User login
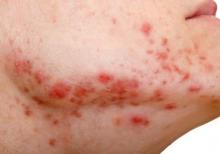
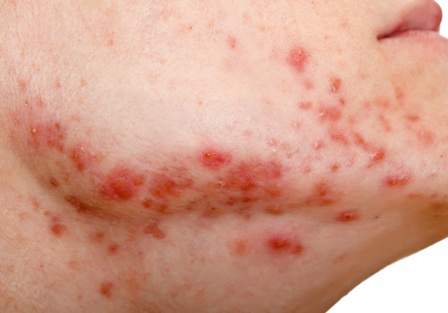
Workplace Interactions for Rosacea Patients
A new survey from the National Rosacea Society of 794 rosacea patients revealed that the majority of respondents indicated the disease had affected interactions with others in the workplace. More than 82% of respondents said they would notice people staring when they were experiencing a flare-up, and nearly 54% reported hearing rude or inappropriate comments about their facial appearance. More than 66% of the survey respondents said rosacea had negatively impacted interactions with customers or coworkers. Twenty-nine percent of patients with mild symptoms and 43% of those with severe symptoms reported they had missed work because of the condition.
Although rosacea may impact workplace interactions, 76.5% of respondents did not feel their appearance had cost them a promotion or new responsibilities, and 77.5% indicated that it had not kept them from landing a new job. Most respondents indicated that workplace problems were resolved when medical therapy was started, with nearly 67% reporting that effective treatment had improved their interactions with others at work.
In a January 2015 Cutis article, “The Rosacea Patient Journey: A Novel Approach to Conceptualizing Patient Experiences,” Kuo and colleagues discussed how patients can be educated to prepare for the rosacea patient experience. “Rosacea patients are faced with confusing and aggravating symptoms that can cause anxiety and may lead them to seek treatment from a physician,” the authors said. Rosacea can be a socially stigmatizing disease because the facial flushing and phymatous changes may be mistaken for alcohol abuse. It can also disrupt social and professional interactions, leading to quality-of-life effects such as difficulty functioning on a day-to-day basis.
Because there is no cure for rosacea, the patient and dermatologist must work together to devise a treatment plan that will help control the symptoms of rosacea. “Ultimately, with the alleviation of visible symptoms, the patient’s quality of life also can improve,” Kuo and colleagues reported. “Better understanding of the rosacea patient perspective can lead to a more efficient health care system, improved patient care, and better patient satisfaction.”
Share a copy of the Cutis rosacea patient journey guide with your patients today.
A new survey from the National Rosacea Society of 794 rosacea patients revealed that the majority of respondents indicated the disease had affected interactions with others in the workplace. More than 82% of respondents said they would notice people staring when they were experiencing a flare-up, and nearly 54% reported hearing rude or inappropriate comments about their facial appearance. More than 66% of the survey respondents said rosacea had negatively impacted interactions with customers or coworkers. Twenty-nine percent of patients with mild symptoms and 43% of those with severe symptoms reported they had missed work because of the condition.
Although rosacea may impact workplace interactions, 76.5% of respondents did not feel their appearance had cost them a promotion or new responsibilities, and 77.5% indicated that it had not kept them from landing a new job. Most respondents indicated that workplace problems were resolved when medical therapy was started, with nearly 67% reporting that effective treatment had improved their interactions with others at work.
In a January 2015 Cutis article, “The Rosacea Patient Journey: A Novel Approach to Conceptualizing Patient Experiences,” Kuo and colleagues discussed how patients can be educated to prepare for the rosacea patient experience. “Rosacea patients are faced with confusing and aggravating symptoms that can cause anxiety and may lead them to seek treatment from a physician,” the authors said. Rosacea can be a socially stigmatizing disease because the facial flushing and phymatous changes may be mistaken for alcohol abuse. It can also disrupt social and professional interactions, leading to quality-of-life effects such as difficulty functioning on a day-to-day basis.
Because there is no cure for rosacea, the patient and dermatologist must work together to devise a treatment plan that will help control the symptoms of rosacea. “Ultimately, with the alleviation of visible symptoms, the patient’s quality of life also can improve,” Kuo and colleagues reported. “Better understanding of the rosacea patient perspective can lead to a more efficient health care system, improved patient care, and better patient satisfaction.”
Share a copy of the Cutis rosacea patient journey guide with your patients today.
A new survey from the National Rosacea Society of 794 rosacea patients revealed that the majority of respondents indicated the disease had affected interactions with others in the workplace. More than 82% of respondents said they would notice people staring when they were experiencing a flare-up, and nearly 54% reported hearing rude or inappropriate comments about their facial appearance. More than 66% of the survey respondents said rosacea had negatively impacted interactions with customers or coworkers. Twenty-nine percent of patients with mild symptoms and 43% of those with severe symptoms reported they had missed work because of the condition.
Although rosacea may impact workplace interactions, 76.5% of respondents did not feel their appearance had cost them a promotion or new responsibilities, and 77.5% indicated that it had not kept them from landing a new job. Most respondents indicated that workplace problems were resolved when medical therapy was started, with nearly 67% reporting that effective treatment had improved their interactions with others at work.
In a January 2015 Cutis article, “The Rosacea Patient Journey: A Novel Approach to Conceptualizing Patient Experiences,” Kuo and colleagues discussed how patients can be educated to prepare for the rosacea patient experience. “Rosacea patients are faced with confusing and aggravating symptoms that can cause anxiety and may lead them to seek treatment from a physician,” the authors said. Rosacea can be a socially stigmatizing disease because the facial flushing and phymatous changes may be mistaken for alcohol abuse. It can also disrupt social and professional interactions, leading to quality-of-life effects such as difficulty functioning on a day-to-day basis.
Because there is no cure for rosacea, the patient and dermatologist must work together to devise a treatment plan that will help control the symptoms of rosacea. “Ultimately, with the alleviation of visible symptoms, the patient’s quality of life also can improve,” Kuo and colleagues reported. “Better understanding of the rosacea patient perspective can lead to a more efficient health care system, improved patient care, and better patient satisfaction.”
Share a copy of the Cutis rosacea patient journey guide with your patients today.
Pretreatment hydroquinone for nonablative laser resurfacing of acne scars?
Pretreatment of skin prior to nonablative or ablative laser resurfacing is common practice, particularly in darker skin types. Treatment regimens include using hydroquinone 4% (and other hydroquinone-containing combinations) once to twice daily for 1-2 weeks prior to the laser procedure. The rationale makes sense. Quieting melanin production by inhibiting tyrosinase would seem to decrease the incidence of postinflammatory hyperpigmentation after laser resurfacing procedures. But is this common practice effective?
For ablative CO2 resurfacing in 100 patients Fitzpatrick Skin Types (FST) I-III, there was no significant difference in the incidence of hyperpigmentation in those randomized to be pretreated with either hydroquinone, glycolic acid, tretinoin, or to no treatment.1 The thought was that the follicular melanocytes involved in re-epithelialization were not affected by the pretreatment. This is the only published laser resurfacing today to date examining various pretreatment protocols with hyperpigmentation as a primary study outcome. From this study, it seems as though pretreatment before laser resurfacing is not helpful, but what about for nonablative resurfacing in darker skin types (FST IV-VI)?
In darker skin types (FST IV-VI), the risk of postinflammatory hyperpigmentation (PIH) is inherently higher and the incidence after laser resurfacing is greater. While the incidence of PIH is lower with nonablative fractional resurfacing, compared with ablative resurfacing, PIH can still occur whether pretreatment hydroquinone is used or not.2,3,4 To date, there are no published studies looking at the incidence of PIH when comparing pretreatment antipigment agents versus no pretreatment for laser resurfacing for acne scars in darker skin types. A split-face study comparing pretreatment on one side and no pretreatment on the other could help delineate whether this practice is evidence based.
For nonablative fractional laser resurfacing of acne scars, lower densities in darker skin types are recommended and may help reduce PIH risk. There is no statistically significant difference in improvement of acne scars in using low versus high densities using the same fluences. However, some studies note that higher densities clinically resulted in a mild improvement of acne scars over lower densities (not statistically significant); thus, if lower densities are used, it is possible that more treatments may be needed.4,5
Vigorous sun protection before and after treatment is prudent, with sun avoidance and physical sunscreens reducing the risk of PIH in darker skin from irritant or allergic contact dermatitis, compared with chemical sunscreens. If PIH occurs, it is often self limited (up to 1-2 months). Sun protection and posttreatment regimens of hydroquinone (or other lightening agent) aid in hastening improvement.
If the patient is undergoing nonablative laser resurfacing to treat pigmentation, such as melasma, then hydroquinone pre- and postlaser is appropriate. In my opinion, laser treatment of melasma should not be first line because of safety and efficacy concerns. However, in these cases, hydroquinone prior to laser has shown benefit.6 In addition, hydroquinone after nonablative fractional resurfacing may enhance penetration of the topical and improve efficacy.
In summary, the evidence shows that pretreatment with antipigment agents is not warranted in skin types I-III for ablative laser resurfacing. Pretreatment with antipigment agents for nonablative laser resurfacing for melasma (which should not be considered a first line treatment for melasma) is warranted. However, at this time, it is not clear whether pretreatment with antipigments for nonablative laser resurfacing for acne scars in darker skin types is useful. Lower densities should be used and if PIH does occur, it is usually self limited, and posttreatment hydroquinone or other antipigment agents may be useful.
References
1. Dermatol Surg. 1999 Jan;25(1):15-7.
2. Dermatol Surg. 2010 May;36(5):602-9.
3. Br J Dermatol. 2012 Jun;166(6):1160-9.
4.Lasers Surg Med. 2007 Jun;39(5):381-5.
5. Lasers Surg Med. 2007 Apr;39(4):311-4.
6. Dermatol Surg. 2010 Jun;36(6):909-18.
Dr. Wesley and Dr. Talakoub are co-contributors to the monthly Aesthetic Dermatology column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
Pretreatment of skin prior to nonablative or ablative laser resurfacing is common practice, particularly in darker skin types. Treatment regimens include using hydroquinone 4% (and other hydroquinone-containing combinations) once to twice daily for 1-2 weeks prior to the laser procedure. The rationale makes sense. Quieting melanin production by inhibiting tyrosinase would seem to decrease the incidence of postinflammatory hyperpigmentation after laser resurfacing procedures. But is this common practice effective?
For ablative CO2 resurfacing in 100 patients Fitzpatrick Skin Types (FST) I-III, there was no significant difference in the incidence of hyperpigmentation in those randomized to be pretreated with either hydroquinone, glycolic acid, tretinoin, or to no treatment.1 The thought was that the follicular melanocytes involved in re-epithelialization were not affected by the pretreatment. This is the only published laser resurfacing today to date examining various pretreatment protocols with hyperpigmentation as a primary study outcome. From this study, it seems as though pretreatment before laser resurfacing is not helpful, but what about for nonablative resurfacing in darker skin types (FST IV-VI)?
In darker skin types (FST IV-VI), the risk of postinflammatory hyperpigmentation (PIH) is inherently higher and the incidence after laser resurfacing is greater. While the incidence of PIH is lower with nonablative fractional resurfacing, compared with ablative resurfacing, PIH can still occur whether pretreatment hydroquinone is used or not.2,3,4 To date, there are no published studies looking at the incidence of PIH when comparing pretreatment antipigment agents versus no pretreatment for laser resurfacing for acne scars in darker skin types. A split-face study comparing pretreatment on one side and no pretreatment on the other could help delineate whether this practice is evidence based.
For nonablative fractional laser resurfacing of acne scars, lower densities in darker skin types are recommended and may help reduce PIH risk. There is no statistically significant difference in improvement of acne scars in using low versus high densities using the same fluences. However, some studies note that higher densities clinically resulted in a mild improvement of acne scars over lower densities (not statistically significant); thus, if lower densities are used, it is possible that more treatments may be needed.4,5
Vigorous sun protection before and after treatment is prudent, with sun avoidance and physical sunscreens reducing the risk of PIH in darker skin from irritant or allergic contact dermatitis, compared with chemical sunscreens. If PIH occurs, it is often self limited (up to 1-2 months). Sun protection and posttreatment regimens of hydroquinone (or other lightening agent) aid in hastening improvement.
If the patient is undergoing nonablative laser resurfacing to treat pigmentation, such as melasma, then hydroquinone pre- and postlaser is appropriate. In my opinion, laser treatment of melasma should not be first line because of safety and efficacy concerns. However, in these cases, hydroquinone prior to laser has shown benefit.6 In addition, hydroquinone after nonablative fractional resurfacing may enhance penetration of the topical and improve efficacy.
In summary, the evidence shows that pretreatment with antipigment agents is not warranted in skin types I-III for ablative laser resurfacing. Pretreatment with antipigment agents for nonablative laser resurfacing for melasma (which should not be considered a first line treatment for melasma) is warranted. However, at this time, it is not clear whether pretreatment with antipigments for nonablative laser resurfacing for acne scars in darker skin types is useful. Lower densities should be used and if PIH does occur, it is usually self limited, and posttreatment hydroquinone or other antipigment agents may be useful.
References
1. Dermatol Surg. 1999 Jan;25(1):15-7.
2. Dermatol Surg. 2010 May;36(5):602-9.
3. Br J Dermatol. 2012 Jun;166(6):1160-9.
4.Lasers Surg Med. 2007 Jun;39(5):381-5.
5. Lasers Surg Med. 2007 Apr;39(4):311-4.
6. Dermatol Surg. 2010 Jun;36(6):909-18.
Dr. Wesley and Dr. Talakoub are co-contributors to the monthly Aesthetic Dermatology column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
Pretreatment of skin prior to nonablative or ablative laser resurfacing is common practice, particularly in darker skin types. Treatment regimens include using hydroquinone 4% (and other hydroquinone-containing combinations) once to twice daily for 1-2 weeks prior to the laser procedure. The rationale makes sense. Quieting melanin production by inhibiting tyrosinase would seem to decrease the incidence of postinflammatory hyperpigmentation after laser resurfacing procedures. But is this common practice effective?
For ablative CO2 resurfacing in 100 patients Fitzpatrick Skin Types (FST) I-III, there was no significant difference in the incidence of hyperpigmentation in those randomized to be pretreated with either hydroquinone, glycolic acid, tretinoin, or to no treatment.1 The thought was that the follicular melanocytes involved in re-epithelialization were not affected by the pretreatment. This is the only published laser resurfacing today to date examining various pretreatment protocols with hyperpigmentation as a primary study outcome. From this study, it seems as though pretreatment before laser resurfacing is not helpful, but what about for nonablative resurfacing in darker skin types (FST IV-VI)?
In darker skin types (FST IV-VI), the risk of postinflammatory hyperpigmentation (PIH) is inherently higher and the incidence after laser resurfacing is greater. While the incidence of PIH is lower with nonablative fractional resurfacing, compared with ablative resurfacing, PIH can still occur whether pretreatment hydroquinone is used or not.2,3,4 To date, there are no published studies looking at the incidence of PIH when comparing pretreatment antipigment agents versus no pretreatment for laser resurfacing for acne scars in darker skin types. A split-face study comparing pretreatment on one side and no pretreatment on the other could help delineate whether this practice is evidence based.
For nonablative fractional laser resurfacing of acne scars, lower densities in darker skin types are recommended and may help reduce PIH risk. There is no statistically significant difference in improvement of acne scars in using low versus high densities using the same fluences. However, some studies note that higher densities clinically resulted in a mild improvement of acne scars over lower densities (not statistically significant); thus, if lower densities are used, it is possible that more treatments may be needed.4,5
Vigorous sun protection before and after treatment is prudent, with sun avoidance and physical sunscreens reducing the risk of PIH in darker skin from irritant or allergic contact dermatitis, compared with chemical sunscreens. If PIH occurs, it is often self limited (up to 1-2 months). Sun protection and posttreatment regimens of hydroquinone (or other lightening agent) aid in hastening improvement.
If the patient is undergoing nonablative laser resurfacing to treat pigmentation, such as melasma, then hydroquinone pre- and postlaser is appropriate. In my opinion, laser treatment of melasma should not be first line because of safety and efficacy concerns. However, in these cases, hydroquinone prior to laser has shown benefit.6 In addition, hydroquinone after nonablative fractional resurfacing may enhance penetration of the topical and improve efficacy.
In summary, the evidence shows that pretreatment with antipigment agents is not warranted in skin types I-III for ablative laser resurfacing. Pretreatment with antipigment agents for nonablative laser resurfacing for melasma (which should not be considered a first line treatment for melasma) is warranted. However, at this time, it is not clear whether pretreatment with antipigments for nonablative laser resurfacing for acne scars in darker skin types is useful. Lower densities should be used and if PIH does occur, it is usually self limited, and posttreatment hydroquinone or other antipigment agents may be useful.
References
1. Dermatol Surg. 1999 Jan;25(1):15-7.
2. Dermatol Surg. 2010 May;36(5):602-9.
3. Br J Dermatol. 2012 Jun;166(6):1160-9.
4.Lasers Surg Med. 2007 Jun;39(5):381-5.
5. Lasers Surg Med. 2007 Apr;39(4):311-4.
6. Dermatol Surg. 2010 Jun;36(6):909-18.
Dr. Wesley and Dr. Talakoub are co-contributors to the monthly Aesthetic Dermatology column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
Study: PDT with methyl aminolevulinate promising for severe acne
Methyl aminolevulinate plus photodynamic therapy shows promise as a treatment for severe acne vulgaris, according to a U.S. study published in the British Journal of Dermatology.
Dr. David M. Pariser of Eastern Virginia Medical School, Norfolk, and his associates conducted the randomized double-blind, vehicle-controlled trial of photodynamic therapy (PDT) with methyl aminolevulinate (MAL) as a photosensitizer in 153 males and females aged 12-35 years with severe facial acne. Inclusion criteria comprised an Investigator Global Assessment (IGA) rating score of 4, 20-100 noninflammatory lesions, and 25-75 inflammatory lesions with no more than three nodules (Br J Dermatol. 2015 December. doi: 10.1111/bjd.14345).
Participants received topical MAL 80 mg/g (100) or vehicle cream (53) and PDT every 2 weeks for four treatments, and were evaluated at each treatment and at 12 weeks. The MAL or vehicle cream was applied and covered for 1.5 hours, followed by PDT (635 nm of red light for a total dose of 37 J/cm2). Most completed the study (85% in MAL group and 91% in vehicle group).
Those in the MAL PDT group demonstrated a significant reduction in inflammatory lesions based on the percentage change at 12 weeks (a mean reduction of 37.3% vs. 16.2%; P = .003), and absolute change (a mean reduction of 15.6 lesions vs. 7.8; P = .006). However, the reduction in noninflammatory lesions was not significantly different between the two groups (a mean reduction of 11.8 lesions among those on MAL PDT vs. 10.7 with vehicle PDT) .
The rates of treatment success, defined as improvement of 2 or more IGA grades at 12 weeks, were higher among those in the MAL PDT group (44% vs. 26.4%; OR, 3.24; P = .013).
Pain scores were higher during PDT for the MAL group and remained similar with subsequent treatments. The MAL treatment was discontinued in six participants because of pain and PDT was paused briefly in 15 participants. More participants in the MAL group reported moderate erythema after the first PDT session (46% versus 15%) and three reported severe erythema.
The most commonly reported adverse events in the MAL treatment group were a sensation of skin burning and pain, mostly mild to moderate, lasting a median of 3 days. Further, 12% of those in the MAL group withdrew secondary to adverse events.
“This large, controlled randomized clinical study shows the potential of PDT using 80 mg/g MAL cream for treatment of severe acne” in patients aged 12 years and older, with all skin types, the authors concluded, noting that more follow-up data are needed on the duration of treatment response and long-term effect on scarring. “Severe acne has limited therapeutic options with problematic side effects and bacterial resistance and 80 mg/g MAL PDT could be an alternative approach with improved tolerability for these patients,” they added.
The authors noted that for severe cases of acne, treatment is limited, and while oral isotretinoin is often used, it is teratogenic, has side effects, and is associated with reimbursement difficulties.
Dr. Pariser and two colleagues disclosed receiving honoraria from Photocure ASA, which markets MAL as Visonac. The company funded the study.
Methyl aminolevulinate plus photodynamic therapy shows promise as a treatment for severe acne vulgaris, according to a U.S. study published in the British Journal of Dermatology.
Dr. David M. Pariser of Eastern Virginia Medical School, Norfolk, and his associates conducted the randomized double-blind, vehicle-controlled trial of photodynamic therapy (PDT) with methyl aminolevulinate (MAL) as a photosensitizer in 153 males and females aged 12-35 years with severe facial acne. Inclusion criteria comprised an Investigator Global Assessment (IGA) rating score of 4, 20-100 noninflammatory lesions, and 25-75 inflammatory lesions with no more than three nodules (Br J Dermatol. 2015 December. doi: 10.1111/bjd.14345).
Participants received topical MAL 80 mg/g (100) or vehicle cream (53) and PDT every 2 weeks for four treatments, and were evaluated at each treatment and at 12 weeks. The MAL or vehicle cream was applied and covered for 1.5 hours, followed by PDT (635 nm of red light for a total dose of 37 J/cm2). Most completed the study (85% in MAL group and 91% in vehicle group).
Those in the MAL PDT group demonstrated a significant reduction in inflammatory lesions based on the percentage change at 12 weeks (a mean reduction of 37.3% vs. 16.2%; P = .003), and absolute change (a mean reduction of 15.6 lesions vs. 7.8; P = .006). However, the reduction in noninflammatory lesions was not significantly different between the two groups (a mean reduction of 11.8 lesions among those on MAL PDT vs. 10.7 with vehicle PDT) .
The rates of treatment success, defined as improvement of 2 or more IGA grades at 12 weeks, were higher among those in the MAL PDT group (44% vs. 26.4%; OR, 3.24; P = .013).
Pain scores were higher during PDT for the MAL group and remained similar with subsequent treatments. The MAL treatment was discontinued in six participants because of pain and PDT was paused briefly in 15 participants. More participants in the MAL group reported moderate erythema after the first PDT session (46% versus 15%) and three reported severe erythema.
The most commonly reported adverse events in the MAL treatment group were a sensation of skin burning and pain, mostly mild to moderate, lasting a median of 3 days. Further, 12% of those in the MAL group withdrew secondary to adverse events.
“This large, controlled randomized clinical study shows the potential of PDT using 80 mg/g MAL cream for treatment of severe acne” in patients aged 12 years and older, with all skin types, the authors concluded, noting that more follow-up data are needed on the duration of treatment response and long-term effect on scarring. “Severe acne has limited therapeutic options with problematic side effects and bacterial resistance and 80 mg/g MAL PDT could be an alternative approach with improved tolerability for these patients,” they added.
The authors noted that for severe cases of acne, treatment is limited, and while oral isotretinoin is often used, it is teratogenic, has side effects, and is associated with reimbursement difficulties.
Dr. Pariser and two colleagues disclosed receiving honoraria from Photocure ASA, which markets MAL as Visonac. The company funded the study.
Methyl aminolevulinate plus photodynamic therapy shows promise as a treatment for severe acne vulgaris, according to a U.S. study published in the British Journal of Dermatology.
Dr. David M. Pariser of Eastern Virginia Medical School, Norfolk, and his associates conducted the randomized double-blind, vehicle-controlled trial of photodynamic therapy (PDT) with methyl aminolevulinate (MAL) as a photosensitizer in 153 males and females aged 12-35 years with severe facial acne. Inclusion criteria comprised an Investigator Global Assessment (IGA) rating score of 4, 20-100 noninflammatory lesions, and 25-75 inflammatory lesions with no more than three nodules (Br J Dermatol. 2015 December. doi: 10.1111/bjd.14345).
Participants received topical MAL 80 mg/g (100) or vehicle cream (53) and PDT every 2 weeks for four treatments, and were evaluated at each treatment and at 12 weeks. The MAL or vehicle cream was applied and covered for 1.5 hours, followed by PDT (635 nm of red light for a total dose of 37 J/cm2). Most completed the study (85% in MAL group and 91% in vehicle group).
Those in the MAL PDT group demonstrated a significant reduction in inflammatory lesions based on the percentage change at 12 weeks (a mean reduction of 37.3% vs. 16.2%; P = .003), and absolute change (a mean reduction of 15.6 lesions vs. 7.8; P = .006). However, the reduction in noninflammatory lesions was not significantly different between the two groups (a mean reduction of 11.8 lesions among those on MAL PDT vs. 10.7 with vehicle PDT) .
The rates of treatment success, defined as improvement of 2 or more IGA grades at 12 weeks, were higher among those in the MAL PDT group (44% vs. 26.4%; OR, 3.24; P = .013).
Pain scores were higher during PDT for the MAL group and remained similar with subsequent treatments. The MAL treatment was discontinued in six participants because of pain and PDT was paused briefly in 15 participants. More participants in the MAL group reported moderate erythema after the first PDT session (46% versus 15%) and three reported severe erythema.
The most commonly reported adverse events in the MAL treatment group were a sensation of skin burning and pain, mostly mild to moderate, lasting a median of 3 days. Further, 12% of those in the MAL group withdrew secondary to adverse events.
“This large, controlled randomized clinical study shows the potential of PDT using 80 mg/g MAL cream for treatment of severe acne” in patients aged 12 years and older, with all skin types, the authors concluded, noting that more follow-up data are needed on the duration of treatment response and long-term effect on scarring. “Severe acne has limited therapeutic options with problematic side effects and bacterial resistance and 80 mg/g MAL PDT could be an alternative approach with improved tolerability for these patients,” they added.
The authors noted that for severe cases of acne, treatment is limited, and while oral isotretinoin is often used, it is teratogenic, has side effects, and is associated with reimbursement difficulties.
Dr. Pariser and two colleagues disclosed receiving honoraria from Photocure ASA, which markets MAL as Visonac. The company funded the study.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Methyl aminolevulinate (MAL) with photodynamic therapy (PDT) shows promise as an alternative treatment for severe acne vulgaris.
Major finding: The MAL/PDT group had a significantly higher rate of treatment success compared with the vehicle/PDT group at 12 weeks (44% vs. 26.4%; P = .013).
Data source: A randomized, double-blind, vehicle-controlled study evaluating the safety and efficacy of PDT with MAL in 153 people aged 12-35 years, with severe facial acne, at 15 outpatient dermatology centers in the United States.
Disclosures: Dr. Pariser and two colleagues disclosed receiving honoraria from MAL manufacturer Photocure ASA, which funded the study.
Insulin resistance in 22% of men with acne
Young adult men with acne were more likely to have insulin resistance and to have higher fasting plasma glucose levels than were men of the same age who did not have acne, in a cross-sectional study of 20 to 32 year old men in India.
In a study published online in JAMA Dermatology, on Dec. 23 (doi: 10.1001/jamadermatol.2015.4499), Dr. Mohit Nagpal, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India, and associates, wrote that “Insulin resistance may be a stage of prediabetes, and the patients may develop hyperinsulinemia or type 2 diabetes in the future. These patients should be followed up to determine whether they develop conditions associated with insulin resistance.”
The researchers compared 100 men with acne, aged 20 to 32 years, with 100 age-matched men who did not have acne and were being treated for non-acne dermatoses; all were being treated at the Institute’s dermatology outpatient department. Insulin resistance, as defined by a Homeostasis Model Assessment–Insulin Resistance (HOMA-IR) value greater than 2.5, was present in 22% of those with acne, vs. 11% of those without acne, a significant difference (P = .036). Metabolic syndrome, based on criteria of the modified National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATP III), was more common among those with acne (17% vs. 9%), but the difference was not significant (P = .09).
The mean diastolic and systolic blood pressure values were also significantly higher among those with acne, compared with controls, as were mean fasting plasma glucose levels.
When evaluated by acne severity (mild, moderate, severe, or very severe), there were no significant differences in the prevalence of insulin resistance or metabolic syndrome between the four groups. However, the mean body mass index and the mean weights among those with very severe acne were significantly higher than among those with mild acne (P = .04).
The cross-sectional design of the study was a limitation, the authors noted, and future studies will “follow up patients with acne to assess the development of clinical conditions associated with insulin resistance,” such as acanthosis nigricans and metabolic syndrome.
In an accompanying editorial, Dr. Rachel V. Reynolds of Beth Israel Deaconess Medical Center, Boston, wrote that this study, and another study published in the same issue regarding insulin resistance and polycystic ovary syndrome, “highlight the important role that the dermatologist plays in identifying and characterizing patients with common skin disorders who may be at risk for metabolic and androgen-mediated disease.” The study, “to our knowledge, [is] the largest cohort to date examining the prevalence of insulin resistance and metabolic syndrome in postadolescent males with acne of varying severity,” she added (doi: 10.1001/jamadermatol.2015.4500).
The authors of the study had no disclosures.
Young adult men with acne were more likely to have insulin resistance and to have higher fasting plasma glucose levels than were men of the same age who did not have acne, in a cross-sectional study of 20 to 32 year old men in India.
In a study published online in JAMA Dermatology, on Dec. 23 (doi: 10.1001/jamadermatol.2015.4499), Dr. Mohit Nagpal, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India, and associates, wrote that “Insulin resistance may be a stage of prediabetes, and the patients may develop hyperinsulinemia or type 2 diabetes in the future. These patients should be followed up to determine whether they develop conditions associated with insulin resistance.”
The researchers compared 100 men with acne, aged 20 to 32 years, with 100 age-matched men who did not have acne and were being treated for non-acne dermatoses; all were being treated at the Institute’s dermatology outpatient department. Insulin resistance, as defined by a Homeostasis Model Assessment–Insulin Resistance (HOMA-IR) value greater than 2.5, was present in 22% of those with acne, vs. 11% of those without acne, a significant difference (P = .036). Metabolic syndrome, based on criteria of the modified National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATP III), was more common among those with acne (17% vs. 9%), but the difference was not significant (P = .09).
The mean diastolic and systolic blood pressure values were also significantly higher among those with acne, compared with controls, as were mean fasting plasma glucose levels.
When evaluated by acne severity (mild, moderate, severe, or very severe), there were no significant differences in the prevalence of insulin resistance or metabolic syndrome between the four groups. However, the mean body mass index and the mean weights among those with very severe acne were significantly higher than among those with mild acne (P = .04).
The cross-sectional design of the study was a limitation, the authors noted, and future studies will “follow up patients with acne to assess the development of clinical conditions associated with insulin resistance,” such as acanthosis nigricans and metabolic syndrome.
In an accompanying editorial, Dr. Rachel V. Reynolds of Beth Israel Deaconess Medical Center, Boston, wrote that this study, and another study published in the same issue regarding insulin resistance and polycystic ovary syndrome, “highlight the important role that the dermatologist plays in identifying and characterizing patients with common skin disorders who may be at risk for metabolic and androgen-mediated disease.” The study, “to our knowledge, [is] the largest cohort to date examining the prevalence of insulin resistance and metabolic syndrome in postadolescent males with acne of varying severity,” she added (doi: 10.1001/jamadermatol.2015.4500).
The authors of the study had no disclosures.
Young adult men with acne were more likely to have insulin resistance and to have higher fasting plasma glucose levels than were men of the same age who did not have acne, in a cross-sectional study of 20 to 32 year old men in India.
In a study published online in JAMA Dermatology, on Dec. 23 (doi: 10.1001/jamadermatol.2015.4499), Dr. Mohit Nagpal, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India, and associates, wrote that “Insulin resistance may be a stage of prediabetes, and the patients may develop hyperinsulinemia or type 2 diabetes in the future. These patients should be followed up to determine whether they develop conditions associated with insulin resistance.”
The researchers compared 100 men with acne, aged 20 to 32 years, with 100 age-matched men who did not have acne and were being treated for non-acne dermatoses; all were being treated at the Institute’s dermatology outpatient department. Insulin resistance, as defined by a Homeostasis Model Assessment–Insulin Resistance (HOMA-IR) value greater than 2.5, was present in 22% of those with acne, vs. 11% of those without acne, a significant difference (P = .036). Metabolic syndrome, based on criteria of the modified National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATP III), was more common among those with acne (17% vs. 9%), but the difference was not significant (P = .09).
The mean diastolic and systolic blood pressure values were also significantly higher among those with acne, compared with controls, as were mean fasting plasma glucose levels.
When evaluated by acne severity (mild, moderate, severe, or very severe), there were no significant differences in the prevalence of insulin resistance or metabolic syndrome between the four groups. However, the mean body mass index and the mean weights among those with very severe acne were significantly higher than among those with mild acne (P = .04).
The cross-sectional design of the study was a limitation, the authors noted, and future studies will “follow up patients with acne to assess the development of clinical conditions associated with insulin resistance,” such as acanthosis nigricans and metabolic syndrome.
In an accompanying editorial, Dr. Rachel V. Reynolds of Beth Israel Deaconess Medical Center, Boston, wrote that this study, and another study published in the same issue regarding insulin resistance and polycystic ovary syndrome, “highlight the important role that the dermatologist plays in identifying and characterizing patients with common skin disorders who may be at risk for metabolic and androgen-mediated disease.” The study, “to our knowledge, [is] the largest cohort to date examining the prevalence of insulin resistance and metabolic syndrome in postadolescent males with acne of varying severity,” she added (doi: 10.1001/jamadermatol.2015.4500).
The authors of the study had no disclosures.
FROM JAMA DERMATOLOGY
Key clinical point: Acne in young men may be a sign of insulin resistance.
Major finding: 22% of the young men with acne had insulin resistance, compared with 11% of the age-matched controls, a significant difference (P = .036).
Data source: The cross-sectional study compared the prevalence of insulin resistance and metabolic syndrome in 100 men aged 20-32 years with acne and 100 age-matched controls without acne.
Disclosures: The authors had no disclosures.
Botanical over-the-counter regimen reduces acne lesions
An over-the-counter botanical acne regimen outperformed a conventional acne regimen, with improved skin appearance and fewer lesions after 12 weeks of treatment, in a double-blind randomized controlled trial.
Eighty individuals aged 12 years and older with mild to moderate acne were randomized either to a three-step botanical-based acne treatment regimen (Receutics) consisting of a skin cleanser, breakout treatment, and tone and complexion corrector twice daily, or the currently marketed acne treatment kit, Proactiv (Guthy-Renker).
The botanical-based acne treatment contains a range of botanical ingredients, including algae and lentil seed extracts; cranberry seed, grape seed, and pumpkin seed oils; and allantoin; with 3.4% benzoyl peroxide as the active ingredient. The active ingredient in the cleanser is 2% salicylic acid, and niacinamide is the active ingredient in the tone and complexion corrector; both also contain botanical ingredients.
In the study, published in December, the investigator, Dr. Zoe Diana Draelos, reported that the botanical regimen achieved a significantly greater reduction in lesion count, in terms of closed comedones and inflammatory lesions, by week four, compared with the control treatment (J Drugs Dermatol. 2015 Dec; 14 [12]:1418-21).
This effect persisted at 12 weeks, with fewer closed comedones (P = .006). The botanical regimen also achieved greater reductions in pus, erythema, lesion height, and inflammation at weeks 2 and 4; although this difference disappeared by week 12. By week four and onwards, the botanical regimen also outperformed the conventional treatment on all blinded, investigator-assessed cosmetic appearance parameters, including skin tone, blemishes, erythema, and overall appearance.
While both treatments were effective at improving acne, Dr. Draelos, of Dermatology Consulting Services, High Point, N.C., said the botanical three-step regimen had the advantage of cosmetic ingredients such as emollients, anti-inflammatory/antioxidants, and “sensitive skin modulators.”
“This study demonstrates the value of combining monographed acne ingredients with advanced cosmeceutical technology,” she wrote.
The author received a grant from manufacturer Receutics to conduct the study.
An over-the-counter botanical acne regimen outperformed a conventional acne regimen, with improved skin appearance and fewer lesions after 12 weeks of treatment, in a double-blind randomized controlled trial.
Eighty individuals aged 12 years and older with mild to moderate acne were randomized either to a three-step botanical-based acne treatment regimen (Receutics) consisting of a skin cleanser, breakout treatment, and tone and complexion corrector twice daily, or the currently marketed acne treatment kit, Proactiv (Guthy-Renker).
The botanical-based acne treatment contains a range of botanical ingredients, including algae and lentil seed extracts; cranberry seed, grape seed, and pumpkin seed oils; and allantoin; with 3.4% benzoyl peroxide as the active ingredient. The active ingredient in the cleanser is 2% salicylic acid, and niacinamide is the active ingredient in the tone and complexion corrector; both also contain botanical ingredients.
In the study, published in December, the investigator, Dr. Zoe Diana Draelos, reported that the botanical regimen achieved a significantly greater reduction in lesion count, in terms of closed comedones and inflammatory lesions, by week four, compared with the control treatment (J Drugs Dermatol. 2015 Dec; 14 [12]:1418-21).
This effect persisted at 12 weeks, with fewer closed comedones (P = .006). The botanical regimen also achieved greater reductions in pus, erythema, lesion height, and inflammation at weeks 2 and 4; although this difference disappeared by week 12. By week four and onwards, the botanical regimen also outperformed the conventional treatment on all blinded, investigator-assessed cosmetic appearance parameters, including skin tone, blemishes, erythema, and overall appearance.
While both treatments were effective at improving acne, Dr. Draelos, of Dermatology Consulting Services, High Point, N.C., said the botanical three-step regimen had the advantage of cosmetic ingredients such as emollients, anti-inflammatory/antioxidants, and “sensitive skin modulators.”
“This study demonstrates the value of combining monographed acne ingredients with advanced cosmeceutical technology,” she wrote.
The author received a grant from manufacturer Receutics to conduct the study.
An over-the-counter botanical acne regimen outperformed a conventional acne regimen, with improved skin appearance and fewer lesions after 12 weeks of treatment, in a double-blind randomized controlled trial.
Eighty individuals aged 12 years and older with mild to moderate acne were randomized either to a three-step botanical-based acne treatment regimen (Receutics) consisting of a skin cleanser, breakout treatment, and tone and complexion corrector twice daily, or the currently marketed acne treatment kit, Proactiv (Guthy-Renker).
The botanical-based acne treatment contains a range of botanical ingredients, including algae and lentil seed extracts; cranberry seed, grape seed, and pumpkin seed oils; and allantoin; with 3.4% benzoyl peroxide as the active ingredient. The active ingredient in the cleanser is 2% salicylic acid, and niacinamide is the active ingredient in the tone and complexion corrector; both also contain botanical ingredients.
In the study, published in December, the investigator, Dr. Zoe Diana Draelos, reported that the botanical regimen achieved a significantly greater reduction in lesion count, in terms of closed comedones and inflammatory lesions, by week four, compared with the control treatment (J Drugs Dermatol. 2015 Dec; 14 [12]:1418-21).
This effect persisted at 12 weeks, with fewer closed comedones (P = .006). The botanical regimen also achieved greater reductions in pus, erythema, lesion height, and inflammation at weeks 2 and 4; although this difference disappeared by week 12. By week four and onwards, the botanical regimen also outperformed the conventional treatment on all blinded, investigator-assessed cosmetic appearance parameters, including skin tone, blemishes, erythema, and overall appearance.
While both treatments were effective at improving acne, Dr. Draelos, of Dermatology Consulting Services, High Point, N.C., said the botanical three-step regimen had the advantage of cosmetic ingredients such as emollients, anti-inflammatory/antioxidants, and “sensitive skin modulators.”
“This study demonstrates the value of combining monographed acne ingredients with advanced cosmeceutical technology,” she wrote.
The author received a grant from manufacturer Receutics to conduct the study.
FROM THE JOURNAL OF DRUGS IN DERMATOLOGY
Key clinical point: A botanical over-the-counter acne regimen achieved greater reductions in lesions than a currently marketed acne treatment.
Major finding: A three-step botanical-based acne regimen achieved significantly greater reduction in lesion counts and improvements in skin appearance than a conventional acne treatment.
Data source: A randomized, double-blind, controlled trial of 80 patients with mild to moderate acne.
Disclosures: The author received a grant from treatment manufacturer Receutics to conduct the study.
New low-dose antibiotics, topicals offer options for acne
LAS VEGAS – New acne treatment strategies that address the issue of antibiotic resistance include subantimicrobial dosing; new, narrower-spectrum antibiotics; and topical use of tetracycline-family antibiotics, according to Dr. Linda Stein Gold, a dermatologist at Henry Ford Hospital, Detroit.
Oral antibiotics have long been a mainstay of acne treatment, but long-term use of low-dose antibiotics may be contributing to the global crisis of antibiotic resistance. At least 2 million people become infected with resistant bacteria yearly in the United States alone, and at least 23,000 people die yearly from these infections, she noted.
“In dermatology we use antibiotics quite a bit, and we want to make sure when we’re utilizing drugs, we’re utilizing them in the best possible way,” Dr. Stein Gold said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. Finding the right antibiotic dose for effective treatment of acne can be a challenge, she noted. “Is more better? Is too little bad?”
In a review of new treatment strategies that address these concerns without compromising efficacy, Dr. Stein Gold said that the rationale for using subantimicrobial antibiotic dosing comes from the anti-inflammatory effect seen with many antibiotics, even with doses lower than needed for antimicrobial action.
For example, a study of a subantimicrobial-dose of doxycycline found that when adults with moderate acne were treated with the antibiotic (20 mg, twice daily) for 6 months, their acne significantly improved. The number of comedones, inflammatory lesions, and noninflammatory lesions improved significantly compared with those on placebo (Arch Dermatol. 2003 Apr; 139:459-64).
In another head-to-head trial that compared low-dose modified-release doxycycline with placebo or 100 mg of doxycycline, the lower dose outperformed both placebo and full-strength antibiotics. No resistant organisms were found among skin flora in the subjects, and the microbiota of the patients’ skin did not change significantly during the study period, she said.
Dr. Stein Gold’s work also suggests that systemic antibiotics may not be necessary for all patients with acne: In a study, after 12 weeks of treatment, adapalene plus benzoyl peroxide, in combination with doxycycline, resulted in significantly more patients with clear or almost-clear skin than with vehicle alone plus doxycycline. “Antibiotics are not always the golden nugget in the treatment of acne,” she commented.
Another tactic is to treat with antibiotics for a period of 3-6 months along with potent topicals, to get skin clear or almost clear, then discontinue the antibiotic and continue topical treatment. Many patients will be able to maintain clear skin on this regime, she noted.
A new tetracycline-family antibiotic, sarecycline, is in phase III trials for acne vulgaris and in phase II trials for acne rosacea. Sarecycline, “compared with existing tetracycline antibiotics, showed improved anti-inflammatory properties and a narrower spectrum of activity,” Dr. Stein Gold said.
A topical minocycline in a foam formulation shows promising results for tolerability and efficacy in phase II trials for moderate and severe acne, she added. Dapsone as a 7.5% topical gel formulation is in phase III clinical trials as well.
Another antibiotic with a long history of systemic use for acne, clindamycin, is also showing promising results in combination with benzoyl peroxide (1.2%/3.75% gel). A 12-week double-blind study of the combination, compared with vehicle alone for individuals with moderate or severe acne, showed significant improvement in comedonal and inflammatory lesions, as well as overall global improvement in severity, for the treatment arm, she said (J Drugs Dermatol. 2014 Sep;13[9]:1083-9).
Dr. Stein Gold reports being a consultant and investigator for Galderma, Stiefel Laboratories, and Allergan; a consultant and speaker for Valeant; a speaker for Ranbaxy Laboratories, Promius Pharma, and Actavis; and a medical/legal consultant for Roche.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – New acne treatment strategies that address the issue of antibiotic resistance include subantimicrobial dosing; new, narrower-spectrum antibiotics; and topical use of tetracycline-family antibiotics, according to Dr. Linda Stein Gold, a dermatologist at Henry Ford Hospital, Detroit.
Oral antibiotics have long been a mainstay of acne treatment, but long-term use of low-dose antibiotics may be contributing to the global crisis of antibiotic resistance. At least 2 million people become infected with resistant bacteria yearly in the United States alone, and at least 23,000 people die yearly from these infections, she noted.
“In dermatology we use antibiotics quite a bit, and we want to make sure when we’re utilizing drugs, we’re utilizing them in the best possible way,” Dr. Stein Gold said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. Finding the right antibiotic dose for effective treatment of acne can be a challenge, she noted. “Is more better? Is too little bad?”
In a review of new treatment strategies that address these concerns without compromising efficacy, Dr. Stein Gold said that the rationale for using subantimicrobial antibiotic dosing comes from the anti-inflammatory effect seen with many antibiotics, even with doses lower than needed for antimicrobial action.
For example, a study of a subantimicrobial-dose of doxycycline found that when adults with moderate acne were treated with the antibiotic (20 mg, twice daily) for 6 months, their acne significantly improved. The number of comedones, inflammatory lesions, and noninflammatory lesions improved significantly compared with those on placebo (Arch Dermatol. 2003 Apr; 139:459-64).
In another head-to-head trial that compared low-dose modified-release doxycycline with placebo or 100 mg of doxycycline, the lower dose outperformed both placebo and full-strength antibiotics. No resistant organisms were found among skin flora in the subjects, and the microbiota of the patients’ skin did not change significantly during the study period, she said.
Dr. Stein Gold’s work also suggests that systemic antibiotics may not be necessary for all patients with acne: In a study, after 12 weeks of treatment, adapalene plus benzoyl peroxide, in combination with doxycycline, resulted in significantly more patients with clear or almost-clear skin than with vehicle alone plus doxycycline. “Antibiotics are not always the golden nugget in the treatment of acne,” she commented.
Another tactic is to treat with antibiotics for a period of 3-6 months along with potent topicals, to get skin clear or almost clear, then discontinue the antibiotic and continue topical treatment. Many patients will be able to maintain clear skin on this regime, she noted.
A new tetracycline-family antibiotic, sarecycline, is in phase III trials for acne vulgaris and in phase II trials for acne rosacea. Sarecycline, “compared with existing tetracycline antibiotics, showed improved anti-inflammatory properties and a narrower spectrum of activity,” Dr. Stein Gold said.
A topical minocycline in a foam formulation shows promising results for tolerability and efficacy in phase II trials for moderate and severe acne, she added. Dapsone as a 7.5% topical gel formulation is in phase III clinical trials as well.
Another antibiotic with a long history of systemic use for acne, clindamycin, is also showing promising results in combination with benzoyl peroxide (1.2%/3.75% gel). A 12-week double-blind study of the combination, compared with vehicle alone for individuals with moderate or severe acne, showed significant improvement in comedonal and inflammatory lesions, as well as overall global improvement in severity, for the treatment arm, she said (J Drugs Dermatol. 2014 Sep;13[9]:1083-9).
Dr. Stein Gold reports being a consultant and investigator for Galderma, Stiefel Laboratories, and Allergan; a consultant and speaker for Valeant; a speaker for Ranbaxy Laboratories, Promius Pharma, and Actavis; and a medical/legal consultant for Roche.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – New acne treatment strategies that address the issue of antibiotic resistance include subantimicrobial dosing; new, narrower-spectrum antibiotics; and topical use of tetracycline-family antibiotics, according to Dr. Linda Stein Gold, a dermatologist at Henry Ford Hospital, Detroit.
Oral antibiotics have long been a mainstay of acne treatment, but long-term use of low-dose antibiotics may be contributing to the global crisis of antibiotic resistance. At least 2 million people become infected with resistant bacteria yearly in the United States alone, and at least 23,000 people die yearly from these infections, she noted.
“In dermatology we use antibiotics quite a bit, and we want to make sure when we’re utilizing drugs, we’re utilizing them in the best possible way,” Dr. Stein Gold said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. Finding the right antibiotic dose for effective treatment of acne can be a challenge, she noted. “Is more better? Is too little bad?”
In a review of new treatment strategies that address these concerns without compromising efficacy, Dr. Stein Gold said that the rationale for using subantimicrobial antibiotic dosing comes from the anti-inflammatory effect seen with many antibiotics, even with doses lower than needed for antimicrobial action.
For example, a study of a subantimicrobial-dose of doxycycline found that when adults with moderate acne were treated with the antibiotic (20 mg, twice daily) for 6 months, their acne significantly improved. The number of comedones, inflammatory lesions, and noninflammatory lesions improved significantly compared with those on placebo (Arch Dermatol. 2003 Apr; 139:459-64).
In another head-to-head trial that compared low-dose modified-release doxycycline with placebo or 100 mg of doxycycline, the lower dose outperformed both placebo and full-strength antibiotics. No resistant organisms were found among skin flora in the subjects, and the microbiota of the patients’ skin did not change significantly during the study period, she said.
Dr. Stein Gold’s work also suggests that systemic antibiotics may not be necessary for all patients with acne: In a study, after 12 weeks of treatment, adapalene plus benzoyl peroxide, in combination with doxycycline, resulted in significantly more patients with clear or almost-clear skin than with vehicle alone plus doxycycline. “Antibiotics are not always the golden nugget in the treatment of acne,” she commented.
Another tactic is to treat with antibiotics for a period of 3-6 months along with potent topicals, to get skin clear or almost clear, then discontinue the antibiotic and continue topical treatment. Many patients will be able to maintain clear skin on this regime, she noted.
A new tetracycline-family antibiotic, sarecycline, is in phase III trials for acne vulgaris and in phase II trials for acne rosacea. Sarecycline, “compared with existing tetracycline antibiotics, showed improved anti-inflammatory properties and a narrower spectrum of activity,” Dr. Stein Gold said.
A topical minocycline in a foam formulation shows promising results for tolerability and efficacy in phase II trials for moderate and severe acne, she added. Dapsone as a 7.5% topical gel formulation is in phase III clinical trials as well.
Another antibiotic with a long history of systemic use for acne, clindamycin, is also showing promising results in combination with benzoyl peroxide (1.2%/3.75% gel). A 12-week double-blind study of the combination, compared with vehicle alone for individuals with moderate or severe acne, showed significant improvement in comedonal and inflammatory lesions, as well as overall global improvement in severity, for the treatment arm, she said (J Drugs Dermatol. 2014 Sep;13[9]:1083-9).
Dr. Stein Gold reports being a consultant and investigator for Galderma, Stiefel Laboratories, and Allergan; a consultant and speaker for Valeant; a speaker for Ranbaxy Laboratories, Promius Pharma, and Actavis; and a medical/legal consultant for Roche.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
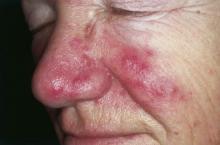
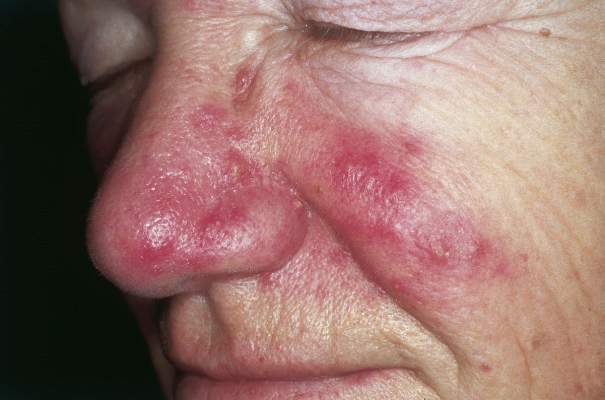
Inflammatory loop drives rosacea
NEW YORK – Rosacea seems to represent a Möbius strip of an overly enthusiastic immune response, with chemicals that recruit neutrophils, which, in turn, activate even more immunomodulators.
Researchers have been pointing the finger at trigger-happy neutrophils for more than a decade, Dr. David E. Cohen said at the summer meeting of the American Academy of Dermatology. But this group of cells is really more of an accomplice than a mastermind.
“Neutrophils were once the alpha and omega of rosacea,” said Dr. Cohen, the Charles C. and Dorothea E. Harris Professor of Dermatology, New York University. “But what are they doing on the skin and why are they there? They’re doing exactly what they’re supposed to do – defending against invasion by dumping out a whole poisonous soup of chemicals,” designed to protect and defend against microbes.
In the chronic disease of rosacea, however, these frontline soldiers are constantly recruited, even when there’s no invader to fight. They continue to carry out their preprogrammed attacks, releasing proteins that activate enzymes that stimulate immunomodulators that, in turn, call more neutrophils to the battlefield. Now, new research suggests that cathelicidins – antimicrobial peptides that are especially present in epithelial cells – are giving neutrophils their marching orders.
“Cathelicidins sit fully engaged on the skin and, the minute there’s a breach, they are turned on as the first defense,” Dr. Cohen said. “We need that kind of help right away – we can’t afford to wait around” for the second wave of defenders: neutrophils and lymphocytes.
This command seems to be bidirectional, though. Neutrophils release matrix metalloproteases (MMPs), enzymes critical for wound healing. MMPs remove dead extracellular matrix and microbes, prepare the wound bed for angiogenesis, and stimulate epidermal cell migration. But these benefits also exacerbate the things that make rosacea such a tough problem. By dissolving tissue, MMPs ramp up inflammation. By stimulating angiogenesis, they contribute to vasodilation, erythema, and telangiectasias.
And, Dr. Cohen noted, MMPs also just happen to recruit more neutrophils, which are capable of activating triptych enzymes, which turn on cathelicidins. “Now, a loop is being theorized,” he said. “The recruitment tools are kept on and this keeps cycling and cycling allowing the neutrophils to stay in the skin” for years.
The exaggerated presence of MMPs is likely the very reason that the tetracycline class of antibiotics can be so effective during rosacea exacerbations, Dr. Cohen said. The drugs aren’t necessarily fighting bacteria – instead, their anti-MMP properties function as a temporary break in this inflammatory loop. “They interfere with MMP, and you can’t turn on cathelicidin without MMP. If you lock MMP down, you can break the cycle because there is no neutrophil recruitment.”
Interestingly, he said, antibiotics that target MMP appear to suppress the enzymes at subantimicrobial doses. “This opens the possibility that we might be able to treat with these drugs and not exert antibiotic effects,” lessening the problems associated with indiscriminate antibiotic usage.
Inflammatory overdrive makes rosacea skin hypersensitive to any kind of inflammatory trigger, said Dr. Hilary Baldwin, medical director of the Acne Treatment and Research Center in Morristown, N.J. At the meeting, Dr. Baldwin discussed several new topical medications aimed at breaking the cycle:
• 1% Ivermectin: People with rosacea can harbor up to six times more demodex mites than can people without the disorder. The explanation behind this relationship is unclear: Is rosacea skin somehow more hospitable to the mite, or is an excess of mites a direct causative factor? Whatever the interaction, topical ivermectin breaks the link as both an antimicrobial and an anti-inflammatory agent. Ivermectin directly inhibits cellular and humoral immune response, and regulates tumor necrosis factor–alpha and interleukins 1-beta and 10.
Two randomized, double-blind vehicle-controlled studies evaluating 1% ivermectin cream in patients with papulopustular rosacea found that almost 80% of rosacea patients were clear or nearly clear on the Investigator’s Global Assessment (IGA) scale at 12 weeks – significantly more than those who were using a vehicle placebo. Inflammatory lesions significantly decreased and adverse events were similar to those associated with the vehicle (J Drugs Dermatol. 2014 Mar;13[3]:316-23). Another study found that ivermectin was significantly more effective than metronidazole, she said.
• Azelaic acid: A phase III study examined azelaic acid foam in almost 1,000 patients with moderate to severe papulopustular rosacea. IGA was clear or near-clear in 32% of the active group at 12 weeks, and 23.5% of the vehicle control group – a significant difference. There were significantly fewer inflammatory lesions among those in the active treatment group as well. Drug-related cutaneous adverse events were significantly more common among those treated with azelaic acid (7% vs. about 4%) and were mostly pain, pruritus, and dryness at application site (Cutis. 2015 Jul;96[1]:54-61).
• Oxymetazoline and brimonidine: These two alpha-adrenergic receptor agonists are under investigation for their ability to decrease erythema. Both cause a transient vasoconstriction. Two studies of patients with moderate or severe erythema of rosacea found that a topical brimonidine gel formulation significantly decreased erythema for up to 12 hours after application, compared with a vehicle gel (J Drugs Dermatol. 2013 Jun 1;12[6]:650-6). However, there have been several case reports of severe rebound, with redness not only returning, but returning at a more severe grade (J Am Acad Dermatol. 2014 Feb;70[2]:e37-8).
• Isotretinoin: New investigations are looking at topical retinoids for rosacea. They do appear effective – but not only for the reason they work in acne. While they decrease the size of sebaceous glands and the amount of sebum produced, they seem to exert their benefit through an anti-inflammatory pathway, as well. Isotretinoin significantly decreases toll-like receptor-2 expression on monocytes.
Isotretinoin has been shown to be more effective than doxycycline in clearing skin (J Dtsch Dermatol Ges. 2010 Jul;8[7]:505-15) but has also seemed helpful in preventing and slowing phyma development and improving ocular disease.
Both Dr. Cohen and Dr. Baldwin disclosed relationships with multiple pharmaceutical companies.
NEW YORK – Rosacea seems to represent a Möbius strip of an overly enthusiastic immune response, with chemicals that recruit neutrophils, which, in turn, activate even more immunomodulators.
Researchers have been pointing the finger at trigger-happy neutrophils for more than a decade, Dr. David E. Cohen said at the summer meeting of the American Academy of Dermatology. But this group of cells is really more of an accomplice than a mastermind.
“Neutrophils were once the alpha and omega of rosacea,” said Dr. Cohen, the Charles C. and Dorothea E. Harris Professor of Dermatology, New York University. “But what are they doing on the skin and why are they there? They’re doing exactly what they’re supposed to do – defending against invasion by dumping out a whole poisonous soup of chemicals,” designed to protect and defend against microbes.
In the chronic disease of rosacea, however, these frontline soldiers are constantly recruited, even when there’s no invader to fight. They continue to carry out their preprogrammed attacks, releasing proteins that activate enzymes that stimulate immunomodulators that, in turn, call more neutrophils to the battlefield. Now, new research suggests that cathelicidins – antimicrobial peptides that are especially present in epithelial cells – are giving neutrophils their marching orders.
“Cathelicidins sit fully engaged on the skin and, the minute there’s a breach, they are turned on as the first defense,” Dr. Cohen said. “We need that kind of help right away – we can’t afford to wait around” for the second wave of defenders: neutrophils and lymphocytes.
This command seems to be bidirectional, though. Neutrophils release matrix metalloproteases (MMPs), enzymes critical for wound healing. MMPs remove dead extracellular matrix and microbes, prepare the wound bed for angiogenesis, and stimulate epidermal cell migration. But these benefits also exacerbate the things that make rosacea such a tough problem. By dissolving tissue, MMPs ramp up inflammation. By stimulating angiogenesis, they contribute to vasodilation, erythema, and telangiectasias.
And, Dr. Cohen noted, MMPs also just happen to recruit more neutrophils, which are capable of activating triptych enzymes, which turn on cathelicidins. “Now, a loop is being theorized,” he said. “The recruitment tools are kept on and this keeps cycling and cycling allowing the neutrophils to stay in the skin” for years.
The exaggerated presence of MMPs is likely the very reason that the tetracycline class of antibiotics can be so effective during rosacea exacerbations, Dr. Cohen said. The drugs aren’t necessarily fighting bacteria – instead, their anti-MMP properties function as a temporary break in this inflammatory loop. “They interfere with MMP, and you can’t turn on cathelicidin without MMP. If you lock MMP down, you can break the cycle because there is no neutrophil recruitment.”
Interestingly, he said, antibiotics that target MMP appear to suppress the enzymes at subantimicrobial doses. “This opens the possibility that we might be able to treat with these drugs and not exert antibiotic effects,” lessening the problems associated with indiscriminate antibiotic usage.
Inflammatory overdrive makes rosacea skin hypersensitive to any kind of inflammatory trigger, said Dr. Hilary Baldwin, medical director of the Acne Treatment and Research Center in Morristown, N.J. At the meeting, Dr. Baldwin discussed several new topical medications aimed at breaking the cycle:
• 1% Ivermectin: People with rosacea can harbor up to six times more demodex mites than can people without the disorder. The explanation behind this relationship is unclear: Is rosacea skin somehow more hospitable to the mite, or is an excess of mites a direct causative factor? Whatever the interaction, topical ivermectin breaks the link as both an antimicrobial and an anti-inflammatory agent. Ivermectin directly inhibits cellular and humoral immune response, and regulates tumor necrosis factor–alpha and interleukins 1-beta and 10.
Two randomized, double-blind vehicle-controlled studies evaluating 1% ivermectin cream in patients with papulopustular rosacea found that almost 80% of rosacea patients were clear or nearly clear on the Investigator’s Global Assessment (IGA) scale at 12 weeks – significantly more than those who were using a vehicle placebo. Inflammatory lesions significantly decreased and adverse events were similar to those associated with the vehicle (J Drugs Dermatol. 2014 Mar;13[3]:316-23). Another study found that ivermectin was significantly more effective than metronidazole, she said.
• Azelaic acid: A phase III study examined azelaic acid foam in almost 1,000 patients with moderate to severe papulopustular rosacea. IGA was clear or near-clear in 32% of the active group at 12 weeks, and 23.5% of the vehicle control group – a significant difference. There were significantly fewer inflammatory lesions among those in the active treatment group as well. Drug-related cutaneous adverse events were significantly more common among those treated with azelaic acid (7% vs. about 4%) and were mostly pain, pruritus, and dryness at application site (Cutis. 2015 Jul;96[1]:54-61).
• Oxymetazoline and brimonidine: These two alpha-adrenergic receptor agonists are under investigation for their ability to decrease erythema. Both cause a transient vasoconstriction. Two studies of patients with moderate or severe erythema of rosacea found that a topical brimonidine gel formulation significantly decreased erythema for up to 12 hours after application, compared with a vehicle gel (J Drugs Dermatol. 2013 Jun 1;12[6]:650-6). However, there have been several case reports of severe rebound, with redness not only returning, but returning at a more severe grade (J Am Acad Dermatol. 2014 Feb;70[2]:e37-8).
• Isotretinoin: New investigations are looking at topical retinoids for rosacea. They do appear effective – but not only for the reason they work in acne. While they decrease the size of sebaceous glands and the amount of sebum produced, they seem to exert their benefit through an anti-inflammatory pathway, as well. Isotretinoin significantly decreases toll-like receptor-2 expression on monocytes.
Isotretinoin has been shown to be more effective than doxycycline in clearing skin (J Dtsch Dermatol Ges. 2010 Jul;8[7]:505-15) but has also seemed helpful in preventing and slowing phyma development and improving ocular disease.
Both Dr. Cohen and Dr. Baldwin disclosed relationships with multiple pharmaceutical companies.
NEW YORK – Rosacea seems to represent a Möbius strip of an overly enthusiastic immune response, with chemicals that recruit neutrophils, which, in turn, activate even more immunomodulators.
Researchers have been pointing the finger at trigger-happy neutrophils for more than a decade, Dr. David E. Cohen said at the summer meeting of the American Academy of Dermatology. But this group of cells is really more of an accomplice than a mastermind.
“Neutrophils were once the alpha and omega of rosacea,” said Dr. Cohen, the Charles C. and Dorothea E. Harris Professor of Dermatology, New York University. “But what are they doing on the skin and why are they there? They’re doing exactly what they’re supposed to do – defending against invasion by dumping out a whole poisonous soup of chemicals,” designed to protect and defend against microbes.
In the chronic disease of rosacea, however, these frontline soldiers are constantly recruited, even when there’s no invader to fight. They continue to carry out their preprogrammed attacks, releasing proteins that activate enzymes that stimulate immunomodulators that, in turn, call more neutrophils to the battlefield. Now, new research suggests that cathelicidins – antimicrobial peptides that are especially present in epithelial cells – are giving neutrophils their marching orders.
“Cathelicidins sit fully engaged on the skin and, the minute there’s a breach, they are turned on as the first defense,” Dr. Cohen said. “We need that kind of help right away – we can’t afford to wait around” for the second wave of defenders: neutrophils and lymphocytes.
This command seems to be bidirectional, though. Neutrophils release matrix metalloproteases (MMPs), enzymes critical for wound healing. MMPs remove dead extracellular matrix and microbes, prepare the wound bed for angiogenesis, and stimulate epidermal cell migration. But these benefits also exacerbate the things that make rosacea such a tough problem. By dissolving tissue, MMPs ramp up inflammation. By stimulating angiogenesis, they contribute to vasodilation, erythema, and telangiectasias.
And, Dr. Cohen noted, MMPs also just happen to recruit more neutrophils, which are capable of activating triptych enzymes, which turn on cathelicidins. “Now, a loop is being theorized,” he said. “The recruitment tools are kept on and this keeps cycling and cycling allowing the neutrophils to stay in the skin” for years.
The exaggerated presence of MMPs is likely the very reason that the tetracycline class of antibiotics can be so effective during rosacea exacerbations, Dr. Cohen said. The drugs aren’t necessarily fighting bacteria – instead, their anti-MMP properties function as a temporary break in this inflammatory loop. “They interfere with MMP, and you can’t turn on cathelicidin without MMP. If you lock MMP down, you can break the cycle because there is no neutrophil recruitment.”
Interestingly, he said, antibiotics that target MMP appear to suppress the enzymes at subantimicrobial doses. “This opens the possibility that we might be able to treat with these drugs and not exert antibiotic effects,” lessening the problems associated with indiscriminate antibiotic usage.
Inflammatory overdrive makes rosacea skin hypersensitive to any kind of inflammatory trigger, said Dr. Hilary Baldwin, medical director of the Acne Treatment and Research Center in Morristown, N.J. At the meeting, Dr. Baldwin discussed several new topical medications aimed at breaking the cycle:
• 1% Ivermectin: People with rosacea can harbor up to six times more demodex mites than can people without the disorder. The explanation behind this relationship is unclear: Is rosacea skin somehow more hospitable to the mite, or is an excess of mites a direct causative factor? Whatever the interaction, topical ivermectin breaks the link as both an antimicrobial and an anti-inflammatory agent. Ivermectin directly inhibits cellular and humoral immune response, and regulates tumor necrosis factor–alpha and interleukins 1-beta and 10.
Two randomized, double-blind vehicle-controlled studies evaluating 1% ivermectin cream in patients with papulopustular rosacea found that almost 80% of rosacea patients were clear or nearly clear on the Investigator’s Global Assessment (IGA) scale at 12 weeks – significantly more than those who were using a vehicle placebo. Inflammatory lesions significantly decreased and adverse events were similar to those associated with the vehicle (J Drugs Dermatol. 2014 Mar;13[3]:316-23). Another study found that ivermectin was significantly more effective than metronidazole, she said.
• Azelaic acid: A phase III study examined azelaic acid foam in almost 1,000 patients with moderate to severe papulopustular rosacea. IGA was clear or near-clear in 32% of the active group at 12 weeks, and 23.5% of the vehicle control group – a significant difference. There were significantly fewer inflammatory lesions among those in the active treatment group as well. Drug-related cutaneous adverse events were significantly more common among those treated with azelaic acid (7% vs. about 4%) and were mostly pain, pruritus, and dryness at application site (Cutis. 2015 Jul;96[1]:54-61).
• Oxymetazoline and brimonidine: These two alpha-adrenergic receptor agonists are under investigation for their ability to decrease erythema. Both cause a transient vasoconstriction. Two studies of patients with moderate or severe erythema of rosacea found that a topical brimonidine gel formulation significantly decreased erythema for up to 12 hours after application, compared with a vehicle gel (J Drugs Dermatol. 2013 Jun 1;12[6]:650-6). However, there have been several case reports of severe rebound, with redness not only returning, but returning at a more severe grade (J Am Acad Dermatol. 2014 Feb;70[2]:e37-8).
• Isotretinoin: New investigations are looking at topical retinoids for rosacea. They do appear effective – but not only for the reason they work in acne. While they decrease the size of sebaceous glands and the amount of sebum produced, they seem to exert their benefit through an anti-inflammatory pathway, as well. Isotretinoin significantly decreases toll-like receptor-2 expression on monocytes.
Isotretinoin has been shown to be more effective than doxycycline in clearing skin (J Dtsch Dermatol Ges. 2010 Jul;8[7]:505-15) but has also seemed helpful in preventing and slowing phyma development and improving ocular disease.
Both Dr. Cohen and Dr. Baldwin disclosed relationships with multiple pharmaceutical companies.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY
Severe acne patients stay on antibiotics too long
Patients with severe acne often remained on antibiotics longer than recommended before beginning treatment with isotretinoin, in a retrospective chart review of patients treated for acne at New York University.
The medical records analysis of 137 patients with severe acne who eventually received isotretinoin found that the average duration of antibiotic use in these patients was 331.3 days, far exceeding expert recommendations to limit use to 3 months, reported Dr. Arielle R. Nagler and her associates in the department of dermatology, New York University.
In total, 15.3% of patients in the study were treated with antibiotics for 3 months or less, and 64.2% were treated with antibiotics for 6 months or longer. Almost 34% were treated with antibiotics for 1 year or longer.
The mean time elapsed between the first recorded mention of possible isotretinoin use and actual initiation of treatment was 155.8 days, Dr. Nagler and her colleagues reported. The mean age of initiation of isotretinoin was 19.6 years.
“Prolonged courses of systemic antibiotics are discouraged for several reasons including increasing resistance of P. acnes [Propionibacterium acnes] to antibiotics,” the authors wrote. “Courses 6 months or longer are highly likely to induce resistance.”
Patients who received antibiotic treatment only at the study site took antibiotics for a mean duration of 283.1 days, whereas those treated at multiple institutions had a mean duration of 380.2 days, they added (P = .054).
“Dermatologists should be aware that patients presenting to them who have been cared for by other providers are at particular risk for extended courses of antibiotics,” they said.
To help reduce unnecessary antibiotic use, providers should recognize patients who are not improving after 6-8 weeks, and consider starting isotretinoin therapy “at an earlier time point, especially in those with severe acne,” Dr. Nagler and her coauthors advised.
Read the full report in the Journal of the American Academy of Dermatology.
Patients with severe acne often remained on antibiotics longer than recommended before beginning treatment with isotretinoin, in a retrospective chart review of patients treated for acne at New York University.
The medical records analysis of 137 patients with severe acne who eventually received isotretinoin found that the average duration of antibiotic use in these patients was 331.3 days, far exceeding expert recommendations to limit use to 3 months, reported Dr. Arielle R. Nagler and her associates in the department of dermatology, New York University.
In total, 15.3% of patients in the study were treated with antibiotics for 3 months or less, and 64.2% were treated with antibiotics for 6 months or longer. Almost 34% were treated with antibiotics for 1 year or longer.
The mean time elapsed between the first recorded mention of possible isotretinoin use and actual initiation of treatment was 155.8 days, Dr. Nagler and her colleagues reported. The mean age of initiation of isotretinoin was 19.6 years.
“Prolonged courses of systemic antibiotics are discouraged for several reasons including increasing resistance of P. acnes [Propionibacterium acnes] to antibiotics,” the authors wrote. “Courses 6 months or longer are highly likely to induce resistance.”
Patients who received antibiotic treatment only at the study site took antibiotics for a mean duration of 283.1 days, whereas those treated at multiple institutions had a mean duration of 380.2 days, they added (P = .054).
“Dermatologists should be aware that patients presenting to them who have been cared for by other providers are at particular risk for extended courses of antibiotics,” they said.
To help reduce unnecessary antibiotic use, providers should recognize patients who are not improving after 6-8 weeks, and consider starting isotretinoin therapy “at an earlier time point, especially in those with severe acne,” Dr. Nagler and her coauthors advised.
Read the full report in the Journal of the American Academy of Dermatology.
Patients with severe acne often remained on antibiotics longer than recommended before beginning treatment with isotretinoin, in a retrospective chart review of patients treated for acne at New York University.
The medical records analysis of 137 patients with severe acne who eventually received isotretinoin found that the average duration of antibiotic use in these patients was 331.3 days, far exceeding expert recommendations to limit use to 3 months, reported Dr. Arielle R. Nagler and her associates in the department of dermatology, New York University.
In total, 15.3% of patients in the study were treated with antibiotics for 3 months or less, and 64.2% were treated with antibiotics for 6 months or longer. Almost 34% were treated with antibiotics for 1 year or longer.
The mean time elapsed between the first recorded mention of possible isotretinoin use and actual initiation of treatment was 155.8 days, Dr. Nagler and her colleagues reported. The mean age of initiation of isotretinoin was 19.6 years.
“Prolonged courses of systemic antibiotics are discouraged for several reasons including increasing resistance of P. acnes [Propionibacterium acnes] to antibiotics,” the authors wrote. “Courses 6 months or longer are highly likely to induce resistance.”
Patients who received antibiotic treatment only at the study site took antibiotics for a mean duration of 283.1 days, whereas those treated at multiple institutions had a mean duration of 380.2 days, they added (P = .054).
“Dermatologists should be aware that patients presenting to them who have been cared for by other providers are at particular risk for extended courses of antibiotics,” they said.
To help reduce unnecessary antibiotic use, providers should recognize patients who are not improving after 6-8 weeks, and consider starting isotretinoin therapy “at an earlier time point, especially in those with severe acne,” Dr. Nagler and her coauthors advised.
Read the full report in the Journal of the American Academy of Dermatology.
BMI negatively associated with acne lesion counts in postadolescent women
Body mass index was negatively associated with the number of acne lesions in a study of Taiwanese women, report Dr. P.H. Lu and coauthors at National Yang-Ming University in Taipei, Taiwan.
A study of 104 Taiwanese women aged 25-45 years with moderate to severe postadolescent acne vulgaris found a negative association between body mass index (BMI) and acne lesion count (P = .001).
In addition to BMI, other recorded measurements included blood pressure, fasting glucose, triglyceride levels, cholesterol levels, waist circumference, and hip circumference. Subjects were classified into four categories: BMI less than 18.5 kg/m2, BMI of 18.5 kg/m2–23.9 kg/m2, BMI of 24 kg/m2–26.9 kg/m2), or BMI greater than or equal to 27.
Acne severity was determined by the Investigator’s Global Assessment (IGA), with inclusion criteria being a score of 3 or 4 on a scale of 0 to 5. Inflammatory lesion counts were recorded by counting papules, pustules, nodules, and cysts. Noninflammatory lesions were recorded by counting comedones.
Women in the lowest BMI category had a mean of 35.5 inflammatory lesions, 7.4 noninflammatory lesions, and 42.9 total lesions, compared with patients in the 18.5-23.9 range (27.9 inflammatory, 6.6 noninflammatory, 34.0 total), and those who were in the two higher categories (18.1 inflammatory, 4.4 noninflammatory, 22.0 total), the authors reported.
“Further investigation is warranted into the association between BMI and acne in this subset of women,” Dr. Lu and associates concluded.
Read the full study in the Journal of the European Academy of Dermatology and Venereology.
Body mass index was negatively associated with the number of acne lesions in a study of Taiwanese women, report Dr. P.H. Lu and coauthors at National Yang-Ming University in Taipei, Taiwan.
A study of 104 Taiwanese women aged 25-45 years with moderate to severe postadolescent acne vulgaris found a negative association between body mass index (BMI) and acne lesion count (P = .001).
In addition to BMI, other recorded measurements included blood pressure, fasting glucose, triglyceride levels, cholesterol levels, waist circumference, and hip circumference. Subjects were classified into four categories: BMI less than 18.5 kg/m2, BMI of 18.5 kg/m2–23.9 kg/m2, BMI of 24 kg/m2–26.9 kg/m2), or BMI greater than or equal to 27.
Acne severity was determined by the Investigator’s Global Assessment (IGA), with inclusion criteria being a score of 3 or 4 on a scale of 0 to 5. Inflammatory lesion counts were recorded by counting papules, pustules, nodules, and cysts. Noninflammatory lesions were recorded by counting comedones.
Women in the lowest BMI category had a mean of 35.5 inflammatory lesions, 7.4 noninflammatory lesions, and 42.9 total lesions, compared with patients in the 18.5-23.9 range (27.9 inflammatory, 6.6 noninflammatory, 34.0 total), and those who were in the two higher categories (18.1 inflammatory, 4.4 noninflammatory, 22.0 total), the authors reported.
“Further investigation is warranted into the association between BMI and acne in this subset of women,” Dr. Lu and associates concluded.
Read the full study in the Journal of the European Academy of Dermatology and Venereology.
Body mass index was negatively associated with the number of acne lesions in a study of Taiwanese women, report Dr. P.H. Lu and coauthors at National Yang-Ming University in Taipei, Taiwan.
A study of 104 Taiwanese women aged 25-45 years with moderate to severe postadolescent acne vulgaris found a negative association between body mass index (BMI) and acne lesion count (P = .001).
In addition to BMI, other recorded measurements included blood pressure, fasting glucose, triglyceride levels, cholesterol levels, waist circumference, and hip circumference. Subjects were classified into four categories: BMI less than 18.5 kg/m2, BMI of 18.5 kg/m2–23.9 kg/m2, BMI of 24 kg/m2–26.9 kg/m2), or BMI greater than or equal to 27.
Acne severity was determined by the Investigator’s Global Assessment (IGA), with inclusion criteria being a score of 3 or 4 on a scale of 0 to 5. Inflammatory lesion counts were recorded by counting papules, pustules, nodules, and cysts. Noninflammatory lesions were recorded by counting comedones.
Women in the lowest BMI category had a mean of 35.5 inflammatory lesions, 7.4 noninflammatory lesions, and 42.9 total lesions, compared with patients in the 18.5-23.9 range (27.9 inflammatory, 6.6 noninflammatory, 34.0 total), and those who were in the two higher categories (18.1 inflammatory, 4.4 noninflammatory, 22.0 total), the authors reported.
“Further investigation is warranted into the association between BMI and acne in this subset of women,” Dr. Lu and associates concluded.
Read the full study in the Journal of the European Academy of Dermatology and Venereology.
Tolerance of Fragranced and Fragrance-Free Facial Cleansers in Adults With Clinically Sensitive Skin
For thousands of years, humans have used fragrances to change or affect their mood and enhance an “aura of beauty.”1 Fragrance is a primary driver in consumer choice and purchasing decisions, especially when considering personal care products.2 In addition to fragrance, consumers choose cleanser products based on compatibility with skin, cleansing properties, and sensory attributes such as viscosity and foaming.3,4 However, fragrance sensitivity is among the most common causes of allergic contact dermatitis from cosmetics and personal care products,5 and estimates of the prevalence of fragrance sensitivity range from 1.8% to 4.2%.6
A panel of 26 fragrance ingredients that frequently induce contact dermatitis in sensitive individuals has been identified.7 Since 2003, regulatory authorities in the European Union require these compounds to be listed on the labels of consumer products to protect presensitized consumers.7,8 However, manufacturers of cosmetics are not required to specify allergenic fragrance ingredients outside the European Union, and therefore it is difficult for consumers in the United States to avoid fragrance allergens.
Creation of a fragranced product for fragrance-sensitive individuals begins with careful selection of ingredients and extensive formulation testing and evaluation. This process usually is followed by testing in normal individuals to confirm that the fragranced product is well accepted and then evaluation is done in clinically confirmed fragrance-sensitive patients and those with a compromised skin barrier from atopic dermatitis, rosacea, or eczema.
Sensitive skin may be due to increased immune responsiveness, altered neurosensory input, and/or decreased skin barrier function, and presents a complex challenge for dermatologists.9 Subjective perceptions of sensitive skin include stinging, burning, pruritus, and tightness following product application. Clinically sensitive skin is defined by the presence of erythema, stratum corneum desquamation, papules, pustules, wheals, vesicles, bullae, and/or erosions.9 Although some of these symptoms may be observed immediately, others may be delayed by minutes, hours, or days following the use of an irritating product. Patients who present with subjective symptoms of sensitive skin may or may not show objective symptoms.
Gentle skin cleansing is particularly important for patients with compromised skin barrier integrity, such as those with acne, atopic dermatitis, eczema, or rosacea. Standard alkaline surfactants in skin cleansers help to remove dirt and oily soil and produce lather but can impair the skin barrier function and facilitate development of irritation.10-13 The tolerability of a cleanser is influenced by its pH, the type and amount of surfactant ingredients, the presence of moisturizing agents, and the amount of residue left on the skin after washing.11,12 Mild cleansers have been developed for patients with sensitive skin conditions and are expected to provide cleansing benefits without negatively affecting the hydration and viscoelastic properties of skin.11 Mild cleansers interact minimally with skin proteins and lipids because they usually contain nonionic synthetic surfactant mixtures; they also have a pH value close to the slightly acidic pH of normal skin, contain moisturizing agents,11,14,15 and usually produce less foam.10,16 In patients with sensitive skin, mild and fragrance-free cleansers often are recommended.17,18 Because fragrances often affect consumers’ perception of product performance19 and enhance the cleaning experience of the user, consumer compliance with clinical recommendations to use fragrance-free cleansers often is poor.
Low–molecular-weight, water-soluble, hydrophobically modified polymers (HMPs) have been used to create gentle foaming cleansers with reduced impact on the skin barrier.12,16,20 In the presence of HMPs, surfactants assemble into larger, more stable polymer-surfactant structures that are less likely to penetrate the skin.16 Hydrophobically modified polymers can potentially reduce skin irritation by lowering the concentration of free micelles in solution. Additionally, both HMPs and HMP-surfactant complexes stabilize newly formed air-water interfaces, leading to thicker, denser, and longer-lasting foams.16 A gentle, fragrance-free, foaming liquid facial test cleanser with HMPs has been shown to be well tolerated in women with sensitive skin.20
This report describes 2 studies of a new mild, HMP-containing, foaming facial cleanser with a fragrance that was free of common allergens and irritating essential oils in patients with sensitive skin. Study 1 was designed to evaluate the tolerance and acceptability of 2 variations of the HMP-containing cleanser—one fragrance free and the other with fragrance—in a small sample of healthy adults with clinically diagnosed fragrance-sensitive skin. Study 2 was a large, 2-center study of the tolerability and effectiveness of the fragranced HMP-containing cleanser compared with a benchmark dermatologist-recommended, gentle, fragrance-free, nonfoaming cleanser in women with clinically diagnosed sensitive skin.
Methods
Study 1 Design
The primary objective of this prospective, randomized, single-center, crossover study was to evaluate the tolerability of fragranced versus fragrance-free formulations of a mild, HMP-containing liquid facial cleanser in healthy male and female adults with Fitzpatrick skin types I to IV who were clinically diagnosed as having fragrance sensitivity. Fragrance sensitivity was defined as a history of positive reactions to a fragrance mixture of 8 components (fragrance mixture I) and/or a fragrance mixture of 14 fragrances (fragrance mixture II) that included balsam of Peru (Myroxylonpereirae), geraniol, jasmine oil, and oakmoss.5 All participants provided written informed consent prior to enrolling in the study, and both the study protocol and informed consent agreement were approved by an institutional review board.
Participants were instructed to wash their face twice daily, noting the time of cleansing and providing commentary about their cleansing experience in a diary. The liquid facial test cleansers contained the HMP potassium acrylates copolymer, glycerin, and a surfactant system primarily containing cocamidopropyl betaine and lauryl glucoside prepared without added fragrance (as previously described20) or with a fragrance free of common allergens and irritating essential oils.
Half of the participants used the fragranced test cleanser and half used the fragrance-free test cleanser for a 3-week treatment period (weeks 1–3). Each treatment group subsequently switched to the other test cleanser for a second 3-week treatment period (weeks 4–6). Clinicians assessed global disease severity (an overall assessment of skin condition that was independent of other evaluation criteria), itching/burning, visible irritation, erythema, and desquamation at weekly time points throughout the study and graded each clinical tolerance attribute on a 5-point scale (0=none; 1=minimal; 2=mild; 3=moderate; 4=severe). Ordinal scores at baseline and at weeks 1 and 3 were used to calculate change from baseline.
A 7-item questionnaire also was administered to participants at each visit to assess skin condition, smoothness, softness, cleanliness, radiance, satisfaction with cleansing experience, and lathering. Each item was scored on a 5-point ordinal scale (0=none; 1=minimal; 2=good; 3=excellent; 4=superior). The scores for all parameters were statistically compared with baseline values using a paired t test with a significance level of P≤.05.
Study 2 Design
This prospective, 3-week, double-blind, randomized, comparative, 2-center study to evaluate the tolerability of the fragranced, HMP-containing test cleanser from study 1 versus a benchmark gentle, fragrance-free, nonfoaming cleanser in a large population of otherwise healthy females who had been clinically diagnosed with sensitive skin (not limited to fragrance sensitivity). The study sponsor provided blinded test materials, and neither the examiner nor the recorder knew which investigational product was administered to which participants. Additionally, personnel who dispensed the test cleansers to participants or supervised their use did not participate in the evaluation to minimize potential bias. All participants provided written informed consent prior to enrolling in the study, and the study protocol and informed consent agreement were approved by an institutional review board.
Participants included women aged 18 to 65 years with mild to moderate clinical symptoms of atopic dermatitis, eczema, acne, or rosacea within the 90 days prior to the study period. They were randomized into 2 balanced treatment groups: group 1 received the mild, fragranced, HMP-containing liquid facial cleanser from study 1 and group 2 received a leading, dermatologist-recommended, gentle, fragrance-free, nonfoaming cleanser. Each treatment group used the test cleansers at least once daily for 3 weeks.
Clinicians evaluated facial skin for softness and smoothness, global disease severity (rated visually by the investigator as an overall assessment of skin condition that was independent of other evaluation criteria [as previously described20]), itching, irritation, erythema, and desquamation at baseline and at weeks 1 and 3. The effectiveness of each product to remove facial dirt, cosmetics, and sebum also was assessed; clinical grading was performed as described for study 1 using the same grading scale as in study 1 and percentage change from baseline (improvement) was calculated.
The study also included a self-assessment of skin irritation in which participants responded yes or no to the following question: Have you experienced irritation using this product? Participants also completed a questionnaire in which they were asked to select the most appropriate answer—agree strongly, agree somewhat, neither, disagree somewhat, and disagree strongly— to the following statements: the cleanser leaves no residue; cleanses deep to remove dirt, oil, and makeup; the cleanser effectively removes makeup; the cleanser leaves my skin smooth; the cleanser leaves my skin soft; the cleanser rinses completely clean; cleanser does not over dry my skin; and my skin is completely clean.
The statistical analysis was performed using a nonparametric, 2-tailed, paired Mann-Whitney U test, and statistical significance was set at P≤.05.
Results
Study 1 Assessment
Eight female participants aged 22 to 60 years with clinically diagnosed fragrance sensitivity were enrolled in the study. After 3 weeks of use, clinician assessment showed that both the fragranced and fragrance-free test cleansers with HMPs improved several skin tolerance attributes, including global disease severity, irritation, and erythema (Figure 1). No notable differences in skin tolerance attributes were reported in the fragranced versus the fragrance-free formulations.
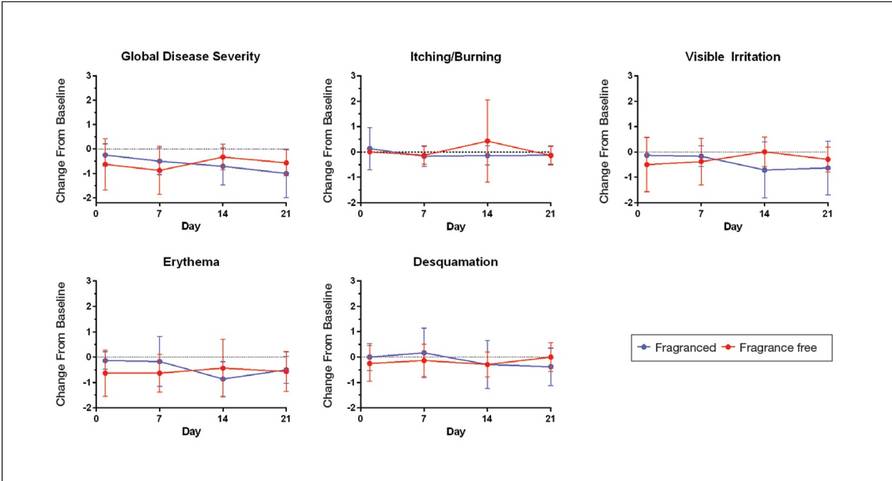
There were no reported differences in participant-reported cleanser effectiveness for the fragranced versus the fragrance-free cleanser either at baseline or weeks 1 or 3 (data not shown).
Study 2 Assessment
A total of 153 women aged 25 to 54 years with sensitive skin were enrolled in the study. Seventy-three participants were randomized to receive the fragranced test cleanser and 80 were randomized to receive the benchmark fragrance-free cleanser.
At week 3, there were no differences between the fragranced test cleanser and the benchmark cleanser in any of the clinician-assessed skin parameters (Figure 2). Of the parameters assessed, itching, irritation, and desquamation were the most improved from baseline in both treatment groups. Similar results were observed at week 1 (data not shown).
There were no apparent differences in subjective self-assessment of skin irritation between the test and benchmark cleansers at week 1 (15.7% vs 13.0%) or week 3 (24.3% vs 12.3%). When asked to respond to a series of 8 statements related to cleanser effectiveness, most participants either agreed strongly or agreed somewhat with the statements (Figure 3). There were no statistically significant differences between treatment groups, and responses to all statements indicated that participants were as satisfied with the test cleanser as they were with the benchmark cleanser.
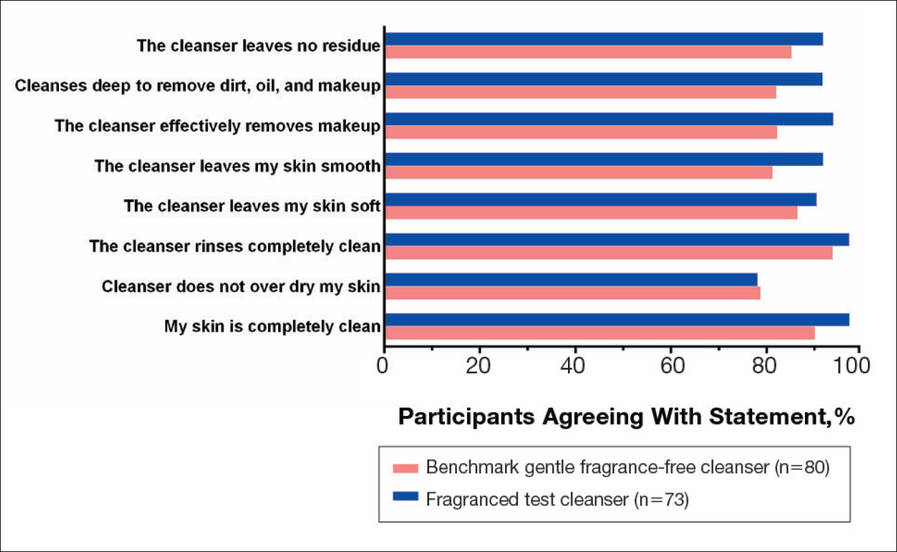
Comment
Consumers value cleansing, fragrance, viscosity, and foaming attributes in skin care products very highly.3,4,10 Fragrances are added to personal care products to positively affect consumers’ perception of product performance and to add emotional benefits by implying social or economic prestige to the use of a product.19 In one study, shampoo formulations that varied only in the added fragrance received different consumer evaluations for cleansing effectiveness and foaming.4
Although mild nonfoaming cleansers can be effective, adult consumers generally use cleansers that foam10,16 and often judge the performance of a cleansing product based on its foaming properties.3,10 Mild cleansers with HMPs maintain the ability to foam while also reducing the likelihood of skin irritation.16 One study showed that a mild, fragrance-free, foaming cleanser containing HMPs was as effective, well tolerated, and nonirritating in patients with sensitive skin as a benchmark nonfoaming gentle cleanser.20
Results from study 1 presented here show that fragranced and fragrance-free formulations of a mild, HMP-containing cleanser are equally efficacious and well tolerated in a small sample of participants with clinically diagnosed fragrance sensitivity. Skin tolerance attributes improved with both cleansers over a 3-week period, particularly global disease severity, irritation, and erythema. These results suggest that a fragrance free of common allergens and irritating essential oils could be introduced into a mild foaming cleanser containing HMPs without causing adverse reactions, even in patients who are fragrance sensitive.
Although the populations of studies 1 and 2 both included female participants with sensitive skin, they were not identical. While study 1 assessed a limited number of participants with clinically diagnosed fragrance sensitivity, study 2 was larger and included a broader range of participants with clinically diagnosed skin sensitivity, which could include fragrance sensitivity. The well-chosen fragrance of the test cleanser containing HMPs was well tolerated; however, this does not imply that any other fragrances added to this cleanser formulation would be as well tolerated.
Conclusion
The current studies indicate that a gentle fragranced foaming cleanser with HMPs was well tolerated in a small population of participants with clinically diagnosed fragrance sensitivity. In a larger population of female participants with sensitive skin, the gentle fragranced foaming cleanser with HMPs was as effective as a leading dermatologist-recommended, fragrance-free, gentle, nonfoaming cleanser. The gentle, HMP-containing, foaming cleanser with a fragrance that does not contain common allergens and irritating essential oils offers a new cleansing option for adults with sensitive skin who may prefer to use a fragranced and foaming product.
Acknowledgments—The authors are grateful to the patients and clinicians who participated in these studies. Editorial and medical writing support was provided by Tove Anderson, PhD, and Alex Loeb, PhD, both from Evidence Scientific Solutions, Inc, Philadelphia, Pennsylvania, and was funded by Johnson & Johnson Consumer Inc.
- Draelos ZD. To smell or not to smell? that is the question! J Cosmet Dermatol. 2013;12:1-2.
- Milotic D. The impact of fragrance on consumer choice. J Consumer Behaviour. 2003;3:179-191.
- Klein K. Evaluating shampoo foam. Cosmetics & Toiletries. 2004;119:32-36.
- Herman S. Skin care: the importance of feel. GCI Magazine. December 2007:70-74.
- Larsen WG. How to test for fragrance allergy. Cutis. 2000;65:39-41.
- Schnuch A, Uter W, Geier J, et al. Epidemiology of contact allergy: an estimation of morbidity employing the clinical epidemiology and drug-utilization research (CE-DUR) approach. Contact Dermatitis. 2002;47:32-39.
- Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products. Official Journal of the European Communities. 2003;L66:26-35.
- Guidance note: labelling of ingredients in Cosmetics Directive 76/768/EEC. European Commission Web site. http: //ec.europa.eu/consumers/sectors/cosmetics/files/doc/guide _labelling200802_en.pdf. Updated February 2008. Accessed September 2, 2015.
- Draelos ZD. Sensitive skin: perceptions, evaluation, and treatment. Am J Contact Dermatitis. 1997;8:67-78.
- Abbas S, Goldberg JW, Massaro M. Personal cleanser technology and clinical performance. Dermatol Ther. 2004;17(suppl 1):35-42.
- Ananthapadmanabhan KP, Moore DJ, Subramanyan K, et al. Cleansing without compromise: the impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol Ther. 2004;17(suppl 1):16-25.
- Walters RM, Mao G, Gunn ET, et al. Cleansing formulations that respect skin barrier integrity. Dermatol Res Pract. 2012;2012:495917.
- Saad P, Flach CR, Walters RM, et al. Infrared spectroscopic studies of sodium dodecyl sulphate permeation and interaction with stratum corneum lipids in skin. Int J Cosmet Sci. 2012;34:36-43.
- Bikowski J. The use of cleansers as therapeutic concomitants in various dermatologic disorders. Cutis. 2001;68(suppl 5):12-19.
- Walters RM, Fevola MJ, LiBrizzi JJ, et al. Designing cleansers for the unique needs of baby skin. Cosmetics & Toiletries. 2008;123:53-60.
- Fevola MJ, Walters RM, LiBrizzi JJ. A new approach to formulating mild cleansers: hydrophobically-modified polymers for irritation mitigation. In: Morgan SE, Lochhead RY, eds. Polymeric Delivery of Therapeutics. Vol 1053. Washington, DC: American Chemical Society; 2011:221-242.
- Nelson SA, Yiannias JA. Relevance and avoidance of skin-care product allergens: pearls and pitfalls. Dermatol Clin. 2009;27:329-336.
- Arribas MP, Soro P, Silvestre JF. Allergic contact dermatitis to fragrances: part 2. Actas Dermosifiliogr. 2013;104:29-37.
- Schroeder W. Understanding fragrance in personal care. Cosmetics & Toiletries. 2009;124:36-44.
- Draelos Z, Hornby S, Walters RM, et al. Hydrophobically-modified polymers can minimize skin irritation potential caused by surfactant-based cleansers. J Cosmet Dermatol. 2013;12:314-321.
For thousands of years, humans have used fragrances to change or affect their mood and enhance an “aura of beauty.”1 Fragrance is a primary driver in consumer choice and purchasing decisions, especially when considering personal care products.2 In addition to fragrance, consumers choose cleanser products based on compatibility with skin, cleansing properties, and sensory attributes such as viscosity and foaming.3,4 However, fragrance sensitivity is among the most common causes of allergic contact dermatitis from cosmetics and personal care products,5 and estimates of the prevalence of fragrance sensitivity range from 1.8% to 4.2%.6
A panel of 26 fragrance ingredients that frequently induce contact dermatitis in sensitive individuals has been identified.7 Since 2003, regulatory authorities in the European Union require these compounds to be listed on the labels of consumer products to protect presensitized consumers.7,8 However, manufacturers of cosmetics are not required to specify allergenic fragrance ingredients outside the European Union, and therefore it is difficult for consumers in the United States to avoid fragrance allergens.
Creation of a fragranced product for fragrance-sensitive individuals begins with careful selection of ingredients and extensive formulation testing and evaluation. This process usually is followed by testing in normal individuals to confirm that the fragranced product is well accepted and then evaluation is done in clinically confirmed fragrance-sensitive patients and those with a compromised skin barrier from atopic dermatitis, rosacea, or eczema.
Sensitive skin may be due to increased immune responsiveness, altered neurosensory input, and/or decreased skin barrier function, and presents a complex challenge for dermatologists.9 Subjective perceptions of sensitive skin include stinging, burning, pruritus, and tightness following product application. Clinically sensitive skin is defined by the presence of erythema, stratum corneum desquamation, papules, pustules, wheals, vesicles, bullae, and/or erosions.9 Although some of these symptoms may be observed immediately, others may be delayed by minutes, hours, or days following the use of an irritating product. Patients who present with subjective symptoms of sensitive skin may or may not show objective symptoms.
Gentle skin cleansing is particularly important for patients with compromised skin barrier integrity, such as those with acne, atopic dermatitis, eczema, or rosacea. Standard alkaline surfactants in skin cleansers help to remove dirt and oily soil and produce lather but can impair the skin barrier function and facilitate development of irritation.10-13 The tolerability of a cleanser is influenced by its pH, the type and amount of surfactant ingredients, the presence of moisturizing agents, and the amount of residue left on the skin after washing.11,12 Mild cleansers have been developed for patients with sensitive skin conditions and are expected to provide cleansing benefits without negatively affecting the hydration and viscoelastic properties of skin.11 Mild cleansers interact minimally with skin proteins and lipids because they usually contain nonionic synthetic surfactant mixtures; they also have a pH value close to the slightly acidic pH of normal skin, contain moisturizing agents,11,14,15 and usually produce less foam.10,16 In patients with sensitive skin, mild and fragrance-free cleansers often are recommended.17,18 Because fragrances often affect consumers’ perception of product performance19 and enhance the cleaning experience of the user, consumer compliance with clinical recommendations to use fragrance-free cleansers often is poor.
Low–molecular-weight, water-soluble, hydrophobically modified polymers (HMPs) have been used to create gentle foaming cleansers with reduced impact on the skin barrier.12,16,20 In the presence of HMPs, surfactants assemble into larger, more stable polymer-surfactant structures that are less likely to penetrate the skin.16 Hydrophobically modified polymers can potentially reduce skin irritation by lowering the concentration of free micelles in solution. Additionally, both HMPs and HMP-surfactant complexes stabilize newly formed air-water interfaces, leading to thicker, denser, and longer-lasting foams.16 A gentle, fragrance-free, foaming liquid facial test cleanser with HMPs has been shown to be well tolerated in women with sensitive skin.20
This report describes 2 studies of a new mild, HMP-containing, foaming facial cleanser with a fragrance that was free of common allergens and irritating essential oils in patients with sensitive skin. Study 1 was designed to evaluate the tolerance and acceptability of 2 variations of the HMP-containing cleanser—one fragrance free and the other with fragrance—in a small sample of healthy adults with clinically diagnosed fragrance-sensitive skin. Study 2 was a large, 2-center study of the tolerability and effectiveness of the fragranced HMP-containing cleanser compared with a benchmark dermatologist-recommended, gentle, fragrance-free, nonfoaming cleanser in women with clinically diagnosed sensitive skin.
Methods
Study 1 Design
The primary objective of this prospective, randomized, single-center, crossover study was to evaluate the tolerability of fragranced versus fragrance-free formulations of a mild, HMP-containing liquid facial cleanser in healthy male and female adults with Fitzpatrick skin types I to IV who were clinically diagnosed as having fragrance sensitivity. Fragrance sensitivity was defined as a history of positive reactions to a fragrance mixture of 8 components (fragrance mixture I) and/or a fragrance mixture of 14 fragrances (fragrance mixture II) that included balsam of Peru (Myroxylonpereirae), geraniol, jasmine oil, and oakmoss.5 All participants provided written informed consent prior to enrolling in the study, and both the study protocol and informed consent agreement were approved by an institutional review board.
Participants were instructed to wash their face twice daily, noting the time of cleansing and providing commentary about their cleansing experience in a diary. The liquid facial test cleansers contained the HMP potassium acrylates copolymer, glycerin, and a surfactant system primarily containing cocamidopropyl betaine and lauryl glucoside prepared without added fragrance (as previously described20) or with a fragrance free of common allergens and irritating essential oils.
Half of the participants used the fragranced test cleanser and half used the fragrance-free test cleanser for a 3-week treatment period (weeks 1–3). Each treatment group subsequently switched to the other test cleanser for a second 3-week treatment period (weeks 4–6). Clinicians assessed global disease severity (an overall assessment of skin condition that was independent of other evaluation criteria), itching/burning, visible irritation, erythema, and desquamation at weekly time points throughout the study and graded each clinical tolerance attribute on a 5-point scale (0=none; 1=minimal; 2=mild; 3=moderate; 4=severe). Ordinal scores at baseline and at weeks 1 and 3 were used to calculate change from baseline.
A 7-item questionnaire also was administered to participants at each visit to assess skin condition, smoothness, softness, cleanliness, radiance, satisfaction with cleansing experience, and lathering. Each item was scored on a 5-point ordinal scale (0=none; 1=minimal; 2=good; 3=excellent; 4=superior). The scores for all parameters were statistically compared with baseline values using a paired t test with a significance level of P≤.05.
Study 2 Design
This prospective, 3-week, double-blind, randomized, comparative, 2-center study to evaluate the tolerability of the fragranced, HMP-containing test cleanser from study 1 versus a benchmark gentle, fragrance-free, nonfoaming cleanser in a large population of otherwise healthy females who had been clinically diagnosed with sensitive skin (not limited to fragrance sensitivity). The study sponsor provided blinded test materials, and neither the examiner nor the recorder knew which investigational product was administered to which participants. Additionally, personnel who dispensed the test cleansers to participants or supervised their use did not participate in the evaluation to minimize potential bias. All participants provided written informed consent prior to enrolling in the study, and the study protocol and informed consent agreement were approved by an institutional review board.
Participants included women aged 18 to 65 years with mild to moderate clinical symptoms of atopic dermatitis, eczema, acne, or rosacea within the 90 days prior to the study period. They were randomized into 2 balanced treatment groups: group 1 received the mild, fragranced, HMP-containing liquid facial cleanser from study 1 and group 2 received a leading, dermatologist-recommended, gentle, fragrance-free, nonfoaming cleanser. Each treatment group used the test cleansers at least once daily for 3 weeks.
Clinicians evaluated facial skin for softness and smoothness, global disease severity (rated visually by the investigator as an overall assessment of skin condition that was independent of other evaluation criteria [as previously described20]), itching, irritation, erythema, and desquamation at baseline and at weeks 1 and 3. The effectiveness of each product to remove facial dirt, cosmetics, and sebum also was assessed; clinical grading was performed as described for study 1 using the same grading scale as in study 1 and percentage change from baseline (improvement) was calculated.
The study also included a self-assessment of skin irritation in which participants responded yes or no to the following question: Have you experienced irritation using this product? Participants also completed a questionnaire in which they were asked to select the most appropriate answer—agree strongly, agree somewhat, neither, disagree somewhat, and disagree strongly— to the following statements: the cleanser leaves no residue; cleanses deep to remove dirt, oil, and makeup; the cleanser effectively removes makeup; the cleanser leaves my skin smooth; the cleanser leaves my skin soft; the cleanser rinses completely clean; cleanser does not over dry my skin; and my skin is completely clean.
The statistical analysis was performed using a nonparametric, 2-tailed, paired Mann-Whitney U test, and statistical significance was set at P≤.05.
Results
Study 1 Assessment
Eight female participants aged 22 to 60 years with clinically diagnosed fragrance sensitivity were enrolled in the study. After 3 weeks of use, clinician assessment showed that both the fragranced and fragrance-free test cleansers with HMPs improved several skin tolerance attributes, including global disease severity, irritation, and erythema (Figure 1). No notable differences in skin tolerance attributes were reported in the fragranced versus the fragrance-free formulations.

There were no reported differences in participant-reported cleanser effectiveness for the fragranced versus the fragrance-free cleanser either at baseline or weeks 1 or 3 (data not shown).
Study 2 Assessment
A total of 153 women aged 25 to 54 years with sensitive skin were enrolled in the study. Seventy-three participants were randomized to receive the fragranced test cleanser and 80 were randomized to receive the benchmark fragrance-free cleanser.
At week 3, there were no differences between the fragranced test cleanser and the benchmark cleanser in any of the clinician-assessed skin parameters (Figure 2). Of the parameters assessed, itching, irritation, and desquamation were the most improved from baseline in both treatment groups. Similar results were observed at week 1 (data not shown).
There were no apparent differences in subjective self-assessment of skin irritation between the test and benchmark cleansers at week 1 (15.7% vs 13.0%) or week 3 (24.3% vs 12.3%). When asked to respond to a series of 8 statements related to cleanser effectiveness, most participants either agreed strongly or agreed somewhat with the statements (Figure 3). There were no statistically significant differences between treatment groups, and responses to all statements indicated that participants were as satisfied with the test cleanser as they were with the benchmark cleanser.

Comment
Consumers value cleansing, fragrance, viscosity, and foaming attributes in skin care products very highly.3,4,10 Fragrances are added to personal care products to positively affect consumers’ perception of product performance and to add emotional benefits by implying social or economic prestige to the use of a product.19 In one study, shampoo formulations that varied only in the added fragrance received different consumer evaluations for cleansing effectiveness and foaming.4
Although mild nonfoaming cleansers can be effective, adult consumers generally use cleansers that foam10,16 and often judge the performance of a cleansing product based on its foaming properties.3,10 Mild cleansers with HMPs maintain the ability to foam while also reducing the likelihood of skin irritation.16 One study showed that a mild, fragrance-free, foaming cleanser containing HMPs was as effective, well tolerated, and nonirritating in patients with sensitive skin as a benchmark nonfoaming gentle cleanser.20
Results from study 1 presented here show that fragranced and fragrance-free formulations of a mild, HMP-containing cleanser are equally efficacious and well tolerated in a small sample of participants with clinically diagnosed fragrance sensitivity. Skin tolerance attributes improved with both cleansers over a 3-week period, particularly global disease severity, irritation, and erythema. These results suggest that a fragrance free of common allergens and irritating essential oils could be introduced into a mild foaming cleanser containing HMPs without causing adverse reactions, even in patients who are fragrance sensitive.
Although the populations of studies 1 and 2 both included female participants with sensitive skin, they were not identical. While study 1 assessed a limited number of participants with clinically diagnosed fragrance sensitivity, study 2 was larger and included a broader range of participants with clinically diagnosed skin sensitivity, which could include fragrance sensitivity. The well-chosen fragrance of the test cleanser containing HMPs was well tolerated; however, this does not imply that any other fragrances added to this cleanser formulation would be as well tolerated.
Conclusion
The current studies indicate that a gentle fragranced foaming cleanser with HMPs was well tolerated in a small population of participants with clinically diagnosed fragrance sensitivity. In a larger population of female participants with sensitive skin, the gentle fragranced foaming cleanser with HMPs was as effective as a leading dermatologist-recommended, fragrance-free, gentle, nonfoaming cleanser. The gentle, HMP-containing, foaming cleanser with a fragrance that does not contain common allergens and irritating essential oils offers a new cleansing option for adults with sensitive skin who may prefer to use a fragranced and foaming product.
Acknowledgments—The authors are grateful to the patients and clinicians who participated in these studies. Editorial and medical writing support was provided by Tove Anderson, PhD, and Alex Loeb, PhD, both from Evidence Scientific Solutions, Inc, Philadelphia, Pennsylvania, and was funded by Johnson & Johnson Consumer Inc.
For thousands of years, humans have used fragrances to change or affect their mood and enhance an “aura of beauty.”1 Fragrance is a primary driver in consumer choice and purchasing decisions, especially when considering personal care products.2 In addition to fragrance, consumers choose cleanser products based on compatibility with skin, cleansing properties, and sensory attributes such as viscosity and foaming.3,4 However, fragrance sensitivity is among the most common causes of allergic contact dermatitis from cosmetics and personal care products,5 and estimates of the prevalence of fragrance sensitivity range from 1.8% to 4.2%.6
A panel of 26 fragrance ingredients that frequently induce contact dermatitis in sensitive individuals has been identified.7 Since 2003, regulatory authorities in the European Union require these compounds to be listed on the labels of consumer products to protect presensitized consumers.7,8 However, manufacturers of cosmetics are not required to specify allergenic fragrance ingredients outside the European Union, and therefore it is difficult for consumers in the United States to avoid fragrance allergens.
Creation of a fragranced product for fragrance-sensitive individuals begins with careful selection of ingredients and extensive formulation testing and evaluation. This process usually is followed by testing in normal individuals to confirm that the fragranced product is well accepted and then evaluation is done in clinically confirmed fragrance-sensitive patients and those with a compromised skin barrier from atopic dermatitis, rosacea, or eczema.
Sensitive skin may be due to increased immune responsiveness, altered neurosensory input, and/or decreased skin barrier function, and presents a complex challenge for dermatologists.9 Subjective perceptions of sensitive skin include stinging, burning, pruritus, and tightness following product application. Clinically sensitive skin is defined by the presence of erythema, stratum corneum desquamation, papules, pustules, wheals, vesicles, bullae, and/or erosions.9 Although some of these symptoms may be observed immediately, others may be delayed by minutes, hours, or days following the use of an irritating product. Patients who present with subjective symptoms of sensitive skin may or may not show objective symptoms.
Gentle skin cleansing is particularly important for patients with compromised skin barrier integrity, such as those with acne, atopic dermatitis, eczema, or rosacea. Standard alkaline surfactants in skin cleansers help to remove dirt and oily soil and produce lather but can impair the skin barrier function and facilitate development of irritation.10-13 The tolerability of a cleanser is influenced by its pH, the type and amount of surfactant ingredients, the presence of moisturizing agents, and the amount of residue left on the skin after washing.11,12 Mild cleansers have been developed for patients with sensitive skin conditions and are expected to provide cleansing benefits without negatively affecting the hydration and viscoelastic properties of skin.11 Mild cleansers interact minimally with skin proteins and lipids because they usually contain nonionic synthetic surfactant mixtures; they also have a pH value close to the slightly acidic pH of normal skin, contain moisturizing agents,11,14,15 and usually produce less foam.10,16 In patients with sensitive skin, mild and fragrance-free cleansers often are recommended.17,18 Because fragrances often affect consumers’ perception of product performance19 and enhance the cleaning experience of the user, consumer compliance with clinical recommendations to use fragrance-free cleansers often is poor.
Low–molecular-weight, water-soluble, hydrophobically modified polymers (HMPs) have been used to create gentle foaming cleansers with reduced impact on the skin barrier.12,16,20 In the presence of HMPs, surfactants assemble into larger, more stable polymer-surfactant structures that are less likely to penetrate the skin.16 Hydrophobically modified polymers can potentially reduce skin irritation by lowering the concentration of free micelles in solution. Additionally, both HMPs and HMP-surfactant complexes stabilize newly formed air-water interfaces, leading to thicker, denser, and longer-lasting foams.16 A gentle, fragrance-free, foaming liquid facial test cleanser with HMPs has been shown to be well tolerated in women with sensitive skin.20
This report describes 2 studies of a new mild, HMP-containing, foaming facial cleanser with a fragrance that was free of common allergens and irritating essential oils in patients with sensitive skin. Study 1 was designed to evaluate the tolerance and acceptability of 2 variations of the HMP-containing cleanser—one fragrance free and the other with fragrance—in a small sample of healthy adults with clinically diagnosed fragrance-sensitive skin. Study 2 was a large, 2-center study of the tolerability and effectiveness of the fragranced HMP-containing cleanser compared with a benchmark dermatologist-recommended, gentle, fragrance-free, nonfoaming cleanser in women with clinically diagnosed sensitive skin.
Methods
Study 1 Design
The primary objective of this prospective, randomized, single-center, crossover study was to evaluate the tolerability of fragranced versus fragrance-free formulations of a mild, HMP-containing liquid facial cleanser in healthy male and female adults with Fitzpatrick skin types I to IV who were clinically diagnosed as having fragrance sensitivity. Fragrance sensitivity was defined as a history of positive reactions to a fragrance mixture of 8 components (fragrance mixture I) and/or a fragrance mixture of 14 fragrances (fragrance mixture II) that included balsam of Peru (Myroxylonpereirae), geraniol, jasmine oil, and oakmoss.5 All participants provided written informed consent prior to enrolling in the study, and both the study protocol and informed consent agreement were approved by an institutional review board.
Participants were instructed to wash their face twice daily, noting the time of cleansing and providing commentary about their cleansing experience in a diary. The liquid facial test cleansers contained the HMP potassium acrylates copolymer, glycerin, and a surfactant system primarily containing cocamidopropyl betaine and lauryl glucoside prepared without added fragrance (as previously described20) or with a fragrance free of common allergens and irritating essential oils.
Half of the participants used the fragranced test cleanser and half used the fragrance-free test cleanser for a 3-week treatment period (weeks 1–3). Each treatment group subsequently switched to the other test cleanser for a second 3-week treatment period (weeks 4–6). Clinicians assessed global disease severity (an overall assessment of skin condition that was independent of other evaluation criteria), itching/burning, visible irritation, erythema, and desquamation at weekly time points throughout the study and graded each clinical tolerance attribute on a 5-point scale (0=none; 1=minimal; 2=mild; 3=moderate; 4=severe). Ordinal scores at baseline and at weeks 1 and 3 were used to calculate change from baseline.
A 7-item questionnaire also was administered to participants at each visit to assess skin condition, smoothness, softness, cleanliness, radiance, satisfaction with cleansing experience, and lathering. Each item was scored on a 5-point ordinal scale (0=none; 1=minimal; 2=good; 3=excellent; 4=superior). The scores for all parameters were statistically compared with baseline values using a paired t test with a significance level of P≤.05.
Study 2 Design
This prospective, 3-week, double-blind, randomized, comparative, 2-center study to evaluate the tolerability of the fragranced, HMP-containing test cleanser from study 1 versus a benchmark gentle, fragrance-free, nonfoaming cleanser in a large population of otherwise healthy females who had been clinically diagnosed with sensitive skin (not limited to fragrance sensitivity). The study sponsor provided blinded test materials, and neither the examiner nor the recorder knew which investigational product was administered to which participants. Additionally, personnel who dispensed the test cleansers to participants or supervised their use did not participate in the evaluation to minimize potential bias. All participants provided written informed consent prior to enrolling in the study, and the study protocol and informed consent agreement were approved by an institutional review board.
Participants included women aged 18 to 65 years with mild to moderate clinical symptoms of atopic dermatitis, eczema, acne, or rosacea within the 90 days prior to the study period. They were randomized into 2 balanced treatment groups: group 1 received the mild, fragranced, HMP-containing liquid facial cleanser from study 1 and group 2 received a leading, dermatologist-recommended, gentle, fragrance-free, nonfoaming cleanser. Each treatment group used the test cleansers at least once daily for 3 weeks.
Clinicians evaluated facial skin for softness and smoothness, global disease severity (rated visually by the investigator as an overall assessment of skin condition that was independent of other evaluation criteria [as previously described20]), itching, irritation, erythema, and desquamation at baseline and at weeks 1 and 3. The effectiveness of each product to remove facial dirt, cosmetics, and sebum also was assessed; clinical grading was performed as described for study 1 using the same grading scale as in study 1 and percentage change from baseline (improvement) was calculated.
The study also included a self-assessment of skin irritation in which participants responded yes or no to the following question: Have you experienced irritation using this product? Participants also completed a questionnaire in which they were asked to select the most appropriate answer—agree strongly, agree somewhat, neither, disagree somewhat, and disagree strongly— to the following statements: the cleanser leaves no residue; cleanses deep to remove dirt, oil, and makeup; the cleanser effectively removes makeup; the cleanser leaves my skin smooth; the cleanser leaves my skin soft; the cleanser rinses completely clean; cleanser does not over dry my skin; and my skin is completely clean.
The statistical analysis was performed using a nonparametric, 2-tailed, paired Mann-Whitney U test, and statistical significance was set at P≤.05.
Results
Study 1 Assessment
Eight female participants aged 22 to 60 years with clinically diagnosed fragrance sensitivity were enrolled in the study. After 3 weeks of use, clinician assessment showed that both the fragranced and fragrance-free test cleansers with HMPs improved several skin tolerance attributes, including global disease severity, irritation, and erythema (Figure 1). No notable differences in skin tolerance attributes were reported in the fragranced versus the fragrance-free formulations.

There were no reported differences in participant-reported cleanser effectiveness for the fragranced versus the fragrance-free cleanser either at baseline or weeks 1 or 3 (data not shown).
Study 2 Assessment
A total of 153 women aged 25 to 54 years with sensitive skin were enrolled in the study. Seventy-three participants were randomized to receive the fragranced test cleanser and 80 were randomized to receive the benchmark fragrance-free cleanser.
At week 3, there were no differences between the fragranced test cleanser and the benchmark cleanser in any of the clinician-assessed skin parameters (Figure 2). Of the parameters assessed, itching, irritation, and desquamation were the most improved from baseline in both treatment groups. Similar results were observed at week 1 (data not shown).
There were no apparent differences in subjective self-assessment of skin irritation between the test and benchmark cleansers at week 1 (15.7% vs 13.0%) or week 3 (24.3% vs 12.3%). When asked to respond to a series of 8 statements related to cleanser effectiveness, most participants either agreed strongly or agreed somewhat with the statements (Figure 3). There were no statistically significant differences between treatment groups, and responses to all statements indicated that participants were as satisfied with the test cleanser as they were with the benchmark cleanser.

Comment
Consumers value cleansing, fragrance, viscosity, and foaming attributes in skin care products very highly.3,4,10 Fragrances are added to personal care products to positively affect consumers’ perception of product performance and to add emotional benefits by implying social or economic prestige to the use of a product.19 In one study, shampoo formulations that varied only in the added fragrance received different consumer evaluations for cleansing effectiveness and foaming.4
Although mild nonfoaming cleansers can be effective, adult consumers generally use cleansers that foam10,16 and often judge the performance of a cleansing product based on its foaming properties.3,10 Mild cleansers with HMPs maintain the ability to foam while also reducing the likelihood of skin irritation.16 One study showed that a mild, fragrance-free, foaming cleanser containing HMPs was as effective, well tolerated, and nonirritating in patients with sensitive skin as a benchmark nonfoaming gentle cleanser.20
Results from study 1 presented here show that fragranced and fragrance-free formulations of a mild, HMP-containing cleanser are equally efficacious and well tolerated in a small sample of participants with clinically diagnosed fragrance sensitivity. Skin tolerance attributes improved with both cleansers over a 3-week period, particularly global disease severity, irritation, and erythema. These results suggest that a fragrance free of common allergens and irritating essential oils could be introduced into a mild foaming cleanser containing HMPs without causing adverse reactions, even in patients who are fragrance sensitive.
Although the populations of studies 1 and 2 both included female participants with sensitive skin, they were not identical. While study 1 assessed a limited number of participants with clinically diagnosed fragrance sensitivity, study 2 was larger and included a broader range of participants with clinically diagnosed skin sensitivity, which could include fragrance sensitivity. The well-chosen fragrance of the test cleanser containing HMPs was well tolerated; however, this does not imply that any other fragrances added to this cleanser formulation would be as well tolerated.
Conclusion
The current studies indicate that a gentle fragranced foaming cleanser with HMPs was well tolerated in a small population of participants with clinically diagnosed fragrance sensitivity. In a larger population of female participants with sensitive skin, the gentle fragranced foaming cleanser with HMPs was as effective as a leading dermatologist-recommended, fragrance-free, gentle, nonfoaming cleanser. The gentle, HMP-containing, foaming cleanser with a fragrance that does not contain common allergens and irritating essential oils offers a new cleansing option for adults with sensitive skin who may prefer to use a fragranced and foaming product.
Acknowledgments—The authors are grateful to the patients and clinicians who participated in these studies. Editorial and medical writing support was provided by Tove Anderson, PhD, and Alex Loeb, PhD, both from Evidence Scientific Solutions, Inc, Philadelphia, Pennsylvania, and was funded by Johnson & Johnson Consumer Inc.
- Draelos ZD. To smell or not to smell? that is the question! J Cosmet Dermatol. 2013;12:1-2.
- Milotic D. The impact of fragrance on consumer choice. J Consumer Behaviour. 2003;3:179-191.
- Klein K. Evaluating shampoo foam. Cosmetics & Toiletries. 2004;119:32-36.
- Herman S. Skin care: the importance of feel. GCI Magazine. December 2007:70-74.
- Larsen WG. How to test for fragrance allergy. Cutis. 2000;65:39-41.
- Schnuch A, Uter W, Geier J, et al. Epidemiology of contact allergy: an estimation of morbidity employing the clinical epidemiology and drug-utilization research (CE-DUR) approach. Contact Dermatitis. 2002;47:32-39.
- Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products. Official Journal of the European Communities. 2003;L66:26-35.
- Guidance note: labelling of ingredients in Cosmetics Directive 76/768/EEC. European Commission Web site. http: //ec.europa.eu/consumers/sectors/cosmetics/files/doc/guide _labelling200802_en.pdf. Updated February 2008. Accessed September 2, 2015.
- Draelos ZD. Sensitive skin: perceptions, evaluation, and treatment. Am J Contact Dermatitis. 1997;8:67-78.
- Abbas S, Goldberg JW, Massaro M. Personal cleanser technology and clinical performance. Dermatol Ther. 2004;17(suppl 1):35-42.
- Ananthapadmanabhan KP, Moore DJ, Subramanyan K, et al. Cleansing without compromise: the impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol Ther. 2004;17(suppl 1):16-25.
- Walters RM, Mao G, Gunn ET, et al. Cleansing formulations that respect skin barrier integrity. Dermatol Res Pract. 2012;2012:495917.
- Saad P, Flach CR, Walters RM, et al. Infrared spectroscopic studies of sodium dodecyl sulphate permeation and interaction with stratum corneum lipids in skin. Int J Cosmet Sci. 2012;34:36-43.
- Bikowski J. The use of cleansers as therapeutic concomitants in various dermatologic disorders. Cutis. 2001;68(suppl 5):12-19.
- Walters RM, Fevola MJ, LiBrizzi JJ, et al. Designing cleansers for the unique needs of baby skin. Cosmetics & Toiletries. 2008;123:53-60.
- Fevola MJ, Walters RM, LiBrizzi JJ. A new approach to formulating mild cleansers: hydrophobically-modified polymers for irritation mitigation. In: Morgan SE, Lochhead RY, eds. Polymeric Delivery of Therapeutics. Vol 1053. Washington, DC: American Chemical Society; 2011:221-242.
- Nelson SA, Yiannias JA. Relevance and avoidance of skin-care product allergens: pearls and pitfalls. Dermatol Clin. 2009;27:329-336.
- Arribas MP, Soro P, Silvestre JF. Allergic contact dermatitis to fragrances: part 2. Actas Dermosifiliogr. 2013;104:29-37.
- Schroeder W. Understanding fragrance in personal care. Cosmetics & Toiletries. 2009;124:36-44.
- Draelos Z, Hornby S, Walters RM, et al. Hydrophobically-modified polymers can minimize skin irritation potential caused by surfactant-based cleansers. J Cosmet Dermatol. 2013;12:314-321.
- Draelos ZD. To smell or not to smell? that is the question! J Cosmet Dermatol. 2013;12:1-2.
- Milotic D. The impact of fragrance on consumer choice. J Consumer Behaviour. 2003;3:179-191.
- Klein K. Evaluating shampoo foam. Cosmetics & Toiletries. 2004;119:32-36.
- Herman S. Skin care: the importance of feel. GCI Magazine. December 2007:70-74.
- Larsen WG. How to test for fragrance allergy. Cutis. 2000;65:39-41.
- Schnuch A, Uter W, Geier J, et al. Epidemiology of contact allergy: an estimation of morbidity employing the clinical epidemiology and drug-utilization research (CE-DUR) approach. Contact Dermatitis. 2002;47:32-39.
- Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products. Official Journal of the European Communities. 2003;L66:26-35.
- Guidance note: labelling of ingredients in Cosmetics Directive 76/768/EEC. European Commission Web site. http: //ec.europa.eu/consumers/sectors/cosmetics/files/doc/guide _labelling200802_en.pdf. Updated February 2008. Accessed September 2, 2015.
- Draelos ZD. Sensitive skin: perceptions, evaluation, and treatment. Am J Contact Dermatitis. 1997;8:67-78.
- Abbas S, Goldberg JW, Massaro M. Personal cleanser technology and clinical performance. Dermatol Ther. 2004;17(suppl 1):35-42.
- Ananthapadmanabhan KP, Moore DJ, Subramanyan K, et al. Cleansing without compromise: the impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol Ther. 2004;17(suppl 1):16-25.
- Walters RM, Mao G, Gunn ET, et al. Cleansing formulations that respect skin barrier integrity. Dermatol Res Pract. 2012;2012:495917.
- Saad P, Flach CR, Walters RM, et al. Infrared spectroscopic studies of sodium dodecyl sulphate permeation and interaction with stratum corneum lipids in skin. Int J Cosmet Sci. 2012;34:36-43.
- Bikowski J. The use of cleansers as therapeutic concomitants in various dermatologic disorders. Cutis. 2001;68(suppl 5):12-19.
- Walters RM, Fevola MJ, LiBrizzi JJ, et al. Designing cleansers for the unique needs of baby skin. Cosmetics & Toiletries. 2008;123:53-60.
- Fevola MJ, Walters RM, LiBrizzi JJ. A new approach to formulating mild cleansers: hydrophobically-modified polymers for irritation mitigation. In: Morgan SE, Lochhead RY, eds. Polymeric Delivery of Therapeutics. Vol 1053. Washington, DC: American Chemical Society; 2011:221-242.
- Nelson SA, Yiannias JA. Relevance and avoidance of skin-care product allergens: pearls and pitfalls. Dermatol Clin. 2009;27:329-336.
- Arribas MP, Soro P, Silvestre JF. Allergic contact dermatitis to fragrances: part 2. Actas Dermosifiliogr. 2013;104:29-37.
- Schroeder W. Understanding fragrance in personal care. Cosmetics & Toiletries. 2009;124:36-44.
- Draelos Z, Hornby S, Walters RM, et al. Hydrophobically-modified polymers can minimize skin irritation potential caused by surfactant-based cleansers. J Cosmet Dermatol. 2013;12:314-321.
Practice Points
- Fragranced and fragrance-free versions of a gentle foaming cleanser with hydrophobically modified polymers (HMPs) were similarly well tolerated in participants with clinically diagnosed fragrance sensitivity.
- In a large population of female participants with sensitive skin, the fragranced gentle foaming cleanser with HMPs was as effective as a leading dermatologist-recommended, fragrance-free, gentle, nonfoaming cleanser.
- The gentle, HMP-containing, foaming cleanser with a fragrance offers a new cleansing option for adults with sensitive skin who may prefer to use a fragranced and foaming product.