User login
New uterine compression technique controls postpartum hemorrhage
A newly described uterine compression technique that uses simple supplies and does not require hysterotomy was successful in controlling postpartum hemorrhage in 16 of 18 (89%) women in two teaching hospitals in Nigeria, averting the need for hysterectomy in these women.
Each of the women had severe postpartum hemorrhage attributable to uterine atony and had undergone local protocols for medical management “to no avail,” Chidi Ochu Uzoma Esike, MD, who developed the technique, wrote in a report published in Obstetrics and Gynecology.
The technique involves placing six polyglactin (Vicryl) #2 or chromic #2 sutures in the lower uterine segment – three anteriorly and three posteriorly – and could be particularly useful in developing countries, where many women die from postpartum hemorrhage “because most of the medical officers who attend the majority of births in health facilities can perform cesarean delivery but cannot perform hysterectomy and find existing compression suture techniques too complex to perform,” Dr. Esike wrote in the case series report.
In addition, “specialized sutures and needles required for some of the known compression techniques are not readily available,” said Dr. Esike of the department of obstetrics and gynecology at Alex Ekwueme Federal University Hospital and Ebyonyi State University in Abakaliki, Nigeria.
Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas Medical Center in Kansas City, said that “having a quick and effective surgical technique [for uncontrollable postpartum hemorrhage] is essential.”
“I love that Esike’s technique uses polyglactin (Vicryl) or chromic sutures. These are familiar to most surgeons, cheap, and typically available even in most resource-deficient settings,” said Dr. Martin, who was asked to comment on the report, adding that several of the known surgical techniques for uterine atony require a skilled operator and are indeed not universally feasible.
“If successful,” Dr. Martin said in an interview, “compression sutures can be lifesaving and fertility preserving.”
The technique involves tying the two middle sutures (one placed anteriorly and one posteriorly) at the fundus as an assistant slowly and continuously compresses the uterus. The more laterally placed sutures are tied similarly, with each pair tied at about 4 cm from the lateral edge of the uterus. “As the uterus is compressed, the slack should be taken up by the sutures before tying,” said Dr. Esike, whose report features both diagrammatic and photographic representations of suture insertion and tying.
For patients who delivered vaginally – nine in this case series – the technique involves performing a laparotomy and exteriorizing the uterus. The technique’s “suture placement,” Dr. Esike wrote, “took 11-25 minutes from the onset of laparotomy to completion.” There were no short or long-term complications in any of the 18 patients.
B-Lynch compression sutures are more complex to perform and require a larger curved needle, Dr. Esike wrote, and the Hayman technique similarly requires a longer needle that may not be available in resource-constrained countries. The hysterotomy required in the B-Lynch technique, Dr. Esike added, “leads to the uterus not contracting maximally until it is repaired,” which increases blood loss from the procedure.
Dr. Martin said the small size of the case series is not discouraging. “The B-Lynch suture was widely adopted after it was described in five cases in 1997,” she said. There are no randomized controlled trials to suggest that one method of uterine compression sutures is better than another. “Ultimately,” she said, “the technique chosen will depend on the surgeon’s training and available supplies.”
Dr. Esike had no relevant financial disclosures. Dr. Martin had no relevant financial disclosures.
SOURCE: Esike COU. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000003947.
A newly described uterine compression technique that uses simple supplies and does not require hysterotomy was successful in controlling postpartum hemorrhage in 16 of 18 (89%) women in two teaching hospitals in Nigeria, averting the need for hysterectomy in these women.
Each of the women had severe postpartum hemorrhage attributable to uterine atony and had undergone local protocols for medical management “to no avail,” Chidi Ochu Uzoma Esike, MD, who developed the technique, wrote in a report published in Obstetrics and Gynecology.
The technique involves placing six polyglactin (Vicryl) #2 or chromic #2 sutures in the lower uterine segment – three anteriorly and three posteriorly – and could be particularly useful in developing countries, where many women die from postpartum hemorrhage “because most of the medical officers who attend the majority of births in health facilities can perform cesarean delivery but cannot perform hysterectomy and find existing compression suture techniques too complex to perform,” Dr. Esike wrote in the case series report.
In addition, “specialized sutures and needles required for some of the known compression techniques are not readily available,” said Dr. Esike of the department of obstetrics and gynecology at Alex Ekwueme Federal University Hospital and Ebyonyi State University in Abakaliki, Nigeria.
Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas Medical Center in Kansas City, said that “having a quick and effective surgical technique [for uncontrollable postpartum hemorrhage] is essential.”
“I love that Esike’s technique uses polyglactin (Vicryl) or chromic sutures. These are familiar to most surgeons, cheap, and typically available even in most resource-deficient settings,” said Dr. Martin, who was asked to comment on the report, adding that several of the known surgical techniques for uterine atony require a skilled operator and are indeed not universally feasible.
“If successful,” Dr. Martin said in an interview, “compression sutures can be lifesaving and fertility preserving.”
The technique involves tying the two middle sutures (one placed anteriorly and one posteriorly) at the fundus as an assistant slowly and continuously compresses the uterus. The more laterally placed sutures are tied similarly, with each pair tied at about 4 cm from the lateral edge of the uterus. “As the uterus is compressed, the slack should be taken up by the sutures before tying,” said Dr. Esike, whose report features both diagrammatic and photographic representations of suture insertion and tying.
For patients who delivered vaginally – nine in this case series – the technique involves performing a laparotomy and exteriorizing the uterus. The technique’s “suture placement,” Dr. Esike wrote, “took 11-25 minutes from the onset of laparotomy to completion.” There were no short or long-term complications in any of the 18 patients.
B-Lynch compression sutures are more complex to perform and require a larger curved needle, Dr. Esike wrote, and the Hayman technique similarly requires a longer needle that may not be available in resource-constrained countries. The hysterotomy required in the B-Lynch technique, Dr. Esike added, “leads to the uterus not contracting maximally until it is repaired,” which increases blood loss from the procedure.
Dr. Martin said the small size of the case series is not discouraging. “The B-Lynch suture was widely adopted after it was described in five cases in 1997,” she said. There are no randomized controlled trials to suggest that one method of uterine compression sutures is better than another. “Ultimately,” she said, “the technique chosen will depend on the surgeon’s training and available supplies.”
Dr. Esike had no relevant financial disclosures. Dr. Martin had no relevant financial disclosures.
SOURCE: Esike COU. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000003947.
A newly described uterine compression technique that uses simple supplies and does not require hysterotomy was successful in controlling postpartum hemorrhage in 16 of 18 (89%) women in two teaching hospitals in Nigeria, averting the need for hysterectomy in these women.
Each of the women had severe postpartum hemorrhage attributable to uterine atony and had undergone local protocols for medical management “to no avail,” Chidi Ochu Uzoma Esike, MD, who developed the technique, wrote in a report published in Obstetrics and Gynecology.
The technique involves placing six polyglactin (Vicryl) #2 or chromic #2 sutures in the lower uterine segment – three anteriorly and three posteriorly – and could be particularly useful in developing countries, where many women die from postpartum hemorrhage “because most of the medical officers who attend the majority of births in health facilities can perform cesarean delivery but cannot perform hysterectomy and find existing compression suture techniques too complex to perform,” Dr. Esike wrote in the case series report.
In addition, “specialized sutures and needles required for some of the known compression techniques are not readily available,” said Dr. Esike of the department of obstetrics and gynecology at Alex Ekwueme Federal University Hospital and Ebyonyi State University in Abakaliki, Nigeria.
Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas Medical Center in Kansas City, said that “having a quick and effective surgical technique [for uncontrollable postpartum hemorrhage] is essential.”
“I love that Esike’s technique uses polyglactin (Vicryl) or chromic sutures. These are familiar to most surgeons, cheap, and typically available even in most resource-deficient settings,” said Dr. Martin, who was asked to comment on the report, adding that several of the known surgical techniques for uterine atony require a skilled operator and are indeed not universally feasible.
“If successful,” Dr. Martin said in an interview, “compression sutures can be lifesaving and fertility preserving.”
The technique involves tying the two middle sutures (one placed anteriorly and one posteriorly) at the fundus as an assistant slowly and continuously compresses the uterus. The more laterally placed sutures are tied similarly, with each pair tied at about 4 cm from the lateral edge of the uterus. “As the uterus is compressed, the slack should be taken up by the sutures before tying,” said Dr. Esike, whose report features both diagrammatic and photographic representations of suture insertion and tying.
For patients who delivered vaginally – nine in this case series – the technique involves performing a laparotomy and exteriorizing the uterus. The technique’s “suture placement,” Dr. Esike wrote, “took 11-25 minutes from the onset of laparotomy to completion.” There were no short or long-term complications in any of the 18 patients.
B-Lynch compression sutures are more complex to perform and require a larger curved needle, Dr. Esike wrote, and the Hayman technique similarly requires a longer needle that may not be available in resource-constrained countries. The hysterotomy required in the B-Lynch technique, Dr. Esike added, “leads to the uterus not contracting maximally until it is repaired,” which increases blood loss from the procedure.
Dr. Martin said the small size of the case series is not discouraging. “The B-Lynch suture was widely adopted after it was described in five cases in 1997,” she said. There are no randomized controlled trials to suggest that one method of uterine compression sutures is better than another. “Ultimately,” she said, “the technique chosen will depend on the surgeon’s training and available supplies.”
Dr. Esike had no relevant financial disclosures. Dr. Martin had no relevant financial disclosures.
SOURCE: Esike COU. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000003947.
FROM OBSTETRICS & GYNECOLOGY
Treatment for a tobacco-dependent adult
Applying American Thoracic Society’s new clinical practice guideline
Complications from tobacco use are the most common preventable cause of death, disability, and disease in the United States. Tobacco use causes 480,000 premature deaths every year. In pregnancy, tobacco use causes complications such as premature birth, intrauterine growth restriction, and placental abruption. In the perinatal period, it is associated with sudden infant death syndrome. While cigarette smoking is decreasing in adolescents, e-cigarette use in on the rise. Approximately 1,600 children aged 12-17 smoke their first cigarette every day and it is estimated that 5.6 million children and adolescents will die of a tobacco use–related death.1 For these reasons it is important to address tobacco use and cessation with patients whenever it is possible.
Case
A forty-five-year-old male who rarely comes to the office is here today for a physical exam at the urging of his partner. He has been smoking a pack a day since age 17. You have tried at past visits to discuss quitting, but he had been in the precontemplative stage and had been unwilling to consider any change. This visit, however, he is ready to try to quit. What can you offer him?
Core recommendations from ATS guidelines
This patient can be offered varenicline plus nicotine replacement therapy rather than nicotine replacement therapy, bupropion, e-cigarettes, or varenicline alone. His course of therapy should extend beyond 12 weeks instead of the standard 6- to 12-week therapy. Alternatively, he could be offered varenicline alone, rather than nicotine replacement.2
A change from previous guidelines
What makes this recommendation so interesting and new is the emphasis it places on varenicline. The United States Preventive Services Task Force released a recommendation statement in 2015 that stressed a combination of pharmacological and behavioral interventions. It discussed nicotine replacement therapy, bupropion, and varenicline, but did not recommend any one over any of the others.3 The new recommendation from the American Thoracic Society favors varenicline over other pharmacologic interventions. It is based on an independent systematic review of the literature that showed higher rates of tobacco use abstinence at the 6-month follow-up with varenicline alone versus nicotine replacement therapy alone, bupropion alone, or e-cigarette use only.
A review of 14 randomized controlled trials showed that varenicline improves abstinence rates during treatment by approximately 40% compared with nicotine replacement, and by 20% at the end of 6 months of treatment. The review found that varenicline plus nicotine replacement therapy is more effective than varenicline alone. In this comparison, based on three trials, there was a 36% higher abstinence rate at 6 months using varenicline plus nicotine replacement. When varenicline use was compared with use of a nicotine patch, bupropion, or e-cigarettes, there was a reduction in serious adverse events – changes in mood, suicidal ideation, and neurological side effects such as seizures.2 Clinicians may remember a black box warning on the varenicline label citing neuropsychiatric effects and it is important to note that the Food and Drug Administration removed this boxed warning in 2016.4
Opinion
This recommendation represents an important, evidence-based change from previous guidelines. It presents the opportunity for better outcomes, but will likely take a while to filter into practice, as clinicians need to become more comfortable with the use of varenicline and insurance supports the cost of varenicline.
The average cost of varenicline for 12 weeks is between $1,220 and $1,584. For comparison, nicotine replacement therapy costs $170 to $240 for the same number of weeks. To put those costs in perspective, the 12-week cost of cigarettes for a two-pack-a-day smoker is approximately $1,000.
For some patients, the motivation to quit smoking comes from the realization of how much they are spending on cigarettes each month. That said, if a patient does not have insurance or their insurance does not cover the cost of varenicline, nicotine replacement therapy might be more appealing. It should be noted that better abstinence rates have been seen in patients taking varenicline plus nicotine replacement therapy versus varenicline alone.
Suggested treatment
Based on a systematic review of randomized controlled trials, the American Thoracic Society’s guideline on pharmacological treatment in tobacco-dependent adults concludes that varenicline plus nicotine patch is the preferred pharmacological treatment for tobacco cessation when compared with varenicline alone, bupropion alone, nicotine replacement therapy alone, and e-cigarettes alone. If the patient does not want to start two medicines at once, then varenicline alone would be the preferred choice.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Sprogell is a third-year resident in the family medicine residency program at Abington Jefferson Health. They have no conflicts related to the content of this piece. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. U.S. Preventive Services Task Force. Primary care interventions for prevention and cessation of tobacco use in children and adolescents: U.S. Preventive Services Task Force Recommendation Statement. JAMA.2020;323(16):1590-8. doi: 10.1001/jama.2020.4679.
2. Leone FT et al. Initiating pharmacologic treatment in tobacco-dependent adults: An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(2):e5–e31.
3. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. U.S. Preventive Services Task Force 2015 Sep 21.
4. FDA Drug Safety Communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. 2016 Dec. 16.
Applying American Thoracic Society’s new clinical practice guideline
Applying American Thoracic Society’s new clinical practice guideline
Complications from tobacco use are the most common preventable cause of death, disability, and disease in the United States. Tobacco use causes 480,000 premature deaths every year. In pregnancy, tobacco use causes complications such as premature birth, intrauterine growth restriction, and placental abruption. In the perinatal period, it is associated with sudden infant death syndrome. While cigarette smoking is decreasing in adolescents, e-cigarette use in on the rise. Approximately 1,600 children aged 12-17 smoke their first cigarette every day and it is estimated that 5.6 million children and adolescents will die of a tobacco use–related death.1 For these reasons it is important to address tobacco use and cessation with patients whenever it is possible.
Case
A forty-five-year-old male who rarely comes to the office is here today for a physical exam at the urging of his partner. He has been smoking a pack a day since age 17. You have tried at past visits to discuss quitting, but he had been in the precontemplative stage and had been unwilling to consider any change. This visit, however, he is ready to try to quit. What can you offer him?
Core recommendations from ATS guidelines
This patient can be offered varenicline plus nicotine replacement therapy rather than nicotine replacement therapy, bupropion, e-cigarettes, or varenicline alone. His course of therapy should extend beyond 12 weeks instead of the standard 6- to 12-week therapy. Alternatively, he could be offered varenicline alone, rather than nicotine replacement.2
A change from previous guidelines
What makes this recommendation so interesting and new is the emphasis it places on varenicline. The United States Preventive Services Task Force released a recommendation statement in 2015 that stressed a combination of pharmacological and behavioral interventions. It discussed nicotine replacement therapy, bupropion, and varenicline, but did not recommend any one over any of the others.3 The new recommendation from the American Thoracic Society favors varenicline over other pharmacologic interventions. It is based on an independent systematic review of the literature that showed higher rates of tobacco use abstinence at the 6-month follow-up with varenicline alone versus nicotine replacement therapy alone, bupropion alone, or e-cigarette use only.
A review of 14 randomized controlled trials showed that varenicline improves abstinence rates during treatment by approximately 40% compared with nicotine replacement, and by 20% at the end of 6 months of treatment. The review found that varenicline plus nicotine replacement therapy is more effective than varenicline alone. In this comparison, based on three trials, there was a 36% higher abstinence rate at 6 months using varenicline plus nicotine replacement. When varenicline use was compared with use of a nicotine patch, bupropion, or e-cigarettes, there was a reduction in serious adverse events – changes in mood, suicidal ideation, and neurological side effects such as seizures.2 Clinicians may remember a black box warning on the varenicline label citing neuropsychiatric effects and it is important to note that the Food and Drug Administration removed this boxed warning in 2016.4
Opinion
This recommendation represents an important, evidence-based change from previous guidelines. It presents the opportunity for better outcomes, but will likely take a while to filter into practice, as clinicians need to become more comfortable with the use of varenicline and insurance supports the cost of varenicline.
The average cost of varenicline for 12 weeks is between $1,220 and $1,584. For comparison, nicotine replacement therapy costs $170 to $240 for the same number of weeks. To put those costs in perspective, the 12-week cost of cigarettes for a two-pack-a-day smoker is approximately $1,000.
For some patients, the motivation to quit smoking comes from the realization of how much they are spending on cigarettes each month. That said, if a patient does not have insurance or their insurance does not cover the cost of varenicline, nicotine replacement therapy might be more appealing. It should be noted that better abstinence rates have been seen in patients taking varenicline plus nicotine replacement therapy versus varenicline alone.
Suggested treatment
Based on a systematic review of randomized controlled trials, the American Thoracic Society’s guideline on pharmacological treatment in tobacco-dependent adults concludes that varenicline plus nicotine patch is the preferred pharmacological treatment for tobacco cessation when compared with varenicline alone, bupropion alone, nicotine replacement therapy alone, and e-cigarettes alone. If the patient does not want to start two medicines at once, then varenicline alone would be the preferred choice.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Sprogell is a third-year resident in the family medicine residency program at Abington Jefferson Health. They have no conflicts related to the content of this piece. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. U.S. Preventive Services Task Force. Primary care interventions for prevention and cessation of tobacco use in children and adolescents: U.S. Preventive Services Task Force Recommendation Statement. JAMA.2020;323(16):1590-8. doi: 10.1001/jama.2020.4679.
2. Leone FT et al. Initiating pharmacologic treatment in tobacco-dependent adults: An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(2):e5–e31.
3. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. U.S. Preventive Services Task Force 2015 Sep 21.
4. FDA Drug Safety Communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. 2016 Dec. 16.
Complications from tobacco use are the most common preventable cause of death, disability, and disease in the United States. Tobacco use causes 480,000 premature deaths every year. In pregnancy, tobacco use causes complications such as premature birth, intrauterine growth restriction, and placental abruption. In the perinatal period, it is associated with sudden infant death syndrome. While cigarette smoking is decreasing in adolescents, e-cigarette use in on the rise. Approximately 1,600 children aged 12-17 smoke their first cigarette every day and it is estimated that 5.6 million children and adolescents will die of a tobacco use–related death.1 For these reasons it is important to address tobacco use and cessation with patients whenever it is possible.
Case
A forty-five-year-old male who rarely comes to the office is here today for a physical exam at the urging of his partner. He has been smoking a pack a day since age 17. You have tried at past visits to discuss quitting, but he had been in the precontemplative stage and had been unwilling to consider any change. This visit, however, he is ready to try to quit. What can you offer him?
Core recommendations from ATS guidelines
This patient can be offered varenicline plus nicotine replacement therapy rather than nicotine replacement therapy, bupropion, e-cigarettes, or varenicline alone. His course of therapy should extend beyond 12 weeks instead of the standard 6- to 12-week therapy. Alternatively, he could be offered varenicline alone, rather than nicotine replacement.2
A change from previous guidelines
What makes this recommendation so interesting and new is the emphasis it places on varenicline. The United States Preventive Services Task Force released a recommendation statement in 2015 that stressed a combination of pharmacological and behavioral interventions. It discussed nicotine replacement therapy, bupropion, and varenicline, but did not recommend any one over any of the others.3 The new recommendation from the American Thoracic Society favors varenicline over other pharmacologic interventions. It is based on an independent systematic review of the literature that showed higher rates of tobacco use abstinence at the 6-month follow-up with varenicline alone versus nicotine replacement therapy alone, bupropion alone, or e-cigarette use only.
A review of 14 randomized controlled trials showed that varenicline improves abstinence rates during treatment by approximately 40% compared with nicotine replacement, and by 20% at the end of 6 months of treatment. The review found that varenicline plus nicotine replacement therapy is more effective than varenicline alone. In this comparison, based on three trials, there was a 36% higher abstinence rate at 6 months using varenicline plus nicotine replacement. When varenicline use was compared with use of a nicotine patch, bupropion, or e-cigarettes, there was a reduction in serious adverse events – changes in mood, suicidal ideation, and neurological side effects such as seizures.2 Clinicians may remember a black box warning on the varenicline label citing neuropsychiatric effects and it is important to note that the Food and Drug Administration removed this boxed warning in 2016.4
Opinion
This recommendation represents an important, evidence-based change from previous guidelines. It presents the opportunity for better outcomes, but will likely take a while to filter into practice, as clinicians need to become more comfortable with the use of varenicline and insurance supports the cost of varenicline.
The average cost of varenicline for 12 weeks is between $1,220 and $1,584. For comparison, nicotine replacement therapy costs $170 to $240 for the same number of weeks. To put those costs in perspective, the 12-week cost of cigarettes for a two-pack-a-day smoker is approximately $1,000.
For some patients, the motivation to quit smoking comes from the realization of how much they are spending on cigarettes each month. That said, if a patient does not have insurance or their insurance does not cover the cost of varenicline, nicotine replacement therapy might be more appealing. It should be noted that better abstinence rates have been seen in patients taking varenicline plus nicotine replacement therapy versus varenicline alone.
Suggested treatment
Based on a systematic review of randomized controlled trials, the American Thoracic Society’s guideline on pharmacological treatment in tobacco-dependent adults concludes that varenicline plus nicotine patch is the preferred pharmacological treatment for tobacco cessation when compared with varenicline alone, bupropion alone, nicotine replacement therapy alone, and e-cigarettes alone. If the patient does not want to start two medicines at once, then varenicline alone would be the preferred choice.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Sprogell is a third-year resident in the family medicine residency program at Abington Jefferson Health. They have no conflicts related to the content of this piece. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. U.S. Preventive Services Task Force. Primary care interventions for prevention and cessation of tobacco use in children and adolescents: U.S. Preventive Services Task Force Recommendation Statement. JAMA.2020;323(16):1590-8. doi: 10.1001/jama.2020.4679.
2. Leone FT et al. Initiating pharmacologic treatment in tobacco-dependent adults: An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(2):e5–e31.
3. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. U.S. Preventive Services Task Force 2015 Sep 21.
4. FDA Drug Safety Communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. 2016 Dec. 16.
Comment & Controversy
How do you feel about expectantly managing a well-dated pregnancy past 41 weeks’ gestation?
ROBERT L. BARBIERI, MD
(EDITORIAL; FEBRUARY 2019)
Is it reasonable to choose the age of 40 for proposing an anticipation of labor induction?
In physiologic ongoing pregnancies (whether they are spontaneous or autologous in vitro fertilization [IVF] or heterologous IVF), the evidence for anticipating labor induction based upon the only factor of age (after 40 years) is missing. Nonetheless, the number of women becoming pregnant at an older age is expected to increase, and from my perspective, to induce all physiologic pregnancies at term by 41 weeks and 5 days’ gestation does not appear to be best practice. I favor the idea of all women aged 40 and older to start labor induction earlier (for instance, to offer labor induction, with proper informed consent, by 41+ 0 and not 41+ 5 through 42+ 0 weeks of pregnancy).
Luca Bernardini, MD
La Spezia, Italy
Dr. Barbieri responds
At Brigham and Women’s Hospital in Boston, Massachusetts, our approach is to offer women ≥40 years of age induction of labor (IOL) at 39 weeks’ gestation, unless there is an obstetric contraindication to IOL. We believe that IOL at 39 weeks’ gestation is associated with a reduced risk of both cesarean delivery and a new diagnosis of hypertension.1
Reference
- Grobman WA, Rice MM, Reddy, UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
What is the optimal hormonal treatment for women with polycystic ovary syndrome?
ROBERT L. BARBIERI, MD
(EDITORIAL; JANUARY 2020)
OCs and spironolactone study
I often recommend oral contraceptives (OCs) containing drospirenone for patients with polycyctic ovary syndrome (PCOS)-associated mild acne and hirsutism—since OCs are already approved by the US Food and Drug Administration for acne, with similar effects as spironolactone. My patients seem to do well on an OC, and require only one medication. Of course, I would add spironolactone to the treatment regimen and switch OCs if she was not responding well.
Michael T. Cane, MD
Arlington, Texas
Dr. Barbieri responds
The Endocrine Society agrees with Dr. Cane’s approach, recommending the initiation of monotherapy with an estrogen-progestin followed by the addition of spironolactone if 6 months of monotherapy produces insufficient improvement in dermatologic symptoms of PCOS, including hirsutism and acne. Most contraceptives contain 3 mg or 4 mg of drospirenone, which is thought to have antiandrogenic effects similar to spironolactone 25 mg. I believe that spironolactone 100 mg provides more complete and rapid resolution of the dermatologic symptoms caused by PCOS. Hence, I initiate both an estrogen-progestin contraceptive with spironolactone.
How do you feel about expectantly managing a well-dated pregnancy past 41 weeks’ gestation?
ROBERT L. BARBIERI, MD
(EDITORIAL; FEBRUARY 2019)
Is it reasonable to choose the age of 40 for proposing an anticipation of labor induction?
In physiologic ongoing pregnancies (whether they are spontaneous or autologous in vitro fertilization [IVF] or heterologous IVF), the evidence for anticipating labor induction based upon the only factor of age (after 40 years) is missing. Nonetheless, the number of women becoming pregnant at an older age is expected to increase, and from my perspective, to induce all physiologic pregnancies at term by 41 weeks and 5 days’ gestation does not appear to be best practice. I favor the idea of all women aged 40 and older to start labor induction earlier (for instance, to offer labor induction, with proper informed consent, by 41+ 0 and not 41+ 5 through 42+ 0 weeks of pregnancy).
Luca Bernardini, MD
La Spezia, Italy
Dr. Barbieri responds
At Brigham and Women’s Hospital in Boston, Massachusetts, our approach is to offer women ≥40 years of age induction of labor (IOL) at 39 weeks’ gestation, unless there is an obstetric contraindication to IOL. We believe that IOL at 39 weeks’ gestation is associated with a reduced risk of both cesarean delivery and a new diagnosis of hypertension.1
Reference
- Grobman WA, Rice MM, Reddy, UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
What is the optimal hormonal treatment for women with polycystic ovary syndrome?
ROBERT L. BARBIERI, MD
(EDITORIAL; JANUARY 2020)
OCs and spironolactone study
I often recommend oral contraceptives (OCs) containing drospirenone for patients with polycyctic ovary syndrome (PCOS)-associated mild acne and hirsutism—since OCs are already approved by the US Food and Drug Administration for acne, with similar effects as spironolactone. My patients seem to do well on an OC, and require only one medication. Of course, I would add spironolactone to the treatment regimen and switch OCs if she was not responding well.
Michael T. Cane, MD
Arlington, Texas
Dr. Barbieri responds
The Endocrine Society agrees with Dr. Cane’s approach, recommending the initiation of monotherapy with an estrogen-progestin followed by the addition of spironolactone if 6 months of monotherapy produces insufficient improvement in dermatologic symptoms of PCOS, including hirsutism and acne. Most contraceptives contain 3 mg or 4 mg of drospirenone, which is thought to have antiandrogenic effects similar to spironolactone 25 mg. I believe that spironolactone 100 mg provides more complete and rapid resolution of the dermatologic symptoms caused by PCOS. Hence, I initiate both an estrogen-progestin contraceptive with spironolactone.
How do you feel about expectantly managing a well-dated pregnancy past 41 weeks’ gestation?
ROBERT L. BARBIERI, MD
(EDITORIAL; FEBRUARY 2019)
Is it reasonable to choose the age of 40 for proposing an anticipation of labor induction?
In physiologic ongoing pregnancies (whether they are spontaneous or autologous in vitro fertilization [IVF] or heterologous IVF), the evidence for anticipating labor induction based upon the only factor of age (after 40 years) is missing. Nonetheless, the number of women becoming pregnant at an older age is expected to increase, and from my perspective, to induce all physiologic pregnancies at term by 41 weeks and 5 days’ gestation does not appear to be best practice. I favor the idea of all women aged 40 and older to start labor induction earlier (for instance, to offer labor induction, with proper informed consent, by 41+ 0 and not 41+ 5 through 42+ 0 weeks of pregnancy).
Luca Bernardini, MD
La Spezia, Italy
Dr. Barbieri responds
At Brigham and Women’s Hospital in Boston, Massachusetts, our approach is to offer women ≥40 years of age induction of labor (IOL) at 39 weeks’ gestation, unless there is an obstetric contraindication to IOL. We believe that IOL at 39 weeks’ gestation is associated with a reduced risk of both cesarean delivery and a new diagnosis of hypertension.1
Reference
- Grobman WA, Rice MM, Reddy, UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
What is the optimal hormonal treatment for women with polycystic ovary syndrome?
ROBERT L. BARBIERI, MD
(EDITORIAL; JANUARY 2020)
OCs and spironolactone study
I often recommend oral contraceptives (OCs) containing drospirenone for patients with polycyctic ovary syndrome (PCOS)-associated mild acne and hirsutism—since OCs are already approved by the US Food and Drug Administration for acne, with similar effects as spironolactone. My patients seem to do well on an OC, and require only one medication. Of course, I would add spironolactone to the treatment regimen and switch OCs if she was not responding well.
Michael T. Cane, MD
Arlington, Texas
Dr. Barbieri responds
The Endocrine Society agrees with Dr. Cane’s approach, recommending the initiation of monotherapy with an estrogen-progestin followed by the addition of spironolactone if 6 months of monotherapy produces insufficient improvement in dermatologic symptoms of PCOS, including hirsutism and acne. Most contraceptives contain 3 mg or 4 mg of drospirenone, which is thought to have antiandrogenic effects similar to spironolactone 25 mg. I believe that spironolactone 100 mg provides more complete and rapid resolution of the dermatologic symptoms caused by PCOS. Hence, I initiate both an estrogen-progestin contraceptive with spironolactone.
Pregnancy of unknown location: Evidence-based evaluation and management
CASE Woman with bleeding in early pregnancy
A 31-year-old woman (G1P0) presents to the local emergency department (ED) due to bleeding in pregnancy. She reports a prior open appendectomy for ruptured appendix; she denies a history of sexually transmitted infections, smoking, and contraception use. She reports having regular menstrual cycles and trying to conceive with her husband for 18 months without success until now.
The patient reports that the previous week she took a home pregnancy test that was positive; she endorses having dark brown spotting for the past 2 days but denies pain. Based on the date of her last menstrual period, gestational age is estimated to be 5 weeks and 1 day. Her human chorionic gonadotropin (hCG) level is 1,670 mIU/mL. Transvaginal ultrasonography demonstrates a normal uterus with an endometrial thickness of 10 mm, no evidence of an intrauterine pregnancy (IUP), normal adnexa bilaterally, and scant free fluid in the pelvis.
Identifying and evaluating pregnancy of unknown location
A pregnancy of unknown location (PUL) is defined by a positive serum hCG level in the absence of a visualized IUP or ectopic pregnancy (EP) by pelvic ultrasonography.
Because of variations in screening tools and clinical practices between institutions and care settings (for example, EDs versus specialized outpatient offices), the incidence of PUL is difficult to capture. In specialized early pregnancy clinics, the rate is 8% to 10%, whereas in the ED setting, the PUL rate has been reported to be as high as 42%.1-6 While approximately 98% to 99% of all pregnancies are intrauterine, only 30% of PULs will continue to develop as viable ongoing intrauterine gestations.7-9 The remainder are revealed as failing IUPs or EPs. To counsel patients, set expectations, and triage to appropriate management, it is critical to diagnose pregnancy location as efficiently as possible.
Ectopic pregnancy
Ectopic pregnancies represent only 1% to 2% of conceptions (both spontaneous and through assisted reproduction) and occur most commonly in the fallopian tube, although EPs also can implant in the cornua of the uterus, the cervix, cesarean scar, and more rarely on the ovary or abdominal viscera.10,11 Least common, heterotopic pregnancies—in which an IUP coexists with an EP—occur in 1 in 4,000 to 30,000 pregnancies, more commonly in women who used assisted reproduction.11
Major risk factors for EP include a history of tubal surgery, sexually transmitted infections (particularly Chlamydia trachomatis), pelvic inflammatory disease, conception with an intrauterine device in situ, and a history of prior EP or tubal surgery, particularly prior tubal ligation; minor risk factors include a history of infertility (excluding known tubal factor infertility) or smoking (in a dose-dependent manner).11,12 The concern for an EP is heightened in patients with these risk factors.
Because of the possibility of rupture and life-threatening hemorrhage, EP carries a risk of significant morbidity and mortality.13 Ruptured EPs account for approximately 2.7% of all maternal deaths each year.14 When diagnosed sufficiently early in a stable patient, most EPs can be managed medically with methotrexate, a folic acid antagonist.15 Ectopic pregnancies also may be managed surgically, and emergency surgery is indicated in women with evidence of EP rupture and intraperitoneal bleeding.
Continue to: Intrauterine pregnancy...
Intrauterine pregnancy
While excluding EP is critical, it is equally important to diagnose an IUP as expeditiously as possible to avoid inadvertent, destructive intervention. Diagnosis and management of a PUL can involve endometrial aspiration, which would interrupt an IUP and should be avoided until the possibility of a viable IUP has been eliminated in desired pregnancies. The inadvertent administration of methotrexate, a known teratogen, to a patient with an undiagnosed viable IUP can result in miscarriage, elective termination, or a live-born infant with significant malformations, all of which expose the administering physician to malpractice litigation.16,17
In desired pregnancies, it is essential to differentiate between a viable IUP, a nonviable IUP, and an EP to guide appropriate management and ensure patient safety, whereas exclusion of EP is the priority in undesired pregnancies.
Tools for diagnosing pregnancy location
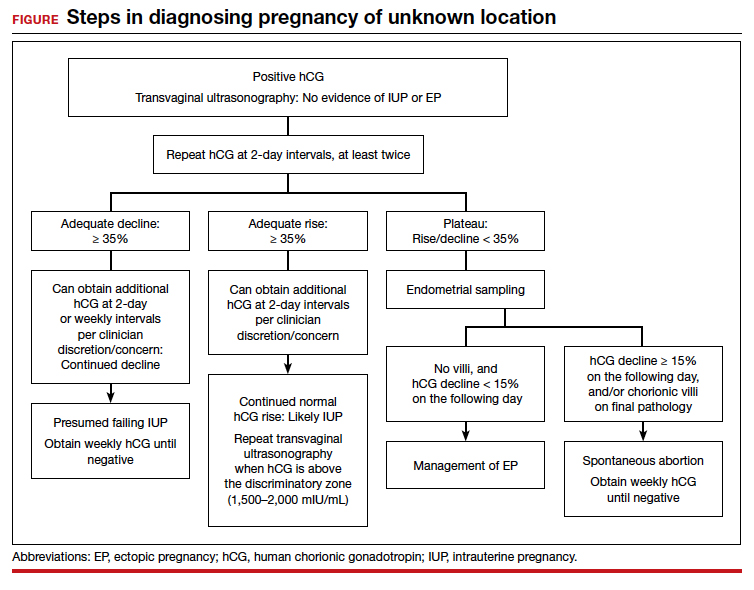
For diagnosing pregnancy location, serial hCG measurement, transvaginal pelvic ultrasonography, and outpatient endometrial aspiration are all relevant clinical tools. Pregnancy location can be diagnosed with either direct visualization of an IUP or EP by ultrasonography or with confirmed pathology (chorionic villi or trophoblast cells) from endometrial aspiration (FIGURE). A decline in hCG to an undetectable level following endometrial aspiration also is considered sufficient to diagnose a failed IUP, even in the absence of a confirmatory ultrasonography.
Trending hCG values
In stable patients with PUL, serum hCG levels are commonly measured at 2-day intervals, ideally for a minimum of 3 values. Conventional wisdom dictates that in viable IUPs, the hCG level should roughly double every 2 days. However, more recent data suggest that the threshold for minimum expected hCG rise for an ongoing IUP should be far lower when the pregnancy is desired.18 A less conservative cutoff can be considered when a pregnancy is not desired.
In a multisite cohort study of 1,005 women with PUL, a minimum hCG rise of 35% in 2 days captured the majority of IUPs, with a negative predictive value of 97.2% for IUP.19 Of note, although the cutoff of 35% was selected to reduce the risk of misdiagnosing an IUP as an EP, 7.7% of IUPs (and 16.8% of EPs) were still misclassified, showing that hCG trends must be interpreted in the context of other clinical data, including ultrasonography findings and patient symptoms and history.
A follow-up study demonstrated that hCG rises are lower (but still within this normal range) when the initial hCG value is higher, particularly greater than 3,000 mIU/mL.20
Studies show that the rate of spontaneous hCG decline in failing IUPs ranges from 12% to 47% in 2 days, falling more rapidly from higher starting hCG values.19,21 In a retrospective review of 443 women with spontaneously resolving PUL (presumed to be failing IUPs), the minimum 2-day decline in hCG was 35%.22 Any spontaneous hCG decline less than 35% in 2 days in a PUL should raise physician concern for EP.
Conversely, EPs do not demonstrate predictable hCG trends and can mimic the hCG trends of viable or failing IUPs. Although typically half of EPs present with an increasing hCG value and half present with a decreasing hCG value, the majority (71%) demonstrate a slower rate of change than either a viable IUP or a miscarriage.11 This slower change (plateau) should heighten the clinician’s suspicion for an EP.
Continue to: Progesterone levels...
Progesterone levels
A progesterone level often is used to attempt to determine pregnancy viability in women who are not receiving progesterone supplementation, although it ultimately has limited utility. While far less sensitive than an hCG value trend, a serum progesterone level of less than 5 to 10 ng/mL is a rough marker of nonviable pregnancy.23
In a large meta-analysis of women with pain and bleeding, 96.8% of pregnancies with a single progesterone level of less than 10 ng/mL were nonviable.23 When an inconclusive ultrasonography was documented in addition to symptoms of pain and bleeding, 99.2% of pregnancies with a progesterone level of less than 3.2 to 6 ng/mL were nonviable.
Progesterone’s usefulness in assessing for a PUL is limited: While progesterone levels may indicate nonviability, they provide no indication of pregnancy location (intrauterine or ectopic).
Alternative serologic markers
Various other reproductive and pregnancy-related hormones have been investigated for use in the diagnosis of pregnancy location in PULs, including activin A, inhibin A, pregnancy-associated plasma protein A (PAPP-A), placental-like growth factor, vascular endothelial growth factor, follistatin, and various microRNAs.24,25 While research into these biomarkers is ongoing, none have been studied in prospective trials, and they are not for use in current clinical care.
Pelvic ultrasonography
Pelvic ultrasonography is a crucial part of PUL assessment. Transvaginal ultrasonography should be interpreted in the context of the estimated gestational age of the pregnancy and serial hCG values, if available; the patient’s symptoms; and the sensitivity of the ultrasonography equipment, which also may be affected by variables that can reduce visualization, such as uterine fibroids and obesity.
The “discriminatory zone” refers to the hCG value above which an IUP should be visualized by ultrasonography. Generally, with an hCG value of 1,500 to 2,000 mIU/mL or greater, an IUP is expected to be seen with transvaginal sonography.3,26 Many exceptions to the discriminatory zone have been reported, however, including multiple pregnancies, which will have a higher hCG value at an earlier gestational age. Even in singleton pregnancies, viable IUPs have been documented as developing from PULs with an elevated initial hCG value as high as 4,300 mIU/mL.27 The discriminatory zone may vary among clinical hCG assays, and it also is affected by the quality and modernity of the ultrasonography equipment as well as by the ultrasonography operator’s experience and skill.28,29
The estimated gestational age, based on either the last menstrual period or assisted reproduction procedure, provides a helpful data point to guide expectations for ultrasonography findings.30 Using transvaginal ultrasonography in a normally progressing IUP, a gestational sac—typically measuring 2 to 3 mm—should be visualized at 5 weeks.15,30 At approximately 5.5 weeks, a yolk sac measuring 3 to 5 mm should appear. At 6 weeks, an embryo with cardiac activity should be visualized.
In a pregnancy reliably dated beyond 5 weeks, the lack of an intrauterine gestational sac is suspicious for, but not diagnostic of, an EP. Conversely, the visualization of a gestational sac alone (without a yolk sac) is insufficient to definitively exclude an EP, since a small fluid collection in the endometrium (a “pseudosac”) can convincingly mimic the appearance of a gestational sac, and a follow-up ultrasonography should be performed in such cases.
Among patients without ultrasonographic evidence of an IUP, endometrial thickness has been posited as a way to differentiate between IUP and EP.31,32 Evidence suggests that an endometrial stripe of at least 8 to 10 mm may be somewhat predictive of an IUP, while endometrial thickness below 8 mm is more concerning for EP. This clinical variable, however, has been shown repeatedly to lack sufficient sensitivity and specificity for IUP and should be considered only within the entire clinical context.
Continue to: Endometrial aspiration...
Endometrial aspiration
A persistently abnormal hCG trend and an ultrasonography without evidence of an IUP—particularly with an hCG value above the discriminatory zone and/or with reliable pregnancy dating beyond 5 to 6 weeks—is highly concerning for either a failing IUP or an EP. Once a viable desired IUP is excluded beyond reasonable doubt through these measures, endometrial aspiration to determine pregnancy location is a reasonable next step in PUL management.
Endometrial aspiration can identify a failing IUP by detection of trophoblasts or chorionic villi on pathology and/or by a decline of at least 15% in hCG, measured on the day of endometrial aspiration and again the following day. Endometrial aspiration is effective even in clinical care settings that do not have rapid pathologic analysis available, as hCG measurement before and within 24 hours after the procedure still can be performed.
Vacuum aspiration (electric or manual) in an operating room or office setting is an effective tool for diagnosing pregnancy location.33,34 The use of an endometrial Pipelle for endometrial sampling (typically used for an office endometrial biopsy to diagnose hyperplasia or malignancy) is insufficient for determining pregnancy location.35 For all patients managed with this protocol, the hCG value ideally should be followed until it is undetectable, regardless of whether an EP or failing IUP was diagnosed. In rare cases, an EP may be diagnosed by a late plateau in hCG values, following an initial decline consistent with a failing IUP.
Utility for diagnosis. Retrospective studies in patients with PUL following in vitro fertilization have established the utility of outpatient endometrial aspiration with a Karman cannula, followed by a repeat hCG measurement on the day after the procedure.34,36 These data demonstrate that between 42% and 69% of women were ultimately diagnosed with a failed IUP following endometrial aspiration, thereby sparing them unnecessary exposure to methotrexate.
A decline in hCG levels of at least 15% within 24 hours after the procedure indicates that a failed IUP is the most likely diagnosis and further intervention is not indicated (although falling hCG values should be monitored for confirmation); confirmatory pathology with chorionic villi or trophoblasts was present in less than half of these women and is not necessary to diagnose a failed IUP.36 Women diagnosed with a failed IUP after endometrial aspiration also benefitted from a shorter time to resolution of the nonviable pregnancy by approximately 2 weeks.36
Despite the efficacy of endometrial aspiration for the diagnosis of pregnancy location, recent data show that physicians have highly variable approaches to PUL with an hCG plateaued above the discriminatory zone: One-third would first perform endometrial aspiration, while one-third would give methotrexate without further diagnostics.37 Academic physicians were 4 times more likely to recommend endometrial aspiration.37
Presumed EP. Following endometrial aspiration, if pathology does not confirm an intrauterine gestation and the hCG fails to decline by at least 15%, the diagnosis of a presumed EP is made.
For stable patients with neither evidence of intra-abdominal bleeding nor contraindications to methotrexate (such as blood dyscrasias, hepatic or renal insufficiency, active pulmonary or peptic ulcer disease, breastfeeding, or a known intolerance to the medication), methotrexate is recommended for medical management.26 Following screening blood work that includes a complete blood count and liver function and renal function tests, the typical methotrexate dose is 50 mg/m2 of body surface area. The single-dose regimen entails checking hCG on the day of methotrexate administration and again on days 4 and 7 thereafter. A minimum decline in hCG of 15% between days 4 and 7 indicates successful treatment; if the hCG decline is below 15%, the patient should receive an additional dose of methotrexate.
There are several published alternative regimens for methotrexate administration, including 2-dose and multidose regimens; the 2-dose protocol (2 doses within 7 days) may be more effective in women with higher hCG (> 3,000 mIU/mL) or known adnexal mass.26,38
Continue to: Contraindications to methotrexate...
Contraindications to methotrexate. In addition to strict medical contraindications to methotrexate, relative contraindications that indicate a higher risk of methotrexate failure include the presence of fetal cardiac activity, EP mass greater than 4 cm, and serum hCG above 5,000 mIU/mL.26 Because of the potential risk of tubal rupture during medical management, relative contraindications also include patient inability to follow up as an outpatient and patient refusal of blood transfusion.26 Patients with contraindications to methotrexate, hemodynamic instability, ultrasonographic or clinical evidence of EP rupture, or those electing for surgical management may be managed with laparoscopy.11 Discussion of surgical management of EP is beyond the scope of this article.
Follow the hCG level. In patients with a failing IUP or an EP treated with methotrexate or salpingostomy, the hCG level should always be followed until it is negative, usually by weekly measurements once the diagnosis is made. In some cases, the hCG level may plateau after an initial decline, alerting the clinician to failed treatment for a known EP or the need for recategorization of a failed IUP as an EP.
CASE Concluded
The patient’s second and third hCG measurements at 2-day intervals were 1,903 mIU/mL (14% rise) and 2,264 mIU/mL (16% rise). At that point, a repeat transvaginal ultrasonography showed no IUP, adnexal mass, or free fluid. The patient was counseled for outpatient endometrial aspiration, which was performed using manual vacuum aspiration. The serum hCG level on the morning of the procedure was 2,420 mIU/mL. On postprocedure day 1, the serum hCG level fell to 1,615 mIU/mL, a 33% decline. The patient was counseled that this decline in hCG indicated a failing IUP. The final pathologic analysis was returned 3 days later, showing no evidence of trophoblasts and chorionic villi. Regardless, the diagnosis of failing IUP remained given the rapid hCG decline; the tissue from the disrupted failing IUP was likely very scant or simply not drawn into the cannula. Serum hCG levels repeated at weekly intervals revealed ongoing decline, and after 4 weeks, the serum hCG was negative.
In summary
For women diagnosed with PUL, the primary goal is to distinguish an IUP from an EP to reduce the risk of EP rupture through expeditious diagnosis and treatment. In women for whom the pregnancy is desired, distinguishing a viable IUP from a nonviable IUP or an EP is the more specific goal to avoid intervention on a viable IUP (with methotrexate or endometrial aspiration). In women with abnormal hCG trends and indeterminate ultrasonography results (particularly with a serum hCG above the discriminatory zone), outpatient endometrial aspiration is a highly effective way to determine pregnancy location, which dictates further treatment. ●
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2014;20:250-261.
- Kirk E, Condous G, Bourne T. Pregnancies of unknown location. Best Pract Res Clin Obstet Gynaecol. 2009;23:493-499.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Banerjee S, Aslam N, Zosmer N, et al. The expectant management of women with early pregnancy of unknown location. Ultrasound Obstet Gynecol. 1999;14:231-236.
- Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
- Mol BW, Hajenius PJ, Engelsbel S, et al. Serum human chorionic gonadotropin measurement in the diagnosis of ectopic pregnancy when transvaginal sonography is inconclusive. Fertil Steril. 1998;70:972-981.
- Kirk E, Condous G, Van Calster B, et al. Rationalizing the follow-up of pregnancies of unknown location. Hum Reprod. 2007;22:1744-1750.
- Stulberg DB, Cain LR, Dahlquist I, et al. Ectopic pregnancy rates and racial disparities in the Medicaid population, 2004-2008. Fertil Steril. 2014;102:1671-1676.
- Zeng MF, Li LM. Frozen blastocyst transfer reduces incidence of ectopic pregnancy compared with fresh blastocyst transfer: a meta-analysis. Gynecol Endocrinol. 2019;35:93-99.
- Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583-591.
- Barnhart KT. Ectopic pregnancy. N Engl J Med. 2009;361:379-387.
- Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373.
- Brady PC. Handbook of Consult and Inpatient Gynecology. Switzerland: Springer International Publishing; 2016.
- Fridman D, Hawkins E, Dar P, et al. Methotrexate administration to patients with presumed ectopic pregnancy leads to methotrexate exposure of intrauterine pregnancies. J Ultrasound Med. 2019;38:675-684.
- Nurmohamed L, Moretti ME, Schechter T, et al. Outcome following high-dose methotrexate in pregnancies misdiagnosed as ectopic. Am J Obstet Gynecol. 2011;205:533.e1-533.e3.
- Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104:50-55.
- Morse CB, Sammel MD, Shaunik A, et al. Performance of human chorionic gonadotropin curves in women at risk for ectopic pregnancy: exceptions to the rules. Fertil Steril. 2012;97:101-6.e2.
- Barnhart KT, Guo W, Cary MS, et al. Differences in serum human chorionic gonadotropin rise in early pregnancy by race and value at presentation. Obstet Gynecol. 2016;128:504-511.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5, pt 1):975-981.
- Butts SF, Guo W, Cary MS, et al. Predicting the decline in human chorionic gonadotropin in a resolving pregnancy of unknown location. Obstet Gynecol. 2013;122(2 pt 1):337-343.
- Verhaegen J, Gallos ID, van Mello NM, et al. Accuracy of single progesterone test to predict early pregnancy outcome in women with pain or bleeding: meta-analysis of cohort studies. BMJ. 2012;345:e6077.
- Senapati S, Sammel MD, Butts SF, et al. Predicting first trimester pregnancy outcome: derivation of a multiple marker test. Fertil Steril. 2016;106:1725-1732.e3.
- Refaat B, Bahathiq AO. The performances of serum activins and follistatin in the diagnosis of ectopic pregnancy: a prospective case-control study. Clin Chim Acta. 2020;500:69-74.
- Practice Committee of American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100:638-644.
- Doubilet PM, Benson CB. Further evidence against the reliability of the human chorionic gonadotropin discriminatory level. J Ultrasound Med. 2011;30:1637-1642.
- Desai D, Lu J, Wyness SP, et al. Human chorionic gonadotropin discriminatory zone in ectopic pregnancy: does assay harmonization matter? Fertil Steril. 2014;101:1671-1674.
- Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33:465-471.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Moschos E, Twickler DM. Endometrial thickness predicts intrauterine pregnancy in patients with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2008;32:929-934.
- Ellaithy M, Abdelaziz A, Hassan MF. Outcome prediction in pregnancies of unknown location using endometrial thickness measurement: is this of real clinical value? Eur J Obstet Gynecol Reprod Biol. 2013;168:68-74.
- Shaunik A, Kulp J, Appleby DH, et al. Utility of dilation and curettage in the diagnosis of pregnancy of unknown location. Am J Obstet Gynecol. 2011;204:130.e1-130.e6.
- Brady P, Imudia AN, Awonuga AO, et al. Pregnancies of unknown location after in vitro fertilization: minimally invasive management with Karman cannula aspiration. Fertil Steril. 2014;101:420-426.
- Barnhart KT, Gracia CR, Reindl B, et al. Usefulness of pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol. 2003;188:906-909.
- Insogna IG, Farland LV, Missmer SA, et al. Outpatient endometrial aspiration: an alternative to methotrexate for pregnancy of unknown location. Am J Obstet Gynecol. 2017;217:185.e1-185.e9.
- Parks MA, Barnhart KT, Howard DL. Trends in the management of nonviable pregnancies of unknown location in the United States. Gynecol Obstet Invest. 2018;83:552-557.
- Alur-Gupta S, Cooney LG, Senapati S, et al. Two-dose versus single-dose methotrexate for treatment of ectopic pregnancy: a meta-analysis. Am J Obstet Gynecol. 2019;221:95-108.e2.
CASE Woman with bleeding in early pregnancy
A 31-year-old woman (G1P0) presents to the local emergency department (ED) due to bleeding in pregnancy. She reports a prior open appendectomy for ruptured appendix; she denies a history of sexually transmitted infections, smoking, and contraception use. She reports having regular menstrual cycles and trying to conceive with her husband for 18 months without success until now.
The patient reports that the previous week she took a home pregnancy test that was positive; she endorses having dark brown spotting for the past 2 days but denies pain. Based on the date of her last menstrual period, gestational age is estimated to be 5 weeks and 1 day. Her human chorionic gonadotropin (hCG) level is 1,670 mIU/mL. Transvaginal ultrasonography demonstrates a normal uterus with an endometrial thickness of 10 mm, no evidence of an intrauterine pregnancy (IUP), normal adnexa bilaterally, and scant free fluid in the pelvis.
Identifying and evaluating pregnancy of unknown location
A pregnancy of unknown location (PUL) is defined by a positive serum hCG level in the absence of a visualized IUP or ectopic pregnancy (EP) by pelvic ultrasonography.
Because of variations in screening tools and clinical practices between institutions and care settings (for example, EDs versus specialized outpatient offices), the incidence of PUL is difficult to capture. In specialized early pregnancy clinics, the rate is 8% to 10%, whereas in the ED setting, the PUL rate has been reported to be as high as 42%.1-6 While approximately 98% to 99% of all pregnancies are intrauterine, only 30% of PULs will continue to develop as viable ongoing intrauterine gestations.7-9 The remainder are revealed as failing IUPs or EPs. To counsel patients, set expectations, and triage to appropriate management, it is critical to diagnose pregnancy location as efficiently as possible.
Ectopic pregnancy
Ectopic pregnancies represent only 1% to 2% of conceptions (both spontaneous and through assisted reproduction) and occur most commonly in the fallopian tube, although EPs also can implant in the cornua of the uterus, the cervix, cesarean scar, and more rarely on the ovary or abdominal viscera.10,11 Least common, heterotopic pregnancies—in which an IUP coexists with an EP—occur in 1 in 4,000 to 30,000 pregnancies, more commonly in women who used assisted reproduction.11
Major risk factors for EP include a history of tubal surgery, sexually transmitted infections (particularly Chlamydia trachomatis), pelvic inflammatory disease, conception with an intrauterine device in situ, and a history of prior EP or tubal surgery, particularly prior tubal ligation; minor risk factors include a history of infertility (excluding known tubal factor infertility) or smoking (in a dose-dependent manner).11,12 The concern for an EP is heightened in patients with these risk factors.
Because of the possibility of rupture and life-threatening hemorrhage, EP carries a risk of significant morbidity and mortality.13 Ruptured EPs account for approximately 2.7% of all maternal deaths each year.14 When diagnosed sufficiently early in a stable patient, most EPs can be managed medically with methotrexate, a folic acid antagonist.15 Ectopic pregnancies also may be managed surgically, and emergency surgery is indicated in women with evidence of EP rupture and intraperitoneal bleeding.

Continue to: Intrauterine pregnancy...
Intrauterine pregnancy
While excluding EP is critical, it is equally important to diagnose an IUP as expeditiously as possible to avoid inadvertent, destructive intervention. Diagnosis and management of a PUL can involve endometrial aspiration, which would interrupt an IUP and should be avoided until the possibility of a viable IUP has been eliminated in desired pregnancies. The inadvertent administration of methotrexate, a known teratogen, to a patient with an undiagnosed viable IUP can result in miscarriage, elective termination, or a live-born infant with significant malformations, all of which expose the administering physician to malpractice litigation.16,17
In desired pregnancies, it is essential to differentiate between a viable IUP, a nonviable IUP, and an EP to guide appropriate management and ensure patient safety, whereas exclusion of EP is the priority in undesired pregnancies.
Tools for diagnosing pregnancy location
For diagnosing pregnancy location, serial hCG measurement, transvaginal pelvic ultrasonography, and outpatient endometrial aspiration are all relevant clinical tools. Pregnancy location can be diagnosed with either direct visualization of an IUP or EP by ultrasonography or with confirmed pathology (chorionic villi or trophoblast cells) from endometrial aspiration (FIGURE). A decline in hCG to an undetectable level following endometrial aspiration also is considered sufficient to diagnose a failed IUP, even in the absence of a confirmatory ultrasonography.

Trending hCG values
In stable patients with PUL, serum hCG levels are commonly measured at 2-day intervals, ideally for a minimum of 3 values. Conventional wisdom dictates that in viable IUPs, the hCG level should roughly double every 2 days. However, more recent data suggest that the threshold for minimum expected hCG rise for an ongoing IUP should be far lower when the pregnancy is desired.18 A less conservative cutoff can be considered when a pregnancy is not desired.
In a multisite cohort study of 1,005 women with PUL, a minimum hCG rise of 35% in 2 days captured the majority of IUPs, with a negative predictive value of 97.2% for IUP.19 Of note, although the cutoff of 35% was selected to reduce the risk of misdiagnosing an IUP as an EP, 7.7% of IUPs (and 16.8% of EPs) were still misclassified, showing that hCG trends must be interpreted in the context of other clinical data, including ultrasonography findings and patient symptoms and history.
A follow-up study demonstrated that hCG rises are lower (but still within this normal range) when the initial hCG value is higher, particularly greater than 3,000 mIU/mL.20
Studies show that the rate of spontaneous hCG decline in failing IUPs ranges from 12% to 47% in 2 days, falling more rapidly from higher starting hCG values.19,21 In a retrospective review of 443 women with spontaneously resolving PUL (presumed to be failing IUPs), the minimum 2-day decline in hCG was 35%.22 Any spontaneous hCG decline less than 35% in 2 days in a PUL should raise physician concern for EP.
Conversely, EPs do not demonstrate predictable hCG trends and can mimic the hCG trends of viable or failing IUPs. Although typically half of EPs present with an increasing hCG value and half present with a decreasing hCG value, the majority (71%) demonstrate a slower rate of change than either a viable IUP or a miscarriage.11 This slower change (plateau) should heighten the clinician’s suspicion for an EP.
Continue to: Progesterone levels...
Progesterone levels
A progesterone level often is used to attempt to determine pregnancy viability in women who are not receiving progesterone supplementation, although it ultimately has limited utility. While far less sensitive than an hCG value trend, a serum progesterone level of less than 5 to 10 ng/mL is a rough marker of nonviable pregnancy.23
In a large meta-analysis of women with pain and bleeding, 96.8% of pregnancies with a single progesterone level of less than 10 ng/mL were nonviable.23 When an inconclusive ultrasonography was documented in addition to symptoms of pain and bleeding, 99.2% of pregnancies with a progesterone level of less than 3.2 to 6 ng/mL were nonviable.
Progesterone’s usefulness in assessing for a PUL is limited: While progesterone levels may indicate nonviability, they provide no indication of pregnancy location (intrauterine or ectopic).
Alternative serologic markers
Various other reproductive and pregnancy-related hormones have been investigated for use in the diagnosis of pregnancy location in PULs, including activin A, inhibin A, pregnancy-associated plasma protein A (PAPP-A), placental-like growth factor, vascular endothelial growth factor, follistatin, and various microRNAs.24,25 While research into these biomarkers is ongoing, none have been studied in prospective trials, and they are not for use in current clinical care.
Pelvic ultrasonography
Pelvic ultrasonography is a crucial part of PUL assessment. Transvaginal ultrasonography should be interpreted in the context of the estimated gestational age of the pregnancy and serial hCG values, if available; the patient’s symptoms; and the sensitivity of the ultrasonography equipment, which also may be affected by variables that can reduce visualization, such as uterine fibroids and obesity.
The “discriminatory zone” refers to the hCG value above which an IUP should be visualized by ultrasonography. Generally, with an hCG value of 1,500 to 2,000 mIU/mL or greater, an IUP is expected to be seen with transvaginal sonography.3,26 Many exceptions to the discriminatory zone have been reported, however, including multiple pregnancies, which will have a higher hCG value at an earlier gestational age. Even in singleton pregnancies, viable IUPs have been documented as developing from PULs with an elevated initial hCG value as high as 4,300 mIU/mL.27 The discriminatory zone may vary among clinical hCG assays, and it also is affected by the quality and modernity of the ultrasonography equipment as well as by the ultrasonography operator’s experience and skill.28,29
The estimated gestational age, based on either the last menstrual period or assisted reproduction procedure, provides a helpful data point to guide expectations for ultrasonography findings.30 Using transvaginal ultrasonography in a normally progressing IUP, a gestational sac—typically measuring 2 to 3 mm—should be visualized at 5 weeks.15,30 At approximately 5.5 weeks, a yolk sac measuring 3 to 5 mm should appear. At 6 weeks, an embryo with cardiac activity should be visualized.
In a pregnancy reliably dated beyond 5 weeks, the lack of an intrauterine gestational sac is suspicious for, but not diagnostic of, an EP. Conversely, the visualization of a gestational sac alone (without a yolk sac) is insufficient to definitively exclude an EP, since a small fluid collection in the endometrium (a “pseudosac”) can convincingly mimic the appearance of a gestational sac, and a follow-up ultrasonography should be performed in such cases.
Among patients without ultrasonographic evidence of an IUP, endometrial thickness has been posited as a way to differentiate between IUP and EP.31,32 Evidence suggests that an endometrial stripe of at least 8 to 10 mm may be somewhat predictive of an IUP, while endometrial thickness below 8 mm is more concerning for EP. This clinical variable, however, has been shown repeatedly to lack sufficient sensitivity and specificity for IUP and should be considered only within the entire clinical context.
Continue to: Endometrial aspiration...
Endometrial aspiration
A persistently abnormal hCG trend and an ultrasonography without evidence of an IUP—particularly with an hCG value above the discriminatory zone and/or with reliable pregnancy dating beyond 5 to 6 weeks—is highly concerning for either a failing IUP or an EP. Once a viable desired IUP is excluded beyond reasonable doubt through these measures, endometrial aspiration to determine pregnancy location is a reasonable next step in PUL management.
Endometrial aspiration can identify a failing IUP by detection of trophoblasts or chorionic villi on pathology and/or by a decline of at least 15% in hCG, measured on the day of endometrial aspiration and again the following day. Endometrial aspiration is effective even in clinical care settings that do not have rapid pathologic analysis available, as hCG measurement before and within 24 hours after the procedure still can be performed.
Vacuum aspiration (electric or manual) in an operating room or office setting is an effective tool for diagnosing pregnancy location.33,34 The use of an endometrial Pipelle for endometrial sampling (typically used for an office endometrial biopsy to diagnose hyperplasia or malignancy) is insufficient for determining pregnancy location.35 For all patients managed with this protocol, the hCG value ideally should be followed until it is undetectable, regardless of whether an EP or failing IUP was diagnosed. In rare cases, an EP may be diagnosed by a late plateau in hCG values, following an initial decline consistent with a failing IUP.
Utility for diagnosis. Retrospective studies in patients with PUL following in vitro fertilization have established the utility of outpatient endometrial aspiration with a Karman cannula, followed by a repeat hCG measurement on the day after the procedure.34,36 These data demonstrate that between 42% and 69% of women were ultimately diagnosed with a failed IUP following endometrial aspiration, thereby sparing them unnecessary exposure to methotrexate.
A decline in hCG levels of at least 15% within 24 hours after the procedure indicates that a failed IUP is the most likely diagnosis and further intervention is not indicated (although falling hCG values should be monitored for confirmation); confirmatory pathology with chorionic villi or trophoblasts was present in less than half of these women and is not necessary to diagnose a failed IUP.36 Women diagnosed with a failed IUP after endometrial aspiration also benefitted from a shorter time to resolution of the nonviable pregnancy by approximately 2 weeks.36
Despite the efficacy of endometrial aspiration for the diagnosis of pregnancy location, recent data show that physicians have highly variable approaches to PUL with an hCG plateaued above the discriminatory zone: One-third would first perform endometrial aspiration, while one-third would give methotrexate without further diagnostics.37 Academic physicians were 4 times more likely to recommend endometrial aspiration.37
Presumed EP. Following endometrial aspiration, if pathology does not confirm an intrauterine gestation and the hCG fails to decline by at least 15%, the diagnosis of a presumed EP is made.
For stable patients with neither evidence of intra-abdominal bleeding nor contraindications to methotrexate (such as blood dyscrasias, hepatic or renal insufficiency, active pulmonary or peptic ulcer disease, breastfeeding, or a known intolerance to the medication), methotrexate is recommended for medical management.26 Following screening blood work that includes a complete blood count and liver function and renal function tests, the typical methotrexate dose is 50 mg/m2 of body surface area. The single-dose regimen entails checking hCG on the day of methotrexate administration and again on days 4 and 7 thereafter. A minimum decline in hCG of 15% between days 4 and 7 indicates successful treatment; if the hCG decline is below 15%, the patient should receive an additional dose of methotrexate.
There are several published alternative regimens for methotrexate administration, including 2-dose and multidose regimens; the 2-dose protocol (2 doses within 7 days) may be more effective in women with higher hCG (> 3,000 mIU/mL) or known adnexal mass.26,38
Continue to: Contraindications to methotrexate...
Contraindications to methotrexate. In addition to strict medical contraindications to methotrexate, relative contraindications that indicate a higher risk of methotrexate failure include the presence of fetal cardiac activity, EP mass greater than 4 cm, and serum hCG above 5,000 mIU/mL.26 Because of the potential risk of tubal rupture during medical management, relative contraindications also include patient inability to follow up as an outpatient and patient refusal of blood transfusion.26 Patients with contraindications to methotrexate, hemodynamic instability, ultrasonographic or clinical evidence of EP rupture, or those electing for surgical management may be managed with laparoscopy.11 Discussion of surgical management of EP is beyond the scope of this article.
Follow the hCG level. In patients with a failing IUP or an EP treated with methotrexate or salpingostomy, the hCG level should always be followed until it is negative, usually by weekly measurements once the diagnosis is made. In some cases, the hCG level may plateau after an initial decline, alerting the clinician to failed treatment for a known EP or the need for recategorization of a failed IUP as an EP.
CASE Concluded
The patient’s second and third hCG measurements at 2-day intervals were 1,903 mIU/mL (14% rise) and 2,264 mIU/mL (16% rise). At that point, a repeat transvaginal ultrasonography showed no IUP, adnexal mass, or free fluid. The patient was counseled for outpatient endometrial aspiration, which was performed using manual vacuum aspiration. The serum hCG level on the morning of the procedure was 2,420 mIU/mL. On postprocedure day 1, the serum hCG level fell to 1,615 mIU/mL, a 33% decline. The patient was counseled that this decline in hCG indicated a failing IUP. The final pathologic analysis was returned 3 days later, showing no evidence of trophoblasts and chorionic villi. Regardless, the diagnosis of failing IUP remained given the rapid hCG decline; the tissue from the disrupted failing IUP was likely very scant or simply not drawn into the cannula. Serum hCG levels repeated at weekly intervals revealed ongoing decline, and after 4 weeks, the serum hCG was negative.
In summary
For women diagnosed with PUL, the primary goal is to distinguish an IUP from an EP to reduce the risk of EP rupture through expeditious diagnosis and treatment. In women for whom the pregnancy is desired, distinguishing a viable IUP from a nonviable IUP or an EP is the more specific goal to avoid intervention on a viable IUP (with methotrexate or endometrial aspiration). In women with abnormal hCG trends and indeterminate ultrasonography results (particularly with a serum hCG above the discriminatory zone), outpatient endometrial aspiration is a highly effective way to determine pregnancy location, which dictates further treatment. ●
CASE Woman with bleeding in early pregnancy
A 31-year-old woman (G1P0) presents to the local emergency department (ED) due to bleeding in pregnancy. She reports a prior open appendectomy for ruptured appendix; she denies a history of sexually transmitted infections, smoking, and contraception use. She reports having regular menstrual cycles and trying to conceive with her husband for 18 months without success until now.
The patient reports that the previous week she took a home pregnancy test that was positive; she endorses having dark brown spotting for the past 2 days but denies pain. Based on the date of her last menstrual period, gestational age is estimated to be 5 weeks and 1 day. Her human chorionic gonadotropin (hCG) level is 1,670 mIU/mL. Transvaginal ultrasonography demonstrates a normal uterus with an endometrial thickness of 10 mm, no evidence of an intrauterine pregnancy (IUP), normal adnexa bilaterally, and scant free fluid in the pelvis.
Identifying and evaluating pregnancy of unknown location
A pregnancy of unknown location (PUL) is defined by a positive serum hCG level in the absence of a visualized IUP or ectopic pregnancy (EP) by pelvic ultrasonography.
Because of variations in screening tools and clinical practices between institutions and care settings (for example, EDs versus specialized outpatient offices), the incidence of PUL is difficult to capture. In specialized early pregnancy clinics, the rate is 8% to 10%, whereas in the ED setting, the PUL rate has been reported to be as high as 42%.1-6 While approximately 98% to 99% of all pregnancies are intrauterine, only 30% of PULs will continue to develop as viable ongoing intrauterine gestations.7-9 The remainder are revealed as failing IUPs or EPs. To counsel patients, set expectations, and triage to appropriate management, it is critical to diagnose pregnancy location as efficiently as possible.
Ectopic pregnancy
Ectopic pregnancies represent only 1% to 2% of conceptions (both spontaneous and through assisted reproduction) and occur most commonly in the fallopian tube, although EPs also can implant in the cornua of the uterus, the cervix, cesarean scar, and more rarely on the ovary or abdominal viscera.10,11 Least common, heterotopic pregnancies—in which an IUP coexists with an EP—occur in 1 in 4,000 to 30,000 pregnancies, more commonly in women who used assisted reproduction.11
Major risk factors for EP include a history of tubal surgery, sexually transmitted infections (particularly Chlamydia trachomatis), pelvic inflammatory disease, conception with an intrauterine device in situ, and a history of prior EP or tubal surgery, particularly prior tubal ligation; minor risk factors include a history of infertility (excluding known tubal factor infertility) or smoking (in a dose-dependent manner).11,12 The concern for an EP is heightened in patients with these risk factors.
Because of the possibility of rupture and life-threatening hemorrhage, EP carries a risk of significant morbidity and mortality.13 Ruptured EPs account for approximately 2.7% of all maternal deaths each year.14 When diagnosed sufficiently early in a stable patient, most EPs can be managed medically with methotrexate, a folic acid antagonist.15 Ectopic pregnancies also may be managed surgically, and emergency surgery is indicated in women with evidence of EP rupture and intraperitoneal bleeding.

Continue to: Intrauterine pregnancy...
Intrauterine pregnancy
While excluding EP is critical, it is equally important to diagnose an IUP as expeditiously as possible to avoid inadvertent, destructive intervention. Diagnosis and management of a PUL can involve endometrial aspiration, which would interrupt an IUP and should be avoided until the possibility of a viable IUP has been eliminated in desired pregnancies. The inadvertent administration of methotrexate, a known teratogen, to a patient with an undiagnosed viable IUP can result in miscarriage, elective termination, or a live-born infant with significant malformations, all of which expose the administering physician to malpractice litigation.16,17
In desired pregnancies, it is essential to differentiate between a viable IUP, a nonviable IUP, and an EP to guide appropriate management and ensure patient safety, whereas exclusion of EP is the priority in undesired pregnancies.
Tools for diagnosing pregnancy location
For diagnosing pregnancy location, serial hCG measurement, transvaginal pelvic ultrasonography, and outpatient endometrial aspiration are all relevant clinical tools. Pregnancy location can be diagnosed with either direct visualization of an IUP or EP by ultrasonography or with confirmed pathology (chorionic villi or trophoblast cells) from endometrial aspiration (FIGURE). A decline in hCG to an undetectable level following endometrial aspiration also is considered sufficient to diagnose a failed IUP, even in the absence of a confirmatory ultrasonography.

Trending hCG values
In stable patients with PUL, serum hCG levels are commonly measured at 2-day intervals, ideally for a minimum of 3 values. Conventional wisdom dictates that in viable IUPs, the hCG level should roughly double every 2 days. However, more recent data suggest that the threshold for minimum expected hCG rise for an ongoing IUP should be far lower when the pregnancy is desired.18 A less conservative cutoff can be considered when a pregnancy is not desired.
In a multisite cohort study of 1,005 women with PUL, a minimum hCG rise of 35% in 2 days captured the majority of IUPs, with a negative predictive value of 97.2% for IUP.19 Of note, although the cutoff of 35% was selected to reduce the risk of misdiagnosing an IUP as an EP, 7.7% of IUPs (and 16.8% of EPs) were still misclassified, showing that hCG trends must be interpreted in the context of other clinical data, including ultrasonography findings and patient symptoms and history.
A follow-up study demonstrated that hCG rises are lower (but still within this normal range) when the initial hCG value is higher, particularly greater than 3,000 mIU/mL.20
Studies show that the rate of spontaneous hCG decline in failing IUPs ranges from 12% to 47% in 2 days, falling more rapidly from higher starting hCG values.19,21 In a retrospective review of 443 women with spontaneously resolving PUL (presumed to be failing IUPs), the minimum 2-day decline in hCG was 35%.22 Any spontaneous hCG decline less than 35% in 2 days in a PUL should raise physician concern for EP.
Conversely, EPs do not demonstrate predictable hCG trends and can mimic the hCG trends of viable or failing IUPs. Although typically half of EPs present with an increasing hCG value and half present with a decreasing hCG value, the majority (71%) demonstrate a slower rate of change than either a viable IUP or a miscarriage.11 This slower change (plateau) should heighten the clinician’s suspicion for an EP.
Continue to: Progesterone levels...
Progesterone levels
A progesterone level often is used to attempt to determine pregnancy viability in women who are not receiving progesterone supplementation, although it ultimately has limited utility. While far less sensitive than an hCG value trend, a serum progesterone level of less than 5 to 10 ng/mL is a rough marker of nonviable pregnancy.23
In a large meta-analysis of women with pain and bleeding, 96.8% of pregnancies with a single progesterone level of less than 10 ng/mL were nonviable.23 When an inconclusive ultrasonography was documented in addition to symptoms of pain and bleeding, 99.2% of pregnancies with a progesterone level of less than 3.2 to 6 ng/mL were nonviable.
Progesterone’s usefulness in assessing for a PUL is limited: While progesterone levels may indicate nonviability, they provide no indication of pregnancy location (intrauterine or ectopic).
Alternative serologic markers
Various other reproductive and pregnancy-related hormones have been investigated for use in the diagnosis of pregnancy location in PULs, including activin A, inhibin A, pregnancy-associated plasma protein A (PAPP-A), placental-like growth factor, vascular endothelial growth factor, follistatin, and various microRNAs.24,25 While research into these biomarkers is ongoing, none have been studied in prospective trials, and they are not for use in current clinical care.
Pelvic ultrasonography
Pelvic ultrasonography is a crucial part of PUL assessment. Transvaginal ultrasonography should be interpreted in the context of the estimated gestational age of the pregnancy and serial hCG values, if available; the patient’s symptoms; and the sensitivity of the ultrasonography equipment, which also may be affected by variables that can reduce visualization, such as uterine fibroids and obesity.
The “discriminatory zone” refers to the hCG value above which an IUP should be visualized by ultrasonography. Generally, with an hCG value of 1,500 to 2,000 mIU/mL or greater, an IUP is expected to be seen with transvaginal sonography.3,26 Many exceptions to the discriminatory zone have been reported, however, including multiple pregnancies, which will have a higher hCG value at an earlier gestational age. Even in singleton pregnancies, viable IUPs have been documented as developing from PULs with an elevated initial hCG value as high as 4,300 mIU/mL.27 The discriminatory zone may vary among clinical hCG assays, and it also is affected by the quality and modernity of the ultrasonography equipment as well as by the ultrasonography operator’s experience and skill.28,29
The estimated gestational age, based on either the last menstrual period or assisted reproduction procedure, provides a helpful data point to guide expectations for ultrasonography findings.30 Using transvaginal ultrasonography in a normally progressing IUP, a gestational sac—typically measuring 2 to 3 mm—should be visualized at 5 weeks.15,30 At approximately 5.5 weeks, a yolk sac measuring 3 to 5 mm should appear. At 6 weeks, an embryo with cardiac activity should be visualized.
In a pregnancy reliably dated beyond 5 weeks, the lack of an intrauterine gestational sac is suspicious for, but not diagnostic of, an EP. Conversely, the visualization of a gestational sac alone (without a yolk sac) is insufficient to definitively exclude an EP, since a small fluid collection in the endometrium (a “pseudosac”) can convincingly mimic the appearance of a gestational sac, and a follow-up ultrasonography should be performed in such cases.
Among patients without ultrasonographic evidence of an IUP, endometrial thickness has been posited as a way to differentiate between IUP and EP.31,32 Evidence suggests that an endometrial stripe of at least 8 to 10 mm may be somewhat predictive of an IUP, while endometrial thickness below 8 mm is more concerning for EP. This clinical variable, however, has been shown repeatedly to lack sufficient sensitivity and specificity for IUP and should be considered only within the entire clinical context.
Continue to: Endometrial aspiration...
Endometrial aspiration
A persistently abnormal hCG trend and an ultrasonography without evidence of an IUP—particularly with an hCG value above the discriminatory zone and/or with reliable pregnancy dating beyond 5 to 6 weeks—is highly concerning for either a failing IUP or an EP. Once a viable desired IUP is excluded beyond reasonable doubt through these measures, endometrial aspiration to determine pregnancy location is a reasonable next step in PUL management.
Endometrial aspiration can identify a failing IUP by detection of trophoblasts or chorionic villi on pathology and/or by a decline of at least 15% in hCG, measured on the day of endometrial aspiration and again the following day. Endometrial aspiration is effective even in clinical care settings that do not have rapid pathologic analysis available, as hCG measurement before and within 24 hours after the procedure still can be performed.
Vacuum aspiration (electric or manual) in an operating room or office setting is an effective tool for diagnosing pregnancy location.33,34 The use of an endometrial Pipelle for endometrial sampling (typically used for an office endometrial biopsy to diagnose hyperplasia or malignancy) is insufficient for determining pregnancy location.35 For all patients managed with this protocol, the hCG value ideally should be followed until it is undetectable, regardless of whether an EP or failing IUP was diagnosed. In rare cases, an EP may be diagnosed by a late plateau in hCG values, following an initial decline consistent with a failing IUP.
Utility for diagnosis. Retrospective studies in patients with PUL following in vitro fertilization have established the utility of outpatient endometrial aspiration with a Karman cannula, followed by a repeat hCG measurement on the day after the procedure.34,36 These data demonstrate that between 42% and 69% of women were ultimately diagnosed with a failed IUP following endometrial aspiration, thereby sparing them unnecessary exposure to methotrexate.
A decline in hCG levels of at least 15% within 24 hours after the procedure indicates that a failed IUP is the most likely diagnosis and further intervention is not indicated (although falling hCG values should be monitored for confirmation); confirmatory pathology with chorionic villi or trophoblasts was present in less than half of these women and is not necessary to diagnose a failed IUP.36 Women diagnosed with a failed IUP after endometrial aspiration also benefitted from a shorter time to resolution of the nonviable pregnancy by approximately 2 weeks.36
Despite the efficacy of endometrial aspiration for the diagnosis of pregnancy location, recent data show that physicians have highly variable approaches to PUL with an hCG plateaued above the discriminatory zone: One-third would first perform endometrial aspiration, while one-third would give methotrexate without further diagnostics.37 Academic physicians were 4 times more likely to recommend endometrial aspiration.37
Presumed EP. Following endometrial aspiration, if pathology does not confirm an intrauterine gestation and the hCG fails to decline by at least 15%, the diagnosis of a presumed EP is made.
For stable patients with neither evidence of intra-abdominal bleeding nor contraindications to methotrexate (such as blood dyscrasias, hepatic or renal insufficiency, active pulmonary or peptic ulcer disease, breastfeeding, or a known intolerance to the medication), methotrexate is recommended for medical management.26 Following screening blood work that includes a complete blood count and liver function and renal function tests, the typical methotrexate dose is 50 mg/m2 of body surface area. The single-dose regimen entails checking hCG on the day of methotrexate administration and again on days 4 and 7 thereafter. A minimum decline in hCG of 15% between days 4 and 7 indicates successful treatment; if the hCG decline is below 15%, the patient should receive an additional dose of methotrexate.
There are several published alternative regimens for methotrexate administration, including 2-dose and multidose regimens; the 2-dose protocol (2 doses within 7 days) may be more effective in women with higher hCG (> 3,000 mIU/mL) or known adnexal mass.26,38
Continue to: Contraindications to methotrexate...
Contraindications to methotrexate. In addition to strict medical contraindications to methotrexate, relative contraindications that indicate a higher risk of methotrexate failure include the presence of fetal cardiac activity, EP mass greater than 4 cm, and serum hCG above 5,000 mIU/mL.26 Because of the potential risk of tubal rupture during medical management, relative contraindications also include patient inability to follow up as an outpatient and patient refusal of blood transfusion.26 Patients with contraindications to methotrexate, hemodynamic instability, ultrasonographic or clinical evidence of EP rupture, or those electing for surgical management may be managed with laparoscopy.11 Discussion of surgical management of EP is beyond the scope of this article.
Follow the hCG level. In patients with a failing IUP or an EP treated with methotrexate or salpingostomy, the hCG level should always be followed until it is negative, usually by weekly measurements once the diagnosis is made. In some cases, the hCG level may plateau after an initial decline, alerting the clinician to failed treatment for a known EP or the need for recategorization of a failed IUP as an EP.
CASE Concluded
The patient’s second and third hCG measurements at 2-day intervals were 1,903 mIU/mL (14% rise) and 2,264 mIU/mL (16% rise). At that point, a repeat transvaginal ultrasonography showed no IUP, adnexal mass, or free fluid. The patient was counseled for outpatient endometrial aspiration, which was performed using manual vacuum aspiration. The serum hCG level on the morning of the procedure was 2,420 mIU/mL. On postprocedure day 1, the serum hCG level fell to 1,615 mIU/mL, a 33% decline. The patient was counseled that this decline in hCG indicated a failing IUP. The final pathologic analysis was returned 3 days later, showing no evidence of trophoblasts and chorionic villi. Regardless, the diagnosis of failing IUP remained given the rapid hCG decline; the tissue from the disrupted failing IUP was likely very scant or simply not drawn into the cannula. Serum hCG levels repeated at weekly intervals revealed ongoing decline, and after 4 weeks, the serum hCG was negative.
In summary
For women diagnosed with PUL, the primary goal is to distinguish an IUP from an EP to reduce the risk of EP rupture through expeditious diagnosis and treatment. In women for whom the pregnancy is desired, distinguishing a viable IUP from a nonviable IUP or an EP is the more specific goal to avoid intervention on a viable IUP (with methotrexate or endometrial aspiration). In women with abnormal hCG trends and indeterminate ultrasonography results (particularly with a serum hCG above the discriminatory zone), outpatient endometrial aspiration is a highly effective way to determine pregnancy location, which dictates further treatment. ●
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2014;20:250-261.
- Kirk E, Condous G, Bourne T. Pregnancies of unknown location. Best Pract Res Clin Obstet Gynaecol. 2009;23:493-499.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Banerjee S, Aslam N, Zosmer N, et al. The expectant management of women with early pregnancy of unknown location. Ultrasound Obstet Gynecol. 1999;14:231-236.
- Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
- Mol BW, Hajenius PJ, Engelsbel S, et al. Serum human chorionic gonadotropin measurement in the diagnosis of ectopic pregnancy when transvaginal sonography is inconclusive. Fertil Steril. 1998;70:972-981.
- Kirk E, Condous G, Van Calster B, et al. Rationalizing the follow-up of pregnancies of unknown location. Hum Reprod. 2007;22:1744-1750.
- Stulberg DB, Cain LR, Dahlquist I, et al. Ectopic pregnancy rates and racial disparities in the Medicaid population, 2004-2008. Fertil Steril. 2014;102:1671-1676.
- Zeng MF, Li LM. Frozen blastocyst transfer reduces incidence of ectopic pregnancy compared with fresh blastocyst transfer: a meta-analysis. Gynecol Endocrinol. 2019;35:93-99.
- Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583-591.
- Barnhart KT. Ectopic pregnancy. N Engl J Med. 2009;361:379-387.
- Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373.
- Brady PC. Handbook of Consult and Inpatient Gynecology. Switzerland: Springer International Publishing; 2016.
- Fridman D, Hawkins E, Dar P, et al. Methotrexate administration to patients with presumed ectopic pregnancy leads to methotrexate exposure of intrauterine pregnancies. J Ultrasound Med. 2019;38:675-684.
- Nurmohamed L, Moretti ME, Schechter T, et al. Outcome following high-dose methotrexate in pregnancies misdiagnosed as ectopic. Am J Obstet Gynecol. 2011;205:533.e1-533.e3.
- Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104:50-55.
- Morse CB, Sammel MD, Shaunik A, et al. Performance of human chorionic gonadotropin curves in women at risk for ectopic pregnancy: exceptions to the rules. Fertil Steril. 2012;97:101-6.e2.
- Barnhart KT, Guo W, Cary MS, et al. Differences in serum human chorionic gonadotropin rise in early pregnancy by race and value at presentation. Obstet Gynecol. 2016;128:504-511.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5, pt 1):975-981.
- Butts SF, Guo W, Cary MS, et al. Predicting the decline in human chorionic gonadotropin in a resolving pregnancy of unknown location. Obstet Gynecol. 2013;122(2 pt 1):337-343.
- Verhaegen J, Gallos ID, van Mello NM, et al. Accuracy of single progesterone test to predict early pregnancy outcome in women with pain or bleeding: meta-analysis of cohort studies. BMJ. 2012;345:e6077.
- Senapati S, Sammel MD, Butts SF, et al. Predicting first trimester pregnancy outcome: derivation of a multiple marker test. Fertil Steril. 2016;106:1725-1732.e3.
- Refaat B, Bahathiq AO. The performances of serum activins and follistatin in the diagnosis of ectopic pregnancy: a prospective case-control study. Clin Chim Acta. 2020;500:69-74.
- Practice Committee of American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100:638-644.
- Doubilet PM, Benson CB. Further evidence against the reliability of the human chorionic gonadotropin discriminatory level. J Ultrasound Med. 2011;30:1637-1642.
- Desai D, Lu J, Wyness SP, et al. Human chorionic gonadotropin discriminatory zone in ectopic pregnancy: does assay harmonization matter? Fertil Steril. 2014;101:1671-1674.
- Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33:465-471.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Moschos E, Twickler DM. Endometrial thickness predicts intrauterine pregnancy in patients with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2008;32:929-934.
- Ellaithy M, Abdelaziz A, Hassan MF. Outcome prediction in pregnancies of unknown location using endometrial thickness measurement: is this of real clinical value? Eur J Obstet Gynecol Reprod Biol. 2013;168:68-74.
- Shaunik A, Kulp J, Appleby DH, et al. Utility of dilation and curettage in the diagnosis of pregnancy of unknown location. Am J Obstet Gynecol. 2011;204:130.e1-130.e6.
- Brady P, Imudia AN, Awonuga AO, et al. Pregnancies of unknown location after in vitro fertilization: minimally invasive management with Karman cannula aspiration. Fertil Steril. 2014;101:420-426.
- Barnhart KT, Gracia CR, Reindl B, et al. Usefulness of pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol. 2003;188:906-909.
- Insogna IG, Farland LV, Missmer SA, et al. Outpatient endometrial aspiration: an alternative to methotrexate for pregnancy of unknown location. Am J Obstet Gynecol. 2017;217:185.e1-185.e9.
- Parks MA, Barnhart KT, Howard DL. Trends in the management of nonviable pregnancies of unknown location in the United States. Gynecol Obstet Invest. 2018;83:552-557.
- Alur-Gupta S, Cooney LG, Senapati S, et al. Two-dose versus single-dose methotrexate for treatment of ectopic pregnancy: a meta-analysis. Am J Obstet Gynecol. 2019;221:95-108.e2.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2014;20:250-261.
- Kirk E, Condous G, Bourne T. Pregnancies of unknown location. Best Pract Res Clin Obstet Gynaecol. 2009;23:493-499.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Banerjee S, Aslam N, Zosmer N, et al. The expectant management of women with early pregnancy of unknown location. Ultrasound Obstet Gynecol. 1999;14:231-236.
- Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
- Mol BW, Hajenius PJ, Engelsbel S, et al. Serum human chorionic gonadotropin measurement in the diagnosis of ectopic pregnancy when transvaginal sonography is inconclusive. Fertil Steril. 1998;70:972-981.
- Kirk E, Condous G, Van Calster B, et al. Rationalizing the follow-up of pregnancies of unknown location. Hum Reprod. 2007;22:1744-1750.
- Stulberg DB, Cain LR, Dahlquist I, et al. Ectopic pregnancy rates and racial disparities in the Medicaid population, 2004-2008. Fertil Steril. 2014;102:1671-1676.
- Zeng MF, Li LM. Frozen blastocyst transfer reduces incidence of ectopic pregnancy compared with fresh blastocyst transfer: a meta-analysis. Gynecol Endocrinol. 2019;35:93-99.
- Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583-591.
- Barnhart KT. Ectopic pregnancy. N Engl J Med. 2009;361:379-387.
- Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373.
- Brady PC. Handbook of Consult and Inpatient Gynecology. Switzerland: Springer International Publishing; 2016.
- Fridman D, Hawkins E, Dar P, et al. Methotrexate administration to patients with presumed ectopic pregnancy leads to methotrexate exposure of intrauterine pregnancies. J Ultrasound Med. 2019;38:675-684.
- Nurmohamed L, Moretti ME, Schechter T, et al. Outcome following high-dose methotrexate in pregnancies misdiagnosed as ectopic. Am J Obstet Gynecol. 2011;205:533.e1-533.e3.
- Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104:50-55.
- Morse CB, Sammel MD, Shaunik A, et al. Performance of human chorionic gonadotropin curves in women at risk for ectopic pregnancy: exceptions to the rules. Fertil Steril. 2012;97:101-6.e2.
- Barnhart KT, Guo W, Cary MS, et al. Differences in serum human chorionic gonadotropin rise in early pregnancy by race and value at presentation. Obstet Gynecol. 2016;128:504-511.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5, pt 1):975-981.
- Butts SF, Guo W, Cary MS, et al. Predicting the decline in human chorionic gonadotropin in a resolving pregnancy of unknown location. Obstet Gynecol. 2013;122(2 pt 1):337-343.
- Verhaegen J, Gallos ID, van Mello NM, et al. Accuracy of single progesterone test to predict early pregnancy outcome in women with pain or bleeding: meta-analysis of cohort studies. BMJ. 2012;345:e6077.
- Senapati S, Sammel MD, Butts SF, et al. Predicting first trimester pregnancy outcome: derivation of a multiple marker test. Fertil Steril. 2016;106:1725-1732.e3.
- Refaat B, Bahathiq AO. The performances of serum activins and follistatin in the diagnosis of ectopic pregnancy: a prospective case-control study. Clin Chim Acta. 2020;500:69-74.
- Practice Committee of American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100:638-644.
- Doubilet PM, Benson CB. Further evidence against the reliability of the human chorionic gonadotropin discriminatory level. J Ultrasound Med. 2011;30:1637-1642.
- Desai D, Lu J, Wyness SP, et al. Human chorionic gonadotropin discriminatory zone in ectopic pregnancy: does assay harmonization matter? Fertil Steril. 2014;101:1671-1674.
- Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33:465-471.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Moschos E, Twickler DM. Endometrial thickness predicts intrauterine pregnancy in patients with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2008;32:929-934.
- Ellaithy M, Abdelaziz A, Hassan MF. Outcome prediction in pregnancies of unknown location using endometrial thickness measurement: is this of real clinical value? Eur J Obstet Gynecol Reprod Biol. 2013;168:68-74.
- Shaunik A, Kulp J, Appleby DH, et al. Utility of dilation and curettage in the diagnosis of pregnancy of unknown location. Am J Obstet Gynecol. 2011;204:130.e1-130.e6.
- Brady P, Imudia AN, Awonuga AO, et al. Pregnancies of unknown location after in vitro fertilization: minimally invasive management with Karman cannula aspiration. Fertil Steril. 2014;101:420-426.
- Barnhart KT, Gracia CR, Reindl B, et al. Usefulness of pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol. 2003;188:906-909.
- Insogna IG, Farland LV, Missmer SA, et al. Outpatient endometrial aspiration: an alternative to methotrexate for pregnancy of unknown location. Am J Obstet Gynecol. 2017;217:185.e1-185.e9.
- Parks MA, Barnhart KT, Howard DL. Trends in the management of nonviable pregnancies of unknown location in the United States. Gynecol Obstet Invest. 2018;83:552-557.
- Alur-Gupta S, Cooney LG, Senapati S, et al. Two-dose versus single-dose methotrexate for treatment of ectopic pregnancy: a meta-analysis. Am J Obstet Gynecol. 2019;221:95-108.e2.
Fetal movement education: Time to change the status quo
Every antepartum record, whether it is on paper or EMR, has a space asking whether the patient feels fetal movement at the visit. Every provider inherently knows that fetal movement is important and worth asking about at each visit. Yet the education for patients about fetal movement and when to alert a provider to changes is not currently standardized in the United States. There is no practice bulletin or guideline from the American College of Obstetricians and Gynecologists and, therefore, there is a wide variation in clinical practice. An Australian study found that 97% of women were asked about fetal movement, but only 62% reported formal education regarding fetal movement. More concerning, only 40% were advised to call immediately if concerned about fetal movement change. A quarter were told to call only if baby moved fewer than 10 times in an hour.1
We have a standardized approach to most aspects of prenatal care. We know what to do if the patient has contractions, or protein in their urine, or an increased blood pressure. Our management and education regarding fetal movement must be standardized as well. In this article I will go through the incorrect education that often is given and the data that do not support this. We need a similar care plan or model for fetal movement education in the United States.
Myth one: Kick counts
When education is done, kick counts are far and away what providers and nurses advise in the clinic and hospital triage when women present with complaint of decreased fetal movement. The standard approach to this is advising the patient to perform a kick count several times per day to check in on the baby and call if less than 10 kicks per hour. This is not bad advice as it may help create awareness for the mom about what is “normal” for her baby and may help her to “check in” on the baby when she is occupied at work or with older children. However, advising that a kick count should be done to reassure a patient about a concerning change in fetal movement is not supported in the literature. A meta-analysis in the February 2020 issue of the Green Journal found that advised kick count monitoring did not significantly reduce stillbirth risk.2 Research shows that most moms will get 10 kicks normally within an hour, but there are no data showing what percentage of moms with perceived decreased fetal movement also will get a “passing” result despite their concern. For example, take a patient who normally feels 50 movements in an hour and is not reassured by 10 movements in an hour, but because she is told that 10 movements is okay, she tries not to worry about the concerning change. Many mothers in the stillbirth community report “passing kick counts” in the days leading up to the diagnosis. We need to move away from kick count education to a much simpler plan. We must tell patients if they are worried about a concerning change in fetal movement, they should call their provider.
Myth 2: Fetuses slow down at the end of pregnancy
There is a very common myth that fetuses slow down at the end of pregnancy, especially once labor has started. A study in the Journal of Physiology continuously monitored term fetuses when mom was both awake and asleep. The study also looked at the effect on fetal heart rate and fetal activity based on different maternal positions. The study found the fetuses spent around 90% of the day with active movements and with reactive nonstress tests (NSTs).3 A 2019 study looking at fetal movement at term and preterm in third-trimester patients illustrated that fetal movement does not decrease in frequency or strength at term. It found that only 6% of patients noted decreased strength and 14% decreased frequency of movements at term. Furthermore, 59% reported an increase in strength, and nearly 39% reported an increase in frequency of fetal movements at term.4 We must educate patients that a change in frequency or strength of movements is not normal or expected, and they must call if concerned about a change.
Myth 3: Try juice, ice water, or food before coming in for evaluation
A common set of advice when a patient calls with a complaint of decreased fetal movement is to suggest a meal or something sugary, although there is little or no evidence to support this. A randomized controlled trial found maternal perception of increased fetal movement was similar among the two groups. Giving something sugary at NST also was not shown in this study to improve reactivity.5 Another randomized, double placebo blind study was done to answer the question of whether glucose via IV helped improve fetal movements and decreased the need for admission for induction or further monitoring. In this study, no difference in outcome is found.6
When a patient calls with decreased fetal movement, advice should be to come and be evaluated, not recommendation of measures like ice water, orange juice, or sugary meal because it is not supported by the literature. This incorrect message also may further the false impression that a baby who is not moving is most likely sleeping or is simply in need of sugar, not that the baby may be at risk for impending stillbirth. The Perinatal Society of Australia and New Zealand and Royal College of Obstetricians and Gynecologists have fetal movement protocol that both discourage this advice and encourage immediate evaluation of patients with complaint of concerning fetal movement change.7,8
Myth 4: An increase in fetal movement is not of concern
I used to believe that increased fetal movement is never of concern. However, the STARS study illustrated that a concerning increase in fetal movement often is noted just before the diagnosis of stillbirth. A single episode of excessively vigorous activity which often is described as frantic or crazy is associated with an odds ratio for stillbirth of 4.3. In the study, 30% of cases reported this, compared with 7% of controls.9 In our practice, we manage mothers who call with this concern the same way as a decreased fetal movement complaint, and bring the mother in immediately for evaluation.
Myth 5: Patients all know that a concerning change in fetal movement is a risk factor for stillbirth
Decreased fetal movement has been associated with an increased OR for stillbirth of 4.51.10 However, patients often do not know of this association. A study in the United States of providers and stillbirth families showed fear of anxiety kept providers from talking about stillbirth and that it still happens. Because of this patients were completely surprised by the diagnosis.11 We tell patients that stillbirth still happens because research by Dr Suzanne Pullen found that 77% of families said they never worried their baby could die outside of the first trimester. Our patients have received this information without increased anxiety and are very appreciative and reassured about the education and protocol (based on the U.K. Saving Babies Lives Care Bundle Version 2) that we have implemented in our practice.
Fact: Fetal movement education guidelines exist and are easy to implement
The practice I am a partner at has been using a formalized method for educating patients about fetal movement over the past year. As mentioned earlier the U.K. and Australia have formal fetal movement education and management guidelines.7,8 Both protocols encourage formal education around 20-24 weeks and education for the patient to call immediately with concerns; the patient should be evaluated within 2 hours of the complaint. The formal education we provide is quite simple. The Star Legacy Foundation (United States) and Still Aware (Australia) have created a simple card to educate patients.
These patient-centric materials were devised from the results of the case/control cohort STARS study by Heazell et al. The STARS study demonstrated that patient report of reduced fetal movement in the 2 weeks prior to loss was associated with an OR of 12.9 for stillbirth, that decreased strength of fetal movement was associated with stillbirth OR of 2.83, and that decreased night time activity was strongly associated with impending stillbirth (74% of cases felt their fetuses died at night).12 This card also addresses sleep position data, supported by a 2018 meta-analysis in the journal Sleep Medicine. The study identified an OR for stillbirth of 2.45 for supine sleepers with LGA or average sized babies. Furthermore, if the baby was SGA and the mother slept supine, the OR for stillbirth increased to 15.66.13
Conclusions
When I think about the patients I have cared for who have presented with a stillborn baby, I think often that they usually presented for a complaint other than decreased fetal movement such as labor check or routine prenatal visit. When asked when they last felt fetal movement they will often say days before. This does not need to happen. Protocols in Norway for fetal movement education have shown that patients call sooner with decreased fetal movement when they have received a formal education.14
Not all stillbirth can be prevented but proper education about fetal movement and not perpetuating dangerous myths about fetal movement, may keep presentations like this from happening. I hope we may soon have a formal protocol for fetal movement education, but until then, I hope some will take these educational tips to heart.
Dr. Heather Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, NY. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures.
References
1. Aust N Z J Obstet Gynaecol. 2012 Oct;52(5):445-9.
2. Obstet Gynecol. 2020 Feb;135(2):453-62.
3. J Physiol. 2017 Feb 15;595(4):1213-21.
4. PLOS One. 2019 Jun 12. doi: 10.1371/journal.pone.0217583.
5. J Matern Fetal Neonatal Med. 2013 Jun;26(9):915-9.
6. J Perinatol. 2016 Aug;36(8):598-600.
7. Aust N Z J Obstet Gynaecol. 2018 Aug;58(4):463-8.
8. Reduced fetal movements: Green top #57, Royal College of Obstetricians and Gynaecologists.
9. BMC Pregnancy Childb. 2017. doi: 10.1186/s12884-017-1555-6.
10. BMJ Open. 2018. doi: 10.1136/bmjopen-2017-020031.
11. BMC Pregnancy Childb. 2012. doi: 10.1186/1471-2393-12-137.
12. BMC Pregnancy Childb. 2015. doi: 10.1186/s12884-015-0602-4.
13. EClinicalMedicine. 2019 Apr. doi: 10.1016/j.eclinm.2019.03.014.
14. BMC Pregnancy Childb. 2009. doi: 10.1186/1471-2393-9-32.
Every antepartum record, whether it is on paper or EMR, has a space asking whether the patient feels fetal movement at the visit. Every provider inherently knows that fetal movement is important and worth asking about at each visit. Yet the education for patients about fetal movement and when to alert a provider to changes is not currently standardized in the United States. There is no practice bulletin or guideline from the American College of Obstetricians and Gynecologists and, therefore, there is a wide variation in clinical practice. An Australian study found that 97% of women were asked about fetal movement, but only 62% reported formal education regarding fetal movement. More concerning, only 40% were advised to call immediately if concerned about fetal movement change. A quarter were told to call only if baby moved fewer than 10 times in an hour.1
We have a standardized approach to most aspects of prenatal care. We know what to do if the patient has contractions, or protein in their urine, or an increased blood pressure. Our management and education regarding fetal movement must be standardized as well. In this article I will go through the incorrect education that often is given and the data that do not support this. We need a similar care plan or model for fetal movement education in the United States.
Myth one: Kick counts
When education is done, kick counts are far and away what providers and nurses advise in the clinic and hospital triage when women present with complaint of decreased fetal movement. The standard approach to this is advising the patient to perform a kick count several times per day to check in on the baby and call if less than 10 kicks per hour. This is not bad advice as it may help create awareness for the mom about what is “normal” for her baby and may help her to “check in” on the baby when she is occupied at work or with older children. However, advising that a kick count should be done to reassure a patient about a concerning change in fetal movement is not supported in the literature. A meta-analysis in the February 2020 issue of the Green Journal found that advised kick count monitoring did not significantly reduce stillbirth risk.2 Research shows that most moms will get 10 kicks normally within an hour, but there are no data showing what percentage of moms with perceived decreased fetal movement also will get a “passing” result despite their concern. For example, take a patient who normally feels 50 movements in an hour and is not reassured by 10 movements in an hour, but because she is told that 10 movements is okay, she tries not to worry about the concerning change. Many mothers in the stillbirth community report “passing kick counts” in the days leading up to the diagnosis. We need to move away from kick count education to a much simpler plan. We must tell patients if they are worried about a concerning change in fetal movement, they should call their provider.
Myth 2: Fetuses slow down at the end of pregnancy
There is a very common myth that fetuses slow down at the end of pregnancy, especially once labor has started. A study in the Journal of Physiology continuously monitored term fetuses when mom was both awake and asleep. The study also looked at the effect on fetal heart rate and fetal activity based on different maternal positions. The study found the fetuses spent around 90% of the day with active movements and with reactive nonstress tests (NSTs).3 A 2019 study looking at fetal movement at term and preterm in third-trimester patients illustrated that fetal movement does not decrease in frequency or strength at term. It found that only 6% of patients noted decreased strength and 14% decreased frequency of movements at term. Furthermore, 59% reported an increase in strength, and nearly 39% reported an increase in frequency of fetal movements at term.4 We must educate patients that a change in frequency or strength of movements is not normal or expected, and they must call if concerned about a change.
Myth 3: Try juice, ice water, or food before coming in for evaluation
A common set of advice when a patient calls with a complaint of decreased fetal movement is to suggest a meal or something sugary, although there is little or no evidence to support this. A randomized controlled trial found maternal perception of increased fetal movement was similar among the two groups. Giving something sugary at NST also was not shown in this study to improve reactivity.5 Another randomized, double placebo blind study was done to answer the question of whether glucose via IV helped improve fetal movements and decreased the need for admission for induction or further monitoring. In this study, no difference in outcome is found.6
When a patient calls with decreased fetal movement, advice should be to come and be evaluated, not recommendation of measures like ice water, orange juice, or sugary meal because it is not supported by the literature. This incorrect message also may further the false impression that a baby who is not moving is most likely sleeping or is simply in need of sugar, not that the baby may be at risk for impending stillbirth. The Perinatal Society of Australia and New Zealand and Royal College of Obstetricians and Gynecologists have fetal movement protocol that both discourage this advice and encourage immediate evaluation of patients with complaint of concerning fetal movement change.7,8
Myth 4: An increase in fetal movement is not of concern
I used to believe that increased fetal movement is never of concern. However, the STARS study illustrated that a concerning increase in fetal movement often is noted just before the diagnosis of stillbirth. A single episode of excessively vigorous activity which often is described as frantic or crazy is associated with an odds ratio for stillbirth of 4.3. In the study, 30% of cases reported this, compared with 7% of controls.9 In our practice, we manage mothers who call with this concern the same way as a decreased fetal movement complaint, and bring the mother in immediately for evaluation.
Myth 5: Patients all know that a concerning change in fetal movement is a risk factor for stillbirth
Decreased fetal movement has been associated with an increased OR for stillbirth of 4.51.10 However, patients often do not know of this association. A study in the United States of providers and stillbirth families showed fear of anxiety kept providers from talking about stillbirth and that it still happens. Because of this patients were completely surprised by the diagnosis.11 We tell patients that stillbirth still happens because research by Dr Suzanne Pullen found that 77% of families said they never worried their baby could die outside of the first trimester. Our patients have received this information without increased anxiety and are very appreciative and reassured about the education and protocol (based on the U.K. Saving Babies Lives Care Bundle Version 2) that we have implemented in our practice.
Fact: Fetal movement education guidelines exist and are easy to implement
The practice I am a partner at has been using a formalized method for educating patients about fetal movement over the past year. As mentioned earlier the U.K. and Australia have formal fetal movement education and management guidelines.7,8 Both protocols encourage formal education around 20-24 weeks and education for the patient to call immediately with concerns; the patient should be evaluated within 2 hours of the complaint. The formal education we provide is quite simple. The Star Legacy Foundation (United States) and Still Aware (Australia) have created a simple card to educate patients.
These patient-centric materials were devised from the results of the case/control cohort STARS study by Heazell et al. The STARS study demonstrated that patient report of reduced fetal movement in the 2 weeks prior to loss was associated with an OR of 12.9 for stillbirth, that decreased strength of fetal movement was associated with stillbirth OR of 2.83, and that decreased night time activity was strongly associated with impending stillbirth (74% of cases felt their fetuses died at night).12 This card also addresses sleep position data, supported by a 2018 meta-analysis in the journal Sleep Medicine. The study identified an OR for stillbirth of 2.45 for supine sleepers with LGA or average sized babies. Furthermore, if the baby was SGA and the mother slept supine, the OR for stillbirth increased to 15.66.13
Conclusions
When I think about the patients I have cared for who have presented with a stillborn baby, I think often that they usually presented for a complaint other than decreased fetal movement such as labor check or routine prenatal visit. When asked when they last felt fetal movement they will often say days before. This does not need to happen. Protocols in Norway for fetal movement education have shown that patients call sooner with decreased fetal movement when they have received a formal education.14
Not all stillbirth can be prevented but proper education about fetal movement and not perpetuating dangerous myths about fetal movement, may keep presentations like this from happening. I hope we may soon have a formal protocol for fetal movement education, but until then, I hope some will take these educational tips to heart.
Dr. Heather Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, NY. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures.
References
1. Aust N Z J Obstet Gynaecol. 2012 Oct;52(5):445-9.
2. Obstet Gynecol. 2020 Feb;135(2):453-62.
3. J Physiol. 2017 Feb 15;595(4):1213-21.
4. PLOS One. 2019 Jun 12. doi: 10.1371/journal.pone.0217583.
5. J Matern Fetal Neonatal Med. 2013 Jun;26(9):915-9.
6. J Perinatol. 2016 Aug;36(8):598-600.
7. Aust N Z J Obstet Gynaecol. 2018 Aug;58(4):463-8.
8. Reduced fetal movements: Green top #57, Royal College of Obstetricians and Gynaecologists.
9. BMC Pregnancy Childb. 2017. doi: 10.1186/s12884-017-1555-6.
10. BMJ Open. 2018. doi: 10.1136/bmjopen-2017-020031.
11. BMC Pregnancy Childb. 2012. doi: 10.1186/1471-2393-12-137.
12. BMC Pregnancy Childb. 2015. doi: 10.1186/s12884-015-0602-4.
13. EClinicalMedicine. 2019 Apr. doi: 10.1016/j.eclinm.2019.03.014.
14. BMC Pregnancy Childb. 2009. doi: 10.1186/1471-2393-9-32.
Every antepartum record, whether it is on paper or EMR, has a space asking whether the patient feels fetal movement at the visit. Every provider inherently knows that fetal movement is important and worth asking about at each visit. Yet the education for patients about fetal movement and when to alert a provider to changes is not currently standardized in the United States. There is no practice bulletin or guideline from the American College of Obstetricians and Gynecologists and, therefore, there is a wide variation in clinical practice. An Australian study found that 97% of women were asked about fetal movement, but only 62% reported formal education regarding fetal movement. More concerning, only 40% were advised to call immediately if concerned about fetal movement change. A quarter were told to call only if baby moved fewer than 10 times in an hour.1
We have a standardized approach to most aspects of prenatal care. We know what to do if the patient has contractions, or protein in their urine, or an increased blood pressure. Our management and education regarding fetal movement must be standardized as well. In this article I will go through the incorrect education that often is given and the data that do not support this. We need a similar care plan or model for fetal movement education in the United States.
Myth one: Kick counts
When education is done, kick counts are far and away what providers and nurses advise in the clinic and hospital triage when women present with complaint of decreased fetal movement. The standard approach to this is advising the patient to perform a kick count several times per day to check in on the baby and call if less than 10 kicks per hour. This is not bad advice as it may help create awareness for the mom about what is “normal” for her baby and may help her to “check in” on the baby when she is occupied at work or with older children. However, advising that a kick count should be done to reassure a patient about a concerning change in fetal movement is not supported in the literature. A meta-analysis in the February 2020 issue of the Green Journal found that advised kick count monitoring did not significantly reduce stillbirth risk.2 Research shows that most moms will get 10 kicks normally within an hour, but there are no data showing what percentage of moms with perceived decreased fetal movement also will get a “passing” result despite their concern. For example, take a patient who normally feels 50 movements in an hour and is not reassured by 10 movements in an hour, but because she is told that 10 movements is okay, she tries not to worry about the concerning change. Many mothers in the stillbirth community report “passing kick counts” in the days leading up to the diagnosis. We need to move away from kick count education to a much simpler plan. We must tell patients if they are worried about a concerning change in fetal movement, they should call their provider.
Myth 2: Fetuses slow down at the end of pregnancy
There is a very common myth that fetuses slow down at the end of pregnancy, especially once labor has started. A study in the Journal of Physiology continuously monitored term fetuses when mom was both awake and asleep. The study also looked at the effect on fetal heart rate and fetal activity based on different maternal positions. The study found the fetuses spent around 90% of the day with active movements and with reactive nonstress tests (NSTs).3 A 2019 study looking at fetal movement at term and preterm in third-trimester patients illustrated that fetal movement does not decrease in frequency or strength at term. It found that only 6% of patients noted decreased strength and 14% decreased frequency of movements at term. Furthermore, 59% reported an increase in strength, and nearly 39% reported an increase in frequency of fetal movements at term.4 We must educate patients that a change in frequency or strength of movements is not normal or expected, and they must call if concerned about a change.
Myth 3: Try juice, ice water, or food before coming in for evaluation
A common set of advice when a patient calls with a complaint of decreased fetal movement is to suggest a meal or something sugary, although there is little or no evidence to support this. A randomized controlled trial found maternal perception of increased fetal movement was similar among the two groups. Giving something sugary at NST also was not shown in this study to improve reactivity.5 Another randomized, double placebo blind study was done to answer the question of whether glucose via IV helped improve fetal movements and decreased the need for admission for induction or further monitoring. In this study, no difference in outcome is found.6
When a patient calls with decreased fetal movement, advice should be to come and be evaluated, not recommendation of measures like ice water, orange juice, or sugary meal because it is not supported by the literature. This incorrect message also may further the false impression that a baby who is not moving is most likely sleeping or is simply in need of sugar, not that the baby may be at risk for impending stillbirth. The Perinatal Society of Australia and New Zealand and Royal College of Obstetricians and Gynecologists have fetal movement protocol that both discourage this advice and encourage immediate evaluation of patients with complaint of concerning fetal movement change.7,8
Myth 4: An increase in fetal movement is not of concern
I used to believe that increased fetal movement is never of concern. However, the STARS study illustrated that a concerning increase in fetal movement often is noted just before the diagnosis of stillbirth. A single episode of excessively vigorous activity which often is described as frantic or crazy is associated with an odds ratio for stillbirth of 4.3. In the study, 30% of cases reported this, compared with 7% of controls.9 In our practice, we manage mothers who call with this concern the same way as a decreased fetal movement complaint, and bring the mother in immediately for evaluation.
Myth 5: Patients all know that a concerning change in fetal movement is a risk factor for stillbirth
Decreased fetal movement has been associated with an increased OR for stillbirth of 4.51.10 However, patients often do not know of this association. A study in the United States of providers and stillbirth families showed fear of anxiety kept providers from talking about stillbirth and that it still happens. Because of this patients were completely surprised by the diagnosis.11 We tell patients that stillbirth still happens because research by Dr Suzanne Pullen found that 77% of families said they never worried their baby could die outside of the first trimester. Our patients have received this information without increased anxiety and are very appreciative and reassured about the education and protocol (based on the U.K. Saving Babies Lives Care Bundle Version 2) that we have implemented in our practice.
Fact: Fetal movement education guidelines exist and are easy to implement
The practice I am a partner at has been using a formalized method for educating patients about fetal movement over the past year. As mentioned earlier the U.K. and Australia have formal fetal movement education and management guidelines.7,8 Both protocols encourage formal education around 20-24 weeks and education for the patient to call immediately with concerns; the patient should be evaluated within 2 hours of the complaint. The formal education we provide is quite simple. The Star Legacy Foundation (United States) and Still Aware (Australia) have created a simple card to educate patients.
These patient-centric materials were devised from the results of the case/control cohort STARS study by Heazell et al. The STARS study demonstrated that patient report of reduced fetal movement in the 2 weeks prior to loss was associated with an OR of 12.9 for stillbirth, that decreased strength of fetal movement was associated with stillbirth OR of 2.83, and that decreased night time activity was strongly associated with impending stillbirth (74% of cases felt their fetuses died at night).12 This card also addresses sleep position data, supported by a 2018 meta-analysis in the journal Sleep Medicine. The study identified an OR for stillbirth of 2.45 for supine sleepers with LGA or average sized babies. Furthermore, if the baby was SGA and the mother slept supine, the OR for stillbirth increased to 15.66.13
Conclusions
When I think about the patients I have cared for who have presented with a stillborn baby, I think often that they usually presented for a complaint other than decreased fetal movement such as labor check or routine prenatal visit. When asked when they last felt fetal movement they will often say days before. This does not need to happen. Protocols in Norway for fetal movement education have shown that patients call sooner with decreased fetal movement when they have received a formal education.14
Not all stillbirth can be prevented but proper education about fetal movement and not perpetuating dangerous myths about fetal movement, may keep presentations like this from happening. I hope we may soon have a formal protocol for fetal movement education, but until then, I hope some will take these educational tips to heart.
Dr. Heather Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, NY. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures.
References
1. Aust N Z J Obstet Gynaecol. 2012 Oct;52(5):445-9.
2. Obstet Gynecol. 2020 Feb;135(2):453-62.
3. J Physiol. 2017 Feb 15;595(4):1213-21.
4. PLOS One. 2019 Jun 12. doi: 10.1371/journal.pone.0217583.
5. J Matern Fetal Neonatal Med. 2013 Jun;26(9):915-9.
6. J Perinatol. 2016 Aug;36(8):598-600.
7. Aust N Z J Obstet Gynaecol. 2018 Aug;58(4):463-8.
8. Reduced fetal movements: Green top #57, Royal College of Obstetricians and Gynaecologists.
9. BMC Pregnancy Childb. 2017. doi: 10.1186/s12884-017-1555-6.
10. BMJ Open. 2018. doi: 10.1136/bmjopen-2017-020031.
11. BMC Pregnancy Childb. 2012. doi: 10.1186/1471-2393-12-137.
12. BMC Pregnancy Childb. 2015. doi: 10.1186/s12884-015-0602-4.
13. EClinicalMedicine. 2019 Apr. doi: 10.1016/j.eclinm.2019.03.014.
14. BMC Pregnancy Childb. 2009. doi: 10.1186/1471-2393-9-32.
Health disparity: Race, mortality, and infants of teenage mothers
according to a new analysis from the National Center for Health Statistics.

In 2017-2018, overall mortality rates were 12.5 per 100,000 live births for infants born to Black mothers aged 15-19 years, 8.4 per 100,000 for infants born to White teenagers, and 6.5 per 100,000 for those born to Hispanic teens, Ashley M. Woodall, MPH, and Anne K. Driscoll, PhD, of the NCHS said in a data brief.
Looking at the five leading causes of those deaths shows that deaths of Black infants were the highest by significant margins in four, although, when it comes to “disorders related to short gestation and low birth weight,” significant may be an understatement.
The rate of preterm/low-birth-weight deaths for white infants in 2017-2018 was 119 per 100,000 live births; for Hispanic infants it was 94 per 100,000. Among infants born to Black teenagers, however, it was 284 deaths per 100,000, they reported based on data from the National Vital Statistics System’s linked birth/infant death file.
The numbers for congenital malformations and accidents were closer but still significantly different, and with each of the three most common causes, the rates for infants of Hispanic mothers also were significantly lower than those of White infants, the researchers said.
The situation changes for mortality-cause No. 4, sudden infant death syndrome, which was significantly more common among infants born to White teenagers, with a rate of 91 deaths per 100,000 live births, compared with either black (77) or Hispanic (44) infants, Ms. Woodall and Dr. Driscoll said.
Infants born to Black teens had the highest death rate again (68 per 100,000) for maternal complications of pregnancy, the fifth-leading cause of mortality, but for the first time Hispanic infants had a higher rate (36) than did those of White teenagers (29), they reported.
according to a new analysis from the National Center for Health Statistics.

In 2017-2018, overall mortality rates were 12.5 per 100,000 live births for infants born to Black mothers aged 15-19 years, 8.4 per 100,000 for infants born to White teenagers, and 6.5 per 100,000 for those born to Hispanic teens, Ashley M. Woodall, MPH, and Anne K. Driscoll, PhD, of the NCHS said in a data brief.
Looking at the five leading causes of those deaths shows that deaths of Black infants were the highest by significant margins in four, although, when it comes to “disorders related to short gestation and low birth weight,” significant may be an understatement.
The rate of preterm/low-birth-weight deaths for white infants in 2017-2018 was 119 per 100,000 live births; for Hispanic infants it was 94 per 100,000. Among infants born to Black teenagers, however, it was 284 deaths per 100,000, they reported based on data from the National Vital Statistics System’s linked birth/infant death file.
The numbers for congenital malformations and accidents were closer but still significantly different, and with each of the three most common causes, the rates for infants of Hispanic mothers also were significantly lower than those of White infants, the researchers said.
The situation changes for mortality-cause No. 4, sudden infant death syndrome, which was significantly more common among infants born to White teenagers, with a rate of 91 deaths per 100,000 live births, compared with either black (77) or Hispanic (44) infants, Ms. Woodall and Dr. Driscoll said.
Infants born to Black teens had the highest death rate again (68 per 100,000) for maternal complications of pregnancy, the fifth-leading cause of mortality, but for the first time Hispanic infants had a higher rate (36) than did those of White teenagers (29), they reported.
according to a new analysis from the National Center for Health Statistics.

In 2017-2018, overall mortality rates were 12.5 per 100,000 live births for infants born to Black mothers aged 15-19 years, 8.4 per 100,000 for infants born to White teenagers, and 6.5 per 100,000 for those born to Hispanic teens, Ashley M. Woodall, MPH, and Anne K. Driscoll, PhD, of the NCHS said in a data brief.
Looking at the five leading causes of those deaths shows that deaths of Black infants were the highest by significant margins in four, although, when it comes to “disorders related to short gestation and low birth weight,” significant may be an understatement.
The rate of preterm/low-birth-weight deaths for white infants in 2017-2018 was 119 per 100,000 live births; for Hispanic infants it was 94 per 100,000. Among infants born to Black teenagers, however, it was 284 deaths per 100,000, they reported based on data from the National Vital Statistics System’s linked birth/infant death file.
The numbers for congenital malformations and accidents were closer but still significantly different, and with each of the three most common causes, the rates for infants of Hispanic mothers also were significantly lower than those of White infants, the researchers said.
The situation changes for mortality-cause No. 4, sudden infant death syndrome, which was significantly more common among infants born to White teenagers, with a rate of 91 deaths per 100,000 live births, compared with either black (77) or Hispanic (44) infants, Ms. Woodall and Dr. Driscoll said.
Infants born to Black teens had the highest death rate again (68 per 100,000) for maternal complications of pregnancy, the fifth-leading cause of mortality, but for the first time Hispanic infants had a higher rate (36) than did those of White teenagers (29), they reported.
Vaccines for maternal and fetal health
Biomedical science is ever changing, and what may be believed in one era – for instance, bloodletting can cure disease or lobotomies can treat psychiatric disorders – may not be accepted in the next. However, one medical advance stands out in terms of maintaining and sustaining our health: vaccines. The data comparing morbidity and mortality before and after widespread vaccination are staggering. Before the smallpox vaccine, nearly 49,000 people were infected and more than 1,500 died annually from smallpox; by 1977, the vaccine eradicated the disease in the United States.1 Polio caused paralytic disease in more than 16,000 people per year in the United States, including, perhaps most famously, President Franklin Roosevelt. After development of the polio vaccine, cases and deaths dropped to zero.2
Despite the evidence indicating the effectiveness of vaccines to reduce disease and death, rates of vaccination in the United States remain low among adults, ranging from about 23% for pneumococcal disease to 45% for seasonal influenza.3 Childhood immunization in 2017 hovered around 70% for those receiving all the recommended vaccines.4 Clearly there is room for improvement.
A woman’s ob.gyn. may be the only medical professional she sees regularly, and her annual well visit may be the only time she receives information regarding her weight and blood pressure, or reviews her current medications. For women who are planning pregnancy, pregnant, or post partum, ob.gyn. consultations present unique opportunities to increase patient engagement in healthy behaviors, such as diet, exercise, and regular sleep, because women are highly motivated to do what is best for their babies.
Immunization during pregnancy not only reduces the mother’s risk of severe disease, which can lead to complications, defects, and fetal or perinatal death, but also has been shown to improve the neonate’s ability to fight infection and may reduce vertical transmission of certain diseases. In this era of COVID-19 where we have no vaccine but we have evidence that pregnant women may be at greater risk for severe disease,5 routine immunizations are vital to maternal and fetal health.
We have invited Laura E. Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York, to address the importance of vaccination and the role of the ob.gyn. in advocating for this life-saving preventive health measure. Dr. Riley disclosed she is an author for Up to Date and was a consultant to GlaxoSmithKline about a cytomegalovirus vaccine.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore County, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
References
1. MMWR Morb Mortal Wkly Rep. 1999 Apr 2;48(12);243-8.
2. JAMA. 2007 Nov 14;298(18):2155-63.
3. MMWR Morb Mortal Wkly Rep. 2017 May 5;66(11);1-28.
4. CDC National Center for Health Statistics FastStats on Immunization.
5. MMWR Morb Mortal Wkly Rep. 2020 Jun 26;69(25);769-75.
Biomedical science is ever changing, and what may be believed in one era – for instance, bloodletting can cure disease or lobotomies can treat psychiatric disorders – may not be accepted in the next. However, one medical advance stands out in terms of maintaining and sustaining our health: vaccines. The data comparing morbidity and mortality before and after widespread vaccination are staggering. Before the smallpox vaccine, nearly 49,000 people were infected and more than 1,500 died annually from smallpox; by 1977, the vaccine eradicated the disease in the United States.1 Polio caused paralytic disease in more than 16,000 people per year in the United States, including, perhaps most famously, President Franklin Roosevelt. After development of the polio vaccine, cases and deaths dropped to zero.2
Despite the evidence indicating the effectiveness of vaccines to reduce disease and death, rates of vaccination in the United States remain low among adults, ranging from about 23% for pneumococcal disease to 45% for seasonal influenza.3 Childhood immunization in 2017 hovered around 70% for those receiving all the recommended vaccines.4 Clearly there is room for improvement.
A woman’s ob.gyn. may be the only medical professional she sees regularly, and her annual well visit may be the only time she receives information regarding her weight and blood pressure, or reviews her current medications. For women who are planning pregnancy, pregnant, or post partum, ob.gyn. consultations present unique opportunities to increase patient engagement in healthy behaviors, such as diet, exercise, and regular sleep, because women are highly motivated to do what is best for their babies.
Immunization during pregnancy not only reduces the mother’s risk of severe disease, which can lead to complications, defects, and fetal or perinatal death, but also has been shown to improve the neonate’s ability to fight infection and may reduce vertical transmission of certain diseases. In this era of COVID-19 where we have no vaccine but we have evidence that pregnant women may be at greater risk for severe disease,5 routine immunizations are vital to maternal and fetal health.
We have invited Laura E. Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York, to address the importance of vaccination and the role of the ob.gyn. in advocating for this life-saving preventive health measure. Dr. Riley disclosed she is an author for Up to Date and was a consultant to GlaxoSmithKline about a cytomegalovirus vaccine.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore County, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
References
1. MMWR Morb Mortal Wkly Rep. 1999 Apr 2;48(12);243-8.
2. JAMA. 2007 Nov 14;298(18):2155-63.
3. MMWR Morb Mortal Wkly Rep. 2017 May 5;66(11);1-28.
4. CDC National Center for Health Statistics FastStats on Immunization.
5. MMWR Morb Mortal Wkly Rep. 2020 Jun 26;69(25);769-75.
Biomedical science is ever changing, and what may be believed in one era – for instance, bloodletting can cure disease or lobotomies can treat psychiatric disorders – may not be accepted in the next. However, one medical advance stands out in terms of maintaining and sustaining our health: vaccines. The data comparing morbidity and mortality before and after widespread vaccination are staggering. Before the smallpox vaccine, nearly 49,000 people were infected and more than 1,500 died annually from smallpox; by 1977, the vaccine eradicated the disease in the United States.1 Polio caused paralytic disease in more than 16,000 people per year in the United States, including, perhaps most famously, President Franklin Roosevelt. After development of the polio vaccine, cases and deaths dropped to zero.2
Despite the evidence indicating the effectiveness of vaccines to reduce disease and death, rates of vaccination in the United States remain low among adults, ranging from about 23% for pneumococcal disease to 45% for seasonal influenza.3 Childhood immunization in 2017 hovered around 70% for those receiving all the recommended vaccines.4 Clearly there is room for improvement.
A woman’s ob.gyn. may be the only medical professional she sees regularly, and her annual well visit may be the only time she receives information regarding her weight and blood pressure, or reviews her current medications. For women who are planning pregnancy, pregnant, or post partum, ob.gyn. consultations present unique opportunities to increase patient engagement in healthy behaviors, such as diet, exercise, and regular sleep, because women are highly motivated to do what is best for their babies.
Immunization during pregnancy not only reduces the mother’s risk of severe disease, which can lead to complications, defects, and fetal or perinatal death, but also has been shown to improve the neonate’s ability to fight infection and may reduce vertical transmission of certain diseases. In this era of COVID-19 where we have no vaccine but we have evidence that pregnant women may be at greater risk for severe disease,5 routine immunizations are vital to maternal and fetal health.
We have invited Laura E. Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York, to address the importance of vaccination and the role of the ob.gyn. in advocating for this life-saving preventive health measure. Dr. Riley disclosed she is an author for Up to Date and was a consultant to GlaxoSmithKline about a cytomegalovirus vaccine.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore County, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
References
1. MMWR Morb Mortal Wkly Rep. 1999 Apr 2;48(12);243-8.
2. JAMA. 2007 Nov 14;298(18):2155-63.
3. MMWR Morb Mortal Wkly Rep. 2017 May 5;66(11);1-28.
4. CDC National Center for Health Statistics FastStats on Immunization.
5. MMWR Morb Mortal Wkly Rep. 2020 Jun 26;69(25);769-75.
Maternal immunization is a priority
Maternal immunization remains a priority for ob.gyns. – an opportunity to provide protection against serious infectious diseases for both the mother and the baby. With influenza vaccination rates in pregnant women still hovering around 50% and the emerging public health problem of vaccine hesitancy, we must fully embrace our responsibility to recommend immunizations and to effectively communicate what is known about their efficacy and safety. Ideally, we should offer them as well.
One reason for the low rates of influenza vaccination – one of the two vaccinations routinely recommended for all pregnant women in the United States – is that pregnant women do not always know the importance of the vaccine. This is actionable: Data clearly show that the physician’s recommendation makes a difference and that a clinician’s offer to administer the vaccination has an even greater impact.
A 2017 Centers for Disease Control and Prevention analysis of data from Internet panel surveys1 shows that women who reported receiving both a clinician recommendation and offer of vaccination had higher coverage during the 2015-2016 and 2016-2017 influenza seasons (63.7% and 70.5%) than did women who reported receiving a clinician recommendation but no offer (37.5% and 43.7%) and women who reported receiving no recommendation for vaccination (12.8% and 14.8%).
The analysis suggests there are consistently missed opportunities: Fewer than 70% (67.3%) of pregnant women in the 2016-2017 flu season reported receiving a clinician recommendation for and offer of vaccination. This is similar to the prior three flu seasons, according to the CDC.
This year, with the COVID-19 pandemic ensuing, the prevention of severe influenza illness – and other vaccine-preventable illnesses – takes on even greater importance. It is not known what the impact of two potentially devastating respiratory infections could be for pregnant individuals. Therefore, maximal protection against at least influenza will be critical.
Influenza and Tdap
Poor outcomes and disproportionately high death rates for pregnant women were observed in both the influenza pandemic of 1918-1919 and the 1957 “Asian flu” pandemic. Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), but it was the H1N1 influenza pandemic of 2009 that reinforced its value and led our field to more fully embrace influenza vaccination as a priority for prenatal care.
Surprisingly, most of the pregnant women who became severely ill from the H1N1 virus were young and healthy and did not have a coexisting condition known to increase risk, such as asthma or diabetes. In an analysis of California epidemiologic data, 2 only one-third of 94 pregnant women who were hospitalized with 2009 H1N1 influenza had established risk factors for complications from influenza, compared with almost two-thirds of nonpregnant women of reproductive age.
Nationally, 75 deaths of pregnant women were confirmed as because of H1N1 and 34 were possibly related to H1N1, most of which (64.3%) occurred in the third trimester.3 Records of the 1957 pandemic similarly show that pregnant women in the second and third trimesters were particularly affected.
That healthy pregnant women became so ill during the H1N1 pandemic raised several flags. For one, it became clearer that pregnancy is its own significant risk factor for severe illness from the influenza virus. Physiological changes believed to make a pregnant woman more susceptible to becoming ill include decreased lung capacity, increased nasal congestion, reduced colloid oncotic pressure, and changes in the immune system. The morbidity and mortality from H1N1 influenza also increased our drive as a specialty to convince women that vaccination is an important strategy in each influenza season.
The flu vaccine can be administered at any point during pregnancy. There is no evidence that the safety profile is any different during one trimester than another.
Patients should be reassured that vaccines recommended in pregnancy have undergone rigorous testing and that the influenza vaccine has been given to millions of pregnant women over decades. They also should understand that contracting influenza has risks for the fetus; research has demonstrated that pregnant women who contract influenza are at greater risk of spontaneous abortion as well as preterm birth and low birth weight.4
In addition, the issue of flu vaccine efficacy needs to be properly teased apart. Women read every year that the vaccine is not effective, so we need to discuss with patients what efficacy means. Does the vaccine prevent illness altogether, or does it prevent severe illness? For the most part, whereas influenza vaccines often do not offer an exact match for the year’s circulating strains – and therefore may not prevent all illness – data show that the vaccine can prevent severe illness.5 That is a worthy outcome.
Also worthy is the impact of influenza vaccination on the newborn. That maternal immunization also protects the baby – it can reduce the risk for influenza in infants under 6 months of age – is underappreciated and should be part of patient counseling. There is clear evidence that maternal immunization boosts the concentration of maternal antibodies that can cross the placenta and that infants benefit from this passive antibody protection.6
The Tdap vaccine (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis), the second vaccine routinely recommended during each pregnancy, is administered as early as possible during the third trimester precisely for this reason – to boost maternal immune response and maximize the passive transfer of antibodies to the newborn. The target is the prevention of pertussis and associated hospitalizations and death during the first 2 months of life in an era when sporadic and unpredictable outbreaks of the infection are occurring.
Data from the CDC of morbidity and mortality from pertussis in children (2001-2011) prior to routine maternal vaccination show that the highest rates of pediatric hospitalizations and deaths occurred in newborns. Research has demonstrated that the Tdap vaccine is highly effective in preventing infections and hospitalizations in newborns: Case-control and cohort studies in the United Kingdom7,8 have shown vaccine effectiveness of 91%-93%, and similar research9 done in the U.S. has demonstrated effectiveness of 78%-85%.
The Tdap vaccine is recommended for pregnant women at 27-36 weeks of gestation – in each pregnancy. The reason for revaccination with each pregnancy is that antibody levels do not remain high for too long; at 8 months post immunization, research has shown, maternal antibody levels have begun to wane.
The vaccine also is recommended for all individuals who will be in close contact with infants younger than 12 months (for example, parents, grandparents, and child-care providers) and who have not previously received it. However, “cocooning” the newborn is effective only when the mother also is immunized – a point that ob.gyns. need to better explain to their patients so that they understand the purpose of this strategy.
Other vaccines in pregnancy and post partum
As described in the American College of Obstetricians and Gynecologists’ committee opinion on maternal immunization, 4 it is the responsibility of the ob.gyn. or obstetric care provider to routinely assess the immunization status of every pregnant patient and recommend additional vaccines for those patients who have conditions or social/behavioral practices that put them at higher risk of acquiring vaccine-preventable diseases.
Patients who have asthma or diabetes, who smoke, or who have never been vaccinated for the prevention of pneumococcal disease should receive the PPV23 pneumococcal vaccine, for instance. For pregnant women with immune deficiencies such as HIV, the PCV13 vaccine followed by PPV23 is recommended. There are approximately 500,000 cases of invasive pneumococcal disease in the United States each year, resulting in 40,000 deaths, and many multidrug-resistant strains of Streptococcus pneumoniae.
Hepatitis A and B vaccines – both recombinant vaccines with no safety concerns – also can be given during pregnancy and are officially recommended for women who have high-risk exposures. In the case of hepatitis A, high risk entails traveling to countries where the disease is endemic. High-risk behavior for hepatitis B includes sex work or being the household contact or sexual partner of a person positive for hepatitis B surface antigen.
Other travel-related vaccines, such as Japanese encephalitis, yellow fever, smallpox, and inactivated polio vaccine, can be considered in pregnancy, but decisions should be driven by more in-depth conversations about potential risks and benefits. Unlike for other vaccinations, there are limited data on the safety of travel-related immunizations in pregnancy. Sometimes, the question of whether travel is advisable in the middle of pregnancy – whether potential risks are worth taking – is a valid question to pose in conversations with patients.
Standard obstetric practice includes assessment of rubella susceptibility at the beginning of pregnancy. In some locations such as New York, measles susceptibility is also routinely evaluated. After delivery, seronegative women should be vaccinated with MMR (measles, mumps, and rubella) vaccine prior to discharge. In recent years, with the growing problem of vaccine refusal and an increasingly mobile and global society, we’re seeing sporadic outbreaks of measles and rubella – diseases that were once eradicated.
Measles in particular is highly contagious and requires a herd immunity threshold of 92%-94% to prevent sustained spread of the disease. Postpartum immunization has important maternal and pediatric implications for subsequent pregnancies, before which vaccination is often missed.
Both the MMR vaccine and the varicella vaccine (another vaccine that can be initiated post partum) are live vaccines and therefore contraindicated during pregnancy but should be administered post partum, including to people who are breastfeeding.
Other immunizations that hold some promise to protect either the mother or fetus/neonate or both are in various stages of development or testing. These include vaccines for cytomegalovirus, malaria, respiratory syncytial virus, and group B streptococcus.
A word about COVID-19
In mid-July there were more than 120 vaccine candidates for COVID-19 in various phases of study and a host of questions. Will a vaccine be efficacious? Will it prevent severe illness, or illness altogether? And will it be safe for pregnant women?
Vaccines work by manipulating the immune system, and it is important to appreciate the possibility that there may be unique pregnancy-related issues to consider with future COVID-19 vaccines – issues that could influence the effectiveness, safety, and timing of vaccination – and to understand that with any new immunization, there will likely be reluctance on the part of pregnant women who routinely prioritize fetal safety over their own health.
Pregnant women have been excluded from COVID-19 vaccine trials, but there may come a time when experts decide that a vaccine against COVID-19 is beneficial in pregnancy. Thus far, we know that the disease is clearly different from influenza. A growing knowledge of the impact of COVID-19 on the health of pregnant women, particularly the risk of developing severe illness, will be important for the future of COVID-19 immunization, as many women will not want to accept any potential risk of a vaccine unless they believe there is a significant benefit.
References
1. MMWR Morb Mortal Wkly Rep. 2017 Sep 29;66(38):1016-22.
2. N Engl J Med. 2010 Jan 7;362(1):27-35.
3. Obstet Gynecol. 2015 Sep;126(3):486-90.
4. Obstet Gynecol. 2018 Jun;131(6):e214-e217.
5. MMWR Morb Mortal Wkly Rep. 2019 Feb 15;68(6):135-9.
6. Obstet Gynecol. 2019 Apr;133(4):739-53.
7. Lancet. 2014 Oct 25;384(9953):1521-8.
8. Clin Infect Dis. 2015 Feb 1;60(3):333-7.
9. Clin Infect Dis. 2017 Jan 1;64(1):9-14.
Maternal immunization remains a priority for ob.gyns. – an opportunity to provide protection against serious infectious diseases for both the mother and the baby. With influenza vaccination rates in pregnant women still hovering around 50% and the emerging public health problem of vaccine hesitancy, we must fully embrace our responsibility to recommend immunizations and to effectively communicate what is known about their efficacy and safety. Ideally, we should offer them as well.
One reason for the low rates of influenza vaccination – one of the two vaccinations routinely recommended for all pregnant women in the United States – is that pregnant women do not always know the importance of the vaccine. This is actionable: Data clearly show that the physician’s recommendation makes a difference and that a clinician’s offer to administer the vaccination has an even greater impact.
A 2017 Centers for Disease Control and Prevention analysis of data from Internet panel surveys1 shows that women who reported receiving both a clinician recommendation and offer of vaccination had higher coverage during the 2015-2016 and 2016-2017 influenza seasons (63.7% and 70.5%) than did women who reported receiving a clinician recommendation but no offer (37.5% and 43.7%) and women who reported receiving no recommendation for vaccination (12.8% and 14.8%).
The analysis suggests there are consistently missed opportunities: Fewer than 70% (67.3%) of pregnant women in the 2016-2017 flu season reported receiving a clinician recommendation for and offer of vaccination. This is similar to the prior three flu seasons, according to the CDC.
This year, with the COVID-19 pandemic ensuing, the prevention of severe influenza illness – and other vaccine-preventable illnesses – takes on even greater importance. It is not known what the impact of two potentially devastating respiratory infections could be for pregnant individuals. Therefore, maximal protection against at least influenza will be critical.
Influenza and Tdap
Poor outcomes and disproportionately high death rates for pregnant women were observed in both the influenza pandemic of 1918-1919 and the 1957 “Asian flu” pandemic. Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), but it was the H1N1 influenza pandemic of 2009 that reinforced its value and led our field to more fully embrace influenza vaccination as a priority for prenatal care.
Surprisingly, most of the pregnant women who became severely ill from the H1N1 virus were young and healthy and did not have a coexisting condition known to increase risk, such as asthma or diabetes. In an analysis of California epidemiologic data, 2 only one-third of 94 pregnant women who were hospitalized with 2009 H1N1 influenza had established risk factors for complications from influenza, compared with almost two-thirds of nonpregnant women of reproductive age.
Nationally, 75 deaths of pregnant women were confirmed as because of H1N1 and 34 were possibly related to H1N1, most of which (64.3%) occurred in the third trimester.3 Records of the 1957 pandemic similarly show that pregnant women in the second and third trimesters were particularly affected.
That healthy pregnant women became so ill during the H1N1 pandemic raised several flags. For one, it became clearer that pregnancy is its own significant risk factor for severe illness from the influenza virus. Physiological changes believed to make a pregnant woman more susceptible to becoming ill include decreased lung capacity, increased nasal congestion, reduced colloid oncotic pressure, and changes in the immune system. The morbidity and mortality from H1N1 influenza also increased our drive as a specialty to convince women that vaccination is an important strategy in each influenza season.
The flu vaccine can be administered at any point during pregnancy. There is no evidence that the safety profile is any different during one trimester than another.
Patients should be reassured that vaccines recommended in pregnancy have undergone rigorous testing and that the influenza vaccine has been given to millions of pregnant women over decades. They also should understand that contracting influenza has risks for the fetus; research has demonstrated that pregnant women who contract influenza are at greater risk of spontaneous abortion as well as preterm birth and low birth weight.4
In addition, the issue of flu vaccine efficacy needs to be properly teased apart. Women read every year that the vaccine is not effective, so we need to discuss with patients what efficacy means. Does the vaccine prevent illness altogether, or does it prevent severe illness? For the most part, whereas influenza vaccines often do not offer an exact match for the year’s circulating strains – and therefore may not prevent all illness – data show that the vaccine can prevent severe illness.5 That is a worthy outcome.
Also worthy is the impact of influenza vaccination on the newborn. That maternal immunization also protects the baby – it can reduce the risk for influenza in infants under 6 months of age – is underappreciated and should be part of patient counseling. There is clear evidence that maternal immunization boosts the concentration of maternal antibodies that can cross the placenta and that infants benefit from this passive antibody protection.6
The Tdap vaccine (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis), the second vaccine routinely recommended during each pregnancy, is administered as early as possible during the third trimester precisely for this reason – to boost maternal immune response and maximize the passive transfer of antibodies to the newborn. The target is the prevention of pertussis and associated hospitalizations and death during the first 2 months of life in an era when sporadic and unpredictable outbreaks of the infection are occurring.
Data from the CDC of morbidity and mortality from pertussis in children (2001-2011) prior to routine maternal vaccination show that the highest rates of pediatric hospitalizations and deaths occurred in newborns. Research has demonstrated that the Tdap vaccine is highly effective in preventing infections and hospitalizations in newborns: Case-control and cohort studies in the United Kingdom7,8 have shown vaccine effectiveness of 91%-93%, and similar research9 done in the U.S. has demonstrated effectiveness of 78%-85%.
The Tdap vaccine is recommended for pregnant women at 27-36 weeks of gestation – in each pregnancy. The reason for revaccination with each pregnancy is that antibody levels do not remain high for too long; at 8 months post immunization, research has shown, maternal antibody levels have begun to wane.
The vaccine also is recommended for all individuals who will be in close contact with infants younger than 12 months (for example, parents, grandparents, and child-care providers) and who have not previously received it. However, “cocooning” the newborn is effective only when the mother also is immunized – a point that ob.gyns. need to better explain to their patients so that they understand the purpose of this strategy.
Other vaccines in pregnancy and post partum
As described in the American College of Obstetricians and Gynecologists’ committee opinion on maternal immunization, 4 it is the responsibility of the ob.gyn. or obstetric care provider to routinely assess the immunization status of every pregnant patient and recommend additional vaccines for those patients who have conditions or social/behavioral practices that put them at higher risk of acquiring vaccine-preventable diseases.
Patients who have asthma or diabetes, who smoke, or who have never been vaccinated for the prevention of pneumococcal disease should receive the PPV23 pneumococcal vaccine, for instance. For pregnant women with immune deficiencies such as HIV, the PCV13 vaccine followed by PPV23 is recommended. There are approximately 500,000 cases of invasive pneumococcal disease in the United States each year, resulting in 40,000 deaths, and many multidrug-resistant strains of Streptococcus pneumoniae.
Hepatitis A and B vaccines – both recombinant vaccines with no safety concerns – also can be given during pregnancy and are officially recommended for women who have high-risk exposures. In the case of hepatitis A, high risk entails traveling to countries where the disease is endemic. High-risk behavior for hepatitis B includes sex work or being the household contact or sexual partner of a person positive for hepatitis B surface antigen.
Other travel-related vaccines, such as Japanese encephalitis, yellow fever, smallpox, and inactivated polio vaccine, can be considered in pregnancy, but decisions should be driven by more in-depth conversations about potential risks and benefits. Unlike for other vaccinations, there are limited data on the safety of travel-related immunizations in pregnancy. Sometimes, the question of whether travel is advisable in the middle of pregnancy – whether potential risks are worth taking – is a valid question to pose in conversations with patients.
Standard obstetric practice includes assessment of rubella susceptibility at the beginning of pregnancy. In some locations such as New York, measles susceptibility is also routinely evaluated. After delivery, seronegative women should be vaccinated with MMR (measles, mumps, and rubella) vaccine prior to discharge. In recent years, with the growing problem of vaccine refusal and an increasingly mobile and global society, we’re seeing sporadic outbreaks of measles and rubella – diseases that were once eradicated.
Measles in particular is highly contagious and requires a herd immunity threshold of 92%-94% to prevent sustained spread of the disease. Postpartum immunization has important maternal and pediatric implications for subsequent pregnancies, before which vaccination is often missed.
Both the MMR vaccine and the varicella vaccine (another vaccine that can be initiated post partum) are live vaccines and therefore contraindicated during pregnancy but should be administered post partum, including to people who are breastfeeding.
Other immunizations that hold some promise to protect either the mother or fetus/neonate or both are in various stages of development or testing. These include vaccines for cytomegalovirus, malaria, respiratory syncytial virus, and group B streptococcus.
A word about COVID-19
In mid-July there were more than 120 vaccine candidates for COVID-19 in various phases of study and a host of questions. Will a vaccine be efficacious? Will it prevent severe illness, or illness altogether? And will it be safe for pregnant women?
Vaccines work by manipulating the immune system, and it is important to appreciate the possibility that there may be unique pregnancy-related issues to consider with future COVID-19 vaccines – issues that could influence the effectiveness, safety, and timing of vaccination – and to understand that with any new immunization, there will likely be reluctance on the part of pregnant women who routinely prioritize fetal safety over their own health.
Pregnant women have been excluded from COVID-19 vaccine trials, but there may come a time when experts decide that a vaccine against COVID-19 is beneficial in pregnancy. Thus far, we know that the disease is clearly different from influenza. A growing knowledge of the impact of COVID-19 on the health of pregnant women, particularly the risk of developing severe illness, will be important for the future of COVID-19 immunization, as many women will not want to accept any potential risk of a vaccine unless they believe there is a significant benefit.
References
1. MMWR Morb Mortal Wkly Rep. 2017 Sep 29;66(38):1016-22.
2. N Engl J Med. 2010 Jan 7;362(1):27-35.
3. Obstet Gynecol. 2015 Sep;126(3):486-90.
4. Obstet Gynecol. 2018 Jun;131(6):e214-e217.
5. MMWR Morb Mortal Wkly Rep. 2019 Feb 15;68(6):135-9.
6. Obstet Gynecol. 2019 Apr;133(4):739-53.
7. Lancet. 2014 Oct 25;384(9953):1521-8.
8. Clin Infect Dis. 2015 Feb 1;60(3):333-7.
9. Clin Infect Dis. 2017 Jan 1;64(1):9-14.
Maternal immunization remains a priority for ob.gyns. – an opportunity to provide protection against serious infectious diseases for both the mother and the baby. With influenza vaccination rates in pregnant women still hovering around 50% and the emerging public health problem of vaccine hesitancy, we must fully embrace our responsibility to recommend immunizations and to effectively communicate what is known about their efficacy and safety. Ideally, we should offer them as well.
One reason for the low rates of influenza vaccination – one of the two vaccinations routinely recommended for all pregnant women in the United States – is that pregnant women do not always know the importance of the vaccine. This is actionable: Data clearly show that the physician’s recommendation makes a difference and that a clinician’s offer to administer the vaccination has an even greater impact.
A 2017 Centers for Disease Control and Prevention analysis of data from Internet panel surveys1 shows that women who reported receiving both a clinician recommendation and offer of vaccination had higher coverage during the 2015-2016 and 2016-2017 influenza seasons (63.7% and 70.5%) than did women who reported receiving a clinician recommendation but no offer (37.5% and 43.7%) and women who reported receiving no recommendation for vaccination (12.8% and 14.8%).
The analysis suggests there are consistently missed opportunities: Fewer than 70% (67.3%) of pregnant women in the 2016-2017 flu season reported receiving a clinician recommendation for and offer of vaccination. This is similar to the prior three flu seasons, according to the CDC.
This year, with the COVID-19 pandemic ensuing, the prevention of severe influenza illness – and other vaccine-preventable illnesses – takes on even greater importance. It is not known what the impact of two potentially devastating respiratory infections could be for pregnant individuals. Therefore, maximal protection against at least influenza will be critical.
Influenza and Tdap
Poor outcomes and disproportionately high death rates for pregnant women were observed in both the influenza pandemic of 1918-1919 and the 1957 “Asian flu” pandemic. Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), but it was the H1N1 influenza pandemic of 2009 that reinforced its value and led our field to more fully embrace influenza vaccination as a priority for prenatal care.
Surprisingly, most of the pregnant women who became severely ill from the H1N1 virus were young and healthy and did not have a coexisting condition known to increase risk, such as asthma or diabetes. In an analysis of California epidemiologic data, 2 only one-third of 94 pregnant women who were hospitalized with 2009 H1N1 influenza had established risk factors for complications from influenza, compared with almost two-thirds of nonpregnant women of reproductive age.
Nationally, 75 deaths of pregnant women were confirmed as because of H1N1 and 34 were possibly related to H1N1, most of which (64.3%) occurred in the third trimester.3 Records of the 1957 pandemic similarly show that pregnant women in the second and third trimesters were particularly affected.
That healthy pregnant women became so ill during the H1N1 pandemic raised several flags. For one, it became clearer that pregnancy is its own significant risk factor for severe illness from the influenza virus. Physiological changes believed to make a pregnant woman more susceptible to becoming ill include decreased lung capacity, increased nasal congestion, reduced colloid oncotic pressure, and changes in the immune system. The morbidity and mortality from H1N1 influenza also increased our drive as a specialty to convince women that vaccination is an important strategy in each influenza season.
The flu vaccine can be administered at any point during pregnancy. There is no evidence that the safety profile is any different during one trimester than another.
Patients should be reassured that vaccines recommended in pregnancy have undergone rigorous testing and that the influenza vaccine has been given to millions of pregnant women over decades. They also should understand that contracting influenza has risks for the fetus; research has demonstrated that pregnant women who contract influenza are at greater risk of spontaneous abortion as well as preterm birth and low birth weight.4
In addition, the issue of flu vaccine efficacy needs to be properly teased apart. Women read every year that the vaccine is not effective, so we need to discuss with patients what efficacy means. Does the vaccine prevent illness altogether, or does it prevent severe illness? For the most part, whereas influenza vaccines often do not offer an exact match for the year’s circulating strains – and therefore may not prevent all illness – data show that the vaccine can prevent severe illness.5 That is a worthy outcome.
Also worthy is the impact of influenza vaccination on the newborn. That maternal immunization also protects the baby – it can reduce the risk for influenza in infants under 6 months of age – is underappreciated and should be part of patient counseling. There is clear evidence that maternal immunization boosts the concentration of maternal antibodies that can cross the placenta and that infants benefit from this passive antibody protection.6
The Tdap vaccine (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis), the second vaccine routinely recommended during each pregnancy, is administered as early as possible during the third trimester precisely for this reason – to boost maternal immune response and maximize the passive transfer of antibodies to the newborn. The target is the prevention of pertussis and associated hospitalizations and death during the first 2 months of life in an era when sporadic and unpredictable outbreaks of the infection are occurring.
Data from the CDC of morbidity and mortality from pertussis in children (2001-2011) prior to routine maternal vaccination show that the highest rates of pediatric hospitalizations and deaths occurred in newborns. Research has demonstrated that the Tdap vaccine is highly effective in preventing infections and hospitalizations in newborns: Case-control and cohort studies in the United Kingdom7,8 have shown vaccine effectiveness of 91%-93%, and similar research9 done in the U.S. has demonstrated effectiveness of 78%-85%.
The Tdap vaccine is recommended for pregnant women at 27-36 weeks of gestation – in each pregnancy. The reason for revaccination with each pregnancy is that antibody levels do not remain high for too long; at 8 months post immunization, research has shown, maternal antibody levels have begun to wane.
The vaccine also is recommended for all individuals who will be in close contact with infants younger than 12 months (for example, parents, grandparents, and child-care providers) and who have not previously received it. However, “cocooning” the newborn is effective only when the mother also is immunized – a point that ob.gyns. need to better explain to their patients so that they understand the purpose of this strategy.
Other vaccines in pregnancy and post partum
As described in the American College of Obstetricians and Gynecologists’ committee opinion on maternal immunization, 4 it is the responsibility of the ob.gyn. or obstetric care provider to routinely assess the immunization status of every pregnant patient and recommend additional vaccines for those patients who have conditions or social/behavioral practices that put them at higher risk of acquiring vaccine-preventable diseases.
Patients who have asthma or diabetes, who smoke, or who have never been vaccinated for the prevention of pneumococcal disease should receive the PPV23 pneumococcal vaccine, for instance. For pregnant women with immune deficiencies such as HIV, the PCV13 vaccine followed by PPV23 is recommended. There are approximately 500,000 cases of invasive pneumococcal disease in the United States each year, resulting in 40,000 deaths, and many multidrug-resistant strains of Streptococcus pneumoniae.
Hepatitis A and B vaccines – both recombinant vaccines with no safety concerns – also can be given during pregnancy and are officially recommended for women who have high-risk exposures. In the case of hepatitis A, high risk entails traveling to countries where the disease is endemic. High-risk behavior for hepatitis B includes sex work or being the household contact or sexual partner of a person positive for hepatitis B surface antigen.
Other travel-related vaccines, such as Japanese encephalitis, yellow fever, smallpox, and inactivated polio vaccine, can be considered in pregnancy, but decisions should be driven by more in-depth conversations about potential risks and benefits. Unlike for other vaccinations, there are limited data on the safety of travel-related immunizations in pregnancy. Sometimes, the question of whether travel is advisable in the middle of pregnancy – whether potential risks are worth taking – is a valid question to pose in conversations with patients.
Standard obstetric practice includes assessment of rubella susceptibility at the beginning of pregnancy. In some locations such as New York, measles susceptibility is also routinely evaluated. After delivery, seronegative women should be vaccinated with MMR (measles, mumps, and rubella) vaccine prior to discharge. In recent years, with the growing problem of vaccine refusal and an increasingly mobile and global society, we’re seeing sporadic outbreaks of measles and rubella – diseases that were once eradicated.
Measles in particular is highly contagious and requires a herd immunity threshold of 92%-94% to prevent sustained spread of the disease. Postpartum immunization has important maternal and pediatric implications for subsequent pregnancies, before which vaccination is often missed.
Both the MMR vaccine and the varicella vaccine (another vaccine that can be initiated post partum) are live vaccines and therefore contraindicated during pregnancy but should be administered post partum, including to people who are breastfeeding.
Other immunizations that hold some promise to protect either the mother or fetus/neonate or both are in various stages of development or testing. These include vaccines for cytomegalovirus, malaria, respiratory syncytial virus, and group B streptococcus.
A word about COVID-19
In mid-July there were more than 120 vaccine candidates for COVID-19 in various phases of study and a host of questions. Will a vaccine be efficacious? Will it prevent severe illness, or illness altogether? And will it be safe for pregnant women?
Vaccines work by manipulating the immune system, and it is important to appreciate the possibility that there may be unique pregnancy-related issues to consider with future COVID-19 vaccines – issues that could influence the effectiveness, safety, and timing of vaccination – and to understand that with any new immunization, there will likely be reluctance on the part of pregnant women who routinely prioritize fetal safety over their own health.
Pregnant women have been excluded from COVID-19 vaccine trials, but there may come a time when experts decide that a vaccine against COVID-19 is beneficial in pregnancy. Thus far, we know that the disease is clearly different from influenza. A growing knowledge of the impact of COVID-19 on the health of pregnant women, particularly the risk of developing severe illness, will be important for the future of COVID-19 immunization, as many women will not want to accept any potential risk of a vaccine unless they believe there is a significant benefit.
References
1. MMWR Morb Mortal Wkly Rep. 2017 Sep 29;66(38):1016-22.
2. N Engl J Med. 2010 Jan 7;362(1):27-35.
3. Obstet Gynecol. 2015 Sep;126(3):486-90.
4. Obstet Gynecol. 2018 Jun;131(6):e214-e217.
5. MMWR Morb Mortal Wkly Rep. 2019 Feb 15;68(6):135-9.
6. Obstet Gynecol. 2019 Apr;133(4):739-53.
7. Lancet. 2014 Oct 25;384(9953):1521-8.
8. Clin Infect Dis. 2015 Feb 1;60(3):333-7.
9. Clin Infect Dis. 2017 Jan 1;64(1):9-14.
The fix is in: AIM bundles to combat maternal morbidity and mortality
“Anytime you have a maternal death, it sticks with you for life,” said Elliott Main, MD, a maternal fetal medicine specialist at Stanford (Calif.) University and one of the nation’s leaders in combating maternal mortality.
Dr. Main has had two maternal deaths in his career, years ago. One woman had a fatal stroke because of severe hypertension, and another died of cardiac complications. “We tried to do everything we possibly could, but you scrounge your memory for years and years [afterward]. To have a young healthy person go into labor and delivery and not come out is a tragedy at all levels. It charged me to not ever want to see that happen again,” he said.
Today, Dr. Main is the medical director of the California Maternal Quality Care Collaborative (CMQCC), a wide-ranging group of clinicians, state officials, hospitals, and others who have come together to address the issue. About 30 states have similar perinatal quality collaboratives (PQCs), and other states are forming them.
They work in collaboration with maternal mortality review committees (MMRCs), state-level groups that review maternal deaths, identify problems to address, and make recommendations to the quality collaboratives on how to prevent maternal deaths.
About 600-800 women die in the United States each year due to pregnancy-related complications, which ranks the United States behind other industrialized nations. Leading causes include hemorrhage and hemorrhagic strokes secondary to hypertension. It’s estimated that the majority of maternal deaths could be prevented with proper care.
To that end, states are enacting safety bundles from the Alliance for Innovation on Maternal Health (AIM), which was established by the American College of Obstetricians and Gynecologist several years ago. There are bundles that address obstetric hypertension, hemorrhage, mental health, venous thromboembolism, opioid use, racial disparities, and other problems. They were developed by experts in the field and published in multiple journals. California and other states have issued toolkits on how to implement them based on local circumstances.
Dr. Main said.
AIM bundle implementation is “what’s happening in New Mexico and a lot of states, mostly through the efforts of state level quality care collaboratives. Some [states] are further ahead than others,” said Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, and president of the New Mexico PQC.
“Most states now have a [MMRC] that collects maternal mortality and near-miss data. Those data are used by the action arm,” which is the PQC. “If the review committee says” opioid use disorder is a significant contributor “like in our state, the collaborative rolls out the opioid use disorder bundle,” she said.
Beginning next January, the Joint Commission, formerly known as the Joint Commission on Accreditation of Healthcare Organizations, will require that accredited hospitals enact key elements of the AIM bundles for both obstetric hemorrhage and severe hypertension. “Everyone’s [now] motivated to get on that bandwagon,” Dr. Espey said.
“The bundles are here to stay,” and the Joint Commission requirements are “a really important step for sustainability and basic implementation. We really want to get them adopted everywhere,” said Dr. Main, who is also the national implementation director for the AIM initiative.
“The key thing is to work on implementing the hemorrhage and hypertension bundles in your hospital. I would suggest contacting [your] state” PQC, he said.
The California model
California, which has been working to reduce maternal mortality and morbidity since the mid 2000s, has produced among the strongest evidence to date that the efforts make a difference.
By 2013, the state had halved its maternal mortality rate to a 3-year average of 7 deaths per 100,000 live births, which is comparable with the average Western Europe rate of 7.2 deaths. Nationwide, the rate was about 17.4 deaths per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention
The reasons are multifactorial, but “we think” the quality improvement efforts have been “an important contributor,” Dr. Main said.
Improvements especially for Black women
Among the success stories has been California’s implementation of the AIM obstetric hemorrhage bundle about 5 years ago. Among other steps, the 17 evidence-based recommendations included early recognition, immediate access to oxytocin and other medications, immediate access to a hemorrhage cart with instructions for intrauterine balloons and compression stitches, the establishment of a hemorrhage response protocol and team, and regular unit-based drills with debriefing sessions afterward.
Mentoring teams consisting of a physician and nurse with maternal quality improvement experience were created to help hospitals come on board, with each team working with five to eight hospitals. Efforts included monthly telephone calls and face-to-face meetings, and providers were held accountable for progress. Hospitals shared data and tips on implementation, under the aegis of the CMQCC.
When the baseline period of 2011-2014 to the postintervention period of October 2015 to December 2016 were compared, the rate of severe maternal morbidity from hemorrhage fell from 22.1% to 18.5% across 99 hospitals and 73,476 women.
The benefit among Black women exceeded that among White women, with a 9% absolute rate reduction versus 2.1%. “If you adjusted for risk factors, [we found] you could eliminate [racial differences] completely,” which is something that hadn’t been shown before. “This is a really big deal,” Dr. Main said, because the risk of maternal morbidity and mortality is three to four times higher among Black women, compared with White women.
Dr. Main and his team found that the biggest clinical risk factor that accounted for racial differences was a higher rate of cesarean deliveries among Black women, followed by higher rates of anemia at hospital admission. “If you have a C-section when you are anemic, you are going to have a transfusion,” he explained.
More recently, there’s been a push in California to reduce the rate of primary cesarean deliveries by enacting the associated AIM bundle with use of the same approach as with the hemorrhage bundle. Dr. Main and his team recently reported a rate reduction from 29.3% to 25% without compromising birth outcomes.
However, “some changes are easier than others. Hemorrhage was an easy one to change because it didn’t deal with physician autonomy as much, and you saw more immediate results” with fewer hemorrhages. Reducing cesarean delivery rates is “a bigger lift” because “it’s really changing the culture of labor and delivery. It involves more group pressure and more reinforcing, but we were able to do that,” he said.
Problems in the Show Me State
“We’ve patterned a lot” of what’s being done in New Mexico “after California,” Dr. Espey said.
The AIM hemorrhage bundle, for instance, is being rolled out to New Mexico hospitals, with the help of virtual meetings and mentoring programs, plus outreach to the Navajo and others reservations because, as with Black women, rates of maternal morbidity and mortality are higher among Native American women.
It’s been tougher going, however, in states such as Missouri, which recently ranked 44th in the country for maternal mortality.
“We started a little bit late, and we are a little bit behind,” said ob.gyn. Karen L. Florio, DO, at the University of Missouri–Kansas City and also a leader of the state MMRC and member of its PQC.
The main problem is money. California’s efforts are funded by the Centers for Disease Control and Prevention, the state health department, and hospitals, among others.
But Missouri is “not as well funded as California for our mortality review board, and our [PQC] is mostly not funded. If we could get that funding, we would have more resources to implement these AIM bundles,” she said.
In addition to the issue, Missouri didn’t expand Medicaid under the Accountable Care Act – something that’s been linked to reduced maternal morbidity and mortality – and there are entire rural areas with no maternity care. Plus after generations of mistreatment, “our African American population has a valid distrust of the medical system that contributes to maternal mortality,” she said.
Obesity-related heart disease is also prevalent in Missouri, even among young people. “I cannot tell you how many women I have had who have had a heart attack at the age of 30 and who have had stents placed,” Dr. Florio said.
Dr. Florio and her colleagues are currently using teleconferences and other means to roll out the AIM hypertension bundle but can do so only selectively. “We don’t have the resources to reach every single rural hospital all over the state,” she said; they are working to address the funding issues.
For rural hospitals, implementation is “daunting”
Meanwhile, rural hospitals have been a particular concern in South Dakota, said Kimberlee McKay, MD, an ob.gyn. who is the clinical vice president of the ob.gyn. service line at Avera Health, a hospital system based in Sioux Falls, S.D.
She’s been overseeing Avera’s implementation of the hypertension, hemorrhage, and venous thromboembolism bundles. “What’s hard is that” the AIM protocols come “out of academic centers. Implementation of complex algorithms is daunting” for hospitals that only do a couple hundred deliveries a year, she said.
For small hospitals, the approach she’s found that works is to first assess what they can offer, and then have them “do what’s reasonable” for their resources. The second part is making sure high-risk women get to a regional center – with an adequate blood supply, in the case of hemorrhage, for instance – for complications. Dr. McKay and colleagues are working on a system by which regional centers can monitor smaller hospitals for potential maternity problems, and contact them proactively before they emerge.
They’ve also made access to hemorrhage and hypertension drugs easier on labor and delivery units with the help of close-by dedicated medicine boxes, and standardized protocols and order sets across Avera. “We try to make the right thing the easy thing to do,” Dr. McKay said.
Dr. Espey is an editorial adviser for Ob.Gyn. News. The physicians have no relevant financial disclosures.
“Anytime you have a maternal death, it sticks with you for life,” said Elliott Main, MD, a maternal fetal medicine specialist at Stanford (Calif.) University and one of the nation’s leaders in combating maternal mortality.
Dr. Main has had two maternal deaths in his career, years ago. One woman had a fatal stroke because of severe hypertension, and another died of cardiac complications. “We tried to do everything we possibly could, but you scrounge your memory for years and years [afterward]. To have a young healthy person go into labor and delivery and not come out is a tragedy at all levels. It charged me to not ever want to see that happen again,” he said.
Today, Dr. Main is the medical director of the California Maternal Quality Care Collaborative (CMQCC), a wide-ranging group of clinicians, state officials, hospitals, and others who have come together to address the issue. About 30 states have similar perinatal quality collaboratives (PQCs), and other states are forming them.
They work in collaboration with maternal mortality review committees (MMRCs), state-level groups that review maternal deaths, identify problems to address, and make recommendations to the quality collaboratives on how to prevent maternal deaths.
About 600-800 women die in the United States each year due to pregnancy-related complications, which ranks the United States behind other industrialized nations. Leading causes include hemorrhage and hemorrhagic strokes secondary to hypertension. It’s estimated that the majority of maternal deaths could be prevented with proper care.
To that end, states are enacting safety bundles from the Alliance for Innovation on Maternal Health (AIM), which was established by the American College of Obstetricians and Gynecologist several years ago. There are bundles that address obstetric hypertension, hemorrhage, mental health, venous thromboembolism, opioid use, racial disparities, and other problems. They were developed by experts in the field and published in multiple journals. California and other states have issued toolkits on how to implement them based on local circumstances.
Dr. Main said.
AIM bundle implementation is “what’s happening in New Mexico and a lot of states, mostly through the efforts of state level quality care collaboratives. Some [states] are further ahead than others,” said Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, and president of the New Mexico PQC.
“Most states now have a [MMRC] that collects maternal mortality and near-miss data. Those data are used by the action arm,” which is the PQC. “If the review committee says” opioid use disorder is a significant contributor “like in our state, the collaborative rolls out the opioid use disorder bundle,” she said.
Beginning next January, the Joint Commission, formerly known as the Joint Commission on Accreditation of Healthcare Organizations, will require that accredited hospitals enact key elements of the AIM bundles for both obstetric hemorrhage and severe hypertension. “Everyone’s [now] motivated to get on that bandwagon,” Dr. Espey said.
“The bundles are here to stay,” and the Joint Commission requirements are “a really important step for sustainability and basic implementation. We really want to get them adopted everywhere,” said Dr. Main, who is also the national implementation director for the AIM initiative.
“The key thing is to work on implementing the hemorrhage and hypertension bundles in your hospital. I would suggest contacting [your] state” PQC, he said.
The California model
California, which has been working to reduce maternal mortality and morbidity since the mid 2000s, has produced among the strongest evidence to date that the efforts make a difference.
By 2013, the state had halved its maternal mortality rate to a 3-year average of 7 deaths per 100,000 live births, which is comparable with the average Western Europe rate of 7.2 deaths. Nationwide, the rate was about 17.4 deaths per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention
The reasons are multifactorial, but “we think” the quality improvement efforts have been “an important contributor,” Dr. Main said.
Improvements especially for Black women
Among the success stories has been California’s implementation of the AIM obstetric hemorrhage bundle about 5 years ago. Among other steps, the 17 evidence-based recommendations included early recognition, immediate access to oxytocin and other medications, immediate access to a hemorrhage cart with instructions for intrauterine balloons and compression stitches, the establishment of a hemorrhage response protocol and team, and regular unit-based drills with debriefing sessions afterward.
Mentoring teams consisting of a physician and nurse with maternal quality improvement experience were created to help hospitals come on board, with each team working with five to eight hospitals. Efforts included monthly telephone calls and face-to-face meetings, and providers were held accountable for progress. Hospitals shared data and tips on implementation, under the aegis of the CMQCC.
When the baseline period of 2011-2014 to the postintervention period of October 2015 to December 2016 were compared, the rate of severe maternal morbidity from hemorrhage fell from 22.1% to 18.5% across 99 hospitals and 73,476 women.
The benefit among Black women exceeded that among White women, with a 9% absolute rate reduction versus 2.1%. “If you adjusted for risk factors, [we found] you could eliminate [racial differences] completely,” which is something that hadn’t been shown before. “This is a really big deal,” Dr. Main said, because the risk of maternal morbidity and mortality is three to four times higher among Black women, compared with White women.
Dr. Main and his team found that the biggest clinical risk factor that accounted for racial differences was a higher rate of cesarean deliveries among Black women, followed by higher rates of anemia at hospital admission. “If you have a C-section when you are anemic, you are going to have a transfusion,” he explained.
More recently, there’s been a push in California to reduce the rate of primary cesarean deliveries by enacting the associated AIM bundle with use of the same approach as with the hemorrhage bundle. Dr. Main and his team recently reported a rate reduction from 29.3% to 25% without compromising birth outcomes.
However, “some changes are easier than others. Hemorrhage was an easy one to change because it didn’t deal with physician autonomy as much, and you saw more immediate results” with fewer hemorrhages. Reducing cesarean delivery rates is “a bigger lift” because “it’s really changing the culture of labor and delivery. It involves more group pressure and more reinforcing, but we were able to do that,” he said.
Problems in the Show Me State
“We’ve patterned a lot” of what’s being done in New Mexico “after California,” Dr. Espey said.
The AIM hemorrhage bundle, for instance, is being rolled out to New Mexico hospitals, with the help of virtual meetings and mentoring programs, plus outreach to the Navajo and others reservations because, as with Black women, rates of maternal morbidity and mortality are higher among Native American women.
It’s been tougher going, however, in states such as Missouri, which recently ranked 44th in the country for maternal mortality.
“We started a little bit late, and we are a little bit behind,” said ob.gyn. Karen L. Florio, DO, at the University of Missouri–Kansas City and also a leader of the state MMRC and member of its PQC.
The main problem is money. California’s efforts are funded by the Centers for Disease Control and Prevention, the state health department, and hospitals, among others.
But Missouri is “not as well funded as California for our mortality review board, and our [PQC] is mostly not funded. If we could get that funding, we would have more resources to implement these AIM bundles,” she said.
In addition to the issue, Missouri didn’t expand Medicaid under the Accountable Care Act – something that’s been linked to reduced maternal morbidity and mortality – and there are entire rural areas with no maternity care. Plus after generations of mistreatment, “our African American population has a valid distrust of the medical system that contributes to maternal mortality,” she said.
Obesity-related heart disease is also prevalent in Missouri, even among young people. “I cannot tell you how many women I have had who have had a heart attack at the age of 30 and who have had stents placed,” Dr. Florio said.
Dr. Florio and her colleagues are currently using teleconferences and other means to roll out the AIM hypertension bundle but can do so only selectively. “We don’t have the resources to reach every single rural hospital all over the state,” she said; they are working to address the funding issues.
For rural hospitals, implementation is “daunting”
Meanwhile, rural hospitals have been a particular concern in South Dakota, said Kimberlee McKay, MD, an ob.gyn. who is the clinical vice president of the ob.gyn. service line at Avera Health, a hospital system based in Sioux Falls, S.D.
She’s been overseeing Avera’s implementation of the hypertension, hemorrhage, and venous thromboembolism bundles. “What’s hard is that” the AIM protocols come “out of academic centers. Implementation of complex algorithms is daunting” for hospitals that only do a couple hundred deliveries a year, she said.
For small hospitals, the approach she’s found that works is to first assess what they can offer, and then have them “do what’s reasonable” for their resources. The second part is making sure high-risk women get to a regional center – with an adequate blood supply, in the case of hemorrhage, for instance – for complications. Dr. McKay and colleagues are working on a system by which regional centers can monitor smaller hospitals for potential maternity problems, and contact them proactively before they emerge.
They’ve also made access to hemorrhage and hypertension drugs easier on labor and delivery units with the help of close-by dedicated medicine boxes, and standardized protocols and order sets across Avera. “We try to make the right thing the easy thing to do,” Dr. McKay said.
Dr. Espey is an editorial adviser for Ob.Gyn. News. The physicians have no relevant financial disclosures.
“Anytime you have a maternal death, it sticks with you for life,” said Elliott Main, MD, a maternal fetal medicine specialist at Stanford (Calif.) University and one of the nation’s leaders in combating maternal mortality.
Dr. Main has had two maternal deaths in his career, years ago. One woman had a fatal stroke because of severe hypertension, and another died of cardiac complications. “We tried to do everything we possibly could, but you scrounge your memory for years and years [afterward]. To have a young healthy person go into labor and delivery and not come out is a tragedy at all levels. It charged me to not ever want to see that happen again,” he said.
Today, Dr. Main is the medical director of the California Maternal Quality Care Collaborative (CMQCC), a wide-ranging group of clinicians, state officials, hospitals, and others who have come together to address the issue. About 30 states have similar perinatal quality collaboratives (PQCs), and other states are forming them.
They work in collaboration with maternal mortality review committees (MMRCs), state-level groups that review maternal deaths, identify problems to address, and make recommendations to the quality collaboratives on how to prevent maternal deaths.
About 600-800 women die in the United States each year due to pregnancy-related complications, which ranks the United States behind other industrialized nations. Leading causes include hemorrhage and hemorrhagic strokes secondary to hypertension. It’s estimated that the majority of maternal deaths could be prevented with proper care.
To that end, states are enacting safety bundles from the Alliance for Innovation on Maternal Health (AIM), which was established by the American College of Obstetricians and Gynecologist several years ago. There are bundles that address obstetric hypertension, hemorrhage, mental health, venous thromboembolism, opioid use, racial disparities, and other problems. They were developed by experts in the field and published in multiple journals. California and other states have issued toolkits on how to implement them based on local circumstances.
Dr. Main said.
AIM bundle implementation is “what’s happening in New Mexico and a lot of states, mostly through the efforts of state level quality care collaboratives. Some [states] are further ahead than others,” said Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, and president of the New Mexico PQC.
“Most states now have a [MMRC] that collects maternal mortality and near-miss data. Those data are used by the action arm,” which is the PQC. “If the review committee says” opioid use disorder is a significant contributor “like in our state, the collaborative rolls out the opioid use disorder bundle,” she said.
Beginning next January, the Joint Commission, formerly known as the Joint Commission on Accreditation of Healthcare Organizations, will require that accredited hospitals enact key elements of the AIM bundles for both obstetric hemorrhage and severe hypertension. “Everyone’s [now] motivated to get on that bandwagon,” Dr. Espey said.
“The bundles are here to stay,” and the Joint Commission requirements are “a really important step for sustainability and basic implementation. We really want to get them adopted everywhere,” said Dr. Main, who is also the national implementation director for the AIM initiative.
“The key thing is to work on implementing the hemorrhage and hypertension bundles in your hospital. I would suggest contacting [your] state” PQC, he said.
The California model
California, which has been working to reduce maternal mortality and morbidity since the mid 2000s, has produced among the strongest evidence to date that the efforts make a difference.
By 2013, the state had halved its maternal mortality rate to a 3-year average of 7 deaths per 100,000 live births, which is comparable with the average Western Europe rate of 7.2 deaths. Nationwide, the rate was about 17.4 deaths per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention
The reasons are multifactorial, but “we think” the quality improvement efforts have been “an important contributor,” Dr. Main said.
Improvements especially for Black women
Among the success stories has been California’s implementation of the AIM obstetric hemorrhage bundle about 5 years ago. Among other steps, the 17 evidence-based recommendations included early recognition, immediate access to oxytocin and other medications, immediate access to a hemorrhage cart with instructions for intrauterine balloons and compression stitches, the establishment of a hemorrhage response protocol and team, and regular unit-based drills with debriefing sessions afterward.
Mentoring teams consisting of a physician and nurse with maternal quality improvement experience were created to help hospitals come on board, with each team working with five to eight hospitals. Efforts included monthly telephone calls and face-to-face meetings, and providers were held accountable for progress. Hospitals shared data and tips on implementation, under the aegis of the CMQCC.
When the baseline period of 2011-2014 to the postintervention period of October 2015 to December 2016 were compared, the rate of severe maternal morbidity from hemorrhage fell from 22.1% to 18.5% across 99 hospitals and 73,476 women.
The benefit among Black women exceeded that among White women, with a 9% absolute rate reduction versus 2.1%. “If you adjusted for risk factors, [we found] you could eliminate [racial differences] completely,” which is something that hadn’t been shown before. “This is a really big deal,” Dr. Main said, because the risk of maternal morbidity and mortality is three to four times higher among Black women, compared with White women.
Dr. Main and his team found that the biggest clinical risk factor that accounted for racial differences was a higher rate of cesarean deliveries among Black women, followed by higher rates of anemia at hospital admission. “If you have a C-section when you are anemic, you are going to have a transfusion,” he explained.
More recently, there’s been a push in California to reduce the rate of primary cesarean deliveries by enacting the associated AIM bundle with use of the same approach as with the hemorrhage bundle. Dr. Main and his team recently reported a rate reduction from 29.3% to 25% without compromising birth outcomes.
However, “some changes are easier than others. Hemorrhage was an easy one to change because it didn’t deal with physician autonomy as much, and you saw more immediate results” with fewer hemorrhages. Reducing cesarean delivery rates is “a bigger lift” because “it’s really changing the culture of labor and delivery. It involves more group pressure and more reinforcing, but we were able to do that,” he said.
Problems in the Show Me State
“We’ve patterned a lot” of what’s being done in New Mexico “after California,” Dr. Espey said.
The AIM hemorrhage bundle, for instance, is being rolled out to New Mexico hospitals, with the help of virtual meetings and mentoring programs, plus outreach to the Navajo and others reservations because, as with Black women, rates of maternal morbidity and mortality are higher among Native American women.
It’s been tougher going, however, in states such as Missouri, which recently ranked 44th in the country for maternal mortality.
“We started a little bit late, and we are a little bit behind,” said ob.gyn. Karen L. Florio, DO, at the University of Missouri–Kansas City and also a leader of the state MMRC and member of its PQC.
The main problem is money. California’s efforts are funded by the Centers for Disease Control and Prevention, the state health department, and hospitals, among others.
But Missouri is “not as well funded as California for our mortality review board, and our [PQC] is mostly not funded. If we could get that funding, we would have more resources to implement these AIM bundles,” she said.
In addition to the issue, Missouri didn’t expand Medicaid under the Accountable Care Act – something that’s been linked to reduced maternal morbidity and mortality – and there are entire rural areas with no maternity care. Plus after generations of mistreatment, “our African American population has a valid distrust of the medical system that contributes to maternal mortality,” she said.
Obesity-related heart disease is also prevalent in Missouri, even among young people. “I cannot tell you how many women I have had who have had a heart attack at the age of 30 and who have had stents placed,” Dr. Florio said.
Dr. Florio and her colleagues are currently using teleconferences and other means to roll out the AIM hypertension bundle but can do so only selectively. “We don’t have the resources to reach every single rural hospital all over the state,” she said; they are working to address the funding issues.
For rural hospitals, implementation is “daunting”
Meanwhile, rural hospitals have been a particular concern in South Dakota, said Kimberlee McKay, MD, an ob.gyn. who is the clinical vice president of the ob.gyn. service line at Avera Health, a hospital system based in Sioux Falls, S.D.
She’s been overseeing Avera’s implementation of the hypertension, hemorrhage, and venous thromboembolism bundles. “What’s hard is that” the AIM protocols come “out of academic centers. Implementation of complex algorithms is daunting” for hospitals that only do a couple hundred deliveries a year, she said.
For small hospitals, the approach she’s found that works is to first assess what they can offer, and then have them “do what’s reasonable” for their resources. The second part is making sure high-risk women get to a regional center – with an adequate blood supply, in the case of hemorrhage, for instance – for complications. Dr. McKay and colleagues are working on a system by which regional centers can monitor smaller hospitals for potential maternity problems, and contact them proactively before they emerge.
They’ve also made access to hemorrhage and hypertension drugs easier on labor and delivery units with the help of close-by dedicated medicine boxes, and standardized protocols and order sets across Avera. “We try to make the right thing the easy thing to do,” Dr. McKay said.
Dr. Espey is an editorial adviser for Ob.Gyn. News. The physicians have no relevant financial disclosures.
Updated EULAR/ACR criteria identify more lupus patients
Use of the 2019 EULAR/ACR criteria for systemic lupus erythematosus identified an additional 17% of lupus patients in a cohort of 133 women with undifferentiated connective tissue disease.
Several studies have applied the 2019 EULAR/ACR criteria for systemic lupus erythematosus (SLE) to different patient populations, wrote Massimo Radin, MD, of S. Giovanni Bosco Hospital, Turin, Italy, and colleagues.
“However, it is unknown if the new classifications criteria for SLE might impact on the categorization of patients previously diagnosed with undifferentiated connective tissue disease (UCTD),” they said in a brief report published in Arthritis Care & Research.
In addition, “being classified or not as having SLE may pose clinical and logistic consequences, as patients with a diagnosis of ‘SLE’ might be followed up according to a specific local protocol and have in-label access to certain medications (such as biologics) or may be eligible for the participation in clinical trials,” they wrote.
The investigators applied the 2019 EULAR/ACR criteria to a cohort of 133 women with UCTD but no other diagnosis. The average age of the women was 38 years; the average disease duration was 10 years. Patients who scored 10 points or more on positive clinical and immunological domains at the start of the study were classified as SLE under the 2019 EULAR/ACR criteria.
Overall, 22 patients (17%) met the classification criteria for SLE at the time of their first pregnancy.
Compared with the other patients in the cohort who were not classified as SLE, patients classified as SLE under the 2019 EULAR/ACR criteria had significantly higher frequency of mucocutaneous manifestations (5% vs. 23%), arthritis (17% vs. 59%), isolated urine abnormalities (1% vs. 18%), and highly specific antibodies (15% vs. 50%).
In addition, patients who met the 2019 EULAR/ACR SLE criteria were significantly more likely to meet the ACR 1997 and SLICC criteria after an average follow-up of 9 years compared with the rest of the cohort (18.2% vs. 1.8%). Patients who met the 2019 EULAR/ACR criteria also had significantly shorter disease duration than that of the other patients in the UCTD cohort (8.23 years vs. 10.7 years) and were significantly more likely to develop preeclampsia during pregnancy (18% vs. 0%).
The findings were limited by several factors including the retrospective design of the study and possible lack of generalizability to male patients, the researchers noted.
The results support the need for improved classification criteria for UCTD, as early identification of specific conditions can help guide treatment and reduce the risk of more severe symptoms and complications, the authors said.
“When discriminating between conditions with a marked overlap, such as SLE and UCTD, the proposal of new classification criteria should balance specificity and sensitivity,” the researchers wrote. “When developing new classification criteria, one approach is to select patients and the control groups as representative as possible of the settings (the medical practices) in which these criteria will be used.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Radin M et al. Art
Use of the 2019 EULAR/ACR criteria for systemic lupus erythematosus identified an additional 17% of lupus patients in a cohort of 133 women with undifferentiated connective tissue disease.
Several studies have applied the 2019 EULAR/ACR criteria for systemic lupus erythematosus (SLE) to different patient populations, wrote Massimo Radin, MD, of S. Giovanni Bosco Hospital, Turin, Italy, and colleagues.
“However, it is unknown if the new classifications criteria for SLE might impact on the categorization of patients previously diagnosed with undifferentiated connective tissue disease (UCTD),” they said in a brief report published in Arthritis Care & Research.
In addition, “being classified or not as having SLE may pose clinical and logistic consequences, as patients with a diagnosis of ‘SLE’ might be followed up according to a specific local protocol and have in-label access to certain medications (such as biologics) or may be eligible for the participation in clinical trials,” they wrote.
The investigators applied the 2019 EULAR/ACR criteria to a cohort of 133 women with UCTD but no other diagnosis. The average age of the women was 38 years; the average disease duration was 10 years. Patients who scored 10 points or more on positive clinical and immunological domains at the start of the study were classified as SLE under the 2019 EULAR/ACR criteria.
Overall, 22 patients (17%) met the classification criteria for SLE at the time of their first pregnancy.
Compared with the other patients in the cohort who were not classified as SLE, patients classified as SLE under the 2019 EULAR/ACR criteria had significantly higher frequency of mucocutaneous manifestations (5% vs. 23%), arthritis (17% vs. 59%), isolated urine abnormalities (1% vs. 18%), and highly specific antibodies (15% vs. 50%).
In addition, patients who met the 2019 EULAR/ACR SLE criteria were significantly more likely to meet the ACR 1997 and SLICC criteria after an average follow-up of 9 years compared with the rest of the cohort (18.2% vs. 1.8%). Patients who met the 2019 EULAR/ACR criteria also had significantly shorter disease duration than that of the other patients in the UCTD cohort (8.23 years vs. 10.7 years) and were significantly more likely to develop preeclampsia during pregnancy (18% vs. 0%).
The findings were limited by several factors including the retrospective design of the study and possible lack of generalizability to male patients, the researchers noted.
The results support the need for improved classification criteria for UCTD, as early identification of specific conditions can help guide treatment and reduce the risk of more severe symptoms and complications, the authors said.
“When discriminating between conditions with a marked overlap, such as SLE and UCTD, the proposal of new classification criteria should balance specificity and sensitivity,” the researchers wrote. “When developing new classification criteria, one approach is to select patients and the control groups as representative as possible of the settings (the medical practices) in which these criteria will be used.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Radin M et al. Art
Use of the 2019 EULAR/ACR criteria for systemic lupus erythematosus identified an additional 17% of lupus patients in a cohort of 133 women with undifferentiated connective tissue disease.
Several studies have applied the 2019 EULAR/ACR criteria for systemic lupus erythematosus (SLE) to different patient populations, wrote Massimo Radin, MD, of S. Giovanni Bosco Hospital, Turin, Italy, and colleagues.
“However, it is unknown if the new classifications criteria for SLE might impact on the categorization of patients previously diagnosed with undifferentiated connective tissue disease (UCTD),” they said in a brief report published in Arthritis Care & Research.
In addition, “being classified or not as having SLE may pose clinical and logistic consequences, as patients with a diagnosis of ‘SLE’ might be followed up according to a specific local protocol and have in-label access to certain medications (such as biologics) or may be eligible for the participation in clinical trials,” they wrote.
The investigators applied the 2019 EULAR/ACR criteria to a cohort of 133 women with UCTD but no other diagnosis. The average age of the women was 38 years; the average disease duration was 10 years. Patients who scored 10 points or more on positive clinical and immunological domains at the start of the study were classified as SLE under the 2019 EULAR/ACR criteria.
Overall, 22 patients (17%) met the classification criteria for SLE at the time of their first pregnancy.
Compared with the other patients in the cohort who were not classified as SLE, patients classified as SLE under the 2019 EULAR/ACR criteria had significantly higher frequency of mucocutaneous manifestations (5% vs. 23%), arthritis (17% vs. 59%), isolated urine abnormalities (1% vs. 18%), and highly specific antibodies (15% vs. 50%).
In addition, patients who met the 2019 EULAR/ACR SLE criteria were significantly more likely to meet the ACR 1997 and SLICC criteria after an average follow-up of 9 years compared with the rest of the cohort (18.2% vs. 1.8%). Patients who met the 2019 EULAR/ACR criteria also had significantly shorter disease duration than that of the other patients in the UCTD cohort (8.23 years vs. 10.7 years) and were significantly more likely to develop preeclampsia during pregnancy (18% vs. 0%).
The findings were limited by several factors including the retrospective design of the study and possible lack of generalizability to male patients, the researchers noted.
The results support the need for improved classification criteria for UCTD, as early identification of specific conditions can help guide treatment and reduce the risk of more severe symptoms and complications, the authors said.
“When discriminating between conditions with a marked overlap, such as SLE and UCTD, the proposal of new classification criteria should balance specificity and sensitivity,” the researchers wrote. “When developing new classification criteria, one approach is to select patients and the control groups as representative as possible of the settings (the medical practices) in which these criteria will be used.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Radin M et al. Art
FROM ARTHRITIS CARE & RESEARCH