User login
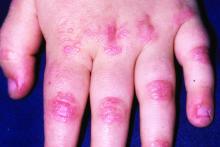
Novel therapy shows promise for treating skin-predominant dermatomyositis
NEW ORLEANS – in a double-blind, placebo-controlled phase 2 trial, according to results presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
“These findings support the inhibition of IFN-beta as a promising therapeutic strategy in skin-predominant disease,” said principal investigator Aaron Mangold, MD, associate professor of dermatology, Mayo Clinic, Scottsdale, Ariz.
Dermatomyositis, a rare autoimmune inflammatory condition that typically involves both skeletal muscles and skin, is a challenging disease with a diverse set of potential complications.
Immunosuppressive and immunomodulatory agents are used with mixed success for myositis, but skin manifestations, which include papular eruptions, heliotrope rash, photoerythema, burning, and pruritus, are often the most troublesome and the most difficult to control. Treatment options other than immunomodulators that target cutaneous involvement – which include steroids, emollients, and photoprotection – are generally modestly effective, according to Dr. Mangold.
Targeting an elevated cytokine
Interest in IFN-beta, which is elevated in the blood of individuals with dermatomyositis, was triggered by evidence that this cytokine plays an important role in driving the skin inflammation, Dr. Mangold explained.
“The blood concentrations of IFN-beta are positively correlated with cutaneous disease activity and severity,” he said.
The study drug, currently known as PF-06823859 (Dazukibart), “is a potent, selective humanized IgG1-neutralizing antibody directed at IFN-beta,” Dr. Mangold said. A dose-ranging phase 1 study published 2 years ago provided evidence of acceptable pharmacokinetics and safety in healthy individuals to support treatment studies for disorders associated with elevated IFN-beta levels. In addition to dermatomyositis, this includes systemic lupus erythematosus.
In this phase 2 trial, patients whose condition was not improved by at least one standard-care therapy for skin manifestations of dermatomyositis were eligible if they had moderate to severe disease as measured with the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), according to Dr. Mangold. During the study, patients were allowed to remain on a disease modifying antirheumatic drug and/or prednisone if they had been on stable doses and did not change the dose.
After a screening run-in, the trial had two blinded stages. In stage 1, 30 patients were randomly assigned either to 600 mg of PF-06823859 or to placebo, both administered intravenously every 4 weeks. A second cohort of 25 patients was randomly assigned in stage 2 to placebo, 150 mg of PF-06823859, or 600 mg of PF-06823859. The primary endpoint assessed at 12 weeks was a greater than 5-point reduction in CDASI score or greater than 40% CDASI improvement from baseline.
Both endpoints are associated with a clinically meaningful response in regard to an improved quality of life, Dr. Mangold noted.
Both doses better than placebo
In results from the stage 1 portion, the mean reduction in CDASI at 12 weeks after three doses of the assigned therapy was 18.8 points in the active-treatment group versus 3.9 points in the placebo group. In pooled data from stage 1 and 2, the reductions were 16.6 points, 19.2 points, and 2.9 points for the 150-mg, 600-mg, and placebo arms, respectively. Both doses achieved a highly significant advantage over placebo.
For both stages and doses, the response curves of the active-treatment groups and the placebo group diverged almost immediately. By 4 weeks, both measures of CDASI reductions on active therapy were significantly improved relative to placebo, and the response curves had a consistent downward slope through the end of the 12-week study, Dr. Mangold reported.
The majority of patients responded by either of the primary endpoint criteria. For a CDASI reduction of greater than 5 points, the response rates were 100% and 96% for the 150-mg and 600-mg doses of PF-06823859, respectively. The placebo response was 35.7%. For the CDASI reduction of greater than 40%, the rates were 80%, 82.1%, and 7.1% for the 150-mg, 600-mg, and placebo arms, respectively.
“There were no major safety concerns. Most of the treatment-emergent adverse events were mild, and adverse events did not have a relationship to dose,” Dr. Mangold said. Notably, there were no cases of herpes zoster, and infections of any kind were low in all study groups.
A phase 3 study is being planned with the 600-mg dose, according to Dr. Mangold, but he acknowledged that regulatory authorities have generally required endpoints for both cutaneous and muscle manifestations in previous trials of therapies for dermatomyositis.
It is not yet certain that “there will be a carve-out for skin,” he said in answer to a question about investigations moving forward. So far, studies have been focused on skin response. However, a meaningful degree of benefit against muscle involvement, which has not yet been well studied, has not been ruled out.
Even though this is a phase 2 trial with small numbers, it was controlled and blinded, and the potential of an inhibitor of IFN-beta to control the skin manifestations of dermatomyositis “is kind of a big deal,” said Paul Nghiem, MD, PhD, professor of dermatology, University of Washington, Seattle.
“There is definitely an unmet need for better therapies to control the skin involvement,” Dr. Nghiem said.
Hensin Tsao, MD, PhD, clinical director of the Melanoma and Pigmented Lesion Center at Massachusetts General Hospital, Boston, agreed. Like Dr. Nghiem, Dr. Tsao was a panelist during the late-breaker session where the study was presented, and he was impressed by the data.
“This is something that is definitely newsworthy,” Dr. Tsao said.
Dr. Mangold reports financial relationships with Actelion, Amgen, Corbus, Eli Lilly, Incyte, miRagen, Novartis, Regeneron, Solagenix, Sun Pharmaceuticals, Teva, and Pfizer, which provided funding for this trial. Both Dr. Nghiem and Dr. Tsao reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – in a double-blind, placebo-controlled phase 2 trial, according to results presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
“These findings support the inhibition of IFN-beta as a promising therapeutic strategy in skin-predominant disease,” said principal investigator Aaron Mangold, MD, associate professor of dermatology, Mayo Clinic, Scottsdale, Ariz.
Dermatomyositis, a rare autoimmune inflammatory condition that typically involves both skeletal muscles and skin, is a challenging disease with a diverse set of potential complications.
Immunosuppressive and immunomodulatory agents are used with mixed success for myositis, but skin manifestations, which include papular eruptions, heliotrope rash, photoerythema, burning, and pruritus, are often the most troublesome and the most difficult to control. Treatment options other than immunomodulators that target cutaneous involvement – which include steroids, emollients, and photoprotection – are generally modestly effective, according to Dr. Mangold.
Targeting an elevated cytokine
Interest in IFN-beta, which is elevated in the blood of individuals with dermatomyositis, was triggered by evidence that this cytokine plays an important role in driving the skin inflammation, Dr. Mangold explained.
“The blood concentrations of IFN-beta are positively correlated with cutaneous disease activity and severity,” he said.
The study drug, currently known as PF-06823859 (Dazukibart), “is a potent, selective humanized IgG1-neutralizing antibody directed at IFN-beta,” Dr. Mangold said. A dose-ranging phase 1 study published 2 years ago provided evidence of acceptable pharmacokinetics and safety in healthy individuals to support treatment studies for disorders associated with elevated IFN-beta levels. In addition to dermatomyositis, this includes systemic lupus erythematosus.
In this phase 2 trial, patients whose condition was not improved by at least one standard-care therapy for skin manifestations of dermatomyositis were eligible if they had moderate to severe disease as measured with the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), according to Dr. Mangold. During the study, patients were allowed to remain on a disease modifying antirheumatic drug and/or prednisone if they had been on stable doses and did not change the dose.
After a screening run-in, the trial had two blinded stages. In stage 1, 30 patients were randomly assigned either to 600 mg of PF-06823859 or to placebo, both administered intravenously every 4 weeks. A second cohort of 25 patients was randomly assigned in stage 2 to placebo, 150 mg of PF-06823859, or 600 mg of PF-06823859. The primary endpoint assessed at 12 weeks was a greater than 5-point reduction in CDASI score or greater than 40% CDASI improvement from baseline.
Both endpoints are associated with a clinically meaningful response in regard to an improved quality of life, Dr. Mangold noted.
Both doses better than placebo
In results from the stage 1 portion, the mean reduction in CDASI at 12 weeks after three doses of the assigned therapy was 18.8 points in the active-treatment group versus 3.9 points in the placebo group. In pooled data from stage 1 and 2, the reductions were 16.6 points, 19.2 points, and 2.9 points for the 150-mg, 600-mg, and placebo arms, respectively. Both doses achieved a highly significant advantage over placebo.
For both stages and doses, the response curves of the active-treatment groups and the placebo group diverged almost immediately. By 4 weeks, both measures of CDASI reductions on active therapy were significantly improved relative to placebo, and the response curves had a consistent downward slope through the end of the 12-week study, Dr. Mangold reported.
The majority of patients responded by either of the primary endpoint criteria. For a CDASI reduction of greater than 5 points, the response rates were 100% and 96% for the 150-mg and 600-mg doses of PF-06823859, respectively. The placebo response was 35.7%. For the CDASI reduction of greater than 40%, the rates were 80%, 82.1%, and 7.1% for the 150-mg, 600-mg, and placebo arms, respectively.
“There were no major safety concerns. Most of the treatment-emergent adverse events were mild, and adverse events did not have a relationship to dose,” Dr. Mangold said. Notably, there were no cases of herpes zoster, and infections of any kind were low in all study groups.
A phase 3 study is being planned with the 600-mg dose, according to Dr. Mangold, but he acknowledged that regulatory authorities have generally required endpoints for both cutaneous and muscle manifestations in previous trials of therapies for dermatomyositis.
It is not yet certain that “there will be a carve-out for skin,” he said in answer to a question about investigations moving forward. So far, studies have been focused on skin response. However, a meaningful degree of benefit against muscle involvement, which has not yet been well studied, has not been ruled out.
Even though this is a phase 2 trial with small numbers, it was controlled and blinded, and the potential of an inhibitor of IFN-beta to control the skin manifestations of dermatomyositis “is kind of a big deal,” said Paul Nghiem, MD, PhD, professor of dermatology, University of Washington, Seattle.
“There is definitely an unmet need for better therapies to control the skin involvement,” Dr. Nghiem said.
Hensin Tsao, MD, PhD, clinical director of the Melanoma and Pigmented Lesion Center at Massachusetts General Hospital, Boston, agreed. Like Dr. Nghiem, Dr. Tsao was a panelist during the late-breaker session where the study was presented, and he was impressed by the data.
“This is something that is definitely newsworthy,” Dr. Tsao said.
Dr. Mangold reports financial relationships with Actelion, Amgen, Corbus, Eli Lilly, Incyte, miRagen, Novartis, Regeneron, Solagenix, Sun Pharmaceuticals, Teva, and Pfizer, which provided funding for this trial. Both Dr. Nghiem and Dr. Tsao reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – in a double-blind, placebo-controlled phase 2 trial, according to results presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
“These findings support the inhibition of IFN-beta as a promising therapeutic strategy in skin-predominant disease,” said principal investigator Aaron Mangold, MD, associate professor of dermatology, Mayo Clinic, Scottsdale, Ariz.
Dermatomyositis, a rare autoimmune inflammatory condition that typically involves both skeletal muscles and skin, is a challenging disease with a diverse set of potential complications.
Immunosuppressive and immunomodulatory agents are used with mixed success for myositis, but skin manifestations, which include papular eruptions, heliotrope rash, photoerythema, burning, and pruritus, are often the most troublesome and the most difficult to control. Treatment options other than immunomodulators that target cutaneous involvement – which include steroids, emollients, and photoprotection – are generally modestly effective, according to Dr. Mangold.
Targeting an elevated cytokine
Interest in IFN-beta, which is elevated in the blood of individuals with dermatomyositis, was triggered by evidence that this cytokine plays an important role in driving the skin inflammation, Dr. Mangold explained.
“The blood concentrations of IFN-beta are positively correlated with cutaneous disease activity and severity,” he said.
The study drug, currently known as PF-06823859 (Dazukibart), “is a potent, selective humanized IgG1-neutralizing antibody directed at IFN-beta,” Dr. Mangold said. A dose-ranging phase 1 study published 2 years ago provided evidence of acceptable pharmacokinetics and safety in healthy individuals to support treatment studies for disorders associated with elevated IFN-beta levels. In addition to dermatomyositis, this includes systemic lupus erythematosus.
In this phase 2 trial, patients whose condition was not improved by at least one standard-care therapy for skin manifestations of dermatomyositis were eligible if they had moderate to severe disease as measured with the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), according to Dr. Mangold. During the study, patients were allowed to remain on a disease modifying antirheumatic drug and/or prednisone if they had been on stable doses and did not change the dose.
After a screening run-in, the trial had two blinded stages. In stage 1, 30 patients were randomly assigned either to 600 mg of PF-06823859 or to placebo, both administered intravenously every 4 weeks. A second cohort of 25 patients was randomly assigned in stage 2 to placebo, 150 mg of PF-06823859, or 600 mg of PF-06823859. The primary endpoint assessed at 12 weeks was a greater than 5-point reduction in CDASI score or greater than 40% CDASI improvement from baseline.
Both endpoints are associated with a clinically meaningful response in regard to an improved quality of life, Dr. Mangold noted.
Both doses better than placebo
In results from the stage 1 portion, the mean reduction in CDASI at 12 weeks after three doses of the assigned therapy was 18.8 points in the active-treatment group versus 3.9 points in the placebo group. In pooled data from stage 1 and 2, the reductions were 16.6 points, 19.2 points, and 2.9 points for the 150-mg, 600-mg, and placebo arms, respectively. Both doses achieved a highly significant advantage over placebo.
For both stages and doses, the response curves of the active-treatment groups and the placebo group diverged almost immediately. By 4 weeks, both measures of CDASI reductions on active therapy were significantly improved relative to placebo, and the response curves had a consistent downward slope through the end of the 12-week study, Dr. Mangold reported.
The majority of patients responded by either of the primary endpoint criteria. For a CDASI reduction of greater than 5 points, the response rates were 100% and 96% for the 150-mg and 600-mg doses of PF-06823859, respectively. The placebo response was 35.7%. For the CDASI reduction of greater than 40%, the rates were 80%, 82.1%, and 7.1% for the 150-mg, 600-mg, and placebo arms, respectively.
“There were no major safety concerns. Most of the treatment-emergent adverse events were mild, and adverse events did not have a relationship to dose,” Dr. Mangold said. Notably, there were no cases of herpes zoster, and infections of any kind were low in all study groups.
A phase 3 study is being planned with the 600-mg dose, according to Dr. Mangold, but he acknowledged that regulatory authorities have generally required endpoints for both cutaneous and muscle manifestations in previous trials of therapies for dermatomyositis.
It is not yet certain that “there will be a carve-out for skin,” he said in answer to a question about investigations moving forward. So far, studies have been focused on skin response. However, a meaningful degree of benefit against muscle involvement, which has not yet been well studied, has not been ruled out.
Even though this is a phase 2 trial with small numbers, it was controlled and blinded, and the potential of an inhibitor of IFN-beta to control the skin manifestations of dermatomyositis “is kind of a big deal,” said Paul Nghiem, MD, PhD, professor of dermatology, University of Washington, Seattle.
“There is definitely an unmet need for better therapies to control the skin involvement,” Dr. Nghiem said.
Hensin Tsao, MD, PhD, clinical director of the Melanoma and Pigmented Lesion Center at Massachusetts General Hospital, Boston, agreed. Like Dr. Nghiem, Dr. Tsao was a panelist during the late-breaker session where the study was presented, and he was impressed by the data.
“This is something that is definitely newsworthy,” Dr. Tsao said.
Dr. Mangold reports financial relationships with Actelion, Amgen, Corbus, Eli Lilly, Incyte, miRagen, Novartis, Regeneron, Solagenix, Sun Pharmaceuticals, Teva, and Pfizer, which provided funding for this trial. Both Dr. Nghiem and Dr. Tsao reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAD 2023
Early exercise intervention improves knee osteoarthritis
DENVER – Initiating exercise therapy early on in people who develop symptoms of knee osteoarthritis – even within their first year of pain or reduced function – is associated with modestly lower pain scores and modestly better function than in those whose symptoms have lasted longer, according to a study presented at the OARSI 2023 World Congress.
Although the benefits of exercise therapy for advanced knee osteoarthritis had already been well established, this study looked specifically at benefits from exercise therapy earlier on, in patients with a shorter duration of symptoms.
“Exercise indeed seems especially beneficial in patients with shorter symptom duration and should therefore be encouraged at first symptom presentation,” Marienke van Middelkoop, PhD, of Erasmus MC Medical University in Rotterdam, the Netherlands, told attendees at the meeting, sponsored by Osteoarthritis Research Society International. “It is, however, still a challenge how we can identify patients but also how we can motivate these patients with early symptoms of osteoarthritis.” She noted that a separate pilot study had experienced difficulty recruiting people with short-term symptom duration.
The researchers compared the effect of exercise therapy and no exercise among adults at least 45 years old with knee osteoarthritis, relying on individual participant data from the STEER OA study, a meta-analysis of 31 studies that involved 4,241 participants. After excluding studies that didn’t report symptom duration, lacked a control group or consent, or focused on hip osteoarthritis, the researchers ended up with 10 studies involving 1,895 participants. These participants were stratified based on the duration of their symptoms: up to 1 year (14.4%), 1-2 years (11%), and 2 years or longer (74%).
About two-thirds of the participants were women (65.9%), with an average age of 65 years and an average body mass index (BMI) of 30.7 kg/m2. Any land-based or water-based therapeutic exercise counted for the 62% of participants in the intervention group, while the control group had no exercise. Outcomes were assessed based on self-reported pain or physical function at short-term and long-term follow-up, which were as close as possible to 3 months for short-term and the closest date to 12 months for longer term. At baseline, the participants reported an average pain score of 41.7 on a 0-to-100 scale and an average physical function score of 37.4 on a 0-to-100 scale where lower scores indicate better function.
Among those doing exercise therapy, average pain scores dropped 4.56 points in the short term and 7.43 points in the long term. Short-term and long-term pain scores were lower among those whose symptom durations were shorter. For example, those with symptoms for less than a year reported a short-term pain score of 29, compared with 30 for those with 1-2 years of pain and 32 for those with at least 2 years of pain. Results were similar for long-term pain (a score of 26, compared with 28 and 33, respectively).
Participants engaging in exercise therapy also improved average function scores, with a pattern of improvement that was similar to pain scores based on patients’ symptom duration. The average short-term function score was 26 among those with less than a year of symptoms, compared with 28 for those with symptoms for 1-2 years, and 30 for those with symptoms for at least 2 years. Longer-term function scores were 21, 24, and 29, respectively, based on increasing symptom durations.
Chris Yun Lane, PT, DPT, a physical therapist and a fourth-year PhD student at the University of North Carolina at Chapel Hill, was not surprised at the exercise benefit given the extensive evidence already showing that exercise is beneficial for patients with osteoarthritis whose symptoms have lasted longer.
“Just spending a little bit of time on education, designing kind of simple exercise programs, such as walking programs, can be very helpful,” Dr. Lane said in an interview. “Of course, some of it is dependent on the patient itself, but strengthening range of motion is often very helpful.” Dr. Lane said it’s particularly important for physicians and physical therapists to emphasize the importance of exercise to their patients because that guidance doesn’t always occur as often as it should.
Ron Ellis Jr., DO, MBA, chief strategy officer of Pacira BioSciences in Tampa, Fla., noted that a lot of patients with knee osteoarthritis have weakness in their quads, so quad strengthening is “a typical part of our improvement program for patients with osteoarthritis,” he said in an interview. Dr. Ellis also referenced a session he attended the previous day that showed exercise results in reduced inflammation.
“So you may not have weight loss, but you can lower the inflammatory state of the overall body and of the specific joints,” Dr. Ellis said, “so that would support [this study’s] conclusion.”
The STEER OA study was funded by the Chartered Society of Physiotherapy Charitable Trust and the National Institute for Health Research School of Primary Care Research. Dr. van Middelkoop and Dr. Lane both reported having no relevant financial relationships.
DENVER – Initiating exercise therapy early on in people who develop symptoms of knee osteoarthritis – even within their first year of pain or reduced function – is associated with modestly lower pain scores and modestly better function than in those whose symptoms have lasted longer, according to a study presented at the OARSI 2023 World Congress.
Although the benefits of exercise therapy for advanced knee osteoarthritis had already been well established, this study looked specifically at benefits from exercise therapy earlier on, in patients with a shorter duration of symptoms.
“Exercise indeed seems especially beneficial in patients with shorter symptom duration and should therefore be encouraged at first symptom presentation,” Marienke van Middelkoop, PhD, of Erasmus MC Medical University in Rotterdam, the Netherlands, told attendees at the meeting, sponsored by Osteoarthritis Research Society International. “It is, however, still a challenge how we can identify patients but also how we can motivate these patients with early symptoms of osteoarthritis.” She noted that a separate pilot study had experienced difficulty recruiting people with short-term symptom duration.
The researchers compared the effect of exercise therapy and no exercise among adults at least 45 years old with knee osteoarthritis, relying on individual participant data from the STEER OA study, a meta-analysis of 31 studies that involved 4,241 participants. After excluding studies that didn’t report symptom duration, lacked a control group or consent, or focused on hip osteoarthritis, the researchers ended up with 10 studies involving 1,895 participants. These participants were stratified based on the duration of their symptoms: up to 1 year (14.4%), 1-2 years (11%), and 2 years or longer (74%).
About two-thirds of the participants were women (65.9%), with an average age of 65 years and an average body mass index (BMI) of 30.7 kg/m2. Any land-based or water-based therapeutic exercise counted for the 62% of participants in the intervention group, while the control group had no exercise. Outcomes were assessed based on self-reported pain or physical function at short-term and long-term follow-up, which were as close as possible to 3 months for short-term and the closest date to 12 months for longer term. At baseline, the participants reported an average pain score of 41.7 on a 0-to-100 scale and an average physical function score of 37.4 on a 0-to-100 scale where lower scores indicate better function.
Among those doing exercise therapy, average pain scores dropped 4.56 points in the short term and 7.43 points in the long term. Short-term and long-term pain scores were lower among those whose symptom durations were shorter. For example, those with symptoms for less than a year reported a short-term pain score of 29, compared with 30 for those with 1-2 years of pain and 32 for those with at least 2 years of pain. Results were similar for long-term pain (a score of 26, compared with 28 and 33, respectively).
Participants engaging in exercise therapy also improved average function scores, with a pattern of improvement that was similar to pain scores based on patients’ symptom duration. The average short-term function score was 26 among those with less than a year of symptoms, compared with 28 for those with symptoms for 1-2 years, and 30 for those with symptoms for at least 2 years. Longer-term function scores were 21, 24, and 29, respectively, based on increasing symptom durations.
Chris Yun Lane, PT, DPT, a physical therapist and a fourth-year PhD student at the University of North Carolina at Chapel Hill, was not surprised at the exercise benefit given the extensive evidence already showing that exercise is beneficial for patients with osteoarthritis whose symptoms have lasted longer.
“Just spending a little bit of time on education, designing kind of simple exercise programs, such as walking programs, can be very helpful,” Dr. Lane said in an interview. “Of course, some of it is dependent on the patient itself, but strengthening range of motion is often very helpful.” Dr. Lane said it’s particularly important for physicians and physical therapists to emphasize the importance of exercise to their patients because that guidance doesn’t always occur as often as it should.
Ron Ellis Jr., DO, MBA, chief strategy officer of Pacira BioSciences in Tampa, Fla., noted that a lot of patients with knee osteoarthritis have weakness in their quads, so quad strengthening is “a typical part of our improvement program for patients with osteoarthritis,” he said in an interview. Dr. Ellis also referenced a session he attended the previous day that showed exercise results in reduced inflammation.
“So you may not have weight loss, but you can lower the inflammatory state of the overall body and of the specific joints,” Dr. Ellis said, “so that would support [this study’s] conclusion.”
The STEER OA study was funded by the Chartered Society of Physiotherapy Charitable Trust and the National Institute for Health Research School of Primary Care Research. Dr. van Middelkoop and Dr. Lane both reported having no relevant financial relationships.
DENVER – Initiating exercise therapy early on in people who develop symptoms of knee osteoarthritis – even within their first year of pain or reduced function – is associated with modestly lower pain scores and modestly better function than in those whose symptoms have lasted longer, according to a study presented at the OARSI 2023 World Congress.
Although the benefits of exercise therapy for advanced knee osteoarthritis had already been well established, this study looked specifically at benefits from exercise therapy earlier on, in patients with a shorter duration of symptoms.
“Exercise indeed seems especially beneficial in patients with shorter symptom duration and should therefore be encouraged at first symptom presentation,” Marienke van Middelkoop, PhD, of Erasmus MC Medical University in Rotterdam, the Netherlands, told attendees at the meeting, sponsored by Osteoarthritis Research Society International. “It is, however, still a challenge how we can identify patients but also how we can motivate these patients with early symptoms of osteoarthritis.” She noted that a separate pilot study had experienced difficulty recruiting people with short-term symptom duration.
The researchers compared the effect of exercise therapy and no exercise among adults at least 45 years old with knee osteoarthritis, relying on individual participant data from the STEER OA study, a meta-analysis of 31 studies that involved 4,241 participants. After excluding studies that didn’t report symptom duration, lacked a control group or consent, or focused on hip osteoarthritis, the researchers ended up with 10 studies involving 1,895 participants. These participants were stratified based on the duration of their symptoms: up to 1 year (14.4%), 1-2 years (11%), and 2 years or longer (74%).
About two-thirds of the participants were women (65.9%), with an average age of 65 years and an average body mass index (BMI) of 30.7 kg/m2. Any land-based or water-based therapeutic exercise counted for the 62% of participants in the intervention group, while the control group had no exercise. Outcomes were assessed based on self-reported pain or physical function at short-term and long-term follow-up, which were as close as possible to 3 months for short-term and the closest date to 12 months for longer term. At baseline, the participants reported an average pain score of 41.7 on a 0-to-100 scale and an average physical function score of 37.4 on a 0-to-100 scale where lower scores indicate better function.
Among those doing exercise therapy, average pain scores dropped 4.56 points in the short term and 7.43 points in the long term. Short-term and long-term pain scores were lower among those whose symptom durations were shorter. For example, those with symptoms for less than a year reported a short-term pain score of 29, compared with 30 for those with 1-2 years of pain and 32 for those with at least 2 years of pain. Results were similar for long-term pain (a score of 26, compared with 28 and 33, respectively).
Participants engaging in exercise therapy also improved average function scores, with a pattern of improvement that was similar to pain scores based on patients’ symptom duration. The average short-term function score was 26 among those with less than a year of symptoms, compared with 28 for those with symptoms for 1-2 years, and 30 for those with symptoms for at least 2 years. Longer-term function scores were 21, 24, and 29, respectively, based on increasing symptom durations.
Chris Yun Lane, PT, DPT, a physical therapist and a fourth-year PhD student at the University of North Carolina at Chapel Hill, was not surprised at the exercise benefit given the extensive evidence already showing that exercise is beneficial for patients with osteoarthritis whose symptoms have lasted longer.
“Just spending a little bit of time on education, designing kind of simple exercise programs, such as walking programs, can be very helpful,” Dr. Lane said in an interview. “Of course, some of it is dependent on the patient itself, but strengthening range of motion is often very helpful.” Dr. Lane said it’s particularly important for physicians and physical therapists to emphasize the importance of exercise to their patients because that guidance doesn’t always occur as often as it should.
Ron Ellis Jr., DO, MBA, chief strategy officer of Pacira BioSciences in Tampa, Fla., noted that a lot of patients with knee osteoarthritis have weakness in their quads, so quad strengthening is “a typical part of our improvement program for patients with osteoarthritis,” he said in an interview. Dr. Ellis also referenced a session he attended the previous day that showed exercise results in reduced inflammation.
“So you may not have weight loss, but you can lower the inflammatory state of the overall body and of the specific joints,” Dr. Ellis said, “so that would support [this study’s] conclusion.”
The STEER OA study was funded by the Chartered Society of Physiotherapy Charitable Trust and the National Institute for Health Research School of Primary Care Research. Dr. van Middelkoop and Dr. Lane both reported having no relevant financial relationships.
AT OARSI 2023
Holy smoke: Air pollution link to bone damage confirmed
Air pollution appears to contribute independently to bone damage in postmenopausal women, new data suggest.
The findings come from a new analysis of data from the Women’s Health Initiative (WHI) and location-specific air particulate information from the U.S. Environmental Protection Agency.
“Our findings confirm that poor air quality may be a risk factor for bone loss, independent of socioeconomic or demographic factors, and expands previous findings to postmenopausal women. Indeed, to our knowledge, this is the first study of the impact of criteria air pollutants on bone health in postmenopausal women,” Diddier Prada, MD, PhD, Columbia University, New York, and colleagues wrote.
The results are also the first to show that “nitrogen oxides contribute the most to bone damage and that the lumbar spine is one of the most susceptible sites,” they added.
Public health policies should aim to reduce air pollution in general, they wrote, and reducing nitrogen oxides, in particular, will reduce bone damage in postmenopausal women, prevent bone fractures, and reduce the health cost burden associated with osteoporosis in this population.
The findings were recently published in eClinicalMedicine.
Asked to comment, Giovanni Adami, MD, PhD, said in an interview that the study “adds to the body of literature on air pollution and bone health. The study confirms and provides further evidence linking air pollution exposure and osteoporosis.”
Dr. Adami, of the University of Verona (Italy), who also studies this topic, said that these new findings align with those from his group and others.
“The scientific literature in the field is clearly pointing toward a negative effect of chronic pollution exposure on bone health.”
He pointed to one study from his group that found chronic exposure to ultrafine particulate matter is associated with low BMD, and consequently, bone fragility, and another study that showed acute exposure to high levels of pollutants could actually cause fractures.
As for what might be done clinically, Dr. Adami said: “It is difficult to extrapolate direct and immediate recommendations for patients.
“However, it might be acceptable to say that patients at risk of osteoporosis, such as older women or those with prior bone fractures, should avoid chronic exposure to air pollution, perhaps using masks when walking in traffic or using air filters for indoor ventilation.”
Dr. Adami also said that this evidence so far might spur the future inclusion of chronic exposure to air pollution in fracture risk assessment tools, although this isn’t likely to come about in the near future.
Particulates linked to whole-body, hip, lumbar, and femoral neck BMD
The prospective observational study included 9,041 WHI participants seen over 32,663 visits who were an average of 63 years old at baseline. More than 70% were White, and just under half were college graduates.
With geocoded address data used to estimate particulate matter concentrations, mean levels of particulate matter of 10 mcm or less, nitrogen oxide nitrogen dioxide, and sulfur dioxide over 1, 3, and 5 years were all negatively associated with whole-body, total hip, femoral neck, and lumbar spine BMD.
In the multivariate analysis, the highest correlations were found between nitrogen oxide and nitrogen dioxide. For example, lumbar spine BMD decreased by 0.026 g/cm2 per year per 10% increase in 3-year mean nitrogen dioxide concentration.
“Our findings show that both particulate matter and gases may adversely impact BMD and that nitrogen oxides may play a critical role in bone damage and osteoporosis risk,” Dr. Prada and colleagues wrote.
Dr. Adami added: “We need more data to understand the precise magnitude of effect of air pollution on fractures, which might depend on levels of exposure but also on genetics and lifestyle.”
The study was funded by the National Institutes of Health. The authors reported no relevant financial relationships. Dr. Adami reported receiving fees from Amgen, Eli Lilly, UCB, Fresenius Kabi, Galapagos, and Theramex.
A version of this article originally appeared on Medscape.com.
Air pollution appears to contribute independently to bone damage in postmenopausal women, new data suggest.
The findings come from a new analysis of data from the Women’s Health Initiative (WHI) and location-specific air particulate information from the U.S. Environmental Protection Agency.
“Our findings confirm that poor air quality may be a risk factor for bone loss, independent of socioeconomic or demographic factors, and expands previous findings to postmenopausal women. Indeed, to our knowledge, this is the first study of the impact of criteria air pollutants on bone health in postmenopausal women,” Diddier Prada, MD, PhD, Columbia University, New York, and colleagues wrote.
The results are also the first to show that “nitrogen oxides contribute the most to bone damage and that the lumbar spine is one of the most susceptible sites,” they added.
Public health policies should aim to reduce air pollution in general, they wrote, and reducing nitrogen oxides, in particular, will reduce bone damage in postmenopausal women, prevent bone fractures, and reduce the health cost burden associated with osteoporosis in this population.
The findings were recently published in eClinicalMedicine.
Asked to comment, Giovanni Adami, MD, PhD, said in an interview that the study “adds to the body of literature on air pollution and bone health. The study confirms and provides further evidence linking air pollution exposure and osteoporosis.”
Dr. Adami, of the University of Verona (Italy), who also studies this topic, said that these new findings align with those from his group and others.
“The scientific literature in the field is clearly pointing toward a negative effect of chronic pollution exposure on bone health.”
He pointed to one study from his group that found chronic exposure to ultrafine particulate matter is associated with low BMD, and consequently, bone fragility, and another study that showed acute exposure to high levels of pollutants could actually cause fractures.
As for what might be done clinically, Dr. Adami said: “It is difficult to extrapolate direct and immediate recommendations for patients.
“However, it might be acceptable to say that patients at risk of osteoporosis, such as older women or those with prior bone fractures, should avoid chronic exposure to air pollution, perhaps using masks when walking in traffic or using air filters for indoor ventilation.”
Dr. Adami also said that this evidence so far might spur the future inclusion of chronic exposure to air pollution in fracture risk assessment tools, although this isn’t likely to come about in the near future.
Particulates linked to whole-body, hip, lumbar, and femoral neck BMD
The prospective observational study included 9,041 WHI participants seen over 32,663 visits who were an average of 63 years old at baseline. More than 70% were White, and just under half were college graduates.
With geocoded address data used to estimate particulate matter concentrations, mean levels of particulate matter of 10 mcm or less, nitrogen oxide nitrogen dioxide, and sulfur dioxide over 1, 3, and 5 years were all negatively associated with whole-body, total hip, femoral neck, and lumbar spine BMD.
In the multivariate analysis, the highest correlations were found between nitrogen oxide and nitrogen dioxide. For example, lumbar spine BMD decreased by 0.026 g/cm2 per year per 10% increase in 3-year mean nitrogen dioxide concentration.
“Our findings show that both particulate matter and gases may adversely impact BMD and that nitrogen oxides may play a critical role in bone damage and osteoporosis risk,” Dr. Prada and colleagues wrote.
Dr. Adami added: “We need more data to understand the precise magnitude of effect of air pollution on fractures, which might depend on levels of exposure but also on genetics and lifestyle.”
The study was funded by the National Institutes of Health. The authors reported no relevant financial relationships. Dr. Adami reported receiving fees from Amgen, Eli Lilly, UCB, Fresenius Kabi, Galapagos, and Theramex.
A version of this article originally appeared on Medscape.com.
Air pollution appears to contribute independently to bone damage in postmenopausal women, new data suggest.
The findings come from a new analysis of data from the Women’s Health Initiative (WHI) and location-specific air particulate information from the U.S. Environmental Protection Agency.
“Our findings confirm that poor air quality may be a risk factor for bone loss, independent of socioeconomic or demographic factors, and expands previous findings to postmenopausal women. Indeed, to our knowledge, this is the first study of the impact of criteria air pollutants on bone health in postmenopausal women,” Diddier Prada, MD, PhD, Columbia University, New York, and colleagues wrote.
The results are also the first to show that “nitrogen oxides contribute the most to bone damage and that the lumbar spine is one of the most susceptible sites,” they added.
Public health policies should aim to reduce air pollution in general, they wrote, and reducing nitrogen oxides, in particular, will reduce bone damage in postmenopausal women, prevent bone fractures, and reduce the health cost burden associated with osteoporosis in this population.
The findings were recently published in eClinicalMedicine.
Asked to comment, Giovanni Adami, MD, PhD, said in an interview that the study “adds to the body of literature on air pollution and bone health. The study confirms and provides further evidence linking air pollution exposure and osteoporosis.”
Dr. Adami, of the University of Verona (Italy), who also studies this topic, said that these new findings align with those from his group and others.
“The scientific literature in the field is clearly pointing toward a negative effect of chronic pollution exposure on bone health.”
He pointed to one study from his group that found chronic exposure to ultrafine particulate matter is associated with low BMD, and consequently, bone fragility, and another study that showed acute exposure to high levels of pollutants could actually cause fractures.
As for what might be done clinically, Dr. Adami said: “It is difficult to extrapolate direct and immediate recommendations for patients.
“However, it might be acceptable to say that patients at risk of osteoporosis, such as older women or those with prior bone fractures, should avoid chronic exposure to air pollution, perhaps using masks when walking in traffic or using air filters for indoor ventilation.”
Dr. Adami also said that this evidence so far might spur the future inclusion of chronic exposure to air pollution in fracture risk assessment tools, although this isn’t likely to come about in the near future.
Particulates linked to whole-body, hip, lumbar, and femoral neck BMD
The prospective observational study included 9,041 WHI participants seen over 32,663 visits who were an average of 63 years old at baseline. More than 70% were White, and just under half were college graduates.
With geocoded address data used to estimate particulate matter concentrations, mean levels of particulate matter of 10 mcm or less, nitrogen oxide nitrogen dioxide, and sulfur dioxide over 1, 3, and 5 years were all negatively associated with whole-body, total hip, femoral neck, and lumbar spine BMD.
In the multivariate analysis, the highest correlations were found between nitrogen oxide and nitrogen dioxide. For example, lumbar spine BMD decreased by 0.026 g/cm2 per year per 10% increase in 3-year mean nitrogen dioxide concentration.
“Our findings show that both particulate matter and gases may adversely impact BMD and that nitrogen oxides may play a critical role in bone damage and osteoporosis risk,” Dr. Prada and colleagues wrote.
Dr. Adami added: “We need more data to understand the precise magnitude of effect of air pollution on fractures, which might depend on levels of exposure but also on genetics and lifestyle.”
The study was funded by the National Institutes of Health. The authors reported no relevant financial relationships. Dr. Adami reported receiving fees from Amgen, Eli Lilly, UCB, Fresenius Kabi, Galapagos, and Theramex.
A version of this article originally appeared on Medscape.com.
FROM ECLINICALMEDICINE
Guidelines: Don’t delay total joint arthroplasty for additional nonoperative therapies
Patients with moderate to severe osteoarthritis (OA) or osteonecrosis (ON) eligible for total joint arthroplasty (TJA) who have failed one or more nonoperative therapies should proceed directly to surgery, according to new guidelines from the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“One of the reasons for creating this guideline was that many patients have been subjected to delays for surgery after completing nonoperative therapy, despite persistent moderate to severe pain, loss of function, and moderate to severe radiographic OA or ON,” said coauthors Susan M. Goodman, MD, a rheumatologist at Hospital for Special Surgery in New York, and Charles Hannon, MD, an orthopedic surgeon at Washington University in St. Louis, in an email interview with this news organization. “This guideline supports surgery being performed in an expeditious fashion after the decision has been made to proceed with surgery by both the physician and patient through a shared decision-making process,” they said.
The guidelines also state that obesity by itself should not be a reason to delay TJA. “We could not find a rationale for a strict cut off for weight/body mass index (BMI). Our literature review revealed that though many adverse events were, in fact, increased in patients with morbid obesity, there is also an increase in adverse events for those who had bariatric surgery prior to their arthroplasty,” they added, noting that patients need to be made aware of the increased risk for adverse events in patients with obesity. Though the guidelines do not pose any BMI cutoffs, they state that weight loss should be “strongly encouraged.” These new recommendations are conditional, and all had a “low” to “very low” certainty of evidence; however, there was high consensus on the recommendations from the expert panel.
The guidelines also recommended:
- Delaying TJA to achieve smoking and nicotine cessation or reduction.
- Delaying TJA to improve glycemic control in patients with diabetes, although the group did not recommend any specific measure or threshold.
- Not delaying TJA in patients with a severe deformity, bone loss, or a neuropathic joint.
The new guidelines formalize what many surgeons have already been doing for the past few years, said Arjun Saxena, MD, MBA, an orthopedic surgeon in Philadelphia who was not involved with the guidelines. “A lot of total joint programs have really focused on patient optimization, including smoking cessation, glycemic control, and weight loss prior to surgery,” he said.
Most importantly, the guidelines put an emphasis on how the decision to proceed with TJA should be a shared decision between a physician and patient, he added. Some insurance companies with prior authorization policies may require a patient to try additional nonoperative therapies before approving surgery, creating barriers to care, he said. “Hopefully [these new recommendations] will help third parties understand that joint replacement is a big decision – most doctors aren’t going to recommend that unless it’s necessary or something that is going to help patients,” he said. “I understand that there is a certain need for preauthorization, but just having strict guidelines isn’t appropriate. You really need to look at the whole picture,” he added.
The full manuscript has been submitted for review and is expected to be jointly published in American College of Rheumatology and the American Association of Hip and Knee Surgeons journals later this year.
Dr. Saxena consults for the orthopedic implant company Corin.
A version of this article originally appeared on Medscape.com.
Patients with moderate to severe osteoarthritis (OA) or osteonecrosis (ON) eligible for total joint arthroplasty (TJA) who have failed one or more nonoperative therapies should proceed directly to surgery, according to new guidelines from the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“One of the reasons for creating this guideline was that many patients have been subjected to delays for surgery after completing nonoperative therapy, despite persistent moderate to severe pain, loss of function, and moderate to severe radiographic OA or ON,” said coauthors Susan M. Goodman, MD, a rheumatologist at Hospital for Special Surgery in New York, and Charles Hannon, MD, an orthopedic surgeon at Washington University in St. Louis, in an email interview with this news organization. “This guideline supports surgery being performed in an expeditious fashion after the decision has been made to proceed with surgery by both the physician and patient through a shared decision-making process,” they said.
The guidelines also state that obesity by itself should not be a reason to delay TJA. “We could not find a rationale for a strict cut off for weight/body mass index (BMI). Our literature review revealed that though many adverse events were, in fact, increased in patients with morbid obesity, there is also an increase in adverse events for those who had bariatric surgery prior to their arthroplasty,” they added, noting that patients need to be made aware of the increased risk for adverse events in patients with obesity. Though the guidelines do not pose any BMI cutoffs, they state that weight loss should be “strongly encouraged.” These new recommendations are conditional, and all had a “low” to “very low” certainty of evidence; however, there was high consensus on the recommendations from the expert panel.
The guidelines also recommended:
- Delaying TJA to achieve smoking and nicotine cessation or reduction.
- Delaying TJA to improve glycemic control in patients with diabetes, although the group did not recommend any specific measure or threshold.
- Not delaying TJA in patients with a severe deformity, bone loss, or a neuropathic joint.
The new guidelines formalize what many surgeons have already been doing for the past few years, said Arjun Saxena, MD, MBA, an orthopedic surgeon in Philadelphia who was not involved with the guidelines. “A lot of total joint programs have really focused on patient optimization, including smoking cessation, glycemic control, and weight loss prior to surgery,” he said.
Most importantly, the guidelines put an emphasis on how the decision to proceed with TJA should be a shared decision between a physician and patient, he added. Some insurance companies with prior authorization policies may require a patient to try additional nonoperative therapies before approving surgery, creating barriers to care, he said. “Hopefully [these new recommendations] will help third parties understand that joint replacement is a big decision – most doctors aren’t going to recommend that unless it’s necessary or something that is going to help patients,” he said. “I understand that there is a certain need for preauthorization, but just having strict guidelines isn’t appropriate. You really need to look at the whole picture,” he added.
The full manuscript has been submitted for review and is expected to be jointly published in American College of Rheumatology and the American Association of Hip and Knee Surgeons journals later this year.
Dr. Saxena consults for the orthopedic implant company Corin.
A version of this article originally appeared on Medscape.com.
Patients with moderate to severe osteoarthritis (OA) or osteonecrosis (ON) eligible for total joint arthroplasty (TJA) who have failed one or more nonoperative therapies should proceed directly to surgery, according to new guidelines from the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“One of the reasons for creating this guideline was that many patients have been subjected to delays for surgery after completing nonoperative therapy, despite persistent moderate to severe pain, loss of function, and moderate to severe radiographic OA or ON,” said coauthors Susan M. Goodman, MD, a rheumatologist at Hospital for Special Surgery in New York, and Charles Hannon, MD, an orthopedic surgeon at Washington University in St. Louis, in an email interview with this news organization. “This guideline supports surgery being performed in an expeditious fashion after the decision has been made to proceed with surgery by both the physician and patient through a shared decision-making process,” they said.
The guidelines also state that obesity by itself should not be a reason to delay TJA. “We could not find a rationale for a strict cut off for weight/body mass index (BMI). Our literature review revealed that though many adverse events were, in fact, increased in patients with morbid obesity, there is also an increase in adverse events for those who had bariatric surgery prior to their arthroplasty,” they added, noting that patients need to be made aware of the increased risk for adverse events in patients with obesity. Though the guidelines do not pose any BMI cutoffs, they state that weight loss should be “strongly encouraged.” These new recommendations are conditional, and all had a “low” to “very low” certainty of evidence; however, there was high consensus on the recommendations from the expert panel.
The guidelines also recommended:
- Delaying TJA to achieve smoking and nicotine cessation or reduction.
- Delaying TJA to improve glycemic control in patients with diabetes, although the group did not recommend any specific measure or threshold.
- Not delaying TJA in patients with a severe deformity, bone loss, or a neuropathic joint.
The new guidelines formalize what many surgeons have already been doing for the past few years, said Arjun Saxena, MD, MBA, an orthopedic surgeon in Philadelphia who was not involved with the guidelines. “A lot of total joint programs have really focused on patient optimization, including smoking cessation, glycemic control, and weight loss prior to surgery,” he said.
Most importantly, the guidelines put an emphasis on how the decision to proceed with TJA should be a shared decision between a physician and patient, he added. Some insurance companies with prior authorization policies may require a patient to try additional nonoperative therapies before approving surgery, creating barriers to care, he said. “Hopefully [these new recommendations] will help third parties understand that joint replacement is a big decision – most doctors aren’t going to recommend that unless it’s necessary or something that is going to help patients,” he said. “I understand that there is a certain need for preauthorization, but just having strict guidelines isn’t appropriate. You really need to look at the whole picture,” he added.
The full manuscript has been submitted for review and is expected to be jointly published in American College of Rheumatology and the American Association of Hip and Knee Surgeons journals later this year.
Dr. Saxena consults for the orthopedic implant company Corin.
A version of this article originally appeared on Medscape.com.
JAK inhibitor safety warnings drawn from rheumatologic data may be misleading in dermatology
NEW ORLEANS – , even though the basis for all the risks is a rheumatoid arthritis study, according to a critical review at the annual meeting of the American Academy of Dermatology.
Given the fact that the postmarketing RA study was specifically enriched with high-risk patients by requiring an age at enrollment of at least 50 years and the presence of at least one cardiovascular risk factor, the extrapolation of these risks to dermatologic indications is “not necessarily data-driven,” said Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
The recently approved deucravacitinib is the only JAK inhibitor that has so far been exempt from these warnings. Instead, based on the ORAL Surveillance study, published in the New England Journal of Medicine, the Food and Drug Administration requires a boxed warning in nearly identical language for all the other JAK inhibitors. Relative to tofacitinib, the JAK inhibitor tested in ORAL Surveillance, many of these drugs differ by JAK selectivity and other characteristics that are likely relevant to risk of adverse events, Dr. King said. The same language has even been applied to topical ruxolitinib cream.
Basis of boxed warnings
In ORAL Surveillance, about 4,300 high-risk patients with RA were randomized to one of two doses of tofacitinib (5 mg or 10 mg) twice daily or a tumor necrosis factor (TNF) inhibitor. All patients in the trial were taking methotrexate, and almost 60% were taking concomitant corticosteroids. The average body mass index of the study population was about 30 kg/m2.
After a median 4 years of follow-up (about 5,000 patient-years), the incidence of many of the adverse events tracked in the study were higher in the tofacitinib groups, including serious infections, MACE, thromboembolic events, and cancer. Dr. King did not challenge the importance of these data, but he questioned whether they are reasonably extrapolated to dermatologic indications, particularly as many of those treated are younger than those common to an RA population.
In fact, despite a study enriched for a higher risk of many events tracked, most adverse events were only slightly elevated, Dr. King pointed out. For example, the incidence of MACE over the 4 years of follow-up was 3.4% among those taking any dose of tofacitinib versus 2.5% of those randomized to TNF inhibitor. Rates of cancer were 4.2% versus 2.9%, respectively. There were also absolute increases in the number of serious infections and thromboembolic events for tofacitinib relative to TNF inhibitor.
Dr. King acknowledged that the numbers in ORAL Surveillance associated tofacitinib with a higher risk of serious events than TNF inhibitor in patients with RA, but he believes that “JAK inhibitor safety is almost certainly not the same in dermatology as it is in rheumatology patients.”
Evidence of difference in dermatology
There is some evidence to back this up. Dr. King cited a recently published study in RMD Open that evaluated the safety profile of the JAK inhibitor upadacitinib in nearly 7,000 patients over 15,000 patient-years of follow-up. Drug safety data were evaluated with up to 5.5 years of follow-up from 12 clinical trials of the four diseases for which upadacitinib is now indicated. Three were rheumatologic (RA, psoriatic arthritis, and ankylosing spondylitis), and the fourth was atopic dermatitis (AD). Fourteen outcomes, including numerous types of infection, MACE, hepatic complications, and malignancy, were compared with methotrexate and the TNF inhibitor adalimumab.
For the RA diseases, upadacitinib was associated with a greater risk than comparators for several outcomes, including serious infections. But in AD, there was a smaller increased risk of adverse outcomes for the JAK inhibitor relative to comparators.
When evaluated by risk of adverse events across indications, for MACE, the exposure-adjusted event rates for upadacitinib were less than 0.1 in patients treated for AD over the observation period versus 0.3 and 0.4 for RA and psoriatic arthritis, respectively. Similarly, for venous thromboembolism, the rates for upadacitinib were again less than 0.1 in patients with AD versus 0.4 and 0.2 in RA and psoriatic arthritis, respectively.
Referring back to the postmarketing study, Dr. King emphasized that it is essential to consider how the boxed warning for JAK inhibitors was generated before applying them to dermatologic indications.
“Is a 30-year-old patient with a dermatologic disorder possibly at the same risk as the patients in the study from which we got the boxed warning? The answer is simply no,” he said.
Like the tofacitinib data in the ORAL Surveillance study, the upadacitinib clinical trial data are not necessarily relevant to other JAK inhibitors. In fact, Dr. King pointed out that the safety profiles of the available JAK inhibitors are not identical, an observation that is consistent with differences in JAK inhibitor selectivity that has implications for off-target events.
Dr. King does not dismiss the potential risks outlined in the current regulatory cautions about the use of JAK inhibitors, but he believes that dermatologists should be cognizant of “where the black box warning comes from.”
“We need to think carefully about the risk-to-benefit ratio in older patients or patients with risk factors, such as obesity and diabetes,” he said. But the safety profile of JAK inhibitors “is almost certainly better” than the profile suggested in boxed warnings applied to JAK inhibitors for dermatologic indications, he advised.
Risk-benefit considerations in dermatology
This position was supported by numerous other experts when asked for their perspectives. “I fully agree,” said Emma Guttman-Yassky, MD, PhD, system chair of dermatology and immunology, Icahn School of Medicine, Mount Sinai, New York.
Like Dr. King, Dr. Guttman-Yassky did not dismiss the potential risks of JAK inhibitors when treating dermatologic diseases.
“While JAK inhibitors need monitoring as advised, adopting a boxed warning from an RA study for patients who are older [is problematic],” she commented. A study with the nonselective tofacitinib in this population “cannot be compared to more selective inhibitors in a much younger population, such as those treated [for] alopecia areata or atopic dermatitis.”
George Z. Han, MD, PhD, an associate professor of dermatology, Zucker School of Medicine, Hofstra, Northwell Medical Center, New Hyde Park, New York, also agreed but added some caveats.
“The comments about the ORAL Surveillance study are salient,” he said in an interview. “This kind of data should not directly be extrapolated to other patient types or to other medications.” However, one of Dr. Han’s most important caveats involves long-term use.
“JAK inhibitors are still relatively narrow-therapeutic-window drugs that in a dose-dependent fashion could lead to negative effects, including thromboembolic events, abnormalities in red blood cells, white blood cells, platelets, and lipids,” he said. While doses used in dermatology “are generally below the level of any major concern,” Dr. Han cautioned that “we lack definitive data” on long-term use, and this is important for understanding “any potential small risk of rare events, such as malignancy or thromboembolism.”
Saakshi Khattri, MD, a colleague of Dr. Guttman-Yassky at Mount Sinai, said the risks of JAK inhibitors should not be underestimated, but she also agreed that risk “needs to be delivered in the right context.” Dr. Khattri, who is board certified in both dermatology and rheumatology, noted the safety profiles of available JAK inhibitors differ and that extrapolating safety from an RA study to dermatologic indications does not make sense. “Different diseases, different age groups,” she said.
Dr. King has reported financial relationships with more than 15 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Han reports financial relationships with Amgen, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, PellePharm, Pfizer, and UCB. Dr. Khattri has reported financial relationships with AbbVie, Arcutis, Bristol-Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, and UCB.
A version of this article originally appeared on Medscape.com.
NEW ORLEANS – , even though the basis for all the risks is a rheumatoid arthritis study, according to a critical review at the annual meeting of the American Academy of Dermatology.
Given the fact that the postmarketing RA study was specifically enriched with high-risk patients by requiring an age at enrollment of at least 50 years and the presence of at least one cardiovascular risk factor, the extrapolation of these risks to dermatologic indications is “not necessarily data-driven,” said Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
The recently approved deucravacitinib is the only JAK inhibitor that has so far been exempt from these warnings. Instead, based on the ORAL Surveillance study, published in the New England Journal of Medicine, the Food and Drug Administration requires a boxed warning in nearly identical language for all the other JAK inhibitors. Relative to tofacitinib, the JAK inhibitor tested in ORAL Surveillance, many of these drugs differ by JAK selectivity and other characteristics that are likely relevant to risk of adverse events, Dr. King said. The same language has even been applied to topical ruxolitinib cream.
Basis of boxed warnings
In ORAL Surveillance, about 4,300 high-risk patients with RA were randomized to one of two doses of tofacitinib (5 mg or 10 mg) twice daily or a tumor necrosis factor (TNF) inhibitor. All patients in the trial were taking methotrexate, and almost 60% were taking concomitant corticosteroids. The average body mass index of the study population was about 30 kg/m2.
After a median 4 years of follow-up (about 5,000 patient-years), the incidence of many of the adverse events tracked in the study were higher in the tofacitinib groups, including serious infections, MACE, thromboembolic events, and cancer. Dr. King did not challenge the importance of these data, but he questioned whether they are reasonably extrapolated to dermatologic indications, particularly as many of those treated are younger than those common to an RA population.
In fact, despite a study enriched for a higher risk of many events tracked, most adverse events were only slightly elevated, Dr. King pointed out. For example, the incidence of MACE over the 4 years of follow-up was 3.4% among those taking any dose of tofacitinib versus 2.5% of those randomized to TNF inhibitor. Rates of cancer were 4.2% versus 2.9%, respectively. There were also absolute increases in the number of serious infections and thromboembolic events for tofacitinib relative to TNF inhibitor.
Dr. King acknowledged that the numbers in ORAL Surveillance associated tofacitinib with a higher risk of serious events than TNF inhibitor in patients with RA, but he believes that “JAK inhibitor safety is almost certainly not the same in dermatology as it is in rheumatology patients.”
Evidence of difference in dermatology
There is some evidence to back this up. Dr. King cited a recently published study in RMD Open that evaluated the safety profile of the JAK inhibitor upadacitinib in nearly 7,000 patients over 15,000 patient-years of follow-up. Drug safety data were evaluated with up to 5.5 years of follow-up from 12 clinical trials of the four diseases for which upadacitinib is now indicated. Three were rheumatologic (RA, psoriatic arthritis, and ankylosing spondylitis), and the fourth was atopic dermatitis (AD). Fourteen outcomes, including numerous types of infection, MACE, hepatic complications, and malignancy, were compared with methotrexate and the TNF inhibitor adalimumab.
For the RA diseases, upadacitinib was associated with a greater risk than comparators for several outcomes, including serious infections. But in AD, there was a smaller increased risk of adverse outcomes for the JAK inhibitor relative to comparators.
When evaluated by risk of adverse events across indications, for MACE, the exposure-adjusted event rates for upadacitinib were less than 0.1 in patients treated for AD over the observation period versus 0.3 and 0.4 for RA and psoriatic arthritis, respectively. Similarly, for venous thromboembolism, the rates for upadacitinib were again less than 0.1 in patients with AD versus 0.4 and 0.2 in RA and psoriatic arthritis, respectively.
Referring back to the postmarketing study, Dr. King emphasized that it is essential to consider how the boxed warning for JAK inhibitors was generated before applying them to dermatologic indications.
“Is a 30-year-old patient with a dermatologic disorder possibly at the same risk as the patients in the study from which we got the boxed warning? The answer is simply no,” he said.
Like the tofacitinib data in the ORAL Surveillance study, the upadacitinib clinical trial data are not necessarily relevant to other JAK inhibitors. In fact, Dr. King pointed out that the safety profiles of the available JAK inhibitors are not identical, an observation that is consistent with differences in JAK inhibitor selectivity that has implications for off-target events.
Dr. King does not dismiss the potential risks outlined in the current regulatory cautions about the use of JAK inhibitors, but he believes that dermatologists should be cognizant of “where the black box warning comes from.”
“We need to think carefully about the risk-to-benefit ratio in older patients or patients with risk factors, such as obesity and diabetes,” he said. But the safety profile of JAK inhibitors “is almost certainly better” than the profile suggested in boxed warnings applied to JAK inhibitors for dermatologic indications, he advised.
Risk-benefit considerations in dermatology
This position was supported by numerous other experts when asked for their perspectives. “I fully agree,” said Emma Guttman-Yassky, MD, PhD, system chair of dermatology and immunology, Icahn School of Medicine, Mount Sinai, New York.
Like Dr. King, Dr. Guttman-Yassky did not dismiss the potential risks of JAK inhibitors when treating dermatologic diseases.
“While JAK inhibitors need monitoring as advised, adopting a boxed warning from an RA study for patients who are older [is problematic],” she commented. A study with the nonselective tofacitinib in this population “cannot be compared to more selective inhibitors in a much younger population, such as those treated [for] alopecia areata or atopic dermatitis.”
George Z. Han, MD, PhD, an associate professor of dermatology, Zucker School of Medicine, Hofstra, Northwell Medical Center, New Hyde Park, New York, also agreed but added some caveats.
“The comments about the ORAL Surveillance study are salient,” he said in an interview. “This kind of data should not directly be extrapolated to other patient types or to other medications.” However, one of Dr. Han’s most important caveats involves long-term use.
“JAK inhibitors are still relatively narrow-therapeutic-window drugs that in a dose-dependent fashion could lead to negative effects, including thromboembolic events, abnormalities in red blood cells, white blood cells, platelets, and lipids,” he said. While doses used in dermatology “are generally below the level of any major concern,” Dr. Han cautioned that “we lack definitive data” on long-term use, and this is important for understanding “any potential small risk of rare events, such as malignancy or thromboembolism.”
Saakshi Khattri, MD, a colleague of Dr. Guttman-Yassky at Mount Sinai, said the risks of JAK inhibitors should not be underestimated, but she also agreed that risk “needs to be delivered in the right context.” Dr. Khattri, who is board certified in both dermatology and rheumatology, noted the safety profiles of available JAK inhibitors differ and that extrapolating safety from an RA study to dermatologic indications does not make sense. “Different diseases, different age groups,” she said.
Dr. King has reported financial relationships with more than 15 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Han reports financial relationships with Amgen, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, PellePharm, Pfizer, and UCB. Dr. Khattri has reported financial relationships with AbbVie, Arcutis, Bristol-Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, and UCB.
A version of this article originally appeared on Medscape.com.
NEW ORLEANS – , even though the basis for all the risks is a rheumatoid arthritis study, according to a critical review at the annual meeting of the American Academy of Dermatology.
Given the fact that the postmarketing RA study was specifically enriched with high-risk patients by requiring an age at enrollment of at least 50 years and the presence of at least one cardiovascular risk factor, the extrapolation of these risks to dermatologic indications is “not necessarily data-driven,” said Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
The recently approved deucravacitinib is the only JAK inhibitor that has so far been exempt from these warnings. Instead, based on the ORAL Surveillance study, published in the New England Journal of Medicine, the Food and Drug Administration requires a boxed warning in nearly identical language for all the other JAK inhibitors. Relative to tofacitinib, the JAK inhibitor tested in ORAL Surveillance, many of these drugs differ by JAK selectivity and other characteristics that are likely relevant to risk of adverse events, Dr. King said. The same language has even been applied to topical ruxolitinib cream.
Basis of boxed warnings
In ORAL Surveillance, about 4,300 high-risk patients with RA were randomized to one of two doses of tofacitinib (5 mg or 10 mg) twice daily or a tumor necrosis factor (TNF) inhibitor. All patients in the trial were taking methotrexate, and almost 60% were taking concomitant corticosteroids. The average body mass index of the study population was about 30 kg/m2.
After a median 4 years of follow-up (about 5,000 patient-years), the incidence of many of the adverse events tracked in the study were higher in the tofacitinib groups, including serious infections, MACE, thromboembolic events, and cancer. Dr. King did not challenge the importance of these data, but he questioned whether they are reasonably extrapolated to dermatologic indications, particularly as many of those treated are younger than those common to an RA population.
In fact, despite a study enriched for a higher risk of many events tracked, most adverse events were only slightly elevated, Dr. King pointed out. For example, the incidence of MACE over the 4 years of follow-up was 3.4% among those taking any dose of tofacitinib versus 2.5% of those randomized to TNF inhibitor. Rates of cancer were 4.2% versus 2.9%, respectively. There were also absolute increases in the number of serious infections and thromboembolic events for tofacitinib relative to TNF inhibitor.
Dr. King acknowledged that the numbers in ORAL Surveillance associated tofacitinib with a higher risk of serious events than TNF inhibitor in patients with RA, but he believes that “JAK inhibitor safety is almost certainly not the same in dermatology as it is in rheumatology patients.”
Evidence of difference in dermatology
There is some evidence to back this up. Dr. King cited a recently published study in RMD Open that evaluated the safety profile of the JAK inhibitor upadacitinib in nearly 7,000 patients over 15,000 patient-years of follow-up. Drug safety data were evaluated with up to 5.5 years of follow-up from 12 clinical trials of the four diseases for which upadacitinib is now indicated. Three were rheumatologic (RA, psoriatic arthritis, and ankylosing spondylitis), and the fourth was atopic dermatitis (AD). Fourteen outcomes, including numerous types of infection, MACE, hepatic complications, and malignancy, were compared with methotrexate and the TNF inhibitor adalimumab.
For the RA diseases, upadacitinib was associated with a greater risk than comparators for several outcomes, including serious infections. But in AD, there was a smaller increased risk of adverse outcomes for the JAK inhibitor relative to comparators.
When evaluated by risk of adverse events across indications, for MACE, the exposure-adjusted event rates for upadacitinib were less than 0.1 in patients treated for AD over the observation period versus 0.3 and 0.4 for RA and psoriatic arthritis, respectively. Similarly, for venous thromboembolism, the rates for upadacitinib were again less than 0.1 in patients with AD versus 0.4 and 0.2 in RA and psoriatic arthritis, respectively.
Referring back to the postmarketing study, Dr. King emphasized that it is essential to consider how the boxed warning for JAK inhibitors was generated before applying them to dermatologic indications.
“Is a 30-year-old patient with a dermatologic disorder possibly at the same risk as the patients in the study from which we got the boxed warning? The answer is simply no,” he said.
Like the tofacitinib data in the ORAL Surveillance study, the upadacitinib clinical trial data are not necessarily relevant to other JAK inhibitors. In fact, Dr. King pointed out that the safety profiles of the available JAK inhibitors are not identical, an observation that is consistent with differences in JAK inhibitor selectivity that has implications for off-target events.
Dr. King does not dismiss the potential risks outlined in the current regulatory cautions about the use of JAK inhibitors, but he believes that dermatologists should be cognizant of “where the black box warning comes from.”
“We need to think carefully about the risk-to-benefit ratio in older patients or patients with risk factors, such as obesity and diabetes,” he said. But the safety profile of JAK inhibitors “is almost certainly better” than the profile suggested in boxed warnings applied to JAK inhibitors for dermatologic indications, he advised.
Risk-benefit considerations in dermatology
This position was supported by numerous other experts when asked for their perspectives. “I fully agree,” said Emma Guttman-Yassky, MD, PhD, system chair of dermatology and immunology, Icahn School of Medicine, Mount Sinai, New York.
Like Dr. King, Dr. Guttman-Yassky did not dismiss the potential risks of JAK inhibitors when treating dermatologic diseases.
“While JAK inhibitors need monitoring as advised, adopting a boxed warning from an RA study for patients who are older [is problematic],” she commented. A study with the nonselective tofacitinib in this population “cannot be compared to more selective inhibitors in a much younger population, such as those treated [for] alopecia areata or atopic dermatitis.”
George Z. Han, MD, PhD, an associate professor of dermatology, Zucker School of Medicine, Hofstra, Northwell Medical Center, New Hyde Park, New York, also agreed but added some caveats.
“The comments about the ORAL Surveillance study are salient,” he said in an interview. “This kind of data should not directly be extrapolated to other patient types or to other medications.” However, one of Dr. Han’s most important caveats involves long-term use.
“JAK inhibitors are still relatively narrow-therapeutic-window drugs that in a dose-dependent fashion could lead to negative effects, including thromboembolic events, abnormalities in red blood cells, white blood cells, platelets, and lipids,” he said. While doses used in dermatology “are generally below the level of any major concern,” Dr. Han cautioned that “we lack definitive data” on long-term use, and this is important for understanding “any potential small risk of rare events, such as malignancy or thromboembolism.”
Saakshi Khattri, MD, a colleague of Dr. Guttman-Yassky at Mount Sinai, said the risks of JAK inhibitors should not be underestimated, but she also agreed that risk “needs to be delivered in the right context.” Dr. Khattri, who is board certified in both dermatology and rheumatology, noted the safety profiles of available JAK inhibitors differ and that extrapolating safety from an RA study to dermatologic indications does not make sense. “Different diseases, different age groups,” she said.
Dr. King has reported financial relationships with more than 15 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Han reports financial relationships with Amgen, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, PellePharm, Pfizer, and UCB. Dr. Khattri has reported financial relationships with AbbVie, Arcutis, Bristol-Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, and UCB.
A version of this article originally appeared on Medscape.com.
AT AAD 2023
Experts share early details prescribing avacopan for ANCA-associated vasculitis
When the Food and Drug Administration approved avacopan (Tavneos) as an adjunctive treatment for severe, active antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis (AAV) in October 2021, the oral complement C5a receptor inhibitor was hailed by its developer, ChemoCentryx, as a “new hope” for patients with the disease.
But avacopan’s novelty as a new drug for the rare diseases granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), coupled with its approval as an adjunctive to standard therapy, including glucocorticoids, rather than strictly as a glucocorticoid-sparing agent as it was tested, has so far led to little reported real-world experience with the drug.
In the phase 3 ADVOCATE trial, the pivotal trial that served as the basis for avacopan’s approval, 331 patients with active newly diagnosed or relapsing GPA or MPA received either avacopan or an oral prednisone taper over 20 weeks on a background of cyclophosphamide followed by azathioprine or rituximab. The results of the trial showed avacopan was noninferior to the group that received prednisone taper for remission at 26 weeks and superior to prednisone taper for sustained remission at 52 weeks, but the FDA was concerned that its complex design made it difficult to define the clinically meaningful benefit of avacopan and its role in the management of AAV.
The FDA noted that, in the avacopan arm of the trial, 86% of patients received glucocorticoids outside of the study protocol. Despite this, avacopan reduced the cumulative glucocorticoid dose over the trial’s 52 weeks by nearly two-thirds, compared with the prednisone group (1,349 mg vs. 3,655 mg).
The data also indicate a higher sustained remission rate at 52 weeks in patients who received induction with rituximab, compared with cyclophosphamide. But trial did not include a maintenance therapy dose of rituximab and is thereby not a good comparison against the standard of care, the FDA said. (ADVOCATE began enrolling patients prior to the FDA's 2018 approval of an expanded indication for patients with GPA or MPA who have achieved disease control after induction treatment.)
At the FDA’s Arthritis Advisory Committee meeting in May 2021, committee members were split on whether to recommend avacopan for approval. The committee voted 9-9 on whether the ADVOCATE trial showed efficacy supporting approval of avacopan, 10-8 in favor of whether the drug’s safety profile supported approval, and 10-8 in favor of the overall benefit-risk profile of avacopan for approval. But rather than give an indication to avacopan to reduce the use of glucocorticoids in adults with GPA or MPA, the agency approved avacopan as an adjunctive treatment for severe, active disease, noting in particular that avacopan “does not eliminate glucocorticoid use.”
The European Union’s marketing authorization for avacopan states its indication for use in combination with a rituximab or cyclophosphamide regimen for the treatment of adult patients with severe, active GPA or MPA and does not mention a role for reducing glucocorticoids. Avacopan will appear in forthcoming guidelines on management of AAV released by the European Alliance of Associations for Rheumatology.
In North America, the Canadian Vasculitis Research Network recently released an addendum to their guidelines on AAV specifically for avacopan, which includes recommendations to consider adding oral avacopan (30 mg twice daily) for induction of remission in patients with new or relapsing GPA or MPA who are also receiving cyclophosphamide or rituximab. The guidelines also recommend clinicians consider a glucocorticoid tapering schedule that aims for discontinuation at 4 weeks, and continuing avacopan for at least 1 year after induction therapy. The American College of Rheumatology guideline for AAV management, updated in 2021, acknowledges avacopan but did not consider its inclusion prior to FDA approval.
There have been few real-world studies of how patients with AAV are responding to avacopan, but recent studies from researchers in the Netherlands and in France have evaluated prednisone tapering and clinical outcomes.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, said those real-world studies “seemed to re-enforce the findings from the ADVOCATE study demonstrating the efficacy of avacopan in severe disease with steroid-sparing effects.”
However, Carol Langford, MD, MHS, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, emphasized caution is needed when drawing conclusions about avacopan use outside formal studies.
“We are all interested in what other settings this might be used. I think those are things that really require formal investigation to really try and understand better as far as through a study process,” she said.
Prescribing experience with avacopan
A spokesperson from Amgen, which recently acquired ChemoCentryx, said in an interview that over 800 physicians in the United States have prescribed avacopan to patients with new or relapsing ANCA-associated vasculitis as induction or maintenance treatment, and physicians have reported outcomes consistent with the ADVOCATE trial.
Many rheumatologists are likely familiar with avacopan but are not used to prescribing it, said Lindsay S. Lally, MD, a rheumatologist with Hospital for Special Surgery in New York.
“Rituximab was approved for GPA and MPA a decade ago at this point. It was a drug that we as rheumatologists were used to using. We used it for other indications. Avacopan is a totally new drug, a new mechanism of action, so there’s not a lot of extractable data that we have in terms of comfort with the drug, and so I think that’s one of the biggest hurdles,” she said.
Mehrnaz Hojjati, MD, a rheumatologist with Loma Linda (Calif.) University Health, said that, when the FDA approved avacopan, it was an “exciting time” in her practice. “I have used avacopan now in a handful of my patients with severe ANCA-associated vasculitis, and the results are similar to what [was] reported in the ADVOCATE trial.”
Amgen offers help for clinicians in obtaining avacopan for patients, financial assistance for patients, and support in navigating insurance, which several rheumatologists noted was important for patients. Dr. Langford said the process of working with the manufacturer to get avacopan while insurance information is being processed has been “fairly smooth.”
“Certainly, the ability to get a very rapid 30-day supply with the goal of trying to initiate this as early as possible in the disease process has been helpful,” she said.
In Dr. Dua’s experience, while there were “some glitches or difficulty for providers early on” in how to access and prescribe avacopan, since then “it has been much easier to obtain the medication with the first month being provided to patients free while the authorization process is managed.”
Prescribing avacopan from inpatient pharmacies has been more challenging, she said. “The inpatient side is trickier because each hospital system has their own pharmacy system and regulations that have to be navigated. For outpatients, all the provider needs to do is fill out the start form available on their website, have the patients sign it, and then have it sent in.”
Concerns about affordability, insurance approval
Another consideration is cost, with avacopan having an estimated price of $150,000-$200,000 per patient per year.
Dr. Hojjati noted that, while it is easy to prescribe, avacopan is hard to get approved through insurance. “We face the same challenge every time a new medication comes to the market on how to convince the payers to pay for it given higher prices,” she said.
Rheumatologist Michael Putman, MD, MSCI, assistant professor of medicine at the Medical College of Wisconsin, Milwaukee, also acknowledged some difficulties in prescribing the medication. “The insurance companies have no interest in spending $150,000 on a drug that they know nothing about, and patients are a little hesitant to take it because it’s just so new,” he said.
While Dr. Lally said avacopan has not been difficult to get for patients with commercial insurance, reimbursement through Medicare has been problematic. “In many of the Medicare patients it has not really been a feasible option for them to be on the drug for the year of therapy.”
Patient response
Dr. Dua said almost all her patients with new or relapsing AAV who require induction are being prescribed avacopan, and that the medication is well tolerated. “The remission and ability to wean prednisone has really paralleled the findings from the clinical trial.”
In her practice, Dr. Hojjati starts patients on avacopan immediately after discharge from the hospital after a major vasculitis flare requiring high-dose glucocorticoids. “Avacopan does not eliminate/replace GC [glucocorticoid] use but has a notable GC-sparing effect and assists in rapid tapering of the GC while treating our severe ANCA-associated vasculitis patients,” she said.
Dr. Lally said her patients are tolerating avacopan well and hasn’t seen any of the safety signals seen in the trial, including liver function abnormalities. She has treated about 20-25 patients with avacopan.
Dr. Putman noted that he has treated about five patients with avacopan but hasn’t seen dramatic efficacy or side effects in his practice, compared with standard therapy.
Unanswered questions about avacopan
A key unanswered question with avacopan is the timeline for tapering glucocorticoids once patients start treatment. “I would like to see much more data on how prednisone is being tapered in clinical practice as well as outcomes in patients who are treated with the standard of care second dose of rituximab at 6 months,” Dr. Dua said.
Dr. Lally noted she has tried to expedite the steroid taper in her patients. “That’s really where I feel this drug is going to have most relevance, is getting it started early in active disease and getting patients off of the reliance on high doses of oral steroids. I have been able to see that in practice, and I do think ultimately that’s going to lead to better outcomes and quality of life for these patients.”
Of the rheumatologists Dr. Lally has spoken to about avacopan, there is “some confusion about what type of patients are appropriate, [and] how sick or not sick the patient needs to be.”
Dr. Putman noted he is unsure which of his patients should be receiving avacopan. “I don’t totally have a sense for where avacopan stands and how often we should be using it” outside of patients with severe disease. He added that the drug is still trying to find a niche because most patients with AAV who take rituximab and steroids get better without additional treatments.
“I think we do a pretty good job treating these diseases even in the preavacopan era. But it’s really a matter of how to really optimize these outcomes, reduce damage, reduce steroid-related and treatment-related toxicity for our patients,” Dr. Lally said.
Dr. Dua reported being a consultant and serving on advisory boards for ChemoCentryx; she was also a site principal investigator for the ADVOCATE trial. Dr. Hojjati reported being on the speaker’s bureau for Amgen. Dr. Langford reported being an investigator in the ADVOCATE trial, and her institution received funding to conduct the trial. Dr. Lally reported being a consultant for Amgen on avacopan. Dr. Putman reported no relevant financial disclosures.
*This story was updated 3/15/2023.
When the Food and Drug Administration approved avacopan (Tavneos) as an adjunctive treatment for severe, active antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis (AAV) in October 2021, the oral complement C5a receptor inhibitor was hailed by its developer, ChemoCentryx, as a “new hope” for patients with the disease.
But avacopan’s novelty as a new drug for the rare diseases granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), coupled with its approval as an adjunctive to standard therapy, including glucocorticoids, rather than strictly as a glucocorticoid-sparing agent as it was tested, has so far led to little reported real-world experience with the drug.
In the phase 3 ADVOCATE trial, the pivotal trial that served as the basis for avacopan’s approval, 331 patients with active newly diagnosed or relapsing GPA or MPA received either avacopan or an oral prednisone taper over 20 weeks on a background of cyclophosphamide followed by azathioprine or rituximab. The results of the trial showed avacopan was noninferior to the group that received prednisone taper for remission at 26 weeks and superior to prednisone taper for sustained remission at 52 weeks, but the FDA was concerned that its complex design made it difficult to define the clinically meaningful benefit of avacopan and its role in the management of AAV.
The FDA noted that, in the avacopan arm of the trial, 86% of patients received glucocorticoids outside of the study protocol. Despite this, avacopan reduced the cumulative glucocorticoid dose over the trial’s 52 weeks by nearly two-thirds, compared with the prednisone group (1,349 mg vs. 3,655 mg).
The data also indicate a higher sustained remission rate at 52 weeks in patients who received induction with rituximab, compared with cyclophosphamide. But trial did not include a maintenance therapy dose of rituximab and is thereby not a good comparison against the standard of care, the FDA said. (ADVOCATE began enrolling patients prior to the FDA's 2018 approval of an expanded indication for patients with GPA or MPA who have achieved disease control after induction treatment.)
At the FDA’s Arthritis Advisory Committee meeting in May 2021, committee members were split on whether to recommend avacopan for approval. The committee voted 9-9 on whether the ADVOCATE trial showed efficacy supporting approval of avacopan, 10-8 in favor of whether the drug’s safety profile supported approval, and 10-8 in favor of the overall benefit-risk profile of avacopan for approval. But rather than give an indication to avacopan to reduce the use of glucocorticoids in adults with GPA or MPA, the agency approved avacopan as an adjunctive treatment for severe, active disease, noting in particular that avacopan “does not eliminate glucocorticoid use.”
The European Union’s marketing authorization for avacopan states its indication for use in combination with a rituximab or cyclophosphamide regimen for the treatment of adult patients with severe, active GPA or MPA and does not mention a role for reducing glucocorticoids. Avacopan will appear in forthcoming guidelines on management of AAV released by the European Alliance of Associations for Rheumatology.
In North America, the Canadian Vasculitis Research Network recently released an addendum to their guidelines on AAV specifically for avacopan, which includes recommendations to consider adding oral avacopan (30 mg twice daily) for induction of remission in patients with new or relapsing GPA or MPA who are also receiving cyclophosphamide or rituximab. The guidelines also recommend clinicians consider a glucocorticoid tapering schedule that aims for discontinuation at 4 weeks, and continuing avacopan for at least 1 year after induction therapy. The American College of Rheumatology guideline for AAV management, updated in 2021, acknowledges avacopan but did not consider its inclusion prior to FDA approval.
There have been few real-world studies of how patients with AAV are responding to avacopan, but recent studies from researchers in the Netherlands and in France have evaluated prednisone tapering and clinical outcomes.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, said those real-world studies “seemed to re-enforce the findings from the ADVOCATE study demonstrating the efficacy of avacopan in severe disease with steroid-sparing effects.”
However, Carol Langford, MD, MHS, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, emphasized caution is needed when drawing conclusions about avacopan use outside formal studies.
“We are all interested in what other settings this might be used. I think those are things that really require formal investigation to really try and understand better as far as through a study process,” she said.
Prescribing experience with avacopan
A spokesperson from Amgen, which recently acquired ChemoCentryx, said in an interview that over 800 physicians in the United States have prescribed avacopan to patients with new or relapsing ANCA-associated vasculitis as induction or maintenance treatment, and physicians have reported outcomes consistent with the ADVOCATE trial.
Many rheumatologists are likely familiar with avacopan but are not used to prescribing it, said Lindsay S. Lally, MD, a rheumatologist with Hospital for Special Surgery in New York.
“Rituximab was approved for GPA and MPA a decade ago at this point. It was a drug that we as rheumatologists were used to using. We used it for other indications. Avacopan is a totally new drug, a new mechanism of action, so there’s not a lot of extractable data that we have in terms of comfort with the drug, and so I think that’s one of the biggest hurdles,” she said.
Mehrnaz Hojjati, MD, a rheumatologist with Loma Linda (Calif.) University Health, said that, when the FDA approved avacopan, it was an “exciting time” in her practice. “I have used avacopan now in a handful of my patients with severe ANCA-associated vasculitis, and the results are similar to what [was] reported in the ADVOCATE trial.”
Amgen offers help for clinicians in obtaining avacopan for patients, financial assistance for patients, and support in navigating insurance, which several rheumatologists noted was important for patients. Dr. Langford said the process of working with the manufacturer to get avacopan while insurance information is being processed has been “fairly smooth.”
“Certainly, the ability to get a very rapid 30-day supply with the goal of trying to initiate this as early as possible in the disease process has been helpful,” she said.
In Dr. Dua’s experience, while there were “some glitches or difficulty for providers early on” in how to access and prescribe avacopan, since then “it has been much easier to obtain the medication with the first month being provided to patients free while the authorization process is managed.”
Prescribing avacopan from inpatient pharmacies has been more challenging, she said. “The inpatient side is trickier because each hospital system has their own pharmacy system and regulations that have to be navigated. For outpatients, all the provider needs to do is fill out the start form available on their website, have the patients sign it, and then have it sent in.”
Concerns about affordability, insurance approval
Another consideration is cost, with avacopan having an estimated price of $150,000-$200,000 per patient per year.
Dr. Hojjati noted that, while it is easy to prescribe, avacopan is hard to get approved through insurance. “We face the same challenge every time a new medication comes to the market on how to convince the payers to pay for it given higher prices,” she said.
Rheumatologist Michael Putman, MD, MSCI, assistant professor of medicine at the Medical College of Wisconsin, Milwaukee, also acknowledged some difficulties in prescribing the medication. “The insurance companies have no interest in spending $150,000 on a drug that they know nothing about, and patients are a little hesitant to take it because it’s just so new,” he said.
While Dr. Lally said avacopan has not been difficult to get for patients with commercial insurance, reimbursement through Medicare has been problematic. “In many of the Medicare patients it has not really been a feasible option for them to be on the drug for the year of therapy.”
Patient response
Dr. Dua said almost all her patients with new or relapsing AAV who require induction are being prescribed avacopan, and that the medication is well tolerated. “The remission and ability to wean prednisone has really paralleled the findings from the clinical trial.”
In her practice, Dr. Hojjati starts patients on avacopan immediately after discharge from the hospital after a major vasculitis flare requiring high-dose glucocorticoids. “Avacopan does not eliminate/replace GC [glucocorticoid] use but has a notable GC-sparing effect and assists in rapid tapering of the GC while treating our severe ANCA-associated vasculitis patients,” she said.
Dr. Lally said her patients are tolerating avacopan well and hasn’t seen any of the safety signals seen in the trial, including liver function abnormalities. She has treated about 20-25 patients with avacopan.
Dr. Putman noted that he has treated about five patients with avacopan but hasn’t seen dramatic efficacy or side effects in his practice, compared with standard therapy.
Unanswered questions about avacopan
A key unanswered question with avacopan is the timeline for tapering glucocorticoids once patients start treatment. “I would like to see much more data on how prednisone is being tapered in clinical practice as well as outcomes in patients who are treated with the standard of care second dose of rituximab at 6 months,” Dr. Dua said.
Dr. Lally noted she has tried to expedite the steroid taper in her patients. “That’s really where I feel this drug is going to have most relevance, is getting it started early in active disease and getting patients off of the reliance on high doses of oral steroids. I have been able to see that in practice, and I do think ultimately that’s going to lead to better outcomes and quality of life for these patients.”
Of the rheumatologists Dr. Lally has spoken to about avacopan, there is “some confusion about what type of patients are appropriate, [and] how sick or not sick the patient needs to be.”
Dr. Putman noted he is unsure which of his patients should be receiving avacopan. “I don’t totally have a sense for where avacopan stands and how often we should be using it” outside of patients with severe disease. He added that the drug is still trying to find a niche because most patients with AAV who take rituximab and steroids get better without additional treatments.
“I think we do a pretty good job treating these diseases even in the preavacopan era. But it’s really a matter of how to really optimize these outcomes, reduce damage, reduce steroid-related and treatment-related toxicity for our patients,” Dr. Lally said.
Dr. Dua reported being a consultant and serving on advisory boards for ChemoCentryx; she was also a site principal investigator for the ADVOCATE trial. Dr. Hojjati reported being on the speaker’s bureau for Amgen. Dr. Langford reported being an investigator in the ADVOCATE trial, and her institution received funding to conduct the trial. Dr. Lally reported being a consultant for Amgen on avacopan. Dr. Putman reported no relevant financial disclosures.
*This story was updated 3/15/2023.
When the Food and Drug Administration approved avacopan (Tavneos) as an adjunctive treatment for severe, active antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis (AAV) in October 2021, the oral complement C5a receptor inhibitor was hailed by its developer, ChemoCentryx, as a “new hope” for patients with the disease.
But avacopan’s novelty as a new drug for the rare diseases granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), coupled with its approval as an adjunctive to standard therapy, including glucocorticoids, rather than strictly as a glucocorticoid-sparing agent as it was tested, has so far led to little reported real-world experience with the drug.
In the phase 3 ADVOCATE trial, the pivotal trial that served as the basis for avacopan’s approval, 331 patients with active newly diagnosed or relapsing GPA or MPA received either avacopan or an oral prednisone taper over 20 weeks on a background of cyclophosphamide followed by azathioprine or rituximab. The results of the trial showed avacopan was noninferior to the group that received prednisone taper for remission at 26 weeks and superior to prednisone taper for sustained remission at 52 weeks, but the FDA was concerned that its complex design made it difficult to define the clinically meaningful benefit of avacopan and its role in the management of AAV.
The FDA noted that, in the avacopan arm of the trial, 86% of patients received glucocorticoids outside of the study protocol. Despite this, avacopan reduced the cumulative glucocorticoid dose over the trial’s 52 weeks by nearly two-thirds, compared with the prednisone group (1,349 mg vs. 3,655 mg).
The data also indicate a higher sustained remission rate at 52 weeks in patients who received induction with rituximab, compared with cyclophosphamide. But trial did not include a maintenance therapy dose of rituximab and is thereby not a good comparison against the standard of care, the FDA said. (ADVOCATE began enrolling patients prior to the FDA's 2018 approval of an expanded indication for patients with GPA or MPA who have achieved disease control after induction treatment.)
At the FDA’s Arthritis Advisory Committee meeting in May 2021, committee members were split on whether to recommend avacopan for approval. The committee voted 9-9 on whether the ADVOCATE trial showed efficacy supporting approval of avacopan, 10-8 in favor of whether the drug’s safety profile supported approval, and 10-8 in favor of the overall benefit-risk profile of avacopan for approval. But rather than give an indication to avacopan to reduce the use of glucocorticoids in adults with GPA or MPA, the agency approved avacopan as an adjunctive treatment for severe, active disease, noting in particular that avacopan “does not eliminate glucocorticoid use.”
The European Union’s marketing authorization for avacopan states its indication for use in combination with a rituximab or cyclophosphamide regimen for the treatment of adult patients with severe, active GPA or MPA and does not mention a role for reducing glucocorticoids. Avacopan will appear in forthcoming guidelines on management of AAV released by the European Alliance of Associations for Rheumatology.
In North America, the Canadian Vasculitis Research Network recently released an addendum to their guidelines on AAV specifically for avacopan, which includes recommendations to consider adding oral avacopan (30 mg twice daily) for induction of remission in patients with new or relapsing GPA or MPA who are also receiving cyclophosphamide or rituximab. The guidelines also recommend clinicians consider a glucocorticoid tapering schedule that aims for discontinuation at 4 weeks, and continuing avacopan for at least 1 year after induction therapy. The American College of Rheumatology guideline for AAV management, updated in 2021, acknowledges avacopan but did not consider its inclusion prior to FDA approval.
There have been few real-world studies of how patients with AAV are responding to avacopan, but recent studies from researchers in the Netherlands and in France have evaluated prednisone tapering and clinical outcomes.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, said those real-world studies “seemed to re-enforce the findings from the ADVOCATE study demonstrating the efficacy of avacopan in severe disease with steroid-sparing effects.”
However, Carol Langford, MD, MHS, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, emphasized caution is needed when drawing conclusions about avacopan use outside formal studies.
“We are all interested in what other settings this might be used. I think those are things that really require formal investigation to really try and understand better as far as through a study process,” she said.
Prescribing experience with avacopan
A spokesperson from Amgen, which recently acquired ChemoCentryx, said in an interview that over 800 physicians in the United States have prescribed avacopan to patients with new or relapsing ANCA-associated vasculitis as induction or maintenance treatment, and physicians have reported outcomes consistent with the ADVOCATE trial.
Many rheumatologists are likely familiar with avacopan but are not used to prescribing it, said Lindsay S. Lally, MD, a rheumatologist with Hospital for Special Surgery in New York.
“Rituximab was approved for GPA and MPA a decade ago at this point. It was a drug that we as rheumatologists were used to using. We used it for other indications. Avacopan is a totally new drug, a new mechanism of action, so there’s not a lot of extractable data that we have in terms of comfort with the drug, and so I think that’s one of the biggest hurdles,” she said.
Mehrnaz Hojjati, MD, a rheumatologist with Loma Linda (Calif.) University Health, said that, when the FDA approved avacopan, it was an “exciting time” in her practice. “I have used avacopan now in a handful of my patients with severe ANCA-associated vasculitis, and the results are similar to what [was] reported in the ADVOCATE trial.”
Amgen offers help for clinicians in obtaining avacopan for patients, financial assistance for patients, and support in navigating insurance, which several rheumatologists noted was important for patients. Dr. Langford said the process of working with the manufacturer to get avacopan while insurance information is being processed has been “fairly smooth.”
“Certainly, the ability to get a very rapid 30-day supply with the goal of trying to initiate this as early as possible in the disease process has been helpful,” she said.
In Dr. Dua’s experience, while there were “some glitches or difficulty for providers early on” in how to access and prescribe avacopan, since then “it has been much easier to obtain the medication with the first month being provided to patients free while the authorization process is managed.”
Prescribing avacopan from inpatient pharmacies has been more challenging, she said. “The inpatient side is trickier because each hospital system has their own pharmacy system and regulations that have to be navigated. For outpatients, all the provider needs to do is fill out the start form available on their website, have the patients sign it, and then have it sent in.”
Concerns about affordability, insurance approval
Another consideration is cost, with avacopan having an estimated price of $150,000-$200,000 per patient per year.
Dr. Hojjati noted that, while it is easy to prescribe, avacopan is hard to get approved through insurance. “We face the same challenge every time a new medication comes to the market on how to convince the payers to pay for it given higher prices,” she said.
Rheumatologist Michael Putman, MD, MSCI, assistant professor of medicine at the Medical College of Wisconsin, Milwaukee, also acknowledged some difficulties in prescribing the medication. “The insurance companies have no interest in spending $150,000 on a drug that they know nothing about, and patients are a little hesitant to take it because it’s just so new,” he said.
While Dr. Lally said avacopan has not been difficult to get for patients with commercial insurance, reimbursement through Medicare has been problematic. “In many of the Medicare patients it has not really been a feasible option for them to be on the drug for the year of therapy.”
Patient response
Dr. Dua said almost all her patients with new or relapsing AAV who require induction are being prescribed avacopan, and that the medication is well tolerated. “The remission and ability to wean prednisone has really paralleled the findings from the clinical trial.”
In her practice, Dr. Hojjati starts patients on avacopan immediately after discharge from the hospital after a major vasculitis flare requiring high-dose glucocorticoids. “Avacopan does not eliminate/replace GC [glucocorticoid] use but has a notable GC-sparing effect and assists in rapid tapering of the GC while treating our severe ANCA-associated vasculitis patients,” she said.
Dr. Lally said her patients are tolerating avacopan well and hasn’t seen any of the safety signals seen in the trial, including liver function abnormalities. She has treated about 20-25 patients with avacopan.
Dr. Putman noted that he has treated about five patients with avacopan but hasn’t seen dramatic efficacy or side effects in his practice, compared with standard therapy.
Unanswered questions about avacopan
A key unanswered question with avacopan is the timeline for tapering glucocorticoids once patients start treatment. “I would like to see much more data on how prednisone is being tapered in clinical practice as well as outcomes in patients who are treated with the standard of care second dose of rituximab at 6 months,” Dr. Dua said.
Dr. Lally noted she has tried to expedite the steroid taper in her patients. “That’s really where I feel this drug is going to have most relevance, is getting it started early in active disease and getting patients off of the reliance on high doses of oral steroids. I have been able to see that in practice, and I do think ultimately that’s going to lead to better outcomes and quality of life for these patients.”
Of the rheumatologists Dr. Lally has spoken to about avacopan, there is “some confusion about what type of patients are appropriate, [and] how sick or not sick the patient needs to be.”
Dr. Putman noted he is unsure which of his patients should be receiving avacopan. “I don’t totally have a sense for where avacopan stands and how often we should be using it” outside of patients with severe disease. He added that the drug is still trying to find a niche because most patients with AAV who take rituximab and steroids get better without additional treatments.
“I think we do a pretty good job treating these diseases even in the preavacopan era. But it’s really a matter of how to really optimize these outcomes, reduce damage, reduce steroid-related and treatment-related toxicity for our patients,” Dr. Lally said.
Dr. Dua reported being a consultant and serving on advisory boards for ChemoCentryx; she was also a site principal investigator for the ADVOCATE trial. Dr. Hojjati reported being on the speaker’s bureau for Amgen. Dr. Langford reported being an investigator in the ADVOCATE trial, and her institution received funding to conduct the trial. Dr. Lally reported being a consultant for Amgen on avacopan. Dr. Putman reported no relevant financial disclosures.
*This story was updated 3/15/2023.
Biologics show signs of delaying arthritis in psoriasis patients
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
FROM LANCET RHEUMATOLOGY
Experts share real-world experience prescribing voclosporin, belimumab for lupus nephritis
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
Call it preclinical or subclinical, ILD in RA needs to be tracked
More clinical guidance is needed for monitoring interstitial lung disease (ILD) in patients with rheumatoid arthritis, according to a new commentary.
Though ILD is a leading cause of death among patients with RA, these patients are not routinely screened for ILD, the authors say, and there are currently no guidelines on how to monitor ILD progression in patients with RA.
“ILD associated with rheumatoid arthritis is a disease for which there’s been very little research done, so it’s an area of rheumatology where there are many unknowns,” lead author Elizabeth R. Volkmann, MD, who codirects the connective tissue disease–related interstitial lung disease (CTD-ILD) program at University of California, Los Angeles, told this news organization.
The commentary was published in The Lancet Rheumatology.
Defining disease
One of the major unknowns is how to define the disease, she said. RA patients sometimes undergo imaging for other medical reasons, and interstitial lung abnormalities are incidentally detected. These patients can be classified as having “preclinical” or “subclinical” ILD, as they do not yet have symptoms; however, there is no consensus as to what these terms mean, the commentary authors write. “The other problem that we have with these terms is that it sometimes creates the perception that this is a nonworrisome feature of rheumatoid arthritis,” Dr. Volkmann said, although the condition should be followed closely.
“We know we can detect imaging features of ILD in people who may not yet have symptoms, and we need to know when to define a clinically important informality that requires follow-up or treatment,” added John M. Davis III, MD, a rheumatologist at the Mayo Clinic, Rochester, Minn. He was not involved with the work.
Dr. Volkmann proposed eliminating the prefixes “pre” and “sub” when referring to ILD. “In other connective tissue diseases, like systemic sclerosis, for example, we can use the term ‘limited’ or ‘extensive’ ILD, based on the extent of involvement of the ILD on high-resolution computed tomography (HRCT) imaging,” she said. “This could potentially be something that is applied to how we classify patients with RA-ILD.”
Tracking ILD progression
Once ILD is identified, monitoring its progression poses challenges, as respiratory symptoms may be difficult to detect. RA patients may already be avoiding exercise because of joint pain, so they may not notice shortness of breath during physical activity, noted Jessica K. Gordon, MD, of the Hospital for Special Surgery, New York, in an interview with this news organization. She was not involved with the commentary. Cough is a potential symptom of ILD, but cough can also be the result of allergies, postnasal drip, or reflux, she said. Making the distinction between “preclinical” and symptomatic disease can be “complicated,” she added; “you may have to really dig.”
Additionally, there has been little research on the outcomes of patients with preclinical or subclinical ILD and clinical ILD, the commentary authors write. “It is therefore conceivable that some patients with rheumatoid arthritis diagnosed with preclinical or subclinical ILD could potentially have worse outcomes if both the rheumatoid arthritis and ILD are not monitored closely,” they note.
To better track RA-associated ILD for patients with and those without symptoms, the authors advocate for monitoring patients using pulmonary testing and CT scanning, as well as evaluating symptoms. How often these assessments should be conducted depends on the individual, they note. In her own practice, Dr. Volkmann sees patients every 3 months to evaluate their symptoms and conduct pulmonary function tests (PFTs). For patients early in the course of ILD, she orders HRCT imaging once per year.
For Dr. Davis, the frequency of follow-up depends on the severity of ILD. “For minimally symptomatic patients without compromised lung function, we would generally follow annually. For patients with symptomatic ILD on stable therapy, we may monitor every 6 months. For patients with active/progressive ILD, we would generally be following at least every 1-3 months,” he said.
Screening and future research
While there is no evidence to recommend screening patients for ILD using CT, there are certain risk factors for ILD in RA patients, including a history of smoking, male sex, and high RA disease activity despite antirheumatic treatment, Dr. Volkmann said. In both of their practices, Dr. Davis and Dr. Volkmann screen with RA via HRCT and PFTs for ILD for patients with known risk factors that predispose them to the lung condition and/or for patients who report respiratory symptoms.
“We still don’t have an algorithm [for screening patients], and that is a desperate need in this field,” added Joshua J. Solomon, MD, a pulmonologist at National Jewish Health, Denver, whose research focuses on RA-associated ILD. While recommendations state that all patients with scleroderma should be screened with CT, ILD incidence is lower among patients with RA, and thus these screening recommendations need to be narrowed, he said. But more research is needed to better fine tune recommendations, he said; “The only thing you can do is give some expert consensus until there are good data.”
Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and institutional support for performing studies on systemic sclerosis for Kadmon, Forbius, Boehringer Ingelheim, Horizon, and Prometheus. Dr. Gordon, Dr. Davis, and Dr. Solomon report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More clinical guidance is needed for monitoring interstitial lung disease (ILD) in patients with rheumatoid arthritis, according to a new commentary.
Though ILD is a leading cause of death among patients with RA, these patients are not routinely screened for ILD, the authors say, and there are currently no guidelines on how to monitor ILD progression in patients with RA.
“ILD associated with rheumatoid arthritis is a disease for which there’s been very little research done, so it’s an area of rheumatology where there are many unknowns,” lead author Elizabeth R. Volkmann, MD, who codirects the connective tissue disease–related interstitial lung disease (CTD-ILD) program at University of California, Los Angeles, told this news organization.
The commentary was published in The Lancet Rheumatology.
Defining disease
One of the major unknowns is how to define the disease, she said. RA patients sometimes undergo imaging for other medical reasons, and interstitial lung abnormalities are incidentally detected. These patients can be classified as having “preclinical” or “subclinical” ILD, as they do not yet have symptoms; however, there is no consensus as to what these terms mean, the commentary authors write. “The other problem that we have with these terms is that it sometimes creates the perception that this is a nonworrisome feature of rheumatoid arthritis,” Dr. Volkmann said, although the condition should be followed closely.
“We know we can detect imaging features of ILD in people who may not yet have symptoms, and we need to know when to define a clinically important informality that requires follow-up or treatment,” added John M. Davis III, MD, a rheumatologist at the Mayo Clinic, Rochester, Minn. He was not involved with the work.
Dr. Volkmann proposed eliminating the prefixes “pre” and “sub” when referring to ILD. “In other connective tissue diseases, like systemic sclerosis, for example, we can use the term ‘limited’ or ‘extensive’ ILD, based on the extent of involvement of the ILD on high-resolution computed tomography (HRCT) imaging,” she said. “This could potentially be something that is applied to how we classify patients with RA-ILD.”
Tracking ILD progression
Once ILD is identified, monitoring its progression poses challenges, as respiratory symptoms may be difficult to detect. RA patients may already be avoiding exercise because of joint pain, so they may not notice shortness of breath during physical activity, noted Jessica K. Gordon, MD, of the Hospital for Special Surgery, New York, in an interview with this news organization. She was not involved with the commentary. Cough is a potential symptom of ILD, but cough can also be the result of allergies, postnasal drip, or reflux, she said. Making the distinction between “preclinical” and symptomatic disease can be “complicated,” she added; “you may have to really dig.”
Additionally, there has been little research on the outcomes of patients with preclinical or subclinical ILD and clinical ILD, the commentary authors write. “It is therefore conceivable that some patients with rheumatoid arthritis diagnosed with preclinical or subclinical ILD could potentially have worse outcomes if both the rheumatoid arthritis and ILD are not monitored closely,” they note.
To better track RA-associated ILD for patients with and those without symptoms, the authors advocate for monitoring patients using pulmonary testing and CT scanning, as well as evaluating symptoms. How often these assessments should be conducted depends on the individual, they note. In her own practice, Dr. Volkmann sees patients every 3 months to evaluate their symptoms and conduct pulmonary function tests (PFTs). For patients early in the course of ILD, she orders HRCT imaging once per year.
For Dr. Davis, the frequency of follow-up depends on the severity of ILD. “For minimally symptomatic patients without compromised lung function, we would generally follow annually. For patients with symptomatic ILD on stable therapy, we may monitor every 6 months. For patients with active/progressive ILD, we would generally be following at least every 1-3 months,” he said.
Screening and future research
While there is no evidence to recommend screening patients for ILD using CT, there are certain risk factors for ILD in RA patients, including a history of smoking, male sex, and high RA disease activity despite antirheumatic treatment, Dr. Volkmann said. In both of their practices, Dr. Davis and Dr. Volkmann screen with RA via HRCT and PFTs for ILD for patients with known risk factors that predispose them to the lung condition and/or for patients who report respiratory symptoms.
“We still don’t have an algorithm [for screening patients], and that is a desperate need in this field,” added Joshua J. Solomon, MD, a pulmonologist at National Jewish Health, Denver, whose research focuses on RA-associated ILD. While recommendations state that all patients with scleroderma should be screened with CT, ILD incidence is lower among patients with RA, and thus these screening recommendations need to be narrowed, he said. But more research is needed to better fine tune recommendations, he said; “The only thing you can do is give some expert consensus until there are good data.”
Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and institutional support for performing studies on systemic sclerosis for Kadmon, Forbius, Boehringer Ingelheim, Horizon, and Prometheus. Dr. Gordon, Dr. Davis, and Dr. Solomon report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More clinical guidance is needed for monitoring interstitial lung disease (ILD) in patients with rheumatoid arthritis, according to a new commentary.
Though ILD is a leading cause of death among patients with RA, these patients are not routinely screened for ILD, the authors say, and there are currently no guidelines on how to monitor ILD progression in patients with RA.
“ILD associated with rheumatoid arthritis is a disease for which there’s been very little research done, so it’s an area of rheumatology where there are many unknowns,” lead author Elizabeth R. Volkmann, MD, who codirects the connective tissue disease–related interstitial lung disease (CTD-ILD) program at University of California, Los Angeles, told this news organization.
The commentary was published in The Lancet Rheumatology.
Defining disease
One of the major unknowns is how to define the disease, she said. RA patients sometimes undergo imaging for other medical reasons, and interstitial lung abnormalities are incidentally detected. These patients can be classified as having “preclinical” or “subclinical” ILD, as they do not yet have symptoms; however, there is no consensus as to what these terms mean, the commentary authors write. “The other problem that we have with these terms is that it sometimes creates the perception that this is a nonworrisome feature of rheumatoid arthritis,” Dr. Volkmann said, although the condition should be followed closely.
“We know we can detect imaging features of ILD in people who may not yet have symptoms, and we need to know when to define a clinically important informality that requires follow-up or treatment,” added John M. Davis III, MD, a rheumatologist at the Mayo Clinic, Rochester, Minn. He was not involved with the work.
Dr. Volkmann proposed eliminating the prefixes “pre” and “sub” when referring to ILD. “In other connective tissue diseases, like systemic sclerosis, for example, we can use the term ‘limited’ or ‘extensive’ ILD, based on the extent of involvement of the ILD on high-resolution computed tomography (HRCT) imaging,” she said. “This could potentially be something that is applied to how we classify patients with RA-ILD.”
Tracking ILD progression
Once ILD is identified, monitoring its progression poses challenges, as respiratory symptoms may be difficult to detect. RA patients may already be avoiding exercise because of joint pain, so they may not notice shortness of breath during physical activity, noted Jessica K. Gordon, MD, of the Hospital for Special Surgery, New York, in an interview with this news organization. She was not involved with the commentary. Cough is a potential symptom of ILD, but cough can also be the result of allergies, postnasal drip, or reflux, she said. Making the distinction between “preclinical” and symptomatic disease can be “complicated,” she added; “you may have to really dig.”
Additionally, there has been little research on the outcomes of patients with preclinical or subclinical ILD and clinical ILD, the commentary authors write. “It is therefore conceivable that some patients with rheumatoid arthritis diagnosed with preclinical or subclinical ILD could potentially have worse outcomes if both the rheumatoid arthritis and ILD are not monitored closely,” they note.
To better track RA-associated ILD for patients with and those without symptoms, the authors advocate for monitoring patients using pulmonary testing and CT scanning, as well as evaluating symptoms. How often these assessments should be conducted depends on the individual, they note. In her own practice, Dr. Volkmann sees patients every 3 months to evaluate their symptoms and conduct pulmonary function tests (PFTs). For patients early in the course of ILD, she orders HRCT imaging once per year.
For Dr. Davis, the frequency of follow-up depends on the severity of ILD. “For minimally symptomatic patients without compromised lung function, we would generally follow annually. For patients with symptomatic ILD on stable therapy, we may monitor every 6 months. For patients with active/progressive ILD, we would generally be following at least every 1-3 months,” he said.
Screening and future research
While there is no evidence to recommend screening patients for ILD using CT, there are certain risk factors for ILD in RA patients, including a history of smoking, male sex, and high RA disease activity despite antirheumatic treatment, Dr. Volkmann said. In both of their practices, Dr. Davis and Dr. Volkmann screen with RA via HRCT and PFTs for ILD for patients with known risk factors that predispose them to the lung condition and/or for patients who report respiratory symptoms.
“We still don’t have an algorithm [for screening patients], and that is a desperate need in this field,” added Joshua J. Solomon, MD, a pulmonologist at National Jewish Health, Denver, whose research focuses on RA-associated ILD. While recommendations state that all patients with scleroderma should be screened with CT, ILD incidence is lower among patients with RA, and thus these screening recommendations need to be narrowed, he said. But more research is needed to better fine tune recommendations, he said; “The only thing you can do is give some expert consensus until there are good data.”
Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and institutional support for performing studies on systemic sclerosis for Kadmon, Forbius, Boehringer Ingelheim, Horizon, and Prometheus. Dr. Gordon, Dr. Davis, and Dr. Solomon report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves first biologic treatment for polymyalgia rheumatica
The Food and Drug Administration approved sarilumab (Kevzara) on March 1 for the treatment of polymyalgia rheumatica (PMR) in adults who have had an inadequate response to corticosteroids or could not tolerate a corticosteroid taper, joint developers Sanofi and Regeneron announced. The drug is the first and only FDA-approved biologic treatment for this inflammatory rheumatic disease.
The FDA previously approved sarilumab, an interleukin-6 receptor antagonist, in May 2017 for the treatment of moderate to severe active rheumatoid arthritis in adults who do not respond well or have an intolerance to disease-modifying antirheumatic drugs (DMARDs), like methotrexate.
The FDA approval for this new indication was based on results from the multicenter, phase 3 SAPHYR trial in patients with corticosteroid-resistant, active PMR. In the randomized, double-blind, placebo-controlled study, 59 participants received 200 mg of sarilumab plus a 14-week taper of corticosteroid treatment and 58 participants received placebo every 2 weeks along with a 52-week taper of corticosteroid treatment.
After 1 year, 28% of sarilumab patients achieved sustained remission, compared with 10% of the placebo group (P = .0193). This news organization previously reported these trial results in November when they were presented at the 2022 annual meeting of the American College of Rheumatology.
The most common adverse events in the sarilumab group were neutropenia (15%), leukopenia (7%), constipation (7%), pruritic rash (5%), myalgia (7%), fatigue (5%), and injection-site pruritus (5%). Two patients had serious adverse reactions of neutropenia, which resolved after discontinuing treatment.
“Polymyalgia rheumatica can be an incapacitating disease, causing painful disease flares in multiple parts of the bodies that leave people fatigued and unable to fully perform everyday activities. Corticosteroids have been the primary treatment to date, but many patients do not adequately respond to steroids or cannot be tapered off steroids, which puts such patients at risk of complications from long-term steroid therapy,” George D. Yancopolous, MD, PhD, president and chief scientific officer at Regeneron, said in the announcement. “With the approval of Kevzara for polymyalgia rheumatica, patients now have an FDA-approved treatment to help offer relief from the disabling symptoms of this disease and long-term dependence on steroids.”
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration approved sarilumab (Kevzara) on March 1 for the treatment of polymyalgia rheumatica (PMR) in adults who have had an inadequate response to corticosteroids or could not tolerate a corticosteroid taper, joint developers Sanofi and Regeneron announced. The drug is the first and only FDA-approved biologic treatment for this inflammatory rheumatic disease.
The FDA previously approved sarilumab, an interleukin-6 receptor antagonist, in May 2017 for the treatment of moderate to severe active rheumatoid arthritis in adults who do not respond well or have an intolerance to disease-modifying antirheumatic drugs (DMARDs), like methotrexate.
The FDA approval for this new indication was based on results from the multicenter, phase 3 SAPHYR trial in patients with corticosteroid-resistant, active PMR. In the randomized, double-blind, placebo-controlled study, 59 participants received 200 mg of sarilumab plus a 14-week taper of corticosteroid treatment and 58 participants received placebo every 2 weeks along with a 52-week taper of corticosteroid treatment.
After 1 year, 28% of sarilumab patients achieved sustained remission, compared with 10% of the placebo group (P = .0193). This news organization previously reported these trial results in November when they were presented at the 2022 annual meeting of the American College of Rheumatology.
The most common adverse events in the sarilumab group were neutropenia (15%), leukopenia (7%), constipation (7%), pruritic rash (5%), myalgia (7%), fatigue (5%), and injection-site pruritus (5%). Two patients had serious adverse reactions of neutropenia, which resolved after discontinuing treatment.
“Polymyalgia rheumatica can be an incapacitating disease, causing painful disease flares in multiple parts of the bodies that leave people fatigued and unable to fully perform everyday activities. Corticosteroids have been the primary treatment to date, but many patients do not adequately respond to steroids or cannot be tapered off steroids, which puts such patients at risk of complications from long-term steroid therapy,” George D. Yancopolous, MD, PhD, president and chief scientific officer at Regeneron, said in the announcement. “With the approval of Kevzara for polymyalgia rheumatica, patients now have an FDA-approved treatment to help offer relief from the disabling symptoms of this disease and long-term dependence on steroids.”
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration approved sarilumab (Kevzara) on March 1 for the treatment of polymyalgia rheumatica (PMR) in adults who have had an inadequate response to corticosteroids or could not tolerate a corticosteroid taper, joint developers Sanofi and Regeneron announced. The drug is the first and only FDA-approved biologic treatment for this inflammatory rheumatic disease.
The FDA previously approved sarilumab, an interleukin-6 receptor antagonist, in May 2017 for the treatment of moderate to severe active rheumatoid arthritis in adults who do not respond well or have an intolerance to disease-modifying antirheumatic drugs (DMARDs), like methotrexate.
The FDA approval for this new indication was based on results from the multicenter, phase 3 SAPHYR trial in patients with corticosteroid-resistant, active PMR. In the randomized, double-blind, placebo-controlled study, 59 participants received 200 mg of sarilumab plus a 14-week taper of corticosteroid treatment and 58 participants received placebo every 2 weeks along with a 52-week taper of corticosteroid treatment.
After 1 year, 28% of sarilumab patients achieved sustained remission, compared with 10% of the placebo group (P = .0193). This news organization previously reported these trial results in November when they were presented at the 2022 annual meeting of the American College of Rheumatology.
The most common adverse events in the sarilumab group were neutropenia (15%), leukopenia (7%), constipation (7%), pruritic rash (5%), myalgia (7%), fatigue (5%), and injection-site pruritus (5%). Two patients had serious adverse reactions of neutropenia, which resolved after discontinuing treatment.
“Polymyalgia rheumatica can be an incapacitating disease, causing painful disease flares in multiple parts of the bodies that leave people fatigued and unable to fully perform everyday activities. Corticosteroids have been the primary treatment to date, but many patients do not adequately respond to steroids or cannot be tapered off steroids, which puts such patients at risk of complications from long-term steroid therapy,” George D. Yancopolous, MD, PhD, president and chief scientific officer at Regeneron, said in the announcement. “With the approval of Kevzara for polymyalgia rheumatica, patients now have an FDA-approved treatment to help offer relief from the disabling symptoms of this disease and long-term dependence on steroids.”
A version of this article originally appeared on Medscape.com.