User login
Clinical Guidelines Hub only
Guidelines for children’s bronchiolitis treatment issued by AAP
The main treatment for bronchiolitis in young children should be support and observation, according to new clinical practice guidelines for diagnosing, managing, and preventing bronchiolitis.
The guidelines apply to children aged 1-23 months and emphasize clinical diagnosis and no medications except nebulized hypertonic saline for infants hospitalized with bronchiolitis, wrote Dr. Shawn L. Ralston, Dr. Allan S. Lieberthal, and their associates (Pediatrics 2014 October 27 [doi:10.1542/peds.2014-2742]). These guidelines update and replace the ones issued by the American Academy of Pediatrics in 2006 (Pediatrics 2006 118:1774-93). The findings are based on a review of the evidence in the Cochrane Library, Medline, and the Cumulative Index of Nursing and Allied Health Literature (CINAHL) from 2004 through May 2014.
The most notable change to these updated guidelines, according to Dr. Lieberthal, is the preventive recommendation for palivizumab, which is now not indicated for children born at 29 weeks’ gestation or older unless they have hemodynamically significant heart disease or chronic lung disease of prematurity (those born at less than 32 weeks’ gestation who needed at least 21% oxygen for their first month). Infants who qualify for prophylactic palivizumab should receive five monthly doses during respiratory syncytial virus season.
Dr. Lieberthal noted in an interview that several other recommendations state that certain treatments should not be used at all rather than simply not being routinely used. These include albuterol, epinephrine, corticosteroids, chest physiotherapy, and antibiotics.
“Bronchiolitis is a self-limited viral illness,” he said. Because it is diagnosed by signs and symptoms, no lab tests, oximetry, imaging, or other tests are needed, and treatment involves only support and observation. “None of the treatments that have been tested have been shown to affect the outcome of the illness,” said Dr. Lieberthal, who practices general pediatrics and clinical pediatric pulmonology at Kaiser-Permanente in Panorama City, Calif.
Dr. Ralston noted in an interview that a new recommendation exists for using hypertonic saline to children who are hospitalized for bronchiolitis (although not in the emergency department), but the evidence for it is weak and its therapeutic value limited.
“This medication appears to have a slow onset and to provide a favorable response only in settings where patients are hospitalized for longer than is typical in most U.S. hospitals, as most of the studies were performed outside the U.S.,” said Dr. Ralston, a pediatrician at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
The guidelines also note that clinicians “may choose not to administer supplemental oxygen if the oxyhemoglobin saturation exceeds 90%” in children, although the evidence for this recommendation is also weak. Children should receive nasogastric or intravenous fluids if they cannot maintain oral hydration.
Parents should be advised that children who avoid secondhand tobacco smoke and are exclusively breastfed for at least 6 months have a reduced risk of bronchiolitis. Further, anyone caring for a child with bronchiolitis should disinfect their hands using an alcohol-based rub or soap and water after direct contact with the child and the child’s immediate environment.
Dr. Ralston said that important points stressed in both this recommendation and in the previous one include clinical diagnosis and avoiding exposure to tobacco smoke to reduce children’s risk of bronchiolitis.
“This guideline is mostly about what you shouldn’t do for the disease since because of the high volume of disease bronchiolitis represents a major area of unnecessary medical intervention in children,” she said. “We know that the vast majority of children will suffer only side effects from the medications or testing typically used in bronchiolitis care.”
Funding was provided by the American Academy of Pediatrics with travel support from the American Academy of Family Physicians, the American College of Chest Physicians, the American Thoracic Society, and the American College of Emergency Physicians for their representatives.
These guidelines, written with clarity, give incredibly direct and helpful direction on the diagnosis and treatment of bronchiolitis. It is great that they are coming out now, prior to RSV season. Bronchiolitis is a clinical diagnosis and these guidelines reaffirm that there is not usually any need for x-ray or laboratory confirmation of the diagnosis. The guidelines are primarily important for clarifying, based on the evidence, that many commonly used treatments, including albuterol, epinephrine, and steroids are not recommended for treatment of bronchiolitis as they are simply not helpful.
The guidance on administration of palivizumab is also important. It should not be administered in infants with a gestational age of > 29 weeks, and it should be reserved for infants in the first year of life who had a gestational age < 32 weeks and who had hemodynamically significant heart disease or chronic lung disease of prematurity.
Neil Skolnik, M.D., is the associate director of the family medicine program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
These guidelines, written with clarity, give incredibly direct and helpful direction on the diagnosis and treatment of bronchiolitis. It is great that they are coming out now, prior to RSV season. Bronchiolitis is a clinical diagnosis and these guidelines reaffirm that there is not usually any need for x-ray or laboratory confirmation of the diagnosis. The guidelines are primarily important for clarifying, based on the evidence, that many commonly used treatments, including albuterol, epinephrine, and steroids are not recommended for treatment of bronchiolitis as they are simply not helpful.
The guidance on administration of palivizumab is also important. It should not be administered in infants with a gestational age of > 29 weeks, and it should be reserved for infants in the first year of life who had a gestational age < 32 weeks and who had hemodynamically significant heart disease or chronic lung disease of prematurity.
Neil Skolnik, M.D., is the associate director of the family medicine program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
These guidelines, written with clarity, give incredibly direct and helpful direction on the diagnosis and treatment of bronchiolitis. It is great that they are coming out now, prior to RSV season. Bronchiolitis is a clinical diagnosis and these guidelines reaffirm that there is not usually any need for x-ray or laboratory confirmation of the diagnosis. The guidelines are primarily important for clarifying, based on the evidence, that many commonly used treatments, including albuterol, epinephrine, and steroids are not recommended for treatment of bronchiolitis as they are simply not helpful.
The guidance on administration of palivizumab is also important. It should not be administered in infants with a gestational age of > 29 weeks, and it should be reserved for infants in the first year of life who had a gestational age < 32 weeks and who had hemodynamically significant heart disease or chronic lung disease of prematurity.
Neil Skolnik, M.D., is the associate director of the family medicine program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
The main treatment for bronchiolitis in young children should be support and observation, according to new clinical practice guidelines for diagnosing, managing, and preventing bronchiolitis.
The guidelines apply to children aged 1-23 months and emphasize clinical diagnosis and no medications except nebulized hypertonic saline for infants hospitalized with bronchiolitis, wrote Dr. Shawn L. Ralston, Dr. Allan S. Lieberthal, and their associates (Pediatrics 2014 October 27 [doi:10.1542/peds.2014-2742]). These guidelines update and replace the ones issued by the American Academy of Pediatrics in 2006 (Pediatrics 2006 118:1774-93). The findings are based on a review of the evidence in the Cochrane Library, Medline, and the Cumulative Index of Nursing and Allied Health Literature (CINAHL) from 2004 through May 2014.
The most notable change to these updated guidelines, according to Dr. Lieberthal, is the preventive recommendation for palivizumab, which is now not indicated for children born at 29 weeks’ gestation or older unless they have hemodynamically significant heart disease or chronic lung disease of prematurity (those born at less than 32 weeks’ gestation who needed at least 21% oxygen for their first month). Infants who qualify for prophylactic palivizumab should receive five monthly doses during respiratory syncytial virus season.
Dr. Lieberthal noted in an interview that several other recommendations state that certain treatments should not be used at all rather than simply not being routinely used. These include albuterol, epinephrine, corticosteroids, chest physiotherapy, and antibiotics.
“Bronchiolitis is a self-limited viral illness,” he said. Because it is diagnosed by signs and symptoms, no lab tests, oximetry, imaging, or other tests are needed, and treatment involves only support and observation. “None of the treatments that have been tested have been shown to affect the outcome of the illness,” said Dr. Lieberthal, who practices general pediatrics and clinical pediatric pulmonology at Kaiser-Permanente in Panorama City, Calif.
Dr. Ralston noted in an interview that a new recommendation exists for using hypertonic saline to children who are hospitalized for bronchiolitis (although not in the emergency department), but the evidence for it is weak and its therapeutic value limited.
“This medication appears to have a slow onset and to provide a favorable response only in settings where patients are hospitalized for longer than is typical in most U.S. hospitals, as most of the studies were performed outside the U.S.,” said Dr. Ralston, a pediatrician at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
The guidelines also note that clinicians “may choose not to administer supplemental oxygen if the oxyhemoglobin saturation exceeds 90%” in children, although the evidence for this recommendation is also weak. Children should receive nasogastric or intravenous fluids if they cannot maintain oral hydration.
Parents should be advised that children who avoid secondhand tobacco smoke and are exclusively breastfed for at least 6 months have a reduced risk of bronchiolitis. Further, anyone caring for a child with bronchiolitis should disinfect their hands using an alcohol-based rub or soap and water after direct contact with the child and the child’s immediate environment.
Dr. Ralston said that important points stressed in both this recommendation and in the previous one include clinical diagnosis and avoiding exposure to tobacco smoke to reduce children’s risk of bronchiolitis.
“This guideline is mostly about what you shouldn’t do for the disease since because of the high volume of disease bronchiolitis represents a major area of unnecessary medical intervention in children,” she said. “We know that the vast majority of children will suffer only side effects from the medications or testing typically used in bronchiolitis care.”
Funding was provided by the American Academy of Pediatrics with travel support from the American Academy of Family Physicians, the American College of Chest Physicians, the American Thoracic Society, and the American College of Emergency Physicians for their representatives.
The main treatment for bronchiolitis in young children should be support and observation, according to new clinical practice guidelines for diagnosing, managing, and preventing bronchiolitis.
The guidelines apply to children aged 1-23 months and emphasize clinical diagnosis and no medications except nebulized hypertonic saline for infants hospitalized with bronchiolitis, wrote Dr. Shawn L. Ralston, Dr. Allan S. Lieberthal, and their associates (Pediatrics 2014 October 27 [doi:10.1542/peds.2014-2742]). These guidelines update and replace the ones issued by the American Academy of Pediatrics in 2006 (Pediatrics 2006 118:1774-93). The findings are based on a review of the evidence in the Cochrane Library, Medline, and the Cumulative Index of Nursing and Allied Health Literature (CINAHL) from 2004 through May 2014.
The most notable change to these updated guidelines, according to Dr. Lieberthal, is the preventive recommendation for palivizumab, which is now not indicated for children born at 29 weeks’ gestation or older unless they have hemodynamically significant heart disease or chronic lung disease of prematurity (those born at less than 32 weeks’ gestation who needed at least 21% oxygen for their first month). Infants who qualify for prophylactic palivizumab should receive five monthly doses during respiratory syncytial virus season.
Dr. Lieberthal noted in an interview that several other recommendations state that certain treatments should not be used at all rather than simply not being routinely used. These include albuterol, epinephrine, corticosteroids, chest physiotherapy, and antibiotics.
“Bronchiolitis is a self-limited viral illness,” he said. Because it is diagnosed by signs and symptoms, no lab tests, oximetry, imaging, or other tests are needed, and treatment involves only support and observation. “None of the treatments that have been tested have been shown to affect the outcome of the illness,” said Dr. Lieberthal, who practices general pediatrics and clinical pediatric pulmonology at Kaiser-Permanente in Panorama City, Calif.
Dr. Ralston noted in an interview that a new recommendation exists for using hypertonic saline to children who are hospitalized for bronchiolitis (although not in the emergency department), but the evidence for it is weak and its therapeutic value limited.
“This medication appears to have a slow onset and to provide a favorable response only in settings where patients are hospitalized for longer than is typical in most U.S. hospitals, as most of the studies were performed outside the U.S.,” said Dr. Ralston, a pediatrician at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
The guidelines also note that clinicians “may choose not to administer supplemental oxygen if the oxyhemoglobin saturation exceeds 90%” in children, although the evidence for this recommendation is also weak. Children should receive nasogastric or intravenous fluids if they cannot maintain oral hydration.
Parents should be advised that children who avoid secondhand tobacco smoke and are exclusively breastfed for at least 6 months have a reduced risk of bronchiolitis. Further, anyone caring for a child with bronchiolitis should disinfect their hands using an alcohol-based rub or soap and water after direct contact with the child and the child’s immediate environment.
Dr. Ralston said that important points stressed in both this recommendation and in the previous one include clinical diagnosis and avoiding exposure to tobacco smoke to reduce children’s risk of bronchiolitis.
“This guideline is mostly about what you shouldn’t do for the disease since because of the high volume of disease bronchiolitis represents a major area of unnecessary medical intervention in children,” she said. “We know that the vast majority of children will suffer only side effects from the medications or testing typically used in bronchiolitis care.”
Funding was provided by the American Academy of Pediatrics with travel support from the American Academy of Family Physicians, the American College of Chest Physicians, the American Thoracic Society, and the American College of Emergency Physicians for their representatives.
FROM PEDIATRICS
Key clinical point: Bronchiolitis should be diagnosed clinically and treated with support.
Major finding: Most treatments should not be administered because outcomes are not improved.
Data source: The findings are based on a review of the evidence in the Cochrane Library, Medline, and CINAHL from 2004 through May 2014.
Disclosures: Funding was provided by the American Academy of Pediatrics with travel support from the American Academy of Family Physicians, the American College of Chest Physicians, the American Thoracic Society, and the American College of Emergency Physicians for their representatives.
Positive CvLPRIT results lead ACC to change guidelines
BARCELONA – Heart attack patients who had complete revascularization of all blocked arteries had better outcomes than those who had only the “culprit” artery unblocked, according to results from the CvLPRIT (Complete Versus Lesion-Only Primary PCI Trial) study.
The open label, randomized trial showed that among patients with acute ST-segment elevation myocardial infarction (STEMI), those who had stenting of significant coronary stenoses not responsible for the infarction as well as the infarct-producing lesion had a 55% reduction in major adverse cardiac events (MACE) at 1 year, compared with the group that had only the infarct-related artery treated. The results were presented at the annual congress of the European Society of Cardiology.
The positive results mirror the results of the PRAMI trial presented at last year’s ESC annual congress, and seem to be the tipping point for the American College of Cardiology to withdraw one of its Choosing Wisely recommendations, which had questioned any intervention beyond unblocking just the artery responsible for the heart attack.
“The newest findings regarding coronary revascularization are great examples of science on the move, and we are responding accordingly,” wrote ACC President Patrick T. O’Gara in a statement issued on Sept. 22, not too long after the results of CvLPRIT were presented.
Dr. Anthony Gershlick, who presented the results of CvLPRIT at ESC, also concluded that “this strategy may be needed to be considered for future STEMI guidelines committees.”
But the topic remains controversial, and not all experts agree that it’s time for a change in clinical practice.
Dr. Shamir R. Mehta of McMaster University in Hamilton, Ont., said that both the CvLPRIT and PRAMI trials are still relatively small to measure up to the results of large meta-analyses, which show that revascularization of nonculprit arteries at the time of primary percutaneous coronary intervention (PCI) could be associated with higher mortality rates.
“The important question is, was there a significant hazard with doing revascularization at a later time point, and unfortunately this trial was too small to answer that question,” Dr. Mehta said at ESC. Dr. Gershlick, of University Hospitals of Leicester NHS Trust in England, disagreed.
“One question for me was, if a clinician is presented with angiographically significant stenoses in a non–infarct-related artery, should these be treated on that admission?” said Dr. Gershlick in a press conference. He said although retrospective registry data suggest otherwise, the results of PRAMI showed a 65% reduction in MACE with total revascularization at the time of primary PCI.
For CvLPRIT, he and his colleagues randomized 296 heart attack patients to receive either revascularization of only the infarct-related artery (146 patients), or have complete revascularization at the time of primary PCI.
The primary endpoint was MACE, which is a composite of total mortality, recurrent myocardial infarction (MI), heart failure, and ischemia-driven revascularization at 12 months.
Patients were on average 65 years old and mostly male. More than 80% had stenoses of a non–infarct-related artery, and more than 70% were treated via the radial approach.
In the complete revascularization group, the non–infarct-related arteries were treated after the infarct-related artery during the same sitting or during the same hospital admission.
At 12 months, there was a 55% reduction in MACE among patients who had complete revascularization. All components of the composite endpoint also showed a decrease, although they didn’t reach significance, compared with the group that received stenting of only the infarct-related artery.
There also was a reduction in all-cause mortality, recurrent MI, heart failure, and repeat revascularization in the complete revascularization group.
In addition, there were no safety signals, Dr. Gershlick said.
The study had several limitations, including its small size, combined endpoint, and loss to follow-up.
Experts agreed that there’s a need for larger randomized trials, such as the COMPLETE trial, which is currently enrolling patients.
Dr. Gershlick and Dr. Mehta had no disclosures.
On Twitter @naseemmiller
BARCELONA – Heart attack patients who had complete revascularization of all blocked arteries had better outcomes than those who had only the “culprit” artery unblocked, according to results from the CvLPRIT (Complete Versus Lesion-Only Primary PCI Trial) study.
The open label, randomized trial showed that among patients with acute ST-segment elevation myocardial infarction (STEMI), those who had stenting of significant coronary stenoses not responsible for the infarction as well as the infarct-producing lesion had a 55% reduction in major adverse cardiac events (MACE) at 1 year, compared with the group that had only the infarct-related artery treated. The results were presented at the annual congress of the European Society of Cardiology.
The positive results mirror the results of the PRAMI trial presented at last year’s ESC annual congress, and seem to be the tipping point for the American College of Cardiology to withdraw one of its Choosing Wisely recommendations, which had questioned any intervention beyond unblocking just the artery responsible for the heart attack.
“The newest findings regarding coronary revascularization are great examples of science on the move, and we are responding accordingly,” wrote ACC President Patrick T. O’Gara in a statement issued on Sept. 22, not too long after the results of CvLPRIT were presented.
Dr. Anthony Gershlick, who presented the results of CvLPRIT at ESC, also concluded that “this strategy may be needed to be considered for future STEMI guidelines committees.”
But the topic remains controversial, and not all experts agree that it’s time for a change in clinical practice.
Dr. Shamir R. Mehta of McMaster University in Hamilton, Ont., said that both the CvLPRIT and PRAMI trials are still relatively small to measure up to the results of large meta-analyses, which show that revascularization of nonculprit arteries at the time of primary percutaneous coronary intervention (PCI) could be associated with higher mortality rates.
“The important question is, was there a significant hazard with doing revascularization at a later time point, and unfortunately this trial was too small to answer that question,” Dr. Mehta said at ESC. Dr. Gershlick, of University Hospitals of Leicester NHS Trust in England, disagreed.
“One question for me was, if a clinician is presented with angiographically significant stenoses in a non–infarct-related artery, should these be treated on that admission?” said Dr. Gershlick in a press conference. He said although retrospective registry data suggest otherwise, the results of PRAMI showed a 65% reduction in MACE with total revascularization at the time of primary PCI.
For CvLPRIT, he and his colleagues randomized 296 heart attack patients to receive either revascularization of only the infarct-related artery (146 patients), or have complete revascularization at the time of primary PCI.
The primary endpoint was MACE, which is a composite of total mortality, recurrent myocardial infarction (MI), heart failure, and ischemia-driven revascularization at 12 months.
Patients were on average 65 years old and mostly male. More than 80% had stenoses of a non–infarct-related artery, and more than 70% were treated via the radial approach.
In the complete revascularization group, the non–infarct-related arteries were treated after the infarct-related artery during the same sitting or during the same hospital admission.
At 12 months, there was a 55% reduction in MACE among patients who had complete revascularization. All components of the composite endpoint also showed a decrease, although they didn’t reach significance, compared with the group that received stenting of only the infarct-related artery.
There also was a reduction in all-cause mortality, recurrent MI, heart failure, and repeat revascularization in the complete revascularization group.
In addition, there were no safety signals, Dr. Gershlick said.
The study had several limitations, including its small size, combined endpoint, and loss to follow-up.
Experts agreed that there’s a need for larger randomized trials, such as the COMPLETE trial, which is currently enrolling patients.
Dr. Gershlick and Dr. Mehta had no disclosures.
On Twitter @naseemmiller
BARCELONA – Heart attack patients who had complete revascularization of all blocked arteries had better outcomes than those who had only the “culprit” artery unblocked, according to results from the CvLPRIT (Complete Versus Lesion-Only Primary PCI Trial) study.
The open label, randomized trial showed that among patients with acute ST-segment elevation myocardial infarction (STEMI), those who had stenting of significant coronary stenoses not responsible for the infarction as well as the infarct-producing lesion had a 55% reduction in major adverse cardiac events (MACE) at 1 year, compared with the group that had only the infarct-related artery treated. The results were presented at the annual congress of the European Society of Cardiology.
The positive results mirror the results of the PRAMI trial presented at last year’s ESC annual congress, and seem to be the tipping point for the American College of Cardiology to withdraw one of its Choosing Wisely recommendations, which had questioned any intervention beyond unblocking just the artery responsible for the heart attack.
“The newest findings regarding coronary revascularization are great examples of science on the move, and we are responding accordingly,” wrote ACC President Patrick T. O’Gara in a statement issued on Sept. 22, not too long after the results of CvLPRIT were presented.
Dr. Anthony Gershlick, who presented the results of CvLPRIT at ESC, also concluded that “this strategy may be needed to be considered for future STEMI guidelines committees.”
But the topic remains controversial, and not all experts agree that it’s time for a change in clinical practice.
Dr. Shamir R. Mehta of McMaster University in Hamilton, Ont., said that both the CvLPRIT and PRAMI trials are still relatively small to measure up to the results of large meta-analyses, which show that revascularization of nonculprit arteries at the time of primary percutaneous coronary intervention (PCI) could be associated with higher mortality rates.
“The important question is, was there a significant hazard with doing revascularization at a later time point, and unfortunately this trial was too small to answer that question,” Dr. Mehta said at ESC. Dr. Gershlick, of University Hospitals of Leicester NHS Trust in England, disagreed.
“One question for me was, if a clinician is presented with angiographically significant stenoses in a non–infarct-related artery, should these be treated on that admission?” said Dr. Gershlick in a press conference. He said although retrospective registry data suggest otherwise, the results of PRAMI showed a 65% reduction in MACE with total revascularization at the time of primary PCI.
For CvLPRIT, he and his colleagues randomized 296 heart attack patients to receive either revascularization of only the infarct-related artery (146 patients), or have complete revascularization at the time of primary PCI.
The primary endpoint was MACE, which is a composite of total mortality, recurrent myocardial infarction (MI), heart failure, and ischemia-driven revascularization at 12 months.
Patients were on average 65 years old and mostly male. More than 80% had stenoses of a non–infarct-related artery, and more than 70% were treated via the radial approach.
In the complete revascularization group, the non–infarct-related arteries were treated after the infarct-related artery during the same sitting or during the same hospital admission.
At 12 months, there was a 55% reduction in MACE among patients who had complete revascularization. All components of the composite endpoint also showed a decrease, although they didn’t reach significance, compared with the group that received stenting of only the infarct-related artery.
There also was a reduction in all-cause mortality, recurrent MI, heart failure, and repeat revascularization in the complete revascularization group.
In addition, there were no safety signals, Dr. Gershlick said.
The study had several limitations, including its small size, combined endpoint, and loss to follow-up.
Experts agreed that there’s a need for larger randomized trials, such as the COMPLETE trial, which is currently enrolling patients.
Dr. Gershlick and Dr. Mehta had no disclosures.
On Twitter @naseemmiller
AT THE ESC CONGRESS 2014
Key clinical point: Complete revascularization at the time of primary PCI may be considered by future STEMI guidelines committees.
Major finding: There was a 55% reduction in MACE among heart attack patients who received complete revascularization at the time of primary PCI.
Data source: An open-label, randomized trial of 296 heart attack patients.
Disclosures: Dr. Gershlick and Dr. Mehta had no disclosures.
Mitral valve guidelines stress early intervention at experienced centers
CHICAGO – Early repair and greater reliance on experienced surgical centers are key to the new guidelines on the management of mitral valve disease.
It’s been 8 years since the last American Heart Association/American College of Cardiology guideline on valvular heart disease in 2006, with little change in the 2008 update.
The 2014 guidelines, however, have substantiative changes, including the decision to begin talking about valvular disease and at-risk patients much as we do for heart failure, guideline committee member Robert Bonow said at the Heart Valve Summit 2014.
The 2014 guidelines, published earlier this year, include four stages of valvular heart disease:
Stage A, for people at risk of valvular disease such as those with bicuspid valves, a history of rheumatic heart disease, or mitral valve prolapse without regurgitation.
Stage B, for mild to moderate, asymptomatic disease.
Stage C, for severe, asymptomatic disease, including those with normal left ventricular function (stage C1) or depressed LV function (stage C2).
Stage D, for severe, symptomatic valve disease.
The new guidelines also drive home the point that primary and secondary mitral regurgitation (MR), while they can be difficult to distinguish, are separate diseases with different pathophysiologies, natural histories, management strategies, and outcomes, said Dr. Bonow, director of the Center for Cardiovascular Innovation, Northwestern University, Chicago.
Class 1 surgical indications for primary MR, or diseases of the valve, are symptomatic patients and asymptomatic patients with LV systolic dysfunction. This continues to be defined as an ejection fraction of < 60% or an end-systolic dimension > 40 mm, although new data have suggested that even smaller systolic dimensions may have prognostic importance, he noted.
Pulmonary hypertension and atrial fibrillation are class IIa indications for surgery in asymptomatic, primary MR.
Critics would argue that patients shouldn’t be allowed to develop these indications because they may be irreversible, but the reality is that many patients arrive in your office with one or more indications already in place, Dr. Bonow said. The real issue is whether mitral valve repair is feasible and can improve survival in patients who have normal LV function and none of these indications, with the guidelines clearly tipping in favor of early surgery for asymptomatic MR patients.
Dr. Bonow highlighted recent long-term outcomes data from Dr. Tirone David’s group (Circulation 2013;127:1485-92) showing that overall survival among patients undergoing mitral valve repair for degenerative diseases is 75% at 20 years for those with functional class (FC) I disease, 66% with FC II, 52% with FC III, and only 32% for those with FC IV.
“I think these data, along with many other series, are quite important in identifying the risks we have for our patients for waiting too long, and if we can refer our patients to an expert surgical team for these valves to be repaired, their outcomes will be much better,” Dr. Bonow said.
The guidelines include the class I indication that repair is better than mitral valve replacement for primary MR and that patients should be referred to “centers experienced in repair.” Instead of stating that there should be a 90% or greater likelihood of a durable repair without residual MR for a patient undergoing elective surgery at that center, the 2014 threshold is now set at more than 95%.
“We really want to make sure patients are going to an experienced center,” he said.
Despite the emphasis on a heart team approach and referral to experienced centers, the term “experienced” has not been fully defined, Dr. Bonow acknowledged.
“Our medical and surgical societies need to be working together to start defining what we mean by ‘experienced,’ what we mean by ‘centers of excellence,’ and that process is already underway,” he added.
Dr. David H. Adams, chair of cardiovascular surgery at Mount Sinai Hospital, New York City, said that there’s no question asymptomatic patients need to be treated in experienced repair centers, but questioned whether the 95% threshold is realistic. Although repair rates are increasing worldwide, Society of Thoracic Surgery published data show a wide disparity in mitral repair that would be troublesome in an asymptomatic population. Mandatory reporting data from New York State, home to several experienced heart programs, also show that 45% of the latest 4,325 mitral valves with interventions were replaced, rather than repaired. Dr. Adams added that data are similar across the world.
He also urged caution about an “asymptomatic surgery for all” attitude, emphasizing judgment is necessary, particularly in elderly patients or in those who are very early in the course of severe regurgitation with no evidence of ventricular dilation or declining systolic function.
Finally, the new guidelines include recommendations for using transcatheter valves and the mitral clip to treat patients with secondary MR with LV dysfunction. This is not yet an approved indication from the Food and Drug Administration, pending the results of three ongoing trials, but in Europe, more than 70% of patients getting a mitral clip do so for secondary MR rather than primary mitral valve prolapse, Dr. Bonow said. The European guidelines came out 2 years ahead of the new AHA/ACC guidelines because writing was delayed until these devices were approved in the United States.
Secondary MR, or disease of the heart muscle, remains “problematic” because of a lack of outcomes data indicating that surgery leads to a better outcome than medical management in patients with LV dysfunction and because of questions raised by the Cardiothoracic Surgical Network about whether these valves should be repaired rather than replaced, he said.
What remains is a solid class 1 recommendation for guideline-directed medical therapy for heart failure including cardiac resynchronization therapy (CRT).
The surgical indications in secondary MR are class IIa for patients with severe MR undergoing coronary artery bypass grafting or aortic valve replacement and class IIb for those not undergoing such surgeries, but with severe MR and persistent symptoms, despite medical therapy, including CRT.
“There’s no data we’re going to improve survival ... but clearly some patients will have a dramatic improvement in symptoms,” Dr. Bonow said.
Dr. Bonow disclosed reviewing grant applications for the Gilead (Sciences) Scholars Program. Dr. Adams disclosed royalties as an inventor for Edwards Lifesciences and Medtronic, and serving as a Medtronic national coprimary investigator for the CoreValve Trial.
CHICAGO – Early repair and greater reliance on experienced surgical centers are key to the new guidelines on the management of mitral valve disease.
It’s been 8 years since the last American Heart Association/American College of Cardiology guideline on valvular heart disease in 2006, with little change in the 2008 update.
The 2014 guidelines, however, have substantiative changes, including the decision to begin talking about valvular disease and at-risk patients much as we do for heart failure, guideline committee member Robert Bonow said at the Heart Valve Summit 2014.
The 2014 guidelines, published earlier this year, include four stages of valvular heart disease:
Stage A, for people at risk of valvular disease such as those with bicuspid valves, a history of rheumatic heart disease, or mitral valve prolapse without regurgitation.
Stage B, for mild to moderate, asymptomatic disease.
Stage C, for severe, asymptomatic disease, including those with normal left ventricular function (stage C1) or depressed LV function (stage C2).
Stage D, for severe, symptomatic valve disease.
The new guidelines also drive home the point that primary and secondary mitral regurgitation (MR), while they can be difficult to distinguish, are separate diseases with different pathophysiologies, natural histories, management strategies, and outcomes, said Dr. Bonow, director of the Center for Cardiovascular Innovation, Northwestern University, Chicago.
Class 1 surgical indications for primary MR, or diseases of the valve, are symptomatic patients and asymptomatic patients with LV systolic dysfunction. This continues to be defined as an ejection fraction of < 60% or an end-systolic dimension > 40 mm, although new data have suggested that even smaller systolic dimensions may have prognostic importance, he noted.
Pulmonary hypertension and atrial fibrillation are class IIa indications for surgery in asymptomatic, primary MR.
Critics would argue that patients shouldn’t be allowed to develop these indications because they may be irreversible, but the reality is that many patients arrive in your office with one or more indications already in place, Dr. Bonow said. The real issue is whether mitral valve repair is feasible and can improve survival in patients who have normal LV function and none of these indications, with the guidelines clearly tipping in favor of early surgery for asymptomatic MR patients.
Dr. Bonow highlighted recent long-term outcomes data from Dr. Tirone David’s group (Circulation 2013;127:1485-92) showing that overall survival among patients undergoing mitral valve repair for degenerative diseases is 75% at 20 years for those with functional class (FC) I disease, 66% with FC II, 52% with FC III, and only 32% for those with FC IV.
“I think these data, along with many other series, are quite important in identifying the risks we have for our patients for waiting too long, and if we can refer our patients to an expert surgical team for these valves to be repaired, their outcomes will be much better,” Dr. Bonow said.
The guidelines include the class I indication that repair is better than mitral valve replacement for primary MR and that patients should be referred to “centers experienced in repair.” Instead of stating that there should be a 90% or greater likelihood of a durable repair without residual MR for a patient undergoing elective surgery at that center, the 2014 threshold is now set at more than 95%.
“We really want to make sure patients are going to an experienced center,” he said.
Despite the emphasis on a heart team approach and referral to experienced centers, the term “experienced” has not been fully defined, Dr. Bonow acknowledged.
“Our medical and surgical societies need to be working together to start defining what we mean by ‘experienced,’ what we mean by ‘centers of excellence,’ and that process is already underway,” he added.
Dr. David H. Adams, chair of cardiovascular surgery at Mount Sinai Hospital, New York City, said that there’s no question asymptomatic patients need to be treated in experienced repair centers, but questioned whether the 95% threshold is realistic. Although repair rates are increasing worldwide, Society of Thoracic Surgery published data show a wide disparity in mitral repair that would be troublesome in an asymptomatic population. Mandatory reporting data from New York State, home to several experienced heart programs, also show that 45% of the latest 4,325 mitral valves with interventions were replaced, rather than repaired. Dr. Adams added that data are similar across the world.
He also urged caution about an “asymptomatic surgery for all” attitude, emphasizing judgment is necessary, particularly in elderly patients or in those who are very early in the course of severe regurgitation with no evidence of ventricular dilation or declining systolic function.
Finally, the new guidelines include recommendations for using transcatheter valves and the mitral clip to treat patients with secondary MR with LV dysfunction. This is not yet an approved indication from the Food and Drug Administration, pending the results of three ongoing trials, but in Europe, more than 70% of patients getting a mitral clip do so for secondary MR rather than primary mitral valve prolapse, Dr. Bonow said. The European guidelines came out 2 years ahead of the new AHA/ACC guidelines because writing was delayed until these devices were approved in the United States.
Secondary MR, or disease of the heart muscle, remains “problematic” because of a lack of outcomes data indicating that surgery leads to a better outcome than medical management in patients with LV dysfunction and because of questions raised by the Cardiothoracic Surgical Network about whether these valves should be repaired rather than replaced, he said.
What remains is a solid class 1 recommendation for guideline-directed medical therapy for heart failure including cardiac resynchronization therapy (CRT).
The surgical indications in secondary MR are class IIa for patients with severe MR undergoing coronary artery bypass grafting or aortic valve replacement and class IIb for those not undergoing such surgeries, but with severe MR and persistent symptoms, despite medical therapy, including CRT.
“There’s no data we’re going to improve survival ... but clearly some patients will have a dramatic improvement in symptoms,” Dr. Bonow said.
Dr. Bonow disclosed reviewing grant applications for the Gilead (Sciences) Scholars Program. Dr. Adams disclosed royalties as an inventor for Edwards Lifesciences and Medtronic, and serving as a Medtronic national coprimary investigator for the CoreValve Trial.
CHICAGO – Early repair and greater reliance on experienced surgical centers are key to the new guidelines on the management of mitral valve disease.
It’s been 8 years since the last American Heart Association/American College of Cardiology guideline on valvular heart disease in 2006, with little change in the 2008 update.
The 2014 guidelines, however, have substantiative changes, including the decision to begin talking about valvular disease and at-risk patients much as we do for heart failure, guideline committee member Robert Bonow said at the Heart Valve Summit 2014.
The 2014 guidelines, published earlier this year, include four stages of valvular heart disease:
Stage A, for people at risk of valvular disease such as those with bicuspid valves, a history of rheumatic heart disease, or mitral valve prolapse without regurgitation.
Stage B, for mild to moderate, asymptomatic disease.
Stage C, for severe, asymptomatic disease, including those with normal left ventricular function (stage C1) or depressed LV function (stage C2).
Stage D, for severe, symptomatic valve disease.
The new guidelines also drive home the point that primary and secondary mitral regurgitation (MR), while they can be difficult to distinguish, are separate diseases with different pathophysiologies, natural histories, management strategies, and outcomes, said Dr. Bonow, director of the Center for Cardiovascular Innovation, Northwestern University, Chicago.
Class 1 surgical indications for primary MR, or diseases of the valve, are symptomatic patients and asymptomatic patients with LV systolic dysfunction. This continues to be defined as an ejection fraction of < 60% or an end-systolic dimension > 40 mm, although new data have suggested that even smaller systolic dimensions may have prognostic importance, he noted.
Pulmonary hypertension and atrial fibrillation are class IIa indications for surgery in asymptomatic, primary MR.
Critics would argue that patients shouldn’t be allowed to develop these indications because they may be irreversible, but the reality is that many patients arrive in your office with one or more indications already in place, Dr. Bonow said. The real issue is whether mitral valve repair is feasible and can improve survival in patients who have normal LV function and none of these indications, with the guidelines clearly tipping in favor of early surgery for asymptomatic MR patients.
Dr. Bonow highlighted recent long-term outcomes data from Dr. Tirone David’s group (Circulation 2013;127:1485-92) showing that overall survival among patients undergoing mitral valve repair for degenerative diseases is 75% at 20 years for those with functional class (FC) I disease, 66% with FC II, 52% with FC III, and only 32% for those with FC IV.
“I think these data, along with many other series, are quite important in identifying the risks we have for our patients for waiting too long, and if we can refer our patients to an expert surgical team for these valves to be repaired, their outcomes will be much better,” Dr. Bonow said.
The guidelines include the class I indication that repair is better than mitral valve replacement for primary MR and that patients should be referred to “centers experienced in repair.” Instead of stating that there should be a 90% or greater likelihood of a durable repair without residual MR for a patient undergoing elective surgery at that center, the 2014 threshold is now set at more than 95%.
“We really want to make sure patients are going to an experienced center,” he said.
Despite the emphasis on a heart team approach and referral to experienced centers, the term “experienced” has not been fully defined, Dr. Bonow acknowledged.
“Our medical and surgical societies need to be working together to start defining what we mean by ‘experienced,’ what we mean by ‘centers of excellence,’ and that process is already underway,” he added.
Dr. David H. Adams, chair of cardiovascular surgery at Mount Sinai Hospital, New York City, said that there’s no question asymptomatic patients need to be treated in experienced repair centers, but questioned whether the 95% threshold is realistic. Although repair rates are increasing worldwide, Society of Thoracic Surgery published data show a wide disparity in mitral repair that would be troublesome in an asymptomatic population. Mandatory reporting data from New York State, home to several experienced heart programs, also show that 45% of the latest 4,325 mitral valves with interventions were replaced, rather than repaired. Dr. Adams added that data are similar across the world.
He also urged caution about an “asymptomatic surgery for all” attitude, emphasizing judgment is necessary, particularly in elderly patients or in those who are very early in the course of severe regurgitation with no evidence of ventricular dilation or declining systolic function.
Finally, the new guidelines include recommendations for using transcatheter valves and the mitral clip to treat patients with secondary MR with LV dysfunction. This is not yet an approved indication from the Food and Drug Administration, pending the results of three ongoing trials, but in Europe, more than 70% of patients getting a mitral clip do so for secondary MR rather than primary mitral valve prolapse, Dr. Bonow said. The European guidelines came out 2 years ahead of the new AHA/ACC guidelines because writing was delayed until these devices were approved in the United States.
Secondary MR, or disease of the heart muscle, remains “problematic” because of a lack of outcomes data indicating that surgery leads to a better outcome than medical management in patients with LV dysfunction and because of questions raised by the Cardiothoracic Surgical Network about whether these valves should be repaired rather than replaced, he said.
What remains is a solid class 1 recommendation for guideline-directed medical therapy for heart failure including cardiac resynchronization therapy (CRT).
The surgical indications in secondary MR are class IIa for patients with severe MR undergoing coronary artery bypass grafting or aortic valve replacement and class IIb for those not undergoing such surgeries, but with severe MR and persistent symptoms, despite medical therapy, including CRT.
“There’s no data we’re going to improve survival ... but clearly some patients will have a dramatic improvement in symptoms,” Dr. Bonow said.
Dr. Bonow disclosed reviewing grant applications for the Gilead (Sciences) Scholars Program. Dr. Adams disclosed royalties as an inventor for Edwards Lifesciences and Medtronic, and serving as a Medtronic national coprimary investigator for the CoreValve Trial.
AT THE HEART VALVE SUMMIT 2014
Efficacy, not tolerability, of bowel prep is primary
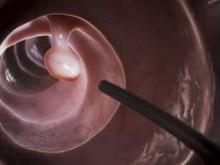
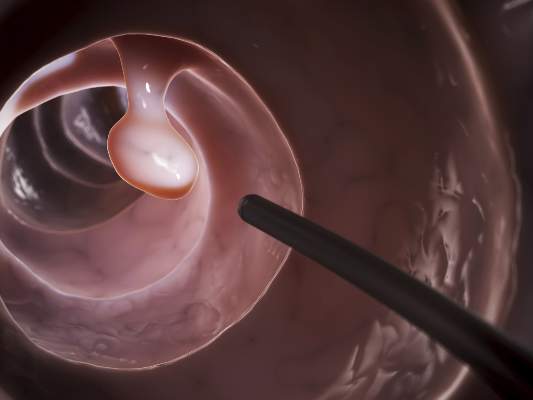
The efficacy, not the tolerability, of bowel cleansing is the primary concern in patients undergoing colonoscopy, because of the substantial adverse consequences of inadequate bowel preparation, according to a consensus report published simultaneously in Gastroenterology, the American Journal of Gastroenterology, and Gastrointestinal Endoscopy.
As many as 20%-25% of all colonoscopies reportedly have inadequate bowel cleansing, which is associated with lower rates of lesion detection, longer procedure times, and increased electrocautery risks, in addition to the excess costs and risks of repeat procedures. “Efficacy should be a higher priority than tolerability, [so] the choice of a bowel-cleansing regimen should be based on cleansing efficacy first and patient tolerability second,” said Dr. David A. Johnson of Eastern Veterans Administration Medical School, Norfolk (Va.) and his associates on the U.S. Multi-Society Task Force on Colorectal Cancer.
The USMSTF comprised representatives from the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy, who compiled recommendations and wrote this report based on a systematic review and meta-analysis of the literature concerning bowel preparation from 1980 to the present.
When selecting a bowel-cleansing regimen for patients, physicians should consider the patient’s medical history, medications, and, if applicable, the adequacy of bowel preparation for previous colonoscopies. High-quality evidence shows that a split-dose regimen of 4 liters of polyethylene glycol–electrolyte lavage solution (PEG-ELS) provides superior bowel cleansing to other doses and solutions. The second dose ideally should begin 4-6 hours and conclude at least 2 hours before the procedure time. However, same-day regimens are acceptable, especially for patients scheduled for an afternoon colonoscopy.
Using a split-dose regimen allows some flexibility with the diet on the day before the procedure. Instead of ingesting only clear liquids, patients can consume either a full liquid or a low-residue diet for part or all of the day preceding colonoscopy, which improves their tolerance of the bowel preparation.
Over-the-counter, nonapproved (by the FDA) bowel-cleansing agents have widely varying efficacy, ranging from adequate to excellent. They generally are safe, but “caution is required when using these agents in certain populations” such as patients with chronic kidney disease. The routine use of adjunctive agents intended to enhance purgation or visualization of the mucosa is not recommended, Dr. Johnson and his associates said (Gastroenterology 2014;147:903-24).
At present, the evidence is insufficient to allow recommendation of specific bowel-preparation regimens for special patient populations such as the elderly, children and adolescents, people with inflammatory bowel disease, patients who have undergone bariatric surgery, and people with spinal cord injury. However, sodium phosphate preparations should be avoided in the elderly, children, and people with IBD. Additional bowel purgatives can be considered for patients at risk for inadequate preparation, such as those with a history of constipation, those using opioids or other constipating medications, those who have undergone previous colon resection, and those with spinal cord injury.
The report also includes recommendations concerning patient education, the risk factors for inadequate bowel preparation, assessing the adequacy of bowel preparation before and during the procedure, and salvage options for cases in which preparation is inadequate. It is available at http://dx.doi.org/10.1053/j.gastro.2014.07.002.
The efficacy, not the tolerability, of bowel cleansing is the primary concern in patients undergoing colonoscopy, because of the substantial adverse consequences of inadequate bowel preparation, according to a consensus report published simultaneously in Gastroenterology, the American Journal of Gastroenterology, and Gastrointestinal Endoscopy.
As many as 20%-25% of all colonoscopies reportedly have inadequate bowel cleansing, which is associated with lower rates of lesion detection, longer procedure times, and increased electrocautery risks, in addition to the excess costs and risks of repeat procedures. “Efficacy should be a higher priority than tolerability, [so] the choice of a bowel-cleansing regimen should be based on cleansing efficacy first and patient tolerability second,” said Dr. David A. Johnson of Eastern Veterans Administration Medical School, Norfolk (Va.) and his associates on the U.S. Multi-Society Task Force on Colorectal Cancer.
The USMSTF comprised representatives from the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy, who compiled recommendations and wrote this report based on a systematic review and meta-analysis of the literature concerning bowel preparation from 1980 to the present.
When selecting a bowel-cleansing regimen for patients, physicians should consider the patient’s medical history, medications, and, if applicable, the adequacy of bowel preparation for previous colonoscopies. High-quality evidence shows that a split-dose regimen of 4 liters of polyethylene glycol–electrolyte lavage solution (PEG-ELS) provides superior bowel cleansing to other doses and solutions. The second dose ideally should begin 4-6 hours and conclude at least 2 hours before the procedure time. However, same-day regimens are acceptable, especially for patients scheduled for an afternoon colonoscopy.
Using a split-dose regimen allows some flexibility with the diet on the day before the procedure. Instead of ingesting only clear liquids, patients can consume either a full liquid or a low-residue diet for part or all of the day preceding colonoscopy, which improves their tolerance of the bowel preparation.
Over-the-counter, nonapproved (by the FDA) bowel-cleansing agents have widely varying efficacy, ranging from adequate to excellent. They generally are safe, but “caution is required when using these agents in certain populations” such as patients with chronic kidney disease. The routine use of adjunctive agents intended to enhance purgation or visualization of the mucosa is not recommended, Dr. Johnson and his associates said (Gastroenterology 2014;147:903-24).
At present, the evidence is insufficient to allow recommendation of specific bowel-preparation regimens for special patient populations such as the elderly, children and adolescents, people with inflammatory bowel disease, patients who have undergone bariatric surgery, and people with spinal cord injury. However, sodium phosphate preparations should be avoided in the elderly, children, and people with IBD. Additional bowel purgatives can be considered for patients at risk for inadequate preparation, such as those with a history of constipation, those using opioids or other constipating medications, those who have undergone previous colon resection, and those with spinal cord injury.
The report also includes recommendations concerning patient education, the risk factors for inadequate bowel preparation, assessing the adequacy of bowel preparation before and during the procedure, and salvage options for cases in which preparation is inadequate. It is available at http://dx.doi.org/10.1053/j.gastro.2014.07.002.
The efficacy, not the tolerability, of bowel cleansing is the primary concern in patients undergoing colonoscopy, because of the substantial adverse consequences of inadequate bowel preparation, according to a consensus report published simultaneously in Gastroenterology, the American Journal of Gastroenterology, and Gastrointestinal Endoscopy.
As many as 20%-25% of all colonoscopies reportedly have inadequate bowel cleansing, which is associated with lower rates of lesion detection, longer procedure times, and increased electrocautery risks, in addition to the excess costs and risks of repeat procedures. “Efficacy should be a higher priority than tolerability, [so] the choice of a bowel-cleansing regimen should be based on cleansing efficacy first and patient tolerability second,” said Dr. David A. Johnson of Eastern Veterans Administration Medical School, Norfolk (Va.) and his associates on the U.S. Multi-Society Task Force on Colorectal Cancer.
The USMSTF comprised representatives from the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy, who compiled recommendations and wrote this report based on a systematic review and meta-analysis of the literature concerning bowel preparation from 1980 to the present.
When selecting a bowel-cleansing regimen for patients, physicians should consider the patient’s medical history, medications, and, if applicable, the adequacy of bowel preparation for previous colonoscopies. High-quality evidence shows that a split-dose regimen of 4 liters of polyethylene glycol–electrolyte lavage solution (PEG-ELS) provides superior bowel cleansing to other doses and solutions. The second dose ideally should begin 4-6 hours and conclude at least 2 hours before the procedure time. However, same-day regimens are acceptable, especially for patients scheduled for an afternoon colonoscopy.
Using a split-dose regimen allows some flexibility with the diet on the day before the procedure. Instead of ingesting only clear liquids, patients can consume either a full liquid or a low-residue diet for part or all of the day preceding colonoscopy, which improves their tolerance of the bowel preparation.
Over-the-counter, nonapproved (by the FDA) bowel-cleansing agents have widely varying efficacy, ranging from adequate to excellent. They generally are safe, but “caution is required when using these agents in certain populations” such as patients with chronic kidney disease. The routine use of adjunctive agents intended to enhance purgation or visualization of the mucosa is not recommended, Dr. Johnson and his associates said (Gastroenterology 2014;147:903-24).
At present, the evidence is insufficient to allow recommendation of specific bowel-preparation regimens for special patient populations such as the elderly, children and adolescents, people with inflammatory bowel disease, patients who have undergone bariatric surgery, and people with spinal cord injury. However, sodium phosphate preparations should be avoided in the elderly, children, and people with IBD. Additional bowel purgatives can be considered for patients at risk for inadequate preparation, such as those with a history of constipation, those using opioids or other constipating medications, those who have undergone previous colon resection, and those with spinal cord injury.
The report also includes recommendations concerning patient education, the risk factors for inadequate bowel preparation, assessing the adequacy of bowel preparation before and during the procedure, and salvage options for cases in which preparation is inadequate. It is available at http://dx.doi.org/10.1053/j.gastro.2014.07.002.
FROM GASTROENTEROLOGY
Key clinical point: Efficacy, not tolerability, of bowel preparation is the primary concern before colonoscopy.
Major finding: Up to 20%-25% of all colonoscopies have inadequate bowel preparation, which lowers detection rates, lengthens procedure time, raises electrocautery risks, and raises overall costs and risks by requiring repeat procedures.
Data source: A consensus statement based on a systematic review and meta-analysis of the literature concerning bowel preparation for colonoscopy.
Disclosures: This report was supported by the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy, with further support provided by the U.S. Veterans Health Administration. Dr. Johnson reported serving as a consultant and clinical investigator for Epigenomics, Given Imaging, and Exact Sciences; his associates reported numerous ties to industry sources.
Should LCZ696 receive a level I indication?
LAS VEGAS – Additional findings from the landmark PARADIGM-HF trial presented at the annual meeting of the Heart Failure Society of America provided what many observers deemed a persuasive case for the novel angiotensin receptor neprilysin inhibitor known for now as LCZ696 as deserving of a level I indication in the next update of the major heart failure management guidelines.
At a special session added late to the meeting program in the wake of the spectacularly positive top-line results of PARADIGM-HF presented just a few weeks earlier at the European Society of Cardiology meeting in Barcelona, an international panel of heart failure heavyweights tackled questions about the study’s implications, including whether the results need replication in a second randomized controlled trial before LCZ696 can win regulatory approval. And once approved, should guidelines committees give it a level I, must-use indication? How applicable are the PARADIGM-HF results to the broader population of heart failure patients, and in particular black patients and older individuals with class III/IV heart failure? And what about patients with heart failure with preserved ejection fraction (HFpEF) ?
In other words, does PARADIGM-HF, with more than 8,400 randomized subjects, represent a true paradigm shift in heart failure management?
For coprincipal investigator Dr. Milton Packer, the answer is a resounding yes.
“For the past 25 years, the magnitude of the effect of ACE inhibitors on cardiovascular mortality – about an 18% reduction – has created an ethical mandate for their use in all patients with chronic heart failure who could tolerate treatment with these drugs. The finding that LCZ696 has a 20% greater effect on cardiovascular mortality than ACE inhibitors strongly supports the conclusion that LCZ696 should replace the current use of ACE inhibitors and angiotensin receptor blockers in the management of chronic heart failure,” said Dr. Packer, professor and chair of the department of clinical sciences at University of Texas Southwestern Medical Center, Dallas.
His coprincipal investigator, Dr. John J.V. McMurray, cited the statistical strength of the PARADIGM-HF results for the primary composite outcome of cardiovascular death or heart failure hospitalization, which had an extraordinary P value of .0000004, in making the case that the trial findings are sufficient to win regulatory approval without a confirmatory study.
He noted that the regulatory standard in the United States and Europe is that a positive clinical trial having a P value of less than .05 requires replication in a second study that also yields outcomes with a P value of less than .05.
“If, however, you have a large single trial, you can win approval by meeting a standard of P less than .00125. The strength of the result of PARADIGM-HF, with a P of .0000004, is equivalent to between four and five single trials replicated at P less than .05. And for the endpoint of cardiovascular mortality, where the PARADIGM-HF result was significant at a P of .00008, that’s equivalent to between two and three trials replicated at P less than .05. So in my view PARADIGM-HF easily meets the criteria for a level IA indication,” said Dr. McMurray, professor of cardiology at the University of Glasgow.
He presented for the first time a new analysis with a major wow factor. This was an imputed placebo analysis providing the answer to a question many cardiologists have asked him since the presentation of the top-line PARADIGM-HF results in Barcelona: namely, how would LCZ696 have stacked up in a placebo-controlled trial?
Such a study wouldn’t be ethical now, of course, but it’s possible to make inferences by comparing LCZ696’s superiority to enalapril at 10 mg b.i.d. in PARADIGM-HF to enalapril’s performance at the same dose relative to placebo in the earlier 2,569-patient SOLVD-Treatment trial, which featured the same composite primary endpoint (N. Engl. J. Med. 1991;325:293-302).
In SOLVD-Treatment, enalapril resulted in a 28% relative risk reduction in the composite endpoint, compared with placebo. Through indirect comparison, LCZ696 would have an imputed 43% relative risk reduction, compared with placebo. For the endpoint of cardiovascular mortality, enalapril showed a 17% risk reduction relative to placebo; when the PARADIGM-HF results are factored in, this translates to an inferred 34% relative risk reduction for LCZ696 versus placebo.
Similarly, in the CHARM-Alternative trial (Lancet 2003;362:772-6), which featured 2,028 patients on more contemporary guideline–recommended background therapy than in SOLVD-Treatment, patients on the angiotensin receptor blocker candesartan showed a 23% relative risk reduction in the composite endpoint, compared with placebo, along with a 15% reduction in cardiovascular mortality. In the imputed placebo analysis, this translated to relative risk reductions of 49% and 34%, respectively, for LCZ696 versus placebo.
“We see a doubling in the reduction in cardiovascular mortality with this new therapy over and above that obtained with an ACE inhibitor or ARB [angiotensin receptor blocker],” Dr. McMurray emphasized.
Panelist Dr. Lynne W. Stevenson wasn’t convinced.
“I don’t believe it is time to replace ACE inhibitors and ARBs. I don’t think LCZ696 is ready for a level I [treatment should be performed] indication; that is a higher bar. ... I think we could see a level IIa [treatment is reasonable to perform] indication based on the strong results that we’ve seen,” said Dr. Stevenson, director of the heart failure and cardiomyopathy program at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
She estimated that fewer than 10% of U.S. heart failure patients fit the description of PARADIGM-HF participants, with mild to moderate heart failure with reduced ejection fraction. Importantly, the run-in process employed in the study ensured that only patients with a demonstrated ability to tolerate enalapril in therapeutic doses were enrolled. And even in that filtered population, there was a substantial dropout rate in the LCZ696 arm due to hypotension during follow-up.
“I certainly don’t think we have any information about patients newly diagnosed with heart failure. I don’t think if you put new heart failure patients on LCZ696, they’d necessarily be able to stand up, and if they could stand up I’m not sure we could get them on the appropriate dose of beta blockers,” Dr. Stevenson added.
Noting that only 5% of PARADIGM-HF participants were black, she said that “clearly this is something we will need to watch as we get more experience with this drug, but there was no signal of concern.”
Dr. Marvin A. Konstam, professor of medicine at Tufts University, Boston, shared one of Dr. Stevenson’s concerns: “How do we know what will happen with ACE inhibitor virgins in the real world where you don’t get a run-in period?”
Panelist Dr. John G.F. Cleland said, “I don’t want to second-guess the guideline committees, but surely this must be a IA [data derived from multiple clinical trials or meta-analyses] indication. What intrigues me is what will the indication for ACE inhibitors look like in future guidelines? Is it also going to be IA in the same group of patients? That’s something the guidelines committees are going to have to sort out.”
“A lot of these questions and people’s concerns will either be increased or reduced once we start to get the medicine into clinical practice. What I find quite distressing is that we might be sitting here at this time next year and still not be in a position to prescribe this agent because it may still be going through the regulatory process,” said Dr. Cleland, professor of cardiology at the University of Hull (England).
LAS VEGAS – Additional findings from the landmark PARADIGM-HF trial presented at the annual meeting of the Heart Failure Society of America provided what many observers deemed a persuasive case for the novel angiotensin receptor neprilysin inhibitor known for now as LCZ696 as deserving of a level I indication in the next update of the major heart failure management guidelines.
At a special session added late to the meeting program in the wake of the spectacularly positive top-line results of PARADIGM-HF presented just a few weeks earlier at the European Society of Cardiology meeting in Barcelona, an international panel of heart failure heavyweights tackled questions about the study’s implications, including whether the results need replication in a second randomized controlled trial before LCZ696 can win regulatory approval. And once approved, should guidelines committees give it a level I, must-use indication? How applicable are the PARADIGM-HF results to the broader population of heart failure patients, and in particular black patients and older individuals with class III/IV heart failure? And what about patients with heart failure with preserved ejection fraction (HFpEF) ?
In other words, does PARADIGM-HF, with more than 8,400 randomized subjects, represent a true paradigm shift in heart failure management?
For coprincipal investigator Dr. Milton Packer, the answer is a resounding yes.
“For the past 25 years, the magnitude of the effect of ACE inhibitors on cardiovascular mortality – about an 18% reduction – has created an ethical mandate for their use in all patients with chronic heart failure who could tolerate treatment with these drugs. The finding that LCZ696 has a 20% greater effect on cardiovascular mortality than ACE inhibitors strongly supports the conclusion that LCZ696 should replace the current use of ACE inhibitors and angiotensin receptor blockers in the management of chronic heart failure,” said Dr. Packer, professor and chair of the department of clinical sciences at University of Texas Southwestern Medical Center, Dallas.
His coprincipal investigator, Dr. John J.V. McMurray, cited the statistical strength of the PARADIGM-HF results for the primary composite outcome of cardiovascular death or heart failure hospitalization, which had an extraordinary P value of .0000004, in making the case that the trial findings are sufficient to win regulatory approval without a confirmatory study.
He noted that the regulatory standard in the United States and Europe is that a positive clinical trial having a P value of less than .05 requires replication in a second study that also yields outcomes with a P value of less than .05.
“If, however, you have a large single trial, you can win approval by meeting a standard of P less than .00125. The strength of the result of PARADIGM-HF, with a P of .0000004, is equivalent to between four and five single trials replicated at P less than .05. And for the endpoint of cardiovascular mortality, where the PARADIGM-HF result was significant at a P of .00008, that’s equivalent to between two and three trials replicated at P less than .05. So in my view PARADIGM-HF easily meets the criteria for a level IA indication,” said Dr. McMurray, professor of cardiology at the University of Glasgow.
He presented for the first time a new analysis with a major wow factor. This was an imputed placebo analysis providing the answer to a question many cardiologists have asked him since the presentation of the top-line PARADIGM-HF results in Barcelona: namely, how would LCZ696 have stacked up in a placebo-controlled trial?
Such a study wouldn’t be ethical now, of course, but it’s possible to make inferences by comparing LCZ696’s superiority to enalapril at 10 mg b.i.d. in PARADIGM-HF to enalapril’s performance at the same dose relative to placebo in the earlier 2,569-patient SOLVD-Treatment trial, which featured the same composite primary endpoint (N. Engl. J. Med. 1991;325:293-302).
In SOLVD-Treatment, enalapril resulted in a 28% relative risk reduction in the composite endpoint, compared with placebo. Through indirect comparison, LCZ696 would have an imputed 43% relative risk reduction, compared with placebo. For the endpoint of cardiovascular mortality, enalapril showed a 17% risk reduction relative to placebo; when the PARADIGM-HF results are factored in, this translates to an inferred 34% relative risk reduction for LCZ696 versus placebo.
Similarly, in the CHARM-Alternative trial (Lancet 2003;362:772-6), which featured 2,028 patients on more contemporary guideline–recommended background therapy than in SOLVD-Treatment, patients on the angiotensin receptor blocker candesartan showed a 23% relative risk reduction in the composite endpoint, compared with placebo, along with a 15% reduction in cardiovascular mortality. In the imputed placebo analysis, this translated to relative risk reductions of 49% and 34%, respectively, for LCZ696 versus placebo.
“We see a doubling in the reduction in cardiovascular mortality with this new therapy over and above that obtained with an ACE inhibitor or ARB [angiotensin receptor blocker],” Dr. McMurray emphasized.
Panelist Dr. Lynne W. Stevenson wasn’t convinced.
“I don’t believe it is time to replace ACE inhibitors and ARBs. I don’t think LCZ696 is ready for a level I [treatment should be performed] indication; that is a higher bar. ... I think we could see a level IIa [treatment is reasonable to perform] indication based on the strong results that we’ve seen,” said Dr. Stevenson, director of the heart failure and cardiomyopathy program at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
She estimated that fewer than 10% of U.S. heart failure patients fit the description of PARADIGM-HF participants, with mild to moderate heart failure with reduced ejection fraction. Importantly, the run-in process employed in the study ensured that only patients with a demonstrated ability to tolerate enalapril in therapeutic doses were enrolled. And even in that filtered population, there was a substantial dropout rate in the LCZ696 arm due to hypotension during follow-up.
“I certainly don’t think we have any information about patients newly diagnosed with heart failure. I don’t think if you put new heart failure patients on LCZ696, they’d necessarily be able to stand up, and if they could stand up I’m not sure we could get them on the appropriate dose of beta blockers,” Dr. Stevenson added.
Noting that only 5% of PARADIGM-HF participants were black, she said that “clearly this is something we will need to watch as we get more experience with this drug, but there was no signal of concern.”
Dr. Marvin A. Konstam, professor of medicine at Tufts University, Boston, shared one of Dr. Stevenson’s concerns: “How do we know what will happen with ACE inhibitor virgins in the real world where you don’t get a run-in period?”
Panelist Dr. John G.F. Cleland said, “I don’t want to second-guess the guideline committees, but surely this must be a IA [data derived from multiple clinical trials or meta-analyses] indication. What intrigues me is what will the indication for ACE inhibitors look like in future guidelines? Is it also going to be IA in the same group of patients? That’s something the guidelines committees are going to have to sort out.”
“A lot of these questions and people’s concerns will either be increased or reduced once we start to get the medicine into clinical practice. What I find quite distressing is that we might be sitting here at this time next year and still not be in a position to prescribe this agent because it may still be going through the regulatory process,” said Dr. Cleland, professor of cardiology at the University of Hull (England).
LAS VEGAS – Additional findings from the landmark PARADIGM-HF trial presented at the annual meeting of the Heart Failure Society of America provided what many observers deemed a persuasive case for the novel angiotensin receptor neprilysin inhibitor known for now as LCZ696 as deserving of a level I indication in the next update of the major heart failure management guidelines.
At a special session added late to the meeting program in the wake of the spectacularly positive top-line results of PARADIGM-HF presented just a few weeks earlier at the European Society of Cardiology meeting in Barcelona, an international panel of heart failure heavyweights tackled questions about the study’s implications, including whether the results need replication in a second randomized controlled trial before LCZ696 can win regulatory approval. And once approved, should guidelines committees give it a level I, must-use indication? How applicable are the PARADIGM-HF results to the broader population of heart failure patients, and in particular black patients and older individuals with class III/IV heart failure? And what about patients with heart failure with preserved ejection fraction (HFpEF) ?
In other words, does PARADIGM-HF, with more than 8,400 randomized subjects, represent a true paradigm shift in heart failure management?
For coprincipal investigator Dr. Milton Packer, the answer is a resounding yes.
“For the past 25 years, the magnitude of the effect of ACE inhibitors on cardiovascular mortality – about an 18% reduction – has created an ethical mandate for their use in all patients with chronic heart failure who could tolerate treatment with these drugs. The finding that LCZ696 has a 20% greater effect on cardiovascular mortality than ACE inhibitors strongly supports the conclusion that LCZ696 should replace the current use of ACE inhibitors and angiotensin receptor blockers in the management of chronic heart failure,” said Dr. Packer, professor and chair of the department of clinical sciences at University of Texas Southwestern Medical Center, Dallas.
His coprincipal investigator, Dr. John J.V. McMurray, cited the statistical strength of the PARADIGM-HF results for the primary composite outcome of cardiovascular death or heart failure hospitalization, which had an extraordinary P value of .0000004, in making the case that the trial findings are sufficient to win regulatory approval without a confirmatory study.
He noted that the regulatory standard in the United States and Europe is that a positive clinical trial having a P value of less than .05 requires replication in a second study that also yields outcomes with a P value of less than .05.
“If, however, you have a large single trial, you can win approval by meeting a standard of P less than .00125. The strength of the result of PARADIGM-HF, with a P of .0000004, is equivalent to between four and five single trials replicated at P less than .05. And for the endpoint of cardiovascular mortality, where the PARADIGM-HF result was significant at a P of .00008, that’s equivalent to between two and three trials replicated at P less than .05. So in my view PARADIGM-HF easily meets the criteria for a level IA indication,” said Dr. McMurray, professor of cardiology at the University of Glasgow.
He presented for the first time a new analysis with a major wow factor. This was an imputed placebo analysis providing the answer to a question many cardiologists have asked him since the presentation of the top-line PARADIGM-HF results in Barcelona: namely, how would LCZ696 have stacked up in a placebo-controlled trial?
Such a study wouldn’t be ethical now, of course, but it’s possible to make inferences by comparing LCZ696’s superiority to enalapril at 10 mg b.i.d. in PARADIGM-HF to enalapril’s performance at the same dose relative to placebo in the earlier 2,569-patient SOLVD-Treatment trial, which featured the same composite primary endpoint (N. Engl. J. Med. 1991;325:293-302).
In SOLVD-Treatment, enalapril resulted in a 28% relative risk reduction in the composite endpoint, compared with placebo. Through indirect comparison, LCZ696 would have an imputed 43% relative risk reduction, compared with placebo. For the endpoint of cardiovascular mortality, enalapril showed a 17% risk reduction relative to placebo; when the PARADIGM-HF results are factored in, this translates to an inferred 34% relative risk reduction for LCZ696 versus placebo.
Similarly, in the CHARM-Alternative trial (Lancet 2003;362:772-6), which featured 2,028 patients on more contemporary guideline–recommended background therapy than in SOLVD-Treatment, patients on the angiotensin receptor blocker candesartan showed a 23% relative risk reduction in the composite endpoint, compared with placebo, along with a 15% reduction in cardiovascular mortality. In the imputed placebo analysis, this translated to relative risk reductions of 49% and 34%, respectively, for LCZ696 versus placebo.
“We see a doubling in the reduction in cardiovascular mortality with this new therapy over and above that obtained with an ACE inhibitor or ARB [angiotensin receptor blocker],” Dr. McMurray emphasized.
Panelist Dr. Lynne W. Stevenson wasn’t convinced.
“I don’t believe it is time to replace ACE inhibitors and ARBs. I don’t think LCZ696 is ready for a level I [treatment should be performed] indication; that is a higher bar. ... I think we could see a level IIa [treatment is reasonable to perform] indication based on the strong results that we’ve seen,” said Dr. Stevenson, director of the heart failure and cardiomyopathy program at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
She estimated that fewer than 10% of U.S. heart failure patients fit the description of PARADIGM-HF participants, with mild to moderate heart failure with reduced ejection fraction. Importantly, the run-in process employed in the study ensured that only patients with a demonstrated ability to tolerate enalapril in therapeutic doses were enrolled. And even in that filtered population, there was a substantial dropout rate in the LCZ696 arm due to hypotension during follow-up.
“I certainly don’t think we have any information about patients newly diagnosed with heart failure. I don’t think if you put new heart failure patients on LCZ696, they’d necessarily be able to stand up, and if they could stand up I’m not sure we could get them on the appropriate dose of beta blockers,” Dr. Stevenson added.
Noting that only 5% of PARADIGM-HF participants were black, she said that “clearly this is something we will need to watch as we get more experience with this drug, but there was no signal of concern.”
Dr. Marvin A. Konstam, professor of medicine at Tufts University, Boston, shared one of Dr. Stevenson’s concerns: “How do we know what will happen with ACE inhibitor virgins in the real world where you don’t get a run-in period?”
Panelist Dr. John G.F. Cleland said, “I don’t want to second-guess the guideline committees, but surely this must be a IA [data derived from multiple clinical trials or meta-analyses] indication. What intrigues me is what will the indication for ACE inhibitors look like in future guidelines? Is it also going to be IA in the same group of patients? That’s something the guidelines committees are going to have to sort out.”
“A lot of these questions and people’s concerns will either be increased or reduced once we start to get the medicine into clinical practice. What I find quite distressing is that we might be sitting here at this time next year and still not be in a position to prescribe this agent because it may still be going through the regulatory process,” said Dr. Cleland, professor of cardiology at the University of Hull (England).
EXPERT OPINION FROM THE HFSA ANNUAL SCIENTIFIC MEETING
Pediatric IBD rose by more than 40% in 15 years
Pediatric inflammatory bowel disease grew by more than 40% in a 15-year period in Ontario, Canada, according to a retrospective cohort study published in the October issue of Gastroenterology.
Although rates of inflammatory bowel disease (IBD) rose in children and adolescents of all ages, the steepest increase occurred in children with very-early-onset IBD (VEO-IBD), defined as disease diagnosed before they were 10 years old, said Dr. Eric Benchimol at the University of Ottawa and his associates. But these patients also tended to use fewer health services and have fewer surgeries for IBD, compared with older children with the disease, the investigators said (Gastroenterology 2014 October [doi.org/10.1053/j.gastro.2014.06.023]).
Source: American Gastroenterological Association
The findings add to research indicating that VEO-IBD is a distinct form of IBD and indicate the need to assess subgroups of these patients to look at phenotype, genotype, intestinal microbiome, and treatment response, the investigators said.
For the study, researchers created a cohort based on an algorithm of health care visits that identified all children and adolescents in Ontario diagnosed with IBD before age 18 years. The analysis included 7,143 patients with IBD, among whom about 14% had VEO-IBD, the investigators reported.
The overall rate of IBD in children up to 18 years old increased from 9.4 to 13.2 cases per 100,000 population from 1994 through 2009 (P less than .0001), the researchers said. And the yearly increase in VEO-IBD averaged 7.4% – more than three times greater than the 2.2% average annual rise among children diagnosed at 10 years and older, the investigators reported.
But health care utilization trends did not mirror changes in incidence, Dr. Benchimol and associates reported. For example, children diagnosed before they were 6 years old had significantly fewer outpatient visits for IBD, compared with children diagnosed at 10 years and older (odds ratio for girls, 0.67; 95% confidence interval, 0.58-0.78; OR for boys, 0.86; 95% CI, 0.75-0.98). Furthermore, patients diagnosed before age 6 years were less likely to be hospitalized for IBD than were older children with the disease (hazard ratio for girls, 0.70; 95% CI, 0.56-0.87; HR for boys, 1.12; 95% CI, 0.94-1.33), the investigators said.
The likelihood of undergoing intestinal resection also was lower for children diagnosed before age 6 years with Crohn’s disease, compared with older girls (HR, 0.35; 95% CI, 0.16-0.78) and boys (HR, 0.59; 95% CI, 0.34-0.99), said the researchers. And patients diagnosed before age 6 years with ulcerative colitis were less likely to undergo colectomy than were older girls (HR, 0.88; 95% CI, 0.47-1.63) and boys (HR, 0.42; 95% CI, 0.21-0.85). In contrast, rates of IBD-related surgery and hospitalization were similar between children diagnosed at 6-9.9 years of age and those diagnosed at age 10 up to 18 years, the investigators said.
A cohort study from the United States also found a lower likelihood of surgery in children with VEO-IBD, the researchers noted. Large-bowel involvement without ileal disease is prominent in young children with IBD, and these patients might be unlikely to undergo resection because colectomy requires a permanent ostotomy, they added.
The work was supported by the American College of Gastroenterology, the Ontario Ministry of Health and Long-Term Care, the Canadian Institutes of Health Research, the Crohn’s and Colitis Foundation of Canada, the National Institutes of Health, the Wolpow Family Chair in IBD Treatment and Research, the Ontario Ministry of Research and Innovation, and the Leona M. and Harry B. Helmsley Charitable Trust. The authors reported no conflicts of interest.
Pediatric inflammatory bowel disease grew by more than 40% in a 15-year period in Ontario, Canada, according to a retrospective cohort study published in the October issue of Gastroenterology.
Although rates of inflammatory bowel disease (IBD) rose in children and adolescents of all ages, the steepest increase occurred in children with very-early-onset IBD (VEO-IBD), defined as disease diagnosed before they were 10 years old, said Dr. Eric Benchimol at the University of Ottawa and his associates. But these patients also tended to use fewer health services and have fewer surgeries for IBD, compared with older children with the disease, the investigators said (Gastroenterology 2014 October [doi.org/10.1053/j.gastro.2014.06.023]).
Source: American Gastroenterological Association
The findings add to research indicating that VEO-IBD is a distinct form of IBD and indicate the need to assess subgroups of these patients to look at phenotype, genotype, intestinal microbiome, and treatment response, the investigators said.
For the study, researchers created a cohort based on an algorithm of health care visits that identified all children and adolescents in Ontario diagnosed with IBD before age 18 years. The analysis included 7,143 patients with IBD, among whom about 14% had VEO-IBD, the investigators reported.
The overall rate of IBD in children up to 18 years old increased from 9.4 to 13.2 cases per 100,000 population from 1994 through 2009 (P less than .0001), the researchers said. And the yearly increase in VEO-IBD averaged 7.4% – more than three times greater than the 2.2% average annual rise among children diagnosed at 10 years and older, the investigators reported.
But health care utilization trends did not mirror changes in incidence, Dr. Benchimol and associates reported. For example, children diagnosed before they were 6 years old had significantly fewer outpatient visits for IBD, compared with children diagnosed at 10 years and older (odds ratio for girls, 0.67; 95% confidence interval, 0.58-0.78; OR for boys, 0.86; 95% CI, 0.75-0.98). Furthermore, patients diagnosed before age 6 years were less likely to be hospitalized for IBD than were older children with the disease (hazard ratio for girls, 0.70; 95% CI, 0.56-0.87; HR for boys, 1.12; 95% CI, 0.94-1.33), the investigators said.
The likelihood of undergoing intestinal resection also was lower for children diagnosed before age 6 years with Crohn’s disease, compared with older girls (HR, 0.35; 95% CI, 0.16-0.78) and boys (HR, 0.59; 95% CI, 0.34-0.99), said the researchers. And patients diagnosed before age 6 years with ulcerative colitis were less likely to undergo colectomy than were older girls (HR, 0.88; 95% CI, 0.47-1.63) and boys (HR, 0.42; 95% CI, 0.21-0.85). In contrast, rates of IBD-related surgery and hospitalization were similar between children diagnosed at 6-9.9 years of age and those diagnosed at age 10 up to 18 years, the investigators said.
A cohort study from the United States also found a lower likelihood of surgery in children with VEO-IBD, the researchers noted. Large-bowel involvement without ileal disease is prominent in young children with IBD, and these patients might be unlikely to undergo resection because colectomy requires a permanent ostotomy, they added.
The work was supported by the American College of Gastroenterology, the Ontario Ministry of Health and Long-Term Care, the Canadian Institutes of Health Research, the Crohn’s and Colitis Foundation of Canada, the National Institutes of Health, the Wolpow Family Chair in IBD Treatment and Research, the Ontario Ministry of Research and Innovation, and the Leona M. and Harry B. Helmsley Charitable Trust. The authors reported no conflicts of interest.
Pediatric inflammatory bowel disease grew by more than 40% in a 15-year period in Ontario, Canada, according to a retrospective cohort study published in the October issue of Gastroenterology.
Although rates of inflammatory bowel disease (IBD) rose in children and adolescents of all ages, the steepest increase occurred in children with very-early-onset IBD (VEO-IBD), defined as disease diagnosed before they were 10 years old, said Dr. Eric Benchimol at the University of Ottawa and his associates. But these patients also tended to use fewer health services and have fewer surgeries for IBD, compared with older children with the disease, the investigators said (Gastroenterology 2014 October [doi.org/10.1053/j.gastro.2014.06.023]).
Source: American Gastroenterological Association
The findings add to research indicating that VEO-IBD is a distinct form of IBD and indicate the need to assess subgroups of these patients to look at phenotype, genotype, intestinal microbiome, and treatment response, the investigators said.
For the study, researchers created a cohort based on an algorithm of health care visits that identified all children and adolescents in Ontario diagnosed with IBD before age 18 years. The analysis included 7,143 patients with IBD, among whom about 14% had VEO-IBD, the investigators reported.
The overall rate of IBD in children up to 18 years old increased from 9.4 to 13.2 cases per 100,000 population from 1994 through 2009 (P less than .0001), the researchers said. And the yearly increase in VEO-IBD averaged 7.4% – more than three times greater than the 2.2% average annual rise among children diagnosed at 10 years and older, the investigators reported.
But health care utilization trends did not mirror changes in incidence, Dr. Benchimol and associates reported. For example, children diagnosed before they were 6 years old had significantly fewer outpatient visits for IBD, compared with children diagnosed at 10 years and older (odds ratio for girls, 0.67; 95% confidence interval, 0.58-0.78; OR for boys, 0.86; 95% CI, 0.75-0.98). Furthermore, patients diagnosed before age 6 years were less likely to be hospitalized for IBD than were older children with the disease (hazard ratio for girls, 0.70; 95% CI, 0.56-0.87; HR for boys, 1.12; 95% CI, 0.94-1.33), the investigators said.
The likelihood of undergoing intestinal resection also was lower for children diagnosed before age 6 years with Crohn’s disease, compared with older girls (HR, 0.35; 95% CI, 0.16-0.78) and boys (HR, 0.59; 95% CI, 0.34-0.99), said the researchers. And patients diagnosed before age 6 years with ulcerative colitis were less likely to undergo colectomy than were older girls (HR, 0.88; 95% CI, 0.47-1.63) and boys (HR, 0.42; 95% CI, 0.21-0.85). In contrast, rates of IBD-related surgery and hospitalization were similar between children diagnosed at 6-9.9 years of age and those diagnosed at age 10 up to 18 years, the investigators said.
A cohort study from the United States also found a lower likelihood of surgery in children with VEO-IBD, the researchers noted. Large-bowel involvement without ileal disease is prominent in young children with IBD, and these patients might be unlikely to undergo resection because colectomy requires a permanent ostotomy, they added.
The work was supported by the American College of Gastroenterology, the Ontario Ministry of Health and Long-Term Care, the Canadian Institutes of Health Research, the Crohn’s and Colitis Foundation of Canada, the National Institutes of Health, the Wolpow Family Chair in IBD Treatment and Research, the Ontario Ministry of Research and Innovation, and the Leona M. and Harry B. Helmsley Charitable Trust. The authors reported no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Although the steepest rise in inflammatory bowel disease occurred in children diagnosed before age 10 years, children diagnosed before age 6 years had the lowest rates of IBD-related outpatient visits, hospitalizations, and surgeries.
Major finding: Rates of pediatric IBD increased by more than 40% between 1994 and 2009 in Ontario, Canada. Rates rose by an average of 7.4% annually in children diagnosed before age 10 years, compared with 2.2% for children diagnosed from 10 years to before 18 years of age. Rates of outpatient visits, hospitalizations, and IBD-related surgeries were significantly lower in children diagnosed before age 6 years, compared with children diagnosed at 10 years or older.
Data Source: Retrospective study of the Ontario Crohn’s and Colitis Cohort, which included 7,143 children and adolescents with IBD diagnosed between 1994 and 2009 in Ontario, Canada.
Disclosures: The work was supported by grants and researcher awards from the American College of Gastroenterology, the Ontario Ministry of Health and Long-Term Care, the Canadian Institutes of Health Research, the Crohn’s and Colitis Foundation of Canada, the National Institutes of Health, the Wolpow Family Chair in IBD Treatment and Research, the Ontario Ministry of Research and Innovation, and the Leona M. and Harry B. Helmsley Charitable Trust. The authors reported no conflicts of interest.
ESC issues cascade of new cardiology practice guidelines
BARCELONA – Routine use of a new risk calculator tool to estimate the 5-year risk of sudden cardiac death is recommended in all patients with hypertrophic cardiomyopathy in updated guidelines launched at the annual congress of the European Society of Cardiology.
“Based on that estimate, you can now have an intelligent conversation with the patient about what the threshold is for implantable cardioverter-defibrillator placement given an individual’s risk/benefit ratio,” explained Dr. Perry M. Elliott, chair of the guidelines task force and professor of inherited cardiovascular disease at The Heart Hospital, London.
The hypertrophic cardiomyopathy guidelines were among five new practice guidelines introduced at the annual congress. Others addressed acute pulmonary embolism, noncardiac surgery, myocardial revascularization, and aortic diseases.
Here are selected highlights of the new guidelines:
• Hypertrophic cardiomyopathy. The novel sudden cardiac death risk prognostication tool, known as HCM Risk-SCD, is a major innovation in the new guidelines. The risk calculator grew out of a recent multicenter study of nearly 3,700 patients conducted by Dr. Elliott and his coinvestigators (Eur. Heart J. 2014;35:2010-20).
Now physicians can plug a series of validated risk factors into an online calculator and receive an estimated 5-year risk figure. The input factors include maximal wall thickness, left atrial diameter, family history of sudden cardiac death, unexplained syncope, and maximal left ventricular outflow gradient. If the estimated 5-year risk is 6% or more and the patient has a life expectancy of more than a year, implantation of a cardioverter-defibrillator should be seriously considered, according to the guidelines.
“What we’re trying to do in these guidelines is to change a mind-set, because hypertrophic cardiomyopathy is not really a diagnosis; it actually represents a family of diseases. Running throughout this entire document is an emphasis on individualization, from diagnosis all the way through treatment,” Dr. Elliott said. “There’s a strong emphasis on making a specific diagnosis if you possibly can, because these subtypes of cardiomyopathy have totally different natural histories and in the future will have very different treatments.”
Other highlights of the new guidelines include a stepwise approach to management of left ventricular outflow tract obstruction and heart failure, advice on reproduction, suggestions regarding simple laboratory tests with diagnostic utility, and guidance on the effective utilization of ECG, echocardiography, and cardiac magnetic resonance imaging.
“We show a number of echocardiographic red flags, some of which are perhaps not readily appreciated in everyday practice,” Dr. Elliott said. “For example, the presence of hypertrophy with impaired systolic function immediately narrows down your diagnosis to one of five or six different conditions.”
• Pulmonary embolism. The new guidelines place the novel oral anticoagulants rivaroxaban (Xarelto), apixaban (Eliquis), dabigatran (Pradaxa), and edoxaban (Savaysa) on equal footing with once-standard warfarin.
Task force chair Dr. Stavros Konstantinides highlighted as new in the 2014 guidelines a strong recommendation that patients who do not have a high-risk pulmonary embolism – that is, those who are not in shock or hypotensive – should be scored using the Pulmonary Embolism Severity Index or another validated clinical risk prediction score. The purpose is to distinguish between those at low versus intermediate risk.
Those at intermediate risk should undergo right ventricular imaging via CT or echocardiography, along with a biomarker test for myocardial injury. If both the imaging study and biomarker are positive, the patient is classified as being at intermediate-high risk; if not, intermediate-low. This has treatment implications, since it’s recommended that systemic thrombolysis be considered only in those with intermediate-high-risk pulmonary embolism and clinical signs of hemodynamic decompensation, explained Dr. Konstantinides of University Medical Center in Mainz (Germany).
• Noncardiac surgery. Perioperative initiation of beta-blocker therapy is no longer recommended routinely, but may be considered in patients scheduled for high-risk noncardiac surgery who also have two or more cardiovascular risk factors or known ischemic heart disease. Atenolol and bisoprolol are recommended as the perioperative beta-blockers of choice. If a patient is already on a beta-blocker prior to surgery, the drug can be continued perioperatively, according to task force cochair Dr. Juhani Knuuti of the University of Turku (Finland).
The guidelines emphasize that prophylactic coronary revascularization is seldom indicated in patients undergoing noncardiac surgery that has a low or intermediate cardiovascular risk.
• Myocardial revascularization. These guidelines, with more than 300 recommendations, are the lengthiest of the five new sets of guidelines. The myocardial revascularization guidelines rely heavily upon the findings of a recent meta-analysis conducted by task force cochairs Dr. Stephan Windecker of Bern (Switzerland) University and Dr. Philippe Kohl of the University of Liege (Belgium) and their coinvestigators. The meta-analysis encompassed 100 randomized controlled trials with nearly 94,000 randomized patients and more than 262,000 patient-years of follow-u p (BMJ 2014 June 23 [doi: 10.1136.bmj.g3859]).
The guidelines introduce substantial changes in the recommended method of revascularization in various situations. There is a strong emphasis on risk stratification using the SYNTAX score; in fact, the guidelines include a primer on how to calculate it.
Based largely on the results of the meta-analysis as well as 5-year follow-up in the SYNTAX trial (Lancet 2013;381:629-38), the guidelines now regard percutaneous coronary intervention as equivalent to coronary artery bypass graft surgery in several patient subsets where CABG was previously preferred. These include patients with left main or triple-vessel disease and a SYNTAX score below 22. In patients with one- or two-vessel disease and proximal left anterior descending coronary artery stenosis, both PCI and CABG get a Class I recommendation.
The new guidelines emphasize the value of intracoronary fractional flow reserve measurement to identify hemodynamically relevant lesions warranting revascularization in patients with stable coronary artery disease lacking noninvasive evidence of ischemia.
• Aortic diseases. Routine screening for abdominal aortic aneurysm via ultrasound is recommended in all men over age 65 and “may be considered” in women over 65 with a history of smoking, according to the new guidelines. That’s a much stronger proscreening stance than has been taken by the U.S. Preventive Services Task Force.
Former ESC guidelines were confined to aortic dissection and focused on the thoracic aorta. The new guidelines expand in scope to include intramural hematomas, thoracic and abdominal aneurysms, aortic valve lesions, and penetrating ulcers.
“We are not only dealing with the thoracic aorta, but are taking the holistic view of the aorta as one organ,” according to guidelines task force cochair Dr. Raimund Erbel, professor of medicine at the University of Essen (Germany).
“When an aortic aneurysm is identified at any location, assessment of the entire aorta and aortic valve is recommended at baseline and during follow-up,” he added.
All of the new guidelines can be downloaded in their entirely at the ESC website (escardio.org/guidelines).
BARCELONA – Routine use of a new risk calculator tool to estimate the 5-year risk of sudden cardiac death is recommended in all patients with hypertrophic cardiomyopathy in updated guidelines launched at the annual congress of the European Society of Cardiology.
“Based on that estimate, you can now have an intelligent conversation with the patient about what the threshold is for implantable cardioverter-defibrillator placement given an individual’s risk/benefit ratio,” explained Dr. Perry M. Elliott, chair of the guidelines task force and professor of inherited cardiovascular disease at The Heart Hospital, London.
The hypertrophic cardiomyopathy guidelines were among five new practice guidelines introduced at the annual congress. Others addressed acute pulmonary embolism, noncardiac surgery, myocardial revascularization, and aortic diseases.
Here are selected highlights of the new guidelines:
• Hypertrophic cardiomyopathy. The novel sudden cardiac death risk prognostication tool, known as HCM Risk-SCD, is a major innovation in the new guidelines. The risk calculator grew out of a recent multicenter study of nearly 3,700 patients conducted by Dr. Elliott and his coinvestigators (Eur. Heart J. 2014;35:2010-20).
Now physicians can plug a series of validated risk factors into an online calculator and receive an estimated 5-year risk figure. The input factors include maximal wall thickness, left atrial diameter, family history of sudden cardiac death, unexplained syncope, and maximal left ventricular outflow gradient. If the estimated 5-year risk is 6% or more and the patient has a life expectancy of more than a year, implantation of a cardioverter-defibrillator should be seriously considered, according to the guidelines.
“What we’re trying to do in these guidelines is to change a mind-set, because hypertrophic cardiomyopathy is not really a diagnosis; it actually represents a family of diseases. Running throughout this entire document is an emphasis on individualization, from diagnosis all the way through treatment,” Dr. Elliott said. “There’s a strong emphasis on making a specific diagnosis if you possibly can, because these subtypes of cardiomyopathy have totally different natural histories and in the future will have very different treatments.”
Other highlights of the new guidelines include a stepwise approach to management of left ventricular outflow tract obstruction and heart failure, advice on reproduction, suggestions regarding simple laboratory tests with diagnostic utility, and guidance on the effective utilization of ECG, echocardiography, and cardiac magnetic resonance imaging.
“We show a number of echocardiographic red flags, some of which are perhaps not readily appreciated in everyday practice,” Dr. Elliott said. “For example, the presence of hypertrophy with impaired systolic function immediately narrows down your diagnosis to one of five or six different conditions.”
• Pulmonary embolism. The new guidelines place the novel oral anticoagulants rivaroxaban (Xarelto), apixaban (Eliquis), dabigatran (Pradaxa), and edoxaban (Savaysa) on equal footing with once-standard warfarin.
Task force chair Dr. Stavros Konstantinides highlighted as new in the 2014 guidelines a strong recommendation that patients who do not have a high-risk pulmonary embolism – that is, those who are not in shock or hypotensive – should be scored using the Pulmonary Embolism Severity Index or another validated clinical risk prediction score. The purpose is to distinguish between those at low versus intermediate risk.
Those at intermediate risk should undergo right ventricular imaging via CT or echocardiography, along with a biomarker test for myocardial injury. If both the imaging study and biomarker are positive, the patient is classified as being at intermediate-high risk; if not, intermediate-low. This has treatment implications, since it’s recommended that systemic thrombolysis be considered only in those with intermediate-high-risk pulmonary embolism and clinical signs of hemodynamic decompensation, explained Dr. Konstantinides of University Medical Center in Mainz (Germany).
• Noncardiac surgery. Perioperative initiation of beta-blocker therapy is no longer recommended routinely, but may be considered in patients scheduled for high-risk noncardiac surgery who also have two or more cardiovascular risk factors or known ischemic heart disease. Atenolol and bisoprolol are recommended as the perioperative beta-blockers of choice. If a patient is already on a beta-blocker prior to surgery, the drug can be continued perioperatively, according to task force cochair Dr. Juhani Knuuti of the University of Turku (Finland).
The guidelines emphasize that prophylactic coronary revascularization is seldom indicated in patients undergoing noncardiac surgery that has a low or intermediate cardiovascular risk.
• Myocardial revascularization. These guidelines, with more than 300 recommendations, are the lengthiest of the five new sets of guidelines. The myocardial revascularization guidelines rely heavily upon the findings of a recent meta-analysis conducted by task force cochairs Dr. Stephan Windecker of Bern (Switzerland) University and Dr. Philippe Kohl of the University of Liege (Belgium) and their coinvestigators. The meta-analysis encompassed 100 randomized controlled trials with nearly 94,000 randomized patients and more than 262,000 patient-years of follow-u p (BMJ 2014 June 23 [doi: 10.1136.bmj.g3859]).
The guidelines introduce substantial changes in the recommended method of revascularization in various situations. There is a strong emphasis on risk stratification using the SYNTAX score; in fact, the guidelines include a primer on how to calculate it.
Based largely on the results of the meta-analysis as well as 5-year follow-up in the SYNTAX trial (Lancet 2013;381:629-38), the guidelines now regard percutaneous coronary intervention as equivalent to coronary artery bypass graft surgery in several patient subsets where CABG was previously preferred. These include patients with left main or triple-vessel disease and a SYNTAX score below 22. In patients with one- or two-vessel disease and proximal left anterior descending coronary artery stenosis, both PCI and CABG get a Class I recommendation.
The new guidelines emphasize the value of intracoronary fractional flow reserve measurement to identify hemodynamically relevant lesions warranting revascularization in patients with stable coronary artery disease lacking noninvasive evidence of ischemia.
• Aortic diseases. Routine screening for abdominal aortic aneurysm via ultrasound is recommended in all men over age 65 and “may be considered” in women over 65 with a history of smoking, according to the new guidelines. That’s a much stronger proscreening stance than has been taken by the U.S. Preventive Services Task Force.
Former ESC guidelines were confined to aortic dissection and focused on the thoracic aorta. The new guidelines expand in scope to include intramural hematomas, thoracic and abdominal aneurysms, aortic valve lesions, and penetrating ulcers.
“We are not only dealing with the thoracic aorta, but are taking the holistic view of the aorta as one organ,” according to guidelines task force cochair Dr. Raimund Erbel, professor of medicine at the University of Essen (Germany).
“When an aortic aneurysm is identified at any location, assessment of the entire aorta and aortic valve is recommended at baseline and during follow-up,” he added.
All of the new guidelines can be downloaded in their entirely at the ESC website (escardio.org/guidelines).
BARCELONA – Routine use of a new risk calculator tool to estimate the 5-year risk of sudden cardiac death is recommended in all patients with hypertrophic cardiomyopathy in updated guidelines launched at the annual congress of the European Society of Cardiology.
“Based on that estimate, you can now have an intelligent conversation with the patient about what the threshold is for implantable cardioverter-defibrillator placement given an individual’s risk/benefit ratio,” explained Dr. Perry M. Elliott, chair of the guidelines task force and professor of inherited cardiovascular disease at The Heart Hospital, London.
The hypertrophic cardiomyopathy guidelines were among five new practice guidelines introduced at the annual congress. Others addressed acute pulmonary embolism, noncardiac surgery, myocardial revascularization, and aortic diseases.
Here are selected highlights of the new guidelines:
• Hypertrophic cardiomyopathy. The novel sudden cardiac death risk prognostication tool, known as HCM Risk-SCD, is a major innovation in the new guidelines. The risk calculator grew out of a recent multicenter study of nearly 3,700 patients conducted by Dr. Elliott and his coinvestigators (Eur. Heart J. 2014;35:2010-20).
Now physicians can plug a series of validated risk factors into an online calculator and receive an estimated 5-year risk figure. The input factors include maximal wall thickness, left atrial diameter, family history of sudden cardiac death, unexplained syncope, and maximal left ventricular outflow gradient. If the estimated 5-year risk is 6% or more and the patient has a life expectancy of more than a year, implantation of a cardioverter-defibrillator should be seriously considered, according to the guidelines.
“What we’re trying to do in these guidelines is to change a mind-set, because hypertrophic cardiomyopathy is not really a diagnosis; it actually represents a family of diseases. Running throughout this entire document is an emphasis on individualization, from diagnosis all the way through treatment,” Dr. Elliott said. “There’s a strong emphasis on making a specific diagnosis if you possibly can, because these subtypes of cardiomyopathy have totally different natural histories and in the future will have very different treatments.”
Other highlights of the new guidelines include a stepwise approach to management of left ventricular outflow tract obstruction and heart failure, advice on reproduction, suggestions regarding simple laboratory tests with diagnostic utility, and guidance on the effective utilization of ECG, echocardiography, and cardiac magnetic resonance imaging.
“We show a number of echocardiographic red flags, some of which are perhaps not readily appreciated in everyday practice,” Dr. Elliott said. “For example, the presence of hypertrophy with impaired systolic function immediately narrows down your diagnosis to one of five or six different conditions.”
• Pulmonary embolism. The new guidelines place the novel oral anticoagulants rivaroxaban (Xarelto), apixaban (Eliquis), dabigatran (Pradaxa), and edoxaban (Savaysa) on equal footing with once-standard warfarin.
Task force chair Dr. Stavros Konstantinides highlighted as new in the 2014 guidelines a strong recommendation that patients who do not have a high-risk pulmonary embolism – that is, those who are not in shock or hypotensive – should be scored using the Pulmonary Embolism Severity Index or another validated clinical risk prediction score. The purpose is to distinguish between those at low versus intermediate risk.
Those at intermediate risk should undergo right ventricular imaging via CT or echocardiography, along with a biomarker test for myocardial injury. If both the imaging study and biomarker are positive, the patient is classified as being at intermediate-high risk; if not, intermediate-low. This has treatment implications, since it’s recommended that systemic thrombolysis be considered only in those with intermediate-high-risk pulmonary embolism and clinical signs of hemodynamic decompensation, explained Dr. Konstantinides of University Medical Center in Mainz (Germany).
• Noncardiac surgery. Perioperative initiation of beta-blocker therapy is no longer recommended routinely, but may be considered in patients scheduled for high-risk noncardiac surgery who also have two or more cardiovascular risk factors or known ischemic heart disease. Atenolol and bisoprolol are recommended as the perioperative beta-blockers of choice. If a patient is already on a beta-blocker prior to surgery, the drug can be continued perioperatively, according to task force cochair Dr. Juhani Knuuti of the University of Turku (Finland).
The guidelines emphasize that prophylactic coronary revascularization is seldom indicated in patients undergoing noncardiac surgery that has a low or intermediate cardiovascular risk.
• Myocardial revascularization. These guidelines, with more than 300 recommendations, are the lengthiest of the five new sets of guidelines. The myocardial revascularization guidelines rely heavily upon the findings of a recent meta-analysis conducted by task force cochairs Dr. Stephan Windecker of Bern (Switzerland) University and Dr. Philippe Kohl of the University of Liege (Belgium) and their coinvestigators. The meta-analysis encompassed 100 randomized controlled trials with nearly 94,000 randomized patients and more than 262,000 patient-years of follow-u p (BMJ 2014 June 23 [doi: 10.1136.bmj.g3859]).
The guidelines introduce substantial changes in the recommended method of revascularization in various situations. There is a strong emphasis on risk stratification using the SYNTAX score; in fact, the guidelines include a primer on how to calculate it.
Based largely on the results of the meta-analysis as well as 5-year follow-up in the SYNTAX trial (Lancet 2013;381:629-38), the guidelines now regard percutaneous coronary intervention as equivalent to coronary artery bypass graft surgery in several patient subsets where CABG was previously preferred. These include patients with left main or triple-vessel disease and a SYNTAX score below 22. In patients with one- or two-vessel disease and proximal left anterior descending coronary artery stenosis, both PCI and CABG get a Class I recommendation.
The new guidelines emphasize the value of intracoronary fractional flow reserve measurement to identify hemodynamically relevant lesions warranting revascularization in patients with stable coronary artery disease lacking noninvasive evidence of ischemia.
• Aortic diseases. Routine screening for abdominal aortic aneurysm via ultrasound is recommended in all men over age 65 and “may be considered” in women over 65 with a history of smoking, according to the new guidelines. That’s a much stronger proscreening stance than has been taken by the U.S. Preventive Services Task Force.
Former ESC guidelines were confined to aortic dissection and focused on the thoracic aorta. The new guidelines expand in scope to include intramural hematomas, thoracic and abdominal aneurysms, aortic valve lesions, and penetrating ulcers.
“We are not only dealing with the thoracic aorta, but are taking the holistic view of the aorta as one organ,” according to guidelines task force cochair Dr. Raimund Erbel, professor of medicine at the University of Essen (Germany).
“When an aortic aneurysm is identified at any location, assessment of the entire aorta and aortic valve is recommended at baseline and during follow-up,” he added.
All of the new guidelines can be downloaded in their entirely at the ESC website (escardio.org/guidelines).
AT THE ESC CONGRESS 2014
AHA/ACC: No to universal ECG screen in healthy young people
Twelve-lead ECG should not be used to screen healthy young people in the general population for occult cardiovascular abnormalities, according to a scientific statement jointly released Sept. 15 by the American Heart Association and the American College of Cardiology
Such screening has been advocated as a way to identify young people at risk for sudden death. Those at risk then could avoid participating in sports that could trigger a fatal CV event, and cardioverter-defibrillators could be implanted in young patients who would benefit.
The issue is ontroversial, however. Opponents argue that existing screening technology produces too many false-positive and false-negative results to be useful on a large scale. In addition, such screening would be exhorbitantly expensive, diverting scarce health care resources away from other, more practical programs that would be more beneficial to the approximately 60 million young people in this patient population.
“[We] acknowledge the tragic nature of sudden deaths in the young, but do not believe the available data support a significant public health benefit from using the 12-lead ECG as a universal screening tool,” said Dr. Barry J. Maron, chair, and Dr. Richard A. Friedman, cochair, of the AHA/ACC writing committee that presented the scientific statement published online in Circulation and the Journal of the American College of Cardiology (Circulation 2014 Sept. 15 [doi: 10.1161/CIR.0000000000000025]).
The Pediatric and Congenital Electrophysiology Society and the American College of Sports Medicine also endorsed the statement.
The investigators reviewed the evidence both for and against ECG screening for all young people and for the subgroup of young athletes, who number an estimated 10 million in the United States. They found that universal screening using ECGs “would be an undertaking of enormous magnitude, with massive resource demands.” Moreover, the net benefit would be “trivial,” given the very low prevalence of abnormalities that cause sudden death in youths and the extremely low risk of sudden death even in these at-risk people.
The nationwide registry of sudden deaths among athletes documents approximately 75 cardiovascular deaths per year – a frequency that is much lower than that for virtually every other cause of death in this age group. By comparison, motor vehicle accidents cause approximately 2,500-fold more deaths per year than cardiovascular events during sports, they noted.
In addition, 12-lead ECG would make an “imperfect” screening test, especially “in a real-world mass screening setting [when] readers and technicians [who have] vastly different expertise and efficiency are confronted with large numbers of studies to perform and interpret rapidly,” the authors noted.
The overlap between normal and abnormal ECG measurements is “a major obstacle,” with readings from people with high-risk cardiovascular disease sometimes indistinguishable from those of healthy patients. Misplacement of electrodes, selection of inadequate bandwidth, inadvertent lead reversal, and imprecise measurement of the QT interval are common operator-related difficulties.
At present, the rates of both false-positive and false-negative ECG results are unacceptably high for large-scale screening, the committee said. False-positive results lead to unnecessary and expensive further testing; unwarranted restriction from sports and other activities; anxiety and other adverse psychological consequences; and impediments to insurability or employment.
Even if the false-positive rate could be reduced to only 5% of all ECGs, the authors added, in a population of 10 million athletes, this would disqualify 500,000 healthy people from sports until they underwent further testing to exclude heart abnormalities.
Restricting ECG screening only to athletes would lessen these problems but would introduce others. For example, confining mass screening to a certain segment of the population necessarily excludes other segments. In the case of restricting ECG screening to college athletes, it could appear that this process moves from being exclusionary to being discriminatory and even elitist.
Overall, the statement’s authors concluded that “currently there is insufficient information available to support the view that universal screening ECGs in asymptomatic young people for CVD is appropriate or possible on a national basis for the United States, in competitive athletes or in the general youthful population, and practical issues essentially exclude either strategy from any realistic consideration.”
The statement is available from the AHA at http://my.americanheart.org and from the ACC at http://cardiosource.org.
This scientific statement was supported by the American Heart Association and the American College of Cardiology. The authors’ financial disclosures were not available.
Twelve-lead ECG should not be used to screen healthy young people in the general population for occult cardiovascular abnormalities, according to a scientific statement jointly released Sept. 15 by the American Heart Association and the American College of Cardiology
Such screening has been advocated as a way to identify young people at risk for sudden death. Those at risk then could avoid participating in sports that could trigger a fatal CV event, and cardioverter-defibrillators could be implanted in young patients who would benefit.
The issue is ontroversial, however. Opponents argue that existing screening technology produces too many false-positive and false-negative results to be useful on a large scale. In addition, such screening would be exhorbitantly expensive, diverting scarce health care resources away from other, more practical programs that would be more beneficial to the approximately 60 million young people in this patient population.
“[We] acknowledge the tragic nature of sudden deaths in the young, but do not believe the available data support a significant public health benefit from using the 12-lead ECG as a universal screening tool,” said Dr. Barry J. Maron, chair, and Dr. Richard A. Friedman, cochair, of the AHA/ACC writing committee that presented the scientific statement published online in Circulation and the Journal of the American College of Cardiology (Circulation 2014 Sept. 15 [doi: 10.1161/CIR.0000000000000025]).
The Pediatric and Congenital Electrophysiology Society and the American College of Sports Medicine also endorsed the statement.
The investigators reviewed the evidence both for and against ECG screening for all young people and for the subgroup of young athletes, who number an estimated 10 million in the United States. They found that universal screening using ECGs “would be an undertaking of enormous magnitude, with massive resource demands.” Moreover, the net benefit would be “trivial,” given the very low prevalence of abnormalities that cause sudden death in youths and the extremely low risk of sudden death even in these at-risk people.
The nationwide registry of sudden deaths among athletes documents approximately 75 cardiovascular deaths per year – a frequency that is much lower than that for virtually every other cause of death in this age group. By comparison, motor vehicle accidents cause approximately 2,500-fold more deaths per year than cardiovascular events during sports, they noted.
In addition, 12-lead ECG would make an “imperfect” screening test, especially “in a real-world mass screening setting [when] readers and technicians [who have] vastly different expertise and efficiency are confronted with large numbers of studies to perform and interpret rapidly,” the authors noted.
The overlap between normal and abnormal ECG measurements is “a major obstacle,” with readings from people with high-risk cardiovascular disease sometimes indistinguishable from those of healthy patients. Misplacement of electrodes, selection of inadequate bandwidth, inadvertent lead reversal, and imprecise measurement of the QT interval are common operator-related difficulties.
At present, the rates of both false-positive and false-negative ECG results are unacceptably high for large-scale screening, the committee said. False-positive results lead to unnecessary and expensive further testing; unwarranted restriction from sports and other activities; anxiety and other adverse psychological consequences; and impediments to insurability or employment.
Even if the false-positive rate could be reduced to only 5% of all ECGs, the authors added, in a population of 10 million athletes, this would disqualify 500,000 healthy people from sports until they underwent further testing to exclude heart abnormalities.
Restricting ECG screening only to athletes would lessen these problems but would introduce others. For example, confining mass screening to a certain segment of the population necessarily excludes other segments. In the case of restricting ECG screening to college athletes, it could appear that this process moves from being exclusionary to being discriminatory and even elitist.
Overall, the statement’s authors concluded that “currently there is insufficient information available to support the view that universal screening ECGs in asymptomatic young people for CVD is appropriate or possible on a national basis for the United States, in competitive athletes or in the general youthful population, and practical issues essentially exclude either strategy from any realistic consideration.”
The statement is available from the AHA at http://my.americanheart.org and from the ACC at http://cardiosource.org.
This scientific statement was supported by the American Heart Association and the American College of Cardiology. The authors’ financial disclosures were not available.
Twelve-lead ECG should not be used to screen healthy young people in the general population for occult cardiovascular abnormalities, according to a scientific statement jointly released Sept. 15 by the American Heart Association and the American College of Cardiology
Such screening has been advocated as a way to identify young people at risk for sudden death. Those at risk then could avoid participating in sports that could trigger a fatal CV event, and cardioverter-defibrillators could be implanted in young patients who would benefit.
The issue is ontroversial, however. Opponents argue that existing screening technology produces too many false-positive and false-negative results to be useful on a large scale. In addition, such screening would be exhorbitantly expensive, diverting scarce health care resources away from other, more practical programs that would be more beneficial to the approximately 60 million young people in this patient population.
“[We] acknowledge the tragic nature of sudden deaths in the young, but do not believe the available data support a significant public health benefit from using the 12-lead ECG as a universal screening tool,” said Dr. Barry J. Maron, chair, and Dr. Richard A. Friedman, cochair, of the AHA/ACC writing committee that presented the scientific statement published online in Circulation and the Journal of the American College of Cardiology (Circulation 2014 Sept. 15 [doi: 10.1161/CIR.0000000000000025]).
The Pediatric and Congenital Electrophysiology Society and the American College of Sports Medicine also endorsed the statement.
The investigators reviewed the evidence both for and against ECG screening for all young people and for the subgroup of young athletes, who number an estimated 10 million in the United States. They found that universal screening using ECGs “would be an undertaking of enormous magnitude, with massive resource demands.” Moreover, the net benefit would be “trivial,” given the very low prevalence of abnormalities that cause sudden death in youths and the extremely low risk of sudden death even in these at-risk people.
The nationwide registry of sudden deaths among athletes documents approximately 75 cardiovascular deaths per year – a frequency that is much lower than that for virtually every other cause of death in this age group. By comparison, motor vehicle accidents cause approximately 2,500-fold more deaths per year than cardiovascular events during sports, they noted.
In addition, 12-lead ECG would make an “imperfect” screening test, especially “in a real-world mass screening setting [when] readers and technicians [who have] vastly different expertise and efficiency are confronted with large numbers of studies to perform and interpret rapidly,” the authors noted.
The overlap between normal and abnormal ECG measurements is “a major obstacle,” with readings from people with high-risk cardiovascular disease sometimes indistinguishable from those of healthy patients. Misplacement of electrodes, selection of inadequate bandwidth, inadvertent lead reversal, and imprecise measurement of the QT interval are common operator-related difficulties.
At present, the rates of both false-positive and false-negative ECG results are unacceptably high for large-scale screening, the committee said. False-positive results lead to unnecessary and expensive further testing; unwarranted restriction from sports and other activities; anxiety and other adverse psychological consequences; and impediments to insurability or employment.
Even if the false-positive rate could be reduced to only 5% of all ECGs, the authors added, in a population of 10 million athletes, this would disqualify 500,000 healthy people from sports until they underwent further testing to exclude heart abnormalities.
Restricting ECG screening only to athletes would lessen these problems but would introduce others. For example, confining mass screening to a certain segment of the population necessarily excludes other segments. In the case of restricting ECG screening to college athletes, it could appear that this process moves from being exclusionary to being discriminatory and even elitist.
Overall, the statement’s authors concluded that “currently there is insufficient information available to support the view that universal screening ECGs in asymptomatic young people for CVD is appropriate or possible on a national basis for the United States, in competitive athletes or in the general youthful population, and practical issues essentially exclude either strategy from any realistic consideration.”
The statement is available from the AHA at http://my.americanheart.org and from the ACC at http://cardiosource.org.
This scientific statement was supported by the American Heart Association and the American College of Cardiology. The authors’ financial disclosures were not available.
FROM CIRCULATION
Key clinical point: Don’t use 12-lead ECG to screen healthy young people for cardiovascular abnormalities.
Major finding: Insufficient information is available to support universal screening ECGs in asymptomatic young people for cardiovascular abnormalities, either competitive athletes or the general youthful population – and practical issues essentially exclude ECG screening from any realistic consideration.
Data source: A scientific statement based on a review of the available evidence regarding the use of 12-lead ECG to screen healthy people in the general population aged 12-25 years for occult CVD.
Disclosures: This scientific statement was supported by the American Heart Association and the American College of Cardiology. The authors’ financial disclosures were not available.
NHLBI expert panel issues guideline on sickle cell disease
The "much anticipated" guideline to help primary care and emergency clinicians improve the management of sickle cell disease includes a consensus treatment protocol for implementing hydroxyurea therapy and more detailed guidance regarding long-term transfusion therapy, according to a summary report published online September 9 in Journal of the American Medical Association.
Sickle cell disease (SCD), a life-threatening genetically transmitted disorder affecting 70,000-100,000 Americans, is associated with a wide array of complex acute and chronic complications that require immediate medical attention. But high-quality data on which to base management decisions are sorely lacking, and clinicians get little in the way of guidance from existing recommendations. One result is that "the two most widely available disease-modifying therapies, hydroxyurea and long-term transfusions, are underused, and hematopoietic stem cell transplantation, the only curative approach, has been used in only a small proportion of affected individuals," said Dr. Barbara P. Yawn and her associates on the National Heart, Lung, and Blood Institute expert panel that issued the summary report.
Even this guideline is somewhat rudimentary due to the dearth of good data "in virtually every area related to SCD management," and cannot help but leave "many uncertainties for health professionals caring for individuals with SCD." But it is hoped that this guideline will furnish a critical foundation for future research and will now begin "to facilitate improved and more accessible care for all affected individuals," said Dr. Yawn, director of research at Olmsted Medical Center, Rochester, Minn., and her associates.
The guideline is based on an extensive literature review of more than 13,000 abstracts and articles, which was winnowed to 1,583 original studies regarding SCD. From this, a team of health care professionals in family medicine, internal medicine, pediatric and adult hematology, psychiatry and mental health, transfusion medicine, obstetrics and gynecology, maternal/fetal medicine, and emergency department nursing compiled the guideline as well as the summary, entitled Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014 (JAMA 2014 September 9 [doi:10.1001/jama.2014.10517]).
In addition to establishing a protocol for implementing hydroxyurea therapy, the guideline addresses changes in pneumococcal vaccination recommendations for adults and children; annual transcranial Doppler screening coupled with long-term transfusion therapy when necessary to prevent stroke in children aged 2-16 years; rapid initiation of opioids for severe pain during vasoocclusive crises; analgesics and physical therapy for avascular necrosis; ACE inhibitor treatment for adults with microalbuminuria; referral to specialists for screening and treatment of proliferative retinopathy; echocardiography to assess signs of pulmonary hypertension; and monitoring for iron overload in patients receiving transfusion therapy.
Both the summary report and the full guideline are available at http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/.
Yawn et al. have made a monumental effort to produce practical, evidence-based guidelines, but they were hampered at every turn by a pervasive lack of good quality evidence on which to base their recommendations. Still missing from this guideline are suggestions for how often and when to screen for kidney disease, how to screen for and treat the common clinical problem of asthma-like symptoms (when standard therapies are contraindicated in SCD), how to advocate for patients with the common sequelae of silent cerebral infarcts, or when to consider hematopoietic stem-cell transplantation.
The expert panel also failed to include representatives from the people most affected by SCD: patients and their families. Failure to listen to the perspective of the families, understand which of these recommendations are important to them, and deal with the obstacles families face in implementing the recommendations is a critically important omission.
Dr. Michael R. DeBaun is in the department of pediatrics at the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Nashville. He made his remarks in an editorial accompanying Dr. Yawn’s report (JAMA 2014:312;1004-5). Dr. DeBaun reported no financial conflicts of interest.
Yawn et al. have made a monumental effort to produce practical, evidence-based guidelines, but they were hampered at every turn by a pervasive lack of good quality evidence on which to base their recommendations. Still missing from this guideline are suggestions for how often and when to screen for kidney disease, how to screen for and treat the common clinical problem of asthma-like symptoms (when standard therapies are contraindicated in SCD), how to advocate for patients with the common sequelae of silent cerebral infarcts, or when to consider hematopoietic stem-cell transplantation.
The expert panel also failed to include representatives from the people most affected by SCD: patients and their families. Failure to listen to the perspective of the families, understand which of these recommendations are important to them, and deal with the obstacles families face in implementing the recommendations is a critically important omission.
Dr. Michael R. DeBaun is in the department of pediatrics at the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Nashville. He made his remarks in an editorial accompanying Dr. Yawn’s report (JAMA 2014:312;1004-5). Dr. DeBaun reported no financial conflicts of interest.
Yawn et al. have made a monumental effort to produce practical, evidence-based guidelines, but they were hampered at every turn by a pervasive lack of good quality evidence on which to base their recommendations. Still missing from this guideline are suggestions for how often and when to screen for kidney disease, how to screen for and treat the common clinical problem of asthma-like symptoms (when standard therapies are contraindicated in SCD), how to advocate for patients with the common sequelae of silent cerebral infarcts, or when to consider hematopoietic stem-cell transplantation.
The expert panel also failed to include representatives from the people most affected by SCD: patients and their families. Failure to listen to the perspective of the families, understand which of these recommendations are important to them, and deal with the obstacles families face in implementing the recommendations is a critically important omission.
Dr. Michael R. DeBaun is in the department of pediatrics at the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Nashville. He made his remarks in an editorial accompanying Dr. Yawn’s report (JAMA 2014:312;1004-5). Dr. DeBaun reported no financial conflicts of interest.
The "much anticipated" guideline to help primary care and emergency clinicians improve the management of sickle cell disease includes a consensus treatment protocol for implementing hydroxyurea therapy and more detailed guidance regarding long-term transfusion therapy, according to a summary report published online September 9 in Journal of the American Medical Association.
Sickle cell disease (SCD), a life-threatening genetically transmitted disorder affecting 70,000-100,000 Americans, is associated with a wide array of complex acute and chronic complications that require immediate medical attention. But high-quality data on which to base management decisions are sorely lacking, and clinicians get little in the way of guidance from existing recommendations. One result is that "the two most widely available disease-modifying therapies, hydroxyurea and long-term transfusions, are underused, and hematopoietic stem cell transplantation, the only curative approach, has been used in only a small proportion of affected individuals," said Dr. Barbara P. Yawn and her associates on the National Heart, Lung, and Blood Institute expert panel that issued the summary report.
Even this guideline is somewhat rudimentary due to the dearth of good data "in virtually every area related to SCD management," and cannot help but leave "many uncertainties for health professionals caring for individuals with SCD." But it is hoped that this guideline will furnish a critical foundation for future research and will now begin "to facilitate improved and more accessible care for all affected individuals," said Dr. Yawn, director of research at Olmsted Medical Center, Rochester, Minn., and her associates.
The guideline is based on an extensive literature review of more than 13,000 abstracts and articles, which was winnowed to 1,583 original studies regarding SCD. From this, a team of health care professionals in family medicine, internal medicine, pediatric and adult hematology, psychiatry and mental health, transfusion medicine, obstetrics and gynecology, maternal/fetal medicine, and emergency department nursing compiled the guideline as well as the summary, entitled Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014 (JAMA 2014 September 9 [doi:10.1001/jama.2014.10517]).
In addition to establishing a protocol for implementing hydroxyurea therapy, the guideline addresses changes in pneumococcal vaccination recommendations for adults and children; annual transcranial Doppler screening coupled with long-term transfusion therapy when necessary to prevent stroke in children aged 2-16 years; rapid initiation of opioids for severe pain during vasoocclusive crises; analgesics and physical therapy for avascular necrosis; ACE inhibitor treatment for adults with microalbuminuria; referral to specialists for screening and treatment of proliferative retinopathy; echocardiography to assess signs of pulmonary hypertension; and monitoring for iron overload in patients receiving transfusion therapy.
Both the summary report and the full guideline are available at http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/.
The "much anticipated" guideline to help primary care and emergency clinicians improve the management of sickle cell disease includes a consensus treatment protocol for implementing hydroxyurea therapy and more detailed guidance regarding long-term transfusion therapy, according to a summary report published online September 9 in Journal of the American Medical Association.
Sickle cell disease (SCD), a life-threatening genetically transmitted disorder affecting 70,000-100,000 Americans, is associated with a wide array of complex acute and chronic complications that require immediate medical attention. But high-quality data on which to base management decisions are sorely lacking, and clinicians get little in the way of guidance from existing recommendations. One result is that "the two most widely available disease-modifying therapies, hydroxyurea and long-term transfusions, are underused, and hematopoietic stem cell transplantation, the only curative approach, has been used in only a small proportion of affected individuals," said Dr. Barbara P. Yawn and her associates on the National Heart, Lung, and Blood Institute expert panel that issued the summary report.
Even this guideline is somewhat rudimentary due to the dearth of good data "in virtually every area related to SCD management," and cannot help but leave "many uncertainties for health professionals caring for individuals with SCD." But it is hoped that this guideline will furnish a critical foundation for future research and will now begin "to facilitate improved and more accessible care for all affected individuals," said Dr. Yawn, director of research at Olmsted Medical Center, Rochester, Minn., and her associates.
The guideline is based on an extensive literature review of more than 13,000 abstracts and articles, which was winnowed to 1,583 original studies regarding SCD. From this, a team of health care professionals in family medicine, internal medicine, pediatric and adult hematology, psychiatry and mental health, transfusion medicine, obstetrics and gynecology, maternal/fetal medicine, and emergency department nursing compiled the guideline as well as the summary, entitled Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014 (JAMA 2014 September 9 [doi:10.1001/jama.2014.10517]).
In addition to establishing a protocol for implementing hydroxyurea therapy, the guideline addresses changes in pneumococcal vaccination recommendations for adults and children; annual transcranial Doppler screening coupled with long-term transfusion therapy when necessary to prevent stroke in children aged 2-16 years; rapid initiation of opioids for severe pain during vasoocclusive crises; analgesics and physical therapy for avascular necrosis; ACE inhibitor treatment for adults with microalbuminuria; referral to specialists for screening and treatment of proliferative retinopathy; echocardiography to assess signs of pulmonary hypertension; and monitoring for iron overload in patients receiving transfusion therapy.
Both the summary report and the full guideline are available at http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/.
FROM JAMA
Major finding: The two most widely available disease-modifying therapies for SCD, hydroxyurea and long-term transfusions, are underused, in large part because no evidence-based treatment protocols have been devised until now.
Data source: A review of the literature and compilation of management guidelines "to assist health care professionals in the management of common issues of sickle cell disease."
Disclosures: The National Heart, Lung, and Blood Institute sponsored the development of this guideline. All expert panel members served voluntarily. Many reported numerous ties to industry sources.
USPSTF recommends low-dose aspirin for preeclampsia prevention
The use of low-dose aspirin is advisable after 12 weeks of gestation in asymptomatic pregnant women at high risk for developing preeclampsia, according to a recommendation from the U.S. Preventive Services Task Force.
The recommendation, published online Sept. 8 in the Annals of Internal Medicine, is based on a review of new evidence suggesting that the net benefit of low-dose aspirin for preventing preeclampsia is of substantial magnitude. It updates a 1996 recommendation from the USPSTF, which concluded that there was insufficient evidence at that time to recommend for or against the routine use of aspirin for the prevention of preeclampsia.
The current evidence – including 15 randomized controlled trials used to assess the health benefits of low-dose aspirin, 13 randomized controlled trials used to evaluate preeclampsia incidence, and 19 randomized controlled trials and 2 good-quality observational studies used to evaluate harms associated with low-dose aspirin use – suggests that women at risk may benefit from low-dose aspirin beginning after 12 weeks of gestation.
Preeclampsia complicates 2%-8% of pregnancies worldwide, and accounts for 15% of preterm births and 12% of maternal deaths in the United States, according to the task force.
"The USPSTF found adequate evidence of a reduction in risk for preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit. Low-dose aspirin (range, 60-150 mg/day) reduced the risk for preeclampsia by 24% in clinical trials [pooled relative risk, 0.76] and reduced the risk for preterm birth by 14% and IUGR by 20% [pooled relative risk, 0.86 and 0.80, respectively]," the updated recommendation stated (Ann. Intern. Med. 2014 Sept. 8 [doi:10.7326/m14-1884]).
Adequate evidence also indicates that low-dose aspirin is not associated with any increase in the risk of placental abruption, postpartum hemorrhage, fetal intracranial bleeding, or perinatal mortality.
"Evidence on long-term outcomes in offspring exposed in utero to low-dose aspirin is limited, but no developmental harms were identified by age 18 months in the one study reviewed," the task force wrote, concluding – with moderate certainty – that there is a substantial net benefit of daily low-dose aspirin use to reduce the risk for preeclampsia, preterm birth, and IUGR in women at high risk.
The decision to initiate low-dose aspirin therapy in this population is typically based on medical history; there are no validated methods for identifying women at high risk based on biomarkers, clinical diagnostic tests, or medical history. However, as part of the recommendation, the USPSTF provided a pragmatic approach that may help identify those at risk.
"Women with one or more risk factors should receive low-dose aspirin. Women with several moderate risk factors may also benefit from low-dose aspirin," the task force noted, adding that the evidence for the latter approach is less certain, and that clinicians should use clinical judgment and discuss the risks and benefits with patients.
The recommendation applies to asymptomatic women at risk in whom low-dose aspirin is not contraindicated, and defines women at high risk as those with a history of preeclampsia, especially those with an adverse outcome; chronic hypertension, renal disease, type 1 or 2 diabetes, or an autoimmune disease; and those with multifetal gestation, according to the updated recommendation.
Moderate risk factors include nulliparity, obesity, a family history of preeclampsia, age greater than or equal to 35 years, African American race, low socioeconomic status, low birth rate or small for gestational age, greater than 10-year pregnancy interval, or previous adverse pregnancy outcome.
As for appropriate dosing, the most common dosage across studies was 100 mg, but the two largest trials contributing to benefit estimates used 60 mg.
An 81-mg dose was not specifically evaluated, but is commonly available in the United States in tablet form, and is a reasonable dosage for preeclampsia prophylaxis, the task force said.
The updated recommendation is generally in keeping with those of other organizations, including the American College of Obstetricians and Gynecologists, the World Health Organization, the National Institute for Health and Clinical Excellence, the American Heart Association/American Stroke Association, and the American Academy of Family Physicians. For example, ACOG recommends initiating daily low-dose aspirin during the late first trimester in those with a history of early-onset preeclampsia and preterm delivery, or with a history of preeclampsia in more than one prior pregnancy (<cf number="\"2\"">“</cf>American College of Obstetricians and Gynecologists: Hypertension in Pregnancy [Washington, D.C.: American College of Obstetricians and Gynecologists, 2013]), and WHO recommends daily low-dose aspirin as early as 12 weeks for those at high risk ("WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia" [Geneva: World Health Organization, 2011]).
The review by the USPSTF identified several research needs. For example, additional study is needed on the effects of low-dose aspirin on the development of preeclampsia and how patient response is affected by various risk factors. Research is also needed on how to improve clinicians’ ability to identify those at risk, and particularly those who would benefit most from prophylaxis. Study is needed on risk assessment tools, and on populations at particular risk, such as African American and nulliparous women.
Future trials should recruit adequate numbers of women from racial/ethnic populations that are at disproportionate risk.
"Larger studies on aspirin use in the first or early second trimester may improve the evidence base on optimal timing of low-dose aspirin preventive medication. Other areas of research include optimal therapies that individualize the aspirin dosage and timing of administration (e.g., morning vs. bedtime)," they concluded, noting that research is also needed to explore less-well-established risk factors, and to investigate whether preeclampsia prevention with low-dose aspirin affects long-term risk for cardiovascular disease, and whether there is any benefit to continuing low-dose aspirin after delivery in those at high risk.
The use of low-dose aspirin is advisable after 12 weeks of gestation in asymptomatic pregnant women at high risk for developing preeclampsia, according to a recommendation from the U.S. Preventive Services Task Force.
The recommendation, published online Sept. 8 in the Annals of Internal Medicine, is based on a review of new evidence suggesting that the net benefit of low-dose aspirin for preventing preeclampsia is of substantial magnitude. It updates a 1996 recommendation from the USPSTF, which concluded that there was insufficient evidence at that time to recommend for or against the routine use of aspirin for the prevention of preeclampsia.
The current evidence – including 15 randomized controlled trials used to assess the health benefits of low-dose aspirin, 13 randomized controlled trials used to evaluate preeclampsia incidence, and 19 randomized controlled trials and 2 good-quality observational studies used to evaluate harms associated with low-dose aspirin use – suggests that women at risk may benefit from low-dose aspirin beginning after 12 weeks of gestation.
Preeclampsia complicates 2%-8% of pregnancies worldwide, and accounts for 15% of preterm births and 12% of maternal deaths in the United States, according to the task force.
"The USPSTF found adequate evidence of a reduction in risk for preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit. Low-dose aspirin (range, 60-150 mg/day) reduced the risk for preeclampsia by 24% in clinical trials [pooled relative risk, 0.76] and reduced the risk for preterm birth by 14% and IUGR by 20% [pooled relative risk, 0.86 and 0.80, respectively]," the updated recommendation stated (Ann. Intern. Med. 2014 Sept. 8 [doi:10.7326/m14-1884]).
Adequate evidence also indicates that low-dose aspirin is not associated with any increase in the risk of placental abruption, postpartum hemorrhage, fetal intracranial bleeding, or perinatal mortality.
"Evidence on long-term outcomes in offspring exposed in utero to low-dose aspirin is limited, but no developmental harms were identified by age 18 months in the one study reviewed," the task force wrote, concluding – with moderate certainty – that there is a substantial net benefit of daily low-dose aspirin use to reduce the risk for preeclampsia, preterm birth, and IUGR in women at high risk.
The decision to initiate low-dose aspirin therapy in this population is typically based on medical history; there are no validated methods for identifying women at high risk based on biomarkers, clinical diagnostic tests, or medical history. However, as part of the recommendation, the USPSTF provided a pragmatic approach that may help identify those at risk.
"Women with one or more risk factors should receive low-dose aspirin. Women with several moderate risk factors may also benefit from low-dose aspirin," the task force noted, adding that the evidence for the latter approach is less certain, and that clinicians should use clinical judgment and discuss the risks and benefits with patients.
The recommendation applies to asymptomatic women at risk in whom low-dose aspirin is not contraindicated, and defines women at high risk as those with a history of preeclampsia, especially those with an adverse outcome; chronic hypertension, renal disease, type 1 or 2 diabetes, or an autoimmune disease; and those with multifetal gestation, according to the updated recommendation.
Moderate risk factors include nulliparity, obesity, a family history of preeclampsia, age greater than or equal to 35 years, African American race, low socioeconomic status, low birth rate or small for gestational age, greater than 10-year pregnancy interval, or previous adverse pregnancy outcome.
As for appropriate dosing, the most common dosage across studies was 100 mg, but the two largest trials contributing to benefit estimates used 60 mg.
An 81-mg dose was not specifically evaluated, but is commonly available in the United States in tablet form, and is a reasonable dosage for preeclampsia prophylaxis, the task force said.
The updated recommendation is generally in keeping with those of other organizations, including the American College of Obstetricians and Gynecologists, the World Health Organization, the National Institute for Health and Clinical Excellence, the American Heart Association/American Stroke Association, and the American Academy of Family Physicians. For example, ACOG recommends initiating daily low-dose aspirin during the late first trimester in those with a history of early-onset preeclampsia and preterm delivery, or with a history of preeclampsia in more than one prior pregnancy (<cf number="\"2\"">“</cf>American College of Obstetricians and Gynecologists: Hypertension in Pregnancy [Washington, D.C.: American College of Obstetricians and Gynecologists, 2013]), and WHO recommends daily low-dose aspirin as early as 12 weeks for those at high risk ("WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia" [Geneva: World Health Organization, 2011]).
The review by the USPSTF identified several research needs. For example, additional study is needed on the effects of low-dose aspirin on the development of preeclampsia and how patient response is affected by various risk factors. Research is also needed on how to improve clinicians’ ability to identify those at risk, and particularly those who would benefit most from prophylaxis. Study is needed on risk assessment tools, and on populations at particular risk, such as African American and nulliparous women.
Future trials should recruit adequate numbers of women from racial/ethnic populations that are at disproportionate risk.
"Larger studies on aspirin use in the first or early second trimester may improve the evidence base on optimal timing of low-dose aspirin preventive medication. Other areas of research include optimal therapies that individualize the aspirin dosage and timing of administration (e.g., morning vs. bedtime)," they concluded, noting that research is also needed to explore less-well-established risk factors, and to investigate whether preeclampsia prevention with low-dose aspirin affects long-term risk for cardiovascular disease, and whether there is any benefit to continuing low-dose aspirin after delivery in those at high risk.
The use of low-dose aspirin is advisable after 12 weeks of gestation in asymptomatic pregnant women at high risk for developing preeclampsia, according to a recommendation from the U.S. Preventive Services Task Force.
The recommendation, published online Sept. 8 in the Annals of Internal Medicine, is based on a review of new evidence suggesting that the net benefit of low-dose aspirin for preventing preeclampsia is of substantial magnitude. It updates a 1996 recommendation from the USPSTF, which concluded that there was insufficient evidence at that time to recommend for or against the routine use of aspirin for the prevention of preeclampsia.
The current evidence – including 15 randomized controlled trials used to assess the health benefits of low-dose aspirin, 13 randomized controlled trials used to evaluate preeclampsia incidence, and 19 randomized controlled trials and 2 good-quality observational studies used to evaluate harms associated with low-dose aspirin use – suggests that women at risk may benefit from low-dose aspirin beginning after 12 weeks of gestation.
Preeclampsia complicates 2%-8% of pregnancies worldwide, and accounts for 15% of preterm births and 12% of maternal deaths in the United States, according to the task force.
"The USPSTF found adequate evidence of a reduction in risk for preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit. Low-dose aspirin (range, 60-150 mg/day) reduced the risk for preeclampsia by 24% in clinical trials [pooled relative risk, 0.76] and reduced the risk for preterm birth by 14% and IUGR by 20% [pooled relative risk, 0.86 and 0.80, respectively]," the updated recommendation stated (Ann. Intern. Med. 2014 Sept. 8 [doi:10.7326/m14-1884]).
Adequate evidence also indicates that low-dose aspirin is not associated with any increase in the risk of placental abruption, postpartum hemorrhage, fetal intracranial bleeding, or perinatal mortality.
"Evidence on long-term outcomes in offspring exposed in utero to low-dose aspirin is limited, but no developmental harms were identified by age 18 months in the one study reviewed," the task force wrote, concluding – with moderate certainty – that there is a substantial net benefit of daily low-dose aspirin use to reduce the risk for preeclampsia, preterm birth, and IUGR in women at high risk.
The decision to initiate low-dose aspirin therapy in this population is typically based on medical history; there are no validated methods for identifying women at high risk based on biomarkers, clinical diagnostic tests, or medical history. However, as part of the recommendation, the USPSTF provided a pragmatic approach that may help identify those at risk.
"Women with one or more risk factors should receive low-dose aspirin. Women with several moderate risk factors may also benefit from low-dose aspirin," the task force noted, adding that the evidence for the latter approach is less certain, and that clinicians should use clinical judgment and discuss the risks and benefits with patients.
The recommendation applies to asymptomatic women at risk in whom low-dose aspirin is not contraindicated, and defines women at high risk as those with a history of preeclampsia, especially those with an adverse outcome; chronic hypertension, renal disease, type 1 or 2 diabetes, or an autoimmune disease; and those with multifetal gestation, according to the updated recommendation.
Moderate risk factors include nulliparity, obesity, a family history of preeclampsia, age greater than or equal to 35 years, African American race, low socioeconomic status, low birth rate or small for gestational age, greater than 10-year pregnancy interval, or previous adverse pregnancy outcome.
As for appropriate dosing, the most common dosage across studies was 100 mg, but the two largest trials contributing to benefit estimates used 60 mg.
An 81-mg dose was not specifically evaluated, but is commonly available in the United States in tablet form, and is a reasonable dosage for preeclampsia prophylaxis, the task force said.
The updated recommendation is generally in keeping with those of other organizations, including the American College of Obstetricians and Gynecologists, the World Health Organization, the National Institute for Health and Clinical Excellence, the American Heart Association/American Stroke Association, and the American Academy of Family Physicians. For example, ACOG recommends initiating daily low-dose aspirin during the late first trimester in those with a history of early-onset preeclampsia and preterm delivery, or with a history of preeclampsia in more than one prior pregnancy (<cf number="\"2\"">“</cf>American College of Obstetricians and Gynecologists: Hypertension in Pregnancy [Washington, D.C.: American College of Obstetricians and Gynecologists, 2013]), and WHO recommends daily low-dose aspirin as early as 12 weeks for those at high risk ("WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia" [Geneva: World Health Organization, 2011]).
The review by the USPSTF identified several research needs. For example, additional study is needed on the effects of low-dose aspirin on the development of preeclampsia and how patient response is affected by various risk factors. Research is also needed on how to improve clinicians’ ability to identify those at risk, and particularly those who would benefit most from prophylaxis. Study is needed on risk assessment tools, and on populations at particular risk, such as African American and nulliparous women.
Future trials should recruit adequate numbers of women from racial/ethnic populations that are at disproportionate risk.
"Larger studies on aspirin use in the first or early second trimester may improve the evidence base on optimal timing of low-dose aspirin preventive medication. Other areas of research include optimal therapies that individualize the aspirin dosage and timing of administration (e.g., morning vs. bedtime)," they concluded, noting that research is also needed to explore less-well-established risk factors, and to investigate whether preeclampsia prevention with low-dose aspirin affects long-term risk for cardiovascular disease, and whether there is any benefit to continuing low-dose aspirin after delivery in those at high risk.
FROM ANNALS OF INTERNAL MEDICINE