospital, her ObGyn could not find a fetal heartbeat or detectable fetal movement on ultrasonography. A radiologist performed another ultrasound 30 minutes later and detected a fetal heart rate of 47 bpm. An emergency cesarean delivery was performed. Soon after birth, the child had seizures and was found to have hypoxic ischemic encephalopathy and diffuse brain injury. The child is profoundly disabled.
Parents’ claim The ObGyn was negligent for failing to detect the fetal heart rate and in failing to respond properly to placental abruption. Cesarean delivery should have been performed immediately after placental abruption was identified.
Defendant’s defense The case was settled at trial.
Verdict A $13 million Illinois settlement was reached, including $5 million in cash and $8 million placed in trust for the child.
Large fetus, shoulder dystocia: Erb’s palsy
Labor was induced at 39 weeks’ gestation because the fetus was anticipated to be large. During vaginal delivery, shoulder dystocia was encountered. At birth, the baby weighed 9 lb 2 oz. She sustained a brachial plexus injury to the posterior shoulder with permanent nerve root damage and Erb’s palsy. The child continues to have limited use of her left arm and hand even after 3 corrective operations.
Parents’ claim While performing maneuvers to relieve shoulder dystocia, the ObGyn exerted excessive traction on the baby’s head, causing a C-5 nerve root injury and complete avulsion at C-8. A cesarean delivery should have been performed.
Physician’s defense There was no negligence. The nerve injury was caused by the natural forces of labor and the mother’s pushing while the posterior shoulder was wedged behind the mother’s sa-
cral promontory.
Verdict A $1 million Illinois verdict was returned.
Woman dies from toxemia
A 22-year-old woman was seen by her ObGyn 4 days after vaginal delivery. Early the next day, the patient had a seizure at home and was transported by ambulance to the hospital. She could not be resuscitated and died. At autopsy, the cause of death was determined to be toxemia from pregnancy.
Estate’s claim The ObGyn failed to properly diagnose and treat the patient’s hypertension.
Defendant’s defense The case was settled during trial.
Verdict A $775,000 New York settlement was reached.
Blood transfusion delayed for hours: $14.75M net award
After emergency cesarean delivery, the baby was extremely anemic. The physicians determined that a fetal-maternal hemorrhage had started days before, causing the fetus to lose most of her blood.
An hour after birth, the attending neonatologist ordered blood from the hospital’s blood bank and arranged for emergency transport to a neonatal intensive care unit (NICU). Blood transfusion did not occur prior to transport. The child has severe cerebral palsy (CP) and cannot walk or talk at age 8 years.
Parents’ claim The neonatologist ordered cross-matched blood, which, because it is tested for compatibility, takes longer to supply. Universal donor blood could have been delivered in 20 minutes or less because it is readily available. The ambulance from the receiving hospital took an hour to drive 9 miles between the facilities, a trip that should have taken 12 minutes. The ambulance staff did not call ahead to the medical center to have blood ready for the baby. It took 4.5 hours before the newborn received a blood transfusion, a delay that caused severe injury to the child.
Defendants’ defense The matter went to trial against the neonatologist and his employer after the other defendants settled.
Verdict Before trial, an ObGyn and the hospital settled for a combined $750,000, and the county agreed to a $12 million settlement. During trial, a $2 million Illinois settlement was reached.
Pregnant woman has a massive stroke: $10.9M
Pregnant with her third child and at 26 weeks’ gestation, a 35-year-old woman had a massive intracerebral hemorrhage at home.
The day before, she had contacted her ObGyn’s office to report severe headache and abdominal pain. The call was taken by an associate of her ObGyn, who told her there was no need to go to the hospital and suggested that she had a gastrointestinal virus.
The stroke caused severe cognitive impairment, loss of memory, partial vision loss, dysphasia, and partial paralysis on her right side. At trial, she was still undergoing therapy to regain mobility, speech, and memory. She uses a wheelchair.
Patient’s claim The covering ObGyn was negligent for not sending the patient to the hospital when she reported severe headache.
Defendants’ defense The ObGyn and medical practice denied negligence, contending that the patient’s pregnancy was normal and that there was no indication that she was at risk for a stroke.
Verdict A $10,928,188 Ohio verdict was returned.
Was the fetus properly monitored?
One month before her due date, a woman was found to have premature rupture of membranes. She had gestational diabetes controlled by diet. She was admitted for induction of labor.
For more than 12 hours, external fetal monitor heart-rate tracings were reassuring. Then tracings began to show variable decelerations. For a period of 90 minutes, it was impossible to evaluate the fetal heart rate because the monitor was not working. An internal monitor was not placed. Just prior to birth, the tracings showed a 15-minute period of fetal tachycardia with the heart rate at 180 bpm. The physician’s notes indicated that the baby’s head had crowned for a prolonged period of time.
The baby was floppy at birth with Apgar scores of 2, 4, and 6 at 1, 5, and 10 minutes, respectively. The child was resuscitated and transferred to the NICU. She was found to have perinatal asphyxia, severe metabolic acidosis, multiorgan injury, hypoxic ischemic encephalopathy, and seizures. She stayed in the NICU for 1 month. At age 9 years, she has developmental delays and memory problems, but no motor injuries.
Parents’ claim During the 90 minutes in which the fetal heart-rate monitor was not working properly, the fetus was in distress. An emergency cesarean delivery should have been performed when variable decelerations were seen on tracings.
Physician’s defense The lack of motor injury indicates that the injury was not related to birth.
Verdict A $2 million Michigan settlement was reached.
Rectal tear after vacuum extraction
Vacuum extraction was used to deliver a 47-year-old woman’s child. Later, the mother developed a rectovaginal fistula that became inflamed and involved vaginal passage of stool. The patient required 2 operations and still has residual complications.
Patient’s claim The ObGyn should have found and repaired the rectal tear at delivery. Vacuum extraction was used after only 2 pushes. The mother did not consent to the use of the vacuum extractor.
Physician’s defense The ObGyn admitted that he did not specifically remember this delivery. He claimed that there was informed consent and that the rectal injury was small and easy to overlook.
Verdict A $1.02 million New York verdict was returned.
Preeclamptic mother dies after giving birth
A 24-year-old woman developed preeclampsia when under prenatal care at a hospital clinic. At 36 weeks’ gestation, she presented to the clinic with a headache, “seeing spots,” and feeling ill; her blood pressure (BP) was 169/89 mm Hg. She was admitted for induction of labor and treated for preeclampsia with magnesium sulfate. A healthy baby was born 2 days later. The mother continued to have high BP and was prescribed nifedipine.
Her BP was 148/88 mm Hg at discharge. No antihypertensive medications were prescribed. She was given standard postpartum instructions and told to schedule a follow-up appointment in 6 to 8 weeks.
Five days after discharge, she experienced shortness of breath and swelling in her extremities, but did not seek medical attention until the next day, when breathing became labored. When emergency medical services arrived, she was in cardiac arrest. Prolonged resuscitation was required with intubation and artificial respiration. A computed tomog-raphy (CT) scan revealed cerebral edema from prolonged hypoxia. She was transferred to another hospital where a neurologist determined that she had suffered a profound anoxic brain injury. She died 3 days later.
Estate’s claim The hospital staff was negligent for failing to inform the patient of the signs and symptoms of continuing preeclampsia and for not prescribing antihypertensive medication at discharge. Her follow-up appointment should have been scheduled for 1 week.
Defendant’s defense The patient was given oral instructions regarding postpartum preeclampsia. The case was settled during trial.
Verdict A $50,000 North Carolina settlement was reached.
Was delivery properly managed?
When a 16-year-old woman was found to have preeclampsia, she was admitted and labor was induced using oxytocin. An external fetal heart-rate monitor was placed.
Three hours later, her ObGyn took over her care from the attending physician. He saw the patient once in the evening, then left to deliver a baby at another hospital. He maintained telephone contact with labor and delivery nurses, who told him that the mother’s labor was progressing as planned. Early the next morning, the nurse called the ObGyn to report that the mother was fully dilated and ready to deliver. The ObGyn was at the patient’s bedside within 30 minutes. After the mother pushed once, the ObGyn determined that a cesarean delivery was necessary.
After birth, the child suffered seizures in the NICU and was transferred to another facility. With CP and microcephaly, he cannot speak, is incontinent, has motor difficulties, and will require 24-hour care for life.
Parent’s claim Labor was not properly monitored. Oxytocin doses were too large and continued for too long.
Defendants’ defense The mother’s treatment was appropriate and timely. There was no negligence.
Verdict A confidential Kansas settlement was reached with another defendant during the trial. A defense verdict was returned for the ObGyn.
Evidence of CMV on ultrasonography
During her pregnancy in 2012, a woman contracted congenital cytomegalovirus (CMV), although she did not have any symptoms. The child has CP, a hearing deficiency, and other complications caused by the virus.
Parents’ claim The ObGyn failed to identify CMV, despite ultrasound evidence that the virus was affecting the fetus. Studies available at the time of the pregnancy show considerable success in treating the condition in utero with hyperimmune globulin antiviral agents.
Defendant’s defense The case was settled during trial.
Verdict A confidential Idaho settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements, & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Was the ObGyn’s dexterity compromised?
A woman underwent a hysterectomy. During surgery, the patient’s bladder was injured; the ObGyn called in a urologist to make the repair.
Patient’s claim The ObGyn failed to inform the patient about the possible complications from hysterectomy. The patient also claimed fraudulent concealment because the ObGyn had suffered a serious injury 3 years earlier that affected his dexterity. At the time of surgery, the ObGyn had a pending lawsuit against the owner of the premises where he fell in which he claimed that he was unable to continue his surgical practice because of the injury. The ObGyn never informed the patient of the extent of his injury or any associated risks related to his injury.
Defendants’ defense The patient was fully informed that bladder injury is a known risk of the procedure. The ObGyn maintained that his injury only affected his ability to stand for many hours while operating. The hospital settled during trial.
Verdict A $12,000 Louisiana settlement was reached with the hospital. Summary judgment was granted to the ObGyn on the informed consent claim. A $30,000 verdict was returned on the fraud count.
Placental abruption: Was child dead?
At 32 weeks’ gestation, a woman was found to have placental abruption. At the hospital, her ObGyn could not find a fetal heartbeat or detectable fetal movement on ultrasonography. A radiologist performed another ultrasound 30 minutes later and detected a fetal heart rate of 47 bpm. An emergency cesarean delivery was performed. Soon after birth, the child had seizures and was found to have hypoxic ischemic encephalopathy and diffuse brain injury. The child is profoundly disabled.
Parents’ claim The ObGyn was negligent for failing to detect the fetal heart rate and in failing to respond properly to placental abruption. Cesarean delivery should have been performed immediately after placental abruption was identified.
Defendant’s defense The case was settled at trial.
Verdict A $13 million Illinois settlement was reached, including $5 million in cash and $8 million placed in trust for the child.
Large fetus, shoulder dystocia: Erb’s palsy
Labor was induced at 39 weeks’ gestation because the fetus was anticipated to be large. During vaginal delivery, shoulder dystocia was encountered. At birth, the baby weighed 9 lb 2 oz. She sustained a brachial plexus injury to the posterior shoulder with permanent nerve root damage and Erb’s palsy. The child continues to have limited use of her left arm and hand even after 3 corrective operations.
Parents’ claim While performing maneuvers to relieve shoulder dystocia, the ObGyn exerted excessive traction on the baby’s head, causing a C-5 nerve root injury and complete avulsion at C-8. A cesarean delivery should have been performed.
Physician’s defense There was no negligence. The nerve injury was caused by the natural forces of labor and the mother’s pushing while the posterior shoulder was wedged behind the mother’s sa-
cral promontory.
Verdict A $1 million Illinois verdict was returned.
Woman dies from toxemia
A 22-year-old woman was seen by her ObGyn 4 days after vaginal delivery. Early the next day, the patient had a seizure at home and was transported by ambulance to the hospital. She could not be resuscitated and died. At autopsy, the cause of death was determined to be toxemia from pregnancy.
Estate’s claim The ObGyn failed to properly diagnose and treat the patient’s hypertension.
Defendant’s defense The case was settled during trial.
Verdict A $775,000 New York settlement was reached.
Blood transfusion delayed for hours: $14.75M net award
After emergency cesarean delivery, the baby was extremely anemic. The physicians determined that a fetal-maternal hemorrhage had started days before, causing the fetus to lose most of her blood.
An hour after birth, the attending neonatologist ordered blood from the hospital’s blood bank and arranged for emergency transport to a neonatal intensive care unit (NICU). Blood transfusion did not occur prior to transport. The child has severe cerebral palsy (CP) and cannot walk or talk at age 8 years.
Parents’ claim The neonatologist ordered cross-matched blood, which, because it is tested for compatibility, takes longer to supply. Universal donor blood could have been delivered in 20 minutes or less because it is readily available. The ambulance from the receiving hospital took an hour to drive 9 miles between the facilities, a trip that should have taken 12 minutes. The ambulance staff did not call ahead to the medical center to have blood ready for the baby. It took 4.5 hours before the newborn received a blood transfusion, a delay that caused severe injury to the child.
Defendants’ defense The matter went to trial against the neonatologist and his employer after the other defendants settled.
Verdict Before trial, an ObGyn and the hospital settled for a combined $750,000, and the county agreed to a $12 million settlement. During trial, a $2 million Illinois settlement was reached.
Pregnant woman has a massive stroke: $10.9M
Pregnant with her third child and at 26 weeks’ gestation, a 35-year-old woman had a massive intracerebral hemorrhage at home.
The day before, she had contacted her ObGyn’s office to report severe headache and abdominal pain. The call was taken by an associate of her ObGyn, who told her there was no need to go to the hospital and suggested that she had a gastrointestinal virus.
The stroke caused severe cognitive impairment, loss of memory, partial vision loss, dysphasia, and partial paralysis on her right side. At trial, she was still undergoing therapy to regain mobility, speech, and memory. She uses a wheelchair.
Patient’s claim The covering ObGyn was negligent for not sending the patient to the hospital when she reported severe headache.
Defendants’ defense The ObGyn and medical practice denied negligence, contending that the patient’s pregnancy was normal and that there was no indication that she was at risk for a stroke.
Verdict A $10,928,188 Ohio verdict was returned.
Was the fetus properly monitored?
One month before her due date, a woman was found to have premature rupture of membranes. She had gestational diabetes controlled by diet. She was admitted for induction of labor.
For more than 12 hours, external fetal monitor heart-rate tracings were reassuring. Then tracings began to show variable decelerations. For a period of 90 minutes, it was impossible to evaluate the fetal heart rate because the monitor was not working. An internal monitor was not placed. Just prior to birth, the tracings showed a 15-minute period of fetal tachycardia with the heart rate at 180 bpm. The physician’s notes indicated that the baby’s head had crowned for a prolonged period of time.
The baby was floppy at birth with Apgar scores of 2, 4, and 6 at 1, 5, and 10 minutes, respectively. The child was resuscitated and transferred to the NICU. She was found to have perinatal asphyxia, severe metabolic acidosis, multiorgan injury, hypoxic ischemic encephalopathy, and seizures. She stayed in the NICU for 1 month. At age 9 years, she has developmental delays and memory problems, but no motor injuries.
Parents’ claim During the 90 minutes in which the fetal heart-rate monitor was not working properly, the fetus was in distress. An emergency cesarean delivery should have been performed when variable decelerations were seen on tracings.
Physician’s defense The lack of motor injury indicates that the injury was not related to birth.
Verdict A $2 million Michigan settlement was reached.
Rectal tear after vacuum extraction
Vacuum extraction was used to deliver a 47-year-old woman’s child. Later, the mother developed a rectovaginal fistula that became inflamed and involved vaginal passage of stool. The patient required 2 operations and still has residual complications.
Patient’s claim The ObGyn should have found and repaired the rectal tear at delivery. Vacuum extraction was used after only 2 pushes. The mother did not consent to the use of the vacuum extractor.
Physician’s defense The ObGyn admitted that he did not specifically remember this delivery. He claimed that there was informed consent and that the rectal injury was small and easy to overlook.
Verdict A $1.02 million New York verdict was returned.
Preeclamptic mother dies after giving birth
A 24-year-old woman developed preeclampsia when under prenatal care at a hospital clinic. At 36 weeks’ gestation, she presented to the clinic with a headache, “seeing spots,” and feeling ill; her blood pressure (BP) was 169/89 mm Hg. She was admitted for induction of labor and treated for preeclampsia with magnesium sulfate. A healthy baby was born 2 days later. The mother continued to have high BP and was prescribed nifedipine.
Her BP was 148/88 mm Hg at discharge. No antihypertensive medications were prescribed. She was given standard postpartum instructions and told to schedule a follow-up appointment in 6 to 8 weeks.
Five days after discharge, she experienced shortness of breath and swelling in her extremities, but did not seek medical attention until the next day, when breathing became labored. When emergency medical services arrived, she was in cardiac arrest. Prolonged resuscitation was required with intubation and artificial respiration. A computed tomog-raphy (CT) scan revealed cerebral edema from prolonged hypoxia. She was transferred to another hospital where a neurologist determined that she had suffered a profound anoxic brain injury. She died 3 days later.
Estate’s claim The hospital staff was negligent for failing to inform the patient of the signs and symptoms of continuing preeclampsia and for not prescribing antihypertensive medication at discharge. Her follow-up appointment should have been scheduled for 1 week.
Defendant’s defense The patient was given oral instructions regarding postpartum preeclampsia. The case was settled during trial.
Verdict A $50,000 North Carolina settlement was reached.
Was delivery properly managed?
When a 16-year-old woman was found to have preeclampsia, she was admitted and labor was induced using oxytocin. An external fetal heart-rate monitor was placed.
Three hours later, her ObGyn took over her care from the attending physician. He saw the patient once in the evening, then left to deliver a baby at another hospital. He maintained telephone contact with labor and delivery nurses, who told him that the mother’s labor was progressing as planned. Early the next morning, the nurse called the ObGyn to report that the mother was fully dilated and ready to deliver. The ObGyn was at the patient’s bedside within 30 minutes. After the mother pushed once, the ObGyn determined that a cesarean delivery was necessary.
After birth, the child suffered seizures in the NICU and was transferred to another facility. With CP and microcephaly, he cannot speak, is incontinent, has motor difficulties, and will require 24-hour care for life.
Parent’s claim Labor was not properly monitored. Oxytocin doses were too large and continued for too long.
Defendants’ defense The mother’s treatment was appropriate and timely. There was no negligence.
Verdict A confidential Kansas settlement was reached with another defendant during the trial. A defense verdict was returned for the ObGyn.
Evidence of CMV on ultrasonography
During her pregnancy in 2012, a woman contracted congenital cytomegalovirus (CMV), although she did not have any symptoms. The child has CP, a hearing deficiency, and other complications caused by the virus.
Parents’ claim The ObGyn failed to identify CMV, despite ultrasound evidence that the virus was affecting the fetus. Studies available at the time of the pregnancy show considerable success in treating the condition in utero with hyperimmune globulin antiviral agents.
Defendant’s defense The case was settled during trial.
Verdict A confidential Idaho settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements, & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Was the ObGyn’s dexterity compromised?
A woman underwent a hysterectomy. During surgery, the patient’s bladder was injured; the ObGyn called in a urologist to make the repair.
Patient’s claim The ObGyn failed to inform the patient about the possible complications from hysterectomy. The patient also claimed fraudulent concealment because the ObGyn had suffered a serious injury 3 years earlier that affected his dexterity. At the time of surgery, the ObGyn had a pending lawsuit against the owner of the premises where he fell in which he claimed that he was unable to continue his surgical practice because of the injury. The ObGyn never informed the patient of the extent of his injury or any associated risks related to his injury.
Defendants’ defense The patient was fully informed that bladder injury is a known risk of the procedure. The ObGyn maintained that his injury only affected his ability to stand for many hours while operating. The hospital settled during trial.
Verdict A $12,000 Louisiana settlement was reached with the hospital. Summary judgment was granted to the ObGyn on the informed consent claim. A $30,000 verdict was returned on the fraud count.
Placental abruption: Was child dead?
At 32 weeks’ gestation, a woman was found to have placental abruption. At the hospital, her ObGyn could not find a fetal heartbeat or detectable fetal movement on ultrasonography. A radiologist performed another ultrasound 30 minutes later and detected a fetal heart rate of 47 bpm. An emergency cesarean delivery was performed. Soon after birth, the child had seizures and was found to have hypoxic ischemic encephalopathy and diffuse brain injury. The child is profoundly disabled.
Parents’ claim The ObGyn was negligent for failing to detect the fetal heart rate and in failing to respond properly to placental abruption. Cesarean delivery should have been performed immediately after placental abruption was identified.
Defendant’s defense The case was settled at trial.
Verdict A $13 million Illinois settlement was reached, including $5 million in cash and $8 million placed in trust for the child.
Large fetus, shoulder dystocia: Erb’s palsy
Labor was induced at 39 weeks’ gestation because the fetus was anticipated to be large. During vaginal delivery, shoulder dystocia was encountered. At birth, the baby weighed 9 lb 2 oz. She sustained a brachial plexus injury to the posterior shoulder with permanent nerve root damage and Erb’s palsy. The child continues to have limited use of her left arm and hand even after 3 corrective operations.
Parents’ claim While performing maneuvers to relieve shoulder dystocia, the ObGyn exerted excessive traction on the baby’s head, causing a C-5 nerve root injury and complete avulsion at C-8. A cesarean delivery should have been performed.
Physician’s defense There was no negligence. The nerve injury was caused by the natural forces of labor and the mother’s pushing while the posterior shoulder was wedged behind the mother’s sa-
cral promontory.
Verdict A $1 million Illinois verdict was returned.
Woman dies from toxemia
A 22-year-old woman was seen by her ObGyn 4 days after vaginal delivery. Early the next day, the patient had a seizure at home and was transported by ambulance to the hospital. She could not be resuscitated and died. At autopsy, the cause of death was determined to be toxemia from pregnancy.
Estate’s claim The ObGyn failed to properly diagnose and treat the patient’s hypertension.
Defendant’s defense The case was settled during trial.
Verdict A $775,000 New York settlement was reached.
Blood transfusion delayed for hours: $14.75M net award
After emergency cesarean delivery, the baby was extremely anemic. The physicians determined that a fetal-maternal hemorrhage had started days before, causing the fetus to lose most of her blood.
An hour after birth, the attending neonatologist ordered blood from the hospital’s blood bank and arranged for emergency transport to a neonatal intensive care unit (NICU). Blood transfusion did not occur prior to transport. The child has severe cerebral palsy (CP) and cannot walk or talk at age 8 years.
Parents’ claim The neonatologist ordered cross-matched blood, which, because it is tested for compatibility, takes longer to supply. Universal donor blood could have been delivered in 20 minutes or less because it is readily available. The ambulance from the receiving hospital took an hour to drive 9 miles between the facilities, a trip that should have taken 12 minutes. The ambulance staff did not call ahead to the medical center to have blood ready for the baby. It took 4.5 hours before the newborn received a blood transfusion, a delay that caused severe injury to the child.
Defendants’ defense The matter went to trial against the neonatologist and his employer after the other defendants settled.
Verdict Before trial, an ObGyn and the hospital settled for a combined $750,000, and the county agreed to a $12 million settlement. During trial, a $2 million Illinois settlement was reached.
Pregnant woman has a massive stroke: $10.9M
Pregnant with her third child and at 26 weeks’ gestation, a 35-year-old woman had a massive intracerebral hemorrhage at home.
The day before, she had contacted her ObGyn’s office to report severe headache and abdominal pain. The call was taken by an associate of her ObGyn, who told her there was no need to go to the hospital and suggested that she had a gastrointestinal virus.
The stroke caused severe cognitive impairment, loss of memory, partial vision loss, dysphasia, and partial paralysis on her right side. At trial, she was still undergoing therapy to regain mobility, speech, and memory. She uses a wheelchair.
Patient’s claim The covering ObGyn was negligent for not sending the patient to the hospital when she reported severe headache.
Defendants’ defense The ObGyn and medical practice denied negligence, contending that the patient’s pregnancy was normal and that there was no indication that she was at risk for a stroke.
Verdict A $10,928,188 Ohio verdict was returned.
Was the fetus properly monitored?
One month before her due date, a woman was found to have premature rupture of membranes. She had gestational diabetes controlled by diet. She was admitted for induction of labor.
For more than 12 hours, external fetal monitor heart-rate tracings were reassuring. Then tracings began to show variable decelerations. For a period of 90 minutes, it was impossible to evaluate the fetal heart rate because the monitor was not working. An internal monitor was not placed. Just prior to birth, the tracings showed a 15-minute period of fetal tachycardia with the heart rate at 180 bpm. The physician’s notes indicated that the baby’s head had crowned for a prolonged period of time.
The baby was floppy at birth with Apgar scores of 2, 4, and 6 at 1, 5, and 10 minutes, respectively. The child was resuscitated and transferred to the NICU. She was found to have perinatal asphyxia, severe metabolic acidosis, multiorgan injury, hypoxic ischemic encephalopathy, and seizures. She stayed in the NICU for 1 month. At age 9 years, she has developmental delays and memory problems, but no motor injuries.
Parents’ claim During the 90 minutes in which the fetal heart-rate monitor was not working properly, the fetus was in distress. An emergency cesarean delivery should have been performed when variable decelerations were seen on tracings.
Physician’s defense The lack of motor injury indicates that the injury was not related to birth.
Verdict A $2 million Michigan settlement was reached.
Rectal tear after vacuum extraction
Vacuum extraction was used to deliver a 47-year-old woman’s child. Later, the mother developed a rectovaginal fistula that became inflamed and involved vaginal passage of stool. The patient required 2 operations and still has residual complications.
Patient’s claim The ObGyn should have found and repaired the rectal tear at delivery. Vacuum extraction was used after only 2 pushes. The mother did not consent to the use of the vacuum extractor.
Physician’s defense The ObGyn admitted that he did not specifically remember this delivery. He claimed that there was informed consent and that the rectal injury was small and easy to overlook.
Verdict A $1.02 million New York verdict was returned.
Preeclamptic mother dies after giving birth
A 24-year-old woman developed preeclampsia when under prenatal care at a hospital clinic. At 36 weeks’ gestation, she presented to the clinic with a headache, “seeing spots,” and feeling ill; her blood pressure (BP) was 169/89 mm Hg. She was admitted for induction of labor and treated for preeclampsia with magnesium sulfate. A healthy baby was born 2 days later. The mother continued to have high BP and was prescribed nifedipine.
Her BP was 148/88 mm Hg at discharge. No antihypertensive medications were prescribed. She was given standard postpartum instructions and told to schedule a follow-up appointment in 6 to 8 weeks.
Five days after discharge, she experienced shortness of breath and swelling in her extremities, but did not seek medical attention until the next day, when breathing became labored. When emergency medical services arrived, she was in cardiac arrest. Prolonged resuscitation was required with intubation and artificial respiration. A computed tomog-raphy (CT) scan revealed cerebral edema from prolonged hypoxia. She was transferred to another hospital where a neurologist determined that she had suffered a profound anoxic brain injury. She died 3 days later.
Estate’s claim The hospital staff was negligent for failing to inform the patient of the signs and symptoms of continuing preeclampsia and for not prescribing antihypertensive medication at discharge. Her follow-up appointment should have been scheduled for 1 week.
Defendant’s defense The patient was given oral instructions regarding postpartum preeclampsia. The case was settled during trial.
Verdict A $50,000 North Carolina settlement was reached.
Was delivery properly managed?
When a 16-year-old woman was found to have preeclampsia, she was admitted and labor was induced using oxytocin. An external fetal heart-rate monitor was placed.
Three hours later, her ObGyn took over her care from the attending physician. He saw the patient once in the evening, then left to deliver a baby at another hospital. He maintained telephone contact with labor and delivery nurses, who told him that the mother’s labor was progressing as planned. Early the next morning, the nurse called the ObGyn to report that the mother was fully dilated and ready to deliver. The ObGyn was at the patient’s bedside within 30 minutes. After the mother pushed once, the ObGyn determined that a cesarean delivery was necessary.
After birth, the child suffered seizures in the NICU and was transferred to another facility. With CP and microcephaly, he cannot speak, is incontinent, has motor difficulties, and will require 24-hour care for life.
Parent’s claim Labor was not properly monitored. Oxytocin doses were too large and continued for too long.
Defendants’ defense The mother’s treatment was appropriate and timely. There was no negligence.
Verdict A confidential Kansas settlement was reached with another defendant during the trial. A defense verdict was returned for the ObGyn.
Evidence of CMV on ultrasonography
During her pregnancy in 2012, a woman contracted congenital cytomegalovirus (CMV), although she did not have any symptoms. The child has CP, a hearing deficiency, and other complications caused by the virus.
Parents’ claim The ObGyn failed to identify CMV, despite ultrasound evidence that the virus was affecting the fetus. Studies available at the time of the pregnancy show considerable success in treating the condition in utero with hyperimmune globulin antiviral agents.
Defendant’s defense The case was settled during trial.
Verdict A confidential Idaho settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements, & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Article
- Placental abruption: Was child dead?
- Large fetus, shoulder dystocia: Erb’s palsy
- Woman dies from toxemia
- Blood transfusion delayed for hours: $14.75M net award
- Pregnant woman has a massive stroke: $10.9M
- Was the fetus properly monitored?
- Rectal tear after vacuum extraction
- Preeclamptic mother dies after giving birth
- Was delivery properly managed?
- Evidence of CMV on ultrasonography
Joint Commission offers perinatal care certification
Joint Commission-accredited health care organizations looking to up their perinatal care credentials now can, thanks to a new Perinatal Care Certification program.
The program was developed by the Joint Commission in response to data from the World Health Organization that shows at least 1,200 American women die during pregnancy or in childbirth annually. An additional 60,000 women in the U.S. suffer near-fatal perinatal complications.
“The certification will give providers an unparalleled advantage when it comes to preparing mothers for labor and delivery, while helping mothers and newborns if complications arise,” Wendi Roberts, executive director of The Joint Commission’s Certification Program, said in a statement.
The certification program, which covers prenatal care through postpartum care, requires early identification of high-risk pregnancies and births, transfers for women and babies who require a higher level of care, and ongoing quality improvement processes.
The program is aimed at reducing maternal and infant mortality and complications, unnecessary induction of labor, elective deliveries, and prematurity rates.
Along with improving patient outcomes, the program could also drive down the cost of maternity care, which the Joint Commission said reached $60 billion in 2012 the United States alone. Hospitals may be able to reduce costs associated with longer length of stay due to complications, according to the Joint Commission.
On Twitter @whitneymcknight
Joint Commission-accredited health care organizations looking to up their perinatal care credentials now can, thanks to a new Perinatal Care Certification program.
The program was developed by the Joint Commission in response to data from the World Health Organization that shows at least 1,200 American women die during pregnancy or in childbirth annually. An additional 60,000 women in the U.S. suffer near-fatal perinatal complications.
“The certification will give providers an unparalleled advantage when it comes to preparing mothers for labor and delivery, while helping mothers and newborns if complications arise,” Wendi Roberts, executive director of The Joint Commission’s Certification Program, said in a statement.
The certification program, which covers prenatal care through postpartum care, requires early identification of high-risk pregnancies and births, transfers for women and babies who require a higher level of care, and ongoing quality improvement processes.
The program is aimed at reducing maternal and infant mortality and complications, unnecessary induction of labor, elective deliveries, and prematurity rates.
Along with improving patient outcomes, the program could also drive down the cost of maternity care, which the Joint Commission said reached $60 billion in 2012 the United States alone. Hospitals may be able to reduce costs associated with longer length of stay due to complications, according to the Joint Commission.
On Twitter @whitneymcknight
Joint Commission-accredited health care organizations looking to up their perinatal care credentials now can, thanks to a new Perinatal Care Certification program.
The program was developed by the Joint Commission in response to data from the World Health Organization that shows at least 1,200 American women die during pregnancy or in childbirth annually. An additional 60,000 women in the U.S. suffer near-fatal perinatal complications.
“The certification will give providers an unparalleled advantage when it comes to preparing mothers for labor and delivery, while helping mothers and newborns if complications arise,” Wendi Roberts, executive director of The Joint Commission’s Certification Program, said in a statement.
The certification program, which covers prenatal care through postpartum care, requires early identification of high-risk pregnancies and births, transfers for women and babies who require a higher level of care, and ongoing quality improvement processes.
The program is aimed at reducing maternal and infant mortality and complications, unnecessary induction of labor, elective deliveries, and prematurity rates.
Along with improving patient outcomes, the program could also drive down the cost of maternity care, which the Joint Commission said reached $60 billion in 2012 the United States alone. Hospitals may be able to reduce costs associated with longer length of stay due to complications, according to the Joint Commission.
On Twitter @whitneymcknight
Pros and cons of cfDNA testing
WASHINGTON – The high trisomy 21 detection rate is among the selling points of noninvasive prenatal testing using cell-free DNA screening, though the test detects only a small number of abnormalities, Dr. Mary Norton said at the International Conference on Prenatal Diagnosis and Therapy.
During a symposium on introducing cfDNA screening into routine prenatal care, Dr. Norton, professor of obstetrics and gynecology at the University of California, San Francisco, said physicians are debating not only whether the cfDNA test should be used to screen low- versus high-risk women, but also whether cfDNA screening is appropriate as a primary screen for all pregnant women.
The use of noninvasive prenatal testing (NIPT) is growing in popularity, but its usefulness is narrow, focusing on three chromosomal abnormalities (trisomy 13, 21, and 18), while traditional diagnostic tests provide a broad screen for a wide array of abnormalities, she said.
One of the pros of using cfDNA as a primary screen is that “there’s no question” the detection rate for Down syndrome is better than with other methods, she said.
Other benefits include a lower false positive rate and a reduction in diagnostic tests.
In a prospective, international study published earlier this year that compared cfDNA testing to standard screening for trisomy 21, cfDNA testing identified trisomy 21 in all 38 women with the abnormality, compared to 30 of 38 with standard screening.
In the cfDNA group, the false positive rate was significantly lower (0.06% vs. 5.4%), and the positive predictive value was significantly higher (almost 81% vs. 3.4%) when compared with standard screening, according to Dr. Norton, who was the lead author of the study (N. Engl. J. Med. 2015;372:1589-97).
Another issue is positive predictive value, which is driven by prevalence of the abnormality screened, with lower prevalence corresponding to a lower PPV, she said. In a study of about 1,900 low-risk women whose mean maternal age was 30 years, cfDNA testing detected all cases of trisomy 21. The PPV for trisomy 21 was 45.5% compared with 4.2% with standard screening (N. Engl. J. Med. 2014;370:799-808).
But there are downsides to using the NIPT. For instance, cfDNA testing does not detect many other chromosome abnormalities.
In a study of more than 1.3 million women who had traditional prenatal screening between 2009 and 2012, about 17% of chromosome abnormalities identified were not detectable by current NIPT methods (Obset. Gynecol. 2014;124:979-86).
Other issues include test failure, which can occur because of a low fetal fraction (the amount of cfDNA in the maternal blood of fetal origin), as well as providers who offer cfDNA testing without a full understanding about the implications of positive results, Dr. Norton said.
In many communities, general ob.gyns. are confused about the meaning of the results, she said.
She pointed to a December 2014 investigative report on NIPT in the Boston Globe that said some women have terminated their pregnancies based on NIPT screening results without having a confirmatory diagnostic test.
And when using cfDNA screening, about 3% of samples do not provide a result, which can be associated with aneuploidy and cannot be ignored, Dr. Norton said.
The cfDNA testing is also expensive, Dr. Norton said. For patients who are young with a low risk for Down syndrome but higher risk for other abnormalities, it could be argued that a screening test should be “cheap and broad,” she said.
In the United States, there are many different ways serum and ultrasound screening is currently practiced, and cfDNA screening is being “integrated in very different ways in different settings,” she noted.
A recent American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine statement on cfDNA screening states that considering the performance of conventional screening methods, “the limitations of cell-free DNA screening performance, and the limited data on cost-effectiveness in the low-risk obstetric population, conventional screening methods remain the most appropriate choice for first line screening for most women in the general obstetric population.”
The statement also notes that while any patient, regardless of her risk, can choose cfDNA testing, she should understand the “limitations and benefits of this screening paradigm” in the context of alternative screening and diagnostic options.
The ACOG/SMFM opinion adds that the cfDNA technology for prenatal aneuploidy screening is rapidly changing, and that “any recommendations regarding its use in screening also will likely evolve quickly.”
Dr. Norton disclosed having received research support from Natera and Ariosa.
WASHINGTON – The high trisomy 21 detection rate is among the selling points of noninvasive prenatal testing using cell-free DNA screening, though the test detects only a small number of abnormalities, Dr. Mary Norton said at the International Conference on Prenatal Diagnosis and Therapy.
During a symposium on introducing cfDNA screening into routine prenatal care, Dr. Norton, professor of obstetrics and gynecology at the University of California, San Francisco, said physicians are debating not only whether the cfDNA test should be used to screen low- versus high-risk women, but also whether cfDNA screening is appropriate as a primary screen for all pregnant women.
The use of noninvasive prenatal testing (NIPT) is growing in popularity, but its usefulness is narrow, focusing on three chromosomal abnormalities (trisomy 13, 21, and 18), while traditional diagnostic tests provide a broad screen for a wide array of abnormalities, she said.
One of the pros of using cfDNA as a primary screen is that “there’s no question” the detection rate for Down syndrome is better than with other methods, she said.
Other benefits include a lower false positive rate and a reduction in diagnostic tests.
In a prospective, international study published earlier this year that compared cfDNA testing to standard screening for trisomy 21, cfDNA testing identified trisomy 21 in all 38 women with the abnormality, compared to 30 of 38 with standard screening.
In the cfDNA group, the false positive rate was significantly lower (0.06% vs. 5.4%), and the positive predictive value was significantly higher (almost 81% vs. 3.4%) when compared with standard screening, according to Dr. Norton, who was the lead author of the study (N. Engl. J. Med. 2015;372:1589-97).
Another issue is positive predictive value, which is driven by prevalence of the abnormality screened, with lower prevalence corresponding to a lower PPV, she said. In a study of about 1,900 low-risk women whose mean maternal age was 30 years, cfDNA testing detected all cases of trisomy 21. The PPV for trisomy 21 was 45.5% compared with 4.2% with standard screening (N. Engl. J. Med. 2014;370:799-808).
But there are downsides to using the NIPT. For instance, cfDNA testing does not detect many other chromosome abnormalities.
In a study of more than 1.3 million women who had traditional prenatal screening between 2009 and 2012, about 17% of chromosome abnormalities identified were not detectable by current NIPT methods (Obset. Gynecol. 2014;124:979-86).
Other issues include test failure, which can occur because of a low fetal fraction (the amount of cfDNA in the maternal blood of fetal origin), as well as providers who offer cfDNA testing without a full understanding about the implications of positive results, Dr. Norton said.
In many communities, general ob.gyns. are confused about the meaning of the results, she said.
She pointed to a December 2014 investigative report on NIPT in the Boston Globe that said some women have terminated their pregnancies based on NIPT screening results without having a confirmatory diagnostic test.
And when using cfDNA screening, about 3% of samples do not provide a result, which can be associated with aneuploidy and cannot be ignored, Dr. Norton said.
The cfDNA testing is also expensive, Dr. Norton said. For patients who are young with a low risk for Down syndrome but higher risk for other abnormalities, it could be argued that a screening test should be “cheap and broad,” she said.
In the United States, there are many different ways serum and ultrasound screening is currently practiced, and cfDNA screening is being “integrated in very different ways in different settings,” she noted.
A recent American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine statement on cfDNA screening states that considering the performance of conventional screening methods, “the limitations of cell-free DNA screening performance, and the limited data on cost-effectiveness in the low-risk obstetric population, conventional screening methods remain the most appropriate choice for first line screening for most women in the general obstetric population.”
The statement also notes that while any patient, regardless of her risk, can choose cfDNA testing, she should understand the “limitations and benefits of this screening paradigm” in the context of alternative screening and diagnostic options.
The ACOG/SMFM opinion adds that the cfDNA technology for prenatal aneuploidy screening is rapidly changing, and that “any recommendations regarding its use in screening also will likely evolve quickly.”
Dr. Norton disclosed having received research support from Natera and Ariosa.
WASHINGTON – The high trisomy 21 detection rate is among the selling points of noninvasive prenatal testing using cell-free DNA screening, though the test detects only a small number of abnormalities, Dr. Mary Norton said at the International Conference on Prenatal Diagnosis and Therapy.
During a symposium on introducing cfDNA screening into routine prenatal care, Dr. Norton, professor of obstetrics and gynecology at the University of California, San Francisco, said physicians are debating not only whether the cfDNA test should be used to screen low- versus high-risk women, but also whether cfDNA screening is appropriate as a primary screen for all pregnant women.
The use of noninvasive prenatal testing (NIPT) is growing in popularity, but its usefulness is narrow, focusing on three chromosomal abnormalities (trisomy 13, 21, and 18), while traditional diagnostic tests provide a broad screen for a wide array of abnormalities, she said.
One of the pros of using cfDNA as a primary screen is that “there’s no question” the detection rate for Down syndrome is better than with other methods, she said.
Other benefits include a lower false positive rate and a reduction in diagnostic tests.
In a prospective, international study published earlier this year that compared cfDNA testing to standard screening for trisomy 21, cfDNA testing identified trisomy 21 in all 38 women with the abnormality, compared to 30 of 38 with standard screening.
In the cfDNA group, the false positive rate was significantly lower (0.06% vs. 5.4%), and the positive predictive value was significantly higher (almost 81% vs. 3.4%) when compared with standard screening, according to Dr. Norton, who was the lead author of the study (N. Engl. J. Med. 2015;372:1589-97).
Another issue is positive predictive value, which is driven by prevalence of the abnormality screened, with lower prevalence corresponding to a lower PPV, she said. In a study of about 1,900 low-risk women whose mean maternal age was 30 years, cfDNA testing detected all cases of trisomy 21. The PPV for trisomy 21 was 45.5% compared with 4.2% with standard screening (N. Engl. J. Med. 2014;370:799-808).
But there are downsides to using the NIPT. For instance, cfDNA testing does not detect many other chromosome abnormalities.
In a study of more than 1.3 million women who had traditional prenatal screening between 2009 and 2012, about 17% of chromosome abnormalities identified were not detectable by current NIPT methods (Obset. Gynecol. 2014;124:979-86).
Other issues include test failure, which can occur because of a low fetal fraction (the amount of cfDNA in the maternal blood of fetal origin), as well as providers who offer cfDNA testing without a full understanding about the implications of positive results, Dr. Norton said.
In many communities, general ob.gyns. are confused about the meaning of the results, she said.
She pointed to a December 2014 investigative report on NIPT in the Boston Globe that said some women have terminated their pregnancies based on NIPT screening results without having a confirmatory diagnostic test.
And when using cfDNA screening, about 3% of samples do not provide a result, which can be associated with aneuploidy and cannot be ignored, Dr. Norton said.
The cfDNA testing is also expensive, Dr. Norton said. For patients who are young with a low risk for Down syndrome but higher risk for other abnormalities, it could be argued that a screening test should be “cheap and broad,” she said.
In the United States, there are many different ways serum and ultrasound screening is currently practiced, and cfDNA screening is being “integrated in very different ways in different settings,” she noted.
A recent American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine statement on cfDNA screening states that considering the performance of conventional screening methods, “the limitations of cell-free DNA screening performance, and the limited data on cost-effectiveness in the low-risk obstetric population, conventional screening methods remain the most appropriate choice for first line screening for most women in the general obstetric population.”
The statement also notes that while any patient, regardless of her risk, can choose cfDNA testing, she should understand the “limitations and benefits of this screening paradigm” in the context of alternative screening and diagnostic options.
The ACOG/SMFM opinion adds that the cfDNA technology for prenatal aneuploidy screening is rapidly changing, and that “any recommendations regarding its use in screening also will likely evolve quickly.”
Dr. Norton disclosed having received research support from Natera and Ariosa.
EXPERT ANALYSIS FROM ISPD 2015
Pregnancy registries add to the clinical picture
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of human pregnancy experience. The new Food and Drug Administration prescription drug information format may increase the amount of information available, at least from registries conducted by drug manufacturers, because they will be required to list all substantive changes made within the year. Although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of pregnancy registries are their prospective nature and enrollment across a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions).
Registries can identify early signals of teratogenicity, but they have several limitations, including voluntary reporting that results in selection bias, pregnancies lost to follow-up that may have had different outcomes than those with documented outcomes, and a lack of control groups (with some exceptions).
Other limitations are that registry cases are not representative of target populations, and pregnancies lost to follow-up lack details on elective terminations and fetal deaths without birth defects, as well as all spontaneous abortions. Additionally, publication of results may be delayed and does not appear in a peer-reviewed journal.
Since the total number of exposed pregnancies is unknown, registry data cannot be used to calculate prevalence, but can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports, which are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. However, they may be helpful in detecting unusual patterns of birth defects.
MothertoBaby Registry
The large Organization of Teratology Information Specialists (OTIS) MothertoBaby registry (877-311-8972) involves four different categories: autoimmune diseases(rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma; antiviral agents; and vaccines.
The drugs for autoimmune diseases are tocilizumab (Actemra), leflunomide (Arava), teriflunomide (Aubagio), certolizumab pegol (Cimzia), etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), apremilast (Otezla), ustekinumab (Stelara), tofacitinib (Xeljanz), infliximab (Remicade), and methotrexate.
The asthma drugs are formoterol (Foradil, Perforomist, Symbicort, Dulera), albuterol, levalbuterol, metaproterenol, pirbuterol (Maxair), and terbutaline.
The antiviral agents are oseltamivir (Tamiflu) and zanamivir (Relenza).
The vaccines include pertussis (Tdap), seasonal influenza, and meningococcal disease (Menveo). In addition to a control group in each category, a unique aspect of this registry is that many exposed infants will undergo dysmorphology examinations.
Vaccine registries
A second vaccine for meningococcal disease (Menactra) is being studied in the Menactra vaccine Pregnancy Registry (800-822-2463 / www.sanofipasteurpregnancy.com).
Two other registries also include influenza vaccines: Flu vaccine Pregnancy Registry, PPD (Flucelvax) (877-413-4759 / email: [email protected]) and the GSK Seasonal Influenza Vaccine Pregnancy Registry, GlaxoSmithKline (888-825-5249 / pregnancyregistry.gsk.com/seasonalinfluenzavaccines.html), which includes the Fluarix and FluLaval vaccines.
Asthma
Women with asthma who are being treated with omalizumab (Xolair) are the target population for the EXPECT Pregnancy Registry (866-496-5247 Option 3 / www.xolairpregnancyregistry.com).
GSK registries
GlaxoSmithKline is also conducting three other registries: the Belimumab (Benlysta) Pregnancy Registry for patients with systemic lupus erythematosus treated with belimumab (877-681-6296 / [email protected]); and the Twinrix Pregnancy Registry for women who have received the Twinrix hepatitis A and B vaccine (888-825-5249).
Atypical antipsychotics
TheNational Pregnancy Registry for Atypical Antipsychotics (866-961-2388 / registry@womens mentalhealth.org) is studying 10 drugs: aripiprazole (Abilify), clozapine (Clozaril), iloperidone (Fanapt), paliperidone (Invega), lurasidone (Latuda), risperidone (Risperdal), asenapine (Saphris), quetiapine (Seroquel), ziprasidone (Geodon), and olanzapine (Zyprexa).
TAPP registry
Acitretin is under study by the Take Action to Prevent Pregnancy (T.A.P.P.) registry (855-850-2138 / www.tevagenerics.com/acitretin).
MPS I registry
The MPS I (Mucopolysaccharidosis I) Registry, Genzyme Corp (617-591-5500 / [email protected]), is studying the use of laronidase (Aldurazyme) for Hurler syndrome, Scheie syndrome, and Hurler-Scheie syndrome.
MPS VI registry
The use of galsulfase (Naglazyme) for Maroteaux-Lamy syndrome during pregnancy is under study by the Mucopolysaccharidosis VI (MPS VI) Clinical Surveillance Program(415-506-6849 or 415-506-6703)
Epilepsy drugs
The Antiepileptic Drug Pregnancy Registry (888-233-2334 / www.aedpregnancyregistry.org) is studying antiepileptic drugs.
Type 2 diabetes
The Exenatide Pregnancy Registry, conducted by INC Research for AstraZeneca (800-633-9081 / www.exenatidepregnancyregistry.com), examines the use of exenatide (Byetta, Bydureon) for the treatment of type 2 diabetes.
Renal transplant
Renal transplant patients exposed to mycophenolate (Cellcept) can be enrolled in the Mycophenolate Pregnancy Registry (800-617-8191) or the National Transplantation Pregnancy Registry (877-955-6877). The NTPR is enrolling renal transplant patients exposed to belatacept (Nulojix).
Cymbalta
The antidepressant duloxetine (Cymbalta), when used for major depressive or generalized anxiety disorders, diabetic peripheral neuropathic pain, or fibromyalgia is being studied by the Cymbalta Pregnancy Registry, INC Research ([email protected] / www.cymbaltapregancyregistry.com).
Fabry disease
The Fabry Registry, Genzyme Corp (617-581-5500 / [email protected]) is studying the use in pregnancy of agalsidase beta (Fabrazyme) for Fabry disease.
MS registry
Novartis Pharmaceuticals is conducting the Gilenya (fingolimod) Pregnancy Registry (877-598-7237 / [email protected]) for patients with multiple sclerosis who are taking fingolimod (Gilenya).
Cancer
The MotHER Pregnancy Registry, INC Research (800-690-6720 / [email protected] / themotherpregnancyregistry.com) is enrolling breast cancer patients who have been treated during pregnancy with ado-trastuzumab (Kadcyla), trastuzumab (Herceptin), or pertuzumab (Perjeta).
Merck registries
Merck Pregnancy Registries (800-986-8999) include a registry for type 2 diabetes – sitagliptin/metformin (Janumet) or sitagliptin (Januvia) (www.merckpregancyregistries.com/januvia.html); a registry for rizatriptan (Maxalt) (www.merckpregancyregistries.com/maxalt.html), for migraine headaches; and a registry for the 9-valent human papillomavirus (HPV) (Gardasil 9) vaccine (www.merckpregnancyregistries.com/gardasil9.html).
The registry for the asthma drug montelukast (Singulair) and the registry for Gardasil, the quadrivalent HPV vaccine, are now closed to new enrollment, but new cases of pregnancy exposures can be reported to the company at: 877-888-4231.
Osteoporosis
Amgen’s Pregnancy Surveillance Program (800-772-6436) is enrolling pregnant subjects with osteoporosis who are being treated with denosumab (Prolia).
Hepatitis C
The Ribavirin Pregnancy Registry, INC Research (800-593-2214/ [email protected] / ribavirinpregnancyregistry.com) is looking for subjects with hepatitis C who have been treated with ribavirin (Copegus).
Fibromyalgia
The Savella Pregnancy Registry (877-643-3010/ [email protected] / savellapregnancyregistry.com) is looking for patients with fibromyalgia who are being treated with milnacipran (Savella).
Skin infections
An antibacterial, telavancin (Vibativ), indicated for skin infections is being studied by the Vibativ Pregnancy Registry (888-658-4228 / www.clinicaltrial.gov).
Pompe disease
Pregnant women treated with alglucosidase alfa (Myozyme) for Pompe disease can enroll in the Pompe Registry (800-745-4447 x 15500 / www.pompe.com/en/healthcare-professionals/pompe-registry.aspx).
Sleep disorders
Armodafinil (Nuvigil) used to treat excessive sleepiness associated with narcolepsy and other sleep disorders is being studied in the Nuvigil Pregnancy Registry (866-404-4106 / www.nuvigilpregnancyregistry.com). A second drug with the same indication and telephone number, modafinil (Provigil), is in the Provigil Pregnancy Registry (provigilpregnancyregistry.com).
Additional details, including fax numbers and addresses of the registries reviewed above, can be obtained from the FDA website, List of Pregnancy Exposure Registries. Since the strength of a registry is based on numbers, health care professionals are encouraged to enroll potential subjects or have their patients call to enroll themselves.
Before retirement, Mr. Briggs was a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; he remains a clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He had no relevant financial disclosures. Contact him at [email protected].
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of human pregnancy experience. The new Food and Drug Administration prescription drug information format may increase the amount of information available, at least from registries conducted by drug manufacturers, because they will be required to list all substantive changes made within the year. Although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of pregnancy registries are their prospective nature and enrollment across a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions).
Registries can identify early signals of teratogenicity, but they have several limitations, including voluntary reporting that results in selection bias, pregnancies lost to follow-up that may have had different outcomes than those with documented outcomes, and a lack of control groups (with some exceptions).
Other limitations are that registry cases are not representative of target populations, and pregnancies lost to follow-up lack details on elective terminations and fetal deaths without birth defects, as well as all spontaneous abortions. Additionally, publication of results may be delayed and does not appear in a peer-reviewed journal.
Since the total number of exposed pregnancies is unknown, registry data cannot be used to calculate prevalence, but can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports, which are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. However, they may be helpful in detecting unusual patterns of birth defects.
MothertoBaby Registry
The large Organization of Teratology Information Specialists (OTIS) MothertoBaby registry (877-311-8972) involves four different categories: autoimmune diseases(rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma; antiviral agents; and vaccines.
The drugs for autoimmune diseases are tocilizumab (Actemra), leflunomide (Arava), teriflunomide (Aubagio), certolizumab pegol (Cimzia), etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), apremilast (Otezla), ustekinumab (Stelara), tofacitinib (Xeljanz), infliximab (Remicade), and methotrexate.
The asthma drugs are formoterol (Foradil, Perforomist, Symbicort, Dulera), albuterol, levalbuterol, metaproterenol, pirbuterol (Maxair), and terbutaline.
The antiviral agents are oseltamivir (Tamiflu) and zanamivir (Relenza).
The vaccines include pertussis (Tdap), seasonal influenza, and meningococcal disease (Menveo). In addition to a control group in each category, a unique aspect of this registry is that many exposed infants will undergo dysmorphology examinations.
Vaccine registries
A second vaccine for meningococcal disease (Menactra) is being studied in the Menactra vaccine Pregnancy Registry (800-822-2463 / www.sanofipasteurpregnancy.com).
Two other registries also include influenza vaccines: Flu vaccine Pregnancy Registry, PPD (Flucelvax) (877-413-4759 / email: [email protected]) and the GSK Seasonal Influenza Vaccine Pregnancy Registry, GlaxoSmithKline (888-825-5249 / pregnancyregistry.gsk.com/seasonalinfluenzavaccines.html), which includes the Fluarix and FluLaval vaccines.
Asthma
Women with asthma who are being treated with omalizumab (Xolair) are the target population for the EXPECT Pregnancy Registry (866-496-5247 Option 3 / www.xolairpregnancyregistry.com).
GSK registries
GlaxoSmithKline is also conducting three other registries: the Belimumab (Benlysta) Pregnancy Registry for patients with systemic lupus erythematosus treated with belimumab (877-681-6296 / [email protected]); and the Twinrix Pregnancy Registry for women who have received the Twinrix hepatitis A and B vaccine (888-825-5249).
Atypical antipsychotics
TheNational Pregnancy Registry for Atypical Antipsychotics (866-961-2388 / registry@womens mentalhealth.org) is studying 10 drugs: aripiprazole (Abilify), clozapine (Clozaril), iloperidone (Fanapt), paliperidone (Invega), lurasidone (Latuda), risperidone (Risperdal), asenapine (Saphris), quetiapine (Seroquel), ziprasidone (Geodon), and olanzapine (Zyprexa).
TAPP registry
Acitretin is under study by the Take Action to Prevent Pregnancy (T.A.P.P.) registry (855-850-2138 / www.tevagenerics.com/acitretin).
MPS I registry
The MPS I (Mucopolysaccharidosis I) Registry, Genzyme Corp (617-591-5500 / [email protected]), is studying the use of laronidase (Aldurazyme) for Hurler syndrome, Scheie syndrome, and Hurler-Scheie syndrome.
MPS VI registry
The use of galsulfase (Naglazyme) for Maroteaux-Lamy syndrome during pregnancy is under study by the Mucopolysaccharidosis VI (MPS VI) Clinical Surveillance Program(415-506-6849 or 415-506-6703)
Epilepsy drugs
The Antiepileptic Drug Pregnancy Registry (888-233-2334 / www.aedpregnancyregistry.org) is studying antiepileptic drugs.
Type 2 diabetes
The Exenatide Pregnancy Registry, conducted by INC Research for AstraZeneca (800-633-9081 / www.exenatidepregnancyregistry.com), examines the use of exenatide (Byetta, Bydureon) for the treatment of type 2 diabetes.
Renal transplant
Renal transplant patients exposed to mycophenolate (Cellcept) can be enrolled in the Mycophenolate Pregnancy Registry (800-617-8191) or the National Transplantation Pregnancy Registry (877-955-6877). The NTPR is enrolling renal transplant patients exposed to belatacept (Nulojix).
Cymbalta
The antidepressant duloxetine (Cymbalta), when used for major depressive or generalized anxiety disorders, diabetic peripheral neuropathic pain, or fibromyalgia is being studied by the Cymbalta Pregnancy Registry, INC Research ([email protected] / www.cymbaltapregancyregistry.com).
Fabry disease
The Fabry Registry, Genzyme Corp (617-581-5500 / [email protected]) is studying the use in pregnancy of agalsidase beta (Fabrazyme) for Fabry disease.
MS registry
Novartis Pharmaceuticals is conducting the Gilenya (fingolimod) Pregnancy Registry (877-598-7237 / [email protected]) for patients with multiple sclerosis who are taking fingolimod (Gilenya).
Cancer
The MotHER Pregnancy Registry, INC Research (800-690-6720 / [email protected] / themotherpregnancyregistry.com) is enrolling breast cancer patients who have been treated during pregnancy with ado-trastuzumab (Kadcyla), trastuzumab (Herceptin), or pertuzumab (Perjeta).
Merck registries
Merck Pregnancy Registries (800-986-8999) include a registry for type 2 diabetes – sitagliptin/metformin (Janumet) or sitagliptin (Januvia) (www.merckpregancyregistries.com/januvia.html); a registry for rizatriptan (Maxalt) (www.merckpregancyregistries.com/maxalt.html), for migraine headaches; and a registry for the 9-valent human papillomavirus (HPV) (Gardasil 9) vaccine (www.merckpregnancyregistries.com/gardasil9.html).
The registry for the asthma drug montelukast (Singulair) and the registry for Gardasil, the quadrivalent HPV vaccine, are now closed to new enrollment, but new cases of pregnancy exposures can be reported to the company at: 877-888-4231.
Osteoporosis
Amgen’s Pregnancy Surveillance Program (800-772-6436) is enrolling pregnant subjects with osteoporosis who are being treated with denosumab (Prolia).
Hepatitis C
The Ribavirin Pregnancy Registry, INC Research (800-593-2214/ [email protected] / ribavirinpregnancyregistry.com) is looking for subjects with hepatitis C who have been treated with ribavirin (Copegus).
Fibromyalgia
The Savella Pregnancy Registry (877-643-3010/ [email protected] / savellapregnancyregistry.com) is looking for patients with fibromyalgia who are being treated with milnacipran (Savella).
Skin infections
An antibacterial, telavancin (Vibativ), indicated for skin infections is being studied by the Vibativ Pregnancy Registry (888-658-4228 / www.clinicaltrial.gov).
Pompe disease
Pregnant women treated with alglucosidase alfa (Myozyme) for Pompe disease can enroll in the Pompe Registry (800-745-4447 x 15500 / www.pompe.com/en/healthcare-professionals/pompe-registry.aspx).
Sleep disorders
Armodafinil (Nuvigil) used to treat excessive sleepiness associated with narcolepsy and other sleep disorders is being studied in the Nuvigil Pregnancy Registry (866-404-4106 / www.nuvigilpregnancyregistry.com). A second drug with the same indication and telephone number, modafinil (Provigil), is in the Provigil Pregnancy Registry (provigilpregnancyregistry.com).
Additional details, including fax numbers and addresses of the registries reviewed above, can be obtained from the FDA website, List of Pregnancy Exposure Registries. Since the strength of a registry is based on numbers, health care professionals are encouraged to enroll potential subjects or have their patients call to enroll themselves.
Before retirement, Mr. Briggs was a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; he remains a clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He had no relevant financial disclosures. Contact him at [email protected].
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of human pregnancy experience. The new Food and Drug Administration prescription drug information format may increase the amount of information available, at least from registries conducted by drug manufacturers, because they will be required to list all substantive changes made within the year. Although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of pregnancy registries are their prospective nature and enrollment across a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions).
Registries can identify early signals of teratogenicity, but they have several limitations, including voluntary reporting that results in selection bias, pregnancies lost to follow-up that may have had different outcomes than those with documented outcomes, and a lack of control groups (with some exceptions).
Other limitations are that registry cases are not representative of target populations, and pregnancies lost to follow-up lack details on elective terminations and fetal deaths without birth defects, as well as all spontaneous abortions. Additionally, publication of results may be delayed and does not appear in a peer-reviewed journal.
Since the total number of exposed pregnancies is unknown, registry data cannot be used to calculate prevalence, but can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports, which are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. However, they may be helpful in detecting unusual patterns of birth defects.
MothertoBaby Registry
The large Organization of Teratology Information Specialists (OTIS) MothertoBaby registry (877-311-8972) involves four different categories: autoimmune diseases(rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma; antiviral agents; and vaccines.
The drugs for autoimmune diseases are tocilizumab (Actemra), leflunomide (Arava), teriflunomide (Aubagio), certolizumab pegol (Cimzia), etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), apremilast (Otezla), ustekinumab (Stelara), tofacitinib (Xeljanz), infliximab (Remicade), and methotrexate.
The asthma drugs are formoterol (Foradil, Perforomist, Symbicort, Dulera), albuterol, levalbuterol, metaproterenol, pirbuterol (Maxair), and terbutaline.
The antiviral agents are oseltamivir (Tamiflu) and zanamivir (Relenza).
The vaccines include pertussis (Tdap), seasonal influenza, and meningococcal disease (Menveo). In addition to a control group in each category, a unique aspect of this registry is that many exposed infants will undergo dysmorphology examinations.
Vaccine registries
A second vaccine for meningococcal disease (Menactra) is being studied in the Menactra vaccine Pregnancy Registry (800-822-2463 / www.sanofipasteurpregnancy.com).
Two other registries also include influenza vaccines: Flu vaccine Pregnancy Registry, PPD (Flucelvax) (877-413-4759 / email: [email protected]) and the GSK Seasonal Influenza Vaccine Pregnancy Registry, GlaxoSmithKline (888-825-5249 / pregnancyregistry.gsk.com/seasonalinfluenzavaccines.html), which includes the Fluarix and FluLaval vaccines.
Asthma
Women with asthma who are being treated with omalizumab (Xolair) are the target population for the EXPECT Pregnancy Registry (866-496-5247 Option 3 / www.xolairpregnancyregistry.com).
GSK registries
GlaxoSmithKline is also conducting three other registries: the Belimumab (Benlysta) Pregnancy Registry for patients with systemic lupus erythematosus treated with belimumab (877-681-6296 / [email protected]); and the Twinrix Pregnancy Registry for women who have received the Twinrix hepatitis A and B vaccine (888-825-5249).
Atypical antipsychotics
TheNational Pregnancy Registry for Atypical Antipsychotics (866-961-2388 / registry@womens mentalhealth.org) is studying 10 drugs: aripiprazole (Abilify), clozapine (Clozaril), iloperidone (Fanapt), paliperidone (Invega), lurasidone (Latuda), risperidone (Risperdal), asenapine (Saphris), quetiapine (Seroquel), ziprasidone (Geodon), and olanzapine (Zyprexa).
TAPP registry
Acitretin is under study by the Take Action to Prevent Pregnancy (T.A.P.P.) registry (855-850-2138 / www.tevagenerics.com/acitretin).
MPS I registry
The MPS I (Mucopolysaccharidosis I) Registry, Genzyme Corp (617-591-5500 / [email protected]), is studying the use of laronidase (Aldurazyme) for Hurler syndrome, Scheie syndrome, and Hurler-Scheie syndrome.
MPS VI registry
The use of galsulfase (Naglazyme) for Maroteaux-Lamy syndrome during pregnancy is under study by the Mucopolysaccharidosis VI (MPS VI) Clinical Surveillance Program(415-506-6849 or 415-506-6703)
Epilepsy drugs
The Antiepileptic Drug Pregnancy Registry (888-233-2334 / www.aedpregnancyregistry.org) is studying antiepileptic drugs.
Type 2 diabetes
The Exenatide Pregnancy Registry, conducted by INC Research for AstraZeneca (800-633-9081 / www.exenatidepregnancyregistry.com), examines the use of exenatide (Byetta, Bydureon) for the treatment of type 2 diabetes.
Renal transplant
Renal transplant patients exposed to mycophenolate (Cellcept) can be enrolled in the Mycophenolate Pregnancy Registry (800-617-8191) or the National Transplantation Pregnancy Registry (877-955-6877). The NTPR is enrolling renal transplant patients exposed to belatacept (Nulojix).
Cymbalta
The antidepressant duloxetine (Cymbalta), when used for major depressive or generalized anxiety disorders, diabetic peripheral neuropathic pain, or fibromyalgia is being studied by the Cymbalta Pregnancy Registry, INC Research ([email protected] / www.cymbaltapregancyregistry.com).
Fabry disease
The Fabry Registry, Genzyme Corp (617-581-5500 / [email protected]) is studying the use in pregnancy of agalsidase beta (Fabrazyme) for Fabry disease.
MS registry
Novartis Pharmaceuticals is conducting the Gilenya (fingolimod) Pregnancy Registry (877-598-7237 / [email protected]) for patients with multiple sclerosis who are taking fingolimod (Gilenya).
Cancer
The MotHER Pregnancy Registry, INC Research (800-690-6720 / [email protected] / themotherpregnancyregistry.com) is enrolling breast cancer patients who have been treated during pregnancy with ado-trastuzumab (Kadcyla), trastuzumab (Herceptin), or pertuzumab (Perjeta).
Merck registries
Merck Pregnancy Registries (800-986-8999) include a registry for type 2 diabetes – sitagliptin/metformin (Janumet) or sitagliptin (Januvia) (www.merckpregancyregistries.com/januvia.html); a registry for rizatriptan (Maxalt) (www.merckpregancyregistries.com/maxalt.html), for migraine headaches; and a registry for the 9-valent human papillomavirus (HPV) (Gardasil 9) vaccine (www.merckpregnancyregistries.com/gardasil9.html).
The registry for the asthma drug montelukast (Singulair) and the registry for Gardasil, the quadrivalent HPV vaccine, are now closed to new enrollment, but new cases of pregnancy exposures can be reported to the company at: 877-888-4231.
Osteoporosis
Amgen’s Pregnancy Surveillance Program (800-772-6436) is enrolling pregnant subjects with osteoporosis who are being treated with denosumab (Prolia).
Hepatitis C
The Ribavirin Pregnancy Registry, INC Research (800-593-2214/ [email protected] / ribavirinpregnancyregistry.com) is looking for subjects with hepatitis C who have been treated with ribavirin (Copegus).
Fibromyalgia
The Savella Pregnancy Registry (877-643-3010/ [email protected] / savellapregnancyregistry.com) is looking for patients with fibromyalgia who are being treated with milnacipran (Savella).
Skin infections
An antibacterial, telavancin (Vibativ), indicated for skin infections is being studied by the Vibativ Pregnancy Registry (888-658-4228 / www.clinicaltrial.gov).
Pompe disease
Pregnant women treated with alglucosidase alfa (Myozyme) for Pompe disease can enroll in the Pompe Registry (800-745-4447 x 15500 / www.pompe.com/en/healthcare-professionals/pompe-registry.aspx).
Sleep disorders
Armodafinil (Nuvigil) used to treat excessive sleepiness associated with narcolepsy and other sleep disorders is being studied in the Nuvigil Pregnancy Registry (866-404-4106 / www.nuvigilpregnancyregistry.com). A second drug with the same indication and telephone number, modafinil (Provigil), is in the Provigil Pregnancy Registry (provigilpregnancyregistry.com).
Additional details, including fax numbers and addresses of the registries reviewed above, can be obtained from the FDA website, List of Pregnancy Exposure Registries. Since the strength of a registry is based on numbers, health care professionals are encouraged to enroll potential subjects or have their patients call to enroll themselves.
Before retirement, Mr. Briggs was a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; he remains a clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He had no relevant financial disclosures. Contact him at [email protected].
Preterm birth rate continues to drop
For the 7th straight year, the rate of preterm births in the United States has decreased, with 11.4% of babies being born preterm in 2013, compared with 11.5% in 2012, according to a new federal report.
The report, America’s Children: Key National Indicators of Well-Being, 2015, was compiled by the Federal Interagency Forum on Child and Family Statistics, and includes participants from 23 federal agencies. The report attempts to quantify children’s well-being based on key indicators in seven domains: family and social environment, economic circumstances, health care, physical environment and safety, behavior, education, and health.
The percentage of preterm births – babies born before 37 weeks’ gestation – along with low-birth-weight infants had been rising for several decades, but the latest report shows progress in both areas. The preterm birth rate fell from a high of 12.8% in 2006 to 11.4% in 2013; the percentage of infants born with low birth weight (less than 5 pounds 8 ounces) was 8.0% in 2013, compared with 8.3% in 2006.
In addition, in 2013, the teen birth rate hit 12/1,000 adolescents aged 15-17 years, a record low, according to the report. In 2007, the teen birth rate was more than 40% higher at 22 births/1,000 adolescents.
But major depressive disorder among adolescents is on the rise, with 11% of youth aged 12-17 years experiencing a major depressive episode in 2013, compared with 9% in 2004. And only 38% of young adults in 2013 received treatment for such an episode, compared to 40% in 2004.
For the 7th straight year, the rate of preterm births in the United States has decreased, with 11.4% of babies being born preterm in 2013, compared with 11.5% in 2012, according to a new federal report.
The report, America’s Children: Key National Indicators of Well-Being, 2015, was compiled by the Federal Interagency Forum on Child and Family Statistics, and includes participants from 23 federal agencies. The report attempts to quantify children’s well-being based on key indicators in seven domains: family and social environment, economic circumstances, health care, physical environment and safety, behavior, education, and health.
The percentage of preterm births – babies born before 37 weeks’ gestation – along with low-birth-weight infants had been rising for several decades, but the latest report shows progress in both areas. The preterm birth rate fell from a high of 12.8% in 2006 to 11.4% in 2013; the percentage of infants born with low birth weight (less than 5 pounds 8 ounces) was 8.0% in 2013, compared with 8.3% in 2006.
In addition, in 2013, the teen birth rate hit 12/1,000 adolescents aged 15-17 years, a record low, according to the report. In 2007, the teen birth rate was more than 40% higher at 22 births/1,000 adolescents.
But major depressive disorder among adolescents is on the rise, with 11% of youth aged 12-17 years experiencing a major depressive episode in 2013, compared with 9% in 2004. And only 38% of young adults in 2013 received treatment for such an episode, compared to 40% in 2004.
For the 7th straight year, the rate of preterm births in the United States has decreased, with 11.4% of babies being born preterm in 2013, compared with 11.5% in 2012, according to a new federal report.
The report, America’s Children: Key National Indicators of Well-Being, 2015, was compiled by the Federal Interagency Forum on Child and Family Statistics, and includes participants from 23 federal agencies. The report attempts to quantify children’s well-being based on key indicators in seven domains: family and social environment, economic circumstances, health care, physical environment and safety, behavior, education, and health.
The percentage of preterm births – babies born before 37 weeks’ gestation – along with low-birth-weight infants had been rising for several decades, but the latest report shows progress in both areas. The preterm birth rate fell from a high of 12.8% in 2006 to 11.4% in 2013; the percentage of infants born with low birth weight (less than 5 pounds 8 ounces) was 8.0% in 2013, compared with 8.3% in 2006.
In addition, in 2013, the teen birth rate hit 12/1,000 adolescents aged 15-17 years, a record low, according to the report. In 2007, the teen birth rate was more than 40% higher at 22 births/1,000 adolescents.
But major depressive disorder among adolescents is on the rise, with 11% of youth aged 12-17 years experiencing a major depressive episode in 2013, compared with 9% in 2004. And only 38% of young adults in 2013 received treatment for such an episode, compared to 40% in 2004.