User login
Systemic Agents on the Horizon for Psoriasis
For more information, read Dr. Han's Resident Corner column from March 2014, "In the Pipeline for Psoriasis: Upcoming Psoriasis Treatments."
For more information, read Dr. Han's Resident Corner column from March 2014, "In the Pipeline for Psoriasis: Upcoming Psoriasis Treatments."
For more information, read Dr. Han's Resident Corner column from March 2014, "In the Pipeline for Psoriasis: Upcoming Psoriasis Treatments."
Is There a Potential Benefit of Expressive Writing for Dermatology Patients?

A diagnosis of a chronic and/or serious medical condition can be a traumatic experience. Patients may experience not only intrusive thoughts but also avoidance behaviors. The affected individuals can express these trauma-associated symptoms as depression, fatigue, and sleep disturbance.
Milbury et al (J Clin Oncol. 2014;32:663-670) included 277 patients who had been diagnosed with renal cell carcinoma in a study to evaluate if writing about their deepest thoughts and feelings regarding their cancer (expressive writing) offered a quality-of-life benefit compared to writing about neutral topics (neutral writing). They found that expressive writing not only reduced the patients’ cancer-related symptoms but also improved their physical functioning.
What’s the issue?
Many of the conditions that affect dermatology patients are chronic, serious, or both. For example, psoriasis is a chronic skin condition with the potential for serious associated rheumatologic or metabolic sequelae. In addition, skin malignancies (eg, basal cell carcinoma, squamous cell carcinoma, malignant melanoma) and cutaneous lymphoma (eg, mycosis fungoides) are conditions that require chronic surveillance and possibly may have serious consequences.
Expressive writing for dermatology patients might result in remarkable improvement in emotional and physical health. Perhaps dermatologists should recommend this intervention for their patients.

A diagnosis of a chronic and/or serious medical condition can be a traumatic experience. Patients may experience not only intrusive thoughts but also avoidance behaviors. The affected individuals can express these trauma-associated symptoms as depression, fatigue, and sleep disturbance.
Milbury et al (J Clin Oncol. 2014;32:663-670) included 277 patients who had been diagnosed with renal cell carcinoma in a study to evaluate if writing about their deepest thoughts and feelings regarding their cancer (expressive writing) offered a quality-of-life benefit compared to writing about neutral topics (neutral writing). They found that expressive writing not only reduced the patients’ cancer-related symptoms but also improved their physical functioning.
What’s the issue?
Many of the conditions that affect dermatology patients are chronic, serious, or both. For example, psoriasis is a chronic skin condition with the potential for serious associated rheumatologic or metabolic sequelae. In addition, skin malignancies (eg, basal cell carcinoma, squamous cell carcinoma, malignant melanoma) and cutaneous lymphoma (eg, mycosis fungoides) are conditions that require chronic surveillance and possibly may have serious consequences.
Expressive writing for dermatology patients might result in remarkable improvement in emotional and physical health. Perhaps dermatologists should recommend this intervention for their patients.

A diagnosis of a chronic and/or serious medical condition can be a traumatic experience. Patients may experience not only intrusive thoughts but also avoidance behaviors. The affected individuals can express these trauma-associated symptoms as depression, fatigue, and sleep disturbance.
Milbury et al (J Clin Oncol. 2014;32:663-670) included 277 patients who had been diagnosed with renal cell carcinoma in a study to evaluate if writing about their deepest thoughts and feelings regarding their cancer (expressive writing) offered a quality-of-life benefit compared to writing about neutral topics (neutral writing). They found that expressive writing not only reduced the patients’ cancer-related symptoms but also improved their physical functioning.
What’s the issue?
Many of the conditions that affect dermatology patients are chronic, serious, or both. For example, psoriasis is a chronic skin condition with the potential for serious associated rheumatologic or metabolic sequelae. In addition, skin malignancies (eg, basal cell carcinoma, squamous cell carcinoma, malignant melanoma) and cutaneous lymphoma (eg, mycosis fungoides) are conditions that require chronic surveillance and possibly may have serious consequences.
Expressive writing for dermatology patients might result in remarkable improvement in emotional and physical health. Perhaps dermatologists should recommend this intervention for their patients.
Biologics in Dermatology Beyond Psoriasis
New treatments for psoriatic arthritis are changing management
DESTIN, FLA. – Revolutionary advances in the treatment of psoriatic arthritis in recent years – particularly the advent of highly effective biologic therapies like tumor necrosis factor inhibitors – are opening other doors in the management of the disease, as well, according to Dr. Arthur Kavanaugh.
"One of the tangible benefits that came from the increased research in psoriatic arthritis is that it got all of us thinking not just about new therapies, but also about the disease itself," Dr. Kavanaugh, professor of medicine at the University of California, San Diego, said at the annual Congress of Clinical Rheumatology.
The "tremendous uptick" in interest in psoriatic arthritis – as evidenced by a surge in the annual number of related articles available at PubMed over the past decade or so – has helped drive collaborative efforts to further improve the care of patients with this complex condition. Rheumatologists and dermatologists came together, for example, to form the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), which has developed treatment recommendations in light of the new therapies, he said.
"All the different domains of disease, which all of us really assess and take into account as we see patients in our clinic – we needed to consider these in ways separate [from the disease as a whole], and see what the data were for them," he said, adding that psoriatic arthritis doesn’t lend itself to a very strict algorithm.
"Psoriatic arthritis is still an art; you still have to talk to the patient. Some patients have horrible peripheral arthritis and have never had dactylitis; some people have really bad dactylitis, and that’s really their main complaint. It’s been really exciting to learn more about how to capture all of these elements," he said.
The success with new treatments has opened up more conversation about how to approach the disease. Dr. Kavanaugh reviewed a few of the changes in the approach to psoriatic arthritis that highlight recent advances.
"We’ve come pretty far, and now we’re talking about how to manage people in a more comprehensive way," he said, explaining that:
• More attention is being paid to other areas of disease involvement.
Management is no longer just about skin disease. Attention is being paid both in trials and in the clinic to other disease characteristics, such as nail involvement.
"It’s not life threatening, but it is life changing," he said, noting that nail involvement in psoriatic arthritis can be painful, may be associated with distal interphalangeal joint arthritis, and can cause patients to feel self-conscious.
"So this is an important area of involvement, and we’re starting to say that in clinical trials we really ought to capture this, we really ought to look at what effect a new drug has on the skin and nails," said.
Similarly, dactylitis, enthesitis, and structural damage are garnering more attention, he said.
"We’re using adjunctive measures better. We’re using disease-modifying antirheumatic drugs more. We’re seeing patients early. We’re treating patients a little earlier," he said.
• More information is emerging about factors that affect treatment response.
Currently there are five tumor necrosis factor (TNF) inhibitors available, all of which are approved for psoriatic arthritis. What has been unclear is whether switching TNF inhibitors is an effective treatment strategy.
"It looks as if it can work," he said referring to findings from the RAPID-PsA study, which showed that certolizumab pegol (Cimzia) was effective in psoriatic arthritis – including in patients with prior TNF inhibitor therapy (Ann. Rheum. Dis. 2014;73:48-55).
Information from the Scandinavian NOR-DMARD (Ann. Rheum. Dis. 2013;72:1840-4) and DANBIO (Arthritis Rheum. 2013;65:1213-23) registries also provide some guidance on switching TNF inhibitors. The data from the two registries were not so different, but the interpretation was different, Dr. Kavanaugh said, explaining that the former’s findings were interpreted to mean that another mechanism should be tried if an initial TNF inhibitor fails; the latter was interpreted to suggest that trying a second TNF inhibitor is reasonable.
"I think both are correct. ... A second TNF inhibitor certainly can work. Maybe it doesn’t work as well as the first, maybe the third doesn’t work as well as the second, but switching certainly does seem to be a viable idea," he said, adding that nonetheless, "additional mechanisms of action certainly give us something to look forward to."
Another issue is the effect of obesity on treatment outcomes. Multiple studies show that losing weight has beneficial effects not only on psoriatic arthritis, but on response to TNF inhibitors.
"Obesity is an incredibly important factor. ... Study after study shows that," he said, noting that in the CORRONA (Consortium of Rheumatology Researchers of North America Inc.) study of patients starting a TNF inhibitor, the only factor that predicted treatment duration and response was obesity.
This has been shown to be true for both weight-based and fix-dosed treatment.
"So it’s not just that fat people aren’t getting enough of the drug, compared to the skinny people. It’s that obesity – as we’ve learned from our dermatology colleagues – is inflammatory and it’s something to be reckoned with," he said.
Weight loss really needs to be stressed to overweight patients in the clinic, he said.
• Important questions are being asked.
"Unlike for rheumatoid arthritis, we still don’t know if methotrexate and TNF inhibitors are synergistic. ... That’s a gap I would love to see filled in our psoriatic arthritis understanding," he said.
Another important idea that is being addressed involves thinking of how different means of attacking the immune system have disparate results in various immune diseases, thus showing that the diseases may be similar but are not the same.
Dr. Kavanaugh called this a "bedside-to-bench" phenomenon, in which treatment outcomes provide improved understanding of the disease processes.
Targeting interleukin (IL)-6, for example, works very well in RA, juvenile idiopathic arthritis (JIA), and systemic JIA, but it doesn’t work so well in ankylosing spondylitis, and it doesn’t appear that it will work well in several other conditions, based on anecdotal reports.
Similarly, drugs that target IL-17 – a very exciting prospect in psoriasis – don’t seem to work so well in RA. IL-17 inhibition theoretically should work well in inflammatory bowel disease, but it actually appears to make Crohn’s disease worse, he noted.
"We now have newer therapeutic approaches, and it’s very exciting, because we’re going to think of these diseases a little bit differently and almost define the diseases by how they respond to different focal immunomodulatory interventions," he said.
• New treatment targets and approaches are emerging.
There has been some question as to whether IL-23, which is known to be an important driver of IL-17, will be the mechanism by which IL-17 works.
Three IL-17 inhibitors are currently in development. Results in skin psoriasis have been remarkable, and there is a great deal of excitement about them, but data are just beginning to emerge for psoriatic arthritis, Dr. Kavanaugh said.
In an extension study reported at the 2013 annual meeting of the American College of Rheumatology (ACR), for example, the anti-IL-17 receptor A monoclonal antibody brodalumab demonstrated possible increased efficacy through 24 weeks of treatment.
"It’s very exciting to see new data. These drugs will come almost certainly to the clinic first for psoriasis, but I think if they are shown to have good effects in psoriatic arthritis, they will be available to us as another option in our patients," he said.
As for new treatment strategies, psoriatic arthritis is catching up with rheumatoid arthritis with respect to attention to tight control.
"The idea is to evaluate patients, and if they are not reaching a goal, you change treatment. That is the basis for TICOPA – tight control of early psoriatic arthritis," he said.
Joint and skin outcomes were significantly improved in patients in the 48-week, open-label, randomized controlled trial who were treated using a treat-to-target approach, compared with those treated with usual care, according to findings presented at ACR 2013.
However, more serious adverse events occurred in the tight control group (14 vs. 6 in the usual care group), Dr. Kavanaugh noted.
"So I think it’s a thought exercise. I think it shows us that tight control works in psoriatic arthritis. If you follow people very regularly, if you demand that they achieve a good goal like minimal disease activity, they are going to do better," he said.
The downside of increased adverse events raises interesting issues of value and pharmacoeconomics, he noted.
Another "superhot issue in psoriatic arthritis" is whether therapy – and particularly biologic therapy – can be stopped or tapered in patients who are doing very well. A number of studies are looking at this, and it’s an important issue that payers are interested in considering, but data are currently limited.
Based on data that are available, it appears that discontinuing therapy abruptly is not a good idea. In one small study, 77% of patients who discontinued therapy had a disease flare, with greater risk among those with longer treatment duration. Restart of treatment was effective in all cases.
"Certainly there is going to be a lot more interest in this in rheumatoid arthritis, and I think it’s going to spill over and we’ll see more studies in this in psoriatic arthritis as well," he said.
Dr. Kavanaugh reported having no financial disclosures.
DESTIN, FLA. – Revolutionary advances in the treatment of psoriatic arthritis in recent years – particularly the advent of highly effective biologic therapies like tumor necrosis factor inhibitors – are opening other doors in the management of the disease, as well, according to Dr. Arthur Kavanaugh.
"One of the tangible benefits that came from the increased research in psoriatic arthritis is that it got all of us thinking not just about new therapies, but also about the disease itself," Dr. Kavanaugh, professor of medicine at the University of California, San Diego, said at the annual Congress of Clinical Rheumatology.
The "tremendous uptick" in interest in psoriatic arthritis – as evidenced by a surge in the annual number of related articles available at PubMed over the past decade or so – has helped drive collaborative efforts to further improve the care of patients with this complex condition. Rheumatologists and dermatologists came together, for example, to form the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), which has developed treatment recommendations in light of the new therapies, he said.
"All the different domains of disease, which all of us really assess and take into account as we see patients in our clinic – we needed to consider these in ways separate [from the disease as a whole], and see what the data were for them," he said, adding that psoriatic arthritis doesn’t lend itself to a very strict algorithm.
"Psoriatic arthritis is still an art; you still have to talk to the patient. Some patients have horrible peripheral arthritis and have never had dactylitis; some people have really bad dactylitis, and that’s really their main complaint. It’s been really exciting to learn more about how to capture all of these elements," he said.
The success with new treatments has opened up more conversation about how to approach the disease. Dr. Kavanaugh reviewed a few of the changes in the approach to psoriatic arthritis that highlight recent advances.
"We’ve come pretty far, and now we’re talking about how to manage people in a more comprehensive way," he said, explaining that:
• More attention is being paid to other areas of disease involvement.
Management is no longer just about skin disease. Attention is being paid both in trials and in the clinic to other disease characteristics, such as nail involvement.
"It’s not life threatening, but it is life changing," he said, noting that nail involvement in psoriatic arthritis can be painful, may be associated with distal interphalangeal joint arthritis, and can cause patients to feel self-conscious.
"So this is an important area of involvement, and we’re starting to say that in clinical trials we really ought to capture this, we really ought to look at what effect a new drug has on the skin and nails," said.
Similarly, dactylitis, enthesitis, and structural damage are garnering more attention, he said.
"We’re using adjunctive measures better. We’re using disease-modifying antirheumatic drugs more. We’re seeing patients early. We’re treating patients a little earlier," he said.
• More information is emerging about factors that affect treatment response.
Currently there are five tumor necrosis factor (TNF) inhibitors available, all of which are approved for psoriatic arthritis. What has been unclear is whether switching TNF inhibitors is an effective treatment strategy.
"It looks as if it can work," he said referring to findings from the RAPID-PsA study, which showed that certolizumab pegol (Cimzia) was effective in psoriatic arthritis – including in patients with prior TNF inhibitor therapy (Ann. Rheum. Dis. 2014;73:48-55).
Information from the Scandinavian NOR-DMARD (Ann. Rheum. Dis. 2013;72:1840-4) and DANBIO (Arthritis Rheum. 2013;65:1213-23) registries also provide some guidance on switching TNF inhibitors. The data from the two registries were not so different, but the interpretation was different, Dr. Kavanaugh said, explaining that the former’s findings were interpreted to mean that another mechanism should be tried if an initial TNF inhibitor fails; the latter was interpreted to suggest that trying a second TNF inhibitor is reasonable.
"I think both are correct. ... A second TNF inhibitor certainly can work. Maybe it doesn’t work as well as the first, maybe the third doesn’t work as well as the second, but switching certainly does seem to be a viable idea," he said, adding that nonetheless, "additional mechanisms of action certainly give us something to look forward to."
Another issue is the effect of obesity on treatment outcomes. Multiple studies show that losing weight has beneficial effects not only on psoriatic arthritis, but on response to TNF inhibitors.
"Obesity is an incredibly important factor. ... Study after study shows that," he said, noting that in the CORRONA (Consortium of Rheumatology Researchers of North America Inc.) study of patients starting a TNF inhibitor, the only factor that predicted treatment duration and response was obesity.
This has been shown to be true for both weight-based and fix-dosed treatment.
"So it’s not just that fat people aren’t getting enough of the drug, compared to the skinny people. It’s that obesity – as we’ve learned from our dermatology colleagues – is inflammatory and it’s something to be reckoned with," he said.
Weight loss really needs to be stressed to overweight patients in the clinic, he said.
• Important questions are being asked.
"Unlike for rheumatoid arthritis, we still don’t know if methotrexate and TNF inhibitors are synergistic. ... That’s a gap I would love to see filled in our psoriatic arthritis understanding," he said.
Another important idea that is being addressed involves thinking of how different means of attacking the immune system have disparate results in various immune diseases, thus showing that the diseases may be similar but are not the same.
Dr. Kavanaugh called this a "bedside-to-bench" phenomenon, in which treatment outcomes provide improved understanding of the disease processes.
Targeting interleukin (IL)-6, for example, works very well in RA, juvenile idiopathic arthritis (JIA), and systemic JIA, but it doesn’t work so well in ankylosing spondylitis, and it doesn’t appear that it will work well in several other conditions, based on anecdotal reports.
Similarly, drugs that target IL-17 – a very exciting prospect in psoriasis – don’t seem to work so well in RA. IL-17 inhibition theoretically should work well in inflammatory bowel disease, but it actually appears to make Crohn’s disease worse, he noted.
"We now have newer therapeutic approaches, and it’s very exciting, because we’re going to think of these diseases a little bit differently and almost define the diseases by how they respond to different focal immunomodulatory interventions," he said.
• New treatment targets and approaches are emerging.
There has been some question as to whether IL-23, which is known to be an important driver of IL-17, will be the mechanism by which IL-17 works.
Three IL-17 inhibitors are currently in development. Results in skin psoriasis have been remarkable, and there is a great deal of excitement about them, but data are just beginning to emerge for psoriatic arthritis, Dr. Kavanaugh said.
In an extension study reported at the 2013 annual meeting of the American College of Rheumatology (ACR), for example, the anti-IL-17 receptor A monoclonal antibody brodalumab demonstrated possible increased efficacy through 24 weeks of treatment.
"It’s very exciting to see new data. These drugs will come almost certainly to the clinic first for psoriasis, but I think if they are shown to have good effects in psoriatic arthritis, they will be available to us as another option in our patients," he said.
As for new treatment strategies, psoriatic arthritis is catching up with rheumatoid arthritis with respect to attention to tight control.
"The idea is to evaluate patients, and if they are not reaching a goal, you change treatment. That is the basis for TICOPA – tight control of early psoriatic arthritis," he said.
Joint and skin outcomes were significantly improved in patients in the 48-week, open-label, randomized controlled trial who were treated using a treat-to-target approach, compared with those treated with usual care, according to findings presented at ACR 2013.
However, more serious adverse events occurred in the tight control group (14 vs. 6 in the usual care group), Dr. Kavanaugh noted.
"So I think it’s a thought exercise. I think it shows us that tight control works in psoriatic arthritis. If you follow people very regularly, if you demand that they achieve a good goal like minimal disease activity, they are going to do better," he said.
The downside of increased adverse events raises interesting issues of value and pharmacoeconomics, he noted.
Another "superhot issue in psoriatic arthritis" is whether therapy – and particularly biologic therapy – can be stopped or tapered in patients who are doing very well. A number of studies are looking at this, and it’s an important issue that payers are interested in considering, but data are currently limited.
Based on data that are available, it appears that discontinuing therapy abruptly is not a good idea. In one small study, 77% of patients who discontinued therapy had a disease flare, with greater risk among those with longer treatment duration. Restart of treatment was effective in all cases.
"Certainly there is going to be a lot more interest in this in rheumatoid arthritis, and I think it’s going to spill over and we’ll see more studies in this in psoriatic arthritis as well," he said.
Dr. Kavanaugh reported having no financial disclosures.
DESTIN, FLA. – Revolutionary advances in the treatment of psoriatic arthritis in recent years – particularly the advent of highly effective biologic therapies like tumor necrosis factor inhibitors – are opening other doors in the management of the disease, as well, according to Dr. Arthur Kavanaugh.
"One of the tangible benefits that came from the increased research in psoriatic arthritis is that it got all of us thinking not just about new therapies, but also about the disease itself," Dr. Kavanaugh, professor of medicine at the University of California, San Diego, said at the annual Congress of Clinical Rheumatology.
The "tremendous uptick" in interest in psoriatic arthritis – as evidenced by a surge in the annual number of related articles available at PubMed over the past decade or so – has helped drive collaborative efforts to further improve the care of patients with this complex condition. Rheumatologists and dermatologists came together, for example, to form the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), which has developed treatment recommendations in light of the new therapies, he said.
"All the different domains of disease, which all of us really assess and take into account as we see patients in our clinic – we needed to consider these in ways separate [from the disease as a whole], and see what the data were for them," he said, adding that psoriatic arthritis doesn’t lend itself to a very strict algorithm.
"Psoriatic arthritis is still an art; you still have to talk to the patient. Some patients have horrible peripheral arthritis and have never had dactylitis; some people have really bad dactylitis, and that’s really their main complaint. It’s been really exciting to learn more about how to capture all of these elements," he said.
The success with new treatments has opened up more conversation about how to approach the disease. Dr. Kavanaugh reviewed a few of the changes in the approach to psoriatic arthritis that highlight recent advances.
"We’ve come pretty far, and now we’re talking about how to manage people in a more comprehensive way," he said, explaining that:
• More attention is being paid to other areas of disease involvement.
Management is no longer just about skin disease. Attention is being paid both in trials and in the clinic to other disease characteristics, such as nail involvement.
"It’s not life threatening, but it is life changing," he said, noting that nail involvement in psoriatic arthritis can be painful, may be associated with distal interphalangeal joint arthritis, and can cause patients to feel self-conscious.
"So this is an important area of involvement, and we’re starting to say that in clinical trials we really ought to capture this, we really ought to look at what effect a new drug has on the skin and nails," said.
Similarly, dactylitis, enthesitis, and structural damage are garnering more attention, he said.
"We’re using adjunctive measures better. We’re using disease-modifying antirheumatic drugs more. We’re seeing patients early. We’re treating patients a little earlier," he said.
• More information is emerging about factors that affect treatment response.
Currently there are five tumor necrosis factor (TNF) inhibitors available, all of which are approved for psoriatic arthritis. What has been unclear is whether switching TNF inhibitors is an effective treatment strategy.
"It looks as if it can work," he said referring to findings from the RAPID-PsA study, which showed that certolizumab pegol (Cimzia) was effective in psoriatic arthritis – including in patients with prior TNF inhibitor therapy (Ann. Rheum. Dis. 2014;73:48-55).
Information from the Scandinavian NOR-DMARD (Ann. Rheum. Dis. 2013;72:1840-4) and DANBIO (Arthritis Rheum. 2013;65:1213-23) registries also provide some guidance on switching TNF inhibitors. The data from the two registries were not so different, but the interpretation was different, Dr. Kavanaugh said, explaining that the former’s findings were interpreted to mean that another mechanism should be tried if an initial TNF inhibitor fails; the latter was interpreted to suggest that trying a second TNF inhibitor is reasonable.
"I think both are correct. ... A second TNF inhibitor certainly can work. Maybe it doesn’t work as well as the first, maybe the third doesn’t work as well as the second, but switching certainly does seem to be a viable idea," he said, adding that nonetheless, "additional mechanisms of action certainly give us something to look forward to."
Another issue is the effect of obesity on treatment outcomes. Multiple studies show that losing weight has beneficial effects not only on psoriatic arthritis, but on response to TNF inhibitors.
"Obesity is an incredibly important factor. ... Study after study shows that," he said, noting that in the CORRONA (Consortium of Rheumatology Researchers of North America Inc.) study of patients starting a TNF inhibitor, the only factor that predicted treatment duration and response was obesity.
This has been shown to be true for both weight-based and fix-dosed treatment.
"So it’s not just that fat people aren’t getting enough of the drug, compared to the skinny people. It’s that obesity – as we’ve learned from our dermatology colleagues – is inflammatory and it’s something to be reckoned with," he said.
Weight loss really needs to be stressed to overweight patients in the clinic, he said.
• Important questions are being asked.
"Unlike for rheumatoid arthritis, we still don’t know if methotrexate and TNF inhibitors are synergistic. ... That’s a gap I would love to see filled in our psoriatic arthritis understanding," he said.
Another important idea that is being addressed involves thinking of how different means of attacking the immune system have disparate results in various immune diseases, thus showing that the diseases may be similar but are not the same.
Dr. Kavanaugh called this a "bedside-to-bench" phenomenon, in which treatment outcomes provide improved understanding of the disease processes.
Targeting interleukin (IL)-6, for example, works very well in RA, juvenile idiopathic arthritis (JIA), and systemic JIA, but it doesn’t work so well in ankylosing spondylitis, and it doesn’t appear that it will work well in several other conditions, based on anecdotal reports.
Similarly, drugs that target IL-17 – a very exciting prospect in psoriasis – don’t seem to work so well in RA. IL-17 inhibition theoretically should work well in inflammatory bowel disease, but it actually appears to make Crohn’s disease worse, he noted.
"We now have newer therapeutic approaches, and it’s very exciting, because we’re going to think of these diseases a little bit differently and almost define the diseases by how they respond to different focal immunomodulatory interventions," he said.
• New treatment targets and approaches are emerging.
There has been some question as to whether IL-23, which is known to be an important driver of IL-17, will be the mechanism by which IL-17 works.
Three IL-17 inhibitors are currently in development. Results in skin psoriasis have been remarkable, and there is a great deal of excitement about them, but data are just beginning to emerge for psoriatic arthritis, Dr. Kavanaugh said.
In an extension study reported at the 2013 annual meeting of the American College of Rheumatology (ACR), for example, the anti-IL-17 receptor A monoclonal antibody brodalumab demonstrated possible increased efficacy through 24 weeks of treatment.
"It’s very exciting to see new data. These drugs will come almost certainly to the clinic first for psoriasis, but I think if they are shown to have good effects in psoriatic arthritis, they will be available to us as another option in our patients," he said.
As for new treatment strategies, psoriatic arthritis is catching up with rheumatoid arthritis with respect to attention to tight control.
"The idea is to evaluate patients, and if they are not reaching a goal, you change treatment. That is the basis for TICOPA – tight control of early psoriatic arthritis," he said.
Joint and skin outcomes were significantly improved in patients in the 48-week, open-label, randomized controlled trial who were treated using a treat-to-target approach, compared with those treated with usual care, according to findings presented at ACR 2013.
However, more serious adverse events occurred in the tight control group (14 vs. 6 in the usual care group), Dr. Kavanaugh noted.
"So I think it’s a thought exercise. I think it shows us that tight control works in psoriatic arthritis. If you follow people very regularly, if you demand that they achieve a good goal like minimal disease activity, they are going to do better," he said.
The downside of increased adverse events raises interesting issues of value and pharmacoeconomics, he noted.
Another "superhot issue in psoriatic arthritis" is whether therapy – and particularly biologic therapy – can be stopped or tapered in patients who are doing very well. A number of studies are looking at this, and it’s an important issue that payers are interested in considering, but data are currently limited.
Based on data that are available, it appears that discontinuing therapy abruptly is not a good idea. In one small study, 77% of patients who discontinued therapy had a disease flare, with greater risk among those with longer treatment duration. Restart of treatment was effective in all cases.
"Certainly there is going to be a lot more interest in this in rheumatoid arthritis, and I think it’s going to spill over and we’ll see more studies in this in psoriatic arthritis as well," he said.
Dr. Kavanaugh reported having no financial disclosures.
EXPERT ANALYSIS FROM CCR 14
Unmet Needs in Psoriasis
The concept of unmet needs in psoriasis is not new. We are aware that both patients and physicians feel there are gaps in the management and treatment of the condition. Lebwohl et al (J Am Acad Dermatol. 2014;70:871-881) performed an extensive survey to further the understanding of the unmet needs of psoriasis and psoriatic arthritis (PsA) patients. The survey was a large, multinational, population-based analysis of psoriasis and/or PsA patients in North America and Europe. In terms of methodology, surveyed individuals were selected by list-assisted random digit dialing and did not have to currently be under the care of a health care provider, a patient organization member, or receiving treatment. Overall, the survey screened 139,948 households, and 3426 patients completed the survey.
The population prevalence of psoriasis and/or PsA ranged from 1.4% in Spain to 3.3% in Canada, with an overall prevalence of 1.9%. Seventy-nine percent of patients had psoriasis alone, while 21% experienced PsA with or without psoriasis.
When rating disease severity at its worst, 27% of those with psoriasis and 53% with psoriasis and/or PsA rated it as severe. Itching (43%), scales (23%), and flaking (20%) were considered the most bothersome signs or symptoms by psoriasis patients, and 45% of them had not seen a physician in 1 year. More than 80% of psoriasis patients with at least 4 palms body surface area and 59% of the PsA cohort were receiving no treatment or only topical treatment. Of patients who had received oral or biologic therapy, 57% and 45%, respectively, discontinued therapy, most often for safety or tolerability reasons and a lack or loss of efficacy.
The authors concluded that the following identified unmet needs warrant additional attention and action: improved severity assessment, PsA screening, patient awareness, and treatment options. They noted that their findings suggest there is a high rate of undertreatment of both psoriasis and PsA and a mismatch between patient and physician assessment of severity.
What’s the issue?
Again we are faced with data suggesting that our patients perceive deficiencies in their psoriasis care. Therefore, it is important that we continue to educate our patients and ourselves so that we can narrow these gaps. How will you react to these findings in your management of psoriasis?
The concept of unmet needs in psoriasis is not new. We are aware that both patients and physicians feel there are gaps in the management and treatment of the condition. Lebwohl et al (J Am Acad Dermatol. 2014;70:871-881) performed an extensive survey to further the understanding of the unmet needs of psoriasis and psoriatic arthritis (PsA) patients. The survey was a large, multinational, population-based analysis of psoriasis and/or PsA patients in North America and Europe. In terms of methodology, surveyed individuals were selected by list-assisted random digit dialing and did not have to currently be under the care of a health care provider, a patient organization member, or receiving treatment. Overall, the survey screened 139,948 households, and 3426 patients completed the survey.
The population prevalence of psoriasis and/or PsA ranged from 1.4% in Spain to 3.3% in Canada, with an overall prevalence of 1.9%. Seventy-nine percent of patients had psoriasis alone, while 21% experienced PsA with or without psoriasis.
When rating disease severity at its worst, 27% of those with psoriasis and 53% with psoriasis and/or PsA rated it as severe. Itching (43%), scales (23%), and flaking (20%) were considered the most bothersome signs or symptoms by psoriasis patients, and 45% of them had not seen a physician in 1 year. More than 80% of psoriasis patients with at least 4 palms body surface area and 59% of the PsA cohort were receiving no treatment or only topical treatment. Of patients who had received oral or biologic therapy, 57% and 45%, respectively, discontinued therapy, most often for safety or tolerability reasons and a lack or loss of efficacy.
The authors concluded that the following identified unmet needs warrant additional attention and action: improved severity assessment, PsA screening, patient awareness, and treatment options. They noted that their findings suggest there is a high rate of undertreatment of both psoriasis and PsA and a mismatch between patient and physician assessment of severity.
What’s the issue?
Again we are faced with data suggesting that our patients perceive deficiencies in their psoriasis care. Therefore, it is important that we continue to educate our patients and ourselves so that we can narrow these gaps. How will you react to these findings in your management of psoriasis?
The concept of unmet needs in psoriasis is not new. We are aware that both patients and physicians feel there are gaps in the management and treatment of the condition. Lebwohl et al (J Am Acad Dermatol. 2014;70:871-881) performed an extensive survey to further the understanding of the unmet needs of psoriasis and psoriatic arthritis (PsA) patients. The survey was a large, multinational, population-based analysis of psoriasis and/or PsA patients in North America and Europe. In terms of methodology, surveyed individuals were selected by list-assisted random digit dialing and did not have to currently be under the care of a health care provider, a patient organization member, or receiving treatment. Overall, the survey screened 139,948 households, and 3426 patients completed the survey.
The population prevalence of psoriasis and/or PsA ranged from 1.4% in Spain to 3.3% in Canada, with an overall prevalence of 1.9%. Seventy-nine percent of patients had psoriasis alone, while 21% experienced PsA with or without psoriasis.
When rating disease severity at its worst, 27% of those with psoriasis and 53% with psoriasis and/or PsA rated it as severe. Itching (43%), scales (23%), and flaking (20%) were considered the most bothersome signs or symptoms by psoriasis patients, and 45% of them had not seen a physician in 1 year. More than 80% of psoriasis patients with at least 4 palms body surface area and 59% of the PsA cohort were receiving no treatment or only topical treatment. Of patients who had received oral or biologic therapy, 57% and 45%, respectively, discontinued therapy, most often for safety or tolerability reasons and a lack or loss of efficacy.
The authors concluded that the following identified unmet needs warrant additional attention and action: improved severity assessment, PsA screening, patient awareness, and treatment options. They noted that their findings suggest there is a high rate of undertreatment of both psoriasis and PsA and a mismatch between patient and physician assessment of severity.
What’s the issue?
Again we are faced with data suggesting that our patients perceive deficiencies in their psoriasis care. Therefore, it is important that we continue to educate our patients and ourselves so that we can narrow these gaps. How will you react to these findings in your management of psoriasis?
Apremilast appears to have multiple, lasting benefits in psoriatic arthritis
LIVERPOOL, ENGLAND – Further evidence that apremilast has multiple and sustained effects in psoriatic arthritis for at least 1 year were reported at the British Society for Rheumatology annual conference.
Data from two of the phase III studies that make up the PALACE (Psoriatic Arthritis Long-term Assessment of Clinical Efficacy) clinical trials program showed that the oral phosphodiesterase 4 inhibitor continued to suppress disease activity and improved pain and physical functioning associated with the skin and joint condition.
In the PALACE 1 (Psoriatic Arthritis Long-term Assessment of Clinical Efficacy 1) trial, 75 of 119 (63%) patients treated with apremilast 20 mg twice daily and 71 of 130 (55%) patients treated with apremilast 30 mg twice daily still had an American College of Rheumatology 20% (ACR20) response at 52 weeks.
Corresponding 52-week data from the PALACE 3 trial showed ACR20 responses in 56% of 116 patients and 62% of 127 patients for the two doses of apremilast, respectively. ACR50 and ACR70 were achieved by 25%-30% of patients, and 9%-10% achieved an ACR70.
Apremilast is marketed by Celgene as Otezla and gained approval from the Food and Drug Administration for the treatment of adults with active psoriatic arthritis in March. The FDA approval came with a caution that patients’ weight should be monitored regularly and that there had been increased reports of depression.
The long-term safety data from these two PALACE trials, however, showed no new safety concerns, according to the studies’ authors. The most common side effects seen were as reported previously and included diarrhea, nausea, and headache.
PALACE 1 and PALACE 3 were designed to assess the efficacy and safety of apremilast versus placebo in patients who had active psoriatic arthritis despite treatment with conventional nonbiologic disease-modifying antirheumatic drugs (nbDMARDs) with or without additional biologic treatment.
For inclusion in the trials, patients had to have disease duration of at least 6 months, meet Classification of Psoriatic Arthritis (CASPAR) criteria, and have three or more tender or swollen joints involved. Patients enrolled in PALACE 3 also had to have skin involvement, with at least plaque psoriasis of 2 cm in size or larger.
A total of 504 patients were enrolled into PALACE 1 (Ann. Rheum. Dis. 2013;72[Suppl. 3]:163) and 505 into PALACE 3 and initially randomized to twice-daily treatment with placebo or one of two doses of apremilast for 24 weeks. At this point, all patients in the placebo arm who had not already been rerandomized to active treatment were randomized to apremilast 20 mg or 30 mg. Patients originally randomized to active therapy continued with their treatment if they continued to respond.
"I think what comes out of these [data], and looking over time, is that the effect is sustained with all the caveats that go with that because, of course, people only stay on [the drug] if they are doing well," PALACE 3 investigator Dr. Christopher Edwards observed in an interview.
Dr. Edwards, who is a clinical rheumatologist at University Hospital Southampton, England, noted that the statistical power of long-term extension studies is perhaps a little less robust than the initial study phases, but that these long-term data from the PALACE trials do seem to show that the effects of treatment with apremilast are sustained and maybe even that the effect size improves slightly over time.
Physical function, assessed via the Health Assessment Questionnaire–Disability Index (HAQ-DI), was improved with apremilast treatment in both the PALACE 1 and PALACE 3 trials. In the latter trial, mean change in HAQ-DI scores at 16 weeks declined by 0.13 and 0.19, compared with baseline values in the apremilast 20-mg and 30-mg groups. In contrast, scores increased by 0.07 in the placebo group. Over the following year, HAQ-DI scores continued to improve, with the mean change in scores from baseline crossing the threshold of –0.30, which many think signifies a clinically meaningful change, Dr. Edwards explained.
HAQ-DI scores in PALACE 1 at 52 weeks were a respective –0.369 and –0.318 in the 20-mg and 30-mg groups, and at the 16-week assessment point, they had been –0.20 and –0.24, respectively, and –0.09 for placebo.
Furthermore, in the PALACE 3 trial, 29%-39% of patients treated with apremilast achieved a 75% improvement in skin involvement, assessed via the Psoriasis Area and Severity Index (PASI) at 1 year. PASI50 was achieved in 49%-54%.
Dr. Edwards observed that some of the additional data now available from PALACE 3 showed that there was a beneficial effect of apremilast on enthesitis and dactylitis – two unique features of psoriatic arthritis that are often not treated by the use of the nbDMARDS, such as methotrexate and sulfasalazine.
At 24 weeks, he noted that in other comparisons of patients treated with apremilast or placebo, active therapy yielded significant improvements in pain (assessed using a visual analog scale), improved Functional Assessment of Chronic Illness Therapy-Fatigue scores, a higher rate of achieving ‘good’ or ‘moderate’ European League Against Rheumatism responses, and greater modified Psoriatic Arthritis Response Criteria results.
Dr. Edwards has acted as a consultant to Celgene, the company that sponsored the studies. He has also received research support from Pfizer and honoraria from Roche, Abbott, and GlaxoSmithKline.
LIVERPOOL, ENGLAND – Further evidence that apremilast has multiple and sustained effects in psoriatic arthritis for at least 1 year were reported at the British Society for Rheumatology annual conference.
Data from two of the phase III studies that make up the PALACE (Psoriatic Arthritis Long-term Assessment of Clinical Efficacy) clinical trials program showed that the oral phosphodiesterase 4 inhibitor continued to suppress disease activity and improved pain and physical functioning associated with the skin and joint condition.
In the PALACE 1 (Psoriatic Arthritis Long-term Assessment of Clinical Efficacy 1) trial, 75 of 119 (63%) patients treated with apremilast 20 mg twice daily and 71 of 130 (55%) patients treated with apremilast 30 mg twice daily still had an American College of Rheumatology 20% (ACR20) response at 52 weeks.
Corresponding 52-week data from the PALACE 3 trial showed ACR20 responses in 56% of 116 patients and 62% of 127 patients for the two doses of apremilast, respectively. ACR50 and ACR70 were achieved by 25%-30% of patients, and 9%-10% achieved an ACR70.
Apremilast is marketed by Celgene as Otezla and gained approval from the Food and Drug Administration for the treatment of adults with active psoriatic arthritis in March. The FDA approval came with a caution that patients’ weight should be monitored regularly and that there had been increased reports of depression.
The long-term safety data from these two PALACE trials, however, showed no new safety concerns, according to the studies’ authors. The most common side effects seen were as reported previously and included diarrhea, nausea, and headache.
PALACE 1 and PALACE 3 were designed to assess the efficacy and safety of apremilast versus placebo in patients who had active psoriatic arthritis despite treatment with conventional nonbiologic disease-modifying antirheumatic drugs (nbDMARDs) with or without additional biologic treatment.
For inclusion in the trials, patients had to have disease duration of at least 6 months, meet Classification of Psoriatic Arthritis (CASPAR) criteria, and have three or more tender or swollen joints involved. Patients enrolled in PALACE 3 also had to have skin involvement, with at least plaque psoriasis of 2 cm in size or larger.
A total of 504 patients were enrolled into PALACE 1 (Ann. Rheum. Dis. 2013;72[Suppl. 3]:163) and 505 into PALACE 3 and initially randomized to twice-daily treatment with placebo or one of two doses of apremilast for 24 weeks. At this point, all patients in the placebo arm who had not already been rerandomized to active treatment were randomized to apremilast 20 mg or 30 mg. Patients originally randomized to active therapy continued with their treatment if they continued to respond.
"I think what comes out of these [data], and looking over time, is that the effect is sustained with all the caveats that go with that because, of course, people only stay on [the drug] if they are doing well," PALACE 3 investigator Dr. Christopher Edwards observed in an interview.
Dr. Edwards, who is a clinical rheumatologist at University Hospital Southampton, England, noted that the statistical power of long-term extension studies is perhaps a little less robust than the initial study phases, but that these long-term data from the PALACE trials do seem to show that the effects of treatment with apremilast are sustained and maybe even that the effect size improves slightly over time.
Physical function, assessed via the Health Assessment Questionnaire–Disability Index (HAQ-DI), was improved with apremilast treatment in both the PALACE 1 and PALACE 3 trials. In the latter trial, mean change in HAQ-DI scores at 16 weeks declined by 0.13 and 0.19, compared with baseline values in the apremilast 20-mg and 30-mg groups. In contrast, scores increased by 0.07 in the placebo group. Over the following year, HAQ-DI scores continued to improve, with the mean change in scores from baseline crossing the threshold of –0.30, which many think signifies a clinically meaningful change, Dr. Edwards explained.
HAQ-DI scores in PALACE 1 at 52 weeks were a respective –0.369 and –0.318 in the 20-mg and 30-mg groups, and at the 16-week assessment point, they had been –0.20 and –0.24, respectively, and –0.09 for placebo.
Furthermore, in the PALACE 3 trial, 29%-39% of patients treated with apremilast achieved a 75% improvement in skin involvement, assessed via the Psoriasis Area and Severity Index (PASI) at 1 year. PASI50 was achieved in 49%-54%.
Dr. Edwards observed that some of the additional data now available from PALACE 3 showed that there was a beneficial effect of apremilast on enthesitis and dactylitis – two unique features of psoriatic arthritis that are often not treated by the use of the nbDMARDS, such as methotrexate and sulfasalazine.
At 24 weeks, he noted that in other comparisons of patients treated with apremilast or placebo, active therapy yielded significant improvements in pain (assessed using a visual analog scale), improved Functional Assessment of Chronic Illness Therapy-Fatigue scores, a higher rate of achieving ‘good’ or ‘moderate’ European League Against Rheumatism responses, and greater modified Psoriatic Arthritis Response Criteria results.
Dr. Edwards has acted as a consultant to Celgene, the company that sponsored the studies. He has also received research support from Pfizer and honoraria from Roche, Abbott, and GlaxoSmithKline.
LIVERPOOL, ENGLAND – Further evidence that apremilast has multiple and sustained effects in psoriatic arthritis for at least 1 year were reported at the British Society for Rheumatology annual conference.
Data from two of the phase III studies that make up the PALACE (Psoriatic Arthritis Long-term Assessment of Clinical Efficacy) clinical trials program showed that the oral phosphodiesterase 4 inhibitor continued to suppress disease activity and improved pain and physical functioning associated with the skin and joint condition.
In the PALACE 1 (Psoriatic Arthritis Long-term Assessment of Clinical Efficacy 1) trial, 75 of 119 (63%) patients treated with apremilast 20 mg twice daily and 71 of 130 (55%) patients treated with apremilast 30 mg twice daily still had an American College of Rheumatology 20% (ACR20) response at 52 weeks.
Corresponding 52-week data from the PALACE 3 trial showed ACR20 responses in 56% of 116 patients and 62% of 127 patients for the two doses of apremilast, respectively. ACR50 and ACR70 were achieved by 25%-30% of patients, and 9%-10% achieved an ACR70.
Apremilast is marketed by Celgene as Otezla and gained approval from the Food and Drug Administration for the treatment of adults with active psoriatic arthritis in March. The FDA approval came with a caution that patients’ weight should be monitored regularly and that there had been increased reports of depression.
The long-term safety data from these two PALACE trials, however, showed no new safety concerns, according to the studies’ authors. The most common side effects seen were as reported previously and included diarrhea, nausea, and headache.
PALACE 1 and PALACE 3 were designed to assess the efficacy and safety of apremilast versus placebo in patients who had active psoriatic arthritis despite treatment with conventional nonbiologic disease-modifying antirheumatic drugs (nbDMARDs) with or without additional biologic treatment.
For inclusion in the trials, patients had to have disease duration of at least 6 months, meet Classification of Psoriatic Arthritis (CASPAR) criteria, and have three or more tender or swollen joints involved. Patients enrolled in PALACE 3 also had to have skin involvement, with at least plaque psoriasis of 2 cm in size or larger.
A total of 504 patients were enrolled into PALACE 1 (Ann. Rheum. Dis. 2013;72[Suppl. 3]:163) and 505 into PALACE 3 and initially randomized to twice-daily treatment with placebo or one of two doses of apremilast for 24 weeks. At this point, all patients in the placebo arm who had not already been rerandomized to active treatment were randomized to apremilast 20 mg or 30 mg. Patients originally randomized to active therapy continued with their treatment if they continued to respond.
"I think what comes out of these [data], and looking over time, is that the effect is sustained with all the caveats that go with that because, of course, people only stay on [the drug] if they are doing well," PALACE 3 investigator Dr. Christopher Edwards observed in an interview.
Dr. Edwards, who is a clinical rheumatologist at University Hospital Southampton, England, noted that the statistical power of long-term extension studies is perhaps a little less robust than the initial study phases, but that these long-term data from the PALACE trials do seem to show that the effects of treatment with apremilast are sustained and maybe even that the effect size improves slightly over time.
Physical function, assessed via the Health Assessment Questionnaire–Disability Index (HAQ-DI), was improved with apremilast treatment in both the PALACE 1 and PALACE 3 trials. In the latter trial, mean change in HAQ-DI scores at 16 weeks declined by 0.13 and 0.19, compared with baseline values in the apremilast 20-mg and 30-mg groups. In contrast, scores increased by 0.07 in the placebo group. Over the following year, HAQ-DI scores continued to improve, with the mean change in scores from baseline crossing the threshold of –0.30, which many think signifies a clinically meaningful change, Dr. Edwards explained.
HAQ-DI scores in PALACE 1 at 52 weeks were a respective –0.369 and –0.318 in the 20-mg and 30-mg groups, and at the 16-week assessment point, they had been –0.20 and –0.24, respectively, and –0.09 for placebo.
Furthermore, in the PALACE 3 trial, 29%-39% of patients treated with apremilast achieved a 75% improvement in skin involvement, assessed via the Psoriasis Area and Severity Index (PASI) at 1 year. PASI50 was achieved in 49%-54%.
Dr. Edwards observed that some of the additional data now available from PALACE 3 showed that there was a beneficial effect of apremilast on enthesitis and dactylitis – two unique features of psoriatic arthritis that are often not treated by the use of the nbDMARDS, such as methotrexate and sulfasalazine.
At 24 weeks, he noted that in other comparisons of patients treated with apremilast or placebo, active therapy yielded significant improvements in pain (assessed using a visual analog scale), improved Functional Assessment of Chronic Illness Therapy-Fatigue scores, a higher rate of achieving ‘good’ or ‘moderate’ European League Against Rheumatism responses, and greater modified Psoriatic Arthritis Response Criteria results.
Dr. Edwards has acted as a consultant to Celgene, the company that sponsored the studies. He has also received research support from Pfizer and honoraria from Roche, Abbott, and GlaxoSmithKline.
AT RHEUMATOLOGY 2014
Key clinical point: Apremilast maintained its efficacy and safety profile through 1 year of treatment.
Major finding: At 52 weeks, 55%-63% of patients treated with apremilast 20 mg or 30 mg had an ACR20, 25%-30% had an ACR50, and 9%-10% an ACR70.
Data source: Two phase III, multicenter, randomized, clinical trials of 1,009 patients with psoriatic arthritis treated with apremilast, 20 mg or 30 mg, or placebo.
Disclosures: Dr. Edwards has acted as a consultant to Celgene, the company that sponsored the studies. He has also received research support from Pfizer and honoraria from Roche, Abbott, and GlaxoSmithKline.
Humira, Enbrel costs highest in the United States
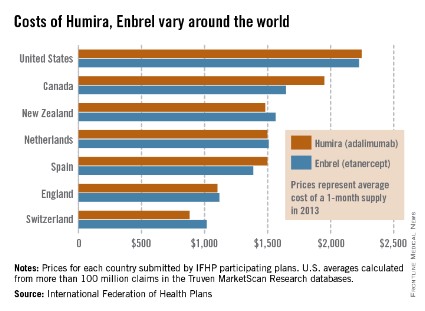
The costs of Humira and Enbrel are higher in the United States, compared with other industrialized countries included in the International Federation of Health Plans’ 2013 Comparative Price Report.
The average cost of a 1-month supply was $2,246 for Humira (adalimumab) in 2013 and $2,225 for Enbrel (etanercept). In Canada, which had second-highest cost for both drugs, Humira cost $1,950 per month and Enbrel cost $1,646 per month, the IFHP reported.
Switzerland had the lowest cost for both drugs among the countries included in the comparison: $881 for Humira and $1,017 for Enbrel.
In the United States, Humira is approved for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. Enbrel is approved for RA, polyarticular juvenile idiopathic arthritis in patients aged 2 years or older, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis.
The IFHP comprises more than 100 member companies in 25 countries. For the survey, the price for each country was submitted by participating member plans. Some prices are drawn from the public sector, some from the private, and some from both. U.S. averages were calculated from more than 100 million claims in the Truven MarketScan Research databases.
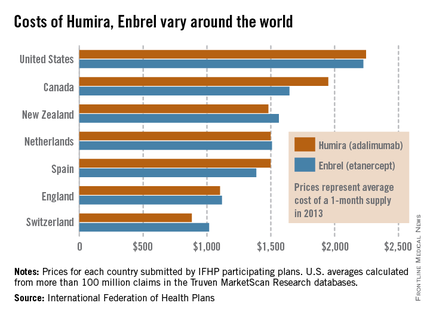
The costs of Humira and Enbrel are higher in the United States, compared with other industrialized countries included in the International Federation of Health Plans’ 2013 Comparative Price Report.
The average cost of a 1-month supply was $2,246 for Humira (adalimumab) in 2013 and $2,225 for Enbrel (etanercept). In Canada, which had second-highest cost for both drugs, Humira cost $1,950 per month and Enbrel cost $1,646 per month, the IFHP reported.
Switzerland had the lowest cost for both drugs among the countries included in the comparison: $881 for Humira and $1,017 for Enbrel.
In the United States, Humira is approved for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. Enbrel is approved for RA, polyarticular juvenile idiopathic arthritis in patients aged 2 years or older, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis.
The IFHP comprises more than 100 member companies in 25 countries. For the survey, the price for each country was submitted by participating member plans. Some prices are drawn from the public sector, some from the private, and some from both. U.S. averages were calculated from more than 100 million claims in the Truven MarketScan Research databases.

The costs of Humira and Enbrel are higher in the United States, compared with other industrialized countries included in the International Federation of Health Plans’ 2013 Comparative Price Report.
The average cost of a 1-month supply was $2,246 for Humira (adalimumab) in 2013 and $2,225 for Enbrel (etanercept). In Canada, which had second-highest cost for both drugs, Humira cost $1,950 per month and Enbrel cost $1,646 per month, the IFHP reported.
Switzerland had the lowest cost for both drugs among the countries included in the comparison: $881 for Humira and $1,017 for Enbrel.
In the United States, Humira is approved for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. Enbrel is approved for RA, polyarticular juvenile idiopathic arthritis in patients aged 2 years or older, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis.
The IFHP comprises more than 100 member companies in 25 countries. For the survey, the price for each country was submitted by participating member plans. Some prices are drawn from the public sector, some from the private, and some from both. U.S. averages were calculated from more than 100 million claims in the Truven MarketScan Research databases.

Alopecia Areata Universalis Complicating Daclizumab Therapy for Uveitis
Practice Question Answers: Medications in Dermatology, Part 2
1. A 40-year-old woman is diagnosed with systemic lupus erythematosus. You discuss treatment options and decide to start hydroxychloroquine. What laboratory tests and monitoring are required prior to starting this medication?
a. complete blood cell count with differential and glucose-6-phosphate dehydrogenase
b. complete blood cell count with differential and complete metabolic profile
c. ophthalmology evaluation and glucose-6-phosphate dehydrogenase
d. b and c
2. Two months ago you saw a 30-year-old woman with a history of severe atopic dermatitis. She had been using topical steroids with not much improvement. You decided to start a systemic medication. Within 1 month of drug initiation, she called your office to tell you that she is much better but has noticed unwanted hair on her face lately. Which medication is most likely implicated?
a. cyclosporine
b. dapsone
c. hydroxychloroquine
d. methotrexate
3. A 70-year-old man with type 2 diabetes mellitus who drinks 10 cans of beer per week presents to the emergency department with a 3-day history of diffuse tense bullae and pruritus on the legs and trunk. Direct immunofluorescence displayed linear deposition of IgG and C3 at the dermoepidermal junction, confirming your clinical diagnosis. What is the best long-term treatment option for this patient?
a. combination of oral steroids plus methotrexate
b. oral steroids and mycophenolate mofetil
c. oral steroids only
d. topical steroids only
4. A 45-year-old Venezuelan man presents with painful nodules on his bilateral lower legs. A biopsy demonstrates acid-fast bacilli, and a multidrug regimen is initiated for erythema nodosum leprosum. Which of the following is the mechanism of action of the treatment that is US Food and Drug Administration approved for this condition?
a. inhibits chemotaxis
b. inhibits dihydrofolate reductase
c. inhibits tumor necrosis factor α
d. suppresses T-cell function and B-cell antibody production
5. A patient consults her physician because of several side effects from a medication she started 2 weeks ago due to erythematous to violaceous papules on the legs from palpable purpura. She reports diarrhea, abdominal pain, and fatigue. Which medication is she taking?
a. azathioprine
b. colchicine
c. dapsone
d. methotrexate
1. A 40-year-old woman is diagnosed with systemic lupus erythematosus. You discuss treatment options and decide to start hydroxychloroquine. What laboratory tests and monitoring are required prior to starting this medication?
a. complete blood cell count with differential and glucose-6-phosphate dehydrogenase
b. complete blood cell count with differential and complete metabolic profile
c. ophthalmology evaluation and glucose-6-phosphate dehydrogenase
d. b and c
2. Two months ago you saw a 30-year-old woman with a history of severe atopic dermatitis. She had been using topical steroids with not much improvement. You decided to start a systemic medication. Within 1 month of drug initiation, she called your office to tell you that she is much better but has noticed unwanted hair on her face lately. Which medication is most likely implicated?
a. cyclosporine
b. dapsone
c. hydroxychloroquine
d. methotrexate
3. A 70-year-old man with type 2 diabetes mellitus who drinks 10 cans of beer per week presents to the emergency department with a 3-day history of diffuse tense bullae and pruritus on the legs and trunk. Direct immunofluorescence displayed linear deposition of IgG and C3 at the dermoepidermal junction, confirming your clinical diagnosis. What is the best long-term treatment option for this patient?
a. combination of oral steroids plus methotrexate
b. oral steroids and mycophenolate mofetil
c. oral steroids only
d. topical steroids only
4. A 45-year-old Venezuelan man presents with painful nodules on his bilateral lower legs. A biopsy demonstrates acid-fast bacilli, and a multidrug regimen is initiated for erythema nodosum leprosum. Which of the following is the mechanism of action of the treatment that is US Food and Drug Administration approved for this condition?
a. inhibits chemotaxis
b. inhibits dihydrofolate reductase
c. inhibits tumor necrosis factor α
d. suppresses T-cell function and B-cell antibody production
5. A patient consults her physician because of several side effects from a medication she started 2 weeks ago due to erythematous to violaceous papules on the legs from palpable purpura. She reports diarrhea, abdominal pain, and fatigue. Which medication is she taking?
a. azathioprine
b. colchicine
c. dapsone
d. methotrexate
1. A 40-year-old woman is diagnosed with systemic lupus erythematosus. You discuss treatment options and decide to start hydroxychloroquine. What laboratory tests and monitoring are required prior to starting this medication?
a. complete blood cell count with differential and glucose-6-phosphate dehydrogenase
b. complete blood cell count with differential and complete metabolic profile
c. ophthalmology evaluation and glucose-6-phosphate dehydrogenase
d. b and c
2. Two months ago you saw a 30-year-old woman with a history of severe atopic dermatitis. She had been using topical steroids with not much improvement. You decided to start a systemic medication. Within 1 month of drug initiation, she called your office to tell you that she is much better but has noticed unwanted hair on her face lately. Which medication is most likely implicated?
a. cyclosporine
b. dapsone
c. hydroxychloroquine
d. methotrexate
3. A 70-year-old man with type 2 diabetes mellitus who drinks 10 cans of beer per week presents to the emergency department with a 3-day history of diffuse tense bullae and pruritus on the legs and trunk. Direct immunofluorescence displayed linear deposition of IgG and C3 at the dermoepidermal junction, confirming your clinical diagnosis. What is the best long-term treatment option for this patient?
a. combination of oral steroids plus methotrexate
b. oral steroids and mycophenolate mofetil
c. oral steroids only
d. topical steroids only
4. A 45-year-old Venezuelan man presents with painful nodules on his bilateral lower legs. A biopsy demonstrates acid-fast bacilli, and a multidrug regimen is initiated for erythema nodosum leprosum. Which of the following is the mechanism of action of the treatment that is US Food and Drug Administration approved for this condition?
a. inhibits chemotaxis
b. inhibits dihydrofolate reductase
c. inhibits tumor necrosis factor α
d. suppresses T-cell function and B-cell antibody production
5. A patient consults her physician because of several side effects from a medication she started 2 weeks ago due to erythematous to violaceous papules on the legs from palpable purpura. She reports diarrhea, abdominal pain, and fatigue. Which medication is she taking?
a. azathioprine
b. colchicine
c. dapsone
d. methotrexate