User login
Roe v. Wade: Medical groups react to Supreme Court decision
The country’s top medical organizations condemned the overturning of Roe v. Wade, saying the removal of federal protections for women to access abortion services marks a “dark day.”
“It is unfathomable. It is unfair. It is wrong,” said the President of the American College of Obstetricians and Gynecologists (ACOG) Iffath Abbasi Hoskins, MD.
“Today is a very dark day in health care. It is a dark day, indeed, for the tens of millions of patients who have suddenly and unfairly lost access to safe legal and evidence-based abortion care,” Dr. Hoskins said at a press conference June 24 sponsored by ACOG.
“It is dark for the thousands of clinicians who now, instead of focusing on providing health care to their patients, have to live with the threats of legal, civil, and even professional penalties,” Dr. Hoskins added.
ACOG has 62,000 members and is the leading group of doctors that provides obstetric and gynecologic care.
Dilemma for some doctors?
“I’d like to take a moment to talk about the future of the medical profession,” said ACOG Chief Executive Officer Maureen G. Phipps, MD, MPH. “[The] decision is, as Dr. Hoskins clearly said, a tragic one for our patients in states across the country, but the harm does not end there.”
Dr. Phipps described overturning Roe v. Wade as “the boldest act of legislative interference that we have seen in this country. It will allow state legislators to tell physicians what care they can and cannot provide to their patients.”
“It will leave physicians looking over our shoulders, wondering if a patient is in enough of a crisis to permit an exception to a law,” Dr. Phipps added. “This is an affront to all that drew my colleagues and me into medicine.”
Although the impact on doctor training remains to be seen, she said 44% of ob.gyn. residents are trained in states now empowered to ban abortions.
The effect of the Supreme Court decision on miscarriage management is another unknown.
“It’s going to be very difficult for us, the clinicians, to manage miscarriage,” Dr. Hoskins said. “Many miscarriages could be what we call ‘incomplete’ in the beginning,” where there is still a heartbeat and the patient is cramping and/or bleeding.
In that instance, Dr. Hoskins said, clinicians may be thinking that they have to wait.
“They may be needing to get additional opinions, whether it’s a legal opinion ... or another medical opinion.”
“It’s going to have a devastating effect on every aspect of a woman’s health care, including if she is spontaneously miscarrying,” Dr. Hoskins predicted.
Physician protect thyself?
To what extent doctors can shield themselves from potential prosecution “is a hard question to answer,” Molly Meegan, JD, ACOG’s chief legal officer and general counsel, said.
Ms. Meegan recommended members speak to the risk managers at their individual institutions for guidance.
“It is a real patchwork [of laws] out there, she said. “And that patchwork itself is a danger to people as they seek essential reproductive health care.”
Also, she added, “If a doctor can’t tell what the law is at the time they’re trying to provide the care, it has a terribly chilling effect on medical care.”
Another potential threat to doctors in states that still allow abortion services is action from a neighboring state.
“We are going to be advocating very strongly that states do not have extra-territorial jurisdiction to reach beyond the edges of their state.”
The worry is if a doctor in New Mexico, where abortion is legal, performs an abortion for a person from Texas, where it will soon be illegal, is then prosecuted by Texas, for example.
Medication abortion
Asked about any potential effects on medication abortions, ACOG’s Jen Villavicencio, MD, said it remains to be seen.
“Certainly many of the laws that we have seen, including trigger ban laws, encompass medication abortion,” she said. Several states have these so-called trigger laws, which put into effect laws passed to ban abortion in case Roe was overturned.
This means, she said, that any abortion option, whether it’s procedural or medication, could be and will be banned in some of these states.
Ms. Meegan added that ACOG will continue to support access to medication abortion and that it should be decided by the U.S. Food and Drug Administration and not individual states.
Maternal mortality may rise
“Maternal mortality in and of itself is a very difficult topic,” Dr. Hoskins said, but [the] decision amplifies the implications. “I think of the patients who will have to manage severe complications and mental health challenges while they are carrying a pregnancy that they are forced to carry.”
“I also think of the patients who need to end their pregnancies in order to save their own lives,” Dr. Hoskins added.
Dr. Hoskins said the United States already has a high maternal mortality rate. This new law, she added, could force women into higher-risk situations if they experience high blood pressure, preeclampsia, or bleeding after the birth of the baby.
Growing inequality possible?
“The grievous inequities that exist in this country will grow and expand unchecked without safe access to legal abortion,” Dr. Phipps said.
She noted that women, based on location, will continue “to have protected access to safe evidence-based abortion. Others will have the means and resources and opportunities to secure the care.”
But the same may not be true for women in underserved or disadvantaged communities, Dr. Phipps added.
American Medical Association
ACOG was not the only group to react. “The American Medical Association is deeply disturbed by the U.S. Supreme Court’s decision to overturn nearly a half century of precedent protecting patients’ right to critical reproductive health care,” President Jack Resneck Jr., MD, said in a statement.
The decision represents “an egregious allowance of government intrusion into the medical examination room, a direct attack on the practice of medicine and the patient-physician relationship, and a brazen violation of patients’ rights to evidence-based reproductive health services.”
American Academy of Family Physicians
“The American Academy of Family Physicians is disappointed and disheartened by the Supreme Court’s decision to strike down longstanding protections afforded by Roe v. Wade and Planned Parenthood v. Casey,” President Sterling N. Ransone Jr., MD, said in a statement.
The organization has 127,600 physician and medical student members.
“This decision negatively impacts our practices and our patients by undermining the patient-physician relationship and potentially criminalizing evidence-based medical care,” added Dr. Ransone.
American College of Physicians
“A patient’s decision about whether to continue a pregnancy should be a private decision made in consultation with a physician or other health care professional, without interference from the government,” President Ryan D. Mire, MD, said in a statement. “We strongly oppose medically unnecessary government restrictions on any health care services,” added Dr. Mire on behalf of the group’s 161,000 members.
American Academy of Pediatrics
“This decision carries grave consequences for our adolescent patients, who already face many more barriers than adults in accessing comprehensive reproductive health care services and abortion care,” President Moira Szilagyi, MD, PhD, said in a statement.
“In the wake of this ruling, the American Academy of Pediatrics will continue to support our chapters as states consider policies affecting access to abortion care, and pediatricians will continue to support our patients,” Dr. Szilagyi added.
American Public Health Association
The court’s decision “is a catastrophic judicial failure that will reverberate differently in each state and portends to jeopardize the health and lives of all Americans,” Executive Director Georges C. Benjamin, MD, said in a statement.
American Urogynecologic Society
“The American Urogynecologic Society opposes any ruling that restricts a person’s access to health care and criminalizes the practice of medicine,” the group said in a statement. “This ruling ultimately poses a serious threat to the patient-provider relationship and subsequent decisionmaking necessary to ensure optimal outcomes for patients. As practitioners, we should be free to provide what is in the best interest of our patients.”
A version of this article first appeared on Medscape.com.
The country’s top medical organizations condemned the overturning of Roe v. Wade, saying the removal of federal protections for women to access abortion services marks a “dark day.”
“It is unfathomable. It is unfair. It is wrong,” said the President of the American College of Obstetricians and Gynecologists (ACOG) Iffath Abbasi Hoskins, MD.
“Today is a very dark day in health care. It is a dark day, indeed, for the tens of millions of patients who have suddenly and unfairly lost access to safe legal and evidence-based abortion care,” Dr. Hoskins said at a press conference June 24 sponsored by ACOG.
“It is dark for the thousands of clinicians who now, instead of focusing on providing health care to their patients, have to live with the threats of legal, civil, and even professional penalties,” Dr. Hoskins added.
ACOG has 62,000 members and is the leading group of doctors that provides obstetric and gynecologic care.
Dilemma for some doctors?
“I’d like to take a moment to talk about the future of the medical profession,” said ACOG Chief Executive Officer Maureen G. Phipps, MD, MPH. “[The] decision is, as Dr. Hoskins clearly said, a tragic one for our patients in states across the country, but the harm does not end there.”
Dr. Phipps described overturning Roe v. Wade as “the boldest act of legislative interference that we have seen in this country. It will allow state legislators to tell physicians what care they can and cannot provide to their patients.”
“It will leave physicians looking over our shoulders, wondering if a patient is in enough of a crisis to permit an exception to a law,” Dr. Phipps added. “This is an affront to all that drew my colleagues and me into medicine.”
Although the impact on doctor training remains to be seen, she said 44% of ob.gyn. residents are trained in states now empowered to ban abortions.
The effect of the Supreme Court decision on miscarriage management is another unknown.
“It’s going to be very difficult for us, the clinicians, to manage miscarriage,” Dr. Hoskins said. “Many miscarriages could be what we call ‘incomplete’ in the beginning,” where there is still a heartbeat and the patient is cramping and/or bleeding.
In that instance, Dr. Hoskins said, clinicians may be thinking that they have to wait.
“They may be needing to get additional opinions, whether it’s a legal opinion ... or another medical opinion.”
“It’s going to have a devastating effect on every aspect of a woman’s health care, including if she is spontaneously miscarrying,” Dr. Hoskins predicted.
Physician protect thyself?
To what extent doctors can shield themselves from potential prosecution “is a hard question to answer,” Molly Meegan, JD, ACOG’s chief legal officer and general counsel, said.
Ms. Meegan recommended members speak to the risk managers at their individual institutions for guidance.
“It is a real patchwork [of laws] out there, she said. “And that patchwork itself is a danger to people as they seek essential reproductive health care.”
Also, she added, “If a doctor can’t tell what the law is at the time they’re trying to provide the care, it has a terribly chilling effect on medical care.”
Another potential threat to doctors in states that still allow abortion services is action from a neighboring state.
“We are going to be advocating very strongly that states do not have extra-territorial jurisdiction to reach beyond the edges of their state.”
The worry is if a doctor in New Mexico, where abortion is legal, performs an abortion for a person from Texas, where it will soon be illegal, is then prosecuted by Texas, for example.
Medication abortion
Asked about any potential effects on medication abortions, ACOG’s Jen Villavicencio, MD, said it remains to be seen.
“Certainly many of the laws that we have seen, including trigger ban laws, encompass medication abortion,” she said. Several states have these so-called trigger laws, which put into effect laws passed to ban abortion in case Roe was overturned.
This means, she said, that any abortion option, whether it’s procedural or medication, could be and will be banned in some of these states.
Ms. Meegan added that ACOG will continue to support access to medication abortion and that it should be decided by the U.S. Food and Drug Administration and not individual states.
Maternal mortality may rise
“Maternal mortality in and of itself is a very difficult topic,” Dr. Hoskins said, but [the] decision amplifies the implications. “I think of the patients who will have to manage severe complications and mental health challenges while they are carrying a pregnancy that they are forced to carry.”
“I also think of the patients who need to end their pregnancies in order to save their own lives,” Dr. Hoskins added.
Dr. Hoskins said the United States already has a high maternal mortality rate. This new law, she added, could force women into higher-risk situations if they experience high blood pressure, preeclampsia, or bleeding after the birth of the baby.
Growing inequality possible?
“The grievous inequities that exist in this country will grow and expand unchecked without safe access to legal abortion,” Dr. Phipps said.
She noted that women, based on location, will continue “to have protected access to safe evidence-based abortion. Others will have the means and resources and opportunities to secure the care.”
But the same may not be true for women in underserved or disadvantaged communities, Dr. Phipps added.
American Medical Association
ACOG was not the only group to react. “The American Medical Association is deeply disturbed by the U.S. Supreme Court’s decision to overturn nearly a half century of precedent protecting patients’ right to critical reproductive health care,” President Jack Resneck Jr., MD, said in a statement.
The decision represents “an egregious allowance of government intrusion into the medical examination room, a direct attack on the practice of medicine and the patient-physician relationship, and a brazen violation of patients’ rights to evidence-based reproductive health services.”
American Academy of Family Physicians
“The American Academy of Family Physicians is disappointed and disheartened by the Supreme Court’s decision to strike down longstanding protections afforded by Roe v. Wade and Planned Parenthood v. Casey,” President Sterling N. Ransone Jr., MD, said in a statement.
The organization has 127,600 physician and medical student members.
“This decision negatively impacts our practices and our patients by undermining the patient-physician relationship and potentially criminalizing evidence-based medical care,” added Dr. Ransone.
American College of Physicians
“A patient’s decision about whether to continue a pregnancy should be a private decision made in consultation with a physician or other health care professional, without interference from the government,” President Ryan D. Mire, MD, said in a statement. “We strongly oppose medically unnecessary government restrictions on any health care services,” added Dr. Mire on behalf of the group’s 161,000 members.
American Academy of Pediatrics
“This decision carries grave consequences for our adolescent patients, who already face many more barriers than adults in accessing comprehensive reproductive health care services and abortion care,” President Moira Szilagyi, MD, PhD, said in a statement.
“In the wake of this ruling, the American Academy of Pediatrics will continue to support our chapters as states consider policies affecting access to abortion care, and pediatricians will continue to support our patients,” Dr. Szilagyi added.
American Public Health Association
The court’s decision “is a catastrophic judicial failure that will reverberate differently in each state and portends to jeopardize the health and lives of all Americans,” Executive Director Georges C. Benjamin, MD, said in a statement.
American Urogynecologic Society
“The American Urogynecologic Society opposes any ruling that restricts a person’s access to health care and criminalizes the practice of medicine,” the group said in a statement. “This ruling ultimately poses a serious threat to the patient-provider relationship and subsequent decisionmaking necessary to ensure optimal outcomes for patients. As practitioners, we should be free to provide what is in the best interest of our patients.”
A version of this article first appeared on Medscape.com.
The country’s top medical organizations condemned the overturning of Roe v. Wade, saying the removal of federal protections for women to access abortion services marks a “dark day.”
“It is unfathomable. It is unfair. It is wrong,” said the President of the American College of Obstetricians and Gynecologists (ACOG) Iffath Abbasi Hoskins, MD.
“Today is a very dark day in health care. It is a dark day, indeed, for the tens of millions of patients who have suddenly and unfairly lost access to safe legal and evidence-based abortion care,” Dr. Hoskins said at a press conference June 24 sponsored by ACOG.
“It is dark for the thousands of clinicians who now, instead of focusing on providing health care to their patients, have to live with the threats of legal, civil, and even professional penalties,” Dr. Hoskins added.
ACOG has 62,000 members and is the leading group of doctors that provides obstetric and gynecologic care.
Dilemma for some doctors?
“I’d like to take a moment to talk about the future of the medical profession,” said ACOG Chief Executive Officer Maureen G. Phipps, MD, MPH. “[The] decision is, as Dr. Hoskins clearly said, a tragic one for our patients in states across the country, but the harm does not end there.”
Dr. Phipps described overturning Roe v. Wade as “the boldest act of legislative interference that we have seen in this country. It will allow state legislators to tell physicians what care they can and cannot provide to their patients.”
“It will leave physicians looking over our shoulders, wondering if a patient is in enough of a crisis to permit an exception to a law,” Dr. Phipps added. “This is an affront to all that drew my colleagues and me into medicine.”
Although the impact on doctor training remains to be seen, she said 44% of ob.gyn. residents are trained in states now empowered to ban abortions.
The effect of the Supreme Court decision on miscarriage management is another unknown.
“It’s going to be very difficult for us, the clinicians, to manage miscarriage,” Dr. Hoskins said. “Many miscarriages could be what we call ‘incomplete’ in the beginning,” where there is still a heartbeat and the patient is cramping and/or bleeding.
In that instance, Dr. Hoskins said, clinicians may be thinking that they have to wait.
“They may be needing to get additional opinions, whether it’s a legal opinion ... or another medical opinion.”
“It’s going to have a devastating effect on every aspect of a woman’s health care, including if she is spontaneously miscarrying,” Dr. Hoskins predicted.
Physician protect thyself?
To what extent doctors can shield themselves from potential prosecution “is a hard question to answer,” Molly Meegan, JD, ACOG’s chief legal officer and general counsel, said.
Ms. Meegan recommended members speak to the risk managers at their individual institutions for guidance.
“It is a real patchwork [of laws] out there, she said. “And that patchwork itself is a danger to people as they seek essential reproductive health care.”
Also, she added, “If a doctor can’t tell what the law is at the time they’re trying to provide the care, it has a terribly chilling effect on medical care.”
Another potential threat to doctors in states that still allow abortion services is action from a neighboring state.
“We are going to be advocating very strongly that states do not have extra-territorial jurisdiction to reach beyond the edges of their state.”
The worry is if a doctor in New Mexico, where abortion is legal, performs an abortion for a person from Texas, where it will soon be illegal, is then prosecuted by Texas, for example.
Medication abortion
Asked about any potential effects on medication abortions, ACOG’s Jen Villavicencio, MD, said it remains to be seen.
“Certainly many of the laws that we have seen, including trigger ban laws, encompass medication abortion,” she said. Several states have these so-called trigger laws, which put into effect laws passed to ban abortion in case Roe was overturned.
This means, she said, that any abortion option, whether it’s procedural or medication, could be and will be banned in some of these states.
Ms. Meegan added that ACOG will continue to support access to medication abortion and that it should be decided by the U.S. Food and Drug Administration and not individual states.
Maternal mortality may rise
“Maternal mortality in and of itself is a very difficult topic,” Dr. Hoskins said, but [the] decision amplifies the implications. “I think of the patients who will have to manage severe complications and mental health challenges while they are carrying a pregnancy that they are forced to carry.”
“I also think of the patients who need to end their pregnancies in order to save their own lives,” Dr. Hoskins added.
Dr. Hoskins said the United States already has a high maternal mortality rate. This new law, she added, could force women into higher-risk situations if they experience high blood pressure, preeclampsia, or bleeding after the birth of the baby.
Growing inequality possible?
“The grievous inequities that exist in this country will grow and expand unchecked without safe access to legal abortion,” Dr. Phipps said.
She noted that women, based on location, will continue “to have protected access to safe evidence-based abortion. Others will have the means and resources and opportunities to secure the care.”
But the same may not be true for women in underserved or disadvantaged communities, Dr. Phipps added.
American Medical Association
ACOG was not the only group to react. “The American Medical Association is deeply disturbed by the U.S. Supreme Court’s decision to overturn nearly a half century of precedent protecting patients’ right to critical reproductive health care,” President Jack Resneck Jr., MD, said in a statement.
The decision represents “an egregious allowance of government intrusion into the medical examination room, a direct attack on the practice of medicine and the patient-physician relationship, and a brazen violation of patients’ rights to evidence-based reproductive health services.”
American Academy of Family Physicians
“The American Academy of Family Physicians is disappointed and disheartened by the Supreme Court’s decision to strike down longstanding protections afforded by Roe v. Wade and Planned Parenthood v. Casey,” President Sterling N. Ransone Jr., MD, said in a statement.
The organization has 127,600 physician and medical student members.
“This decision negatively impacts our practices and our patients by undermining the patient-physician relationship and potentially criminalizing evidence-based medical care,” added Dr. Ransone.
American College of Physicians
“A patient’s decision about whether to continue a pregnancy should be a private decision made in consultation with a physician or other health care professional, without interference from the government,” President Ryan D. Mire, MD, said in a statement. “We strongly oppose medically unnecessary government restrictions on any health care services,” added Dr. Mire on behalf of the group’s 161,000 members.
American Academy of Pediatrics
“This decision carries grave consequences for our adolescent patients, who already face many more barriers than adults in accessing comprehensive reproductive health care services and abortion care,” President Moira Szilagyi, MD, PhD, said in a statement.
“In the wake of this ruling, the American Academy of Pediatrics will continue to support our chapters as states consider policies affecting access to abortion care, and pediatricians will continue to support our patients,” Dr. Szilagyi added.
American Public Health Association
The court’s decision “is a catastrophic judicial failure that will reverberate differently in each state and portends to jeopardize the health and lives of all Americans,” Executive Director Georges C. Benjamin, MD, said in a statement.
American Urogynecologic Society
“The American Urogynecologic Society opposes any ruling that restricts a person’s access to health care and criminalizes the practice of medicine,” the group said in a statement. “This ruling ultimately poses a serious threat to the patient-provider relationship and subsequent decisionmaking necessary to ensure optimal outcomes for patients. As practitioners, we should be free to provide what is in the best interest of our patients.”
A version of this article first appeared on Medscape.com.
Roe reversal may go well beyond abortion
Kami, a mother of one daughter in central Texas, lost three pregnancies in 2008. The third one nearly killed her.
The embryo became implanted in one of the fallopian tubes connecting her ovaries to her uterus. Because fallopian tubes can’t stretch to accommodate a fetus, patients must undergo surgery to remove the embryo before the tube ruptures. Failure to do so can result in internal bleeding and death.
But when Kami – who did not want to use her last name to avoid harassment – underwent an ultrasound to start the process of extracting the embryo, her doctor miscalculated how far along in the pregnancy she was and told her to come back in a few weeks.
She eventually did return, but only after passing out in the bathtub and waking up in a pool of her own blood. The tube had ruptured, and to remove it, emergency surgery was necessary.
Stories such as Kami’s could become more common in the aftermath of the U.S. Supreme Court’s decision to overturn Roe v. Wade, the 1973 case that created a right to an abortion.
Experts fear that antiabortion laws that take effect in the United States following the court’s decision will lead to a medical and legal limbo for thousands of people like Kami – people with uncommon reproductive conditions whose treatments involve the termination of pregnancies or the destruction of embryos.
Vague exceptions prompt concerns
According to the Guttmacher Institute, a nonprofit group for reproductive health, 13 states currently have trigger laws on the books that make abortion illegal in the absence of Roe. Nine other states have laws that would outlaw or severely restrict abortion without a federal right to the procedure.
Each of these laws carves out exceptions that allow the termination of a pregnancy to prevent the death of the pregnant individual. But the language of the provisions is not always precise in describing what those exceptions mean in practice, according to Elizabeth Nash, the principal policy associate for state issues at the Guttmacher Institute.
“These exceptions are designed to be extraordinarily narrow. These aren’t really designed to be usable exceptions,” Ms. Nash said. “There’s so much misinformation about abortion that there are probably legislators out there who think that it’s never needed to save a life.”
Tubal pregnancies
One of the best examples of a pregnancy termination that’s necessary to avoid death is in the case of an ectopic pregnancy such as Kami experienced. Without treatment to end the pregnancy, the embryo will eventually grow so large that the tube ruptures, causing massive bleeding that can kill the mother.
Most state laws regarding abortion exclude treatment of ectopic pregnancy, according to Ms. Nash. But, “if the state does not exclude ectopic pregnancy from all the regulations, then people might not be able to get the care that they need when they need it.”
The current abortion law in Texas, for example, prohibits ending a pregnancy after 6 weeks, or after cardiac activity becomes present. Cardiac activity can be present in cases of ectopic pregnancies, which account for between 1% and 2% of all pregnancies and are the leading cause of maternal deaths in the first trimester. And treatment definitely ends the life of the embryo or fetus in the fallopian tube, said Lisa Harris, MD, PhD, an ob.gyn. and medical ethicist at the University of Michigan, Ann Arbor.
Dr. Harris said she has never doubted that an ectopic pregnancy cannot possibly result in a live birth. But she recalled an encounter with another clinician on a surgical team for an ectopic pregnancy who said: “So you’re going to take it out of the tube and put it in the uterus, right?”
“It was a startling moment,” Dr. Harris recalled. She regarded the procedure as a “lifesaving, obvious surgery,” but her colleague, whose suggestion was a medical impossibility since the window of implantation is very brief after fertilization, viewed it “as an abortion, as killing an embryo or fetus.”
Dr. Harris said she isn’t concerned that physicians would stop treating ectopic pregnancies in a post-Roe world. Rather, she worries about two other possibilities: an overzealous prosecutor might not believe it was an ectopic pregnancy and press charges; or laws will cause physicians to second guess their clinical decisions for patients.
“What it means, in the middle of the night, when someone comes in with a 10-week ectopic pregnancy with a heartbeat, is the doctor may hesitate,” Dr. Harris said. Despite knowing the appropriate treatment, the doctor may want to speak with a lawyer or ethicist first to ensure they are covered legally. “And as that process unfolds, which could take hours or days, the person might have a complication.” s
Not treating an ectopic pregnancy would be malpractice, but “some doctors may not provide the standard of care that they would have ordinarily provided because they don’t want to risk breaking the law,” she said.
Even more ambiguous are cornual ectopic pregnancies, in which the implantation occurs at the junction of a fallopian tube and the uterus. These pregnancies, which make up 2%-4% of all tubal pregnancies, are immediately adjacent to the uterus. If an abortion is defined as the termination of an embryo or fetus in the womb, how such a legal definition would apply to these pregnancies is unclear.
An ob.gyn. wouldn’t regard ending an ectopic pregnancy as an abortion, but “this is not about logic or clinical meaning,” Dr. Harris said. “This is people outside of medicine making determinations that all pregnancies must continue, and when you think of a ban that way, you could see why a doctor would be frightened to end a pregnancy, whether it might be viable in the future or not.”
That’s true even if the pregnancy is located fully in the uterus. Dr. Harris described a pregnant patient she saw who had traveled from Texas to Michigan with a fetus that had a lethal defect.
The fetus had “an anomaly where the lungs couldn’t develop, where there were no kidneys. There was no chance this baby could be born and live. Her doctors were very clear that there will never be a baby that [she could] take home at the end of this pregnancy, yet they would not end her pregnancy because that would be an abortion,” Dr. Harris said.
Texas law “doesn’t make any allowances for whether a pregnancy will ever actually result in a baby or not,” Dr. Harris said. “The law, in effect, just says all pregnancies must continue.”
Selective reduction
How abortion laws in different states might affect selective reduction, which is used in some pregnancies to reduce the total number of fetuses a person is carrying, is even more ambiguous. The goal of selective reduction is to decrease health risks to the pregnant individual and increase the likelihood of survival for the remaining fetuses. Current Texas law prohibits these procedures.
Someone pregnant with quintuplets, for example, might seek selection reduction to reduce the pregnancy outcome to triplets or twins. A related procedure, selective termination, is used to terminate the life of a fetus with abnormalities while the pregnancy of the fetus’ in utero siblings continues.
The advent of assisted reproduction methods, such as in vitro fertilization (IVF), greatly increased the incidence of higher-order multiples, those with three or more fetuses. The first IVF baby was born in 1978. By 1998, the rate of higher-order multiple births was 1.9 per 1,000 births, five times the figure in 1980. The rate has since decreased by nearly half, to 1 per 1,000 births, but with 3.75 million live births a year, that’s still a lot of pregnancies with higher-order multiples.
The American College of Obstetricians and Gynecologists does not provide explicit guidance on when selective reduction is warranted, but its committee opinion on multifetal pregnancy reduction provides an ethical framework for providers to use when counseling people with pregnancies of three or more fetuses. How would various state laws that outlaw abortion affect these decisions? No one knows.
“Selective reduction ends the life of a fetus or embryo, but it doesn’t end the pregnancy,” Dr. Harris said. “So, if the pregnancy continues but it kills an embryo or fetus, is that an abortion?”
‘The question of the hour’
Dr. Harris and other doctors are haunted by potential medical cases in which continuing a pregnancy may result in the death of the person carrying the fetus but in which such death may not be so imminent that the law would allow immediate termination of the pregnancy.
Michael Northrup, MD, an intensive care pediatrician in Winston-Salem, N.C., recalled a particularly harrowing case that illustrates the peril in deciding when someone’s life is “enough” in danger to qualify as an exception to abortion bans.
The 14-year-old girl had severe lupus and kidney failure that required treatment with methotrexate and immediate dialysis to replace her electrolytes. A standard pretreatment pregnancy test revealed that she had been carrying a child for at least 10 weeks. Her pregnancy presented two problems. Methotrexate is so severely toxic that it’s sometimes used to end pregnancies. Even at low doses, fetuses that survive usually have severe deformities. In addition, dialysis requires administration of a blood thinner. If the teen miscarried while taking a blood thinner during dialysis, she risked bleeding to death.
Treatment could be delayed until week 24 of pregnancy, at which time delivery could be attempted, but the patient likely wouldn’t have any kidney function left by then. In addition, at 24 weeks, it was unlikely that the baby would survive anyway.
Dr. Northrup said that, had she chosen that route, “I’m not sure she would have made it. This was a religious family, people who very much were believers. They had their head of church come in, who fairly quickly determined that the best thing for her health was to terminate this pregnancy immediately and get the treatment she needed for her body.”
Would such a situation qualify for an emergency termination? The girl wasn’t going to die within 24 or 48 hours, but it may not have been possible to pinpoint the time of death within a day or 2.
“The family was sad, but they made that choice, and I wonder, would we have to justify that with these new laws?” Dr. Northrup said. “You definitely worry, being in the hot seat, ‘Does this count enough? Is she close enough to death?’ ”
The same question comes up when someone’s water breaks early in the second trimester. Since a live-birth delivery would be highly unlikely, given the age of the fetus, the standard of care is to offer to terminate the pregnancy to avoid a serious infection, Dr. Harris said. But if the infection hasn’t yet developed – even if it’s likely to develop soon – doctors in a state that outlaws abortion would not be able to offer termination. But as providers wait for an infection to develop, the person’s risk of dying from infection rapidly increases.
“How likely does someone need to be to die for it to count to get a life-preserving abortion?” Dr. Harris asked. “That, I think, is the question of the hour.”
Different institutions may decide to determine their own risk thresholds. One hospital, for example, may decide that any health threat that is associated with a 10% risk of death qualifies for a lifesaving abortion. But for many people, a 1 in 10 chance of dying is quite high.
“Who gets to decide what’s meaningful?” Dr. Harris asked, especially if the patient is already a parent of living children and doesn’t want to take any risk at all of orphaning them for a pregnancy with severe complications.
“The point is that this is way more complicated than anybody really knows, way more complicated than any legislator or justice could possibly know, and it creates all kinds of complicated ambiguities, some of which could result in harm to women,” she said. “I’ve been a doctor almost 30 years, and every week, sometimes every day, I’m humbled by how complicated pregnancy is and how complicated people’s bodies and life situations are.”
That’s what makes it so dangerous for policymakers to “insert themselves into medical practice,” Ms. Nash said. She worries about the legal ramifications of overturning Roe, such as prosecution of people who illegally undergo an abortion or of physicians who perform a procedure that a judge deems to be in violation of abortion law.
“There are already local prosecutors who have misused the law to go after people who have managed their own abortions,” Ms. Nash said. “Criminal abortion law, fetal homicide, child neglect, practicing medicine without a license – these are things people have actually been arrested and convicted under.”
Some laws may target the person seeking an abortion, whereas others may target clinicians providing abortions, or even people who simply help someone obtain an abortion, as the Texas law does. In Dr. Harris’s own state of Michigan, a group of Republican lawmakers recently introduced a bill that would imprison abortion providers for up to 10 years and anyone creating or distributing abortion medication for up to 20 years.
Michigan Gov. Gretchen Whitmer, a Democrat who called the proposed legislation “disturbing” and “infuriating,” would almost certainly veto the bill, but it’s just one of dozens already filed or that are expected to be filed across the United States.
The antiabortion organization National Right to Life has published a “post-Roe model abortion law” for states to adopt. The model includes an exemption for abortions that, “based on reasonable medical judgment, [were] necessary to prevent the death of the pregnant woman” – but, again, it does not clarify what that means in practice.
Lectures from strangers
Four years after nearly dying, Kami gave birth to a healthy girl following an uncomplicated pregnancy. But her journey to having more children presented more challenges.
Two years after the birth of her child, she had another ectopic pregnancy. Her doctor sent her prescriptions for medication that would end this pregnancy, but a pharmacist refused to fill the prescription.
“Do you know these are very serious medications?” the pharmacist asked her. She did – she had taken them once before for another ectopic pregnancy. She was with her daughter, feeling devastated about losing yet another desired pregnancy. She simply wanted to get the medication and go home.
“‘So you’re trying to have a cheap abortion,’ he said, and 30 heads turned and looked at me. The whole pharmacy heard,” Kami said.
She told the pharmacist that she’d miscarried. She said he responded with: “So you have a dead baby in your body.”
Even after her doctor called to insist on filling the order, the man refused to fill it.
Kami left without the prescription, and her doctor performed a surgical dilation and curettage to remove the embryo from her fallopian tube for no fee.
Kami later tried again to have more children. She experienced another ruptured tube that she said nearly killed her.
“There was such a sense of pain knowing that I couldn’t have any more kids, but also the relief of knowing that I don’t have to go through this again,” Kami said. Now, however, with the Supreme Court having overturned Roe v. Wade, she has a new worry: “That my daughter will not have the same rights and access to health care that I did.”
A version of this article first appeared on Medscape.com.
Kami, a mother of one daughter in central Texas, lost three pregnancies in 2008. The third one nearly killed her.
The embryo became implanted in one of the fallopian tubes connecting her ovaries to her uterus. Because fallopian tubes can’t stretch to accommodate a fetus, patients must undergo surgery to remove the embryo before the tube ruptures. Failure to do so can result in internal bleeding and death.
But when Kami – who did not want to use her last name to avoid harassment – underwent an ultrasound to start the process of extracting the embryo, her doctor miscalculated how far along in the pregnancy she was and told her to come back in a few weeks.
She eventually did return, but only after passing out in the bathtub and waking up in a pool of her own blood. The tube had ruptured, and to remove it, emergency surgery was necessary.
Stories such as Kami’s could become more common in the aftermath of the U.S. Supreme Court’s decision to overturn Roe v. Wade, the 1973 case that created a right to an abortion.
Experts fear that antiabortion laws that take effect in the United States following the court’s decision will lead to a medical and legal limbo for thousands of people like Kami – people with uncommon reproductive conditions whose treatments involve the termination of pregnancies or the destruction of embryos.
Vague exceptions prompt concerns
According to the Guttmacher Institute, a nonprofit group for reproductive health, 13 states currently have trigger laws on the books that make abortion illegal in the absence of Roe. Nine other states have laws that would outlaw or severely restrict abortion without a federal right to the procedure.
Each of these laws carves out exceptions that allow the termination of a pregnancy to prevent the death of the pregnant individual. But the language of the provisions is not always precise in describing what those exceptions mean in practice, according to Elizabeth Nash, the principal policy associate for state issues at the Guttmacher Institute.
“These exceptions are designed to be extraordinarily narrow. These aren’t really designed to be usable exceptions,” Ms. Nash said. “There’s so much misinformation about abortion that there are probably legislators out there who think that it’s never needed to save a life.”
Tubal pregnancies
One of the best examples of a pregnancy termination that’s necessary to avoid death is in the case of an ectopic pregnancy such as Kami experienced. Without treatment to end the pregnancy, the embryo will eventually grow so large that the tube ruptures, causing massive bleeding that can kill the mother.
Most state laws regarding abortion exclude treatment of ectopic pregnancy, according to Ms. Nash. But, “if the state does not exclude ectopic pregnancy from all the regulations, then people might not be able to get the care that they need when they need it.”
The current abortion law in Texas, for example, prohibits ending a pregnancy after 6 weeks, or after cardiac activity becomes present. Cardiac activity can be present in cases of ectopic pregnancies, which account for between 1% and 2% of all pregnancies and are the leading cause of maternal deaths in the first trimester. And treatment definitely ends the life of the embryo or fetus in the fallopian tube, said Lisa Harris, MD, PhD, an ob.gyn. and medical ethicist at the University of Michigan, Ann Arbor.
Dr. Harris said she has never doubted that an ectopic pregnancy cannot possibly result in a live birth. But she recalled an encounter with another clinician on a surgical team for an ectopic pregnancy who said: “So you’re going to take it out of the tube and put it in the uterus, right?”
“It was a startling moment,” Dr. Harris recalled. She regarded the procedure as a “lifesaving, obvious surgery,” but her colleague, whose suggestion was a medical impossibility since the window of implantation is very brief after fertilization, viewed it “as an abortion, as killing an embryo or fetus.”
Dr. Harris said she isn’t concerned that physicians would stop treating ectopic pregnancies in a post-Roe world. Rather, she worries about two other possibilities: an overzealous prosecutor might not believe it was an ectopic pregnancy and press charges; or laws will cause physicians to second guess their clinical decisions for patients.
“What it means, in the middle of the night, when someone comes in with a 10-week ectopic pregnancy with a heartbeat, is the doctor may hesitate,” Dr. Harris said. Despite knowing the appropriate treatment, the doctor may want to speak with a lawyer or ethicist first to ensure they are covered legally. “And as that process unfolds, which could take hours or days, the person might have a complication.” s
Not treating an ectopic pregnancy would be malpractice, but “some doctors may not provide the standard of care that they would have ordinarily provided because they don’t want to risk breaking the law,” she said.
Even more ambiguous are cornual ectopic pregnancies, in which the implantation occurs at the junction of a fallopian tube and the uterus. These pregnancies, which make up 2%-4% of all tubal pregnancies, are immediately adjacent to the uterus. If an abortion is defined as the termination of an embryo or fetus in the womb, how such a legal definition would apply to these pregnancies is unclear.
An ob.gyn. wouldn’t regard ending an ectopic pregnancy as an abortion, but “this is not about logic or clinical meaning,” Dr. Harris said. “This is people outside of medicine making determinations that all pregnancies must continue, and when you think of a ban that way, you could see why a doctor would be frightened to end a pregnancy, whether it might be viable in the future or not.”
That’s true even if the pregnancy is located fully in the uterus. Dr. Harris described a pregnant patient she saw who had traveled from Texas to Michigan with a fetus that had a lethal defect.
The fetus had “an anomaly where the lungs couldn’t develop, where there were no kidneys. There was no chance this baby could be born and live. Her doctors were very clear that there will never be a baby that [she could] take home at the end of this pregnancy, yet they would not end her pregnancy because that would be an abortion,” Dr. Harris said.
Texas law “doesn’t make any allowances for whether a pregnancy will ever actually result in a baby or not,” Dr. Harris said. “The law, in effect, just says all pregnancies must continue.”
Selective reduction
How abortion laws in different states might affect selective reduction, which is used in some pregnancies to reduce the total number of fetuses a person is carrying, is even more ambiguous. The goal of selective reduction is to decrease health risks to the pregnant individual and increase the likelihood of survival for the remaining fetuses. Current Texas law prohibits these procedures.
Someone pregnant with quintuplets, for example, might seek selection reduction to reduce the pregnancy outcome to triplets or twins. A related procedure, selective termination, is used to terminate the life of a fetus with abnormalities while the pregnancy of the fetus’ in utero siblings continues.
The advent of assisted reproduction methods, such as in vitro fertilization (IVF), greatly increased the incidence of higher-order multiples, those with three or more fetuses. The first IVF baby was born in 1978. By 1998, the rate of higher-order multiple births was 1.9 per 1,000 births, five times the figure in 1980. The rate has since decreased by nearly half, to 1 per 1,000 births, but with 3.75 million live births a year, that’s still a lot of pregnancies with higher-order multiples.
The American College of Obstetricians and Gynecologists does not provide explicit guidance on when selective reduction is warranted, but its committee opinion on multifetal pregnancy reduction provides an ethical framework for providers to use when counseling people with pregnancies of three or more fetuses. How would various state laws that outlaw abortion affect these decisions? No one knows.
“Selective reduction ends the life of a fetus or embryo, but it doesn’t end the pregnancy,” Dr. Harris said. “So, if the pregnancy continues but it kills an embryo or fetus, is that an abortion?”
‘The question of the hour’
Dr. Harris and other doctors are haunted by potential medical cases in which continuing a pregnancy may result in the death of the person carrying the fetus but in which such death may not be so imminent that the law would allow immediate termination of the pregnancy.
Michael Northrup, MD, an intensive care pediatrician in Winston-Salem, N.C., recalled a particularly harrowing case that illustrates the peril in deciding when someone’s life is “enough” in danger to qualify as an exception to abortion bans.
The 14-year-old girl had severe lupus and kidney failure that required treatment with methotrexate and immediate dialysis to replace her electrolytes. A standard pretreatment pregnancy test revealed that she had been carrying a child for at least 10 weeks. Her pregnancy presented two problems. Methotrexate is so severely toxic that it’s sometimes used to end pregnancies. Even at low doses, fetuses that survive usually have severe deformities. In addition, dialysis requires administration of a blood thinner. If the teen miscarried while taking a blood thinner during dialysis, she risked bleeding to death.
Treatment could be delayed until week 24 of pregnancy, at which time delivery could be attempted, but the patient likely wouldn’t have any kidney function left by then. In addition, at 24 weeks, it was unlikely that the baby would survive anyway.
Dr. Northrup said that, had she chosen that route, “I’m not sure she would have made it. This was a religious family, people who very much were believers. They had their head of church come in, who fairly quickly determined that the best thing for her health was to terminate this pregnancy immediately and get the treatment she needed for her body.”
Would such a situation qualify for an emergency termination? The girl wasn’t going to die within 24 or 48 hours, but it may not have been possible to pinpoint the time of death within a day or 2.
“The family was sad, but they made that choice, and I wonder, would we have to justify that with these new laws?” Dr. Northrup said. “You definitely worry, being in the hot seat, ‘Does this count enough? Is she close enough to death?’ ”
The same question comes up when someone’s water breaks early in the second trimester. Since a live-birth delivery would be highly unlikely, given the age of the fetus, the standard of care is to offer to terminate the pregnancy to avoid a serious infection, Dr. Harris said. But if the infection hasn’t yet developed – even if it’s likely to develop soon – doctors in a state that outlaws abortion would not be able to offer termination. But as providers wait for an infection to develop, the person’s risk of dying from infection rapidly increases.
“How likely does someone need to be to die for it to count to get a life-preserving abortion?” Dr. Harris asked. “That, I think, is the question of the hour.”
Different institutions may decide to determine their own risk thresholds. One hospital, for example, may decide that any health threat that is associated with a 10% risk of death qualifies for a lifesaving abortion. But for many people, a 1 in 10 chance of dying is quite high.
“Who gets to decide what’s meaningful?” Dr. Harris asked, especially if the patient is already a parent of living children and doesn’t want to take any risk at all of orphaning them for a pregnancy with severe complications.
“The point is that this is way more complicated than anybody really knows, way more complicated than any legislator or justice could possibly know, and it creates all kinds of complicated ambiguities, some of which could result in harm to women,” she said. “I’ve been a doctor almost 30 years, and every week, sometimes every day, I’m humbled by how complicated pregnancy is and how complicated people’s bodies and life situations are.”
That’s what makes it so dangerous for policymakers to “insert themselves into medical practice,” Ms. Nash said. She worries about the legal ramifications of overturning Roe, such as prosecution of people who illegally undergo an abortion or of physicians who perform a procedure that a judge deems to be in violation of abortion law.
“There are already local prosecutors who have misused the law to go after people who have managed their own abortions,” Ms. Nash said. “Criminal abortion law, fetal homicide, child neglect, practicing medicine without a license – these are things people have actually been arrested and convicted under.”
Some laws may target the person seeking an abortion, whereas others may target clinicians providing abortions, or even people who simply help someone obtain an abortion, as the Texas law does. In Dr. Harris’s own state of Michigan, a group of Republican lawmakers recently introduced a bill that would imprison abortion providers for up to 10 years and anyone creating or distributing abortion medication for up to 20 years.
Michigan Gov. Gretchen Whitmer, a Democrat who called the proposed legislation “disturbing” and “infuriating,” would almost certainly veto the bill, but it’s just one of dozens already filed or that are expected to be filed across the United States.
The antiabortion organization National Right to Life has published a “post-Roe model abortion law” for states to adopt. The model includes an exemption for abortions that, “based on reasonable medical judgment, [were] necessary to prevent the death of the pregnant woman” – but, again, it does not clarify what that means in practice.
Lectures from strangers
Four years after nearly dying, Kami gave birth to a healthy girl following an uncomplicated pregnancy. But her journey to having more children presented more challenges.
Two years after the birth of her child, she had another ectopic pregnancy. Her doctor sent her prescriptions for medication that would end this pregnancy, but a pharmacist refused to fill the prescription.
“Do you know these are very serious medications?” the pharmacist asked her. She did – she had taken them once before for another ectopic pregnancy. She was with her daughter, feeling devastated about losing yet another desired pregnancy. She simply wanted to get the medication and go home.
“‘So you’re trying to have a cheap abortion,’ he said, and 30 heads turned and looked at me. The whole pharmacy heard,” Kami said.
She told the pharmacist that she’d miscarried. She said he responded with: “So you have a dead baby in your body.”
Even after her doctor called to insist on filling the order, the man refused to fill it.
Kami left without the prescription, and her doctor performed a surgical dilation and curettage to remove the embryo from her fallopian tube for no fee.
Kami later tried again to have more children. She experienced another ruptured tube that she said nearly killed her.
“There was such a sense of pain knowing that I couldn’t have any more kids, but also the relief of knowing that I don’t have to go through this again,” Kami said. Now, however, with the Supreme Court having overturned Roe v. Wade, she has a new worry: “That my daughter will not have the same rights and access to health care that I did.”
A version of this article first appeared on Medscape.com.
Kami, a mother of one daughter in central Texas, lost three pregnancies in 2008. The third one nearly killed her.
The embryo became implanted in one of the fallopian tubes connecting her ovaries to her uterus. Because fallopian tubes can’t stretch to accommodate a fetus, patients must undergo surgery to remove the embryo before the tube ruptures. Failure to do so can result in internal bleeding and death.
But when Kami – who did not want to use her last name to avoid harassment – underwent an ultrasound to start the process of extracting the embryo, her doctor miscalculated how far along in the pregnancy she was and told her to come back in a few weeks.
She eventually did return, but only after passing out in the bathtub and waking up in a pool of her own blood. The tube had ruptured, and to remove it, emergency surgery was necessary.
Stories such as Kami’s could become more common in the aftermath of the U.S. Supreme Court’s decision to overturn Roe v. Wade, the 1973 case that created a right to an abortion.
Experts fear that antiabortion laws that take effect in the United States following the court’s decision will lead to a medical and legal limbo for thousands of people like Kami – people with uncommon reproductive conditions whose treatments involve the termination of pregnancies or the destruction of embryos.
Vague exceptions prompt concerns
According to the Guttmacher Institute, a nonprofit group for reproductive health, 13 states currently have trigger laws on the books that make abortion illegal in the absence of Roe. Nine other states have laws that would outlaw or severely restrict abortion without a federal right to the procedure.
Each of these laws carves out exceptions that allow the termination of a pregnancy to prevent the death of the pregnant individual. But the language of the provisions is not always precise in describing what those exceptions mean in practice, according to Elizabeth Nash, the principal policy associate for state issues at the Guttmacher Institute.
“These exceptions are designed to be extraordinarily narrow. These aren’t really designed to be usable exceptions,” Ms. Nash said. “There’s so much misinformation about abortion that there are probably legislators out there who think that it’s never needed to save a life.”
Tubal pregnancies
One of the best examples of a pregnancy termination that’s necessary to avoid death is in the case of an ectopic pregnancy such as Kami experienced. Without treatment to end the pregnancy, the embryo will eventually grow so large that the tube ruptures, causing massive bleeding that can kill the mother.
Most state laws regarding abortion exclude treatment of ectopic pregnancy, according to Ms. Nash. But, “if the state does not exclude ectopic pregnancy from all the regulations, then people might not be able to get the care that they need when they need it.”
The current abortion law in Texas, for example, prohibits ending a pregnancy after 6 weeks, or after cardiac activity becomes present. Cardiac activity can be present in cases of ectopic pregnancies, which account for between 1% and 2% of all pregnancies and are the leading cause of maternal deaths in the first trimester. And treatment definitely ends the life of the embryo or fetus in the fallopian tube, said Lisa Harris, MD, PhD, an ob.gyn. and medical ethicist at the University of Michigan, Ann Arbor.
Dr. Harris said she has never doubted that an ectopic pregnancy cannot possibly result in a live birth. But she recalled an encounter with another clinician on a surgical team for an ectopic pregnancy who said: “So you’re going to take it out of the tube and put it in the uterus, right?”
“It was a startling moment,” Dr. Harris recalled. She regarded the procedure as a “lifesaving, obvious surgery,” but her colleague, whose suggestion was a medical impossibility since the window of implantation is very brief after fertilization, viewed it “as an abortion, as killing an embryo or fetus.”
Dr. Harris said she isn’t concerned that physicians would stop treating ectopic pregnancies in a post-Roe world. Rather, she worries about two other possibilities: an overzealous prosecutor might not believe it was an ectopic pregnancy and press charges; or laws will cause physicians to second guess their clinical decisions for patients.
“What it means, in the middle of the night, when someone comes in with a 10-week ectopic pregnancy with a heartbeat, is the doctor may hesitate,” Dr. Harris said. Despite knowing the appropriate treatment, the doctor may want to speak with a lawyer or ethicist first to ensure they are covered legally. “And as that process unfolds, which could take hours or days, the person might have a complication.” s
Not treating an ectopic pregnancy would be malpractice, but “some doctors may not provide the standard of care that they would have ordinarily provided because they don’t want to risk breaking the law,” she said.
Even more ambiguous are cornual ectopic pregnancies, in which the implantation occurs at the junction of a fallopian tube and the uterus. These pregnancies, which make up 2%-4% of all tubal pregnancies, are immediately adjacent to the uterus. If an abortion is defined as the termination of an embryo or fetus in the womb, how such a legal definition would apply to these pregnancies is unclear.
An ob.gyn. wouldn’t regard ending an ectopic pregnancy as an abortion, but “this is not about logic or clinical meaning,” Dr. Harris said. “This is people outside of medicine making determinations that all pregnancies must continue, and when you think of a ban that way, you could see why a doctor would be frightened to end a pregnancy, whether it might be viable in the future or not.”
That’s true even if the pregnancy is located fully in the uterus. Dr. Harris described a pregnant patient she saw who had traveled from Texas to Michigan with a fetus that had a lethal defect.
The fetus had “an anomaly where the lungs couldn’t develop, where there were no kidneys. There was no chance this baby could be born and live. Her doctors were very clear that there will never be a baby that [she could] take home at the end of this pregnancy, yet they would not end her pregnancy because that would be an abortion,” Dr. Harris said.
Texas law “doesn’t make any allowances for whether a pregnancy will ever actually result in a baby or not,” Dr. Harris said. “The law, in effect, just says all pregnancies must continue.”
Selective reduction
How abortion laws in different states might affect selective reduction, which is used in some pregnancies to reduce the total number of fetuses a person is carrying, is even more ambiguous. The goal of selective reduction is to decrease health risks to the pregnant individual and increase the likelihood of survival for the remaining fetuses. Current Texas law prohibits these procedures.
Someone pregnant with quintuplets, for example, might seek selection reduction to reduce the pregnancy outcome to triplets or twins. A related procedure, selective termination, is used to terminate the life of a fetus with abnormalities while the pregnancy of the fetus’ in utero siblings continues.
The advent of assisted reproduction methods, such as in vitro fertilization (IVF), greatly increased the incidence of higher-order multiples, those with three or more fetuses. The first IVF baby was born in 1978. By 1998, the rate of higher-order multiple births was 1.9 per 1,000 births, five times the figure in 1980. The rate has since decreased by nearly half, to 1 per 1,000 births, but with 3.75 million live births a year, that’s still a lot of pregnancies with higher-order multiples.
The American College of Obstetricians and Gynecologists does not provide explicit guidance on when selective reduction is warranted, but its committee opinion on multifetal pregnancy reduction provides an ethical framework for providers to use when counseling people with pregnancies of three or more fetuses. How would various state laws that outlaw abortion affect these decisions? No one knows.
“Selective reduction ends the life of a fetus or embryo, but it doesn’t end the pregnancy,” Dr. Harris said. “So, if the pregnancy continues but it kills an embryo or fetus, is that an abortion?”
‘The question of the hour’
Dr. Harris and other doctors are haunted by potential medical cases in which continuing a pregnancy may result in the death of the person carrying the fetus but in which such death may not be so imminent that the law would allow immediate termination of the pregnancy.
Michael Northrup, MD, an intensive care pediatrician in Winston-Salem, N.C., recalled a particularly harrowing case that illustrates the peril in deciding when someone’s life is “enough” in danger to qualify as an exception to abortion bans.
The 14-year-old girl had severe lupus and kidney failure that required treatment with methotrexate and immediate dialysis to replace her electrolytes. A standard pretreatment pregnancy test revealed that she had been carrying a child for at least 10 weeks. Her pregnancy presented two problems. Methotrexate is so severely toxic that it’s sometimes used to end pregnancies. Even at low doses, fetuses that survive usually have severe deformities. In addition, dialysis requires administration of a blood thinner. If the teen miscarried while taking a blood thinner during dialysis, she risked bleeding to death.
Treatment could be delayed until week 24 of pregnancy, at which time delivery could be attempted, but the patient likely wouldn’t have any kidney function left by then. In addition, at 24 weeks, it was unlikely that the baby would survive anyway.
Dr. Northrup said that, had she chosen that route, “I’m not sure she would have made it. This was a religious family, people who very much were believers. They had their head of church come in, who fairly quickly determined that the best thing for her health was to terminate this pregnancy immediately and get the treatment she needed for her body.”
Would such a situation qualify for an emergency termination? The girl wasn’t going to die within 24 or 48 hours, but it may not have been possible to pinpoint the time of death within a day or 2.
“The family was sad, but they made that choice, and I wonder, would we have to justify that with these new laws?” Dr. Northrup said. “You definitely worry, being in the hot seat, ‘Does this count enough? Is she close enough to death?’ ”
The same question comes up when someone’s water breaks early in the second trimester. Since a live-birth delivery would be highly unlikely, given the age of the fetus, the standard of care is to offer to terminate the pregnancy to avoid a serious infection, Dr. Harris said. But if the infection hasn’t yet developed – even if it’s likely to develop soon – doctors in a state that outlaws abortion would not be able to offer termination. But as providers wait for an infection to develop, the person’s risk of dying from infection rapidly increases.
“How likely does someone need to be to die for it to count to get a life-preserving abortion?” Dr. Harris asked. “That, I think, is the question of the hour.”
Different institutions may decide to determine their own risk thresholds. One hospital, for example, may decide that any health threat that is associated with a 10% risk of death qualifies for a lifesaving abortion. But for many people, a 1 in 10 chance of dying is quite high.
“Who gets to decide what’s meaningful?” Dr. Harris asked, especially if the patient is already a parent of living children and doesn’t want to take any risk at all of orphaning them for a pregnancy with severe complications.
“The point is that this is way more complicated than anybody really knows, way more complicated than any legislator or justice could possibly know, and it creates all kinds of complicated ambiguities, some of which could result in harm to women,” she said. “I’ve been a doctor almost 30 years, and every week, sometimes every day, I’m humbled by how complicated pregnancy is and how complicated people’s bodies and life situations are.”
That’s what makes it so dangerous for policymakers to “insert themselves into medical practice,” Ms. Nash said. She worries about the legal ramifications of overturning Roe, such as prosecution of people who illegally undergo an abortion or of physicians who perform a procedure that a judge deems to be in violation of abortion law.
“There are already local prosecutors who have misused the law to go after people who have managed their own abortions,” Ms. Nash said. “Criminal abortion law, fetal homicide, child neglect, practicing medicine without a license – these are things people have actually been arrested and convicted under.”
Some laws may target the person seeking an abortion, whereas others may target clinicians providing abortions, or even people who simply help someone obtain an abortion, as the Texas law does. In Dr. Harris’s own state of Michigan, a group of Republican lawmakers recently introduced a bill that would imprison abortion providers for up to 10 years and anyone creating or distributing abortion medication for up to 20 years.
Michigan Gov. Gretchen Whitmer, a Democrat who called the proposed legislation “disturbing” and “infuriating,” would almost certainly veto the bill, but it’s just one of dozens already filed or that are expected to be filed across the United States.
The antiabortion organization National Right to Life has published a “post-Roe model abortion law” for states to adopt. The model includes an exemption for abortions that, “based on reasonable medical judgment, [were] necessary to prevent the death of the pregnant woman” – but, again, it does not clarify what that means in practice.
Lectures from strangers
Four years after nearly dying, Kami gave birth to a healthy girl following an uncomplicated pregnancy. But her journey to having more children presented more challenges.
Two years after the birth of her child, she had another ectopic pregnancy. Her doctor sent her prescriptions for medication that would end this pregnancy, but a pharmacist refused to fill the prescription.
“Do you know these are very serious medications?” the pharmacist asked her. She did – she had taken them once before for another ectopic pregnancy. She was with her daughter, feeling devastated about losing yet another desired pregnancy. She simply wanted to get the medication and go home.
“‘So you’re trying to have a cheap abortion,’ he said, and 30 heads turned and looked at me. The whole pharmacy heard,” Kami said.
She told the pharmacist that she’d miscarried. She said he responded with: “So you have a dead baby in your body.”
Even after her doctor called to insist on filling the order, the man refused to fill it.
Kami left without the prescription, and her doctor performed a surgical dilation and curettage to remove the embryo from her fallopian tube for no fee.
Kami later tried again to have more children. She experienced another ruptured tube that she said nearly killed her.
“There was such a sense of pain knowing that I couldn’t have any more kids, but also the relief of knowing that I don’t have to go through this again,” Kami said. Now, however, with the Supreme Court having overturned Roe v. Wade, she has a new worry: “That my daughter will not have the same rights and access to health care that I did.”
A version of this article first appeared on Medscape.com.
Antibiotics during pregnancy may increase child’s risk for asthma and other atopic diseases
, a systematic review and meta-analysis reports.
“Antibiotic use during pregnancy is significantly associated with the development of asthma in children. Additionally prenatal antibiotic exposure is also associated with disorders present in the atopic march including atopic sensitization, dermatitis/eczema, food allergy, allergic rhinitis, and wheeze,” lead study author Alissa Cait, PhD, of Malaghan Institute of Medical Research in Wellington, New Zealand, and colleagues write in Allergy.
“Antibiotics account for 80% of prescribed medications during pregnancy, and it is estimated that 20%-25% of pregnant women receive at least one course of an antibiotic during this time period,” they add.
The researchers evaluated prenatal antibiotic exposure and the risk for childhood wheeze or asthma, as well as for diseases associated with the atopic march, by searching standard medical databases for controlled trials in English, German, French, Dutch, or Arabic involving the use of any antibiotic at any time during pregnancy and for atopic disease incidence in children with asthma or wheeze as primary outcome. They excluded reviews, preclinical data, and descriptive studies.
From the 6,060 citations the search returned, 11 prospective and 16 retrospective studies met the authors’ selection criteria. For each study, they evaluated risk of bias using the Newcastle-Ottawa Quality Assessment Scale, and they rated certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) protocol.
The studies, published between 2002 and 2020, were conducted in Europe, North America, Asia, and South America. Exposure to antibiotics during the prenatal period was assessed through unsupervised questionnaires, interviews by medical professionals, or extraction from official medical databases.
The results showed that:
- Antibiotic use during pregnancy was linked with increased relative risk of developing wheeze (relative risk, 1.51; 95% confidence interval, 1.17-1.94) or asthma (RR, 1.28; 95% CI, 1.22-1.34) during childhood.
- Antibiotic use during pregnancy also increased a child’s risk for eczema or dermatitis (RR, 1.28; 95% CI, 1.06-1.53) and allergic rhinitis (RR, 1.13; 95% CI, 1.02-1.25).
- Food allergy increased in one study (RR, 1.81; 95% CI, 1.11-2.95).
Quality of studies
“These results have importance for antibiotic stewardship throughout the prenatal period,” the authors write. However, due to issues including high heterogeneity, publication bias, and lack of population numbers in some studies, the overall quality of the evidence presented in the studies was low. Other limitations include mainly White and European study populations, underpowered studies, and study protocol inconsistencies.
“Though there is evidence that antibiotic treatment during pregnancy is a driver of the atopic march, due to a large heterogeneity between studies more research is needed to draw firm conclusions on this matter,” the authors add. “Future studies should employ and report more direct and objective measurement methods rather than self-reported questionnaires.”
Dustin D. Flannery, DO, MSCE, a neonatologist and clinical researcher in perinatal infectious diseases and neonatal antimicrobial resistance and stewardship at Children’s Hospital of Philadelphia, said in an email that the study was well done.
He noted, though, that “although the study reports an association, it cannot prove causation. The relationship between prenatal antibiotics and childhood allergic disorders is likely multifactorial and quite complex.”
He joins the authors in recommending further related research. “Due to the variation in how exposures and outcomes were defined across the studies, more rigorous research will be needed in this area.”
Despite the study’s limitations, “given that some studies have found associations between prenatal antibiotic exposure and childhood atopic and allergic disorders, including asthma, while other studies have not, this systematic review and meta-analysis asks an important question,” Dr. Flannery, who was not involved in the study, said in an interview.
“Investigators found a strong association between prenatal antibiotic exposure and risk of childhood asthma and other disorders,” he said. “This finding supports efforts to safely reduce antibiotic use during pregnancy.”
The study was supported by the Deutsche Forschungsgemeinschaft and by the Konrad Adenauer Foundation. The authors and Dr. Flannery have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a systematic review and meta-analysis reports.
“Antibiotic use during pregnancy is significantly associated with the development of asthma in children. Additionally prenatal antibiotic exposure is also associated with disorders present in the atopic march including atopic sensitization, dermatitis/eczema, food allergy, allergic rhinitis, and wheeze,” lead study author Alissa Cait, PhD, of Malaghan Institute of Medical Research in Wellington, New Zealand, and colleagues write in Allergy.
“Antibiotics account for 80% of prescribed medications during pregnancy, and it is estimated that 20%-25% of pregnant women receive at least one course of an antibiotic during this time period,” they add.
The researchers evaluated prenatal antibiotic exposure and the risk for childhood wheeze or asthma, as well as for diseases associated with the atopic march, by searching standard medical databases for controlled trials in English, German, French, Dutch, or Arabic involving the use of any antibiotic at any time during pregnancy and for atopic disease incidence in children with asthma or wheeze as primary outcome. They excluded reviews, preclinical data, and descriptive studies.
From the 6,060 citations the search returned, 11 prospective and 16 retrospective studies met the authors’ selection criteria. For each study, they evaluated risk of bias using the Newcastle-Ottawa Quality Assessment Scale, and they rated certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) protocol.
The studies, published between 2002 and 2020, were conducted in Europe, North America, Asia, and South America. Exposure to antibiotics during the prenatal period was assessed through unsupervised questionnaires, interviews by medical professionals, or extraction from official medical databases.
The results showed that:
- Antibiotic use during pregnancy was linked with increased relative risk of developing wheeze (relative risk, 1.51; 95% confidence interval, 1.17-1.94) or asthma (RR, 1.28; 95% CI, 1.22-1.34) during childhood.
- Antibiotic use during pregnancy also increased a child’s risk for eczema or dermatitis (RR, 1.28; 95% CI, 1.06-1.53) and allergic rhinitis (RR, 1.13; 95% CI, 1.02-1.25).
- Food allergy increased in one study (RR, 1.81; 95% CI, 1.11-2.95).
Quality of studies
“These results have importance for antibiotic stewardship throughout the prenatal period,” the authors write. However, due to issues including high heterogeneity, publication bias, and lack of population numbers in some studies, the overall quality of the evidence presented in the studies was low. Other limitations include mainly White and European study populations, underpowered studies, and study protocol inconsistencies.
“Though there is evidence that antibiotic treatment during pregnancy is a driver of the atopic march, due to a large heterogeneity between studies more research is needed to draw firm conclusions on this matter,” the authors add. “Future studies should employ and report more direct and objective measurement methods rather than self-reported questionnaires.”
Dustin D. Flannery, DO, MSCE, a neonatologist and clinical researcher in perinatal infectious diseases and neonatal antimicrobial resistance and stewardship at Children’s Hospital of Philadelphia, said in an email that the study was well done.
He noted, though, that “although the study reports an association, it cannot prove causation. The relationship between prenatal antibiotics and childhood allergic disorders is likely multifactorial and quite complex.”
He joins the authors in recommending further related research. “Due to the variation in how exposures and outcomes were defined across the studies, more rigorous research will be needed in this area.”
Despite the study’s limitations, “given that some studies have found associations between prenatal antibiotic exposure and childhood atopic and allergic disorders, including asthma, while other studies have not, this systematic review and meta-analysis asks an important question,” Dr. Flannery, who was not involved in the study, said in an interview.
“Investigators found a strong association between prenatal antibiotic exposure and risk of childhood asthma and other disorders,” he said. “This finding supports efforts to safely reduce antibiotic use during pregnancy.”
The study was supported by the Deutsche Forschungsgemeinschaft and by the Konrad Adenauer Foundation. The authors and Dr. Flannery have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a systematic review and meta-analysis reports.
“Antibiotic use during pregnancy is significantly associated with the development of asthma in children. Additionally prenatal antibiotic exposure is also associated with disorders present in the atopic march including atopic sensitization, dermatitis/eczema, food allergy, allergic rhinitis, and wheeze,” lead study author Alissa Cait, PhD, of Malaghan Institute of Medical Research in Wellington, New Zealand, and colleagues write in Allergy.
“Antibiotics account for 80% of prescribed medications during pregnancy, and it is estimated that 20%-25% of pregnant women receive at least one course of an antibiotic during this time period,” they add.
The researchers evaluated prenatal antibiotic exposure and the risk for childhood wheeze or asthma, as well as for diseases associated with the atopic march, by searching standard medical databases for controlled trials in English, German, French, Dutch, or Arabic involving the use of any antibiotic at any time during pregnancy and for atopic disease incidence in children with asthma or wheeze as primary outcome. They excluded reviews, preclinical data, and descriptive studies.
From the 6,060 citations the search returned, 11 prospective and 16 retrospective studies met the authors’ selection criteria. For each study, they evaluated risk of bias using the Newcastle-Ottawa Quality Assessment Scale, and they rated certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) protocol.
The studies, published between 2002 and 2020, were conducted in Europe, North America, Asia, and South America. Exposure to antibiotics during the prenatal period was assessed through unsupervised questionnaires, interviews by medical professionals, or extraction from official medical databases.
The results showed that:
- Antibiotic use during pregnancy was linked with increased relative risk of developing wheeze (relative risk, 1.51; 95% confidence interval, 1.17-1.94) or asthma (RR, 1.28; 95% CI, 1.22-1.34) during childhood.
- Antibiotic use during pregnancy also increased a child’s risk for eczema or dermatitis (RR, 1.28; 95% CI, 1.06-1.53) and allergic rhinitis (RR, 1.13; 95% CI, 1.02-1.25).
- Food allergy increased in one study (RR, 1.81; 95% CI, 1.11-2.95).
Quality of studies
“These results have importance for antibiotic stewardship throughout the prenatal period,” the authors write. However, due to issues including high heterogeneity, publication bias, and lack of population numbers in some studies, the overall quality of the evidence presented in the studies was low. Other limitations include mainly White and European study populations, underpowered studies, and study protocol inconsistencies.
“Though there is evidence that antibiotic treatment during pregnancy is a driver of the atopic march, due to a large heterogeneity between studies more research is needed to draw firm conclusions on this matter,” the authors add. “Future studies should employ and report more direct and objective measurement methods rather than self-reported questionnaires.”
Dustin D. Flannery, DO, MSCE, a neonatologist and clinical researcher in perinatal infectious diseases and neonatal antimicrobial resistance and stewardship at Children’s Hospital of Philadelphia, said in an email that the study was well done.
He noted, though, that “although the study reports an association, it cannot prove causation. The relationship between prenatal antibiotics and childhood allergic disorders is likely multifactorial and quite complex.”
He joins the authors in recommending further related research. “Due to the variation in how exposures and outcomes were defined across the studies, more rigorous research will be needed in this area.”
Despite the study’s limitations, “given that some studies have found associations between prenatal antibiotic exposure and childhood atopic and allergic disorders, including asthma, while other studies have not, this systematic review and meta-analysis asks an important question,” Dr. Flannery, who was not involved in the study, said in an interview.
“Investigators found a strong association between prenatal antibiotic exposure and risk of childhood asthma and other disorders,” he said. “This finding supports efforts to safely reduce antibiotic use during pregnancy.”
The study was supported by the Deutsche Forschungsgemeinschaft and by the Konrad Adenauer Foundation. The authors and Dr. Flannery have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALLERGY
Roe v. Wade overturned, ending 50 years of abortion protections
According to some estimates, about 25 million women of reproductive age will now live in states that ban or severely restrict abortion. Twenty-six states are “certain or likely” to ban abortion, according to the Guttmacher Institute, which supports abortion rights.
Thirteen states have so-called trigger laws that will ban abortion almost immediately, while nine other states are now likely to try to enforce near-total bans or severe restrictions that have been blocked by courts pending the outcome of the just-issued decision in Dobbs v. Jackson Women’s Health Organization. Four states also have a history or have shown a recent desire to prohibit abortion, according to the Guttmacher Institute.
Doctors and others who provide abortion services, or in some states “aid or abet” an abortion, could be fined thousands of dollars or sent to prison.
The court voted in favor of Mississippi and its 2018 law that outlawed abortion after 15 weeks. Jackson Women’s Health, the state’s sole remaining abortion provider, sued to block the law soon after it passed.
The Supreme Court decision is not a surprise, as the justices indicated they were leaning that way during oral arguments in December. The majority’s thoughts were further revealed when a draft of the opinion was leaked to the news outlet Politico on May 2.
In the final opinion, Justice Samuel Alito, writing for the majority, “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
The decision strikes down both precedent-setting rulings that established a right to abortion until the point of viability, long considered to be 24 weeks: Roe v. Wade (1973) and Planned Parenthood v. Casey (1992).
Twenty-five medical professional societies – representing OB/GYNs, family medicine doctors, fertility specialists, geneticists, hospitalists, internists, pediatricians, psychiatrists, nurses, nurse practitioners, and midwives – had urged the court to throw out the Mississippi law. And more than 2,500 medical professionals signed on to a petition in June, urging the court to uphold the right to abortion.
The number of abortions has recently increased from what had been a long decline. The Guttmacher Institute estimates there were there were 930,160 abortion procedures in 2020 (compared to 3.6 million births), an 8% increase from 2017. The number does not include self-managed abortions. The organization said the increase was potentially due to expanded Medicaid coverage and reduced access to contraception due to Trump administration policies.
Trigger laws and bans
When trigger laws and new restrictions go into effect, women in the South, Midwest, and Inter-Mountain West will likely have to drive hundreds of miles for an abortion, according to Guttmacher. Women in Louisiana, for instance, would have to drive 660 miles to get to the nearest provider in Illinois.
University of Utah researchers estimated that almost half of women will see a big increase in the distance to abortion care, from a median distance of 39 miles to 113 miles. State bans will disproportionately impact women of color, those living in poverty, and people with less education, they said.
The CDC has reported that Black women are three times more likely to die from a pregnancy-related cause than white women.
Doctors and other abortion providers could face serious penalties. The maximum penalty in Texas is life in prison, and the sentence could be 10 to 15 years in 11 other states, according to an article in the medical journal JAMA by attorneys Rebecca B. Reingold and Lawrence O. Gostin.
“Threats of prosecution undermine clinicians’ ability to provide safe, evidence-based care and to counsel patients honestly, impeding the patient-physician relationship,” they wrote. “Given harsh penalties, physicians may cease treating pregnancy loss, with no clear line between treating miscarriages and abortions.”
In preparing for these attacks on patients and doctors, New York Gov. Kathy Hochul on June 13 signed a bill that immediately protects anyone who has an abortion and medical professionals in the state who provide them from legal retaliation by states that restrict or prohibit abortion.
Even while Roe was still the law, Mississippi had banned most abortions after 20 weeks, and 16 states prohibited abortion after 22 weeks. A Texas ban on abortion after 6 weeks – which also allows private citizens to sue abortion providers – was allowed to stay in place while it was being challenged.
On May 26, Oklahoma Gov. Kevin Stitt signed a bill banning abortion from the moment of conception. Just as in Texas, the Oklahoma law allows what critics have called “bounty hunting” of abortion providers.
Four states have a constitutional amendment declaring that the state constitution does not secure or protect the right to abortion or allow the use of public funds for abortion: Alabama, Louisiana, Tennessee, and West Virginia.
Some states protecting rights
At least 16 states have proactively protected a right to an abortion, according to Guttmacher, while The New York Times reports that Washington, DC, has laws that protect abortion, along with 20 states: Alaska, Colorado, Illinois, Maine, Massachusetts, Minnesota, Nevada, New Hampshire, New Mexico, Rhode Island, California, Connecticut, Delaware, Hawaii, Maryland, New Jersey, New York, Oregon, Vermont, and Washington.
Some of these states are gearing up for a potential influx of patients. Washington Gov. Jay Inslee signed a law that authorizes physician assistants, advanced registered nurse practitioners, and other providers acting within their scope of practice to perform abortions. And the Maryland Legislature overrode a veto by Gov. Larry Hogan of a law that expands who can perform abortions.
Wisconsin Gov. Tony Evers in early June called a special legislative session to repeal the state’s 173-year-old dormant ban on abortion. But the majority Republican legislature vowed to take no action.
B. Jessie Hill, JD, associate dean for academic affairs and a professor at the Case Western Reserve University School of Law, says she expects anti-abortion groups to challenge these protective laws, “by saying that fetuses are persons under the Constitution with a right to life and therefore that the state has to protect them.”
But, she says, “there’s going to be big, big challenges with those lawsuits,” and they will not be “winners off the bat.”
Medication abortions, travel next battle
Some states are also trying to outlaw or severely restrict the use of RU-486, the abortion pill. A Tennessee law that goes into effect in 2023 would ban delivery of pills by mail and require a patient to have two doctor visits – one consultation and one to pick up the pills.
Mississippi has also enacted restrictions including the requirement that women meet with a doctor first – and is being sued by pill maker GenBioPro.
Guttmacher estimates that medication abortion accounted for 39% of all abortions in the U.S. in 2017 and 60% of all abortions that occurred before 10 weeks’ gestation.
Some states have floated the idea of prohibiting anyone from traveling to another state for an abortion.
George Mason University law professor Ilya Somin, JD, has written that such a law would likely violate the Dormant Commerce Clause, “which forbids state regulations that specifically restrict interstate commerce or discriminate against it.”
He also wrote that states lack the authority to regulate activity that takes place beyond their borders and that such bans “are open to challenge because they violate the constitutional right to travel.”
Hill also said a travel ban would be problematic, noting that it might be difficult to prosecute someone for “something you did completely in another state.”
A version of this article first appeared on Medscape.com.
According to some estimates, about 25 million women of reproductive age will now live in states that ban or severely restrict abortion. Twenty-six states are “certain or likely” to ban abortion, according to the Guttmacher Institute, which supports abortion rights.
Thirteen states have so-called trigger laws that will ban abortion almost immediately, while nine other states are now likely to try to enforce near-total bans or severe restrictions that have been blocked by courts pending the outcome of the just-issued decision in Dobbs v. Jackson Women’s Health Organization. Four states also have a history or have shown a recent desire to prohibit abortion, according to the Guttmacher Institute.
Doctors and others who provide abortion services, or in some states “aid or abet” an abortion, could be fined thousands of dollars or sent to prison.
The court voted in favor of Mississippi and its 2018 law that outlawed abortion after 15 weeks. Jackson Women’s Health, the state’s sole remaining abortion provider, sued to block the law soon after it passed.
The Supreme Court decision is not a surprise, as the justices indicated they were leaning that way during oral arguments in December. The majority’s thoughts were further revealed when a draft of the opinion was leaked to the news outlet Politico on May 2.
In the final opinion, Justice Samuel Alito, writing for the majority, “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
The decision strikes down both precedent-setting rulings that established a right to abortion until the point of viability, long considered to be 24 weeks: Roe v. Wade (1973) and Planned Parenthood v. Casey (1992).
Twenty-five medical professional societies – representing OB/GYNs, family medicine doctors, fertility specialists, geneticists, hospitalists, internists, pediatricians, psychiatrists, nurses, nurse practitioners, and midwives – had urged the court to throw out the Mississippi law. And more than 2,500 medical professionals signed on to a petition in June, urging the court to uphold the right to abortion.
The number of abortions has recently increased from what had been a long decline. The Guttmacher Institute estimates there were there were 930,160 abortion procedures in 2020 (compared to 3.6 million births), an 8% increase from 2017. The number does not include self-managed abortions. The organization said the increase was potentially due to expanded Medicaid coverage and reduced access to contraception due to Trump administration policies.
Trigger laws and bans
When trigger laws and new restrictions go into effect, women in the South, Midwest, and Inter-Mountain West will likely have to drive hundreds of miles for an abortion, according to Guttmacher. Women in Louisiana, for instance, would have to drive 660 miles to get to the nearest provider in Illinois.
University of Utah researchers estimated that almost half of women will see a big increase in the distance to abortion care, from a median distance of 39 miles to 113 miles. State bans will disproportionately impact women of color, those living in poverty, and people with less education, they said.
The CDC has reported that Black women are three times more likely to die from a pregnancy-related cause than white women.
Doctors and other abortion providers could face serious penalties. The maximum penalty in Texas is life in prison, and the sentence could be 10 to 15 years in 11 other states, according to an article in the medical journal JAMA by attorneys Rebecca B. Reingold and Lawrence O. Gostin.
“Threats of prosecution undermine clinicians’ ability to provide safe, evidence-based care and to counsel patients honestly, impeding the patient-physician relationship,” they wrote. “Given harsh penalties, physicians may cease treating pregnancy loss, with no clear line between treating miscarriages and abortions.”
In preparing for these attacks on patients and doctors, New York Gov. Kathy Hochul on June 13 signed a bill that immediately protects anyone who has an abortion and medical professionals in the state who provide them from legal retaliation by states that restrict or prohibit abortion.
Even while Roe was still the law, Mississippi had banned most abortions after 20 weeks, and 16 states prohibited abortion after 22 weeks. A Texas ban on abortion after 6 weeks – which also allows private citizens to sue abortion providers – was allowed to stay in place while it was being challenged.
On May 26, Oklahoma Gov. Kevin Stitt signed a bill banning abortion from the moment of conception. Just as in Texas, the Oklahoma law allows what critics have called “bounty hunting” of abortion providers.
Four states have a constitutional amendment declaring that the state constitution does not secure or protect the right to abortion or allow the use of public funds for abortion: Alabama, Louisiana, Tennessee, and West Virginia.
Some states protecting rights
At least 16 states have proactively protected a right to an abortion, according to Guttmacher, while The New York Times reports that Washington, DC, has laws that protect abortion, along with 20 states: Alaska, Colorado, Illinois, Maine, Massachusetts, Minnesota, Nevada, New Hampshire, New Mexico, Rhode Island, California, Connecticut, Delaware, Hawaii, Maryland, New Jersey, New York, Oregon, Vermont, and Washington.
Some of these states are gearing up for a potential influx of patients. Washington Gov. Jay Inslee signed a law that authorizes physician assistants, advanced registered nurse practitioners, and other providers acting within their scope of practice to perform abortions. And the Maryland Legislature overrode a veto by Gov. Larry Hogan of a law that expands who can perform abortions.
Wisconsin Gov. Tony Evers in early June called a special legislative session to repeal the state’s 173-year-old dormant ban on abortion. But the majority Republican legislature vowed to take no action.
B. Jessie Hill, JD, associate dean for academic affairs and a professor at the Case Western Reserve University School of Law, says she expects anti-abortion groups to challenge these protective laws, “by saying that fetuses are persons under the Constitution with a right to life and therefore that the state has to protect them.”
But, she says, “there’s going to be big, big challenges with those lawsuits,” and they will not be “winners off the bat.”
Medication abortions, travel next battle
Some states are also trying to outlaw or severely restrict the use of RU-486, the abortion pill. A Tennessee law that goes into effect in 2023 would ban delivery of pills by mail and require a patient to have two doctor visits – one consultation and one to pick up the pills.
Mississippi has also enacted restrictions including the requirement that women meet with a doctor first – and is being sued by pill maker GenBioPro.
Guttmacher estimates that medication abortion accounted for 39% of all abortions in the U.S. in 2017 and 60% of all abortions that occurred before 10 weeks’ gestation.
Some states have floated the idea of prohibiting anyone from traveling to another state for an abortion.
George Mason University law professor Ilya Somin, JD, has written that such a law would likely violate the Dormant Commerce Clause, “which forbids state regulations that specifically restrict interstate commerce or discriminate against it.”
He also wrote that states lack the authority to regulate activity that takes place beyond their borders and that such bans “are open to challenge because they violate the constitutional right to travel.”
Hill also said a travel ban would be problematic, noting that it might be difficult to prosecute someone for “something you did completely in another state.”
A version of this article first appeared on Medscape.com.
According to some estimates, about 25 million women of reproductive age will now live in states that ban or severely restrict abortion. Twenty-six states are “certain or likely” to ban abortion, according to the Guttmacher Institute, which supports abortion rights.
Thirteen states have so-called trigger laws that will ban abortion almost immediately, while nine other states are now likely to try to enforce near-total bans or severe restrictions that have been blocked by courts pending the outcome of the just-issued decision in Dobbs v. Jackson Women’s Health Organization. Four states also have a history or have shown a recent desire to prohibit abortion, according to the Guttmacher Institute.
Doctors and others who provide abortion services, or in some states “aid or abet” an abortion, could be fined thousands of dollars or sent to prison.
The court voted in favor of Mississippi and its 2018 law that outlawed abortion after 15 weeks. Jackson Women’s Health, the state’s sole remaining abortion provider, sued to block the law soon after it passed.
The Supreme Court decision is not a surprise, as the justices indicated they were leaning that way during oral arguments in December. The majority’s thoughts were further revealed when a draft of the opinion was leaked to the news outlet Politico on May 2.
In the final opinion, Justice Samuel Alito, writing for the majority, “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
The decision strikes down both precedent-setting rulings that established a right to abortion until the point of viability, long considered to be 24 weeks: Roe v. Wade (1973) and Planned Parenthood v. Casey (1992).
Twenty-five medical professional societies – representing OB/GYNs, family medicine doctors, fertility specialists, geneticists, hospitalists, internists, pediatricians, psychiatrists, nurses, nurse practitioners, and midwives – had urged the court to throw out the Mississippi law. And more than 2,500 medical professionals signed on to a petition in June, urging the court to uphold the right to abortion.
The number of abortions has recently increased from what had been a long decline. The Guttmacher Institute estimates there were there were 930,160 abortion procedures in 2020 (compared to 3.6 million births), an 8% increase from 2017. The number does not include self-managed abortions. The organization said the increase was potentially due to expanded Medicaid coverage and reduced access to contraception due to Trump administration policies.
Trigger laws and bans
When trigger laws and new restrictions go into effect, women in the South, Midwest, and Inter-Mountain West will likely have to drive hundreds of miles for an abortion, according to Guttmacher. Women in Louisiana, for instance, would have to drive 660 miles to get to the nearest provider in Illinois.
University of Utah researchers estimated that almost half of women will see a big increase in the distance to abortion care, from a median distance of 39 miles to 113 miles. State bans will disproportionately impact women of color, those living in poverty, and people with less education, they said.
The CDC has reported that Black women are three times more likely to die from a pregnancy-related cause than white women.
Doctors and other abortion providers could face serious penalties. The maximum penalty in Texas is life in prison, and the sentence could be 10 to 15 years in 11 other states, according to an article in the medical journal JAMA by attorneys Rebecca B. Reingold and Lawrence O. Gostin.
“Threats of prosecution undermine clinicians’ ability to provide safe, evidence-based care and to counsel patients honestly, impeding the patient-physician relationship,” they wrote. “Given harsh penalties, physicians may cease treating pregnancy loss, with no clear line between treating miscarriages and abortions.”
In preparing for these attacks on patients and doctors, New York Gov. Kathy Hochul on June 13 signed a bill that immediately protects anyone who has an abortion and medical professionals in the state who provide them from legal retaliation by states that restrict or prohibit abortion.
Even while Roe was still the law, Mississippi had banned most abortions after 20 weeks, and 16 states prohibited abortion after 22 weeks. A Texas ban on abortion after 6 weeks – which also allows private citizens to sue abortion providers – was allowed to stay in place while it was being challenged.
On May 26, Oklahoma Gov. Kevin Stitt signed a bill banning abortion from the moment of conception. Just as in Texas, the Oklahoma law allows what critics have called “bounty hunting” of abortion providers.
Four states have a constitutional amendment declaring that the state constitution does not secure or protect the right to abortion or allow the use of public funds for abortion: Alabama, Louisiana, Tennessee, and West Virginia.
Some states protecting rights
At least 16 states have proactively protected a right to an abortion, according to Guttmacher, while The New York Times reports that Washington, DC, has laws that protect abortion, along with 20 states: Alaska, Colorado, Illinois, Maine, Massachusetts, Minnesota, Nevada, New Hampshire, New Mexico, Rhode Island, California, Connecticut, Delaware, Hawaii, Maryland, New Jersey, New York, Oregon, Vermont, and Washington.
Some of these states are gearing up for a potential influx of patients. Washington Gov. Jay Inslee signed a law that authorizes physician assistants, advanced registered nurse practitioners, and other providers acting within their scope of practice to perform abortions. And the Maryland Legislature overrode a veto by Gov. Larry Hogan of a law that expands who can perform abortions.
Wisconsin Gov. Tony Evers in early June called a special legislative session to repeal the state’s 173-year-old dormant ban on abortion. But the majority Republican legislature vowed to take no action.
B. Jessie Hill, JD, associate dean for academic affairs and a professor at the Case Western Reserve University School of Law, says she expects anti-abortion groups to challenge these protective laws, “by saying that fetuses are persons under the Constitution with a right to life and therefore that the state has to protect them.”
But, she says, “there’s going to be big, big challenges with those lawsuits,” and they will not be “winners off the bat.”
Medication abortions, travel next battle
Some states are also trying to outlaw or severely restrict the use of RU-486, the abortion pill. A Tennessee law that goes into effect in 2023 would ban delivery of pills by mail and require a patient to have two doctor visits – one consultation and one to pick up the pills.
Mississippi has also enacted restrictions including the requirement that women meet with a doctor first – and is being sued by pill maker GenBioPro.
Guttmacher estimates that medication abortion accounted for 39% of all abortions in the U.S. in 2017 and 60% of all abortions that occurred before 10 weeks’ gestation.
Some states have floated the idea of prohibiting anyone from traveling to another state for an abortion.
George Mason University law professor Ilya Somin, JD, has written that such a law would likely violate the Dormant Commerce Clause, “which forbids state regulations that specifically restrict interstate commerce or discriminate against it.”
He also wrote that states lack the authority to regulate activity that takes place beyond their borders and that such bans “are open to challenge because they violate the constitutional right to travel.”
Hill also said a travel ban would be problematic, noting that it might be difficult to prosecute someone for “something you did completely in another state.”
A version of this article first appeared on Medscape.com.
Hypertensive pregnancy disorders tied to double hypertension risk
Hypertensive disorders of pregnancy (HDP) are associated with a greater than twofold risk of developing hypertension a decade later, new research suggests.
Investigators prospectively studied patients who had and who had not experienced HDP 10 years earlier; most self-identified as Black. They found that those with a history of HDP had a 2.4-fold higher risk for new hypertension than those without such a history.
Patients who developed hypertension showed greater left ventricular (LV) remodeling (including greater relative wall thickness), worse diastolic function, more abnormal longitudinal strain, and higher effective arterial elastance than those without hypertension, regardless of the presence or absence of an HDP history.
“We know that patients with preeclampsia are at a higher risk for heart disease later in life, and it seems to be driven by the development of new hypertension,” lead author Lisa Levine, MD, MSCE, director, pregnancy and heart disease program, Hospital of the University of Pennsylvania, Philadelphia, told this news organization.
It is critically important to “study a more diverse population, including a larger percentage of Black patients, since HDP and CVD both disproportionately affect Black women,” Dr. Levine said. “And it is important to screen patients for hypertension, getting them into primary care for visits, getting them diagnosed sooner, and treating them early for hypertension.”
The study was published in the Journal of the American College of Cardiology.
Understudied population
HDP includes gestational hypertension and preeclampsia, Dr. Levine explained. “We already know that patients who have had preeclampsia are at higher risk for stroke, heart failure [HF], and myocardial infarction later in life,” she said. The goal of this study was to see whether, instead of waiting 20-30 years, they could look only 10 years later to see which patients would be at highest risk for future heart disease, Dr. Levine said.
In particular, it’s known that cardiovascular disease (CVD) and HDP “disproportionately affect Black women,” Dr. Levine continued. “What makes our study different from other studies is that we focused predominantly on the Black African American population, since it’s understudied and also at highest risk for preeclampsia and heart disease,” she said.
They set out to “evaluate differences in CV risk factors as well as subclinical CVD among a well-characterized group of racially diverse patients with and without a history of HDP 10 years earlier,” the authors state.
To investigate the question, the researchers performed a prospective, cross-sectional study between April 2016 and December 2019 of patients with and without a diagnosis of HDP during a previous pregnancy at least 10 years earlier (from 2005 to 2007). Patients were drawn from a parent cohort in a previously performed observational study of patients with preeclampsia or HDP and normotensive control subjects.
The current study focused on 135 patients (85% Black), 84 with a history of HDP and 51 without. Of the Black patients, 91.7% had a history of HDP, compared with 8.3% of the White patients.
During an in-person visit, the researchers assessed participants’ blood pressure and other clinical risk factors for CVD, including fasting glucose and lipids. They also used noninvasive means to measure cardiac and vascular structure and function.
Importance of routine screening
The risk for new hypertension was 2.4 times higher in patients with a history of HDP than in those without HDP, with stage 2 hypertension noted in 56.0% of patients with and in 23.5% without HDP (P < .001). This equates to a relative risk of 2.4 (95% confidence interval, 1.39-4.14), even after adjustment for race, maternal age, body mass index, and history of preterm birth.
“Importantly, 18% of patients with a history of HDP met criteria for a new diagnosis of hypertension identified through the study visit,” the authors report.
There were no differences in many cardiac measures (left ventricular (LV) structure, global longitudinal strain, diastolic function, arterial stiffness, or endothelial function) between patients with and without a history of HDP.
However, patients with chronic hypertension (CHTN), regardless of HDP history, had other cardiac abnormalities, including greater LV remodeling, worse diastolic function, and higher effective arterial elastance.
“The data regarding increased risk of hypertension after HDP is not a novel finding, however our cohort is unique in the high baseline rate of stage 2 hypertension, even among patients without a history of HDP,” the authors comment.
In fact, when they looked at the diagnosis of either stage 1 or stage 2 hypertension, they found that more than 80% of patients with and 60% of patients without a history of HDP had hypertension. Notably, among patients with a history of HDP, only 39% had a formal diagnosis of either stage 1 or stage 2 hypertension, further highlighting “the importance of routine screening for CHTN in this population,” they state.
“Further studies should evaluate the optimal time period to screen for postpartum hypertension and a monitoring plan for these at-risk women,” Dr. Levine added.
‘Opportunity of a lifetime’
Commenting for this news organization, Malamo Countouris, MD, MS, assistant professor of medicine and codirector, postpartum hypertension program, University of Pittsburgh Medical Center, said hypertension is “underrecognized and undertreated among young, premenopausal, Black women.”
Pregnancy “gives us a clue, through HDP, as to who is high risk to develop chronic hypertension and subsequent subclinical structural cardiac changes in the decade after delivery,” said Dr. Countouris, who was not involved with the study.
“The jury is still out on whether HDP contributes independently to cardiovascular changes in the years after delivery. Ongoing research is needed to clarify the unique or compounding contributions of pregnancy complications and hypertension,” she added.
In an accompanying editorial , Josephine Chou, MD, MS, director of cardio-obstetrics and codirector of maternal cardiology, Yale University, New Haven, Conn., called the study a “laudable contribution to understanding of HDP and hypertension within the first decade after pregnancy,” saying that it “paves the way for future efforts to improve postpartum CV care, enabling us to grasp this opportunity of a lifetime to ultimately reduce maternal and pregnancy-related morbidity and mortality.”
This study was supported by the National Institutes of Health, the National Heart, Lung, and Blood Institute, and the American Association of Obstetricians and Gynecologists Foundation. Dr. Levine reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Countouris reports receiving funding from the American Heart Association. Dr. Chou reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hypertensive disorders of pregnancy (HDP) are associated with a greater than twofold risk of developing hypertension a decade later, new research suggests.
Investigators prospectively studied patients who had and who had not experienced HDP 10 years earlier; most self-identified as Black. They found that those with a history of HDP had a 2.4-fold higher risk for new hypertension than those without such a history.
Patients who developed hypertension showed greater left ventricular (LV) remodeling (including greater relative wall thickness), worse diastolic function, more abnormal longitudinal strain, and higher effective arterial elastance than those without hypertension, regardless of the presence or absence of an HDP history.
“We know that patients with preeclampsia are at a higher risk for heart disease later in life, and it seems to be driven by the development of new hypertension,” lead author Lisa Levine, MD, MSCE, director, pregnancy and heart disease program, Hospital of the University of Pennsylvania, Philadelphia, told this news organization.
It is critically important to “study a more diverse population, including a larger percentage of Black patients, since HDP and CVD both disproportionately affect Black women,” Dr. Levine said. “And it is important to screen patients for hypertension, getting them into primary care for visits, getting them diagnosed sooner, and treating them early for hypertension.”
The study was published in the Journal of the American College of Cardiology.
Understudied population
HDP includes gestational hypertension and preeclampsia, Dr. Levine explained. “We already know that patients who have had preeclampsia are at higher risk for stroke, heart failure [HF], and myocardial infarction later in life,” she said. The goal of this study was to see whether, instead of waiting 20-30 years, they could look only 10 years later to see which patients would be at highest risk for future heart disease, Dr. Levine said.
In particular, it’s known that cardiovascular disease (CVD) and HDP “disproportionately affect Black women,” Dr. Levine continued. “What makes our study different from other studies is that we focused predominantly on the Black African American population, since it’s understudied and also at highest risk for preeclampsia and heart disease,” she said.
They set out to “evaluate differences in CV risk factors as well as subclinical CVD among a well-characterized group of racially diverse patients with and without a history of HDP 10 years earlier,” the authors state.
To investigate the question, the researchers performed a prospective, cross-sectional study between April 2016 and December 2019 of patients with and without a diagnosis of HDP during a previous pregnancy at least 10 years earlier (from 2005 to 2007). Patients were drawn from a parent cohort in a previously performed observational study of patients with preeclampsia or HDP and normotensive control subjects.
The current study focused on 135 patients (85% Black), 84 with a history of HDP and 51 without. Of the Black patients, 91.7% had a history of HDP, compared with 8.3% of the White patients.
During an in-person visit, the researchers assessed participants’ blood pressure and other clinical risk factors for CVD, including fasting glucose and lipids. They also used noninvasive means to measure cardiac and vascular structure and function.
Importance of routine screening
The risk for new hypertension was 2.4 times higher in patients with a history of HDP than in those without HDP, with stage 2 hypertension noted in 56.0% of patients with and in 23.5% without HDP (P < .001). This equates to a relative risk of 2.4 (95% confidence interval, 1.39-4.14), even after adjustment for race, maternal age, body mass index, and history of preterm birth.
“Importantly, 18% of patients with a history of HDP met criteria for a new diagnosis of hypertension identified through the study visit,” the authors report.
There were no differences in many cardiac measures (left ventricular (LV) structure, global longitudinal strain, diastolic function, arterial stiffness, or endothelial function) between patients with and without a history of HDP.
However, patients with chronic hypertension (CHTN), regardless of HDP history, had other cardiac abnormalities, including greater LV remodeling, worse diastolic function, and higher effective arterial elastance.
“The data regarding increased risk of hypertension after HDP is not a novel finding, however our cohort is unique in the high baseline rate of stage 2 hypertension, even among patients without a history of HDP,” the authors comment.
In fact, when they looked at the diagnosis of either stage 1 or stage 2 hypertension, they found that more than 80% of patients with and 60% of patients without a history of HDP had hypertension. Notably, among patients with a history of HDP, only 39% had a formal diagnosis of either stage 1 or stage 2 hypertension, further highlighting “the importance of routine screening for CHTN in this population,” they state.
“Further studies should evaluate the optimal time period to screen for postpartum hypertension and a monitoring plan for these at-risk women,” Dr. Levine added.
‘Opportunity of a lifetime’
Commenting for this news organization, Malamo Countouris, MD, MS, assistant professor of medicine and codirector, postpartum hypertension program, University of Pittsburgh Medical Center, said hypertension is “underrecognized and undertreated among young, premenopausal, Black women.”
Pregnancy “gives us a clue, through HDP, as to who is high risk to develop chronic hypertension and subsequent subclinical structural cardiac changes in the decade after delivery,” said Dr. Countouris, who was not involved with the study.
“The jury is still out on whether HDP contributes independently to cardiovascular changes in the years after delivery. Ongoing research is needed to clarify the unique or compounding contributions of pregnancy complications and hypertension,” she added.
In an accompanying editorial , Josephine Chou, MD, MS, director of cardio-obstetrics and codirector of maternal cardiology, Yale University, New Haven, Conn., called the study a “laudable contribution to understanding of HDP and hypertension within the first decade after pregnancy,” saying that it “paves the way for future efforts to improve postpartum CV care, enabling us to grasp this opportunity of a lifetime to ultimately reduce maternal and pregnancy-related morbidity and mortality.”
This study was supported by the National Institutes of Health, the National Heart, Lung, and Blood Institute, and the American Association of Obstetricians and Gynecologists Foundation. Dr. Levine reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Countouris reports receiving funding from the American Heart Association. Dr. Chou reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hypertensive disorders of pregnancy (HDP) are associated with a greater than twofold risk of developing hypertension a decade later, new research suggests.
Investigators prospectively studied patients who had and who had not experienced HDP 10 years earlier; most self-identified as Black. They found that those with a history of HDP had a 2.4-fold higher risk for new hypertension than those without such a history.
Patients who developed hypertension showed greater left ventricular (LV) remodeling (including greater relative wall thickness), worse diastolic function, more abnormal longitudinal strain, and higher effective arterial elastance than those without hypertension, regardless of the presence or absence of an HDP history.
“We know that patients with preeclampsia are at a higher risk for heart disease later in life, and it seems to be driven by the development of new hypertension,” lead author Lisa Levine, MD, MSCE, director, pregnancy and heart disease program, Hospital of the University of Pennsylvania, Philadelphia, told this news organization.
It is critically important to “study a more diverse population, including a larger percentage of Black patients, since HDP and CVD both disproportionately affect Black women,” Dr. Levine said. “And it is important to screen patients for hypertension, getting them into primary care for visits, getting them diagnosed sooner, and treating them early for hypertension.”
The study was published in the Journal of the American College of Cardiology.
Understudied population
HDP includes gestational hypertension and preeclampsia, Dr. Levine explained. “We already know that patients who have had preeclampsia are at higher risk for stroke, heart failure [HF], and myocardial infarction later in life,” she said. The goal of this study was to see whether, instead of waiting 20-30 years, they could look only 10 years later to see which patients would be at highest risk for future heart disease, Dr. Levine said.
In particular, it’s known that cardiovascular disease (CVD) and HDP “disproportionately affect Black women,” Dr. Levine continued. “What makes our study different from other studies is that we focused predominantly on the Black African American population, since it’s understudied and also at highest risk for preeclampsia and heart disease,” she said.
They set out to “evaluate differences in CV risk factors as well as subclinical CVD among a well-characterized group of racially diverse patients with and without a history of HDP 10 years earlier,” the authors state.
To investigate the question, the researchers performed a prospective, cross-sectional study between April 2016 and December 2019 of patients with and without a diagnosis of HDP during a previous pregnancy at least 10 years earlier (from 2005 to 2007). Patients were drawn from a parent cohort in a previously performed observational study of patients with preeclampsia or HDP and normotensive control subjects.
The current study focused on 135 patients (85% Black), 84 with a history of HDP and 51 without. Of the Black patients, 91.7% had a history of HDP, compared with 8.3% of the White patients.
During an in-person visit, the researchers assessed participants’ blood pressure and other clinical risk factors for CVD, including fasting glucose and lipids. They also used noninvasive means to measure cardiac and vascular structure and function.
Importance of routine screening
The risk for new hypertension was 2.4 times higher in patients with a history of HDP than in those without HDP, with stage 2 hypertension noted in 56.0% of patients with and in 23.5% without HDP (P < .001). This equates to a relative risk of 2.4 (95% confidence interval, 1.39-4.14), even after adjustment for race, maternal age, body mass index, and history of preterm birth.
“Importantly, 18% of patients with a history of HDP met criteria for a new diagnosis of hypertension identified through the study visit,” the authors report.
There were no differences in many cardiac measures (left ventricular (LV) structure, global longitudinal strain, diastolic function, arterial stiffness, or endothelial function) between patients with and without a history of HDP.
However, patients with chronic hypertension (CHTN), regardless of HDP history, had other cardiac abnormalities, including greater LV remodeling, worse diastolic function, and higher effective arterial elastance.
“The data regarding increased risk of hypertension after HDP is not a novel finding, however our cohort is unique in the high baseline rate of stage 2 hypertension, even among patients without a history of HDP,” the authors comment.
In fact, when they looked at the diagnosis of either stage 1 or stage 2 hypertension, they found that more than 80% of patients with and 60% of patients without a history of HDP had hypertension. Notably, among patients with a history of HDP, only 39% had a formal diagnosis of either stage 1 or stage 2 hypertension, further highlighting “the importance of routine screening for CHTN in this population,” they state.
“Further studies should evaluate the optimal time period to screen for postpartum hypertension and a monitoring plan for these at-risk women,” Dr. Levine added.
‘Opportunity of a lifetime’
Commenting for this news organization, Malamo Countouris, MD, MS, assistant professor of medicine and codirector, postpartum hypertension program, University of Pittsburgh Medical Center, said hypertension is “underrecognized and undertreated among young, premenopausal, Black women.”
Pregnancy “gives us a clue, through HDP, as to who is high risk to develop chronic hypertension and subsequent subclinical structural cardiac changes in the decade after delivery,” said Dr. Countouris, who was not involved with the study.
“The jury is still out on whether HDP contributes independently to cardiovascular changes in the years after delivery. Ongoing research is needed to clarify the unique or compounding contributions of pregnancy complications and hypertension,” she added.
In an accompanying editorial , Josephine Chou, MD, MS, director of cardio-obstetrics and codirector of maternal cardiology, Yale University, New Haven, Conn., called the study a “laudable contribution to understanding of HDP and hypertension within the first decade after pregnancy,” saying that it “paves the way for future efforts to improve postpartum CV care, enabling us to grasp this opportunity of a lifetime to ultimately reduce maternal and pregnancy-related morbidity and mortality.”
This study was supported by the National Institutes of Health, the National Heart, Lung, and Blood Institute, and the American Association of Obstetricians and Gynecologists Foundation. Dr. Levine reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Countouris reports receiving funding from the American Heart Association. Dr. Chou reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More Black mothers deliver by cesarean, not always by choice
When 29-year-old Sakeenah Fowler was pregnant with her first child, doctors kept a close watch. Ms. Fowler has lupus, high blood pressure, a history of blood clotting, and kidney problems that all could have endangered her or the health of her unborn baby.
She saw maternal-fetal specialists who could keep watch of her high-risk pregnancy, and she collected urine samples every 24 hours to make sure her kidneys were functioning properly from her home in Roebuck, S.C.
But the pregnancy ultimately proved uneventful; even her kidneys remained stable. So Ms. Fowler said she was shocked when her doctors ordered an emergency cesarean delivery after she had gone into active labor.
“I was already dilated all the way to 6 cm,” but the baby’s heart rate had decreased by a small amount, she says. “They thought it was best to just go ahead with a C-section.”
Ms. Fowler, who is Black, said she believes the surgical intervention was unnecessary and that she wasn’t given a chance to discuss her options for a vaginal childbirth.
“They already had it in their minds that I wasn’t going to make it through the pregnancy without any issues; then when I did, it was like they wanted to find something that made me have to have a C-section,” Ms. Fowler said. “It was close to the holidays; everybody was ready to go home. It was just like I was pushed to do what they wanted me to do.”
Ms. Fowler’s sense of a lack of choice is important beyond the measure of patient experience. While cesarean deliveries can be a lifeline for mother and baby, they can put up massive roadblocks to maternal and infant health when not necessary.
“The risk of hemorrhage, infection – on average, all of these go up when you have surgery instead of a vaginal delivery,” says Kimberly B. Glazer, PhD, a perinatal epidemiologist at the Icahn School of Medicine at Mount Sinai, New York.
“Birth is one of the most salient experiences you can have. People want to feel like their values and preferences – whatever they may be – were honored and respected. Even if the delivery goes a different way than you wanted, feeling like your values were taken into account is very important.”
More than 1 million women undergo cesarean deliveries in the United States every year, composing over 31% of all births in 2020, according to the Centers for Disease Control and Prevention.
The World Health Organization, meanwhile, recommends a rate of cesarean delivery of no more than 15% per region. Whether or not all the U.S. procedures were medically warranted is unclear, however.
Black women have higher odds of undergoing a cesarean: 36% undergo surgical deliveries annually, compared with about 30% of White women. Black women are also about three times more likely to die of pregnancy-related causes than White women.
Risk becomes reality
Ms. Fowler eventually developed an infection in her cesarean surgical wound, but her doctors initially insisted her alternating chills and fever were merely postpartum hormonal swings, she says.
“I thought something had to be wrong, but they just kept saying nothing was wrong,” she says.
By the time her doctors caught the infection, Ms. Fowler was readmitted to the hospital for several days of IV antibiotic therapy. The infection “almost got into my bloodstream and could have killed me,” she says.
While cesarean deliveries are associated with decreases in maternal, neonatal, and infant mortality, the benefits are only seen up to a certain threshold. The WHO, for instance, has reported that over the 15% threshold, that lower mortality benefit disappears.
“When medically necessary, cesarean delivery can improve outcomes for mother and baby. But the fact that cesarean section rates have increased in recent years without a corresponding improvement in health outcomes indicates overreliance on the procedure,” Dr. Glazer says.
Clinical discretion leads to biased judgment calls
Rates of cesarean deliveries are even higher among low-risk pregnancies in women of color than in White women. Between 2016 and 2019, the overall rate of cesarean deliveries for low-risk births was 23%, according to a recent analysis. But the rate was almost 18% higher among Black women than among White women (27% vs. 22%).
“When you see data about these subjective indications varying by race and ethnicity, I think that’s pointing us toward some answers,” Dr. Glazer says. “Once you adjust for all these measures, prepregnancy characteristics, and risk factors, the research identifies variation in quality and outcomes that is rooted in structural and systemic racism in health care, implicit bias from clinicians.”
Researchers investigating cesarean deliveries have found that Black women are more likely to undergo the surgery for reasons that are highly subjective, such as fetal distress.
“There is a huge range of how concerning a fetal heart rate can be, and some health providers might perform a C-section for only minor changes in the fetal heart rate, while others might wait until it is much worse,” said Rebecca Hamm, MD, an assistant professor of obstetrics and gynecology at the Perelman School of Medicine at the University of Pennsylvania.
At least some of the differences in care can be explained by where women deliver their babies, studies have shown. Women of color disproportionately deliver at hospitals with poorer quality outcomes for moms and babies.
Dealing with the aftermath
There can be costs that reverberate throughout the life of a mother, child, and their family as the result of surgical delivery.
“Cesarean sections cost a lot more,” says Jamila Taylor, PhD, director of health care reform and a senior fellow with The Century Foundation, a progressive policy think tank in Washington, D.C. The cost of a cesarean delivery averages about $17,000, compared with about $12,200 for a vaginal birth; for uninsured patients, surgical deliveries cost about $9,000 more than vaginal deliveries.
Dr. Taylor, who has studied the historical mistreatment of Black women in obstetrics, noted that this cost includes not just the bill for surgery but also a prolonged recovery time that is often spent in a hospital bed.
Beyond the detrimental effect that a large hospital bill for delivery and aftercare can have on families, other costs can crop up later. Infants delivered by cesarean surgery are more likely to develop an infection, breathing problems, and to spend time in the neonatal intensive care unit than babies born vaginally. Although studies suggest these outcomes may result from a medically necessary health concern that spurred the cesarean surgery, they often stem from the delivery itself.
Babies born surgically also miss out on the benefits of passing through the birth canal, such as supporting a newborn’s immune system and preparing their lungs to breathe oxygen after birth.
Most of the efforts to reduce inequities in maternal care are happening at the clinical level, aimed at both patients and providers, Dr. Taylor says.
“As advocates, we’re talking about how we can help Black women be advocates for themselves in the health care system – if the physician suggests a C-section, getting a second opinion, or walking through what a [surgical delivery] will mean and what their recovery will look like,” she says.
Women are also increasingly choosing non-hospital settings to deliver when possible, Dr. Taylor says. Including doulas or midwife practitioners in the maternal care team can reduce unnecessary cesarean deliveries among Black women, according to Camille Clare, MD, chair of the New York chapter of the American College of Obstetricians and Gynecologists.
Also, last year, race was removed from the vaginal birth after C-section (VBAC) calculator, which is used to gauge the safety of vaginal delivery in women with a history of surgical birth. The original calculator included race-based correction factors for Black women and Hispanic women. It predicted a lower likelihood of successful vaginal deliveries for women who already had a C-section and who identify as Black or Hispanic than for White women with otherwise identical characteristics, such as age, weight, and a history of cesarean delivery.
“Those are things that over time should reduce the high rates of cesarean section for Black women in particular,” Dr. Clare says.
In addition to embracing the updated calculator and including nurse-midwives and doulas in their obstetrics services, Penn Medicine, Philadelphia received a federal grant to study the impact of creating a standard plan for deliveries. This includes standardizing the induction of labor and any effect that might have on reducing C-section rates.
“This idea that biases lead to difference in decisionmaking, and that by standardizing practices we could address these differences – people were somewhat resistant at first,” Dr. Hamm says. “They didn’t believe there were differences in their practices.”
People struggle to recognize those differences, she says, and “it takes active participation in reducing disparities to make that happen.”
At the community level, Synergistic Sisters in Science (SIS), a group of maternal health experts and health equity advocates, is working on a project called PM3, to reduce maternal mortality through mobile technology.
The smartphone app will provide information for new moms to empower them to start conversations with health care providers. It also connects users to social support and resources. SIS is especially hoping to engage Black women living in rural areas.
“There is so much mistrust due to things like unnecessary C-sections and the fact that Black women feel they aren’t heard,” said Natalie Hernandez, PhD, executive director of the Center for Maternal Health Equity at Morehouse School of Medicine, Atlanta. “Here is a tool that gives a woman information that’s culturally centered, looks like her, and was informed by her voice.”
A version of this article first appeared on WebMD.com.
When 29-year-old Sakeenah Fowler was pregnant with her first child, doctors kept a close watch. Ms. Fowler has lupus, high blood pressure, a history of blood clotting, and kidney problems that all could have endangered her or the health of her unborn baby.
She saw maternal-fetal specialists who could keep watch of her high-risk pregnancy, and she collected urine samples every 24 hours to make sure her kidneys were functioning properly from her home in Roebuck, S.C.
But the pregnancy ultimately proved uneventful; even her kidneys remained stable. So Ms. Fowler said she was shocked when her doctors ordered an emergency cesarean delivery after she had gone into active labor.
“I was already dilated all the way to 6 cm,” but the baby’s heart rate had decreased by a small amount, she says. “They thought it was best to just go ahead with a C-section.”
Ms. Fowler, who is Black, said she believes the surgical intervention was unnecessary and that she wasn’t given a chance to discuss her options for a vaginal childbirth.
“They already had it in their minds that I wasn’t going to make it through the pregnancy without any issues; then when I did, it was like they wanted to find something that made me have to have a C-section,” Ms. Fowler said. “It was close to the holidays; everybody was ready to go home. It was just like I was pushed to do what they wanted me to do.”
Ms. Fowler’s sense of a lack of choice is important beyond the measure of patient experience. While cesarean deliveries can be a lifeline for mother and baby, they can put up massive roadblocks to maternal and infant health when not necessary.
“The risk of hemorrhage, infection – on average, all of these go up when you have surgery instead of a vaginal delivery,” says Kimberly B. Glazer, PhD, a perinatal epidemiologist at the Icahn School of Medicine at Mount Sinai, New York.
“Birth is one of the most salient experiences you can have. People want to feel like their values and preferences – whatever they may be – were honored and respected. Even if the delivery goes a different way than you wanted, feeling like your values were taken into account is very important.”
More than 1 million women undergo cesarean deliveries in the United States every year, composing over 31% of all births in 2020, according to the Centers for Disease Control and Prevention.
The World Health Organization, meanwhile, recommends a rate of cesarean delivery of no more than 15% per region. Whether or not all the U.S. procedures were medically warranted is unclear, however.
Black women have higher odds of undergoing a cesarean: 36% undergo surgical deliveries annually, compared with about 30% of White women. Black women are also about three times more likely to die of pregnancy-related causes than White women.
Risk becomes reality
Ms. Fowler eventually developed an infection in her cesarean surgical wound, but her doctors initially insisted her alternating chills and fever were merely postpartum hormonal swings, she says.
“I thought something had to be wrong, but they just kept saying nothing was wrong,” she says.
By the time her doctors caught the infection, Ms. Fowler was readmitted to the hospital for several days of IV antibiotic therapy. The infection “almost got into my bloodstream and could have killed me,” she says.
While cesarean deliveries are associated with decreases in maternal, neonatal, and infant mortality, the benefits are only seen up to a certain threshold. The WHO, for instance, has reported that over the 15% threshold, that lower mortality benefit disappears.
“When medically necessary, cesarean delivery can improve outcomes for mother and baby. But the fact that cesarean section rates have increased in recent years without a corresponding improvement in health outcomes indicates overreliance on the procedure,” Dr. Glazer says.
Clinical discretion leads to biased judgment calls
Rates of cesarean deliveries are even higher among low-risk pregnancies in women of color than in White women. Between 2016 and 2019, the overall rate of cesarean deliveries for low-risk births was 23%, according to a recent analysis. But the rate was almost 18% higher among Black women than among White women (27% vs. 22%).
“When you see data about these subjective indications varying by race and ethnicity, I think that’s pointing us toward some answers,” Dr. Glazer says. “Once you adjust for all these measures, prepregnancy characteristics, and risk factors, the research identifies variation in quality and outcomes that is rooted in structural and systemic racism in health care, implicit bias from clinicians.”
Researchers investigating cesarean deliveries have found that Black women are more likely to undergo the surgery for reasons that are highly subjective, such as fetal distress.
“There is a huge range of how concerning a fetal heart rate can be, and some health providers might perform a C-section for only minor changes in the fetal heart rate, while others might wait until it is much worse,” said Rebecca Hamm, MD, an assistant professor of obstetrics and gynecology at the Perelman School of Medicine at the University of Pennsylvania.
At least some of the differences in care can be explained by where women deliver their babies, studies have shown. Women of color disproportionately deliver at hospitals with poorer quality outcomes for moms and babies.
Dealing with the aftermath
There can be costs that reverberate throughout the life of a mother, child, and their family as the result of surgical delivery.
“Cesarean sections cost a lot more,” says Jamila Taylor, PhD, director of health care reform and a senior fellow with The Century Foundation, a progressive policy think tank in Washington, D.C. The cost of a cesarean delivery averages about $17,000, compared with about $12,200 for a vaginal birth; for uninsured patients, surgical deliveries cost about $9,000 more than vaginal deliveries.
Dr. Taylor, who has studied the historical mistreatment of Black women in obstetrics, noted that this cost includes not just the bill for surgery but also a prolonged recovery time that is often spent in a hospital bed.
Beyond the detrimental effect that a large hospital bill for delivery and aftercare can have on families, other costs can crop up later. Infants delivered by cesarean surgery are more likely to develop an infection, breathing problems, and to spend time in the neonatal intensive care unit than babies born vaginally. Although studies suggest these outcomes may result from a medically necessary health concern that spurred the cesarean surgery, they often stem from the delivery itself.
Babies born surgically also miss out on the benefits of passing through the birth canal, such as supporting a newborn’s immune system and preparing their lungs to breathe oxygen after birth.
Most of the efforts to reduce inequities in maternal care are happening at the clinical level, aimed at both patients and providers, Dr. Taylor says.
“As advocates, we’re talking about how we can help Black women be advocates for themselves in the health care system – if the physician suggests a C-section, getting a second opinion, or walking through what a [surgical delivery] will mean and what their recovery will look like,” she says.
Women are also increasingly choosing non-hospital settings to deliver when possible, Dr. Taylor says. Including doulas or midwife practitioners in the maternal care team can reduce unnecessary cesarean deliveries among Black women, according to Camille Clare, MD, chair of the New York chapter of the American College of Obstetricians and Gynecologists.
Also, last year, race was removed from the vaginal birth after C-section (VBAC) calculator, which is used to gauge the safety of vaginal delivery in women with a history of surgical birth. The original calculator included race-based correction factors for Black women and Hispanic women. It predicted a lower likelihood of successful vaginal deliveries for women who already had a C-section and who identify as Black or Hispanic than for White women with otherwise identical characteristics, such as age, weight, and a history of cesarean delivery.
“Those are things that over time should reduce the high rates of cesarean section for Black women in particular,” Dr. Clare says.
In addition to embracing the updated calculator and including nurse-midwives and doulas in their obstetrics services, Penn Medicine, Philadelphia received a federal grant to study the impact of creating a standard plan for deliveries. This includes standardizing the induction of labor and any effect that might have on reducing C-section rates.
“This idea that biases lead to difference in decisionmaking, and that by standardizing practices we could address these differences – people were somewhat resistant at first,” Dr. Hamm says. “They didn’t believe there were differences in their practices.”
People struggle to recognize those differences, she says, and “it takes active participation in reducing disparities to make that happen.”
At the community level, Synergistic Sisters in Science (SIS), a group of maternal health experts and health equity advocates, is working on a project called PM3, to reduce maternal mortality through mobile technology.
The smartphone app will provide information for new moms to empower them to start conversations with health care providers. It also connects users to social support and resources. SIS is especially hoping to engage Black women living in rural areas.
“There is so much mistrust due to things like unnecessary C-sections and the fact that Black women feel they aren’t heard,” said Natalie Hernandez, PhD, executive director of the Center for Maternal Health Equity at Morehouse School of Medicine, Atlanta. “Here is a tool that gives a woman information that’s culturally centered, looks like her, and was informed by her voice.”
A version of this article first appeared on WebMD.com.
When 29-year-old Sakeenah Fowler was pregnant with her first child, doctors kept a close watch. Ms. Fowler has lupus, high blood pressure, a history of blood clotting, and kidney problems that all could have endangered her or the health of her unborn baby.
She saw maternal-fetal specialists who could keep watch of her high-risk pregnancy, and she collected urine samples every 24 hours to make sure her kidneys were functioning properly from her home in Roebuck, S.C.
But the pregnancy ultimately proved uneventful; even her kidneys remained stable. So Ms. Fowler said she was shocked when her doctors ordered an emergency cesarean delivery after she had gone into active labor.
“I was already dilated all the way to 6 cm,” but the baby’s heart rate had decreased by a small amount, she says. “They thought it was best to just go ahead with a C-section.”
Ms. Fowler, who is Black, said she believes the surgical intervention was unnecessary and that she wasn’t given a chance to discuss her options for a vaginal childbirth.
“They already had it in their minds that I wasn’t going to make it through the pregnancy without any issues; then when I did, it was like they wanted to find something that made me have to have a C-section,” Ms. Fowler said. “It was close to the holidays; everybody was ready to go home. It was just like I was pushed to do what they wanted me to do.”
Ms. Fowler’s sense of a lack of choice is important beyond the measure of patient experience. While cesarean deliveries can be a lifeline for mother and baby, they can put up massive roadblocks to maternal and infant health when not necessary.
“The risk of hemorrhage, infection – on average, all of these go up when you have surgery instead of a vaginal delivery,” says Kimberly B. Glazer, PhD, a perinatal epidemiologist at the Icahn School of Medicine at Mount Sinai, New York.
“Birth is one of the most salient experiences you can have. People want to feel like their values and preferences – whatever they may be – were honored and respected. Even if the delivery goes a different way than you wanted, feeling like your values were taken into account is very important.”
More than 1 million women undergo cesarean deliveries in the United States every year, composing over 31% of all births in 2020, according to the Centers for Disease Control and Prevention.
The World Health Organization, meanwhile, recommends a rate of cesarean delivery of no more than 15% per region. Whether or not all the U.S. procedures were medically warranted is unclear, however.
Black women have higher odds of undergoing a cesarean: 36% undergo surgical deliveries annually, compared with about 30% of White women. Black women are also about three times more likely to die of pregnancy-related causes than White women.
Risk becomes reality
Ms. Fowler eventually developed an infection in her cesarean surgical wound, but her doctors initially insisted her alternating chills and fever were merely postpartum hormonal swings, she says.
“I thought something had to be wrong, but they just kept saying nothing was wrong,” she says.
By the time her doctors caught the infection, Ms. Fowler was readmitted to the hospital for several days of IV antibiotic therapy. The infection “almost got into my bloodstream and could have killed me,” she says.
While cesarean deliveries are associated with decreases in maternal, neonatal, and infant mortality, the benefits are only seen up to a certain threshold. The WHO, for instance, has reported that over the 15% threshold, that lower mortality benefit disappears.
“When medically necessary, cesarean delivery can improve outcomes for mother and baby. But the fact that cesarean section rates have increased in recent years without a corresponding improvement in health outcomes indicates overreliance on the procedure,” Dr. Glazer says.
Clinical discretion leads to biased judgment calls
Rates of cesarean deliveries are even higher among low-risk pregnancies in women of color than in White women. Between 2016 and 2019, the overall rate of cesarean deliveries for low-risk births was 23%, according to a recent analysis. But the rate was almost 18% higher among Black women than among White women (27% vs. 22%).
“When you see data about these subjective indications varying by race and ethnicity, I think that’s pointing us toward some answers,” Dr. Glazer says. “Once you adjust for all these measures, prepregnancy characteristics, and risk factors, the research identifies variation in quality and outcomes that is rooted in structural and systemic racism in health care, implicit bias from clinicians.”
Researchers investigating cesarean deliveries have found that Black women are more likely to undergo the surgery for reasons that are highly subjective, such as fetal distress.
“There is a huge range of how concerning a fetal heart rate can be, and some health providers might perform a C-section for only minor changes in the fetal heart rate, while others might wait until it is much worse,” said Rebecca Hamm, MD, an assistant professor of obstetrics and gynecology at the Perelman School of Medicine at the University of Pennsylvania.
At least some of the differences in care can be explained by where women deliver their babies, studies have shown. Women of color disproportionately deliver at hospitals with poorer quality outcomes for moms and babies.
Dealing with the aftermath
There can be costs that reverberate throughout the life of a mother, child, and their family as the result of surgical delivery.
“Cesarean sections cost a lot more,” says Jamila Taylor, PhD, director of health care reform and a senior fellow with The Century Foundation, a progressive policy think tank in Washington, D.C. The cost of a cesarean delivery averages about $17,000, compared with about $12,200 for a vaginal birth; for uninsured patients, surgical deliveries cost about $9,000 more than vaginal deliveries.
Dr. Taylor, who has studied the historical mistreatment of Black women in obstetrics, noted that this cost includes not just the bill for surgery but also a prolonged recovery time that is often spent in a hospital bed.
Beyond the detrimental effect that a large hospital bill for delivery and aftercare can have on families, other costs can crop up later. Infants delivered by cesarean surgery are more likely to develop an infection, breathing problems, and to spend time in the neonatal intensive care unit than babies born vaginally. Although studies suggest these outcomes may result from a medically necessary health concern that spurred the cesarean surgery, they often stem from the delivery itself.
Babies born surgically also miss out on the benefits of passing through the birth canal, such as supporting a newborn’s immune system and preparing their lungs to breathe oxygen after birth.
Most of the efforts to reduce inequities in maternal care are happening at the clinical level, aimed at both patients and providers, Dr. Taylor says.
“As advocates, we’re talking about how we can help Black women be advocates for themselves in the health care system – if the physician suggests a C-section, getting a second opinion, or walking through what a [surgical delivery] will mean and what their recovery will look like,” she says.
Women are also increasingly choosing non-hospital settings to deliver when possible, Dr. Taylor says. Including doulas or midwife practitioners in the maternal care team can reduce unnecessary cesarean deliveries among Black women, according to Camille Clare, MD, chair of the New York chapter of the American College of Obstetricians and Gynecologists.
Also, last year, race was removed from the vaginal birth after C-section (VBAC) calculator, which is used to gauge the safety of vaginal delivery in women with a history of surgical birth. The original calculator included race-based correction factors for Black women and Hispanic women. It predicted a lower likelihood of successful vaginal deliveries for women who already had a C-section and who identify as Black or Hispanic than for White women with otherwise identical characteristics, such as age, weight, and a history of cesarean delivery.
“Those are things that over time should reduce the high rates of cesarean section for Black women in particular,” Dr. Clare says.
In addition to embracing the updated calculator and including nurse-midwives and doulas in their obstetrics services, Penn Medicine, Philadelphia received a federal grant to study the impact of creating a standard plan for deliveries. This includes standardizing the induction of labor and any effect that might have on reducing C-section rates.
“This idea that biases lead to difference in decisionmaking, and that by standardizing practices we could address these differences – people were somewhat resistant at first,” Dr. Hamm says. “They didn’t believe there were differences in their practices.”
People struggle to recognize those differences, she says, and “it takes active participation in reducing disparities to make that happen.”
At the community level, Synergistic Sisters in Science (SIS), a group of maternal health experts and health equity advocates, is working on a project called PM3, to reduce maternal mortality through mobile technology.
The smartphone app will provide information for new moms to empower them to start conversations with health care providers. It also connects users to social support and resources. SIS is especially hoping to engage Black women living in rural areas.
“There is so much mistrust due to things like unnecessary C-sections and the fact that Black women feel they aren’t heard,” said Natalie Hernandez, PhD, executive director of the Center for Maternal Health Equity at Morehouse School of Medicine, Atlanta. “Here is a tool that gives a woman information that’s culturally centered, looks like her, and was informed by her voice.”
A version of this article first appeared on WebMD.com.
Care gaps common after anal sphincter injuries from childbirth
Postpartum complications may go unrecognized in women who incur anal sphincter injuries during childbirth, a review of electronic medical records at one academic health system suggests.
In the first 3 months after delivery, few patients with an obstetric anal sphincter injury (OASI) had documented pelvic floor problems, compared with higher rates documented in medical literature, the researchers found.
“Lack of identified pelvic floor dysfunction in this population differs from the incidence in previously published data and may reflect lack of identification by obstetric providers,” the researchers reported. The findings “highlight a gap in health care that, when addressed, could significantly improve postpartum quality of life.”
The findings are scheduled to be presented at the annual scientific meeting of the American Urogynecologic Society and International Urogynecological Association.
Anal sphincter injuries occur in about 4.4% of vaginal deliveries and are the most common cause of anal incontinence in women of reproductive age.
For the new study, researchers reviewed records of 287 women who underwent a vaginal birth that resulted in an anal sphincter injury at five Ohio hospitals affiliated with Cleveland Clinic from 2013 to 2015.
Of those who met eligibility criteria, 209 (72.8%) were White, 262 (91.3%) were non-Hispanic, and 249 (86.8%) were aged 20-34 years. Most had an epidural (92%), did not require a blood transfusion (97.9%), did not develop a vaginal hematoma (98.9%), and did not have their injury repaired in an operating room (97.2%), the researchers reported.
Among pelvic floor disorders, urinary incontinence was not reported in 96% of patients, fecal incontinence was not reported in 97.1%, and pelvic organ prolapse was not reported in 99.3%. Most had no recorded complications from their lacerations (87.8%) or postpartum depression (92%), the researchers found.
However, a 2015 study found that, 12 weeks after delivery, women with OASIs commonly reported symptoms of incontinence, with 26% reporting urinary stress incontinence, 21.4% urinary urgency incontinence, 59% anal incontinence, and 15% fecal incontinence.
Depression was also seldom identified despite higher risk of mood disorders among women with OASI, the researchers found.
The team also examined interpregnancy intervals, defined as the time between a woman’s first vaginal delivery and conception of a subsequent pregnancy. Of 178 women for whom data were available, the median interval was 26.4 months (95% confidence interval, 23.7-29.9), similar to the median for births nationally.
Lead researcher Alexandra Nutaitis, DO, a resident in obstetrics and gynecology at Cleveland Clinic Akron General, said in an interview that it’s unclear whether physicians did not inquire about symptoms or didn’t record them. She noted that anal sphincter injuries are a “stigmatized topic.”
Not asked, not told
Carolyn Swenson, MD, an associate professor in urogynecology at the University of Utah, Salt Lake City, said physicians in the study may have relied on patients to bring up their symptoms rather than using questionnaires to screen for problems.
“What we know is that if you don’t ask women about pelvic floor disorders, they often don’t tell you that they are experiencing symptoms,” said Dr. Swenson, who was not involved in the new research.
Dr. Swenson called for validated questionnaires to assess pelvic floor symptoms in postpartum patients.
Regarding interpregnancy intervals, Dr. Nutaitis said she would be surprised if women who experienced an OASI didn’t delay having another child longer than women who did not undergo that physical and psychological trauma – but other factors such as societal pressures may override any reluctance to proceed with another pregnancy.
Dr. Swenson said it’s possible that a subgroup of women who have severe complications, such as those with a fourth-degree tear, might put off having another child. However, more research is needed to find out, she said.
Dr. Nutaitis and Dr. Swenson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Postpartum complications may go unrecognized in women who incur anal sphincter injuries during childbirth, a review of electronic medical records at one academic health system suggests.
In the first 3 months after delivery, few patients with an obstetric anal sphincter injury (OASI) had documented pelvic floor problems, compared with higher rates documented in medical literature, the researchers found.
“Lack of identified pelvic floor dysfunction in this population differs from the incidence in previously published data and may reflect lack of identification by obstetric providers,” the researchers reported. The findings “highlight a gap in health care that, when addressed, could significantly improve postpartum quality of life.”
The findings are scheduled to be presented at the annual scientific meeting of the American Urogynecologic Society and International Urogynecological Association.
Anal sphincter injuries occur in about 4.4% of vaginal deliveries and are the most common cause of anal incontinence in women of reproductive age.
For the new study, researchers reviewed records of 287 women who underwent a vaginal birth that resulted in an anal sphincter injury at five Ohio hospitals affiliated with Cleveland Clinic from 2013 to 2015.
Of those who met eligibility criteria, 209 (72.8%) were White, 262 (91.3%) were non-Hispanic, and 249 (86.8%) were aged 20-34 years. Most had an epidural (92%), did not require a blood transfusion (97.9%), did not develop a vaginal hematoma (98.9%), and did not have their injury repaired in an operating room (97.2%), the researchers reported.
Among pelvic floor disorders, urinary incontinence was not reported in 96% of patients, fecal incontinence was not reported in 97.1%, and pelvic organ prolapse was not reported in 99.3%. Most had no recorded complications from their lacerations (87.8%) or postpartum depression (92%), the researchers found.
However, a 2015 study found that, 12 weeks after delivery, women with OASIs commonly reported symptoms of incontinence, with 26% reporting urinary stress incontinence, 21.4% urinary urgency incontinence, 59% anal incontinence, and 15% fecal incontinence.
Depression was also seldom identified despite higher risk of mood disorders among women with OASI, the researchers found.
The team also examined interpregnancy intervals, defined as the time between a woman’s first vaginal delivery and conception of a subsequent pregnancy. Of 178 women for whom data were available, the median interval was 26.4 months (95% confidence interval, 23.7-29.9), similar to the median for births nationally.
Lead researcher Alexandra Nutaitis, DO, a resident in obstetrics and gynecology at Cleveland Clinic Akron General, said in an interview that it’s unclear whether physicians did not inquire about symptoms or didn’t record them. She noted that anal sphincter injuries are a “stigmatized topic.”
Not asked, not told
Carolyn Swenson, MD, an associate professor in urogynecology at the University of Utah, Salt Lake City, said physicians in the study may have relied on patients to bring up their symptoms rather than using questionnaires to screen for problems.
“What we know is that if you don’t ask women about pelvic floor disorders, they often don’t tell you that they are experiencing symptoms,” said Dr. Swenson, who was not involved in the new research.
Dr. Swenson called for validated questionnaires to assess pelvic floor symptoms in postpartum patients.
Regarding interpregnancy intervals, Dr. Nutaitis said she would be surprised if women who experienced an OASI didn’t delay having another child longer than women who did not undergo that physical and psychological trauma – but other factors such as societal pressures may override any reluctance to proceed with another pregnancy.
Dr. Swenson said it’s possible that a subgroup of women who have severe complications, such as those with a fourth-degree tear, might put off having another child. However, more research is needed to find out, she said.
Dr. Nutaitis and Dr. Swenson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Postpartum complications may go unrecognized in women who incur anal sphincter injuries during childbirth, a review of electronic medical records at one academic health system suggests.
In the first 3 months after delivery, few patients with an obstetric anal sphincter injury (OASI) had documented pelvic floor problems, compared with higher rates documented in medical literature, the researchers found.
“Lack of identified pelvic floor dysfunction in this population differs from the incidence in previously published data and may reflect lack of identification by obstetric providers,” the researchers reported. The findings “highlight a gap in health care that, when addressed, could significantly improve postpartum quality of life.”
The findings are scheduled to be presented at the annual scientific meeting of the American Urogynecologic Society and International Urogynecological Association.
Anal sphincter injuries occur in about 4.4% of vaginal deliveries and are the most common cause of anal incontinence in women of reproductive age.
For the new study, researchers reviewed records of 287 women who underwent a vaginal birth that resulted in an anal sphincter injury at five Ohio hospitals affiliated with Cleveland Clinic from 2013 to 2015.
Of those who met eligibility criteria, 209 (72.8%) were White, 262 (91.3%) were non-Hispanic, and 249 (86.8%) were aged 20-34 years. Most had an epidural (92%), did not require a blood transfusion (97.9%), did not develop a vaginal hematoma (98.9%), and did not have their injury repaired in an operating room (97.2%), the researchers reported.
Among pelvic floor disorders, urinary incontinence was not reported in 96% of patients, fecal incontinence was not reported in 97.1%, and pelvic organ prolapse was not reported in 99.3%. Most had no recorded complications from their lacerations (87.8%) or postpartum depression (92%), the researchers found.
However, a 2015 study found that, 12 weeks after delivery, women with OASIs commonly reported symptoms of incontinence, with 26% reporting urinary stress incontinence, 21.4% urinary urgency incontinence, 59% anal incontinence, and 15% fecal incontinence.
Depression was also seldom identified despite higher risk of mood disorders among women with OASI, the researchers found.
The team also examined interpregnancy intervals, defined as the time between a woman’s first vaginal delivery and conception of a subsequent pregnancy. Of 178 women for whom data were available, the median interval was 26.4 months (95% confidence interval, 23.7-29.9), similar to the median for births nationally.
Lead researcher Alexandra Nutaitis, DO, a resident in obstetrics and gynecology at Cleveland Clinic Akron General, said in an interview that it’s unclear whether physicians did not inquire about symptoms or didn’t record them. She noted that anal sphincter injuries are a “stigmatized topic.”
Not asked, not told
Carolyn Swenson, MD, an associate professor in urogynecology at the University of Utah, Salt Lake City, said physicians in the study may have relied on patients to bring up their symptoms rather than using questionnaires to screen for problems.
“What we know is that if you don’t ask women about pelvic floor disorders, they often don’t tell you that they are experiencing symptoms,” said Dr. Swenson, who was not involved in the new research.
Dr. Swenson called for validated questionnaires to assess pelvic floor symptoms in postpartum patients.
Regarding interpregnancy intervals, Dr. Nutaitis said she would be surprised if women who experienced an OASI didn’t delay having another child longer than women who did not undergo that physical and psychological trauma – but other factors such as societal pressures may override any reluctance to proceed with another pregnancy.
Dr. Swenson said it’s possible that a subgroup of women who have severe complications, such as those with a fourth-degree tear, might put off having another child. However, more research is needed to find out, she said.
Dr. Nutaitis and Dr. Swenson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AUGS 2022
About one in five U.S. pregnancies ended in abortion in 2020: Report
The number and rate of U.S. abortions increased between 2017 and 2020 after a 30-year decline, according to a new report from the Guttmacher Institute.
More than 930,000 abortions took place in the United States in 2020, up 8% from 862,000 abortions in 2017. About one in five pregnancies ended in abortion in 2020, the report said.
The Guttmacher Institute, a research organization that supports abortion rights, said the trend shows a rising need for abortion care as the Supreme Court is poised to overturn the Roe v. Wade decision in coming weeks.
National abortion numbers reached the lowest point in 2017 since the landmark 1973 ruling that legalized the procedure. In the years following the ruling, abortion numbers rose above 1.5 million annually throughout the 1980s and then began declining in the 1990s, though they remained above 1 million annually through the early 2010s.
The latest data show that the abortion rate increased from 13.5 abortions per 1,000 women between ages 15 and 44 in 2017 to 14.4 abortions per 1,000 women in 2020, marking a 7% increase.
Similarly, the abortion ratio – or the number of abortions per 100 pregnancies – increased from 18.4% in 2017 to 20.6% in 2020, marking a 12% increase.
The increase in abortions was accompanied by a 6% decline in births between 2017 and 2020, the Guttmacher Institute said.
“Because there were many more births (3.6 million) than abortions (930,000) in 2020, these patterns mean that fewer people were getting pregnant and, among those who did, a larger proportion chose to have an abortion,” the institute wrote.
Medication-related abortions accounted for 54% of U.S. abortions in 2020, according to the report, which was the first time they made up more than half of abortions.
The number of abortions increased in every region of the country between 2017 and 2020, the report shows. The increases were largest in the West (12%) and Midwest (10%), followed by 8% in the South and 2% in the Northeast.
In some states – Illinois, Mississippi, and Oklahoma – there were substantial increases in the number of abortions, the institute said. In others – such as Missouri, Oregon, and North Dakota – there were substantially fewer abortions in 2020, compared with 2017.
The COVID-19 pandemic may have led to a decline in some states. In New York, abortions increased 5% from 2017 to 2019 and then fell 6% between 2019 and 2020. About 10% of clinics in New York paused or stopped abortion care in 2020 when the pandemic started.
New laws likely affected the numbers as well. Texas had a 7% increase between 2017 and 2019, followed by a 2% decrease between 2019 and 2020, which overlapped with restrictions that deemed abortions “nonessential” health care at the beginning of the pandemic.
In contrast, some numbers may have increased because of expanded Medicaid funding. Illinois began allowing state Medicaid funds to pay for abortions in January 2018, and abortions increased 25% between 2017 and 2020.
In Missouri, abortions decreased substantially from 4,710 in 2017 to 170 in 2020, the report shows, but the number of Missouri residents who traveled to Illinois for abortions increased to more than 6,500.
Every 3 years, the Guttmacher Institute contacts U.S. facilities that provide abortions to collect information about services, including the total number of abortions. The most recent count was completed in May, representing 1,687 health care facilities that provided abortions in 2019 or 2020. A full summary of the data will be published later this year in a peer-reviewed journal article.
A version of this article first appeared on WebMD.com.
The number and rate of U.S. abortions increased between 2017 and 2020 after a 30-year decline, according to a new report from the Guttmacher Institute.
More than 930,000 abortions took place in the United States in 2020, up 8% from 862,000 abortions in 2017. About one in five pregnancies ended in abortion in 2020, the report said.
The Guttmacher Institute, a research organization that supports abortion rights, said the trend shows a rising need for abortion care as the Supreme Court is poised to overturn the Roe v. Wade decision in coming weeks.
National abortion numbers reached the lowest point in 2017 since the landmark 1973 ruling that legalized the procedure. In the years following the ruling, abortion numbers rose above 1.5 million annually throughout the 1980s and then began declining in the 1990s, though they remained above 1 million annually through the early 2010s.
The latest data show that the abortion rate increased from 13.5 abortions per 1,000 women between ages 15 and 44 in 2017 to 14.4 abortions per 1,000 women in 2020, marking a 7% increase.
Similarly, the abortion ratio – or the number of abortions per 100 pregnancies – increased from 18.4% in 2017 to 20.6% in 2020, marking a 12% increase.
The increase in abortions was accompanied by a 6% decline in births between 2017 and 2020, the Guttmacher Institute said.
“Because there were many more births (3.6 million) than abortions (930,000) in 2020, these patterns mean that fewer people were getting pregnant and, among those who did, a larger proportion chose to have an abortion,” the institute wrote.
Medication-related abortions accounted for 54% of U.S. abortions in 2020, according to the report, which was the first time they made up more than half of abortions.
The number of abortions increased in every region of the country between 2017 and 2020, the report shows. The increases were largest in the West (12%) and Midwest (10%), followed by 8% in the South and 2% in the Northeast.
In some states – Illinois, Mississippi, and Oklahoma – there were substantial increases in the number of abortions, the institute said. In others – such as Missouri, Oregon, and North Dakota – there were substantially fewer abortions in 2020, compared with 2017.
The COVID-19 pandemic may have led to a decline in some states. In New York, abortions increased 5% from 2017 to 2019 and then fell 6% between 2019 and 2020. About 10% of clinics in New York paused or stopped abortion care in 2020 when the pandemic started.
New laws likely affected the numbers as well. Texas had a 7% increase between 2017 and 2019, followed by a 2% decrease between 2019 and 2020, which overlapped with restrictions that deemed abortions “nonessential” health care at the beginning of the pandemic.
In contrast, some numbers may have increased because of expanded Medicaid funding. Illinois began allowing state Medicaid funds to pay for abortions in January 2018, and abortions increased 25% between 2017 and 2020.
In Missouri, abortions decreased substantially from 4,710 in 2017 to 170 in 2020, the report shows, but the number of Missouri residents who traveled to Illinois for abortions increased to more than 6,500.
Every 3 years, the Guttmacher Institute contacts U.S. facilities that provide abortions to collect information about services, including the total number of abortions. The most recent count was completed in May, representing 1,687 health care facilities that provided abortions in 2019 or 2020. A full summary of the data will be published later this year in a peer-reviewed journal article.
A version of this article first appeared on WebMD.com.
The number and rate of U.S. abortions increased between 2017 and 2020 after a 30-year decline, according to a new report from the Guttmacher Institute.
More than 930,000 abortions took place in the United States in 2020, up 8% from 862,000 abortions in 2017. About one in five pregnancies ended in abortion in 2020, the report said.
The Guttmacher Institute, a research organization that supports abortion rights, said the trend shows a rising need for abortion care as the Supreme Court is poised to overturn the Roe v. Wade decision in coming weeks.
National abortion numbers reached the lowest point in 2017 since the landmark 1973 ruling that legalized the procedure. In the years following the ruling, abortion numbers rose above 1.5 million annually throughout the 1980s and then began declining in the 1990s, though they remained above 1 million annually through the early 2010s.
The latest data show that the abortion rate increased from 13.5 abortions per 1,000 women between ages 15 and 44 in 2017 to 14.4 abortions per 1,000 women in 2020, marking a 7% increase.
Similarly, the abortion ratio – or the number of abortions per 100 pregnancies – increased from 18.4% in 2017 to 20.6% in 2020, marking a 12% increase.
The increase in abortions was accompanied by a 6% decline in births between 2017 and 2020, the Guttmacher Institute said.
“Because there were many more births (3.6 million) than abortions (930,000) in 2020, these patterns mean that fewer people were getting pregnant and, among those who did, a larger proportion chose to have an abortion,” the institute wrote.
Medication-related abortions accounted for 54% of U.S. abortions in 2020, according to the report, which was the first time they made up more than half of abortions.
The number of abortions increased in every region of the country between 2017 and 2020, the report shows. The increases were largest in the West (12%) and Midwest (10%), followed by 8% in the South and 2% in the Northeast.
In some states – Illinois, Mississippi, and Oklahoma – there were substantial increases in the number of abortions, the institute said. In others – such as Missouri, Oregon, and North Dakota – there were substantially fewer abortions in 2020, compared with 2017.
The COVID-19 pandemic may have led to a decline in some states. In New York, abortions increased 5% from 2017 to 2019 and then fell 6% between 2019 and 2020. About 10% of clinics in New York paused or stopped abortion care in 2020 when the pandemic started.
New laws likely affected the numbers as well. Texas had a 7% increase between 2017 and 2019, followed by a 2% decrease between 2019 and 2020, which overlapped with restrictions that deemed abortions “nonessential” health care at the beginning of the pandemic.
In contrast, some numbers may have increased because of expanded Medicaid funding. Illinois began allowing state Medicaid funds to pay for abortions in January 2018, and abortions increased 25% between 2017 and 2020.
In Missouri, abortions decreased substantially from 4,710 in 2017 to 170 in 2020, the report shows, but the number of Missouri residents who traveled to Illinois for abortions increased to more than 6,500.
Every 3 years, the Guttmacher Institute contacts U.S. facilities that provide abortions to collect information about services, including the total number of abortions. The most recent count was completed in May, representing 1,687 health care facilities that provided abortions in 2019 or 2020. A full summary of the data will be published later this year in a peer-reviewed journal article.
A version of this article first appeared on WebMD.com.
MS and family planning: Bring it up at every office visit
NATIONAL HARBOR, MD – Just 2 days before she spoke in a presentation at the annual meeting of the Consortium of Multiple Sclerosis Centers, University of Colorado neurologist Anna Shah, MD, asked a 26-year-old patient with MS about whether she planned to have children. Absolutely not, the young woman replied. “I read online that I can give birth to a baby with MS, which is crazy.”
The patient didn’t understand the risk of having a child with MS – it’s thought to be 2%-5% if one parent has the condition – but she wouldn’t have learned the facts if Dr. Shah hadn’t asked the right questions. “It’s really important for us as a community to know how to be proactive with discussions [about pregnancy],” she said.
As she noted, an estimated 75% of patients with MS are women, most are diagnosed during prime child-bearing years, and many pregnancies in general – an estimated half – are not planned. And while a higher percentage of women with MS are having children than in the past, she said, misinformation remains common. In fact, physicians can be part of the problem.
Dr. Shaw highlighted a 2019 Italian survey that found that 16% of 395 people with MS reported that they were discouraged from having children, mainly by medical professionals, after their diagnosis. Seven percent said they never wanted to become parents because of their MS. A 2021 survey of 332 patients with MS in the United States, United Kingdom, France, Germany, Italy, and Spain, found that 56% reported that MS played a role in their decisions about family planning, and 14% of those decided not to have children.
In regard to women of child-bearing age, Dr. Shah recommends that Open-ended, individualized questions are key. “We don’t know what patients don’t share with us,” she said.
Make sure to consider the timing of any plans to have children, she said. If the patient wants to have children within a year, talk about matters such as whether disease activity is well-controlled (6-12 months of good control is ideal) and whether current disease-modifying therapies are safe. Make sure to get a baseline prepartum MRI scan, she said.
If the patients don’t want to have children, make sure they are using a reliable strategy to avoid conception. Be aware that modafinil – “not one that immediately comes to mind” – may decrease the efficacy of oral contraceptives, she said, as can anticonvulsants (phenytoin, carbamazepine, oxcarbazepine, topiramate, and primidone). Oral contraceptives, meanwhile, may decrease levels of lamotrigine.
What if a patient has trouble conceiving? There are some hints in research that MS may boost the risk of infertility in women, Dr. Shah said. That’s why she recommends that colleagues consider referring a patient to an infertility specialist after attempting conception for 6 months as opposed to the general recommendation for 12 months.
Dr. Shah disclosed advisory board service (Genentech) and development of nonbranded educational programming through Novartis and the National Committee for Quality Assurance.
NATIONAL HARBOR, MD – Just 2 days before she spoke in a presentation at the annual meeting of the Consortium of Multiple Sclerosis Centers, University of Colorado neurologist Anna Shah, MD, asked a 26-year-old patient with MS about whether she planned to have children. Absolutely not, the young woman replied. “I read online that I can give birth to a baby with MS, which is crazy.”
The patient didn’t understand the risk of having a child with MS – it’s thought to be 2%-5% if one parent has the condition – but she wouldn’t have learned the facts if Dr. Shah hadn’t asked the right questions. “It’s really important for us as a community to know how to be proactive with discussions [about pregnancy],” she said.
As she noted, an estimated 75% of patients with MS are women, most are diagnosed during prime child-bearing years, and many pregnancies in general – an estimated half – are not planned. And while a higher percentage of women with MS are having children than in the past, she said, misinformation remains common. In fact, physicians can be part of the problem.
Dr. Shaw highlighted a 2019 Italian survey that found that 16% of 395 people with MS reported that they were discouraged from having children, mainly by medical professionals, after their diagnosis. Seven percent said they never wanted to become parents because of their MS. A 2021 survey of 332 patients with MS in the United States, United Kingdom, France, Germany, Italy, and Spain, found that 56% reported that MS played a role in their decisions about family planning, and 14% of those decided not to have children.
In regard to women of child-bearing age, Dr. Shah recommends that Open-ended, individualized questions are key. “We don’t know what patients don’t share with us,” she said.
Make sure to consider the timing of any plans to have children, she said. If the patient wants to have children within a year, talk about matters such as whether disease activity is well-controlled (6-12 months of good control is ideal) and whether current disease-modifying therapies are safe. Make sure to get a baseline prepartum MRI scan, she said.
If the patients don’t want to have children, make sure they are using a reliable strategy to avoid conception. Be aware that modafinil – “not one that immediately comes to mind” – may decrease the efficacy of oral contraceptives, she said, as can anticonvulsants (phenytoin, carbamazepine, oxcarbazepine, topiramate, and primidone). Oral contraceptives, meanwhile, may decrease levels of lamotrigine.
What if a patient has trouble conceiving? There are some hints in research that MS may boost the risk of infertility in women, Dr. Shah said. That’s why she recommends that colleagues consider referring a patient to an infertility specialist after attempting conception for 6 months as opposed to the general recommendation for 12 months.
Dr. Shah disclosed advisory board service (Genentech) and development of nonbranded educational programming through Novartis and the National Committee for Quality Assurance.
NATIONAL HARBOR, MD – Just 2 days before she spoke in a presentation at the annual meeting of the Consortium of Multiple Sclerosis Centers, University of Colorado neurologist Anna Shah, MD, asked a 26-year-old patient with MS about whether she planned to have children. Absolutely not, the young woman replied. “I read online that I can give birth to a baby with MS, which is crazy.”
The patient didn’t understand the risk of having a child with MS – it’s thought to be 2%-5% if one parent has the condition – but she wouldn’t have learned the facts if Dr. Shah hadn’t asked the right questions. “It’s really important for us as a community to know how to be proactive with discussions [about pregnancy],” she said.
As she noted, an estimated 75% of patients with MS are women, most are diagnosed during prime child-bearing years, and many pregnancies in general – an estimated half – are not planned. And while a higher percentage of women with MS are having children than in the past, she said, misinformation remains common. In fact, physicians can be part of the problem.
Dr. Shaw highlighted a 2019 Italian survey that found that 16% of 395 people with MS reported that they were discouraged from having children, mainly by medical professionals, after their diagnosis. Seven percent said they never wanted to become parents because of their MS. A 2021 survey of 332 patients with MS in the United States, United Kingdom, France, Germany, Italy, and Spain, found that 56% reported that MS played a role in their decisions about family planning, and 14% of those decided not to have children.
In regard to women of child-bearing age, Dr. Shah recommends that Open-ended, individualized questions are key. “We don’t know what patients don’t share with us,” she said.
Make sure to consider the timing of any plans to have children, she said. If the patient wants to have children within a year, talk about matters such as whether disease activity is well-controlled (6-12 months of good control is ideal) and whether current disease-modifying therapies are safe. Make sure to get a baseline prepartum MRI scan, she said.
If the patients don’t want to have children, make sure they are using a reliable strategy to avoid conception. Be aware that modafinil – “not one that immediately comes to mind” – may decrease the efficacy of oral contraceptives, she said, as can anticonvulsants (phenytoin, carbamazepine, oxcarbazepine, topiramate, and primidone). Oral contraceptives, meanwhile, may decrease levels of lamotrigine.
What if a patient has trouble conceiving? There are some hints in research that MS may boost the risk of infertility in women, Dr. Shah said. That’s why she recommends that colleagues consider referring a patient to an infertility specialist after attempting conception for 6 months as opposed to the general recommendation for 12 months.
Dr. Shah disclosed advisory board service (Genentech) and development of nonbranded educational programming through Novartis and the National Committee for Quality Assurance.
At CMSC 2022
2022 Update: Beyond prenatal exome sequencing
Last year, our Update focused on the expansion of sequencing in prenatal diagnosis. This year, we are taking a step sideways to remember the many diagnoses we may miss if we rely on exome sequencing alone. A recent case report in Prenatal Diagnosis describes a pregnancy affected by fetal akinesia sequence and polyhydramnios in which sequencing did not reveal a diagnosis. Expansion of the differential to include congenital myotonic dystrophy and subsequent triplet repeat testing led the clinicians to the diagnosis and identification of a triplet repeat expansion in the DMPK gene. This case serves as our first example of how complementary testing and technologies should continue to help us make critical diagnoses.
What is the yield of exome sequencing vs panels in nonimmune hydrops?
Rogers R, Moyer K, Moise KJ Jr. Congenital myotonic dystrophy: an overlooked diagnosis not amenable to detection by sequencing. Prenat Diagn. 2022;42:233-235. doi:10.1002/pd.6105.
Norton ME, Ziffle JV, Lianoglou BR, et al. Exome sequencing vs targeted gene panels for the evaluation of nonimmune hydrops fetalis. Am J Obstet Gynecol. 2021;28:S0002-9378(21)00828-0. doi:10.1016/j.ajog.2021.07.014.
We have had several illuminating discussions with our colleagues about the merits of exome sequencing (ES) versus panels and other modalities for fetal diagnosis. Many obstetricians practicing at the leading edge may feel like ES should be utilized uniformly for fetal anomalies with nondiagnostic karyotype or microarray. However, for well-defined phenotypes with clear and narrow lists of implicated genes (eg, skeletal dysplasias) or patients without insurance coverage, panel sequencing still has utility in prenatal diagnosis. The question of which phenotypes most benefit from ES versus panel sequencing is an area of interesting, ongoing research for several investigators.
Secondary analysis of nonimmune hydrops cohort
Norton and colleagues tackled one such cohort in a study presented in the American Journal of Obstetrics and Gynecology. They compared the proportion of diagnoses that would have been identified in commercial lab panels with their research of phenotype-driven ES in a cohort of 127 fetuses with features of nonimmune hydrops fetalis (NIHF). NIHF can be caused by a variety of single-gene disorders in addition to chromosomal disorders and copy number variants on chromosomal microarray. Patients were eligible for inclusion in the cohort if they had a nondiagnostic karyotype or microarray and any of the following features: nuchal translucency of 3.5 mm or greater, cystic hygroma, pleural effusion, pericardial effusion, ascites, or skin edema. Standard sequencing methods and variant analysis were performed. They assumed 100% analytical sensitivity and specificity of the panels for variant detection and collected cost information on the targeted gene panels.
Study outcomes
In the ES analysis of cases, 37 of 127 cases (29%) had a pathogenic or likely pathogenic variant in 1 of 29 genes, and another 12 of 127 cases (9%) had variants of uncertain significance that were strongly suspected to be the etiology during clinical analysis. The types of disorders that were identified are listed in the TABLE. In addition to a feature of NIHF, 50% of the cases had a structural anomaly.
There were 10 identified clinical panels from 7 clinical laboratories. These panels ranged in size from 11 to 128 genes. The highest simulated yield of any commercial panel was only 62% of the pathogenic variants identified by ES. The other commercial laboratory panels detection yield ranged from 11% to 62% of pathogenic variants detected by ES. For overall yield, the largest panel would have a diagnostic yield of 18% of diagnoses relative to the 29% diagnostic yield from ES.
The largest panel included 128 genes prior to the publication of the original cohort and was updated after publication to include 148 genes. The larger updated panel would have identified all of the patients in the ES cohort. However, many of the other panels listed would have identified a smaller fraction of the variants identified by ES (range, 11%-62%). At the time of publication, the cost of the panels ranged from $640 to $3,500, and the cost of prenatal ES ranged from $2,458 to $7,500.
Continue to: Strengths and limitations...
Strengths and limitations
Twenty-three percent of the patients who were sequenced had an increased fetal nuchal translucency or cystic hygroma, and another 17% had a single fetal effusion. This inclusivity makes this study more applicable to broader fetal anomaly populations. However, it is worth noting that only 61% of patients had NIHF by the definition of 2 or more fluid collections or skin thickening.
The authors assumed 100% sensitivity and specificity for the panel tests relative to diagnostic ES results, but this may not reflect real-life analysis. There is inherent subjectivity and subsequent differences in variant calling (deciding which genetic changes are pathogenic) between institutions and companies despite efforts to standardize this process. Due to the simulated nature of this study, these differences are not captured. Additionally, although the authors note that the research ES had at least 30 times the coverage (an adequate number of sequence reads for accurate testing) than did the commercial lab panels, some gene panels have additional sequencing of intronic regions, copy number analysis, and up to 10 times more coverage than ES, which could lead to more diagnoses.
This study illustrates that there is nuance involved in selecting which type of gene sequencing and which clinical laboratory to use for prenatal diagnosis. Labs with more updated literature searches and more inclusive gene panels may be excellent options for patients in whom ES is not covered by insurance or with phenotypes with a narrow range of suspected causative genes. However, there is a lag time in updating the genes offered on each panel, and new genedisease associations will not be captured by existing panels.
From a cost, speed-of-analysis, and depth-of-sequencing perspective, panel sequencing can have advantages that should be considered in some patients, particularly if the panels are large and regularly updated. However, the authors summarize our sentiments and their findings with the following:
“For disorders, such as NIHF with marked genetic heterogeneity and less clear in utero phenotypes of underlying genetic diseases, the broader coverage of exome sequencing makes it a superior option to targeted panel testing.”
We look forward to the publication of further anomaly-specific cohorts and secondary analyses of the utility of current panels and ES that may follow.
Frequency of Beckwith-Widemann syndrome in prenatally diagnosed omphaloceles
Abbasi N, Moore A, Chiu P, et al. Prenatally diagnosed omphaloceles: report of 92 cases and association with Beckwith-Wiedemann syndrome. Prenat Diagn. 2021;41:798-816. doi:10.1002/pd.5930.
An omphalocele is diagnosed prenatally on ultrasound when an anterior midline mass, often containing abdominal contents, is seen herniating into the base of the umbilical cord. Omphaloceles are often associated with additional structural abnormalities and underlying genetic syndromes, thus a thorough fetal assessment is required for accurate prenatal counseling and neonatal care.
Identification of Beckwith-Widemann syndrome (BWS) in the setting of a prenatally diagnosed omphalocele is difficult because of its wide range of clinical features and its unique genetic basis. Unlike many genetic disorders that are caused by specific genetic variants, or spelling changes in the genes, BWS results from a change in the expression of one or more of the genes in a specific region of chromosome 11. A high index of clinical suspicion as well as an understanding of the various genetic and epigenetics alterations that cause BWS is required for prenatal diagnosis.
Retrospective cohort at a single center
The authors in this study reviewed all pregnancies in which an omphalocele was diagnosed prenatally at a single center between 2010 and 2015. They describe a standard prenatal evaluation following identification of an omphalocele including echocardiogram, detailed anatomic survey, and availability of an amniocentesis to facilitate aneuploidy screening and testing for BWS. This review also includes an overview of perinatal and long-term outcomes for cases of BWS diagnosed at their center between 2000 and 2015.
Study outcomes
Results of prenatal genetic testing in this cohort were divided between cases of an isolated omphalocele (without other structural changes) and cases of nonisolated omphaloceles. In the group of pregnancies with an isolated omphalocele, 2 of 27 pregnancies (7.4%) were found to have an abnormal karyotype, and 6 of 16 of the remaining pregnancies (37.5%) were diagnosed with BWS. Among the group of pregnancies with a nonisolated omphalocele, 23 of 59 pregnancies (39%) were found to have an abnormal karyotype, and 1 of 20 pregnancies (5%) were diagnosed with BWS.
Prenatal sonographic features associated with cases of BWS included polyhydramnios in 12 of 19 cases (63%) and macrosomia in 8 of 19 cases (42%). Macroglossia is another characteristic feature of the disorder, which was identified in 4 of 19 cases (21%) prenatally and in an additional 10 of 19 cases (52.6%) postnatally. Interestingly, only 1 of the cases of BWS was caused by a microdeletion at 11p15.4—a change that was identified on microarray. The additional 6 cases of BWS were caused by imprinting changes in the region, which are only detectable with a specific methylation-analysis technique.
Among the 19 cases of BWS identified over a 15-year period, there was 1 intrauterine demise. Preterm birth occurred in 10 of 19 cases (52.6%), including 8 of 19 cases (42.1%) of spontaneous preterm labor. Respiratory distress (27.8%), hypoglycemia (61%), and gastrointestinal reflux (59%) were common neonatal complications. Embryonal tumors were diagnosed in 2 of 16 patients (12.5%). Although neurodevelopmental outcomes were incomplete, their data suggested normal development in 75% of children. There were 2 neonatal deaths in this cohort and 1 childhood death at age 2 years.
Study strengths and limitations
As with many studies investigating a rare disorder, this study is limited by its retrospective nature and small sample size. Nevertheless, it adds an important cohort of patients with a prenatally diagnosed omphalocele to the literature and illuminates the utility of a standardized approach to testing for BWS in this population.
In this cohort with prenatally diagnosed omphaloceles with standardized testing for BWS, the prevalence of the disorder was approximately 8% and more common in cases of an isolated omphalocele. The most common supporting sonographic features of BWS may not be detected until later in gestation, including polyhydramnios and macrosomia. This demonstrates the importance of both sonographic follow-up as well as universal testing for BWS in euploid cases of a prenatally diagnosed omphalocele. Almost all cases of BWS in this cohort required specialized molecular techniques for diagnosis, and the diagnosis would have been missed on karyotype, microarray, and ES.
Continue to: Genetic diagnoses that could have been identified by expanded carrier screening...
Genetic diagnoses that could have been identified by expanded carrier screening
Stevens BK, Nunley PB, Wagner C, et al. Utility of expanded carrier screening in pregnancies with ultrasound abnormalities. Prenat Diagn. 2022;42:60-78. doi:10.1002/pd.6069.
This series is a thorough retrospective review of patients evaluated in a pediatric genetics clinic from 2014 through 2017. Patients were included if they were evaluated in the first 6 months of life and had a structural abnormality that might be detected on prenatal ultrasonography. The genetic testing results were analyzed and categorized according to types of genetic disorders, with the goal of identifying how many patients might have been identified by expanded carrier screening (ECS) panels.
Study outcomes
A total of 931 charts were reviewed, and 85% (791 of 931) of patients evaluated in the first 6 months of life were determined to have a structural anomaly that might be detected on prenatal ultrasonography. Of those patients, 691 went on to have genetic testing and 32.1% (222 of 691) of them had a diagnostic (pathogenic) genetic testing result related to the phenotype. The types of diagnostic testing results are shown in the FIGURE. Notably, 42 single-gene disorders were detected.
FIGURE Diagnostic test result in pediatric patients evaluated under age 6 months
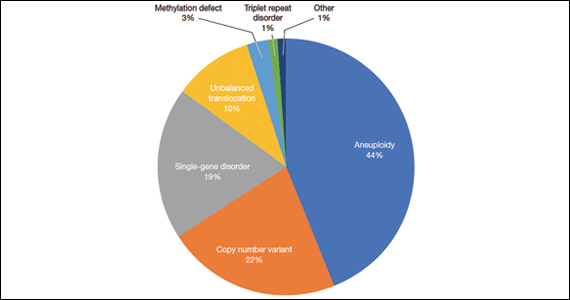
Of those 222 patients with diagnostic results, there were 8 patients with autosomal recessive and X-linked conditions that could be detected using a 500-gene ECS panel. Five patients could be detected with a 271-gene panel. After nondiagnostic microarray, 11.3% of patients had a condition that could be detected by using a 500-gene ECS panel. The identified conditions included cystic fibrosis, CYP21‐related congenital adrenal hyperplasia, autosomal recessive polycystic kidney disease, Antley‐Bixler syndrome, and Morquio syndrome type A.
Furthermore, the authors conducted a literature review of 271 conditions and found that 32% (88 of 271) of conditions may be associated with ultrasound findings.
Study strengths and limitations
When applying these data to prenatal populations, the authors acknowledge several notable limitations. There is a selection bias toward less-severe phenotypes for many patients choosing to continue rather than to interrupt a pregnancy. Additionally, only 23% of the patients in the study had a microarray and ES, which may lead to an underrepresentation of single-gene disorders and an underestimation of the utility of ECS. Finally, a retrospective classification of structural abnormalities that may be detectable by ultrasonography may not always reflect what is actually reported in prenatal imaging.
However, the work that the authors put forth to evaluate and categorize 931 participants by the results of genetic testing and structural anomalies is appreciated, and the level of detail is impressive for this retrospective chart review. Additionally, the tables itemizing the authors’ review of 271 ECS disorders that may have ultrasonography findings categorized by disorder and system are helpful and quick diagnostic references for clinicians providing prenatal care. ●
This study of potentially detectable prenatal findings from the lens of a pediatric genetics clinic lends an interesting perspective: Exome sequencing is not the primary route to establish a diagnosis; karyotype, microarray, methylation disorders, and triplet repeat disorders all have an established role in the diagnostic toolkit. Keeping in mind the contribution of these modalities to pediatric testing may shorten the diagnostic odyssey to continue pregnancies or help to fully counsel patients on expectations and decision-making after birth.
Carrier screening is not a substitute for diagnostic testing in pregnancy. However, in appropriately selected patients, a broad carrier screening panel may have added utility. ECS can be conducted while awaiting microarray results to help target testing and may be particularly useful for patients who decline diagnostic testing until the postnatal period. It is important to counsel patients that carrier screening is not a diagnostic test, and results will only report likely pathogenic or pathogenic variants, not variants of uncertain significance that may be of clinical relevance. However, our practice has had several insightful diagnoses reached through ECS, in conjunction with microarray testing that allowed for faster and more targeted sequencing and precise fetal diagnosis. This readily available molecular tool (often covered by insurance) deserves a spot in your fetal diagnosis tool belt based on available evidence.
Last year, our Update focused on the expansion of sequencing in prenatal diagnosis. This year, we are taking a step sideways to remember the many diagnoses we may miss if we rely on exome sequencing alone. A recent case report in Prenatal Diagnosis describes a pregnancy affected by fetal akinesia sequence and polyhydramnios in which sequencing did not reveal a diagnosis. Expansion of the differential to include congenital myotonic dystrophy and subsequent triplet repeat testing led the clinicians to the diagnosis and identification of a triplet repeat expansion in the DMPK gene. This case serves as our first example of how complementary testing and technologies should continue to help us make critical diagnoses.
What is the yield of exome sequencing vs panels in nonimmune hydrops?
Rogers R, Moyer K, Moise KJ Jr. Congenital myotonic dystrophy: an overlooked diagnosis not amenable to detection by sequencing. Prenat Diagn. 2022;42:233-235. doi:10.1002/pd.6105.
Norton ME, Ziffle JV, Lianoglou BR, et al. Exome sequencing vs targeted gene panels for the evaluation of nonimmune hydrops fetalis. Am J Obstet Gynecol. 2021;28:S0002-9378(21)00828-0. doi:10.1016/j.ajog.2021.07.014.
We have had several illuminating discussions with our colleagues about the merits of exome sequencing (ES) versus panels and other modalities for fetal diagnosis. Many obstetricians practicing at the leading edge may feel like ES should be utilized uniformly for fetal anomalies with nondiagnostic karyotype or microarray. However, for well-defined phenotypes with clear and narrow lists of implicated genes (eg, skeletal dysplasias) or patients without insurance coverage, panel sequencing still has utility in prenatal diagnosis. The question of which phenotypes most benefit from ES versus panel sequencing is an area of interesting, ongoing research for several investigators.
Secondary analysis of nonimmune hydrops cohort
Norton and colleagues tackled one such cohort in a study presented in the American Journal of Obstetrics and Gynecology. They compared the proportion of diagnoses that would have been identified in commercial lab panels with their research of phenotype-driven ES in a cohort of 127 fetuses with features of nonimmune hydrops fetalis (NIHF). NIHF can be caused by a variety of single-gene disorders in addition to chromosomal disorders and copy number variants on chromosomal microarray. Patients were eligible for inclusion in the cohort if they had a nondiagnostic karyotype or microarray and any of the following features: nuchal translucency of 3.5 mm or greater, cystic hygroma, pleural effusion, pericardial effusion, ascites, or skin edema. Standard sequencing methods and variant analysis were performed. They assumed 100% analytical sensitivity and specificity of the panels for variant detection and collected cost information on the targeted gene panels.
Study outcomes
In the ES analysis of cases, 37 of 127 cases (29%) had a pathogenic or likely pathogenic variant in 1 of 29 genes, and another 12 of 127 cases (9%) had variants of uncertain significance that were strongly suspected to be the etiology during clinical analysis. The types of disorders that were identified are listed in the TABLE. In addition to a feature of NIHF, 50% of the cases had a structural anomaly.
There were 10 identified clinical panels from 7 clinical laboratories. These panels ranged in size from 11 to 128 genes. The highest simulated yield of any commercial panel was only 62% of the pathogenic variants identified by ES. The other commercial laboratory panels detection yield ranged from 11% to 62% of pathogenic variants detected by ES. For overall yield, the largest panel would have a diagnostic yield of 18% of diagnoses relative to the 29% diagnostic yield from ES.
The largest panel included 128 genes prior to the publication of the original cohort and was updated after publication to include 148 genes. The larger updated panel would have identified all of the patients in the ES cohort. However, many of the other panels listed would have identified a smaller fraction of the variants identified by ES (range, 11%-62%). At the time of publication, the cost of the panels ranged from $640 to $3,500, and the cost of prenatal ES ranged from $2,458 to $7,500.
Continue to: Strengths and limitations...
Strengths and limitations
Twenty-three percent of the patients who were sequenced had an increased fetal nuchal translucency or cystic hygroma, and another 17% had a single fetal effusion. This inclusivity makes this study more applicable to broader fetal anomaly populations. However, it is worth noting that only 61% of patients had NIHF by the definition of 2 or more fluid collections or skin thickening.
The authors assumed 100% sensitivity and specificity for the panel tests relative to diagnostic ES results, but this may not reflect real-life analysis. There is inherent subjectivity and subsequent differences in variant calling (deciding which genetic changes are pathogenic) between institutions and companies despite efforts to standardize this process. Due to the simulated nature of this study, these differences are not captured. Additionally, although the authors note that the research ES had at least 30 times the coverage (an adequate number of sequence reads for accurate testing) than did the commercial lab panels, some gene panels have additional sequencing of intronic regions, copy number analysis, and up to 10 times more coverage than ES, which could lead to more diagnoses.
This study illustrates that there is nuance involved in selecting which type of gene sequencing and which clinical laboratory to use for prenatal diagnosis. Labs with more updated literature searches and more inclusive gene panels may be excellent options for patients in whom ES is not covered by insurance or with phenotypes with a narrow range of suspected causative genes. However, there is a lag time in updating the genes offered on each panel, and new genedisease associations will not be captured by existing panels.
From a cost, speed-of-analysis, and depth-of-sequencing perspective, panel sequencing can have advantages that should be considered in some patients, particularly if the panels are large and regularly updated. However, the authors summarize our sentiments and their findings with the following:
“For disorders, such as NIHF with marked genetic heterogeneity and less clear in utero phenotypes of underlying genetic diseases, the broader coverage of exome sequencing makes it a superior option to targeted panel testing.”
We look forward to the publication of further anomaly-specific cohorts and secondary analyses of the utility of current panels and ES that may follow.
Frequency of Beckwith-Widemann syndrome in prenatally diagnosed omphaloceles
Abbasi N, Moore A, Chiu P, et al. Prenatally diagnosed omphaloceles: report of 92 cases and association with Beckwith-Wiedemann syndrome. Prenat Diagn. 2021;41:798-816. doi:10.1002/pd.5930.
An omphalocele is diagnosed prenatally on ultrasound when an anterior midline mass, often containing abdominal contents, is seen herniating into the base of the umbilical cord. Omphaloceles are often associated with additional structural abnormalities and underlying genetic syndromes, thus a thorough fetal assessment is required for accurate prenatal counseling and neonatal care.
Identification of Beckwith-Widemann syndrome (BWS) in the setting of a prenatally diagnosed omphalocele is difficult because of its wide range of clinical features and its unique genetic basis. Unlike many genetic disorders that are caused by specific genetic variants, or spelling changes in the genes, BWS results from a change in the expression of one or more of the genes in a specific region of chromosome 11. A high index of clinical suspicion as well as an understanding of the various genetic and epigenetics alterations that cause BWS is required for prenatal diagnosis.
Retrospective cohort at a single center
The authors in this study reviewed all pregnancies in which an omphalocele was diagnosed prenatally at a single center between 2010 and 2015. They describe a standard prenatal evaluation following identification of an omphalocele including echocardiogram, detailed anatomic survey, and availability of an amniocentesis to facilitate aneuploidy screening and testing for BWS. This review also includes an overview of perinatal and long-term outcomes for cases of BWS diagnosed at their center between 2000 and 2015.
Study outcomes
Results of prenatal genetic testing in this cohort were divided between cases of an isolated omphalocele (without other structural changes) and cases of nonisolated omphaloceles. In the group of pregnancies with an isolated omphalocele, 2 of 27 pregnancies (7.4%) were found to have an abnormal karyotype, and 6 of 16 of the remaining pregnancies (37.5%) were diagnosed with BWS. Among the group of pregnancies with a nonisolated omphalocele, 23 of 59 pregnancies (39%) were found to have an abnormal karyotype, and 1 of 20 pregnancies (5%) were diagnosed with BWS.
Prenatal sonographic features associated with cases of BWS included polyhydramnios in 12 of 19 cases (63%) and macrosomia in 8 of 19 cases (42%). Macroglossia is another characteristic feature of the disorder, which was identified in 4 of 19 cases (21%) prenatally and in an additional 10 of 19 cases (52.6%) postnatally. Interestingly, only 1 of the cases of BWS was caused by a microdeletion at 11p15.4—a change that was identified on microarray. The additional 6 cases of BWS were caused by imprinting changes in the region, which are only detectable with a specific methylation-analysis technique.
Among the 19 cases of BWS identified over a 15-year period, there was 1 intrauterine demise. Preterm birth occurred in 10 of 19 cases (52.6%), including 8 of 19 cases (42.1%) of spontaneous preterm labor. Respiratory distress (27.8%), hypoglycemia (61%), and gastrointestinal reflux (59%) were common neonatal complications. Embryonal tumors were diagnosed in 2 of 16 patients (12.5%). Although neurodevelopmental outcomes were incomplete, their data suggested normal development in 75% of children. There were 2 neonatal deaths in this cohort and 1 childhood death at age 2 years.
Study strengths and limitations
As with many studies investigating a rare disorder, this study is limited by its retrospective nature and small sample size. Nevertheless, it adds an important cohort of patients with a prenatally diagnosed omphalocele to the literature and illuminates the utility of a standardized approach to testing for BWS in this population.
In this cohort with prenatally diagnosed omphaloceles with standardized testing for BWS, the prevalence of the disorder was approximately 8% and more common in cases of an isolated omphalocele. The most common supporting sonographic features of BWS may not be detected until later in gestation, including polyhydramnios and macrosomia. This demonstrates the importance of both sonographic follow-up as well as universal testing for BWS in euploid cases of a prenatally diagnosed omphalocele. Almost all cases of BWS in this cohort required specialized molecular techniques for diagnosis, and the diagnosis would have been missed on karyotype, microarray, and ES.
Continue to: Genetic diagnoses that could have been identified by expanded carrier screening...
Genetic diagnoses that could have been identified by expanded carrier screening
Stevens BK, Nunley PB, Wagner C, et al. Utility of expanded carrier screening in pregnancies with ultrasound abnormalities. Prenat Diagn. 2022;42:60-78. doi:10.1002/pd.6069.
This series is a thorough retrospective review of patients evaluated in a pediatric genetics clinic from 2014 through 2017. Patients were included if they were evaluated in the first 6 months of life and had a structural abnormality that might be detected on prenatal ultrasonography. The genetic testing results were analyzed and categorized according to types of genetic disorders, with the goal of identifying how many patients might have been identified by expanded carrier screening (ECS) panels.
Study outcomes
A total of 931 charts were reviewed, and 85% (791 of 931) of patients evaluated in the first 6 months of life were determined to have a structural anomaly that might be detected on prenatal ultrasonography. Of those patients, 691 went on to have genetic testing and 32.1% (222 of 691) of them had a diagnostic (pathogenic) genetic testing result related to the phenotype. The types of diagnostic testing results are shown in the FIGURE. Notably, 42 single-gene disorders were detected.
FIGURE Diagnostic test result in pediatric patients evaluated under age 6 months

Of those 222 patients with diagnostic results, there were 8 patients with autosomal recessive and X-linked conditions that could be detected using a 500-gene ECS panel. Five patients could be detected with a 271-gene panel. After nondiagnostic microarray, 11.3% of patients had a condition that could be detected by using a 500-gene ECS panel. The identified conditions included cystic fibrosis, CYP21‐related congenital adrenal hyperplasia, autosomal recessive polycystic kidney disease, Antley‐Bixler syndrome, and Morquio syndrome type A.
Furthermore, the authors conducted a literature review of 271 conditions and found that 32% (88 of 271) of conditions may be associated with ultrasound findings.
Study strengths and limitations
When applying these data to prenatal populations, the authors acknowledge several notable limitations. There is a selection bias toward less-severe phenotypes for many patients choosing to continue rather than to interrupt a pregnancy. Additionally, only 23% of the patients in the study had a microarray and ES, which may lead to an underrepresentation of single-gene disorders and an underestimation of the utility of ECS. Finally, a retrospective classification of structural abnormalities that may be detectable by ultrasonography may not always reflect what is actually reported in prenatal imaging.
However, the work that the authors put forth to evaluate and categorize 931 participants by the results of genetic testing and structural anomalies is appreciated, and the level of detail is impressive for this retrospective chart review. Additionally, the tables itemizing the authors’ review of 271 ECS disorders that may have ultrasonography findings categorized by disorder and system are helpful and quick diagnostic references for clinicians providing prenatal care. ●
This study of potentially detectable prenatal findings from the lens of a pediatric genetics clinic lends an interesting perspective: Exome sequencing is not the primary route to establish a diagnosis; karyotype, microarray, methylation disorders, and triplet repeat disorders all have an established role in the diagnostic toolkit. Keeping in mind the contribution of these modalities to pediatric testing may shorten the diagnostic odyssey to continue pregnancies or help to fully counsel patients on expectations and decision-making after birth.
Carrier screening is not a substitute for diagnostic testing in pregnancy. However, in appropriately selected patients, a broad carrier screening panel may have added utility. ECS can be conducted while awaiting microarray results to help target testing and may be particularly useful for patients who decline diagnostic testing until the postnatal period. It is important to counsel patients that carrier screening is not a diagnostic test, and results will only report likely pathogenic or pathogenic variants, not variants of uncertain significance that may be of clinical relevance. However, our practice has had several insightful diagnoses reached through ECS, in conjunction with microarray testing that allowed for faster and more targeted sequencing and precise fetal diagnosis. This readily available molecular tool (often covered by insurance) deserves a spot in your fetal diagnosis tool belt based on available evidence.
Last year, our Update focused on the expansion of sequencing in prenatal diagnosis. This year, we are taking a step sideways to remember the many diagnoses we may miss if we rely on exome sequencing alone. A recent case report in Prenatal Diagnosis describes a pregnancy affected by fetal akinesia sequence and polyhydramnios in which sequencing did not reveal a diagnosis. Expansion of the differential to include congenital myotonic dystrophy and subsequent triplet repeat testing led the clinicians to the diagnosis and identification of a triplet repeat expansion in the DMPK gene. This case serves as our first example of how complementary testing and technologies should continue to help us make critical diagnoses.
What is the yield of exome sequencing vs panels in nonimmune hydrops?
Rogers R, Moyer K, Moise KJ Jr. Congenital myotonic dystrophy: an overlooked diagnosis not amenable to detection by sequencing. Prenat Diagn. 2022;42:233-235. doi:10.1002/pd.6105.
Norton ME, Ziffle JV, Lianoglou BR, et al. Exome sequencing vs targeted gene panels for the evaluation of nonimmune hydrops fetalis. Am J Obstet Gynecol. 2021;28:S0002-9378(21)00828-0. doi:10.1016/j.ajog.2021.07.014.
We have had several illuminating discussions with our colleagues about the merits of exome sequencing (ES) versus panels and other modalities for fetal diagnosis. Many obstetricians practicing at the leading edge may feel like ES should be utilized uniformly for fetal anomalies with nondiagnostic karyotype or microarray. However, for well-defined phenotypes with clear and narrow lists of implicated genes (eg, skeletal dysplasias) or patients without insurance coverage, panel sequencing still has utility in prenatal diagnosis. The question of which phenotypes most benefit from ES versus panel sequencing is an area of interesting, ongoing research for several investigators.
Secondary analysis of nonimmune hydrops cohort
Norton and colleagues tackled one such cohort in a study presented in the American Journal of Obstetrics and Gynecology. They compared the proportion of diagnoses that would have been identified in commercial lab panels with their research of phenotype-driven ES in a cohort of 127 fetuses with features of nonimmune hydrops fetalis (NIHF). NIHF can be caused by a variety of single-gene disorders in addition to chromosomal disorders and copy number variants on chromosomal microarray. Patients were eligible for inclusion in the cohort if they had a nondiagnostic karyotype or microarray and any of the following features: nuchal translucency of 3.5 mm or greater, cystic hygroma, pleural effusion, pericardial effusion, ascites, or skin edema. Standard sequencing methods and variant analysis were performed. They assumed 100% analytical sensitivity and specificity of the panels for variant detection and collected cost information on the targeted gene panels.
Study outcomes
In the ES analysis of cases, 37 of 127 cases (29%) had a pathogenic or likely pathogenic variant in 1 of 29 genes, and another 12 of 127 cases (9%) had variants of uncertain significance that were strongly suspected to be the etiology during clinical analysis. The types of disorders that were identified are listed in the TABLE. In addition to a feature of NIHF, 50% of the cases had a structural anomaly.
There were 10 identified clinical panels from 7 clinical laboratories. These panels ranged in size from 11 to 128 genes. The highest simulated yield of any commercial panel was only 62% of the pathogenic variants identified by ES. The other commercial laboratory panels detection yield ranged from 11% to 62% of pathogenic variants detected by ES. For overall yield, the largest panel would have a diagnostic yield of 18% of diagnoses relative to the 29% diagnostic yield from ES.
The largest panel included 128 genes prior to the publication of the original cohort and was updated after publication to include 148 genes. The larger updated panel would have identified all of the patients in the ES cohort. However, many of the other panels listed would have identified a smaller fraction of the variants identified by ES (range, 11%-62%). At the time of publication, the cost of the panels ranged from $640 to $3,500, and the cost of prenatal ES ranged from $2,458 to $7,500.
Continue to: Strengths and limitations...
Strengths and limitations
Twenty-three percent of the patients who were sequenced had an increased fetal nuchal translucency or cystic hygroma, and another 17% had a single fetal effusion. This inclusivity makes this study more applicable to broader fetal anomaly populations. However, it is worth noting that only 61% of patients had NIHF by the definition of 2 or more fluid collections or skin thickening.
The authors assumed 100% sensitivity and specificity for the panel tests relative to diagnostic ES results, but this may not reflect real-life analysis. There is inherent subjectivity and subsequent differences in variant calling (deciding which genetic changes are pathogenic) between institutions and companies despite efforts to standardize this process. Due to the simulated nature of this study, these differences are not captured. Additionally, although the authors note that the research ES had at least 30 times the coverage (an adequate number of sequence reads for accurate testing) than did the commercial lab panels, some gene panels have additional sequencing of intronic regions, copy number analysis, and up to 10 times more coverage than ES, which could lead to more diagnoses.
This study illustrates that there is nuance involved in selecting which type of gene sequencing and which clinical laboratory to use for prenatal diagnosis. Labs with more updated literature searches and more inclusive gene panels may be excellent options for patients in whom ES is not covered by insurance or with phenotypes with a narrow range of suspected causative genes. However, there is a lag time in updating the genes offered on each panel, and new genedisease associations will not be captured by existing panels.
From a cost, speed-of-analysis, and depth-of-sequencing perspective, panel sequencing can have advantages that should be considered in some patients, particularly if the panels are large and regularly updated. However, the authors summarize our sentiments and their findings with the following:
“For disorders, such as NIHF with marked genetic heterogeneity and less clear in utero phenotypes of underlying genetic diseases, the broader coverage of exome sequencing makes it a superior option to targeted panel testing.”
We look forward to the publication of further anomaly-specific cohorts and secondary analyses of the utility of current panels and ES that may follow.
Frequency of Beckwith-Widemann syndrome in prenatally diagnosed omphaloceles
Abbasi N, Moore A, Chiu P, et al. Prenatally diagnosed omphaloceles: report of 92 cases and association with Beckwith-Wiedemann syndrome. Prenat Diagn. 2021;41:798-816. doi:10.1002/pd.5930.
An omphalocele is diagnosed prenatally on ultrasound when an anterior midline mass, often containing abdominal contents, is seen herniating into the base of the umbilical cord. Omphaloceles are often associated with additional structural abnormalities and underlying genetic syndromes, thus a thorough fetal assessment is required for accurate prenatal counseling and neonatal care.
Identification of Beckwith-Widemann syndrome (BWS) in the setting of a prenatally diagnosed omphalocele is difficult because of its wide range of clinical features and its unique genetic basis. Unlike many genetic disorders that are caused by specific genetic variants, or spelling changes in the genes, BWS results from a change in the expression of one or more of the genes in a specific region of chromosome 11. A high index of clinical suspicion as well as an understanding of the various genetic and epigenetics alterations that cause BWS is required for prenatal diagnosis.
Retrospective cohort at a single center
The authors in this study reviewed all pregnancies in which an omphalocele was diagnosed prenatally at a single center between 2010 and 2015. They describe a standard prenatal evaluation following identification of an omphalocele including echocardiogram, detailed anatomic survey, and availability of an amniocentesis to facilitate aneuploidy screening and testing for BWS. This review also includes an overview of perinatal and long-term outcomes for cases of BWS diagnosed at their center between 2000 and 2015.
Study outcomes
Results of prenatal genetic testing in this cohort were divided between cases of an isolated omphalocele (without other structural changes) and cases of nonisolated omphaloceles. In the group of pregnancies with an isolated omphalocele, 2 of 27 pregnancies (7.4%) were found to have an abnormal karyotype, and 6 of 16 of the remaining pregnancies (37.5%) were diagnosed with BWS. Among the group of pregnancies with a nonisolated omphalocele, 23 of 59 pregnancies (39%) were found to have an abnormal karyotype, and 1 of 20 pregnancies (5%) were diagnosed with BWS.
Prenatal sonographic features associated with cases of BWS included polyhydramnios in 12 of 19 cases (63%) and macrosomia in 8 of 19 cases (42%). Macroglossia is another characteristic feature of the disorder, which was identified in 4 of 19 cases (21%) prenatally and in an additional 10 of 19 cases (52.6%) postnatally. Interestingly, only 1 of the cases of BWS was caused by a microdeletion at 11p15.4—a change that was identified on microarray. The additional 6 cases of BWS were caused by imprinting changes in the region, which are only detectable with a specific methylation-analysis technique.
Among the 19 cases of BWS identified over a 15-year period, there was 1 intrauterine demise. Preterm birth occurred in 10 of 19 cases (52.6%), including 8 of 19 cases (42.1%) of spontaneous preterm labor. Respiratory distress (27.8%), hypoglycemia (61%), and gastrointestinal reflux (59%) were common neonatal complications. Embryonal tumors were diagnosed in 2 of 16 patients (12.5%). Although neurodevelopmental outcomes were incomplete, their data suggested normal development in 75% of children. There were 2 neonatal deaths in this cohort and 1 childhood death at age 2 years.
Study strengths and limitations
As with many studies investigating a rare disorder, this study is limited by its retrospective nature and small sample size. Nevertheless, it adds an important cohort of patients with a prenatally diagnosed omphalocele to the literature and illuminates the utility of a standardized approach to testing for BWS in this population.
In this cohort with prenatally diagnosed omphaloceles with standardized testing for BWS, the prevalence of the disorder was approximately 8% and more common in cases of an isolated omphalocele. The most common supporting sonographic features of BWS may not be detected until later in gestation, including polyhydramnios and macrosomia. This demonstrates the importance of both sonographic follow-up as well as universal testing for BWS in euploid cases of a prenatally diagnosed omphalocele. Almost all cases of BWS in this cohort required specialized molecular techniques for diagnosis, and the diagnosis would have been missed on karyotype, microarray, and ES.
Continue to: Genetic diagnoses that could have been identified by expanded carrier screening...
Genetic diagnoses that could have been identified by expanded carrier screening
Stevens BK, Nunley PB, Wagner C, et al. Utility of expanded carrier screening in pregnancies with ultrasound abnormalities. Prenat Diagn. 2022;42:60-78. doi:10.1002/pd.6069.
This series is a thorough retrospective review of patients evaluated in a pediatric genetics clinic from 2014 through 2017. Patients were included if they were evaluated in the first 6 months of life and had a structural abnormality that might be detected on prenatal ultrasonography. The genetic testing results were analyzed and categorized according to types of genetic disorders, with the goal of identifying how many patients might have been identified by expanded carrier screening (ECS) panels.
Study outcomes
A total of 931 charts were reviewed, and 85% (791 of 931) of patients evaluated in the first 6 months of life were determined to have a structural anomaly that might be detected on prenatal ultrasonography. Of those patients, 691 went on to have genetic testing and 32.1% (222 of 691) of them had a diagnostic (pathogenic) genetic testing result related to the phenotype. The types of diagnostic testing results are shown in the FIGURE. Notably, 42 single-gene disorders were detected.
FIGURE Diagnostic test result in pediatric patients evaluated under age 6 months

Of those 222 patients with diagnostic results, there were 8 patients with autosomal recessive and X-linked conditions that could be detected using a 500-gene ECS panel. Five patients could be detected with a 271-gene panel. After nondiagnostic microarray, 11.3% of patients had a condition that could be detected by using a 500-gene ECS panel. The identified conditions included cystic fibrosis, CYP21‐related congenital adrenal hyperplasia, autosomal recessive polycystic kidney disease, Antley‐Bixler syndrome, and Morquio syndrome type A.
Furthermore, the authors conducted a literature review of 271 conditions and found that 32% (88 of 271) of conditions may be associated with ultrasound findings.
Study strengths and limitations
When applying these data to prenatal populations, the authors acknowledge several notable limitations. There is a selection bias toward less-severe phenotypes for many patients choosing to continue rather than to interrupt a pregnancy. Additionally, only 23% of the patients in the study had a microarray and ES, which may lead to an underrepresentation of single-gene disorders and an underestimation of the utility of ECS. Finally, a retrospective classification of structural abnormalities that may be detectable by ultrasonography may not always reflect what is actually reported in prenatal imaging.
However, the work that the authors put forth to evaluate and categorize 931 participants by the results of genetic testing and structural anomalies is appreciated, and the level of detail is impressive for this retrospective chart review. Additionally, the tables itemizing the authors’ review of 271 ECS disorders that may have ultrasonography findings categorized by disorder and system are helpful and quick diagnostic references for clinicians providing prenatal care. ●
This study of potentially detectable prenatal findings from the lens of a pediatric genetics clinic lends an interesting perspective: Exome sequencing is not the primary route to establish a diagnosis; karyotype, microarray, methylation disorders, and triplet repeat disorders all have an established role in the diagnostic toolkit. Keeping in mind the contribution of these modalities to pediatric testing may shorten the diagnostic odyssey to continue pregnancies or help to fully counsel patients on expectations and decision-making after birth.
Carrier screening is not a substitute for diagnostic testing in pregnancy. However, in appropriately selected patients, a broad carrier screening panel may have added utility. ECS can be conducted while awaiting microarray results to help target testing and may be particularly useful for patients who decline diagnostic testing until the postnatal period. It is important to counsel patients that carrier screening is not a diagnostic test, and results will only report likely pathogenic or pathogenic variants, not variants of uncertain significance that may be of clinical relevance. However, our practice has had several insightful diagnoses reached through ECS, in conjunction with microarray testing that allowed for faster and more targeted sequencing and precise fetal diagnosis. This readily available molecular tool (often covered by insurance) deserves a spot in your fetal diagnosis tool belt based on available evidence.