User login
Small NY study: Mother-baby transmission of COVID-19 not seen
according to a study out of New York-Presbyterian Hospital.
“It is suggested in the cumulative data that the virus does not confer additional risk to the fetus during labor or during the early postnatal period in both preterm and term infants,” concluded Jeffrey Perlman, MB ChB, and colleagues in Pediatrics.
But other experts suggest substantial gaps remain in our understanding of maternal transmission of SARS-CoV-2.
“Much more needs to be known,” Munish Gupta, MD, and colleagues from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, said in an accompanying editorial.
The prospective study is the first to describe a cohort of U.S. COVID-19–related deliveries, with the prior neonatal impact of COVID-19 “almost exclusively” reported from China, noted the authors. They included a cohort of 326 women who were tested for SARS-CoV-2 on admission to labor and delivery at New York-Presbyterian Hospital between March 22 and April 15th, 2020. Of the 31 (10%) mothers who tested positive, 15 (48%) were asymptomatic and 16 (52%) were symptomatic.
Two babies were born prematurely (one by Cesarean) and were isolated in negative pressure rooms with continuous positive airway pressure. Both were moved out of isolation after two negative test results and “have exhibited an unremarkable clinical course,” the authors reported.
The other 29 term babies were cared for in their mothers’ rooms, with breastfeeding allowed, if desired. These babies and their mothers were discharged from the hospital between 24 and 48 hours after delivery.
“Visitor restriction for mothers who were positive for COVID-19 included 14 days of no visitation from the start of symptoms,” noted the team.
They added “since the prepublication release there have been a total of 47 mothers positive for COVID-19, resulting in 47 infants; 4 have been admitted to neonatal intensive care. In addition, 32 other infants have been tested for a variety of indications within the unit. All infants test results have been negative.”
The brief report outlined the institution’s checklist for delivery preparedness in either the operating room or labor delivery room, including personal protective equipment, resuscitation, transportation to the neonatal intensive care unit, and early postresuscitation care. “Suspected or confirmed COVID-19 alone in an otherwise uncomplicated pregnancy is not an indication for the resuscitation team or the neonatal fellow,” they noted, adding delivery room preparation and management should include contact precautions. “With scrupulous attention to infectious precautions, horizontal viral transmission should be minimized,” they advised.
Dr. Perlman and associates emphasized that rapid turnaround SARSCoV-2 testing is “crucial to minimize the likelihood of a provider becoming infected and/or infecting the infant.”
Although the findings are “clearly reassuring,” Dr. Gupta and colleagues have reservations. “To what extent does this report address concerns for infection risk with a rooming-in approach to care?” they asked in their accompanying editorial. “The answer is likely some, but not much.”
Many questions remain, they said, including: “What precautions were used to minimize infection risk during the postbirth hospital course? What was the approach to skin-to-skin care and direct mother-newborn contact? Were restrictions placed on family members? Were changes made to routine interventions such as hearing screens or circumcisions? What practices were in place around environmental cleaning? Most important, how did the newborns do after discharge?”
The current uncertainty around neonatal COVID-19 infection risk has led to “disparate” variations in care recommendations, they pointed out. Whereas China’s consensus guidelines recommend a 14-day separation of COVID-19–positive mothers from their healthy infants, a practice supported by the American Academy of Pediatrics “when possible,” the Italian Society of Neonatology, the Royal College of Paediatrics and Child Health, and the Canadian Paediatric Society advise “rooming-in and breastfeeding with appropriate infection prevention measures.”
Dr. Gupta and colleagues pointed to the following as at least three “critical and time-sensitive needs for research around neonatal care and outcomes related to COVID-19”:
- Studies need to have much larger sample sizes and include diverse populations. This will allow for reliable measurement of outcomes.
- Descriptions of care practices must be in detail, especially about infection prevention; these should be presented in a way to compare the efficacy of different approaches.
- There needs to be follow-up information on outcomes of both the mother and the neonate after the birth hospitalization.
Asked to comment, Lillian Beard, MD, of George Washington University in Washington welcomed the data as “good news.”
“Although small, the study was done during a 3-week peak period at the hottest spot of the pandemic in the United States during that period. It illustrates how delivery room preparedness, adequate personal protective equipment, and carefully planned infection control precautions can positively impact outcomes even during a seemingly impossible period,” she said.
“Although there are many uncertainties about maternal COVID-19 transmission and neonatal infection risks ... in my opinion, during the after birth hospitalization, the inherent benefits of rooming in for breast feeding and the opportunities for the demonstration and teaching of infection prevention practices for the family home, far outweigh the risks of disease transmission,” said Dr. Beard, who was not involved with the study.
The study and the commentary emphasize the likely low risk of vertical transmission of the virus, with horizontal transmission being the greater risk. However, cases of transplacental transmission have been reported, and the lead investigator of one recent placental study cautions against complacency.
“Neonates can get infected in both ways. The majority of cases seem to be horizontal, but those who have been infected or highly suspected to be vertically infected are not a small percentage either,” said Daniele de Luca, MD, PhD, president-elect of the European Society for Pediatric and Neonatal Intensive Care (ESPNIC) and a neonatologist at Antoine Béclère Hospital in Clamart, France.
“Perlman’s data are interesting and consistent with other reports around the world. However, two things must be remembered,” he said in an interview. “First, newborn infants are at relatively low risk from SARS-CoV-2 infections, but this is very far from zero risk. Neonatal SARS-CoV-2 infections do exist and have been described around the world. While they have a mild course in the majority of cases, neonatologists should not forget them and should be prepared to offer the best care to these babies.”
“Second, how this can be balanced with the need to promote breastfeeding and avoid overtreatment or separation from the mother is a question far from being answered. Gupta et al. in their commentary are right in saying that we have more questions than answers. While waiting for the results of large initiatives (such as the ESPNIC EPICENTRE Registry that they cite) to answer these open points, the best we can do is to provide a personalised case by case approach, transparent information to parents, and an open counselling informing clinical decisions.”
The study received no external funding. Dr. Perlman and associates had no financial disclosures. Dr. Gupta and colleagues had no relevant financial disclosures. Neither Dr. Beard nor Dr. de Luca had any relevant financial disclosures.
SOURCE: Perlman J et al. Pediatrics. 2020;146(2):e20201567.
according to a study out of New York-Presbyterian Hospital.
“It is suggested in the cumulative data that the virus does not confer additional risk to the fetus during labor or during the early postnatal period in both preterm and term infants,” concluded Jeffrey Perlman, MB ChB, and colleagues in Pediatrics.
But other experts suggest substantial gaps remain in our understanding of maternal transmission of SARS-CoV-2.
“Much more needs to be known,” Munish Gupta, MD, and colleagues from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, said in an accompanying editorial.
The prospective study is the first to describe a cohort of U.S. COVID-19–related deliveries, with the prior neonatal impact of COVID-19 “almost exclusively” reported from China, noted the authors. They included a cohort of 326 women who were tested for SARS-CoV-2 on admission to labor and delivery at New York-Presbyterian Hospital between March 22 and April 15th, 2020. Of the 31 (10%) mothers who tested positive, 15 (48%) were asymptomatic and 16 (52%) were symptomatic.
Two babies were born prematurely (one by Cesarean) and were isolated in negative pressure rooms with continuous positive airway pressure. Both were moved out of isolation after two negative test results and “have exhibited an unremarkable clinical course,” the authors reported.
The other 29 term babies were cared for in their mothers’ rooms, with breastfeeding allowed, if desired. These babies and their mothers were discharged from the hospital between 24 and 48 hours after delivery.
“Visitor restriction for mothers who were positive for COVID-19 included 14 days of no visitation from the start of symptoms,” noted the team.
They added “since the prepublication release there have been a total of 47 mothers positive for COVID-19, resulting in 47 infants; 4 have been admitted to neonatal intensive care. In addition, 32 other infants have been tested for a variety of indications within the unit. All infants test results have been negative.”
The brief report outlined the institution’s checklist for delivery preparedness in either the operating room or labor delivery room, including personal protective equipment, resuscitation, transportation to the neonatal intensive care unit, and early postresuscitation care. “Suspected or confirmed COVID-19 alone in an otherwise uncomplicated pregnancy is not an indication for the resuscitation team or the neonatal fellow,” they noted, adding delivery room preparation and management should include contact precautions. “With scrupulous attention to infectious precautions, horizontal viral transmission should be minimized,” they advised.
Dr. Perlman and associates emphasized that rapid turnaround SARSCoV-2 testing is “crucial to minimize the likelihood of a provider becoming infected and/or infecting the infant.”
Although the findings are “clearly reassuring,” Dr. Gupta and colleagues have reservations. “To what extent does this report address concerns for infection risk with a rooming-in approach to care?” they asked in their accompanying editorial. “The answer is likely some, but not much.”
Many questions remain, they said, including: “What precautions were used to minimize infection risk during the postbirth hospital course? What was the approach to skin-to-skin care and direct mother-newborn contact? Were restrictions placed on family members? Were changes made to routine interventions such as hearing screens or circumcisions? What practices were in place around environmental cleaning? Most important, how did the newborns do after discharge?”
The current uncertainty around neonatal COVID-19 infection risk has led to “disparate” variations in care recommendations, they pointed out. Whereas China’s consensus guidelines recommend a 14-day separation of COVID-19–positive mothers from their healthy infants, a practice supported by the American Academy of Pediatrics “when possible,” the Italian Society of Neonatology, the Royal College of Paediatrics and Child Health, and the Canadian Paediatric Society advise “rooming-in and breastfeeding with appropriate infection prevention measures.”
Dr. Gupta and colleagues pointed to the following as at least three “critical and time-sensitive needs for research around neonatal care and outcomes related to COVID-19”:
- Studies need to have much larger sample sizes and include diverse populations. This will allow for reliable measurement of outcomes.
- Descriptions of care practices must be in detail, especially about infection prevention; these should be presented in a way to compare the efficacy of different approaches.
- There needs to be follow-up information on outcomes of both the mother and the neonate after the birth hospitalization.
Asked to comment, Lillian Beard, MD, of George Washington University in Washington welcomed the data as “good news.”
“Although small, the study was done during a 3-week peak period at the hottest spot of the pandemic in the United States during that period. It illustrates how delivery room preparedness, adequate personal protective equipment, and carefully planned infection control precautions can positively impact outcomes even during a seemingly impossible period,” she said.
“Although there are many uncertainties about maternal COVID-19 transmission and neonatal infection risks ... in my opinion, during the after birth hospitalization, the inherent benefits of rooming in for breast feeding and the opportunities for the demonstration and teaching of infection prevention practices for the family home, far outweigh the risks of disease transmission,” said Dr. Beard, who was not involved with the study.
The study and the commentary emphasize the likely low risk of vertical transmission of the virus, with horizontal transmission being the greater risk. However, cases of transplacental transmission have been reported, and the lead investigator of one recent placental study cautions against complacency.
“Neonates can get infected in both ways. The majority of cases seem to be horizontal, but those who have been infected or highly suspected to be vertically infected are not a small percentage either,” said Daniele de Luca, MD, PhD, president-elect of the European Society for Pediatric and Neonatal Intensive Care (ESPNIC) and a neonatologist at Antoine Béclère Hospital in Clamart, France.
“Perlman’s data are interesting and consistent with other reports around the world. However, two things must be remembered,” he said in an interview. “First, newborn infants are at relatively low risk from SARS-CoV-2 infections, but this is very far from zero risk. Neonatal SARS-CoV-2 infections do exist and have been described around the world. While they have a mild course in the majority of cases, neonatologists should not forget them and should be prepared to offer the best care to these babies.”
“Second, how this can be balanced with the need to promote breastfeeding and avoid overtreatment or separation from the mother is a question far from being answered. Gupta et al. in their commentary are right in saying that we have more questions than answers. While waiting for the results of large initiatives (such as the ESPNIC EPICENTRE Registry that they cite) to answer these open points, the best we can do is to provide a personalised case by case approach, transparent information to parents, and an open counselling informing clinical decisions.”
The study received no external funding. Dr. Perlman and associates had no financial disclosures. Dr. Gupta and colleagues had no relevant financial disclosures. Neither Dr. Beard nor Dr. de Luca had any relevant financial disclosures.
SOURCE: Perlman J et al. Pediatrics. 2020;146(2):e20201567.
according to a study out of New York-Presbyterian Hospital.
“It is suggested in the cumulative data that the virus does not confer additional risk to the fetus during labor or during the early postnatal period in both preterm and term infants,” concluded Jeffrey Perlman, MB ChB, and colleagues in Pediatrics.
But other experts suggest substantial gaps remain in our understanding of maternal transmission of SARS-CoV-2.
“Much more needs to be known,” Munish Gupta, MD, and colleagues from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, said in an accompanying editorial.
The prospective study is the first to describe a cohort of U.S. COVID-19–related deliveries, with the prior neonatal impact of COVID-19 “almost exclusively” reported from China, noted the authors. They included a cohort of 326 women who were tested for SARS-CoV-2 on admission to labor and delivery at New York-Presbyterian Hospital between March 22 and April 15th, 2020. Of the 31 (10%) mothers who tested positive, 15 (48%) were asymptomatic and 16 (52%) were symptomatic.
Two babies were born prematurely (one by Cesarean) and were isolated in negative pressure rooms with continuous positive airway pressure. Both were moved out of isolation after two negative test results and “have exhibited an unremarkable clinical course,” the authors reported.
The other 29 term babies were cared for in their mothers’ rooms, with breastfeeding allowed, if desired. These babies and their mothers were discharged from the hospital between 24 and 48 hours after delivery.
“Visitor restriction for mothers who were positive for COVID-19 included 14 days of no visitation from the start of symptoms,” noted the team.
They added “since the prepublication release there have been a total of 47 mothers positive for COVID-19, resulting in 47 infants; 4 have been admitted to neonatal intensive care. In addition, 32 other infants have been tested for a variety of indications within the unit. All infants test results have been negative.”
The brief report outlined the institution’s checklist for delivery preparedness in either the operating room or labor delivery room, including personal protective equipment, resuscitation, transportation to the neonatal intensive care unit, and early postresuscitation care. “Suspected or confirmed COVID-19 alone in an otherwise uncomplicated pregnancy is not an indication for the resuscitation team or the neonatal fellow,” they noted, adding delivery room preparation and management should include contact precautions. “With scrupulous attention to infectious precautions, horizontal viral transmission should be minimized,” they advised.
Dr. Perlman and associates emphasized that rapid turnaround SARSCoV-2 testing is “crucial to minimize the likelihood of a provider becoming infected and/or infecting the infant.”
Although the findings are “clearly reassuring,” Dr. Gupta and colleagues have reservations. “To what extent does this report address concerns for infection risk with a rooming-in approach to care?” they asked in their accompanying editorial. “The answer is likely some, but not much.”
Many questions remain, they said, including: “What precautions were used to minimize infection risk during the postbirth hospital course? What was the approach to skin-to-skin care and direct mother-newborn contact? Were restrictions placed on family members? Were changes made to routine interventions such as hearing screens or circumcisions? What practices were in place around environmental cleaning? Most important, how did the newborns do after discharge?”
The current uncertainty around neonatal COVID-19 infection risk has led to “disparate” variations in care recommendations, they pointed out. Whereas China’s consensus guidelines recommend a 14-day separation of COVID-19–positive mothers from their healthy infants, a practice supported by the American Academy of Pediatrics “when possible,” the Italian Society of Neonatology, the Royal College of Paediatrics and Child Health, and the Canadian Paediatric Society advise “rooming-in and breastfeeding with appropriate infection prevention measures.”
Dr. Gupta and colleagues pointed to the following as at least three “critical and time-sensitive needs for research around neonatal care and outcomes related to COVID-19”:
- Studies need to have much larger sample sizes and include diverse populations. This will allow for reliable measurement of outcomes.
- Descriptions of care practices must be in detail, especially about infection prevention; these should be presented in a way to compare the efficacy of different approaches.
- There needs to be follow-up information on outcomes of both the mother and the neonate after the birth hospitalization.
Asked to comment, Lillian Beard, MD, of George Washington University in Washington welcomed the data as “good news.”
“Although small, the study was done during a 3-week peak period at the hottest spot of the pandemic in the United States during that period. It illustrates how delivery room preparedness, adequate personal protective equipment, and carefully planned infection control precautions can positively impact outcomes even during a seemingly impossible period,” she said.
“Although there are many uncertainties about maternal COVID-19 transmission and neonatal infection risks ... in my opinion, during the after birth hospitalization, the inherent benefits of rooming in for breast feeding and the opportunities for the demonstration and teaching of infection prevention practices for the family home, far outweigh the risks of disease transmission,” said Dr. Beard, who was not involved with the study.
The study and the commentary emphasize the likely low risk of vertical transmission of the virus, with horizontal transmission being the greater risk. However, cases of transplacental transmission have been reported, and the lead investigator of one recent placental study cautions against complacency.
“Neonates can get infected in both ways. The majority of cases seem to be horizontal, but those who have been infected or highly suspected to be vertically infected are not a small percentage either,” said Daniele de Luca, MD, PhD, president-elect of the European Society for Pediatric and Neonatal Intensive Care (ESPNIC) and a neonatologist at Antoine Béclère Hospital in Clamart, France.
“Perlman’s data are interesting and consistent with other reports around the world. However, two things must be remembered,” he said in an interview. “First, newborn infants are at relatively low risk from SARS-CoV-2 infections, but this is very far from zero risk. Neonatal SARS-CoV-2 infections do exist and have been described around the world. While they have a mild course in the majority of cases, neonatologists should not forget them and should be prepared to offer the best care to these babies.”
“Second, how this can be balanced with the need to promote breastfeeding and avoid overtreatment or separation from the mother is a question far from being answered. Gupta et al. in their commentary are right in saying that we have more questions than answers. While waiting for the results of large initiatives (such as the ESPNIC EPICENTRE Registry that they cite) to answer these open points, the best we can do is to provide a personalised case by case approach, transparent information to parents, and an open counselling informing clinical decisions.”
The study received no external funding. Dr. Perlman and associates had no financial disclosures. Dr. Gupta and colleagues had no relevant financial disclosures. Neither Dr. Beard nor Dr. de Luca had any relevant financial disclosures.
SOURCE: Perlman J et al. Pediatrics. 2020;146(2):e20201567.
FROM PEDIATRICS
Ob.gyns. struggle to keep pace with changing COVID-19 knowledge
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
Some women use prescription opioids during pregnancy
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.
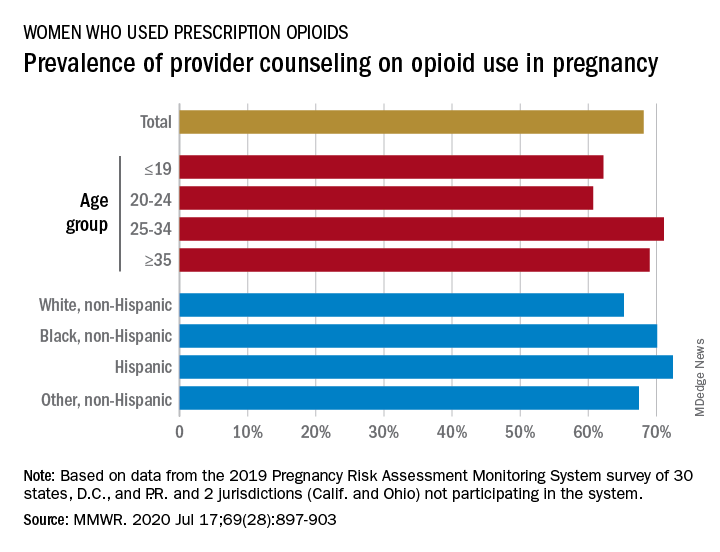
Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.

Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.

Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
FROM MMWR
Confronting the epidemic of racism in ObGyn practice
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
Cerclage in twin pregnancies reduces perinatal mortality in randomized trial
according to a randomized controlled trial. The trial, which was published in the American Journal of Obstetrics and Gynecology, included 30 patients at 8 centers. The investigators stopped the trial early because perinatal mortality occurred more often in the group that did not receive the intervention.
The research suggests that a combination of physical exam–indicated cerclage, indomethacin, and antibiotics decreased the incidence of spontaneous preterm birth and prolonged the period from diagnosis to delivery by an average of 5.6 weeks, compared with no cerclage.
“We’ve already incorporated this cerclage into our practice and have been able to offer this to pregnant mothers with twins with great success,” senior author Vincenzo Berghella, MD, said in a news release.
“These results have the potential to change practice and help many more women have healthy twin babies,” said Dr. Berghella, director of the division of maternal fetal medicine at Thomas Jefferson University in Philadelphia.
A shift in perspective
More research is needed to establish a standardized approach, but the trial should “open physicians’ perspectives to think about how, in selected cases and with the proper approach, cerclage can work well,” said Ozhan M. Turan, MD, PhD, director of the division of maternal and fetal medicine and director of fetal therapy and complex obstetric surgery at University of Maryland in Baltimore.
Although many physicians use cerclage for twin pregnancies in select situations, the practice is not well established. “If you look at the guidelines or books, mostly everyone thinks that doing a cerclage in twins is not a good idea,” Dr. Turan said in an interview.
In the present trial, the researchers controlled for many factors and carefully selected patients with no signs of preterm labor or infection. It is not simply a matter of saying, “Do the stitch,” he said. “But it is proven: if you select patients well and use the appropriate approach, then you could improve the outcome.”
The study is the first randomized controlled trial of physical exam–indicated cerclage focused on twins, according to its authors. It enrolled patients between July 2015 and July 2019. In the end, the researchers analyzed data from 30 pregnancies, rather than the originally intended 52. They stopped the trial after a data and safety monitoring board considered it “unethical to continue the study due to the considerable perinatal mortality in one of the arms ... and requested to unmask the arms of the study,” the researchers said.
Perinatal mortality occurred in 18% of neonates in the cerclage group (6 of 34), compared with 77% in the group without cerclage (20 of 26). All perinatal mortality cases were associated with delivery before 24 weeks.
“The small number of participants reflects how rare this condition is among all pregnancies,” first author Amanda Roman, MD, of the division of maternal fetal medicine at Thomas Jefferson University, Philadelphia, said in the news release. “But because women were randomized to treatment and nontreatment groups, the results are strong, as confirmed by the independent data and safety monitoring board.”
The researchers enrolled women with twin pregnancies and asymptomatic cervical dilation from 1 to 4 cm before 24 weeks. Exclusion criteria included monochorionic-monoamniotic pregnancy, selective fetal growth restriction, twin-twin transfusion syndrome, major fetal malformation, known genetic anomaly, placenta previa, signs of labor, or clinical chorioamnionitis.
In all, 17 women were randomized to cerclage and 13 to the no-cerclage group. Both groups had similar patient characteristics. About 93% of the twin gestations were diamniotic-dichorionic. Assisted reproductive technology was used by about 36% of the participants, and 20% had a history of singleton preterm birth. Four women assigned to cerclage did not undergo the procedure but were included in the intention-to-treat analysis. Two of the four patients had contraindications that occurred soon after randomization (rupture of amniotic membranes and vaginal bleeding), one had a friable cervix, and one declined cerclage after being randomized.
Spontaneous preterm birth before 34 weeks of gestation, the primary outcome, occurred in 12 of 17 women in the cerclage group and in all 13 women in the no-cerclage group (70% vs. 100%).
Trial to assess ultrasound indicated cerclage
“Expectant management with no cerclage is the current standard of care for these women,” Dr. Roman and coauthors wrote. “Despite small sample size, we were able to show a significant benefit to physical exam–indicated cerclage.”
Inability to place the cerclage in one patient due to friable cervix was the only intraoperative complication. “Larger cohorts in singleton pregnancies have informed a 10%-20% risk of intraoperative rupture of the membranes, cervical laceration, and bleeding during the procedure,” the researchers noted.
All women who received cerclage also received indomethacin and antibiotics, although these elements of management were not prespecified. Given the relatively small sample size, it is unclear what role factors such as indomethacin, which was administered to 82% of the cerclage group versus 31% of the no-cerclage group, and antibiotics may have played, said Dr. Turan.
Prospective studies may help clarify how the degree of cervical dilation, gestational age, use of progesterone, or surgical techniques may influence outcomes. In addition, the researchers are enrolling patients in another trial. That study aims to assess whether cerclage reduces the incidence of spontaneous preterm birth in asymptomatic women with twin gestations and cervical length of 15 mm or less diagnosed by transvaginal ultrasound between 16 and 24 weeks of gestation.
The study had no external financial support. The authors had no conflicts of interest. Dr. Turan said he had no relevant financial disclosures.
SOURCE: Roman A et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.047.
according to a randomized controlled trial. The trial, which was published in the American Journal of Obstetrics and Gynecology, included 30 patients at 8 centers. The investigators stopped the trial early because perinatal mortality occurred more often in the group that did not receive the intervention.
The research suggests that a combination of physical exam–indicated cerclage, indomethacin, and antibiotics decreased the incidence of spontaneous preterm birth and prolonged the period from diagnosis to delivery by an average of 5.6 weeks, compared with no cerclage.
“We’ve already incorporated this cerclage into our practice and have been able to offer this to pregnant mothers with twins with great success,” senior author Vincenzo Berghella, MD, said in a news release.
“These results have the potential to change practice and help many more women have healthy twin babies,” said Dr. Berghella, director of the division of maternal fetal medicine at Thomas Jefferson University in Philadelphia.
A shift in perspective
More research is needed to establish a standardized approach, but the trial should “open physicians’ perspectives to think about how, in selected cases and with the proper approach, cerclage can work well,” said Ozhan M. Turan, MD, PhD, director of the division of maternal and fetal medicine and director of fetal therapy and complex obstetric surgery at University of Maryland in Baltimore.
Although many physicians use cerclage for twin pregnancies in select situations, the practice is not well established. “If you look at the guidelines or books, mostly everyone thinks that doing a cerclage in twins is not a good idea,” Dr. Turan said in an interview.
In the present trial, the researchers controlled for many factors and carefully selected patients with no signs of preterm labor or infection. It is not simply a matter of saying, “Do the stitch,” he said. “But it is proven: if you select patients well and use the appropriate approach, then you could improve the outcome.”
The study is the first randomized controlled trial of physical exam–indicated cerclage focused on twins, according to its authors. It enrolled patients between July 2015 and July 2019. In the end, the researchers analyzed data from 30 pregnancies, rather than the originally intended 52. They stopped the trial after a data and safety monitoring board considered it “unethical to continue the study due to the considerable perinatal mortality in one of the arms ... and requested to unmask the arms of the study,” the researchers said.
Perinatal mortality occurred in 18% of neonates in the cerclage group (6 of 34), compared with 77% in the group without cerclage (20 of 26). All perinatal mortality cases were associated with delivery before 24 weeks.
“The small number of participants reflects how rare this condition is among all pregnancies,” first author Amanda Roman, MD, of the division of maternal fetal medicine at Thomas Jefferson University, Philadelphia, said in the news release. “But because women were randomized to treatment and nontreatment groups, the results are strong, as confirmed by the independent data and safety monitoring board.”
The researchers enrolled women with twin pregnancies and asymptomatic cervical dilation from 1 to 4 cm before 24 weeks. Exclusion criteria included monochorionic-monoamniotic pregnancy, selective fetal growth restriction, twin-twin transfusion syndrome, major fetal malformation, known genetic anomaly, placenta previa, signs of labor, or clinical chorioamnionitis.
In all, 17 women were randomized to cerclage and 13 to the no-cerclage group. Both groups had similar patient characteristics. About 93% of the twin gestations were diamniotic-dichorionic. Assisted reproductive technology was used by about 36% of the participants, and 20% had a history of singleton preterm birth. Four women assigned to cerclage did not undergo the procedure but were included in the intention-to-treat analysis. Two of the four patients had contraindications that occurred soon after randomization (rupture of amniotic membranes and vaginal bleeding), one had a friable cervix, and one declined cerclage after being randomized.
Spontaneous preterm birth before 34 weeks of gestation, the primary outcome, occurred in 12 of 17 women in the cerclage group and in all 13 women in the no-cerclage group (70% vs. 100%).
Trial to assess ultrasound indicated cerclage
“Expectant management with no cerclage is the current standard of care for these women,” Dr. Roman and coauthors wrote. “Despite small sample size, we were able to show a significant benefit to physical exam–indicated cerclage.”
Inability to place the cerclage in one patient due to friable cervix was the only intraoperative complication. “Larger cohorts in singleton pregnancies have informed a 10%-20% risk of intraoperative rupture of the membranes, cervical laceration, and bleeding during the procedure,” the researchers noted.
All women who received cerclage also received indomethacin and antibiotics, although these elements of management were not prespecified. Given the relatively small sample size, it is unclear what role factors such as indomethacin, which was administered to 82% of the cerclage group versus 31% of the no-cerclage group, and antibiotics may have played, said Dr. Turan.
Prospective studies may help clarify how the degree of cervical dilation, gestational age, use of progesterone, or surgical techniques may influence outcomes. In addition, the researchers are enrolling patients in another trial. That study aims to assess whether cerclage reduces the incidence of spontaneous preterm birth in asymptomatic women with twin gestations and cervical length of 15 mm or less diagnosed by transvaginal ultrasound between 16 and 24 weeks of gestation.
The study had no external financial support. The authors had no conflicts of interest. Dr. Turan said he had no relevant financial disclosures.
SOURCE: Roman A et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.047.
according to a randomized controlled trial. The trial, which was published in the American Journal of Obstetrics and Gynecology, included 30 patients at 8 centers. The investigators stopped the trial early because perinatal mortality occurred more often in the group that did not receive the intervention.
The research suggests that a combination of physical exam–indicated cerclage, indomethacin, and antibiotics decreased the incidence of spontaneous preterm birth and prolonged the period from diagnosis to delivery by an average of 5.6 weeks, compared with no cerclage.
“We’ve already incorporated this cerclage into our practice and have been able to offer this to pregnant mothers with twins with great success,” senior author Vincenzo Berghella, MD, said in a news release.
“These results have the potential to change practice and help many more women have healthy twin babies,” said Dr. Berghella, director of the division of maternal fetal medicine at Thomas Jefferson University in Philadelphia.
A shift in perspective
More research is needed to establish a standardized approach, but the trial should “open physicians’ perspectives to think about how, in selected cases and with the proper approach, cerclage can work well,” said Ozhan M. Turan, MD, PhD, director of the division of maternal and fetal medicine and director of fetal therapy and complex obstetric surgery at University of Maryland in Baltimore.
Although many physicians use cerclage for twin pregnancies in select situations, the practice is not well established. “If you look at the guidelines or books, mostly everyone thinks that doing a cerclage in twins is not a good idea,” Dr. Turan said in an interview.
In the present trial, the researchers controlled for many factors and carefully selected patients with no signs of preterm labor or infection. It is not simply a matter of saying, “Do the stitch,” he said. “But it is proven: if you select patients well and use the appropriate approach, then you could improve the outcome.”
The study is the first randomized controlled trial of physical exam–indicated cerclage focused on twins, according to its authors. It enrolled patients between July 2015 and July 2019. In the end, the researchers analyzed data from 30 pregnancies, rather than the originally intended 52. They stopped the trial after a data and safety monitoring board considered it “unethical to continue the study due to the considerable perinatal mortality in one of the arms ... and requested to unmask the arms of the study,” the researchers said.
Perinatal mortality occurred in 18% of neonates in the cerclage group (6 of 34), compared with 77% in the group without cerclage (20 of 26). All perinatal mortality cases were associated with delivery before 24 weeks.
“The small number of participants reflects how rare this condition is among all pregnancies,” first author Amanda Roman, MD, of the division of maternal fetal medicine at Thomas Jefferson University, Philadelphia, said in the news release. “But because women were randomized to treatment and nontreatment groups, the results are strong, as confirmed by the independent data and safety monitoring board.”
The researchers enrolled women with twin pregnancies and asymptomatic cervical dilation from 1 to 4 cm before 24 weeks. Exclusion criteria included monochorionic-monoamniotic pregnancy, selective fetal growth restriction, twin-twin transfusion syndrome, major fetal malformation, known genetic anomaly, placenta previa, signs of labor, or clinical chorioamnionitis.
In all, 17 women were randomized to cerclage and 13 to the no-cerclage group. Both groups had similar patient characteristics. About 93% of the twin gestations were diamniotic-dichorionic. Assisted reproductive technology was used by about 36% of the participants, and 20% had a history of singleton preterm birth. Four women assigned to cerclage did not undergo the procedure but were included in the intention-to-treat analysis. Two of the four patients had contraindications that occurred soon after randomization (rupture of amniotic membranes and vaginal bleeding), one had a friable cervix, and one declined cerclage after being randomized.
Spontaneous preterm birth before 34 weeks of gestation, the primary outcome, occurred in 12 of 17 women in the cerclage group and in all 13 women in the no-cerclage group (70% vs. 100%).
Trial to assess ultrasound indicated cerclage
“Expectant management with no cerclage is the current standard of care for these women,” Dr. Roman and coauthors wrote. “Despite small sample size, we were able to show a significant benefit to physical exam–indicated cerclage.”
Inability to place the cerclage in one patient due to friable cervix was the only intraoperative complication. “Larger cohorts in singleton pregnancies have informed a 10%-20% risk of intraoperative rupture of the membranes, cervical laceration, and bleeding during the procedure,” the researchers noted.
All women who received cerclage also received indomethacin and antibiotics, although these elements of management were not prespecified. Given the relatively small sample size, it is unclear what role factors such as indomethacin, which was administered to 82% of the cerclage group versus 31% of the no-cerclage group, and antibiotics may have played, said Dr. Turan.
Prospective studies may help clarify how the degree of cervical dilation, gestational age, use of progesterone, or surgical techniques may influence outcomes. In addition, the researchers are enrolling patients in another trial. That study aims to assess whether cerclage reduces the incidence of spontaneous preterm birth in asymptomatic women with twin gestations and cervical length of 15 mm or less diagnosed by transvaginal ultrasound between 16 and 24 weeks of gestation.
The study had no external financial support. The authors had no conflicts of interest. Dr. Turan said he had no relevant financial disclosures.
SOURCE: Roman A et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.047.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Stillbirth incidence increases during COVID-19 pandemic
The incidence of stillbirth has increased since the COVID-19 pandemic began, according to a comparative study of pregnancy outcomes in a London hospital.
“The increase in stillbirths may have resulted from indirect effects such as reluctance to attend hospital when needed (e.g., with reduced fetal movements), fear of contracting infection, or not wanting to add to the National Health Service burden,” Asma Khalil, MD, of St George’s University of London and coauthors reported in JAMA.
To further assess reported changes in stillbirth and preterm delivery rates during the pandemic, the researchers began a retrospective study of pregnancy outcomes at St George’s University Hospital in London. They compared two periods: from Oct. 1, 2019, to Jan. 31, 2020 as the pre–COVID-19 period and from Feb. 1, 2020, to June 14, 2020 as the pandemic period. The median age of the mother at time of birth in both periods was 33 years. The prepandemic period had 1,681 births, and the pandemic period had 1,718 births.
Although there were found to be fewer nulliparous women and fewer women with hypertension in the pandemic period, the incidence of stillbirth in that period was significantly higher (n = 16 [9 per 1,000 births]) than in the prepandemic period (n = 4 [2 per 1,000 births]) (difference, 7 per 1,000 births; 95% confidence interval, 1.83-12.0; P = .01). The pandemic rate remained higher when late terminations for fetal abnormality were excluded (difference 6 per 1,000 births; 95% CI 1.54-10.1; P = .01).
None of the pregnant women who experienced stillbirth had COVID-19 symptoms, and none of the postmortems or placental exams indicated infection. There were no significant differences between the two periods in regard to births before 37 weeks’ gestation, births after 34 weeks’ gestation, neonatal unit admission, or cesarean delivery.
“It’s very important to highlight the effects of the pandemic on pregnant patients, even if they’re not infected with COVID-19,” Shannon Clark, MD, of the University of Texas Medical Branch in Galveston said in an interview.
She noted several COVID-related considerations that could have contributed to this increase: the reluctance of both low-risk and high-risk patients to enter a hospital setting during a pandemic, along with safety-centered changes made in antenatal services and care, which includes a reduced number of ultrasounds and screening exams.
“Checking a patient’s blood pressure, checking their weight changes, checking how the baby is growing,” she said. “They’re all simple things that just can’t be done via telemedicine.”
“We’ve thought a lot about the potential effects of getting COVID in pregnancy,” she added, “but it’s just as important to think about what might happen to those who don’t have it and are considered low risk otherwise.”
The study authors noted its limitations, including it being retrospective, analyzing a short time frame, and focusing on a single medical center. It also didn’t factor in the causes of the stillbirths, nor were the time periods precisely comparable, although they did add that “there is no seasonality to stillbirths in the UK.”
One doctor reported receiving grants outside of the submitted work. No other potential conflicts of interest were noted. Dr. Clark said she had no relevant financial disclosures.
SOURCE: Khalil A et al. JAMA. 2020 Jul. doi: 10.1001/jama.2020.12746.
The incidence of stillbirth has increased since the COVID-19 pandemic began, according to a comparative study of pregnancy outcomes in a London hospital.
“The increase in stillbirths may have resulted from indirect effects such as reluctance to attend hospital when needed (e.g., with reduced fetal movements), fear of contracting infection, or not wanting to add to the National Health Service burden,” Asma Khalil, MD, of St George’s University of London and coauthors reported in JAMA.
To further assess reported changes in stillbirth and preterm delivery rates during the pandemic, the researchers began a retrospective study of pregnancy outcomes at St George’s University Hospital in London. They compared two periods: from Oct. 1, 2019, to Jan. 31, 2020 as the pre–COVID-19 period and from Feb. 1, 2020, to June 14, 2020 as the pandemic period. The median age of the mother at time of birth in both periods was 33 years. The prepandemic period had 1,681 births, and the pandemic period had 1,718 births.
Although there were found to be fewer nulliparous women and fewer women with hypertension in the pandemic period, the incidence of stillbirth in that period was significantly higher (n = 16 [9 per 1,000 births]) than in the prepandemic period (n = 4 [2 per 1,000 births]) (difference, 7 per 1,000 births; 95% confidence interval, 1.83-12.0; P = .01). The pandemic rate remained higher when late terminations for fetal abnormality were excluded (difference 6 per 1,000 births; 95% CI 1.54-10.1; P = .01).
None of the pregnant women who experienced stillbirth had COVID-19 symptoms, and none of the postmortems or placental exams indicated infection. There were no significant differences between the two periods in regard to births before 37 weeks’ gestation, births after 34 weeks’ gestation, neonatal unit admission, or cesarean delivery.
“It’s very important to highlight the effects of the pandemic on pregnant patients, even if they’re not infected with COVID-19,” Shannon Clark, MD, of the University of Texas Medical Branch in Galveston said in an interview.
She noted several COVID-related considerations that could have contributed to this increase: the reluctance of both low-risk and high-risk patients to enter a hospital setting during a pandemic, along with safety-centered changes made in antenatal services and care, which includes a reduced number of ultrasounds and screening exams.
“Checking a patient’s blood pressure, checking their weight changes, checking how the baby is growing,” she said. “They’re all simple things that just can’t be done via telemedicine.”
“We’ve thought a lot about the potential effects of getting COVID in pregnancy,” she added, “but it’s just as important to think about what might happen to those who don’t have it and are considered low risk otherwise.”
The study authors noted its limitations, including it being retrospective, analyzing a short time frame, and focusing on a single medical center. It also didn’t factor in the causes of the stillbirths, nor were the time periods precisely comparable, although they did add that “there is no seasonality to stillbirths in the UK.”
One doctor reported receiving grants outside of the submitted work. No other potential conflicts of interest were noted. Dr. Clark said she had no relevant financial disclosures.
SOURCE: Khalil A et al. JAMA. 2020 Jul. doi: 10.1001/jama.2020.12746.
The incidence of stillbirth has increased since the COVID-19 pandemic began, according to a comparative study of pregnancy outcomes in a London hospital.
“The increase in stillbirths may have resulted from indirect effects such as reluctance to attend hospital when needed (e.g., with reduced fetal movements), fear of contracting infection, or not wanting to add to the National Health Service burden,” Asma Khalil, MD, of St George’s University of London and coauthors reported in JAMA.
To further assess reported changes in stillbirth and preterm delivery rates during the pandemic, the researchers began a retrospective study of pregnancy outcomes at St George’s University Hospital in London. They compared two periods: from Oct. 1, 2019, to Jan. 31, 2020 as the pre–COVID-19 period and from Feb. 1, 2020, to June 14, 2020 as the pandemic period. The median age of the mother at time of birth in both periods was 33 years. The prepandemic period had 1,681 births, and the pandemic period had 1,718 births.
Although there were found to be fewer nulliparous women and fewer women with hypertension in the pandemic period, the incidence of stillbirth in that period was significantly higher (n = 16 [9 per 1,000 births]) than in the prepandemic period (n = 4 [2 per 1,000 births]) (difference, 7 per 1,000 births; 95% confidence interval, 1.83-12.0; P = .01). The pandemic rate remained higher when late terminations for fetal abnormality were excluded (difference 6 per 1,000 births; 95% CI 1.54-10.1; P = .01).
None of the pregnant women who experienced stillbirth had COVID-19 symptoms, and none of the postmortems or placental exams indicated infection. There were no significant differences between the two periods in regard to births before 37 weeks’ gestation, births after 34 weeks’ gestation, neonatal unit admission, or cesarean delivery.
“It’s very important to highlight the effects of the pandemic on pregnant patients, even if they’re not infected with COVID-19,” Shannon Clark, MD, of the University of Texas Medical Branch in Galveston said in an interview.
She noted several COVID-related considerations that could have contributed to this increase: the reluctance of both low-risk and high-risk patients to enter a hospital setting during a pandemic, along with safety-centered changes made in antenatal services and care, which includes a reduced number of ultrasounds and screening exams.
“Checking a patient’s blood pressure, checking their weight changes, checking how the baby is growing,” she said. “They’re all simple things that just can’t be done via telemedicine.”
“We’ve thought a lot about the potential effects of getting COVID in pregnancy,” she added, “but it’s just as important to think about what might happen to those who don’t have it and are considered low risk otherwise.”
The study authors noted its limitations, including it being retrospective, analyzing a short time frame, and focusing on a single medical center. It also didn’t factor in the causes of the stillbirths, nor were the time periods precisely comparable, although they did add that “there is no seasonality to stillbirths in the UK.”
One doctor reported receiving grants outside of the submitted work. No other potential conflicts of interest were noted. Dr. Clark said she had no relevant financial disclosures.
SOURCE: Khalil A et al. JAMA. 2020 Jul. doi: 10.1001/jama.2020.12746.
FROM JAMA
Novel program cuts weight retention after gestational diabetes
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ADA 2020
Neural tube defect risk from dolutegravir drops as clinical experience grows
The newest data, based on 3,591 deliveries among women in Botswana infected by HIV and treated with dolutegravir at the time of conception during a little more than 5.5 years through April 2020, showed that dolutegravir use at conception linked with 7 cases of neonatal neural tube defects (NTDs), a 0.19% rate that exceeded comparator rates by about 1 in every 1,000 deliveries, far below the 0.94% rate initially found and that raised a red flag 2 years ago (New Engl J Med. 2018 Sep 6;379[10]:979-81). “The prevalence of NTDs among infants born to women on dolutegravir at conception may be stabilizing at approximately 2 per 1,000,” said Rebecca Zash, MD, during the virtual meeting of the International AIDS conference.
“This small absolute risk for neural tube defects is far outweighed by the potential benefits from dolutegravir” for better tolerability than alternative drugs and fewer drug-drug interactions. “This should allow for broader use of dolutegravir in women,” added Dr. Zash, an HIV specialist at Beth Israel Deaconess Medical Center and codirector of the Placental Scientific Working Group of the Harvard University Center for AIDS Research, both in Boston.
“What this has taught us is that women are not a niche population” of people infected with HIV, but rather constitute about half of HIV patients worldwide. “Maintaining gender equity in HIV treatment requires safety data for treatments during pregnancy,” she said during a press briefing.
The new findings mean that it’s “time to lay to rest” concerns about neural tube defects (NTDs) in infants born to women treated with dolutegravir, “given the incredible benefits of dolutegravir,” commented Monica Gandhi, MD, professor of medicine and associate chief of the division of HIV, infectious disease, and global medicine at the University of California, San Francisco. Another benefit from removing any caveats about use of dolutegravir in women who could become pregnant is that it would simplify treatment recommendations and make dolutegravir the unqualified first-line agent for treating HIV infection, Dr. Gandhi said during the briefing. “It’s super reassuring to have these data, as the incidence of NTDs goes down and down,” she added.
Following the alarm raised by initial findings from the Tsepamo study in 2018, Dr. Zash and associates first updated their data through March 2019, when they reported a revised cumulative NTD incidence rate of 0.3% (New Engl J Med. 2019 Aug 29;381[9]:827-40). The Tsepamo study began by following the pregnancy outcomes of women at eight Botswana sites during August 2014–July 2018, representing 45% of the country’s deliveries. This expanded to 18 sites and 72% of deliveries during July-September 2018, and then starting in September 2019 the scope slightly reduced to 16 Botswana sites with 70% of the nation’s deliveries.
Folate supplementation to women who might conceive is vital, but remains spotty in Botswana. “Folate supplementation is a no-brainer, but has had really slow adoption in many countries,” Dr. Zash said. “Folate supplementation, especially in food so that everyone gets it, will reduce NTDs by half.” The two most recent cases of infants born with a NTD to mothers who had been on dolutegravir at conception occurred in mothers who had received no folate supplementation, Dr. Zash reported.
The most recent HIV treatment guidelines for adults from the Department of Health & Human Services, which date from late 2019, designated dolutegravir plus lamivudine as a first-line regimen for most, but flagged it as an “alternative” antiretroviral drug when treating women who have childbearing potential and are either trying to conceive or are sexually active but not using contraception.
The study had no commercial funding. Dr. Zash has been a researcher in studies funded by CytoDyn, Fulcrum, and Gilead. Dr. Gandhi had no commercial disclosures.
The newest data, based on 3,591 deliveries among women in Botswana infected by HIV and treated with dolutegravir at the time of conception during a little more than 5.5 years through April 2020, showed that dolutegravir use at conception linked with 7 cases of neonatal neural tube defects (NTDs), a 0.19% rate that exceeded comparator rates by about 1 in every 1,000 deliveries, far below the 0.94% rate initially found and that raised a red flag 2 years ago (New Engl J Med. 2018 Sep 6;379[10]:979-81). “The prevalence of NTDs among infants born to women on dolutegravir at conception may be stabilizing at approximately 2 per 1,000,” said Rebecca Zash, MD, during the virtual meeting of the International AIDS conference.
“This small absolute risk for neural tube defects is far outweighed by the potential benefits from dolutegravir” for better tolerability than alternative drugs and fewer drug-drug interactions. “This should allow for broader use of dolutegravir in women,” added Dr. Zash, an HIV specialist at Beth Israel Deaconess Medical Center and codirector of the Placental Scientific Working Group of the Harvard University Center for AIDS Research, both in Boston.
“What this has taught us is that women are not a niche population” of people infected with HIV, but rather constitute about half of HIV patients worldwide. “Maintaining gender equity in HIV treatment requires safety data for treatments during pregnancy,” she said during a press briefing.
The new findings mean that it’s “time to lay to rest” concerns about neural tube defects (NTDs) in infants born to women treated with dolutegravir, “given the incredible benefits of dolutegravir,” commented Monica Gandhi, MD, professor of medicine and associate chief of the division of HIV, infectious disease, and global medicine at the University of California, San Francisco. Another benefit from removing any caveats about use of dolutegravir in women who could become pregnant is that it would simplify treatment recommendations and make dolutegravir the unqualified first-line agent for treating HIV infection, Dr. Gandhi said during the briefing. “It’s super reassuring to have these data, as the incidence of NTDs goes down and down,” she added.
Following the alarm raised by initial findings from the Tsepamo study in 2018, Dr. Zash and associates first updated their data through March 2019, when they reported a revised cumulative NTD incidence rate of 0.3% (New Engl J Med. 2019 Aug 29;381[9]:827-40). The Tsepamo study began by following the pregnancy outcomes of women at eight Botswana sites during August 2014–July 2018, representing 45% of the country’s deliveries. This expanded to 18 sites and 72% of deliveries during July-September 2018, and then starting in September 2019 the scope slightly reduced to 16 Botswana sites with 70% of the nation’s deliveries.
Folate supplementation to women who might conceive is vital, but remains spotty in Botswana. “Folate supplementation is a no-brainer, but has had really slow adoption in many countries,” Dr. Zash said. “Folate supplementation, especially in food so that everyone gets it, will reduce NTDs by half.” The two most recent cases of infants born with a NTD to mothers who had been on dolutegravir at conception occurred in mothers who had received no folate supplementation, Dr. Zash reported.
The most recent HIV treatment guidelines for adults from the Department of Health & Human Services, which date from late 2019, designated dolutegravir plus lamivudine as a first-line regimen for most, but flagged it as an “alternative” antiretroviral drug when treating women who have childbearing potential and are either trying to conceive or are sexually active but not using contraception.
The study had no commercial funding. Dr. Zash has been a researcher in studies funded by CytoDyn, Fulcrum, and Gilead. Dr. Gandhi had no commercial disclosures.
The newest data, based on 3,591 deliveries among women in Botswana infected by HIV and treated with dolutegravir at the time of conception during a little more than 5.5 years through April 2020, showed that dolutegravir use at conception linked with 7 cases of neonatal neural tube defects (NTDs), a 0.19% rate that exceeded comparator rates by about 1 in every 1,000 deliveries, far below the 0.94% rate initially found and that raised a red flag 2 years ago (New Engl J Med. 2018 Sep 6;379[10]:979-81). “The prevalence of NTDs among infants born to women on dolutegravir at conception may be stabilizing at approximately 2 per 1,000,” said Rebecca Zash, MD, during the virtual meeting of the International AIDS conference.
“This small absolute risk for neural tube defects is far outweighed by the potential benefits from dolutegravir” for better tolerability than alternative drugs and fewer drug-drug interactions. “This should allow for broader use of dolutegravir in women,” added Dr. Zash, an HIV specialist at Beth Israel Deaconess Medical Center and codirector of the Placental Scientific Working Group of the Harvard University Center for AIDS Research, both in Boston.
“What this has taught us is that women are not a niche population” of people infected with HIV, but rather constitute about half of HIV patients worldwide. “Maintaining gender equity in HIV treatment requires safety data for treatments during pregnancy,” she said during a press briefing.
The new findings mean that it’s “time to lay to rest” concerns about neural tube defects (NTDs) in infants born to women treated with dolutegravir, “given the incredible benefits of dolutegravir,” commented Monica Gandhi, MD, professor of medicine and associate chief of the division of HIV, infectious disease, and global medicine at the University of California, San Francisco. Another benefit from removing any caveats about use of dolutegravir in women who could become pregnant is that it would simplify treatment recommendations and make dolutegravir the unqualified first-line agent for treating HIV infection, Dr. Gandhi said during the briefing. “It’s super reassuring to have these data, as the incidence of NTDs goes down and down,” she added.
Following the alarm raised by initial findings from the Tsepamo study in 2018, Dr. Zash and associates first updated their data through March 2019, when they reported a revised cumulative NTD incidence rate of 0.3% (New Engl J Med. 2019 Aug 29;381[9]:827-40). The Tsepamo study began by following the pregnancy outcomes of women at eight Botswana sites during August 2014–July 2018, representing 45% of the country’s deliveries. This expanded to 18 sites and 72% of deliveries during July-September 2018, and then starting in September 2019 the scope slightly reduced to 16 Botswana sites with 70% of the nation’s deliveries.
Folate supplementation to women who might conceive is vital, but remains spotty in Botswana. “Folate supplementation is a no-brainer, but has had really slow adoption in many countries,” Dr. Zash said. “Folate supplementation, especially in food so that everyone gets it, will reduce NTDs by half.” The two most recent cases of infants born with a NTD to mothers who had been on dolutegravir at conception occurred in mothers who had received no folate supplementation, Dr. Zash reported.
The most recent HIV treatment guidelines for adults from the Department of Health & Human Services, which date from late 2019, designated dolutegravir plus lamivudine as a first-line regimen for most, but flagged it as an “alternative” antiretroviral drug when treating women who have childbearing potential and are either trying to conceive or are sexually active but not using contraception.
The study had no commercial funding. Dr. Zash has been a researcher in studies funded by CytoDyn, Fulcrum, and Gilead. Dr. Gandhi had no commercial disclosures.
FROM AIDS 2020
Physician leadership: Racial disparities and racism. Where do we go from here?
The destructive toll COVID-19 has caused worldwide is devastating. In the United States, the disproportionate deaths of Black, Indigenous, and Latinx people due to structural racism, amplified by economic adversity, is unacceptable. Meanwhile, the continued murder of Black people by those sworn to protect the public is abhorrent and can no longer be ignored. Black lives matter. These crises have rightly gripped our attention, and should galvanize physicians individually and collectively to use our privileged voices and relative power for justice. We must strive for engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.
The COVID-19 pandemic has illuminated the vast inequities in our country. It has highlighted the continued poor outcomes our health and health care systems create for Black, Indigenous, and Latinx communities. It also has demonstrated clearly that we are all connected—one large community, interdependent yet rife with differential power, privilege, and oppression. We must address these racial disparities—not only in the name of justice and good health for all but also because it is a moral and ethical imperative for us as physicians—and SARS-CoV-2 clearly shows us that it is in the best interest of everyone to do so.
First step: A deep dive look at systemic racism
What is first needed is an examination and acknowledgement by medicine and health care at large of the deeply entrenched roots of systemic and institutional racism in our profession and care systems, and their disproportionate and unjust impact on the health and livelihood of communities of color. The COVID-19 pandemic is only a recent example that highlights the perpetuation of a system that harms people of color. Racism, sexism, gender discrimination, economic and social injustice, religious persecution, and violence against women and children are age-old. We have yet to see health care institutions implement system-wide intersectional and antiracist practices to address them. Mandatory implicit bias training, policies for inclusion and diversity, and position statements are necessary first steps; however, they are not a panacea. They are insufficient to create the bold changes we need. The time for words has long passed. It is time to listen, to hear the cries of anguish and outrage, to examine our privileged position, to embrace change and discomfort, and most importantly to act, and to lead in dismantling the structures around us that perpetuate racial inequity.
How can we, as physicians and leaders, join in action and make an impact?
Dr. Camara Jones, past president of the American Public Health Association, describes 3 levels of racism:
- structural or systemic
- individual or personally mediated
- internalized.
Interventions at each level are important if we are to promote equity in health and health care. This framework can help us think about the following strategic initiatives.
Continue to: 1. Commit to becoming an antiracist and engage in independent study...
1. Commit to becoming antiracist and engage in independent study. This is an important first step as it will form the foundations for interventions—one cannot facilitate change without understanding the matter at hand. This step also may be the most personally challenging step forcing all of us to wrestle with discomfort, sadness, fear, guilt, and a host of other emotional responses. Remember that great change has never been born out of comfort, and the discomfort physicians may experience while unlearning racism and learning antiracism pales in comparison to what communities of color experience daily. We must actively work to unlearn the racist and anti-Black culture that is so deeply woven into every aspect of our existence.
Learn the history that was not given to us as kids in school. Read the brilliant literary works of Black, Indigenous, and Latinx artists and scholars on dismantling racism. Expand our vocabulary and knowledge of core concepts in racism, racial justice, and equity. Examine and reflect on our day-to-day practices. Be vocal in our commitment to antiracism—the time has passed for staying silent. If you are white, facilitate conversations about race with your white colleagues; the inherent power of racism relegates it to an issue that can never be on the table, but it is time to dismantle that power. Learn what acts of meaningful and intentional alliances are and when we need to give up power or privilege to a person of color. We also need to recognize that we as physicians, while leaders in many spaces, are not leaders in the powerful racial justice grassroots movements. We should learn from these movements, follow their lead, and use our privilege to uplift racial justice in our settings.
2. Embrace the current complexities with empathy and humility, finding ways to exercise our civic responsibility to the public with compassion. During the COVID-19 pandemic we have seen the devastation that social isolation, job loss, and illness can create. Suddenly those who could never have imagined themselves without food are waiting hours in their cars for food bank donations or are finding empty shelves in stores. Those who were not safe at home were suddenly imprisoned indefinitely in unsafe situations. Those who were comfortable, well-insured, and healthy are facing an invisible health threat, insecurity, fear, anxiety, and loss. Additionally, our civic institutions are failing. Those of us who always took our right to vote for granted are being forced to stand in hours’-long lines to exercise that right; while those who have been systematically disenfranchised are enduring even greater threats to their constitutional right to exercise their political power, disallowing them to speak for their families and communities and to vote for the justice they deserve. This may be an opportunity to stop blaming victims and recognize the toll that structural and systemic contributions to inequity have created over generations.
3. Meaningfully engage with and advocate for patients. In health and health care, we must begin to engage with the communities we serve and truly listen to their needs, desires, and barriers to care, and respond accordingly. Policies that try to address the social determinants of health without that engagement, and without the acknowledgement of the structural issues that cause them, however well-intentioned, are unlikely to accomplish their goals. We need to advocate as physicians and leaders in our settings for every policy, practice, and procedure to be scrutinized using an antiracist lens. To execute this, we need to:
- ask why clinic and hospital practices are built the way they are and how to make them more reflexive and responsive to individual patient’s needs
- examine what the disproportionate impacts might be on different groups of patients from a systems-level
- be ready to dismantle and/or rebuild something that is exacerbating disparate outcomes and experiences
- advocate for change that is built upon the narratives of patients and their communities.
We should include patients in the creation of hospital policies and guidelines in order to shift power toward them and to be transparent about how the system operates in order to facilitate trust and collaboration that centers patients and communities in the systems created to serve them.
Continue to: 4. Intentionally repair and build trust...
4. Intentionally repair and build trust. To create a safe environment, we must repair what we have broken and earn the trust of communities by uplifting their voices and redistributing our power to them in changing the systems and structures that have, for generations, kept Black, Indigenous, and Latinx people oppressed. Building trust requires first owning our histories of colonization, genocide, and slavery—now turned mass incarceration, debasement, and exploitation—that has existed for centuries. We as physicians need to do an honest examination of how we have eroded the trust of the very communities we care for since our profession’s creation. We need to acknowledge, as a white-dominant profession, the medical experimentation on and exploitation of Black and Brown bodies, and how this formed the foundation for a very valid deep distrust and fear of the medical establishment. We need to recognize how our inherent racial biases continue to feed this distrust, like when we don’t treat patients’ pain adequately or make them feel like we believe and listen to their needs and concerns. We must acknowledge our complicity in perpetuating the racial inequities in health, again highlighted by the COVID-19 pandemic.
5. Increase Black, Indigenous, and Latinx representation in physician and other health care professions’ workforce. Racism impacts not only patients but also our colleagues of color. The lack of racial diversity is a symptom of racism and a representation of the continued exclusion and devaluing of physicians of color. We must recognize this legacy of exclusion and facilitate intentional recruitment, retention, inclusion, and belonging of people of color into our workforce. Tokenism, the act of symbolically including one or few people from underrepresented groups, has been a weapon used by our workforce against physicians of color, resulting in isolation, “othering,” demoralization, and other deleterious impacts. We need to reverse this history and diversify our training programs and workforce to ensure justice in our own community.
6. Design multifaceted interventions. Multilevel problems require multilevel solutions. Interventions targeted solely at one level, while helpful, are unlikely to result in the larger scale changes our society needs to implement if we are to eradicate the impact of racism on health. We have long known that it is not just “preexisting conditions” or “poor” individual behaviors that lead to negative and disparate health outcomes—these are impacted by social and structural determinants much larger and more deleterious than that. It is critically important that we allocate and redistribute resources to create safe and affordable housing; childcare and preschool facilities; healthy, available, and affordable food; equitable and affordable educational opportunities; and a clean environment to support the health of all communities—not only those with the highest tax base. It is imperative that we strive to understand the lives of our fellow human beings who have been subjected to intergenerational social injustices and oppressions that have continued to place them at the margins of society. We need to center the lived experiences of communities of color in the design of multilevel interventions, especially Black and Indigenous communities. While we as physicians cannot individually impact education, economic, or food/environment systems, we can use our power to advocate for providing resources for the patients we care for and can create strategies within the health care system to address these needs in order to achieve optimal health. Robust and equitable social structures are the foundations for health, and ensuring equitable access to them is critical to reducing disparities.
Commit to lead
We must commit to unlearning our internalized racism, rebuilding relationships with communities of color, and engaging in antiracist practices. As a profession dedicated to healing, we have an obligation to be leaders in advocating for these changes, and dismantling the inequitable structure of our health care system.
Our challenge now is to articulate solutions. While antiracism should be informed by the lived experiences of communities of color, the work of antiracism is not their responsibility. In fact, it is the responsibility of our white-dominated systems and institutions to change.
There are some solutions that are easier to enumerate because they have easily measurable outcomes or activities, such as:
- collecting data transparently
- identifying inequities in access, treatment, and care
- conducting rigorous root cause analysis of those barriers to care
- increasing diverse racial and gender representation on decision-making bodies, from board rooms to committees, from leadership teams to research participants
- redistribute power by paving the way for underrepresented colleagues to participate in clinical, administrative, educational, executive, and health policy spaces
- mentoring new leaders who come from marginalized communities.
Every patient deserves our expertise and access to high-quality care. We should review our patient panels to ensure we are taking steps personally to be just and eliminate disparities, and we should monitor the results of those efforts.
Continue to: Be open to solutions that may make us “uncomfortable”...
Be open to solutions that may make us “uncomfortable”
There are other solutions, perhaps those that would be more effective on a larger scale, which may be harder to measure using our traditional ways of inquiry or measurement. Solutions that may create discomfort, anger, or fear for those who have held their power or positions for a long time. We need to begin to engage in developing, cultivating, and valuing innovative strategies that produce equally valid knowledge, evidence, and solutions without engaging in a randomized controlled trial. We need to reinvent the way inquiry, investigation, and implementation are done, and utilize novel, justice-informed strategies that include real-world evidence to produce results that are applicable to all (not just those willing to participate in sponsored trials). Only then will we be able to provide equitable health outcomes for all.
We also must accept responsibility for the past and humbly ask communities to work with us as we struggle to eliminate racism and dehumanization of Black lives by calling out our actions or inaction, recognizing the impact of our privileged status, and stepping down or stepping aside to allow others to lead. Sometimes it is as simple as turning off the Zoom camera so others can talk. By redistributing power and focusing this work upon the narratives of marginalized communities, we can improve our system for everyone. We must lead with action within our practices and systems; become advocates within our communities, institutions, and profession; strategize and organize interventions at both structural and individual levels to first recognize and name—then change—the systems; and unlearn behaviors that perpetuate racism.
Inaction is shirking our responsibility among the medical community
Benign inaction and unintentional acquiescence with “the way things are and have always been” abdicates our responsibility as physicians to improve the health of our patients and our communities. The modern Hippocratic Oath reminds us: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.” We have a professional and ethical responsibility to ensure health equity, and thus racial equity. As physicians, as healers, as leaders we must address racial inequities at all levels as we commit to improving the health of our nation. We can no longer stand silent in the face of the violence, brutality, and injustices our patients, friends, family, neighbors, communities, and society as a whole live through daily. It is unjust and inhumane to do so.
To be silent is to be complicit. As Gandhi said so long ago, we must “be the change we wish to see in the world.” And as Ijeoma Olua teaches us, “Anti-racism is the commitment to fight racism wherever you find it, including in yourself. And it’s the only way forward.”
- “So You Want to Talk about Race” Ijeoma Oluo
- “How to Be an Antiracist” Ibram X. Kendi
- “Between the World and Me” Ta-Nehisi Coates
- A conversation on race and privilege (Angela Davis and Jane Elliot) https://www.youtube.com/watch?reload=9&v=S0jf8D5WHoo
- Uncomfortable conversations with a Black man (Emmanuel Acho) https://www.youtube.com/watch?v=h8jUA7JBkF4
Antiracism – defined as the work of actively opposing racism by advocating for changes in political, economic, and social life. Antiracism tends to be an individualized approach, and set up in opposition to individual racist behaviors and impacts
Black Lives Matter – a political movement to address systemic and state violence against African Americans. Per the Black Lives Matter organizers: “In 2013, three radical Black organizers—Alicia Garza, Patrisse Cullors, and Opal Tometi—created a Black-centered political will and movement building project called BlackLivesMatter. It was in response to the acquittal of Trayvon Martin’s murderer, George Zimmerman. The project is now a member-led global network of more than 40 chapters. Members organize and build local power to intervene in violence inflicted on Black communities by the state and vigilantes. Black Lives Matter is an ideological and political intervention in a world where Black lives are systematically and intentionally targeted for demise. It is an affirmation of Black folks’ humanity, our contributions to this society, and our resilience in the face of deadly oppression.”
Implicit bias – also known as unconscious or hidden bias, implicit biases are negative associations that people unknowingly hold. They are expressed automatically, without conscious awareness. Many studies have indicated that implicit biases affect individuals’ attitudes and actions, thus creating real-world implications, even though individuals may not even be aware that those biases exist within themselves. Notably, implicit biases have been shown to trump individuals stated commitments to equality and fairness, thereby producing behavior that diverges from the explicit attitudes that many people profess.
Othering – view or treat (a person or group of people) as intrinsically different from and alien to oneself. (From https://lexico.com.)
For a full glossary of terms, visit RacialEquityTools.org (https://www.racialequitytools.org/glossary#anti-black)
The destructive toll COVID-19 has caused worldwide is devastating. In the United States, the disproportionate deaths of Black, Indigenous, and Latinx people due to structural racism, amplified by economic adversity, is unacceptable. Meanwhile, the continued murder of Black people by those sworn to protect the public is abhorrent and can no longer be ignored. Black lives matter. These crises have rightly gripped our attention, and should galvanize physicians individually and collectively to use our privileged voices and relative power for justice. We must strive for engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.
The COVID-19 pandemic has illuminated the vast inequities in our country. It has highlighted the continued poor outcomes our health and health care systems create for Black, Indigenous, and Latinx communities. It also has demonstrated clearly that we are all connected—one large community, interdependent yet rife with differential power, privilege, and oppression. We must address these racial disparities—not only in the name of justice and good health for all but also because it is a moral and ethical imperative for us as physicians—and SARS-CoV-2 clearly shows us that it is in the best interest of everyone to do so.
First step: A deep dive look at systemic racism
What is first needed is an examination and acknowledgement by medicine and health care at large of the deeply entrenched roots of systemic and institutional racism in our profession and care systems, and their disproportionate and unjust impact on the health and livelihood of communities of color. The COVID-19 pandemic is only a recent example that highlights the perpetuation of a system that harms people of color. Racism, sexism, gender discrimination, economic and social injustice, religious persecution, and violence against women and children are age-old. We have yet to see health care institutions implement system-wide intersectional and antiracist practices to address them. Mandatory implicit bias training, policies for inclusion and diversity, and position statements are necessary first steps; however, they are not a panacea. They are insufficient to create the bold changes we need. The time for words has long passed. It is time to listen, to hear the cries of anguish and outrage, to examine our privileged position, to embrace change and discomfort, and most importantly to act, and to lead in dismantling the structures around us that perpetuate racial inequity.
How can we, as physicians and leaders, join in action and make an impact?
Dr. Camara Jones, past president of the American Public Health Association, describes 3 levels of racism:
- structural or systemic
- individual or personally mediated
- internalized.
Interventions at each level are important if we are to promote equity in health and health care. This framework can help us think about the following strategic initiatives.
Continue to: 1. Commit to becoming an antiracist and engage in independent study...
1. Commit to becoming antiracist and engage in independent study. This is an important first step as it will form the foundations for interventions—one cannot facilitate change without understanding the matter at hand. This step also may be the most personally challenging step forcing all of us to wrestle with discomfort, sadness, fear, guilt, and a host of other emotional responses. Remember that great change has never been born out of comfort, and the discomfort physicians may experience while unlearning racism and learning antiracism pales in comparison to what communities of color experience daily. We must actively work to unlearn the racist and anti-Black culture that is so deeply woven into every aspect of our existence.
Learn the history that was not given to us as kids in school. Read the brilliant literary works of Black, Indigenous, and Latinx artists and scholars on dismantling racism. Expand our vocabulary and knowledge of core concepts in racism, racial justice, and equity. Examine and reflect on our day-to-day practices. Be vocal in our commitment to antiracism—the time has passed for staying silent. If you are white, facilitate conversations about race with your white colleagues; the inherent power of racism relegates it to an issue that can never be on the table, but it is time to dismantle that power. Learn what acts of meaningful and intentional alliances are and when we need to give up power or privilege to a person of color. We also need to recognize that we as physicians, while leaders in many spaces, are not leaders in the powerful racial justice grassroots movements. We should learn from these movements, follow their lead, and use our privilege to uplift racial justice in our settings.
2. Embrace the current complexities with empathy and humility, finding ways to exercise our civic responsibility to the public with compassion. During the COVID-19 pandemic we have seen the devastation that social isolation, job loss, and illness can create. Suddenly those who could never have imagined themselves without food are waiting hours in their cars for food bank donations or are finding empty shelves in stores. Those who were not safe at home were suddenly imprisoned indefinitely in unsafe situations. Those who were comfortable, well-insured, and healthy are facing an invisible health threat, insecurity, fear, anxiety, and loss. Additionally, our civic institutions are failing. Those of us who always took our right to vote for granted are being forced to stand in hours’-long lines to exercise that right; while those who have been systematically disenfranchised are enduring even greater threats to their constitutional right to exercise their political power, disallowing them to speak for their families and communities and to vote for the justice they deserve. This may be an opportunity to stop blaming victims and recognize the toll that structural and systemic contributions to inequity have created over generations.
3. Meaningfully engage with and advocate for patients. In health and health care, we must begin to engage with the communities we serve and truly listen to their needs, desires, and barriers to care, and respond accordingly. Policies that try to address the social determinants of health without that engagement, and without the acknowledgement of the structural issues that cause them, however well-intentioned, are unlikely to accomplish their goals. We need to advocate as physicians and leaders in our settings for every policy, practice, and procedure to be scrutinized using an antiracist lens. To execute this, we need to:
- ask why clinic and hospital practices are built the way they are and how to make them more reflexive and responsive to individual patient’s needs
- examine what the disproportionate impacts might be on different groups of patients from a systems-level
- be ready to dismantle and/or rebuild something that is exacerbating disparate outcomes and experiences
- advocate for change that is built upon the narratives of patients and their communities.
We should include patients in the creation of hospital policies and guidelines in order to shift power toward them and to be transparent about how the system operates in order to facilitate trust and collaboration that centers patients and communities in the systems created to serve them.
Continue to: 4. Intentionally repair and build trust...
4. Intentionally repair and build trust. To create a safe environment, we must repair what we have broken and earn the trust of communities by uplifting their voices and redistributing our power to them in changing the systems and structures that have, for generations, kept Black, Indigenous, and Latinx people oppressed. Building trust requires first owning our histories of colonization, genocide, and slavery—now turned mass incarceration, debasement, and exploitation—that has existed for centuries. We as physicians need to do an honest examination of how we have eroded the trust of the very communities we care for since our profession’s creation. We need to acknowledge, as a white-dominant profession, the medical experimentation on and exploitation of Black and Brown bodies, and how this formed the foundation for a very valid deep distrust and fear of the medical establishment. We need to recognize how our inherent racial biases continue to feed this distrust, like when we don’t treat patients’ pain adequately or make them feel like we believe and listen to their needs and concerns. We must acknowledge our complicity in perpetuating the racial inequities in health, again highlighted by the COVID-19 pandemic.
5. Increase Black, Indigenous, and Latinx representation in physician and other health care professions’ workforce. Racism impacts not only patients but also our colleagues of color. The lack of racial diversity is a symptom of racism and a representation of the continued exclusion and devaluing of physicians of color. We must recognize this legacy of exclusion and facilitate intentional recruitment, retention, inclusion, and belonging of people of color into our workforce. Tokenism, the act of symbolically including one or few people from underrepresented groups, has been a weapon used by our workforce against physicians of color, resulting in isolation, “othering,” demoralization, and other deleterious impacts. We need to reverse this history and diversify our training programs and workforce to ensure justice in our own community.
6. Design multifaceted interventions. Multilevel problems require multilevel solutions. Interventions targeted solely at one level, while helpful, are unlikely to result in the larger scale changes our society needs to implement if we are to eradicate the impact of racism on health. We have long known that it is not just “preexisting conditions” or “poor” individual behaviors that lead to negative and disparate health outcomes—these are impacted by social and structural determinants much larger and more deleterious than that. It is critically important that we allocate and redistribute resources to create safe and affordable housing; childcare and preschool facilities; healthy, available, and affordable food; equitable and affordable educational opportunities; and a clean environment to support the health of all communities—not only those with the highest tax base. It is imperative that we strive to understand the lives of our fellow human beings who have been subjected to intergenerational social injustices and oppressions that have continued to place them at the margins of society. We need to center the lived experiences of communities of color in the design of multilevel interventions, especially Black and Indigenous communities. While we as physicians cannot individually impact education, economic, or food/environment systems, we can use our power to advocate for providing resources for the patients we care for and can create strategies within the health care system to address these needs in order to achieve optimal health. Robust and equitable social structures are the foundations for health, and ensuring equitable access to them is critical to reducing disparities.
Commit to lead
We must commit to unlearning our internalized racism, rebuilding relationships with communities of color, and engaging in antiracist practices. As a profession dedicated to healing, we have an obligation to be leaders in advocating for these changes, and dismantling the inequitable structure of our health care system.
Our challenge now is to articulate solutions. While antiracism should be informed by the lived experiences of communities of color, the work of antiracism is not their responsibility. In fact, it is the responsibility of our white-dominated systems and institutions to change.
There are some solutions that are easier to enumerate because they have easily measurable outcomes or activities, such as:
- collecting data transparently
- identifying inequities in access, treatment, and care
- conducting rigorous root cause analysis of those barriers to care
- increasing diverse racial and gender representation on decision-making bodies, from board rooms to committees, from leadership teams to research participants
- redistribute power by paving the way for underrepresented colleagues to participate in clinical, administrative, educational, executive, and health policy spaces
- mentoring new leaders who come from marginalized communities.
Every patient deserves our expertise and access to high-quality care. We should review our patient panels to ensure we are taking steps personally to be just and eliminate disparities, and we should monitor the results of those efforts.
Continue to: Be open to solutions that may make us “uncomfortable”...
Be open to solutions that may make us “uncomfortable”
There are other solutions, perhaps those that would be more effective on a larger scale, which may be harder to measure using our traditional ways of inquiry or measurement. Solutions that may create discomfort, anger, or fear for those who have held their power or positions for a long time. We need to begin to engage in developing, cultivating, and valuing innovative strategies that produce equally valid knowledge, evidence, and solutions without engaging in a randomized controlled trial. We need to reinvent the way inquiry, investigation, and implementation are done, and utilize novel, justice-informed strategies that include real-world evidence to produce results that are applicable to all (not just those willing to participate in sponsored trials). Only then will we be able to provide equitable health outcomes for all.
We also must accept responsibility for the past and humbly ask communities to work with us as we struggle to eliminate racism and dehumanization of Black lives by calling out our actions or inaction, recognizing the impact of our privileged status, and stepping down or stepping aside to allow others to lead. Sometimes it is as simple as turning off the Zoom camera so others can talk. By redistributing power and focusing this work upon the narratives of marginalized communities, we can improve our system for everyone. We must lead with action within our practices and systems; become advocates within our communities, institutions, and profession; strategize and organize interventions at both structural and individual levels to first recognize and name—then change—the systems; and unlearn behaviors that perpetuate racism.
Inaction is shirking our responsibility among the medical community
Benign inaction and unintentional acquiescence with “the way things are and have always been” abdicates our responsibility as physicians to improve the health of our patients and our communities. The modern Hippocratic Oath reminds us: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.” We have a professional and ethical responsibility to ensure health equity, and thus racial equity. As physicians, as healers, as leaders we must address racial inequities at all levels as we commit to improving the health of our nation. We can no longer stand silent in the face of the violence, brutality, and injustices our patients, friends, family, neighbors, communities, and society as a whole live through daily. It is unjust and inhumane to do so.
To be silent is to be complicit. As Gandhi said so long ago, we must “be the change we wish to see in the world.” And as Ijeoma Olua teaches us, “Anti-racism is the commitment to fight racism wherever you find it, including in yourself. And it’s the only way forward.”
- “So You Want to Talk about Race” Ijeoma Oluo
- “How to Be an Antiracist” Ibram X. Kendi
- “Between the World and Me” Ta-Nehisi Coates
- A conversation on race and privilege (Angela Davis and Jane Elliot) https://www.youtube.com/watch?reload=9&v=S0jf8D5WHoo
- Uncomfortable conversations with a Black man (Emmanuel Acho) https://www.youtube.com/watch?v=h8jUA7JBkF4
Antiracism – defined as the work of actively opposing racism by advocating for changes in political, economic, and social life. Antiracism tends to be an individualized approach, and set up in opposition to individual racist behaviors and impacts
Black Lives Matter – a political movement to address systemic and state violence against African Americans. Per the Black Lives Matter organizers: “In 2013, three radical Black organizers—Alicia Garza, Patrisse Cullors, and Opal Tometi—created a Black-centered political will and movement building project called BlackLivesMatter. It was in response to the acquittal of Trayvon Martin’s murderer, George Zimmerman. The project is now a member-led global network of more than 40 chapters. Members organize and build local power to intervene in violence inflicted on Black communities by the state and vigilantes. Black Lives Matter is an ideological and political intervention in a world where Black lives are systematically and intentionally targeted for demise. It is an affirmation of Black folks’ humanity, our contributions to this society, and our resilience in the face of deadly oppression.”
Implicit bias – also known as unconscious or hidden bias, implicit biases are negative associations that people unknowingly hold. They are expressed automatically, without conscious awareness. Many studies have indicated that implicit biases affect individuals’ attitudes and actions, thus creating real-world implications, even though individuals may not even be aware that those biases exist within themselves. Notably, implicit biases have been shown to trump individuals stated commitments to equality and fairness, thereby producing behavior that diverges from the explicit attitudes that many people profess.
Othering – view or treat (a person or group of people) as intrinsically different from and alien to oneself. (From https://lexico.com.)
For a full glossary of terms, visit RacialEquityTools.org (https://www.racialequitytools.org/glossary#anti-black)
The destructive toll COVID-19 has caused worldwide is devastating. In the United States, the disproportionate deaths of Black, Indigenous, and Latinx people due to structural racism, amplified by economic adversity, is unacceptable. Meanwhile, the continued murder of Black people by those sworn to protect the public is abhorrent and can no longer be ignored. Black lives matter. These crises have rightly gripped our attention, and should galvanize physicians individually and collectively to use our privileged voices and relative power for justice. We must strive for engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.
The COVID-19 pandemic has illuminated the vast inequities in our country. It has highlighted the continued poor outcomes our health and health care systems create for Black, Indigenous, and Latinx communities. It also has demonstrated clearly that we are all connected—one large community, interdependent yet rife with differential power, privilege, and oppression. We must address these racial disparities—not only in the name of justice and good health for all but also because it is a moral and ethical imperative for us as physicians—and SARS-CoV-2 clearly shows us that it is in the best interest of everyone to do so.
First step: A deep dive look at systemic racism
What is first needed is an examination and acknowledgement by medicine and health care at large of the deeply entrenched roots of systemic and institutional racism in our profession and care systems, and their disproportionate and unjust impact on the health and livelihood of communities of color. The COVID-19 pandemic is only a recent example that highlights the perpetuation of a system that harms people of color. Racism, sexism, gender discrimination, economic and social injustice, religious persecution, and violence against women and children are age-old. We have yet to see health care institutions implement system-wide intersectional and antiracist practices to address them. Mandatory implicit bias training, policies for inclusion and diversity, and position statements are necessary first steps; however, they are not a panacea. They are insufficient to create the bold changes we need. The time for words has long passed. It is time to listen, to hear the cries of anguish and outrage, to examine our privileged position, to embrace change and discomfort, and most importantly to act, and to lead in dismantling the structures around us that perpetuate racial inequity.
How can we, as physicians and leaders, join in action and make an impact?
Dr. Camara Jones, past president of the American Public Health Association, describes 3 levels of racism:
- structural or systemic
- individual or personally mediated
- internalized.
Interventions at each level are important if we are to promote equity in health and health care. This framework can help us think about the following strategic initiatives.
Continue to: 1. Commit to becoming an antiracist and engage in independent study...
1. Commit to becoming antiracist and engage in independent study. This is an important first step as it will form the foundations for interventions—one cannot facilitate change without understanding the matter at hand. This step also may be the most personally challenging step forcing all of us to wrestle with discomfort, sadness, fear, guilt, and a host of other emotional responses. Remember that great change has never been born out of comfort, and the discomfort physicians may experience while unlearning racism and learning antiracism pales in comparison to what communities of color experience daily. We must actively work to unlearn the racist and anti-Black culture that is so deeply woven into every aspect of our existence.
Learn the history that was not given to us as kids in school. Read the brilliant literary works of Black, Indigenous, and Latinx artists and scholars on dismantling racism. Expand our vocabulary and knowledge of core concepts in racism, racial justice, and equity. Examine and reflect on our day-to-day practices. Be vocal in our commitment to antiracism—the time has passed for staying silent. If you are white, facilitate conversations about race with your white colleagues; the inherent power of racism relegates it to an issue that can never be on the table, but it is time to dismantle that power. Learn what acts of meaningful and intentional alliances are and when we need to give up power or privilege to a person of color. We also need to recognize that we as physicians, while leaders in many spaces, are not leaders in the powerful racial justice grassroots movements. We should learn from these movements, follow their lead, and use our privilege to uplift racial justice in our settings.
2. Embrace the current complexities with empathy and humility, finding ways to exercise our civic responsibility to the public with compassion. During the COVID-19 pandemic we have seen the devastation that social isolation, job loss, and illness can create. Suddenly those who could never have imagined themselves without food are waiting hours in their cars for food bank donations or are finding empty shelves in stores. Those who were not safe at home were suddenly imprisoned indefinitely in unsafe situations. Those who were comfortable, well-insured, and healthy are facing an invisible health threat, insecurity, fear, anxiety, and loss. Additionally, our civic institutions are failing. Those of us who always took our right to vote for granted are being forced to stand in hours’-long lines to exercise that right; while those who have been systematically disenfranchised are enduring even greater threats to their constitutional right to exercise their political power, disallowing them to speak for their families and communities and to vote for the justice they deserve. This may be an opportunity to stop blaming victims and recognize the toll that structural and systemic contributions to inequity have created over generations.
3. Meaningfully engage with and advocate for patients. In health and health care, we must begin to engage with the communities we serve and truly listen to their needs, desires, and barriers to care, and respond accordingly. Policies that try to address the social determinants of health without that engagement, and without the acknowledgement of the structural issues that cause them, however well-intentioned, are unlikely to accomplish their goals. We need to advocate as physicians and leaders in our settings for every policy, practice, and procedure to be scrutinized using an antiracist lens. To execute this, we need to:
- ask why clinic and hospital practices are built the way they are and how to make them more reflexive and responsive to individual patient’s needs
- examine what the disproportionate impacts might be on different groups of patients from a systems-level
- be ready to dismantle and/or rebuild something that is exacerbating disparate outcomes and experiences
- advocate for change that is built upon the narratives of patients and their communities.
We should include patients in the creation of hospital policies and guidelines in order to shift power toward them and to be transparent about how the system operates in order to facilitate trust and collaboration that centers patients and communities in the systems created to serve them.
Continue to: 4. Intentionally repair and build trust...
4. Intentionally repair and build trust. To create a safe environment, we must repair what we have broken and earn the trust of communities by uplifting their voices and redistributing our power to them in changing the systems and structures that have, for generations, kept Black, Indigenous, and Latinx people oppressed. Building trust requires first owning our histories of colonization, genocide, and slavery—now turned mass incarceration, debasement, and exploitation—that has existed for centuries. We as physicians need to do an honest examination of how we have eroded the trust of the very communities we care for since our profession’s creation. We need to acknowledge, as a white-dominant profession, the medical experimentation on and exploitation of Black and Brown bodies, and how this formed the foundation for a very valid deep distrust and fear of the medical establishment. We need to recognize how our inherent racial biases continue to feed this distrust, like when we don’t treat patients’ pain adequately or make them feel like we believe and listen to their needs and concerns. We must acknowledge our complicity in perpetuating the racial inequities in health, again highlighted by the COVID-19 pandemic.
5. Increase Black, Indigenous, and Latinx representation in physician and other health care professions’ workforce. Racism impacts not only patients but also our colleagues of color. The lack of racial diversity is a symptom of racism and a representation of the continued exclusion and devaluing of physicians of color. We must recognize this legacy of exclusion and facilitate intentional recruitment, retention, inclusion, and belonging of people of color into our workforce. Tokenism, the act of symbolically including one or few people from underrepresented groups, has been a weapon used by our workforce against physicians of color, resulting in isolation, “othering,” demoralization, and other deleterious impacts. We need to reverse this history and diversify our training programs and workforce to ensure justice in our own community.
6. Design multifaceted interventions. Multilevel problems require multilevel solutions. Interventions targeted solely at one level, while helpful, are unlikely to result in the larger scale changes our society needs to implement if we are to eradicate the impact of racism on health. We have long known that it is not just “preexisting conditions” or “poor” individual behaviors that lead to negative and disparate health outcomes—these are impacted by social and structural determinants much larger and more deleterious than that. It is critically important that we allocate and redistribute resources to create safe and affordable housing; childcare and preschool facilities; healthy, available, and affordable food; equitable and affordable educational opportunities; and a clean environment to support the health of all communities—not only those with the highest tax base. It is imperative that we strive to understand the lives of our fellow human beings who have been subjected to intergenerational social injustices and oppressions that have continued to place them at the margins of society. We need to center the lived experiences of communities of color in the design of multilevel interventions, especially Black and Indigenous communities. While we as physicians cannot individually impact education, economic, or food/environment systems, we can use our power to advocate for providing resources for the patients we care for and can create strategies within the health care system to address these needs in order to achieve optimal health. Robust and equitable social structures are the foundations for health, and ensuring equitable access to them is critical to reducing disparities.
Commit to lead
We must commit to unlearning our internalized racism, rebuilding relationships with communities of color, and engaging in antiracist practices. As a profession dedicated to healing, we have an obligation to be leaders in advocating for these changes, and dismantling the inequitable structure of our health care system.
Our challenge now is to articulate solutions. While antiracism should be informed by the lived experiences of communities of color, the work of antiracism is not their responsibility. In fact, it is the responsibility of our white-dominated systems and institutions to change.
There are some solutions that are easier to enumerate because they have easily measurable outcomes or activities, such as:
- collecting data transparently
- identifying inequities in access, treatment, and care
- conducting rigorous root cause analysis of those barriers to care
- increasing diverse racial and gender representation on decision-making bodies, from board rooms to committees, from leadership teams to research participants
- redistribute power by paving the way for underrepresented colleagues to participate in clinical, administrative, educational, executive, and health policy spaces
- mentoring new leaders who come from marginalized communities.
Every patient deserves our expertise and access to high-quality care. We should review our patient panels to ensure we are taking steps personally to be just and eliminate disparities, and we should monitor the results of those efforts.
Continue to: Be open to solutions that may make us “uncomfortable”...
Be open to solutions that may make us “uncomfortable”
There are other solutions, perhaps those that would be more effective on a larger scale, which may be harder to measure using our traditional ways of inquiry or measurement. Solutions that may create discomfort, anger, or fear for those who have held their power or positions for a long time. We need to begin to engage in developing, cultivating, and valuing innovative strategies that produce equally valid knowledge, evidence, and solutions without engaging in a randomized controlled trial. We need to reinvent the way inquiry, investigation, and implementation are done, and utilize novel, justice-informed strategies that include real-world evidence to produce results that are applicable to all (not just those willing to participate in sponsored trials). Only then will we be able to provide equitable health outcomes for all.
We also must accept responsibility for the past and humbly ask communities to work with us as we struggle to eliminate racism and dehumanization of Black lives by calling out our actions or inaction, recognizing the impact of our privileged status, and stepping down or stepping aside to allow others to lead. Sometimes it is as simple as turning off the Zoom camera so others can talk. By redistributing power and focusing this work upon the narratives of marginalized communities, we can improve our system for everyone. We must lead with action within our practices and systems; become advocates within our communities, institutions, and profession; strategize and organize interventions at both structural and individual levels to first recognize and name—then change—the systems; and unlearn behaviors that perpetuate racism.
Inaction is shirking our responsibility among the medical community
Benign inaction and unintentional acquiescence with “the way things are and have always been” abdicates our responsibility as physicians to improve the health of our patients and our communities. The modern Hippocratic Oath reminds us: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.” We have a professional and ethical responsibility to ensure health equity, and thus racial equity. As physicians, as healers, as leaders we must address racial inequities at all levels as we commit to improving the health of our nation. We can no longer stand silent in the face of the violence, brutality, and injustices our patients, friends, family, neighbors, communities, and society as a whole live through daily. It is unjust and inhumane to do so.
To be silent is to be complicit. As Gandhi said so long ago, we must “be the change we wish to see in the world.” And as Ijeoma Olua teaches us, “Anti-racism is the commitment to fight racism wherever you find it, including in yourself. And it’s the only way forward.”
- “So You Want to Talk about Race” Ijeoma Oluo
- “How to Be an Antiracist” Ibram X. Kendi
- “Between the World and Me” Ta-Nehisi Coates
- A conversation on race and privilege (Angela Davis and Jane Elliot) https://www.youtube.com/watch?reload=9&v=S0jf8D5WHoo
- Uncomfortable conversations with a Black man (Emmanuel Acho) https://www.youtube.com/watch?v=h8jUA7JBkF4
Antiracism – defined as the work of actively opposing racism by advocating for changes in political, economic, and social life. Antiracism tends to be an individualized approach, and set up in opposition to individual racist behaviors and impacts
Black Lives Matter – a political movement to address systemic and state violence against African Americans. Per the Black Lives Matter organizers: “In 2013, three radical Black organizers—Alicia Garza, Patrisse Cullors, and Opal Tometi—created a Black-centered political will and movement building project called BlackLivesMatter. It was in response to the acquittal of Trayvon Martin’s murderer, George Zimmerman. The project is now a member-led global network of more than 40 chapters. Members organize and build local power to intervene in violence inflicted on Black communities by the state and vigilantes. Black Lives Matter is an ideological and political intervention in a world where Black lives are systematically and intentionally targeted for demise. It is an affirmation of Black folks’ humanity, our contributions to this society, and our resilience in the face of deadly oppression.”
Implicit bias – also known as unconscious or hidden bias, implicit biases are negative associations that people unknowingly hold. They are expressed automatically, without conscious awareness. Many studies have indicated that implicit biases affect individuals’ attitudes and actions, thus creating real-world implications, even though individuals may not even be aware that those biases exist within themselves. Notably, implicit biases have been shown to trump individuals stated commitments to equality and fairness, thereby producing behavior that diverges from the explicit attitudes that many people profess.
Othering – view or treat (a person or group of people) as intrinsically different from and alien to oneself. (From https://lexico.com.)
For a full glossary of terms, visit RacialEquityTools.org (https://www.racialequitytools.org/glossary#anti-black)
The in-person postpartum blood pressure check: For whose benefit?
CASE Patient questions need for postpartum BP check
Ms. P presents at 28 weeks’ gestation with superimposed preeclampsia. She receives antenatal corticosteroids and titration of her nifedipine, but she is delivered at 29 weeks because of worsening fetal status. Her physician recommends a blood pressure (BP) visit in the office at 7 days postpartum.
She asks, “But can’t I just call you with the BP reading? And what do I do in the meantime?”
Hypertensive disorders of pregnancy and chronic hypertension remain among the leading causes of maternal morbidity and mortality in the United States and worldwide.1 The postpartum period remains a particularly high-risk time since up to 40% of maternal mortality can occur after delivery. To that end, the 2013 American College of Obstetricians and Gynecologists Hypertension in Pregnancy Task Force recommends postpartum follow-up 7 to 10 days after delivery in women with a hypertensive disorder of pregnancy.2
Why we need to find an alternative approach
Unfortunately, these guidelines are both cumbersome and insufficient. Up to one-third of patients do not attend their postpartum visit, particularly those who are young, uninsured, and nonwhite, a list uncomfortably similar to that for women most at risk for adverse outcomes after a high-risk pregnancy. In addition, the 7- to 10-day visit still represents only a single snapshot of the patient’s BP values rather than an ongoing assessment of symptoms or BP elevation over time. Moreover, studies also have shown that BP in both normotensive and hypertensive women often rises by the fifth day postpartum, suggesting that leaving this large window of time without surveillance may miss an opportunity to detect elevated BP in a more timely manner.3
It is time to break the habit of the in-office postpartum BP check and to evaluate the patient where she is and when she needs it. Research in the last 2 years shows that there are several solutions to our case patient’s question.

Solution 1: The provider-driven system
“Of course. Text us your numbers, and you will hear from the doctor if you need to do anything differently.”
One method that addresses both the communication and safety issues inherent in the 7- to 10-day routine in-office BP check is to have the patient send in her BP measurements for direct clinician review.
Researchers at the University of Pennsylvania developed a robust program using their Way to Health platform.4 Participating patients text their BP values twice daily, and they receive automated feedback for all values, with additional human feedback in real time from a clinician for severe-range values (>160 mm Hg systolic or >110 mm Hg diastolic). As an added safety measure, a physician reviews all inputted BPs daily and assesses the need for antihypertensive medication for BPs in the high mild range. Using this protocol, the researchers achieved a significant increase in adherence with the recommendation for reporting a BP value in the first 10 days after discharge (from 44% to 92%) as well as having fewer readmissions in the text-messaging arm (4% vs 0%).
Perhaps most impressive, though, is that the technology use eliminated pre-existing racial disparities in adherence. Black participants were as likely as nonblack participants to report a postpartum BP in the text-messaging system (93% vs 91%) despite being less than half as likely to keep a BP check visit (33% vs 70%).5
A similar solution is in place at the University of Pittsburgh, where a text message system on the Vivify platform is used to deliver patient BP measurements to a centralized monitoring team.6 This program is unique in that, rather than relying on a single physician, it is run through a nurse “call center” that allowed them to expand to 3 hospitals with the use of a single centralized monitoring team. To date, the program has enrolled more than 2,000 patients and achieved patient satisfaction rates greater than 94%.
A final program to consider was developed and piloted at the University of Wisconsin with an added technological advance: the use of a Bluetooth-enabled BP cuff that permits values to be automatically transmitted to a tablet that then uploads the information to a centralized database.7 This database was in turn monitored by trained nurses for safety and initiation or titration of antihypertensive medication as needed. Similar to the experience at the University of Pennsylvania, the researchers found improved adherence with monitoring and a notable reduction in readmissions (3.7% in controls vs 0.5% in the intervention arm). Of note, among those who did receive the ongoing monitoring, severe hypertension occurred in 56 (26.2%) of those patients and did so a mean of 6 days after discharge (that is, prior to when they typically would have seen a provider.)
The promise of such provider-driven systems is that they represent a true chronicle of a patient’s ongoing clinical course rather than a single snapshot of her BP in an artificial environment (and often after the highest risk time period!). In addition, direct monitoring by clinicians ensures an optimal safety profile.
Such systems, however, are also extremely resource intense in terms of both upfront information technology investment and ongoing provider surveillance. The systems above also relied on giving the patients a BP cuff, so it is unclear whether it was the technology support or this simple intervention that yielded the benefits. Nonetheless, the benefits were undeniable, and the financial costs saved by reducing even 1 hospital admission as well as the costs of outpatient surveillance may in the end justify these upfront expenditures.
Continue to: Solution 2: The algorithm-driven system...
Solution 2: The algorithm-driven system
“Sure. Plug your numbers into our system, and you’ll receive an automated response as to what to do next.”
One way to alleviate both the financial and opportunity cost of constant clinician surveillance would be to offload some tasks to algorithmic support. This approach—home BP monitoring accompanied by self-titration of antihypertensive medication—has been validated in outpatient primary care hypertension management in nonpregnant adults and more recently for postpartum patients as well.
In the SNAP-HT trial, investigators randomly assigned women to either usual care or algorithm-driven outpatient BP management.8 While both groups had serial visits (for safety monitoring), those in the experimental arm were advised only by the algorithm for any ongoing titration of medication. At 6 weeks, the investigators found that BPs were lower in the intervention group, and diastolic BPs remained lower at 6 months.
This methodology emphasizes the potential utility of true self-management of hypertension in the postpartum period. It relies, however, on having a highly developed system in place that can receive the data, respond with recommendations, and safely monitor for any aberrations in the feed. Still, this hybrid method may represent the sweet spot: a combination that ensures adequate surveillance while not overburdening the clinician with the simpler, initial steps in postpartum antihypertensive management.
Solution 3: The DIY system
“That’s a good point. I want to hear about your blood pressure readings in the meantime. Here’s what we can do.”
What about the 99% of practicing ObGyns who do not have an entire connected system for remote hypertension monitoring? A number of options can be put in place today with little cost and even less tech know-how (see “Do-it-yourself options for remote blood pressure monitoring,” below). Note that since many of these options would not be monitored in “real time” like the connected systems discussed above, the patient should be given strict parameters for contacting her clinician directly. These do-it-yourself, or DIY, methods are instead best for the purpose of chronic monitoring and medication titration but are still an improvement in communication over the single-serve BP check.
The bottom line
Pregnant women represent one of the most connected, Internet-savvy demographic groups of any patient population: More than three-quarters of pregnant women turn to the Internet for advice during their pregnancy.9,10 In addition, unlike most social determinants of health, such as housing, food access, and health care coverage, access to connected electronic devices differs little across racial lines, suggesting the potential for targeting health care inequities by implementing more—not less—technology into prenatal and postpartum care.
For this generation of new mothers, the in-office postpartum BP check is insufficient, artificial, and simply a waste of everyone’s time. While there is no one-size-fits-all approach, there are many options, and it is up to us as health care providers to facilitate the right care, in the right place, at the right time for our patients. ●
Acknowledgements: The authors would like to thank Haritha Pavuluri, Margaret Oliver, and Samantha Boniface for their assistance in the preparation of this manuscript.
Electronic health record (EHR) messaging
Most EHR systems have some form of patient messaging built in. Consider asking your patient to:
- message her blood pressure measurements every 1 to 2 days
- send a photo of handwritten blood pressure measurements
Vendor text messaging platforms
The year 2020 has seen the entire telehealth space grow tremendously, and platforms such as Doxy.me (https://doxy.me) and Updox (https://www.updox.com) allow secure text messaging with patients.
All-in-one connected vendor solutions
Third-party solutions are available that give the patient a connected blood pressure cuff, scale, and personalized app. For the clinician, these data then can be accessed either independently through a portal or can be integrated into the EHR. Examples of 2 companies include:
- Babyscripts (https://www.getbabyscripts.com)
- Wildflower Health (https://www.wildflowerhealth.com)
Telehealth visits
Scheduling weekly telephone or video visits (while not near the frequency of the above) would still yield greater engagement, and many payors currently reimburse for these visits at rates on par with in-person visits.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin summary, No. 222. Gestational hypertension and preeclampsia. Obstet Gynecol. 2020;135:1492-1495.
- American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122:1122-1131.
- Walters BN, Thompson ME, Lee A, et al. Blood pressure in the puerperium. Clin Sci. 1986;71:589-594.
- Hirshberg A, Downes K, Srinivas S. Comparing standard office-based follow-up with text-based remote monitoring in the management of postpartum hypertension: a randomised clinical trial. BMJ Qual Saf. 2018;27:871-877.
- Hirshberg A, Sammel MD, Srinivas SK. Text message remote monitoring reduced racial disparities in postpartum blood pressure ascertainment. Am J Obstet Gynecol. 2019;221:283-285.
- Hauspurg A, Lemon LS, Quinn BA, et al. A postpartum remote hypertension monitoring protocol implemented at the hospital level. Obstet Gynecol. 2019;134:685-691.
- Hoppe KK, Thomas N, Zernick M, et al. Telehealth with remote blood pressure monitoring compared to standard care for postpartum hypertension. Am J Obstet Gynecol. 2020;S0002-9378(20)30554-doi:10.1016/j.ajog.2020.05.027.
- Cairns AE, Tucker KL, Leeson P, et al. Self-management of postnatal hypertension. Hypertension. 2018;72:425-432.
- Pew Research Center. Mobile fact sheet, 2019. https://www.pewresearch.org/internet/fact-sheet/mobile/. Accessed June 16, 2020.
- Sayakhot P, Carolan-Olah M. Internet use by pregnant women seeking pregnancy-related information: a systematic review. BMC Pregnancy Childbirth. 2016;16:65
CASE Patient questions need for postpartum BP check
Ms. P presents at 28 weeks’ gestation with superimposed preeclampsia. She receives antenatal corticosteroids and titration of her nifedipine, but she is delivered at 29 weeks because of worsening fetal status. Her physician recommends a blood pressure (BP) visit in the office at 7 days postpartum.
She asks, “But can’t I just call you with the BP reading? And what do I do in the meantime?”
Hypertensive disorders of pregnancy and chronic hypertension remain among the leading causes of maternal morbidity and mortality in the United States and worldwide.1 The postpartum period remains a particularly high-risk time since up to 40% of maternal mortality can occur after delivery. To that end, the 2013 American College of Obstetricians and Gynecologists Hypertension in Pregnancy Task Force recommends postpartum follow-up 7 to 10 days after delivery in women with a hypertensive disorder of pregnancy.2
Why we need to find an alternative approach
Unfortunately, these guidelines are both cumbersome and insufficient. Up to one-third of patients do not attend their postpartum visit, particularly those who are young, uninsured, and nonwhite, a list uncomfortably similar to that for women most at risk for adverse outcomes after a high-risk pregnancy. In addition, the 7- to 10-day visit still represents only a single snapshot of the patient’s BP values rather than an ongoing assessment of symptoms or BP elevation over time. Moreover, studies also have shown that BP in both normotensive and hypertensive women often rises by the fifth day postpartum, suggesting that leaving this large window of time without surveillance may miss an opportunity to detect elevated BP in a more timely manner.3
It is time to break the habit of the in-office postpartum BP check and to evaluate the patient where she is and when she needs it. Research in the last 2 years shows that there are several solutions to our case patient’s question.

Solution 1: The provider-driven system
“Of course. Text us your numbers, and you will hear from the doctor if you need to do anything differently.”
One method that addresses both the communication and safety issues inherent in the 7- to 10-day routine in-office BP check is to have the patient send in her BP measurements for direct clinician review.
Researchers at the University of Pennsylvania developed a robust program using their Way to Health platform.4 Participating patients text their BP values twice daily, and they receive automated feedback for all values, with additional human feedback in real time from a clinician for severe-range values (>160 mm Hg systolic or >110 mm Hg diastolic). As an added safety measure, a physician reviews all inputted BPs daily and assesses the need for antihypertensive medication for BPs in the high mild range. Using this protocol, the researchers achieved a significant increase in adherence with the recommendation for reporting a BP value in the first 10 days after discharge (from 44% to 92%) as well as having fewer readmissions in the text-messaging arm (4% vs 0%).
Perhaps most impressive, though, is that the technology use eliminated pre-existing racial disparities in adherence. Black participants were as likely as nonblack participants to report a postpartum BP in the text-messaging system (93% vs 91%) despite being less than half as likely to keep a BP check visit (33% vs 70%).5
A similar solution is in place at the University of Pittsburgh, where a text message system on the Vivify platform is used to deliver patient BP measurements to a centralized monitoring team.6 This program is unique in that, rather than relying on a single physician, it is run through a nurse “call center” that allowed them to expand to 3 hospitals with the use of a single centralized monitoring team. To date, the program has enrolled more than 2,000 patients and achieved patient satisfaction rates greater than 94%.
A final program to consider was developed and piloted at the University of Wisconsin with an added technological advance: the use of a Bluetooth-enabled BP cuff that permits values to be automatically transmitted to a tablet that then uploads the information to a centralized database.7 This database was in turn monitored by trained nurses for safety and initiation or titration of antihypertensive medication as needed. Similar to the experience at the University of Pennsylvania, the researchers found improved adherence with monitoring and a notable reduction in readmissions (3.7% in controls vs 0.5% in the intervention arm). Of note, among those who did receive the ongoing monitoring, severe hypertension occurred in 56 (26.2%) of those patients and did so a mean of 6 days after discharge (that is, prior to when they typically would have seen a provider.)
The promise of such provider-driven systems is that they represent a true chronicle of a patient’s ongoing clinical course rather than a single snapshot of her BP in an artificial environment (and often after the highest risk time period!). In addition, direct monitoring by clinicians ensures an optimal safety profile.
Such systems, however, are also extremely resource intense in terms of both upfront information technology investment and ongoing provider surveillance. The systems above also relied on giving the patients a BP cuff, so it is unclear whether it was the technology support or this simple intervention that yielded the benefits. Nonetheless, the benefits were undeniable, and the financial costs saved by reducing even 1 hospital admission as well as the costs of outpatient surveillance may in the end justify these upfront expenditures.
Continue to: Solution 2: The algorithm-driven system...
Solution 2: The algorithm-driven system
“Sure. Plug your numbers into our system, and you’ll receive an automated response as to what to do next.”
One way to alleviate both the financial and opportunity cost of constant clinician surveillance would be to offload some tasks to algorithmic support. This approach—home BP monitoring accompanied by self-titration of antihypertensive medication—has been validated in outpatient primary care hypertension management in nonpregnant adults and more recently for postpartum patients as well.
In the SNAP-HT trial, investigators randomly assigned women to either usual care or algorithm-driven outpatient BP management.8 While both groups had serial visits (for safety monitoring), those in the experimental arm were advised only by the algorithm for any ongoing titration of medication. At 6 weeks, the investigators found that BPs were lower in the intervention group, and diastolic BPs remained lower at 6 months.
This methodology emphasizes the potential utility of true self-management of hypertension in the postpartum period. It relies, however, on having a highly developed system in place that can receive the data, respond with recommendations, and safely monitor for any aberrations in the feed. Still, this hybrid method may represent the sweet spot: a combination that ensures adequate surveillance while not overburdening the clinician with the simpler, initial steps in postpartum antihypertensive management.
Solution 3: The DIY system
“That’s a good point. I want to hear about your blood pressure readings in the meantime. Here’s what we can do.”
What about the 99% of practicing ObGyns who do not have an entire connected system for remote hypertension monitoring? A number of options can be put in place today with little cost and even less tech know-how (see “Do-it-yourself options for remote blood pressure monitoring,” below). Note that since many of these options would not be monitored in “real time” like the connected systems discussed above, the patient should be given strict parameters for contacting her clinician directly. These do-it-yourself, or DIY, methods are instead best for the purpose of chronic monitoring and medication titration but are still an improvement in communication over the single-serve BP check.
The bottom line
Pregnant women represent one of the most connected, Internet-savvy demographic groups of any patient population: More than three-quarters of pregnant women turn to the Internet for advice during their pregnancy.9,10 In addition, unlike most social determinants of health, such as housing, food access, and health care coverage, access to connected electronic devices differs little across racial lines, suggesting the potential for targeting health care inequities by implementing more—not less—technology into prenatal and postpartum care.
For this generation of new mothers, the in-office postpartum BP check is insufficient, artificial, and simply a waste of everyone’s time. While there is no one-size-fits-all approach, there are many options, and it is up to us as health care providers to facilitate the right care, in the right place, at the right time for our patients. ●
Acknowledgements: The authors would like to thank Haritha Pavuluri, Margaret Oliver, and Samantha Boniface for their assistance in the preparation of this manuscript.
Electronic health record (EHR) messaging
Most EHR systems have some form of patient messaging built in. Consider asking your patient to:
- message her blood pressure measurements every 1 to 2 days
- send a photo of handwritten blood pressure measurements
Vendor text messaging platforms
The year 2020 has seen the entire telehealth space grow tremendously, and platforms such as Doxy.me (https://doxy.me) and Updox (https://www.updox.com) allow secure text messaging with patients.
All-in-one connected vendor solutions
Third-party solutions are available that give the patient a connected blood pressure cuff, scale, and personalized app. For the clinician, these data then can be accessed either independently through a portal or can be integrated into the EHR. Examples of 2 companies include:
- Babyscripts (https://www.getbabyscripts.com)
- Wildflower Health (https://www.wildflowerhealth.com)
Telehealth visits
Scheduling weekly telephone or video visits (while not near the frequency of the above) would still yield greater engagement, and many payors currently reimburse for these visits at rates on par with in-person visits.
CASE Patient questions need for postpartum BP check
Ms. P presents at 28 weeks’ gestation with superimposed preeclampsia. She receives antenatal corticosteroids and titration of her nifedipine, but she is delivered at 29 weeks because of worsening fetal status. Her physician recommends a blood pressure (BP) visit in the office at 7 days postpartum.
She asks, “But can’t I just call you with the BP reading? And what do I do in the meantime?”
Hypertensive disorders of pregnancy and chronic hypertension remain among the leading causes of maternal morbidity and mortality in the United States and worldwide.1 The postpartum period remains a particularly high-risk time since up to 40% of maternal mortality can occur after delivery. To that end, the 2013 American College of Obstetricians and Gynecologists Hypertension in Pregnancy Task Force recommends postpartum follow-up 7 to 10 days after delivery in women with a hypertensive disorder of pregnancy.2
Why we need to find an alternative approach
Unfortunately, these guidelines are both cumbersome and insufficient. Up to one-third of patients do not attend their postpartum visit, particularly those who are young, uninsured, and nonwhite, a list uncomfortably similar to that for women most at risk for adverse outcomes after a high-risk pregnancy. In addition, the 7- to 10-day visit still represents only a single snapshot of the patient’s BP values rather than an ongoing assessment of symptoms or BP elevation over time. Moreover, studies also have shown that BP in both normotensive and hypertensive women often rises by the fifth day postpartum, suggesting that leaving this large window of time without surveillance may miss an opportunity to detect elevated BP in a more timely manner.3
It is time to break the habit of the in-office postpartum BP check and to evaluate the patient where she is and when she needs it. Research in the last 2 years shows that there are several solutions to our case patient’s question.

Solution 1: The provider-driven system
“Of course. Text us your numbers, and you will hear from the doctor if you need to do anything differently.”
One method that addresses both the communication and safety issues inherent in the 7- to 10-day routine in-office BP check is to have the patient send in her BP measurements for direct clinician review.
Researchers at the University of Pennsylvania developed a robust program using their Way to Health platform.4 Participating patients text their BP values twice daily, and they receive automated feedback for all values, with additional human feedback in real time from a clinician for severe-range values (>160 mm Hg systolic or >110 mm Hg diastolic). As an added safety measure, a physician reviews all inputted BPs daily and assesses the need for antihypertensive medication for BPs in the high mild range. Using this protocol, the researchers achieved a significant increase in adherence with the recommendation for reporting a BP value in the first 10 days after discharge (from 44% to 92%) as well as having fewer readmissions in the text-messaging arm (4% vs 0%).
Perhaps most impressive, though, is that the technology use eliminated pre-existing racial disparities in adherence. Black participants were as likely as nonblack participants to report a postpartum BP in the text-messaging system (93% vs 91%) despite being less than half as likely to keep a BP check visit (33% vs 70%).5
A similar solution is in place at the University of Pittsburgh, where a text message system on the Vivify platform is used to deliver patient BP measurements to a centralized monitoring team.6 This program is unique in that, rather than relying on a single physician, it is run through a nurse “call center” that allowed them to expand to 3 hospitals with the use of a single centralized monitoring team. To date, the program has enrolled more than 2,000 patients and achieved patient satisfaction rates greater than 94%.
A final program to consider was developed and piloted at the University of Wisconsin with an added technological advance: the use of a Bluetooth-enabled BP cuff that permits values to be automatically transmitted to a tablet that then uploads the information to a centralized database.7 This database was in turn monitored by trained nurses for safety and initiation or titration of antihypertensive medication as needed. Similar to the experience at the University of Pennsylvania, the researchers found improved adherence with monitoring and a notable reduction in readmissions (3.7% in controls vs 0.5% in the intervention arm). Of note, among those who did receive the ongoing monitoring, severe hypertension occurred in 56 (26.2%) of those patients and did so a mean of 6 days after discharge (that is, prior to when they typically would have seen a provider.)
The promise of such provider-driven systems is that they represent a true chronicle of a patient’s ongoing clinical course rather than a single snapshot of her BP in an artificial environment (and often after the highest risk time period!). In addition, direct monitoring by clinicians ensures an optimal safety profile.
Such systems, however, are also extremely resource intense in terms of both upfront information technology investment and ongoing provider surveillance. The systems above also relied on giving the patients a BP cuff, so it is unclear whether it was the technology support or this simple intervention that yielded the benefits. Nonetheless, the benefits were undeniable, and the financial costs saved by reducing even 1 hospital admission as well as the costs of outpatient surveillance may in the end justify these upfront expenditures.
Continue to: Solution 2: The algorithm-driven system...
Solution 2: The algorithm-driven system
“Sure. Plug your numbers into our system, and you’ll receive an automated response as to what to do next.”
One way to alleviate both the financial and opportunity cost of constant clinician surveillance would be to offload some tasks to algorithmic support. This approach—home BP monitoring accompanied by self-titration of antihypertensive medication—has been validated in outpatient primary care hypertension management in nonpregnant adults and more recently for postpartum patients as well.
In the SNAP-HT trial, investigators randomly assigned women to either usual care or algorithm-driven outpatient BP management.8 While both groups had serial visits (for safety monitoring), those in the experimental arm were advised only by the algorithm for any ongoing titration of medication. At 6 weeks, the investigators found that BPs were lower in the intervention group, and diastolic BPs remained lower at 6 months.
This methodology emphasizes the potential utility of true self-management of hypertension in the postpartum period. It relies, however, on having a highly developed system in place that can receive the data, respond with recommendations, and safely monitor for any aberrations in the feed. Still, this hybrid method may represent the sweet spot: a combination that ensures adequate surveillance while not overburdening the clinician with the simpler, initial steps in postpartum antihypertensive management.
Solution 3: The DIY system
“That’s a good point. I want to hear about your blood pressure readings in the meantime. Here’s what we can do.”
What about the 99% of practicing ObGyns who do not have an entire connected system for remote hypertension monitoring? A number of options can be put in place today with little cost and even less tech know-how (see “Do-it-yourself options for remote blood pressure monitoring,” below). Note that since many of these options would not be monitored in “real time” like the connected systems discussed above, the patient should be given strict parameters for contacting her clinician directly. These do-it-yourself, or DIY, methods are instead best for the purpose of chronic monitoring and medication titration but are still an improvement in communication over the single-serve BP check.
The bottom line
Pregnant women represent one of the most connected, Internet-savvy demographic groups of any patient population: More than three-quarters of pregnant women turn to the Internet for advice during their pregnancy.9,10 In addition, unlike most social determinants of health, such as housing, food access, and health care coverage, access to connected electronic devices differs little across racial lines, suggesting the potential for targeting health care inequities by implementing more—not less—technology into prenatal and postpartum care.
For this generation of new mothers, the in-office postpartum BP check is insufficient, artificial, and simply a waste of everyone’s time. While there is no one-size-fits-all approach, there are many options, and it is up to us as health care providers to facilitate the right care, in the right place, at the right time for our patients. ●
Acknowledgements: The authors would like to thank Haritha Pavuluri, Margaret Oliver, and Samantha Boniface for their assistance in the preparation of this manuscript.
Electronic health record (EHR) messaging
Most EHR systems have some form of patient messaging built in. Consider asking your patient to:
- message her blood pressure measurements every 1 to 2 days
- send a photo of handwritten blood pressure measurements
Vendor text messaging platforms
The year 2020 has seen the entire telehealth space grow tremendously, and platforms such as Doxy.me (https://doxy.me) and Updox (https://www.updox.com) allow secure text messaging with patients.
All-in-one connected vendor solutions
Third-party solutions are available that give the patient a connected blood pressure cuff, scale, and personalized app. For the clinician, these data then can be accessed either independently through a portal or can be integrated into the EHR. Examples of 2 companies include:
- Babyscripts (https://www.getbabyscripts.com)
- Wildflower Health (https://www.wildflowerhealth.com)
Telehealth visits
Scheduling weekly telephone or video visits (while not near the frequency of the above) would still yield greater engagement, and many payors currently reimburse for these visits at rates on par with in-person visits.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin summary, No. 222. Gestational hypertension and preeclampsia. Obstet Gynecol. 2020;135:1492-1495.
- American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122:1122-1131.
- Walters BN, Thompson ME, Lee A, et al. Blood pressure in the puerperium. Clin Sci. 1986;71:589-594.
- Hirshberg A, Downes K, Srinivas S. Comparing standard office-based follow-up with text-based remote monitoring in the management of postpartum hypertension: a randomised clinical trial. BMJ Qual Saf. 2018;27:871-877.
- Hirshberg A, Sammel MD, Srinivas SK. Text message remote monitoring reduced racial disparities in postpartum blood pressure ascertainment. Am J Obstet Gynecol. 2019;221:283-285.
- Hauspurg A, Lemon LS, Quinn BA, et al. A postpartum remote hypertension monitoring protocol implemented at the hospital level. Obstet Gynecol. 2019;134:685-691.
- Hoppe KK, Thomas N, Zernick M, et al. Telehealth with remote blood pressure monitoring compared to standard care for postpartum hypertension. Am J Obstet Gynecol. 2020;S0002-9378(20)30554-doi:10.1016/j.ajog.2020.05.027.
- Cairns AE, Tucker KL, Leeson P, et al. Self-management of postnatal hypertension. Hypertension. 2018;72:425-432.
- Pew Research Center. Mobile fact sheet, 2019. https://www.pewresearch.org/internet/fact-sheet/mobile/. Accessed June 16, 2020.
- Sayakhot P, Carolan-Olah M. Internet use by pregnant women seeking pregnancy-related information: a systematic review. BMC Pregnancy Childbirth. 2016;16:65
- American College of Obstetricians and Gynecologists. ACOG practice bulletin summary, No. 222. Gestational hypertension and preeclampsia. Obstet Gynecol. 2020;135:1492-1495.
- American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122:1122-1131.
- Walters BN, Thompson ME, Lee A, et al. Blood pressure in the puerperium. Clin Sci. 1986;71:589-594.
- Hirshberg A, Downes K, Srinivas S. Comparing standard office-based follow-up with text-based remote monitoring in the management of postpartum hypertension: a randomised clinical trial. BMJ Qual Saf. 2018;27:871-877.
- Hirshberg A, Sammel MD, Srinivas SK. Text message remote monitoring reduced racial disparities in postpartum blood pressure ascertainment. Am J Obstet Gynecol. 2019;221:283-285.
- Hauspurg A, Lemon LS, Quinn BA, et al. A postpartum remote hypertension monitoring protocol implemented at the hospital level. Obstet Gynecol. 2019;134:685-691.
- Hoppe KK, Thomas N, Zernick M, et al. Telehealth with remote blood pressure monitoring compared to standard care for postpartum hypertension. Am J Obstet Gynecol. 2020;S0002-9378(20)30554-doi:10.1016/j.ajog.2020.05.027.
- Cairns AE, Tucker KL, Leeson P, et al. Self-management of postnatal hypertension. Hypertension. 2018;72:425-432.
- Pew Research Center. Mobile fact sheet, 2019. https://www.pewresearch.org/internet/fact-sheet/mobile/. Accessed June 16, 2020.
- Sayakhot P, Carolan-Olah M. Internet use by pregnant women seeking pregnancy-related information: a systematic review. BMC Pregnancy Childbirth. 2016;16:65











