User login
Operative Versus Nonoperative Treatment of Jones Fractures: A Decision Analysis Model
The optimal management strategy for acute fractures of the metadiaphyseal fifth metatarsal (Jones fractures) is controversial. Patients can be successfully treated nonoperatively with non-weight-bearing and immobilization in a short leg cast1-7 or operatively with placement of an intramedullary screw.8-10 The primary advantage of nonoperative treatment is avoiding the risks and discomfort of surgery; disadvantages include the need for prolonged immobilization and protected weight-bearing as well as a decreased union rate.8,9 Advantages of operative treatment include accelerated functional recovery and an improved union rate; disadvantages include exposure to the risks, inconvenience, and discomfort of surgery. Clear, definitive evidence for guiding treatment is not available in the orthopedic literature, and treatment strategies vary substantially according to surgeon and patient preference.
Expected-value decision analysis, a research tool that helps guide decision-making in situations of uncertainty, has been effectively applied to other areas of uncertainty in the orthopedic literature.11-14 Borrowed from gaming theory, the technique involves creating a decision tree to define the clinical problem, determining outcome probabilities and utilities, performing a fold-back analysis to determine the optimal decision-making strategy, and performing a sensitivity analysis to model the effect of varying outcome probabilities and utilities on decision-making. Decision analysis may therefore allow the clinician and the patient to optimize decision-making based on best available evidence and patient preferences. It also helps determine the most important factors affecting management strategies and the decision-making process, which may not always be intuitive.
In the present study, we used expected-value decision analysis to determine the optimal management strategy, operative or nonoperative, for acute Jones fracture. We also explored factors with the most influence on the model and identified important questions for future research.
Materials and Methods
Institutional review board approval was obtained for this study. Analysis was performed with Treeage Pro statistical software (Treeage Software).
Outcome Probabilities
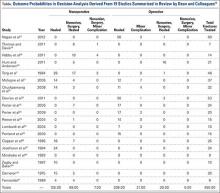
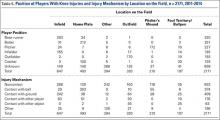
Outcome probabilities were determined by reviewing the literature for articles on Jones fractures. This body of literature was summarized in a comprehensive review by Dean and colleagues15, who extracted data from 19 studies: 1 randomized controlled trial, 1 prospective case series, and 17 retrospective case series.15 We used data from these studies to determine outcome probabilities (Table).
Outcome Utilities
Utilities represent patient preferences for various disease states. Outcome utility values were obtained from 32 adults (25 women, 7 men) with no history of foot injury. Mean age was 32.4 years (range, 20-69 years). The questionnaire presented scenarios for the different outcomes and asked patients to rate these outcomes on a scale ranging from 0 (worst possible outcome) to 10 (best possible outcome). The Sports subscale of the Foot and Ankle Ability Measure (FAAM) 16 was used to quantify patient activity level.
Decision Tree and Fold-Back Analysis
A decision tree was constructed with 1 decision node, 4 chance nodes, and 7 terminal nodes (Figure 1). The decision tree demonstrates 2 different strategies for managing a Jones fracture. The decision node divides the tree into 2 branches: initial operative or nonoperative treatment. Both branches are followed by various chance nodes, each terminating in a discrete clinical outcome. Per convention, utility data were placed to the right of the terminal nodes, and probability data were placed under the terminal nodes.
Fold-back analysis was performed to identify the optimal strategy. Fold-back analysis involves multiplying each outcome utility by its associated probability, thereby providing an “expected value” for each clinical endpoint. Then, the expected values for each endpoint can be summed for a given management strategy, and the ultimate expected values of the different strategies can be compared. The management strategy associated with the highest expected value is optimal for the given outcome utilities and probabilities.
Sensitivity Analysis
One-way sensitivity analysis was performed to model the effect on decision-making of changing the values for utility for uncomplicated surgery, utility for healing with nonoperative treatment, utility for uncomplicated treatment of nonunion, likelihood of healing with nonoperative treatment, likelihood of healing with surgery, and likelihood of minor complication with surgery. These were the variables found to affect the decision-making strategy within their clinically plausible ranges.
Results
Outcome Probabilities and Utilities
Outcome probabilities and utilities are illustrated in Figure 1. By convention, probabilities appear below the corresponding branches of the decision tree, and utilities appear at the end of each branch. Mean (SD) FAAM Sports subscale score was 84.6 (27.4). This subscale is scored as a percentage from 0% to 100%, with higher scores indicating a higher level of physical function.
Decision Analysis
The expected value for nonoperative treatment was 7.74, and the expected value for intramedullary screw fixation was 7.88 (Figure 1). Therefore, operative treatment was identified as the optimal treatment strategy.
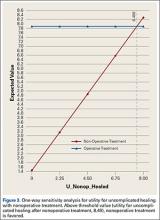
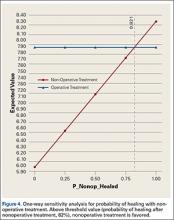
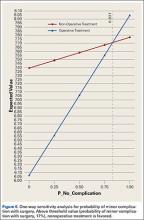
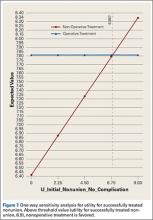
Sensitivity analyses revealed that the optimal decision making strategy was very sensitive to small changes in several variables. Nonoperative treatment becomes the preferred strategy when the utility value for uncomplicated surgery falls below 8.04 (Figure 2), when the utility for healing with nonoperative treatment rises above 8.49 (Figure 3), when the likelihood of healing with nonoperative treatment rises above 82% (Figure 4), or when the probability of healing after surgery falls below 92% (Figure 5). Nonoperative treatment is also favored when the probability of minor complication with surgery is above 17% (Figure 6) and when utility for a successfully treated nonunion is higher than 6.9 (Figure 7).
Discussion
Optimal management of a metadiaphyseal fracture of the fifth metatarsal (Jones fracture) remains controversial. The decision between initial operative or nonoperative treatment lends itself to expected-value decision analysis because of well-defined treatment options and relatively discrete outcomes. The principal advantages of nonoperative treatment are that it allows the patient to avoid the risks and discomfort of surgery, and the principal advantages of operative treatment are that it maximizes the chance of fracture union and may accelerate functional recovery.
Our decision analysis determined that operative fixation is the optimal decision path, given the outcome probabilities derived from the literature and the utilities obtained from surveys. This finding is in accordance with several expert opinions in foot and ankle fracture surgery.17,18 However, the expected values of the operative and nonoperative treatment strategies differed by only 0.3 on a 10-point scale. Such similar expected values in our model are not surprising given the controversy surrounding clinical decision making in the treatment of these fractures.19
In addition, our analysis identified the important variables in the decision-making process. Patients averse to surgery, patients not averse to successful nonoperative treatment, and patients who view successful nonunion surgery after initial nonoperative treatment as a relatively positive outcome may be best treated nonoperatively. These findings emphasize the importance of patient preferences and shared decision-making. Higher rates of healing with nonoperative treatment, lower rates of healing with surgery, and higher complication rates with surgery also favor nonoperative management. It would therefore be valuable to identify risk factors for nonunion with nonoperative treatment and to identify the technical details of surgery that maximize rates of healing and minimize the risk of complications.
The limitations of decision analysis involve the methods by which probabilities and utilities are obtained. In general, the most accurate, stable, and robust estimates of outcome probabilities are derived from a meta-analytic synthesis of randomized clinical trials, the highest level of clinical evidence. In our model, data were extracted primarily from level IV studies; only 1 level III study20 and 1 level II study21 were available for analysis. Thus, as is the case with many foot and ankle disorders22, the information on treatment of Jones fractures is very limited in its level of clinical evidence.
Determination of outcome utility also has limitations. Utility is a subjective value that an individual places on a specific outcome. This can be very difficult to quantify. In general, the most robust estimates of patient-derived utilities are derived from complex qualitative methods, such as the standard reference gamble or time trade-offs, in which patients are asked to gamble or choose between health states usually referenced to death. In this study, we determined patient-derived utility values from a direct scaling method using a Likert scale because of the complexity of the standard reference gamble and the difficulty of referencing to death for metatarsal fracture. Although use of a direct scale to determine utility values is less rigorous than the standard reference gamble, this technique has been corroborated methodologically,23 is advantageous in terms of feasibility and reliability,24 and has been successfully used in other orthopedic decision analysis models.12,25,26 In our estimation, generally active patients without a history of foot pathology constituted a sample of convenience but also were representative of individuals at risk for Jones fracture. Although specific scenarios were presented, the patients who completed the questionnaire may not have had deep insights into the subtleties and implications of the various disease states and treatments. Regardless of how outcome probabilities and utilities are determined, they are considered point estimates in decision analysis, and sensitivity analyses are therefore performed to assess how decision making changes over a range of values.
Conclusion
The results of this study may help optimize the process of deciding between operative and nonoperative treatment for Jones fracture. For a given patient, the optimal strategy depends not only on the probabilities of the various outcomes but also on personal preference. Thus, there may not be one right answer for all patients. Patients who value a higher chance of fracture healing with initial treatment or an earlier return to sports are best treated operatively, whereas patients who are risk-averse and place a high value on fracture healing without surgery should be managed nonoperatively. We therefore advocate a model of shared medical decision-making in which the physician and the patient are jointly involved, considering both outcome probabilities and patient preferences. Ongoing research efforts should focus on predictors of nonunion with nonoperative treatment.
1. Dameron TB Jr. Fractures of the proximal fifth metatarsal: selecting the best treatment option. J Am Acad Orthop Surg. 1995;3(2):110-114.
2. Fetzer GB, Wright RW. Metatarsal shaft fractures and fractures of the proximal fifth metatarsal. Clin Sports Med. 2006;25(1):139-150, x.
3. Konkel KF, Menger AG, Retzlaff SA. Nonoperative treatment of fifth metatarsal fractures in an orthopaedic suburban private multispeciality practice. Foot Ankle Int. 2005;26(9):704-707.
4. Lawrence SJ, Botte MJ. Jones’ fractures and related fractures of the proximal fifth metatarsal. Foot Ankle. 1993;14(6):358-365.
5. Nunley JA. Fractures of the base of the fifth metatarsal: the Jones fracture. Orthop Clin North Am. 2001;32(1):171-180.
6. Quill GE Jr. Fractures of the proximal fifth metatarsal. Orthop Clin North Am. 1995;26(2):353-361.
7. Torg JS, Balduini FC, Zelko RR, Pavlov H, Peff TC, Das M. Fractures of the base of the fifth metatarsal distal to the tuberosity. Classification and guidelines for non-surgical and surgical management. J Bone Joint Surg Am. 1984;66(2):209-214.
8. DeLee JC, Evans JP, Julian J. Stress fracture of the fifth metatarsal. Am J Sports Med. 1983;11(5):349-353.
9. Kavanaugh JH, Brower TD, Mann RV. The Jones fracture revisited. J Bone Joint Surg Am. 1978;60(6):776-782.
10. Porter DA, Duncan M, Meyer SJ. Fifth metatarsal Jones fracture fixation with a 4.5-mm cannulated stainless steel screw in the competitive and recreational athlete: a clinical and radiographic evaluation. Am J Sports Med. 2005;33(5):726-733.
11. Aleem IS, Jalal H, Sheikh AA, Bhandari M. Clinical decision analysis: Incorporating the evidence with patient p. Patient Prefer Adherence. 2009;3:21-24.
12. Bishop J, Ring D. Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surg Am. 2009;34(6):991-996.e1.
13. Chen NC, Shauver MJ, Chung KC. A primer on use of decision analysis methodology in hand surgery. J Hand Surg Am. 2009;34(6):983-990.
14. Kocher MS, Henley MB. It is money that matters: decision analysis and cost-effectiveness analysis. Clin Orthop Relat Res. 2003(413):106-116.
15. Dean BJ, Kothari A, Uppal H, Kankate R. The jones fracture classification, management, outcome, and complications: a systematic review. Foot Ankle Spec. 2012;5(4):256-259.
16. Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the Foot and Ankle Ability Measure (FAAM). Foot Ankle Int. 2005;26(11):968-983.
17. Roche AJ, Calder JD. Treatment and return to sport following a Jones fracture of the fifth metatarsal: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1307-1315.
18. Zwitser EW, Breederveld RS. Fractures of the fifth metatarsal; diagnosis and treatment. Injury. 2010;41(6):555-562.
19. McBryde AM Jr. The complicated Jones fracture, including revision and malalignment. Foot Ankle Clin. 2009;14(2):151-168.
20. Porter DA, Rund AM, Dobslaw R, Duncan M. Comparison of 4.5- and 5.5-mm cannulated stainless steel screws for fifth metatarsal Jones fracture fixation. Foot Ankle Int. 2009;30(1):27-33.
21. Mologne TS, Lundeen JM, Clapper MF, O’Brien TJ. Early screw fixation versus casting in the treatment of acute Jones fractures. Am J Sports Med. 2005;33(7):970-975.
22. Hunt KJ, Hurwit D. Use of patient-reported outcome measures in foot and ankle research. J Bone Joint Surg Am. 2013;95(16):e118(1-9).
23. Stiggelbout AM, Eijkemans MJ, Kiebert GM, Kievit J, Leer JW, De Haes HJ. The ‘utility’ of the visual analog scale in medical decision making and technology assessment. Is it an alternative to the time trade-off? International journal of technology assessment in health care. Spring. 1996;12(2):291-298.
24. Parkin D, Devlin N. Is there a case for using visual analogue scale valuations in cost-utility analysis? Health Econ. 2006;15(7):653-664.
25. Bishop JA, Crall TS, Kocher MS. Operative versus nonoperative treatment after primary traumatic anterior glenohumeral dislocation: expected-value decision analysis. J Shoulder Elbow Surg. 2011;20(7):1087-1094.
26. Kocher MS, Bishop J, Marshall R, Briggs KK, Hawkins RJ. Operative versus nonoperative management of acute Achilles tendon rupture: expected-value decision analysis. Am J Sports Med. 2002;30(6):783-790.
27. Nagao M, Saita Y, Kameda S, et al. Headless compression screw fixation of jones fractures: an outcomes study in Japanese athletes. Am J Sports Med. 2012;40(11):2578-2582.
28. Thomas JL, Davis BC. Treatment of Jones fracture nonunion with isolated intramedullary screw fixation. J Foot Ankle Surg. 2011;50(5):566-568.
29. Habbu RA, Marsh RS, Anderson JG, Bohay DR. Closed intramedullary screw fixation for nonunion of fifth metatarsal Jones fracture. Foot Ankle Int. 2011;32(6):603-608.
30. Hunt KJ, Anderson RB. Treatment of Jones fracture nonunions and refractures in the elite athlete: outcomes of intramedullary screw fixation with bone grafting. Am J Sports Med. 2011;39(9):1948-1954.
31. Chuckpaiwong B, Queen RM, Easley ME, Nunley JA. Distinguishing Jones and proximal diaphyseal fractures of the fifth metatarsal. Clin Orthop Relat Res. 2008;466(8):1966-1970.
32. DeVries JG, Cuttica DJ, Hyer CF. Cannulated screw fixation of Jones fifth metatarsal fractures: a comparison of titanium and stainless steel screw fixation. J Foot Ankle Surg. 2011;50(2):207-212.
33. Reese K, Litsky A, Kaeding C, Pedroza A, Shah N. Cannulated screw fixation of Jones fractures: a clinical and biomechanical study. Am J Sports Med. 2004;32(7):1736-1742.
34. Lombardi CM, Connolly FG, Silhanek AD. The use of external fixation for treatment of the acute Jones fracture: a retrospective review of 10 cases. J Foot Ankle Surg. 2004;43(3):173-178.
35. Portland G, Kelikian A, Kodros S. Acute surgical management of Jones’ fractures. Foot Ankle Int. 2003;24(11):829-833.
36. Clapper MF, O’Brien TJ, Lyons PM. Fractures of the fifth metatarsal. Analysis of a fracture registry. Clin Orthop Relat Res. 1995(315):238-241.
37. Josefsson PO, Karlsson M, Redlund-Johnell I, Wendeberg B. Closed treatment of Jones fracture. Good results in 40 cases after 11-26 years. Orthop Scand. 1994;65(5):545-547.
38. Mindrebo N, Shelbourne KD, Van Meter CD, Rettig AC. Outpatient percutaneous screw fixation of the acute Jones fracture. Am J Sports Med. 1993;21(5):720-723.
39. Zogby RG, Baker BE. A review of nonoperative treatment of Jones’ fracture. Am J Sports Med. 1987;15(4):304-307.
40. Dameron TB Jr. Fractures and anatomical variations of the proximal portion of the fifth metatarsal. J Bone Joint Surg Am. 1975;57(6):788-792.
41. Fernandez Fairen M, Guillen J, Busto JM, Roura J. Fractures of the fifth metatarsal in basketball players. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):373-377.
The optimal management strategy for acute fractures of the metadiaphyseal fifth metatarsal (Jones fractures) is controversial. Patients can be successfully treated nonoperatively with non-weight-bearing and immobilization in a short leg cast1-7 or operatively with placement of an intramedullary screw.8-10 The primary advantage of nonoperative treatment is avoiding the risks and discomfort of surgery; disadvantages include the need for prolonged immobilization and protected weight-bearing as well as a decreased union rate.8,9 Advantages of operative treatment include accelerated functional recovery and an improved union rate; disadvantages include exposure to the risks, inconvenience, and discomfort of surgery. Clear, definitive evidence for guiding treatment is not available in the orthopedic literature, and treatment strategies vary substantially according to surgeon and patient preference.
Expected-value decision analysis, a research tool that helps guide decision-making in situations of uncertainty, has been effectively applied to other areas of uncertainty in the orthopedic literature.11-14 Borrowed from gaming theory, the technique involves creating a decision tree to define the clinical problem, determining outcome probabilities and utilities, performing a fold-back analysis to determine the optimal decision-making strategy, and performing a sensitivity analysis to model the effect of varying outcome probabilities and utilities on decision-making. Decision analysis may therefore allow the clinician and the patient to optimize decision-making based on best available evidence and patient preferences. It also helps determine the most important factors affecting management strategies and the decision-making process, which may not always be intuitive.
In the present study, we used expected-value decision analysis to determine the optimal management strategy, operative or nonoperative, for acute Jones fracture. We also explored factors with the most influence on the model and identified important questions for future research.
Materials and Methods
Institutional review board approval was obtained for this study. Analysis was performed with Treeage Pro statistical software (Treeage Software).
Outcome Probabilities
Outcome probabilities were determined by reviewing the literature for articles on Jones fractures. This body of literature was summarized in a comprehensive review by Dean and colleagues15, who extracted data from 19 studies: 1 randomized controlled trial, 1 prospective case series, and 17 retrospective case series.15 We used data from these studies to determine outcome probabilities (Table).
Outcome Utilities
Utilities represent patient preferences for various disease states. Outcome utility values were obtained from 32 adults (25 women, 7 men) with no history of foot injury. Mean age was 32.4 years (range, 20-69 years). The questionnaire presented scenarios for the different outcomes and asked patients to rate these outcomes on a scale ranging from 0 (worst possible outcome) to 10 (best possible outcome). The Sports subscale of the Foot and Ankle Ability Measure (FAAM) 16 was used to quantify patient activity level.
Decision Tree and Fold-Back Analysis
A decision tree was constructed with 1 decision node, 4 chance nodes, and 7 terminal nodes (Figure 1). The decision tree demonstrates 2 different strategies for managing a Jones fracture. The decision node divides the tree into 2 branches: initial operative or nonoperative treatment. Both branches are followed by various chance nodes, each terminating in a discrete clinical outcome. Per convention, utility data were placed to the right of the terminal nodes, and probability data were placed under the terminal nodes.
Fold-back analysis was performed to identify the optimal strategy. Fold-back analysis involves multiplying each outcome utility by its associated probability, thereby providing an “expected value” for each clinical endpoint. Then, the expected values for each endpoint can be summed for a given management strategy, and the ultimate expected values of the different strategies can be compared. The management strategy associated with the highest expected value is optimal for the given outcome utilities and probabilities.
Sensitivity Analysis
One-way sensitivity analysis was performed to model the effect on decision-making of changing the values for utility for uncomplicated surgery, utility for healing with nonoperative treatment, utility for uncomplicated treatment of nonunion, likelihood of healing with nonoperative treatment, likelihood of healing with surgery, and likelihood of minor complication with surgery. These were the variables found to affect the decision-making strategy within their clinically plausible ranges.
Results
Outcome Probabilities and Utilities
Outcome probabilities and utilities are illustrated in Figure 1. By convention, probabilities appear below the corresponding branches of the decision tree, and utilities appear at the end of each branch. Mean (SD) FAAM Sports subscale score was 84.6 (27.4). This subscale is scored as a percentage from 0% to 100%, with higher scores indicating a higher level of physical function.
Decision Analysis
The expected value for nonoperative treatment was 7.74, and the expected value for intramedullary screw fixation was 7.88 (Figure 1). Therefore, operative treatment was identified as the optimal treatment strategy.
Sensitivity analyses revealed that the optimal decision making strategy was very sensitive to small changes in several variables. Nonoperative treatment becomes the preferred strategy when the utility value for uncomplicated surgery falls below 8.04 (Figure 2), when the utility for healing with nonoperative treatment rises above 8.49 (Figure 3), when the likelihood of healing with nonoperative treatment rises above 82% (Figure 4), or when the probability of healing after surgery falls below 92% (Figure 5). Nonoperative treatment is also favored when the probability of minor complication with surgery is above 17% (Figure 6) and when utility for a successfully treated nonunion is higher than 6.9 (Figure 7).
Discussion
Optimal management of a metadiaphyseal fracture of the fifth metatarsal (Jones fracture) remains controversial. The decision between initial operative or nonoperative treatment lends itself to expected-value decision analysis because of well-defined treatment options and relatively discrete outcomes. The principal advantages of nonoperative treatment are that it allows the patient to avoid the risks and discomfort of surgery, and the principal advantages of operative treatment are that it maximizes the chance of fracture union and may accelerate functional recovery.
Our decision analysis determined that operative fixation is the optimal decision path, given the outcome probabilities derived from the literature and the utilities obtained from surveys. This finding is in accordance with several expert opinions in foot and ankle fracture surgery.17,18 However, the expected values of the operative and nonoperative treatment strategies differed by only 0.3 on a 10-point scale. Such similar expected values in our model are not surprising given the controversy surrounding clinical decision making in the treatment of these fractures.19
In addition, our analysis identified the important variables in the decision-making process. Patients averse to surgery, patients not averse to successful nonoperative treatment, and patients who view successful nonunion surgery after initial nonoperative treatment as a relatively positive outcome may be best treated nonoperatively. These findings emphasize the importance of patient preferences and shared decision-making. Higher rates of healing with nonoperative treatment, lower rates of healing with surgery, and higher complication rates with surgery also favor nonoperative management. It would therefore be valuable to identify risk factors for nonunion with nonoperative treatment and to identify the technical details of surgery that maximize rates of healing and minimize the risk of complications.
The limitations of decision analysis involve the methods by which probabilities and utilities are obtained. In general, the most accurate, stable, and robust estimates of outcome probabilities are derived from a meta-analytic synthesis of randomized clinical trials, the highest level of clinical evidence. In our model, data were extracted primarily from level IV studies; only 1 level III study20 and 1 level II study21 were available for analysis. Thus, as is the case with many foot and ankle disorders22, the information on treatment of Jones fractures is very limited in its level of clinical evidence.
Determination of outcome utility also has limitations. Utility is a subjective value that an individual places on a specific outcome. This can be very difficult to quantify. In general, the most robust estimates of patient-derived utilities are derived from complex qualitative methods, such as the standard reference gamble or time trade-offs, in which patients are asked to gamble or choose between health states usually referenced to death. In this study, we determined patient-derived utility values from a direct scaling method using a Likert scale because of the complexity of the standard reference gamble and the difficulty of referencing to death for metatarsal fracture. Although use of a direct scale to determine utility values is less rigorous than the standard reference gamble, this technique has been corroborated methodologically,23 is advantageous in terms of feasibility and reliability,24 and has been successfully used in other orthopedic decision analysis models.12,25,26 In our estimation, generally active patients without a history of foot pathology constituted a sample of convenience but also were representative of individuals at risk for Jones fracture. Although specific scenarios were presented, the patients who completed the questionnaire may not have had deep insights into the subtleties and implications of the various disease states and treatments. Regardless of how outcome probabilities and utilities are determined, they are considered point estimates in decision analysis, and sensitivity analyses are therefore performed to assess how decision making changes over a range of values.
Conclusion
The results of this study may help optimize the process of deciding between operative and nonoperative treatment for Jones fracture. For a given patient, the optimal strategy depends not only on the probabilities of the various outcomes but also on personal preference. Thus, there may not be one right answer for all patients. Patients who value a higher chance of fracture healing with initial treatment or an earlier return to sports are best treated operatively, whereas patients who are risk-averse and place a high value on fracture healing without surgery should be managed nonoperatively. We therefore advocate a model of shared medical decision-making in which the physician and the patient are jointly involved, considering both outcome probabilities and patient preferences. Ongoing research efforts should focus on predictors of nonunion with nonoperative treatment.
The optimal management strategy for acute fractures of the metadiaphyseal fifth metatarsal (Jones fractures) is controversial. Patients can be successfully treated nonoperatively with non-weight-bearing and immobilization in a short leg cast1-7 or operatively with placement of an intramedullary screw.8-10 The primary advantage of nonoperative treatment is avoiding the risks and discomfort of surgery; disadvantages include the need for prolonged immobilization and protected weight-bearing as well as a decreased union rate.8,9 Advantages of operative treatment include accelerated functional recovery and an improved union rate; disadvantages include exposure to the risks, inconvenience, and discomfort of surgery. Clear, definitive evidence for guiding treatment is not available in the orthopedic literature, and treatment strategies vary substantially according to surgeon and patient preference.
Expected-value decision analysis, a research tool that helps guide decision-making in situations of uncertainty, has been effectively applied to other areas of uncertainty in the orthopedic literature.11-14 Borrowed from gaming theory, the technique involves creating a decision tree to define the clinical problem, determining outcome probabilities and utilities, performing a fold-back analysis to determine the optimal decision-making strategy, and performing a sensitivity analysis to model the effect of varying outcome probabilities and utilities on decision-making. Decision analysis may therefore allow the clinician and the patient to optimize decision-making based on best available evidence and patient preferences. It also helps determine the most important factors affecting management strategies and the decision-making process, which may not always be intuitive.
In the present study, we used expected-value decision analysis to determine the optimal management strategy, operative or nonoperative, for acute Jones fracture. We also explored factors with the most influence on the model and identified important questions for future research.
Materials and Methods
Institutional review board approval was obtained for this study. Analysis was performed with Treeage Pro statistical software (Treeage Software).
Outcome Probabilities
Outcome probabilities were determined by reviewing the literature for articles on Jones fractures. This body of literature was summarized in a comprehensive review by Dean and colleagues15, who extracted data from 19 studies: 1 randomized controlled trial, 1 prospective case series, and 17 retrospective case series.15 We used data from these studies to determine outcome probabilities (Table).
Outcome Utilities
Utilities represent patient preferences for various disease states. Outcome utility values were obtained from 32 adults (25 women, 7 men) with no history of foot injury. Mean age was 32.4 years (range, 20-69 years). The questionnaire presented scenarios for the different outcomes and asked patients to rate these outcomes on a scale ranging from 0 (worst possible outcome) to 10 (best possible outcome). The Sports subscale of the Foot and Ankle Ability Measure (FAAM) 16 was used to quantify patient activity level.
Decision Tree and Fold-Back Analysis
A decision tree was constructed with 1 decision node, 4 chance nodes, and 7 terminal nodes (Figure 1). The decision tree demonstrates 2 different strategies for managing a Jones fracture. The decision node divides the tree into 2 branches: initial operative or nonoperative treatment. Both branches are followed by various chance nodes, each terminating in a discrete clinical outcome. Per convention, utility data were placed to the right of the terminal nodes, and probability data were placed under the terminal nodes.
Fold-back analysis was performed to identify the optimal strategy. Fold-back analysis involves multiplying each outcome utility by its associated probability, thereby providing an “expected value” for each clinical endpoint. Then, the expected values for each endpoint can be summed for a given management strategy, and the ultimate expected values of the different strategies can be compared. The management strategy associated with the highest expected value is optimal for the given outcome utilities and probabilities.
Sensitivity Analysis
One-way sensitivity analysis was performed to model the effect on decision-making of changing the values for utility for uncomplicated surgery, utility for healing with nonoperative treatment, utility for uncomplicated treatment of nonunion, likelihood of healing with nonoperative treatment, likelihood of healing with surgery, and likelihood of minor complication with surgery. These were the variables found to affect the decision-making strategy within their clinically plausible ranges.
Results
Outcome Probabilities and Utilities
Outcome probabilities and utilities are illustrated in Figure 1. By convention, probabilities appear below the corresponding branches of the decision tree, and utilities appear at the end of each branch. Mean (SD) FAAM Sports subscale score was 84.6 (27.4). This subscale is scored as a percentage from 0% to 100%, with higher scores indicating a higher level of physical function.
Decision Analysis
The expected value for nonoperative treatment was 7.74, and the expected value for intramedullary screw fixation was 7.88 (Figure 1). Therefore, operative treatment was identified as the optimal treatment strategy.
Sensitivity analyses revealed that the optimal decision making strategy was very sensitive to small changes in several variables. Nonoperative treatment becomes the preferred strategy when the utility value for uncomplicated surgery falls below 8.04 (Figure 2), when the utility for healing with nonoperative treatment rises above 8.49 (Figure 3), when the likelihood of healing with nonoperative treatment rises above 82% (Figure 4), or when the probability of healing after surgery falls below 92% (Figure 5). Nonoperative treatment is also favored when the probability of minor complication with surgery is above 17% (Figure 6) and when utility for a successfully treated nonunion is higher than 6.9 (Figure 7).
Discussion
Optimal management of a metadiaphyseal fracture of the fifth metatarsal (Jones fracture) remains controversial. The decision between initial operative or nonoperative treatment lends itself to expected-value decision analysis because of well-defined treatment options and relatively discrete outcomes. The principal advantages of nonoperative treatment are that it allows the patient to avoid the risks and discomfort of surgery, and the principal advantages of operative treatment are that it maximizes the chance of fracture union and may accelerate functional recovery.
Our decision analysis determined that operative fixation is the optimal decision path, given the outcome probabilities derived from the literature and the utilities obtained from surveys. This finding is in accordance with several expert opinions in foot and ankle fracture surgery.17,18 However, the expected values of the operative and nonoperative treatment strategies differed by only 0.3 on a 10-point scale. Such similar expected values in our model are not surprising given the controversy surrounding clinical decision making in the treatment of these fractures.19
In addition, our analysis identified the important variables in the decision-making process. Patients averse to surgery, patients not averse to successful nonoperative treatment, and patients who view successful nonunion surgery after initial nonoperative treatment as a relatively positive outcome may be best treated nonoperatively. These findings emphasize the importance of patient preferences and shared decision-making. Higher rates of healing with nonoperative treatment, lower rates of healing with surgery, and higher complication rates with surgery also favor nonoperative management. It would therefore be valuable to identify risk factors for nonunion with nonoperative treatment and to identify the technical details of surgery that maximize rates of healing and minimize the risk of complications.
The limitations of decision analysis involve the methods by which probabilities and utilities are obtained. In general, the most accurate, stable, and robust estimates of outcome probabilities are derived from a meta-analytic synthesis of randomized clinical trials, the highest level of clinical evidence. In our model, data were extracted primarily from level IV studies; only 1 level III study20 and 1 level II study21 were available for analysis. Thus, as is the case with many foot and ankle disorders22, the information on treatment of Jones fractures is very limited in its level of clinical evidence.
Determination of outcome utility also has limitations. Utility is a subjective value that an individual places on a specific outcome. This can be very difficult to quantify. In general, the most robust estimates of patient-derived utilities are derived from complex qualitative methods, such as the standard reference gamble or time trade-offs, in which patients are asked to gamble or choose between health states usually referenced to death. In this study, we determined patient-derived utility values from a direct scaling method using a Likert scale because of the complexity of the standard reference gamble and the difficulty of referencing to death for metatarsal fracture. Although use of a direct scale to determine utility values is less rigorous than the standard reference gamble, this technique has been corroborated methodologically,23 is advantageous in terms of feasibility and reliability,24 and has been successfully used in other orthopedic decision analysis models.12,25,26 In our estimation, generally active patients without a history of foot pathology constituted a sample of convenience but also were representative of individuals at risk for Jones fracture. Although specific scenarios were presented, the patients who completed the questionnaire may not have had deep insights into the subtleties and implications of the various disease states and treatments. Regardless of how outcome probabilities and utilities are determined, they are considered point estimates in decision analysis, and sensitivity analyses are therefore performed to assess how decision making changes over a range of values.
Conclusion
The results of this study may help optimize the process of deciding between operative and nonoperative treatment for Jones fracture. For a given patient, the optimal strategy depends not only on the probabilities of the various outcomes but also on personal preference. Thus, there may not be one right answer for all patients. Patients who value a higher chance of fracture healing with initial treatment or an earlier return to sports are best treated operatively, whereas patients who are risk-averse and place a high value on fracture healing without surgery should be managed nonoperatively. We therefore advocate a model of shared medical decision-making in which the physician and the patient are jointly involved, considering both outcome probabilities and patient preferences. Ongoing research efforts should focus on predictors of nonunion with nonoperative treatment.
1. Dameron TB Jr. Fractures of the proximal fifth metatarsal: selecting the best treatment option. J Am Acad Orthop Surg. 1995;3(2):110-114.
2. Fetzer GB, Wright RW. Metatarsal shaft fractures and fractures of the proximal fifth metatarsal. Clin Sports Med. 2006;25(1):139-150, x.
3. Konkel KF, Menger AG, Retzlaff SA. Nonoperative treatment of fifth metatarsal fractures in an orthopaedic suburban private multispeciality practice. Foot Ankle Int. 2005;26(9):704-707.
4. Lawrence SJ, Botte MJ. Jones’ fractures and related fractures of the proximal fifth metatarsal. Foot Ankle. 1993;14(6):358-365.
5. Nunley JA. Fractures of the base of the fifth metatarsal: the Jones fracture. Orthop Clin North Am. 2001;32(1):171-180.
6. Quill GE Jr. Fractures of the proximal fifth metatarsal. Orthop Clin North Am. 1995;26(2):353-361.
7. Torg JS, Balduini FC, Zelko RR, Pavlov H, Peff TC, Das M. Fractures of the base of the fifth metatarsal distal to the tuberosity. Classification and guidelines for non-surgical and surgical management. J Bone Joint Surg Am. 1984;66(2):209-214.
8. DeLee JC, Evans JP, Julian J. Stress fracture of the fifth metatarsal. Am J Sports Med. 1983;11(5):349-353.
9. Kavanaugh JH, Brower TD, Mann RV. The Jones fracture revisited. J Bone Joint Surg Am. 1978;60(6):776-782.
10. Porter DA, Duncan M, Meyer SJ. Fifth metatarsal Jones fracture fixation with a 4.5-mm cannulated stainless steel screw in the competitive and recreational athlete: a clinical and radiographic evaluation. Am J Sports Med. 2005;33(5):726-733.
11. Aleem IS, Jalal H, Sheikh AA, Bhandari M. Clinical decision analysis: Incorporating the evidence with patient p. Patient Prefer Adherence. 2009;3:21-24.
12. Bishop J, Ring D. Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surg Am. 2009;34(6):991-996.e1.
13. Chen NC, Shauver MJ, Chung KC. A primer on use of decision analysis methodology in hand surgery. J Hand Surg Am. 2009;34(6):983-990.
14. Kocher MS, Henley MB. It is money that matters: decision analysis and cost-effectiveness analysis. Clin Orthop Relat Res. 2003(413):106-116.
15. Dean BJ, Kothari A, Uppal H, Kankate R. The jones fracture classification, management, outcome, and complications: a systematic review. Foot Ankle Spec. 2012;5(4):256-259.
16. Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the Foot and Ankle Ability Measure (FAAM). Foot Ankle Int. 2005;26(11):968-983.
17. Roche AJ, Calder JD. Treatment and return to sport following a Jones fracture of the fifth metatarsal: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1307-1315.
18. Zwitser EW, Breederveld RS. Fractures of the fifth metatarsal; diagnosis and treatment. Injury. 2010;41(6):555-562.
19. McBryde AM Jr. The complicated Jones fracture, including revision and malalignment. Foot Ankle Clin. 2009;14(2):151-168.
20. Porter DA, Rund AM, Dobslaw R, Duncan M. Comparison of 4.5- and 5.5-mm cannulated stainless steel screws for fifth metatarsal Jones fracture fixation. Foot Ankle Int. 2009;30(1):27-33.
21. Mologne TS, Lundeen JM, Clapper MF, O’Brien TJ. Early screw fixation versus casting in the treatment of acute Jones fractures. Am J Sports Med. 2005;33(7):970-975.
22. Hunt KJ, Hurwit D. Use of patient-reported outcome measures in foot and ankle research. J Bone Joint Surg Am. 2013;95(16):e118(1-9).
23. Stiggelbout AM, Eijkemans MJ, Kiebert GM, Kievit J, Leer JW, De Haes HJ. The ‘utility’ of the visual analog scale in medical decision making and technology assessment. Is it an alternative to the time trade-off? International journal of technology assessment in health care. Spring. 1996;12(2):291-298.
24. Parkin D, Devlin N. Is there a case for using visual analogue scale valuations in cost-utility analysis? Health Econ. 2006;15(7):653-664.
25. Bishop JA, Crall TS, Kocher MS. Operative versus nonoperative treatment after primary traumatic anterior glenohumeral dislocation: expected-value decision analysis. J Shoulder Elbow Surg. 2011;20(7):1087-1094.
26. Kocher MS, Bishop J, Marshall R, Briggs KK, Hawkins RJ. Operative versus nonoperative management of acute Achilles tendon rupture: expected-value decision analysis. Am J Sports Med. 2002;30(6):783-790.
27. Nagao M, Saita Y, Kameda S, et al. Headless compression screw fixation of jones fractures: an outcomes study in Japanese athletes. Am J Sports Med. 2012;40(11):2578-2582.
28. Thomas JL, Davis BC. Treatment of Jones fracture nonunion with isolated intramedullary screw fixation. J Foot Ankle Surg. 2011;50(5):566-568.
29. Habbu RA, Marsh RS, Anderson JG, Bohay DR. Closed intramedullary screw fixation for nonunion of fifth metatarsal Jones fracture. Foot Ankle Int. 2011;32(6):603-608.
30. Hunt KJ, Anderson RB. Treatment of Jones fracture nonunions and refractures in the elite athlete: outcomes of intramedullary screw fixation with bone grafting. Am J Sports Med. 2011;39(9):1948-1954.
31. Chuckpaiwong B, Queen RM, Easley ME, Nunley JA. Distinguishing Jones and proximal diaphyseal fractures of the fifth metatarsal. Clin Orthop Relat Res. 2008;466(8):1966-1970.
32. DeVries JG, Cuttica DJ, Hyer CF. Cannulated screw fixation of Jones fifth metatarsal fractures: a comparison of titanium and stainless steel screw fixation. J Foot Ankle Surg. 2011;50(2):207-212.
33. Reese K, Litsky A, Kaeding C, Pedroza A, Shah N. Cannulated screw fixation of Jones fractures: a clinical and biomechanical study. Am J Sports Med. 2004;32(7):1736-1742.
34. Lombardi CM, Connolly FG, Silhanek AD. The use of external fixation for treatment of the acute Jones fracture: a retrospective review of 10 cases. J Foot Ankle Surg. 2004;43(3):173-178.
35. Portland G, Kelikian A, Kodros S. Acute surgical management of Jones’ fractures. Foot Ankle Int. 2003;24(11):829-833.
36. Clapper MF, O’Brien TJ, Lyons PM. Fractures of the fifth metatarsal. Analysis of a fracture registry. Clin Orthop Relat Res. 1995(315):238-241.
37. Josefsson PO, Karlsson M, Redlund-Johnell I, Wendeberg B. Closed treatment of Jones fracture. Good results in 40 cases after 11-26 years. Orthop Scand. 1994;65(5):545-547.
38. Mindrebo N, Shelbourne KD, Van Meter CD, Rettig AC. Outpatient percutaneous screw fixation of the acute Jones fracture. Am J Sports Med. 1993;21(5):720-723.
39. Zogby RG, Baker BE. A review of nonoperative treatment of Jones’ fracture. Am J Sports Med. 1987;15(4):304-307.
40. Dameron TB Jr. Fractures and anatomical variations of the proximal portion of the fifth metatarsal. J Bone Joint Surg Am. 1975;57(6):788-792.
41. Fernandez Fairen M, Guillen J, Busto JM, Roura J. Fractures of the fifth metatarsal in basketball players. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):373-377.
1. Dameron TB Jr. Fractures of the proximal fifth metatarsal: selecting the best treatment option. J Am Acad Orthop Surg. 1995;3(2):110-114.
2. Fetzer GB, Wright RW. Metatarsal shaft fractures and fractures of the proximal fifth metatarsal. Clin Sports Med. 2006;25(1):139-150, x.
3. Konkel KF, Menger AG, Retzlaff SA. Nonoperative treatment of fifth metatarsal fractures in an orthopaedic suburban private multispeciality practice. Foot Ankle Int. 2005;26(9):704-707.
4. Lawrence SJ, Botte MJ. Jones’ fractures and related fractures of the proximal fifth metatarsal. Foot Ankle. 1993;14(6):358-365.
5. Nunley JA. Fractures of the base of the fifth metatarsal: the Jones fracture. Orthop Clin North Am. 2001;32(1):171-180.
6. Quill GE Jr. Fractures of the proximal fifth metatarsal. Orthop Clin North Am. 1995;26(2):353-361.
7. Torg JS, Balduini FC, Zelko RR, Pavlov H, Peff TC, Das M. Fractures of the base of the fifth metatarsal distal to the tuberosity. Classification and guidelines for non-surgical and surgical management. J Bone Joint Surg Am. 1984;66(2):209-214.
8. DeLee JC, Evans JP, Julian J. Stress fracture of the fifth metatarsal. Am J Sports Med. 1983;11(5):349-353.
9. Kavanaugh JH, Brower TD, Mann RV. The Jones fracture revisited. J Bone Joint Surg Am. 1978;60(6):776-782.
10. Porter DA, Duncan M, Meyer SJ. Fifth metatarsal Jones fracture fixation with a 4.5-mm cannulated stainless steel screw in the competitive and recreational athlete: a clinical and radiographic evaluation. Am J Sports Med. 2005;33(5):726-733.
11. Aleem IS, Jalal H, Sheikh AA, Bhandari M. Clinical decision analysis: Incorporating the evidence with patient p. Patient Prefer Adherence. 2009;3:21-24.
12. Bishop J, Ring D. Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surg Am. 2009;34(6):991-996.e1.
13. Chen NC, Shauver MJ, Chung KC. A primer on use of decision analysis methodology in hand surgery. J Hand Surg Am. 2009;34(6):983-990.
14. Kocher MS, Henley MB. It is money that matters: decision analysis and cost-effectiveness analysis. Clin Orthop Relat Res. 2003(413):106-116.
15. Dean BJ, Kothari A, Uppal H, Kankate R. The jones fracture classification, management, outcome, and complications: a systematic review. Foot Ankle Spec. 2012;5(4):256-259.
16. Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the Foot and Ankle Ability Measure (FAAM). Foot Ankle Int. 2005;26(11):968-983.
17. Roche AJ, Calder JD. Treatment and return to sport following a Jones fracture of the fifth metatarsal: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1307-1315.
18. Zwitser EW, Breederveld RS. Fractures of the fifth metatarsal; diagnosis and treatment. Injury. 2010;41(6):555-562.
19. McBryde AM Jr. The complicated Jones fracture, including revision and malalignment. Foot Ankle Clin. 2009;14(2):151-168.
20. Porter DA, Rund AM, Dobslaw R, Duncan M. Comparison of 4.5- and 5.5-mm cannulated stainless steel screws for fifth metatarsal Jones fracture fixation. Foot Ankle Int. 2009;30(1):27-33.
21. Mologne TS, Lundeen JM, Clapper MF, O’Brien TJ. Early screw fixation versus casting in the treatment of acute Jones fractures. Am J Sports Med. 2005;33(7):970-975.
22. Hunt KJ, Hurwit D. Use of patient-reported outcome measures in foot and ankle research. J Bone Joint Surg Am. 2013;95(16):e118(1-9).
23. Stiggelbout AM, Eijkemans MJ, Kiebert GM, Kievit J, Leer JW, De Haes HJ. The ‘utility’ of the visual analog scale in medical decision making and technology assessment. Is it an alternative to the time trade-off? International journal of technology assessment in health care. Spring. 1996;12(2):291-298.
24. Parkin D, Devlin N. Is there a case for using visual analogue scale valuations in cost-utility analysis? Health Econ. 2006;15(7):653-664.
25. Bishop JA, Crall TS, Kocher MS. Operative versus nonoperative treatment after primary traumatic anterior glenohumeral dislocation: expected-value decision analysis. J Shoulder Elbow Surg. 2011;20(7):1087-1094.
26. Kocher MS, Bishop J, Marshall R, Briggs KK, Hawkins RJ. Operative versus nonoperative management of acute Achilles tendon rupture: expected-value decision analysis. Am J Sports Med. 2002;30(6):783-790.
27. Nagao M, Saita Y, Kameda S, et al. Headless compression screw fixation of jones fractures: an outcomes study in Japanese athletes. Am J Sports Med. 2012;40(11):2578-2582.
28. Thomas JL, Davis BC. Treatment of Jones fracture nonunion with isolated intramedullary screw fixation. J Foot Ankle Surg. 2011;50(5):566-568.
29. Habbu RA, Marsh RS, Anderson JG, Bohay DR. Closed intramedullary screw fixation for nonunion of fifth metatarsal Jones fracture. Foot Ankle Int. 2011;32(6):603-608.
30. Hunt KJ, Anderson RB. Treatment of Jones fracture nonunions and refractures in the elite athlete: outcomes of intramedullary screw fixation with bone grafting. Am J Sports Med. 2011;39(9):1948-1954.
31. Chuckpaiwong B, Queen RM, Easley ME, Nunley JA. Distinguishing Jones and proximal diaphyseal fractures of the fifth metatarsal. Clin Orthop Relat Res. 2008;466(8):1966-1970.
32. DeVries JG, Cuttica DJ, Hyer CF. Cannulated screw fixation of Jones fifth metatarsal fractures: a comparison of titanium and stainless steel screw fixation. J Foot Ankle Surg. 2011;50(2):207-212.
33. Reese K, Litsky A, Kaeding C, Pedroza A, Shah N. Cannulated screw fixation of Jones fractures: a clinical and biomechanical study. Am J Sports Med. 2004;32(7):1736-1742.
34. Lombardi CM, Connolly FG, Silhanek AD. The use of external fixation for treatment of the acute Jones fracture: a retrospective review of 10 cases. J Foot Ankle Surg. 2004;43(3):173-178.
35. Portland G, Kelikian A, Kodros S. Acute surgical management of Jones’ fractures. Foot Ankle Int. 2003;24(11):829-833.
36. Clapper MF, O’Brien TJ, Lyons PM. Fractures of the fifth metatarsal. Analysis of a fracture registry. Clin Orthop Relat Res. 1995(315):238-241.
37. Josefsson PO, Karlsson M, Redlund-Johnell I, Wendeberg B. Closed treatment of Jones fracture. Good results in 40 cases after 11-26 years. Orthop Scand. 1994;65(5):545-547.
38. Mindrebo N, Shelbourne KD, Van Meter CD, Rettig AC. Outpatient percutaneous screw fixation of the acute Jones fracture. Am J Sports Med. 1993;21(5):720-723.
39. Zogby RG, Baker BE. A review of nonoperative treatment of Jones’ fracture. Am J Sports Med. 1987;15(4):304-307.
40. Dameron TB Jr. Fractures and anatomical variations of the proximal portion of the fifth metatarsal. J Bone Joint Surg Am. 1975;57(6):788-792.
41. Fernandez Fairen M, Guillen J, Busto JM, Roura J. Fractures of the fifth metatarsal in basketball players. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):373-377.
Neurocognitive Deficits and Cerebral Desaturation During Shoulder Arthroscopy With Patient in Beach-Chair Position: A Review of the Current Literature
The beach-chair position (BCP) is commonly used for both arthroscopic and open shoulder surgery. This technique positions the shoulder in an anatomical upright position, facilitating shoulder access and visualization.1 Compared with the lateral decubitus position, the BCP also improves airway access, reduces bleeding, and lessens the risk for brachial plexus injury.2
Despite the advantages of using the BCP, there have been multiple reports of catastrophic neurologic complications, including severe brain damage and death, in relatively healthy patients without any known risk factors.3-6 The definitive etiology of these complications remains unclear, but it has been hypothesized that BCP use may be an independent risk factor for cerebrovascular ischemia,1,5-16 as the upright position can cause hypotension leading to increased risk for cerebral hypoperfusion.7-11,17 Reducing cerebral perfusion pressure below critical thresholds may result in permanent neurologic injury.4-6,14 Therefore, monitoring of cerebral perfusion and optimization of intraoperative cerebral oxygenation have been recommended to help avoid potential neurologic complications. However, a direct relationship between intraoperative cerebral desaturation events (CDEs) and postoperative neurocognitive deficits has not been definitively established.1,9-12
To put into perspective the importance of detecting and preventing CDEs and neurologic complications, we can consider the incidence of fatal pulmonary embolism associated with total joint arthroplasty. Although the incidence is very low, about 0.1% to 2.0%, some form of venous thromboembolism prophylaxis is the standard of care for helping prevent this serious complication. Similarly, catastrophic neurologic complications of upright shoulder arthroscopy are very rare, but it is still important to consider measures that help minimize them.
We reviewed the literature for the incidence of postoperative neurocognitive deficits, number of reported neurocognitive complications, and incidence of intraoperative CDEs in patients who underwent arthroscopic shoulder surgery in the BCP.
Methods
Dr. Salazar and Dr. Hazel independently searched the Medline, Cochrane, and Embase databases for case series, prospective studies, and cohort studies that reported neurocognitive complications associated with the BCP and the incidence of intraoperative CDEs. The authors used beach chair, desaturation, near infrared spectroscopy, and shoulder as medical subject headings (MeSH). In addition, bibliographies of retrieved articles were checked for studies that the search terms may have missed. Eighty-one publications were identified and reviewed for possible inclusion.
Next, the same 2 authors reviewed the titles and abstracts for relevance and determined which articles had potential to contribute to the study. Only English-language publications were considered for inclusion. To review the incidence of postoperative neurocognitive deficits, we included only those studies with more than 25 patients, documentation of postoperative complications, and arthroscopic shoulder surgery performed with the patient in the seated, semi-upright, or BCP. Only studies with at least 25 patients were used in order to increase the power and improve the level of evidence. To review reported cases of neurocognitive complications, we included all relevant case reports and case series. To review the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive deficits, we included studies that reported on use of intraoperative cerebral perfusion monitoring. Modalities used in these studies included near infrared spectroscopy, electroencephalography, and invasive blood pressure monitoring calculated at the brain level. Studies were excluded if they did not involve arthroscopic shoulder surgery or were not conducted with human subjects.
Information recorded for each study included general information such as author and publication year, type of study, number of patients enrolled, type of intraoperative monitoring, anesthesia protocol, number of patients with CDEs, and number of patients with neurocognitive complications after surgery.
Results
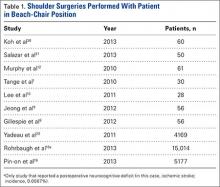
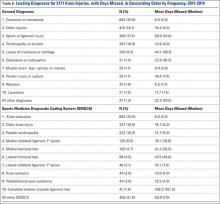
Our search identified 81 publications for potential inclusion. Our first aim was to identify the overall incidence of reported neurocognitive deficits after arthroscopic shoulder surgery with the patient in the BCP. We identified 10 studies (Table 1) that met the inclusion criteria. Among the 24,701 patients in these 10 studies, there was only 1 reported case of neurocognitive deficit after surgery, in a mixed prospective-retrospective study of 15,014 cases by Rohrbaugh and colleagues.18 The deficit they reported was an ischemic cerebral vascular accident. The 0.0067% incidence in their study demonstrates how rare the complication is. Two large retrospective studies (Ns = 4169 and 5177 patients) found no postoperative neurocognitive complications.19,20 Only 3 studies performed formal postoperative cognitive testing. Salazar and colleagues21 used the Repeatable Battery for the Assessment of Neuropsychological Status before and after surgery, and Gillespie and colleagues8 and Lee and colleagues10 used the Mini–Mental State Examination before and after surgery. Total incidence of reported neurocognitive deficits from our review was 0.004% (1/24,701).
Our second aim was to review all reported cases of neurocognitive complications after arthroscopic shoulder surgery with the patient in the BCP. We identified 4 publications that fit our inclusion criteria (Table 2). Pohl and Cullen6 described 4 cases of ischemic brain injury after arthroscopic shoulder surgery with the patient in the BCP. Age range was 47 to 57 years. Specific intraoperative cerebral monitoring was not used. However, these patients had several episodes of intraoperative hypotension (systolic blood pressures, 80-90 mm Hg), measured with a traditional blood pressure cuff on the arm. In general, these patients had minimal cerebrovascular risk factors and no known preexisting cerebrovascular disease. Drummond and colleagues22 described an ischemic stroke in a 50-year-old man after arthroscopic subacromial decompression and open rotator cuff repair that resulted in unresolved right hemiplegia. Subsequent diagnostic investigation revealed an asymmetry of the circle of Willis resulting in limited flow to the left anterior and middle cerebral artery distributions. Bhatti and Enneking3 reported the case of a 64-year-old man who lost vision in the right eye immediately after arthroscopic rotator cuff repair. His vision improved spontaneously the next morning and continued to improve over the next 6 months—he regained 20/20 vision with some residual optic neuropathy.
Our third aim was to determine the incidence of intraoperative CDEs during arthroscopic shoulder surgery with the patient in the BCP. Incidence of CDEs varied widely among the 7 studies reviewed (Table 3). Minimum incidence of intraoperative CDE was 0% in a cohort of 30 patients,1 and maximum incidence was 80% in a study of 61 patients,12 all of whom underwent elective arthroscopic shoulder surgery in the BCP. Although there was wide variability in CDE incidence, the studies were consistent with respect to their definition of a CDE. Most authors used a decrease in regional cerebral tissue oxygen saturation of 20% or more from baseline, or an absolute value up to 55%, to define a CDE. None of the 7 studies reviewed reported a clinically significant adverse neurocognitive event.
Discussion
Of concern, there have been several surveys, case reports, and small case series of previously healthy patients who had no known risk factors, underwent arthroscopic shoulder surgery in the BCP, and developed unanticipated postoperative neurologic complications.4-6,14 Beach-chair positioning during surgical procedures has been implicated as a contributing factor leading to cerebral hypoperfusion with potential for cerebral ischemia.1,12,23 These changes in cerebral perfusion pressure are thought to be the major determinant of poor neurologic outcomes. Such reports have exposed the potential need for heightened vigilance, alternative anesthesia techniques, and improved monitoring, though the exact etiology of the central nervous system injuries in this patient population is incompletely understood and is likely multifactorial. Therefore, in this study we wanted to determine the incidence of postoperative neurocognitive deficits and review all reported cases of neurocognitive complications in patients who have undergone arthroscopic shoulder surgery in the BCP. In addition, we wanted to define the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive complications.
According to our review, the incidence of postoperative neurocognitive complications after surgery in the BCP is 0.004% (1/24,701). However, this finding is based only on what has been reported; the true incidence is not known. It is also important to note that the incidence of neurocognitive deficits after many other types of surgery is not known and that surgery itself may be a risk factor for postoperative neurocognitive deficits.24 In their retrospective review of 15,014 patients who underwent arthroscopic shoulder surgery in the BCP at a single institution over an 11-year period, Rohrbaugh and colleagues18 found an overall postoperative complication rate of 0.37% and a 0.0067% incidence of neurocognitive deficits. One patient in the series was given a diagnosis of ischemic stroke on the basis of neurologic deficits that occurred 24 hours after surgery. Yadeau and colleagues20 found no postoperative neurocognitive complications in a mixed prospective-retrospective study of 4169 patients—3000 identified retrospectively, 1169 prospectively—who underwent arthroscopic shoulder surgery in the BCP at an ambulatory surgery center. Pin-on and colleagues19 reported on a series of 5177 orthopedic and neurosurgical patients who underwent surgery in the BCP. In those who had arthroscopic shoulder surgery, intraoperative systolic blood pressures obtained from an arterial line referenced to heart level decreased a mean (SD) of 14.4% (12.7%), whereas in those whose pressures were obtained from a noninvasive blood pressure cuff referenced to heart level decreased 19.3% (12.6%). However, the authors reported no incidence of postoperative stroke or neurologic deficits.
Although uncommon, perioperative cerebral ischemic accidents are potentially devastating for patients, their families, and the health care professionals involved. These events have tremendous economic, social, professional, and medicolegal implications, with perioperative stroke being particularly morbid. Perioperative stroke has a mortality rate of 60%, versus 15% to 46% for stroke in general.25,26 In 2005, Pohl and Cullen6 published a landmark article on a series of 4 relatively healthy middle-aged patients who were at low risk for stroke but had catastrophic neurocognitive complications (including 1 death) after arthroscopic shoulder surgery in the BCP. Bhatti and Enneking3 described a case of acute postoperative vision loss and ophthalmoplegia attributed to intraoperative hypotension leading to ischemia in a patient who underwent an elective shoulder arthroscopic procedure in the BCP. These reports prompted multiple investigations into the physiologic hemodynamic changes associated with surgery in the BCP and the treatment strategies used to improve patient safety.
In the normal physiologic state, the sympathetic nervous system is activated when a person assumes the seated position. The result is increased systemic vascular resistance and heart rate alterations to maintain cardiac output and mean arterial pressure. In anesthetized patients, this response is blunted by the vasodilatory effects of intravenous and volatile anesthetics. Multiple studies have demonstrated substantial hemodynamic changes in both awake and anesthetized patients during the maneuver from the supine position to the seated position1,27,28; these changes include diminished cardiac index, stroke volume, and arterial pressure.17 The data underscore the need for attentiveness and accurate monitoring of cerebral perfusion when the transition is made from the supine position to the BCP, particularly in the early phase of surgery and in high-risk patients.
Knowledge of these hemodynamic changes has led several authors to recommend additional intraoperative monitoring of cerebral perfusion. Monitoring techniques have included use of invasive blood pressure monitoring adjusted to brain level, cerebral oximetry using near infrared spectroscopy, and electroencephalography. However, the clinical relevance of intraoperative CDEs in isolation is not well understood.1,6,7,23 In addition, cost and availability of additional advanced monitoring likely factor into why it is not more commonly used. For this patient population, the severity, frequency, and duration of desaturation that causes cerebral ischemia and the relationship with postoperative neurocognitive deficits remain undefined.
The incidence of CDEs in patients being monitored with near infrared spectroscopy while undergoing elective arthroscopic shoulder surgery in the BCP varies widely, from 0% to 80% (mean, 41%).1,4,7,10,12,21 Magnitude and duration of cerebral ischemia required to produce neurocognitive dysfunction in this patient population remain unidentified as well. In conscious patients, a 20% reduction in frontal lobe oxygenation is associated with clinical manifestations of cerebral hypoperfusion, such as syncope.15,29 As none of the patients in the studies we reviewed experienced any sort of deficit, we cannot definitively state there is a correlation between CDE occurrence and neurocognitive deficit.
One limitation of our investigation is that it was a systemic review, and thus there was substantial heterogeneity in the methods and designs of the studies included in the analysis. Among the different series, there was variability in multiple aspects of the study design, including type of anesthetic, patient inclusion criteria, type of surgery, type of intraoperative cerebral perfusion monitoring, and type of neurocognitive testing. As a result, comparing the groups was difficult, and the generalizability of our findings may be limited. In addition, it is difficult to accurately establish incidence and comprehensively review these events because of the paucity of literature.
Conclusion
Neurocognitive complications after shoulder arthroscopy with the patient in the BCP are extremely rare but potentially devastating events that can affect healthy patients with no preexisting cerebrovascular risk factors. Our review indicated the incidence of permanent neurologic deficit after arthroscopy in the BCP may be as low as 0.004%. The exact etiology of such complications is not clear. Basic science research and large prospective studies are needed to identify the clinically relevant thresholds of magnitude, duration, and frequency of intraoperative CDEs in order to establish their relationship with postoperative neurocognitive complications. Such large studies may also elucidate modifiable patient-specific risk factors and establish the most sensitive, safe, and cost-effective intraoperative monitoring tools. Current literature suggests that accurate intraoperative monitoring of cerebral perfusion, alternatives to general anesthesia, and prudent use of intraoperative blood pressure control may improve patient safety.
1. Tange K, Kinoshita H, Minonishi T, et al. Cerebral oxygenation in the beach chair position before and during general anesthesia. Minerva Anestesiol. 2010;76(7):485-490.
2. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
3. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96(3):899-902.
4. Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32(4).
5. Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24(4):481-482.
6. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463-469.
7. Dippmann C, Winge S, Nielsen HB. Severe cerebral desaturation during shoulder arthroscopy in the beach-chair position. Arthroscopy. 2010;26(9 suppl):S148-S150.
8. Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94(14):1284-1290.
9. Jeong H, Lee SH, Jang EA, Chung SS, Lee J, Yoo KY. Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 2012;56(7):872-879.
10. Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889-894.
11. Moerman AT, De Hert SG, Jacobs TF, De Wilde LF, Wouters PF. Cerebral oxygen desaturation during beach chair position. Eur J Anaesthesiol. 2012;29(2):82-87.
12. Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496-505.
13. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009;25(8):891-896.
14. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
15. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93(4):964-970.
16. Smythe PR, Samra SK. Monitors of cerebral oxygenation. Anesthesiol Clin North Am. 2002;20(2):293-313.
17. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34(5):375-386.
18. Rohrbaugh M, Kentor ML, Orebaugh SL, Williams B. Outcomes of shoulder surgery in the sitting position with interscalene nerve block: a single-center series. Reg Anesth Pain Med. 2013;38(1):28-33.
19. Pin-on P, Schroeder D, Munis J. The hemodynamic management of 5177 neurosurgical and orthopedic patients who underwent surgery in the sitting or “beach chair” position without incidence of adverse neurologic events. Anesth Analg. 2013;116(6):1317-1324.
20. Yadeau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;36(5):430-435.
21. Salazar D, Sears BW, Aghdasi B, et al. Cerebral desaturation events during shoulder arthroscopy in the beach chair position: patient risk factors and neurocognitive effects. J Shoulder Elbow Surg. 2013;22(9):1228-1235.
22. Drummond JC, Lee RR, Howell JP Jr. Focal cerebral ischemia after surgery in the “beach chair” position: the role of a congenital variation of circle of Willis anatomy. Anesth Analg. 2012;114(6):1301-1303.
23. Fischer GW, Torrillo TM, Weiner MM, Rosenblatt MA. The use of cerebral oximetry as a monitor of the adequacy of cerebral perfusion in a patient undergoing shoulder surgery in the beach chair position. Pain Pract. 2009;9(4):304-307.
24. Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92(2):425-432.
25. Knapp RB, Topkins MJ, Artusio JF Jr. The cerebrovascular accident and coronary occlusion in anesthesia. JAMA. 1962;182:332-334.
26. Landercasper J, Merz BJ, Cogbill TH, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125(8):986-989.
27. Fuchs G, Schwarz G, Kulier A, Litscher G. The influence of positioning on spectroscopic measurements of brain oxygenation. J Neurosurg Anesthesiol. 2000;12(2):75-80.
28. Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554-558.
29. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58(6):541-560.
30. Koh JL, Levin SD, Chehab EL, Murphy GS. Neer award 2012: cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22:1325-1331.
The beach-chair position (BCP) is commonly used for both arthroscopic and open shoulder surgery. This technique positions the shoulder in an anatomical upright position, facilitating shoulder access and visualization.1 Compared with the lateral decubitus position, the BCP also improves airway access, reduces bleeding, and lessens the risk for brachial plexus injury.2
Despite the advantages of using the BCP, there have been multiple reports of catastrophic neurologic complications, including severe brain damage and death, in relatively healthy patients without any known risk factors.3-6 The definitive etiology of these complications remains unclear, but it has been hypothesized that BCP use may be an independent risk factor for cerebrovascular ischemia,1,5-16 as the upright position can cause hypotension leading to increased risk for cerebral hypoperfusion.7-11,17 Reducing cerebral perfusion pressure below critical thresholds may result in permanent neurologic injury.4-6,14 Therefore, monitoring of cerebral perfusion and optimization of intraoperative cerebral oxygenation have been recommended to help avoid potential neurologic complications. However, a direct relationship between intraoperative cerebral desaturation events (CDEs) and postoperative neurocognitive deficits has not been definitively established.1,9-12
To put into perspective the importance of detecting and preventing CDEs and neurologic complications, we can consider the incidence of fatal pulmonary embolism associated with total joint arthroplasty. Although the incidence is very low, about 0.1% to 2.0%, some form of venous thromboembolism prophylaxis is the standard of care for helping prevent this serious complication. Similarly, catastrophic neurologic complications of upright shoulder arthroscopy are very rare, but it is still important to consider measures that help minimize them.
We reviewed the literature for the incidence of postoperative neurocognitive deficits, number of reported neurocognitive complications, and incidence of intraoperative CDEs in patients who underwent arthroscopic shoulder surgery in the BCP.
Methods
Dr. Salazar and Dr. Hazel independently searched the Medline, Cochrane, and Embase databases for case series, prospective studies, and cohort studies that reported neurocognitive complications associated with the BCP and the incidence of intraoperative CDEs. The authors used beach chair, desaturation, near infrared spectroscopy, and shoulder as medical subject headings (MeSH). In addition, bibliographies of retrieved articles were checked for studies that the search terms may have missed. Eighty-one publications were identified and reviewed for possible inclusion.
Next, the same 2 authors reviewed the titles and abstracts for relevance and determined which articles had potential to contribute to the study. Only English-language publications were considered for inclusion. To review the incidence of postoperative neurocognitive deficits, we included only those studies with more than 25 patients, documentation of postoperative complications, and arthroscopic shoulder surgery performed with the patient in the seated, semi-upright, or BCP. Only studies with at least 25 patients were used in order to increase the power and improve the level of evidence. To review reported cases of neurocognitive complications, we included all relevant case reports and case series. To review the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive deficits, we included studies that reported on use of intraoperative cerebral perfusion monitoring. Modalities used in these studies included near infrared spectroscopy, electroencephalography, and invasive blood pressure monitoring calculated at the brain level. Studies were excluded if they did not involve arthroscopic shoulder surgery or were not conducted with human subjects.
Information recorded for each study included general information such as author and publication year, type of study, number of patients enrolled, type of intraoperative monitoring, anesthesia protocol, number of patients with CDEs, and number of patients with neurocognitive complications after surgery.
Results
Our search identified 81 publications for potential inclusion. Our first aim was to identify the overall incidence of reported neurocognitive deficits after arthroscopic shoulder surgery with the patient in the BCP. We identified 10 studies (Table 1) that met the inclusion criteria. Among the 24,701 patients in these 10 studies, there was only 1 reported case of neurocognitive deficit after surgery, in a mixed prospective-retrospective study of 15,014 cases by Rohrbaugh and colleagues.18 The deficit they reported was an ischemic cerebral vascular accident. The 0.0067% incidence in their study demonstrates how rare the complication is. Two large retrospective studies (Ns = 4169 and 5177 patients) found no postoperative neurocognitive complications.19,20 Only 3 studies performed formal postoperative cognitive testing. Salazar and colleagues21 used the Repeatable Battery for the Assessment of Neuropsychological Status before and after surgery, and Gillespie and colleagues8 and Lee and colleagues10 used the Mini–Mental State Examination before and after surgery. Total incidence of reported neurocognitive deficits from our review was 0.004% (1/24,701).
Our second aim was to review all reported cases of neurocognitive complications after arthroscopic shoulder surgery with the patient in the BCP. We identified 4 publications that fit our inclusion criteria (Table 2). Pohl and Cullen6 described 4 cases of ischemic brain injury after arthroscopic shoulder surgery with the patient in the BCP. Age range was 47 to 57 years. Specific intraoperative cerebral monitoring was not used. However, these patients had several episodes of intraoperative hypotension (systolic blood pressures, 80-90 mm Hg), measured with a traditional blood pressure cuff on the arm. In general, these patients had minimal cerebrovascular risk factors and no known preexisting cerebrovascular disease. Drummond and colleagues22 described an ischemic stroke in a 50-year-old man after arthroscopic subacromial decompression and open rotator cuff repair that resulted in unresolved right hemiplegia. Subsequent diagnostic investigation revealed an asymmetry of the circle of Willis resulting in limited flow to the left anterior and middle cerebral artery distributions. Bhatti and Enneking3 reported the case of a 64-year-old man who lost vision in the right eye immediately after arthroscopic rotator cuff repair. His vision improved spontaneously the next morning and continued to improve over the next 6 months—he regained 20/20 vision with some residual optic neuropathy.
Our third aim was to determine the incidence of intraoperative CDEs during arthroscopic shoulder surgery with the patient in the BCP. Incidence of CDEs varied widely among the 7 studies reviewed (Table 3). Minimum incidence of intraoperative CDE was 0% in a cohort of 30 patients,1 and maximum incidence was 80% in a study of 61 patients,12 all of whom underwent elective arthroscopic shoulder surgery in the BCP. Although there was wide variability in CDE incidence, the studies were consistent with respect to their definition of a CDE. Most authors used a decrease in regional cerebral tissue oxygen saturation of 20% or more from baseline, or an absolute value up to 55%, to define a CDE. None of the 7 studies reviewed reported a clinically significant adverse neurocognitive event.
Discussion
Of concern, there have been several surveys, case reports, and small case series of previously healthy patients who had no known risk factors, underwent arthroscopic shoulder surgery in the BCP, and developed unanticipated postoperative neurologic complications.4-6,14 Beach-chair positioning during surgical procedures has been implicated as a contributing factor leading to cerebral hypoperfusion with potential for cerebral ischemia.1,12,23 These changes in cerebral perfusion pressure are thought to be the major determinant of poor neurologic outcomes. Such reports have exposed the potential need for heightened vigilance, alternative anesthesia techniques, and improved monitoring, though the exact etiology of the central nervous system injuries in this patient population is incompletely understood and is likely multifactorial. Therefore, in this study we wanted to determine the incidence of postoperative neurocognitive deficits and review all reported cases of neurocognitive complications in patients who have undergone arthroscopic shoulder surgery in the BCP. In addition, we wanted to define the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive complications.
According to our review, the incidence of postoperative neurocognitive complications after surgery in the BCP is 0.004% (1/24,701). However, this finding is based only on what has been reported; the true incidence is not known. It is also important to note that the incidence of neurocognitive deficits after many other types of surgery is not known and that surgery itself may be a risk factor for postoperative neurocognitive deficits.24 In their retrospective review of 15,014 patients who underwent arthroscopic shoulder surgery in the BCP at a single institution over an 11-year period, Rohrbaugh and colleagues18 found an overall postoperative complication rate of 0.37% and a 0.0067% incidence of neurocognitive deficits. One patient in the series was given a diagnosis of ischemic stroke on the basis of neurologic deficits that occurred 24 hours after surgery. Yadeau and colleagues20 found no postoperative neurocognitive complications in a mixed prospective-retrospective study of 4169 patients—3000 identified retrospectively, 1169 prospectively—who underwent arthroscopic shoulder surgery in the BCP at an ambulatory surgery center. Pin-on and colleagues19 reported on a series of 5177 orthopedic and neurosurgical patients who underwent surgery in the BCP. In those who had arthroscopic shoulder surgery, intraoperative systolic blood pressures obtained from an arterial line referenced to heart level decreased a mean (SD) of 14.4% (12.7%), whereas in those whose pressures were obtained from a noninvasive blood pressure cuff referenced to heart level decreased 19.3% (12.6%). However, the authors reported no incidence of postoperative stroke or neurologic deficits.
Although uncommon, perioperative cerebral ischemic accidents are potentially devastating for patients, their families, and the health care professionals involved. These events have tremendous economic, social, professional, and medicolegal implications, with perioperative stroke being particularly morbid. Perioperative stroke has a mortality rate of 60%, versus 15% to 46% for stroke in general.25,26 In 2005, Pohl and Cullen6 published a landmark article on a series of 4 relatively healthy middle-aged patients who were at low risk for stroke but had catastrophic neurocognitive complications (including 1 death) after arthroscopic shoulder surgery in the BCP. Bhatti and Enneking3 described a case of acute postoperative vision loss and ophthalmoplegia attributed to intraoperative hypotension leading to ischemia in a patient who underwent an elective shoulder arthroscopic procedure in the BCP. These reports prompted multiple investigations into the physiologic hemodynamic changes associated with surgery in the BCP and the treatment strategies used to improve patient safety.
In the normal physiologic state, the sympathetic nervous system is activated when a person assumes the seated position. The result is increased systemic vascular resistance and heart rate alterations to maintain cardiac output and mean arterial pressure. In anesthetized patients, this response is blunted by the vasodilatory effects of intravenous and volatile anesthetics. Multiple studies have demonstrated substantial hemodynamic changes in both awake and anesthetized patients during the maneuver from the supine position to the seated position1,27,28; these changes include diminished cardiac index, stroke volume, and arterial pressure.17 The data underscore the need for attentiveness and accurate monitoring of cerebral perfusion when the transition is made from the supine position to the BCP, particularly in the early phase of surgery and in high-risk patients.
Knowledge of these hemodynamic changes has led several authors to recommend additional intraoperative monitoring of cerebral perfusion. Monitoring techniques have included use of invasive blood pressure monitoring adjusted to brain level, cerebral oximetry using near infrared spectroscopy, and electroencephalography. However, the clinical relevance of intraoperative CDEs in isolation is not well understood.1,6,7,23 In addition, cost and availability of additional advanced monitoring likely factor into why it is not more commonly used. For this patient population, the severity, frequency, and duration of desaturation that causes cerebral ischemia and the relationship with postoperative neurocognitive deficits remain undefined.
The incidence of CDEs in patients being monitored with near infrared spectroscopy while undergoing elective arthroscopic shoulder surgery in the BCP varies widely, from 0% to 80% (mean, 41%).1,4,7,10,12,21 Magnitude and duration of cerebral ischemia required to produce neurocognitive dysfunction in this patient population remain unidentified as well. In conscious patients, a 20% reduction in frontal lobe oxygenation is associated with clinical manifestations of cerebral hypoperfusion, such as syncope.15,29 As none of the patients in the studies we reviewed experienced any sort of deficit, we cannot definitively state there is a correlation between CDE occurrence and neurocognitive deficit.
One limitation of our investigation is that it was a systemic review, and thus there was substantial heterogeneity in the methods and designs of the studies included in the analysis. Among the different series, there was variability in multiple aspects of the study design, including type of anesthetic, patient inclusion criteria, type of surgery, type of intraoperative cerebral perfusion monitoring, and type of neurocognitive testing. As a result, comparing the groups was difficult, and the generalizability of our findings may be limited. In addition, it is difficult to accurately establish incidence and comprehensively review these events because of the paucity of literature.
Conclusion
Neurocognitive complications after shoulder arthroscopy with the patient in the BCP are extremely rare but potentially devastating events that can affect healthy patients with no preexisting cerebrovascular risk factors. Our review indicated the incidence of permanent neurologic deficit after arthroscopy in the BCP may be as low as 0.004%. The exact etiology of such complications is not clear. Basic science research and large prospective studies are needed to identify the clinically relevant thresholds of magnitude, duration, and frequency of intraoperative CDEs in order to establish their relationship with postoperative neurocognitive complications. Such large studies may also elucidate modifiable patient-specific risk factors and establish the most sensitive, safe, and cost-effective intraoperative monitoring tools. Current literature suggests that accurate intraoperative monitoring of cerebral perfusion, alternatives to general anesthesia, and prudent use of intraoperative blood pressure control may improve patient safety.
The beach-chair position (BCP) is commonly used for both arthroscopic and open shoulder surgery. This technique positions the shoulder in an anatomical upright position, facilitating shoulder access and visualization.1 Compared with the lateral decubitus position, the BCP also improves airway access, reduces bleeding, and lessens the risk for brachial plexus injury.2
Despite the advantages of using the BCP, there have been multiple reports of catastrophic neurologic complications, including severe brain damage and death, in relatively healthy patients without any known risk factors.3-6 The definitive etiology of these complications remains unclear, but it has been hypothesized that BCP use may be an independent risk factor for cerebrovascular ischemia,1,5-16 as the upright position can cause hypotension leading to increased risk for cerebral hypoperfusion.7-11,17 Reducing cerebral perfusion pressure below critical thresholds may result in permanent neurologic injury.4-6,14 Therefore, monitoring of cerebral perfusion and optimization of intraoperative cerebral oxygenation have been recommended to help avoid potential neurologic complications. However, a direct relationship between intraoperative cerebral desaturation events (CDEs) and postoperative neurocognitive deficits has not been definitively established.1,9-12
To put into perspective the importance of detecting and preventing CDEs and neurologic complications, we can consider the incidence of fatal pulmonary embolism associated with total joint arthroplasty. Although the incidence is very low, about 0.1% to 2.0%, some form of venous thromboembolism prophylaxis is the standard of care for helping prevent this serious complication. Similarly, catastrophic neurologic complications of upright shoulder arthroscopy are very rare, but it is still important to consider measures that help minimize them.
We reviewed the literature for the incidence of postoperative neurocognitive deficits, number of reported neurocognitive complications, and incidence of intraoperative CDEs in patients who underwent arthroscopic shoulder surgery in the BCP.
Methods
Dr. Salazar and Dr. Hazel independently searched the Medline, Cochrane, and Embase databases for case series, prospective studies, and cohort studies that reported neurocognitive complications associated with the BCP and the incidence of intraoperative CDEs. The authors used beach chair, desaturation, near infrared spectroscopy, and shoulder as medical subject headings (MeSH). In addition, bibliographies of retrieved articles were checked for studies that the search terms may have missed. Eighty-one publications were identified and reviewed for possible inclusion.
Next, the same 2 authors reviewed the titles and abstracts for relevance and determined which articles had potential to contribute to the study. Only English-language publications were considered for inclusion. To review the incidence of postoperative neurocognitive deficits, we included only those studies with more than 25 patients, documentation of postoperative complications, and arthroscopic shoulder surgery performed with the patient in the seated, semi-upright, or BCP. Only studies with at least 25 patients were used in order to increase the power and improve the level of evidence. To review reported cases of neurocognitive complications, we included all relevant case reports and case series. To review the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive deficits, we included studies that reported on use of intraoperative cerebral perfusion monitoring. Modalities used in these studies included near infrared spectroscopy, electroencephalography, and invasive blood pressure monitoring calculated at the brain level. Studies were excluded if they did not involve arthroscopic shoulder surgery or were not conducted with human subjects.
Information recorded for each study included general information such as author and publication year, type of study, number of patients enrolled, type of intraoperative monitoring, anesthesia protocol, number of patients with CDEs, and number of patients with neurocognitive complications after surgery.
Results
Our search identified 81 publications for potential inclusion. Our first aim was to identify the overall incidence of reported neurocognitive deficits after arthroscopic shoulder surgery with the patient in the BCP. We identified 10 studies (Table 1) that met the inclusion criteria. Among the 24,701 patients in these 10 studies, there was only 1 reported case of neurocognitive deficit after surgery, in a mixed prospective-retrospective study of 15,014 cases by Rohrbaugh and colleagues.18 The deficit they reported was an ischemic cerebral vascular accident. The 0.0067% incidence in their study demonstrates how rare the complication is. Two large retrospective studies (Ns = 4169 and 5177 patients) found no postoperative neurocognitive complications.19,20 Only 3 studies performed formal postoperative cognitive testing. Salazar and colleagues21 used the Repeatable Battery for the Assessment of Neuropsychological Status before and after surgery, and Gillespie and colleagues8 and Lee and colleagues10 used the Mini–Mental State Examination before and after surgery. Total incidence of reported neurocognitive deficits from our review was 0.004% (1/24,701).
Our second aim was to review all reported cases of neurocognitive complications after arthroscopic shoulder surgery with the patient in the BCP. We identified 4 publications that fit our inclusion criteria (Table 2). Pohl and Cullen6 described 4 cases of ischemic brain injury after arthroscopic shoulder surgery with the patient in the BCP. Age range was 47 to 57 years. Specific intraoperative cerebral monitoring was not used. However, these patients had several episodes of intraoperative hypotension (systolic blood pressures, 80-90 mm Hg), measured with a traditional blood pressure cuff on the arm. In general, these patients had minimal cerebrovascular risk factors and no known preexisting cerebrovascular disease. Drummond and colleagues22 described an ischemic stroke in a 50-year-old man after arthroscopic subacromial decompression and open rotator cuff repair that resulted in unresolved right hemiplegia. Subsequent diagnostic investigation revealed an asymmetry of the circle of Willis resulting in limited flow to the left anterior and middle cerebral artery distributions. Bhatti and Enneking3 reported the case of a 64-year-old man who lost vision in the right eye immediately after arthroscopic rotator cuff repair. His vision improved spontaneously the next morning and continued to improve over the next 6 months—he regained 20/20 vision with some residual optic neuropathy.
Our third aim was to determine the incidence of intraoperative CDEs during arthroscopic shoulder surgery with the patient in the BCP. Incidence of CDEs varied widely among the 7 studies reviewed (Table 3). Minimum incidence of intraoperative CDE was 0% in a cohort of 30 patients,1 and maximum incidence was 80% in a study of 61 patients,12 all of whom underwent elective arthroscopic shoulder surgery in the BCP. Although there was wide variability in CDE incidence, the studies were consistent with respect to their definition of a CDE. Most authors used a decrease in regional cerebral tissue oxygen saturation of 20% or more from baseline, or an absolute value up to 55%, to define a CDE. None of the 7 studies reviewed reported a clinically significant adverse neurocognitive event.
Discussion
Of concern, there have been several surveys, case reports, and small case series of previously healthy patients who had no known risk factors, underwent arthroscopic shoulder surgery in the BCP, and developed unanticipated postoperative neurologic complications.4-6,14 Beach-chair positioning during surgical procedures has been implicated as a contributing factor leading to cerebral hypoperfusion with potential for cerebral ischemia.1,12,23 These changes in cerebral perfusion pressure are thought to be the major determinant of poor neurologic outcomes. Such reports have exposed the potential need for heightened vigilance, alternative anesthesia techniques, and improved monitoring, though the exact etiology of the central nervous system injuries in this patient population is incompletely understood and is likely multifactorial. Therefore, in this study we wanted to determine the incidence of postoperative neurocognitive deficits and review all reported cases of neurocognitive complications in patients who have undergone arthroscopic shoulder surgery in the BCP. In addition, we wanted to define the incidence of intraoperative CDEs and investigate their relationship with postoperative neurocognitive complications.
According to our review, the incidence of postoperative neurocognitive complications after surgery in the BCP is 0.004% (1/24,701). However, this finding is based only on what has been reported; the true incidence is not known. It is also important to note that the incidence of neurocognitive deficits after many other types of surgery is not known and that surgery itself may be a risk factor for postoperative neurocognitive deficits.24 In their retrospective review of 15,014 patients who underwent arthroscopic shoulder surgery in the BCP at a single institution over an 11-year period, Rohrbaugh and colleagues18 found an overall postoperative complication rate of 0.37% and a 0.0067% incidence of neurocognitive deficits. One patient in the series was given a diagnosis of ischemic stroke on the basis of neurologic deficits that occurred 24 hours after surgery. Yadeau and colleagues20 found no postoperative neurocognitive complications in a mixed prospective-retrospective study of 4169 patients—3000 identified retrospectively, 1169 prospectively—who underwent arthroscopic shoulder surgery in the BCP at an ambulatory surgery center. Pin-on and colleagues19 reported on a series of 5177 orthopedic and neurosurgical patients who underwent surgery in the BCP. In those who had arthroscopic shoulder surgery, intraoperative systolic blood pressures obtained from an arterial line referenced to heart level decreased a mean (SD) of 14.4% (12.7%), whereas in those whose pressures were obtained from a noninvasive blood pressure cuff referenced to heart level decreased 19.3% (12.6%). However, the authors reported no incidence of postoperative stroke or neurologic deficits.
Although uncommon, perioperative cerebral ischemic accidents are potentially devastating for patients, their families, and the health care professionals involved. These events have tremendous economic, social, professional, and medicolegal implications, with perioperative stroke being particularly morbid. Perioperative stroke has a mortality rate of 60%, versus 15% to 46% for stroke in general.25,26 In 2005, Pohl and Cullen6 published a landmark article on a series of 4 relatively healthy middle-aged patients who were at low risk for stroke but had catastrophic neurocognitive complications (including 1 death) after arthroscopic shoulder surgery in the BCP. Bhatti and Enneking3 described a case of acute postoperative vision loss and ophthalmoplegia attributed to intraoperative hypotension leading to ischemia in a patient who underwent an elective shoulder arthroscopic procedure in the BCP. These reports prompted multiple investigations into the physiologic hemodynamic changes associated with surgery in the BCP and the treatment strategies used to improve patient safety.
In the normal physiologic state, the sympathetic nervous system is activated when a person assumes the seated position. The result is increased systemic vascular resistance and heart rate alterations to maintain cardiac output and mean arterial pressure. In anesthetized patients, this response is blunted by the vasodilatory effects of intravenous and volatile anesthetics. Multiple studies have demonstrated substantial hemodynamic changes in both awake and anesthetized patients during the maneuver from the supine position to the seated position1,27,28; these changes include diminished cardiac index, stroke volume, and arterial pressure.17 The data underscore the need for attentiveness and accurate monitoring of cerebral perfusion when the transition is made from the supine position to the BCP, particularly in the early phase of surgery and in high-risk patients.
Knowledge of these hemodynamic changes has led several authors to recommend additional intraoperative monitoring of cerebral perfusion. Monitoring techniques have included use of invasive blood pressure monitoring adjusted to brain level, cerebral oximetry using near infrared spectroscopy, and electroencephalography. However, the clinical relevance of intraoperative CDEs in isolation is not well understood.1,6,7,23 In addition, cost and availability of additional advanced monitoring likely factor into why it is not more commonly used. For this patient population, the severity, frequency, and duration of desaturation that causes cerebral ischemia and the relationship with postoperative neurocognitive deficits remain undefined.
The incidence of CDEs in patients being monitored with near infrared spectroscopy while undergoing elective arthroscopic shoulder surgery in the BCP varies widely, from 0% to 80% (mean, 41%).1,4,7,10,12,21 Magnitude and duration of cerebral ischemia required to produce neurocognitive dysfunction in this patient population remain unidentified as well. In conscious patients, a 20% reduction in frontal lobe oxygenation is associated with clinical manifestations of cerebral hypoperfusion, such as syncope.15,29 As none of the patients in the studies we reviewed experienced any sort of deficit, we cannot definitively state there is a correlation between CDE occurrence and neurocognitive deficit.
One limitation of our investigation is that it was a systemic review, and thus there was substantial heterogeneity in the methods and designs of the studies included in the analysis. Among the different series, there was variability in multiple aspects of the study design, including type of anesthetic, patient inclusion criteria, type of surgery, type of intraoperative cerebral perfusion monitoring, and type of neurocognitive testing. As a result, comparing the groups was difficult, and the generalizability of our findings may be limited. In addition, it is difficult to accurately establish incidence and comprehensively review these events because of the paucity of literature.
Conclusion
Neurocognitive complications after shoulder arthroscopy with the patient in the BCP are extremely rare but potentially devastating events that can affect healthy patients with no preexisting cerebrovascular risk factors. Our review indicated the incidence of permanent neurologic deficit after arthroscopy in the BCP may be as low as 0.004%. The exact etiology of such complications is not clear. Basic science research and large prospective studies are needed to identify the clinically relevant thresholds of magnitude, duration, and frequency of intraoperative CDEs in order to establish their relationship with postoperative neurocognitive complications. Such large studies may also elucidate modifiable patient-specific risk factors and establish the most sensitive, safe, and cost-effective intraoperative monitoring tools. Current literature suggests that accurate intraoperative monitoring of cerebral perfusion, alternatives to general anesthesia, and prudent use of intraoperative blood pressure control may improve patient safety.
1. Tange K, Kinoshita H, Minonishi T, et al. Cerebral oxygenation in the beach chair position before and during general anesthesia. Minerva Anestesiol. 2010;76(7):485-490.
2. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
3. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96(3):899-902.
4. Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32(4).
5. Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24(4):481-482.
6. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463-469.
7. Dippmann C, Winge S, Nielsen HB. Severe cerebral desaturation during shoulder arthroscopy in the beach-chair position. Arthroscopy. 2010;26(9 suppl):S148-S150.
8. Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94(14):1284-1290.
9. Jeong H, Lee SH, Jang EA, Chung SS, Lee J, Yoo KY. Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 2012;56(7):872-879.
10. Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889-894.
11. Moerman AT, De Hert SG, Jacobs TF, De Wilde LF, Wouters PF. Cerebral oxygen desaturation during beach chair position. Eur J Anaesthesiol. 2012;29(2):82-87.
12. Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496-505.
13. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009;25(8):891-896.
14. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
15. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93(4):964-970.
16. Smythe PR, Samra SK. Monitors of cerebral oxygenation. Anesthesiol Clin North Am. 2002;20(2):293-313.
17. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34(5):375-386.
18. Rohrbaugh M, Kentor ML, Orebaugh SL, Williams B. Outcomes of shoulder surgery in the sitting position with interscalene nerve block: a single-center series. Reg Anesth Pain Med. 2013;38(1):28-33.
19. Pin-on P, Schroeder D, Munis J. The hemodynamic management of 5177 neurosurgical and orthopedic patients who underwent surgery in the sitting or “beach chair” position without incidence of adverse neurologic events. Anesth Analg. 2013;116(6):1317-1324.
20. Yadeau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;36(5):430-435.
21. Salazar D, Sears BW, Aghdasi B, et al. Cerebral desaturation events during shoulder arthroscopy in the beach chair position: patient risk factors and neurocognitive effects. J Shoulder Elbow Surg. 2013;22(9):1228-1235.
22. Drummond JC, Lee RR, Howell JP Jr. Focal cerebral ischemia after surgery in the “beach chair” position: the role of a congenital variation of circle of Willis anatomy. Anesth Analg. 2012;114(6):1301-1303.
23. Fischer GW, Torrillo TM, Weiner MM, Rosenblatt MA. The use of cerebral oximetry as a monitor of the adequacy of cerebral perfusion in a patient undergoing shoulder surgery in the beach chair position. Pain Pract. 2009;9(4):304-307.
24. Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92(2):425-432.
25. Knapp RB, Topkins MJ, Artusio JF Jr. The cerebrovascular accident and coronary occlusion in anesthesia. JAMA. 1962;182:332-334.
26. Landercasper J, Merz BJ, Cogbill TH, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125(8):986-989.
27. Fuchs G, Schwarz G, Kulier A, Litscher G. The influence of positioning on spectroscopic measurements of brain oxygenation. J Neurosurg Anesthesiol. 2000;12(2):75-80.
28. Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554-558.
29. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58(6):541-560.
30. Koh JL, Levin SD, Chehab EL, Murphy GS. Neer award 2012: cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22:1325-1331.
1. Tange K, Kinoshita H, Minonishi T, et al. Cerebral oxygenation in the beach chair position before and during general anesthesia. Minerva Anestesiol. 2010;76(7):485-490.
2. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
3. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96(3):899-902.
4. Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32(4).
5. Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24(4):481-482.
6. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463-469.
7. Dippmann C, Winge S, Nielsen HB. Severe cerebral desaturation during shoulder arthroscopy in the beach-chair position. Arthroscopy. 2010;26(9 suppl):S148-S150.
8. Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94(14):1284-1290.
9. Jeong H, Lee SH, Jang EA, Chung SS, Lee J, Yoo KY. Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 2012;56(7):872-879.
10. Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889-894.
11. Moerman AT, De Hert SG, Jacobs TF, De Wilde LF, Wouters PF. Cerebral oxygen desaturation during beach chair position. Eur J Anaesthesiol. 2012;29(2):82-87.
12. Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496-505.
13. Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009;25(8):891-896.
14. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
15. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93(4):964-970.
16. Smythe PR, Samra SK. Monitors of cerebral oxygenation. Anesthesiol Clin North Am. 2002;20(2):293-313.
17. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34(5):375-386.
18. Rohrbaugh M, Kentor ML, Orebaugh SL, Williams B. Outcomes of shoulder surgery in the sitting position with interscalene nerve block: a single-center series. Reg Anesth Pain Med. 2013;38(1):28-33.
19. Pin-on P, Schroeder D, Munis J. The hemodynamic management of 5177 neurosurgical and orthopedic patients who underwent surgery in the sitting or “beach chair” position without incidence of adverse neurologic events. Anesth Analg. 2013;116(6):1317-1324.
20. Yadeau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;36(5):430-435.
21. Salazar D, Sears BW, Aghdasi B, et al. Cerebral desaturation events during shoulder arthroscopy in the beach chair position: patient risk factors and neurocognitive effects. J Shoulder Elbow Surg. 2013;22(9):1228-1235.
22. Drummond JC, Lee RR, Howell JP Jr. Focal cerebral ischemia after surgery in the “beach chair” position: the role of a congenital variation of circle of Willis anatomy. Anesth Analg. 2012;114(6):1301-1303.
23. Fischer GW, Torrillo TM, Weiner MM, Rosenblatt MA. The use of cerebral oximetry as a monitor of the adequacy of cerebral perfusion in a patient undergoing shoulder surgery in the beach chair position. Pain Pract. 2009;9(4):304-307.
24. Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92(2):425-432.
25. Knapp RB, Topkins MJ, Artusio JF Jr. The cerebrovascular accident and coronary occlusion in anesthesia. JAMA. 1962;182:332-334.
26. Landercasper J, Merz BJ, Cogbill TH, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125(8):986-989.
27. Fuchs G, Schwarz G, Kulier A, Litscher G. The influence of positioning on spectroscopic measurements of brain oxygenation. J Neurosurg Anesthesiol. 2000;12(2):75-80.
28. Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554-558.
29. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58(6):541-560.
30. Koh JL, Levin SD, Chehab EL, Murphy GS. Neer award 2012: cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22:1325-1331.
Epidemiology and Impact of Knee Injuries in Major and Minor League Baseball Players
Injuries among professional baseball players have been on the rise for several years.1,2 From 1989 to 1999, the number of disabled list (DL) reports increased 38% (266 to 367 annual reports),1 and a similar increase in injury rates was noted from the 2002 to the 2008 seasons (37%).2 These injuries have important implications for future injury risk and time away from play. Identifying these injuries and determining correlates and risk factors is important for targeted prevention efforts.
Several studies have explored the prevalence of upper extremity injuries in professional and collegiate baseball players;2-4 however, detailed epidemiology of knee injuries in Major League Baseball (MLB) and Minor League Baseball (MiLB) players is lacking. Much more is known about the prevalence, treatment, and outcomes of knee injuries in other professional sporting organizations, such as the National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL).4-12 A recent meta-analysis exploring injuries in professional athletes found that studies on lower extremity injuries comprised approximately 12% of the literature reporting injuries in MLB players.4 In other professional leagues, publications on lower extremity injuries comprise approximately 56% of the sports medicine literature in the NFL, 54% in the NBA, and 62% in the NHL.4 Since few studies have investigated lower extremity injuries among professional baseball players, there is an opportunity for additional research to guide evidence-based prevention strategies.
A better understanding of the nature of these injuries is one of the first steps towards developing targeted injury prevention programs and treatment algorithms. The study of injury epidemiology among professional baseball players has been aided by the creation of an injury tracking system initiated by the MLB, its minor league affiliates, and the Major League Baseball Players Association.5,13,14 This surveillance system allows for the tracking of medical histories and injuries to players as they move across major and minor league organizations. Similar systems have been utilized in the National Collegiate Athletic Association and other professional sports organizations.3,15-17 A unique advantage of the MLB surveillance system is the required participation of all major and minor league teams, which allows for investigation of the entire population of players rather than simply a sample of players from select teams. This system has propelled an effort to identify injury patterns as a means of developing appropriate targets for potential preventative measures.5
The purpose of this descriptive epidemiologic study is to better understand the distribution and characteristics of knee injuries in these elite athletes by reporting on all knee injuries occurring over a span of 4 seasons (2011-2014). Additionally, this study seeks to characterize the impact of these injuries by analyzing the time required for return to play and the treatments rendered (surgical and nonsurgical).
Materials and Methods
After approval from the Johns Hopkins Bloomberg School of Public Health Institutional Review Board, detailed data regarding knee injuries in both MLB and MiLB baseball players were extracted from the de-identified MLB Health and Injury Tracking System (HITS). The HITS database is a centralized database that contains data on injuries from an electronic medical record (EMR). All players provided consent to have their data included in this EMR. HITS system captures injuries reported by the athletic trainers for all professional baseball players from 30 MLB clubs and their 230 minor league affiliates. Additional details on this population of professional baseball players have been published elsewhere.5 Only injuries that result in time out of play (≥1 day missed) are included in the database, and they are logged with basic information such as region of the body, diagnosis, date, player position, activity leading to injury, and general treatment. Any injury that affects participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training) is captured in HITS.
All baseball-related knee injuries occurring during the 2011-2014 seasons that resulted in time out of sport were included in the study. These injuries were identified based on the Sports Medicine Diagnostic Coding System (SMDCS) to capture injuries by diagnostic groups.18 Knee injuries were included if they occurred during spring training, regular season, or postseason play. Offseason injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of days missed because many of these players may not have been cleared to play until the beginning of the following season. To determine the proportion of knee injuries during the study period, all injuries were included for comparative purposes (subdivided based on 30 anatomic regions or types).
For each knee injury, a number of variables were analyzed, including diagnosis, level of play (MLB vs. MiLB), age, player position at the time of injury (pitcher, catcher, infield, outfield, base runner, or batter), field location where the injury occurred (home plate, pitcher’s mound, infield, outfield, foul territory or bullpen, or other), mechanism of injury, days missed, and treatment rendered (conservative vs surgical). The classification used to describe the mechanism of injury consisted of contact with ball, contact with ground, contact with another player, contact with another object, or noncontact.
Statistical Analysis Epidemiologic data are presented with descriptive statistics such as mean, median, frequency, and percentage where appropriate. When comparing player age, days missed, and surgical vs nonsurgical treatment between MLB and MiLB players, t-tests and tests for difference in proportions were applied as appropriate. Statistical significance was established for P values < .05.
The distribution of days missed for the variables considered was often skewed to the right (ie, days missed mostly concentrated on the low to moderate number of days, with fewer values in the much higher days missed range), even after excluding the season-ending injuries; hence the mean (or average) days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when knee injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those knee injuries that occurred during the regular season. It should be noted that the number of regular season knee injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training knee injuries.
RESULTS
Overall Summary
Of the 30 general body regions/systems included in the HITS database, injuries to the knee were the fifth most common reason for days missed in all of professional baseball from 2011-2014 (Table 1). Injuries to the knee represented 6.5% of the nearly 34,000 injuries sustained during the study period. Knee injuries were the fifth most common reason for time out of play for players in both the MiLB and MLB.
A total of 2171 isolated knee injuries resulted in time out of sport for professional baseball players (Table 2). Of these, 410 (19%) occurred in MLB players and 1761 (81%) occurred in MiLB players. MLB players were older than MiLB players at the time of injury (29.5 vs 22.8 years, respectively). Overall mean number of days missed was 16.2 days per knee injury, with MLB players missing an approximately 7 days more per injury than MiLB athletes (21.8 vs. 14.9 days respectively; P = .001).Over the course of the 4 seasons, a total of 30,449 days were missed due to knee injuries in professional baseball, giving an average rate of 7612 days lost per season. Surgery was performed for 263 (12.1%) of the 2171 knee injuries, with a greater proportion of MLB players requiring surgery than MiLB players (17.3% vs 10.9%) (P < .001). With respect to number of days missed per injury, 26% of knee injuries in the minor leagues resulted in greater than 30 days missed, while this number rose to 32% for knee injuries in MLB players (Table 3).
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE, respectively, over the course of the 4 seasons (2011-2014). The overall knee injury rate across both the MiLB and MLB was 1.2 per 1000 AE, based on the subset of 308 and 1473 regular season knee injuries in MiLB and MLB, respectively. The rate of knee injury was similar and not significantly different between the MiLB and MLB (1.2 per 1000 AE in the MiLB and 1.1 per 1000 AE in the MLB).
Characteristics of Injuries
When considering the position of the player during injury, defensive players were most frequently injured (n = 742, 56.5%), with pitchers (n = 227, 17.3%), infielders (n =193, 14.7%), outfielders (n = 193, 14.7%), and catchers (n = 129, 9.8%) sustaining injuries in decreasing frequency. Injuries while on offense (n = 571, 43.5%) were most frequent in base runners (n = 320, 24.4%) followed by batters (n = 251, 19.1%) (Table 4). Injuries while on defense occurring in infielders and catchers resulted in the longest period of time away from play (average of 22.4 and 20.8 days missed, respectively), while those occurring in batters resulted in the least average days missed (8.9 days).
The most common field location for knee injuries to occur was the infield, which was responsible for n = 647 (29.8%) of the total knee injuries (Table 4). This was followed by home plate (n = 493, 22.7%), other locations outside those specified (n = 394, 18.1%), outfield (n = 320, 14.7%), pitcher’s mound (n = 210, 9.7%), and foul territory or the bullpen (n = 107, 4.9%). Of the knee injuries with a specified location, those occurring in foul territory or the bullpen resulted in the highest mean days missed (18.4), while those occurring at home plate resulted in the least mean days missed (13.4 days).
When analyzed by mechanism of injury, noncontact injuries (n = 953, 43.9%) were more common than being hit with the ball (n = 374, 17.2%), striking the ground (n = 409, 18.8%), other mechanisms not listed (n = 196, 9%), contact with another player (n = 176, 8.1%), or contact with other objects (n = 63, 2.9%) (Table 4). Noncontact injuries and player to player collisions resulted in the greatest number of missed days (21.6 and 17.1 days, respectively) while being struck by the ball resulted in the least mean days missed (5.1).
Of the n = 493 knee injuries occurring at home plate, n = 212 (43%) occurred to the batter, n = 100 (20%) to the catcher, n = 34 (6.9%) to base runners, and n = 7 (1.4%) to pitchers (Table 5). The majority of knee injuries in the infield occurred to base runners (n = 283, 43.7%). Player-to-player collisions at home plate were responsible for 51 (2.3%) knee injuries, while 163 (24%) were noncontact injuries and 376 (56%) were the result of a player being hit by the ball (Table 5).
Injury Diagnosis
By diagnosis, the most common knee injuries observed were contusions or hematomas (n = 662, 30.5%), other injuries (n = 415, 19.1%), sprains or ligament injuries (n = 380, 17.5%), tendinopathies or bursitis (n = 367, 16.9%), and meniscal or cartilage injury (n = 200, 9.2%) (Table 6). Injuries resulting in the greatest mean number of days missed included meniscal or cartilage injuries (44 days), sprains or ligament injuries (30 days), or dislocations (22 days).
Based on specific SMDCS descriptors, the most frequent knee injuries reported were contusion (n = 662, 30.5%), patella tendinopathy (n = 222, 10.2%), and meniscal tears (n = 200, 9.2%) (Table 6). Complete anterior cruciate ligament tears, although infrequent, were responsible for the greatest mean days missed (156.2 days). This was followed by lateral meniscus tears (47.5 days) and medial meniscus tears (41.2 days). Knee contusions, although very common, resulted in the least number of days missed (6.0 days).
Discussion
Although much is known about knee injuries in other professional athletic leagues, little is known about knee injuries in professional baseball players.2-4 The majority of epidemiologic studies regarding baseball players at any level emphasizes the study of shoulder and elbow injuries.3,4,19 Since the implementation of the electronic medical record and the HITS database in professional baseball, there has been increased effort to document injuries that have received less attention in the existing literature. Understanding the epidemiology of these injuries is important for the development of targeted prevention efforts.
Prior studies of injuries in professional baseball relied on data captured by the publicly available DL. Posner and colleagues2 provide one of the most comprehensive reports on MLB injuries in a report utilizing DL assignment data over a period of 7 seasons.They demonstrated that knee injuries were responsible for 7.7% (12.5% for fielders and 3.7% for pitchers) of assignments to the DL. The current study utilized a comprehensive surveillance and builds on this existing knowledge. The present study found similar trends to Posner and colleagues2 in that knee injuries were responsible for 6.5% of injuries in professional baseball players that resulted in missed games. From the 2002 season to the 2008 season, knee injuries were the fifth most common reason MLB players were placed on the DL,2 and the current study indicates that they remain the fifth most common reason for missed time from play based on the HITS data. Since the prevalence of these injuries have remained constant since the 2002 season, efforts to better understand these injuries are warranted in order to identify strategies to prevent them. These analyses have generated important data towards achieving this understanding.
As with most injuries in professional sports, goals for treatment are aimed at maximizing patient function and performance while minimizing time out of play. For the 2011-2014 professional baseball seasons, a total of 2171 players sustained knee injuries and missed an average of 16.2 days per injury. Knee injuries were responsible for a total of 7612 days of missed work for MLB and MiLB players per season (30,449 days over the 4-season study period). This is equivalent to a total of 20.9 years of players’ time lost in professional baseball per season over the last 4 years. The implications of this amount of time away from sport are significant, and further study should be targeted at prevention of these injuries and optimizing return to play times.
When attempting to reduce the burden of knee injuries in professional baseball, it may prove beneficial to first understand how the injuries occur, where on the field, and who is at greatest risk. From 2011 to 2014, nearly 44% of knee injuries occurred by noncontact mechanisms. Among all locations on the field where knee injuries occurred, those occurring in the infield were responsible for the greatest mean days missed. The players who seem to be at greatest risk for knee injuries appear to be base runners. These data suggest the need for prevention efforts targeting base runners and infield players, as well as players in MiLB, where the largest number of injuries occurred.
Recently, playing rules implemented by MLB after consultation with players have focused on reducing the number of player-to-player collisions at home plate in an attempt to decrease the injury burden to catchers and base runners.20 This present analysis suggests that this rule change may also reduce the occurrence of knee injuries, as player collisions at home plate were responsible for a total of 51 knee injuries during the study period. The impact of this rule change on injury rates should also be explored. Interestingly, of the 51 knees injuries occurring due to contact at home plate, 23 occurred in 2011, and only 2 occurred in 2014—the first year of the new rule. Additional areas that resulted in high numbers of knee injuries were player-to-player contact in the infield and player contact with the ground in the infield.
Attempting to reduce injury burden and time out of play related to knee injuries in professional baseball players will likely prove to be a difficult task. In order to generate meaningful improvement, a comprehensive approach that involves players, management, trainers, therapists, and physicians will likely be required. As the first report of the epidemiology of knee injuries in professional baseball players, this study is one important step in that process. The strengths of this study are its comprehensive nature that analyzes injuries from an entire population of players on more than 200 teams over a 3-year period. Also, this research is strengthened by its focus on one particular region of the body that has received limited attention in the empirical literature, but represents a significant source of lost time during the baseball season.
There are some limitations to this study. As with any injury surveillance system, there is the possibility that not all cases were captured. Additionally, since the surveillance system is based on data from multiple teams, data entry discrepancy is possible; however, the presence of dropdown boxes and systematic definitions for injuries reduces this risk. Finally, this study did not investigate the various treatments for knee injuries beyond whether or not the injury required surgery. Since this was the first comprehensive exploration of knee injuries in professional baseball, future studies are needed to explore additional facets including outcomes related to treatment, return to play, and performance.
Conclusion
Knee injuries represent 6.5% of all injuries in professional baseball, occurring at a rate of 1.3 per 1000 AE. The burden of these injuries is significant for professional baseball players. This study fills a critical gap in sports injury research by contributing to the knowledge about the effect of knee injuries in professional baseball. It also provides an important foundation for future epidemiologic inquiry to identify modifiable risk factors and interventions that may reduce the impact of these injuries in athletes.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Dick R, Sauers EL, Agel J, et al. Descriptive epidemiology of collegiate men’s baseball injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athletic Training. 2007;42(2):183-193.
4. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: A comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
5. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
6. Aune KT, Andrews JR, Dugas JR, Cain EL Jr. Return to play after partial lateral meniscectomy in National Football League Athletes. Am J Sports Med. 2014;42(8):1865-1872.
7. Brophy RH, Gill CS, Lyman S, Barnes RP, Rodeo SA, Warren RF. Effect of anterior cruciate ligament reconstruction and meniscectomy on length of career in National Football League athletes: a case control study. Am J Sports Med. 2009;37(11):2102-2107.
8. Brophy RH, Rodeo SA, Barnes RP, Powell JW, Warren RF. Knee articular cartilage injuries in the National Football League: epidemiology and treatment approach by team physicians. J Knee Surg. 2009;22(4):331-338.
9. Cerynik DL, Lewullis GE, Joves BC, Palmer MP, Tom JA. Outcomes of microfracture in professional basketball players. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1135-1139.
10. Hershman EB, Anderson R, Bergfeld JA, et al; National Football League Injury and Safety Panel. An analysis of specific lower extremity injury rates on grass and FieldTurf playing surfaces in National Football League Games: 2000-2009 seasons. Am J Sports Med. 2012;40(10):2200-2205.
11. Namdari S, Baldwin K, Anakwenze O, Park MJ, Huffman GR, Sennett BJ. Results and performance after microfracture in National Basketball Association athletes. Am J Sports Med. 2009;37(5):943-948.
12. Yeh PC, Starkey C, Lombardo S, Vitti G, Kharrazi FD. Epidemiology of isolated meniscal injury and its effect on performance in athletes from the National Basketball Association. Am J Sports Med. 2012;40(3):589-594.
13. Pollack KM, D’Angelo J, Green G, et al. Developing and implementing major league baseball’s health and injury tracking system. Am J Epidem. (accepted), 2016.
14. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
15. Dick R, Agel J, Marshall SW. National Collegiate Athletic Association Injury Surveillance System commentaries: introduction and methods. J Athletic Training. 2007;42(2):173-182.
16. Pellman EJ, Viano DC, Casson IR, Arfken C, Feuer H. Concussion in professional football players returning to the same game—part 7. Neurosurg. 2005;56(1):79-90.
17. Stevens ST, Lassonde M, De Beaumont L, Keenan JP. The effect of visors on head and facial injury in national hockey league players. J Sci Med Sport. 2006;9(3):238-242.
18. Meeuwisse WH, Wiley JP. The sport medicine diagnostic coding system. Clin J Sport Med. 2007;17(3):205-207.
19. Mcfarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
20. Hagen P. New rule on home-plate collisions put into effect. Major League Baseball website. http://m.mlb.com/news/article/68267610/mlb-institutes-new-rule-on-home-plate-collisions. Accessed December 5, 2014.
Injuries among professional baseball players have been on the rise for several years.1,2 From 1989 to 1999, the number of disabled list (DL) reports increased 38% (266 to 367 annual reports),1 and a similar increase in injury rates was noted from the 2002 to the 2008 seasons (37%).2 These injuries have important implications for future injury risk and time away from play. Identifying these injuries and determining correlates and risk factors is important for targeted prevention efforts.
Several studies have explored the prevalence of upper extremity injuries in professional and collegiate baseball players;2-4 however, detailed epidemiology of knee injuries in Major League Baseball (MLB) and Minor League Baseball (MiLB) players is lacking. Much more is known about the prevalence, treatment, and outcomes of knee injuries in other professional sporting organizations, such as the National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL).4-12 A recent meta-analysis exploring injuries in professional athletes found that studies on lower extremity injuries comprised approximately 12% of the literature reporting injuries in MLB players.4 In other professional leagues, publications on lower extremity injuries comprise approximately 56% of the sports medicine literature in the NFL, 54% in the NBA, and 62% in the NHL.4 Since few studies have investigated lower extremity injuries among professional baseball players, there is an opportunity for additional research to guide evidence-based prevention strategies.
A better understanding of the nature of these injuries is one of the first steps towards developing targeted injury prevention programs and treatment algorithms. The study of injury epidemiology among professional baseball players has been aided by the creation of an injury tracking system initiated by the MLB, its minor league affiliates, and the Major League Baseball Players Association.5,13,14 This surveillance system allows for the tracking of medical histories and injuries to players as they move across major and minor league organizations. Similar systems have been utilized in the National Collegiate Athletic Association and other professional sports organizations.3,15-17 A unique advantage of the MLB surveillance system is the required participation of all major and minor league teams, which allows for investigation of the entire population of players rather than simply a sample of players from select teams. This system has propelled an effort to identify injury patterns as a means of developing appropriate targets for potential preventative measures.5
The purpose of this descriptive epidemiologic study is to better understand the distribution and characteristics of knee injuries in these elite athletes by reporting on all knee injuries occurring over a span of 4 seasons (2011-2014). Additionally, this study seeks to characterize the impact of these injuries by analyzing the time required for return to play and the treatments rendered (surgical and nonsurgical).
Materials and Methods
After approval from the Johns Hopkins Bloomberg School of Public Health Institutional Review Board, detailed data regarding knee injuries in both MLB and MiLB baseball players were extracted from the de-identified MLB Health and Injury Tracking System (HITS). The HITS database is a centralized database that contains data on injuries from an electronic medical record (EMR). All players provided consent to have their data included in this EMR. HITS system captures injuries reported by the athletic trainers for all professional baseball players from 30 MLB clubs and their 230 minor league affiliates. Additional details on this population of professional baseball players have been published elsewhere.5 Only injuries that result in time out of play (≥1 day missed) are included in the database, and they are logged with basic information such as region of the body, diagnosis, date, player position, activity leading to injury, and general treatment. Any injury that affects participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training) is captured in HITS.
All baseball-related knee injuries occurring during the 2011-2014 seasons that resulted in time out of sport were included in the study. These injuries were identified based on the Sports Medicine Diagnostic Coding System (SMDCS) to capture injuries by diagnostic groups.18 Knee injuries were included if they occurred during spring training, regular season, or postseason play. Offseason injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of days missed because many of these players may not have been cleared to play until the beginning of the following season. To determine the proportion of knee injuries during the study period, all injuries were included for comparative purposes (subdivided based on 30 anatomic regions or types).
For each knee injury, a number of variables were analyzed, including diagnosis, level of play (MLB vs. MiLB), age, player position at the time of injury (pitcher, catcher, infield, outfield, base runner, or batter), field location where the injury occurred (home plate, pitcher’s mound, infield, outfield, foul territory or bullpen, or other), mechanism of injury, days missed, and treatment rendered (conservative vs surgical). The classification used to describe the mechanism of injury consisted of contact with ball, contact with ground, contact with another player, contact with another object, or noncontact.
Statistical Analysis Epidemiologic data are presented with descriptive statistics such as mean, median, frequency, and percentage where appropriate. When comparing player age, days missed, and surgical vs nonsurgical treatment between MLB and MiLB players, t-tests and tests for difference in proportions were applied as appropriate. Statistical significance was established for P values < .05.
The distribution of days missed for the variables considered was often skewed to the right (ie, days missed mostly concentrated on the low to moderate number of days, with fewer values in the much higher days missed range), even after excluding the season-ending injuries; hence the mean (or average) days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when knee injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those knee injuries that occurred during the regular season. It should be noted that the number of regular season knee injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training knee injuries.
RESULTS
Overall Summary
Of the 30 general body regions/systems included in the HITS database, injuries to the knee were the fifth most common reason for days missed in all of professional baseball from 2011-2014 (Table 1). Injuries to the knee represented 6.5% of the nearly 34,000 injuries sustained during the study period. Knee injuries were the fifth most common reason for time out of play for players in both the MiLB and MLB.
A total of 2171 isolated knee injuries resulted in time out of sport for professional baseball players (Table 2). Of these, 410 (19%) occurred in MLB players and 1761 (81%) occurred in MiLB players. MLB players were older than MiLB players at the time of injury (29.5 vs 22.8 years, respectively). Overall mean number of days missed was 16.2 days per knee injury, with MLB players missing an approximately 7 days more per injury than MiLB athletes (21.8 vs. 14.9 days respectively; P = .001).Over the course of the 4 seasons, a total of 30,449 days were missed due to knee injuries in professional baseball, giving an average rate of 7612 days lost per season. Surgery was performed for 263 (12.1%) of the 2171 knee injuries, with a greater proportion of MLB players requiring surgery than MiLB players (17.3% vs 10.9%) (P < .001). With respect to number of days missed per injury, 26% of knee injuries in the minor leagues resulted in greater than 30 days missed, while this number rose to 32% for knee injuries in MLB players (Table 3).
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE, respectively, over the course of the 4 seasons (2011-2014). The overall knee injury rate across both the MiLB and MLB was 1.2 per 1000 AE, based on the subset of 308 and 1473 regular season knee injuries in MiLB and MLB, respectively. The rate of knee injury was similar and not significantly different between the MiLB and MLB (1.2 per 1000 AE in the MiLB and 1.1 per 1000 AE in the MLB).
Characteristics of Injuries
When considering the position of the player during injury, defensive players were most frequently injured (n = 742, 56.5%), with pitchers (n = 227, 17.3%), infielders (n =193, 14.7%), outfielders (n = 193, 14.7%), and catchers (n = 129, 9.8%) sustaining injuries in decreasing frequency. Injuries while on offense (n = 571, 43.5%) were most frequent in base runners (n = 320, 24.4%) followed by batters (n = 251, 19.1%) (Table 4). Injuries while on defense occurring in infielders and catchers resulted in the longest period of time away from play (average of 22.4 and 20.8 days missed, respectively), while those occurring in batters resulted in the least average days missed (8.9 days).
The most common field location for knee injuries to occur was the infield, which was responsible for n = 647 (29.8%) of the total knee injuries (Table 4). This was followed by home plate (n = 493, 22.7%), other locations outside those specified (n = 394, 18.1%), outfield (n = 320, 14.7%), pitcher’s mound (n = 210, 9.7%), and foul territory or the bullpen (n = 107, 4.9%). Of the knee injuries with a specified location, those occurring in foul territory or the bullpen resulted in the highest mean days missed (18.4), while those occurring at home plate resulted in the least mean days missed (13.4 days).
When analyzed by mechanism of injury, noncontact injuries (n = 953, 43.9%) were more common than being hit with the ball (n = 374, 17.2%), striking the ground (n = 409, 18.8%), other mechanisms not listed (n = 196, 9%), contact with another player (n = 176, 8.1%), or contact with other objects (n = 63, 2.9%) (Table 4). Noncontact injuries and player to player collisions resulted in the greatest number of missed days (21.6 and 17.1 days, respectively) while being struck by the ball resulted in the least mean days missed (5.1).
Of the n = 493 knee injuries occurring at home plate, n = 212 (43%) occurred to the batter, n = 100 (20%) to the catcher, n = 34 (6.9%) to base runners, and n = 7 (1.4%) to pitchers (Table 5). The majority of knee injuries in the infield occurred to base runners (n = 283, 43.7%). Player-to-player collisions at home plate were responsible for 51 (2.3%) knee injuries, while 163 (24%) were noncontact injuries and 376 (56%) were the result of a player being hit by the ball (Table 5).
Injury Diagnosis
By diagnosis, the most common knee injuries observed were contusions or hematomas (n = 662, 30.5%), other injuries (n = 415, 19.1%), sprains or ligament injuries (n = 380, 17.5%), tendinopathies or bursitis (n = 367, 16.9%), and meniscal or cartilage injury (n = 200, 9.2%) (Table 6). Injuries resulting in the greatest mean number of days missed included meniscal or cartilage injuries (44 days), sprains or ligament injuries (30 days), or dislocations (22 days).
Based on specific SMDCS descriptors, the most frequent knee injuries reported were contusion (n = 662, 30.5%), patella tendinopathy (n = 222, 10.2%), and meniscal tears (n = 200, 9.2%) (Table 6). Complete anterior cruciate ligament tears, although infrequent, were responsible for the greatest mean days missed (156.2 days). This was followed by lateral meniscus tears (47.5 days) and medial meniscus tears (41.2 days). Knee contusions, although very common, resulted in the least number of days missed (6.0 days).
Discussion
Although much is known about knee injuries in other professional athletic leagues, little is known about knee injuries in professional baseball players.2-4 The majority of epidemiologic studies regarding baseball players at any level emphasizes the study of shoulder and elbow injuries.3,4,19 Since the implementation of the electronic medical record and the HITS database in professional baseball, there has been increased effort to document injuries that have received less attention in the existing literature. Understanding the epidemiology of these injuries is important for the development of targeted prevention efforts.
Prior studies of injuries in professional baseball relied on data captured by the publicly available DL. Posner and colleagues2 provide one of the most comprehensive reports on MLB injuries in a report utilizing DL assignment data over a period of 7 seasons.They demonstrated that knee injuries were responsible for 7.7% (12.5% for fielders and 3.7% for pitchers) of assignments to the DL. The current study utilized a comprehensive surveillance and builds on this existing knowledge. The present study found similar trends to Posner and colleagues2 in that knee injuries were responsible for 6.5% of injuries in professional baseball players that resulted in missed games. From the 2002 season to the 2008 season, knee injuries were the fifth most common reason MLB players were placed on the DL,2 and the current study indicates that they remain the fifth most common reason for missed time from play based on the HITS data. Since the prevalence of these injuries have remained constant since the 2002 season, efforts to better understand these injuries are warranted in order to identify strategies to prevent them. These analyses have generated important data towards achieving this understanding.
As with most injuries in professional sports, goals for treatment are aimed at maximizing patient function and performance while minimizing time out of play. For the 2011-2014 professional baseball seasons, a total of 2171 players sustained knee injuries and missed an average of 16.2 days per injury. Knee injuries were responsible for a total of 7612 days of missed work for MLB and MiLB players per season (30,449 days over the 4-season study period). This is equivalent to a total of 20.9 years of players’ time lost in professional baseball per season over the last 4 years. The implications of this amount of time away from sport are significant, and further study should be targeted at prevention of these injuries and optimizing return to play times.
When attempting to reduce the burden of knee injuries in professional baseball, it may prove beneficial to first understand how the injuries occur, where on the field, and who is at greatest risk. From 2011 to 2014, nearly 44% of knee injuries occurred by noncontact mechanisms. Among all locations on the field where knee injuries occurred, those occurring in the infield were responsible for the greatest mean days missed. The players who seem to be at greatest risk for knee injuries appear to be base runners. These data suggest the need for prevention efforts targeting base runners and infield players, as well as players in MiLB, where the largest number of injuries occurred.
Recently, playing rules implemented by MLB after consultation with players have focused on reducing the number of player-to-player collisions at home plate in an attempt to decrease the injury burden to catchers and base runners.20 This present analysis suggests that this rule change may also reduce the occurrence of knee injuries, as player collisions at home plate were responsible for a total of 51 knee injuries during the study period. The impact of this rule change on injury rates should also be explored. Interestingly, of the 51 knees injuries occurring due to contact at home plate, 23 occurred in 2011, and only 2 occurred in 2014—the first year of the new rule. Additional areas that resulted in high numbers of knee injuries were player-to-player contact in the infield and player contact with the ground in the infield.
Attempting to reduce injury burden and time out of play related to knee injuries in professional baseball players will likely prove to be a difficult task. In order to generate meaningful improvement, a comprehensive approach that involves players, management, trainers, therapists, and physicians will likely be required. As the first report of the epidemiology of knee injuries in professional baseball players, this study is one important step in that process. The strengths of this study are its comprehensive nature that analyzes injuries from an entire population of players on more than 200 teams over a 3-year period. Also, this research is strengthened by its focus on one particular region of the body that has received limited attention in the empirical literature, but represents a significant source of lost time during the baseball season.
There are some limitations to this study. As with any injury surveillance system, there is the possibility that not all cases were captured. Additionally, since the surveillance system is based on data from multiple teams, data entry discrepancy is possible; however, the presence of dropdown boxes and systematic definitions for injuries reduces this risk. Finally, this study did not investigate the various treatments for knee injuries beyond whether or not the injury required surgery. Since this was the first comprehensive exploration of knee injuries in professional baseball, future studies are needed to explore additional facets including outcomes related to treatment, return to play, and performance.
Conclusion
Knee injuries represent 6.5% of all injuries in professional baseball, occurring at a rate of 1.3 per 1000 AE. The burden of these injuries is significant for professional baseball players. This study fills a critical gap in sports injury research by contributing to the knowledge about the effect of knee injuries in professional baseball. It also provides an important foundation for future epidemiologic inquiry to identify modifiable risk factors and interventions that may reduce the impact of these injuries in athletes.
Injuries among professional baseball players have been on the rise for several years.1,2 From 1989 to 1999, the number of disabled list (DL) reports increased 38% (266 to 367 annual reports),1 and a similar increase in injury rates was noted from the 2002 to the 2008 seasons (37%).2 These injuries have important implications for future injury risk and time away from play. Identifying these injuries and determining correlates and risk factors is important for targeted prevention efforts.
Several studies have explored the prevalence of upper extremity injuries in professional and collegiate baseball players;2-4 however, detailed epidemiology of knee injuries in Major League Baseball (MLB) and Minor League Baseball (MiLB) players is lacking. Much more is known about the prevalence, treatment, and outcomes of knee injuries in other professional sporting organizations, such as the National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL).4-12 A recent meta-analysis exploring injuries in professional athletes found that studies on lower extremity injuries comprised approximately 12% of the literature reporting injuries in MLB players.4 In other professional leagues, publications on lower extremity injuries comprise approximately 56% of the sports medicine literature in the NFL, 54% in the NBA, and 62% in the NHL.4 Since few studies have investigated lower extremity injuries among professional baseball players, there is an opportunity for additional research to guide evidence-based prevention strategies.
A better understanding of the nature of these injuries is one of the first steps towards developing targeted injury prevention programs and treatment algorithms. The study of injury epidemiology among professional baseball players has been aided by the creation of an injury tracking system initiated by the MLB, its minor league affiliates, and the Major League Baseball Players Association.5,13,14 This surveillance system allows for the tracking of medical histories and injuries to players as they move across major and minor league organizations. Similar systems have been utilized in the National Collegiate Athletic Association and other professional sports organizations.3,15-17 A unique advantage of the MLB surveillance system is the required participation of all major and minor league teams, which allows for investigation of the entire population of players rather than simply a sample of players from select teams. This system has propelled an effort to identify injury patterns as a means of developing appropriate targets for potential preventative measures.5
The purpose of this descriptive epidemiologic study is to better understand the distribution and characteristics of knee injuries in these elite athletes by reporting on all knee injuries occurring over a span of 4 seasons (2011-2014). Additionally, this study seeks to characterize the impact of these injuries by analyzing the time required for return to play and the treatments rendered (surgical and nonsurgical).
Materials and Methods
After approval from the Johns Hopkins Bloomberg School of Public Health Institutional Review Board, detailed data regarding knee injuries in both MLB and MiLB baseball players were extracted from the de-identified MLB Health and Injury Tracking System (HITS). The HITS database is a centralized database that contains data on injuries from an electronic medical record (EMR). All players provided consent to have their data included in this EMR. HITS system captures injuries reported by the athletic trainers for all professional baseball players from 30 MLB clubs and their 230 minor league affiliates. Additional details on this population of professional baseball players have been published elsewhere.5 Only injuries that result in time out of play (≥1 day missed) are included in the database, and they are logged with basic information such as region of the body, diagnosis, date, player position, activity leading to injury, and general treatment. Any injury that affects participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training) is captured in HITS.
All baseball-related knee injuries occurring during the 2011-2014 seasons that resulted in time out of sport were included in the study. These injuries were identified based on the Sports Medicine Diagnostic Coding System (SMDCS) to capture injuries by diagnostic groups.18 Knee injuries were included if they occurred during spring training, regular season, or postseason play. Offseason injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of days missed because many of these players may not have been cleared to play until the beginning of the following season. To determine the proportion of knee injuries during the study period, all injuries were included for comparative purposes (subdivided based on 30 anatomic regions or types).
For each knee injury, a number of variables were analyzed, including diagnosis, level of play (MLB vs. MiLB), age, player position at the time of injury (pitcher, catcher, infield, outfield, base runner, or batter), field location where the injury occurred (home plate, pitcher’s mound, infield, outfield, foul territory or bullpen, or other), mechanism of injury, days missed, and treatment rendered (conservative vs surgical). The classification used to describe the mechanism of injury consisted of contact with ball, contact with ground, contact with another player, contact with another object, or noncontact.
Statistical Analysis Epidemiologic data are presented with descriptive statistics such as mean, median, frequency, and percentage where appropriate. When comparing player age, days missed, and surgical vs nonsurgical treatment between MLB and MiLB players, t-tests and tests for difference in proportions were applied as appropriate. Statistical significance was established for P values < .05.
The distribution of days missed for the variables considered was often skewed to the right (ie, days missed mostly concentrated on the low to moderate number of days, with fewer values in the much higher days missed range), even after excluding the season-ending injuries; hence the mean (or average) days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when knee injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those knee injuries that occurred during the regular season. It should be noted that the number of regular season knee injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training knee injuries.
RESULTS
Overall Summary
Of the 30 general body regions/systems included in the HITS database, injuries to the knee were the fifth most common reason for days missed in all of professional baseball from 2011-2014 (Table 1). Injuries to the knee represented 6.5% of the nearly 34,000 injuries sustained during the study period. Knee injuries were the fifth most common reason for time out of play for players in both the MiLB and MLB.
A total of 2171 isolated knee injuries resulted in time out of sport for professional baseball players (Table 2). Of these, 410 (19%) occurred in MLB players and 1761 (81%) occurred in MiLB players. MLB players were older than MiLB players at the time of injury (29.5 vs 22.8 years, respectively). Overall mean number of days missed was 16.2 days per knee injury, with MLB players missing an approximately 7 days more per injury than MiLB athletes (21.8 vs. 14.9 days respectively; P = .001).Over the course of the 4 seasons, a total of 30,449 days were missed due to knee injuries in professional baseball, giving an average rate of 7612 days lost per season. Surgery was performed for 263 (12.1%) of the 2171 knee injuries, with a greater proportion of MLB players requiring surgery than MiLB players (17.3% vs 10.9%) (P < .001). With respect to number of days missed per injury, 26% of knee injuries in the minor leagues resulted in greater than 30 days missed, while this number rose to 32% for knee injuries in MLB players (Table 3).
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE, respectively, over the course of the 4 seasons (2011-2014). The overall knee injury rate across both the MiLB and MLB was 1.2 per 1000 AE, based on the subset of 308 and 1473 regular season knee injuries in MiLB and MLB, respectively. The rate of knee injury was similar and not significantly different between the MiLB and MLB (1.2 per 1000 AE in the MiLB and 1.1 per 1000 AE in the MLB).
Characteristics of Injuries
When considering the position of the player during injury, defensive players were most frequently injured (n = 742, 56.5%), with pitchers (n = 227, 17.3%), infielders (n =193, 14.7%), outfielders (n = 193, 14.7%), and catchers (n = 129, 9.8%) sustaining injuries in decreasing frequency. Injuries while on offense (n = 571, 43.5%) were most frequent in base runners (n = 320, 24.4%) followed by batters (n = 251, 19.1%) (Table 4). Injuries while on defense occurring in infielders and catchers resulted in the longest period of time away from play (average of 22.4 and 20.8 days missed, respectively), while those occurring in batters resulted in the least average days missed (8.9 days).
The most common field location for knee injuries to occur was the infield, which was responsible for n = 647 (29.8%) of the total knee injuries (Table 4). This was followed by home plate (n = 493, 22.7%), other locations outside those specified (n = 394, 18.1%), outfield (n = 320, 14.7%), pitcher’s mound (n = 210, 9.7%), and foul territory or the bullpen (n = 107, 4.9%). Of the knee injuries with a specified location, those occurring in foul territory or the bullpen resulted in the highest mean days missed (18.4), while those occurring at home plate resulted in the least mean days missed (13.4 days).
When analyzed by mechanism of injury, noncontact injuries (n = 953, 43.9%) were more common than being hit with the ball (n = 374, 17.2%), striking the ground (n = 409, 18.8%), other mechanisms not listed (n = 196, 9%), contact with another player (n = 176, 8.1%), or contact with other objects (n = 63, 2.9%) (Table 4). Noncontact injuries and player to player collisions resulted in the greatest number of missed days (21.6 and 17.1 days, respectively) while being struck by the ball resulted in the least mean days missed (5.1).
Of the n = 493 knee injuries occurring at home plate, n = 212 (43%) occurred to the batter, n = 100 (20%) to the catcher, n = 34 (6.9%) to base runners, and n = 7 (1.4%) to pitchers (Table 5). The majority of knee injuries in the infield occurred to base runners (n = 283, 43.7%). Player-to-player collisions at home plate were responsible for 51 (2.3%) knee injuries, while 163 (24%) were noncontact injuries and 376 (56%) were the result of a player being hit by the ball (Table 5).
Injury Diagnosis
By diagnosis, the most common knee injuries observed were contusions or hematomas (n = 662, 30.5%), other injuries (n = 415, 19.1%), sprains or ligament injuries (n = 380, 17.5%), tendinopathies or bursitis (n = 367, 16.9%), and meniscal or cartilage injury (n = 200, 9.2%) (Table 6). Injuries resulting in the greatest mean number of days missed included meniscal or cartilage injuries (44 days), sprains or ligament injuries (30 days), or dislocations (22 days).
Based on specific SMDCS descriptors, the most frequent knee injuries reported were contusion (n = 662, 30.5%), patella tendinopathy (n = 222, 10.2%), and meniscal tears (n = 200, 9.2%) (Table 6). Complete anterior cruciate ligament tears, although infrequent, were responsible for the greatest mean days missed (156.2 days). This was followed by lateral meniscus tears (47.5 days) and medial meniscus tears (41.2 days). Knee contusions, although very common, resulted in the least number of days missed (6.0 days).
Discussion
Although much is known about knee injuries in other professional athletic leagues, little is known about knee injuries in professional baseball players.2-4 The majority of epidemiologic studies regarding baseball players at any level emphasizes the study of shoulder and elbow injuries.3,4,19 Since the implementation of the electronic medical record and the HITS database in professional baseball, there has been increased effort to document injuries that have received less attention in the existing literature. Understanding the epidemiology of these injuries is important for the development of targeted prevention efforts.
Prior studies of injuries in professional baseball relied on data captured by the publicly available DL. Posner and colleagues2 provide one of the most comprehensive reports on MLB injuries in a report utilizing DL assignment data over a period of 7 seasons.They demonstrated that knee injuries were responsible for 7.7% (12.5% for fielders and 3.7% for pitchers) of assignments to the DL. The current study utilized a comprehensive surveillance and builds on this existing knowledge. The present study found similar trends to Posner and colleagues2 in that knee injuries were responsible for 6.5% of injuries in professional baseball players that resulted in missed games. From the 2002 season to the 2008 season, knee injuries were the fifth most common reason MLB players were placed on the DL,2 and the current study indicates that they remain the fifth most common reason for missed time from play based on the HITS data. Since the prevalence of these injuries have remained constant since the 2002 season, efforts to better understand these injuries are warranted in order to identify strategies to prevent them. These analyses have generated important data towards achieving this understanding.
As with most injuries in professional sports, goals for treatment are aimed at maximizing patient function and performance while minimizing time out of play. For the 2011-2014 professional baseball seasons, a total of 2171 players sustained knee injuries and missed an average of 16.2 days per injury. Knee injuries were responsible for a total of 7612 days of missed work for MLB and MiLB players per season (30,449 days over the 4-season study period). This is equivalent to a total of 20.9 years of players’ time lost in professional baseball per season over the last 4 years. The implications of this amount of time away from sport are significant, and further study should be targeted at prevention of these injuries and optimizing return to play times.
When attempting to reduce the burden of knee injuries in professional baseball, it may prove beneficial to first understand how the injuries occur, where on the field, and who is at greatest risk. From 2011 to 2014, nearly 44% of knee injuries occurred by noncontact mechanisms. Among all locations on the field where knee injuries occurred, those occurring in the infield were responsible for the greatest mean days missed. The players who seem to be at greatest risk for knee injuries appear to be base runners. These data suggest the need for prevention efforts targeting base runners and infield players, as well as players in MiLB, where the largest number of injuries occurred.
Recently, playing rules implemented by MLB after consultation with players have focused on reducing the number of player-to-player collisions at home plate in an attempt to decrease the injury burden to catchers and base runners.20 This present analysis suggests that this rule change may also reduce the occurrence of knee injuries, as player collisions at home plate were responsible for a total of 51 knee injuries during the study period. The impact of this rule change on injury rates should also be explored. Interestingly, of the 51 knees injuries occurring due to contact at home plate, 23 occurred in 2011, and only 2 occurred in 2014—the first year of the new rule. Additional areas that resulted in high numbers of knee injuries were player-to-player contact in the infield and player contact with the ground in the infield.
Attempting to reduce injury burden and time out of play related to knee injuries in professional baseball players will likely prove to be a difficult task. In order to generate meaningful improvement, a comprehensive approach that involves players, management, trainers, therapists, and physicians will likely be required. As the first report of the epidemiology of knee injuries in professional baseball players, this study is one important step in that process. The strengths of this study are its comprehensive nature that analyzes injuries from an entire population of players on more than 200 teams over a 3-year period. Also, this research is strengthened by its focus on one particular region of the body that has received limited attention in the empirical literature, but represents a significant source of lost time during the baseball season.
There are some limitations to this study. As with any injury surveillance system, there is the possibility that not all cases were captured. Additionally, since the surveillance system is based on data from multiple teams, data entry discrepancy is possible; however, the presence of dropdown boxes and systematic definitions for injuries reduces this risk. Finally, this study did not investigate the various treatments for knee injuries beyond whether or not the injury required surgery. Since this was the first comprehensive exploration of knee injuries in professional baseball, future studies are needed to explore additional facets including outcomes related to treatment, return to play, and performance.
Conclusion
Knee injuries represent 6.5% of all injuries in professional baseball, occurring at a rate of 1.3 per 1000 AE. The burden of these injuries is significant for professional baseball players. This study fills a critical gap in sports injury research by contributing to the knowledge about the effect of knee injuries in professional baseball. It also provides an important foundation for future epidemiologic inquiry to identify modifiable risk factors and interventions that may reduce the impact of these injuries in athletes.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Dick R, Sauers EL, Agel J, et al. Descriptive epidemiology of collegiate men’s baseball injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athletic Training. 2007;42(2):183-193.
4. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: A comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
5. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
6. Aune KT, Andrews JR, Dugas JR, Cain EL Jr. Return to play after partial lateral meniscectomy in National Football League Athletes. Am J Sports Med. 2014;42(8):1865-1872.
7. Brophy RH, Gill CS, Lyman S, Barnes RP, Rodeo SA, Warren RF. Effect of anterior cruciate ligament reconstruction and meniscectomy on length of career in National Football League athletes: a case control study. Am J Sports Med. 2009;37(11):2102-2107.
8. Brophy RH, Rodeo SA, Barnes RP, Powell JW, Warren RF. Knee articular cartilage injuries in the National Football League: epidemiology and treatment approach by team physicians. J Knee Surg. 2009;22(4):331-338.
9. Cerynik DL, Lewullis GE, Joves BC, Palmer MP, Tom JA. Outcomes of microfracture in professional basketball players. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1135-1139.
10. Hershman EB, Anderson R, Bergfeld JA, et al; National Football League Injury and Safety Panel. An analysis of specific lower extremity injury rates on grass and FieldTurf playing surfaces in National Football League Games: 2000-2009 seasons. Am J Sports Med. 2012;40(10):2200-2205.
11. Namdari S, Baldwin K, Anakwenze O, Park MJ, Huffman GR, Sennett BJ. Results and performance after microfracture in National Basketball Association athletes. Am J Sports Med. 2009;37(5):943-948.
12. Yeh PC, Starkey C, Lombardo S, Vitti G, Kharrazi FD. Epidemiology of isolated meniscal injury and its effect on performance in athletes from the National Basketball Association. Am J Sports Med. 2012;40(3):589-594.
13. Pollack KM, D’Angelo J, Green G, et al. Developing and implementing major league baseball’s health and injury tracking system. Am J Epidem. (accepted), 2016.
14. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
15. Dick R, Agel J, Marshall SW. National Collegiate Athletic Association Injury Surveillance System commentaries: introduction and methods. J Athletic Training. 2007;42(2):173-182.
16. Pellman EJ, Viano DC, Casson IR, Arfken C, Feuer H. Concussion in professional football players returning to the same game—part 7. Neurosurg. 2005;56(1):79-90.
17. Stevens ST, Lassonde M, De Beaumont L, Keenan JP. The effect of visors on head and facial injury in national hockey league players. J Sci Med Sport. 2006;9(3):238-242.
18. Meeuwisse WH, Wiley JP. The sport medicine diagnostic coding system. Clin J Sport Med. 2007;17(3):205-207.
19. Mcfarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
20. Hagen P. New rule on home-plate collisions put into effect. Major League Baseball website. http://m.mlb.com/news/article/68267610/mlb-institutes-new-rule-on-home-plate-collisions. Accessed December 5, 2014.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Dick R, Sauers EL, Agel J, et al. Descriptive epidemiology of collegiate men’s baseball injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athletic Training. 2007;42(2):183-193.
4. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: A comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
5. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
6. Aune KT, Andrews JR, Dugas JR, Cain EL Jr. Return to play after partial lateral meniscectomy in National Football League Athletes. Am J Sports Med. 2014;42(8):1865-1872.
7. Brophy RH, Gill CS, Lyman S, Barnes RP, Rodeo SA, Warren RF. Effect of anterior cruciate ligament reconstruction and meniscectomy on length of career in National Football League athletes: a case control study. Am J Sports Med. 2009;37(11):2102-2107.
8. Brophy RH, Rodeo SA, Barnes RP, Powell JW, Warren RF. Knee articular cartilage injuries in the National Football League: epidemiology and treatment approach by team physicians. J Knee Surg. 2009;22(4):331-338.
9. Cerynik DL, Lewullis GE, Joves BC, Palmer MP, Tom JA. Outcomes of microfracture in professional basketball players. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1135-1139.
10. Hershman EB, Anderson R, Bergfeld JA, et al; National Football League Injury and Safety Panel. An analysis of specific lower extremity injury rates on grass and FieldTurf playing surfaces in National Football League Games: 2000-2009 seasons. Am J Sports Med. 2012;40(10):2200-2205.
11. Namdari S, Baldwin K, Anakwenze O, Park MJ, Huffman GR, Sennett BJ. Results and performance after microfracture in National Basketball Association athletes. Am J Sports Med. 2009;37(5):943-948.
12. Yeh PC, Starkey C, Lombardo S, Vitti G, Kharrazi FD. Epidemiology of isolated meniscal injury and its effect on performance in athletes from the National Basketball Association. Am J Sports Med. 2012;40(3):589-594.
13. Pollack KM, D’Angelo J, Green G, et al. Developing and implementing major league baseball’s health and injury tracking system. Am J Epidem. (accepted), 2016.
14. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
15. Dick R, Agel J, Marshall SW. National Collegiate Athletic Association Injury Surveillance System commentaries: introduction and methods. J Athletic Training. 2007;42(2):173-182.
16. Pellman EJ, Viano DC, Casson IR, Arfken C, Feuer H. Concussion in professional football players returning to the same game—part 7. Neurosurg. 2005;56(1):79-90.
17. Stevens ST, Lassonde M, De Beaumont L, Keenan JP. The effect of visors on head and facial injury in national hockey league players. J Sci Med Sport. 2006;9(3):238-242.
18. Meeuwisse WH, Wiley JP. The sport medicine diagnostic coding system. Clin J Sport Med. 2007;17(3):205-207.
19. Mcfarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
20. Hagen P. New rule on home-plate collisions put into effect. Major League Baseball website. http://m.mlb.com/news/article/68267610/mlb-institutes-new-rule-on-home-plate-collisions. Accessed December 5, 2014.
Editorial Board Biographies
Asheesh Bedi, MD
Deputy Editor-in-Chief

Dr. Bedi is the Harold and Helen W. Gehring Professor of Orthopaedic Surgery; chief of sports medicine and shoulder surgery at the University of Michigan and MedSport program; team physician for the University of Michigan Athletic Department and the Detroit Lions; and consultant for the NBA, NFL, and NHL Players Associations. He completed his undergraduate training at Northwestern University, where he graduated Summa Cum Laude. He graduated from the University of Michigan Medical School with AOA recognition, and completed his residency training in orthopaedic surgery at the University of Michigan. He completed a 2-year fellowship in sports medicine and shoulder surgery at the Hospital for Special Surgery and Weill Cornell Medical College in New York. His research interests include shoulder, elbow, knee, and hip injuries in athletes.
Joshua S. Dines, MD
Deputy Editor-in-Chief

Dr. Dines is an orthopedic surgeon specializing in sports medicine at the Hospital for Special Surgery in New York; associate professor of orthopedic surgery at Weill Cornell Medical College; assistant team physician for the NY Mets; sports medicine consultant for the NY Rangers; and consultant for the LA Dodgers and LI Ducks. He attended Dartmouth as an undergraduate, completed medical school at Cornell, and completed his residency at the Hospital for Special Surgery. He also completed a sports medicine fellowship at Kerlan-Jobe Orthopaedic Clinic in Los Angeles, California, where he worked as part of the medical staff for the LA Dodgers and the LA Lakers. Previously he served as head team physician for the United States Davis Cup Tennis Team. He is a member of American Academy of Orthopaedic Surgeons, American Shoulder and Elbow Surgeons, The Council on Sports Medicine, The Interurban Orthopedic Association, the American Orthopedic Association, Arthroscopy Association of North America (AANA), and the American Orthopaedic Society for Sports Medicine.
Shane J. Nho, MD, MS
Deputy Editor-in-Chief

Dr. Nho is the director of the Hip Preservation Center, co-head of the Hip Study Group, and assistant professor, Department of Orthopedic Surgery, Division of Sports Medicine at Rush University Medical Center in Chicago, Illinois; and team physician for the Chicago White Sox. He graduated from Northwestern University and enrolled in the MD/MS program at Rush Medical College and the Graduate College of Rush University. He completed his surgical internship at New York Presbyterian Hospital of Weill Cornell Medical College, and completed his residency in orthopedic surgery at the Hospital for Special Surgery in New York. He completed his fellowship in sports medicine at Rush University Medical Center in Chicago, Illinois, where he was the recipient of the Herodicus Society Traveling Fellowship. His research interests include hip, shoulder, and knee reconstruction.
Robin V. West, MD
Deputy Editor-in-Chief

Dr. West is the chairman of sports medicine at Inova Health System; lead team physician for the Washington Nationals; and associate professor at Georgetown University Medical Center and Virginia Commonwealth University School of Medicine in Virginia. She attended Johns Hopkins University as an undergraduate, and completed medical school and an orthopedic surgery residency at George Washington University. Previously, she served as a team physician for the Pittsburgh Steelers; head team physician for the University of Pittsburgh Men’s Basketball team; head team physician for Carnegie Mellon University; and was a former member of the NFL Physician’s Society. She currently is an active member of American Academy of Orthopaedic Surgeons (AAOS), American Orthopaedic Society for Sports Medicine (AOSSM), Major League Baseball Team Physicians Society, and Arthroscopy Association of North America (AANA).
Lisa A. Fortier, DVM, PhD, DACVS
Associate Editor for Translation Science and Animal Research

Dr. Fortier is professor of surgery at Cornell University in Ithaca, New York; board-certified equine surgeon with practices at Cornell University and Ruffian Center in Elmont, New York; vice president of the International Veterinary Regenerative Medicine Society; faculty director of the Cornell Equine Park; staff surgeon at Cornell Ruffian Equine Specialists in Long Island, New York; and executive board (treasurer) of the International Cartilage Repair Society (ICRS). She received her DVM from Colorado State University, and completed her PhD and surgical residency training at Cornell University. She has received the Jacques Lemans Award from the ICRS, the New Investigator Research Award from the ORS, the Pfizer Research Award for Research Excellence from Cornell University, and was elected as a Distinguished Graduate from Drayton High School. She was also the first veterinarian to be elected as president of the ICRS. Her research interests include osteoarthritis, biologics, cartilage repair, and tendonosis.
Alan M. Hirahara, MD, FRCSC
Associate Editor for Sports Medicine/Ultrasound and Biologics

Dr. Hirahara is an orthopaedic surgeon specializing in sports medicine. He runs a private practice in Sacramento, CA. He speaks and teaches nationally and internationally, and does research on arthroscopic shoulder and knee surgery, orthobiologics, and the use of ultrasound. He is board-certified in orthopaedic surgery and orthopaedic sports medicine in the U.S. and Canada. He is the medical director and team physician for California State University, Sacramento Athletics; and head team physician for the Sacramento River Cats. He has been the team physician for the NCAA championships and Olympic Trials in Sacramento since 2001. He did his fellowship training in orthopaedic sports medicine at the University of Toronto. He completed his residency training in orthopaedic surgery in French at l’Université de Montréal. He attended medical school at UCSF and completed his BS in Kinesiology at UCLA with College Honors, Departmental Honors, Phi Beta Kappa, and graduated Magna Cum Laude.
Thay Q. Lee, PhD
Associate Editor for Biomechanics

Dr. Lee is the director and senior research career scientist at VA Long Beach Healthcare System; professor and vice chair for research in the Department of Orthopaedic Surgery; and professor in the Department of Biomedical Engineering at the University of California, Irvine. He received his undergraduate degree in Bioengineering and his Master of Science degree in applied mechanics from the University of California, San Diego. He completed his doctorate degree in biomaterials at Gothenburg University in Sweden. He was elected Fellow for the American Society of Mechanical Engineers (ASME) and the American Institute for Medical and Biological Engineering (AIMBE). He is also an elected member of the American Shoulder and Elbow Surgeons (ASES), Orthopaedic Research Society (ORS), American Society of Biomechanics (ASB), California Orthopaedic Association (COA), Society for Biomaterials (SFB), Biomedical Engineering Society (BMES), and American Orthopaedic Society for Sports Medicine (AOSSM). His research interests include joint biomechanics, shoulder, and knee.
Raffy Mirzayan, MD
Associate Editor for Biologics

Dr. Mirzayan is a board-certified orthopedic surgeon with a subspecialty in sports medicine at Kaiser Permanente in Baldwin Park, California; and founder and director of the Advanced Concepts course held in Las Vegas. He received his Bachelor of Science degree from University of California, Los Angeles (UCLA) and graduated from the Keck School of Medicine of University of Southern California (USC) with AOA Honors. He completed his residency at the Los Angeles County/USC Medical Center, and his fellowship at the Kerlan-Jobe Orthopedic Clinic. He belongs to several orthopedic and sports medicine societies, and was chosen as an American Academy of Orthopaedic Surgeons (AAOS) Leadership Fellow and an American Orthopaedic Society for Sports Medicine (AOSSM) Traveling Fellow. His research interests include shoulder, elbow, knee, cartilage reconstruction, osteotomies, meniscal transplantation, and orthobiologics.
Asheesh Bedi, MD
Deputy Editor-in-Chief

Dr. Bedi is the Harold and Helen W. Gehring Professor of Orthopaedic Surgery; chief of sports medicine and shoulder surgery at the University of Michigan and MedSport program; team physician for the University of Michigan Athletic Department and the Detroit Lions; and consultant for the NBA, NFL, and NHL Players Associations. He completed his undergraduate training at Northwestern University, where he graduated Summa Cum Laude. He graduated from the University of Michigan Medical School with AOA recognition, and completed his residency training in orthopaedic surgery at the University of Michigan. He completed a 2-year fellowship in sports medicine and shoulder surgery at the Hospital for Special Surgery and Weill Cornell Medical College in New York. His research interests include shoulder, elbow, knee, and hip injuries in athletes.
Joshua S. Dines, MD
Deputy Editor-in-Chief

Dr. Dines is an orthopedic surgeon specializing in sports medicine at the Hospital for Special Surgery in New York; associate professor of orthopedic surgery at Weill Cornell Medical College; assistant team physician for the NY Mets; sports medicine consultant for the NY Rangers; and consultant for the LA Dodgers and LI Ducks. He attended Dartmouth as an undergraduate, completed medical school at Cornell, and completed his residency at the Hospital for Special Surgery. He also completed a sports medicine fellowship at Kerlan-Jobe Orthopaedic Clinic in Los Angeles, California, where he worked as part of the medical staff for the LA Dodgers and the LA Lakers. Previously he served as head team physician for the United States Davis Cup Tennis Team. He is a member of American Academy of Orthopaedic Surgeons, American Shoulder and Elbow Surgeons, The Council on Sports Medicine, The Interurban Orthopedic Association, the American Orthopedic Association, Arthroscopy Association of North America (AANA), and the American Orthopaedic Society for Sports Medicine.
Shane J. Nho, MD, MS
Deputy Editor-in-Chief

Dr. Nho is the director of the Hip Preservation Center, co-head of the Hip Study Group, and assistant professor, Department of Orthopedic Surgery, Division of Sports Medicine at Rush University Medical Center in Chicago, Illinois; and team physician for the Chicago White Sox. He graduated from Northwestern University and enrolled in the MD/MS program at Rush Medical College and the Graduate College of Rush University. He completed his surgical internship at New York Presbyterian Hospital of Weill Cornell Medical College, and completed his residency in orthopedic surgery at the Hospital for Special Surgery in New York. He completed his fellowship in sports medicine at Rush University Medical Center in Chicago, Illinois, where he was the recipient of the Herodicus Society Traveling Fellowship. His research interests include hip, shoulder, and knee reconstruction.
Robin V. West, MD
Deputy Editor-in-Chief

Dr. West is the chairman of sports medicine at Inova Health System; lead team physician for the Washington Nationals; and associate professor at Georgetown University Medical Center and Virginia Commonwealth University School of Medicine in Virginia. She attended Johns Hopkins University as an undergraduate, and completed medical school and an orthopedic surgery residency at George Washington University. Previously, she served as a team physician for the Pittsburgh Steelers; head team physician for the University of Pittsburgh Men’s Basketball team; head team physician for Carnegie Mellon University; and was a former member of the NFL Physician’s Society. She currently is an active member of American Academy of Orthopaedic Surgeons (AAOS), American Orthopaedic Society for Sports Medicine (AOSSM), Major League Baseball Team Physicians Society, and Arthroscopy Association of North America (AANA).
Lisa A. Fortier, DVM, PhD, DACVS
Associate Editor for Translation Science and Animal Research

Dr. Fortier is professor of surgery at Cornell University in Ithaca, New York; board-certified equine surgeon with practices at Cornell University and Ruffian Center in Elmont, New York; vice president of the International Veterinary Regenerative Medicine Society; faculty director of the Cornell Equine Park; staff surgeon at Cornell Ruffian Equine Specialists in Long Island, New York; and executive board (treasurer) of the International Cartilage Repair Society (ICRS). She received her DVM from Colorado State University, and completed her PhD and surgical residency training at Cornell University. She has received the Jacques Lemans Award from the ICRS, the New Investigator Research Award from the ORS, the Pfizer Research Award for Research Excellence from Cornell University, and was elected as a Distinguished Graduate from Drayton High School. She was also the first veterinarian to be elected as president of the ICRS. Her research interests include osteoarthritis, biologics, cartilage repair, and tendonosis.
Alan M. Hirahara, MD, FRCSC
Associate Editor for Sports Medicine/Ultrasound and Biologics

Dr. Hirahara is an orthopaedic surgeon specializing in sports medicine. He runs a private practice in Sacramento, CA. He speaks and teaches nationally and internationally, and does research on arthroscopic shoulder and knee surgery, orthobiologics, and the use of ultrasound. He is board-certified in orthopaedic surgery and orthopaedic sports medicine in the U.S. and Canada. He is the medical director and team physician for California State University, Sacramento Athletics; and head team physician for the Sacramento River Cats. He has been the team physician for the NCAA championships and Olympic Trials in Sacramento since 2001. He did his fellowship training in orthopaedic sports medicine at the University of Toronto. He completed his residency training in orthopaedic surgery in French at l’Université de Montréal. He attended medical school at UCSF and completed his BS in Kinesiology at UCLA with College Honors, Departmental Honors, Phi Beta Kappa, and graduated Magna Cum Laude.
Thay Q. Lee, PhD
Associate Editor for Biomechanics

Dr. Lee is the director and senior research career scientist at VA Long Beach Healthcare System; professor and vice chair for research in the Department of Orthopaedic Surgery; and professor in the Department of Biomedical Engineering at the University of California, Irvine. He received his undergraduate degree in Bioengineering and his Master of Science degree in applied mechanics from the University of California, San Diego. He completed his doctorate degree in biomaterials at Gothenburg University in Sweden. He was elected Fellow for the American Society of Mechanical Engineers (ASME) and the American Institute for Medical and Biological Engineering (AIMBE). He is also an elected member of the American Shoulder and Elbow Surgeons (ASES), Orthopaedic Research Society (ORS), American Society of Biomechanics (ASB), California Orthopaedic Association (COA), Society for Biomaterials (SFB), Biomedical Engineering Society (BMES), and American Orthopaedic Society for Sports Medicine (AOSSM). His research interests include joint biomechanics, shoulder, and knee.
Raffy Mirzayan, MD
Associate Editor for Biologics

Dr. Mirzayan is a board-certified orthopedic surgeon with a subspecialty in sports medicine at Kaiser Permanente in Baldwin Park, California; and founder and director of the Advanced Concepts course held in Las Vegas. He received his Bachelor of Science degree from University of California, Los Angeles (UCLA) and graduated from the Keck School of Medicine of University of Southern California (USC) with AOA Honors. He completed his residency at the Los Angeles County/USC Medical Center, and his fellowship at the Kerlan-Jobe Orthopedic Clinic. He belongs to several orthopedic and sports medicine societies, and was chosen as an American Academy of Orthopaedic Surgeons (AAOS) Leadership Fellow and an American Orthopaedic Society for Sports Medicine (AOSSM) Traveling Fellow. His research interests include shoulder, elbow, knee, cartilage reconstruction, osteotomies, meniscal transplantation, and orthobiologics.
Asheesh Bedi, MD
Deputy Editor-in-Chief

Dr. Bedi is the Harold and Helen W. Gehring Professor of Orthopaedic Surgery; chief of sports medicine and shoulder surgery at the University of Michigan and MedSport program; team physician for the University of Michigan Athletic Department and the Detroit Lions; and consultant for the NBA, NFL, and NHL Players Associations. He completed his undergraduate training at Northwestern University, where he graduated Summa Cum Laude. He graduated from the University of Michigan Medical School with AOA recognition, and completed his residency training in orthopaedic surgery at the University of Michigan. He completed a 2-year fellowship in sports medicine and shoulder surgery at the Hospital for Special Surgery and Weill Cornell Medical College in New York. His research interests include shoulder, elbow, knee, and hip injuries in athletes.
Joshua S. Dines, MD
Deputy Editor-in-Chief

Dr. Dines is an orthopedic surgeon specializing in sports medicine at the Hospital for Special Surgery in New York; associate professor of orthopedic surgery at Weill Cornell Medical College; assistant team physician for the NY Mets; sports medicine consultant for the NY Rangers; and consultant for the LA Dodgers and LI Ducks. He attended Dartmouth as an undergraduate, completed medical school at Cornell, and completed his residency at the Hospital for Special Surgery. He also completed a sports medicine fellowship at Kerlan-Jobe Orthopaedic Clinic in Los Angeles, California, where he worked as part of the medical staff for the LA Dodgers and the LA Lakers. Previously he served as head team physician for the United States Davis Cup Tennis Team. He is a member of American Academy of Orthopaedic Surgeons, American Shoulder and Elbow Surgeons, The Council on Sports Medicine, The Interurban Orthopedic Association, the American Orthopedic Association, Arthroscopy Association of North America (AANA), and the American Orthopaedic Society for Sports Medicine.
Shane J. Nho, MD, MS
Deputy Editor-in-Chief

Dr. Nho is the director of the Hip Preservation Center, co-head of the Hip Study Group, and assistant professor, Department of Orthopedic Surgery, Division of Sports Medicine at Rush University Medical Center in Chicago, Illinois; and team physician for the Chicago White Sox. He graduated from Northwestern University and enrolled in the MD/MS program at Rush Medical College and the Graduate College of Rush University. He completed his surgical internship at New York Presbyterian Hospital of Weill Cornell Medical College, and completed his residency in orthopedic surgery at the Hospital for Special Surgery in New York. He completed his fellowship in sports medicine at Rush University Medical Center in Chicago, Illinois, where he was the recipient of the Herodicus Society Traveling Fellowship. His research interests include hip, shoulder, and knee reconstruction.
Robin V. West, MD
Deputy Editor-in-Chief

Dr. West is the chairman of sports medicine at Inova Health System; lead team physician for the Washington Nationals; and associate professor at Georgetown University Medical Center and Virginia Commonwealth University School of Medicine in Virginia. She attended Johns Hopkins University as an undergraduate, and completed medical school and an orthopedic surgery residency at George Washington University. Previously, she served as a team physician for the Pittsburgh Steelers; head team physician for the University of Pittsburgh Men’s Basketball team; head team physician for Carnegie Mellon University; and was a former member of the NFL Physician’s Society. She currently is an active member of American Academy of Orthopaedic Surgeons (AAOS), American Orthopaedic Society for Sports Medicine (AOSSM), Major League Baseball Team Physicians Society, and Arthroscopy Association of North America (AANA).
Lisa A. Fortier, DVM, PhD, DACVS
Associate Editor for Translation Science and Animal Research

Dr. Fortier is professor of surgery at Cornell University in Ithaca, New York; board-certified equine surgeon with practices at Cornell University and Ruffian Center in Elmont, New York; vice president of the International Veterinary Regenerative Medicine Society; faculty director of the Cornell Equine Park; staff surgeon at Cornell Ruffian Equine Specialists in Long Island, New York; and executive board (treasurer) of the International Cartilage Repair Society (ICRS). She received her DVM from Colorado State University, and completed her PhD and surgical residency training at Cornell University. She has received the Jacques Lemans Award from the ICRS, the New Investigator Research Award from the ORS, the Pfizer Research Award for Research Excellence from Cornell University, and was elected as a Distinguished Graduate from Drayton High School. She was also the first veterinarian to be elected as president of the ICRS. Her research interests include osteoarthritis, biologics, cartilage repair, and tendonosis.
Alan M. Hirahara, MD, FRCSC
Associate Editor for Sports Medicine/Ultrasound and Biologics

Dr. Hirahara is an orthopaedic surgeon specializing in sports medicine. He runs a private practice in Sacramento, CA. He speaks and teaches nationally and internationally, and does research on arthroscopic shoulder and knee surgery, orthobiologics, and the use of ultrasound. He is board-certified in orthopaedic surgery and orthopaedic sports medicine in the U.S. and Canada. He is the medical director and team physician for California State University, Sacramento Athletics; and head team physician for the Sacramento River Cats. He has been the team physician for the NCAA championships and Olympic Trials in Sacramento since 2001. He did his fellowship training in orthopaedic sports medicine at the University of Toronto. He completed his residency training in orthopaedic surgery in French at l’Université de Montréal. He attended medical school at UCSF and completed his BS in Kinesiology at UCLA with College Honors, Departmental Honors, Phi Beta Kappa, and graduated Magna Cum Laude.
Thay Q. Lee, PhD
Associate Editor for Biomechanics

Dr. Lee is the director and senior research career scientist at VA Long Beach Healthcare System; professor and vice chair for research in the Department of Orthopaedic Surgery; and professor in the Department of Biomedical Engineering at the University of California, Irvine. He received his undergraduate degree in Bioengineering and his Master of Science degree in applied mechanics from the University of California, San Diego. He completed his doctorate degree in biomaterials at Gothenburg University in Sweden. He was elected Fellow for the American Society of Mechanical Engineers (ASME) and the American Institute for Medical and Biological Engineering (AIMBE). He is also an elected member of the American Shoulder and Elbow Surgeons (ASES), Orthopaedic Research Society (ORS), American Society of Biomechanics (ASB), California Orthopaedic Association (COA), Society for Biomaterials (SFB), Biomedical Engineering Society (BMES), and American Orthopaedic Society for Sports Medicine (AOSSM). His research interests include joint biomechanics, shoulder, and knee.
Raffy Mirzayan, MD
Associate Editor for Biologics

Dr. Mirzayan is a board-certified orthopedic surgeon with a subspecialty in sports medicine at Kaiser Permanente in Baldwin Park, California; and founder and director of the Advanced Concepts course held in Las Vegas. He received his Bachelor of Science degree from University of California, Los Angeles (UCLA) and graduated from the Keck School of Medicine of University of Southern California (USC) with AOA Honors. He completed his residency at the Los Angeles County/USC Medical Center, and his fellowship at the Kerlan-Jobe Orthopedic Clinic. He belongs to several orthopedic and sports medicine societies, and was chosen as an American Academy of Orthopaedic Surgeons (AAOS) Leadership Fellow and an American Orthopaedic Society for Sports Medicine (AOSSM) Traveling Fellow. His research interests include shoulder, elbow, knee, cartilage reconstruction, osteotomies, meniscal transplantation, and orthobiologics.
Cryo-Compression Therapy
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
All-Inside Meniscal Repair Devices
Tools of the Trade features reviews of the hottest new products, with surgical pearls written by surgeons who know these products best.
Arthrex, Inc. (http://www.arthrex.com)
Knee Scorpion
The Arthrex Knee Scorpion allows for simple passage of suture at the root to repair the tissue. The mechanism of the Knee Scorpion self-retrieving the suture after passage eliminates the need for another step in the procedure, which saves time. There are various types of suture configurations that can be incorporated into meniscus repairs with the Knee Scorpion. Depending on tissue quality and location, I will either pass the center of the suture to create a cinch or luggage tag type of stitch, or sometimes a simple stitch.
A challenging meniscal tear pattern that repair is particularly made easier by use of the Knee Scorpion is the radial tear of the lateral meniscus. Here it is easy to place a side-to-side “spanning” circumferential suture pattern, which is the strongest suture configuration, and it reduces the tissue very well. This approach also is ideal for variant root type tears, those that are 3 to 4 mm from the root commonly seen with the lateral meniscus associated with anterior cruciate ligament tears.
Benefits: The Knee Scorpion has a very low profile to facilitate placement in the joint with a 5º upcurve to avoid injury to the femoral condyle. The needle captures the suture after passage, which saves time and an additional surgical step. It can be used with both 0 FiberWire and 2-0 FiberWire.
Surgical pearl: Using a cannula through the working portal prevents any tissue bridge when passing and tying sutures. I prefer a Passport button cannula since its flexibility allows more access in different planes compared to a rigid type cannula.
Patrick A. Smith, MD, Columbia Orthopaedic Group, Head Section of Sports Medicine University of Missouri, Head Team Physician University of Missouri
Arthrex, Inc. (http://www.arthrex.com)
SpeedCinch
The SpeedCinch’s pistol grip design permits ergonomic, one-handed all-inside meniscal repair. It is best suited for meniscal tears of the posterior horn and body. The posterior horn of the meniscus can be repaired with the SpeedCinch inserted through either the ipsilateral or contralateral portal. Meniscal body tears, however, are best approached via a contralateral portal. The end of the device contains a 15g needle that should be advanced across the meniscus. After passage of the needle, the first implant is pushed through the needle when the trigger is fully deployed.
Next, the SpeedCinch needle should be moved at least 1 cm for placement of the second implant in either a horizontal or vertical mattress fashion. After the needle is brought to the second insertion site, the implant selector button is moved to 2 and the trigger is depressed until the first click is felt and heard. Next, the trigger is held in place after the first click and the needle is advanced across the meniscus before fully depressing the trigger, which will advance the second implant. The device is then removed and the pre-tied knot is secured with a knot pusher and then cut.
Vic Goradia, MD, G2 Orthopedics and Sports Medicine, Glen Allen, VA
Cayenne Medical, Inc. (www.cayennemedical.com)
The CrossFix II System
The CrossFix II System offers a unique, all-arthroscopic, suture-only meniscal repair that reduces the risk of chondral injury. Its “all-inside” technique uses 2 parallel suture delivery needles, available in both curved and straight designs, inserted through a single incision to provide an all-inside meniscus repair. This simple procedure can be performed in minutes via the device’s pre-tied, sliding knot, creating a “suture only” mattress stitch that replicates the repair of standard open suturing techniques. The speed and reproducibility of the repair dramatically improves operating room efficiency. Even complex tears requiring multiple sutures can be repaired in minutes.
Benefits: All-suture, no implants; single insertion; pre-tied, sliding knot. Parallel needles may be difficult to use in very tight knees.
Surgical pearl: Avoid torqueing the needles upon insertion into the meniscus.
Kenneth Montgomery, MD, Tri-County Orthopedics, Cedar Knolls, NJ.
Ceterix Orthopaedics, Inc. (www.ceterix.com)
NovoStitch Plus
The Ceterix NovoStitch Plus enables surgeons to place circumferential compression stitches around meniscus tears. These stitch patterns are designed to provide anatomical reduction and uniform compression of the tear edges, and repair the femoral surface (top) and tibial surface (bottom) of the tear with each stitch. It is also designed to eliminate neurovascular risk and avoid excessive entrapment or extrusion of the meniscus to capsule. The NovoStitch Plus passes circumferential stitches via an all-inside arthroscopic technique. In addition to anatomically repairing vertical tears, circumferential stitches also enable repair of tear types that were previously considered difficult to adequately sew.
Benefits: Passes both sides of the stitch with 1 insertion (avoids tissue bridges and girth hitches). Retractable lower jaw (allows access to tight knees, and allows removal from knees with mobile menisci). Needle extends into the posterior gutter through the tip of the lower jaw (does not touch chondral surfaces). Smaller lower jaw tooth (easier insertion of the lower jaw under the meniscus). Upper jaw curved to follow the shape contour of the femoral side of the meniscus and femoral condyle. Compared to hybrid all-inside devices, inside-out and outside-in repairs. Enables anatomical reduction and uniform compression. Each stitch repairs the top and bottom of the tear. Designed to eliminate neurovascular risk. Avoids excessive capsular entrapment. Repairs effectively in front of the popliteal hiatus. Enables side-to-side radial tear repair, and top-to-bottom horizontal tear repair. Significantly smaller needle than used by hybrid all-inside devices.
Surgical pearl: A spinal needle should be used to establish the skin incision location of the working portal. The optimal skin incision location is one where a spinal needle can be inserted such that the distal end of the needle is parallel to the region of the tibial plateau under the meniscus.
Justin Saliman, MD, Cedars-Sinai Orthopaedic Center, Los Angeles CA
Mitek Sports Medicine (www.depuysynthes.com)
Mitek Omnispan
The Mitek Omnispan Meniscal Repair System is an all-inside arthroscopic meniscal repair device. It consists of a low profile needle, pre-loaded with 2 PEEK backstops and No. 2/0 Orthocord High Strength Orthopaedic Suture, which is delivered using the Omnispan System Applier. The needles come in 3 angles (0°, 12°, and 27°) and the Applier is a single-patient, multi-use design, meaning the same Applier can be used for multiple implants with a single patient. The needles also have a safety sleeve over each set of implants to prevent delivery failure. Once the needle is attached to the Applier, the device is inserted into the knee. Using the gray trigger, the first implant is delivered into the meniscocapsular region. The red trigger is then pulled, advancing the second implant. Once appropriate positioning of the second implant is determined, the gray trigger is fired again. The surgeon pulls on the sliding knot advancing the suture through the implants creating a suture bridge over the tear. The final repair consists of Orthocord Suture spanning the meniscus, while the PEEK backstops are embedded in the meniscocapsular area only.
Surgical pearls:
An adequate portal opening is paramount for successful implementation. I widen my portal with an instrument clamp to allow easy passage of the Applier. It is usually necessary to switch the camera back and forth between both portals to gain access to the angles needed to create the repair. I also always use a skid when inserting the Applier to prevent soft tissue impingement.
The surgeon should always check to make sure the implant is properly loaded on the back table, ensuring it is fully inserted into the locking mechanism and loaded in the correct direction.
The surgeon should hold the Applier firmly when firing the gray trigger, as some kick-back does occur.
The Applier should remain in the joint throughout the advancing and deployment of implants, so the surgeon can maintain visualization with the scope. Once the implants are in position, I then remove the Applier from the joint space.
Use a probe to place counter-pressure on the meniscus when tightening the suture. The sliding suture system is a double-loop design. In order to smoothly advance the suture, I recommend that a probe be placed on the smaller loop closest to the meniscus, so that when the suture is tightened, the probe is under the smaller loop. A gentle back-and-forth pulling maneuver between the free suture and the probe creates a smooth transition during tightening.
Scott A. Sigman, MD, Chief of Orthopedics, Lowell General Hospital; Team Physician, UMass Lowell
Smith & Nephew Inc. (www.smith-nephew.com)
FAST-FIX 360
The FAST-FIX360 design enables you to deploy implants in any hand position—vertically, horizontally on either side of the meniscus—with a fast, smooth advancing motion. This spring-action design facilitates the advancement of each implant into the capsule. Smaller implants and pre-tied, self-sliding knots made of ULTRABRAID 2-0 Suture create smaller needle insertions, reducing disruption to the meniscus. Low-profile, stiffer needle shaft improves control while enabling access and visibility to hard-to-reach areas of the meniscus. Set needle depth penetration from 10 mm to 18 mm with the push of a button. The FAST-FIX 360 System has biomechanical properties that best reproduce the vertical mattress suture technique.
Surgical pearl: Assess tear pattern and reparability, then reduce and template repair construct and suture points. Precisely select portal insertion sites, ensuring perpendicular vector needle delivery at the repair site. Measure the meniscal fragment size and rim width, setting the depth penetration limiter (usually set to 15 to 18 mm). Insert first anchor posteriorly and superiorly or away from the insertion portal and then after deployment, insert the second anchor anteriorly and inferiorly or into the “near” tear fragment. Tension the suture using the knot pusher/cutter as a “suture stent” to manually “pull and push” the suture, compressing the repair construct and coapting the tear. Avoid “over-repairing” the tear by spacing out sutures at 3 mm to 5 mm and alternating femoral and tibial undersurface placement.
Nicholas A. Sgaglione, MD (pictured), and Ryan A. Harrell, DO
Tools of the Trade features reviews of the hottest new products, with surgical pearls written by surgeons who know these products best.
Arthrex, Inc. (http://www.arthrex.com)
Knee Scorpion
The Arthrex Knee Scorpion allows for simple passage of suture at the root to repair the tissue. The mechanism of the Knee Scorpion self-retrieving the suture after passage eliminates the need for another step in the procedure, which saves time. There are various types of suture configurations that can be incorporated into meniscus repairs with the Knee Scorpion. Depending on tissue quality and location, I will either pass the center of the suture to create a cinch or luggage tag type of stitch, or sometimes a simple stitch.
A challenging meniscal tear pattern that repair is particularly made easier by use of the Knee Scorpion is the radial tear of the lateral meniscus. Here it is easy to place a side-to-side “spanning” circumferential suture pattern, which is the strongest suture configuration, and it reduces the tissue very well. This approach also is ideal for variant root type tears, those that are 3 to 4 mm from the root commonly seen with the lateral meniscus associated with anterior cruciate ligament tears.
Benefits: The Knee Scorpion has a very low profile to facilitate placement in the joint with a 5º upcurve to avoid injury to the femoral condyle. The needle captures the suture after passage, which saves time and an additional surgical step. It can be used with both 0 FiberWire and 2-0 FiberWire.
Surgical pearl: Using a cannula through the working portal prevents any tissue bridge when passing and tying sutures. I prefer a Passport button cannula since its flexibility allows more access in different planes compared to a rigid type cannula.
Patrick A. Smith, MD, Columbia Orthopaedic Group, Head Section of Sports Medicine University of Missouri, Head Team Physician University of Missouri
Arthrex, Inc. (http://www.arthrex.com)
SpeedCinch
The SpeedCinch’s pistol grip design permits ergonomic, one-handed all-inside meniscal repair. It is best suited for meniscal tears of the posterior horn and body. The posterior horn of the meniscus can be repaired with the SpeedCinch inserted through either the ipsilateral or contralateral portal. Meniscal body tears, however, are best approached via a contralateral portal. The end of the device contains a 15g needle that should be advanced across the meniscus. After passage of the needle, the first implant is pushed through the needle when the trigger is fully deployed.
Next, the SpeedCinch needle should be moved at least 1 cm for placement of the second implant in either a horizontal or vertical mattress fashion. After the needle is brought to the second insertion site, the implant selector button is moved to 2 and the trigger is depressed until the first click is felt and heard. Next, the trigger is held in place after the first click and the needle is advanced across the meniscus before fully depressing the trigger, which will advance the second implant. The device is then removed and the pre-tied knot is secured with a knot pusher and then cut.
Vic Goradia, MD, G2 Orthopedics and Sports Medicine, Glen Allen, VA
Cayenne Medical, Inc. (www.cayennemedical.com)
The CrossFix II System
The CrossFix II System offers a unique, all-arthroscopic, suture-only meniscal repair that reduces the risk of chondral injury. Its “all-inside” technique uses 2 parallel suture delivery needles, available in both curved and straight designs, inserted through a single incision to provide an all-inside meniscus repair. This simple procedure can be performed in minutes via the device’s pre-tied, sliding knot, creating a “suture only” mattress stitch that replicates the repair of standard open suturing techniques. The speed and reproducibility of the repair dramatically improves operating room efficiency. Even complex tears requiring multiple sutures can be repaired in minutes.
Benefits: All-suture, no implants; single insertion; pre-tied, sliding knot. Parallel needles may be difficult to use in very tight knees.
Surgical pearl: Avoid torqueing the needles upon insertion into the meniscus.
Kenneth Montgomery, MD, Tri-County Orthopedics, Cedar Knolls, NJ.
Ceterix Orthopaedics, Inc. (www.ceterix.com)
NovoStitch Plus
The Ceterix NovoStitch Plus enables surgeons to place circumferential compression stitches around meniscus tears. These stitch patterns are designed to provide anatomical reduction and uniform compression of the tear edges, and repair the femoral surface (top) and tibial surface (bottom) of the tear with each stitch. It is also designed to eliminate neurovascular risk and avoid excessive entrapment or extrusion of the meniscus to capsule. The NovoStitch Plus passes circumferential stitches via an all-inside arthroscopic technique. In addition to anatomically repairing vertical tears, circumferential stitches also enable repair of tear types that were previously considered difficult to adequately sew.
Benefits: Passes both sides of the stitch with 1 insertion (avoids tissue bridges and girth hitches). Retractable lower jaw (allows access to tight knees, and allows removal from knees with mobile menisci). Needle extends into the posterior gutter through the tip of the lower jaw (does not touch chondral surfaces). Smaller lower jaw tooth (easier insertion of the lower jaw under the meniscus). Upper jaw curved to follow the shape contour of the femoral side of the meniscus and femoral condyle. Compared to hybrid all-inside devices, inside-out and outside-in repairs. Enables anatomical reduction and uniform compression. Each stitch repairs the top and bottom of the tear. Designed to eliminate neurovascular risk. Avoids excessive capsular entrapment. Repairs effectively in front of the popliteal hiatus. Enables side-to-side radial tear repair, and top-to-bottom horizontal tear repair. Significantly smaller needle than used by hybrid all-inside devices.
Surgical pearl: A spinal needle should be used to establish the skin incision location of the working portal. The optimal skin incision location is one where a spinal needle can be inserted such that the distal end of the needle is parallel to the region of the tibial plateau under the meniscus.
Justin Saliman, MD, Cedars-Sinai Orthopaedic Center, Los Angeles CA
Mitek Sports Medicine (www.depuysynthes.com)
Mitek Omnispan
The Mitek Omnispan Meniscal Repair System is an all-inside arthroscopic meniscal repair device. It consists of a low profile needle, pre-loaded with 2 PEEK backstops and No. 2/0 Orthocord High Strength Orthopaedic Suture, which is delivered using the Omnispan System Applier. The needles come in 3 angles (0°, 12°, and 27°) and the Applier is a single-patient, multi-use design, meaning the same Applier can be used for multiple implants with a single patient. The needles also have a safety sleeve over each set of implants to prevent delivery failure. Once the needle is attached to the Applier, the device is inserted into the knee. Using the gray trigger, the first implant is delivered into the meniscocapsular region. The red trigger is then pulled, advancing the second implant. Once appropriate positioning of the second implant is determined, the gray trigger is fired again. The surgeon pulls on the sliding knot advancing the suture through the implants creating a suture bridge over the tear. The final repair consists of Orthocord Suture spanning the meniscus, while the PEEK backstops are embedded in the meniscocapsular area only.
Surgical pearls:
An adequate portal opening is paramount for successful implementation. I widen my portal with an instrument clamp to allow easy passage of the Applier. It is usually necessary to switch the camera back and forth between both portals to gain access to the angles needed to create the repair. I also always use a skid when inserting the Applier to prevent soft tissue impingement.
The surgeon should always check to make sure the implant is properly loaded on the back table, ensuring it is fully inserted into the locking mechanism and loaded in the correct direction.
The surgeon should hold the Applier firmly when firing the gray trigger, as some kick-back does occur.
The Applier should remain in the joint throughout the advancing and deployment of implants, so the surgeon can maintain visualization with the scope. Once the implants are in position, I then remove the Applier from the joint space.
Use a probe to place counter-pressure on the meniscus when tightening the suture. The sliding suture system is a double-loop design. In order to smoothly advance the suture, I recommend that a probe be placed on the smaller loop closest to the meniscus, so that when the suture is tightened, the probe is under the smaller loop. A gentle back-and-forth pulling maneuver between the free suture and the probe creates a smooth transition during tightening.
Scott A. Sigman, MD, Chief of Orthopedics, Lowell General Hospital; Team Physician, UMass Lowell
Smith & Nephew Inc. (www.smith-nephew.com)
FAST-FIX 360
The FAST-FIX360 design enables you to deploy implants in any hand position—vertically, horizontally on either side of the meniscus—with a fast, smooth advancing motion. This spring-action design facilitates the advancement of each implant into the capsule. Smaller implants and pre-tied, self-sliding knots made of ULTRABRAID 2-0 Suture create smaller needle insertions, reducing disruption to the meniscus. Low-profile, stiffer needle shaft improves control while enabling access and visibility to hard-to-reach areas of the meniscus. Set needle depth penetration from 10 mm to 18 mm with the push of a button. The FAST-FIX 360 System has biomechanical properties that best reproduce the vertical mattress suture technique.
Surgical pearl: Assess tear pattern and reparability, then reduce and template repair construct and suture points. Precisely select portal insertion sites, ensuring perpendicular vector needle delivery at the repair site. Measure the meniscal fragment size and rim width, setting the depth penetration limiter (usually set to 15 to 18 mm). Insert first anchor posteriorly and superiorly or away from the insertion portal and then after deployment, insert the second anchor anteriorly and inferiorly or into the “near” tear fragment. Tension the suture using the knot pusher/cutter as a “suture stent” to manually “pull and push” the suture, compressing the repair construct and coapting the tear. Avoid “over-repairing” the tear by spacing out sutures at 3 mm to 5 mm and alternating femoral and tibial undersurface placement.
Nicholas A. Sgaglione, MD (pictured), and Ryan A. Harrell, DO
Tools of the Trade features reviews of the hottest new products, with surgical pearls written by surgeons who know these products best.
Arthrex, Inc. (http://www.arthrex.com)
Knee Scorpion
The Arthrex Knee Scorpion allows for simple passage of suture at the root to repair the tissue. The mechanism of the Knee Scorpion self-retrieving the suture after passage eliminates the need for another step in the procedure, which saves time. There are various types of suture configurations that can be incorporated into meniscus repairs with the Knee Scorpion. Depending on tissue quality and location, I will either pass the center of the suture to create a cinch or luggage tag type of stitch, or sometimes a simple stitch.
A challenging meniscal tear pattern that repair is particularly made easier by use of the Knee Scorpion is the radial tear of the lateral meniscus. Here it is easy to place a side-to-side “spanning” circumferential suture pattern, which is the strongest suture configuration, and it reduces the tissue very well. This approach also is ideal for variant root type tears, those that are 3 to 4 mm from the root commonly seen with the lateral meniscus associated with anterior cruciate ligament tears.
Benefits: The Knee Scorpion has a very low profile to facilitate placement in the joint with a 5º upcurve to avoid injury to the femoral condyle. The needle captures the suture after passage, which saves time and an additional surgical step. It can be used with both 0 FiberWire and 2-0 FiberWire.
Surgical pearl: Using a cannula through the working portal prevents any tissue bridge when passing and tying sutures. I prefer a Passport button cannula since its flexibility allows more access in different planes compared to a rigid type cannula.
Patrick A. Smith, MD, Columbia Orthopaedic Group, Head Section of Sports Medicine University of Missouri, Head Team Physician University of Missouri
Arthrex, Inc. (http://www.arthrex.com)
SpeedCinch
The SpeedCinch’s pistol grip design permits ergonomic, one-handed all-inside meniscal repair. It is best suited for meniscal tears of the posterior horn and body. The posterior horn of the meniscus can be repaired with the SpeedCinch inserted through either the ipsilateral or contralateral portal. Meniscal body tears, however, are best approached via a contralateral portal. The end of the device contains a 15g needle that should be advanced across the meniscus. After passage of the needle, the first implant is pushed through the needle when the trigger is fully deployed.
Next, the SpeedCinch needle should be moved at least 1 cm for placement of the second implant in either a horizontal or vertical mattress fashion. After the needle is brought to the second insertion site, the implant selector button is moved to 2 and the trigger is depressed until the first click is felt and heard. Next, the trigger is held in place after the first click and the needle is advanced across the meniscus before fully depressing the trigger, which will advance the second implant. The device is then removed and the pre-tied knot is secured with a knot pusher and then cut.
Vic Goradia, MD, G2 Orthopedics and Sports Medicine, Glen Allen, VA
Cayenne Medical, Inc. (www.cayennemedical.com)
The CrossFix II System
The CrossFix II System offers a unique, all-arthroscopic, suture-only meniscal repair that reduces the risk of chondral injury. Its “all-inside” technique uses 2 parallel suture delivery needles, available in both curved and straight designs, inserted through a single incision to provide an all-inside meniscus repair. This simple procedure can be performed in minutes via the device’s pre-tied, sliding knot, creating a “suture only” mattress stitch that replicates the repair of standard open suturing techniques. The speed and reproducibility of the repair dramatically improves operating room efficiency. Even complex tears requiring multiple sutures can be repaired in minutes.
Benefits: All-suture, no implants; single insertion; pre-tied, sliding knot. Parallel needles may be difficult to use in very tight knees.
Surgical pearl: Avoid torqueing the needles upon insertion into the meniscus.
Kenneth Montgomery, MD, Tri-County Orthopedics, Cedar Knolls, NJ.
Ceterix Orthopaedics, Inc. (www.ceterix.com)
NovoStitch Plus
The Ceterix NovoStitch Plus enables surgeons to place circumferential compression stitches around meniscus tears. These stitch patterns are designed to provide anatomical reduction and uniform compression of the tear edges, and repair the femoral surface (top) and tibial surface (bottom) of the tear with each stitch. It is also designed to eliminate neurovascular risk and avoid excessive entrapment or extrusion of the meniscus to capsule. The NovoStitch Plus passes circumferential stitches via an all-inside arthroscopic technique. In addition to anatomically repairing vertical tears, circumferential stitches also enable repair of tear types that were previously considered difficult to adequately sew.
Benefits: Passes both sides of the stitch with 1 insertion (avoids tissue bridges and girth hitches). Retractable lower jaw (allows access to tight knees, and allows removal from knees with mobile menisci). Needle extends into the posterior gutter through the tip of the lower jaw (does not touch chondral surfaces). Smaller lower jaw tooth (easier insertion of the lower jaw under the meniscus). Upper jaw curved to follow the shape contour of the femoral side of the meniscus and femoral condyle. Compared to hybrid all-inside devices, inside-out and outside-in repairs. Enables anatomical reduction and uniform compression. Each stitch repairs the top and bottom of the tear. Designed to eliminate neurovascular risk. Avoids excessive capsular entrapment. Repairs effectively in front of the popliteal hiatus. Enables side-to-side radial tear repair, and top-to-bottom horizontal tear repair. Significantly smaller needle than used by hybrid all-inside devices.
Surgical pearl: A spinal needle should be used to establish the skin incision location of the working portal. The optimal skin incision location is one where a spinal needle can be inserted such that the distal end of the needle is parallel to the region of the tibial plateau under the meniscus.
Justin Saliman, MD, Cedars-Sinai Orthopaedic Center, Los Angeles CA
Mitek Sports Medicine (www.depuysynthes.com)
Mitek Omnispan
The Mitek Omnispan Meniscal Repair System is an all-inside arthroscopic meniscal repair device. It consists of a low profile needle, pre-loaded with 2 PEEK backstops and No. 2/0 Orthocord High Strength Orthopaedic Suture, which is delivered using the Omnispan System Applier. The needles come in 3 angles (0°, 12°, and 27°) and the Applier is a single-patient, multi-use design, meaning the same Applier can be used for multiple implants with a single patient. The needles also have a safety sleeve over each set of implants to prevent delivery failure. Once the needle is attached to the Applier, the device is inserted into the knee. Using the gray trigger, the first implant is delivered into the meniscocapsular region. The red trigger is then pulled, advancing the second implant. Once appropriate positioning of the second implant is determined, the gray trigger is fired again. The surgeon pulls on the sliding knot advancing the suture through the implants creating a suture bridge over the tear. The final repair consists of Orthocord Suture spanning the meniscus, while the PEEK backstops are embedded in the meniscocapsular area only.
Surgical pearls:
An adequate portal opening is paramount for successful implementation. I widen my portal with an instrument clamp to allow easy passage of the Applier. It is usually necessary to switch the camera back and forth between both portals to gain access to the angles needed to create the repair. I also always use a skid when inserting the Applier to prevent soft tissue impingement.
The surgeon should always check to make sure the implant is properly loaded on the back table, ensuring it is fully inserted into the locking mechanism and loaded in the correct direction.
The surgeon should hold the Applier firmly when firing the gray trigger, as some kick-back does occur.
The Applier should remain in the joint throughout the advancing and deployment of implants, so the surgeon can maintain visualization with the scope. Once the implants are in position, I then remove the Applier from the joint space.
Use a probe to place counter-pressure on the meniscus when tightening the suture. The sliding suture system is a double-loop design. In order to smoothly advance the suture, I recommend that a probe be placed on the smaller loop closest to the meniscus, so that when the suture is tightened, the probe is under the smaller loop. A gentle back-and-forth pulling maneuver between the free suture and the probe creates a smooth transition during tightening.
Scott A. Sigman, MD, Chief of Orthopedics, Lowell General Hospital; Team Physician, UMass Lowell
Smith & Nephew Inc. (www.smith-nephew.com)
FAST-FIX 360
The FAST-FIX360 design enables you to deploy implants in any hand position—vertically, horizontally on either side of the meniscus—with a fast, smooth advancing motion. This spring-action design facilitates the advancement of each implant into the capsule. Smaller implants and pre-tied, self-sliding knots made of ULTRABRAID 2-0 Suture create smaller needle insertions, reducing disruption to the meniscus. Low-profile, stiffer needle shaft improves control while enabling access and visibility to hard-to-reach areas of the meniscus. Set needle depth penetration from 10 mm to 18 mm with the push of a button. The FAST-FIX 360 System has biomechanical properties that best reproduce the vertical mattress suture technique.
Surgical pearl: Assess tear pattern and reparability, then reduce and template repair construct and suture points. Precisely select portal insertion sites, ensuring perpendicular vector needle delivery at the repair site. Measure the meniscal fragment size and rim width, setting the depth penetration limiter (usually set to 15 to 18 mm). Insert first anchor posteriorly and superiorly or away from the insertion portal and then after deployment, insert the second anchor anteriorly and inferiorly or into the “near” tear fragment. Tension the suture using the knot pusher/cutter as a “suture stent” to manually “pull and push” the suture, compressing the repair construct and coapting the tear. Avoid “over-repairing” the tear by spacing out sutures at 3 mm to 5 mm and alternating femoral and tibial undersurface placement.
Nicholas A. Sgaglione, MD (pictured), and Ryan A. Harrell, DO
Meniscal Root Tears: Identification and Repair
Intact and well functioning menisci are essential for optimal knee function. Articular cartilage damage and rapid joint degeneration have been observed in knees after meniscectomy.1-5 Meniscal root tears and avulsions are now increasingly recognized as a functional equivalent to total meniscectomy, and will follow a similar course if left untreated.6-8
The menisci provide shock absorption and stability through their unique anatomy and physiology. Their essential role in dissipation of the axial load encountered during daily activities is accomplished via generation of circumferential hoop stress.4,5,9 Tears of the horn or body may diminish this ability depending on the size and location, but a tear or an avulsion that renders the root incompetent will leave the meniscus unable to generate hoop stress.10 Likewise, as the menisci have been shown to be important secondary stabilizers for both translation and rotation, this function is lost or significantly diminished in the setting of a root tear.6,11,12
Despite their clinical and biomechanical implications, meniscal root tears can be difficult to identify, particularly when they are not actively sought. The goal of this article is to highlight the current diagnostic workup and treatment in patients with suspected meniscal root pathology. We will also aim to emphasize important anatomic and biomechanical considerations when attempting a meniscal root repair.
Anatomy
The menisci are 2 fibrocartilage wedge-shaped structures that surround the medial and lateral tibial plateau’s weight-bearing surfaces. They are attached at many points along their periphery via coronary ligaments that comprise a continuous junction of the meniscus to the capsule to the tibial plateau. Each meniscus has an anterior and a posterior horn that are securely anchored to the tibial intercondylar region via strong ligaments known as the roots.
The anterior medial root attaches just anterior and medial to the medial tibial spine. The anterior lateral root attaches just anterior to the lateral tibial spine. The medial and lateral anterior horns of the menisci are also connected via the anterior intermeniscal ligament (AIML).13-15 Recent cadaveric biomechanical studies have questioned the importance of the AIML, demonstrating no significant change in contact pressure or area before and after sectioning.16 Another important consideration with respect to the anterior root insertion of the lateral meniscus is its intimate relationship with the tibial insertion of the anterior cruciate ligament (ACL). The anterior lateral root and the ACL share over 60% of their tibial footprints.13,17
When the menisci are competent, they absorb between 40% to 70% of the contact force generated between the femur and tibia.1 By providing strong anchor points, the meniscal roots allow the horns and bodies of the menisci to maintain a stable position that maximizes congruency with the femoral condyles.
Pathology
The conversion of axial load to circumferential hoop stresses occur as the resilient, yet pliable, menisci are squeezed between the femoral condyle and tibial plateau. However, this function is dependent on secure attachment sites at the roots. In the setting of root tear, there is no restraint to the peripheral distortion of the menisci, and meniscal extrusion can occur.18
Clinical evidence and biomechanical evidence strongly show the consequences of meniscectomy. Multiple studies have shown similar findings and have proven that a meniscal root tear or avulsion is the biomechanical equivalent to total meniscectomy.3 With meniscectomy, not only do peak pressures within compartments increase significantly, it has been demonstrated that other compartments within the knee with intact menisci do not have increases in compartment pressures, lending more evidence to the menisci functioning as separate units.16 It has also been found that anterior/posterior translation is increased with medial meniscal root tears. When lateral meniscus root tears were studied with associated ACL tear, the pivot shift motion was found to be exaggerated.6
However, the finding of utmost importance in these biomechanical studies is that peak pressures and excessive tibiofemoral motion are restored to normal levels after meniscal root repair. Therefore, repair of meniscal root tears restores native knee biomechanics and will potentially prevent arthritic sequelae from developing.3,4,7,19
Epidemiology
Tears of the posterior root of either menisci are more common than their anterior counterparts, and have been more extensively studied. However, there are situations that can lead to anterior root tears, specifically during ACL reconstruction and during medullary nailing of the tibia.20,21 Barring iatrogenic injury, the anterior horn is less at risk for injury than the posterior horn given the biomechanical environment of the knee.3
Medial meniscus posterior root tears are more common than lateral tears. However, these are often more chronic in nature and not associated with an acute event. Risk factors for medial meniscus root tear include increased body mass index, varus mechanical axis, female gender, and low activity level.22
Lateral meniscus root tears more commonly occur during trauma with sprains and/or tears of knee ligaments.23 Along with increased recognition of meniscal root injuries associated with knee ligamentous injury comes the recognition that certain ligamentous reconstructions—namely the ACL—are more prone to failure and have higher stresses when a root tear is left untreated.17,24
Diagnosis
The gold standard for diagnosis of a meniscal root lesion is under direct visualization during arthroscopy.18 The meniscal roots must be probed and stressed to assess their integrity regardless of the initial indication for knee arthroscopy. In most cases, however, the diagnosis of meniscal root tears should occur prior to proceeding to the operating room.
Magnetic resonance imaging (MRI) has been used to aid in diagnosis of meniscal root tears since the early 1990s.25 Now, with the widespread use of MRI, understanding and diagnosis of meniscal root pathology has increased. All sequences should be reviewed, but T2 weighted coronal sections should provide the best visualization of the posterior roots (Figures 1A, 1B). Sagittal sections may also be helpful in this diagnosis. Increased signal within the root or horn may represent partial or full thickness tears, or may show a more degenerative process with fraying.14,15,26,27
MRI does have limitations, however. When compared to arthroscopy, the sensitivity of 3T MRI to identify posterior root tears is 77%, and specificity is 73%. Medial root tears are more readily identified on MRI than lateral tears.28 This further highlights the need for high suspicion during arthroscopy with the requisite equipment on standby should it be needed.
A concerning finding that may be observed on MRI includes meniscal extrusion (Figures 2A, 2B). Most often seen with the medial meniscus, extrusion is diagnosed when the meniscal body displaces greater than 3 mm past the tibial articular surface on a midcoronal image.26,27 Over 50% of patients with medial meniscal extrusion on MRI will have medial meniscal root tears.26,27 Conversely, meniscal extrusion is less common in lateral menisci for multiple reasons. The lateral compartment of the knee does not have as high contact pressure as the medial compartment, so the lateral meniscus is not as likely to be extruded from the joint. Additionally, the posterior lateral root has the added benefit of further stability from meniscofemoral ligaments.11 They provide a restraint to meniscal extrusion, with a reported rate of 14% lateral meniscus extrusion when they are intact. If the meniscofemoral ligaments are not present or torn in the setting of posterior root tear, the lateral meniscus extrusion rate quadruples and approaches that of medial meniscal extrusion.15
Another finding indicative of meniscal root tear is the “ghost meniscus” (Figure 3). The posterior horn and anterior horn should both be visible in sagittal cuts on MRI. When the anterior horn is present, but the posterior horn is not visualized, it is termed a “ghost meniscus.” This MRI finding is highly associated with meniscal root tears, and will often be found along with meniscal extrusion on coronal sequencing.27,28
Treatment
Historically, large meniscal tears, extruded menisci, or root avulsions have been treated with conservative observation if asymptomatic, or with meniscectomy when symptomatic. With a meniscal root tear, both forms of treatment will not provide lasting benefit and rapid joint degeneration ensues. Evidence now supports repair over meniscectomy when treating root tears.7,8,19,29
Patients who have meniscal root tears that are likely sequelae of an arthritic process are not candidates for meniscal root repair. These patients will often have known arthritis with an intact meniscus and then progress to meniscal pathology, most often medially. Because arthritis is the cause of these meniscal tears, a repair will not reverse this process; such repairs will likely fail, and the patient will re-tear the meniscus. For this subset of patients, physical therapy and activity modification are appropriate treatment.
Repair is indicated for patients with acute tears, with or without associated soft tissue injury to the knee, and those with chronic or acute on chronic tears with minimal arthritis within the knee. The authors’ preferred method of repair is via suture fixation through transosseous tunnel (Figures 4A-4F).
Once a root tear has been identified during arthroscopy, it should be probed and/or grasped and pulled to confirm its integrity. A shaver is then used to debride any fraying of the meniscus and to debride the anatomic footprint of the root. Curettes and rasps are used to prepare the meniscal bed at the center of its insertion and the undersurface of the meniscal root. Once the attachment site of the root insertion has been prepared, an ACL tip-to-tip drill guide is placed over the prepared bed. For repair of a medial meniscus posterior root, a 2.4-mm drill tip guide pin is inserted through the guide via an incision made at the anteromedial tibia. For repair of the lateral meniscus posterior root, the pin is inserted through an incision at the anterolateral aspect of the tibia.
Once the guide pin has been inserted and is visualized at the center of the root footprint, it is held in place by a hemostat or grasper placed intra-articularly. Next, the guide pin is overreamed with a 4.5-mm cannulated drill bit. The transosseous tunnel is then further prepared using a shaver to remove excess soft tissue surrounding the tunnel entrance at the tibial plateau. Further rasping around the edges of the tunnel is performed to make final preparations.
Attention is then turned back to the meniscal root. Using a FastPass Scorpion (Arthrex), 2 or 3 size 0 fiber wire sutures are passed through the root, and a cinch stitch is then secured leaving four to six stands (2 from each Scorpion pass) in the root. A FiberStick is then introduced into the tibial bone tunnel and each strand of the 0 fiberwire is retrieved. Once the FiberWire attached to the meniscal root is in the tunnel, the meniscus should be directly visualized as the appropriate tension is toggled to reduce the meniscal root into its footprint. In order to securely fasten the meniscal root, an Arthrex SwiveLock 4.75-mm suture anchor is used. The meniscus is again probed to assess the integrity of the repair. Of note, an alternative method of fixation is accomplished by tying the fiberwire over an Arthrex suture button at the anterior tibia.
Postoperatively, weight bearing restriction is warranted, along with range of motion restrictions. During the first 2 weeks, patients will be counseled to be touch down weight bearing with the use of crutches or a walker. During this period, range of motion will be restricted by hinged knee brace to 30° of flexion and full extension. The next 2-week period will advance to progressive partial weight bearing, again with crutches or a walker. Range of motion will also be expanded to 60° of flexion. After a month, the patient will then be allowed to be full weight bearing as tolerated and be weaned from assistive ambulation devices. Range of motion will then be 90° of flexion. It is paramount that full extension be achieved and maintained in the early postoperative period. Quadriceps strengthening should also proceed with unlimited straight leg raises throughout this period as well.
1. Kidron A, Thein R. Radial tears associated with cleavage tears of the medial meniscus in athletes. Arthroscopy. 2002;18(3):254-256.
2. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664-670.
3. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Joint Surg. 2008;90(9):1922-1931.
4. Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124-129.
5. Hein CN, Deperio JG, Ehrensberger MT, Marzo JM. Effects of medial meniscal posterior horn avulsion and repair on meniscal displacement. Knee. 2011;18(3):189-192.
6. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43(4):905-911.
7. Vyas D, Harner CD. Meniscus root repair. Sports Med Arthrosc Rev. 2012;20(2):86-94.
8. Koenig JH, Ranawat AS, Umans HR, Difelice GS. Meniscal root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025-1032.
9. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990;(252):19-31.
10. Weaver JB. Ossification of the internal semilunar cartilage. J Bone Joint Surg. 1935;17(1):195-198.
11. Ahn JH, Lee YS, Chang JY, Chang MJ, Eun SS, Kim SM. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77-80.
12. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate–deficient knee: a review of the literature. Am J Orthop. 1997;26(1):18-23.
13. LaPrade CM, Ellman MB, Rasmussen MT, et al. Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med. 2014;42(10):2386-2392.
14. Brody JM, Hulstyn MJ, Fleming BC, Tung GA. The meniscal roots: Gross anatomic correlation with 3-T MRI findings. AJR Am J Roentgenol. 2007;188(5):W446-W450.
15. Brody JM, Lin HM, Hulstyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-810.
16. Poh S-Y, Yew K-SA, Wong P-LK, et al. Role of the anterior intermeniscal ligament in tibiofemoral contact mechanics during axial joint loading. Knee. 2012;19(2):135-139.
17. Naranje S, Mittal R, Nag H, Sharma R. Arthroscopic and magnetic resonance imaging evaluation of meniscus lesions in the chronic anterior ligament–deficient knee. Arthroscopy. 2008;24(9):1045-1051.
18. Magee T. MR findings of meniscal extrusion correlated with arthroscopy. J Magn Reson Imaging. 2008;28(2):466-470.
19. Kim SB, Ha JK, Lee SW, et al. Medial meniscus root tear refixation: comparison of clinical, radiologic, and arthroscopic findings with medial meniscectomy. Arthroscopy. 2011;27(3):346-354.
20. LaPrade CM, Smith SD, Rasmussen MT, et al. Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 1: the anterior cruciate ligament. Am J Sports Med. 2015;43(1):200-206.
21. Ellman MB, James EW, Laprade CM, Laprade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191.
22. Hwang BY, Kim SJ, Lee SW, et al. Risk factors for medial meniscus posterior root tear. Am J Sports Med. 2012;40(7):1606-1610.
23. Binfield PM, Maffulli N, King JB. Patterns of meniscal tears associated with anterior cruciate ligament lesions in athletes. Injury. 1993;24(8):557-561.
24. Wu WH, Hackett T, Richmond JC. Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med. 2002;30(6):845-850.
25. Pagnani MJ, Cooper DE, Warren RF. Extrusion of the medial meniscus. Arthroscopy. 1991;7(3):297-300.
26. Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33(10):569-574.
27. Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol. 2004;183(1):17-23.
28. LaPrade RF, Ho CP, James E, Crespo B, LaPrade CM, Matheny LM. Diagnostic accuracy of 3.0 T magnetic resonance imaging for the detection of meniscus posterior root pathology. Knee Surg Sports Traumatol Arthroscopy. 2015;23(1):152-157.
29. Chung KS, Ha JK, Yeom CH, et al. Comparison of clinical and radiologic results between partial meniscectomy and refixation of medial mensicus posterior root tears: a minimum 5-year follow-up. Arthroscopy. 2015;31(10):1941-1950.
Intact and well functioning menisci are essential for optimal knee function. Articular cartilage damage and rapid joint degeneration have been observed in knees after meniscectomy.1-5 Meniscal root tears and avulsions are now increasingly recognized as a functional equivalent to total meniscectomy, and will follow a similar course if left untreated.6-8
The menisci provide shock absorption and stability through their unique anatomy and physiology. Their essential role in dissipation of the axial load encountered during daily activities is accomplished via generation of circumferential hoop stress.4,5,9 Tears of the horn or body may diminish this ability depending on the size and location, but a tear or an avulsion that renders the root incompetent will leave the meniscus unable to generate hoop stress.10 Likewise, as the menisci have been shown to be important secondary stabilizers for both translation and rotation, this function is lost or significantly diminished in the setting of a root tear.6,11,12
Despite their clinical and biomechanical implications, meniscal root tears can be difficult to identify, particularly when they are not actively sought. The goal of this article is to highlight the current diagnostic workup and treatment in patients with suspected meniscal root pathology. We will also aim to emphasize important anatomic and biomechanical considerations when attempting a meniscal root repair.
Anatomy
The menisci are 2 fibrocartilage wedge-shaped structures that surround the medial and lateral tibial plateau’s weight-bearing surfaces. They are attached at many points along their periphery via coronary ligaments that comprise a continuous junction of the meniscus to the capsule to the tibial plateau. Each meniscus has an anterior and a posterior horn that are securely anchored to the tibial intercondylar region via strong ligaments known as the roots.
The anterior medial root attaches just anterior and medial to the medial tibial spine. The anterior lateral root attaches just anterior to the lateral tibial spine. The medial and lateral anterior horns of the menisci are also connected via the anterior intermeniscal ligament (AIML).13-15 Recent cadaveric biomechanical studies have questioned the importance of the AIML, demonstrating no significant change in contact pressure or area before and after sectioning.16 Another important consideration with respect to the anterior root insertion of the lateral meniscus is its intimate relationship with the tibial insertion of the anterior cruciate ligament (ACL). The anterior lateral root and the ACL share over 60% of their tibial footprints.13,17
When the menisci are competent, they absorb between 40% to 70% of the contact force generated between the femur and tibia.1 By providing strong anchor points, the meniscal roots allow the horns and bodies of the menisci to maintain a stable position that maximizes congruency with the femoral condyles.
Pathology
The conversion of axial load to circumferential hoop stresses occur as the resilient, yet pliable, menisci are squeezed between the femoral condyle and tibial plateau. However, this function is dependent on secure attachment sites at the roots. In the setting of root tear, there is no restraint to the peripheral distortion of the menisci, and meniscal extrusion can occur.18
Clinical evidence and biomechanical evidence strongly show the consequences of meniscectomy. Multiple studies have shown similar findings and have proven that a meniscal root tear or avulsion is the biomechanical equivalent to total meniscectomy.3 With meniscectomy, not only do peak pressures within compartments increase significantly, it has been demonstrated that other compartments within the knee with intact menisci do not have increases in compartment pressures, lending more evidence to the menisci functioning as separate units.16 It has also been found that anterior/posterior translation is increased with medial meniscal root tears. When lateral meniscus root tears were studied with associated ACL tear, the pivot shift motion was found to be exaggerated.6
However, the finding of utmost importance in these biomechanical studies is that peak pressures and excessive tibiofemoral motion are restored to normal levels after meniscal root repair. Therefore, repair of meniscal root tears restores native knee biomechanics and will potentially prevent arthritic sequelae from developing.3,4,7,19
Epidemiology
Tears of the posterior root of either menisci are more common than their anterior counterparts, and have been more extensively studied. However, there are situations that can lead to anterior root tears, specifically during ACL reconstruction and during medullary nailing of the tibia.20,21 Barring iatrogenic injury, the anterior horn is less at risk for injury than the posterior horn given the biomechanical environment of the knee.3
Medial meniscus posterior root tears are more common than lateral tears. However, these are often more chronic in nature and not associated with an acute event. Risk factors for medial meniscus root tear include increased body mass index, varus mechanical axis, female gender, and low activity level.22
Lateral meniscus root tears more commonly occur during trauma with sprains and/or tears of knee ligaments.23 Along with increased recognition of meniscal root injuries associated with knee ligamentous injury comes the recognition that certain ligamentous reconstructions—namely the ACL—are more prone to failure and have higher stresses when a root tear is left untreated.17,24
Diagnosis
The gold standard for diagnosis of a meniscal root lesion is under direct visualization during arthroscopy.18 The meniscal roots must be probed and stressed to assess their integrity regardless of the initial indication for knee arthroscopy. In most cases, however, the diagnosis of meniscal root tears should occur prior to proceeding to the operating room.
Magnetic resonance imaging (MRI) has been used to aid in diagnosis of meniscal root tears since the early 1990s.25 Now, with the widespread use of MRI, understanding and diagnosis of meniscal root pathology has increased. All sequences should be reviewed, but T2 weighted coronal sections should provide the best visualization of the posterior roots (Figures 1A, 1B). Sagittal sections may also be helpful in this diagnosis. Increased signal within the root or horn may represent partial or full thickness tears, or may show a more degenerative process with fraying.14,15,26,27
MRI does have limitations, however. When compared to arthroscopy, the sensitivity of 3T MRI to identify posterior root tears is 77%, and specificity is 73%. Medial root tears are more readily identified on MRI than lateral tears.28 This further highlights the need for high suspicion during arthroscopy with the requisite equipment on standby should it be needed.
A concerning finding that may be observed on MRI includes meniscal extrusion (Figures 2A, 2B). Most often seen with the medial meniscus, extrusion is diagnosed when the meniscal body displaces greater than 3 mm past the tibial articular surface on a midcoronal image.26,27 Over 50% of patients with medial meniscal extrusion on MRI will have medial meniscal root tears.26,27 Conversely, meniscal extrusion is less common in lateral menisci for multiple reasons. The lateral compartment of the knee does not have as high contact pressure as the medial compartment, so the lateral meniscus is not as likely to be extruded from the joint. Additionally, the posterior lateral root has the added benefit of further stability from meniscofemoral ligaments.11 They provide a restraint to meniscal extrusion, with a reported rate of 14% lateral meniscus extrusion when they are intact. If the meniscofemoral ligaments are not present or torn in the setting of posterior root tear, the lateral meniscus extrusion rate quadruples and approaches that of medial meniscal extrusion.15
Another finding indicative of meniscal root tear is the “ghost meniscus” (Figure 3). The posterior horn and anterior horn should both be visible in sagittal cuts on MRI. When the anterior horn is present, but the posterior horn is not visualized, it is termed a “ghost meniscus.” This MRI finding is highly associated with meniscal root tears, and will often be found along with meniscal extrusion on coronal sequencing.27,28
Treatment
Historically, large meniscal tears, extruded menisci, or root avulsions have been treated with conservative observation if asymptomatic, or with meniscectomy when symptomatic. With a meniscal root tear, both forms of treatment will not provide lasting benefit and rapid joint degeneration ensues. Evidence now supports repair over meniscectomy when treating root tears.7,8,19,29
Patients who have meniscal root tears that are likely sequelae of an arthritic process are not candidates for meniscal root repair. These patients will often have known arthritis with an intact meniscus and then progress to meniscal pathology, most often medially. Because arthritis is the cause of these meniscal tears, a repair will not reverse this process; such repairs will likely fail, and the patient will re-tear the meniscus. For this subset of patients, physical therapy and activity modification are appropriate treatment.
Repair is indicated for patients with acute tears, with or without associated soft tissue injury to the knee, and those with chronic or acute on chronic tears with minimal arthritis within the knee. The authors’ preferred method of repair is via suture fixation through transosseous tunnel (Figures 4A-4F).
Once a root tear has been identified during arthroscopy, it should be probed and/or grasped and pulled to confirm its integrity. A shaver is then used to debride any fraying of the meniscus and to debride the anatomic footprint of the root. Curettes and rasps are used to prepare the meniscal bed at the center of its insertion and the undersurface of the meniscal root. Once the attachment site of the root insertion has been prepared, an ACL tip-to-tip drill guide is placed over the prepared bed. For repair of a medial meniscus posterior root, a 2.4-mm drill tip guide pin is inserted through the guide via an incision made at the anteromedial tibia. For repair of the lateral meniscus posterior root, the pin is inserted through an incision at the anterolateral aspect of the tibia.
Once the guide pin has been inserted and is visualized at the center of the root footprint, it is held in place by a hemostat or grasper placed intra-articularly. Next, the guide pin is overreamed with a 4.5-mm cannulated drill bit. The transosseous tunnel is then further prepared using a shaver to remove excess soft tissue surrounding the tunnel entrance at the tibial plateau. Further rasping around the edges of the tunnel is performed to make final preparations.
Attention is then turned back to the meniscal root. Using a FastPass Scorpion (Arthrex), 2 or 3 size 0 fiber wire sutures are passed through the root, and a cinch stitch is then secured leaving four to six stands (2 from each Scorpion pass) in the root. A FiberStick is then introduced into the tibial bone tunnel and each strand of the 0 fiberwire is retrieved. Once the FiberWire attached to the meniscal root is in the tunnel, the meniscus should be directly visualized as the appropriate tension is toggled to reduce the meniscal root into its footprint. In order to securely fasten the meniscal root, an Arthrex SwiveLock 4.75-mm suture anchor is used. The meniscus is again probed to assess the integrity of the repair. Of note, an alternative method of fixation is accomplished by tying the fiberwire over an Arthrex suture button at the anterior tibia.
Postoperatively, weight bearing restriction is warranted, along with range of motion restrictions. During the first 2 weeks, patients will be counseled to be touch down weight bearing with the use of crutches or a walker. During this period, range of motion will be restricted by hinged knee brace to 30° of flexion and full extension. The next 2-week period will advance to progressive partial weight bearing, again with crutches or a walker. Range of motion will also be expanded to 60° of flexion. After a month, the patient will then be allowed to be full weight bearing as tolerated and be weaned from assistive ambulation devices. Range of motion will then be 90° of flexion. It is paramount that full extension be achieved and maintained in the early postoperative period. Quadriceps strengthening should also proceed with unlimited straight leg raises throughout this period as well.
Intact and well functioning menisci are essential for optimal knee function. Articular cartilage damage and rapid joint degeneration have been observed in knees after meniscectomy.1-5 Meniscal root tears and avulsions are now increasingly recognized as a functional equivalent to total meniscectomy, and will follow a similar course if left untreated.6-8
The menisci provide shock absorption and stability through their unique anatomy and physiology. Their essential role in dissipation of the axial load encountered during daily activities is accomplished via generation of circumferential hoop stress.4,5,9 Tears of the horn or body may diminish this ability depending on the size and location, but a tear or an avulsion that renders the root incompetent will leave the meniscus unable to generate hoop stress.10 Likewise, as the menisci have been shown to be important secondary stabilizers for both translation and rotation, this function is lost or significantly diminished in the setting of a root tear.6,11,12
Despite their clinical and biomechanical implications, meniscal root tears can be difficult to identify, particularly when they are not actively sought. The goal of this article is to highlight the current diagnostic workup and treatment in patients with suspected meniscal root pathology. We will also aim to emphasize important anatomic and biomechanical considerations when attempting a meniscal root repair.
Anatomy
The menisci are 2 fibrocartilage wedge-shaped structures that surround the medial and lateral tibial plateau’s weight-bearing surfaces. They are attached at many points along their periphery via coronary ligaments that comprise a continuous junction of the meniscus to the capsule to the tibial plateau. Each meniscus has an anterior and a posterior horn that are securely anchored to the tibial intercondylar region via strong ligaments known as the roots.
The anterior medial root attaches just anterior and medial to the medial tibial spine. The anterior lateral root attaches just anterior to the lateral tibial spine. The medial and lateral anterior horns of the menisci are also connected via the anterior intermeniscal ligament (AIML).13-15 Recent cadaveric biomechanical studies have questioned the importance of the AIML, demonstrating no significant change in contact pressure or area before and after sectioning.16 Another important consideration with respect to the anterior root insertion of the lateral meniscus is its intimate relationship with the tibial insertion of the anterior cruciate ligament (ACL). The anterior lateral root and the ACL share over 60% of their tibial footprints.13,17
When the menisci are competent, they absorb between 40% to 70% of the contact force generated between the femur and tibia.1 By providing strong anchor points, the meniscal roots allow the horns and bodies of the menisci to maintain a stable position that maximizes congruency with the femoral condyles.
Pathology
The conversion of axial load to circumferential hoop stresses occur as the resilient, yet pliable, menisci are squeezed between the femoral condyle and tibial plateau. However, this function is dependent on secure attachment sites at the roots. In the setting of root tear, there is no restraint to the peripheral distortion of the menisci, and meniscal extrusion can occur.18
Clinical evidence and biomechanical evidence strongly show the consequences of meniscectomy. Multiple studies have shown similar findings and have proven that a meniscal root tear or avulsion is the biomechanical equivalent to total meniscectomy.3 With meniscectomy, not only do peak pressures within compartments increase significantly, it has been demonstrated that other compartments within the knee with intact menisci do not have increases in compartment pressures, lending more evidence to the menisci functioning as separate units.16 It has also been found that anterior/posterior translation is increased with medial meniscal root tears. When lateral meniscus root tears were studied with associated ACL tear, the pivot shift motion was found to be exaggerated.6
However, the finding of utmost importance in these biomechanical studies is that peak pressures and excessive tibiofemoral motion are restored to normal levels after meniscal root repair. Therefore, repair of meniscal root tears restores native knee biomechanics and will potentially prevent arthritic sequelae from developing.3,4,7,19
Epidemiology
Tears of the posterior root of either menisci are more common than their anterior counterparts, and have been more extensively studied. However, there are situations that can lead to anterior root tears, specifically during ACL reconstruction and during medullary nailing of the tibia.20,21 Barring iatrogenic injury, the anterior horn is less at risk for injury than the posterior horn given the biomechanical environment of the knee.3
Medial meniscus posterior root tears are more common than lateral tears. However, these are often more chronic in nature and not associated with an acute event. Risk factors for medial meniscus root tear include increased body mass index, varus mechanical axis, female gender, and low activity level.22
Lateral meniscus root tears more commonly occur during trauma with sprains and/or tears of knee ligaments.23 Along with increased recognition of meniscal root injuries associated with knee ligamentous injury comes the recognition that certain ligamentous reconstructions—namely the ACL—are more prone to failure and have higher stresses when a root tear is left untreated.17,24
Diagnosis
The gold standard for diagnosis of a meniscal root lesion is under direct visualization during arthroscopy.18 The meniscal roots must be probed and stressed to assess their integrity regardless of the initial indication for knee arthroscopy. In most cases, however, the diagnosis of meniscal root tears should occur prior to proceeding to the operating room.
Magnetic resonance imaging (MRI) has been used to aid in diagnosis of meniscal root tears since the early 1990s.25 Now, with the widespread use of MRI, understanding and diagnosis of meniscal root pathology has increased. All sequences should be reviewed, but T2 weighted coronal sections should provide the best visualization of the posterior roots (Figures 1A, 1B). Sagittal sections may also be helpful in this diagnosis. Increased signal within the root or horn may represent partial or full thickness tears, or may show a more degenerative process with fraying.14,15,26,27
MRI does have limitations, however. When compared to arthroscopy, the sensitivity of 3T MRI to identify posterior root tears is 77%, and specificity is 73%. Medial root tears are more readily identified on MRI than lateral tears.28 This further highlights the need for high suspicion during arthroscopy with the requisite equipment on standby should it be needed.
A concerning finding that may be observed on MRI includes meniscal extrusion (Figures 2A, 2B). Most often seen with the medial meniscus, extrusion is diagnosed when the meniscal body displaces greater than 3 mm past the tibial articular surface on a midcoronal image.26,27 Over 50% of patients with medial meniscal extrusion on MRI will have medial meniscal root tears.26,27 Conversely, meniscal extrusion is less common in lateral menisci for multiple reasons. The lateral compartment of the knee does not have as high contact pressure as the medial compartment, so the lateral meniscus is not as likely to be extruded from the joint. Additionally, the posterior lateral root has the added benefit of further stability from meniscofemoral ligaments.11 They provide a restraint to meniscal extrusion, with a reported rate of 14% lateral meniscus extrusion when they are intact. If the meniscofemoral ligaments are not present or torn in the setting of posterior root tear, the lateral meniscus extrusion rate quadruples and approaches that of medial meniscal extrusion.15
Another finding indicative of meniscal root tear is the “ghost meniscus” (Figure 3). The posterior horn and anterior horn should both be visible in sagittal cuts on MRI. When the anterior horn is present, but the posterior horn is not visualized, it is termed a “ghost meniscus.” This MRI finding is highly associated with meniscal root tears, and will often be found along with meniscal extrusion on coronal sequencing.27,28
Treatment
Historically, large meniscal tears, extruded menisci, or root avulsions have been treated with conservative observation if asymptomatic, or with meniscectomy when symptomatic. With a meniscal root tear, both forms of treatment will not provide lasting benefit and rapid joint degeneration ensues. Evidence now supports repair over meniscectomy when treating root tears.7,8,19,29
Patients who have meniscal root tears that are likely sequelae of an arthritic process are not candidates for meniscal root repair. These patients will often have known arthritis with an intact meniscus and then progress to meniscal pathology, most often medially. Because arthritis is the cause of these meniscal tears, a repair will not reverse this process; such repairs will likely fail, and the patient will re-tear the meniscus. For this subset of patients, physical therapy and activity modification are appropriate treatment.
Repair is indicated for patients with acute tears, with or without associated soft tissue injury to the knee, and those with chronic or acute on chronic tears with minimal arthritis within the knee. The authors’ preferred method of repair is via suture fixation through transosseous tunnel (Figures 4A-4F).
Once a root tear has been identified during arthroscopy, it should be probed and/or grasped and pulled to confirm its integrity. A shaver is then used to debride any fraying of the meniscus and to debride the anatomic footprint of the root. Curettes and rasps are used to prepare the meniscal bed at the center of its insertion and the undersurface of the meniscal root. Once the attachment site of the root insertion has been prepared, an ACL tip-to-tip drill guide is placed over the prepared bed. For repair of a medial meniscus posterior root, a 2.4-mm drill tip guide pin is inserted through the guide via an incision made at the anteromedial tibia. For repair of the lateral meniscus posterior root, the pin is inserted through an incision at the anterolateral aspect of the tibia.
Once the guide pin has been inserted and is visualized at the center of the root footprint, it is held in place by a hemostat or grasper placed intra-articularly. Next, the guide pin is overreamed with a 4.5-mm cannulated drill bit. The transosseous tunnel is then further prepared using a shaver to remove excess soft tissue surrounding the tunnel entrance at the tibial plateau. Further rasping around the edges of the tunnel is performed to make final preparations.
Attention is then turned back to the meniscal root. Using a FastPass Scorpion (Arthrex), 2 or 3 size 0 fiber wire sutures are passed through the root, and a cinch stitch is then secured leaving four to six stands (2 from each Scorpion pass) in the root. A FiberStick is then introduced into the tibial bone tunnel and each strand of the 0 fiberwire is retrieved. Once the FiberWire attached to the meniscal root is in the tunnel, the meniscus should be directly visualized as the appropriate tension is toggled to reduce the meniscal root into its footprint. In order to securely fasten the meniscal root, an Arthrex SwiveLock 4.75-mm suture anchor is used. The meniscus is again probed to assess the integrity of the repair. Of note, an alternative method of fixation is accomplished by tying the fiberwire over an Arthrex suture button at the anterior tibia.
Postoperatively, weight bearing restriction is warranted, along with range of motion restrictions. During the first 2 weeks, patients will be counseled to be touch down weight bearing with the use of crutches or a walker. During this period, range of motion will be restricted by hinged knee brace to 30° of flexion and full extension. The next 2-week period will advance to progressive partial weight bearing, again with crutches or a walker. Range of motion will also be expanded to 60° of flexion. After a month, the patient will then be allowed to be full weight bearing as tolerated and be weaned from assistive ambulation devices. Range of motion will then be 90° of flexion. It is paramount that full extension be achieved and maintained in the early postoperative period. Quadriceps strengthening should also proceed with unlimited straight leg raises throughout this period as well.
1. Kidron A, Thein R. Radial tears associated with cleavage tears of the medial meniscus in athletes. Arthroscopy. 2002;18(3):254-256.
2. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664-670.
3. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Joint Surg. 2008;90(9):1922-1931.
4. Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124-129.
5. Hein CN, Deperio JG, Ehrensberger MT, Marzo JM. Effects of medial meniscal posterior horn avulsion and repair on meniscal displacement. Knee. 2011;18(3):189-192.
6. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43(4):905-911.
7. Vyas D, Harner CD. Meniscus root repair. Sports Med Arthrosc Rev. 2012;20(2):86-94.
8. Koenig JH, Ranawat AS, Umans HR, Difelice GS. Meniscal root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025-1032.
9. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990;(252):19-31.
10. Weaver JB. Ossification of the internal semilunar cartilage. J Bone Joint Surg. 1935;17(1):195-198.
11. Ahn JH, Lee YS, Chang JY, Chang MJ, Eun SS, Kim SM. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77-80.
12. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate–deficient knee: a review of the literature. Am J Orthop. 1997;26(1):18-23.
13. LaPrade CM, Ellman MB, Rasmussen MT, et al. Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med. 2014;42(10):2386-2392.
14. Brody JM, Hulstyn MJ, Fleming BC, Tung GA. The meniscal roots: Gross anatomic correlation with 3-T MRI findings. AJR Am J Roentgenol. 2007;188(5):W446-W450.
15. Brody JM, Lin HM, Hulstyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-810.
16. Poh S-Y, Yew K-SA, Wong P-LK, et al. Role of the anterior intermeniscal ligament in tibiofemoral contact mechanics during axial joint loading. Knee. 2012;19(2):135-139.
17. Naranje S, Mittal R, Nag H, Sharma R. Arthroscopic and magnetic resonance imaging evaluation of meniscus lesions in the chronic anterior ligament–deficient knee. Arthroscopy. 2008;24(9):1045-1051.
18. Magee T. MR findings of meniscal extrusion correlated with arthroscopy. J Magn Reson Imaging. 2008;28(2):466-470.
19. Kim SB, Ha JK, Lee SW, et al. Medial meniscus root tear refixation: comparison of clinical, radiologic, and arthroscopic findings with medial meniscectomy. Arthroscopy. 2011;27(3):346-354.
20. LaPrade CM, Smith SD, Rasmussen MT, et al. Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 1: the anterior cruciate ligament. Am J Sports Med. 2015;43(1):200-206.
21. Ellman MB, James EW, Laprade CM, Laprade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191.
22. Hwang BY, Kim SJ, Lee SW, et al. Risk factors for medial meniscus posterior root tear. Am J Sports Med. 2012;40(7):1606-1610.
23. Binfield PM, Maffulli N, King JB. Patterns of meniscal tears associated with anterior cruciate ligament lesions in athletes. Injury. 1993;24(8):557-561.
24. Wu WH, Hackett T, Richmond JC. Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med. 2002;30(6):845-850.
25. Pagnani MJ, Cooper DE, Warren RF. Extrusion of the medial meniscus. Arthroscopy. 1991;7(3):297-300.
26. Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33(10):569-574.
27. Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol. 2004;183(1):17-23.
28. LaPrade RF, Ho CP, James E, Crespo B, LaPrade CM, Matheny LM. Diagnostic accuracy of 3.0 T magnetic resonance imaging for the detection of meniscus posterior root pathology. Knee Surg Sports Traumatol Arthroscopy. 2015;23(1):152-157.
29. Chung KS, Ha JK, Yeom CH, et al. Comparison of clinical and radiologic results between partial meniscectomy and refixation of medial mensicus posterior root tears: a minimum 5-year follow-up. Arthroscopy. 2015;31(10):1941-1950.
1. Kidron A, Thein R. Radial tears associated with cleavage tears of the medial meniscus in athletes. Arthroscopy. 2002;18(3):254-256.
2. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664-670.
3. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Joint Surg. 2008;90(9):1922-1931.
4. Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124-129.
5. Hein CN, Deperio JG, Ehrensberger MT, Marzo JM. Effects of medial meniscal posterior horn avulsion and repair on meniscal displacement. Knee. 2011;18(3):189-192.
6. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43(4):905-911.
7. Vyas D, Harner CD. Meniscus root repair. Sports Med Arthrosc Rev. 2012;20(2):86-94.
8. Koenig JH, Ranawat AS, Umans HR, Difelice GS. Meniscal root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025-1032.
9. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990;(252):19-31.
10. Weaver JB. Ossification of the internal semilunar cartilage. J Bone Joint Surg. 1935;17(1):195-198.
11. Ahn JH, Lee YS, Chang JY, Chang MJ, Eun SS, Kim SM. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77-80.
12. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate–deficient knee: a review of the literature. Am J Orthop. 1997;26(1):18-23.
13. LaPrade CM, Ellman MB, Rasmussen MT, et al. Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med. 2014;42(10):2386-2392.
14. Brody JM, Hulstyn MJ, Fleming BC, Tung GA. The meniscal roots: Gross anatomic correlation with 3-T MRI findings. AJR Am J Roentgenol. 2007;188(5):W446-W450.
15. Brody JM, Lin HM, Hulstyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-810.
16. Poh S-Y, Yew K-SA, Wong P-LK, et al. Role of the anterior intermeniscal ligament in tibiofemoral contact mechanics during axial joint loading. Knee. 2012;19(2):135-139.
17. Naranje S, Mittal R, Nag H, Sharma R. Arthroscopic and magnetic resonance imaging evaluation of meniscus lesions in the chronic anterior ligament–deficient knee. Arthroscopy. 2008;24(9):1045-1051.
18. Magee T. MR findings of meniscal extrusion correlated with arthroscopy. J Magn Reson Imaging. 2008;28(2):466-470.
19. Kim SB, Ha JK, Lee SW, et al. Medial meniscus root tear refixation: comparison of clinical, radiologic, and arthroscopic findings with medial meniscectomy. Arthroscopy. 2011;27(3):346-354.
20. LaPrade CM, Smith SD, Rasmussen MT, et al. Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 1: the anterior cruciate ligament. Am J Sports Med. 2015;43(1):200-206.
21. Ellman MB, James EW, Laprade CM, Laprade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191.
22. Hwang BY, Kim SJ, Lee SW, et al. Risk factors for medial meniscus posterior root tear. Am J Sports Med. 2012;40(7):1606-1610.
23. Binfield PM, Maffulli N, King JB. Patterns of meniscal tears associated with anterior cruciate ligament lesions in athletes. Injury. 1993;24(8):557-561.
24. Wu WH, Hackett T, Richmond JC. Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med. 2002;30(6):845-850.
25. Pagnani MJ, Cooper DE, Warren RF. Extrusion of the medial meniscus. Arthroscopy. 1991;7(3):297-300.
26. Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33(10):569-574.
27. Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol. 2004;183(1):17-23.
28. LaPrade RF, Ho CP, James E, Crespo B, LaPrade CM, Matheny LM. Diagnostic accuracy of 3.0 T magnetic resonance imaging for the detection of meniscus posterior root pathology. Knee Surg Sports Traumatol Arthroscopy. 2015;23(1):152-157.
29. Chung KS, Ha JK, Yeom CH, et al. Comparison of clinical and radiologic results between partial meniscectomy and refixation of medial mensicus posterior root tears: a minimum 5-year follow-up. Arthroscopy. 2015;31(10):1941-1950.
A Guide to Ultrasound of the Shoulder, Part 1: Coding and Reimbursement
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
Arthroscopic Management of Full-Thickness Rotator Cuff Tears in Major League Baseball Pitchers: The Lateralized Footprint Repair Technique
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Ulnar Collateral Ligament Repair: An Old Idea With a New Wrinkle
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].