User login
Study challenges role of birth canal exposure in newborn microbiome establishment
During parturient transmission of gut bacteria from mothers to infants, the dominant maternal source of bacteria is rectal, according to investigators.
This challenges the hypothesis that exposure to the birth canal explains major differences in gut bacteria between infants born vaginally and those born via C-section, reported Moran Yassour, PhD, of Hebrew University in Jerusalem.
“It’s not how and if you entered the birth canal, but rather how you exited it,” Dr. Yassour said during a presentation at the annual Gut Microbiota for Health World Summit.
According to Dr. Yassour, a number of investigators have evaluated vertical transmission of gut bacteria from mothers to newborns, but most began collecting data a week or more after birth, potentially missing critical information.
“We wanted to generate large-scale, paired, longitudinal data, which means that we had [samples from] both mothers and children, and we wanted to start at birth,” Dr. Yassour said at the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
Dr. Yassour explained that newborns delivered vaginally often exhibit Bacteroides in their gut, whereas babies born via C-section do not exhibit these bacteria until 6-18 months of age; however, the vaginal microbiome typically lacks Bacteroides, making the birth canal an unlikely source. This disconnect served as the impetus for the present investigation, Dr. Yassour said.
The study, which is available as a preprint, involved 73 mothers and their infants. To determine the impact of birth canal exposure, the investigators compared gut bacteria of infants born vaginally with those born via pre-labor C-section (no exposure to the birth canal), and those born via post-labor C-section (exposure to the birth canal).
Initial results were surprising, Dr. Yassour said, as 54% of babies delivered via C-section had Bacteroides in their stool during the first week. But in the second week, 94% of the C-section group lacked Bacteroides, which aligns with characteristic findings and suggests failure of colonization, rather than complete lack of exposure.
Out of the 24 infants with persistent Bacteroides colonization, 22 (92%) were born vaginally, compared with 2 (8%) born via pre-labor C-section, and none born via post-labor C-section. This pattern was maintained in a multivariate analysis that accounted for antibiotic use and exposure to formula, both of which are more common among mothers that give birth via C-section.
The investigators also conducted a strain-level analysis of mothers and infants using metagenomic sequencing. Across all time points, 90% of matched maternal-infant strains were detected in babies delivered vaginally.
“[W]e found evidence for mother-to-child transmission of rectal rather than vaginal strains,” the investigators wrote. “These results challenge birth canal exposure as the dominant factor in infant gut microbiome establishment and implicate colonization efficiency rather than exposure as a dictating factor of the newborn gut microbiome composition.”
Dr. Yassour said that these findings may have an immediate effect on clinical practice.
“People have reported the practice of smearing babies that were born by C-section with vaginal fluids in the sense of trying to recapitulate the microbial signature that we find in kids born vaginally,” Dr. Yassour said. “But it’s probably not the vaginal fluid that we need to smear; it’s probably the proximity to the rectum and the bowel movements that happen during delivery ... and that is what’s causing this initial seeding from mother to child.”
Dr. Yassour disclosed no conflicts of interest.
SOURCE: Yassour M et al. GMFH 2020.
During parturient transmission of gut bacteria from mothers to infants, the dominant maternal source of bacteria is rectal, according to investigators.
This challenges the hypothesis that exposure to the birth canal explains major differences in gut bacteria between infants born vaginally and those born via C-section, reported Moran Yassour, PhD, of Hebrew University in Jerusalem.
“It’s not how and if you entered the birth canal, but rather how you exited it,” Dr. Yassour said during a presentation at the annual Gut Microbiota for Health World Summit.
According to Dr. Yassour, a number of investigators have evaluated vertical transmission of gut bacteria from mothers to newborns, but most began collecting data a week or more after birth, potentially missing critical information.
“We wanted to generate large-scale, paired, longitudinal data, which means that we had [samples from] both mothers and children, and we wanted to start at birth,” Dr. Yassour said at the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
Dr. Yassour explained that newborns delivered vaginally often exhibit Bacteroides in their gut, whereas babies born via C-section do not exhibit these bacteria until 6-18 months of age; however, the vaginal microbiome typically lacks Bacteroides, making the birth canal an unlikely source. This disconnect served as the impetus for the present investigation, Dr. Yassour said.
The study, which is available as a preprint, involved 73 mothers and their infants. To determine the impact of birth canal exposure, the investigators compared gut bacteria of infants born vaginally with those born via pre-labor C-section (no exposure to the birth canal), and those born via post-labor C-section (exposure to the birth canal).
Initial results were surprising, Dr. Yassour said, as 54% of babies delivered via C-section had Bacteroides in their stool during the first week. But in the second week, 94% of the C-section group lacked Bacteroides, which aligns with characteristic findings and suggests failure of colonization, rather than complete lack of exposure.
Out of the 24 infants with persistent Bacteroides colonization, 22 (92%) were born vaginally, compared with 2 (8%) born via pre-labor C-section, and none born via post-labor C-section. This pattern was maintained in a multivariate analysis that accounted for antibiotic use and exposure to formula, both of which are more common among mothers that give birth via C-section.
The investigators also conducted a strain-level analysis of mothers and infants using metagenomic sequencing. Across all time points, 90% of matched maternal-infant strains were detected in babies delivered vaginally.
“[W]e found evidence for mother-to-child transmission of rectal rather than vaginal strains,” the investigators wrote. “These results challenge birth canal exposure as the dominant factor in infant gut microbiome establishment and implicate colonization efficiency rather than exposure as a dictating factor of the newborn gut microbiome composition.”
Dr. Yassour said that these findings may have an immediate effect on clinical practice.
“People have reported the practice of smearing babies that were born by C-section with vaginal fluids in the sense of trying to recapitulate the microbial signature that we find in kids born vaginally,” Dr. Yassour said. “But it’s probably not the vaginal fluid that we need to smear; it’s probably the proximity to the rectum and the bowel movements that happen during delivery ... and that is what’s causing this initial seeding from mother to child.”
Dr. Yassour disclosed no conflicts of interest.
SOURCE: Yassour M et al. GMFH 2020.
During parturient transmission of gut bacteria from mothers to infants, the dominant maternal source of bacteria is rectal, according to investigators.
This challenges the hypothesis that exposure to the birth canal explains major differences in gut bacteria between infants born vaginally and those born via C-section, reported Moran Yassour, PhD, of Hebrew University in Jerusalem.
“It’s not how and if you entered the birth canal, but rather how you exited it,” Dr. Yassour said during a presentation at the annual Gut Microbiota for Health World Summit.
According to Dr. Yassour, a number of investigators have evaluated vertical transmission of gut bacteria from mothers to newborns, but most began collecting data a week or more after birth, potentially missing critical information.
“We wanted to generate large-scale, paired, longitudinal data, which means that we had [samples from] both mothers and children, and we wanted to start at birth,” Dr. Yassour said at the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
Dr. Yassour explained that newborns delivered vaginally often exhibit Bacteroides in their gut, whereas babies born via C-section do not exhibit these bacteria until 6-18 months of age; however, the vaginal microbiome typically lacks Bacteroides, making the birth canal an unlikely source. This disconnect served as the impetus for the present investigation, Dr. Yassour said.
The study, which is available as a preprint, involved 73 mothers and their infants. To determine the impact of birth canal exposure, the investigators compared gut bacteria of infants born vaginally with those born via pre-labor C-section (no exposure to the birth canal), and those born via post-labor C-section (exposure to the birth canal).
Initial results were surprising, Dr. Yassour said, as 54% of babies delivered via C-section had Bacteroides in their stool during the first week. But in the second week, 94% of the C-section group lacked Bacteroides, which aligns with characteristic findings and suggests failure of colonization, rather than complete lack of exposure.
Out of the 24 infants with persistent Bacteroides colonization, 22 (92%) were born vaginally, compared with 2 (8%) born via pre-labor C-section, and none born via post-labor C-section. This pattern was maintained in a multivariate analysis that accounted for antibiotic use and exposure to formula, both of which are more common among mothers that give birth via C-section.
The investigators also conducted a strain-level analysis of mothers and infants using metagenomic sequencing. Across all time points, 90% of matched maternal-infant strains were detected in babies delivered vaginally.
“[W]e found evidence for mother-to-child transmission of rectal rather than vaginal strains,” the investigators wrote. “These results challenge birth canal exposure as the dominant factor in infant gut microbiome establishment and implicate colonization efficiency rather than exposure as a dictating factor of the newborn gut microbiome composition.”
Dr. Yassour said that these findings may have an immediate effect on clinical practice.
“People have reported the practice of smearing babies that were born by C-section with vaginal fluids in the sense of trying to recapitulate the microbial signature that we find in kids born vaginally,” Dr. Yassour said. “But it’s probably not the vaginal fluid that we need to smear; it’s probably the proximity to the rectum and the bowel movements that happen during delivery ... and that is what’s causing this initial seeding from mother to child.”
Dr. Yassour disclosed no conflicts of interest.
SOURCE: Yassour M et al. GMFH 2020.
FROM GMFH 2020
HCV screening risk factors in pregnant women need updating
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
FROM OBSTETRICS & GYNECOLOGY
Cervical pessary didn’t prevent preterm birth in selected women
GRAPEVINE, TEX. – or a composite measure of adverse neonatal outcomes, according to a randomized, open-label study.
“The Arabin pessary should not be used to prevent preterm birth in women with twin pregnancy,” Jane E. Norman, MD, dean of health sciences at the University of Bristol (England), said at the Pregnancy Meeting.
“Preterm birth is very common in twin pregnancy” and leads to “excess neonatal mortality amongst twins,” Dr. Norman said at the meeting, which is sponsored by the Society for Maternal-Fetal Medicine. “Preventing preterm birth is important for both singletons and twins, but it could have even more benefits in twins.”
Emerging evidence has suggested that the Arabin cervical pessary may be useful for the prevention of preterm birth in women with singleton pregnancy and a short cervix. In twin pregnancy, data are more limited.
The ProTwin study randomized 813 women with twin pregnancy to a cervical pessary or standard care. Although the pessary had no impact on preterm birth overall, among women with cervical length of less than 38 mm, those who received a pessary were less likely to have preterm birth. The sample size was small, however, and the average length of the cervix in ProTwin differed from that in a previous U.K. study, Dr. Norman said.
Inspired by the study, Dr. Norman and coinvestigators conducted STOPPIT-2, a multicenter, open-label, randomized, controlled trial to further study whether a certain cervical length threshold was associated with benefit of a cervical pessary in preventing preterm birth. The trial included women with twin pregnancy who had a cervical length ultrasound between 18 and 20 weeks and 6 days of gestation. Women with a cervical length of 35 mm or less were eligible for randomization. Patients received an Arabin pessary plus standard care or standard care alone.
The primary obstetric outcome was spontaneous onset of labor leading to delivery before 34 weeks and 6 days of gestation. The primary neonatal outcome was a composite of outcomes – stillbirth, neonatal death, periventricular leukomalacia, respiratory morbidity, intraventricular hemorrhage, necrotizing enterocolitis, or sepsis – measured up to 28 days after the expected date of delivery.
The investigators randomized 503 women in all, including 250 to the pessary and 253 to standard care. Both groups had similar baseline characteristics, Dr. Norman said. The average age was about 33 years, and the average cervical length was about 29 mm. A total of 20% had monochorionic diamniotic pregnancies, and 80% had dichorionic pregnancies. The researchers excluded women with monochorionic monoamniotic pregnancies. In the pessary group, 16 patients declined the intervention, and 4 were unable to have the pessary inserted.
Spontaneous preterm birth occurred in 18% of patients in the Arabin pessary group, compared with 21% in the standard treatment group. The adjusted odds ratio, 0.87, was not statistically significant. In subgroups of patients with monochorionic pregnancy, cervical length less than 28 mm, or cervical length less than 25 mm, there was no significant benefit.
The composite measure of adverse neonatal outcomes also did not significantly differ between the groups. None of the individual components indicated benefit of the pessary either, Dr. Norman said.
In subgroup analyses, odds ratios for adverse neonatal outcomes were “tending towards harm for the Arabin pessary group ... although clearly none of them conferring statistical significance,” she said. Among women with cervical length less than 28 mm, a primary neonatal outcome – at least one of the adverse outcomes – occurred in 23% of patients in the Arabin pessary group, compared with 20% of patients in the standard care group.
Approximately two-thirds of patients found pessary insertion painless or slightly uncomfortable, whereas about 10% described the experience of device fitting as very uncomfortable, and about 1% described it as the worse pain imaginable.
“Since we started STOPPIT-2, in addition to ProTwin, another three studies have been published on the efficacy of the Arabin pessary in twins,” said Dr. Norman. Combined data show no significant effect of the pessary on preventing preterm birth in twin pregnancy. Still, the meta-analysis does not rule out the possibility that there could subgroups of patients who may benefit from the intervention, Dr. Norman said.
STOPPIT-2 was funded by the National Institute for Health Research (NIHR) in the United Kingdom. Dr. Norman chaired the UK National Institute for Health and Care Excellence guidelines on preterm labor and birth in 2015. In addition, Dr. Norman was a member of a GlaxoSmithKline data safety and monitoring group for a trial of a preterm birth prevention agent, has consulted for Dilafor, and has received research grants for preterm birth prevention from the U.K. Medical Research Council, NIHR, and Tommy’s: Together, for every baby charity.
SOURCE: Norman JE et al. Am J Obstet Gynecol. 2020 Jan;222(1):S756. Abstract LB 1.
GRAPEVINE, TEX. – or a composite measure of adverse neonatal outcomes, according to a randomized, open-label study.
“The Arabin pessary should not be used to prevent preterm birth in women with twin pregnancy,” Jane E. Norman, MD, dean of health sciences at the University of Bristol (England), said at the Pregnancy Meeting.
“Preterm birth is very common in twin pregnancy” and leads to “excess neonatal mortality amongst twins,” Dr. Norman said at the meeting, which is sponsored by the Society for Maternal-Fetal Medicine. “Preventing preterm birth is important for both singletons and twins, but it could have even more benefits in twins.”
Emerging evidence has suggested that the Arabin cervical pessary may be useful for the prevention of preterm birth in women with singleton pregnancy and a short cervix. In twin pregnancy, data are more limited.
The ProTwin study randomized 813 women with twin pregnancy to a cervical pessary or standard care. Although the pessary had no impact on preterm birth overall, among women with cervical length of less than 38 mm, those who received a pessary were less likely to have preterm birth. The sample size was small, however, and the average length of the cervix in ProTwin differed from that in a previous U.K. study, Dr. Norman said.
Inspired by the study, Dr. Norman and coinvestigators conducted STOPPIT-2, a multicenter, open-label, randomized, controlled trial to further study whether a certain cervical length threshold was associated with benefit of a cervical pessary in preventing preterm birth. The trial included women with twin pregnancy who had a cervical length ultrasound between 18 and 20 weeks and 6 days of gestation. Women with a cervical length of 35 mm or less were eligible for randomization. Patients received an Arabin pessary plus standard care or standard care alone.
The primary obstetric outcome was spontaneous onset of labor leading to delivery before 34 weeks and 6 days of gestation. The primary neonatal outcome was a composite of outcomes – stillbirth, neonatal death, periventricular leukomalacia, respiratory morbidity, intraventricular hemorrhage, necrotizing enterocolitis, or sepsis – measured up to 28 days after the expected date of delivery.
The investigators randomized 503 women in all, including 250 to the pessary and 253 to standard care. Both groups had similar baseline characteristics, Dr. Norman said. The average age was about 33 years, and the average cervical length was about 29 mm. A total of 20% had monochorionic diamniotic pregnancies, and 80% had dichorionic pregnancies. The researchers excluded women with monochorionic monoamniotic pregnancies. In the pessary group, 16 patients declined the intervention, and 4 were unable to have the pessary inserted.
Spontaneous preterm birth occurred in 18% of patients in the Arabin pessary group, compared with 21% in the standard treatment group. The adjusted odds ratio, 0.87, was not statistically significant. In subgroups of patients with monochorionic pregnancy, cervical length less than 28 mm, or cervical length less than 25 mm, there was no significant benefit.
The composite measure of adverse neonatal outcomes also did not significantly differ between the groups. None of the individual components indicated benefit of the pessary either, Dr. Norman said.
In subgroup analyses, odds ratios for adverse neonatal outcomes were “tending towards harm for the Arabin pessary group ... although clearly none of them conferring statistical significance,” she said. Among women with cervical length less than 28 mm, a primary neonatal outcome – at least one of the adverse outcomes – occurred in 23% of patients in the Arabin pessary group, compared with 20% of patients in the standard care group.
Approximately two-thirds of patients found pessary insertion painless or slightly uncomfortable, whereas about 10% described the experience of device fitting as very uncomfortable, and about 1% described it as the worse pain imaginable.
“Since we started STOPPIT-2, in addition to ProTwin, another three studies have been published on the efficacy of the Arabin pessary in twins,” said Dr. Norman. Combined data show no significant effect of the pessary on preventing preterm birth in twin pregnancy. Still, the meta-analysis does not rule out the possibility that there could subgroups of patients who may benefit from the intervention, Dr. Norman said.
STOPPIT-2 was funded by the National Institute for Health Research (NIHR) in the United Kingdom. Dr. Norman chaired the UK National Institute for Health and Care Excellence guidelines on preterm labor and birth in 2015. In addition, Dr. Norman was a member of a GlaxoSmithKline data safety and monitoring group for a trial of a preterm birth prevention agent, has consulted for Dilafor, and has received research grants for preterm birth prevention from the U.K. Medical Research Council, NIHR, and Tommy’s: Together, for every baby charity.
SOURCE: Norman JE et al. Am J Obstet Gynecol. 2020 Jan;222(1):S756. Abstract LB 1.
GRAPEVINE, TEX. – or a composite measure of adverse neonatal outcomes, according to a randomized, open-label study.
“The Arabin pessary should not be used to prevent preterm birth in women with twin pregnancy,” Jane E. Norman, MD, dean of health sciences at the University of Bristol (England), said at the Pregnancy Meeting.
“Preterm birth is very common in twin pregnancy” and leads to “excess neonatal mortality amongst twins,” Dr. Norman said at the meeting, which is sponsored by the Society for Maternal-Fetal Medicine. “Preventing preterm birth is important for both singletons and twins, but it could have even more benefits in twins.”
Emerging evidence has suggested that the Arabin cervical pessary may be useful for the prevention of preterm birth in women with singleton pregnancy and a short cervix. In twin pregnancy, data are more limited.
The ProTwin study randomized 813 women with twin pregnancy to a cervical pessary or standard care. Although the pessary had no impact on preterm birth overall, among women with cervical length of less than 38 mm, those who received a pessary were less likely to have preterm birth. The sample size was small, however, and the average length of the cervix in ProTwin differed from that in a previous U.K. study, Dr. Norman said.
Inspired by the study, Dr. Norman and coinvestigators conducted STOPPIT-2, a multicenter, open-label, randomized, controlled trial to further study whether a certain cervical length threshold was associated with benefit of a cervical pessary in preventing preterm birth. The trial included women with twin pregnancy who had a cervical length ultrasound between 18 and 20 weeks and 6 days of gestation. Women with a cervical length of 35 mm or less were eligible for randomization. Patients received an Arabin pessary plus standard care or standard care alone.
The primary obstetric outcome was spontaneous onset of labor leading to delivery before 34 weeks and 6 days of gestation. The primary neonatal outcome was a composite of outcomes – stillbirth, neonatal death, periventricular leukomalacia, respiratory morbidity, intraventricular hemorrhage, necrotizing enterocolitis, or sepsis – measured up to 28 days after the expected date of delivery.
The investigators randomized 503 women in all, including 250 to the pessary and 253 to standard care. Both groups had similar baseline characteristics, Dr. Norman said. The average age was about 33 years, and the average cervical length was about 29 mm. A total of 20% had monochorionic diamniotic pregnancies, and 80% had dichorionic pregnancies. The researchers excluded women with monochorionic monoamniotic pregnancies. In the pessary group, 16 patients declined the intervention, and 4 were unable to have the pessary inserted.
Spontaneous preterm birth occurred in 18% of patients in the Arabin pessary group, compared with 21% in the standard treatment group. The adjusted odds ratio, 0.87, was not statistically significant. In subgroups of patients with monochorionic pregnancy, cervical length less than 28 mm, or cervical length less than 25 mm, there was no significant benefit.
The composite measure of adverse neonatal outcomes also did not significantly differ between the groups. None of the individual components indicated benefit of the pessary either, Dr. Norman said.
In subgroup analyses, odds ratios for adverse neonatal outcomes were “tending towards harm for the Arabin pessary group ... although clearly none of them conferring statistical significance,” she said. Among women with cervical length less than 28 mm, a primary neonatal outcome – at least one of the adverse outcomes – occurred in 23% of patients in the Arabin pessary group, compared with 20% of patients in the standard care group.
Approximately two-thirds of patients found pessary insertion painless or slightly uncomfortable, whereas about 10% described the experience of device fitting as very uncomfortable, and about 1% described it as the worse pain imaginable.
“Since we started STOPPIT-2, in addition to ProTwin, another three studies have been published on the efficacy of the Arabin pessary in twins,” said Dr. Norman. Combined data show no significant effect of the pessary on preventing preterm birth in twin pregnancy. Still, the meta-analysis does not rule out the possibility that there could subgroups of patients who may benefit from the intervention, Dr. Norman said.
STOPPIT-2 was funded by the National Institute for Health Research (NIHR) in the United Kingdom. Dr. Norman chaired the UK National Institute for Health and Care Excellence guidelines on preterm labor and birth in 2015. In addition, Dr. Norman was a member of a GlaxoSmithKline data safety and monitoring group for a trial of a preterm birth prevention agent, has consulted for Dilafor, and has received research grants for preterm birth prevention from the U.K. Medical Research Council, NIHR, and Tommy’s: Together, for every baby charity.
SOURCE: Norman JE et al. Am J Obstet Gynecol. 2020 Jan;222(1):S756. Abstract LB 1.
REPORTING FROM THE PREGNANCY MEETING
Maternal methadone opioid maintenance therapy may be tied to smaller postnatal head circumference
GRAPEVINE, TEX. – compared with opioid maintenance therapy with buprenorphine, according to a study presented at the Pregnancy Meeting.
Antenatal ultrasound measurements do not differ by treatment, however, the researchers said. A separate study suggests that serial ultrasound examinations of fetal brain and biometry measurements may not be helpful in patients who receive these medications for opioid use disorder.
To examine the effects of methadone and buprenorphine opioid maintenance therapy on prenatal and postnatal growth parameters, Jay Davis, MD, a maternal-fetal medicine fellow at Stony Brook University in New York, and coinvestigators conducted a retrospective cohort study using medical records from an academic center during 2007-2017. They included women with singleton pregnancies receiving opioid maintenance therapy with methadone or buprenorphine. They compared head circumference percentile, abdominal circumference percentile, head circumference/abdominal circumference ratio, and postnatal head circumference percentile between the two groups. The investigators analyzed the data using the Wilcoxon–Mann–Whitney test, chi-square test, and logistic regression.
The researchers studied 282 cases, including 120 patients who received buprenorphine and 162 who received methadone. Patients who received buprenorphine delivered at a later average gestational age (39 weeks vs. 37.8 weeks) and had newborns with greater average birth weights (3,206 g vs. 2,877 g). Compared with patients who received methadone, patients who received buprenorphine were significantly more likely to have a larger postnatal head circumference percentile (39 vs. 30), Dr. Davis and colleagues reported. This difference remained significant after controlling for race, prescriber, gestational age at delivery, and birth weight.
In a separate study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine, Jose M. Perez Yordan, MD, of the University of New Mexico, Albuquerque, and colleagues examined effects of medications for opioid use disorder on fetal brain and body measurements.
They found that maternal medications for opioid use disorder do not have a clinically significant effect on fetal brain and body measurements, compared with controls. “No consistent pattern of decreased fetal growth was identified, since the body measurement affected did not persist with serial ultrasounds,” they said. “Serial ultrasound examinations do not appear to be helpful in patients” who take medications of opioid use disorder, with or without alcohol coexposure, unless other risk factors are present.
To evaluate the effects of medications of opioid use disorder and alcohol coexposure on fetal brain and biometric measurements at the second- and third-trimester ultrasound measurements, the investigators are conducting a prospective study known as ENRICH-1. The study includes healthy controls, patients taking medications of opioid use disorder (that is, buprenorphine or methadone), and patients taking medications of opioid use disorder with alcohol coexposure.
Ultrasound measurements from the second and third trimesters evaluated biparietal diameter, femur length, frontal lobe width and length, front-thalamic distance, and caval-calvarial distance. Univariate and multivariate analyses assessed differences in measurements adjusting for gestational age and other factors.
The present analysis included data from 171 participants, including 56 healthy controls, 75 patients taking medications of opioid use disorder, and 40 patients taking medications of opioid use disorder with alcohol coexposure. There was no consistent pattern of decreased fetal growth. Affected measurements did not persist over time.
The study presented by Dr. Perez Yordan was supported by a National Institute on Alcohol Abuse and Alcoholism grant. The remaining investigators in both studies had no relevant financial disclosures.
SOURCE: Perez Yordan JM et al. Am J Obstet Gynecol. 2020 Jan;222(1):S110, Abstract 149; Davis J et al. Am J Obstet Gynecol. 2020 Jan;222(1):S430, Abstract 678.
GRAPEVINE, TEX. – compared with opioid maintenance therapy with buprenorphine, according to a study presented at the Pregnancy Meeting.
Antenatal ultrasound measurements do not differ by treatment, however, the researchers said. A separate study suggests that serial ultrasound examinations of fetal brain and biometry measurements may not be helpful in patients who receive these medications for opioid use disorder.
To examine the effects of methadone and buprenorphine opioid maintenance therapy on prenatal and postnatal growth parameters, Jay Davis, MD, a maternal-fetal medicine fellow at Stony Brook University in New York, and coinvestigators conducted a retrospective cohort study using medical records from an academic center during 2007-2017. They included women with singleton pregnancies receiving opioid maintenance therapy with methadone or buprenorphine. They compared head circumference percentile, abdominal circumference percentile, head circumference/abdominal circumference ratio, and postnatal head circumference percentile between the two groups. The investigators analyzed the data using the Wilcoxon–Mann–Whitney test, chi-square test, and logistic regression.
The researchers studied 282 cases, including 120 patients who received buprenorphine and 162 who received methadone. Patients who received buprenorphine delivered at a later average gestational age (39 weeks vs. 37.8 weeks) and had newborns with greater average birth weights (3,206 g vs. 2,877 g). Compared with patients who received methadone, patients who received buprenorphine were significantly more likely to have a larger postnatal head circumference percentile (39 vs. 30), Dr. Davis and colleagues reported. This difference remained significant after controlling for race, prescriber, gestational age at delivery, and birth weight.
In a separate study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine, Jose M. Perez Yordan, MD, of the University of New Mexico, Albuquerque, and colleagues examined effects of medications for opioid use disorder on fetal brain and body measurements.
They found that maternal medications for opioid use disorder do not have a clinically significant effect on fetal brain and body measurements, compared with controls. “No consistent pattern of decreased fetal growth was identified, since the body measurement affected did not persist with serial ultrasounds,” they said. “Serial ultrasound examinations do not appear to be helpful in patients” who take medications of opioid use disorder, with or without alcohol coexposure, unless other risk factors are present.
To evaluate the effects of medications of opioid use disorder and alcohol coexposure on fetal brain and biometric measurements at the second- and third-trimester ultrasound measurements, the investigators are conducting a prospective study known as ENRICH-1. The study includes healthy controls, patients taking medications of opioid use disorder (that is, buprenorphine or methadone), and patients taking medications of opioid use disorder with alcohol coexposure.
Ultrasound measurements from the second and third trimesters evaluated biparietal diameter, femur length, frontal lobe width and length, front-thalamic distance, and caval-calvarial distance. Univariate and multivariate analyses assessed differences in measurements adjusting for gestational age and other factors.
The present analysis included data from 171 participants, including 56 healthy controls, 75 patients taking medications of opioid use disorder, and 40 patients taking medications of opioid use disorder with alcohol coexposure. There was no consistent pattern of decreased fetal growth. Affected measurements did not persist over time.
The study presented by Dr. Perez Yordan was supported by a National Institute on Alcohol Abuse and Alcoholism grant. The remaining investigators in both studies had no relevant financial disclosures.
SOURCE: Perez Yordan JM et al. Am J Obstet Gynecol. 2020 Jan;222(1):S110, Abstract 149; Davis J et al. Am J Obstet Gynecol. 2020 Jan;222(1):S430, Abstract 678.
GRAPEVINE, TEX. – compared with opioid maintenance therapy with buprenorphine, according to a study presented at the Pregnancy Meeting.
Antenatal ultrasound measurements do not differ by treatment, however, the researchers said. A separate study suggests that serial ultrasound examinations of fetal brain and biometry measurements may not be helpful in patients who receive these medications for opioid use disorder.
To examine the effects of methadone and buprenorphine opioid maintenance therapy on prenatal and postnatal growth parameters, Jay Davis, MD, a maternal-fetal medicine fellow at Stony Brook University in New York, and coinvestigators conducted a retrospective cohort study using medical records from an academic center during 2007-2017. They included women with singleton pregnancies receiving opioid maintenance therapy with methadone or buprenorphine. They compared head circumference percentile, abdominal circumference percentile, head circumference/abdominal circumference ratio, and postnatal head circumference percentile between the two groups. The investigators analyzed the data using the Wilcoxon–Mann–Whitney test, chi-square test, and logistic regression.
The researchers studied 282 cases, including 120 patients who received buprenorphine and 162 who received methadone. Patients who received buprenorphine delivered at a later average gestational age (39 weeks vs. 37.8 weeks) and had newborns with greater average birth weights (3,206 g vs. 2,877 g). Compared with patients who received methadone, patients who received buprenorphine were significantly more likely to have a larger postnatal head circumference percentile (39 vs. 30), Dr. Davis and colleagues reported. This difference remained significant after controlling for race, prescriber, gestational age at delivery, and birth weight.
In a separate study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine, Jose M. Perez Yordan, MD, of the University of New Mexico, Albuquerque, and colleagues examined effects of medications for opioid use disorder on fetal brain and body measurements.
They found that maternal medications for opioid use disorder do not have a clinically significant effect on fetal brain and body measurements, compared with controls. “No consistent pattern of decreased fetal growth was identified, since the body measurement affected did not persist with serial ultrasounds,” they said. “Serial ultrasound examinations do not appear to be helpful in patients” who take medications of opioid use disorder, with or without alcohol coexposure, unless other risk factors are present.
To evaluate the effects of medications of opioid use disorder and alcohol coexposure on fetal brain and biometric measurements at the second- and third-trimester ultrasound measurements, the investigators are conducting a prospective study known as ENRICH-1. The study includes healthy controls, patients taking medications of opioid use disorder (that is, buprenorphine or methadone), and patients taking medications of opioid use disorder with alcohol coexposure.
Ultrasound measurements from the second and third trimesters evaluated biparietal diameter, femur length, frontal lobe width and length, front-thalamic distance, and caval-calvarial distance. Univariate and multivariate analyses assessed differences in measurements adjusting for gestational age and other factors.
The present analysis included data from 171 participants, including 56 healthy controls, 75 patients taking medications of opioid use disorder, and 40 patients taking medications of opioid use disorder with alcohol coexposure. There was no consistent pattern of decreased fetal growth. Affected measurements did not persist over time.
The study presented by Dr. Perez Yordan was supported by a National Institute on Alcohol Abuse and Alcoholism grant. The remaining investigators in both studies had no relevant financial disclosures.
SOURCE: Perez Yordan JM et al. Am J Obstet Gynecol. 2020 Jan;222(1):S110, Abstract 149; Davis J et al. Am J Obstet Gynecol. 2020 Jan;222(1):S430, Abstract 678.
REPORTING FROM THE PREGNANCY MEETING
CVH in pregnant women: Ample room for improvement
Cardiovascular disease is both common and chronic, and it remains the leading cause of death in women. Because it is a life-long condition, cardiovascular disease must be managed over the entire lifespan. In recognition of the important role of obstetricians and gynecologists in monitoring women’s health, the American Heart Association/American College of Obstetricians and Gynecologists 2018 guidelines1 promoted the use of “Life’s Simple 7”2 for assessing cardiovascular health (CVH) in women.
These seven metrics include diet, physical activity, smoking status, body mass index (BMI), blood pressure, total cholesterol, and fasting blood glucose levels. They have been shown to predict positive health outcomes in nonpregnant adults. However, until now, CVH had not been assessed in pregnant women.
Perak et al. recently performed the first cross-sectional study of the prevalence of CVH metrics in pregnant women using the AHA definition.3 Using data from the National Health and Nutrition Examination Surveys (NHANES), they used the Life’s Simple 7 metrics to assess CVH in 1,117 pregnant and 8,200 nonpregnant women in the United States aged 20-44 years. Each of the Life’s Simple 7 metrics was scored 0, 1, or 2 points, corresponding to a rating of poor, intermediate, or ideal, respectively. Thus, the total CVH score ranged from 0-14 points, with total scores of 0-7 indicating low CVH, 8-11 indicating moderate CVH, and 12-14 indicating high CVH.
which was even worse than in nonpregnant women, of whom only 13% were scored as having ideal CVH. Ideal scores were observed for 0.1% of pregnant women for diet, 27% for physical activity, 39% for cholesterol levels, 51% for BMI, 78% for smoking, 90% for blood pressure, and 92% for fasting blood glucose. Physical activity and cholesterol levels appeared to be the major drivers of the lower CVH scores in pregnant women.
Although further studies are warranted to determine the relevance of CVH during pregnancy to outcomes for both mother and offspring, the study by Perak et al. is an important step toward the development of pregnancy-specific guidelines and definitions for CVH metrics. These are stated goals of the AHA/ACOG that will help promote CVH in women across their lifespans, but which have not been possible due to scant data.
Emerging data suggest that cumulative lifetime exposure is a significant factor in cardiovascular disease outcomes; therefore, earlier intervention would have a more significant impact. Just as gestational diabetes is a predictor of future type 2 diabetes, CVH earlier in a woman’s life predicts cardiovascular disease later in life.4-7 The best data in this regard come from genetic and other studies of hyperlipidemia, which suggest that lowering lipid levels before symptoms develop may prevent cardiovascular disease. In contrast, treatment of patients with clinically manifest disease neither offers a cure nor prevents the occurrence of most cardiovascular events.
It is a particularly salient point in this regard that there currently are no guidelines on treatment of hypercholesterolemia during pregnancy. Notably, the study by Perak et al. suggested that cholesterol levels may have a significant impact on CVH in pregnant women. There also is emerging data supporting the importance of controlling blood pressure across the lifespan,7,8 including during pregnancy.9
For many women, their ob.gyn. is their primary care physician, and pregnancy is often the first time that a woman will have a substantial interaction with the health care system. The AHA/ACOG advisory panel described pregnancy as a “physiological stress test” for women that offers the opportunity to identify those at increased risk of cardiovascular disease.1
As pregnancy is a time when women particularly are motivated to improve their health,10 it also presents a valuable opportunity for physicians, including ob.gyns., to make a lifelong impact on the CVH of their patients through early identification, education, and intervention.
Dr. Charles Hong is the Melvin Sharoky, MD, Professor of Medicine and director of cardiovascular research in the department of medicine at the University of Maryland School of Medicine. Dr. E. Albert Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Neither physician had any relevant financial disclosures. Contact him at [email protected].
References
1. Circulation. 2018;137:e843–e852.
2. Circulation. 2010 Jan 20;121(4):586–613.
3. J Am Heart Assoc. 2020 Feb 17;9:e015123.
4. J Am Coll Cardiol. 2018 Sep 4;72(10):1141-56.
5. N Engl J Med. 2016 Dec 1;375:2144-53.
6. Nat Rev Cardiol. 2011 Nov 1;8(12):721-5.
7. J Am Coll Cardiol. 2019 Jul 23;74(3):330-41.
8. Circulation. 2020 Mar 2:141:725-7.
9. Circulation. 2013 Feb 12;127(6):681-90.
10. Nutrients. 2018 Aug 8. doi: 10.3390/nu10081032.
Cardiovascular disease is both common and chronic, and it remains the leading cause of death in women. Because it is a life-long condition, cardiovascular disease must be managed over the entire lifespan. In recognition of the important role of obstetricians and gynecologists in monitoring women’s health, the American Heart Association/American College of Obstetricians and Gynecologists 2018 guidelines1 promoted the use of “Life’s Simple 7”2 for assessing cardiovascular health (CVH) in women.
These seven metrics include diet, physical activity, smoking status, body mass index (BMI), blood pressure, total cholesterol, and fasting blood glucose levels. They have been shown to predict positive health outcomes in nonpregnant adults. However, until now, CVH had not been assessed in pregnant women.
Perak et al. recently performed the first cross-sectional study of the prevalence of CVH metrics in pregnant women using the AHA definition.3 Using data from the National Health and Nutrition Examination Surveys (NHANES), they used the Life’s Simple 7 metrics to assess CVH in 1,117 pregnant and 8,200 nonpregnant women in the United States aged 20-44 years. Each of the Life’s Simple 7 metrics was scored 0, 1, or 2 points, corresponding to a rating of poor, intermediate, or ideal, respectively. Thus, the total CVH score ranged from 0-14 points, with total scores of 0-7 indicating low CVH, 8-11 indicating moderate CVH, and 12-14 indicating high CVH.
which was even worse than in nonpregnant women, of whom only 13% were scored as having ideal CVH. Ideal scores were observed for 0.1% of pregnant women for diet, 27% for physical activity, 39% for cholesterol levels, 51% for BMI, 78% for smoking, 90% for blood pressure, and 92% for fasting blood glucose. Physical activity and cholesterol levels appeared to be the major drivers of the lower CVH scores in pregnant women.
Although further studies are warranted to determine the relevance of CVH during pregnancy to outcomes for both mother and offspring, the study by Perak et al. is an important step toward the development of pregnancy-specific guidelines and definitions for CVH metrics. These are stated goals of the AHA/ACOG that will help promote CVH in women across their lifespans, but which have not been possible due to scant data.
Emerging data suggest that cumulative lifetime exposure is a significant factor in cardiovascular disease outcomes; therefore, earlier intervention would have a more significant impact. Just as gestational diabetes is a predictor of future type 2 diabetes, CVH earlier in a woman’s life predicts cardiovascular disease later in life.4-7 The best data in this regard come from genetic and other studies of hyperlipidemia, which suggest that lowering lipid levels before symptoms develop may prevent cardiovascular disease. In contrast, treatment of patients with clinically manifest disease neither offers a cure nor prevents the occurrence of most cardiovascular events.
It is a particularly salient point in this regard that there currently are no guidelines on treatment of hypercholesterolemia during pregnancy. Notably, the study by Perak et al. suggested that cholesterol levels may have a significant impact on CVH in pregnant women. There also is emerging data supporting the importance of controlling blood pressure across the lifespan,7,8 including during pregnancy.9
For many women, their ob.gyn. is their primary care physician, and pregnancy is often the first time that a woman will have a substantial interaction with the health care system. The AHA/ACOG advisory panel described pregnancy as a “physiological stress test” for women that offers the opportunity to identify those at increased risk of cardiovascular disease.1
As pregnancy is a time when women particularly are motivated to improve their health,10 it also presents a valuable opportunity for physicians, including ob.gyns., to make a lifelong impact on the CVH of their patients through early identification, education, and intervention.
Dr. Charles Hong is the Melvin Sharoky, MD, Professor of Medicine and director of cardiovascular research in the department of medicine at the University of Maryland School of Medicine. Dr. E. Albert Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Neither physician had any relevant financial disclosures. Contact him at [email protected].
References
1. Circulation. 2018;137:e843–e852.
2. Circulation. 2010 Jan 20;121(4):586–613.
3. J Am Heart Assoc. 2020 Feb 17;9:e015123.
4. J Am Coll Cardiol. 2018 Sep 4;72(10):1141-56.
5. N Engl J Med. 2016 Dec 1;375:2144-53.
6. Nat Rev Cardiol. 2011 Nov 1;8(12):721-5.
7. J Am Coll Cardiol. 2019 Jul 23;74(3):330-41.
8. Circulation. 2020 Mar 2:141:725-7.
9. Circulation. 2013 Feb 12;127(6):681-90.
10. Nutrients. 2018 Aug 8. doi: 10.3390/nu10081032.
Cardiovascular disease is both common and chronic, and it remains the leading cause of death in women. Because it is a life-long condition, cardiovascular disease must be managed over the entire lifespan. In recognition of the important role of obstetricians and gynecologists in monitoring women’s health, the American Heart Association/American College of Obstetricians and Gynecologists 2018 guidelines1 promoted the use of “Life’s Simple 7”2 for assessing cardiovascular health (CVH) in women.
These seven metrics include diet, physical activity, smoking status, body mass index (BMI), blood pressure, total cholesterol, and fasting blood glucose levels. They have been shown to predict positive health outcomes in nonpregnant adults. However, until now, CVH had not been assessed in pregnant women.
Perak et al. recently performed the first cross-sectional study of the prevalence of CVH metrics in pregnant women using the AHA definition.3 Using data from the National Health and Nutrition Examination Surveys (NHANES), they used the Life’s Simple 7 metrics to assess CVH in 1,117 pregnant and 8,200 nonpregnant women in the United States aged 20-44 years. Each of the Life’s Simple 7 metrics was scored 0, 1, or 2 points, corresponding to a rating of poor, intermediate, or ideal, respectively. Thus, the total CVH score ranged from 0-14 points, with total scores of 0-7 indicating low CVH, 8-11 indicating moderate CVH, and 12-14 indicating high CVH.
which was even worse than in nonpregnant women, of whom only 13% were scored as having ideal CVH. Ideal scores were observed for 0.1% of pregnant women for diet, 27% for physical activity, 39% for cholesterol levels, 51% for BMI, 78% for smoking, 90% for blood pressure, and 92% for fasting blood glucose. Physical activity and cholesterol levels appeared to be the major drivers of the lower CVH scores in pregnant women.
Although further studies are warranted to determine the relevance of CVH during pregnancy to outcomes for both mother and offspring, the study by Perak et al. is an important step toward the development of pregnancy-specific guidelines and definitions for CVH metrics. These are stated goals of the AHA/ACOG that will help promote CVH in women across their lifespans, but which have not been possible due to scant data.
Emerging data suggest that cumulative lifetime exposure is a significant factor in cardiovascular disease outcomes; therefore, earlier intervention would have a more significant impact. Just as gestational diabetes is a predictor of future type 2 diabetes, CVH earlier in a woman’s life predicts cardiovascular disease later in life.4-7 The best data in this regard come from genetic and other studies of hyperlipidemia, which suggest that lowering lipid levels before symptoms develop may prevent cardiovascular disease. In contrast, treatment of patients with clinically manifest disease neither offers a cure nor prevents the occurrence of most cardiovascular events.
It is a particularly salient point in this regard that there currently are no guidelines on treatment of hypercholesterolemia during pregnancy. Notably, the study by Perak et al. suggested that cholesterol levels may have a significant impact on CVH in pregnant women. There also is emerging data supporting the importance of controlling blood pressure across the lifespan,7,8 including during pregnancy.9
For many women, their ob.gyn. is their primary care physician, and pregnancy is often the first time that a woman will have a substantial interaction with the health care system. The AHA/ACOG advisory panel described pregnancy as a “physiological stress test” for women that offers the opportunity to identify those at increased risk of cardiovascular disease.1
As pregnancy is a time when women particularly are motivated to improve their health,10 it also presents a valuable opportunity for physicians, including ob.gyns., to make a lifelong impact on the CVH of their patients through early identification, education, and intervention.
Dr. Charles Hong is the Melvin Sharoky, MD, Professor of Medicine and director of cardiovascular research in the department of medicine at the University of Maryland School of Medicine. Dr. E. Albert Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Neither physician had any relevant financial disclosures. Contact him at [email protected].
References
1. Circulation. 2018;137:e843–e852.
2. Circulation. 2010 Jan 20;121(4):586–613.
3. J Am Heart Assoc. 2020 Feb 17;9:e015123.
4. J Am Coll Cardiol. 2018 Sep 4;72(10):1141-56.
5. N Engl J Med. 2016 Dec 1;375:2144-53.
6. Nat Rev Cardiol. 2011 Nov 1;8(12):721-5.
7. J Am Coll Cardiol. 2019 Jul 23;74(3):330-41.
8. Circulation. 2020 Mar 2:141:725-7.
9. Circulation. 2013 Feb 12;127(6):681-90.
10. Nutrients. 2018 Aug 8. doi: 10.3390/nu10081032.
Chlamydia trachomatis infections
CASE Pregnant woman with symptoms of genital infection
A 23-year-old primigravid woman at 15 weeks and 2 days’ gestation reported having a 2-week history of increased urinary frequency and vaginal discharge. She said she experienced similar symptoms 6 weeks previously that resolved within a week. The patient has had 3 sexual partners in the past year. Her current partner was experiencing a yellow urethral discharge and dysuria. On the patient’s speculum examination, the clinician noted a yellow-green discharge emanating from the cervix as well as cervical motion tenderness.
What is the most likely diagnosis, and how would you treat this patient?
The culprit was chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium that does not stain with Gram staining. A rigid cell wall encloses its intracellular component. C trachomatis infection begins when the chlamydial elementary body enters a susceptible host cell.
Once ingested, the organism’s surface antigens (major outer membrane protein and lipopolysaccharide antigens) provide intracellular sanctuary for the bacterium by inhibiting phagolysosomal fusion. Subsequently, the elementary body morphs into a reticular body, which replicates through adenosine triphosphate (ATP)–dependent binary fission. After approximately 48 hours of replication, the organism again morphs into an elementary body and is released to infect additional cells and acquire new ATP stores for further replication.
Chlamydia can be transmitted horizontally during oral, vaginal, or anal intercourse or vertically to the infant during vaginal delivery.
The US’s most common notifiable disease
According to the Centers for Disease Control and Prevention (CDC), the incidence of chlamydia infection in the United States increased considerably in recent years: from 976,455 cases in 2005 to 1,758,668 cases in 2018.1 In 2018, rates of chlamydia infection in women were nearly double the rates in men, with an incidence of 688.2 versus 377.5 per 100,000 cases, and a prevalence of 1,150,672 versus 612,020.1
Young adults have a higher frequency of chlamydia infection than any other age group. From 2017 to 2018, reported cases in women aged 15–19 years increased by 1.3%, to 3,306.8 per 100,000; in women aged 20–24 years, cases increased by 0.8%, to 4,064.6 per 100,000. In young men in the same age ranges, reported cases increased by 3.7%, to 959.0 cases per 100,000, and by 3.3%, to 1,784.5 per 100,000 cases, respectively.1
Both the incidence and prevalence of chlamydia infection are higher in African Americans than in whites, while Asians have the lowest rates.1 The prevalence of infection also is increased with incarceration, lower socioeconomic status, and residence in the southern United States.
The prevalence of chlamydia infection in pregnant women is approximately 2% to 3%, but it may be as high as 30% in high-risk populations, such as women who are unmarried, have multiple sex partners, are coinfected with another sexually transmitted disease (STD), have partners with nongonococcal urethritis, have mucopurulent discharge, have acute urethral syndrome, and have late or no prenatal care.2 Since chlamydia infection often is asymptomatic and some infections resolve spontaneously, the true prevalence of infection probably is underreported.
Continue to: Chlamydia infection can cause serious clinical manifestations...
Chlamydia infection can cause serious clinical manifestations
The 15 serotypes of C trachomatis are grouped into 3 categories according to clinical manifestations:
- Serotypes A, B, Ba, and C cause endemic trachoma, characterized by bilateral irritation of the eyelids that progresses to eyelid thickening and scarring, eventually leading to corneal abrasion and blindness.
- Serotypes D–K manifest as conjunctivitis and pneumonia in newborns, proctitis in men (especially in men who have sex with men), and genitourinary infections in women. Reactive arthritis and inclusion conjunctivitis also can occur with D–K serotypes.
- Serotypes L1–L3 cause lymphogranuloma venereum.
About 70% of women with chlamydia infection are asymptomatic. Those who have symptoms often present with endocervicitis or acute urethral syndrome (acute urethritis). Manifestations of these 2 conditions include a frothy yellow-green vaginal and/or urethral discharge, dysuria, and frequency. Women who engage in rectal intercourse also may notice a purulent discharge from the anus. Untreated, C trachomatis organisms may ascend the reproductive tract, causing both endometritis and pelvic inflammatory disease (PID).
While a single episode of PID increases tubal infertility risk by 10%, a second episode increases the risk by 40%.3 Over time, recurrent and/or chronic PID causes scarring and adhesion formation, which may result in chronic pelvic pain. In addition, chronic infection is the single most important risk factor for ectopic pregnancy. Finally, chlamydia infection is a risk factor for Fitz-Hugh-Cutis syndrome (perihepatitis). In this condition, organisms ascend from the site of pelvic infection along the pericolic gutter to ultimately infect the liver capsule.
Specific complications in pregnancy
Chlamydia infection in pregnant women is associated with preterm delivery and preterm premature rupture of membranes. Infants born to mothers with untreated chlamydia infection are at risk for pneumonia, conjunctivitis, and even perinatal death.2 Acquisition of infection occurs at the time of delivery rather than in the antepartum period.
The significant morbidity associated with chlamydia infection underscores the importance of regular screening, especially in pregnant women. The current United States Preventive Service Task Force guidelines recommend annual screening of all sexually active women who are 24 years of age or younger, as well as of older, high-risk women.
The CDC recommends routine screening of all pregnant women for chlamydia at the first prenatal visit. Repeat screening is recommended in the third trimester for all pregnant women younger than 25 years, those at increased risk, and those infected within the past 3 to 6 months or during the first trimester. Those who test positive should be retested 3 weeks after completion of treatment.1
Chlamydia screening strategies
Historically, a chlamydia diagnosis was made by isolating the organisms in tissue culture. In the 1990s, however, that extremely time-consuming and resource-intensive procedure was replaced by nucleic acid amplification testing (NAAT).
NAAT methodology. NAAT is the gold standard for diagnosing C trachomatis infection; this methodology utilizes various assays, including polymerase chain reaction, ligase chain reaction, and transcription-mediated amplification.
Continue to: Compared with previous culture and antigen detection techniques...
Compared with previous culture and antigen detection techniques, NAAT’s advantages include excellent sensitivity and specificity (>90% and ≥99%, respectively), enabling detection of a low inoculum of organisms in a sample obtained by noninvasive methods, such as first-void urine collection or vaginal swab.2,4,5 Furthermore, NAAT does not impose any specific storage regulations on collected specimens, is cost effective, and can jointly test for Neisseria gonorrhoeae, which commonly co-infects with C trachomatis.6
Screening in pregnancy. In 2012, Blatt and colleagues examined testing patterns in nearly 1.3 million obstetric patients and found that only 59% (761,315) of women were tested for chlamydia at least once in pregnancy.7 Only 1 in 3 women were tested during the first prenatal visit, as CDC guidelines recommend. Testing rates declined with increasing age. Of women screened, 3.5% tested positive for chlamydia.7 Of these, 3 of 4 were retested at least once, with almost 20% having at least 1 subsequent positive result.7
Of note, in a study of women who reported receptive anal intercourse (n = 2,818), 292 women tested positive for chlamydia; 10.4% tested positive in genital-only sites, 58.6% in genital and rectal sites, and 20.5% at the rectal site only.8
It is alarming that only 59% of pregnant women are screened for chlamydia given the significant perinatal complications associated with this infection. Barriers to screening pregnant women may include clinician discomfort in discussing STDs and patient refusal of screening. Furthermore, clinicians should routinely ask women about receptive anal sex. Women who report this risk factor should be tested for chlamydia in both the endocervix and rectum.
Retesting and follow-up. After the initial diagnosis of chlamydia, a test of cure 3 weeks after treatment is an important aspect of care. Thus, identifying and overcoming barriers to retesting is important. Clinicians should educate patients about the importance of follow-up. Also consider incorporating the use of home-based, self-obtained vaginal swabs for retesting. Results from 2 randomized trials showed that eliminating a patient’s transportation barriers and providing a home-based alternative to a follow-up visit significantly increased rescreening rates by 33% in STD clinic patients and by 59.2% in family planning clinic patients.9
Reinfection risk. The rate of venereal chlamydia transmission in heterosexual partners is 70%. Since sexually active chlamydia-positive patients are at risk for reinfection by their partner after treatment completion, clinicians should refer the sex partners for evaluation. If the sex partners are reluctant to have testing, it is reasonable to provide empiric antibiotic treatment to decrease the risk of re-infection in the patient.7 Before doing so, however, make certain that state law permits this practice, and be sure to document the prescribed treatment in the patient’s record.
Treatment options
Prompt treatment of C trachomatis infection is essential to decrease the risk of disease sequelae. Nonpregnant adults can be treated with oral doxycycline 100 mg twice daily for 7 days.
In a head-to-head study performed in a controlled environment that ensured treatment adherence, 97% efficacy was achieved with one oral dose of azithromycin (1 g) compared with 100% efficacy with doxycycline.10 However, in the real-world setting, imperfect adherence to the multi-day doxycycline regimen is associated with treatment failures. Thus, a single dose of azithromycin is preferable for patients with questionable compliance.11
In obstetric patients, azithromycin and amoxicillin are preferred as first-line agents for treatment of C trachomatis due to their improved safety profile in this demographic. Amoxicillin 500 mg orally 3 times daily for 7 days has 95% efficacy.2
Women allergic to these agents may be treated with an alternative regimen of erythromycin base, 500 mg orally 4 times daily for 7 days, or erythromycin ethylsuccinate, 800 mg orally 4 times daily for 7 days. Erythromycin should be reserved for second-line therapy because of its lower efficacy (64%) and frequent gastrointestinal adverse effects.2 Doxycycline is contraindicated in pregnancy because of possible teratogenic effects on the teeth and bone of the fetus.
- Centers for Disease Control and Prevention Division of STD Prevention. Sexually transmitted disease surveillance 2018. October 2019. https://www.cdc.gov/std/stats18/default.htm. 2019. Accessed January 4, 2020.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams JD, et al, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2019:869.
- Ljubin-Sternak S, Meštrović T. Chlamydia trachomatis and genital mycoplasmas: pathogens with an impact on human reproductive health. J Pathog. 2014. doi: 10.1155/2014/183167.
- Meyer T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms. 2016:4(3).
- Centers for Disease Control and Prevention.. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep. 2014;63:1-19.
- Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017;376:765-773.
- Blatt AJ, Lieberman JM, Hoover DR, et al. Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol. 2012;207:55.e1-8.
- Llata E, Braxton J, Asbel L, et al. Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse. Obstet Gynecol. 2018;132:692-697.
- Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol. 2011;118(2 pt 1):231-239.
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373:2512-2521.
- Quinn TC, Gaydos CA. Treatment for chlamydia infection—doxycycline versus azithromycin. N Engl J Med. 2015;373:2573-2575.
CASE Pregnant woman with symptoms of genital infection
A 23-year-old primigravid woman at 15 weeks and 2 days’ gestation reported having a 2-week history of increased urinary frequency and vaginal discharge. She said she experienced similar symptoms 6 weeks previously that resolved within a week. The patient has had 3 sexual partners in the past year. Her current partner was experiencing a yellow urethral discharge and dysuria. On the patient’s speculum examination, the clinician noted a yellow-green discharge emanating from the cervix as well as cervical motion tenderness.
What is the most likely diagnosis, and how would you treat this patient?
The culprit was chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium that does not stain with Gram staining. A rigid cell wall encloses its intracellular component. C trachomatis infection begins when the chlamydial elementary body enters a susceptible host cell.
Once ingested, the organism’s surface antigens (major outer membrane protein and lipopolysaccharide antigens) provide intracellular sanctuary for the bacterium by inhibiting phagolysosomal fusion. Subsequently, the elementary body morphs into a reticular body, which replicates through adenosine triphosphate (ATP)–dependent binary fission. After approximately 48 hours of replication, the organism again morphs into an elementary body and is released to infect additional cells and acquire new ATP stores for further replication.
Chlamydia can be transmitted horizontally during oral, vaginal, or anal intercourse or vertically to the infant during vaginal delivery.
The US’s most common notifiable disease
According to the Centers for Disease Control and Prevention (CDC), the incidence of chlamydia infection in the United States increased considerably in recent years: from 976,455 cases in 2005 to 1,758,668 cases in 2018.1 In 2018, rates of chlamydia infection in women were nearly double the rates in men, with an incidence of 688.2 versus 377.5 per 100,000 cases, and a prevalence of 1,150,672 versus 612,020.1
Young adults have a higher frequency of chlamydia infection than any other age group. From 2017 to 2018, reported cases in women aged 15–19 years increased by 1.3%, to 3,306.8 per 100,000; in women aged 20–24 years, cases increased by 0.8%, to 4,064.6 per 100,000. In young men in the same age ranges, reported cases increased by 3.7%, to 959.0 cases per 100,000, and by 3.3%, to 1,784.5 per 100,000 cases, respectively.1
Both the incidence and prevalence of chlamydia infection are higher in African Americans than in whites, while Asians have the lowest rates.1 The prevalence of infection also is increased with incarceration, lower socioeconomic status, and residence in the southern United States.
The prevalence of chlamydia infection in pregnant women is approximately 2% to 3%, but it may be as high as 30% in high-risk populations, such as women who are unmarried, have multiple sex partners, are coinfected with another sexually transmitted disease (STD), have partners with nongonococcal urethritis, have mucopurulent discharge, have acute urethral syndrome, and have late or no prenatal care.2 Since chlamydia infection often is asymptomatic and some infections resolve spontaneously, the true prevalence of infection probably is underreported.
Continue to: Chlamydia infection can cause serious clinical manifestations...
Chlamydia infection can cause serious clinical manifestations
The 15 serotypes of C trachomatis are grouped into 3 categories according to clinical manifestations:
- Serotypes A, B, Ba, and C cause endemic trachoma, characterized by bilateral irritation of the eyelids that progresses to eyelid thickening and scarring, eventually leading to corneal abrasion and blindness.
- Serotypes D–K manifest as conjunctivitis and pneumonia in newborns, proctitis in men (especially in men who have sex with men), and genitourinary infections in women. Reactive arthritis and inclusion conjunctivitis also can occur with D–K serotypes.
- Serotypes L1–L3 cause lymphogranuloma venereum.
About 70% of women with chlamydia infection are asymptomatic. Those who have symptoms often present with endocervicitis or acute urethral syndrome (acute urethritis). Manifestations of these 2 conditions include a frothy yellow-green vaginal and/or urethral discharge, dysuria, and frequency. Women who engage in rectal intercourse also may notice a purulent discharge from the anus. Untreated, C trachomatis organisms may ascend the reproductive tract, causing both endometritis and pelvic inflammatory disease (PID).
While a single episode of PID increases tubal infertility risk by 10%, a second episode increases the risk by 40%.3 Over time, recurrent and/or chronic PID causes scarring and adhesion formation, which may result in chronic pelvic pain. In addition, chronic infection is the single most important risk factor for ectopic pregnancy. Finally, chlamydia infection is a risk factor for Fitz-Hugh-Cutis syndrome (perihepatitis). In this condition, organisms ascend from the site of pelvic infection along the pericolic gutter to ultimately infect the liver capsule.
Specific complications in pregnancy
Chlamydia infection in pregnant women is associated with preterm delivery and preterm premature rupture of membranes. Infants born to mothers with untreated chlamydia infection are at risk for pneumonia, conjunctivitis, and even perinatal death.2 Acquisition of infection occurs at the time of delivery rather than in the antepartum period.
The significant morbidity associated with chlamydia infection underscores the importance of regular screening, especially in pregnant women. The current United States Preventive Service Task Force guidelines recommend annual screening of all sexually active women who are 24 years of age or younger, as well as of older, high-risk women.
The CDC recommends routine screening of all pregnant women for chlamydia at the first prenatal visit. Repeat screening is recommended in the third trimester for all pregnant women younger than 25 years, those at increased risk, and those infected within the past 3 to 6 months or during the first trimester. Those who test positive should be retested 3 weeks after completion of treatment.1
Chlamydia screening strategies
Historically, a chlamydia diagnosis was made by isolating the organisms in tissue culture. In the 1990s, however, that extremely time-consuming and resource-intensive procedure was replaced by nucleic acid amplification testing (NAAT).
NAAT methodology. NAAT is the gold standard for diagnosing C trachomatis infection; this methodology utilizes various assays, including polymerase chain reaction, ligase chain reaction, and transcription-mediated amplification.
Continue to: Compared with previous culture and antigen detection techniques...
Compared with previous culture and antigen detection techniques, NAAT’s advantages include excellent sensitivity and specificity (>90% and ≥99%, respectively), enabling detection of a low inoculum of organisms in a sample obtained by noninvasive methods, such as first-void urine collection or vaginal swab.2,4,5 Furthermore, NAAT does not impose any specific storage regulations on collected specimens, is cost effective, and can jointly test for Neisseria gonorrhoeae, which commonly co-infects with C trachomatis.6
Screening in pregnancy. In 2012, Blatt and colleagues examined testing patterns in nearly 1.3 million obstetric patients and found that only 59% (761,315) of women were tested for chlamydia at least once in pregnancy.7 Only 1 in 3 women were tested during the first prenatal visit, as CDC guidelines recommend. Testing rates declined with increasing age. Of women screened, 3.5% tested positive for chlamydia.7 Of these, 3 of 4 were retested at least once, with almost 20% having at least 1 subsequent positive result.7
Of note, in a study of women who reported receptive anal intercourse (n = 2,818), 292 women tested positive for chlamydia; 10.4% tested positive in genital-only sites, 58.6% in genital and rectal sites, and 20.5% at the rectal site only.8
It is alarming that only 59% of pregnant women are screened for chlamydia given the significant perinatal complications associated with this infection. Barriers to screening pregnant women may include clinician discomfort in discussing STDs and patient refusal of screening. Furthermore, clinicians should routinely ask women about receptive anal sex. Women who report this risk factor should be tested for chlamydia in both the endocervix and rectum.
Retesting and follow-up. After the initial diagnosis of chlamydia, a test of cure 3 weeks after treatment is an important aspect of care. Thus, identifying and overcoming barriers to retesting is important. Clinicians should educate patients about the importance of follow-up. Also consider incorporating the use of home-based, self-obtained vaginal swabs for retesting. Results from 2 randomized trials showed that eliminating a patient’s transportation barriers and providing a home-based alternative to a follow-up visit significantly increased rescreening rates by 33% in STD clinic patients and by 59.2% in family planning clinic patients.9
Reinfection risk. The rate of venereal chlamydia transmission in heterosexual partners is 70%. Since sexually active chlamydia-positive patients are at risk for reinfection by their partner after treatment completion, clinicians should refer the sex partners for evaluation. If the sex partners are reluctant to have testing, it is reasonable to provide empiric antibiotic treatment to decrease the risk of re-infection in the patient.7 Before doing so, however, make certain that state law permits this practice, and be sure to document the prescribed treatment in the patient’s record.
Treatment options
Prompt treatment of C trachomatis infection is essential to decrease the risk of disease sequelae. Nonpregnant adults can be treated with oral doxycycline 100 mg twice daily for 7 days.
In a head-to-head study performed in a controlled environment that ensured treatment adherence, 97% efficacy was achieved with one oral dose of azithromycin (1 g) compared with 100% efficacy with doxycycline.10 However, in the real-world setting, imperfect adherence to the multi-day doxycycline regimen is associated with treatment failures. Thus, a single dose of azithromycin is preferable for patients with questionable compliance.11
In obstetric patients, azithromycin and amoxicillin are preferred as first-line agents for treatment of C trachomatis due to their improved safety profile in this demographic. Amoxicillin 500 mg orally 3 times daily for 7 days has 95% efficacy.2
Women allergic to these agents may be treated with an alternative regimen of erythromycin base, 500 mg orally 4 times daily for 7 days, or erythromycin ethylsuccinate, 800 mg orally 4 times daily for 7 days. Erythromycin should be reserved for second-line therapy because of its lower efficacy (64%) and frequent gastrointestinal adverse effects.2 Doxycycline is contraindicated in pregnancy because of possible teratogenic effects on the teeth and bone of the fetus.
CASE Pregnant woman with symptoms of genital infection
A 23-year-old primigravid woman at 15 weeks and 2 days’ gestation reported having a 2-week history of increased urinary frequency and vaginal discharge. She said she experienced similar symptoms 6 weeks previously that resolved within a week. The patient has had 3 sexual partners in the past year. Her current partner was experiencing a yellow urethral discharge and dysuria. On the patient’s speculum examination, the clinician noted a yellow-green discharge emanating from the cervix as well as cervical motion tenderness.
What is the most likely diagnosis, and how would you treat this patient?
The culprit was chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium that does not stain with Gram staining. A rigid cell wall encloses its intracellular component. C trachomatis infection begins when the chlamydial elementary body enters a susceptible host cell.
Once ingested, the organism’s surface antigens (major outer membrane protein and lipopolysaccharide antigens) provide intracellular sanctuary for the bacterium by inhibiting phagolysosomal fusion. Subsequently, the elementary body morphs into a reticular body, which replicates through adenosine triphosphate (ATP)–dependent binary fission. After approximately 48 hours of replication, the organism again morphs into an elementary body and is released to infect additional cells and acquire new ATP stores for further replication.
Chlamydia can be transmitted horizontally during oral, vaginal, or anal intercourse or vertically to the infant during vaginal delivery.
The US’s most common notifiable disease
According to the Centers for Disease Control and Prevention (CDC), the incidence of chlamydia infection in the United States increased considerably in recent years: from 976,455 cases in 2005 to 1,758,668 cases in 2018.1 In 2018, rates of chlamydia infection in women were nearly double the rates in men, with an incidence of 688.2 versus 377.5 per 100,000 cases, and a prevalence of 1,150,672 versus 612,020.1
Young adults have a higher frequency of chlamydia infection than any other age group. From 2017 to 2018, reported cases in women aged 15–19 years increased by 1.3%, to 3,306.8 per 100,000; in women aged 20–24 years, cases increased by 0.8%, to 4,064.6 per 100,000. In young men in the same age ranges, reported cases increased by 3.7%, to 959.0 cases per 100,000, and by 3.3%, to 1,784.5 per 100,000 cases, respectively.1
Both the incidence and prevalence of chlamydia infection are higher in African Americans than in whites, while Asians have the lowest rates.1 The prevalence of infection also is increased with incarceration, lower socioeconomic status, and residence in the southern United States.
The prevalence of chlamydia infection in pregnant women is approximately 2% to 3%, but it may be as high as 30% in high-risk populations, such as women who are unmarried, have multiple sex partners, are coinfected with another sexually transmitted disease (STD), have partners with nongonococcal urethritis, have mucopurulent discharge, have acute urethral syndrome, and have late or no prenatal care.2 Since chlamydia infection often is asymptomatic and some infections resolve spontaneously, the true prevalence of infection probably is underreported.
Continue to: Chlamydia infection can cause serious clinical manifestations...
Chlamydia infection can cause serious clinical manifestations
The 15 serotypes of C trachomatis are grouped into 3 categories according to clinical manifestations:
- Serotypes A, B, Ba, and C cause endemic trachoma, characterized by bilateral irritation of the eyelids that progresses to eyelid thickening and scarring, eventually leading to corneal abrasion and blindness.
- Serotypes D–K manifest as conjunctivitis and pneumonia in newborns, proctitis in men (especially in men who have sex with men), and genitourinary infections in women. Reactive arthritis and inclusion conjunctivitis also can occur with D–K serotypes.
- Serotypes L1–L3 cause lymphogranuloma venereum.
About 70% of women with chlamydia infection are asymptomatic. Those who have symptoms often present with endocervicitis or acute urethral syndrome (acute urethritis). Manifestations of these 2 conditions include a frothy yellow-green vaginal and/or urethral discharge, dysuria, and frequency. Women who engage in rectal intercourse also may notice a purulent discharge from the anus. Untreated, C trachomatis organisms may ascend the reproductive tract, causing both endometritis and pelvic inflammatory disease (PID).
While a single episode of PID increases tubal infertility risk by 10%, a second episode increases the risk by 40%.3 Over time, recurrent and/or chronic PID causes scarring and adhesion formation, which may result in chronic pelvic pain. In addition, chronic infection is the single most important risk factor for ectopic pregnancy. Finally, chlamydia infection is a risk factor for Fitz-Hugh-Cutis syndrome (perihepatitis). In this condition, organisms ascend from the site of pelvic infection along the pericolic gutter to ultimately infect the liver capsule.
Specific complications in pregnancy
Chlamydia infection in pregnant women is associated with preterm delivery and preterm premature rupture of membranes. Infants born to mothers with untreated chlamydia infection are at risk for pneumonia, conjunctivitis, and even perinatal death.2 Acquisition of infection occurs at the time of delivery rather than in the antepartum period.
The significant morbidity associated with chlamydia infection underscores the importance of regular screening, especially in pregnant women. The current United States Preventive Service Task Force guidelines recommend annual screening of all sexually active women who are 24 years of age or younger, as well as of older, high-risk women.
The CDC recommends routine screening of all pregnant women for chlamydia at the first prenatal visit. Repeat screening is recommended in the third trimester for all pregnant women younger than 25 years, those at increased risk, and those infected within the past 3 to 6 months or during the first trimester. Those who test positive should be retested 3 weeks after completion of treatment.1
Chlamydia screening strategies
Historically, a chlamydia diagnosis was made by isolating the organisms in tissue culture. In the 1990s, however, that extremely time-consuming and resource-intensive procedure was replaced by nucleic acid amplification testing (NAAT).
NAAT methodology. NAAT is the gold standard for diagnosing C trachomatis infection; this methodology utilizes various assays, including polymerase chain reaction, ligase chain reaction, and transcription-mediated amplification.
Continue to: Compared with previous culture and antigen detection techniques...
Compared with previous culture and antigen detection techniques, NAAT’s advantages include excellent sensitivity and specificity (>90% and ≥99%, respectively), enabling detection of a low inoculum of organisms in a sample obtained by noninvasive methods, such as first-void urine collection or vaginal swab.2,4,5 Furthermore, NAAT does not impose any specific storage regulations on collected specimens, is cost effective, and can jointly test for Neisseria gonorrhoeae, which commonly co-infects with C trachomatis.6
Screening in pregnancy. In 2012, Blatt and colleagues examined testing patterns in nearly 1.3 million obstetric patients and found that only 59% (761,315) of women were tested for chlamydia at least once in pregnancy.7 Only 1 in 3 women were tested during the first prenatal visit, as CDC guidelines recommend. Testing rates declined with increasing age. Of women screened, 3.5% tested positive for chlamydia.7 Of these, 3 of 4 were retested at least once, with almost 20% having at least 1 subsequent positive result.7
Of note, in a study of women who reported receptive anal intercourse (n = 2,818), 292 women tested positive for chlamydia; 10.4% tested positive in genital-only sites, 58.6% in genital and rectal sites, and 20.5% at the rectal site only.8
It is alarming that only 59% of pregnant women are screened for chlamydia given the significant perinatal complications associated with this infection. Barriers to screening pregnant women may include clinician discomfort in discussing STDs and patient refusal of screening. Furthermore, clinicians should routinely ask women about receptive anal sex. Women who report this risk factor should be tested for chlamydia in both the endocervix and rectum.
Retesting and follow-up. After the initial diagnosis of chlamydia, a test of cure 3 weeks after treatment is an important aspect of care. Thus, identifying and overcoming barriers to retesting is important. Clinicians should educate patients about the importance of follow-up. Also consider incorporating the use of home-based, self-obtained vaginal swabs for retesting. Results from 2 randomized trials showed that eliminating a patient’s transportation barriers and providing a home-based alternative to a follow-up visit significantly increased rescreening rates by 33% in STD clinic patients and by 59.2% in family planning clinic patients.9
Reinfection risk. The rate of venereal chlamydia transmission in heterosexual partners is 70%. Since sexually active chlamydia-positive patients are at risk for reinfection by their partner after treatment completion, clinicians should refer the sex partners for evaluation. If the sex partners are reluctant to have testing, it is reasonable to provide empiric antibiotic treatment to decrease the risk of re-infection in the patient.7 Before doing so, however, make certain that state law permits this practice, and be sure to document the prescribed treatment in the patient’s record.
Treatment options
Prompt treatment of C trachomatis infection is essential to decrease the risk of disease sequelae. Nonpregnant adults can be treated with oral doxycycline 100 mg twice daily for 7 days.
In a head-to-head study performed in a controlled environment that ensured treatment adherence, 97% efficacy was achieved with one oral dose of azithromycin (1 g) compared with 100% efficacy with doxycycline.10 However, in the real-world setting, imperfect adherence to the multi-day doxycycline regimen is associated with treatment failures. Thus, a single dose of azithromycin is preferable for patients with questionable compliance.11
In obstetric patients, azithromycin and amoxicillin are preferred as first-line agents for treatment of C trachomatis due to their improved safety profile in this demographic. Amoxicillin 500 mg orally 3 times daily for 7 days has 95% efficacy.2
Women allergic to these agents may be treated with an alternative regimen of erythromycin base, 500 mg orally 4 times daily for 7 days, or erythromycin ethylsuccinate, 800 mg orally 4 times daily for 7 days. Erythromycin should be reserved for second-line therapy because of its lower efficacy (64%) and frequent gastrointestinal adverse effects.2 Doxycycline is contraindicated in pregnancy because of possible teratogenic effects on the teeth and bone of the fetus.
- Centers for Disease Control and Prevention Division of STD Prevention. Sexually transmitted disease surveillance 2018. October 2019. https://www.cdc.gov/std/stats18/default.htm. 2019. Accessed January 4, 2020.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams JD, et al, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2019:869.
- Ljubin-Sternak S, Meštrović T. Chlamydia trachomatis and genital mycoplasmas: pathogens with an impact on human reproductive health. J Pathog. 2014. doi: 10.1155/2014/183167.
- Meyer T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms. 2016:4(3).
- Centers for Disease Control and Prevention.. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep. 2014;63:1-19.
- Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017;376:765-773.
- Blatt AJ, Lieberman JM, Hoover DR, et al. Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol. 2012;207:55.e1-8.
- Llata E, Braxton J, Asbel L, et al. Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse. Obstet Gynecol. 2018;132:692-697.
- Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol. 2011;118(2 pt 1):231-239.
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373:2512-2521.
- Quinn TC, Gaydos CA. Treatment for chlamydia infection—doxycycline versus azithromycin. N Engl J Med. 2015;373:2573-2575.
- Centers for Disease Control and Prevention Division of STD Prevention. Sexually transmitted disease surveillance 2018. October 2019. https://www.cdc.gov/std/stats18/default.htm. 2019. Accessed January 4, 2020.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams JD, et al, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2019:869.
- Ljubin-Sternak S, Meštrović T. Chlamydia trachomatis and genital mycoplasmas: pathogens with an impact on human reproductive health. J Pathog. 2014. doi: 10.1155/2014/183167.
- Meyer T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms. 2016:4(3).
- Centers for Disease Control and Prevention.. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep. 2014;63:1-19.
- Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017;376:765-773.
- Blatt AJ, Lieberman JM, Hoover DR, et al. Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol. 2012;207:55.e1-8.
- Llata E, Braxton J, Asbel L, et al. Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse. Obstet Gynecol. 2018;132:692-697.
- Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol. 2011;118(2 pt 1):231-239.
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373:2512-2521.
- Quinn TC, Gaydos CA. Treatment for chlamydia infection—doxycycline versus azithromycin. N Engl J Med. 2015;373:2573-2575.
COVID-19 during pregnancy: How would you proceed in this case of a novel and ominous emerging pathogen?
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).
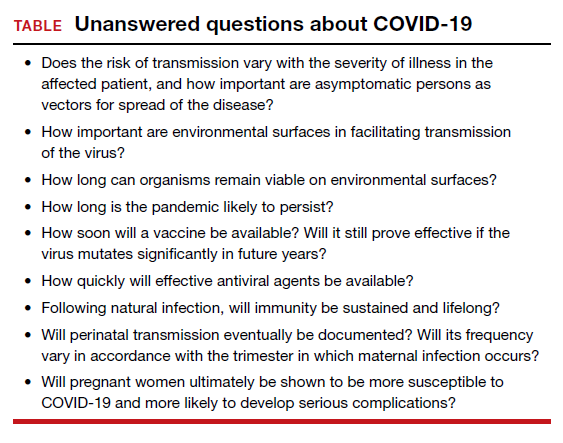
Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
Maternal mortality: A national crisis
This article is the first in a series on maternal mortality.
“You’re in really bad shape, kid. I don’t know if you’re gonna live through the night. I’m going to do everything I can to save your life, but the truth is you might die.”
If Timoria McQueen Saba imagined the words she would hear in the moments after she gave birth, those likely weren’t among them. But then she started to bleed. The energy around her shifted; she felt the urgency and intensity in the room, and she could see it – reflected from the television monitor over her bed – in the faces of her care team. After her husband and newborn daughter were led from the room, she did, in fact, hear those words.
They were spoken by a surgeon called in after efforts to control the bleeding failed – emetic words that joined forces with her hemorrhaging and confusion and fear, and as she began to vomit, her eyelids felt heavy. She fought to keep them open, sensing that if she closed them they might never open again.
In 2018 alone, similar words perhaps were spoken to the 658 U.S. women who suffered maternal complications and whose eyes never did open again. This is the latest official maternal mortality data from the Centers for Disease Control and Prevention.
Ms. Saba’s eyes, however, remained open through her birth trauma and through the PTSD that followed. A fierce advocate for maternal health, she shares her story often, as she did during a panel discussion at the American College of Obstetricians and Gynecologists’ annual meeting in May 2019, in an effort to improve outcomes for other women and families.
But her story unfolded nearly a decade ago and those eyes still are seeing women die from childbirth. Despite her efforts and the efforts of countless other individuals and organizations working to improve maternal outcomes, the new CDC data show that the United States has the highest maternal mortality rate of any similarly wealthy industrialized nation.
“I cannot believe I’m still talking about this issue,” Ms. Saba told a standing-room-only crowd and her copanelists Neel T. Shah, MD, and Charles S. Johnson IV, whose wife, Kira, died in 2016 during surgery for bleeding complications following the birth of their second child. “If all the people who I’d written to had just listened maybe once and tried to propel my message forward back then, Charles would be in a much better situation and so would his children.”
Mr. Johnson said that for 10 hours he and other family members pleaded for help for Kira, a healthy, vibrant women he described as “sunshine personified.”
She showed signs of postpartum bleeding after delivering a healthy baby boy by C-section, but a “STAT CT” order went unheeded for hours before she was finally taken for surgery.
“You’re walking down this corridor, you get to this point, these double doors open, and you just can’t go any further – and that was the last time I saw my wife alive,” he said. “When they took Kira back into the operating room, there were three-and-a-half liters of blood in her abdomen, and her heart stopped immediately.
Kira Johnson died April 13, 2016.
“I’m not here to tell you what I think, I’m here to tell you what I know, and that’s that Kira deserved so much better, and that Kira’s not alone, and that women all over this country deserve so much better.”
The U.S. maternal mortality crisis
Dr. Shah, an ob.gyn. at Beth Israel Deaconess Medical Center and director of the Delivery Decisions Initiative at Harvard Medical School’s Ariadne Labs, both in Boston, where he has “been on this mission to improve safety in childbirth for years now,” echoed Ms. Saba’s dismay regarding the pace of progress.
“It’s not just about the present, it’s about the future, it’s about the pact that every generation ought to have with the next one to leave things at least as well as they found them. And when it comes to the health of our moms in this country, we are not doing that,” he said. “An American mom today is 50% more likely to die in childbirth than her own mother was, and 3-4 times more likely to die if she’s black than if she’s white.”
Indeed, the data released Jan. 30 by the CDC’s National Center for Health Statistics (NCHS) – the first on maternal mortality released by the agency since 2007 – show a U.S. maternal mortality rate of 17.4 maternal deaths per 100,000 live births in 2018.
The rate is higher than the 12.7 per 100,000 live births reported in 2007, but the increase is attributable mostly to changes in data collection and reporting methods. In 2003, “a consensus process recommended that all states add a standardized ‘checkbox’ to improve the identification of maternal deaths,” and implementation wasn’t complete until 2017 as “funding, technology, and state laws allowed,” meaning 2018 was the first year that data were reported in a standardized fashion across states, the CDC explained in a press release.
The data demonstrate ongoing wide racial/ethnic disparities: the maternal mortality rates for non-Hispanic black women, non-Hispanic white women, and Hispanic women were 37.1, 14.7, and 11.8 per 100,000 live births, consistent with earlier data.
Further, the rates for women aged 40 years and over were nearly eightfold higher than for those under age 25 years (81.9 vs. 10.6 per 100,000 live births).
CDC officials noted, however, that inconsistencies in reporting still leave some question about the accuracy of the data, stating in the release that “NCHS has identified instances where application of the checkbox information according to coding rules led to misclassification of maternal deaths.”
The agency is making changes in rules and reporting to ensure greater accuracy, but the numbers nevertheless reveal a startling truth: “The United States is the most dangerous place to deliver a baby in the industrialized world.”
Progress and challenges
Rebekah Gee, MD, an ob.gyn. who served for 4 years as Secretary of the Louisiana Department of Health before leaving the position in January, made that statement during another panel discussion at ACOG 2019 – The President’s Panel: Maternal Mortality: Progress Toward Prevention – which was moderated by Lisa M. Hollier, MD, now the immediate past president of ACOG.
That’s not to say progress hasn’t been or can’t be made, Dr. Gee said.
In fact, quality improvement measures she facilitated in Louisiana led to a 25% reduction in infant mortality and a 10% reduction in neonatal intensive care unit admissions, demonstrating the potential for improvement with such initiatives, but addressing maternal issues is a greater challenge, she said.
“I think part of the sad truth is that we really focus on babies first, not moms ... and that needs to change,” Dr. Gee said.
Dr. Hollier focused much of her attention during her tenure as ACOG president on doing just that, particularly through an emphasis on heart disease, which is the leading cause of U.S. maternal deaths in pregnancy and the postpartum period.
In an interview, she shared her thoughts on the progress achieved and the work that remains.
ACOG was instrumental in the enactment of the Preventing Maternal Deaths Act of 2018, which appropriated funding for Enhancing Reviews and Surveillance to Eliminate Maternal Mortality (ERASE MM), a CDC initiative to support state-based maternal mortality review committees, said Dr. Hollier, professor of obstetrics and gynecology at Baylor College of Medicine, Houston.
“The really great news is that almost immediately after passage of the legislation, the CDC put out the notice of the funding opportunity, and they were able to provide 24 awards supporting 25 states,” she said.
ERASE MM will enhance state data collection and availability and enable a level of data sharing that “will really add strength and depth to reporting from the maternal mortality review committees, which really provides us with the best information we have to truly understand the causes, the contributing factors, and the strategies that can be put in place to prevent future maternal deaths.”
Further, the Alliance for Innovation on Maternal Health (AIM) program, a cooperative agreement with the Health Resources and Services Administration (HRSA) Maternal and Child Health Bureau to improve safety and outcomes through evidence-based patient safety bundles, was extended, and in May 2019, ACOG updated its guidance on managing cardiac contributors to maternal mortality, releasing its “Pregnancy and Heart Disease” Practice Bulletin, she said.
Dr. Hollier continues in her quest for improved maternal outcomes. She is slated to deliver a keynote address at the American College of Cardiology/World Congress of Cardiology conference March 28 in Chicago.
“I’m so excited ... to talk about the new guidelines that we’ve put out and to really talk about how cardiologists and ob.gyns. can work together to improve women’s health outcomes,” she said, adding that she already is seeing a strengthening of such partnerships.
A number of academic institutions are developing “pregnancy heart teams” to identify and care for women who have or develop heart disease during pregnancy.
“This type of collaboration ... is going to be essential to address mortality from cardiovascular causes and from cardiomyopathy, which accounts for about 25% of all maternal mortality,” she said. “The next area where we really need some buy-in is from our emergency physicians.”
Enhanced collaboration with emergency physicians and other specialties present opportunities to better identify and address pregnancy-related complications and sequelae, she said.
“Women are dying because they’re not being diagnosed,” she added. “We have to raise that level of awareness – it’s just absolutely critical.”
Identifying and addressing drivers of the crisis
Dr. Gee further emphasized the importance of addressing maternal health, noting that for every woman who dies from maternal causes, 100 experience maternal morbidity.
“It’s startling and it’s scary,” she said. “We are looking at this not just as a problem of outcomes, but a problem of racial inequity and racial bias and implicit bias.”
When she and her team assessed maternal mortality in Louisiana, they looked specifically at whether each death could have been prevented if, for example, blood was given sooner, cardiomyopathy was recognized sooner, or hypertension was treated on time.
“When we looked at these numbers ... when we looked at white women, 9% of the time we could have done better with our medical care; with black women, 59% of the time we could have saved her life with better care,” said Dr Gee, who is a gratis assistant professor of obstetrics and gynecology at Louisiana State University, New Orleans. “And if that doesn’t convince you that racial bias is an incredibly important thing to address – that we need to have a conversation about and address at a national level – I don’t know what would.”
In fact, numerous health, societal, socioeconomic, and other factors – some known, some yet to be identified, and many inter-related – are among the drivers of the U.S. maternal mortality crisis. In the coming months, an Ob.Gyn. News team will examine several of these drivers in depth. We’ll look specifically at the role of racism and bias, and at urban-rural disparities in access and outcomes – especially for women of color and indigenous women. We’ll address the scope and impact of each, successes and failures in addressing the problems, and ongoing initiatives.
Follow us for insights from experts, researchers, practicing physicians, and patients and families affected by the maternal mortality crisis, and stay with us through coverage of ACOG 2020 for perspective on what, specifically, ob.gyns. can do about it.
Mr. Johnson proposed a starting point:
“Here’s the good news – you guys ready for this? We can fix this,” he said, adding that the solution starts with “speaking Timoria’s name ... speaking the name of Kira Dixon Johnson ... speaking the names of these women and then asking the people that are around you, ‘What are we prepared to do to make sure that this doesn’t happen to other women.’ ”
This article is the first in a series on maternal mortality.
“You’re in really bad shape, kid. I don’t know if you’re gonna live through the night. I’m going to do everything I can to save your life, but the truth is you might die.”
If Timoria McQueen Saba imagined the words she would hear in the moments after she gave birth, those likely weren’t among them. But then she started to bleed. The energy around her shifted; she felt the urgency and intensity in the room, and she could see it – reflected from the television monitor over her bed – in the faces of her care team. After her husband and newborn daughter were led from the room, she did, in fact, hear those words.
They were spoken by a surgeon called in after efforts to control the bleeding failed – emetic words that joined forces with her hemorrhaging and confusion and fear, and as she began to vomit, her eyelids felt heavy. She fought to keep them open, sensing that if she closed them they might never open again.
In 2018 alone, similar words perhaps were spoken to the 658 U.S. women who suffered maternal complications and whose eyes never did open again. This is the latest official maternal mortality data from the Centers for Disease Control and Prevention.
Ms. Saba’s eyes, however, remained open through her birth trauma and through the PTSD that followed. A fierce advocate for maternal health, she shares her story often, as she did during a panel discussion at the American College of Obstetricians and Gynecologists’ annual meeting in May 2019, in an effort to improve outcomes for other women and families.
But her story unfolded nearly a decade ago and those eyes still are seeing women die from childbirth. Despite her efforts and the efforts of countless other individuals and organizations working to improve maternal outcomes, the new CDC data show that the United States has the highest maternal mortality rate of any similarly wealthy industrialized nation.
“I cannot believe I’m still talking about this issue,” Ms. Saba told a standing-room-only crowd and her copanelists Neel T. Shah, MD, and Charles S. Johnson IV, whose wife, Kira, died in 2016 during surgery for bleeding complications following the birth of their second child. “If all the people who I’d written to had just listened maybe once and tried to propel my message forward back then, Charles would be in a much better situation and so would his children.”
Mr. Johnson said that for 10 hours he and other family members pleaded for help for Kira, a healthy, vibrant women he described as “sunshine personified.”
She showed signs of postpartum bleeding after delivering a healthy baby boy by C-section, but a “STAT CT” order went unheeded for hours before she was finally taken for surgery.
“You’re walking down this corridor, you get to this point, these double doors open, and you just can’t go any further – and that was the last time I saw my wife alive,” he said. “When they took Kira back into the operating room, there were three-and-a-half liters of blood in her abdomen, and her heart stopped immediately.
Kira Johnson died April 13, 2016.
“I’m not here to tell you what I think, I’m here to tell you what I know, and that’s that Kira deserved so much better, and that Kira’s not alone, and that women all over this country deserve so much better.”
The U.S. maternal mortality crisis
Dr. Shah, an ob.gyn. at Beth Israel Deaconess Medical Center and director of the Delivery Decisions Initiative at Harvard Medical School’s Ariadne Labs, both in Boston, where he has “been on this mission to improve safety in childbirth for years now,” echoed Ms. Saba’s dismay regarding the pace of progress.
“It’s not just about the present, it’s about the future, it’s about the pact that every generation ought to have with the next one to leave things at least as well as they found them. And when it comes to the health of our moms in this country, we are not doing that,” he said. “An American mom today is 50% more likely to die in childbirth than her own mother was, and 3-4 times more likely to die if she’s black than if she’s white.”
Indeed, the data released Jan. 30 by the CDC’s National Center for Health Statistics (NCHS) – the first on maternal mortality released by the agency since 2007 – show a U.S. maternal mortality rate of 17.4 maternal deaths per 100,000 live births in 2018.
The rate is higher than the 12.7 per 100,000 live births reported in 2007, but the increase is attributable mostly to changes in data collection and reporting methods. In 2003, “a consensus process recommended that all states add a standardized ‘checkbox’ to improve the identification of maternal deaths,” and implementation wasn’t complete until 2017 as “funding, technology, and state laws allowed,” meaning 2018 was the first year that data were reported in a standardized fashion across states, the CDC explained in a press release.
The data demonstrate ongoing wide racial/ethnic disparities: the maternal mortality rates for non-Hispanic black women, non-Hispanic white women, and Hispanic women were 37.1, 14.7, and 11.8 per 100,000 live births, consistent with earlier data.
Further, the rates for women aged 40 years and over were nearly eightfold higher than for those under age 25 years (81.9 vs. 10.6 per 100,000 live births).
CDC officials noted, however, that inconsistencies in reporting still leave some question about the accuracy of the data, stating in the release that “NCHS has identified instances where application of the checkbox information according to coding rules led to misclassification of maternal deaths.”
The agency is making changes in rules and reporting to ensure greater accuracy, but the numbers nevertheless reveal a startling truth: “The United States is the most dangerous place to deliver a baby in the industrialized world.”
Progress and challenges
Rebekah Gee, MD, an ob.gyn. who served for 4 years as Secretary of the Louisiana Department of Health before leaving the position in January, made that statement during another panel discussion at ACOG 2019 – The President’s Panel: Maternal Mortality: Progress Toward Prevention – which was moderated by Lisa M. Hollier, MD, now the immediate past president of ACOG.
That’s not to say progress hasn’t been or can’t be made, Dr. Gee said.
In fact, quality improvement measures she facilitated in Louisiana led to a 25% reduction in infant mortality and a 10% reduction in neonatal intensive care unit admissions, demonstrating the potential for improvement with such initiatives, but addressing maternal issues is a greater challenge, she said.
“I think part of the sad truth is that we really focus on babies first, not moms ... and that needs to change,” Dr. Gee said.
Dr. Hollier focused much of her attention during her tenure as ACOG president on doing just that, particularly through an emphasis on heart disease, which is the leading cause of U.S. maternal deaths in pregnancy and the postpartum period.
In an interview, she shared her thoughts on the progress achieved and the work that remains.
ACOG was instrumental in the enactment of the Preventing Maternal Deaths Act of 2018, which appropriated funding for Enhancing Reviews and Surveillance to Eliminate Maternal Mortality (ERASE MM), a CDC initiative to support state-based maternal mortality review committees, said Dr. Hollier, professor of obstetrics and gynecology at Baylor College of Medicine, Houston.
“The really great news is that almost immediately after passage of the legislation, the CDC put out the notice of the funding opportunity, and they were able to provide 24 awards supporting 25 states,” she said.
ERASE MM will enhance state data collection and availability and enable a level of data sharing that “will really add strength and depth to reporting from the maternal mortality review committees, which really provides us with the best information we have to truly understand the causes, the contributing factors, and the strategies that can be put in place to prevent future maternal deaths.”
Further, the Alliance for Innovation on Maternal Health (AIM) program, a cooperative agreement with the Health Resources and Services Administration (HRSA) Maternal and Child Health Bureau to improve safety and outcomes through evidence-based patient safety bundles, was extended, and in May 2019, ACOG updated its guidance on managing cardiac contributors to maternal mortality, releasing its “Pregnancy and Heart Disease” Practice Bulletin, she said.
Dr. Hollier continues in her quest for improved maternal outcomes. She is slated to deliver a keynote address at the American College of Cardiology/World Congress of Cardiology conference March 28 in Chicago.
“I’m so excited ... to talk about the new guidelines that we’ve put out and to really talk about how cardiologists and ob.gyns. can work together to improve women’s health outcomes,” she said, adding that she already is seeing a strengthening of such partnerships.
A number of academic institutions are developing “pregnancy heart teams” to identify and care for women who have or develop heart disease during pregnancy.
“This type of collaboration ... is going to be essential to address mortality from cardiovascular causes and from cardiomyopathy, which accounts for about 25% of all maternal mortality,” she said. “The next area where we really need some buy-in is from our emergency physicians.”
Enhanced collaboration with emergency physicians and other specialties present opportunities to better identify and address pregnancy-related complications and sequelae, she said.
“Women are dying because they’re not being diagnosed,” she added. “We have to raise that level of awareness – it’s just absolutely critical.”
Identifying and addressing drivers of the crisis
Dr. Gee further emphasized the importance of addressing maternal health, noting that for every woman who dies from maternal causes, 100 experience maternal morbidity.
“It’s startling and it’s scary,” she said. “We are looking at this not just as a problem of outcomes, but a problem of racial inequity and racial bias and implicit bias.”
When she and her team assessed maternal mortality in Louisiana, they looked specifically at whether each death could have been prevented if, for example, blood was given sooner, cardiomyopathy was recognized sooner, or hypertension was treated on time.
“When we looked at these numbers ... when we looked at white women, 9% of the time we could have done better with our medical care; with black women, 59% of the time we could have saved her life with better care,” said Dr Gee, who is a gratis assistant professor of obstetrics and gynecology at Louisiana State University, New Orleans. “And if that doesn’t convince you that racial bias is an incredibly important thing to address – that we need to have a conversation about and address at a national level – I don’t know what would.”
In fact, numerous health, societal, socioeconomic, and other factors – some known, some yet to be identified, and many inter-related – are among the drivers of the U.S. maternal mortality crisis. In the coming months, an Ob.Gyn. News team will examine several of these drivers in depth. We’ll look specifically at the role of racism and bias, and at urban-rural disparities in access and outcomes – especially for women of color and indigenous women. We’ll address the scope and impact of each, successes and failures in addressing the problems, and ongoing initiatives.
Follow us for insights from experts, researchers, practicing physicians, and patients and families affected by the maternal mortality crisis, and stay with us through coverage of ACOG 2020 for perspective on what, specifically, ob.gyns. can do about it.
Mr. Johnson proposed a starting point:
“Here’s the good news – you guys ready for this? We can fix this,” he said, adding that the solution starts with “speaking Timoria’s name ... speaking the name of Kira Dixon Johnson ... speaking the names of these women and then asking the people that are around you, ‘What are we prepared to do to make sure that this doesn’t happen to other women.’ ”
This article is the first in a series on maternal mortality.
“You’re in really bad shape, kid. I don’t know if you’re gonna live through the night. I’m going to do everything I can to save your life, but the truth is you might die.”
If Timoria McQueen Saba imagined the words she would hear in the moments after she gave birth, those likely weren’t among them. But then she started to bleed. The energy around her shifted; she felt the urgency and intensity in the room, and she could see it – reflected from the television monitor over her bed – in the faces of her care team. After her husband and newborn daughter were led from the room, she did, in fact, hear those words.
They were spoken by a surgeon called in after efforts to control the bleeding failed – emetic words that joined forces with her hemorrhaging and confusion and fear, and as she began to vomit, her eyelids felt heavy. She fought to keep them open, sensing that if she closed them they might never open again.
In 2018 alone, similar words perhaps were spoken to the 658 U.S. women who suffered maternal complications and whose eyes never did open again. This is the latest official maternal mortality data from the Centers for Disease Control and Prevention.
Ms. Saba’s eyes, however, remained open through her birth trauma and through the PTSD that followed. A fierce advocate for maternal health, she shares her story often, as she did during a panel discussion at the American College of Obstetricians and Gynecologists’ annual meeting in May 2019, in an effort to improve outcomes for other women and families.
But her story unfolded nearly a decade ago and those eyes still are seeing women die from childbirth. Despite her efforts and the efforts of countless other individuals and organizations working to improve maternal outcomes, the new CDC data show that the United States has the highest maternal mortality rate of any similarly wealthy industrialized nation.
“I cannot believe I’m still talking about this issue,” Ms. Saba told a standing-room-only crowd and her copanelists Neel T. Shah, MD, and Charles S. Johnson IV, whose wife, Kira, died in 2016 during surgery for bleeding complications following the birth of their second child. “If all the people who I’d written to had just listened maybe once and tried to propel my message forward back then, Charles would be in a much better situation and so would his children.”
Mr. Johnson said that for 10 hours he and other family members pleaded for help for Kira, a healthy, vibrant women he described as “sunshine personified.”
She showed signs of postpartum bleeding after delivering a healthy baby boy by C-section, but a “STAT CT” order went unheeded for hours before she was finally taken for surgery.
“You’re walking down this corridor, you get to this point, these double doors open, and you just can’t go any further – and that was the last time I saw my wife alive,” he said. “When they took Kira back into the operating room, there were three-and-a-half liters of blood in her abdomen, and her heart stopped immediately.
Kira Johnson died April 13, 2016.
“I’m not here to tell you what I think, I’m here to tell you what I know, and that’s that Kira deserved so much better, and that Kira’s not alone, and that women all over this country deserve so much better.”
The U.S. maternal mortality crisis
Dr. Shah, an ob.gyn. at Beth Israel Deaconess Medical Center and director of the Delivery Decisions Initiative at Harvard Medical School’s Ariadne Labs, both in Boston, where he has “been on this mission to improve safety in childbirth for years now,” echoed Ms. Saba’s dismay regarding the pace of progress.
“It’s not just about the present, it’s about the future, it’s about the pact that every generation ought to have with the next one to leave things at least as well as they found them. And when it comes to the health of our moms in this country, we are not doing that,” he said. “An American mom today is 50% more likely to die in childbirth than her own mother was, and 3-4 times more likely to die if she’s black than if she’s white.”
Indeed, the data released Jan. 30 by the CDC’s National Center for Health Statistics (NCHS) – the first on maternal mortality released by the agency since 2007 – show a U.S. maternal mortality rate of 17.4 maternal deaths per 100,000 live births in 2018.
The rate is higher than the 12.7 per 100,000 live births reported in 2007, but the increase is attributable mostly to changes in data collection and reporting methods. In 2003, “a consensus process recommended that all states add a standardized ‘checkbox’ to improve the identification of maternal deaths,” and implementation wasn’t complete until 2017 as “funding, technology, and state laws allowed,” meaning 2018 was the first year that data were reported in a standardized fashion across states, the CDC explained in a press release.
The data demonstrate ongoing wide racial/ethnic disparities: the maternal mortality rates for non-Hispanic black women, non-Hispanic white women, and Hispanic women were 37.1, 14.7, and 11.8 per 100,000 live births, consistent with earlier data.
Further, the rates for women aged 40 years and over were nearly eightfold higher than for those under age 25 years (81.9 vs. 10.6 per 100,000 live births).
CDC officials noted, however, that inconsistencies in reporting still leave some question about the accuracy of the data, stating in the release that “NCHS has identified instances where application of the checkbox information according to coding rules led to misclassification of maternal deaths.”
The agency is making changes in rules and reporting to ensure greater accuracy, but the numbers nevertheless reveal a startling truth: “The United States is the most dangerous place to deliver a baby in the industrialized world.”
Progress and challenges
Rebekah Gee, MD, an ob.gyn. who served for 4 years as Secretary of the Louisiana Department of Health before leaving the position in January, made that statement during another panel discussion at ACOG 2019 – The President’s Panel: Maternal Mortality: Progress Toward Prevention – which was moderated by Lisa M. Hollier, MD, now the immediate past president of ACOG.
That’s not to say progress hasn’t been or can’t be made, Dr. Gee said.
In fact, quality improvement measures she facilitated in Louisiana led to a 25% reduction in infant mortality and a 10% reduction in neonatal intensive care unit admissions, demonstrating the potential for improvement with such initiatives, but addressing maternal issues is a greater challenge, she said.
“I think part of the sad truth is that we really focus on babies first, not moms ... and that needs to change,” Dr. Gee said.
Dr. Hollier focused much of her attention during her tenure as ACOG president on doing just that, particularly through an emphasis on heart disease, which is the leading cause of U.S. maternal deaths in pregnancy and the postpartum period.
In an interview, she shared her thoughts on the progress achieved and the work that remains.
ACOG was instrumental in the enactment of the Preventing Maternal Deaths Act of 2018, which appropriated funding for Enhancing Reviews and Surveillance to Eliminate Maternal Mortality (ERASE MM), a CDC initiative to support state-based maternal mortality review committees, said Dr. Hollier, professor of obstetrics and gynecology at Baylor College of Medicine, Houston.
“The really great news is that almost immediately after passage of the legislation, the CDC put out the notice of the funding opportunity, and they were able to provide 24 awards supporting 25 states,” she said.
ERASE MM will enhance state data collection and availability and enable a level of data sharing that “will really add strength and depth to reporting from the maternal mortality review committees, which really provides us with the best information we have to truly understand the causes, the contributing factors, and the strategies that can be put in place to prevent future maternal deaths.”
Further, the Alliance for Innovation on Maternal Health (AIM) program, a cooperative agreement with the Health Resources and Services Administration (HRSA) Maternal and Child Health Bureau to improve safety and outcomes through evidence-based patient safety bundles, was extended, and in May 2019, ACOG updated its guidance on managing cardiac contributors to maternal mortality, releasing its “Pregnancy and Heart Disease” Practice Bulletin, she said.
Dr. Hollier continues in her quest for improved maternal outcomes. She is slated to deliver a keynote address at the American College of Cardiology/World Congress of Cardiology conference March 28 in Chicago.
“I’m so excited ... to talk about the new guidelines that we’ve put out and to really talk about how cardiologists and ob.gyns. can work together to improve women’s health outcomes,” she said, adding that she already is seeing a strengthening of such partnerships.
A number of academic institutions are developing “pregnancy heart teams” to identify and care for women who have or develop heart disease during pregnancy.
“This type of collaboration ... is going to be essential to address mortality from cardiovascular causes and from cardiomyopathy, which accounts for about 25% of all maternal mortality,” she said. “The next area where we really need some buy-in is from our emergency physicians.”
Enhanced collaboration with emergency physicians and other specialties present opportunities to better identify and address pregnancy-related complications and sequelae, she said.
“Women are dying because they’re not being diagnosed,” she added. “We have to raise that level of awareness – it’s just absolutely critical.”
Identifying and addressing drivers of the crisis
Dr. Gee further emphasized the importance of addressing maternal health, noting that for every woman who dies from maternal causes, 100 experience maternal morbidity.
“It’s startling and it’s scary,” she said. “We are looking at this not just as a problem of outcomes, but a problem of racial inequity and racial bias and implicit bias.”
When she and her team assessed maternal mortality in Louisiana, they looked specifically at whether each death could have been prevented if, for example, blood was given sooner, cardiomyopathy was recognized sooner, or hypertension was treated on time.
“When we looked at these numbers ... when we looked at white women, 9% of the time we could have done better with our medical care; with black women, 59% of the time we could have saved her life with better care,” said Dr Gee, who is a gratis assistant professor of obstetrics and gynecology at Louisiana State University, New Orleans. “And if that doesn’t convince you that racial bias is an incredibly important thing to address – that we need to have a conversation about and address at a national level – I don’t know what would.”
In fact, numerous health, societal, socioeconomic, and other factors – some known, some yet to be identified, and many inter-related – are among the drivers of the U.S. maternal mortality crisis. In the coming months, an Ob.Gyn. News team will examine several of these drivers in depth. We’ll look specifically at the role of racism and bias, and at urban-rural disparities in access and outcomes – especially for women of color and indigenous women. We’ll address the scope and impact of each, successes and failures in addressing the problems, and ongoing initiatives.
Follow us for insights from experts, researchers, practicing physicians, and patients and families affected by the maternal mortality crisis, and stay with us through coverage of ACOG 2020 for perspective on what, specifically, ob.gyns. can do about it.
Mr. Johnson proposed a starting point:
“Here’s the good news – you guys ready for this? We can fix this,” he said, adding that the solution starts with “speaking Timoria’s name ... speaking the name of Kira Dixon Johnson ... speaking the names of these women and then asking the people that are around you, ‘What are we prepared to do to make sure that this doesn’t happen to other women.’ ”
CV health in pregnancy improves outcomes for mother and infant
PHOENIX – according to results from a multinational cohort study.
“Over the past 10 years, cardiovascular health [CVH] has been characterized across most of the life course and is associated with a variety of health outcomes, but CVH as a whole has not been well studied during pregnancy,” Amanda M. Perak, MD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
In an effort to examine the associations of maternal gestational CVH with adverse maternal and newborn outcomes, Dr. Perak of the departments of pediatrics and preventive medicine at Northwestern University and Lurie Children’s Hospital, both in Chicago, and colleagues drew from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study, which examined pregnant women at a target of 28 weeks’ gestation and assessed the associations of glycemia with pregnancy outcomes. The researchers analyzed data from an ancillary study of 2,230 mother-child dyads to characterize clinical gestational CVH with use of five metrics: body mass index, blood pressure, cholesterol, glucose, and smoking. The study excluded women with prepregnancy diabetes, preterm births, and cases of fetal death/major malformations.
Each maternal CVH metric was classified as ideal, intermediate, or poor according to modified definitions based on pregnancy guidelines. “For lipids, it’s known that levels change substantially during pregnancy, but there are no pregnancy guidelines,” Dr. Perak said. “We and others have also shown that higher triglycerides in pregnancy are associated with adverse pregnancy outcomes. We selected thresholds of less than 250 mg/dL for ideal and at least 500 mg/dL for poor, based on triglyceride distribution and clinical relevance.”
Total CVH was scored by assigning 2 points for ideal, 1 for intermediate, and 0 for each poor metric, for a total possible 10 points, with 10 being most favorable. They also created four CVH categories, ranging from all ideal to two or more poor metrics. Maternal adverse pregnancy outcomes included preeclampsia and unplanned primary cesarean section. Newborn adverse pregnancy outcomes included birth weight above the 90th percentile and a cord blood insulin sensitivity index lower than the 10th percentile.
The researchers used logistic and multinomial logistic regression of pregnancy outcomes on maternal gestational CVH in two adjusted models. Secondarily, they examined associations of individual CVH metrics with outcomes, with adjustment for the other metrics.
The cohort comprised mother-child dyads from nine field centers in six countries: the United States (25%), Barbados (23%), United Kingdom (21%), China (18%), Thailand (7%), and Canada (7%). The mothers’ mean age was 30 years, and the mean gestational age was 28 weeks. The mean gestational CVH score was 8.8 out of 10. Nearly half of mothers (42%) had ideal metrics, while 4% had two or more poor metrics. Delivery occurred at a mean of 39.8 weeks, and adverse pregnancy outcomes occurred in 4.7%-17.9% of pregnancies.
In the fully adjusted model, which accounted for maternal age, height, alcohol use, gestational age at pregnancy exam, maternal parity, and newborn sex and race/ethnicity, odds ratios per 1-point higher (better) CVH score were 0.61 (95% confidence interval, 0.53-0.70) for preeclampsia, 0.85 (95% CI, 0.76-0.95) for unplanned primary cesarean section (among primiparous mothers), 0.83 (95% CI, 0.77-0.91) for large for gestational age infant, and 0.79 (95% CI, 0.72-0.87) for infant insulin sensitivity index below the 10th percentile. CVH categories were also associated with outcomes. For example, odds ratios for preeclampsia were 4.61 (95% CI, 2.13-11.14) for mothers with one or more intermediate metrics, 7.62 (95% CI, 3.60-18.13) for mothers with one poor metric, and 12.02 (95% CI, 4.70-32.50) for mothers with two or more poor metrics, compared with mothers with all metrics ideal.
“Except for smoking, each CVH metric was independently associated with adverse outcomes,” Dr. Perak said. “However, total CVH was associated with a wider range of outcomes than any single metric. This suggests that CVH provides health insights beyond single risk factors.”
Strengths of the study, she continued, included geographic and racial diversity of participants and high-quality research measurements of CVH. Limitations were that the cohort excluded prepregnancy diabetes and preterm births. “Diet and exercise data were not available, and CVH was measured once at 28 weeks,” she said. “Further study is needed across pregnancy and in other settings, but this study provides the first data on the relevance of gestational CVH for pregnancy outcomes.”
In an interview, Stephen S. Rich, PhD, who directs the Center for Public Health Genomics at the University of Virginia, said that the data “provide strong epidemiologic support to focus on the full range of cardiovascular health. In my view, the primary limitation of the study is that there may be significant differences in how one achieves ideal CHV across a single country, not to mention across the world, particularly in absence of a highly controlled, research environment. It is not clear that the approach used in this study at nine selected sites in six relatively highly developed countries could be translated into primary care – particularly in the U.S. with different regulatory and reimbursement plans and payers. Nonetheless, the evidence suggests a way to reduce adverse outcomes in pregnancy and the area deserves greater research.”
According to Dr. Perak, gestational diabetes is associated with a twofold higher maternal risk for cardiovascular disease (Diabetologia. 2019;62:905-14), while diabetes is also associated with higher offspring risk for CVD (BMJ. 2019;367:16398). However, a paucity of data exists on gestational CVH. In one report, better gestational CVH was associated with less subclinical CVD for the mother 10 years later (J Am Heart Assoc. 2019 Jul 23. doi:10.1161/JAHA.118.011394). In a separate analysis, Dr. Perak and her colleagues found that better gestational CVH was associated with better offspring CVH in childhood. “Unfortunately, we also reported that, among pregnant women in the United States, fewer than 1 in 10 had high CVH,” she said (J Am Heart Assoc. 2020 Feb 17. doi:10.1161/JAHA.119.015123). “However, the relevance of gestational CVH for pregnancy outcomes is unknown, but a it’s key question when considering CVH monitoring in prenatal care.”
Dr. Perak reported having received grant support from the National Heart, Lung, and Blood Institute, the American Heart Association, and Northwestern University. The HAPO Study was supported by NHLBI and the National Institute of Diabetes and Digestive and Kidney Diseases.
The meeting was sponsored by the American Heart Association.
SOURCE: Perak A et al. Epi/Lifestyle 2020, Abstract 33.
PHOENIX – according to results from a multinational cohort study.
“Over the past 10 years, cardiovascular health [CVH] has been characterized across most of the life course and is associated with a variety of health outcomes, but CVH as a whole has not been well studied during pregnancy,” Amanda M. Perak, MD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
In an effort to examine the associations of maternal gestational CVH with adverse maternal and newborn outcomes, Dr. Perak of the departments of pediatrics and preventive medicine at Northwestern University and Lurie Children’s Hospital, both in Chicago, and colleagues drew from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study, which examined pregnant women at a target of 28 weeks’ gestation and assessed the associations of glycemia with pregnancy outcomes. The researchers analyzed data from an ancillary study of 2,230 mother-child dyads to characterize clinical gestational CVH with use of five metrics: body mass index, blood pressure, cholesterol, glucose, and smoking. The study excluded women with prepregnancy diabetes, preterm births, and cases of fetal death/major malformations.
Each maternal CVH metric was classified as ideal, intermediate, or poor according to modified definitions based on pregnancy guidelines. “For lipids, it’s known that levels change substantially during pregnancy, but there are no pregnancy guidelines,” Dr. Perak said. “We and others have also shown that higher triglycerides in pregnancy are associated with adverse pregnancy outcomes. We selected thresholds of less than 250 mg/dL for ideal and at least 500 mg/dL for poor, based on triglyceride distribution and clinical relevance.”
Total CVH was scored by assigning 2 points for ideal, 1 for intermediate, and 0 for each poor metric, for a total possible 10 points, with 10 being most favorable. They also created four CVH categories, ranging from all ideal to two or more poor metrics. Maternal adverse pregnancy outcomes included preeclampsia and unplanned primary cesarean section. Newborn adverse pregnancy outcomes included birth weight above the 90th percentile and a cord blood insulin sensitivity index lower than the 10th percentile.
The researchers used logistic and multinomial logistic regression of pregnancy outcomes on maternal gestational CVH in two adjusted models. Secondarily, they examined associations of individual CVH metrics with outcomes, with adjustment for the other metrics.
The cohort comprised mother-child dyads from nine field centers in six countries: the United States (25%), Barbados (23%), United Kingdom (21%), China (18%), Thailand (7%), and Canada (7%). The mothers’ mean age was 30 years, and the mean gestational age was 28 weeks. The mean gestational CVH score was 8.8 out of 10. Nearly half of mothers (42%) had ideal metrics, while 4% had two or more poor metrics. Delivery occurred at a mean of 39.8 weeks, and adverse pregnancy outcomes occurred in 4.7%-17.9% of pregnancies.
In the fully adjusted model, which accounted for maternal age, height, alcohol use, gestational age at pregnancy exam, maternal parity, and newborn sex and race/ethnicity, odds ratios per 1-point higher (better) CVH score were 0.61 (95% confidence interval, 0.53-0.70) for preeclampsia, 0.85 (95% CI, 0.76-0.95) for unplanned primary cesarean section (among primiparous mothers), 0.83 (95% CI, 0.77-0.91) for large for gestational age infant, and 0.79 (95% CI, 0.72-0.87) for infant insulin sensitivity index below the 10th percentile. CVH categories were also associated with outcomes. For example, odds ratios for preeclampsia were 4.61 (95% CI, 2.13-11.14) for mothers with one or more intermediate metrics, 7.62 (95% CI, 3.60-18.13) for mothers with one poor metric, and 12.02 (95% CI, 4.70-32.50) for mothers with two or more poor metrics, compared with mothers with all metrics ideal.
“Except for smoking, each CVH metric was independently associated with adverse outcomes,” Dr. Perak said. “However, total CVH was associated with a wider range of outcomes than any single metric. This suggests that CVH provides health insights beyond single risk factors.”
Strengths of the study, she continued, included geographic and racial diversity of participants and high-quality research measurements of CVH. Limitations were that the cohort excluded prepregnancy diabetes and preterm births. “Diet and exercise data were not available, and CVH was measured once at 28 weeks,” she said. “Further study is needed across pregnancy and in other settings, but this study provides the first data on the relevance of gestational CVH for pregnancy outcomes.”
In an interview, Stephen S. Rich, PhD, who directs the Center for Public Health Genomics at the University of Virginia, said that the data “provide strong epidemiologic support to focus on the full range of cardiovascular health. In my view, the primary limitation of the study is that there may be significant differences in how one achieves ideal CHV across a single country, not to mention across the world, particularly in absence of a highly controlled, research environment. It is not clear that the approach used in this study at nine selected sites in six relatively highly developed countries could be translated into primary care – particularly in the U.S. with different regulatory and reimbursement plans and payers. Nonetheless, the evidence suggests a way to reduce adverse outcomes in pregnancy and the area deserves greater research.”
According to Dr. Perak, gestational diabetes is associated with a twofold higher maternal risk for cardiovascular disease (Diabetologia. 2019;62:905-14), while diabetes is also associated with higher offspring risk for CVD (BMJ. 2019;367:16398). However, a paucity of data exists on gestational CVH. In one report, better gestational CVH was associated with less subclinical CVD for the mother 10 years later (J Am Heart Assoc. 2019 Jul 23. doi:10.1161/JAHA.118.011394). In a separate analysis, Dr. Perak and her colleagues found that better gestational CVH was associated with better offspring CVH in childhood. “Unfortunately, we also reported that, among pregnant women in the United States, fewer than 1 in 10 had high CVH,” she said (J Am Heart Assoc. 2020 Feb 17. doi:10.1161/JAHA.119.015123). “However, the relevance of gestational CVH for pregnancy outcomes is unknown, but a it’s key question when considering CVH monitoring in prenatal care.”
Dr. Perak reported having received grant support from the National Heart, Lung, and Blood Institute, the American Heart Association, and Northwestern University. The HAPO Study was supported by NHLBI and the National Institute of Diabetes and Digestive and Kidney Diseases.
The meeting was sponsored by the American Heart Association.
SOURCE: Perak A et al. Epi/Lifestyle 2020, Abstract 33.
PHOENIX – according to results from a multinational cohort study.
“Over the past 10 years, cardiovascular health [CVH] has been characterized across most of the life course and is associated with a variety of health outcomes, but CVH as a whole has not been well studied during pregnancy,” Amanda M. Perak, MD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
In an effort to examine the associations of maternal gestational CVH with adverse maternal and newborn outcomes, Dr. Perak of the departments of pediatrics and preventive medicine at Northwestern University and Lurie Children’s Hospital, both in Chicago, and colleagues drew from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study, which examined pregnant women at a target of 28 weeks’ gestation and assessed the associations of glycemia with pregnancy outcomes. The researchers analyzed data from an ancillary study of 2,230 mother-child dyads to characterize clinical gestational CVH with use of five metrics: body mass index, blood pressure, cholesterol, glucose, and smoking. The study excluded women with prepregnancy diabetes, preterm births, and cases of fetal death/major malformations.
Each maternal CVH metric was classified as ideal, intermediate, or poor according to modified definitions based on pregnancy guidelines. “For lipids, it’s known that levels change substantially during pregnancy, but there are no pregnancy guidelines,” Dr. Perak said. “We and others have also shown that higher triglycerides in pregnancy are associated with adverse pregnancy outcomes. We selected thresholds of less than 250 mg/dL for ideal and at least 500 mg/dL for poor, based on triglyceride distribution and clinical relevance.”
Total CVH was scored by assigning 2 points for ideal, 1 for intermediate, and 0 for each poor metric, for a total possible 10 points, with 10 being most favorable. They also created four CVH categories, ranging from all ideal to two or more poor metrics. Maternal adverse pregnancy outcomes included preeclampsia and unplanned primary cesarean section. Newborn adverse pregnancy outcomes included birth weight above the 90th percentile and a cord blood insulin sensitivity index lower than the 10th percentile.
The researchers used logistic and multinomial logistic regression of pregnancy outcomes on maternal gestational CVH in two adjusted models. Secondarily, they examined associations of individual CVH metrics with outcomes, with adjustment for the other metrics.
The cohort comprised mother-child dyads from nine field centers in six countries: the United States (25%), Barbados (23%), United Kingdom (21%), China (18%), Thailand (7%), and Canada (7%). The mothers’ mean age was 30 years, and the mean gestational age was 28 weeks. The mean gestational CVH score was 8.8 out of 10. Nearly half of mothers (42%) had ideal metrics, while 4% had two or more poor metrics. Delivery occurred at a mean of 39.8 weeks, and adverse pregnancy outcomes occurred in 4.7%-17.9% of pregnancies.
In the fully adjusted model, which accounted for maternal age, height, alcohol use, gestational age at pregnancy exam, maternal parity, and newborn sex and race/ethnicity, odds ratios per 1-point higher (better) CVH score were 0.61 (95% confidence interval, 0.53-0.70) for preeclampsia, 0.85 (95% CI, 0.76-0.95) for unplanned primary cesarean section (among primiparous mothers), 0.83 (95% CI, 0.77-0.91) for large for gestational age infant, and 0.79 (95% CI, 0.72-0.87) for infant insulin sensitivity index below the 10th percentile. CVH categories were also associated with outcomes. For example, odds ratios for preeclampsia were 4.61 (95% CI, 2.13-11.14) for mothers with one or more intermediate metrics, 7.62 (95% CI, 3.60-18.13) for mothers with one poor metric, and 12.02 (95% CI, 4.70-32.50) for mothers with two or more poor metrics, compared with mothers with all metrics ideal.
“Except for smoking, each CVH metric was independently associated with adverse outcomes,” Dr. Perak said. “However, total CVH was associated with a wider range of outcomes than any single metric. This suggests that CVH provides health insights beyond single risk factors.”
Strengths of the study, she continued, included geographic and racial diversity of participants and high-quality research measurements of CVH. Limitations were that the cohort excluded prepregnancy diabetes and preterm births. “Diet and exercise data were not available, and CVH was measured once at 28 weeks,” she said. “Further study is needed across pregnancy and in other settings, but this study provides the first data on the relevance of gestational CVH for pregnancy outcomes.”
In an interview, Stephen S. Rich, PhD, who directs the Center for Public Health Genomics at the University of Virginia, said that the data “provide strong epidemiologic support to focus on the full range of cardiovascular health. In my view, the primary limitation of the study is that there may be significant differences in how one achieves ideal CHV across a single country, not to mention across the world, particularly in absence of a highly controlled, research environment. It is not clear that the approach used in this study at nine selected sites in six relatively highly developed countries could be translated into primary care – particularly in the U.S. with different regulatory and reimbursement plans and payers. Nonetheless, the evidence suggests a way to reduce adverse outcomes in pregnancy and the area deserves greater research.”
According to Dr. Perak, gestational diabetes is associated with a twofold higher maternal risk for cardiovascular disease (Diabetologia. 2019;62:905-14), while diabetes is also associated with higher offspring risk for CVD (BMJ. 2019;367:16398). However, a paucity of data exists on gestational CVH. In one report, better gestational CVH was associated with less subclinical CVD for the mother 10 years later (J Am Heart Assoc. 2019 Jul 23. doi:10.1161/JAHA.118.011394). In a separate analysis, Dr. Perak and her colleagues found that better gestational CVH was associated with better offspring CVH in childhood. “Unfortunately, we also reported that, among pregnant women in the United States, fewer than 1 in 10 had high CVH,” she said (J Am Heart Assoc. 2020 Feb 17. doi:10.1161/JAHA.119.015123). “However, the relevance of gestational CVH for pregnancy outcomes is unknown, but a it’s key question when considering CVH monitoring in prenatal care.”
Dr. Perak reported having received grant support from the National Heart, Lung, and Blood Institute, the American Heart Association, and Northwestern University. The HAPO Study was supported by NHLBI and the National Institute of Diabetes and Digestive and Kidney Diseases.
The meeting was sponsored by the American Heart Association.
SOURCE: Perak A et al. Epi/Lifestyle 2020, Abstract 33.
REPORTING FROM EPI/LIFESTYLE 2020
ERAS for cesarean delivery: Postoperative care