User login
Treatment Consideration for US Military Members With Skin Disease
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
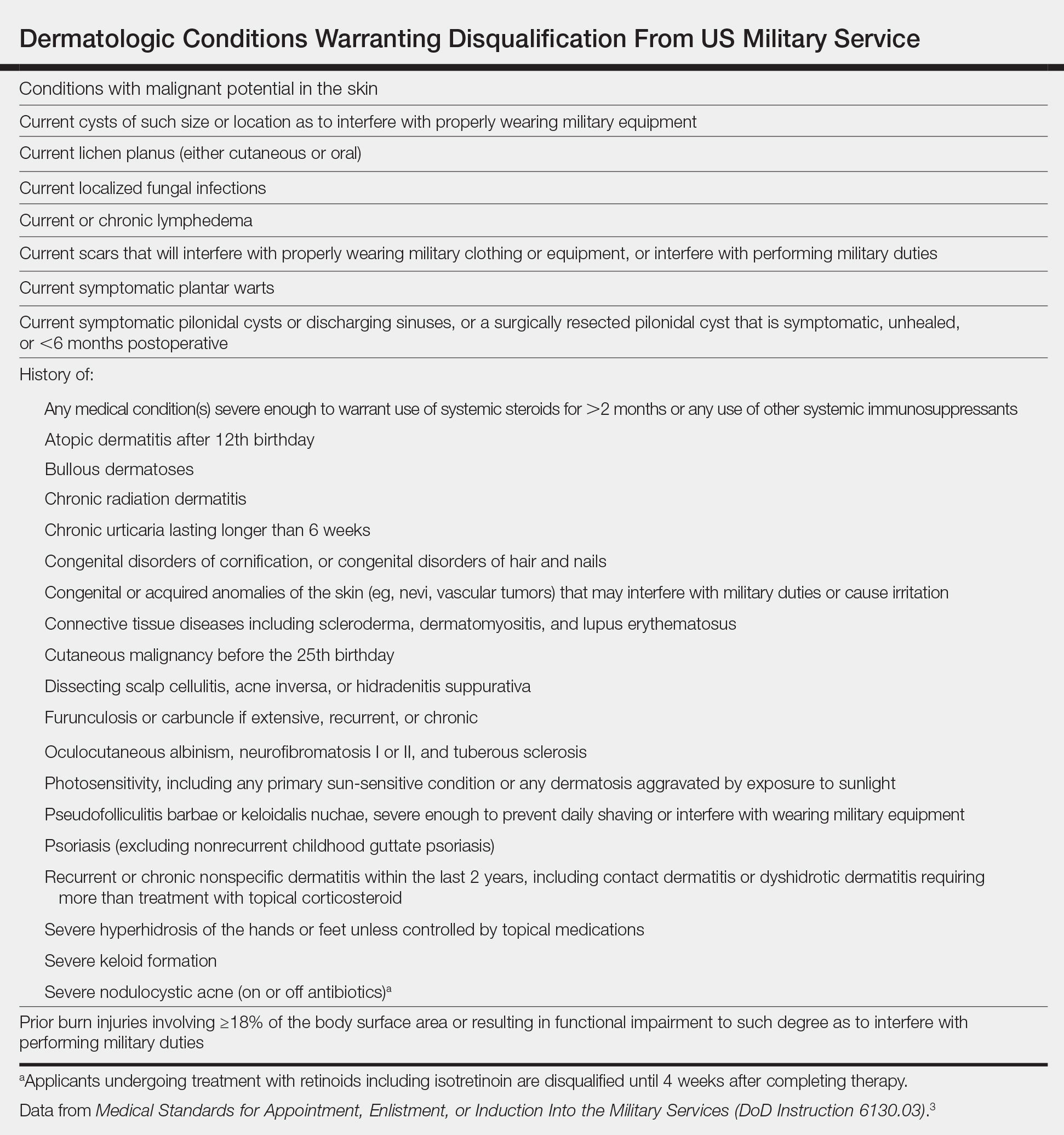
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4
Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4

Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4

Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
Practice Points
- Certain conditions and treatments are incompatible with military service and may result in separation.
- Dermatologists must consider a patient’s profession when choosing a treatment modality.
Carotid ultrasound may aid cardiovascular risk stratification of patients with psoriatic disease
according to findings from a retrospective study.
When added to the Framingham risk score, the measurement significantly improved its predictive ability, Curtis Sobchak, MD, and colleagues wrote in Arthritis & Rheumatology.
The findings indicate that carotid ultrasound could be a useful addition to cardiovascular risk stratification among these patients.
“Traditional algorithms do not consider other factors that may contribute to increased cardiovascular risk in rheumatic disease patients and tend to underestimate cardiovascular risk,” wrote Dr. Sobchak of the University of Toronto and coauthors.
“The advantage of ultrasound over other modalities for vascular imaging includes lack of radiation, low cost of the examination, and its widespread use in rheumatology for joint evaluation. Thus, this assessment could potentially be performed ‘at the bedside’ during consultation to provide immediate valuable information to complement clinical data from history, physical examination, and laboratory data,” they added.
The study retrospectively examined a prospective, observational cohort of 559 patients with psoriasis alone or psoriasis and psoriatic arthritis enrolled in the University of Toronto Psoriatic Disease Program. The investigators evaluated five ultrasound measures of atherosclerosis, including total plaque area (TPA), mean carotid intima-media thickness (cIMT), maximal cIMT, plaque category, and TPA category. Then they analyzed the risk relationship with major cardiovascular events (CVEs) classified as myocardial infarction, unstable angina, ischemic stroke, revascularization procedures, or cardiovascular-related death. Minor CVEs included stable angina, exacerbation of congestive heart failure, and transient ischemic attack over a mean follow-up close to 4 years.
The mean baseline TPA was 0.18 cm2 and mean cIMT was 639 mcm. Most patients had plaques, including 27.0% with unilateral and 31.5% with bilateral plaques.
The rate of a first CVE during the study period was 1.11 per 100 patient-years, and the rate of a first major CVE was 0.91 per 100 patient-years. The risk of each was significantly related to a higher baseline burden of atherosclerosis.
A multivariate analysis determined that increased TPA at baseline increased the risk of an event by nearly 200% (hazard ratio, 2.85). Mean cIMT was not an independent predictor in the final analysis, “suggesting that TPA is a stronger predictor for CVE than cIMT,” the authors wrote.
Finally, they examined the predictive value of atherosclerosis alone, as well as combined with the Framingham risk score. The 5-year model indicated that the bivariate model was slightly more accurate than the Framingham score alone (area under the curve, 0.84 vs. 0.81), although this was not a significant difference. The predictive value of the Framingham risk score plus maximal cIMT, mean cIMT, or TPA all significantly improved when they were calculated using only high-risk patients (those above the treatment threshold for dyslipidemia).
“To the best of our knowledge this is the first study to assess the utility of various measures of carotid atherosclerosis to predict CVE in patients with psoriasis and PsA [psoriatic arthritis]. ... Combining vascular imaging data with clinical and laboratory measures of traditional cardiovascular risk factors could improve accuracy of cardiovascular risk stratification in patients with psoriatic disease and facilitate earlier initiation of appropriate treatment to reduce CVE in this population,” the investigators wrote.
The study was supported in part by a Young Investigator Operating Grant from the Arthritis Society. Dr. Sobchak had no financial disclosures.
SOURCE: Sobchak C et al. Arthritis Rheumatol. 2019 Jun 5. doi: 10.1002/art.40925.
according to findings from a retrospective study.
When added to the Framingham risk score, the measurement significantly improved its predictive ability, Curtis Sobchak, MD, and colleagues wrote in Arthritis & Rheumatology.
The findings indicate that carotid ultrasound could be a useful addition to cardiovascular risk stratification among these patients.
“Traditional algorithms do not consider other factors that may contribute to increased cardiovascular risk in rheumatic disease patients and tend to underestimate cardiovascular risk,” wrote Dr. Sobchak of the University of Toronto and coauthors.
“The advantage of ultrasound over other modalities for vascular imaging includes lack of radiation, low cost of the examination, and its widespread use in rheumatology for joint evaluation. Thus, this assessment could potentially be performed ‘at the bedside’ during consultation to provide immediate valuable information to complement clinical data from history, physical examination, and laboratory data,” they added.
The study retrospectively examined a prospective, observational cohort of 559 patients with psoriasis alone or psoriasis and psoriatic arthritis enrolled in the University of Toronto Psoriatic Disease Program. The investigators evaluated five ultrasound measures of atherosclerosis, including total plaque area (TPA), mean carotid intima-media thickness (cIMT), maximal cIMT, plaque category, and TPA category. Then they analyzed the risk relationship with major cardiovascular events (CVEs) classified as myocardial infarction, unstable angina, ischemic stroke, revascularization procedures, or cardiovascular-related death. Minor CVEs included stable angina, exacerbation of congestive heart failure, and transient ischemic attack over a mean follow-up close to 4 years.
The mean baseline TPA was 0.18 cm2 and mean cIMT was 639 mcm. Most patients had plaques, including 27.0% with unilateral and 31.5% with bilateral plaques.
The rate of a first CVE during the study period was 1.11 per 100 patient-years, and the rate of a first major CVE was 0.91 per 100 patient-years. The risk of each was significantly related to a higher baseline burden of atherosclerosis.
A multivariate analysis determined that increased TPA at baseline increased the risk of an event by nearly 200% (hazard ratio, 2.85). Mean cIMT was not an independent predictor in the final analysis, “suggesting that TPA is a stronger predictor for CVE than cIMT,” the authors wrote.
Finally, they examined the predictive value of atherosclerosis alone, as well as combined with the Framingham risk score. The 5-year model indicated that the bivariate model was slightly more accurate than the Framingham score alone (area under the curve, 0.84 vs. 0.81), although this was not a significant difference. The predictive value of the Framingham risk score plus maximal cIMT, mean cIMT, or TPA all significantly improved when they were calculated using only high-risk patients (those above the treatment threshold for dyslipidemia).
“To the best of our knowledge this is the first study to assess the utility of various measures of carotid atherosclerosis to predict CVE in patients with psoriasis and PsA [psoriatic arthritis]. ... Combining vascular imaging data with clinical and laboratory measures of traditional cardiovascular risk factors could improve accuracy of cardiovascular risk stratification in patients with psoriatic disease and facilitate earlier initiation of appropriate treatment to reduce CVE in this population,” the investigators wrote.
The study was supported in part by a Young Investigator Operating Grant from the Arthritis Society. Dr. Sobchak had no financial disclosures.
SOURCE: Sobchak C et al. Arthritis Rheumatol. 2019 Jun 5. doi: 10.1002/art.40925.
according to findings from a retrospective study.
When added to the Framingham risk score, the measurement significantly improved its predictive ability, Curtis Sobchak, MD, and colleagues wrote in Arthritis & Rheumatology.
The findings indicate that carotid ultrasound could be a useful addition to cardiovascular risk stratification among these patients.
“Traditional algorithms do not consider other factors that may contribute to increased cardiovascular risk in rheumatic disease patients and tend to underestimate cardiovascular risk,” wrote Dr. Sobchak of the University of Toronto and coauthors.
“The advantage of ultrasound over other modalities for vascular imaging includes lack of radiation, low cost of the examination, and its widespread use in rheumatology for joint evaluation. Thus, this assessment could potentially be performed ‘at the bedside’ during consultation to provide immediate valuable information to complement clinical data from history, physical examination, and laboratory data,” they added.
The study retrospectively examined a prospective, observational cohort of 559 patients with psoriasis alone or psoriasis and psoriatic arthritis enrolled in the University of Toronto Psoriatic Disease Program. The investigators evaluated five ultrasound measures of atherosclerosis, including total plaque area (TPA), mean carotid intima-media thickness (cIMT), maximal cIMT, plaque category, and TPA category. Then they analyzed the risk relationship with major cardiovascular events (CVEs) classified as myocardial infarction, unstable angina, ischemic stroke, revascularization procedures, or cardiovascular-related death. Minor CVEs included stable angina, exacerbation of congestive heart failure, and transient ischemic attack over a mean follow-up close to 4 years.
The mean baseline TPA was 0.18 cm2 and mean cIMT was 639 mcm. Most patients had plaques, including 27.0% with unilateral and 31.5% with bilateral plaques.
The rate of a first CVE during the study period was 1.11 per 100 patient-years, and the rate of a first major CVE was 0.91 per 100 patient-years. The risk of each was significantly related to a higher baseline burden of atherosclerosis.
A multivariate analysis determined that increased TPA at baseline increased the risk of an event by nearly 200% (hazard ratio, 2.85). Mean cIMT was not an independent predictor in the final analysis, “suggesting that TPA is a stronger predictor for CVE than cIMT,” the authors wrote.
Finally, they examined the predictive value of atherosclerosis alone, as well as combined with the Framingham risk score. The 5-year model indicated that the bivariate model was slightly more accurate than the Framingham score alone (area under the curve, 0.84 vs. 0.81), although this was not a significant difference. The predictive value of the Framingham risk score plus maximal cIMT, mean cIMT, or TPA all significantly improved when they were calculated using only high-risk patients (those above the treatment threshold for dyslipidemia).
“To the best of our knowledge this is the first study to assess the utility of various measures of carotid atherosclerosis to predict CVE in patients with psoriasis and PsA [psoriatic arthritis]. ... Combining vascular imaging data with clinical and laboratory measures of traditional cardiovascular risk factors could improve accuracy of cardiovascular risk stratification in patients with psoriatic disease and facilitate earlier initiation of appropriate treatment to reduce CVE in this population,” the investigators wrote.
The study was supported in part by a Young Investigator Operating Grant from the Arthritis Society. Dr. Sobchak had no financial disclosures.
SOURCE: Sobchak C et al. Arthritis Rheumatol. 2019 Jun 5. doi: 10.1002/art.40925.
FROM ARTHRITIS & RHEUMATOLOGY
VIDEO: Did You Know? Psoriasis and mental health
The American Academy of Dermatology and the National Psoriasis Foundation recently issued a joint guideline on the management and treatment of psoriasis, with a focus on comorbidities. The guideline offers information and recommendations on mental health in patients with psoriasis.

The American Academy of Dermatology and the National Psoriasis Foundation recently issued a joint guideline on the management and treatment of psoriasis, with a focus on comorbidities. The guideline offers information and recommendations on mental health in patients with psoriasis.

The American Academy of Dermatology and the National Psoriasis Foundation recently issued a joint guideline on the management and treatment of psoriasis, with a focus on comorbidities. The guideline offers information and recommendations on mental health in patients with psoriasis.

Psoriasis Journal Scan: May 2019
The Broad-Spectrum Impact of Hidradenitis Suppurativa on Quality of Life: A Comparison with Psoriasis.
Sampogna F, Fania L, Mazzanti C, et al. Dermatology. 2019 May 23:1-7.
The aim of this study was to evaluate in detail the QoL impact of HS comparing it with other skin conditions, and in particular with psoriasis. HS had the worst QoL among several skin conditions. Compared to psoriasis the mean symptom score was 69.4 versus 53.7, and the mean psychosocial score was 56.1 versus 32.7. Overall, the scores of patients with HS were higher than those of psoriasis patients on 16 of the 17 items of the Skindex-17.
Suicidality and risk of suicidality in psoriasis: A critical appraisal of two systematic reviews and meta-analyses.
Matterne U, Baumeister SE, Apfelbacher C. Br J Dermatol. 2019 May 10.
Chi et al. and Singh et al each conducted a systematic review and meta-analysis of observational studies examining the relationship between suicidality and psoriasis. Singh et al. concluded that patients with psoriasis have a significantly higher risk of suicidal ideation, suicide attempts, and completed suicides, while Chi et al concluded that the available limited, very low-quality evidence does not support the notion of an association between psoriasis on the one hand, and suicide, suicidal ideation and attempts on the other.
Psoriasis and Inflammatory Bowel Disease.
Cottone M, Sapienza C, Macaluso FS, Cannizzaro M. Dig Dis. 2019 May 10:1-7.
Inflammatory bowel disease (IBD) and psoriasis (PS) are associated conditions. The reason for this association lies in the sharing of predisposition genes and common immunological mechanisms. This review will focus on the interplay between IBD and PS, with details on prevalence and phenotype of PS in IBD, genetics, pathogenetic pathways, and therapy.
Psoriasis in HIV infection: an update.
Alpalhão M, Borges-Costa J, Filipe P. Int J STD AIDS. 2019 May;30(6):596-604.
A review of the available literature to highlight the updated evidence on psoriasis in HIV-infected individuals, particularly in regards to its epidemiology, proposed pathophysiology, clinical presentation, currently available therapeutic options, and future perspectives.
All-cause and cause-specific mortality in psoriasis: A systematic review and meta-analysis.
Dhana A, Yen H, Yen H, Cho E. J Am Acad Dermatol. 2019 May;80(5):1332-1343.
A systematic review and meta-analysis of mortality risk in psoriasis that included studies reporting all-cause or cause-specific mortality risk estimates in psoriasis patients compared with general population or subjects free of psoriasis. The pooled RRs for cardiovascular mortality were 1.15 (95% CI 1.09-1.21) in psoriasis, 1.05 (95% CI 0.92-1.20) in mild psoriasis, and 1.38 (95% CI 1.09-1.74) in severe psoriasis. For noncardiovascular causes, mortality risk from liver disease, kidney disease, and infection was significantly increased in psoriasis, regardless of disease severity.
The Broad-Spectrum Impact of Hidradenitis Suppurativa on Quality of Life: A Comparison with Psoriasis.
Sampogna F, Fania L, Mazzanti C, et al. Dermatology. 2019 May 23:1-7.
The aim of this study was to evaluate in detail the QoL impact of HS comparing it with other skin conditions, and in particular with psoriasis. HS had the worst QoL among several skin conditions. Compared to psoriasis the mean symptom score was 69.4 versus 53.7, and the mean psychosocial score was 56.1 versus 32.7. Overall, the scores of patients with HS were higher than those of psoriasis patients on 16 of the 17 items of the Skindex-17.
Suicidality and risk of suicidality in psoriasis: A critical appraisal of two systematic reviews and meta-analyses.
Matterne U, Baumeister SE, Apfelbacher C. Br J Dermatol. 2019 May 10.
Chi et al. and Singh et al each conducted a systematic review and meta-analysis of observational studies examining the relationship between suicidality and psoriasis. Singh et al. concluded that patients with psoriasis have a significantly higher risk of suicidal ideation, suicide attempts, and completed suicides, while Chi et al concluded that the available limited, very low-quality evidence does not support the notion of an association between psoriasis on the one hand, and suicide, suicidal ideation and attempts on the other.
Psoriasis and Inflammatory Bowel Disease.
Cottone M, Sapienza C, Macaluso FS, Cannizzaro M. Dig Dis. 2019 May 10:1-7.
Inflammatory bowel disease (IBD) and psoriasis (PS) are associated conditions. The reason for this association lies in the sharing of predisposition genes and common immunological mechanisms. This review will focus on the interplay between IBD and PS, with details on prevalence and phenotype of PS in IBD, genetics, pathogenetic pathways, and therapy.
Psoriasis in HIV infection: an update.
Alpalhão M, Borges-Costa J, Filipe P. Int J STD AIDS. 2019 May;30(6):596-604.
A review of the available literature to highlight the updated evidence on psoriasis in HIV-infected individuals, particularly in regards to its epidemiology, proposed pathophysiology, clinical presentation, currently available therapeutic options, and future perspectives.
All-cause and cause-specific mortality in psoriasis: A systematic review and meta-analysis.
Dhana A, Yen H, Yen H, Cho E. J Am Acad Dermatol. 2019 May;80(5):1332-1343.
A systematic review and meta-analysis of mortality risk in psoriasis that included studies reporting all-cause or cause-specific mortality risk estimates in psoriasis patients compared with general population or subjects free of psoriasis. The pooled RRs for cardiovascular mortality were 1.15 (95% CI 1.09-1.21) in psoriasis, 1.05 (95% CI 0.92-1.20) in mild psoriasis, and 1.38 (95% CI 1.09-1.74) in severe psoriasis. For noncardiovascular causes, mortality risk from liver disease, kidney disease, and infection was significantly increased in psoriasis, regardless of disease severity.
The Broad-Spectrum Impact of Hidradenitis Suppurativa on Quality of Life: A Comparison with Psoriasis.
Sampogna F, Fania L, Mazzanti C, et al. Dermatology. 2019 May 23:1-7.
The aim of this study was to evaluate in detail the QoL impact of HS comparing it with other skin conditions, and in particular with psoriasis. HS had the worst QoL among several skin conditions. Compared to psoriasis the mean symptom score was 69.4 versus 53.7, and the mean psychosocial score was 56.1 versus 32.7. Overall, the scores of patients with HS were higher than those of psoriasis patients on 16 of the 17 items of the Skindex-17.
Suicidality and risk of suicidality in psoriasis: A critical appraisal of two systematic reviews and meta-analyses.
Matterne U, Baumeister SE, Apfelbacher C. Br J Dermatol. 2019 May 10.
Chi et al. and Singh et al each conducted a systematic review and meta-analysis of observational studies examining the relationship between suicidality and psoriasis. Singh et al. concluded that patients with psoriasis have a significantly higher risk of suicidal ideation, suicide attempts, and completed suicides, while Chi et al concluded that the available limited, very low-quality evidence does not support the notion of an association between psoriasis on the one hand, and suicide, suicidal ideation and attempts on the other.
Psoriasis and Inflammatory Bowel Disease.
Cottone M, Sapienza C, Macaluso FS, Cannizzaro M. Dig Dis. 2019 May 10:1-7.
Inflammatory bowel disease (IBD) and psoriasis (PS) are associated conditions. The reason for this association lies in the sharing of predisposition genes and common immunological mechanisms. This review will focus on the interplay between IBD and PS, with details on prevalence and phenotype of PS in IBD, genetics, pathogenetic pathways, and therapy.
Psoriasis in HIV infection: an update.
Alpalhão M, Borges-Costa J, Filipe P. Int J STD AIDS. 2019 May;30(6):596-604.
A review of the available literature to highlight the updated evidence on psoriasis in HIV-infected individuals, particularly in regards to its epidemiology, proposed pathophysiology, clinical presentation, currently available therapeutic options, and future perspectives.
All-cause and cause-specific mortality in psoriasis: A systematic review and meta-analysis.
Dhana A, Yen H, Yen H, Cho E. J Am Acad Dermatol. 2019 May;80(5):1332-1343.
A systematic review and meta-analysis of mortality risk in psoriasis that included studies reporting all-cause or cause-specific mortality risk estimates in psoriasis patients compared with general population or subjects free of psoriasis. The pooled RRs for cardiovascular mortality were 1.15 (95% CI 1.09-1.21) in psoriasis, 1.05 (95% CI 0.92-1.20) in mild psoriasis, and 1.38 (95% CI 1.09-1.74) in severe psoriasis. For noncardiovascular causes, mortality risk from liver disease, kidney disease, and infection was significantly increased in psoriasis, regardless of disease severity.
Etanercept biosimilar SB4 a cost-effective alternative for psoriasis, PsA
The development of biosimilars such as etanercept SB4 offers a “significant opportunity to decrease medical care cost and increase treatment options,” Alessandro Giunta, MD, of the department of dermatology at the University of Rome Tor Vergata, and associates reported in a letter to the editor in the British Journal of Dermatology.
Dr. Giunta and his associates performed an observational, retrospective, single-center study to investigate etanercept biosimilar SB4 in patients being treated for plaque type psoriasis and psoriatic arthritis (PsA). They evaluated 40 patients – 21 men and 19 women – mean age 55, ranging from 19 to 79 years. The patients received the etanercept biosimilar SB4 between Oct. 21, 2016, and March 31, 2017, at University of Rome Tor Vergata’s department of dermatology. (The etanercept biosimilar SB4 was approved April 29 by the Food and Drug Administration under the brand name Eticovo [etanercept-ykro]. It is also approved in other countries under the names Benepali and Brenzys.)
Accounting for erythrocyte sedimentation rate as a variable, Dr. Giunta and colleagues calculated disease activity scores based on 28 joints; 14 patients (35%) had plaque psoriasis (mean Psoriasis Area Severity Index [PASI] of 9.61 at baseline), while 26 (65%) had psoriatic arthritis (mean PASI, 4.69). All patients reported prior treatment with systemic conventional and biologic treatments. A group of 10 patients (25%) who had been previously treated with etanercept originator underwent an intermittent treatment regimen of 24 weeks with etanercept biosimilar, which was interrupted once clinical resolution was achieved. No treatments were prescribed between etanercept originator and etanercept biosimilar. Mean exposure was 50.4 weeks, ranging from 24 to 96 weeks, with an average washout period of 12.1 weeks from originator to biosimilar (range 8-24).
A significant improvement in mean PASI score was observed in plaque type psoriasis patients as well as psoriatic arthritis patients at week 24 (P less than .0001 and P less than .001, respectively), noted Dr. Giunta and associates.
“All scores achieved a statistical significant improvement with the exception of [swollen joint count] that markedly improved but not significantly,” they added. One patient experienced injection site reaction, but no serious adverse events were observed.
Despite low sample size and limited follow-up time, the authors concluded that etanercept biosimilar achieved effectiveness as a treatment for psoriatic patients even in cases involving previous exposure to originator etanercept. Cost savings of 61.58% for 50-mg treatment and 62.55% for 25-mg treatment respectively guaranteed “the continuity of etanercept-treated patients’ care and gave us the opportunity to allocate patients in innovative but more expensive agents with marginal increase in our annual budget,” they noted.
The authors reported serving as consultants and speakers for AbbVie, Biogen, Eli Lilly, Janssen, Pfizer, and Novartis.
SOURCE: Giunta A et al. Br J Dermatol. 2019 May 3. doi: 10.1111/bjd.18090.
The development of biosimilars such as etanercept SB4 offers a “significant opportunity to decrease medical care cost and increase treatment options,” Alessandro Giunta, MD, of the department of dermatology at the University of Rome Tor Vergata, and associates reported in a letter to the editor in the British Journal of Dermatology.
Dr. Giunta and his associates performed an observational, retrospective, single-center study to investigate etanercept biosimilar SB4 in patients being treated for plaque type psoriasis and psoriatic arthritis (PsA). They evaluated 40 patients – 21 men and 19 women – mean age 55, ranging from 19 to 79 years. The patients received the etanercept biosimilar SB4 between Oct. 21, 2016, and March 31, 2017, at University of Rome Tor Vergata’s department of dermatology. (The etanercept biosimilar SB4 was approved April 29 by the Food and Drug Administration under the brand name Eticovo [etanercept-ykro]. It is also approved in other countries under the names Benepali and Brenzys.)
Accounting for erythrocyte sedimentation rate as a variable, Dr. Giunta and colleagues calculated disease activity scores based on 28 joints; 14 patients (35%) had plaque psoriasis (mean Psoriasis Area Severity Index [PASI] of 9.61 at baseline), while 26 (65%) had psoriatic arthritis (mean PASI, 4.69). All patients reported prior treatment with systemic conventional and biologic treatments. A group of 10 patients (25%) who had been previously treated with etanercept originator underwent an intermittent treatment regimen of 24 weeks with etanercept biosimilar, which was interrupted once clinical resolution was achieved. No treatments were prescribed between etanercept originator and etanercept biosimilar. Mean exposure was 50.4 weeks, ranging from 24 to 96 weeks, with an average washout period of 12.1 weeks from originator to biosimilar (range 8-24).
A significant improvement in mean PASI score was observed in plaque type psoriasis patients as well as psoriatic arthritis patients at week 24 (P less than .0001 and P less than .001, respectively), noted Dr. Giunta and associates.
“All scores achieved a statistical significant improvement with the exception of [swollen joint count] that markedly improved but not significantly,” they added. One patient experienced injection site reaction, but no serious adverse events were observed.
Despite low sample size and limited follow-up time, the authors concluded that etanercept biosimilar achieved effectiveness as a treatment for psoriatic patients even in cases involving previous exposure to originator etanercept. Cost savings of 61.58% for 50-mg treatment and 62.55% for 25-mg treatment respectively guaranteed “the continuity of etanercept-treated patients’ care and gave us the opportunity to allocate patients in innovative but more expensive agents with marginal increase in our annual budget,” they noted.
The authors reported serving as consultants and speakers for AbbVie, Biogen, Eli Lilly, Janssen, Pfizer, and Novartis.
SOURCE: Giunta A et al. Br J Dermatol. 2019 May 3. doi: 10.1111/bjd.18090.
The development of biosimilars such as etanercept SB4 offers a “significant opportunity to decrease medical care cost and increase treatment options,” Alessandro Giunta, MD, of the department of dermatology at the University of Rome Tor Vergata, and associates reported in a letter to the editor in the British Journal of Dermatology.
Dr. Giunta and his associates performed an observational, retrospective, single-center study to investigate etanercept biosimilar SB4 in patients being treated for plaque type psoriasis and psoriatic arthritis (PsA). They evaluated 40 patients – 21 men and 19 women – mean age 55, ranging from 19 to 79 years. The patients received the etanercept biosimilar SB4 between Oct. 21, 2016, and March 31, 2017, at University of Rome Tor Vergata’s department of dermatology. (The etanercept biosimilar SB4 was approved April 29 by the Food and Drug Administration under the brand name Eticovo [etanercept-ykro]. It is also approved in other countries under the names Benepali and Brenzys.)
Accounting for erythrocyte sedimentation rate as a variable, Dr. Giunta and colleagues calculated disease activity scores based on 28 joints; 14 patients (35%) had plaque psoriasis (mean Psoriasis Area Severity Index [PASI] of 9.61 at baseline), while 26 (65%) had psoriatic arthritis (mean PASI, 4.69). All patients reported prior treatment with systemic conventional and biologic treatments. A group of 10 patients (25%) who had been previously treated with etanercept originator underwent an intermittent treatment regimen of 24 weeks with etanercept biosimilar, which was interrupted once clinical resolution was achieved. No treatments were prescribed between etanercept originator and etanercept biosimilar. Mean exposure was 50.4 weeks, ranging from 24 to 96 weeks, with an average washout period of 12.1 weeks from originator to biosimilar (range 8-24).
A significant improvement in mean PASI score was observed in plaque type psoriasis patients as well as psoriatic arthritis patients at week 24 (P less than .0001 and P less than .001, respectively), noted Dr. Giunta and associates.
“All scores achieved a statistical significant improvement with the exception of [swollen joint count] that markedly improved but not significantly,” they added. One patient experienced injection site reaction, but no serious adverse events were observed.
Despite low sample size and limited follow-up time, the authors concluded that etanercept biosimilar achieved effectiveness as a treatment for psoriatic patients even in cases involving previous exposure to originator etanercept. Cost savings of 61.58% for 50-mg treatment and 62.55% for 25-mg treatment respectively guaranteed “the continuity of etanercept-treated patients’ care and gave us the opportunity to allocate patients in innovative but more expensive agents with marginal increase in our annual budget,” they noted.
The authors reported serving as consultants and speakers for AbbVie, Biogen, Eli Lilly, Janssen, Pfizer, and Novartis.
SOURCE: Giunta A et al. Br J Dermatol. 2019 May 3. doi: 10.1111/bjd.18090.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Apremilast and Phototherapy for Treatment of Psoriasis in a Patient With Human Immunodeficiency Virus
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
Practice Point
- Apremilast may be considered as a first-line therapy in the human immunodeficiency virus population due to decreased immunosuppression.
Positive psoriatic arthritis screens occur often in psoriasis patients
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
FDA approves new etanercept biosimilar, Eticovo
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
FDA approves corticosteroid-retinoid lotion for plaque psoriasis
The in a lotion formulation, for the treatment of plaque psoriasis in adults, the manufacturer announced on April 25.
A press release from the manufacturer, Ortho Dermatologics, summarized the results of two phase 3 studies that compared treatment with the combination product, in 418 adults with moderate to severe plaque psoriasis. At week 8, the proportion of patients who had achieved treatment success – defined as at least a two-grade improvement in Investigator Global Assessment score and a score of “clear” or “almost clear” – was 36% and 45% in the treatment groups, compared with 7% and 13% of those on vehicle, respectively (P less than .001 for both).
The release also refers to a phase 2 study of 212 patients, which found that the combination treatment was more effective in treating plaque psoriasis than was either component separately (J Drugs Dermatol. 2017 Mar 1;16[1]:197-204). By week 8, 52.5% of patients treated with the combination lotion had shown treatment success – compared with 33.3% of those treated with halobetasol, 18.6% of those treated with tazarotene, and 9.7% of those treated with vehicle (P = .033).
Common treatment-related adverse events included treatment site reactions, such as irritation, pain, itching, and folliculitis. Treatment may cause birth defects if used during pregnancy, so a negative pregnancy test should be obtained before treatment begins and effective birth control should be used during treatment.
It will be marketed under the trade name Duobrii and is priced at $825 for a 100-gram tube, according to the press release.
The in a lotion formulation, for the treatment of plaque psoriasis in adults, the manufacturer announced on April 25.
A press release from the manufacturer, Ortho Dermatologics, summarized the results of two phase 3 studies that compared treatment with the combination product, in 418 adults with moderate to severe plaque psoriasis. At week 8, the proportion of patients who had achieved treatment success – defined as at least a two-grade improvement in Investigator Global Assessment score and a score of “clear” or “almost clear” – was 36% and 45% in the treatment groups, compared with 7% and 13% of those on vehicle, respectively (P less than .001 for both).
The release also refers to a phase 2 study of 212 patients, which found that the combination treatment was more effective in treating plaque psoriasis than was either component separately (J Drugs Dermatol. 2017 Mar 1;16[1]:197-204). By week 8, 52.5% of patients treated with the combination lotion had shown treatment success – compared with 33.3% of those treated with halobetasol, 18.6% of those treated with tazarotene, and 9.7% of those treated with vehicle (P = .033).
Common treatment-related adverse events included treatment site reactions, such as irritation, pain, itching, and folliculitis. Treatment may cause birth defects if used during pregnancy, so a negative pregnancy test should be obtained before treatment begins and effective birth control should be used during treatment.
It will be marketed under the trade name Duobrii and is priced at $825 for a 100-gram tube, according to the press release.
The in a lotion formulation, for the treatment of plaque psoriasis in adults, the manufacturer announced on April 25.
A press release from the manufacturer, Ortho Dermatologics, summarized the results of two phase 3 studies that compared treatment with the combination product, in 418 adults with moderate to severe plaque psoriasis. At week 8, the proportion of patients who had achieved treatment success – defined as at least a two-grade improvement in Investigator Global Assessment score and a score of “clear” or “almost clear” – was 36% and 45% in the treatment groups, compared with 7% and 13% of those on vehicle, respectively (P less than .001 for both).
The release also refers to a phase 2 study of 212 patients, which found that the combination treatment was more effective in treating plaque psoriasis than was either component separately (J Drugs Dermatol. 2017 Mar 1;16[1]:197-204). By week 8, 52.5% of patients treated with the combination lotion had shown treatment success – compared with 33.3% of those treated with halobetasol, 18.6% of those treated with tazarotene, and 9.7% of those treated with vehicle (P = .033).
Common treatment-related adverse events included treatment site reactions, such as irritation, pain, itching, and folliculitis. Treatment may cause birth defects if used during pregnancy, so a negative pregnancy test should be obtained before treatment begins and effective birth control should be used during treatment.
It will be marketed under the trade name Duobrii and is priced at $825 for a 100-gram tube, according to the press release.
FDA approves IL-23 inhibitor risankizumab for treating plaque psoriasis
Risankizumab, an interleukin-23 inhibitor, has been approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, the manufacturer announced on April 23.
Risankizumab selectively inhibits interleukin-23 (IL-23), a key inflammatory protein, by binding to its p19 subunit. The drug is administered at a dose of 150 mg, in two subcutaneous injections, every 12 weeks, after starting doses at weeks 0 and 4. It will be available in early May, according to an AbbVie press release announcing the approval.
The approval was based in part on data from two phase 3, 2-year studies, In UltIMMA-1 and UltIMMA-2, at 16 weeks, 75% of risankizumab patients in both studies achieved a Psoriasis Area and Severity Index (PASI 90), compared with 5% and 2% of those on placebo, respectively. These results were published in 2018 (Lancet. 2018 Aug 25;392[10148]:650-61).
At 1 year, 82% and 81% of those treated with risankizumab in the two studies achieved a PASI 90, and 56% and 60% achieved a PASI 100, respectively, according to the company.
Approval was also based on additional phase 3 studies, IMMhance and IMMvent.
Upper respiratory infections were among the most common adverse events associated with risankizumab in trials, reported in 13%, according to the company. Other adverse events associated with treatment included headache (3.5 %), fatigue (2.5 %), injection site reactions (1.5%) and tinea infections (1.1%). The AbbVie release states that candidates for treatment should be evaluated for tuberculosis before starting therapy, and patients should be instructed to report signs and symptoms of infection.
Risankizumab, which will be marketed as Skyrizi, was recently approved in Canada for the same indication, and in Japan, for plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adults. It currently is under review in Europe.
AbbVie and Boehringer Ingelheim are collaborating on the development of risankizumab, according to an AbbVie press release. Studies of risankizumab for treatment of psoriatic arthritis and Crohn’s disease are underway.
Risankizumab, an interleukin-23 inhibitor, has been approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, the manufacturer announced on April 23.
Risankizumab selectively inhibits interleukin-23 (IL-23), a key inflammatory protein, by binding to its p19 subunit. The drug is administered at a dose of 150 mg, in two subcutaneous injections, every 12 weeks, after starting doses at weeks 0 and 4. It will be available in early May, according to an AbbVie press release announcing the approval.
The approval was based in part on data from two phase 3, 2-year studies, In UltIMMA-1 and UltIMMA-2, at 16 weeks, 75% of risankizumab patients in both studies achieved a Psoriasis Area and Severity Index (PASI 90), compared with 5% and 2% of those on placebo, respectively. These results were published in 2018 (Lancet. 2018 Aug 25;392[10148]:650-61).
At 1 year, 82% and 81% of those treated with risankizumab in the two studies achieved a PASI 90, and 56% and 60% achieved a PASI 100, respectively, according to the company.
Approval was also based on additional phase 3 studies, IMMhance and IMMvent.
Upper respiratory infections were among the most common adverse events associated with risankizumab in trials, reported in 13%, according to the company. Other adverse events associated with treatment included headache (3.5 %), fatigue (2.5 %), injection site reactions (1.5%) and tinea infections (1.1%). The AbbVie release states that candidates for treatment should be evaluated for tuberculosis before starting therapy, and patients should be instructed to report signs and symptoms of infection.
Risankizumab, which will be marketed as Skyrizi, was recently approved in Canada for the same indication, and in Japan, for plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adults. It currently is under review in Europe.
AbbVie and Boehringer Ingelheim are collaborating on the development of risankizumab, according to an AbbVie press release. Studies of risankizumab for treatment of psoriatic arthritis and Crohn’s disease are underway.
Risankizumab, an interleukin-23 inhibitor, has been approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, the manufacturer announced on April 23.
Risankizumab selectively inhibits interleukin-23 (IL-23), a key inflammatory protein, by binding to its p19 subunit. The drug is administered at a dose of 150 mg, in two subcutaneous injections, every 12 weeks, after starting doses at weeks 0 and 4. It will be available in early May, according to an AbbVie press release announcing the approval.
The approval was based in part on data from two phase 3, 2-year studies, In UltIMMA-1 and UltIMMA-2, at 16 weeks, 75% of risankizumab patients in both studies achieved a Psoriasis Area and Severity Index (PASI 90), compared with 5% and 2% of those on placebo, respectively. These results were published in 2018 (Lancet. 2018 Aug 25;392[10148]:650-61).
At 1 year, 82% and 81% of those treated with risankizumab in the two studies achieved a PASI 90, and 56% and 60% achieved a PASI 100, respectively, according to the company.
Approval was also based on additional phase 3 studies, IMMhance and IMMvent.
Upper respiratory infections were among the most common adverse events associated with risankizumab in trials, reported in 13%, according to the company. Other adverse events associated with treatment included headache (3.5 %), fatigue (2.5 %), injection site reactions (1.5%) and tinea infections (1.1%). The AbbVie release states that candidates for treatment should be evaluated for tuberculosis before starting therapy, and patients should be instructed to report signs and symptoms of infection.
Risankizumab, which will be marketed as Skyrizi, was recently approved in Canada for the same indication, and in Japan, for plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adults. It currently is under review in Europe.
AbbVie and Boehringer Ingelheim are collaborating on the development of risankizumab, according to an AbbVie press release. Studies of risankizumab for treatment of psoriatic arthritis and Crohn’s disease are underway.