User login
Botulinum Injections Might Help Relieve Anterolateral Knee Pain
A single injection of botulinum toxin type A into the tensor fasciae latae can improve symptoms of lateral patellaofemoral overload syndrome (LPOS), which is characterized by pain in the anterior and lateral parts of the knee during exercise, according to a study published online ahead of print in American Journal of Sports Medicine.
Researchers gave a botulinum injection to 45 patients who’d had LPOS for at least 3 months and hadn’t improved after a course of physical therapy. Patients reported on their symptoms before the injection; at 1, 4, and 12 weeks after the injection; and at a mean of 5 years post-injection.
There was significant improvement in pain scores from before the injection to 1, 4, and 12 weeks after treatment, and in 87% of patients, this improvement was maintained at the 5-year follow-up.
Suggested Reading
Stephen JM, Urquhart DW, van Arkel RJ, et al. The use of sonographically guided botulinum toxin type a (Dysport) injections into the tensor fasciae latae for the treatment of lateral patellofemoral overload syndrome. Am J Sports Med. 2016 Feb 22 [Epub ahead of print].
A single injection of botulinum toxin type A into the tensor fasciae latae can improve symptoms of lateral patellaofemoral overload syndrome (LPOS), which is characterized by pain in the anterior and lateral parts of the knee during exercise, according to a study published online ahead of print in American Journal of Sports Medicine.
Researchers gave a botulinum injection to 45 patients who’d had LPOS for at least 3 months and hadn’t improved after a course of physical therapy. Patients reported on their symptoms before the injection; at 1, 4, and 12 weeks after the injection; and at a mean of 5 years post-injection.
There was significant improvement in pain scores from before the injection to 1, 4, and 12 weeks after treatment, and in 87% of patients, this improvement was maintained at the 5-year follow-up.
A single injection of botulinum toxin type A into the tensor fasciae latae can improve symptoms of lateral patellaofemoral overload syndrome (LPOS), which is characterized by pain in the anterior and lateral parts of the knee during exercise, according to a study published online ahead of print in American Journal of Sports Medicine.
Researchers gave a botulinum injection to 45 patients who’d had LPOS for at least 3 months and hadn’t improved after a course of physical therapy. Patients reported on their symptoms before the injection; at 1, 4, and 12 weeks after the injection; and at a mean of 5 years post-injection.
There was significant improvement in pain scores from before the injection to 1, 4, and 12 weeks after treatment, and in 87% of patients, this improvement was maintained at the 5-year follow-up.
Suggested Reading
Stephen JM, Urquhart DW, van Arkel RJ, et al. The use of sonographically guided botulinum toxin type a (Dysport) injections into the tensor fasciae latae for the treatment of lateral patellofemoral overload syndrome. Am J Sports Med. 2016 Feb 22 [Epub ahead of print].
Suggested Reading
Stephen JM, Urquhart DW, van Arkel RJ, et al. The use of sonographically guided botulinum toxin type a (Dysport) injections into the tensor fasciae latae for the treatment of lateral patellofemoral overload syndrome. Am J Sports Med. 2016 Feb 22 [Epub ahead of print].
Do Genetics Influence Knee Osteoarthritis Patients’ Sensitivity to Pain?
Preliminary evidence suggests that patients with knee osteoarthritis (OA) who have certain alleles of the catechol-O-methyltransferase (COMT) and mu-opioid receptor (OPRM1) genes experience more variability in their day-to-day pain and exacerbation of pain after daily physical activity, compared with patients with other genotypes, according to a study published in Scandinavian Journal of Pain.
Researchers looked at variability in day-to-day knee OA pain among patients with different variants of the COMT and OPRM1 genes. They assigned 120 patients with knee OA to a 22-day assessment protocol in which they wore an accelerometer to measure daily physical activity and completed a pain questionnaire 3 times a day. Multilevel modeling was used to examine the magnitude of within-person variability in pain by genetic group.
Patients with two copies of the Asn40 allele of OPRM1 rs 1799971 showed the greatest variability in day-to-day pain. Patients with the Val/Val genotype of COMT rs4680 showed the greatest pain variability and also experienced the greatest increase in pain as a result of physical activity.
Suggested Reading
Martire LM, Wilson SJ, Small BJ, et al. COMT and OPRM1 genotype associations with daily knee pain variability and activity induced pain. Scand J Pain. 2016 Jan 1;10:6-12.
Preliminary evidence suggests that patients with knee osteoarthritis (OA) who have certain alleles of the catechol-O-methyltransferase (COMT) and mu-opioid receptor (OPRM1) genes experience more variability in their day-to-day pain and exacerbation of pain after daily physical activity, compared with patients with other genotypes, according to a study published in Scandinavian Journal of Pain.
Researchers looked at variability in day-to-day knee OA pain among patients with different variants of the COMT and OPRM1 genes. They assigned 120 patients with knee OA to a 22-day assessment protocol in which they wore an accelerometer to measure daily physical activity and completed a pain questionnaire 3 times a day. Multilevel modeling was used to examine the magnitude of within-person variability in pain by genetic group.
Patients with two copies of the Asn40 allele of OPRM1 rs 1799971 showed the greatest variability in day-to-day pain. Patients with the Val/Val genotype of COMT rs4680 showed the greatest pain variability and also experienced the greatest increase in pain as a result of physical activity.
Preliminary evidence suggests that patients with knee osteoarthritis (OA) who have certain alleles of the catechol-O-methyltransferase (COMT) and mu-opioid receptor (OPRM1) genes experience more variability in their day-to-day pain and exacerbation of pain after daily physical activity, compared with patients with other genotypes, according to a study published in Scandinavian Journal of Pain.
Researchers looked at variability in day-to-day knee OA pain among patients with different variants of the COMT and OPRM1 genes. They assigned 120 patients with knee OA to a 22-day assessment protocol in which they wore an accelerometer to measure daily physical activity and completed a pain questionnaire 3 times a day. Multilevel modeling was used to examine the magnitude of within-person variability in pain by genetic group.
Patients with two copies of the Asn40 allele of OPRM1 rs 1799971 showed the greatest variability in day-to-day pain. Patients with the Val/Val genotype of COMT rs4680 showed the greatest pain variability and also experienced the greatest increase in pain as a result of physical activity.
Suggested Reading
Martire LM, Wilson SJ, Small BJ, et al. COMT and OPRM1 genotype associations with daily knee pain variability and activity induced pain. Scand J Pain. 2016 Jan 1;10:6-12.
Suggested Reading
Martire LM, Wilson SJ, Small BJ, et al. COMT and OPRM1 genotype associations with daily knee pain variability and activity induced pain. Scand J Pain. 2016 Jan 1;10:6-12.
For Men, Exercise-related Bone Loading During Adolescence Reaps Benefits Later in Life
Men who continuously participate in high-impact activities, such as jogging and tennis, during adolescence and young adulthood have greater hip and lumbar spine bone mineral density than those who do not take part in such activities, according to a study published in American Journal of Men’s Health.
In a cross-sectional study, researchers analyzed the physical histories of 203 healthy, physically active males ages 30 to 65. Participants’ sports and exercise histories varied in the type and level of activity and the length of time spent doing various physical activities.
Exercise-associated bone loading scores were calculated based on the biomechanical ground-reaction forces of the patients’ past and current physical activities. Current bone mineral density (BMD) was measured using dual-energy x-ray absorptiometry. In addition, participants were grouped based on current participation in a high-impact activity, resistance training, both, or neither.
Bone loading during adolescence and young adulthood were significant, positive predictors of BMD of the whole body, total hip, and lumbar spine, adjusting for lean body mass and/or age Individuals who currently participate in a high-impact activity had greater lumbar spine BMD than nonparticipants. Men who continuously participated in a high-impact activity had greater hip and lumbar spine BMD than those who did not.
Suggested Reading
Matthew A. Strope, Peggy Nigh, Melissa I. Carter, et al. Physical activity–associated bone loading during adolescence and young adulthood is positively associated with adult bone mineral density in men. Am J Mens Health. 2015 November. [Epub ahead of print].
Men who continuously participate in high-impact activities, such as jogging and tennis, during adolescence and young adulthood have greater hip and lumbar spine bone mineral density than those who do not take part in such activities, according to a study published in American Journal of Men’s Health.
In a cross-sectional study, researchers analyzed the physical histories of 203 healthy, physically active males ages 30 to 65. Participants’ sports and exercise histories varied in the type and level of activity and the length of time spent doing various physical activities.
Exercise-associated bone loading scores were calculated based on the biomechanical ground-reaction forces of the patients’ past and current physical activities. Current bone mineral density (BMD) was measured using dual-energy x-ray absorptiometry. In addition, participants were grouped based on current participation in a high-impact activity, resistance training, both, or neither.
Bone loading during adolescence and young adulthood were significant, positive predictors of BMD of the whole body, total hip, and lumbar spine, adjusting for lean body mass and/or age Individuals who currently participate in a high-impact activity had greater lumbar spine BMD than nonparticipants. Men who continuously participated in a high-impact activity had greater hip and lumbar spine BMD than those who did not.
Men who continuously participate in high-impact activities, such as jogging and tennis, during adolescence and young adulthood have greater hip and lumbar spine bone mineral density than those who do not take part in such activities, according to a study published in American Journal of Men’s Health.
In a cross-sectional study, researchers analyzed the physical histories of 203 healthy, physically active males ages 30 to 65. Participants’ sports and exercise histories varied in the type and level of activity and the length of time spent doing various physical activities.
Exercise-associated bone loading scores were calculated based on the biomechanical ground-reaction forces of the patients’ past and current physical activities. Current bone mineral density (BMD) was measured using dual-energy x-ray absorptiometry. In addition, participants were grouped based on current participation in a high-impact activity, resistance training, both, or neither.
Bone loading during adolescence and young adulthood were significant, positive predictors of BMD of the whole body, total hip, and lumbar spine, adjusting for lean body mass and/or age Individuals who currently participate in a high-impact activity had greater lumbar spine BMD than nonparticipants. Men who continuously participated in a high-impact activity had greater hip and lumbar spine BMD than those who did not.
Suggested Reading
Matthew A. Strope, Peggy Nigh, Melissa I. Carter, et al. Physical activity–associated bone loading during adolescence and young adulthood is positively associated with adult bone mineral density in men. Am J Mens Health. 2015 November. [Epub ahead of print].
Suggested Reading
Matthew A. Strope, Peggy Nigh, Melissa I. Carter, et al. Physical activity–associated bone loading during adolescence and young adulthood is positively associated with adult bone mineral density in men. Am J Mens Health. 2015 November. [Epub ahead of print].
Optimizing Outcomes of Total Joint Arthroplasty Under the Comprehensive Care for Joint Replacement
On July 9, 2015, the Centers for Medicare and Medicaid Services announced the Comprehensive Care for Joint Replacement model, which aims to improve coordination of the whole episode of care for total hip and knee replacement.1 At stake is the fact that hip and knee replacements are the most common inpatient procedures among Medicare beneficiaries, costing over $7 billion in 20141 and projected to grow to $50 billion by 2030.2 Under Medicare’s new initiative, hospitals and physicians are held accountable for the quality and cost of care delivered from the time of surgery through 90 days after discharge. For the first time in the history of our profession, large-scale reimbursement is based on outcomes and value rather than fee-for-service. As a result, a hospital can either earn a reward or be held liable for added expenses related to events such as prolonged hospitalization, readmissions, and complications.
How can we optimize outcomes for total joint arthroplasty (TJA) patients in this era of Medicare (r)evolution? A good outcome starts with good patient selection. Numerous studies have been published on patient-related risk factors for postoperative TJA complications including obesity, congestive heart failure, lung disease, and depression.3,4 The risks and benefits of TJA should be carefully weighed in high-risk patients and surgery delayed until appropriate medical optimization has been achieved. Following the famous saying, “Good surgeons know how to operate, better surgeons know when to operate, and the best surgeons know when not to operate,” one cannot overemphasize the need for an objective assessment of the likelihood of patient outcome weighed against patient risk factors.
Moderating patient expectation is another crucial component given the changing demographics of our country. Patients seeking TJA today are younger, more obese, and better educated; live longer; and have higher expectations.5 Unrealistic expectations can have a profound impact on surgical outcomes, leading to frustration, dissatisfaction, and unnecessary resource utilization. For example, despite alleviating pain and restoring function in a severely degenerative joint, TJA does not necessarily translate to weight loss. There is currently conflicting evidence on this topic,6-8 and the expectation of weight loss after TJA cannot be supported. There is also a paucity of data regarding return to athletic activity after TJA and the effect of athletic activity on TJA survivorship.9 Communication and transparency are needed to moderate unrealistic expectations before surgery, outlining clear and achievable goals.
Clinical pathways for TJA have seen tremendous improvements in the past decade with the advent of multimodal analgesia, rapid recovery programs, use of spinal and regional anesthesia, and evidence-based guidelines for prevention of venous thromboembolic disease. Adequate pain control is critical to recovery. In a prospective, randomized controlled trial, Lamplot and colleagues10 showed that the use of multimodal analgesia correlated with improved pain scores, decreased narcotic usage, faster functional recovery, and higher patient satisfaction after total knee arthroplasty (TKA). In another study, Quack and colleagues11 performed a systematic review of the literature on fast-track rehabilitation and found that it reduced both inpatient length of stay and costs after TKA. With respect to anesthetic choice, Pugely and colleagues12 reviewed a national database of 14,052 cases of primary TKA and found that patients with multiple comorbidities were at higher risk of complications after general anesthesia when compared with spinal anesthesia. We should continue to invest in safer and more effective modalities for pain control and functional recovery.
Last but not least, in today’s era of Medicare’s Comprehensive Care for Joint Replacement, the role of low-volume orthopedic surgeons performing TJA deserves special mention. Over the next few years, we could likely see a decline in the role of low-volume surgeons in favor of high-volume surgeons. While most orthopedic surgeons are comfortable doing primary TJA, failed cases and complications are frequently referred to larger centers, which may create frustration among patients owing to fragmentation of care. The economic pressures related to bundled payments could further influence this transition. Given the lack of a widespread, long-standing national joint registry, the incidence of failed TJA performed by low-volume orthopedic surgeons compared with high-volume orthopedic surgeons is unknown. However, multiple studies have shown surgeon volume to be associated with lower rates of complication, mortality, readmission, reoperation, and discharge to postacute facilities.13-16 As hospitals assume further financial risk, considerable data on physician performance will undoubtedly be gathered and leveraged. Time and data will determine the value of this transition of care.
Today, more than ever, we are challenged to provide efficient, high-quality, patient-centered care. As our nation grapples with reforming a broken health care system, initiatives like the Comprehensive Care for Joint Replacement will continue to emerge in the future. Orthopedic surgeons are the gatekeepers of the system and therefore hold significant responsibility to patients and society. Ensuring good outcomes should be a top priority not just from a financial standpoint, but as a moral obligation. We shall continue to be leaders in the face of challenges, using innovation and integrity to produce the best results and advance our profession.
1. Comprehensive Care for Joint Replacement model. Centers for Medicare and Medicaid Services website. https://innovation.cms.gov/initiatives/cjr. Updated December 21, 2015. Accessed December 30, 2015.
2. Wilson NA, Schneller ES, Montgomery K, Bozic KJ. Hip and knee implants: current trends and policy considerations. Health Aff. 2008;27(6):1587-1598.
3. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary total hip arthroplasty in Medicare patients. Clin Orthop Relat Res. 2014;472(2):449-454.
4. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary TKA in Medicare patients. Clin Orthop Relat Res. 2014;472(1):232-237.
5. Mason JB. The new demands by patients in the modern era of total joint arthroplasty: a point of view. Clin Orthop Relat Res. 2008;466(1):146-152.
6. Riddle DL, Singh JA, Harmsen WS, Schleck CD, Lewallen DG. Clinically important body weight gain following knee arthroplasty: a five-year comparative cohort study. Arthritis Care Res. 2013;65(5):669-677.
7. Zeni JA Jr, Snyder-Mackler L. Most patients gain weight in the 2 years after total knee arthroplasty: comparison to a healthy control group. Osteoarthritis Cartilage. 2010;18(4):510-514.
8. Ast MP, Abdel MP, Lee YY, Lyman S, Ruel AV, Westrich GH. Weight changes after total hip or knee arthroplasty: prevalence, predictors, and effects on outcomes. J Bone Joint Surg Am. 2015;97(11):911-919.
9. Healy WL, Sharma S, Schwartz B, Iorio R. Athletic activity after total joint arthroplasty. J Bone Joint Surg Am. 2008;90(10):2245-2252.
10. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
11. Quack V, Ippendorf AV, Betsch M, et al. Multidisciplinary rehabilitation and fast-track rehabilitation after knee replacement: faster, better, cheaper? A survey and systematic review of literature [in German]. Rehabilitation (Stuttg). 2015;54(4):245-251.
12. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
13. Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629.
14. Manley M, Ong K, Lau E, Kurtz SM. Effect of volume on total hip arthroplasty revision rates in the United States Medicare population. J Bone Joint Surg Am. 2008;90(11):2446-2451.
15. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
16. Lau RL, Perruccio AV, Gandhi R, Mahomed NN. The role of surgeon volume on patient outcome in total knee arthroplasty: a systematic review of the literature. BMC Musculoskelet Disord. 2012;13:250.
On July 9, 2015, the Centers for Medicare and Medicaid Services announced the Comprehensive Care for Joint Replacement model, which aims to improve coordination of the whole episode of care for total hip and knee replacement.1 At stake is the fact that hip and knee replacements are the most common inpatient procedures among Medicare beneficiaries, costing over $7 billion in 20141 and projected to grow to $50 billion by 2030.2 Under Medicare’s new initiative, hospitals and physicians are held accountable for the quality and cost of care delivered from the time of surgery through 90 days after discharge. For the first time in the history of our profession, large-scale reimbursement is based on outcomes and value rather than fee-for-service. As a result, a hospital can either earn a reward or be held liable for added expenses related to events such as prolonged hospitalization, readmissions, and complications.
How can we optimize outcomes for total joint arthroplasty (TJA) patients in this era of Medicare (r)evolution? A good outcome starts with good patient selection. Numerous studies have been published on patient-related risk factors for postoperative TJA complications including obesity, congestive heart failure, lung disease, and depression.3,4 The risks and benefits of TJA should be carefully weighed in high-risk patients and surgery delayed until appropriate medical optimization has been achieved. Following the famous saying, “Good surgeons know how to operate, better surgeons know when to operate, and the best surgeons know when not to operate,” one cannot overemphasize the need for an objective assessment of the likelihood of patient outcome weighed against patient risk factors.
Moderating patient expectation is another crucial component given the changing demographics of our country. Patients seeking TJA today are younger, more obese, and better educated; live longer; and have higher expectations.5 Unrealistic expectations can have a profound impact on surgical outcomes, leading to frustration, dissatisfaction, and unnecessary resource utilization. For example, despite alleviating pain and restoring function in a severely degenerative joint, TJA does not necessarily translate to weight loss. There is currently conflicting evidence on this topic,6-8 and the expectation of weight loss after TJA cannot be supported. There is also a paucity of data regarding return to athletic activity after TJA and the effect of athletic activity on TJA survivorship.9 Communication and transparency are needed to moderate unrealistic expectations before surgery, outlining clear and achievable goals.
Clinical pathways for TJA have seen tremendous improvements in the past decade with the advent of multimodal analgesia, rapid recovery programs, use of spinal and regional anesthesia, and evidence-based guidelines for prevention of venous thromboembolic disease. Adequate pain control is critical to recovery. In a prospective, randomized controlled trial, Lamplot and colleagues10 showed that the use of multimodal analgesia correlated with improved pain scores, decreased narcotic usage, faster functional recovery, and higher patient satisfaction after total knee arthroplasty (TKA). In another study, Quack and colleagues11 performed a systematic review of the literature on fast-track rehabilitation and found that it reduced both inpatient length of stay and costs after TKA. With respect to anesthetic choice, Pugely and colleagues12 reviewed a national database of 14,052 cases of primary TKA and found that patients with multiple comorbidities were at higher risk of complications after general anesthesia when compared with spinal anesthesia. We should continue to invest in safer and more effective modalities for pain control and functional recovery.
Last but not least, in today’s era of Medicare’s Comprehensive Care for Joint Replacement, the role of low-volume orthopedic surgeons performing TJA deserves special mention. Over the next few years, we could likely see a decline in the role of low-volume surgeons in favor of high-volume surgeons. While most orthopedic surgeons are comfortable doing primary TJA, failed cases and complications are frequently referred to larger centers, which may create frustration among patients owing to fragmentation of care. The economic pressures related to bundled payments could further influence this transition. Given the lack of a widespread, long-standing national joint registry, the incidence of failed TJA performed by low-volume orthopedic surgeons compared with high-volume orthopedic surgeons is unknown. However, multiple studies have shown surgeon volume to be associated with lower rates of complication, mortality, readmission, reoperation, and discharge to postacute facilities.13-16 As hospitals assume further financial risk, considerable data on physician performance will undoubtedly be gathered and leveraged. Time and data will determine the value of this transition of care.
Today, more than ever, we are challenged to provide efficient, high-quality, patient-centered care. As our nation grapples with reforming a broken health care system, initiatives like the Comprehensive Care for Joint Replacement will continue to emerge in the future. Orthopedic surgeons are the gatekeepers of the system and therefore hold significant responsibility to patients and society. Ensuring good outcomes should be a top priority not just from a financial standpoint, but as a moral obligation. We shall continue to be leaders in the face of challenges, using innovation and integrity to produce the best results and advance our profession.
On July 9, 2015, the Centers for Medicare and Medicaid Services announced the Comprehensive Care for Joint Replacement model, which aims to improve coordination of the whole episode of care for total hip and knee replacement.1 At stake is the fact that hip and knee replacements are the most common inpatient procedures among Medicare beneficiaries, costing over $7 billion in 20141 and projected to grow to $50 billion by 2030.2 Under Medicare’s new initiative, hospitals and physicians are held accountable for the quality and cost of care delivered from the time of surgery through 90 days after discharge. For the first time in the history of our profession, large-scale reimbursement is based on outcomes and value rather than fee-for-service. As a result, a hospital can either earn a reward or be held liable for added expenses related to events such as prolonged hospitalization, readmissions, and complications.
How can we optimize outcomes for total joint arthroplasty (TJA) patients in this era of Medicare (r)evolution? A good outcome starts with good patient selection. Numerous studies have been published on patient-related risk factors for postoperative TJA complications including obesity, congestive heart failure, lung disease, and depression.3,4 The risks and benefits of TJA should be carefully weighed in high-risk patients and surgery delayed until appropriate medical optimization has been achieved. Following the famous saying, “Good surgeons know how to operate, better surgeons know when to operate, and the best surgeons know when not to operate,” one cannot overemphasize the need for an objective assessment of the likelihood of patient outcome weighed against patient risk factors.
Moderating patient expectation is another crucial component given the changing demographics of our country. Patients seeking TJA today are younger, more obese, and better educated; live longer; and have higher expectations.5 Unrealistic expectations can have a profound impact on surgical outcomes, leading to frustration, dissatisfaction, and unnecessary resource utilization. For example, despite alleviating pain and restoring function in a severely degenerative joint, TJA does not necessarily translate to weight loss. There is currently conflicting evidence on this topic,6-8 and the expectation of weight loss after TJA cannot be supported. There is also a paucity of data regarding return to athletic activity after TJA and the effect of athletic activity on TJA survivorship.9 Communication and transparency are needed to moderate unrealistic expectations before surgery, outlining clear and achievable goals.
Clinical pathways for TJA have seen tremendous improvements in the past decade with the advent of multimodal analgesia, rapid recovery programs, use of spinal and regional anesthesia, and evidence-based guidelines for prevention of venous thromboembolic disease. Adequate pain control is critical to recovery. In a prospective, randomized controlled trial, Lamplot and colleagues10 showed that the use of multimodal analgesia correlated with improved pain scores, decreased narcotic usage, faster functional recovery, and higher patient satisfaction after total knee arthroplasty (TKA). In another study, Quack and colleagues11 performed a systematic review of the literature on fast-track rehabilitation and found that it reduced both inpatient length of stay and costs after TKA. With respect to anesthetic choice, Pugely and colleagues12 reviewed a national database of 14,052 cases of primary TKA and found that patients with multiple comorbidities were at higher risk of complications after general anesthesia when compared with spinal anesthesia. We should continue to invest in safer and more effective modalities for pain control and functional recovery.
Last but not least, in today’s era of Medicare’s Comprehensive Care for Joint Replacement, the role of low-volume orthopedic surgeons performing TJA deserves special mention. Over the next few years, we could likely see a decline in the role of low-volume surgeons in favor of high-volume surgeons. While most orthopedic surgeons are comfortable doing primary TJA, failed cases and complications are frequently referred to larger centers, which may create frustration among patients owing to fragmentation of care. The economic pressures related to bundled payments could further influence this transition. Given the lack of a widespread, long-standing national joint registry, the incidence of failed TJA performed by low-volume orthopedic surgeons compared with high-volume orthopedic surgeons is unknown. However, multiple studies have shown surgeon volume to be associated with lower rates of complication, mortality, readmission, reoperation, and discharge to postacute facilities.13-16 As hospitals assume further financial risk, considerable data on physician performance will undoubtedly be gathered and leveraged. Time and data will determine the value of this transition of care.
Today, more than ever, we are challenged to provide efficient, high-quality, patient-centered care. As our nation grapples with reforming a broken health care system, initiatives like the Comprehensive Care for Joint Replacement will continue to emerge in the future. Orthopedic surgeons are the gatekeepers of the system and therefore hold significant responsibility to patients and society. Ensuring good outcomes should be a top priority not just from a financial standpoint, but as a moral obligation. We shall continue to be leaders in the face of challenges, using innovation and integrity to produce the best results and advance our profession.
1. Comprehensive Care for Joint Replacement model. Centers for Medicare and Medicaid Services website. https://innovation.cms.gov/initiatives/cjr. Updated December 21, 2015. Accessed December 30, 2015.
2. Wilson NA, Schneller ES, Montgomery K, Bozic KJ. Hip and knee implants: current trends and policy considerations. Health Aff. 2008;27(6):1587-1598.
3. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary total hip arthroplasty in Medicare patients. Clin Orthop Relat Res. 2014;472(2):449-454.
4. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary TKA in Medicare patients. Clin Orthop Relat Res. 2014;472(1):232-237.
5. Mason JB. The new demands by patients in the modern era of total joint arthroplasty: a point of view. Clin Orthop Relat Res. 2008;466(1):146-152.
6. Riddle DL, Singh JA, Harmsen WS, Schleck CD, Lewallen DG. Clinically important body weight gain following knee arthroplasty: a five-year comparative cohort study. Arthritis Care Res. 2013;65(5):669-677.
7. Zeni JA Jr, Snyder-Mackler L. Most patients gain weight in the 2 years after total knee arthroplasty: comparison to a healthy control group. Osteoarthritis Cartilage. 2010;18(4):510-514.
8. Ast MP, Abdel MP, Lee YY, Lyman S, Ruel AV, Westrich GH. Weight changes after total hip or knee arthroplasty: prevalence, predictors, and effects on outcomes. J Bone Joint Surg Am. 2015;97(11):911-919.
9. Healy WL, Sharma S, Schwartz B, Iorio R. Athletic activity after total joint arthroplasty. J Bone Joint Surg Am. 2008;90(10):2245-2252.
10. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
11. Quack V, Ippendorf AV, Betsch M, et al. Multidisciplinary rehabilitation and fast-track rehabilitation after knee replacement: faster, better, cheaper? A survey and systematic review of literature [in German]. Rehabilitation (Stuttg). 2015;54(4):245-251.
12. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
13. Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629.
14. Manley M, Ong K, Lau E, Kurtz SM. Effect of volume on total hip arthroplasty revision rates in the United States Medicare population. J Bone Joint Surg Am. 2008;90(11):2446-2451.
15. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
16. Lau RL, Perruccio AV, Gandhi R, Mahomed NN. The role of surgeon volume on patient outcome in total knee arthroplasty: a systematic review of the literature. BMC Musculoskelet Disord. 2012;13:250.
1. Comprehensive Care for Joint Replacement model. Centers for Medicare and Medicaid Services website. https://innovation.cms.gov/initiatives/cjr. Updated December 21, 2015. Accessed December 30, 2015.
2. Wilson NA, Schneller ES, Montgomery K, Bozic KJ. Hip and knee implants: current trends and policy considerations. Health Aff. 2008;27(6):1587-1598.
3. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary total hip arthroplasty in Medicare patients. Clin Orthop Relat Res. 2014;472(2):449-454.
4. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary TKA in Medicare patients. Clin Orthop Relat Res. 2014;472(1):232-237.
5. Mason JB. The new demands by patients in the modern era of total joint arthroplasty: a point of view. Clin Orthop Relat Res. 2008;466(1):146-152.
6. Riddle DL, Singh JA, Harmsen WS, Schleck CD, Lewallen DG. Clinically important body weight gain following knee arthroplasty: a five-year comparative cohort study. Arthritis Care Res. 2013;65(5):669-677.
7. Zeni JA Jr, Snyder-Mackler L. Most patients gain weight in the 2 years after total knee arthroplasty: comparison to a healthy control group. Osteoarthritis Cartilage. 2010;18(4):510-514.
8. Ast MP, Abdel MP, Lee YY, Lyman S, Ruel AV, Westrich GH. Weight changes after total hip or knee arthroplasty: prevalence, predictors, and effects on outcomes. J Bone Joint Surg Am. 2015;97(11):911-919.
9. Healy WL, Sharma S, Schwartz B, Iorio R. Athletic activity after total joint arthroplasty. J Bone Joint Surg Am. 2008;90(10):2245-2252.
10. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
11. Quack V, Ippendorf AV, Betsch M, et al. Multidisciplinary rehabilitation and fast-track rehabilitation after knee replacement: faster, better, cheaper? A survey and systematic review of literature [in German]. Rehabilitation (Stuttg). 2015;54(4):245-251.
12. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
13. Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629.
14. Manley M, Ong K, Lau E, Kurtz SM. Effect of volume on total hip arthroplasty revision rates in the United States Medicare population. J Bone Joint Surg Am. 2008;90(11):2446-2451.
15. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
16. Lau RL, Perruccio AV, Gandhi R, Mahomed NN. The role of surgeon volume on patient outcome in total knee arthroplasty: a systematic review of the literature. BMC Musculoskelet Disord. 2012;13:250.
Extreme Postinjection Flare in Response to Intra-Articular Triamcinolone Acetonide (Kenalog)
Intra-articular corticosteroid injections (CSIs) have been a common treatment for osteoarthritis since the 1950s and continue to be an option for patients who prefer nonoperative management.1 Although CSIs may improve pain secondary to osteoarthritis temporarily, they do not slow articular cartilage degradation, and many patients request multiple CSIs before total joint arthroplasty ultimately is required.1,2 Therefore, acute and chronic side effects of CSI must be considered when repeatedly administering corticosteroids.
A postinjection flare, the most common acute side effect of intra-articular CSI, is characterized by a localized inflammatory response that can last 2 to 3 days. The flare occurs in 2% to 25% of CSI cases.3-5 Symptoms can range from mild joint effusion to disabling pain.6 In the present case, a severe postinjection flare occurred after intra-articular administration of triamcinolone acetonide (Kenalog). This case is novel in that its acuity of onset, severity of symptoms, and synovial fluid analysis mimicked septic arthritis, which was ultimately ruled out with negative cultures and confirmation of triamcinolone acetonide crystals in the synovial aspirate, viewed by polarized light microscopy. To date, only one other case of an immediate (<2 hours) and severe postinjection flare in response to triamcinolone has been reported.7 As CSIs are often used in the nonoperative treatment of osteoarthritis, it is imperative for the treating physician to be aware of this potential side effect in order to appropriately inform the patient of this risk and guide treatment should the scenario arise. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman with a history of hypertension, hypothyroidism, and moderate bilateral knee osteoarthritis presented with left knee pain. She had been receiving annual hylan injections for 5 years and had no adverse reactions, but the pain gradually worsened over the past 3 months. She was given an intra-articular injection of 2 mL of 1% lidocaine and 2 mL (40 mg) of triamcinolone acetonide in the left knee.
Two hours later, she experienced swelling and intense pain in the knee and was unable to ambulate. Physical examination revealed she was afebrile but was having severe pain in the knee through all range of motion. The knee had no appreciable erythema or warmth. Laboratory data were significant: White blood cell (WBC) count was 14,600, and erythrocyte sedimentation rate was 1 mm/h. The knee was aspirated with a return of 25 mL of “butterscotch”-colored fluid (Figure 1). The patient was admitted to rule out iatrogenic septic arthritis, or chronic, indolent septic arthritis acutely worsened by CSI, until synovial fluid analysis and cultures could be performed (Table 1).
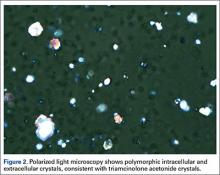
She was treated overnight with a compressive wrap, elevation, ice, and nonsteroidal anti-inflammatory drugs, which provided significant pain relief. Polarized light microscopy revealed polymorphic intracellular and extracellular crystals with crystal morphology consistent with the injection of triamcinolone ester (Figure 2). Gram stain showed many WBCs but no organisms. These findings were thought to represent an exogenous crystal-induced acute inflammatory response. Given the patient’s improving clinical course, she was discharged the next morning.
Twelve days later, at clinic follow-up, she was still experiencing pain above her baseline level. Given the continued effusion, 8 mL of synovial fluid was aspirated, which appeared clear and only slightly blood-tinged. Synovial analysis showed resolution of leukocytosis, confirming a severe postinjection flare in response to triamcinolone acetonide.
Discussion
Although rare, side effects from repeated intra-articular CSIs include hypothalamic-pituitary-adrenal axis dysfunction and steroid-induced myopathy.8,9 Acute side effects are more common and include postinjection flare, iatrogenic septic arthritis, local tissue atrophy, cartilage damage, tendon rupture, nerve atrophy, increased blood glucose, and osteonecrosis.10,11 The present case report describes an extreme example of a postinjection flare in response to triamcinolone acetonide and summarizes the characteristics of injections that cause flares.
The physical properties of corticosteroids have a significant impact on their efficacy and on their potential for adverse events. Corticosteroid preparations can be water-soluble or water-insoluble. Most commonly, water-insoluble preparations that contain insoluble corticosteroid esters (eg, triamcinolone, methylprednisolone) are used in intra-articular injections. These form microcrystalline aggregates in solution, which require the patient’s own hydrolytic enzymes (esterases) to release the active moiety and thus have a longer duration of action. However, they are more commonly associated with postinjection flares compared with their more soluble and faster- acting counterparts (eg. dexamethasone, betamethasone).10 Microcrystalline aggregates, which are larger in size, induce a stronger inflammatory response, and in a dose-dependent manner.6A sterile inflammatory reaction to hydrocortisone, cortisone, dexamethasone, triamcinolone, and prednisolone crystals in normal joints has been previously described,6,12,13 and crystals of the various preparations have been demonstrated within leukocytes by both polarized light and electron microscopy.12,13 Table 2 summarizes previous synovial fluid analyses after intra-articular injections of various corticosteroid preparations in normal healthy joints and in patients experiencing a postinjection flare. To date, there have been no reports of an immediate (<2 hours) and severe postinjection flare in response to triamcinolone acetonide, though there was a report of a postinjection flare in response to triamcinolone hexacetonide (Aristospan),7 and here the synovial fluid WBC count (30,000) was much lower.
Although many cases of corticosteroid hypersensitivity have been reported, in rare cases intra-articular administration of triamcinolone has caused anaphylactic reactions and shock.14,15 Multiple case studies have determined that the specific excipient carboxymethylcellulose (found in many triamcinolone preparations), and not the corticosteroid itself, can cause an immunoglobulin E–mediated anaphylactic reaction.16-18 Therefore, performing skin-prick tests for potential corticosteroids and their excipients in patients with known postinjection flares might help prevent serious adverse reactions.18,19
The present case involved an extreme postinjection flare in response to intra-articular administration of triamcinolone acetonide. Postinjection flares are rare but significant events, and physicians using CSIs in the treatment of arthritis need to be aware of this potential reaction in order to appropriately inform patients of this risk and guide treatment should the scenario arise.
1. Hollander JL, Brown EM Jr, Jessar RA, Brown CY. Hydrocortisone and cortisone injected into arthritic joints; comparative effects of and use of hydrocortisone as a local antiarthritic agent. J Am Med Assoc. 1951;147(17):1629-1635.
2. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;19(2):CD005328.
3. Friedman DM, Moore ME. The efficacy of intraarticular steroids in osteoarthritis: a double-blind study. J Rheumatol. 1980;7(6):850-856.
4. Brown EM Jr, Frain JB, Udell L, Hollander JL. Locally administered hydrocortisone in the rheumatic diseases; a summary of its use in 547 patients. Am J Med. 1953;15(5):656-665.
5. Hollander JL, Jessar RA, Brown EM Jr. Intra-synovial corticosteroid therapy: a decade of use. Bull Rheum Dis. 1961;11:239-240.
6. McCarty DJ Jr, Hogan JM. Inflammatory reaction after intrasynovial injection of microcrystalline adrenocorticosteroid esters. Arthritis Rheum. 1964;7(4):359-367.
7. Berger RG, Yount WJ. Immediate “steroid flare” from intraarticular triamcinolone hexacetonide injection: case report and review of the literature. Arthritis Rheum. 1990;33(8):1284-1286.
8. Mader R, Lavi I, Luboshitzky R. Evaluation of the pituitary-adrenal axis function following single intraarticular injection of methylprednisolone. Arthritis Rheum. 2005;52(3):924-928.
9. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370-377.
10. MacMahon PJ, Eustace SJ, Kavanagh EC. Injectable corticosteroid and local anesthetic preparations: a review for radiologists. Radiology. 2009;252(3):647-661.
11. Sparling M, Malleson P, Wood B, Petty R. Radiographic followup of joints injected with triamcinolone hexacetonide for the management of childhood arthritis. Arthritis Rheum. 1990;33(6):821-826.
12. Eymontt MJ, Gordon GV, Schumacher HR, Hansell JR. The effects on synovial permeability and synovial fluid leukocyte counts in symptomatic osteoarthritis after intraarticular corticosteroid administration. J Rheumatol. 1982;9(2):198-203.
13. Gordon GV, Schumacher HR. Electron microscopic study of depot corticosteroid crystals with clinical studies after intra-articular injection. J Rheumatol. 1979;6(1):7-14.
14. Karsh J, Yang WH. An anaphylactic reaction to intra-articular triamcinolone: a case report and review of the literature. Ann Allergy Asthma Immunol. 2003;90(2):254-258.
15. Larsson LG. Anaphylactic shock after i.a. administration of triamcinolone acetonide in a 35-year-old female. Scand J Rheumatol. 1989;18(6):441-442.
16. García-Ortega P, Corominas M, Badia M. Carboxymethylcellulose allergy as a cause of suspected corticosteroid anaphylaxis. Ann Allergy Asthma Immunol. 2003;91(4):421.
17. Patterson DL, Yunginger JW, Dunn WF, Jones RT, Hunt LW. Anaphylaxis induced by the carboxymethylcellulose component of injectable triamcinolone acetonide suspension (Kenalog). Ann Allergy Asthma Immunol. 1995;74(2):163-166.
18. Steiner UC, Gentinetta T, Hausmann O, Pichler WJ. IgE-mediated anaphylaxis to intraarticular glucocorticoid preparations. AJR Am J Roentgenol. 2009;193(2):W156-W157.
19. Ijsselmuiden OE, Knegt-Junk KJ, van Wijk RG, van Joost T. Cutaneous adverse reactions after intra-articular injection of triamcinolone acetonide. Acta Derm Venereol. 1995;75(1):57-58.
20. Pullman-Mooar S, Mooar P, Sieck M, Clayburne G, Schumacher HR. Are there distinctive inflammatory flares after hylan g-f 20 intraarticular injections? J Rheumatol. 2002;29(12):2611-2614.
Intra-articular corticosteroid injections (CSIs) have been a common treatment for osteoarthritis since the 1950s and continue to be an option for patients who prefer nonoperative management.1 Although CSIs may improve pain secondary to osteoarthritis temporarily, they do not slow articular cartilage degradation, and many patients request multiple CSIs before total joint arthroplasty ultimately is required.1,2 Therefore, acute and chronic side effects of CSI must be considered when repeatedly administering corticosteroids.
A postinjection flare, the most common acute side effect of intra-articular CSI, is characterized by a localized inflammatory response that can last 2 to 3 days. The flare occurs in 2% to 25% of CSI cases.3-5 Symptoms can range from mild joint effusion to disabling pain.6 In the present case, a severe postinjection flare occurred after intra-articular administration of triamcinolone acetonide (Kenalog). This case is novel in that its acuity of onset, severity of symptoms, and synovial fluid analysis mimicked septic arthritis, which was ultimately ruled out with negative cultures and confirmation of triamcinolone acetonide crystals in the synovial aspirate, viewed by polarized light microscopy. To date, only one other case of an immediate (<2 hours) and severe postinjection flare in response to triamcinolone has been reported.7 As CSIs are often used in the nonoperative treatment of osteoarthritis, it is imperative for the treating physician to be aware of this potential side effect in order to appropriately inform the patient of this risk and guide treatment should the scenario arise. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman with a history of hypertension, hypothyroidism, and moderate bilateral knee osteoarthritis presented with left knee pain. She had been receiving annual hylan injections for 5 years and had no adverse reactions, but the pain gradually worsened over the past 3 months. She was given an intra-articular injection of 2 mL of 1% lidocaine and 2 mL (40 mg) of triamcinolone acetonide in the left knee.
Two hours later, she experienced swelling and intense pain in the knee and was unable to ambulate. Physical examination revealed she was afebrile but was having severe pain in the knee through all range of motion. The knee had no appreciable erythema or warmth. Laboratory data were significant: White blood cell (WBC) count was 14,600, and erythrocyte sedimentation rate was 1 mm/h. The knee was aspirated with a return of 25 mL of “butterscotch”-colored fluid (Figure 1). The patient was admitted to rule out iatrogenic septic arthritis, or chronic, indolent septic arthritis acutely worsened by CSI, until synovial fluid analysis and cultures could be performed (Table 1).
She was treated overnight with a compressive wrap, elevation, ice, and nonsteroidal anti-inflammatory drugs, which provided significant pain relief. Polarized light microscopy revealed polymorphic intracellular and extracellular crystals with crystal morphology consistent with the injection of triamcinolone ester (Figure 2). Gram stain showed many WBCs but no organisms. These findings were thought to represent an exogenous crystal-induced acute inflammatory response. Given the patient’s improving clinical course, she was discharged the next morning.
Twelve days later, at clinic follow-up, she was still experiencing pain above her baseline level. Given the continued effusion, 8 mL of synovial fluid was aspirated, which appeared clear and only slightly blood-tinged. Synovial analysis showed resolution of leukocytosis, confirming a severe postinjection flare in response to triamcinolone acetonide.
Discussion
Although rare, side effects from repeated intra-articular CSIs include hypothalamic-pituitary-adrenal axis dysfunction and steroid-induced myopathy.8,9 Acute side effects are more common and include postinjection flare, iatrogenic septic arthritis, local tissue atrophy, cartilage damage, tendon rupture, nerve atrophy, increased blood glucose, and osteonecrosis.10,11 The present case report describes an extreme example of a postinjection flare in response to triamcinolone acetonide and summarizes the characteristics of injections that cause flares.
The physical properties of corticosteroids have a significant impact on their efficacy and on their potential for adverse events. Corticosteroid preparations can be water-soluble or water-insoluble. Most commonly, water-insoluble preparations that contain insoluble corticosteroid esters (eg, triamcinolone, methylprednisolone) are used in intra-articular injections. These form microcrystalline aggregates in solution, which require the patient’s own hydrolytic enzymes (esterases) to release the active moiety and thus have a longer duration of action. However, they are more commonly associated with postinjection flares compared with their more soluble and faster- acting counterparts (eg. dexamethasone, betamethasone).10 Microcrystalline aggregates, which are larger in size, induce a stronger inflammatory response, and in a dose-dependent manner.6A sterile inflammatory reaction to hydrocortisone, cortisone, dexamethasone, triamcinolone, and prednisolone crystals in normal joints has been previously described,6,12,13 and crystals of the various preparations have been demonstrated within leukocytes by both polarized light and electron microscopy.12,13 Table 2 summarizes previous synovial fluid analyses after intra-articular injections of various corticosteroid preparations in normal healthy joints and in patients experiencing a postinjection flare. To date, there have been no reports of an immediate (<2 hours) and severe postinjection flare in response to triamcinolone acetonide, though there was a report of a postinjection flare in response to triamcinolone hexacetonide (Aristospan),7 and here the synovial fluid WBC count (30,000) was much lower.
Although many cases of corticosteroid hypersensitivity have been reported, in rare cases intra-articular administration of triamcinolone has caused anaphylactic reactions and shock.14,15 Multiple case studies have determined that the specific excipient carboxymethylcellulose (found in many triamcinolone preparations), and not the corticosteroid itself, can cause an immunoglobulin E–mediated anaphylactic reaction.16-18 Therefore, performing skin-prick tests for potential corticosteroids and their excipients in patients with known postinjection flares might help prevent serious adverse reactions.18,19
The present case involved an extreme postinjection flare in response to intra-articular administration of triamcinolone acetonide. Postinjection flares are rare but significant events, and physicians using CSIs in the treatment of arthritis need to be aware of this potential reaction in order to appropriately inform patients of this risk and guide treatment should the scenario arise.
Intra-articular corticosteroid injections (CSIs) have been a common treatment for osteoarthritis since the 1950s and continue to be an option for patients who prefer nonoperative management.1 Although CSIs may improve pain secondary to osteoarthritis temporarily, they do not slow articular cartilage degradation, and many patients request multiple CSIs before total joint arthroplasty ultimately is required.1,2 Therefore, acute and chronic side effects of CSI must be considered when repeatedly administering corticosteroids.
A postinjection flare, the most common acute side effect of intra-articular CSI, is characterized by a localized inflammatory response that can last 2 to 3 days. The flare occurs in 2% to 25% of CSI cases.3-5 Symptoms can range from mild joint effusion to disabling pain.6 In the present case, a severe postinjection flare occurred after intra-articular administration of triamcinolone acetonide (Kenalog). This case is novel in that its acuity of onset, severity of symptoms, and synovial fluid analysis mimicked septic arthritis, which was ultimately ruled out with negative cultures and confirmation of triamcinolone acetonide crystals in the synovial aspirate, viewed by polarized light microscopy. To date, only one other case of an immediate (<2 hours) and severe postinjection flare in response to triamcinolone has been reported.7 As CSIs are often used in the nonoperative treatment of osteoarthritis, it is imperative for the treating physician to be aware of this potential side effect in order to appropriately inform the patient of this risk and guide treatment should the scenario arise. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman with a history of hypertension, hypothyroidism, and moderate bilateral knee osteoarthritis presented with left knee pain. She had been receiving annual hylan injections for 5 years and had no adverse reactions, but the pain gradually worsened over the past 3 months. She was given an intra-articular injection of 2 mL of 1% lidocaine and 2 mL (40 mg) of triamcinolone acetonide in the left knee.
Two hours later, she experienced swelling and intense pain in the knee and was unable to ambulate. Physical examination revealed she was afebrile but was having severe pain in the knee through all range of motion. The knee had no appreciable erythema or warmth. Laboratory data were significant: White blood cell (WBC) count was 14,600, and erythrocyte sedimentation rate was 1 mm/h. The knee was aspirated with a return of 25 mL of “butterscotch”-colored fluid (Figure 1). The patient was admitted to rule out iatrogenic septic arthritis, or chronic, indolent septic arthritis acutely worsened by CSI, until synovial fluid analysis and cultures could be performed (Table 1).
She was treated overnight with a compressive wrap, elevation, ice, and nonsteroidal anti-inflammatory drugs, which provided significant pain relief. Polarized light microscopy revealed polymorphic intracellular and extracellular crystals with crystal morphology consistent with the injection of triamcinolone ester (Figure 2). Gram stain showed many WBCs but no organisms. These findings were thought to represent an exogenous crystal-induced acute inflammatory response. Given the patient’s improving clinical course, she was discharged the next morning.
Twelve days later, at clinic follow-up, she was still experiencing pain above her baseline level. Given the continued effusion, 8 mL of synovial fluid was aspirated, which appeared clear and only slightly blood-tinged. Synovial analysis showed resolution of leukocytosis, confirming a severe postinjection flare in response to triamcinolone acetonide.
Discussion
Although rare, side effects from repeated intra-articular CSIs include hypothalamic-pituitary-adrenal axis dysfunction and steroid-induced myopathy.8,9 Acute side effects are more common and include postinjection flare, iatrogenic septic arthritis, local tissue atrophy, cartilage damage, tendon rupture, nerve atrophy, increased blood glucose, and osteonecrosis.10,11 The present case report describes an extreme example of a postinjection flare in response to triamcinolone acetonide and summarizes the characteristics of injections that cause flares.
The physical properties of corticosteroids have a significant impact on their efficacy and on their potential for adverse events. Corticosteroid preparations can be water-soluble or water-insoluble. Most commonly, water-insoluble preparations that contain insoluble corticosteroid esters (eg, triamcinolone, methylprednisolone) are used in intra-articular injections. These form microcrystalline aggregates in solution, which require the patient’s own hydrolytic enzymes (esterases) to release the active moiety and thus have a longer duration of action. However, they are more commonly associated with postinjection flares compared with their more soluble and faster- acting counterparts (eg. dexamethasone, betamethasone).10 Microcrystalline aggregates, which are larger in size, induce a stronger inflammatory response, and in a dose-dependent manner.6A sterile inflammatory reaction to hydrocortisone, cortisone, dexamethasone, triamcinolone, and prednisolone crystals in normal joints has been previously described,6,12,13 and crystals of the various preparations have been demonstrated within leukocytes by both polarized light and electron microscopy.12,13 Table 2 summarizes previous synovial fluid analyses after intra-articular injections of various corticosteroid preparations in normal healthy joints and in patients experiencing a postinjection flare. To date, there have been no reports of an immediate (<2 hours) and severe postinjection flare in response to triamcinolone acetonide, though there was a report of a postinjection flare in response to triamcinolone hexacetonide (Aristospan),7 and here the synovial fluid WBC count (30,000) was much lower.
Although many cases of corticosteroid hypersensitivity have been reported, in rare cases intra-articular administration of triamcinolone has caused anaphylactic reactions and shock.14,15 Multiple case studies have determined that the specific excipient carboxymethylcellulose (found in many triamcinolone preparations), and not the corticosteroid itself, can cause an immunoglobulin E–mediated anaphylactic reaction.16-18 Therefore, performing skin-prick tests for potential corticosteroids and their excipients in patients with known postinjection flares might help prevent serious adverse reactions.18,19
The present case involved an extreme postinjection flare in response to intra-articular administration of triamcinolone acetonide. Postinjection flares are rare but significant events, and physicians using CSIs in the treatment of arthritis need to be aware of this potential reaction in order to appropriately inform patients of this risk and guide treatment should the scenario arise.
1. Hollander JL, Brown EM Jr, Jessar RA, Brown CY. Hydrocortisone and cortisone injected into arthritic joints; comparative effects of and use of hydrocortisone as a local antiarthritic agent. J Am Med Assoc. 1951;147(17):1629-1635.
2. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;19(2):CD005328.
3. Friedman DM, Moore ME. The efficacy of intraarticular steroids in osteoarthritis: a double-blind study. J Rheumatol. 1980;7(6):850-856.
4. Brown EM Jr, Frain JB, Udell L, Hollander JL. Locally administered hydrocortisone in the rheumatic diseases; a summary of its use in 547 patients. Am J Med. 1953;15(5):656-665.
5. Hollander JL, Jessar RA, Brown EM Jr. Intra-synovial corticosteroid therapy: a decade of use. Bull Rheum Dis. 1961;11:239-240.
6. McCarty DJ Jr, Hogan JM. Inflammatory reaction after intrasynovial injection of microcrystalline adrenocorticosteroid esters. Arthritis Rheum. 1964;7(4):359-367.
7. Berger RG, Yount WJ. Immediate “steroid flare” from intraarticular triamcinolone hexacetonide injection: case report and review of the literature. Arthritis Rheum. 1990;33(8):1284-1286.
8. Mader R, Lavi I, Luboshitzky R. Evaluation of the pituitary-adrenal axis function following single intraarticular injection of methylprednisolone. Arthritis Rheum. 2005;52(3):924-928.
9. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370-377.
10. MacMahon PJ, Eustace SJ, Kavanagh EC. Injectable corticosteroid and local anesthetic preparations: a review for radiologists. Radiology. 2009;252(3):647-661.
11. Sparling M, Malleson P, Wood B, Petty R. Radiographic followup of joints injected with triamcinolone hexacetonide for the management of childhood arthritis. Arthritis Rheum. 1990;33(6):821-826.
12. Eymontt MJ, Gordon GV, Schumacher HR, Hansell JR. The effects on synovial permeability and synovial fluid leukocyte counts in symptomatic osteoarthritis after intraarticular corticosteroid administration. J Rheumatol. 1982;9(2):198-203.
13. Gordon GV, Schumacher HR. Electron microscopic study of depot corticosteroid crystals with clinical studies after intra-articular injection. J Rheumatol. 1979;6(1):7-14.
14. Karsh J, Yang WH. An anaphylactic reaction to intra-articular triamcinolone: a case report and review of the literature. Ann Allergy Asthma Immunol. 2003;90(2):254-258.
15. Larsson LG. Anaphylactic shock after i.a. administration of triamcinolone acetonide in a 35-year-old female. Scand J Rheumatol. 1989;18(6):441-442.
16. García-Ortega P, Corominas M, Badia M. Carboxymethylcellulose allergy as a cause of suspected corticosteroid anaphylaxis. Ann Allergy Asthma Immunol. 2003;91(4):421.
17. Patterson DL, Yunginger JW, Dunn WF, Jones RT, Hunt LW. Anaphylaxis induced by the carboxymethylcellulose component of injectable triamcinolone acetonide suspension (Kenalog). Ann Allergy Asthma Immunol. 1995;74(2):163-166.
18. Steiner UC, Gentinetta T, Hausmann O, Pichler WJ. IgE-mediated anaphylaxis to intraarticular glucocorticoid preparations. AJR Am J Roentgenol. 2009;193(2):W156-W157.
19. Ijsselmuiden OE, Knegt-Junk KJ, van Wijk RG, van Joost T. Cutaneous adverse reactions after intra-articular injection of triamcinolone acetonide. Acta Derm Venereol. 1995;75(1):57-58.
20. Pullman-Mooar S, Mooar P, Sieck M, Clayburne G, Schumacher HR. Are there distinctive inflammatory flares after hylan g-f 20 intraarticular injections? J Rheumatol. 2002;29(12):2611-2614.
1. Hollander JL, Brown EM Jr, Jessar RA, Brown CY. Hydrocortisone and cortisone injected into arthritic joints; comparative effects of and use of hydrocortisone as a local antiarthritic agent. J Am Med Assoc. 1951;147(17):1629-1635.
2. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;19(2):CD005328.
3. Friedman DM, Moore ME. The efficacy of intraarticular steroids in osteoarthritis: a double-blind study. J Rheumatol. 1980;7(6):850-856.
4. Brown EM Jr, Frain JB, Udell L, Hollander JL. Locally administered hydrocortisone in the rheumatic diseases; a summary of its use in 547 patients. Am J Med. 1953;15(5):656-665.
5. Hollander JL, Jessar RA, Brown EM Jr. Intra-synovial corticosteroid therapy: a decade of use. Bull Rheum Dis. 1961;11:239-240.
6. McCarty DJ Jr, Hogan JM. Inflammatory reaction after intrasynovial injection of microcrystalline adrenocorticosteroid esters. Arthritis Rheum. 1964;7(4):359-367.
7. Berger RG, Yount WJ. Immediate “steroid flare” from intraarticular triamcinolone hexacetonide injection: case report and review of the literature. Arthritis Rheum. 1990;33(8):1284-1286.
8. Mader R, Lavi I, Luboshitzky R. Evaluation of the pituitary-adrenal axis function following single intraarticular injection of methylprednisolone. Arthritis Rheum. 2005;52(3):924-928.
9. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370-377.
10. MacMahon PJ, Eustace SJ, Kavanagh EC. Injectable corticosteroid and local anesthetic preparations: a review for radiologists. Radiology. 2009;252(3):647-661.
11. Sparling M, Malleson P, Wood B, Petty R. Radiographic followup of joints injected with triamcinolone hexacetonide for the management of childhood arthritis. Arthritis Rheum. 1990;33(6):821-826.
12. Eymontt MJ, Gordon GV, Schumacher HR, Hansell JR. The effects on synovial permeability and synovial fluid leukocyte counts in symptomatic osteoarthritis after intraarticular corticosteroid administration. J Rheumatol. 1982;9(2):198-203.
13. Gordon GV, Schumacher HR. Electron microscopic study of depot corticosteroid crystals with clinical studies after intra-articular injection. J Rheumatol. 1979;6(1):7-14.
14. Karsh J, Yang WH. An anaphylactic reaction to intra-articular triamcinolone: a case report and review of the literature. Ann Allergy Asthma Immunol. 2003;90(2):254-258.
15. Larsson LG. Anaphylactic shock after i.a. administration of triamcinolone acetonide in a 35-year-old female. Scand J Rheumatol. 1989;18(6):441-442.
16. García-Ortega P, Corominas M, Badia M. Carboxymethylcellulose allergy as a cause of suspected corticosteroid anaphylaxis. Ann Allergy Asthma Immunol. 2003;91(4):421.
17. Patterson DL, Yunginger JW, Dunn WF, Jones RT, Hunt LW. Anaphylaxis induced by the carboxymethylcellulose component of injectable triamcinolone acetonide suspension (Kenalog). Ann Allergy Asthma Immunol. 1995;74(2):163-166.
18. Steiner UC, Gentinetta T, Hausmann O, Pichler WJ. IgE-mediated anaphylaxis to intraarticular glucocorticoid preparations. AJR Am J Roentgenol. 2009;193(2):W156-W157.
19. Ijsselmuiden OE, Knegt-Junk KJ, van Wijk RG, van Joost T. Cutaneous adverse reactions after intra-articular injection of triamcinolone acetonide. Acta Derm Venereol. 1995;75(1):57-58.
20. Pullman-Mooar S, Mooar P, Sieck M, Clayburne G, Schumacher HR. Are there distinctive inflammatory flares after hylan g-f 20 intraarticular injections? J Rheumatol. 2002;29(12):2611-2614.
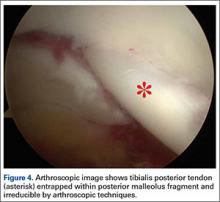
Tibialis Posterior Tendon Entrapment Within Posterior Malleolar Fracture Fragment
Irreducible ankle fracture-dislocation secondary to tibialis posterior tendon interposition is a rare but documented complication most commonly associated with Lauge-Hansen classification pronation–external rotation ankle fractures.1-4 Entrapment of the tibialis posterior tendon has been documented in the syndesmosis (tibiotalar joint)1,2,4 and within a medial malleolus fracture.5 To our knowledge, however, there are no case reports of entrapment of the tibialis posterior tendon in a posterior malleolus fracture.
Ankle arthroscopy performed at time of fracture fixation is gaining in popularity because of its enhanced ability to document and treat intra-articular pathology associated with the initial injury.6,7 In addition, percutaneous fixation of a posterior malleolar fragment with arthroscopic assessment of the articular surface reduction may be valuable, as evaluation of tibial plafond fracture reduction by plain radiographs and fluoroscopy has proved to have limitations.8,9
In this article, we present the case of a patient who underwent attempted arthroscopy-assisted reduction of the posterior malleolus with entrapment of the tibialis posterior tendon within the posterior malleolar fracture fragment. The tendon was irreducible with arthroscopic techniques, necessitating posteromedial incision and subsequent open reduction of the incarcerated structure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
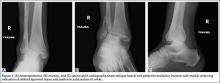
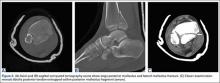
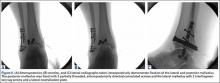
A 67-year-old man slipped and fell on ice while jogging and subsequently presented to the emergency department with a closed bimalleolar ankle fracture-dislocation. Plain radiography (Figure 1) and computed tomography (CT) showed an oblique lateral malleolar fracture and a large posterior malleolar fracture. Further examination of the CT scan revealed entrapment of the tibialis posterior tendon within the posterior malleolar fracture (Figure 2).
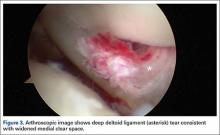
Two days after injury, the patient was taken to the operating room for ankle arthroscopy with planned extrication of the entrapped tibialis posterior tendon and possible arthroscopy-assisted percutaneous fixation of the posterior malleolar fracture and open fixation of the distal fibula fracture. Diagnostic arthroscopy revealed a deltoid ligament injury (Figure 3) and a loose piece of articular cartilage (~1 cm in diameter), which was excised. No donor site for this cartilage fragment was identified with further arthroscopic evaluation. During arthroscopic examination, the tibialis posterior tendon was visualized within the joint, incarcerated within the posterior malleolar fracture (Figure 4). Attempts to release the tibialis posterior tendon from the fracture site using arthroscopic instruments and closed reduction techniques were unsuccessful, both with and without noninvasive skeletal traction applied to the ankle.
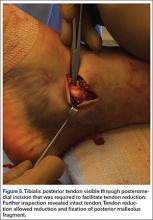
After multiple unsuccessful attempts to extract the tibialis posterior tendon arthroscopically, traction was removed, and a separate incision was made over the posteromedial aspect of the ankle. The tibialis posterior tendon was identified within the fracture site and was removed using an angled clamp (Figure 5). The fracture was reduced and held provisionally with a large tenaculum clamp. Two anterior-to-posterior, partially threaded cannulated screws were placed for fixation after adequate fracture reduction was confirmed on fluoroscopy. As a medial incision was made to extract the tibialis posterior tendon, the joint could not retain arthroscopic fluid, and visualization of the posterior fracture fragment after tendon removal was difficult. Therefore, arthroscopy-assisted reduction could not be completed.
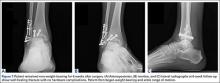
Next, the lateral malleolus was open-reduced, and fixation was achieved using a standard interfragmentary lag screw and a lateral neutralization plate technique (Figure 6). After surgery, the patient was immobilized in a posterior splint with side gussets. Two weeks later, the incisions were healing well, and the tibialis posterior tendon was functioning normally. The sutures were removed, the patient was transitioned to a controlled ankle movement (CAM) boot, and ankle and subtalar range-of-motion exercises were initiated. The patient remained non-weight-bearing for 6 weeks. Radiographs 6 weeks after surgery showed healing fractures with stable hardware (Figure 7). The patient demonstrated 5/5 strength of the tibialis posterior tendon without subluxation or dislocation. There was no tenderness to palpation over the fracture sites or tibialis posterior tendon. The patient began progressive weight-bearing in a CAM boot and physical therapy for range of motion and strengthening.
Discussion
Tibialis posterior tendon injuries—including rupture, dislocation, and entrapment—are well-described complications of ankle injuries.1,2,5,10 Most commonly, the tibialis posterior tendon has been reported to cause a mechanical block to reduction in lateral subtalar dislocations.11-13 In addition, there are case reports of isolated traumatic dislocations of the tibialis posterior tendon without rupture, requiring operative stabilization and retinaculum repair with or without deepening of the posterior groove.14,15
Posterior malleolar ankle fractures remain controversial, with respect to both need for fixation and fixation methods. Although multiple investigators have advocated operative treatment for such fractures that exceed 25% to 33% of the anteroposterior dimension of the tibial plafond, there are no conclusive studies or evidence-based guidelines for treating these fractures.16,17 Anatomical reduction and plating are important to restore articular congruity and increase syndesmotic stability; recent studies have demonstrated that fixation of posterior malleolar fractures provides more syndesmotic stability than trans-syndesmotic screws do.18,19 Indirect reduction of the posterior malleolar fragment after fibula fixation is often accepted as adequate. Whether indirect or direct reduction is attempted, close attention should be given to plain radiographs after attempted reduction, and consideration should be given to possible soft-tissue or bony interposition if malreduction is identified.16,17 Plain radiographs are unreliable in assessing posterior malleolar fragment size as well as amount of comminution and impaction.8,9 Therefore, an arthroscopy-assisted approach coupled with percutaneous fixation may provide more reliable fracture reduction over indirect fracture reduction with fibular fixation, with less dissection than a formal posterolateral approach with posterior plating.
Not all ankle fractures require CT. However, for posterior malleolus fractures thought to require fixation, preoperative CT may help in determining if percutaneous fixation with or without arthroscopic guidance is a feasible treatment option. Ideally, percutaneous reduction can obviate the need for a larger posterolateral incision and buttress plate and, with arthroscopic assistance, may be superior to indirect reduction with fluoroscopy.
In our patient’s case, arthroscopic assistance facilitated diagnosis of an entrapped structure that would have been difficult to identify, particularly without preoperative CT. It may be difficult to identify imperfect reduction of the posterior malleolus on plain radiographs alone, and arthroscopy-assisted fixation enhances the surgeon’s ability to consider reduction, view incarcerated structures within the joint, and treat articular injuries. We do not routinely use ankle arthroscopy as an adjunct to ankle fracture fixation, but judicious use in select cases can facilitate treatment of intra-articular injuries and facilitate visualization and reduction of posterior malleolar fracture fragments before percutaneous anterior-to-posterior cannulated screw fixation. If an open incision is required, as in the present case, visualization becomes difficult secondary to fluid extravasation. However, we think avoiding the morbidity associated with an open incision is worthwhile for fixation of posterior malleolus fractures.
Conclusion
Close inspection of both preoperative and intraoperative radiographs is required to ensure adequate reduction of a posterior malleolar fragment without soft-tissue or bony interposition in the reduction of ankle fractures. Although not previously reported, posterior tendon entrapment within the posterior malleolus fracture may occur and may require arthroscopic or open techniques to ensure adequate extrication of the tendon to allow for posterior malleolar fracture reduction and fixation. This case report highlights one indication for arthroscopy in the treatment of ankle fractures despite the fact that the tibialis posterior tendon was openly removed. Arthroscopic assistance in acute ankle injuries allows the surgeon to evaluate articular cartilage injuries and ensure there are no interposed structures while checking reduction of the posterior malleolar fracture fragment when present.
1. Ermis MN, Yagmurlu MF, Kilinc AS, Karakas ES. Irreducible fracture dislocation of the ankle caused by tibialis posterior tendon interposition. J Foot Ankle Surg. 2010;49(2):166-171.
2. Curry EE, O’Brien TS, Johnson JE. Fibular nonunion and equinovarus deformity secondary to posterior tibial tendon incarceration in the syndesmosis: a case report after a bimalleolar fracture-dislocation. Foot Ankle Int. 1999;20(8):527-531.
3. Coonrad RW, Bugg EI Jr. Trapping of the posterior tibial tendon and interposition of soft tissue in severe fractures about the ankle joint. J Bone Joint Surg Am. 1954;36(4):744-750.
4. Pankovich AM. Fracture-dislocation of the ankle. Trapping of the postero-medial ankle tendons and neurovascular bundle in the tibiofibular interosseous space: a case report. J Trauma. 1976;16(11):927-929.
5. Khamaisy S, Leibner ED, Elishoov O. Tibialis posterior entrapment: case report. Foot Ankle Int. 2012;33(5):441-443.
6. Hsu AR, Gross CE, Lee S, Carreira DS. Extended indications for foot and ankle arthroscopy. J Am Acad Orthop Surg. 2014;22(1):10-19.
7. Stufkens SA, Knupp M, Horisberger M, Lampert C, Hintermann B. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. 2010;92(2):279-286.
8. Büchler L, Tannast M, Bonel HM, Weber M. Reliability of radiologic assessment of the fracture anatomy at the posterior tibial plafond in malleolar fractures. J Orthop Trauma. 2009;23(3):208-212.
9. Ferries JS, DeCoster TA, Firoozbakhsh KK, Garcia JF, Miller RA. Plain radiographic interpretation in trimalleolar ankle fractures poorly assesses posterior fragment size. J Orthop Trauma. 1994;8(4):328-331.
10. Jarvis HC, Cannada LK. Acute tibialis posterior tendon rupture associated with a distal tibial fracture. Orthopedics. 2012;35(4):e595-e597.
11. Woodruff MJ, Brown JN, Mountney J. A mechanism for entrapment of the tibialis posterior tendon in lateral subtalar dislocation. Injury. 1996;27(3):193-194.
12. Leitner B. Obstacles to reduction in subtalar dislocations. J Bone Joint Surg Am. 1954;36(2):299-306.
13. Waldrop J, Ebraheim NA, Shapiro P, Jackson WT. Anatomical considerations of posterior tibialis tendon entrapment in irreducible lateral subtalar dislocation. Foot Ankle. 1992;13(8):458-461.
14. Goucher NR, Coughlin MJ, Kristensen RM. Dislocation of the posterior tibial tendon: a literature review and presentation of two cases. Iowa Orthop J. 2006;26:122-126.
15. Olivé Vilás R, Redón Montojo N, Pino Sorroche S. Traumatic dislocation of tibialis posterior tendon: a case report in a tae-kwon-do athlete. Clin J Sport Med. 2009;19(1):68-69.
16. Gardner MJ, Streubel PN, McCormick JJ, Klein SE, Johnson JE, Ricci WM. Surgeon practices regarding operative treatment of posterior malleolus fractures. Foot Ankle Int. 2011;32(4):385-393.
17. Irwin TA, Lien J, Kadakia AR. Posterior malleolus fracture. J Am Acad Orthop Surg. 2013;21(1):32-40.
18. Gardner MJ, Brodsky A, Briggs SM, Nielson JH, Lorich DG. Fixation of posterior malleolar fractures provides greater syndesmotic stability. Clin Orthop Relat Res. 2006;(447):165-171.
19. Miller AN, Carroll EA, Parker RJ, Helfet DL, Lorich DG. Posterior malleolar stabilization of syndesmotic injuries is equivalent to screw fixation. Clin Orthop Relat Res. 2010;468(4):1129-1135.
Irreducible ankle fracture-dislocation secondary to tibialis posterior tendon interposition is a rare but documented complication most commonly associated with Lauge-Hansen classification pronation–external rotation ankle fractures.1-4 Entrapment of the tibialis posterior tendon has been documented in the syndesmosis (tibiotalar joint)1,2,4 and within a medial malleolus fracture.5 To our knowledge, however, there are no case reports of entrapment of the tibialis posterior tendon in a posterior malleolus fracture.
Ankle arthroscopy performed at time of fracture fixation is gaining in popularity because of its enhanced ability to document and treat intra-articular pathology associated with the initial injury.6,7 In addition, percutaneous fixation of a posterior malleolar fragment with arthroscopic assessment of the articular surface reduction may be valuable, as evaluation of tibial plafond fracture reduction by plain radiographs and fluoroscopy has proved to have limitations.8,9
In this article, we present the case of a patient who underwent attempted arthroscopy-assisted reduction of the posterior malleolus with entrapment of the tibialis posterior tendon within the posterior malleolar fracture fragment. The tendon was irreducible with arthroscopic techniques, necessitating posteromedial incision and subsequent open reduction of the incarcerated structure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old man slipped and fell on ice while jogging and subsequently presented to the emergency department with a closed bimalleolar ankle fracture-dislocation. Plain radiography (Figure 1) and computed tomography (CT) showed an oblique lateral malleolar fracture and a large posterior malleolar fracture. Further examination of the CT scan revealed entrapment of the tibialis posterior tendon within the posterior malleolar fracture (Figure 2).
Two days after injury, the patient was taken to the operating room for ankle arthroscopy with planned extrication of the entrapped tibialis posterior tendon and possible arthroscopy-assisted percutaneous fixation of the posterior malleolar fracture and open fixation of the distal fibula fracture. Diagnostic arthroscopy revealed a deltoid ligament injury (Figure 3) and a loose piece of articular cartilage (~1 cm in diameter), which was excised. No donor site for this cartilage fragment was identified with further arthroscopic evaluation. During arthroscopic examination, the tibialis posterior tendon was visualized within the joint, incarcerated within the posterior malleolar fracture (Figure 4). Attempts to release the tibialis posterior tendon from the fracture site using arthroscopic instruments and closed reduction techniques were unsuccessful, both with and without noninvasive skeletal traction applied to the ankle.
After multiple unsuccessful attempts to extract the tibialis posterior tendon arthroscopically, traction was removed, and a separate incision was made over the posteromedial aspect of the ankle. The tibialis posterior tendon was identified within the fracture site and was removed using an angled clamp (Figure 5). The fracture was reduced and held provisionally with a large tenaculum clamp. Two anterior-to-posterior, partially threaded cannulated screws were placed for fixation after adequate fracture reduction was confirmed on fluoroscopy. As a medial incision was made to extract the tibialis posterior tendon, the joint could not retain arthroscopic fluid, and visualization of the posterior fracture fragment after tendon removal was difficult. Therefore, arthroscopy-assisted reduction could not be completed.
Next, the lateral malleolus was open-reduced, and fixation was achieved using a standard interfragmentary lag screw and a lateral neutralization plate technique (Figure 6). After surgery, the patient was immobilized in a posterior splint with side gussets. Two weeks later, the incisions were healing well, and the tibialis posterior tendon was functioning normally. The sutures were removed, the patient was transitioned to a controlled ankle movement (CAM) boot, and ankle and subtalar range-of-motion exercises were initiated. The patient remained non-weight-bearing for 6 weeks. Radiographs 6 weeks after surgery showed healing fractures with stable hardware (Figure 7). The patient demonstrated 5/5 strength of the tibialis posterior tendon without subluxation or dislocation. There was no tenderness to palpation over the fracture sites or tibialis posterior tendon. The patient began progressive weight-bearing in a CAM boot and physical therapy for range of motion and strengthening.
Discussion
Tibialis posterior tendon injuries—including rupture, dislocation, and entrapment—are well-described complications of ankle injuries.1,2,5,10 Most commonly, the tibialis posterior tendon has been reported to cause a mechanical block to reduction in lateral subtalar dislocations.11-13 In addition, there are case reports of isolated traumatic dislocations of the tibialis posterior tendon without rupture, requiring operative stabilization and retinaculum repair with or without deepening of the posterior groove.14,15
Posterior malleolar ankle fractures remain controversial, with respect to both need for fixation and fixation methods. Although multiple investigators have advocated operative treatment for such fractures that exceed 25% to 33% of the anteroposterior dimension of the tibial plafond, there are no conclusive studies or evidence-based guidelines for treating these fractures.16,17 Anatomical reduction and plating are important to restore articular congruity and increase syndesmotic stability; recent studies have demonstrated that fixation of posterior malleolar fractures provides more syndesmotic stability than trans-syndesmotic screws do.18,19 Indirect reduction of the posterior malleolar fragment after fibula fixation is often accepted as adequate. Whether indirect or direct reduction is attempted, close attention should be given to plain radiographs after attempted reduction, and consideration should be given to possible soft-tissue or bony interposition if malreduction is identified.16,17 Plain radiographs are unreliable in assessing posterior malleolar fragment size as well as amount of comminution and impaction.8,9 Therefore, an arthroscopy-assisted approach coupled with percutaneous fixation may provide more reliable fracture reduction over indirect fracture reduction with fibular fixation, with less dissection than a formal posterolateral approach with posterior plating.
Not all ankle fractures require CT. However, for posterior malleolus fractures thought to require fixation, preoperative CT may help in determining if percutaneous fixation with or without arthroscopic guidance is a feasible treatment option. Ideally, percutaneous reduction can obviate the need for a larger posterolateral incision and buttress plate and, with arthroscopic assistance, may be superior to indirect reduction with fluoroscopy.
In our patient’s case, arthroscopic assistance facilitated diagnosis of an entrapped structure that would have been difficult to identify, particularly without preoperative CT. It may be difficult to identify imperfect reduction of the posterior malleolus on plain radiographs alone, and arthroscopy-assisted fixation enhances the surgeon’s ability to consider reduction, view incarcerated structures within the joint, and treat articular injuries. We do not routinely use ankle arthroscopy as an adjunct to ankle fracture fixation, but judicious use in select cases can facilitate treatment of intra-articular injuries and facilitate visualization and reduction of posterior malleolar fracture fragments before percutaneous anterior-to-posterior cannulated screw fixation. If an open incision is required, as in the present case, visualization becomes difficult secondary to fluid extravasation. However, we think avoiding the morbidity associated with an open incision is worthwhile for fixation of posterior malleolus fractures.
Conclusion
Close inspection of both preoperative and intraoperative radiographs is required to ensure adequate reduction of a posterior malleolar fragment without soft-tissue or bony interposition in the reduction of ankle fractures. Although not previously reported, posterior tendon entrapment within the posterior malleolus fracture may occur and may require arthroscopic or open techniques to ensure adequate extrication of the tendon to allow for posterior malleolar fracture reduction and fixation. This case report highlights one indication for arthroscopy in the treatment of ankle fractures despite the fact that the tibialis posterior tendon was openly removed. Arthroscopic assistance in acute ankle injuries allows the surgeon to evaluate articular cartilage injuries and ensure there are no interposed structures while checking reduction of the posterior malleolar fracture fragment when present.
Irreducible ankle fracture-dislocation secondary to tibialis posterior tendon interposition is a rare but documented complication most commonly associated with Lauge-Hansen classification pronation–external rotation ankle fractures.1-4 Entrapment of the tibialis posterior tendon has been documented in the syndesmosis (tibiotalar joint)1,2,4 and within a medial malleolus fracture.5 To our knowledge, however, there are no case reports of entrapment of the tibialis posterior tendon in a posterior malleolus fracture.
Ankle arthroscopy performed at time of fracture fixation is gaining in popularity because of its enhanced ability to document and treat intra-articular pathology associated with the initial injury.6,7 In addition, percutaneous fixation of a posterior malleolar fragment with arthroscopic assessment of the articular surface reduction may be valuable, as evaluation of tibial plafond fracture reduction by plain radiographs and fluoroscopy has proved to have limitations.8,9
In this article, we present the case of a patient who underwent attempted arthroscopy-assisted reduction of the posterior malleolus with entrapment of the tibialis posterior tendon within the posterior malleolar fracture fragment. The tendon was irreducible with arthroscopic techniques, necessitating posteromedial incision and subsequent open reduction of the incarcerated structure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old man slipped and fell on ice while jogging and subsequently presented to the emergency department with a closed bimalleolar ankle fracture-dislocation. Plain radiography (Figure 1) and computed tomography (CT) showed an oblique lateral malleolar fracture and a large posterior malleolar fracture. Further examination of the CT scan revealed entrapment of the tibialis posterior tendon within the posterior malleolar fracture (Figure 2).
Two days after injury, the patient was taken to the operating room for ankle arthroscopy with planned extrication of the entrapped tibialis posterior tendon and possible arthroscopy-assisted percutaneous fixation of the posterior malleolar fracture and open fixation of the distal fibula fracture. Diagnostic arthroscopy revealed a deltoid ligament injury (Figure 3) and a loose piece of articular cartilage (~1 cm in diameter), which was excised. No donor site for this cartilage fragment was identified with further arthroscopic evaluation. During arthroscopic examination, the tibialis posterior tendon was visualized within the joint, incarcerated within the posterior malleolar fracture (Figure 4). Attempts to release the tibialis posterior tendon from the fracture site using arthroscopic instruments and closed reduction techniques were unsuccessful, both with and without noninvasive skeletal traction applied to the ankle.
After multiple unsuccessful attempts to extract the tibialis posterior tendon arthroscopically, traction was removed, and a separate incision was made over the posteromedial aspect of the ankle. The tibialis posterior tendon was identified within the fracture site and was removed using an angled clamp (Figure 5). The fracture was reduced and held provisionally with a large tenaculum clamp. Two anterior-to-posterior, partially threaded cannulated screws were placed for fixation after adequate fracture reduction was confirmed on fluoroscopy. As a medial incision was made to extract the tibialis posterior tendon, the joint could not retain arthroscopic fluid, and visualization of the posterior fracture fragment after tendon removal was difficult. Therefore, arthroscopy-assisted reduction could not be completed.
Next, the lateral malleolus was open-reduced, and fixation was achieved using a standard interfragmentary lag screw and a lateral neutralization plate technique (Figure 6). After surgery, the patient was immobilized in a posterior splint with side gussets. Two weeks later, the incisions were healing well, and the tibialis posterior tendon was functioning normally. The sutures were removed, the patient was transitioned to a controlled ankle movement (CAM) boot, and ankle and subtalar range-of-motion exercises were initiated. The patient remained non-weight-bearing for 6 weeks. Radiographs 6 weeks after surgery showed healing fractures with stable hardware (Figure 7). The patient demonstrated 5/5 strength of the tibialis posterior tendon without subluxation or dislocation. There was no tenderness to palpation over the fracture sites or tibialis posterior tendon. The patient began progressive weight-bearing in a CAM boot and physical therapy for range of motion and strengthening.
Discussion
Tibialis posterior tendon injuries—including rupture, dislocation, and entrapment—are well-described complications of ankle injuries.1,2,5,10 Most commonly, the tibialis posterior tendon has been reported to cause a mechanical block to reduction in lateral subtalar dislocations.11-13 In addition, there are case reports of isolated traumatic dislocations of the tibialis posterior tendon without rupture, requiring operative stabilization and retinaculum repair with or without deepening of the posterior groove.14,15
Posterior malleolar ankle fractures remain controversial, with respect to both need for fixation and fixation methods. Although multiple investigators have advocated operative treatment for such fractures that exceed 25% to 33% of the anteroposterior dimension of the tibial plafond, there are no conclusive studies or evidence-based guidelines for treating these fractures.16,17 Anatomical reduction and plating are important to restore articular congruity and increase syndesmotic stability; recent studies have demonstrated that fixation of posterior malleolar fractures provides more syndesmotic stability than trans-syndesmotic screws do.18,19 Indirect reduction of the posterior malleolar fragment after fibula fixation is often accepted as adequate. Whether indirect or direct reduction is attempted, close attention should be given to plain radiographs after attempted reduction, and consideration should be given to possible soft-tissue or bony interposition if malreduction is identified.16,17 Plain radiographs are unreliable in assessing posterior malleolar fragment size as well as amount of comminution and impaction.8,9 Therefore, an arthroscopy-assisted approach coupled with percutaneous fixation may provide more reliable fracture reduction over indirect fracture reduction with fibular fixation, with less dissection than a formal posterolateral approach with posterior plating.
Not all ankle fractures require CT. However, for posterior malleolus fractures thought to require fixation, preoperative CT may help in determining if percutaneous fixation with or without arthroscopic guidance is a feasible treatment option. Ideally, percutaneous reduction can obviate the need for a larger posterolateral incision and buttress plate and, with arthroscopic assistance, may be superior to indirect reduction with fluoroscopy.
In our patient’s case, arthroscopic assistance facilitated diagnosis of an entrapped structure that would have been difficult to identify, particularly without preoperative CT. It may be difficult to identify imperfect reduction of the posterior malleolus on plain radiographs alone, and arthroscopy-assisted fixation enhances the surgeon’s ability to consider reduction, view incarcerated structures within the joint, and treat articular injuries. We do not routinely use ankle arthroscopy as an adjunct to ankle fracture fixation, but judicious use in select cases can facilitate treatment of intra-articular injuries and facilitate visualization and reduction of posterior malleolar fracture fragments before percutaneous anterior-to-posterior cannulated screw fixation. If an open incision is required, as in the present case, visualization becomes difficult secondary to fluid extravasation. However, we think avoiding the morbidity associated with an open incision is worthwhile for fixation of posterior malleolus fractures.
Conclusion
Close inspection of both preoperative and intraoperative radiographs is required to ensure adequate reduction of a posterior malleolar fragment without soft-tissue or bony interposition in the reduction of ankle fractures. Although not previously reported, posterior tendon entrapment within the posterior malleolus fracture may occur and may require arthroscopic or open techniques to ensure adequate extrication of the tendon to allow for posterior malleolar fracture reduction and fixation. This case report highlights one indication for arthroscopy in the treatment of ankle fractures despite the fact that the tibialis posterior tendon was openly removed. Arthroscopic assistance in acute ankle injuries allows the surgeon to evaluate articular cartilage injuries and ensure there are no interposed structures while checking reduction of the posterior malleolar fracture fragment when present.
1. Ermis MN, Yagmurlu MF, Kilinc AS, Karakas ES. Irreducible fracture dislocation of the ankle caused by tibialis posterior tendon interposition. J Foot Ankle Surg. 2010;49(2):166-171.
2. Curry EE, O’Brien TS, Johnson JE. Fibular nonunion and equinovarus deformity secondary to posterior tibial tendon incarceration in the syndesmosis: a case report after a bimalleolar fracture-dislocation. Foot Ankle Int. 1999;20(8):527-531.
3. Coonrad RW, Bugg EI Jr. Trapping of the posterior tibial tendon and interposition of soft tissue in severe fractures about the ankle joint. J Bone Joint Surg Am. 1954;36(4):744-750.
4. Pankovich AM. Fracture-dislocation of the ankle. Trapping of the postero-medial ankle tendons and neurovascular bundle in the tibiofibular interosseous space: a case report. J Trauma. 1976;16(11):927-929.
5. Khamaisy S, Leibner ED, Elishoov O. Tibialis posterior entrapment: case report. Foot Ankle Int. 2012;33(5):441-443.
6. Hsu AR, Gross CE, Lee S, Carreira DS. Extended indications for foot and ankle arthroscopy. J Am Acad Orthop Surg. 2014;22(1):10-19.
7. Stufkens SA, Knupp M, Horisberger M, Lampert C, Hintermann B. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. 2010;92(2):279-286.
8. Büchler L, Tannast M, Bonel HM, Weber M. Reliability of radiologic assessment of the fracture anatomy at the posterior tibial plafond in malleolar fractures. J Orthop Trauma. 2009;23(3):208-212.
9. Ferries JS, DeCoster TA, Firoozbakhsh KK, Garcia JF, Miller RA. Plain radiographic interpretation in trimalleolar ankle fractures poorly assesses posterior fragment size. J Orthop Trauma. 1994;8(4):328-331.
10. Jarvis HC, Cannada LK. Acute tibialis posterior tendon rupture associated with a distal tibial fracture. Orthopedics. 2012;35(4):e595-e597.
11. Woodruff MJ, Brown JN, Mountney J. A mechanism for entrapment of the tibialis posterior tendon in lateral subtalar dislocation. Injury. 1996;27(3):193-194.
12. Leitner B. Obstacles to reduction in subtalar dislocations. J Bone Joint Surg Am. 1954;36(2):299-306.
13. Waldrop J, Ebraheim NA, Shapiro P, Jackson WT. Anatomical considerations of posterior tibialis tendon entrapment in irreducible lateral subtalar dislocation. Foot Ankle. 1992;13(8):458-461.
14. Goucher NR, Coughlin MJ, Kristensen RM. Dislocation of the posterior tibial tendon: a literature review and presentation of two cases. Iowa Orthop J. 2006;26:122-126.
15. Olivé Vilás R, Redón Montojo N, Pino Sorroche S. Traumatic dislocation of tibialis posterior tendon: a case report in a tae-kwon-do athlete. Clin J Sport Med. 2009;19(1):68-69.
16. Gardner MJ, Streubel PN, McCormick JJ, Klein SE, Johnson JE, Ricci WM. Surgeon practices regarding operative treatment of posterior malleolus fractures. Foot Ankle Int. 2011;32(4):385-393.
17. Irwin TA, Lien J, Kadakia AR. Posterior malleolus fracture. J Am Acad Orthop Surg. 2013;21(1):32-40.
18. Gardner MJ, Brodsky A, Briggs SM, Nielson JH, Lorich DG. Fixation of posterior malleolar fractures provides greater syndesmotic stability. Clin Orthop Relat Res. 2006;(447):165-171.
19. Miller AN, Carroll EA, Parker RJ, Helfet DL, Lorich DG. Posterior malleolar stabilization of syndesmotic injuries is equivalent to screw fixation. Clin Orthop Relat Res. 2010;468(4):1129-1135.
1. Ermis MN, Yagmurlu MF, Kilinc AS, Karakas ES. Irreducible fracture dislocation of the ankle caused by tibialis posterior tendon interposition. J Foot Ankle Surg. 2010;49(2):166-171.
2. Curry EE, O’Brien TS, Johnson JE. Fibular nonunion and equinovarus deformity secondary to posterior tibial tendon incarceration in the syndesmosis: a case report after a bimalleolar fracture-dislocation. Foot Ankle Int. 1999;20(8):527-531.
3. Coonrad RW, Bugg EI Jr. Trapping of the posterior tibial tendon and interposition of soft tissue in severe fractures about the ankle joint. J Bone Joint Surg Am. 1954;36(4):744-750.
4. Pankovich AM. Fracture-dislocation of the ankle. Trapping of the postero-medial ankle tendons and neurovascular bundle in the tibiofibular interosseous space: a case report. J Trauma. 1976;16(11):927-929.
5. Khamaisy S, Leibner ED, Elishoov O. Tibialis posterior entrapment: case report. Foot Ankle Int. 2012;33(5):441-443.
6. Hsu AR, Gross CE, Lee S, Carreira DS. Extended indications for foot and ankle arthroscopy. J Am Acad Orthop Surg. 2014;22(1):10-19.
7. Stufkens SA, Knupp M, Horisberger M, Lampert C, Hintermann B. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. 2010;92(2):279-286.
8. Büchler L, Tannast M, Bonel HM, Weber M. Reliability of radiologic assessment of the fracture anatomy at the posterior tibial plafond in malleolar fractures. J Orthop Trauma. 2009;23(3):208-212.
9. Ferries JS, DeCoster TA, Firoozbakhsh KK, Garcia JF, Miller RA. Plain radiographic interpretation in trimalleolar ankle fractures poorly assesses posterior fragment size. J Orthop Trauma. 1994;8(4):328-331.
10. Jarvis HC, Cannada LK. Acute tibialis posterior tendon rupture associated with a distal tibial fracture. Orthopedics. 2012;35(4):e595-e597.
11. Woodruff MJ, Brown JN, Mountney J. A mechanism for entrapment of the tibialis posterior tendon in lateral subtalar dislocation. Injury. 1996;27(3):193-194.
12. Leitner B. Obstacles to reduction in subtalar dislocations. J Bone Joint Surg Am. 1954;36(2):299-306.
13. Waldrop J, Ebraheim NA, Shapiro P, Jackson WT. Anatomical considerations of posterior tibialis tendon entrapment in irreducible lateral subtalar dislocation. Foot Ankle. 1992;13(8):458-461.
14. Goucher NR, Coughlin MJ, Kristensen RM. Dislocation of the posterior tibial tendon: a literature review and presentation of two cases. Iowa Orthop J. 2006;26:122-126.
15. Olivé Vilás R, Redón Montojo N, Pino Sorroche S. Traumatic dislocation of tibialis posterior tendon: a case report in a tae-kwon-do athlete. Clin J Sport Med. 2009;19(1):68-69.
16. Gardner MJ, Streubel PN, McCormick JJ, Klein SE, Johnson JE, Ricci WM. Surgeon practices regarding operative treatment of posterior malleolus fractures. Foot Ankle Int. 2011;32(4):385-393.
17. Irwin TA, Lien J, Kadakia AR. Posterior malleolus fracture. J Am Acad Orthop Surg. 2013;21(1):32-40.
18. Gardner MJ, Brodsky A, Briggs SM, Nielson JH, Lorich DG. Fixation of posterior malleolar fractures provides greater syndesmotic stability. Clin Orthop Relat Res. 2006;(447):165-171.
19. Miller AN, Carroll EA, Parker RJ, Helfet DL, Lorich DG. Posterior malleolar stabilization of syndesmotic injuries is equivalent to screw fixation. Clin Orthop Relat Res. 2010;468(4):1129-1135.
Are Hook Plates Advantageous Compared to Antiglide Plates for Vertical Shear Malleolar Fractures?
Supination-adduction (SAD)-type fractures of the ankle comprise approximately 5% to 20% of ankle fractures.1-3 As the name describes, this fracture is caused by forceful adduction of the supinated foot. There are 2 stages of the fracture pattern: the injury usually occurs first on the lateral side of the ankle with injury to the soft tissues or a low transverse fracture of the distal fibula. With continued force, in the second stage, the talus causes a shearing of the medial malleolus, creating the vertical shear fracture pattern.4-7 The vertical shear medial malleolus fracture pattern is the subject of this investigation.
Several techniques have been traditionally recommended for fixation of SAD-type ankle fracture, including: a 2-screw construct without plate fixation, oriented perpendicular to the fracture; and an AG plate construct with variable positioning and numbers of screws for fixation. There have been, however, only 2 published articles about the biomechanical properties of fixation of vertical shear medial malleolar fractures, which reported conflicting results.8,9 The most recent of these studies argued that one-third tubular plate fixation offers significant mechanical advantage over screw-only fixation, supporting the use of AG plates for fixation of SAD ankle fractures.8
An additional design for fixation of medial malleolus fractures has been introduced, consisting of a hook plate (HP) contoured for the medial malleolus. To our knowledge, no studies have investigated HP’s biomechanical properties. Thus, the objective of this study was to investigate and compare the biomechanical properties of 3 constructs for fixation of SAD-ankle fractures: an antiglide (AG) plate, an AG plate with an additional lag-screw across the fracture, and a precontoured HP.
Materials and Methods
Thirty 4th-generation–composite polyurethane models of the left tibia were obtained (Sawbones, Pacific Research Laboratories, Inc.). Largely, our methods accorded with the precedent set by other studies on these fracture types.8,9
Prior to creation of the fractures, each model was individually evaluated for pretest stiffness by using the slope of the linear portion of the load-displacement curve during offset-axial loading. This demonstrated the baseline elasticity of the models during loading. Assessing pretest stiffness was performed to reduce potential variables in the stiffness of individual models in the analysis of the testing data.
The models were numbered 1 through 30 on the shaft and on the medial malleolus. A custom jig was constructed with a table saw to create identical vertical shear medial malleolar fracture patterns in each model. The jig created the vertical shear SAD fracture described by Lauge-Hansen.7 All models were randomly assigned to 1 of 3 groups; each group consisted of 10 models (Figures 1A, 1B).
The 10 specimens in group 1 were fixed with a 5-hole, 3.5-mm, one-third tubular plate (Smith & Nephew) in a traditional AG fashion. The plates were placed at the same location on all tibiae. The proximal hole and the hole closest to the fracture line were filled with 3.5-mm cortical screws, which were long enough to achieve bicortical fixation. No lag screws were placed in this specimen group. In group 2, specimens were fixed with the same plate used in group 1 (Smith & Nephew). In this modified AG (MAG) construct, specimens were fixed identically to group 1 for plate placement and fixation of the 2 proximal screws. In this group, an additional screw was placed perpendicular to the fracture and parallel to the distal tibial articular surface. In both groups (AG and MAG), the plates were not bent before application.
Group 3 consisted of specimens fixed with a 5-hole, precontoured medial malleolar HP (Arthrex). This HP construct was fixed with two 3.5-mm cortical screws long enough to achieve bicortical fixation. The plate also engaged the bone at the tip of the medial malleolus by using 2 sharp prongs. The screws were placed in the most proximal hole and the hole just proximal to the fracture line. No lag screws were placed in the HP construct.
All models were tested in offset-axial loading to replicate a SAD moment similar to previous studies. To test offset-axial loading, a vice held each model identically with a 17º angle from the longitudinal axis (Figure 2). Loading was performed with a material testing system; a material testing system plunger was directed at the inferior articulating cartilage surface of the medial malleolus. The specimens were loaded at a rate of 1 mm/sec until 2 mm of displacement was reached (Figure 3) or catastrophic failure occurred. The raw data analyzed consisted of the initial stiffness of the construct and the overall load-to-failure. The slope of the linear portion of the load-displacement curve of stiffness determined stiffness of the construct.
One-way analysis of variance with post hoc Tukey HSD data analysis was performed to determine if there were statistical differences among the different fixation constructs during load-to-failure. To prevent skewing of results by different values of model elasticity, pretest stiffness was accounted for by calculating a ratio of construct stiffness as a function of pretest model stiffness. Total force-to-failure was the recorded maximum force (in N) to cause failure. A P value of < .05 was set for significance. All data were analyzed using SPSS software (SPSS Version 15.0; SPSS Inc.).
Results
Analysis of pretest stiffness showed no significant difference among models (P = .490). All models failed by a gap of 2 mm at the distal fracture site except for 3 models in the MAG group. These 3 models failed at a much higher load than the remainder of the models and failed by fracture of the models.
The MAG group demonstrated significantly superior stiffness to the 2 other models tested (Figure 4). On average, this group required 753.5 N of force before failure. This was 530 N higher than the HP (P < .05) and 638 N higher than the AG constructs, respectively (P < .05). The HP and AG groups required forces of 223.2 N and 115.5 N for failure, respectively. These numbers were not significant (P= .063).
The absolute construct stiffness and construct stiffness as a function of pretest stiffness of the MAG group was the highest of all groups, 271.7 N/mm and 57.2%, respectively (Figure 5). These numbers showed significance when compared with the values of the HP group (P < .05 for both) and the AG group (P < .05 for both). The average stiffness of the HP group was 159.7 N/mm, which was 36.8% of pretest stiffness.
The AG group had the lowest construct stiffness and percent of pretest stiffness (128.1 N/mm and 29.6%). The HP and AG groups were not statistically different in these comparisons, P = .350 for construct stiffness and P = .395 for percent of pretest stiffness.
Discussion
These results support the use of a one-third tubular plate and lag-screw construct for fixation of vertical shear medial malleolus fractures. This is clinically important because one-third tubular plates with 3.5-mm screws are readily available and cost significantly less than a precountoured anatomic-specific type of fixation. These results are based on the biomechanical properties of the constructs tested in this study.
The previous 2 studies8,9showed conflicting results about the most biomechanically sound fixation for SAD medial malleolar fractures. The study by Toolan and colleagues9 reported that 2 screws placed perpendicular to the fracture demonstrated the strongest overall construct. This study compared 3 separate types of 2-screw–only fixations and 2 plate-and-screw fixations. One construct was similar to the AG group in our study, and the other construct had a lag screw at the apex of the fracture. This previous study,9 however, did not investigate a similar construct to the MAG group that was tested in our study.
According to Dumigan and associates,8 a construct that consisted of a 4-hole plate with 2 screws proximal to the fracture and 2 lag screws showed the strongest fixation. This study, however, did not include a group like our study’s AG group, which is the traditional AG form of fixation.
In our study, we examined the biomechanic properties of a traditional fixation (AG construct), a commonly used fixation (MAG construct), and a newer construct (HP construct). The HP group is unique to this study and, to our knowledge, there is no literature on its use as fixation for this fracture. We did not include a 2-screw–only group, which is a limitation, because this fixation type is not common for the SAD fracture. This study also did not include an HP construct with an additional lag screw, which is an available option as well.
The current investigation used synthetic bone models constructed for biomechanical testing. The models were thought to provide a consistent model for fixation as opposed to using potentially osteopenic cadaveric bone. Each model was the same size and laterality. The stiffness as determined by pretest stiffness was not significantly different among models. Because all models were similar in composition and size, this allowed for more consistent osteotomies and similarly sized malleolar fragments. Theoretically, this allowed a more uniform comparison of all specimens and constructs.
Using models, however, is a limit of this study. While the models were of similar biomechanical quality, it is possible that a model may not reproduce the biology of a cavaderic specimen or the physiology of a construct in vivo. Of the 2 studies that investigated SAD fractures, the Dumigan study8 used cadaveric specimens. The fact that these models were all mildly osteoporotic and were embalmed specimens were study limits. The Toolan study9 used synthetic models. Although these models were consistent, they were models of bones and not intended for biomechanical studies, thereby increasing the potential for skewed results.
Our study investigated loading only in the offset-axial direction, a difference when compared to the Dumigan and colleagues8 and Toolan and colleagues9 studies. The offest transverse loading previously investigated would most likely represent an external rotation moment. While fixation in vivo could experience an external rotation moment, the specific fracture pattern of interest fails in offset-axial loading. In the original discription of the SAD fracture, Lauge-Hanson7 stated that the talus causes the vertically oriented medial malleolar fracture in the extreme of ankle supination with an adduction moment. Considering this, we investigated failure with a force in the direction that causes this type of fracture.
There are some additional limitations. This study demonstrated superiority of a one-third tubular plate with 2 screws proximally and 1 lag screw. While this was shown in the laboratory under pure offset-axial loading conditions, this may not reproduce daily forces experienced by the constructs. Additionally, this study examined load-to-failure of the constructs and did not investigate cyclic loading that a construct would experience in vivo. Because the testing is not recognizably consistent with day-to-day stresses of these constructs in vivo, this confounds the clinical application of our study.
The stiffness required for clinical healing is undetermined and, therefore, all 3 types of fixation could be adequate clinically. Patients are typically instructed to adhere to weight-bearing limitations on the affected extremity, and casts or splints are applied postoperatively for extended periods of time. Clinical studies would have significant benefit in the evaluation of fixation of vertical shear medial malleolar fractures.
Conclusion
AG plating technique with lag-screw placement is biomechanically superior to the other 2 constructs investigated. The clinical applications of these results are not known, and clinical trials are suggested to determine the best type of fixation for SAD-type medial malleolar fractures.
1. Hak DJ, Egol KA, Gardner MJ, Haskell A. The “not so simple” ankle fracture: avoiding problems and pitfalls to improve patient outcomes. Instr Course Lect. 2011;60:73-88.
2. Hamilton WC. Supination-adduction injuries. In: Hamilton WC, ed. Traumatic Disorders of the Ankle. 1st ed. New York, NY: Springer-Verlag; 1984:101-112.
3. McConnell T, Tornetta P. Marginal plafond impaction in association with supination-adduction ankle fractures: a report of eight cases. J Orthop Trauma. 2001;15(6):447-449.
4. Arimoto HK, Forrester DM. Classification of ankle fractures: an algorithm. AJR Am J Roentgenol. 1980;135(5):1057-1063.
5. Carr JB. Malleolar fractures and soft tissue injuries of the ankle. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma: Basic Science, Management and Reconstruction. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:2515-2584.
6. Davidovitch RI, Egol KA. Ankle fractures. In: Bucholz RW HJ, Court-Brown CM, Tornetta P III, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2010:1975-2021.
7. Lauge-Hansen N. Fractures of the ankle. II. Combined experimental-surgical and experimental-roentgenologic investigations. Arch Surg. 1950;60(5):957-985.
8. Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691.
9. Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489.
Supination-adduction (SAD)-type fractures of the ankle comprise approximately 5% to 20% of ankle fractures.1-3 As the name describes, this fracture is caused by forceful adduction of the supinated foot. There are 2 stages of the fracture pattern: the injury usually occurs first on the lateral side of the ankle with injury to the soft tissues or a low transverse fracture of the distal fibula. With continued force, in the second stage, the talus causes a shearing of the medial malleolus, creating the vertical shear fracture pattern.4-7 The vertical shear medial malleolus fracture pattern is the subject of this investigation.
Several techniques have been traditionally recommended for fixation of SAD-type ankle fracture, including: a 2-screw construct without plate fixation, oriented perpendicular to the fracture; and an AG plate construct with variable positioning and numbers of screws for fixation. There have been, however, only 2 published articles about the biomechanical properties of fixation of vertical shear medial malleolar fractures, which reported conflicting results.8,9 The most recent of these studies argued that one-third tubular plate fixation offers significant mechanical advantage over screw-only fixation, supporting the use of AG plates for fixation of SAD ankle fractures.8
An additional design for fixation of medial malleolus fractures has been introduced, consisting of a hook plate (HP) contoured for the medial malleolus. To our knowledge, no studies have investigated HP’s biomechanical properties. Thus, the objective of this study was to investigate and compare the biomechanical properties of 3 constructs for fixation of SAD-ankle fractures: an antiglide (AG) plate, an AG plate with an additional lag-screw across the fracture, and a precontoured HP.
Materials and Methods
Thirty 4th-generation–composite polyurethane models of the left tibia were obtained (Sawbones, Pacific Research Laboratories, Inc.). Largely, our methods accorded with the precedent set by other studies on these fracture types.8,9
Prior to creation of the fractures, each model was individually evaluated for pretest stiffness by using the slope of the linear portion of the load-displacement curve during offset-axial loading. This demonstrated the baseline elasticity of the models during loading. Assessing pretest stiffness was performed to reduce potential variables in the stiffness of individual models in the analysis of the testing data.
The models were numbered 1 through 30 on the shaft and on the medial malleolus. A custom jig was constructed with a table saw to create identical vertical shear medial malleolar fracture patterns in each model. The jig created the vertical shear SAD fracture described by Lauge-Hansen.7 All models were randomly assigned to 1 of 3 groups; each group consisted of 10 models (Figures 1A, 1B).
The 10 specimens in group 1 were fixed with a 5-hole, 3.5-mm, one-third tubular plate (Smith & Nephew) in a traditional AG fashion. The plates were placed at the same location on all tibiae. The proximal hole and the hole closest to the fracture line were filled with 3.5-mm cortical screws, which were long enough to achieve bicortical fixation. No lag screws were placed in this specimen group. In group 2, specimens were fixed with the same plate used in group 1 (Smith & Nephew). In this modified AG (MAG) construct, specimens were fixed identically to group 1 for plate placement and fixation of the 2 proximal screws. In this group, an additional screw was placed perpendicular to the fracture and parallel to the distal tibial articular surface. In both groups (AG and MAG), the plates were not bent before application.
Group 3 consisted of specimens fixed with a 5-hole, precontoured medial malleolar HP (Arthrex). This HP construct was fixed with two 3.5-mm cortical screws long enough to achieve bicortical fixation. The plate also engaged the bone at the tip of the medial malleolus by using 2 sharp prongs. The screws were placed in the most proximal hole and the hole just proximal to the fracture line. No lag screws were placed in the HP construct.
All models were tested in offset-axial loading to replicate a SAD moment similar to previous studies. To test offset-axial loading, a vice held each model identically with a 17º angle from the longitudinal axis (Figure 2). Loading was performed with a material testing system; a material testing system plunger was directed at the inferior articulating cartilage surface of the medial malleolus. The specimens were loaded at a rate of 1 mm/sec until 2 mm of displacement was reached (Figure 3) or catastrophic failure occurred. The raw data analyzed consisted of the initial stiffness of the construct and the overall load-to-failure. The slope of the linear portion of the load-displacement curve of stiffness determined stiffness of the construct.
One-way analysis of variance with post hoc Tukey HSD data analysis was performed to determine if there were statistical differences among the different fixation constructs during load-to-failure. To prevent skewing of results by different values of model elasticity, pretest stiffness was accounted for by calculating a ratio of construct stiffness as a function of pretest model stiffness. Total force-to-failure was the recorded maximum force (in N) to cause failure. A P value of < .05 was set for significance. All data were analyzed using SPSS software (SPSS Version 15.0; SPSS Inc.).
Results
Analysis of pretest stiffness showed no significant difference among models (P = .490). All models failed by a gap of 2 mm at the distal fracture site except for 3 models in the MAG group. These 3 models failed at a much higher load than the remainder of the models and failed by fracture of the models.
The MAG group demonstrated significantly superior stiffness to the 2 other models tested (Figure 4). On average, this group required 753.5 N of force before failure. This was 530 N higher than the HP (P < .05) and 638 N higher than the AG constructs, respectively (P < .05). The HP and AG groups required forces of 223.2 N and 115.5 N for failure, respectively. These numbers were not significant (P= .063).
The absolute construct stiffness and construct stiffness as a function of pretest stiffness of the MAG group was the highest of all groups, 271.7 N/mm and 57.2%, respectively (Figure 5). These numbers showed significance when compared with the values of the HP group (P < .05 for both) and the AG group (P < .05 for both). The average stiffness of the HP group was 159.7 N/mm, which was 36.8% of pretest stiffness.
The AG group had the lowest construct stiffness and percent of pretest stiffness (128.1 N/mm and 29.6%). The HP and AG groups were not statistically different in these comparisons, P = .350 for construct stiffness and P = .395 for percent of pretest stiffness.
Discussion
These results support the use of a one-third tubular plate and lag-screw construct for fixation of vertical shear medial malleolus fractures. This is clinically important because one-third tubular plates with 3.5-mm screws are readily available and cost significantly less than a precountoured anatomic-specific type of fixation. These results are based on the biomechanical properties of the constructs tested in this study.
The previous 2 studies8,9showed conflicting results about the most biomechanically sound fixation for SAD medial malleolar fractures. The study by Toolan and colleagues9 reported that 2 screws placed perpendicular to the fracture demonstrated the strongest overall construct. This study compared 3 separate types of 2-screw–only fixations and 2 plate-and-screw fixations. One construct was similar to the AG group in our study, and the other construct had a lag screw at the apex of the fracture. This previous study,9 however, did not investigate a similar construct to the MAG group that was tested in our study.
According to Dumigan and associates,8 a construct that consisted of a 4-hole plate with 2 screws proximal to the fracture and 2 lag screws showed the strongest fixation. This study, however, did not include a group like our study’s AG group, which is the traditional AG form of fixation.
In our study, we examined the biomechanic properties of a traditional fixation (AG construct), a commonly used fixation (MAG construct), and a newer construct (HP construct). The HP group is unique to this study and, to our knowledge, there is no literature on its use as fixation for this fracture. We did not include a 2-screw–only group, which is a limitation, because this fixation type is not common for the SAD fracture. This study also did not include an HP construct with an additional lag screw, which is an available option as well.
The current investigation used synthetic bone models constructed for biomechanical testing. The models were thought to provide a consistent model for fixation as opposed to using potentially osteopenic cadaveric bone. Each model was the same size and laterality. The stiffness as determined by pretest stiffness was not significantly different among models. Because all models were similar in composition and size, this allowed for more consistent osteotomies and similarly sized malleolar fragments. Theoretically, this allowed a more uniform comparison of all specimens and constructs.
Using models, however, is a limit of this study. While the models were of similar biomechanical quality, it is possible that a model may not reproduce the biology of a cavaderic specimen or the physiology of a construct in vivo. Of the 2 studies that investigated SAD fractures, the Dumigan study8 used cadaveric specimens. The fact that these models were all mildly osteoporotic and were embalmed specimens were study limits. The Toolan study9 used synthetic models. Although these models were consistent, they were models of bones and not intended for biomechanical studies, thereby increasing the potential for skewed results.
Our study investigated loading only in the offset-axial direction, a difference when compared to the Dumigan and colleagues8 and Toolan and colleagues9 studies. The offest transverse loading previously investigated would most likely represent an external rotation moment. While fixation in vivo could experience an external rotation moment, the specific fracture pattern of interest fails in offset-axial loading. In the original discription of the SAD fracture, Lauge-Hanson7 stated that the talus causes the vertically oriented medial malleolar fracture in the extreme of ankle supination with an adduction moment. Considering this, we investigated failure with a force in the direction that causes this type of fracture.
There are some additional limitations. This study demonstrated superiority of a one-third tubular plate with 2 screws proximally and 1 lag screw. While this was shown in the laboratory under pure offset-axial loading conditions, this may not reproduce daily forces experienced by the constructs. Additionally, this study examined load-to-failure of the constructs and did not investigate cyclic loading that a construct would experience in vivo. Because the testing is not recognizably consistent with day-to-day stresses of these constructs in vivo, this confounds the clinical application of our study.
The stiffness required for clinical healing is undetermined and, therefore, all 3 types of fixation could be adequate clinically. Patients are typically instructed to adhere to weight-bearing limitations on the affected extremity, and casts or splints are applied postoperatively for extended periods of time. Clinical studies would have significant benefit in the evaluation of fixation of vertical shear medial malleolar fractures.
Conclusion
AG plating technique with lag-screw placement is biomechanically superior to the other 2 constructs investigated. The clinical applications of these results are not known, and clinical trials are suggested to determine the best type of fixation for SAD-type medial malleolar fractures.
Supination-adduction (SAD)-type fractures of the ankle comprise approximately 5% to 20% of ankle fractures.1-3 As the name describes, this fracture is caused by forceful adduction of the supinated foot. There are 2 stages of the fracture pattern: the injury usually occurs first on the lateral side of the ankle with injury to the soft tissues or a low transverse fracture of the distal fibula. With continued force, in the second stage, the talus causes a shearing of the medial malleolus, creating the vertical shear fracture pattern.4-7 The vertical shear medial malleolus fracture pattern is the subject of this investigation.
Several techniques have been traditionally recommended for fixation of SAD-type ankle fracture, including: a 2-screw construct without plate fixation, oriented perpendicular to the fracture; and an AG plate construct with variable positioning and numbers of screws for fixation. There have been, however, only 2 published articles about the biomechanical properties of fixation of vertical shear medial malleolar fractures, which reported conflicting results.8,9 The most recent of these studies argued that one-third tubular plate fixation offers significant mechanical advantage over screw-only fixation, supporting the use of AG plates for fixation of SAD ankle fractures.8
An additional design for fixation of medial malleolus fractures has been introduced, consisting of a hook plate (HP) contoured for the medial malleolus. To our knowledge, no studies have investigated HP’s biomechanical properties. Thus, the objective of this study was to investigate and compare the biomechanical properties of 3 constructs for fixation of SAD-ankle fractures: an antiglide (AG) plate, an AG plate with an additional lag-screw across the fracture, and a precontoured HP.
Materials and Methods
Thirty 4th-generation–composite polyurethane models of the left tibia were obtained (Sawbones, Pacific Research Laboratories, Inc.). Largely, our methods accorded with the precedent set by other studies on these fracture types.8,9
Prior to creation of the fractures, each model was individually evaluated for pretest stiffness by using the slope of the linear portion of the load-displacement curve during offset-axial loading. This demonstrated the baseline elasticity of the models during loading. Assessing pretest stiffness was performed to reduce potential variables in the stiffness of individual models in the analysis of the testing data.
The models were numbered 1 through 30 on the shaft and on the medial malleolus. A custom jig was constructed with a table saw to create identical vertical shear medial malleolar fracture patterns in each model. The jig created the vertical shear SAD fracture described by Lauge-Hansen.7 All models were randomly assigned to 1 of 3 groups; each group consisted of 10 models (Figures 1A, 1B).
The 10 specimens in group 1 were fixed with a 5-hole, 3.5-mm, one-third tubular plate (Smith & Nephew) in a traditional AG fashion. The plates were placed at the same location on all tibiae. The proximal hole and the hole closest to the fracture line were filled with 3.5-mm cortical screws, which were long enough to achieve bicortical fixation. No lag screws were placed in this specimen group. In group 2, specimens were fixed with the same plate used in group 1 (Smith & Nephew). In this modified AG (MAG) construct, specimens were fixed identically to group 1 for plate placement and fixation of the 2 proximal screws. In this group, an additional screw was placed perpendicular to the fracture and parallel to the distal tibial articular surface. In both groups (AG and MAG), the plates were not bent before application.
Group 3 consisted of specimens fixed with a 5-hole, precontoured medial malleolar HP (Arthrex). This HP construct was fixed with two 3.5-mm cortical screws long enough to achieve bicortical fixation. The plate also engaged the bone at the tip of the medial malleolus by using 2 sharp prongs. The screws were placed in the most proximal hole and the hole just proximal to the fracture line. No lag screws were placed in the HP construct.
All models were tested in offset-axial loading to replicate a SAD moment similar to previous studies. To test offset-axial loading, a vice held each model identically with a 17º angle from the longitudinal axis (Figure 2). Loading was performed with a material testing system; a material testing system plunger was directed at the inferior articulating cartilage surface of the medial malleolus. The specimens were loaded at a rate of 1 mm/sec until 2 mm of displacement was reached (Figure 3) or catastrophic failure occurred. The raw data analyzed consisted of the initial stiffness of the construct and the overall load-to-failure. The slope of the linear portion of the load-displacement curve of stiffness determined stiffness of the construct.
One-way analysis of variance with post hoc Tukey HSD data analysis was performed to determine if there were statistical differences among the different fixation constructs during load-to-failure. To prevent skewing of results by different values of model elasticity, pretest stiffness was accounted for by calculating a ratio of construct stiffness as a function of pretest model stiffness. Total force-to-failure was the recorded maximum force (in N) to cause failure. A P value of < .05 was set for significance. All data were analyzed using SPSS software (SPSS Version 15.0; SPSS Inc.).
Results
Analysis of pretest stiffness showed no significant difference among models (P = .490). All models failed by a gap of 2 mm at the distal fracture site except for 3 models in the MAG group. These 3 models failed at a much higher load than the remainder of the models and failed by fracture of the models.
The MAG group demonstrated significantly superior stiffness to the 2 other models tested (Figure 4). On average, this group required 753.5 N of force before failure. This was 530 N higher than the HP (P < .05) and 638 N higher than the AG constructs, respectively (P < .05). The HP and AG groups required forces of 223.2 N and 115.5 N for failure, respectively. These numbers were not significant (P= .063).
The absolute construct stiffness and construct stiffness as a function of pretest stiffness of the MAG group was the highest of all groups, 271.7 N/mm and 57.2%, respectively (Figure 5). These numbers showed significance when compared with the values of the HP group (P < .05 for both) and the AG group (P < .05 for both). The average stiffness of the HP group was 159.7 N/mm, which was 36.8% of pretest stiffness.
The AG group had the lowest construct stiffness and percent of pretest stiffness (128.1 N/mm and 29.6%). The HP and AG groups were not statistically different in these comparisons, P = .350 for construct stiffness and P = .395 for percent of pretest stiffness.
Discussion
These results support the use of a one-third tubular plate and lag-screw construct for fixation of vertical shear medial malleolus fractures. This is clinically important because one-third tubular plates with 3.5-mm screws are readily available and cost significantly less than a precountoured anatomic-specific type of fixation. These results are based on the biomechanical properties of the constructs tested in this study.
The previous 2 studies8,9showed conflicting results about the most biomechanically sound fixation for SAD medial malleolar fractures. The study by Toolan and colleagues9 reported that 2 screws placed perpendicular to the fracture demonstrated the strongest overall construct. This study compared 3 separate types of 2-screw–only fixations and 2 plate-and-screw fixations. One construct was similar to the AG group in our study, and the other construct had a lag screw at the apex of the fracture. This previous study,9 however, did not investigate a similar construct to the MAG group that was tested in our study.
According to Dumigan and associates,8 a construct that consisted of a 4-hole plate with 2 screws proximal to the fracture and 2 lag screws showed the strongest fixation. This study, however, did not include a group like our study’s AG group, which is the traditional AG form of fixation.
In our study, we examined the biomechanic properties of a traditional fixation (AG construct), a commonly used fixation (MAG construct), and a newer construct (HP construct). The HP group is unique to this study and, to our knowledge, there is no literature on its use as fixation for this fracture. We did not include a 2-screw–only group, which is a limitation, because this fixation type is not common for the SAD fracture. This study also did not include an HP construct with an additional lag screw, which is an available option as well.
The current investigation used synthetic bone models constructed for biomechanical testing. The models were thought to provide a consistent model for fixation as opposed to using potentially osteopenic cadaveric bone. Each model was the same size and laterality. The stiffness as determined by pretest stiffness was not significantly different among models. Because all models were similar in composition and size, this allowed for more consistent osteotomies and similarly sized malleolar fragments. Theoretically, this allowed a more uniform comparison of all specimens and constructs.
Using models, however, is a limit of this study. While the models were of similar biomechanical quality, it is possible that a model may not reproduce the biology of a cavaderic specimen or the physiology of a construct in vivo. Of the 2 studies that investigated SAD fractures, the Dumigan study8 used cadaveric specimens. The fact that these models were all mildly osteoporotic and were embalmed specimens were study limits. The Toolan study9 used synthetic models. Although these models were consistent, they were models of bones and not intended for biomechanical studies, thereby increasing the potential for skewed results.
Our study investigated loading only in the offset-axial direction, a difference when compared to the Dumigan and colleagues8 and Toolan and colleagues9 studies. The offest transverse loading previously investigated would most likely represent an external rotation moment. While fixation in vivo could experience an external rotation moment, the specific fracture pattern of interest fails in offset-axial loading. In the original discription of the SAD fracture, Lauge-Hanson7 stated that the talus causes the vertically oriented medial malleolar fracture in the extreme of ankle supination with an adduction moment. Considering this, we investigated failure with a force in the direction that causes this type of fracture.
There are some additional limitations. This study demonstrated superiority of a one-third tubular plate with 2 screws proximally and 1 lag screw. While this was shown in the laboratory under pure offset-axial loading conditions, this may not reproduce daily forces experienced by the constructs. Additionally, this study examined load-to-failure of the constructs and did not investigate cyclic loading that a construct would experience in vivo. Because the testing is not recognizably consistent with day-to-day stresses of these constructs in vivo, this confounds the clinical application of our study.
The stiffness required for clinical healing is undetermined and, therefore, all 3 types of fixation could be adequate clinically. Patients are typically instructed to adhere to weight-bearing limitations on the affected extremity, and casts or splints are applied postoperatively for extended periods of time. Clinical studies would have significant benefit in the evaluation of fixation of vertical shear medial malleolar fractures.
Conclusion
AG plating technique with lag-screw placement is biomechanically superior to the other 2 constructs investigated. The clinical applications of these results are not known, and clinical trials are suggested to determine the best type of fixation for SAD-type medial malleolar fractures.
1. Hak DJ, Egol KA, Gardner MJ, Haskell A. The “not so simple” ankle fracture: avoiding problems and pitfalls to improve patient outcomes. Instr Course Lect. 2011;60:73-88.
2. Hamilton WC. Supination-adduction injuries. In: Hamilton WC, ed. Traumatic Disorders of the Ankle. 1st ed. New York, NY: Springer-Verlag; 1984:101-112.
3. McConnell T, Tornetta P. Marginal plafond impaction in association with supination-adduction ankle fractures: a report of eight cases. J Orthop Trauma. 2001;15(6):447-449.
4. Arimoto HK, Forrester DM. Classification of ankle fractures: an algorithm. AJR Am J Roentgenol. 1980;135(5):1057-1063.
5. Carr JB. Malleolar fractures and soft tissue injuries of the ankle. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma: Basic Science, Management and Reconstruction. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:2515-2584.
6. Davidovitch RI, Egol KA. Ankle fractures. In: Bucholz RW HJ, Court-Brown CM, Tornetta P III, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2010:1975-2021.
7. Lauge-Hansen N. Fractures of the ankle. II. Combined experimental-surgical and experimental-roentgenologic investigations. Arch Surg. 1950;60(5):957-985.
8. Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691.
9. Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489.
1. Hak DJ, Egol KA, Gardner MJ, Haskell A. The “not so simple” ankle fracture: avoiding problems and pitfalls to improve patient outcomes. Instr Course Lect. 2011;60:73-88.
2. Hamilton WC. Supination-adduction injuries. In: Hamilton WC, ed. Traumatic Disorders of the Ankle. 1st ed. New York, NY: Springer-Verlag; 1984:101-112.
3. McConnell T, Tornetta P. Marginal plafond impaction in association with supination-adduction ankle fractures: a report of eight cases. J Orthop Trauma. 2001;15(6):447-449.
4. Arimoto HK, Forrester DM. Classification of ankle fractures: an algorithm. AJR Am J Roentgenol. 1980;135(5):1057-1063.
5. Carr JB. Malleolar fractures and soft tissue injuries of the ankle. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma: Basic Science, Management and Reconstruction. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:2515-2584.
6. Davidovitch RI, Egol KA. Ankle fractures. In: Bucholz RW HJ, Court-Brown CM, Tornetta P III, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2010:1975-2021.
7. Lauge-Hansen N. Fractures of the ankle. II. Combined experimental-surgical and experimental-roentgenologic investigations. Arch Surg. 1950;60(5):957-985.
8. Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691.
9. Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489.
Shoulder Instability Management: A Survey of the American Shoulder and Elbow Surgeons
Despite an abundance of peer-reviewed resources, there is wide variation in the surgical management of shoulder instability.1,2 Current American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines regarding the shoulder address only generalized shoulder pain, glenohumeral osteoarthritis, and rotator cuff injuries,3,4 and treatment algorithms focus on conservative treatment, rather than surgical recommendations.4-7
Shoulder instability most commonly results from 1 or more of 4 common lesions (capsular laxity, glenoid bone loss, humeral bone loss, and capsulolabral insufficiency).8 While it is a relatively common condition that represents 1% to 2% of all athletic injuries,9,10 little consensus exists about surgical indications, ideal treatment algorithms, or optimal operative technique. This is a critical issue because more than 50% of patients with glenohumeral instability will undergo surgical intervention.11 Chahal and associates6 surveyed 44 shoulder experts and reported strong consensus about diagnosis, but little agreement regarding surgical management. Owens and colleagues1 have also evaluated current trends for surgical treatment of this pathology. Randelli and associates5 attempted to categorize operative management based upon case-specific shoulder scenarios through online surveys. Their survey, however, covered a broad range of shoulder injuries rather than instability in particular. In this study, we assess trends for surgical management of glenohumeral instability in a case-based survey of shoulder experts.
Materials and Methods
Survey Information
An online survey (Survey Monkey) of 417 active members of the American Shoulder and Elbow Surgeons (ASES) was administered on May 1, 2014. Respondents were blinded to the institution and co-investigators conducting the survey. The survey link was distributed via email because it has been shown to be a more efficacious conduit than standard postal mail.12 The case-based, 25-question survey (Appendix) was designed to assess respondents’ selection of surgical intervention. Section 1 determined member demographics, including fellowship training, arthroscopy experience, and years of practice. Section 2 involved the presentation of 5 case scenarios. For each case, respondents were asked to identify the optimal surgical procedure in both primary and revision settings. Section 3 posed several general questions regarding shoulder-instability management.
Statistical Analysis
Data were stored using Microsoft Excel (Microsoft) and analyzed using SAS Software version 9.3 (SAS Institute, Inc.). Demographic survey responses were reported using descriptive statistics. Responses to clinical survey questions were reported using frequencies and percentages. To identify when a majority consensus was achieved for a given question, responses were flagged as reaching consensus when more than 50% of participants gave the same response.13In the event that only 2 response options were available, reaching consensus required 67% of respondents to choose a single answer (since, by default, a consensus would be reached with only 2 response options). Because this was an analysis of all respondents, an a priori power calculation was not performed. Associations between training and practice demographics and responses to clinical questions were investigated using chi-square analyses. All comparative analyses were two-tailed and used P = .05 as the threshold for statistical significance.
Results
Demographics
One hundred and twenty-five (29.9%) ASES members responded to the survey. Of the respondents, 71.2% reported at least 15 years of experience, and 71% performed more than 150 shoulder cases annually. Surgeons came from academic institutions (41.6%), private practice (24.8%), or mixed (33.6%). The majority of respondents were fellowship-trained in shoulder/elbow surgery (52.8%), while fewer had completed a sports-medicine fellowship (24.0%). For arthroscopic procedures, responses were nearly divided between those who preferred beach-chair positioning (47.2%) and those who preferred the lateral decubitus position (46.4%). The majority (70.4%) of respondents practiced in the United States and with a relatively even distribution among states and region. The remaining 29.6% of those surveyed practiced abroad.
Degree of Consensus Responses and Cases
Of the 25 survey questions, 6 questions were omitted from consensus calculations because these were designed for demographic categorization rather than professional opinion (questions 1-5, 8). Thirteen of the remaining 19 questions (68%) reached consensus response. All clinical case scenarios (5 of 5) reached consensus for selection of technique for the primary procedure; however, only 40% (2 of 5) of cases had a consensus in the revision setting.
In case 1, a young soccer player (noncontact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 81.6% of respondents. In the event of revision surgery, only 22.4% recommended arthroscopic Bankart repair, and the remainder split between open Bankart repair with possible capsular shift (36%) or Latarjet procedure (32.8%).
In case 2, a college American football player (contact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 56.8%. In the event of revision surgery, a majority of members (51.2%) suggested a Latarjet procedure.
In case 3, the weekend warrior with significant bone loss, most respondents recommended a Latarjet procedure for both primary (72.8%) and revision surgery (79.0%).
In case 4, a weekend warrior with multidirectional instability, 60% of respondents suggested arthroscopic Bankart repair, 21.6% recommended rotator interval closure, and 10.4% chose a capsular shift. As a revision procedure, there was less agreement, with a split between open Bankart repair (39.2%) and capsular shift only (39.2%).
In case 5, the weekend warrior with large engaging Hill-Sachs lesions, 60% of respondent selected a remplissage procedure. If revision was required, a Latarjet procedure was the choice of 48.8% of respondents (Table).
General Questions
For contact athletes, most respondents (87.2%) would allow return to play in the same season and recommended surgery after the end of the season. After surgical intervention, 56.8% prescribed 4 weeks of immobilization. When counseling a return to contact sports, 51.2% recommended waiting for 4 to 6 months.
The ASES members were divided on conservative management of instability injuries. Responses included immobilization in internal rotation (39.2%), no immobilization (39.2%), and external-rotation bracing (21.6%).
Finally, members thought the most important factor in choosing surgical technique was the patient’s pathology, then age; the least influential criteria was the patient’s sports participation.
Analysis of Training Demographics and Surgical Technique Preferences
Chi-square analyses demonstrated that respondents who completed a sports fellowship were more likely to do at least 50% of cases arthroscopically (odds ratio [OR], 15.3; P < .001) and were more likely to use the lateral decubitus position (OR, 2.8; P < .021). Furthermore, American respondents had a higher likelihood of having completed either a sports fellowship (OR, 12.8; P < .001) or a shoulder/elbow fellowship (OR, 4.6; P = .002) when compared with foreign respondents.
Discussion
In the absence of formal clinical practice guidelines, most surgeons formulate treatment strategy based upon a combination of experience and peer-reviewed evidence. The cohort analyzed in the current study was highly experienced, with more than 70% performing 150 shoulder cases annually and having more than 15 years of experience. We found a consensus response in 68% of questions and all primary surgical techniques for our shoulder instability scenarios. While expert consensus reported here is not equivalent to evidence-based clinical practice guidelines, it does provide important information to consider when treating anterior shoulder instability.
Specific responses to our case scenarios invite further reflection. Considering young (both noncontact and contact) athletes without bony pathology (cases 1 and 2, respectively), the ASES surgeons recommended arthroscopic Bankart repair for both. Randelli and associates5 found 71% of survey respondents recommended arthroscopic Bankart repair in a similar setting. It is interesting to note that consensus persisted regardless of the sport in which they engaged. Contact athletes have the highest rates of dislocation (up to 7 times higher incidence) compared with the general population.14 In addition, they have a higher recurrence rate after surgery.15 It should be noted, however, that although both cases reached consensus, the percentage of experts who recommended an arthroscopic procedure fell from 82% in the noncontact athlete to 57% in the contact athlete. This concurs with a recent review by Harris and Romeo,16 who recommended similar treatments for athletes without bony defects. In an older patient population with recurrent instability (case 3), responses varied more widely but still reached a consensus on primary surgical techniques. Respondents agreed that, even for patients with multidirectional instability, initial management should consist of arthroscopic capsulolabral repair. Overall, the agreement for arthroscopy for cases 1 through 3 mimics recent US practice patterns, showing 90% of stabilizations are being performed arthroscopically.17 Additionally, a recent meta-analysis by Harris and associates18 favored arthroscopic Bankart repair, showing no significant difference vs open stabilization even on long-term follow-up.
Glenoid bone loss is a difficult clinical scenario and that is reflected in this study’s findings. The literature suggests that arthroscopic Bankart repair, in this setting, is usually not sufficient and may result in a recurrence rate up to 75%, if bone loss greater than 20% is unaddressed.19 Our study supports this trend because ASES members recommended a Latarjet procedure when there is substantial bone loss.
While open Latarjet procedure was the consensus for dealing with glenoid bone loss, arthroscopic techniques were strongly favored for humeral head defects. This change in practice patterns results from the introduction of the arthroscopic remplissage technique.20 Two recent systemic reviews have supported this technique, reporting good functional outcomes for engaging Hill-Sachs lesions.21,22 Our study had similar agreement, with most respondents recommending remplissage for these patients.
This study found the lowest rates of expert consensus in the setting of revision surgery, likely caused, in part, by the paucity of available large cohort studies. This is a major void in the literature, and more studies are needed to help guide surgeons on the best techniques to deal with this difficult patient population.
Conservative bracing technique was 1 of the survey questions lacking a consensus response. Interestingly, 39% of members recommended no immobilization after an instability event. This contrasts with recent literature concerning the best position for bracing. We also found twice as many surgeons recommended internal rotation immobilization over external rotation. This is a subject of debate, with some studies stating improvement with external rotation immobilization,23 while other studies reported no difference.24 Overall, recommendations regarding type of immobilization are unclear, which will likely continue until larger studies can be performed.
The literature describing surgical trends in the treatment of shoulder instability is sparse and variable. With regard to other shoulder etiologies, only rotator cuff pathology has used expert consensus. Acevedo and colleagues13 reported agreement of ASES members surveyed regarding rotator cuff management. There was no consensus among surgeons in more than 50% of questions, despite AAOS published guidelines for rotator cuff treatment.25 Despite the lack of guidelines for our topic, we found a consensus among respondents with 68% of survey questions.
To date, only 2 studies of shoulder instability management have elicited the opinion of experts in shoulder surgery. Chahal and associates6 surveyed 42 members of ASES and JOINTS (Joined Orthopaedic Initiatives for National Trials of the Shoulder) Canada on shoulder instability cases and found substantial agreement on diagnosis but little consensus regarding surgical technique. This lack of agreement on procedures differs from our findings and may be related to their complicated case scenarios that generated a wide array of treatment recommendations. Randelli and colleagues5 surveyed more than 1000 European Society of Sports Traumatology, Knee Surgery, and Arthroscopy members and reported similar agreement on arthroscopic Bankart repair in young male shoulder-dislocation patients, although no other instability scenarios were investigated. Our study is the first to report responses from expert shoulder surgeons on surgical-treatment strategies for an array of common shoulder instability pathologies.
This study had several limitations. First, while our study suffered from a low response rate (29.9%), it was similar to other published studies.5,13 Second, because the case series included in the survey attempted to capture the most common instability scenarios, they were limited in their scope and failed to address additional etiologies or pathologic permutations. We believe, however, that a more comprehensive survey would have resulted in respondent fatigue and lowered the response rate. It is unlikely that any survey could capture all variables that come into play during clinical decision-making, and we sought to evaluate the most common shoulder instability scenarios. Third, 30% of respondents were from outside the United States, where the Latarjet procedure is much more popular. While this was not a majority, Latarjet’s regional preference may have decreased the consensus response in some scenarios if only the United States was included. Finally, there is inherent bias in a respondent pool that is heavily weighted to shoulder-surgery experts (ASES members) and does not consider the responses of the general orthopedic surgery community as have other studies.7
Conclusion
This study demonstrates that expert shoulder surgeons often agreed on shoulder-treatment principles for anterior shoulder instability. In the setting of primary repair, arthroscopic Bankart repair was favored in the absence of bony pathology, regardless of age (20 to 35 years) or nature of sport (contact versus noncontact). Latarjet procedures were favored in the setting of glenoid bone loss, and remplissage for an engaging Hill-Sachs lesion. Less agreement was observed for revision stabilization. It should be noted that, while consensus was often reached for our cases, there was a wide distribution of technical considerations and surgical preferences even among those who are fellowship-trained and high-volume surgeons, and who can be considered experts in the field of shoulder surgery.
1. Owens BD, Harrast JJ, Hurwitz SR, Thompson TL, Wolf JM. Surgical trends in bankart repair: an analysis of data from the American Board of Orthopaedic Surgery certification examination. Am J Sports Med. 2011;39(9):1865-1869.
2. Loebenberg MI, Rosen JE, Ishak C, Jazrawi LM, Zuckerman JD. A survey of decision-making processes in the treatment of common shoulder ailments among primary care physicians. Bull Hosp Jt Dis. 2006;63(3-4):137-144.
3. American Academy of Orthopaedic Surgeons. AAOS clinical practice guidelines (CPG). www.aaos.org/research/guidelines/guide.asp. Updated December 30, 2013. Accessed May 1, 2015.
4. Sanders JO, Bozic KJ, Glassman SD, Jevsevar DS, Weber KL. Clinical practice guidelines: their use, misuse, and future directions. J Am Acad Orthop Surg. 2014;22(3):135-144.
5. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2011;20(5):803-815.
6. Chahal J, Kassiri K, Dion A, MacDonald P, Leiter J. Diagnostic and treatment differences among experienced shoulder surgeons for instability conditions of the shoulder. Clin J Sport Med. 2007;17(1):5-9.
7. Redfern J, Burks R. 2009 survey results: surgeon practice patterns regarding arthroscopic surgery. Arthroscopy. 2009;25(12):1447-1452.
8. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic bankart repairs: significance of the inverted-pear glenoid and the humeral engaging hill-sachs lesion. Arthroscopy. 2000;16(7):677-694.
9. Owens BD, Agel J, Mountcastle SB, Cameron KL, Nelson BJ. Incidence of glenohumeral instability in collegiate athletics. Am J Sports Med. 2009;37(9):1750-1754.
10. Owens MBD, Duffey ML, Nelson BJ, et al. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
11. Hovelius L, Olofsson A, Sandström B, et al. Nonoperative treatment of primary anterior shoulder dislocation in patients forty years of age and younger. A prospective twenty-five-year follow-up. J Bone Joint Surg Am. 2008;90(5):945-952.
12. Raziano DB, Jayadevappa R, Valenzula D, Weiner M, Lavizzo-Mourey R. E-mail versus conventional postal mail survey of geriatric chiefs. Gerontologist. 2001;41(6):799-804.
13. Acevedo DC, Paxton ES, Williams GR, Abboud JA. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96(14):e123.
14. Kaplan LD, Flanigan DC, Norwig J, Jost P, Bradley J. Prevalence and variance of shoulder injuries in elite collegiate football players. Am J Sports Med. 2005;33(8):1142-1146.
15. Petrera M, Dwyer T, Tsuji MR, Theodoropoulos JS. Outcomes of arthroscopic Bankart repair in collision versus noncollision athletes. Orthopedics. 2013;36(5):e621-e626.
16. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
17. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the united states. Arthroscopy. 2014;30(4):436-443.
18. Harris JD, Gupta AK, Mall NA, et al. Long-term outcomes after Bankart shoulder stabilization. Arthroscopy. 2013;29(5):920-933.
19. Boileau P, Villalba M, Héry J, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88(8):1755-1763.
20. Purchase RJ, Wolf EM, Hobgood ER, Pollock ME, Smalley CC. Hill-sachs ”remplissage”: an arthroscopic solution for the engaging hill-sachs lesion. Arthroscopy. 2008;24(6):723-726.
21. Buza JA 3rd, Iyengar JJ, Anakwenze OA, Ahmad CS, Levine WN. Arthroscopic Hill-Sachs remplissage: a systematic review. J Bone Joint Surg Am. 2014;96(7):549-555.
22. Rashid MS, Crichton J, Butt U, Akimau PI, Charalambous CP. Arthroscopic “Remplissage” for shoulder instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014:1-7.
23. Itoi E, Hatakeyama Y, Kido T, et al. A new method of immobilization after traumatic anterior dislocation of the shoulder: a preliminary study. J Shoulder Elbow Surg. 2003;12(5):413-415.
24. Whelan DB, Litchfield R, Wambolt E, Dainty KN; Joint Orthopaedic Initiative for National Trials of the Shoulder (JOINTS). External rotation immobilization for primary shoulder dislocation: A randomized controlled trial. Clin Orthop Relat Res. 2014;472(8):2380-2386.
25. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
Despite an abundance of peer-reviewed resources, there is wide variation in the surgical management of shoulder instability.1,2 Current American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines regarding the shoulder address only generalized shoulder pain, glenohumeral osteoarthritis, and rotator cuff injuries,3,4 and treatment algorithms focus on conservative treatment, rather than surgical recommendations.4-7
Shoulder instability most commonly results from 1 or more of 4 common lesions (capsular laxity, glenoid bone loss, humeral bone loss, and capsulolabral insufficiency).8 While it is a relatively common condition that represents 1% to 2% of all athletic injuries,9,10 little consensus exists about surgical indications, ideal treatment algorithms, or optimal operative technique. This is a critical issue because more than 50% of patients with glenohumeral instability will undergo surgical intervention.11 Chahal and associates6 surveyed 44 shoulder experts and reported strong consensus about diagnosis, but little agreement regarding surgical management. Owens and colleagues1 have also evaluated current trends for surgical treatment of this pathology. Randelli and associates5 attempted to categorize operative management based upon case-specific shoulder scenarios through online surveys. Their survey, however, covered a broad range of shoulder injuries rather than instability in particular. In this study, we assess trends for surgical management of glenohumeral instability in a case-based survey of shoulder experts.
Materials and Methods
Survey Information
An online survey (Survey Monkey) of 417 active members of the American Shoulder and Elbow Surgeons (ASES) was administered on May 1, 2014. Respondents were blinded to the institution and co-investigators conducting the survey. The survey link was distributed via email because it has been shown to be a more efficacious conduit than standard postal mail.12 The case-based, 25-question survey (Appendix) was designed to assess respondents’ selection of surgical intervention. Section 1 determined member demographics, including fellowship training, arthroscopy experience, and years of practice. Section 2 involved the presentation of 5 case scenarios. For each case, respondents were asked to identify the optimal surgical procedure in both primary and revision settings. Section 3 posed several general questions regarding shoulder-instability management.
Statistical Analysis
Data were stored using Microsoft Excel (Microsoft) and analyzed using SAS Software version 9.3 (SAS Institute, Inc.). Demographic survey responses were reported using descriptive statistics. Responses to clinical survey questions were reported using frequencies and percentages. To identify when a majority consensus was achieved for a given question, responses were flagged as reaching consensus when more than 50% of participants gave the same response.13In the event that only 2 response options were available, reaching consensus required 67% of respondents to choose a single answer (since, by default, a consensus would be reached with only 2 response options). Because this was an analysis of all respondents, an a priori power calculation was not performed. Associations between training and practice demographics and responses to clinical questions were investigated using chi-square analyses. All comparative analyses were two-tailed and used P = .05 as the threshold for statistical significance.
Results
Demographics
One hundred and twenty-five (29.9%) ASES members responded to the survey. Of the respondents, 71.2% reported at least 15 years of experience, and 71% performed more than 150 shoulder cases annually. Surgeons came from academic institutions (41.6%), private practice (24.8%), or mixed (33.6%). The majority of respondents were fellowship-trained in shoulder/elbow surgery (52.8%), while fewer had completed a sports-medicine fellowship (24.0%). For arthroscopic procedures, responses were nearly divided between those who preferred beach-chair positioning (47.2%) and those who preferred the lateral decubitus position (46.4%). The majority (70.4%) of respondents practiced in the United States and with a relatively even distribution among states and region. The remaining 29.6% of those surveyed practiced abroad.
Degree of Consensus Responses and Cases
Of the 25 survey questions, 6 questions were omitted from consensus calculations because these were designed for demographic categorization rather than professional opinion (questions 1-5, 8). Thirteen of the remaining 19 questions (68%) reached consensus response. All clinical case scenarios (5 of 5) reached consensus for selection of technique for the primary procedure; however, only 40% (2 of 5) of cases had a consensus in the revision setting.
In case 1, a young soccer player (noncontact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 81.6% of respondents. In the event of revision surgery, only 22.4% recommended arthroscopic Bankart repair, and the remainder split between open Bankart repair with possible capsular shift (36%) or Latarjet procedure (32.8%).
In case 2, a college American football player (contact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 56.8%. In the event of revision surgery, a majority of members (51.2%) suggested a Latarjet procedure.
In case 3, the weekend warrior with significant bone loss, most respondents recommended a Latarjet procedure for both primary (72.8%) and revision surgery (79.0%).
In case 4, a weekend warrior with multidirectional instability, 60% of respondents suggested arthroscopic Bankart repair, 21.6% recommended rotator interval closure, and 10.4% chose a capsular shift. As a revision procedure, there was less agreement, with a split between open Bankart repair (39.2%) and capsular shift only (39.2%).
In case 5, the weekend warrior with large engaging Hill-Sachs lesions, 60% of respondent selected a remplissage procedure. If revision was required, a Latarjet procedure was the choice of 48.8% of respondents (Table).
General Questions
For contact athletes, most respondents (87.2%) would allow return to play in the same season and recommended surgery after the end of the season. After surgical intervention, 56.8% prescribed 4 weeks of immobilization. When counseling a return to contact sports, 51.2% recommended waiting for 4 to 6 months.
The ASES members were divided on conservative management of instability injuries. Responses included immobilization in internal rotation (39.2%), no immobilization (39.2%), and external-rotation bracing (21.6%).
Finally, members thought the most important factor in choosing surgical technique was the patient’s pathology, then age; the least influential criteria was the patient’s sports participation.
Analysis of Training Demographics and Surgical Technique Preferences
Chi-square analyses demonstrated that respondents who completed a sports fellowship were more likely to do at least 50% of cases arthroscopically (odds ratio [OR], 15.3; P < .001) and were more likely to use the lateral decubitus position (OR, 2.8; P < .021). Furthermore, American respondents had a higher likelihood of having completed either a sports fellowship (OR, 12.8; P < .001) or a shoulder/elbow fellowship (OR, 4.6; P = .002) when compared with foreign respondents.
Discussion
In the absence of formal clinical practice guidelines, most surgeons formulate treatment strategy based upon a combination of experience and peer-reviewed evidence. The cohort analyzed in the current study was highly experienced, with more than 70% performing 150 shoulder cases annually and having more than 15 years of experience. We found a consensus response in 68% of questions and all primary surgical techniques for our shoulder instability scenarios. While expert consensus reported here is not equivalent to evidence-based clinical practice guidelines, it does provide important information to consider when treating anterior shoulder instability.
Specific responses to our case scenarios invite further reflection. Considering young (both noncontact and contact) athletes without bony pathology (cases 1 and 2, respectively), the ASES surgeons recommended arthroscopic Bankart repair for both. Randelli and associates5 found 71% of survey respondents recommended arthroscopic Bankart repair in a similar setting. It is interesting to note that consensus persisted regardless of the sport in which they engaged. Contact athletes have the highest rates of dislocation (up to 7 times higher incidence) compared with the general population.14 In addition, they have a higher recurrence rate after surgery.15 It should be noted, however, that although both cases reached consensus, the percentage of experts who recommended an arthroscopic procedure fell from 82% in the noncontact athlete to 57% in the contact athlete. This concurs with a recent review by Harris and Romeo,16 who recommended similar treatments for athletes without bony defects. In an older patient population with recurrent instability (case 3), responses varied more widely but still reached a consensus on primary surgical techniques. Respondents agreed that, even for patients with multidirectional instability, initial management should consist of arthroscopic capsulolabral repair. Overall, the agreement for arthroscopy for cases 1 through 3 mimics recent US practice patterns, showing 90% of stabilizations are being performed arthroscopically.17 Additionally, a recent meta-analysis by Harris and associates18 favored arthroscopic Bankart repair, showing no significant difference vs open stabilization even on long-term follow-up.
Glenoid bone loss is a difficult clinical scenario and that is reflected in this study’s findings. The literature suggests that arthroscopic Bankart repair, in this setting, is usually not sufficient and may result in a recurrence rate up to 75%, if bone loss greater than 20% is unaddressed.19 Our study supports this trend because ASES members recommended a Latarjet procedure when there is substantial bone loss.
While open Latarjet procedure was the consensus for dealing with glenoid bone loss, arthroscopic techniques were strongly favored for humeral head defects. This change in practice patterns results from the introduction of the arthroscopic remplissage technique.20 Two recent systemic reviews have supported this technique, reporting good functional outcomes for engaging Hill-Sachs lesions.21,22 Our study had similar agreement, with most respondents recommending remplissage for these patients.
This study found the lowest rates of expert consensus in the setting of revision surgery, likely caused, in part, by the paucity of available large cohort studies. This is a major void in the literature, and more studies are needed to help guide surgeons on the best techniques to deal with this difficult patient population.
Conservative bracing technique was 1 of the survey questions lacking a consensus response. Interestingly, 39% of members recommended no immobilization after an instability event. This contrasts with recent literature concerning the best position for bracing. We also found twice as many surgeons recommended internal rotation immobilization over external rotation. This is a subject of debate, with some studies stating improvement with external rotation immobilization,23 while other studies reported no difference.24 Overall, recommendations regarding type of immobilization are unclear, which will likely continue until larger studies can be performed.
The literature describing surgical trends in the treatment of shoulder instability is sparse and variable. With regard to other shoulder etiologies, only rotator cuff pathology has used expert consensus. Acevedo and colleagues13 reported agreement of ASES members surveyed regarding rotator cuff management. There was no consensus among surgeons in more than 50% of questions, despite AAOS published guidelines for rotator cuff treatment.25 Despite the lack of guidelines for our topic, we found a consensus among respondents with 68% of survey questions.
To date, only 2 studies of shoulder instability management have elicited the opinion of experts in shoulder surgery. Chahal and associates6 surveyed 42 members of ASES and JOINTS (Joined Orthopaedic Initiatives for National Trials of the Shoulder) Canada on shoulder instability cases and found substantial agreement on diagnosis but little consensus regarding surgical technique. This lack of agreement on procedures differs from our findings and may be related to their complicated case scenarios that generated a wide array of treatment recommendations. Randelli and colleagues5 surveyed more than 1000 European Society of Sports Traumatology, Knee Surgery, and Arthroscopy members and reported similar agreement on arthroscopic Bankart repair in young male shoulder-dislocation patients, although no other instability scenarios were investigated. Our study is the first to report responses from expert shoulder surgeons on surgical-treatment strategies for an array of common shoulder instability pathologies.
This study had several limitations. First, while our study suffered from a low response rate (29.9%), it was similar to other published studies.5,13 Second, because the case series included in the survey attempted to capture the most common instability scenarios, they were limited in their scope and failed to address additional etiologies or pathologic permutations. We believe, however, that a more comprehensive survey would have resulted in respondent fatigue and lowered the response rate. It is unlikely that any survey could capture all variables that come into play during clinical decision-making, and we sought to evaluate the most common shoulder instability scenarios. Third, 30% of respondents were from outside the United States, where the Latarjet procedure is much more popular. While this was not a majority, Latarjet’s regional preference may have decreased the consensus response in some scenarios if only the United States was included. Finally, there is inherent bias in a respondent pool that is heavily weighted to shoulder-surgery experts (ASES members) and does not consider the responses of the general orthopedic surgery community as have other studies.7
Conclusion
This study demonstrates that expert shoulder surgeons often agreed on shoulder-treatment principles for anterior shoulder instability. In the setting of primary repair, arthroscopic Bankart repair was favored in the absence of bony pathology, regardless of age (20 to 35 years) or nature of sport (contact versus noncontact). Latarjet procedures were favored in the setting of glenoid bone loss, and remplissage for an engaging Hill-Sachs lesion. Less agreement was observed for revision stabilization. It should be noted that, while consensus was often reached for our cases, there was a wide distribution of technical considerations and surgical preferences even among those who are fellowship-trained and high-volume surgeons, and who can be considered experts in the field of shoulder surgery.
Despite an abundance of peer-reviewed resources, there is wide variation in the surgical management of shoulder instability.1,2 Current American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines regarding the shoulder address only generalized shoulder pain, glenohumeral osteoarthritis, and rotator cuff injuries,3,4 and treatment algorithms focus on conservative treatment, rather than surgical recommendations.4-7
Shoulder instability most commonly results from 1 or more of 4 common lesions (capsular laxity, glenoid bone loss, humeral bone loss, and capsulolabral insufficiency).8 While it is a relatively common condition that represents 1% to 2% of all athletic injuries,9,10 little consensus exists about surgical indications, ideal treatment algorithms, or optimal operative technique. This is a critical issue because more than 50% of patients with glenohumeral instability will undergo surgical intervention.11 Chahal and associates6 surveyed 44 shoulder experts and reported strong consensus about diagnosis, but little agreement regarding surgical management. Owens and colleagues1 have also evaluated current trends for surgical treatment of this pathology. Randelli and associates5 attempted to categorize operative management based upon case-specific shoulder scenarios through online surveys. Their survey, however, covered a broad range of shoulder injuries rather than instability in particular. In this study, we assess trends for surgical management of glenohumeral instability in a case-based survey of shoulder experts.
Materials and Methods
Survey Information
An online survey (Survey Monkey) of 417 active members of the American Shoulder and Elbow Surgeons (ASES) was administered on May 1, 2014. Respondents were blinded to the institution and co-investigators conducting the survey. The survey link was distributed via email because it has been shown to be a more efficacious conduit than standard postal mail.12 The case-based, 25-question survey (Appendix) was designed to assess respondents’ selection of surgical intervention. Section 1 determined member demographics, including fellowship training, arthroscopy experience, and years of practice. Section 2 involved the presentation of 5 case scenarios. For each case, respondents were asked to identify the optimal surgical procedure in both primary and revision settings. Section 3 posed several general questions regarding shoulder-instability management.
Statistical Analysis
Data were stored using Microsoft Excel (Microsoft) and analyzed using SAS Software version 9.3 (SAS Institute, Inc.). Demographic survey responses were reported using descriptive statistics. Responses to clinical survey questions were reported using frequencies and percentages. To identify when a majority consensus was achieved for a given question, responses were flagged as reaching consensus when more than 50% of participants gave the same response.13In the event that only 2 response options were available, reaching consensus required 67% of respondents to choose a single answer (since, by default, a consensus would be reached with only 2 response options). Because this was an analysis of all respondents, an a priori power calculation was not performed. Associations between training and practice demographics and responses to clinical questions were investigated using chi-square analyses. All comparative analyses were two-tailed and used P = .05 as the threshold for statistical significance.
Results
Demographics
One hundred and twenty-five (29.9%) ASES members responded to the survey. Of the respondents, 71.2% reported at least 15 years of experience, and 71% performed more than 150 shoulder cases annually. Surgeons came from academic institutions (41.6%), private practice (24.8%), or mixed (33.6%). The majority of respondents were fellowship-trained in shoulder/elbow surgery (52.8%), while fewer had completed a sports-medicine fellowship (24.0%). For arthroscopic procedures, responses were nearly divided between those who preferred beach-chair positioning (47.2%) and those who preferred the lateral decubitus position (46.4%). The majority (70.4%) of respondents practiced in the United States and with a relatively even distribution among states and region. The remaining 29.6% of those surveyed practiced abroad.
Degree of Consensus Responses and Cases
Of the 25 survey questions, 6 questions were omitted from consensus calculations because these were designed for demographic categorization rather than professional opinion (questions 1-5, 8). Thirteen of the remaining 19 questions (68%) reached consensus response. All clinical case scenarios (5 of 5) reached consensus for selection of technique for the primary procedure; however, only 40% (2 of 5) of cases had a consensus in the revision setting.
In case 1, a young soccer player (noncontact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 81.6% of respondents. In the event of revision surgery, only 22.4% recommended arthroscopic Bankart repair, and the remainder split between open Bankart repair with possible capsular shift (36%) or Latarjet procedure (32.8%).
In case 2, a college American football player (contact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 56.8%. In the event of revision surgery, a majority of members (51.2%) suggested a Latarjet procedure.
In case 3, the weekend warrior with significant bone loss, most respondents recommended a Latarjet procedure for both primary (72.8%) and revision surgery (79.0%).
In case 4, a weekend warrior with multidirectional instability, 60% of respondents suggested arthroscopic Bankart repair, 21.6% recommended rotator interval closure, and 10.4% chose a capsular shift. As a revision procedure, there was less agreement, with a split between open Bankart repair (39.2%) and capsular shift only (39.2%).
In case 5, the weekend warrior with large engaging Hill-Sachs lesions, 60% of respondent selected a remplissage procedure. If revision was required, a Latarjet procedure was the choice of 48.8% of respondents (Table).
General Questions
For contact athletes, most respondents (87.2%) would allow return to play in the same season and recommended surgery after the end of the season. After surgical intervention, 56.8% prescribed 4 weeks of immobilization. When counseling a return to contact sports, 51.2% recommended waiting for 4 to 6 months.
The ASES members were divided on conservative management of instability injuries. Responses included immobilization in internal rotation (39.2%), no immobilization (39.2%), and external-rotation bracing (21.6%).
Finally, members thought the most important factor in choosing surgical technique was the patient’s pathology, then age; the least influential criteria was the patient’s sports participation.
Analysis of Training Demographics and Surgical Technique Preferences
Chi-square analyses demonstrated that respondents who completed a sports fellowship were more likely to do at least 50% of cases arthroscopically (odds ratio [OR], 15.3; P < .001) and were more likely to use the lateral decubitus position (OR, 2.8; P < .021). Furthermore, American respondents had a higher likelihood of having completed either a sports fellowship (OR, 12.8; P < .001) or a shoulder/elbow fellowship (OR, 4.6; P = .002) when compared with foreign respondents.
Discussion
In the absence of formal clinical practice guidelines, most surgeons formulate treatment strategy based upon a combination of experience and peer-reviewed evidence. The cohort analyzed in the current study was highly experienced, with more than 70% performing 150 shoulder cases annually and having more than 15 years of experience. We found a consensus response in 68% of questions and all primary surgical techniques for our shoulder instability scenarios. While expert consensus reported here is not equivalent to evidence-based clinical practice guidelines, it does provide important information to consider when treating anterior shoulder instability.
Specific responses to our case scenarios invite further reflection. Considering young (both noncontact and contact) athletes without bony pathology (cases 1 and 2, respectively), the ASES surgeons recommended arthroscopic Bankart repair for both. Randelli and associates5 found 71% of survey respondents recommended arthroscopic Bankart repair in a similar setting. It is interesting to note that consensus persisted regardless of the sport in which they engaged. Contact athletes have the highest rates of dislocation (up to 7 times higher incidence) compared with the general population.14 In addition, they have a higher recurrence rate after surgery.15 It should be noted, however, that although both cases reached consensus, the percentage of experts who recommended an arthroscopic procedure fell from 82% in the noncontact athlete to 57% in the contact athlete. This concurs with a recent review by Harris and Romeo,16 who recommended similar treatments for athletes without bony defects. In an older patient population with recurrent instability (case 3), responses varied more widely but still reached a consensus on primary surgical techniques. Respondents agreed that, even for patients with multidirectional instability, initial management should consist of arthroscopic capsulolabral repair. Overall, the agreement for arthroscopy for cases 1 through 3 mimics recent US practice patterns, showing 90% of stabilizations are being performed arthroscopically.17 Additionally, a recent meta-analysis by Harris and associates18 favored arthroscopic Bankart repair, showing no significant difference vs open stabilization even on long-term follow-up.
Glenoid bone loss is a difficult clinical scenario and that is reflected in this study’s findings. The literature suggests that arthroscopic Bankart repair, in this setting, is usually not sufficient and may result in a recurrence rate up to 75%, if bone loss greater than 20% is unaddressed.19 Our study supports this trend because ASES members recommended a Latarjet procedure when there is substantial bone loss.
While open Latarjet procedure was the consensus for dealing with glenoid bone loss, arthroscopic techniques were strongly favored for humeral head defects. This change in practice patterns results from the introduction of the arthroscopic remplissage technique.20 Two recent systemic reviews have supported this technique, reporting good functional outcomes for engaging Hill-Sachs lesions.21,22 Our study had similar agreement, with most respondents recommending remplissage for these patients.
This study found the lowest rates of expert consensus in the setting of revision surgery, likely caused, in part, by the paucity of available large cohort studies. This is a major void in the literature, and more studies are needed to help guide surgeons on the best techniques to deal with this difficult patient population.
Conservative bracing technique was 1 of the survey questions lacking a consensus response. Interestingly, 39% of members recommended no immobilization after an instability event. This contrasts with recent literature concerning the best position for bracing. We also found twice as many surgeons recommended internal rotation immobilization over external rotation. This is a subject of debate, with some studies stating improvement with external rotation immobilization,23 while other studies reported no difference.24 Overall, recommendations regarding type of immobilization are unclear, which will likely continue until larger studies can be performed.
The literature describing surgical trends in the treatment of shoulder instability is sparse and variable. With regard to other shoulder etiologies, only rotator cuff pathology has used expert consensus. Acevedo and colleagues13 reported agreement of ASES members surveyed regarding rotator cuff management. There was no consensus among surgeons in more than 50% of questions, despite AAOS published guidelines for rotator cuff treatment.25 Despite the lack of guidelines for our topic, we found a consensus among respondents with 68% of survey questions.
To date, only 2 studies of shoulder instability management have elicited the opinion of experts in shoulder surgery. Chahal and associates6 surveyed 42 members of ASES and JOINTS (Joined Orthopaedic Initiatives for National Trials of the Shoulder) Canada on shoulder instability cases and found substantial agreement on diagnosis but little consensus regarding surgical technique. This lack of agreement on procedures differs from our findings and may be related to their complicated case scenarios that generated a wide array of treatment recommendations. Randelli and colleagues5 surveyed more than 1000 European Society of Sports Traumatology, Knee Surgery, and Arthroscopy members and reported similar agreement on arthroscopic Bankart repair in young male shoulder-dislocation patients, although no other instability scenarios were investigated. Our study is the first to report responses from expert shoulder surgeons on surgical-treatment strategies for an array of common shoulder instability pathologies.
This study had several limitations. First, while our study suffered from a low response rate (29.9%), it was similar to other published studies.5,13 Second, because the case series included in the survey attempted to capture the most common instability scenarios, they were limited in their scope and failed to address additional etiologies or pathologic permutations. We believe, however, that a more comprehensive survey would have resulted in respondent fatigue and lowered the response rate. It is unlikely that any survey could capture all variables that come into play during clinical decision-making, and we sought to evaluate the most common shoulder instability scenarios. Third, 30% of respondents were from outside the United States, where the Latarjet procedure is much more popular. While this was not a majority, Latarjet’s regional preference may have decreased the consensus response in some scenarios if only the United States was included. Finally, there is inherent bias in a respondent pool that is heavily weighted to shoulder-surgery experts (ASES members) and does not consider the responses of the general orthopedic surgery community as have other studies.7
Conclusion
This study demonstrates that expert shoulder surgeons often agreed on shoulder-treatment principles for anterior shoulder instability. In the setting of primary repair, arthroscopic Bankart repair was favored in the absence of bony pathology, regardless of age (20 to 35 years) or nature of sport (contact versus noncontact). Latarjet procedures were favored in the setting of glenoid bone loss, and remplissage for an engaging Hill-Sachs lesion. Less agreement was observed for revision stabilization. It should be noted that, while consensus was often reached for our cases, there was a wide distribution of technical considerations and surgical preferences even among those who are fellowship-trained and high-volume surgeons, and who can be considered experts in the field of shoulder surgery.
1. Owens BD, Harrast JJ, Hurwitz SR, Thompson TL, Wolf JM. Surgical trends in bankart repair: an analysis of data from the American Board of Orthopaedic Surgery certification examination. Am J Sports Med. 2011;39(9):1865-1869.
2. Loebenberg MI, Rosen JE, Ishak C, Jazrawi LM, Zuckerman JD. A survey of decision-making processes in the treatment of common shoulder ailments among primary care physicians. Bull Hosp Jt Dis. 2006;63(3-4):137-144.
3. American Academy of Orthopaedic Surgeons. AAOS clinical practice guidelines (CPG). www.aaos.org/research/guidelines/guide.asp. Updated December 30, 2013. Accessed May 1, 2015.
4. Sanders JO, Bozic KJ, Glassman SD, Jevsevar DS, Weber KL. Clinical practice guidelines: their use, misuse, and future directions. J Am Acad Orthop Surg. 2014;22(3):135-144.
5. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2011;20(5):803-815.
6. Chahal J, Kassiri K, Dion A, MacDonald P, Leiter J. Diagnostic and treatment differences among experienced shoulder surgeons for instability conditions of the shoulder. Clin J Sport Med. 2007;17(1):5-9.
7. Redfern J, Burks R. 2009 survey results: surgeon practice patterns regarding arthroscopic surgery. Arthroscopy. 2009;25(12):1447-1452.
8. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic bankart repairs: significance of the inverted-pear glenoid and the humeral engaging hill-sachs lesion. Arthroscopy. 2000;16(7):677-694.
9. Owens BD, Agel J, Mountcastle SB, Cameron KL, Nelson BJ. Incidence of glenohumeral instability in collegiate athletics. Am J Sports Med. 2009;37(9):1750-1754.
10. Owens MBD, Duffey ML, Nelson BJ, et al. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
11. Hovelius L, Olofsson A, Sandström B, et al. Nonoperative treatment of primary anterior shoulder dislocation in patients forty years of age and younger. A prospective twenty-five-year follow-up. J Bone Joint Surg Am. 2008;90(5):945-952.
12. Raziano DB, Jayadevappa R, Valenzula D, Weiner M, Lavizzo-Mourey R. E-mail versus conventional postal mail survey of geriatric chiefs. Gerontologist. 2001;41(6):799-804.
13. Acevedo DC, Paxton ES, Williams GR, Abboud JA. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96(14):e123.
14. Kaplan LD, Flanigan DC, Norwig J, Jost P, Bradley J. Prevalence and variance of shoulder injuries in elite collegiate football players. Am J Sports Med. 2005;33(8):1142-1146.
15. Petrera M, Dwyer T, Tsuji MR, Theodoropoulos JS. Outcomes of arthroscopic Bankart repair in collision versus noncollision athletes. Orthopedics. 2013;36(5):e621-e626.
16. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
17. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the united states. Arthroscopy. 2014;30(4):436-443.
18. Harris JD, Gupta AK, Mall NA, et al. Long-term outcomes after Bankart shoulder stabilization. Arthroscopy. 2013;29(5):920-933.
19. Boileau P, Villalba M, Héry J, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88(8):1755-1763.
20. Purchase RJ, Wolf EM, Hobgood ER, Pollock ME, Smalley CC. Hill-sachs ”remplissage”: an arthroscopic solution for the engaging hill-sachs lesion. Arthroscopy. 2008;24(6):723-726.
21. Buza JA 3rd, Iyengar JJ, Anakwenze OA, Ahmad CS, Levine WN. Arthroscopic Hill-Sachs remplissage: a systematic review. J Bone Joint Surg Am. 2014;96(7):549-555.
22. Rashid MS, Crichton J, Butt U, Akimau PI, Charalambous CP. Arthroscopic “Remplissage” for shoulder instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014:1-7.
23. Itoi E, Hatakeyama Y, Kido T, et al. A new method of immobilization after traumatic anterior dislocation of the shoulder: a preliminary study. J Shoulder Elbow Surg. 2003;12(5):413-415.
24. Whelan DB, Litchfield R, Wambolt E, Dainty KN; Joint Orthopaedic Initiative for National Trials of the Shoulder (JOINTS). External rotation immobilization for primary shoulder dislocation: A randomized controlled trial. Clin Orthop Relat Res. 2014;472(8):2380-2386.
25. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
1. Owens BD, Harrast JJ, Hurwitz SR, Thompson TL, Wolf JM. Surgical trends in bankart repair: an analysis of data from the American Board of Orthopaedic Surgery certification examination. Am J Sports Med. 2011;39(9):1865-1869.
2. Loebenberg MI, Rosen JE, Ishak C, Jazrawi LM, Zuckerman JD. A survey of decision-making processes in the treatment of common shoulder ailments among primary care physicians. Bull Hosp Jt Dis. 2006;63(3-4):137-144.
3. American Academy of Orthopaedic Surgeons. AAOS clinical practice guidelines (CPG). www.aaos.org/research/guidelines/guide.asp. Updated December 30, 2013. Accessed May 1, 2015.
4. Sanders JO, Bozic KJ, Glassman SD, Jevsevar DS, Weber KL. Clinical practice guidelines: their use, misuse, and future directions. J Am Acad Orthop Surg. 2014;22(3):135-144.
5. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2011;20(5):803-815.
6. Chahal J, Kassiri K, Dion A, MacDonald P, Leiter J. Diagnostic and treatment differences among experienced shoulder surgeons for instability conditions of the shoulder. Clin J Sport Med. 2007;17(1):5-9.
7. Redfern J, Burks R. 2009 survey results: surgeon practice patterns regarding arthroscopic surgery. Arthroscopy. 2009;25(12):1447-1452.
8. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic bankart repairs: significance of the inverted-pear glenoid and the humeral engaging hill-sachs lesion. Arthroscopy. 2000;16(7):677-694.
9. Owens BD, Agel J, Mountcastle SB, Cameron KL, Nelson BJ. Incidence of glenohumeral instability in collegiate athletics. Am J Sports Med. 2009;37(9):1750-1754.
10. Owens MBD, Duffey ML, Nelson BJ, et al. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
11. Hovelius L, Olofsson A, Sandström B, et al. Nonoperative treatment of primary anterior shoulder dislocation in patients forty years of age and younger. A prospective twenty-five-year follow-up. J Bone Joint Surg Am. 2008;90(5):945-952.
12. Raziano DB, Jayadevappa R, Valenzula D, Weiner M, Lavizzo-Mourey R. E-mail versus conventional postal mail survey of geriatric chiefs. Gerontologist. 2001;41(6):799-804.
13. Acevedo DC, Paxton ES, Williams GR, Abboud JA. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96(14):e123.
14. Kaplan LD, Flanigan DC, Norwig J, Jost P, Bradley J. Prevalence and variance of shoulder injuries in elite collegiate football players. Am J Sports Med. 2005;33(8):1142-1146.
15. Petrera M, Dwyer T, Tsuji MR, Theodoropoulos JS. Outcomes of arthroscopic Bankart repair in collision versus noncollision athletes. Orthopedics. 2013;36(5):e621-e626.
16. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
17. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the united states. Arthroscopy. 2014;30(4):436-443.
18. Harris JD, Gupta AK, Mall NA, et al. Long-term outcomes after Bankart shoulder stabilization. Arthroscopy. 2013;29(5):920-933.
19. Boileau P, Villalba M, Héry J, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88(8):1755-1763.
20. Purchase RJ, Wolf EM, Hobgood ER, Pollock ME, Smalley CC. Hill-sachs ”remplissage”: an arthroscopic solution for the engaging hill-sachs lesion. Arthroscopy. 2008;24(6):723-726.
21. Buza JA 3rd, Iyengar JJ, Anakwenze OA, Ahmad CS, Levine WN. Arthroscopic Hill-Sachs remplissage: a systematic review. J Bone Joint Surg Am. 2014;96(7):549-555.
22. Rashid MS, Crichton J, Butt U, Akimau PI, Charalambous CP. Arthroscopic “Remplissage” for shoulder instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014:1-7.
23. Itoi E, Hatakeyama Y, Kido T, et al. A new method of immobilization after traumatic anterior dislocation of the shoulder: a preliminary study. J Shoulder Elbow Surg. 2003;12(5):413-415.
24. Whelan DB, Litchfield R, Wambolt E, Dainty KN; Joint Orthopaedic Initiative for National Trials of the Shoulder (JOINTS). External rotation immobilization for primary shoulder dislocation: A randomized controlled trial. Clin Orthop Relat Res. 2014;472(8):2380-2386.
25. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
In Vivo Measurement of Rotator Cuff Tear Tension: Medial Versus Lateral Footprint Position
Although recent clinical results of arthroscopic rotator cuff repair (RCR) have been encouraging, achieving anatomical healing of full-thickness rotator cuff tears remains a challenge.1-4 Several factors influence rotator cuff healing after repair.1,3-8 Patient-related factors include advanced patient age, tear size, tear chronicity, and amount of fatty infiltration.1,3,5,6,8-10 Tension applied to the repair construct is a significant factor as well.11,12
In the literature, limited consideration has been given to repair tension.13 The majority of studies have focused on other factors, mainly repair technique. Some surgeons advocate use of a double-row repair construct in which the rotator cuff tendon is pulled to the lateral margin of the footprint.14-19 Double-row techniques, which include the transosseous-equivalent (TOE) construct, are biomechanically superior to other repairs.20-26 Another purported benefit of double-row repair is more complete restoration and pressurization of the rotator cuff footprint.21,24,27,28
Rotator cuff tears typically occur near the dysvascular region of the diseased musculotendinous unit, often leaving a stump of tissue attached to the tuberosity and ultimately a shortened tendon.29 In addition, full-thickness tears often retract over time. Meyer and colleagues29 recently demonstrated that this shortening is irreversible. Snyder30 and Sostak and colleagues31 suggested that pulling a shortened, degenerative rotator cuff tendon to the lateral margin of the footprint results in increased tissue tension compared with that produced with a more medially based repair just off the articular margin. In our opinion, the possible increase in tension during a laterally based repair, whether single- or double-row, may place excessive strain on the diseased tissue as well as the surgical construct, potentially contributing to repair failure.
We conducted a study to evaluate the difference, if any, in tension applied to the rotator cuff tendon positioned at the medial versus lateral margin of the footprint during arthroscopic RCR. We hypothesized significantly more tension would be placed on the rotator cuff tendon when positioned at the lateral versus medial footprint.
Methods
After obtaining Institutional Review Board approval for this study, we collected data on a consecutive series of patients who underwent arthroscopic RCR performed by Dr. Getelman at a single institution. Only patients with primary full-thickness tears of the supraspinatus and/or infraspinatus were included. Exclusion criteria included revision rotator cuff surgeries, partial-thickness tears, concurrent subscapularis tears requiring anchor fixation, and any tears that could not be mobilized to the lateral footprint without interval slides or margin convergence. The 20 identified patients constituted the study group.
Demographic factors, including age and preoperative length of symptoms, were recorded after chart review. Magnetic resonance imaging (MRI) was performed for all patients before surgery and was retrospectively reviewed. Dr. Getelman assigned each patient a modified Goutallier score, based on MRI, to assess for fatty infiltration/atrophy.32 Each patient was placed in the lateral decubitus position with the operative arm in balanced suspension at 70° of abduction. Standard glenohumeral and subacromial diagnostic arthroscopy was performed. The rotator cuff tear was gently debrided back to a healthy-appearing margin in preparation for repair. The tear was then measured in the anterior-posterior (A-P) and medial-lateral (M-L) planes using a premeasured, marked suture, as previously described.33 Complete bursal and articular-sided releases were performed to allow for appropriate mobilization of the tendon. The tear was classified as crescent-shaped, U-shaped, or L-shaped.
Viewing from the posterior portal, the surgeon inserted a tissue grasper through the lateral portal. The tendon was grasped at multiple points along its edge, anterior to posterior, and was translated laterally to assess its reducibility; the apex of the tear correlated with the point of maximal excursion and coverage of the footprint. Once confirmed, the rotator cuff tear apex was clamped with a tissue grasper. After placement in a sterile arthroscopic camera sleeve (DeRoyal camera drape with perforated tip), a calibrated digital weigh scale (American Weigh Scales model H22 portable electronic hanging scale, with accuracy of 0.01 lb) was attached to the tissue grasper with an S-hook (Figure 1). The tendon edge was first translated about 3 mm lateral to the articular margin (the medial footprint position), and tension was recorded (Figures 2A, 2B). After a 1-minute relaxation period, the tendon edge was translated to the lateral edge of the rotator cuff footprint (the lateral footprint position), and tension was recorded again (Figures 2C, 2D). A medially based single-row RCR with triple-loaded sutures and bone marrow vents placed in the lateral tuberosity was then completed, regardless of tension, tear size, or tear morphology.31 Typically, 1 anchor was used for every 10 to 15 mm of A-P tear length.
SAS software was used for statistical analysis, the Wilcoxon signed rank test for continuous or ordinal data comparisons between paired groups, and the Mann-Whitney test for continuous or ordinal data comparisons between independent, unmatched groups. One-way analysis of variance (ANOVA) was used to compare means among the 3 groups of morphology subtypes. Linear regression was performed to assess the simultaneous relationship between potential predictors (age, sex, length of symptoms, Goutallier score, tear size) and medial or lateral tension, where medial tension was included as an additional potential predictor for lateral tension. Restricted cubic splines were fit to assess linearity. Predictors were retained in multivariate regression using backward variable retention. Because of inadequate sample size, additivity was assumed except for sex. Statistical significance was set at P < .05.
Results
Of the 20 rotator cuff tears evaluated (Table 1), 13 were crescent-shaped, 5 were U-shaped, and 2 were L-shaped. Mean (SD) A-P tear size was 17.7 (5.8) mm, and mean (SD) M-L tear size was 19.1 (8.6) mm. Mean age of the 20 patients (15 men, 5 women) was 57.9 years (range, 44-72 years). Mean (SD) length of symptoms was 12.9 (12.4) months (range, 3-48 months). Mean (SD) modified Goutallier score was 1.4 (0.7; range, 0-3).
Mean (SD) rotator cuff tension for all tears approximated to the medial footprint was 0.41 (0.33) pound, and mean (SD) cuff tension for all tears approximated to the lateral footprint was 2.21 (1.20) pounds—representing a 5.4-fold difference (P < .0001).
No statistically significant differences were detected in the ANOVA comparing tensions at medial and lateral positions among tear morphologic subtypes (all Ps >.05).
Subgroup analysis (Table 2) was performed for smaller (≤20 mm A-P) and larger (>20 mm A-P) tears. For smaller tears, mean (SD) tension was 0.27 (0.24) pound applied with the cuff tendon pulled to the medial footprint and 2.06 (1.06) pounds applied with the tendon pulled to the lateral footprint—a 7.6-fold difference (P < .0018). For larger tears, mean (SD) tension was 0.58 (0.37) pound applied with the tendon pulled to the medial footprint and 2.38 (1.4) pounds applied with the tendon pulled to the lateral footprint—a 4.1-fold difference (P < .005).
A statistically significant difference in tensions was found between small and large cuff tears positioned at the medial footprint (0.27 vs 0.58 lb; P = .0367); no difference was found between groups with the tendon at the lateral footprint (2.06 vs 2.38 lb; P = .284).
Univariate and multivariate analyses were performed using linear regression analysis (Table 3). During univariate analysis for medial footprint position, A-P tear size and Goutallier score both positively correlated with increasing tension; for lateral footprint position, no factors statistically correlated with lateral tension, though there was a positive trend for medial tension and female sex. During multivariate analysis for medial footprint position, only A-P tear size positively correlated with increasing tension; for lateral footprint position, both age (in nonlinear fashion as function of age + age2) and medial tension positively correlated with increasing tension.
Discussion
Our results indicated that significantly more tension is placed on the torn rotator cuff tendon when it is reduced across the footprint from a medial to a more lateral position in vivo. More tension was required for all tears to be reduced to the lateral footprint compared with the medial footprint. As expected, compared with smaller tears, larger tears required significantly more tension in order to be reduced to the medial footprint. Interestingly, no statistical difference was found between tensions required to reduce either small or large tears to the lateral footprint, which suggests that, regardless of tear size, more force must be applied to reduce the torn tendon to the lateral footprint compared with the medial footprint.
Hersche and Gerber34 were the first to report rotator cuff tension measurements in vivo. Although their study did not specifically compare cuff tensions reducing the tear to the medial versus lateral footprint, it did examine tension at displacement of 10 and 20 mm. Tension increased from 27 N to 60 N, correlating with a 2.2-fold difference between the 2 distances. Domb and colleagues35 also compared in vivo rotator cuff tension differences between the medial footprint and the lateral footprint in 4 patients. Mean tension applied to the cuff during reduction to the articular margin was 27 N, or 6 pounds. Mean tension needed to reduce the cuff to the lateral tuberosity was 76 N, or 17 pounds, for a 2.8-fold difference. Tears were not measured but were described as massive and retracted.
Although repair tension has long been recognized as a crucial factor in RCR healing, little clinical research has focused on the effects of excess tension. Davidson and Rivenburgh11 prospectively followed the clinical outcomes of 67 consecutive cuff repairs after intraoperative tension measurement and found that high-tension repairs (>8 lb) had significantly lower clinical outcome measures. However, the authors did not report on correlations with radiologic healing and stated, “Functional outcome is inversely proportional to rotator cuff repair tension.” Further study of the in vivo effects of increased tension on clinical and radiologic outcomes is needed.
Several animal studies have been conducted on the effects of tension on RCRs. Gerber and colleagues36 reported that the force needed to produce 1 cm of sheep supraspinatus tendon excursion increased 7-fold, from 6.8 N to 47.8 N, after 40 weeks of tendon tear. Coleman and colleagues37 compared the modulus of elasticity in sheep supraspinatus tendon after 6 weeks and 18 weeks of detachment and reported increases of 60% and 70%, respectively. Gimbel and colleagues38 showed that, in a rat model, “repair tension rapidly increased initially after injury followed by a progressive, but less dramatic, increase with additional time.” Of note, we did not identify any correlation between chronicity of symptoms and the tension needed to reduce the tendon medially or to a more lateral position on the footprint.
In acute tears, the cuff tissue is more compliant and mobile and can be pulled laterally across its anatomical footprint with minimal tension.39 In contrast, cuff tissue in the more commonly encountered chronic tear is less compliant and is not mobile enough to be pulled to the lateral margin of the footprint without added stress.30,34,35 In large, acute tears in which there are minimal tissue degeneration and retraction, a laterally based footprint-restoring technique may be performed with minimal tension. This technique may have advantages over a medially based repair. In the literature, more attention needs to be directed toward the biomechanics and biology of chronic rotator cuff tears, as these are more commonly encountered.
Almost all of the prospective studies that have compared single- and double-row RCR have found no significant differences in MRI healing rates or clinical results at follow-up up to 2 years.14,16,40-45 Detailed analysis of the surgical techniques used in all these studies revealed that the rotator cuff tendons were repaired back to the lateral footprint in both the single- and double-row constructs.14,16,40-45 Although no clinical studies have compared medially and laterally based single-row repairs, our data suggest that medially based repairs have lower tensions and therefore should not be considered equivalent. Sostak and colleagues31 and Murray and colleagues46 have shown that a medially based single-row RCR can achieve excellent clinical and anatomical results, likely partly because of the lower tension applied to the torn cuff tissue.31,46 Studies are needed to compare medially and laterally based repairs, including single- and double-row repairs.
The vast majority of recent research has aimed to counteract construct tension with stronger biomechanical constructs.20-26 Surgeons have also aimed to improve biological healing by pulling the tendon laterally across the footprint to achieve complete footprint coverage, ultimately increasing the surface area for tendon–bone healing. This has led to the development of various double-row repair techniques, in which the cuff tendon is pulled to the lateral margin of its footprint. One row of anchors is placed in the medial aspect of the footprint, while a second is placed in the lateral aspect; the cuff is reduced and compressed to the tuberosity with various suture configurations. The TOE technique was developed to improve pressurization of the cuff tendon across the footprint by linking the 2 rows with bridging sutures. In doing so, however, the potentially deleterious effects of increased tension introduced by pulling the tendon laterally may have been overlooked. Nevertheless, the biomechanics and stress distribution likely differ between single-row repair and TOE repairs, and direct comparisons cannot be made at this time. The medial row of a double-row or TOE construct may stress-shield or “unload” the more lateral tissue. Studies are needed in order to better understand the tension differential and stress distribution of various double-row constructs.
Recognizing tear morphology is crucial in maximizing chances of healing after cuff repair. For example, a crescent-shaped tear is reduced to the tuberosity with direct lateral translation of the apex of the tear, which is also the deepest or most displaced part of the tear. On the other hand, reducing an L- or reverse L-shaped tear to the tuberosity is not as direct; reducing the deepest or most displaced part of the tear would lead to overreduction and overtensioning of the tendon. However, often the exact “elbow” of the tear is not obvious and appears more rounded; therefore, it is crucial for the surgeon to examine the mobility of the torn tendon along its entire length to minimize tension. Study is needed to assess tension along the entire length of the tear for different tear morphologies and sizes.
Although our results showed that increased tension was needed to reduce a torn tendon to its lateral footprint, no study has indicated exactly how much is “too much” tension. As stated earlier, use of stronger biomechanical constructs, including TOE constructs, may overcome the increased tension associated with laterally based repairs. In addition, laterally based repairs, either single- or double-row, may be best suited for tears with lower tension, whereas medially based repairs may be best suited for higher tension tears. It is also possible that the difference in tensions noted in this study is not significant enough to have a clinical impact on choice of construct or on anatomical healing. We need studies that correlate anatomical healing rates with repair tension in order to better guide surgeons on when to use a medially or laterally based repair.
Other possible effects of increased tension associated with laterally based repairs, including beneficial effects, must be considered as well. Viscoelastic properties of human rotator cuff tendon may dissipate increased tension over time through a variety of mechanisms. Stress relaxation, gap formation, creep, and the hysteresis effect, all associated with cyclical loading in the early healing period, may lead to dissipation of force over time.47,48 These more complex biomechanical properties of RCR constructs are yet to be clearly defined.
This study had several weaknesses. Its data represent a static measurement of time-zero rotator cuff tension, which greatly simplifies the biomechanics of the torn rotator cuff and repair construct as well as changes that occur with healing. During cuff repair, forces typically are distributed through several fixation points in stepwise process and are not focused on a single point of tissue with a grasper. Therefore, the findings of this study may not directly correlate with medially versus laterally based repairs in vivo. Furthermore, as this is a time-zero measurement, we could not determine whether the tension differential between the 2 repair positions remained static over time. Current literature suggests that muscle atrophy, fatty infiltration, and loss of elasticity of the musculotendinous unit are relatively irreversible.35,37,49 In addition, determining the precise apex of a cuff tear can be difficult, so error may have been introduced during this process. Last, although placement of the cuff tissue at the medial or lateral footprint position was based on visual estimation by an experienced and skilled arthroscopist, error may have been introduced based on this imprecise technique.
Conclusion
This study demonstrated a significant, 5.4-fold increase in in vivo time-zero rotator cuff tension with the tendon edge reduced to the lateral footprint rather than the medial footprint.
1. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
2. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
5. Gulotta LV, Nho SJ, Dodson CC, Adler RS, Altchek DW, MacGillivray JD; HSS Arthroscopic Rotator Cuff Registry. Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part II—prognostic factors for clinical and radiographic outcomes. J Shoulder Elbow Surg. 2011;20(6):941-946.
6. Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009;1(2):96-104.
7. Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719-728.
8. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
9. Tashjian RZ, Hollins AM, Kim HM, et al. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2435-2442.
10. Burkhart SS, Lo IK. Arthroscopic rotator cuff repair. J Am Acad Orthop Surg. 2006;14(6):333-346.
11. Davidson PA, Rivenburgh DW. Rotator cuff repair tension as a determinant of functional outcome. J Shoulder Elbow Surg. 2000;9(6):502-506.
12. Goutallier D, Postel JM, Van Driessche S, Godefroy D, Radier C. Tension-free cuff repairs with excision of macroscopic tendon lesions and muscular advancement: results in a prospective series with limited fatty muscular degeneration. J Shoulder Elbow Surg. 2006;15(2):164-172.
13. Gimbel JA, Van Kleunen JP, Lake SP, Williams GR, Soslowsky LJ. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40(3):561-568.
14. Ma HL, Chiang ER, Wu HT, et al. Clinical outcome and imaging of arthroscopic single-row and double-row rotator cuff repair: a prospective randomized trial. Arthroscopy. 2012;28(1):16-24.
15. Mihata T, Watanabe C, Fukunishi K, et al. Functional and structural outcomes of single-row versus double-row versus combined double-row and suture-bridge repair for rotator cuff tears. Am J Sports Med. 2011;39(10):2091-2098.
16. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
17. Voigt C, Bosse C, Vosshenrich R, Schulz AP, Lill H. Arthroscopic supraspinatus tendon repair with suture-bridging technique: functional outcome and magnetic resonance imaging. Am J Sports Med. 2010;38(5):983-991.
18. Lafosse L, Brzoska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 2):275-286.
19. Park JY, Lhee SH, Choi JH, Park HK, Yu JW, Seo JB. Comparison of the clinical outcomes of single- and double-row repairs in rotator cuff tears. Am J Sports Med. 2008;36(7):1310-1316.
20. Kim DH, ElAttrache NS, Tibone JE, et al. Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med. 2006;34(3):407-414.
21. Mazzocca AD, Bollier MJ, Ciminiello AM, et al. Biomechanical evaluation of arthroscopic rotator cuff repairs over time. Arthroscopy. 2010;26(5):592-599.
22. Grimberg J, Diop A, Kalra K, Charousset C, Duranthon LD, Maurel N. In vitro biomechanical comparison of three different types of single- and double-row arthroscopic rotator cuff repairs: analysis of continuous bone–tendon contact pressure and surface during different simulated joint positions. J Shoulder Elbow Surg. 2010;19(2):236-243.
23. Nelson CO, Sileo MJ, Grossman MG, Serra-Hsu F. Single-row modified Mason-Allen versus double-row arthroscopic rotator cuff repair: a biomechanical and surface area comparison. Arthroscopy. 2008;24(8):941-948.
24. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
25. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
26. Ma CB, Comerford L, Wilson J, Puttlitz CM. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88(2):403-410.
27. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
28. Tuoheti Y, Itoi E, Yamamoto N, et al. Contact area, contact pressure, and pressure patterns of the tendon–bone interface after rotator cuff repair. Am J Sports Med. 2005;33(12):1869-1874.
29. Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606-610.
30. Snyder SJ. Single vs. double row suture anchor fixation rotator cuff repair. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; March 8, 2007; San Francisco, CA.
31. Sostak JP, Bahk MS, Getelman MH, Wong IH, Snyder SJ, Burns JP. Arthroscopic single row rotator cuff repair using the “SCOI row”: structural and clinical outcomes. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; February 7-11, 2012; San Francisco, CA.
32. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599-605.
33. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
34. Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998;7(4):393-396.
35. Domb BG, Glousman RE, Brooks A, Hansen M, Lee TQ, ElAttrache NS. High-tension double-row footprint repair compared with reduced-tension single-row repair for massive rotator cuff tears. J Bone Joint Surg Am. 2008;90(suppl 4):35-39.
36. Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86(9):1973-1982.
37. Coleman SH, Fealy S, Ehteshami JR, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am. 2003;85(12):2391-2402.
38. Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res. 2004;(426):258-265.
39. Mannava S, Plate JF, Whitlock PW, et al. Evaluation of in vivo rotator cuff muscle function after acute and chronic detachment of the supraspinatus tendon: an experimental study in an animal model. J Bone Joint Surg Am. 2011;93(18):1702-1711.
40. Burks RT, Crim J, Brown N, Fink B, Greis PE. A prospective randomized clinical trial comparing arthroscopic single- and double-row rotator cuff repair: magnetic resonance imaging and early clinical evaluation. Am J Sports Med. 2009;37(4):674-682.
41. Grasso A, Milano G, Salvatore M, Falcone G, Deriu L, Fabbriciani C. Single-row versus double-row arthroscopic rotator cuff repair: a prospective randomized clinical study. Arthroscopy. 2009;25(1):4-12.
42. Franceschi F, Ruzzini L, Longo UG, et al. Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2007;35(8):1254-1260.
43. Carbonel I, Martinez AA, Calvo A, Ripalda J, Herrera A. Single-row versus double-row arthroscopic repair in the treatment of rotator cuff tears: a prospective randomized clinical study. Int Orthop. 2012;36(9):1877-1883.
44. Lapner PL, Sabri E, Rakhra K, et al. A multicenter randomized controlled trial comparing single-row with double-row fixation in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(14):1249-1257.
45. Gartsman GM, Drake G, Edwards TB, et al. Ultrasound evaluation of arthroscopic full-thickness supraspinatus rotator cuff repair: single-row versus double-row suture bridge (transosseous equivalent) fixation. Results of a prospective, randomized study. J Shoulder Elbow Surg. 2013;22(11):1480-1487.
46. Murray TF Jr, Lajtai G, Mileski RM, Snyder SJ. Arthroscopic repair of medium to large full-thickness rotator cuff tears: outcome at 2- to 6-year follow-up. J Shoulder Elbow Surg. 2002;11(1):19-24.
47. Szczesny SE, Peloquin JM, Cortes DH, Kadlowec JA, Soslowsky LJ, Elliott DM. Biaxial tensile testing and constitutive modeling of human supraspinatus tendon. J Biomech Eng. 2012;134(2):021004.
48. Chaudhury S, Holland C, Vollrath F, Carr AJ. Comparing normal and torn rotator cuff tendons using dynamic shear analysis. J Bone Joint Surg Br. 2011;93(7):942-948.
49. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
Although recent clinical results of arthroscopic rotator cuff repair (RCR) have been encouraging, achieving anatomical healing of full-thickness rotator cuff tears remains a challenge.1-4 Several factors influence rotator cuff healing after repair.1,3-8 Patient-related factors include advanced patient age, tear size, tear chronicity, and amount of fatty infiltration.1,3,5,6,8-10 Tension applied to the repair construct is a significant factor as well.11,12
In the literature, limited consideration has been given to repair tension.13 The majority of studies have focused on other factors, mainly repair technique. Some surgeons advocate use of a double-row repair construct in which the rotator cuff tendon is pulled to the lateral margin of the footprint.14-19 Double-row techniques, which include the transosseous-equivalent (TOE) construct, are biomechanically superior to other repairs.20-26 Another purported benefit of double-row repair is more complete restoration and pressurization of the rotator cuff footprint.21,24,27,28
Rotator cuff tears typically occur near the dysvascular region of the diseased musculotendinous unit, often leaving a stump of tissue attached to the tuberosity and ultimately a shortened tendon.29 In addition, full-thickness tears often retract over time. Meyer and colleagues29 recently demonstrated that this shortening is irreversible. Snyder30 and Sostak and colleagues31 suggested that pulling a shortened, degenerative rotator cuff tendon to the lateral margin of the footprint results in increased tissue tension compared with that produced with a more medially based repair just off the articular margin. In our opinion, the possible increase in tension during a laterally based repair, whether single- or double-row, may place excessive strain on the diseased tissue as well as the surgical construct, potentially contributing to repair failure.
We conducted a study to evaluate the difference, if any, in tension applied to the rotator cuff tendon positioned at the medial versus lateral margin of the footprint during arthroscopic RCR. We hypothesized significantly more tension would be placed on the rotator cuff tendon when positioned at the lateral versus medial footprint.
Methods
After obtaining Institutional Review Board approval for this study, we collected data on a consecutive series of patients who underwent arthroscopic RCR performed by Dr. Getelman at a single institution. Only patients with primary full-thickness tears of the supraspinatus and/or infraspinatus were included. Exclusion criteria included revision rotator cuff surgeries, partial-thickness tears, concurrent subscapularis tears requiring anchor fixation, and any tears that could not be mobilized to the lateral footprint without interval slides or margin convergence. The 20 identified patients constituted the study group.
Demographic factors, including age and preoperative length of symptoms, were recorded after chart review. Magnetic resonance imaging (MRI) was performed for all patients before surgery and was retrospectively reviewed. Dr. Getelman assigned each patient a modified Goutallier score, based on MRI, to assess for fatty infiltration/atrophy.32 Each patient was placed in the lateral decubitus position with the operative arm in balanced suspension at 70° of abduction. Standard glenohumeral and subacromial diagnostic arthroscopy was performed. The rotator cuff tear was gently debrided back to a healthy-appearing margin in preparation for repair. The tear was then measured in the anterior-posterior (A-P) and medial-lateral (M-L) planes using a premeasured, marked suture, as previously described.33 Complete bursal and articular-sided releases were performed to allow for appropriate mobilization of the tendon. The tear was classified as crescent-shaped, U-shaped, or L-shaped.
Viewing from the posterior portal, the surgeon inserted a tissue grasper through the lateral portal. The tendon was grasped at multiple points along its edge, anterior to posterior, and was translated laterally to assess its reducibility; the apex of the tear correlated with the point of maximal excursion and coverage of the footprint. Once confirmed, the rotator cuff tear apex was clamped with a tissue grasper. After placement in a sterile arthroscopic camera sleeve (DeRoyal camera drape with perforated tip), a calibrated digital weigh scale (American Weigh Scales model H22 portable electronic hanging scale, with accuracy of 0.01 lb) was attached to the tissue grasper with an S-hook (Figure 1). The tendon edge was first translated about 3 mm lateral to the articular margin (the medial footprint position), and tension was recorded (Figures 2A, 2B). After a 1-minute relaxation period, the tendon edge was translated to the lateral edge of the rotator cuff footprint (the lateral footprint position), and tension was recorded again (Figures 2C, 2D). A medially based single-row RCR with triple-loaded sutures and bone marrow vents placed in the lateral tuberosity was then completed, regardless of tension, tear size, or tear morphology.31 Typically, 1 anchor was used for every 10 to 15 mm of A-P tear length.
SAS software was used for statistical analysis, the Wilcoxon signed rank test for continuous or ordinal data comparisons between paired groups, and the Mann-Whitney test for continuous or ordinal data comparisons between independent, unmatched groups. One-way analysis of variance (ANOVA) was used to compare means among the 3 groups of morphology subtypes. Linear regression was performed to assess the simultaneous relationship between potential predictors (age, sex, length of symptoms, Goutallier score, tear size) and medial or lateral tension, where medial tension was included as an additional potential predictor for lateral tension. Restricted cubic splines were fit to assess linearity. Predictors were retained in multivariate regression using backward variable retention. Because of inadequate sample size, additivity was assumed except for sex. Statistical significance was set at P < .05.
Results
Of the 20 rotator cuff tears evaluated (Table 1), 13 were crescent-shaped, 5 were U-shaped, and 2 were L-shaped. Mean (SD) A-P tear size was 17.7 (5.8) mm, and mean (SD) M-L tear size was 19.1 (8.6) mm. Mean age of the 20 patients (15 men, 5 women) was 57.9 years (range, 44-72 years). Mean (SD) length of symptoms was 12.9 (12.4) months (range, 3-48 months). Mean (SD) modified Goutallier score was 1.4 (0.7; range, 0-3).
Mean (SD) rotator cuff tension for all tears approximated to the medial footprint was 0.41 (0.33) pound, and mean (SD) cuff tension for all tears approximated to the lateral footprint was 2.21 (1.20) pounds—representing a 5.4-fold difference (P < .0001).
No statistically significant differences were detected in the ANOVA comparing tensions at medial and lateral positions among tear morphologic subtypes (all Ps >.05).
Subgroup analysis (Table 2) was performed for smaller (≤20 mm A-P) and larger (>20 mm A-P) tears. For smaller tears, mean (SD) tension was 0.27 (0.24) pound applied with the cuff tendon pulled to the medial footprint and 2.06 (1.06) pounds applied with the tendon pulled to the lateral footprint—a 7.6-fold difference (P < .0018). For larger tears, mean (SD) tension was 0.58 (0.37) pound applied with the tendon pulled to the medial footprint and 2.38 (1.4) pounds applied with the tendon pulled to the lateral footprint—a 4.1-fold difference (P < .005).
A statistically significant difference in tensions was found between small and large cuff tears positioned at the medial footprint (0.27 vs 0.58 lb; P = .0367); no difference was found between groups with the tendon at the lateral footprint (2.06 vs 2.38 lb; P = .284).
Univariate and multivariate analyses were performed using linear regression analysis (Table 3). During univariate analysis for medial footprint position, A-P tear size and Goutallier score both positively correlated with increasing tension; for lateral footprint position, no factors statistically correlated with lateral tension, though there was a positive trend for medial tension and female sex. During multivariate analysis for medial footprint position, only A-P tear size positively correlated with increasing tension; for lateral footprint position, both age (in nonlinear fashion as function of age + age2) and medial tension positively correlated with increasing tension.
Discussion
Our results indicated that significantly more tension is placed on the torn rotator cuff tendon when it is reduced across the footprint from a medial to a more lateral position in vivo. More tension was required for all tears to be reduced to the lateral footprint compared with the medial footprint. As expected, compared with smaller tears, larger tears required significantly more tension in order to be reduced to the medial footprint. Interestingly, no statistical difference was found between tensions required to reduce either small or large tears to the lateral footprint, which suggests that, regardless of tear size, more force must be applied to reduce the torn tendon to the lateral footprint compared with the medial footprint.
Hersche and Gerber34 were the first to report rotator cuff tension measurements in vivo. Although their study did not specifically compare cuff tensions reducing the tear to the medial versus lateral footprint, it did examine tension at displacement of 10 and 20 mm. Tension increased from 27 N to 60 N, correlating with a 2.2-fold difference between the 2 distances. Domb and colleagues35 also compared in vivo rotator cuff tension differences between the medial footprint and the lateral footprint in 4 patients. Mean tension applied to the cuff during reduction to the articular margin was 27 N, or 6 pounds. Mean tension needed to reduce the cuff to the lateral tuberosity was 76 N, or 17 pounds, for a 2.8-fold difference. Tears were not measured but were described as massive and retracted.
Although repair tension has long been recognized as a crucial factor in RCR healing, little clinical research has focused on the effects of excess tension. Davidson and Rivenburgh11 prospectively followed the clinical outcomes of 67 consecutive cuff repairs after intraoperative tension measurement and found that high-tension repairs (>8 lb) had significantly lower clinical outcome measures. However, the authors did not report on correlations with radiologic healing and stated, “Functional outcome is inversely proportional to rotator cuff repair tension.” Further study of the in vivo effects of increased tension on clinical and radiologic outcomes is needed.
Several animal studies have been conducted on the effects of tension on RCRs. Gerber and colleagues36 reported that the force needed to produce 1 cm of sheep supraspinatus tendon excursion increased 7-fold, from 6.8 N to 47.8 N, after 40 weeks of tendon tear. Coleman and colleagues37 compared the modulus of elasticity in sheep supraspinatus tendon after 6 weeks and 18 weeks of detachment and reported increases of 60% and 70%, respectively. Gimbel and colleagues38 showed that, in a rat model, “repair tension rapidly increased initially after injury followed by a progressive, but less dramatic, increase with additional time.” Of note, we did not identify any correlation between chronicity of symptoms and the tension needed to reduce the tendon medially or to a more lateral position on the footprint.
In acute tears, the cuff tissue is more compliant and mobile and can be pulled laterally across its anatomical footprint with minimal tension.39 In contrast, cuff tissue in the more commonly encountered chronic tear is less compliant and is not mobile enough to be pulled to the lateral margin of the footprint without added stress.30,34,35 In large, acute tears in which there are minimal tissue degeneration and retraction, a laterally based footprint-restoring technique may be performed with minimal tension. This technique may have advantages over a medially based repair. In the literature, more attention needs to be directed toward the biomechanics and biology of chronic rotator cuff tears, as these are more commonly encountered.
Almost all of the prospective studies that have compared single- and double-row RCR have found no significant differences in MRI healing rates or clinical results at follow-up up to 2 years.14,16,40-45 Detailed analysis of the surgical techniques used in all these studies revealed that the rotator cuff tendons were repaired back to the lateral footprint in both the single- and double-row constructs.14,16,40-45 Although no clinical studies have compared medially and laterally based single-row repairs, our data suggest that medially based repairs have lower tensions and therefore should not be considered equivalent. Sostak and colleagues31 and Murray and colleagues46 have shown that a medially based single-row RCR can achieve excellent clinical and anatomical results, likely partly because of the lower tension applied to the torn cuff tissue.31,46 Studies are needed to compare medially and laterally based repairs, including single- and double-row repairs.
The vast majority of recent research has aimed to counteract construct tension with stronger biomechanical constructs.20-26 Surgeons have also aimed to improve biological healing by pulling the tendon laterally across the footprint to achieve complete footprint coverage, ultimately increasing the surface area for tendon–bone healing. This has led to the development of various double-row repair techniques, in which the cuff tendon is pulled to the lateral margin of its footprint. One row of anchors is placed in the medial aspect of the footprint, while a second is placed in the lateral aspect; the cuff is reduced and compressed to the tuberosity with various suture configurations. The TOE technique was developed to improve pressurization of the cuff tendon across the footprint by linking the 2 rows with bridging sutures. In doing so, however, the potentially deleterious effects of increased tension introduced by pulling the tendon laterally may have been overlooked. Nevertheless, the biomechanics and stress distribution likely differ between single-row repair and TOE repairs, and direct comparisons cannot be made at this time. The medial row of a double-row or TOE construct may stress-shield or “unload” the more lateral tissue. Studies are needed in order to better understand the tension differential and stress distribution of various double-row constructs.
Recognizing tear morphology is crucial in maximizing chances of healing after cuff repair. For example, a crescent-shaped tear is reduced to the tuberosity with direct lateral translation of the apex of the tear, which is also the deepest or most displaced part of the tear. On the other hand, reducing an L- or reverse L-shaped tear to the tuberosity is not as direct; reducing the deepest or most displaced part of the tear would lead to overreduction and overtensioning of the tendon. However, often the exact “elbow” of the tear is not obvious and appears more rounded; therefore, it is crucial for the surgeon to examine the mobility of the torn tendon along its entire length to minimize tension. Study is needed to assess tension along the entire length of the tear for different tear morphologies and sizes.
Although our results showed that increased tension was needed to reduce a torn tendon to its lateral footprint, no study has indicated exactly how much is “too much” tension. As stated earlier, use of stronger biomechanical constructs, including TOE constructs, may overcome the increased tension associated with laterally based repairs. In addition, laterally based repairs, either single- or double-row, may be best suited for tears with lower tension, whereas medially based repairs may be best suited for higher tension tears. It is also possible that the difference in tensions noted in this study is not significant enough to have a clinical impact on choice of construct or on anatomical healing. We need studies that correlate anatomical healing rates with repair tension in order to better guide surgeons on when to use a medially or laterally based repair.
Other possible effects of increased tension associated with laterally based repairs, including beneficial effects, must be considered as well. Viscoelastic properties of human rotator cuff tendon may dissipate increased tension over time through a variety of mechanisms. Stress relaxation, gap formation, creep, and the hysteresis effect, all associated with cyclical loading in the early healing period, may lead to dissipation of force over time.47,48 These more complex biomechanical properties of RCR constructs are yet to be clearly defined.
This study had several weaknesses. Its data represent a static measurement of time-zero rotator cuff tension, which greatly simplifies the biomechanics of the torn rotator cuff and repair construct as well as changes that occur with healing. During cuff repair, forces typically are distributed through several fixation points in stepwise process and are not focused on a single point of tissue with a grasper. Therefore, the findings of this study may not directly correlate with medially versus laterally based repairs in vivo. Furthermore, as this is a time-zero measurement, we could not determine whether the tension differential between the 2 repair positions remained static over time. Current literature suggests that muscle atrophy, fatty infiltration, and loss of elasticity of the musculotendinous unit are relatively irreversible.35,37,49 In addition, determining the precise apex of a cuff tear can be difficult, so error may have been introduced during this process. Last, although placement of the cuff tissue at the medial or lateral footprint position was based on visual estimation by an experienced and skilled arthroscopist, error may have been introduced based on this imprecise technique.
Conclusion
This study demonstrated a significant, 5.4-fold increase in in vivo time-zero rotator cuff tension with the tendon edge reduced to the lateral footprint rather than the medial footprint.
Although recent clinical results of arthroscopic rotator cuff repair (RCR) have been encouraging, achieving anatomical healing of full-thickness rotator cuff tears remains a challenge.1-4 Several factors influence rotator cuff healing after repair.1,3-8 Patient-related factors include advanced patient age, tear size, tear chronicity, and amount of fatty infiltration.1,3,5,6,8-10 Tension applied to the repair construct is a significant factor as well.11,12
In the literature, limited consideration has been given to repair tension.13 The majority of studies have focused on other factors, mainly repair technique. Some surgeons advocate use of a double-row repair construct in which the rotator cuff tendon is pulled to the lateral margin of the footprint.14-19 Double-row techniques, which include the transosseous-equivalent (TOE) construct, are biomechanically superior to other repairs.20-26 Another purported benefit of double-row repair is more complete restoration and pressurization of the rotator cuff footprint.21,24,27,28
Rotator cuff tears typically occur near the dysvascular region of the diseased musculotendinous unit, often leaving a stump of tissue attached to the tuberosity and ultimately a shortened tendon.29 In addition, full-thickness tears often retract over time. Meyer and colleagues29 recently demonstrated that this shortening is irreversible. Snyder30 and Sostak and colleagues31 suggested that pulling a shortened, degenerative rotator cuff tendon to the lateral margin of the footprint results in increased tissue tension compared with that produced with a more medially based repair just off the articular margin. In our opinion, the possible increase in tension during a laterally based repair, whether single- or double-row, may place excessive strain on the diseased tissue as well as the surgical construct, potentially contributing to repair failure.
We conducted a study to evaluate the difference, if any, in tension applied to the rotator cuff tendon positioned at the medial versus lateral margin of the footprint during arthroscopic RCR. We hypothesized significantly more tension would be placed on the rotator cuff tendon when positioned at the lateral versus medial footprint.
Methods
After obtaining Institutional Review Board approval for this study, we collected data on a consecutive series of patients who underwent arthroscopic RCR performed by Dr. Getelman at a single institution. Only patients with primary full-thickness tears of the supraspinatus and/or infraspinatus were included. Exclusion criteria included revision rotator cuff surgeries, partial-thickness tears, concurrent subscapularis tears requiring anchor fixation, and any tears that could not be mobilized to the lateral footprint without interval slides or margin convergence. The 20 identified patients constituted the study group.
Demographic factors, including age and preoperative length of symptoms, were recorded after chart review. Magnetic resonance imaging (MRI) was performed for all patients before surgery and was retrospectively reviewed. Dr. Getelman assigned each patient a modified Goutallier score, based on MRI, to assess for fatty infiltration/atrophy.32 Each patient was placed in the lateral decubitus position with the operative arm in balanced suspension at 70° of abduction. Standard glenohumeral and subacromial diagnostic arthroscopy was performed. The rotator cuff tear was gently debrided back to a healthy-appearing margin in preparation for repair. The tear was then measured in the anterior-posterior (A-P) and medial-lateral (M-L) planes using a premeasured, marked suture, as previously described.33 Complete bursal and articular-sided releases were performed to allow for appropriate mobilization of the tendon. The tear was classified as crescent-shaped, U-shaped, or L-shaped.
Viewing from the posterior portal, the surgeon inserted a tissue grasper through the lateral portal. The tendon was grasped at multiple points along its edge, anterior to posterior, and was translated laterally to assess its reducibility; the apex of the tear correlated with the point of maximal excursion and coverage of the footprint. Once confirmed, the rotator cuff tear apex was clamped with a tissue grasper. After placement in a sterile arthroscopic camera sleeve (DeRoyal camera drape with perforated tip), a calibrated digital weigh scale (American Weigh Scales model H22 portable electronic hanging scale, with accuracy of 0.01 lb) was attached to the tissue grasper with an S-hook (Figure 1). The tendon edge was first translated about 3 mm lateral to the articular margin (the medial footprint position), and tension was recorded (Figures 2A, 2B). After a 1-minute relaxation period, the tendon edge was translated to the lateral edge of the rotator cuff footprint (the lateral footprint position), and tension was recorded again (Figures 2C, 2D). A medially based single-row RCR with triple-loaded sutures and bone marrow vents placed in the lateral tuberosity was then completed, regardless of tension, tear size, or tear morphology.31 Typically, 1 anchor was used for every 10 to 15 mm of A-P tear length.
SAS software was used for statistical analysis, the Wilcoxon signed rank test for continuous or ordinal data comparisons between paired groups, and the Mann-Whitney test for continuous or ordinal data comparisons between independent, unmatched groups. One-way analysis of variance (ANOVA) was used to compare means among the 3 groups of morphology subtypes. Linear regression was performed to assess the simultaneous relationship between potential predictors (age, sex, length of symptoms, Goutallier score, tear size) and medial or lateral tension, where medial tension was included as an additional potential predictor for lateral tension. Restricted cubic splines were fit to assess linearity. Predictors were retained in multivariate regression using backward variable retention. Because of inadequate sample size, additivity was assumed except for sex. Statistical significance was set at P < .05.
Results
Of the 20 rotator cuff tears evaluated (Table 1), 13 were crescent-shaped, 5 were U-shaped, and 2 were L-shaped. Mean (SD) A-P tear size was 17.7 (5.8) mm, and mean (SD) M-L tear size was 19.1 (8.6) mm. Mean age of the 20 patients (15 men, 5 women) was 57.9 years (range, 44-72 years). Mean (SD) length of symptoms was 12.9 (12.4) months (range, 3-48 months). Mean (SD) modified Goutallier score was 1.4 (0.7; range, 0-3).
Mean (SD) rotator cuff tension for all tears approximated to the medial footprint was 0.41 (0.33) pound, and mean (SD) cuff tension for all tears approximated to the lateral footprint was 2.21 (1.20) pounds—representing a 5.4-fold difference (P < .0001).
No statistically significant differences were detected in the ANOVA comparing tensions at medial and lateral positions among tear morphologic subtypes (all Ps >.05).
Subgroup analysis (Table 2) was performed for smaller (≤20 mm A-P) and larger (>20 mm A-P) tears. For smaller tears, mean (SD) tension was 0.27 (0.24) pound applied with the cuff tendon pulled to the medial footprint and 2.06 (1.06) pounds applied with the tendon pulled to the lateral footprint—a 7.6-fold difference (P < .0018). For larger tears, mean (SD) tension was 0.58 (0.37) pound applied with the tendon pulled to the medial footprint and 2.38 (1.4) pounds applied with the tendon pulled to the lateral footprint—a 4.1-fold difference (P < .005).
A statistically significant difference in tensions was found between small and large cuff tears positioned at the medial footprint (0.27 vs 0.58 lb; P = .0367); no difference was found between groups with the tendon at the lateral footprint (2.06 vs 2.38 lb; P = .284).
Univariate and multivariate analyses were performed using linear regression analysis (Table 3). During univariate analysis for medial footprint position, A-P tear size and Goutallier score both positively correlated with increasing tension; for lateral footprint position, no factors statistically correlated with lateral tension, though there was a positive trend for medial tension and female sex. During multivariate analysis for medial footprint position, only A-P tear size positively correlated with increasing tension; for lateral footprint position, both age (in nonlinear fashion as function of age + age2) and medial tension positively correlated with increasing tension.
Discussion
Our results indicated that significantly more tension is placed on the torn rotator cuff tendon when it is reduced across the footprint from a medial to a more lateral position in vivo. More tension was required for all tears to be reduced to the lateral footprint compared with the medial footprint. As expected, compared with smaller tears, larger tears required significantly more tension in order to be reduced to the medial footprint. Interestingly, no statistical difference was found between tensions required to reduce either small or large tears to the lateral footprint, which suggests that, regardless of tear size, more force must be applied to reduce the torn tendon to the lateral footprint compared with the medial footprint.
Hersche and Gerber34 were the first to report rotator cuff tension measurements in vivo. Although their study did not specifically compare cuff tensions reducing the tear to the medial versus lateral footprint, it did examine tension at displacement of 10 and 20 mm. Tension increased from 27 N to 60 N, correlating with a 2.2-fold difference between the 2 distances. Domb and colleagues35 also compared in vivo rotator cuff tension differences between the medial footprint and the lateral footprint in 4 patients. Mean tension applied to the cuff during reduction to the articular margin was 27 N, or 6 pounds. Mean tension needed to reduce the cuff to the lateral tuberosity was 76 N, or 17 pounds, for a 2.8-fold difference. Tears were not measured but were described as massive and retracted.
Although repair tension has long been recognized as a crucial factor in RCR healing, little clinical research has focused on the effects of excess tension. Davidson and Rivenburgh11 prospectively followed the clinical outcomes of 67 consecutive cuff repairs after intraoperative tension measurement and found that high-tension repairs (>8 lb) had significantly lower clinical outcome measures. However, the authors did not report on correlations with radiologic healing and stated, “Functional outcome is inversely proportional to rotator cuff repair tension.” Further study of the in vivo effects of increased tension on clinical and radiologic outcomes is needed.
Several animal studies have been conducted on the effects of tension on RCRs. Gerber and colleagues36 reported that the force needed to produce 1 cm of sheep supraspinatus tendon excursion increased 7-fold, from 6.8 N to 47.8 N, after 40 weeks of tendon tear. Coleman and colleagues37 compared the modulus of elasticity in sheep supraspinatus tendon after 6 weeks and 18 weeks of detachment and reported increases of 60% and 70%, respectively. Gimbel and colleagues38 showed that, in a rat model, “repair tension rapidly increased initially after injury followed by a progressive, but less dramatic, increase with additional time.” Of note, we did not identify any correlation between chronicity of symptoms and the tension needed to reduce the tendon medially or to a more lateral position on the footprint.
In acute tears, the cuff tissue is more compliant and mobile and can be pulled laterally across its anatomical footprint with minimal tension.39 In contrast, cuff tissue in the more commonly encountered chronic tear is less compliant and is not mobile enough to be pulled to the lateral margin of the footprint without added stress.30,34,35 In large, acute tears in which there are minimal tissue degeneration and retraction, a laterally based footprint-restoring technique may be performed with minimal tension. This technique may have advantages over a medially based repair. In the literature, more attention needs to be directed toward the biomechanics and biology of chronic rotator cuff tears, as these are more commonly encountered.
Almost all of the prospective studies that have compared single- and double-row RCR have found no significant differences in MRI healing rates or clinical results at follow-up up to 2 years.14,16,40-45 Detailed analysis of the surgical techniques used in all these studies revealed that the rotator cuff tendons were repaired back to the lateral footprint in both the single- and double-row constructs.14,16,40-45 Although no clinical studies have compared medially and laterally based single-row repairs, our data suggest that medially based repairs have lower tensions and therefore should not be considered equivalent. Sostak and colleagues31 and Murray and colleagues46 have shown that a medially based single-row RCR can achieve excellent clinical and anatomical results, likely partly because of the lower tension applied to the torn cuff tissue.31,46 Studies are needed to compare medially and laterally based repairs, including single- and double-row repairs.
The vast majority of recent research has aimed to counteract construct tension with stronger biomechanical constructs.20-26 Surgeons have also aimed to improve biological healing by pulling the tendon laterally across the footprint to achieve complete footprint coverage, ultimately increasing the surface area for tendon–bone healing. This has led to the development of various double-row repair techniques, in which the cuff tendon is pulled to the lateral margin of its footprint. One row of anchors is placed in the medial aspect of the footprint, while a second is placed in the lateral aspect; the cuff is reduced and compressed to the tuberosity with various suture configurations. The TOE technique was developed to improve pressurization of the cuff tendon across the footprint by linking the 2 rows with bridging sutures. In doing so, however, the potentially deleterious effects of increased tension introduced by pulling the tendon laterally may have been overlooked. Nevertheless, the biomechanics and stress distribution likely differ between single-row repair and TOE repairs, and direct comparisons cannot be made at this time. The medial row of a double-row or TOE construct may stress-shield or “unload” the more lateral tissue. Studies are needed in order to better understand the tension differential and stress distribution of various double-row constructs.
Recognizing tear morphology is crucial in maximizing chances of healing after cuff repair. For example, a crescent-shaped tear is reduced to the tuberosity with direct lateral translation of the apex of the tear, which is also the deepest or most displaced part of the tear. On the other hand, reducing an L- or reverse L-shaped tear to the tuberosity is not as direct; reducing the deepest or most displaced part of the tear would lead to overreduction and overtensioning of the tendon. However, often the exact “elbow” of the tear is not obvious and appears more rounded; therefore, it is crucial for the surgeon to examine the mobility of the torn tendon along its entire length to minimize tension. Study is needed to assess tension along the entire length of the tear for different tear morphologies and sizes.
Although our results showed that increased tension was needed to reduce a torn tendon to its lateral footprint, no study has indicated exactly how much is “too much” tension. As stated earlier, use of stronger biomechanical constructs, including TOE constructs, may overcome the increased tension associated with laterally based repairs. In addition, laterally based repairs, either single- or double-row, may be best suited for tears with lower tension, whereas medially based repairs may be best suited for higher tension tears. It is also possible that the difference in tensions noted in this study is not significant enough to have a clinical impact on choice of construct or on anatomical healing. We need studies that correlate anatomical healing rates with repair tension in order to better guide surgeons on when to use a medially or laterally based repair.
Other possible effects of increased tension associated with laterally based repairs, including beneficial effects, must be considered as well. Viscoelastic properties of human rotator cuff tendon may dissipate increased tension over time through a variety of mechanisms. Stress relaxation, gap formation, creep, and the hysteresis effect, all associated with cyclical loading in the early healing period, may lead to dissipation of force over time.47,48 These more complex biomechanical properties of RCR constructs are yet to be clearly defined.
This study had several weaknesses. Its data represent a static measurement of time-zero rotator cuff tension, which greatly simplifies the biomechanics of the torn rotator cuff and repair construct as well as changes that occur with healing. During cuff repair, forces typically are distributed through several fixation points in stepwise process and are not focused on a single point of tissue with a grasper. Therefore, the findings of this study may not directly correlate with medially versus laterally based repairs in vivo. Furthermore, as this is a time-zero measurement, we could not determine whether the tension differential between the 2 repair positions remained static over time. Current literature suggests that muscle atrophy, fatty infiltration, and loss of elasticity of the musculotendinous unit are relatively irreversible.35,37,49 In addition, determining the precise apex of a cuff tear can be difficult, so error may have been introduced during this process. Last, although placement of the cuff tissue at the medial or lateral footprint position was based on visual estimation by an experienced and skilled arthroscopist, error may have been introduced based on this imprecise technique.
Conclusion
This study demonstrated a significant, 5.4-fold increase in in vivo time-zero rotator cuff tension with the tendon edge reduced to the lateral footprint rather than the medial footprint.
1. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
2. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
5. Gulotta LV, Nho SJ, Dodson CC, Adler RS, Altchek DW, MacGillivray JD; HSS Arthroscopic Rotator Cuff Registry. Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part II—prognostic factors for clinical and radiographic outcomes. J Shoulder Elbow Surg. 2011;20(6):941-946.
6. Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009;1(2):96-104.
7. Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719-728.
8. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
9. Tashjian RZ, Hollins AM, Kim HM, et al. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2435-2442.
10. Burkhart SS, Lo IK. Arthroscopic rotator cuff repair. J Am Acad Orthop Surg. 2006;14(6):333-346.
11. Davidson PA, Rivenburgh DW. Rotator cuff repair tension as a determinant of functional outcome. J Shoulder Elbow Surg. 2000;9(6):502-506.
12. Goutallier D, Postel JM, Van Driessche S, Godefroy D, Radier C. Tension-free cuff repairs with excision of macroscopic tendon lesions and muscular advancement: results in a prospective series with limited fatty muscular degeneration. J Shoulder Elbow Surg. 2006;15(2):164-172.
13. Gimbel JA, Van Kleunen JP, Lake SP, Williams GR, Soslowsky LJ. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40(3):561-568.
14. Ma HL, Chiang ER, Wu HT, et al. Clinical outcome and imaging of arthroscopic single-row and double-row rotator cuff repair: a prospective randomized trial. Arthroscopy. 2012;28(1):16-24.
15. Mihata T, Watanabe C, Fukunishi K, et al. Functional and structural outcomes of single-row versus double-row versus combined double-row and suture-bridge repair for rotator cuff tears. Am J Sports Med. 2011;39(10):2091-2098.
16. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
17. Voigt C, Bosse C, Vosshenrich R, Schulz AP, Lill H. Arthroscopic supraspinatus tendon repair with suture-bridging technique: functional outcome and magnetic resonance imaging. Am J Sports Med. 2010;38(5):983-991.
18. Lafosse L, Brzoska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 2):275-286.
19. Park JY, Lhee SH, Choi JH, Park HK, Yu JW, Seo JB. Comparison of the clinical outcomes of single- and double-row repairs in rotator cuff tears. Am J Sports Med. 2008;36(7):1310-1316.
20. Kim DH, ElAttrache NS, Tibone JE, et al. Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med. 2006;34(3):407-414.
21. Mazzocca AD, Bollier MJ, Ciminiello AM, et al. Biomechanical evaluation of arthroscopic rotator cuff repairs over time. Arthroscopy. 2010;26(5):592-599.
22. Grimberg J, Diop A, Kalra K, Charousset C, Duranthon LD, Maurel N. In vitro biomechanical comparison of three different types of single- and double-row arthroscopic rotator cuff repairs: analysis of continuous bone–tendon contact pressure and surface during different simulated joint positions. J Shoulder Elbow Surg. 2010;19(2):236-243.
23. Nelson CO, Sileo MJ, Grossman MG, Serra-Hsu F. Single-row modified Mason-Allen versus double-row arthroscopic rotator cuff repair: a biomechanical and surface area comparison. Arthroscopy. 2008;24(8):941-948.
24. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
25. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
26. Ma CB, Comerford L, Wilson J, Puttlitz CM. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88(2):403-410.
27. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
28. Tuoheti Y, Itoi E, Yamamoto N, et al. Contact area, contact pressure, and pressure patterns of the tendon–bone interface after rotator cuff repair. Am J Sports Med. 2005;33(12):1869-1874.
29. Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606-610.
30. Snyder SJ. Single vs. double row suture anchor fixation rotator cuff repair. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; March 8, 2007; San Francisco, CA.
31. Sostak JP, Bahk MS, Getelman MH, Wong IH, Snyder SJ, Burns JP. Arthroscopic single row rotator cuff repair using the “SCOI row”: structural and clinical outcomes. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; February 7-11, 2012; San Francisco, CA.
32. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599-605.
33. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
34. Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998;7(4):393-396.
35. Domb BG, Glousman RE, Brooks A, Hansen M, Lee TQ, ElAttrache NS. High-tension double-row footprint repair compared with reduced-tension single-row repair for massive rotator cuff tears. J Bone Joint Surg Am. 2008;90(suppl 4):35-39.
36. Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86(9):1973-1982.
37. Coleman SH, Fealy S, Ehteshami JR, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am. 2003;85(12):2391-2402.
38. Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res. 2004;(426):258-265.
39. Mannava S, Plate JF, Whitlock PW, et al. Evaluation of in vivo rotator cuff muscle function after acute and chronic detachment of the supraspinatus tendon: an experimental study in an animal model. J Bone Joint Surg Am. 2011;93(18):1702-1711.
40. Burks RT, Crim J, Brown N, Fink B, Greis PE. A prospective randomized clinical trial comparing arthroscopic single- and double-row rotator cuff repair: magnetic resonance imaging and early clinical evaluation. Am J Sports Med. 2009;37(4):674-682.
41. Grasso A, Milano G, Salvatore M, Falcone G, Deriu L, Fabbriciani C. Single-row versus double-row arthroscopic rotator cuff repair: a prospective randomized clinical study. Arthroscopy. 2009;25(1):4-12.
42. Franceschi F, Ruzzini L, Longo UG, et al. Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2007;35(8):1254-1260.
43. Carbonel I, Martinez AA, Calvo A, Ripalda J, Herrera A. Single-row versus double-row arthroscopic repair in the treatment of rotator cuff tears: a prospective randomized clinical study. Int Orthop. 2012;36(9):1877-1883.
44. Lapner PL, Sabri E, Rakhra K, et al. A multicenter randomized controlled trial comparing single-row with double-row fixation in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(14):1249-1257.
45. Gartsman GM, Drake G, Edwards TB, et al. Ultrasound evaluation of arthroscopic full-thickness supraspinatus rotator cuff repair: single-row versus double-row suture bridge (transosseous equivalent) fixation. Results of a prospective, randomized study. J Shoulder Elbow Surg. 2013;22(11):1480-1487.
46. Murray TF Jr, Lajtai G, Mileski RM, Snyder SJ. Arthroscopic repair of medium to large full-thickness rotator cuff tears: outcome at 2- to 6-year follow-up. J Shoulder Elbow Surg. 2002;11(1):19-24.
47. Szczesny SE, Peloquin JM, Cortes DH, Kadlowec JA, Soslowsky LJ, Elliott DM. Biaxial tensile testing and constitutive modeling of human supraspinatus tendon. J Biomech Eng. 2012;134(2):021004.
48. Chaudhury S, Holland C, Vollrath F, Carr AJ. Comparing normal and torn rotator cuff tendons using dynamic shear analysis. J Bone Joint Surg Br. 2011;93(7):942-948.
49. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
1. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
2. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
5. Gulotta LV, Nho SJ, Dodson CC, Adler RS, Altchek DW, MacGillivray JD; HSS Arthroscopic Rotator Cuff Registry. Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part II—prognostic factors for clinical and radiographic outcomes. J Shoulder Elbow Surg. 2011;20(6):941-946.
6. Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009;1(2):96-104.
7. Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719-728.
8. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
9. Tashjian RZ, Hollins AM, Kim HM, et al. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2435-2442.
10. Burkhart SS, Lo IK. Arthroscopic rotator cuff repair. J Am Acad Orthop Surg. 2006;14(6):333-346.
11. Davidson PA, Rivenburgh DW. Rotator cuff repair tension as a determinant of functional outcome. J Shoulder Elbow Surg. 2000;9(6):502-506.
12. Goutallier D, Postel JM, Van Driessche S, Godefroy D, Radier C. Tension-free cuff repairs with excision of macroscopic tendon lesions and muscular advancement: results in a prospective series with limited fatty muscular degeneration. J Shoulder Elbow Surg. 2006;15(2):164-172.
13. Gimbel JA, Van Kleunen JP, Lake SP, Williams GR, Soslowsky LJ. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40(3):561-568.
14. Ma HL, Chiang ER, Wu HT, et al. Clinical outcome and imaging of arthroscopic single-row and double-row rotator cuff repair: a prospective randomized trial. Arthroscopy. 2012;28(1):16-24.
15. Mihata T, Watanabe C, Fukunishi K, et al. Functional and structural outcomes of single-row versus double-row versus combined double-row and suture-bridge repair for rotator cuff tears. Am J Sports Med. 2011;39(10):2091-2098.
16. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
17. Voigt C, Bosse C, Vosshenrich R, Schulz AP, Lill H. Arthroscopic supraspinatus tendon repair with suture-bridging technique: functional outcome and magnetic resonance imaging. Am J Sports Med. 2010;38(5):983-991.
18. Lafosse L, Brzoska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 2):275-286.
19. Park JY, Lhee SH, Choi JH, Park HK, Yu JW, Seo JB. Comparison of the clinical outcomes of single- and double-row repairs in rotator cuff tears. Am J Sports Med. 2008;36(7):1310-1316.
20. Kim DH, ElAttrache NS, Tibone JE, et al. Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med. 2006;34(3):407-414.
21. Mazzocca AD, Bollier MJ, Ciminiello AM, et al. Biomechanical evaluation of arthroscopic rotator cuff repairs over time. Arthroscopy. 2010;26(5):592-599.
22. Grimberg J, Diop A, Kalra K, Charousset C, Duranthon LD, Maurel N. In vitro biomechanical comparison of three different types of single- and double-row arthroscopic rotator cuff repairs: analysis of continuous bone–tendon contact pressure and surface during different simulated joint positions. J Shoulder Elbow Surg. 2010;19(2):236-243.
23. Nelson CO, Sileo MJ, Grossman MG, Serra-Hsu F. Single-row modified Mason-Allen versus double-row arthroscopic rotator cuff repair: a biomechanical and surface area comparison. Arthroscopy. 2008;24(8):941-948.
24. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
25. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
26. Ma CB, Comerford L, Wilson J, Puttlitz CM. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88(2):403-410.
27. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
28. Tuoheti Y, Itoi E, Yamamoto N, et al. Contact area, contact pressure, and pressure patterns of the tendon–bone interface after rotator cuff repair. Am J Sports Med. 2005;33(12):1869-1874.
29. Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606-610.
30. Snyder SJ. Single vs. double row suture anchor fixation rotator cuff repair. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; March 8, 2007; San Francisco, CA.
31. Sostak JP, Bahk MS, Getelman MH, Wong IH, Snyder SJ, Burns JP. Arthroscopic single row rotator cuff repair using the “SCOI row”: structural and clinical outcomes. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; February 7-11, 2012; San Francisco, CA.
32. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599-605.
33. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
34. Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998;7(4):393-396.
35. Domb BG, Glousman RE, Brooks A, Hansen M, Lee TQ, ElAttrache NS. High-tension double-row footprint repair compared with reduced-tension single-row repair for massive rotator cuff tears. J Bone Joint Surg Am. 2008;90(suppl 4):35-39.
36. Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86(9):1973-1982.
37. Coleman SH, Fealy S, Ehteshami JR, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am. 2003;85(12):2391-2402.
38. Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res. 2004;(426):258-265.
39. Mannava S, Plate JF, Whitlock PW, et al. Evaluation of in vivo rotator cuff muscle function after acute and chronic detachment of the supraspinatus tendon: an experimental study in an animal model. J Bone Joint Surg Am. 2011;93(18):1702-1711.
40. Burks RT, Crim J, Brown N, Fink B, Greis PE. A prospective randomized clinical trial comparing arthroscopic single- and double-row rotator cuff repair: magnetic resonance imaging and early clinical evaluation. Am J Sports Med. 2009;37(4):674-682.
41. Grasso A, Milano G, Salvatore M, Falcone G, Deriu L, Fabbriciani C. Single-row versus double-row arthroscopic rotator cuff repair: a prospective randomized clinical study. Arthroscopy. 2009;25(1):4-12.
42. Franceschi F, Ruzzini L, Longo UG, et al. Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2007;35(8):1254-1260.
43. Carbonel I, Martinez AA, Calvo A, Ripalda J, Herrera A. Single-row versus double-row arthroscopic repair in the treatment of rotator cuff tears: a prospective randomized clinical study. Int Orthop. 2012;36(9):1877-1883.
44. Lapner PL, Sabri E, Rakhra K, et al. A multicenter randomized controlled trial comparing single-row with double-row fixation in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(14):1249-1257.
45. Gartsman GM, Drake G, Edwards TB, et al. Ultrasound evaluation of arthroscopic full-thickness supraspinatus rotator cuff repair: single-row versus double-row suture bridge (transosseous equivalent) fixation. Results of a prospective, randomized study. J Shoulder Elbow Surg. 2013;22(11):1480-1487.
46. Murray TF Jr, Lajtai G, Mileski RM, Snyder SJ. Arthroscopic repair of medium to large full-thickness rotator cuff tears: outcome at 2- to 6-year follow-up. J Shoulder Elbow Surg. 2002;11(1):19-24.
47. Szczesny SE, Peloquin JM, Cortes DH, Kadlowec JA, Soslowsky LJ, Elliott DM. Biaxial tensile testing and constitutive modeling of human supraspinatus tendon. J Biomech Eng. 2012;134(2):021004.
48. Chaudhury S, Holland C, Vollrath F, Carr AJ. Comparing normal and torn rotator cuff tendons using dynamic shear analysis. J Bone Joint Surg Br. 2011;93(7):942-948.
49. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
Clinical Outcomes of Minimally Invasive Versus Open TLIF: A Propensity-Matched Cohort Study
Transforaminal lumbar interbody fusion (TLIF) has become an increasingly popular method of lumbar fusion, since its introduction by Harms and Rolinger in 1982.1 The procedure allows for a circumferential fusion through a posterior-only approach, with improved sagittal alignment2 and minimal risk for iatrogenic nerve injury. In the past decade, a minimally invasive surgical method of TLIF (MIS TLIF) has been introduced3-5 and involves neural decompression and interbody fusion through a tubular retractor, and percutaneous placement of pedicle-screw instrumentation. This technique uses muscle dilation rather than large-scale detachment of muscle. Proponents of the MIS technique have postulated that decreased muscle damage would lead to better short-term, and possibly long-term, clinical outcomes, because of less iatrogenic soft-tissue damage.
Studies that have compared results of MIS TLIF with open TLIF have shown improved perioperative outcomes, but most have shown similar intermediate-term clinical outcomes.6 In the short term, multiple studies demonstrate that MIS TLIF is associated with decreased blood loss, less postoperative pain and narcotic requirements, and shorter hospital length of stay.7-13 However, changes in pain score and disease-specific and generic health-related quality of life measures have been similar for the 2 procedures, beyond 6 months postoperatively.10,13-15 These studies have generally involved retrospective reviews of unmatched patient groups, with small sample sizes and significant heterogeneity in surgical indications and case complexity. In our study, we compared intermediate-term clinical outcomes of MIS TLIF with open TLIF, using propensity matching to optimize baseline similarity of the groups.
Methods
This retrospective study was conducted after receiving approval from the Institutional Review Board. Surgical and clinical databases of 2 centers from 2008 to 2012 were reviewed for eligible subjects. Cases in 2007 were excluded because this was the year that MIS was introduced as a new technique in the practice. Inclusion criteria consisted of patients who underwent 1- to 2-level MIS TLIF and had complete baseline, 1- and 2-year postoperative outcome measures. Patients who had surgery for trauma, tumor, or osteomyelitis were excluded. Outcome measures collected and reviewed in this study included the Oswestry Disability Index (ODI),16,17 the Medical Outcomes Study Short-Form 36 (SF-36),18 and numeric rating scales for back and leg pain (0-100 scale).19 The Physical Composite Summary (PCS) and Mental Composite Summary of the SF-36 were reviewed separately. We recorded the following patient demographic data: age, gender, American Society of Anesthesiologists (ASA) grade, body mass index, indication for surgery, workers’ compensation, and smoking status. Surgical data included number of levels fused, operative time, estimated blood loss, and length of hospital stay.
Propensity-scoring technique20,21 was used to match the MIS TLIF patients to a control group of patients who underwent TLIF using an open approach (open TLIF), matching for multiple characteristics to produce 2 similar comparison groups. Propensity matching was performed to control for bias. In controlling for known confounders or biases, propensity matching, in theory, should also control for unknown confounders. Gender, age, body mass index, smoking status, indication for fusion, as well as preoperative ODI, SF-36 PCS, SF-36 Mental Composite Summary, and pain scores were used to generate a control open TLIF group.
MIS TLIF Surgical Technique
Patients in the MIS TLIF group underwent neural decompression and interbody fusion through a tubular retractor system (METRx, Medtronic Inc.), followed by percutaneous pedicle-screw fixation under fluoroscopic guidance (Sextant, Medtronic Inc.). After successful induction of general endotracheal anesthesia, patients were positioned prone on a radiolucent table. Posteroanterior (PA) and lateral fluoroscopic images were used to localize 2 paramedian incisions, approximately 3-cm to 5-cm lateral to midline, over the pedicles of interest. Modified Jamshidi needles (Medtronic Inc.) were used to cannulate the pedicles under PA, posterior-oblique, PA, and lateral fluoroscopic guidance. The pedicles were tapped with a cannulated tap. Pedicle screws and rods were introduced on the side contralateral to the TLIF and were used as needed to maintain intradiscal distraction during the TLIF portion of the procedure.
Decompression and TLIF were carried out on the side of the patient’s radicular pain or bilaterally, according to the surgeon’s discretion. A K-wire was advanced to the facet joint complex, after which sequential dilators were used to dilate through the muscles to establish an intramuscular corridor to the facet. A 26-mm fixed tubular retractor was docked over the facet and locked in place, using a post attached to the operating room table. Neural decompression was obtained by removal of the entire facet-joint complex and lamina to the base of the spinous process, using a combination of high-speed drills and Kerrison rongeurs. The ligamentum flavum was completely resected. The superior articular process of the caudal vertebra was removed all the way to the pedicle below. Ball-tipped probes were used to confirm that traversing and exiting nerve roots were completely free. An annulotomy was performed, and all disc material was removed from the disc through a combination of rotating shavers, serrated curettes, endplate scrapers, and rasps. Bone graft was placed anterior and contralateral to the interbody cage. (Bone grafts included autogenous iliac crest, local bone obtained from the decompression, recombinant human bone morphogenetic protein 2, or allograft demineralized bone matrix at the surgeon’s discretion.) After placement of the interbody cage, the ipsilateral pedicle-screw instrumentation was put over the remaining guide wires and compression applied across the construct to lock the interbody cage and restore lordosis. Wounds were closed without drains.
Open TLIF Surgical Technique
In patients undergoing open TLIF, a midline incision was made over the vertebrae of interest, and paraspinal muscles were subperiosteally dissected to the tips of the transverse processes. The appropriate level was confirmed with intraoperative radiograph. Pedicle screws were placed free-hand using anatomic landmarks, and appropriate placement was confirmed with intraoperative radiograph and evoked electromyography stimulation. Laminectomy and facetectomy were performed, and the disc was entered on the side of the facetectomy. After thorough disc-space preparation, bone graft and an interbody cage were placed, rods inserted, and compression carried out. A supplemental posterolateral fusion was also performed after decortication of the transverse processes and cartilaginous surface of the contralateral facet. Layered wound closure was performed over drains.
Analysis
Statistical analysis was carried out using SPSS Statistics version 17.0 (IBM) with significance set at the P < .01 level. A small, conservative P-value threshold was used to minimize type II error that resulted from the multiple comparisons performed. Student t test was used to determine any significant differences between continuous demographic variables, and to compare preoperative and postoperative outcome measure scores within and between study groups. Fisher’s exact test was used to compare categorical variables between the 2 groups.
Results
The MIS TLIF group consisted of 64 patients (average age, 52 years), and included 22 patients with degenerative spondylolisthesis, 33 with disc pathology, 8 with postdecompression, and 1 non-union patient. The open TLIF group consisted of 64 patients (average age, 54 years), and included 39 degenerative spondylolisthesis, 15 disc pathology, 7 postdecompression, and 3 nonunion patients (Table 1). All 64 open and 19 MIS cases were from a spine practice with 6 surgeons, and 45 MIS cases came from a spine practice with 2 surgeons. There was also an unequal distribution of the specific levels fused between the open and MIS groups.
Although the operative time was similar in both groups, the MIS TLIF group had a statistically significantly lower blood loss compared with the open TLIF group (Table 2). Both MIS TLIF and open TLIF lead to significant improvements in pain, ODI, and SF-36 PCS (P < .01) (Table 3). At 1 year, both groups had similar improvements in pain (36.9 vs 30.8, P = .178) and SF-36 PCS (9.9 vs 7.5, P = .231), but the MIS TLIF group had a statistically significantly greater improvement in ODI compared with the open TLIF group (30.4 vs 15.1, P < .000). At 2 years, both groups had similar improvements in SF-36 PCS (12.1 vs 7.5, P = .033), but the MIS TLIF group had a statistically significantly greater improvement in pain (40.2 vs 27.0, P = .005) and ODI (33.1 vs 15.4, P < .000) compared with the open TLIF group (Table 4).
Discussion
The current study compared intermediate-term clinical outcomes of MIS TLIF to open TLIF. We used propensity matching to identify a control group of open TLIFs that were comparable to the MIS TLIF group across a variety of covariates that are known to influence the results of lumbar fusion. This created comparison groups that were as closely matched at baseline as possible. We found that, at 2-year follow-up, MIS TLIF patients had less pain and less low-back pain–related disability as measured by ODI. There was also a trend toward better generic health-related quality of life in the MIS TLIF group.
These data suggest that the decreased soft-tissue trauma of the minimally invasive surgical technique, which leads to improved perioperative parameters in the short term, may also lead to some advantages that translate to improved intermediate-term clinical outcomes. Traditional lumbar fusion procedures have shown excellent clinical results when used for accepted clinical indications.22 However, the procedure requires extensive dissection of the paraspinal muscles, which causes significant muscle damage as evidenced by muscle breakdown products that can be detected in the bloodstream postoperatively.23,24 The lateral dissection also transects the dorsal ramus of the segmental nerves, which innervate the paraspinal muscles, leading to significant scarring and atrophy on postoperative imaging studies.23 Some authors have used the term “fusion disease” to describe the constellation of soft-tissue degradation seen after open lumbar fusion.5
An MIS version of the TLIF procedure that was described in 20033 avoids much of this iatrogenic soft-tissue trauma. It involves intramuscular dilation to approach the spine and to carry out neural decompression and interbody fusion, in conjunction with percutaneous pedicle-screw instrumentation. Proponents of this technique point to diminished iatrogenic soft-tissue and muscle damage as an advantage. Multiple studies have, in fact, confirmed improved short-term perioperative parameters, such as less blood loss, lower narcotic requirements, and decreased length-of-hospital stay.25 Economic analyses have also shown lower direct and indirect costs with the MIS technique.26
Several studies have compared patient-reported outcome measures of MIS and open TLIF, and the results have been mixed. Most of these studies have shown similar improvement in clinical outcomes between the 2 procedures, but the MIS technique demonstrated short-term perioperative advantages, such as lower blood loss, less narcotic requirements, and shorter length of stay.7-15 The authors of these studies conclude that the MIS technique can provide similar long-term results with lower short-term morbidity when compared with open TLIF. In contrast, some studies have shown better short- and intermediate-term clinical outcomes with the MIS technique.23,27-29 As a whole, the literature comparing the 2 procedures consists of mostly small retrospective studies with nonrandomized patient samples, heterogeneous surgical indications, and differing surgical techniques, making it difficult to draw conclusions.
The current study suggests that MIS TLIF may lead to improved clinical results at 2-year follow-up, compared with open TLIF. Our study used propensity-score matching to minimize the effects of nonrandom assignment of subjects to MIS TLIF or open TLIF. A limitation of observational studies is that bias in assignment of subjects to treatment groups can lead to overestimation or underestimation of the effect of the treatment itself. Propensity-score matching attempts to reduce this bias by accounting for several covariates that predict whether a subject will receive a certain treatment. These covariates are used in a logistic regression to produce a propensity score, which can be used to match subjects to controls across multiple dimensions, thus ensuring groups are as comparable as possible at baseline.
Our study still has several limitations. Sample size is relatively small, and follow-up is still only intermediate, at 2 years. There was unequal distribution of specific levels of surgery. Because patients were not blinded to the treatment they received, it is possible that patient perception of receiving a newer, less-invasive treatment method may influence their subjective improvement. The study sample was drawn from 2 different centers, with one center providing mostly MIS cases and the other providing mostly open cases. Because of this, undetected differences in how patients were selected for surgery could also affect outcomes. Any latent confounding variables, which are not identified a priori, will not be accounted for in the matching process. Only a prospective, randomized study with large numbers can control for observed and unobserved confounding patient characteristics.
In summary, our study shows that MIS TLIF is associated with improved low back pain and low back–related disability at 2 years compared with open TLIF. Other studies comparing the 2 techniques have come to different conclusions regarding whether the short-term benefits of MIS TLIF translate into long-term differences in clinical outcome. This study adds to this evidence and suggests there may be longer term advantages to the MIS approach, but prospective randomized trials are needed to confirm this finding and determine the true magnitude of these differences.
1. Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolisthesis: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb. 1982;120(3):343-347.
2. Jagannathan J, Sansur CA, Oskouian RJ Jr, Fu KM, Shaffrey CI. Radiographic restoration of lumbar alignment after transforaminal lumbar interbody fusion. Neurosurgery. 2009;64(5):955-963.
3. Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28(15 suppl):S26-S35.
4. Rouben D, Casnellie M, Ferguson M. Long-term durability of minimally invasive posterior transforaminal lumbar interbody fusion: a clinical and radiographic follow-up. J Spinal Disord Tech. 2011;24(5):288-296.
5. Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(suppl):S1-S6.
6. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res. 2014;472(6):1727-1737.
7. Adogwa O, Parker SL, Bydon A, Cheng J, McGirt MJ. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech. 2011;24(8):479-484.
8. Ghahreman A, Ferch RD, Rao PJ, Bogduk N. Minimal access versus open posterior lumbar interbody fusion in the treatment of spondylolisthesis. Neurosurgery. 2010;66(2):296-304.
9. Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32(5):537-543.
10. Saetia K, Phankhongsab A, Kuansongtham V, Paiboonsirijit S. Comparison between minimally invasive and open transforaminal lumbar interbody fusion. J Med Assoc Thai. 2013;96(1):41-46.
11. Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Ortop. 2009;33(6):1683-1688.
12. Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Eur Spine J. 2010;19(1):1780-1784.
13. Lee KH, Yue WM, Yeo W, Soeharno H, Tan SB. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21(11):2265-2270.
14. Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine. 2009;34(13):1385-1389.
15. Seng C, Siddiqui MA, Wong KP, et al. Five-year outcomes of minimally invasive versus open transforaminal lumbar interbody fusion: a matched-pair comparison study. Spine. 2013;38(23):2049-2055.
16. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940-2953.
17. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273.
18. Ware JE, Kosinski M, Keller SK. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute, 1994.
19. McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Baltimore, MD: V.V. Mosby Company, 1993.
20. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281.
21. Rosenbaum PR. Model-based direct adjustment. J Am Stat Assn. 1987;82:387-394.
22. Glassman SD, Carreon LY, Djurasovic M, et al. Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J. 2009;9(1):13-21.
23. Fan S, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19(2):316-324.
24. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine. 1996;21(8):941-944.
25. Sun ZJ, Li WJ, Zhao Y, Qui GX. Comparing minimally invasive and open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: a meta-analysis. Chin Med J. 2013;126(2):3962-3971.
26. Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg. 2014;82(1-2):230-238.
27. Kotani Y, Abumi K, Ito M, Sudo H, Abe Y, Minami A. Mid-term clinical results of minimally invasive decompression and posterolateral fusion with percutaneous pedicle screws versus conventional approach for degenerative spondylolisthesis with spinal stenosis. Eur Spine J. 2012;21(6):1171-1177.
28. Pelton MA, Phillips FM, Singh K. A comparison of perioperative costs and outcomes in patients with and without worker’s compensation claims treated with MIS or open TLIF. Spine. 2012;37(22):1914-1919.
29. Wong AP, Smith ZA, Stadler JA 3rd, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF). Surgical technique, long-term 4 year prospective outcomes and complications compared with an open TLIF cohort. Neurosurg Clin N Am. 2014;25(2):279-304.
Transforaminal lumbar interbody fusion (TLIF) has become an increasingly popular method of lumbar fusion, since its introduction by Harms and Rolinger in 1982.1 The procedure allows for a circumferential fusion through a posterior-only approach, with improved sagittal alignment2 and minimal risk for iatrogenic nerve injury. In the past decade, a minimally invasive surgical method of TLIF (MIS TLIF) has been introduced3-5 and involves neural decompression and interbody fusion through a tubular retractor, and percutaneous placement of pedicle-screw instrumentation. This technique uses muscle dilation rather than large-scale detachment of muscle. Proponents of the MIS technique have postulated that decreased muscle damage would lead to better short-term, and possibly long-term, clinical outcomes, because of less iatrogenic soft-tissue damage.
Studies that have compared results of MIS TLIF with open TLIF have shown improved perioperative outcomes, but most have shown similar intermediate-term clinical outcomes.6 In the short term, multiple studies demonstrate that MIS TLIF is associated with decreased blood loss, less postoperative pain and narcotic requirements, and shorter hospital length of stay.7-13 However, changes in pain score and disease-specific and generic health-related quality of life measures have been similar for the 2 procedures, beyond 6 months postoperatively.10,13-15 These studies have generally involved retrospective reviews of unmatched patient groups, with small sample sizes and significant heterogeneity in surgical indications and case complexity. In our study, we compared intermediate-term clinical outcomes of MIS TLIF with open TLIF, using propensity matching to optimize baseline similarity of the groups.
Methods
This retrospective study was conducted after receiving approval from the Institutional Review Board. Surgical and clinical databases of 2 centers from 2008 to 2012 were reviewed for eligible subjects. Cases in 2007 were excluded because this was the year that MIS was introduced as a new technique in the practice. Inclusion criteria consisted of patients who underwent 1- to 2-level MIS TLIF and had complete baseline, 1- and 2-year postoperative outcome measures. Patients who had surgery for trauma, tumor, or osteomyelitis were excluded. Outcome measures collected and reviewed in this study included the Oswestry Disability Index (ODI),16,17 the Medical Outcomes Study Short-Form 36 (SF-36),18 and numeric rating scales for back and leg pain (0-100 scale).19 The Physical Composite Summary (PCS) and Mental Composite Summary of the SF-36 were reviewed separately. We recorded the following patient demographic data: age, gender, American Society of Anesthesiologists (ASA) grade, body mass index, indication for surgery, workers’ compensation, and smoking status. Surgical data included number of levels fused, operative time, estimated blood loss, and length of hospital stay.
Propensity-scoring technique20,21 was used to match the MIS TLIF patients to a control group of patients who underwent TLIF using an open approach (open TLIF), matching for multiple characteristics to produce 2 similar comparison groups. Propensity matching was performed to control for bias. In controlling for known confounders or biases, propensity matching, in theory, should also control for unknown confounders. Gender, age, body mass index, smoking status, indication for fusion, as well as preoperative ODI, SF-36 PCS, SF-36 Mental Composite Summary, and pain scores were used to generate a control open TLIF group.
MIS TLIF Surgical Technique
Patients in the MIS TLIF group underwent neural decompression and interbody fusion through a tubular retractor system (METRx, Medtronic Inc.), followed by percutaneous pedicle-screw fixation under fluoroscopic guidance (Sextant, Medtronic Inc.). After successful induction of general endotracheal anesthesia, patients were positioned prone on a radiolucent table. Posteroanterior (PA) and lateral fluoroscopic images were used to localize 2 paramedian incisions, approximately 3-cm to 5-cm lateral to midline, over the pedicles of interest. Modified Jamshidi needles (Medtronic Inc.) were used to cannulate the pedicles under PA, posterior-oblique, PA, and lateral fluoroscopic guidance. The pedicles were tapped with a cannulated tap. Pedicle screws and rods were introduced on the side contralateral to the TLIF and were used as needed to maintain intradiscal distraction during the TLIF portion of the procedure.
Decompression and TLIF were carried out on the side of the patient’s radicular pain or bilaterally, according to the surgeon’s discretion. A K-wire was advanced to the facet joint complex, after which sequential dilators were used to dilate through the muscles to establish an intramuscular corridor to the facet. A 26-mm fixed tubular retractor was docked over the facet and locked in place, using a post attached to the operating room table. Neural decompression was obtained by removal of the entire facet-joint complex and lamina to the base of the spinous process, using a combination of high-speed drills and Kerrison rongeurs. The ligamentum flavum was completely resected. The superior articular process of the caudal vertebra was removed all the way to the pedicle below. Ball-tipped probes were used to confirm that traversing and exiting nerve roots were completely free. An annulotomy was performed, and all disc material was removed from the disc through a combination of rotating shavers, serrated curettes, endplate scrapers, and rasps. Bone graft was placed anterior and contralateral to the interbody cage. (Bone grafts included autogenous iliac crest, local bone obtained from the decompression, recombinant human bone morphogenetic protein 2, or allograft demineralized bone matrix at the surgeon’s discretion.) After placement of the interbody cage, the ipsilateral pedicle-screw instrumentation was put over the remaining guide wires and compression applied across the construct to lock the interbody cage and restore lordosis. Wounds were closed without drains.
Open TLIF Surgical Technique
In patients undergoing open TLIF, a midline incision was made over the vertebrae of interest, and paraspinal muscles were subperiosteally dissected to the tips of the transverse processes. The appropriate level was confirmed with intraoperative radiograph. Pedicle screws were placed free-hand using anatomic landmarks, and appropriate placement was confirmed with intraoperative radiograph and evoked electromyography stimulation. Laminectomy and facetectomy were performed, and the disc was entered on the side of the facetectomy. After thorough disc-space preparation, bone graft and an interbody cage were placed, rods inserted, and compression carried out. A supplemental posterolateral fusion was also performed after decortication of the transverse processes and cartilaginous surface of the contralateral facet. Layered wound closure was performed over drains.
Analysis
Statistical analysis was carried out using SPSS Statistics version 17.0 (IBM) with significance set at the P < .01 level. A small, conservative P-value threshold was used to minimize type II error that resulted from the multiple comparisons performed. Student t test was used to determine any significant differences between continuous demographic variables, and to compare preoperative and postoperative outcome measure scores within and between study groups. Fisher’s exact test was used to compare categorical variables between the 2 groups.
Results
The MIS TLIF group consisted of 64 patients (average age, 52 years), and included 22 patients with degenerative spondylolisthesis, 33 with disc pathology, 8 with postdecompression, and 1 non-union patient. The open TLIF group consisted of 64 patients (average age, 54 years), and included 39 degenerative spondylolisthesis, 15 disc pathology, 7 postdecompression, and 3 nonunion patients (Table 1). All 64 open and 19 MIS cases were from a spine practice with 6 surgeons, and 45 MIS cases came from a spine practice with 2 surgeons. There was also an unequal distribution of the specific levels fused between the open and MIS groups.
Although the operative time was similar in both groups, the MIS TLIF group had a statistically significantly lower blood loss compared with the open TLIF group (Table 2). Both MIS TLIF and open TLIF lead to significant improvements in pain, ODI, and SF-36 PCS (P < .01) (Table 3). At 1 year, both groups had similar improvements in pain (36.9 vs 30.8, P = .178) and SF-36 PCS (9.9 vs 7.5, P = .231), but the MIS TLIF group had a statistically significantly greater improvement in ODI compared with the open TLIF group (30.4 vs 15.1, P < .000). At 2 years, both groups had similar improvements in SF-36 PCS (12.1 vs 7.5, P = .033), but the MIS TLIF group had a statistically significantly greater improvement in pain (40.2 vs 27.0, P = .005) and ODI (33.1 vs 15.4, P < .000) compared with the open TLIF group (Table 4).
Discussion
The current study compared intermediate-term clinical outcomes of MIS TLIF to open TLIF. We used propensity matching to identify a control group of open TLIFs that were comparable to the MIS TLIF group across a variety of covariates that are known to influence the results of lumbar fusion. This created comparison groups that were as closely matched at baseline as possible. We found that, at 2-year follow-up, MIS TLIF patients had less pain and less low-back pain–related disability as measured by ODI. There was also a trend toward better generic health-related quality of life in the MIS TLIF group.
These data suggest that the decreased soft-tissue trauma of the minimally invasive surgical technique, which leads to improved perioperative parameters in the short term, may also lead to some advantages that translate to improved intermediate-term clinical outcomes. Traditional lumbar fusion procedures have shown excellent clinical results when used for accepted clinical indications.22 However, the procedure requires extensive dissection of the paraspinal muscles, which causes significant muscle damage as evidenced by muscle breakdown products that can be detected in the bloodstream postoperatively.23,24 The lateral dissection also transects the dorsal ramus of the segmental nerves, which innervate the paraspinal muscles, leading to significant scarring and atrophy on postoperative imaging studies.23 Some authors have used the term “fusion disease” to describe the constellation of soft-tissue degradation seen after open lumbar fusion.5
An MIS version of the TLIF procedure that was described in 20033 avoids much of this iatrogenic soft-tissue trauma. It involves intramuscular dilation to approach the spine and to carry out neural decompression and interbody fusion, in conjunction with percutaneous pedicle-screw instrumentation. Proponents of this technique point to diminished iatrogenic soft-tissue and muscle damage as an advantage. Multiple studies have, in fact, confirmed improved short-term perioperative parameters, such as less blood loss, lower narcotic requirements, and decreased length-of-hospital stay.25 Economic analyses have also shown lower direct and indirect costs with the MIS technique.26
Several studies have compared patient-reported outcome measures of MIS and open TLIF, and the results have been mixed. Most of these studies have shown similar improvement in clinical outcomes between the 2 procedures, but the MIS technique demonstrated short-term perioperative advantages, such as lower blood loss, less narcotic requirements, and shorter length of stay.7-15 The authors of these studies conclude that the MIS technique can provide similar long-term results with lower short-term morbidity when compared with open TLIF. In contrast, some studies have shown better short- and intermediate-term clinical outcomes with the MIS technique.23,27-29 As a whole, the literature comparing the 2 procedures consists of mostly small retrospective studies with nonrandomized patient samples, heterogeneous surgical indications, and differing surgical techniques, making it difficult to draw conclusions.
The current study suggests that MIS TLIF may lead to improved clinical results at 2-year follow-up, compared with open TLIF. Our study used propensity-score matching to minimize the effects of nonrandom assignment of subjects to MIS TLIF or open TLIF. A limitation of observational studies is that bias in assignment of subjects to treatment groups can lead to overestimation or underestimation of the effect of the treatment itself. Propensity-score matching attempts to reduce this bias by accounting for several covariates that predict whether a subject will receive a certain treatment. These covariates are used in a logistic regression to produce a propensity score, which can be used to match subjects to controls across multiple dimensions, thus ensuring groups are as comparable as possible at baseline.
Our study still has several limitations. Sample size is relatively small, and follow-up is still only intermediate, at 2 years. There was unequal distribution of specific levels of surgery. Because patients were not blinded to the treatment they received, it is possible that patient perception of receiving a newer, less-invasive treatment method may influence their subjective improvement. The study sample was drawn from 2 different centers, with one center providing mostly MIS cases and the other providing mostly open cases. Because of this, undetected differences in how patients were selected for surgery could also affect outcomes. Any latent confounding variables, which are not identified a priori, will not be accounted for in the matching process. Only a prospective, randomized study with large numbers can control for observed and unobserved confounding patient characteristics.
In summary, our study shows that MIS TLIF is associated with improved low back pain and low back–related disability at 2 years compared with open TLIF. Other studies comparing the 2 techniques have come to different conclusions regarding whether the short-term benefits of MIS TLIF translate into long-term differences in clinical outcome. This study adds to this evidence and suggests there may be longer term advantages to the MIS approach, but prospective randomized trials are needed to confirm this finding and determine the true magnitude of these differences.
Transforaminal lumbar interbody fusion (TLIF) has become an increasingly popular method of lumbar fusion, since its introduction by Harms and Rolinger in 1982.1 The procedure allows for a circumferential fusion through a posterior-only approach, with improved sagittal alignment2 and minimal risk for iatrogenic nerve injury. In the past decade, a minimally invasive surgical method of TLIF (MIS TLIF) has been introduced3-5 and involves neural decompression and interbody fusion through a tubular retractor, and percutaneous placement of pedicle-screw instrumentation. This technique uses muscle dilation rather than large-scale detachment of muscle. Proponents of the MIS technique have postulated that decreased muscle damage would lead to better short-term, and possibly long-term, clinical outcomes, because of less iatrogenic soft-tissue damage.
Studies that have compared results of MIS TLIF with open TLIF have shown improved perioperative outcomes, but most have shown similar intermediate-term clinical outcomes.6 In the short term, multiple studies demonstrate that MIS TLIF is associated with decreased blood loss, less postoperative pain and narcotic requirements, and shorter hospital length of stay.7-13 However, changes in pain score and disease-specific and generic health-related quality of life measures have been similar for the 2 procedures, beyond 6 months postoperatively.10,13-15 These studies have generally involved retrospective reviews of unmatched patient groups, with small sample sizes and significant heterogeneity in surgical indications and case complexity. In our study, we compared intermediate-term clinical outcomes of MIS TLIF with open TLIF, using propensity matching to optimize baseline similarity of the groups.
Methods
This retrospective study was conducted after receiving approval from the Institutional Review Board. Surgical and clinical databases of 2 centers from 2008 to 2012 were reviewed for eligible subjects. Cases in 2007 were excluded because this was the year that MIS was introduced as a new technique in the practice. Inclusion criteria consisted of patients who underwent 1- to 2-level MIS TLIF and had complete baseline, 1- and 2-year postoperative outcome measures. Patients who had surgery for trauma, tumor, or osteomyelitis were excluded. Outcome measures collected and reviewed in this study included the Oswestry Disability Index (ODI),16,17 the Medical Outcomes Study Short-Form 36 (SF-36),18 and numeric rating scales for back and leg pain (0-100 scale).19 The Physical Composite Summary (PCS) and Mental Composite Summary of the SF-36 were reviewed separately. We recorded the following patient demographic data: age, gender, American Society of Anesthesiologists (ASA) grade, body mass index, indication for surgery, workers’ compensation, and smoking status. Surgical data included number of levels fused, operative time, estimated blood loss, and length of hospital stay.
Propensity-scoring technique20,21 was used to match the MIS TLIF patients to a control group of patients who underwent TLIF using an open approach (open TLIF), matching for multiple characteristics to produce 2 similar comparison groups. Propensity matching was performed to control for bias. In controlling for known confounders or biases, propensity matching, in theory, should also control for unknown confounders. Gender, age, body mass index, smoking status, indication for fusion, as well as preoperative ODI, SF-36 PCS, SF-36 Mental Composite Summary, and pain scores were used to generate a control open TLIF group.
MIS TLIF Surgical Technique
Patients in the MIS TLIF group underwent neural decompression and interbody fusion through a tubular retractor system (METRx, Medtronic Inc.), followed by percutaneous pedicle-screw fixation under fluoroscopic guidance (Sextant, Medtronic Inc.). After successful induction of general endotracheal anesthesia, patients were positioned prone on a radiolucent table. Posteroanterior (PA) and lateral fluoroscopic images were used to localize 2 paramedian incisions, approximately 3-cm to 5-cm lateral to midline, over the pedicles of interest. Modified Jamshidi needles (Medtronic Inc.) were used to cannulate the pedicles under PA, posterior-oblique, PA, and lateral fluoroscopic guidance. The pedicles were tapped with a cannulated tap. Pedicle screws and rods were introduced on the side contralateral to the TLIF and were used as needed to maintain intradiscal distraction during the TLIF portion of the procedure.
Decompression and TLIF were carried out on the side of the patient’s radicular pain or bilaterally, according to the surgeon’s discretion. A K-wire was advanced to the facet joint complex, after which sequential dilators were used to dilate through the muscles to establish an intramuscular corridor to the facet. A 26-mm fixed tubular retractor was docked over the facet and locked in place, using a post attached to the operating room table. Neural decompression was obtained by removal of the entire facet-joint complex and lamina to the base of the spinous process, using a combination of high-speed drills and Kerrison rongeurs. The ligamentum flavum was completely resected. The superior articular process of the caudal vertebra was removed all the way to the pedicle below. Ball-tipped probes were used to confirm that traversing and exiting nerve roots were completely free. An annulotomy was performed, and all disc material was removed from the disc through a combination of rotating shavers, serrated curettes, endplate scrapers, and rasps. Bone graft was placed anterior and contralateral to the interbody cage. (Bone grafts included autogenous iliac crest, local bone obtained from the decompression, recombinant human bone morphogenetic protein 2, or allograft demineralized bone matrix at the surgeon’s discretion.) After placement of the interbody cage, the ipsilateral pedicle-screw instrumentation was put over the remaining guide wires and compression applied across the construct to lock the interbody cage and restore lordosis. Wounds were closed without drains.
Open TLIF Surgical Technique
In patients undergoing open TLIF, a midline incision was made over the vertebrae of interest, and paraspinal muscles were subperiosteally dissected to the tips of the transverse processes. The appropriate level was confirmed with intraoperative radiograph. Pedicle screws were placed free-hand using anatomic landmarks, and appropriate placement was confirmed with intraoperative radiograph and evoked electromyography stimulation. Laminectomy and facetectomy were performed, and the disc was entered on the side of the facetectomy. After thorough disc-space preparation, bone graft and an interbody cage were placed, rods inserted, and compression carried out. A supplemental posterolateral fusion was also performed after decortication of the transverse processes and cartilaginous surface of the contralateral facet. Layered wound closure was performed over drains.
Analysis
Statistical analysis was carried out using SPSS Statistics version 17.0 (IBM) with significance set at the P < .01 level. A small, conservative P-value threshold was used to minimize type II error that resulted from the multiple comparisons performed. Student t test was used to determine any significant differences between continuous demographic variables, and to compare preoperative and postoperative outcome measure scores within and between study groups. Fisher’s exact test was used to compare categorical variables between the 2 groups.
Results
The MIS TLIF group consisted of 64 patients (average age, 52 years), and included 22 patients with degenerative spondylolisthesis, 33 with disc pathology, 8 with postdecompression, and 1 non-union patient. The open TLIF group consisted of 64 patients (average age, 54 years), and included 39 degenerative spondylolisthesis, 15 disc pathology, 7 postdecompression, and 3 nonunion patients (Table 1). All 64 open and 19 MIS cases were from a spine practice with 6 surgeons, and 45 MIS cases came from a spine practice with 2 surgeons. There was also an unequal distribution of the specific levels fused between the open and MIS groups.
Although the operative time was similar in both groups, the MIS TLIF group had a statistically significantly lower blood loss compared with the open TLIF group (Table 2). Both MIS TLIF and open TLIF lead to significant improvements in pain, ODI, and SF-36 PCS (P < .01) (Table 3). At 1 year, both groups had similar improvements in pain (36.9 vs 30.8, P = .178) and SF-36 PCS (9.9 vs 7.5, P = .231), but the MIS TLIF group had a statistically significantly greater improvement in ODI compared with the open TLIF group (30.4 vs 15.1, P < .000). At 2 years, both groups had similar improvements in SF-36 PCS (12.1 vs 7.5, P = .033), but the MIS TLIF group had a statistically significantly greater improvement in pain (40.2 vs 27.0, P = .005) and ODI (33.1 vs 15.4, P < .000) compared with the open TLIF group (Table 4).
Discussion
The current study compared intermediate-term clinical outcomes of MIS TLIF to open TLIF. We used propensity matching to identify a control group of open TLIFs that were comparable to the MIS TLIF group across a variety of covariates that are known to influence the results of lumbar fusion. This created comparison groups that were as closely matched at baseline as possible. We found that, at 2-year follow-up, MIS TLIF patients had less pain and less low-back pain–related disability as measured by ODI. There was also a trend toward better generic health-related quality of life in the MIS TLIF group.
These data suggest that the decreased soft-tissue trauma of the minimally invasive surgical technique, which leads to improved perioperative parameters in the short term, may also lead to some advantages that translate to improved intermediate-term clinical outcomes. Traditional lumbar fusion procedures have shown excellent clinical results when used for accepted clinical indications.22 However, the procedure requires extensive dissection of the paraspinal muscles, which causes significant muscle damage as evidenced by muscle breakdown products that can be detected in the bloodstream postoperatively.23,24 The lateral dissection also transects the dorsal ramus of the segmental nerves, which innervate the paraspinal muscles, leading to significant scarring and atrophy on postoperative imaging studies.23 Some authors have used the term “fusion disease” to describe the constellation of soft-tissue degradation seen after open lumbar fusion.5
An MIS version of the TLIF procedure that was described in 20033 avoids much of this iatrogenic soft-tissue trauma. It involves intramuscular dilation to approach the spine and to carry out neural decompression and interbody fusion, in conjunction with percutaneous pedicle-screw instrumentation. Proponents of this technique point to diminished iatrogenic soft-tissue and muscle damage as an advantage. Multiple studies have, in fact, confirmed improved short-term perioperative parameters, such as less blood loss, lower narcotic requirements, and decreased length-of-hospital stay.25 Economic analyses have also shown lower direct and indirect costs with the MIS technique.26
Several studies have compared patient-reported outcome measures of MIS and open TLIF, and the results have been mixed. Most of these studies have shown similar improvement in clinical outcomes between the 2 procedures, but the MIS technique demonstrated short-term perioperative advantages, such as lower blood loss, less narcotic requirements, and shorter length of stay.7-15 The authors of these studies conclude that the MIS technique can provide similar long-term results with lower short-term morbidity when compared with open TLIF. In contrast, some studies have shown better short- and intermediate-term clinical outcomes with the MIS technique.23,27-29 As a whole, the literature comparing the 2 procedures consists of mostly small retrospective studies with nonrandomized patient samples, heterogeneous surgical indications, and differing surgical techniques, making it difficult to draw conclusions.
The current study suggests that MIS TLIF may lead to improved clinical results at 2-year follow-up, compared with open TLIF. Our study used propensity-score matching to minimize the effects of nonrandom assignment of subjects to MIS TLIF or open TLIF. A limitation of observational studies is that bias in assignment of subjects to treatment groups can lead to overestimation or underestimation of the effect of the treatment itself. Propensity-score matching attempts to reduce this bias by accounting for several covariates that predict whether a subject will receive a certain treatment. These covariates are used in a logistic regression to produce a propensity score, which can be used to match subjects to controls across multiple dimensions, thus ensuring groups are as comparable as possible at baseline.
Our study still has several limitations. Sample size is relatively small, and follow-up is still only intermediate, at 2 years. There was unequal distribution of specific levels of surgery. Because patients were not blinded to the treatment they received, it is possible that patient perception of receiving a newer, less-invasive treatment method may influence their subjective improvement. The study sample was drawn from 2 different centers, with one center providing mostly MIS cases and the other providing mostly open cases. Because of this, undetected differences in how patients were selected for surgery could also affect outcomes. Any latent confounding variables, which are not identified a priori, will not be accounted for in the matching process. Only a prospective, randomized study with large numbers can control for observed and unobserved confounding patient characteristics.
In summary, our study shows that MIS TLIF is associated with improved low back pain and low back–related disability at 2 years compared with open TLIF. Other studies comparing the 2 techniques have come to different conclusions regarding whether the short-term benefits of MIS TLIF translate into long-term differences in clinical outcome. This study adds to this evidence and suggests there may be longer term advantages to the MIS approach, but prospective randomized trials are needed to confirm this finding and determine the true magnitude of these differences.
1. Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolisthesis: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb. 1982;120(3):343-347.
2. Jagannathan J, Sansur CA, Oskouian RJ Jr, Fu KM, Shaffrey CI. Radiographic restoration of lumbar alignment after transforaminal lumbar interbody fusion. Neurosurgery. 2009;64(5):955-963.
3. Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28(15 suppl):S26-S35.
4. Rouben D, Casnellie M, Ferguson M. Long-term durability of minimally invasive posterior transforaminal lumbar interbody fusion: a clinical and radiographic follow-up. J Spinal Disord Tech. 2011;24(5):288-296.
5. Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(suppl):S1-S6.
6. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res. 2014;472(6):1727-1737.
7. Adogwa O, Parker SL, Bydon A, Cheng J, McGirt MJ. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech. 2011;24(8):479-484.
8. Ghahreman A, Ferch RD, Rao PJ, Bogduk N. Minimal access versus open posterior lumbar interbody fusion in the treatment of spondylolisthesis. Neurosurgery. 2010;66(2):296-304.
9. Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32(5):537-543.
10. Saetia K, Phankhongsab A, Kuansongtham V, Paiboonsirijit S. Comparison between minimally invasive and open transforaminal lumbar interbody fusion. J Med Assoc Thai. 2013;96(1):41-46.
11. Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Ortop. 2009;33(6):1683-1688.
12. Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Eur Spine J. 2010;19(1):1780-1784.
13. Lee KH, Yue WM, Yeo W, Soeharno H, Tan SB. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21(11):2265-2270.
14. Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine. 2009;34(13):1385-1389.
15. Seng C, Siddiqui MA, Wong KP, et al. Five-year outcomes of minimally invasive versus open transforaminal lumbar interbody fusion: a matched-pair comparison study. Spine. 2013;38(23):2049-2055.
16. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940-2953.
17. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273.
18. Ware JE, Kosinski M, Keller SK. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute, 1994.
19. McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Baltimore, MD: V.V. Mosby Company, 1993.
20. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281.
21. Rosenbaum PR. Model-based direct adjustment. J Am Stat Assn. 1987;82:387-394.
22. Glassman SD, Carreon LY, Djurasovic M, et al. Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J. 2009;9(1):13-21.
23. Fan S, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19(2):316-324.
24. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine. 1996;21(8):941-944.
25. Sun ZJ, Li WJ, Zhao Y, Qui GX. Comparing minimally invasive and open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: a meta-analysis. Chin Med J. 2013;126(2):3962-3971.
26. Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg. 2014;82(1-2):230-238.
27. Kotani Y, Abumi K, Ito M, Sudo H, Abe Y, Minami A. Mid-term clinical results of minimally invasive decompression and posterolateral fusion with percutaneous pedicle screws versus conventional approach for degenerative spondylolisthesis with spinal stenosis. Eur Spine J. 2012;21(6):1171-1177.
28. Pelton MA, Phillips FM, Singh K. A comparison of perioperative costs and outcomes in patients with and without worker’s compensation claims treated with MIS or open TLIF. Spine. 2012;37(22):1914-1919.
29. Wong AP, Smith ZA, Stadler JA 3rd, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF). Surgical technique, long-term 4 year prospective outcomes and complications compared with an open TLIF cohort. Neurosurg Clin N Am. 2014;25(2):279-304.
1. Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolisthesis: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb. 1982;120(3):343-347.
2. Jagannathan J, Sansur CA, Oskouian RJ Jr, Fu KM, Shaffrey CI. Radiographic restoration of lumbar alignment after transforaminal lumbar interbody fusion. Neurosurgery. 2009;64(5):955-963.
3. Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28(15 suppl):S26-S35.
4. Rouben D, Casnellie M, Ferguson M. Long-term durability of minimally invasive posterior transforaminal lumbar interbody fusion: a clinical and radiographic follow-up. J Spinal Disord Tech. 2011;24(5):288-296.
5. Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(suppl):S1-S6.
6. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res. 2014;472(6):1727-1737.
7. Adogwa O, Parker SL, Bydon A, Cheng J, McGirt MJ. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech. 2011;24(8):479-484.
8. Ghahreman A, Ferch RD, Rao PJ, Bogduk N. Minimal access versus open posterior lumbar interbody fusion in the treatment of spondylolisthesis. Neurosurgery. 2010;66(2):296-304.
9. Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32(5):537-543.
10. Saetia K, Phankhongsab A, Kuansongtham V, Paiboonsirijit S. Comparison between minimally invasive and open transforaminal lumbar interbody fusion. J Med Assoc Thai. 2013;96(1):41-46.
11. Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Ortop. 2009;33(6):1683-1688.
12. Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Eur Spine J. 2010;19(1):1780-1784.
13. Lee KH, Yue WM, Yeo W, Soeharno H, Tan SB. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21(11):2265-2270.
14. Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine. 2009;34(13):1385-1389.
15. Seng C, Siddiqui MA, Wong KP, et al. Five-year outcomes of minimally invasive versus open transforaminal lumbar interbody fusion: a matched-pair comparison study. Spine. 2013;38(23):2049-2055.
16. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940-2953.
17. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273.
18. Ware JE, Kosinski M, Keller SK. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute, 1994.
19. McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Baltimore, MD: V.V. Mosby Company, 1993.
20. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281.
21. Rosenbaum PR. Model-based direct adjustment. J Am Stat Assn. 1987;82:387-394.
22. Glassman SD, Carreon LY, Djurasovic M, et al. Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J. 2009;9(1):13-21.
23. Fan S, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19(2):316-324.
24. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine. 1996;21(8):941-944.
25. Sun ZJ, Li WJ, Zhao Y, Qui GX. Comparing minimally invasive and open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: a meta-analysis. Chin Med J. 2013;126(2):3962-3971.
26. Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg. 2014;82(1-2):230-238.
27. Kotani Y, Abumi K, Ito M, Sudo H, Abe Y, Minami A. Mid-term clinical results of minimally invasive decompression and posterolateral fusion with percutaneous pedicle screws versus conventional approach for degenerative spondylolisthesis with spinal stenosis. Eur Spine J. 2012;21(6):1171-1177.
28. Pelton MA, Phillips FM, Singh K. A comparison of perioperative costs and outcomes in patients with and without worker’s compensation claims treated with MIS or open TLIF. Spine. 2012;37(22):1914-1919.
29. Wong AP, Smith ZA, Stadler JA 3rd, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF). Surgical technique, long-term 4 year prospective outcomes and complications compared with an open TLIF cohort. Neurosurg Clin N Am. 2014;25(2):279-304.