User login
Role of Diet in Treating Skin Conditions



TNF-alpha, adiponectin potential biomarkers for PsA, psoriasis differentiation
High plasma levels of tumor necrosis factor (TNF)–alpha and adiponectin can be used to differentiate patients with psoriasis and psoriatic arthritis, according to Wen-Qing Li, PhD, of Brown University, Providence, R.I., and his associates.
In a research letter published in the British Journal of Dermatology, the investigators detailed an analysis of 180 patients with psoriasis only and 143 patients with psoriatic arthritis (PsA) from the Psoriatic Arthritis and Psoriasis Follow-up Study. Patients in both groups had a mean age of 51 years. Plasma levels of interleukin-6, C-reactive protein, TNF-alpha, leptin, total adiponectin, and high-molecular-weight (HMW) adiponectin were assessed as potential biomarkers by ultrasensitive enzyme-linked immunosorbent assay or immunoturbidimetric assay.
Median TNF-alpha plasma levels were higher in patients with PsA, compared with those with psoriasis (3.27 vs. 1.32 pg/mL–1), while total and HMW adiponectin levels were lower in patients with PsA, compared with those with psoriasis (4.66 vs. 5.36 mcg/mL–1; 2.58 vs. 3.01 mcg/mL–1). After logistic regression, TNF-alpha (adjusted odds ratio, 2.25; 95% confidence interval, 1.41-3.61) and total adiponectin (aOR, 0.61; 95% CI, 0.39-0.96) remained significantly associated as biomarkers. HMW adiponectin maintained marginal significance (aOR, 0.64; 95% CI, 0.41-1.01).
“Further large-scale investigation in a prospective setting of patients with PsO [psoriasis] would be warranted, if a clinically useful screening test is to be developed for risk prediction of PsA based on circulating biomarkers,” the investigators concluded.
Two study authors reported consulting with or advising numerous pharmaceutical companies.
SOURCE: Li W-Q et al. Br J Dermatol. 2019 Jan 29. doi: 10.1111/bjd.17700.
High plasma levels of tumor necrosis factor (TNF)–alpha and adiponectin can be used to differentiate patients with psoriasis and psoriatic arthritis, according to Wen-Qing Li, PhD, of Brown University, Providence, R.I., and his associates.
In a research letter published in the British Journal of Dermatology, the investigators detailed an analysis of 180 patients with psoriasis only and 143 patients with psoriatic arthritis (PsA) from the Psoriatic Arthritis and Psoriasis Follow-up Study. Patients in both groups had a mean age of 51 years. Plasma levels of interleukin-6, C-reactive protein, TNF-alpha, leptin, total adiponectin, and high-molecular-weight (HMW) adiponectin were assessed as potential biomarkers by ultrasensitive enzyme-linked immunosorbent assay or immunoturbidimetric assay.
Median TNF-alpha plasma levels were higher in patients with PsA, compared with those with psoriasis (3.27 vs. 1.32 pg/mL–1), while total and HMW adiponectin levels were lower in patients with PsA, compared with those with psoriasis (4.66 vs. 5.36 mcg/mL–1; 2.58 vs. 3.01 mcg/mL–1). After logistic regression, TNF-alpha (adjusted odds ratio, 2.25; 95% confidence interval, 1.41-3.61) and total adiponectin (aOR, 0.61; 95% CI, 0.39-0.96) remained significantly associated as biomarkers. HMW adiponectin maintained marginal significance (aOR, 0.64; 95% CI, 0.41-1.01).
“Further large-scale investigation in a prospective setting of patients with PsO [psoriasis] would be warranted, if a clinically useful screening test is to be developed for risk prediction of PsA based on circulating biomarkers,” the investigators concluded.
Two study authors reported consulting with or advising numerous pharmaceutical companies.
SOURCE: Li W-Q et al. Br J Dermatol. 2019 Jan 29. doi: 10.1111/bjd.17700.
High plasma levels of tumor necrosis factor (TNF)–alpha and adiponectin can be used to differentiate patients with psoriasis and psoriatic arthritis, according to Wen-Qing Li, PhD, of Brown University, Providence, R.I., and his associates.
In a research letter published in the British Journal of Dermatology, the investigators detailed an analysis of 180 patients with psoriasis only and 143 patients with psoriatic arthritis (PsA) from the Psoriatic Arthritis and Psoriasis Follow-up Study. Patients in both groups had a mean age of 51 years. Plasma levels of interleukin-6, C-reactive protein, TNF-alpha, leptin, total adiponectin, and high-molecular-weight (HMW) adiponectin were assessed as potential biomarkers by ultrasensitive enzyme-linked immunosorbent assay or immunoturbidimetric assay.
Median TNF-alpha plasma levels were higher in patients with PsA, compared with those with psoriasis (3.27 vs. 1.32 pg/mL–1), while total and HMW adiponectin levels were lower in patients with PsA, compared with those with psoriasis (4.66 vs. 5.36 mcg/mL–1; 2.58 vs. 3.01 mcg/mL–1). After logistic regression, TNF-alpha (adjusted odds ratio, 2.25; 95% confidence interval, 1.41-3.61) and total adiponectin (aOR, 0.61; 95% CI, 0.39-0.96) remained significantly associated as biomarkers. HMW adiponectin maintained marginal significance (aOR, 0.64; 95% CI, 0.41-1.01).
“Further large-scale investigation in a prospective setting of patients with PsO [psoriasis] would be warranted, if a clinically useful screening test is to be developed for risk prediction of PsA based on circulating biomarkers,” the investigators concluded.
Two study authors reported consulting with or advising numerous pharmaceutical companies.
SOURCE: Li W-Q et al. Br J Dermatol. 2019 Jan 29. doi: 10.1111/bjd.17700.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Survey finds psoriasis patients seek relief with alternative therapies
Treatment (CAMs), despite limited documentation supporting their efficacy, reported Emily C. Murphy and her associates, in the department of dermatology, George Washington University, Washington.
They performed a survey-based statistical analysis to identify specific types of commonly used CAMs, and to explore reasons patients increasingly turn to alternative therapies. The survey was distributed in the National Psoriasis Foundation’s (NPF) October 2018 newsletter to its 100,927 members. Their results were published in a letter to the editor of the Journal of the American Academy of Dermatology.
Of the 6,101 NPF members who opened the newsletter, 324 clicked on the survey link. Of the 219 who completed the survey, almost 70% were women. The majority were white (84.1%), compared with Hispanic (6.2%), Asian (3.1%), and black (2.6%) participants. Most of the survey respondents had a dermatologist diagnosis of psoriasis, as well as access to health insurance to cover any prescribed medicines needed.
Of the 41% of respondents who reported using alternative therapies, usage was especially high among those who considered their psoriasis as severe (50% vs. 33.6% of those with nonsevere disease). Among the respondents, women were more likely than were men to use CAMs (45.6% vs. 26.5%, P = .002).
Only 4% cited access to care as a reason for choosing alternative therapies; the majority said they used CAMs because “traditional medications did not help or had side effects.”
While men were more likely than were women to use vitamins (24% vs. 18.9%, respectively), Dead Sea bath salts (17% vs. 7.8%), and cupping (3% vs. 0.8%), women were more likely to use herbals/botanicals (17% vs. 14%) and yoga (9.6% vs. 2%).
Patients with moderate psoriasis were significantly more likely than were those with mild or severe cases of the disease to recommend CAMs, regardless of insurance status (52.4% vs. 35% among those with mild disease and 40.4% for those with severe disease).
For some of the commonly used treatments, such as vitamins D and B12, there is insufficient evidence documenting their efficacy, although Dead Sea treatments have been shown to have therapeutic effects. And while there is efficacy evidence for indigo naturalis and meditation, these were not mentioned or were not commonly reported by respondents, the authors pointed out.
Although just 43% of patients said they would recommend a CAM to other people with psoriasis, its use remains widespread. For this reason, “educational initiatives that enable physicians to discuss evidence-based CAMs may improve patient satisfaction and outcomes,” observed Ms. Murphy, a research fellow, and her associates.
Previous studies have cited rates of CAM usage among patients with psoriasis as high as 62%, but researchers have failed to examine the reasons motivating usage. Not surprisingly, patients often use but misunderstand the benefits of alternative therapies.
“The onus is on us as physicians to not only ask our patients if they are using nonallopathic therapies for their psoriasis, but also to create an accepting environment that enables further discussion regarding said treatments to ensure patient safety and ultimately good outcomes,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, said in an interview.
The authors had no financial sources or conflicts of interest to disclose; there was no funding source.
SOURCE: Murphy E et al. J Am Acad Dermatol. 2019 Mar 29. pii: S0190-9622(19)30503-1. doi: 10.1016/j.jaad.2019.03.059.
Treatment (CAMs), despite limited documentation supporting their efficacy, reported Emily C. Murphy and her associates, in the department of dermatology, George Washington University, Washington.
They performed a survey-based statistical analysis to identify specific types of commonly used CAMs, and to explore reasons patients increasingly turn to alternative therapies. The survey was distributed in the National Psoriasis Foundation’s (NPF) October 2018 newsletter to its 100,927 members. Their results were published in a letter to the editor of the Journal of the American Academy of Dermatology.
Of the 6,101 NPF members who opened the newsletter, 324 clicked on the survey link. Of the 219 who completed the survey, almost 70% were women. The majority were white (84.1%), compared with Hispanic (6.2%), Asian (3.1%), and black (2.6%) participants. Most of the survey respondents had a dermatologist diagnosis of psoriasis, as well as access to health insurance to cover any prescribed medicines needed.
Of the 41% of respondents who reported using alternative therapies, usage was especially high among those who considered their psoriasis as severe (50% vs. 33.6% of those with nonsevere disease). Among the respondents, women were more likely than were men to use CAMs (45.6% vs. 26.5%, P = .002).
Only 4% cited access to care as a reason for choosing alternative therapies; the majority said they used CAMs because “traditional medications did not help or had side effects.”
While men were more likely than were women to use vitamins (24% vs. 18.9%, respectively), Dead Sea bath salts (17% vs. 7.8%), and cupping (3% vs. 0.8%), women were more likely to use herbals/botanicals (17% vs. 14%) and yoga (9.6% vs. 2%).
Patients with moderate psoriasis were significantly more likely than were those with mild or severe cases of the disease to recommend CAMs, regardless of insurance status (52.4% vs. 35% among those with mild disease and 40.4% for those with severe disease).
For some of the commonly used treatments, such as vitamins D and B12, there is insufficient evidence documenting their efficacy, although Dead Sea treatments have been shown to have therapeutic effects. And while there is efficacy evidence for indigo naturalis and meditation, these were not mentioned or were not commonly reported by respondents, the authors pointed out.
Although just 43% of patients said they would recommend a CAM to other people with psoriasis, its use remains widespread. For this reason, “educational initiatives that enable physicians to discuss evidence-based CAMs may improve patient satisfaction and outcomes,” observed Ms. Murphy, a research fellow, and her associates.
Previous studies have cited rates of CAM usage among patients with psoriasis as high as 62%, but researchers have failed to examine the reasons motivating usage. Not surprisingly, patients often use but misunderstand the benefits of alternative therapies.
“The onus is on us as physicians to not only ask our patients if they are using nonallopathic therapies for their psoriasis, but also to create an accepting environment that enables further discussion regarding said treatments to ensure patient safety and ultimately good outcomes,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, said in an interview.
The authors had no financial sources or conflicts of interest to disclose; there was no funding source.
SOURCE: Murphy E et al. J Am Acad Dermatol. 2019 Mar 29. pii: S0190-9622(19)30503-1. doi: 10.1016/j.jaad.2019.03.059.
Treatment (CAMs), despite limited documentation supporting their efficacy, reported Emily C. Murphy and her associates, in the department of dermatology, George Washington University, Washington.
They performed a survey-based statistical analysis to identify specific types of commonly used CAMs, and to explore reasons patients increasingly turn to alternative therapies. The survey was distributed in the National Psoriasis Foundation’s (NPF) October 2018 newsletter to its 100,927 members. Their results were published in a letter to the editor of the Journal of the American Academy of Dermatology.
Of the 6,101 NPF members who opened the newsletter, 324 clicked on the survey link. Of the 219 who completed the survey, almost 70% were women. The majority were white (84.1%), compared with Hispanic (6.2%), Asian (3.1%), and black (2.6%) participants. Most of the survey respondents had a dermatologist diagnosis of psoriasis, as well as access to health insurance to cover any prescribed medicines needed.
Of the 41% of respondents who reported using alternative therapies, usage was especially high among those who considered their psoriasis as severe (50% vs. 33.6% of those with nonsevere disease). Among the respondents, women were more likely than were men to use CAMs (45.6% vs. 26.5%, P = .002).
Only 4% cited access to care as a reason for choosing alternative therapies; the majority said they used CAMs because “traditional medications did not help or had side effects.”
While men were more likely than were women to use vitamins (24% vs. 18.9%, respectively), Dead Sea bath salts (17% vs. 7.8%), and cupping (3% vs. 0.8%), women were more likely to use herbals/botanicals (17% vs. 14%) and yoga (9.6% vs. 2%).
Patients with moderate psoriasis were significantly more likely than were those with mild or severe cases of the disease to recommend CAMs, regardless of insurance status (52.4% vs. 35% among those with mild disease and 40.4% for those with severe disease).
For some of the commonly used treatments, such as vitamins D and B12, there is insufficient evidence documenting their efficacy, although Dead Sea treatments have been shown to have therapeutic effects. And while there is efficacy evidence for indigo naturalis and meditation, these were not mentioned or were not commonly reported by respondents, the authors pointed out.
Although just 43% of patients said they would recommend a CAM to other people with psoriasis, its use remains widespread. For this reason, “educational initiatives that enable physicians to discuss evidence-based CAMs may improve patient satisfaction and outcomes,” observed Ms. Murphy, a research fellow, and her associates.
Previous studies have cited rates of CAM usage among patients with psoriasis as high as 62%, but researchers have failed to examine the reasons motivating usage. Not surprisingly, patients often use but misunderstand the benefits of alternative therapies.
“The onus is on us as physicians to not only ask our patients if they are using nonallopathic therapies for their psoriasis, but also to create an accepting environment that enables further discussion regarding said treatments to ensure patient safety and ultimately good outcomes,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, said in an interview.
The authors had no financial sources or conflicts of interest to disclose; there was no funding source.
SOURCE: Murphy E et al. J Am Acad Dermatol. 2019 Mar 29. pii: S0190-9622(19)30503-1. doi: 10.1016/j.jaad.2019.03.059.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
AFib, CVD risks similar after ustekinumab and TNF inhibitors in psoriatic patients
Patients with psoriasis or psoriatic arthritis who started ustekinumab (Stelara) versus a tumor necrosis factor inhibitor had no differences in the overall risks of incident atrial fibrillation (AFib) or major adverse cardiovascular events (MACE), according to authors of a retrospective cohort study of two commercial insurance databases.
Subgroup analyses in the study also revealed “no statistically significant heterogeneity” in risk of AFib or MACE by age, sex, or presence of diabetes, Moa P. Lee, PharmD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, and her coauthors reported in JAMA Dermatology.
These findings provide additional evidence on cardiovascular risks with ustekinumab versus other treatments.
The findings are consistent with previous observations of a small but nonsignificant increase in cardiovascular disease among patients with psoriatic disease and provide new insight into the risk of AFib with psoriatic treatments with the two therapies, Dr. Lee and her colleagues wrote.
The retrospective study of two U.S. commercial health care claims databases included 60,028 adult patients with psoriasis or psoriatic arthritis who initiated therapy with ustekinumab (n = 9,071) or a tumor necrosis factor (TNF) inhibitor (n = 50,957), including adalimumab, certolizumab pegol, golimumab, etanercept, or infliximab. The investigators excluded any patient with an AFib diagnosis at baseline and those receiving any antiarrhythmic or anticoagulant treatment.
The incidence of AFib was 5.0 and 4.1 per 1,000 person-years in the ustekinumab and TNF inhibitor groups, respectively, with an adjusted hazard ratio of 1.08 (95% CI, 0.76-1.54). The incidence of MACE (a composite endpoint of MI, stroke, and coronary revascularization) was 6.2 and 6.1 per 1,000 person-years in the ustekinumab and TNF inhibitor groups, with an adjusted hazard ratio of 1.10 (95% CI, 0.80-1.52).
In subgroup analyses, the adjusted HR for AFib with ustekinumab versus TNF inhibitor was 1.46 (95% CI, 0.98-2.18) for patients aged 60 years and older and 1.47 (95% CI, 0.93-2.31) in patients with diabetes, the investigators wrote.
The adjusted HR for AFib with ustekinumab versus TNF inhibitors was 1.21 in men (95% CI, 0.87-1.69) and 0.82 in women (95% CI, 0.49-1.39), while for MACE, the HRs were 1.31 in men (95% CI, 0.97-1.76) and 0.91 in women (95% CI, 0.56-1.47).
“Although the risk of these cardiovascular outcomes appeared to be similar across the subpopulations included in our study, further investigations on potentially modifying treatment effects stratified by important risk factors may be warranted,” Dr. Lee and her coauthors wrote.
The study was supported by the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital. Several authors reported financial relationships with pharmaceutical companies marketing biologics for psoriasis and psoriatic arthritis.
SOURCE: Lee MP et al. JAMA Dermatol. 2019 Mar 27. doi: 10.1001/jamadermatol.2019.0001.
Patients with psoriasis or psoriatic arthritis who started ustekinumab (Stelara) versus a tumor necrosis factor inhibitor had no differences in the overall risks of incident atrial fibrillation (AFib) or major adverse cardiovascular events (MACE), according to authors of a retrospective cohort study of two commercial insurance databases.
Subgroup analyses in the study also revealed “no statistically significant heterogeneity” in risk of AFib or MACE by age, sex, or presence of diabetes, Moa P. Lee, PharmD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, and her coauthors reported in JAMA Dermatology.
These findings provide additional evidence on cardiovascular risks with ustekinumab versus other treatments.
The findings are consistent with previous observations of a small but nonsignificant increase in cardiovascular disease among patients with psoriatic disease and provide new insight into the risk of AFib with psoriatic treatments with the two therapies, Dr. Lee and her colleagues wrote.
The retrospective study of two U.S. commercial health care claims databases included 60,028 adult patients with psoriasis or psoriatic arthritis who initiated therapy with ustekinumab (n = 9,071) or a tumor necrosis factor (TNF) inhibitor (n = 50,957), including adalimumab, certolizumab pegol, golimumab, etanercept, or infliximab. The investigators excluded any patient with an AFib diagnosis at baseline and those receiving any antiarrhythmic or anticoagulant treatment.
The incidence of AFib was 5.0 and 4.1 per 1,000 person-years in the ustekinumab and TNF inhibitor groups, respectively, with an adjusted hazard ratio of 1.08 (95% CI, 0.76-1.54). The incidence of MACE (a composite endpoint of MI, stroke, and coronary revascularization) was 6.2 and 6.1 per 1,000 person-years in the ustekinumab and TNF inhibitor groups, with an adjusted hazard ratio of 1.10 (95% CI, 0.80-1.52).
In subgroup analyses, the adjusted HR for AFib with ustekinumab versus TNF inhibitor was 1.46 (95% CI, 0.98-2.18) for patients aged 60 years and older and 1.47 (95% CI, 0.93-2.31) in patients with diabetes, the investigators wrote.
The adjusted HR for AFib with ustekinumab versus TNF inhibitors was 1.21 in men (95% CI, 0.87-1.69) and 0.82 in women (95% CI, 0.49-1.39), while for MACE, the HRs were 1.31 in men (95% CI, 0.97-1.76) and 0.91 in women (95% CI, 0.56-1.47).
“Although the risk of these cardiovascular outcomes appeared to be similar across the subpopulations included in our study, further investigations on potentially modifying treatment effects stratified by important risk factors may be warranted,” Dr. Lee and her coauthors wrote.
The study was supported by the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital. Several authors reported financial relationships with pharmaceutical companies marketing biologics for psoriasis and psoriatic arthritis.
SOURCE: Lee MP et al. JAMA Dermatol. 2019 Mar 27. doi: 10.1001/jamadermatol.2019.0001.
Patients with psoriasis or psoriatic arthritis who started ustekinumab (Stelara) versus a tumor necrosis factor inhibitor had no differences in the overall risks of incident atrial fibrillation (AFib) or major adverse cardiovascular events (MACE), according to authors of a retrospective cohort study of two commercial insurance databases.
Subgroup analyses in the study also revealed “no statistically significant heterogeneity” in risk of AFib or MACE by age, sex, or presence of diabetes, Moa P. Lee, PharmD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, and her coauthors reported in JAMA Dermatology.
These findings provide additional evidence on cardiovascular risks with ustekinumab versus other treatments.
The findings are consistent with previous observations of a small but nonsignificant increase in cardiovascular disease among patients with psoriatic disease and provide new insight into the risk of AFib with psoriatic treatments with the two therapies, Dr. Lee and her colleagues wrote.
The retrospective study of two U.S. commercial health care claims databases included 60,028 adult patients with psoriasis or psoriatic arthritis who initiated therapy with ustekinumab (n = 9,071) or a tumor necrosis factor (TNF) inhibitor (n = 50,957), including adalimumab, certolizumab pegol, golimumab, etanercept, or infliximab. The investigators excluded any patient with an AFib diagnosis at baseline and those receiving any antiarrhythmic or anticoagulant treatment.
The incidence of AFib was 5.0 and 4.1 per 1,000 person-years in the ustekinumab and TNF inhibitor groups, respectively, with an adjusted hazard ratio of 1.08 (95% CI, 0.76-1.54). The incidence of MACE (a composite endpoint of MI, stroke, and coronary revascularization) was 6.2 and 6.1 per 1,000 person-years in the ustekinumab and TNF inhibitor groups, with an adjusted hazard ratio of 1.10 (95% CI, 0.80-1.52).
In subgroup analyses, the adjusted HR for AFib with ustekinumab versus TNF inhibitor was 1.46 (95% CI, 0.98-2.18) for patients aged 60 years and older and 1.47 (95% CI, 0.93-2.31) in patients with diabetes, the investigators wrote.
The adjusted HR for AFib with ustekinumab versus TNF inhibitors was 1.21 in men (95% CI, 0.87-1.69) and 0.82 in women (95% CI, 0.49-1.39), while for MACE, the HRs were 1.31 in men (95% CI, 0.97-1.76) and 0.91 in women (95% CI, 0.56-1.47).
“Although the risk of these cardiovascular outcomes appeared to be similar across the subpopulations included in our study, further investigations on potentially modifying treatment effects stratified by important risk factors may be warranted,” Dr. Lee and her coauthors wrote.
The study was supported by the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital. Several authors reported financial relationships with pharmaceutical companies marketing biologics for psoriasis and psoriatic arthritis.
SOURCE: Lee MP et al. JAMA Dermatol. 2019 Mar 27. doi: 10.1001/jamadermatol.2019.0001.
FROM JAMA DERMATOLOGY
Proinflammatory diet may not trigger adult psoriasis, PsA, or AD
reported Alanna C. Bridgman of Queen’s University, Kingston, Ont., and her associates.
In a large, retrospective cohort study among women from the Nurses’ Health Study II (NHS-II), including 85,185 psoriasis participants and 63,443 atopic dermatitis participants, Ms. Bridgman and her associates sought to determine whether proinflammatory diet increased the risk of incident psoriasis, psoriatic arthritis, or atopic dermatitis. Clinicians administered food frequency questionnaires every 4 years beginning in 1991 among female nurses aged 25-42 years.
Food groups included in the evaluation were those most predictive of three plasma markers of inflammation: interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor–alpha R2 (TNF-R2). Proinflammatory foods included processed meat, red meat, organ meat, white fish, vegetables other than leafy green and dark yellow, refined grains, low- and high-energy drinks, and tomatoes. Anti-inflammatory foods included beer, wine, tea, coffee, dark yellow and green leafy vegetables, snacks such as popcorn and crackers, fruit juice, and pizza.
No association was found between proinflammatory diet and increased likelihood for incident psoriasis, psoriatic arthritis, or atopic dermatitis. Although proinflammatory dietary patterns were associated with psoriatic arthritis in the age-adjusted model, the hazard ratio was attenuated and found to be no longer statistically significant after adjustment for important confounders such as body mass index. In addition, no significant relationship between atopic dermatitis and proinflammatory diet was observed, they reported. The study was published in the Journal of the American Academy of Dermatology.
Ms. Bridgman and her associates measured dietary patterns using the Empirical Dietary Inflammatory Pattern (EDIP); dietary patterns measuring high on the EDIP scale were associated with higher levels of TNF-alpha, TNF-alpha R1, TNF-alpha R2, CRP, IL-6, and adiponectin. Psoriasis and psoriatic arthritis are Th1- and Th17-mediated diseases that exhibit higher serum levels of IL-6, CRP, and TNF-alpha, unlike atopic dermatitis, which is primarily a Th2-mediated condition featuring reduced involvement of the Th1/Th17 inflammatory cytokines.
Because a goal of the EDIP score was to “account for the overall effect of dietary patterns,” the researchers included in their analysis only those food groups that “explain the maximal variation in the three noted inflammatory biomarkers.”
All patients included in the study were questioned at baseline regarding their height and race/ethnicity. Weight, smoking status, and physical activity, and diagnoses of hypercholesterolemia, type 2 diabetes, cardiovascular disease, and asthma were monitored biennially.
Overall, patients with higher EDIP scores were found to have higher BMI, lower physical activity, and alcohol use, as well as increased rates of hypercholesterolemia and hypertension.
“Though we found no convincing evidence for an association with EDIP score for any of the investigated diseases, the results followed an internal pattern consistent with our hypotheses that higher EDIP scores would have more of an association with psoriatic disease than with atopic dermatitis,” the researchers wrote.
Citing recent evidence gathered in studies, such as the French NutriNet-Santé study, which demonstrated proinflammatory effects similar to those measured with the EDIP in cases where there was low adherence to the Mediterranean diet, the authors attributed their contradictory findings to “important methodological differences.” Unlike the NutriNet-Santé study, which classified psoriasis by severity, Ms. Bridgman and her colleagues examined the overall risk of incident psoriasis. “It is possible that a dietary index associated with more Th-2 inflammation would yield different results,” they noted.
The large sample size, prospectively collected dietary, and psoriatic disease data, as well as the ability to adjust for important confounding factors, were included among the strengths of the study.
That the participants were limited to U.S. women could be considered a limitation because the results may not be generalizable to other populations. The results also may not be relevant to child-onset disease because the patient population included only cases of adult-onset atopic dermatitis. Questionnaire-based diagnoses increase the likelihood of misclassification, so “dilution of the case pool with false-positive cases would bias our results towards the null,” they added.
Ultimately, the authors noted that proinflammatory diet may be associated with other health risks, but these do not warrant counseling patients concerning their possible impact in cases of psoriatic disease or atopic dermatitis.
The study was funded by Brown University department of dermatology and from Regeneron, Sanofi, the National Institutes of Health, and the National Cancer Institute. Two coauthors, one of whom has a patent pending for the nix-tix tick remover, disclosed ties with various companies.
SOURCE: Bridgman AC et al. J Am Acad Dermatol. 2019 Feb 21. pii: S0190-9622(19)30329-9.
reported Alanna C. Bridgman of Queen’s University, Kingston, Ont., and her associates.
In a large, retrospective cohort study among women from the Nurses’ Health Study II (NHS-II), including 85,185 psoriasis participants and 63,443 atopic dermatitis participants, Ms. Bridgman and her associates sought to determine whether proinflammatory diet increased the risk of incident psoriasis, psoriatic arthritis, or atopic dermatitis. Clinicians administered food frequency questionnaires every 4 years beginning in 1991 among female nurses aged 25-42 years.
Food groups included in the evaluation were those most predictive of three plasma markers of inflammation: interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor–alpha R2 (TNF-R2). Proinflammatory foods included processed meat, red meat, organ meat, white fish, vegetables other than leafy green and dark yellow, refined grains, low- and high-energy drinks, and tomatoes. Anti-inflammatory foods included beer, wine, tea, coffee, dark yellow and green leafy vegetables, snacks such as popcorn and crackers, fruit juice, and pizza.
No association was found between proinflammatory diet and increased likelihood for incident psoriasis, psoriatic arthritis, or atopic dermatitis. Although proinflammatory dietary patterns were associated with psoriatic arthritis in the age-adjusted model, the hazard ratio was attenuated and found to be no longer statistically significant after adjustment for important confounders such as body mass index. In addition, no significant relationship between atopic dermatitis and proinflammatory diet was observed, they reported. The study was published in the Journal of the American Academy of Dermatology.
Ms. Bridgman and her associates measured dietary patterns using the Empirical Dietary Inflammatory Pattern (EDIP); dietary patterns measuring high on the EDIP scale were associated with higher levels of TNF-alpha, TNF-alpha R1, TNF-alpha R2, CRP, IL-6, and adiponectin. Psoriasis and psoriatic arthritis are Th1- and Th17-mediated diseases that exhibit higher serum levels of IL-6, CRP, and TNF-alpha, unlike atopic dermatitis, which is primarily a Th2-mediated condition featuring reduced involvement of the Th1/Th17 inflammatory cytokines.
Because a goal of the EDIP score was to “account for the overall effect of dietary patterns,” the researchers included in their analysis only those food groups that “explain the maximal variation in the three noted inflammatory biomarkers.”
All patients included in the study were questioned at baseline regarding their height and race/ethnicity. Weight, smoking status, and physical activity, and diagnoses of hypercholesterolemia, type 2 diabetes, cardiovascular disease, and asthma were monitored biennially.
Overall, patients with higher EDIP scores were found to have higher BMI, lower physical activity, and alcohol use, as well as increased rates of hypercholesterolemia and hypertension.
“Though we found no convincing evidence for an association with EDIP score for any of the investigated diseases, the results followed an internal pattern consistent with our hypotheses that higher EDIP scores would have more of an association with psoriatic disease than with atopic dermatitis,” the researchers wrote.
Citing recent evidence gathered in studies, such as the French NutriNet-Santé study, which demonstrated proinflammatory effects similar to those measured with the EDIP in cases where there was low adherence to the Mediterranean diet, the authors attributed their contradictory findings to “important methodological differences.” Unlike the NutriNet-Santé study, which classified psoriasis by severity, Ms. Bridgman and her colleagues examined the overall risk of incident psoriasis. “It is possible that a dietary index associated with more Th-2 inflammation would yield different results,” they noted.
The large sample size, prospectively collected dietary, and psoriatic disease data, as well as the ability to adjust for important confounding factors, were included among the strengths of the study.
That the participants were limited to U.S. women could be considered a limitation because the results may not be generalizable to other populations. The results also may not be relevant to child-onset disease because the patient population included only cases of adult-onset atopic dermatitis. Questionnaire-based diagnoses increase the likelihood of misclassification, so “dilution of the case pool with false-positive cases would bias our results towards the null,” they added.
Ultimately, the authors noted that proinflammatory diet may be associated with other health risks, but these do not warrant counseling patients concerning their possible impact in cases of psoriatic disease or atopic dermatitis.
The study was funded by Brown University department of dermatology and from Regeneron, Sanofi, the National Institutes of Health, and the National Cancer Institute. Two coauthors, one of whom has a patent pending for the nix-tix tick remover, disclosed ties with various companies.
SOURCE: Bridgman AC et al. J Am Acad Dermatol. 2019 Feb 21. pii: S0190-9622(19)30329-9.
reported Alanna C. Bridgman of Queen’s University, Kingston, Ont., and her associates.
In a large, retrospective cohort study among women from the Nurses’ Health Study II (NHS-II), including 85,185 psoriasis participants and 63,443 atopic dermatitis participants, Ms. Bridgman and her associates sought to determine whether proinflammatory diet increased the risk of incident psoriasis, psoriatic arthritis, or atopic dermatitis. Clinicians administered food frequency questionnaires every 4 years beginning in 1991 among female nurses aged 25-42 years.
Food groups included in the evaluation were those most predictive of three plasma markers of inflammation: interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor–alpha R2 (TNF-R2). Proinflammatory foods included processed meat, red meat, organ meat, white fish, vegetables other than leafy green and dark yellow, refined grains, low- and high-energy drinks, and tomatoes. Anti-inflammatory foods included beer, wine, tea, coffee, dark yellow and green leafy vegetables, snacks such as popcorn and crackers, fruit juice, and pizza.
No association was found between proinflammatory diet and increased likelihood for incident psoriasis, psoriatic arthritis, or atopic dermatitis. Although proinflammatory dietary patterns were associated with psoriatic arthritis in the age-adjusted model, the hazard ratio was attenuated and found to be no longer statistically significant after adjustment for important confounders such as body mass index. In addition, no significant relationship between atopic dermatitis and proinflammatory diet was observed, they reported. The study was published in the Journal of the American Academy of Dermatology.
Ms. Bridgman and her associates measured dietary patterns using the Empirical Dietary Inflammatory Pattern (EDIP); dietary patterns measuring high on the EDIP scale were associated with higher levels of TNF-alpha, TNF-alpha R1, TNF-alpha R2, CRP, IL-6, and adiponectin. Psoriasis and psoriatic arthritis are Th1- and Th17-mediated diseases that exhibit higher serum levels of IL-6, CRP, and TNF-alpha, unlike atopic dermatitis, which is primarily a Th2-mediated condition featuring reduced involvement of the Th1/Th17 inflammatory cytokines.
Because a goal of the EDIP score was to “account for the overall effect of dietary patterns,” the researchers included in their analysis only those food groups that “explain the maximal variation in the three noted inflammatory biomarkers.”
All patients included in the study were questioned at baseline regarding their height and race/ethnicity. Weight, smoking status, and physical activity, and diagnoses of hypercholesterolemia, type 2 diabetes, cardiovascular disease, and asthma were monitored biennially.
Overall, patients with higher EDIP scores were found to have higher BMI, lower physical activity, and alcohol use, as well as increased rates of hypercholesterolemia and hypertension.
“Though we found no convincing evidence for an association with EDIP score for any of the investigated diseases, the results followed an internal pattern consistent with our hypotheses that higher EDIP scores would have more of an association with psoriatic disease than with atopic dermatitis,” the researchers wrote.
Citing recent evidence gathered in studies, such as the French NutriNet-Santé study, which demonstrated proinflammatory effects similar to those measured with the EDIP in cases where there was low adherence to the Mediterranean diet, the authors attributed their contradictory findings to “important methodological differences.” Unlike the NutriNet-Santé study, which classified psoriasis by severity, Ms. Bridgman and her colleagues examined the overall risk of incident psoriasis. “It is possible that a dietary index associated with more Th-2 inflammation would yield different results,” they noted.
The large sample size, prospectively collected dietary, and psoriatic disease data, as well as the ability to adjust for important confounding factors, were included among the strengths of the study.
That the participants were limited to U.S. women could be considered a limitation because the results may not be generalizable to other populations. The results also may not be relevant to child-onset disease because the patient population included only cases of adult-onset atopic dermatitis. Questionnaire-based diagnoses increase the likelihood of misclassification, so “dilution of the case pool with false-positive cases would bias our results towards the null,” they added.
Ultimately, the authors noted that proinflammatory diet may be associated with other health risks, but these do not warrant counseling patients concerning their possible impact in cases of psoriatic disease or atopic dermatitis.
The study was funded by Brown University department of dermatology and from Regeneron, Sanofi, the National Institutes of Health, and the National Cancer Institute. Two coauthors, one of whom has a patent pending for the nix-tix tick remover, disclosed ties with various companies.
SOURCE: Bridgman AC et al. J Am Acad Dermatol. 2019 Feb 21. pii: S0190-9622(19)30329-9.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Study results may not be generalizable to other study populations.
Major finding: No association was found between proinflammatory diet and increased likelihood for incident psoriasis, psoriatic arthritis, or atopic dermatitis in adult women.
Study details: Large retrospective cohort study of 85,185 psoriasis subjects and 63,443 atopic dermatitis subjects.
Disclosures: The study was funded by Brown University department of dermatology and from Regeneron, Sanofi, the National Institutes of Health, and the National Cancer Institute. Two coauthors, one of whom has a patent pending for the nix-tix tick remover, disclosed ties with various companies. Source: Bridgman AC et al. J Am Acad Dermatol. 2019 Feb 21. pii: S0190-9622(19)30329-9.
TNF inhibitor–induced psoriasis in IBD patients a consideration
WASHINGTON – (IBD), Sophia Delano, MD, said during a session on the cutaneous effects of IBD at the annual meeting of the American Academy of Dermatology.
This is a paradoxical reaction, which can happen “weeks to years after starting a TNF blocker,” with about 70% of cases occurring during the first year of therapy, said Dr. Delano, an attending physician in the dermatology program at Boston Children’s Hospital.
Those receiving infliximab are more likely to develop TNF inhibitor–induced psoriasis, compared with those on adalimumab or etanercept. TNF inhibitor–induced psoriasis may not track with gastrointestinal activity, and some patients whose gastrointestinal disease is responding to treatment can begin to develop psoriasis, she noted.
The clinical presentation of TNF inhibitor–induced psoriasis can also vary. In one study of 216 cases, 26.9% of patients had a mixed morphology, with the most common presentations including plaque psoriasis (44.8%) and palmoplantar pustular psoriasis (36.3%). Other presentations were psoriasiform dermatitis (19.9%), scalp involvement with alopecia (7.5%), and generalized pustular psoriasis (10.9%). Locations affected were the soles of the feet (45.8%), extremities (45.4%), palms (44.9%), scalp (36.1%), and trunk (32.4%), Dr. Delano said.
TNF inhibitor–induced psoriasis is likely a class effect, she said, noting that, in the same review, symptoms resolved in 47.7% of patients who discontinued TNF inhibitors, in 36.7% of patients who switched to another TNF inhibitor, and in 32.9% of patients who continued their original therapy (J Am Acad Dermatol. 2017 Feb;76[2]:334-41). In the study, Crohn’s disease and RA were the most common diseases, in 40.7% and 37.0% of the patients, respectively.
There have been case reports of TNF antagonist–induced lupus-like syndrome (TAILS), which is more common in patients with RA and ulcerative colitis. TAILS occurs more often in women than in men; can present similarly to systemic lupus erythematosus, subacute cutaneous lupus erythematosus, and chronic cutaneous lupus; and resolves by stopping TNF inhibitor treatment, Dr. Delano said.
Skin cancer risk, infections, and injection site reactions
Both adult and pediatric patients treated with TNF inhibitors for IBD may be at increased risk for lymphoma, visceral tumors, melanoma, and nonmelanoma skin cancers. Dr. Delano referred to a study published in 2014, which identified 972 reports of melanoma in the Food and Drug Administration’s Adverse Event Reporting System database associated with TNF inhibitor use; of these, 69 cases involved patients using more than one TNF inhibitor. Infliximab, golimumab, etanercept, and adalimumab were associated with a safety signal for melanoma, but not certolizumab (Br J Dermatol. 2014 May;170[5]:1170-2).
Dr. Delano observed that thiopurines such as azathioprine are also associated with an increased cancer risk, as noted in one retrospective study that found that the risk of nonmelanoma skin cancer was 2.1 times higher in a mostly white male cohort with ulcerative colitis during treatment with thiopurines, compared with patients not treated with thiopurines (Am J Gastroenterol. 2014 Nov;109[11]:1781-93). A greater duration of treatment (more than 6 months) and higher doses were associated with higher risks.
Adalimumab, golimumab, and certolizumab can also cause injection site reactions, typically within 1- 2 days of injection, said Dr. Delano. In these cases, symptoms of erythema, warmth, burning, or pruritus are worse at the beginning of treatment and can be relieved by rotating the injection site as well as providing cool compresses, topical steroids, antihistamines, and supportive care.
“If you have a patient with a worsening reaction, consider it may represent the type 1 IgE-related hypersensitivity requiring desensitization to continue that systemic,” she noted.
Cutaneous bacterial, fungal, and viral infections such as molluscum contagiosum, verruca vulgaris, herpes simplex, and varicella zoster can occur as a result of TNF inhibition as well, and can be difficult to clear because of immunosuppression, she added.
Dr. Delano reported no relevant conflicts of interest.
WASHINGTON – (IBD), Sophia Delano, MD, said during a session on the cutaneous effects of IBD at the annual meeting of the American Academy of Dermatology.
This is a paradoxical reaction, which can happen “weeks to years after starting a TNF blocker,” with about 70% of cases occurring during the first year of therapy, said Dr. Delano, an attending physician in the dermatology program at Boston Children’s Hospital.
Those receiving infliximab are more likely to develop TNF inhibitor–induced psoriasis, compared with those on adalimumab or etanercept. TNF inhibitor–induced psoriasis may not track with gastrointestinal activity, and some patients whose gastrointestinal disease is responding to treatment can begin to develop psoriasis, she noted.
The clinical presentation of TNF inhibitor–induced psoriasis can also vary. In one study of 216 cases, 26.9% of patients had a mixed morphology, with the most common presentations including plaque psoriasis (44.8%) and palmoplantar pustular psoriasis (36.3%). Other presentations were psoriasiform dermatitis (19.9%), scalp involvement with alopecia (7.5%), and generalized pustular psoriasis (10.9%). Locations affected were the soles of the feet (45.8%), extremities (45.4%), palms (44.9%), scalp (36.1%), and trunk (32.4%), Dr. Delano said.
TNF inhibitor–induced psoriasis is likely a class effect, she said, noting that, in the same review, symptoms resolved in 47.7% of patients who discontinued TNF inhibitors, in 36.7% of patients who switched to another TNF inhibitor, and in 32.9% of patients who continued their original therapy (J Am Acad Dermatol. 2017 Feb;76[2]:334-41). In the study, Crohn’s disease and RA were the most common diseases, in 40.7% and 37.0% of the patients, respectively.
There have been case reports of TNF antagonist–induced lupus-like syndrome (TAILS), which is more common in patients with RA and ulcerative colitis. TAILS occurs more often in women than in men; can present similarly to systemic lupus erythematosus, subacute cutaneous lupus erythematosus, and chronic cutaneous lupus; and resolves by stopping TNF inhibitor treatment, Dr. Delano said.
Skin cancer risk, infections, and injection site reactions
Both adult and pediatric patients treated with TNF inhibitors for IBD may be at increased risk for lymphoma, visceral tumors, melanoma, and nonmelanoma skin cancers. Dr. Delano referred to a study published in 2014, which identified 972 reports of melanoma in the Food and Drug Administration’s Adverse Event Reporting System database associated with TNF inhibitor use; of these, 69 cases involved patients using more than one TNF inhibitor. Infliximab, golimumab, etanercept, and adalimumab were associated with a safety signal for melanoma, but not certolizumab (Br J Dermatol. 2014 May;170[5]:1170-2).
Dr. Delano observed that thiopurines such as azathioprine are also associated with an increased cancer risk, as noted in one retrospective study that found that the risk of nonmelanoma skin cancer was 2.1 times higher in a mostly white male cohort with ulcerative colitis during treatment with thiopurines, compared with patients not treated with thiopurines (Am J Gastroenterol. 2014 Nov;109[11]:1781-93). A greater duration of treatment (more than 6 months) and higher doses were associated with higher risks.
Adalimumab, golimumab, and certolizumab can also cause injection site reactions, typically within 1- 2 days of injection, said Dr. Delano. In these cases, symptoms of erythema, warmth, burning, or pruritus are worse at the beginning of treatment and can be relieved by rotating the injection site as well as providing cool compresses, topical steroids, antihistamines, and supportive care.
“If you have a patient with a worsening reaction, consider it may represent the type 1 IgE-related hypersensitivity requiring desensitization to continue that systemic,” she noted.
Cutaneous bacterial, fungal, and viral infections such as molluscum contagiosum, verruca vulgaris, herpes simplex, and varicella zoster can occur as a result of TNF inhibition as well, and can be difficult to clear because of immunosuppression, she added.
Dr. Delano reported no relevant conflicts of interest.
WASHINGTON – (IBD), Sophia Delano, MD, said during a session on the cutaneous effects of IBD at the annual meeting of the American Academy of Dermatology.
This is a paradoxical reaction, which can happen “weeks to years after starting a TNF blocker,” with about 70% of cases occurring during the first year of therapy, said Dr. Delano, an attending physician in the dermatology program at Boston Children’s Hospital.
Those receiving infliximab are more likely to develop TNF inhibitor–induced psoriasis, compared with those on adalimumab or etanercept. TNF inhibitor–induced psoriasis may not track with gastrointestinal activity, and some patients whose gastrointestinal disease is responding to treatment can begin to develop psoriasis, she noted.
The clinical presentation of TNF inhibitor–induced psoriasis can also vary. In one study of 216 cases, 26.9% of patients had a mixed morphology, with the most common presentations including plaque psoriasis (44.8%) and palmoplantar pustular psoriasis (36.3%). Other presentations were psoriasiform dermatitis (19.9%), scalp involvement with alopecia (7.5%), and generalized pustular psoriasis (10.9%). Locations affected were the soles of the feet (45.8%), extremities (45.4%), palms (44.9%), scalp (36.1%), and trunk (32.4%), Dr. Delano said.
TNF inhibitor–induced psoriasis is likely a class effect, she said, noting that, in the same review, symptoms resolved in 47.7% of patients who discontinued TNF inhibitors, in 36.7% of patients who switched to another TNF inhibitor, and in 32.9% of patients who continued their original therapy (J Am Acad Dermatol. 2017 Feb;76[2]:334-41). In the study, Crohn’s disease and RA were the most common diseases, in 40.7% and 37.0% of the patients, respectively.
There have been case reports of TNF antagonist–induced lupus-like syndrome (TAILS), which is more common in patients with RA and ulcerative colitis. TAILS occurs more often in women than in men; can present similarly to systemic lupus erythematosus, subacute cutaneous lupus erythematosus, and chronic cutaneous lupus; and resolves by stopping TNF inhibitor treatment, Dr. Delano said.
Skin cancer risk, infections, and injection site reactions
Both adult and pediatric patients treated with TNF inhibitors for IBD may be at increased risk for lymphoma, visceral tumors, melanoma, and nonmelanoma skin cancers. Dr. Delano referred to a study published in 2014, which identified 972 reports of melanoma in the Food and Drug Administration’s Adverse Event Reporting System database associated with TNF inhibitor use; of these, 69 cases involved patients using more than one TNF inhibitor. Infliximab, golimumab, etanercept, and adalimumab were associated with a safety signal for melanoma, but not certolizumab (Br J Dermatol. 2014 May;170[5]:1170-2).
Dr. Delano observed that thiopurines such as azathioprine are also associated with an increased cancer risk, as noted in one retrospective study that found that the risk of nonmelanoma skin cancer was 2.1 times higher in a mostly white male cohort with ulcerative colitis during treatment with thiopurines, compared with patients not treated with thiopurines (Am J Gastroenterol. 2014 Nov;109[11]:1781-93). A greater duration of treatment (more than 6 months) and higher doses were associated with higher risks.
Adalimumab, golimumab, and certolizumab can also cause injection site reactions, typically within 1- 2 days of injection, said Dr. Delano. In these cases, symptoms of erythema, warmth, burning, or pruritus are worse at the beginning of treatment and can be relieved by rotating the injection site as well as providing cool compresses, topical steroids, antihistamines, and supportive care.
“If you have a patient with a worsening reaction, consider it may represent the type 1 IgE-related hypersensitivity requiring desensitization to continue that systemic,” she noted.
Cutaneous bacterial, fungal, and viral infections such as molluscum contagiosum, verruca vulgaris, herpes simplex, and varicella zoster can occur as a result of TNF inhibition as well, and can be difficult to clear because of immunosuppression, she added.
Dr. Delano reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM AAD 2019
Herpes zoster risk increased with some psoriasis, psoriatic arthritis treatments
All individuals with psoriasis or psoriatic arthritis aged over 50 years should receive the recombinant herpes zoster vaccine, according to a systematic review and consensus recommendations from the National Psoriasis Foundation.
Emily Baumrin, MD, of Brigham and Women’s Hospital, Boston, and her coauthors reviewed 41 studies of herpes zoster in people with psoriasis or psoriatic arthritis according to treatment modality. Their report is in the Journal of the American Academy of Dermatology.
Overall, psoriasis was associated with an increased rate of herpes zoster when compared with the general population: 13.3 cases per 1,000 patient-years for psoriasis and 15.9 for psoriatic arthritis, compared with 8.5 in healthy controls after adjustment for age, sex, and systemic medications. Most of this increased incidence was seen in patients with more severe disease: Those with mild disease who were not receiving systemic therapy had a risk similar to that of healthy controls.
However, one study suggested much of the increased risk of herpes zoster in psoriasis was accounted for by immunosuppressive therapy; when those patients were excluded, there was an 8% increase in risk.
The authors found that people whose psoriasis was treated with tofacitinib (Xeljanz) had a two- to threefold increased risk of herpes zoster, compared with those treated with tumor necrosis factor (TNF) inhibitors or conventional synthetic disease-modifying antirheumatic drugs (DMARDs).
Corticosteroids – either alone or in combination with DMARDs – were also associated with significant increases in the risk of herpes zoster. Patients treated with TNF inhibitor monotherapy had a risk of herpes zoster similar to that of those treated with conventional synthetic DMARDs or no synthetic therapy.
On the question of immunization, the authors pointed to guidelines recommending use of the live attenuated zoster vaccine (Zostavax) in immunocompetent patients or those on low-dose immunosuppression, although they noted that the vaccine is currently contraindicated for patients on biologic DMARDs.
They also examined the evidence for the use of the recently-released non-live recombinant herpes zoster vaccine (Shingrix) in immunocompromised patients, which found no evidence of vaccine-related serious adverse events in individuals with HIV and low CD4 cell counts and in autologous hematopoietic stem cell transplant recipients.
Given this, they recommended that the recombinant vaccine be administered to all patients aged over 50 years with psoriasis or psoriatic arthritis, and to those aged under 50 years who were being treated with tofacitinib, systemic corticosteroids, or combination systemic therapy.
There were insufficient data to draw conclusions about the impact of treatment with the interleukin-12/23 blocker ustekinumab (Stelara) on herpes zoster risk, but the authors noted that there was a trend toward an increased risk. They found no increase in the risk of herpes zoster with interleukin-17 inhibitors (ixekizumab [Taltz], secukinumab [Cosentyx], and brodalumab [Siliq]) and interleukin-23 (p19 subunit) inhibitors (guselkumab [Tremfya], tildrakizumab [Ilumya], and risankizumab) but noted an absence of long-term safety data for these drugs.
Four authors declared advisory, consultancy, or speaker positions with the pharmaceutical sector.
SOURCE: Baumrin E et al. J Am Acad Dermatol. 2019 March 15. doi: 10.1016/j.jaad.2019.03.017.
All individuals with psoriasis or psoriatic arthritis aged over 50 years should receive the recombinant herpes zoster vaccine, according to a systematic review and consensus recommendations from the National Psoriasis Foundation.
Emily Baumrin, MD, of Brigham and Women’s Hospital, Boston, and her coauthors reviewed 41 studies of herpes zoster in people with psoriasis or psoriatic arthritis according to treatment modality. Their report is in the Journal of the American Academy of Dermatology.
Overall, psoriasis was associated with an increased rate of herpes zoster when compared with the general population: 13.3 cases per 1,000 patient-years for psoriasis and 15.9 for psoriatic arthritis, compared with 8.5 in healthy controls after adjustment for age, sex, and systemic medications. Most of this increased incidence was seen in patients with more severe disease: Those with mild disease who were not receiving systemic therapy had a risk similar to that of healthy controls.
However, one study suggested much of the increased risk of herpes zoster in psoriasis was accounted for by immunosuppressive therapy; when those patients were excluded, there was an 8% increase in risk.
The authors found that people whose psoriasis was treated with tofacitinib (Xeljanz) had a two- to threefold increased risk of herpes zoster, compared with those treated with tumor necrosis factor (TNF) inhibitors or conventional synthetic disease-modifying antirheumatic drugs (DMARDs).
Corticosteroids – either alone or in combination with DMARDs – were also associated with significant increases in the risk of herpes zoster. Patients treated with TNF inhibitor monotherapy had a risk of herpes zoster similar to that of those treated with conventional synthetic DMARDs or no synthetic therapy.
On the question of immunization, the authors pointed to guidelines recommending use of the live attenuated zoster vaccine (Zostavax) in immunocompetent patients or those on low-dose immunosuppression, although they noted that the vaccine is currently contraindicated for patients on biologic DMARDs.
They also examined the evidence for the use of the recently-released non-live recombinant herpes zoster vaccine (Shingrix) in immunocompromised patients, which found no evidence of vaccine-related serious adverse events in individuals with HIV and low CD4 cell counts and in autologous hematopoietic stem cell transplant recipients.
Given this, they recommended that the recombinant vaccine be administered to all patients aged over 50 years with psoriasis or psoriatic arthritis, and to those aged under 50 years who were being treated with tofacitinib, systemic corticosteroids, or combination systemic therapy.
There were insufficient data to draw conclusions about the impact of treatment with the interleukin-12/23 blocker ustekinumab (Stelara) on herpes zoster risk, but the authors noted that there was a trend toward an increased risk. They found no increase in the risk of herpes zoster with interleukin-17 inhibitors (ixekizumab [Taltz], secukinumab [Cosentyx], and brodalumab [Siliq]) and interleukin-23 (p19 subunit) inhibitors (guselkumab [Tremfya], tildrakizumab [Ilumya], and risankizumab) but noted an absence of long-term safety data for these drugs.
Four authors declared advisory, consultancy, or speaker positions with the pharmaceutical sector.
SOURCE: Baumrin E et al. J Am Acad Dermatol. 2019 March 15. doi: 10.1016/j.jaad.2019.03.017.
All individuals with psoriasis or psoriatic arthritis aged over 50 years should receive the recombinant herpes zoster vaccine, according to a systematic review and consensus recommendations from the National Psoriasis Foundation.
Emily Baumrin, MD, of Brigham and Women’s Hospital, Boston, and her coauthors reviewed 41 studies of herpes zoster in people with psoriasis or psoriatic arthritis according to treatment modality. Their report is in the Journal of the American Academy of Dermatology.
Overall, psoriasis was associated with an increased rate of herpes zoster when compared with the general population: 13.3 cases per 1,000 patient-years for psoriasis and 15.9 for psoriatic arthritis, compared with 8.5 in healthy controls after adjustment for age, sex, and systemic medications. Most of this increased incidence was seen in patients with more severe disease: Those with mild disease who were not receiving systemic therapy had a risk similar to that of healthy controls.
However, one study suggested much of the increased risk of herpes zoster in psoriasis was accounted for by immunosuppressive therapy; when those patients were excluded, there was an 8% increase in risk.
The authors found that people whose psoriasis was treated with tofacitinib (Xeljanz) had a two- to threefold increased risk of herpes zoster, compared with those treated with tumor necrosis factor (TNF) inhibitors or conventional synthetic disease-modifying antirheumatic drugs (DMARDs).
Corticosteroids – either alone or in combination with DMARDs – were also associated with significant increases in the risk of herpes zoster. Patients treated with TNF inhibitor monotherapy had a risk of herpes zoster similar to that of those treated with conventional synthetic DMARDs or no synthetic therapy.
On the question of immunization, the authors pointed to guidelines recommending use of the live attenuated zoster vaccine (Zostavax) in immunocompetent patients or those on low-dose immunosuppression, although they noted that the vaccine is currently contraindicated for patients on biologic DMARDs.
They also examined the evidence for the use of the recently-released non-live recombinant herpes zoster vaccine (Shingrix) in immunocompromised patients, which found no evidence of vaccine-related serious adverse events in individuals with HIV and low CD4 cell counts and in autologous hematopoietic stem cell transplant recipients.
Given this, they recommended that the recombinant vaccine be administered to all patients aged over 50 years with psoriasis or psoriatic arthritis, and to those aged under 50 years who were being treated with tofacitinib, systemic corticosteroids, or combination systemic therapy.
There were insufficient data to draw conclusions about the impact of treatment with the interleukin-12/23 blocker ustekinumab (Stelara) on herpes zoster risk, but the authors noted that there was a trend toward an increased risk. They found no increase in the risk of herpes zoster with interleukin-17 inhibitors (ixekizumab [Taltz], secukinumab [Cosentyx], and brodalumab [Siliq]) and interleukin-23 (p19 subunit) inhibitors (guselkumab [Tremfya], tildrakizumab [Ilumya], and risankizumab) but noted an absence of long-term safety data for these drugs.
Four authors declared advisory, consultancy, or speaker positions with the pharmaceutical sector.
SOURCE: Baumrin E et al. J Am Acad Dermatol. 2019 March 15. doi: 10.1016/j.jaad.2019.03.017.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Immunomodulators for pediatric skin diseases
WASHINGTON – At the annual meeting of the American Academy of Dermatology, colleagues A. Yasmine Kirkorian, MD, a pediatric dermatologist at George Washington University, Washington, and interim chief of pediatric dermatology at Children’s National Health System, and Adam Friedman, MD, professor and interim chair of dermatology at the university, sat down with Dermatology News and discussed their presentations at a session on the use of immunomodulators for inflammatory and neoplastic skin diseases.
In this video, , with her clinical pearls and practical considerations for treating atopic dermatitis, psoriasis, and hidradenitis suppurativa in pediatric patients, covering both on- and off-label treatments.
“Children sometimes require systemic treatment and we shouldn’t hold it back from them because of their age; if they’re severely ill ... they need to be treated,” she said, summing up one of her main points.
During the interview immediately after the AAD meeting, she mentioned dupilumab, which was approved by the Food and Drug Administration for treatment of moderate to severe AD in patients aged 12-17 years.
Dr. Friedman and Dr. Kirkorian reported having no financial disclosures.
WASHINGTON – At the annual meeting of the American Academy of Dermatology, colleagues A. Yasmine Kirkorian, MD, a pediatric dermatologist at George Washington University, Washington, and interim chief of pediatric dermatology at Children’s National Health System, and Adam Friedman, MD, professor and interim chair of dermatology at the university, sat down with Dermatology News and discussed their presentations at a session on the use of immunomodulators for inflammatory and neoplastic skin diseases.
In this video, , with her clinical pearls and practical considerations for treating atopic dermatitis, psoriasis, and hidradenitis suppurativa in pediatric patients, covering both on- and off-label treatments.
“Children sometimes require systemic treatment and we shouldn’t hold it back from them because of their age; if they’re severely ill ... they need to be treated,” she said, summing up one of her main points.
During the interview immediately after the AAD meeting, she mentioned dupilumab, which was approved by the Food and Drug Administration for treatment of moderate to severe AD in patients aged 12-17 years.
Dr. Friedman and Dr. Kirkorian reported having no financial disclosures.
WASHINGTON – At the annual meeting of the American Academy of Dermatology, colleagues A. Yasmine Kirkorian, MD, a pediatric dermatologist at George Washington University, Washington, and interim chief of pediatric dermatology at Children’s National Health System, and Adam Friedman, MD, professor and interim chair of dermatology at the university, sat down with Dermatology News and discussed their presentations at a session on the use of immunomodulators for inflammatory and neoplastic skin diseases.
In this video, , with her clinical pearls and practical considerations for treating atopic dermatitis, psoriasis, and hidradenitis suppurativa in pediatric patients, covering both on- and off-label treatments.
“Children sometimes require systemic treatment and we shouldn’t hold it back from them because of their age; if they’re severely ill ... they need to be treated,” she said, summing up one of her main points.
During the interview immediately after the AAD meeting, she mentioned dupilumab, which was approved by the Food and Drug Administration for treatment of moderate to severe AD in patients aged 12-17 years.
Dr. Friedman and Dr. Kirkorian reported having no financial disclosures.
FDA approves patient-controlled injector for guselkumab
The Food and Drug Administration has in adults, the manufacturer announced.
FDA approval is based on results from the phase 3, multicenter, randomized ORION trial, according to a press release issued by Janssen. In a Self-Injection Assessment Questionnaire, patients who received the One-Press injection rated their satisfaction with self-injection a mean score of 9.18 (0 indicated least satisfaction, 10 indicated highest satisfaction) and rated ease of use at 9.24 (10 indicated “very easy”).
In addition, 81% of patients who received One-Press achieved a Investigator’s Global Assessment score of 0 or 1, and 76% achieved a Psoriasis Area Severity Index (PASI) 90 response after 16 weeks; no patients who received the placebo achieved either.
The most common adverse event during the ORION study was injection-site reaction; the most common adverse events associated with guselkumab, an interleukin-23 blocker, include upper respiratory infections, headache, injection-site reactions, joint pain, diarrhea, gastroenteritis, fungal skin infections, and herpes simplex infections.
The Food and Drug Administration has in adults, the manufacturer announced.
FDA approval is based on results from the phase 3, multicenter, randomized ORION trial, according to a press release issued by Janssen. In a Self-Injection Assessment Questionnaire, patients who received the One-Press injection rated their satisfaction with self-injection a mean score of 9.18 (0 indicated least satisfaction, 10 indicated highest satisfaction) and rated ease of use at 9.24 (10 indicated “very easy”).
In addition, 81% of patients who received One-Press achieved a Investigator’s Global Assessment score of 0 or 1, and 76% achieved a Psoriasis Area Severity Index (PASI) 90 response after 16 weeks; no patients who received the placebo achieved either.
The most common adverse event during the ORION study was injection-site reaction; the most common adverse events associated with guselkumab, an interleukin-23 blocker, include upper respiratory infections, headache, injection-site reactions, joint pain, diarrhea, gastroenteritis, fungal skin infections, and herpes simplex infections.
The Food and Drug Administration has in adults, the manufacturer announced.
FDA approval is based on results from the phase 3, multicenter, randomized ORION trial, according to a press release issued by Janssen. In a Self-Injection Assessment Questionnaire, patients who received the One-Press injection rated their satisfaction with self-injection a mean score of 9.18 (0 indicated least satisfaction, 10 indicated highest satisfaction) and rated ease of use at 9.24 (10 indicated “very easy”).
In addition, 81% of patients who received One-Press achieved a Investigator’s Global Assessment score of 0 or 1, and 76% achieved a Psoriasis Area Severity Index (PASI) 90 response after 16 weeks; no patients who received the placebo achieved either.
The most common adverse event during the ORION study was injection-site reaction; the most common adverse events associated with guselkumab, an interleukin-23 blocker, include upper respiratory infections, headache, injection-site reactions, joint pain, diarrhea, gastroenteritis, fungal skin infections, and herpes simplex infections.
Ixekizumab psoriasis outcomes, sliced and diced
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
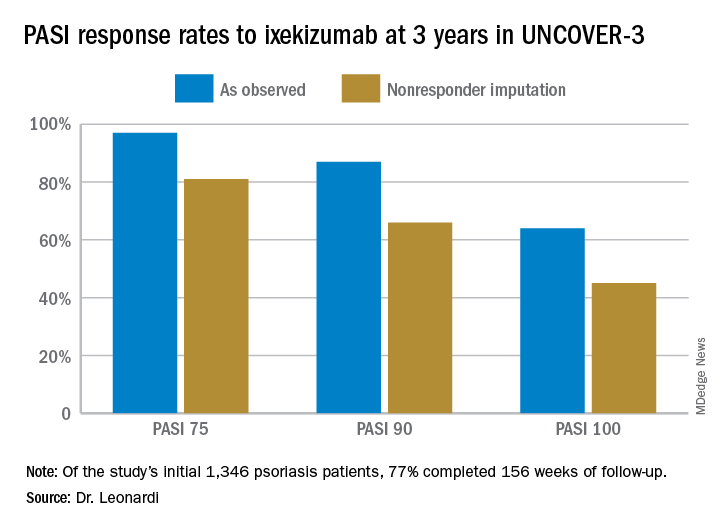
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.

However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.

However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR