User login
Robot-assisted surgery: Twice the price
HOUSTON – Robot-assisted operations for inguinal hernia repair (IHR) and cholecystectomy have grown steadily in recent years, but these procedures can be done equally well by traditional operations at a fraction of the cost, according to a study from Geisinger Medical Center in Pennsylvania.
Ellen Vogels, DO, of Geisinger, reported results of a study of 1,248 cholecystectomies and 723 initial IHRs from 2007 to 2016. The cholecystectomies were done via robot-assisted surgery or laparoscopy in the hospital or via laparoscopy in an ambulatory surgery center (ASC). The IHRs were done robotically, open, or laparoscopically in the hospital, or open or laparoscopically in an ASC.
Dr. Vogels quoted statistics from the ECRI Institute that showed robotic surgery procedures have increased 178% between 2009 and 2014, and the two procedures the group studied are the most frequently performed robotic procedures.
Within the Geisinger system, the study found a 3:1 cost disparity for IHR: $6,292 total cost for hospital-based robotic surgery vs. $3,421 for ASC-based laparoscopy IHR and $1,853 for ASC-based open repair. For cholecystectomy, the disparity isn’t as wide – it’s 2:1 – but is still significant: Total costs for hospital-based robotic surgery are $6,057 vs. $3,443 for ASC-based cholecystectomy and $3,270 for hospital-based laparoscopic cholecystectomy (the study did not include any open cholecystectomies).
Total costs not only include costs for the procedure but also all related pre- and postoperative care. The cost analysis did not account for the cost of the robot, including maintenance contracts, or costs for laparoscopic instruments. Variable costs also ranged from about $3,000 for robotic IHR to $942 for ASC open repair – which means the lowest per-procedure cost for the latter was around $900.
“Translating this into the fact that cholecystectomies and inguinal hernia repairs are the most often performed general surgery procedures, ambulatory surgery centers can save over $60 billion over the next 10 years in just overhead costs as well as increased efficiency,” Dr. Vogels said.
The study also found access issues depending on where patients had their operations. “As far as service and access in our institution alone, we found that patients going to the main hospital spent as much as two times longer getting these procedures done as compared to the ambulatory surgery centers,” Dr. Vogels said.
Robotic procedures also required longer operative times, the study found – an average of 109 minutes for IHR vs. about an hour for ASC procedures and hospital-based open surgery (but averaging 78 minutes for in-hospital laparoscopy); and 73 minutes for robotic cholecystectomy, 60 minutes for hospital laparoscopy, and 45 minutes for ASC laparoscopy.
Robotic session moderator Dmitry Oleynikov, MD, FACS, of the University of Nebraska Medical Center, Omaha, asked Dr. Vogels if putting a robotic platform in an ambulatory surgery setting would make it more cost effective.
That’s not practical from a cost or efficiency perspective, she said.
“When you look at the cost of the ASCs, specifically in the hernia group, the lowest-cost hernia repair is about $800; with the robot it’s going to be significantly higher than that, up to three times higher than that,” Dr. Vogels replied. “Then you’re also changing all those simple ambulatory surgery procedures to more involved robotic procedures, so it’s hard to justify doing that in the ASC.”
Dr. Vogels and her coauthors had no relevant financial disclosures.
HOUSTON – Robot-assisted operations for inguinal hernia repair (IHR) and cholecystectomy have grown steadily in recent years, but these procedures can be done equally well by traditional operations at a fraction of the cost, according to a study from Geisinger Medical Center in Pennsylvania.
Ellen Vogels, DO, of Geisinger, reported results of a study of 1,248 cholecystectomies and 723 initial IHRs from 2007 to 2016. The cholecystectomies were done via robot-assisted surgery or laparoscopy in the hospital or via laparoscopy in an ambulatory surgery center (ASC). The IHRs were done robotically, open, or laparoscopically in the hospital, or open or laparoscopically in an ASC.
Dr. Vogels quoted statistics from the ECRI Institute that showed robotic surgery procedures have increased 178% between 2009 and 2014, and the two procedures the group studied are the most frequently performed robotic procedures.
Within the Geisinger system, the study found a 3:1 cost disparity for IHR: $6,292 total cost for hospital-based robotic surgery vs. $3,421 for ASC-based laparoscopy IHR and $1,853 for ASC-based open repair. For cholecystectomy, the disparity isn’t as wide – it’s 2:1 – but is still significant: Total costs for hospital-based robotic surgery are $6,057 vs. $3,443 for ASC-based cholecystectomy and $3,270 for hospital-based laparoscopic cholecystectomy (the study did not include any open cholecystectomies).
Total costs not only include costs for the procedure but also all related pre- and postoperative care. The cost analysis did not account for the cost of the robot, including maintenance contracts, or costs for laparoscopic instruments. Variable costs also ranged from about $3,000 for robotic IHR to $942 for ASC open repair – which means the lowest per-procedure cost for the latter was around $900.
“Translating this into the fact that cholecystectomies and inguinal hernia repairs are the most often performed general surgery procedures, ambulatory surgery centers can save over $60 billion over the next 10 years in just overhead costs as well as increased efficiency,” Dr. Vogels said.
The study also found access issues depending on where patients had their operations. “As far as service and access in our institution alone, we found that patients going to the main hospital spent as much as two times longer getting these procedures done as compared to the ambulatory surgery centers,” Dr. Vogels said.
Robotic procedures also required longer operative times, the study found – an average of 109 minutes for IHR vs. about an hour for ASC procedures and hospital-based open surgery (but averaging 78 minutes for in-hospital laparoscopy); and 73 minutes for robotic cholecystectomy, 60 minutes for hospital laparoscopy, and 45 minutes for ASC laparoscopy.
Robotic session moderator Dmitry Oleynikov, MD, FACS, of the University of Nebraska Medical Center, Omaha, asked Dr. Vogels if putting a robotic platform in an ambulatory surgery setting would make it more cost effective.
That’s not practical from a cost or efficiency perspective, she said.
“When you look at the cost of the ASCs, specifically in the hernia group, the lowest-cost hernia repair is about $800; with the robot it’s going to be significantly higher than that, up to three times higher than that,” Dr. Vogels replied. “Then you’re also changing all those simple ambulatory surgery procedures to more involved robotic procedures, so it’s hard to justify doing that in the ASC.”
Dr. Vogels and her coauthors had no relevant financial disclosures.
HOUSTON – Robot-assisted operations for inguinal hernia repair (IHR) and cholecystectomy have grown steadily in recent years, but these procedures can be done equally well by traditional operations at a fraction of the cost, according to a study from Geisinger Medical Center in Pennsylvania.
Ellen Vogels, DO, of Geisinger, reported results of a study of 1,248 cholecystectomies and 723 initial IHRs from 2007 to 2016. The cholecystectomies were done via robot-assisted surgery or laparoscopy in the hospital or via laparoscopy in an ambulatory surgery center (ASC). The IHRs were done robotically, open, or laparoscopically in the hospital, or open or laparoscopically in an ASC.
Dr. Vogels quoted statistics from the ECRI Institute that showed robotic surgery procedures have increased 178% between 2009 and 2014, and the two procedures the group studied are the most frequently performed robotic procedures.
Within the Geisinger system, the study found a 3:1 cost disparity for IHR: $6,292 total cost for hospital-based robotic surgery vs. $3,421 for ASC-based laparoscopy IHR and $1,853 for ASC-based open repair. For cholecystectomy, the disparity isn’t as wide – it’s 2:1 – but is still significant: Total costs for hospital-based robotic surgery are $6,057 vs. $3,443 for ASC-based cholecystectomy and $3,270 for hospital-based laparoscopic cholecystectomy (the study did not include any open cholecystectomies).
Total costs not only include costs for the procedure but also all related pre- and postoperative care. The cost analysis did not account for the cost of the robot, including maintenance contracts, or costs for laparoscopic instruments. Variable costs also ranged from about $3,000 for robotic IHR to $942 for ASC open repair – which means the lowest per-procedure cost for the latter was around $900.
“Translating this into the fact that cholecystectomies and inguinal hernia repairs are the most often performed general surgery procedures, ambulatory surgery centers can save over $60 billion over the next 10 years in just overhead costs as well as increased efficiency,” Dr. Vogels said.
The study also found access issues depending on where patients had their operations. “As far as service and access in our institution alone, we found that patients going to the main hospital spent as much as two times longer getting these procedures done as compared to the ambulatory surgery centers,” Dr. Vogels said.
Robotic procedures also required longer operative times, the study found – an average of 109 minutes for IHR vs. about an hour for ASC procedures and hospital-based open surgery (but averaging 78 minutes for in-hospital laparoscopy); and 73 minutes for robotic cholecystectomy, 60 minutes for hospital laparoscopy, and 45 minutes for ASC laparoscopy.
Robotic session moderator Dmitry Oleynikov, MD, FACS, of the University of Nebraska Medical Center, Omaha, asked Dr. Vogels if putting a robotic platform in an ambulatory surgery setting would make it more cost effective.
That’s not practical from a cost or efficiency perspective, she said.
“When you look at the cost of the ASCs, specifically in the hernia group, the lowest-cost hernia repair is about $800; with the robot it’s going to be significantly higher than that, up to three times higher than that,” Dr. Vogels replied. “Then you’re also changing all those simple ambulatory surgery procedures to more involved robotic procedures, so it’s hard to justify doing that in the ASC.”
Dr. Vogels and her coauthors had no relevant financial disclosures.
AT SAGES 2017
Key clinical point: Outcomes for robot-assisted inguinal hernia repair and cholecystectomy are similar to those for outpatient open and laparoscopic procedures.
Major finding: Robotic IHR costs up to three times more than open outpatient surgery, and robotic cholecystectomy costs twice as much as outpatient surgery.
Data source: Study of 1,971 in-hospital robotic, laparoscopic, and open procedures, and outpatient laparoscopic and open operations done from 2007 to 2016 at Geisinger Medical Center.
Disclosures: Dr. Vogels and coauthors reported having no financial disclosures.
Robotics: General surgery goes its own way
HOUSTON – Subspecialties such as urology and gynecology have seen a steady increase in robot-assisted surgery and an offsetting decline in open procedures, but in general surgery, robot-assisted procedures seem to be making gains at the expense of laparoscopy, according to researchers from the University of Nebraska.
In two specific operations, ventral and inguinal hernia repairs (VHR and IHR), the percentage of open procedures has increased or held steady over the 7-year study period while the share of laparoscopic operations declined and robot-assisted surgeries (RAS) increased, Priscila Rodrigues Armijo, MD, reported at the annual meeting of the Society of American Gastrointestinal and Endoscopic Surgeons.
This shift to RAS rather than laparoscopy could have significant implications because RAS is significantly more costly than laparoscopy, Dr. Armijo said. “In our study, the open procedures were the most expensive, followed by the robot-assisted surgeries and then laparoscopy,” she said. Median direct costs were $14,364 for open procedures, $11,376 for RAS and $7,945 for laparoscopy.
The Nebraska study retrospectively analyzed five different general surgery procedures: colectomy, cholecystectomy, and bariatric procedures in addition to VHR and IHR. The researchers analyzed 857,468 operations entered into the University HealthSystem Consortium Clinical Database Resource Manager from October 2008 to September 2015.
Dr. Armijo explained that the goal was to study trends in general surgery because while several studies have examined trends in urologic and gynecologic surgery, few studies have done so in general surgery.
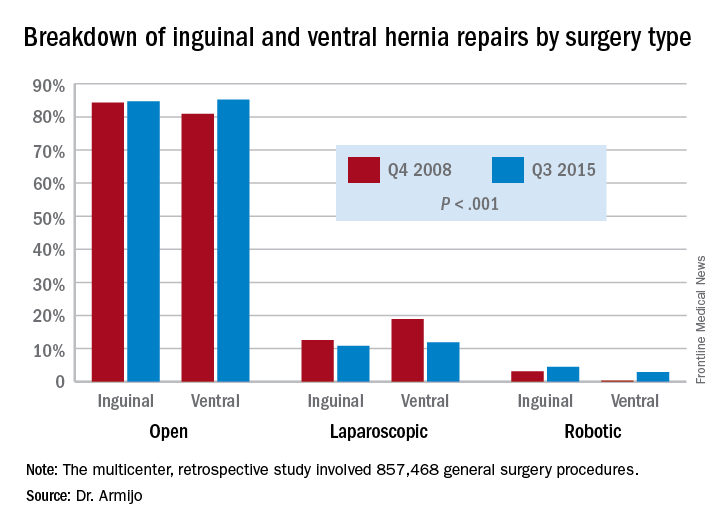
“There was a significant increase in minimally invasive utilizations over time, and robotic surgery increased disproportionately compared to the laparoscope counterpart,” Dr. Armijo said. “And although we cannot prove where those patients are coming from, we believe that, especially for inguinal and ventral hernia repairs, they are coming from laparoscopic surgeons who now are adopting robotic techniques and not from open surgeons switching to the robotic approach.”
In 7 years, the study showed a significant decrease in the share of open procedures in colectomy (from 71.8% to 61.9%), cholecystectomy (35.7% to 27.1%), and bariatric surgery (20.1% to 10.1%), but an increase in both laparoscopic and RAS approaches in these surgeries.
However, in IHR, open procedures held steady at around 84% through the study period, while laparoscopic procedures declined from 12.6% to 10.8% and RAS jumped 3.1% to 4.5%. For VHR, the share of open procedures actually jumped from 80.9% to 85.2%, while the proportion of laparoscopic procedures fell from 18.9% to 11.9% and RAS operations jumped more than tenfold, from 0.2% to 2.9%.
“For ventral hernia repair there was a significant decrease in the laparoscopic approach with a significant increase in both open and robotic procedures, which may be due to new open techniques, including component separation, that have been shown to be more durable as a repair,” Dr. Armijo said. “In addition, those repair techniques are more easily performed with the robotic approach. Laparoscopic surgeons are finding that robotic technology is enabling them to execute surgical tasks, such as suturing mesh.”
Coauthor Dmitry Oleynikov, MD, FACS, disclosed he is a stockholder in Virtual Incision Corp. Dr. Armijo and other coauthors had no financial relationships to disclose.
HOUSTON – Subspecialties such as urology and gynecology have seen a steady increase in robot-assisted surgery and an offsetting decline in open procedures, but in general surgery, robot-assisted procedures seem to be making gains at the expense of laparoscopy, according to researchers from the University of Nebraska.
In two specific operations, ventral and inguinal hernia repairs (VHR and IHR), the percentage of open procedures has increased or held steady over the 7-year study period while the share of laparoscopic operations declined and robot-assisted surgeries (RAS) increased, Priscila Rodrigues Armijo, MD, reported at the annual meeting of the Society of American Gastrointestinal and Endoscopic Surgeons.
This shift to RAS rather than laparoscopy could have significant implications because RAS is significantly more costly than laparoscopy, Dr. Armijo said. “In our study, the open procedures were the most expensive, followed by the robot-assisted surgeries and then laparoscopy,” she said. Median direct costs were $14,364 for open procedures, $11,376 for RAS and $7,945 for laparoscopy.
The Nebraska study retrospectively analyzed five different general surgery procedures: colectomy, cholecystectomy, and bariatric procedures in addition to VHR and IHR. The researchers analyzed 857,468 operations entered into the University HealthSystem Consortium Clinical Database Resource Manager from October 2008 to September 2015.
Dr. Armijo explained that the goal was to study trends in general surgery because while several studies have examined trends in urologic and gynecologic surgery, few studies have done so in general surgery.

“There was a significant increase in minimally invasive utilizations over time, and robotic surgery increased disproportionately compared to the laparoscope counterpart,” Dr. Armijo said. “And although we cannot prove where those patients are coming from, we believe that, especially for inguinal and ventral hernia repairs, they are coming from laparoscopic surgeons who now are adopting robotic techniques and not from open surgeons switching to the robotic approach.”
In 7 years, the study showed a significant decrease in the share of open procedures in colectomy (from 71.8% to 61.9%), cholecystectomy (35.7% to 27.1%), and bariatric surgery (20.1% to 10.1%), but an increase in both laparoscopic and RAS approaches in these surgeries.
However, in IHR, open procedures held steady at around 84% through the study period, while laparoscopic procedures declined from 12.6% to 10.8% and RAS jumped 3.1% to 4.5%. For VHR, the share of open procedures actually jumped from 80.9% to 85.2%, while the proportion of laparoscopic procedures fell from 18.9% to 11.9% and RAS operations jumped more than tenfold, from 0.2% to 2.9%.
“For ventral hernia repair there was a significant decrease in the laparoscopic approach with a significant increase in both open and robotic procedures, which may be due to new open techniques, including component separation, that have been shown to be more durable as a repair,” Dr. Armijo said. “In addition, those repair techniques are more easily performed with the robotic approach. Laparoscopic surgeons are finding that robotic technology is enabling them to execute surgical tasks, such as suturing mesh.”
Coauthor Dmitry Oleynikov, MD, FACS, disclosed he is a stockholder in Virtual Incision Corp. Dr. Armijo and other coauthors had no financial relationships to disclose.
HOUSTON – Subspecialties such as urology and gynecology have seen a steady increase in robot-assisted surgery and an offsetting decline in open procedures, but in general surgery, robot-assisted procedures seem to be making gains at the expense of laparoscopy, according to researchers from the University of Nebraska.
In two specific operations, ventral and inguinal hernia repairs (VHR and IHR), the percentage of open procedures has increased or held steady over the 7-year study period while the share of laparoscopic operations declined and robot-assisted surgeries (RAS) increased, Priscila Rodrigues Armijo, MD, reported at the annual meeting of the Society of American Gastrointestinal and Endoscopic Surgeons.
This shift to RAS rather than laparoscopy could have significant implications because RAS is significantly more costly than laparoscopy, Dr. Armijo said. “In our study, the open procedures were the most expensive, followed by the robot-assisted surgeries and then laparoscopy,” she said. Median direct costs were $14,364 for open procedures, $11,376 for RAS and $7,945 for laparoscopy.
The Nebraska study retrospectively analyzed five different general surgery procedures: colectomy, cholecystectomy, and bariatric procedures in addition to VHR and IHR. The researchers analyzed 857,468 operations entered into the University HealthSystem Consortium Clinical Database Resource Manager from October 2008 to September 2015.
Dr. Armijo explained that the goal was to study trends in general surgery because while several studies have examined trends in urologic and gynecologic surgery, few studies have done so in general surgery.

“There was a significant increase in minimally invasive utilizations over time, and robotic surgery increased disproportionately compared to the laparoscope counterpart,” Dr. Armijo said. “And although we cannot prove where those patients are coming from, we believe that, especially for inguinal and ventral hernia repairs, they are coming from laparoscopic surgeons who now are adopting robotic techniques and not from open surgeons switching to the robotic approach.”
In 7 years, the study showed a significant decrease in the share of open procedures in colectomy (from 71.8% to 61.9%), cholecystectomy (35.7% to 27.1%), and bariatric surgery (20.1% to 10.1%), but an increase in both laparoscopic and RAS approaches in these surgeries.
However, in IHR, open procedures held steady at around 84% through the study period, while laparoscopic procedures declined from 12.6% to 10.8% and RAS jumped 3.1% to 4.5%. For VHR, the share of open procedures actually jumped from 80.9% to 85.2%, while the proportion of laparoscopic procedures fell from 18.9% to 11.9% and RAS operations jumped more than tenfold, from 0.2% to 2.9%.
“For ventral hernia repair there was a significant decrease in the laparoscopic approach with a significant increase in both open and robotic procedures, which may be due to new open techniques, including component separation, that have been shown to be more durable as a repair,” Dr. Armijo said. “In addition, those repair techniques are more easily performed with the robotic approach. Laparoscopic surgeons are finding that robotic technology is enabling them to execute surgical tasks, such as suturing mesh.”
Coauthor Dmitry Oleynikov, MD, FACS, disclosed he is a stockholder in Virtual Incision Corp. Dr. Armijo and other coauthors had no financial relationships to disclose.
AT SAGES 2017
Key clinical point: In inguinal and ventral hernia repair, laparoscopic surgeons are more likely than are open surgery counterparts to move to surgical robot.
Major finding: Over the 7-year study period, the share of open ventral hernia repair procedures increased from 80.9% to 85.2%, while the proportion of laparoscopic procedures fell from 18.9% to 11.9% and RAS operations increased from 0.2% to 2.9%.
Data source: Multicenter, retrospective study of 857,468 general surgery procedures from 2008 to 2015 in the University HealthSystem Consortium Clinical Database Resource Manager.
Disclosures: Dr. Armijo reported having no financial disclosures. Coauthor Dmitry Oleynikov, MD, disclosed stock holding in Virtual Incision Corp.
VIDEO: Pain and impaired QOL persist after open endometrial cancer surgery
NATIONAL HARBOR, MD. – Patient-reported outcomes from a prospective cohort study of minimally invasive versus open surgery for women with endometrial cancer showed that the disability from open surgery persisted for longer than had previously been recognized. Further, for a subset of patients, impairment in sexual functioning was significant, and persistent, regardless of the type of surgery.
At 3 weeks after surgery, patients who had open surgery had greater pain as measured by the Brief Pain Inventory (minimally important difference greater than 1, P = .0004). By 3 months post surgery, responses on the Functional Assessment of Cancer Therapy–General were still significantly lower for the open-surgery group, compared with the minimally invasive group (P = .0011).
Although patients’ pain and overall state of health were better at 3 weeks post surgery, regardless of whether women had open, laparoscopic, or robotic surgery, the reduced overall quality of life experienced by patients who had open surgery persisted.
“What was a bit different from other studies … is that we found that this is maintained even at 3 months, and it was clinically and statistically different,” Sarah Ferguson, MD, said in a video interview at the annual meeting of the Society of Gynecologic Oncology. “So that was really, I think, an interesting finding, that this doesn’t just impact the very short term. Three months is a fairly long time after a primary surgery, and [it’s] important for women to know this.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Patients in the eight-center study had histologically confirmed clinical stage I or II endometrial cancer. The open-surgery arm of the study involved 106 patients, and 414 had minimally invasive surgery (168 laparascopic, 246 robotic).
The robotic and laparoscopic arms showed no statistically significant differences for any patient-reported outcome, even after adjusting for potentially confounding variables, said Dr. Ferguson of Princess Margaret Cancer Centre at the University of Toronto. Accordingly, investigators compared both minimally invasive arms grouped together against open surgery.
Overall, about 80% of patients completed the quality-of-life questionnaires. The response rate for the sexual-functioning questionnaires, however, was much lower, ranging from about a quarter to a half of the participants.
When Dr. Ferguson and her colleagues examined the characteristics of the patients who did complete the sexual-functioning questionnaires, they found that these women were more likely to be younger, partnered, premenopausal and sexually active at the time of diagnosis. Both of the surgical groups “met the clinical cutoff for sexual dysfunction” on the Female Sexual Function Index questionnaire, she said.
For the sexual function questionnaires, differences between the open and minimally invasive groups were not significant at any time point throughout the 26 weeks that patients were studied. “Though it’s a small population, I think these results are important,” said Dr. Ferguson. “These variables may be helpful for us to target patients in our practice, or in future studies, who require intervention.”
Though the study was not randomized, Dr. Ferguson said that the baseline characteristics were similar between groups, and the investigators’ intention-to-treat analysis used a statistical model that adjusted for many potential confounding variables.
Dr. Ferguson reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
NATIONAL HARBOR, MD. – Patient-reported outcomes from a prospective cohort study of minimally invasive versus open surgery for women with endometrial cancer showed that the disability from open surgery persisted for longer than had previously been recognized. Further, for a subset of patients, impairment in sexual functioning was significant, and persistent, regardless of the type of surgery.
At 3 weeks after surgery, patients who had open surgery had greater pain as measured by the Brief Pain Inventory (minimally important difference greater than 1, P = .0004). By 3 months post surgery, responses on the Functional Assessment of Cancer Therapy–General were still significantly lower for the open-surgery group, compared with the minimally invasive group (P = .0011).
Although patients’ pain and overall state of health were better at 3 weeks post surgery, regardless of whether women had open, laparoscopic, or robotic surgery, the reduced overall quality of life experienced by patients who had open surgery persisted.
“What was a bit different from other studies … is that we found that this is maintained even at 3 months, and it was clinically and statistically different,” Sarah Ferguson, MD, said in a video interview at the annual meeting of the Society of Gynecologic Oncology. “So that was really, I think, an interesting finding, that this doesn’t just impact the very short term. Three months is a fairly long time after a primary surgery, and [it’s] important for women to know this.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Patients in the eight-center study had histologically confirmed clinical stage I or II endometrial cancer. The open-surgery arm of the study involved 106 patients, and 414 had minimally invasive surgery (168 laparascopic, 246 robotic).
The robotic and laparoscopic arms showed no statistically significant differences for any patient-reported outcome, even after adjusting for potentially confounding variables, said Dr. Ferguson of Princess Margaret Cancer Centre at the University of Toronto. Accordingly, investigators compared both minimally invasive arms grouped together against open surgery.
Overall, about 80% of patients completed the quality-of-life questionnaires. The response rate for the sexual-functioning questionnaires, however, was much lower, ranging from about a quarter to a half of the participants.
When Dr. Ferguson and her colleagues examined the characteristics of the patients who did complete the sexual-functioning questionnaires, they found that these women were more likely to be younger, partnered, premenopausal and sexually active at the time of diagnosis. Both of the surgical groups “met the clinical cutoff for sexual dysfunction” on the Female Sexual Function Index questionnaire, she said.
For the sexual function questionnaires, differences between the open and minimally invasive groups were not significant at any time point throughout the 26 weeks that patients were studied. “Though it’s a small population, I think these results are important,” said Dr. Ferguson. “These variables may be helpful for us to target patients in our practice, or in future studies, who require intervention.”
Though the study was not randomized, Dr. Ferguson said that the baseline characteristics were similar between groups, and the investigators’ intention-to-treat analysis used a statistical model that adjusted for many potential confounding variables.
Dr. Ferguson reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
NATIONAL HARBOR, MD. – Patient-reported outcomes from a prospective cohort study of minimally invasive versus open surgery for women with endometrial cancer showed that the disability from open surgery persisted for longer than had previously been recognized. Further, for a subset of patients, impairment in sexual functioning was significant, and persistent, regardless of the type of surgery.
At 3 weeks after surgery, patients who had open surgery had greater pain as measured by the Brief Pain Inventory (minimally important difference greater than 1, P = .0004). By 3 months post surgery, responses on the Functional Assessment of Cancer Therapy–General were still significantly lower for the open-surgery group, compared with the minimally invasive group (P = .0011).
Although patients’ pain and overall state of health were better at 3 weeks post surgery, regardless of whether women had open, laparoscopic, or robotic surgery, the reduced overall quality of life experienced by patients who had open surgery persisted.
“What was a bit different from other studies … is that we found that this is maintained even at 3 months, and it was clinically and statistically different,” Sarah Ferguson, MD, said in a video interview at the annual meeting of the Society of Gynecologic Oncology. “So that was really, I think, an interesting finding, that this doesn’t just impact the very short term. Three months is a fairly long time after a primary surgery, and [it’s] important for women to know this.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Patients in the eight-center study had histologically confirmed clinical stage I or II endometrial cancer. The open-surgery arm of the study involved 106 patients, and 414 had minimally invasive surgery (168 laparascopic, 246 robotic).
The robotic and laparoscopic arms showed no statistically significant differences for any patient-reported outcome, even after adjusting for potentially confounding variables, said Dr. Ferguson of Princess Margaret Cancer Centre at the University of Toronto. Accordingly, investigators compared both minimally invasive arms grouped together against open surgery.
Overall, about 80% of patients completed the quality-of-life questionnaires. The response rate for the sexual-functioning questionnaires, however, was much lower, ranging from about a quarter to a half of the participants.
When Dr. Ferguson and her colleagues examined the characteristics of the patients who did complete the sexual-functioning questionnaires, they found that these women were more likely to be younger, partnered, premenopausal and sexually active at the time of diagnosis. Both of the surgical groups “met the clinical cutoff for sexual dysfunction” on the Female Sexual Function Index questionnaire, she said.
For the sexual function questionnaires, differences between the open and minimally invasive groups were not significant at any time point throughout the 26 weeks that patients were studied. “Though it’s a small population, I think these results are important,” said Dr. Ferguson. “These variables may be helpful for us to target patients in our practice, or in future studies, who require intervention.”
Though the study was not randomized, Dr. Ferguson said that the baseline characteristics were similar between groups, and the investigators’ intention-to-treat analysis used a statistical model that adjusted for many potential confounding variables.
Dr. Ferguson reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
AT THE ANNUAL MEETING ON WOMEN'S CANCER
Robotic PCI success rates higher with radial access
WASHINGTON – The clinical and technical success rates are higher among patients undergoing robotic percutaneous coronary interventions through radial than femoral access, according to registry data presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Although both the clinical and technical success rates were high with either type of access, the advantage for radial over femoral access was significant for each, reported Ali Pourdjabbar, MD, an interventional cardiologist completing his fellowship at the University of California, San Diego. However, as this was not a randomized trial, he placed emphasis on the message that robotic percutaneous coronary intervention (PCI) is safe and effective when performed through either access point.
Clinical success, defined as less than 30% residual occlusion with TIMI3 flow and no major adverse cardiovascular events, such as myocardial infarction, cardiovascular death, or revascularization, was achieved in 99.4% of the 310 patients treated through radial access and 94.7% of the 191 patients treated through femoral access (P = .002). Technical success, defined as PCI performed without any manual assistance, was achieved in 92.4% of procedures performed through radial access and 86.7% of those performed through femoral access (P = .03).
There were no significant differences in the two groups for contrast use or fluoroscopy time, but the time to completing PCI was shorter with the radial approach (57 vs. 66 minutes; P less than .04).
However, the groups did differ in baseline characteristics, according to Dr. Pourdjabbar. Patients undergoing robotic PCI through a radial approach were younger, less likely to have diabetes, and less likely to have received a prior PCI. Most importantly, they were less likely to have complex lesions. Patients treated with radial access had higher average body mass indexes.
“It is important to recognize that this was a nonrandomized, retrospective analysis,” Dr. Pourdjabbar emphasized. He noted that one reason for this analysis was to confirm that efficacy and safety was just as good with radial access, which although an approved robotic approach, was supported with fewer data at the time that the device became available.
However, it is notable that 60% of the robotic procedures were done with the radial approach, which is approximately double the proportion currently performed in the United States when done manually, according to data presented by Dr. Pourdjabbar. He noted that radial access has been more commonly used outside of the United States, but rates have also started climbing in this country, rising from less than 5% of cases in 2005 to nearly one third of cases in the most recent analysis. It is unclear why robotic procedures are performed more frequently through radial access, but Dr. Pourdjabbar speculated that centers innovating with robots might also be in the vanguard of the movement toward radial PCI.
Of reasons to consider robots, Dr. Pourdjabbar suggested that the safety advantages for the interventionalist are particularly compelling. Citing a variety of data associating cath lab radiation exposure to health risks for physicians and staff, Dr. Pourdjabbar explained that the operator performs robotic PCI from a shielded cockpit that completely eliminates exposure to radiation. A next generation robotic device, called the CorPath GRX System, is expected to further reduce opportunities for radiation exposure by allowing the operator to disengage the guide catheter in cases when this had to be done manually with the first generation CorPath 200 system.
Asked about the learning curve of PCI robotics, Dr. Pourdjabbar said that the principles appear to be grasped quickly by interventionalists, but he acknowledged that his experience as a training fellow has been limited. However, Rajesh V. Swaminathan, MD, an interventionalist affiliated with Duke University, Durham, N.C., who has experience with robotic PCI, reported that although the tactile sense of the guide wire is lost in robotic PCI, the procedure has typically proceeded more quickly in his hands once access is achieved.
“The greatest learning curve may with the staff that has to get used to not having the interventionalist at the table,” observed Dr. Swaminathan, who was a moderator of the session in which these data were presented.
WASHINGTON – The clinical and technical success rates are higher among patients undergoing robotic percutaneous coronary interventions through radial than femoral access, according to registry data presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Although both the clinical and technical success rates were high with either type of access, the advantage for radial over femoral access was significant for each, reported Ali Pourdjabbar, MD, an interventional cardiologist completing his fellowship at the University of California, San Diego. However, as this was not a randomized trial, he placed emphasis on the message that robotic percutaneous coronary intervention (PCI) is safe and effective when performed through either access point.
Clinical success, defined as less than 30% residual occlusion with TIMI3 flow and no major adverse cardiovascular events, such as myocardial infarction, cardiovascular death, or revascularization, was achieved in 99.4% of the 310 patients treated through radial access and 94.7% of the 191 patients treated through femoral access (P = .002). Technical success, defined as PCI performed without any manual assistance, was achieved in 92.4% of procedures performed through radial access and 86.7% of those performed through femoral access (P = .03).
There were no significant differences in the two groups for contrast use or fluoroscopy time, but the time to completing PCI was shorter with the radial approach (57 vs. 66 minutes; P less than .04).
However, the groups did differ in baseline characteristics, according to Dr. Pourdjabbar. Patients undergoing robotic PCI through a radial approach were younger, less likely to have diabetes, and less likely to have received a prior PCI. Most importantly, they were less likely to have complex lesions. Patients treated with radial access had higher average body mass indexes.
“It is important to recognize that this was a nonrandomized, retrospective analysis,” Dr. Pourdjabbar emphasized. He noted that one reason for this analysis was to confirm that efficacy and safety was just as good with radial access, which although an approved robotic approach, was supported with fewer data at the time that the device became available.
However, it is notable that 60% of the robotic procedures were done with the radial approach, which is approximately double the proportion currently performed in the United States when done manually, according to data presented by Dr. Pourdjabbar. He noted that radial access has been more commonly used outside of the United States, but rates have also started climbing in this country, rising from less than 5% of cases in 2005 to nearly one third of cases in the most recent analysis. It is unclear why robotic procedures are performed more frequently through radial access, but Dr. Pourdjabbar speculated that centers innovating with robots might also be in the vanguard of the movement toward radial PCI.
Of reasons to consider robots, Dr. Pourdjabbar suggested that the safety advantages for the interventionalist are particularly compelling. Citing a variety of data associating cath lab radiation exposure to health risks for physicians and staff, Dr. Pourdjabbar explained that the operator performs robotic PCI from a shielded cockpit that completely eliminates exposure to radiation. A next generation robotic device, called the CorPath GRX System, is expected to further reduce opportunities for radiation exposure by allowing the operator to disengage the guide catheter in cases when this had to be done manually with the first generation CorPath 200 system.
Asked about the learning curve of PCI robotics, Dr. Pourdjabbar said that the principles appear to be grasped quickly by interventionalists, but he acknowledged that his experience as a training fellow has been limited. However, Rajesh V. Swaminathan, MD, an interventionalist affiliated with Duke University, Durham, N.C., who has experience with robotic PCI, reported that although the tactile sense of the guide wire is lost in robotic PCI, the procedure has typically proceeded more quickly in his hands once access is achieved.
“The greatest learning curve may with the staff that has to get used to not having the interventionalist at the table,” observed Dr. Swaminathan, who was a moderator of the session in which these data were presented.
WASHINGTON – The clinical and technical success rates are higher among patients undergoing robotic percutaneous coronary interventions through radial than femoral access, according to registry data presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Although both the clinical and technical success rates were high with either type of access, the advantage for radial over femoral access was significant for each, reported Ali Pourdjabbar, MD, an interventional cardiologist completing his fellowship at the University of California, San Diego. However, as this was not a randomized trial, he placed emphasis on the message that robotic percutaneous coronary intervention (PCI) is safe and effective when performed through either access point.
Clinical success, defined as less than 30% residual occlusion with TIMI3 flow and no major adverse cardiovascular events, such as myocardial infarction, cardiovascular death, or revascularization, was achieved in 99.4% of the 310 patients treated through radial access and 94.7% of the 191 patients treated through femoral access (P = .002). Technical success, defined as PCI performed without any manual assistance, was achieved in 92.4% of procedures performed through radial access and 86.7% of those performed through femoral access (P = .03).
There were no significant differences in the two groups for contrast use or fluoroscopy time, but the time to completing PCI was shorter with the radial approach (57 vs. 66 minutes; P less than .04).
However, the groups did differ in baseline characteristics, according to Dr. Pourdjabbar. Patients undergoing robotic PCI through a radial approach were younger, less likely to have diabetes, and less likely to have received a prior PCI. Most importantly, they were less likely to have complex lesions. Patients treated with radial access had higher average body mass indexes.
“It is important to recognize that this was a nonrandomized, retrospective analysis,” Dr. Pourdjabbar emphasized. He noted that one reason for this analysis was to confirm that efficacy and safety was just as good with radial access, which although an approved robotic approach, was supported with fewer data at the time that the device became available.
However, it is notable that 60% of the robotic procedures were done with the radial approach, which is approximately double the proportion currently performed in the United States when done manually, according to data presented by Dr. Pourdjabbar. He noted that radial access has been more commonly used outside of the United States, but rates have also started climbing in this country, rising from less than 5% of cases in 2005 to nearly one third of cases in the most recent analysis. It is unclear why robotic procedures are performed more frequently through radial access, but Dr. Pourdjabbar speculated that centers innovating with robots might also be in the vanguard of the movement toward radial PCI.
Of reasons to consider robots, Dr. Pourdjabbar suggested that the safety advantages for the interventionalist are particularly compelling. Citing a variety of data associating cath lab radiation exposure to health risks for physicians and staff, Dr. Pourdjabbar explained that the operator performs robotic PCI from a shielded cockpit that completely eliminates exposure to radiation. A next generation robotic device, called the CorPath GRX System, is expected to further reduce opportunities for radiation exposure by allowing the operator to disengage the guide catheter in cases when this had to be done manually with the first generation CorPath 200 system.
Asked about the learning curve of PCI robotics, Dr. Pourdjabbar said that the principles appear to be grasped quickly by interventionalists, but he acknowledged that his experience as a training fellow has been limited. However, Rajesh V. Swaminathan, MD, an interventionalist affiliated with Duke University, Durham, N.C., who has experience with robotic PCI, reported that although the tactile sense of the guide wire is lost in robotic PCI, the procedure has typically proceeded more quickly in his hands once access is achieved.
“The greatest learning curve may with the staff that has to get used to not having the interventionalist at the table,” observed Dr. Swaminathan, who was a moderator of the session in which these data were presented.
AT CRT 2017
Key clinical point: Registry data shows higher success rate for radial versus femoral access in robotic percutaneous coronary interventions.
Major finding: In robotic PCI, the clinical success rate was 99.4% with radial access and 94.7% (P = .002) with femoral access.
Data source: A nonrandomized, retrospective analysis.
Disclosures: Dr. Pourdjabbar reported no financial relationships to disclose.
Lower analgesic use after robotic pelvic surgery
Postoperative use of both opioid and nonopioid analgesics was lower after robotic surgery than after laparotomy for endometrial cancer, according to a report published in Gynecologic Oncology.
Researchers assessed the use of postoperative pain medication in a single-center retrospective study involving 340 consecutive robotically assisted surgeries for endometrial cancer during a 6-year period. The mean patient age was 65 years, and more than one-third of the women were aged 70 or older. Slightly more than half were obese, and 19% were morbidly obese, said Jeremie Abitbol, PhD, of the division of gynecologic oncology, Jewish General Hospital and McGill University, Montreal, and his associates.
This benefit in the use of pain medication occurred regardless of the patient’s obesity status or age, which is particularly helpful in view of the increased risk of adverse events in these two patient populations, Dr. Abitbol and his associates reported (Gynecol Oncol. 2016. doi: 10.1016/jgyno.2016.11.014).
The direct costs associated with postoperative analgesia also were commensurately lower for robotically assisted surgery ($2.52 per day) than for laparotomy ($7.89 per day).
This study was supported by grants from the Israel Cancer Research Foundation, the Gloria’s Girls Fund, the Levi Family Fund, and the Weekend to End Women’s Cancers. Dr. Abitbol reported having no relevant financial disclosures; one of his associates reported receiving a grant from Intuitive Surgical.
Postoperative use of both opioid and nonopioid analgesics was lower after robotic surgery than after laparotomy for endometrial cancer, according to a report published in Gynecologic Oncology.
Researchers assessed the use of postoperative pain medication in a single-center retrospective study involving 340 consecutive robotically assisted surgeries for endometrial cancer during a 6-year period. The mean patient age was 65 years, and more than one-third of the women were aged 70 or older. Slightly more than half were obese, and 19% were morbidly obese, said Jeremie Abitbol, PhD, of the division of gynecologic oncology, Jewish General Hospital and McGill University, Montreal, and his associates.
This benefit in the use of pain medication occurred regardless of the patient’s obesity status or age, which is particularly helpful in view of the increased risk of adverse events in these two patient populations, Dr. Abitbol and his associates reported (Gynecol Oncol. 2016. doi: 10.1016/jgyno.2016.11.014).
The direct costs associated with postoperative analgesia also were commensurately lower for robotically assisted surgery ($2.52 per day) than for laparotomy ($7.89 per day).
This study was supported by grants from the Israel Cancer Research Foundation, the Gloria’s Girls Fund, the Levi Family Fund, and the Weekend to End Women’s Cancers. Dr. Abitbol reported having no relevant financial disclosures; one of his associates reported receiving a grant from Intuitive Surgical.
Postoperative use of both opioid and nonopioid analgesics was lower after robotic surgery than after laparotomy for endometrial cancer, according to a report published in Gynecologic Oncology.
Researchers assessed the use of postoperative pain medication in a single-center retrospective study involving 340 consecutive robotically assisted surgeries for endometrial cancer during a 6-year period. The mean patient age was 65 years, and more than one-third of the women were aged 70 or older. Slightly more than half were obese, and 19% were morbidly obese, said Jeremie Abitbol, PhD, of the division of gynecologic oncology, Jewish General Hospital and McGill University, Montreal, and his associates.
This benefit in the use of pain medication occurred regardless of the patient’s obesity status or age, which is particularly helpful in view of the increased risk of adverse events in these two patient populations, Dr. Abitbol and his associates reported (Gynecol Oncol. 2016. doi: 10.1016/jgyno.2016.11.014).
The direct costs associated with postoperative analgesia also were commensurately lower for robotically assisted surgery ($2.52 per day) than for laparotomy ($7.89 per day).
This study was supported by grants from the Israel Cancer Research Foundation, the Gloria’s Girls Fund, the Levi Family Fund, and the Weekend to End Women’s Cancers. Dr. Abitbol reported having no relevant financial disclosures; one of his associates reported receiving a grant from Intuitive Surgical.
FROM GYNECOLOGIC ONCOLOGY
Key clinical point: Postoperative use of both opioid and nonopioid analgesics was lower after robotic surgery than after laparotomy for endometrial cancer.
Major finding: The robotic surgery cohort required significantly less opioids (12 mg vs. 71 mg), acetaminophen (2,151 mg vs. 4,810 mg), ibuprofen (377 mg vs. 1,892 mg), and naproxen (393 mg vs 1,470 mg), compared with an historical cohort of 59 women who underwent laparotomy.
Data source: A single-center retrospective cohort study involving 340 consecutive robotically assisted pelvic surgeries during a 6-year period.
Disclosures: This study was supported by grants from the Israel Cancer Research Foundation, the Gloria’s Girls Fund, the Levi Family Fund, and the Weekend to End Women’s Cancers. Dr. Abitbol reported having no relevant financial disclosures; one of his associates reported receiving a grant from Intuitive Surgical.
Open, laparoscopic, robotic approaches all sound for distal pancreatectomy
WASHINGTON – The three approaches to distal pancreatectomy – open, laparoscopic, and robotic – are equally safe and oncologically sound methods in properly selected patients, findings from a NSQIP database study show.
“The three approaches have specific indications for use and advantages in well-selected patients; therefore, demonstrating the superiority of one technique over another remains challenging,” said Dimitrios Xourafas, MD, in his presentation at the annual clinical congress of the American College of Surgeons.
Laparoscopic surgeries are more likely to result in open conversions than are robotic surgeries – but robotic surgeries take more time to complete. Open surgeries may be best for advanced disease. But all in all, there are no overriding advantages or disadvantages to any of them, reported Dr. Xourafas, a research fellow in surgery at Brigham and Women’s Hospital, Boston.
He and his colleagues used the American College of Surgeons National Surgical Quality Improvement (NSQIP) database for detailed information on 1,815 distal pancreatectomies performed in 2014. These they separated into three groups: open (921), laparoscopic (694) and robotic (200).
There were no differences in baseline characteristics. Mean age of the patients was 62 years. More than 70% of each group had serious comorbidities. Significantly more in the open surgery group had lost 10% or more of their body weight since diagnosis; this was probably related to a higher rate of preoperative chemotherapy in this group, Dr. Xourafas said. Patients having open surgery also were more likely to have undergone radiation therapy.
There were significant, but mixed, differences in operative outcomes. Surgery was longest in the robotic group (243 minutes vs. 205 minutes in the laparoscopic group and 222 minutes in the open group.) Laparoscopic procedures were more likely to convert to open than were robotic (16% vs. 8%). Blood loss was significantly greater in the open group, with 21% needing transfusion vs. 5% laparoscopic and 6% robotic. Close to 100% of patients in the robotic and open groups needed no vascular resection and no reconstruction. In the open group, fewer patients (88%) had no vascular resection, and 84% required no reconstruction, suggesting that these cases were more advanced disease. This finding was also borne out by the larger percentage of tumors that staged at T3 or higher (34% in the open group vs. about 20% in the other groups). However, about half of each group had a malignant tumor subtype and lesion sizes were similar.
Surgical site infections were more common in the open group (10% vs. 7% of the other groups). The length of stay was longest in the open group (7 days vs. 5 in the other groups). The robotic group had the highest rate of pancreatic fistula (21%) although this was not significantly different from the open and laparoscopic rates (16% and 17%, respectively).
Overall morbidity was 45% in the open group, 36% in the laparoscopic group, and 37% in the robotic group. Mortality was 1.5% in the open group, 1% in the laparoscopic group, and 0.5% in the robotic group – not significantly different.
Dr. Xourafas had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
WASHINGTON – The three approaches to distal pancreatectomy – open, laparoscopic, and robotic – are equally safe and oncologically sound methods in properly selected patients, findings from a NSQIP database study show.
“The three approaches have specific indications for use and advantages in well-selected patients; therefore, demonstrating the superiority of one technique over another remains challenging,” said Dimitrios Xourafas, MD, in his presentation at the annual clinical congress of the American College of Surgeons.
Laparoscopic surgeries are more likely to result in open conversions than are robotic surgeries – but robotic surgeries take more time to complete. Open surgeries may be best for advanced disease. But all in all, there are no overriding advantages or disadvantages to any of them, reported Dr. Xourafas, a research fellow in surgery at Brigham and Women’s Hospital, Boston.
He and his colleagues used the American College of Surgeons National Surgical Quality Improvement (NSQIP) database for detailed information on 1,815 distal pancreatectomies performed in 2014. These they separated into three groups: open (921), laparoscopic (694) and robotic (200).
There were no differences in baseline characteristics. Mean age of the patients was 62 years. More than 70% of each group had serious comorbidities. Significantly more in the open surgery group had lost 10% or more of their body weight since diagnosis; this was probably related to a higher rate of preoperative chemotherapy in this group, Dr. Xourafas said. Patients having open surgery also were more likely to have undergone radiation therapy.
There were significant, but mixed, differences in operative outcomes. Surgery was longest in the robotic group (243 minutes vs. 205 minutes in the laparoscopic group and 222 minutes in the open group.) Laparoscopic procedures were more likely to convert to open than were robotic (16% vs. 8%). Blood loss was significantly greater in the open group, with 21% needing transfusion vs. 5% laparoscopic and 6% robotic. Close to 100% of patients in the robotic and open groups needed no vascular resection and no reconstruction. In the open group, fewer patients (88%) had no vascular resection, and 84% required no reconstruction, suggesting that these cases were more advanced disease. This finding was also borne out by the larger percentage of tumors that staged at T3 or higher (34% in the open group vs. about 20% in the other groups). However, about half of each group had a malignant tumor subtype and lesion sizes were similar.
Surgical site infections were more common in the open group (10% vs. 7% of the other groups). The length of stay was longest in the open group (7 days vs. 5 in the other groups). The robotic group had the highest rate of pancreatic fistula (21%) although this was not significantly different from the open and laparoscopic rates (16% and 17%, respectively).
Overall morbidity was 45% in the open group, 36% in the laparoscopic group, and 37% in the robotic group. Mortality was 1.5% in the open group, 1% in the laparoscopic group, and 0.5% in the robotic group – not significantly different.
Dr. Xourafas had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
WASHINGTON – The three approaches to distal pancreatectomy – open, laparoscopic, and robotic – are equally safe and oncologically sound methods in properly selected patients, findings from a NSQIP database study show.
“The three approaches have specific indications for use and advantages in well-selected patients; therefore, demonstrating the superiority of one technique over another remains challenging,” said Dimitrios Xourafas, MD, in his presentation at the annual clinical congress of the American College of Surgeons.
Laparoscopic surgeries are more likely to result in open conversions than are robotic surgeries – but robotic surgeries take more time to complete. Open surgeries may be best for advanced disease. But all in all, there are no overriding advantages or disadvantages to any of them, reported Dr. Xourafas, a research fellow in surgery at Brigham and Women’s Hospital, Boston.
He and his colleagues used the American College of Surgeons National Surgical Quality Improvement (NSQIP) database for detailed information on 1,815 distal pancreatectomies performed in 2014. These they separated into three groups: open (921), laparoscopic (694) and robotic (200).
There were no differences in baseline characteristics. Mean age of the patients was 62 years. More than 70% of each group had serious comorbidities. Significantly more in the open surgery group had lost 10% or more of their body weight since diagnosis; this was probably related to a higher rate of preoperative chemotherapy in this group, Dr. Xourafas said. Patients having open surgery also were more likely to have undergone radiation therapy.
There were significant, but mixed, differences in operative outcomes. Surgery was longest in the robotic group (243 minutes vs. 205 minutes in the laparoscopic group and 222 minutes in the open group.) Laparoscopic procedures were more likely to convert to open than were robotic (16% vs. 8%). Blood loss was significantly greater in the open group, with 21% needing transfusion vs. 5% laparoscopic and 6% robotic. Close to 100% of patients in the robotic and open groups needed no vascular resection and no reconstruction. In the open group, fewer patients (88%) had no vascular resection, and 84% required no reconstruction, suggesting that these cases were more advanced disease. This finding was also borne out by the larger percentage of tumors that staged at T3 or higher (34% in the open group vs. about 20% in the other groups). However, about half of each group had a malignant tumor subtype and lesion sizes were similar.
Surgical site infections were more common in the open group (10% vs. 7% of the other groups). The length of stay was longest in the open group (7 days vs. 5 in the other groups). The robotic group had the highest rate of pancreatic fistula (21%) although this was not significantly different from the open and laparoscopic rates (16% and 17%, respectively).
Overall morbidity was 45% in the open group, 36% in the laparoscopic group, and 37% in the robotic group. Mortality was 1.5% in the open group, 1% in the laparoscopic group, and 0.5% in the robotic group – not significantly different.
Dr. Xourafas had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
AT THE ACS CLINICAL CONGRESS
Key clinical point:
Major finding: Surgery was longest in the robotic group (243 minutes), but more patients in the open group needed transfusions (21% vs. 5% of the other groups).
Data source: The database review comprised 1,815 patients.
Disclosures: Dr. Xourafas had no financial disclosures.
Robotic surgery instruments ‘virtually impossible’ to clean completely
Instruments used for robotic surgery are “virtually impossible” to clean completely, according to a report published in Infection Control & Hospital Epidemiology.
“A new standard for the cleaning of complex surgical instruments needs to be established, especially for those used in robotic surgery,” wrote Yuhei Saito of the surgical center at the University of Tokyo Hospital and associates.
They assessed the residual contamination of both robotic and regular surgical instruments at their medical center because “hospital staff in central sterile supply departments are troubled by the reprocessing of robotic instruments because they cannot be disassembled for cleaning like other endoscopic instruments. Their complex structure impairs brushing the inner surface of narrow lumens, resulting in failure to [completely] remove contaminants,” the researchers wrote.
In the first phase of the study, the researchers examined 41 instruments immediately after they were used in robotic surgery (7 radical prostatectomies and 2 anterior resections of the rectum) and 27 regular instruments immediately after they were used for open surgery (gastrectomy and colectomy). The robotic instruments were contaminated with 72.3 × 103 mcg of protein each, compared with 5.5 × 103 mcg of protein on the regular instruments, the investigators reported (Infect Control Hosp Epidemiol. 2016 Oct 31. doi: 10.1017/ice.2016.249).
In the second phase of the study, the researchers examined another 24 robotic instruments and 40 regular instruments after they were used in surgery and then cleaned according to the manufacturers’ instructions three successive times. For the robotic instruments, this involved manually brushing the outer surface while moving the instrument “wrists” through their full range of motion, followed by 15 minutes of ultrasonication with enzymatic detergent, flushing the lumen with a water gun through flush ports, and rinsing the entire instrument. For regular instruments, cleaning involved washer-disinfectors and included 5 minutes of ultrasonication, 10 minutes of spraying with an alkaline detergent, and 10 minutes of disinfection via heating.
The level of contamination declined with each successive cleaning but still remained comparatively high for the robotic instruments. The amount of protein contaminants released in the three cleanings was 650, 550, and 530 mcg per robotic instrument, compared with 16, 17, and 17 mcg per ordinary instrument.
The efficacy of cleaning was 97.6% for robotic instruments and 99.1% for regular instruments, the researchers reported.
This study was not designed to assess whether residual contamination is associated with adverse events such as infection in subsequent patients, and there are few data available on this topic.
“We have to recognize that there might be a considerable volume of insufficient cleaning or occult surgical site infections,” the investigators wrote.
New instrument washers equipped with a specific cleaning function for narrow lumens are becoming available, they noted, and “further study should be conducted using these washers with improved cleaning efficacy.”
The study was supported by the Japan Society for the Promotion of Science. The investigators reported having no relevant financial disclosures.
Instruments used for robotic surgery are “virtually impossible” to clean completely, according to a report published in Infection Control & Hospital Epidemiology.
“A new standard for the cleaning of complex surgical instruments needs to be established, especially for those used in robotic surgery,” wrote Yuhei Saito of the surgical center at the University of Tokyo Hospital and associates.
They assessed the residual contamination of both robotic and regular surgical instruments at their medical center because “hospital staff in central sterile supply departments are troubled by the reprocessing of robotic instruments because they cannot be disassembled for cleaning like other endoscopic instruments. Their complex structure impairs brushing the inner surface of narrow lumens, resulting in failure to [completely] remove contaminants,” the researchers wrote.
In the first phase of the study, the researchers examined 41 instruments immediately after they were used in robotic surgery (7 radical prostatectomies and 2 anterior resections of the rectum) and 27 regular instruments immediately after they were used for open surgery (gastrectomy and colectomy). The robotic instruments were contaminated with 72.3 × 103 mcg of protein each, compared with 5.5 × 103 mcg of protein on the regular instruments, the investigators reported (Infect Control Hosp Epidemiol. 2016 Oct 31. doi: 10.1017/ice.2016.249).
In the second phase of the study, the researchers examined another 24 robotic instruments and 40 regular instruments after they were used in surgery and then cleaned according to the manufacturers’ instructions three successive times. For the robotic instruments, this involved manually brushing the outer surface while moving the instrument “wrists” through their full range of motion, followed by 15 minutes of ultrasonication with enzymatic detergent, flushing the lumen with a water gun through flush ports, and rinsing the entire instrument. For regular instruments, cleaning involved washer-disinfectors and included 5 minutes of ultrasonication, 10 minutes of spraying with an alkaline detergent, and 10 minutes of disinfection via heating.
The level of contamination declined with each successive cleaning but still remained comparatively high for the robotic instruments. The amount of protein contaminants released in the three cleanings was 650, 550, and 530 mcg per robotic instrument, compared with 16, 17, and 17 mcg per ordinary instrument.
The efficacy of cleaning was 97.6% for robotic instruments and 99.1% for regular instruments, the researchers reported.
This study was not designed to assess whether residual contamination is associated with adverse events such as infection in subsequent patients, and there are few data available on this topic.
“We have to recognize that there might be a considerable volume of insufficient cleaning or occult surgical site infections,” the investigators wrote.
New instrument washers equipped with a specific cleaning function for narrow lumens are becoming available, they noted, and “further study should be conducted using these washers with improved cleaning efficacy.”
The study was supported by the Japan Society for the Promotion of Science. The investigators reported having no relevant financial disclosures.
Instruments used for robotic surgery are “virtually impossible” to clean completely, according to a report published in Infection Control & Hospital Epidemiology.
“A new standard for the cleaning of complex surgical instruments needs to be established, especially for those used in robotic surgery,” wrote Yuhei Saito of the surgical center at the University of Tokyo Hospital and associates.
They assessed the residual contamination of both robotic and regular surgical instruments at their medical center because “hospital staff in central sterile supply departments are troubled by the reprocessing of robotic instruments because they cannot be disassembled for cleaning like other endoscopic instruments. Their complex structure impairs brushing the inner surface of narrow lumens, resulting in failure to [completely] remove contaminants,” the researchers wrote.
In the first phase of the study, the researchers examined 41 instruments immediately after they were used in robotic surgery (7 radical prostatectomies and 2 anterior resections of the rectum) and 27 regular instruments immediately after they were used for open surgery (gastrectomy and colectomy). The robotic instruments were contaminated with 72.3 × 103 mcg of protein each, compared with 5.5 × 103 mcg of protein on the regular instruments, the investigators reported (Infect Control Hosp Epidemiol. 2016 Oct 31. doi: 10.1017/ice.2016.249).
In the second phase of the study, the researchers examined another 24 robotic instruments and 40 regular instruments after they were used in surgery and then cleaned according to the manufacturers’ instructions three successive times. For the robotic instruments, this involved manually brushing the outer surface while moving the instrument “wrists” through their full range of motion, followed by 15 minutes of ultrasonication with enzymatic detergent, flushing the lumen with a water gun through flush ports, and rinsing the entire instrument. For regular instruments, cleaning involved washer-disinfectors and included 5 minutes of ultrasonication, 10 minutes of spraying with an alkaline detergent, and 10 minutes of disinfection via heating.
The level of contamination declined with each successive cleaning but still remained comparatively high for the robotic instruments. The amount of protein contaminants released in the three cleanings was 650, 550, and 530 mcg per robotic instrument, compared with 16, 17, and 17 mcg per ordinary instrument.
The efficacy of cleaning was 97.6% for robotic instruments and 99.1% for regular instruments, the researchers reported.
This study was not designed to assess whether residual contamination is associated with adverse events such as infection in subsequent patients, and there are few data available on this topic.
“We have to recognize that there might be a considerable volume of insufficient cleaning or occult surgical site infections,” the investigators wrote.
New instrument washers equipped with a specific cleaning function for narrow lumens are becoming available, they noted, and “further study should be conducted using these washers with improved cleaning efficacy.”
The study was supported by the Japan Society for the Promotion of Science. The investigators reported having no relevant financial disclosures.
FROM INFECTION CONTROL AND HOSPITAL EPIDEMIOLOGY
Key clinical point:
Major finding: Immediately after surgery, robotic instruments were contaminated with 72.3 × 103 mcg of protein each, compared with 5.5 × 103 mcg of protein on regular instruments.
Data source: A single-center analysis of protein contamination before and after three successive cleanings on 65 instruments used for robotic surgery and 67 regular instruments used for open surgery.
Disclosures: This study was supported by the Japan Society for the Promotion of Science. The investigators reported having no relevant financial disclosures.
‘Stepping’ up to a better way to teach robotic lobectomy
Teaching minimally invasive robotic surgery to residents can be difficult in a health care environment obsessed with quality outcome measures and under scrutiny by hospital administrators and payers, but researchers at the University of Alabama at Birmingham may have devised a method to instruct residents in robotic lobectomy without compromising patient outcomes, according to a study published in the October issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152:991-7).
Robert J. Cerfolio, MD, MBA, and his coauthors divided the procedure into 19 sequential, teachable steps and allowed residents to perform selected steps during operations that Dr. Cerfolio directed. “We then applied simulation training, coaching techniques, and video review of each step to help improve the steps that residents could not complete,” Dr. Cerfolio and his coauthors said.
Surgeons in academic centers face the challenge of teaching “the art and science of surgery,” Dr. Cerfolio and his colleagues said, while maintaining quality outcomes. “Teaching minimally invasive surgery, especially robotic surgery, is challenging given the risks and the limited availability of the robot.”
The researchers acknowledged that other groups have taken a similar approach to training, but this is the first study that included video review, coaching, and instruction tied to time constraints, they said. “A major concern is that while teaching robotic surgery, patients can be injured, care is worse, and metrics that are increasingly used as surrogates for quality outcomes suffer,” they noted.
They allotted each step in the procedure a set amount of time in which the resident had to complete it, totaling 80 minutes for all 19 steps and ranging from 1 minute to inspect the pleura after placing ports (9 minutes) to 20 minutes to close the five incisions. If the resident completed the task in the allotted time, it was recorded as “performed.”
Between February 2010 and December 2010 Dr. Cerfolio performed 520 robotic lobectomies, and over time the percentage of successful steps per resident improved. For example, in the first year, 50% of thoracic surgery residents completed the first five steps (mark and place ports, inspect pleura, resect the inferior pulmonary ligament, and remove three lymph nodes), but by the last year of the study 90% of them successfully completed the five steps.
Dr. Cerfolio and coauthors acknowledged “many flaws” in their study, but the study also had strengths: It involved only one operation and corroborated the database with each resident’s own surgical logs.
“Operations such as robotic lobectomy can be successfully taught by dividing them into a series of surgical maneuvers or steps,” the researchers noted. Recording what residents can and can’t do, reviewing video, and coaching contribute to the process to improve their skills. “Further studies that scientifically measure ‘ways to teach’ and ways to coach and mentor are needed,” they said.
Dr. Cerfolio disclosed relationships with Intuitive Surgical, Ethicon, Community Health Services, KCL, Bovie and C-SATS. Coauthor Douglas Minnich, MD, is a consultant to Medtronic. The other co-authors had no financial relationships to disclose.
Inderpal S. Sarkaria, MD, of the University of Pittsburgh acknowledged in his invited commentary how “metric-driven patient outcomes” have changed cardiothoracic surgical training (J Thorac Cardiovasc Surg. 2016;152:998).
But Dr. Sarkaria questioned the validity of using time performed as a metric in this study to evaluate a trainee’s competency. “Although ‘time’ is an important component, should not the primary focus be on ‘quality’ of the trainee’s work?” Dr. Sarkaria asked.
Despite these questions and the limitations of the study, he found the approach to surgical training “laudable.” Said Dr. Sarkaria: “It is arguable that the limitations of the study speak more to a common wisdom that certain aspects of surgical education remain an art to a greater or lesser extent, not easily amenable to our efforts to discretely compartmentalize and quantify the process.”
While the premise demands further study, Dr. Cerfolio and his coauthors “have laid a solid foundation on which further to build, explore, and potentially improve the science and art of teaching complex operations to our surgical residents,” Dr. Sarkaria said.
Dr. Sarkaria had no relationships to disclose.
Inderpal S. Sarkaria, MD, of the University of Pittsburgh acknowledged in his invited commentary how “metric-driven patient outcomes” have changed cardiothoracic surgical training (J Thorac Cardiovasc Surg. 2016;152:998).
But Dr. Sarkaria questioned the validity of using time performed as a metric in this study to evaluate a trainee’s competency. “Although ‘time’ is an important component, should not the primary focus be on ‘quality’ of the trainee’s work?” Dr. Sarkaria asked.
Despite these questions and the limitations of the study, he found the approach to surgical training “laudable.” Said Dr. Sarkaria: “It is arguable that the limitations of the study speak more to a common wisdom that certain aspects of surgical education remain an art to a greater or lesser extent, not easily amenable to our efforts to discretely compartmentalize and quantify the process.”
While the premise demands further study, Dr. Cerfolio and his coauthors “have laid a solid foundation on which further to build, explore, and potentially improve the science and art of teaching complex operations to our surgical residents,” Dr. Sarkaria said.
Dr. Sarkaria had no relationships to disclose.
Inderpal S. Sarkaria, MD, of the University of Pittsburgh acknowledged in his invited commentary how “metric-driven patient outcomes” have changed cardiothoracic surgical training (J Thorac Cardiovasc Surg. 2016;152:998).
But Dr. Sarkaria questioned the validity of using time performed as a metric in this study to evaluate a trainee’s competency. “Although ‘time’ is an important component, should not the primary focus be on ‘quality’ of the trainee’s work?” Dr. Sarkaria asked.
Despite these questions and the limitations of the study, he found the approach to surgical training “laudable.” Said Dr. Sarkaria: “It is arguable that the limitations of the study speak more to a common wisdom that certain aspects of surgical education remain an art to a greater or lesser extent, not easily amenable to our efforts to discretely compartmentalize and quantify the process.”
While the premise demands further study, Dr. Cerfolio and his coauthors “have laid a solid foundation on which further to build, explore, and potentially improve the science and art of teaching complex operations to our surgical residents,” Dr. Sarkaria said.
Dr. Sarkaria had no relationships to disclose.
Teaching minimally invasive robotic surgery to residents can be difficult in a health care environment obsessed with quality outcome measures and under scrutiny by hospital administrators and payers, but researchers at the University of Alabama at Birmingham may have devised a method to instruct residents in robotic lobectomy without compromising patient outcomes, according to a study published in the October issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152:991-7).
Robert J. Cerfolio, MD, MBA, and his coauthors divided the procedure into 19 sequential, teachable steps and allowed residents to perform selected steps during operations that Dr. Cerfolio directed. “We then applied simulation training, coaching techniques, and video review of each step to help improve the steps that residents could not complete,” Dr. Cerfolio and his coauthors said.
Surgeons in academic centers face the challenge of teaching “the art and science of surgery,” Dr. Cerfolio and his colleagues said, while maintaining quality outcomes. “Teaching minimally invasive surgery, especially robotic surgery, is challenging given the risks and the limited availability of the robot.”
The researchers acknowledged that other groups have taken a similar approach to training, but this is the first study that included video review, coaching, and instruction tied to time constraints, they said. “A major concern is that while teaching robotic surgery, patients can be injured, care is worse, and metrics that are increasingly used as surrogates for quality outcomes suffer,” they noted.
They allotted each step in the procedure a set amount of time in which the resident had to complete it, totaling 80 minutes for all 19 steps and ranging from 1 minute to inspect the pleura after placing ports (9 minutes) to 20 minutes to close the five incisions. If the resident completed the task in the allotted time, it was recorded as “performed.”
Between February 2010 and December 2010 Dr. Cerfolio performed 520 robotic lobectomies, and over time the percentage of successful steps per resident improved. For example, in the first year, 50% of thoracic surgery residents completed the first five steps (mark and place ports, inspect pleura, resect the inferior pulmonary ligament, and remove three lymph nodes), but by the last year of the study 90% of them successfully completed the five steps.
Dr. Cerfolio and coauthors acknowledged “many flaws” in their study, but the study also had strengths: It involved only one operation and corroborated the database with each resident’s own surgical logs.
“Operations such as robotic lobectomy can be successfully taught by dividing them into a series of surgical maneuvers or steps,” the researchers noted. Recording what residents can and can’t do, reviewing video, and coaching contribute to the process to improve their skills. “Further studies that scientifically measure ‘ways to teach’ and ways to coach and mentor are needed,” they said.
Dr. Cerfolio disclosed relationships with Intuitive Surgical, Ethicon, Community Health Services, KCL, Bovie and C-SATS. Coauthor Douglas Minnich, MD, is a consultant to Medtronic. The other co-authors had no financial relationships to disclose.
Teaching minimally invasive robotic surgery to residents can be difficult in a health care environment obsessed with quality outcome measures and under scrutiny by hospital administrators and payers, but researchers at the University of Alabama at Birmingham may have devised a method to instruct residents in robotic lobectomy without compromising patient outcomes, according to a study published in the October issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152:991-7).
Robert J. Cerfolio, MD, MBA, and his coauthors divided the procedure into 19 sequential, teachable steps and allowed residents to perform selected steps during operations that Dr. Cerfolio directed. “We then applied simulation training, coaching techniques, and video review of each step to help improve the steps that residents could not complete,” Dr. Cerfolio and his coauthors said.
Surgeons in academic centers face the challenge of teaching “the art and science of surgery,” Dr. Cerfolio and his colleagues said, while maintaining quality outcomes. “Teaching minimally invasive surgery, especially robotic surgery, is challenging given the risks and the limited availability of the robot.”
The researchers acknowledged that other groups have taken a similar approach to training, but this is the first study that included video review, coaching, and instruction tied to time constraints, they said. “A major concern is that while teaching robotic surgery, patients can be injured, care is worse, and metrics that are increasingly used as surrogates for quality outcomes suffer,” they noted.
They allotted each step in the procedure a set amount of time in which the resident had to complete it, totaling 80 minutes for all 19 steps and ranging from 1 minute to inspect the pleura after placing ports (9 minutes) to 20 minutes to close the five incisions. If the resident completed the task in the allotted time, it was recorded as “performed.”
Between February 2010 and December 2010 Dr. Cerfolio performed 520 robotic lobectomies, and over time the percentage of successful steps per resident improved. For example, in the first year, 50% of thoracic surgery residents completed the first five steps (mark and place ports, inspect pleura, resect the inferior pulmonary ligament, and remove three lymph nodes), but by the last year of the study 90% of them successfully completed the five steps.
Dr. Cerfolio and coauthors acknowledged “many flaws” in their study, but the study also had strengths: It involved only one operation and corroborated the database with each resident’s own surgical logs.
“Operations such as robotic lobectomy can be successfully taught by dividing them into a series of surgical maneuvers or steps,” the researchers noted. Recording what residents can and can’t do, reviewing video, and coaching contribute to the process to improve their skills. “Further studies that scientifically measure ‘ways to teach’ and ways to coach and mentor are needed,” they said.
Dr. Cerfolio disclosed relationships with Intuitive Surgical, Ethicon, Community Health Services, KCL, Bovie and C-SATS. Coauthor Douglas Minnich, MD, is a consultant to Medtronic. The other co-authors had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Surgical residents learn and safely perform robotic lobectomy by dividing the procedure into a series of surgical maneuvers.
Major finding: The percentage of thoracic surgery residents who completed the first 5 of 19 procedural steps of the operation improved from 50% in the first year to 90% in the fifth year.
Data source: Single-center study of 520 consecutive lobectomies over 5 years by 35 general surgery residents and 7 cardiothoracic residents from February 2010 to December 2015.
Disclosures: Dr. Cerfolio disclosed relationships with Intuitive Surgical, Ethicon, Community Health Services, KCL, Bovie and C-SATS. Coauthor Douglas Minnich, MD, is a consultant to Medtronic. The other coauthors had no financial relationships to disclose.
VIDEO: Open, robotic, laparoscopic approaches equally effective in pancreatectomy
WASHINGTON – Minimally invasive surgery – whether robotic or laparoscopic – is just as effective as open surgery in pancreatectomy.
Both minimally invasive approaches had perioperative and oncologic outcomes that were similar to open approaches, as well as to each other, Katelin Mirkin, MD, reported at the annual clinical congress of the American College of Surgeons. And while minimally invasive surgery (MIS) techniques were associated with a slightly faster move to neoadjuvant chemotherapy, survival outcomes in all three surgical approaches were similar.
Dr. Mirkin, a surgery resident at Penn State Milton S. Hershey Medical Center, Hershey, Pa., plumbed the National Cancer Database for patients with stage I-III pancreatic cancer who were treated by surgical resection from 2010 to 2012. Her cohort comprised 9,047 patients; of these, 7,924 were treated with open surgery, 992 with laparoscopic surgery, and 131 with robotic surgery. She examined a number of factors including lymph node harvest and surgical margins, length of stay and time to adjuvant chemotherapy, and survival.
Patients who had MIS were older (67 vs. 66 years) and more often treated at an academic center, but otherwise there were no significant baseline differences.
Dr. Mirkin first compared the open surgeries with MIS. There were no significant associations with surgical approach and cancer stage. However, distal resections were significantly more likely to be dealt with by MIS, and Whipple procedures by open approaches. There were also more open than MIS total resections.
MIS was more likely to conclude with negative surgical margins (79% vs. 75%), and open surgery more likely to end with positive margins (22% vs. 19%).
Perioperative outcomes favored MIS approaches for all types of surgery, with a mean overall stay of 9.5 days vs. 11.3 days for open surgery. The mean length of stay for a distal resection was 7 days for MIS vs. 8 for open. For a Whipple procedure, the mean stay was 10.7 vs. 11.9 days. For a total resection, it was 10 vs. 11.8 days.
MIS was also associated with a significantly shorter time to the initiation of adjuvant chemotherapy overall (56 vs. 59 days). For a Whipple, time to chemotherapy was 58 vs. 60 days, respectively. For a distal resection, it was 52 vs. 56 days, and for a total resection, 52 vs. 58 days.
Neither approach offered a survival benefit over the other, Dr. Mirkin noted. For stage I cancers, less than 50% of MIS patients and less than 25% of open patients were alive by 50 months. For those with stage II tumors, less than 25% of each group was alive by 40 months. For stage III tumors, the 40-month survival rates were about 10% for MIS patients and 15% for open patients.
Dr. Mirkin then examined perioperative, oncologic, and survival outcomes among those who underwent laparoscopic and robotic surgeries. There were no demographic differences between these groups.
Oncologic outcomes were almost identical with regard to the number of positive regional nodes harvested (six), and surgical margins. Nodes were negative in 82% of robotic cases vs. 78% of laparoscopic cases and positive in 17.6% of robotic cases and 19.4% of laparoscopic cases.
Length of stay was significantly shorter for a laparoscopic approach overall (10 vs. 9.4 days) and particularly in distal resection (7 vs. 10 days). However, there were no differences in length of stay in any other surgery type. Nor was there any difference in the time to neoadjuvant chemotherapy.
Survival outcomes were similar as well. For stage I cancers, 40-month survival was about 40% in the laparoscopic group and 25% in the robotic group. For stage II cancers, 40-month survival was about 15% and 25%, respectively. For stage III tumors, 20-month survival in the robotic group was near 0 and 25% in the laparoscopic group. By 40 months almost all patients were deceased.
A multivariate survival analysis controlled for age, sex, race, comorbidities, facility type and location, surgery type, surgical margins, pathologic stage, and systemic therapy. It found only one significant association: Patients with 12 or more lymph nodes harvested were 19% more likely to die than those with fewer than 12 nodes harvested.
Time to chemotherapy (longer or shorter than 57 days) did not significantly impact survival, Dr. Mirkin said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
WASHINGTON – Minimally invasive surgery – whether robotic or laparoscopic – is just as effective as open surgery in pancreatectomy.
Both minimally invasive approaches had perioperative and oncologic outcomes that were similar to open approaches, as well as to each other, Katelin Mirkin, MD, reported at the annual clinical congress of the American College of Surgeons. And while minimally invasive surgery (MIS) techniques were associated with a slightly faster move to neoadjuvant chemotherapy, survival outcomes in all three surgical approaches were similar.
Dr. Mirkin, a surgery resident at Penn State Milton S. Hershey Medical Center, Hershey, Pa., plumbed the National Cancer Database for patients with stage I-III pancreatic cancer who were treated by surgical resection from 2010 to 2012. Her cohort comprised 9,047 patients; of these, 7,924 were treated with open surgery, 992 with laparoscopic surgery, and 131 with robotic surgery. She examined a number of factors including lymph node harvest and surgical margins, length of stay and time to adjuvant chemotherapy, and survival.
Patients who had MIS were older (67 vs. 66 years) and more often treated at an academic center, but otherwise there were no significant baseline differences.
Dr. Mirkin first compared the open surgeries with MIS. There were no significant associations with surgical approach and cancer stage. However, distal resections were significantly more likely to be dealt with by MIS, and Whipple procedures by open approaches. There were also more open than MIS total resections.
MIS was more likely to conclude with negative surgical margins (79% vs. 75%), and open surgery more likely to end with positive margins (22% vs. 19%).
Perioperative outcomes favored MIS approaches for all types of surgery, with a mean overall stay of 9.5 days vs. 11.3 days for open surgery. The mean length of stay for a distal resection was 7 days for MIS vs. 8 for open. For a Whipple procedure, the mean stay was 10.7 vs. 11.9 days. For a total resection, it was 10 vs. 11.8 days.
MIS was also associated with a significantly shorter time to the initiation of adjuvant chemotherapy overall (56 vs. 59 days). For a Whipple, time to chemotherapy was 58 vs. 60 days, respectively. For a distal resection, it was 52 vs. 56 days, and for a total resection, 52 vs. 58 days.
Neither approach offered a survival benefit over the other, Dr. Mirkin noted. For stage I cancers, less than 50% of MIS patients and less than 25% of open patients were alive by 50 months. For those with stage II tumors, less than 25% of each group was alive by 40 months. For stage III tumors, the 40-month survival rates were about 10% for MIS patients and 15% for open patients.
Dr. Mirkin then examined perioperative, oncologic, and survival outcomes among those who underwent laparoscopic and robotic surgeries. There were no demographic differences between these groups.
Oncologic outcomes were almost identical with regard to the number of positive regional nodes harvested (six), and surgical margins. Nodes were negative in 82% of robotic cases vs. 78% of laparoscopic cases and positive in 17.6% of robotic cases and 19.4% of laparoscopic cases.
Length of stay was significantly shorter for a laparoscopic approach overall (10 vs. 9.4 days) and particularly in distal resection (7 vs. 10 days). However, there were no differences in length of stay in any other surgery type. Nor was there any difference in the time to neoadjuvant chemotherapy.
Survival outcomes were similar as well. For stage I cancers, 40-month survival was about 40% in the laparoscopic group and 25% in the robotic group. For stage II cancers, 40-month survival was about 15% and 25%, respectively. For stage III tumors, 20-month survival in the robotic group was near 0 and 25% in the laparoscopic group. By 40 months almost all patients were deceased.
A multivariate survival analysis controlled for age, sex, race, comorbidities, facility type and location, surgery type, surgical margins, pathologic stage, and systemic therapy. It found only one significant association: Patients with 12 or more lymph nodes harvested were 19% more likely to die than those with fewer than 12 nodes harvested.
Time to chemotherapy (longer or shorter than 57 days) did not significantly impact survival, Dr. Mirkin said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
WASHINGTON – Minimally invasive surgery – whether robotic or laparoscopic – is just as effective as open surgery in pancreatectomy.
Both minimally invasive approaches had perioperative and oncologic outcomes that were similar to open approaches, as well as to each other, Katelin Mirkin, MD, reported at the annual clinical congress of the American College of Surgeons. And while minimally invasive surgery (MIS) techniques were associated with a slightly faster move to neoadjuvant chemotherapy, survival outcomes in all three surgical approaches were similar.
Dr. Mirkin, a surgery resident at Penn State Milton S. Hershey Medical Center, Hershey, Pa., plumbed the National Cancer Database for patients with stage I-III pancreatic cancer who were treated by surgical resection from 2010 to 2012. Her cohort comprised 9,047 patients; of these, 7,924 were treated with open surgery, 992 with laparoscopic surgery, and 131 with robotic surgery. She examined a number of factors including lymph node harvest and surgical margins, length of stay and time to adjuvant chemotherapy, and survival.
Patients who had MIS were older (67 vs. 66 years) and more often treated at an academic center, but otherwise there were no significant baseline differences.
Dr. Mirkin first compared the open surgeries with MIS. There were no significant associations with surgical approach and cancer stage. However, distal resections were significantly more likely to be dealt with by MIS, and Whipple procedures by open approaches. There were also more open than MIS total resections.
MIS was more likely to conclude with negative surgical margins (79% vs. 75%), and open surgery more likely to end with positive margins (22% vs. 19%).
Perioperative outcomes favored MIS approaches for all types of surgery, with a mean overall stay of 9.5 days vs. 11.3 days for open surgery. The mean length of stay for a distal resection was 7 days for MIS vs. 8 for open. For a Whipple procedure, the mean stay was 10.7 vs. 11.9 days. For a total resection, it was 10 vs. 11.8 days.
MIS was also associated with a significantly shorter time to the initiation of adjuvant chemotherapy overall (56 vs. 59 days). For a Whipple, time to chemotherapy was 58 vs. 60 days, respectively. For a distal resection, it was 52 vs. 56 days, and for a total resection, 52 vs. 58 days.
Neither approach offered a survival benefit over the other, Dr. Mirkin noted. For stage I cancers, less than 50% of MIS patients and less than 25% of open patients were alive by 50 months. For those with stage II tumors, less than 25% of each group was alive by 40 months. For stage III tumors, the 40-month survival rates were about 10% for MIS patients and 15% for open patients.
Dr. Mirkin then examined perioperative, oncologic, and survival outcomes among those who underwent laparoscopic and robotic surgeries. There were no demographic differences between these groups.
Oncologic outcomes were almost identical with regard to the number of positive regional nodes harvested (six), and surgical margins. Nodes were negative in 82% of robotic cases vs. 78% of laparoscopic cases and positive in 17.6% of robotic cases and 19.4% of laparoscopic cases.
Length of stay was significantly shorter for a laparoscopic approach overall (10 vs. 9.4 days) and particularly in distal resection (7 vs. 10 days). However, there were no differences in length of stay in any other surgery type. Nor was there any difference in the time to neoadjuvant chemotherapy.
Survival outcomes were similar as well. For stage I cancers, 40-month survival was about 40% in the laparoscopic group and 25% in the robotic group. For stage II cancers, 40-month survival was about 15% and 25%, respectively. For stage III tumors, 20-month survival in the robotic group was near 0 and 25% in the laparoscopic group. By 40 months almost all patients were deceased.
A multivariate survival analysis controlled for age, sex, race, comorbidities, facility type and location, surgery type, surgical margins, pathologic stage, and systemic therapy. It found only one significant association: Patients with 12 or more lymph nodes harvested were 19% more likely to die than those with fewer than 12 nodes harvested.
Time to chemotherapy (longer or shorter than 57 days) did not significantly impact survival, Dr. Mirkin said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
AT THE ACS CLINICAL CONGRESS
Key clinical point:
Major finding: For stage I cancers, less than 50% of minimally invasive surgery patients and less than 25% of open surgery patients were alive by 50 months. For those with stage II tumors, less than 25% of each group was alive by 40 months.
Data source: The database review comprised 9,047 cases.
Disclosures: Dr. Mirkin had no financial disclosures.
Robotic surgery boasts fewer postoperative complications in radical hysterectomy
BOSTON – Robot-assisted radical hysterectomy is just as safe, or perhaps safer, than open surgery, according to a new study that examined perioperative and postoperative outcomes with long-term follow-ups for both types of procedures.
“Robotic surgery has been expanding for the last 20 years, but still the recurrence rate with cancer patients is missing data because very few studies are published; they don’t have long-term oncologic outcomes, and if [the technology] works properly we have to put it into the literature,” M. Bilal Sert, MD, of Oslo University, said at the annual Minimally Invasive Surgery Week.
Dr. Sert and his coinvestigators identified 215 women who underwent either open or robot-assisted radical hysterectomy between November 2005 and December 2012. All of the procedures were elective and the robot-assisted operations were performed using the da Vinci robotic surgical platform. After excluding neoadjuvant cases, which totaled 19, the researchers looked at data on 196 patients (122 open radical hysterectomy cases and 74 robot-assisted radical hysterectomy cases).
On average, operating time for open radical hysterectomy was 171 minutes, versus 263 minutes for robot-assisted radical hysterectomy. However, the robotic surgery arm had lower mean estimated blood loss than the open surgery cohort: 80 milliliters versus 468 milliliters, respectively (P = .003). Follow-up time frames were shorter in the robotic surgery cohort by 6 months: 46 months reported for robotic surgery, compared with a 52-month average experienced by those in the open surgery cohort.
Both groups experienced recurrences, including 12 patients in the open surgery cohort (9.8%) and 9 patients in the robotic surgery cohort (12.1%) (P = .3), indicating a statistically insignificant difference. Similarly, rates of perioperative complications were 8% for open surgery and 11% for robotic surgery (P = .3), which was not significantly different.
However, rates of postoperative complications were 36% for open surgery and 12% for robotic surgery (P = .001), which was statistically significant.
“Based on our data, I can say that [robot-assisted radical hysterectomy] is safe, and in fact I prefer to use the robot,” Dr. Sert said at the meeting, which was held by the Society of Laparoendoscopic Surgeons. “Of course, robot-assisted surgery will not automatically make you a better surgeon, but on more complicated radical hysterectomy patients, it will help make the surgeon more precise.”
No funding source was disclosed for this study. Dr. Sert reported having no relevant financial disclosures.
BOSTON – Robot-assisted radical hysterectomy is just as safe, or perhaps safer, than open surgery, according to a new study that examined perioperative and postoperative outcomes with long-term follow-ups for both types of procedures.
“Robotic surgery has been expanding for the last 20 years, but still the recurrence rate with cancer patients is missing data because very few studies are published; they don’t have long-term oncologic outcomes, and if [the technology] works properly we have to put it into the literature,” M. Bilal Sert, MD, of Oslo University, said at the annual Minimally Invasive Surgery Week.
Dr. Sert and his coinvestigators identified 215 women who underwent either open or robot-assisted radical hysterectomy between November 2005 and December 2012. All of the procedures were elective and the robot-assisted operations were performed using the da Vinci robotic surgical platform. After excluding neoadjuvant cases, which totaled 19, the researchers looked at data on 196 patients (122 open radical hysterectomy cases and 74 robot-assisted radical hysterectomy cases).
On average, operating time for open radical hysterectomy was 171 minutes, versus 263 minutes for robot-assisted radical hysterectomy. However, the robotic surgery arm had lower mean estimated blood loss than the open surgery cohort: 80 milliliters versus 468 milliliters, respectively (P = .003). Follow-up time frames were shorter in the robotic surgery cohort by 6 months: 46 months reported for robotic surgery, compared with a 52-month average experienced by those in the open surgery cohort.
Both groups experienced recurrences, including 12 patients in the open surgery cohort (9.8%) and 9 patients in the robotic surgery cohort (12.1%) (P = .3), indicating a statistically insignificant difference. Similarly, rates of perioperative complications were 8% for open surgery and 11% for robotic surgery (P = .3), which was not significantly different.
However, rates of postoperative complications were 36% for open surgery and 12% for robotic surgery (P = .001), which was statistically significant.
“Based on our data, I can say that [robot-assisted radical hysterectomy] is safe, and in fact I prefer to use the robot,” Dr. Sert said at the meeting, which was held by the Society of Laparoendoscopic Surgeons. “Of course, robot-assisted surgery will not automatically make you a better surgeon, but on more complicated radical hysterectomy patients, it will help make the surgeon more precise.”
No funding source was disclosed for this study. Dr. Sert reported having no relevant financial disclosures.
BOSTON – Robot-assisted radical hysterectomy is just as safe, or perhaps safer, than open surgery, according to a new study that examined perioperative and postoperative outcomes with long-term follow-ups for both types of procedures.
“Robotic surgery has been expanding for the last 20 years, but still the recurrence rate with cancer patients is missing data because very few studies are published; they don’t have long-term oncologic outcomes, and if [the technology] works properly we have to put it into the literature,” M. Bilal Sert, MD, of Oslo University, said at the annual Minimally Invasive Surgery Week.
Dr. Sert and his coinvestigators identified 215 women who underwent either open or robot-assisted radical hysterectomy between November 2005 and December 2012. All of the procedures were elective and the robot-assisted operations were performed using the da Vinci robotic surgical platform. After excluding neoadjuvant cases, which totaled 19, the researchers looked at data on 196 patients (122 open radical hysterectomy cases and 74 robot-assisted radical hysterectomy cases).
On average, operating time for open radical hysterectomy was 171 minutes, versus 263 minutes for robot-assisted radical hysterectomy. However, the robotic surgery arm had lower mean estimated blood loss than the open surgery cohort: 80 milliliters versus 468 milliliters, respectively (P = .003). Follow-up time frames were shorter in the robotic surgery cohort by 6 months: 46 months reported for robotic surgery, compared with a 52-month average experienced by those in the open surgery cohort.
Both groups experienced recurrences, including 12 patients in the open surgery cohort (9.8%) and 9 patients in the robotic surgery cohort (12.1%) (P = .3), indicating a statistically insignificant difference. Similarly, rates of perioperative complications were 8% for open surgery and 11% for robotic surgery (P = .3), which was not significantly different.
However, rates of postoperative complications were 36% for open surgery and 12% for robotic surgery (P = .001), which was statistically significant.
“Based on our data, I can say that [robot-assisted radical hysterectomy] is safe, and in fact I prefer to use the robot,” Dr. Sert said at the meeting, which was held by the Society of Laparoendoscopic Surgeons. “Of course, robot-assisted surgery will not automatically make you a better surgeon, but on more complicated radical hysterectomy patients, it will help make the surgeon more precise.”
No funding source was disclosed for this study. Dr. Sert reported having no relevant financial disclosures.
Key clinical point:
Major finding: Postoperative complications were 36% for patients who underwent open radical hysterectomy, compared with 12% for those undergoing robot-assisted radical hysterectomy (P = .001).
Data source: Retrospective review of data on 215 patients who underwent open or robot-assisted radical hysterectomy between November 2005 and December 2012.
Disclosures: Dr. Sert reported having no relevant financial disclosures.