User login
Inadequate sleep & obesity: Breaking the vicious cycle
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
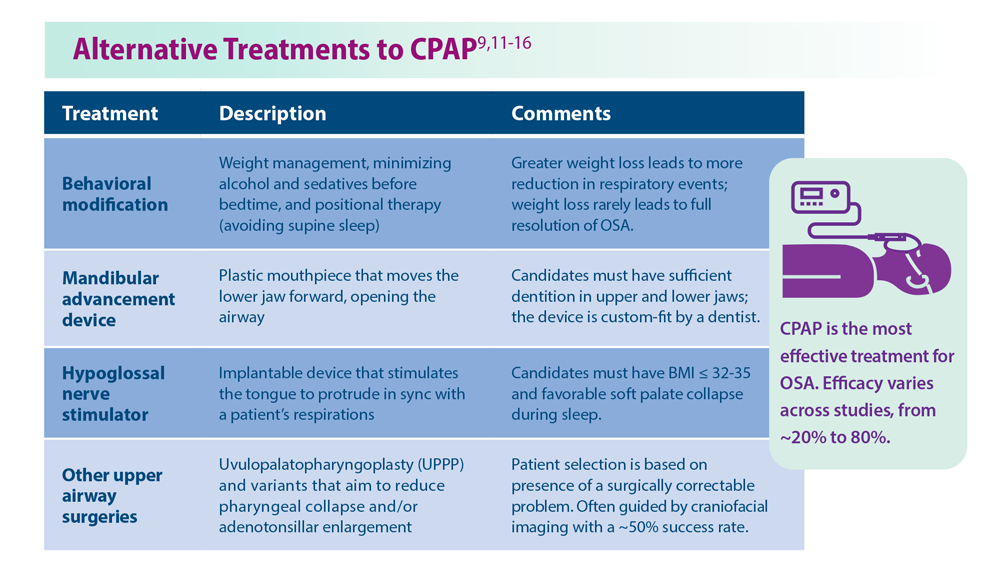
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45
Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene (TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52
Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; [email protected]
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
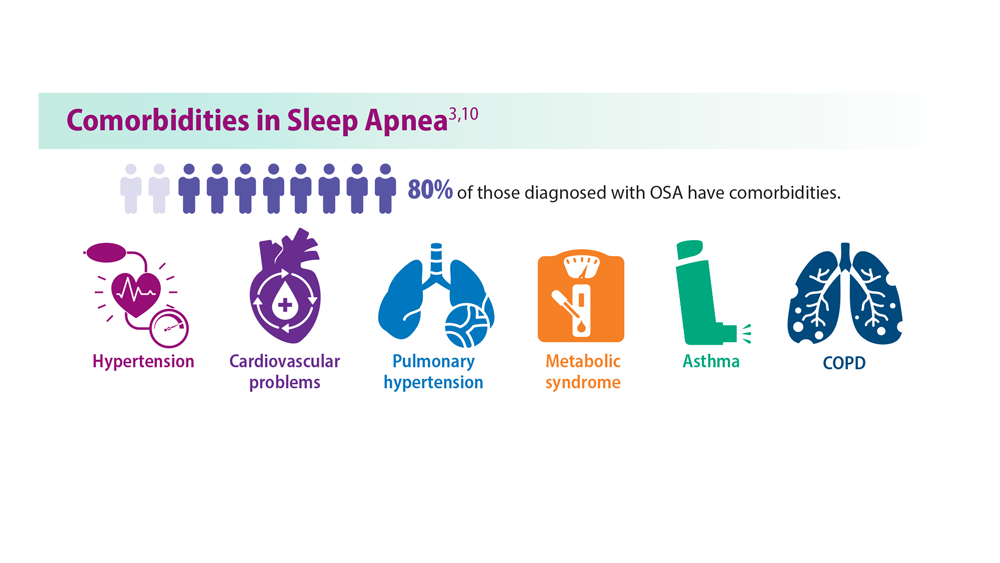
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
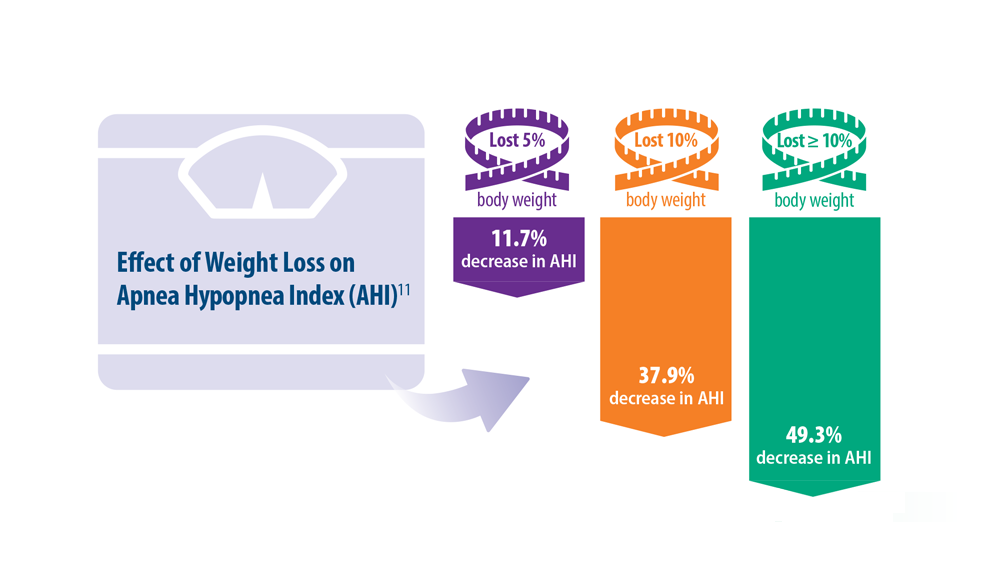
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45

Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
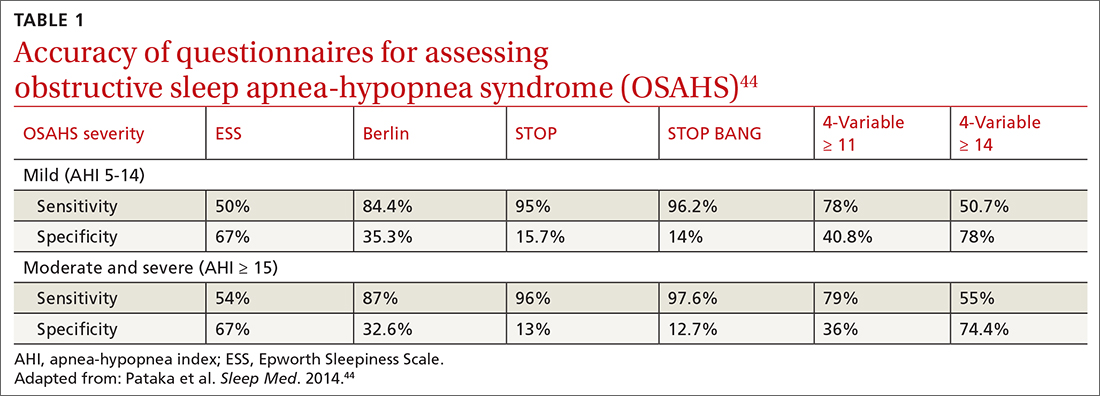
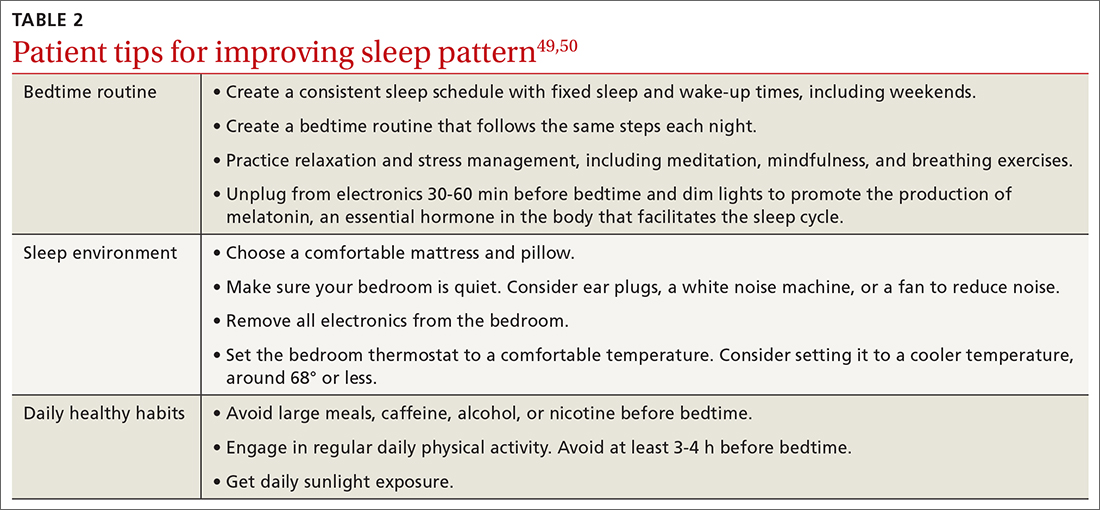
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene (TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52

Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; [email protected]
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45

Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene (TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52

Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; [email protected]
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
PRACTICE RECOMMENDATIONS
› Consider cognitive behaviorial therapy for insomnia (CBT-I) first-line treatment for insomnia. A
› Carefully review patients’ medication lists, as many pharmaceuticals can affect weight and sleep. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Take a closer look at sleep’s role in GERD
This transcript has been edited for clarity.
The ongoing longitudinal Nurses’ Health Study has served as an incredible database for evaluating disease states prospectively over decades, thanks to the robust input of its participants. Most recently, this allowed for an important analysis of the association between gastroesophageal reflux (GER) symptoms and sleep quality, the results of which were published in JAMA Network Open.
Approximately 49,000 women with a median age of 59 years (range, 48-69 years) provided data for this analysis. Starting in 2005, they were asked about their experience of GER symptoms. In 2017, they were also asked to respond to a questionnaire, a modified Pittsburgh Sleep Quality Index (PSQI). This is a tool we’ve used a lot in prospective studies looking at gastrointestinal diseases and sleep-related abnormalities. It’s unique in that it looks not only at sleep but also at next-day function and daytime sleepiness, which is important here for its implications related to reflux disease and sleep fragmentation.
For those with GER symptoms occurring once a week and more than once a week, the approximate relative risk increased by 30% and 53%, respectively. Clearly, the association of GER symptoms and relative sleep quality was really important.
It should be noted that the PSQI is a disease-independent, validated instrument. It’s not specific to GER disease or any diseases. It’s cross validated across 17 different languages. I think what’s most important about its use in the assessment here is the incorporation of next-day function and asking participants about daytime sleepiness, which we’ll discuss in more detail shortly.
The many causes of interrupted sleep
We’ve all experienced sleep fragmentation, whether in the form of having been on call during our medical training or common experiences like hearing a child cry in the night, a noisy truck pass by, or a dog barking. You may or may not remember that these happened the next day, but they’ve nonetheless interrupted your sleep efficiency.
When you transition laterally across the stages of sleep, that’s what establishes the circadian rhythm and ensures sleep hygiene. Typically, we require approximately 7 hours of restful sleep to do that. But if you fragment or interrupt this process, you more or less move your way erratically through the night, disrupting sleep hygiene and efficiency.
If you have a cognitive awakening during those disruptions, you may recall those events the next day. Or, you may not remember it at all, and such amnestic events are normal for some people with sleep disruptions.
You may also have a sensory arousal, whether it’s due to GER symptoms, auditory stimuli, bumping your toe, or whatever disruptive event. Any of these can cause you to lose that laterality of smooth transition through sleep.
Approximately 20% of the U.S. population have reported GER symptoms at least once a week. Incident data indicate that number may be increasing by as much as 5% a year. Much of that increase is tied to obesity. But nonetheless, it’s a problem on the rise.
It’s important to know this as we start to look at sleep. If GER is acting as a trigger to sleep disruption, you need to ask your patients with this condition about next-day function.
In particular, the next-day function questions to ask are, “How do you feel when you get up? Are you awake and refreshed? Do you have early fatigue? Do you drag yourself out of bed, have daytime somnolence, loss of concentration, or irritability?”
Those are key parameters we can use for looking back to the night before and gauging sleep efficiency. If you’re not asking those questions, you may miss out on identifying a patient having sleep fragmentation.
Sleep’s role in inflammatory disease processes
I now perform an interval assessment of this type not just in my patients with GER disease but across all my patients. I do so because sleep is physiologically important in so many ways.
In patients who have nonalcoholic fatty liver disease and a variety of other liver diseases, we’re finding an increased association with sleep fragmentation outside of sleep apnea.
The same is true with irritable bowel and other functional diseases.
When you have sleep fragmentation in inflammatory bowel disease, you turn on a variety of inflammatory proteins (e.g., C-reactive protein) and cytokines, such as interleukins and tumor necrosis factor alpha. These processes may actually tip somebody over to a pro-inflammatory state.
When it comes to what might be considered a relatively simpler condition like GER disease, Ronnie Fass and colleagues showed a number of years ago via Bernstein testing performed in patients with both fragmented and normal sleep that the sensory thresholds all get lowered in the former group. This is irrespective of whether you have a functional symptom or you’re awakened by bumping your toe, a headache, or having heartburn; your sensory thresholds are lower. As a result, the same stimulus provides a higher sense of awareness. By ramping up that awareness, you increase the interference with the next-day function.
We’ve shown that sleep fragmentation affects a variety of things, including immune function. This may be why many people get sick when they travel in between time zones.
There are also implications relating to things like obesity. When you have sleep dysfunction, you have effects on leptin and ghrelin, contrary to what you would normally want to have. This, in turn, causes adverse effects on stimulation or suppression of satiety or appetite. These are things that I counsel my patients about when I talk about reflux as well as those trying to lose weight.
Sleep disruption affects cortisol stimulation and has a significant correlation with type 2 diabetes, cardiovascular diseases, and even mortality statistics.
Advice for counseling patients
This latest analysis from the Nurses’ Health Study reminds us that a lot of people have reflux and a lot of people have sleep fragmentation. We need to do better in asking our patients if they have symptoms specific not only to reflux but also to potentially sleep-related complications.
The more we do that, the more we individualize patient treatment rather than treating them as a disease state. This, in turn, will allow us to practice personalized medicine. The more we can engage our patients with reflux disease by asking the right questions about next-day function, the better we can do in improving their outcomes.
It’s time for us all to open our eyes to the value of closing them. Let’s talk to our patients with reflux disease in a little bit of a different light, providing a new perspective on strategies we can use to mitigate and deal with those symptoms, thereby preventing the consequences of sleep fragmentation.
Hopefully, this overview gives you some guidance the next time you have a conversation with your patients. It will apply across many, many disease states, and in almost everything we do in gastroenterology.
David A. Johnson, MD, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, Va., and a past president of the American College of Gastroenterology. He reported advising with ISOTHRIVE and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
The ongoing longitudinal Nurses’ Health Study has served as an incredible database for evaluating disease states prospectively over decades, thanks to the robust input of its participants. Most recently, this allowed for an important analysis of the association between gastroesophageal reflux (GER) symptoms and sleep quality, the results of which were published in JAMA Network Open.
Approximately 49,000 women with a median age of 59 years (range, 48-69 years) provided data for this analysis. Starting in 2005, they were asked about their experience of GER symptoms. In 2017, they were also asked to respond to a questionnaire, a modified Pittsburgh Sleep Quality Index (PSQI). This is a tool we’ve used a lot in prospective studies looking at gastrointestinal diseases and sleep-related abnormalities. It’s unique in that it looks not only at sleep but also at next-day function and daytime sleepiness, which is important here for its implications related to reflux disease and sleep fragmentation.
For those with GER symptoms occurring once a week and more than once a week, the approximate relative risk increased by 30% and 53%, respectively. Clearly, the association of GER symptoms and relative sleep quality was really important.
It should be noted that the PSQI is a disease-independent, validated instrument. It’s not specific to GER disease or any diseases. It’s cross validated across 17 different languages. I think what’s most important about its use in the assessment here is the incorporation of next-day function and asking participants about daytime sleepiness, which we’ll discuss in more detail shortly.
The many causes of interrupted sleep
We’ve all experienced sleep fragmentation, whether in the form of having been on call during our medical training or common experiences like hearing a child cry in the night, a noisy truck pass by, or a dog barking. You may or may not remember that these happened the next day, but they’ve nonetheless interrupted your sleep efficiency.
When you transition laterally across the stages of sleep, that’s what establishes the circadian rhythm and ensures sleep hygiene. Typically, we require approximately 7 hours of restful sleep to do that. But if you fragment or interrupt this process, you more or less move your way erratically through the night, disrupting sleep hygiene and efficiency.
If you have a cognitive awakening during those disruptions, you may recall those events the next day. Or, you may not remember it at all, and such amnestic events are normal for some people with sleep disruptions.
You may also have a sensory arousal, whether it’s due to GER symptoms, auditory stimuli, bumping your toe, or whatever disruptive event. Any of these can cause you to lose that laterality of smooth transition through sleep.
Approximately 20% of the U.S. population have reported GER symptoms at least once a week. Incident data indicate that number may be increasing by as much as 5% a year. Much of that increase is tied to obesity. But nonetheless, it’s a problem on the rise.
It’s important to know this as we start to look at sleep. If GER is acting as a trigger to sleep disruption, you need to ask your patients with this condition about next-day function.
In particular, the next-day function questions to ask are, “How do you feel when you get up? Are you awake and refreshed? Do you have early fatigue? Do you drag yourself out of bed, have daytime somnolence, loss of concentration, or irritability?”
Those are key parameters we can use for looking back to the night before and gauging sleep efficiency. If you’re not asking those questions, you may miss out on identifying a patient having sleep fragmentation.
Sleep’s role in inflammatory disease processes
I now perform an interval assessment of this type not just in my patients with GER disease but across all my patients. I do so because sleep is physiologically important in so many ways.
In patients who have nonalcoholic fatty liver disease and a variety of other liver diseases, we’re finding an increased association with sleep fragmentation outside of sleep apnea.
The same is true with irritable bowel and other functional diseases.
When you have sleep fragmentation in inflammatory bowel disease, you turn on a variety of inflammatory proteins (e.g., C-reactive protein) and cytokines, such as interleukins and tumor necrosis factor alpha. These processes may actually tip somebody over to a pro-inflammatory state.
When it comes to what might be considered a relatively simpler condition like GER disease, Ronnie Fass and colleagues showed a number of years ago via Bernstein testing performed in patients with both fragmented and normal sleep that the sensory thresholds all get lowered in the former group. This is irrespective of whether you have a functional symptom or you’re awakened by bumping your toe, a headache, or having heartburn; your sensory thresholds are lower. As a result, the same stimulus provides a higher sense of awareness. By ramping up that awareness, you increase the interference with the next-day function.
We’ve shown that sleep fragmentation affects a variety of things, including immune function. This may be why many people get sick when they travel in between time zones.
There are also implications relating to things like obesity. When you have sleep dysfunction, you have effects on leptin and ghrelin, contrary to what you would normally want to have. This, in turn, causes adverse effects on stimulation or suppression of satiety or appetite. These are things that I counsel my patients about when I talk about reflux as well as those trying to lose weight.
Sleep disruption affects cortisol stimulation and has a significant correlation with type 2 diabetes, cardiovascular diseases, and even mortality statistics.
Advice for counseling patients
This latest analysis from the Nurses’ Health Study reminds us that a lot of people have reflux and a lot of people have sleep fragmentation. We need to do better in asking our patients if they have symptoms specific not only to reflux but also to potentially sleep-related complications.
The more we do that, the more we individualize patient treatment rather than treating them as a disease state. This, in turn, will allow us to practice personalized medicine. The more we can engage our patients with reflux disease by asking the right questions about next-day function, the better we can do in improving their outcomes.
It’s time for us all to open our eyes to the value of closing them. Let’s talk to our patients with reflux disease in a little bit of a different light, providing a new perspective on strategies we can use to mitigate and deal with those symptoms, thereby preventing the consequences of sleep fragmentation.
Hopefully, this overview gives you some guidance the next time you have a conversation with your patients. It will apply across many, many disease states, and in almost everything we do in gastroenterology.
David A. Johnson, MD, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, Va., and a past president of the American College of Gastroenterology. He reported advising with ISOTHRIVE and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
The ongoing longitudinal Nurses’ Health Study has served as an incredible database for evaluating disease states prospectively over decades, thanks to the robust input of its participants. Most recently, this allowed for an important analysis of the association between gastroesophageal reflux (GER) symptoms and sleep quality, the results of which were published in JAMA Network Open.
Approximately 49,000 women with a median age of 59 years (range, 48-69 years) provided data for this analysis. Starting in 2005, they were asked about their experience of GER symptoms. In 2017, they were also asked to respond to a questionnaire, a modified Pittsburgh Sleep Quality Index (PSQI). This is a tool we’ve used a lot in prospective studies looking at gastrointestinal diseases and sleep-related abnormalities. It’s unique in that it looks not only at sleep but also at next-day function and daytime sleepiness, which is important here for its implications related to reflux disease and sleep fragmentation.
For those with GER symptoms occurring once a week and more than once a week, the approximate relative risk increased by 30% and 53%, respectively. Clearly, the association of GER symptoms and relative sleep quality was really important.
It should be noted that the PSQI is a disease-independent, validated instrument. It’s not specific to GER disease or any diseases. It’s cross validated across 17 different languages. I think what’s most important about its use in the assessment here is the incorporation of next-day function and asking participants about daytime sleepiness, which we’ll discuss in more detail shortly.
The many causes of interrupted sleep
We’ve all experienced sleep fragmentation, whether in the form of having been on call during our medical training or common experiences like hearing a child cry in the night, a noisy truck pass by, or a dog barking. You may or may not remember that these happened the next day, but they’ve nonetheless interrupted your sleep efficiency.
When you transition laterally across the stages of sleep, that’s what establishes the circadian rhythm and ensures sleep hygiene. Typically, we require approximately 7 hours of restful sleep to do that. But if you fragment or interrupt this process, you more or less move your way erratically through the night, disrupting sleep hygiene and efficiency.
If you have a cognitive awakening during those disruptions, you may recall those events the next day. Or, you may not remember it at all, and such amnestic events are normal for some people with sleep disruptions.
You may also have a sensory arousal, whether it’s due to GER symptoms, auditory stimuli, bumping your toe, or whatever disruptive event. Any of these can cause you to lose that laterality of smooth transition through sleep.
Approximately 20% of the U.S. population have reported GER symptoms at least once a week. Incident data indicate that number may be increasing by as much as 5% a year. Much of that increase is tied to obesity. But nonetheless, it’s a problem on the rise.
It’s important to know this as we start to look at sleep. If GER is acting as a trigger to sleep disruption, you need to ask your patients with this condition about next-day function.
In particular, the next-day function questions to ask are, “How do you feel when you get up? Are you awake and refreshed? Do you have early fatigue? Do you drag yourself out of bed, have daytime somnolence, loss of concentration, or irritability?”
Those are key parameters we can use for looking back to the night before and gauging sleep efficiency. If you’re not asking those questions, you may miss out on identifying a patient having sleep fragmentation.
Sleep’s role in inflammatory disease processes
I now perform an interval assessment of this type not just in my patients with GER disease but across all my patients. I do so because sleep is physiologically important in so many ways.
In patients who have nonalcoholic fatty liver disease and a variety of other liver diseases, we’re finding an increased association with sleep fragmentation outside of sleep apnea.
The same is true with irritable bowel and other functional diseases.
When you have sleep fragmentation in inflammatory bowel disease, you turn on a variety of inflammatory proteins (e.g., C-reactive protein) and cytokines, such as interleukins and tumor necrosis factor alpha. These processes may actually tip somebody over to a pro-inflammatory state.
When it comes to what might be considered a relatively simpler condition like GER disease, Ronnie Fass and colleagues showed a number of years ago via Bernstein testing performed in patients with both fragmented and normal sleep that the sensory thresholds all get lowered in the former group. This is irrespective of whether you have a functional symptom or you’re awakened by bumping your toe, a headache, or having heartburn; your sensory thresholds are lower. As a result, the same stimulus provides a higher sense of awareness. By ramping up that awareness, you increase the interference with the next-day function.
We’ve shown that sleep fragmentation affects a variety of things, including immune function. This may be why many people get sick when they travel in between time zones.
There are also implications relating to things like obesity. When you have sleep dysfunction, you have effects on leptin and ghrelin, contrary to what you would normally want to have. This, in turn, causes adverse effects on stimulation or suppression of satiety or appetite. These are things that I counsel my patients about when I talk about reflux as well as those trying to lose weight.
Sleep disruption affects cortisol stimulation and has a significant correlation with type 2 diabetes, cardiovascular diseases, and even mortality statistics.
Advice for counseling patients
This latest analysis from the Nurses’ Health Study reminds us that a lot of people have reflux and a lot of people have sleep fragmentation. We need to do better in asking our patients if they have symptoms specific not only to reflux but also to potentially sleep-related complications.
The more we do that, the more we individualize patient treatment rather than treating them as a disease state. This, in turn, will allow us to practice personalized medicine. The more we can engage our patients with reflux disease by asking the right questions about next-day function, the better we can do in improving their outcomes.
It’s time for us all to open our eyes to the value of closing them. Let’s talk to our patients with reflux disease in a little bit of a different light, providing a new perspective on strategies we can use to mitigate and deal with those symptoms, thereby preventing the consequences of sleep fragmentation.
Hopefully, this overview gives you some guidance the next time you have a conversation with your patients. It will apply across many, many disease states, and in almost everything we do in gastroenterology.
David A. Johnson, MD, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, Va., and a past president of the American College of Gastroenterology. He reported advising with ISOTHRIVE and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
Sleep irregularity
In discussions between health care providers and patients, the words “regularity” and “irregularity” come up primarily in reference to either constipation or menstrual cycles. However, the participants in a recent panel convened by the National Sleep Foundation think we should also be discussing irregularity when we are discussing sleep with our patients.
The sleep experts on the panel began by considering 40,000 papers that directly or tangentially dealt with the topic of irregular sleep patterns. The reviewers uncovered numerous references to an association between sleep irregularity and a wide variety of adverse health outcomes, including obesity and metabolic disorders, hypertension and other cardiovascular disorders, and elevations in several inflammatory markers. Not surprisingly, the investigators also found an abundance of references supporting an association between irregular sleep and a suite of mental health problems, including depression, mood disorders, lower self esteem, poor academic performance, and deficits in attention. For example, several of the studies the panel reviewed found that in college students, GPA was lower when their sleep pattern was irregular. There were some papers that found no significant association between irregular sleep and other adverse health outcomes, but none of the studies demonstrated an association with better or improved health outcomes.
There is currently no universally accepted definition of an irregular sleep pattern. The experts pointed to some papers that used a standard deviation of 1 hour from the patient’s usual bed time determined by averaging over an interval measured in weeks. You and I shouldn’t be surprised that irregular sleep is unhealthy, but the breadth of the panel’s findings is impressive.
Although it has been long in coming, sleep is finally beginning to get some attention by the media. The focus is usually on the optimal number of hours we need each night. This panel’s findings suggest that total sleep time is only part of the story, and may even be less important than the regularity of our sleep patterns.
For those of us in pediatrics, the place where irregularity raises its ugly head is with teenagers and weekends. Although the numbers are far from clear, the question remains of how effective is catch-up sleep after a week of too-early mornings and too-late bedtimes for the chronically under-slept adolescent.
In some studies in which patients had the demonstrable effects of sleep deprivation (e.g., metabolic and cardiovascular) there was some improvement when weekend sleep was extended by 1 or 2 hours, but none beyond 2 hours.
The panel’s findings, while certainly significant, merely add weight and nuance to the existing evidence of importance of sleep and the damage done by sleep deprivation. As one of the panel members has said, “Sleep is the third pillar of health, equally important as diet and exercise, if not more.” However, this message is not getting out, or at least it is not being heeded. Like obesity, our efforts as advisers to our patients isn’t working. Unfortunately, this is because our advice is often whispered and given halfheartedly.
There was some evidence of improvement as a result of the pandemic, when those fortunate enough to be able to work from home were taking advantage of the flexibility in their schedules and getting more sleep. But health care providers certainly can’t take responsibility for what was an accident of nature.
Those of you who have been reading Letters from Maine for the last 3 decades may tire of my beating the tired horse of sleep deprivation. But I will not be deterred. I see very little evidence among health care professionals in taking the importance of sleep seriously. Sure, they may include it buried in the list of potential contributors to their patient’s complaint, but I see very little effort to move it higher on their list of priorities and almost no movement toward making substantive recommendations and then reinforcing them with follow-up.
Like obesity, sleep deprivation is a societal problem. We can lay some of the blame on Thomas Edison, but Until that time you will continue to read columns like this one when I encounter significant studies on the importance of sleep.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In discussions between health care providers and patients, the words “regularity” and “irregularity” come up primarily in reference to either constipation or menstrual cycles. However, the participants in a recent panel convened by the National Sleep Foundation think we should also be discussing irregularity when we are discussing sleep with our patients.
The sleep experts on the panel began by considering 40,000 papers that directly or tangentially dealt with the topic of irregular sleep patterns. The reviewers uncovered numerous references to an association between sleep irregularity and a wide variety of adverse health outcomes, including obesity and metabolic disorders, hypertension and other cardiovascular disorders, and elevations in several inflammatory markers. Not surprisingly, the investigators also found an abundance of references supporting an association between irregular sleep and a suite of mental health problems, including depression, mood disorders, lower self esteem, poor academic performance, and deficits in attention. For example, several of the studies the panel reviewed found that in college students, GPA was lower when their sleep pattern was irregular. There were some papers that found no significant association between irregular sleep and other adverse health outcomes, but none of the studies demonstrated an association with better or improved health outcomes.
There is currently no universally accepted definition of an irregular sleep pattern. The experts pointed to some papers that used a standard deviation of 1 hour from the patient’s usual bed time determined by averaging over an interval measured in weeks. You and I shouldn’t be surprised that irregular sleep is unhealthy, but the breadth of the panel’s findings is impressive.
Although it has been long in coming, sleep is finally beginning to get some attention by the media. The focus is usually on the optimal number of hours we need each night. This panel’s findings suggest that total sleep time is only part of the story, and may even be less important than the regularity of our sleep patterns.
For those of us in pediatrics, the place where irregularity raises its ugly head is with teenagers and weekends. Although the numbers are far from clear, the question remains of how effective is catch-up sleep after a week of too-early mornings and too-late bedtimes for the chronically under-slept adolescent.
In some studies in which patients had the demonstrable effects of sleep deprivation (e.g., metabolic and cardiovascular) there was some improvement when weekend sleep was extended by 1 or 2 hours, but none beyond 2 hours.
The panel’s findings, while certainly significant, merely add weight and nuance to the existing evidence of importance of sleep and the damage done by sleep deprivation. As one of the panel members has said, “Sleep is the third pillar of health, equally important as diet and exercise, if not more.” However, this message is not getting out, or at least it is not being heeded. Like obesity, our efforts as advisers to our patients isn’t working. Unfortunately, this is because our advice is often whispered and given halfheartedly.
There was some evidence of improvement as a result of the pandemic, when those fortunate enough to be able to work from home were taking advantage of the flexibility in their schedules and getting more sleep. But health care providers certainly can’t take responsibility for what was an accident of nature.
Those of you who have been reading Letters from Maine for the last 3 decades may tire of my beating the tired horse of sleep deprivation. But I will not be deterred. I see very little evidence among health care professionals in taking the importance of sleep seriously. Sure, they may include it buried in the list of potential contributors to their patient’s complaint, but I see very little effort to move it higher on their list of priorities and almost no movement toward making substantive recommendations and then reinforcing them with follow-up.
Like obesity, sleep deprivation is a societal problem. We can lay some of the blame on Thomas Edison, but Until that time you will continue to read columns like this one when I encounter significant studies on the importance of sleep.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In discussions between health care providers and patients, the words “regularity” and “irregularity” come up primarily in reference to either constipation or menstrual cycles. However, the participants in a recent panel convened by the National Sleep Foundation think we should also be discussing irregularity when we are discussing sleep with our patients.
The sleep experts on the panel began by considering 40,000 papers that directly or tangentially dealt with the topic of irregular sleep patterns. The reviewers uncovered numerous references to an association between sleep irregularity and a wide variety of adverse health outcomes, including obesity and metabolic disorders, hypertension and other cardiovascular disorders, and elevations in several inflammatory markers. Not surprisingly, the investigators also found an abundance of references supporting an association between irregular sleep and a suite of mental health problems, including depression, mood disorders, lower self esteem, poor academic performance, and deficits in attention. For example, several of the studies the panel reviewed found that in college students, GPA was lower when their sleep pattern was irregular. There were some papers that found no significant association between irregular sleep and other adverse health outcomes, but none of the studies demonstrated an association with better or improved health outcomes.
There is currently no universally accepted definition of an irregular sleep pattern. The experts pointed to some papers that used a standard deviation of 1 hour from the patient’s usual bed time determined by averaging over an interval measured in weeks. You and I shouldn’t be surprised that irregular sleep is unhealthy, but the breadth of the panel’s findings is impressive.
Although it has been long in coming, sleep is finally beginning to get some attention by the media. The focus is usually on the optimal number of hours we need each night. This panel’s findings suggest that total sleep time is only part of the story, and may even be less important than the regularity of our sleep patterns.
For those of us in pediatrics, the place where irregularity raises its ugly head is with teenagers and weekends. Although the numbers are far from clear, the question remains of how effective is catch-up sleep after a week of too-early mornings and too-late bedtimes for the chronically under-slept adolescent.
In some studies in which patients had the demonstrable effects of sleep deprivation (e.g., metabolic and cardiovascular) there was some improvement when weekend sleep was extended by 1 or 2 hours, but none beyond 2 hours.
The panel’s findings, while certainly significant, merely add weight and nuance to the existing evidence of importance of sleep and the damage done by sleep deprivation. As one of the panel members has said, “Sleep is the third pillar of health, equally important as diet and exercise, if not more.” However, this message is not getting out, or at least it is not being heeded. Like obesity, our efforts as advisers to our patients isn’t working. Unfortunately, this is because our advice is often whispered and given halfheartedly.
There was some evidence of improvement as a result of the pandemic, when those fortunate enough to be able to work from home were taking advantage of the flexibility in their schedules and getting more sleep. But health care providers certainly can’t take responsibility for what was an accident of nature.
Those of you who have been reading Letters from Maine for the last 3 decades may tire of my beating the tired horse of sleep deprivation. But I will not be deterred. I see very little evidence among health care professionals in taking the importance of sleep seriously. Sure, they may include it buried in the list of potential contributors to their patient’s complaint, but I see very little effort to move it higher on their list of priorities and almost no movement toward making substantive recommendations and then reinforcing them with follow-up.
Like obesity, sleep deprivation is a societal problem. We can lay some of the blame on Thomas Edison, but Until that time you will continue to read columns like this one when I encounter significant studies on the importance of sleep.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Sleep Apnea: Comorbidities, Racial Disparities, Weight Guidelines, and Alternatives to CPAP
1. Gottlieb DJ, Punjabi NM. JAMA. 2020;323(14):1389-1400. doi:10.1001/jama.2020.3514
2. Slowik JM et al. Obstructive Sleep Apnea. In: StatPearls. Treasure Island (FL): StatPearls Publishing; December 11, 2022.
3. Bonsignore MR et al. Multidiscip Respir Med. 2019;14:8. doi:10.1186/s40248-019-0172-9
4. Schwartz SW et al. Sleep Breath. 2016;20(3):947-955. doi:10.1007/s11325-016-1316-1
5. Grandner MA et al. Sleep Med. 2016;18:7-18. doi:10.1016/j.sleep.2015.01.020
6. Lee YC et al. Sleep Med. 2022;90:204-213. doi:10.1016/j.sleep.2021.11.014
7. Hudgel DW et al. Am J Respir Crit Care Med. 2018;198(6):e70-e87. doi:10.1164/rccm.201807-1326ST
8. Lloyd R et al. J Clin Sleep Med. 2022;18(11):2673-2680. doi:10.5664/jcsm.10244
9. Nokes B et al. Expert Rev Respir Med. 2022;16(8):917-929. doi:10.1080/17476348.2022.2112669
10. Pinto JA et al. Int Arch Otorhinolaryngol. 2016;20(2):145-150.doi:10.1055/s-0036-1579546
11. Georgoulis M et al. J Clin Sleep Med. 2022;18(5):1251-1261. doi:10.5664/jcsm.9834
12. Askland K et al. Cochrane Database Syst Rev. 2020;4(4):CD007736. doi:10.1002/14651858.CD007736.pub3
13. Jugé L et al. Sleep. 2022;45(6):zsac044. doi:10.1093/sleep/zsac044
14. Strollo PJ Jr et al. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
15. Fattal D et al. J Clin Sleep Med. 2022;18(12):2723-2729. doi:10.5664/jcsm.10190
16. He M et al. Otolaryngol Head Neck Surg. 2019;161(3):401-411. doi:10.1177/0194599819840356
1. Gottlieb DJ, Punjabi NM. JAMA. 2020;323(14):1389-1400. doi:10.1001/jama.2020.3514
2. Slowik JM et al. Obstructive Sleep Apnea. In: StatPearls. Treasure Island (FL): StatPearls Publishing; December 11, 2022.
3. Bonsignore MR et al. Multidiscip Respir Med. 2019;14:8. doi:10.1186/s40248-019-0172-9
4. Schwartz SW et al. Sleep Breath. 2016;20(3):947-955. doi:10.1007/s11325-016-1316-1
5. Grandner MA et al. Sleep Med. 2016;18:7-18. doi:10.1016/j.sleep.2015.01.020
6. Lee YC et al. Sleep Med. 2022;90:204-213. doi:10.1016/j.sleep.2021.11.014
7. Hudgel DW et al. Am J Respir Crit Care Med. 2018;198(6):e70-e87. doi:10.1164/rccm.201807-1326ST
8. Lloyd R et al. J Clin Sleep Med. 2022;18(11):2673-2680. doi:10.5664/jcsm.10244
9. Nokes B et al. Expert Rev Respir Med. 2022;16(8):917-929. doi:10.1080/17476348.2022.2112669
10. Pinto JA et al. Int Arch Otorhinolaryngol. 2016;20(2):145-150.doi:10.1055/s-0036-1579546
11. Georgoulis M et al. J Clin Sleep Med. 2022;18(5):1251-1261. doi:10.5664/jcsm.9834
12. Askland K et al. Cochrane Database Syst Rev. 2020;4(4):CD007736. doi:10.1002/14651858.CD007736.pub3
13. Jugé L et al. Sleep. 2022;45(6):zsac044. doi:10.1093/sleep/zsac044
14. Strollo PJ Jr et al. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
15. Fattal D et al. J Clin Sleep Med. 2022;18(12):2723-2729. doi:10.5664/jcsm.10190
16. He M et al. Otolaryngol Head Neck Surg. 2019;161(3):401-411. doi:10.1177/0194599819840356
1. Gottlieb DJ, Punjabi NM. JAMA. 2020;323(14):1389-1400. doi:10.1001/jama.2020.3514
2. Slowik JM et al. Obstructive Sleep Apnea. In: StatPearls. Treasure Island (FL): StatPearls Publishing; December 11, 2022.
3. Bonsignore MR et al. Multidiscip Respir Med. 2019;14:8. doi:10.1186/s40248-019-0172-9
4. Schwartz SW et al. Sleep Breath. 2016;20(3):947-955. doi:10.1007/s11325-016-1316-1
5. Grandner MA et al. Sleep Med. 2016;18:7-18. doi:10.1016/j.sleep.2015.01.020
6. Lee YC et al. Sleep Med. 2022;90:204-213. doi:10.1016/j.sleep.2021.11.014
7. Hudgel DW et al. Am J Respir Crit Care Med. 2018;198(6):e70-e87. doi:10.1164/rccm.201807-1326ST
8. Lloyd R et al. J Clin Sleep Med. 2022;18(11):2673-2680. doi:10.5664/jcsm.10244
9. Nokes B et al. Expert Rev Respir Med. 2022;16(8):917-929. doi:10.1080/17476348.2022.2112669
10. Pinto JA et al. Int Arch Otorhinolaryngol. 2016;20(2):145-150.doi:10.1055/s-0036-1579546
11. Georgoulis M et al. J Clin Sleep Med. 2022;18(5):1251-1261. doi:10.5664/jcsm.9834
12. Askland K et al. Cochrane Database Syst Rev. 2020;4(4):CD007736. doi:10.1002/14651858.CD007736.pub3
13. Jugé L et al. Sleep. 2022;45(6):zsac044. doi:10.1093/sleep/zsac044
14. Strollo PJ Jr et al. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
15. Fattal D et al. J Clin Sleep Med. 2022;18(12):2723-2729. doi:10.5664/jcsm.10190
16. He M et al. Otolaryngol Head Neck Surg. 2019;161(3):401-411. doi:10.1177/0194599819840356
CPAP adherence curbs severe cardiovascular disease outcomes
, based on data from more than 4,000 individuals.
Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular diseases, but the association between management of OSA with a continuous positive-airway pressure device (CPAP) and major adverse cardiac or cerebrovascular events (MACCEs) remains unclear, wrote Manuel Sánchez-de-la-Torre, PhD, of the University of Lleida, Spain, and colleagues.
In a meta-analysis published in JAMA, the researchers reviewed data from 4,186 individuals with a mean age of 61.2 years; 82.1% were men. The study population included 2,097 patients who used CPAP and 2,089 who did not. The mean apnea-hypopnea index (AHI) was 31.2 events per hour, and OSA was defined as an oxygen desaturation index of 12 events or more per hour or an AHI of 15 events or more per hour. The composite primary outcome included the first MACCE, or death from cardiovascular causes, myocardial infarction, stroke, revascularization procedure, hospital admission for heart failure, hospital admission for unstable angina, or hospital admission for transient ischemic attack. Each of these components was a secondary endpoint.
Overall, the primary outcome of MACCE was similar for CPAP and non-CPAP using patients (hazard ratio, 1.01) with a total of 349 MACCE events in the CPAP group and 342 in the non-CPAP group. The mean adherence to CPAP was 3.1 hours per day. A total of 38.5% of patients in the CPAP group met the criteria for good adherence, defined as a mean of 4 or more hours per day.
However, as defined, good adherence to CPAP significantly reduced the risk of MACCE, compared with no CPAP use (HR, 0.69), and a sensitivity analysis showed a significant risk reduction, compared with patients who did not meet the criteria for good adherence (HR, 0.55; P = .005).
“Adherence to treatment is complex to determine and there are other potential factors that could affect patient adherence, such as health education, motivation, attitude, self-efficacy, psychosocial factors, and other health care system–related features,” the researchers wrote in their discussion.
The findings were limited by several factors including the evaluation only of CPAP as a treatment for OSA, and the inability to assess separate components of the composite endpoint, the researchers noted. Other limitations included the relatively small number of female patients, reliance mainly on at-home sleep apnea tests, and the potential for selection bias, they said.
However, the results suggest that CPAP adherence is important to prevention of secondary cardiovascular outcomes in OSA patients, and that implementation of specific and personalized strategies to improve adherence to treatment should be a clinical priority, they concluded.
The study was funded by the Instituto de Salud Carlos III, the European Union and FEDER, IRBLleida–Fundació Dr Pifarré, SEPAR, ResMed Ltd. (Australia), Associació Lleidatana de Respiratori, and CIBERES. Dr Sánchez-de-la-Torre also disclosed financial support from a Ramón y Cajal grant.
, based on data from more than 4,000 individuals.
Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular diseases, but the association between management of OSA with a continuous positive-airway pressure device (CPAP) and major adverse cardiac or cerebrovascular events (MACCEs) remains unclear, wrote Manuel Sánchez-de-la-Torre, PhD, of the University of Lleida, Spain, and colleagues.
In a meta-analysis published in JAMA, the researchers reviewed data from 4,186 individuals with a mean age of 61.2 years; 82.1% were men. The study population included 2,097 patients who used CPAP and 2,089 who did not. The mean apnea-hypopnea index (AHI) was 31.2 events per hour, and OSA was defined as an oxygen desaturation index of 12 events or more per hour or an AHI of 15 events or more per hour. The composite primary outcome included the first MACCE, or death from cardiovascular causes, myocardial infarction, stroke, revascularization procedure, hospital admission for heart failure, hospital admission for unstable angina, or hospital admission for transient ischemic attack. Each of these components was a secondary endpoint.
Overall, the primary outcome of MACCE was similar for CPAP and non-CPAP using patients (hazard ratio, 1.01) with a total of 349 MACCE events in the CPAP group and 342 in the non-CPAP group. The mean adherence to CPAP was 3.1 hours per day. A total of 38.5% of patients in the CPAP group met the criteria for good adherence, defined as a mean of 4 or more hours per day.
However, as defined, good adherence to CPAP significantly reduced the risk of MACCE, compared with no CPAP use (HR, 0.69), and a sensitivity analysis showed a significant risk reduction, compared with patients who did not meet the criteria for good adherence (HR, 0.55; P = .005).
“Adherence to treatment is complex to determine and there are other potential factors that could affect patient adherence, such as health education, motivation, attitude, self-efficacy, psychosocial factors, and other health care system–related features,” the researchers wrote in their discussion.
The findings were limited by several factors including the evaluation only of CPAP as a treatment for OSA, and the inability to assess separate components of the composite endpoint, the researchers noted. Other limitations included the relatively small number of female patients, reliance mainly on at-home sleep apnea tests, and the potential for selection bias, they said.
However, the results suggest that CPAP adherence is important to prevention of secondary cardiovascular outcomes in OSA patients, and that implementation of specific and personalized strategies to improve adherence to treatment should be a clinical priority, they concluded.
The study was funded by the Instituto de Salud Carlos III, the European Union and FEDER, IRBLleida–Fundació Dr Pifarré, SEPAR, ResMed Ltd. (Australia), Associació Lleidatana de Respiratori, and CIBERES. Dr Sánchez-de-la-Torre also disclosed financial support from a Ramón y Cajal grant.
, based on data from more than 4,000 individuals.
Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular diseases, but the association between management of OSA with a continuous positive-airway pressure device (CPAP) and major adverse cardiac or cerebrovascular events (MACCEs) remains unclear, wrote Manuel Sánchez-de-la-Torre, PhD, of the University of Lleida, Spain, and colleagues.
In a meta-analysis published in JAMA, the researchers reviewed data from 4,186 individuals with a mean age of 61.2 years; 82.1% were men. The study population included 2,097 patients who used CPAP and 2,089 who did not. The mean apnea-hypopnea index (AHI) was 31.2 events per hour, and OSA was defined as an oxygen desaturation index of 12 events or more per hour or an AHI of 15 events or more per hour. The composite primary outcome included the first MACCE, or death from cardiovascular causes, myocardial infarction, stroke, revascularization procedure, hospital admission for heart failure, hospital admission for unstable angina, or hospital admission for transient ischemic attack. Each of these components was a secondary endpoint.
Overall, the primary outcome of MACCE was similar for CPAP and non-CPAP using patients (hazard ratio, 1.01) with a total of 349 MACCE events in the CPAP group and 342 in the non-CPAP group. The mean adherence to CPAP was 3.1 hours per day. A total of 38.5% of patients in the CPAP group met the criteria for good adherence, defined as a mean of 4 or more hours per day.
However, as defined, good adherence to CPAP significantly reduced the risk of MACCE, compared with no CPAP use (HR, 0.69), and a sensitivity analysis showed a significant risk reduction, compared with patients who did not meet the criteria for good adherence (HR, 0.55; P = .005).
“Adherence to treatment is complex to determine and there are other potential factors that could affect patient adherence, such as health education, motivation, attitude, self-efficacy, psychosocial factors, and other health care system–related features,” the researchers wrote in their discussion.
The findings were limited by several factors including the evaluation only of CPAP as a treatment for OSA, and the inability to assess separate components of the composite endpoint, the researchers noted. Other limitations included the relatively small number of female patients, reliance mainly on at-home sleep apnea tests, and the potential for selection bias, they said.
However, the results suggest that CPAP adherence is important to prevention of secondary cardiovascular outcomes in OSA patients, and that implementation of specific and personalized strategies to improve adherence to treatment should be a clinical priority, they concluded.
The study was funded by the Instituto de Salud Carlos III, the European Union and FEDER, IRBLleida–Fundació Dr Pifarré, SEPAR, ResMed Ltd. (Australia), Associació Lleidatana de Respiratori, and CIBERES. Dr Sánchez-de-la-Torre also disclosed financial support from a Ramón y Cajal grant.
FROM JAMA
Sentinel central events prevalent during DISE for obstructive sleep apnea
DISE has become the top choice for surgical selection in patients with OSA, but it has a variable effect on surgical outcomes, Julianna G. Rodin, MD, of the University of Pennsylvania, Philadelphia, and colleagues explained.
The University of Pennsylvania sleep surgery team developed a comprehensive DISE platform that includes simultaneous collection of respiratory airflow and effort measurements, airway collapsibility, and videoendoscopy.
“This home sleep study-style setup has allowed us to better characterize the upper airway during DISE, and even helped our team diagnose a patient with Cheyne-Stokes breathing/central sleep apnea,” Dr. Rodin said in an interview.
“With it, we also began to notice relatively frequent central and/or mixed sleep disordered breathing events during DISE after propofol dosing initiation,” she said.
In a study presented at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery, Dr. Rodin and colleagues measured both the frequency and timing of sentinel central and/or mixed events (SCent) in adults undergoing DISE to assess the prevalence and impact on DISE.
The researchers also assessed differences in VOTE classification (velum, oropharynx, tongue base, and epiglottis) in sentinel central events, compared with obstructive events. VOTE scores were calculated using a grade of 0 for no obstruction, 1 for partial obstruction, and 2 for total obstruction.
The study population included 103 adults with OSA who underwent DISE with propofol sedation at a single tertiary academic medical center between June 2020 and November 2022. The mean age of the participants was 53.5 years, the mean body mass index (BMI) was 29.7 kg/m2, and 67% were male. The average apnea-hypopnea index (AHI) was 30.7 events per hour. The researchers used a polysomnography platform to capture data on nasal airflow, thoraco-abdominal effort belt signals, and videoendoscopy.
A total of 47 patients (46%) had at least one SCent. The average time to the first SCent was just under 6 minutes, and average transition to obstructive pathology in these patients occurred between 7 and 8 minutes. Using the one-sided prediction interval, at least 95% of patients were expected to transition to obstructive pathology within 12-13 minutes, Dr. Rodin said.
In addition, 29 of the 46 patients with SCent (63%) showed significant variability between central/mixed VOTE scores and obstructive VOTE scores.
No statistically significant differences were noted between patients with and without SCent in terms of demographics or AHI.
Surprising prevalence of SCents
“We anecdotally noted that SCents seemed to be somewhat common during the initial period of DISE, but were surprised that we saw at least one SCent in almost 50% of our DISE population,” Dr. Rodin said. “We also saw that the majority of these SCents eventually transitioned to obstructive events after approximately 12 minutes, which is often past the average duration of normal DISE exams.”
The high frequency of differing VOTE scores between SCents and obstructive events also was unexpected, she added. Within the changes in VOTE scores as defined in the study, “there was a higher tendency for SCents to have more complete tongue base collapse compared to no or partial collapse in obstructive events, and to transition from anterior-posterior velum to concentric velum collapse during the obstructive event.”
This outcome could potentially affect a patient’s candidacy for hypoglossal nerve stimulator therapy, she explained.
The takeaway from the current study is an increased awareness of the prevalence and timing of SCents in OSA patients, said Dr. Rodin. Clinicians who offer DISE and PAP alternatives also should be mindful of clinical signs of effort, by monitoring the chest and abdomen during DISE in the absence of respiratory effort belts.
The study findings also suggest that clinicians consider extending the minimum DISE duration to 10 minutes to ensure that the majority of SCents have passed, and delay VOTE scoring until patients transition to obstructive events, she added.
As for additional research, Dr. Rodin said: “If we could repeat the study with a standardized protocol of target-controlled infusion (TCI) of propofol, that would further bolster the data.” However, TCI is not approved in the United States.
“Our propofol dosing technique was not standardized across all patients, which in theory could account for more SCents if patients were more sedated,” Dr. Rodin noted. “However, we did not see a difference in average bispectral index levels across all patients.”
Other limitations of the current study included an inability to visualize the entire upper airway to achieve a complete VOTE score for every patient, which could have led to underestimation of the VOTE difference frequency, she added.
Data inform team approaches to DISE
As DISE procedures become more widespread, “it is paramount that we understand the risks associated with these procedures to increase safety, improve shared decision-making, and encourage a team-based approach in the operating room with our anesthesia colleagues,” said Daniel M. Zeitler, MD, from the University of Washington and Virgina Mason Medical Center, both in Seattle, who served as a moderator for the session in which the study was presented.
“I was surprised by these data for two reasons,” Dr. Zeitler said in an interview. “We typically don’t wait more than a few minutes between induction of anesthesia and the initiation of the airway procedure. This study calls that practice into question, and the duration of time before the onset of a sentinel event was much longer than I would have expected,” he said.
Second, “I was quite surprised that there were no differences in the demographics or AHI between the two groups; this reminds us that AHI and BMI alone may not be themselves predictive of risk and all patients should be assessed similarly.”
“Otolaryngologists performing DISE need to be aware of these data, communicate them to the involved teams, including anesthesia, nursing, and postanesthesia care units, and remember to delay the manipulation of the airway long enough to minimize the risk of a sentinel event,” Dr. Zeitler said. “Perhaps this also means we need improved intraoperative monitoring for these patients, including respiratory airflow and effort monitoring.”
For further research, “we need to increase the number of patients, perform a multicenter study, and expand the study to a wider range of ages, BMI, and AHI,” he added. A recommended algorithm for these cases in order to standardize the practice would be useful.
The study received no outside funding. Dr. Rodin and Dr. Zeitler reported no relevant financial relationships. Several coauthors disclosed funding and relationships with multiple companies unrelated to the current study.
A version of this article appeared on Medscape.com.
DISE has become the top choice for surgical selection in patients with OSA, but it has a variable effect on surgical outcomes, Julianna G. Rodin, MD, of the University of Pennsylvania, Philadelphia, and colleagues explained.
The University of Pennsylvania sleep surgery team developed a comprehensive DISE platform that includes simultaneous collection of respiratory airflow and effort measurements, airway collapsibility, and videoendoscopy.
“This home sleep study-style setup has allowed us to better characterize the upper airway during DISE, and even helped our team diagnose a patient with Cheyne-Stokes breathing/central sleep apnea,” Dr. Rodin said in an interview.
“With it, we also began to notice relatively frequent central and/or mixed sleep disordered breathing events during DISE after propofol dosing initiation,” she said.
In a study presented at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery, Dr. Rodin and colleagues measured both the frequency and timing of sentinel central and/or mixed events (SCent) in adults undergoing DISE to assess the prevalence and impact on DISE.
The researchers also assessed differences in VOTE classification (velum, oropharynx, tongue base, and epiglottis) in sentinel central events, compared with obstructive events. VOTE scores were calculated using a grade of 0 for no obstruction, 1 for partial obstruction, and 2 for total obstruction.
The study population included 103 adults with OSA who underwent DISE with propofol sedation at a single tertiary academic medical center between June 2020 and November 2022. The mean age of the participants was 53.5 years, the mean body mass index (BMI) was 29.7 kg/m2, and 67% were male. The average apnea-hypopnea index (AHI) was 30.7 events per hour. The researchers used a polysomnography platform to capture data on nasal airflow, thoraco-abdominal effort belt signals, and videoendoscopy.
A total of 47 patients (46%) had at least one SCent. The average time to the first SCent was just under 6 minutes, and average transition to obstructive pathology in these patients occurred between 7 and 8 minutes. Using the one-sided prediction interval, at least 95% of patients were expected to transition to obstructive pathology within 12-13 minutes, Dr. Rodin said.
In addition, 29 of the 46 patients with SCent (63%) showed significant variability between central/mixed VOTE scores and obstructive VOTE scores.
No statistically significant differences were noted between patients with and without SCent in terms of demographics or AHI.
Surprising prevalence of SCents
“We anecdotally noted that SCents seemed to be somewhat common during the initial period of DISE, but were surprised that we saw at least one SCent in almost 50% of our DISE population,” Dr. Rodin said. “We also saw that the majority of these SCents eventually transitioned to obstructive events after approximately 12 minutes, which is often past the average duration of normal DISE exams.”
The high frequency of differing VOTE scores between SCents and obstructive events also was unexpected, she added. Within the changes in VOTE scores as defined in the study, “there was a higher tendency for SCents to have more complete tongue base collapse compared to no or partial collapse in obstructive events, and to transition from anterior-posterior velum to concentric velum collapse during the obstructive event.”
This outcome could potentially affect a patient’s candidacy for hypoglossal nerve stimulator therapy, she explained.
The takeaway from the current study is an increased awareness of the prevalence and timing of SCents in OSA patients, said Dr. Rodin. Clinicians who offer DISE and PAP alternatives also should be mindful of clinical signs of effort, by monitoring the chest and abdomen during DISE in the absence of respiratory effort belts.
The study findings also suggest that clinicians consider extending the minimum DISE duration to 10 minutes to ensure that the majority of SCents have passed, and delay VOTE scoring until patients transition to obstructive events, she added.
As for additional research, Dr. Rodin said: “If we could repeat the study with a standardized protocol of target-controlled infusion (TCI) of propofol, that would further bolster the data.” However, TCI is not approved in the United States.
“Our propofol dosing technique was not standardized across all patients, which in theory could account for more SCents if patients were more sedated,” Dr. Rodin noted. “However, we did not see a difference in average bispectral index levels across all patients.”
Other limitations of the current study included an inability to visualize the entire upper airway to achieve a complete VOTE score for every patient, which could have led to underestimation of the VOTE difference frequency, she added.
Data inform team approaches to DISE
As DISE procedures become more widespread, “it is paramount that we understand the risks associated with these procedures to increase safety, improve shared decision-making, and encourage a team-based approach in the operating room with our anesthesia colleagues,” said Daniel M. Zeitler, MD, from the University of Washington and Virgina Mason Medical Center, both in Seattle, who served as a moderator for the session in which the study was presented.
“I was surprised by these data for two reasons,” Dr. Zeitler said in an interview. “We typically don’t wait more than a few minutes between induction of anesthesia and the initiation of the airway procedure. This study calls that practice into question, and the duration of time before the onset of a sentinel event was much longer than I would have expected,” he said.
Second, “I was quite surprised that there were no differences in the demographics or AHI between the two groups; this reminds us that AHI and BMI alone may not be themselves predictive of risk and all patients should be assessed similarly.”
“Otolaryngologists performing DISE need to be aware of these data, communicate them to the involved teams, including anesthesia, nursing, and postanesthesia care units, and remember to delay the manipulation of the airway long enough to minimize the risk of a sentinel event,” Dr. Zeitler said. “Perhaps this also means we need improved intraoperative monitoring for these patients, including respiratory airflow and effort monitoring.”
For further research, “we need to increase the number of patients, perform a multicenter study, and expand the study to a wider range of ages, BMI, and AHI,” he added. A recommended algorithm for these cases in order to standardize the practice would be useful.
The study received no outside funding. Dr. Rodin and Dr. Zeitler reported no relevant financial relationships. Several coauthors disclosed funding and relationships with multiple companies unrelated to the current study.
A version of this article appeared on Medscape.com.
DISE has become the top choice for surgical selection in patients with OSA, but it has a variable effect on surgical outcomes, Julianna G. Rodin, MD, of the University of Pennsylvania, Philadelphia, and colleagues explained.
The University of Pennsylvania sleep surgery team developed a comprehensive DISE platform that includes simultaneous collection of respiratory airflow and effort measurements, airway collapsibility, and videoendoscopy.
“This home sleep study-style setup has allowed us to better characterize the upper airway during DISE, and even helped our team diagnose a patient with Cheyne-Stokes breathing/central sleep apnea,” Dr. Rodin said in an interview.
“With it, we also began to notice relatively frequent central and/or mixed sleep disordered breathing events during DISE after propofol dosing initiation,” she said.
In a study presented at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery, Dr. Rodin and colleagues measured both the frequency and timing of sentinel central and/or mixed events (SCent) in adults undergoing DISE to assess the prevalence and impact on DISE.
The researchers also assessed differences in VOTE classification (velum, oropharynx, tongue base, and epiglottis) in sentinel central events, compared with obstructive events. VOTE scores were calculated using a grade of 0 for no obstruction, 1 for partial obstruction, and 2 for total obstruction.
The study population included 103 adults with OSA who underwent DISE with propofol sedation at a single tertiary academic medical center between June 2020 and November 2022. The mean age of the participants was 53.5 years, the mean body mass index (BMI) was 29.7 kg/m2, and 67% were male. The average apnea-hypopnea index (AHI) was 30.7 events per hour. The researchers used a polysomnography platform to capture data on nasal airflow, thoraco-abdominal effort belt signals, and videoendoscopy.
A total of 47 patients (46%) had at least one SCent. The average time to the first SCent was just under 6 minutes, and average transition to obstructive pathology in these patients occurred between 7 and 8 minutes. Using the one-sided prediction interval, at least 95% of patients were expected to transition to obstructive pathology within 12-13 minutes, Dr. Rodin said.
In addition, 29 of the 46 patients with SCent (63%) showed significant variability between central/mixed VOTE scores and obstructive VOTE scores.
No statistically significant differences were noted between patients with and without SCent in terms of demographics or AHI.
Surprising prevalence of SCents
“We anecdotally noted that SCents seemed to be somewhat common during the initial period of DISE, but were surprised that we saw at least one SCent in almost 50% of our DISE population,” Dr. Rodin said. “We also saw that the majority of these SCents eventually transitioned to obstructive events after approximately 12 minutes, which is often past the average duration of normal DISE exams.”
The high frequency of differing VOTE scores between SCents and obstructive events also was unexpected, she added. Within the changes in VOTE scores as defined in the study, “there was a higher tendency for SCents to have more complete tongue base collapse compared to no or partial collapse in obstructive events, and to transition from anterior-posterior velum to concentric velum collapse during the obstructive event.”
This outcome could potentially affect a patient’s candidacy for hypoglossal nerve stimulator therapy, she explained.
The takeaway from the current study is an increased awareness of the prevalence and timing of SCents in OSA patients, said Dr. Rodin. Clinicians who offer DISE and PAP alternatives also should be mindful of clinical signs of effort, by monitoring the chest and abdomen during DISE in the absence of respiratory effort belts.
The study findings also suggest that clinicians consider extending the minimum DISE duration to 10 minutes to ensure that the majority of SCents have passed, and delay VOTE scoring until patients transition to obstructive events, she added.
As for additional research, Dr. Rodin said: “If we could repeat the study with a standardized protocol of target-controlled infusion (TCI) of propofol, that would further bolster the data.” However, TCI is not approved in the United States.
“Our propofol dosing technique was not standardized across all patients, which in theory could account for more SCents if patients were more sedated,” Dr. Rodin noted. “However, we did not see a difference in average bispectral index levels across all patients.”
Other limitations of the current study included an inability to visualize the entire upper airway to achieve a complete VOTE score for every patient, which could have led to underestimation of the VOTE difference frequency, she added.
Data inform team approaches to DISE
As DISE procedures become more widespread, “it is paramount that we understand the risks associated with these procedures to increase safety, improve shared decision-making, and encourage a team-based approach in the operating room with our anesthesia colleagues,” said Daniel M. Zeitler, MD, from the University of Washington and Virgina Mason Medical Center, both in Seattle, who served as a moderator for the session in which the study was presented.
“I was surprised by these data for two reasons,” Dr. Zeitler said in an interview. “We typically don’t wait more than a few minutes between induction of anesthesia and the initiation of the airway procedure. This study calls that practice into question, and the duration of time before the onset of a sentinel event was much longer than I would have expected,” he said.
Second, “I was quite surprised that there were no differences in the demographics or AHI between the two groups; this reminds us that AHI and BMI alone may not be themselves predictive of risk and all patients should be assessed similarly.”
“Otolaryngologists performing DISE need to be aware of these data, communicate them to the involved teams, including anesthesia, nursing, and postanesthesia care units, and remember to delay the manipulation of the airway long enough to minimize the risk of a sentinel event,” Dr. Zeitler said. “Perhaps this also means we need improved intraoperative monitoring for these patients, including respiratory airflow and effort monitoring.”
For further research, “we need to increase the number of patients, perform a multicenter study, and expand the study to a wider range of ages, BMI, and AHI,” he added. A recommended algorithm for these cases in order to standardize the practice would be useful.
The study received no outside funding. Dr. Rodin and Dr. Zeitler reported no relevant financial relationships. Several coauthors disclosed funding and relationships with multiple companies unrelated to the current study.
A version of this article appeared on Medscape.com.
FROM THE AAOH-HNS MEETING
Treating chronic insomnia: An alternating medication strategy
Patients with chronic insomnia that does not improve with nonpharmacologic techniques often develop tolerance to sedative medications (benzodiazepines) prescribed for nightly use. When nonbenzodiazepine medications are used, tachyphylaxis can develop and these medications no longer initiate or maintain sleep. Strategies that alternate between these 2 types of agents are simple to follow and may allow patients to maintain sensitivity to both types of medications. In this article, I review the types, causes, evaluation, and treatment of insomnia; describe an alternating medication strategy to help patients avoid developing tolerance/tachyphylaxis; and present 3 fictional case vignettes to illustrate this approach.
A common, troubling condition
Insomnia is a common problem among psychiatric patients. Approximately 30% to 50% of adults experience occasional, short-term (<3 months) insomnia, and 5% to 10% experience chronic (≥3 months) insomnia,1 with associated negative impacts on health and quality of life. Insomnia is sometimes primary and may have a hereditary component, but more often is associated with medical, neurologic, or psychiatric disorders.
Patterns of insomnia include difficulty falling asleep (initial or sleep-onset insomnia), remaining asleep (middle or sleep-maintenance insomnia), or falling back asleep after early awakening (late or sleep-offset insomnia). Sleep-onset insomnia correlates with high levels of anxiety and worrying, but once asleep, patients usually stay asleep. Sleep-maintenance problems involve multiple awakenings after falling asleep and taking hours to fall back to sleep. These patients experience inadequate sleep when they must wake up early for school or work. Early-awakening patients report feeling wide awake by 4 to 5
Caffeine is an important consideration for patients with sleep difficulties. Its use is widespread in much of the world, whether ingested as coffee, tea, in soft drinks, or in “energy” drinks that may contain as much as 200 mg of caffeine (twice the amount in a typical cup of brewed coffee). Caffeine may also be ingested as an ingredient of medications for headache or migraine. While some individuals maintain that they can fall asleep easily after drinking caffeinated coffee, many may not recognize the amount of caffeine they consume and its negative impact on sleep.2 Author Michael Pollan stopped use of all caffeine and reported on the surprising positive effect on his sleep.3
Patients with mood, anxiety, or psychotic disorders are likely to experience insomnia intermittently or chronically, and insomnia predisposes some individuals to develop mood and anxiety symptoms.4 Patients with insomnia often experience anxiety focused on a fear of not getting adequate sleep, which creates a vicious cycle in which hyperarousal associated with fear of not sleeping complicates other causes of insomnia. A patient’s chronotype (preference for the time of day in which they carry out activities vs sleeping) also may play a role in sleep difficulties (Box5).
Box
Chronotypes—the expression of circadian rhythmicity in an individual—have been studied extensively.5 Psychiatrists may encounter patients who sleep most of the day and stay awake at night, those who sleep up to 20 hours per day, and those who sleep <4 hours in 24 hours. Patients typically know which category they fall into. The early bird typically is awake by 6 or 7 am, remains alert through most of the day, and feels sleepy by 10 pm. The usual diurnal variation in cortisol, with peaks at 7 am and 7 pm and nadirs at 1 pm and 1 am, correspond with the early bird’s habits.
Night owls typically report feeling exhausted and irritable in the early morning; prefer to sleep past noon; feel energized around dark, when they can do their best studying, concentrating, etc; and do not feel sleepy until early morning. While this night owl pattern is a natural variation and not necessarily associated with psychiatric illness, patients with mood disorders frequently have chaotic sleep patterns that may not conform to a pattern. Night owls maintain the same diurnal pattern of cortisol secretion as early birds.
Certain medications may contribute to insomnia, particularly stimulants. It is important to understand and explain to patients the time frame during which immediate-release or extended-release (ER) stimulants are active, which varies in individuals depending on liver enzyme activity. Other commonly used psychotropic medications—including bupropion, modafinil, armodafinil, atomoxetine, amphetamine salts, and methylphenidate—may interfere with sleep if used later in the day.6
Patients typically do not mention their use of alcohol and/or marijuana unless asked. Those who are binge drinkers or alcohol-dependent may expect alcohol to help them fall asleep, but usually find their sleep is disrupted and difficult to maintain. Patients may use marijuana to help them sleep, particularly marijuana high in tetrahydrocannabinol (THC). While it may help with sleep initiation, THC can disrupt sleep maintenance. Cannabidiol does not have intrinsic sedating effects and may even interfere with sleep.7,8
Continue to: Women may be more likely...
Women may be more likely than men to experience insomnia.9 The onset of menopause can bring hot flashes that interfere with sleep.
Women with a history of mood disorders are more likely to have a history of premenstrual dysphoric disorder, postpartum depression, and unusual responses to oral contraceptives.10 These women are more likely to report problems with mood, energy, and sleep at perimenopause. Treatment with estrogen replacement may be an option for women without risk factors, such as clotting disorders, smoking history, or a personal or family history of breast or uterine cancer. For many who are not candidates for or who refuse estrogen replacement, use of a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor at low doses may help with vasomotor symptoms but not with insomnia.
Insomnia symptoms typically increase with age.11 When sleep is adequate early in life but becomes a problem in midlife, an individual’s eating habits, obesity, and lack of exercise may be contributing factors. The typical American diet includes highly refined carbohydrates with excess salt; such foods are often readily available to the exclusion of healthy options. Overweight and obese patients may insist they eat a healthy diet with 3 meals per day, but a careful history often uncovers nighttime binge eating. Nighttime binge eating is rarely reported. This not only maintains obesity, but also interferes with sleep, since patients stay up late to avoid discovery by family members.12 This lack of sleep can lead to an endless loop because insufficient sleep is a risk factor for obesity.13
Evaluating sleep difficulties
New patient evaluations should include a careful history beginning with childhood, including personal early childhood history and family psychiatric history. Patients often report the onset of sleep difficulty and anxiety during childhood, which should raise further questions about aspects of mood regulation from early life such as concentration, energy, motivation, appetite, and academic performance. While many children and adolescents are diagnosed with attention-deficit/hyperactivity disorder due to concentration problems that cause difficulties at school, be aware this might be part of a syndrome related to mood regulation.14 Unexpected responses to an SSRI—such as agitation, euphoria, or an immediate response with the first dose—should also raise suspicion of a mood disorder. Once the underlying mood disorder is stabilized, many patients report improved sleep.15
If a patient reports having difficulty falling and remaining asleep but is not sure if there is a pattern, keeping a sleep diary can help. Further questioning may uncover the cause. Does the patient have spontaneous jerks of lower extremities (restless leg syndrome) that interfere with falling asleep or wake them up? Have they noticed problems with dreams/nightmares that wake them, which could be associated with posttraumatic stress, anxiety, or depression? Have they been told by a partner that they act out dreams or are seemingly awake but not responsive, which could point to REM sleep behavior disorder or early Parkinson’s disease? Referral to a sleep laboratory and a neurologist can help establish the correct diagnosis and point to appropriate treatment.16-18
Treatment options
Several cognitive-behavioral techniques, including cognitive-behavioral therapy for insomnia (CBT-I), yogic breathing, progressive relaxation, mindfulness meditation, and sleep hygiene techniques may help considerably,19,20 but insomnia often remains difficult to treat. Pharmacotherapy is not necessarily more effective than nonpharmacologic approaches. Both options require the patient to take initiative to either find nonpharmacologic approaches or discuss the problem with a physician and agree to take medication.21 A trial comparing CBT-I to sedatives or the combination of CBT-I plus sedatives found higher rates of sleep with CBT-I for 3 months, after which improvement fluctuated; the combination showed sustained improvement for the entire 6-month trial.22 CBT-I has also been shown to be as effective with patients who do not have psychiatric illness as for those who are depressed, anxious, or stressed.23 However, behavioral techniques that require regular practice may be difficult for individuals to maintain, particularly when they are depressed or anxious.
Continue to: Clinicians should understand...
Clinicians should understand the distinctions among the various types of pharmacotherapy for insomnia. Sedative-hypnotics include medications with varying half-lives and metabolic pathways. Short-acting benzodiazepines such as triazolam or alprazolam and the “z-drugs” zolpidem or zaleplon may help initiate sleep in patients with sleep-onset insomnia. Longer-acting benzodiazepines such as diazepam, clonazepam, or temazepam and the z-drug eszopiclone may also help with sleep maintenance.23 Based on my clinical experience, individual patients may respond better to 1 type of medication over another, or even to different agents within the same class of sedative-hypnotics.
Some clinicians prescribe nonbenzodiazepine medications for sleep, such as doxepin (which is FDA-approved for treating insomnia) or off-label trazodone, mirtazapine, or quetiapine. Their antihistaminic properties confer sedating effects. Virtually all over-the-counter (OTC) medications for insomnia are antihistaminic. These OTC medications are not designed to treat insomnia, and the optimal dosage to maintain sleep without daytime sedation must be determined by trial and error. Sedating nonbenzodiazepine medications may be slowly absorbed if taken at bedtime (depending on whether they are taken with or without food) and cause daytime sedation and cognitive slowness in patients with sleep-onset and maintenance insomnia who must wake up early. Starting trazodone at 50 to 75 mg may cause slow metabolizers to wake up with considerable sedation, while fast metabolizers might never feel soundly asleep.24
Patients with mood and anxiety disorders that complicate insomnia are often prescribed second-generation antipsychotics such as quetiapine, lurasidone, or olanzapine, which are sedating as well as mood-stabilizing. These approaches require careful attention to titrating doses and timing their use.
Problems with pharmacotherapy
When either benzodiazepines or nonbenzodiazepine medications are used on a long-standing, nightly basis, they often stop working well. It is not unusual that after days to weeks of taking a benzodiazepine, patients find they no longer stay asleep but can’t fall asleep if they don’t take them. Once tolerance develops, the individual experiences pharmacologic withdrawal with an inability to fall asleep or stay asleep. The medication becomes necessary but ineffective, and many patients increase their use to higher doses to fall asleep, and sometimes in early morning to maintain sleep. This leads to negative effects on cognition, coordination/balance, and mood during the day, especially in older patients.
Nonbenzodiazepine sedating medications do not lead to pharmacologic tolerance but do lead to tachyphylaxis as the CNS attempts to downregulate sedation to keep the organism safe. For some patients, this happens quickly, within a matter of days.25 Others increase doses to stay asleep. For example, a patient with a starting dose of trazodone 75 mg/d might increase the dosage to 300 mg/d. While trazodone is approved in doses of 300 to 600 mg as an antidepressant, it is preferable to keep doses lower when used only for sedation.
Continue to: An alternating medication strategy
An alternating medication strategy
Alternating between medications from different classes can help patients avoid developing tolerance with benzodiazepines or tachyphylaxis as occurs with antihistaminic medications. It can be effective for patients with primary insomnia as well as for those whose sleep problems are associated with mood or anxiety disorders. Patients typically maintain sensitivity to any form of pharmacologic sedation for several nights without loss of effect but need to take a break to maintain the sedation effect. For example, in 1 case study, a 30-year-old female who rapidly developed tachyphylaxis to the sedative action of mirtazapine experienced a return of the medication’s sedative effects after taking a 3-day break.25
To initiate an alternating strategy, the clinician must first help the patient establish a sedating dose of 2 medications from different classes, such as trazodone and zolpidem, and then instruct the patient to use each for 2 to 3 consecutive nights on an alternating basis. Patients can use calendars or pillboxes to avoid confusion about which medication to take on a given night. In many cases, this approach can work indefinitely.
The following 3 case vignettes illustrate how this alternating medication strategy can work.
CASE 1
Mr. B, age 58, is a married salesman whose territory includes 3 states. He drives from client to client from Monday through Thursday each week, staying overnight in hotels. He is comfortable talking to clients, has a close and supportive relationship with his wife, and enjoys socializing with friends. Mr. B has a high level of trait anxiety and perfectionism and is proud of his sales record throughout his career, but this leads to insomnia during his nights on the road, and often on Sunday night as he starts anticipating the week ahead. Mr. B denies having a depressed mood or cognitive problems. When on vacation with his wife he has no trouble sleeping. He has no psychiatric family history or any substantial medical problems. He simply wishes that he could sleep on work nights.
We set up an alternating medication approach. Mr. B takes trazodone 100 mg on the first night and 150 mg on the second and third nights. He then takes triazolam 0.25 mg for 2 nights; previously, he had found that zolpidem did not work as well for maintaining sleep. He can sleep adequately for the 2 weekend nights, then restarts the alternating pattern. Mr. B has done well with this regimen for >10 years.
Continue to: CASE 2
CASE 2
Ms. C, age 60, is widowed and has a successful career as a corporate attorney. She has been anxious since early childhood and has had trouble falling asleep for much of her life. Once she falls asleep on her sofa—often between 1 and 2
Ms. C denies having depression, but experienced appropriate grief related to her husband’s illness and death from metastatic cancer 3 years ago. At the time, her internist prescribed escitalopram and zolpidem; escitalopram caused greater agitation and distress, so she stopped it after 10 days. Zolpidem 10 mg/d allowed her to sleep but she worried about taking it because her mother had long-standing sedative dependence. Ms. C lives alone, but her adult children live nearby, and she has a strong support system that includes colleagues at her firm, friends at her book club, and a support group for partners of cancer patients.
Ms. C tries trazodone, starting with 50 mg, but reports feeling agitated rather than sleepy and has cognitive fogginess in the morning. She is switched to quetiapine 50 mg, which she tolerates well and allows her to sleep soundly. To avoid developing tachyphylaxis with quetiapine, she takes eszopiclone 3 mg for 2 nights, alternating with quetiapine for 3 nights. This strategy allows her to reliably fall asleep by 11
CASE 3
Ms. D, age 55, is married with a long-standing diagnosis of generalized anxiety disorder (GAD), panic disorder, and depression so severe she is unable to work as a preschool teacher. She notes that past clinicians have prescribed a wide array of antidepressants and benzodiazepines but she remains anxious, agitated, and unable to sleep. She worries constantly about running out of benzodiazepines, which are “the only medication that helps me.” At the time of evaluation, her medications are venlafaxine ER 150 mg/d, lorazepam 1 mg 3 times daily and 2 mg at bedtime, and buspirone 15 mg 3 times daily, which she admits to not taking. She is overweight and does not exercise. She spends her days snacking and watching television. She can’t settle down enough to read and feels overwhelmed most of the time. Her adult children won’t allow her to babysit their young children because she dozes during the day.
Ms. D has a strong family history of psychiatric illness, including a father with bipolar I disorder and alcohol use disorder and a sister with schizoaffective disorder. Ms. D has never felt overtly manic, but has spent most of her life feeling depressed, anxious, and hopeless, and at times she has wished she was dead. She has had poor responses to many antidepressants, with transient euphoria followed by more anxiety.
Continue to: Rather than major depressive disorder...
Rather than major depressive disorder or GAD, Ms. D’s symptoms better meet the criteria for bipolar II disorder. She agrees to a slow taper of venlafaxine and a slow increase of divalproex, starting with 125 mg each evening. While taking venlafaxine 75 mg/d and divalproex 375 mg/d, she experiences distinct improvement in anxiety and agitation, which further improve after venlafaxine is stopped and divalproex is increased to 750 mg in the evening. She finds that she forgets daytime doses of lorazepam but depends on it to fall asleep. While taking quetiapine 50 mg and lorazepam 1 mg at bedtime, Ms. D reports sleeping soundly and feeling alert in the morning. Over several weeks, she tapers lorazepam slowly by 0.5 mg every 2 weeks. She finds she needs a higher dose of quetiapine to stay asleep, eventually requiring 400 mg each night. Ms. D says overall she feels better but is distressed because she has gained 25 lbs since starting divalproex and quetiapine.
To avoid further increases in quetiapine and maintain its sedating effect, Ms. D is switched to an alternating schedule of clonazepam 1.5 mg for 2 nights and quetiapine 300 mg for 3 nights. She agrees to begin exercising by walking in her neighborhood daily, and gradually increases this to 1 hour per day. After starting to exercise regularly, she finds she is oversedated by quetiapine at night, so she is gradually decreased to a dose of 150 mg, while still alternating with clonazepam 1.5 mg. Ms. D loses most of the weight she had gained and begins volunteering as a reading coach in the elementary school in her neighborhood.
Bottom Line
Patients with chronic insomnia can often maintain adequate sedation without developing tolerance to benzodiazepines or tachyphylaxis with nonsedating agents by using 2 sleep medications that have different mechanisms of action on an alternating schedule.
Related Resources
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2): 307-349. doi:10.5664/jcsm.6470
- Muppavarapu K, Muthukanagaraj M, Saeed SA. Cognitive-behavioral therapy for insomnia: a review of 8 studies. Current Psychiatry. 2020;19(9):40-46. doi:10.12788/cp.0040
Drug Brand Names
Alprazolam • Xanax
Armodafinil • Nuvigil
Atomoxetine • Strattera
Bupropion • Wellbutrin
Clonazepam • Klonopin
Diazepam • Valium
Divalproex • Depakote
Doxepin • Sinequan
Escitalopram • Lexapro
Eszopiclone • Lunesta
Lorazepam • Ativan
Lurasidone • Latuda
Methylphenidate • Concerta
Mirtazapine • Remeron
Modafinil • Provigil
Olanzapine • Zyprexa
Quetiapine • Seroquel
Temazepam • Restoril
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Zaleplon • Sonata
Zolpidem • Ambien
1. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
2. Drake C, Roehrs T, Shambroom J, et al. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J Clin Sleep Med. 2013;9(11):1195-1200.
3. Pollan M. Caffeine: How Coffee and Tea Created the Modern World. 2023; Audible Audiobooks.
4. Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021:17:2549-2566.
5. Vitale JA, Roveda E, Montaruli A, et al. Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32(3):405-415.
6. Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9(3):509-517.
7. Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19(4):23.
8. Monti JM, Pandi-Perumal SR. Clinical management of sleep and sleep disorders with cannabis and cannabinoids: implications to practicing psychiatrists. Clin Neuropharmacol. 2022;45(2):27-31.
9. Dockray S, Steptoe A. Chronotype and diurnal cortisol profile in working women: differences between work and leisure days. Psychoneuroendocrinology. 2011;36(5):649-655.
10. Parry BL, Newton RP. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology. 2001;25(5 Suppl):S102-S108.
11. Rosenberg RP, Krystal AD. Diagnosing and treating insomnia in adults and older adults. J Clin Psychiatry. 2021;82(6):59-66.
12. Stunkard A. Eating disorders and obesity. Psychiatr Clin North Am. 2011; 34(4):765-771.
13. Crönlein T. Insomnia and obesity. Curr Opin Psychiatry. 2016;29(6):409-412.
14. Gillberg C, Gillberg IC, Rasmussen P, et al. Co-existing disorders in ADHD -- implications for diagnosis and intervention. Eur Child Adolesc Psychiatry. 2004; 1(Suppl 1):i80-i92.
15. Goldberg JF, Nierenberg AA, Iosifescu DV. Wrestling with antidepressant use in bipolar disorder: the ongoing debate. J Clin Psychiatry. 2021;82(1):19. doi:10.4088/JCP.19ac13181
16. Baltzan M, Yao C, Rizzo D, et al. Dream enactment behavior: review for the clinician. J Clin Sleep Med. 2020;16(11):1949-1969.
17. Barone DA. Dream enactment behavior—a real nightmare: a review of post-traumatic stress disorder, REM sleep behavior disorder, and trauma-associated sleep disorder. J Clin Sleep Med. 2020;16(11):1943-1948.
18. Figorilli M, Meloni M, Lanza G, et al. Considering REM sleep behavior disorder in the management of Parkinson’s disease. Nat Sci Sleep. 2023;15:333-352.
19. Rios P, Cardoso R, Morra D, et al. Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: an overview of reviews. Syst Rev. 2019;8(1):281-297.
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139.
21. Lu M, Zhang Y, Zhang J, et al. Comparative effectiveness of digital cognitive behavioral therapy vs. medication therapy among patients with insomnia. JAMA Network Open. 2023;6(4):e237597.
22. Sweetman A, McEvoy RD, Catcheside PG, et al. Effect of depression, anxiety, and stress symptoms on response to cognitive behavioral therapy for insomnia in patients with comorbid insomnia and sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2021;17(3):545-554.
23. O’Brien CP. Benzodiazepine use, abuse and dependence. J Clin Psychiatry. 2005;66(Suppl 2):28-33.
24. Wichniak A, Wierzbicka AE, Jarema M. Treatment of insomnia - effect of trazodone and hypnotics on sleep. Psychiatr Pol. 2021;55(4):743-755.
25. Papazisis G, Siafis S, Tzachanis D. Tachyphylaxis to the sedative action of mirtazapine. Am J Case Rep. 2018;19:410-412.
Patients with chronic insomnia that does not improve with nonpharmacologic techniques often develop tolerance to sedative medications (benzodiazepines) prescribed for nightly use. When nonbenzodiazepine medications are used, tachyphylaxis can develop and these medications no longer initiate or maintain sleep. Strategies that alternate between these 2 types of agents are simple to follow and may allow patients to maintain sensitivity to both types of medications. In this article, I review the types, causes, evaluation, and treatment of insomnia; describe an alternating medication strategy to help patients avoid developing tolerance/tachyphylaxis; and present 3 fictional case vignettes to illustrate this approach.
A common, troubling condition
Insomnia is a common problem among psychiatric patients. Approximately 30% to 50% of adults experience occasional, short-term (<3 months) insomnia, and 5% to 10% experience chronic (≥3 months) insomnia,1 with associated negative impacts on health and quality of life. Insomnia is sometimes primary and may have a hereditary component, but more often is associated with medical, neurologic, or psychiatric disorders.
Patterns of insomnia include difficulty falling asleep (initial or sleep-onset insomnia), remaining asleep (middle or sleep-maintenance insomnia), or falling back asleep after early awakening (late or sleep-offset insomnia). Sleep-onset insomnia correlates with high levels of anxiety and worrying, but once asleep, patients usually stay asleep. Sleep-maintenance problems involve multiple awakenings after falling asleep and taking hours to fall back to sleep. These patients experience inadequate sleep when they must wake up early for school or work. Early-awakening patients report feeling wide awake by 4 to 5
Caffeine is an important consideration for patients with sleep difficulties. Its use is widespread in much of the world, whether ingested as coffee, tea, in soft drinks, or in “energy” drinks that may contain as much as 200 mg of caffeine (twice the amount in a typical cup of brewed coffee). Caffeine may also be ingested as an ingredient of medications for headache or migraine. While some individuals maintain that they can fall asleep easily after drinking caffeinated coffee, many may not recognize the amount of caffeine they consume and its negative impact on sleep.2 Author Michael Pollan stopped use of all caffeine and reported on the surprising positive effect on his sleep.3
Patients with mood, anxiety, or psychotic disorders are likely to experience insomnia intermittently or chronically, and insomnia predisposes some individuals to develop mood and anxiety symptoms.4 Patients with insomnia often experience anxiety focused on a fear of not getting adequate sleep, which creates a vicious cycle in which hyperarousal associated with fear of not sleeping complicates other causes of insomnia. A patient’s chronotype (preference for the time of day in which they carry out activities vs sleeping) also may play a role in sleep difficulties (Box5).
Box
Chronotypes—the expression of circadian rhythmicity in an individual—have been studied extensively.5 Psychiatrists may encounter patients who sleep most of the day and stay awake at night, those who sleep up to 20 hours per day, and those who sleep <4 hours in 24 hours. Patients typically know which category they fall into. The early bird typically is awake by 6 or 7 am, remains alert through most of the day, and feels sleepy by 10 pm. The usual diurnal variation in cortisol, with peaks at 7 am and 7 pm and nadirs at 1 pm and 1 am, correspond with the early bird’s habits.
Night owls typically report feeling exhausted and irritable in the early morning; prefer to sleep past noon; feel energized around dark, when they can do their best studying, concentrating, etc; and do not feel sleepy until early morning. While this night owl pattern is a natural variation and not necessarily associated with psychiatric illness, patients with mood disorders frequently have chaotic sleep patterns that may not conform to a pattern. Night owls maintain the same diurnal pattern of cortisol secretion as early birds.
Certain medications may contribute to insomnia, particularly stimulants. It is important to understand and explain to patients the time frame during which immediate-release or extended-release (ER) stimulants are active, which varies in individuals depending on liver enzyme activity. Other commonly used psychotropic medications—including bupropion, modafinil, armodafinil, atomoxetine, amphetamine salts, and methylphenidate—may interfere with sleep if used later in the day.6
Patients typically do not mention their use of alcohol and/or marijuana unless asked. Those who are binge drinkers or alcohol-dependent may expect alcohol to help them fall asleep, but usually find their sleep is disrupted and difficult to maintain. Patients may use marijuana to help them sleep, particularly marijuana high in tetrahydrocannabinol (THC). While it may help with sleep initiation, THC can disrupt sleep maintenance. Cannabidiol does not have intrinsic sedating effects and may even interfere with sleep.7,8
Continue to: Women may be more likely...
Women may be more likely than men to experience insomnia.9 The onset of menopause can bring hot flashes that interfere with sleep.
Women with a history of mood disorders are more likely to have a history of premenstrual dysphoric disorder, postpartum depression, and unusual responses to oral contraceptives.10 These women are more likely to report problems with mood, energy, and sleep at perimenopause. Treatment with estrogen replacement may be an option for women without risk factors, such as clotting disorders, smoking history, or a personal or family history of breast or uterine cancer. For many who are not candidates for or who refuse estrogen replacement, use of a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor at low doses may help with vasomotor symptoms but not with insomnia.
Insomnia symptoms typically increase with age.11 When sleep is adequate early in life but becomes a problem in midlife, an individual’s eating habits, obesity, and lack of exercise may be contributing factors. The typical American diet includes highly refined carbohydrates with excess salt; such foods are often readily available to the exclusion of healthy options. Overweight and obese patients may insist they eat a healthy diet with 3 meals per day, but a careful history often uncovers nighttime binge eating. Nighttime binge eating is rarely reported. This not only maintains obesity, but also interferes with sleep, since patients stay up late to avoid discovery by family members.12 This lack of sleep can lead to an endless loop because insufficient sleep is a risk factor for obesity.13
Evaluating sleep difficulties
New patient evaluations should include a careful history beginning with childhood, including personal early childhood history and family psychiatric history. Patients often report the onset of sleep difficulty and anxiety during childhood, which should raise further questions about aspects of mood regulation from early life such as concentration, energy, motivation, appetite, and academic performance. While many children and adolescents are diagnosed with attention-deficit/hyperactivity disorder due to concentration problems that cause difficulties at school, be aware this might be part of a syndrome related to mood regulation.14 Unexpected responses to an SSRI—such as agitation, euphoria, or an immediate response with the first dose—should also raise suspicion of a mood disorder. Once the underlying mood disorder is stabilized, many patients report improved sleep.15
If a patient reports having difficulty falling and remaining asleep but is not sure if there is a pattern, keeping a sleep diary can help. Further questioning may uncover the cause. Does the patient have spontaneous jerks of lower extremities (restless leg syndrome) that interfere with falling asleep or wake them up? Have they noticed problems with dreams/nightmares that wake them, which could be associated with posttraumatic stress, anxiety, or depression? Have they been told by a partner that they act out dreams or are seemingly awake but not responsive, which could point to REM sleep behavior disorder or early Parkinson’s disease? Referral to a sleep laboratory and a neurologist can help establish the correct diagnosis and point to appropriate treatment.16-18
Treatment options
Several cognitive-behavioral techniques, including cognitive-behavioral therapy for insomnia (CBT-I), yogic breathing, progressive relaxation, mindfulness meditation, and sleep hygiene techniques may help considerably,19,20 but insomnia often remains difficult to treat. Pharmacotherapy is not necessarily more effective than nonpharmacologic approaches. Both options require the patient to take initiative to either find nonpharmacologic approaches or discuss the problem with a physician and agree to take medication.21 A trial comparing CBT-I to sedatives or the combination of CBT-I plus sedatives found higher rates of sleep with CBT-I for 3 months, after which improvement fluctuated; the combination showed sustained improvement for the entire 6-month trial.22 CBT-I has also been shown to be as effective with patients who do not have psychiatric illness as for those who are depressed, anxious, or stressed.23 However, behavioral techniques that require regular practice may be difficult for individuals to maintain, particularly when they are depressed or anxious.
Continue to: Clinicians should understand...
Clinicians should understand the distinctions among the various types of pharmacotherapy for insomnia. Sedative-hypnotics include medications with varying half-lives and metabolic pathways. Short-acting benzodiazepines such as triazolam or alprazolam and the “z-drugs” zolpidem or zaleplon may help initiate sleep in patients with sleep-onset insomnia. Longer-acting benzodiazepines such as diazepam, clonazepam, or temazepam and the z-drug eszopiclone may also help with sleep maintenance.23 Based on my clinical experience, individual patients may respond better to 1 type of medication over another, or even to different agents within the same class of sedative-hypnotics.
Some clinicians prescribe nonbenzodiazepine medications for sleep, such as doxepin (which is FDA-approved for treating insomnia) or off-label trazodone, mirtazapine, or quetiapine. Their antihistaminic properties confer sedating effects. Virtually all over-the-counter (OTC) medications for insomnia are antihistaminic. These OTC medications are not designed to treat insomnia, and the optimal dosage to maintain sleep without daytime sedation must be determined by trial and error. Sedating nonbenzodiazepine medications may be slowly absorbed if taken at bedtime (depending on whether they are taken with or without food) and cause daytime sedation and cognitive slowness in patients with sleep-onset and maintenance insomnia who must wake up early. Starting trazodone at 50 to 75 mg may cause slow metabolizers to wake up with considerable sedation, while fast metabolizers might never feel soundly asleep.24
Patients with mood and anxiety disorders that complicate insomnia are often prescribed second-generation antipsychotics such as quetiapine, lurasidone, or olanzapine, which are sedating as well as mood-stabilizing. These approaches require careful attention to titrating doses and timing their use.
Problems with pharmacotherapy
When either benzodiazepines or nonbenzodiazepine medications are used on a long-standing, nightly basis, they often stop working well. It is not unusual that after days to weeks of taking a benzodiazepine, patients find they no longer stay asleep but can’t fall asleep if they don’t take them. Once tolerance develops, the individual experiences pharmacologic withdrawal with an inability to fall asleep or stay asleep. The medication becomes necessary but ineffective, and many patients increase their use to higher doses to fall asleep, and sometimes in early morning to maintain sleep. This leads to negative effects on cognition, coordination/balance, and mood during the day, especially in older patients.
Nonbenzodiazepine sedating medications do not lead to pharmacologic tolerance but do lead to tachyphylaxis as the CNS attempts to downregulate sedation to keep the organism safe. For some patients, this happens quickly, within a matter of days.25 Others increase doses to stay asleep. For example, a patient with a starting dose of trazodone 75 mg/d might increase the dosage to 300 mg/d. While trazodone is approved in doses of 300 to 600 mg as an antidepressant, it is preferable to keep doses lower when used only for sedation.
Continue to: An alternating medication strategy
An alternating medication strategy
Alternating between medications from different classes can help patients avoid developing tolerance with benzodiazepines or tachyphylaxis as occurs with antihistaminic medications. It can be effective for patients with primary insomnia as well as for those whose sleep problems are associated with mood or anxiety disorders. Patients typically maintain sensitivity to any form of pharmacologic sedation for several nights without loss of effect but need to take a break to maintain the sedation effect. For example, in 1 case study, a 30-year-old female who rapidly developed tachyphylaxis to the sedative action of mirtazapine experienced a return of the medication’s sedative effects after taking a 3-day break.25
To initiate an alternating strategy, the clinician must first help the patient establish a sedating dose of 2 medications from different classes, such as trazodone and zolpidem, and then instruct the patient to use each for 2 to 3 consecutive nights on an alternating basis. Patients can use calendars or pillboxes to avoid confusion about which medication to take on a given night. In many cases, this approach can work indefinitely.
The following 3 case vignettes illustrate how this alternating medication strategy can work.
CASE 1
Mr. B, age 58, is a married salesman whose territory includes 3 states. He drives from client to client from Monday through Thursday each week, staying overnight in hotels. He is comfortable talking to clients, has a close and supportive relationship with his wife, and enjoys socializing with friends. Mr. B has a high level of trait anxiety and perfectionism and is proud of his sales record throughout his career, but this leads to insomnia during his nights on the road, and often on Sunday night as he starts anticipating the week ahead. Mr. B denies having a depressed mood or cognitive problems. When on vacation with his wife he has no trouble sleeping. He has no psychiatric family history or any substantial medical problems. He simply wishes that he could sleep on work nights.
We set up an alternating medication approach. Mr. B takes trazodone 100 mg on the first night and 150 mg on the second and third nights. He then takes triazolam 0.25 mg for 2 nights; previously, he had found that zolpidem did not work as well for maintaining sleep. He can sleep adequately for the 2 weekend nights, then restarts the alternating pattern. Mr. B has done well with this regimen for >10 years.
Continue to: CASE 2
CASE 2
Ms. C, age 60, is widowed and has a successful career as a corporate attorney. She has been anxious since early childhood and has had trouble falling asleep for much of her life. Once she falls asleep on her sofa—often between 1 and 2
Ms. C denies having depression, but experienced appropriate grief related to her husband’s illness and death from metastatic cancer 3 years ago. At the time, her internist prescribed escitalopram and zolpidem; escitalopram caused greater agitation and distress, so she stopped it after 10 days. Zolpidem 10 mg/d allowed her to sleep but she worried about taking it because her mother had long-standing sedative dependence. Ms. C lives alone, but her adult children live nearby, and she has a strong support system that includes colleagues at her firm, friends at her book club, and a support group for partners of cancer patients.
Ms. C tries trazodone, starting with 50 mg, but reports feeling agitated rather than sleepy and has cognitive fogginess in the morning. She is switched to quetiapine 50 mg, which she tolerates well and allows her to sleep soundly. To avoid developing tachyphylaxis with quetiapine, she takes eszopiclone 3 mg for 2 nights, alternating with quetiapine for 3 nights. This strategy allows her to reliably fall asleep by 11
CASE 3
Ms. D, age 55, is married with a long-standing diagnosis of generalized anxiety disorder (GAD), panic disorder, and depression so severe she is unable to work as a preschool teacher. She notes that past clinicians have prescribed a wide array of antidepressants and benzodiazepines but she remains anxious, agitated, and unable to sleep. She worries constantly about running out of benzodiazepines, which are “the only medication that helps me.” At the time of evaluation, her medications are venlafaxine ER 150 mg/d, lorazepam 1 mg 3 times daily and 2 mg at bedtime, and buspirone 15 mg 3 times daily, which she admits to not taking. She is overweight and does not exercise. She spends her days snacking and watching television. She can’t settle down enough to read and feels overwhelmed most of the time. Her adult children won’t allow her to babysit their young children because she dozes during the day.
Ms. D has a strong family history of psychiatric illness, including a father with bipolar I disorder and alcohol use disorder and a sister with schizoaffective disorder. Ms. D has never felt overtly manic, but has spent most of her life feeling depressed, anxious, and hopeless, and at times she has wished she was dead. She has had poor responses to many antidepressants, with transient euphoria followed by more anxiety.
Continue to: Rather than major depressive disorder...
Rather than major depressive disorder or GAD, Ms. D’s symptoms better meet the criteria for bipolar II disorder. She agrees to a slow taper of venlafaxine and a slow increase of divalproex, starting with 125 mg each evening. While taking venlafaxine 75 mg/d and divalproex 375 mg/d, she experiences distinct improvement in anxiety and agitation, which further improve after venlafaxine is stopped and divalproex is increased to 750 mg in the evening. She finds that she forgets daytime doses of lorazepam but depends on it to fall asleep. While taking quetiapine 50 mg and lorazepam 1 mg at bedtime, Ms. D reports sleeping soundly and feeling alert in the morning. Over several weeks, she tapers lorazepam slowly by 0.5 mg every 2 weeks. She finds she needs a higher dose of quetiapine to stay asleep, eventually requiring 400 mg each night. Ms. D says overall she feels better but is distressed because she has gained 25 lbs since starting divalproex and quetiapine.
To avoid further increases in quetiapine and maintain its sedating effect, Ms. D is switched to an alternating schedule of clonazepam 1.5 mg for 2 nights and quetiapine 300 mg for 3 nights. She agrees to begin exercising by walking in her neighborhood daily, and gradually increases this to 1 hour per day. After starting to exercise regularly, she finds she is oversedated by quetiapine at night, so she is gradually decreased to a dose of 150 mg, while still alternating with clonazepam 1.5 mg. Ms. D loses most of the weight she had gained and begins volunteering as a reading coach in the elementary school in her neighborhood.
Bottom Line
Patients with chronic insomnia can often maintain adequate sedation without developing tolerance to benzodiazepines or tachyphylaxis with nonsedating agents by using 2 sleep medications that have different mechanisms of action on an alternating schedule.
Related Resources
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2): 307-349. doi:10.5664/jcsm.6470
- Muppavarapu K, Muthukanagaraj M, Saeed SA. Cognitive-behavioral therapy for insomnia: a review of 8 studies. Current Psychiatry. 2020;19(9):40-46. doi:10.12788/cp.0040
Drug Brand Names
Alprazolam • Xanax
Armodafinil • Nuvigil
Atomoxetine • Strattera
Bupropion • Wellbutrin
Clonazepam • Klonopin
Diazepam • Valium
Divalproex • Depakote
Doxepin • Sinequan
Escitalopram • Lexapro
Eszopiclone • Lunesta
Lorazepam • Ativan
Lurasidone • Latuda
Methylphenidate • Concerta
Mirtazapine • Remeron
Modafinil • Provigil
Olanzapine • Zyprexa
Quetiapine • Seroquel
Temazepam • Restoril
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Zaleplon • Sonata
Zolpidem • Ambien
Patients with chronic insomnia that does not improve with nonpharmacologic techniques often develop tolerance to sedative medications (benzodiazepines) prescribed for nightly use. When nonbenzodiazepine medications are used, tachyphylaxis can develop and these medications no longer initiate or maintain sleep. Strategies that alternate between these 2 types of agents are simple to follow and may allow patients to maintain sensitivity to both types of medications. In this article, I review the types, causes, evaluation, and treatment of insomnia; describe an alternating medication strategy to help patients avoid developing tolerance/tachyphylaxis; and present 3 fictional case vignettes to illustrate this approach.
A common, troubling condition
Insomnia is a common problem among psychiatric patients. Approximately 30% to 50% of adults experience occasional, short-term (<3 months) insomnia, and 5% to 10% experience chronic (≥3 months) insomnia,1 with associated negative impacts on health and quality of life. Insomnia is sometimes primary and may have a hereditary component, but more often is associated with medical, neurologic, or psychiatric disorders.
Patterns of insomnia include difficulty falling asleep (initial or sleep-onset insomnia), remaining asleep (middle or sleep-maintenance insomnia), or falling back asleep after early awakening (late or sleep-offset insomnia). Sleep-onset insomnia correlates with high levels of anxiety and worrying, but once asleep, patients usually stay asleep. Sleep-maintenance problems involve multiple awakenings after falling asleep and taking hours to fall back to sleep. These patients experience inadequate sleep when they must wake up early for school or work. Early-awakening patients report feeling wide awake by 4 to 5
Caffeine is an important consideration for patients with sleep difficulties. Its use is widespread in much of the world, whether ingested as coffee, tea, in soft drinks, or in “energy” drinks that may contain as much as 200 mg of caffeine (twice the amount in a typical cup of brewed coffee). Caffeine may also be ingested as an ingredient of medications for headache or migraine. While some individuals maintain that they can fall asleep easily after drinking caffeinated coffee, many may not recognize the amount of caffeine they consume and its negative impact on sleep.2 Author Michael Pollan stopped use of all caffeine and reported on the surprising positive effect on his sleep.3
Patients with mood, anxiety, or psychotic disorders are likely to experience insomnia intermittently or chronically, and insomnia predisposes some individuals to develop mood and anxiety symptoms.4 Patients with insomnia often experience anxiety focused on a fear of not getting adequate sleep, which creates a vicious cycle in which hyperarousal associated with fear of not sleeping complicates other causes of insomnia. A patient’s chronotype (preference for the time of day in which they carry out activities vs sleeping) also may play a role in sleep difficulties (Box5).
Box
Chronotypes—the expression of circadian rhythmicity in an individual—have been studied extensively.5 Psychiatrists may encounter patients who sleep most of the day and stay awake at night, those who sleep up to 20 hours per day, and those who sleep <4 hours in 24 hours. Patients typically know which category they fall into. The early bird typically is awake by 6 or 7 am, remains alert through most of the day, and feels sleepy by 10 pm. The usual diurnal variation in cortisol, with peaks at 7 am and 7 pm and nadirs at 1 pm and 1 am, correspond with the early bird’s habits.
Night owls typically report feeling exhausted and irritable in the early morning; prefer to sleep past noon; feel energized around dark, when they can do their best studying, concentrating, etc; and do not feel sleepy until early morning. While this night owl pattern is a natural variation and not necessarily associated with psychiatric illness, patients with mood disorders frequently have chaotic sleep patterns that may not conform to a pattern. Night owls maintain the same diurnal pattern of cortisol secretion as early birds.
Certain medications may contribute to insomnia, particularly stimulants. It is important to understand and explain to patients the time frame during which immediate-release or extended-release (ER) stimulants are active, which varies in individuals depending on liver enzyme activity. Other commonly used psychotropic medications—including bupropion, modafinil, armodafinil, atomoxetine, amphetamine salts, and methylphenidate—may interfere with sleep if used later in the day.6
Patients typically do not mention their use of alcohol and/or marijuana unless asked. Those who are binge drinkers or alcohol-dependent may expect alcohol to help them fall asleep, but usually find their sleep is disrupted and difficult to maintain. Patients may use marijuana to help them sleep, particularly marijuana high in tetrahydrocannabinol (THC). While it may help with sleep initiation, THC can disrupt sleep maintenance. Cannabidiol does not have intrinsic sedating effects and may even interfere with sleep.7,8
Continue to: Women may be more likely...
Women may be more likely than men to experience insomnia.9 The onset of menopause can bring hot flashes that interfere with sleep.
Women with a history of mood disorders are more likely to have a history of premenstrual dysphoric disorder, postpartum depression, and unusual responses to oral contraceptives.10 These women are more likely to report problems with mood, energy, and sleep at perimenopause. Treatment with estrogen replacement may be an option for women without risk factors, such as clotting disorders, smoking history, or a personal or family history of breast or uterine cancer. For many who are not candidates for or who refuse estrogen replacement, use of a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor at low doses may help with vasomotor symptoms but not with insomnia.
Insomnia symptoms typically increase with age.11 When sleep is adequate early in life but becomes a problem in midlife, an individual’s eating habits, obesity, and lack of exercise may be contributing factors. The typical American diet includes highly refined carbohydrates with excess salt; such foods are often readily available to the exclusion of healthy options. Overweight and obese patients may insist they eat a healthy diet with 3 meals per day, but a careful history often uncovers nighttime binge eating. Nighttime binge eating is rarely reported. This not only maintains obesity, but also interferes with sleep, since patients stay up late to avoid discovery by family members.12 This lack of sleep can lead to an endless loop because insufficient sleep is a risk factor for obesity.13
Evaluating sleep difficulties
New patient evaluations should include a careful history beginning with childhood, including personal early childhood history and family psychiatric history. Patients often report the onset of sleep difficulty and anxiety during childhood, which should raise further questions about aspects of mood regulation from early life such as concentration, energy, motivation, appetite, and academic performance. While many children and adolescents are diagnosed with attention-deficit/hyperactivity disorder due to concentration problems that cause difficulties at school, be aware this might be part of a syndrome related to mood regulation.14 Unexpected responses to an SSRI—such as agitation, euphoria, or an immediate response with the first dose—should also raise suspicion of a mood disorder. Once the underlying mood disorder is stabilized, many patients report improved sleep.15
If a patient reports having difficulty falling and remaining asleep but is not sure if there is a pattern, keeping a sleep diary can help. Further questioning may uncover the cause. Does the patient have spontaneous jerks of lower extremities (restless leg syndrome) that interfere with falling asleep or wake them up? Have they noticed problems with dreams/nightmares that wake them, which could be associated with posttraumatic stress, anxiety, or depression? Have they been told by a partner that they act out dreams or are seemingly awake but not responsive, which could point to REM sleep behavior disorder or early Parkinson’s disease? Referral to a sleep laboratory and a neurologist can help establish the correct diagnosis and point to appropriate treatment.16-18
Treatment options
Several cognitive-behavioral techniques, including cognitive-behavioral therapy for insomnia (CBT-I), yogic breathing, progressive relaxation, mindfulness meditation, and sleep hygiene techniques may help considerably,19,20 but insomnia often remains difficult to treat. Pharmacotherapy is not necessarily more effective than nonpharmacologic approaches. Both options require the patient to take initiative to either find nonpharmacologic approaches or discuss the problem with a physician and agree to take medication.21 A trial comparing CBT-I to sedatives or the combination of CBT-I plus sedatives found higher rates of sleep with CBT-I for 3 months, after which improvement fluctuated; the combination showed sustained improvement for the entire 6-month trial.22 CBT-I has also been shown to be as effective with patients who do not have psychiatric illness as for those who are depressed, anxious, or stressed.23 However, behavioral techniques that require regular practice may be difficult for individuals to maintain, particularly when they are depressed or anxious.
Continue to: Clinicians should understand...
Clinicians should understand the distinctions among the various types of pharmacotherapy for insomnia. Sedative-hypnotics include medications with varying half-lives and metabolic pathways. Short-acting benzodiazepines such as triazolam or alprazolam and the “z-drugs” zolpidem or zaleplon may help initiate sleep in patients with sleep-onset insomnia. Longer-acting benzodiazepines such as diazepam, clonazepam, or temazepam and the z-drug eszopiclone may also help with sleep maintenance.23 Based on my clinical experience, individual patients may respond better to 1 type of medication over another, or even to different agents within the same class of sedative-hypnotics.
Some clinicians prescribe nonbenzodiazepine medications for sleep, such as doxepin (which is FDA-approved for treating insomnia) or off-label trazodone, mirtazapine, or quetiapine. Their antihistaminic properties confer sedating effects. Virtually all over-the-counter (OTC) medications for insomnia are antihistaminic. These OTC medications are not designed to treat insomnia, and the optimal dosage to maintain sleep without daytime sedation must be determined by trial and error. Sedating nonbenzodiazepine medications may be slowly absorbed if taken at bedtime (depending on whether they are taken with or without food) and cause daytime sedation and cognitive slowness in patients with sleep-onset and maintenance insomnia who must wake up early. Starting trazodone at 50 to 75 mg may cause slow metabolizers to wake up with considerable sedation, while fast metabolizers might never feel soundly asleep.24
Patients with mood and anxiety disorders that complicate insomnia are often prescribed second-generation antipsychotics such as quetiapine, lurasidone, or olanzapine, which are sedating as well as mood-stabilizing. These approaches require careful attention to titrating doses and timing their use.
Problems with pharmacotherapy
When either benzodiazepines or nonbenzodiazepine medications are used on a long-standing, nightly basis, they often stop working well. It is not unusual that after days to weeks of taking a benzodiazepine, patients find they no longer stay asleep but can’t fall asleep if they don’t take them. Once tolerance develops, the individual experiences pharmacologic withdrawal with an inability to fall asleep or stay asleep. The medication becomes necessary but ineffective, and many patients increase their use to higher doses to fall asleep, and sometimes in early morning to maintain sleep. This leads to negative effects on cognition, coordination/balance, and mood during the day, especially in older patients.
Nonbenzodiazepine sedating medications do not lead to pharmacologic tolerance but do lead to tachyphylaxis as the CNS attempts to downregulate sedation to keep the organism safe. For some patients, this happens quickly, within a matter of days.25 Others increase doses to stay asleep. For example, a patient with a starting dose of trazodone 75 mg/d might increase the dosage to 300 mg/d. While trazodone is approved in doses of 300 to 600 mg as an antidepressant, it is preferable to keep doses lower when used only for sedation.
Continue to: An alternating medication strategy
An alternating medication strategy
Alternating between medications from different classes can help patients avoid developing tolerance with benzodiazepines or tachyphylaxis as occurs with antihistaminic medications. It can be effective for patients with primary insomnia as well as for those whose sleep problems are associated with mood or anxiety disorders. Patients typically maintain sensitivity to any form of pharmacologic sedation for several nights without loss of effect but need to take a break to maintain the sedation effect. For example, in 1 case study, a 30-year-old female who rapidly developed tachyphylaxis to the sedative action of mirtazapine experienced a return of the medication’s sedative effects after taking a 3-day break.25
To initiate an alternating strategy, the clinician must first help the patient establish a sedating dose of 2 medications from different classes, such as trazodone and zolpidem, and then instruct the patient to use each for 2 to 3 consecutive nights on an alternating basis. Patients can use calendars or pillboxes to avoid confusion about which medication to take on a given night. In many cases, this approach can work indefinitely.
The following 3 case vignettes illustrate how this alternating medication strategy can work.
CASE 1
Mr. B, age 58, is a married salesman whose territory includes 3 states. He drives from client to client from Monday through Thursday each week, staying overnight in hotels. He is comfortable talking to clients, has a close and supportive relationship with his wife, and enjoys socializing with friends. Mr. B has a high level of trait anxiety and perfectionism and is proud of his sales record throughout his career, but this leads to insomnia during his nights on the road, and often on Sunday night as he starts anticipating the week ahead. Mr. B denies having a depressed mood or cognitive problems. When on vacation with his wife he has no trouble sleeping. He has no psychiatric family history or any substantial medical problems. He simply wishes that he could sleep on work nights.
We set up an alternating medication approach. Mr. B takes trazodone 100 mg on the first night and 150 mg on the second and third nights. He then takes triazolam 0.25 mg for 2 nights; previously, he had found that zolpidem did not work as well for maintaining sleep. He can sleep adequately for the 2 weekend nights, then restarts the alternating pattern. Mr. B has done well with this regimen for >10 years.
Continue to: CASE 2
CASE 2
Ms. C, age 60, is widowed and has a successful career as a corporate attorney. She has been anxious since early childhood and has had trouble falling asleep for much of her life. Once she falls asleep on her sofa—often between 1 and 2
Ms. C denies having depression, but experienced appropriate grief related to her husband’s illness and death from metastatic cancer 3 years ago. At the time, her internist prescribed escitalopram and zolpidem; escitalopram caused greater agitation and distress, so she stopped it after 10 days. Zolpidem 10 mg/d allowed her to sleep but she worried about taking it because her mother had long-standing sedative dependence. Ms. C lives alone, but her adult children live nearby, and she has a strong support system that includes colleagues at her firm, friends at her book club, and a support group for partners of cancer patients.
Ms. C tries trazodone, starting with 50 mg, but reports feeling agitated rather than sleepy and has cognitive fogginess in the morning. She is switched to quetiapine 50 mg, which she tolerates well and allows her to sleep soundly. To avoid developing tachyphylaxis with quetiapine, she takes eszopiclone 3 mg for 2 nights, alternating with quetiapine for 3 nights. This strategy allows her to reliably fall asleep by 11
CASE 3
Ms. D, age 55, is married with a long-standing diagnosis of generalized anxiety disorder (GAD), panic disorder, and depression so severe she is unable to work as a preschool teacher. She notes that past clinicians have prescribed a wide array of antidepressants and benzodiazepines but she remains anxious, agitated, and unable to sleep. She worries constantly about running out of benzodiazepines, which are “the only medication that helps me.” At the time of evaluation, her medications are venlafaxine ER 150 mg/d, lorazepam 1 mg 3 times daily and 2 mg at bedtime, and buspirone 15 mg 3 times daily, which she admits to not taking. She is overweight and does not exercise. She spends her days snacking and watching television. She can’t settle down enough to read and feels overwhelmed most of the time. Her adult children won’t allow her to babysit their young children because she dozes during the day.
Ms. D has a strong family history of psychiatric illness, including a father with bipolar I disorder and alcohol use disorder and a sister with schizoaffective disorder. Ms. D has never felt overtly manic, but has spent most of her life feeling depressed, anxious, and hopeless, and at times she has wished she was dead. She has had poor responses to many antidepressants, with transient euphoria followed by more anxiety.
Continue to: Rather than major depressive disorder...
Rather than major depressive disorder or GAD, Ms. D’s symptoms better meet the criteria for bipolar II disorder. She agrees to a slow taper of venlafaxine and a slow increase of divalproex, starting with 125 mg each evening. While taking venlafaxine 75 mg/d and divalproex 375 mg/d, she experiences distinct improvement in anxiety and agitation, which further improve after venlafaxine is stopped and divalproex is increased to 750 mg in the evening. She finds that she forgets daytime doses of lorazepam but depends on it to fall asleep. While taking quetiapine 50 mg and lorazepam 1 mg at bedtime, Ms. D reports sleeping soundly and feeling alert in the morning. Over several weeks, she tapers lorazepam slowly by 0.5 mg every 2 weeks. She finds she needs a higher dose of quetiapine to stay asleep, eventually requiring 400 mg each night. Ms. D says overall she feels better but is distressed because she has gained 25 lbs since starting divalproex and quetiapine.
To avoid further increases in quetiapine and maintain its sedating effect, Ms. D is switched to an alternating schedule of clonazepam 1.5 mg for 2 nights and quetiapine 300 mg for 3 nights. She agrees to begin exercising by walking in her neighborhood daily, and gradually increases this to 1 hour per day. After starting to exercise regularly, she finds she is oversedated by quetiapine at night, so she is gradually decreased to a dose of 150 mg, while still alternating with clonazepam 1.5 mg. Ms. D loses most of the weight she had gained and begins volunteering as a reading coach in the elementary school in her neighborhood.
Bottom Line
Patients with chronic insomnia can often maintain adequate sedation without developing tolerance to benzodiazepines or tachyphylaxis with nonsedating agents by using 2 sleep medications that have different mechanisms of action on an alternating schedule.
Related Resources
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2): 307-349. doi:10.5664/jcsm.6470
- Muppavarapu K, Muthukanagaraj M, Saeed SA. Cognitive-behavioral therapy for insomnia: a review of 8 studies. Current Psychiatry. 2020;19(9):40-46. doi:10.12788/cp.0040
Drug Brand Names
Alprazolam • Xanax
Armodafinil • Nuvigil
Atomoxetine • Strattera
Bupropion • Wellbutrin
Clonazepam • Klonopin
Diazepam • Valium
Divalproex • Depakote
Doxepin • Sinequan
Escitalopram • Lexapro
Eszopiclone • Lunesta
Lorazepam • Ativan
Lurasidone • Latuda
Methylphenidate • Concerta
Mirtazapine • Remeron
Modafinil • Provigil
Olanzapine • Zyprexa
Quetiapine • Seroquel
Temazepam • Restoril
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Zaleplon • Sonata
Zolpidem • Ambien
1. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
2. Drake C, Roehrs T, Shambroom J, et al. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J Clin Sleep Med. 2013;9(11):1195-1200.
3. Pollan M. Caffeine: How Coffee and Tea Created the Modern World. 2023; Audible Audiobooks.
4. Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021:17:2549-2566.
5. Vitale JA, Roveda E, Montaruli A, et al. Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32(3):405-415.
6. Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9(3):509-517.
7. Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19(4):23.
8. Monti JM, Pandi-Perumal SR. Clinical management of sleep and sleep disorders with cannabis and cannabinoids: implications to practicing psychiatrists. Clin Neuropharmacol. 2022;45(2):27-31.
9. Dockray S, Steptoe A. Chronotype and diurnal cortisol profile in working women: differences between work and leisure days. Psychoneuroendocrinology. 2011;36(5):649-655.
10. Parry BL, Newton RP. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology. 2001;25(5 Suppl):S102-S108.
11. Rosenberg RP, Krystal AD. Diagnosing and treating insomnia in adults and older adults. J Clin Psychiatry. 2021;82(6):59-66.
12. Stunkard A. Eating disorders and obesity. Psychiatr Clin North Am. 2011; 34(4):765-771.
13. Crönlein T. Insomnia and obesity. Curr Opin Psychiatry. 2016;29(6):409-412.
14. Gillberg C, Gillberg IC, Rasmussen P, et al. Co-existing disorders in ADHD -- implications for diagnosis and intervention. Eur Child Adolesc Psychiatry. 2004; 1(Suppl 1):i80-i92.
15. Goldberg JF, Nierenberg AA, Iosifescu DV. Wrestling with antidepressant use in bipolar disorder: the ongoing debate. J Clin Psychiatry. 2021;82(1):19. doi:10.4088/JCP.19ac13181
16. Baltzan M, Yao C, Rizzo D, et al. Dream enactment behavior: review for the clinician. J Clin Sleep Med. 2020;16(11):1949-1969.
17. Barone DA. Dream enactment behavior—a real nightmare: a review of post-traumatic stress disorder, REM sleep behavior disorder, and trauma-associated sleep disorder. J Clin Sleep Med. 2020;16(11):1943-1948.
18. Figorilli M, Meloni M, Lanza G, et al. Considering REM sleep behavior disorder in the management of Parkinson’s disease. Nat Sci Sleep. 2023;15:333-352.
19. Rios P, Cardoso R, Morra D, et al. Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: an overview of reviews. Syst Rev. 2019;8(1):281-297.
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139.
21. Lu M, Zhang Y, Zhang J, et al. Comparative effectiveness of digital cognitive behavioral therapy vs. medication therapy among patients with insomnia. JAMA Network Open. 2023;6(4):e237597.
22. Sweetman A, McEvoy RD, Catcheside PG, et al. Effect of depression, anxiety, and stress symptoms on response to cognitive behavioral therapy for insomnia in patients with comorbid insomnia and sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2021;17(3):545-554.
23. O’Brien CP. Benzodiazepine use, abuse and dependence. J Clin Psychiatry. 2005;66(Suppl 2):28-33.
24. Wichniak A, Wierzbicka AE, Jarema M. Treatment of insomnia - effect of trazodone and hypnotics on sleep. Psychiatr Pol. 2021;55(4):743-755.
25. Papazisis G, Siafis S, Tzachanis D. Tachyphylaxis to the sedative action of mirtazapine. Am J Case Rep. 2018;19:410-412.
1. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
2. Drake C, Roehrs T, Shambroom J, et al. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J Clin Sleep Med. 2013;9(11):1195-1200.
3. Pollan M. Caffeine: How Coffee and Tea Created the Modern World. 2023; Audible Audiobooks.
4. Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021:17:2549-2566.
5. Vitale JA, Roveda E, Montaruli A, et al. Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32(3):405-415.
6. Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9(3):509-517.
7. Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19(4):23.
8. Monti JM, Pandi-Perumal SR. Clinical management of sleep and sleep disorders with cannabis and cannabinoids: implications to practicing psychiatrists. Clin Neuropharmacol. 2022;45(2):27-31.
9. Dockray S, Steptoe A. Chronotype and diurnal cortisol profile in working women: differences between work and leisure days. Psychoneuroendocrinology. 2011;36(5):649-655.
10. Parry BL, Newton RP. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology. 2001;25(5 Suppl):S102-S108.
11. Rosenberg RP, Krystal AD. Diagnosing and treating insomnia in adults and older adults. J Clin Psychiatry. 2021;82(6):59-66.
12. Stunkard A. Eating disorders and obesity. Psychiatr Clin North Am. 2011; 34(4):765-771.
13. Crönlein T. Insomnia and obesity. Curr Opin Psychiatry. 2016;29(6):409-412.
14. Gillberg C, Gillberg IC, Rasmussen P, et al. Co-existing disorders in ADHD -- implications for diagnosis and intervention. Eur Child Adolesc Psychiatry. 2004; 1(Suppl 1):i80-i92.
15. Goldberg JF, Nierenberg AA, Iosifescu DV. Wrestling with antidepressant use in bipolar disorder: the ongoing debate. J Clin Psychiatry. 2021;82(1):19. doi:10.4088/JCP.19ac13181
16. Baltzan M, Yao C, Rizzo D, et al. Dream enactment behavior: review for the clinician. J Clin Sleep Med. 2020;16(11):1949-1969.
17. Barone DA. Dream enactment behavior—a real nightmare: a review of post-traumatic stress disorder, REM sleep behavior disorder, and trauma-associated sleep disorder. J Clin Sleep Med. 2020;16(11):1943-1948.
18. Figorilli M, Meloni M, Lanza G, et al. Considering REM sleep behavior disorder in the management of Parkinson’s disease. Nat Sci Sleep. 2023;15:333-352.
19. Rios P, Cardoso R, Morra D, et al. Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: an overview of reviews. Syst Rev. 2019;8(1):281-297.
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139.
21. Lu M, Zhang Y, Zhang J, et al. Comparative effectiveness of digital cognitive behavioral therapy vs. medication therapy among patients with insomnia. JAMA Network Open. 2023;6(4):e237597.
22. Sweetman A, McEvoy RD, Catcheside PG, et al. Effect of depression, anxiety, and stress symptoms on response to cognitive behavioral therapy for insomnia in patients with comorbid insomnia and sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2021;17(3):545-554.
23. O’Brien CP. Benzodiazepine use, abuse and dependence. J Clin Psychiatry. 2005;66(Suppl 2):28-33.
24. Wichniak A, Wierzbicka AE, Jarema M. Treatment of insomnia - effect of trazodone and hypnotics on sleep. Psychiatr Pol. 2021;55(4):743-755.
25. Papazisis G, Siafis S, Tzachanis D. Tachyphylaxis to the sedative action of mirtazapine. Am J Case Rep. 2018;19:410-412.
Sleep apnea diagnosis: Awareness and tools
Obstructive sleep apnea (OSA) remains a significantly underdiagnosed condition, despite its high prevalence. Primary care physicians play a pivotal role in identifying patients afflicted by this condition. To effectively diagnose OSA in primary care, increasing awareness and enhancing communication are imperative. Fortunately, several straightforward diagnostic tools are readily available, and even more sophisticated ones, driven by artificial intelligence, are on the horizon.
Recognize the problem
At the annual congress of the European Respiratory Society, Cláudia Sofia De Almeida Vicente Ferreira, MD, a family physician from Coimbra, Portugal, and coordinator of the Respiratory Diseases Interest Group of the Portuguese Association of General and Family Medicine, highlighted the challenges of diagnosing OSA.
People do not come to the doctor and complain about it. Sometimes you catch it in the middle of other things,” she said in an interview.
Moreover, physicians’ busy schedules and limited appointment times often lead to a focus on the symptoms reported by patients, and insufficient attention is paid to the quality of sleep. This may be compounded by a tendency among medical professionals to underestimate the risks associated with OSA, as it is not directly linked to mortality, despite its clear connection to cardiovascular risks.
Identifying and recognizing risk factors can facilitate OSA suspicion during patient evaluations. These factors encompass both structural (for example, craniofacial and upper airway anomalies) and nonstructural elements (for example, smoking, alcohol use, or sedative consumption). While men are at higher risk, postmenopausal women who are not receiving hormone replacement therapy face similar risks. Certain medical conditions, such as hypothyroidism, acromegaly, amyloidosis, Cushing syndrome, and Down syndrome, have also been associated with OSA. A comprehensive physical examination can provide additional clues. Factors might include obesity, neck circumference, Mallampati score, and nasal and pharyngeal problems.
Inquire actively
Once the possibility of OSA is considered, the next step is to ask patients about their symptoms. Questionnaires are simple yet valuable tools for this purpose. The STOP questionnaire comprises four key questions:
- Do you SNORE loudly (louder than talking or loud enough to be heard through closed doors)?
- Do you often feel TIRED, fatigued, or sleepy during daytime?
- Has anyone OBSERVED you stop breathing during your sleep?
- Do you have or are you being treated for high blood PRESSURE?
The STOP-BANG questionnaire adds four clinical attributes: obesity (body mass index > 35 kg/m2), age (> 50 years), neck size (> 40 cm, or 16 inches), and sex.
Patients are classified as being at low, intermediate, or high risk for OSA.
The Epworth Sleepiness Scale, which is self-administered, is also useful: patients rate the likelihood of falling asleep in various daytime contexts. These questionnaires can be seamlessly integrated into routine patient appointments.
Comorbidities and occupation
Primary care physicians should carefully assess comorbidities, especially those linked to cardiovascular risk. Patients with resistant hypertension, pulmonary hypertension, and recurrent atrial fibrillation following cardioversion/ablation should be prioritized for diagnostic testing for OSA. Patients with other conditions, such as coronary artery disease or cerebrovascular disease, should also be referred to a sleep center if OSA is suspected on the basis of comprehensive sleep assessment. OSA has also been associated with type 2 diabetes, metabolic syndrome, and asthma.
Gaining access to sleep study services and subsequent therapy, such as continuous positive airway pressure (CPAP), can be challenging. Primary care physicians should prioritize patients on the basis of their risk levels. Occupation plays a significant role in this prioritization, as sleep fragmentation and daytime sleepiness can lead to workplace and vehicular accidents.
“You should include the occupation in the patient’s profile. What is he doing? Is he sitting at a desk, or is he working at height, driving, or operating machines? These workers are high-risk patients,” continued Dr. De Almeida Vicente Ferreira.
“I think that the family physician has a key role in the follow-up. Nobody else will look for CPAP compliance and will verify if CPAP is working or not. If the patient is not using it or if it is not effective, still there is someone paying for the machine (the national health care system or an insurance company). More importantly, if CPAP is not working, we are not improving our patient’s life in terms of reducing cardiovascular risk and ameliorating the quality of life.”
Is home testing a viable option?
Diagnosing OSA typically relies on overnight polysomnography in specialized sleep clinics, which is often associated with long waiting lists. Researchers are actively working on innovative sensors and digital solutions for home-based sleep testing, but according to Dr. De Almeida Vicente Ferreira, they are not yet ready for prime time: “Home-based studies with fewer evaluation parameters (such as pulse and oxygen levels) are not so secure or sensitive to establish a correct and complete diagnosis. Actually, the architecture of sleep is very complex. The test must be performed and read by a specialized team.”
Still, according to Renaud Tamisier, MD, PhD, professor of clinical physiology at the Université Grenoble Alpes in La Tronche, France, simplified sleep testing could be very useful. “There are many patients that still are not diagnosed despite having severe sleep apnea, with symptoms and comorbidities. These patients usually are not aware of their disease but complain about changes in their quality of life with excessive tiredness and sleepiness. Also, they are not connected to the healthcare system, for different reasons, including no time for consulting a sleep physician and performing a polysomnography, health cost, negligence. Therefore, providing through primary care a simple diagnostic approach deserves efforts and research,” he said in an interview.
New technologies could enable diagnostic sleep tests to be conducted at home, with the added benefit of multiple-night recordings to overcome the challenges of night-to-night variability in the apnea-hypopnea index. These novel testing methods should be cost effective, easy to install, and user friendly. Dr. Tamisier continued: “The issue about sleep diagnosis is that up to now, there was no such devices available. Many physicians use type III sleep recording that are dedicated to highly trained sleep scorers, but they use automatic analysis which in many cases is unsuccessful. For a trained sleep physician, it is easy to see that the result is inaccurate. New devices are being built for automatic analysis using artificial intelligence algorithms. Because by design they are automatic, the rate of success is very high, and if used with the right purpose, they could be highly effective and quick.”
In conclusion, the diagnosis of sleep apnea in primary care is becoming more feasible with advancements in diagnostic tools and technology. However, it is crucial for primary care physicians to exercise caution in cases in which the clinical presentation is not straightforward or when OSA is associated with comorbidities. Care management and clear boundaries are vital to ensure effective treatment and improve patient outcomes.
Dr. De Almeida Vicente Ferreira and Dr. Tamisier disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obstructive sleep apnea (OSA) remains a significantly underdiagnosed condition, despite its high prevalence. Primary care physicians play a pivotal role in identifying patients afflicted by this condition. To effectively diagnose OSA in primary care, increasing awareness and enhancing communication are imperative. Fortunately, several straightforward diagnostic tools are readily available, and even more sophisticated ones, driven by artificial intelligence, are on the horizon.
Recognize the problem
At the annual congress of the European Respiratory Society, Cláudia Sofia De Almeida Vicente Ferreira, MD, a family physician from Coimbra, Portugal, and coordinator of the Respiratory Diseases Interest Group of the Portuguese Association of General and Family Medicine, highlighted the challenges of diagnosing OSA.
People do not come to the doctor and complain about it. Sometimes you catch it in the middle of other things,” she said in an interview.
Moreover, physicians’ busy schedules and limited appointment times often lead to a focus on the symptoms reported by patients, and insufficient attention is paid to the quality of sleep. This may be compounded by a tendency among medical professionals to underestimate the risks associated with OSA, as it is not directly linked to mortality, despite its clear connection to cardiovascular risks.
Identifying and recognizing risk factors can facilitate OSA suspicion during patient evaluations. These factors encompass both structural (for example, craniofacial and upper airway anomalies) and nonstructural elements (for example, smoking, alcohol use, or sedative consumption). While men are at higher risk, postmenopausal women who are not receiving hormone replacement therapy face similar risks. Certain medical conditions, such as hypothyroidism, acromegaly, amyloidosis, Cushing syndrome, and Down syndrome, have also been associated with OSA. A comprehensive physical examination can provide additional clues. Factors might include obesity, neck circumference, Mallampati score, and nasal and pharyngeal problems.
Inquire actively
Once the possibility of OSA is considered, the next step is to ask patients about their symptoms. Questionnaires are simple yet valuable tools for this purpose. The STOP questionnaire comprises four key questions:
- Do you SNORE loudly (louder than talking or loud enough to be heard through closed doors)?
- Do you often feel TIRED, fatigued, or sleepy during daytime?
- Has anyone OBSERVED you stop breathing during your sleep?
- Do you have or are you being treated for high blood PRESSURE?
The STOP-BANG questionnaire adds four clinical attributes: obesity (body mass index > 35 kg/m2), age (> 50 years), neck size (> 40 cm, or 16 inches), and sex.
Patients are classified as being at low, intermediate, or high risk for OSA.
The Epworth Sleepiness Scale, which is self-administered, is also useful: patients rate the likelihood of falling asleep in various daytime contexts. These questionnaires can be seamlessly integrated into routine patient appointments.
Comorbidities and occupation
Primary care physicians should carefully assess comorbidities, especially those linked to cardiovascular risk. Patients with resistant hypertension, pulmonary hypertension, and recurrent atrial fibrillation following cardioversion/ablation should be prioritized for diagnostic testing for OSA. Patients with other conditions, such as coronary artery disease or cerebrovascular disease, should also be referred to a sleep center if OSA is suspected on the basis of comprehensive sleep assessment. OSA has also been associated with type 2 diabetes, metabolic syndrome, and asthma.
Gaining access to sleep study services and subsequent therapy, such as continuous positive airway pressure (CPAP), can be challenging. Primary care physicians should prioritize patients on the basis of their risk levels. Occupation plays a significant role in this prioritization, as sleep fragmentation and daytime sleepiness can lead to workplace and vehicular accidents.
“You should include the occupation in the patient’s profile. What is he doing? Is he sitting at a desk, or is he working at height, driving, or operating machines? These workers are high-risk patients,” continued Dr. De Almeida Vicente Ferreira.
“I think that the family physician has a key role in the follow-up. Nobody else will look for CPAP compliance and will verify if CPAP is working or not. If the patient is not using it or if it is not effective, still there is someone paying for the machine (the national health care system or an insurance company). More importantly, if CPAP is not working, we are not improving our patient’s life in terms of reducing cardiovascular risk and ameliorating the quality of life.”
Is home testing a viable option?
Diagnosing OSA typically relies on overnight polysomnography in specialized sleep clinics, which is often associated with long waiting lists. Researchers are actively working on innovative sensors and digital solutions for home-based sleep testing, but according to Dr. De Almeida Vicente Ferreira, they are not yet ready for prime time: “Home-based studies with fewer evaluation parameters (such as pulse and oxygen levels) are not so secure or sensitive to establish a correct and complete diagnosis. Actually, the architecture of sleep is very complex. The test must be performed and read by a specialized team.”
Still, according to Renaud Tamisier, MD, PhD, professor of clinical physiology at the Université Grenoble Alpes in La Tronche, France, simplified sleep testing could be very useful. “There are many patients that still are not diagnosed despite having severe sleep apnea, with symptoms and comorbidities. These patients usually are not aware of their disease but complain about changes in their quality of life with excessive tiredness and sleepiness. Also, they are not connected to the healthcare system, for different reasons, including no time for consulting a sleep physician and performing a polysomnography, health cost, negligence. Therefore, providing through primary care a simple diagnostic approach deserves efforts and research,” he said in an interview.
New technologies could enable diagnostic sleep tests to be conducted at home, with the added benefit of multiple-night recordings to overcome the challenges of night-to-night variability in the apnea-hypopnea index. These novel testing methods should be cost effective, easy to install, and user friendly. Dr. Tamisier continued: “The issue about sleep diagnosis is that up to now, there was no such devices available. Many physicians use type III sleep recording that are dedicated to highly trained sleep scorers, but they use automatic analysis which in many cases is unsuccessful. For a trained sleep physician, it is easy to see that the result is inaccurate. New devices are being built for automatic analysis using artificial intelligence algorithms. Because by design they are automatic, the rate of success is very high, and if used with the right purpose, they could be highly effective and quick.”
In conclusion, the diagnosis of sleep apnea in primary care is becoming more feasible with advancements in diagnostic tools and technology. However, it is crucial for primary care physicians to exercise caution in cases in which the clinical presentation is not straightforward or when OSA is associated with comorbidities. Care management and clear boundaries are vital to ensure effective treatment and improve patient outcomes.
Dr. De Almeida Vicente Ferreira and Dr. Tamisier disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obstructive sleep apnea (OSA) remains a significantly underdiagnosed condition, despite its high prevalence. Primary care physicians play a pivotal role in identifying patients afflicted by this condition. To effectively diagnose OSA in primary care, increasing awareness and enhancing communication are imperative. Fortunately, several straightforward diagnostic tools are readily available, and even more sophisticated ones, driven by artificial intelligence, are on the horizon.
Recognize the problem
At the annual congress of the European Respiratory Society, Cláudia Sofia De Almeida Vicente Ferreira, MD, a family physician from Coimbra, Portugal, and coordinator of the Respiratory Diseases Interest Group of the Portuguese Association of General and Family Medicine, highlighted the challenges of diagnosing OSA.
People do not come to the doctor and complain about it. Sometimes you catch it in the middle of other things,” she said in an interview.
Moreover, physicians’ busy schedules and limited appointment times often lead to a focus on the symptoms reported by patients, and insufficient attention is paid to the quality of sleep. This may be compounded by a tendency among medical professionals to underestimate the risks associated with OSA, as it is not directly linked to mortality, despite its clear connection to cardiovascular risks.
Identifying and recognizing risk factors can facilitate OSA suspicion during patient evaluations. These factors encompass both structural (for example, craniofacial and upper airway anomalies) and nonstructural elements (for example, smoking, alcohol use, or sedative consumption). While men are at higher risk, postmenopausal women who are not receiving hormone replacement therapy face similar risks. Certain medical conditions, such as hypothyroidism, acromegaly, amyloidosis, Cushing syndrome, and Down syndrome, have also been associated with OSA. A comprehensive physical examination can provide additional clues. Factors might include obesity, neck circumference, Mallampati score, and nasal and pharyngeal problems.
Inquire actively
Once the possibility of OSA is considered, the next step is to ask patients about their symptoms. Questionnaires are simple yet valuable tools for this purpose. The STOP questionnaire comprises four key questions:
- Do you SNORE loudly (louder than talking or loud enough to be heard through closed doors)?
- Do you often feel TIRED, fatigued, or sleepy during daytime?
- Has anyone OBSERVED you stop breathing during your sleep?
- Do you have or are you being treated for high blood PRESSURE?
The STOP-BANG questionnaire adds four clinical attributes: obesity (body mass index > 35 kg/m2), age (> 50 years), neck size (> 40 cm, or 16 inches), and sex.
Patients are classified as being at low, intermediate, or high risk for OSA.
The Epworth Sleepiness Scale, which is self-administered, is also useful: patients rate the likelihood of falling asleep in various daytime contexts. These questionnaires can be seamlessly integrated into routine patient appointments.
Comorbidities and occupation
Primary care physicians should carefully assess comorbidities, especially those linked to cardiovascular risk. Patients with resistant hypertension, pulmonary hypertension, and recurrent atrial fibrillation following cardioversion/ablation should be prioritized for diagnostic testing for OSA. Patients with other conditions, such as coronary artery disease or cerebrovascular disease, should also be referred to a sleep center if OSA is suspected on the basis of comprehensive sleep assessment. OSA has also been associated with type 2 diabetes, metabolic syndrome, and asthma.
Gaining access to sleep study services and subsequent therapy, such as continuous positive airway pressure (CPAP), can be challenging. Primary care physicians should prioritize patients on the basis of their risk levels. Occupation plays a significant role in this prioritization, as sleep fragmentation and daytime sleepiness can lead to workplace and vehicular accidents.
“You should include the occupation in the patient’s profile. What is he doing? Is he sitting at a desk, or is he working at height, driving, or operating machines? These workers are high-risk patients,” continued Dr. De Almeida Vicente Ferreira.
“I think that the family physician has a key role in the follow-up. Nobody else will look for CPAP compliance and will verify if CPAP is working or not. If the patient is not using it or if it is not effective, still there is someone paying for the machine (the national health care system or an insurance company). More importantly, if CPAP is not working, we are not improving our patient’s life in terms of reducing cardiovascular risk and ameliorating the quality of life.”
Is home testing a viable option?
Diagnosing OSA typically relies on overnight polysomnography in specialized sleep clinics, which is often associated with long waiting lists. Researchers are actively working on innovative sensors and digital solutions for home-based sleep testing, but according to Dr. De Almeida Vicente Ferreira, they are not yet ready for prime time: “Home-based studies with fewer evaluation parameters (such as pulse and oxygen levels) are not so secure or sensitive to establish a correct and complete diagnosis. Actually, the architecture of sleep is very complex. The test must be performed and read by a specialized team.”
Still, according to Renaud Tamisier, MD, PhD, professor of clinical physiology at the Université Grenoble Alpes in La Tronche, France, simplified sleep testing could be very useful. “There are many patients that still are not diagnosed despite having severe sleep apnea, with symptoms and comorbidities. These patients usually are not aware of their disease but complain about changes in their quality of life with excessive tiredness and sleepiness. Also, they are not connected to the healthcare system, for different reasons, including no time for consulting a sleep physician and performing a polysomnography, health cost, negligence. Therefore, providing through primary care a simple diagnostic approach deserves efforts and research,” he said in an interview.
New technologies could enable diagnostic sleep tests to be conducted at home, with the added benefit of multiple-night recordings to overcome the challenges of night-to-night variability in the apnea-hypopnea index. These novel testing methods should be cost effective, easy to install, and user friendly. Dr. Tamisier continued: “The issue about sleep diagnosis is that up to now, there was no such devices available. Many physicians use type III sleep recording that are dedicated to highly trained sleep scorers, but they use automatic analysis which in many cases is unsuccessful. For a trained sleep physician, it is easy to see that the result is inaccurate. New devices are being built for automatic analysis using artificial intelligence algorithms. Because by design they are automatic, the rate of success is very high, and if used with the right purpose, they could be highly effective and quick.”
In conclusion, the diagnosis of sleep apnea in primary care is becoming more feasible with advancements in diagnostic tools and technology. However, it is crucial for primary care physicians to exercise caution in cases in which the clinical presentation is not straightforward or when OSA is associated with comorbidities. Care management and clear boundaries are vital to ensure effective treatment and improve patient outcomes.
Dr. De Almeida Vicente Ferreira and Dr. Tamisier disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ERS 2023
Stress, insomnia tied to increased AFib risk for older women
TOPLINE:
Eight psychosocial factors, grouped into two distinct clusters, are significantly associated with risk for atrial fibrillation in postmenopausal women, with insomnia and stressful life events (SLEs) being the most strongly associated with AFib, a large new study has found.
METHODOLOGY:
- In addition to traditional risk factors such as obesity, advanced age, ethnicity, smoking, alcohol, hypertension, diabetes, coronary artery disease, heart failure, and emotional and psychological distress may also affect AFib.
- The study included 83,736 postmenopausal women in the Women’s Health Initiative (mean age, 63.9 years; 88.1% White) who did not have AFib at baseline.
- From questionnaires, researchers collected information on psychosocial stressors and used hierarchical cluster analysis to identify patterns of psychosocial predictors.
- They separated these clusters into quartiles, identified associations between psychosocial exposure variables, and adjusted for traditional risk factors.
- Over an average follow-up of 10.5 years, 23,954 participants (28.6%) developed incident AFib.
TAKEAWAY:
- The analysis generated two clusters of distinct psychosocial variables that were significantly associated with AFib: the Stress Cluster, including SLEs, depressive symptoms, and insomnia; and the Strain Cluster, including three personality traits: optimism, cynical hostility, and emotional expressiveness; and two social measures: social support, and social strain.
- Those in the highest quartiles of both the Stress Cluster and the Strain Cluster had greater rates of AFib, compared with those in the lowest quartiles.
- In a final model, the association between SLEs (hazard ratio, 1.02; 95% confidence interval, 1.01-1.04) and insomnia (HR, 1.04; 95% CI, 1.03-1.06) were most strongly linked to increased incidence of AFib, and a sensitivity analysis using snoring as a surrogate marker for sleep apnea didn’t change this outcome, supporting the independent effect of insomnia on AFib.
- In subgroup analyses, the Stress Cluster had a stronger association with AFib incidence in younger (50-69 years) versus older women (70-79 years), and in non-Hispanic White and Asian women versus Hispanic and non-Hispanic Black women.
IN PRACTICE:
The results support the hypothesis that psychosocial predictors account for additional risk for AFib “above and beyond” traditional risk factors, the authors wrote. Identifying and addressing sex-specific, modifiable risk factors, including insomnia, “may help reduce the burden of AF[ib] in aging women.”
SOURCE:
The study was conducted by Susan X. Zhao, MD, division of cardiology, department of medicine, Santa Clara Valley Medical Center, San Jose, Calif., and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
The psychometric questionnaires were administered only at study entry, but psychosocial variables may change over time. Data on sleep apnea and other sleep disorders, which may confound the relationship between insomnia and AFib, were not available, and although the study included a sensitivity analysis controlling for snoring, this is an imperfect surrogate for sleep apnea. Generalizability to other demographic, racial, and ethnic groups is limited.
DISCLOSURES:
The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Eight psychosocial factors, grouped into two distinct clusters, are significantly associated with risk for atrial fibrillation in postmenopausal women, with insomnia and stressful life events (SLEs) being the most strongly associated with AFib, a large new study has found.
METHODOLOGY:
- In addition to traditional risk factors such as obesity, advanced age, ethnicity, smoking, alcohol, hypertension, diabetes, coronary artery disease, heart failure, and emotional and psychological distress may also affect AFib.
- The study included 83,736 postmenopausal women in the Women’s Health Initiative (mean age, 63.9 years; 88.1% White) who did not have AFib at baseline.
- From questionnaires, researchers collected information on psychosocial stressors and used hierarchical cluster analysis to identify patterns of psychosocial predictors.
- They separated these clusters into quartiles, identified associations between psychosocial exposure variables, and adjusted for traditional risk factors.
- Over an average follow-up of 10.5 years, 23,954 participants (28.6%) developed incident AFib.
TAKEAWAY:
- The analysis generated two clusters of distinct psychosocial variables that were significantly associated with AFib: the Stress Cluster, including SLEs, depressive symptoms, and insomnia; and the Strain Cluster, including three personality traits: optimism, cynical hostility, and emotional expressiveness; and two social measures: social support, and social strain.
- Those in the highest quartiles of both the Stress Cluster and the Strain Cluster had greater rates of AFib, compared with those in the lowest quartiles.
- In a final model, the association between SLEs (hazard ratio, 1.02; 95% confidence interval, 1.01-1.04) and insomnia (HR, 1.04; 95% CI, 1.03-1.06) were most strongly linked to increased incidence of AFib, and a sensitivity analysis using snoring as a surrogate marker for sleep apnea didn’t change this outcome, supporting the independent effect of insomnia on AFib.
- In subgroup analyses, the Stress Cluster had a stronger association with AFib incidence in younger (50-69 years) versus older women (70-79 years), and in non-Hispanic White and Asian women versus Hispanic and non-Hispanic Black women.
IN PRACTICE:
The results support the hypothesis that psychosocial predictors account for additional risk for AFib “above and beyond” traditional risk factors, the authors wrote. Identifying and addressing sex-specific, modifiable risk factors, including insomnia, “may help reduce the burden of AF[ib] in aging women.”
SOURCE:
The study was conducted by Susan X. Zhao, MD, division of cardiology, department of medicine, Santa Clara Valley Medical Center, San Jose, Calif., and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
The psychometric questionnaires were administered only at study entry, but psychosocial variables may change over time. Data on sleep apnea and other sleep disorders, which may confound the relationship between insomnia and AFib, were not available, and although the study included a sensitivity analysis controlling for snoring, this is an imperfect surrogate for sleep apnea. Generalizability to other demographic, racial, and ethnic groups is limited.
DISCLOSURES:
The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Eight psychosocial factors, grouped into two distinct clusters, are significantly associated with risk for atrial fibrillation in postmenopausal women, with insomnia and stressful life events (SLEs) being the most strongly associated with AFib, a large new study has found.
METHODOLOGY:
- In addition to traditional risk factors such as obesity, advanced age, ethnicity, smoking, alcohol, hypertension, diabetes, coronary artery disease, heart failure, and emotional and psychological distress may also affect AFib.
- The study included 83,736 postmenopausal women in the Women’s Health Initiative (mean age, 63.9 years; 88.1% White) who did not have AFib at baseline.
- From questionnaires, researchers collected information on psychosocial stressors and used hierarchical cluster analysis to identify patterns of psychosocial predictors.
- They separated these clusters into quartiles, identified associations between psychosocial exposure variables, and adjusted for traditional risk factors.
- Over an average follow-up of 10.5 years, 23,954 participants (28.6%) developed incident AFib.
TAKEAWAY:
- The analysis generated two clusters of distinct psychosocial variables that were significantly associated with AFib: the Stress Cluster, including SLEs, depressive symptoms, and insomnia; and the Strain Cluster, including three personality traits: optimism, cynical hostility, and emotional expressiveness; and two social measures: social support, and social strain.
- Those in the highest quartiles of both the Stress Cluster and the Strain Cluster had greater rates of AFib, compared with those in the lowest quartiles.
- In a final model, the association between SLEs (hazard ratio, 1.02; 95% confidence interval, 1.01-1.04) and insomnia (HR, 1.04; 95% CI, 1.03-1.06) were most strongly linked to increased incidence of AFib, and a sensitivity analysis using snoring as a surrogate marker for sleep apnea didn’t change this outcome, supporting the independent effect of insomnia on AFib.
- In subgroup analyses, the Stress Cluster had a stronger association with AFib incidence in younger (50-69 years) versus older women (70-79 years), and in non-Hispanic White and Asian women versus Hispanic and non-Hispanic Black women.
IN PRACTICE:
The results support the hypothesis that psychosocial predictors account for additional risk for AFib “above and beyond” traditional risk factors, the authors wrote. Identifying and addressing sex-specific, modifiable risk factors, including insomnia, “may help reduce the burden of AF[ib] in aging women.”
SOURCE:
The study was conducted by Susan X. Zhao, MD, division of cardiology, department of medicine, Santa Clara Valley Medical Center, San Jose, Calif., and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
The psychometric questionnaires were administered only at study entry, but psychosocial variables may change over time. Data on sleep apnea and other sleep disorders, which may confound the relationship between insomnia and AFib, were not available, and although the study included a sensitivity analysis controlling for snoring, this is an imperfect surrogate for sleep apnea. Generalizability to other demographic, racial, and ethnic groups is limited.
DISCLOSURES:
The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Night owls have higher risk of developing type 2 diabetes
, compared with their “early bird” counterparts, according to a new study, published in Annals of Internal Medicine.
The work focused on participants’ self-assessed chronotype – an individuals’ circadian preference, or natural preference to sleep and wake up earlier or later, commonly known as being an early bird or a night owl.
Analyzing the self-reported lifestyle behaviors and sleeping habits of more than 60,000 middle-aged female nurses, researchers from Brigham and Women’s Hospital and Harvard Medical School, both in Boston, found that those with a preference for waking up later had a 72% higher risk for diabetes and were 54% more likely to have unhealthy lifestyle behaviors, compared with participants who tended to wake up earlier.
After adjustment for six lifestyle factors – diet, alcohol use, body mass index (BMI), physical activity, smoking status, and sleep duration – the association between diabetes risk and evening chronotype weakened to a 19% higher risk of developing type 2 diabetes.
In a subgroup analysis, this association was stronger among women who either had had no night shifts over the previous 2 years or had worked night shifts for less than 10 years in their careers. For nurses who had worked night shifts recently, the study found no association between evening chronotype and diabetes risk.
The participants, drawn from the Nurses’ Health Study II, were between 45 and 62 years age, with no history of cancer, cardiovascular disease, or diabetes. Researchers followed the group from 2009 until 2017.
Is there a mismatch between natural circadian rhythm and work schedule?
The authors, led by Sina Kianersi, DVM, PhD, of Harvard Medical School, Boston, suggest that their results may be linked to a mismatch between a person’s circadian rhythm and their physical and social environment – for example, if someone lives on a schedule opposite to their circadian preference.
In one 2015 study, female nurses who had worked daytime shifts for more than 10 years but had an evening chronotype had the highest diabetes risk, compared with early chronotypes (51% more likely to develop type 2 diabetes).
In a 2022 study, an evening chronotype was associated with a 30% elevated risk for type 2 diabetes. The authors speculated that circadian misalignment could be to blame – for example, being a night owl but working early morning – which can disrupt glycemic and lipid metabolism.
Previous studies have found that shorter or irregular sleep habits are associated with a higher risk of type 2 diabetes. Other studies have also found that people with an evening chronotype are more likely than early birds to have unhealthy eating habits, have lower levels of physical activity, and smoke and drink.
This new study did not find that an evening chronotype was associated with unhealthy drinking, which the authors defined as having one or more drinks per day.
In an accompanying editorial, two physicians from the Harvard T.H. Chan School of Public Health in Boston caution that the statistical design of the study limits its ability to establish causation.
“Chronotype could change later, which might correlate with lifestyle changes,” write Kehuan Lin, MS, Mingyang Song, MBBS, and Edward Giovannucci, MD. “Experimental trials are required to determine whether chronotype is a marker of unhealthy lifestyle or an independent determinant.”
They also suggest that psychological factors and the type of work being performed by the participants could be potential confounders.
The authors of the study note that their findings might not be generalizable to groups other than middle-aged White female nurses. The study population also had a relatively high level of education and were socioeconomically advantaged.
Self-reporting chronotypes with a single question could also result in misclassification and measurement error, the authors acknowledge.
The findings underscore the value of assessing an individuals’ chronotype for scheduling shift work – for example, assigning night owls to night shifts may improve their metabolic health and sleeping habits, according to the authors of the study.
“Given the importance of lifestyle modification in diabetes prevention, future research is warranted to investigate whether improving lifestyle behaviors could effectively reduce diabetes risk in persons with an evening chronotype,” the authors conclude.
The study was supported by grants from the National Institutes of Health and the European Research Council.
A version of this article first appeared on Medscape.com.
, compared with their “early bird” counterparts, according to a new study, published in Annals of Internal Medicine.
The work focused on participants’ self-assessed chronotype – an individuals’ circadian preference, or natural preference to sleep and wake up earlier or later, commonly known as being an early bird or a night owl.
Analyzing the self-reported lifestyle behaviors and sleeping habits of more than 60,000 middle-aged female nurses, researchers from Brigham and Women’s Hospital and Harvard Medical School, both in Boston, found that those with a preference for waking up later had a 72% higher risk for diabetes and were 54% more likely to have unhealthy lifestyle behaviors, compared with participants who tended to wake up earlier.
After adjustment for six lifestyle factors – diet, alcohol use, body mass index (BMI), physical activity, smoking status, and sleep duration – the association between diabetes risk and evening chronotype weakened to a 19% higher risk of developing type 2 diabetes.
In a subgroup analysis, this association was stronger among women who either had had no night shifts over the previous 2 years or had worked night shifts for less than 10 years in their careers. For nurses who had worked night shifts recently, the study found no association between evening chronotype and diabetes risk.
The participants, drawn from the Nurses’ Health Study II, were between 45 and 62 years age, with no history of cancer, cardiovascular disease, or diabetes. Researchers followed the group from 2009 until 2017.
Is there a mismatch between natural circadian rhythm and work schedule?
The authors, led by Sina Kianersi, DVM, PhD, of Harvard Medical School, Boston, suggest that their results may be linked to a mismatch between a person’s circadian rhythm and their physical and social environment – for example, if someone lives on a schedule opposite to their circadian preference.
In one 2015 study, female nurses who had worked daytime shifts for more than 10 years but had an evening chronotype had the highest diabetes risk, compared with early chronotypes (51% more likely to develop type 2 diabetes).
In a 2022 study, an evening chronotype was associated with a 30% elevated risk for type 2 diabetes. The authors speculated that circadian misalignment could be to blame – for example, being a night owl but working early morning – which can disrupt glycemic and lipid metabolism.
Previous studies have found that shorter or irregular sleep habits are associated with a higher risk of type 2 diabetes. Other studies have also found that people with an evening chronotype are more likely than early birds to have unhealthy eating habits, have lower levels of physical activity, and smoke and drink.
This new study did not find that an evening chronotype was associated with unhealthy drinking, which the authors defined as having one or more drinks per day.
In an accompanying editorial, two physicians from the Harvard T.H. Chan School of Public Health in Boston caution that the statistical design of the study limits its ability to establish causation.
“Chronotype could change later, which might correlate with lifestyle changes,” write Kehuan Lin, MS, Mingyang Song, MBBS, and Edward Giovannucci, MD. “Experimental trials are required to determine whether chronotype is a marker of unhealthy lifestyle or an independent determinant.”
They also suggest that psychological factors and the type of work being performed by the participants could be potential confounders.
The authors of the study note that their findings might not be generalizable to groups other than middle-aged White female nurses. The study population also had a relatively high level of education and were socioeconomically advantaged.
Self-reporting chronotypes with a single question could also result in misclassification and measurement error, the authors acknowledge.
The findings underscore the value of assessing an individuals’ chronotype for scheduling shift work – for example, assigning night owls to night shifts may improve their metabolic health and sleeping habits, according to the authors of the study.
“Given the importance of lifestyle modification in diabetes prevention, future research is warranted to investigate whether improving lifestyle behaviors could effectively reduce diabetes risk in persons with an evening chronotype,” the authors conclude.
The study was supported by grants from the National Institutes of Health and the European Research Council.
A version of this article first appeared on Medscape.com.
, compared with their “early bird” counterparts, according to a new study, published in Annals of Internal Medicine.
The work focused on participants’ self-assessed chronotype – an individuals’ circadian preference, or natural preference to sleep and wake up earlier or later, commonly known as being an early bird or a night owl.
Analyzing the self-reported lifestyle behaviors and sleeping habits of more than 60,000 middle-aged female nurses, researchers from Brigham and Women’s Hospital and Harvard Medical School, both in Boston, found that those with a preference for waking up later had a 72% higher risk for diabetes and were 54% more likely to have unhealthy lifestyle behaviors, compared with participants who tended to wake up earlier.
After adjustment for six lifestyle factors – diet, alcohol use, body mass index (BMI), physical activity, smoking status, and sleep duration – the association between diabetes risk and evening chronotype weakened to a 19% higher risk of developing type 2 diabetes.
In a subgroup analysis, this association was stronger among women who either had had no night shifts over the previous 2 years or had worked night shifts for less than 10 years in their careers. For nurses who had worked night shifts recently, the study found no association between evening chronotype and diabetes risk.
The participants, drawn from the Nurses’ Health Study II, were between 45 and 62 years age, with no history of cancer, cardiovascular disease, or diabetes. Researchers followed the group from 2009 until 2017.
Is there a mismatch between natural circadian rhythm and work schedule?
The authors, led by Sina Kianersi, DVM, PhD, of Harvard Medical School, Boston, suggest that their results may be linked to a mismatch between a person’s circadian rhythm and their physical and social environment – for example, if someone lives on a schedule opposite to their circadian preference.
In one 2015 study, female nurses who had worked daytime shifts for more than 10 years but had an evening chronotype had the highest diabetes risk, compared with early chronotypes (51% more likely to develop type 2 diabetes).
In a 2022 study, an evening chronotype was associated with a 30% elevated risk for type 2 diabetes. The authors speculated that circadian misalignment could be to blame – for example, being a night owl but working early morning – which can disrupt glycemic and lipid metabolism.
Previous studies have found that shorter or irregular sleep habits are associated with a higher risk of type 2 diabetes. Other studies have also found that people with an evening chronotype are more likely than early birds to have unhealthy eating habits, have lower levels of physical activity, and smoke and drink.
This new study did not find that an evening chronotype was associated with unhealthy drinking, which the authors defined as having one or more drinks per day.
In an accompanying editorial, two physicians from the Harvard T.H. Chan School of Public Health in Boston caution that the statistical design of the study limits its ability to establish causation.
“Chronotype could change later, which might correlate with lifestyle changes,” write Kehuan Lin, MS, Mingyang Song, MBBS, and Edward Giovannucci, MD. “Experimental trials are required to determine whether chronotype is a marker of unhealthy lifestyle or an independent determinant.”
They also suggest that psychological factors and the type of work being performed by the participants could be potential confounders.
The authors of the study note that their findings might not be generalizable to groups other than middle-aged White female nurses. The study population also had a relatively high level of education and were socioeconomically advantaged.
Self-reporting chronotypes with a single question could also result in misclassification and measurement error, the authors acknowledge.
The findings underscore the value of assessing an individuals’ chronotype for scheduling shift work – for example, assigning night owls to night shifts may improve their metabolic health and sleeping habits, according to the authors of the study.
“Given the importance of lifestyle modification in diabetes prevention, future research is warranted to investigate whether improving lifestyle behaviors could effectively reduce diabetes risk in persons with an evening chronotype,” the authors conclude.
The study was supported by grants from the National Institutes of Health and the European Research Council.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE