User login
‘Where does it hurt?’: Primary care tips for common ortho problems
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2022
Wearable sensors deemed reliable for home gait assessment in knee OA
Remote gait assessment in people with knee osteoarthritis using wearable sensors appears reliable but yields results slightly different from those achieved in the laboratory, researchers from Boston University have found.
As reported at the OARSI 2022 World Congress, there was “good to excellent reliability” in repeated measures collected by patients at home while being instructed via video teleconferencing.
Agreement was “moderate to excellent” when the findings were compared with those recorded in the lab, Michael J. Rose of Boston University reported at the congress, sponsored by the Osteoarthritis Research Society International.
“People walked faster and stood up faster in the lab,” Mr. Rose said. “Later we found that the difference in gait speed was statistically significant between the lab and home environment.”
This has been suggested previously and implies that data collected at home may have “greater ecological validity,” he observed.
Accelerated adoption of telehealth
Assessing how well someone walks or can stand from a seated position are well known and important assessments in knee OA, but these have but have traditionally only been done in large and expensive gait labs, Mr. Rose said.
Wearable technologies, such as the ones used in the study he presented, could help move these assessments out into the community. This is particularly timely considering the increased adoption of telehealth practices during the COVID-19 pandemic.
To look at the reliability measurements obtained via wearable sensors versus lab assessments, Mr. Rose and associates set up a substudy within a larger ongoing, single-arm trial looking at the use of digital assessments to measure the efficacy of an exercise intervention in reducing knee pain and improving knee function.
For inclusion in the main trial (n = 60), and hence the substudy (n = 20), participants had to have physician-diagnosed knee OA, be 50 years of age or older, have a body mass index of 40 kg/m2 or lower, be able to walk at for a least 20 minutes, and have a score of three or higher on the Knee Injury and Osteoarthritis Outcome Score pain subscale for weight-bearing items.
Acceptance of in-lab versus home testing
The substudy participants (mean age, 70.5 years) all underwent in-person lab visits in which a wearable sensor was placed on each foot and one around the lower back and the participant asked to perform walking and chair stand tests. The latter involved standing from a seated position as quickly as possible without using the arms five times, while the former involved walking 28 meters in two laps of a 7-meter path defined by two cones. These tests were repeated twice.
Participants were then given the equipment to repeat these tests at home; this included the three sensors, a tablet computer, and chair and cones. The home assessments were conducted via video conferencing, with the researchers reminding how to place the sensors correctly. The walking and chair stand tests were then each performed four times: Twice in a row and then a 15-minute rest period before being performed twice in a row again.
The researchers collected participants’ feedback about the process on questionnaires and Likert scales that showed an overall positive experience for the remote home visit, with the median rating being “very likely” to participate in another home visit and that the time commitment required was “very manageable.”
Good correlation found
To determine the correlation and the test-retest reliability of the data obtained during the repeated home tasks, Mr. Rose and collaborators used Pearson’s correlation R2 and the intra-class correlation coefficients (ICC).
ICCs for various gait and chair stand variables obtained with the sensors were between 0.85 and 0.96 for the test-retest reliability during the remote home visit, and R2 ranged between 0.81 and 0.95. Variables include stance, cadence (steps per minute), step duration and length, speed, and chair stand duration.
With regard to the agreement between the home versus lab results, ICCs ranged between 0.63 and 0.9.
“There were some logistical and technological challenges with the approach,” Mr. Rose conceded. “Despite written and verbal instructions, 2 of the 20 participants ended up having gait data that was unusable in the home visit.”
Another limitation is that the study population, while “representative,” contained a higher number of individuals than the general population who identified as being White (95%) and female (85%), and 90% had a college degree.
“Individuals typically representative of an OA population were generally accepting and willing to participate in remote visits showing the feasibility of our approach,” Mr. Rose said.
“We need to determine the responsiveness of gait and chair stand outcomes from wearable sensors at home to change over time.”
The study was sponsored by Boston University with funding from Pfizer and Eli Lilly. The researchers used the OPAL inertial sensor (APDM Wearable Technologies) in the study. Mr. Rose made no personal disclosures. Four of his collaborators were employees of Pfizer and one is an employee of Eli Lilly & Company, all with stock or stock options.
Remote gait assessment in people with knee osteoarthritis using wearable sensors appears reliable but yields results slightly different from those achieved in the laboratory, researchers from Boston University have found.
As reported at the OARSI 2022 World Congress, there was “good to excellent reliability” in repeated measures collected by patients at home while being instructed via video teleconferencing.
Agreement was “moderate to excellent” when the findings were compared with those recorded in the lab, Michael J. Rose of Boston University reported at the congress, sponsored by the Osteoarthritis Research Society International.
“People walked faster and stood up faster in the lab,” Mr. Rose said. “Later we found that the difference in gait speed was statistically significant between the lab and home environment.”
This has been suggested previously and implies that data collected at home may have “greater ecological validity,” he observed.
Accelerated adoption of telehealth
Assessing how well someone walks or can stand from a seated position are well known and important assessments in knee OA, but these have but have traditionally only been done in large and expensive gait labs, Mr. Rose said.
Wearable technologies, such as the ones used in the study he presented, could help move these assessments out into the community. This is particularly timely considering the increased adoption of telehealth practices during the COVID-19 pandemic.
To look at the reliability measurements obtained via wearable sensors versus lab assessments, Mr. Rose and associates set up a substudy within a larger ongoing, single-arm trial looking at the use of digital assessments to measure the efficacy of an exercise intervention in reducing knee pain and improving knee function.
For inclusion in the main trial (n = 60), and hence the substudy (n = 20), participants had to have physician-diagnosed knee OA, be 50 years of age or older, have a body mass index of 40 kg/m2 or lower, be able to walk at for a least 20 minutes, and have a score of three or higher on the Knee Injury and Osteoarthritis Outcome Score pain subscale for weight-bearing items.
Acceptance of in-lab versus home testing
The substudy participants (mean age, 70.5 years) all underwent in-person lab visits in which a wearable sensor was placed on each foot and one around the lower back and the participant asked to perform walking and chair stand tests. The latter involved standing from a seated position as quickly as possible without using the arms five times, while the former involved walking 28 meters in two laps of a 7-meter path defined by two cones. These tests were repeated twice.
Participants were then given the equipment to repeat these tests at home; this included the three sensors, a tablet computer, and chair and cones. The home assessments were conducted via video conferencing, with the researchers reminding how to place the sensors correctly. The walking and chair stand tests were then each performed four times: Twice in a row and then a 15-minute rest period before being performed twice in a row again.
The researchers collected participants’ feedback about the process on questionnaires and Likert scales that showed an overall positive experience for the remote home visit, with the median rating being “very likely” to participate in another home visit and that the time commitment required was “very manageable.”
Good correlation found
To determine the correlation and the test-retest reliability of the data obtained during the repeated home tasks, Mr. Rose and collaborators used Pearson’s correlation R2 and the intra-class correlation coefficients (ICC).
ICCs for various gait and chair stand variables obtained with the sensors were between 0.85 and 0.96 for the test-retest reliability during the remote home visit, and R2 ranged between 0.81 and 0.95. Variables include stance, cadence (steps per minute), step duration and length, speed, and chair stand duration.
With regard to the agreement between the home versus lab results, ICCs ranged between 0.63 and 0.9.
“There were some logistical and technological challenges with the approach,” Mr. Rose conceded. “Despite written and verbal instructions, 2 of the 20 participants ended up having gait data that was unusable in the home visit.”
Another limitation is that the study population, while “representative,” contained a higher number of individuals than the general population who identified as being White (95%) and female (85%), and 90% had a college degree.
“Individuals typically representative of an OA population were generally accepting and willing to participate in remote visits showing the feasibility of our approach,” Mr. Rose said.
“We need to determine the responsiveness of gait and chair stand outcomes from wearable sensors at home to change over time.”
The study was sponsored by Boston University with funding from Pfizer and Eli Lilly. The researchers used the OPAL inertial sensor (APDM Wearable Technologies) in the study. Mr. Rose made no personal disclosures. Four of his collaborators were employees of Pfizer and one is an employee of Eli Lilly & Company, all with stock or stock options.
Remote gait assessment in people with knee osteoarthritis using wearable sensors appears reliable but yields results slightly different from those achieved in the laboratory, researchers from Boston University have found.
As reported at the OARSI 2022 World Congress, there was “good to excellent reliability” in repeated measures collected by patients at home while being instructed via video teleconferencing.
Agreement was “moderate to excellent” when the findings were compared with those recorded in the lab, Michael J. Rose of Boston University reported at the congress, sponsored by the Osteoarthritis Research Society International.
“People walked faster and stood up faster in the lab,” Mr. Rose said. “Later we found that the difference in gait speed was statistically significant between the lab and home environment.”
This has been suggested previously and implies that data collected at home may have “greater ecological validity,” he observed.
Accelerated adoption of telehealth
Assessing how well someone walks or can stand from a seated position are well known and important assessments in knee OA, but these have but have traditionally only been done in large and expensive gait labs, Mr. Rose said.
Wearable technologies, such as the ones used in the study he presented, could help move these assessments out into the community. This is particularly timely considering the increased adoption of telehealth practices during the COVID-19 pandemic.
To look at the reliability measurements obtained via wearable sensors versus lab assessments, Mr. Rose and associates set up a substudy within a larger ongoing, single-arm trial looking at the use of digital assessments to measure the efficacy of an exercise intervention in reducing knee pain and improving knee function.
For inclusion in the main trial (n = 60), and hence the substudy (n = 20), participants had to have physician-diagnosed knee OA, be 50 years of age or older, have a body mass index of 40 kg/m2 or lower, be able to walk at for a least 20 minutes, and have a score of three or higher on the Knee Injury and Osteoarthritis Outcome Score pain subscale for weight-bearing items.
Acceptance of in-lab versus home testing
The substudy participants (mean age, 70.5 years) all underwent in-person lab visits in which a wearable sensor was placed on each foot and one around the lower back and the participant asked to perform walking and chair stand tests. The latter involved standing from a seated position as quickly as possible without using the arms five times, while the former involved walking 28 meters in two laps of a 7-meter path defined by two cones. These tests were repeated twice.
Participants were then given the equipment to repeat these tests at home; this included the three sensors, a tablet computer, and chair and cones. The home assessments were conducted via video conferencing, with the researchers reminding how to place the sensors correctly. The walking and chair stand tests were then each performed four times: Twice in a row and then a 15-minute rest period before being performed twice in a row again.
The researchers collected participants’ feedback about the process on questionnaires and Likert scales that showed an overall positive experience for the remote home visit, with the median rating being “very likely” to participate in another home visit and that the time commitment required was “very manageable.”
Good correlation found
To determine the correlation and the test-retest reliability of the data obtained during the repeated home tasks, Mr. Rose and collaborators used Pearson’s correlation R2 and the intra-class correlation coefficients (ICC).
ICCs for various gait and chair stand variables obtained with the sensors were between 0.85 and 0.96 for the test-retest reliability during the remote home visit, and R2 ranged between 0.81 and 0.95. Variables include stance, cadence (steps per minute), step duration and length, speed, and chair stand duration.
With regard to the agreement between the home versus lab results, ICCs ranged between 0.63 and 0.9.
“There were some logistical and technological challenges with the approach,” Mr. Rose conceded. “Despite written and verbal instructions, 2 of the 20 participants ended up having gait data that was unusable in the home visit.”
Another limitation is that the study population, while “representative,” contained a higher number of individuals than the general population who identified as being White (95%) and female (85%), and 90% had a college degree.
“Individuals typically representative of an OA population were generally accepting and willing to participate in remote visits showing the feasibility of our approach,” Mr. Rose said.
“We need to determine the responsiveness of gait and chair stand outcomes from wearable sensors at home to change over time.”
The study was sponsored by Boston University with funding from Pfizer and Eli Lilly. The researchers used the OPAL inertial sensor (APDM Wearable Technologies) in the study. Mr. Rose made no personal disclosures. Four of his collaborators were employees of Pfizer and one is an employee of Eli Lilly & Company, all with stock or stock options.
FROM OARSI 2022
Inadequate pain relief in OA, high opioid use before TKA
Inadequate pain relief was recorded in 68.8% of a sample of people with hip or knee OA who participated in the population-based EpiReumaPt study, researchers reported at the OARSI 2022 World Congress.
“This can be explained by a lack of effectiveness of current management strategies, low uptake of recommended interventions by health care professionals, and also by low adherence by patients to medication and lifestyle interventions,” said Daniela Sofia Albino Costa, MSc, a PhD student at NOVA University Lisbon.
In addition to looking at the prevalence of inadequate pain relief – defined as a score of 5 or higher on the Numeric Pain Rating Scale (NPRS) – the study she presented at the congress, which was sponsored by the Osteoarthritis Research Society International, looked at the predictors for inadequate pain control.
It was found that being female, obesity, and having multimorbidity doubled the risk of inadequate versus adequate pain control, with respective odds ratios of 2.32 (P < .001), 2.26 (P = .006), and 2.07 (P = .001). Overweight was also associated with an increased odds ratio for poor pain control (OR, 1.84; P = .0035).
“We found that patients with inadequate pain relief also have a low performance on activities of daily living and a low quality of life,” Ms. Costa said.
Nearly one-third (29%) of patients in the inadequate pain relief group (n = 765) took medication, versus 15% of patients in the adequate pain relief group (n = 270). This was mostly NSAIDs, but also included analgesics and antipyretics, and in a few cases (4.8% vs. 1.3%), simple opioids.
“We know that current care is not concordant with recommendations,” said Ms. Costa, noting that medication being used as first-line treatment and core nonpharmacologic interventions are being offered to less than half of patients who are eligible.
In addition, the rate for total joint replacement has increased globally, and pain is an important predictor for this.
“So, we need to evaluate pain control and current management offered to people with hip or knee arthritis to identify to identify areas for improvement,” Ms. Costa said.
High rates of prescription opioid use before TKA
In a separate study also presented at the congress, Daniel Rhon, DPT, DSc, director of musculoskeletal research in primary care at Brooke Army Medical Center in San Antonio, gave a worrying glimpse of high rates of opioid use in the 4 years before total knee arthroplasty (TKA).
Using data from the U.S. Military Health System, the records of all individuals who had a knee replacement procedure between January 2017 and December 2018 were studied, to identify and characterize the use of prescription opioids.
Of the 46,362 individuals, 52.9% had prior opioid use, despite the fact that “opioids are not recommended for the management of knee OA,” said Dr. Rhon.
He also reported that as many as 40% of those who had at least one prescription for opioids had received a high-potency drug, such as fentanyl or oxycodone. The mean age of participants overall was 65 years, with a higher mean for those receiving opioids than those who did not (68 vs. 61.5 years). Data on sex and ethnicity were not available in time for presentation at the congress.
“Most of these individuals are getting these opioid prescriptions probably within 6 months, which maybe aligns with escalation of pain and maybe the decision to have that knee replacement,” Dr. Rhon said. Individuals that used opioids filled their most recent prescription a median of 146 days before TKA to surgery, with a mean of 317 days.
“You can’t always link the reason for the opioid prescription, that’s not really clear in the database,” he admitted; however, an analysis was performed to check if other surgeries had been performed that may have warranted the opioid treatment. The results revealed that very few of the opioid users (4%-7%) had undergone another type of surgical procedure.
“So, we feel a little bit better, that these findings weren’t for other surgical procedures,” said Dr. Rhon. He added that future qualitative research was needed to understand why health care professionals were prescribing opioids, and why patients felt like they needed them.
“That’s bad,” Haxby Abbott, PhD, DPT, a research professor at the University of Otago, Dunedin, New Zealand, commented on Twitter.
Dr. Abbott, who was not involved in the study, added: “We’ve done a similar study of the whole NZ population [currently under review] – similar to Australia and not nearly as bad as you found. That needs urgent attention.”
Sharp rise in opioid use 2 years before TKA
Lower rates of opioid use before TKA were seen in two European cohorts, at 43% in England and 33% in Sweden, as reported by Clara Hellberg, PhD, MD, of Lund (Sweden) University. However, rates had increased over a 10-year study period from a respective 23% and 16%, with a sharp increase in use in the 2 years before knee replacement.
The analysis was based on 49,043 patients from the English national database Clinical Practice Research Datalink, and 5,955 patients from the Swedish Skåne Healthcare register who had undergone total knee replacement between 2015 and 2019 and were matched by age, sex and general practice to individuals not undergoing knee replacement.
The prevalence ratio for using opioids over a 10-year period increased from 1.6 to 2.7 in England, and from 1.6 to 2.6 in Sweden.
“While the overall prevalence of opioid use was higher in England, the majority of both cases and controls were using weak opioids,” Dr. Hellberg said.
“Codeine was classified as a weak opioid, whereas morphine was classified as a strong opioid,” she added.
In contrast, the proportion of people using strong opioids in Sweden was greater than in England, she said.
The high opioid use found in the study highlights “the need for better opioid stewardship, and the availability of acceptable, effective alternatives,” Dr. Hellberg and associates concluded in their abstract.
The study presented by Ms. Costa was funded by the Portuguese national funding agency for science, research and technology and by an independent research grant from Pfizer. Dr. Rhon acknowledged grant funding from the National Institutes of Health and the U.S. Department of Defense. Dr. Hellberg had no conflicts of interest to disclose.
Inadequate pain relief was recorded in 68.8% of a sample of people with hip or knee OA who participated in the population-based EpiReumaPt study, researchers reported at the OARSI 2022 World Congress.
“This can be explained by a lack of effectiveness of current management strategies, low uptake of recommended interventions by health care professionals, and also by low adherence by patients to medication and lifestyle interventions,” said Daniela Sofia Albino Costa, MSc, a PhD student at NOVA University Lisbon.
In addition to looking at the prevalence of inadequate pain relief – defined as a score of 5 or higher on the Numeric Pain Rating Scale (NPRS) – the study she presented at the congress, which was sponsored by the Osteoarthritis Research Society International, looked at the predictors for inadequate pain control.
It was found that being female, obesity, and having multimorbidity doubled the risk of inadequate versus adequate pain control, with respective odds ratios of 2.32 (P < .001), 2.26 (P = .006), and 2.07 (P = .001). Overweight was also associated with an increased odds ratio for poor pain control (OR, 1.84; P = .0035).
“We found that patients with inadequate pain relief also have a low performance on activities of daily living and a low quality of life,” Ms. Costa said.
Nearly one-third (29%) of patients in the inadequate pain relief group (n = 765) took medication, versus 15% of patients in the adequate pain relief group (n = 270). This was mostly NSAIDs, but also included analgesics and antipyretics, and in a few cases (4.8% vs. 1.3%), simple opioids.
“We know that current care is not concordant with recommendations,” said Ms. Costa, noting that medication being used as first-line treatment and core nonpharmacologic interventions are being offered to less than half of patients who are eligible.
In addition, the rate for total joint replacement has increased globally, and pain is an important predictor for this.
“So, we need to evaluate pain control and current management offered to people with hip or knee arthritis to identify to identify areas for improvement,” Ms. Costa said.
High rates of prescription opioid use before TKA
In a separate study also presented at the congress, Daniel Rhon, DPT, DSc, director of musculoskeletal research in primary care at Brooke Army Medical Center in San Antonio, gave a worrying glimpse of high rates of opioid use in the 4 years before total knee arthroplasty (TKA).
Using data from the U.S. Military Health System, the records of all individuals who had a knee replacement procedure between January 2017 and December 2018 were studied, to identify and characterize the use of prescription opioids.
Of the 46,362 individuals, 52.9% had prior opioid use, despite the fact that “opioids are not recommended for the management of knee OA,” said Dr. Rhon.
He also reported that as many as 40% of those who had at least one prescription for opioids had received a high-potency drug, such as fentanyl or oxycodone. The mean age of participants overall was 65 years, with a higher mean for those receiving opioids than those who did not (68 vs. 61.5 years). Data on sex and ethnicity were not available in time for presentation at the congress.
“Most of these individuals are getting these opioid prescriptions probably within 6 months, which maybe aligns with escalation of pain and maybe the decision to have that knee replacement,” Dr. Rhon said. Individuals that used opioids filled their most recent prescription a median of 146 days before TKA to surgery, with a mean of 317 days.
“You can’t always link the reason for the opioid prescription, that’s not really clear in the database,” he admitted; however, an analysis was performed to check if other surgeries had been performed that may have warranted the opioid treatment. The results revealed that very few of the opioid users (4%-7%) had undergone another type of surgical procedure.
“So, we feel a little bit better, that these findings weren’t for other surgical procedures,” said Dr. Rhon. He added that future qualitative research was needed to understand why health care professionals were prescribing opioids, and why patients felt like they needed them.
“That’s bad,” Haxby Abbott, PhD, DPT, a research professor at the University of Otago, Dunedin, New Zealand, commented on Twitter.
Dr. Abbott, who was not involved in the study, added: “We’ve done a similar study of the whole NZ population [currently under review] – similar to Australia and not nearly as bad as you found. That needs urgent attention.”
Sharp rise in opioid use 2 years before TKA
Lower rates of opioid use before TKA were seen in two European cohorts, at 43% in England and 33% in Sweden, as reported by Clara Hellberg, PhD, MD, of Lund (Sweden) University. However, rates had increased over a 10-year study period from a respective 23% and 16%, with a sharp increase in use in the 2 years before knee replacement.
The analysis was based on 49,043 patients from the English national database Clinical Practice Research Datalink, and 5,955 patients from the Swedish Skåne Healthcare register who had undergone total knee replacement between 2015 and 2019 and were matched by age, sex and general practice to individuals not undergoing knee replacement.
The prevalence ratio for using opioids over a 10-year period increased from 1.6 to 2.7 in England, and from 1.6 to 2.6 in Sweden.
“While the overall prevalence of opioid use was higher in England, the majority of both cases and controls were using weak opioids,” Dr. Hellberg said.
“Codeine was classified as a weak opioid, whereas morphine was classified as a strong opioid,” she added.
In contrast, the proportion of people using strong opioids in Sweden was greater than in England, she said.
The high opioid use found in the study highlights “the need for better opioid stewardship, and the availability of acceptable, effective alternatives,” Dr. Hellberg and associates concluded in their abstract.
The study presented by Ms. Costa was funded by the Portuguese national funding agency for science, research and technology and by an independent research grant from Pfizer. Dr. Rhon acknowledged grant funding from the National Institutes of Health and the U.S. Department of Defense. Dr. Hellberg had no conflicts of interest to disclose.
Inadequate pain relief was recorded in 68.8% of a sample of people with hip or knee OA who participated in the population-based EpiReumaPt study, researchers reported at the OARSI 2022 World Congress.
“This can be explained by a lack of effectiveness of current management strategies, low uptake of recommended interventions by health care professionals, and also by low adherence by patients to medication and lifestyle interventions,” said Daniela Sofia Albino Costa, MSc, a PhD student at NOVA University Lisbon.
In addition to looking at the prevalence of inadequate pain relief – defined as a score of 5 or higher on the Numeric Pain Rating Scale (NPRS) – the study she presented at the congress, which was sponsored by the Osteoarthritis Research Society International, looked at the predictors for inadequate pain control.
It was found that being female, obesity, and having multimorbidity doubled the risk of inadequate versus adequate pain control, with respective odds ratios of 2.32 (P < .001), 2.26 (P = .006), and 2.07 (P = .001). Overweight was also associated with an increased odds ratio for poor pain control (OR, 1.84; P = .0035).
“We found that patients with inadequate pain relief also have a low performance on activities of daily living and a low quality of life,” Ms. Costa said.
Nearly one-third (29%) of patients in the inadequate pain relief group (n = 765) took medication, versus 15% of patients in the adequate pain relief group (n = 270). This was mostly NSAIDs, but also included analgesics and antipyretics, and in a few cases (4.8% vs. 1.3%), simple opioids.
“We know that current care is not concordant with recommendations,” said Ms. Costa, noting that medication being used as first-line treatment and core nonpharmacologic interventions are being offered to less than half of patients who are eligible.
In addition, the rate for total joint replacement has increased globally, and pain is an important predictor for this.
“So, we need to evaluate pain control and current management offered to people with hip or knee arthritis to identify to identify areas for improvement,” Ms. Costa said.
High rates of prescription opioid use before TKA
In a separate study also presented at the congress, Daniel Rhon, DPT, DSc, director of musculoskeletal research in primary care at Brooke Army Medical Center in San Antonio, gave a worrying glimpse of high rates of opioid use in the 4 years before total knee arthroplasty (TKA).
Using data from the U.S. Military Health System, the records of all individuals who had a knee replacement procedure between January 2017 and December 2018 were studied, to identify and characterize the use of prescription opioids.
Of the 46,362 individuals, 52.9% had prior opioid use, despite the fact that “opioids are not recommended for the management of knee OA,” said Dr. Rhon.
He also reported that as many as 40% of those who had at least one prescription for opioids had received a high-potency drug, such as fentanyl or oxycodone. The mean age of participants overall was 65 years, with a higher mean for those receiving opioids than those who did not (68 vs. 61.5 years). Data on sex and ethnicity were not available in time for presentation at the congress.
“Most of these individuals are getting these opioid prescriptions probably within 6 months, which maybe aligns with escalation of pain and maybe the decision to have that knee replacement,” Dr. Rhon said. Individuals that used opioids filled their most recent prescription a median of 146 days before TKA to surgery, with a mean of 317 days.
“You can’t always link the reason for the opioid prescription, that’s not really clear in the database,” he admitted; however, an analysis was performed to check if other surgeries had been performed that may have warranted the opioid treatment. The results revealed that very few of the opioid users (4%-7%) had undergone another type of surgical procedure.
“So, we feel a little bit better, that these findings weren’t for other surgical procedures,” said Dr. Rhon. He added that future qualitative research was needed to understand why health care professionals were prescribing opioids, and why patients felt like they needed them.
“That’s bad,” Haxby Abbott, PhD, DPT, a research professor at the University of Otago, Dunedin, New Zealand, commented on Twitter.
Dr. Abbott, who was not involved in the study, added: “We’ve done a similar study of the whole NZ population [currently under review] – similar to Australia and not nearly as bad as you found. That needs urgent attention.”
Sharp rise in opioid use 2 years before TKA
Lower rates of opioid use before TKA were seen in two European cohorts, at 43% in England and 33% in Sweden, as reported by Clara Hellberg, PhD, MD, of Lund (Sweden) University. However, rates had increased over a 10-year study period from a respective 23% and 16%, with a sharp increase in use in the 2 years before knee replacement.
The analysis was based on 49,043 patients from the English national database Clinical Practice Research Datalink, and 5,955 patients from the Swedish Skåne Healthcare register who had undergone total knee replacement between 2015 and 2019 and were matched by age, sex and general practice to individuals not undergoing knee replacement.
The prevalence ratio for using opioids over a 10-year period increased from 1.6 to 2.7 in England, and from 1.6 to 2.6 in Sweden.
“While the overall prevalence of opioid use was higher in England, the majority of both cases and controls were using weak opioids,” Dr. Hellberg said.
“Codeine was classified as a weak opioid, whereas morphine was classified as a strong opioid,” she added.
In contrast, the proportion of people using strong opioids in Sweden was greater than in England, she said.
The high opioid use found in the study highlights “the need for better opioid stewardship, and the availability of acceptable, effective alternatives,” Dr. Hellberg and associates concluded in their abstract.
The study presented by Ms. Costa was funded by the Portuguese national funding agency for science, research and technology and by an independent research grant from Pfizer. Dr. Rhon acknowledged grant funding from the National Institutes of Health and the U.S. Department of Defense. Dr. Hellberg had no conflicts of interest to disclose.
FROM OARSI 2022
Early meniscal surgery on par with active rehab in under 40s
according to the results of the randomized controlled DREAM trial presented at the OARSI 2022 World Congress.
Indeed, similar clinically relevant improvements in knee pain, function, and quality of life at 12 months were seen among participants in both study arms.
“Our results highlight that decisions on surgery or nonsurgical treatment must depend on preferences and values and needs of the individuals consulting their surgeon,” Søren T. Skou, PT, MSc, PhD, reported during one of the opening sessions at the meeting sponsored by the Osteoarthritis Research Society International.
The lack of superiority was contrary to the expectations of the researchers who hypothesized that early surgical intervention in adults aged between 18 and 40 years would be more beneficial than an active rehabilitation program with later surgery if needed.
Although the results do tie in with the results of other trials and systematic reviews in older adults the reason for looking at young adults specifically, aside from the obvious differences and the origin of meniscal tears, was that no study had previously looked at this population, Dr. Skou explained.
Assembling the DREAM team
The DREAM (Danish RCT on Exercise versus Arthroscopic Meniscal Surgery for Young Adults) trial “was a collaborative effort among many clinicians in Denmark – physical therapists, exercise physiologists, and surgeons,” Dr. Skou observed.
In total, 121 adults with MRI-verified meniscal tears who were eligible for surgery were recruited and randomized to either the early meniscal surgery group (n = 60) or to the exercise and education group (n = 61). The mean age was just below 30 years and 28% were female
Meniscal surgery, which was either an arthroscopic partial meniscectomy or meniscal repair, was performed at seven specialist centers. The exercise and education program was delivered by trained physical therapists working at 19 participating centers. The latter consisted of 24 sessions of group-based exercise therapy and education held over a period of 12 weeks.
Participants randomized to the exercise and education arm had the option of later meniscal surgery, with one in four eventually undergoing this procedure.
No gain in pain
The primary outcome measure was the difference in the mean of four of the subscales of the Knee Injury and Osteoarthritis Outcome Score (KOOS4) from baseline to 12-month assessment. The KOOS4 looks at knee pain, symptoms, function in sport and recreation, and quality of life.
“We considered a 10-point difference between groups as clinically relevant,” said Dr. Skou, but “adjusting for the baseline differences, we found no [statistical] differences or clinically relevant differences between groups.”
Improvement was seen in both groups. In an intention-to-treat analysis the KOOS4 scores improved by 19.2 points and 16.4 points respectively in the surgery and exercise and education groups, with a mean adjusted difference of 5.4 (95% confidence interval, –0.7 to 11.4). There was also no difference in a per protocol analysis, which considered only those participants who received the treatment strategy they were allocated (mean adjusted difference, 5.7; 95% CI, –0.9 to 12.4).
Secondary outcomes were also similarly improved in both groups with clinically relevant increases in all four KOOS subscale scores and in the Western Ontario Meniscal Evaluation Tool (WOMET).
While there were some statistical differences between the groups, such as better KOOS pain, symptoms, and WOMET scores in the surgery group, these were felt unlikely to be clinically relevant. Likewise, there was a statistically greater improvement in muscle strength in the exercise and education group than surgery group.
There was no statistical difference in the number of serious adverse events, including worsening of symptoms with or without acute onset during activity and lateral meniscal cysts, with four reported in the surgical group and seven in the exercise and education group.
Views on results
The results of the trial, published in NEJM Evidence, garnered attention on Twitter with several physiotherapists noting the data were positive for the nonsurgical management of meniscal tears in younger adults.
During discussion at the meeting, Nadine Foster, PhD, NIHR Professor of Musculoskeletal Health in Primary Care at Keele (England) University, asked if a larger cohort might not swing the results in favor of surgery.
She said: “Congratulations on this trial. The challenge: Your 95% CIs suggest a larger trial would have concluded superiority of surgery?”
Dr. Skou responded: “Most likely the true difference is outside the clinically relevant difference, but obviously, we cannot exclude that there is actually a clinically relevant difference between groups.”
Martin Englund, MD, Phd, of Lund (Sweden) University Hospital in Sweden, pointed out that 16 patients in the exercise and education group had “crossed over” and undergone surgery. “Were there any differences for those patients?” he asked.
“We looked at whether there was a difference between those – obviously only having 16 participants, we’re not able to do any statistical comparisons – but looking just visually at the data, they seem to improve to the same extent as those undergoing nonsurgical only,” Dr. Skou said.
The 2-year MRI data are currently being examined and will “obviously also be very interesting,” he added.
The DREAM trial was funded by the Danish Council for Independent Research, IMK Almene Fond, Lundbeck Foundation, Spar Nord Foundation, Danish Rheumatism Association, Association of Danish Physiotherapists Research Fund, Research Council at Næstved-Slagelse-Ringsted Hospitals, and Region Zealand. Dr. Skou had no financial or other conflicts of interest to disclose.
according to the results of the randomized controlled DREAM trial presented at the OARSI 2022 World Congress.
Indeed, similar clinically relevant improvements in knee pain, function, and quality of life at 12 months were seen among participants in both study arms.
“Our results highlight that decisions on surgery or nonsurgical treatment must depend on preferences and values and needs of the individuals consulting their surgeon,” Søren T. Skou, PT, MSc, PhD, reported during one of the opening sessions at the meeting sponsored by the Osteoarthritis Research Society International.
The lack of superiority was contrary to the expectations of the researchers who hypothesized that early surgical intervention in adults aged between 18 and 40 years would be more beneficial than an active rehabilitation program with later surgery if needed.
Although the results do tie in with the results of other trials and systematic reviews in older adults the reason for looking at young adults specifically, aside from the obvious differences and the origin of meniscal tears, was that no study had previously looked at this population, Dr. Skou explained.
Assembling the DREAM team
The DREAM (Danish RCT on Exercise versus Arthroscopic Meniscal Surgery for Young Adults) trial “was a collaborative effort among many clinicians in Denmark – physical therapists, exercise physiologists, and surgeons,” Dr. Skou observed.
In total, 121 adults with MRI-verified meniscal tears who were eligible for surgery were recruited and randomized to either the early meniscal surgery group (n = 60) or to the exercise and education group (n = 61). The mean age was just below 30 years and 28% were female
Meniscal surgery, which was either an arthroscopic partial meniscectomy or meniscal repair, was performed at seven specialist centers. The exercise and education program was delivered by trained physical therapists working at 19 participating centers. The latter consisted of 24 sessions of group-based exercise therapy and education held over a period of 12 weeks.
Participants randomized to the exercise and education arm had the option of later meniscal surgery, with one in four eventually undergoing this procedure.
No gain in pain
The primary outcome measure was the difference in the mean of four of the subscales of the Knee Injury and Osteoarthritis Outcome Score (KOOS4) from baseline to 12-month assessment. The KOOS4 looks at knee pain, symptoms, function in sport and recreation, and quality of life.
“We considered a 10-point difference between groups as clinically relevant,” said Dr. Skou, but “adjusting for the baseline differences, we found no [statistical] differences or clinically relevant differences between groups.”
Improvement was seen in both groups. In an intention-to-treat analysis the KOOS4 scores improved by 19.2 points and 16.4 points respectively in the surgery and exercise and education groups, with a mean adjusted difference of 5.4 (95% confidence interval, –0.7 to 11.4). There was also no difference in a per protocol analysis, which considered only those participants who received the treatment strategy they were allocated (mean adjusted difference, 5.7; 95% CI, –0.9 to 12.4).
Secondary outcomes were also similarly improved in both groups with clinically relevant increases in all four KOOS subscale scores and in the Western Ontario Meniscal Evaluation Tool (WOMET).
While there were some statistical differences between the groups, such as better KOOS pain, symptoms, and WOMET scores in the surgery group, these were felt unlikely to be clinically relevant. Likewise, there was a statistically greater improvement in muscle strength in the exercise and education group than surgery group.
There was no statistical difference in the number of serious adverse events, including worsening of symptoms with or without acute onset during activity and lateral meniscal cysts, with four reported in the surgical group and seven in the exercise and education group.
Views on results
The results of the trial, published in NEJM Evidence, garnered attention on Twitter with several physiotherapists noting the data were positive for the nonsurgical management of meniscal tears in younger adults.
During discussion at the meeting, Nadine Foster, PhD, NIHR Professor of Musculoskeletal Health in Primary Care at Keele (England) University, asked if a larger cohort might not swing the results in favor of surgery.
She said: “Congratulations on this trial. The challenge: Your 95% CIs suggest a larger trial would have concluded superiority of surgery?”
Dr. Skou responded: “Most likely the true difference is outside the clinically relevant difference, but obviously, we cannot exclude that there is actually a clinically relevant difference between groups.”
Martin Englund, MD, Phd, of Lund (Sweden) University Hospital in Sweden, pointed out that 16 patients in the exercise and education group had “crossed over” and undergone surgery. “Were there any differences for those patients?” he asked.
“We looked at whether there was a difference between those – obviously only having 16 participants, we’re not able to do any statistical comparisons – but looking just visually at the data, they seem to improve to the same extent as those undergoing nonsurgical only,” Dr. Skou said.
The 2-year MRI data are currently being examined and will “obviously also be very interesting,” he added.
The DREAM trial was funded by the Danish Council for Independent Research, IMK Almene Fond, Lundbeck Foundation, Spar Nord Foundation, Danish Rheumatism Association, Association of Danish Physiotherapists Research Fund, Research Council at Næstved-Slagelse-Ringsted Hospitals, and Region Zealand. Dr. Skou had no financial or other conflicts of interest to disclose.
according to the results of the randomized controlled DREAM trial presented at the OARSI 2022 World Congress.
Indeed, similar clinically relevant improvements in knee pain, function, and quality of life at 12 months were seen among participants in both study arms.
“Our results highlight that decisions on surgery or nonsurgical treatment must depend on preferences and values and needs of the individuals consulting their surgeon,” Søren T. Skou, PT, MSc, PhD, reported during one of the opening sessions at the meeting sponsored by the Osteoarthritis Research Society International.
The lack of superiority was contrary to the expectations of the researchers who hypothesized that early surgical intervention in adults aged between 18 and 40 years would be more beneficial than an active rehabilitation program with later surgery if needed.
Although the results do tie in with the results of other trials and systematic reviews in older adults the reason for looking at young adults specifically, aside from the obvious differences and the origin of meniscal tears, was that no study had previously looked at this population, Dr. Skou explained.
Assembling the DREAM team
The DREAM (Danish RCT on Exercise versus Arthroscopic Meniscal Surgery for Young Adults) trial “was a collaborative effort among many clinicians in Denmark – physical therapists, exercise physiologists, and surgeons,” Dr. Skou observed.
In total, 121 adults with MRI-verified meniscal tears who were eligible for surgery were recruited and randomized to either the early meniscal surgery group (n = 60) or to the exercise and education group (n = 61). The mean age was just below 30 years and 28% were female
Meniscal surgery, which was either an arthroscopic partial meniscectomy or meniscal repair, was performed at seven specialist centers. The exercise and education program was delivered by trained physical therapists working at 19 participating centers. The latter consisted of 24 sessions of group-based exercise therapy and education held over a period of 12 weeks.
Participants randomized to the exercise and education arm had the option of later meniscal surgery, with one in four eventually undergoing this procedure.
No gain in pain
The primary outcome measure was the difference in the mean of four of the subscales of the Knee Injury and Osteoarthritis Outcome Score (KOOS4) from baseline to 12-month assessment. The KOOS4 looks at knee pain, symptoms, function in sport and recreation, and quality of life.
“We considered a 10-point difference between groups as clinically relevant,” said Dr. Skou, but “adjusting for the baseline differences, we found no [statistical] differences or clinically relevant differences between groups.”
Improvement was seen in both groups. In an intention-to-treat analysis the KOOS4 scores improved by 19.2 points and 16.4 points respectively in the surgery and exercise and education groups, with a mean adjusted difference of 5.4 (95% confidence interval, –0.7 to 11.4). There was also no difference in a per protocol analysis, which considered only those participants who received the treatment strategy they were allocated (mean adjusted difference, 5.7; 95% CI, –0.9 to 12.4).
Secondary outcomes were also similarly improved in both groups with clinically relevant increases in all four KOOS subscale scores and in the Western Ontario Meniscal Evaluation Tool (WOMET).
While there were some statistical differences between the groups, such as better KOOS pain, symptoms, and WOMET scores in the surgery group, these were felt unlikely to be clinically relevant. Likewise, there was a statistically greater improvement in muscle strength in the exercise and education group than surgery group.
There was no statistical difference in the number of serious adverse events, including worsening of symptoms with or without acute onset during activity and lateral meniscal cysts, with four reported in the surgical group and seven in the exercise and education group.
Views on results
The results of the trial, published in NEJM Evidence, garnered attention on Twitter with several physiotherapists noting the data were positive for the nonsurgical management of meniscal tears in younger adults.
During discussion at the meeting, Nadine Foster, PhD, NIHR Professor of Musculoskeletal Health in Primary Care at Keele (England) University, asked if a larger cohort might not swing the results in favor of surgery.
She said: “Congratulations on this trial. The challenge: Your 95% CIs suggest a larger trial would have concluded superiority of surgery?”
Dr. Skou responded: “Most likely the true difference is outside the clinically relevant difference, but obviously, we cannot exclude that there is actually a clinically relevant difference between groups.”
Martin Englund, MD, Phd, of Lund (Sweden) University Hospital in Sweden, pointed out that 16 patients in the exercise and education group had “crossed over” and undergone surgery. “Were there any differences for those patients?” he asked.
“We looked at whether there was a difference between those – obviously only having 16 participants, we’re not able to do any statistical comparisons – but looking just visually at the data, they seem to improve to the same extent as those undergoing nonsurgical only,” Dr. Skou said.
The 2-year MRI data are currently being examined and will “obviously also be very interesting,” he added.
The DREAM trial was funded by the Danish Council for Independent Research, IMK Almene Fond, Lundbeck Foundation, Spar Nord Foundation, Danish Rheumatism Association, Association of Danish Physiotherapists Research Fund, Research Council at Næstved-Slagelse-Ringsted Hospitals, and Region Zealand. Dr. Skou had no financial or other conflicts of interest to disclose.
FROM OARSI 2022
Elective Total Hip Arthroplasty: Which Surgical Approach Is Optimal?
Total hip arthroplasty (THA) is one of the most successful orthopedic interventions performed today in terms of pain relief, cost effectiveness, and clinical outcomes.1 As a definitive treatment for end-stage arthritis of the hip, more than 330,000 procedures are performed in the Unites States each year. The number performed is growing by > 5% per year and is predicted to double by 2030, partly due to patients living longer, older individuals seeking a higher level of functionality than did previous generations, and better access to health care.2,3
The THA procedure also has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early-onset secondary arthritis, such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoroacetabular impingement.4 Younger patients are more likely to need a revision. According to a study by Evans and colleagues using available arthroplasty registry data, about three-quarters of hip replacements last 15 to 20 years, and 58% of hip replacements last 25 years in patients with osteoarthritis.5
For decades, the THA procedure of choice has been a standard posterior approach (PA). The PA was used because it allowed excellent intraoperative exposure and was applicable to a wide range of hip problems.6 In the past several years, modified muscle-sparing surgical approaches have been introduced. Two performed frequently are the mini PA (MPA) and the direct anterior approach (DAA).
The MPA is a modification of the PA. Surgeons perform the THA through a small incision without cutting the abductor muscles that are critical to hip stability and gait. A study published in 2010 concluded that the MPA was associated with less pain, shorter hospital length of stay (LOS) (therefore, an economic saving), and an earlier return to walking postoperatively.7
The DAA has been around since the early days of THA. Carl Hueter first described the anterior approach to the hip in 1881 (referred to as the Hueter approach). Smith-Peterson is frequently credited with popularizing the DAA technique during his career after publishing his first description of the approach in 1917.8 About 10 years ago, the DAA showed a resurgence as another muscle-sparing alternative for THAs. The DAA is considered to be a true intermuscular approach that preserves the soft tissues around the hip joint, thereby preserving the stability of the joint.9-11 The optimal surgical approach is still the subject of debate.
We present a male with right hip end-stage degenerative joint disease (DJD) and review some medical literature. Although other approaches to THA can be used (lateral, anterolateral), the discussion focuses on 2 muscle-sparing approaches performed frequently, the MPA and the DAA, and can be of value to primary care practitioners in their discussion with patients.
Case Presentation
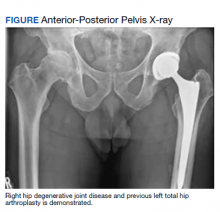
A 61-year-old male patient presented with progressive right hip pain. At age 37, he had a left THA via a PA due to hip dysplasia and a revision on the same hip at age 55 (the polyethylene liner was replaced and the cobalt chromium head was changed to ceramic), again through a PA. An orthopedic clinical evaluation and X-rays confirmed end-stage DJD of the right hip (Figure). He was informed to return to plan an elective THA when the “bad days were significantly greater than the good days” and/or when his functionality or quality of life was unacceptable. The orthopedic surgeon favored an MPA but offered a hand-off to colleagues who preferred the DAA. The patient was given information to review.
Discussion
No matter which approach is used, one study concluded that surgeons who perform > 50 hip replacements each year have better overall outcomes.12
The MPA emerged in the past decade as a muscle-sparing modification of the PA. The incision length (< 10 cm) is the simplest way of categorizing the surgery as an MPA. However, the amount of deep surgical dissection is a more important consideration for sparing muscle (for improved postoperative functionality, recovery, and joint stability) due to the gluteus maximus insertion, the quadratus femoris, and the piriformis tendons being left intact.13-16
Multiple studies have directly compared the MPA and PA, with variable results. One study concluded that the MPA was associated with lower surgical blood loss, lower pain at rest, and a faster recovery compared with that of the PA. Still, the study found no significant difference in postoperative laboratory values of possible markers of increased tissue damage and surgical invasiveness, such as creatinine phosphokinase (CPK) levels.15 Another randomized controlled trial (RCT) of 100 patients concluded that there was a trend for improved walking times and patient satisfaction at 6 weeks post-MPA vs PA.16 Other studies have found that the MPA and PA were essentially equivalent to each other regarding operative time, early postoperative outcomes, transfusion rate, hospital LOS, and postoperative complications.14 However, a recent meta-analysis found positive trends in favor of the MPA. The MPA was associated with a slight decrease in operating time, blood loss, hospital LOS, and earlier improvement in Harris hip scores. The meta-analysis found no significant decrease in the rate of dislocation or femoral fracture.13 Studies are still needed to evaluate long-term implant survival and outcomes for MPA and PA.
The DAA has received renewed attention as surgeons seek minimally invasive techniques and more rapid recoveries.6 The DAA involves a 3- to 4-inch incision on the front of the hip and enters the hip joint through the intermuscular interval between the tensor fasciae latae and gluteus medius muscles laterally and the sartorius muscle and rectus fascia medially.9 The DAA is considered a true intermuscular approach that preserves the soft tissues around the hip joint (including the posterior capsule), thereby presumably preserving the stability of the joint.9 The popularity for this approach has been attributed primarily to claims of improved recovery times, lower pain levels, improved patient satisfaction, as well as improved accuracy on both implant placement/alignment and leg length restoration.17 Orthopedic surgeons are increasingly being trained in the DAA during their residency and fellowship training.
There are many potential disadvantages to DAA. For example, DAA may present intraoperative radiation exposure for patients and surgeons during a fluoroscopy-assisted procedure. In addition, neuropraxia, particularly to the lateral femoral cutaneous nerve, can cause transient or permanent meralgia paresthetica. Wound healing may also present problems for female and obese patients, particularly those with a body mass index > 39 who are at increased risk of wound complications. DAA also increases time under anesthesia. Patients may experience proximal femoral fractures and dislocations and complex/challenging femoral exposure and bone preparation. Finally, sagittal malalignment of the stem could lead to loosening and an increased need for revision surgery.18
Another disadvantage of the DAA compared with the PA and MPA is the steep learning curve. Most studies find that the complication rate decreases only when the surgeon performs a significant number of DAA procedures. DeSteiger and colleagues noted a learning curve of 50 to 100 cases needed, and Masonis and colleagues concluded that at least 100 cases needed to be done to decrease operating and fluoroscopy times.19,20 Many orthopedic surgeons perform < 25 THA procedures a year.21
With the recent surge in popularity of the DAA, several studies have evaluated the DAA vs the MPA. A prospective RCT of 54 patients comparing the 2 approaches found that DAA patients walked without assistive devices sooner than did MPA patients: 22 days for DAA and 28 days for MPA.22 Improved cup position and a faster return of functionality were found in another study. DAA patients transitioned to a cane at 12 days vs 15.5 days for MPA patients and had a negative Trendelenburg sign at 16.7 days vs 24.8 days for MPA patients.23
Comparing DAA and MPA for inflammatory markers (serum CPK, C-reactive protein, interleukin-6, interleukin-1 β and tumor necrosis factor-α), the level of CPK postoperatively was 5.5 times higher in MPA patients, consistent with significantly more muscle damage. However, the overall physiologic burden as demonstrated by the measurement of all inflammatory markers was similar between the MPA and the DAA. This suggests that the inflammatory cascade associated with THA may be influenced more by the osteotomy and prosthesis implantation than by the surgical approach.24
Of note, some surgeons who perform the DAA recommend fewer postoperative precautions and suggest that physical therapy may not be necessary after discharge.25,26 Nevertheless, physiotherapeutic rehabilitation after all THA surgery is recommended as the standard treatment to minimize postoperative complications, such as hip dislocation, wound infection, deep venous thrombosis, and pulmonary embolism, and to maximize the patient’s functionality.27-29 RCTs are needed to look at long-term data on clinical outcomes between the MPA and DAA. Dislocation is a risk regardless of the approach used. Nevertheless, rates of dislocation, in general, are now very low, given the use of larger femoral head implants for all approaches.
Conclusions
THA is one of the most successful surgical procedures performed today. Patients desire hip pain relief and a return to function with as little interruption in their life as possible. Additionally, health care systems and insurers require THA procedures to be as efficient and cost-effective as possible. The debate regarding the most effective or preferable approach for THA continues. Although some prospective RCTs found that patients who underwent the DAA had objectively faster recovery than patients who had the MPA, it is also acknowledged that the results were dependent on surgeons who are very skilled in performing DAAs. The hope of both approaches is to get the individual moving as quickly and safely as possible to avoid a cascade of deterioration in the postoperative period. Factors other than the surgical approach, including patient selection, surgical volume and experience, careful preoperative assessments, attentive pain management, and rapid rehabilitation protocols, may be just as important as to which procedure is performed.30 The final decision should still be dependent on the patient-surgeon relationship and informed decision making.
In this case, the patient reviewed all the information he was given and independently researched the 2 procedures over many months. Ultimately, he decided to undergo a right THA via the DAA.
1. Elmallah RK, Chughtai M, Khlopas A. et al. Determining cost-effectiveness of total hip and knee arthroplasty using the Short Form-6D utility measure. J Arthroplasty. 2017;32(2):351-354. doi:10.1016/j.arth.2016.08.006
2. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
3. Kurtz, S, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
4. Sheahan WT, Parvataneni HK. Asymptomatic but time for a hip revision. Fed Pract. 2016;33(2):39-43.
5. Evans, JT, Evans JP, Walker RW, et al. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet. 2019;393(10172):647-654. doi:10.1016/S0140-6736(18)31665-9
6. Yang X, Huang H-F, Sun L , Yang Z, Deng C-Y, Tian XB. Direct anterior approach versus posterolateral approach in total hip arthroplasty: a systematic review and meta-analysis of randomized controlled studies. Orthop Surg. 2020;12:1065-1073. doi:10.1111/os.12669
7. Varela Egocheaga JR, Suárez-Suárez MA, Fernández-Villán M, González-Sastre V, Varela-Gómez JR, Murcia-Mazón A. Minimally invasive posterior approach in total hip arthroplasty. Prospective randomized trial. An Sist Sanit Navar. 2010:33(2):133-143. doi:10.4321/s1137-66272010000300002
8. Raxhbauer F, Kain MS, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320. doi:10.1016/j.ocl.2009.02.007
9. Jia F, Guo B, Xu F, Hou Y, Tang X, Huang L. A comparison of clinical, radiographic and surgical outcomes of total hip arthroplasty between direct anterior and posterior approaches: a systematic review and meta-analysis. Hip Int. 2019;29(6):584-596. doi:10.1177/1120700018820652
10. Kennon RE Keggi JM, Wetmore RS, Zatorski LE, Huo MH, Keggi KJ. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am. 2003;85-A(suppl 4):39-48. doi:10.2106/00004623-200300004-00005
11. Bal BS, Vallurupalli S. Minimally invasive total hip arthroplasty with the anterior approach. Indian J Orthop. 2008;42(3):301-308. doi:10.4103/0019-5413.41853
12. Katz JN, Losina E, Barrett E. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629. doi:10.2106/00004623-200111000-00002
13. Berstock JR, Blom AW, Beswick AD. A systematic review and meta-analysis of the standard versus mini-incision approach to a total hip arthroplasty. J Arthroplasty. 2014;29(10):1970-1982. doi:10.1016/j.arth.2014.05.021
14. Chimento GF, Pavone V, Sharrock S, Kahn K, Cahill J, Sculco TP. Minimally invasive total hip arthroplasty: a prospective randomized study. J Arthroplasty. 2005;20(2):139-144. doi:10.1016/j.arth.2004.09.061
15. Fink B, Mittelstaedt A, Schulz MS, Sebena P, Sing J. Comparison of a minimally invasive posterior approach and the standard posterior approach for total hip arthroplasty. A prospective and comparative study. J Orthop Surg Res. 2010;5:46. doi:10.1186/1749-799X-5-46
16. Khan RJ, Maor D, Hofmann M, Haebich S. A comparison of a less invasive piriformis-sparing approach versus the standard approach to the hip: a randomized controlled trial. J Bone Joint Surg Br. 2012;94:43-50. doi:10.1302/0301-620X.94B1.27001
17. Galakatos GR. Direct anterior total hip arthroplasty. Missouri Med. 2018;115(6):537-541.
18. Flevas, DA, Tsantes AG, Mavrogenis, AE. Direct anterior approach total hip arthroplasty revisited. JBJS Rev. 2020;8(4):e0144. doi:10.2106/JBJS.RVW.19.00144
19. DeSteiger RN, Lorimer M, Solomon M. What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res. 2015;473(12):3860-3866. doi:10.1007/s11999-015-4565-6
20. Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics. 2008;31(12)(suppl 2).
21. Bal BS. Clinical faceoff: anterior total hip versus mini-posterior: Which one is better? Clin Orthop Relat Res. 2015;473(4):1192-1196. doi:10.1007/s11999-014-3684-9
22. Taunton MJ, Mason JB, Odum SM, Bryan D, Springer BD. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: a prospective, randomized clinical trial. J Arthroplasty. 2014;29;(suppl 9):169-172. doi:10.1016/j.arth.2014.03.05
23. Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24(5):698-704. doi:10.1016/j.arth.2008.04.012
24. Bergin PF, Doppelt JD, Kephart CJ. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. Bone Joint Surg Am. 2011; 93(15):1392-1398. doi:10.2106/JBJS.J.00557
25. Carli AV, Poitras S, Clohisy JC, Beaule PE. Variation in use of postoperative precautions and equipment following total hip arthroplasty: a survey of the AAHKS and CAS membership. J Arthroplasty. 2018;33(10):3201-3205. doi:10.1016/j.arth.2018.05.043
26. Kavcˇicˇ G, Mirt PK, Tumpej J, Bedenčič. The direct anterior approach for total hip arthroplasty without specific table: surgical approach and our seven years of experience. Published June 14, 2019. Accessed March 4, 2022. https://crimsonăpublishers.com/rabs/fulltext/RABS.000520.php27. American Academy of Orthopedic Surgeons. Total hip replacement exercise guide. Published 2017. Updated February 2022. Accessed March 4, 2022. https://orthoinfo.aaos.org/en/recovery/total-hip-replacement-exercise-guide
28. Medical Advisory Secretariat. Physiotherapy rehabilitation after total knee or hip replacement: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5(8):1-91.
29. Pa˘unescu F, Didilescu A, Antonescu DM. Factors that may influence the functional outcome after primary total hip arthroplasty. Clujul Med. 2013;86(2):121-127.
30. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631. doi:10.1007/s11999-014-3827-z
Total hip arthroplasty (THA) is one of the most successful orthopedic interventions performed today in terms of pain relief, cost effectiveness, and clinical outcomes.1 As a definitive treatment for end-stage arthritis of the hip, more than 330,000 procedures are performed in the Unites States each year. The number performed is growing by > 5% per year and is predicted to double by 2030, partly due to patients living longer, older individuals seeking a higher level of functionality than did previous generations, and better access to health care.2,3
The THA procedure also has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early-onset secondary arthritis, such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoroacetabular impingement.4 Younger patients are more likely to need a revision. According to a study by Evans and colleagues using available arthroplasty registry data, about three-quarters of hip replacements last 15 to 20 years, and 58% of hip replacements last 25 years in patients with osteoarthritis.5
For decades, the THA procedure of choice has been a standard posterior approach (PA). The PA was used because it allowed excellent intraoperative exposure and was applicable to a wide range of hip problems.6 In the past several years, modified muscle-sparing surgical approaches have been introduced. Two performed frequently are the mini PA (MPA) and the direct anterior approach (DAA).
The MPA is a modification of the PA. Surgeons perform the THA through a small incision without cutting the abductor muscles that are critical to hip stability and gait. A study published in 2010 concluded that the MPA was associated with less pain, shorter hospital length of stay (LOS) (therefore, an economic saving), and an earlier return to walking postoperatively.7
The DAA has been around since the early days of THA. Carl Hueter first described the anterior approach to the hip in 1881 (referred to as the Hueter approach). Smith-Peterson is frequently credited with popularizing the DAA technique during his career after publishing his first description of the approach in 1917.8 About 10 years ago, the DAA showed a resurgence as another muscle-sparing alternative for THAs. The DAA is considered to be a true intermuscular approach that preserves the soft tissues around the hip joint, thereby preserving the stability of the joint.9-11 The optimal surgical approach is still the subject of debate.
We present a male with right hip end-stage degenerative joint disease (DJD) and review some medical literature. Although other approaches to THA can be used (lateral, anterolateral), the discussion focuses on 2 muscle-sparing approaches performed frequently, the MPA and the DAA, and can be of value to primary care practitioners in their discussion with patients.
Case Presentation
A 61-year-old male patient presented with progressive right hip pain. At age 37, he had a left THA via a PA due to hip dysplasia and a revision on the same hip at age 55 (the polyethylene liner was replaced and the cobalt chromium head was changed to ceramic), again through a PA. An orthopedic clinical evaluation and X-rays confirmed end-stage DJD of the right hip (Figure). He was informed to return to plan an elective THA when the “bad days were significantly greater than the good days” and/or when his functionality or quality of life was unacceptable. The orthopedic surgeon favored an MPA but offered a hand-off to colleagues who preferred the DAA. The patient was given information to review.
Discussion
No matter which approach is used, one study concluded that surgeons who perform > 50 hip replacements each year have better overall outcomes.12
The MPA emerged in the past decade as a muscle-sparing modification of the PA. The incision length (< 10 cm) is the simplest way of categorizing the surgery as an MPA. However, the amount of deep surgical dissection is a more important consideration for sparing muscle (for improved postoperative functionality, recovery, and joint stability) due to the gluteus maximus insertion, the quadratus femoris, and the piriformis tendons being left intact.13-16
Multiple studies have directly compared the MPA and PA, with variable results. One study concluded that the MPA was associated with lower surgical blood loss, lower pain at rest, and a faster recovery compared with that of the PA. Still, the study found no significant difference in postoperative laboratory values of possible markers of increased tissue damage and surgical invasiveness, such as creatinine phosphokinase (CPK) levels.15 Another randomized controlled trial (RCT) of 100 patients concluded that there was a trend for improved walking times and patient satisfaction at 6 weeks post-MPA vs PA.16 Other studies have found that the MPA and PA were essentially equivalent to each other regarding operative time, early postoperative outcomes, transfusion rate, hospital LOS, and postoperative complications.14 However, a recent meta-analysis found positive trends in favor of the MPA. The MPA was associated with a slight decrease in operating time, blood loss, hospital LOS, and earlier improvement in Harris hip scores. The meta-analysis found no significant decrease in the rate of dislocation or femoral fracture.13 Studies are still needed to evaluate long-term implant survival and outcomes for MPA and PA.
The DAA has received renewed attention as surgeons seek minimally invasive techniques and more rapid recoveries.6 The DAA involves a 3- to 4-inch incision on the front of the hip and enters the hip joint through the intermuscular interval between the tensor fasciae latae and gluteus medius muscles laterally and the sartorius muscle and rectus fascia medially.9 The DAA is considered a true intermuscular approach that preserves the soft tissues around the hip joint (including the posterior capsule), thereby presumably preserving the stability of the joint.9 The popularity for this approach has been attributed primarily to claims of improved recovery times, lower pain levels, improved patient satisfaction, as well as improved accuracy on both implant placement/alignment and leg length restoration.17 Orthopedic surgeons are increasingly being trained in the DAA during their residency and fellowship training.
There are many potential disadvantages to DAA. For example, DAA may present intraoperative radiation exposure for patients and surgeons during a fluoroscopy-assisted procedure. In addition, neuropraxia, particularly to the lateral femoral cutaneous nerve, can cause transient or permanent meralgia paresthetica. Wound healing may also present problems for female and obese patients, particularly those with a body mass index > 39 who are at increased risk of wound complications. DAA also increases time under anesthesia. Patients may experience proximal femoral fractures and dislocations and complex/challenging femoral exposure and bone preparation. Finally, sagittal malalignment of the stem could lead to loosening and an increased need for revision surgery.18
Another disadvantage of the DAA compared with the PA and MPA is the steep learning curve. Most studies find that the complication rate decreases only when the surgeon performs a significant number of DAA procedures. DeSteiger and colleagues noted a learning curve of 50 to 100 cases needed, and Masonis and colleagues concluded that at least 100 cases needed to be done to decrease operating and fluoroscopy times.19,20 Many orthopedic surgeons perform < 25 THA procedures a year.21
With the recent surge in popularity of the DAA, several studies have evaluated the DAA vs the MPA. A prospective RCT of 54 patients comparing the 2 approaches found that DAA patients walked without assistive devices sooner than did MPA patients: 22 days for DAA and 28 days for MPA.22 Improved cup position and a faster return of functionality were found in another study. DAA patients transitioned to a cane at 12 days vs 15.5 days for MPA patients and had a negative Trendelenburg sign at 16.7 days vs 24.8 days for MPA patients.23
Comparing DAA and MPA for inflammatory markers (serum CPK, C-reactive protein, interleukin-6, interleukin-1 β and tumor necrosis factor-α), the level of CPK postoperatively was 5.5 times higher in MPA patients, consistent with significantly more muscle damage. However, the overall physiologic burden as demonstrated by the measurement of all inflammatory markers was similar between the MPA and the DAA. This suggests that the inflammatory cascade associated with THA may be influenced more by the osteotomy and prosthesis implantation than by the surgical approach.24
Of note, some surgeons who perform the DAA recommend fewer postoperative precautions and suggest that physical therapy may not be necessary after discharge.25,26 Nevertheless, physiotherapeutic rehabilitation after all THA surgery is recommended as the standard treatment to minimize postoperative complications, such as hip dislocation, wound infection, deep venous thrombosis, and pulmonary embolism, and to maximize the patient’s functionality.27-29 RCTs are needed to look at long-term data on clinical outcomes between the MPA and DAA. Dislocation is a risk regardless of the approach used. Nevertheless, rates of dislocation, in general, are now very low, given the use of larger femoral head implants for all approaches.
Conclusions
THA is one of the most successful surgical procedures performed today. Patients desire hip pain relief and a return to function with as little interruption in their life as possible. Additionally, health care systems and insurers require THA procedures to be as efficient and cost-effective as possible. The debate regarding the most effective or preferable approach for THA continues. Although some prospective RCTs found that patients who underwent the DAA had objectively faster recovery than patients who had the MPA, it is also acknowledged that the results were dependent on surgeons who are very skilled in performing DAAs. The hope of both approaches is to get the individual moving as quickly and safely as possible to avoid a cascade of deterioration in the postoperative period. Factors other than the surgical approach, including patient selection, surgical volume and experience, careful preoperative assessments, attentive pain management, and rapid rehabilitation protocols, may be just as important as to which procedure is performed.30 The final decision should still be dependent on the patient-surgeon relationship and informed decision making.
In this case, the patient reviewed all the information he was given and independently researched the 2 procedures over many months. Ultimately, he decided to undergo a right THA via the DAA.
Total hip arthroplasty (THA) is one of the most successful orthopedic interventions performed today in terms of pain relief, cost effectiveness, and clinical outcomes.1 As a definitive treatment for end-stage arthritis of the hip, more than 330,000 procedures are performed in the Unites States each year. The number performed is growing by > 5% per year and is predicted to double by 2030, partly due to patients living longer, older individuals seeking a higher level of functionality than did previous generations, and better access to health care.2,3
The THA procedure also has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early-onset secondary arthritis, such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoroacetabular impingement.4 Younger patients are more likely to need a revision. According to a study by Evans and colleagues using available arthroplasty registry data, about three-quarters of hip replacements last 15 to 20 years, and 58% of hip replacements last 25 years in patients with osteoarthritis.5
For decades, the THA procedure of choice has been a standard posterior approach (PA). The PA was used because it allowed excellent intraoperative exposure and was applicable to a wide range of hip problems.6 In the past several years, modified muscle-sparing surgical approaches have been introduced. Two performed frequently are the mini PA (MPA) and the direct anterior approach (DAA).
The MPA is a modification of the PA. Surgeons perform the THA through a small incision without cutting the abductor muscles that are critical to hip stability and gait. A study published in 2010 concluded that the MPA was associated with less pain, shorter hospital length of stay (LOS) (therefore, an economic saving), and an earlier return to walking postoperatively.7
The DAA has been around since the early days of THA. Carl Hueter first described the anterior approach to the hip in 1881 (referred to as the Hueter approach). Smith-Peterson is frequently credited with popularizing the DAA technique during his career after publishing his first description of the approach in 1917.8 About 10 years ago, the DAA showed a resurgence as another muscle-sparing alternative for THAs. The DAA is considered to be a true intermuscular approach that preserves the soft tissues around the hip joint, thereby preserving the stability of the joint.9-11 The optimal surgical approach is still the subject of debate.
We present a male with right hip end-stage degenerative joint disease (DJD) and review some medical literature. Although other approaches to THA can be used (lateral, anterolateral), the discussion focuses on 2 muscle-sparing approaches performed frequently, the MPA and the DAA, and can be of value to primary care practitioners in their discussion with patients.
Case Presentation
A 61-year-old male patient presented with progressive right hip pain. At age 37, he had a left THA via a PA due to hip dysplasia and a revision on the same hip at age 55 (the polyethylene liner was replaced and the cobalt chromium head was changed to ceramic), again through a PA. An orthopedic clinical evaluation and X-rays confirmed end-stage DJD of the right hip (Figure). He was informed to return to plan an elective THA when the “bad days were significantly greater than the good days” and/or when his functionality or quality of life was unacceptable. The orthopedic surgeon favored an MPA but offered a hand-off to colleagues who preferred the DAA. The patient was given information to review.
Discussion
No matter which approach is used, one study concluded that surgeons who perform > 50 hip replacements each year have better overall outcomes.12
The MPA emerged in the past decade as a muscle-sparing modification of the PA. The incision length (< 10 cm) is the simplest way of categorizing the surgery as an MPA. However, the amount of deep surgical dissection is a more important consideration for sparing muscle (for improved postoperative functionality, recovery, and joint stability) due to the gluteus maximus insertion, the quadratus femoris, and the piriformis tendons being left intact.13-16
Multiple studies have directly compared the MPA and PA, with variable results. One study concluded that the MPA was associated with lower surgical blood loss, lower pain at rest, and a faster recovery compared with that of the PA. Still, the study found no significant difference in postoperative laboratory values of possible markers of increased tissue damage and surgical invasiveness, such as creatinine phosphokinase (CPK) levels.15 Another randomized controlled trial (RCT) of 100 patients concluded that there was a trend for improved walking times and patient satisfaction at 6 weeks post-MPA vs PA.16 Other studies have found that the MPA and PA were essentially equivalent to each other regarding operative time, early postoperative outcomes, transfusion rate, hospital LOS, and postoperative complications.14 However, a recent meta-analysis found positive trends in favor of the MPA. The MPA was associated with a slight decrease in operating time, blood loss, hospital LOS, and earlier improvement in Harris hip scores. The meta-analysis found no significant decrease in the rate of dislocation or femoral fracture.13 Studies are still needed to evaluate long-term implant survival and outcomes for MPA and PA.
The DAA has received renewed attention as surgeons seek minimally invasive techniques and more rapid recoveries.6 The DAA involves a 3- to 4-inch incision on the front of the hip and enters the hip joint through the intermuscular interval between the tensor fasciae latae and gluteus medius muscles laterally and the sartorius muscle and rectus fascia medially.9 The DAA is considered a true intermuscular approach that preserves the soft tissues around the hip joint (including the posterior capsule), thereby presumably preserving the stability of the joint.9 The popularity for this approach has been attributed primarily to claims of improved recovery times, lower pain levels, improved patient satisfaction, as well as improved accuracy on both implant placement/alignment and leg length restoration.17 Orthopedic surgeons are increasingly being trained in the DAA during their residency and fellowship training.
There are many potential disadvantages to DAA. For example, DAA may present intraoperative radiation exposure for patients and surgeons during a fluoroscopy-assisted procedure. In addition, neuropraxia, particularly to the lateral femoral cutaneous nerve, can cause transient or permanent meralgia paresthetica. Wound healing may also present problems for female and obese patients, particularly those with a body mass index > 39 who are at increased risk of wound complications. DAA also increases time under anesthesia. Patients may experience proximal femoral fractures and dislocations and complex/challenging femoral exposure and bone preparation. Finally, sagittal malalignment of the stem could lead to loosening and an increased need for revision surgery.18
Another disadvantage of the DAA compared with the PA and MPA is the steep learning curve. Most studies find that the complication rate decreases only when the surgeon performs a significant number of DAA procedures. DeSteiger and colleagues noted a learning curve of 50 to 100 cases needed, and Masonis and colleagues concluded that at least 100 cases needed to be done to decrease operating and fluoroscopy times.19,20 Many orthopedic surgeons perform < 25 THA procedures a year.21
With the recent surge in popularity of the DAA, several studies have evaluated the DAA vs the MPA. A prospective RCT of 54 patients comparing the 2 approaches found that DAA patients walked without assistive devices sooner than did MPA patients: 22 days for DAA and 28 days for MPA.22 Improved cup position and a faster return of functionality were found in another study. DAA patients transitioned to a cane at 12 days vs 15.5 days for MPA patients and had a negative Trendelenburg sign at 16.7 days vs 24.8 days for MPA patients.23
Comparing DAA and MPA for inflammatory markers (serum CPK, C-reactive protein, interleukin-6, interleukin-1 β and tumor necrosis factor-α), the level of CPK postoperatively was 5.5 times higher in MPA patients, consistent with significantly more muscle damage. However, the overall physiologic burden as demonstrated by the measurement of all inflammatory markers was similar between the MPA and the DAA. This suggests that the inflammatory cascade associated with THA may be influenced more by the osteotomy and prosthesis implantation than by the surgical approach.24
Of note, some surgeons who perform the DAA recommend fewer postoperative precautions and suggest that physical therapy may not be necessary after discharge.25,26 Nevertheless, physiotherapeutic rehabilitation after all THA surgery is recommended as the standard treatment to minimize postoperative complications, such as hip dislocation, wound infection, deep venous thrombosis, and pulmonary embolism, and to maximize the patient’s functionality.27-29 RCTs are needed to look at long-term data on clinical outcomes between the MPA and DAA. Dislocation is a risk regardless of the approach used. Nevertheless, rates of dislocation, in general, are now very low, given the use of larger femoral head implants for all approaches.
Conclusions
THA is one of the most successful surgical procedures performed today. Patients desire hip pain relief and a return to function with as little interruption in their life as possible. Additionally, health care systems and insurers require THA procedures to be as efficient and cost-effective as possible. The debate regarding the most effective or preferable approach for THA continues. Although some prospective RCTs found that patients who underwent the DAA had objectively faster recovery than patients who had the MPA, it is also acknowledged that the results were dependent on surgeons who are very skilled in performing DAAs. The hope of both approaches is to get the individual moving as quickly and safely as possible to avoid a cascade of deterioration in the postoperative period. Factors other than the surgical approach, including patient selection, surgical volume and experience, careful preoperative assessments, attentive pain management, and rapid rehabilitation protocols, may be just as important as to which procedure is performed.30 The final decision should still be dependent on the patient-surgeon relationship and informed decision making.
In this case, the patient reviewed all the information he was given and independently researched the 2 procedures over many months. Ultimately, he decided to undergo a right THA via the DAA.
1. Elmallah RK, Chughtai M, Khlopas A. et al. Determining cost-effectiveness of total hip and knee arthroplasty using the Short Form-6D utility measure. J Arthroplasty. 2017;32(2):351-354. doi:10.1016/j.arth.2016.08.006
2. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
3. Kurtz, S, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
4. Sheahan WT, Parvataneni HK. Asymptomatic but time for a hip revision. Fed Pract. 2016;33(2):39-43.
5. Evans, JT, Evans JP, Walker RW, et al. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet. 2019;393(10172):647-654. doi:10.1016/S0140-6736(18)31665-9
6. Yang X, Huang H-F, Sun L , Yang Z, Deng C-Y, Tian XB. Direct anterior approach versus posterolateral approach in total hip arthroplasty: a systematic review and meta-analysis of randomized controlled studies. Orthop Surg. 2020;12:1065-1073. doi:10.1111/os.12669
7. Varela Egocheaga JR, Suárez-Suárez MA, Fernández-Villán M, González-Sastre V, Varela-Gómez JR, Murcia-Mazón A. Minimally invasive posterior approach in total hip arthroplasty. Prospective randomized trial. An Sist Sanit Navar. 2010:33(2):133-143. doi:10.4321/s1137-66272010000300002
8. Raxhbauer F, Kain MS, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320. doi:10.1016/j.ocl.2009.02.007
9. Jia F, Guo B, Xu F, Hou Y, Tang X, Huang L. A comparison of clinical, radiographic and surgical outcomes of total hip arthroplasty between direct anterior and posterior approaches: a systematic review and meta-analysis. Hip Int. 2019;29(6):584-596. doi:10.1177/1120700018820652
10. Kennon RE Keggi JM, Wetmore RS, Zatorski LE, Huo MH, Keggi KJ. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am. 2003;85-A(suppl 4):39-48. doi:10.2106/00004623-200300004-00005
11. Bal BS, Vallurupalli S. Minimally invasive total hip arthroplasty with the anterior approach. Indian J Orthop. 2008;42(3):301-308. doi:10.4103/0019-5413.41853
12. Katz JN, Losina E, Barrett E. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629. doi:10.2106/00004623-200111000-00002
13. Berstock JR, Blom AW, Beswick AD. A systematic review and meta-analysis of the standard versus mini-incision approach to a total hip arthroplasty. J Arthroplasty. 2014;29(10):1970-1982. doi:10.1016/j.arth.2014.05.021
14. Chimento GF, Pavone V, Sharrock S, Kahn K, Cahill J, Sculco TP. Minimally invasive total hip arthroplasty: a prospective randomized study. J Arthroplasty. 2005;20(2):139-144. doi:10.1016/j.arth.2004.09.061
15. Fink B, Mittelstaedt A, Schulz MS, Sebena P, Sing J. Comparison of a minimally invasive posterior approach and the standard posterior approach for total hip arthroplasty. A prospective and comparative study. J Orthop Surg Res. 2010;5:46. doi:10.1186/1749-799X-5-46
16. Khan RJ, Maor D, Hofmann M, Haebich S. A comparison of a less invasive piriformis-sparing approach versus the standard approach to the hip: a randomized controlled trial. J Bone Joint Surg Br. 2012;94:43-50. doi:10.1302/0301-620X.94B1.27001
17. Galakatos GR. Direct anterior total hip arthroplasty. Missouri Med. 2018;115(6):537-541.
18. Flevas, DA, Tsantes AG, Mavrogenis, AE. Direct anterior approach total hip arthroplasty revisited. JBJS Rev. 2020;8(4):e0144. doi:10.2106/JBJS.RVW.19.00144
19. DeSteiger RN, Lorimer M, Solomon M. What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res. 2015;473(12):3860-3866. doi:10.1007/s11999-015-4565-6
20. Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics. 2008;31(12)(suppl 2).
21. Bal BS. Clinical faceoff: anterior total hip versus mini-posterior: Which one is better? Clin Orthop Relat Res. 2015;473(4):1192-1196. doi:10.1007/s11999-014-3684-9
22. Taunton MJ, Mason JB, Odum SM, Bryan D, Springer BD. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: a prospective, randomized clinical trial. J Arthroplasty. 2014;29;(suppl 9):169-172. doi:10.1016/j.arth.2014.03.05
23. Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24(5):698-704. doi:10.1016/j.arth.2008.04.012
24. Bergin PF, Doppelt JD, Kephart CJ. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. Bone Joint Surg Am. 2011; 93(15):1392-1398. doi:10.2106/JBJS.J.00557
25. Carli AV, Poitras S, Clohisy JC, Beaule PE. Variation in use of postoperative precautions and equipment following total hip arthroplasty: a survey of the AAHKS and CAS membership. J Arthroplasty. 2018;33(10):3201-3205. doi:10.1016/j.arth.2018.05.043
26. Kavcˇicˇ G, Mirt PK, Tumpej J, Bedenčič. The direct anterior approach for total hip arthroplasty without specific table: surgical approach and our seven years of experience. Published June 14, 2019. Accessed March 4, 2022. https://crimsonăpublishers.com/rabs/fulltext/RABS.000520.php27. American Academy of Orthopedic Surgeons. Total hip replacement exercise guide. Published 2017. Updated February 2022. Accessed March 4, 2022. https://orthoinfo.aaos.org/en/recovery/total-hip-replacement-exercise-guide
28. Medical Advisory Secretariat. Physiotherapy rehabilitation after total knee or hip replacement: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5(8):1-91.
29. Pa˘unescu F, Didilescu A, Antonescu DM. Factors that may influence the functional outcome after primary total hip arthroplasty. Clujul Med. 2013;86(2):121-127.
30. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631. doi:10.1007/s11999-014-3827-z
1. Elmallah RK, Chughtai M, Khlopas A. et al. Determining cost-effectiveness of total hip and knee arthroplasty using the Short Form-6D utility measure. J Arthroplasty. 2017;32(2):351-354. doi:10.1016/j.arth.2016.08.006
2. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
3. Kurtz, S, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
4. Sheahan WT, Parvataneni HK. Asymptomatic but time for a hip revision. Fed Pract. 2016;33(2):39-43.
5. Evans, JT, Evans JP, Walker RW, et al. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet. 2019;393(10172):647-654. doi:10.1016/S0140-6736(18)31665-9
6. Yang X, Huang H-F, Sun L , Yang Z, Deng C-Y, Tian XB. Direct anterior approach versus posterolateral approach in total hip arthroplasty: a systematic review and meta-analysis of randomized controlled studies. Orthop Surg. 2020;12:1065-1073. doi:10.1111/os.12669
7. Varela Egocheaga JR, Suárez-Suárez MA, Fernández-Villán M, González-Sastre V, Varela-Gómez JR, Murcia-Mazón A. Minimally invasive posterior approach in total hip arthroplasty. Prospective randomized trial. An Sist Sanit Navar. 2010:33(2):133-143. doi:10.4321/s1137-66272010000300002
8. Raxhbauer F, Kain MS, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320. doi:10.1016/j.ocl.2009.02.007
9. Jia F, Guo B, Xu F, Hou Y, Tang X, Huang L. A comparison of clinical, radiographic and surgical outcomes of total hip arthroplasty between direct anterior and posterior approaches: a systematic review and meta-analysis. Hip Int. 2019;29(6):584-596. doi:10.1177/1120700018820652
10. Kennon RE Keggi JM, Wetmore RS, Zatorski LE, Huo MH, Keggi KJ. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am. 2003;85-A(suppl 4):39-48. doi:10.2106/00004623-200300004-00005
11. Bal BS, Vallurupalli S. Minimally invasive total hip arthroplasty with the anterior approach. Indian J Orthop. 2008;42(3):301-308. doi:10.4103/0019-5413.41853
12. Katz JN, Losina E, Barrett E. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629. doi:10.2106/00004623-200111000-00002
13. Berstock JR, Blom AW, Beswick AD. A systematic review and meta-analysis of the standard versus mini-incision approach to a total hip arthroplasty. J Arthroplasty. 2014;29(10):1970-1982. doi:10.1016/j.arth.2014.05.021
14. Chimento GF, Pavone V, Sharrock S, Kahn K, Cahill J, Sculco TP. Minimally invasive total hip arthroplasty: a prospective randomized study. J Arthroplasty. 2005;20(2):139-144. doi:10.1016/j.arth.2004.09.061
15. Fink B, Mittelstaedt A, Schulz MS, Sebena P, Sing J. Comparison of a minimally invasive posterior approach and the standard posterior approach for total hip arthroplasty. A prospective and comparative study. J Orthop Surg Res. 2010;5:46. doi:10.1186/1749-799X-5-46
16. Khan RJ, Maor D, Hofmann M, Haebich S. A comparison of a less invasive piriformis-sparing approach versus the standard approach to the hip: a randomized controlled trial. J Bone Joint Surg Br. 2012;94:43-50. doi:10.1302/0301-620X.94B1.27001
17. Galakatos GR. Direct anterior total hip arthroplasty. Missouri Med. 2018;115(6):537-541.
18. Flevas, DA, Tsantes AG, Mavrogenis, AE. Direct anterior approach total hip arthroplasty revisited. JBJS Rev. 2020;8(4):e0144. doi:10.2106/JBJS.RVW.19.00144
19. DeSteiger RN, Lorimer M, Solomon M. What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res. 2015;473(12):3860-3866. doi:10.1007/s11999-015-4565-6
20. Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics. 2008;31(12)(suppl 2).
21. Bal BS. Clinical faceoff: anterior total hip versus mini-posterior: Which one is better? Clin Orthop Relat Res. 2015;473(4):1192-1196. doi:10.1007/s11999-014-3684-9
22. Taunton MJ, Mason JB, Odum SM, Bryan D, Springer BD. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: a prospective, randomized clinical trial. J Arthroplasty. 2014;29;(suppl 9):169-172. doi:10.1016/j.arth.2014.03.05
23. Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24(5):698-704. doi:10.1016/j.arth.2008.04.012
24. Bergin PF, Doppelt JD, Kephart CJ. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. Bone Joint Surg Am. 2011; 93(15):1392-1398. doi:10.2106/JBJS.J.00557
25. Carli AV, Poitras S, Clohisy JC, Beaule PE. Variation in use of postoperative precautions and equipment following total hip arthroplasty: a survey of the AAHKS and CAS membership. J Arthroplasty. 2018;33(10):3201-3205. doi:10.1016/j.arth.2018.05.043
26. Kavcˇicˇ G, Mirt PK, Tumpej J, Bedenčič. The direct anterior approach for total hip arthroplasty without specific table: surgical approach and our seven years of experience. Published June 14, 2019. Accessed March 4, 2022. https://crimsonăpublishers.com/rabs/fulltext/RABS.000520.php27. American Academy of Orthopedic Surgeons. Total hip replacement exercise guide. Published 2017. Updated February 2022. Accessed March 4, 2022. https://orthoinfo.aaos.org/en/recovery/total-hip-replacement-exercise-guide
28. Medical Advisory Secretariat. Physiotherapy rehabilitation after total knee or hip replacement: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5(8):1-91.
29. Pa˘unescu F, Didilescu A, Antonescu DM. Factors that may influence the functional outcome after primary total hip arthroplasty. Clujul Med. 2013;86(2):121-127.
30. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631. doi:10.1007/s11999-014-3827-z
POISE-3 backs wider use of tranexamic acid in noncardiac surgery
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
Is aspirin the best way to prevent blood clots after THA/TKA?
CHICAGO – Patients discharged to facilities rather than to home after total hip arthroplasty (THA) or total knee arthroplasty (TKA) may need more potent chemoprophylaxis than aspirin to prevent blood clots, new data suggest.
Researchers led by Stefano Muscatelli, MD, an orthopedist at Michigan Medicine, Ann Arbor, first aimed to determine whether there was an increase in risk of venous thromboembolism (VTE) in patients who were discharged to facilities such as a skilled nursing facility or inpatient rehabilitation facility, compared with those discharged to home after THA or TKA.
The second aim was to determine whether VTE risk differed between home- and non–home-discharge patients when stratified by the chemoprophylaxis prescribed to prevent VTE.
Findings were presented at the annual meeting of the American Academy of Orthopaedic Surgeons by coauthor Michael McHugh, MD, also an orthopedist at Michigan Medicine in Ann Arbor.
The agents were categorized in three groups: aspirin only; more aggressive anticoagulants, including warfarin, factor Xa inhibitor, direct thrombin inhibitor, low-molecular-weight heparin, pentasaccharide, or antiplatelet agents, with or without concurrent aspirin; and other regimens.
The researchers found that rates of VTE were higher among patients discharged to facilities.
Of 6,411 patients included in the study, the overall rate of VTE was 1.05%. Among home-discharge patients (n = 5445), rates of VTE were significantly lower than among patients discharged to facilities (n = 966) (0.83% vs. 2.26%; P < .001).
However, the researchers found there was no difference in VTE rates between non-home and home discharge in patients who received more aggressive chemoprophylaxis.
Among discharged patients who received only aspirin, rates of VTE among those discharged to home were significantly lower compared to those discharged to facilities (0.76% vs. 3.83%; P < .001).
“Smoking, BMI [body mass index], procedure type, and preoperative anticoagulation were not associated with the outcome of VTE,” Dr. McHugh said.
“Although we found VTE to continue to be an uncommon complication, non-home discharge is independently associated with higher rates of VTE. Patients should be encouraged to discharge home, but those discharged to non-home facilities after total joint arthroplasty should be considered for more potent chemoprophylaxis than aspirin,” he concluded.
Stuart J. Fischer, MD, with Summit (N.J.) Orthopaedics and Sports Medicine, who was not part of the study, told this news organization that he found the results inconclusive.
He said there is the potential for confounding because “the people who are sent to a facility after total hip or total knee are inherently less mobile and less able to take care of themselves, so they are at a higher risk for VTE. They are going to be more static.”
Dr. Fischer noted that over the past few years, there has been a movement away from anticoagulation with more aggressive agents toward aspirin, for several reasons. Providers don’t have to monitor aspirin use and can instruct patients to take it once or twice a day. Initial data seem to show that it protects well against VTE.
“The question is, in certain population of patients, is it enough? And that’s where the data are unclear,” Dr. Fischer said.
“It’s certainly a useful study, and we need to find out which methods of anticoagulation are most effective in each setting,” he said.
Limitations include that it was a retrospective review and that adverse events from more aggressive chemoprophylaxis agents were not assessed. Prophylactic regimens were chosen at the discretion of the treating surgeon.
The researchers excluded bilateral cases, conversion arthroplasty, hip hemiarthroplasty, unicompartmental knee arthroplasty, and deaths.
Dr. Muscatelli and Dr. McHugh reported no relevant financial relationships. A coauthor reported being a paid consultant for DePuy and Zimmer. Dr. Fischer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – Patients discharged to facilities rather than to home after total hip arthroplasty (THA) or total knee arthroplasty (TKA) may need more potent chemoprophylaxis than aspirin to prevent blood clots, new data suggest.
Researchers led by Stefano Muscatelli, MD, an orthopedist at Michigan Medicine, Ann Arbor, first aimed to determine whether there was an increase in risk of venous thromboembolism (VTE) in patients who were discharged to facilities such as a skilled nursing facility or inpatient rehabilitation facility, compared with those discharged to home after THA or TKA.
The second aim was to determine whether VTE risk differed between home- and non–home-discharge patients when stratified by the chemoprophylaxis prescribed to prevent VTE.
Findings were presented at the annual meeting of the American Academy of Orthopaedic Surgeons by coauthor Michael McHugh, MD, also an orthopedist at Michigan Medicine in Ann Arbor.
The agents were categorized in three groups: aspirin only; more aggressive anticoagulants, including warfarin, factor Xa inhibitor, direct thrombin inhibitor, low-molecular-weight heparin, pentasaccharide, or antiplatelet agents, with or without concurrent aspirin; and other regimens.
The researchers found that rates of VTE were higher among patients discharged to facilities.
Of 6,411 patients included in the study, the overall rate of VTE was 1.05%. Among home-discharge patients (n = 5445), rates of VTE were significantly lower than among patients discharged to facilities (n = 966) (0.83% vs. 2.26%; P < .001).
However, the researchers found there was no difference in VTE rates between non-home and home discharge in patients who received more aggressive chemoprophylaxis.
Among discharged patients who received only aspirin, rates of VTE among those discharged to home were significantly lower compared to those discharged to facilities (0.76% vs. 3.83%; P < .001).
“Smoking, BMI [body mass index], procedure type, and preoperative anticoagulation were not associated with the outcome of VTE,” Dr. McHugh said.
“Although we found VTE to continue to be an uncommon complication, non-home discharge is independently associated with higher rates of VTE. Patients should be encouraged to discharge home, but those discharged to non-home facilities after total joint arthroplasty should be considered for more potent chemoprophylaxis than aspirin,” he concluded.
Stuart J. Fischer, MD, with Summit (N.J.) Orthopaedics and Sports Medicine, who was not part of the study, told this news organization that he found the results inconclusive.
He said there is the potential for confounding because “the people who are sent to a facility after total hip or total knee are inherently less mobile and less able to take care of themselves, so they are at a higher risk for VTE. They are going to be more static.”
Dr. Fischer noted that over the past few years, there has been a movement away from anticoagulation with more aggressive agents toward aspirin, for several reasons. Providers don’t have to monitor aspirin use and can instruct patients to take it once or twice a day. Initial data seem to show that it protects well against VTE.
“The question is, in certain population of patients, is it enough? And that’s where the data are unclear,” Dr. Fischer said.
“It’s certainly a useful study, and we need to find out which methods of anticoagulation are most effective in each setting,” he said.
Limitations include that it was a retrospective review and that adverse events from more aggressive chemoprophylaxis agents were not assessed. Prophylactic regimens were chosen at the discretion of the treating surgeon.
The researchers excluded bilateral cases, conversion arthroplasty, hip hemiarthroplasty, unicompartmental knee arthroplasty, and deaths.
Dr. Muscatelli and Dr. McHugh reported no relevant financial relationships. A coauthor reported being a paid consultant for DePuy and Zimmer. Dr. Fischer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – Patients discharged to facilities rather than to home after total hip arthroplasty (THA) or total knee arthroplasty (TKA) may need more potent chemoprophylaxis than aspirin to prevent blood clots, new data suggest.
Researchers led by Stefano Muscatelli, MD, an orthopedist at Michigan Medicine, Ann Arbor, first aimed to determine whether there was an increase in risk of venous thromboembolism (VTE) in patients who were discharged to facilities such as a skilled nursing facility or inpatient rehabilitation facility, compared with those discharged to home after THA or TKA.
The second aim was to determine whether VTE risk differed between home- and non–home-discharge patients when stratified by the chemoprophylaxis prescribed to prevent VTE.
Findings were presented at the annual meeting of the American Academy of Orthopaedic Surgeons by coauthor Michael McHugh, MD, also an orthopedist at Michigan Medicine in Ann Arbor.
The agents were categorized in three groups: aspirin only; more aggressive anticoagulants, including warfarin, factor Xa inhibitor, direct thrombin inhibitor, low-molecular-weight heparin, pentasaccharide, or antiplatelet agents, with or without concurrent aspirin; and other regimens.
The researchers found that rates of VTE were higher among patients discharged to facilities.
Of 6,411 patients included in the study, the overall rate of VTE was 1.05%. Among home-discharge patients (n = 5445), rates of VTE were significantly lower than among patients discharged to facilities (n = 966) (0.83% vs. 2.26%; P < .001).
However, the researchers found there was no difference in VTE rates between non-home and home discharge in patients who received more aggressive chemoprophylaxis.
Among discharged patients who received only aspirin, rates of VTE among those discharged to home were significantly lower compared to those discharged to facilities (0.76% vs. 3.83%; P < .001).
“Smoking, BMI [body mass index], procedure type, and preoperative anticoagulation were not associated with the outcome of VTE,” Dr. McHugh said.
“Although we found VTE to continue to be an uncommon complication, non-home discharge is independently associated with higher rates of VTE. Patients should be encouraged to discharge home, but those discharged to non-home facilities after total joint arthroplasty should be considered for more potent chemoprophylaxis than aspirin,” he concluded.
Stuart J. Fischer, MD, with Summit (N.J.) Orthopaedics and Sports Medicine, who was not part of the study, told this news organization that he found the results inconclusive.
He said there is the potential for confounding because “the people who are sent to a facility after total hip or total knee are inherently less mobile and less able to take care of themselves, so they are at a higher risk for VTE. They are going to be more static.”
Dr. Fischer noted that over the past few years, there has been a movement away from anticoagulation with more aggressive agents toward aspirin, for several reasons. Providers don’t have to monitor aspirin use and can instruct patients to take it once or twice a day. Initial data seem to show that it protects well against VTE.
“The question is, in certain population of patients, is it enough? And that’s where the data are unclear,” Dr. Fischer said.
“It’s certainly a useful study, and we need to find out which methods of anticoagulation are most effective in each setting,” he said.
Limitations include that it was a retrospective review and that adverse events from more aggressive chemoprophylaxis agents were not assessed. Prophylactic regimens were chosen at the discretion of the treating surgeon.
The researchers excluded bilateral cases, conversion arthroplasty, hip hemiarthroplasty, unicompartmental knee arthroplasty, and deaths.
Dr. Muscatelli and Dr. McHugh reported no relevant financial relationships. A coauthor reported being a paid consultant for DePuy and Zimmer. Dr. Fischer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAOS 2022
Medical cannabis may cut opioid use for back pain, OA
CHICAGO – Access to medical cannabis (MC) cut opioid prescriptions for patients with chronic noncancer back pain and patients with osteoarthritis, according to preliminary data presented at the annual meeting of the American Academy of Orthopaedic Surgeons.
For those with chronic back pain, the average morphine milligram equivalents (MME) per day dropped from 15.1 to 11.0 (n = 186; P < .01). More than one-third of the patients (38.7%) stopped taking morphine after they filled prescriptions for medical cannabis.
Opioid prescriptions were filled 6 months before access to MC and then were compared with 6 months after access to MC.
In analyzing subgroups, the researchers found that patients who started at less than 15 MME/day and more than 15 MME/day showed significant decreases after filling the MC prescription.
Almost half (48.5%) of the patients in the group that started at less than 15 MME daily dropped to 0 MME/day, and 13.5% of patients who were getting more than 15 MME/day stopped using opioids.
Data on filled opioid prescriptions were gathered from a Prescription Drug Monitoring Program (PDMP) system for patients diagnosed with chronic musculoskeletal noncancer back pain who were eligible for MC access between February 2018 and July 2019.
Medical cannabis has shown benefit in treating chronic pain, but evidence has been limited on whether it can reduce opioid use, which can lead to substance abuse, addiction, overdose, and death, the researchers noted.
Researchers found that using MC via multiple routes of administration seemed to be important.
Patients who used only a single administration route showed a statistically insignificant decrease in MME/day from 20.0 to 15.1 (n = 68; P = .054), whereas patients who used two or more routes showed a significant decrease from 13.2 to 9.5 (n = 76; P < .01).
“We have many patients who are benefiting from a single route of delivery for chronic orthopedic pain,” Ari Greis, DO, a physical medicine and rehabilitation specialist in Bryn Mawr, Pa., and a coauthor of the MC studies for both back pain and OA, said in an interview. “However, our data shows a greater reduction in opioid consumption in patients using more than one route of delivery.”
He said delivery modes in the studies included vaporized cannabis oil or flower; sublingual tinctures; capsules or tablets; and topical lotions, creams, and salves.
Dr. Greis is the director of the medical cannabis department at Rothman Orthopaedic Institute in Bryn Mawr, and is a senior fellow in the Institute of Emerging Health Professions and the Lambert Center for the Study of Medicinal Cannabis and Hemp, both in Philadelphia.
Medical cannabis also reduces opioids for OA
The same team of researchers, using the data from the PDMP system, showed that medical cannabis also helped reduce opioid use for osteoarthritis.
For patients using opioids for OA, there was a significant decrease in average MME/day of prescriptions filled by patients following MC access – from 18.2 to 9.8 (n = 40; P < .05). The average drop in MME/day was 46.3%. The percentage of patients who stopped using opioids was 37.5%. Pain score on a 0-10 visual analog scale decreased significantly from 6.6 (n = 36) to 5.0 (n = 26; P < .01) at 3 months and 5.4 (n = 16; P < .05) at 6 months.
Gary Stewart, MD, an orthopedic surgeon in Morrow, Ga., who was not part of the studies, told this news organization that the studies offer good preliminary data to offer help with the opioid issue.
“I sometimes feel that we, as orthopedic surgeons and physicians in general, are working with one hand behind our back. We’re taking something that is a heroin or morphine derivative and giving it to our patients when we know it has a high risk of building tolerance and addiction. But at the same time, we have no alternative,” he said.
He said it’s important to remember the results from the relatively small study are preliminary and observational. People used different forms and amounts of MC and the data show only that prescriptions were filled, but not whether the cannabis was used. Prospective, controlled studies where opioids go head-to-head with MC are needed, he said.
“Still, this can lead us to more studies to give us an option [apart from] an opioid that we know is highly addictive,” he said.
Dr. Stewart is a member of the AAOS Opioid Task Force. Dr. Greis and several coauthors have disclosed no relevant financial relationships, and other coauthors report financial ties to companies unrelated to the research presented.
A version of this article first appeared on Medscape.com.
CHICAGO – Access to medical cannabis (MC) cut opioid prescriptions for patients with chronic noncancer back pain and patients with osteoarthritis, according to preliminary data presented at the annual meeting of the American Academy of Orthopaedic Surgeons.
For those with chronic back pain, the average morphine milligram equivalents (MME) per day dropped from 15.1 to 11.0 (n = 186; P < .01). More than one-third of the patients (38.7%) stopped taking morphine after they filled prescriptions for medical cannabis.
Opioid prescriptions were filled 6 months before access to MC and then were compared with 6 months after access to MC.
In analyzing subgroups, the researchers found that patients who started at less than 15 MME/day and more than 15 MME/day showed significant decreases after filling the MC prescription.
Almost half (48.5%) of the patients in the group that started at less than 15 MME daily dropped to 0 MME/day, and 13.5% of patients who were getting more than 15 MME/day stopped using opioids.
Data on filled opioid prescriptions were gathered from a Prescription Drug Monitoring Program (PDMP) system for patients diagnosed with chronic musculoskeletal noncancer back pain who were eligible for MC access between February 2018 and July 2019.
Medical cannabis has shown benefit in treating chronic pain, but evidence has been limited on whether it can reduce opioid use, which can lead to substance abuse, addiction, overdose, and death, the researchers noted.
Researchers found that using MC via multiple routes of administration seemed to be important.
Patients who used only a single administration route showed a statistically insignificant decrease in MME/day from 20.0 to 15.1 (n = 68; P = .054), whereas patients who used two or more routes showed a significant decrease from 13.2 to 9.5 (n = 76; P < .01).
“We have many patients who are benefiting from a single route of delivery for chronic orthopedic pain,” Ari Greis, DO, a physical medicine and rehabilitation specialist in Bryn Mawr, Pa., and a coauthor of the MC studies for both back pain and OA, said in an interview. “However, our data shows a greater reduction in opioid consumption in patients using more than one route of delivery.”
He said delivery modes in the studies included vaporized cannabis oil or flower; sublingual tinctures; capsules or tablets; and topical lotions, creams, and salves.
Dr. Greis is the director of the medical cannabis department at Rothman Orthopaedic Institute in Bryn Mawr, and is a senior fellow in the Institute of Emerging Health Professions and the Lambert Center for the Study of Medicinal Cannabis and Hemp, both in Philadelphia.
Medical cannabis also reduces opioids for OA
The same team of researchers, using the data from the PDMP system, showed that medical cannabis also helped reduce opioid use for osteoarthritis.
For patients using opioids for OA, there was a significant decrease in average MME/day of prescriptions filled by patients following MC access – from 18.2 to 9.8 (n = 40; P < .05). The average drop in MME/day was 46.3%. The percentage of patients who stopped using opioids was 37.5%. Pain score on a 0-10 visual analog scale decreased significantly from 6.6 (n = 36) to 5.0 (n = 26; P < .01) at 3 months and 5.4 (n = 16; P < .05) at 6 months.
Gary Stewart, MD, an orthopedic surgeon in Morrow, Ga., who was not part of the studies, told this news organization that the studies offer good preliminary data to offer help with the opioid issue.
“I sometimes feel that we, as orthopedic surgeons and physicians in general, are working with one hand behind our back. We’re taking something that is a heroin or morphine derivative and giving it to our patients when we know it has a high risk of building tolerance and addiction. But at the same time, we have no alternative,” he said.
He said it’s important to remember the results from the relatively small study are preliminary and observational. People used different forms and amounts of MC and the data show only that prescriptions were filled, but not whether the cannabis was used. Prospective, controlled studies where opioids go head-to-head with MC are needed, he said.
“Still, this can lead us to more studies to give us an option [apart from] an opioid that we know is highly addictive,” he said.
Dr. Stewart is a member of the AAOS Opioid Task Force. Dr. Greis and several coauthors have disclosed no relevant financial relationships, and other coauthors report financial ties to companies unrelated to the research presented.
A version of this article first appeared on Medscape.com.
CHICAGO – Access to medical cannabis (MC) cut opioid prescriptions for patients with chronic noncancer back pain and patients with osteoarthritis, according to preliminary data presented at the annual meeting of the American Academy of Orthopaedic Surgeons.
For those with chronic back pain, the average morphine milligram equivalents (MME) per day dropped from 15.1 to 11.0 (n = 186; P < .01). More than one-third of the patients (38.7%) stopped taking morphine after they filled prescriptions for medical cannabis.
Opioid prescriptions were filled 6 months before access to MC and then were compared with 6 months after access to MC.
In analyzing subgroups, the researchers found that patients who started at less than 15 MME/day and more than 15 MME/day showed significant decreases after filling the MC prescription.
Almost half (48.5%) of the patients in the group that started at less than 15 MME daily dropped to 0 MME/day, and 13.5% of patients who were getting more than 15 MME/day stopped using opioids.
Data on filled opioid prescriptions were gathered from a Prescription Drug Monitoring Program (PDMP) system for patients diagnosed with chronic musculoskeletal noncancer back pain who were eligible for MC access between February 2018 and July 2019.
Medical cannabis has shown benefit in treating chronic pain, but evidence has been limited on whether it can reduce opioid use, which can lead to substance abuse, addiction, overdose, and death, the researchers noted.
Researchers found that using MC via multiple routes of administration seemed to be important.
Patients who used only a single administration route showed a statistically insignificant decrease in MME/day from 20.0 to 15.1 (n = 68; P = .054), whereas patients who used two or more routes showed a significant decrease from 13.2 to 9.5 (n = 76; P < .01).
“We have many patients who are benefiting from a single route of delivery for chronic orthopedic pain,” Ari Greis, DO, a physical medicine and rehabilitation specialist in Bryn Mawr, Pa., and a coauthor of the MC studies for both back pain and OA, said in an interview. “However, our data shows a greater reduction in opioid consumption in patients using more than one route of delivery.”
He said delivery modes in the studies included vaporized cannabis oil or flower; sublingual tinctures; capsules or tablets; and topical lotions, creams, and salves.
Dr. Greis is the director of the medical cannabis department at Rothman Orthopaedic Institute in Bryn Mawr, and is a senior fellow in the Institute of Emerging Health Professions and the Lambert Center for the Study of Medicinal Cannabis and Hemp, both in Philadelphia.
Medical cannabis also reduces opioids for OA
The same team of researchers, using the data from the PDMP system, showed that medical cannabis also helped reduce opioid use for osteoarthritis.
For patients using opioids for OA, there was a significant decrease in average MME/day of prescriptions filled by patients following MC access – from 18.2 to 9.8 (n = 40; P < .05). The average drop in MME/day was 46.3%. The percentage of patients who stopped using opioids was 37.5%. Pain score on a 0-10 visual analog scale decreased significantly from 6.6 (n = 36) to 5.0 (n = 26; P < .01) at 3 months and 5.4 (n = 16; P < .05) at 6 months.
Gary Stewart, MD, an orthopedic surgeon in Morrow, Ga., who was not part of the studies, told this news organization that the studies offer good preliminary data to offer help with the opioid issue.
“I sometimes feel that we, as orthopedic surgeons and physicians in general, are working with one hand behind our back. We’re taking something that is a heroin or morphine derivative and giving it to our patients when we know it has a high risk of building tolerance and addiction. But at the same time, we have no alternative,” he said.
He said it’s important to remember the results from the relatively small study are preliminary and observational. People used different forms and amounts of MC and the data show only that prescriptions were filled, but not whether the cannabis was used. Prospective, controlled studies where opioids go head-to-head with MC are needed, he said.
“Still, this can lead us to more studies to give us an option [apart from] an opioid that we know is highly addictive,” he said.
Dr. Stewart is a member of the AAOS Opioid Task Force. Dr. Greis and several coauthors have disclosed no relevant financial relationships, and other coauthors report financial ties to companies unrelated to the research presented.
A version of this article first appeared on Medscape.com.
AT AAOS 2022
Shoulder arthritis surgery: Depression complicates care
CHICAGO – new data show.
The abstract was presented at the annual meeting of the American Academy of Orthopedic Surgeons.
Researchers, led by Keith Diamond, MD, an orthopedic surgeon at Maimonides Medical Center in New York, queried a private payer database looking for patients who had primary RSA for treatment of glenohumeral OA and also had a diagnosis of depressive disorder (DD) from 2010 to 2019. Patients without DD served as the controls.
After the randomized matching with controls at a 1:5 ratio, the study consisted of 28,410 patients: 4,084 in the DD group and 24,326 in the control group.
Researchers found that patients with depression had longer hospital stays (3 vs. 2 days, P = .0007). They also had higher frequency and odds of developing side effects within the period of care (47.4% vs. 14.7%; odds ratio, 2.27; 95% CI, 2.10-2.45, P < .0001).
Patients with depression also had significantly higher rates of medical complications surrounding the surgery and costs were higher ($19,363 vs. $17,927, P < .0001).
Pneumonia rates were much higher in patients with DD (10% vs. 1.8%; OR, 2.88; P < .0001).
Patients with depression had higher odds of cerebrovascular accident (3.1% vs. 0.7%; OR, 2.69, P < .0001); myocardial infarctions (2% vs. 0.4%; OR, 2.54; P < .0001); acute kidney injuries (11.1% vs. 2.3%; OR, 2.11, P < .0001); surgical site infections (4.4% vs. 2.4%; OR, 1.52, P < .0001); and other complications, the authors wrote.
Dr. Diamond said in an interview that there may be a few potential reasons for the associations.
In regard to the strong association with pneumonia, Dr. Diamond hypothesized, “patients with depression can be shown to have lower respiratory drive. If a patient isn’t motivated to get out of bed, that can lead to decreased inflation of the lungs.”
Acute kidney injury could be linked with depression-related lack of self-care in properly hydrating, he said. Surgical site infections could come from suboptimal hygiene related to managing the cast after surgery, which may be more difficult when patients also struggle with depression.
Asked to comment on Dr. Diamond’s study, Grant Garrigues, MD, an associate professor at Rush University Medical Center, Chicago, and director of upper extremity research, told this news organization the study helps confirm known associations between depression and arthritis.
“We know that people with depression and anxiety feel pain differently,” he said. “It might have to do with your outlook – are you catastrophizing or thinking it’s a minor inconvenience? It’s not that it’s just in your head – you physically feel it differently. That is something we’re certainly attuned to. We want to make sure the mental health part of the picture is optimized as much as possible.”
He added that there is increasing evidence of links between depression and the development of arthritis.
“I’m not saying that everyone with arthritis has depression, but with arthritis being multifactorial, there’s a relatively high incidence of symptomatic arthritis in patients with depression,” Dr. Garrigues said.
“We think it may have something to do with the fight-or-flight hormones in your body that may be revved up if you are living in a stressful environment or are living with a mental health problem. Those will actually change – on a cellular and biochemical basis – some of the things that affect arthritis.”
Stronger emphasis on mental health
Dr. Diamond said the field needs more emphasis on perioperative state of mind.
“As orthopedic surgeons, we are preoccupied with the mechanical, the structural aspects of health care as we try to fix bones, ligaments, and tendons. But I think we need to recognize and explore the connection between the psychiatric and psychological health with our musculoskeletal health.”
He noted that, in the preoperative setting, providers look for hypertension, diabetes, smoking status, and other conditions that could complicate surgical outcomes and said mental health should be a factor in whether a surgery proceeds.
“If someone’s diabetes isn’t controlled you can delay an elective case until their [hemoglobin] A1c is under the recommended limit and you get clearance from their primary care doctor. I think that’s something that should be applied to patients with depressive disorders,” Dr. Diamond said.
This study did not distinguish between patients who were being treated for depression at the time of surgery and those not on treatment. More study related to whether treatment affects depression’s association with RSA outcomes is needed, Dr. Diamond added.
Dr. Garrigues said he talks candidly with patients considering surgery about how they are managing their mental health struggles.
“If they say they haven’t seen their psychiatrist or are off their medications, that’s a nonstarter,” he said.
“Anything outside of the surgery you can optimize, whether it’s mental health, medical, social situations – you want to have all your ducks in a row before you dive into surgery,” Dr. Garrigues said.
He added that patients’ mental health status may even affect the venue for the patient – whether outpatient or inpatient, where they can get more supervision and help in making transitions after surgery.
Dr. Diamond and coauthors and Dr. Garrigues disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – new data show.
The abstract was presented at the annual meeting of the American Academy of Orthopedic Surgeons.
Researchers, led by Keith Diamond, MD, an orthopedic surgeon at Maimonides Medical Center in New York, queried a private payer database looking for patients who had primary RSA for treatment of glenohumeral OA and also had a diagnosis of depressive disorder (DD) from 2010 to 2019. Patients without DD served as the controls.
After the randomized matching with controls at a 1:5 ratio, the study consisted of 28,410 patients: 4,084 in the DD group and 24,326 in the control group.
Researchers found that patients with depression had longer hospital stays (3 vs. 2 days, P = .0007). They also had higher frequency and odds of developing side effects within the period of care (47.4% vs. 14.7%; odds ratio, 2.27; 95% CI, 2.10-2.45, P < .0001).
Patients with depression also had significantly higher rates of medical complications surrounding the surgery and costs were higher ($19,363 vs. $17,927, P < .0001).
Pneumonia rates were much higher in patients with DD (10% vs. 1.8%; OR, 2.88; P < .0001).
Patients with depression had higher odds of cerebrovascular accident (3.1% vs. 0.7%; OR, 2.69, P < .0001); myocardial infarctions (2% vs. 0.4%; OR, 2.54; P < .0001); acute kidney injuries (11.1% vs. 2.3%; OR, 2.11, P < .0001); surgical site infections (4.4% vs. 2.4%; OR, 1.52, P < .0001); and other complications, the authors wrote.
Dr. Diamond said in an interview that there may be a few potential reasons for the associations.
In regard to the strong association with pneumonia, Dr. Diamond hypothesized, “patients with depression can be shown to have lower respiratory drive. If a patient isn’t motivated to get out of bed, that can lead to decreased inflation of the lungs.”
Acute kidney injury could be linked with depression-related lack of self-care in properly hydrating, he said. Surgical site infections could come from suboptimal hygiene related to managing the cast after surgery, which may be more difficult when patients also struggle with depression.
Asked to comment on Dr. Diamond’s study, Grant Garrigues, MD, an associate professor at Rush University Medical Center, Chicago, and director of upper extremity research, told this news organization the study helps confirm known associations between depression and arthritis.
“We know that people with depression and anxiety feel pain differently,” he said. “It might have to do with your outlook – are you catastrophizing or thinking it’s a minor inconvenience? It’s not that it’s just in your head – you physically feel it differently. That is something we’re certainly attuned to. We want to make sure the mental health part of the picture is optimized as much as possible.”
He added that there is increasing evidence of links between depression and the development of arthritis.
“I’m not saying that everyone with arthritis has depression, but with arthritis being multifactorial, there’s a relatively high incidence of symptomatic arthritis in patients with depression,” Dr. Garrigues said.
“We think it may have something to do with the fight-or-flight hormones in your body that may be revved up if you are living in a stressful environment or are living with a mental health problem. Those will actually change – on a cellular and biochemical basis – some of the things that affect arthritis.”
Stronger emphasis on mental health
Dr. Diamond said the field needs more emphasis on perioperative state of mind.
“As orthopedic surgeons, we are preoccupied with the mechanical, the structural aspects of health care as we try to fix bones, ligaments, and tendons. But I think we need to recognize and explore the connection between the psychiatric and psychological health with our musculoskeletal health.”
He noted that, in the preoperative setting, providers look for hypertension, diabetes, smoking status, and other conditions that could complicate surgical outcomes and said mental health should be a factor in whether a surgery proceeds.
“If someone’s diabetes isn’t controlled you can delay an elective case until their [hemoglobin] A1c is under the recommended limit and you get clearance from their primary care doctor. I think that’s something that should be applied to patients with depressive disorders,” Dr. Diamond said.
This study did not distinguish between patients who were being treated for depression at the time of surgery and those not on treatment. More study related to whether treatment affects depression’s association with RSA outcomes is needed, Dr. Diamond added.
Dr. Garrigues said he talks candidly with patients considering surgery about how they are managing their mental health struggles.
“If they say they haven’t seen their psychiatrist or are off their medications, that’s a nonstarter,” he said.
“Anything outside of the surgery you can optimize, whether it’s mental health, medical, social situations – you want to have all your ducks in a row before you dive into surgery,” Dr. Garrigues said.
He added that patients’ mental health status may even affect the venue for the patient – whether outpatient or inpatient, where they can get more supervision and help in making transitions after surgery.
Dr. Diamond and coauthors and Dr. Garrigues disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – new data show.
The abstract was presented at the annual meeting of the American Academy of Orthopedic Surgeons.
Researchers, led by Keith Diamond, MD, an orthopedic surgeon at Maimonides Medical Center in New York, queried a private payer database looking for patients who had primary RSA for treatment of glenohumeral OA and also had a diagnosis of depressive disorder (DD) from 2010 to 2019. Patients without DD served as the controls.
After the randomized matching with controls at a 1:5 ratio, the study consisted of 28,410 patients: 4,084 in the DD group and 24,326 in the control group.
Researchers found that patients with depression had longer hospital stays (3 vs. 2 days, P = .0007). They also had higher frequency and odds of developing side effects within the period of care (47.4% vs. 14.7%; odds ratio, 2.27; 95% CI, 2.10-2.45, P < .0001).
Patients with depression also had significantly higher rates of medical complications surrounding the surgery and costs were higher ($19,363 vs. $17,927, P < .0001).
Pneumonia rates were much higher in patients with DD (10% vs. 1.8%; OR, 2.88; P < .0001).
Patients with depression had higher odds of cerebrovascular accident (3.1% vs. 0.7%; OR, 2.69, P < .0001); myocardial infarctions (2% vs. 0.4%; OR, 2.54; P < .0001); acute kidney injuries (11.1% vs. 2.3%; OR, 2.11, P < .0001); surgical site infections (4.4% vs. 2.4%; OR, 1.52, P < .0001); and other complications, the authors wrote.
Dr. Diamond said in an interview that there may be a few potential reasons for the associations.
In regard to the strong association with pneumonia, Dr. Diamond hypothesized, “patients with depression can be shown to have lower respiratory drive. If a patient isn’t motivated to get out of bed, that can lead to decreased inflation of the lungs.”
Acute kidney injury could be linked with depression-related lack of self-care in properly hydrating, he said. Surgical site infections could come from suboptimal hygiene related to managing the cast after surgery, which may be more difficult when patients also struggle with depression.
Asked to comment on Dr. Diamond’s study, Grant Garrigues, MD, an associate professor at Rush University Medical Center, Chicago, and director of upper extremity research, told this news organization the study helps confirm known associations between depression and arthritis.
“We know that people with depression and anxiety feel pain differently,” he said. “It might have to do with your outlook – are you catastrophizing or thinking it’s a minor inconvenience? It’s not that it’s just in your head – you physically feel it differently. That is something we’re certainly attuned to. We want to make sure the mental health part of the picture is optimized as much as possible.”
He added that there is increasing evidence of links between depression and the development of arthritis.
“I’m not saying that everyone with arthritis has depression, but with arthritis being multifactorial, there’s a relatively high incidence of symptomatic arthritis in patients with depression,” Dr. Garrigues said.
“We think it may have something to do with the fight-or-flight hormones in your body that may be revved up if you are living in a stressful environment or are living with a mental health problem. Those will actually change – on a cellular and biochemical basis – some of the things that affect arthritis.”
Stronger emphasis on mental health
Dr. Diamond said the field needs more emphasis on perioperative state of mind.
“As orthopedic surgeons, we are preoccupied with the mechanical, the structural aspects of health care as we try to fix bones, ligaments, and tendons. But I think we need to recognize and explore the connection between the psychiatric and psychological health with our musculoskeletal health.”
He noted that, in the preoperative setting, providers look for hypertension, diabetes, smoking status, and other conditions that could complicate surgical outcomes and said mental health should be a factor in whether a surgery proceeds.
“If someone’s diabetes isn’t controlled you can delay an elective case until their [hemoglobin] A1c is under the recommended limit and you get clearance from their primary care doctor. I think that’s something that should be applied to patients with depressive disorders,” Dr. Diamond said.
This study did not distinguish between patients who were being treated for depression at the time of surgery and those not on treatment. More study related to whether treatment affects depression’s association with RSA outcomes is needed, Dr. Diamond added.
Dr. Garrigues said he talks candidly with patients considering surgery about how they are managing their mental health struggles.
“If they say they haven’t seen their psychiatrist or are off their medications, that’s a nonstarter,” he said.
“Anything outside of the surgery you can optimize, whether it’s mental health, medical, social situations – you want to have all your ducks in a row before you dive into surgery,” Dr. Garrigues said.
He added that patients’ mental health status may even affect the venue for the patient – whether outpatient or inpatient, where they can get more supervision and help in making transitions after surgery.
Dr. Diamond and coauthors and Dr. Garrigues disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAOS 2022
TKA: Posterior-stabilized bearing design ups revision risk
CHICAGO - Posterior-stabilized (PS) bearings used in total knee arthroplasty (TKA) may increase the risk of revision compared with bearings of other design, new data suggest.
That possibility has previously been reported in studies outside the United States, and now an analysis of more than 300,000 cases in the American Joint Replacement Registry (AJRR) suggests it’s the case in the United States as well.
Principal investigator Ryland Kagan, MD, of the department of orthopedic surgery at Oregon Health & Science University in Portland, told this news organization, “What’s unique about our experience in the U.S. is our overall high use of PS implants.”
More than half of TKAs in the United States use the PS bearings; in comparison, in Australia and European countries, PS use is closer to 20%, he said. Because of this disparity, previous studies have not been seen as generalizable to the United States, he said.
Researchers used AJRR data from 2012 to 2019 and identified all primary TKA procedures performed during that period. Cases were linked to supplemental Centers for Medicare & Medicaid Services data to find revision procedures that may not have been included in the AJRR database.
Jamil Kendall, MD, an orthopedic resident at OHSU, was first author on the study. The team evaluated patient demographics, polyethylene characteristics, procedure dates, and cause for revision in the 305,279 cases.
Of those cases in which implant characteristics were reported, 161,486 (52.9%) patients received PS bearings, and 143,793 (47.1%) received minimally stabilized bearings.
The researchers compared three minimally stabilized implants (cruciate retaining [CR], anterior stabilized [AS], or pivot bearing designs) with each other for risk and then compared minimally stabilized options as a group with the PS bearings.
They found no significant differences among the three minimally stabilized options.
But revision risk was higher when they compared the minimally stabilized implants with the PS bearing implants. Use of PS bearings had a hazard ratio of 1.25 (95% confidence interval, 1.2-1.3; P < .0001) for all-cause revision and an HR of 1.18 (95% CI, 1.0-1.4; P = .02) for infection.
Among the patients with minimally stabilized bearings, 1,693 (1.2%) underwent revision for any cause, and 334 (0.2%) underwent revision for infection. For patients with PS bearings, 2,406 (1.5%) underwent revision for any cause, and 446 (0.3%) underwent revision for infection.
Even a small difference significant
Dr. Kagan said, “The difference isn’t dramatic, but when you think of the total number of total knee arthroplasties done, you’re talking about millions of procedures. Even with a small increased risk, you’re going to see a large influence for a population.”
Richard Lynn Illgen, MD, director of the University of Wisconsin-Madison joint replacement program, told this news organization this work identifies a trend, but he pointed out that registry-based studies have important limitations.
“They cannot establish causality,” he said. “There are many potential confounding variables and potential selection biases that could affect the study. Specifically, the study did not control for degree of deformity or medical comorbidities. Although some surgeons routinely use PS designs for all primary TKAs, others use PS designs for patients with more severe deformities. It is possible that PS designs were used more frequently in patients with a greater degree of deformity, and this could introduce a selection bias.”
He added that no data were included to enable stratification of groups according to medical comorbidities.
“It is possible that selection bias exists comparing the relative degree of medical comorbidities between patients in the PS TKA and minimally constrained TKA groups,” Dr. Illgen said.
He said further prospective, randomized studies are needed to eliminate selection bias and to better determine whether the observed pattern of increased risk of revision holds up, compared with the minimally supported versions.
The authors acknowledged those limitations, but Dr. Kagan said the high percentage of PS procedures in the United States helps mitigate potential bias.
Dr. Illgen serves as a consultant and developer for Stryker, is chair of the AAOS AJRR Research Projects Subcommittee, and is a member of the AJRR Steering Committee. Dr. Kagan receives research support from KCI, Ortho Development, and Smith & Nephew, where he is also a paid consultant. Dr. Kendall reports no relevant financial relationships. Another coauthor of the study is a paid consultant for 3M, Heraeus, Immunis, Smith & Nephew, Zimmer Biomet, and Total Joint Orthopedics and has stock or stock options in Joint Development.
A version of this article first appeared on Medscape.com.
CHICAGO - Posterior-stabilized (PS) bearings used in total knee arthroplasty (TKA) may increase the risk of revision compared with bearings of other design, new data suggest.
That possibility has previously been reported in studies outside the United States, and now an analysis of more than 300,000 cases in the American Joint Replacement Registry (AJRR) suggests it’s the case in the United States as well.
Principal investigator Ryland Kagan, MD, of the department of orthopedic surgery at Oregon Health & Science University in Portland, told this news organization, “What’s unique about our experience in the U.S. is our overall high use of PS implants.”
More than half of TKAs in the United States use the PS bearings; in comparison, in Australia and European countries, PS use is closer to 20%, he said. Because of this disparity, previous studies have not been seen as generalizable to the United States, he said.
Researchers used AJRR data from 2012 to 2019 and identified all primary TKA procedures performed during that period. Cases were linked to supplemental Centers for Medicare & Medicaid Services data to find revision procedures that may not have been included in the AJRR database.
Jamil Kendall, MD, an orthopedic resident at OHSU, was first author on the study. The team evaluated patient demographics, polyethylene characteristics, procedure dates, and cause for revision in the 305,279 cases.
Of those cases in which implant characteristics were reported, 161,486 (52.9%) patients received PS bearings, and 143,793 (47.1%) received minimally stabilized bearings.
The researchers compared three minimally stabilized implants (cruciate retaining [CR], anterior stabilized [AS], or pivot bearing designs) with each other for risk and then compared minimally stabilized options as a group with the PS bearings.
They found no significant differences among the three minimally stabilized options.
But revision risk was higher when they compared the minimally stabilized implants with the PS bearing implants. Use of PS bearings had a hazard ratio of 1.25 (95% confidence interval, 1.2-1.3; P < .0001) for all-cause revision and an HR of 1.18 (95% CI, 1.0-1.4; P = .02) for infection.
Among the patients with minimally stabilized bearings, 1,693 (1.2%) underwent revision for any cause, and 334 (0.2%) underwent revision for infection. For patients with PS bearings, 2,406 (1.5%) underwent revision for any cause, and 446 (0.3%) underwent revision for infection.
Even a small difference significant
Dr. Kagan said, “The difference isn’t dramatic, but when you think of the total number of total knee arthroplasties done, you’re talking about millions of procedures. Even with a small increased risk, you’re going to see a large influence for a population.”
Richard Lynn Illgen, MD, director of the University of Wisconsin-Madison joint replacement program, told this news organization this work identifies a trend, but he pointed out that registry-based studies have important limitations.
“They cannot establish causality,” he said. “There are many potential confounding variables and potential selection biases that could affect the study. Specifically, the study did not control for degree of deformity or medical comorbidities. Although some surgeons routinely use PS designs for all primary TKAs, others use PS designs for patients with more severe deformities. It is possible that PS designs were used more frequently in patients with a greater degree of deformity, and this could introduce a selection bias.”
He added that no data were included to enable stratification of groups according to medical comorbidities.
“It is possible that selection bias exists comparing the relative degree of medical comorbidities between patients in the PS TKA and minimally constrained TKA groups,” Dr. Illgen said.
He said further prospective, randomized studies are needed to eliminate selection bias and to better determine whether the observed pattern of increased risk of revision holds up, compared with the minimally supported versions.
The authors acknowledged those limitations, but Dr. Kagan said the high percentage of PS procedures in the United States helps mitigate potential bias.
Dr. Illgen serves as a consultant and developer for Stryker, is chair of the AAOS AJRR Research Projects Subcommittee, and is a member of the AJRR Steering Committee. Dr. Kagan receives research support from KCI, Ortho Development, and Smith & Nephew, where he is also a paid consultant. Dr. Kendall reports no relevant financial relationships. Another coauthor of the study is a paid consultant for 3M, Heraeus, Immunis, Smith & Nephew, Zimmer Biomet, and Total Joint Orthopedics and has stock or stock options in Joint Development.
A version of this article first appeared on Medscape.com.
CHICAGO - Posterior-stabilized (PS) bearings used in total knee arthroplasty (TKA) may increase the risk of revision compared with bearings of other design, new data suggest.
That possibility has previously been reported in studies outside the United States, and now an analysis of more than 300,000 cases in the American Joint Replacement Registry (AJRR) suggests it’s the case in the United States as well.
Principal investigator Ryland Kagan, MD, of the department of orthopedic surgery at Oregon Health & Science University in Portland, told this news organization, “What’s unique about our experience in the U.S. is our overall high use of PS implants.”
More than half of TKAs in the United States use the PS bearings; in comparison, in Australia and European countries, PS use is closer to 20%, he said. Because of this disparity, previous studies have not been seen as generalizable to the United States, he said.
Researchers used AJRR data from 2012 to 2019 and identified all primary TKA procedures performed during that period. Cases were linked to supplemental Centers for Medicare & Medicaid Services data to find revision procedures that may not have been included in the AJRR database.
Jamil Kendall, MD, an orthopedic resident at OHSU, was first author on the study. The team evaluated patient demographics, polyethylene characteristics, procedure dates, and cause for revision in the 305,279 cases.
Of those cases in which implant characteristics were reported, 161,486 (52.9%) patients received PS bearings, and 143,793 (47.1%) received minimally stabilized bearings.
The researchers compared three minimally stabilized implants (cruciate retaining [CR], anterior stabilized [AS], or pivot bearing designs) with each other for risk and then compared minimally stabilized options as a group with the PS bearings.
They found no significant differences among the three minimally stabilized options.
But revision risk was higher when they compared the minimally stabilized implants with the PS bearing implants. Use of PS bearings had a hazard ratio of 1.25 (95% confidence interval, 1.2-1.3; P < .0001) for all-cause revision and an HR of 1.18 (95% CI, 1.0-1.4; P = .02) for infection.
Among the patients with minimally stabilized bearings, 1,693 (1.2%) underwent revision for any cause, and 334 (0.2%) underwent revision for infection. For patients with PS bearings, 2,406 (1.5%) underwent revision for any cause, and 446 (0.3%) underwent revision for infection.
Even a small difference significant
Dr. Kagan said, “The difference isn’t dramatic, but when you think of the total number of total knee arthroplasties done, you’re talking about millions of procedures. Even with a small increased risk, you’re going to see a large influence for a population.”
Richard Lynn Illgen, MD, director of the University of Wisconsin-Madison joint replacement program, told this news organization this work identifies a trend, but he pointed out that registry-based studies have important limitations.
“They cannot establish causality,” he said. “There are many potential confounding variables and potential selection biases that could affect the study. Specifically, the study did not control for degree of deformity or medical comorbidities. Although some surgeons routinely use PS designs for all primary TKAs, others use PS designs for patients with more severe deformities. It is possible that PS designs were used more frequently in patients with a greater degree of deformity, and this could introduce a selection bias.”
He added that no data were included to enable stratification of groups according to medical comorbidities.
“It is possible that selection bias exists comparing the relative degree of medical comorbidities between patients in the PS TKA and minimally constrained TKA groups,” Dr. Illgen said.
He said further prospective, randomized studies are needed to eliminate selection bias and to better determine whether the observed pattern of increased risk of revision holds up, compared with the minimally supported versions.
The authors acknowledged those limitations, but Dr. Kagan said the high percentage of PS procedures in the United States helps mitigate potential bias.
Dr. Illgen serves as a consultant and developer for Stryker, is chair of the AAOS AJRR Research Projects Subcommittee, and is a member of the AJRR Steering Committee. Dr. Kagan receives research support from KCI, Ortho Development, and Smith & Nephew, where he is also a paid consultant. Dr. Kendall reports no relevant financial relationships. Another coauthor of the study is a paid consultant for 3M, Heraeus, Immunis, Smith & Nephew, Zimmer Biomet, and Total Joint Orthopedics and has stock or stock options in Joint Development.
A version of this article first appeared on Medscape.com.