User login
Hypereosinophilia in Erythrodermic Psoriasis: Superimposed Scabies
Erythrodermic psoriasis is a severe form of psoriasis associated with higher morbidity and mortality rates compared to other forms of psoriasis. Cutaneous signs of erythrodermic psoriasis include erythema, edema, and superficial desquamation. Scabies is a common ectoparasitic disease that is diagnosed by the presence of pruritus and typical clinical signs including burrows, vesicles, and erythematous papules. Erythematous papules usually are distributed on the abdomen, thoracic region, axillae, and medial thighs and are characterized by more intense pruritus, especially at night. The course of scabies may be altered in patients with desquamative inflammatory skin disease such as psoriasis. Pruritus may be absent and typical scabies lesions may be concealed due to the preexisting disease, resulting in delayed diagnosis.
Case Reports
Patient 1
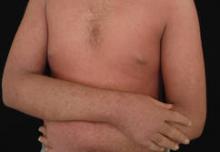
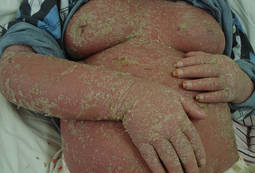
A 13-year-old adolescent boy with psoriasis of 4 years’ duration presented to our outpatient clinic with severe widespread erythema and mild desquamation (Figure 1). The patient was hospitalized following a diagnosis of erythrodermic psoriasis. His medical history was remarkable for recurrent episodes of bronchitis, and his family history included 2 siblings with psoriasis. Physical examination revealed no abnormalities, except for a fever (temperature, 38.0°C). A complete blood cell count revealed an elevated absolute eosinophil count (9260 cells/µL [reference range, 0–700 cells/µL]) corresponding to 48.4% (reference range, 0%–7%) of blood cells. There were no pathological findings in the serum biochemistry; complete urine analysis; throat, sputum, and urine cultures; stool analysis for parasites; or chest radiography. A VDRL test, hepatitis markers, and anti–human immunodeficiency virus test were negative. Antistreptolysin O titer; thyroid function tests; hormone profile; and IgG, IgA, IgM, C3, C4, and total IgE levels were within reference range. Peripheral blood smear, abdominal ultrasonography, electrocardiography, and echocardiography were performed; no cardiac pathology was observed. Cyclosporine 200 mg daily was initiated for treatment of erythrodermic psoriasis.
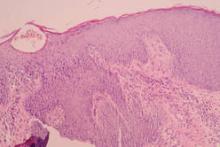
One week after the initiation of treatment the patient’s fever improved. At 2-week follow-up, a complete blood cell count demonstrated more marked eosinophilia, and the percentage of eosinophils at weekly intervals over the next 3 weeks increased to 51.2%, 63.0%, and 71.7%, respectively. The patient presented 2 weeks later with a chief concern of pruritus. Histologic examination of a lesional biopsy specimen revealed psoriasiform epithelial hyperplasia with scabies mites in the stratum corneum (Figure 2). Mites also were noted on direct microscopic examination of scrapings performed with the suspicion of scabies. The patient was treated with permethrin cream 5%. Although all of the patients and staff in the ward also were administered topical permethrin to prevent a scabies epidemic, 2 inpatients who had been discharged before the diagnosis of scabies presented to our outpatient clinic approximately 1 month later with scabies. At 6-month follow-up, the patient’s eosinophil count was within reference range (0.237cells/µL; 3.78%); pruritus and lesions were not observed.
Patient 2
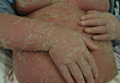
A 26-year-old woman with psoriasis of 5 years’ duration was hospitalized for treatment of erythrodermic psoriasis at the same time as patient 1, her brother. On dermatologic examination, severe widespread erythema, scaling, and edema were noted (Figure 3). Physical examination revealed a fever (temperature, 38.5°C). Hypoalbuminemia and high C-reactive protein levels were present in serum biochemistry. Eosinophil counts were within reference range (0.346 cells/µL; 1.88%). No pathological findings were noted in the complete urine analysis; throat, sputum, and urine cultures; stool analysis for parasites; or chest radiography. A VDRL test, hepatitis markers, and anti–human immunodeficiency virus test were negative. Antistreptolysin O titer; thyroid function tests; hormone profile; and IgG, IgA, IgM, C3, C4, and total IgE levels were within reference range. Cyclosporine 200 mg daily was initiated for treatment of erythrodermic psoriasis. On days 20 and 45 of treatment, eosinophil levels were 8.26% (0.994 cells/mL) and 17.5% (1620 cells/mL), respectively. The patient’s erythema and edema remarkably decreased at the end of the first month of treatment with cyclosporine, but simultaneous onset of pruritus and increasing eosinophil levels despite treatment with cyclosporine were noted. Scabies mites were demonstrated on microscopic examination of skin scrapings from the dorsal aspect of the hand (Figure 4), and the patient was treated with permethrin cream 5%. At 6-month follow-up, eosinophil levels were within reference range (0.317 cells/mL; 4.75%); pruritus and lesions were not observed.
|
| Figure 3. Severe widespread erythema, scaling, and edema in a 26-year-old woman with psoriasis. |

|
Figure 4. A mite and an egg were noted on direct microscopic examination of skin scrapings from the dorsal aspect of the hand (original magnification ×400). |
Comment
Erythrodermic psoriasis is a severe form of psoriasis. In the 2010 consensus of the National Psoriasis Foundation medical board, it was reported that cyclosporine and infliximab are the fastest and most effective agents in treating erythrodermic psoriasis.1
Progressive increases in the number of eosinophils prompted us to screen our patients for causes of hypereosinophilia. Increased eosinophil counts have not been linked to treatment with cyclosporine. In contrast, it has been detected that cyclosporine reduces the number of eosinophils in many eosinophilic dermatoses.2
There is no hematologic finding for scabies; therefore, clinical findings are most important in the diagnosis. Crusted scabies is a special form of scabies seen in immunocompromised patients that is characterized by excessive numbers of scabies mites. Peripheral eosinophilia may be observed in this form of the disease.3 In classic scabies, eosinophilia is uncommon in peripheral blood. In contrast with these data, there are 2 cases in the literature of scabies secondary to disorders of keratinization without immune deficiency with different clinical presentations.4 In these patients, the most striking and only finding at the time of diagnosis was substantial eosinophilia. These cases were reported with emphasis on eosinophilia as the first sign of scabies infestation in patients with severe hyperkeratosis.4 In our patients, the spread of infection may have been facilitated by the immunosuppressive effects of cyclosporine in addition to the existing disease. Crusted scabies after use of cyclosporine for atopic dermatitis has been reported. It was emphasized that suppression of scratching and immunosuppression due to cyclospor-ine caused the spread of scabies mites in the skin.5
Burrows, vesicles, and erythematous papules are typical lesions seen in scabies. Erythematous papules usually are distributed on the abdomen, thoracic region, axillae, and medial thighs and are characterized by more intense pruritus, especially at night. In our patients, widespread erythema and scaling were noted, and pruritus was thought to be due to psoriasis lesions. Because of excessive scaling in the stratum corneum from psoriasis, the clinical features of scabies were concealed and the classic clinical signs of scabies were not present. The patients’ hypereosinophilia led us to investigate the cause. A lesional biopsy and direct microscopy demonstrated scabies mites.
Conclusion
The relationship between psoriasis and scabies previously has been reported in the literature as scabies with crusts mimicking rupioid psoriasis.6 However, our patients developed scabies in the setting of psoriasis. Severe scabies can present as erythroderma.7 We believe the diagnosis of scabies in our patients would have been more complicated without the preexisting psoriasis, as biopsies of erythrodermic psoriasis often are nonspecific and may contain eosinophils in the inflammatory infiltrate. Although pruritus may be interpreted as a result of the primary dermatologic disease, the presence of hypereosinophilia may suggest scabies in erythrodermic patients. For this reason, peripheral eosinophilia may suggest scabies in patients with erythematous scaly inflammatory skin diseases who are treated with immunosuppressive agents, and a search for scabies mites in skin scrapings should be undertaken.
1. Rosenbach M, Hsu S, Korman NJ, et al. Treatment of erythrodermic psoriasis: from the medical board of the National Psoriasis Foundation [published online ahead of print August 8, 2009]. J Am Acad Dermatol. 2010;62:655-662.
2. Maleki D, Sayyah A, Rahimi-Rad MH, et al. Kimura’s disease with eosinophilic panniculitis–treated with cyclosporine: a case report. Allergy Asthma Clin Immunol. 2010;6:5.
3. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381.
4. Sluzevich JC, Sheth AP, Lucky AV. Persistent eosinophilia as a presenting sign of scabies in patients with disorders of keratinization. Arch Dermatol. 2007;143:670-673.
5. Monari P, Sala R, Calzavara-Pinton P. Norwegian scabies in a healthy woman during oral cyclosporine therapy [published online ahead of print March 2, 2007]. Eur J Dermatol. 2007;17:173.
6. Costa JB, Rocha de Sousa VL, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913.
7. Mehta V, Balachandran C, Monga P, et al. Images in clinical practice. Norwegian scabies presenting as erythroderma. Indian J Dermatol Venereol Leprol. 2009;75:609-610.
Erythrodermic psoriasis is a severe form of psoriasis associated with higher morbidity and mortality rates compared to other forms of psoriasis. Cutaneous signs of erythrodermic psoriasis include erythema, edema, and superficial desquamation. Scabies is a common ectoparasitic disease that is diagnosed by the presence of pruritus and typical clinical signs including burrows, vesicles, and erythematous papules. Erythematous papules usually are distributed on the abdomen, thoracic region, axillae, and medial thighs and are characterized by more intense pruritus, especially at night. The course of scabies may be altered in patients with desquamative inflammatory skin disease such as psoriasis. Pruritus may be absent and typical scabies lesions may be concealed due to the preexisting disease, resulting in delayed diagnosis.
Case Reports
Patient 1
A 13-year-old adolescent boy with psoriasis of 4 years’ duration presented to our outpatient clinic with severe widespread erythema and mild desquamation (Figure 1). The patient was hospitalized following a diagnosis of erythrodermic psoriasis. His medical history was remarkable for recurrent episodes of bronchitis, and his family history included 2 siblings with psoriasis. Physical examination revealed no abnormalities, except for a fever (temperature, 38.0°C). A complete blood cell count revealed an elevated absolute eosinophil count (9260 cells/µL [reference range, 0–700 cells/µL]) corresponding to 48.4% (reference range, 0%–7%) of blood cells. There were no pathological findings in the serum biochemistry; complete urine analysis; throat, sputum, and urine cultures; stool analysis for parasites; or chest radiography. A VDRL test, hepatitis markers, and anti–human immunodeficiency virus test were negative. Antistreptolysin O titer; thyroid function tests; hormone profile; and IgG, IgA, IgM, C3, C4, and total IgE levels were within reference range. Peripheral blood smear, abdominal ultrasonography, electrocardiography, and echocardiography were performed; no cardiac pathology was observed. Cyclosporine 200 mg daily was initiated for treatment of erythrodermic psoriasis.
One week after the initiation of treatment the patient’s fever improved. At 2-week follow-up, a complete blood cell count demonstrated more marked eosinophilia, and the percentage of eosinophils at weekly intervals over the next 3 weeks increased to 51.2%, 63.0%, and 71.7%, respectively. The patient presented 2 weeks later with a chief concern of pruritus. Histologic examination of a lesional biopsy specimen revealed psoriasiform epithelial hyperplasia with scabies mites in the stratum corneum (Figure 2). Mites also were noted on direct microscopic examination of scrapings performed with the suspicion of scabies. The patient was treated with permethrin cream 5%. Although all of the patients and staff in the ward also were administered topical permethrin to prevent a scabies epidemic, 2 inpatients who had been discharged before the diagnosis of scabies presented to our outpatient clinic approximately 1 month later with scabies. At 6-month follow-up, the patient’s eosinophil count was within reference range (0.237cells/µL; 3.78%); pruritus and lesions were not observed.
Patient 2
A 26-year-old woman with psoriasis of 5 years’ duration was hospitalized for treatment of erythrodermic psoriasis at the same time as patient 1, her brother. On dermatologic examination, severe widespread erythema, scaling, and edema were noted (Figure 3). Physical examination revealed a fever (temperature, 38.5°C). Hypoalbuminemia and high C-reactive protein levels were present in serum biochemistry. Eosinophil counts were within reference range (0.346 cells/µL; 1.88%). No pathological findings were noted in the complete urine analysis; throat, sputum, and urine cultures; stool analysis for parasites; or chest radiography. A VDRL test, hepatitis markers, and anti–human immunodeficiency virus test were negative. Antistreptolysin O titer; thyroid function tests; hormone profile; and IgG, IgA, IgM, C3, C4, and total IgE levels were within reference range. Cyclosporine 200 mg daily was initiated for treatment of erythrodermic psoriasis. On days 20 and 45 of treatment, eosinophil levels were 8.26% (0.994 cells/mL) and 17.5% (1620 cells/mL), respectively. The patient’s erythema and edema remarkably decreased at the end of the first month of treatment with cyclosporine, but simultaneous onset of pruritus and increasing eosinophil levels despite treatment with cyclosporine were noted. Scabies mites were demonstrated on microscopic examination of skin scrapings from the dorsal aspect of the hand (Figure 4), and the patient was treated with permethrin cream 5%. At 6-month follow-up, eosinophil levels were within reference range (0.317 cells/mL; 4.75%); pruritus and lesions were not observed.

|
| Figure 3. Severe widespread erythema, scaling, and edema in a 26-year-old woman with psoriasis. |

|
Figure 4. A mite and an egg were noted on direct microscopic examination of skin scrapings from the dorsal aspect of the hand (original magnification ×400). |
Comment
Erythrodermic psoriasis is a severe form of psoriasis. In the 2010 consensus of the National Psoriasis Foundation medical board, it was reported that cyclosporine and infliximab are the fastest and most effective agents in treating erythrodermic psoriasis.1
Progressive increases in the number of eosinophils prompted us to screen our patients for causes of hypereosinophilia. Increased eosinophil counts have not been linked to treatment with cyclosporine. In contrast, it has been detected that cyclosporine reduces the number of eosinophils in many eosinophilic dermatoses.2
There is no hematologic finding for scabies; therefore, clinical findings are most important in the diagnosis. Crusted scabies is a special form of scabies seen in immunocompromised patients that is characterized by excessive numbers of scabies mites. Peripheral eosinophilia may be observed in this form of the disease.3 In classic scabies, eosinophilia is uncommon in peripheral blood. In contrast with these data, there are 2 cases in the literature of scabies secondary to disorders of keratinization without immune deficiency with different clinical presentations.4 In these patients, the most striking and only finding at the time of diagnosis was substantial eosinophilia. These cases were reported with emphasis on eosinophilia as the first sign of scabies infestation in patients with severe hyperkeratosis.4 In our patients, the spread of infection may have been facilitated by the immunosuppressive effects of cyclosporine in addition to the existing disease. Crusted scabies after use of cyclosporine for atopic dermatitis has been reported. It was emphasized that suppression of scratching and immunosuppression due to cyclospor-ine caused the spread of scabies mites in the skin.5
Burrows, vesicles, and erythematous papules are typical lesions seen in scabies. Erythematous papules usually are distributed on the abdomen, thoracic region, axillae, and medial thighs and are characterized by more intense pruritus, especially at night. In our patients, widespread erythema and scaling were noted, and pruritus was thought to be due to psoriasis lesions. Because of excessive scaling in the stratum corneum from psoriasis, the clinical features of scabies were concealed and the classic clinical signs of scabies were not present. The patients’ hypereosinophilia led us to investigate the cause. A lesional biopsy and direct microscopy demonstrated scabies mites.
Conclusion
The relationship between psoriasis and scabies previously has been reported in the literature as scabies with crusts mimicking rupioid psoriasis.6 However, our patients developed scabies in the setting of psoriasis. Severe scabies can present as erythroderma.7 We believe the diagnosis of scabies in our patients would have been more complicated without the preexisting psoriasis, as biopsies of erythrodermic psoriasis often are nonspecific and may contain eosinophils in the inflammatory infiltrate. Although pruritus may be interpreted as a result of the primary dermatologic disease, the presence of hypereosinophilia may suggest scabies in erythrodermic patients. For this reason, peripheral eosinophilia may suggest scabies in patients with erythematous scaly inflammatory skin diseases who are treated with immunosuppressive agents, and a search for scabies mites in skin scrapings should be undertaken.
Erythrodermic psoriasis is a severe form of psoriasis associated with higher morbidity and mortality rates compared to other forms of psoriasis. Cutaneous signs of erythrodermic psoriasis include erythema, edema, and superficial desquamation. Scabies is a common ectoparasitic disease that is diagnosed by the presence of pruritus and typical clinical signs including burrows, vesicles, and erythematous papules. Erythematous papules usually are distributed on the abdomen, thoracic region, axillae, and medial thighs and are characterized by more intense pruritus, especially at night. The course of scabies may be altered in patients with desquamative inflammatory skin disease such as psoriasis. Pruritus may be absent and typical scabies lesions may be concealed due to the preexisting disease, resulting in delayed diagnosis.
Case Reports
Patient 1
A 13-year-old adolescent boy with psoriasis of 4 years’ duration presented to our outpatient clinic with severe widespread erythema and mild desquamation (Figure 1). The patient was hospitalized following a diagnosis of erythrodermic psoriasis. His medical history was remarkable for recurrent episodes of bronchitis, and his family history included 2 siblings with psoriasis. Physical examination revealed no abnormalities, except for a fever (temperature, 38.0°C). A complete blood cell count revealed an elevated absolute eosinophil count (9260 cells/µL [reference range, 0–700 cells/µL]) corresponding to 48.4% (reference range, 0%–7%) of blood cells. There were no pathological findings in the serum biochemistry; complete urine analysis; throat, sputum, and urine cultures; stool analysis for parasites; or chest radiography. A VDRL test, hepatitis markers, and anti–human immunodeficiency virus test were negative. Antistreptolysin O titer; thyroid function tests; hormone profile; and IgG, IgA, IgM, C3, C4, and total IgE levels were within reference range. Peripheral blood smear, abdominal ultrasonography, electrocardiography, and echocardiography were performed; no cardiac pathology was observed. Cyclosporine 200 mg daily was initiated for treatment of erythrodermic psoriasis.
One week after the initiation of treatment the patient’s fever improved. At 2-week follow-up, a complete blood cell count demonstrated more marked eosinophilia, and the percentage of eosinophils at weekly intervals over the next 3 weeks increased to 51.2%, 63.0%, and 71.7%, respectively. The patient presented 2 weeks later with a chief concern of pruritus. Histologic examination of a lesional biopsy specimen revealed psoriasiform epithelial hyperplasia with scabies mites in the stratum corneum (Figure 2). Mites also were noted on direct microscopic examination of scrapings performed with the suspicion of scabies. The patient was treated with permethrin cream 5%. Although all of the patients and staff in the ward also were administered topical permethrin to prevent a scabies epidemic, 2 inpatients who had been discharged before the diagnosis of scabies presented to our outpatient clinic approximately 1 month later with scabies. At 6-month follow-up, the patient’s eosinophil count was within reference range (0.237cells/µL; 3.78%); pruritus and lesions were not observed.
Patient 2
A 26-year-old woman with psoriasis of 5 years’ duration was hospitalized for treatment of erythrodermic psoriasis at the same time as patient 1, her brother. On dermatologic examination, severe widespread erythema, scaling, and edema were noted (Figure 3). Physical examination revealed a fever (temperature, 38.5°C). Hypoalbuminemia and high C-reactive protein levels were present in serum biochemistry. Eosinophil counts were within reference range (0.346 cells/µL; 1.88%). No pathological findings were noted in the complete urine analysis; throat, sputum, and urine cultures; stool analysis for parasites; or chest radiography. A VDRL test, hepatitis markers, and anti–human immunodeficiency virus test were negative. Antistreptolysin O titer; thyroid function tests; hormone profile; and IgG, IgA, IgM, C3, C4, and total IgE levels were within reference range. Cyclosporine 200 mg daily was initiated for treatment of erythrodermic psoriasis. On days 20 and 45 of treatment, eosinophil levels were 8.26% (0.994 cells/mL) and 17.5% (1620 cells/mL), respectively. The patient’s erythema and edema remarkably decreased at the end of the first month of treatment with cyclosporine, but simultaneous onset of pruritus and increasing eosinophil levels despite treatment with cyclosporine were noted. Scabies mites were demonstrated on microscopic examination of skin scrapings from the dorsal aspect of the hand (Figure 4), and the patient was treated with permethrin cream 5%. At 6-month follow-up, eosinophil levels were within reference range (0.317 cells/mL; 4.75%); pruritus and lesions were not observed.

|
| Figure 3. Severe widespread erythema, scaling, and edema in a 26-year-old woman with psoriasis. |

|
Figure 4. A mite and an egg were noted on direct microscopic examination of skin scrapings from the dorsal aspect of the hand (original magnification ×400). |
Comment
Erythrodermic psoriasis is a severe form of psoriasis. In the 2010 consensus of the National Psoriasis Foundation medical board, it was reported that cyclosporine and infliximab are the fastest and most effective agents in treating erythrodermic psoriasis.1
Progressive increases in the number of eosinophils prompted us to screen our patients for causes of hypereosinophilia. Increased eosinophil counts have not been linked to treatment with cyclosporine. In contrast, it has been detected that cyclosporine reduces the number of eosinophils in many eosinophilic dermatoses.2
There is no hematologic finding for scabies; therefore, clinical findings are most important in the diagnosis. Crusted scabies is a special form of scabies seen in immunocompromised patients that is characterized by excessive numbers of scabies mites. Peripheral eosinophilia may be observed in this form of the disease.3 In classic scabies, eosinophilia is uncommon in peripheral blood. In contrast with these data, there are 2 cases in the literature of scabies secondary to disorders of keratinization without immune deficiency with different clinical presentations.4 In these patients, the most striking and only finding at the time of diagnosis was substantial eosinophilia. These cases were reported with emphasis on eosinophilia as the first sign of scabies infestation in patients with severe hyperkeratosis.4 In our patients, the spread of infection may have been facilitated by the immunosuppressive effects of cyclosporine in addition to the existing disease. Crusted scabies after use of cyclosporine for atopic dermatitis has been reported. It was emphasized that suppression of scratching and immunosuppression due to cyclospor-ine caused the spread of scabies mites in the skin.5
Burrows, vesicles, and erythematous papules are typical lesions seen in scabies. Erythematous papules usually are distributed on the abdomen, thoracic region, axillae, and medial thighs and are characterized by more intense pruritus, especially at night. In our patients, widespread erythema and scaling were noted, and pruritus was thought to be due to psoriasis lesions. Because of excessive scaling in the stratum corneum from psoriasis, the clinical features of scabies were concealed and the classic clinical signs of scabies were not present. The patients’ hypereosinophilia led us to investigate the cause. A lesional biopsy and direct microscopy demonstrated scabies mites.
Conclusion
The relationship between psoriasis and scabies previously has been reported in the literature as scabies with crusts mimicking rupioid psoriasis.6 However, our patients developed scabies in the setting of psoriasis. Severe scabies can present as erythroderma.7 We believe the diagnosis of scabies in our patients would have been more complicated without the preexisting psoriasis, as biopsies of erythrodermic psoriasis often are nonspecific and may contain eosinophils in the inflammatory infiltrate. Although pruritus may be interpreted as a result of the primary dermatologic disease, the presence of hypereosinophilia may suggest scabies in erythrodermic patients. For this reason, peripheral eosinophilia may suggest scabies in patients with erythematous scaly inflammatory skin diseases who are treated with immunosuppressive agents, and a search for scabies mites in skin scrapings should be undertaken.
1. Rosenbach M, Hsu S, Korman NJ, et al. Treatment of erythrodermic psoriasis: from the medical board of the National Psoriasis Foundation [published online ahead of print August 8, 2009]. J Am Acad Dermatol. 2010;62:655-662.
2. Maleki D, Sayyah A, Rahimi-Rad MH, et al. Kimura’s disease with eosinophilic panniculitis–treated with cyclosporine: a case report. Allergy Asthma Clin Immunol. 2010;6:5.
3. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381.
4. Sluzevich JC, Sheth AP, Lucky AV. Persistent eosinophilia as a presenting sign of scabies in patients with disorders of keratinization. Arch Dermatol. 2007;143:670-673.
5. Monari P, Sala R, Calzavara-Pinton P. Norwegian scabies in a healthy woman during oral cyclosporine therapy [published online ahead of print March 2, 2007]. Eur J Dermatol. 2007;17:173.
6. Costa JB, Rocha de Sousa VL, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913.
7. Mehta V, Balachandran C, Monga P, et al. Images in clinical practice. Norwegian scabies presenting as erythroderma. Indian J Dermatol Venereol Leprol. 2009;75:609-610.
1. Rosenbach M, Hsu S, Korman NJ, et al. Treatment of erythrodermic psoriasis: from the medical board of the National Psoriasis Foundation [published online ahead of print August 8, 2009]. J Am Acad Dermatol. 2010;62:655-662.
2. Maleki D, Sayyah A, Rahimi-Rad MH, et al. Kimura’s disease with eosinophilic panniculitis–treated with cyclosporine: a case report. Allergy Asthma Clin Immunol. 2010;6:5.
3. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381.
4. Sluzevich JC, Sheth AP, Lucky AV. Persistent eosinophilia as a presenting sign of scabies in patients with disorders of keratinization. Arch Dermatol. 2007;143:670-673.
5. Monari P, Sala R, Calzavara-Pinton P. Norwegian scabies in a healthy woman during oral cyclosporine therapy [published online ahead of print March 2, 2007]. Eur J Dermatol. 2007;17:173.
6. Costa JB, Rocha de Sousa VL, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913.
7. Mehta V, Balachandran C, Monga P, et al. Images in clinical practice. Norwegian scabies presenting as erythroderma. Indian J Dermatol Venereol Leprol. 2009;75:609-610.
- If a desquamative disease such as psoriasis precedes scabies, then the disease course may be altered. Pruritus may be absent and typical scabies lesions may be concealed due to the preexisting disease, resulting in delayed diagnosis.
- The presence of hypereosinophilia may suggest scabies in patients with erythematous scaly inflammatory skin diseases who are treated with immunosuppressive agents; therefore, a search for sarcoptic mites in skin scrapings should be undertaken.
Palmoplantar Pustular Psoriasis Following Initiation of a Beta-blocker: Disease Control With Low-Dose Methotrexate
Psoriasis affects 1% to 2% of individuals in the United States, typically within the third decade of life.1,2 Psoriasis lesions may be persistent or relapsing plaques or pustules. The epidermal thickening that often is noted in psoriasis is secondary to the elongation of rete ridges. Parakeratosis, which also is often noted in psoriasis, is the accumulation of cells with retained nuclei within the cornified layer. Localized pustular psoriasis is a variant of psoriasis that displays scaling erythematous plaques studded with pustules. The pustules are most frequently observed on the palms, soles, and nails of affected individuals.1 Palmoplantar pustular psoriasis is most commonly seen in women in their fifth and sixth decades of life.3 One agent commonly used in the treatment of psoriasis is methotrexate, a prodrug that is converted to polyglutamyl derivatives and acts as a dihydrofolate reductase inhibitor.4,5 We report a case of palmoplantar pustular psoriasis that was triggered by initiation of a beta-blocker. The patient’s condition was controlled with a low-dose methotrexate regimen.
Case Report
A 76-year-old woman with a history of hypertension, hyperlipidemia, and hypothyroidism presented with erythema and pustules on the bilateral palms and soles 6 weeks following initiation of a beta-blocker. On discontinuation of the beta-blocker, the lesions showed minimal improvement without resolution. The patient then was started on fluocinonide ointment 0.05% and acitretin 25 mg 3 times weekly. Improvement (25%) was noted over the course of 9 months; acitretin then was increased to 25 mg 4 times weekly, but no change was noted (Figure). Acitretin then was discontinued and she was started on methotrexate 2.5 mg weekly, followed by improvement of the lesions on the palms and soles. This regimen was continued and the patient was stable at 2-year follow-up with moderate hyperpigmentation of the palms and minimal hyperpigmentation of the soles, both without erythema or exudates.
Comment
Palmoplantar pustular psoriasis is a rare form of psoriasis; it may, however, be induced by a variety of medications.6 A causal relationship to psoriasis has been documented with beta-blockers, lithium, tetracyclines, nonsteroidal anti-inflammatory drugs, adalimumab, and synthetic antimalarials. Other drugs linked to psoriasis are angiotensin-converting enzyme inhibitors, interferons, and terbinafine.7 Anti–tumor necrosis factor a agents such as in-fliximab and etanercept also have been reported to induce pustular psoriasis.6 These drugs have been reported to aggravate preexisting psoriasis, provoke lesions in uninvolved skin in individuals with psoriasis, and induce psoriasis in patients without a personal or family history of psoriasis.8 The pathogenesis of psoriasis triggered by beta-blockers is thought to be due to decreased intraepidermal cyclic adenosine monophosphate, leading to an increase in epidermal cell turnover.7
Palmoplantar pustular psoriasis is a debilitating chronic illness that can span decades.9 Not only can it be socially stigmatizing, but it also interferes with patients’ quality of life.10 Various therapies are used to treat this condition including coal tar, topical corticosteroids with or without polythene occlusion, photochemotherapy, tetracyclines, systemic retinoids, cyclosporine, biologics, and methotrexate.9 There currently is no therapeutic standard for controlling this disease, as treatment often is fraught with medication resistance and intolerance as well as frequent relapses. Many medications also are used without firm evidence proving they are beneficial.11
Despite the advent of biologics, methotrexate remains commonly used in the treatment of psoriasis as monotherapy or in combination with other drugs. In comparison to biologics, methotrexate is less expensive, has established efficacy data, and can be administered orally.12 Although it was previously believed that the antiproliferative action of methotrexate via antifolate metabolism led to improvement of psoriatic lesions, in vitro data point to the anti-inflammatory activity of methotrexate playing the more dominant role in disease improvement. Methotrexate also inhibits 5-aminoimidazole-4-carboxamide ribonucleotide transformylase, leading to the buildup of adeno-sine in tissue and consequently contributing to its anti-inflammatory properties.12
In psoriasis patients, methotrexate is commonly used in dosages up to 30 mg weekly.5 Our patient demonstrates a rare case of palmoplantar pustular psoriasis that was well controlled using low-dose methotrexate (2.5 mg weekly). Some cases report low doses of 15 to 20 mg for long-term control in psoriasis.13 However, the successful use of doses as low as 2.5 mg for control of any variant of psoriasis is rare.
Conclusion
Although it has been shown to be effective in the treatment of psoriasis, the use of methotrexate is not benign; it has been associated with hepatotoxicity and bone marrow toxicity.12 It is important for dermatologists to recognize that pustular psoriasis can be treated with low-dose methotrexate to avoid potentially toxic effects of higher doses of methotrexate, which is especially true in cases of drug-induced disease, as seen in our patient.
1. Timothy H. Diseases of the skin. In: McPhee SJ, Hammer GD, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 6th ed. New York City, NY: McGraw-Hill Professional; 2009:183-208.
2. Chlapek BH. Dermatologic emergencies. In: Stone CK, Humphries RL, eds. Current Diagnosis & Treatment: Emergency Medicine. 6th ed. New York City, NY: McGraw-Hill Companies; 2007:270-284.
3. Adişen E, Gürer MA. Therapeutic options for palmoplantar pustulosis. Clin Exp Dermatol. 2009;35:219-222.
4. Imboden JB, Donald FA, Stone JH, et al. Medications. In: Imboden JB, Hellmann DB, Stone JH, eds. Current Rheumatology Diagnosis & Treatment. 2nd ed. New York City, NY: Lange Medical Books/McGraw-Hill; 2004:355-383.
5. Warren RB, Chalmers RJG, Griffiths EM, et al. Methotrexate for psoriasis in the era of biological therapy. Clin Exp Dermatol. 2008;33:551-554.
6. Park J, Lee S. A case of tumor necrosis factor-alpha inhibitors-induced pustular psoriasis. Ann Dermatol. 2010;22:212-215.
7. Tsankov N, Angelova I, Kazandjieva J. Drug-induced psoriasis. Am J Clin Dermatol. 2000;1:159-165.
8. Basavaraj K, Ashok N, Rashmi R, et al. The role of drugs in the induction and/or exacerbation of psoriasis. Int J Dermatol. 2010;49:1351-1361.
9. Chalmers R, Hollis S, Leonardi-Bee J, et al. Interventions for chronic palmoplantar pustulosis (review). Cochrane Database Syst Rev. 2009;1:1-51.
10. Spuls P, Hadi S, Rivera L, et al. Retrospective analysis of the treatment of psoriasis of the palms and soles. J Dermatolog Treat. 2003;14:21-25.
11. Mrowietz U, van de Kerkhof PCM. Management of palmoplantar pustulosis: do we need to change? Br J Dermatol. 2011;164:942-946.
12. Kanwar A, Yanav S, Dogra S. Psoriasis: what is new in nonbiologic systemic therapy in the era of biologics? Indian J Dermatol. 2010;76:622-633.
13. Haustein UF, Rytter M. Methotrexate in psoriasis: 26 years’ experience with low-dose long-term treatment. J Eur Acad Dermatol Venereol. 2000;14:382-388.
Psoriasis affects 1% to 2% of individuals in the United States, typically within the third decade of life.1,2 Psoriasis lesions may be persistent or relapsing plaques or pustules. The epidermal thickening that often is noted in psoriasis is secondary to the elongation of rete ridges. Parakeratosis, which also is often noted in psoriasis, is the accumulation of cells with retained nuclei within the cornified layer. Localized pustular psoriasis is a variant of psoriasis that displays scaling erythematous plaques studded with pustules. The pustules are most frequently observed on the palms, soles, and nails of affected individuals.1 Palmoplantar pustular psoriasis is most commonly seen in women in their fifth and sixth decades of life.3 One agent commonly used in the treatment of psoriasis is methotrexate, a prodrug that is converted to polyglutamyl derivatives and acts as a dihydrofolate reductase inhibitor.4,5 We report a case of palmoplantar pustular psoriasis that was triggered by initiation of a beta-blocker. The patient’s condition was controlled with a low-dose methotrexate regimen.
Case Report
A 76-year-old woman with a history of hypertension, hyperlipidemia, and hypothyroidism presented with erythema and pustules on the bilateral palms and soles 6 weeks following initiation of a beta-blocker. On discontinuation of the beta-blocker, the lesions showed minimal improvement without resolution. The patient then was started on fluocinonide ointment 0.05% and acitretin 25 mg 3 times weekly. Improvement (25%) was noted over the course of 9 months; acitretin then was increased to 25 mg 4 times weekly, but no change was noted (Figure). Acitretin then was discontinued and she was started on methotrexate 2.5 mg weekly, followed by improvement of the lesions on the palms and soles. This regimen was continued and the patient was stable at 2-year follow-up with moderate hyperpigmentation of the palms and minimal hyperpigmentation of the soles, both without erythema or exudates.
Comment
Palmoplantar pustular psoriasis is a rare form of psoriasis; it may, however, be induced by a variety of medications.6 A causal relationship to psoriasis has been documented with beta-blockers, lithium, tetracyclines, nonsteroidal anti-inflammatory drugs, adalimumab, and synthetic antimalarials. Other drugs linked to psoriasis are angiotensin-converting enzyme inhibitors, interferons, and terbinafine.7 Anti–tumor necrosis factor a agents such as in-fliximab and etanercept also have been reported to induce pustular psoriasis.6 These drugs have been reported to aggravate preexisting psoriasis, provoke lesions in uninvolved skin in individuals with psoriasis, and induce psoriasis in patients without a personal or family history of psoriasis.8 The pathogenesis of psoriasis triggered by beta-blockers is thought to be due to decreased intraepidermal cyclic adenosine monophosphate, leading to an increase in epidermal cell turnover.7
Palmoplantar pustular psoriasis is a debilitating chronic illness that can span decades.9 Not only can it be socially stigmatizing, but it also interferes with patients’ quality of life.10 Various therapies are used to treat this condition including coal tar, topical corticosteroids with or without polythene occlusion, photochemotherapy, tetracyclines, systemic retinoids, cyclosporine, biologics, and methotrexate.9 There currently is no therapeutic standard for controlling this disease, as treatment often is fraught with medication resistance and intolerance as well as frequent relapses. Many medications also are used without firm evidence proving they are beneficial.11
Despite the advent of biologics, methotrexate remains commonly used in the treatment of psoriasis as monotherapy or in combination with other drugs. In comparison to biologics, methotrexate is less expensive, has established efficacy data, and can be administered orally.12 Although it was previously believed that the antiproliferative action of methotrexate via antifolate metabolism led to improvement of psoriatic lesions, in vitro data point to the anti-inflammatory activity of methotrexate playing the more dominant role in disease improvement. Methotrexate also inhibits 5-aminoimidazole-4-carboxamide ribonucleotide transformylase, leading to the buildup of adeno-sine in tissue and consequently contributing to its anti-inflammatory properties.12
In psoriasis patients, methotrexate is commonly used in dosages up to 30 mg weekly.5 Our patient demonstrates a rare case of palmoplantar pustular psoriasis that was well controlled using low-dose methotrexate (2.5 mg weekly). Some cases report low doses of 15 to 20 mg for long-term control in psoriasis.13 However, the successful use of doses as low as 2.5 mg for control of any variant of psoriasis is rare.
Conclusion
Although it has been shown to be effective in the treatment of psoriasis, the use of methotrexate is not benign; it has been associated with hepatotoxicity and bone marrow toxicity.12 It is important for dermatologists to recognize that pustular psoriasis can be treated with low-dose methotrexate to avoid potentially toxic effects of higher doses of methotrexate, which is especially true in cases of drug-induced disease, as seen in our patient.
Psoriasis affects 1% to 2% of individuals in the United States, typically within the third decade of life.1,2 Psoriasis lesions may be persistent or relapsing plaques or pustules. The epidermal thickening that often is noted in psoriasis is secondary to the elongation of rete ridges. Parakeratosis, which also is often noted in psoriasis, is the accumulation of cells with retained nuclei within the cornified layer. Localized pustular psoriasis is a variant of psoriasis that displays scaling erythematous plaques studded with pustules. The pustules are most frequently observed on the palms, soles, and nails of affected individuals.1 Palmoplantar pustular psoriasis is most commonly seen in women in their fifth and sixth decades of life.3 One agent commonly used in the treatment of psoriasis is methotrexate, a prodrug that is converted to polyglutamyl derivatives and acts as a dihydrofolate reductase inhibitor.4,5 We report a case of palmoplantar pustular psoriasis that was triggered by initiation of a beta-blocker. The patient’s condition was controlled with a low-dose methotrexate regimen.
Case Report
A 76-year-old woman with a history of hypertension, hyperlipidemia, and hypothyroidism presented with erythema and pustules on the bilateral palms and soles 6 weeks following initiation of a beta-blocker. On discontinuation of the beta-blocker, the lesions showed minimal improvement without resolution. The patient then was started on fluocinonide ointment 0.05% and acitretin 25 mg 3 times weekly. Improvement (25%) was noted over the course of 9 months; acitretin then was increased to 25 mg 4 times weekly, but no change was noted (Figure). Acitretin then was discontinued and she was started on methotrexate 2.5 mg weekly, followed by improvement of the lesions on the palms and soles. This regimen was continued and the patient was stable at 2-year follow-up with moderate hyperpigmentation of the palms and minimal hyperpigmentation of the soles, both without erythema or exudates.
Comment
Palmoplantar pustular psoriasis is a rare form of psoriasis; it may, however, be induced by a variety of medications.6 A causal relationship to psoriasis has been documented with beta-blockers, lithium, tetracyclines, nonsteroidal anti-inflammatory drugs, adalimumab, and synthetic antimalarials. Other drugs linked to psoriasis are angiotensin-converting enzyme inhibitors, interferons, and terbinafine.7 Anti–tumor necrosis factor a agents such as in-fliximab and etanercept also have been reported to induce pustular psoriasis.6 These drugs have been reported to aggravate preexisting psoriasis, provoke lesions in uninvolved skin in individuals with psoriasis, and induce psoriasis in patients without a personal or family history of psoriasis.8 The pathogenesis of psoriasis triggered by beta-blockers is thought to be due to decreased intraepidermal cyclic adenosine monophosphate, leading to an increase in epidermal cell turnover.7
Palmoplantar pustular psoriasis is a debilitating chronic illness that can span decades.9 Not only can it be socially stigmatizing, but it also interferes with patients’ quality of life.10 Various therapies are used to treat this condition including coal tar, topical corticosteroids with or without polythene occlusion, photochemotherapy, tetracyclines, systemic retinoids, cyclosporine, biologics, and methotrexate.9 There currently is no therapeutic standard for controlling this disease, as treatment often is fraught with medication resistance and intolerance as well as frequent relapses. Many medications also are used without firm evidence proving they are beneficial.11
Despite the advent of biologics, methotrexate remains commonly used in the treatment of psoriasis as monotherapy or in combination with other drugs. In comparison to biologics, methotrexate is less expensive, has established efficacy data, and can be administered orally.12 Although it was previously believed that the antiproliferative action of methotrexate via antifolate metabolism led to improvement of psoriatic lesions, in vitro data point to the anti-inflammatory activity of methotrexate playing the more dominant role in disease improvement. Methotrexate also inhibits 5-aminoimidazole-4-carboxamide ribonucleotide transformylase, leading to the buildup of adeno-sine in tissue and consequently contributing to its anti-inflammatory properties.12
In psoriasis patients, methotrexate is commonly used in dosages up to 30 mg weekly.5 Our patient demonstrates a rare case of palmoplantar pustular psoriasis that was well controlled using low-dose methotrexate (2.5 mg weekly). Some cases report low doses of 15 to 20 mg for long-term control in psoriasis.13 However, the successful use of doses as low as 2.5 mg for control of any variant of psoriasis is rare.
Conclusion
Although it has been shown to be effective in the treatment of psoriasis, the use of methotrexate is not benign; it has been associated with hepatotoxicity and bone marrow toxicity.12 It is important for dermatologists to recognize that pustular psoriasis can be treated with low-dose methotrexate to avoid potentially toxic effects of higher doses of methotrexate, which is especially true in cases of drug-induced disease, as seen in our patient.
1. Timothy H. Diseases of the skin. In: McPhee SJ, Hammer GD, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 6th ed. New York City, NY: McGraw-Hill Professional; 2009:183-208.
2. Chlapek BH. Dermatologic emergencies. In: Stone CK, Humphries RL, eds. Current Diagnosis & Treatment: Emergency Medicine. 6th ed. New York City, NY: McGraw-Hill Companies; 2007:270-284.
3. Adişen E, Gürer MA. Therapeutic options for palmoplantar pustulosis. Clin Exp Dermatol. 2009;35:219-222.
4. Imboden JB, Donald FA, Stone JH, et al. Medications. In: Imboden JB, Hellmann DB, Stone JH, eds. Current Rheumatology Diagnosis & Treatment. 2nd ed. New York City, NY: Lange Medical Books/McGraw-Hill; 2004:355-383.
5. Warren RB, Chalmers RJG, Griffiths EM, et al. Methotrexate for psoriasis in the era of biological therapy. Clin Exp Dermatol. 2008;33:551-554.
6. Park J, Lee S. A case of tumor necrosis factor-alpha inhibitors-induced pustular psoriasis. Ann Dermatol. 2010;22:212-215.
7. Tsankov N, Angelova I, Kazandjieva J. Drug-induced psoriasis. Am J Clin Dermatol. 2000;1:159-165.
8. Basavaraj K, Ashok N, Rashmi R, et al. The role of drugs in the induction and/or exacerbation of psoriasis. Int J Dermatol. 2010;49:1351-1361.
9. Chalmers R, Hollis S, Leonardi-Bee J, et al. Interventions for chronic palmoplantar pustulosis (review). Cochrane Database Syst Rev. 2009;1:1-51.
10. Spuls P, Hadi S, Rivera L, et al. Retrospective analysis of the treatment of psoriasis of the palms and soles. J Dermatolog Treat. 2003;14:21-25.
11. Mrowietz U, van de Kerkhof PCM. Management of palmoplantar pustulosis: do we need to change? Br J Dermatol. 2011;164:942-946.
12. Kanwar A, Yanav S, Dogra S. Psoriasis: what is new in nonbiologic systemic therapy in the era of biologics? Indian J Dermatol. 2010;76:622-633.
13. Haustein UF, Rytter M. Methotrexate in psoriasis: 26 years’ experience with low-dose long-term treatment. J Eur Acad Dermatol Venereol. 2000;14:382-388.
1. Timothy H. Diseases of the skin. In: McPhee SJ, Hammer GD, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 6th ed. New York City, NY: McGraw-Hill Professional; 2009:183-208.
2. Chlapek BH. Dermatologic emergencies. In: Stone CK, Humphries RL, eds. Current Diagnosis & Treatment: Emergency Medicine. 6th ed. New York City, NY: McGraw-Hill Companies; 2007:270-284.
3. Adişen E, Gürer MA. Therapeutic options for palmoplantar pustulosis. Clin Exp Dermatol. 2009;35:219-222.
4. Imboden JB, Donald FA, Stone JH, et al. Medications. In: Imboden JB, Hellmann DB, Stone JH, eds. Current Rheumatology Diagnosis & Treatment. 2nd ed. New York City, NY: Lange Medical Books/McGraw-Hill; 2004:355-383.
5. Warren RB, Chalmers RJG, Griffiths EM, et al. Methotrexate for psoriasis in the era of biological therapy. Clin Exp Dermatol. 2008;33:551-554.
6. Park J, Lee S. A case of tumor necrosis factor-alpha inhibitors-induced pustular psoriasis. Ann Dermatol. 2010;22:212-215.
7. Tsankov N, Angelova I, Kazandjieva J. Drug-induced psoriasis. Am J Clin Dermatol. 2000;1:159-165.
8. Basavaraj K, Ashok N, Rashmi R, et al. The role of drugs in the induction and/or exacerbation of psoriasis. Int J Dermatol. 2010;49:1351-1361.
9. Chalmers R, Hollis S, Leonardi-Bee J, et al. Interventions for chronic palmoplantar pustulosis (review). Cochrane Database Syst Rev. 2009;1:1-51.
10. Spuls P, Hadi S, Rivera L, et al. Retrospective analysis of the treatment of psoriasis of the palms and soles. J Dermatolog Treat. 2003;14:21-25.
11. Mrowietz U, van de Kerkhof PCM. Management of palmoplantar pustulosis: do we need to change? Br J Dermatol. 2011;164:942-946.
12. Kanwar A, Yanav S, Dogra S. Psoriasis: what is new in nonbiologic systemic therapy in the era of biologics? Indian J Dermatol. 2010;76:622-633.
13. Haustein UF, Rytter M. Methotrexate in psoriasis: 26 years’ experience with low-dose long-term treatment. J Eur Acad Dermatol Venereol. 2000;14:382-388.
- Beta-blockers, lithium, tetracyclines, nonsteroidal anti-inflammatory drugs, adalimumab, synthetic antimalarials, angiotensin-converting enzyme inhibitors, interferons, terbinafine, infliximab, and etanercept can aggravate preexisting psoriasis, provoke lesions in uninvolved skin in individuals with psoriasis, and induce psoriasis in patients without a personal or family history of psoriasis.
- Methotrexate can be effective and safe in treating palmoplantar pustular psoriasis when prescribed at a low dose.
Rupioid Psoriasis and Other Skin Diseases With Rupioid Manifestations
Case Report
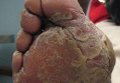
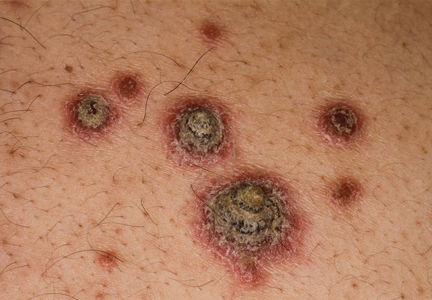
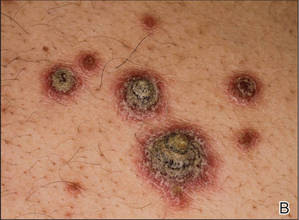
A 28-year-old man presented to the dermatology department with cone-shaped, oyster shell–like skin lesions on the scalp, trunk, arms, and legs of 1 month’s duration. He denied any fever, pruritus, pain, joint stiffness, or arthralgia. His family history was remarkable for psoriasis in his paternal grandfather and uncle.
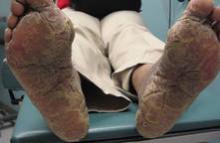
A few years prior to the eruption, the patient developed a rash in the bilateral inguinal area but did not seek medical attention. One month prior to presentation, the rash began to spread to the scalp, trunk, arms, and legs. He was treated in the emergency department with a 5-day course of oral prednisone without any noticeable improvement. At the time of presentation to the dermatology clinic, he was found to have multiple well-demarcated erythematous plaques with conical, oyster shell–like, dirty-appearing, hyperkeratotic crusts (Figure 1). Rapid plasma reagin testing was negative. A 4-mm punch biopsy specimen from the right upper arm demonstrated thick parakeratosis with a remarkable Munro microabscess, regular psoriasiform acanthosis with thin suprapapillary epidermal plates, absent granular layer, and prominent papillary dermal edema (Figure 2). In the stratum corneum, there was seroexudate with numerous red blood cells between the parakeratosis.

|
|
| Figure 1. Multiple well-demarcated erythematous plaques with hyperkeratotic crust on the back (A). Closer view of erythematous plaques with conical, oyster shell–like, dirty-appearing, hyperkeratotic crust (B). |
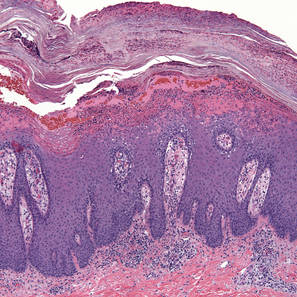
|
| Figure 2. Thick parakeratosis with a remarkable Munro microabscess, regular psoriasiform acanthosis with thin suprapapillary epidermal plates, absent granular layer, and prominent papillary dermal edema (H&E, original magnification ×100). |
The patient was diagnosed with rupioid psoriasis. The lesions dramatically improved with methotrexate 10 mg weekly and topical steroids. Two months following diagnosis the patient presented with persistent hyperkeratotic lesions on the back, as he had difficulty reaching the lesions to apply topical medications; intralesional steroid injections were added. This regimen resulted in near-complete resolution maintained at his most recent follow-up 2 years following diagnosis in our clinic.
Comment
Rupia is based on the Greek word rhupos, which means dirt or filth. The term rupioid has been used to describe well-demarcated, cone-shaped plaques with thick, dark, lamellate, and adherent crusts on the skin somewhat resembling oyster or limpet shells. Histologically, a serosanguineous exudate along with thick skin helps to impart a “dirty” appearance to rupioid lesions. Rupioid manifestations have been clinically observed in a variety of diseases, including rupioid psoriasis,1-3 reactive arthritis,4 disseminated histoplasmosis,5 keratotic scabies,6 secondary syphilis,7 and photosensitive skin lesions in association with aminoaciduria.8 To diagnose the underlying infectious or inflammatory diseases beneath the thick crusts, skin biopsy and a blood test for syphilis may be necessary.
Rupioid psoriasis is a morphologic subtype of plaque psoriasis with hyperkeratotic lesions that resemble an oyster or limpet shell. Patients with thick plaque psoriasis are more likely to be male with a higher incidence of nail disease and psoriatic arthritis as well as a greater body surface area affected than patients with thin plaque psoriasis.1 Although most cases of rupioid psoriasis were associated with psoriatic arthritis,3 our patient showed no evidence of psoriatic arthritis or nail changes.
Reactive arthritis may have a similar appearance to rupioid psoriasis but may be distinguished by a geographic relief map configuration with coalescing, keratotic and desquamating lesions, as well as associated urethritis, arthritis, and conjunctivitis.4 A rupioid eruption was reported as a manifestation of disseminated histoplasmosis with dirty-appearing, heaped-up, crusted lesions present on the cheeks, nose, and forehead on clinical examination and several intracellular and extracellular oval structures on histologic examination with periodic acid–Schiff and Gomori methenamine-silver stain.5 Malignant or rupioid syphilis refers to the stage in which papulopustules of pustular syphilis undergo central necrosis due to endarteritis obliterans and intravascular thrombosis.7
In our case, the patient’s psoriasis could have flared after discontinuation of the prednisone that was administered by the emergency department physician. Most cases have been treated with combined systemic and topical therapy.9 For systemic treatment, cyclosporine, intramuscular or oral methotrexate, adalimumab, and ustekinumab3 have been used with remarkable improvement. Hyperkeratotic types of psoriasis are generally thought to be resistant to topical therapy because of poor penetration of applied agents; however, a case of rupioid psoriasis without arthritis was successfully treated with topical steroids without concomitant systemic medications.2
Conclusion
Rupioid psoriasis is a morphological subtype of plaque psoriasis with hyperkeratotic lesions that resemble a limpet shell. Rupioid skin manifestations may be seen in a variety of diseases including rupioid psoriasis, reactive arthritis, disseminated histoplasmosis, keratotic scabies, secondary syphilis, and photosensitive skin lesions associated with aminoaciduria. Diagnosis of rupioid psoriasis often requires additional testing such as skin biopsy, skin scraping, and blood tests, and it typically requires systemic therapy for treatment.
1. Christensen TE, Callis KP, Papenfuss J, et al. Observations of psoriasis in the absence of therapeutic intervention identifies two unappreciated morphologic variants, thin-plaque and thick-plaque psoriasis, and their associated phenotypes. J Invest Dermatol. 2006;126:2397-2403.
2. Feldman SR, Brown KL, Heald P. ‘Coral reef’ psoriasis: a marker of resistance to topical treatment. J Dermatolog Treat. 2008;19:257-258.
3. Necas M, Vasku V. Ustekinumab in the treatment of severe rupioid psoriasis: a case report. Acta Dermatovenerol Alp Panonica Adriat. 2010;19:23-27.
4. Sehgal VN, Koranne RV, Shyam Prasad AL. Unusual manifestations of Reiter’s disease in a child. Dermatologica. 1985;170:77-79.
5. Corti M, Villafañe MF, Palmieri O, et al. Rupioid histoplasmosis: first case reported in an AIDS patient in Argentina. Rev Inst Med Trop Sao Paulo. 2010;52:279-280.
6. Costa JB, Rocha de Sousa VL, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913.
7. Bhagwat PV, Tophakhane RS, Rathod RM, et al. Rupioid syphilis in an HIV patient. Indian J Dermatol Venereol. 2009;75:201-202.
8. Haim S, Gilhar A, Cohen A. Cutaneous manifestations associated with aminoaciduria. report of two cases. Dermatologica. 1978;156:244-250.
9. Murakami T, Ohtsuki M, Nakagawa H. Rupioid psoriasis with arthropathy. Clin Exp Dermatol. 2000;25:409-412.
Case Report
A 28-year-old man presented to the dermatology department with cone-shaped, oyster shell–like skin lesions on the scalp, trunk, arms, and legs of 1 month’s duration. He denied any fever, pruritus, pain, joint stiffness, or arthralgia. His family history was remarkable for psoriasis in his paternal grandfather and uncle.
A few years prior to the eruption, the patient developed a rash in the bilateral inguinal area but did not seek medical attention. One month prior to presentation, the rash began to spread to the scalp, trunk, arms, and legs. He was treated in the emergency department with a 5-day course of oral prednisone without any noticeable improvement. At the time of presentation to the dermatology clinic, he was found to have multiple well-demarcated erythematous plaques with conical, oyster shell–like, dirty-appearing, hyperkeratotic crusts (Figure 1). Rapid plasma reagin testing was negative. A 4-mm punch biopsy specimen from the right upper arm demonstrated thick parakeratosis with a remarkable Munro microabscess, regular psoriasiform acanthosis with thin suprapapillary epidermal plates, absent granular layer, and prominent papillary dermal edema (Figure 2). In the stratum corneum, there was seroexudate with numerous red blood cells between the parakeratosis.

|

|
| Figure 1. Multiple well-demarcated erythematous plaques with hyperkeratotic crust on the back (A). Closer view of erythematous plaques with conical, oyster shell–like, dirty-appearing, hyperkeratotic crust (B). |

|
| Figure 2. Thick parakeratosis with a remarkable Munro microabscess, regular psoriasiform acanthosis with thin suprapapillary epidermal plates, absent granular layer, and prominent papillary dermal edema (H&E, original magnification ×100). |
The patient was diagnosed with rupioid psoriasis. The lesions dramatically improved with methotrexate 10 mg weekly and topical steroids. Two months following diagnosis the patient presented with persistent hyperkeratotic lesions on the back, as he had difficulty reaching the lesions to apply topical medications; intralesional steroid injections were added. This regimen resulted in near-complete resolution maintained at his most recent follow-up 2 years following diagnosis in our clinic.
Comment
Rupia is based on the Greek word rhupos, which means dirt or filth. The term rupioid has been used to describe well-demarcated, cone-shaped plaques with thick, dark, lamellate, and adherent crusts on the skin somewhat resembling oyster or limpet shells. Histologically, a serosanguineous exudate along with thick skin helps to impart a “dirty” appearance to rupioid lesions. Rupioid manifestations have been clinically observed in a variety of diseases, including rupioid psoriasis,1-3 reactive arthritis,4 disseminated histoplasmosis,5 keratotic scabies,6 secondary syphilis,7 and photosensitive skin lesions in association with aminoaciduria.8 To diagnose the underlying infectious or inflammatory diseases beneath the thick crusts, skin biopsy and a blood test for syphilis may be necessary.
Rupioid psoriasis is a morphologic subtype of plaque psoriasis with hyperkeratotic lesions that resemble an oyster or limpet shell. Patients with thick plaque psoriasis are more likely to be male with a higher incidence of nail disease and psoriatic arthritis as well as a greater body surface area affected than patients with thin plaque psoriasis.1 Although most cases of rupioid psoriasis were associated with psoriatic arthritis,3 our patient showed no evidence of psoriatic arthritis or nail changes.
Reactive arthritis may have a similar appearance to rupioid psoriasis but may be distinguished by a geographic relief map configuration with coalescing, keratotic and desquamating lesions, as well as associated urethritis, arthritis, and conjunctivitis.4 A rupioid eruption was reported as a manifestation of disseminated histoplasmosis with dirty-appearing, heaped-up, crusted lesions present on the cheeks, nose, and forehead on clinical examination and several intracellular and extracellular oval structures on histologic examination with periodic acid–Schiff and Gomori methenamine-silver stain.5 Malignant or rupioid syphilis refers to the stage in which papulopustules of pustular syphilis undergo central necrosis due to endarteritis obliterans and intravascular thrombosis.7
In our case, the patient’s psoriasis could have flared after discontinuation of the prednisone that was administered by the emergency department physician. Most cases have been treated with combined systemic and topical therapy.9 For systemic treatment, cyclosporine, intramuscular or oral methotrexate, adalimumab, and ustekinumab3 have been used with remarkable improvement. Hyperkeratotic types of psoriasis are generally thought to be resistant to topical therapy because of poor penetration of applied agents; however, a case of rupioid psoriasis without arthritis was successfully treated with topical steroids without concomitant systemic medications.2
Conclusion
Rupioid psoriasis is a morphological subtype of plaque psoriasis with hyperkeratotic lesions that resemble a limpet shell. Rupioid skin manifestations may be seen in a variety of diseases including rupioid psoriasis, reactive arthritis, disseminated histoplasmosis, keratotic scabies, secondary syphilis, and photosensitive skin lesions associated with aminoaciduria. Diagnosis of rupioid psoriasis often requires additional testing such as skin biopsy, skin scraping, and blood tests, and it typically requires systemic therapy for treatment.
Case Report
A 28-year-old man presented to the dermatology department with cone-shaped, oyster shell–like skin lesions on the scalp, trunk, arms, and legs of 1 month’s duration. He denied any fever, pruritus, pain, joint stiffness, or arthralgia. His family history was remarkable for psoriasis in his paternal grandfather and uncle.
A few years prior to the eruption, the patient developed a rash in the bilateral inguinal area but did not seek medical attention. One month prior to presentation, the rash began to spread to the scalp, trunk, arms, and legs. He was treated in the emergency department with a 5-day course of oral prednisone without any noticeable improvement. At the time of presentation to the dermatology clinic, he was found to have multiple well-demarcated erythematous plaques with conical, oyster shell–like, dirty-appearing, hyperkeratotic crusts (Figure 1). Rapid plasma reagin testing was negative. A 4-mm punch biopsy specimen from the right upper arm demonstrated thick parakeratosis with a remarkable Munro microabscess, regular psoriasiform acanthosis with thin suprapapillary epidermal plates, absent granular layer, and prominent papillary dermal edema (Figure 2). In the stratum corneum, there was seroexudate with numerous red blood cells between the parakeratosis.

|

|
| Figure 1. Multiple well-demarcated erythematous plaques with hyperkeratotic crust on the back (A). Closer view of erythematous plaques with conical, oyster shell–like, dirty-appearing, hyperkeratotic crust (B). |

|
| Figure 2. Thick parakeratosis with a remarkable Munro microabscess, regular psoriasiform acanthosis with thin suprapapillary epidermal plates, absent granular layer, and prominent papillary dermal edema (H&E, original magnification ×100). |
The patient was diagnosed with rupioid psoriasis. The lesions dramatically improved with methotrexate 10 mg weekly and topical steroids. Two months following diagnosis the patient presented with persistent hyperkeratotic lesions on the back, as he had difficulty reaching the lesions to apply topical medications; intralesional steroid injections were added. This regimen resulted in near-complete resolution maintained at his most recent follow-up 2 years following diagnosis in our clinic.
Comment
Rupia is based on the Greek word rhupos, which means dirt or filth. The term rupioid has been used to describe well-demarcated, cone-shaped plaques with thick, dark, lamellate, and adherent crusts on the skin somewhat resembling oyster or limpet shells. Histologically, a serosanguineous exudate along with thick skin helps to impart a “dirty” appearance to rupioid lesions. Rupioid manifestations have been clinically observed in a variety of diseases, including rupioid psoriasis,1-3 reactive arthritis,4 disseminated histoplasmosis,5 keratotic scabies,6 secondary syphilis,7 and photosensitive skin lesions in association with aminoaciduria.8 To diagnose the underlying infectious or inflammatory diseases beneath the thick crusts, skin biopsy and a blood test for syphilis may be necessary.
Rupioid psoriasis is a morphologic subtype of plaque psoriasis with hyperkeratotic lesions that resemble an oyster or limpet shell. Patients with thick plaque psoriasis are more likely to be male with a higher incidence of nail disease and psoriatic arthritis as well as a greater body surface area affected than patients with thin plaque psoriasis.1 Although most cases of rupioid psoriasis were associated with psoriatic arthritis,3 our patient showed no evidence of psoriatic arthritis or nail changes.
Reactive arthritis may have a similar appearance to rupioid psoriasis but may be distinguished by a geographic relief map configuration with coalescing, keratotic and desquamating lesions, as well as associated urethritis, arthritis, and conjunctivitis.4 A rupioid eruption was reported as a manifestation of disseminated histoplasmosis with dirty-appearing, heaped-up, crusted lesions present on the cheeks, nose, and forehead on clinical examination and several intracellular and extracellular oval structures on histologic examination with periodic acid–Schiff and Gomori methenamine-silver stain.5 Malignant or rupioid syphilis refers to the stage in which papulopustules of pustular syphilis undergo central necrosis due to endarteritis obliterans and intravascular thrombosis.7
In our case, the patient’s psoriasis could have flared after discontinuation of the prednisone that was administered by the emergency department physician. Most cases have been treated with combined systemic and topical therapy.9 For systemic treatment, cyclosporine, intramuscular or oral methotrexate, adalimumab, and ustekinumab3 have been used with remarkable improvement. Hyperkeratotic types of psoriasis are generally thought to be resistant to topical therapy because of poor penetration of applied agents; however, a case of rupioid psoriasis without arthritis was successfully treated with topical steroids without concomitant systemic medications.2
Conclusion
Rupioid psoriasis is a morphological subtype of plaque psoriasis with hyperkeratotic lesions that resemble a limpet shell. Rupioid skin manifestations may be seen in a variety of diseases including rupioid psoriasis, reactive arthritis, disseminated histoplasmosis, keratotic scabies, secondary syphilis, and photosensitive skin lesions associated with aminoaciduria. Diagnosis of rupioid psoriasis often requires additional testing such as skin biopsy, skin scraping, and blood tests, and it typically requires systemic therapy for treatment.
1. Christensen TE, Callis KP, Papenfuss J, et al. Observations of psoriasis in the absence of therapeutic intervention identifies two unappreciated morphologic variants, thin-plaque and thick-plaque psoriasis, and their associated phenotypes. J Invest Dermatol. 2006;126:2397-2403.
2. Feldman SR, Brown KL, Heald P. ‘Coral reef’ psoriasis: a marker of resistance to topical treatment. J Dermatolog Treat. 2008;19:257-258.
3. Necas M, Vasku V. Ustekinumab in the treatment of severe rupioid psoriasis: a case report. Acta Dermatovenerol Alp Panonica Adriat. 2010;19:23-27.
4. Sehgal VN, Koranne RV, Shyam Prasad AL. Unusual manifestations of Reiter’s disease in a child. Dermatologica. 1985;170:77-79.
5. Corti M, Villafañe MF, Palmieri O, et al. Rupioid histoplasmosis: first case reported in an AIDS patient in Argentina. Rev Inst Med Trop Sao Paulo. 2010;52:279-280.
6. Costa JB, Rocha de Sousa VL, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913.
7. Bhagwat PV, Tophakhane RS, Rathod RM, et al. Rupioid syphilis in an HIV patient. Indian J Dermatol Venereol. 2009;75:201-202.
8. Haim S, Gilhar A, Cohen A. Cutaneous manifestations associated with aminoaciduria. report of two cases. Dermatologica. 1978;156:244-250.
9. Murakami T, Ohtsuki M, Nakagawa H. Rupioid psoriasis with arthropathy. Clin Exp Dermatol. 2000;25:409-412.
1. Christensen TE, Callis KP, Papenfuss J, et al. Observations of psoriasis in the absence of therapeutic intervention identifies two unappreciated morphologic variants, thin-plaque and thick-plaque psoriasis, and their associated phenotypes. J Invest Dermatol. 2006;126:2397-2403.
2. Feldman SR, Brown KL, Heald P. ‘Coral reef’ psoriasis: a marker of resistance to topical treatment. J Dermatolog Treat. 2008;19:257-258.
3. Necas M, Vasku V. Ustekinumab in the treatment of severe rupioid psoriasis: a case report. Acta Dermatovenerol Alp Panonica Adriat. 2010;19:23-27.
4. Sehgal VN, Koranne RV, Shyam Prasad AL. Unusual manifestations of Reiter’s disease in a child. Dermatologica. 1985;170:77-79.
5. Corti M, Villafañe MF, Palmieri O, et al. Rupioid histoplasmosis: first case reported in an AIDS patient in Argentina. Rev Inst Med Trop Sao Paulo. 2010;52:279-280.
6. Costa JB, Rocha de Sousa VL, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913.
7. Bhagwat PV, Tophakhane RS, Rathod RM, et al. Rupioid syphilis in an HIV patient. Indian J Dermatol Venereol. 2009;75:201-202.
8. Haim S, Gilhar A, Cohen A. Cutaneous manifestations associated with aminoaciduria. report of two cases. Dermatologica. 1978;156:244-250.
9. Murakami T, Ohtsuki M, Nakagawa H. Rupioid psoriasis with arthropathy. Clin Exp Dermatol. 2000;25:409-412.
- Diseases with rupioid manifestations include rupioid psoriasis, reactive arthritis, disseminated histoplasmosis, keratotic scabies, secondary syphilis, and photosensitive skin lesions associated with aminoaciduria.
- Skin biopsy, skin scraping, and blood tests may be necessary to diagnose the underlying diseases beneath the thick crusts and to rule out other diagnoses within the differential.
- Treatment of rupioid psoriasis is no different than typical plaque psoriasis, except for the need for systemic therapy in most cases due to the thick scale.
Recent Findings About Diet/Obesity and Psoriasis
Psoriasis Associated With Obesity in Adults
The National Health and Nutrition Examination Surveys were reviewed to better understand the burden of psoriasis. Helmick et al (Am J Prev Med. 2014;47:37-45) examined psoriasis prevalence, severity, disparities, health-related quality of life, and selected comorbidities in 10,676 adults aged 20 to 59 years from 2003-2006 and 2009-2010. Related to patient diet and weight, they noted that psoriasis was associated with obesity.
Practice Point: The association between psoriasis and obesity warrants further research, as the disease is a large public health concern.
>>Read more at American Journal of Preventive Medicine
Dietary Plan With Physical Exercise Reduces Psoriasis Severity
Among the risk factors for psoriasis are increased body mass index and weight gain. The prevalence of obesity in patients with psoriasis is higher than in the general population. Naldi et al (Br J Dermatol. 2014;170:634-642) assessed the impact of a dietary intervention combined with physical exercise for weight loss on improving psoriasis in overweight or obese patients. Patients were randomized to receive either a 20-week quantitative and qualitative dietary plan associated with physical exercise for weight loss or simple informative counseling at baseline about the utility of weight loss for clinical control of psoriatic disease. They reported that the median reduction in psoriasis area and severity index scores was significantly higher in the dietary intervention arm compared with the information-only arm (P=.02).
Practice Point: A dietary plan associated with physical exercise for obese or overweight psoriasis patients may help reduce disease severity.
>>Read more at British Journal of Dermatology
Improvement in Psoriasis With a Low-Energy Diet
Psoriasis severity increases with weight gain. Jensen et al (JAMA Dermatol. 2013;149:795-801) sought to measure the effect of weight reduction on the severity of psoriasis in obese patients. The intervention group received a low-energy diet for 8 weeks to induce weight loss, followed by 8 weeks of reintroduction of normal food intake. The control group was instructed to continue eating ordinary healthy foods. Results based on psoriasis area and severity index and dermatology life quality index scores were in favor of the low-energy diet group.
Practice Point: A low-energy diet may improve the severity of psoriasis in obese patients.
>>Read more at JAMA Dermatology
Psoriasis Associated With Obesity in Adults
The National Health and Nutrition Examination Surveys were reviewed to better understand the burden of psoriasis. Helmick et al (Am J Prev Med. 2014;47:37-45) examined psoriasis prevalence, severity, disparities, health-related quality of life, and selected comorbidities in 10,676 adults aged 20 to 59 years from 2003-2006 and 2009-2010. Related to patient diet and weight, they noted that psoriasis was associated with obesity.
Practice Point: The association between psoriasis and obesity warrants further research, as the disease is a large public health concern.
>>Read more at American Journal of Preventive Medicine
Dietary Plan With Physical Exercise Reduces Psoriasis Severity
Among the risk factors for psoriasis are increased body mass index and weight gain. The prevalence of obesity in patients with psoriasis is higher than in the general population. Naldi et al (Br J Dermatol. 2014;170:634-642) assessed the impact of a dietary intervention combined with physical exercise for weight loss on improving psoriasis in overweight or obese patients. Patients were randomized to receive either a 20-week quantitative and qualitative dietary plan associated with physical exercise for weight loss or simple informative counseling at baseline about the utility of weight loss for clinical control of psoriatic disease. They reported that the median reduction in psoriasis area and severity index scores was significantly higher in the dietary intervention arm compared with the information-only arm (P=.02).
Practice Point: A dietary plan associated with physical exercise for obese or overweight psoriasis patients may help reduce disease severity.
>>Read more at British Journal of Dermatology
Improvement in Psoriasis With a Low-Energy Diet
Psoriasis severity increases with weight gain. Jensen et al (JAMA Dermatol. 2013;149:795-801) sought to measure the effect of weight reduction on the severity of psoriasis in obese patients. The intervention group received a low-energy diet for 8 weeks to induce weight loss, followed by 8 weeks of reintroduction of normal food intake. The control group was instructed to continue eating ordinary healthy foods. Results based on psoriasis area and severity index and dermatology life quality index scores were in favor of the low-energy diet group.
Practice Point: A low-energy diet may improve the severity of psoriasis in obese patients.
>>Read more at JAMA Dermatology
Psoriasis Associated With Obesity in Adults
The National Health and Nutrition Examination Surveys were reviewed to better understand the burden of psoriasis. Helmick et al (Am J Prev Med. 2014;47:37-45) examined psoriasis prevalence, severity, disparities, health-related quality of life, and selected comorbidities in 10,676 adults aged 20 to 59 years from 2003-2006 and 2009-2010. Related to patient diet and weight, they noted that psoriasis was associated with obesity.
Practice Point: The association between psoriasis and obesity warrants further research, as the disease is a large public health concern.
>>Read more at American Journal of Preventive Medicine
Dietary Plan With Physical Exercise Reduces Psoriasis Severity
Among the risk factors for psoriasis are increased body mass index and weight gain. The prevalence of obesity in patients with psoriasis is higher than in the general population. Naldi et al (Br J Dermatol. 2014;170:634-642) assessed the impact of a dietary intervention combined with physical exercise for weight loss on improving psoriasis in overweight or obese patients. Patients were randomized to receive either a 20-week quantitative and qualitative dietary plan associated with physical exercise for weight loss or simple informative counseling at baseline about the utility of weight loss for clinical control of psoriatic disease. They reported that the median reduction in psoriasis area and severity index scores was significantly higher in the dietary intervention arm compared with the information-only arm (P=.02).
Practice Point: A dietary plan associated with physical exercise for obese or overweight psoriasis patients may help reduce disease severity.
>>Read more at British Journal of Dermatology
Improvement in Psoriasis With a Low-Energy Diet
Psoriasis severity increases with weight gain. Jensen et al (JAMA Dermatol. 2013;149:795-801) sought to measure the effect of weight reduction on the severity of psoriasis in obese patients. The intervention group received a low-energy diet for 8 weeks to induce weight loss, followed by 8 weeks of reintroduction of normal food intake. The control group was instructed to continue eating ordinary healthy foods. Results based on psoriasis area and severity index and dermatology life quality index scores were in favor of the low-energy diet group.
Practice Point: A low-energy diet may improve the severity of psoriasis in obese patients.
>>Read more at JAMA Dermatology
Psoriasis patients post above-average cancer rates
CHICAGO – Malignancy rates in patients with psoriasis outstrip those in the general population, based on data from a retrospective analysis of commercial claims.
Rates for all cancers were similar among patients undergoing different psoriasis treatments, with the exception of nonmelanoma skin cancer and lymphoma.
Rates for these two cancers were more variable across treatment groups, but were still above those in the general public, Dr. Alexa B. Kimball reported at the American Academy of Dermatology summer meeting.
The increased cancer risk may be associated with chronic inflammation, a hallmark of psoriasis, and exposure to some psoriasis therapies such as phototherapy with psoralen plus ultraviolet, cyclosporine, and methotrexate, she noted in the study’s background information.
Previous studies also have suggested that patients with psoriasis may be at increased risk for some cancers such as respiratory tract, urinary tract, and liver cancers, non-Hodgkin’s lymphoma, and skin cancers.
To evaluate the incidence of malignancy, the investigators obtained data from the MarketScan Commercial and Medicare Supplemental claims databases for patients with a diagnosis of psoriasis on or before Dec. 31, 2006, and at least one prescription claim for etanercept (Enbrel), adalimumab (Humira), infliximab (Remicade), ustekinumab (Stelara), nonbiologic therapies, or phototherapy.
The general population cohort included patients at least 18 years old as of 2005 or at enrollment in the health care plan. Both cohorts had 12 months’ continuous enrollment in the health care plan from Jan. 1, 2005, through Dec. 31, 2006.
Follow-up for a patient ended at the first cancer event, disenrollment from the health care plan, or after 5 years from the index date.
Patients with psoriasis had a 5-year malignancy rate of 115.5 cases/10,000 person-years, compared with 96/10,000 person-years for the general population, reported Dr. Kimball of Massachusetts General Hospital, Boston.
Excluding nonmelanoma skin cancer and lymphoma, incidence rates were similar across treatment groups: etanercept (100.2/10,000), adalimumab (94.6/10,000), infliximab (138.1/10,000), ustekinumab (100.6/10,000), nonbiologics (116.8/10,000), and phototherapy (117.3/10,000).
"These large database queries continue to be reassuring that most systemic therapies are not changing the risk for common cancers, excluding lymphoma and skin cancer, which we continue to examine separately," Dr. Kimball said in an interview.
Nonmelanoma skin cancer was far and away the most common malignancy in psoriasis patients, occurring at a rate of 147.2/10,000 person-years vs. 94.2/10,000 person-years among the general public. Rates were highest in patients treated with adalimumab (234.2/10,000 person-years) and ustekinumab (233.3/10,000) and lowest in those treated with etanercept (155.9/10,000).
"Even with the large size of the database, the number of skin cancers remains small, so making conclusions about specific therapies may be premature," she said.
Incidence rates of lymphoma were considerably lower, but again higher in psoriasis patients than the general public (11.1/10,000 vs. 6.6/10,000). Rates of this hematologic cancer were highest with ustekinumab (25.1/10,000) and, once again, lowest with etanercept (6.9/10,000), according to the poster.
In all, 5,857 patients received nonbiologic therapies, 6,856 received etanercept, 3,314 adalimumab, 1,044 infliximab, 526 ustekinumab, and 5,156 were treated with phototherapy.
Dr. Kimball is a consultant for several pharmaceutical companies including Amgen, the study sponsor.
CHICAGO – Malignancy rates in patients with psoriasis outstrip those in the general population, based on data from a retrospective analysis of commercial claims.
Rates for all cancers were similar among patients undergoing different psoriasis treatments, with the exception of nonmelanoma skin cancer and lymphoma.
Rates for these two cancers were more variable across treatment groups, but were still above those in the general public, Dr. Alexa B. Kimball reported at the American Academy of Dermatology summer meeting.
The increased cancer risk may be associated with chronic inflammation, a hallmark of psoriasis, and exposure to some psoriasis therapies such as phototherapy with psoralen plus ultraviolet, cyclosporine, and methotrexate, she noted in the study’s background information.
Previous studies also have suggested that patients with psoriasis may be at increased risk for some cancers such as respiratory tract, urinary tract, and liver cancers, non-Hodgkin’s lymphoma, and skin cancers.
To evaluate the incidence of malignancy, the investigators obtained data from the MarketScan Commercial and Medicare Supplemental claims databases for patients with a diagnosis of psoriasis on or before Dec. 31, 2006, and at least one prescription claim for etanercept (Enbrel), adalimumab (Humira), infliximab (Remicade), ustekinumab (Stelara), nonbiologic therapies, or phototherapy.
The general population cohort included patients at least 18 years old as of 2005 or at enrollment in the health care plan. Both cohorts had 12 months’ continuous enrollment in the health care plan from Jan. 1, 2005, through Dec. 31, 2006.
Follow-up for a patient ended at the first cancer event, disenrollment from the health care plan, or after 5 years from the index date.
Patients with psoriasis had a 5-year malignancy rate of 115.5 cases/10,000 person-years, compared with 96/10,000 person-years for the general population, reported Dr. Kimball of Massachusetts General Hospital, Boston.
Excluding nonmelanoma skin cancer and lymphoma, incidence rates were similar across treatment groups: etanercept (100.2/10,000), adalimumab (94.6/10,000), infliximab (138.1/10,000), ustekinumab (100.6/10,000), nonbiologics (116.8/10,000), and phototherapy (117.3/10,000).
"These large database queries continue to be reassuring that most systemic therapies are not changing the risk for common cancers, excluding lymphoma and skin cancer, which we continue to examine separately," Dr. Kimball said in an interview.
Nonmelanoma skin cancer was far and away the most common malignancy in psoriasis patients, occurring at a rate of 147.2/10,000 person-years vs. 94.2/10,000 person-years among the general public. Rates were highest in patients treated with adalimumab (234.2/10,000 person-years) and ustekinumab (233.3/10,000) and lowest in those treated with etanercept (155.9/10,000).
"Even with the large size of the database, the number of skin cancers remains small, so making conclusions about specific therapies may be premature," she said.
Incidence rates of lymphoma were considerably lower, but again higher in psoriasis patients than the general public (11.1/10,000 vs. 6.6/10,000). Rates of this hematologic cancer were highest with ustekinumab (25.1/10,000) and, once again, lowest with etanercept (6.9/10,000), according to the poster.
In all, 5,857 patients received nonbiologic therapies, 6,856 received etanercept, 3,314 adalimumab, 1,044 infliximab, 526 ustekinumab, and 5,156 were treated with phototherapy.
Dr. Kimball is a consultant for several pharmaceutical companies including Amgen, the study sponsor.
CHICAGO – Malignancy rates in patients with psoriasis outstrip those in the general population, based on data from a retrospective analysis of commercial claims.
Rates for all cancers were similar among patients undergoing different psoriasis treatments, with the exception of nonmelanoma skin cancer and lymphoma.
Rates for these two cancers were more variable across treatment groups, but were still above those in the general public, Dr. Alexa B. Kimball reported at the American Academy of Dermatology summer meeting.
The increased cancer risk may be associated with chronic inflammation, a hallmark of psoriasis, and exposure to some psoriasis therapies such as phototherapy with psoralen plus ultraviolet, cyclosporine, and methotrexate, she noted in the study’s background information.
Previous studies also have suggested that patients with psoriasis may be at increased risk for some cancers such as respiratory tract, urinary tract, and liver cancers, non-Hodgkin’s lymphoma, and skin cancers.
To evaluate the incidence of malignancy, the investigators obtained data from the MarketScan Commercial and Medicare Supplemental claims databases for patients with a diagnosis of psoriasis on or before Dec. 31, 2006, and at least one prescription claim for etanercept (Enbrel), adalimumab (Humira), infliximab (Remicade), ustekinumab (Stelara), nonbiologic therapies, or phototherapy.
The general population cohort included patients at least 18 years old as of 2005 or at enrollment in the health care plan. Both cohorts had 12 months’ continuous enrollment in the health care plan from Jan. 1, 2005, through Dec. 31, 2006.
Follow-up for a patient ended at the first cancer event, disenrollment from the health care plan, or after 5 years from the index date.
Patients with psoriasis had a 5-year malignancy rate of 115.5 cases/10,000 person-years, compared with 96/10,000 person-years for the general population, reported Dr. Kimball of Massachusetts General Hospital, Boston.
Excluding nonmelanoma skin cancer and lymphoma, incidence rates were similar across treatment groups: etanercept (100.2/10,000), adalimumab (94.6/10,000), infliximab (138.1/10,000), ustekinumab (100.6/10,000), nonbiologics (116.8/10,000), and phototherapy (117.3/10,000).
"These large database queries continue to be reassuring that most systemic therapies are not changing the risk for common cancers, excluding lymphoma and skin cancer, which we continue to examine separately," Dr. Kimball said in an interview.
Nonmelanoma skin cancer was far and away the most common malignancy in psoriasis patients, occurring at a rate of 147.2/10,000 person-years vs. 94.2/10,000 person-years among the general public. Rates were highest in patients treated with adalimumab (234.2/10,000 person-years) and ustekinumab (233.3/10,000) and lowest in those treated with etanercept (155.9/10,000).
"Even with the large size of the database, the number of skin cancers remains small, so making conclusions about specific therapies may be premature," she said.
Incidence rates of lymphoma were considerably lower, but again higher in psoriasis patients than the general public (11.1/10,000 vs. 6.6/10,000). Rates of this hematologic cancer were highest with ustekinumab (25.1/10,000) and, once again, lowest with etanercept (6.9/10,000), according to the poster.
In all, 5,857 patients received nonbiologic therapies, 6,856 received etanercept, 3,314 adalimumab, 1,044 infliximab, 526 ustekinumab, and 5,156 were treated with phototherapy.
Dr. Kimball is a consultant for several pharmaceutical companies including Amgen, the study sponsor.
AT THE AAD SUMMER ACADEMY 2014
Key clinical point: Rates of malignancy were higher among patients with psoriasis than in the general population.
Major finding: The 5-year malignancy rate in patients with psoriasis was 115.5 cases/10,000 person-years vs. 96/10,000 person-years in the general population.
Data source: Retrospective database analysis of 22,753 patients with psoriasis.
Disclosures: Dr. Kimball is a consultant for several pharmaceutical companies including Amgen, the study sponsor.
Anti-adalimumab antibodies mean poorer outcomes in psoriatic arthritis
Psoriatic arthritis patients with detectable anti-adalimumab antibodies have significantly lower serum adalimumab concentrations and poorer clinical outcomes at 28 weeks and 52 weeks of treatment, than did patients without antibodies in a prospective cohort study of 103 patients.
At week 52, the 23 patients with detectable anti-adalimumab antibodies had a median adalimumab concentration of 0.9 mg/L, compared with 9.4 mg/L in the 80 patients without detectable antibodies, as well as significantly higher C-reactive protein, Psoriasis Area Severity Index score, and 28-joint Disease Activity Score.
The researchers, led by Erik H. Vogelzang of the Jan van Breemen Research Institute/Reade in Amsterdam, also found that patients on adalimumab monotherapy had significantly lower median adalimumab concentrations at 28 and 52 weeks, compared with patients taking adalimumab and concomitant methotrexate (Ann. Rheum. Dis. 2014 [doi:10.1136/annrheumdis-2014-205554]).
"Further studies regarding measuring drug concentrations would be relevant, since this could give more insight on the cause of inadequate response, especially since treatment options in PsA [psoriatic arthritis] are limited," the investigators wrote.
The study was partly supported by AbbVie and Pfizer. The authors declared a range of consultancies, lecture fees, and research grants from the pharmaceutical industry.
Psoriatic arthritis patients with detectable anti-adalimumab antibodies have significantly lower serum adalimumab concentrations and poorer clinical outcomes at 28 weeks and 52 weeks of treatment, than did patients without antibodies in a prospective cohort study of 103 patients.
At week 52, the 23 patients with detectable anti-adalimumab antibodies had a median adalimumab concentration of 0.9 mg/L, compared with 9.4 mg/L in the 80 patients without detectable antibodies, as well as significantly higher C-reactive protein, Psoriasis Area Severity Index score, and 28-joint Disease Activity Score.
The researchers, led by Erik H. Vogelzang of the Jan van Breemen Research Institute/Reade in Amsterdam, also found that patients on adalimumab monotherapy had significantly lower median adalimumab concentrations at 28 and 52 weeks, compared with patients taking adalimumab and concomitant methotrexate (Ann. Rheum. Dis. 2014 [doi:10.1136/annrheumdis-2014-205554]).
"Further studies regarding measuring drug concentrations would be relevant, since this could give more insight on the cause of inadequate response, especially since treatment options in PsA [psoriatic arthritis] are limited," the investigators wrote.
The study was partly supported by AbbVie and Pfizer. The authors declared a range of consultancies, lecture fees, and research grants from the pharmaceutical industry.
Psoriatic arthritis patients with detectable anti-adalimumab antibodies have significantly lower serum adalimumab concentrations and poorer clinical outcomes at 28 weeks and 52 weeks of treatment, than did patients without antibodies in a prospective cohort study of 103 patients.
At week 52, the 23 patients with detectable anti-adalimumab antibodies had a median adalimumab concentration of 0.9 mg/L, compared with 9.4 mg/L in the 80 patients without detectable antibodies, as well as significantly higher C-reactive protein, Psoriasis Area Severity Index score, and 28-joint Disease Activity Score.
The researchers, led by Erik H. Vogelzang of the Jan van Breemen Research Institute/Reade in Amsterdam, also found that patients on adalimumab monotherapy had significantly lower median adalimumab concentrations at 28 and 52 weeks, compared with patients taking adalimumab and concomitant methotrexate (Ann. Rheum. Dis. 2014 [doi:10.1136/annrheumdis-2014-205554]).
"Further studies regarding measuring drug concentrations would be relevant, since this could give more insight on the cause of inadequate response, especially since treatment options in PsA [psoriatic arthritis] are limited," the investigators wrote.
The study was partly supported by AbbVie and Pfizer. The authors declared a range of consultancies, lecture fees, and research grants from the pharmaceutical industry.
FROM ANNALS OF RHEUMATIC DISEASES
Key clinical point: The presence of anti-adalimumab antibodies results in lower adalimumab concentrations and poorer clinical outcome.
Major finding: Patients with detectable anti-adalimumab antibodies had a median adalimumab concentration of 0.9 mg/L, compared with 9.4 mg/L in patients without detectable antibodies.
Data source: Prospective cohort study in 103 patients with psoriatic arthritis.
Disclosures: The study was partly supported by AbbVie and Pfizer. The authors declared a range of consultancies, lecture fees, and research grants from the pharmaceutical industry.
Think methotrexate for juvenile localized scleroderma
COEUR D’ALENE, IDAHO – Long-term use of methotrexate has a lot going for it as first-line therapy for active juvenile localized scleroderma, according to Dr. Francesco Zulian, chief of pediatric rheumatology at the University of Padua (Italy).
"It’s a drug that’s very old, it’s not expensive, it’s used in many dermatologic and rheumatologic conditions – and it is very useful in patients with scleroderma," he observed in his Sidney Hurwitz Memorial Lecture at the annual meeting of the Society for Pediatric Dermatology.
The initial studies of methotrexate in scleroderma were conducted in adults. Dr. Zulian and colleagues are credited with performing the first randomized, double-blind, prospective clinical trial in pediatric patients, building upon other investigators’ favorable earlier nonrandomized results.
Based upon the randomized trial findings and the subsequent long-term follow-up study, his recommendation for patients with active juvenile localized scleroderma – whether of the linear, pansclerotic, or generalized morphea subtype – is 3 months of initial bridging therapy with a combination of methotrexate plus systemic corticosteroids, followed by at least 24 months of methotrexate without systemic steroids.
In the long-term follow-up study involving 65 patients, treatment was associated with a 74% clinical remission rate. This broke down as approximately a 54% complete remission rate maintained for at least 6 months without treatment, and a 20% clinical remission rate on treatment. Treatment for less than 24 months yielded lesser long-term benefit. Adverse effects were seen in nearly half of patients; however, they were typically mild, and no patients discontinued treatment as a result (J. Am. Acad. Dermatol. 2012;67:1151-6).
"This study shows there is a large group of patients who get better with a relatively mild treatment," Dr. Zulian noted.
The bridging therapy regimen employed in the landmark double-blind, placebo-controlled trial involved oral methotrexate at 15 mg/m2 or a maximum of 20 mg per week, along with prednisone at 1 mg/kg/day or a maximum of 50 mg daily for 3 months (Arthritis Rheum. 2011;63:1998-2006).
In his own clinical practice, Dr. Zulian said, he turns to mycophenolate mofetil (CellCept) in the minority of cases in which bridging therapy with methotrexate and prednisone proves inadequate.
In patients with circumscribed morphea as defined in the international Padua consensus conference guidelines, subsequently formalized by the American College of Rheumatology, the European League Against Rheumatism, and the Pediatric Rheumatism European Society (Arthritis Rheum. 2007;203-12), his recommended treatment is topical corticosteroids, calcipotriol, or phototherapy.
While scleroderma is a rare condition in children, it is nonetheless the third most frequent condition within pediatric rheumatology. For physicians with affected patients who are interested in collaborative research to advance the treatment and understanding of this disease, Dr. Zulian recommended contacting the Juvenile Scleroderma International Network (www.jusinet.org).
Dr. Zulian reported having no financial conflicts with regard to his presentation.
COEUR D’ALENE, IDAHO – Long-term use of methotrexate has a lot going for it as first-line therapy for active juvenile localized scleroderma, according to Dr. Francesco Zulian, chief of pediatric rheumatology at the University of Padua (Italy).
"It’s a drug that’s very old, it’s not expensive, it’s used in many dermatologic and rheumatologic conditions – and it is very useful in patients with scleroderma," he observed in his Sidney Hurwitz Memorial Lecture at the annual meeting of the Society for Pediatric Dermatology.
The initial studies of methotrexate in scleroderma were conducted in adults. Dr. Zulian and colleagues are credited with performing the first randomized, double-blind, prospective clinical trial in pediatric patients, building upon other investigators’ favorable earlier nonrandomized results.
Based upon the randomized trial findings and the subsequent long-term follow-up study, his recommendation for patients with active juvenile localized scleroderma – whether of the linear, pansclerotic, or generalized morphea subtype – is 3 months of initial bridging therapy with a combination of methotrexate plus systemic corticosteroids, followed by at least 24 months of methotrexate without systemic steroids.
In the long-term follow-up study involving 65 patients, treatment was associated with a 74% clinical remission rate. This broke down as approximately a 54% complete remission rate maintained for at least 6 months without treatment, and a 20% clinical remission rate on treatment. Treatment for less than 24 months yielded lesser long-term benefit. Adverse effects were seen in nearly half of patients; however, they were typically mild, and no patients discontinued treatment as a result (J. Am. Acad. Dermatol. 2012;67:1151-6).
"This study shows there is a large group of patients who get better with a relatively mild treatment," Dr. Zulian noted.
The bridging therapy regimen employed in the landmark double-blind, placebo-controlled trial involved oral methotrexate at 15 mg/m2 or a maximum of 20 mg per week, along with prednisone at 1 mg/kg/day or a maximum of 50 mg daily for 3 months (Arthritis Rheum. 2011;63:1998-2006).
In his own clinical practice, Dr. Zulian said, he turns to mycophenolate mofetil (CellCept) in the minority of cases in which bridging therapy with methotrexate and prednisone proves inadequate.
In patients with circumscribed morphea as defined in the international Padua consensus conference guidelines, subsequently formalized by the American College of Rheumatology, the European League Against Rheumatism, and the Pediatric Rheumatism European Society (Arthritis Rheum. 2007;203-12), his recommended treatment is topical corticosteroids, calcipotriol, or phototherapy.
While scleroderma is a rare condition in children, it is nonetheless the third most frequent condition within pediatric rheumatology. For physicians with affected patients who are interested in collaborative research to advance the treatment and understanding of this disease, Dr. Zulian recommended contacting the Juvenile Scleroderma International Network (www.jusinet.org).
Dr. Zulian reported having no financial conflicts with regard to his presentation.
COEUR D’ALENE, IDAHO – Long-term use of methotrexate has a lot going for it as first-line therapy for active juvenile localized scleroderma, according to Dr. Francesco Zulian, chief of pediatric rheumatology at the University of Padua (Italy).
"It’s a drug that’s very old, it’s not expensive, it’s used in many dermatologic and rheumatologic conditions – and it is very useful in patients with scleroderma," he observed in his Sidney Hurwitz Memorial Lecture at the annual meeting of the Society for Pediatric Dermatology.
The initial studies of methotrexate in scleroderma were conducted in adults. Dr. Zulian and colleagues are credited with performing the first randomized, double-blind, prospective clinical trial in pediatric patients, building upon other investigators’ favorable earlier nonrandomized results.
Based upon the randomized trial findings and the subsequent long-term follow-up study, his recommendation for patients with active juvenile localized scleroderma – whether of the linear, pansclerotic, or generalized morphea subtype – is 3 months of initial bridging therapy with a combination of methotrexate plus systemic corticosteroids, followed by at least 24 months of methotrexate without systemic steroids.
In the long-term follow-up study involving 65 patients, treatment was associated with a 74% clinical remission rate. This broke down as approximately a 54% complete remission rate maintained for at least 6 months without treatment, and a 20% clinical remission rate on treatment. Treatment for less than 24 months yielded lesser long-term benefit. Adverse effects were seen in nearly half of patients; however, they were typically mild, and no patients discontinued treatment as a result (J. Am. Acad. Dermatol. 2012;67:1151-6).
"This study shows there is a large group of patients who get better with a relatively mild treatment," Dr. Zulian noted.
The bridging therapy regimen employed in the landmark double-blind, placebo-controlled trial involved oral methotrexate at 15 mg/m2 or a maximum of 20 mg per week, along with prednisone at 1 mg/kg/day or a maximum of 50 mg daily for 3 months (Arthritis Rheum. 2011;63:1998-2006).
In his own clinical practice, Dr. Zulian said, he turns to mycophenolate mofetil (CellCept) in the minority of cases in which bridging therapy with methotrexate and prednisone proves inadequate.
In patients with circumscribed morphea as defined in the international Padua consensus conference guidelines, subsequently formalized by the American College of Rheumatology, the European League Against Rheumatism, and the Pediatric Rheumatism European Society (Arthritis Rheum. 2007;203-12), his recommended treatment is topical corticosteroids, calcipotriol, or phototherapy.
While scleroderma is a rare condition in children, it is nonetheless the third most frequent condition within pediatric rheumatology. For physicians with affected patients who are interested in collaborative research to advance the treatment and understanding of this disease, Dr. Zulian recommended contacting the Juvenile Scleroderma International Network (www.jusinet.org).
Dr. Zulian reported having no financial conflicts with regard to his presentation.
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Patching Psoriasis
Patch testing is one of the major diagnostic tools in the evaluation of allergic contact dermatitis. One limitation of patch testing is the use of steroids prior to testing. Because steroids may suppress a positive test reaction, the use of topical steroids on the test site or oral steroids should be discontinued for at least 2 weeks prior to testing. Therefore, it is interesting to consider the effect of biologics on the reliability of patch testing.
Kim et al (Dermatitis. 2014;25:182-190) evaluated the prevalence of positive patch tests in psoriasis patients receiving biologics and whether these results differed from those of psoriasis patients who were not receiving biologics. An institutional review board–approved retrospective chart review was performed for individuals with psoriasis who were patch tested from January 2002 to 2012 at Tufts Medical Center in Boston, Massachusetts. Patients were selected if they had a history of psoriasis, psoriatic arthritis, and patch testing as identified by International Classification of Diseases, Ninth Revision, codes 696.1, 696.0, and 95044, respectively, in their medical records. Patients were patch tested using a modified North American Contact Dermatitis Group standard and cosmetics series. Readings were performed at 48 hours and 72 to 96 hours. The North American Contact Dermatitis Group grading system was used to grade reactions.
The chart review included 15 psoriasis patients who were on biologics (cases) and 16 psoriasis patients who were not on biologics (control subjects). The biologics used were ustekinumab (n=7), etanercept (n=4), adalimumab (n=3), and infliximab (n=1). The authors determined that 80% (12/15) of cases had at least 1 positive reaction compared with 81% (13/16) of control subjects, 67% (10/15) of cases had 2+ positive reactions compared with 63% (10/16) of control subjects, and 27% (4/15) of cases had 3+ positive reactions compared with 38% (6/16) of control subjects. These differences were not statistically significant.
Given the limitation of the small number of patients evaluated, the authors concluded that biologics do not appear to influence the abilities of patients with psoriasis to mount a positive patch test.
What’s the issue?
This study is small, but the findings do give an indication that the biologic agents utilized for psoriasis do not suppress patch test reactions. These data are not typically what we collect in this population, but it is nice to know. What has been your experience in patch testing patients on biologic therapy?
Patch testing is one of the major diagnostic tools in the evaluation of allergic contact dermatitis. One limitation of patch testing is the use of steroids prior to testing. Because steroids may suppress a positive test reaction, the use of topical steroids on the test site or oral steroids should be discontinued for at least 2 weeks prior to testing. Therefore, it is interesting to consider the effect of biologics on the reliability of patch testing.
Kim et al (Dermatitis. 2014;25:182-190) evaluated the prevalence of positive patch tests in psoriasis patients receiving biologics and whether these results differed from those of psoriasis patients who were not receiving biologics. An institutional review board–approved retrospective chart review was performed for individuals with psoriasis who were patch tested from January 2002 to 2012 at Tufts Medical Center in Boston, Massachusetts. Patients were selected if they had a history of psoriasis, psoriatic arthritis, and patch testing as identified by International Classification of Diseases, Ninth Revision, codes 696.1, 696.0, and 95044, respectively, in their medical records. Patients were patch tested using a modified North American Contact Dermatitis Group standard and cosmetics series. Readings were performed at 48 hours and 72 to 96 hours. The North American Contact Dermatitis Group grading system was used to grade reactions.
The chart review included 15 psoriasis patients who were on biologics (cases) and 16 psoriasis patients who were not on biologics (control subjects). The biologics used were ustekinumab (n=7), etanercept (n=4), adalimumab (n=3), and infliximab (n=1). The authors determined that 80% (12/15) of cases had at least 1 positive reaction compared with 81% (13/16) of control subjects, 67% (10/15) of cases had 2+ positive reactions compared with 63% (10/16) of control subjects, and 27% (4/15) of cases had 3+ positive reactions compared with 38% (6/16) of control subjects. These differences were not statistically significant.
Given the limitation of the small number of patients evaluated, the authors concluded that biologics do not appear to influence the abilities of patients with psoriasis to mount a positive patch test.
What’s the issue?
This study is small, but the findings do give an indication that the biologic agents utilized for psoriasis do not suppress patch test reactions. These data are not typically what we collect in this population, but it is nice to know. What has been your experience in patch testing patients on biologic therapy?
Patch testing is one of the major diagnostic tools in the evaluation of allergic contact dermatitis. One limitation of patch testing is the use of steroids prior to testing. Because steroids may suppress a positive test reaction, the use of topical steroids on the test site or oral steroids should be discontinued for at least 2 weeks prior to testing. Therefore, it is interesting to consider the effect of biologics on the reliability of patch testing.
Kim et al (Dermatitis. 2014;25:182-190) evaluated the prevalence of positive patch tests in psoriasis patients receiving biologics and whether these results differed from those of psoriasis patients who were not receiving biologics. An institutional review board–approved retrospective chart review was performed for individuals with psoriasis who were patch tested from January 2002 to 2012 at Tufts Medical Center in Boston, Massachusetts. Patients were selected if they had a history of psoriasis, psoriatic arthritis, and patch testing as identified by International Classification of Diseases, Ninth Revision, codes 696.1, 696.0, and 95044, respectively, in their medical records. Patients were patch tested using a modified North American Contact Dermatitis Group standard and cosmetics series. Readings were performed at 48 hours and 72 to 96 hours. The North American Contact Dermatitis Group grading system was used to grade reactions.
The chart review included 15 psoriasis patients who were on biologics (cases) and 16 psoriasis patients who were not on biologics (control subjects). The biologics used were ustekinumab (n=7), etanercept (n=4), adalimumab (n=3), and infliximab (n=1). The authors determined that 80% (12/15) of cases had at least 1 positive reaction compared with 81% (13/16) of control subjects, 67% (10/15) of cases had 2+ positive reactions compared with 63% (10/16) of control subjects, and 27% (4/15) of cases had 3+ positive reactions compared with 38% (6/16) of control subjects. These differences were not statistically significant.
Given the limitation of the small number of patients evaluated, the authors concluded that biologics do not appear to influence the abilities of patients with psoriasis to mount a positive patch test.
What’s the issue?
This study is small, but the findings do give an indication that the biologic agents utilized for psoriasis do not suppress patch test reactions. These data are not typically what we collect in this population, but it is nice to know. What has been your experience in patch testing patients on biologic therapy?
Infliximab may carry a higher infection rate in psoriasis patients
CHICAGO – Patients with psoriasis treated with infliximab had the highest incidence of serious infections in a retrospective analysis of commercial claims data.
After 5 years of follow-up among 22,753 patients, there were 554.7 hospitalized infectious events (HIEs) per 10,000 patient-years of exposure to infliximab (Remicade).
Among the other treatment groups, the incidence of HIEs varied within a comparatively narrow range of 261.2 for phototherapy to 341.4 per 10,000 patient-years of exposure to nonbiologic therapies, Dr. Alexa B. Kimball reported at the American Academy of Dermatology summer meeting.
The biologics etanercept (Enbrel), ustekinumab (Stelara), and adalimumab (Humira) fell somewhere in between with HIE incidence rates of 261.5, 287.1, and 321.9 per 10,000 patient years, respectively.
"It is reassuring that some of the rates we reported for patients on biologics were similar to that found in the phototherapy-treated population, which is not expected to have a reduced immune response to infection," Dr. Kimball of Massachusetts General Hospital, Boston, said in an interview. "Still, in any given patient, individual risk and benefits are important to assess when starting or continuing therapy."
In one other study evaluating the safety of anti–tumor necrosis factor agents, rates of serious infections and lymphoma were higher among patients with psoriasis and psoriatic arthritis treated with infliximab and adalimumab, compared with etanercept (Immunopharmacol. Immunotoxicol. 2012;34:548-60).
In the current analysis, hospitalizations for an infectious event were also higher in patients on concomitant corticosteroid therapy at baseline, Dr. Kimball reported.
Five-year HIE incidence rates were higher in patients with systemic corticosteroid exposure versus those without, regardless of treatment group: nonbiologics (649.2 vs. 251.6); etanercept (676.9 vs. 198.1); adalimumab (424.5 vs. 260.9); infliximab (990.2 vs. 443.3); ustekinumab (542.4 vs. 201.2); and phototherapy (320.3 vs. 235.3).
"This finding reinforces that combination therapy, especially with systemic steroids, may confer additional risk," Dr. Kimball said. "Patients on infliximab were also more likely to have psoriatic arthritis and may have a different underlying level of disease severity and risk, which we could not ascertain from these data."
The retrospective analysis was based on data obtained from the MarketScan Commercial Claims and Encounters and Medicare Supplemental and COB databases for patients with a diagnosis of psoriasis on or before Dec. 31, 2006, and at least one prescription claim for one of the above-mentioned therapies. Patients could belong to more than one treatment group.
A new HIE was defined as at least one overnight hospitalization with an ICD-9 infection code. Patients hospitalized in the previous 3 months for the same ICD-9 code were not eligible.
In all, there were 5,857 patients with any exposure to nonbiologics, 6,856 to etanercept, 3,314 to adalimumab, 1,044 to infliximab, 526 to ustekinumab, and 5,156 to phototherapy.
Unique exposures to these agents totaled 2,700; 3,433; 719; 301; 0; and 3,482, respectively, according to the poster.
Amgen sponsored the study. Dr. Kimball is a consultant for Amgen and several other pharmaceutical companies.
CHICAGO – Patients with psoriasis treated with infliximab had the highest incidence of serious infections in a retrospective analysis of commercial claims data.
After 5 years of follow-up among 22,753 patients, there were 554.7 hospitalized infectious events (HIEs) per 10,000 patient-years of exposure to infliximab (Remicade).
Among the other treatment groups, the incidence of HIEs varied within a comparatively narrow range of 261.2 for phototherapy to 341.4 per 10,000 patient-years of exposure to nonbiologic therapies, Dr. Alexa B. Kimball reported at the American Academy of Dermatology summer meeting.
The biologics etanercept (Enbrel), ustekinumab (Stelara), and adalimumab (Humira) fell somewhere in between with HIE incidence rates of 261.5, 287.1, and 321.9 per 10,000 patient years, respectively.
"It is reassuring that some of the rates we reported for patients on biologics were similar to that found in the phototherapy-treated population, which is not expected to have a reduced immune response to infection," Dr. Kimball of Massachusetts General Hospital, Boston, said in an interview. "Still, in any given patient, individual risk and benefits are important to assess when starting or continuing therapy."
In one other study evaluating the safety of anti–tumor necrosis factor agents, rates of serious infections and lymphoma were higher among patients with psoriasis and psoriatic arthritis treated with infliximab and adalimumab, compared with etanercept (Immunopharmacol. Immunotoxicol. 2012;34:548-60).
In the current analysis, hospitalizations for an infectious event were also higher in patients on concomitant corticosteroid therapy at baseline, Dr. Kimball reported.
Five-year HIE incidence rates were higher in patients with systemic corticosteroid exposure versus those without, regardless of treatment group: nonbiologics (649.2 vs. 251.6); etanercept (676.9 vs. 198.1); adalimumab (424.5 vs. 260.9); infliximab (990.2 vs. 443.3); ustekinumab (542.4 vs. 201.2); and phototherapy (320.3 vs. 235.3).
"This finding reinforces that combination therapy, especially with systemic steroids, may confer additional risk," Dr. Kimball said. "Patients on infliximab were also more likely to have psoriatic arthritis and may have a different underlying level of disease severity and risk, which we could not ascertain from these data."
The retrospective analysis was based on data obtained from the MarketScan Commercial Claims and Encounters and Medicare Supplemental and COB databases for patients with a diagnosis of psoriasis on or before Dec. 31, 2006, and at least one prescription claim for one of the above-mentioned therapies. Patients could belong to more than one treatment group.
A new HIE was defined as at least one overnight hospitalization with an ICD-9 infection code. Patients hospitalized in the previous 3 months for the same ICD-9 code were not eligible.
In all, there were 5,857 patients with any exposure to nonbiologics, 6,856 to etanercept, 3,314 to adalimumab, 1,044 to infliximab, 526 to ustekinumab, and 5,156 to phototherapy.
Unique exposures to these agents totaled 2,700; 3,433; 719; 301; 0; and 3,482, respectively, according to the poster.
Amgen sponsored the study. Dr. Kimball is a consultant for Amgen and several other pharmaceutical companies.
CHICAGO – Patients with psoriasis treated with infliximab had the highest incidence of serious infections in a retrospective analysis of commercial claims data.
After 5 years of follow-up among 22,753 patients, there were 554.7 hospitalized infectious events (HIEs) per 10,000 patient-years of exposure to infliximab (Remicade).
Among the other treatment groups, the incidence of HIEs varied within a comparatively narrow range of 261.2 for phototherapy to 341.4 per 10,000 patient-years of exposure to nonbiologic therapies, Dr. Alexa B. Kimball reported at the American Academy of Dermatology summer meeting.
The biologics etanercept (Enbrel), ustekinumab (Stelara), and adalimumab (Humira) fell somewhere in between with HIE incidence rates of 261.5, 287.1, and 321.9 per 10,000 patient years, respectively.
"It is reassuring that some of the rates we reported for patients on biologics were similar to that found in the phototherapy-treated population, which is not expected to have a reduced immune response to infection," Dr. Kimball of Massachusetts General Hospital, Boston, said in an interview. "Still, in any given patient, individual risk and benefits are important to assess when starting or continuing therapy."
In one other study evaluating the safety of anti–tumor necrosis factor agents, rates of serious infections and lymphoma were higher among patients with psoriasis and psoriatic arthritis treated with infliximab and adalimumab, compared with etanercept (Immunopharmacol. Immunotoxicol. 2012;34:548-60).
In the current analysis, hospitalizations for an infectious event were also higher in patients on concomitant corticosteroid therapy at baseline, Dr. Kimball reported.
Five-year HIE incidence rates were higher in patients with systemic corticosteroid exposure versus those without, regardless of treatment group: nonbiologics (649.2 vs. 251.6); etanercept (676.9 vs. 198.1); adalimumab (424.5 vs. 260.9); infliximab (990.2 vs. 443.3); ustekinumab (542.4 vs. 201.2); and phototherapy (320.3 vs. 235.3).
"This finding reinforces that combination therapy, especially with systemic steroids, may confer additional risk," Dr. Kimball said. "Patients on infliximab were also more likely to have psoriatic arthritis and may have a different underlying level of disease severity and risk, which we could not ascertain from these data."
The retrospective analysis was based on data obtained from the MarketScan Commercial Claims and Encounters and Medicare Supplemental and COB databases for patients with a diagnosis of psoriasis on or before Dec. 31, 2006, and at least one prescription claim for one of the above-mentioned therapies. Patients could belong to more than one treatment group.
A new HIE was defined as at least one overnight hospitalization with an ICD-9 infection code. Patients hospitalized in the previous 3 months for the same ICD-9 code were not eligible.
In all, there were 5,857 patients with any exposure to nonbiologics, 6,856 to etanercept, 3,314 to adalimumab, 1,044 to infliximab, 526 to ustekinumab, and 5,156 to phototherapy.
Unique exposures to these agents totaled 2,700; 3,433; 719; 301; 0; and 3,482, respectively, according to the poster.
Amgen sponsored the study. Dr. Kimball is a consultant for Amgen and several other pharmaceutical companies.
AT THE AAD SUMMER ACADEMY 2014
Key clinical point: The risk of being hospitalized for an infectious event was higher with exposure to infliximab.
Major finding: The incidence of hospitalized infectious events was 554.7 per 10,000 patient-years of exposure to infliximab, compared with 261.2-341.4 events per 10,000 patient-years of exposure to other therapies.
Data source: Retrospective database analysis of 22,753 patients with psoriasis.
Disclosures: Amgen sponsored the study. Dr. Kimball is a consultant for Amgen and several other pharmaceutical companies.
VIDEO: Stress and inflammatory skin diseases – Does the science prove a link?
CHICAGO – The data are still being developed, but evidence of a direct link between stress and inflammatory skin conditions continues to mount.
The research is especially compelling for psoriasis; experimental data suggest that stress triggers the nerves to release elevated levels of neuropeptides and neurotransmitters, which in turn affect the nervous system.
Dr. Richard Granstein, chairman of the dermatology department at Weill Cornell Medical College in New York, gave an exclusive interview to Frontline Medical News in which he described studies of how calming the nerves clearly interrupts psoriatic and other inflammatory skin conditions. Dr. Granstein discussed the implications of this new, and still controversial, line of research, and what this means for clinicians: Should they prescribe stress management programs to their patients with these skin conditions?
Dr. Granstein disclosed he has financial relationships with Velius and Clinique.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
CHICAGO – The data are still being developed, but evidence of a direct link between stress and inflammatory skin conditions continues to mount.
The research is especially compelling for psoriasis; experimental data suggest that stress triggers the nerves to release elevated levels of neuropeptides and neurotransmitters, which in turn affect the nervous system.
Dr. Richard Granstein, chairman of the dermatology department at Weill Cornell Medical College in New York, gave an exclusive interview to Frontline Medical News in which he described studies of how calming the nerves clearly interrupts psoriatic and other inflammatory skin conditions. Dr. Granstein discussed the implications of this new, and still controversial, line of research, and what this means for clinicians: Should they prescribe stress management programs to their patients with these skin conditions?
Dr. Granstein disclosed he has financial relationships with Velius and Clinique.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
CHICAGO – The data are still being developed, but evidence of a direct link between stress and inflammatory skin conditions continues to mount.
The research is especially compelling for psoriasis; experimental data suggest that stress triggers the nerves to release elevated levels of neuropeptides and neurotransmitters, which in turn affect the nervous system.
Dr. Richard Granstein, chairman of the dermatology department at Weill Cornell Medical College in New York, gave an exclusive interview to Frontline Medical News in which he described studies of how calming the nerves clearly interrupts psoriatic and other inflammatory skin conditions. Dr. Granstein discussed the implications of this new, and still controversial, line of research, and what this means for clinicians: Should they prescribe stress management programs to their patients with these skin conditions?
Dr. Granstein disclosed he has financial relationships with Velius and Clinique.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2014