User login
Clindamycin–benzoyl peroxide combo effective in moderate and severe acne
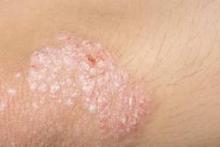
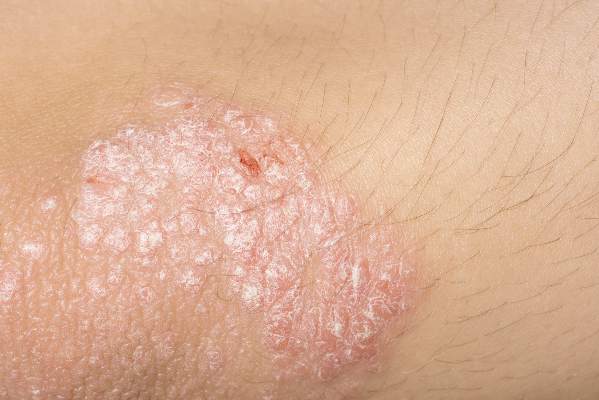
A fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% (clindamycin-BP 3.75%) aqueous gel was effective and well tolerated in the treatment of moderate and severe acne vulgaris, reported Dr. Linda Stein Gold of Henry Ford Hospital in Detroit (J Drugs Dermatol. 2015;14[9]:969-74).
A multicenter, double-blind, randomized trial of 498 patients with moderate to severe acne showed that inflammatory and noninflammatory lesions were significantly reduced in patients who received treatment with clindamycin-BP 3.75% topical gel, compared with those in the vehicle group.
In patients with severe acne, 55.1% had a reduction of two grades or more in severity score at 12 weeks. In addition, 30.6% of patients assessed their acne as “clear” or “almost clear,” Dr. Stein Gold wrote. No participants withdrew from the study because of adverse events.
Read the article here.
A fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% (clindamycin-BP 3.75%) aqueous gel was effective and well tolerated in the treatment of moderate and severe acne vulgaris, reported Dr. Linda Stein Gold of Henry Ford Hospital in Detroit (J Drugs Dermatol. 2015;14[9]:969-74).
A multicenter, double-blind, randomized trial of 498 patients with moderate to severe acne showed that inflammatory and noninflammatory lesions were significantly reduced in patients who received treatment with clindamycin-BP 3.75% topical gel, compared with those in the vehicle group.
In patients with severe acne, 55.1% had a reduction of two grades or more in severity score at 12 weeks. In addition, 30.6% of patients assessed their acne as “clear” or “almost clear,” Dr. Stein Gold wrote. No participants withdrew from the study because of adverse events.
Read the article here.
A fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% (clindamycin-BP 3.75%) aqueous gel was effective and well tolerated in the treatment of moderate and severe acne vulgaris, reported Dr. Linda Stein Gold of Henry Ford Hospital in Detroit (J Drugs Dermatol. 2015;14[9]:969-74).
A multicenter, double-blind, randomized trial of 498 patients with moderate to severe acne showed that inflammatory and noninflammatory lesions were significantly reduced in patients who received treatment with clindamycin-BP 3.75% topical gel, compared with those in the vehicle group.
In patients with severe acne, 55.1% had a reduction of two grades or more in severity score at 12 weeks. In addition, 30.6% of patients assessed their acne as “clear” or “almost clear,” Dr. Stein Gold wrote. No participants withdrew from the study because of adverse events.
Read the article here.
Acne scars improved with topical epidermal growth factor serum
Topical synthetic epidermal growth factor serum moderately improved the appearance of atrophic acne scars in a small pilot study.
At the end of 12 weeks of twice-daily application, scar appearance improved from 2.875 to 2.38 points on a 5-point investigator global assessment scale. Mean Goodman and Baron acne scar grade fell from 3.00 to 2.75, with 3 representing moderate disease and 2 mild disease. Of eight pairs of before and after photographs given to a blinded investigator, posttreatment images were correctly identified in five. Two were assessed as 76%-100% improved, and three were assessed as 50%-75% improved (J Drugs Dermatol. 2015;14[9]:1005-1010).
The patients were an average of 38 years old, split about equally between the sexes, and racially diverse. They used a basic facial cleanser during the study, but were banned from using tretinoin and other topicals.
Previously studied topicals don’t do much for acne scars, so the usual go-to treatments are chemical peels, dermabrasion, resurfacing lasers, and percutaneous collagen needling. They all work in part by promoting collagen synthesis, but at the cost of pain and side effects. Epidermal growth factor (EGF) also promotes collagen synthesis, so the investigators thought it might help. The EGF used in the study – DNA Regeneration Serum, derived from barley – was supplied by its maker, DNA EGF Renewal in Los Angeles.
“The findings suggest EGF serum has the potential to be a modern, noninvasive treatment for an otherwise highly refractory condition. Whereas resurfacing procedures rely on skin injury to trigger [EGF] release, direct topical application offers the effects of EGF without the associated discomfort and recovery time,” said Dr. Ronald L. Moy of the University of Southern California, Los Angeles, and Rachel Seidel, a medical student at Georgetown University in Washington, D.C.
“All subjects in this study [also] noted improvements in skin texture, fine lines, and wrinkles, while the vast majority also saw a reduction in brown and age spots,” they said.
The investigators said they are interested next in seeing if topical EGF prevents scars in active acne. “We believe that, by counteracting collagen degradation during the course of the inflammatory response, significant tissue atrophy capable of causing visible scarring may be prevented,” they said.
Dr. Moy owns stock in DNA EGF Renewal and is the company’s scientific adviser.
Topical synthetic epidermal growth factor serum moderately improved the appearance of atrophic acne scars in a small pilot study.
At the end of 12 weeks of twice-daily application, scar appearance improved from 2.875 to 2.38 points on a 5-point investigator global assessment scale. Mean Goodman and Baron acne scar grade fell from 3.00 to 2.75, with 3 representing moderate disease and 2 mild disease. Of eight pairs of before and after photographs given to a blinded investigator, posttreatment images were correctly identified in five. Two were assessed as 76%-100% improved, and three were assessed as 50%-75% improved (J Drugs Dermatol. 2015;14[9]:1005-1010).
The patients were an average of 38 years old, split about equally between the sexes, and racially diverse. They used a basic facial cleanser during the study, but were banned from using tretinoin and other topicals.
Previously studied topicals don’t do much for acne scars, so the usual go-to treatments are chemical peels, dermabrasion, resurfacing lasers, and percutaneous collagen needling. They all work in part by promoting collagen synthesis, but at the cost of pain and side effects. Epidermal growth factor (EGF) also promotes collagen synthesis, so the investigators thought it might help. The EGF used in the study – DNA Regeneration Serum, derived from barley – was supplied by its maker, DNA EGF Renewal in Los Angeles.
“The findings suggest EGF serum has the potential to be a modern, noninvasive treatment for an otherwise highly refractory condition. Whereas resurfacing procedures rely on skin injury to trigger [EGF] release, direct topical application offers the effects of EGF without the associated discomfort and recovery time,” said Dr. Ronald L. Moy of the University of Southern California, Los Angeles, and Rachel Seidel, a medical student at Georgetown University in Washington, D.C.
“All subjects in this study [also] noted improvements in skin texture, fine lines, and wrinkles, while the vast majority also saw a reduction in brown and age spots,” they said.
The investigators said they are interested next in seeing if topical EGF prevents scars in active acne. “We believe that, by counteracting collagen degradation during the course of the inflammatory response, significant tissue atrophy capable of causing visible scarring may be prevented,” they said.
Dr. Moy owns stock in DNA EGF Renewal and is the company’s scientific adviser.
Topical synthetic epidermal growth factor serum moderately improved the appearance of atrophic acne scars in a small pilot study.
At the end of 12 weeks of twice-daily application, scar appearance improved from 2.875 to 2.38 points on a 5-point investigator global assessment scale. Mean Goodman and Baron acne scar grade fell from 3.00 to 2.75, with 3 representing moderate disease and 2 mild disease. Of eight pairs of before and after photographs given to a blinded investigator, posttreatment images were correctly identified in five. Two were assessed as 76%-100% improved, and three were assessed as 50%-75% improved (J Drugs Dermatol. 2015;14[9]:1005-1010).
The patients were an average of 38 years old, split about equally between the sexes, and racially diverse. They used a basic facial cleanser during the study, but were banned from using tretinoin and other topicals.
Previously studied topicals don’t do much for acne scars, so the usual go-to treatments are chemical peels, dermabrasion, resurfacing lasers, and percutaneous collagen needling. They all work in part by promoting collagen synthesis, but at the cost of pain and side effects. Epidermal growth factor (EGF) also promotes collagen synthesis, so the investigators thought it might help. The EGF used in the study – DNA Regeneration Serum, derived from barley – was supplied by its maker, DNA EGF Renewal in Los Angeles.
“The findings suggest EGF serum has the potential to be a modern, noninvasive treatment for an otherwise highly refractory condition. Whereas resurfacing procedures rely on skin injury to trigger [EGF] release, direct topical application offers the effects of EGF without the associated discomfort and recovery time,” said Dr. Ronald L. Moy of the University of Southern California, Los Angeles, and Rachel Seidel, a medical student at Georgetown University in Washington, D.C.
“All subjects in this study [also] noted improvements in skin texture, fine lines, and wrinkles, while the vast majority also saw a reduction in brown and age spots,” they said.
The investigators said they are interested next in seeing if topical EGF prevents scars in active acne. “We believe that, by counteracting collagen degradation during the course of the inflammatory response, significant tissue atrophy capable of causing visible scarring may be prevented,” they said.
Dr. Moy owns stock in DNA EGF Renewal and is the company’s scientific adviser.
FROM THE JOURNAL OF DRUGS AND DERMATOLOGY
Key clinical point: Topical synthetic epidermal growth factor serum may be a noninvasive way to improve the appearance of atrophic acne scars.
Major finding: Five of the 8 pairs of before and after photographs given to a blinded investigator were correctly identified as the posttreatment image.
Data source: A pilot study of eight patients with atrophic acne scars.
Disclosures: The EGF serum used in the study was supplied by its maker, DNA EGF Renewal. Dr. Moy owns stock in the company and is its scientific adviser.
Psoriasis and acne worse in winter, milder in summer
Psoriasis and acne appear to be susceptible to seasonal variations of clearing and worsening, with an analysis revealing both conditions maintained a significant trend of summer clearing and winter worsening, according to a research letter published in the Journal of the American Academy of Dermatology.
Using Physician’s Global Assessment scales for psoriasis and acne, Dr. Vanessa Lindsay Pascoe and Dr. Alexandra Boer Kimball, both of Massachusetts General Hospital, Boston, collected data from 5,468 psoriasis patients and 9,301 acne patients between June 2011 and May 2014 in the New England area. Among the psoriasis patient group, 16% were seen in the summer, 25% in the fall, 31% in the winter, and 28% in the spring. The trend was similar for acne patients, with 18% seen in the summer, 25% in the fall, 28% in the winter, and 29% in the spring. There were no significant seasonal differences in age or sex for either group.
The percentage of psoriasis patients with clear/almost clear disease was highest in the summer at 20.4%, while the percentage of patients with moderate/severe disease was highest in the winter at 40.5%. For acne, the percentage of patients with clear/almost clear disease was highest in the fall at 17.5%, and the percentage of patients with moderate/severe disease was highest in the winter at 45.9%. Fewer psoriasis and acne patients presented to the clinic in the summer, which the researchers suggested could be due to disease improvement.
“Although the climate of the Northeastern United States may not generalize to regions with less seasonal variation, providers may consider seasonal adjustment of acne plans as they have traditionally done for psoriasis,” the authors wrote. “For example, they may wait until after winter to taper a systemic antibiotic for acne, just as some providers may wait until spring to change systemic psoriasis treatments.”
Read the full article in the Journal of the American Academy of Dermatology.
Psoriasis and acne appear to be susceptible to seasonal variations of clearing and worsening, with an analysis revealing both conditions maintained a significant trend of summer clearing and winter worsening, according to a research letter published in the Journal of the American Academy of Dermatology.
Using Physician’s Global Assessment scales for psoriasis and acne, Dr. Vanessa Lindsay Pascoe and Dr. Alexandra Boer Kimball, both of Massachusetts General Hospital, Boston, collected data from 5,468 psoriasis patients and 9,301 acne patients between June 2011 and May 2014 in the New England area. Among the psoriasis patient group, 16% were seen in the summer, 25% in the fall, 31% in the winter, and 28% in the spring. The trend was similar for acne patients, with 18% seen in the summer, 25% in the fall, 28% in the winter, and 29% in the spring. There were no significant seasonal differences in age or sex for either group.
The percentage of psoriasis patients with clear/almost clear disease was highest in the summer at 20.4%, while the percentage of patients with moderate/severe disease was highest in the winter at 40.5%. For acne, the percentage of patients with clear/almost clear disease was highest in the fall at 17.5%, and the percentage of patients with moderate/severe disease was highest in the winter at 45.9%. Fewer psoriasis and acne patients presented to the clinic in the summer, which the researchers suggested could be due to disease improvement.
“Although the climate of the Northeastern United States may not generalize to regions with less seasonal variation, providers may consider seasonal adjustment of acne plans as they have traditionally done for psoriasis,” the authors wrote. “For example, they may wait until after winter to taper a systemic antibiotic for acne, just as some providers may wait until spring to change systemic psoriasis treatments.”
Read the full article in the Journal of the American Academy of Dermatology.
Psoriasis and acne appear to be susceptible to seasonal variations of clearing and worsening, with an analysis revealing both conditions maintained a significant trend of summer clearing and winter worsening, according to a research letter published in the Journal of the American Academy of Dermatology.
Using Physician’s Global Assessment scales for psoriasis and acne, Dr. Vanessa Lindsay Pascoe and Dr. Alexandra Boer Kimball, both of Massachusetts General Hospital, Boston, collected data from 5,468 psoriasis patients and 9,301 acne patients between June 2011 and May 2014 in the New England area. Among the psoriasis patient group, 16% were seen in the summer, 25% in the fall, 31% in the winter, and 28% in the spring. The trend was similar for acne patients, with 18% seen in the summer, 25% in the fall, 28% in the winter, and 29% in the spring. There were no significant seasonal differences in age or sex for either group.
The percentage of psoriasis patients with clear/almost clear disease was highest in the summer at 20.4%, while the percentage of patients with moderate/severe disease was highest in the winter at 40.5%. For acne, the percentage of patients with clear/almost clear disease was highest in the fall at 17.5%, and the percentage of patients with moderate/severe disease was highest in the winter at 45.9%. Fewer psoriasis and acne patients presented to the clinic in the summer, which the researchers suggested could be due to disease improvement.
“Although the climate of the Northeastern United States may not generalize to regions with less seasonal variation, providers may consider seasonal adjustment of acne plans as they have traditionally done for psoriasis,” the authors wrote. “For example, they may wait until after winter to taper a systemic antibiotic for acne, just as some providers may wait until spring to change systemic psoriasis treatments.”
Read the full article in the Journal of the American Academy of Dermatology.
Head for oral contraceptives to target women’s acne
NEW YORK – Almost all women with acne will have at least a fair response to therapy with oral contraceptive pills.
Most should experience at least a 50% reduction in lesions, Dr. Bethanee Schlosser said at the American Academy of Dermatology summer meeting.
“From baseline, you are generally speaking about a 50% decrease in inflammatory and noninflammatory lesions and total lesion count,” said Dr. Schlosser of Northwestern University, Chicago. “The important thing, though, is that you have to tell a patient this is not an overnight thing. You have to wait at least three cycles before you make any kind of judgment on whether it’s working.”
The improvement will be seen on all affected areas, not just the face, she said.
“This is important. It’s not just facial acne that’s hormonally sensitive. For us to say it’s just the facial distribution that’s hormonally sensitive is ridiculous. We all know as dermatologists that all acne is androgen driven, and it’s all hormonally sensitive.”
Oral contraceptives can be used alone, as Dr. Schlosser usually initiates treatment, or they can be used in conjunction with spironolactone or antibiotics. Three OCs are approved by the FDA for the treatment of acne.
“I often get asked which OC is the best,” she said. “Just because the FDA approved some for acne doesn’t mean they are better. It means the company had the studies done and basically paid for this labeling indication.”
A 2012 Cochrane review examined 31 studies that compared different OCs to placebo and to each other. The investigators found that all the OCs were consistently more effective than placebo (Cochrane Database Syst Rev. doi: 10.1002/14651858.CD004425.pub6). The head-to-head comparisons produced conflicting results with no clear advantage of one formulation over another.
“I would say use what you are comfortable with,” Dr. Schlosser said.
Some personal and family history and health screenings are necessary before prescribing OCs, although leading women’s health associations, as well as the FDA have said there’s no need for a pelvic exam and Pap smear. “You do have to make sure they are not pregnant, hypertensive, or at risk for stroke or heart disease.”
Spironolactone is usually prescribed at 100-150 mg/day and rarely up to 200 mg/day, she noted. It can be added to an OC regimen if the patient has not adequately responded to monotherapy. It can also be combined with drospirenone, an antibiotic, or with both OCs and antibiotics.
Since spironolactone is a diuretic, women should be monitored for increased thirst and urination, and signs of hypokalemia (lethargy, muscle cramps, dizziness, and increased heart rate). In utero exposure can cause feminization of a male fetus, so reliable contraception is a must.
The drug does carry a boxed warning, as it was carcinogenic in rat studies – but only when given at 50-100 times the usual human dose.
Dr. Schlosser disclosed that she is an investigator with Galderma and Allergan.
On Twitter @Alz_Gal
NEW YORK – Almost all women with acne will have at least a fair response to therapy with oral contraceptive pills.
Most should experience at least a 50% reduction in lesions, Dr. Bethanee Schlosser said at the American Academy of Dermatology summer meeting.
“From baseline, you are generally speaking about a 50% decrease in inflammatory and noninflammatory lesions and total lesion count,” said Dr. Schlosser of Northwestern University, Chicago. “The important thing, though, is that you have to tell a patient this is not an overnight thing. You have to wait at least three cycles before you make any kind of judgment on whether it’s working.”
The improvement will be seen on all affected areas, not just the face, she said.
“This is important. It’s not just facial acne that’s hormonally sensitive. For us to say it’s just the facial distribution that’s hormonally sensitive is ridiculous. We all know as dermatologists that all acne is androgen driven, and it’s all hormonally sensitive.”
Oral contraceptives can be used alone, as Dr. Schlosser usually initiates treatment, or they can be used in conjunction with spironolactone or antibiotics. Three OCs are approved by the FDA for the treatment of acne.
“I often get asked which OC is the best,” she said. “Just because the FDA approved some for acne doesn’t mean they are better. It means the company had the studies done and basically paid for this labeling indication.”
A 2012 Cochrane review examined 31 studies that compared different OCs to placebo and to each other. The investigators found that all the OCs were consistently more effective than placebo (Cochrane Database Syst Rev. doi: 10.1002/14651858.CD004425.pub6). The head-to-head comparisons produced conflicting results with no clear advantage of one formulation over another.
“I would say use what you are comfortable with,” Dr. Schlosser said.
Some personal and family history and health screenings are necessary before prescribing OCs, although leading women’s health associations, as well as the FDA have said there’s no need for a pelvic exam and Pap smear. “You do have to make sure they are not pregnant, hypertensive, or at risk for stroke or heart disease.”
Spironolactone is usually prescribed at 100-150 mg/day and rarely up to 200 mg/day, she noted. It can be added to an OC regimen if the patient has not adequately responded to monotherapy. It can also be combined with drospirenone, an antibiotic, or with both OCs and antibiotics.
Since spironolactone is a diuretic, women should be monitored for increased thirst and urination, and signs of hypokalemia (lethargy, muscle cramps, dizziness, and increased heart rate). In utero exposure can cause feminization of a male fetus, so reliable contraception is a must.
The drug does carry a boxed warning, as it was carcinogenic in rat studies – but only when given at 50-100 times the usual human dose.
Dr. Schlosser disclosed that she is an investigator with Galderma and Allergan.
On Twitter @Alz_Gal
NEW YORK – Almost all women with acne will have at least a fair response to therapy with oral contraceptive pills.
Most should experience at least a 50% reduction in lesions, Dr. Bethanee Schlosser said at the American Academy of Dermatology summer meeting.
“From baseline, you are generally speaking about a 50% decrease in inflammatory and noninflammatory lesions and total lesion count,” said Dr. Schlosser of Northwestern University, Chicago. “The important thing, though, is that you have to tell a patient this is not an overnight thing. You have to wait at least three cycles before you make any kind of judgment on whether it’s working.”
The improvement will be seen on all affected areas, not just the face, she said.
“This is important. It’s not just facial acne that’s hormonally sensitive. For us to say it’s just the facial distribution that’s hormonally sensitive is ridiculous. We all know as dermatologists that all acne is androgen driven, and it’s all hormonally sensitive.”
Oral contraceptives can be used alone, as Dr. Schlosser usually initiates treatment, or they can be used in conjunction with spironolactone or antibiotics. Three OCs are approved by the FDA for the treatment of acne.
“I often get asked which OC is the best,” she said. “Just because the FDA approved some for acne doesn’t mean they are better. It means the company had the studies done and basically paid for this labeling indication.”
A 2012 Cochrane review examined 31 studies that compared different OCs to placebo and to each other. The investigators found that all the OCs were consistently more effective than placebo (Cochrane Database Syst Rev. doi: 10.1002/14651858.CD004425.pub6). The head-to-head comparisons produced conflicting results with no clear advantage of one formulation over another.
“I would say use what you are comfortable with,” Dr. Schlosser said.
Some personal and family history and health screenings are necessary before prescribing OCs, although leading women’s health associations, as well as the FDA have said there’s no need for a pelvic exam and Pap smear. “You do have to make sure they are not pregnant, hypertensive, or at risk for stroke or heart disease.”
Spironolactone is usually prescribed at 100-150 mg/day and rarely up to 200 mg/day, she noted. It can be added to an OC regimen if the patient has not adequately responded to monotherapy. It can also be combined with drospirenone, an antibiotic, or with both OCs and antibiotics.
Since spironolactone is a diuretic, women should be monitored for increased thirst and urination, and signs of hypokalemia (lethargy, muscle cramps, dizziness, and increased heart rate). In utero exposure can cause feminization of a male fetus, so reliable contraception is a must.
The drug does carry a boxed warning, as it was carcinogenic in rat studies – but only when given at 50-100 times the usual human dose.
Dr. Schlosser disclosed that she is an investigator with Galderma and Allergan.
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
The Use of Sodium Sulfacetamide in Dermatology
Sodium sulfacetamide has various uses in the field of dermatology due to its anti-inflammatory and antibacterial properties. It has been shown to be effective in the management of a variety of inflammatory facial dermatoses, including papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis. We review the mechanism of action, pharmacology and formulations, clinical uses, and adverse effects of sodium sulfacetamide as a dermatologic treatment.
Mechanism of Action
Sodium sulfacetamide is a sulfonamide-type antibacterial agent. Its mechanism of action is the inhibition of bacterial dihydropteroate synthetase, which prevents the conversion of p-aminobenzoic acid to folic acid. This process causes a bacteriostatic effect on the growth of several gram-negative and gram-positive organisms, including Propionibacterium acnes.1,2
The effectiveness of sodium sulfacetamide is increased when used in combination with sulfur, which has keratolytic, antibacterial, antifungal, and antiparasitic effects. The addition of hydrocortisone has been reported to increase the effectiveness of both agents.3
Pharmacology
Sodium sulfacetamide is highly soluble at the physiologic pH of 7.4, which contributes to its high level of penetration and absorption.4 An in vitro study showed percutaneous absorption of sodium sulfacetamide to be around 4%.5 Sulfonamides are metabolized mainly by the liver and are excreted by the kidneys.
Formulations
The most common concentrations of sodium sulfacetamide and sulfur are 10% and 5%, respectively. A wide variety of sulfacetamide-containing products are available, many of which are marketed to treat specific conditions depending on additional ingredients or the type of delivery system.
Clinical Uses
Topical formulations of sodium sulfacetamide and sulfur have proven to be efficacious in the management of rosacea, with a typical regimen consisting of twice-daily application for 8 weeks.6 The sulfur in the formulation has the additional benefit of targeting Demodex mites, which are implicated as a contributing factor in some cases of rosacea.7 Sodium sulfacetamide 10%–sulfur 5% lotion was more effective in improving the erythema, papulopustules, and overall severity of rosacea as compared to metronidazole gel 0.75%.8 Other studies have reported increased efficacy when sodium sulfacetamide and topical sulfur are used along with metronidazole.9,10
Sodium sulfacetamide also has shown efficacy against acne. Its antibacterial and drying properties have been shown to decrease the number of inflammatory lesions and comedones, and in the treatment of acne vulgaris, no sensitivity reactions have been observed.2 Also, unlike topical antibiotics, cases of P acnes resistance to topical sulfur products have not been widely reported. Studies have demonstrated that twice-daily use of sodium sulfacetamide 10%–sulfur 5% for 12 weeks decreases inflammatory acne lesions by 80.4% to 83%.11,12
Seborrheic dermatitis is a common chronic infection of the skin caused by Malassezia species. One study investigated the use of sodium sulfacetamide ointment and soap to treat seborrheic dermatitis and found that the condition was either improved or completely controlled in 93% (71/76) of cases.4 Sodium sulfacetamide lotion was an effective treatment of seborrheic dermatitis in 89% (54/61) of patients with scalp involvement and 68% (30/44) of patients with glabrous skin involvement.13
Perioral dermatitis is characterized by groups of erythematous papules and pustules localized around the mouth. The use of topical sodium sulfacetamide along with oral tetracyclines has been demonstrated to consistently clear lesions in most patients with perioral dermatitis.14 Sodium sulfacetamide is unique in that it is not associated with the excessive erythema and irritation often found with retinoic acid and benzoyl peroxide.15 Unfortunately, however, there have been no well-controlled trials to compare the efficacy of sodium sulfacetamide to other topical therapies for this condition.
Adverse Effects
Adverse effects from sodium sulfacetamide are rare and generally are limited to cutaneous reactions including dryness, erythema, pruritus, and discomfort.1 Periocular use of sodium sulfacetamide can cause conjunctival irritation. One study reported that 19% (6/31) of patients experienced local reactions but most were considered mild.9 Rare but serious reactions including erythema multiforme and Stevens-Johnson syndrome have been reported from ophthalmic use.16,17
A common limiting factor to sodium sulfacetamide preparations that include elemental sulfur is the offensive smell, which has hindered patient compliance in the past; however, pharmaceutical companies have attempted to create more tolerable products without the odor.10 One study found that the tolerability of a sodium sulfacetamide 10%–sulfur 5% foam using a rinse-off method of application was excellent, with only 33% (8/24) of participants commenting on the smell.18 Another limiting factor of sodium sulfacetamide preparations containing sulfur is orange-brown discoloration when combined with benzoyl peroxide, which does not affect the skin but may stain clothing.19
Sodium sulfacetamide is rendered less effective when combined with silver-containing products.20 No other notable drug interactions are known; however, oral sulfonamides are known to interact with several drugs, including cyclosporine and phenytoin.21,22
Contraindications
Sodium sulfacetamide is contraindicated in patients with known hypersensitivity to sulfonamides, sulfur, or any other component of the preparation. It is a pregnancy category C drug, and pregnant women should only use sodium sulfacetamide if it is the only modality to treat the condition or the benefits outweigh the risks. Although there are no known reports of problems related to topical sodium sulfacetamide during pregnancy, the use of oral sulfonamides during pregnancy can increase the risk for neonatal jaundice.23 Likewise, caution should be exercised in prescribing this product to nursing women, as systemic sulfonamide antibacterials are well known to cause kernicterus in nursing neonates.1
Conclusion
The efficacy and safety of sodium sulfacetamide, used alone or in combination with sulfur, has been demonstrated in the treatment of rosacea, acne, seborrheic dermatitis, and perioral dermatitis. Advances in formulation technology to decrease odor and irritation have allowed for more use of this product. Further studies will help elucidate the role that sodium sulfacetamide should play in the treatment of inflammatory dermatoses in comparison to other available products.
1. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003;4:473-492.
2. Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
3. Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders; 2012:445-459.
4. Duemling WM. Sodium sulfacetamide in topical therapy. AMA Arch Derm Syphilol. 1954;69:75-82.
5. Sodium sulfacetamide. Drugs.com Web site. http://drugs.com/pro/sodium-sulfacetamide.html. Revised December 2012. Accessed June 16, 2015.
6. Sauder DN, Miller R, Gratton D, et al. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatol Treat. 1997;8:79-85.
7. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
8. Lebwohl MG, Medansky RS, Russo CL, et al. The comparative efficacy of sodium sulfacetamide 10%/sulfur 5% lotion and metronidazole 0.75% gel in the treatment of rosacea. J Geriatr Dermatol. 1995;3:183-185.
9. Nally JB, Berson DS. Topical therapies for rosacea. J Drugs Dermatol. 2006;5:23-26.
10. Pelle MT, Crawford GH, James WD. Rosacea II: therapy. J Am Acad Dermatol. 2004;51:499-512.
11. Tarimci N, Sener S, Kilinç T. Topical sodium sulfacetamide/sulfur lotion. J Clin Pharm Ther. 1997;22:301.
12. Breneman DL, Ariano MC. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. Int J Dermatol. 1993;32:365-367.
13. Whelan ST. Sodium sulfacetamide for seborrheic dermatitis. AMA Arch Derm. 1955;71:724.
14. Bendl BJ. Perioral dermatitis: etiology and treatment. Cutis. 1976;17:903-908.
15. Olansky S. Old drug—in a new system—revisited. Cutis. 1977;19:852-854.
16. Genvert GI, Cohen EJ, Donnenfeld ED, et al. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985;99:465-468.
17. Rubin Z. Ophthalmic sulfonamide-induced Stevens-Johnson syndrome. Arch Dermatol. 1977;113:235-236.
18. Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-246.
19. Dubina MI, Fleischer AB. Interaction of topical sulfacetamide and topical dapsone with benzoyl peroxide. Arch Dermatol. 2009;145:1027-1029.
20. Sodium sulfacetamide – sulfacetamide sodium liquid. DailyMed Web site. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0d92c55b-5b54-4f5d-8921-24e4e877ae50. Accessed June 17, 2015.
21. Spes CH, Angermann CE, Stempfle HU, et al. Sulfadiazine therapy for toxoplasmosis in heart transplant recipients decreases cyclosporine concentration. Clin Investig. 1992;70:752-754.
22. Hansen JM, Kampmann JP, Siersbaek-Nielsen K, et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl. 1979;624:106-110.
23. Bradley JS, Sauberan JB. Antimicrobial agents. In: Long SS, Pickering LK, Prober CG. Principles and Practices of Pediatric Infectious Diseases. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1453-1483.
Sodium sulfacetamide has various uses in the field of dermatology due to its anti-inflammatory and antibacterial properties. It has been shown to be effective in the management of a variety of inflammatory facial dermatoses, including papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis. We review the mechanism of action, pharmacology and formulations, clinical uses, and adverse effects of sodium sulfacetamide as a dermatologic treatment.
Mechanism of Action
Sodium sulfacetamide is a sulfonamide-type antibacterial agent. Its mechanism of action is the inhibition of bacterial dihydropteroate synthetase, which prevents the conversion of p-aminobenzoic acid to folic acid. This process causes a bacteriostatic effect on the growth of several gram-negative and gram-positive organisms, including Propionibacterium acnes.1,2
The effectiveness of sodium sulfacetamide is increased when used in combination with sulfur, which has keratolytic, antibacterial, antifungal, and antiparasitic effects. The addition of hydrocortisone has been reported to increase the effectiveness of both agents.3
Pharmacology
Sodium sulfacetamide is highly soluble at the physiologic pH of 7.4, which contributes to its high level of penetration and absorption.4 An in vitro study showed percutaneous absorption of sodium sulfacetamide to be around 4%.5 Sulfonamides are metabolized mainly by the liver and are excreted by the kidneys.
Formulations
The most common concentrations of sodium sulfacetamide and sulfur are 10% and 5%, respectively. A wide variety of sulfacetamide-containing products are available, many of which are marketed to treat specific conditions depending on additional ingredients or the type of delivery system.
Clinical Uses
Topical formulations of sodium sulfacetamide and sulfur have proven to be efficacious in the management of rosacea, with a typical regimen consisting of twice-daily application for 8 weeks.6 The sulfur in the formulation has the additional benefit of targeting Demodex mites, which are implicated as a contributing factor in some cases of rosacea.7 Sodium sulfacetamide 10%–sulfur 5% lotion was more effective in improving the erythema, papulopustules, and overall severity of rosacea as compared to metronidazole gel 0.75%.8 Other studies have reported increased efficacy when sodium sulfacetamide and topical sulfur are used along with metronidazole.9,10
Sodium sulfacetamide also has shown efficacy against acne. Its antibacterial and drying properties have been shown to decrease the number of inflammatory lesions and comedones, and in the treatment of acne vulgaris, no sensitivity reactions have been observed.2 Also, unlike topical antibiotics, cases of P acnes resistance to topical sulfur products have not been widely reported. Studies have demonstrated that twice-daily use of sodium sulfacetamide 10%–sulfur 5% for 12 weeks decreases inflammatory acne lesions by 80.4% to 83%.11,12
Seborrheic dermatitis is a common chronic infection of the skin caused by Malassezia species. One study investigated the use of sodium sulfacetamide ointment and soap to treat seborrheic dermatitis and found that the condition was either improved or completely controlled in 93% (71/76) of cases.4 Sodium sulfacetamide lotion was an effective treatment of seborrheic dermatitis in 89% (54/61) of patients with scalp involvement and 68% (30/44) of patients with glabrous skin involvement.13
Perioral dermatitis is characterized by groups of erythematous papules and pustules localized around the mouth. The use of topical sodium sulfacetamide along with oral tetracyclines has been demonstrated to consistently clear lesions in most patients with perioral dermatitis.14 Sodium sulfacetamide is unique in that it is not associated with the excessive erythema and irritation often found with retinoic acid and benzoyl peroxide.15 Unfortunately, however, there have been no well-controlled trials to compare the efficacy of sodium sulfacetamide to other topical therapies for this condition.
Adverse Effects
Adverse effects from sodium sulfacetamide are rare and generally are limited to cutaneous reactions including dryness, erythema, pruritus, and discomfort.1 Periocular use of sodium sulfacetamide can cause conjunctival irritation. One study reported that 19% (6/31) of patients experienced local reactions but most were considered mild.9 Rare but serious reactions including erythema multiforme and Stevens-Johnson syndrome have been reported from ophthalmic use.16,17
A common limiting factor to sodium sulfacetamide preparations that include elemental sulfur is the offensive smell, which has hindered patient compliance in the past; however, pharmaceutical companies have attempted to create more tolerable products without the odor.10 One study found that the tolerability of a sodium sulfacetamide 10%–sulfur 5% foam using a rinse-off method of application was excellent, with only 33% (8/24) of participants commenting on the smell.18 Another limiting factor of sodium sulfacetamide preparations containing sulfur is orange-brown discoloration when combined with benzoyl peroxide, which does not affect the skin but may stain clothing.19
Sodium sulfacetamide is rendered less effective when combined with silver-containing products.20 No other notable drug interactions are known; however, oral sulfonamides are known to interact with several drugs, including cyclosporine and phenytoin.21,22
Contraindications
Sodium sulfacetamide is contraindicated in patients with known hypersensitivity to sulfonamides, sulfur, or any other component of the preparation. It is a pregnancy category C drug, and pregnant women should only use sodium sulfacetamide if it is the only modality to treat the condition or the benefits outweigh the risks. Although there are no known reports of problems related to topical sodium sulfacetamide during pregnancy, the use of oral sulfonamides during pregnancy can increase the risk for neonatal jaundice.23 Likewise, caution should be exercised in prescribing this product to nursing women, as systemic sulfonamide antibacterials are well known to cause kernicterus in nursing neonates.1
Conclusion
The efficacy and safety of sodium sulfacetamide, used alone or in combination with sulfur, has been demonstrated in the treatment of rosacea, acne, seborrheic dermatitis, and perioral dermatitis. Advances in formulation technology to decrease odor and irritation have allowed for more use of this product. Further studies will help elucidate the role that sodium sulfacetamide should play in the treatment of inflammatory dermatoses in comparison to other available products.
Sodium sulfacetamide has various uses in the field of dermatology due to its anti-inflammatory and antibacterial properties. It has been shown to be effective in the management of a variety of inflammatory facial dermatoses, including papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis. We review the mechanism of action, pharmacology and formulations, clinical uses, and adverse effects of sodium sulfacetamide as a dermatologic treatment.
Mechanism of Action
Sodium sulfacetamide is a sulfonamide-type antibacterial agent. Its mechanism of action is the inhibition of bacterial dihydropteroate synthetase, which prevents the conversion of p-aminobenzoic acid to folic acid. This process causes a bacteriostatic effect on the growth of several gram-negative and gram-positive organisms, including Propionibacterium acnes.1,2
The effectiveness of sodium sulfacetamide is increased when used in combination with sulfur, which has keratolytic, antibacterial, antifungal, and antiparasitic effects. The addition of hydrocortisone has been reported to increase the effectiveness of both agents.3
Pharmacology
Sodium sulfacetamide is highly soluble at the physiologic pH of 7.4, which contributes to its high level of penetration and absorption.4 An in vitro study showed percutaneous absorption of sodium sulfacetamide to be around 4%.5 Sulfonamides are metabolized mainly by the liver and are excreted by the kidneys.
Formulations
The most common concentrations of sodium sulfacetamide and sulfur are 10% and 5%, respectively. A wide variety of sulfacetamide-containing products are available, many of which are marketed to treat specific conditions depending on additional ingredients or the type of delivery system.
Clinical Uses
Topical formulations of sodium sulfacetamide and sulfur have proven to be efficacious in the management of rosacea, with a typical regimen consisting of twice-daily application for 8 weeks.6 The sulfur in the formulation has the additional benefit of targeting Demodex mites, which are implicated as a contributing factor in some cases of rosacea.7 Sodium sulfacetamide 10%–sulfur 5% lotion was more effective in improving the erythema, papulopustules, and overall severity of rosacea as compared to metronidazole gel 0.75%.8 Other studies have reported increased efficacy when sodium sulfacetamide and topical sulfur are used along with metronidazole.9,10
Sodium sulfacetamide also has shown efficacy against acne. Its antibacterial and drying properties have been shown to decrease the number of inflammatory lesions and comedones, and in the treatment of acne vulgaris, no sensitivity reactions have been observed.2 Also, unlike topical antibiotics, cases of P acnes resistance to topical sulfur products have not been widely reported. Studies have demonstrated that twice-daily use of sodium sulfacetamide 10%–sulfur 5% for 12 weeks decreases inflammatory acne lesions by 80.4% to 83%.11,12
Seborrheic dermatitis is a common chronic infection of the skin caused by Malassezia species. One study investigated the use of sodium sulfacetamide ointment and soap to treat seborrheic dermatitis and found that the condition was either improved or completely controlled in 93% (71/76) of cases.4 Sodium sulfacetamide lotion was an effective treatment of seborrheic dermatitis in 89% (54/61) of patients with scalp involvement and 68% (30/44) of patients with glabrous skin involvement.13
Perioral dermatitis is characterized by groups of erythematous papules and pustules localized around the mouth. The use of topical sodium sulfacetamide along with oral tetracyclines has been demonstrated to consistently clear lesions in most patients with perioral dermatitis.14 Sodium sulfacetamide is unique in that it is not associated with the excessive erythema and irritation often found with retinoic acid and benzoyl peroxide.15 Unfortunately, however, there have been no well-controlled trials to compare the efficacy of sodium sulfacetamide to other topical therapies for this condition.
Adverse Effects
Adverse effects from sodium sulfacetamide are rare and generally are limited to cutaneous reactions including dryness, erythema, pruritus, and discomfort.1 Periocular use of sodium sulfacetamide can cause conjunctival irritation. One study reported that 19% (6/31) of patients experienced local reactions but most were considered mild.9 Rare but serious reactions including erythema multiforme and Stevens-Johnson syndrome have been reported from ophthalmic use.16,17
A common limiting factor to sodium sulfacetamide preparations that include elemental sulfur is the offensive smell, which has hindered patient compliance in the past; however, pharmaceutical companies have attempted to create more tolerable products without the odor.10 One study found that the tolerability of a sodium sulfacetamide 10%–sulfur 5% foam using a rinse-off method of application was excellent, with only 33% (8/24) of participants commenting on the smell.18 Another limiting factor of sodium sulfacetamide preparations containing sulfur is orange-brown discoloration when combined with benzoyl peroxide, which does not affect the skin but may stain clothing.19
Sodium sulfacetamide is rendered less effective when combined with silver-containing products.20 No other notable drug interactions are known; however, oral sulfonamides are known to interact with several drugs, including cyclosporine and phenytoin.21,22
Contraindications
Sodium sulfacetamide is contraindicated in patients with known hypersensitivity to sulfonamides, sulfur, or any other component of the preparation. It is a pregnancy category C drug, and pregnant women should only use sodium sulfacetamide if it is the only modality to treat the condition or the benefits outweigh the risks. Although there are no known reports of problems related to topical sodium sulfacetamide during pregnancy, the use of oral sulfonamides during pregnancy can increase the risk for neonatal jaundice.23 Likewise, caution should be exercised in prescribing this product to nursing women, as systemic sulfonamide antibacterials are well known to cause kernicterus in nursing neonates.1
Conclusion
The efficacy and safety of sodium sulfacetamide, used alone or in combination with sulfur, has been demonstrated in the treatment of rosacea, acne, seborrheic dermatitis, and perioral dermatitis. Advances in formulation technology to decrease odor and irritation have allowed for more use of this product. Further studies will help elucidate the role that sodium sulfacetamide should play in the treatment of inflammatory dermatoses in comparison to other available products.
1. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003;4:473-492.
2. Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
3. Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders; 2012:445-459.
4. Duemling WM. Sodium sulfacetamide in topical therapy. AMA Arch Derm Syphilol. 1954;69:75-82.
5. Sodium sulfacetamide. Drugs.com Web site. http://drugs.com/pro/sodium-sulfacetamide.html. Revised December 2012. Accessed June 16, 2015.
6. Sauder DN, Miller R, Gratton D, et al. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatol Treat. 1997;8:79-85.
7. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
8. Lebwohl MG, Medansky RS, Russo CL, et al. The comparative efficacy of sodium sulfacetamide 10%/sulfur 5% lotion and metronidazole 0.75% gel in the treatment of rosacea. J Geriatr Dermatol. 1995;3:183-185.
9. Nally JB, Berson DS. Topical therapies for rosacea. J Drugs Dermatol. 2006;5:23-26.
10. Pelle MT, Crawford GH, James WD. Rosacea II: therapy. J Am Acad Dermatol. 2004;51:499-512.
11. Tarimci N, Sener S, Kilinç T. Topical sodium sulfacetamide/sulfur lotion. J Clin Pharm Ther. 1997;22:301.
12. Breneman DL, Ariano MC. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. Int J Dermatol. 1993;32:365-367.
13. Whelan ST. Sodium sulfacetamide for seborrheic dermatitis. AMA Arch Derm. 1955;71:724.
14. Bendl BJ. Perioral dermatitis: etiology and treatment. Cutis. 1976;17:903-908.
15. Olansky S. Old drug—in a new system—revisited. Cutis. 1977;19:852-854.
16. Genvert GI, Cohen EJ, Donnenfeld ED, et al. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985;99:465-468.
17. Rubin Z. Ophthalmic sulfonamide-induced Stevens-Johnson syndrome. Arch Dermatol. 1977;113:235-236.
18. Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-246.
19. Dubina MI, Fleischer AB. Interaction of topical sulfacetamide and topical dapsone with benzoyl peroxide. Arch Dermatol. 2009;145:1027-1029.
20. Sodium sulfacetamide – sulfacetamide sodium liquid. DailyMed Web site. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0d92c55b-5b54-4f5d-8921-24e4e877ae50. Accessed June 17, 2015.
21. Spes CH, Angermann CE, Stempfle HU, et al. Sulfadiazine therapy for toxoplasmosis in heart transplant recipients decreases cyclosporine concentration. Clin Investig. 1992;70:752-754.
22. Hansen JM, Kampmann JP, Siersbaek-Nielsen K, et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl. 1979;624:106-110.
23. Bradley JS, Sauberan JB. Antimicrobial agents. In: Long SS, Pickering LK, Prober CG. Principles and Practices of Pediatric Infectious Diseases. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1453-1483.
1. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003;4:473-492.
2. Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
3. Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders; 2012:445-459.
4. Duemling WM. Sodium sulfacetamide in topical therapy. AMA Arch Derm Syphilol. 1954;69:75-82.
5. Sodium sulfacetamide. Drugs.com Web site. http://drugs.com/pro/sodium-sulfacetamide.html. Revised December 2012. Accessed June 16, 2015.
6. Sauder DN, Miller R, Gratton D, et al. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatol Treat. 1997;8:79-85.
7. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
8. Lebwohl MG, Medansky RS, Russo CL, et al. The comparative efficacy of sodium sulfacetamide 10%/sulfur 5% lotion and metronidazole 0.75% gel in the treatment of rosacea. J Geriatr Dermatol. 1995;3:183-185.
9. Nally JB, Berson DS. Topical therapies for rosacea. J Drugs Dermatol. 2006;5:23-26.
10. Pelle MT, Crawford GH, James WD. Rosacea II: therapy. J Am Acad Dermatol. 2004;51:499-512.
11. Tarimci N, Sener S, Kilinç T. Topical sodium sulfacetamide/sulfur lotion. J Clin Pharm Ther. 1997;22:301.
12. Breneman DL, Ariano MC. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. Int J Dermatol. 1993;32:365-367.
13. Whelan ST. Sodium sulfacetamide for seborrheic dermatitis. AMA Arch Derm. 1955;71:724.
14. Bendl BJ. Perioral dermatitis: etiology and treatment. Cutis. 1976;17:903-908.
15. Olansky S. Old drug—in a new system—revisited. Cutis. 1977;19:852-854.
16. Genvert GI, Cohen EJ, Donnenfeld ED, et al. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985;99:465-468.
17. Rubin Z. Ophthalmic sulfonamide-induced Stevens-Johnson syndrome. Arch Dermatol. 1977;113:235-236.
18. Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-246.
19. Dubina MI, Fleischer AB. Interaction of topical sulfacetamide and topical dapsone with benzoyl peroxide. Arch Dermatol. 2009;145:1027-1029.
20. Sodium sulfacetamide – sulfacetamide sodium liquid. DailyMed Web site. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0d92c55b-5b54-4f5d-8921-24e4e877ae50. Accessed June 17, 2015.
21. Spes CH, Angermann CE, Stempfle HU, et al. Sulfadiazine therapy for toxoplasmosis in heart transplant recipients decreases cyclosporine concentration. Clin Investig. 1992;70:752-754.
22. Hansen JM, Kampmann JP, Siersbaek-Nielsen K, et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl. 1979;624:106-110.
23. Bradley JS, Sauberan JB. Antimicrobial agents. In: Long SS, Pickering LK, Prober CG. Principles and Practices of Pediatric Infectious Diseases. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1453-1483.
Practice Points
- Sodium sulfacetamide is a useful agent in the management of papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis.
- Adverse effects are rare and generally are limited to dryness, erythema, pruritus, and discomfort.
Cosmetic Corner: Dermatologists Weigh in on Products for Sensitive Skin
To improve patient care and outcomes, leading dermatologists offered their recommendations on top products for sensitive skin. Consideration must be given to:
- Aveeno Eczema Therapy Moisturizing Cream
- Cetaphil Restoraderm
- PRESCRIBEDsolutions Don’t Be So Sensitive Post-Procedure Cleanser
- Rosaliac AR Intense
- Vanicream
Cutis invites readers to send us their recommendations. Skin care products for babies, men’s shaving products, eye creams, and OTC dandruff treatments will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on top products for sensitive skin. Consideration must be given to:
- Aveeno Eczema Therapy Moisturizing Cream
- Cetaphil Restoraderm
- PRESCRIBEDsolutions Don’t Be So Sensitive Post-Procedure Cleanser
- Rosaliac AR Intense
- Vanicream
Cutis invites readers to send us their recommendations. Skin care products for babies, men’s shaving products, eye creams, and OTC dandruff treatments will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on top products for sensitive skin. Consideration must be given to:
- Aveeno Eczema Therapy Moisturizing Cream
- Cetaphil Restoraderm
- PRESCRIBEDsolutions Don’t Be So Sensitive Post-Procedure Cleanser
- Rosaliac AR Intense
- Vanicream
Cutis invites readers to send us their recommendations. Skin care products for babies, men’s shaving products, eye creams, and OTC dandruff treatments will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
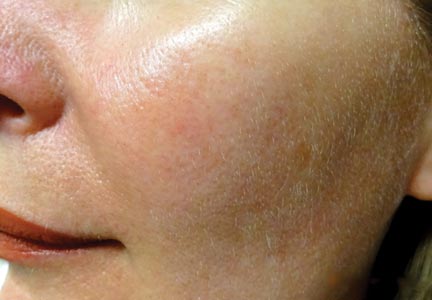
FDA approves azelaic acid foam for rosacea
A foam formulation of azelaic acid has been approved for the topical treatment of the inflammatory papules and pustules of mild to moderate rosacea, the manufacturer announced July 31.
Azelaic acid foam, 15%, was compared with a vehicle foam, applied twice a day for 12 weeks, in two studies with a total of 1,362 people with papulopustular rosacea (mean age 50.6 years) and a mean of about 21 inflammatory papules and pustules at baseline. The success rate was defined as a score of clear or minimal with at least a two-step reduction from baseline on the Investigator’s Global Assessment scale.
At 12 weeks, the success rate in the two studies, respectively, was 32% and 43% among those treated with azelaic foam vs. 23% and 32% among controls, according to the Bayer HealthCare press release announcing the approval.
The most common adverse events associated with treatment were pain at the application site in about 6%, pruritus (2.5%), dryness (0.7%), and erythema (0.7%). Warnings in the prescribing information note that treatment has been associated with “isolated” cases of hypopigmentation and can cause eye irritation, and that the contents are flammable. Patients are instructed to avoid “fire, flame, and smoking during and immediately following application.”
Azelaic acid foam is marketed as Finacea Foam by Bayer; it is the first foam formulation of azelaic acid to be approved, and it will be available in September, according to the company. A gel formulation of azelaic acid, 15%, was approved by the FDA in 2002 for topical treatment of inflammatory papules and pustules of mild to moderate rosacea.
Serious adverse events associated with azelaic acid should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/default.htm.
A foam formulation of azelaic acid has been approved for the topical treatment of the inflammatory papules and pustules of mild to moderate rosacea, the manufacturer announced July 31.
Azelaic acid foam, 15%, was compared with a vehicle foam, applied twice a day for 12 weeks, in two studies with a total of 1,362 people with papulopustular rosacea (mean age 50.6 years) and a mean of about 21 inflammatory papules and pustules at baseline. The success rate was defined as a score of clear or minimal with at least a two-step reduction from baseline on the Investigator’s Global Assessment scale.
At 12 weeks, the success rate in the two studies, respectively, was 32% and 43% among those treated with azelaic foam vs. 23% and 32% among controls, according to the Bayer HealthCare press release announcing the approval.
The most common adverse events associated with treatment were pain at the application site in about 6%, pruritus (2.5%), dryness (0.7%), and erythema (0.7%). Warnings in the prescribing information note that treatment has been associated with “isolated” cases of hypopigmentation and can cause eye irritation, and that the contents are flammable. Patients are instructed to avoid “fire, flame, and smoking during and immediately following application.”
Azelaic acid foam is marketed as Finacea Foam by Bayer; it is the first foam formulation of azelaic acid to be approved, and it will be available in September, according to the company. A gel formulation of azelaic acid, 15%, was approved by the FDA in 2002 for topical treatment of inflammatory papules and pustules of mild to moderate rosacea.
Serious adverse events associated with azelaic acid should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/default.htm.
A foam formulation of azelaic acid has been approved for the topical treatment of the inflammatory papules and pustules of mild to moderate rosacea, the manufacturer announced July 31.
Azelaic acid foam, 15%, was compared with a vehicle foam, applied twice a day for 12 weeks, in two studies with a total of 1,362 people with papulopustular rosacea (mean age 50.6 years) and a mean of about 21 inflammatory papules and pustules at baseline. The success rate was defined as a score of clear or minimal with at least a two-step reduction from baseline on the Investigator’s Global Assessment scale.
At 12 weeks, the success rate in the two studies, respectively, was 32% and 43% among those treated with azelaic foam vs. 23% and 32% among controls, according to the Bayer HealthCare press release announcing the approval.
The most common adverse events associated with treatment were pain at the application site in about 6%, pruritus (2.5%), dryness (0.7%), and erythema (0.7%). Warnings in the prescribing information note that treatment has been associated with “isolated” cases of hypopigmentation and can cause eye irritation, and that the contents are flammable. Patients are instructed to avoid “fire, flame, and smoking during and immediately following application.”
Azelaic acid foam is marketed as Finacea Foam by Bayer; it is the first foam formulation of azelaic acid to be approved, and it will be available in September, according to the company. A gel formulation of azelaic acid, 15%, was approved by the FDA in 2002 for topical treatment of inflammatory papules and pustules of mild to moderate rosacea.
Serious adverse events associated with azelaic acid should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/default.htm.
Chemical Peels
After, test your knowledge by answering the 5 practice questions.
Practice Questions
1. Which one of the following peels produces “frosting” after application?
a. citric acid
b. glycolic acid
c. mandelic acid
d. salicylic acid
e. trichloroacetic acid
2. Which one of the following peels is lipophilic?
a. citric acid
b. glycolic acid
c. mandelic acid
d. salicylic acid
e. trichloroacetic acid
3. A Jessner solution peel contains which of the following 4 components?
a. lactic acid, resorcinol, salicylic acid, ethanol
b. lactic acid, resorcinol, salicylic acid, methanol
c. lactic acid, resorcinol, salicylic acid, retinoic acid
d. retinoic acid, resorcinol, phenol, ethanol
e. retinoic acid, resorcinol, glycolic acid, methanol
4. What is the most serious risk associated with phenol peels?
a. cardiac dysrhythmia
b. hearing loss
c. scarring
d. seizure
e. tinnitus
5. Which one of the following peels self-neutralizes?
a. citric acid
b. glycolic acid
c. lactic acid
d. mandelic acid
e. salicylic acid
The answers appear on the next page.
Practice Question Answers
1. Which one of the following peels produces “frosting” after application?
a. citric acid
b. glycolic acid
c. mandelic acid
d. salicylic acid
e. trichloroacetic acid
2. Which one of the following peels is lipophilic?
a. citric acid
b. glycolic acid
c. mandelic acid
d. salicylic acid
e. trichloroacetic acid
3. A Jessner solution peel contains which of the following 4 components?
a. lactic acid, resorcinol, salicylic acid, ethanol
b. lactic acid, resorcinol, salicylic acid, methanol
c. lactic acid, resorcinol, salicylic acid, retinoic acid
d. retinoic acid, resorcinol, phenol, ethanol
e. retinoic acid, resorcinol, glycolic acid, methanol
4. What is the most serious risk associated with phenol peels?
a. cardiac dysrhythmia
b. hearing loss
c. scarring
d. seizure
e. tinnitus
5. Which one of the following peels self-neutralizes?
a. citric acid
b. glycolic acid
c. lactic acid
d. mandelic acid
e. salicylic acid
After, test your knowledge by answering the 5 practice questions.
Practice Questions
1. Which one of the following peels produces “frosting” after application?
a. citric acid
b. glycolic acid
c. mandelic acid
d. salicylic acid
e. trichloroacetic acid
2. Which one of the following peels is lipophilic?
a. citric acid
b. glycolic acid
c. mandelic acid
d. salicylic acid
e. trichloroacetic acid
3. A Jessner solution peel contains which of the following 4 components?
a. lactic acid, resorcinol, salicylic acid, ethanol
b. lactic acid, resorcinol, salicylic acid, methanol
c. lactic acid, resorcinol, salicylic acid, retinoic acid
d. retinoic acid, resorcinol, phenol, ethanol
e. retinoic acid, resorcinol, glycolic acid, methanol
4. What is the most serious risk associated with phenol peels?
a. cardiac dysrhythmia
b. hearing loss
c. scarring
d. seizure
e. tinnitus
5. Which one of the following peels self-neutralizes?
a. citric acid
b. glycolic acid
c. lactic acid
d. mandelic acid
e. salicylic acid
The answers appear on the next page.
Practice Question Answers
1. Which one of the following peels produces “frosting” after application?
a. citric acid
b. glycolic acid
c. mandelic acid
d. salicylic acid
e. trichloroacetic acid
2. Which one of the following peels is lipophilic?
a. citric acid
b. glycolic acid
c. mandelic acid
d. salicylic acid
e. trichloroacetic acid
3. A Jessner solution peel contains which of the following 4 components?
a. lactic acid, resorcinol, salicylic acid, ethanol
b. lactic acid, resorcinol, salicylic acid, methanol
c. lactic acid, resorcinol, salicylic acid, retinoic acid
d. retinoic acid, resorcinol, phenol, ethanol
e. retinoic acid, resorcinol, glycolic acid, methanol
4. What is the most serious risk associated with phenol peels?
a. cardiac dysrhythmia
b. hearing loss
c. scarring
d. seizure
e. tinnitus
5. Which one of the following peels self-neutralizes?
a. citric acid
b. glycolic acid
c. lactic acid
d. mandelic acid
e. salicylic acid
After, test your knowledge by answering the 5 practice questions.
Practice Questions
1. Which one of the following peels produces “frosting” after application?
a. citric acid
b. glycolic acid
c. mandelic acid
d. salicylic acid
e. trichloroacetic acid
2. Which one of the following peels is lipophilic?
a. citric acid
b. glycolic acid
c. mandelic acid
d. salicylic acid
e. trichloroacetic acid
3. A Jessner solution peel contains which of the following 4 components?
a. lactic acid, resorcinol, salicylic acid, ethanol
b. lactic acid, resorcinol, salicylic acid, methanol
c. lactic acid, resorcinol, salicylic acid, retinoic acid
d. retinoic acid, resorcinol, phenol, ethanol
e. retinoic acid, resorcinol, glycolic acid, methanol
4. What is the most serious risk associated with phenol peels?
a. cardiac dysrhythmia
b. hearing loss
c. scarring
d. seizure
e. tinnitus
5. Which one of the following peels self-neutralizes?
a. citric acid
b. glycolic acid
c. lactic acid
d. mandelic acid
e. salicylic acid
The answers appear on the next page.
Practice Question Answers
1. Which one of the following peels produces “frosting” after application?
a. citric acid
b. glycolic acid
c. mandelic acid
d. salicylic acid
e. trichloroacetic acid
2. Which one of the following peels is lipophilic?
a. citric acid
b. glycolic acid
c. mandelic acid
d. salicylic acid
e. trichloroacetic acid
3. A Jessner solution peel contains which of the following 4 components?
a. lactic acid, resorcinol, salicylic acid, ethanol
b. lactic acid, resorcinol, salicylic acid, methanol
c. lactic acid, resorcinol, salicylic acid, retinoic acid
d. retinoic acid, resorcinol, phenol, ethanol
e. retinoic acid, resorcinol, glycolic acid, methanol
4. What is the most serious risk associated with phenol peels?
a. cardiac dysrhythmia
b. hearing loss
c. scarring
d. seizure
e. tinnitus
5. Which one of the following peels self-neutralizes?
a. citric acid
b. glycolic acid
c. lactic acid
d. mandelic acid
e. salicylic acid
A Phase 3 Randomized, Double-blind, Vehicle-Controlled Trial of Azelaic Acid Foam 15% in the Treatment of Papulopustular Rosacea
Rosacea is a common dermatologic disorder that generally is characterized by erythema as well as papules and pustules on the cheeks, chin, forehead, and nose. Moreover, telangiectasia and burning or stinging sensations often occur.1,2 These clinical manifestations and other related ones frequently lead to the perception of “sensitive skin.” Rosacea patients often experience low self-esteem, anxiety, and social embarrassment.3 Reports of the gender distribution of the disease vary but often show female predominance.4 Although it also occurs in darker skin types, rosacea is more common in individuals with lighter skin.1
The etiology of rosacea is not yet fully understood, but the underlying pathology has been attributed to dysregulated immune responses. Although the flares of a typical fluctuating disease course often are caused by exogenous triggers, there is evidence that an underlying genetic component predisposes some individuals to pathologic changes associated with the condition.5 Augmented immune activity and proinflammatory signaling appear to induce the infiltration of inflammatory elements into affected areas.2 These regions show dilated vasculature and increased cutaneous blood flow secondary to inflammation. Systemic oxidative stress also may contribute to epidermal dysfunction, as the antioxidant capacity of the skin in patients with rosacea is depleted relative to that of healthy individuals. The biochemical and vascular changes characteristic of rosacea coincide with aberrant permeability of the stratum corneum.6 The resulting decreased hydration and water loss across the skin contribute to the sensitivity and irritation typical of the disease.2
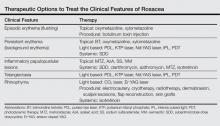
Current guidelines for the optimal management of rosacea with papulopustular lesions recommend skin care, photoprotection, and topical therapy. Depending on the severity of disease and the likelihood of adherence to a topical regimen, use of oral agents may be warranted.7
Azelaic acid (AzA), an unbranched saturated dicarboxylic acid (1,7-heptanedicarboxylic acid) that occurs in plants, is one of several US Food and Drug Administration–approved topical agents for the treatment of inflammatory lesions in rosacea.8 Although the pathophysiology of rosacea is not yet fully understood, there is a growing consensus about the role of proinflammatory molecules (eg, kallikrein 5, cathelicidins) as well as reactive oxygen species (ROS).9 Azelaic acid has been demonstrated to modulate the inflammatory response in normal human keratinocytes through several pathways, including modulation of the signaling pathways of peroxisome proliferator-activated receptor g and nuclear factor kB, concurrent with the observed inhibition of proinflammatory cytokine secretion.10 Additionally, AzA can inhibit the release of ROS from neutrophils and also may reduce ROS by direct scavenging effects.11 Further, AzA shows direct inhibition of kallikrein 5 in human keratinocytes as well as a reduction of the expression of kallikrein 5 and cathelicidin in murine skin and the facial skin of patients with rosacea.12
In a series of randomized trials in patients with papulopustular rosacea (PPR), AzA has shown clinical efficacy and safety as a topical treatment.13-15 Based on these studies, a gel formulation of AzA with a 15% concentration has been approved for treating inflammatory papules and pustules of mild to moderate rosacea.16
Although AzA delivered in a gel matrix is an effective therapy, topical delivery of active pharmaceutical ingredients via foam is often preferred over traditional vehicles in patients with sensitive skin. Patient rationale for favoring foam includes improved appearance and ease of application, namely easier to spread with a reduced need to manipulate inflamed skin.17 Also, data reveal that patients may be more compliant with a treatment that meets their needs such as an optimized foam formulation.18 In addition, the lipid components of an optimized formulation are thought to contribute to an improved skin condition.19 The foam vehicle used in this study is a proprietary oil-in-water formulation that includes fatty alcohols and triglycerides. The novel delivery of AzA in a foam formulation will provide clinicians and patients with a new option for improved individualized care.
We report the primary results of a phase 3 study in patients with PPR comparing the efficacy and safety of twice-daily AzA foam 15% with vehicle foam. The phase 3 study builds on the results of a prior randomized double-blind trial (N=401) that demonstrated significant improvements relative to vehicle in therapeutic success rate (P=.017) and decreased inflammatory lesion count (ILC)(P<.001) among patients treated with AzA foam 15%.8
Methods
Study Design
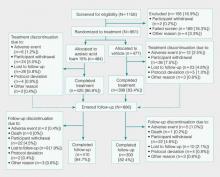
This phase 3 randomized, double-blind, vehicle-controlled, parallel-group, multicenter study was conducted in patients with PPR according to Good Clinical Practice guidelines in 48 study centers in the United States. The objective was to evaluate a 12-week, twice-daily (morning and evening) course of AzA foam 15% versus vehicle.
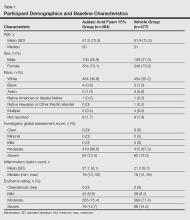
Participants were men and women aged 18 years or older with moderate to severe PPR (as determined by investigator global assessment [IGA]) presenting with 12 to 50 papules and/or pustules and persistent erythema with or without telangiectasia. Informed consent was obtained from all participants before any study-related activities were carried out.
The study products were applied to the entire facial area each morning and evening at a dose of 0.5 g, thus administering 150 mg of AzA daily in the active arm of the trial (computerized randomization 1:1). The treatment period lasted 12 weeks, and participants were evaluated at baseline and weeks 4, 8, and 12. The follow-up period lasted 4 weeks following the end of treatment (EoT) and was concluded with one final end-of-study visit.
Efficacy Evaluations
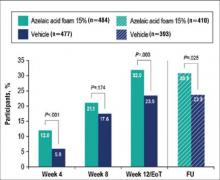
There were 2 coprimary efficacy end points. Therapeutic success rate was evaluated using the IGA scale (clear, minimal, mild, moderate, or severe). Treatment success was defined as an IGA score of either clear or minimal (with at least a 2-step improvement) at EoT, whereas treatment failure was constituted by IGA scores of mild, moderate, or severe.
The second coprimary end point was the nominal change in ILC from baseline to EoT as determined by the total number of facial papules and pustules. Efficacy and safety parameters were evaluated at weeks 4, 8, and 12, as well as at the end of the 4-week follow-up period. Throughout the study, the investigator, participants, and all study personnel remained blinded.
Safety
Information about adverse events (AEs) was collected at each study visit, and AEs were graded according to seriousness (yes or no) and intensity (mild, moderate, or severe).
Statistical Analysis
Efficacy was confirmed by analysis of the treatment success rate at EoT with Cochran-Mantel-Haenszel test statistics, including a point estimate and 95% confidence interval (CI) for the odds ratio. Change in ILC at EoT was analyzed via an analysis of covariance model using treatment, center, and baseline lesion count as factors. (Additional methods can be found in the Appendix below.)
Results
Study Participants
Of the 1156 patients who were screened for eligibility, 961 were randomized to treatment with AzA foam (n=484) or vehicle (n=477)(Figure 1). Sixty-four (13.2%) participants in the AzA foam group and 79 (16.6%) in the vehicle group discontinued treatment before completing the study. The most common reasons for discontinuation were participant withdrawal from the study and lost to follow-up. Six (1.2%) participants from the AzA foam group and 12 (2.5%) from the vehicle group discontinued because of AEs. All safety and efficacy data presented are based on the full analysis set, which consisted of the 961 participants randomized to treatment.
Demographic and baseline characteristics were balanced between the treatment groups (Table 1). The majority of participants were female (73.0%) and white (95.5%), reflecting the patient populations of independent studies that found a higher prevalence of rosacea in women and lighter skin types.4 There were no significant differences in baseline measures of PPR severity between the treatment groups. Participants in the AzA foam and vehicle groups had a mean ILC of 21.7 and 21.2, respectively, and 76.4% of participants had more than 14 lesions. All participants had an IGA score of moderate (86.8%) or severe (13.2%). Moderate or severe erythema was present in 91.5% of participants.
Treatment compliance, as measured by the percentage of expected doses that were actually administered, was 97.1% in the AzA foam group and 95.9% in the vehicle group.
Efficacy
Results from both primary end points demonstrated superior efficacy of AzA foam over vehicle. The AzA foam group achieved a greater IGA success rate at EoT compared with the vehicle group (32.0% vs 23.5%; Cochran-Mantel-Haenszel test center-adjusted P<.001; odds ratio, 1.6; 95% CI, 1.2-2.2). Treatment success rate was higher in the AzA foam group than in the vehicle group at every time point past baseline (Figure 2). Similarly, the decrease in mean nominal ILC values was greater in the AzA foam group at every time point after baseline (Figure 3), and the treatment difference at EoT was statistically significant in favor of AzA foam (-2.7, F1,920=23.7, P<.001; 95% CI, -3.8 to -1.6). The divergence between treatment groups at week 4 reveals an onset of AzA effect early in the study.

|
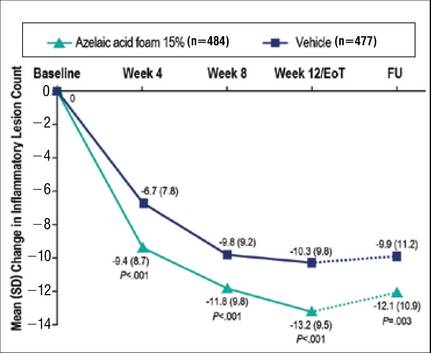
|
Although the AzA foam group showed significantly better efficacy results than the vehicle group for the coprimary end points, participants in the vehicle group did show appreciable IGA success rates (23.5%) and changes in ILC (-10.3) at EoT (Figures 2 and 3).
Notably, the AzA foam group maintained better results than vehicle for both primary end points even at the end of the 4-week follow-up after EoT (Figures 2 and 3). Sensitivity analysis (data not shown) confirmed the findings from the full analysis set.
Safety
Adverse events were experienced by 149 (30.8%) participants in the AzA foam group and 119 (24.9%) in the vehicle group. The most common noncutaneous AEs (>1% of participants) reported during AzA foam treatment were nasopharyngitis, headache, upper respiratory tract infection, and influenza. In the vehicle group, the most common noncutaneous AEs reported were nasopharyngitis and headache. Drug-related AEs (relationship assessed by the investigator) were reported slightly more often in the AzA foam group (7.6%) than in the vehicle group (4.6%). Drug-related AEs were predominantly cutaneous and occurred at the site of application (Table 2). Drug-related cutaneous AEs were more common in the AzA foam group (7.0%) than in the vehicle group (4.4%). Although serious AEs were more common in the vehicle group, all were regarded as unrelated to the study medication. A single death occurred in the vehicle group due to an accident unrelated to the study drug.
The most frequent drug-related AEs in participants treated with AzA foam versus vehicle were application-site pain (3.5% vs 1.3%), application-site pruritus (1.4% vs 0.4%), and application-site dryness (1.0% vs 0.6%). The classical rosacea symptom of stinging is subsumed under the term application-site pain, according to MedDRA (Medical Dictionary for Regulatory Activities).
All other drug-related AEs occurred at a frequency of less than 1% in participants from both groups. Serious AEs were rare and unrelated to treatment, with 3 AEs reported in the AzA foam group and 4 in the vehicle group. Adverse events leading to study drug withdrawal occurred in less than 2% of participants and were more common in the vehicle group (2.5%) than in the AzA foam group (1.2%). Of the 3 drug-related AEs leading to withdrawal in the AzA foam group, 2 were due to cutaneous reaction and 1 was due to a burning sensation. The number of active drug-related cutaneous AEs was highest during the first 4 weeks of treatment and declined over the course of the study (eFigure).
More than 96% of AEs were resolved by the end of the study. Of the participants experiencing AEs that did not resolve during the course of the study, 16 were in the AzA foam group and 10 in the vehicle group. Six unresolved AEs were drug related, with 3 occurring in each treatment group. Unresolved drug-related cutaneous AEs in the AzA foam group were pain, pruritus, and dryness at the application site.
Comment
Overall, the results from this phase 3 trial demonstrate that the new foam formulation of AzA was efficacious and safe in a 12-week, twice-daily course of treatment for moderate to severe PPR. The AzA foam formulation was significantly superior to vehicle (P<.001) for both primary efficacy end points. Participants in the AzA foam group achieved therapeutic success at a higher rate than the vehicle group, and the change in nominal ILC at EoT was significantly greater for participants treated with AzA foam than for those treated with vehicle (P<.001). Differences between the 2 treatment groups for the coprimary end point measures arose early in the study, demonstrating that symptoms were rapidly controlled. Between weeks 8 and 12 (EoT), the rate of increase of beneficial effects in the AzA foam group remained high, while the vehicle group showed a notable slowing. There was no indication of any rebound effect in overall disease severity subsequent to EoT. After 4 weeks of follow-up, there was still a beneficial treatment effect present in favor of the AzA foam group, as indicated by the persistence of improvements in both coprimary end point measures throughout the follow-up period.
Analyses of alternative populations and secondary end points (data not shown) supported the efficacy results reported here. There was no indication of irregular study center effects, and the sensitivity analyses demonstrated robustness of the data for the observed treatment effects.
The use of vehicle foam alone appeared to be beneficial in reducing ILC and improving IGA rating, which suggests that the properties of the new foam formulation are favorable for the inflamed lesional skin of rosacea. Of note, other dermatology studies, including trials in rosacea, have reported therapeutic effects of vehicle treatment that may be attributable to the positive effects of skin care with certain formulations.20
Azelaic acid foam was well tolerated in the current study. More than 93% of AEs in either treatment group were of mild or moderate severity. The incidence of drug-related AEs was low in both groups and mainly occurred at the application site. There were no drug-related severe or serious AEs. The low incidence of reported drug-related noncutaneous AEs in the AzA foam group (dysgeusia in 1 patient and headache in 2 patients) supports the known favorable systemic tolerance profile of AzA.
Although most drug-related AEs occurred at the application site, they were generally transient, with the majority of events in the AzA foam group lasting no more than 1 hour. Most cutaneous AEs developed early in the study. In the AzA foam group, the prevalence of drug-related cutaneous AEs dropped at every time interval as the study progressed (eFigure). Very few AEs of any type persisted through the end of the study. These safety results were accompanied by a high compliance rate and a high participation rate throughout the course of the study. Taken together, the available data for this AzA foam formulation support a favorable tolerability profile. The results of this study are consistent with and expand on data from an earlier investigation of similar design.8
Conclusion
The development of an AzA foam formulation with higher lipid content was intended to expand the treatment options available to physicians and patients who are managing rosacea. Most topical dermatologic treatments are currently delivered in classical formulations such as creams or gels, but patients who use topical therapies have rated messiness and ease of application among the most important characteristics affecting quality of life.17,21 Foam formulations may offer improvements in this regard; ease of application may minimize unnecessary manipulation of inflamed skin and contribute to a high level of user satisfaction.22 However, the design of the current study was limited to evaluating only the AzA foam formulation versus a foam vehicle, and direct comparisons of clinical efficacy and tolerability to other AzA topical preparations were not performed. Nonetheless, patients have previously reported that they would be more likely to comply with a recommended course of dermatologic foam therapy than other topical formulations.18 The proposed foam formulation was designed to attend to the specific needs of the dry and sensitive skin in rosacea by combining the demonstrated efficacy properties exhibited by AzA gel 15% with the good tolerability and acceptability of a lipid-containing foam formulation. Development of this formulation was targeted to obtain a product that would be highly spreadable, dry quickly, and be easy to apply. The available data for this AzA foam formulation support the value of this option in the topical treatment of rosacea. The success in reduction of overall disease severity, lack of any rebound after EoT, and the observed tolerability and high adherence rates suggest that this novel formulation is a useful addition to current treatment options for rosacea.
Addendum
After release of the study data for unblinding and statistical evaluation, the following inconsistency regarding patient distribution was noted: 1 participant was incorrectly evaluated as part of the AzA foam analysis group when in fact this patient was randomized to vehicle and was treated throughout the study with vehicle. This participant did not experience any AE and did not show any IGA improvement at the EoT. As this single case did not have an impact on the statistical conclusions or interpretation of the results, the released study data have not been changed. This deviation was described as a database erratum in the study report.
Acknowledgement—Editorial support through inVentiv Medical Communications, New York, New York, was provided by Bayer HealthCare Pharmaceuticals Inc.
APPENDIX
Supplementary Methods
Supplementary Study Design
This study met all local legal and regulatory requirements and was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Before the start of the study and implementation, the protocol and all amendments were approved by the appropriate independent ethics committee or institutional review board at each study site. Two protocol amendments were implemented before the first participant visit.
Exclusion criteria included the presence of dermatoses that could interfere with rosacea diagnosis or evaluation, facial laser surgery or topical use of any medication to treat rosacea within 6 weeks before randomization, systemic use of any medications to treat rosacea, and known unresponsiveness to AzA treatment. Further standard exclusion criteria included alcohol or drug use or parallel participation in other clinical studies, which were necessary to exclude undue influence on study evaluations and/or participant safety. The study was conducted by qualified investigators at 48 centers in the United States.
The investigational product was filled in identical containers according to the randomization list generated by a computer program using blocks. Complete blocks of study medication were distributed to the centers. Eligible participants were randomized 1:1 into either AzA foam or vehicle treatment groups by assignment of a randomization number at baseline. A blind investigational product under the same randomization number was dispensed to and returned from participants by study personnel who were not involved in the assessments. Blinding was achieved by using labels on the investigational products that did not allow identification of the true medication.
Compliance was evaluated from participant diaries as well as the number of expected doses and actually applied doses.
Additional Efficacy Evaluations
A number of secondary variables (not reported here) were assessed, including changes in other manifestations of PPR, as well as participant assessments of treatment response, tolerability, cosmetic preferences, and quality of life.
Additional Safety
Investigators reported a yes or no response as to whether there was a reasonable causal relationship between AEs and treatment. Moreover, AEs that began at the start of or during treatment were considered treatment emergent. Cutaneous AEs were further assessed regarding location and duration. An AE was deemed local if it occurred at the application site and transient if it subsided within 60 minutes of onset.
Statistical Analysis
The primary efficacy analyses presented here were based on the full analysis set of participants who were randomized and had medication dispensed. For participants with no EoT value, the last nonmissing value was used including baseline (last-observation-carried-forward methodology). Participants who discontinued treatment prematurely because of lack of efficacy were considered to be treatment failures, regardless of the reported IGA score. Statistical significance was needed for both coprimary efficacy variables at a 1-sided 2.5% significance level to show confirmed superiority of AzA foam versus vehicle.
A number of sensitivity analyses were performed, including an analysis of the coprimary end points using observed data, analysis of the per-protocol population of participants who did not prematurely discontinue treatment and had no major protocol deviations, subgroup analyses, and the use of statistical methods to investigate the effect of missing observations. Analyses of success rate and nominal change in ILC were repeated for each postbaseline visit using χ² and t tests, respectively. All summary and statistical analyses were performed according to the study protocol (unchanged after the start of the study) using SAS version 9.2.
Results from a prior study provided the basis for the sample size, which was calculated to show a significant difference in both primary efficacy end points with a power of 90%.8 To allow for dropouts, 480 participants in each treatment group were to be randomized for a total of 960 participants.
1. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
2. Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
3. Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6:348-354.
4. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
5. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
6. Wollina U. Recent advances in the understanding and management of rosacea. F1000Prime Rep. 2014;6:50.
7. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis. 2014;93:134-138.
8. Draelos ZD, Elewski B, Staedtler G, et al. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92:306-317.
9. Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
10. Mastrofrancesco A, Ottaviani M, Aspite N, et al. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARg activation. Exp Dermatol. 2010;19:813-820.
11. Akamatsu H, Komura J, Asada Y, et al. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch Dermatol Res. 1991;283:162-166.
12. Coda AB, Hata T, Miller J, et al. Cathelicidin, kalli-krein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol. 2013;69:570-577.
13. van Zuuren EJ, Kramer SF, Carter BR, et al. Effective and evidence-based management strategies for rosacea: summary of a Cochrane systematic review. Br J Dermatol. 2011;165:760-781.
14. Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48:836-845.
15. Thiboutot DM, Fleischer AB Jr, Del Rosso JQ, et al. Azelaic acid 15% gel once daily versus twice daily in papulopustular rosacea. J Drugs Dermatol. 2008;7:541-546.
16. Finacea [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2015.
17. Zhao Y, Jones SA, Brown MB. Dynamic foams in topical drug delivery. J Pharm Pharmacol. 2010;62:678-684.
18. Gottlieb AB, Ford RO, Spellman MC. The efficacy and tolerability of clobetasol propionate foam 0.05% in the treatment of mild to moderate plaque-type psoriasis of nonscalp regions. J Cutan Med Surg. 2003;7:185-192.
19. Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771-788.
20. Jackson JM, Pelle M. Topical rosacea therapy: the importance of vehicles for efficacy, tolerability and compliance. J Drugs Dermatol. 2011;10:627-633.
21. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
22. Kircik LH, Bikowski JB. Vehicles matter: topical foam formulations. Practical Dermatology. January 2012(suppl):3-18.
Rosacea is a common dermatologic disorder that generally is characterized by erythema as well as papules and pustules on the cheeks, chin, forehead, and nose. Moreover, telangiectasia and burning or stinging sensations often occur.1,2 These clinical manifestations and other related ones frequently lead to the perception of “sensitive skin.” Rosacea patients often experience low self-esteem, anxiety, and social embarrassment.3 Reports of the gender distribution of the disease vary but often show female predominance.4 Although it also occurs in darker skin types, rosacea is more common in individuals with lighter skin.1
The etiology of rosacea is not yet fully understood, but the underlying pathology has been attributed to dysregulated immune responses. Although the flares of a typical fluctuating disease course often are caused by exogenous triggers, there is evidence that an underlying genetic component predisposes some individuals to pathologic changes associated with the condition.5 Augmented immune activity and proinflammatory signaling appear to induce the infiltration of inflammatory elements into affected areas.2 These regions show dilated vasculature and increased cutaneous blood flow secondary to inflammation. Systemic oxidative stress also may contribute to epidermal dysfunction, as the antioxidant capacity of the skin in patients with rosacea is depleted relative to that of healthy individuals. The biochemical and vascular changes characteristic of rosacea coincide with aberrant permeability of the stratum corneum.6 The resulting decreased hydration and water loss across the skin contribute to the sensitivity and irritation typical of the disease.2
Current guidelines for the optimal management of rosacea with papulopustular lesions recommend skin care, photoprotection, and topical therapy. Depending on the severity of disease and the likelihood of adherence to a topical regimen, use of oral agents may be warranted.7
Azelaic acid (AzA), an unbranched saturated dicarboxylic acid (1,7-heptanedicarboxylic acid) that occurs in plants, is one of several US Food and Drug Administration–approved topical agents for the treatment of inflammatory lesions in rosacea.8 Although the pathophysiology of rosacea is not yet fully understood, there is a growing consensus about the role of proinflammatory molecules (eg, kallikrein 5, cathelicidins) as well as reactive oxygen species (ROS).9 Azelaic acid has been demonstrated to modulate the inflammatory response in normal human keratinocytes through several pathways, including modulation of the signaling pathways of peroxisome proliferator-activated receptor g and nuclear factor kB, concurrent with the observed inhibition of proinflammatory cytokine secretion.10 Additionally, AzA can inhibit the release of ROS from neutrophils and also may reduce ROS by direct scavenging effects.11 Further, AzA shows direct inhibition of kallikrein 5 in human keratinocytes as well as a reduction of the expression of kallikrein 5 and cathelicidin in murine skin and the facial skin of patients with rosacea.12
In a series of randomized trials in patients with papulopustular rosacea (PPR), AzA has shown clinical efficacy and safety as a topical treatment.13-15 Based on these studies, a gel formulation of AzA with a 15% concentration has been approved for treating inflammatory papules and pustules of mild to moderate rosacea.16
Although AzA delivered in a gel matrix is an effective therapy, topical delivery of active pharmaceutical ingredients via foam is often preferred over traditional vehicles in patients with sensitive skin. Patient rationale for favoring foam includes improved appearance and ease of application, namely easier to spread with a reduced need to manipulate inflamed skin.17 Also, data reveal that patients may be more compliant with a treatment that meets their needs such as an optimized foam formulation.18 In addition, the lipid components of an optimized formulation are thought to contribute to an improved skin condition.19 The foam vehicle used in this study is a proprietary oil-in-water formulation that includes fatty alcohols and triglycerides. The novel delivery of AzA in a foam formulation will provide clinicians and patients with a new option for improved individualized care.
We report the primary results of a phase 3 study in patients with PPR comparing the efficacy and safety of twice-daily AzA foam 15% with vehicle foam. The phase 3 study builds on the results of a prior randomized double-blind trial (N=401) that demonstrated significant improvements relative to vehicle in therapeutic success rate (P=.017) and decreased inflammatory lesion count (ILC)(P<.001) among patients treated with AzA foam 15%.8
Methods
Study Design
This phase 3 randomized, double-blind, vehicle-controlled, parallel-group, multicenter study was conducted in patients with PPR according to Good Clinical Practice guidelines in 48 study centers in the United States. The objective was to evaluate a 12-week, twice-daily (morning and evening) course of AzA foam 15% versus vehicle.
Participants were men and women aged 18 years or older with moderate to severe PPR (as determined by investigator global assessment [IGA]) presenting with 12 to 50 papules and/or pustules and persistent erythema with or without telangiectasia. Informed consent was obtained from all participants before any study-related activities were carried out.
The study products were applied to the entire facial area each morning and evening at a dose of 0.5 g, thus administering 150 mg of AzA daily in the active arm of the trial (computerized randomization 1:1). The treatment period lasted 12 weeks, and participants were evaluated at baseline and weeks 4, 8, and 12. The follow-up period lasted 4 weeks following the end of treatment (EoT) and was concluded with one final end-of-study visit.
Efficacy Evaluations
There were 2 coprimary efficacy end points. Therapeutic success rate was evaluated using the IGA scale (clear, minimal, mild, moderate, or severe). Treatment success was defined as an IGA score of either clear or minimal (with at least a 2-step improvement) at EoT, whereas treatment failure was constituted by IGA scores of mild, moderate, or severe.
The second coprimary end point was the nominal change in ILC from baseline to EoT as determined by the total number of facial papules and pustules. Efficacy and safety parameters were evaluated at weeks 4, 8, and 12, as well as at the end of the 4-week follow-up period. Throughout the study, the investigator, participants, and all study personnel remained blinded.
Safety
Information about adverse events (AEs) was collected at each study visit, and AEs were graded according to seriousness (yes or no) and intensity (mild, moderate, or severe).
Statistical Analysis
Efficacy was confirmed by analysis of the treatment success rate at EoT with Cochran-Mantel-Haenszel test statistics, including a point estimate and 95% confidence interval (CI) for the odds ratio. Change in ILC at EoT was analyzed via an analysis of covariance model using treatment, center, and baseline lesion count as factors. (Additional methods can be found in the Appendix below.)
Results
Study Participants
Of the 1156 patients who were screened for eligibility, 961 were randomized to treatment with AzA foam (n=484) or vehicle (n=477)(Figure 1). Sixty-four (13.2%) participants in the AzA foam group and 79 (16.6%) in the vehicle group discontinued treatment before completing the study. The most common reasons for discontinuation were participant withdrawal from the study and lost to follow-up. Six (1.2%) participants from the AzA foam group and 12 (2.5%) from the vehicle group discontinued because of AEs. All safety and efficacy data presented are based on the full analysis set, which consisted of the 961 participants randomized to treatment.
Demographic and baseline characteristics were balanced between the treatment groups (Table 1). The majority of participants were female (73.0%) and white (95.5%), reflecting the patient populations of independent studies that found a higher prevalence of rosacea in women and lighter skin types.4 There were no significant differences in baseline measures of PPR severity between the treatment groups. Participants in the AzA foam and vehicle groups had a mean ILC of 21.7 and 21.2, respectively, and 76.4% of participants had more than 14 lesions. All participants had an IGA score of moderate (86.8%) or severe (13.2%). Moderate or severe erythema was present in 91.5% of participants.
Treatment compliance, as measured by the percentage of expected doses that were actually administered, was 97.1% in the AzA foam group and 95.9% in the vehicle group.
Efficacy
Results from both primary end points demonstrated superior efficacy of AzA foam over vehicle. The AzA foam group achieved a greater IGA success rate at EoT compared with the vehicle group (32.0% vs 23.5%; Cochran-Mantel-Haenszel test center-adjusted P<.001; odds ratio, 1.6; 95% CI, 1.2-2.2). Treatment success rate was higher in the AzA foam group than in the vehicle group at every time point past baseline (Figure 2). Similarly, the decrease in mean nominal ILC values was greater in the AzA foam group at every time point after baseline (Figure 3), and the treatment difference at EoT was statistically significant in favor of AzA foam (-2.7, F1,920=23.7, P<.001; 95% CI, -3.8 to -1.6). The divergence between treatment groups at week 4 reveals an onset of AzA effect early in the study.

|

|
Although the AzA foam group showed significantly better efficacy results than the vehicle group for the coprimary end points, participants in the vehicle group did show appreciable IGA success rates (23.5%) and changes in ILC (-10.3) at EoT (Figures 2 and 3).
Notably, the AzA foam group maintained better results than vehicle for both primary end points even at the end of the 4-week follow-up after EoT (Figures 2 and 3). Sensitivity analysis (data not shown) confirmed the findings from the full analysis set.
Safety
Adverse events were experienced by 149 (30.8%) participants in the AzA foam group and 119 (24.9%) in the vehicle group. The most common noncutaneous AEs (>1% of participants) reported during AzA foam treatment were nasopharyngitis, headache, upper respiratory tract infection, and influenza. In the vehicle group, the most common noncutaneous AEs reported were nasopharyngitis and headache. Drug-related AEs (relationship assessed by the investigator) were reported slightly more often in the AzA foam group (7.6%) than in the vehicle group (4.6%). Drug-related AEs were predominantly cutaneous and occurred at the site of application (Table 2). Drug-related cutaneous AEs were more common in the AzA foam group (7.0%) than in the vehicle group (4.4%). Although serious AEs were more common in the vehicle group, all were regarded as unrelated to the study medication. A single death occurred in the vehicle group due to an accident unrelated to the study drug.
The most frequent drug-related AEs in participants treated with AzA foam versus vehicle were application-site pain (3.5% vs 1.3%), application-site pruritus (1.4% vs 0.4%), and application-site dryness (1.0% vs 0.6%). The classical rosacea symptom of stinging is subsumed under the term application-site pain, according to MedDRA (Medical Dictionary for Regulatory Activities).
All other drug-related AEs occurred at a frequency of less than 1% in participants from both groups. Serious AEs were rare and unrelated to treatment, with 3 AEs reported in the AzA foam group and 4 in the vehicle group. Adverse events leading to study drug withdrawal occurred in less than 2% of participants and were more common in the vehicle group (2.5%) than in the AzA foam group (1.2%). Of the 3 drug-related AEs leading to withdrawal in the AzA foam group, 2 were due to cutaneous reaction and 1 was due to a burning sensation. The number of active drug-related cutaneous AEs was highest during the first 4 weeks of treatment and declined over the course of the study (eFigure).
More than 96% of AEs were resolved by the end of the study. Of the participants experiencing AEs that did not resolve during the course of the study, 16 were in the AzA foam group and 10 in the vehicle group. Six unresolved AEs were drug related, with 3 occurring in each treatment group. Unresolved drug-related cutaneous AEs in the AzA foam group were pain, pruritus, and dryness at the application site.
Comment
Overall, the results from this phase 3 trial demonstrate that the new foam formulation of AzA was efficacious and safe in a 12-week, twice-daily course of treatment for moderate to severe PPR. The AzA foam formulation was significantly superior to vehicle (P<.001) for both primary efficacy end points. Participants in the AzA foam group achieved therapeutic success at a higher rate than the vehicle group, and the change in nominal ILC at EoT was significantly greater for participants treated with AzA foam than for those treated with vehicle (P<.001). Differences between the 2 treatment groups for the coprimary end point measures arose early in the study, demonstrating that symptoms were rapidly controlled. Between weeks 8 and 12 (EoT), the rate of increase of beneficial effects in the AzA foam group remained high, while the vehicle group showed a notable slowing. There was no indication of any rebound effect in overall disease severity subsequent to EoT. After 4 weeks of follow-up, there was still a beneficial treatment effect present in favor of the AzA foam group, as indicated by the persistence of improvements in both coprimary end point measures throughout the follow-up period.
Analyses of alternative populations and secondary end points (data not shown) supported the efficacy results reported here. There was no indication of irregular study center effects, and the sensitivity analyses demonstrated robustness of the data for the observed treatment effects.
The use of vehicle foam alone appeared to be beneficial in reducing ILC and improving IGA rating, which suggests that the properties of the new foam formulation are favorable for the inflamed lesional skin of rosacea. Of note, other dermatology studies, including trials in rosacea, have reported therapeutic effects of vehicle treatment that may be attributable to the positive effects of skin care with certain formulations.20
Azelaic acid foam was well tolerated in the current study. More than 93% of AEs in either treatment group were of mild or moderate severity. The incidence of drug-related AEs was low in both groups and mainly occurred at the application site. There were no drug-related severe or serious AEs. The low incidence of reported drug-related noncutaneous AEs in the AzA foam group (dysgeusia in 1 patient and headache in 2 patients) supports the known favorable systemic tolerance profile of AzA.
Although most drug-related AEs occurred at the application site, they were generally transient, with the majority of events in the AzA foam group lasting no more than 1 hour. Most cutaneous AEs developed early in the study. In the AzA foam group, the prevalence of drug-related cutaneous AEs dropped at every time interval as the study progressed (eFigure). Very few AEs of any type persisted through the end of the study. These safety results were accompanied by a high compliance rate and a high participation rate throughout the course of the study. Taken together, the available data for this AzA foam formulation support a favorable tolerability profile. The results of this study are consistent with and expand on data from an earlier investigation of similar design.8
Conclusion
The development of an AzA foam formulation with higher lipid content was intended to expand the treatment options available to physicians and patients who are managing rosacea. Most topical dermatologic treatments are currently delivered in classical formulations such as creams or gels, but patients who use topical therapies have rated messiness and ease of application among the most important characteristics affecting quality of life.17,21 Foam formulations may offer improvements in this regard; ease of application may minimize unnecessary manipulation of inflamed skin and contribute to a high level of user satisfaction.22 However, the design of the current study was limited to evaluating only the AzA foam formulation versus a foam vehicle, and direct comparisons of clinical efficacy and tolerability to other AzA topical preparations were not performed. Nonetheless, patients have previously reported that they would be more likely to comply with a recommended course of dermatologic foam therapy than other topical formulations.18 The proposed foam formulation was designed to attend to the specific needs of the dry and sensitive skin in rosacea by combining the demonstrated efficacy properties exhibited by AzA gel 15% with the good tolerability and acceptability of a lipid-containing foam formulation. Development of this formulation was targeted to obtain a product that would be highly spreadable, dry quickly, and be easy to apply. The available data for this AzA foam formulation support the value of this option in the topical treatment of rosacea. The success in reduction of overall disease severity, lack of any rebound after EoT, and the observed tolerability and high adherence rates suggest that this novel formulation is a useful addition to current treatment options for rosacea.
Addendum
After release of the study data for unblinding and statistical evaluation, the following inconsistency regarding patient distribution was noted: 1 participant was incorrectly evaluated as part of the AzA foam analysis group when in fact this patient was randomized to vehicle and was treated throughout the study with vehicle. This participant did not experience any AE and did not show any IGA improvement at the EoT. As this single case did not have an impact on the statistical conclusions or interpretation of the results, the released study data have not been changed. This deviation was described as a database erratum in the study report.
Acknowledgement—Editorial support through inVentiv Medical Communications, New York, New York, was provided by Bayer HealthCare Pharmaceuticals Inc.
APPENDIX
Supplementary Methods
Supplementary Study Design
This study met all local legal and regulatory requirements and was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Before the start of the study and implementation, the protocol and all amendments were approved by the appropriate independent ethics committee or institutional review board at each study site. Two protocol amendments were implemented before the first participant visit.
Exclusion criteria included the presence of dermatoses that could interfere with rosacea diagnosis or evaluation, facial laser surgery or topical use of any medication to treat rosacea within 6 weeks before randomization, systemic use of any medications to treat rosacea, and known unresponsiveness to AzA treatment. Further standard exclusion criteria included alcohol or drug use or parallel participation in other clinical studies, which were necessary to exclude undue influence on study evaluations and/or participant safety. The study was conducted by qualified investigators at 48 centers in the United States.
The investigational product was filled in identical containers according to the randomization list generated by a computer program using blocks. Complete blocks of study medication were distributed to the centers. Eligible participants were randomized 1:1 into either AzA foam or vehicle treatment groups by assignment of a randomization number at baseline. A blind investigational product under the same randomization number was dispensed to and returned from participants by study personnel who were not involved in the assessments. Blinding was achieved by using labels on the investigational products that did not allow identification of the true medication.
Compliance was evaluated from participant diaries as well as the number of expected doses and actually applied doses.
Additional Efficacy Evaluations
A number of secondary variables (not reported here) were assessed, including changes in other manifestations of PPR, as well as participant assessments of treatment response, tolerability, cosmetic preferences, and quality of life.
Additional Safety
Investigators reported a yes or no response as to whether there was a reasonable causal relationship between AEs and treatment. Moreover, AEs that began at the start of or during treatment were considered treatment emergent. Cutaneous AEs were further assessed regarding location and duration. An AE was deemed local if it occurred at the application site and transient if it subsided within 60 minutes of onset.
Statistical Analysis
The primary efficacy analyses presented here were based on the full analysis set of participants who were randomized and had medication dispensed. For participants with no EoT value, the last nonmissing value was used including baseline (last-observation-carried-forward methodology). Participants who discontinued treatment prematurely because of lack of efficacy were considered to be treatment failures, regardless of the reported IGA score. Statistical significance was needed for both coprimary efficacy variables at a 1-sided 2.5% significance level to show confirmed superiority of AzA foam versus vehicle.
A number of sensitivity analyses were performed, including an analysis of the coprimary end points using observed data, analysis of the per-protocol population of participants who did not prematurely discontinue treatment and had no major protocol deviations, subgroup analyses, and the use of statistical methods to investigate the effect of missing observations. Analyses of success rate and nominal change in ILC were repeated for each postbaseline visit using χ² and t tests, respectively. All summary and statistical analyses were performed according to the study protocol (unchanged after the start of the study) using SAS version 9.2.
Results from a prior study provided the basis for the sample size, which was calculated to show a significant difference in both primary efficacy end points with a power of 90%.8 To allow for dropouts, 480 participants in each treatment group were to be randomized for a total of 960 participants.
Rosacea is a common dermatologic disorder that generally is characterized by erythema as well as papules and pustules on the cheeks, chin, forehead, and nose. Moreover, telangiectasia and burning or stinging sensations often occur.1,2 These clinical manifestations and other related ones frequently lead to the perception of “sensitive skin.” Rosacea patients often experience low self-esteem, anxiety, and social embarrassment.3 Reports of the gender distribution of the disease vary but often show female predominance.4 Although it also occurs in darker skin types, rosacea is more common in individuals with lighter skin.1
The etiology of rosacea is not yet fully understood, but the underlying pathology has been attributed to dysregulated immune responses. Although the flares of a typical fluctuating disease course often are caused by exogenous triggers, there is evidence that an underlying genetic component predisposes some individuals to pathologic changes associated with the condition.5 Augmented immune activity and proinflammatory signaling appear to induce the infiltration of inflammatory elements into affected areas.2 These regions show dilated vasculature and increased cutaneous blood flow secondary to inflammation. Systemic oxidative stress also may contribute to epidermal dysfunction, as the antioxidant capacity of the skin in patients with rosacea is depleted relative to that of healthy individuals. The biochemical and vascular changes characteristic of rosacea coincide with aberrant permeability of the stratum corneum.6 The resulting decreased hydration and water loss across the skin contribute to the sensitivity and irritation typical of the disease.2
Current guidelines for the optimal management of rosacea with papulopustular lesions recommend skin care, photoprotection, and topical therapy. Depending on the severity of disease and the likelihood of adherence to a topical regimen, use of oral agents may be warranted.7
Azelaic acid (AzA), an unbranched saturated dicarboxylic acid (1,7-heptanedicarboxylic acid) that occurs in plants, is one of several US Food and Drug Administration–approved topical agents for the treatment of inflammatory lesions in rosacea.8 Although the pathophysiology of rosacea is not yet fully understood, there is a growing consensus about the role of proinflammatory molecules (eg, kallikrein 5, cathelicidins) as well as reactive oxygen species (ROS).9 Azelaic acid has been demonstrated to modulate the inflammatory response in normal human keratinocytes through several pathways, including modulation of the signaling pathways of peroxisome proliferator-activated receptor g and nuclear factor kB, concurrent with the observed inhibition of proinflammatory cytokine secretion.10 Additionally, AzA can inhibit the release of ROS from neutrophils and also may reduce ROS by direct scavenging effects.11 Further, AzA shows direct inhibition of kallikrein 5 in human keratinocytes as well as a reduction of the expression of kallikrein 5 and cathelicidin in murine skin and the facial skin of patients with rosacea.12
In a series of randomized trials in patients with papulopustular rosacea (PPR), AzA has shown clinical efficacy and safety as a topical treatment.13-15 Based on these studies, a gel formulation of AzA with a 15% concentration has been approved for treating inflammatory papules and pustules of mild to moderate rosacea.16
Although AzA delivered in a gel matrix is an effective therapy, topical delivery of active pharmaceutical ingredients via foam is often preferred over traditional vehicles in patients with sensitive skin. Patient rationale for favoring foam includes improved appearance and ease of application, namely easier to spread with a reduced need to manipulate inflamed skin.17 Also, data reveal that patients may be more compliant with a treatment that meets their needs such as an optimized foam formulation.18 In addition, the lipid components of an optimized formulation are thought to contribute to an improved skin condition.19 The foam vehicle used in this study is a proprietary oil-in-water formulation that includes fatty alcohols and triglycerides. The novel delivery of AzA in a foam formulation will provide clinicians and patients with a new option for improved individualized care.
We report the primary results of a phase 3 study in patients with PPR comparing the efficacy and safety of twice-daily AzA foam 15% with vehicle foam. The phase 3 study builds on the results of a prior randomized double-blind trial (N=401) that demonstrated significant improvements relative to vehicle in therapeutic success rate (P=.017) and decreased inflammatory lesion count (ILC)(P<.001) among patients treated with AzA foam 15%.8
Methods
Study Design
This phase 3 randomized, double-blind, vehicle-controlled, parallel-group, multicenter study was conducted in patients with PPR according to Good Clinical Practice guidelines in 48 study centers in the United States. The objective was to evaluate a 12-week, twice-daily (morning and evening) course of AzA foam 15% versus vehicle.
Participants were men and women aged 18 years or older with moderate to severe PPR (as determined by investigator global assessment [IGA]) presenting with 12 to 50 papules and/or pustules and persistent erythema with or without telangiectasia. Informed consent was obtained from all participants before any study-related activities were carried out.
The study products were applied to the entire facial area each morning and evening at a dose of 0.5 g, thus administering 150 mg of AzA daily in the active arm of the trial (computerized randomization 1:1). The treatment period lasted 12 weeks, and participants were evaluated at baseline and weeks 4, 8, and 12. The follow-up period lasted 4 weeks following the end of treatment (EoT) and was concluded with one final end-of-study visit.
Efficacy Evaluations
There were 2 coprimary efficacy end points. Therapeutic success rate was evaluated using the IGA scale (clear, minimal, mild, moderate, or severe). Treatment success was defined as an IGA score of either clear or minimal (with at least a 2-step improvement) at EoT, whereas treatment failure was constituted by IGA scores of mild, moderate, or severe.
The second coprimary end point was the nominal change in ILC from baseline to EoT as determined by the total number of facial papules and pustules. Efficacy and safety parameters were evaluated at weeks 4, 8, and 12, as well as at the end of the 4-week follow-up period. Throughout the study, the investigator, participants, and all study personnel remained blinded.
Safety
Information about adverse events (AEs) was collected at each study visit, and AEs were graded according to seriousness (yes or no) and intensity (mild, moderate, or severe).
Statistical Analysis
Efficacy was confirmed by analysis of the treatment success rate at EoT with Cochran-Mantel-Haenszel test statistics, including a point estimate and 95% confidence interval (CI) for the odds ratio. Change in ILC at EoT was analyzed via an analysis of covariance model using treatment, center, and baseline lesion count as factors. (Additional methods can be found in the Appendix below.)
Results
Study Participants
Of the 1156 patients who were screened for eligibility, 961 were randomized to treatment with AzA foam (n=484) or vehicle (n=477)(Figure 1). Sixty-four (13.2%) participants in the AzA foam group and 79 (16.6%) in the vehicle group discontinued treatment before completing the study. The most common reasons for discontinuation were participant withdrawal from the study and lost to follow-up. Six (1.2%) participants from the AzA foam group and 12 (2.5%) from the vehicle group discontinued because of AEs. All safety and efficacy data presented are based on the full analysis set, which consisted of the 961 participants randomized to treatment.
Demographic and baseline characteristics were balanced between the treatment groups (Table 1). The majority of participants were female (73.0%) and white (95.5%), reflecting the patient populations of independent studies that found a higher prevalence of rosacea in women and lighter skin types.4 There were no significant differences in baseline measures of PPR severity between the treatment groups. Participants in the AzA foam and vehicle groups had a mean ILC of 21.7 and 21.2, respectively, and 76.4% of participants had more than 14 lesions. All participants had an IGA score of moderate (86.8%) or severe (13.2%). Moderate or severe erythema was present in 91.5% of participants.
Treatment compliance, as measured by the percentage of expected doses that were actually administered, was 97.1% in the AzA foam group and 95.9% in the vehicle group.
Efficacy
Results from both primary end points demonstrated superior efficacy of AzA foam over vehicle. The AzA foam group achieved a greater IGA success rate at EoT compared with the vehicle group (32.0% vs 23.5%; Cochran-Mantel-Haenszel test center-adjusted P<.001; odds ratio, 1.6; 95% CI, 1.2-2.2). Treatment success rate was higher in the AzA foam group than in the vehicle group at every time point past baseline (Figure 2). Similarly, the decrease in mean nominal ILC values was greater in the AzA foam group at every time point after baseline (Figure 3), and the treatment difference at EoT was statistically significant in favor of AzA foam (-2.7, F1,920=23.7, P<.001; 95% CI, -3.8 to -1.6). The divergence between treatment groups at week 4 reveals an onset of AzA effect early in the study.

|

|
Although the AzA foam group showed significantly better efficacy results than the vehicle group for the coprimary end points, participants in the vehicle group did show appreciable IGA success rates (23.5%) and changes in ILC (-10.3) at EoT (Figures 2 and 3).
Notably, the AzA foam group maintained better results than vehicle for both primary end points even at the end of the 4-week follow-up after EoT (Figures 2 and 3). Sensitivity analysis (data not shown) confirmed the findings from the full analysis set.
Safety
Adverse events were experienced by 149 (30.8%) participants in the AzA foam group and 119 (24.9%) in the vehicle group. The most common noncutaneous AEs (>1% of participants) reported during AzA foam treatment were nasopharyngitis, headache, upper respiratory tract infection, and influenza. In the vehicle group, the most common noncutaneous AEs reported were nasopharyngitis and headache. Drug-related AEs (relationship assessed by the investigator) were reported slightly more often in the AzA foam group (7.6%) than in the vehicle group (4.6%). Drug-related AEs were predominantly cutaneous and occurred at the site of application (Table 2). Drug-related cutaneous AEs were more common in the AzA foam group (7.0%) than in the vehicle group (4.4%). Although serious AEs were more common in the vehicle group, all were regarded as unrelated to the study medication. A single death occurred in the vehicle group due to an accident unrelated to the study drug.
The most frequent drug-related AEs in participants treated with AzA foam versus vehicle were application-site pain (3.5% vs 1.3%), application-site pruritus (1.4% vs 0.4%), and application-site dryness (1.0% vs 0.6%). The classical rosacea symptom of stinging is subsumed under the term application-site pain, according to MedDRA (Medical Dictionary for Regulatory Activities).
All other drug-related AEs occurred at a frequency of less than 1% in participants from both groups. Serious AEs were rare and unrelated to treatment, with 3 AEs reported in the AzA foam group and 4 in the vehicle group. Adverse events leading to study drug withdrawal occurred in less than 2% of participants and were more common in the vehicle group (2.5%) than in the AzA foam group (1.2%). Of the 3 drug-related AEs leading to withdrawal in the AzA foam group, 2 were due to cutaneous reaction and 1 was due to a burning sensation. The number of active drug-related cutaneous AEs was highest during the first 4 weeks of treatment and declined over the course of the study (eFigure).
More than 96% of AEs were resolved by the end of the study. Of the participants experiencing AEs that did not resolve during the course of the study, 16 were in the AzA foam group and 10 in the vehicle group. Six unresolved AEs were drug related, with 3 occurring in each treatment group. Unresolved drug-related cutaneous AEs in the AzA foam group were pain, pruritus, and dryness at the application site.
Comment
Overall, the results from this phase 3 trial demonstrate that the new foam formulation of AzA was efficacious and safe in a 12-week, twice-daily course of treatment for moderate to severe PPR. The AzA foam formulation was significantly superior to vehicle (P<.001) for both primary efficacy end points. Participants in the AzA foam group achieved therapeutic success at a higher rate than the vehicle group, and the change in nominal ILC at EoT was significantly greater for participants treated with AzA foam than for those treated with vehicle (P<.001). Differences between the 2 treatment groups for the coprimary end point measures arose early in the study, demonstrating that symptoms were rapidly controlled. Between weeks 8 and 12 (EoT), the rate of increase of beneficial effects in the AzA foam group remained high, while the vehicle group showed a notable slowing. There was no indication of any rebound effect in overall disease severity subsequent to EoT. After 4 weeks of follow-up, there was still a beneficial treatment effect present in favor of the AzA foam group, as indicated by the persistence of improvements in both coprimary end point measures throughout the follow-up period.
Analyses of alternative populations and secondary end points (data not shown) supported the efficacy results reported here. There was no indication of irregular study center effects, and the sensitivity analyses demonstrated robustness of the data for the observed treatment effects.
The use of vehicle foam alone appeared to be beneficial in reducing ILC and improving IGA rating, which suggests that the properties of the new foam formulation are favorable for the inflamed lesional skin of rosacea. Of note, other dermatology studies, including trials in rosacea, have reported therapeutic effects of vehicle treatment that may be attributable to the positive effects of skin care with certain formulations.20
Azelaic acid foam was well tolerated in the current study. More than 93% of AEs in either treatment group were of mild or moderate severity. The incidence of drug-related AEs was low in both groups and mainly occurred at the application site. There were no drug-related severe or serious AEs. The low incidence of reported drug-related noncutaneous AEs in the AzA foam group (dysgeusia in 1 patient and headache in 2 patients) supports the known favorable systemic tolerance profile of AzA.
Although most drug-related AEs occurred at the application site, they were generally transient, with the majority of events in the AzA foam group lasting no more than 1 hour. Most cutaneous AEs developed early in the study. In the AzA foam group, the prevalence of drug-related cutaneous AEs dropped at every time interval as the study progressed (eFigure). Very few AEs of any type persisted through the end of the study. These safety results were accompanied by a high compliance rate and a high participation rate throughout the course of the study. Taken together, the available data for this AzA foam formulation support a favorable tolerability profile. The results of this study are consistent with and expand on data from an earlier investigation of similar design.8
Conclusion
The development of an AzA foam formulation with higher lipid content was intended to expand the treatment options available to physicians and patients who are managing rosacea. Most topical dermatologic treatments are currently delivered in classical formulations such as creams or gels, but patients who use topical therapies have rated messiness and ease of application among the most important characteristics affecting quality of life.17,21 Foam formulations may offer improvements in this regard; ease of application may minimize unnecessary manipulation of inflamed skin and contribute to a high level of user satisfaction.22 However, the design of the current study was limited to evaluating only the AzA foam formulation versus a foam vehicle, and direct comparisons of clinical efficacy and tolerability to other AzA topical preparations were not performed. Nonetheless, patients have previously reported that they would be more likely to comply with a recommended course of dermatologic foam therapy than other topical formulations.18 The proposed foam formulation was designed to attend to the specific needs of the dry and sensitive skin in rosacea by combining the demonstrated efficacy properties exhibited by AzA gel 15% with the good tolerability and acceptability of a lipid-containing foam formulation. Development of this formulation was targeted to obtain a product that would be highly spreadable, dry quickly, and be easy to apply. The available data for this AzA foam formulation support the value of this option in the topical treatment of rosacea. The success in reduction of overall disease severity, lack of any rebound after EoT, and the observed tolerability and high adherence rates suggest that this novel formulation is a useful addition to current treatment options for rosacea.
Addendum
After release of the study data for unblinding and statistical evaluation, the following inconsistency regarding patient distribution was noted: 1 participant was incorrectly evaluated as part of the AzA foam analysis group when in fact this patient was randomized to vehicle and was treated throughout the study with vehicle. This participant did not experience any AE and did not show any IGA improvement at the EoT. As this single case did not have an impact on the statistical conclusions or interpretation of the results, the released study data have not been changed. This deviation was described as a database erratum in the study report.
Acknowledgement—Editorial support through inVentiv Medical Communications, New York, New York, was provided by Bayer HealthCare Pharmaceuticals Inc.
APPENDIX
Supplementary Methods
Supplementary Study Design
This study met all local legal and regulatory requirements and was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Before the start of the study and implementation, the protocol and all amendments were approved by the appropriate independent ethics committee or institutional review board at each study site. Two protocol amendments were implemented before the first participant visit.
Exclusion criteria included the presence of dermatoses that could interfere with rosacea diagnosis or evaluation, facial laser surgery or topical use of any medication to treat rosacea within 6 weeks before randomization, systemic use of any medications to treat rosacea, and known unresponsiveness to AzA treatment. Further standard exclusion criteria included alcohol or drug use or parallel participation in other clinical studies, which were necessary to exclude undue influence on study evaluations and/or participant safety. The study was conducted by qualified investigators at 48 centers in the United States.
The investigational product was filled in identical containers according to the randomization list generated by a computer program using blocks. Complete blocks of study medication were distributed to the centers. Eligible participants were randomized 1:1 into either AzA foam or vehicle treatment groups by assignment of a randomization number at baseline. A blind investigational product under the same randomization number was dispensed to and returned from participants by study personnel who were not involved in the assessments. Blinding was achieved by using labels on the investigational products that did not allow identification of the true medication.
Compliance was evaluated from participant diaries as well as the number of expected doses and actually applied doses.
Additional Efficacy Evaluations
A number of secondary variables (not reported here) were assessed, including changes in other manifestations of PPR, as well as participant assessments of treatment response, tolerability, cosmetic preferences, and quality of life.
Additional Safety
Investigators reported a yes or no response as to whether there was a reasonable causal relationship between AEs and treatment. Moreover, AEs that began at the start of or during treatment were considered treatment emergent. Cutaneous AEs were further assessed regarding location and duration. An AE was deemed local if it occurred at the application site and transient if it subsided within 60 minutes of onset.
Statistical Analysis
The primary efficacy analyses presented here were based on the full analysis set of participants who were randomized and had medication dispensed. For participants with no EoT value, the last nonmissing value was used including baseline (last-observation-carried-forward methodology). Participants who discontinued treatment prematurely because of lack of efficacy were considered to be treatment failures, regardless of the reported IGA score. Statistical significance was needed for both coprimary efficacy variables at a 1-sided 2.5% significance level to show confirmed superiority of AzA foam versus vehicle.
A number of sensitivity analyses were performed, including an analysis of the coprimary end points using observed data, analysis of the per-protocol population of participants who did not prematurely discontinue treatment and had no major protocol deviations, subgroup analyses, and the use of statistical methods to investigate the effect of missing observations. Analyses of success rate and nominal change in ILC were repeated for each postbaseline visit using χ² and t tests, respectively. All summary and statistical analyses were performed according to the study protocol (unchanged after the start of the study) using SAS version 9.2.
Results from a prior study provided the basis for the sample size, which was calculated to show a significant difference in both primary efficacy end points with a power of 90%.8 To allow for dropouts, 480 participants in each treatment group were to be randomized for a total of 960 participants.
1. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
2. Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
3. Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6:348-354.
4. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
5. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
6. Wollina U. Recent advances in the understanding and management of rosacea. F1000Prime Rep. 2014;6:50.
7. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis. 2014;93:134-138.
8. Draelos ZD, Elewski B, Staedtler G, et al. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92:306-317.
9. Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
10. Mastrofrancesco A, Ottaviani M, Aspite N, et al. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARg activation. Exp Dermatol. 2010;19:813-820.
11. Akamatsu H, Komura J, Asada Y, et al. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch Dermatol Res. 1991;283:162-166.
12. Coda AB, Hata T, Miller J, et al. Cathelicidin, kalli-krein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol. 2013;69:570-577.
13. van Zuuren EJ, Kramer SF, Carter BR, et al. Effective and evidence-based management strategies for rosacea: summary of a Cochrane systematic review. Br J Dermatol. 2011;165:760-781.
14. Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48:836-845.
15. Thiboutot DM, Fleischer AB Jr, Del Rosso JQ, et al. Azelaic acid 15% gel once daily versus twice daily in papulopustular rosacea. J Drugs Dermatol. 2008;7:541-546.
16. Finacea [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2015.
17. Zhao Y, Jones SA, Brown MB. Dynamic foams in topical drug delivery. J Pharm Pharmacol. 2010;62:678-684.
18. Gottlieb AB, Ford RO, Spellman MC. The efficacy and tolerability of clobetasol propionate foam 0.05% in the treatment of mild to moderate plaque-type psoriasis of nonscalp regions. J Cutan Med Surg. 2003;7:185-192.
19. Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771-788.
20. Jackson JM, Pelle M. Topical rosacea therapy: the importance of vehicles for efficacy, tolerability and compliance. J Drugs Dermatol. 2011;10:627-633.
21. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
22. Kircik LH, Bikowski JB. Vehicles matter: topical foam formulations. Practical Dermatology. January 2012(suppl):3-18.
1. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
2. Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
3. Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6:348-354.
4. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
5. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
6. Wollina U. Recent advances in the understanding and management of rosacea. F1000Prime Rep. 2014;6:50.
7. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis. 2014;93:134-138.
8. Draelos ZD, Elewski B, Staedtler G, et al. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92:306-317.
9. Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
10. Mastrofrancesco A, Ottaviani M, Aspite N, et al. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARg activation. Exp Dermatol. 2010;19:813-820.
11. Akamatsu H, Komura J, Asada Y, et al. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch Dermatol Res. 1991;283:162-166.
12. Coda AB, Hata T, Miller J, et al. Cathelicidin, kalli-krein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol. 2013;69:570-577.
13. van Zuuren EJ, Kramer SF, Carter BR, et al. Effective and evidence-based management strategies for rosacea: summary of a Cochrane systematic review. Br J Dermatol. 2011;165:760-781.
14. Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48:836-845.
15. Thiboutot DM, Fleischer AB Jr, Del Rosso JQ, et al. Azelaic acid 15% gel once daily versus twice daily in papulopustular rosacea. J Drugs Dermatol. 2008;7:541-546.
16. Finacea [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2015.
17. Zhao Y, Jones SA, Brown MB. Dynamic foams in topical drug delivery. J Pharm Pharmacol. 2010;62:678-684.
18. Gottlieb AB, Ford RO, Spellman MC. The efficacy and tolerability of clobetasol propionate foam 0.05% in the treatment of mild to moderate plaque-type psoriasis of nonscalp regions. J Cutan Med Surg. 2003;7:185-192.
19. Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771-788.
20. Jackson JM, Pelle M. Topical rosacea therapy: the importance of vehicles for efficacy, tolerability and compliance. J Drugs Dermatol. 2011;10:627-633.
21. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
22. Kircik LH, Bikowski JB. Vehicles matter: topical foam formulations. Practical Dermatology. January 2012(suppl):3-18.
Therapies to Improve the Cosmetic Symptoms of Rosacea
Rosacea is a commonly encountered chronic inflammatory skin disease that affects an estimated 16 million Americans and exhibits a particular predilection for the convexities of the central face (eg, forehead, cheeks, nose, chin).1,2 The pathophysiology of rosacea remains poorly understood despite the relatively high prevalence of the disease and substantial ongoing research.3 The current paradigm suggests a complex multifactorial interplay involving aberrations of the innate and adaptive immune system, neurovascular dysregulation, blood and lymphatic vessel changes, genetic predispositions, and overgrowth of commensal organisms such as Demodex.3 Additionally, a variety of external factors may exacerbate clinical symptoms (eg, UV radiation, heat exposure, spicy food, alcohol, stress).
The diagnosis of rosacea is made clinically and rarely requires histologic confirmation. Although rosacea can present with a wide range of clinical features that often wax and wane over time, a near universal finding is diffuse centrofacial erythema.4 This centrofacial redness may symptomatically worsen during a flare period, causing flushing, but it often persists nontransiently between flares as background erythema. Other variable findings of rosacea include the presence of telangiectases, edema, plaques, phymatous changes, dry skin, ocular manifestations, and inflammatory lesions in the form of papules and pustules.5 Patients also may report a stinging or burning sensation in affected areas. It is important to note that most patients will only exhibit some of these clinical features and that symptoms often vary in the timing of their emergence or regression.5 A classification system has been developed for rosacea that categorizes the disease into 4 subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) and one variant (granulomatous).6 These categories are determined by the grouping of clinical features present, but it is not uncommon for patients to exhibit clinical manifestations of more than 1 subtype.7
The detrimental cosmetic effects of rosacea are obvious given its chronic nature and tendency to affect highly visible areas such as the face. As such, rosacea can have a devastating impact on patients’ quality of life.8 Patients with rosacea have been reported to have higher incidence rates of low self-esteem, embarrassment, social anxiety, and depression as compared to the rest of the population. Effective treatment, however, can improve cosmetic appearance and mitigate the negative psychosocial impacts of the disease.8
Treatment of rosacea focuses on relieving cosmetic symptoms, as no curative therapy currently exists. Treatment comes in a wide variety of forms, including topical medications, systemic pharmacologic therapies, light-based modalities, and procedural interventions. Choice of therapy should be determined on a case-by-case basis as guided by the clinical features present, and combination or sequential therapies often are required to achieve optimal cosmetic results. In this article, we review both existing and emerging treatments of rosacea and assess their ability to improve the cosmetic symptoms of rosacea (Table).
Skin Care
Proper skin care is an important aspect of treatment for all patients with rosacea and thus includes the use of over-the-counter cleansers, moisturizers, and sunscreens.9 The choice of skin care products is an important consideration given the often hypersensitive skin of rosacea patients. Moisturizers and cleansers should have an acidic to neutral pH, similar to normal skin. They should not contain emulsifiers that strip moisture from the skin or protective lipids and proteins from the stratum corneum.10 Moisturizers without irritants, abrasives, or allergens should be used following skin cleansing. Protection from UV radiation with sunscreen, ideally with a sun protection factor greater than 30, is particularly important, as it can prevent UV-induced rosacea flares as well as photodamage that can cause additional erythema and telangiectasia.4 Rosacea patients also may find green-tinted makeup to be useful in concealing areas of erythema.8
Topical Therapy
Currently, there are only 5 US Food and Drug Administration (FDA)–approved topical medications for the treatment of rosacea: metronidazole (MTZ) gel 0.75% and 1%, azelaic acid (AzA) gel 15%, sodium sulfacetamide (SS) 10%–sulfur 5% lotion and cream, brimonidine tartrate (BT) gel 0.5%, and the most recently approved ivermectin (IVM) cream 1%.7 Metronidazole, AzA, and SS primarily are used to treat the inflammatory papules and pustules of rosacea, while BT is used to treat persistent background erythema. The exact mechanisms of action by which MTZ, AzA, and SS treat rosacea are unclear, but they are thought to reduce inflammation and/or immune response. Metronidazole and AzA both have demonstrated favorable safety profiles and significant (P<.05) efficacy over vehicle in reducing inflammatory lesions in numerous well-controlled randomized clinical studies.4,11,12 There is some evidence that AzA may be more effective than MTZ; one 15-week multicenter, double-blind, randomized, parallel-group study demonstrated that twice-daily AzA gel 15% showed significant superiority (P=.02) over twice-daily MTZ gel 0.75% in improving the inflammatory lesions and erythema of rosacea.13 Sodium sulfacetamide also has shown good efficacy in the treatment of inflammatory lesions and performed significantly better (P=.04) than MTZ according to one multicenter, investigator-blinded, randomized, parallel-group study,14 but the overall evidence is not as strong as MTZ and AzA.4,11,15 The most common adverse effect for MTZ, AzA, and SS is application-site irritation, but overall most patients report good tolerance to these topical medications.4 Azelaic acid is unique in that patients may report stinging, tingling, or burning after application, but these effects are not associated with visible skin changes and usually are transient, generally remitting after 1 to 2 weeks.4
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist whose mechanism of action in the treatment of rosacea is thought to involve vasoconstriction of superficial skin vasculature and to a lesser extent anti-inflammatory effects.16 In a double-blind, randomized, vehicle-controlled phase 3 trial, application of BT gel 0.5% once daily for 4 weeks demonstrated significant efficacy over vehicle (P<.001) in treating persistent nontransient facial erythema in 553 adult patients with 2 or fewer papulopustular lesions as evaluated over 12 hours on days 1, 15, and 29.17 Notably, a substantial difference in cosmetic appearance was observed in another study as early as 30 minutes after the first gel application on day 1.18 The results of this phase 3 trial17 mirrored those of the phase 2 dose-optimization and safety studies of similar design.18 In addition to another long-term, 1-year, open-label study,19 both phase 2 and 3 studies have shown favorable safety profiles with no reports of tachyphylaxis, rebound erythema, or aggravation of other disease features such as telangiectases or inflammatory lesions.17,18 Recently, however, there have been some reports of considerable rebound erythema with BT use and thus patients should be made aware of this possibility.20,21 Case reports of successful treatment of background erythema and flushing with other topically applied adrenergic receptor modifiers such as oxymetazoline and xylometazoline have been published in the literature,22,23 but additional research will be necessary to validate these claims.
Ivermectin, a decades-old antiparasitic, has recently shown promising results as a treatment of rosacea patients with moderate to severe papulopustular lesions. Its therapeutic effect is believed to be mediated by its activity against Demodex, a natural skin mite that has been found at increased concentrations in a subset of patients with rosacea, as well as by its natural anti-inflammatory properties.24 In 2 identically designed, randomized, double-blind, controlled trials of IVM cream 1% applied once daily for 12 weeks, a significantly larger proportion of patients in the IVM groups achieved an investigator global assessment of clear or almost clear as compared to vehicle (IVM: 38.4% and 40.1%, respectively; vehicle: 11.6% and 18.8%, respectively; P<.001). Both trials also demonstrated that IVM was significantly superior to vehicle in the reduction of inflammatory lesion counts measured at week 12 as compared to baseline (IVM: 76.0% and 75.0%, respectively; vehicle: 50.0% and 50.0%, respectively; P<.001).24 An extension of these original trials demonstrated long-term safety with up to 52 weeks of topical IVM use and reported a low incidence rate of adverse effects, most commonly transient skin burning, pruritus, and dryness. Notably, the incidence rate of these adverse effects was lower than a comparison group receiving AzA gel 15% once daily.25 Once-daily application of IVM cream 1% also has recently demonstrated superiority over twice-daily MTZ cream 0.75% for 16 weeks in a phase 3 investigator-blinded, randomized, parallel-group study. The IVM group was significantly superior to MTZ in the reduction of inflammatory lesions as compared to baseline (83.0% vs 73.7%) and in the number of participants who achieved an investigator global assessment score of clear or almost clear (84.9% vs 75.4%)(both P<.001).26 There also is limited evidence for the use of other antiparasitic topical medications such as crotamiton 10% and permethrin 5%, but such agents frequently cause irritation and may not be well tolerated in rosacea patients.27-29
There are a variety of other non–FDA-approved topical medications that have been used with varying success in the literature, including cyclosporine, macrolides, benzoyl peroxide, retinoids, and calcineurin inhibitors such as tacrolimus and pimecrolimus. Evidence for the use of these medications generally is limited to a few studies with small numbers of patients and will not be discussed further in this article.4,11,30 These agents, however, may be useful in select cases when first-line regimens have failed and also may be good targets for future research.
Systemic Therapy
The mainstay of systemic treatment of rosacea centers around the tetracyclines, a group of antibiotics that have been used off label for rosacea since the 1950s.31 The therapeutic effects of tetracyclines in the treatment of rosacea are thought to revolve around their anti-inflammatory effects rather than their antibacterial properties.32 Currently, the only FDA-approved oral agent for treatment of the inflammatory lesions of rosacea is doxycycline 40-mg modified-release capsules taken once daily. These modified capsules allow for instant release of 30 mg and delayed release of 10 mg of doxycycline. This dosing is considered to be anti-inflammatory rather than antimicrobial, as it does not produce antibiotic selection pressure even with prolonged use.33 Efficacy of 40-mg subantimicrobial-dose doxycycline (SDD) has been demonstrated in 2 phase 3 multicenter, parallel-group, randomized, double-blind, placebo-controlled studies in which SDD demonstrated a significantly greater reduction in the number of total inflammatory lesions at week 16 compared to placebo (P<.001).34 Subantimicrobial-dose doxycycline also has been shown to be equally as efficacious in reducing inflammatory lesions as traditional-dose doxycycline.35 There also is some evidence for the efficacy of SDD in reducing overall erythema, as demonstrated by one open-label, community-based study in which SDD monotherapy resulted in clinician erythema assessment scores of mild or no erythema in 75% of patients with mild to severe rosacea at baseline after 12 weeks of therapy.35 Additionally, SDD is considered to be safe and well-tolerated and does not generally result in the adverse effects that may be seen in antibiotic-level doses of doxycycline (eg, gastrointestinal upset, vaginal candidiasis, photosensitivity).34,36,37 Other antibiotics such as clarithromycin, azithromycin, and MTZ also have been studied as treatments of papulopustular rosacea at antibiotic-level doses with good therapeutic effect.38-40 These therapies, however, generally are not used unless there are contraindications for use of tetracycline antibiotics, such as pregnancy or allergy, as the overall evidence is not as strong and there may be increased risks for serious adverse effects.30
Although it is not FDA approved, isotretinoin is an important therapeutic option for select rosacea patients, as it is the only pharmacologic agent that has shown efficacy for the phymatous changes of rosacea. Its efficacy, however, is limited to early-stage rhinophyma that has not yet progressed to the fibrotic or mucinous stages of disease in which it has been shown to reduce the size and number of cutaneous sebaceous glands.30,41 Isotretinoin at 0.3 mg/kg daily also has shown noninferiority in treatment of the inflammatory papules and pustules of rosacea as compared to antibiotic dosing of doxycycline in one large-scale, placebo-controlled, randomized, 12-week multicenter study.42 Unfortunately, recurrence is highly likely after isotretinoin therapy is discontinued.30,41 However, continuous “microdose” isotretinoin at 0.03 to 0.17 mg/kg daily has shown evidence for efficacy in treatment of recalcitrant papulopustular disease.43 Such dosing may have the added benefit of reduced risk for radiographic changes associated with long-term isotretinoin use.43
Light-Based Therapy
Light-based modalities are an important tool set in the management of rosacea symptoms, as they can treat telangiectases for which medical therapy is not generally effective.9 To a lesser extent, light-based modalities also can help alleviate background erythema. The most commonly used light-based modalities include the pulsed dye laser (PDL)(Figure), potassium titanyl phosphate (KTP) laser, Nd:YAG laser, intense pulsed light, photodynamic therapy, CO2 laser, and erbium-doped YAG (Er:YAG) laser. These treatments produce clinical results by targeting specific chromophores such as oxyhemoglobin, deoxyhemoglobin, methemoglobin, and clotted blood with light of specific wavelengths to induce thermolysis of vasculature while sparing collateral tissue.44 Generally, larger telangiectatic vessels are more amenable to therapy than smaller vessels, which usually require higher energy to be delivered in a shorter period of time, thus predisposing the patient to the development of purpura that may last for 1 to 2 weeks.44
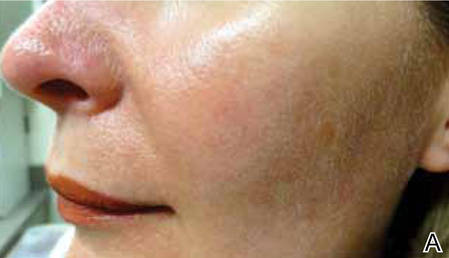
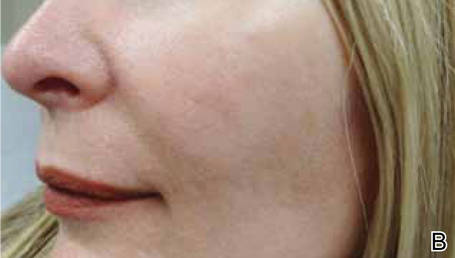
|
Historically, PDL used a light wavelength of 577 nm and was classically associated with posttherapy purpura; however, modern PDLs use wavelengths of 585 or 595 nm and are associated with a reduced risk for purpura through the use of longer pulse durations (ie, 10–40 millisecond), multiple minipulses, multiple passes, and advanced epidermal cooling methods.9,44 In a small, prospective, randomized, controlled, nonblinded study, PDL therapy with fluence sufficiently high enough to induce purpura achieved an approximate 50% improvement in telangiectasia grading scores in most patients after a single treatment.45 Notably, PDL therapy at purpura-inducing settings was reported to be much more efficacious than settings that did not induce purpura (purpura free), especially in the treatment of thicker telangiectases.45
Potassium titanyl phosphate lasers make use of shorter wavelengths (532 nm) than PDL and thus are better able to target superficial vasculature, which translates into a reduced risk for purpura and faster healing times. However, KTP laser therapy typically is only reserved for patients with lighter skin types, as this wavelength of light is more likely to result in higher melanin absorption and possible postinflammatory hyperpigmentation.44 A split-face study comparing the KTP laser with PDL determined that the KTP laser was able to achieve 62% clearing after the first treatment and 85% clearance after the third treatment versus 49% and 75% for PDL treatment, respectively; however, the KTP laser had higher rates of posttherapy erythema lasting at least 1 day (58% vs 8%).46
Conversely, the Nd:YAG laser uses longer wavelengths (1064 nm) and can achieve deeper skin penetration, which may be effective for larger, recalcitrant, or deeper blue-tinted vessels. A split-face, double-blind, randomized, controlled trial found Nd:YAG laser therapy to be an effective treatment of facial erythema, though it was observed to be less effective than purpura-free PDL therapy in reducing redness after 4 treatments (34% vs 52% improvement, respectively); however, treatment with the Nd:YAG laser was found to be significantly (P=.0028) less painful.47
Intense pulsed light is unique from the previously discussed light-based therapies in that it uses noncoherent light with wavelengths between 500 and 1200 nm. Cutoff filters may be used to allow for more selective tissue damage depending on the depth of penetration desired. Intense pulsed light has been shown to be equally as efficacious as purpura-free PDL therapy in the treatment of erythema and telangiectasia in a randomized, controlled, single-blind, split-face trial.48 Additionally, a study of 200 patients with facial vascular lesions, of whom 74 patients had rosacea, showed that intense pulsed light therapy resulted in a 75% to 100% improvement of lesions in 174 of 188 (92.5%) patients who returned for follow-up. Treatment often required at least 2 sessions, but overall adverse effects were reported to be minimal.49
Photodynamic therapy is a well-studied and often utilized treatment of a variety of skin conditions, but there have only been a few studies regarding its use in rosacea. Photodynamic therapy involves the use of topically applied photosensitizing agents such as 5-aminolevulinic acid or methyl aminolevulinate before exposure to red or blue light. This process generates reactive oxygen species, though the exact mechanism of action through which patients achieve cosmetic improvement in rosacea is unclear. In one study of 17 patients with varying rosacea subtypes treated with methyl aminolevulinate and red light, drastic relief of symptoms was seen in 10 (58.8%) patients, marked improvement in 4 (23.5%) patients, and no response in 3 (17.6%) patients. Most patients report a transient irritant skin reaction at the site of therapy.50
Ablative lasers such as the CO2 (10,600 nm) and Er:YAG (2940 nm) lasers also have been shown to be useful in the treatment of rosacea, specifically for the management of rhinophymatous features. Excellent results have been achieved with these lasers given their ability to provide near-bloodless surgical fields. In a 13-year review of 124 patients with rhinophyma receiving a single CO2 laser treatment, good to excellent results were achieved in 118 (95.2%) of patients when evaluated at 3 months posttreatment.51 Patient satisfaction also is reported to be high with few adverse effects reported. The evidence for the Er:YAG laser is not as strong, but the current reports indicate efficacy and safety similar to that of the CO2 laser.52
Procedural Therapies
Procedural therapies in rosacea generally are reserved for management of rhinophyma and include electrocautery, cryotherapy, radiotherapy, dermabrasion, scalpel excisions, flap reconstruction, and skin grafts.30,53 The details and evidence for these methods is beyond the scope of this paper, but it is important to be aware of such modalities. As with most surgical procedures, operator skill and experience may affect treatment outcomes, and there also are definite risks for postprocedural scarring, swelling, erythema, and pigmentation changes. Recently, anecdotal evidence has shown that botulinum toxin injections may be effective for patients with refractory flushing and erythema, but larger studies will be necessary to better assess these claims.54,55
Conclusion
Although recent advances in pharmacology and laser technology have provided physicians with new and effective treatment modalities for rosacea, it remains a poorly understood disease without a definitive cure. The negative impact of rosacea on patients’ quality of life can be substantial, but effective management of cosmetic symptoms can minimize such deleterious effects. Therapy should be individualized and directed at treating the symptoms that are most bothersome to the patient. Additionally, effective treatment often will require a combination of modalities or sequential therapies to achieve optimal cosmetic outcomes.
1. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
2. Rosacea prevalence map. National Rosacea Society Web site. http://rosacea.org/press/prevalencemap. Accessed June 16, 2015.
3. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
4. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
5. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
6. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
7. Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20:623-629.
8. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
9. Mansouri Y, Goldenberg G. Devices and topical agents for rosacea management. Cutis. 2014;94:21-25.
10. Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
11. van Zuuren EJ, Kramer S, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2011;3:CD003262.
12. Liu RH, Smith MK, Basta SA, et al. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006;142:1047-1052.
13. Elewski B, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
14. Torok HM, Webster G, Dunlap FE, et al. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis. 2005;75:357-363.
15. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
16. Piwnica D, Rosignoli C, de Menonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective alpha-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75:49-54.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment of moderate to severe facial erythema of rosacea: results of two multicenter, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
19. Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13:56-61.
20. Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:e37-e38.
21. Ilkovitch D, Pomerantz RG. Brimonidine effective but may lead to significant rebound erythema. J Am Acad Dermatol. 2014;70:e109-e110.
22. Kim JH, Oh YS, Ji JH, et al. Rosacea (erythematotelangiectatic type) effectively improved by topical xylometazoline. J Dermatol. 2011;38:510-513.
23. Shanler SD, Ondo AL. Successful treatment of erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor antagonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
24. Stein-Gold L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13:316-323.
25. Stein-Gold L, Kircik L, Fowler J, et al. Long-term safety of ivermectin 1% cream vs azelaic acid 15% gel in treating inflammatory lesions of rosacea: results of two 40-week controlled, investigator-blinded trials. J Drugs Dermatol. 2014;13:1380-1386.
26. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103-1110.
27. Koçak M, Ya˘gli S, Vahapo˘glu G, et al. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. a randomized double-blind placebo-controlled study. Dermatology (Basel). 2002;205:265-270.
28. Bikowski JB, Del Rosso JQ. Demodex dermatitis: a retrospective analysis of clinical diagnosis and successful treatment with topical crotamiton. J Clin Aesthet Dermatol. 2009;2:20-25.
29. Layton A, Thiboutot D. Emerging therapies in rosacea. J Am Acad Dermatol. 2013;69(6 suppl 1):S57-S65.
30. Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512, quiz 513-514.
31. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
32. Korting HC, Schöllmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22:287-294.
33. Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472-1483.
34. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
35. Webster GF. An open-label, community-based, 12-week assessment of the effectiveness and safety of monotherapy with doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads). Cutis. 2010;86(suppl 5):7-15.
36. Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
37. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93:18-28.
38. Torresani C. Clarithromycin: a new perspective in rosacea treatment. Int J Dermatol. 1998;37:347-349.
39. Bakar O, Demircay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154.
40. Saihan EM, Burton JL. A double-blind trial of metronidazole versus oxytetracycline therapy for rosacea. Br J Dermatol. 1980;102:443-445.
41. Park H, Del Rosso JQ. Use of oral isotretinoin in the management of rosacea. J Clin Aesthet Dermatol. 2011;4:54-61.
42. Gollnick H, Blume-Peytavi U, Szabo EL, et al. Systemic isotretinoin in the treatment of rosacea—doxycycline-and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010;8:505-515.
43. Hofer T. Continuous “microdose” isotretinoin in adult recalcitrant rosacea. Clin Exp Dermatol. 2004;29:204-205.
44. Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
45. Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
46. Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
47. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
48. Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35:920-928.
49. Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
50. Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
51. Maden V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161:814-818.
52. Fincher EF, Gladstone HB. Use of a dual-mode erbium:YAG laser for the surgical correction of rhinophyma. Arch Facial Plast Surg. 2004;6:267-271.
53. Lloyd KM. Surgical correction of rhinophyma. Arch Dermatol. 1990;126:721-723.
54. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012;11:e76-e79.
55. Park KY, Hyun MY, Jeong SY, et al. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230:299-301.
Rosacea is a commonly encountered chronic inflammatory skin disease that affects an estimated 16 million Americans and exhibits a particular predilection for the convexities of the central face (eg, forehead, cheeks, nose, chin).1,2 The pathophysiology of rosacea remains poorly understood despite the relatively high prevalence of the disease and substantial ongoing research.3 The current paradigm suggests a complex multifactorial interplay involving aberrations of the innate and adaptive immune system, neurovascular dysregulation, blood and lymphatic vessel changes, genetic predispositions, and overgrowth of commensal organisms such as Demodex.3 Additionally, a variety of external factors may exacerbate clinical symptoms (eg, UV radiation, heat exposure, spicy food, alcohol, stress).
The diagnosis of rosacea is made clinically and rarely requires histologic confirmation. Although rosacea can present with a wide range of clinical features that often wax and wane over time, a near universal finding is diffuse centrofacial erythema.4 This centrofacial redness may symptomatically worsen during a flare period, causing flushing, but it often persists nontransiently between flares as background erythema. Other variable findings of rosacea include the presence of telangiectases, edema, plaques, phymatous changes, dry skin, ocular manifestations, and inflammatory lesions in the form of papules and pustules.5 Patients also may report a stinging or burning sensation in affected areas. It is important to note that most patients will only exhibit some of these clinical features and that symptoms often vary in the timing of their emergence or regression.5 A classification system has been developed for rosacea that categorizes the disease into 4 subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) and one variant (granulomatous).6 These categories are determined by the grouping of clinical features present, but it is not uncommon for patients to exhibit clinical manifestations of more than 1 subtype.7
The detrimental cosmetic effects of rosacea are obvious given its chronic nature and tendency to affect highly visible areas such as the face. As such, rosacea can have a devastating impact on patients’ quality of life.8 Patients with rosacea have been reported to have higher incidence rates of low self-esteem, embarrassment, social anxiety, and depression as compared to the rest of the population. Effective treatment, however, can improve cosmetic appearance and mitigate the negative psychosocial impacts of the disease.8
Treatment of rosacea focuses on relieving cosmetic symptoms, as no curative therapy currently exists. Treatment comes in a wide variety of forms, including topical medications, systemic pharmacologic therapies, light-based modalities, and procedural interventions. Choice of therapy should be determined on a case-by-case basis as guided by the clinical features present, and combination or sequential therapies often are required to achieve optimal cosmetic results. In this article, we review both existing and emerging treatments of rosacea and assess their ability to improve the cosmetic symptoms of rosacea (Table).
Skin Care
Proper skin care is an important aspect of treatment for all patients with rosacea and thus includes the use of over-the-counter cleansers, moisturizers, and sunscreens.9 The choice of skin care products is an important consideration given the often hypersensitive skin of rosacea patients. Moisturizers and cleansers should have an acidic to neutral pH, similar to normal skin. They should not contain emulsifiers that strip moisture from the skin or protective lipids and proteins from the stratum corneum.10 Moisturizers without irritants, abrasives, or allergens should be used following skin cleansing. Protection from UV radiation with sunscreen, ideally with a sun protection factor greater than 30, is particularly important, as it can prevent UV-induced rosacea flares as well as photodamage that can cause additional erythema and telangiectasia.4 Rosacea patients also may find green-tinted makeup to be useful in concealing areas of erythema.8
Topical Therapy
Currently, there are only 5 US Food and Drug Administration (FDA)–approved topical medications for the treatment of rosacea: metronidazole (MTZ) gel 0.75% and 1%, azelaic acid (AzA) gel 15%, sodium sulfacetamide (SS) 10%–sulfur 5% lotion and cream, brimonidine tartrate (BT) gel 0.5%, and the most recently approved ivermectin (IVM) cream 1%.7 Metronidazole, AzA, and SS primarily are used to treat the inflammatory papules and pustules of rosacea, while BT is used to treat persistent background erythema. The exact mechanisms of action by which MTZ, AzA, and SS treat rosacea are unclear, but they are thought to reduce inflammation and/or immune response. Metronidazole and AzA both have demonstrated favorable safety profiles and significant (P<.05) efficacy over vehicle in reducing inflammatory lesions in numerous well-controlled randomized clinical studies.4,11,12 There is some evidence that AzA may be more effective than MTZ; one 15-week multicenter, double-blind, randomized, parallel-group study demonstrated that twice-daily AzA gel 15% showed significant superiority (P=.02) over twice-daily MTZ gel 0.75% in improving the inflammatory lesions and erythema of rosacea.13 Sodium sulfacetamide also has shown good efficacy in the treatment of inflammatory lesions and performed significantly better (P=.04) than MTZ according to one multicenter, investigator-blinded, randomized, parallel-group study,14 but the overall evidence is not as strong as MTZ and AzA.4,11,15 The most common adverse effect for MTZ, AzA, and SS is application-site irritation, but overall most patients report good tolerance to these topical medications.4 Azelaic acid is unique in that patients may report stinging, tingling, or burning after application, but these effects are not associated with visible skin changes and usually are transient, generally remitting after 1 to 2 weeks.4
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist whose mechanism of action in the treatment of rosacea is thought to involve vasoconstriction of superficial skin vasculature and to a lesser extent anti-inflammatory effects.16 In a double-blind, randomized, vehicle-controlled phase 3 trial, application of BT gel 0.5% once daily for 4 weeks demonstrated significant efficacy over vehicle (P<.001) in treating persistent nontransient facial erythema in 553 adult patients with 2 or fewer papulopustular lesions as evaluated over 12 hours on days 1, 15, and 29.17 Notably, a substantial difference in cosmetic appearance was observed in another study as early as 30 minutes after the first gel application on day 1.18 The results of this phase 3 trial17 mirrored those of the phase 2 dose-optimization and safety studies of similar design.18 In addition to another long-term, 1-year, open-label study,19 both phase 2 and 3 studies have shown favorable safety profiles with no reports of tachyphylaxis, rebound erythema, or aggravation of other disease features such as telangiectases or inflammatory lesions.17,18 Recently, however, there have been some reports of considerable rebound erythema with BT use and thus patients should be made aware of this possibility.20,21 Case reports of successful treatment of background erythema and flushing with other topically applied adrenergic receptor modifiers such as oxymetazoline and xylometazoline have been published in the literature,22,23 but additional research will be necessary to validate these claims.
Ivermectin, a decades-old antiparasitic, has recently shown promising results as a treatment of rosacea patients with moderate to severe papulopustular lesions. Its therapeutic effect is believed to be mediated by its activity against Demodex, a natural skin mite that has been found at increased concentrations in a subset of patients with rosacea, as well as by its natural anti-inflammatory properties.24 In 2 identically designed, randomized, double-blind, controlled trials of IVM cream 1% applied once daily for 12 weeks, a significantly larger proportion of patients in the IVM groups achieved an investigator global assessment of clear or almost clear as compared to vehicle (IVM: 38.4% and 40.1%, respectively; vehicle: 11.6% and 18.8%, respectively; P<.001). Both trials also demonstrated that IVM was significantly superior to vehicle in the reduction of inflammatory lesion counts measured at week 12 as compared to baseline (IVM: 76.0% and 75.0%, respectively; vehicle: 50.0% and 50.0%, respectively; P<.001).24 An extension of these original trials demonstrated long-term safety with up to 52 weeks of topical IVM use and reported a low incidence rate of adverse effects, most commonly transient skin burning, pruritus, and dryness. Notably, the incidence rate of these adverse effects was lower than a comparison group receiving AzA gel 15% once daily.25 Once-daily application of IVM cream 1% also has recently demonstrated superiority over twice-daily MTZ cream 0.75% for 16 weeks in a phase 3 investigator-blinded, randomized, parallel-group study. The IVM group was significantly superior to MTZ in the reduction of inflammatory lesions as compared to baseline (83.0% vs 73.7%) and in the number of participants who achieved an investigator global assessment score of clear or almost clear (84.9% vs 75.4%)(both P<.001).26 There also is limited evidence for the use of other antiparasitic topical medications such as crotamiton 10% and permethrin 5%, but such agents frequently cause irritation and may not be well tolerated in rosacea patients.27-29
There are a variety of other non–FDA-approved topical medications that have been used with varying success in the literature, including cyclosporine, macrolides, benzoyl peroxide, retinoids, and calcineurin inhibitors such as tacrolimus and pimecrolimus. Evidence for the use of these medications generally is limited to a few studies with small numbers of patients and will not be discussed further in this article.4,11,30 These agents, however, may be useful in select cases when first-line regimens have failed and also may be good targets for future research.
Systemic Therapy
The mainstay of systemic treatment of rosacea centers around the tetracyclines, a group of antibiotics that have been used off label for rosacea since the 1950s.31 The therapeutic effects of tetracyclines in the treatment of rosacea are thought to revolve around their anti-inflammatory effects rather than their antibacterial properties.32 Currently, the only FDA-approved oral agent for treatment of the inflammatory lesions of rosacea is doxycycline 40-mg modified-release capsules taken once daily. These modified capsules allow for instant release of 30 mg and delayed release of 10 mg of doxycycline. This dosing is considered to be anti-inflammatory rather than antimicrobial, as it does not produce antibiotic selection pressure even with prolonged use.33 Efficacy of 40-mg subantimicrobial-dose doxycycline (SDD) has been demonstrated in 2 phase 3 multicenter, parallel-group, randomized, double-blind, placebo-controlled studies in which SDD demonstrated a significantly greater reduction in the number of total inflammatory lesions at week 16 compared to placebo (P<.001).34 Subantimicrobial-dose doxycycline also has been shown to be equally as efficacious in reducing inflammatory lesions as traditional-dose doxycycline.35 There also is some evidence for the efficacy of SDD in reducing overall erythema, as demonstrated by one open-label, community-based study in which SDD monotherapy resulted in clinician erythema assessment scores of mild or no erythema in 75% of patients with mild to severe rosacea at baseline after 12 weeks of therapy.35 Additionally, SDD is considered to be safe and well-tolerated and does not generally result in the adverse effects that may be seen in antibiotic-level doses of doxycycline (eg, gastrointestinal upset, vaginal candidiasis, photosensitivity).34,36,37 Other antibiotics such as clarithromycin, azithromycin, and MTZ also have been studied as treatments of papulopustular rosacea at antibiotic-level doses with good therapeutic effect.38-40 These therapies, however, generally are not used unless there are contraindications for use of tetracycline antibiotics, such as pregnancy or allergy, as the overall evidence is not as strong and there may be increased risks for serious adverse effects.30
Although it is not FDA approved, isotretinoin is an important therapeutic option for select rosacea patients, as it is the only pharmacologic agent that has shown efficacy for the phymatous changes of rosacea. Its efficacy, however, is limited to early-stage rhinophyma that has not yet progressed to the fibrotic or mucinous stages of disease in which it has been shown to reduce the size and number of cutaneous sebaceous glands.30,41 Isotretinoin at 0.3 mg/kg daily also has shown noninferiority in treatment of the inflammatory papules and pustules of rosacea as compared to antibiotic dosing of doxycycline in one large-scale, placebo-controlled, randomized, 12-week multicenter study.42 Unfortunately, recurrence is highly likely after isotretinoin therapy is discontinued.30,41 However, continuous “microdose” isotretinoin at 0.03 to 0.17 mg/kg daily has shown evidence for efficacy in treatment of recalcitrant papulopustular disease.43 Such dosing may have the added benefit of reduced risk for radiographic changes associated with long-term isotretinoin use.43
Light-Based Therapy
Light-based modalities are an important tool set in the management of rosacea symptoms, as they can treat telangiectases for which medical therapy is not generally effective.9 To a lesser extent, light-based modalities also can help alleviate background erythema. The most commonly used light-based modalities include the pulsed dye laser (PDL)(Figure), potassium titanyl phosphate (KTP) laser, Nd:YAG laser, intense pulsed light, photodynamic therapy, CO2 laser, and erbium-doped YAG (Er:YAG) laser. These treatments produce clinical results by targeting specific chromophores such as oxyhemoglobin, deoxyhemoglobin, methemoglobin, and clotted blood with light of specific wavelengths to induce thermolysis of vasculature while sparing collateral tissue.44 Generally, larger telangiectatic vessels are more amenable to therapy than smaller vessels, which usually require higher energy to be delivered in a shorter period of time, thus predisposing the patient to the development of purpura that may last for 1 to 2 weeks.44


|
Historically, PDL used a light wavelength of 577 nm and was classically associated with posttherapy purpura; however, modern PDLs use wavelengths of 585 or 595 nm and are associated with a reduced risk for purpura through the use of longer pulse durations (ie, 10–40 millisecond), multiple minipulses, multiple passes, and advanced epidermal cooling methods.9,44 In a small, prospective, randomized, controlled, nonblinded study, PDL therapy with fluence sufficiently high enough to induce purpura achieved an approximate 50% improvement in telangiectasia grading scores in most patients after a single treatment.45 Notably, PDL therapy at purpura-inducing settings was reported to be much more efficacious than settings that did not induce purpura (purpura free), especially in the treatment of thicker telangiectases.45
Potassium titanyl phosphate lasers make use of shorter wavelengths (532 nm) than PDL and thus are better able to target superficial vasculature, which translates into a reduced risk for purpura and faster healing times. However, KTP laser therapy typically is only reserved for patients with lighter skin types, as this wavelength of light is more likely to result in higher melanin absorption and possible postinflammatory hyperpigmentation.44 A split-face study comparing the KTP laser with PDL determined that the KTP laser was able to achieve 62% clearing after the first treatment and 85% clearance after the third treatment versus 49% and 75% for PDL treatment, respectively; however, the KTP laser had higher rates of posttherapy erythema lasting at least 1 day (58% vs 8%).46
Conversely, the Nd:YAG laser uses longer wavelengths (1064 nm) and can achieve deeper skin penetration, which may be effective for larger, recalcitrant, or deeper blue-tinted vessels. A split-face, double-blind, randomized, controlled trial found Nd:YAG laser therapy to be an effective treatment of facial erythema, though it was observed to be less effective than purpura-free PDL therapy in reducing redness after 4 treatments (34% vs 52% improvement, respectively); however, treatment with the Nd:YAG laser was found to be significantly (P=.0028) less painful.47
Intense pulsed light is unique from the previously discussed light-based therapies in that it uses noncoherent light with wavelengths between 500 and 1200 nm. Cutoff filters may be used to allow for more selective tissue damage depending on the depth of penetration desired. Intense pulsed light has been shown to be equally as efficacious as purpura-free PDL therapy in the treatment of erythema and telangiectasia in a randomized, controlled, single-blind, split-face trial.48 Additionally, a study of 200 patients with facial vascular lesions, of whom 74 patients had rosacea, showed that intense pulsed light therapy resulted in a 75% to 100% improvement of lesions in 174 of 188 (92.5%) patients who returned for follow-up. Treatment often required at least 2 sessions, but overall adverse effects were reported to be minimal.49
Photodynamic therapy is a well-studied and often utilized treatment of a variety of skin conditions, but there have only been a few studies regarding its use in rosacea. Photodynamic therapy involves the use of topically applied photosensitizing agents such as 5-aminolevulinic acid or methyl aminolevulinate before exposure to red or blue light. This process generates reactive oxygen species, though the exact mechanism of action through which patients achieve cosmetic improvement in rosacea is unclear. In one study of 17 patients with varying rosacea subtypes treated with methyl aminolevulinate and red light, drastic relief of symptoms was seen in 10 (58.8%) patients, marked improvement in 4 (23.5%) patients, and no response in 3 (17.6%) patients. Most patients report a transient irritant skin reaction at the site of therapy.50
Ablative lasers such as the CO2 (10,600 nm) and Er:YAG (2940 nm) lasers also have been shown to be useful in the treatment of rosacea, specifically for the management of rhinophymatous features. Excellent results have been achieved with these lasers given their ability to provide near-bloodless surgical fields. In a 13-year review of 124 patients with rhinophyma receiving a single CO2 laser treatment, good to excellent results were achieved in 118 (95.2%) of patients when evaluated at 3 months posttreatment.51 Patient satisfaction also is reported to be high with few adverse effects reported. The evidence for the Er:YAG laser is not as strong, but the current reports indicate efficacy and safety similar to that of the CO2 laser.52
Procedural Therapies
Procedural therapies in rosacea generally are reserved for management of rhinophyma and include electrocautery, cryotherapy, radiotherapy, dermabrasion, scalpel excisions, flap reconstruction, and skin grafts.30,53 The details and evidence for these methods is beyond the scope of this paper, but it is important to be aware of such modalities. As with most surgical procedures, operator skill and experience may affect treatment outcomes, and there also are definite risks for postprocedural scarring, swelling, erythema, and pigmentation changes. Recently, anecdotal evidence has shown that botulinum toxin injections may be effective for patients with refractory flushing and erythema, but larger studies will be necessary to better assess these claims.54,55
Conclusion
Although recent advances in pharmacology and laser technology have provided physicians with new and effective treatment modalities for rosacea, it remains a poorly understood disease without a definitive cure. The negative impact of rosacea on patients’ quality of life can be substantial, but effective management of cosmetic symptoms can minimize such deleterious effects. Therapy should be individualized and directed at treating the symptoms that are most bothersome to the patient. Additionally, effective treatment often will require a combination of modalities or sequential therapies to achieve optimal cosmetic outcomes.
Rosacea is a commonly encountered chronic inflammatory skin disease that affects an estimated 16 million Americans and exhibits a particular predilection for the convexities of the central face (eg, forehead, cheeks, nose, chin).1,2 The pathophysiology of rosacea remains poorly understood despite the relatively high prevalence of the disease and substantial ongoing research.3 The current paradigm suggests a complex multifactorial interplay involving aberrations of the innate and adaptive immune system, neurovascular dysregulation, blood and lymphatic vessel changes, genetic predispositions, and overgrowth of commensal organisms such as Demodex.3 Additionally, a variety of external factors may exacerbate clinical symptoms (eg, UV radiation, heat exposure, spicy food, alcohol, stress).
The diagnosis of rosacea is made clinically and rarely requires histologic confirmation. Although rosacea can present with a wide range of clinical features that often wax and wane over time, a near universal finding is diffuse centrofacial erythema.4 This centrofacial redness may symptomatically worsen during a flare period, causing flushing, but it often persists nontransiently between flares as background erythema. Other variable findings of rosacea include the presence of telangiectases, edema, plaques, phymatous changes, dry skin, ocular manifestations, and inflammatory lesions in the form of papules and pustules.5 Patients also may report a stinging or burning sensation in affected areas. It is important to note that most patients will only exhibit some of these clinical features and that symptoms often vary in the timing of their emergence or regression.5 A classification system has been developed for rosacea that categorizes the disease into 4 subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) and one variant (granulomatous).6 These categories are determined by the grouping of clinical features present, but it is not uncommon for patients to exhibit clinical manifestations of more than 1 subtype.7
The detrimental cosmetic effects of rosacea are obvious given its chronic nature and tendency to affect highly visible areas such as the face. As such, rosacea can have a devastating impact on patients’ quality of life.8 Patients with rosacea have been reported to have higher incidence rates of low self-esteem, embarrassment, social anxiety, and depression as compared to the rest of the population. Effective treatment, however, can improve cosmetic appearance and mitigate the negative psychosocial impacts of the disease.8
Treatment of rosacea focuses on relieving cosmetic symptoms, as no curative therapy currently exists. Treatment comes in a wide variety of forms, including topical medications, systemic pharmacologic therapies, light-based modalities, and procedural interventions. Choice of therapy should be determined on a case-by-case basis as guided by the clinical features present, and combination or sequential therapies often are required to achieve optimal cosmetic results. In this article, we review both existing and emerging treatments of rosacea and assess their ability to improve the cosmetic symptoms of rosacea (Table).
Skin Care
Proper skin care is an important aspect of treatment for all patients with rosacea and thus includes the use of over-the-counter cleansers, moisturizers, and sunscreens.9 The choice of skin care products is an important consideration given the often hypersensitive skin of rosacea patients. Moisturizers and cleansers should have an acidic to neutral pH, similar to normal skin. They should not contain emulsifiers that strip moisture from the skin or protective lipids and proteins from the stratum corneum.10 Moisturizers without irritants, abrasives, or allergens should be used following skin cleansing. Protection from UV radiation with sunscreen, ideally with a sun protection factor greater than 30, is particularly important, as it can prevent UV-induced rosacea flares as well as photodamage that can cause additional erythema and telangiectasia.4 Rosacea patients also may find green-tinted makeup to be useful in concealing areas of erythema.8
Topical Therapy
Currently, there are only 5 US Food and Drug Administration (FDA)–approved topical medications for the treatment of rosacea: metronidazole (MTZ) gel 0.75% and 1%, azelaic acid (AzA) gel 15%, sodium sulfacetamide (SS) 10%–sulfur 5% lotion and cream, brimonidine tartrate (BT) gel 0.5%, and the most recently approved ivermectin (IVM) cream 1%.7 Metronidazole, AzA, and SS primarily are used to treat the inflammatory papules and pustules of rosacea, while BT is used to treat persistent background erythema. The exact mechanisms of action by which MTZ, AzA, and SS treat rosacea are unclear, but they are thought to reduce inflammation and/or immune response. Metronidazole and AzA both have demonstrated favorable safety profiles and significant (P<.05) efficacy over vehicle in reducing inflammatory lesions in numerous well-controlled randomized clinical studies.4,11,12 There is some evidence that AzA may be more effective than MTZ; one 15-week multicenter, double-blind, randomized, parallel-group study demonstrated that twice-daily AzA gel 15% showed significant superiority (P=.02) over twice-daily MTZ gel 0.75% in improving the inflammatory lesions and erythema of rosacea.13 Sodium sulfacetamide also has shown good efficacy in the treatment of inflammatory lesions and performed significantly better (P=.04) than MTZ according to one multicenter, investigator-blinded, randomized, parallel-group study,14 but the overall evidence is not as strong as MTZ and AzA.4,11,15 The most common adverse effect for MTZ, AzA, and SS is application-site irritation, but overall most patients report good tolerance to these topical medications.4 Azelaic acid is unique in that patients may report stinging, tingling, or burning after application, but these effects are not associated with visible skin changes and usually are transient, generally remitting after 1 to 2 weeks.4
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist whose mechanism of action in the treatment of rosacea is thought to involve vasoconstriction of superficial skin vasculature and to a lesser extent anti-inflammatory effects.16 In a double-blind, randomized, vehicle-controlled phase 3 trial, application of BT gel 0.5% once daily for 4 weeks demonstrated significant efficacy over vehicle (P<.001) in treating persistent nontransient facial erythema in 553 adult patients with 2 or fewer papulopustular lesions as evaluated over 12 hours on days 1, 15, and 29.17 Notably, a substantial difference in cosmetic appearance was observed in another study as early as 30 minutes after the first gel application on day 1.18 The results of this phase 3 trial17 mirrored those of the phase 2 dose-optimization and safety studies of similar design.18 In addition to another long-term, 1-year, open-label study,19 both phase 2 and 3 studies have shown favorable safety profiles with no reports of tachyphylaxis, rebound erythema, or aggravation of other disease features such as telangiectases or inflammatory lesions.17,18 Recently, however, there have been some reports of considerable rebound erythema with BT use and thus patients should be made aware of this possibility.20,21 Case reports of successful treatment of background erythema and flushing with other topically applied adrenergic receptor modifiers such as oxymetazoline and xylometazoline have been published in the literature,22,23 but additional research will be necessary to validate these claims.
Ivermectin, a decades-old antiparasitic, has recently shown promising results as a treatment of rosacea patients with moderate to severe papulopustular lesions. Its therapeutic effect is believed to be mediated by its activity against Demodex, a natural skin mite that has been found at increased concentrations in a subset of patients with rosacea, as well as by its natural anti-inflammatory properties.24 In 2 identically designed, randomized, double-blind, controlled trials of IVM cream 1% applied once daily for 12 weeks, a significantly larger proportion of patients in the IVM groups achieved an investigator global assessment of clear or almost clear as compared to vehicle (IVM: 38.4% and 40.1%, respectively; vehicle: 11.6% and 18.8%, respectively; P<.001). Both trials also demonstrated that IVM was significantly superior to vehicle in the reduction of inflammatory lesion counts measured at week 12 as compared to baseline (IVM: 76.0% and 75.0%, respectively; vehicle: 50.0% and 50.0%, respectively; P<.001).24 An extension of these original trials demonstrated long-term safety with up to 52 weeks of topical IVM use and reported a low incidence rate of adverse effects, most commonly transient skin burning, pruritus, and dryness. Notably, the incidence rate of these adverse effects was lower than a comparison group receiving AzA gel 15% once daily.25 Once-daily application of IVM cream 1% also has recently demonstrated superiority over twice-daily MTZ cream 0.75% for 16 weeks in a phase 3 investigator-blinded, randomized, parallel-group study. The IVM group was significantly superior to MTZ in the reduction of inflammatory lesions as compared to baseline (83.0% vs 73.7%) and in the number of participants who achieved an investigator global assessment score of clear or almost clear (84.9% vs 75.4%)(both P<.001).26 There also is limited evidence for the use of other antiparasitic topical medications such as crotamiton 10% and permethrin 5%, but such agents frequently cause irritation and may not be well tolerated in rosacea patients.27-29
There are a variety of other non–FDA-approved topical medications that have been used with varying success in the literature, including cyclosporine, macrolides, benzoyl peroxide, retinoids, and calcineurin inhibitors such as tacrolimus and pimecrolimus. Evidence for the use of these medications generally is limited to a few studies with small numbers of patients and will not be discussed further in this article.4,11,30 These agents, however, may be useful in select cases when first-line regimens have failed and also may be good targets for future research.
Systemic Therapy
The mainstay of systemic treatment of rosacea centers around the tetracyclines, a group of antibiotics that have been used off label for rosacea since the 1950s.31 The therapeutic effects of tetracyclines in the treatment of rosacea are thought to revolve around their anti-inflammatory effects rather than their antibacterial properties.32 Currently, the only FDA-approved oral agent for treatment of the inflammatory lesions of rosacea is doxycycline 40-mg modified-release capsules taken once daily. These modified capsules allow for instant release of 30 mg and delayed release of 10 mg of doxycycline. This dosing is considered to be anti-inflammatory rather than antimicrobial, as it does not produce antibiotic selection pressure even with prolonged use.33 Efficacy of 40-mg subantimicrobial-dose doxycycline (SDD) has been demonstrated in 2 phase 3 multicenter, parallel-group, randomized, double-blind, placebo-controlled studies in which SDD demonstrated a significantly greater reduction in the number of total inflammatory lesions at week 16 compared to placebo (P<.001).34 Subantimicrobial-dose doxycycline also has been shown to be equally as efficacious in reducing inflammatory lesions as traditional-dose doxycycline.35 There also is some evidence for the efficacy of SDD in reducing overall erythema, as demonstrated by one open-label, community-based study in which SDD monotherapy resulted in clinician erythema assessment scores of mild or no erythema in 75% of patients with mild to severe rosacea at baseline after 12 weeks of therapy.35 Additionally, SDD is considered to be safe and well-tolerated and does not generally result in the adverse effects that may be seen in antibiotic-level doses of doxycycline (eg, gastrointestinal upset, vaginal candidiasis, photosensitivity).34,36,37 Other antibiotics such as clarithromycin, azithromycin, and MTZ also have been studied as treatments of papulopustular rosacea at antibiotic-level doses with good therapeutic effect.38-40 These therapies, however, generally are not used unless there are contraindications for use of tetracycline antibiotics, such as pregnancy or allergy, as the overall evidence is not as strong and there may be increased risks for serious adverse effects.30
Although it is not FDA approved, isotretinoin is an important therapeutic option for select rosacea patients, as it is the only pharmacologic agent that has shown efficacy for the phymatous changes of rosacea. Its efficacy, however, is limited to early-stage rhinophyma that has not yet progressed to the fibrotic or mucinous stages of disease in which it has been shown to reduce the size and number of cutaneous sebaceous glands.30,41 Isotretinoin at 0.3 mg/kg daily also has shown noninferiority in treatment of the inflammatory papules and pustules of rosacea as compared to antibiotic dosing of doxycycline in one large-scale, placebo-controlled, randomized, 12-week multicenter study.42 Unfortunately, recurrence is highly likely after isotretinoin therapy is discontinued.30,41 However, continuous “microdose” isotretinoin at 0.03 to 0.17 mg/kg daily has shown evidence for efficacy in treatment of recalcitrant papulopustular disease.43 Such dosing may have the added benefit of reduced risk for radiographic changes associated with long-term isotretinoin use.43
Light-Based Therapy
Light-based modalities are an important tool set in the management of rosacea symptoms, as they can treat telangiectases for which medical therapy is not generally effective.9 To a lesser extent, light-based modalities also can help alleviate background erythema. The most commonly used light-based modalities include the pulsed dye laser (PDL)(Figure), potassium titanyl phosphate (KTP) laser, Nd:YAG laser, intense pulsed light, photodynamic therapy, CO2 laser, and erbium-doped YAG (Er:YAG) laser. These treatments produce clinical results by targeting specific chromophores such as oxyhemoglobin, deoxyhemoglobin, methemoglobin, and clotted blood with light of specific wavelengths to induce thermolysis of vasculature while sparing collateral tissue.44 Generally, larger telangiectatic vessels are more amenable to therapy than smaller vessels, which usually require higher energy to be delivered in a shorter period of time, thus predisposing the patient to the development of purpura that may last for 1 to 2 weeks.44


|
Historically, PDL used a light wavelength of 577 nm and was classically associated with posttherapy purpura; however, modern PDLs use wavelengths of 585 or 595 nm and are associated with a reduced risk for purpura through the use of longer pulse durations (ie, 10–40 millisecond), multiple minipulses, multiple passes, and advanced epidermal cooling methods.9,44 In a small, prospective, randomized, controlled, nonblinded study, PDL therapy with fluence sufficiently high enough to induce purpura achieved an approximate 50% improvement in telangiectasia grading scores in most patients after a single treatment.45 Notably, PDL therapy at purpura-inducing settings was reported to be much more efficacious than settings that did not induce purpura (purpura free), especially in the treatment of thicker telangiectases.45
Potassium titanyl phosphate lasers make use of shorter wavelengths (532 nm) than PDL and thus are better able to target superficial vasculature, which translates into a reduced risk for purpura and faster healing times. However, KTP laser therapy typically is only reserved for patients with lighter skin types, as this wavelength of light is more likely to result in higher melanin absorption and possible postinflammatory hyperpigmentation.44 A split-face study comparing the KTP laser with PDL determined that the KTP laser was able to achieve 62% clearing after the first treatment and 85% clearance after the third treatment versus 49% and 75% for PDL treatment, respectively; however, the KTP laser had higher rates of posttherapy erythema lasting at least 1 day (58% vs 8%).46
Conversely, the Nd:YAG laser uses longer wavelengths (1064 nm) and can achieve deeper skin penetration, which may be effective for larger, recalcitrant, or deeper blue-tinted vessels. A split-face, double-blind, randomized, controlled trial found Nd:YAG laser therapy to be an effective treatment of facial erythema, though it was observed to be less effective than purpura-free PDL therapy in reducing redness after 4 treatments (34% vs 52% improvement, respectively); however, treatment with the Nd:YAG laser was found to be significantly (P=.0028) less painful.47
Intense pulsed light is unique from the previously discussed light-based therapies in that it uses noncoherent light with wavelengths between 500 and 1200 nm. Cutoff filters may be used to allow for more selective tissue damage depending on the depth of penetration desired. Intense pulsed light has been shown to be equally as efficacious as purpura-free PDL therapy in the treatment of erythema and telangiectasia in a randomized, controlled, single-blind, split-face trial.48 Additionally, a study of 200 patients with facial vascular lesions, of whom 74 patients had rosacea, showed that intense pulsed light therapy resulted in a 75% to 100% improvement of lesions in 174 of 188 (92.5%) patients who returned for follow-up. Treatment often required at least 2 sessions, but overall adverse effects were reported to be minimal.49
Photodynamic therapy is a well-studied and often utilized treatment of a variety of skin conditions, but there have only been a few studies regarding its use in rosacea. Photodynamic therapy involves the use of topically applied photosensitizing agents such as 5-aminolevulinic acid or methyl aminolevulinate before exposure to red or blue light. This process generates reactive oxygen species, though the exact mechanism of action through which patients achieve cosmetic improvement in rosacea is unclear. In one study of 17 patients with varying rosacea subtypes treated with methyl aminolevulinate and red light, drastic relief of symptoms was seen in 10 (58.8%) patients, marked improvement in 4 (23.5%) patients, and no response in 3 (17.6%) patients. Most patients report a transient irritant skin reaction at the site of therapy.50
Ablative lasers such as the CO2 (10,600 nm) and Er:YAG (2940 nm) lasers also have been shown to be useful in the treatment of rosacea, specifically for the management of rhinophymatous features. Excellent results have been achieved with these lasers given their ability to provide near-bloodless surgical fields. In a 13-year review of 124 patients with rhinophyma receiving a single CO2 laser treatment, good to excellent results were achieved in 118 (95.2%) of patients when evaluated at 3 months posttreatment.51 Patient satisfaction also is reported to be high with few adverse effects reported. The evidence for the Er:YAG laser is not as strong, but the current reports indicate efficacy and safety similar to that of the CO2 laser.52
Procedural Therapies
Procedural therapies in rosacea generally are reserved for management of rhinophyma and include electrocautery, cryotherapy, radiotherapy, dermabrasion, scalpel excisions, flap reconstruction, and skin grafts.30,53 The details and evidence for these methods is beyond the scope of this paper, but it is important to be aware of such modalities. As with most surgical procedures, operator skill and experience may affect treatment outcomes, and there also are definite risks for postprocedural scarring, swelling, erythema, and pigmentation changes. Recently, anecdotal evidence has shown that botulinum toxin injections may be effective for patients with refractory flushing and erythema, but larger studies will be necessary to better assess these claims.54,55
Conclusion
Although recent advances in pharmacology and laser technology have provided physicians with new and effective treatment modalities for rosacea, it remains a poorly understood disease without a definitive cure. The negative impact of rosacea on patients’ quality of life can be substantial, but effective management of cosmetic symptoms can minimize such deleterious effects. Therapy should be individualized and directed at treating the symptoms that are most bothersome to the patient. Additionally, effective treatment often will require a combination of modalities or sequential therapies to achieve optimal cosmetic outcomes.
1. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
2. Rosacea prevalence map. National Rosacea Society Web site. http://rosacea.org/press/prevalencemap. Accessed June 16, 2015.
3. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
4. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
5. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
6. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
7. Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20:623-629.
8. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
9. Mansouri Y, Goldenberg G. Devices and topical agents for rosacea management. Cutis. 2014;94:21-25.
10. Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
11. van Zuuren EJ, Kramer S, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2011;3:CD003262.
12. Liu RH, Smith MK, Basta SA, et al. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006;142:1047-1052.
13. Elewski B, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
14. Torok HM, Webster G, Dunlap FE, et al. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis. 2005;75:357-363.
15. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
16. Piwnica D, Rosignoli C, de Menonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective alpha-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75:49-54.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment of moderate to severe facial erythema of rosacea: results of two multicenter, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
19. Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13:56-61.
20. Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:e37-e38.
21. Ilkovitch D, Pomerantz RG. Brimonidine effective but may lead to significant rebound erythema. J Am Acad Dermatol. 2014;70:e109-e110.
22. Kim JH, Oh YS, Ji JH, et al. Rosacea (erythematotelangiectatic type) effectively improved by topical xylometazoline. J Dermatol. 2011;38:510-513.
23. Shanler SD, Ondo AL. Successful treatment of erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor antagonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
24. Stein-Gold L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13:316-323.
25. Stein-Gold L, Kircik L, Fowler J, et al. Long-term safety of ivermectin 1% cream vs azelaic acid 15% gel in treating inflammatory lesions of rosacea: results of two 40-week controlled, investigator-blinded trials. J Drugs Dermatol. 2014;13:1380-1386.
26. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103-1110.
27. Koçak M, Ya˘gli S, Vahapo˘glu G, et al. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. a randomized double-blind placebo-controlled study. Dermatology (Basel). 2002;205:265-270.
28. Bikowski JB, Del Rosso JQ. Demodex dermatitis: a retrospective analysis of clinical diagnosis and successful treatment with topical crotamiton. J Clin Aesthet Dermatol. 2009;2:20-25.
29. Layton A, Thiboutot D. Emerging therapies in rosacea. J Am Acad Dermatol. 2013;69(6 suppl 1):S57-S65.
30. Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512, quiz 513-514.
31. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
32. Korting HC, Schöllmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22:287-294.
33. Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472-1483.
34. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
35. Webster GF. An open-label, community-based, 12-week assessment of the effectiveness and safety of monotherapy with doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads). Cutis. 2010;86(suppl 5):7-15.
36. Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
37. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93:18-28.
38. Torresani C. Clarithromycin: a new perspective in rosacea treatment. Int J Dermatol. 1998;37:347-349.
39. Bakar O, Demircay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154.
40. Saihan EM, Burton JL. A double-blind trial of metronidazole versus oxytetracycline therapy for rosacea. Br J Dermatol. 1980;102:443-445.
41. Park H, Del Rosso JQ. Use of oral isotretinoin in the management of rosacea. J Clin Aesthet Dermatol. 2011;4:54-61.
42. Gollnick H, Blume-Peytavi U, Szabo EL, et al. Systemic isotretinoin in the treatment of rosacea—doxycycline-and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010;8:505-515.
43. Hofer T. Continuous “microdose” isotretinoin in adult recalcitrant rosacea. Clin Exp Dermatol. 2004;29:204-205.
44. Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
45. Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
46. Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
47. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
48. Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35:920-928.
49. Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
50. Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
51. Maden V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161:814-818.
52. Fincher EF, Gladstone HB. Use of a dual-mode erbium:YAG laser for the surgical correction of rhinophyma. Arch Facial Plast Surg. 2004;6:267-271.
53. Lloyd KM. Surgical correction of rhinophyma. Arch Dermatol. 1990;126:721-723.
54. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012;11:e76-e79.
55. Park KY, Hyun MY, Jeong SY, et al. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230:299-301.
1. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
2. Rosacea prevalence map. National Rosacea Society Web site. http://rosacea.org/press/prevalencemap. Accessed June 16, 2015.
3. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
4. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
5. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
6. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
7. Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20:623-629.
8. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
9. Mansouri Y, Goldenberg G. Devices and topical agents for rosacea management. Cutis. 2014;94:21-25.
10. Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
11. van Zuuren EJ, Kramer S, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2011;3:CD003262.
12. Liu RH, Smith MK, Basta SA, et al. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006;142:1047-1052.
13. Elewski B, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
14. Torok HM, Webster G, Dunlap FE, et al. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis. 2005;75:357-363.
15. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
16. Piwnica D, Rosignoli C, de Menonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective alpha-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75:49-54.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment of moderate to severe facial erythema of rosacea: results of two multicenter, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
19. Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13:56-61.
20. Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:e37-e38.
21. Ilkovitch D, Pomerantz RG. Brimonidine effective but may lead to significant rebound erythema. J Am Acad Dermatol. 2014;70:e109-e110.
22. Kim JH, Oh YS, Ji JH, et al. Rosacea (erythematotelangiectatic type) effectively improved by topical xylometazoline. J Dermatol. 2011;38:510-513.
23. Shanler SD, Ondo AL. Successful treatment of erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor antagonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
24. Stein-Gold L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13:316-323.
25. Stein-Gold L, Kircik L, Fowler J, et al. Long-term safety of ivermectin 1% cream vs azelaic acid 15% gel in treating inflammatory lesions of rosacea: results of two 40-week controlled, investigator-blinded trials. J Drugs Dermatol. 2014;13:1380-1386.
26. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103-1110.
27. Koçak M, Ya˘gli S, Vahapo˘glu G, et al. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. a randomized double-blind placebo-controlled study. Dermatology (Basel). 2002;205:265-270.
28. Bikowski JB, Del Rosso JQ. Demodex dermatitis: a retrospective analysis of clinical diagnosis and successful treatment with topical crotamiton. J Clin Aesthet Dermatol. 2009;2:20-25.
29. Layton A, Thiboutot D. Emerging therapies in rosacea. J Am Acad Dermatol. 2013;69(6 suppl 1):S57-S65.
30. Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512, quiz 513-514.
31. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
32. Korting HC, Schöllmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22:287-294.
33. Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472-1483.
34. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
35. Webster GF. An open-label, community-based, 12-week assessment of the effectiveness and safety of monotherapy with doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads). Cutis. 2010;86(suppl 5):7-15.
36. Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
37. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93:18-28.
38. Torresani C. Clarithromycin: a new perspective in rosacea treatment. Int J Dermatol. 1998;37:347-349.
39. Bakar O, Demircay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154.
40. Saihan EM, Burton JL. A double-blind trial of metronidazole versus oxytetracycline therapy for rosacea. Br J Dermatol. 1980;102:443-445.
41. Park H, Del Rosso JQ. Use of oral isotretinoin in the management of rosacea. J Clin Aesthet Dermatol. 2011;4:54-61.
42. Gollnick H, Blume-Peytavi U, Szabo EL, et al. Systemic isotretinoin in the treatment of rosacea—doxycycline-and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010;8:505-515.
43. Hofer T. Continuous “microdose” isotretinoin in adult recalcitrant rosacea. Clin Exp Dermatol. 2004;29:204-205.
44. Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
45. Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
46. Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
47. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
48. Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35:920-928.
49. Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
50. Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
51. Maden V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161:814-818.
52. Fincher EF, Gladstone HB. Use of a dual-mode erbium:YAG laser for the surgical correction of rhinophyma. Arch Facial Plast Surg. 2004;6:267-271.
53. Lloyd KM. Surgical correction of rhinophyma. Arch Dermatol. 1990;126:721-723.
54. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012;11:e76-e79.
55. Park KY, Hyun MY, Jeong SY, et al. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230:299-301.
Practice Points
- As no definitive cure for rosacea exists, effective treatment is aimed at improving the cosmetic symptoms.
- Choice of therapy should be determined on a case-by-case basis as guided by the clinical features most bothersome to the patient.
- A combination of modalities and/or sequential therapy often is required to achieve optimal cosmetic outcomes.