User login
Should all women with a history of OASI have a mediolateral episiotomy at their subsequent delivery?
Van Bavel J, Ravelli AC, Abu-Hanna A, et al. Risk factors for the recurrence of obstetrical anal sphincter injury and the role of a mediolateral episiotomy: an analysis of a national registry. BJOG. 2020;127:951-956.
EXPERT COMMENTARY
Women with a history of OASI are at increased risk for recurrence in a subsequent delivery. Higher rates of anal and fecal incontinence are reported in women with recurrent OASI (rOASI) compared with women who had an OASI only in their first delivery. Previous studies have reported recurrence rates of 5% to 7%,1 and some suggested that MLE may be protective, but standardized recommendations for mode of delivery and use of MLE currently are not available.
Recently, van Bavel and colleagues sought to determine the rate of rOASI in their population as well as the factors that increase and decrease the risk of this complication.
Details of the study
This cohort study used data from the Dutch Perinatal Registry (Perined) that included 268,607 women who had their first and second deliveries (singleton, term, vertex, < 43 weeks) vaginally in 2000–2009. The study’s primary objective was to determine the rate of rOASI in women who had OASI in their first delivery. The secondary objectives were to identify risk factors for rOASI and to assess the effect of MLE. For the purposes of this study, OASI was defined as subtotal and total rupture of the perineum, or grades 3A-4 as defined by the Royal College of Obstetricians and Gynaecologists.2
Within this cohort, 9,943 women had an OASI in their first delivery (4%), and the rate of rOASI was 5.8% (579 of 9,943). After multivariate analysis, the risk factors for rOASI were birth weight of 4,000 g or greater (odds ratio [OR], 2.1; 95% confidence interval [CI], 1.6–2.6) and duration of the second stage of labor of 30 minutes or longer (OR, 1.8; 95% CI, 1.4–2.3).
The MLE rate was 40.8% (4,054 of 9,943) and was associated with a lower rate of rOASI (OR, 0.3; 95% CI, 0.3–0.4). This association persisted when delivery type was separated into spontaneous and operative vaginal deliveries, with the number of MLEs needed to prevent one rOASI of 22 and 8, respectively. Birth weight of less than 3,000 g also was noted to be protective against rOASI (OR, 0.5; 95% CI, 0.3–0.9).
Based on these findings, as well as comparisons to previous studies, the authors concluded that MLE could be considered for routine use or at least discussed with all women with a prior OASI for prevention of rOASI.
Continue to: Study strengths and limitations...
Study strengths and limitations
A strength of this study was the large number of deliveries and the wide variation of practice included in the registry database, which promotes the generalizability of the results and reduces bias. This also provides an adequate base on which to determine an accurate rate of rOASI in the Dutch population.
One study limitation is that information is not available regarding how the episiotomies were performed (specifically, angle of incision), delivery techniques (“hands on” vs “hands off”), and indication for the episiotomy. Additional limitations suggested are that clinicians who perform an episiotomy may have an inherent bias regarding the protective nature of the procedure and may miss a rOASI due to inadequate examination postprocedure, overestimating its protective effect.
Finally, the relatively high rate of MLE and low rate of cesarean delivery (6.9%) in this study are specific to the Netherlands and do not reflect the obstetric practices used in many other countries. Generalizability of these results in the context of much lower MLE and higher cesarean delivery rates (as in the United States) would therefore be in question.●
Prevention of rOASI is important, as fecal incontinence is debilitating and difficult to treat. While this study provides evidence that MLE may protect against this complication, its results may not be generalizable to all patient or clinician populations. Differences in baseline rate of MLE and cesarean delivery, technique, indication, and comfort with repair—all not evaluated in this study—must be taken into account when counseling OASI patients about their options for delivery and the use of MLE in a subsequent pregnancy.
JAIMEY M. PAULI, MD
- Van Bavel J, Ravelli AC, Abu-Hanna A, et al. Risk factors for the recurrence of obstetrical anal sphincter injury and the role of a mediolateral episiotomy: an analysis of a national registry. BJOG. 2020;127:951-956.
- Royal College of Obstetricians and Gynaecologists. Green-top guideline No. 29: the management of third- and fourth-degree perineal tears. June 2014. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-29.pdf. Accessed June 12, 2020.
Van Bavel J, Ravelli AC, Abu-Hanna A, et al. Risk factors for the recurrence of obstetrical anal sphincter injury and the role of a mediolateral episiotomy: an analysis of a national registry. BJOG. 2020;127:951-956.
EXPERT COMMENTARY
Women with a history of OASI are at increased risk for recurrence in a subsequent delivery. Higher rates of anal and fecal incontinence are reported in women with recurrent OASI (rOASI) compared with women who had an OASI only in their first delivery. Previous studies have reported recurrence rates of 5% to 7%,1 and some suggested that MLE may be protective, but standardized recommendations for mode of delivery and use of MLE currently are not available.
Recently, van Bavel and colleagues sought to determine the rate of rOASI in their population as well as the factors that increase and decrease the risk of this complication.
Details of the study
This cohort study used data from the Dutch Perinatal Registry (Perined) that included 268,607 women who had their first and second deliveries (singleton, term, vertex, < 43 weeks) vaginally in 2000–2009. The study’s primary objective was to determine the rate of rOASI in women who had OASI in their first delivery. The secondary objectives were to identify risk factors for rOASI and to assess the effect of MLE. For the purposes of this study, OASI was defined as subtotal and total rupture of the perineum, or grades 3A-4 as defined by the Royal College of Obstetricians and Gynaecologists.2
Within this cohort, 9,943 women had an OASI in their first delivery (4%), and the rate of rOASI was 5.8% (579 of 9,943). After multivariate analysis, the risk factors for rOASI were birth weight of 4,000 g or greater (odds ratio [OR], 2.1; 95% confidence interval [CI], 1.6–2.6) and duration of the second stage of labor of 30 minutes or longer (OR, 1.8; 95% CI, 1.4–2.3).
The MLE rate was 40.8% (4,054 of 9,943) and was associated with a lower rate of rOASI (OR, 0.3; 95% CI, 0.3–0.4). This association persisted when delivery type was separated into spontaneous and operative vaginal deliveries, with the number of MLEs needed to prevent one rOASI of 22 and 8, respectively. Birth weight of less than 3,000 g also was noted to be protective against rOASI (OR, 0.5; 95% CI, 0.3–0.9).
Based on these findings, as well as comparisons to previous studies, the authors concluded that MLE could be considered for routine use or at least discussed with all women with a prior OASI for prevention of rOASI.
Continue to: Study strengths and limitations...
Study strengths and limitations
A strength of this study was the large number of deliveries and the wide variation of practice included in the registry database, which promotes the generalizability of the results and reduces bias. This also provides an adequate base on which to determine an accurate rate of rOASI in the Dutch population.
One study limitation is that information is not available regarding how the episiotomies were performed (specifically, angle of incision), delivery techniques (“hands on” vs “hands off”), and indication for the episiotomy. Additional limitations suggested are that clinicians who perform an episiotomy may have an inherent bias regarding the protective nature of the procedure and may miss a rOASI due to inadequate examination postprocedure, overestimating its protective effect.
Finally, the relatively high rate of MLE and low rate of cesarean delivery (6.9%) in this study are specific to the Netherlands and do not reflect the obstetric practices used in many other countries. Generalizability of these results in the context of much lower MLE and higher cesarean delivery rates (as in the United States) would therefore be in question.●
Prevention of rOASI is important, as fecal incontinence is debilitating and difficult to treat. While this study provides evidence that MLE may protect against this complication, its results may not be generalizable to all patient or clinician populations. Differences in baseline rate of MLE and cesarean delivery, technique, indication, and comfort with repair—all not evaluated in this study—must be taken into account when counseling OASI patients about their options for delivery and the use of MLE in a subsequent pregnancy.
JAIMEY M. PAULI, MD
Van Bavel J, Ravelli AC, Abu-Hanna A, et al. Risk factors for the recurrence of obstetrical anal sphincter injury and the role of a mediolateral episiotomy: an analysis of a national registry. BJOG. 2020;127:951-956.
EXPERT COMMENTARY
Women with a history of OASI are at increased risk for recurrence in a subsequent delivery. Higher rates of anal and fecal incontinence are reported in women with recurrent OASI (rOASI) compared with women who had an OASI only in their first delivery. Previous studies have reported recurrence rates of 5% to 7%,1 and some suggested that MLE may be protective, but standardized recommendations for mode of delivery and use of MLE currently are not available.
Recently, van Bavel and colleagues sought to determine the rate of rOASI in their population as well as the factors that increase and decrease the risk of this complication.
Details of the study
This cohort study used data from the Dutch Perinatal Registry (Perined) that included 268,607 women who had their first and second deliveries (singleton, term, vertex, < 43 weeks) vaginally in 2000–2009. The study’s primary objective was to determine the rate of rOASI in women who had OASI in their first delivery. The secondary objectives were to identify risk factors for rOASI and to assess the effect of MLE. For the purposes of this study, OASI was defined as subtotal and total rupture of the perineum, or grades 3A-4 as defined by the Royal College of Obstetricians and Gynaecologists.2
Within this cohort, 9,943 women had an OASI in their first delivery (4%), and the rate of rOASI was 5.8% (579 of 9,943). After multivariate analysis, the risk factors for rOASI were birth weight of 4,000 g or greater (odds ratio [OR], 2.1; 95% confidence interval [CI], 1.6–2.6) and duration of the second stage of labor of 30 minutes or longer (OR, 1.8; 95% CI, 1.4–2.3).
The MLE rate was 40.8% (4,054 of 9,943) and was associated with a lower rate of rOASI (OR, 0.3; 95% CI, 0.3–0.4). This association persisted when delivery type was separated into spontaneous and operative vaginal deliveries, with the number of MLEs needed to prevent one rOASI of 22 and 8, respectively. Birth weight of less than 3,000 g also was noted to be protective against rOASI (OR, 0.5; 95% CI, 0.3–0.9).
Based on these findings, as well as comparisons to previous studies, the authors concluded that MLE could be considered for routine use or at least discussed with all women with a prior OASI for prevention of rOASI.
Continue to: Study strengths and limitations...
Study strengths and limitations
A strength of this study was the large number of deliveries and the wide variation of practice included in the registry database, which promotes the generalizability of the results and reduces bias. This also provides an adequate base on which to determine an accurate rate of rOASI in the Dutch population.
One study limitation is that information is not available regarding how the episiotomies were performed (specifically, angle of incision), delivery techniques (“hands on” vs “hands off”), and indication for the episiotomy. Additional limitations suggested are that clinicians who perform an episiotomy may have an inherent bias regarding the protective nature of the procedure and may miss a rOASI due to inadequate examination postprocedure, overestimating its protective effect.
Finally, the relatively high rate of MLE and low rate of cesarean delivery (6.9%) in this study are specific to the Netherlands and do not reflect the obstetric practices used in many other countries. Generalizability of these results in the context of much lower MLE and higher cesarean delivery rates (as in the United States) would therefore be in question.●
Prevention of rOASI is important, as fecal incontinence is debilitating and difficult to treat. While this study provides evidence that MLE may protect against this complication, its results may not be generalizable to all patient or clinician populations. Differences in baseline rate of MLE and cesarean delivery, technique, indication, and comfort with repair—all not evaluated in this study—must be taken into account when counseling OASI patients about their options for delivery and the use of MLE in a subsequent pregnancy.
JAIMEY M. PAULI, MD
- Van Bavel J, Ravelli AC, Abu-Hanna A, et al. Risk factors for the recurrence of obstetrical anal sphincter injury and the role of a mediolateral episiotomy: an analysis of a national registry. BJOG. 2020;127:951-956.
- Royal College of Obstetricians and Gynaecologists. Green-top guideline No. 29: the management of third- and fourth-degree perineal tears. June 2014. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-29.pdf. Accessed June 12, 2020.
- Van Bavel J, Ravelli AC, Abu-Hanna A, et al. Risk factors for the recurrence of obstetrical anal sphincter injury and the role of a mediolateral episiotomy: an analysis of a national registry. BJOG. 2020;127:951-956.
- Royal College of Obstetricians and Gynaecologists. Green-top guideline No. 29: the management of third- and fourth-degree perineal tears. June 2014. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-29.pdf. Accessed June 12, 2020.
The Fetal Pillow: A new option for delivering the deeply impacted fetal head
Obstetricians know that a cesarean delivery (CD) for a woman with a prolonged second stage and a fetal head deeply impacted in the pelvis is challenging. In this situation, extensions of the uterine incision commonly occur, resulting in prolonged operative time and increased blood loss. Even more harrowing is the inability to deliver the fetal head, necessitating emergency assistance from other clinicians. In this situation, interventions that may be helpful include:
- extend or T the uterine incision
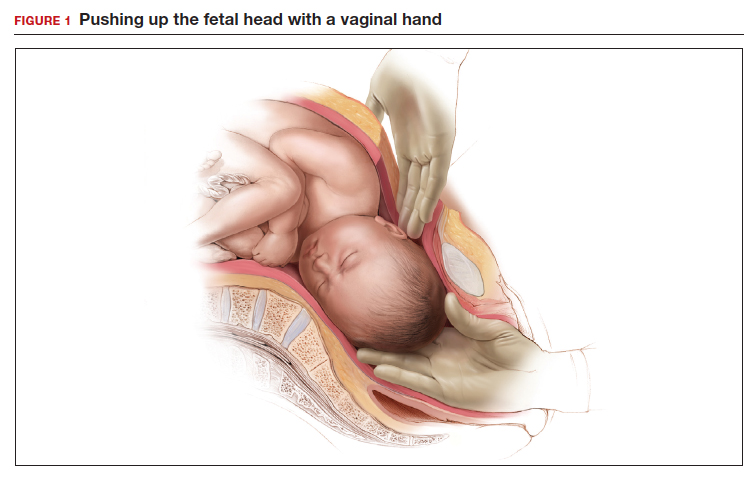
- enlist the aid of a clinician to push up on the fetal head with a vaginal hand (FIGURE 1)
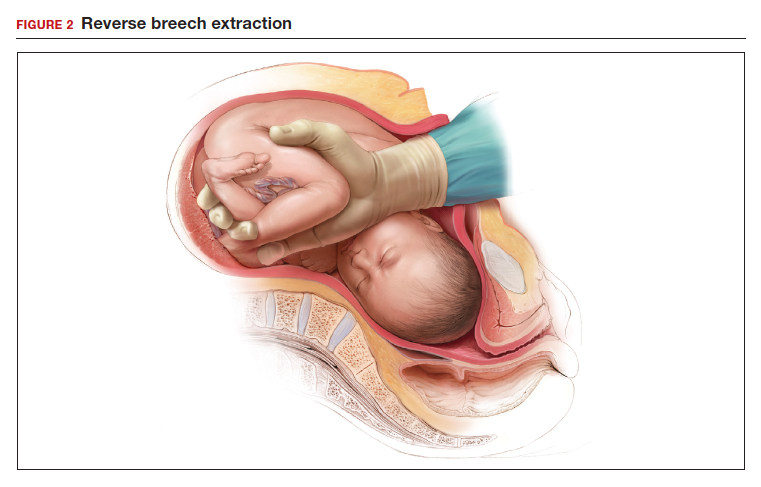
- reverse breech extraction (FIGURE 2), and
- vaginal insertion of a Fetal Pillow prior to starting the delivery.
Evidence from clinical trials indicates that reverse breech extraction or insertion of a Fetal Pillow result in the best clinical outcomes.
Reverse breech extraction vs the push technique
Although the data are limited, most studies report that compared with pushing up with a vaginal hand (as shown in Figure 1), the reverse breech extraction technique (as shown in Figure 2) is associated with a reduction in extensions of the uterine incision, reduced blood loss, and reduced operative time.1 In a randomized trial, 108 women with obstructed labor undergoing CD in the second stage were randomly assigned to reverse breech extraction or pushing up with a vaginal hand.2 Following the uterine incision, the reverse breech extraction technique is performed by immediately reaching into the upper uterus and grasping the lower portion of the fetal leg and applying gentle traction on the leg until the second leg appeared. The lower legs are then pulled out of the uterus. Standard breech delivery maneuvers are used to deliver the shoulders and head. In the trial, compared with the push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (30% vs 11%; P<.05), less blood loss (899 mL vs 1,257 mL; P<.001), and shorter operative time (56 min vs 89 min, P<.001). Fetal injury was similar with the push and breech extraction techniques (6% and 7%).
In another randomized trial, 192 women undergoing CD for obstructed labor were randomly assigned to reverse breech extraction or pushing the head up with a hand in the vagina.3 Compared with the vaginal push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (19% vs 48%; P = .003), fewer cases of wound infection (2% vs 13%; P = .007), and fewer blood transfusions (2 vs 11; P = .012).
Additional options and adjuvants for facilitating delivery of a fetal head deeply impacted in the pelvis include: using a Coyne spoon, using nitroglycerine or terbutaline to relax the myometrium, breaking the vaginal suction on the fetal head before attempting delivery, keeping the wrist of the delivering hand as straight as possible to reduce uterine incision extensions, and incising the ring (if a Bandl’s ring is detected).
Continue to: The Fetal Pillow...
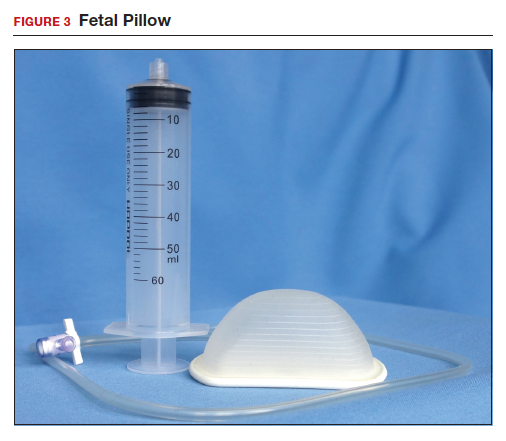
The Fetal Pillow
The Fetal Pillow (Safe Obstetric Systems, New York, New York) is a single-use fetal cephalic elevation device for managing the deeply impacted fetal head (FIGURE 3). The Fetal Pillow has a firm plastic base upon which is attached a soft silicon balloon. The Fetal Pillow is inserted into the vagina prior to initiating CD and the balloon is filled with 180 mL of saline, causing the fetal head to be pushed to a higher station (FIGURE 4). Use of the Fetal Pillow may be indicated prior to CD in the following situations:
- second stage labor with a deeply impacted head
- second stage labor and failed operative delivery
- occiput posterior position or deep transverse arrest
- absent progress in the first stage between 8 cm and 10 cm with a deeply impacted fetal head or excessive caput of the fetal head.
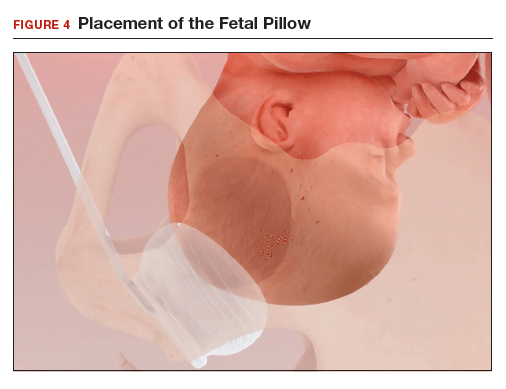
The Fetal Pillow is inserted after completing vaginal preparation for CD and before initiating skin preparation and abdominal draping. The steps for inserting the Fetal Pillow include:
- Use the 60 mL syringe to fully deflate the Fetal Pillow and leave the cock-stop open.
- Fold the Fetal Pillow by squeezing the firm plastic base, and with the patient’s legs in a frog-leg position, place the device in the vagina.
- Allow the firm plastic base to open to a flat position with the base against the posterior vaginal wall and the soft silicon balloon against the fetal head.
- Using pressure on the plastic base, gently push the Fetal Pillow posteriorly toward the sacrum of the mother.
- Use the 60 mL syringe to inflate the balloon with 180 mL of normal saline and close the valve.
- Straighten the patient’s legs and proceed with skin preparation and abdominal draping (FIGURE 4).
When the CD is completed, deflate the balloon by drawing out the saline with the 60 mL syringe and remove the device by hooking a finger around the firm plastic base. The Fetal Pillow is surprisingly easy to use.
Continue to: Effectiveness of the Fetal Pillow...
Effectiveness of the Fetal Pillow
In one randomized trial, 240 women undergoing CD were randomly allocated to a group in which the Fetal Pillow was placed in the vagina and inflated prior to the cesarean and a control group in which the Fetal Pillow was not used.
In another randomized trial, 60 nulliparous women undergoing CD in the second stage of labor had a Fetal Pillow inserted in the vagina and were randomly allocated to inflation of the pillow (Fetal Pillow group) or noninflation of the pillow (control group).5 In this study the mean length of the second stage was 4 hours. Compared with noninflation of the Fetal Pillow, use of the inflated Fetal Pillow was associated with a reduction in grade 3 extension of the uterine incision (extensions into the uterine artery, vagina, or bladder) (0% for inflation vs 13% for noninflation) and fewer difficult plus very difficult deliveries of the fetal head as reported by the surgeon (0% for inflation vs 37% for noninflation). There was no significant difference in blood loss between the two groups (800 mL vs 900 mL). These two randomized studies both reported that the use of the Fetal Pillow was associated with a reduction in grade 3 extensions of the uterine incision and a decrease in the difficulty of delivering the fetal head.
Consider trialing the Fetal Pillow
When a CD is performed after a prolonged second stage of labor, surgical complications are common, including extensions of the uterine incision and difficulty delivering the fetal head. When a grade 3 extension occurs—with tearing of a uterine artery, deep extension into the vagina, or damage to the bladder—the surgical repair can be extraordinarily challenging. Clinical trials report that both reverse breech extraction and the Fetal Pillow can facilitate CD in the setting of a prolonged second stage. For many obstetricians reverse breech extraction is a challenging obstetric maneuver. The insertion and inflation of a Fetal Pillow is a simple procedure. Obstetrician-gynecologists learn by doing. If you have never used the Fetal Pillow, I suggest you consider trialing it in your practice. ●
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123:337-345.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at cesarean section after prolonged obstructed labor: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22:375-378.
- Nooh AM, Abdeldayem HM, Ben-Affan O. Reverse breech extraction versus the standard approach of pushing the impacted fetal head up through the vagina in caesarean section for obstructed labour: a randomised controlled trial. J Obstet Gynaecol. 2017;37:459-463.
- Seal SL, Dey A, Barman SC, et al. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133:178-182.
- Lassey SC, Little SE, Saadeh M,et al. Cephalic elevation device for second-stage cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2020;135:879-884.
Obstetricians know that a cesarean delivery (CD) for a woman with a prolonged second stage and a fetal head deeply impacted in the pelvis is challenging. In this situation, extensions of the uterine incision commonly occur, resulting in prolonged operative time and increased blood loss. Even more harrowing is the inability to deliver the fetal head, necessitating emergency assistance from other clinicians. In this situation, interventions that may be helpful include:
- extend or T the uterine incision
- enlist the aid of a clinician to push up on the fetal head with a vaginal hand (FIGURE 1)
- reverse breech extraction (FIGURE 2), and
- vaginal insertion of a Fetal Pillow prior to starting the delivery.


Evidence from clinical trials indicates that reverse breech extraction or insertion of a Fetal Pillow result in the best clinical outcomes.
Reverse breech extraction vs the push technique
Although the data are limited, most studies report that compared with pushing up with a vaginal hand (as shown in Figure 1), the reverse breech extraction technique (as shown in Figure 2) is associated with a reduction in extensions of the uterine incision, reduced blood loss, and reduced operative time.1 In a randomized trial, 108 women with obstructed labor undergoing CD in the second stage were randomly assigned to reverse breech extraction or pushing up with a vaginal hand.2 Following the uterine incision, the reverse breech extraction technique is performed by immediately reaching into the upper uterus and grasping the lower portion of the fetal leg and applying gentle traction on the leg until the second leg appeared. The lower legs are then pulled out of the uterus. Standard breech delivery maneuvers are used to deliver the shoulders and head. In the trial, compared with the push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (30% vs 11%; P<.05), less blood loss (899 mL vs 1,257 mL; P<.001), and shorter operative time (56 min vs 89 min, P<.001). Fetal injury was similar with the push and breech extraction techniques (6% and 7%).
In another randomized trial, 192 women undergoing CD for obstructed labor were randomly assigned to reverse breech extraction or pushing the head up with a hand in the vagina.3 Compared with the vaginal push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (19% vs 48%; P = .003), fewer cases of wound infection (2% vs 13%; P = .007), and fewer blood transfusions (2 vs 11; P = .012).
Additional options and adjuvants for facilitating delivery of a fetal head deeply impacted in the pelvis include: using a Coyne spoon, using nitroglycerine or terbutaline to relax the myometrium, breaking the vaginal suction on the fetal head before attempting delivery, keeping the wrist of the delivering hand as straight as possible to reduce uterine incision extensions, and incising the ring (if a Bandl’s ring is detected).
Continue to: The Fetal Pillow...
The Fetal Pillow
The Fetal Pillow (Safe Obstetric Systems, New York, New York) is a single-use fetal cephalic elevation device for managing the deeply impacted fetal head (FIGURE 3). The Fetal Pillow has a firm plastic base upon which is attached a soft silicon balloon. The Fetal Pillow is inserted into the vagina prior to initiating CD and the balloon is filled with 180 mL of saline, causing the fetal head to be pushed to a higher station (FIGURE 4). Use of the Fetal Pillow may be indicated prior to CD in the following situations:
- second stage labor with a deeply impacted head
- second stage labor and failed operative delivery
- occiput posterior position or deep transverse arrest
- absent progress in the first stage between 8 cm and 10 cm with a deeply impacted fetal head or excessive caput of the fetal head.


The Fetal Pillow is inserted after completing vaginal preparation for CD and before initiating skin preparation and abdominal draping. The steps for inserting the Fetal Pillow include:
- Use the 60 mL syringe to fully deflate the Fetal Pillow and leave the cock-stop open.
- Fold the Fetal Pillow by squeezing the firm plastic base, and with the patient’s legs in a frog-leg position, place the device in the vagina.
- Allow the firm plastic base to open to a flat position with the base against the posterior vaginal wall and the soft silicon balloon against the fetal head.
- Using pressure on the plastic base, gently push the Fetal Pillow posteriorly toward the sacrum of the mother.
- Use the 60 mL syringe to inflate the balloon with 180 mL of normal saline and close the valve.
- Straighten the patient’s legs and proceed with skin preparation and abdominal draping (FIGURE 4).
When the CD is completed, deflate the balloon by drawing out the saline with the 60 mL syringe and remove the device by hooking a finger around the firm plastic base. The Fetal Pillow is surprisingly easy to use.
Continue to: Effectiveness of the Fetal Pillow...
Effectiveness of the Fetal Pillow
In one randomized trial, 240 women undergoing CD were randomly allocated to a group in which the Fetal Pillow was placed in the vagina and inflated prior to the cesarean and a control group in which the Fetal Pillow was not used.
In another randomized trial, 60 nulliparous women undergoing CD in the second stage of labor had a Fetal Pillow inserted in the vagina and were randomly allocated to inflation of the pillow (Fetal Pillow group) or noninflation of the pillow (control group).5 In this study the mean length of the second stage was 4 hours. Compared with noninflation of the Fetal Pillow, use of the inflated Fetal Pillow was associated with a reduction in grade 3 extension of the uterine incision (extensions into the uterine artery, vagina, or bladder) (0% for inflation vs 13% for noninflation) and fewer difficult plus very difficult deliveries of the fetal head as reported by the surgeon (0% for inflation vs 37% for noninflation). There was no significant difference in blood loss between the two groups (800 mL vs 900 mL). These two randomized studies both reported that the use of the Fetal Pillow was associated with a reduction in grade 3 extensions of the uterine incision and a decrease in the difficulty of delivering the fetal head.
Consider trialing the Fetal Pillow
When a CD is performed after a prolonged second stage of labor, surgical complications are common, including extensions of the uterine incision and difficulty delivering the fetal head. When a grade 3 extension occurs—with tearing of a uterine artery, deep extension into the vagina, or damage to the bladder—the surgical repair can be extraordinarily challenging. Clinical trials report that both reverse breech extraction and the Fetal Pillow can facilitate CD in the setting of a prolonged second stage. For many obstetricians reverse breech extraction is a challenging obstetric maneuver. The insertion and inflation of a Fetal Pillow is a simple procedure. Obstetrician-gynecologists learn by doing. If you have never used the Fetal Pillow, I suggest you consider trialing it in your practice. ●
Obstetricians know that a cesarean delivery (CD) for a woman with a prolonged second stage and a fetal head deeply impacted in the pelvis is challenging. In this situation, extensions of the uterine incision commonly occur, resulting in prolonged operative time and increased blood loss. Even more harrowing is the inability to deliver the fetal head, necessitating emergency assistance from other clinicians. In this situation, interventions that may be helpful include:
- extend or T the uterine incision
- enlist the aid of a clinician to push up on the fetal head with a vaginal hand (FIGURE 1)
- reverse breech extraction (FIGURE 2), and
- vaginal insertion of a Fetal Pillow prior to starting the delivery.


Evidence from clinical trials indicates that reverse breech extraction or insertion of a Fetal Pillow result in the best clinical outcomes.
Reverse breech extraction vs the push technique
Although the data are limited, most studies report that compared with pushing up with a vaginal hand (as shown in Figure 1), the reverse breech extraction technique (as shown in Figure 2) is associated with a reduction in extensions of the uterine incision, reduced blood loss, and reduced operative time.1 In a randomized trial, 108 women with obstructed labor undergoing CD in the second stage were randomly assigned to reverse breech extraction or pushing up with a vaginal hand.2 Following the uterine incision, the reverse breech extraction technique is performed by immediately reaching into the upper uterus and grasping the lower portion of the fetal leg and applying gentle traction on the leg until the second leg appeared. The lower legs are then pulled out of the uterus. Standard breech delivery maneuvers are used to deliver the shoulders and head. In the trial, compared with the push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (30% vs 11%; P<.05), less blood loss (899 mL vs 1,257 mL; P<.001), and shorter operative time (56 min vs 89 min, P<.001). Fetal injury was similar with the push and breech extraction techniques (6% and 7%).
In another randomized trial, 192 women undergoing CD for obstructed labor were randomly assigned to reverse breech extraction or pushing the head up with a hand in the vagina.3 Compared with the vaginal push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (19% vs 48%; P = .003), fewer cases of wound infection (2% vs 13%; P = .007), and fewer blood transfusions (2 vs 11; P = .012).
Additional options and adjuvants for facilitating delivery of a fetal head deeply impacted in the pelvis include: using a Coyne spoon, using nitroglycerine or terbutaline to relax the myometrium, breaking the vaginal suction on the fetal head before attempting delivery, keeping the wrist of the delivering hand as straight as possible to reduce uterine incision extensions, and incising the ring (if a Bandl’s ring is detected).
Continue to: The Fetal Pillow...
The Fetal Pillow
The Fetal Pillow (Safe Obstetric Systems, New York, New York) is a single-use fetal cephalic elevation device for managing the deeply impacted fetal head (FIGURE 3). The Fetal Pillow has a firm plastic base upon which is attached a soft silicon balloon. The Fetal Pillow is inserted into the vagina prior to initiating CD and the balloon is filled with 180 mL of saline, causing the fetal head to be pushed to a higher station (FIGURE 4). Use of the Fetal Pillow may be indicated prior to CD in the following situations:
- second stage labor with a deeply impacted head
- second stage labor and failed operative delivery
- occiput posterior position or deep transverse arrest
- absent progress in the first stage between 8 cm and 10 cm with a deeply impacted fetal head or excessive caput of the fetal head.


The Fetal Pillow is inserted after completing vaginal preparation for CD and before initiating skin preparation and abdominal draping. The steps for inserting the Fetal Pillow include:
- Use the 60 mL syringe to fully deflate the Fetal Pillow and leave the cock-stop open.
- Fold the Fetal Pillow by squeezing the firm plastic base, and with the patient’s legs in a frog-leg position, place the device in the vagina.
- Allow the firm plastic base to open to a flat position with the base against the posterior vaginal wall and the soft silicon balloon against the fetal head.
- Using pressure on the plastic base, gently push the Fetal Pillow posteriorly toward the sacrum of the mother.
- Use the 60 mL syringe to inflate the balloon with 180 mL of normal saline and close the valve.
- Straighten the patient’s legs and proceed with skin preparation and abdominal draping (FIGURE 4).
When the CD is completed, deflate the balloon by drawing out the saline with the 60 mL syringe and remove the device by hooking a finger around the firm plastic base. The Fetal Pillow is surprisingly easy to use.
Continue to: Effectiveness of the Fetal Pillow...
Effectiveness of the Fetal Pillow
In one randomized trial, 240 women undergoing CD were randomly allocated to a group in which the Fetal Pillow was placed in the vagina and inflated prior to the cesarean and a control group in which the Fetal Pillow was not used.
In another randomized trial, 60 nulliparous women undergoing CD in the second stage of labor had a Fetal Pillow inserted in the vagina and were randomly allocated to inflation of the pillow (Fetal Pillow group) or noninflation of the pillow (control group).5 In this study the mean length of the second stage was 4 hours. Compared with noninflation of the Fetal Pillow, use of the inflated Fetal Pillow was associated with a reduction in grade 3 extension of the uterine incision (extensions into the uterine artery, vagina, or bladder) (0% for inflation vs 13% for noninflation) and fewer difficult plus very difficult deliveries of the fetal head as reported by the surgeon (0% for inflation vs 37% for noninflation). There was no significant difference in blood loss between the two groups (800 mL vs 900 mL). These two randomized studies both reported that the use of the Fetal Pillow was associated with a reduction in grade 3 extensions of the uterine incision and a decrease in the difficulty of delivering the fetal head.
Consider trialing the Fetal Pillow
When a CD is performed after a prolonged second stage of labor, surgical complications are common, including extensions of the uterine incision and difficulty delivering the fetal head. When a grade 3 extension occurs—with tearing of a uterine artery, deep extension into the vagina, or damage to the bladder—the surgical repair can be extraordinarily challenging. Clinical trials report that both reverse breech extraction and the Fetal Pillow can facilitate CD in the setting of a prolonged second stage. For many obstetricians reverse breech extraction is a challenging obstetric maneuver. The insertion and inflation of a Fetal Pillow is a simple procedure. Obstetrician-gynecologists learn by doing. If you have never used the Fetal Pillow, I suggest you consider trialing it in your practice. ●
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123:337-345.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at cesarean section after prolonged obstructed labor: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22:375-378.
- Nooh AM, Abdeldayem HM, Ben-Affan O. Reverse breech extraction versus the standard approach of pushing the impacted fetal head up through the vagina in caesarean section for obstructed labour: a randomised controlled trial. J Obstet Gynaecol. 2017;37:459-463.
- Seal SL, Dey A, Barman SC, et al. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133:178-182.
- Lassey SC, Little SE, Saadeh M,et al. Cephalic elevation device for second-stage cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2020;135:879-884.
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123:337-345.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at cesarean section after prolonged obstructed labor: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22:375-378.
- Nooh AM, Abdeldayem HM, Ben-Affan O. Reverse breech extraction versus the standard approach of pushing the impacted fetal head up through the vagina in caesarean section for obstructed labour: a randomised controlled trial. J Obstet Gynaecol. 2017;37:459-463.
- Seal SL, Dey A, Barman SC, et al. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133:178-182.
- Lassey SC, Little SE, Saadeh M,et al. Cephalic elevation device for second-stage cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2020;135:879-884.
Evidence-based management of early pregnancy loss
The American College of Obstetricians and Gynecologists (ACOG) defines early pregnancy loss (EPL) as a nonviable, intrauterine pregnancy up to 12 6/7 weeks’ gestation.1 The term EPL has been used interchangeably with miscarriage, spontaneous abortion, and early pregnancy failure; the preferred terms among US women who experience pregnancy loss are EPL and miscarriage.2 EPL is the most common complication of early pregnancy and accounts for up to 15% to 20% of clinically recognized pregnancies.3
The most common cause of EPL is a chromosomal abnormality (TABLE 1). Other common etiologies include structural abnormalities, such as uterine fibroids or polyps. Risk factors for EPL include maternal age, prior pregnancy loss, and various maternal conditions and medication and substance use (TABLE 2).


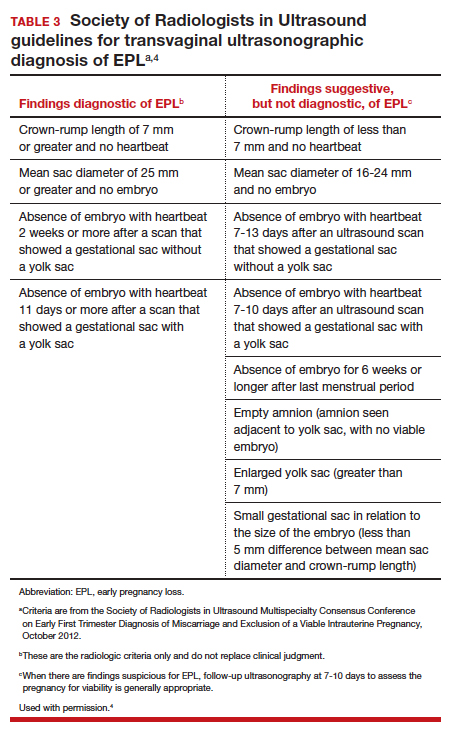
Definitive diagnosis of EPL often requires more than 1 ultrasonography scan or other examination to determine whether a pregnancy is nonviable versus too early to confirm viability. The consensus guidelines from the Society of Radiologists in Ultrasound provide transvaginal ultrasonographic criteria to diagnose EPL (TABLE 3).4 Two of the diagnostic criteria require only 1 ultrasonography scan while the others require repeat ultrasonography.
Note that a definitive diagnosis may be more important to some patients than others due to differing pregnancy intent and/or desirableness. Patients may choose to take action in terms of medication or uterine aspiration based on suspicion of EPL, or they may wish to end the pregnancy regardless of EPL diagnosis.
Management options for EPL
EPL can be managed expectantly, with medication, or with uterine aspiration. These methods have different risks and benefits, and in most cases all should be made available to women who experience EPL.5-7
Expectant management
Expectant management involves waiting for the body to spontaneously expel the nonviable pregnancy. In the absence of any signs of infection, hemodynamic instability, or other medical instability, it is safe and reasonable to wait a month or more before intervening, according to patient choice. Expectant management is up to 80% effective.8
Medication management
Medication management entails using mifepristone and misoprostol, or misoprostol alone, to cause uterine contractions to expel the pregnancy. A landmark study demonstrated that medication management of EPL with the combination of mifepristone and misoprostol is significantly more effective than misoprostol alone.9 While the mean cost of mifepristone is approximately $90 per dose, its addition is cost-effective given the increased efficacy.10
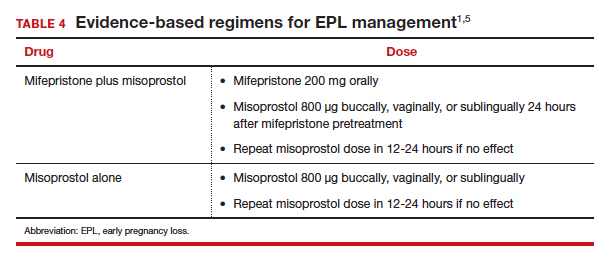
The evidence-based combination regimen is to provide mifepristone 200 mg orally, followed 24 hours later by misoprostol 800 µg vaginally, for a success rate of 87.8% by 8 days, and 91.2% by 30 days posttreatment. Success rates can be increased further by adding a second dose of misoprostol to take as needed.5
We strongly recommend using the combination regimen if you have access to mifepristone. If you do not have access to mifepristone in your clinical setting, perhaps this indication for use can help facilitate getting it onto your formulary. (See “Ordering mifepristone” below.)
Without access to mifepristone, medication abortion still should be offered after discussing the decreased efficacy with patients. The first-trimester misoprostol-only regimen for EPL is to give misoprostol 800 µg buccally, vaginally, or sublingually, with a second dose if there is no effect (TABLE 4).1,5 For losses after 9 weeks, some data suggest adding additional doses of misoprostol 400 µg every 3 hours until expulsion.11
- There are 2 distributors of mifepristone in the United States. Danco (www.earlyoptionpill.com) distributes the branded Mifeprex and GenBioPro (www.genbiopro.com) distributes generic mifepristone.
- To order mifepristone, 1 health care provider from your clinic or facility must read and sign the distributor’s prescriber agreement and account setup form. These forms and instructions can be found on each distributor’s website. Future orders can be made by calling the distributor directly (Danco: 1-877-432-7596; GenBioPro: 1-855-643-3463).
- The shelf life of mifepristone is 18 months.
- Each patient who receives mifepristone needs to read and sign a patient agreement (available on distributor websites), as required by the US Food and Drug Administration–approved Risk Evaluation and Mitigation Strategy (REMS) program.
Continue to: Uterine aspiration...
Uterine aspiration
Uterine aspiration is the third management option for EPL and is virtually 100% successful. Although aspiration is used when expectant or medication management fails, it is also a first-line option based on patient choice or contraindications to the other 2 management options.
We recommend either manual vacuum aspiration (MVA) or electric vacuum aspiration (EVA); sharp curettage almost never should be used. Uterine aspiration can be performed safely in a clinic, emergency department, or operating room (OR) setting, depending on patient characteristics and desires.12-14 For various reasons, many patients prefer outpatient management. These reasons may include avoiding the costs and delays associated with OR management, wanting more control over who performs the procedure, or avoiding more significant/general anesthesia. MVA in the outpatient setting is the most cost-effective approach to uterine aspiration.15
Choosing a management approach
There are virtually no contraindications for uterine aspiration. Expectant and medication management are contraindicated (and uterine aspiration is recommended) in the setting of bleeding disorders, anticoagulation, suspected intrauterine infection, suspected molar pregnancy, significant cardiopulmonary disease, or any condition for which heavy, unsupervised bleeding might be dangerous.1 Uterine aspiration offers immediate resolution, with a procedure usually lasting 3 to 10 minutes. By contrast, expectant and medication management offer a less predictable time to resolution and, often, a more prolonged period of active pregnancy expulsion.
In the absence of a contraindication, patient choice should determine which management option is used. All 3 options are similarly safe and effective, and the differences that do exist are acceptable to patients as long as they are allowed to access their preferred EPL management method.5,6,16 Patient satisfaction is associated directly with the ability to choose the method of preference.
Managing pain
Pain management should be offered to all women diagnosed with EPL. Those who choose expectant or medication management likely will require only oral nonsteroidal anti-inflammatory drugs (NSAIDs). A minority may require the addition of a small number of narcotic pain pills.17
Women who choose uterine aspiration also should be offered pain management. All patients should be given a paracervical block; other medications can include NSAIDs, an oral benzodiazepine, intravenous (IV) sedation, or even general anesthesia/monitored airway care.17
Patients’ expectations about pain management should be addressed directly during initial counseling. This may help patients decide what type of management and treatment location they might prefer.
Checking blood type: Is it necessary?
The ACOG practice bulletin for EPL states, “administration of Rh D immune globulin should be considered in cases of early pregnancy loss, especially those that are later in the first trimester.”1 A growing body of evidence indicates that Rho(D) immune globulin likely is unnecessary in early pregnancy.
A recent prospective cohort study of 42 women who were at 5 to 12 weeks’ gestation found that the fetal red blood cell concentration was below the calculated threshold for Rh sensitization.18 In light of recent evidence, the National Abortion Federation now recommends foregoing Rh testing and provision of Rh immune globulin at less than 8 weeks’ gestation for uterine aspiration and at less than 10 weeks’ gestation for medication abortion.19
We feel there is sufficient evidence to forego Rh testing in EPL at similar gestational ages, although this is not yet reflected in US societal guidelines. (It is already standard practice in some countries.) Although the risk of Rh alloimmunization is low, the risk of significant consequences in the event of Rh alloimmunization is high. Currently, it also is reasonable to continue giving Rho(D) immune globulin to Rh-negative patients who experience EPL at any gestational age. A lower dose (50 µg) is sufficient for EPL; the standard 300-µg dose also is acceptable.20
We anticipate that society and ACOG guidelines will change in the next few years as the body of evidence increases, and practice should change to reflect new guidance.
Continue to: Prophylactic antibiotics...
Prophylactic antibiotics
The risk of infection with EPL is low overall regardless of the management approach.1 Prophylactic antibiotics are recommended for patients undergoing uterine aspiration but are not necessary in the setting of expectant or medication management. We recommend prophylaxis with 1 dose of oral doxycycline 200 mg or oral azithromycin 500 mg approximately 30 minutes to 1 hour prior to uterine aspiration.21 Alternatives include 1 dose of oral metronidazole 500 mg or, if the patient is unable to take oral medications, IV cefazolin 2 g.
A multisite international randomized controlled trial concluded that antibiotic prophylaxis before uterine aspiration for EPL did not significantly reduce the risk of infection.22 However, there was a significant reduction in pelvic infection with antibiotic administration for the subgroup of women who underwent MVA, which is our recommended approach (along with EVA, and opposed to sharp curettage) for outpatient EPL management.
Follow-up after EPL
In-person follow-up after treatment of EPL is not medically necessary. A repeat ultrasonography 1 to 2 weeks after expectant or medication management can be helpful to confirm completion of the process, and clinicians should focus on presence or absence of a gestational sac to determine if further management is needed.1
Follow-up by telemedicine or phone also is an option and may be preferred in the following situations:
- the patient lives far from the clinic
- travel to the clinic is difficult or expensive
- the patient has child-care issues
- there is a global pandemic necessitating physical distancing.
If the patient’s reported history and symptoms are consistent with a completed process, no further intervention is indicated.
If ongoing EPL is a concern, ask the patient to come in for an evaluation and ultrasonography. If visiting the clinic is still a challenge, following with urine or serum human chorionic gonadotropin (HCG) levels also is acceptable. Experts recommend waiting 4 weeks before expecting a negative urine HCG measurement, although up to 25% of women with a completed EPL will still have a positive test at 4 weeks.23,24
A postprocedure serum HCG is more helpful if a preprocedure HCG level already is known. Numerous studies have evaluated phone follow-up after medication abortion and it is reasonable to translate these practices to follow-up after EPL, recognizing that direct data looking at alternative EPL follow-up are much more limited.23,25-30
The benefit of HCG follow-up at a scheduled time (such as 1 week) is less clear for EPL than for medication abortion, as HCG trends are less predictable in the setting of EPL. However, if the pregnancy has passed, a significant drop in the HCG level would be expected. It is important to take into account the patient’s history and clinical symptoms and consider in-person evaluation with possible ultrasonography if there is concern that the pregnancy tissue has not passed.
Pay attention to mental health
It is critical to assess the patient’s mental and emotional health. This should be done both at the time of EPL diagnosis and management and again at follow-up. Both patients and their partners can struggle after experiencing EPL, and they may suffer from prolonged posttraumatic stress.31
Often, EPL occurs before people have shared the news about their pregnancy. This can amplify the sense of isolation and sadness many women report. Equally critical is recognizing that not all women who experience EPL grieve, and clinicians should normalize patient experiences and feelings. Provider language is important. We recommend use of these questions and phrases:
- I’m so sorry for your loss.
- How are you feeling?
- How have you been doing since I saw you last?
- Your friends/family/partner may be grieving differently or at a different pace than you—this is normal.
- Just because the EPL process is complete doesn’t necessarily mean your processing and/or grieving is over.
- Whatever you’re feeling is okay.
Continue to: Address desire for future pregnancy or contraception...
Address desire for future pregnancy or contraception
No additional workup is necessary after EPL unless a patient is experiencing recurrent pregnancy loss. We do recommend discussing plans for future conception. If a patient wants to conceive again as soon as possible, she can start trying when she feels emotionally ready (even before her next menstrual period). One study found that the ability to conceive and those pregnancy outcomes were the same when patients were randomly assigned to start trying immediately versus waiting 3 months after EPL.32
Alternatively, a patient may want to prevent pregnancy after EPL, and this information should be explicitly elicited and addressed with comprehensive contraception counseling as needed. All forms of contraception can be initiated immediately on successful management of EPL. All contraceptive methods, including an intrauterine device, can be initiated immediately following uterine aspiration.1,33,34
Patients should be reminded that if they delay contraception initiation by more than 7 days, they are potentially at risk for pregnancy.35 Most importantly, clinicians should not make assumptions about future pregnancy desires and should ask open-ended questions to provide appropriate patient counseling.
Finally, patients may feel additional anxiety in a subsequent pregnancy. It is helpful to acknowledge this and perhaps even offer earlier and more frequent visits in early pregnancy to help reduce anxiety. EPL is commonly experienced, and unfortunately it is sometimes poorly addressed by clinicians.
We hope this guidance will help you provide excellent, evidence-based, and sensitive care that will not only manage your patient’s EPL but also make the experience as positive as possible. ●
- Early pregnancy loss (EPL) is common, occurring in up to 15% to 20% of clinically recognized pregnancies.
- EPL can be managed expectantly, with medication, or by uterine aspiration.
- There are virtually no contraindications to uterine aspiration.
- Contraindications to expectant or medication management include any situation in which heavy, unsupervised bleeding might be dangerous.
- In the absence of contraindications, patient preference should dictate the management approach.
- Mifepristone-misoprostol is more effective than misoprostol alone.
- Manual uterine aspiration in the outpatient setting is the most cost-effective approach to uterine evacuation.
- Rh testing is not necessary at less than 8 weeks’ gestation if choosing uterine aspiration, or at less than 10 weeks’ gestation if choosing expectant or medication management.
- Antibiotic prophylaxis is indicated for uterine aspiration, but not for expectant or medication management.
- Ultrasonography follow-up should focus on presence or absence of gestational sac.
- There are viable telemedicine and phone follow-up options that do not require repeat ultrasonography or in-person evaluation.
- There is no need to delay future conception once EPL management is confirmed to be complete.
- It is okay to initiate any contraceptive method immediately on completed management of EPL.
- Feelings toward EPL can be complex and varied; it is helpful to normalize your patients’ experiences, ask open-ended questions, and provide support as needed.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 200: early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
- Clement EG, Horvath S, McAllister A, et al. The language of first-trimester nonviable pregnancy: patient-reported preferences and clarity. Obstet Gynecol. 2019;133:149-154.
- Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Uterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Nanda K, Peloggia A, Grimes D, et al. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst Rev. 2006(2):CD003518.
- Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006(3):CD002253.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594.
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd ed. Geneva, Switzerland: World Health Organization; 2012.
- Wiebe E, Janssen P. Management of spontaneous abortion in family practices and hospitals. Fam Med. 1998;30:293-296.
- Harris LH, Dalton VK, Johnson TR. Surgical management of early pregnancy failure: history, politics, and safe, cost-effective care. Am J Obstet Gynecol. 2007;196:445.e1-e5.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (Miscarriage Treatment [MIST] trial). BMJ. 2006;332:1235-1240.
- Calvache JA, Delgado-Noguera MF, Lesaffre E, et al. Anaesthesia for evacuation of incomplete miscarriage. Cochrane Database System Rev. 2012(4):CD008681.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- National Abortion Federation. 2020 clinical policy guidelines for abortion care. https://www.prochoice.org/education-and-advocacy/cpg. Accessed June 9, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 181: prevention of Rh D alloimmunization. Obstet Gynecol. 2017;130:e59-e70.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180-1189.
- Lissauer D, Wilson A, Hewitt CA, et al. A randomized trial of prophylactic antibiotics for miscarriage surgery. N Engl J Med. 2019;380:1012-1021.
- Perriera L, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010:81:143-149.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Clark W, Bracken H, Tanenhaus J, et al. Alternatives to a routine follow-up visit for early medical abortion. Obstet Gynecol. 2010;115(2 pt 1):264-272.
- Jackson AV, Dayananda I, Fortin JM, et al. Can women accurately assess the outcome of medical abortion based on symptoms alone? Contraception. 2012;85:192-197.
- Raymond EG, Tan YL, Grant M, et al. Self-assessment of medical abortion outcome using symptoms and home pregnancy testing. Contraception. 2018;97:324-328.
- Raymond EG, Shochet T, Bracken H. Low-sensitivity urine pregnancy testing to assess medical abortion outcome: a systematic review. Contraception. 2018;98:30-35.
- Raymond EG, Grossman D, Mark A, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361-366.
- Farren J, Jalmbrant M, Ameye L, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open. 2016;6:e011864.
- Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204-212. DOI: 0.1097/AOG.0000000000001159.
- American College of Obstetricians and Gynecologists. Committee opinion No. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126:e44-e48.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria (US MEC) for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
The American College of Obstetricians and Gynecologists (ACOG) defines early pregnancy loss (EPL) as a nonviable, intrauterine pregnancy up to 12 6/7 weeks’ gestation.1 The term EPL has been used interchangeably with miscarriage, spontaneous abortion, and early pregnancy failure; the preferred terms among US women who experience pregnancy loss are EPL and miscarriage.2 EPL is the most common complication of early pregnancy and accounts for up to 15% to 20% of clinically recognized pregnancies.3
The most common cause of EPL is a chromosomal abnormality (TABLE 1). Other common etiologies include structural abnormalities, such as uterine fibroids or polyps. Risk factors for EPL include maternal age, prior pregnancy loss, and various maternal conditions and medication and substance use (TABLE 2).


Definitive diagnosis of EPL often requires more than 1 ultrasonography scan or other examination to determine whether a pregnancy is nonviable versus too early to confirm viability. The consensus guidelines from the Society of Radiologists in Ultrasound provide transvaginal ultrasonographic criteria to diagnose EPL (TABLE 3).4 Two of the diagnostic criteria require only 1 ultrasonography scan while the others require repeat ultrasonography.

Note that a definitive diagnosis may be more important to some patients than others due to differing pregnancy intent and/or desirableness. Patients may choose to take action in terms of medication or uterine aspiration based on suspicion of EPL, or they may wish to end the pregnancy regardless of EPL diagnosis.
Management options for EPL
EPL can be managed expectantly, with medication, or with uterine aspiration. These methods have different risks and benefits, and in most cases all should be made available to women who experience EPL.5-7
Expectant management
Expectant management involves waiting for the body to spontaneously expel the nonviable pregnancy. In the absence of any signs of infection, hemodynamic instability, or other medical instability, it is safe and reasonable to wait a month or more before intervening, according to patient choice. Expectant management is up to 80% effective.8
Medication management
Medication management entails using mifepristone and misoprostol, or misoprostol alone, to cause uterine contractions to expel the pregnancy. A landmark study demonstrated that medication management of EPL with the combination of mifepristone and misoprostol is significantly more effective than misoprostol alone.9 While the mean cost of mifepristone is approximately $90 per dose, its addition is cost-effective given the increased efficacy.10
The evidence-based combination regimen is to provide mifepristone 200 mg orally, followed 24 hours later by misoprostol 800 µg vaginally, for a success rate of 87.8% by 8 days, and 91.2% by 30 days posttreatment. Success rates can be increased further by adding a second dose of misoprostol to take as needed.5
We strongly recommend using the combination regimen if you have access to mifepristone. If you do not have access to mifepristone in your clinical setting, perhaps this indication for use can help facilitate getting it onto your formulary. (See “Ordering mifepristone” below.)
Without access to mifepristone, medication abortion still should be offered after discussing the decreased efficacy with patients. The first-trimester misoprostol-only regimen for EPL is to give misoprostol 800 µg buccally, vaginally, or sublingually, with a second dose if there is no effect (TABLE 4).1,5 For losses after 9 weeks, some data suggest adding additional doses of misoprostol 400 µg every 3 hours until expulsion.11
- There are 2 distributors of mifepristone in the United States. Danco (www.earlyoptionpill.com) distributes the branded Mifeprex and GenBioPro (www.genbiopro.com) distributes generic mifepristone.
- To order mifepristone, 1 health care provider from your clinic or facility must read and sign the distributor’s prescriber agreement and account setup form. These forms and instructions can be found on each distributor’s website. Future orders can be made by calling the distributor directly (Danco: 1-877-432-7596; GenBioPro: 1-855-643-3463).
- The shelf life of mifepristone is 18 months.
- Each patient who receives mifepristone needs to read and sign a patient agreement (available on distributor websites), as required by the US Food and Drug Administration–approved Risk Evaluation and Mitigation Strategy (REMS) program.

Continue to: Uterine aspiration...
Uterine aspiration
Uterine aspiration is the third management option for EPL and is virtually 100% successful. Although aspiration is used when expectant or medication management fails, it is also a first-line option based on patient choice or contraindications to the other 2 management options.
We recommend either manual vacuum aspiration (MVA) or electric vacuum aspiration (EVA); sharp curettage almost never should be used. Uterine aspiration can be performed safely in a clinic, emergency department, or operating room (OR) setting, depending on patient characteristics and desires.12-14 For various reasons, many patients prefer outpatient management. These reasons may include avoiding the costs and delays associated with OR management, wanting more control over who performs the procedure, or avoiding more significant/general anesthesia. MVA in the outpatient setting is the most cost-effective approach to uterine aspiration.15
Choosing a management approach
There are virtually no contraindications for uterine aspiration. Expectant and medication management are contraindicated (and uterine aspiration is recommended) in the setting of bleeding disorders, anticoagulation, suspected intrauterine infection, suspected molar pregnancy, significant cardiopulmonary disease, or any condition for which heavy, unsupervised bleeding might be dangerous.1 Uterine aspiration offers immediate resolution, with a procedure usually lasting 3 to 10 minutes. By contrast, expectant and medication management offer a less predictable time to resolution and, often, a more prolonged period of active pregnancy expulsion.
In the absence of a contraindication, patient choice should determine which management option is used. All 3 options are similarly safe and effective, and the differences that do exist are acceptable to patients as long as they are allowed to access their preferred EPL management method.5,6,16 Patient satisfaction is associated directly with the ability to choose the method of preference.
Managing pain
Pain management should be offered to all women diagnosed with EPL. Those who choose expectant or medication management likely will require only oral nonsteroidal anti-inflammatory drugs (NSAIDs). A minority may require the addition of a small number of narcotic pain pills.17
Women who choose uterine aspiration also should be offered pain management. All patients should be given a paracervical block; other medications can include NSAIDs, an oral benzodiazepine, intravenous (IV) sedation, or even general anesthesia/monitored airway care.17
Patients’ expectations about pain management should be addressed directly during initial counseling. This may help patients decide what type of management and treatment location they might prefer.
Checking blood type: Is it necessary?
The ACOG practice bulletin for EPL states, “administration of Rh D immune globulin should be considered in cases of early pregnancy loss, especially those that are later in the first trimester.”1 A growing body of evidence indicates that Rho(D) immune globulin likely is unnecessary in early pregnancy.
A recent prospective cohort study of 42 women who were at 5 to 12 weeks’ gestation found that the fetal red blood cell concentration was below the calculated threshold for Rh sensitization.18 In light of recent evidence, the National Abortion Federation now recommends foregoing Rh testing and provision of Rh immune globulin at less than 8 weeks’ gestation for uterine aspiration and at less than 10 weeks’ gestation for medication abortion.19
We feel there is sufficient evidence to forego Rh testing in EPL at similar gestational ages, although this is not yet reflected in US societal guidelines. (It is already standard practice in some countries.) Although the risk of Rh alloimmunization is low, the risk of significant consequences in the event of Rh alloimmunization is high. Currently, it also is reasonable to continue giving Rho(D) immune globulin to Rh-negative patients who experience EPL at any gestational age. A lower dose (50 µg) is sufficient for EPL; the standard 300-µg dose also is acceptable.20
We anticipate that society and ACOG guidelines will change in the next few years as the body of evidence increases, and practice should change to reflect new guidance.
Continue to: Prophylactic antibiotics...
Prophylactic antibiotics
The risk of infection with EPL is low overall regardless of the management approach.1 Prophylactic antibiotics are recommended for patients undergoing uterine aspiration but are not necessary in the setting of expectant or medication management. We recommend prophylaxis with 1 dose of oral doxycycline 200 mg or oral azithromycin 500 mg approximately 30 minutes to 1 hour prior to uterine aspiration.21 Alternatives include 1 dose of oral metronidazole 500 mg or, if the patient is unable to take oral medications, IV cefazolin 2 g.
A multisite international randomized controlled trial concluded that antibiotic prophylaxis before uterine aspiration for EPL did not significantly reduce the risk of infection.22 However, there was a significant reduction in pelvic infection with antibiotic administration for the subgroup of women who underwent MVA, which is our recommended approach (along with EVA, and opposed to sharp curettage) for outpatient EPL management.
Follow-up after EPL
In-person follow-up after treatment of EPL is not medically necessary. A repeat ultrasonography 1 to 2 weeks after expectant or medication management can be helpful to confirm completion of the process, and clinicians should focus on presence or absence of a gestational sac to determine if further management is needed.1
Follow-up by telemedicine or phone also is an option and may be preferred in the following situations:
- the patient lives far from the clinic
- travel to the clinic is difficult or expensive
- the patient has child-care issues
- there is a global pandemic necessitating physical distancing.
If the patient’s reported history and symptoms are consistent with a completed process, no further intervention is indicated.
If ongoing EPL is a concern, ask the patient to come in for an evaluation and ultrasonography. If visiting the clinic is still a challenge, following with urine or serum human chorionic gonadotropin (HCG) levels also is acceptable. Experts recommend waiting 4 weeks before expecting a negative urine HCG measurement, although up to 25% of women with a completed EPL will still have a positive test at 4 weeks.23,24
A postprocedure serum HCG is more helpful if a preprocedure HCG level already is known. Numerous studies have evaluated phone follow-up after medication abortion and it is reasonable to translate these practices to follow-up after EPL, recognizing that direct data looking at alternative EPL follow-up are much more limited.23,25-30
The benefit of HCG follow-up at a scheduled time (such as 1 week) is less clear for EPL than for medication abortion, as HCG trends are less predictable in the setting of EPL. However, if the pregnancy has passed, a significant drop in the HCG level would be expected. It is important to take into account the patient’s history and clinical symptoms and consider in-person evaluation with possible ultrasonography if there is concern that the pregnancy tissue has not passed.
Pay attention to mental health
It is critical to assess the patient’s mental and emotional health. This should be done both at the time of EPL diagnosis and management and again at follow-up. Both patients and their partners can struggle after experiencing EPL, and they may suffer from prolonged posttraumatic stress.31
Often, EPL occurs before people have shared the news about their pregnancy. This can amplify the sense of isolation and sadness many women report. Equally critical is recognizing that not all women who experience EPL grieve, and clinicians should normalize patient experiences and feelings. Provider language is important. We recommend use of these questions and phrases:
- I’m so sorry for your loss.
- How are you feeling?
- How have you been doing since I saw you last?
- Your friends/family/partner may be grieving differently or at a different pace than you—this is normal.
- Just because the EPL process is complete doesn’t necessarily mean your processing and/or grieving is over.
- Whatever you’re feeling is okay.
Continue to: Address desire for future pregnancy or contraception...
Address desire for future pregnancy or contraception
No additional workup is necessary after EPL unless a patient is experiencing recurrent pregnancy loss. We do recommend discussing plans for future conception. If a patient wants to conceive again as soon as possible, she can start trying when she feels emotionally ready (even before her next menstrual period). One study found that the ability to conceive and those pregnancy outcomes were the same when patients were randomly assigned to start trying immediately versus waiting 3 months after EPL.32
Alternatively, a patient may want to prevent pregnancy after EPL, and this information should be explicitly elicited and addressed with comprehensive contraception counseling as needed. All forms of contraception can be initiated immediately on successful management of EPL. All contraceptive methods, including an intrauterine device, can be initiated immediately following uterine aspiration.1,33,34
Patients should be reminded that if they delay contraception initiation by more than 7 days, they are potentially at risk for pregnancy.35 Most importantly, clinicians should not make assumptions about future pregnancy desires and should ask open-ended questions to provide appropriate patient counseling.
Finally, patients may feel additional anxiety in a subsequent pregnancy. It is helpful to acknowledge this and perhaps even offer earlier and more frequent visits in early pregnancy to help reduce anxiety. EPL is commonly experienced, and unfortunately it is sometimes poorly addressed by clinicians.
We hope this guidance will help you provide excellent, evidence-based, and sensitive care that will not only manage your patient’s EPL but also make the experience as positive as possible. ●
- Early pregnancy loss (EPL) is common, occurring in up to 15% to 20% of clinically recognized pregnancies.
- EPL can be managed expectantly, with medication, or by uterine aspiration.
- There are virtually no contraindications to uterine aspiration.
- Contraindications to expectant or medication management include any situation in which heavy, unsupervised bleeding might be dangerous.
- In the absence of contraindications, patient preference should dictate the management approach.
- Mifepristone-misoprostol is more effective than misoprostol alone.
- Manual uterine aspiration in the outpatient setting is the most cost-effective approach to uterine evacuation.
- Rh testing is not necessary at less than 8 weeks’ gestation if choosing uterine aspiration, or at less than 10 weeks’ gestation if choosing expectant or medication management.
- Antibiotic prophylaxis is indicated for uterine aspiration, but not for expectant or medication management.
- Ultrasonography follow-up should focus on presence or absence of gestational sac.
- There are viable telemedicine and phone follow-up options that do not require repeat ultrasonography or in-person evaluation.
- There is no need to delay future conception once EPL management is confirmed to be complete.
- It is okay to initiate any contraceptive method immediately on completed management of EPL.
- Feelings toward EPL can be complex and varied; it is helpful to normalize your patients’ experiences, ask open-ended questions, and provide support as needed.
The American College of Obstetricians and Gynecologists (ACOG) defines early pregnancy loss (EPL) as a nonviable, intrauterine pregnancy up to 12 6/7 weeks’ gestation.1 The term EPL has been used interchangeably with miscarriage, spontaneous abortion, and early pregnancy failure; the preferred terms among US women who experience pregnancy loss are EPL and miscarriage.2 EPL is the most common complication of early pregnancy and accounts for up to 15% to 20% of clinically recognized pregnancies.3
The most common cause of EPL is a chromosomal abnormality (TABLE 1). Other common etiologies include structural abnormalities, such as uterine fibroids or polyps. Risk factors for EPL include maternal age, prior pregnancy loss, and various maternal conditions and medication and substance use (TABLE 2).


Definitive diagnosis of EPL often requires more than 1 ultrasonography scan or other examination to determine whether a pregnancy is nonviable versus too early to confirm viability. The consensus guidelines from the Society of Radiologists in Ultrasound provide transvaginal ultrasonographic criteria to diagnose EPL (TABLE 3).4 Two of the diagnostic criteria require only 1 ultrasonography scan while the others require repeat ultrasonography.

Note that a definitive diagnosis may be more important to some patients than others due to differing pregnancy intent and/or desirableness. Patients may choose to take action in terms of medication or uterine aspiration based on suspicion of EPL, or they may wish to end the pregnancy regardless of EPL diagnosis.
Management options for EPL
EPL can be managed expectantly, with medication, or with uterine aspiration. These methods have different risks and benefits, and in most cases all should be made available to women who experience EPL.5-7
Expectant management
Expectant management involves waiting for the body to spontaneously expel the nonviable pregnancy. In the absence of any signs of infection, hemodynamic instability, or other medical instability, it is safe and reasonable to wait a month or more before intervening, according to patient choice. Expectant management is up to 80% effective.8
Medication management
Medication management entails using mifepristone and misoprostol, or misoprostol alone, to cause uterine contractions to expel the pregnancy. A landmark study demonstrated that medication management of EPL with the combination of mifepristone and misoprostol is significantly more effective than misoprostol alone.9 While the mean cost of mifepristone is approximately $90 per dose, its addition is cost-effective given the increased efficacy.10
The evidence-based combination regimen is to provide mifepristone 200 mg orally, followed 24 hours later by misoprostol 800 µg vaginally, for a success rate of 87.8% by 8 days, and 91.2% by 30 days posttreatment. Success rates can be increased further by adding a second dose of misoprostol to take as needed.5
We strongly recommend using the combination regimen if you have access to mifepristone. If you do not have access to mifepristone in your clinical setting, perhaps this indication for use can help facilitate getting it onto your formulary. (See “Ordering mifepristone” below.)
Without access to mifepristone, medication abortion still should be offered after discussing the decreased efficacy with patients. The first-trimester misoprostol-only regimen for EPL is to give misoprostol 800 µg buccally, vaginally, or sublingually, with a second dose if there is no effect (TABLE 4).1,5 For losses after 9 weeks, some data suggest adding additional doses of misoprostol 400 µg every 3 hours until expulsion.11
- There are 2 distributors of mifepristone in the United States. Danco (www.earlyoptionpill.com) distributes the branded Mifeprex and GenBioPro (www.genbiopro.com) distributes generic mifepristone.
- To order mifepristone, 1 health care provider from your clinic or facility must read and sign the distributor’s prescriber agreement and account setup form. These forms and instructions can be found on each distributor’s website. Future orders can be made by calling the distributor directly (Danco: 1-877-432-7596; GenBioPro: 1-855-643-3463).
- The shelf life of mifepristone is 18 months.
- Each patient who receives mifepristone needs to read and sign a patient agreement (available on distributor websites), as required by the US Food and Drug Administration–approved Risk Evaluation and Mitigation Strategy (REMS) program.

Continue to: Uterine aspiration...
Uterine aspiration
Uterine aspiration is the third management option for EPL and is virtually 100% successful. Although aspiration is used when expectant or medication management fails, it is also a first-line option based on patient choice or contraindications to the other 2 management options.
We recommend either manual vacuum aspiration (MVA) or electric vacuum aspiration (EVA); sharp curettage almost never should be used. Uterine aspiration can be performed safely in a clinic, emergency department, or operating room (OR) setting, depending on patient characteristics and desires.12-14 For various reasons, many patients prefer outpatient management. These reasons may include avoiding the costs and delays associated with OR management, wanting more control over who performs the procedure, or avoiding more significant/general anesthesia. MVA in the outpatient setting is the most cost-effective approach to uterine aspiration.15
Choosing a management approach
There are virtually no contraindications for uterine aspiration. Expectant and medication management are contraindicated (and uterine aspiration is recommended) in the setting of bleeding disorders, anticoagulation, suspected intrauterine infection, suspected molar pregnancy, significant cardiopulmonary disease, or any condition for which heavy, unsupervised bleeding might be dangerous.1 Uterine aspiration offers immediate resolution, with a procedure usually lasting 3 to 10 minutes. By contrast, expectant and medication management offer a less predictable time to resolution and, often, a more prolonged period of active pregnancy expulsion.
In the absence of a contraindication, patient choice should determine which management option is used. All 3 options are similarly safe and effective, and the differences that do exist are acceptable to patients as long as they are allowed to access their preferred EPL management method.5,6,16 Patient satisfaction is associated directly with the ability to choose the method of preference.
Managing pain
Pain management should be offered to all women diagnosed with EPL. Those who choose expectant or medication management likely will require only oral nonsteroidal anti-inflammatory drugs (NSAIDs). A minority may require the addition of a small number of narcotic pain pills.17
Women who choose uterine aspiration also should be offered pain management. All patients should be given a paracervical block; other medications can include NSAIDs, an oral benzodiazepine, intravenous (IV) sedation, or even general anesthesia/monitored airway care.17
Patients’ expectations about pain management should be addressed directly during initial counseling. This may help patients decide what type of management and treatment location they might prefer.
Checking blood type: Is it necessary?
The ACOG practice bulletin for EPL states, “administration of Rh D immune globulin should be considered in cases of early pregnancy loss, especially those that are later in the first trimester.”1 A growing body of evidence indicates that Rho(D) immune globulin likely is unnecessary in early pregnancy.
A recent prospective cohort study of 42 women who were at 5 to 12 weeks’ gestation found that the fetal red blood cell concentration was below the calculated threshold for Rh sensitization.18 In light of recent evidence, the National Abortion Federation now recommends foregoing Rh testing and provision of Rh immune globulin at less than 8 weeks’ gestation for uterine aspiration and at less than 10 weeks’ gestation for medication abortion.19
We feel there is sufficient evidence to forego Rh testing in EPL at similar gestational ages, although this is not yet reflected in US societal guidelines. (It is already standard practice in some countries.) Although the risk of Rh alloimmunization is low, the risk of significant consequences in the event of Rh alloimmunization is high. Currently, it also is reasonable to continue giving Rho(D) immune globulin to Rh-negative patients who experience EPL at any gestational age. A lower dose (50 µg) is sufficient for EPL; the standard 300-µg dose also is acceptable.20
We anticipate that society and ACOG guidelines will change in the next few years as the body of evidence increases, and practice should change to reflect new guidance.
Continue to: Prophylactic antibiotics...
Prophylactic antibiotics
The risk of infection with EPL is low overall regardless of the management approach.1 Prophylactic antibiotics are recommended for patients undergoing uterine aspiration but are not necessary in the setting of expectant or medication management. We recommend prophylaxis with 1 dose of oral doxycycline 200 mg or oral azithromycin 500 mg approximately 30 minutes to 1 hour prior to uterine aspiration.21 Alternatives include 1 dose of oral metronidazole 500 mg or, if the patient is unable to take oral medications, IV cefazolin 2 g.
A multisite international randomized controlled trial concluded that antibiotic prophylaxis before uterine aspiration for EPL did not significantly reduce the risk of infection.22 However, there was a significant reduction in pelvic infection with antibiotic administration for the subgroup of women who underwent MVA, which is our recommended approach (along with EVA, and opposed to sharp curettage) for outpatient EPL management.
Follow-up after EPL
In-person follow-up after treatment of EPL is not medically necessary. A repeat ultrasonography 1 to 2 weeks after expectant or medication management can be helpful to confirm completion of the process, and clinicians should focus on presence or absence of a gestational sac to determine if further management is needed.1
Follow-up by telemedicine or phone also is an option and may be preferred in the following situations:
- the patient lives far from the clinic
- travel to the clinic is difficult or expensive
- the patient has child-care issues
- there is a global pandemic necessitating physical distancing.
If the patient’s reported history and symptoms are consistent with a completed process, no further intervention is indicated.
If ongoing EPL is a concern, ask the patient to come in for an evaluation and ultrasonography. If visiting the clinic is still a challenge, following with urine or serum human chorionic gonadotropin (HCG) levels also is acceptable. Experts recommend waiting 4 weeks before expecting a negative urine HCG measurement, although up to 25% of women with a completed EPL will still have a positive test at 4 weeks.23,24
A postprocedure serum HCG is more helpful if a preprocedure HCG level already is known. Numerous studies have evaluated phone follow-up after medication abortion and it is reasonable to translate these practices to follow-up after EPL, recognizing that direct data looking at alternative EPL follow-up are much more limited.23,25-30
The benefit of HCG follow-up at a scheduled time (such as 1 week) is less clear for EPL than for medication abortion, as HCG trends are less predictable in the setting of EPL. However, if the pregnancy has passed, a significant drop in the HCG level would be expected. It is important to take into account the patient’s history and clinical symptoms and consider in-person evaluation with possible ultrasonography if there is concern that the pregnancy tissue has not passed.
Pay attention to mental health
It is critical to assess the patient’s mental and emotional health. This should be done both at the time of EPL diagnosis and management and again at follow-up. Both patients and their partners can struggle after experiencing EPL, and they may suffer from prolonged posttraumatic stress.31
Often, EPL occurs before people have shared the news about their pregnancy. This can amplify the sense of isolation and sadness many women report. Equally critical is recognizing that not all women who experience EPL grieve, and clinicians should normalize patient experiences and feelings. Provider language is important. We recommend use of these questions and phrases:
- I’m so sorry for your loss.
- How are you feeling?
- How have you been doing since I saw you last?
- Your friends/family/partner may be grieving differently or at a different pace than you—this is normal.
- Just because the EPL process is complete doesn’t necessarily mean your processing and/or grieving is over.
- Whatever you’re feeling is okay.
Continue to: Address desire for future pregnancy or contraception...
Address desire for future pregnancy or contraception
No additional workup is necessary after EPL unless a patient is experiencing recurrent pregnancy loss. We do recommend discussing plans for future conception. If a patient wants to conceive again as soon as possible, she can start trying when she feels emotionally ready (even before her next menstrual period). One study found that the ability to conceive and those pregnancy outcomes were the same when patients were randomly assigned to start trying immediately versus waiting 3 months after EPL.32
Alternatively, a patient may want to prevent pregnancy after EPL, and this information should be explicitly elicited and addressed with comprehensive contraception counseling as needed. All forms of contraception can be initiated immediately on successful management of EPL. All contraceptive methods, including an intrauterine device, can be initiated immediately following uterine aspiration.1,33,34
Patients should be reminded that if they delay contraception initiation by more than 7 days, they are potentially at risk for pregnancy.35 Most importantly, clinicians should not make assumptions about future pregnancy desires and should ask open-ended questions to provide appropriate patient counseling.
Finally, patients may feel additional anxiety in a subsequent pregnancy. It is helpful to acknowledge this and perhaps even offer earlier and more frequent visits in early pregnancy to help reduce anxiety. EPL is commonly experienced, and unfortunately it is sometimes poorly addressed by clinicians.
We hope this guidance will help you provide excellent, evidence-based, and sensitive care that will not only manage your patient’s EPL but also make the experience as positive as possible. ●
- Early pregnancy loss (EPL) is common, occurring in up to 15% to 20% of clinically recognized pregnancies.
- EPL can be managed expectantly, with medication, or by uterine aspiration.
- There are virtually no contraindications to uterine aspiration.
- Contraindications to expectant or medication management include any situation in which heavy, unsupervised bleeding might be dangerous.
- In the absence of contraindications, patient preference should dictate the management approach.
- Mifepristone-misoprostol is more effective than misoprostol alone.
- Manual uterine aspiration in the outpatient setting is the most cost-effective approach to uterine evacuation.
- Rh testing is not necessary at less than 8 weeks’ gestation if choosing uterine aspiration, or at less than 10 weeks’ gestation if choosing expectant or medication management.
- Antibiotic prophylaxis is indicated for uterine aspiration, but not for expectant or medication management.
- Ultrasonography follow-up should focus on presence or absence of gestational sac.
- There are viable telemedicine and phone follow-up options that do not require repeat ultrasonography or in-person evaluation.
- There is no need to delay future conception once EPL management is confirmed to be complete.
- It is okay to initiate any contraceptive method immediately on completed management of EPL.
- Feelings toward EPL can be complex and varied; it is helpful to normalize your patients’ experiences, ask open-ended questions, and provide support as needed.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 200: early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
- Clement EG, Horvath S, McAllister A, et al. The language of first-trimester nonviable pregnancy: patient-reported preferences and clarity. Obstet Gynecol. 2019;133:149-154.
- Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Uterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Nanda K, Peloggia A, Grimes D, et al. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst Rev. 2006(2):CD003518.
- Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006(3):CD002253.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594.
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd ed. Geneva, Switzerland: World Health Organization; 2012.
- Wiebe E, Janssen P. Management of spontaneous abortion in family practices and hospitals. Fam Med. 1998;30:293-296.
- Harris LH, Dalton VK, Johnson TR. Surgical management of early pregnancy failure: history, politics, and safe, cost-effective care. Am J Obstet Gynecol. 2007;196:445.e1-e5.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (Miscarriage Treatment [MIST] trial). BMJ. 2006;332:1235-1240.
- Calvache JA, Delgado-Noguera MF, Lesaffre E, et al. Anaesthesia for evacuation of incomplete miscarriage. Cochrane Database System Rev. 2012(4):CD008681.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- National Abortion Federation. 2020 clinical policy guidelines for abortion care. https://www.prochoice.org/education-and-advocacy/cpg. Accessed June 9, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 181: prevention of Rh D alloimmunization. Obstet Gynecol. 2017;130:e59-e70.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180-1189.
- Lissauer D, Wilson A, Hewitt CA, et al. A randomized trial of prophylactic antibiotics for miscarriage surgery. N Engl J Med. 2019;380:1012-1021.
- Perriera L, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010:81:143-149.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Clark W, Bracken H, Tanenhaus J, et al. Alternatives to a routine follow-up visit for early medical abortion. Obstet Gynecol. 2010;115(2 pt 1):264-272.
- Jackson AV, Dayananda I, Fortin JM, et al. Can women accurately assess the outcome of medical abortion based on symptoms alone? Contraception. 2012;85:192-197.
- Raymond EG, Tan YL, Grant M, et al. Self-assessment of medical abortion outcome using symptoms and home pregnancy testing. Contraception. 2018;97:324-328.
- Raymond EG, Shochet T, Bracken H. Low-sensitivity urine pregnancy testing to assess medical abortion outcome: a systematic review. Contraception. 2018;98:30-35.
- Raymond EG, Grossman D, Mark A, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361-366.
- Farren J, Jalmbrant M, Ameye L, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open. 2016;6:e011864.
- Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204-212. DOI: 0.1097/AOG.0000000000001159.
- American College of Obstetricians and Gynecologists. Committee opinion No. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126:e44-e48.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria (US MEC) for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 200: early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
- Clement EG, Horvath S, McAllister A, et al. The language of first-trimester nonviable pregnancy: patient-reported preferences and clarity. Obstet Gynecol. 2019;133:149-154.
- Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Uterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Nanda K, Peloggia A, Grimes D, et al. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst Rev. 2006(2):CD003518.
- Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006(3):CD002253.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594.
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd ed. Geneva, Switzerland: World Health Organization; 2012.
- Wiebe E, Janssen P. Management of spontaneous abortion in family practices and hospitals. Fam Med. 1998;30:293-296.
- Harris LH, Dalton VK, Johnson TR. Surgical management of early pregnancy failure: history, politics, and safe, cost-effective care. Am J Obstet Gynecol. 2007;196:445.e1-e5.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (Miscarriage Treatment [MIST] trial). BMJ. 2006;332:1235-1240.
- Calvache JA, Delgado-Noguera MF, Lesaffre E, et al. Anaesthesia for evacuation of incomplete miscarriage. Cochrane Database System Rev. 2012(4):CD008681.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- National Abortion Federation. 2020 clinical policy guidelines for abortion care. https://www.prochoice.org/education-and-advocacy/cpg. Accessed June 9, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 181: prevention of Rh D alloimmunization. Obstet Gynecol. 2017;130:e59-e70.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180-1189.
- Lissauer D, Wilson A, Hewitt CA, et al. A randomized trial of prophylactic antibiotics for miscarriage surgery. N Engl J Med. 2019;380:1012-1021.
- Perriera L, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010:81:143-149.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Clark W, Bracken H, Tanenhaus J, et al. Alternatives to a routine follow-up visit for early medical abortion. Obstet Gynecol. 2010;115(2 pt 1):264-272.
- Jackson AV, Dayananda I, Fortin JM, et al. Can women accurately assess the outcome of medical abortion based on symptoms alone? Contraception. 2012;85:192-197.
- Raymond EG, Tan YL, Grant M, et al. Self-assessment of medical abortion outcome using symptoms and home pregnancy testing. Contraception. 2018;97:324-328.
- Raymond EG, Shochet T, Bracken H. Low-sensitivity urine pregnancy testing to assess medical abortion outcome: a systematic review. Contraception. 2018;98:30-35.
- Raymond EG, Grossman D, Mark A, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361-366.
- Farren J, Jalmbrant M, Ameye L, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open. 2016;6:e011864.
- Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204-212. DOI: 0.1097/AOG.0000000000001159.
- American College of Obstetricians and Gynecologists. Committee opinion No. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126:e44-e48.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria (US MEC) for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
Telemedicine: Navigating legal issues
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
Declines in infant mortality tempered by disparities
Age-adjusted infant mortality dropped 11% from 2000 to 2017 in the United States, but the even larger decline for infants born to black women still left a death rate more than twice as high as those of white or Hispanic infants, according to a new analysis from the National Center for Health Statistics.

while the crude mortality rate fell 16% from 6.89 to 5.79, reported Anne K. Driscoll, PhD, and Danielle M. Ely, PhD, of the NCHS.
Over that same time period, age-adjusted infant mortality for births to black women went from 13.59 per 1,000 to 11.19, a drop of 18%. By comparison, age-adjusted mortality declined 7% from 5.59 per 1,000 for infants born to Hispanic women to 5.21 in 2017, they said in a National Vital Statistics Report.
Changes in maternal age distribution had an important effect on infant mortality. Women aged under 25 years, who have higher mortality rates, were less likely to give birth in 2017 than in 2000, and women aged 30-39 years, who have the lowest rates, made up a larger share of births in 2017, they pointed out.
It was, however, changes in age-specific mortality rates (ASMRs) that had the largest influence on the overall drop in the crude mortality rate, accounting for about two-thirds of the overall decline, the NCHS researchers said, noting that the effect varied by race and Hispanic origin.
Births to non-Hispanic white women mirrored the national situation: Approximately two-thirds (68.7%) of the decrease in infant mortality came from changes in ASMRs and one-third (31.3%) from changes in maternal age distribution. Among non-Hispanic black women, the distribution was 95.2% ASMRs and 4.8% age distribution, Dr. Driscoll and Dr. Ely reported based on data from the National Vital Statistics System.
The disparity between the two trends went even further for infants born to Hispanic women. Changes in ASMRs were responsible for 133.7% of the overall change in crude mortality versus –33.7% for changes in maternal age distribution. “If no changes occurred in the ASMRs, the changes in the maternal age distribution would have resulted in a higher mortality rate in 2017,” they explained.
The declines in the ASMRs may be related to incremental improved survival of preterm and low-birthweight infants in certain groups. “While little or no progress has been made to lower [these] two key risk factors for poor birth outcomes, progress has been made in lowering the mortality rates of at-risk infants across maternal age and race and Hispanic origin, resulting in lower ASMRs for all age groups,” the investigators suggested.
It also is possible that “changes in other factors, such as maternal education and cigarette smoking during pregnancy, may have indirectly resulted in declining ASMRs for all age groups over time,” they added.
SOURCE: Driscoll AK, Ely DM. National Vital Statistics Reports. 2020;69(5):1-18.
Age-adjusted infant mortality dropped 11% from 2000 to 2017 in the United States, but the even larger decline for infants born to black women still left a death rate more than twice as high as those of white or Hispanic infants, according to a new analysis from the National Center for Health Statistics.

while the crude mortality rate fell 16% from 6.89 to 5.79, reported Anne K. Driscoll, PhD, and Danielle M. Ely, PhD, of the NCHS.
Over that same time period, age-adjusted infant mortality for births to black women went from 13.59 per 1,000 to 11.19, a drop of 18%. By comparison, age-adjusted mortality declined 7% from 5.59 per 1,000 for infants born to Hispanic women to 5.21 in 2017, they said in a National Vital Statistics Report.
Changes in maternal age distribution had an important effect on infant mortality. Women aged under 25 years, who have higher mortality rates, were less likely to give birth in 2017 than in 2000, and women aged 30-39 years, who have the lowest rates, made up a larger share of births in 2017, they pointed out.
It was, however, changes in age-specific mortality rates (ASMRs) that had the largest influence on the overall drop in the crude mortality rate, accounting for about two-thirds of the overall decline, the NCHS researchers said, noting that the effect varied by race and Hispanic origin.
Births to non-Hispanic white women mirrored the national situation: Approximately two-thirds (68.7%) of the decrease in infant mortality came from changes in ASMRs and one-third (31.3%) from changes in maternal age distribution. Among non-Hispanic black women, the distribution was 95.2% ASMRs and 4.8% age distribution, Dr. Driscoll and Dr. Ely reported based on data from the National Vital Statistics System.
The disparity between the two trends went even further for infants born to Hispanic women. Changes in ASMRs were responsible for 133.7% of the overall change in crude mortality versus –33.7% for changes in maternal age distribution. “If no changes occurred in the ASMRs, the changes in the maternal age distribution would have resulted in a higher mortality rate in 2017,” they explained.
The declines in the ASMRs may be related to incremental improved survival of preterm and low-birthweight infants in certain groups. “While little or no progress has been made to lower [these] two key risk factors for poor birth outcomes, progress has been made in lowering the mortality rates of at-risk infants across maternal age and race and Hispanic origin, resulting in lower ASMRs for all age groups,” the investigators suggested.
It also is possible that “changes in other factors, such as maternal education and cigarette smoking during pregnancy, may have indirectly resulted in declining ASMRs for all age groups over time,” they added.
SOURCE: Driscoll AK, Ely DM. National Vital Statistics Reports. 2020;69(5):1-18.
Age-adjusted infant mortality dropped 11% from 2000 to 2017 in the United States, but the even larger decline for infants born to black women still left a death rate more than twice as high as those of white or Hispanic infants, according to a new analysis from the National Center for Health Statistics.

while the crude mortality rate fell 16% from 6.89 to 5.79, reported Anne K. Driscoll, PhD, and Danielle M. Ely, PhD, of the NCHS.
Over that same time period, age-adjusted infant mortality for births to black women went from 13.59 per 1,000 to 11.19, a drop of 18%. By comparison, age-adjusted mortality declined 7% from 5.59 per 1,000 for infants born to Hispanic women to 5.21 in 2017, they said in a National Vital Statistics Report.
Changes in maternal age distribution had an important effect on infant mortality. Women aged under 25 years, who have higher mortality rates, were less likely to give birth in 2017 than in 2000, and women aged 30-39 years, who have the lowest rates, made up a larger share of births in 2017, they pointed out.
It was, however, changes in age-specific mortality rates (ASMRs) that had the largest influence on the overall drop in the crude mortality rate, accounting for about two-thirds of the overall decline, the NCHS researchers said, noting that the effect varied by race and Hispanic origin.
Births to non-Hispanic white women mirrored the national situation: Approximately two-thirds (68.7%) of the decrease in infant mortality came from changes in ASMRs and one-third (31.3%) from changes in maternal age distribution. Among non-Hispanic black women, the distribution was 95.2% ASMRs and 4.8% age distribution, Dr. Driscoll and Dr. Ely reported based on data from the National Vital Statistics System.
The disparity between the two trends went even further for infants born to Hispanic women. Changes in ASMRs were responsible for 133.7% of the overall change in crude mortality versus –33.7% for changes in maternal age distribution. “If no changes occurred in the ASMRs, the changes in the maternal age distribution would have resulted in a higher mortality rate in 2017,” they explained.
The declines in the ASMRs may be related to incremental improved survival of preterm and low-birthweight infants in certain groups. “While little or no progress has been made to lower [these] two key risk factors for poor birth outcomes, progress has been made in lowering the mortality rates of at-risk infants across maternal age and race and Hispanic origin, resulting in lower ASMRs for all age groups,” the investigators suggested.
It also is possible that “changes in other factors, such as maternal education and cigarette smoking during pregnancy, may have indirectly resulted in declining ASMRs for all age groups over time,” they added.
SOURCE: Driscoll AK, Ely DM. National Vital Statistics Reports. 2020;69(5):1-18.
Migraine is often a deciding factor in pregnancy planning
new research shows. Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
Although women with migraine who avoided pregnancy believed their migraines would worsen during pregnancy or make their pregnancy difficult, previous observational research indicates that migraine often improves during pregnancy.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix, Arizona.
The findings were presented at the virtual annual meeting of the American Headache Society.
Plans for the future
There is a paucity of research on the effects of migraine on pregnancy planning, the researchers noted. The few studies that have investigated this issue have focused on women’s previous family planning decisions and experience rather than on plans for the future, the researchers noted.
To evaluate how migraine in women influences pregnancy planning, the investigators analyzed data from the American Registry for Migraine Research (ARMR). The registry, which was established by the American Migraine Foundation, collects clinical data about individuals with migraine and other headache disorders from multiple centers.
Participants eligible for the current analysis were women who had been diagnosed with migraine on the basis of the International Classification of Headache Disorders–3 criteria. All completed the ARMR questionnaire between February 2016 and September 2019. The investigators excluded patients with trigeminal autonomic cephalalgia, secondary headache, painful cranial neuropathies, other facial pain, and other headaches.
They identified 895 eligible women with migraine. Of these, 607 completed the pregnancy question. Among those participants, 121 women (19.9%) reported that migraine was a factor in their decision to not become pregnant. Of this group, 70 (11.5%) reported that migraine was a “significant” factor in deciding to not have children, and 8.4% said it was “somewhat” of a factor. The remainder of the cohort (479) reported that migraine had no influence on their pregnancy plans.
There were no between-group differences by race, marital status, employment, or income. This finding suggests that sociodemographic differences “have less impact on pregnancy planning than migraine-specific characteristics like headache frequency and experience with having migraine attacks triggered by menstruation,” Dr. Ishii said.
“Substantial burden”
Not surprisingly, women who avoided pregnancy had fewer children than the rest of the sample. About 60% of those who made the decision to not become pregnant had no children, and 72% had not been pregnant since they began experiencing migraine.
Compared with women who reported that migraine had no influence on their pregnancy plans, those who avoided pregnancy were more likely to have chronic migraine at 81.8% versus 70.2%. They were also more likely to have menstrual migraine at 4.1% versus 1%. In addition, women who decided to not have children because of migraine were significantly younger at an average age of 37.5 versus 47.2 years.
The number of days with headache per 3-month interval was 53.9 among women who avoided pregnancy versus 42.5 among the other women. The Migraine Disability Assessment score was also higher for women who avoided pregnancy (132.5) than for it was the other women (91.7), indicating more severe disability.
In addition, more of the women who avoided pregnancy had a history of depression (48.8%) compared with the other women (37.7%). The average score on the Patient Health Questionnaire–4 was higher among women who avoided pregnancy (4.0) than among other women (3.1), which indicates greater anxiety or depression. Among women who avoided pregnancy, 72.5% believed their migraine would worsen during pregnancy, and 68.3% believed that migraine would make pregnancy very difficult.
“Clinicians need to recognize that migraine often has a substantial burden on multiple aspects of life, including one’s plans for having children,” Dr. Ishii said.
“Clinicians should educate their patients who are considering pregnancy about the most likely course of migraine during pregnancy, migraine treatment during pregnancy, and the potential impacts of migraine and its treatment on pregnancy outcomes,” he added.
More education needed
Commenting on the study, Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center, Irvine, California, said that not knowing how pregnancy is going to affect patients’ migraines can be “very scary” for women. In addition, patients often wonder what migraine treatments they can safely take once they do become pregnant, said Dr. Hutchinson, who was not involved in the research.
She noted that advantages of the ARMR data are that they are derived from a multicenter study and that migraine diagnoses were made by a headache specialist. A potential limitation of the study is that the population may not reflect outcomes of the millions of women who have migraine and become pregnant but never see a specialist.
“These findings show that more education is needed,” Dr. Hutchinson said.
Most women, especially those who have migraine without aura, note improvement with migraine during pregnancy, primarily because of the high, steady levels of estradiol, especially in the second and third trimesters, she said. In light of this, neurologists should reassure women that migraine is not a contraindication to pregnancy, she added.
There is also a need for additional research to assess how past experience with migraine and pregnancy influences a woman’s comfort level with additional pregnancies. Studies as to which treatments are safest for acute and preventive treatment of migraine during prepregnancy, pregnancy, and lactation are also needed, Dr. Hutchinson noted.
“If women knew they had treatment options that were evidence-based, they might be much more comfortable contemplating a pregnancy,” she said.
Dr. Ishii and Dr. Hutchinson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows. Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
Although women with migraine who avoided pregnancy believed their migraines would worsen during pregnancy or make their pregnancy difficult, previous observational research indicates that migraine often improves during pregnancy.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix, Arizona.
The findings were presented at the virtual annual meeting of the American Headache Society.
Plans for the future
There is a paucity of research on the effects of migraine on pregnancy planning, the researchers noted. The few studies that have investigated this issue have focused on women’s previous family planning decisions and experience rather than on plans for the future, the researchers noted.
To evaluate how migraine in women influences pregnancy planning, the investigators analyzed data from the American Registry for Migraine Research (ARMR). The registry, which was established by the American Migraine Foundation, collects clinical data about individuals with migraine and other headache disorders from multiple centers.
Participants eligible for the current analysis were women who had been diagnosed with migraine on the basis of the International Classification of Headache Disorders–3 criteria. All completed the ARMR questionnaire between February 2016 and September 2019. The investigators excluded patients with trigeminal autonomic cephalalgia, secondary headache, painful cranial neuropathies, other facial pain, and other headaches.
They identified 895 eligible women with migraine. Of these, 607 completed the pregnancy question. Among those participants, 121 women (19.9%) reported that migraine was a factor in their decision to not become pregnant. Of this group, 70 (11.5%) reported that migraine was a “significant” factor in deciding to not have children, and 8.4% said it was “somewhat” of a factor. The remainder of the cohort (479) reported that migraine had no influence on their pregnancy plans.
There were no between-group differences by race, marital status, employment, or income. This finding suggests that sociodemographic differences “have less impact on pregnancy planning than migraine-specific characteristics like headache frequency and experience with having migraine attacks triggered by menstruation,” Dr. Ishii said.
“Substantial burden”
Not surprisingly, women who avoided pregnancy had fewer children than the rest of the sample. About 60% of those who made the decision to not become pregnant had no children, and 72% had not been pregnant since they began experiencing migraine.
Compared with women who reported that migraine had no influence on their pregnancy plans, those who avoided pregnancy were more likely to have chronic migraine at 81.8% versus 70.2%. They were also more likely to have menstrual migraine at 4.1% versus 1%. In addition, women who decided to not have children because of migraine were significantly younger at an average age of 37.5 versus 47.2 years.
The number of days with headache per 3-month interval was 53.9 among women who avoided pregnancy versus 42.5 among the other women. The Migraine Disability Assessment score was also higher for women who avoided pregnancy (132.5) than for it was the other women (91.7), indicating more severe disability.
In addition, more of the women who avoided pregnancy had a history of depression (48.8%) compared with the other women (37.7%). The average score on the Patient Health Questionnaire–4 was higher among women who avoided pregnancy (4.0) than among other women (3.1), which indicates greater anxiety or depression. Among women who avoided pregnancy, 72.5% believed their migraine would worsen during pregnancy, and 68.3% believed that migraine would make pregnancy very difficult.
“Clinicians need to recognize that migraine often has a substantial burden on multiple aspects of life, including one’s plans for having children,” Dr. Ishii said.
“Clinicians should educate their patients who are considering pregnancy about the most likely course of migraine during pregnancy, migraine treatment during pregnancy, and the potential impacts of migraine and its treatment on pregnancy outcomes,” he added.
More education needed
Commenting on the study, Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center, Irvine, California, said that not knowing how pregnancy is going to affect patients’ migraines can be “very scary” for women. In addition, patients often wonder what migraine treatments they can safely take once they do become pregnant, said Dr. Hutchinson, who was not involved in the research.
She noted that advantages of the ARMR data are that they are derived from a multicenter study and that migraine diagnoses were made by a headache specialist. A potential limitation of the study is that the population may not reflect outcomes of the millions of women who have migraine and become pregnant but never see a specialist.
“These findings show that more education is needed,” Dr. Hutchinson said.
Most women, especially those who have migraine without aura, note improvement with migraine during pregnancy, primarily because of the high, steady levels of estradiol, especially in the second and third trimesters, she said. In light of this, neurologists should reassure women that migraine is not a contraindication to pregnancy, she added.
There is also a need for additional research to assess how past experience with migraine and pregnancy influences a woman’s comfort level with additional pregnancies. Studies as to which treatments are safest for acute and preventive treatment of migraine during prepregnancy, pregnancy, and lactation are also needed, Dr. Hutchinson noted.
“If women knew they had treatment options that were evidence-based, they might be much more comfortable contemplating a pregnancy,” she said.
Dr. Ishii and Dr. Hutchinson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows. Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
Although women with migraine who avoided pregnancy believed their migraines would worsen during pregnancy or make their pregnancy difficult, previous observational research indicates that migraine often improves during pregnancy.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix, Arizona.
The findings were presented at the virtual annual meeting of the American Headache Society.
Plans for the future
There is a paucity of research on the effects of migraine on pregnancy planning, the researchers noted. The few studies that have investigated this issue have focused on women’s previous family planning decisions and experience rather than on plans for the future, the researchers noted.
To evaluate how migraine in women influences pregnancy planning, the investigators analyzed data from the American Registry for Migraine Research (ARMR). The registry, which was established by the American Migraine Foundation, collects clinical data about individuals with migraine and other headache disorders from multiple centers.
Participants eligible for the current analysis were women who had been diagnosed with migraine on the basis of the International Classification of Headache Disorders–3 criteria. All completed the ARMR questionnaire between February 2016 and September 2019. The investigators excluded patients with trigeminal autonomic cephalalgia, secondary headache, painful cranial neuropathies, other facial pain, and other headaches.
They identified 895 eligible women with migraine. Of these, 607 completed the pregnancy question. Among those participants, 121 women (19.9%) reported that migraine was a factor in their decision to not become pregnant. Of this group, 70 (11.5%) reported that migraine was a “significant” factor in deciding to not have children, and 8.4% said it was “somewhat” of a factor. The remainder of the cohort (479) reported that migraine had no influence on their pregnancy plans.
There were no between-group differences by race, marital status, employment, or income. This finding suggests that sociodemographic differences “have less impact on pregnancy planning than migraine-specific characteristics like headache frequency and experience with having migraine attacks triggered by menstruation,” Dr. Ishii said.
“Substantial burden”
Not surprisingly, women who avoided pregnancy had fewer children than the rest of the sample. About 60% of those who made the decision to not become pregnant had no children, and 72% had not been pregnant since they began experiencing migraine.
Compared with women who reported that migraine had no influence on their pregnancy plans, those who avoided pregnancy were more likely to have chronic migraine at 81.8% versus 70.2%. They were also more likely to have menstrual migraine at 4.1% versus 1%. In addition, women who decided to not have children because of migraine were significantly younger at an average age of 37.5 versus 47.2 years.
The number of days with headache per 3-month interval was 53.9 among women who avoided pregnancy versus 42.5 among the other women. The Migraine Disability Assessment score was also higher for women who avoided pregnancy (132.5) than for it was the other women (91.7), indicating more severe disability.
In addition, more of the women who avoided pregnancy had a history of depression (48.8%) compared with the other women (37.7%). The average score on the Patient Health Questionnaire–4 was higher among women who avoided pregnancy (4.0) than among other women (3.1), which indicates greater anxiety or depression. Among women who avoided pregnancy, 72.5% believed their migraine would worsen during pregnancy, and 68.3% believed that migraine would make pregnancy very difficult.
“Clinicians need to recognize that migraine often has a substantial burden on multiple aspects of life, including one’s plans for having children,” Dr. Ishii said.
“Clinicians should educate their patients who are considering pregnancy about the most likely course of migraine during pregnancy, migraine treatment during pregnancy, and the potential impacts of migraine and its treatment on pregnancy outcomes,” he added.
More education needed
Commenting on the study, Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center, Irvine, California, said that not knowing how pregnancy is going to affect patients’ migraines can be “very scary” for women. In addition, patients often wonder what migraine treatments they can safely take once they do become pregnant, said Dr. Hutchinson, who was not involved in the research.
She noted that advantages of the ARMR data are that they are derived from a multicenter study and that migraine diagnoses were made by a headache specialist. A potential limitation of the study is that the population may not reflect outcomes of the millions of women who have migraine and become pregnant but never see a specialist.
“These findings show that more education is needed,” Dr. Hutchinson said.
Most women, especially those who have migraine without aura, note improvement with migraine during pregnancy, primarily because of the high, steady levels of estradiol, especially in the second and third trimesters, she said. In light of this, neurologists should reassure women that migraine is not a contraindication to pregnancy, she added.
There is also a need for additional research to assess how past experience with migraine and pregnancy influences a woman’s comfort level with additional pregnancies. Studies as to which treatments are safest for acute and preventive treatment of migraine during prepregnancy, pregnancy, and lactation are also needed, Dr. Hutchinson noted.
“If women knew they had treatment options that were evidence-based, they might be much more comfortable contemplating a pregnancy,” she said.
Dr. Ishii and Dr. Hutchinson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AHS 2020
COVID-19: Haiti is vulnerable, but the international community can help
Doctors Without Borders, other groups urged to mobilize
Do you want to know what keeps us up at night? As 4th-year medical students born, raised, and living in Haiti, we worry about the impact of COVID-19 on our patients.
The pandemic has shaken the world, and Haiti is no exception.
It has taken several months for the disease to spread, and it began with two confirmed cases, one from France and the other from Belgium, on March 19.1 Much of the spread of COVID-19 in Haiti has been tied to workers returning from the Dominican Republic. As of June 29, Haiti had 5,975 confirmed cases and 105 deaths.2 Of course, those numbers sound minuscule, compared with those in the United States, where the number of deaths from COVID-19 surpassed 100,000 several weeks ago. But the population of Haiti is 30 times smaller than that of the United States, and Haiti is the poorest country in the Western Hemisphere. We have watched in horror as the virus has ravaged marginalized groups in the United States and worry that it will do the same in our own country.
Just as the Haitian Ministry of Health worked with various groups to reach the 1-year free of cholera mark in Haiti, groups such as Doctors Without Borders must mobilize to rein in COVID-19.
Community transmission rapid
After the first two cases were confirmed, a state of health emergency was immediately declared. Haitian President Jovenel Moïse and other government officials called for the implementation of several measures aimed at limiting the spread of COVID-19.
Schools, universities, clinical training programs, vocational centers, factories, airports, and ports, except for the transport of goods, were all ordered to close until further notice. Gatherings of larger than 10 people were banned. A curfew from 8 p.m. EST time to 5 a.m. EST was imposed. Measures such as those encouraged by U.S. Centers for Disease Control and Prevention, such as hand washing, physical distancing, and staying at home were also encouraged by the Haitian Ministry of Health. Mask wearing in public places was deemed mandatory.
The latest testing data show that community spread has been occurring among the Haitian population at a rapid rate. According to Jean William Pape, MD, Haiti’s top infectious diseases expert and founder of GHESKIO, an iconic infectious disease center that cares for people with HIV-AIDS and tuberculosis, a COVID-19 simulation from Cornell University in New York shows that about 35% of the Haitian population will be infected by the end of August 2020. A simulation by the University of Oxford (England) paints an even more dire picture. That simulation shows that 86% of the population could be infected, More than 9,000 additional hospital beds would be needed, and 20,000 people would be likely to die from COVID-19, Dr. Pape said in an interview with Haiti’s Nouvelliste newspaper.3
Medical response
We know that there is a global shortage of health care workers,4 and Haiti is no exception. According to a 2018 report from the Haitian Ministry of Health, the country has 11,775 health care professionals, including about 3,354 medical doctors, to care for more than 11 million people. That translates to about 23.4 physicians per 100,000.5
The pandemic has led some members of this already anemic health care workforce to stay home because of a lack of personal protective equipment. Others, because of reduced hospital or clinic budgets, have been furloughed, making the COVID-19 national health emergency even harder to manage.
But a severe health care shortage is not the only challenge facing Haiti. It spends about $131 U.S. per capita, which makes Haiti one of most vulnerable among low- and middle-income countries in the world. As a poor country,7 its health care infrastructure is among the most inadequate and weakest. Prior to COVID-19, medical advocacy groups already had started movements and strikes demanding that the government improve the health care system. The country’s precarious health care infrastructure includes a lack of hospital beds, and basic medical supplies and equipment, such as oxygen and ventilators.8 The emergence of COVID-19 has only exacerbated the situation.
Clinical training programs have been suspended, many doctors and nurses are on quarantine, and some hospitals and clinics are closing. We have witnessed makeshift voodoo clinics built by Haitian voodoo leaders to receive, hospitalize, and treat COVID-19 patients through rituals and herbal remedies. In some areas of the country, residents have protested against the opening of several COVID-19 treatment and management centers.
Unique cultural challenges
Public health officials around the world are facing challenges persuading citizens to engage in behaviors that could protect them from the virus.
Just as in America, where many people opt to not wear face coverings9,10 despite the public health risks, deep distrust of the Haitian government has undermined the messages of President Moïse and public health officials about the role of masks in limiting the spread of COVID. We see large numbers of unmasked people on the streets in the informal markets every day. Crammed tap-taps and overloaded motorcycles are moving everywhere. This also could be tied to cultural attitudes about COVID that persist among some Haitians. For example, many people with signs and symptoms of COVID-19 are afraid of going to the hospital to get tested and receive care, and resort to going to the voodoo clinics. Along with rituals, voodoo priests have been serving up teas with ingredients, including moringa, eucalyptus, ginger, and honey to those seeking COVID-19 care in the centers. The voodoo priests claim that the teas they serve strengthen the immune system.
In addition, it is difficult for poor people who live in small quarters with several other people to adhere to physical distancing.11
Stigma and violence
Other barriers in the fight against COVID-19 in Haiti are stigma and violence. If widespread testing were available, some Haitians would opt not to do so – despite clear signs and symptoms of the infection. Some people who would get tested if they could are afraid to do so because of fears tied to being attacked by neighbors.
When Haitian University professor Bellamy Nelson and his girlfriend returned to Haiti from the United States in March and began experiencing some pain and fever, he experienced attacks from neighbors, he said in an interview. He said neighbors threatened to burn down his house. When an ambulance arrived at his house to transport him to a hospital, it had to drive through back roads to avoid people armed with rocks, fire, and machetes, he told us. No hospital wanted to admit him. Eventually, Professor Nelson self-quarantined at home, he said.
In another incident, a national ambulance center in Gonaïves, a town toward the northern region of Haiti, reportedly was vandalized, because COVID-19 equipment and supplies used to treat people had been stored there. Hospital Bernard Mevs, along with many other hospitals, was forced by the area’s residents to suspend the plan to open a center for COVID-19 management. Threats to burn down the hospitals caused the leaders of the hospitals to back down and give up a plan to build a 20-bed COVID-19 response center.
Maternal health
Another concern we have about the pandemic is the risk it could be to pregnant women. On average, 94,000 deaths occur annually in Haiti. Out of this number, maternal mortality accounts for 1,000. In 2017, for every 100,000 live births for women of reproductive age from 15 to 49 years old, 480 women died. In contrast, in the Dominican Republic, 95 women died per 100,000 that same year. In the United States, 19 died, and in Norway, no more than 2 died that year.12
Some of the primary factors contributing to the crisis are limited accessibility, inadequate health care facilities, and an inadequate number of trained health care practitioners; low percentages of skilled attendants at deliveries and of prenatal and postnatal visits; and high numbers of high-risk deliveries in nonqualified health facilities.
During the COVID-19 national health emergency, with most hospitals reducing their health care personnel either because of budget-related reasons or because they are on quarantine, this maternal-fetal health crisis has escalated.
One of the biggest hospitals in Jacmel, a town in the southern region of Haiti, has stopped its prenatal care program. In Delmas, the city with the highest incidence and prevalence of COVID-19, Hôpital Universitaire de la Paix has reduced this program to 50% of its capacity and gynecologic care has been completely suspended. Hôpital St. Luc, one of the first hospitals in the western region of Haiti to open its doors to care for COVID-19 patients, has recently shut down the entire maternal-fetal department.
So, access to prenatal and postnatal care, including the ability to deliver babies in health care institutions, is significantly reduced because of COVID-19. This leaves thousands of already vulnerable pregnant women at risk and having to deliver domestically with little to no health care professional assistance. We worry that, in light of the data, more women and babies will die because of the COVID-19 pandemic.
A call to action
Despite these conditions, there are reasons for hope. Various groups, both from the international community and locally have mobilized to respond to the pandemic.
International health care organizations such as Doctors Without Borders and Partners in Health, and local groups such as GHESKIO, the St. Luke Foundation for Haiti, and others have been collaborating with the Haitian Ministry of Health to devise and strategic plans and deploy valuable resources with the common goal of saving lives from COVID-19.
GHESKIO, for example, under Dr. Pape’s leadership, currently has one of the three COVID-19 testing centers in the country. It also has two COVID-19 treatment centers in full operation, in Port-au-Prince, the capital city, managing and treating 520 patients with confirmed COVID-19. GHESKIO, which has been in the front lines of previous major infectious disease outbreaks,13 has trained about 200 clinicians from both public and private health care institutions to care for COVID-19 patients.
Doctors Without Borders has been investing in efforts to support the Ministry of Health by converting and renovating its Burn Center in Drouillard, a small section of the city of Cité Soleil, one of the country’s biggest slums. In May, as part of its COVID-19 response, it launched a 20-bed capacity center that can accommodate up to 45 beds to care for patients who have tested positive for COVID-19.
Partners in Health, the Boston-based nonprofit health care organization cofounded in 1987 by American anthropologist and infectious disease specialist, Paul Farmer, MD, and the largest nonprofit health care provider in Haiti, also joined the Ministry of Health through its national and public health efforts to tackle COVID-19 in Haiti. Partners in Health, through its sister organization, Zanmi Lasante, has pioneered the movement of diagnosing and treating people with HIV-AIDS and TB. Since the late 1990s, its efforts against both infectious diseases have helped 15,000 HIV-positive patients begin and remain on treatment. And every year, 1,500 TB patients have started treatment on the path to a cure.
Early in the pandemic in Haiti, Partners in Health, through its state-of-the-art 300-bed university hospital (Hôpital Universitaire de Mirebalais de Mirebalais), was the first to open a COVID-19 center with a 20-bed capacity and has been caring for COVID-19 patients since then. In June, Partners in Health supported and inaugurated the renovation of the internal medicine department at one of its affiliated community hospitals, Hôpital Saint-Nicolas de Saint Marc. That department will have a 24-bed capacity that can extend up to 36 beds to manage and treat COVID-19 patients.
In total, currently, 26 COVID-19 centers with a capacity of 1,011 beds are available to serve, manage, and treat Haitian patients affected with COVID-19. But are those efforts enough? No.
Haiti, as a weak state even before COVID-19, continues to need funding from the international community so it can strengthen its health care infrastructure to be effective and strong in fighting against COVID-19.
In addition, we would like to see preventive initiatives implemented on the local level. Our family has taken on a role that, we think, could help conquer COVID-19 if others followed suit on a large scale.
As part of our contribution in tackling COVID-19, the two of us have launched a small-scale community experiment. We have educated our family in Delmas about COVID-19 and subsequently launched an awareness campaign in the community. We dispatched small groups that go door to door in the community to educate neighbors about the disease in an effort to help them understand that COVID-19 is real and it is normal for people that feel they may have the disease to seek medical care. This approach helps suppress the transmission of the virus. This pilot project could be reproduced in several other communities. It is easy to operate, rapid, effective, and cost-free. The community has been very receptive to and grateful for our efforts.
Like other countries across the world, Haiti was not ready for COVID-19. But we are confident that, with help from the international community, organizations such as GHESKIO,14 and with due diligence on the local level, we are strong and resilient enough to beat COVID. We must act together – quickly.
References
1. Sénat JD. Coronavirus: 2 cas confirmés en Haïti, Jovenel Moïse décrète l’état d’ur-gence sanitaire. 2020 Le Nouvelliste.
2. Haitian Ministry of Health.
3. “Entre appel a la solidarite et de sombres previsions, le Dr William Pape fait le point.” Le Nouvelliste.
4. Darzi A and Evans T. Lancet. 2016 Nov-Dec 26. 388;10060:2576-7.
5. Rapport Statistique 2018. 2019 Republic of Haiti.
6. Sentlinger K. “Water Crisis in Haiti.” The Water Project.
7. The World Bank in Haiti. worldbank.org.
8. Cenat JM. Travel Med Infect Dis. 2020 Mar 28. doi: 10.1016/jtmaid.2020.101684.
9. Block D. “Why some Americans resist wearing face masks.” voanews.com. 2020 May 31.
10. Panceski B and Douglas J. “Masks could help stop coronavirus. So why are they still controversial?” wsj.com. Updated 29 Jun 2020.
11. Bojarski S. “Social distancing: A luxury Haiti’s poor cannot afford. The Haitian Times. 2020 Apr.
12. World Health Organization, UNICEF, World Bank Group, and the U.N. Population Division. Maternal mortality ratio, Haiti.
13. Feliciano I and Kargbo C. “As COVID cases surge, Haiti’s Dr. Pape is on the front line again.” PBS NewsHour Weekend. 2020 Jun 13.
14. Liautaud B and Deschamps MM. New Engl J Med. 2020 Jun 16.
Mr. Dorcela is a senior medical student at Faculté des Sciences de la Santé Université Quisqueya in Port-au-Prince, Haiti. He also is a medical intern at Unité de Médecine Familiale Hôpital Saint Nicolas in Saint-Marc. Mr. Dorcela has no disclosures. Mr. St. Jean, who is Mr. Dorcela’s brother, is also a senior medical student at Faculté des Sciences de la Santé Université Quisqueya in Port-au-Prince. He has no disclosures.
Doctors Without Borders, other groups urged to mobilize
Doctors Without Borders, other groups urged to mobilize
Do you want to know what keeps us up at night? As 4th-year medical students born, raised, and living in Haiti, we worry about the impact of COVID-19 on our patients.
The pandemic has shaken the world, and Haiti is no exception.
It has taken several months for the disease to spread, and it began with two confirmed cases, one from France and the other from Belgium, on March 19.1 Much of the spread of COVID-19 in Haiti has been tied to workers returning from the Dominican Republic. As of June 29, Haiti had 5,975 confirmed cases and 105 deaths.2 Of course, those numbers sound minuscule, compared with those in the United States, where the number of deaths from COVID-19 surpassed 100,000 several weeks ago. But the population of Haiti is 30 times smaller than that of the United States, and Haiti is the poorest country in the Western Hemisphere. We have watched in horror as the virus has ravaged marginalized groups in the United States and worry that it will do the same in our own country.
Just as the Haitian Ministry of Health worked with various groups to reach the 1-year free of cholera mark in Haiti, groups such as Doctors Without Borders must mobilize to rein in COVID-19.
Community transmission rapid
After the first two cases were confirmed, a state of health emergency was immediately declared. Haitian President Jovenel Moïse and other government officials called for the implementation of several measures aimed at limiting the spread of COVID-19.
Schools, universities, clinical training programs, vocational centers, factories, airports, and ports, except for the transport of goods, were all ordered to close until further notice. Gatherings of larger than 10 people were banned. A curfew from 8 p.m. EST time to 5 a.m. EST was imposed. Measures such as those encouraged by U.S. Centers for Disease Control and Prevention, such as hand washing, physical distancing, and staying at home were also encouraged by the Haitian Ministry of Health. Mask wearing in public places was deemed mandatory.
The latest testing data show that community spread has been occurring among the Haitian population at a rapid rate. According to Jean William Pape, MD, Haiti’s top infectious diseases expert and founder of GHESKIO, an iconic infectious disease center that cares for people with HIV-AIDS and tuberculosis, a COVID-19 simulation from Cornell University in New York shows that about 35% of the Haitian population will be infected by the end of August 2020. A simulation by the University of Oxford (England) paints an even more dire picture. That simulation shows that 86% of the population could be infected, More than 9,000 additional hospital beds would be needed, and 20,000 people would be likely to die from COVID-19, Dr. Pape said in an interview with Haiti’s Nouvelliste newspaper.3
Medical response
We know that there is a global shortage of health care workers,4 and Haiti is no exception. According to a 2018 report from the Haitian Ministry of Health, the country has 11,775 health care professionals, including about 3,354 medical doctors, to care for more than 11 million people. That translates to about 23.4 physicians per 100,000.5
The pandemic has led some members of this already anemic health care workforce to stay home because of a lack of personal protective equipment. Others, because of reduced hospital or clinic budgets, have been furloughed, making the COVID-19 national health emergency even harder to manage.
But a severe health care shortage is not the only challenge facing Haiti. It spends about $131 U.S. per capita, which makes Haiti one of most vulnerable among low- and middle-income countries in the world. As a poor country,7 its health care infrastructure is among the most inadequate and weakest. Prior to COVID-19, medical advocacy groups already had started movements and strikes demanding that the government improve the health care system. The country’s precarious health care infrastructure includes a lack of hospital beds, and basic medical supplies and equipment, such as oxygen and ventilators.8 The emergence of COVID-19 has only exacerbated the situation.
Clinical training programs have been suspended, many doctors and nurses are on quarantine, and some hospitals and clinics are closing. We have witnessed makeshift voodoo clinics built by Haitian voodoo leaders to receive, hospitalize, and treat COVID-19 patients through rituals and herbal remedies. In some areas of the country, residents have protested against the opening of several COVID-19 treatment and management centers.
Unique cultural challenges
Public health officials around the world are facing challenges persuading citizens to engage in behaviors that could protect them from the virus.
Just as in America, where many people opt to not wear face coverings9,10 despite the public health risks, deep distrust of the Haitian government has undermined the messages of President Moïse and public health officials about the role of masks in limiting the spread of COVID. We see large numbers of unmasked people on the streets in the informal markets every day. Crammed tap-taps and overloaded motorcycles are moving everywhere. This also could be tied to cultural attitudes about COVID that persist among some Haitians. For example, many people with signs and symptoms of COVID-19 are afraid of going to the hospital to get tested and receive care, and resort to going to the voodoo clinics. Along with rituals, voodoo priests have been serving up teas with ingredients, including moringa, eucalyptus, ginger, and honey to those seeking COVID-19 care in the centers. The voodoo priests claim that the teas they serve strengthen the immune system.
In addition, it is difficult for poor people who live in small quarters with several other people to adhere to physical distancing.11
Stigma and violence
Other barriers in the fight against COVID-19 in Haiti are stigma and violence. If widespread testing were available, some Haitians would opt not to do so – despite clear signs and symptoms of the infection. Some people who would get tested if they could are afraid to do so because of fears tied to being attacked by neighbors.
When Haitian University professor Bellamy Nelson and his girlfriend returned to Haiti from the United States in March and began experiencing some pain and fever, he experienced attacks from neighbors, he said in an interview. He said neighbors threatened to burn down his house. When an ambulance arrived at his house to transport him to a hospital, it had to drive through back roads to avoid people armed with rocks, fire, and machetes, he told us. No hospital wanted to admit him. Eventually, Professor Nelson self-quarantined at home, he said.
In another incident, a national ambulance center in Gonaïves, a town toward the northern region of Haiti, reportedly was vandalized, because COVID-19 equipment and supplies used to treat people had been stored there. Hospital Bernard Mevs, along with many other hospitals, was forced by the area’s residents to suspend the plan to open a center for COVID-19 management. Threats to burn down the hospitals caused the leaders of the hospitals to back down and give up a plan to build a 20-bed COVID-19 response center.
Maternal health
Another concern we have about the pandemic is the risk it could be to pregnant women. On average, 94,000 deaths occur annually in Haiti. Out of this number, maternal mortality accounts for 1,000. In 2017, for every 100,000 live births for women of reproductive age from 15 to 49 years old, 480 women died. In contrast, in the Dominican Republic, 95 women died per 100,000 that same year. In the United States, 19 died, and in Norway, no more than 2 died that year.12
Some of the primary factors contributing to the crisis are limited accessibility, inadequate health care facilities, and an inadequate number of trained health care practitioners; low percentages of skilled attendants at deliveries and of prenatal and postnatal visits; and high numbers of high-risk deliveries in nonqualified health facilities.
During the COVID-19 national health emergency, with most hospitals reducing their health care personnel either because of budget-related reasons or because they are on quarantine, this maternal-fetal health crisis has escalated.
One of the biggest hospitals in Jacmel, a town in the southern region of Haiti, has stopped its prenatal care program. In Delmas, the city with the highest incidence and prevalence of COVID-19, Hôpital Universitaire de la Paix has reduced this program to 50% of its capacity and gynecologic care has been completely suspended. Hôpital St. Luc, one of the first hospitals in the western region of Haiti to open its doors to care for COVID-19 patients, has recently shut down the entire maternal-fetal department.
So, access to prenatal and postnatal care, including the ability to deliver babies in health care institutions, is significantly reduced because of COVID-19. This leaves thousands of already vulnerable pregnant women at risk and having to deliver domestically with little to no health care professional assistance. We worry that, in light of the data, more women and babies will die because of the COVID-19 pandemic.
A call to action
Despite these conditions, there are reasons for hope. Various groups, both from the international community and locally have mobilized to respond to the pandemic.
International health care organizations such as Doctors Without Borders and Partners in Health, and local groups such as GHESKIO, the St. Luke Foundation for Haiti, and others have been collaborating with the Haitian Ministry of Health to devise and strategic plans and deploy valuable resources with the common goal of saving lives from COVID-19.
GHESKIO, for example, under Dr. Pape’s leadership, currently has one of the three COVID-19 testing centers in the country. It also has two COVID-19 treatment centers in full operation, in Port-au-Prince, the capital city, managing and treating 520 patients with confirmed COVID-19. GHESKIO, which has been in the front lines of previous major infectious disease outbreaks,13 has trained about 200 clinicians from both public and private health care institutions to care for COVID-19 patients.
Doctors Without Borders has been investing in efforts to support the Ministry of Health by converting and renovating its Burn Center in Drouillard, a small section of the city of Cité Soleil, one of the country’s biggest slums. In May, as part of its COVID-19 response, it launched a 20-bed capacity center that can accommodate up to 45 beds to care for patients who have tested positive for COVID-19.
Partners in Health, the Boston-based nonprofit health care organization cofounded in 1987 by American anthropologist and infectious disease specialist, Paul Farmer, MD, and the largest nonprofit health care provider in Haiti, also joined the Ministry of Health through its national and public health efforts to tackle COVID-19 in Haiti. Partners in Health, through its sister organization, Zanmi Lasante, has pioneered the movement of diagnosing and treating people with HIV-AIDS and TB. Since the late 1990s, its efforts against both infectious diseases have helped 15,000 HIV-positive patients begin and remain on treatment. And every year, 1,500 TB patients have started treatment on the path to a cure.
Early in the pandemic in Haiti, Partners in Health, through its state-of-the-art 300-bed university hospital (Hôpital Universitaire de Mirebalais de Mirebalais), was the first to open a COVID-19 center with a 20-bed capacity and has been caring for COVID-19 patients since then. In June, Partners in Health supported and inaugurated the renovation of the internal medicine department at one of its affiliated community hospitals, Hôpital Saint-Nicolas de Saint Marc. That department will have a 24-bed capacity that can extend up to 36 beds to manage and treat COVID-19 patients.
In total, currently, 26 COVID-19 centers with a capacity of 1,011 beds are available to serve, manage, and treat Haitian patients affected with COVID-19. But are those efforts enough? No.
Haiti, as a weak state even before COVID-19, continues to need funding from the international community so it can strengthen its health care infrastructure to be effective and strong in fighting against COVID-19.
In addition, we would like to see preventive initiatives implemented on the local level. Our family has taken on a role that, we think, could help conquer COVID-19 if others followed suit on a large scale.
As part of our contribution in tackling COVID-19, the two of us have launched a small-scale community experiment. We have educated our family in Delmas about COVID-19 and subsequently launched an awareness campaign in the community. We dispatched small groups that go door to door in the community to educate neighbors about the disease in an effort to help them understand that COVID-19 is real and it is normal for people that feel they may have the disease to seek medical care. This approach helps suppress the transmission of the virus. This pilot project could be reproduced in several other communities. It is easy to operate, rapid, effective, and cost-free. The community has been very receptive to and grateful for our efforts.
Like other countries across the world, Haiti was not ready for COVID-19. But we are confident that, with help from the international community, organizations such as GHESKIO,14 and with due diligence on the local level, we are strong and resilient enough to beat COVID. We must act together – quickly.
References
1. Sénat JD. Coronavirus: 2 cas confirmés en Haïti, Jovenel Moïse décrète l’état d’ur-gence sanitaire. 2020 Le Nouvelliste.
2. Haitian Ministry of Health.
3. “Entre appel a la solidarite et de sombres previsions, le Dr William Pape fait le point.” Le Nouvelliste.
4. Darzi A and Evans T. Lancet. 2016 Nov-Dec 26. 388;10060:2576-7.
5. Rapport Statistique 2018. 2019 Republic of Haiti.
6. Sentlinger K. “Water Crisis in Haiti.” The Water Project.
7. The World Bank in Haiti. worldbank.org.
8. Cenat JM. Travel Med Infect Dis. 2020 Mar 28. doi: 10.1016/jtmaid.2020.101684.
9. Block D. “Why some Americans resist wearing face masks.” voanews.com. 2020 May 31.
10. Panceski B and Douglas J. “Masks could help stop coronavirus. So why are they still controversial?” wsj.com. Updated 29 Jun 2020.
11. Bojarski S. “Social distancing: A luxury Haiti’s poor cannot afford. The Haitian Times. 2020 Apr.
12. World Health Organization, UNICEF, World Bank Group, and the U.N. Population Division. Maternal mortality ratio, Haiti.
13. Feliciano I and Kargbo C. “As COVID cases surge, Haiti’s Dr. Pape is on the front line again.” PBS NewsHour Weekend. 2020 Jun 13.
14. Liautaud B and Deschamps MM. New Engl J Med. 2020 Jun 16.
Mr. Dorcela is a senior medical student at Faculté des Sciences de la Santé Université Quisqueya in Port-au-Prince, Haiti. He also is a medical intern at Unité de Médecine Familiale Hôpital Saint Nicolas in Saint-Marc. Mr. Dorcela has no disclosures. Mr. St. Jean, who is Mr. Dorcela’s brother, is also a senior medical student at Faculté des Sciences de la Santé Université Quisqueya in Port-au-Prince. He has no disclosures.
Do you want to know what keeps us up at night? As 4th-year medical students born, raised, and living in Haiti, we worry about the impact of COVID-19 on our patients.
The pandemic has shaken the world, and Haiti is no exception.
It has taken several months for the disease to spread, and it began with two confirmed cases, one from France and the other from Belgium, on March 19.1 Much of the spread of COVID-19 in Haiti has been tied to workers returning from the Dominican Republic. As of June 29, Haiti had 5,975 confirmed cases and 105 deaths.2 Of course, those numbers sound minuscule, compared with those in the United States, where the number of deaths from COVID-19 surpassed 100,000 several weeks ago. But the population of Haiti is 30 times smaller than that of the United States, and Haiti is the poorest country in the Western Hemisphere. We have watched in horror as the virus has ravaged marginalized groups in the United States and worry that it will do the same in our own country.
Just as the Haitian Ministry of Health worked with various groups to reach the 1-year free of cholera mark in Haiti, groups such as Doctors Without Borders must mobilize to rein in COVID-19.
Community transmission rapid
After the first two cases were confirmed, a state of health emergency was immediately declared. Haitian President Jovenel Moïse and other government officials called for the implementation of several measures aimed at limiting the spread of COVID-19.
Schools, universities, clinical training programs, vocational centers, factories, airports, and ports, except for the transport of goods, were all ordered to close until further notice. Gatherings of larger than 10 people were banned. A curfew from 8 p.m. EST time to 5 a.m. EST was imposed. Measures such as those encouraged by U.S. Centers for Disease Control and Prevention, such as hand washing, physical distancing, and staying at home were also encouraged by the Haitian Ministry of Health. Mask wearing in public places was deemed mandatory.
The latest testing data show that community spread has been occurring among the Haitian population at a rapid rate. According to Jean William Pape, MD, Haiti’s top infectious diseases expert and founder of GHESKIO, an iconic infectious disease center that cares for people with HIV-AIDS and tuberculosis, a COVID-19 simulation from Cornell University in New York shows that about 35% of the Haitian population will be infected by the end of August 2020. A simulation by the University of Oxford (England) paints an even more dire picture. That simulation shows that 86% of the population could be infected, More than 9,000 additional hospital beds would be needed, and 20,000 people would be likely to die from COVID-19, Dr. Pape said in an interview with Haiti’s Nouvelliste newspaper.3
Medical response
We know that there is a global shortage of health care workers,4 and Haiti is no exception. According to a 2018 report from the Haitian Ministry of Health, the country has 11,775 health care professionals, including about 3,354 medical doctors, to care for more than 11 million people. That translates to about 23.4 physicians per 100,000.5
The pandemic has led some members of this already anemic health care workforce to stay home because of a lack of personal protective equipment. Others, because of reduced hospital or clinic budgets, have been furloughed, making the COVID-19 national health emergency even harder to manage.
But a severe health care shortage is not the only challenge facing Haiti. It spends about $131 U.S. per capita, which makes Haiti one of most vulnerable among low- and middle-income countries in the world. As a poor country,7 its health care infrastructure is among the most inadequate and weakest. Prior to COVID-19, medical advocacy groups already had started movements and strikes demanding that the government improve the health care system. The country’s precarious health care infrastructure includes a lack of hospital beds, and basic medical supplies and equipment, such as oxygen and ventilators.8 The emergence of COVID-19 has only exacerbated the situation.
Clinical training programs have been suspended, many doctors and nurses are on quarantine, and some hospitals and clinics are closing. We have witnessed makeshift voodoo clinics built by Haitian voodoo leaders to receive, hospitalize, and treat COVID-19 patients through rituals and herbal remedies. In some areas of the country, residents have protested against the opening of several COVID-19 treatment and management centers.
Unique cultural challenges
Public health officials around the world are facing challenges persuading citizens to engage in behaviors that could protect them from the virus.
Just as in America, where many people opt to not wear face coverings9,10 despite the public health risks, deep distrust of the Haitian government has undermined the messages of President Moïse and public health officials about the role of masks in limiting the spread of COVID. We see large numbers of unmasked people on the streets in the informal markets every day. Crammed tap-taps and overloaded motorcycles are moving everywhere. This also could be tied to cultural attitudes about COVID that persist among some Haitians. For example, many people with signs and symptoms of COVID-19 are afraid of going to the hospital to get tested and receive care, and resort to going to the voodoo clinics. Along with rituals, voodoo priests have been serving up teas with ingredients, including moringa, eucalyptus, ginger, and honey to those seeking COVID-19 care in the centers. The voodoo priests claim that the teas they serve strengthen the immune system.
In addition, it is difficult for poor people who live in small quarters with several other people to adhere to physical distancing.11
Stigma and violence
Other barriers in the fight against COVID-19 in Haiti are stigma and violence. If widespread testing were available, some Haitians would opt not to do so – despite clear signs and symptoms of the infection. Some people who would get tested if they could are afraid to do so because of fears tied to being attacked by neighbors.
When Haitian University professor Bellamy Nelson and his girlfriend returned to Haiti from the United States in March and began experiencing some pain and fever, he experienced attacks from neighbors, he said in an interview. He said neighbors threatened to burn down his house. When an ambulance arrived at his house to transport him to a hospital, it had to drive through back roads to avoid people armed with rocks, fire, and machetes, he told us. No hospital wanted to admit him. Eventually, Professor Nelson self-quarantined at home, he said.
In another incident, a national ambulance center in Gonaïves, a town toward the northern region of Haiti, reportedly was vandalized, because COVID-19 equipment and supplies used to treat people had been stored there. Hospital Bernard Mevs, along with many other hospitals, was forced by the area’s residents to suspend the plan to open a center for COVID-19 management. Threats to burn down the hospitals caused the leaders of the hospitals to back down and give up a plan to build a 20-bed COVID-19 response center.
Maternal health
Another concern we have about the pandemic is the risk it could be to pregnant women. On average, 94,000 deaths occur annually in Haiti. Out of this number, maternal mortality accounts for 1,000. In 2017, for every 100,000 live births for women of reproductive age from 15 to 49 years old, 480 women died. In contrast, in the Dominican Republic, 95 women died per 100,000 that same year. In the United States, 19 died, and in Norway, no more than 2 died that year.12
Some of the primary factors contributing to the crisis are limited accessibility, inadequate health care facilities, and an inadequate number of trained health care practitioners; low percentages of skilled attendants at deliveries and of prenatal and postnatal visits; and high numbers of high-risk deliveries in nonqualified health facilities.
During the COVID-19 national health emergency, with most hospitals reducing their health care personnel either because of budget-related reasons or because they are on quarantine, this maternal-fetal health crisis has escalated.
One of the biggest hospitals in Jacmel, a town in the southern region of Haiti, has stopped its prenatal care program. In Delmas, the city with the highest incidence and prevalence of COVID-19, Hôpital Universitaire de la Paix has reduced this program to 50% of its capacity and gynecologic care has been completely suspended. Hôpital St. Luc, one of the first hospitals in the western region of Haiti to open its doors to care for COVID-19 patients, has recently shut down the entire maternal-fetal department.
So, access to prenatal and postnatal care, including the ability to deliver babies in health care institutions, is significantly reduced because of COVID-19. This leaves thousands of already vulnerable pregnant women at risk and having to deliver domestically with little to no health care professional assistance. We worry that, in light of the data, more women and babies will die because of the COVID-19 pandemic.
A call to action
Despite these conditions, there are reasons for hope. Various groups, both from the international community and locally have mobilized to respond to the pandemic.
International health care organizations such as Doctors Without Borders and Partners in Health, and local groups such as GHESKIO, the St. Luke Foundation for Haiti, and others have been collaborating with the Haitian Ministry of Health to devise and strategic plans and deploy valuable resources with the common goal of saving lives from COVID-19.
GHESKIO, for example, under Dr. Pape’s leadership, currently has one of the three COVID-19 testing centers in the country. It also has two COVID-19 treatment centers in full operation, in Port-au-Prince, the capital city, managing and treating 520 patients with confirmed COVID-19. GHESKIO, which has been in the front lines of previous major infectious disease outbreaks,13 has trained about 200 clinicians from both public and private health care institutions to care for COVID-19 patients.
Doctors Without Borders has been investing in efforts to support the Ministry of Health by converting and renovating its Burn Center in Drouillard, a small section of the city of Cité Soleil, one of the country’s biggest slums. In May, as part of its COVID-19 response, it launched a 20-bed capacity center that can accommodate up to 45 beds to care for patients who have tested positive for COVID-19.
Partners in Health, the Boston-based nonprofit health care organization cofounded in 1987 by American anthropologist and infectious disease specialist, Paul Farmer, MD, and the largest nonprofit health care provider in Haiti, also joined the Ministry of Health through its national and public health efforts to tackle COVID-19 in Haiti. Partners in Health, through its sister organization, Zanmi Lasante, has pioneered the movement of diagnosing and treating people with HIV-AIDS and TB. Since the late 1990s, its efforts against both infectious diseases have helped 15,000 HIV-positive patients begin and remain on treatment. And every year, 1,500 TB patients have started treatment on the path to a cure.
Early in the pandemic in Haiti, Partners in Health, through its state-of-the-art 300-bed university hospital (Hôpital Universitaire de Mirebalais de Mirebalais), was the first to open a COVID-19 center with a 20-bed capacity and has been caring for COVID-19 patients since then. In June, Partners in Health supported and inaugurated the renovation of the internal medicine department at one of its affiliated community hospitals, Hôpital Saint-Nicolas de Saint Marc. That department will have a 24-bed capacity that can extend up to 36 beds to manage and treat COVID-19 patients.
In total, currently, 26 COVID-19 centers with a capacity of 1,011 beds are available to serve, manage, and treat Haitian patients affected with COVID-19. But are those efforts enough? No.
Haiti, as a weak state even before COVID-19, continues to need funding from the international community so it can strengthen its health care infrastructure to be effective and strong in fighting against COVID-19.
In addition, we would like to see preventive initiatives implemented on the local level. Our family has taken on a role that, we think, could help conquer COVID-19 if others followed suit on a large scale.
As part of our contribution in tackling COVID-19, the two of us have launched a small-scale community experiment. We have educated our family in Delmas about COVID-19 and subsequently launched an awareness campaign in the community. We dispatched small groups that go door to door in the community to educate neighbors about the disease in an effort to help them understand that COVID-19 is real and it is normal for people that feel they may have the disease to seek medical care. This approach helps suppress the transmission of the virus. This pilot project could be reproduced in several other communities. It is easy to operate, rapid, effective, and cost-free. The community has been very receptive to and grateful for our efforts.
Like other countries across the world, Haiti was not ready for COVID-19. But we are confident that, with help from the international community, organizations such as GHESKIO,14 and with due diligence on the local level, we are strong and resilient enough to beat COVID. We must act together – quickly.
References
1. Sénat JD. Coronavirus: 2 cas confirmés en Haïti, Jovenel Moïse décrète l’état d’ur-gence sanitaire. 2020 Le Nouvelliste.
2. Haitian Ministry of Health.
3. “Entre appel a la solidarite et de sombres previsions, le Dr William Pape fait le point.” Le Nouvelliste.
4. Darzi A and Evans T. Lancet. 2016 Nov-Dec 26. 388;10060:2576-7.
5. Rapport Statistique 2018. 2019 Republic of Haiti.
6. Sentlinger K. “Water Crisis in Haiti.” The Water Project.
7. The World Bank in Haiti. worldbank.org.
8. Cenat JM. Travel Med Infect Dis. 2020 Mar 28. doi: 10.1016/jtmaid.2020.101684.
9. Block D. “Why some Americans resist wearing face masks.” voanews.com. 2020 May 31.
10. Panceski B and Douglas J. “Masks could help stop coronavirus. So why are they still controversial?” wsj.com. Updated 29 Jun 2020.
11. Bojarski S. “Social distancing: A luxury Haiti’s poor cannot afford. The Haitian Times. 2020 Apr.
12. World Health Organization, UNICEF, World Bank Group, and the U.N. Population Division. Maternal mortality ratio, Haiti.
13. Feliciano I and Kargbo C. “As COVID cases surge, Haiti’s Dr. Pape is on the front line again.” PBS NewsHour Weekend. 2020 Jun 13.
14. Liautaud B and Deschamps MM. New Engl J Med. 2020 Jun 16.
Mr. Dorcela is a senior medical student at Faculté des Sciences de la Santé Université Quisqueya in Port-au-Prince, Haiti. He also is a medical intern at Unité de Médecine Familiale Hôpital Saint Nicolas in Saint-Marc. Mr. Dorcela has no disclosures. Mr. St. Jean, who is Mr. Dorcela’s brother, is also a senior medical student at Faculté des Sciences de la Santé Université Quisqueya in Port-au-Prince. He has no disclosures.
Subclinical hypothyroidism appears common in women with miscarriage
according to a prospective observational study published in the Journal of Clinical Endocrinology & Metabolism.
Whether asymptomatic patients should be screened for mild subclinical hypothyroidism (SCH) or thyroid peroxidase antibodies (TPOAb) remains an open question, however. “In the absence of evidence of benefit with LT4 [levothyroxine] treatment and possible suggestion of harm ... we pose the question of whether screening should be performed at all in asymptomatic individuals,” wrote Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and colleagues. “Large randomized trials are needed to establish if preconception LT4 treatment of mild SCH with or without TPOAb positivity is beneficial. If treatment is found to be beneficial, this study presents the prevalence of thyroid disorders that can be expected.”
Subclinical hypothyroidism may represent an early stage of thyroid dysfunction. The condition has been associated with subfertility, miscarriage, preterm birth, preeclampsia, and perinatal mortality. Thyroid peroxidase antibodies also have been associated with adverse pregnancy outcomes, and their presence increases the risk of subclinical and overt thyroid disease in pregnancy. “There is international agreement on the treatment of overt thyroid disease,” the researchers wrote. “However, the treatment strategies for SCH or TPOAb preconception and antenatally are debated.”
The Thyroid Antibodies and Levothyroxine (TABLET) trial, to which the present study was linked, “found no improvement in live birth or any secondary pregnancy or neonatal outcomes in euthyroid women with TPOAb taking 50 mcg of LT4, compared with placebo.”
To examine various thyroid-stimulating hormone (TSH) cutoff levels for diagnosing subclinical thyroid disease in preconception asymptomatic women with a history of miscarriage or subfertility, Dr. Dhillon-Smith and colleagues conducted a prospective, observational cohort study at 49 hospitals in the United Kingdom. The study included more than 19,200 patients between November 2011 and January 2016. Participants were aged 16-41 years, had a history of miscarriage or subfertility, and were actively trying to get pregnant.
Using accepted reference ranges, the investigators identified undiagnosed overt hypothyroidism in 0.2%, overt hyperthyroidism in 0.3%, severe SCH (TSH greater than 10 mIU/L) in 0.2%, and SCH (TSH greater than 4.5 mIU/L) in 2.4%. “Lowering the upper limit of TSH to 2.5 mIU/L, as is the recommendation by international societies for ‘high-risk’ women,” such as those with recurrent pregnancy loss or those undergoing assisted reproductive technology, “would class 16%-20% of women as subclinically hypothyroid,” the authors reported.
The prevalence of TPOAb was 9.5%, and the presence of these antibodies “was the factor associated most significantly with any degree of thyroid dysfunction, after adjustment for confounders,” Dr. Dhillon-Smith and colleagues wrote. Multiple regression analyses found that the likelihood of subclinical hypothyroidism (TSH greater than 4.5 mIU/L) was increased for participants with a body mass index of 35 kg/m2 or greater (adjusted odds ratio, 1.71) and Asian ethnicity (aOR, 1.76).
The U.K. rates of thyroid dysfunction appear to be lower than rates in the United States, and it is unclear why higher body mass index and Asian ethnicity were independently associated with higher TSH concentrations, commented Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Women with a history of three or more miscarriages or subfertility were not more likely to be TPOAb positive, compared with women with one or two previous miscarriages, which “underscores the evidence that a recurrent pregnancy loss evaluation yields similar diagnostic findings at two versus three or more losses,” Dr. Trolice said.
According to the American Society for Reproductive Medicine, it is reasonable to test infertile women trying to conceive and to treat SCH with levothyroxine to maintain a TSH within the normal range. Women who have TSH greater than 2.5 mIU/L and/or are TPOAb positive can be considered for treatment with levothyroxine.
The Endocrine Society recommends levothyroxine treatment in women with SCH, especially if they are TPOAb positive.
An American Thyroid Association guideline recommends that subclinically hypothyroid women undergoing in vitro fertilization or intracytoplasmic sperm injection be treated with levothyroxine. A 2019 Cochrane Database review, however, found that low-quality evidence precludes clear conclusions.
“While thyroid autoimmunity has been associated with increased miscarriage, preterm births, and lower live birth rates, the confusion lies in which preconception women to test, when to obtain testing, and how to manage nonovert thyroid disease,” said Dr. Trolice, who is a member of the Ob.Gyn. News editorial advisory board.
The observational study was linked to the TABLET trial, which was funded by the National Institute for Health Research. The researchers had no relevant conflicts of interest.
SOURCE: Dhillon-Smith RK et al. J Clin Endocrinol Metab. 2020 Jun 17. doi: 10.1210/clinem/dgaa302.
according to a prospective observational study published in the Journal of Clinical Endocrinology & Metabolism.
Whether asymptomatic patients should be screened for mild subclinical hypothyroidism (SCH) or thyroid peroxidase antibodies (TPOAb) remains an open question, however. “In the absence of evidence of benefit with LT4 [levothyroxine] treatment and possible suggestion of harm ... we pose the question of whether screening should be performed at all in asymptomatic individuals,” wrote Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and colleagues. “Large randomized trials are needed to establish if preconception LT4 treatment of mild SCH with or without TPOAb positivity is beneficial. If treatment is found to be beneficial, this study presents the prevalence of thyroid disorders that can be expected.”
Subclinical hypothyroidism may represent an early stage of thyroid dysfunction. The condition has been associated with subfertility, miscarriage, preterm birth, preeclampsia, and perinatal mortality. Thyroid peroxidase antibodies also have been associated with adverse pregnancy outcomes, and their presence increases the risk of subclinical and overt thyroid disease in pregnancy. “There is international agreement on the treatment of overt thyroid disease,” the researchers wrote. “However, the treatment strategies for SCH or TPOAb preconception and antenatally are debated.”
The Thyroid Antibodies and Levothyroxine (TABLET) trial, to which the present study was linked, “found no improvement in live birth or any secondary pregnancy or neonatal outcomes in euthyroid women with TPOAb taking 50 mcg of LT4, compared with placebo.”
To examine various thyroid-stimulating hormone (TSH) cutoff levels for diagnosing subclinical thyroid disease in preconception asymptomatic women with a history of miscarriage or subfertility, Dr. Dhillon-Smith and colleagues conducted a prospective, observational cohort study at 49 hospitals in the United Kingdom. The study included more than 19,200 patients between November 2011 and January 2016. Participants were aged 16-41 years, had a history of miscarriage or subfertility, and were actively trying to get pregnant.
Using accepted reference ranges, the investigators identified undiagnosed overt hypothyroidism in 0.2%, overt hyperthyroidism in 0.3%, severe SCH (TSH greater than 10 mIU/L) in 0.2%, and SCH (TSH greater than 4.5 mIU/L) in 2.4%. “Lowering the upper limit of TSH to 2.5 mIU/L, as is the recommendation by international societies for ‘high-risk’ women,” such as those with recurrent pregnancy loss or those undergoing assisted reproductive technology, “would class 16%-20% of women as subclinically hypothyroid,” the authors reported.
The prevalence of TPOAb was 9.5%, and the presence of these antibodies “was the factor associated most significantly with any degree of thyroid dysfunction, after adjustment for confounders,” Dr. Dhillon-Smith and colleagues wrote. Multiple regression analyses found that the likelihood of subclinical hypothyroidism (TSH greater than 4.5 mIU/L) was increased for participants with a body mass index of 35 kg/m2 or greater (adjusted odds ratio, 1.71) and Asian ethnicity (aOR, 1.76).
The U.K. rates of thyroid dysfunction appear to be lower than rates in the United States, and it is unclear why higher body mass index and Asian ethnicity were independently associated with higher TSH concentrations, commented Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Women with a history of three or more miscarriages or subfertility were not more likely to be TPOAb positive, compared with women with one or two previous miscarriages, which “underscores the evidence that a recurrent pregnancy loss evaluation yields similar diagnostic findings at two versus three or more losses,” Dr. Trolice said.
According to the American Society for Reproductive Medicine, it is reasonable to test infertile women trying to conceive and to treat SCH with levothyroxine to maintain a TSH within the normal range. Women who have TSH greater than 2.5 mIU/L and/or are TPOAb positive can be considered for treatment with levothyroxine.
The Endocrine Society recommends levothyroxine treatment in women with SCH, especially if they are TPOAb positive.
An American Thyroid Association guideline recommends that subclinically hypothyroid women undergoing in vitro fertilization or intracytoplasmic sperm injection be treated with levothyroxine. A 2019 Cochrane Database review, however, found that low-quality evidence precludes clear conclusions.
“While thyroid autoimmunity has been associated with increased miscarriage, preterm births, and lower live birth rates, the confusion lies in which preconception women to test, when to obtain testing, and how to manage nonovert thyroid disease,” said Dr. Trolice, who is a member of the Ob.Gyn. News editorial advisory board.
The observational study was linked to the TABLET trial, which was funded by the National Institute for Health Research. The researchers had no relevant conflicts of interest.
SOURCE: Dhillon-Smith RK et al. J Clin Endocrinol Metab. 2020 Jun 17. doi: 10.1210/clinem/dgaa302.
according to a prospective observational study published in the Journal of Clinical Endocrinology & Metabolism.
Whether asymptomatic patients should be screened for mild subclinical hypothyroidism (SCH) or thyroid peroxidase antibodies (TPOAb) remains an open question, however. “In the absence of evidence of benefit with LT4 [levothyroxine] treatment and possible suggestion of harm ... we pose the question of whether screening should be performed at all in asymptomatic individuals,” wrote Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and colleagues. “Large randomized trials are needed to establish if preconception LT4 treatment of mild SCH with or without TPOAb positivity is beneficial. If treatment is found to be beneficial, this study presents the prevalence of thyroid disorders that can be expected.”
Subclinical hypothyroidism may represent an early stage of thyroid dysfunction. The condition has been associated with subfertility, miscarriage, preterm birth, preeclampsia, and perinatal mortality. Thyroid peroxidase antibodies also have been associated with adverse pregnancy outcomes, and their presence increases the risk of subclinical and overt thyroid disease in pregnancy. “There is international agreement on the treatment of overt thyroid disease,” the researchers wrote. “However, the treatment strategies for SCH or TPOAb preconception and antenatally are debated.”
The Thyroid Antibodies and Levothyroxine (TABLET) trial, to which the present study was linked, “found no improvement in live birth or any secondary pregnancy or neonatal outcomes in euthyroid women with TPOAb taking 50 mcg of LT4, compared with placebo.”
To examine various thyroid-stimulating hormone (TSH) cutoff levels for diagnosing subclinical thyroid disease in preconception asymptomatic women with a history of miscarriage or subfertility, Dr. Dhillon-Smith and colleagues conducted a prospective, observational cohort study at 49 hospitals in the United Kingdom. The study included more than 19,200 patients between November 2011 and January 2016. Participants were aged 16-41 years, had a history of miscarriage or subfertility, and were actively trying to get pregnant.
Using accepted reference ranges, the investigators identified undiagnosed overt hypothyroidism in 0.2%, overt hyperthyroidism in 0.3%, severe SCH (TSH greater than 10 mIU/L) in 0.2%, and SCH (TSH greater than 4.5 mIU/L) in 2.4%. “Lowering the upper limit of TSH to 2.5 mIU/L, as is the recommendation by international societies for ‘high-risk’ women,” such as those with recurrent pregnancy loss or those undergoing assisted reproductive technology, “would class 16%-20% of women as subclinically hypothyroid,” the authors reported.
The prevalence of TPOAb was 9.5%, and the presence of these antibodies “was the factor associated most significantly with any degree of thyroid dysfunction, after adjustment for confounders,” Dr. Dhillon-Smith and colleagues wrote. Multiple regression analyses found that the likelihood of subclinical hypothyroidism (TSH greater than 4.5 mIU/L) was increased for participants with a body mass index of 35 kg/m2 or greater (adjusted odds ratio, 1.71) and Asian ethnicity (aOR, 1.76).
The U.K. rates of thyroid dysfunction appear to be lower than rates in the United States, and it is unclear why higher body mass index and Asian ethnicity were independently associated with higher TSH concentrations, commented Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Women with a history of three or more miscarriages or subfertility were not more likely to be TPOAb positive, compared with women with one or two previous miscarriages, which “underscores the evidence that a recurrent pregnancy loss evaluation yields similar diagnostic findings at two versus three or more losses,” Dr. Trolice said.
According to the American Society for Reproductive Medicine, it is reasonable to test infertile women trying to conceive and to treat SCH with levothyroxine to maintain a TSH within the normal range. Women who have TSH greater than 2.5 mIU/L and/or are TPOAb positive can be considered for treatment with levothyroxine.
The Endocrine Society recommends levothyroxine treatment in women with SCH, especially if they are TPOAb positive.
An American Thyroid Association guideline recommends that subclinically hypothyroid women undergoing in vitro fertilization or intracytoplasmic sperm injection be treated with levothyroxine. A 2019 Cochrane Database review, however, found that low-quality evidence precludes clear conclusions.
“While thyroid autoimmunity has been associated with increased miscarriage, preterm births, and lower live birth rates, the confusion lies in which preconception women to test, when to obtain testing, and how to manage nonovert thyroid disease,” said Dr. Trolice, who is a member of the Ob.Gyn. News editorial advisory board.
The observational study was linked to the TABLET trial, which was funded by the National Institute for Health Research. The researchers had no relevant conflicts of interest.
SOURCE: Dhillon-Smith RK et al. J Clin Endocrinol Metab. 2020 Jun 17. doi: 10.1210/clinem/dgaa302.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Pregnant women at greater risk for severe COVID-19, CDC says
Pregnant women may be at increased risk for severe COVID-19 illness, according to a new report published online June 26 in Morbidity and Mortality Weekly Report.
Among reproductive-aged women (15-44 years) infected with SARS-CoV-2, Pregnant women were 5.4 times more likely to be hospitalized, 1.5 times more likely to be admitted to the ICU, and 1.7 times more likely to need mechanical ventilation, after adjustment for age, underlying conditions, and race/ethnicity.
Furthermore, Hispanic and non-Hispanic black pregnant women appear to be disproportionately impacted by the infection.
Sascha Ellington, PhD, of the Centers for Disease Control and Prevention’s COVID-19 Response Pregnancy and Infant Linked Outcomes Team, and colleagues said that preventing COVID-19 infection in pregnant women should be a priority and any potential barriers to compliance with preventive measures need to be removed.
“During pregnancy, women experience immunologic and physiologic changes that could increase their risk for more severe illness from respiratory infections,” they wrote.
As of June 7, a total of 8,207 cases of COVID-19 in pregnant women were reported to the CDC, approximately 9% of COVID-19 cases among reproductive-aged women with known pregnancy status. The authors compared outcomes in these pregnant patients with those in 83,205 nonpregnant women with COVID-19. There was a substantially greater proportion of hospital admissions among pregnant patients (2,587; 31.5%) compared with nonpregnant patients (4,840; 5.8%) with COVID-19.
The authors cautioned that there were no data to differentiate between hospitalizations for COVID-19–related problems as opposed to those arising from pregnancy, including delivery.
For other severity measures, ICU admissions were reported for 1.5% of pregnant women compared with 0.9% for their nonpregnant counterparts, whereas mechanical ventilation was required for 0.5% compared with 0.3%, respectively. Mortality was identical, affecting 0.2% in both groups, with 16 deaths in pregnant patients with COVID-19 and 208 in nonpregnant patients.
Age had an impact as well, with hospitalization more frequent among those aged 35-44 years than among those aged 15-24, regardless of pregnancy status. When stratified by race/ethnicity, ICU admission was most frequently reported among pregnant women who were of non-Hispanic Asian lineage: 3.5% compared with 1.5% in all pregnant women.
Among pregnant women with laboratory-confirmed SARS-CoV-2 infection reporting race/ethnicity, 46% were Hispanic, 22% were black, and 23% were white, whereas among women who gave birth in 2019, 24% were Hispanic, 15% were black, and 51% were white. “Although data on race/ethnicity were missing for 20% of pregnant women in this study, these findings suggest that pregnant women who are Hispanic and black might be disproportionately affected by SARS-CoV-2 infection during pregnancy,” the authors wrote.
They noted that in a recent meta-analysis of influenza, pregnancy was similarly associated with a sevenfold risk for hospitalization, but a lower risk for ICU admission and no increased risk for death. A recent study suggested that COVID-19 severity during pregnancy may be lower than in other respiratory infections such as H1N1.
ACOG responds
In a response to the CDC findings, the American College of Obstetricians and Gynecologists (ACOG) advises calm, noting that the risk of needing the severity-associated interventions in the CDC report remains low and pregnant COVID-19 patients do not appear to have a greater risk for mortality.
Nevertheless, ACOG is reviewing all its COVID-19–related clinical and patient materials and “will make any necessary revisions to recommendations.”
In the meantime, the college advises clinicians to alert patients to the potential increased risk for severe COVID-19 illness during pregnancy. They should also stress to pregnant women and their families the need for precautions to prevent infection, paying particular attention to measures to protect those with greater occupational exposure to the virus.
ACOG also criticized the exclusion of pregnant and lactating women from clinical trials of potential coronavirus vaccines, noting that the new CDC findings underscore the importance of prioritizing pregnant patients to receive coronavirus vaccination when it becomes available.
“ACOG again urges the federal government to use its resources to ensure the safe inclusion of pregnant and lactating patients, including patients of color, in trials for vaccines and therapeutics to ensure that all populations are included in the search for ways to prevent and treat COVID-19,” the statement reads.
The CDC authors said that their report also highlights the need for more complete data to fully understand the risk for severe illness in pregnant women. To address these gaps, the CDC is collaborating with health departments in COVID-19 pregnancy surveillance for the reporting of outcomes in pregnant women with laboratory-confirmed SARS-CoV-2 infection.
A version of article originally appeared on Medscape.com.
Pregnant women may be at increased risk for severe COVID-19 illness, according to a new report published online June 26 in Morbidity and Mortality Weekly Report.
Among reproductive-aged women (15-44 years) infected with SARS-CoV-2, Pregnant women were 5.4 times more likely to be hospitalized, 1.5 times more likely to be admitted to the ICU, and 1.7 times more likely to need mechanical ventilation, after adjustment for age, underlying conditions, and race/ethnicity.
Furthermore, Hispanic and non-Hispanic black pregnant women appear to be disproportionately impacted by the infection.
Sascha Ellington, PhD, of the Centers for Disease Control and Prevention’s COVID-19 Response Pregnancy and Infant Linked Outcomes Team, and colleagues said that preventing COVID-19 infection in pregnant women should be a priority and any potential barriers to compliance with preventive measures need to be removed.
“During pregnancy, women experience immunologic and physiologic changes that could increase their risk for more severe illness from respiratory infections,” they wrote.
As of June 7, a total of 8,207 cases of COVID-19 in pregnant women were reported to the CDC, approximately 9% of COVID-19 cases among reproductive-aged women with known pregnancy status. The authors compared outcomes in these pregnant patients with those in 83,205 nonpregnant women with COVID-19. There was a substantially greater proportion of hospital admissions among pregnant patients (2,587; 31.5%) compared with nonpregnant patients (4,840; 5.8%) with COVID-19.
The authors cautioned that there were no data to differentiate between hospitalizations for COVID-19–related problems as opposed to those arising from pregnancy, including delivery.
For other severity measures, ICU admissions were reported for 1.5% of pregnant women compared with 0.9% for their nonpregnant counterparts, whereas mechanical ventilation was required for 0.5% compared with 0.3%, respectively. Mortality was identical, affecting 0.2% in both groups, with 16 deaths in pregnant patients with COVID-19 and 208 in nonpregnant patients.
Age had an impact as well, with hospitalization more frequent among those aged 35-44 years than among those aged 15-24, regardless of pregnancy status. When stratified by race/ethnicity, ICU admission was most frequently reported among pregnant women who were of non-Hispanic Asian lineage: 3.5% compared with 1.5% in all pregnant women.
Among pregnant women with laboratory-confirmed SARS-CoV-2 infection reporting race/ethnicity, 46% were Hispanic, 22% were black, and 23% were white, whereas among women who gave birth in 2019, 24% were Hispanic, 15% were black, and 51% were white. “Although data on race/ethnicity were missing for 20% of pregnant women in this study, these findings suggest that pregnant women who are Hispanic and black might be disproportionately affected by SARS-CoV-2 infection during pregnancy,” the authors wrote.
They noted that in a recent meta-analysis of influenza, pregnancy was similarly associated with a sevenfold risk for hospitalization, but a lower risk for ICU admission and no increased risk for death. A recent study suggested that COVID-19 severity during pregnancy may be lower than in other respiratory infections such as H1N1.
ACOG responds
In a response to the CDC findings, the American College of Obstetricians and Gynecologists (ACOG) advises calm, noting that the risk of needing the severity-associated interventions in the CDC report remains low and pregnant COVID-19 patients do not appear to have a greater risk for mortality.
Nevertheless, ACOG is reviewing all its COVID-19–related clinical and patient materials and “will make any necessary revisions to recommendations.”
In the meantime, the college advises clinicians to alert patients to the potential increased risk for severe COVID-19 illness during pregnancy. They should also stress to pregnant women and their families the need for precautions to prevent infection, paying particular attention to measures to protect those with greater occupational exposure to the virus.
ACOG also criticized the exclusion of pregnant and lactating women from clinical trials of potential coronavirus vaccines, noting that the new CDC findings underscore the importance of prioritizing pregnant patients to receive coronavirus vaccination when it becomes available.
“ACOG again urges the federal government to use its resources to ensure the safe inclusion of pregnant and lactating patients, including patients of color, in trials for vaccines and therapeutics to ensure that all populations are included in the search for ways to prevent and treat COVID-19,” the statement reads.
The CDC authors said that their report also highlights the need for more complete data to fully understand the risk for severe illness in pregnant women. To address these gaps, the CDC is collaborating with health departments in COVID-19 pregnancy surveillance for the reporting of outcomes in pregnant women with laboratory-confirmed SARS-CoV-2 infection.
A version of article originally appeared on Medscape.com.
Pregnant women may be at increased risk for severe COVID-19 illness, according to a new report published online June 26 in Morbidity and Mortality Weekly Report.
Among reproductive-aged women (15-44 years) infected with SARS-CoV-2, Pregnant women were 5.4 times more likely to be hospitalized, 1.5 times more likely to be admitted to the ICU, and 1.7 times more likely to need mechanical ventilation, after adjustment for age, underlying conditions, and race/ethnicity.
Furthermore, Hispanic and non-Hispanic black pregnant women appear to be disproportionately impacted by the infection.
Sascha Ellington, PhD, of the Centers for Disease Control and Prevention’s COVID-19 Response Pregnancy and Infant Linked Outcomes Team, and colleagues said that preventing COVID-19 infection in pregnant women should be a priority and any potential barriers to compliance with preventive measures need to be removed.
“During pregnancy, women experience immunologic and physiologic changes that could increase their risk for more severe illness from respiratory infections,” they wrote.
As of June 7, a total of 8,207 cases of COVID-19 in pregnant women were reported to the CDC, approximately 9% of COVID-19 cases among reproductive-aged women with known pregnancy status. The authors compared outcomes in these pregnant patients with those in 83,205 nonpregnant women with COVID-19. There was a substantially greater proportion of hospital admissions among pregnant patients (2,587; 31.5%) compared with nonpregnant patients (4,840; 5.8%) with COVID-19.
The authors cautioned that there were no data to differentiate between hospitalizations for COVID-19–related problems as opposed to those arising from pregnancy, including delivery.
For other severity measures, ICU admissions were reported for 1.5% of pregnant women compared with 0.9% for their nonpregnant counterparts, whereas mechanical ventilation was required for 0.5% compared with 0.3%, respectively. Mortality was identical, affecting 0.2% in both groups, with 16 deaths in pregnant patients with COVID-19 and 208 in nonpregnant patients.
Age had an impact as well, with hospitalization more frequent among those aged 35-44 years than among those aged 15-24, regardless of pregnancy status. When stratified by race/ethnicity, ICU admission was most frequently reported among pregnant women who were of non-Hispanic Asian lineage: 3.5% compared with 1.5% in all pregnant women.
Among pregnant women with laboratory-confirmed SARS-CoV-2 infection reporting race/ethnicity, 46% were Hispanic, 22% were black, and 23% were white, whereas among women who gave birth in 2019, 24% were Hispanic, 15% were black, and 51% were white. “Although data on race/ethnicity were missing for 20% of pregnant women in this study, these findings suggest that pregnant women who are Hispanic and black might be disproportionately affected by SARS-CoV-2 infection during pregnancy,” the authors wrote.
They noted that in a recent meta-analysis of influenza, pregnancy was similarly associated with a sevenfold risk for hospitalization, but a lower risk for ICU admission and no increased risk for death. A recent study suggested that COVID-19 severity during pregnancy may be lower than in other respiratory infections such as H1N1.
ACOG responds
In a response to the CDC findings, the American College of Obstetricians and Gynecologists (ACOG) advises calm, noting that the risk of needing the severity-associated interventions in the CDC report remains low and pregnant COVID-19 patients do not appear to have a greater risk for mortality.
Nevertheless, ACOG is reviewing all its COVID-19–related clinical and patient materials and “will make any necessary revisions to recommendations.”
In the meantime, the college advises clinicians to alert patients to the potential increased risk for severe COVID-19 illness during pregnancy. They should also stress to pregnant women and their families the need for precautions to prevent infection, paying particular attention to measures to protect those with greater occupational exposure to the virus.
ACOG also criticized the exclusion of pregnant and lactating women from clinical trials of potential coronavirus vaccines, noting that the new CDC findings underscore the importance of prioritizing pregnant patients to receive coronavirus vaccination when it becomes available.
“ACOG again urges the federal government to use its resources to ensure the safe inclusion of pregnant and lactating patients, including patients of color, in trials for vaccines and therapeutics to ensure that all populations are included in the search for ways to prevent and treat COVID-19,” the statement reads.
The CDC authors said that their report also highlights the need for more complete data to fully understand the risk for severe illness in pregnant women. To address these gaps, the CDC is collaborating with health departments in COVID-19 pregnancy surveillance for the reporting of outcomes in pregnant women with laboratory-confirmed SARS-CoV-2 infection.
A version of article originally appeared on Medscape.com.
Early hypertensive disorders in pregnancy linked to obesity
Rising classes of obesity are linked with progressively increased risk of early-onset hypertensive disorders in pregnant women, as has been established for late-onset hypertensive disorders, according to a U.S.-based retrospective cohort study.
Between 4% and 8% of pregnancies are impacted by hypertensive disorders, and preeclampsia is associated with a doubling of adverse neonatal events and causes 16% of maternal deaths in developed countries, previous studies have found. This study showed a clear risk of early-onset hypertensive disorders (less than 34 weeks’ gestation), which may be more deadly than late-onset disease: Compared with later-developing disorders, early hypertensive disorders are linked to a 400% increased risk of perinatal death and a 100%-300% increased risk of severe cardiovascular, renal, or hepatic maternal morbidity, according to previous studies.
The new research, led by Matthew Bicocca, MD, of the University of Texas Health Science Center, Houston, was published in Obstetrics & Gynecology. The researchers analyzed data from U.S. Vital Statistics, including over 14 million singleton births. The sample excluded women with chronic hypertension and a body mass index (BMI) below 18.5 kg/m2.
Previous studies demonstrated that obesity is a risk factor for late-onset hypertensive disorders, but studies of early-onset hypertensive disorders have yielded conflicting results. That could be because early-onset disorders are rare, representing just 5%-10% of hypertensive disorders during pregnancy, making it difficult to obtain a sufficient sample size to show a relationship.
“We know that obese pregnant women are at increased risk for adverse pregnancy outcomes, and this is of particular importance with the increasing prevalence of obesity in the United States. As this is a nationwide cohort with a large sample size, it allowed for evaluation of the rare outcome of early-onset hypertensive disorders of pregnancy,” said Iris Krishna, MD, MPH, an assistant professor of maternal-fetal medicine at Emory University, Atlanta, who was asked to comment on the study.
The researchers classified the women in the study as nonobese (BMI, 18.5-29.9 kg/m2; 46%), class I obese (BMI, 30.0-34.9; 29%), class II obese (BMI, 35.0-39.9; 15%), or class III obese (BMI, 40 or higher; 10%). About 6% of the participants developed hypertensive disorders during pregnancy (0.3% early onset), and the associated risk was greater with increasing class of obesity. Compared with nonobese women, class III obesity was associated with the highest adjusted risk ratio (2.18; 95% confidence interval, 2.12-2.24) for early-onset hypertensive disorders, followed by class II obesity (aRR, 1.57; 95% CI, 1.53-1.62) and class I (aRR, 1.13; 95% CI, 1.10-1.16). A similar pattern was observed with late-onset hypertensive disorders, with the highest risk associated with class III obese (aRR, 3.93; 95% CI, 3.91-3.96), followed by class II (aRR, 2.60; 95% CI, 2.58-2.62) and class I (aRR, 1.71; 95% CI, 1.70-1.73).
The mechanism underlying any potential link between obesity and risk of hypertensive orders of pregnancy isn’t completely understood, especially because the early-onset and late-onset hypertensive disorders have differing pathophysiology. “Early onset is the result from abnormal placentation [leading to] chronic placental insufficiency, and late onset likely [results from] placental insufficiency paired with oxidative stress from conditions such as obesity and insulin resistance,” Dr. Krishna said.
The new research reinforces the need for obese women to receive early prenatal care and counseling on nutrition and exercise “to mitigate weight gain during pregnancy in hopes of reducing their risk for adverse pregnancy outcomes, such as hypertensive disorders of pregnancy,” she concluded.
No source of funding was disclosed. The authors reported having no potential conflicts of interest.
SOURCE: Bicocca M et al. Obstet Gynecol. 2020 Jun 11. doi: 10.1097/AOG.0000000000003901.
Rising classes of obesity are linked with progressively increased risk of early-onset hypertensive disorders in pregnant women, as has been established for late-onset hypertensive disorders, according to a U.S.-based retrospective cohort study.
Between 4% and 8% of pregnancies are impacted by hypertensive disorders, and preeclampsia is associated with a doubling of adverse neonatal events and causes 16% of maternal deaths in developed countries, previous studies have found. This study showed a clear risk of early-onset hypertensive disorders (less than 34 weeks’ gestation), which may be more deadly than late-onset disease: Compared with later-developing disorders, early hypertensive disorders are linked to a 400% increased risk of perinatal death and a 100%-300% increased risk of severe cardiovascular, renal, or hepatic maternal morbidity, according to previous studies.
The new research, led by Matthew Bicocca, MD, of the University of Texas Health Science Center, Houston, was published in Obstetrics & Gynecology. The researchers analyzed data from U.S. Vital Statistics, including over 14 million singleton births. The sample excluded women with chronic hypertension and a body mass index (BMI) below 18.5 kg/m2.
Previous studies demonstrated that obesity is a risk factor for late-onset hypertensive disorders, but studies of early-onset hypertensive disorders have yielded conflicting results. That could be because early-onset disorders are rare, representing just 5%-10% of hypertensive disorders during pregnancy, making it difficult to obtain a sufficient sample size to show a relationship.
“We know that obese pregnant women are at increased risk for adverse pregnancy outcomes, and this is of particular importance with the increasing prevalence of obesity in the United States. As this is a nationwide cohort with a large sample size, it allowed for evaluation of the rare outcome of early-onset hypertensive disorders of pregnancy,” said Iris Krishna, MD, MPH, an assistant professor of maternal-fetal medicine at Emory University, Atlanta, who was asked to comment on the study.
The researchers classified the women in the study as nonobese (BMI, 18.5-29.9 kg/m2; 46%), class I obese (BMI, 30.0-34.9; 29%), class II obese (BMI, 35.0-39.9; 15%), or class III obese (BMI, 40 or higher; 10%). About 6% of the participants developed hypertensive disorders during pregnancy (0.3% early onset), and the associated risk was greater with increasing class of obesity. Compared with nonobese women, class III obesity was associated with the highest adjusted risk ratio (2.18; 95% confidence interval, 2.12-2.24) for early-onset hypertensive disorders, followed by class II obesity (aRR, 1.57; 95% CI, 1.53-1.62) and class I (aRR, 1.13; 95% CI, 1.10-1.16). A similar pattern was observed with late-onset hypertensive disorders, with the highest risk associated with class III obese (aRR, 3.93; 95% CI, 3.91-3.96), followed by class II (aRR, 2.60; 95% CI, 2.58-2.62) and class I (aRR, 1.71; 95% CI, 1.70-1.73).
The mechanism underlying any potential link between obesity and risk of hypertensive orders of pregnancy isn’t completely understood, especially because the early-onset and late-onset hypertensive disorders have differing pathophysiology. “Early onset is the result from abnormal placentation [leading to] chronic placental insufficiency, and late onset likely [results from] placental insufficiency paired with oxidative stress from conditions such as obesity and insulin resistance,” Dr. Krishna said.
The new research reinforces the need for obese women to receive early prenatal care and counseling on nutrition and exercise “to mitigate weight gain during pregnancy in hopes of reducing their risk for adverse pregnancy outcomes, such as hypertensive disorders of pregnancy,” she concluded.
No source of funding was disclosed. The authors reported having no potential conflicts of interest.
SOURCE: Bicocca M et al. Obstet Gynecol. 2020 Jun 11. doi: 10.1097/AOG.0000000000003901.
Rising classes of obesity are linked with progressively increased risk of early-onset hypertensive disorders in pregnant women, as has been established for late-onset hypertensive disorders, according to a U.S.-based retrospective cohort study.
Between 4% and 8% of pregnancies are impacted by hypertensive disorders, and preeclampsia is associated with a doubling of adverse neonatal events and causes 16% of maternal deaths in developed countries, previous studies have found. This study showed a clear risk of early-onset hypertensive disorders (less than 34 weeks’ gestation), which may be more deadly than late-onset disease: Compared with later-developing disorders, early hypertensive disorders are linked to a 400% increased risk of perinatal death and a 100%-300% increased risk of severe cardiovascular, renal, or hepatic maternal morbidity, according to previous studies.
The new research, led by Matthew Bicocca, MD, of the University of Texas Health Science Center, Houston, was published in Obstetrics & Gynecology. The researchers analyzed data from U.S. Vital Statistics, including over 14 million singleton births. The sample excluded women with chronic hypertension and a body mass index (BMI) below 18.5 kg/m2.
Previous studies demonstrated that obesity is a risk factor for late-onset hypertensive disorders, but studies of early-onset hypertensive disorders have yielded conflicting results. That could be because early-onset disorders are rare, representing just 5%-10% of hypertensive disorders during pregnancy, making it difficult to obtain a sufficient sample size to show a relationship.
“We know that obese pregnant women are at increased risk for adverse pregnancy outcomes, and this is of particular importance with the increasing prevalence of obesity in the United States. As this is a nationwide cohort with a large sample size, it allowed for evaluation of the rare outcome of early-onset hypertensive disorders of pregnancy,” said Iris Krishna, MD, MPH, an assistant professor of maternal-fetal medicine at Emory University, Atlanta, who was asked to comment on the study.
The researchers classified the women in the study as nonobese (BMI, 18.5-29.9 kg/m2; 46%), class I obese (BMI, 30.0-34.9; 29%), class II obese (BMI, 35.0-39.9; 15%), or class III obese (BMI, 40 or higher; 10%). About 6% of the participants developed hypertensive disorders during pregnancy (0.3% early onset), and the associated risk was greater with increasing class of obesity. Compared with nonobese women, class III obesity was associated with the highest adjusted risk ratio (2.18; 95% confidence interval, 2.12-2.24) for early-onset hypertensive disorders, followed by class II obesity (aRR, 1.57; 95% CI, 1.53-1.62) and class I (aRR, 1.13; 95% CI, 1.10-1.16). A similar pattern was observed with late-onset hypertensive disorders, with the highest risk associated with class III obese (aRR, 3.93; 95% CI, 3.91-3.96), followed by class II (aRR, 2.60; 95% CI, 2.58-2.62) and class I (aRR, 1.71; 95% CI, 1.70-1.73).
The mechanism underlying any potential link between obesity and risk of hypertensive orders of pregnancy isn’t completely understood, especially because the early-onset and late-onset hypertensive disorders have differing pathophysiology. “Early onset is the result from abnormal placentation [leading to] chronic placental insufficiency, and late onset likely [results from] placental insufficiency paired with oxidative stress from conditions such as obesity and insulin resistance,” Dr. Krishna said.
The new research reinforces the need for obese women to receive early prenatal care and counseling on nutrition and exercise “to mitigate weight gain during pregnancy in hopes of reducing their risk for adverse pregnancy outcomes, such as hypertensive disorders of pregnancy,” she concluded.
No source of funding was disclosed. The authors reported having no potential conflicts of interest.
SOURCE: Bicocca M et al. Obstet Gynecol. 2020 Jun 11. doi: 10.1097/AOG.0000000000003901.
FROM OBSTETRICS & GYNECOLOGY