User login
Eculizumab benefited pregnant women with paroxysmal nocturnal hemoglobinuria
Eculizumab therapy led to “acceptable” outcomes among pregnant women with paroxysmal nocturnal hemoglobinuria, investigators reported Sept. 9 in the New England Journal of Medicine.
No woman who received eculizumab died while pregnant or within 6 months of delivery; historic mortality rates for these patients are 8%-20%, said Dr. Richard Kelly at St. James’s University Hospital in Leeds, England, and his associates. Treatment with the monoclonal antibody, “has reduced mortality and morbidity associated with PNH and has allowed patients who were previously severely affected to lead a relatively normal life.”
The fetal death rate was 4%, resembling rates of 4%-9% from the era before eculizumab, the researchers said. The rate of premature births also was high (29%) as a result of preeclampsia, suspected intrauterine growth retardation, maternal thrombocytopenia, and slowed fetal movements, they said.
Paroxysmal nocturnal hemoglobinuria is a rare, chronic stem-cell disease in which complement-mediated intravascular hemolysis causes anemia, fatigue, and venous thromboembolism (Adv Exp Med Biol. 2013;735:155-72.). Increased complement activation during pregnancy intensifies the risk of severe hemolytic anemia, fetal morbidity, and fetal mortality for women with PNH. Eculizumab blocks terminal complement activation by binding complement protein C5, and has improved PNH symptoms to the extent that treated women are more likely to consider pregnancy than in the past.
Dr. Kelly and his associates surveyed members of the International PNH Interest Group to study pregnancy outcomes among these women (N Engl J. Med. 2015;373:1032-9). A total of 80% of clinicians responded, reporting data for 75 pregnancies among 61 women, the investigators said. All patients had PNH diagnosed by flow cytometry, and 61% had begun eculizumab therapy before conception. Median age at first pregnancy was 29 years, with a range of 18 to 40 years. The patients received weekly 600-mg IV infusions for 4 weeks, followed by 900 mg every 14 days. Clinicians increased the dose or treatment frequency at their own discretion if patients showed signs of breakthrough intravascular hemolysis.
Two women experienced thrombotic events during treatment, both of which happened soon after delivery. One was a lower-limb deep venous thrombosis, but the other occurred after a patient received a plasma infusion for postpartum hemorrhage. “Plasma contains high levels of complement and can overcome the effects of eculizumab and thereby render the patient susceptible to complications of PNH,” noted the investigators. “The use of plasma should thus be avoided if possible.” Ten samples of breast milk were negative for eculizumab, they added.
Dr. Kelly and 14 of 15 coauthors reported financial relationships outside this work with Alexion Pharmaceuticals, the maker of eculizumab. One coauthor also reported grant support outside this work from Alnylam Pharmaceuticals, Novartis, and Ra Pharma.
Eculizumab therapy led to “acceptable” outcomes among pregnant women with paroxysmal nocturnal hemoglobinuria, investigators reported Sept. 9 in the New England Journal of Medicine.
No woman who received eculizumab died while pregnant or within 6 months of delivery; historic mortality rates for these patients are 8%-20%, said Dr. Richard Kelly at St. James’s University Hospital in Leeds, England, and his associates. Treatment with the monoclonal antibody, “has reduced mortality and morbidity associated with PNH and has allowed patients who were previously severely affected to lead a relatively normal life.”
The fetal death rate was 4%, resembling rates of 4%-9% from the era before eculizumab, the researchers said. The rate of premature births also was high (29%) as a result of preeclampsia, suspected intrauterine growth retardation, maternal thrombocytopenia, and slowed fetal movements, they said.
Paroxysmal nocturnal hemoglobinuria is a rare, chronic stem-cell disease in which complement-mediated intravascular hemolysis causes anemia, fatigue, and venous thromboembolism (Adv Exp Med Biol. 2013;735:155-72.). Increased complement activation during pregnancy intensifies the risk of severe hemolytic anemia, fetal morbidity, and fetal mortality for women with PNH. Eculizumab blocks terminal complement activation by binding complement protein C5, and has improved PNH symptoms to the extent that treated women are more likely to consider pregnancy than in the past.
Dr. Kelly and his associates surveyed members of the International PNH Interest Group to study pregnancy outcomes among these women (N Engl J. Med. 2015;373:1032-9). A total of 80% of clinicians responded, reporting data for 75 pregnancies among 61 women, the investigators said. All patients had PNH diagnosed by flow cytometry, and 61% had begun eculizumab therapy before conception. Median age at first pregnancy was 29 years, with a range of 18 to 40 years. The patients received weekly 600-mg IV infusions for 4 weeks, followed by 900 mg every 14 days. Clinicians increased the dose or treatment frequency at their own discretion if patients showed signs of breakthrough intravascular hemolysis.
Two women experienced thrombotic events during treatment, both of which happened soon after delivery. One was a lower-limb deep venous thrombosis, but the other occurred after a patient received a plasma infusion for postpartum hemorrhage. “Plasma contains high levels of complement and can overcome the effects of eculizumab and thereby render the patient susceptible to complications of PNH,” noted the investigators. “The use of plasma should thus be avoided if possible.” Ten samples of breast milk were negative for eculizumab, they added.
Dr. Kelly and 14 of 15 coauthors reported financial relationships outside this work with Alexion Pharmaceuticals, the maker of eculizumab. One coauthor also reported grant support outside this work from Alnylam Pharmaceuticals, Novartis, and Ra Pharma.
Eculizumab therapy led to “acceptable” outcomes among pregnant women with paroxysmal nocturnal hemoglobinuria, investigators reported Sept. 9 in the New England Journal of Medicine.
No woman who received eculizumab died while pregnant or within 6 months of delivery; historic mortality rates for these patients are 8%-20%, said Dr. Richard Kelly at St. James’s University Hospital in Leeds, England, and his associates. Treatment with the monoclonal antibody, “has reduced mortality and morbidity associated with PNH and has allowed patients who were previously severely affected to lead a relatively normal life.”
The fetal death rate was 4%, resembling rates of 4%-9% from the era before eculizumab, the researchers said. The rate of premature births also was high (29%) as a result of preeclampsia, suspected intrauterine growth retardation, maternal thrombocytopenia, and slowed fetal movements, they said.
Paroxysmal nocturnal hemoglobinuria is a rare, chronic stem-cell disease in which complement-mediated intravascular hemolysis causes anemia, fatigue, and venous thromboembolism (Adv Exp Med Biol. 2013;735:155-72.). Increased complement activation during pregnancy intensifies the risk of severe hemolytic anemia, fetal morbidity, and fetal mortality for women with PNH. Eculizumab blocks terminal complement activation by binding complement protein C5, and has improved PNH symptoms to the extent that treated women are more likely to consider pregnancy than in the past.
Dr. Kelly and his associates surveyed members of the International PNH Interest Group to study pregnancy outcomes among these women (N Engl J. Med. 2015;373:1032-9). A total of 80% of clinicians responded, reporting data for 75 pregnancies among 61 women, the investigators said. All patients had PNH diagnosed by flow cytometry, and 61% had begun eculizumab therapy before conception. Median age at first pregnancy was 29 years, with a range of 18 to 40 years. The patients received weekly 600-mg IV infusions for 4 weeks, followed by 900 mg every 14 days. Clinicians increased the dose or treatment frequency at their own discretion if patients showed signs of breakthrough intravascular hemolysis.
Two women experienced thrombotic events during treatment, both of which happened soon after delivery. One was a lower-limb deep venous thrombosis, but the other occurred after a patient received a plasma infusion for postpartum hemorrhage. “Plasma contains high levels of complement and can overcome the effects of eculizumab and thereby render the patient susceptible to complications of PNH,” noted the investigators. “The use of plasma should thus be avoided if possible.” Ten samples of breast milk were negative for eculizumab, they added.
Dr. Kelly and 14 of 15 coauthors reported financial relationships outside this work with Alexion Pharmaceuticals, the maker of eculizumab. One coauthor also reported grant support outside this work from Alnylam Pharmaceuticals, Novartis, and Ra Pharma.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: None of the pregnant women with paroxysmal nocturnal hemoglobinuria died on eculizumab therapy.
Major finding: The fetal death rate was 4%, and the premature birth rate was 29%.
Data source: A retrospective, survey-based study of 75 pregnancies among 61 women with PNH.
Disclosures: Dr. Kelly and 14 of 15 coauthors reported financial relationships outside this work with Alexion Pharmaceuticals, the maker of eculizumab. One coauthor also reported grant support outside this work from Alnylam Pharmaceuticals, Novartis, and Ra Pharma.
Better outcomes seen for some extremely preterm infants
There has been a significant increase in survival without major neonatal morbidity for infants born at 25-28 weeks’ gestation in the United States in the past 2 decades, researchers reported online Sept. 8 in JAMA.
Survival without major complications improved by about 2% per year for these babies, said Dr. Barbara Stoll of Emory University in Atlanta and her associates. But outcomes for earlier preterm births were mixed. “Although overall survival increased for infants aged 23 and 24 weeks, few infants younger than 25 weeks’ gestational age survived without major neonatal morbidity, underscoring the continued need for interventions to improve outcomes for the most immature infants,” the investigators said.
Despite advances in perinatal care, preterm infants face disproportionate rates of morbidity and mortality. The investigators studied outcomes for 34,636 such babies who were born between 1993 and 2012 at 26 U.S. Neonatal Research Network centers, including 8 that participated for the entire study. All the infants were born at 22-28 weeks’ gestation and weighed 401-1,500 g at birth (JAMA 2015;314[10]:1039-51).
“The percent of infants from a multiple birth increased from 18% in 1993 to 27% in 1998 (P less than .001),with no further increase noted,” Dr. Stoll and her associates reported.
Between 2009 and 2012, survival improved the most for infants born at 23 weeks’ gestation (from 27% to 33%) and at 24 weeks’ gestation (from 63% to 65%). But survival increased only slightly for infants born at 25 and 27 weeks’ gestation, and remained static for infants born at 22, 26, and 28 weeks. Furthermore, although survival without major morbidity improved markedly for infants born at 25-28 weeks, it did not change for those born at 24 weeks.
Rates of late-onset sepsis fell among all gestational age groups, but bronchopulmonary dysplasia rose significantly (P less than .001). The latter “may partly be explained by increased active resuscitation, intensive care, and increased survival, especially for the most immature infants,” the investigators said. Use of prenatal corticosteroids rose from 24% to 87% during the study period (P less than .001), as did rates of cesarean delivery (from 44% to 64%; P less than .001), they added.
The preterm registry used in the study was supported by the National Institutes of Health, National Institute of Child Health and Human Development, National Center for Research Resources, and National Center for Advancing Translational Sciences. The investigators reported no financial disclosures.
This article provides an important historical perspective over the last 2 decades in neonatal-perinatal medicine and the most recent update on trends in neonatal care. Although there has been progress, it is clear that there are still a substantial number of extremely preterm infants who either die or survive after experiencing one or more major neonatal morbidities known to be associated with both short- and long-term adverse consequences. Hence, an additional commitment must be made to further improvements.
There is no obvious breakthrough therapy emerging in the coming years. Perhaps cellular therapy, such as mesenchymal stem cells, will be an important advance in the care of these fragile infants. However, it is more likely that incremental change, such as applying quality improvement practices to outcomes other than nosocomial infection, will lead to improved outcomes.
Dr. Roger F. Soll is at the neonatal-perinatal medicine division, department of pediatrics, University of Vermont Medical Center, Burlington. These comments were excerpted from his accompanying editorial (JAMA 2015;314[10]:1007-8). He reported receiving personal fees from the Vermont Oxford Network outside this work.
This article provides an important historical perspective over the last 2 decades in neonatal-perinatal medicine and the most recent update on trends in neonatal care. Although there has been progress, it is clear that there are still a substantial number of extremely preterm infants who either die or survive after experiencing one or more major neonatal morbidities known to be associated with both short- and long-term adverse consequences. Hence, an additional commitment must be made to further improvements.
There is no obvious breakthrough therapy emerging in the coming years. Perhaps cellular therapy, such as mesenchymal stem cells, will be an important advance in the care of these fragile infants. However, it is more likely that incremental change, such as applying quality improvement practices to outcomes other than nosocomial infection, will lead to improved outcomes.
Dr. Roger F. Soll is at the neonatal-perinatal medicine division, department of pediatrics, University of Vermont Medical Center, Burlington. These comments were excerpted from his accompanying editorial (JAMA 2015;314[10]:1007-8). He reported receiving personal fees from the Vermont Oxford Network outside this work.
This article provides an important historical perspective over the last 2 decades in neonatal-perinatal medicine and the most recent update on trends in neonatal care. Although there has been progress, it is clear that there are still a substantial number of extremely preterm infants who either die or survive after experiencing one or more major neonatal morbidities known to be associated with both short- and long-term adverse consequences. Hence, an additional commitment must be made to further improvements.
There is no obvious breakthrough therapy emerging in the coming years. Perhaps cellular therapy, such as mesenchymal stem cells, will be an important advance in the care of these fragile infants. However, it is more likely that incremental change, such as applying quality improvement practices to outcomes other than nosocomial infection, will lead to improved outcomes.
Dr. Roger F. Soll is at the neonatal-perinatal medicine division, department of pediatrics, University of Vermont Medical Center, Burlington. These comments were excerpted from his accompanying editorial (JAMA 2015;314[10]:1007-8). He reported receiving personal fees from the Vermont Oxford Network outside this work.
There has been a significant increase in survival without major neonatal morbidity for infants born at 25-28 weeks’ gestation in the United States in the past 2 decades, researchers reported online Sept. 8 in JAMA.
Survival without major complications improved by about 2% per year for these babies, said Dr. Barbara Stoll of Emory University in Atlanta and her associates. But outcomes for earlier preterm births were mixed. “Although overall survival increased for infants aged 23 and 24 weeks, few infants younger than 25 weeks’ gestational age survived without major neonatal morbidity, underscoring the continued need for interventions to improve outcomes for the most immature infants,” the investigators said.
Despite advances in perinatal care, preterm infants face disproportionate rates of morbidity and mortality. The investigators studied outcomes for 34,636 such babies who were born between 1993 and 2012 at 26 U.S. Neonatal Research Network centers, including 8 that participated for the entire study. All the infants were born at 22-28 weeks’ gestation and weighed 401-1,500 g at birth (JAMA 2015;314[10]:1039-51).
“The percent of infants from a multiple birth increased from 18% in 1993 to 27% in 1998 (P less than .001),with no further increase noted,” Dr. Stoll and her associates reported.
Between 2009 and 2012, survival improved the most for infants born at 23 weeks’ gestation (from 27% to 33%) and at 24 weeks’ gestation (from 63% to 65%). But survival increased only slightly for infants born at 25 and 27 weeks’ gestation, and remained static for infants born at 22, 26, and 28 weeks. Furthermore, although survival without major morbidity improved markedly for infants born at 25-28 weeks, it did not change for those born at 24 weeks.
Rates of late-onset sepsis fell among all gestational age groups, but bronchopulmonary dysplasia rose significantly (P less than .001). The latter “may partly be explained by increased active resuscitation, intensive care, and increased survival, especially for the most immature infants,” the investigators said. Use of prenatal corticosteroids rose from 24% to 87% during the study period (P less than .001), as did rates of cesarean delivery (from 44% to 64%; P less than .001), they added.
The preterm registry used in the study was supported by the National Institutes of Health, National Institute of Child Health and Human Development, National Center for Research Resources, and National Center for Advancing Translational Sciences. The investigators reported no financial disclosures.
There has been a significant increase in survival without major neonatal morbidity for infants born at 25-28 weeks’ gestation in the United States in the past 2 decades, researchers reported online Sept. 8 in JAMA.
Survival without major complications improved by about 2% per year for these babies, said Dr. Barbara Stoll of Emory University in Atlanta and her associates. But outcomes for earlier preterm births were mixed. “Although overall survival increased for infants aged 23 and 24 weeks, few infants younger than 25 weeks’ gestational age survived without major neonatal morbidity, underscoring the continued need for interventions to improve outcomes for the most immature infants,” the investigators said.
Despite advances in perinatal care, preterm infants face disproportionate rates of morbidity and mortality. The investigators studied outcomes for 34,636 such babies who were born between 1993 and 2012 at 26 U.S. Neonatal Research Network centers, including 8 that participated for the entire study. All the infants were born at 22-28 weeks’ gestation and weighed 401-1,500 g at birth (JAMA 2015;314[10]:1039-51).
“The percent of infants from a multiple birth increased from 18% in 1993 to 27% in 1998 (P less than .001),with no further increase noted,” Dr. Stoll and her associates reported.
Between 2009 and 2012, survival improved the most for infants born at 23 weeks’ gestation (from 27% to 33%) and at 24 weeks’ gestation (from 63% to 65%). But survival increased only slightly for infants born at 25 and 27 weeks’ gestation, and remained static for infants born at 22, 26, and 28 weeks. Furthermore, although survival without major morbidity improved markedly for infants born at 25-28 weeks, it did not change for those born at 24 weeks.
Rates of late-onset sepsis fell among all gestational age groups, but bronchopulmonary dysplasia rose significantly (P less than .001). The latter “may partly be explained by increased active resuscitation, intensive care, and increased survival, especially for the most immature infants,” the investigators said. Use of prenatal corticosteroids rose from 24% to 87% during the study period (P less than .001), as did rates of cesarean delivery (from 44% to 64%; P less than .001), they added.
The preterm registry used in the study was supported by the National Institutes of Health, National Institute of Child Health and Human Development, National Center for Research Resources, and National Center for Advancing Translational Sciences. The investigators reported no financial disclosures.
FROM JAMA
Key clinical point: There has been a significant increase in survival without major neonatal morbidity for infants born at 25-28 weeks’ gestation in the United States in the past 2 decades.
Major finding: Survival without major morbidity rose by about 2% per year for infants born at 25-28 weeks’ gestation.
Data source: A prospective registry study of 34,636 extremely preterm infants born at U.S. academic centers between 1993 and 2012.
Disclosures: The preterm registry used in the study was supported by the National Institutes of Health, National Institute of Child Health and Human Development, National Center for Research Resources, and National Center for Advancing Translational Sciences. The investigators reported no financial disclosures.
USPSTF silent on iron supplementation in pregnancy
The U.S. Preventive Services Task Force announced, in a recommendation on Sept. 7, that it is unsure whether pregnant women who are asymptomatic for iron deficiency anemia should be screened for this condition or take iron supplements.
The recommendation is an update to the 2006 USPSTF recommendation, which also expressed uncertainty on whether iron supplementation is beneficial to pregnant women.
“Both the 2006 and the current recommendation statements found insufficient evidence to determine the balance of the benefits and harms of iron supplementation during pregnancy,” Dr. Albert L. Siu wrote on behalf of members of the USPSTF.
The 2006 recommendation differed from the current one in that it had advocated for routine screening for iron deficiency anemia in pregnant women.
“In its review of the evidence to update the 2006 recommendation, the USPSTF found no good- or fair-quality studies on the benefits or harms of screening that would be applicable to the current U.S. population of pregnant women,” according to the task force recommendation statement published in the Annals of Internal Medicine.
The USPSTF based the updated recommendation on a systematic evidence review, which focused on whether pregnant women and adolescents’ use of oral iron supplementation or treatment was associated with changes in iron status and improvement in maternal and infant health outcomes. It reviewed “studies conducted in settings similar to the United States in rates of malnutrition, hemoparasite burden, and general socioeconomic status.”
The USPSTF’s review included 12 “good- or fair-quality randomized controlled trials,” which evaluated the effects of iron supplementation on various maternal hematologic indexes, including hemoglobin level, serum ferritin level, anemia, iron deficiency, and iron deficiency anemia.
Eight of the studies reported maternal hemoglobin levels at term or delivery, with six of the studies having reported a significantly higher mean hemoglobin level in the supplemented groups than in the control groups (122-139 g/L vs. 115-128 g/L, respectively). Seven studies reported serum ferritin levels at term or delivery, with five of these having reported a significantly higher ferritin level in the supplemented groups, compared with the control groups (12.0-30.0 mcg/L vs. 6.2-24.9 mcg/L, respectively).
“Although adequate evidence shows that [iron] supplementation increases hemoglobin and ferritin levels, the evidence is unclear on whether this increase leads to an improvement in maternal and fetal outcomes. In most of these studies, the supplemented groups had higher mean hemoglobin levels than the nonsupplemented groups; however, both groups reported values within normal limits,” the USPSTF wrote.
Research on the harmful effects of iron supplementation in pregnant women better addressed the USPSTF’s questions; 10 of the trials showed that the harms of iron supplementation during pregnancy were “small to none,” with most of the harms reported having been nausea, constipation, and diarrhea.
While the USPSTF found adequate evidence about the lack of harm from iron supplementation, it failed to find adequate evidence on the benefits of supplementation.
“Reported benefits of supplementation were limited to intermediate outcomes (maternal hematologic indexes), and evidence on the benefits of supplementation on maternal and infant health outcomes was inadequate because of inconsistent results and underpowered studies,” the USPSTF wrote.
The group found less research on the outcomes of screening for iron deficiency anemia in asymptomatic pregnant women and adolescents. “No good- or fair quality studies were found that evaluated the benefits or harms of screening in this population,” according to the recommendation.
The USPSTF concluded that the “current evidence is insufficient to assess the balance of benefits and harms of screening for iron deficiency anemia in pregnant women … [and] of routine iron supplementation for pregnant women to prevent adverse maternal health and birth outcomes.”
Read the full recommendation in Annals of Internal Medicine (doi: 10.7326/M15-1707).
The U.S. Preventive Services Task Force announced, in a recommendation on Sept. 7, that it is unsure whether pregnant women who are asymptomatic for iron deficiency anemia should be screened for this condition or take iron supplements.
The recommendation is an update to the 2006 USPSTF recommendation, which also expressed uncertainty on whether iron supplementation is beneficial to pregnant women.
“Both the 2006 and the current recommendation statements found insufficient evidence to determine the balance of the benefits and harms of iron supplementation during pregnancy,” Dr. Albert L. Siu wrote on behalf of members of the USPSTF.
The 2006 recommendation differed from the current one in that it had advocated for routine screening for iron deficiency anemia in pregnant women.
“In its review of the evidence to update the 2006 recommendation, the USPSTF found no good- or fair-quality studies on the benefits or harms of screening that would be applicable to the current U.S. population of pregnant women,” according to the task force recommendation statement published in the Annals of Internal Medicine.
The USPSTF based the updated recommendation on a systematic evidence review, which focused on whether pregnant women and adolescents’ use of oral iron supplementation or treatment was associated with changes in iron status and improvement in maternal and infant health outcomes. It reviewed “studies conducted in settings similar to the United States in rates of malnutrition, hemoparasite burden, and general socioeconomic status.”
The USPSTF’s review included 12 “good- or fair-quality randomized controlled trials,” which evaluated the effects of iron supplementation on various maternal hematologic indexes, including hemoglobin level, serum ferritin level, anemia, iron deficiency, and iron deficiency anemia.
Eight of the studies reported maternal hemoglobin levels at term or delivery, with six of the studies having reported a significantly higher mean hemoglobin level in the supplemented groups than in the control groups (122-139 g/L vs. 115-128 g/L, respectively). Seven studies reported serum ferritin levels at term or delivery, with five of these having reported a significantly higher ferritin level in the supplemented groups, compared with the control groups (12.0-30.0 mcg/L vs. 6.2-24.9 mcg/L, respectively).
“Although adequate evidence shows that [iron] supplementation increases hemoglobin and ferritin levels, the evidence is unclear on whether this increase leads to an improvement in maternal and fetal outcomes. In most of these studies, the supplemented groups had higher mean hemoglobin levels than the nonsupplemented groups; however, both groups reported values within normal limits,” the USPSTF wrote.
Research on the harmful effects of iron supplementation in pregnant women better addressed the USPSTF’s questions; 10 of the trials showed that the harms of iron supplementation during pregnancy were “small to none,” with most of the harms reported having been nausea, constipation, and diarrhea.
While the USPSTF found adequate evidence about the lack of harm from iron supplementation, it failed to find adequate evidence on the benefits of supplementation.
“Reported benefits of supplementation were limited to intermediate outcomes (maternal hematologic indexes), and evidence on the benefits of supplementation on maternal and infant health outcomes was inadequate because of inconsistent results and underpowered studies,” the USPSTF wrote.
The group found less research on the outcomes of screening for iron deficiency anemia in asymptomatic pregnant women and adolescents. “No good- or fair quality studies were found that evaluated the benefits or harms of screening in this population,” according to the recommendation.
The USPSTF concluded that the “current evidence is insufficient to assess the balance of benefits and harms of screening for iron deficiency anemia in pregnant women … [and] of routine iron supplementation for pregnant women to prevent adverse maternal health and birth outcomes.”
Read the full recommendation in Annals of Internal Medicine (doi: 10.7326/M15-1707).
The U.S. Preventive Services Task Force announced, in a recommendation on Sept. 7, that it is unsure whether pregnant women who are asymptomatic for iron deficiency anemia should be screened for this condition or take iron supplements.
The recommendation is an update to the 2006 USPSTF recommendation, which also expressed uncertainty on whether iron supplementation is beneficial to pregnant women.
“Both the 2006 and the current recommendation statements found insufficient evidence to determine the balance of the benefits and harms of iron supplementation during pregnancy,” Dr. Albert L. Siu wrote on behalf of members of the USPSTF.
The 2006 recommendation differed from the current one in that it had advocated for routine screening for iron deficiency anemia in pregnant women.
“In its review of the evidence to update the 2006 recommendation, the USPSTF found no good- or fair-quality studies on the benefits or harms of screening that would be applicable to the current U.S. population of pregnant women,” according to the task force recommendation statement published in the Annals of Internal Medicine.
The USPSTF based the updated recommendation on a systematic evidence review, which focused on whether pregnant women and adolescents’ use of oral iron supplementation or treatment was associated with changes in iron status and improvement in maternal and infant health outcomes. It reviewed “studies conducted in settings similar to the United States in rates of malnutrition, hemoparasite burden, and general socioeconomic status.”
The USPSTF’s review included 12 “good- or fair-quality randomized controlled trials,” which evaluated the effects of iron supplementation on various maternal hematologic indexes, including hemoglobin level, serum ferritin level, anemia, iron deficiency, and iron deficiency anemia.
Eight of the studies reported maternal hemoglobin levels at term or delivery, with six of the studies having reported a significantly higher mean hemoglobin level in the supplemented groups than in the control groups (122-139 g/L vs. 115-128 g/L, respectively). Seven studies reported serum ferritin levels at term or delivery, with five of these having reported a significantly higher ferritin level in the supplemented groups, compared with the control groups (12.0-30.0 mcg/L vs. 6.2-24.9 mcg/L, respectively).
“Although adequate evidence shows that [iron] supplementation increases hemoglobin and ferritin levels, the evidence is unclear on whether this increase leads to an improvement in maternal and fetal outcomes. In most of these studies, the supplemented groups had higher mean hemoglobin levels than the nonsupplemented groups; however, both groups reported values within normal limits,” the USPSTF wrote.
Research on the harmful effects of iron supplementation in pregnant women better addressed the USPSTF’s questions; 10 of the trials showed that the harms of iron supplementation during pregnancy were “small to none,” with most of the harms reported having been nausea, constipation, and diarrhea.
While the USPSTF found adequate evidence about the lack of harm from iron supplementation, it failed to find adequate evidence on the benefits of supplementation.
“Reported benefits of supplementation were limited to intermediate outcomes (maternal hematologic indexes), and evidence on the benefits of supplementation on maternal and infant health outcomes was inadequate because of inconsistent results and underpowered studies,” the USPSTF wrote.
The group found less research on the outcomes of screening for iron deficiency anemia in asymptomatic pregnant women and adolescents. “No good- or fair quality studies were found that evaluated the benefits or harms of screening in this population,” according to the recommendation.
The USPSTF concluded that the “current evidence is insufficient to assess the balance of benefits and harms of screening for iron deficiency anemia in pregnant women … [and] of routine iron supplementation for pregnant women to prevent adverse maternal health and birth outcomes.”
Read the full recommendation in Annals of Internal Medicine (doi: 10.7326/M15-1707).
FROM ANNALS OF INTERNAL MEDICINE
Reducing maternal mortality in the United States—Let’s get organized!
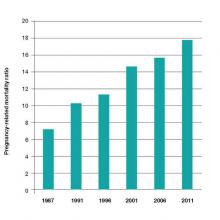
A mother’s untimely death in childbirth is a grave loss that sends shock waves of grief across generations of her family and community. As obstetricians practicing in the United States, we face a terrible problem. We have a continually rising rate of maternal death in a country with exceptional medical resources (FIGURE).1 Our national decentralized approach to dealing with maternal mortality is a factor contributing to the decades-long increase in the maternal mortality ratio. Let’s get organized to better respond to this public health crisis.
Medical education— Let’s get focused on maternal mortality
The 140-page Council on Resident Education in Obstetrics and Gynecology CREOG Educational Objectives: Core Curriculum in Obstetrics and Gynecology provides a detailed enumeration of the key learning objectives for residents in obstetrics and gynecology.2 Surprisingly, the CREOG objectives do not mention reducing maternal mortality as an important curricular goal. Learning clinical processes and practices that decrease the risk of maternal mortality should be an important educational goal for all residents training in obstetrics and gynecology.
Nationwide action is needed to address the problem
Many countries have organized widespread efforts to reduce maternal mortality. In the United Kingdom and France there are nationwide reviews of maternal deaths with detailed analyses of clinical events and identification of areas for future improvement. These reviews result in the dissemination of countrywide clinical recommendations that change practice and hopefully reduce the risk of future maternal deaths. For example, following the identification of pulmonary embolism as a leading cause of maternal death in the United Kingdom there was a nationwide effort to increase the use of mechanical and pharmacologic prophylaxis to prevent deep venous thrombosis.
In the United States, experts have proposed that a national program of clinical review of severe maternal morbidity cases should be mandatory. (There are many more cases of “near misses” with severe maternal morbidity than there are maternal deaths.) The greater number of cases available for review should help institutions to quickly recognize potential areas for clinical improvement. One group of experts has recommended that all deliveries in which a pregnant woman received 4 or more units of blood or was admitted to an intensive care unit should be thoroughly reviewed to identify opportunities for clinical improvement.3
In the United Kingdom a contemporary clinical problem that is being addressed in an organized and systematic manner is how to respond to the rising rate of severe maternal morbidity caused by placenta accreta. Experts have concluded that women with a suspected placenta accreta should deliver in regional centers with advanced clinical resources—including an emergency surgical response team, interventional radiology, a high capacity blood bank, and an intensive care unit.
A similar approach has been proposed for managing placenta accreta in the United States.4 The American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal Fetal Medicine (SMFM) have proposed a tiered system of obstetric care with more complex cases being referred to regional perinatal centers.5 Regionalization of trauma services has been an important part of the US health care system for decades. Cases of severe trauma are brought to regional centers equipped to emergently treat complex injuries. A similar system of regulation and regionalization could be adapted for optimizing maternity care.
High-risk clinical events: Is your unit prepared?
In the United States the leading causes of maternal mortality, in descending order, are6−8:
- cardiovascular diseases
- infection
- hemorrhage
- cardiomyopathy
- pulmonary embolism
- hypertension
- amniotic fluid embolism
- stroke
- anesthesia complications.
Over the last decade, the Joint Commission has recommended that birthing centers develop standardized protocols and use simulation to improve the institution’s ability to respond in a timely manner to clinical events that may result in maternal morbidity or death.
The quality of published protocols dealing with hemorrhage, hypertension, and thromboembolism is continuously improving, and every birthing center should have written protocols that are updated on a regular timetable for these common high-risk events.9,10 Does your birthing unit have written protocols to deal with cardiac diseases, infection, obstetric hemorrhage, thromboembolism, and severe hypertension? Are simulation exercises used to strengthen familiarity with the protocols?
High-risk patients
An amazing fact of today’s medical care is that sexually active women of reproductive age who have high-risk medical problems often have not been counseled to use a highly effective contraceptive, resulting in an increased risk of unintended pregnancy and maternal death. For example, adult women with a history of congenital heart disease are known to be at increased risk of death if they become pregnant. In a recent study, women with a history of congenital heart disease had 178 maternal deaths per 100,000 deliveries—a rate approximately 10-fold higher than the US maternal mortality ratio.11 Yet, many of these women are not using a highly effective contraceptive, and this results in a high rate of unplanned pregnancy.12
In order to reduce the risk of unintended pregnancy in women with high-risk medical problems, health systems could make contraception an important “vital sign” for women with high-risk medical conditions.
Race and age matter greatly when it comes to maternal mortality risk
There are major racial differences in pregnancy-related mortality, with black women having much higher rates than white women. In the United States in 2011, the pregnancy-related mortality ratio for white, black, and women of other races was 12.5, 42.8, and 17.3 deaths per 100,000 live births, respectively. This represents a major racial disparity in pregnancy outcomes.1
The age of the mother is an important determinant of the risk of maternal death. Women younger than age 35 years have the lowest risk of maternal death. From 2006 to 2010, pregnant women older than age 40 had a risk of death approximately 3 times greater than women aged 34 or younger.2
References
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www .cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
Let’s get organized
In a country with a history of embracing the “live free or die” ethic, it is often difficult for physicians to enthusiastically embrace the need for a higher level of organization and a potential reduction in individual freedom in order to improve health outcomes. And with a US maternal mortality ratio of 1 maternal death for every 5,400 births, many obstetricians will never have one of their patients die in childbirth. In fact, most obstetricians will have only 1 maternal death during their entire career. In this reality, when clinical events occur rarely, it is not possible for any single clinician, working alone, to impact the overall outcomes of those rare events. Therefore, teamwork and national efforts, such as the National Partnership for Maternal Safety,13 will be necessary to reverse our alarming trend of increasing maternal mortality. Let’s get organized to stop the rise of maternal deaths in the United States.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chescheir NC. Enough already! Obstet Gynecol. 2015;125(1):2−4.
- Council on Resident Education in Obstetrics and Gynecology (CREOG) Educational Objectives: Core Curriculum in Obstetrics and Gynecology. 10th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2013:140.
- Callaghan WM, Grobman WA, Kilpatrick SJ, Main EK, D’Alton M. Facility-based identification of women with severe maternal morbidity: it is time to start. Obstet Gynecol. 2014;123(5):978−981.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212(5):561−568.
- American College of Obstetricians and Gynecologists and the Society of Maternal Fetal Medicine. Obstetric care consensus No 2: levels of maternal care. Obstet Gynecol. 2015;125(2):502−515.
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998−2005. Obstet Gynecol. 2010;116(6):1302−1309.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006−2010. Obstet Gynecol. 2015;125(1):5−12.
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol. 2015;212(3):272−280.
- James A. Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 123: thromboembolism in pregnancy. ACOG. Obstet Gynecol. 2011;118(3):718−729.
- Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetrical outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015;126(2):346−354.
- Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol. 2015;126(2):363−369.
- D’Alton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstet Gynecol. 2014;123(5):973−977.
A mother’s untimely death in childbirth is a grave loss that sends shock waves of grief across generations of her family and community. As obstetricians practicing in the United States, we face a terrible problem. We have a continually rising rate of maternal death in a country with exceptional medical resources (FIGURE).1 Our national decentralized approach to dealing with maternal mortality is a factor contributing to the decades-long increase in the maternal mortality ratio. Let’s get organized to better respond to this public health crisis.
Medical education— Let’s get focused on maternal mortality
The 140-page Council on Resident Education in Obstetrics and Gynecology CREOG Educational Objectives: Core Curriculum in Obstetrics and Gynecology provides a detailed enumeration of the key learning objectives for residents in obstetrics and gynecology.2 Surprisingly, the CREOG objectives do not mention reducing maternal mortality as an important curricular goal. Learning clinical processes and practices that decrease the risk of maternal mortality should be an important educational goal for all residents training in obstetrics and gynecology.
Nationwide action is needed to address the problem
Many countries have organized widespread efforts to reduce maternal mortality. In the United Kingdom and France there are nationwide reviews of maternal deaths with detailed analyses of clinical events and identification of areas for future improvement. These reviews result in the dissemination of countrywide clinical recommendations that change practice and hopefully reduce the risk of future maternal deaths. For example, following the identification of pulmonary embolism as a leading cause of maternal death in the United Kingdom there was a nationwide effort to increase the use of mechanical and pharmacologic prophylaxis to prevent deep venous thrombosis.
In the United States, experts have proposed that a national program of clinical review of severe maternal morbidity cases should be mandatory. (There are many more cases of “near misses” with severe maternal morbidity than there are maternal deaths.) The greater number of cases available for review should help institutions to quickly recognize potential areas for clinical improvement. One group of experts has recommended that all deliveries in which a pregnant woman received 4 or more units of blood or was admitted to an intensive care unit should be thoroughly reviewed to identify opportunities for clinical improvement.3
In the United Kingdom a contemporary clinical problem that is being addressed in an organized and systematic manner is how to respond to the rising rate of severe maternal morbidity caused by placenta accreta. Experts have concluded that women with a suspected placenta accreta should deliver in regional centers with advanced clinical resources—including an emergency surgical response team, interventional radiology, a high capacity blood bank, and an intensive care unit.
A similar approach has been proposed for managing placenta accreta in the United States.4 The American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal Fetal Medicine (SMFM) have proposed a tiered system of obstetric care with more complex cases being referred to regional perinatal centers.5 Regionalization of trauma services has been an important part of the US health care system for decades. Cases of severe trauma are brought to regional centers equipped to emergently treat complex injuries. A similar system of regulation and regionalization could be adapted for optimizing maternity care.
High-risk clinical events: Is your unit prepared?
In the United States the leading causes of maternal mortality, in descending order, are6−8:
- cardiovascular diseases
- infection
- hemorrhage
- cardiomyopathy
- pulmonary embolism
- hypertension
- amniotic fluid embolism
- stroke
- anesthesia complications.
Over the last decade, the Joint Commission has recommended that birthing centers develop standardized protocols and use simulation to improve the institution’s ability to respond in a timely manner to clinical events that may result in maternal morbidity or death.
The quality of published protocols dealing with hemorrhage, hypertension, and thromboembolism is continuously improving, and every birthing center should have written protocols that are updated on a regular timetable for these common high-risk events.9,10 Does your birthing unit have written protocols to deal with cardiac diseases, infection, obstetric hemorrhage, thromboembolism, and severe hypertension? Are simulation exercises used to strengthen familiarity with the protocols?
High-risk patients
An amazing fact of today’s medical care is that sexually active women of reproductive age who have high-risk medical problems often have not been counseled to use a highly effective contraceptive, resulting in an increased risk of unintended pregnancy and maternal death. For example, adult women with a history of congenital heart disease are known to be at increased risk of death if they become pregnant. In a recent study, women with a history of congenital heart disease had 178 maternal deaths per 100,000 deliveries—a rate approximately 10-fold higher than the US maternal mortality ratio.11 Yet, many of these women are not using a highly effective contraceptive, and this results in a high rate of unplanned pregnancy.12
In order to reduce the risk of unintended pregnancy in women with high-risk medical problems, health systems could make contraception an important “vital sign” for women with high-risk medical conditions.
Race and age matter greatly when it comes to maternal mortality risk
There are major racial differences in pregnancy-related mortality, with black women having much higher rates than white women. In the United States in 2011, the pregnancy-related mortality ratio for white, black, and women of other races was 12.5, 42.8, and 17.3 deaths per 100,000 live births, respectively. This represents a major racial disparity in pregnancy outcomes.1
The age of the mother is an important determinant of the risk of maternal death. Women younger than age 35 years have the lowest risk of maternal death. From 2006 to 2010, pregnant women older than age 40 had a risk of death approximately 3 times greater than women aged 34 or younger.2
References
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www .cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
Let’s get organized
In a country with a history of embracing the “live free or die” ethic, it is often difficult for physicians to enthusiastically embrace the need for a higher level of organization and a potential reduction in individual freedom in order to improve health outcomes. And with a US maternal mortality ratio of 1 maternal death for every 5,400 births, many obstetricians will never have one of their patients die in childbirth. In fact, most obstetricians will have only 1 maternal death during their entire career. In this reality, when clinical events occur rarely, it is not possible for any single clinician, working alone, to impact the overall outcomes of those rare events. Therefore, teamwork and national efforts, such as the National Partnership for Maternal Safety,13 will be necessary to reverse our alarming trend of increasing maternal mortality. Let’s get organized to stop the rise of maternal deaths in the United States.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
A mother’s untimely death in childbirth is a grave loss that sends shock waves of grief across generations of her family and community. As obstetricians practicing in the United States, we face a terrible problem. We have a continually rising rate of maternal death in a country with exceptional medical resources (FIGURE).1 Our national decentralized approach to dealing with maternal mortality is a factor contributing to the decades-long increase in the maternal mortality ratio. Let’s get organized to better respond to this public health crisis.
Medical education— Let’s get focused on maternal mortality
The 140-page Council on Resident Education in Obstetrics and Gynecology CREOG Educational Objectives: Core Curriculum in Obstetrics and Gynecology provides a detailed enumeration of the key learning objectives for residents in obstetrics and gynecology.2 Surprisingly, the CREOG objectives do not mention reducing maternal mortality as an important curricular goal. Learning clinical processes and practices that decrease the risk of maternal mortality should be an important educational goal for all residents training in obstetrics and gynecology.
Nationwide action is needed to address the problem
Many countries have organized widespread efforts to reduce maternal mortality. In the United Kingdom and France there are nationwide reviews of maternal deaths with detailed analyses of clinical events and identification of areas for future improvement. These reviews result in the dissemination of countrywide clinical recommendations that change practice and hopefully reduce the risk of future maternal deaths. For example, following the identification of pulmonary embolism as a leading cause of maternal death in the United Kingdom there was a nationwide effort to increase the use of mechanical and pharmacologic prophylaxis to prevent deep venous thrombosis.
In the United States, experts have proposed that a national program of clinical review of severe maternal morbidity cases should be mandatory. (There are many more cases of “near misses” with severe maternal morbidity than there are maternal deaths.) The greater number of cases available for review should help institutions to quickly recognize potential areas for clinical improvement. One group of experts has recommended that all deliveries in which a pregnant woman received 4 or more units of blood or was admitted to an intensive care unit should be thoroughly reviewed to identify opportunities for clinical improvement.3
In the United Kingdom a contemporary clinical problem that is being addressed in an organized and systematic manner is how to respond to the rising rate of severe maternal morbidity caused by placenta accreta. Experts have concluded that women with a suspected placenta accreta should deliver in regional centers with advanced clinical resources—including an emergency surgical response team, interventional radiology, a high capacity blood bank, and an intensive care unit.
A similar approach has been proposed for managing placenta accreta in the United States.4 The American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal Fetal Medicine (SMFM) have proposed a tiered system of obstetric care with more complex cases being referred to regional perinatal centers.5 Regionalization of trauma services has been an important part of the US health care system for decades. Cases of severe trauma are brought to regional centers equipped to emergently treat complex injuries. A similar system of regulation and regionalization could be adapted for optimizing maternity care.
High-risk clinical events: Is your unit prepared?
In the United States the leading causes of maternal mortality, in descending order, are6−8:
- cardiovascular diseases
- infection
- hemorrhage
- cardiomyopathy
- pulmonary embolism
- hypertension
- amniotic fluid embolism
- stroke
- anesthesia complications.
Over the last decade, the Joint Commission has recommended that birthing centers develop standardized protocols and use simulation to improve the institution’s ability to respond in a timely manner to clinical events that may result in maternal morbidity or death.
The quality of published protocols dealing with hemorrhage, hypertension, and thromboembolism is continuously improving, and every birthing center should have written protocols that are updated on a regular timetable for these common high-risk events.9,10 Does your birthing unit have written protocols to deal with cardiac diseases, infection, obstetric hemorrhage, thromboembolism, and severe hypertension? Are simulation exercises used to strengthen familiarity with the protocols?
High-risk patients
An amazing fact of today’s medical care is that sexually active women of reproductive age who have high-risk medical problems often have not been counseled to use a highly effective contraceptive, resulting in an increased risk of unintended pregnancy and maternal death. For example, adult women with a history of congenital heart disease are known to be at increased risk of death if they become pregnant. In a recent study, women with a history of congenital heart disease had 178 maternal deaths per 100,000 deliveries—a rate approximately 10-fold higher than the US maternal mortality ratio.11 Yet, many of these women are not using a highly effective contraceptive, and this results in a high rate of unplanned pregnancy.12
In order to reduce the risk of unintended pregnancy in women with high-risk medical problems, health systems could make contraception an important “vital sign” for women with high-risk medical conditions.
Race and age matter greatly when it comes to maternal mortality risk
There are major racial differences in pregnancy-related mortality, with black women having much higher rates than white women. In the United States in 2011, the pregnancy-related mortality ratio for white, black, and women of other races was 12.5, 42.8, and 17.3 deaths per 100,000 live births, respectively. This represents a major racial disparity in pregnancy outcomes.1
The age of the mother is an important determinant of the risk of maternal death. Women younger than age 35 years have the lowest risk of maternal death. From 2006 to 2010, pregnant women older than age 40 had a risk of death approximately 3 times greater than women aged 34 or younger.2
References
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www .cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
Let’s get organized
In a country with a history of embracing the “live free or die” ethic, it is often difficult for physicians to enthusiastically embrace the need for a higher level of organization and a potential reduction in individual freedom in order to improve health outcomes. And with a US maternal mortality ratio of 1 maternal death for every 5,400 births, many obstetricians will never have one of their patients die in childbirth. In fact, most obstetricians will have only 1 maternal death during their entire career. In this reality, when clinical events occur rarely, it is not possible for any single clinician, working alone, to impact the overall outcomes of those rare events. Therefore, teamwork and national efforts, such as the National Partnership for Maternal Safety,13 will be necessary to reverse our alarming trend of increasing maternal mortality. Let’s get organized to stop the rise of maternal deaths in the United States.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chescheir NC. Enough already! Obstet Gynecol. 2015;125(1):2−4.
- Council on Resident Education in Obstetrics and Gynecology (CREOG) Educational Objectives: Core Curriculum in Obstetrics and Gynecology. 10th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2013:140.
- Callaghan WM, Grobman WA, Kilpatrick SJ, Main EK, D’Alton M. Facility-based identification of women with severe maternal morbidity: it is time to start. Obstet Gynecol. 2014;123(5):978−981.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212(5):561−568.
- American College of Obstetricians and Gynecologists and the Society of Maternal Fetal Medicine. Obstetric care consensus No 2: levels of maternal care. Obstet Gynecol. 2015;125(2):502−515.
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998−2005. Obstet Gynecol. 2010;116(6):1302−1309.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006−2010. Obstet Gynecol. 2015;125(1):5−12.
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol. 2015;212(3):272−280.
- James A. Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 123: thromboembolism in pregnancy. ACOG. Obstet Gynecol. 2011;118(3):718−729.
- Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetrical outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015;126(2):346−354.
- Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol. 2015;126(2):363−369.
- D’Alton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstet Gynecol. 2014;123(5):973−977.
- Chescheir NC. Enough already! Obstet Gynecol. 2015;125(1):2−4.
- Council on Resident Education in Obstetrics and Gynecology (CREOG) Educational Objectives: Core Curriculum in Obstetrics and Gynecology. 10th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2013:140.
- Callaghan WM, Grobman WA, Kilpatrick SJ, Main EK, D’Alton M. Facility-based identification of women with severe maternal morbidity: it is time to start. Obstet Gynecol. 2014;123(5):978−981.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212(5):561−568.
- American College of Obstetricians and Gynecologists and the Society of Maternal Fetal Medicine. Obstetric care consensus No 2: levels of maternal care. Obstet Gynecol. 2015;125(2):502−515.
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998−2005. Obstet Gynecol. 2010;116(6):1302−1309.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006−2010. Obstet Gynecol. 2015;125(1):5−12.
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol. 2015;212(3):272−280.
- James A. Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 123: thromboembolism in pregnancy. ACOG. Obstet Gynecol. 2011;118(3):718−729.
- Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetrical outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015;126(2):346−354.
- Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol. 2015;126(2):363−369.
- D’Alton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstet Gynecol. 2014;123(5):973−977.
Balance Caution With Necessity When Prescribing Dermatology Drugs in Pregnancy
NEW YORK – Disease exacerbations during pregnancy can be more dangerous for the fetus than some of the medications used to treat them..
No one wants to take unnecessary drugs during pregnancy, Dr. Jenny Murase said at the American Academy of Dermatology summer meeting. But concern about medication safety should never override the need to treat new-onset disease or control existing disorders.
“Often, patients are so worried about the effects of medicines on the baby that they don’t think about the effects of the disease. This is a thing we need to address with each patient. What would happen to you, and your baby, if this disease is not treated?” said Dr. Murase of the Palo Alto Foundation Medical Group, San Francisco.
Primary herpes simplex infections are an excellent example of this dilemma, she said. “Primary HSV has up to a 50% transmission rate to the fetus. In utero infections are associated with microcephaly, hydrocephalus, and chorioretinitis. About a third of neonates have central nervous system disease, and a quarter are born with disseminated disease,” and the neonatal mortality rate is nearly 70%.
“On the other hand, acyclovir is very, very safe,” based on data on thousands of patients, Dr. Murase said. Famciclovir and valacyclovir are also likely safe but data on those drugs are more limited, she added.
Sometimes, ideas about a drug’s safety during pregnancy are based on old or confusing studies. The worries about the teratogenicity of systemic steroids, for example, stem from a 1951 report that cortisone caused orofacial cleft in prenatally exposed mice.
“Since then, no prospective studies have found any evidence of congenital malformations associated with systemic steroids,” Dr. Murase said. However, “epidemiologic studies have suggested a threefold increase, which, interestingly, is in line with the mouse findings.”
The drugs do, however, have an important place in the armamentarium; they should be used judiciously and as part of a careful risk-benefit analysis. Both betamethasone and dexamethasone pass the placental barrier well; prednisone is not as transferable.
Dr. Murase provided a list of some medications commonly employed in dermatologic practice, with considerations for their use during pregnancy.
• Topical steroids. A 2013 study found that pregnancy-long exposure to more than 300 g of a potent or superpotent topical corticosteroid did not increase risk of orofacial cleft in the newborn, but did increase risk of low birth weight. “So if you are giving more than five tubes of 60 g, there is a potential for low birth weight – although this could also be related to the actual severity of the disease. Still, you should discuss this risk with your patient.”
• Antihistamines. These should be avoided in the last month of pregnancy as they can stimulate uterine contractions, especially if they are given intravenously or in very high doses. There have been reports of a doubling of retinopathy in premature infants (22% vs. 11%) associated with antihistamines used within 2 weeks of delivery. Some infants can have withdrawal symptoms, including seizure, if the mother has been taking high doses of the drugs.
• Antibiotics. The first-line antibiotics are penicillin, cephalosporins, and dicloxacillin; all are safe. Of the macrolides, erythromycin is preferred. However, the estolate form of the drug has been associated with reversible abnormalities in liver enzymes; the base and ethylsuccinate forms don’t have this risk. Rifampin may be used, but vitamin K should be given with it. Sulfonamides are a second-line choice up until the third trimester. They shouldn’t be used after that, as they can cause anemia, hyperbilirubinemia, and kernicterus. Tetracycline can be used up to 14 weeks’ gestation but not after that, because of the risks of bone growth inhibition and tooth discoloration in the infant, and maternal hepatitis.
• Antifungals. Topical antifungals are not too concerning. Nystatin is safe, but not as effective as the later-generation medications. Clotrimazole is the first choice, followed by miconazole and ketoconazole. Data are limited, but topical terbinafine, naftifine, and ciclopirox are also probably safe, she said.
Systemic antifungals are a different matter. None of the imidazole derivatives are advised during pregnancy, as they have been associated with craniosynostosis, congenital heart defects, and skeletal anomalies. If they are used, a diagnostic ultrasound at 20 weeks is advisable.
• Light. Light therapy is generally considered safe, although there is some evidence that narrow-band ultraviolet can deplete folic acid levels. Despite that, there are no reports of neural tube or orofacial defects associated with light therapy. Nevertheless, make sure patients are supplementing with folic acid if they receive light therapy.
Light therapy should never be combined with oxsoralen in pregnant women; the drug is a known mutagen. Data on cyclosporine are more limited, but it’s a category C drug in pregnancy, so it’s best to avoid it.
• Biologics. Etanercept, adalimumab, infliximab, and alefacept are all category B drugs in pregnancy. There are some hints that they, as well as tumor necrosis factor (TNF)-inhibitors, may be associated with some fetal anomalies in the VACTERL association cluster (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities). A confirmation of the syndrome requires three signs to be present. Only two infants born to mothers taking a biologic have had three qualifying signs.
However, pregnancy outcome reports for the drugs have recorded malformations. Among the adalimumab cohorts totaling 256 pregnancies, there were 14 fetal anomalies (5.5%); of these, nine were VACTERL-related. In the etanercept cohorts totaling 204 pregnancies, there were 13 anomalies (6.4%); of these, six were VACTERL-related. In the infliximab cohorts totaling 112 pregnancies, there was only one anomaly, which was in the VACTERL cluster. Among the 407 pregnancies in the TNF-inhibitors, there were three anomalies, none of which were related to VACTERL.
Dr. Murase had no financial disclosures relevant to her talk.
The current system of using letter categories to denote risk of prescription drugs during pregnancy and breastfeeding is being replaced by the Food and Drug Administration, with the addition of subsections in drug labeling.
NEW YORK – Disease exacerbations during pregnancy can be more dangerous for the fetus than some of the medications used to treat them..
No one wants to take unnecessary drugs during pregnancy, Dr. Jenny Murase said at the American Academy of Dermatology summer meeting. But concern about medication safety should never override the need to treat new-onset disease or control existing disorders.
“Often, patients are so worried about the effects of medicines on the baby that they don’t think about the effects of the disease. This is a thing we need to address with each patient. What would happen to you, and your baby, if this disease is not treated?” said Dr. Murase of the Palo Alto Foundation Medical Group, San Francisco.
Primary herpes simplex infections are an excellent example of this dilemma, she said. “Primary HSV has up to a 50% transmission rate to the fetus. In utero infections are associated with microcephaly, hydrocephalus, and chorioretinitis. About a third of neonates have central nervous system disease, and a quarter are born with disseminated disease,” and the neonatal mortality rate is nearly 70%.
“On the other hand, acyclovir is very, very safe,” based on data on thousands of patients, Dr. Murase said. Famciclovir and valacyclovir are also likely safe but data on those drugs are more limited, she added.
Sometimes, ideas about a drug’s safety during pregnancy are based on old or confusing studies. The worries about the teratogenicity of systemic steroids, for example, stem from a 1951 report that cortisone caused orofacial cleft in prenatally exposed mice.
“Since then, no prospective studies have found any evidence of congenital malformations associated with systemic steroids,” Dr. Murase said. However, “epidemiologic studies have suggested a threefold increase, which, interestingly, is in line with the mouse findings.”
The drugs do, however, have an important place in the armamentarium; they should be used judiciously and as part of a careful risk-benefit analysis. Both betamethasone and dexamethasone pass the placental barrier well; prednisone is not as transferable.
Dr. Murase provided a list of some medications commonly employed in dermatologic practice, with considerations for their use during pregnancy.
• Topical steroids. A 2013 study found that pregnancy-long exposure to more than 300 g of a potent or superpotent topical corticosteroid did not increase risk of orofacial cleft in the newborn, but did increase risk of low birth weight. “So if you are giving more than five tubes of 60 g, there is a potential for low birth weight – although this could also be related to the actual severity of the disease. Still, you should discuss this risk with your patient.”
• Antihistamines. These should be avoided in the last month of pregnancy as they can stimulate uterine contractions, especially if they are given intravenously or in very high doses. There have been reports of a doubling of retinopathy in premature infants (22% vs. 11%) associated with antihistamines used within 2 weeks of delivery. Some infants can have withdrawal symptoms, including seizure, if the mother has been taking high doses of the drugs.
• Antibiotics. The first-line antibiotics are penicillin, cephalosporins, and dicloxacillin; all are safe. Of the macrolides, erythromycin is preferred. However, the estolate form of the drug has been associated with reversible abnormalities in liver enzymes; the base and ethylsuccinate forms don’t have this risk. Rifampin may be used, but vitamin K should be given with it. Sulfonamides are a second-line choice up until the third trimester. They shouldn’t be used after that, as they can cause anemia, hyperbilirubinemia, and kernicterus. Tetracycline can be used up to 14 weeks’ gestation but not after that, because of the risks of bone growth inhibition and tooth discoloration in the infant, and maternal hepatitis.
• Antifungals. Topical antifungals are not too concerning. Nystatin is safe, but not as effective as the later-generation medications. Clotrimazole is the first choice, followed by miconazole and ketoconazole. Data are limited, but topical terbinafine, naftifine, and ciclopirox are also probably safe, she said.
Systemic antifungals are a different matter. None of the imidazole derivatives are advised during pregnancy, as they have been associated with craniosynostosis, congenital heart defects, and skeletal anomalies. If they are used, a diagnostic ultrasound at 20 weeks is advisable.
• Light. Light therapy is generally considered safe, although there is some evidence that narrow-band ultraviolet can deplete folic acid levels. Despite that, there are no reports of neural tube or orofacial defects associated with light therapy. Nevertheless, make sure patients are supplementing with folic acid if they receive light therapy.
Light therapy should never be combined with oxsoralen in pregnant women; the drug is a known mutagen. Data on cyclosporine are more limited, but it’s a category C drug in pregnancy, so it’s best to avoid it.
• Biologics. Etanercept, adalimumab, infliximab, and alefacept are all category B drugs in pregnancy. There are some hints that they, as well as tumor necrosis factor (TNF)-inhibitors, may be associated with some fetal anomalies in the VACTERL association cluster (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities). A confirmation of the syndrome requires three signs to be present. Only two infants born to mothers taking a biologic have had three qualifying signs.
However, pregnancy outcome reports for the drugs have recorded malformations. Among the adalimumab cohorts totaling 256 pregnancies, there were 14 fetal anomalies (5.5%); of these, nine were VACTERL-related. In the etanercept cohorts totaling 204 pregnancies, there were 13 anomalies (6.4%); of these, six were VACTERL-related. In the infliximab cohorts totaling 112 pregnancies, there was only one anomaly, which was in the VACTERL cluster. Among the 407 pregnancies in the TNF-inhibitors, there were three anomalies, none of which were related to VACTERL.
Dr. Murase had no financial disclosures relevant to her talk.
The current system of using letter categories to denote risk of prescription drugs during pregnancy and breastfeeding is being replaced by the Food and Drug Administration, with the addition of subsections in drug labeling.
NEW YORK – Disease exacerbations during pregnancy can be more dangerous for the fetus than some of the medications used to treat them..
No one wants to take unnecessary drugs during pregnancy, Dr. Jenny Murase said at the American Academy of Dermatology summer meeting. But concern about medication safety should never override the need to treat new-onset disease or control existing disorders.
“Often, patients are so worried about the effects of medicines on the baby that they don’t think about the effects of the disease. This is a thing we need to address with each patient. What would happen to you, and your baby, if this disease is not treated?” said Dr. Murase of the Palo Alto Foundation Medical Group, San Francisco.
Primary herpes simplex infections are an excellent example of this dilemma, she said. “Primary HSV has up to a 50% transmission rate to the fetus. In utero infections are associated with microcephaly, hydrocephalus, and chorioretinitis. About a third of neonates have central nervous system disease, and a quarter are born with disseminated disease,” and the neonatal mortality rate is nearly 70%.
“On the other hand, acyclovir is very, very safe,” based on data on thousands of patients, Dr. Murase said. Famciclovir and valacyclovir are also likely safe but data on those drugs are more limited, she added.
Sometimes, ideas about a drug’s safety during pregnancy are based on old or confusing studies. The worries about the teratogenicity of systemic steroids, for example, stem from a 1951 report that cortisone caused orofacial cleft in prenatally exposed mice.
“Since then, no prospective studies have found any evidence of congenital malformations associated with systemic steroids,” Dr. Murase said. However, “epidemiologic studies have suggested a threefold increase, which, interestingly, is in line with the mouse findings.”
The drugs do, however, have an important place in the armamentarium; they should be used judiciously and as part of a careful risk-benefit analysis. Both betamethasone and dexamethasone pass the placental barrier well; prednisone is not as transferable.
Dr. Murase provided a list of some medications commonly employed in dermatologic practice, with considerations for their use during pregnancy.
• Topical steroids. A 2013 study found that pregnancy-long exposure to more than 300 g of a potent or superpotent topical corticosteroid did not increase risk of orofacial cleft in the newborn, but did increase risk of low birth weight. “So if you are giving more than five tubes of 60 g, there is a potential for low birth weight – although this could also be related to the actual severity of the disease. Still, you should discuss this risk with your patient.”
• Antihistamines. These should be avoided in the last month of pregnancy as they can stimulate uterine contractions, especially if they are given intravenously or in very high doses. There have been reports of a doubling of retinopathy in premature infants (22% vs. 11%) associated with antihistamines used within 2 weeks of delivery. Some infants can have withdrawal symptoms, including seizure, if the mother has been taking high doses of the drugs.
• Antibiotics. The first-line antibiotics are penicillin, cephalosporins, and dicloxacillin; all are safe. Of the macrolides, erythromycin is preferred. However, the estolate form of the drug has been associated with reversible abnormalities in liver enzymes; the base and ethylsuccinate forms don’t have this risk. Rifampin may be used, but vitamin K should be given with it. Sulfonamides are a second-line choice up until the third trimester. They shouldn’t be used after that, as they can cause anemia, hyperbilirubinemia, and kernicterus. Tetracycline can be used up to 14 weeks’ gestation but not after that, because of the risks of bone growth inhibition and tooth discoloration in the infant, and maternal hepatitis.
• Antifungals. Topical antifungals are not too concerning. Nystatin is safe, but not as effective as the later-generation medications. Clotrimazole is the first choice, followed by miconazole and ketoconazole. Data are limited, but topical terbinafine, naftifine, and ciclopirox are also probably safe, she said.
Systemic antifungals are a different matter. None of the imidazole derivatives are advised during pregnancy, as they have been associated with craniosynostosis, congenital heart defects, and skeletal anomalies. If they are used, a diagnostic ultrasound at 20 weeks is advisable.
• Light. Light therapy is generally considered safe, although there is some evidence that narrow-band ultraviolet can deplete folic acid levels. Despite that, there are no reports of neural tube or orofacial defects associated with light therapy. Nevertheless, make sure patients are supplementing with folic acid if they receive light therapy.
Light therapy should never be combined with oxsoralen in pregnant women; the drug is a known mutagen. Data on cyclosporine are more limited, but it’s a category C drug in pregnancy, so it’s best to avoid it.
• Biologics. Etanercept, adalimumab, infliximab, and alefacept are all category B drugs in pregnancy. There are some hints that they, as well as tumor necrosis factor (TNF)-inhibitors, may be associated with some fetal anomalies in the VACTERL association cluster (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities). A confirmation of the syndrome requires three signs to be present. Only two infants born to mothers taking a biologic have had three qualifying signs.
However, pregnancy outcome reports for the drugs have recorded malformations. Among the adalimumab cohorts totaling 256 pregnancies, there were 14 fetal anomalies (5.5%); of these, nine were VACTERL-related. In the etanercept cohorts totaling 204 pregnancies, there were 13 anomalies (6.4%); of these, six were VACTERL-related. In the infliximab cohorts totaling 112 pregnancies, there was only one anomaly, which was in the VACTERL cluster. Among the 407 pregnancies in the TNF-inhibitors, there were three anomalies, none of which were related to VACTERL.
Dr. Murase had no financial disclosures relevant to her talk.
The current system of using letter categories to denote risk of prescription drugs during pregnancy and breastfeeding is being replaced by the Food and Drug Administration, with the addition of subsections in drug labeling.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
Balance caution with necessity when prescribing dermatology drugs in pregnancy
NEW YORK – Disease exacerbations during pregnancy can be more dangerous for the fetus than some of the medications used to treat them..
No one wants to take unnecessary drugs during pregnancy, Dr. Jenny Murase said at the American Academy of Dermatology summer meeting. But concern about medication safety should never override the need to treat new-onset disease or control existing disorders.
“Often, patients are so worried about the effects of medicines on the baby that they don’t think about the effects of the disease. This is a thing we need to address with each patient. What would happen to you, and your baby, if this disease is not treated?” said Dr. Murase of the Palo Alto Foundation Medical Group, San Francisco.
Primary herpes simplex infections are an excellent example of this dilemma, she said. “Primary HSV has up to a 50% transmission rate to the fetus. In utero infections are associated with microcephaly, hydrocephalus, and chorioretinitis. About a third of neonates have central nervous system disease, and a quarter are born with disseminated disease,” and the neonatal mortality rate is nearly 70%.
“On the other hand, acyclovir is very, very safe,” based on data on thousands of patients, Dr. Murase said. Famciclovir and valacyclovir are also likely safe but data on those drugs are more limited, she added.
Sometimes, ideas about a drug’s safety during pregnancy are based on old or confusing studies. The worries about the teratogenicity of systemic steroids, for example, stem from a 1951 report that cortisone caused orofacial cleft in prenatally exposed mice.
“Since then, no prospective studies have found any evidence of congenital malformations associated with systemic steroids,” Dr. Murase said. However, “epidemiologic studies have suggested a threefold increase, which, interestingly, is in line with the mouse findings.”
The drugs do, however, have an important place in the armamentarium; they should be used judiciously and as part of a careful risk-benefit analysis. Both betamethasone and dexamethasone pass the placental barrier well; prednisone is not as transferable.
Dr. Murase provided a list of some medications commonly employed in dermatologic practice, with considerations for their use during pregnancy.
• Topical steroids. A 2013 study found that pregnancy-long exposure to more than 300 g of a potent or superpotent topical corticosteroid did not increase risk of orofacial cleft in the newborn, but did increase risk of low birth weight. “So if you are giving more than five tubes of 60 g, there is a potential for low birth weight – although this could also be related to the actual severity of the disease. Still, you should discuss this risk with your patient.”
• Antihistamines. These should be avoided in the last month of pregnancy as they can stimulate uterine contractions, especially if they are given intravenously or in very high doses. There have been reports of a doubling of retinopathy in premature infants (22% vs. 11%) associated with antihistamines used within 2 weeks of delivery. Some infants can have withdrawal symptoms, including seizure, if the mother has been taking high doses of the drugs.
• Antibiotics. The first-line antibiotics are penicillin, cephalosporins, and dicloxacillin; all are safe. Of the macrolides, erythromycin is preferred. However, the estolate form of the drug has been associated with reversible abnormalities in liver enzymes; the base and ethylsuccinate forms don’t have this risk. Rifampin may be used, but vitamin K should be given with it. Sulfonamides are a second-line choice up until the third trimester. They shouldn’t be used after that, as they can cause anemia, hyperbilirubinemia, and kernicterus. Tetracycline can be used up to 14 weeks’ gestation but not after that, because of the risks of bone growth inhibition and tooth discoloration in the infant, and maternal hepatitis.
• Antifungals. Topical antifungals are not too concerning. Nystatin is safe, but not as effective as the later-generation medications. Clotrimazole is the first choice, followed by miconazole and ketoconazole. Data are limited, but topical terbinafine, naftifine, and ciclopirox are also probably safe, she said.
Systemic antifungals are a different matter. None of the imidazole derivatives are advised during pregnancy, as they have been associated with craniosynostosis, congenital heart defects, and skeletal anomalies. If they are used, a diagnostic ultrasound at 20 weeks is advisable.
• Light. Light therapy is generally considered safe, although there is some evidence that narrow-band ultraviolet can deplete folic acid levels. Despite that, there are no reports of neural tube or orofacial defects associated with light therapy. Nevertheless, make sure patients are supplementing with folic acid if they receive light therapy.
Light therapy should never be combined with oxsoralen in pregnant women; the drug is a known mutagen. Data on cyclosporine are more limited, but it’s a category C drug in pregnancy, so it’s best to avoid it.
• Biologics. Etanercept, adalimumab, infliximab, and alefacept are all category B drugs in pregnancy. There are some hints that they, as well as tumor necrosis factor (TNF)-inhibitors, may be associated with some fetal anomalies in the VACTERL association cluster (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities). A confirmation of the syndrome requires three signs to be present. Only two infants born to mothers taking a biologic have had three qualifying signs.
However, pregnancy outcome reports for the drugs have recorded malformations. Among the adalimumab cohorts totaling 256 pregnancies, there were 14 fetal anomalies (5.5%); of these, nine were VACTERL-related. In the etanercept cohorts totaling 204 pregnancies, there were 13 anomalies (6.4%); of these, six were VACTERL-related. In the infliximab cohorts totaling 112 pregnancies, there was only one anomaly, which was in the VACTERL cluster. Among the 407 pregnancies in the TNF-inhibitors, there were three anomalies, none of which were related to VACTERL.
Dr. Murase had no financial disclosures relevant to her talk.
The current system of using letter categories to denote risk of prescription drugs during pregnancy and breastfeeding is being replaced by the Food and Drug Administration, with the addition of subsections in drug labeling.
On Twitter @Alz_Gal
NEW YORK – Disease exacerbations during pregnancy can be more dangerous for the fetus than some of the medications used to treat them..
No one wants to take unnecessary drugs during pregnancy, Dr. Jenny Murase said at the American Academy of Dermatology summer meeting. But concern about medication safety should never override the need to treat new-onset disease or control existing disorders.
“Often, patients are so worried about the effects of medicines on the baby that they don’t think about the effects of the disease. This is a thing we need to address with each patient. What would happen to you, and your baby, if this disease is not treated?” said Dr. Murase of the Palo Alto Foundation Medical Group, San Francisco.
Primary herpes simplex infections are an excellent example of this dilemma, she said. “Primary HSV has up to a 50% transmission rate to the fetus. In utero infections are associated with microcephaly, hydrocephalus, and chorioretinitis. About a third of neonates have central nervous system disease, and a quarter are born with disseminated disease,” and the neonatal mortality rate is nearly 70%.
“On the other hand, acyclovir is very, very safe,” based on data on thousands of patients, Dr. Murase said. Famciclovir and valacyclovir are also likely safe but data on those drugs are more limited, she added.
Sometimes, ideas about a drug’s safety during pregnancy are based on old or confusing studies. The worries about the teratogenicity of systemic steroids, for example, stem from a 1951 report that cortisone caused orofacial cleft in prenatally exposed mice.
“Since then, no prospective studies have found any evidence of congenital malformations associated with systemic steroids,” Dr. Murase said. However, “epidemiologic studies have suggested a threefold increase, which, interestingly, is in line with the mouse findings.”
The drugs do, however, have an important place in the armamentarium; they should be used judiciously and as part of a careful risk-benefit analysis. Both betamethasone and dexamethasone pass the placental barrier well; prednisone is not as transferable.
Dr. Murase provided a list of some medications commonly employed in dermatologic practice, with considerations for their use during pregnancy.
• Topical steroids. A 2013 study found that pregnancy-long exposure to more than 300 g of a potent or superpotent topical corticosteroid did not increase risk of orofacial cleft in the newborn, but did increase risk of low birth weight. “So if you are giving more than five tubes of 60 g, there is a potential for low birth weight – although this could also be related to the actual severity of the disease. Still, you should discuss this risk with your patient.”
• Antihistamines. These should be avoided in the last month of pregnancy as they can stimulate uterine contractions, especially if they are given intravenously or in very high doses. There have been reports of a doubling of retinopathy in premature infants (22% vs. 11%) associated with antihistamines used within 2 weeks of delivery. Some infants can have withdrawal symptoms, including seizure, if the mother has been taking high doses of the drugs.
• Antibiotics. The first-line antibiotics are penicillin, cephalosporins, and dicloxacillin; all are safe. Of the macrolides, erythromycin is preferred. However, the estolate form of the drug has been associated with reversible abnormalities in liver enzymes; the base and ethylsuccinate forms don’t have this risk. Rifampin may be used, but vitamin K should be given with it. Sulfonamides are a second-line choice up until the third trimester. They shouldn’t be used after that, as they can cause anemia, hyperbilirubinemia, and kernicterus. Tetracycline can be used up to 14 weeks’ gestation but not after that, because of the risks of bone growth inhibition and tooth discoloration in the infant, and maternal hepatitis.
• Antifungals. Topical antifungals are not too concerning. Nystatin is safe, but not as effective as the later-generation medications. Clotrimazole is the first choice, followed by miconazole and ketoconazole. Data are limited, but topical terbinafine, naftifine, and ciclopirox are also probably safe, she said.
Systemic antifungals are a different matter. None of the imidazole derivatives are advised during pregnancy, as they have been associated with craniosynostosis, congenital heart defects, and skeletal anomalies. If they are used, a diagnostic ultrasound at 20 weeks is advisable.
• Light. Light therapy is generally considered safe, although there is some evidence that narrow-band ultraviolet can deplete folic acid levels. Despite that, there are no reports of neural tube or orofacial defects associated with light therapy. Nevertheless, make sure patients are supplementing with folic acid if they receive light therapy.
Light therapy should never be combined with oxsoralen in pregnant women; the drug is a known mutagen. Data on cyclosporine are more limited, but it’s a category C drug in pregnancy, so it’s best to avoid it.
• Biologics. Etanercept, adalimumab, infliximab, and alefacept are all category B drugs in pregnancy. There are some hints that they, as well as tumor necrosis factor (TNF)-inhibitors, may be associated with some fetal anomalies in the VACTERL association cluster (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities). A confirmation of the syndrome requires three signs to be present. Only two infants born to mothers taking a biologic have had three qualifying signs.
However, pregnancy outcome reports for the drugs have recorded malformations. Among the adalimumab cohorts totaling 256 pregnancies, there were 14 fetal anomalies (5.5%); of these, nine were VACTERL-related. In the etanercept cohorts totaling 204 pregnancies, there were 13 anomalies (6.4%); of these, six were VACTERL-related. In the infliximab cohorts totaling 112 pregnancies, there was only one anomaly, which was in the VACTERL cluster. Among the 407 pregnancies in the TNF-inhibitors, there were three anomalies, none of which were related to VACTERL.
Dr. Murase had no financial disclosures relevant to her talk.
The current system of using letter categories to denote risk of prescription drugs during pregnancy and breastfeeding is being replaced by the Food and Drug Administration, with the addition of subsections in drug labeling.
On Twitter @Alz_Gal
NEW YORK – Disease exacerbations during pregnancy can be more dangerous for the fetus than some of the medications used to treat them..
No one wants to take unnecessary drugs during pregnancy, Dr. Jenny Murase said at the American Academy of Dermatology summer meeting. But concern about medication safety should never override the need to treat new-onset disease or control existing disorders.
“Often, patients are so worried about the effects of medicines on the baby that they don’t think about the effects of the disease. This is a thing we need to address with each patient. What would happen to you, and your baby, if this disease is not treated?” said Dr. Murase of the Palo Alto Foundation Medical Group, San Francisco.
Primary herpes simplex infections are an excellent example of this dilemma, she said. “Primary HSV has up to a 50% transmission rate to the fetus. In utero infections are associated with microcephaly, hydrocephalus, and chorioretinitis. About a third of neonates have central nervous system disease, and a quarter are born with disseminated disease,” and the neonatal mortality rate is nearly 70%.
“On the other hand, acyclovir is very, very safe,” based on data on thousands of patients, Dr. Murase said. Famciclovir and valacyclovir are also likely safe but data on those drugs are more limited, she added.
Sometimes, ideas about a drug’s safety during pregnancy are based on old or confusing studies. The worries about the teratogenicity of systemic steroids, for example, stem from a 1951 report that cortisone caused orofacial cleft in prenatally exposed mice.
“Since then, no prospective studies have found any evidence of congenital malformations associated with systemic steroids,” Dr. Murase said. However, “epidemiologic studies have suggested a threefold increase, which, interestingly, is in line with the mouse findings.”
The drugs do, however, have an important place in the armamentarium; they should be used judiciously and as part of a careful risk-benefit analysis. Both betamethasone and dexamethasone pass the placental barrier well; prednisone is not as transferable.
Dr. Murase provided a list of some medications commonly employed in dermatologic practice, with considerations for their use during pregnancy.
• Topical steroids. A 2013 study found that pregnancy-long exposure to more than 300 g of a potent or superpotent topical corticosteroid did not increase risk of orofacial cleft in the newborn, but did increase risk of low birth weight. “So if you are giving more than five tubes of 60 g, there is a potential for low birth weight – although this could also be related to the actual severity of the disease. Still, you should discuss this risk with your patient.”
• Antihistamines. These should be avoided in the last month of pregnancy as they can stimulate uterine contractions, especially if they are given intravenously or in very high doses. There have been reports of a doubling of retinopathy in premature infants (22% vs. 11%) associated with antihistamines used within 2 weeks of delivery. Some infants can have withdrawal symptoms, including seizure, if the mother has been taking high doses of the drugs.
• Antibiotics. The first-line antibiotics are penicillin, cephalosporins, and dicloxacillin; all are safe. Of the macrolides, erythromycin is preferred. However, the estolate form of the drug has been associated with reversible abnormalities in liver enzymes; the base and ethylsuccinate forms don’t have this risk. Rifampin may be used, but vitamin K should be given with it. Sulfonamides are a second-line choice up until the third trimester. They shouldn’t be used after that, as they can cause anemia, hyperbilirubinemia, and kernicterus. Tetracycline can be used up to 14 weeks’ gestation but not after that, because of the risks of bone growth inhibition and tooth discoloration in the infant, and maternal hepatitis.
• Antifungals. Topical antifungals are not too concerning. Nystatin is safe, but not as effective as the later-generation medications. Clotrimazole is the first choice, followed by miconazole and ketoconazole. Data are limited, but topical terbinafine, naftifine, and ciclopirox are also probably safe, she said.
Systemic antifungals are a different matter. None of the imidazole derivatives are advised during pregnancy, as they have been associated with craniosynostosis, congenital heart defects, and skeletal anomalies. If they are used, a diagnostic ultrasound at 20 weeks is advisable.
• Light. Light therapy is generally considered safe, although there is some evidence that narrow-band ultraviolet can deplete folic acid levels. Despite that, there are no reports of neural tube or orofacial defects associated with light therapy. Nevertheless, make sure patients are supplementing with folic acid if they receive light therapy.
Light therapy should never be combined with oxsoralen in pregnant women; the drug is a known mutagen. Data on cyclosporine are more limited, but it’s a category C drug in pregnancy, so it’s best to avoid it.
• Biologics. Etanercept, adalimumab, infliximab, and alefacept are all category B drugs in pregnancy. There are some hints that they, as well as tumor necrosis factor (TNF)-inhibitors, may be associated with some fetal anomalies in the VACTERL association cluster (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities). A confirmation of the syndrome requires three signs to be present. Only two infants born to mothers taking a biologic have had three qualifying signs.
However, pregnancy outcome reports for the drugs have recorded malformations. Among the adalimumab cohorts totaling 256 pregnancies, there were 14 fetal anomalies (5.5%); of these, nine were VACTERL-related. In the etanercept cohorts totaling 204 pregnancies, there were 13 anomalies (6.4%); of these, six were VACTERL-related. In the infliximab cohorts totaling 112 pregnancies, there was only one anomaly, which was in the VACTERL cluster. Among the 407 pregnancies in the TNF-inhibitors, there were three anomalies, none of which were related to VACTERL.
Dr. Murase had no financial disclosures relevant to her talk.
The current system of using letter categories to denote risk of prescription drugs during pregnancy and breastfeeding is being replaced by the Food and Drug Administration, with the addition of subsections in drug labeling.
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
High dose folic acid promising in preventing cardiac malformations
When most physicians and patients hear about folic acid, they automatically think about its role in preventing spina bifida and other neural tube defects. But there are now more than 20 years of data that point toward a larger role for folic acid in preventing common, and in some cases devastating, congenital heart defects.
The various published studies leave little doubt that folic acid can significantly reduce the risk for many common cardiac malformations, but other questions remain. For instance, what is the minimum dose for protection? And should health authorities around the world consider recommending a much higher dose of folic acid for short-term use in pregnant women and those trying to conceive?
Early research on cardiac malformations
One of the early studies showing the promise of folic acid beyond neural tube defects was published in 1996. The randomized, double-blind controlled trial in Hungary compared the effect of periconceptional multivitamin supplements containing 0.8 mg of folic acid versus trace element supplements on neural tube defects and other congenital abnormalities. The multivitamin supplement group had a significant reduction in urinary tract abnormalities, and in the rate of sporadic cardiovascular malformations, specifically ventricular septal defects (Am J Med Genet. 1996 Mar 15;62 [2]:179-83.).
In 2004, the same researchers confirmed their results using a controlled, cohort trial that matched pregnant women from regional antenatal care clinics who did not take vitamin supplements to pregnant women who did take supplements containing 0.8 mg folic acid. The results were similar. From a total of 3,056 offspring evaluated, 31 congenital cardiovascular malformations occurred in the group with folic acid supplementation, compared with 50 in the group without, for a 40% lower risk overall, although the main impact was seen among ventricular septal defects (Birth Defects Res A Clin Mol Teratol. 2004 Nov;70[11]:853-61.).
Our own group examined the evidence in a meta-analysis published in 2006 and found significant support for an expanded role for folic acid (J Obstet Gynaecol Can. 2006 Aug;28[8]:680-9.).
The major issue with the available studies is the lack of highest-quality evidence. While some of the studies were randomized trials, you can no longer conduct a true randomized design and withhold folic acid from women; it’s simply not ethical knowing what we know about the preventive benefits of folic acid for neural tube defects. But the literature – which includes quality cohort and case-controlled studies – is convincing.
High dose best?
While some studies have shown a protective effect of folic acid in congenital heart defects at low levels, such as the 0.8 mg used in the early Hungarian studies, other studies indicate that more is better.
A review of 13 studies published in the Lancet shows that for every 0.1 mg/day increase in folic acid intake in women aged 20-35 years, there is a corresponding 0.94 ng/mL increase in serum folate concentrations and that translates into reduced risk of defects, at least in terms of neural tube defects (Lancet. 2001 Dec 15;358[9298]:2069-73.).
Most recently, some of the same researchers that published the early reports out of Hungary on protection against congenital heart defects showed evidence that a variety of congenital heart defects could be reduced with folic acid supplementation of between 3 mg and 6 mg daily (Eur J Obstet Gynecol Reprod Biol. 2015 Jul 9;193:34-9.).
The study evaluated 3,567 infants with various congenital heart defects and 5,395 matched controls. The researchers excluded women taking multivitamins that contained folic acid and stratified the women taking folic acid alone into three subgroups: those who took folic acid anytime during pregnancy, those who took it during the “critical period” for development of malformations based on medical records and self-reported information, and those who took it during the “critical period” based on medical records alone.
During the study period, there was only one type of folic acid tablet available in Hungary – a 3-mg tablet. On average, the daily dose was 5.6 mg.
Most women began folic acid supplementation around 6-11 weeks’ gestation, coinciding with their first prenatal visit. There was a significant drop in the prevalence of cases with ventricular septal defect (odds ratio, 0.57), tetralogy of Fallot (OR, 0.53), d-transposition of great arteries (OR, 0.47), and atrial septal defect secundum (OR, 0.63) when pregnant women took high doses of folic acid during the critical period for congenital heart defect development.
Overall, the researchers concluded that about 40% of severe congenital heart defects could be prevented using high doses of folic acid during the critical period.
This is a significant reduction for a common and serious problem among pregnant women. While it’s true that there are more surgical solutions available today, cardiac malformations are still a major source of morbidity and mortality among children, and it can be the motivation for parents to terminate a pregnancy in cases where there are serious, complicated malformations.
Prevention is always the best option and this research suggests, once again, that folic acid may offer even more benefits.
The study also shows that there was no clear difference in reduction of congenital heart defects based on either a 3-mg or 6-mg dose, except with atrial septal defect secundum. In that case, the 3-mg dose reduced risk by only 10%, compared with more than 40% at 6 mg. But the researchers were quick to point out that more research is needed to look at the dose-response relationship.
The 5.6-mg average daily dose is significant because it is far above the 0.4-mg level recommended by the Centers for Disease Control and Prevention and the level frequently prescribed for pregnant women – 0.8 mg to 1 mg.
We know that some women may benefit from more folic acid, such as women who have previously given birth to a child with neural tube defects. But there are more subgroups that could benefit from higher doses of folic acid, including women taking antifolate medications such as antiepileptic drugs, methotrexate, and sulfonamides. Women who don’t consume enough vegetables and don’t take a multivitamin could benefit from high doses, as well would obese women, smokers, and women with diabetes.
For years the concern has been that folic acid at higher levels could increase the risk for certain cancers, but the evidence there is uncertain. Additionally, that risk would be triggered only after years of exposure, while the benefits of high-dose folic acid could be achieved in a matter of months. I don’t think the evidence of harm is convincing enough to stop us from considering that high-dose folic acid could be used in the general population.
One of the encouraging aspects of using folic acid to prevent congenital heart defects is that the critical window for influencing malformations is larger than with neural tube defects. While folic acid must be given in the first 28 days of pregnancy to have a benefit, the window of opportunity is 1-2 months longer for cardiac malformations.
The bottom line is that high-dose folic acid to prevent congenital heart defects is a target ripe for further research. While randomized controlled trials aren’t possible, there are plenty of high-quality studies that could be conducted to provide clinicians with the information they need to prevent these devastating malformations.
Dr. Koren is professor of pharmacology and pharmacy at the University of Toronto. He is the founding director of the Motherisk Program. He received grant support to conduct studies on folic acid from Duchesnay. He has been a consultant to several companies that produce vitamins in the context of pregnancy, including Bayer. Email him at [email protected].
When most physicians and patients hear about folic acid, they automatically think about its role in preventing spina bifida and other neural tube defects. But there are now more than 20 years of data that point toward a larger role for folic acid in preventing common, and in some cases devastating, congenital heart defects.
The various published studies leave little doubt that folic acid can significantly reduce the risk for many common cardiac malformations, but other questions remain. For instance, what is the minimum dose for protection? And should health authorities around the world consider recommending a much higher dose of folic acid for short-term use in pregnant women and those trying to conceive?
Early research on cardiac malformations
One of the early studies showing the promise of folic acid beyond neural tube defects was published in 1996. The randomized, double-blind controlled trial in Hungary compared the effect of periconceptional multivitamin supplements containing 0.8 mg of folic acid versus trace element supplements on neural tube defects and other congenital abnormalities. The multivitamin supplement group had a significant reduction in urinary tract abnormalities, and in the rate of sporadic cardiovascular malformations, specifically ventricular septal defects (Am J Med Genet. 1996 Mar 15;62 [2]:179-83.).
In 2004, the same researchers confirmed their results using a controlled, cohort trial that matched pregnant women from regional antenatal care clinics who did not take vitamin supplements to pregnant women who did take supplements containing 0.8 mg folic acid. The results were similar. From a total of 3,056 offspring evaluated, 31 congenital cardiovascular malformations occurred in the group with folic acid supplementation, compared with 50 in the group without, for a 40% lower risk overall, although the main impact was seen among ventricular septal defects (Birth Defects Res A Clin Mol Teratol. 2004 Nov;70[11]:853-61.).
Our own group examined the evidence in a meta-analysis published in 2006 and found significant support for an expanded role for folic acid (J Obstet Gynaecol Can. 2006 Aug;28[8]:680-9.).
The major issue with the available studies is the lack of highest-quality evidence. While some of the studies were randomized trials, you can no longer conduct a true randomized design and withhold folic acid from women; it’s simply not ethical knowing what we know about the preventive benefits of folic acid for neural tube defects. But the literature – which includes quality cohort and case-controlled studies – is convincing.
High dose best?
While some studies have shown a protective effect of folic acid in congenital heart defects at low levels, such as the 0.8 mg used in the early Hungarian studies, other studies indicate that more is better.
A review of 13 studies published in the Lancet shows that for every 0.1 mg/day increase in folic acid intake in women aged 20-35 years, there is a corresponding 0.94 ng/mL increase in serum folate concentrations and that translates into reduced risk of defects, at least in terms of neural tube defects (Lancet. 2001 Dec 15;358[9298]:2069-73.).
Most recently, some of the same researchers that published the early reports out of Hungary on protection against congenital heart defects showed evidence that a variety of congenital heart defects could be reduced with folic acid supplementation of between 3 mg and 6 mg daily (Eur J Obstet Gynecol Reprod Biol. 2015 Jul 9;193:34-9.).
The study evaluated 3,567 infants with various congenital heart defects and 5,395 matched controls. The researchers excluded women taking multivitamins that contained folic acid and stratified the women taking folic acid alone into three subgroups: those who took folic acid anytime during pregnancy, those who took it during the “critical period” for development of malformations based on medical records and self-reported information, and those who took it during the “critical period” based on medical records alone.
During the study period, there was only one type of folic acid tablet available in Hungary – a 3-mg tablet. On average, the daily dose was 5.6 mg.
Most women began folic acid supplementation around 6-11 weeks’ gestation, coinciding with their first prenatal visit. There was a significant drop in the prevalence of cases with ventricular septal defect (odds ratio, 0.57), tetralogy of Fallot (OR, 0.53), d-transposition of great arteries (OR, 0.47), and atrial septal defect secundum (OR, 0.63) when pregnant women took high doses of folic acid during the critical period for congenital heart defect development.
Overall, the researchers concluded that about 40% of severe congenital heart defects could be prevented using high doses of folic acid during the critical period.
This is a significant reduction for a common and serious problem among pregnant women. While it’s true that there are more surgical solutions available today, cardiac malformations are still a major source of morbidity and mortality among children, and it can be the motivation for parents to terminate a pregnancy in cases where there are serious, complicated malformations.
Prevention is always the best option and this research suggests, once again, that folic acid may offer even more benefits.
The study also shows that there was no clear difference in reduction of congenital heart defects based on either a 3-mg or 6-mg dose, except with atrial septal defect secundum. In that case, the 3-mg dose reduced risk by only 10%, compared with more than 40% at 6 mg. But the researchers were quick to point out that more research is needed to look at the dose-response relationship.
The 5.6-mg average daily dose is significant because it is far above the 0.4-mg level recommended by the Centers for Disease Control and Prevention and the level frequently prescribed for pregnant women – 0.8 mg to 1 mg.
We know that some women may benefit from more folic acid, such as women who have previously given birth to a child with neural tube defects. But there are more subgroups that could benefit from higher doses of folic acid, including women taking antifolate medications such as antiepileptic drugs, methotrexate, and sulfonamides. Women who don’t consume enough vegetables and don’t take a multivitamin could benefit from high doses, as well would obese women, smokers, and women with diabetes.
For years the concern has been that folic acid at higher levels could increase the risk for certain cancers, but the evidence there is uncertain. Additionally, that risk would be triggered only after years of exposure, while the benefits of high-dose folic acid could be achieved in a matter of months. I don’t think the evidence of harm is convincing enough to stop us from considering that high-dose folic acid could be used in the general population.
One of the encouraging aspects of using folic acid to prevent congenital heart defects is that the critical window for influencing malformations is larger than with neural tube defects. While folic acid must be given in the first 28 days of pregnancy to have a benefit, the window of opportunity is 1-2 months longer for cardiac malformations.
The bottom line is that high-dose folic acid to prevent congenital heart defects is a target ripe for further research. While randomized controlled trials aren’t possible, there are plenty of high-quality studies that could be conducted to provide clinicians with the information they need to prevent these devastating malformations.
Dr. Koren is professor of pharmacology and pharmacy at the University of Toronto. He is the founding director of the Motherisk Program. He received grant support to conduct studies on folic acid from Duchesnay. He has been a consultant to several companies that produce vitamins in the context of pregnancy, including Bayer. Email him at [email protected].
When most physicians and patients hear about folic acid, they automatically think about its role in preventing spina bifida and other neural tube defects. But there are now more than 20 years of data that point toward a larger role for folic acid in preventing common, and in some cases devastating, congenital heart defects.
The various published studies leave little doubt that folic acid can significantly reduce the risk for many common cardiac malformations, but other questions remain. For instance, what is the minimum dose for protection? And should health authorities around the world consider recommending a much higher dose of folic acid for short-term use in pregnant women and those trying to conceive?
Early research on cardiac malformations
One of the early studies showing the promise of folic acid beyond neural tube defects was published in 1996. The randomized, double-blind controlled trial in Hungary compared the effect of periconceptional multivitamin supplements containing 0.8 mg of folic acid versus trace element supplements on neural tube defects and other congenital abnormalities. The multivitamin supplement group had a significant reduction in urinary tract abnormalities, and in the rate of sporadic cardiovascular malformations, specifically ventricular septal defects (Am J Med Genet. 1996 Mar 15;62 [2]:179-83.).
In 2004, the same researchers confirmed their results using a controlled, cohort trial that matched pregnant women from regional antenatal care clinics who did not take vitamin supplements to pregnant women who did take supplements containing 0.8 mg folic acid. The results were similar. From a total of 3,056 offspring evaluated, 31 congenital cardiovascular malformations occurred in the group with folic acid supplementation, compared with 50 in the group without, for a 40% lower risk overall, although the main impact was seen among ventricular septal defects (Birth Defects Res A Clin Mol Teratol. 2004 Nov;70[11]:853-61.).
Our own group examined the evidence in a meta-analysis published in 2006 and found significant support for an expanded role for folic acid (J Obstet Gynaecol Can. 2006 Aug;28[8]:680-9.).
The major issue with the available studies is the lack of highest-quality evidence. While some of the studies were randomized trials, you can no longer conduct a true randomized design and withhold folic acid from women; it’s simply not ethical knowing what we know about the preventive benefits of folic acid for neural tube defects. But the literature – which includes quality cohort and case-controlled studies – is convincing.
High dose best?
While some studies have shown a protective effect of folic acid in congenital heart defects at low levels, such as the 0.8 mg used in the early Hungarian studies, other studies indicate that more is better.
A review of 13 studies published in the Lancet shows that for every 0.1 mg/day increase in folic acid intake in women aged 20-35 years, there is a corresponding 0.94 ng/mL increase in serum folate concentrations and that translates into reduced risk of defects, at least in terms of neural tube defects (Lancet. 2001 Dec 15;358[9298]:2069-73.).
Most recently, some of the same researchers that published the early reports out of Hungary on protection against congenital heart defects showed evidence that a variety of congenital heart defects could be reduced with folic acid supplementation of between 3 mg and 6 mg daily (Eur J Obstet Gynecol Reprod Biol. 2015 Jul 9;193:34-9.).
The study evaluated 3,567 infants with various congenital heart defects and 5,395 matched controls. The researchers excluded women taking multivitamins that contained folic acid and stratified the women taking folic acid alone into three subgroups: those who took folic acid anytime during pregnancy, those who took it during the “critical period” for development of malformations based on medical records and self-reported information, and those who took it during the “critical period” based on medical records alone.
During the study period, there was only one type of folic acid tablet available in Hungary – a 3-mg tablet. On average, the daily dose was 5.6 mg.
Most women began folic acid supplementation around 6-11 weeks’ gestation, coinciding with their first prenatal visit. There was a significant drop in the prevalence of cases with ventricular septal defect (odds ratio, 0.57), tetralogy of Fallot (OR, 0.53), d-transposition of great arteries (OR, 0.47), and atrial septal defect secundum (OR, 0.63) when pregnant women took high doses of folic acid during the critical period for congenital heart defect development.
Overall, the researchers concluded that about 40% of severe congenital heart defects could be prevented using high doses of folic acid during the critical period.
This is a significant reduction for a common and serious problem among pregnant women. While it’s true that there are more surgical solutions available today, cardiac malformations are still a major source of morbidity and mortality among children, and it can be the motivation for parents to terminate a pregnancy in cases where there are serious, complicated malformations.
Prevention is always the best option and this research suggests, once again, that folic acid may offer even more benefits.
The study also shows that there was no clear difference in reduction of congenital heart defects based on either a 3-mg or 6-mg dose, except with atrial septal defect secundum. In that case, the 3-mg dose reduced risk by only 10%, compared with more than 40% at 6 mg. But the researchers were quick to point out that more research is needed to look at the dose-response relationship.
The 5.6-mg average daily dose is significant because it is far above the 0.4-mg level recommended by the Centers for Disease Control and Prevention and the level frequently prescribed for pregnant women – 0.8 mg to 1 mg.
We know that some women may benefit from more folic acid, such as women who have previously given birth to a child with neural tube defects. But there are more subgroups that could benefit from higher doses of folic acid, including women taking antifolate medications such as antiepileptic drugs, methotrexate, and sulfonamides. Women who don’t consume enough vegetables and don’t take a multivitamin could benefit from high doses, as well would obese women, smokers, and women with diabetes.
For years the concern has been that folic acid at higher levels could increase the risk for certain cancers, but the evidence there is uncertain. Additionally, that risk would be triggered only after years of exposure, while the benefits of high-dose folic acid could be achieved in a matter of months. I don’t think the evidence of harm is convincing enough to stop us from considering that high-dose folic acid could be used in the general population.
One of the encouraging aspects of using folic acid to prevent congenital heart defects is that the critical window for influencing malformations is larger than with neural tube defects. While folic acid must be given in the first 28 days of pregnancy to have a benefit, the window of opportunity is 1-2 months longer for cardiac malformations.
The bottom line is that high-dose folic acid to prevent congenital heart defects is a target ripe for further research. While randomized controlled trials aren’t possible, there are plenty of high-quality studies that could be conducted to provide clinicians with the information they need to prevent these devastating malformations.
Dr. Koren is professor of pharmacology and pharmacy at the University of Toronto. He is the founding director of the Motherisk Program. He received grant support to conduct studies on folic acid from Duchesnay. He has been a consultant to several companies that produce vitamins in the context of pregnancy, including Bayer. Email him at [email protected].
Nipple Raynaud’s can freeze out breastfeeding desire
NEW YORK – The “perfect storm” of pregnancy and lactation can throw breastfeeding moms into a painful deep freeze.
Almost 25% of women with lactation pain may actually be experiencing symptoms of Raynaud’s, Dr. Honor Fullerton Stone said at the American Academy of Dermatology summer meeting.
“Breastfeeding mothers are in a perfect storm,” of physical developments that predispose them to this vasoconstrictive phenomenon, said Dr. Fullerton Stone, who practices dermatology in Menlo Park, Ca. “Estrogen increases the alpha-adrenergic receptors on smooth muscle. Nerve irritation from constant breastfeeding upregulates those receptors. And emotional stress – crying baby, anyone? – increases epinephrine, which contributes further to vasoconstriction.”
She conducted a review of 88 of her own patients with nursing-related breast pain. Of these, about a quarter ended up with a diagnosis of nipple vasoconstriction. None of the women had a history of Raynaud’s disease.
Pain during a nursing session is the presenting complaint, but breastfeeding pain is incredibly nonspecific as a symptom. It’s the quality of the pain, Dr Stone said, that should ring a bell.
“There’s let-down pain, which occurs at latch and then comes on again later, with refill. There’s pain from candidiasis, which is dramatic at latch, like a radiating heat, but goes away rapidly after a few days of antifungals,” she noted. But with Raynaud’s, “the pain is persistent. It’s throbbing, which makes sense since it’s vascular. And it’s constant,” lasting through every nursing session, which she said is the kind of experience that makes mothers stop breastfeeding.
Since pain is such a pervasive symptom in breastfeeding complaints, women with vasoconstriction of the nipple are often misdiagnosed. They can go for months trying to improve latch technique or receiving antifungal therapy with no improvement, she said.
Dr. Stone considers a diagnosis of Raynaud’s if two of the following criteria are met:
• Color change of nipple, cold sensitivity, or color change of acral surfaces with cold exposure.
• Chronic deep breast pain for 4 or more weeks.
• Failure of oral antifungals and or antibiotics.
Treatment is both supportive and systemic and avoiding cold is key. She recalled a young mother who arrived in her office bundled up in a down parka on a warm California spring day. “I don’t know why, but this really seems to help,” she said.
“I suggest taking two hot showers a day and all the better if they can be right before nursing. Hot pads and compresses are not going to help. You need to warm up the entire body to calm this vascular reactivity.”
Women should also avoid consuming anything that can cause vasoconstriction, including caffeine and tobacco, she advised.
Nifedipine is a very effective medication, and is safe for nursing infants, Dr. Stone said. The sustained-released 30 mg/day dose is typically recommended, but she has changed her thinking on this a bit.
Her internal study showed that, although 83% responded very well to the drug, about a third of them also had a vasodilation-related side effect.
“For this reason, I usually now start with the 10 mg nonsustained form, and warn about things like headache, dizziness, postural hypotension, and fainting,” she said. Typically, patients adjust well to the drug’s effects and then the dose can be individually titrated.
Dr. Fullerton Stone had no relevant financial disclosures.
On Twitter @Alz_Gal
NEW YORK – The “perfect storm” of pregnancy and lactation can throw breastfeeding moms into a painful deep freeze.
Almost 25% of women with lactation pain may actually be experiencing symptoms of Raynaud’s, Dr. Honor Fullerton Stone said at the American Academy of Dermatology summer meeting.
“Breastfeeding mothers are in a perfect storm,” of physical developments that predispose them to this vasoconstrictive phenomenon, said Dr. Fullerton Stone, who practices dermatology in Menlo Park, Ca. “Estrogen increases the alpha-adrenergic receptors on smooth muscle. Nerve irritation from constant breastfeeding upregulates those receptors. And emotional stress – crying baby, anyone? – increases epinephrine, which contributes further to vasoconstriction.”
She conducted a review of 88 of her own patients with nursing-related breast pain. Of these, about a quarter ended up with a diagnosis of nipple vasoconstriction. None of the women had a history of Raynaud’s disease.
Pain during a nursing session is the presenting complaint, but breastfeeding pain is incredibly nonspecific as a symptom. It’s the quality of the pain, Dr Stone said, that should ring a bell.
“There’s let-down pain, which occurs at latch and then comes on again later, with refill. There’s pain from candidiasis, which is dramatic at latch, like a radiating heat, but goes away rapidly after a few days of antifungals,” she noted. But with Raynaud’s, “the pain is persistent. It’s throbbing, which makes sense since it’s vascular. And it’s constant,” lasting through every nursing session, which she said is the kind of experience that makes mothers stop breastfeeding.
Since pain is such a pervasive symptom in breastfeeding complaints, women with vasoconstriction of the nipple are often misdiagnosed. They can go for months trying to improve latch technique or receiving antifungal therapy with no improvement, she said.
Dr. Stone considers a diagnosis of Raynaud’s if two of the following criteria are met:
• Color change of nipple, cold sensitivity, or color change of acral surfaces with cold exposure.
• Chronic deep breast pain for 4 or more weeks.
• Failure of oral antifungals and or antibiotics.
Treatment is both supportive and systemic and avoiding cold is key. She recalled a young mother who arrived in her office bundled up in a down parka on a warm California spring day. “I don’t know why, but this really seems to help,” she said.
“I suggest taking two hot showers a day and all the better if they can be right before nursing. Hot pads and compresses are not going to help. You need to warm up the entire body to calm this vascular reactivity.”
Women should also avoid consuming anything that can cause vasoconstriction, including caffeine and tobacco, she advised.
Nifedipine is a very effective medication, and is safe for nursing infants, Dr. Stone said. The sustained-released 30 mg/day dose is typically recommended, but she has changed her thinking on this a bit.
Her internal study showed that, although 83% responded very well to the drug, about a third of them also had a vasodilation-related side effect.
“For this reason, I usually now start with the 10 mg nonsustained form, and warn about things like headache, dizziness, postural hypotension, and fainting,” she said. Typically, patients adjust well to the drug’s effects and then the dose can be individually titrated.
Dr. Fullerton Stone had no relevant financial disclosures.
On Twitter @Alz_Gal
NEW YORK – The “perfect storm” of pregnancy and lactation can throw breastfeeding moms into a painful deep freeze.
Almost 25% of women with lactation pain may actually be experiencing symptoms of Raynaud’s, Dr. Honor Fullerton Stone said at the American Academy of Dermatology summer meeting.
“Breastfeeding mothers are in a perfect storm,” of physical developments that predispose them to this vasoconstrictive phenomenon, said Dr. Fullerton Stone, who practices dermatology in Menlo Park, Ca. “Estrogen increases the alpha-adrenergic receptors on smooth muscle. Nerve irritation from constant breastfeeding upregulates those receptors. And emotional stress – crying baby, anyone? – increases epinephrine, which contributes further to vasoconstriction.”
She conducted a review of 88 of her own patients with nursing-related breast pain. Of these, about a quarter ended up with a diagnosis of nipple vasoconstriction. None of the women had a history of Raynaud’s disease.
Pain during a nursing session is the presenting complaint, but breastfeeding pain is incredibly nonspecific as a symptom. It’s the quality of the pain, Dr Stone said, that should ring a bell.
“There’s let-down pain, which occurs at latch and then comes on again later, with refill. There’s pain from candidiasis, which is dramatic at latch, like a radiating heat, but goes away rapidly after a few days of antifungals,” she noted. But with Raynaud’s, “the pain is persistent. It’s throbbing, which makes sense since it’s vascular. And it’s constant,” lasting through every nursing session, which she said is the kind of experience that makes mothers stop breastfeeding.
Since pain is such a pervasive symptom in breastfeeding complaints, women with vasoconstriction of the nipple are often misdiagnosed. They can go for months trying to improve latch technique or receiving antifungal therapy with no improvement, she said.
Dr. Stone considers a diagnosis of Raynaud’s if two of the following criteria are met:
• Color change of nipple, cold sensitivity, or color change of acral surfaces with cold exposure.
• Chronic deep breast pain for 4 or more weeks.
• Failure of oral antifungals and or antibiotics.
Treatment is both supportive and systemic and avoiding cold is key. She recalled a young mother who arrived in her office bundled up in a down parka on a warm California spring day. “I don’t know why, but this really seems to help,” she said.
“I suggest taking two hot showers a day and all the better if they can be right before nursing. Hot pads and compresses are not going to help. You need to warm up the entire body to calm this vascular reactivity.”
Women should also avoid consuming anything that can cause vasoconstriction, including caffeine and tobacco, she advised.
Nifedipine is a very effective medication, and is safe for nursing infants, Dr. Stone said. The sustained-released 30 mg/day dose is typically recommended, but she has changed her thinking on this a bit.
Her internal study showed that, although 83% responded very well to the drug, about a third of them also had a vasodilation-related side effect.
“For this reason, I usually now start with the 10 mg nonsustained form, and warn about things like headache, dizziness, postural hypotension, and fainting,” she said. Typically, patients adjust well to the drug’s effects and then the dose can be individually titrated.
Dr. Fullerton Stone had no relevant financial disclosures.
On Twitter @Alz_Gal
AT THE AAD SUMMER ACADEMY 2015
Protecting pregnant women, infants from infections
Infectious disease morbidity and mortality continue to disproportionately impact pregnant women and young infants.
In California, the incidence of pertussis approximates 100 cases per 100,000 in infants less than 5 months of age; a rate threefold greater than any other age group. Seven of nine (77%) deaths in 2013/2014 occurred in infants less than 3 months of age (California Department of Public Health Pertussis Report, Aug. 3, 2015).
Influenza severity and mortality is increased in pregnant women, and there is a greater risk of fetal morbidity and wastage. In the 2009 H1N1 pandemic, there was a 20% case fatality rate in women sick enough to be admitted to the ICU. The incidence of low birth weight also was increased among pregnant women delivering while hospitalized for influenza-related illness. These examples highlight the burden of vaccine-preventable disease in two vulnerable populations, pregnant women and infants too young to be protected by vaccines mandated by the U.S.immunization program.
The American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the Centers for Disease Control and Prevention, and many other national and state organizations endorse immunization of pregnant women to improve women’s and infants’ outcomes. Recent studies demonstrate that infants born to women vaccinated with influenza are 45%-48% less likely to be hospitalized for culture-proven influenza.
Benowitz et al. reported a 91.5% effectiveness for maternal influenza vaccination for prevention of hospitalization of infants caused by influenza in the first 6 months of life. The presumed mechanisms of protection are both the transplacental transfer of protective antibody as well as indirect protection from disease prevention in the mother (Clin Infect Dis. 2010 Dec 15;51(12):1355-61). The recommendation is that inactivated influenza vaccine can be given at any time during pregnancy; however, live attenuated influenza vaccine (LAIV; FluMist) is contraindicated, as are all live-virus vaccines. In contrast, Tdap is recommended for use either during pregnancy or post partum.
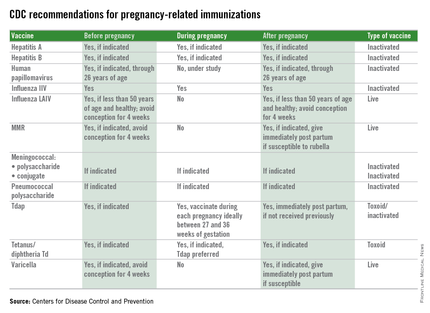
However, Healy et al. (Pediatr Infect Dis J. 2015;34(1):22-60) failed to demonstrate a benefit to postpartum immunization and cocooning for reducing pertussis illness in infants 6 months of age or younger. The likely explanation for this failure is revealed in a recent study in infant baboons where immunization with Tdap failed to decrease colonization or transmission of Bordetella pertussis, compared with natural disease or whole-cell pertussis. Thus, even though protective against disease, Tdap failure to prevent transmission within the community still occurs. The current Advisory Committee on Immunization Practices recommendation, immunization between 27 and 36 weeks, is designed to ensure high antibody concentrations in both mother and newborn at the time of birth and bridge the time period until infant immunization can elicit protective antibody.
The benefits achieved with maternal immunization must be weighed against potential for adverse events. There is no evidence of risk to either mother or infant from inactivated vaccines administered during pregnancy. Still, the recommendations for influenza and Tdap vaccine incorporate the high likelihood of exposure, the risk of morbidity or mortality from the infectious agent, and the likelihood of harm. During the H1N1 epidemic, a cohort study by Chambers et al. of H1N1 vaccine in exposed and unexposed pregnant women concluded that there was no increase in risk for major congenital defects, spontaneous abortion, or small for gestational age (Vaccine. 2013 Oct 17;31(44):5026-32). There was a signal for increase in prematurity, but the difference between H1N1-vaccinated and unvaccinated pregnancies was 3 days. In addition, a review of 11 studies, including one of 10,428 pregnant women, concluded there were no harmful maternal or fetal effects.
Additionally, no adverse risks have been identified in women who were inadvertently vaccinated during pregnancy with live-attenuated rubella, influenza, and yellow fever vaccines. Tetanus vaccination has been administered safely to several millions of pregnant women without documented serious adverse outcomes. Ongoing postmarketing surveillance continues as an important tool for identification of potential adverse effects.
One potential limitation is the blunting of infant immune responses to vaccination due to high serum antibody concentrations at the time of primary immunizations. Some studies have found lower antibody concentrations prior to booster vaccinations at 1 year of age. However, as morbidity and mortality is greater in the first months of life for many infectious diseases, this may be an acceptable trade off if high morbidity and mortality can be reduced in the first months of life.
Immunization during pregnancy represents only one aspect of prevention of vaccine preventable diseases. Preconception, prenatal, and postpartum visits with health care professionals represents an opportune time to discuss the benefits of immunization and their contribution to a healthy pregnancy outcome. Inactivated vaccines are safe for administration during pregnancy, live virus vaccines, despite being attenuated, are a theoretical risk if spread to the fetus occurs and therefore are contraindicated and should be administered during preconception counseling if indicated. The table below outlines vaccines that can be administered before, during, and after pregnancy.
Although once considered potentially contraindicated in pregnant women, evidence now supports specific vaccines as both safe for a pregnant woman and her fetus and effective for preventing serious disease in both. Universal immunization with influenza vaccine and Tdap, as recommended by multiple national professional medical organizations, will improve the outcome of pregnancy by prevention of morbidity and mortality from common community pathogens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. E-mail him at [email protected].
Infectious disease morbidity and mortality continue to disproportionately impact pregnant women and young infants.
In California, the incidence of pertussis approximates 100 cases per 100,000 in infants less than 5 months of age; a rate threefold greater than any other age group. Seven of nine (77%) deaths in 2013/2014 occurred in infants less than 3 months of age (California Department of Public Health Pertussis Report, Aug. 3, 2015).
Influenza severity and mortality is increased in pregnant women, and there is a greater risk of fetal morbidity and wastage. In the 2009 H1N1 pandemic, there was a 20% case fatality rate in women sick enough to be admitted to the ICU. The incidence of low birth weight also was increased among pregnant women delivering while hospitalized for influenza-related illness. These examples highlight the burden of vaccine-preventable disease in two vulnerable populations, pregnant women and infants too young to be protected by vaccines mandated by the U.S.immunization program.
The American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the Centers for Disease Control and Prevention, and many other national and state organizations endorse immunization of pregnant women to improve women’s and infants’ outcomes. Recent studies demonstrate that infants born to women vaccinated with influenza are 45%-48% less likely to be hospitalized for culture-proven influenza.
Benowitz et al. reported a 91.5% effectiveness for maternal influenza vaccination for prevention of hospitalization of infants caused by influenza in the first 6 months of life. The presumed mechanisms of protection are both the transplacental transfer of protective antibody as well as indirect protection from disease prevention in the mother (Clin Infect Dis. 2010 Dec 15;51(12):1355-61). The recommendation is that inactivated influenza vaccine can be given at any time during pregnancy; however, live attenuated influenza vaccine (LAIV; FluMist) is contraindicated, as are all live-virus vaccines. In contrast, Tdap is recommended for use either during pregnancy or post partum.

However, Healy et al. (Pediatr Infect Dis J. 2015;34(1):22-60) failed to demonstrate a benefit to postpartum immunization and cocooning for reducing pertussis illness in infants 6 months of age or younger. The likely explanation for this failure is revealed in a recent study in infant baboons where immunization with Tdap failed to decrease colonization or transmission of Bordetella pertussis, compared with natural disease or whole-cell pertussis. Thus, even though protective against disease, Tdap failure to prevent transmission within the community still occurs. The current Advisory Committee on Immunization Practices recommendation, immunization between 27 and 36 weeks, is designed to ensure high antibody concentrations in both mother and newborn at the time of birth and bridge the time period until infant immunization can elicit protective antibody.
The benefits achieved with maternal immunization must be weighed against potential for adverse events. There is no evidence of risk to either mother or infant from inactivated vaccines administered during pregnancy. Still, the recommendations for influenza and Tdap vaccine incorporate the high likelihood of exposure, the risk of morbidity or mortality from the infectious agent, and the likelihood of harm. During the H1N1 epidemic, a cohort study by Chambers et al. of H1N1 vaccine in exposed and unexposed pregnant women concluded that there was no increase in risk for major congenital defects, spontaneous abortion, or small for gestational age (Vaccine. 2013 Oct 17;31(44):5026-32). There was a signal for increase in prematurity, but the difference between H1N1-vaccinated and unvaccinated pregnancies was 3 days. In addition, a review of 11 studies, including one of 10,428 pregnant women, concluded there were no harmful maternal or fetal effects.
Additionally, no adverse risks have been identified in women who were inadvertently vaccinated during pregnancy with live-attenuated rubella, influenza, and yellow fever vaccines. Tetanus vaccination has been administered safely to several millions of pregnant women without documented serious adverse outcomes. Ongoing postmarketing surveillance continues as an important tool for identification of potential adverse effects.
One potential limitation is the blunting of infant immune responses to vaccination due to high serum antibody concentrations at the time of primary immunizations. Some studies have found lower antibody concentrations prior to booster vaccinations at 1 year of age. However, as morbidity and mortality is greater in the first months of life for many infectious diseases, this may be an acceptable trade off if high morbidity and mortality can be reduced in the first months of life.
Immunization during pregnancy represents only one aspect of prevention of vaccine preventable diseases. Preconception, prenatal, and postpartum visits with health care professionals represents an opportune time to discuss the benefits of immunization and their contribution to a healthy pregnancy outcome. Inactivated vaccines are safe for administration during pregnancy, live virus vaccines, despite being attenuated, are a theoretical risk if spread to the fetus occurs and therefore are contraindicated and should be administered during preconception counseling if indicated. The table below outlines vaccines that can be administered before, during, and after pregnancy.
Although once considered potentially contraindicated in pregnant women, evidence now supports specific vaccines as both safe for a pregnant woman and her fetus and effective for preventing serious disease in both. Universal immunization with influenza vaccine and Tdap, as recommended by multiple national professional medical organizations, will improve the outcome of pregnancy by prevention of morbidity and mortality from common community pathogens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. E-mail him at [email protected].
Infectious disease morbidity and mortality continue to disproportionately impact pregnant women and young infants.
In California, the incidence of pertussis approximates 100 cases per 100,000 in infants less than 5 months of age; a rate threefold greater than any other age group. Seven of nine (77%) deaths in 2013/2014 occurred in infants less than 3 months of age (California Department of Public Health Pertussis Report, Aug. 3, 2015).
Influenza severity and mortality is increased in pregnant women, and there is a greater risk of fetal morbidity and wastage. In the 2009 H1N1 pandemic, there was a 20% case fatality rate in women sick enough to be admitted to the ICU. The incidence of low birth weight also was increased among pregnant women delivering while hospitalized for influenza-related illness. These examples highlight the burden of vaccine-preventable disease in two vulnerable populations, pregnant women and infants too young to be protected by vaccines mandated by the U.S.immunization program.
The American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the Centers for Disease Control and Prevention, and many other national and state organizations endorse immunization of pregnant women to improve women’s and infants’ outcomes. Recent studies demonstrate that infants born to women vaccinated with influenza are 45%-48% less likely to be hospitalized for culture-proven influenza.
Benowitz et al. reported a 91.5% effectiveness for maternal influenza vaccination for prevention of hospitalization of infants caused by influenza in the first 6 months of life. The presumed mechanisms of protection are both the transplacental transfer of protective antibody as well as indirect protection from disease prevention in the mother (Clin Infect Dis. 2010 Dec 15;51(12):1355-61). The recommendation is that inactivated influenza vaccine can be given at any time during pregnancy; however, live attenuated influenza vaccine (LAIV; FluMist) is contraindicated, as are all live-virus vaccines. In contrast, Tdap is recommended for use either during pregnancy or post partum.

However, Healy et al. (Pediatr Infect Dis J. 2015;34(1):22-60) failed to demonstrate a benefit to postpartum immunization and cocooning for reducing pertussis illness in infants 6 months of age or younger. The likely explanation for this failure is revealed in a recent study in infant baboons where immunization with Tdap failed to decrease colonization or transmission of Bordetella pertussis, compared with natural disease or whole-cell pertussis. Thus, even though protective against disease, Tdap failure to prevent transmission within the community still occurs. The current Advisory Committee on Immunization Practices recommendation, immunization between 27 and 36 weeks, is designed to ensure high antibody concentrations in both mother and newborn at the time of birth and bridge the time period until infant immunization can elicit protective antibody.
The benefits achieved with maternal immunization must be weighed against potential for adverse events. There is no evidence of risk to either mother or infant from inactivated vaccines administered during pregnancy. Still, the recommendations for influenza and Tdap vaccine incorporate the high likelihood of exposure, the risk of morbidity or mortality from the infectious agent, and the likelihood of harm. During the H1N1 epidemic, a cohort study by Chambers et al. of H1N1 vaccine in exposed and unexposed pregnant women concluded that there was no increase in risk for major congenital defects, spontaneous abortion, or small for gestational age (Vaccine. 2013 Oct 17;31(44):5026-32). There was a signal for increase in prematurity, but the difference between H1N1-vaccinated and unvaccinated pregnancies was 3 days. In addition, a review of 11 studies, including one of 10,428 pregnant women, concluded there were no harmful maternal or fetal effects.
Additionally, no adverse risks have been identified in women who were inadvertently vaccinated during pregnancy with live-attenuated rubella, influenza, and yellow fever vaccines. Tetanus vaccination has been administered safely to several millions of pregnant women without documented serious adverse outcomes. Ongoing postmarketing surveillance continues as an important tool for identification of potential adverse effects.
One potential limitation is the blunting of infant immune responses to vaccination due to high serum antibody concentrations at the time of primary immunizations. Some studies have found lower antibody concentrations prior to booster vaccinations at 1 year of age. However, as morbidity and mortality is greater in the first months of life for many infectious diseases, this may be an acceptable trade off if high morbidity and mortality can be reduced in the first months of life.
Immunization during pregnancy represents only one aspect of prevention of vaccine preventable diseases. Preconception, prenatal, and postpartum visits with health care professionals represents an opportune time to discuss the benefits of immunization and their contribution to a healthy pregnancy outcome. Inactivated vaccines are safe for administration during pregnancy, live virus vaccines, despite being attenuated, are a theoretical risk if spread to the fetus occurs and therefore are contraindicated and should be administered during preconception counseling if indicated. The table below outlines vaccines that can be administered before, during, and after pregnancy.
Although once considered potentially contraindicated in pregnant women, evidence now supports specific vaccines as both safe for a pregnant woman and her fetus and effective for preventing serious disease in both. Universal immunization with influenza vaccine and Tdap, as recommended by multiple national professional medical organizations, will improve the outcome of pregnancy by prevention of morbidity and mortality from common community pathogens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. E-mail him at [email protected].
Malpractice Counsel: Missed Preeclampsia
Missed Preeclampsia
| A 24-year-old woman, gravida 1, para 1, aborta 0, presented to the ED complaining of a 1-day history of shortness of breath. Four days earlier, she had delivered a healthy baby boy via normal vaginal delivery and without complication. She denied chest pain, fever, or abdominal pain. She was otherwise in good health, stating that she was not taking any medications. She also denied smoking cigarettes. |
On physical examination, the patient’s vital signs were remarkable for the following: heart rate (HR), 86 beats/minute; blood pressure (BP), 164/94 mm Hg; respiratory rate, 18 breaths/minute; temperature, 98.6oF. Oxygen saturation was 96% on room air. The head, eye, ear, nose and throat examination was unremarkable. The lungs were clear to auscultation bilaterally, and HR and heart rhythm were normal. The abdomen was soft and nontender without guarding or rebound. The lower extremities were remarkable for 1+ pedal and pretibial edema bilaterally.
The patient presented to the same ED 2 days later, again with the chief complaint of shortness of breath. On examination, her BP was noted to be elevated and she had 1+ dependent edema bilaterally. Again, the EP was concerned for a PE and ordered a repeat CTA scan of the chest. This study, similar to the first, was read as normal, and showed no evidence of PE. The patient was diagnosed again with “shortness of breath of unknown etiology” and discharged home. The patient’s obstetrician-gynecologist (Ob/Gyn) was not consulted; however, the patient was encouraged to follow up with him.
The next day, the patient presented to the same ED via emergency medical services, this time with seizures; she had no prior history of a seizure disorder. On presentation to the ED, she was noted to be postictal, with an elevated BP and tachycardic with an HR of 104 beats/minute. On examination, the lungs were clear to auscultation and the lower extremities exhibited 1+ pedal and pretibial edema. A urinalysis revealed proteinuria. The patient was given 4 g of magnesium sulfate intravenously (IV) and her Ob/Gyn was consulted.
The patient was admitted to the hospital with a diagnosis of eclampsia. She was given an IV drip of magnesium and labetalol for the high BP. Unfortunately, the patient apparently had suffered an anoxic brain injury from the previous seizures and died on hospital day 3.
The family sued the treating EPs and the hospital for failure to diagnose preeclampsia on two separate ED presentations. They noted the patient’s Ob/Gyn was never consulted; no action was taken to treat the hypertension; and no urinalysis was ordered on either visit. The EPs and hospital settled the case prior to trial for several million dollars.
Discussion
This is an incredibly sad case, and the EPs and hospital were right to settle and not go to trial. While PE was a reasonable diagnosis to consider in this patient on her first ED visit, it should not have been the only one in the differential diagnosis. The EP became anchored to this single diagnosis and refused to consider other alternative diagnoses—even after the CTA scan of the chest ruled out PE. Moreover, it appears the EP either never considered the significance of the elevated BP and dependent edema or just ignored these findings. To repeat essentially the same exact workup on the second visit does not make sense—one should “cast a wider net, not the same net.”
The diagnosis of “shortness of breath of unknown etiology” is similarly unacceptable. While this is a common and accepted diagnosis when it pertains to abdominal pain, the same is not true for dyspnea.
Preeclampsia is characterized by hypertension (BP >140/90 mm Hg) and proteinuria; associated symptoms include edema and hyperreflexia. Postpartum preeclampsia occurs infrequently and can develop up to 4 weeks after delivery.1 In one 10-year retrospective case series, the incidence of preeclampsia in the postpartum period was 5.7%, and nearly 16% went on to develop eclampsia.2 In a retrospective study of 22 postpartum preeclamptic patients, the median time to presentation was 5 days postpartum.1 In a similar retrospective study of 152 patients, 90% of such patients presented within 7 days.3 The patient in this case initially presented on postpartum day 4.
Interestingly, in a study by Al-Safi et al,3 63% of postpartum preeclamptic patients had no antecedent diagnosis of hypertensive disease during pregnancy. These findings are consistent with the findings of others that 33% to 69% of such patients show no evidence of preeclampsia in the ante- or peripartum period.
The clinical presentation of postpartum preeclampsia is similar to preeclampsia complicating pregnancy after gestation week 20. In the study by Al-Safi et al,3 headache was the most common presenting symptom (69%), followed by shortness of breath (30%), blurry vision (21%), nausea (12.5%), and epigastric abdominal pain (5%). Similarly, Yancey et al1 found headache (82%) to be the most common presenting symptom in their series. Unfortunately, it is not known whether the patient in this case complained of headache or blurred vision as the published records note neither their presence nor absence.
The management of patients with preeclampsia includes IV magnesium to prevent seizures (ie, eclampsia) and BP control.1 A bolus of 4 to 6 g IV magnesium sulfate over 15 to 30 minutes is recommended, followed by an infusion of 2 g/h IV. Historically, IV hydralazine has been used to manage preeclamptic patients with a BP greater than 160/110 mm Hg. More recently, however, IV labetalol has become popular.5 All such patients require admission to the hospital with Ob/Gyn involvement.
Missed Subdural Hematoma
A 59-year-old man presented to the ED with a chief complaint of headache, the onset of which he stated started gradually 2 days prior. He noted the headache was worse than normal but without associated nausea, vomiting, fever, chills, or change in vision. His past medical history was significant for a lower extremity deep vein thrombosis 3 months prior, for which he was taking warfarin.
The patient’s vital signs were all normal. The physical examination, including a thorough neurological examination, was also normal. The EP ordered a prothrombin time (PT), an international normalized ratio (INR), and a noncontrast CT scan of the head. The PT/INR results were therapeutic at 22 seconds and 2.3. The CT scan was interpreted by radiology services as normal. The patient’s headache was treated with IV prochlorperazine and diphenhydramine. After treatment, the patient reported feeling better and was discharged home with instructions to follow up with his primary care physician.
Over the next several months, the patient presented to the same ED on seven different occasions, each time with the chief complaint of headache. At each of these presentations, the history and physical examination were documented as unremarkable, with no history of trauma. The thoroughness, however, of the documentation varied considerably for each ED encounter. No head CT scan was ordered on the subsequent seven visits, and at each presentation, the patient was treated symptomatically and discharged home.
Two days after his eighth visit to the same hospital ED, the patient presented to a different ED, again with a chief complaint of headache. The EP at this ED ordered a noncontrast CT of the head, which demonstrated a left subdural hematoma. The patient was admitted to the hospital, given IV vitamin K and fresh frozen plasma, and underwent evacuation of the hematoma by neurosurgery. The patient’s hospital course was unremarkable, and he was discharged home without any focal weakness.
The patient, however, claimed that he suffered cognitive impairment as a result of the missed diagnosis. He sued treating EPs at the first ED as well as the hospital for failure to timely diagnose the subdural hematoma, stating that a CT scan should have been performed at each of his ED visits since he was on warfarin. The defense claimed that a CT scan was not warranted for each visit, and that the timing of when and how the brain bleed started was uncertain. At trial, a defense verdict was returned.
Discussion
It is well known that patients receiving warfarin are at an increased risk for intracranial hemorrhage (ICH) following blunt head trauma.1 The recommendation is that all such patients have a noncontrast CT scan of the head to rule out intracranial bleeding. This is due to the fact that 60% of patients presenting with an immediate traumatic intracranial hemorrhage will have a normal mental status on examination; and 11% will have no history of loss of consciousness, a normal mental status examination, and no physical evidence of trauma above the clavicles.1 In a study by Hart et al,2 subdural hematoma accounted for 44% of all ICH in these types of patients.
More controversial is how to manage patients on warfarin who experience blunt head trauma and have a normal CT scan of the head. Because of the fear for delayed traumatic ICH, many clinicians recommend admitting such patients for neurological observation and repeat head CT scan the next morning.3 Additionally, some clinicians even recommend reversing the warfarin anticoagulation in such patients. 4 These recommendations, though, are based on expert consensus rather than on rigorous, prospective multicenter studies.1 These strategies are also problematic, since such multiple repeat CT scans would not only be incredibly expensive but also would expose the patient to high doses of radiation to the brain. Moreover, the Centers for Medicare and Medicaid Services has now made CT brain scan imaging of patients presenting to the ED with complaint of nontraumatic headache a quality measure they follow. Their goal is to decrease the number of “unnecessary” head CT scans.
The patient in this case denied any history of trauma on the subsequent seven ED visits. Unfortunately, as pointed out, even minor trauma can result in ICH, and patients may not recall the occurrence of the event.
For patients on warfarin who present with headache, a very careful history must be taken—including inquiring about minor traumatic events. Even then, as has been shown, patients may have not experienced a loss of consciousness, have a normal mental status examination, and exhibit no external evidence of head trauma. The clinician is forced to use her or his own best judgment when evaluating such patients in the ED.
Interestingly, the risk of ICH secondary to blunt head trauma in patients on warfarin is increased if they are on concomitant aspirin therapy.2 Similarly, the risk of ICH following head trauma in patients on clopidogrel is greater than for those patients taking warfarin,1 and the risk of ICH in patients taking dabigatran is less than if taking warfarin.2
Reference - Missed Preeclampsia
- Yancey LM, Withers E, Barnes K, Abbott J. Postpartum preeclampsia: emergency department presentation and management. J Emerg Med. 2011;40(4):380-384.
- Matthys LA, Coppage KH, Lambers DS, Barton JR, Sibai BM. Delayed postpartum preeclampsia: an experience of 151 cases. Am J Obstet Gynecol. 2004;190(5):1464-1466.
- Al-Safi Z, Imudia AN, Filetti LC, Hobson DT, Bahado-Singh RO, Awonuga AO. Delayed postpartum preeclampsia and eclampsia: demographics, clinical course, and complications. Obstet Gynecol. 2011;118(5):1102-1107.
- Chames MC, Livingston JC, Ivester TS, Barton JR, Sibai BM. Late postpartum eclampsia: a preventable disease? Am J Obstet Gynecol. 2002;186(6):1174-1177.
- Graeber B, Vanderwal T, Stiller RJ, Werdmann MJ. Late postpartum eclampsia as an obstetric complication seen in the ED. Am J Emerg Med. 2005;23(2):168-170.
Reference - Missed Subdural Hematoma
- Nishijima DK, Offerman SR, Ballard DW, et al; Clinical Research in Emergency Services and Treatment (CREST) Network. Immediate and delayed traumatic intracranial hemorrhage in patients with head trauma and preinjury warfarin or clopidogrel use. Ann Emerg Med. 2012;59(6):460-468.
- Hart RG, Diener HC, Yang S, Connolly SJ, Wallentin L, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43(6): 1511-1517.
- Vos PE, Battistin L, Birbamer G, et al; European Federation of Neurological Societies. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9(3):207-219.
- Coimbra R, Hoyt DB, Anjaria DJ, Potenza BM, Fortlage D, Hollingsworth-Fridlund P. Reversal of anticoagulation in trauma: a North-American survey on clinical practices among trauma surgeons. J Trauma. 2005;59(2):375-382.
Missed Preeclampsia
| A 24-year-old woman, gravida 1, para 1, aborta 0, presented to the ED complaining of a 1-day history of shortness of breath. Four days earlier, she had delivered a healthy baby boy via normal vaginal delivery and without complication. She denied chest pain, fever, or abdominal pain. She was otherwise in good health, stating that she was not taking any medications. She also denied smoking cigarettes. |
On physical examination, the patient’s vital signs were remarkable for the following: heart rate (HR), 86 beats/minute; blood pressure (BP), 164/94 mm Hg; respiratory rate, 18 breaths/minute; temperature, 98.6oF. Oxygen saturation was 96% on room air. The head, eye, ear, nose and throat examination was unremarkable. The lungs were clear to auscultation bilaterally, and HR and heart rhythm were normal. The abdomen was soft and nontender without guarding or rebound. The lower extremities were remarkable for 1+ pedal and pretibial edema bilaterally.
The patient presented to the same ED 2 days later, again with the chief complaint of shortness of breath. On examination, her BP was noted to be elevated and she had 1+ dependent edema bilaterally. Again, the EP was concerned for a PE and ordered a repeat CTA scan of the chest. This study, similar to the first, was read as normal, and showed no evidence of PE. The patient was diagnosed again with “shortness of breath of unknown etiology” and discharged home. The patient’s obstetrician-gynecologist (Ob/Gyn) was not consulted; however, the patient was encouraged to follow up with him.
The next day, the patient presented to the same ED via emergency medical services, this time with seizures; she had no prior history of a seizure disorder. On presentation to the ED, she was noted to be postictal, with an elevated BP and tachycardic with an HR of 104 beats/minute. On examination, the lungs were clear to auscultation and the lower extremities exhibited 1+ pedal and pretibial edema. A urinalysis revealed proteinuria. The patient was given 4 g of magnesium sulfate intravenously (IV) and her Ob/Gyn was consulted.
The patient was admitted to the hospital with a diagnosis of eclampsia. She was given an IV drip of magnesium and labetalol for the high BP. Unfortunately, the patient apparently had suffered an anoxic brain injury from the previous seizures and died on hospital day 3.
The family sued the treating EPs and the hospital for failure to diagnose preeclampsia on two separate ED presentations. They noted the patient’s Ob/Gyn was never consulted; no action was taken to treat the hypertension; and no urinalysis was ordered on either visit. The EPs and hospital settled the case prior to trial for several million dollars.
Discussion
This is an incredibly sad case, and the EPs and hospital were right to settle and not go to trial. While PE was a reasonable diagnosis to consider in this patient on her first ED visit, it should not have been the only one in the differential diagnosis. The EP became anchored to this single diagnosis and refused to consider other alternative diagnoses—even after the CTA scan of the chest ruled out PE. Moreover, it appears the EP either never considered the significance of the elevated BP and dependent edema or just ignored these findings. To repeat essentially the same exact workup on the second visit does not make sense—one should “cast a wider net, not the same net.”
The diagnosis of “shortness of breath of unknown etiology” is similarly unacceptable. While this is a common and accepted diagnosis when it pertains to abdominal pain, the same is not true for dyspnea.
Preeclampsia is characterized by hypertension (BP >140/90 mm Hg) and proteinuria; associated symptoms include edema and hyperreflexia. Postpartum preeclampsia occurs infrequently and can develop up to 4 weeks after delivery.1 In one 10-year retrospective case series, the incidence of preeclampsia in the postpartum period was 5.7%, and nearly 16% went on to develop eclampsia.2 In a retrospective study of 22 postpartum preeclamptic patients, the median time to presentation was 5 days postpartum.1 In a similar retrospective study of 152 patients, 90% of such patients presented within 7 days.3 The patient in this case initially presented on postpartum day 4.
Interestingly, in a study by Al-Safi et al,3 63% of postpartum preeclamptic patients had no antecedent diagnosis of hypertensive disease during pregnancy. These findings are consistent with the findings of others that 33% to 69% of such patients show no evidence of preeclampsia in the ante- or peripartum period.
The clinical presentation of postpartum preeclampsia is similar to preeclampsia complicating pregnancy after gestation week 20. In the study by Al-Safi et al,3 headache was the most common presenting symptom (69%), followed by shortness of breath (30%), blurry vision (21%), nausea (12.5%), and epigastric abdominal pain (5%). Similarly, Yancey et al1 found headache (82%) to be the most common presenting symptom in their series. Unfortunately, it is not known whether the patient in this case complained of headache or blurred vision as the published records note neither their presence nor absence.
The management of patients with preeclampsia includes IV magnesium to prevent seizures (ie, eclampsia) and BP control.1 A bolus of 4 to 6 g IV magnesium sulfate over 15 to 30 minutes is recommended, followed by an infusion of 2 g/h IV. Historically, IV hydralazine has been used to manage preeclamptic patients with a BP greater than 160/110 mm Hg. More recently, however, IV labetalol has become popular.5 All such patients require admission to the hospital with Ob/Gyn involvement.
Missed Subdural Hematoma
A 59-year-old man presented to the ED with a chief complaint of headache, the onset of which he stated started gradually 2 days prior. He noted the headache was worse than normal but without associated nausea, vomiting, fever, chills, or change in vision. His past medical history was significant for a lower extremity deep vein thrombosis 3 months prior, for which he was taking warfarin.
The patient’s vital signs were all normal. The physical examination, including a thorough neurological examination, was also normal. The EP ordered a prothrombin time (PT), an international normalized ratio (INR), and a noncontrast CT scan of the head. The PT/INR results were therapeutic at 22 seconds and 2.3. The CT scan was interpreted by radiology services as normal. The patient’s headache was treated with IV prochlorperazine and diphenhydramine. After treatment, the patient reported feeling better and was discharged home with instructions to follow up with his primary care physician.
Over the next several months, the patient presented to the same ED on seven different occasions, each time with the chief complaint of headache. At each of these presentations, the history and physical examination were documented as unremarkable, with no history of trauma. The thoroughness, however, of the documentation varied considerably for each ED encounter. No head CT scan was ordered on the subsequent seven visits, and at each presentation, the patient was treated symptomatically and discharged home.
Two days after his eighth visit to the same hospital ED, the patient presented to a different ED, again with a chief complaint of headache. The EP at this ED ordered a noncontrast CT of the head, which demonstrated a left subdural hematoma. The patient was admitted to the hospital, given IV vitamin K and fresh frozen plasma, and underwent evacuation of the hematoma by neurosurgery. The patient’s hospital course was unremarkable, and he was discharged home without any focal weakness.
The patient, however, claimed that he suffered cognitive impairment as a result of the missed diagnosis. He sued treating EPs at the first ED as well as the hospital for failure to timely diagnose the subdural hematoma, stating that a CT scan should have been performed at each of his ED visits since he was on warfarin. The defense claimed that a CT scan was not warranted for each visit, and that the timing of when and how the brain bleed started was uncertain. At trial, a defense verdict was returned.
Discussion
It is well known that patients receiving warfarin are at an increased risk for intracranial hemorrhage (ICH) following blunt head trauma.1 The recommendation is that all such patients have a noncontrast CT scan of the head to rule out intracranial bleeding. This is due to the fact that 60% of patients presenting with an immediate traumatic intracranial hemorrhage will have a normal mental status on examination; and 11% will have no history of loss of consciousness, a normal mental status examination, and no physical evidence of trauma above the clavicles.1 In a study by Hart et al,2 subdural hematoma accounted for 44% of all ICH in these types of patients.
More controversial is how to manage patients on warfarin who experience blunt head trauma and have a normal CT scan of the head. Because of the fear for delayed traumatic ICH, many clinicians recommend admitting such patients for neurological observation and repeat head CT scan the next morning.3 Additionally, some clinicians even recommend reversing the warfarin anticoagulation in such patients. 4 These recommendations, though, are based on expert consensus rather than on rigorous, prospective multicenter studies.1 These strategies are also problematic, since such multiple repeat CT scans would not only be incredibly expensive but also would expose the patient to high doses of radiation to the brain. Moreover, the Centers for Medicare and Medicaid Services has now made CT brain scan imaging of patients presenting to the ED with complaint of nontraumatic headache a quality measure they follow. Their goal is to decrease the number of “unnecessary” head CT scans.
The patient in this case denied any history of trauma on the subsequent seven ED visits. Unfortunately, as pointed out, even minor trauma can result in ICH, and patients may not recall the occurrence of the event.
For patients on warfarin who present with headache, a very careful history must be taken—including inquiring about minor traumatic events. Even then, as has been shown, patients may have not experienced a loss of consciousness, have a normal mental status examination, and exhibit no external evidence of head trauma. The clinician is forced to use her or his own best judgment when evaluating such patients in the ED.
Interestingly, the risk of ICH secondary to blunt head trauma in patients on warfarin is increased if they are on concomitant aspirin therapy.2 Similarly, the risk of ICH following head trauma in patients on clopidogrel is greater than for those patients taking warfarin,1 and the risk of ICH in patients taking dabigatran is less than if taking warfarin.2
Missed Preeclampsia
| A 24-year-old woman, gravida 1, para 1, aborta 0, presented to the ED complaining of a 1-day history of shortness of breath. Four days earlier, she had delivered a healthy baby boy via normal vaginal delivery and without complication. She denied chest pain, fever, or abdominal pain. She was otherwise in good health, stating that she was not taking any medications. She also denied smoking cigarettes. |
On physical examination, the patient’s vital signs were remarkable for the following: heart rate (HR), 86 beats/minute; blood pressure (BP), 164/94 mm Hg; respiratory rate, 18 breaths/minute; temperature, 98.6oF. Oxygen saturation was 96% on room air. The head, eye, ear, nose and throat examination was unremarkable. The lungs were clear to auscultation bilaterally, and HR and heart rhythm were normal. The abdomen was soft and nontender without guarding or rebound. The lower extremities were remarkable for 1+ pedal and pretibial edema bilaterally.
The patient presented to the same ED 2 days later, again with the chief complaint of shortness of breath. On examination, her BP was noted to be elevated and she had 1+ dependent edema bilaterally. Again, the EP was concerned for a PE and ordered a repeat CTA scan of the chest. This study, similar to the first, was read as normal, and showed no evidence of PE. The patient was diagnosed again with “shortness of breath of unknown etiology” and discharged home. The patient’s obstetrician-gynecologist (Ob/Gyn) was not consulted; however, the patient was encouraged to follow up with him.
The next day, the patient presented to the same ED via emergency medical services, this time with seizures; she had no prior history of a seizure disorder. On presentation to the ED, she was noted to be postictal, with an elevated BP and tachycardic with an HR of 104 beats/minute. On examination, the lungs were clear to auscultation and the lower extremities exhibited 1+ pedal and pretibial edema. A urinalysis revealed proteinuria. The patient was given 4 g of magnesium sulfate intravenously (IV) and her Ob/Gyn was consulted.
The patient was admitted to the hospital with a diagnosis of eclampsia. She was given an IV drip of magnesium and labetalol for the high BP. Unfortunately, the patient apparently had suffered an anoxic brain injury from the previous seizures and died on hospital day 3.
The family sued the treating EPs and the hospital for failure to diagnose preeclampsia on two separate ED presentations. They noted the patient’s Ob/Gyn was never consulted; no action was taken to treat the hypertension; and no urinalysis was ordered on either visit. The EPs and hospital settled the case prior to trial for several million dollars.
Discussion
This is an incredibly sad case, and the EPs and hospital were right to settle and not go to trial. While PE was a reasonable diagnosis to consider in this patient on her first ED visit, it should not have been the only one in the differential diagnosis. The EP became anchored to this single diagnosis and refused to consider other alternative diagnoses—even after the CTA scan of the chest ruled out PE. Moreover, it appears the EP either never considered the significance of the elevated BP and dependent edema or just ignored these findings. To repeat essentially the same exact workup on the second visit does not make sense—one should “cast a wider net, not the same net.”
The diagnosis of “shortness of breath of unknown etiology” is similarly unacceptable. While this is a common and accepted diagnosis when it pertains to abdominal pain, the same is not true for dyspnea.
Preeclampsia is characterized by hypertension (BP >140/90 mm Hg) and proteinuria; associated symptoms include edema and hyperreflexia. Postpartum preeclampsia occurs infrequently and can develop up to 4 weeks after delivery.1 In one 10-year retrospective case series, the incidence of preeclampsia in the postpartum period was 5.7%, and nearly 16% went on to develop eclampsia.2 In a retrospective study of 22 postpartum preeclamptic patients, the median time to presentation was 5 days postpartum.1 In a similar retrospective study of 152 patients, 90% of such patients presented within 7 days.3 The patient in this case initially presented on postpartum day 4.
Interestingly, in a study by Al-Safi et al,3 63% of postpartum preeclamptic patients had no antecedent diagnosis of hypertensive disease during pregnancy. These findings are consistent with the findings of others that 33% to 69% of such patients show no evidence of preeclampsia in the ante- or peripartum period.
The clinical presentation of postpartum preeclampsia is similar to preeclampsia complicating pregnancy after gestation week 20. In the study by Al-Safi et al,3 headache was the most common presenting symptom (69%), followed by shortness of breath (30%), blurry vision (21%), nausea (12.5%), and epigastric abdominal pain (5%). Similarly, Yancey et al1 found headache (82%) to be the most common presenting symptom in their series. Unfortunately, it is not known whether the patient in this case complained of headache or blurred vision as the published records note neither their presence nor absence.
The management of patients with preeclampsia includes IV magnesium to prevent seizures (ie, eclampsia) and BP control.1 A bolus of 4 to 6 g IV magnesium sulfate over 15 to 30 minutes is recommended, followed by an infusion of 2 g/h IV. Historically, IV hydralazine has been used to manage preeclamptic patients with a BP greater than 160/110 mm Hg. More recently, however, IV labetalol has become popular.5 All such patients require admission to the hospital with Ob/Gyn involvement.
Missed Subdural Hematoma
A 59-year-old man presented to the ED with a chief complaint of headache, the onset of which he stated started gradually 2 days prior. He noted the headache was worse than normal but without associated nausea, vomiting, fever, chills, or change in vision. His past medical history was significant for a lower extremity deep vein thrombosis 3 months prior, for which he was taking warfarin.
The patient’s vital signs were all normal. The physical examination, including a thorough neurological examination, was also normal. The EP ordered a prothrombin time (PT), an international normalized ratio (INR), and a noncontrast CT scan of the head. The PT/INR results were therapeutic at 22 seconds and 2.3. The CT scan was interpreted by radiology services as normal. The patient’s headache was treated with IV prochlorperazine and diphenhydramine. After treatment, the patient reported feeling better and was discharged home with instructions to follow up with his primary care physician.
Over the next several months, the patient presented to the same ED on seven different occasions, each time with the chief complaint of headache. At each of these presentations, the history and physical examination were documented as unremarkable, with no history of trauma. The thoroughness, however, of the documentation varied considerably for each ED encounter. No head CT scan was ordered on the subsequent seven visits, and at each presentation, the patient was treated symptomatically and discharged home.
Two days after his eighth visit to the same hospital ED, the patient presented to a different ED, again with a chief complaint of headache. The EP at this ED ordered a noncontrast CT of the head, which demonstrated a left subdural hematoma. The patient was admitted to the hospital, given IV vitamin K and fresh frozen plasma, and underwent evacuation of the hematoma by neurosurgery. The patient’s hospital course was unremarkable, and he was discharged home without any focal weakness.
The patient, however, claimed that he suffered cognitive impairment as a result of the missed diagnosis. He sued treating EPs at the first ED as well as the hospital for failure to timely diagnose the subdural hematoma, stating that a CT scan should have been performed at each of his ED visits since he was on warfarin. The defense claimed that a CT scan was not warranted for each visit, and that the timing of when and how the brain bleed started was uncertain. At trial, a defense verdict was returned.
Discussion
It is well known that patients receiving warfarin are at an increased risk for intracranial hemorrhage (ICH) following blunt head trauma.1 The recommendation is that all such patients have a noncontrast CT scan of the head to rule out intracranial bleeding. This is due to the fact that 60% of patients presenting with an immediate traumatic intracranial hemorrhage will have a normal mental status on examination; and 11% will have no history of loss of consciousness, a normal mental status examination, and no physical evidence of trauma above the clavicles.1 In a study by Hart et al,2 subdural hematoma accounted for 44% of all ICH in these types of patients.
More controversial is how to manage patients on warfarin who experience blunt head trauma and have a normal CT scan of the head. Because of the fear for delayed traumatic ICH, many clinicians recommend admitting such patients for neurological observation and repeat head CT scan the next morning.3 Additionally, some clinicians even recommend reversing the warfarin anticoagulation in such patients. 4 These recommendations, though, are based on expert consensus rather than on rigorous, prospective multicenter studies.1 These strategies are also problematic, since such multiple repeat CT scans would not only be incredibly expensive but also would expose the patient to high doses of radiation to the brain. Moreover, the Centers for Medicare and Medicaid Services has now made CT brain scan imaging of patients presenting to the ED with complaint of nontraumatic headache a quality measure they follow. Their goal is to decrease the number of “unnecessary” head CT scans.
The patient in this case denied any history of trauma on the subsequent seven ED visits. Unfortunately, as pointed out, even minor trauma can result in ICH, and patients may not recall the occurrence of the event.
For patients on warfarin who present with headache, a very careful history must be taken—including inquiring about minor traumatic events. Even then, as has been shown, patients may have not experienced a loss of consciousness, have a normal mental status examination, and exhibit no external evidence of head trauma. The clinician is forced to use her or his own best judgment when evaluating such patients in the ED.
Interestingly, the risk of ICH secondary to blunt head trauma in patients on warfarin is increased if they are on concomitant aspirin therapy.2 Similarly, the risk of ICH following head trauma in patients on clopidogrel is greater than for those patients taking warfarin,1 and the risk of ICH in patients taking dabigatran is less than if taking warfarin.2
Reference - Missed Preeclampsia
- Yancey LM, Withers E, Barnes K, Abbott J. Postpartum preeclampsia: emergency department presentation and management. J Emerg Med. 2011;40(4):380-384.
- Matthys LA, Coppage KH, Lambers DS, Barton JR, Sibai BM. Delayed postpartum preeclampsia: an experience of 151 cases. Am J Obstet Gynecol. 2004;190(5):1464-1466.
- Al-Safi Z, Imudia AN, Filetti LC, Hobson DT, Bahado-Singh RO, Awonuga AO. Delayed postpartum preeclampsia and eclampsia: demographics, clinical course, and complications. Obstet Gynecol. 2011;118(5):1102-1107.
- Chames MC, Livingston JC, Ivester TS, Barton JR, Sibai BM. Late postpartum eclampsia: a preventable disease? Am J Obstet Gynecol. 2002;186(6):1174-1177.
- Graeber B, Vanderwal T, Stiller RJ, Werdmann MJ. Late postpartum eclampsia as an obstetric complication seen in the ED. Am J Emerg Med. 2005;23(2):168-170.
Reference - Missed Subdural Hematoma
- Nishijima DK, Offerman SR, Ballard DW, et al; Clinical Research in Emergency Services and Treatment (CREST) Network. Immediate and delayed traumatic intracranial hemorrhage in patients with head trauma and preinjury warfarin or clopidogrel use. Ann Emerg Med. 2012;59(6):460-468.
- Hart RG, Diener HC, Yang S, Connolly SJ, Wallentin L, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43(6): 1511-1517.
- Vos PE, Battistin L, Birbamer G, et al; European Federation of Neurological Societies. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9(3):207-219.
- Coimbra R, Hoyt DB, Anjaria DJ, Potenza BM, Fortlage D, Hollingsworth-Fridlund P. Reversal of anticoagulation in trauma: a North-American survey on clinical practices among trauma surgeons. J Trauma. 2005;59(2):375-382.
Reference - Missed Preeclampsia
- Yancey LM, Withers E, Barnes K, Abbott J. Postpartum preeclampsia: emergency department presentation and management. J Emerg Med. 2011;40(4):380-384.
- Matthys LA, Coppage KH, Lambers DS, Barton JR, Sibai BM. Delayed postpartum preeclampsia: an experience of 151 cases. Am J Obstet Gynecol. 2004;190(5):1464-1466.
- Al-Safi Z, Imudia AN, Filetti LC, Hobson DT, Bahado-Singh RO, Awonuga AO. Delayed postpartum preeclampsia and eclampsia: demographics, clinical course, and complications. Obstet Gynecol. 2011;118(5):1102-1107.
- Chames MC, Livingston JC, Ivester TS, Barton JR, Sibai BM. Late postpartum eclampsia: a preventable disease? Am J Obstet Gynecol. 2002;186(6):1174-1177.
- Graeber B, Vanderwal T, Stiller RJ, Werdmann MJ. Late postpartum eclampsia as an obstetric complication seen in the ED. Am J Emerg Med. 2005;23(2):168-170.
Reference - Missed Subdural Hematoma
- Nishijima DK, Offerman SR, Ballard DW, et al; Clinical Research in Emergency Services and Treatment (CREST) Network. Immediate and delayed traumatic intracranial hemorrhage in patients with head trauma and preinjury warfarin or clopidogrel use. Ann Emerg Med. 2012;59(6):460-468.
- Hart RG, Diener HC, Yang S, Connolly SJ, Wallentin L, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43(6): 1511-1517.
- Vos PE, Battistin L, Birbamer G, et al; European Federation of Neurological Societies. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9(3):207-219.
- Coimbra R, Hoyt DB, Anjaria DJ, Potenza BM, Fortlage D, Hollingsworth-Fridlund P. Reversal of anticoagulation in trauma: a North-American survey on clinical practices among trauma surgeons. J Trauma. 2005;59(2):375-382.