User login
BNP testing improves outcomes in evaluation of dyspnea
Data on treating bronchiolitis severity limited
CDC: Five confirmed 2019-nCoV cases in the U.S.
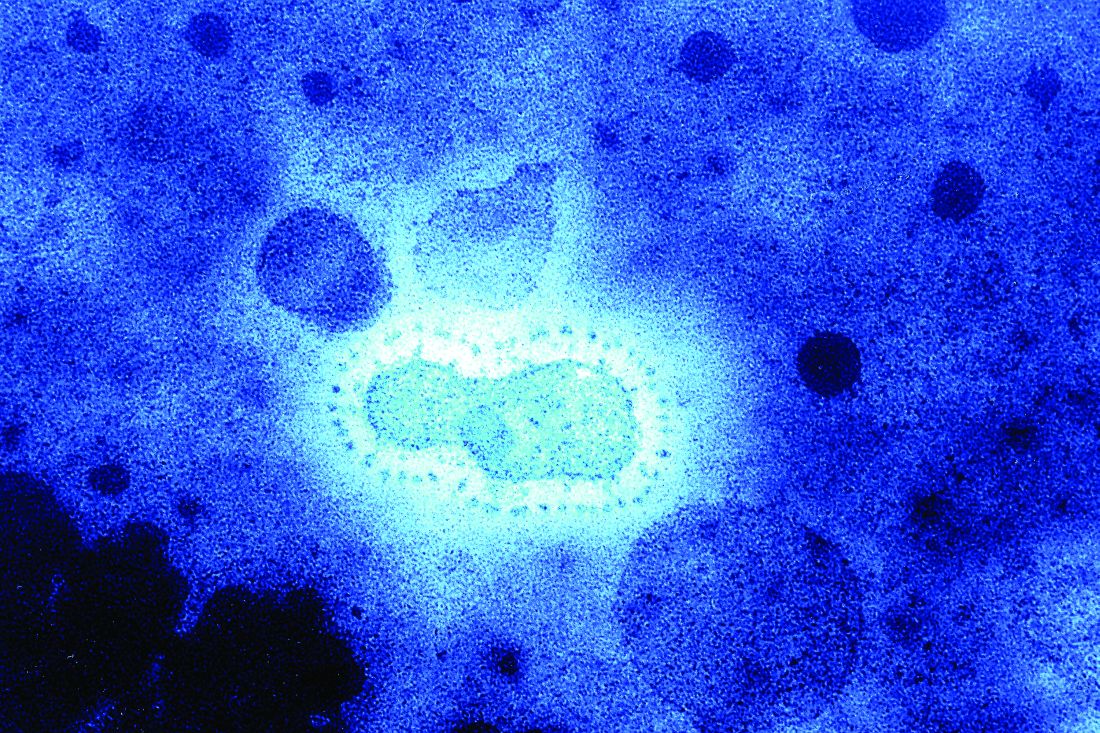
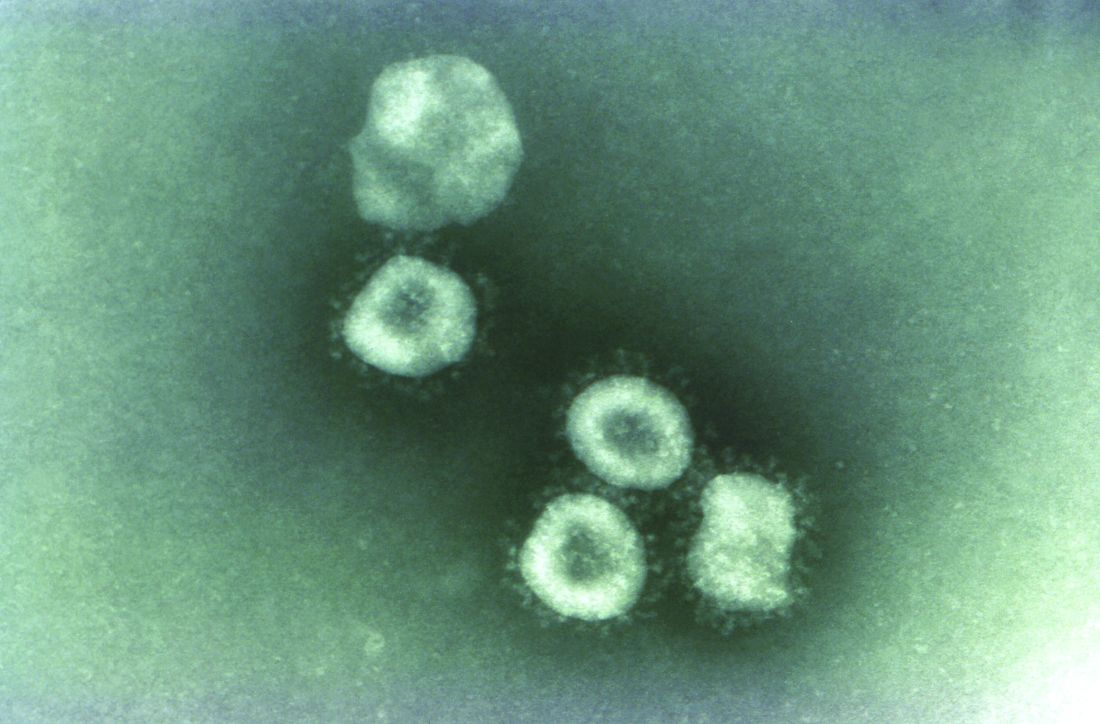
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Wuhan virus: What clinicians need to know
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
EVALI update warns of chemicals in vaping products
A report issued by the Centers for Disease Control and Prevention confirms that 82% of patients presenting with e-cigarette– or vaping product use–associated lung injury (EVALI) used products containing tetrahydrocannabinol (THC).
Another report published in the CDC’s Morbidity and Mortality Weekly Report assessed cases in which the patients reported using only nicotine-containing vaping products.
“As of Jan. 14, 2020, a total of 2,668 hospitalized EVALI cases had been reported to CDC,” based on data from the National Syndromic Surveillance Program (NSSP), wrote Vikram P. Krishnasamy, MD, of the National Center for Injury Prevention and Control at the CDC, Atlanta, and colleagues. Cases have occurred in all 50 states, the District of Columbia, the U.S. Virgin Islands, and Puerto Rico. The age of the patients ranged from 13 to 85 years, with an average age of 24 years; 66% were male, and 73% were non-Hispanic white.
In addition, 57% of the patients reported using any nicotine-containing product, and 14% of these reported use of nicotine products exclusively.
Previous studies have shown that vitamin E acetate is associated with the EVALI outbreak, which peaked during the week of Sept. 15, 2019, with 215 reported hospital admissions, Dr. Krishnasamy and associates noted. “However, evidence is not sufficient to rule out the contribution of other chemicals of concern, including chemicals in either THC- or non-THC–containing products, in some reported EVALI cases,” they said.
The study findings were limited by several factors, including incomplete data on product use, increased reporting of vaping product use at emergency department visits after increased public awareness of risk, and inconsistency in the health care facilities contributing data via the NSSP, the researchers wrote.
The decline in EVALI cases since September 2019 may be related to factors including the rapid public health response to increase awareness of the risks of vaping, and the possible removal of vitamin E acetate as a diluent in THC-containing products, but clinicians and public health professionals should remain on alert for new EVALI cases and continue to discourage the use of THC-containing e-cigarette or vaping products, Dr. Krishnasamy and associates concluded.
Nicotine-only vaping products
In a second report published in MMWR, Isaac Ghinai, MBBS, of the Illinois Department of Public Health and CDC researchers examined characteristics of EVALI patients in Illinois who reported using only nicotine-containing vaping products.
A total of 9 of 121 (7%) EVALI patients surveyed in Illinois reported no indication of THC use. These patients were more likely than those who reported any use of THC-containing products to be female (78% vs. 25%) and aged 45 years and older (33% vs. 2%); P less than .01 in both cases.
In addition, EVALI patients with no indication of THC-containing product use were less likely than THC product users to present with constitutional symptoms (56% vs. 96%) or initial leukocytosis (38% vs. 91%), or to have previously visited an outpatient provider or ED before being hospitalized (25% vs. 80%).
Other presenting characteristics including initial vital signs and lab results, as well as the frequency of severe outcomes such as death or respiratory failure, were not significantly different between users and nonusers of THC-containing vaping products.
The study findings were limited by factors including the use of self-reports, the small sample size, and lack of initial and follow-up interviews for all EVALI patients, the researchers noted. However, the results support the CDC’s recommendation that “persons should not use THC-containing e-cigarette, or vaping, products, particularly those obtained from informal sources such as friends, family members, or from in-person or online dealers,” and should not add vitamin E acetate or other substances to these products, they said.
In addition, users of nicotine-containing e-cigarette or vaping products as an alternative to cigarettes should not return to cigarettes, but should explore other options to help them quit, Dr. Ghinai, and associates said.
The studies were supported by the CDC. The researchers in both studies had no financial conflicts to disclose.
SOURCES: Krishnasamy VP et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e2; Ghinai I et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e1.
A report issued by the Centers for Disease Control and Prevention confirms that 82% of patients presenting with e-cigarette– or vaping product use–associated lung injury (EVALI) used products containing tetrahydrocannabinol (THC).
Another report published in the CDC’s Morbidity and Mortality Weekly Report assessed cases in which the patients reported using only nicotine-containing vaping products.
“As of Jan. 14, 2020, a total of 2,668 hospitalized EVALI cases had been reported to CDC,” based on data from the National Syndromic Surveillance Program (NSSP), wrote Vikram P. Krishnasamy, MD, of the National Center for Injury Prevention and Control at the CDC, Atlanta, and colleagues. Cases have occurred in all 50 states, the District of Columbia, the U.S. Virgin Islands, and Puerto Rico. The age of the patients ranged from 13 to 85 years, with an average age of 24 years; 66% were male, and 73% were non-Hispanic white.
In addition, 57% of the patients reported using any nicotine-containing product, and 14% of these reported use of nicotine products exclusively.
Previous studies have shown that vitamin E acetate is associated with the EVALI outbreak, which peaked during the week of Sept. 15, 2019, with 215 reported hospital admissions, Dr. Krishnasamy and associates noted. “However, evidence is not sufficient to rule out the contribution of other chemicals of concern, including chemicals in either THC- or non-THC–containing products, in some reported EVALI cases,” they said.
The study findings were limited by several factors, including incomplete data on product use, increased reporting of vaping product use at emergency department visits after increased public awareness of risk, and inconsistency in the health care facilities contributing data via the NSSP, the researchers wrote.
The decline in EVALI cases since September 2019 may be related to factors including the rapid public health response to increase awareness of the risks of vaping, and the possible removal of vitamin E acetate as a diluent in THC-containing products, but clinicians and public health professionals should remain on alert for new EVALI cases and continue to discourage the use of THC-containing e-cigarette or vaping products, Dr. Krishnasamy and associates concluded.
Nicotine-only vaping products
In a second report published in MMWR, Isaac Ghinai, MBBS, of the Illinois Department of Public Health and CDC researchers examined characteristics of EVALI patients in Illinois who reported using only nicotine-containing vaping products.
A total of 9 of 121 (7%) EVALI patients surveyed in Illinois reported no indication of THC use. These patients were more likely than those who reported any use of THC-containing products to be female (78% vs. 25%) and aged 45 years and older (33% vs. 2%); P less than .01 in both cases.
In addition, EVALI patients with no indication of THC-containing product use were less likely than THC product users to present with constitutional symptoms (56% vs. 96%) or initial leukocytosis (38% vs. 91%), or to have previously visited an outpatient provider or ED before being hospitalized (25% vs. 80%).
Other presenting characteristics including initial vital signs and lab results, as well as the frequency of severe outcomes such as death or respiratory failure, were not significantly different between users and nonusers of THC-containing vaping products.
The study findings were limited by factors including the use of self-reports, the small sample size, and lack of initial and follow-up interviews for all EVALI patients, the researchers noted. However, the results support the CDC’s recommendation that “persons should not use THC-containing e-cigarette, or vaping, products, particularly those obtained from informal sources such as friends, family members, or from in-person or online dealers,” and should not add vitamin E acetate or other substances to these products, they said.
In addition, users of nicotine-containing e-cigarette or vaping products as an alternative to cigarettes should not return to cigarettes, but should explore other options to help them quit, Dr. Ghinai, and associates said.
The studies were supported by the CDC. The researchers in both studies had no financial conflicts to disclose.
SOURCES: Krishnasamy VP et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e2; Ghinai I et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e1.
A report issued by the Centers for Disease Control and Prevention confirms that 82% of patients presenting with e-cigarette– or vaping product use–associated lung injury (EVALI) used products containing tetrahydrocannabinol (THC).
Another report published in the CDC’s Morbidity and Mortality Weekly Report assessed cases in which the patients reported using only nicotine-containing vaping products.
“As of Jan. 14, 2020, a total of 2,668 hospitalized EVALI cases had been reported to CDC,” based on data from the National Syndromic Surveillance Program (NSSP), wrote Vikram P. Krishnasamy, MD, of the National Center for Injury Prevention and Control at the CDC, Atlanta, and colleagues. Cases have occurred in all 50 states, the District of Columbia, the U.S. Virgin Islands, and Puerto Rico. The age of the patients ranged from 13 to 85 years, with an average age of 24 years; 66% were male, and 73% were non-Hispanic white.
In addition, 57% of the patients reported using any nicotine-containing product, and 14% of these reported use of nicotine products exclusively.
Previous studies have shown that vitamin E acetate is associated with the EVALI outbreak, which peaked during the week of Sept. 15, 2019, with 215 reported hospital admissions, Dr. Krishnasamy and associates noted. “However, evidence is not sufficient to rule out the contribution of other chemicals of concern, including chemicals in either THC- or non-THC–containing products, in some reported EVALI cases,” they said.
The study findings were limited by several factors, including incomplete data on product use, increased reporting of vaping product use at emergency department visits after increased public awareness of risk, and inconsistency in the health care facilities contributing data via the NSSP, the researchers wrote.
The decline in EVALI cases since September 2019 may be related to factors including the rapid public health response to increase awareness of the risks of vaping, and the possible removal of vitamin E acetate as a diluent in THC-containing products, but clinicians and public health professionals should remain on alert for new EVALI cases and continue to discourage the use of THC-containing e-cigarette or vaping products, Dr. Krishnasamy and associates concluded.
Nicotine-only vaping products
In a second report published in MMWR, Isaac Ghinai, MBBS, of the Illinois Department of Public Health and CDC researchers examined characteristics of EVALI patients in Illinois who reported using only nicotine-containing vaping products.
A total of 9 of 121 (7%) EVALI patients surveyed in Illinois reported no indication of THC use. These patients were more likely than those who reported any use of THC-containing products to be female (78% vs. 25%) and aged 45 years and older (33% vs. 2%); P less than .01 in both cases.
In addition, EVALI patients with no indication of THC-containing product use were less likely than THC product users to present with constitutional symptoms (56% vs. 96%) or initial leukocytosis (38% vs. 91%), or to have previously visited an outpatient provider or ED before being hospitalized (25% vs. 80%).
Other presenting characteristics including initial vital signs and lab results, as well as the frequency of severe outcomes such as death or respiratory failure, were not significantly different between users and nonusers of THC-containing vaping products.
The study findings were limited by factors including the use of self-reports, the small sample size, and lack of initial and follow-up interviews for all EVALI patients, the researchers noted. However, the results support the CDC’s recommendation that “persons should not use THC-containing e-cigarette, or vaping, products, particularly those obtained from informal sources such as friends, family members, or from in-person or online dealers,” and should not add vitamin E acetate or other substances to these products, they said.
In addition, users of nicotine-containing e-cigarette or vaping products as an alternative to cigarettes should not return to cigarettes, but should explore other options to help them quit, Dr. Ghinai, and associates said.
The studies were supported by the CDC. The researchers in both studies had no financial conflicts to disclose.
SOURCES: Krishnasamy VP et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e2; Ghinai I et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e1.
FROM MMWR
Second U.S. coronavirus patient confirmed
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
Surgeon General scolds docs for failing to help patients quit smoking
The U.S. Surgeon General is calling on all physicians to help patients stop smoking, noting that two-thirds of adult smokers say they want to quit, but only 40% report that their doctor has advised them to stop.
“I’ve got to own this as the nation’s doctor, and our health providers in this room and in this country need to own this stat,” said Surgeon General Jerome Adams, MD, at a press briefing releasing a new report on smoking cessation.
“Smoking is the No. 1 preventable cause of death, disease, and disability in the United States,” he said. “So why are 40% of our health providers out there not advising smokers to quit when they come in?”
In the first U.S. Surgeon General report on smoking cessation in 30 years, the 700-page report suggests smoking cessation-related quality measures that include physician reimbursement would increase treatment.
The evidence also suggests that using electronic health records to prompt clinicians to inquire about smoking would increase cessation treatment.
EHRs could be used to “empower and enable” physicians to advise people to quit, said Dr. Adams. Physicians also need “the education and the confidence to be able to have that conversation, because too many of them look at someone and say: ‘Nope, too hard, too much effort, no, that’s not what they’re here for today,’ ” he said.
However, “simply asking, advising, and referring can be enough to get someone on the pathway to quitting,” Dr. Adams said.
34 million still smoke
The new report is the first on the topic released since 1990, and the 34th on tobacco control since the first one was issued in 1964, said Dr. Adams. Since that first report, adult smoking has declined 70%, but some 34 million Americans (14%) still smoke, he said.
In addition, Dr. Adams said that many subpopulations have been left behind, noting: “Cigarette smoking remains highest among LGBTQ adults, people with disabilities or limitations, American Indians and Alaska Natives, and people with mental health conditions or substance use disorders.”
He also noted that 40% of cigarettes are consumed by those with a mental illness or a substance use disorder.
Quitting is beneficial at any age and can add as much as a decade to life expectancy, the report notes. Quitting also reduces the risk of 12 cancers, cuts the risk of chronic obstructive pulmonary disease, and reduces cardiovascular and stroke morbidity and mortality.
Pregnant women who quit also reduce their own morbidity and mortality risk and that of unborn children and infants, the report says.
“We know more about the science of quitting than ever before. We can, and must, do more to ensure that evidence-based cessation treatments are reaching the people that need them,” said Dr. Adams.
Less than one-third of those who have quit have used Food and Drug Administration–approved cessation medications or behavioral counseling, Dr. Adams said.
Barriers to care
Despite the existence of five nicotine replacement therapies and two nonnicotine oral medications, and more widespread availability of proven counseling methods – including web- or text-based programs – barriers to access remain.
These include a lack of insurance coverage for comprehensive, evidence-based smoking cessation treatment, which, when offered, increases availability and use.
“These are cost-effective interventions,” said Dr. Adams. “It’s penny wise and pound foolish to not give someone access to what we know works,” he said.
Because of the diversity of e-cigarette products and the variety of ways they are used, coupled with little research, it’s not currently possible to determine whether they are, or are not, useful smoking cessation tools, the report notes.
However, experts who compiled the report found some evidence to suggest that e-cigarettes containing nicotine may be “associated with increased smoking cessation compared with the use of e-cigarettes not containing nicotine.”
Asked whether the report’s conclusions might be interpreted as supportive of e-cigarettes, Dr. Adams said the report focused on smoking cessation, not initiation.
“I’m terribly concerned about the clear data that shows youth are initiating tobacco product use with e-cigarettes,” he said.
The Trump administration’s current proposal to partially restrict sales of some flavored e-cigarettes “reflects the science,” and “a balance between a desire to really make sure that people aren’t initiating with these products, but also a desire to again try to maintain a pathway for adults who want to use these products to quit to use them,” Dr. Adams said.
The focus, said Dr. Adams, should not be on e-cigarettes and whether they do, or do not, work.
“People want to quit,” he said. “We know what works. Not enough of them are getting it, and there are terrible disparities in who is and who is not getting access to effective and evidence-based treatment – that’s the story here.”
This article first appeared on Medscape.com.
The U.S. Surgeon General is calling on all physicians to help patients stop smoking, noting that two-thirds of adult smokers say they want to quit, but only 40% report that their doctor has advised them to stop.
“I’ve got to own this as the nation’s doctor, and our health providers in this room and in this country need to own this stat,” said Surgeon General Jerome Adams, MD, at a press briefing releasing a new report on smoking cessation.
“Smoking is the No. 1 preventable cause of death, disease, and disability in the United States,” he said. “So why are 40% of our health providers out there not advising smokers to quit when they come in?”
In the first U.S. Surgeon General report on smoking cessation in 30 years, the 700-page report suggests smoking cessation-related quality measures that include physician reimbursement would increase treatment.
The evidence also suggests that using electronic health records to prompt clinicians to inquire about smoking would increase cessation treatment.
EHRs could be used to “empower and enable” physicians to advise people to quit, said Dr. Adams. Physicians also need “the education and the confidence to be able to have that conversation, because too many of them look at someone and say: ‘Nope, too hard, too much effort, no, that’s not what they’re here for today,’ ” he said.
However, “simply asking, advising, and referring can be enough to get someone on the pathway to quitting,” Dr. Adams said.
34 million still smoke
The new report is the first on the topic released since 1990, and the 34th on tobacco control since the first one was issued in 1964, said Dr. Adams. Since that first report, adult smoking has declined 70%, but some 34 million Americans (14%) still smoke, he said.
In addition, Dr. Adams said that many subpopulations have been left behind, noting: “Cigarette smoking remains highest among LGBTQ adults, people with disabilities or limitations, American Indians and Alaska Natives, and people with mental health conditions or substance use disorders.”
He also noted that 40% of cigarettes are consumed by those with a mental illness or a substance use disorder.
Quitting is beneficial at any age and can add as much as a decade to life expectancy, the report notes. Quitting also reduces the risk of 12 cancers, cuts the risk of chronic obstructive pulmonary disease, and reduces cardiovascular and stroke morbidity and mortality.
Pregnant women who quit also reduce their own morbidity and mortality risk and that of unborn children and infants, the report says.
“We know more about the science of quitting than ever before. We can, and must, do more to ensure that evidence-based cessation treatments are reaching the people that need them,” said Dr. Adams.
Less than one-third of those who have quit have used Food and Drug Administration–approved cessation medications or behavioral counseling, Dr. Adams said.
Barriers to care
Despite the existence of five nicotine replacement therapies and two nonnicotine oral medications, and more widespread availability of proven counseling methods – including web- or text-based programs – barriers to access remain.
These include a lack of insurance coverage for comprehensive, evidence-based smoking cessation treatment, which, when offered, increases availability and use.
“These are cost-effective interventions,” said Dr. Adams. “It’s penny wise and pound foolish to not give someone access to what we know works,” he said.
Because of the diversity of e-cigarette products and the variety of ways they are used, coupled with little research, it’s not currently possible to determine whether they are, or are not, useful smoking cessation tools, the report notes.
However, experts who compiled the report found some evidence to suggest that e-cigarettes containing nicotine may be “associated with increased smoking cessation compared with the use of e-cigarettes not containing nicotine.”
Asked whether the report’s conclusions might be interpreted as supportive of e-cigarettes, Dr. Adams said the report focused on smoking cessation, not initiation.
“I’m terribly concerned about the clear data that shows youth are initiating tobacco product use with e-cigarettes,” he said.
The Trump administration’s current proposal to partially restrict sales of some flavored e-cigarettes “reflects the science,” and “a balance between a desire to really make sure that people aren’t initiating with these products, but also a desire to again try to maintain a pathway for adults who want to use these products to quit to use them,” Dr. Adams said.
The focus, said Dr. Adams, should not be on e-cigarettes and whether they do, or do not, work.
“People want to quit,” he said. “We know what works. Not enough of them are getting it, and there are terrible disparities in who is and who is not getting access to effective and evidence-based treatment – that’s the story here.”
This article first appeared on Medscape.com.
The U.S. Surgeon General is calling on all physicians to help patients stop smoking, noting that two-thirds of adult smokers say they want to quit, but only 40% report that their doctor has advised them to stop.
“I’ve got to own this as the nation’s doctor, and our health providers in this room and in this country need to own this stat,” said Surgeon General Jerome Adams, MD, at a press briefing releasing a new report on smoking cessation.
“Smoking is the No. 1 preventable cause of death, disease, and disability in the United States,” he said. “So why are 40% of our health providers out there not advising smokers to quit when they come in?”
In the first U.S. Surgeon General report on smoking cessation in 30 years, the 700-page report suggests smoking cessation-related quality measures that include physician reimbursement would increase treatment.
The evidence also suggests that using electronic health records to prompt clinicians to inquire about smoking would increase cessation treatment.
EHRs could be used to “empower and enable” physicians to advise people to quit, said Dr. Adams. Physicians also need “the education and the confidence to be able to have that conversation, because too many of them look at someone and say: ‘Nope, too hard, too much effort, no, that’s not what they’re here for today,’ ” he said.
However, “simply asking, advising, and referring can be enough to get someone on the pathway to quitting,” Dr. Adams said.
34 million still smoke
The new report is the first on the topic released since 1990, and the 34th on tobacco control since the first one was issued in 1964, said Dr. Adams. Since that first report, adult smoking has declined 70%, but some 34 million Americans (14%) still smoke, he said.
In addition, Dr. Adams said that many subpopulations have been left behind, noting: “Cigarette smoking remains highest among LGBTQ adults, people with disabilities or limitations, American Indians and Alaska Natives, and people with mental health conditions or substance use disorders.”
He also noted that 40% of cigarettes are consumed by those with a mental illness or a substance use disorder.
Quitting is beneficial at any age and can add as much as a decade to life expectancy, the report notes. Quitting also reduces the risk of 12 cancers, cuts the risk of chronic obstructive pulmonary disease, and reduces cardiovascular and stroke morbidity and mortality.
Pregnant women who quit also reduce their own morbidity and mortality risk and that of unborn children and infants, the report says.
“We know more about the science of quitting than ever before. We can, and must, do more to ensure that evidence-based cessation treatments are reaching the people that need them,” said Dr. Adams.
Less than one-third of those who have quit have used Food and Drug Administration–approved cessation medications or behavioral counseling, Dr. Adams said.
Barriers to care
Despite the existence of five nicotine replacement therapies and two nonnicotine oral medications, and more widespread availability of proven counseling methods – including web- or text-based programs – barriers to access remain.
These include a lack of insurance coverage for comprehensive, evidence-based smoking cessation treatment, which, when offered, increases availability and use.
“These are cost-effective interventions,” said Dr. Adams. “It’s penny wise and pound foolish to not give someone access to what we know works,” he said.
Because of the diversity of e-cigarette products and the variety of ways they are used, coupled with little research, it’s not currently possible to determine whether they are, or are not, useful smoking cessation tools, the report notes.
However, experts who compiled the report found some evidence to suggest that e-cigarettes containing nicotine may be “associated with increased smoking cessation compared with the use of e-cigarettes not containing nicotine.”
Asked whether the report’s conclusions might be interpreted as supportive of e-cigarettes, Dr. Adams said the report focused on smoking cessation, not initiation.
“I’m terribly concerned about the clear data that shows youth are initiating tobacco product use with e-cigarettes,” he said.
The Trump administration’s current proposal to partially restrict sales of some flavored e-cigarettes “reflects the science,” and “a balance between a desire to really make sure that people aren’t initiating with these products, but also a desire to again try to maintain a pathway for adults who want to use these products to quit to use them,” Dr. Adams said.
The focus, said Dr. Adams, should not be on e-cigarettes and whether they do, or do not, work.
“People want to quit,” he said. “We know what works. Not enough of them are getting it, and there are terrible disparities in who is and who is not getting access to effective and evidence-based treatment – that’s the story here.”
This article first appeared on Medscape.com.
Washington state patient is first U.S. case of novel coronavirus
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
REPORTING FROM CDC
Nontuberculous mycobacterial lung disease cases on the rise across U.S.
To assess the NTM lung disease burden on a national level, Kevin L. Winthrop, MD, of Oregon Health & Science University, Portland, and associates analyzed patient data from a U.S. managed care claims database between 2008 and 2015. Their findings were published in the Annals of the American Thoracic Society.
A case of NTM lung disease was defined as a patient with at least two medical claims with the disease’s diagnostic codes – 031.0 and A31.0 – that were at least 30 days apart. Of the 74,984,596 beneficiaries in the database, 9,476 met the case definition for NTM lung disease; 69% (n = 6,530) were women.
From 2008 to 2015, the annual incidence of NTM lung disease increased from 3.13 (95% confidence interval, 2.88-3.40) to 4.73 (95% CI, 4.43-5.05) per 100,000 person-years, with the average rate of yearly change being +5.2% (95% CI, 4.0%-6.4%; P less than .01).The annual prevalence increased from 6.78 (95% CI, 6.45-7.14) to 11.70 (95% CI, 11.26-12.16) per 100,000 persons, with the average rate of yearly change being +7.5% (95% CI, 6.7-8.2%; P less than .01).
The majority of NTM lung disease in the United States is caused by Mycobacterium avium complex (17), although other species such as M. abscessus, M. kansasii, M. xenopi, and others contribute to this disease burden.
“It’s a classic chicken-or-egg scenario,” said Sachin Gupta, MD, a pulmonologist in San Francisco, in regard to the rising numbers. “Increased awareness of NTM lung disease is, in part, why we’re seeing prevalence and incidence go up. And yet the disease itself may also be growing in clusters and pockets, as the data show, in various places across the nation.
“The worrisome aspect here,” he added, “is that future studies will likely show that, as incidence is increasing, mortality is increasing as well. That speaks to the challenges with these bugs: Very hard to diagnose, very hard to treat.”
The authors acknowledged their study’s limitations, including the lack of microbiologic or radiographic confirmation of the NTM infection and the inherent shortcomings of claims data–based studies overall. They did note a previous report, however, that “claims-based case identification has a high positive predictive value of approximately 82% for NTM lung disease.”
The study was funded by Insmed; the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases; and the National Heart, Lung, and Blood Institute. The authors reported no conflicts of interest.
SOURCE: Winthrop KL et al. Ann Am Thorac Soc. 2019 Dec 13. doi: 10.1513/AnnalsATS.201804-236OC.
To assess the NTM lung disease burden on a national level, Kevin L. Winthrop, MD, of Oregon Health & Science University, Portland, and associates analyzed patient data from a U.S. managed care claims database between 2008 and 2015. Their findings were published in the Annals of the American Thoracic Society.
A case of NTM lung disease was defined as a patient with at least two medical claims with the disease’s diagnostic codes – 031.0 and A31.0 – that were at least 30 days apart. Of the 74,984,596 beneficiaries in the database, 9,476 met the case definition for NTM lung disease; 69% (n = 6,530) were women.
From 2008 to 2015, the annual incidence of NTM lung disease increased from 3.13 (95% confidence interval, 2.88-3.40) to 4.73 (95% CI, 4.43-5.05) per 100,000 person-years, with the average rate of yearly change being +5.2% (95% CI, 4.0%-6.4%; P less than .01).The annual prevalence increased from 6.78 (95% CI, 6.45-7.14) to 11.70 (95% CI, 11.26-12.16) per 100,000 persons, with the average rate of yearly change being +7.5% (95% CI, 6.7-8.2%; P less than .01).
The majority of NTM lung disease in the United States is caused by Mycobacterium avium complex (17), although other species such as M. abscessus, M. kansasii, M. xenopi, and others contribute to this disease burden.
“It’s a classic chicken-or-egg scenario,” said Sachin Gupta, MD, a pulmonologist in San Francisco, in regard to the rising numbers. “Increased awareness of NTM lung disease is, in part, why we’re seeing prevalence and incidence go up. And yet the disease itself may also be growing in clusters and pockets, as the data show, in various places across the nation.
“The worrisome aspect here,” he added, “is that future studies will likely show that, as incidence is increasing, mortality is increasing as well. That speaks to the challenges with these bugs: Very hard to diagnose, very hard to treat.”
The authors acknowledged their study’s limitations, including the lack of microbiologic or radiographic confirmation of the NTM infection and the inherent shortcomings of claims data–based studies overall. They did note a previous report, however, that “claims-based case identification has a high positive predictive value of approximately 82% for NTM lung disease.”
The study was funded by Insmed; the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases; and the National Heart, Lung, and Blood Institute. The authors reported no conflicts of interest.
SOURCE: Winthrop KL et al. Ann Am Thorac Soc. 2019 Dec 13. doi: 10.1513/AnnalsATS.201804-236OC.
To assess the NTM lung disease burden on a national level, Kevin L. Winthrop, MD, of Oregon Health & Science University, Portland, and associates analyzed patient data from a U.S. managed care claims database between 2008 and 2015. Their findings were published in the Annals of the American Thoracic Society.
A case of NTM lung disease was defined as a patient with at least two medical claims with the disease’s diagnostic codes – 031.0 and A31.0 – that were at least 30 days apart. Of the 74,984,596 beneficiaries in the database, 9,476 met the case definition for NTM lung disease; 69% (n = 6,530) were women.
From 2008 to 2015, the annual incidence of NTM lung disease increased from 3.13 (95% confidence interval, 2.88-3.40) to 4.73 (95% CI, 4.43-5.05) per 100,000 person-years, with the average rate of yearly change being +5.2% (95% CI, 4.0%-6.4%; P less than .01).The annual prevalence increased from 6.78 (95% CI, 6.45-7.14) to 11.70 (95% CI, 11.26-12.16) per 100,000 persons, with the average rate of yearly change being +7.5% (95% CI, 6.7-8.2%; P less than .01).
The majority of NTM lung disease in the United States is caused by Mycobacterium avium complex (17), although other species such as M. abscessus, M. kansasii, M. xenopi, and others contribute to this disease burden.
“It’s a classic chicken-or-egg scenario,” said Sachin Gupta, MD, a pulmonologist in San Francisco, in regard to the rising numbers. “Increased awareness of NTM lung disease is, in part, why we’re seeing prevalence and incidence go up. And yet the disease itself may also be growing in clusters and pockets, as the data show, in various places across the nation.
“The worrisome aspect here,” he added, “is that future studies will likely show that, as incidence is increasing, mortality is increasing as well. That speaks to the challenges with these bugs: Very hard to diagnose, very hard to treat.”
The authors acknowledged their study’s limitations, including the lack of microbiologic or radiographic confirmation of the NTM infection and the inherent shortcomings of claims data–based studies overall. They did note a previous report, however, that “claims-based case identification has a high positive predictive value of approximately 82% for NTM lung disease.”
The study was funded by Insmed; the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases; and the National Heart, Lung, and Blood Institute. The authors reported no conflicts of interest.
SOURCE: Winthrop KL et al. Ann Am Thorac Soc. 2019 Dec 13. doi: 10.1513/AnnalsATS.201804-236OC.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Cardiovascular risks associated with cannabis use
Researchers are recommending routine screening of marijuana use in cardiovascular care settings.
A review of current evidence suggests an association between marijuana use and adverse cardiovascular effects, as well as interactions between marijuana and cardiovascular medications.
Although more research is needed, the review authors suggested patients may benefit from marijuana screening and testing as well as discussions about the potential risks of marijuana use in the setting of cardiovascular disease.
Ersilia M. DeFilippis, MD, of Columbia University Irving Medical Center in New York and colleagues conducted this review, which was published in the Journal of the American College of Cardiology.
The authors noted that research on marijuana use and cardiovascular disease is limited. The different forms of cannabis and various routes of administration have made it difficult to draw concrete conclusions about marijuana products. Additionally, there have been no randomized, controlled trials of marijuana products in the United States because such trials are illegal; however, there are observational studies linking marijuana use and adverse cardiovascular effects.
Snapshot of available evidence
One study showed that smoking marijuana produces many of the same cardiotoxic chemicals produced by smoking tobacco (BMJ. 2003 May 3;326[7396]:942-3). Another study suggested marijuana smokers may have greater exposure to harmful chemicals (J Psychoactive Drugs. 1988 Jan-Mar;20[1]:43-6).
More specifically, a meta-analysis suggested that smoking marijuana was one of the top three triggers of myocardial infarction (Lancet. 2011 Feb 26;377[9767]:732-40). And in a systematic analysis, 28 of 33 studies linked marijuana use to an increased risk of acute coronary syndromes (Clin Toxicol [Phila]. 2019 Oct;57[10]:831-41).
Furthermore, a study of 2.5 million marijuana users showed that 3% experienced arrhythmias (Int J Cardiol. 2018 Aug 1;264:91-2). A population survey showed that people who smoked marijuana in the past year experienced a 3.3-fold higher rate of cerebrovascular events (Aust N Z J Public Health. 2016 Jun;40[3]:226-30).
Studies have also indicated that cannabinoids can affect cardiovascular medications, including antiarrhythmics, calcium-channel blockers, isosorbide dinitrate/mononitrate, statins, beta-blockers, warfarin, theophylline, and nonsteroidal anti-inflammatory drugs (Medicines [Basel]. 2018 Dec 23;6[1] pii: E3; Curr Top Behav Neurosci. 2017;32:249-62; Pharmacogenet Genomics. 2009 Jul;19[7]:559-62; Ann Pharmacother. 2009 Jul;43[7]:1347-53; Pharmacol Ther. 2019 Sep;201:25-38).
Reviewer recommendations
Cardiovascular specialists should be informed about regulations governing marijuana products, as well as “potential health consequences of marijuana and its derivatives,” according to Dr. DeFilippis and colleagues.
The authors recommend routinely screening patients for marijuana use, perhaps using the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (PLoS One. 2017 May 26;12[5]:e0178194) or the Cannabis Abuse Screening Test (Int J Methods Psychiatr Res. 2018 Jun;27[2]:e1597).
The authors say urine toxicology “may be reasonable” for patients with myocardial infarction or new-onset heart failure. Such testing is required for patients undergoing a heart transplant because marijuana use may affect their candidacy.
Dr. DeFilippis and colleagues say cardiovascular specialists should inform patients about the risks associated with marijuana use. The authors recommend shared decision making for patients who use marijuana for symptom management or palliative purposes.
Three review authors disclosed relationships with many different pharmaceutical companies. One author disclosed relationships with Medscape Cardiology and WebMD, which are owned by the same parent company as MDedge.
SOURCE: J Am Coll Cardiol. 2020 Jan 20. doi: 10.1016/j.jacc.2019.11.025.
Researchers are recommending routine screening of marijuana use in cardiovascular care settings.
A review of current evidence suggests an association between marijuana use and adverse cardiovascular effects, as well as interactions between marijuana and cardiovascular medications.
Although more research is needed, the review authors suggested patients may benefit from marijuana screening and testing as well as discussions about the potential risks of marijuana use in the setting of cardiovascular disease.
Ersilia M. DeFilippis, MD, of Columbia University Irving Medical Center in New York and colleagues conducted this review, which was published in the Journal of the American College of Cardiology.
The authors noted that research on marijuana use and cardiovascular disease is limited. The different forms of cannabis and various routes of administration have made it difficult to draw concrete conclusions about marijuana products. Additionally, there have been no randomized, controlled trials of marijuana products in the United States because such trials are illegal; however, there are observational studies linking marijuana use and adverse cardiovascular effects.
Snapshot of available evidence
One study showed that smoking marijuana produces many of the same cardiotoxic chemicals produced by smoking tobacco (BMJ. 2003 May 3;326[7396]:942-3). Another study suggested marijuana smokers may have greater exposure to harmful chemicals (J Psychoactive Drugs. 1988 Jan-Mar;20[1]:43-6).
More specifically, a meta-analysis suggested that smoking marijuana was one of the top three triggers of myocardial infarction (Lancet. 2011 Feb 26;377[9767]:732-40). And in a systematic analysis, 28 of 33 studies linked marijuana use to an increased risk of acute coronary syndromes (Clin Toxicol [Phila]. 2019 Oct;57[10]:831-41).
Furthermore, a study of 2.5 million marijuana users showed that 3% experienced arrhythmias (Int J Cardiol. 2018 Aug 1;264:91-2). A population survey showed that people who smoked marijuana in the past year experienced a 3.3-fold higher rate of cerebrovascular events (Aust N Z J Public Health. 2016 Jun;40[3]:226-30).
Studies have also indicated that cannabinoids can affect cardiovascular medications, including antiarrhythmics, calcium-channel blockers, isosorbide dinitrate/mononitrate, statins, beta-blockers, warfarin, theophylline, and nonsteroidal anti-inflammatory drugs (Medicines [Basel]. 2018 Dec 23;6[1] pii: E3; Curr Top Behav Neurosci. 2017;32:249-62; Pharmacogenet Genomics. 2009 Jul;19[7]:559-62; Ann Pharmacother. 2009 Jul;43[7]:1347-53; Pharmacol Ther. 2019 Sep;201:25-38).
Reviewer recommendations
Cardiovascular specialists should be informed about regulations governing marijuana products, as well as “potential health consequences of marijuana and its derivatives,” according to Dr. DeFilippis and colleagues.
The authors recommend routinely screening patients for marijuana use, perhaps using the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (PLoS One. 2017 May 26;12[5]:e0178194) or the Cannabis Abuse Screening Test (Int J Methods Psychiatr Res. 2018 Jun;27[2]:e1597).
The authors say urine toxicology “may be reasonable” for patients with myocardial infarction or new-onset heart failure. Such testing is required for patients undergoing a heart transplant because marijuana use may affect their candidacy.
Dr. DeFilippis and colleagues say cardiovascular specialists should inform patients about the risks associated with marijuana use. The authors recommend shared decision making for patients who use marijuana for symptom management or palliative purposes.
Three review authors disclosed relationships with many different pharmaceutical companies. One author disclosed relationships with Medscape Cardiology and WebMD, which are owned by the same parent company as MDedge.
SOURCE: J Am Coll Cardiol. 2020 Jan 20. doi: 10.1016/j.jacc.2019.11.025.
Researchers are recommending routine screening of marijuana use in cardiovascular care settings.
A review of current evidence suggests an association between marijuana use and adverse cardiovascular effects, as well as interactions between marijuana and cardiovascular medications.
Although more research is needed, the review authors suggested patients may benefit from marijuana screening and testing as well as discussions about the potential risks of marijuana use in the setting of cardiovascular disease.
Ersilia M. DeFilippis, MD, of Columbia University Irving Medical Center in New York and colleagues conducted this review, which was published in the Journal of the American College of Cardiology.
The authors noted that research on marijuana use and cardiovascular disease is limited. The different forms of cannabis and various routes of administration have made it difficult to draw concrete conclusions about marijuana products. Additionally, there have been no randomized, controlled trials of marijuana products in the United States because such trials are illegal; however, there are observational studies linking marijuana use and adverse cardiovascular effects.
Snapshot of available evidence
One study showed that smoking marijuana produces many of the same cardiotoxic chemicals produced by smoking tobacco (BMJ. 2003 May 3;326[7396]:942-3). Another study suggested marijuana smokers may have greater exposure to harmful chemicals (J Psychoactive Drugs. 1988 Jan-Mar;20[1]:43-6).
More specifically, a meta-analysis suggested that smoking marijuana was one of the top three triggers of myocardial infarction (Lancet. 2011 Feb 26;377[9767]:732-40). And in a systematic analysis, 28 of 33 studies linked marijuana use to an increased risk of acute coronary syndromes (Clin Toxicol [Phila]. 2019 Oct;57[10]:831-41).
Furthermore, a study of 2.5 million marijuana users showed that 3% experienced arrhythmias (Int J Cardiol. 2018 Aug 1;264:91-2). A population survey showed that people who smoked marijuana in the past year experienced a 3.3-fold higher rate of cerebrovascular events (Aust N Z J Public Health. 2016 Jun;40[3]:226-30).
Studies have also indicated that cannabinoids can affect cardiovascular medications, including antiarrhythmics, calcium-channel blockers, isosorbide dinitrate/mononitrate, statins, beta-blockers, warfarin, theophylline, and nonsteroidal anti-inflammatory drugs (Medicines [Basel]. 2018 Dec 23;6[1] pii: E3; Curr Top Behav Neurosci. 2017;32:249-62; Pharmacogenet Genomics. 2009 Jul;19[7]:559-62; Ann Pharmacother. 2009 Jul;43[7]:1347-53; Pharmacol Ther. 2019 Sep;201:25-38).
Reviewer recommendations
Cardiovascular specialists should be informed about regulations governing marijuana products, as well as “potential health consequences of marijuana and its derivatives,” according to Dr. DeFilippis and colleagues.
The authors recommend routinely screening patients for marijuana use, perhaps using the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (PLoS One. 2017 May 26;12[5]:e0178194) or the Cannabis Abuse Screening Test (Int J Methods Psychiatr Res. 2018 Jun;27[2]:e1597).
The authors say urine toxicology “may be reasonable” for patients with myocardial infarction or new-onset heart failure. Such testing is required for patients undergoing a heart transplant because marijuana use may affect their candidacy.
Dr. DeFilippis and colleagues say cardiovascular specialists should inform patients about the risks associated with marijuana use. The authors recommend shared decision making for patients who use marijuana for symptom management or palliative purposes.
Three review authors disclosed relationships with many different pharmaceutical companies. One author disclosed relationships with Medscape Cardiology and WebMD, which are owned by the same parent company as MDedge.
SOURCE: J Am Coll Cardiol. 2020 Jan 20. doi: 10.1016/j.jacc.2019.11.025.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY