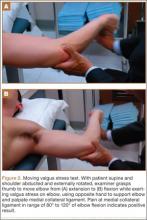
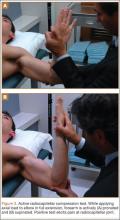
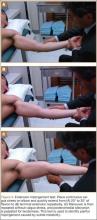
User login
The Importance of Sex of Patient in the Management of Femoroacetabular Impingement
Femoroacetabular impingement (FAI), a recently described hip condition in adolescents and young adults, results from abnormal physical contact between the proximal femur and the acetabulum.1 FAI is usually characterized by the site of the predominant morphologic abnormality—proximal femur (cam-type FAI), acetabulum (pincer-type FAI), or both (mixed impingement). Cam-type FAI is typified by the aspherical extension of the articular surface at the anterosuperior head–neck junction of the proximal femur with loss of the normal offset. With hip motion, especially in the maximal ranges of flexion and internal rotation, the aspherical proximal femur repeatedly contacts the anterosuperior acetabulum, damaging the chondrolabral junction and ultimately the labrum itself. In pincer-type impingement, femoral head overcoverage caused by acetabular retroversion and/or coxa profunda directly damages the anterior labrum when the acetabular rim contacts the proximal femur during physiologic motion. “Contrecoup” injury of the posterior-inferior acetabular cartilage may also occur. Over time, recurrent microtrauma to the acetabular cartilage and/or labrum may lead to degenerative changes of the hip and ultimately to premature osteoarthritis.1,2
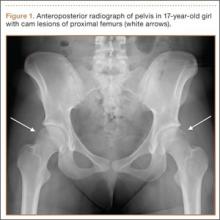
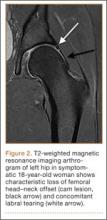
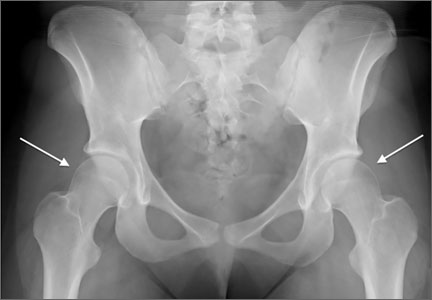
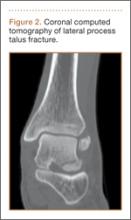
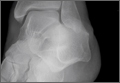
Patients with FAI typically present with groin pain that may be activity-related or that may occur with prolonged sitting with the hip in a flexed position. Physical examination findings suggestive of FAI include decreased passive internal hip rotation and reproducible pain with adduction and internal rotation of the flexed hip—the impingement sign, or the flexion, adduction, and internal rotation (FADIR) test.3 Diagnostic imaging evaluation initially includes radiographs of the pelvis and hips. These radiographs may show a “pistol-grip” deformity and/or decreased head–neck offset (as determined by increased alpha angle) in the setting of cam-type impingement (Figure 1).4 Pincer-type impingement may be associated with a crossover sign, coxa profunda, and an increased center-edge angle (CEA). Advanced imaging studies, such as computed tomography (CT), magnetic resonance imaging (MRI) arthrogram, and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), are commonly used to better delineate bony deformity and concomitant injuries of the labrum and cartilage (Figure 2).
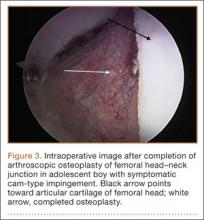
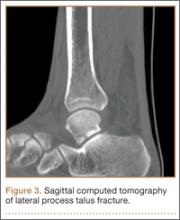
Treatment for FAI often consists initially of activity modification, use of anti-inflammatory medications, and physical therapy. Intra-articular corticosteroid injections may be used both diagnostically and therapeutically. When nonsurgical measures fail to adequately relieve symptoms, surgery may be warranted. Whether performed open or arthroscopically, surgery is directed first at correcting the underlying osseous abnormality—performing an osteoplasty of the proximal femur to remove the cam lesion, performing an acetabular osteoplasty (“rim-trimming”) to address a focal pincer lesion, and/or performing a periacetabular osteotomy to decrease global acetabular overcoverage (Figure 3).5
Sex-Based Differences in FAI Incidence
Traditionally, it was thought that cam-type impingement occurred predominantly in young, athletic males, whereas pincer-type impingement resulting from acetabular overcoverage occurred primarily in females during their fourth decade. However, our understanding of the sex-based differences in the incidence and presentation of FAI has evolved, and it is now clear that the interplay of sex, radiographic signs of impingement, and development of symptoms requiring treatment is more complex.
In recent large population-based studies, investigators have attempted to better characterize the sex-based differences in the incidence of osseous FAI deformity. Gosvig and colleagues2 examined radiographic and questionnaire outcomes of 3620 patients (age range, 21-90 years) and found that males were more likely than females to have a pistol-grip deformity of the hip (19.6% vs 5.2%); that deep acetabular sockets were common in both sexes (15.2% vs 19.4%); and that the presence of pistol-grip deformity or deep socket was significantly associated with development of osteoarthritis, independent of sex.
In a study of 2081 asymptomatic patients (mean age, 18.6 years), Laborie and colleagues4 reported similar radiographic findings. Males were significantly more likely than females to have a cam-type deformity, as evidenced by pistol-grip deformity, focal prominence of the femoral neck, and/or flattening of the lateral aspect of the femoral head. Males were also more likely than females to have a pincer deformity, though radiographic signs of pincer deformity—a crossover sign, excessive acetabular coverage (defined by increased CEA), and a posterior wall sign—were common in both sexes, occurring in 16.6% of females and 34.3% of males. Bilateral findings of FAI-associated deformity were also more common in males than in females, both for cam-type deformity (24.7% vs 6.3%) and pincer-type deformity (21.7% vs 9.7%).
Sex-Based Differences in FAI Presentation
In males and females, the clinical presentation of FAI is similar—insidious onset of deep groin pain, often exacerbated with activity, and physical examination findings of decreased hip motion (particularly internal rotation) and a positive impingement test.3 Nevertheless, the sexes’ clinical presentation differs in several ways. Specifically, in a study using 3-dimensional CT to assess bony deformity in both symptomatic and asymptomatic patients, Beaulé and colleagues6 reported that alpha angles were significantly higher in symptomatic males than in symptomatic females (73.3° vs 58.7°). Hetsroni and colleagues7 recently reported similar results in a study of 217 symptomatic young adults treated arthroscopically for hip pain. Preoperative CT showed that alpha angles were significantly larger in males than in females (63.6° vs 47.8°). The authors postulated that females may be more likely to be symptomatic in the setting of smaller cam lesions because of the increased peak hip flexion and frontal plane motion commonly demonstrated by females during drop landings in sport. The authors further hypothesized that sex differences in muscle mass (which contributes to dynamic hip stability) and ligamentous laxity (a component of static hip stability) may result in larger physiologic ranges of motion for many females. As a result, bony impingement may occur in the setting of smaller anatomical lesions in females. The authors further noted that, compared with their male counterparts, females being treated for symptomatic FAI had significantly more femoral and acetabular anteversion.
Another male–female presentation difference involves symptom bilaterality. Specifically, males are significantly more likely than females to have symptomatic FAI involving both hips. In a recent study of 646 patients who underwent hip arthroscopy for symptomatic FAI during a 2-year period, Klingenstein and colleagues8 found that females constituted 48.2% of unilateral arthroscopy patients but only 34.8% of bilateral arthroscopy patients. The odds ratio of males treated for both hips, compared with females, was 1.7 (95% confidence interval, 1.16–2.54).
Last, it has been reported that, on clinical presentation, hip function scores are significantly lower in females than in males. In a recent study of 612 cases of symptomatic FAI treated with hip arthroscopy, Malviya and colleagues9 found that females had significantly lower quality-of-life scores both before and after surgery. Hetsroni and colleagues7 reported similar findings, with females having significantly lower preoperative modified Harris Hip Scores and lower Hip Outcome Scores in the domains of Activities of Daily Living and Sports.
Sex-Based Differences in FAI Treatment
and Outcomes
Surgical treatment of FAI is focused on identifying the source of hip pain and dysfunction—be it osseous lesion, labral tearing, chondral injury, or iliopsoas tendonitis—and treating it accordingly, regardless of sex. Most studies of this approach find consistent improvement in the short-term and midterm outcome scores for a majority of patients. However, relatively few studies have focused specifically on sex in determining the percentage of patients who require surgical treatment, in deciding the type of surgery that should be performed, or in measuring surgical outcomes in patients with symptomatic FAI.
In their review of 23 studies of FAI surgery, Ng and colleagues10 found that, of 970 patients, 608 (62.7%) were male and 362 (37.3%) were female. Similarly higher rates for males were previously published.5,11 More recently, Clohisy and colleagues12 reported on the descriptive epidemiology of patients having surgery for FAI at 8 different medical centers in North America. Fifty-five percent of the hips surgically treated for symptomatic FAI were females’. The authors speculated that this unexpectedly high rate could have resulted from US and Canadian female athletes’ increasingly higher level of sports participation. The results of this study, one of the largest examining the rate of surgery for males and females with FAI, suggest that females are more likely to have surgery for symptomatic FAI despite being less likely to have radiographic evidence of impingement. Our understanding of this phenomenon continues to advance.
In a recent prospective study, Krych and colleagues13 evaluated the clinical outcomes of FAI surgeries (labral débridement, labral repair) in an all-female patient cohort. Female patients with symptomatic FAI were randomized to undergo either labral débridement or labral repair. There were clinical improvements in both groups, but, compared with labral débridement patients, labral repair patients had more significantly improved Hip Outcome Scores in the domains of Activities of Daily Living and Sports, as well as better subjective outcomes. Although the study did not compare female patients with male patients, it does provide evidence that female patients specifically may benefit more from labral repair than from labral débridement alone.
With respect to different surgical treatments for male and female patients, Hetsroni and colleagues7 introduced the idea of sex-specific treatment when they noted more hip anteversion in their study’s female patients than in its male patients. They suggested that, because the anterosuperior acetabulum is subjected to a high amount of stress during weight-bearing and gait, this area in females with suspected pincer lesions should be rim-trimmed judiciously to avoid increasing the stress and perhaps even hastening the development of degenerative disease. Last, though several authors have noted that hip function scores are lower in females than in males on presentation, it has also been reported that females demonstrate more improvement in functional scores after surgery.9 This may be important information to discuss during preoperative counseling about expected goals and outcomes.
Conclusion
Femoroacetabular impingement is a common clinical entity that affects both males and females. However, sexual dimorphism in FAI incidence, presentation, treatment, and outcomes has recently been described in the literature (Table). Being aware of these sex-based differences and tailoring patient evaluation and management accordingly will likely result in optimal outcomes for each person who presents with symptomatic FAI.
1. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop. 2003;(417):112-120.
2. Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162-1169.
3. Philippon MJ, Maxwell RB, Johnston TL, Schenker M, Briggs KK. Clinical presentation of femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):1041-1047.
4. Laborie LB, Lehmann TG, Engesaeter IO, Eastwood DM, Engesaeter LB, Rosendahl K. Prevalence of radiographic findings thought to be associated with femoroacetabular impingement in a population-based cohort of 2081 healthy young adults. Radiology. 2011;260(2):494-502.
5. Clohisy JC, St John LC, Schutz AL. Surgical treatment of femoroacetabular impingement: a systematic review of the literature. Clin Orthop. 2010;468(2):555-564.
6. Beaulé PE, Zaragoza E, Motamedi K, Copelan N, Dorey FJ. Three-dimensional computed tomography of the hip in the assessment of femoroacetabular impingement. J Orthop Res. 2005;23(6):1286-1292.
7. Hetsroni I, Dela Torre K, Duke G, Lyman S, Kelly BT. Sex differences of hip morphology in young adults with hip pain and labral tears. Arthroscopy. 2013;29(1):54-63.
8. Klingenstein GG, Zbeda RM, Bedi A, Magennis E, Kelly BT. Prevalence and preoperative demographic and radiographic predictors of bilateral femoroacetabular impingement. Am J Sports Med. 2013;41(4):762-768.
9. Malviya A, Stafford GH, Villar RN. Impact of arthroscopy of the hip for femoroacetabular impingement on quality of life at a mean follow-up of 3.2 years. J Bone Joint Surg Br. 2012;94(4):466-470.
10. Ng VY, Arora N, Best TM, Pan X, Ellis TJ. Efficacy of surgery for femoroacetabular impingement: a systematic review. Am J Sports Med. 2010;38(11):2337-2345.
11. Matsuda DK, Carlisle JC, Arthurs SC, Wierks CH, Philippon MJ. Comparative systematic review of the open dislocation, mini-open, and arthroscopic surgeries for femoroacetabular impingement. Arthroscopy. 2011;27(2):252-269.
12. Clohisy JC, Baca G, Beaule PE, et al. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
13. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
Femoroacetabular impingement (FAI), a recently described hip condition in adolescents and young adults, results from abnormal physical contact between the proximal femur and the acetabulum.1 FAI is usually characterized by the site of the predominant morphologic abnormality—proximal femur (cam-type FAI), acetabulum (pincer-type FAI), or both (mixed impingement). Cam-type FAI is typified by the aspherical extension of the articular surface at the anterosuperior head–neck junction of the proximal femur with loss of the normal offset. With hip motion, especially in the maximal ranges of flexion and internal rotation, the aspherical proximal femur repeatedly contacts the anterosuperior acetabulum, damaging the chondrolabral junction and ultimately the labrum itself. In pincer-type impingement, femoral head overcoverage caused by acetabular retroversion and/or coxa profunda directly damages the anterior labrum when the acetabular rim contacts the proximal femur during physiologic motion. “Contrecoup” injury of the posterior-inferior acetabular cartilage may also occur. Over time, recurrent microtrauma to the acetabular cartilage and/or labrum may lead to degenerative changes of the hip and ultimately to premature osteoarthritis.1,2
Patients with FAI typically present with groin pain that may be activity-related or that may occur with prolonged sitting with the hip in a flexed position. Physical examination findings suggestive of FAI include decreased passive internal hip rotation and reproducible pain with adduction and internal rotation of the flexed hip—the impingement sign, or the flexion, adduction, and internal rotation (FADIR) test.3 Diagnostic imaging evaluation initially includes radiographs of the pelvis and hips. These radiographs may show a “pistol-grip” deformity and/or decreased head–neck offset (as determined by increased alpha angle) in the setting of cam-type impingement (Figure 1).4 Pincer-type impingement may be associated with a crossover sign, coxa profunda, and an increased center-edge angle (CEA). Advanced imaging studies, such as computed tomography (CT), magnetic resonance imaging (MRI) arthrogram, and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), are commonly used to better delineate bony deformity and concomitant injuries of the labrum and cartilage (Figure 2).
Treatment for FAI often consists initially of activity modification, use of anti-inflammatory medications, and physical therapy. Intra-articular corticosteroid injections may be used both diagnostically and therapeutically. When nonsurgical measures fail to adequately relieve symptoms, surgery may be warranted. Whether performed open or arthroscopically, surgery is directed first at correcting the underlying osseous abnormality—performing an osteoplasty of the proximal femur to remove the cam lesion, performing an acetabular osteoplasty (“rim-trimming”) to address a focal pincer lesion, and/or performing a periacetabular osteotomy to decrease global acetabular overcoverage (Figure 3).5
Sex-Based Differences in FAI Incidence
Traditionally, it was thought that cam-type impingement occurred predominantly in young, athletic males, whereas pincer-type impingement resulting from acetabular overcoverage occurred primarily in females during their fourth decade. However, our understanding of the sex-based differences in the incidence and presentation of FAI has evolved, and it is now clear that the interplay of sex, radiographic signs of impingement, and development of symptoms requiring treatment is more complex.
In recent large population-based studies, investigators have attempted to better characterize the sex-based differences in the incidence of osseous FAI deformity. Gosvig and colleagues2 examined radiographic and questionnaire outcomes of 3620 patients (age range, 21-90 years) and found that males were more likely than females to have a pistol-grip deformity of the hip (19.6% vs 5.2%); that deep acetabular sockets were common in both sexes (15.2% vs 19.4%); and that the presence of pistol-grip deformity or deep socket was significantly associated with development of osteoarthritis, independent of sex.
In a study of 2081 asymptomatic patients (mean age, 18.6 years), Laborie and colleagues4 reported similar radiographic findings. Males were significantly more likely than females to have a cam-type deformity, as evidenced by pistol-grip deformity, focal prominence of the femoral neck, and/or flattening of the lateral aspect of the femoral head. Males were also more likely than females to have a pincer deformity, though radiographic signs of pincer deformity—a crossover sign, excessive acetabular coverage (defined by increased CEA), and a posterior wall sign—were common in both sexes, occurring in 16.6% of females and 34.3% of males. Bilateral findings of FAI-associated deformity were also more common in males than in females, both for cam-type deformity (24.7% vs 6.3%) and pincer-type deformity (21.7% vs 9.7%).
Sex-Based Differences in FAI Presentation
In males and females, the clinical presentation of FAI is similar—insidious onset of deep groin pain, often exacerbated with activity, and physical examination findings of decreased hip motion (particularly internal rotation) and a positive impingement test.3 Nevertheless, the sexes’ clinical presentation differs in several ways. Specifically, in a study using 3-dimensional CT to assess bony deformity in both symptomatic and asymptomatic patients, Beaulé and colleagues6 reported that alpha angles were significantly higher in symptomatic males than in symptomatic females (73.3° vs 58.7°). Hetsroni and colleagues7 recently reported similar results in a study of 217 symptomatic young adults treated arthroscopically for hip pain. Preoperative CT showed that alpha angles were significantly larger in males than in females (63.6° vs 47.8°). The authors postulated that females may be more likely to be symptomatic in the setting of smaller cam lesions because of the increased peak hip flexion and frontal plane motion commonly demonstrated by females during drop landings in sport. The authors further hypothesized that sex differences in muscle mass (which contributes to dynamic hip stability) and ligamentous laxity (a component of static hip stability) may result in larger physiologic ranges of motion for many females. As a result, bony impingement may occur in the setting of smaller anatomical lesions in females. The authors further noted that, compared with their male counterparts, females being treated for symptomatic FAI had significantly more femoral and acetabular anteversion.
Another male–female presentation difference involves symptom bilaterality. Specifically, males are significantly more likely than females to have symptomatic FAI involving both hips. In a recent study of 646 patients who underwent hip arthroscopy for symptomatic FAI during a 2-year period, Klingenstein and colleagues8 found that females constituted 48.2% of unilateral arthroscopy patients but only 34.8% of bilateral arthroscopy patients. The odds ratio of males treated for both hips, compared with females, was 1.7 (95% confidence interval, 1.16–2.54).
Last, it has been reported that, on clinical presentation, hip function scores are significantly lower in females than in males. In a recent study of 612 cases of symptomatic FAI treated with hip arthroscopy, Malviya and colleagues9 found that females had significantly lower quality-of-life scores both before and after surgery. Hetsroni and colleagues7 reported similar findings, with females having significantly lower preoperative modified Harris Hip Scores and lower Hip Outcome Scores in the domains of Activities of Daily Living and Sports.
Sex-Based Differences in FAI Treatment
and Outcomes
Surgical treatment of FAI is focused on identifying the source of hip pain and dysfunction—be it osseous lesion, labral tearing, chondral injury, or iliopsoas tendonitis—and treating it accordingly, regardless of sex. Most studies of this approach find consistent improvement in the short-term and midterm outcome scores for a majority of patients. However, relatively few studies have focused specifically on sex in determining the percentage of patients who require surgical treatment, in deciding the type of surgery that should be performed, or in measuring surgical outcomes in patients with symptomatic FAI.
In their review of 23 studies of FAI surgery, Ng and colleagues10 found that, of 970 patients, 608 (62.7%) were male and 362 (37.3%) were female. Similarly higher rates for males were previously published.5,11 More recently, Clohisy and colleagues12 reported on the descriptive epidemiology of patients having surgery for FAI at 8 different medical centers in North America. Fifty-five percent of the hips surgically treated for symptomatic FAI were females’. The authors speculated that this unexpectedly high rate could have resulted from US and Canadian female athletes’ increasingly higher level of sports participation. The results of this study, one of the largest examining the rate of surgery for males and females with FAI, suggest that females are more likely to have surgery for symptomatic FAI despite being less likely to have radiographic evidence of impingement. Our understanding of this phenomenon continues to advance.
In a recent prospective study, Krych and colleagues13 evaluated the clinical outcomes of FAI surgeries (labral débridement, labral repair) in an all-female patient cohort. Female patients with symptomatic FAI were randomized to undergo either labral débridement or labral repair. There were clinical improvements in both groups, but, compared with labral débridement patients, labral repair patients had more significantly improved Hip Outcome Scores in the domains of Activities of Daily Living and Sports, as well as better subjective outcomes. Although the study did not compare female patients with male patients, it does provide evidence that female patients specifically may benefit more from labral repair than from labral débridement alone.
With respect to different surgical treatments for male and female patients, Hetsroni and colleagues7 introduced the idea of sex-specific treatment when they noted more hip anteversion in their study’s female patients than in its male patients. They suggested that, because the anterosuperior acetabulum is subjected to a high amount of stress during weight-bearing and gait, this area in females with suspected pincer lesions should be rim-trimmed judiciously to avoid increasing the stress and perhaps even hastening the development of degenerative disease. Last, though several authors have noted that hip function scores are lower in females than in males on presentation, it has also been reported that females demonstrate more improvement in functional scores after surgery.9 This may be important information to discuss during preoperative counseling about expected goals and outcomes.
Conclusion
Femoroacetabular impingement is a common clinical entity that affects both males and females. However, sexual dimorphism in FAI incidence, presentation, treatment, and outcomes has recently been described in the literature (Table). Being aware of these sex-based differences and tailoring patient evaluation and management accordingly will likely result in optimal outcomes for each person who presents with symptomatic FAI.
Femoroacetabular impingement (FAI), a recently described hip condition in adolescents and young adults, results from abnormal physical contact between the proximal femur and the acetabulum.1 FAI is usually characterized by the site of the predominant morphologic abnormality—proximal femur (cam-type FAI), acetabulum (pincer-type FAI), or both (mixed impingement). Cam-type FAI is typified by the aspherical extension of the articular surface at the anterosuperior head–neck junction of the proximal femur with loss of the normal offset. With hip motion, especially in the maximal ranges of flexion and internal rotation, the aspherical proximal femur repeatedly contacts the anterosuperior acetabulum, damaging the chondrolabral junction and ultimately the labrum itself. In pincer-type impingement, femoral head overcoverage caused by acetabular retroversion and/or coxa profunda directly damages the anterior labrum when the acetabular rim contacts the proximal femur during physiologic motion. “Contrecoup” injury of the posterior-inferior acetabular cartilage may also occur. Over time, recurrent microtrauma to the acetabular cartilage and/or labrum may lead to degenerative changes of the hip and ultimately to premature osteoarthritis.1,2
Patients with FAI typically present with groin pain that may be activity-related or that may occur with prolonged sitting with the hip in a flexed position. Physical examination findings suggestive of FAI include decreased passive internal hip rotation and reproducible pain with adduction and internal rotation of the flexed hip—the impingement sign, or the flexion, adduction, and internal rotation (FADIR) test.3 Diagnostic imaging evaluation initially includes radiographs of the pelvis and hips. These radiographs may show a “pistol-grip” deformity and/or decreased head–neck offset (as determined by increased alpha angle) in the setting of cam-type impingement (Figure 1).4 Pincer-type impingement may be associated with a crossover sign, coxa profunda, and an increased center-edge angle (CEA). Advanced imaging studies, such as computed tomography (CT), magnetic resonance imaging (MRI) arthrogram, and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), are commonly used to better delineate bony deformity and concomitant injuries of the labrum and cartilage (Figure 2).
Treatment for FAI often consists initially of activity modification, use of anti-inflammatory medications, and physical therapy. Intra-articular corticosteroid injections may be used both diagnostically and therapeutically. When nonsurgical measures fail to adequately relieve symptoms, surgery may be warranted. Whether performed open or arthroscopically, surgery is directed first at correcting the underlying osseous abnormality—performing an osteoplasty of the proximal femur to remove the cam lesion, performing an acetabular osteoplasty (“rim-trimming”) to address a focal pincer lesion, and/or performing a periacetabular osteotomy to decrease global acetabular overcoverage (Figure 3).5
Sex-Based Differences in FAI Incidence
Traditionally, it was thought that cam-type impingement occurred predominantly in young, athletic males, whereas pincer-type impingement resulting from acetabular overcoverage occurred primarily in females during their fourth decade. However, our understanding of the sex-based differences in the incidence and presentation of FAI has evolved, and it is now clear that the interplay of sex, radiographic signs of impingement, and development of symptoms requiring treatment is more complex.
In recent large population-based studies, investigators have attempted to better characterize the sex-based differences in the incidence of osseous FAI deformity. Gosvig and colleagues2 examined radiographic and questionnaire outcomes of 3620 patients (age range, 21-90 years) and found that males were more likely than females to have a pistol-grip deformity of the hip (19.6% vs 5.2%); that deep acetabular sockets were common in both sexes (15.2% vs 19.4%); and that the presence of pistol-grip deformity or deep socket was significantly associated with development of osteoarthritis, independent of sex.
In a study of 2081 asymptomatic patients (mean age, 18.6 years), Laborie and colleagues4 reported similar radiographic findings. Males were significantly more likely than females to have a cam-type deformity, as evidenced by pistol-grip deformity, focal prominence of the femoral neck, and/or flattening of the lateral aspect of the femoral head. Males were also more likely than females to have a pincer deformity, though radiographic signs of pincer deformity—a crossover sign, excessive acetabular coverage (defined by increased CEA), and a posterior wall sign—were common in both sexes, occurring in 16.6% of females and 34.3% of males. Bilateral findings of FAI-associated deformity were also more common in males than in females, both for cam-type deformity (24.7% vs 6.3%) and pincer-type deformity (21.7% vs 9.7%).
Sex-Based Differences in FAI Presentation
In males and females, the clinical presentation of FAI is similar—insidious onset of deep groin pain, often exacerbated with activity, and physical examination findings of decreased hip motion (particularly internal rotation) and a positive impingement test.3 Nevertheless, the sexes’ clinical presentation differs in several ways. Specifically, in a study using 3-dimensional CT to assess bony deformity in both symptomatic and asymptomatic patients, Beaulé and colleagues6 reported that alpha angles were significantly higher in symptomatic males than in symptomatic females (73.3° vs 58.7°). Hetsroni and colleagues7 recently reported similar results in a study of 217 symptomatic young adults treated arthroscopically for hip pain. Preoperative CT showed that alpha angles were significantly larger in males than in females (63.6° vs 47.8°). The authors postulated that females may be more likely to be symptomatic in the setting of smaller cam lesions because of the increased peak hip flexion and frontal plane motion commonly demonstrated by females during drop landings in sport. The authors further hypothesized that sex differences in muscle mass (which contributes to dynamic hip stability) and ligamentous laxity (a component of static hip stability) may result in larger physiologic ranges of motion for many females. As a result, bony impingement may occur in the setting of smaller anatomical lesions in females. The authors further noted that, compared with their male counterparts, females being treated for symptomatic FAI had significantly more femoral and acetabular anteversion.
Another male–female presentation difference involves symptom bilaterality. Specifically, males are significantly more likely than females to have symptomatic FAI involving both hips. In a recent study of 646 patients who underwent hip arthroscopy for symptomatic FAI during a 2-year period, Klingenstein and colleagues8 found that females constituted 48.2% of unilateral arthroscopy patients but only 34.8% of bilateral arthroscopy patients. The odds ratio of males treated for both hips, compared with females, was 1.7 (95% confidence interval, 1.16–2.54).
Last, it has been reported that, on clinical presentation, hip function scores are significantly lower in females than in males. In a recent study of 612 cases of symptomatic FAI treated with hip arthroscopy, Malviya and colleagues9 found that females had significantly lower quality-of-life scores both before and after surgery. Hetsroni and colleagues7 reported similar findings, with females having significantly lower preoperative modified Harris Hip Scores and lower Hip Outcome Scores in the domains of Activities of Daily Living and Sports.
Sex-Based Differences in FAI Treatment
and Outcomes
Surgical treatment of FAI is focused on identifying the source of hip pain and dysfunction—be it osseous lesion, labral tearing, chondral injury, or iliopsoas tendonitis—and treating it accordingly, regardless of sex. Most studies of this approach find consistent improvement in the short-term and midterm outcome scores for a majority of patients. However, relatively few studies have focused specifically on sex in determining the percentage of patients who require surgical treatment, in deciding the type of surgery that should be performed, or in measuring surgical outcomes in patients with symptomatic FAI.
In their review of 23 studies of FAI surgery, Ng and colleagues10 found that, of 970 patients, 608 (62.7%) were male and 362 (37.3%) were female. Similarly higher rates for males were previously published.5,11 More recently, Clohisy and colleagues12 reported on the descriptive epidemiology of patients having surgery for FAI at 8 different medical centers in North America. Fifty-five percent of the hips surgically treated for symptomatic FAI were females’. The authors speculated that this unexpectedly high rate could have resulted from US and Canadian female athletes’ increasingly higher level of sports participation. The results of this study, one of the largest examining the rate of surgery for males and females with FAI, suggest that females are more likely to have surgery for symptomatic FAI despite being less likely to have radiographic evidence of impingement. Our understanding of this phenomenon continues to advance.
In a recent prospective study, Krych and colleagues13 evaluated the clinical outcomes of FAI surgeries (labral débridement, labral repair) in an all-female patient cohort. Female patients with symptomatic FAI were randomized to undergo either labral débridement or labral repair. There were clinical improvements in both groups, but, compared with labral débridement patients, labral repair patients had more significantly improved Hip Outcome Scores in the domains of Activities of Daily Living and Sports, as well as better subjective outcomes. Although the study did not compare female patients with male patients, it does provide evidence that female patients specifically may benefit more from labral repair than from labral débridement alone.
With respect to different surgical treatments for male and female patients, Hetsroni and colleagues7 introduced the idea of sex-specific treatment when they noted more hip anteversion in their study’s female patients than in its male patients. They suggested that, because the anterosuperior acetabulum is subjected to a high amount of stress during weight-bearing and gait, this area in females with suspected pincer lesions should be rim-trimmed judiciously to avoid increasing the stress and perhaps even hastening the development of degenerative disease. Last, though several authors have noted that hip function scores are lower in females than in males on presentation, it has also been reported that females demonstrate more improvement in functional scores after surgery.9 This may be important information to discuss during preoperative counseling about expected goals and outcomes.
Conclusion
Femoroacetabular impingement is a common clinical entity that affects both males and females. However, sexual dimorphism in FAI incidence, presentation, treatment, and outcomes has recently been described in the literature (Table). Being aware of these sex-based differences and tailoring patient evaluation and management accordingly will likely result in optimal outcomes for each person who presents with symptomatic FAI.
1. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop. 2003;(417):112-120.
2. Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162-1169.
3. Philippon MJ, Maxwell RB, Johnston TL, Schenker M, Briggs KK. Clinical presentation of femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):1041-1047.
4. Laborie LB, Lehmann TG, Engesaeter IO, Eastwood DM, Engesaeter LB, Rosendahl K. Prevalence of radiographic findings thought to be associated with femoroacetabular impingement in a population-based cohort of 2081 healthy young adults. Radiology. 2011;260(2):494-502.
5. Clohisy JC, St John LC, Schutz AL. Surgical treatment of femoroacetabular impingement: a systematic review of the literature. Clin Orthop. 2010;468(2):555-564.
6. Beaulé PE, Zaragoza E, Motamedi K, Copelan N, Dorey FJ. Three-dimensional computed tomography of the hip in the assessment of femoroacetabular impingement. J Orthop Res. 2005;23(6):1286-1292.
7. Hetsroni I, Dela Torre K, Duke G, Lyman S, Kelly BT. Sex differences of hip morphology in young adults with hip pain and labral tears. Arthroscopy. 2013;29(1):54-63.
8. Klingenstein GG, Zbeda RM, Bedi A, Magennis E, Kelly BT. Prevalence and preoperative demographic and radiographic predictors of bilateral femoroacetabular impingement. Am J Sports Med. 2013;41(4):762-768.
9. Malviya A, Stafford GH, Villar RN. Impact of arthroscopy of the hip for femoroacetabular impingement on quality of life at a mean follow-up of 3.2 years. J Bone Joint Surg Br. 2012;94(4):466-470.
10. Ng VY, Arora N, Best TM, Pan X, Ellis TJ. Efficacy of surgery for femoroacetabular impingement: a systematic review. Am J Sports Med. 2010;38(11):2337-2345.
11. Matsuda DK, Carlisle JC, Arthurs SC, Wierks CH, Philippon MJ. Comparative systematic review of the open dislocation, mini-open, and arthroscopic surgeries for femoroacetabular impingement. Arthroscopy. 2011;27(2):252-269.
12. Clohisy JC, Baca G, Beaule PE, et al. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
13. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
1. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop. 2003;(417):112-120.
2. Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162-1169.
3. Philippon MJ, Maxwell RB, Johnston TL, Schenker M, Briggs KK. Clinical presentation of femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):1041-1047.
4. Laborie LB, Lehmann TG, Engesaeter IO, Eastwood DM, Engesaeter LB, Rosendahl K. Prevalence of radiographic findings thought to be associated with femoroacetabular impingement in a population-based cohort of 2081 healthy young adults. Radiology. 2011;260(2):494-502.
5. Clohisy JC, St John LC, Schutz AL. Surgical treatment of femoroacetabular impingement: a systematic review of the literature. Clin Orthop. 2010;468(2):555-564.
6. Beaulé PE, Zaragoza E, Motamedi K, Copelan N, Dorey FJ. Three-dimensional computed tomography of the hip in the assessment of femoroacetabular impingement. J Orthop Res. 2005;23(6):1286-1292.
7. Hetsroni I, Dela Torre K, Duke G, Lyman S, Kelly BT. Sex differences of hip morphology in young adults with hip pain and labral tears. Arthroscopy. 2013;29(1):54-63.
8. Klingenstein GG, Zbeda RM, Bedi A, Magennis E, Kelly BT. Prevalence and preoperative demographic and radiographic predictors of bilateral femoroacetabular impingement. Am J Sports Med. 2013;41(4):762-768.
9. Malviya A, Stafford GH, Villar RN. Impact of arthroscopy of the hip for femoroacetabular impingement on quality of life at a mean follow-up of 3.2 years. J Bone Joint Surg Br. 2012;94(4):466-470.
10. Ng VY, Arora N, Best TM, Pan X, Ellis TJ. Efficacy of surgery for femoroacetabular impingement: a systematic review. Am J Sports Med. 2010;38(11):2337-2345.
11. Matsuda DK, Carlisle JC, Arthurs SC, Wierks CH, Philippon MJ. Comparative systematic review of the open dislocation, mini-open, and arthroscopic surgeries for femoroacetabular impingement. Arthroscopy. 2011;27(2):252-269.
12. Clohisy JC, Baca G, Beaule PE, et al. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
13. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
Greater Auricular Nerve Palsy After Arthroscopic Anterior-Inferior and Posterior-Inferior Labral Tear Repair Using Beach-Chair Positioning and a Standard Universal Headrest
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
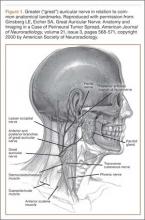
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
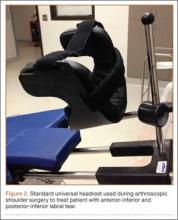
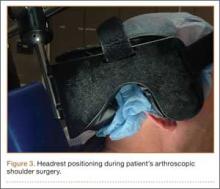
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
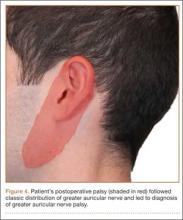
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
Managing gymnasts’ wrist and back overuse injuries
SNOWMASS, COLO. – Overuse injuries in young gymnasts are extremely common, and they cluster at two vulnerable anatomic sites: the wrist and lower back.
“Gymnasts have to be one of the biggest parts of my sports medicine practice. Some of them are the toughest patients I see in terms of their workouts, consistency, and dedication to their sport. And they’ll work through the pain. Gymnastics is truly a sport where it’s been driven into them that no pain is no gain,” Dr. M. Timothy Hresko observed at the Winter Rheumaltogy Symposium sponsored by the American College of Rheumatology.
With a patient mindset like this, it’s little wonder that physicians have their work cut out for them when trying to help gymnasts heal and prevent future setbacks. Gymnasts are loathe to sit back and rest. Yet rest is central to successful treatment of overuse injuries. And therein lies the basis for a frequent locking of horns, added Dr. Hresko, an orthopedic surgeon at Harvard Medical School, Boston, and Boston Children’s Hospital.
“Quite often you need to reinforce to them the rest that’s necessary for this to work,” he said. “Young gymnasts will spend 20 hours a week in the gym: 4 hours, 5 days a week. We see a lot of overuse injuries associated with that. Is that pushing somebody too much? For skill sports, that’s what’s required if you want to develop the skill.”
Gymnast’s wrist: This is a stress fracture through the radial epiphyseal plate. It results from the tremendous weight-bearing loads imposed on the arms and wrists during many hours of repeated activities including tumbling, swinging, mounting, and vaulting.
Affected patients present with a complaint of pain with weight bearing on the wrist. Physical examination reveals tenderness over the distal radial growth plate and loss of range of motion in the wrist. An x-ray will show widening of the radial growth plate and perhaps irregular bone edges.
Treatment entails cessation of all impact activities for 2-3 months to permit the stress fracture to heal, along with wearing a wrist splint for protection during normal daily activities. The patient can return to gymnastics after that rest period, provided the distal radial physis is no longer tender. The return to the sport should be gradual, with resumption of vaulting reserved for last.
“Vaulting is probably the hardest activity to do and to return to,” according to Dr. Hresko.
Spondylolysis: this is a stress fracture of a lumbar vertebra, which, in gymnasts, results from repeated hyperextension. It’s the most common cause of low back pain in adolescent athletes.
“I must see 6-10 patients per week who have some phase of spondylolysis,” the surgeon said.
The diagnostic hallmark on physical examination is lumbar back pain that worsens with lumbar extension. A straight leg raise test is often positive. The stress fracture will show up on a plain film x-ray but therein lies a quandary.
“In a normal population of adolescents without symptoms, 6% will have evidence of spondylolysis on plain film x-ray. So if you see a positive x-ray in a patient with low back pain you have to ask yourself if this is really the cause of their pain,” Dr. Hresko said.
Herniated discs are “pretty rare” in youth, but the clues are the same as in adults: radicular symptoms, burning pain, and a positive straight leg raise test, he noted.
The treatment for spondylolysis is restricted activities, usually for 2-3 months or one season.
“I personally think it’s best to immobilize them with a brace. We use a custom-made, anterior-opening Boston overlap brace to limit extension. That way they can go back to activities sooner, but they’re usually out of their sport for one season, then we try to get them back for their second season, knowing that the injury isn’t going to heal anatomically,” the sports medicine specialist said.
Gymnasts who are hyperlordotic are at increased risk for spondylolysis. For injury prevention in such individuals, he emphasizes abdominal strengthening and pelvic tilting exercises to reduce the lumbar lordosis.
“We haven’t gone quite so far as to use anatomic body markers like lordosis to suggest somebody’s at such high risk that they should not go out for that sport – sort of an East German approach to athletics – but I do think that in gymnastics there are some patients whose bodies are just not up to the stress involved in that sport,” he concluded.
Dr. Hresko serves as a consultant to DePuy Spine.
SNOWMASS, COLO. – Overuse injuries in young gymnasts are extremely common, and they cluster at two vulnerable anatomic sites: the wrist and lower back.
“Gymnasts have to be one of the biggest parts of my sports medicine practice. Some of them are the toughest patients I see in terms of their workouts, consistency, and dedication to their sport. And they’ll work through the pain. Gymnastics is truly a sport where it’s been driven into them that no pain is no gain,” Dr. M. Timothy Hresko observed at the Winter Rheumaltogy Symposium sponsored by the American College of Rheumatology.
With a patient mindset like this, it’s little wonder that physicians have their work cut out for them when trying to help gymnasts heal and prevent future setbacks. Gymnasts are loathe to sit back and rest. Yet rest is central to successful treatment of overuse injuries. And therein lies the basis for a frequent locking of horns, added Dr. Hresko, an orthopedic surgeon at Harvard Medical School, Boston, and Boston Children’s Hospital.
“Quite often you need to reinforce to them the rest that’s necessary for this to work,” he said. “Young gymnasts will spend 20 hours a week in the gym: 4 hours, 5 days a week. We see a lot of overuse injuries associated with that. Is that pushing somebody too much? For skill sports, that’s what’s required if you want to develop the skill.”
Gymnast’s wrist: This is a stress fracture through the radial epiphyseal plate. It results from the tremendous weight-bearing loads imposed on the arms and wrists during many hours of repeated activities including tumbling, swinging, mounting, and vaulting.
Affected patients present with a complaint of pain with weight bearing on the wrist. Physical examination reveals tenderness over the distal radial growth plate and loss of range of motion in the wrist. An x-ray will show widening of the radial growth plate and perhaps irregular bone edges.
Treatment entails cessation of all impact activities for 2-3 months to permit the stress fracture to heal, along with wearing a wrist splint for protection during normal daily activities. The patient can return to gymnastics after that rest period, provided the distal radial physis is no longer tender. The return to the sport should be gradual, with resumption of vaulting reserved for last.
“Vaulting is probably the hardest activity to do and to return to,” according to Dr. Hresko.
Spondylolysis: this is a stress fracture of a lumbar vertebra, which, in gymnasts, results from repeated hyperextension. It’s the most common cause of low back pain in adolescent athletes.
“I must see 6-10 patients per week who have some phase of spondylolysis,” the surgeon said.
The diagnostic hallmark on physical examination is lumbar back pain that worsens with lumbar extension. A straight leg raise test is often positive. The stress fracture will show up on a plain film x-ray but therein lies a quandary.
“In a normal population of adolescents without symptoms, 6% will have evidence of spondylolysis on plain film x-ray. So if you see a positive x-ray in a patient with low back pain you have to ask yourself if this is really the cause of their pain,” Dr. Hresko said.
Herniated discs are “pretty rare” in youth, but the clues are the same as in adults: radicular symptoms, burning pain, and a positive straight leg raise test, he noted.
The treatment for spondylolysis is restricted activities, usually for 2-3 months or one season.
“I personally think it’s best to immobilize them with a brace. We use a custom-made, anterior-opening Boston overlap brace to limit extension. That way they can go back to activities sooner, but they’re usually out of their sport for one season, then we try to get them back for their second season, knowing that the injury isn’t going to heal anatomically,” the sports medicine specialist said.
Gymnasts who are hyperlordotic are at increased risk for spondylolysis. For injury prevention in such individuals, he emphasizes abdominal strengthening and pelvic tilting exercises to reduce the lumbar lordosis.
“We haven’t gone quite so far as to use anatomic body markers like lordosis to suggest somebody’s at such high risk that they should not go out for that sport – sort of an East German approach to athletics – but I do think that in gymnastics there are some patients whose bodies are just not up to the stress involved in that sport,” he concluded.
Dr. Hresko serves as a consultant to DePuy Spine.
SNOWMASS, COLO. – Overuse injuries in young gymnasts are extremely common, and they cluster at two vulnerable anatomic sites: the wrist and lower back.
“Gymnasts have to be one of the biggest parts of my sports medicine practice. Some of them are the toughest patients I see in terms of their workouts, consistency, and dedication to their sport. And they’ll work through the pain. Gymnastics is truly a sport where it’s been driven into them that no pain is no gain,” Dr. M. Timothy Hresko observed at the Winter Rheumaltogy Symposium sponsored by the American College of Rheumatology.
With a patient mindset like this, it’s little wonder that physicians have their work cut out for them when trying to help gymnasts heal and prevent future setbacks. Gymnasts are loathe to sit back and rest. Yet rest is central to successful treatment of overuse injuries. And therein lies the basis for a frequent locking of horns, added Dr. Hresko, an orthopedic surgeon at Harvard Medical School, Boston, and Boston Children’s Hospital.
“Quite often you need to reinforce to them the rest that’s necessary for this to work,” he said. “Young gymnasts will spend 20 hours a week in the gym: 4 hours, 5 days a week. We see a lot of overuse injuries associated with that. Is that pushing somebody too much? For skill sports, that’s what’s required if you want to develop the skill.”
Gymnast’s wrist: This is a stress fracture through the radial epiphyseal plate. It results from the tremendous weight-bearing loads imposed on the arms and wrists during many hours of repeated activities including tumbling, swinging, mounting, and vaulting.
Affected patients present with a complaint of pain with weight bearing on the wrist. Physical examination reveals tenderness over the distal radial growth plate and loss of range of motion in the wrist. An x-ray will show widening of the radial growth plate and perhaps irregular bone edges.
Treatment entails cessation of all impact activities for 2-3 months to permit the stress fracture to heal, along with wearing a wrist splint for protection during normal daily activities. The patient can return to gymnastics after that rest period, provided the distal radial physis is no longer tender. The return to the sport should be gradual, with resumption of vaulting reserved for last.
“Vaulting is probably the hardest activity to do and to return to,” according to Dr. Hresko.
Spondylolysis: this is a stress fracture of a lumbar vertebra, which, in gymnasts, results from repeated hyperextension. It’s the most common cause of low back pain in adolescent athletes.
“I must see 6-10 patients per week who have some phase of spondylolysis,” the surgeon said.
The diagnostic hallmark on physical examination is lumbar back pain that worsens with lumbar extension. A straight leg raise test is often positive. The stress fracture will show up on a plain film x-ray but therein lies a quandary.
“In a normal population of adolescents without symptoms, 6% will have evidence of spondylolysis on plain film x-ray. So if you see a positive x-ray in a patient with low back pain you have to ask yourself if this is really the cause of their pain,” Dr. Hresko said.
Herniated discs are “pretty rare” in youth, but the clues are the same as in adults: radicular symptoms, burning pain, and a positive straight leg raise test, he noted.
The treatment for spondylolysis is restricted activities, usually for 2-3 months or one season.
“I personally think it’s best to immobilize them with a brace. We use a custom-made, anterior-opening Boston overlap brace to limit extension. That way they can go back to activities sooner, but they’re usually out of their sport for one season, then we try to get them back for their second season, knowing that the injury isn’t going to heal anatomically,” the sports medicine specialist said.
Gymnasts who are hyperlordotic are at increased risk for spondylolysis. For injury prevention in such individuals, he emphasizes abdominal strengthening and pelvic tilting exercises to reduce the lumbar lordosis.
“We haven’t gone quite so far as to use anatomic body markers like lordosis to suggest somebody’s at such high risk that they should not go out for that sport – sort of an East German approach to athletics – but I do think that in gymnastics there are some patients whose bodies are just not up to the stress involved in that sport,” he concluded.
Dr. Hresko serves as a consultant to DePuy Spine.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Sports Activity After Reverse Total Shoulder Arthroplasty With Minimum 2-Year Follow-Up
The treatment of patients with severe shoulder pain and disability combined with a nonfunctional rotator cuff was a clinical challenge until the development of the reverse total shoulder arthroplasty (RTSA).1-3 Massive rotator cuff tears can leave patients with a pseudoparalytic upper extremity and may result in advanced arthritis of the joint because of altered mechanical and nutritional factors.4 In this setting, simply replacing the arthritic joint with standard total shoulder arthroplasty (TSA) is not recommended because it does not address the functional deficits, and it has poor long-term outcomes.3,5 RTSA works by changing the center of rotation of the shoulder joint so that the deltoid muscle can be used to elevate the arm.6,7 The 4 rotator cuff muscles are not required for forward elevation or stability of this constrained implant.6,8
Current indications for RTSA are cuff tear arthropathy, complex proximal humerus fractures, and revision from hemiarthroplasty or TSA with rotator cuff dysfunction. Patients with advanced cuff tear arthropathy have minimal forward elevation and pseudoparalysis. Previous studies have shown mean preoperative forward flexion of 55º and mean ASES (American Shoulder and Elbow Surgeons) Standardized Shoulder Assessment Form score of 34.3.9 Thus, minimal overhead activity is possible without RTSA. Advances in the RTSA technique have led to promising results (excellent functional improvement), but there is limited information regarding the activity levels patients can achieve after surgery.7,9-11
We conducted a study of the types of sporting activities in which patients with RTSA could participate. We hypothesized that, relative to historic controls, patients with RTSA could return to low-intensity sporting activities with improvement in motion and ASES scores.
Materials and Methods
After this study received institutional review board approval, patients who had undergone RTSA at our institution between January 1, 2004 and December 31, 2010 were identified by the billing codes used for the procedure. Each patient who had RTSA performed during the study period was included in the study. Charts were then reviewed to extract demographic data, preoperative diagnosis, surgery date, operative side, dominant side, type of implant used, operative complications, and subsequent revisions. A questionnaire (Appendix) was designed and used to assess activity, functional status, pain, and satisfaction levels after RTSA. Patients had to be willing and able to complete this questionnaire in order to be included in the study.
The questionnaire included demographic questions; a list of 42 activities patients could choose from to describe their current activity level, activities they were able to perform before the surgery, and activities they wish they could perform; a list of reasons for any limitations; and questions about overall pain, strength, and satisfaction with the procedure. In addition, there was an open-ended question for activities that may not have been listed. The questionnaire also included a validated method for assessing shoulder range of motion (ROM) at home, where patients rated their overhead motion according to standardized physical landmarks, including the level of the shoulder, chin, eyebrows, top of head, and above head.12-14 Also provided was the ASES Standardized Shoulder Assessment Form, which features a 100-point visual analog scale for pain plus functional ability questions, with higher scores indicating less pain and better function.15,16 The minimal clinical significance in the ASES score is 6.4 points.17,18 Scores were recorded and analyzed. Student t test was used to calculate statistical differences between patients who had primary RTSA performed and patients who underwent revision RTSA.
Study personnel contacted patients by telephone and direct mailing. Patients who could not be reached initially were called at least 4 more times: twice during the weekday, once during the evening, and once on the weekend. Patients who could not be contacted by telephone were then cross-referenced with the Social Security database to see if any were deceased. Response data were tabulated, and patients were stratified into high-, moderate-, and low-intensity activity.
One of the 3 senior authors (Dr. Ahmad, Dr. Bigliani, Dr. Levine) performed the 95 RTSAs: 84 Zimmer (Warsaw, Indiana), 7 DePuy (Warsaw, Indiana), 4 Tornier (Minneapolis, Minnesota). The DePuy and Tornier implants were used when a 30-mm glenoid peg was required (before Zimmer offered this length in its system). The procedure was done with a deltopectoral approach with 20° of retroversion. In revision cases, the same approach was used, the hardware or implants were removed, and the position of the humeral component was determined based on the pectoralis major insertion and the deltoid tension. In 80% of cases, the subscapularis was not repaired; in the other 20%, it was. Whether it was repaired depended on tendon viability and surgeon preference, as subscapularis repair status has been shown not to affect functional outcome.19-21 No combined latissimus transfers were performed. Patients wore a sling the first 4 weeks after surgery (only wrist and elbow motion allowed) and then advanced to active shoulder ROM. Eight weeks after surgery, they began gentle shoulder strengthening.
Results
One hundred nine consecutive patients underwent RTSA at a single institution. Fifteen patients subsequently died, 14 could not be contacted, and 2 declined, leaving 78 patients available for clinical follow-up. Mean follow-up was 4.8 years (range 2-9 years). Mean (SD) age at surgery was 75.3 (7.5) years. Seventy-five percent of the patients were women. Sixty-one percent underwent surgery for cuff tear arthropathy, 31% for revision of previous arthroplasty or internal fixation, 7% for complex fractures, and 1% for tumor. Of the 24 revisions, 15 were for failed hemiarthroplasty, 3 were for failed TSA with rotator cuff dysfunction, 4 were for fracture with failed internal fixation, and 2 were for failed RTSA referred from other institutions. The dominant shoulder was involved 62% of the time. Preoperative active forward shoulder elevation was less than 90° in all patients. There were 10 complications: 2 dislocations that were closed-reduced and remained stable, 1 dislocation that required revision of the liner, 1 aseptic loosening in a patient who has declined revision, 2 dissociated glenosphere baseplates, 2 deep infections that required 2-stage exchanges, 1 deep infection that required a 2-stage exchange that was then complicated by dissociation of the glenosphere baseplate requiring revision, and 1 superficial infection that resolved with oral antibiotics.
After surgery, mean active forward elevation was 140°, mean active external rotation was 48°, and mean active internal rotation was to S1. Mean (SD) postoperative ASES score was 77.5 (23.4). Satisfaction level was high (mean, 8.3/10), and mean pain levels were low: 2.3 out of 10 on the visual analog scale and 44.0 (SD, 11.7) on the ASES pain component. Strength was rated a mean of good. Table 1 lists the clinical data for the primary and revision surgery patients.
Eighteen patients (23.1%) returned to 24 different high-intensity activities, such as hunting, golf, and skiing; 38 patients (48.7%) returned to moderate-intensity activities, such as swimming, bowling, and raking leaves; and 22 patients (28.2%) returned to low-intensity activities, such as riding a stationary bike, playing a musical instrument, and walking (Table 2). Four patients played golf before and after RTSA, but neither of the 2 patients who played tennis before RTSA were able to do so after. Patients reported they engaged in their favorite leisure activity a mean of 4.8 times per week and a mean of 1.5 hours each time.
A medical problem was cited by 58% of patients as the reason for limited activity. These patients reported physical decline resulting from cardiac disease, diabetes, asthma/chronic obstructive pulmonary disease, or arthritis in other joints. Reasons for activity limitation are listed in Table 3. Post-RTSA activities that patients could not do for any reason are listed in Table 4. Activity limitations that patients attributed to the RTSA are listed in Table 5.
The majority of patients (57.7%) reported no change, from before RTSA to after RTSA, in being unable to do certain desired activities (eg, softball, target shooting, horseback riding, running, traveling). Sixteen patients (20.8%) reported being unable to return to an activity (eg, tennis, swimming, baseball, kayaking) they had been able to do before surgery. Most (69%) of those patients reported being unable to return to a moderate- or high-intensity activity after RTSA, but 81.8% were able to return to different moderate- or high-intensity activities.
Revision patients, who reported lower overhead activity levels, constituted 73% of the patients who felt their shoulder mechanically limited their activity, despite the fact that revisions constituted only 25% of the cases overall. Mean active ROM was statistically lower for revision patients than for primary patients (P < .05). Mean ASES score was statistically lower for the revision group (P < .001) and represented a clinically significant difference. Mean pain level was low (3.3) and satisfaction still generally high (7.4), but pain, satisfaction, and strength were about 1 point worse on average in the revision group than in the primary group.
Discussion
In the United States and other countries, RTSA implant survivorship is good.9,22 In this article, we have reported on post-RTSA activity levels, on the significant impact of comorbidities on this group, and on the negative effect of revisions on postoperative activity. Patients in this population reported that concomitant medical problems were the most important factor limiting their post-RTSA activity levels. Understanding and interpreting quality-of-life or functional scores in this elderly group must take into account the impact of comorbidities.23
Patients should have realistic postoperative expectations.24 In this study, some patients engaged in high-intensity overhead activities, such as golf, chopping wood, and shooting. However, the most difficulty was encountered trying to return to activities (eg, tennis, kayaking, archery, combing hair) that required external rotation in abduction.
Patients who had a previous implant (eg, hemiarthroplasty, TSA, failed internal fixation) revised to RTSA had lower activity levels and were 9 times more likely than primary patients to report having a mechanical shoulder limitation affecting their activity. Revision patients also had worse forward elevation, external rotation, pain, and satisfaction.
This study is limited in that it is retrospective. Subsequent prospective studies focused on younger patients who undergo primary RTSA may be useful if indications expand. In addition, subscapularis status and especially infraspinatus status may affect activity levels and could be analyzed in a study. Another limitation is that we did not specifically record detailed preoperative data, though all patients were known to have preoperative forward elevation of less than 90°.
In general, the primary measure of success for RTSA has been pain relief. Some studies have also reported on strength and ROM.2,20,25,26 A recent study using similar methodology demonstrated comparable ROM and low pain after RTSA, though revisions were not included in that study.26 In contrast to the present study, no patient in that study was able to play tennis or golf, but the reasons for the limited activity were not explored. In both studies, post-RTSA sports were generally of lower intensity than those played by patients after anatomical TSA.27
Overall, the majority of patients were very satisfied with their low pain level after RTSA. In addition, many patients not limited by other medical conditions were able to return to their pre-RTSA moderate-intensity recreational activities.
1. Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases [in French]. Acta Orthop Belg. 1995;61(suppl 1):112-119.
2. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
3. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
4. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
5. Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2240-2248.
6. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
7. Nam D, Kepler CK, Neviaser AS, et al. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am. 2010;92(suppl 2):23-35.
8. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221-1230.
9. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
10. Cazeneuve JF, Cristofari DJ. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res. 2011;97(6):583-589.
11. Gerber C, Pennington, SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-295.
12. Brophy RH, Beauvais RL, Jones EC, Cordasco FA, Marx RG. Measurement of shoulder activity level. Clin Orthop. 2005;(439):101-108.
13. Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88(3):508-513.
14. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280.
15. Kocher, MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
16. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
17. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
18. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
19. Molé D, Favard L. Excentered scapulohumeral osteoarthritis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 suppl):37-94.
20. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
21. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25(2):129-133.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Antuña SA, Sperling JW, Sánchez-Sotelo J, Cofield RH. Shoulder arthroplasty for proximal humeral nonunions. J Shoulder Elbow Surg. 2002;11(2):114-121.
24. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
25. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop. 2011;469(9):2476-2482.
26. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
27. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.
The treatment of patients with severe shoulder pain and disability combined with a nonfunctional rotator cuff was a clinical challenge until the development of the reverse total shoulder arthroplasty (RTSA).1-3 Massive rotator cuff tears can leave patients with a pseudoparalytic upper extremity and may result in advanced arthritis of the joint because of altered mechanical and nutritional factors.4 In this setting, simply replacing the arthritic joint with standard total shoulder arthroplasty (TSA) is not recommended because it does not address the functional deficits, and it has poor long-term outcomes.3,5 RTSA works by changing the center of rotation of the shoulder joint so that the deltoid muscle can be used to elevate the arm.6,7 The 4 rotator cuff muscles are not required for forward elevation or stability of this constrained implant.6,8
Current indications for RTSA are cuff tear arthropathy, complex proximal humerus fractures, and revision from hemiarthroplasty or TSA with rotator cuff dysfunction. Patients with advanced cuff tear arthropathy have minimal forward elevation and pseudoparalysis. Previous studies have shown mean preoperative forward flexion of 55º and mean ASES (American Shoulder and Elbow Surgeons) Standardized Shoulder Assessment Form score of 34.3.9 Thus, minimal overhead activity is possible without RTSA. Advances in the RTSA technique have led to promising results (excellent functional improvement), but there is limited information regarding the activity levels patients can achieve after surgery.7,9-11
We conducted a study of the types of sporting activities in which patients with RTSA could participate. We hypothesized that, relative to historic controls, patients with RTSA could return to low-intensity sporting activities with improvement in motion and ASES scores.
Materials and Methods
After this study received institutional review board approval, patients who had undergone RTSA at our institution between January 1, 2004 and December 31, 2010 were identified by the billing codes used for the procedure. Each patient who had RTSA performed during the study period was included in the study. Charts were then reviewed to extract demographic data, preoperative diagnosis, surgery date, operative side, dominant side, type of implant used, operative complications, and subsequent revisions. A questionnaire (Appendix) was designed and used to assess activity, functional status, pain, and satisfaction levels after RTSA. Patients had to be willing and able to complete this questionnaire in order to be included in the study.
The questionnaire included demographic questions; a list of 42 activities patients could choose from to describe their current activity level, activities they were able to perform before the surgery, and activities they wish they could perform; a list of reasons for any limitations; and questions about overall pain, strength, and satisfaction with the procedure. In addition, there was an open-ended question for activities that may not have been listed. The questionnaire also included a validated method for assessing shoulder range of motion (ROM) at home, where patients rated their overhead motion according to standardized physical landmarks, including the level of the shoulder, chin, eyebrows, top of head, and above head.12-14 Also provided was the ASES Standardized Shoulder Assessment Form, which features a 100-point visual analog scale for pain plus functional ability questions, with higher scores indicating less pain and better function.15,16 The minimal clinical significance in the ASES score is 6.4 points.17,18 Scores were recorded and analyzed. Student t test was used to calculate statistical differences between patients who had primary RTSA performed and patients who underwent revision RTSA.
Study personnel contacted patients by telephone and direct mailing. Patients who could not be reached initially were called at least 4 more times: twice during the weekday, once during the evening, and once on the weekend. Patients who could not be contacted by telephone were then cross-referenced with the Social Security database to see if any were deceased. Response data were tabulated, and patients were stratified into high-, moderate-, and low-intensity activity.
One of the 3 senior authors (Dr. Ahmad, Dr. Bigliani, Dr. Levine) performed the 95 RTSAs: 84 Zimmer (Warsaw, Indiana), 7 DePuy (Warsaw, Indiana), 4 Tornier (Minneapolis, Minnesota). The DePuy and Tornier implants were used when a 30-mm glenoid peg was required (before Zimmer offered this length in its system). The procedure was done with a deltopectoral approach with 20° of retroversion. In revision cases, the same approach was used, the hardware or implants were removed, and the position of the humeral component was determined based on the pectoralis major insertion and the deltoid tension. In 80% of cases, the subscapularis was not repaired; in the other 20%, it was. Whether it was repaired depended on tendon viability and surgeon preference, as subscapularis repair status has been shown not to affect functional outcome.19-21 No combined latissimus transfers were performed. Patients wore a sling the first 4 weeks after surgery (only wrist and elbow motion allowed) and then advanced to active shoulder ROM. Eight weeks after surgery, they began gentle shoulder strengthening.
Results
One hundred nine consecutive patients underwent RTSA at a single institution. Fifteen patients subsequently died, 14 could not be contacted, and 2 declined, leaving 78 patients available for clinical follow-up. Mean follow-up was 4.8 years (range 2-9 years). Mean (SD) age at surgery was 75.3 (7.5) years. Seventy-five percent of the patients were women. Sixty-one percent underwent surgery for cuff tear arthropathy, 31% for revision of previous arthroplasty or internal fixation, 7% for complex fractures, and 1% for tumor. Of the 24 revisions, 15 were for failed hemiarthroplasty, 3 were for failed TSA with rotator cuff dysfunction, 4 were for fracture with failed internal fixation, and 2 were for failed RTSA referred from other institutions. The dominant shoulder was involved 62% of the time. Preoperative active forward shoulder elevation was less than 90° in all patients. There were 10 complications: 2 dislocations that were closed-reduced and remained stable, 1 dislocation that required revision of the liner, 1 aseptic loosening in a patient who has declined revision, 2 dissociated glenosphere baseplates, 2 deep infections that required 2-stage exchanges, 1 deep infection that required a 2-stage exchange that was then complicated by dissociation of the glenosphere baseplate requiring revision, and 1 superficial infection that resolved with oral antibiotics.
After surgery, mean active forward elevation was 140°, mean active external rotation was 48°, and mean active internal rotation was to S1. Mean (SD) postoperative ASES score was 77.5 (23.4). Satisfaction level was high (mean, 8.3/10), and mean pain levels were low: 2.3 out of 10 on the visual analog scale and 44.0 (SD, 11.7) on the ASES pain component. Strength was rated a mean of good. Table 1 lists the clinical data for the primary and revision surgery patients.
Eighteen patients (23.1%) returned to 24 different high-intensity activities, such as hunting, golf, and skiing; 38 patients (48.7%) returned to moderate-intensity activities, such as swimming, bowling, and raking leaves; and 22 patients (28.2%) returned to low-intensity activities, such as riding a stationary bike, playing a musical instrument, and walking (Table 2). Four patients played golf before and after RTSA, but neither of the 2 patients who played tennis before RTSA were able to do so after. Patients reported they engaged in their favorite leisure activity a mean of 4.8 times per week and a mean of 1.5 hours each time.
A medical problem was cited by 58% of patients as the reason for limited activity. These patients reported physical decline resulting from cardiac disease, diabetes, asthma/chronic obstructive pulmonary disease, or arthritis in other joints. Reasons for activity limitation are listed in Table 3. Post-RTSA activities that patients could not do for any reason are listed in Table 4. Activity limitations that patients attributed to the RTSA are listed in Table 5.
The majority of patients (57.7%) reported no change, from before RTSA to after RTSA, in being unable to do certain desired activities (eg, softball, target shooting, horseback riding, running, traveling). Sixteen patients (20.8%) reported being unable to return to an activity (eg, tennis, swimming, baseball, kayaking) they had been able to do before surgery. Most (69%) of those patients reported being unable to return to a moderate- or high-intensity activity after RTSA, but 81.8% were able to return to different moderate- or high-intensity activities.
Revision patients, who reported lower overhead activity levels, constituted 73% of the patients who felt their shoulder mechanically limited their activity, despite the fact that revisions constituted only 25% of the cases overall. Mean active ROM was statistically lower for revision patients than for primary patients (P < .05). Mean ASES score was statistically lower for the revision group (P < .001) and represented a clinically significant difference. Mean pain level was low (3.3) and satisfaction still generally high (7.4), but pain, satisfaction, and strength were about 1 point worse on average in the revision group than in the primary group.
Discussion
In the United States and other countries, RTSA implant survivorship is good.9,22 In this article, we have reported on post-RTSA activity levels, on the significant impact of comorbidities on this group, and on the negative effect of revisions on postoperative activity. Patients in this population reported that concomitant medical problems were the most important factor limiting their post-RTSA activity levels. Understanding and interpreting quality-of-life or functional scores in this elderly group must take into account the impact of comorbidities.23
Patients should have realistic postoperative expectations.24 In this study, some patients engaged in high-intensity overhead activities, such as golf, chopping wood, and shooting. However, the most difficulty was encountered trying to return to activities (eg, tennis, kayaking, archery, combing hair) that required external rotation in abduction.
Patients who had a previous implant (eg, hemiarthroplasty, TSA, failed internal fixation) revised to RTSA had lower activity levels and were 9 times more likely than primary patients to report having a mechanical shoulder limitation affecting their activity. Revision patients also had worse forward elevation, external rotation, pain, and satisfaction.
This study is limited in that it is retrospective. Subsequent prospective studies focused on younger patients who undergo primary RTSA may be useful if indications expand. In addition, subscapularis status and especially infraspinatus status may affect activity levels and could be analyzed in a study. Another limitation is that we did not specifically record detailed preoperative data, though all patients were known to have preoperative forward elevation of less than 90°.
In general, the primary measure of success for RTSA has been pain relief. Some studies have also reported on strength and ROM.2,20,25,26 A recent study using similar methodology demonstrated comparable ROM and low pain after RTSA, though revisions were not included in that study.26 In contrast to the present study, no patient in that study was able to play tennis or golf, but the reasons for the limited activity were not explored. In both studies, post-RTSA sports were generally of lower intensity than those played by patients after anatomical TSA.27
Overall, the majority of patients were very satisfied with their low pain level after RTSA. In addition, many patients not limited by other medical conditions were able to return to their pre-RTSA moderate-intensity recreational activities.
The treatment of patients with severe shoulder pain and disability combined with a nonfunctional rotator cuff was a clinical challenge until the development of the reverse total shoulder arthroplasty (RTSA).1-3 Massive rotator cuff tears can leave patients with a pseudoparalytic upper extremity and may result in advanced arthritis of the joint because of altered mechanical and nutritional factors.4 In this setting, simply replacing the arthritic joint with standard total shoulder arthroplasty (TSA) is not recommended because it does not address the functional deficits, and it has poor long-term outcomes.3,5 RTSA works by changing the center of rotation of the shoulder joint so that the deltoid muscle can be used to elevate the arm.6,7 The 4 rotator cuff muscles are not required for forward elevation or stability of this constrained implant.6,8
Current indications for RTSA are cuff tear arthropathy, complex proximal humerus fractures, and revision from hemiarthroplasty or TSA with rotator cuff dysfunction. Patients with advanced cuff tear arthropathy have minimal forward elevation and pseudoparalysis. Previous studies have shown mean preoperative forward flexion of 55º and mean ASES (American Shoulder and Elbow Surgeons) Standardized Shoulder Assessment Form score of 34.3.9 Thus, minimal overhead activity is possible without RTSA. Advances in the RTSA technique have led to promising results (excellent functional improvement), but there is limited information regarding the activity levels patients can achieve after surgery.7,9-11
We conducted a study of the types of sporting activities in which patients with RTSA could participate. We hypothesized that, relative to historic controls, patients with RTSA could return to low-intensity sporting activities with improvement in motion and ASES scores.
Materials and Methods
After this study received institutional review board approval, patients who had undergone RTSA at our institution between January 1, 2004 and December 31, 2010 were identified by the billing codes used for the procedure. Each patient who had RTSA performed during the study period was included in the study. Charts were then reviewed to extract demographic data, preoperative diagnosis, surgery date, operative side, dominant side, type of implant used, operative complications, and subsequent revisions. A questionnaire (Appendix) was designed and used to assess activity, functional status, pain, and satisfaction levels after RTSA. Patients had to be willing and able to complete this questionnaire in order to be included in the study.
The questionnaire included demographic questions; a list of 42 activities patients could choose from to describe their current activity level, activities they were able to perform before the surgery, and activities they wish they could perform; a list of reasons for any limitations; and questions about overall pain, strength, and satisfaction with the procedure. In addition, there was an open-ended question for activities that may not have been listed. The questionnaire also included a validated method for assessing shoulder range of motion (ROM) at home, where patients rated their overhead motion according to standardized physical landmarks, including the level of the shoulder, chin, eyebrows, top of head, and above head.12-14 Also provided was the ASES Standardized Shoulder Assessment Form, which features a 100-point visual analog scale for pain plus functional ability questions, with higher scores indicating less pain and better function.15,16 The minimal clinical significance in the ASES score is 6.4 points.17,18 Scores were recorded and analyzed. Student t test was used to calculate statistical differences between patients who had primary RTSA performed and patients who underwent revision RTSA.
Study personnel contacted patients by telephone and direct mailing. Patients who could not be reached initially were called at least 4 more times: twice during the weekday, once during the evening, and once on the weekend. Patients who could not be contacted by telephone were then cross-referenced with the Social Security database to see if any were deceased. Response data were tabulated, and patients were stratified into high-, moderate-, and low-intensity activity.
One of the 3 senior authors (Dr. Ahmad, Dr. Bigliani, Dr. Levine) performed the 95 RTSAs: 84 Zimmer (Warsaw, Indiana), 7 DePuy (Warsaw, Indiana), 4 Tornier (Minneapolis, Minnesota). The DePuy and Tornier implants were used when a 30-mm glenoid peg was required (before Zimmer offered this length in its system). The procedure was done with a deltopectoral approach with 20° of retroversion. In revision cases, the same approach was used, the hardware or implants were removed, and the position of the humeral component was determined based on the pectoralis major insertion and the deltoid tension. In 80% of cases, the subscapularis was not repaired; in the other 20%, it was. Whether it was repaired depended on tendon viability and surgeon preference, as subscapularis repair status has been shown not to affect functional outcome.19-21 No combined latissimus transfers were performed. Patients wore a sling the first 4 weeks after surgery (only wrist and elbow motion allowed) and then advanced to active shoulder ROM. Eight weeks after surgery, they began gentle shoulder strengthening.
Results
One hundred nine consecutive patients underwent RTSA at a single institution. Fifteen patients subsequently died, 14 could not be contacted, and 2 declined, leaving 78 patients available for clinical follow-up. Mean follow-up was 4.8 years (range 2-9 years). Mean (SD) age at surgery was 75.3 (7.5) years. Seventy-five percent of the patients were women. Sixty-one percent underwent surgery for cuff tear arthropathy, 31% for revision of previous arthroplasty or internal fixation, 7% for complex fractures, and 1% for tumor. Of the 24 revisions, 15 were for failed hemiarthroplasty, 3 were for failed TSA with rotator cuff dysfunction, 4 were for fracture with failed internal fixation, and 2 were for failed RTSA referred from other institutions. The dominant shoulder was involved 62% of the time. Preoperative active forward shoulder elevation was less than 90° in all patients. There were 10 complications: 2 dislocations that were closed-reduced and remained stable, 1 dislocation that required revision of the liner, 1 aseptic loosening in a patient who has declined revision, 2 dissociated glenosphere baseplates, 2 deep infections that required 2-stage exchanges, 1 deep infection that required a 2-stage exchange that was then complicated by dissociation of the glenosphere baseplate requiring revision, and 1 superficial infection that resolved with oral antibiotics.
After surgery, mean active forward elevation was 140°, mean active external rotation was 48°, and mean active internal rotation was to S1. Mean (SD) postoperative ASES score was 77.5 (23.4). Satisfaction level was high (mean, 8.3/10), and mean pain levels were low: 2.3 out of 10 on the visual analog scale and 44.0 (SD, 11.7) on the ASES pain component. Strength was rated a mean of good. Table 1 lists the clinical data for the primary and revision surgery patients.
Eighteen patients (23.1%) returned to 24 different high-intensity activities, such as hunting, golf, and skiing; 38 patients (48.7%) returned to moderate-intensity activities, such as swimming, bowling, and raking leaves; and 22 patients (28.2%) returned to low-intensity activities, such as riding a stationary bike, playing a musical instrument, and walking (Table 2). Four patients played golf before and after RTSA, but neither of the 2 patients who played tennis before RTSA were able to do so after. Patients reported they engaged in their favorite leisure activity a mean of 4.8 times per week and a mean of 1.5 hours each time.
A medical problem was cited by 58% of patients as the reason for limited activity. These patients reported physical decline resulting from cardiac disease, diabetes, asthma/chronic obstructive pulmonary disease, or arthritis in other joints. Reasons for activity limitation are listed in Table 3. Post-RTSA activities that patients could not do for any reason are listed in Table 4. Activity limitations that patients attributed to the RTSA are listed in Table 5.
The majority of patients (57.7%) reported no change, from before RTSA to after RTSA, in being unable to do certain desired activities (eg, softball, target shooting, horseback riding, running, traveling). Sixteen patients (20.8%) reported being unable to return to an activity (eg, tennis, swimming, baseball, kayaking) they had been able to do before surgery. Most (69%) of those patients reported being unable to return to a moderate- or high-intensity activity after RTSA, but 81.8% were able to return to different moderate- or high-intensity activities.
Revision patients, who reported lower overhead activity levels, constituted 73% of the patients who felt their shoulder mechanically limited their activity, despite the fact that revisions constituted only 25% of the cases overall. Mean active ROM was statistically lower for revision patients than for primary patients (P < .05). Mean ASES score was statistically lower for the revision group (P < .001) and represented a clinically significant difference. Mean pain level was low (3.3) and satisfaction still generally high (7.4), but pain, satisfaction, and strength were about 1 point worse on average in the revision group than in the primary group.
Discussion
In the United States and other countries, RTSA implant survivorship is good.9,22 In this article, we have reported on post-RTSA activity levels, on the significant impact of comorbidities on this group, and on the negative effect of revisions on postoperative activity. Patients in this population reported that concomitant medical problems were the most important factor limiting their post-RTSA activity levels. Understanding and interpreting quality-of-life or functional scores in this elderly group must take into account the impact of comorbidities.23
Patients should have realistic postoperative expectations.24 In this study, some patients engaged in high-intensity overhead activities, such as golf, chopping wood, and shooting. However, the most difficulty was encountered trying to return to activities (eg, tennis, kayaking, archery, combing hair) that required external rotation in abduction.
Patients who had a previous implant (eg, hemiarthroplasty, TSA, failed internal fixation) revised to RTSA had lower activity levels and were 9 times more likely than primary patients to report having a mechanical shoulder limitation affecting their activity. Revision patients also had worse forward elevation, external rotation, pain, and satisfaction.
This study is limited in that it is retrospective. Subsequent prospective studies focused on younger patients who undergo primary RTSA may be useful if indications expand. In addition, subscapularis status and especially infraspinatus status may affect activity levels and could be analyzed in a study. Another limitation is that we did not specifically record detailed preoperative data, though all patients were known to have preoperative forward elevation of less than 90°.
In general, the primary measure of success for RTSA has been pain relief. Some studies have also reported on strength and ROM.2,20,25,26 A recent study using similar methodology demonstrated comparable ROM and low pain after RTSA, though revisions were not included in that study.26 In contrast to the present study, no patient in that study was able to play tennis or golf, but the reasons for the limited activity were not explored. In both studies, post-RTSA sports were generally of lower intensity than those played by patients after anatomical TSA.27
Overall, the majority of patients were very satisfied with their low pain level after RTSA. In addition, many patients not limited by other medical conditions were able to return to their pre-RTSA moderate-intensity recreational activities.
1. Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases [in French]. Acta Orthop Belg. 1995;61(suppl 1):112-119.
2. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
3. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
4. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
5. Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2240-2248.
6. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
7. Nam D, Kepler CK, Neviaser AS, et al. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am. 2010;92(suppl 2):23-35.
8. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221-1230.
9. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
10. Cazeneuve JF, Cristofari DJ. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res. 2011;97(6):583-589.
11. Gerber C, Pennington, SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-295.
12. Brophy RH, Beauvais RL, Jones EC, Cordasco FA, Marx RG. Measurement of shoulder activity level. Clin Orthop. 2005;(439):101-108.
13. Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88(3):508-513.
14. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280.
15. Kocher, MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
16. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
17. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
18. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
19. Molé D, Favard L. Excentered scapulohumeral osteoarthritis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 suppl):37-94.
20. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
21. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25(2):129-133.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Antuña SA, Sperling JW, Sánchez-Sotelo J, Cofield RH. Shoulder arthroplasty for proximal humeral nonunions. J Shoulder Elbow Surg. 2002;11(2):114-121.
24. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
25. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop. 2011;469(9):2476-2482.
26. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
27. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.
1. Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases [in French]. Acta Orthop Belg. 1995;61(suppl 1):112-119.
2. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
3. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
4. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
5. Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2240-2248.
6. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
7. Nam D, Kepler CK, Neviaser AS, et al. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am. 2010;92(suppl 2):23-35.
8. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221-1230.
9. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
10. Cazeneuve JF, Cristofari DJ. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res. 2011;97(6):583-589.
11. Gerber C, Pennington, SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-295.
12. Brophy RH, Beauvais RL, Jones EC, Cordasco FA, Marx RG. Measurement of shoulder activity level. Clin Orthop. 2005;(439):101-108.
13. Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88(3):508-513.
14. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280.
15. Kocher, MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
16. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
17. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
18. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
19. Molé D, Favard L. Excentered scapulohumeral osteoarthritis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 suppl):37-94.
20. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
21. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25(2):129-133.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Antuña SA, Sperling JW, Sánchez-Sotelo J, Cofield RH. Shoulder arthroplasty for proximal humeral nonunions. J Shoulder Elbow Surg. 2002;11(2):114-121.
24. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
25. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop. 2011;469(9):2476-2482.
26. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
27. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.
The Epidemic of Tommy John Surgery: The Role of the Orthopedic Surgeon
Ulnar collateral ligament (UCL) reconstruction, commonly referred to as Tommy John surgery, is a well-described surgical treatment for elite athletes with a symptomatic, deficient UCL.1, 2 The procedure was first performed by the late Dr. Frank Jobe in 1974, described in 1986, and has undergone several modifications over the past 30 years.3 Different graft choices, tunnel positions, graft configurations, and tunnel fixation methods are just some of the alterations that have been made to the original Jobe technique.4-6 With time, the index procedure has become more refined, with predictable outcomes in Major League Baseball (MLB) pitchers as well as other elite overhead throwing athletes.2,7,8 However, though this surgery was originally described for elite athletes suffering from UCL deficiency, recent times have seen an increase of over 50% in the number of UCL reconstructions performed on high school–aged and younger athletes.9 Furthermore, in 2000, a total of 13 MLB pitchers underwent UCL reconstruction, while in 2012 this number increased nearly threefold to 32.2 This paradigm shift of performing UCL reconstructions more frequently and on younger athletes raises a very important question: what is the role of the orthopedic surgeon in this epidemic?
UCL reconstruction has become a reliable procedure for MLB pitchers and other overhead throwing athletes.7,10,11 Recent studies have reported that MLB pitchers who undergo UCL reconstruction return to pitch in the MLB 83% of the time, whereas only 3% fail to return to pitch in either MLB or the minor league.2 Furthermore, pitchers who undergo UCL reconstruction perform similarly after surgery as prior to their UCL reconstruction, with fewer innings pitched after surgery, but, more importantly, a lower earned run average (ERA) and walks plus hits per inning pitched (WHIP) after surgery. These last 2 statistics, known as sabermetrics, evaluate the pitcher’s effectiveness; the fact that these are improved after surgery is reassuring for pitchers who undergo this procedure. However, it must be recognized that these pitchers pitched fewer innings after surgery.
There has been a sharp increase in the number of MLB pitchers who have undergone UCL reconstruction in recent years, especially the past 3 seasons, in which over 60 pitchers have had Tommy John surgery.2 This increase, however, is not confined to MLB pitchers. High school–aged pitchers have also been part of this drastic rise in the number of UCL reconstructions performed throughout the country. Dr. James Andrews and colleagues noted a 50% increase from 1988-1994 to 1995-2003 in the proportion of high school–aged pitchers who underwent UCL reconstruction (while the absolute number increased from 7 to 77 in high school–aged players compared with 85 to 609 in adult athletes).9 Given the increase in MLB pitchers over the past few years, it is likely this number has also increased among adolescent pitchers.
This data again raises the question: what is the role of the orthopedic surgeon in this epidemic? There are many plausible responses, but in my opinion, there is one answer that surpasses the others. As a trained professional, surgeons are tasked with the responsibility of looking out for the best interest of their patients, even when this conflicts with the patient’s, or the patient’s parent’s or coach’s desires. This includes injury prevention, such as instituting pitch counts and developing products that allow coaches to determine when a pitcher may be at risk for injury from fatigue, as well as injury treatment.12 It is difficult for a patient to understand the gravity of surgery and the rehabilitation process, specifically a procedure as involved as UCL reconstruction, and especially if the patient is an adolescent who has their outlook clouded by the fact that they believe they will be the next MLB star pitcher. The reality is that the National Collegiate Athletic Association (NCAA)13 has released data that has demonstrated that only 6.8% of high school baseball players will play baseball in college. Furthermore, only 9.4% of college baseball players will reach the professional level. That equates to 0.5%, or 1 in 200 high school players who will eventually play professional baseball.13 However, the reverse of this is also true, that out of every 200 players, 1 will make it to the major leagues, and that 1 player could be the patient in question. Hence, the purpose of this data is to show parents and athletes that, while they do have a chance of playing professional, and certainly collegiate, baseball, that percentage must be weighed against the risks of surgery.
MLB pitchers who have an endless supply of rehabilitation facilities, trainers, etc, do not return to pitching competitively and consistently in the majors for more than 15 months after UCL reconstruction.2 The time commitment and rehabilitation required for these patients is staggering.14,15 Furthermore, parents of these children who are consenting for them also have a difficult time comprehending the workload they are signing their child up for. Some parents believe this surgery will help their child throw faster, longer, and more accurately—beliefs that numerous studies have shown to be flat-out inaccurate. In fact, pitchers tend to lose a slight amount of velocity and accuracy after UCL reconstruction.11,16 Ahmad and colleagues17 administered a questionnaire to 189 players, 15 coaches, and 31 parents about the indications, risks, benefits, etc, regarding UCL reconstruction to determine the public’s perception regarding this surgery. The results demonstrated that the public, including coaches, have a significantly skewed perception of exactly how serious this surgery is. The study showed that 28% of players and 20% of coaches believed the pitcher’s performance would be improved after surgery, and, more strikingly, 26% of collegiate athletes, 30% percent of coaches, 37% of parents, and 51% of high school athletes believed UCL reconstruction should be performed as a prophylactic procedure to enhance performance in an uninjured athlete.17
Henceforth, it becomes the surgeon’s responsibility to ensure that both the patient and the parents understand what the surgery and rehabilitation process entails, to keep the expectations of the patient and his or her family realistic, and to counsel these patients on alternative options with lower risks. As Ahmad and colleagues17 demonstrated, this is not an easy task given the public’s preconceived notions. Many patients, especially patients of the younger generation, seem to be willing to jump to surgery as the first option for treatment without having truly tried any nonoperative measures, because they believe surgery to be a quick, easy, and definitive answer. This is not always the case, and a trial of nonoperative treatment, including rest, ice, physical therapy, and possibly platelet-rich plasma (PRP), should be instituted for high school–aged players who present with UCL insufficiency prior to discussing surgery.18,19
Medial UCL reconstruction is a successful procedure for elite MLB athletes. However, UCL reconstruction is becoming a victim of its own success as younger and younger athletes who will likely never play at the major league level are undergoing this procedure at an alarming rate. This is an epidemic which must be addressed by surgeons, coaches, and parents alike to curb the beliefs that UCL reconstruction will make high school–aged pitchers more successful. This procedure should not be performed prophylactically on an athlete of any age, especially those in high school. Further studies on the effectiveness of both nonoperative rest and rehabilitation and of PRP on partial-thickness UCL tears are warranted. New technology in the form of a compression sleeve with imbedded sensors to track the biomechanics of a pitcher’s elbow has been released and will hopefully provide information to coaches about when pitchers’ elbows begin to fatigue based on several biomechanical parameters.12 The future of UCL reconstruction is still fluid, and with proper prevention strategies, nonoperative treatment, indications, and preoperative discussions, the Tommy John epidemic can be cured. ◾
1. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
2. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
3. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
4. Jackson TJ, Adamson GJ, Peterson A, Patton J, McGarry MH, Lee TQ. Ulnar collateral ligament reconstruction using bisuspensory fixation: a biomechanical comparison with the docking technique. Am J Sports Med. 2013;41(5):1158-1164.
5. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
6. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
7. Dines JS, Jones KJ, Kahlenberg C, Rosenbaum A, Osbahr DC, Altchek DW. Elbow ulnar collateral ligament reconstruction in javelin throwers at a minimum 2-year follow-up. Am J Sports Med. 2012;40(1):148-151.
8. Gibson BW, Webner D, Huffman GR, Sennett BJ. Ulnar collateral ligament reconstruction in major league baseball pitchers. Am J Sports Med. 2007;35(4):575-581.
9. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2004;32(5):1158-1164.
10. Osbahr DC, Cain EL Jr, Raines BT, Fortenbaugh D, Dugas JR, Andrews JR. Long-term outcomes after ulnar collateral ligament reconstruction in competitive baseball players: minimum 10-year follow-up. Am J Sports Med. 2014;42(6):1333-1342.
11. Jiang JJ, Leland JM. Analysis of pitching velocity in major league baseball players before and after ulnar collateral ligament reconstruction. Am J Sports Med. 2014;42(4):880-885.
12. Carroll W. The sleeve that could save baseball: exclusive look at new MLB technology. Bleacher Report. http://bleacherreport.com/articles/2097866-the-sleeve-that-could-save-baseball-exclusive-look-at-new-mlb-technology?utm_campaign=tsipad&utm_medium=referral&utm_source=teamstream. Published July 2, 2014. Accessed November 12, 2014.
13. National Collegiate Athletic Association. Estimated probability of competing in athletics beyond the high school interscholastic level. https://www.ncaa.org/sites/default/files/Probability-of-going-pro-methodology_Update2013.pdf. Updated September 24, 2013. Accessed November 12, 2014.
14. Wilk KE, Macrina LC, Cain EL, Dugas JR, Andrews JR. Rehabilitation of the overhead athlete’s elbow. Sports Health. 2012;4(5):404-414.
15. Wilk KE, Reinold MM, Andrews JR. Rehabilitation of the thrower’s elbow. Tech Hand Up Extrem Surg. 2003;7(4):197-216.
16. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: a review of 147 cases. Am J Sports Med. 2014;42(6):1323-1332.
17. Ahmad CS, Grantham WJ, Greiwe RM. Public perceptions of Tommy John surgery. Phys Sportsmed. 2012;40(2):64-72.
18. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
19. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
Ulnar collateral ligament (UCL) reconstruction, commonly referred to as Tommy John surgery, is a well-described surgical treatment for elite athletes with a symptomatic, deficient UCL.1, 2 The procedure was first performed by the late Dr. Frank Jobe in 1974, described in 1986, and has undergone several modifications over the past 30 years.3 Different graft choices, tunnel positions, graft configurations, and tunnel fixation methods are just some of the alterations that have been made to the original Jobe technique.4-6 With time, the index procedure has become more refined, with predictable outcomes in Major League Baseball (MLB) pitchers as well as other elite overhead throwing athletes.2,7,8 However, though this surgery was originally described for elite athletes suffering from UCL deficiency, recent times have seen an increase of over 50% in the number of UCL reconstructions performed on high school–aged and younger athletes.9 Furthermore, in 2000, a total of 13 MLB pitchers underwent UCL reconstruction, while in 2012 this number increased nearly threefold to 32.2 This paradigm shift of performing UCL reconstructions more frequently and on younger athletes raises a very important question: what is the role of the orthopedic surgeon in this epidemic?
UCL reconstruction has become a reliable procedure for MLB pitchers and other overhead throwing athletes.7,10,11 Recent studies have reported that MLB pitchers who undergo UCL reconstruction return to pitch in the MLB 83% of the time, whereas only 3% fail to return to pitch in either MLB or the minor league.2 Furthermore, pitchers who undergo UCL reconstruction perform similarly after surgery as prior to their UCL reconstruction, with fewer innings pitched after surgery, but, more importantly, a lower earned run average (ERA) and walks plus hits per inning pitched (WHIP) after surgery. These last 2 statistics, known as sabermetrics, evaluate the pitcher’s effectiveness; the fact that these are improved after surgery is reassuring for pitchers who undergo this procedure. However, it must be recognized that these pitchers pitched fewer innings after surgery.
There has been a sharp increase in the number of MLB pitchers who have undergone UCL reconstruction in recent years, especially the past 3 seasons, in which over 60 pitchers have had Tommy John surgery.2 This increase, however, is not confined to MLB pitchers. High school–aged pitchers have also been part of this drastic rise in the number of UCL reconstructions performed throughout the country. Dr. James Andrews and colleagues noted a 50% increase from 1988-1994 to 1995-2003 in the proportion of high school–aged pitchers who underwent UCL reconstruction (while the absolute number increased from 7 to 77 in high school–aged players compared with 85 to 609 in adult athletes).9 Given the increase in MLB pitchers over the past few years, it is likely this number has also increased among adolescent pitchers.
This data again raises the question: what is the role of the orthopedic surgeon in this epidemic? There are many plausible responses, but in my opinion, there is one answer that surpasses the others. As a trained professional, surgeons are tasked with the responsibility of looking out for the best interest of their patients, even when this conflicts with the patient’s, or the patient’s parent’s or coach’s desires. This includes injury prevention, such as instituting pitch counts and developing products that allow coaches to determine when a pitcher may be at risk for injury from fatigue, as well as injury treatment.12 It is difficult for a patient to understand the gravity of surgery and the rehabilitation process, specifically a procedure as involved as UCL reconstruction, and especially if the patient is an adolescent who has their outlook clouded by the fact that they believe they will be the next MLB star pitcher. The reality is that the National Collegiate Athletic Association (NCAA)13 has released data that has demonstrated that only 6.8% of high school baseball players will play baseball in college. Furthermore, only 9.4% of college baseball players will reach the professional level. That equates to 0.5%, or 1 in 200 high school players who will eventually play professional baseball.13 However, the reverse of this is also true, that out of every 200 players, 1 will make it to the major leagues, and that 1 player could be the patient in question. Hence, the purpose of this data is to show parents and athletes that, while they do have a chance of playing professional, and certainly collegiate, baseball, that percentage must be weighed against the risks of surgery.
MLB pitchers who have an endless supply of rehabilitation facilities, trainers, etc, do not return to pitching competitively and consistently in the majors for more than 15 months after UCL reconstruction.2 The time commitment and rehabilitation required for these patients is staggering.14,15 Furthermore, parents of these children who are consenting for them also have a difficult time comprehending the workload they are signing their child up for. Some parents believe this surgery will help their child throw faster, longer, and more accurately—beliefs that numerous studies have shown to be flat-out inaccurate. In fact, pitchers tend to lose a slight amount of velocity and accuracy after UCL reconstruction.11,16 Ahmad and colleagues17 administered a questionnaire to 189 players, 15 coaches, and 31 parents about the indications, risks, benefits, etc, regarding UCL reconstruction to determine the public’s perception regarding this surgery. The results demonstrated that the public, including coaches, have a significantly skewed perception of exactly how serious this surgery is. The study showed that 28% of players and 20% of coaches believed the pitcher’s performance would be improved after surgery, and, more strikingly, 26% of collegiate athletes, 30% percent of coaches, 37% of parents, and 51% of high school athletes believed UCL reconstruction should be performed as a prophylactic procedure to enhance performance in an uninjured athlete.17
Henceforth, it becomes the surgeon’s responsibility to ensure that both the patient and the parents understand what the surgery and rehabilitation process entails, to keep the expectations of the patient and his or her family realistic, and to counsel these patients on alternative options with lower risks. As Ahmad and colleagues17 demonstrated, this is not an easy task given the public’s preconceived notions. Many patients, especially patients of the younger generation, seem to be willing to jump to surgery as the first option for treatment without having truly tried any nonoperative measures, because they believe surgery to be a quick, easy, and definitive answer. This is not always the case, and a trial of nonoperative treatment, including rest, ice, physical therapy, and possibly platelet-rich plasma (PRP), should be instituted for high school–aged players who present with UCL insufficiency prior to discussing surgery.18,19
Medial UCL reconstruction is a successful procedure for elite MLB athletes. However, UCL reconstruction is becoming a victim of its own success as younger and younger athletes who will likely never play at the major league level are undergoing this procedure at an alarming rate. This is an epidemic which must be addressed by surgeons, coaches, and parents alike to curb the beliefs that UCL reconstruction will make high school–aged pitchers more successful. This procedure should not be performed prophylactically on an athlete of any age, especially those in high school. Further studies on the effectiveness of both nonoperative rest and rehabilitation and of PRP on partial-thickness UCL tears are warranted. New technology in the form of a compression sleeve with imbedded sensors to track the biomechanics of a pitcher’s elbow has been released and will hopefully provide information to coaches about when pitchers’ elbows begin to fatigue based on several biomechanical parameters.12 The future of UCL reconstruction is still fluid, and with proper prevention strategies, nonoperative treatment, indications, and preoperative discussions, the Tommy John epidemic can be cured. ◾
Ulnar collateral ligament (UCL) reconstruction, commonly referred to as Tommy John surgery, is a well-described surgical treatment for elite athletes with a symptomatic, deficient UCL.1, 2 The procedure was first performed by the late Dr. Frank Jobe in 1974, described in 1986, and has undergone several modifications over the past 30 years.3 Different graft choices, tunnel positions, graft configurations, and tunnel fixation methods are just some of the alterations that have been made to the original Jobe technique.4-6 With time, the index procedure has become more refined, with predictable outcomes in Major League Baseball (MLB) pitchers as well as other elite overhead throwing athletes.2,7,8 However, though this surgery was originally described for elite athletes suffering from UCL deficiency, recent times have seen an increase of over 50% in the number of UCL reconstructions performed on high school–aged and younger athletes.9 Furthermore, in 2000, a total of 13 MLB pitchers underwent UCL reconstruction, while in 2012 this number increased nearly threefold to 32.2 This paradigm shift of performing UCL reconstructions more frequently and on younger athletes raises a very important question: what is the role of the orthopedic surgeon in this epidemic?
UCL reconstruction has become a reliable procedure for MLB pitchers and other overhead throwing athletes.7,10,11 Recent studies have reported that MLB pitchers who undergo UCL reconstruction return to pitch in the MLB 83% of the time, whereas only 3% fail to return to pitch in either MLB or the minor league.2 Furthermore, pitchers who undergo UCL reconstruction perform similarly after surgery as prior to their UCL reconstruction, with fewer innings pitched after surgery, but, more importantly, a lower earned run average (ERA) and walks plus hits per inning pitched (WHIP) after surgery. These last 2 statistics, known as sabermetrics, evaluate the pitcher’s effectiveness; the fact that these are improved after surgery is reassuring for pitchers who undergo this procedure. However, it must be recognized that these pitchers pitched fewer innings after surgery.
There has been a sharp increase in the number of MLB pitchers who have undergone UCL reconstruction in recent years, especially the past 3 seasons, in which over 60 pitchers have had Tommy John surgery.2 This increase, however, is not confined to MLB pitchers. High school–aged pitchers have also been part of this drastic rise in the number of UCL reconstructions performed throughout the country. Dr. James Andrews and colleagues noted a 50% increase from 1988-1994 to 1995-2003 in the proportion of high school–aged pitchers who underwent UCL reconstruction (while the absolute number increased from 7 to 77 in high school–aged players compared with 85 to 609 in adult athletes).9 Given the increase in MLB pitchers over the past few years, it is likely this number has also increased among adolescent pitchers.
This data again raises the question: what is the role of the orthopedic surgeon in this epidemic? There are many plausible responses, but in my opinion, there is one answer that surpasses the others. As a trained professional, surgeons are tasked with the responsibility of looking out for the best interest of their patients, even when this conflicts with the patient’s, or the patient’s parent’s or coach’s desires. This includes injury prevention, such as instituting pitch counts and developing products that allow coaches to determine when a pitcher may be at risk for injury from fatigue, as well as injury treatment.12 It is difficult for a patient to understand the gravity of surgery and the rehabilitation process, specifically a procedure as involved as UCL reconstruction, and especially if the patient is an adolescent who has their outlook clouded by the fact that they believe they will be the next MLB star pitcher. The reality is that the National Collegiate Athletic Association (NCAA)13 has released data that has demonstrated that only 6.8% of high school baseball players will play baseball in college. Furthermore, only 9.4% of college baseball players will reach the professional level. That equates to 0.5%, or 1 in 200 high school players who will eventually play professional baseball.13 However, the reverse of this is also true, that out of every 200 players, 1 will make it to the major leagues, and that 1 player could be the patient in question. Hence, the purpose of this data is to show parents and athletes that, while they do have a chance of playing professional, and certainly collegiate, baseball, that percentage must be weighed against the risks of surgery.
MLB pitchers who have an endless supply of rehabilitation facilities, trainers, etc, do not return to pitching competitively and consistently in the majors for more than 15 months after UCL reconstruction.2 The time commitment and rehabilitation required for these patients is staggering.14,15 Furthermore, parents of these children who are consenting for them also have a difficult time comprehending the workload they are signing their child up for. Some parents believe this surgery will help their child throw faster, longer, and more accurately—beliefs that numerous studies have shown to be flat-out inaccurate. In fact, pitchers tend to lose a slight amount of velocity and accuracy after UCL reconstruction.11,16 Ahmad and colleagues17 administered a questionnaire to 189 players, 15 coaches, and 31 parents about the indications, risks, benefits, etc, regarding UCL reconstruction to determine the public’s perception regarding this surgery. The results demonstrated that the public, including coaches, have a significantly skewed perception of exactly how serious this surgery is. The study showed that 28% of players and 20% of coaches believed the pitcher’s performance would be improved after surgery, and, more strikingly, 26% of collegiate athletes, 30% percent of coaches, 37% of parents, and 51% of high school athletes believed UCL reconstruction should be performed as a prophylactic procedure to enhance performance in an uninjured athlete.17
Henceforth, it becomes the surgeon’s responsibility to ensure that both the patient and the parents understand what the surgery and rehabilitation process entails, to keep the expectations of the patient and his or her family realistic, and to counsel these patients on alternative options with lower risks. As Ahmad and colleagues17 demonstrated, this is not an easy task given the public’s preconceived notions. Many patients, especially patients of the younger generation, seem to be willing to jump to surgery as the first option for treatment without having truly tried any nonoperative measures, because they believe surgery to be a quick, easy, and definitive answer. This is not always the case, and a trial of nonoperative treatment, including rest, ice, physical therapy, and possibly platelet-rich plasma (PRP), should be instituted for high school–aged players who present with UCL insufficiency prior to discussing surgery.18,19
Medial UCL reconstruction is a successful procedure for elite MLB athletes. However, UCL reconstruction is becoming a victim of its own success as younger and younger athletes who will likely never play at the major league level are undergoing this procedure at an alarming rate. This is an epidemic which must be addressed by surgeons, coaches, and parents alike to curb the beliefs that UCL reconstruction will make high school–aged pitchers more successful. This procedure should not be performed prophylactically on an athlete of any age, especially those in high school. Further studies on the effectiveness of both nonoperative rest and rehabilitation and of PRP on partial-thickness UCL tears are warranted. New technology in the form of a compression sleeve with imbedded sensors to track the biomechanics of a pitcher’s elbow has been released and will hopefully provide information to coaches about when pitchers’ elbows begin to fatigue based on several biomechanical parameters.12 The future of UCL reconstruction is still fluid, and with proper prevention strategies, nonoperative treatment, indications, and preoperative discussions, the Tommy John epidemic can be cured. ◾
1. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
2. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
3. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
4. Jackson TJ, Adamson GJ, Peterson A, Patton J, McGarry MH, Lee TQ. Ulnar collateral ligament reconstruction using bisuspensory fixation: a biomechanical comparison with the docking technique. Am J Sports Med. 2013;41(5):1158-1164.
5. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
6. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
7. Dines JS, Jones KJ, Kahlenberg C, Rosenbaum A, Osbahr DC, Altchek DW. Elbow ulnar collateral ligament reconstruction in javelin throwers at a minimum 2-year follow-up. Am J Sports Med. 2012;40(1):148-151.
8. Gibson BW, Webner D, Huffman GR, Sennett BJ. Ulnar collateral ligament reconstruction in major league baseball pitchers. Am J Sports Med. 2007;35(4):575-581.
9. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2004;32(5):1158-1164.
10. Osbahr DC, Cain EL Jr, Raines BT, Fortenbaugh D, Dugas JR, Andrews JR. Long-term outcomes after ulnar collateral ligament reconstruction in competitive baseball players: minimum 10-year follow-up. Am J Sports Med. 2014;42(6):1333-1342.
11. Jiang JJ, Leland JM. Analysis of pitching velocity in major league baseball players before and after ulnar collateral ligament reconstruction. Am J Sports Med. 2014;42(4):880-885.
12. Carroll W. The sleeve that could save baseball: exclusive look at new MLB technology. Bleacher Report. http://bleacherreport.com/articles/2097866-the-sleeve-that-could-save-baseball-exclusive-look-at-new-mlb-technology?utm_campaign=tsipad&utm_medium=referral&utm_source=teamstream. Published July 2, 2014. Accessed November 12, 2014.
13. National Collegiate Athletic Association. Estimated probability of competing in athletics beyond the high school interscholastic level. https://www.ncaa.org/sites/default/files/Probability-of-going-pro-methodology_Update2013.pdf. Updated September 24, 2013. Accessed November 12, 2014.
14. Wilk KE, Macrina LC, Cain EL, Dugas JR, Andrews JR. Rehabilitation of the overhead athlete’s elbow. Sports Health. 2012;4(5):404-414.
15. Wilk KE, Reinold MM, Andrews JR. Rehabilitation of the thrower’s elbow. Tech Hand Up Extrem Surg. 2003;7(4):197-216.
16. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: a review of 147 cases. Am J Sports Med. 2014;42(6):1323-1332.
17. Ahmad CS, Grantham WJ, Greiwe RM. Public perceptions of Tommy John surgery. Phys Sportsmed. 2012;40(2):64-72.
18. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
19. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
1. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
2. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
3. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
4. Jackson TJ, Adamson GJ, Peterson A, Patton J, McGarry MH, Lee TQ. Ulnar collateral ligament reconstruction using bisuspensory fixation: a biomechanical comparison with the docking technique. Am J Sports Med. 2013;41(5):1158-1164.
5. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
6. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
7. Dines JS, Jones KJ, Kahlenberg C, Rosenbaum A, Osbahr DC, Altchek DW. Elbow ulnar collateral ligament reconstruction in javelin throwers at a minimum 2-year follow-up. Am J Sports Med. 2012;40(1):148-151.
8. Gibson BW, Webner D, Huffman GR, Sennett BJ. Ulnar collateral ligament reconstruction in major league baseball pitchers. Am J Sports Med. 2007;35(4):575-581.
9. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2004;32(5):1158-1164.
10. Osbahr DC, Cain EL Jr, Raines BT, Fortenbaugh D, Dugas JR, Andrews JR. Long-term outcomes after ulnar collateral ligament reconstruction in competitive baseball players: minimum 10-year follow-up. Am J Sports Med. 2014;42(6):1333-1342.
11. Jiang JJ, Leland JM. Analysis of pitching velocity in major league baseball players before and after ulnar collateral ligament reconstruction. Am J Sports Med. 2014;42(4):880-885.
12. Carroll W. The sleeve that could save baseball: exclusive look at new MLB technology. Bleacher Report. http://bleacherreport.com/articles/2097866-the-sleeve-that-could-save-baseball-exclusive-look-at-new-mlb-technology?utm_campaign=tsipad&utm_medium=referral&utm_source=teamstream. Published July 2, 2014. Accessed November 12, 2014.
13. National Collegiate Athletic Association. Estimated probability of competing in athletics beyond the high school interscholastic level. https://www.ncaa.org/sites/default/files/Probability-of-going-pro-methodology_Update2013.pdf. Updated September 24, 2013. Accessed November 12, 2014.
14. Wilk KE, Macrina LC, Cain EL, Dugas JR, Andrews JR. Rehabilitation of the overhead athlete’s elbow. Sports Health. 2012;4(5):404-414.
15. Wilk KE, Reinold MM, Andrews JR. Rehabilitation of the thrower’s elbow. Tech Hand Up Extrem Surg. 2003;7(4):197-216.
16. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: a review of 147 cases. Am J Sports Med. 2014;42(6):1323-1332.
17. Ahmad CS, Grantham WJ, Greiwe RM. Public perceptions of Tommy John surgery. Phys Sportsmed. 2012;40(2):64-72.
18. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
19. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
Biomechanical Comparison of Hamstring Tendon Fixation Devices for Anterior Cruciate Ligament Reconstruction: Part 1. Five Femoral Devices
Anterior cruciate ligament (ACL) reconstruction remains one of the most common orthopedic procedures; almost 100,000 are performed in the United States each year, and they are among the procedures more commonly performed by surgeons specializing in sports medicine and by general orthopedists.1,2 Recent years have seen a trend toward replacing the gold standard of bone–patellar tendon–bone autograft with autograft or allograft hamstring tendon in ACL reconstruction.3 This shift is being made to try to avoid the donor-site morbidity of patellar tendon autografts and decrease the incidence of postoperative anterior knee pain. With increased use of hamstring grafts in ACL reconstruction, graft fixation strength has become a priority in attempts to optimize recovery and rehabilitation.4
Rigid fixation of hamstring grafts is now recognized as a crucial factor in the long-term success of ACL reconstruction. Grafts must withstand both early rehabilitation forces as high as 500 N5 and stresses to the native ACL during healing, which may take up to 12 weeks for soft-tissue incorporation.6
The challenge has been to engineer devices that provide stable, rigid graft fixation that allows expeditious tendon-to-bone healing and increased construct stiffness. Many new fixation devices are being marketed, and there is controversy regarding which provides the best stability and strength.7 Several studies have tested various fixation devices,8-16 but so far several devices have not been compared with one another.
We conducted a study to determine if femoral hamstring fixation devices used in ACL reconstruction differ in fixation strength. We hypothesized we would find no differences.
Materials and Methods
Fifty porcine femurs were harvested after the animals had been euthanized for other studies at our institution. Our study was approved by the institutional animal care and use committee. Specimens were stored at –25°C and, on day of testing, thawed to room temperature. Gracilis and semitendinosus tendon grafts were donated by a tissue bank (LifeNet Health, Virginia Beach, Virginia). The grafts were stored at –25°C; on day of testing, tendons were thawed to room temperature.
We evaluated 5 different femoral fixation devices (Figure 1): Delta screw and Bio-TransFix (Arthrex, Naples, Florida) and Bone Mulch screw, EZLoc, and Zip Loop (Arthrotek, Warsaw, Indiana). For each device, 10 ACL fixation constructs were tested.
Quadrupled human semitendinosus–gracilis tendon grafts were fixed into the femurs using the 5 femoral fixation devices. All fixations were done to manufacturer specifications.
Cyclic loading was followed by testing with the load-to-failure (LTF) protocol described by Kousa and colleagues.13 Specimens were tested in a custom load fixture (Figure 2). The base fixture used an adjustable angle vise mounted on a free rotary stage and a free x-y translation stage. This system allowed the load axis to be oriented to and aligned with the graft tunnel in the porcine femur, preventing off-axis or torsional loading of the grafts.
Pneumatic grips equipped with a custom pincer attachment allowed the graft to be grasped under a constant grip force during testing, regardless of graft thinning under tensile loads. Graft specimens were initially looped over a 3.8-mm horizontal metal shaft, and the 2 strands were double-looped at the graft insertion site. The 2 free strands were then drawn up around the metal shaft, and the shaft was placed above the serrated jaws. The metal shaft with enveloping tendon strands rested on a flat shelf at the top of the grip serrations. This configuration prevented the metal shaft and tendon strands from being pulled through the serrations when compressive force was applied to the jaws.
Before the study, the grip design was tested. There was no detectable relative motion of the strands at the grip end during graft testing to failure. The pincer attachment allowed close approach of the grips to the specimen at all femoral condyle orientations, so that a 25-mm length of exposed graft could be obtained for each specimen under initial conditions.
In the cyclic loading test, the load was applied parallel to the long axis of the femoral tunnel. A 50-N preload was initially applied to each specimen for 10 seconds, and the length of the exposed graft between grips and graft insertion was recorded. Subsequently, 1500 loading cycles between 50 N and 200 N at a rate of 1 cycle per 2 seconds (0.5 Hz) were performed. Standard force-displacement curves were then generated.
Specimens surviving the cyclic loading then underwent a single-cycle LTF test in which the load was applied parallel to the long axis of the drill hole at a rate of 50 mm per minute.
Residual displacement, stiffness, and ultimate LTF data were recorded from the force-displacement curves. Residual displacement data were generated from the cyclic loading test; residual displacement was determined by subtracting preload displacement from displacement at 1, 10, 50, 100, 250, 500, 1000, and 1500 cycles. Stiffness data were generated from the single-cycle LTF test; stiffness was defined as the linear region slope of the force-displacement curve corresponding to the steepest straight-line tangent to the loading curve. Ultimate LTF data were generated from the single-cycle LTF test; ultimate LTF was defined as the maximum load sustained by the specimen during a constant-displacement-rate tensile test for graft pullout.
Statistical analysis generated standard descriptive statistics: means, standard deviations, and proportions. One-way analysis of variance (ANOVA) was used to determine any statistically significant differences in stiffness, yield load, and residual displacement between the different fixation devices. Differences in force (load) between the single cycle and the cyclic loading test were determined by ANOVA. P < .05 was considered statistically significant for all tests.
Results
The modes of failure for the devices differed slightly (Table). Bone Mulch screw failed with a fracture through the femoral condyle extending to the bone tunnel. Zip Loop and EZLoc failed by pulling through their cortical attachment on the lateral femoral condyle. Bio-TransFix broke in the tunnel during LTF. Delta screw failed with slippage of the fixation device, and the tendons pulled out through the tunnel.
For the cyclic loading tests, only 2 of the 10 Delta screws completed the 1500-cycle loading test before failure. Of the 8 Delta screws that did not complete this testing, the majority failed after about 100 cycles. All 10 tests of Bone Mulch, Zip Loop, EZLoc, and Bio-TransFix completed the 1500-cycle loading test.
Residual displacement data were calculated from cyclic loading tests (Table). Mean (SD) residual displacement was lowest for Bio-TransFix at 4.1 (0.4) mm, followed by Bone Mulch at 5.2 (1.0) mm, EZLoc at 6.4 (1.1) mm, and Zip Loop at 6.8 (1.3) mm. Delta screws at 8.2 (1.4) mm had the highest residual displacement, though only 2 completed the cyclic tests. Bio-TransFix had significantly (P < .001) less residual displacement compared with EZLoc, Zip Loop, and Delta. Bone Mulch had significantly less residual displacement compared with Zip Loop (P < .05) and Delta (P < .01).
Stiffness data were calculated from LTF tests (Table). Mean (SD) stiffness was highest for Bone Mulch at 218 (25.9) N/mm, followed by Bio-TransFix at 171 (24.2) N/mm, EZLoc at 122 (24.1) N/mm, Zip Loop at 105 (18.9) N/mm, and Delta at 84 (16.4) N/mm. Bone Mulch had significantly (P < .001) higher stiffness compared with Bio-TransFix, EZLoc, Zip Loop, and Delta. Bio-TransFix had significantly (P < .001) higher stiffness compared with EZLoc, Zip Loop, and Delta.
Mean (SD) ultimate LTF was highest for Bone Mulch at 867 (164) N, followed by Zip Loop at 615 (72.3) N, Bio-TransFix at 552 (141) N, EZLoc at 476 (89.7) N, and Delta at 410 (65.3) N (Table). Bone Mulch failed at a statistically significantly (P < .001) higher load compared with Zip Loop, Bio-TransFix, EZLoc, and Delta. There were no significant differences in mean LTF among Zip Loop, Bio-TransFix, EZLoc, and Delta.
Discussion
In this biomechanical comparison of 5 different femoral fixation devices, the Bone Mulch screw had results superior to those of the other implants. Bone Mulch failed at higher LTF and higher stiffness. Bio-TransFix performed well and had residual displacement similar to that of Bone Mulch, but significantly lower LTF. Overall, EZLoc and Zip Loop were similar to each other in performance. The Delta (interference) screw performed poorly with respect to LTF, residual displacement, and stiffness; a large proportion of these screws failed early into cyclic loading.
Bone Mulch and Bio-TransFix overall outperformed the other fixation devices. These 2 devices are cortical-cancellous suspension devices, which provide transcondylar fixation and resist tensile forces perpendicular to the pullout force. Multiple biomechanical studies have found superior performance for these types of devices compared with various implants.10,13,15,16
Our results were similar to those of Kousa and colleagues,13 who found the Bone Mulch screw to provide highest LTF, highest stiffness, and lowest residual displacement. Another study found significantly higher stiffness for the Bone Mulch screw than for the Endobutton, a cortical suspensory fixation device.14 Bone Mulch failure modes differed, however. In the study by Kousa and colleagues,13 3 specimens failed with bending of the screw tip, and 7 failed with rupture of the tendon loop. All specimens in our study failed with fractures through the condyle. It is unclear why the failure modes differed, as we followed similar manufacturer protocols for inserting the device. It is possible the bone mass density of the porcine femurs differed between studies. This was not reported by Kousa and colleagues,13 and we did not perform testing either. However, all the porcine femurs were about the same age for testing of each device in this study.
Bio-TransFix has also been compared with various implants, but not in the same study. Brown and colleagues8 found the TransFix device significantly stiffer than the Endobutton CL. Shen and colleagues16 determined that TransFix had significantly lower residual displacement compared with Endobutton CL. Milano and colleagues15 compared multiple cortical suspensory fixation devices, including Endobutton CL, with TransFix and Bio-TransFix, and concluded the cortical-cancellous devices (TransFix, Bio-TransFix) offered the best and most predictable results in terms of elongation, fixation strength, and stiffness. TransFix has also been shown to be superior to interference screw fixation in biomechanical studies.10,15
Clinical outcomes of studies using TransFix for femoral fixation have been favorable, with improved Lysholm scores and improved laxity according to the KT-1000 test.17 However, multiple prospective studies have found no clinical difference in knee laxity between interference screw and Endobutton at 1- to 2-year follow-up18-20 and no difference in clinical outcome scores, such as the International Knee Documentation Committee score.11,18-20
Although these studies have shown no major clinical differences at short-term follow-up, the early aggressive rehabilitation period is the larger concern. Our study clearly demonstrated the biomechanical strength of transcondylar devices over other devices. The concern with transcondylar devices (vs other devices) is the increased difficulty that inexperienced surgeons have inserting them. In addition, when removed, transcondylar devices leave a large bone void.
In the present study, an important concern with femoral graft fixation is the poor performance of interference screws. Other authors recently expressed concern with using interference screws in soft-tissue ACL grafts—based on biomechanical study results of increased slippage, bone tunnel widening, and less strength.7 In the present study, Delta screws consistently performed poorest with respect to ultimate LTF, residual displacement, and stiffness. Only 20% of these screws completed 1500 cycles. Poor performance of interference screws has also been seen in other studies in tibial graft fixation21,22 and femoral graft fixation.13-15 Given their poor biomechanical properties, as seen in our study and these other studies, we think use of an interference screw alone is a poor choice for fixation.
Combined fixation techniques—interference screw plus other device(s)—may be used in clinical practice, but the present study did not evaluate any. In a biomechanical study, Yoo and colleagues23 compared an interference screw; an interference screw plus a cortical screw and a spiked washer; and a cortical screw and a spiked washer used alone in the tibia. Stiffness nearly doubled, residual displacement was less, and ultimate LTF was significantly higher in the group with the interference screw plus the cortical screw and the spiked washer. In a similar study involving femoral fixation, Oh and colleagues24 demonstrated improved stiffness, residual displacement, and LTF in cyclic testing with the combination of interference screw and Endobutton CL, compared with Endobutton CL alone. Further studies may include direct comparisons of additional femoral fixation techniques using more than 1 device.
The Zip Loop, or Toggle Loc with Zip Loop technology, is a suspensory cortical fixation device. It was initially designed for use in ACL fixation but has also been used in other surgeries, including distal biceps repair25 and ulnar collateral ligament reconstruction.26 The device itself is easy to use; more important, it allows for adjustment of graft length within the bone tunnel after deployment of the cortical fixation. Few biomechanical studies have been conducted with Zip Loop.9,12 The present study is the first to compare Zip Loop with devices other than suspensory cortical fixation devices. Zip Loop performed very well in LTF testing but had lower stiffness and higher residual displacement compared with the transcondylar fixation devices. Despite these findings, we have continued to use this device for femoral fixation in ACL reconstruction because of its ease of insertion, the ability to adjust graft tension within the bone tunnel, and the difficulties encountered inserting and removing transcondylar fixation.
We recognize the limitations in our study design with respect to how axial and cyclical loading compares with the physiologic orientation of the ACL during ambulation and running activities. This biomechanical study was not able to replicate these types of activities. However, it did provide good data supporting early rehabilitation with various fixation devices, though concern with use of interference screws remains.
Conclusion
Superior strength in fixation of hamstring grafts in the femur was demonstrated by Bone Mulch screws, followed closely by Bio-TransFix. Delta screws demonstrated poor displacement, stiffness, and LTF. When used as the sole femoral fixation device, a device with low LTF, decreased stiffness, and high residual displacement should be used cautiously in patients undergoing aggressive rehabilitation.
1. Dooley PJ, Chan DS, Dainty KN, Mohtadi NGH, Whelan DB. Patellar tendon versus hamstring autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev. 2006;(2):CD005960.
2. Garrett WE Jr, Swiontkowski MF, Weinsten JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
3. West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197-207.
4. Hapa O, Barber FA. ACL fixation devices. Sports Med Arthrosc. 2009;17(4):217-223.
5. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
6. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
7. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
8. Brown CH Jr, Wilson DR, Hecker AT, Ferragamo M. Graft-bone motion and tensile properties of hamstring and patellar tendon anterior cruciate ligament femoral graft fixation under cyclic loading. Arthroscopy. 2004;20(9):922-935.
9. Conner CS, Perez BA, Morris RP, Buckner JW, Buford WL Jr, Ivey FM. Three femoral fixation devices for anterior cruciate ligament reconstruction: comparison of fixation on the lateral cortex versus the anterior cortex. Arthroscopy. 2010;26(6):796-807.
10. Fabbriciani C, Mulas PD, Ziranu F, Deriu L, Zarelli D, Milano G. Mechanical analysis of fixation methods for anterior cruciate ligament reconstruction with hamstring tendon graft. An experimental study in sheep knees. Knee. 2005;12(2):135-138.
11. Harilainen A, Sandelin J, Jansson KA. Cross-pin femoral fixation versus metal interference screw fixation in anterior cruciate ligament reconstruction with hamstring tendons: results of a controlled prospective randomized study with 2-year follow-up. Arthroscopy. 2005;21(1):25-33.
12. Kamelger FS, Onder U, Schmoelz W, Tecklenburg K, Arora R, Fink C. Suspensory fixation of grafts in anterior cruciate ligament reconstruction: a biomechanical comparison of 3 implants. Arthroscopy. 2009;25(7):767-776.
13. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med. 2003;31(2):174-181.
14. Kudo T, Tohyama H, Minami A, Yasuda K. The effect of cyclic loading on the biomechanical characteristics of the femur–graft–tibia complex after anterior cruciate ligament reconstruction using Bone Mulch screw/WasherLoc fixation. Clin Biomech. 2005;20(4):414-420.
15. Milano G, Mulas PD, Ziranu F, Piras S, Manunta A, Fabbriciani C. Comparison between different femoral fixation devices for ACL reconstruction with doubled hamstring tendon graft: a biomechanical analysis. Arthroscopy. 2006;22(6):660-668.
16. Shen HC, Chang JH, Lee CH, et al. Biomechanical comparison of cross-pin and Endobutton-CL femoral fixation of a flexor tendon graft for anterior cruciate ligament reconstruction—a porcine femur–graft–tibia complex study. J Surg Res. 2010;161(2):282-287.
17. Asik M, Sen C, Tuncay I, Erdil M, Avci C, Taser OF. The mid- to long-term results of the anterior cruciate ligament reconstruction with hamstring tendons using Transfix technique. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):965-972.
18. Capuano L, Hardy P, Longo UG, Denaro V, Maffulli N. No difference in clinical results between femoral transfixation and bio-interference screw fixation in hamstring tendon ACL reconstruction. A preliminary study. Knee. 2008;15(3):174-179.
19. Price R, Stoney J, Brown G. Prospective randomized comparison of Endobutton versus cross-pin femoral fixation in hamstring anterior cruciate ligament reconstruction with 2-year follow-up. ANZ J Surg. 2010;80(3):162-165.
20. Rose T, Hepp P, Venus J, Stockmar C, Josten C, Lill H. Prospective randomized clinical comparison of femoral transfixation versus bioscrew fixation in hamstring tendon ACL reconstruction—a preliminary report. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):730-738.
21. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med. 2003;31(2):182-188.
22. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
23. Yoo JC, Ahn JH, Kim JH, et al. Biomechanical testing of hybrid hamstring graft tibial fixation in anterior cruciate ligament reconstruction. Knee. 2006;13(6):455-459.
24. Oh YH, Namkoong S, Strauss EJ, et al. Hybrid femoral fixation of soft-tissue grafts in anterior cruciate ligament reconstruction using the Endobutton CL and bioabsorbable interference screws: a biomechanical study. Arthroscopy. 2006;22(11):1218-1224.
25. DiRaimo MJ Jr, Maney MD, Deitch JR. Distal biceps tendon repair using the Toggle Loc with Zip Loop. Orthopedics. 2008;31(12). doi: 10.3928/01477447-20081201-05.
26. Morgan RJ, Starman JS, Habet NA, et al. A biomechanical evaluation of ulnar collateral ligament reconstruction using a novel technique for ulnar-sided fixation. Am J Sports Med. 2010;38(7):1448-1455.
Anterior cruciate ligament (ACL) reconstruction remains one of the most common orthopedic procedures; almost 100,000 are performed in the United States each year, and they are among the procedures more commonly performed by surgeons specializing in sports medicine and by general orthopedists.1,2 Recent years have seen a trend toward replacing the gold standard of bone–patellar tendon–bone autograft with autograft or allograft hamstring tendon in ACL reconstruction.3 This shift is being made to try to avoid the donor-site morbidity of patellar tendon autografts and decrease the incidence of postoperative anterior knee pain. With increased use of hamstring grafts in ACL reconstruction, graft fixation strength has become a priority in attempts to optimize recovery and rehabilitation.4
Rigid fixation of hamstring grafts is now recognized as a crucial factor in the long-term success of ACL reconstruction. Grafts must withstand both early rehabilitation forces as high as 500 N5 and stresses to the native ACL during healing, which may take up to 12 weeks for soft-tissue incorporation.6
The challenge has been to engineer devices that provide stable, rigid graft fixation that allows expeditious tendon-to-bone healing and increased construct stiffness. Many new fixation devices are being marketed, and there is controversy regarding which provides the best stability and strength.7 Several studies have tested various fixation devices,8-16 but so far several devices have not been compared with one another.
We conducted a study to determine if femoral hamstring fixation devices used in ACL reconstruction differ in fixation strength. We hypothesized we would find no differences.
Materials and Methods
Fifty porcine femurs were harvested after the animals had been euthanized for other studies at our institution. Our study was approved by the institutional animal care and use committee. Specimens were stored at –25°C and, on day of testing, thawed to room temperature. Gracilis and semitendinosus tendon grafts were donated by a tissue bank (LifeNet Health, Virginia Beach, Virginia). The grafts were stored at –25°C; on day of testing, tendons were thawed to room temperature.
We evaluated 5 different femoral fixation devices (Figure 1): Delta screw and Bio-TransFix (Arthrex, Naples, Florida) and Bone Mulch screw, EZLoc, and Zip Loop (Arthrotek, Warsaw, Indiana). For each device, 10 ACL fixation constructs were tested.
Quadrupled human semitendinosus–gracilis tendon grafts were fixed into the femurs using the 5 femoral fixation devices. All fixations were done to manufacturer specifications.
Cyclic loading was followed by testing with the load-to-failure (LTF) protocol described by Kousa and colleagues.13 Specimens were tested in a custom load fixture (Figure 2). The base fixture used an adjustable angle vise mounted on a free rotary stage and a free x-y translation stage. This system allowed the load axis to be oriented to and aligned with the graft tunnel in the porcine femur, preventing off-axis or torsional loading of the grafts.
Pneumatic grips equipped with a custom pincer attachment allowed the graft to be grasped under a constant grip force during testing, regardless of graft thinning under tensile loads. Graft specimens were initially looped over a 3.8-mm horizontal metal shaft, and the 2 strands were double-looped at the graft insertion site. The 2 free strands were then drawn up around the metal shaft, and the shaft was placed above the serrated jaws. The metal shaft with enveloping tendon strands rested on a flat shelf at the top of the grip serrations. This configuration prevented the metal shaft and tendon strands from being pulled through the serrations when compressive force was applied to the jaws.
Before the study, the grip design was tested. There was no detectable relative motion of the strands at the grip end during graft testing to failure. The pincer attachment allowed close approach of the grips to the specimen at all femoral condyle orientations, so that a 25-mm length of exposed graft could be obtained for each specimen under initial conditions.
In the cyclic loading test, the load was applied parallel to the long axis of the femoral tunnel. A 50-N preload was initially applied to each specimen for 10 seconds, and the length of the exposed graft between grips and graft insertion was recorded. Subsequently, 1500 loading cycles between 50 N and 200 N at a rate of 1 cycle per 2 seconds (0.5 Hz) were performed. Standard force-displacement curves were then generated.
Specimens surviving the cyclic loading then underwent a single-cycle LTF test in which the load was applied parallel to the long axis of the drill hole at a rate of 50 mm per minute.
Residual displacement, stiffness, and ultimate LTF data were recorded from the force-displacement curves. Residual displacement data were generated from the cyclic loading test; residual displacement was determined by subtracting preload displacement from displacement at 1, 10, 50, 100, 250, 500, 1000, and 1500 cycles. Stiffness data were generated from the single-cycle LTF test; stiffness was defined as the linear region slope of the force-displacement curve corresponding to the steepest straight-line tangent to the loading curve. Ultimate LTF data were generated from the single-cycle LTF test; ultimate LTF was defined as the maximum load sustained by the specimen during a constant-displacement-rate tensile test for graft pullout.
Statistical analysis generated standard descriptive statistics: means, standard deviations, and proportions. One-way analysis of variance (ANOVA) was used to determine any statistically significant differences in stiffness, yield load, and residual displacement between the different fixation devices. Differences in force (load) between the single cycle and the cyclic loading test were determined by ANOVA. P < .05 was considered statistically significant for all tests.
Results
The modes of failure for the devices differed slightly (Table). Bone Mulch screw failed with a fracture through the femoral condyle extending to the bone tunnel. Zip Loop and EZLoc failed by pulling through their cortical attachment on the lateral femoral condyle. Bio-TransFix broke in the tunnel during LTF. Delta screw failed with slippage of the fixation device, and the tendons pulled out through the tunnel.
For the cyclic loading tests, only 2 of the 10 Delta screws completed the 1500-cycle loading test before failure. Of the 8 Delta screws that did not complete this testing, the majority failed after about 100 cycles. All 10 tests of Bone Mulch, Zip Loop, EZLoc, and Bio-TransFix completed the 1500-cycle loading test.
Residual displacement data were calculated from cyclic loading tests (Table). Mean (SD) residual displacement was lowest for Bio-TransFix at 4.1 (0.4) mm, followed by Bone Mulch at 5.2 (1.0) mm, EZLoc at 6.4 (1.1) mm, and Zip Loop at 6.8 (1.3) mm. Delta screws at 8.2 (1.4) mm had the highest residual displacement, though only 2 completed the cyclic tests. Bio-TransFix had significantly (P < .001) less residual displacement compared with EZLoc, Zip Loop, and Delta. Bone Mulch had significantly less residual displacement compared with Zip Loop (P < .05) and Delta (P < .01).
Stiffness data were calculated from LTF tests (Table). Mean (SD) stiffness was highest for Bone Mulch at 218 (25.9) N/mm, followed by Bio-TransFix at 171 (24.2) N/mm, EZLoc at 122 (24.1) N/mm, Zip Loop at 105 (18.9) N/mm, and Delta at 84 (16.4) N/mm. Bone Mulch had significantly (P < .001) higher stiffness compared with Bio-TransFix, EZLoc, Zip Loop, and Delta. Bio-TransFix had significantly (P < .001) higher stiffness compared with EZLoc, Zip Loop, and Delta.
Mean (SD) ultimate LTF was highest for Bone Mulch at 867 (164) N, followed by Zip Loop at 615 (72.3) N, Bio-TransFix at 552 (141) N, EZLoc at 476 (89.7) N, and Delta at 410 (65.3) N (Table). Bone Mulch failed at a statistically significantly (P < .001) higher load compared with Zip Loop, Bio-TransFix, EZLoc, and Delta. There were no significant differences in mean LTF among Zip Loop, Bio-TransFix, EZLoc, and Delta.
Discussion
In this biomechanical comparison of 5 different femoral fixation devices, the Bone Mulch screw had results superior to those of the other implants. Bone Mulch failed at higher LTF and higher stiffness. Bio-TransFix performed well and had residual displacement similar to that of Bone Mulch, but significantly lower LTF. Overall, EZLoc and Zip Loop were similar to each other in performance. The Delta (interference) screw performed poorly with respect to LTF, residual displacement, and stiffness; a large proportion of these screws failed early into cyclic loading.
Bone Mulch and Bio-TransFix overall outperformed the other fixation devices. These 2 devices are cortical-cancellous suspension devices, which provide transcondylar fixation and resist tensile forces perpendicular to the pullout force. Multiple biomechanical studies have found superior performance for these types of devices compared with various implants.10,13,15,16
Our results were similar to those of Kousa and colleagues,13 who found the Bone Mulch screw to provide highest LTF, highest stiffness, and lowest residual displacement. Another study found significantly higher stiffness for the Bone Mulch screw than for the Endobutton, a cortical suspensory fixation device.14 Bone Mulch failure modes differed, however. In the study by Kousa and colleagues,13 3 specimens failed with bending of the screw tip, and 7 failed with rupture of the tendon loop. All specimens in our study failed with fractures through the condyle. It is unclear why the failure modes differed, as we followed similar manufacturer protocols for inserting the device. It is possible the bone mass density of the porcine femurs differed between studies. This was not reported by Kousa and colleagues,13 and we did not perform testing either. However, all the porcine femurs were about the same age for testing of each device in this study.
Bio-TransFix has also been compared with various implants, but not in the same study. Brown and colleagues8 found the TransFix device significantly stiffer than the Endobutton CL. Shen and colleagues16 determined that TransFix had significantly lower residual displacement compared with Endobutton CL. Milano and colleagues15 compared multiple cortical suspensory fixation devices, including Endobutton CL, with TransFix and Bio-TransFix, and concluded the cortical-cancellous devices (TransFix, Bio-TransFix) offered the best and most predictable results in terms of elongation, fixation strength, and stiffness. TransFix has also been shown to be superior to interference screw fixation in biomechanical studies.10,15
Clinical outcomes of studies using TransFix for femoral fixation have been favorable, with improved Lysholm scores and improved laxity according to the KT-1000 test.17 However, multiple prospective studies have found no clinical difference in knee laxity between interference screw and Endobutton at 1- to 2-year follow-up18-20 and no difference in clinical outcome scores, such as the International Knee Documentation Committee score.11,18-20
Although these studies have shown no major clinical differences at short-term follow-up, the early aggressive rehabilitation period is the larger concern. Our study clearly demonstrated the biomechanical strength of transcondylar devices over other devices. The concern with transcondylar devices (vs other devices) is the increased difficulty that inexperienced surgeons have inserting them. In addition, when removed, transcondylar devices leave a large bone void.
In the present study, an important concern with femoral graft fixation is the poor performance of interference screws. Other authors recently expressed concern with using interference screws in soft-tissue ACL grafts—based on biomechanical study results of increased slippage, bone tunnel widening, and less strength.7 In the present study, Delta screws consistently performed poorest with respect to ultimate LTF, residual displacement, and stiffness. Only 20% of these screws completed 1500 cycles. Poor performance of interference screws has also been seen in other studies in tibial graft fixation21,22 and femoral graft fixation.13-15 Given their poor biomechanical properties, as seen in our study and these other studies, we think use of an interference screw alone is a poor choice for fixation.
Combined fixation techniques—interference screw plus other device(s)—may be used in clinical practice, but the present study did not evaluate any. In a biomechanical study, Yoo and colleagues23 compared an interference screw; an interference screw plus a cortical screw and a spiked washer; and a cortical screw and a spiked washer used alone in the tibia. Stiffness nearly doubled, residual displacement was less, and ultimate LTF was significantly higher in the group with the interference screw plus the cortical screw and the spiked washer. In a similar study involving femoral fixation, Oh and colleagues24 demonstrated improved stiffness, residual displacement, and LTF in cyclic testing with the combination of interference screw and Endobutton CL, compared with Endobutton CL alone. Further studies may include direct comparisons of additional femoral fixation techniques using more than 1 device.
The Zip Loop, or Toggle Loc with Zip Loop technology, is a suspensory cortical fixation device. It was initially designed for use in ACL fixation but has also been used in other surgeries, including distal biceps repair25 and ulnar collateral ligament reconstruction.26 The device itself is easy to use; more important, it allows for adjustment of graft length within the bone tunnel after deployment of the cortical fixation. Few biomechanical studies have been conducted with Zip Loop.9,12 The present study is the first to compare Zip Loop with devices other than suspensory cortical fixation devices. Zip Loop performed very well in LTF testing but had lower stiffness and higher residual displacement compared with the transcondylar fixation devices. Despite these findings, we have continued to use this device for femoral fixation in ACL reconstruction because of its ease of insertion, the ability to adjust graft tension within the bone tunnel, and the difficulties encountered inserting and removing transcondylar fixation.
We recognize the limitations in our study design with respect to how axial and cyclical loading compares with the physiologic orientation of the ACL during ambulation and running activities. This biomechanical study was not able to replicate these types of activities. However, it did provide good data supporting early rehabilitation with various fixation devices, though concern with use of interference screws remains.
Conclusion
Superior strength in fixation of hamstring grafts in the femur was demonstrated by Bone Mulch screws, followed closely by Bio-TransFix. Delta screws demonstrated poor displacement, stiffness, and LTF. When used as the sole femoral fixation device, a device with low LTF, decreased stiffness, and high residual displacement should be used cautiously in patients undergoing aggressive rehabilitation.
Anterior cruciate ligament (ACL) reconstruction remains one of the most common orthopedic procedures; almost 100,000 are performed in the United States each year, and they are among the procedures more commonly performed by surgeons specializing in sports medicine and by general orthopedists.1,2 Recent years have seen a trend toward replacing the gold standard of bone–patellar tendon–bone autograft with autograft or allograft hamstring tendon in ACL reconstruction.3 This shift is being made to try to avoid the donor-site morbidity of patellar tendon autografts and decrease the incidence of postoperative anterior knee pain. With increased use of hamstring grafts in ACL reconstruction, graft fixation strength has become a priority in attempts to optimize recovery and rehabilitation.4
Rigid fixation of hamstring grafts is now recognized as a crucial factor in the long-term success of ACL reconstruction. Grafts must withstand both early rehabilitation forces as high as 500 N5 and stresses to the native ACL during healing, which may take up to 12 weeks for soft-tissue incorporation.6
The challenge has been to engineer devices that provide stable, rigid graft fixation that allows expeditious tendon-to-bone healing and increased construct stiffness. Many new fixation devices are being marketed, and there is controversy regarding which provides the best stability and strength.7 Several studies have tested various fixation devices,8-16 but so far several devices have not been compared with one another.
We conducted a study to determine if femoral hamstring fixation devices used in ACL reconstruction differ in fixation strength. We hypothesized we would find no differences.
Materials and Methods
Fifty porcine femurs were harvested after the animals had been euthanized for other studies at our institution. Our study was approved by the institutional animal care and use committee. Specimens were stored at –25°C and, on day of testing, thawed to room temperature. Gracilis and semitendinosus tendon grafts were donated by a tissue bank (LifeNet Health, Virginia Beach, Virginia). The grafts were stored at –25°C; on day of testing, tendons were thawed to room temperature.
We evaluated 5 different femoral fixation devices (Figure 1): Delta screw and Bio-TransFix (Arthrex, Naples, Florida) and Bone Mulch screw, EZLoc, and Zip Loop (Arthrotek, Warsaw, Indiana). For each device, 10 ACL fixation constructs were tested.
Quadrupled human semitendinosus–gracilis tendon grafts were fixed into the femurs using the 5 femoral fixation devices. All fixations were done to manufacturer specifications.
Cyclic loading was followed by testing with the load-to-failure (LTF) protocol described by Kousa and colleagues.13 Specimens were tested in a custom load fixture (Figure 2). The base fixture used an adjustable angle vise mounted on a free rotary stage and a free x-y translation stage. This system allowed the load axis to be oriented to and aligned with the graft tunnel in the porcine femur, preventing off-axis or torsional loading of the grafts.
Pneumatic grips equipped with a custom pincer attachment allowed the graft to be grasped under a constant grip force during testing, regardless of graft thinning under tensile loads. Graft specimens were initially looped over a 3.8-mm horizontal metal shaft, and the 2 strands were double-looped at the graft insertion site. The 2 free strands were then drawn up around the metal shaft, and the shaft was placed above the serrated jaws. The metal shaft with enveloping tendon strands rested on a flat shelf at the top of the grip serrations. This configuration prevented the metal shaft and tendon strands from being pulled through the serrations when compressive force was applied to the jaws.
Before the study, the grip design was tested. There was no detectable relative motion of the strands at the grip end during graft testing to failure. The pincer attachment allowed close approach of the grips to the specimen at all femoral condyle orientations, so that a 25-mm length of exposed graft could be obtained for each specimen under initial conditions.
In the cyclic loading test, the load was applied parallel to the long axis of the femoral tunnel. A 50-N preload was initially applied to each specimen for 10 seconds, and the length of the exposed graft between grips and graft insertion was recorded. Subsequently, 1500 loading cycles between 50 N and 200 N at a rate of 1 cycle per 2 seconds (0.5 Hz) were performed. Standard force-displacement curves were then generated.
Specimens surviving the cyclic loading then underwent a single-cycle LTF test in which the load was applied parallel to the long axis of the drill hole at a rate of 50 mm per minute.
Residual displacement, stiffness, and ultimate LTF data were recorded from the force-displacement curves. Residual displacement data were generated from the cyclic loading test; residual displacement was determined by subtracting preload displacement from displacement at 1, 10, 50, 100, 250, 500, 1000, and 1500 cycles. Stiffness data were generated from the single-cycle LTF test; stiffness was defined as the linear region slope of the force-displacement curve corresponding to the steepest straight-line tangent to the loading curve. Ultimate LTF data were generated from the single-cycle LTF test; ultimate LTF was defined as the maximum load sustained by the specimen during a constant-displacement-rate tensile test for graft pullout.
Statistical analysis generated standard descriptive statistics: means, standard deviations, and proportions. One-way analysis of variance (ANOVA) was used to determine any statistically significant differences in stiffness, yield load, and residual displacement between the different fixation devices. Differences in force (load) between the single cycle and the cyclic loading test were determined by ANOVA. P < .05 was considered statistically significant for all tests.
Results
The modes of failure for the devices differed slightly (Table). Bone Mulch screw failed with a fracture through the femoral condyle extending to the bone tunnel. Zip Loop and EZLoc failed by pulling through their cortical attachment on the lateral femoral condyle. Bio-TransFix broke in the tunnel during LTF. Delta screw failed with slippage of the fixation device, and the tendons pulled out through the tunnel.
For the cyclic loading tests, only 2 of the 10 Delta screws completed the 1500-cycle loading test before failure. Of the 8 Delta screws that did not complete this testing, the majority failed after about 100 cycles. All 10 tests of Bone Mulch, Zip Loop, EZLoc, and Bio-TransFix completed the 1500-cycle loading test.
Residual displacement data were calculated from cyclic loading tests (Table). Mean (SD) residual displacement was lowest for Bio-TransFix at 4.1 (0.4) mm, followed by Bone Mulch at 5.2 (1.0) mm, EZLoc at 6.4 (1.1) mm, and Zip Loop at 6.8 (1.3) mm. Delta screws at 8.2 (1.4) mm had the highest residual displacement, though only 2 completed the cyclic tests. Bio-TransFix had significantly (P < .001) less residual displacement compared with EZLoc, Zip Loop, and Delta. Bone Mulch had significantly less residual displacement compared with Zip Loop (P < .05) and Delta (P < .01).
Stiffness data were calculated from LTF tests (Table). Mean (SD) stiffness was highest for Bone Mulch at 218 (25.9) N/mm, followed by Bio-TransFix at 171 (24.2) N/mm, EZLoc at 122 (24.1) N/mm, Zip Loop at 105 (18.9) N/mm, and Delta at 84 (16.4) N/mm. Bone Mulch had significantly (P < .001) higher stiffness compared with Bio-TransFix, EZLoc, Zip Loop, and Delta. Bio-TransFix had significantly (P < .001) higher stiffness compared with EZLoc, Zip Loop, and Delta.
Mean (SD) ultimate LTF was highest for Bone Mulch at 867 (164) N, followed by Zip Loop at 615 (72.3) N, Bio-TransFix at 552 (141) N, EZLoc at 476 (89.7) N, and Delta at 410 (65.3) N (Table). Bone Mulch failed at a statistically significantly (P < .001) higher load compared with Zip Loop, Bio-TransFix, EZLoc, and Delta. There were no significant differences in mean LTF among Zip Loop, Bio-TransFix, EZLoc, and Delta.
Discussion
In this biomechanical comparison of 5 different femoral fixation devices, the Bone Mulch screw had results superior to those of the other implants. Bone Mulch failed at higher LTF and higher stiffness. Bio-TransFix performed well and had residual displacement similar to that of Bone Mulch, but significantly lower LTF. Overall, EZLoc and Zip Loop were similar to each other in performance. The Delta (interference) screw performed poorly with respect to LTF, residual displacement, and stiffness; a large proportion of these screws failed early into cyclic loading.
Bone Mulch and Bio-TransFix overall outperformed the other fixation devices. These 2 devices are cortical-cancellous suspension devices, which provide transcondylar fixation and resist tensile forces perpendicular to the pullout force. Multiple biomechanical studies have found superior performance for these types of devices compared with various implants.10,13,15,16
Our results were similar to those of Kousa and colleagues,13 who found the Bone Mulch screw to provide highest LTF, highest stiffness, and lowest residual displacement. Another study found significantly higher stiffness for the Bone Mulch screw than for the Endobutton, a cortical suspensory fixation device.14 Bone Mulch failure modes differed, however. In the study by Kousa and colleagues,13 3 specimens failed with bending of the screw tip, and 7 failed with rupture of the tendon loop. All specimens in our study failed with fractures through the condyle. It is unclear why the failure modes differed, as we followed similar manufacturer protocols for inserting the device. It is possible the bone mass density of the porcine femurs differed between studies. This was not reported by Kousa and colleagues,13 and we did not perform testing either. However, all the porcine femurs were about the same age for testing of each device in this study.
Bio-TransFix has also been compared with various implants, but not in the same study. Brown and colleagues8 found the TransFix device significantly stiffer than the Endobutton CL. Shen and colleagues16 determined that TransFix had significantly lower residual displacement compared with Endobutton CL. Milano and colleagues15 compared multiple cortical suspensory fixation devices, including Endobutton CL, with TransFix and Bio-TransFix, and concluded the cortical-cancellous devices (TransFix, Bio-TransFix) offered the best and most predictable results in terms of elongation, fixation strength, and stiffness. TransFix has also been shown to be superior to interference screw fixation in biomechanical studies.10,15
Clinical outcomes of studies using TransFix for femoral fixation have been favorable, with improved Lysholm scores and improved laxity according to the KT-1000 test.17 However, multiple prospective studies have found no clinical difference in knee laxity between interference screw and Endobutton at 1- to 2-year follow-up18-20 and no difference in clinical outcome scores, such as the International Knee Documentation Committee score.11,18-20
Although these studies have shown no major clinical differences at short-term follow-up, the early aggressive rehabilitation period is the larger concern. Our study clearly demonstrated the biomechanical strength of transcondylar devices over other devices. The concern with transcondylar devices (vs other devices) is the increased difficulty that inexperienced surgeons have inserting them. In addition, when removed, transcondylar devices leave a large bone void.
In the present study, an important concern with femoral graft fixation is the poor performance of interference screws. Other authors recently expressed concern with using interference screws in soft-tissue ACL grafts—based on biomechanical study results of increased slippage, bone tunnel widening, and less strength.7 In the present study, Delta screws consistently performed poorest with respect to ultimate LTF, residual displacement, and stiffness. Only 20% of these screws completed 1500 cycles. Poor performance of interference screws has also been seen in other studies in tibial graft fixation21,22 and femoral graft fixation.13-15 Given their poor biomechanical properties, as seen in our study and these other studies, we think use of an interference screw alone is a poor choice for fixation.
Combined fixation techniques—interference screw plus other device(s)—may be used in clinical practice, but the present study did not evaluate any. In a biomechanical study, Yoo and colleagues23 compared an interference screw; an interference screw plus a cortical screw and a spiked washer; and a cortical screw and a spiked washer used alone in the tibia. Stiffness nearly doubled, residual displacement was less, and ultimate LTF was significantly higher in the group with the interference screw plus the cortical screw and the spiked washer. In a similar study involving femoral fixation, Oh and colleagues24 demonstrated improved stiffness, residual displacement, and LTF in cyclic testing with the combination of interference screw and Endobutton CL, compared with Endobutton CL alone. Further studies may include direct comparisons of additional femoral fixation techniques using more than 1 device.
The Zip Loop, or Toggle Loc with Zip Loop technology, is a suspensory cortical fixation device. It was initially designed for use in ACL fixation but has also been used in other surgeries, including distal biceps repair25 and ulnar collateral ligament reconstruction.26 The device itself is easy to use; more important, it allows for adjustment of graft length within the bone tunnel after deployment of the cortical fixation. Few biomechanical studies have been conducted with Zip Loop.9,12 The present study is the first to compare Zip Loop with devices other than suspensory cortical fixation devices. Zip Loop performed very well in LTF testing but had lower stiffness and higher residual displacement compared with the transcondylar fixation devices. Despite these findings, we have continued to use this device for femoral fixation in ACL reconstruction because of its ease of insertion, the ability to adjust graft tension within the bone tunnel, and the difficulties encountered inserting and removing transcondylar fixation.
We recognize the limitations in our study design with respect to how axial and cyclical loading compares with the physiologic orientation of the ACL during ambulation and running activities. This biomechanical study was not able to replicate these types of activities. However, it did provide good data supporting early rehabilitation with various fixation devices, though concern with use of interference screws remains.
Conclusion
Superior strength in fixation of hamstring grafts in the femur was demonstrated by Bone Mulch screws, followed closely by Bio-TransFix. Delta screws demonstrated poor displacement, stiffness, and LTF. When used as the sole femoral fixation device, a device with low LTF, decreased stiffness, and high residual displacement should be used cautiously in patients undergoing aggressive rehabilitation.
1. Dooley PJ, Chan DS, Dainty KN, Mohtadi NGH, Whelan DB. Patellar tendon versus hamstring autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev. 2006;(2):CD005960.
2. Garrett WE Jr, Swiontkowski MF, Weinsten JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
3. West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197-207.
4. Hapa O, Barber FA. ACL fixation devices. Sports Med Arthrosc. 2009;17(4):217-223.
5. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
6. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
7. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
8. Brown CH Jr, Wilson DR, Hecker AT, Ferragamo M. Graft-bone motion and tensile properties of hamstring and patellar tendon anterior cruciate ligament femoral graft fixation under cyclic loading. Arthroscopy. 2004;20(9):922-935.
9. Conner CS, Perez BA, Morris RP, Buckner JW, Buford WL Jr, Ivey FM. Three femoral fixation devices for anterior cruciate ligament reconstruction: comparison of fixation on the lateral cortex versus the anterior cortex. Arthroscopy. 2010;26(6):796-807.
10. Fabbriciani C, Mulas PD, Ziranu F, Deriu L, Zarelli D, Milano G. Mechanical analysis of fixation methods for anterior cruciate ligament reconstruction with hamstring tendon graft. An experimental study in sheep knees. Knee. 2005;12(2):135-138.
11. Harilainen A, Sandelin J, Jansson KA. Cross-pin femoral fixation versus metal interference screw fixation in anterior cruciate ligament reconstruction with hamstring tendons: results of a controlled prospective randomized study with 2-year follow-up. Arthroscopy. 2005;21(1):25-33.
12. Kamelger FS, Onder U, Schmoelz W, Tecklenburg K, Arora R, Fink C. Suspensory fixation of grafts in anterior cruciate ligament reconstruction: a biomechanical comparison of 3 implants. Arthroscopy. 2009;25(7):767-776.
13. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med. 2003;31(2):174-181.
14. Kudo T, Tohyama H, Minami A, Yasuda K. The effect of cyclic loading on the biomechanical characteristics of the femur–graft–tibia complex after anterior cruciate ligament reconstruction using Bone Mulch screw/WasherLoc fixation. Clin Biomech. 2005;20(4):414-420.
15. Milano G, Mulas PD, Ziranu F, Piras S, Manunta A, Fabbriciani C. Comparison between different femoral fixation devices for ACL reconstruction with doubled hamstring tendon graft: a biomechanical analysis. Arthroscopy. 2006;22(6):660-668.
16. Shen HC, Chang JH, Lee CH, et al. Biomechanical comparison of cross-pin and Endobutton-CL femoral fixation of a flexor tendon graft for anterior cruciate ligament reconstruction—a porcine femur–graft–tibia complex study. J Surg Res. 2010;161(2):282-287.
17. Asik M, Sen C, Tuncay I, Erdil M, Avci C, Taser OF. The mid- to long-term results of the anterior cruciate ligament reconstruction with hamstring tendons using Transfix technique. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):965-972.
18. Capuano L, Hardy P, Longo UG, Denaro V, Maffulli N. No difference in clinical results between femoral transfixation and bio-interference screw fixation in hamstring tendon ACL reconstruction. A preliminary study. Knee. 2008;15(3):174-179.
19. Price R, Stoney J, Brown G. Prospective randomized comparison of Endobutton versus cross-pin femoral fixation in hamstring anterior cruciate ligament reconstruction with 2-year follow-up. ANZ J Surg. 2010;80(3):162-165.
20. Rose T, Hepp P, Venus J, Stockmar C, Josten C, Lill H. Prospective randomized clinical comparison of femoral transfixation versus bioscrew fixation in hamstring tendon ACL reconstruction—a preliminary report. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):730-738.
21. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med. 2003;31(2):182-188.
22. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
23. Yoo JC, Ahn JH, Kim JH, et al. Biomechanical testing of hybrid hamstring graft tibial fixation in anterior cruciate ligament reconstruction. Knee. 2006;13(6):455-459.
24. Oh YH, Namkoong S, Strauss EJ, et al. Hybrid femoral fixation of soft-tissue grafts in anterior cruciate ligament reconstruction using the Endobutton CL and bioabsorbable interference screws: a biomechanical study. Arthroscopy. 2006;22(11):1218-1224.
25. DiRaimo MJ Jr, Maney MD, Deitch JR. Distal biceps tendon repair using the Toggle Loc with Zip Loop. Orthopedics. 2008;31(12). doi: 10.3928/01477447-20081201-05.
26. Morgan RJ, Starman JS, Habet NA, et al. A biomechanical evaluation of ulnar collateral ligament reconstruction using a novel technique for ulnar-sided fixation. Am J Sports Med. 2010;38(7):1448-1455.
1. Dooley PJ, Chan DS, Dainty KN, Mohtadi NGH, Whelan DB. Patellar tendon versus hamstring autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev. 2006;(2):CD005960.
2. Garrett WE Jr, Swiontkowski MF, Weinsten JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
3. West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197-207.
4. Hapa O, Barber FA. ACL fixation devices. Sports Med Arthrosc. 2009;17(4):217-223.
5. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
6. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
7. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
8. Brown CH Jr, Wilson DR, Hecker AT, Ferragamo M. Graft-bone motion and tensile properties of hamstring and patellar tendon anterior cruciate ligament femoral graft fixation under cyclic loading. Arthroscopy. 2004;20(9):922-935.
9. Conner CS, Perez BA, Morris RP, Buckner JW, Buford WL Jr, Ivey FM. Three femoral fixation devices for anterior cruciate ligament reconstruction: comparison of fixation on the lateral cortex versus the anterior cortex. Arthroscopy. 2010;26(6):796-807.
10. Fabbriciani C, Mulas PD, Ziranu F, Deriu L, Zarelli D, Milano G. Mechanical analysis of fixation methods for anterior cruciate ligament reconstruction with hamstring tendon graft. An experimental study in sheep knees. Knee. 2005;12(2):135-138.
11. Harilainen A, Sandelin J, Jansson KA. Cross-pin femoral fixation versus metal interference screw fixation in anterior cruciate ligament reconstruction with hamstring tendons: results of a controlled prospective randomized study with 2-year follow-up. Arthroscopy. 2005;21(1):25-33.
12. Kamelger FS, Onder U, Schmoelz W, Tecklenburg K, Arora R, Fink C. Suspensory fixation of grafts in anterior cruciate ligament reconstruction: a biomechanical comparison of 3 implants. Arthroscopy. 2009;25(7):767-776.
13. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med. 2003;31(2):174-181.
14. Kudo T, Tohyama H, Minami A, Yasuda K. The effect of cyclic loading on the biomechanical characteristics of the femur–graft–tibia complex after anterior cruciate ligament reconstruction using Bone Mulch screw/WasherLoc fixation. Clin Biomech. 2005;20(4):414-420.
15. Milano G, Mulas PD, Ziranu F, Piras S, Manunta A, Fabbriciani C. Comparison between different femoral fixation devices for ACL reconstruction with doubled hamstring tendon graft: a biomechanical analysis. Arthroscopy. 2006;22(6):660-668.
16. Shen HC, Chang JH, Lee CH, et al. Biomechanical comparison of cross-pin and Endobutton-CL femoral fixation of a flexor tendon graft for anterior cruciate ligament reconstruction—a porcine femur–graft–tibia complex study. J Surg Res. 2010;161(2):282-287.
17. Asik M, Sen C, Tuncay I, Erdil M, Avci C, Taser OF. The mid- to long-term results of the anterior cruciate ligament reconstruction with hamstring tendons using Transfix technique. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):965-972.
18. Capuano L, Hardy P, Longo UG, Denaro V, Maffulli N. No difference in clinical results between femoral transfixation and bio-interference screw fixation in hamstring tendon ACL reconstruction. A preliminary study. Knee. 2008;15(3):174-179.
19. Price R, Stoney J, Brown G. Prospective randomized comparison of Endobutton versus cross-pin femoral fixation in hamstring anterior cruciate ligament reconstruction with 2-year follow-up. ANZ J Surg. 2010;80(3):162-165.
20. Rose T, Hepp P, Venus J, Stockmar C, Josten C, Lill H. Prospective randomized clinical comparison of femoral transfixation versus bioscrew fixation in hamstring tendon ACL reconstruction—a preliminary report. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):730-738.
21. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med. 2003;31(2):182-188.
22. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
23. Yoo JC, Ahn JH, Kim JH, et al. Biomechanical testing of hybrid hamstring graft tibial fixation in anterior cruciate ligament reconstruction. Knee. 2006;13(6):455-459.
24. Oh YH, Namkoong S, Strauss EJ, et al. Hybrid femoral fixation of soft-tissue grafts in anterior cruciate ligament reconstruction using the Endobutton CL and bioabsorbable interference screws: a biomechanical study. Arthroscopy. 2006;22(11):1218-1224.
25. DiRaimo MJ Jr, Maney MD, Deitch JR. Distal biceps tendon repair using the Toggle Loc with Zip Loop. Orthopedics. 2008;31(12). doi: 10.3928/01477447-20081201-05.
26. Morgan RJ, Starman JS, Habet NA, et al. A biomechanical evaluation of ulnar collateral ligament reconstruction using a novel technique for ulnar-sided fixation. Am J Sports Med. 2010;38(7):1448-1455.
Physical Examination of the Throwing Athlete’s Elbow
Understanding the pathomechanics of throwing and the accompanying elbow injuries is the groundwork for conducting a directed history taking and a physical examination that produce an accurate diagnosis of elbow injuries in throwing athletes. Advances in physical examination techniques have improved our ability to accurately diagnose and treat throwers’ athletic elbow disorders.
Throwing imposes an extremely high valgus stress (approaching 60-65 Nm) across the elbow. This high stress occurs during the cocking and acceleration phases of the overhead throwing motion.1-3 The valgus stress generates tension on the medial elbow, compression on the lateral elbow, and shear on the posterior aspect of the elbow. These forces cause predictable injury patterns in different parts of throwers’ elbows. Physical examination performed in a systematic anatomical fashion can enhance predictable and accurate elbow injury diagnosis. In this article, we outline 5 points in a systematic approach to physical examination of a throwing athlete’s elbow.
1. Perform a general upper extremity examination
Cervical spine and shoulder girdle
In the initial examination, the cervical spine and the entire affected upper extremity should be quickly assessed. Assessment of the cervical spine should include palpation, range of motion (ROM), and basic provocative testing, such as the Spurling test, to evaluate for radiculopathy caused by foraminal compression. Posture, asymmetry, atrophy, edema, ecchymosis, and any other deformity should be noted. For example, atrophy of the neck and shoulders suggests underlying neuropathy. In addition, fullness of the supraclavicular region and local tenderness or bruit suggest vasculopathy. Symptomatic compression of the subclavian artery and vein between the anterior and middle scalene muscles may present as weakness, fullness, heaviness, and early fatigue. Physical signs include coolness, pallor, claudication, engorgement, and edema in the arm.4 Thoracic outlet syndrome can manifest as effort-induced vague pain at the arm and elbow.5 If this syndrome is suspected, an Adson test should be performed. With the patient’s neck extended and rotated away from the affected side, the examiner, standing next to the patient, palpates the radial pulse with the patient’s elbow extended (Figure 1A). Next, the examiner abducts, extends, and externally rotates the patient’s shoulder (Figure 1B) while the patient alternates between opening and closing the fist (Figure 1C). A decrease or absence in pulse strength from the starting position is a positive test result.
Last, the shoulder and scapulae should be assessed, as an affected shoulder or dyskinetic scapula can lead to improper mechanics of the kinetic chain at the elbow. The shoulder should be palpated, and ROM, strength, and stability should be assessed. Glenohumeral internal rotation deficit is associated with medial collateral ligament (MCL) tears; if present, this deficit should be addressed.6
Elbow
Inspection should reveal a normal carrying angle of about 11° to 14° of valgus in men and 13° to 16° in women. In immature athletes, increased valgus stresses from repetitive overhead throwing can cause medial epicondylar hypertrophy, and carrying angles of more than 15° are common.7-9
Active and passive ROM should be assessed. Normal ROM is about 0° extension and 140° flexion with 80° of supination and pronation. For determination of pathologic differences, ROM should always be compared between the affected and the contralateral sides. Painful loss of motion may be caused by soft-tissue swelling or contracture, effusion, bony impingement, or loose bodies. Crepitus, locking, catching, or another mechanical symptom may indicate loose bodies or chondral injury. Firm, mechanical blocks to ROM during flexion may indicate osteophyte formation in the coronoid fossa, and mechanical blocks to ROM during extension may indicate osteophyte formation in the olecranon fossa. Pain elicited at the end points of motion is caused by osteophytes and impingement, whereas pain elicited during the mid-arc of motion is often caused by osteochondral lesions. Terminal extension, often the first motion lost after injury, may signal intra-articular pathology, if symptomatic. However, throwing athletes may present with developmental flexion contractures of up to 20°.10
2. Examine the medial aspect of the elbow
The medial epicondyle, easy to recognize as a bony prominence on the medial side of the distal humerus, serves as an attachment site for the MCL, pronator teres, and the common flexor tendon. In throwers, assessing the MCL is crucial. The MCL should be palpated from its origin on the inferior aspect of the medial epicondyle moving distally to the sublime tubercle of the proximal ulna. Tenderness at any point along the ligament can indicate a range of ligament pathology, from attenuation to complete rupture.
The MCL is further assessed with stress tests, most commonly the valgus stress test, the milking maneuver, and the moving valgus stress test. Of these 3 procedures, the moving valgus stress test is perhaps the most sensitive and specific for MCL injury, and is the test preferred by the authors.11 This test takes into account shoulder position, simulates the position of throwing, and can account for bony structures that provide stability at more than 120° of flexion. We prefer to position the patient supine on the examining table to help stabilize the shoulder and humerus and to relax the patient. The shoulder is placed in abduction and external rotation while the examiner holds the thumb with one hand and supports the elbow with the other. The elbow is extended (Figure 2A) and flexed (Figure 2B) while valgus stress is applied. A positive test elicits pain localized to the MCL at the arc of motion between 80° to 120°.12 Pain at positions near full extension with the moving valgus stress test may also indicate chondral damage at the posteromedial trochlea.13
During pitching, the tensile demand on the MCL is reduced by the action of the flexor-pronator mass. It is common to see a flexor-pronator mass injury concurrent with MCL injury.14 Medial epicondyle tenderness that increases with resisted wrist flexion may signal flexor-pronator injury, though, classically, flexor-pronator muscle strains and tears produce pain anterior and distal to the medial epicondyle.15
Traction, compression, and friction at the medial elbow can irritate the ulnar nerve. This nerve should be inspected and palpated along its course at the cubital tunnel to determine its location and stability. Ulnar nerve hypermobility, which has been identified in 37% of elbows, can be determined by having the patient actively flex the elbow with the forearm in supination, placing a finger at the posteromedial aspect of the medial humeral epicondyle, and having the patient actively extend the elbow.16 The nerve dislocates if trapped anterior to the examiner’s finger, perches if under the examiner’s finger, or is stable if still palpable in the groove posterior to the medial epicondyle.16
The distal band of the medial triceps tendon may also sublux over the medial epicondyle with elbow flexion. This subluxation, also known as snapping triceps syndrome, may cause pain or ulnar nerve symptoms.17 Bringing the elbow from extension to flexion may produce subluxation, first of the ulnar nerve and then of the medial triceps, in 2 separate “snaps.” Tenderness can be elicited along the medial triceps muscle.
Ulnar neuritis is caused by traction injury, such as with dynamic pitching, nerve subluxation, or compression at the cubital tunnel. With MCL injury and valgus instability, the ulnar nerve can become irritated as it becomes stretched because of medial elbow laxity.18 The nerve can also be damaged during flexion as the cubital tunnel retinaculum tightens, decreasing the space available for the nerve.19 This concept is applied during the elbow flexion compression test. A positive test may elicit tingling radiating toward the small finger or pain at the elbow or medial forearm when manual pressure is directly applied over the ulnar nerve between the posteromedial olecranon and the medial humeral epicondyle as the elbow is maximally flexed.20
3. Examine the lateral aspect of the elbow
Palpation of the lateral epicondyle, the radial head, and the olecranon tip assists in defining injury to the underlying anatomy. The anconeus “soft spot” (infracondylar recess) within the triangle formed by these 3 bony landmarks should be palpated for fullness, indicating a joint effusion, hemarthrosis, or even a subluxed or dislocated radial head.
While the medial elbow endures a large tensile load, throwing imposes a tremendous compressive force at the lateral elbow, particularly at the radiocapitellar joint. This joint may be tender and produce clicking with pronation and supination in patients with radiocapitellar arthrosis, symptomatic posterolateral synovial plica, or an inflamed radial bursa. Tenderness with crepitus that can be exacerbated with forceful flexion and extension may indicate radiocapitellar overload or loose bodies.
Long-term load transmission and subsequent degeneration of the articular surface may advance to osteochondritis dissecans (OCD). Examination for capitellar OCD reveals tenderness over the radiocapitellar joint and commonly a loss of 15° to 20° of extension. The active radiocapitellar compression test is positive for OCD lesions and elicits pain in the lateral compartment of the elbow when the patient pronates (Figure 3A) and supinates (Figure 3B) the forearm with the elbow axially loaded in extension.21
Microtrauma and inflammation may occur with repetitive eccentric overload. Although rare in throwing athletes, “tennis elbow” causes pain with gripping, and decreased grip strength. Tenderness caused by lateral epicondylitis is just anterior and distal to the epicondyle, at the origin of the extensor carpi radialis brevis. Pain is reproducible with passive wrist flexion and resisted wrist extension with the elbow extended (Cozen test).
Less commonly, athletes may complain of mechanical symptoms, such as snapping or catching with posterolateral elbow pain.22 These symptoms may be due to thickened or inflamed synovial plica causing impingement. A posterior radiocapitellar plica can be examined by bringing the elbow to full extension while applying valgus stress with the forearm in supination. Conversely, an anterior radiocapitellar plica can be examined with a valgus load on the elbow and passive flexion with the forearm in pronation.23 A palpable painful snap over the radiocapitellar joint is a positive test.
4. Examine the posterior aspect of the elbow
Posteriorly, palpation is focused on the triceps tendon and the olecranon tip. The elbow should be flexed to 30° to relax the triceps, isolate the olecranon, and allow for palpation of the olecranon fossa on either side of the triceps tendon. Tenderness at the posterolateral or posteromedial aspect of the olecranon should be noted. Warmth, fluctuance, or distension at the elbow may be caused by olecranon bursitis. The 3 heads of the triceps muscle should be palpated where they converge to form an aponeurosis, and tenderness or a palpable gap on any of the heads should be noted.
A combination of valgus force and a rapidly decelerating arm at the follow-through phase of pitching causes a shear force between the medial aspect of the olecranon tip and the olecranon fossa. This shear force can result in chondrolysis, osteophyte formation, and loose bodies, particularly in the posteromedial elbow. This valgus extension overload (VEO) syndrome often results in loss of full extension and symptoms, which may be attributed to osteophytes or fractured and nonunited fragments in the olecranon fossa or the olecranon tip. Frank crepitus may also be present with extension testing caused by loose bodies or synovial reaction over osteophytes. Assessing for VEO using the extension impingement test, the examiner places continuous valgus stress on the elbow while quickly extending from 20° to 30° of flexion (Figure 4A) to terminal extension (Figure 4B) repeatedly. The examiner repeats this without valgus load while palpating the posteromedial olecranon for tenderness to differentiate impingement caused by instability from pain over the medial olecranon without instability (Figure 4C). Particular attention should be focused posteriorly in athletes with medial instability, as MCL injuries and VEO syndrome often occur in conjunction in the throwing athlete.
Repetitive acceleration and deceleration of the arm can also cause stress fractures. With stress fractures, pain is often noted more distal and lateral on the olecranon, but tenderness may be palpable medially from posteromedial impaction that occurs from the valgus load during the overhead throwing motion. In immature athletes, the repetitive sudden snap of full extension in the deceleration phase of throwing can cause olecranon apophysitis. Frank avulsions can occur as well but are usually preceded by chronic posterior elbow pain with possible loss of full extension.
The late cocking phase of the throwing motion (just before throwing) hyperextends the elbow and places significant strain on the elbow. Repetitive strain can cause painful posterior impingement. The arm bar test is extremely sensitive (Figure 5).13 With the patient’s elbow extended, shoulder internally rotated, and hand on the examiner’s shoulder, the examiner pulls down on the olecranon to simulate forced extension and reproduces the pain associated with posteromedial impingement.
Last, though triceps tendon injuries are rare, ruptures most often occur at the origin of the lateral head of the triceps. As the initial swelling and ecchymosis subside, a palpable gap is pathognomonic for rupture. Extensor weakness can often be observed, but extension may still be possible from anconeus triceps expansion with the aid of gravity. With the elbow overhead, the athlete must extend the elbow against gravity and will exhibit weakness against resistance.
5. Examine the anterior aspect of the elbow
Anteriorly, the bulk of the flexor-pronator group restricts the extent of joint palpation, and the soft tissues are usually injured. The antecubital fossa is a triangular area on the anterior aspect of the elbow that is bounded superiorly by a horizontal line connecting the medial epicondyle to the lateral epicondyle of the humerus, medially by the lateral border of the pronator teres muscle and laterally by the medial border of the brachioradialis muscle. From lateral to medial, the antecubital fossa contains the radial nerve, the biceps brachii tendon, the brachial artery, and the median nerve. Evaluating this area is important because a visible defect, change in muscle contour, or proximal retraction of a muscle belly can indicate a muscular rupture. In particular, a distal biceps rupture (rare) may be accompanied by weakness and pain in supination and, to a lesser degree, in flexion. It is important to note that, in the case of a partial biceps rupture, ecchymosis may not appear, as the hematoma is confined by the intact lacertus fibrosis.24 The hook test can be used to evaluate for the presence of an intact distal biceps tendon (Figure 6).25 The patient abducts the shoulder, flexes the elbow to 90°, and actively supinates the forearm while the examiner attempts to hook an index finger laterally under the tendon. The test is negative if the finger can be inserted 1 cm under the tendon and positive if no cordlike structure can be hooked. Partial biceps tendon ruptures or tendinitis may exhibit tenderness of the distal biceps tendon and pain on resisted supination with a negative hook test. Often, resisted elbow flexion with the elbow at maximal extension elicits pain at the biceps insertion. Clicking with forearm rotation near the insertion of the tendon, which may be caused by an inflamed radial bursa between the distal biceps tendon and the radial tuberosity, may be associated with impending rupture.
Conclusion
Physical examination combined with thorough history taking usually provides a solid basis for a diagnosis, which in turn makes the value of surgical treatment more assured.
1. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
4. Aval SM, Durand P Jr, Shankwiler JA. Neurovascular injuries to the athlete’s shoulder: part II. J Am Acad Orthop Surg. 2007;15(5):281-289.
5. Strukel RJ, Garrick JG. Thoracic outlet compression in athletes: a report of four cases. Am J Sports Med. 1978;6(2):35-39.
6. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
7. Adams JE. Injury to the throwing arm. A study of traumatic changes in the elbow joints of boy baseball players. Calif Med. 1965;102:127-132.
8. Hang DW, Chao CM, Hang YS. A clinical and roentgenographic study of Little League elbow. Am J Sports Med. 2004;32(1):79-84.
9. King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop. 1969;(67):116-123.
10. Cain EL Jr, Dugas JR, Wolf RS, Andrews JR. Elbow injuries in throwing athletes: a current concepts review. Am J Sports Med. 2003;31(4):621-635.
11. Safran M, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy. 2005;21(11):1381-1395.
12. O’Driscoll SW, Lawton RL, Smith AM. The “moving valgus stress test” for medial collateral ligament tears of the elbow. Am J Sports Med. 2005;33(2):231-239.
13. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
14. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
15. Andrews JR, Whiteside JA, Buettner CM. Clinical evaluation of the elbow in throwers. Oper Tech Sports Med. 1996;4(2):77-83.
16. Calfee RP, Manske PR, Gelberman RH, Van Steyn MO, Steffen J, Goldfarb CA. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92(17):2801-2808.
17. Spinner RJ, Goldner RD. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. Anatomical and dynamic factors. J Bone Joint Surg Am. 1998;80(2):239-247.
18. Guerra JJ, Timmerman LA. Clinical anatomy, histology, & pathomechanics of the elbow in sports. Oper Tech Sports Med. 1996;4(2):69-76.
19. O’Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73(4):613-617.
20. Novak CB, Lee GW, Mackinnon SE, Lay L. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19(5):817-820.
21. Andrews JR. Bony injuries about the elbow in the throwing athlete. Instr Course Lect. 1985;34:323-331.
22. Kim DH, Gambardella RA, Elattrache NS, Yocum LA, Jobe FW. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am J Sports Med. 2006;34(3):438-444.
23. Antuna SA, O’Driscoll SW. Snapping plicae associated with radiocapitellar chondromalacia. Arthroscopy. 2001;17(5):491-495.
24. Bernstein AD, Breslow MJ, Jazrawi LM. Distal biceps tendon ruptures: a historical perspective and current concepts. Am J Orthop. 2001;30(3):
193-200.
25. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Understanding the pathomechanics of throwing and the accompanying elbow injuries is the groundwork for conducting a directed history taking and a physical examination that produce an accurate diagnosis of elbow injuries in throwing athletes. Advances in physical examination techniques have improved our ability to accurately diagnose and treat throwers’ athletic elbow disorders.
Throwing imposes an extremely high valgus stress (approaching 60-65 Nm) across the elbow. This high stress occurs during the cocking and acceleration phases of the overhead throwing motion.1-3 The valgus stress generates tension on the medial elbow, compression on the lateral elbow, and shear on the posterior aspect of the elbow. These forces cause predictable injury patterns in different parts of throwers’ elbows. Physical examination performed in a systematic anatomical fashion can enhance predictable and accurate elbow injury diagnosis. In this article, we outline 5 points in a systematic approach to physical examination of a throwing athlete’s elbow.
1. Perform a general upper extremity examination
Cervical spine and shoulder girdle
In the initial examination, the cervical spine and the entire affected upper extremity should be quickly assessed. Assessment of the cervical spine should include palpation, range of motion (ROM), and basic provocative testing, such as the Spurling test, to evaluate for radiculopathy caused by foraminal compression. Posture, asymmetry, atrophy, edema, ecchymosis, and any other deformity should be noted. For example, atrophy of the neck and shoulders suggests underlying neuropathy. In addition, fullness of the supraclavicular region and local tenderness or bruit suggest vasculopathy. Symptomatic compression of the subclavian artery and vein between the anterior and middle scalene muscles may present as weakness, fullness, heaviness, and early fatigue. Physical signs include coolness, pallor, claudication, engorgement, and edema in the arm.4 Thoracic outlet syndrome can manifest as effort-induced vague pain at the arm and elbow.5 If this syndrome is suspected, an Adson test should be performed. With the patient’s neck extended and rotated away from the affected side, the examiner, standing next to the patient, palpates the radial pulse with the patient’s elbow extended (Figure 1A). Next, the examiner abducts, extends, and externally rotates the patient’s shoulder (Figure 1B) while the patient alternates between opening and closing the fist (Figure 1C). A decrease or absence in pulse strength from the starting position is a positive test result.
Last, the shoulder and scapulae should be assessed, as an affected shoulder or dyskinetic scapula can lead to improper mechanics of the kinetic chain at the elbow. The shoulder should be palpated, and ROM, strength, and stability should be assessed. Glenohumeral internal rotation deficit is associated with medial collateral ligament (MCL) tears; if present, this deficit should be addressed.6
Elbow
Inspection should reveal a normal carrying angle of about 11° to 14° of valgus in men and 13° to 16° in women. In immature athletes, increased valgus stresses from repetitive overhead throwing can cause medial epicondylar hypertrophy, and carrying angles of more than 15° are common.7-9
Active and passive ROM should be assessed. Normal ROM is about 0° extension and 140° flexion with 80° of supination and pronation. For determination of pathologic differences, ROM should always be compared between the affected and the contralateral sides. Painful loss of motion may be caused by soft-tissue swelling or contracture, effusion, bony impingement, or loose bodies. Crepitus, locking, catching, or another mechanical symptom may indicate loose bodies or chondral injury. Firm, mechanical blocks to ROM during flexion may indicate osteophyte formation in the coronoid fossa, and mechanical blocks to ROM during extension may indicate osteophyte formation in the olecranon fossa. Pain elicited at the end points of motion is caused by osteophytes and impingement, whereas pain elicited during the mid-arc of motion is often caused by osteochondral lesions. Terminal extension, often the first motion lost after injury, may signal intra-articular pathology, if symptomatic. However, throwing athletes may present with developmental flexion contractures of up to 20°.10
2. Examine the medial aspect of the elbow
The medial epicondyle, easy to recognize as a bony prominence on the medial side of the distal humerus, serves as an attachment site for the MCL, pronator teres, and the common flexor tendon. In throwers, assessing the MCL is crucial. The MCL should be palpated from its origin on the inferior aspect of the medial epicondyle moving distally to the sublime tubercle of the proximal ulna. Tenderness at any point along the ligament can indicate a range of ligament pathology, from attenuation to complete rupture.
The MCL is further assessed with stress tests, most commonly the valgus stress test, the milking maneuver, and the moving valgus stress test. Of these 3 procedures, the moving valgus stress test is perhaps the most sensitive and specific for MCL injury, and is the test preferred by the authors.11 This test takes into account shoulder position, simulates the position of throwing, and can account for bony structures that provide stability at more than 120° of flexion. We prefer to position the patient supine on the examining table to help stabilize the shoulder and humerus and to relax the patient. The shoulder is placed in abduction and external rotation while the examiner holds the thumb with one hand and supports the elbow with the other. The elbow is extended (Figure 2A) and flexed (Figure 2B) while valgus stress is applied. A positive test elicits pain localized to the MCL at the arc of motion between 80° to 120°.12 Pain at positions near full extension with the moving valgus stress test may also indicate chondral damage at the posteromedial trochlea.13
During pitching, the tensile demand on the MCL is reduced by the action of the flexor-pronator mass. It is common to see a flexor-pronator mass injury concurrent with MCL injury.14 Medial epicondyle tenderness that increases with resisted wrist flexion may signal flexor-pronator injury, though, classically, flexor-pronator muscle strains and tears produce pain anterior and distal to the medial epicondyle.15
Traction, compression, and friction at the medial elbow can irritate the ulnar nerve. This nerve should be inspected and palpated along its course at the cubital tunnel to determine its location and stability. Ulnar nerve hypermobility, which has been identified in 37% of elbows, can be determined by having the patient actively flex the elbow with the forearm in supination, placing a finger at the posteromedial aspect of the medial humeral epicondyle, and having the patient actively extend the elbow.16 The nerve dislocates if trapped anterior to the examiner’s finger, perches if under the examiner’s finger, or is stable if still palpable in the groove posterior to the medial epicondyle.16
The distal band of the medial triceps tendon may also sublux over the medial epicondyle with elbow flexion. This subluxation, also known as snapping triceps syndrome, may cause pain or ulnar nerve symptoms.17 Bringing the elbow from extension to flexion may produce subluxation, first of the ulnar nerve and then of the medial triceps, in 2 separate “snaps.” Tenderness can be elicited along the medial triceps muscle.
Ulnar neuritis is caused by traction injury, such as with dynamic pitching, nerve subluxation, or compression at the cubital tunnel. With MCL injury and valgus instability, the ulnar nerve can become irritated as it becomes stretched because of medial elbow laxity.18 The nerve can also be damaged during flexion as the cubital tunnel retinaculum tightens, decreasing the space available for the nerve.19 This concept is applied during the elbow flexion compression test. A positive test may elicit tingling radiating toward the small finger or pain at the elbow or medial forearm when manual pressure is directly applied over the ulnar nerve between the posteromedial olecranon and the medial humeral epicondyle as the elbow is maximally flexed.20
3. Examine the lateral aspect of the elbow
Palpation of the lateral epicondyle, the radial head, and the olecranon tip assists in defining injury to the underlying anatomy. The anconeus “soft spot” (infracondylar recess) within the triangle formed by these 3 bony landmarks should be palpated for fullness, indicating a joint effusion, hemarthrosis, or even a subluxed or dislocated radial head.
While the medial elbow endures a large tensile load, throwing imposes a tremendous compressive force at the lateral elbow, particularly at the radiocapitellar joint. This joint may be tender and produce clicking with pronation and supination in patients with radiocapitellar arthrosis, symptomatic posterolateral synovial plica, or an inflamed radial bursa. Tenderness with crepitus that can be exacerbated with forceful flexion and extension may indicate radiocapitellar overload or loose bodies.
Long-term load transmission and subsequent degeneration of the articular surface may advance to osteochondritis dissecans (OCD). Examination for capitellar OCD reveals tenderness over the radiocapitellar joint and commonly a loss of 15° to 20° of extension. The active radiocapitellar compression test is positive for OCD lesions and elicits pain in the lateral compartment of the elbow when the patient pronates (Figure 3A) and supinates (Figure 3B) the forearm with the elbow axially loaded in extension.21
Microtrauma and inflammation may occur with repetitive eccentric overload. Although rare in throwing athletes, “tennis elbow” causes pain with gripping, and decreased grip strength. Tenderness caused by lateral epicondylitis is just anterior and distal to the epicondyle, at the origin of the extensor carpi radialis brevis. Pain is reproducible with passive wrist flexion and resisted wrist extension with the elbow extended (Cozen test).
Less commonly, athletes may complain of mechanical symptoms, such as snapping or catching with posterolateral elbow pain.22 These symptoms may be due to thickened or inflamed synovial plica causing impingement. A posterior radiocapitellar plica can be examined by bringing the elbow to full extension while applying valgus stress with the forearm in supination. Conversely, an anterior radiocapitellar plica can be examined with a valgus load on the elbow and passive flexion with the forearm in pronation.23 A palpable painful snap over the radiocapitellar joint is a positive test.
4. Examine the posterior aspect of the elbow
Posteriorly, palpation is focused on the triceps tendon and the olecranon tip. The elbow should be flexed to 30° to relax the triceps, isolate the olecranon, and allow for palpation of the olecranon fossa on either side of the triceps tendon. Tenderness at the posterolateral or posteromedial aspect of the olecranon should be noted. Warmth, fluctuance, or distension at the elbow may be caused by olecranon bursitis. The 3 heads of the triceps muscle should be palpated where they converge to form an aponeurosis, and tenderness or a palpable gap on any of the heads should be noted.
A combination of valgus force and a rapidly decelerating arm at the follow-through phase of pitching causes a shear force between the medial aspect of the olecranon tip and the olecranon fossa. This shear force can result in chondrolysis, osteophyte formation, and loose bodies, particularly in the posteromedial elbow. This valgus extension overload (VEO) syndrome often results in loss of full extension and symptoms, which may be attributed to osteophytes or fractured and nonunited fragments in the olecranon fossa or the olecranon tip. Frank crepitus may also be present with extension testing caused by loose bodies or synovial reaction over osteophytes. Assessing for VEO using the extension impingement test, the examiner places continuous valgus stress on the elbow while quickly extending from 20° to 30° of flexion (Figure 4A) to terminal extension (Figure 4B) repeatedly. The examiner repeats this without valgus load while palpating the posteromedial olecranon for tenderness to differentiate impingement caused by instability from pain over the medial olecranon without instability (Figure 4C). Particular attention should be focused posteriorly in athletes with medial instability, as MCL injuries and VEO syndrome often occur in conjunction in the throwing athlete.
Repetitive acceleration and deceleration of the arm can also cause stress fractures. With stress fractures, pain is often noted more distal and lateral on the olecranon, but tenderness may be palpable medially from posteromedial impaction that occurs from the valgus load during the overhead throwing motion. In immature athletes, the repetitive sudden snap of full extension in the deceleration phase of throwing can cause olecranon apophysitis. Frank avulsions can occur as well but are usually preceded by chronic posterior elbow pain with possible loss of full extension.
The late cocking phase of the throwing motion (just before throwing) hyperextends the elbow and places significant strain on the elbow. Repetitive strain can cause painful posterior impingement. The arm bar test is extremely sensitive (Figure 5).13 With the patient’s elbow extended, shoulder internally rotated, and hand on the examiner’s shoulder, the examiner pulls down on the olecranon to simulate forced extension and reproduces the pain associated with posteromedial impingement.
Last, though triceps tendon injuries are rare, ruptures most often occur at the origin of the lateral head of the triceps. As the initial swelling and ecchymosis subside, a palpable gap is pathognomonic for rupture. Extensor weakness can often be observed, but extension may still be possible from anconeus triceps expansion with the aid of gravity. With the elbow overhead, the athlete must extend the elbow against gravity and will exhibit weakness against resistance.
5. Examine the anterior aspect of the elbow
Anteriorly, the bulk of the flexor-pronator group restricts the extent of joint palpation, and the soft tissues are usually injured. The antecubital fossa is a triangular area on the anterior aspect of the elbow that is bounded superiorly by a horizontal line connecting the medial epicondyle to the lateral epicondyle of the humerus, medially by the lateral border of the pronator teres muscle and laterally by the medial border of the brachioradialis muscle. From lateral to medial, the antecubital fossa contains the radial nerve, the biceps brachii tendon, the brachial artery, and the median nerve. Evaluating this area is important because a visible defect, change in muscle contour, or proximal retraction of a muscle belly can indicate a muscular rupture. In particular, a distal biceps rupture (rare) may be accompanied by weakness and pain in supination and, to a lesser degree, in flexion. It is important to note that, in the case of a partial biceps rupture, ecchymosis may not appear, as the hematoma is confined by the intact lacertus fibrosis.24 The hook test can be used to evaluate for the presence of an intact distal biceps tendon (Figure 6).25 The patient abducts the shoulder, flexes the elbow to 90°, and actively supinates the forearm while the examiner attempts to hook an index finger laterally under the tendon. The test is negative if the finger can be inserted 1 cm under the tendon and positive if no cordlike structure can be hooked. Partial biceps tendon ruptures or tendinitis may exhibit tenderness of the distal biceps tendon and pain on resisted supination with a negative hook test. Often, resisted elbow flexion with the elbow at maximal extension elicits pain at the biceps insertion. Clicking with forearm rotation near the insertion of the tendon, which may be caused by an inflamed radial bursa between the distal biceps tendon and the radial tuberosity, may be associated with impending rupture.
Conclusion
Physical examination combined with thorough history taking usually provides a solid basis for a diagnosis, which in turn makes the value of surgical treatment more assured.
Understanding the pathomechanics of throwing and the accompanying elbow injuries is the groundwork for conducting a directed history taking and a physical examination that produce an accurate diagnosis of elbow injuries in throwing athletes. Advances in physical examination techniques have improved our ability to accurately diagnose and treat throwers’ athletic elbow disorders.
Throwing imposes an extremely high valgus stress (approaching 60-65 Nm) across the elbow. This high stress occurs during the cocking and acceleration phases of the overhead throwing motion.1-3 The valgus stress generates tension on the medial elbow, compression on the lateral elbow, and shear on the posterior aspect of the elbow. These forces cause predictable injury patterns in different parts of throwers’ elbows. Physical examination performed in a systematic anatomical fashion can enhance predictable and accurate elbow injury diagnosis. In this article, we outline 5 points in a systematic approach to physical examination of a throwing athlete’s elbow.
1. Perform a general upper extremity examination
Cervical spine and shoulder girdle
In the initial examination, the cervical spine and the entire affected upper extremity should be quickly assessed. Assessment of the cervical spine should include palpation, range of motion (ROM), and basic provocative testing, such as the Spurling test, to evaluate for radiculopathy caused by foraminal compression. Posture, asymmetry, atrophy, edema, ecchymosis, and any other deformity should be noted. For example, atrophy of the neck and shoulders suggests underlying neuropathy. In addition, fullness of the supraclavicular region and local tenderness or bruit suggest vasculopathy. Symptomatic compression of the subclavian artery and vein between the anterior and middle scalene muscles may present as weakness, fullness, heaviness, and early fatigue. Physical signs include coolness, pallor, claudication, engorgement, and edema in the arm.4 Thoracic outlet syndrome can manifest as effort-induced vague pain at the arm and elbow.5 If this syndrome is suspected, an Adson test should be performed. With the patient’s neck extended and rotated away from the affected side, the examiner, standing next to the patient, palpates the radial pulse with the patient’s elbow extended (Figure 1A). Next, the examiner abducts, extends, and externally rotates the patient’s shoulder (Figure 1B) while the patient alternates between opening and closing the fist (Figure 1C). A decrease or absence in pulse strength from the starting position is a positive test result.
Last, the shoulder and scapulae should be assessed, as an affected shoulder or dyskinetic scapula can lead to improper mechanics of the kinetic chain at the elbow. The shoulder should be palpated, and ROM, strength, and stability should be assessed. Glenohumeral internal rotation deficit is associated with medial collateral ligament (MCL) tears; if present, this deficit should be addressed.6
Elbow
Inspection should reveal a normal carrying angle of about 11° to 14° of valgus in men and 13° to 16° in women. In immature athletes, increased valgus stresses from repetitive overhead throwing can cause medial epicondylar hypertrophy, and carrying angles of more than 15° are common.7-9
Active and passive ROM should be assessed. Normal ROM is about 0° extension and 140° flexion with 80° of supination and pronation. For determination of pathologic differences, ROM should always be compared between the affected and the contralateral sides. Painful loss of motion may be caused by soft-tissue swelling or contracture, effusion, bony impingement, or loose bodies. Crepitus, locking, catching, or another mechanical symptom may indicate loose bodies or chondral injury. Firm, mechanical blocks to ROM during flexion may indicate osteophyte formation in the coronoid fossa, and mechanical blocks to ROM during extension may indicate osteophyte formation in the olecranon fossa. Pain elicited at the end points of motion is caused by osteophytes and impingement, whereas pain elicited during the mid-arc of motion is often caused by osteochondral lesions. Terminal extension, often the first motion lost after injury, may signal intra-articular pathology, if symptomatic. However, throwing athletes may present with developmental flexion contractures of up to 20°.10
2. Examine the medial aspect of the elbow
The medial epicondyle, easy to recognize as a bony prominence on the medial side of the distal humerus, serves as an attachment site for the MCL, pronator teres, and the common flexor tendon. In throwers, assessing the MCL is crucial. The MCL should be palpated from its origin on the inferior aspect of the medial epicondyle moving distally to the sublime tubercle of the proximal ulna. Tenderness at any point along the ligament can indicate a range of ligament pathology, from attenuation to complete rupture.
The MCL is further assessed with stress tests, most commonly the valgus stress test, the milking maneuver, and the moving valgus stress test. Of these 3 procedures, the moving valgus stress test is perhaps the most sensitive and specific for MCL injury, and is the test preferred by the authors.11 This test takes into account shoulder position, simulates the position of throwing, and can account for bony structures that provide stability at more than 120° of flexion. We prefer to position the patient supine on the examining table to help stabilize the shoulder and humerus and to relax the patient. The shoulder is placed in abduction and external rotation while the examiner holds the thumb with one hand and supports the elbow with the other. The elbow is extended (Figure 2A) and flexed (Figure 2B) while valgus stress is applied. A positive test elicits pain localized to the MCL at the arc of motion between 80° to 120°.12 Pain at positions near full extension with the moving valgus stress test may also indicate chondral damage at the posteromedial trochlea.13
During pitching, the tensile demand on the MCL is reduced by the action of the flexor-pronator mass. It is common to see a flexor-pronator mass injury concurrent with MCL injury.14 Medial epicondyle tenderness that increases with resisted wrist flexion may signal flexor-pronator injury, though, classically, flexor-pronator muscle strains and tears produce pain anterior and distal to the medial epicondyle.15
Traction, compression, and friction at the medial elbow can irritate the ulnar nerve. This nerve should be inspected and palpated along its course at the cubital tunnel to determine its location and stability. Ulnar nerve hypermobility, which has been identified in 37% of elbows, can be determined by having the patient actively flex the elbow with the forearm in supination, placing a finger at the posteromedial aspect of the medial humeral epicondyle, and having the patient actively extend the elbow.16 The nerve dislocates if trapped anterior to the examiner’s finger, perches if under the examiner’s finger, or is stable if still palpable in the groove posterior to the medial epicondyle.16
The distal band of the medial triceps tendon may also sublux over the medial epicondyle with elbow flexion. This subluxation, also known as snapping triceps syndrome, may cause pain or ulnar nerve symptoms.17 Bringing the elbow from extension to flexion may produce subluxation, first of the ulnar nerve and then of the medial triceps, in 2 separate “snaps.” Tenderness can be elicited along the medial triceps muscle.
Ulnar neuritis is caused by traction injury, such as with dynamic pitching, nerve subluxation, or compression at the cubital tunnel. With MCL injury and valgus instability, the ulnar nerve can become irritated as it becomes stretched because of medial elbow laxity.18 The nerve can also be damaged during flexion as the cubital tunnel retinaculum tightens, decreasing the space available for the nerve.19 This concept is applied during the elbow flexion compression test. A positive test may elicit tingling radiating toward the small finger or pain at the elbow or medial forearm when manual pressure is directly applied over the ulnar nerve between the posteromedial olecranon and the medial humeral epicondyle as the elbow is maximally flexed.20
3. Examine the lateral aspect of the elbow
Palpation of the lateral epicondyle, the radial head, and the olecranon tip assists in defining injury to the underlying anatomy. The anconeus “soft spot” (infracondylar recess) within the triangle formed by these 3 bony landmarks should be palpated for fullness, indicating a joint effusion, hemarthrosis, or even a subluxed or dislocated radial head.
While the medial elbow endures a large tensile load, throwing imposes a tremendous compressive force at the lateral elbow, particularly at the radiocapitellar joint. This joint may be tender and produce clicking with pronation and supination in patients with radiocapitellar arthrosis, symptomatic posterolateral synovial plica, or an inflamed radial bursa. Tenderness with crepitus that can be exacerbated with forceful flexion and extension may indicate radiocapitellar overload or loose bodies.
Long-term load transmission and subsequent degeneration of the articular surface may advance to osteochondritis dissecans (OCD). Examination for capitellar OCD reveals tenderness over the radiocapitellar joint and commonly a loss of 15° to 20° of extension. The active radiocapitellar compression test is positive for OCD lesions and elicits pain in the lateral compartment of the elbow when the patient pronates (Figure 3A) and supinates (Figure 3B) the forearm with the elbow axially loaded in extension.21
Microtrauma and inflammation may occur with repetitive eccentric overload. Although rare in throwing athletes, “tennis elbow” causes pain with gripping, and decreased grip strength. Tenderness caused by lateral epicondylitis is just anterior and distal to the epicondyle, at the origin of the extensor carpi radialis brevis. Pain is reproducible with passive wrist flexion and resisted wrist extension with the elbow extended (Cozen test).
Less commonly, athletes may complain of mechanical symptoms, such as snapping or catching with posterolateral elbow pain.22 These symptoms may be due to thickened or inflamed synovial plica causing impingement. A posterior radiocapitellar plica can be examined by bringing the elbow to full extension while applying valgus stress with the forearm in supination. Conversely, an anterior radiocapitellar plica can be examined with a valgus load on the elbow and passive flexion with the forearm in pronation.23 A palpable painful snap over the radiocapitellar joint is a positive test.
4. Examine the posterior aspect of the elbow
Posteriorly, palpation is focused on the triceps tendon and the olecranon tip. The elbow should be flexed to 30° to relax the triceps, isolate the olecranon, and allow for palpation of the olecranon fossa on either side of the triceps tendon. Tenderness at the posterolateral or posteromedial aspect of the olecranon should be noted. Warmth, fluctuance, or distension at the elbow may be caused by olecranon bursitis. The 3 heads of the triceps muscle should be palpated where they converge to form an aponeurosis, and tenderness or a palpable gap on any of the heads should be noted.
A combination of valgus force and a rapidly decelerating arm at the follow-through phase of pitching causes a shear force between the medial aspect of the olecranon tip and the olecranon fossa. This shear force can result in chondrolysis, osteophyte formation, and loose bodies, particularly in the posteromedial elbow. This valgus extension overload (VEO) syndrome often results in loss of full extension and symptoms, which may be attributed to osteophytes or fractured and nonunited fragments in the olecranon fossa or the olecranon tip. Frank crepitus may also be present with extension testing caused by loose bodies or synovial reaction over osteophytes. Assessing for VEO using the extension impingement test, the examiner places continuous valgus stress on the elbow while quickly extending from 20° to 30° of flexion (Figure 4A) to terminal extension (Figure 4B) repeatedly. The examiner repeats this without valgus load while palpating the posteromedial olecranon for tenderness to differentiate impingement caused by instability from pain over the medial olecranon without instability (Figure 4C). Particular attention should be focused posteriorly in athletes with medial instability, as MCL injuries and VEO syndrome often occur in conjunction in the throwing athlete.
Repetitive acceleration and deceleration of the arm can also cause stress fractures. With stress fractures, pain is often noted more distal and lateral on the olecranon, but tenderness may be palpable medially from posteromedial impaction that occurs from the valgus load during the overhead throwing motion. In immature athletes, the repetitive sudden snap of full extension in the deceleration phase of throwing can cause olecranon apophysitis. Frank avulsions can occur as well but are usually preceded by chronic posterior elbow pain with possible loss of full extension.
The late cocking phase of the throwing motion (just before throwing) hyperextends the elbow and places significant strain on the elbow. Repetitive strain can cause painful posterior impingement. The arm bar test is extremely sensitive (Figure 5).13 With the patient’s elbow extended, shoulder internally rotated, and hand on the examiner’s shoulder, the examiner pulls down on the olecranon to simulate forced extension and reproduces the pain associated with posteromedial impingement.
Last, though triceps tendon injuries are rare, ruptures most often occur at the origin of the lateral head of the triceps. As the initial swelling and ecchymosis subside, a palpable gap is pathognomonic for rupture. Extensor weakness can often be observed, but extension may still be possible from anconeus triceps expansion with the aid of gravity. With the elbow overhead, the athlete must extend the elbow against gravity and will exhibit weakness against resistance.
5. Examine the anterior aspect of the elbow
Anteriorly, the bulk of the flexor-pronator group restricts the extent of joint palpation, and the soft tissues are usually injured. The antecubital fossa is a triangular area on the anterior aspect of the elbow that is bounded superiorly by a horizontal line connecting the medial epicondyle to the lateral epicondyle of the humerus, medially by the lateral border of the pronator teres muscle and laterally by the medial border of the brachioradialis muscle. From lateral to medial, the antecubital fossa contains the radial nerve, the biceps brachii tendon, the brachial artery, and the median nerve. Evaluating this area is important because a visible defect, change in muscle contour, or proximal retraction of a muscle belly can indicate a muscular rupture. In particular, a distal biceps rupture (rare) may be accompanied by weakness and pain in supination and, to a lesser degree, in flexion. It is important to note that, in the case of a partial biceps rupture, ecchymosis may not appear, as the hematoma is confined by the intact lacertus fibrosis.24 The hook test can be used to evaluate for the presence of an intact distal biceps tendon (Figure 6).25 The patient abducts the shoulder, flexes the elbow to 90°, and actively supinates the forearm while the examiner attempts to hook an index finger laterally under the tendon. The test is negative if the finger can be inserted 1 cm under the tendon and positive if no cordlike structure can be hooked. Partial biceps tendon ruptures or tendinitis may exhibit tenderness of the distal biceps tendon and pain on resisted supination with a negative hook test. Often, resisted elbow flexion with the elbow at maximal extension elicits pain at the biceps insertion. Clicking with forearm rotation near the insertion of the tendon, which may be caused by an inflamed radial bursa between the distal biceps tendon and the radial tuberosity, may be associated with impending rupture.
Conclusion
Physical examination combined with thorough history taking usually provides a solid basis for a diagnosis, which in turn makes the value of surgical treatment more assured.
1. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
4. Aval SM, Durand P Jr, Shankwiler JA. Neurovascular injuries to the athlete’s shoulder: part II. J Am Acad Orthop Surg. 2007;15(5):281-289.
5. Strukel RJ, Garrick JG. Thoracic outlet compression in athletes: a report of four cases. Am J Sports Med. 1978;6(2):35-39.
6. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
7. Adams JE. Injury to the throwing arm. A study of traumatic changes in the elbow joints of boy baseball players. Calif Med. 1965;102:127-132.
8. Hang DW, Chao CM, Hang YS. A clinical and roentgenographic study of Little League elbow. Am J Sports Med. 2004;32(1):79-84.
9. King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop. 1969;(67):116-123.
10. Cain EL Jr, Dugas JR, Wolf RS, Andrews JR. Elbow injuries in throwing athletes: a current concepts review. Am J Sports Med. 2003;31(4):621-635.
11. Safran M, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy. 2005;21(11):1381-1395.
12. O’Driscoll SW, Lawton RL, Smith AM. The “moving valgus stress test” for medial collateral ligament tears of the elbow. Am J Sports Med. 2005;33(2):231-239.
13. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
14. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
15. Andrews JR, Whiteside JA, Buettner CM. Clinical evaluation of the elbow in throwers. Oper Tech Sports Med. 1996;4(2):77-83.
16. Calfee RP, Manske PR, Gelberman RH, Van Steyn MO, Steffen J, Goldfarb CA. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92(17):2801-2808.
17. Spinner RJ, Goldner RD. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. Anatomical and dynamic factors. J Bone Joint Surg Am. 1998;80(2):239-247.
18. Guerra JJ, Timmerman LA. Clinical anatomy, histology, & pathomechanics of the elbow in sports. Oper Tech Sports Med. 1996;4(2):69-76.
19. O’Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73(4):613-617.
20. Novak CB, Lee GW, Mackinnon SE, Lay L. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19(5):817-820.
21. Andrews JR. Bony injuries about the elbow in the throwing athlete. Instr Course Lect. 1985;34:323-331.
22. Kim DH, Gambardella RA, Elattrache NS, Yocum LA, Jobe FW. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am J Sports Med. 2006;34(3):438-444.
23. Antuna SA, O’Driscoll SW. Snapping plicae associated with radiocapitellar chondromalacia. Arthroscopy. 2001;17(5):491-495.
24. Bernstein AD, Breslow MJ, Jazrawi LM. Distal biceps tendon ruptures: a historical perspective and current concepts. Am J Orthop. 2001;30(3):
193-200.
25. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
1. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
4. Aval SM, Durand P Jr, Shankwiler JA. Neurovascular injuries to the athlete’s shoulder: part II. J Am Acad Orthop Surg. 2007;15(5):281-289.
5. Strukel RJ, Garrick JG. Thoracic outlet compression in athletes: a report of four cases. Am J Sports Med. 1978;6(2):35-39.
6. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
7. Adams JE. Injury to the throwing arm. A study of traumatic changes in the elbow joints of boy baseball players. Calif Med. 1965;102:127-132.
8. Hang DW, Chao CM, Hang YS. A clinical and roentgenographic study of Little League elbow. Am J Sports Med. 2004;32(1):79-84.
9. King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop. 1969;(67):116-123.
10. Cain EL Jr, Dugas JR, Wolf RS, Andrews JR. Elbow injuries in throwing athletes: a current concepts review. Am J Sports Med. 2003;31(4):621-635.
11. Safran M, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy. 2005;21(11):1381-1395.
12. O’Driscoll SW, Lawton RL, Smith AM. The “moving valgus stress test” for medial collateral ligament tears of the elbow. Am J Sports Med. 2005;33(2):231-239.
13. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
14. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
15. Andrews JR, Whiteside JA, Buettner CM. Clinical evaluation of the elbow in throwers. Oper Tech Sports Med. 1996;4(2):77-83.
16. Calfee RP, Manske PR, Gelberman RH, Van Steyn MO, Steffen J, Goldfarb CA. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92(17):2801-2808.
17. Spinner RJ, Goldner RD. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. Anatomical and dynamic factors. J Bone Joint Surg Am. 1998;80(2):239-247.
18. Guerra JJ, Timmerman LA. Clinical anatomy, histology, & pathomechanics of the elbow in sports. Oper Tech Sports Med. 1996;4(2):69-76.
19. O’Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73(4):613-617.
20. Novak CB, Lee GW, Mackinnon SE, Lay L. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19(5):817-820.
21. Andrews JR. Bony injuries about the elbow in the throwing athlete. Instr Course Lect. 1985;34:323-331.
22. Kim DH, Gambardella RA, Elattrache NS, Yocum LA, Jobe FW. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am J Sports Med. 2006;34(3):438-444.
23. Antuna SA, O’Driscoll SW. Snapping plicae associated with radiocapitellar chondromalacia. Arthroscopy. 2001;17(5):491-495.
24. Bernstein AD, Breslow MJ, Jazrawi LM. Distal biceps tendon ruptures: a historical perspective and current concepts. Am J Orthop. 2001;30(3):
193-200.
25. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Don’t think tendinitis in kids, but apophysitis instead
LAS VEGAS – Beware the diagnosis of tendinitis in children and adolescents who present with varying degrees of knee or foot pain.
“If you make the diagnosis of tendinitis, you’re probably wrong,” Dr. Sally S. Harris said at a pediatric update sponsored by the American Academy of Pediatrics California District 9. “Tendinitis rarely occurs in the pediatric and adolescent age group because tendinitis is a degenerative condition. So think of other things, especially apophysitis.”
An apophysis is a secondary center of ossification that contributes to the peripheral prominences around bones, such as around the ankles, elbows, pelvis, and knees: “The bumps, so to speak,” said Dr. Harris, who practices in the departments of sports medicine and pediatrics at Palo Alto (Calif.) Medical Foundation. “It’s a bone-related pain, an inflammation of that softer cartilage turning to bone that isn’t completely formed.”
Osgood-Schlatter disease ranks as the most-common apophysitis injury. This occurs during midpuberty and is marked by a prominent swollen bump on the front of the knee, just below the knee cap, where the patellar tendon attaches to the tibial tubercle, explained Dr. Harris, who founded the AAP section on sports medicine. “It’s a secondary center of ossification that appears at ages 10-12 years and fuses at ages 14-18.” Telltale signs are tenderness with or without swelling at the tibial tubercle that worsens with running, jumping, or impact activities. “If you ask [patients] to extend their knee against the resistance of your hand, you’ll probably reproduce the pain,” she said.
X-rays usually are not required. “You might do it to confirm the presence of an open apophysitis or to rule out other pathology, but other pathology is almost always unheard of at that area.”
Recommended treatment involves decreasing running and jumping activity as needed to keep symptoms manageable, and decreasing inflammation with ice and possibly nonsteroidal anti-inflammatory drugs. Wearing protective knee pads also can help. “Anytime the bump gets hit, kicked, or kneeled on, it will be more irritated,” she said. “Stretching quadriceps and especially hamstrings can help offload this problem.”.
Dr. Harris described the condition as self-limited although it can last 2-3 years. Potential complications considered minor include enlargement of the tibial tubercle, ununited ossicles in the patellar tendon, and avulsion of the tibial tubercle (rare). “Generally, Osgood-Schlatter is a harmless condition that you just want to manage,” she said.
Another common injury is Sinding-Larsen-Johansson syndrome, which is an apophysitis of the inferior pole of the patella that occurs in prepubescent boys and girls. “This is going to be knee pain, but there won’t be anything obvious for you to see or for them to point at,” Dr. Harris said. “You will focus on the inferior pole of the patella and palpate where the patellar tendon attaches to the knee cap. It’s analogous to jumper’s knee in adults.”
Lateral x-rays will reveal a small ossific fragment at the distal portion of the patella. Sinding-Larsen-Johansson disease typically resolves within 1 year. “It rarely interferes with activity; they just need an explanation of what’s going on,” she said.
Severs disease, which occurs in early puberty, is an apophysitis injury that affects the heels of children who participate in soccer and gymnastics. It’s marked by a traction/impact apophysitis at the site of insertion of the Achilles’ tendon at the posterior calcaneus. “At times, it can last for 2-3 years, but it is a self-limited condition,” Dr. Harris said. “Nothing ever bad comes from this other than the ups and downs of the pain. It’s pain at the base of the heel, not the Achilles’ tendon area, but patients will come in and some of them have been told they have Achilles’ tendinitis.”
Telltale signs include pain with heel walking and positive lateral squeeze test of the posterior calcaneus. “That reproduces the symptoms,” she said. “If they’re not symptomatic when you see them in the office, you can ask them to try this test after their next [sports] practice, and this will confirm the diagnosis. X-rays are not needed.”
Treatment involves modifying physical activity to keep symptoms manageable. Insertion of silicone heel cups can help, as can wearing shoes with good padding.
If a child of pubertal age presents with pain localized to the inner side of the arching foot, think tarsal navicular bone apophysitis, which is due to the presence of accessory navicular or open apophysitis. “It doesn’t really matter what the anatomy is; it’s all treated the same way, which is to support pronation with arch support,” Dr. Harris said. “If this doesn’t alleviate symptoms well enough, they need custom orthotics made.”
The final injury Dr. Harris discussed was Iselin’s disease, which is a secondary center of ossification at the site of insertion of the peroneal tendon at the base of the 5th metatarsal. Pain in the region is exacerbated by excessive lateral ankle movement. “On an x-ray, this looks like a small crescent of bone growing that hasn’t completely fused yet,” she said. “It’s often misread as a fracture, but it’s normal development.” Treatment consists of activity modification, icing, and NSAIDs as needed, and an ankle brace to provide lateral ankle support. She described Iselin’s as “harmless and short lived, from 6-10 months at the most.”
Dr. Harris reported having no financial disclosures.
On Twitter @dougbrunk
LAS VEGAS – Beware the diagnosis of tendinitis in children and adolescents who present with varying degrees of knee or foot pain.
“If you make the diagnosis of tendinitis, you’re probably wrong,” Dr. Sally S. Harris said at a pediatric update sponsored by the American Academy of Pediatrics California District 9. “Tendinitis rarely occurs in the pediatric and adolescent age group because tendinitis is a degenerative condition. So think of other things, especially apophysitis.”
An apophysis is a secondary center of ossification that contributes to the peripheral prominences around bones, such as around the ankles, elbows, pelvis, and knees: “The bumps, so to speak,” said Dr. Harris, who practices in the departments of sports medicine and pediatrics at Palo Alto (Calif.) Medical Foundation. “It’s a bone-related pain, an inflammation of that softer cartilage turning to bone that isn’t completely formed.”
Osgood-Schlatter disease ranks as the most-common apophysitis injury. This occurs during midpuberty and is marked by a prominent swollen bump on the front of the knee, just below the knee cap, where the patellar tendon attaches to the tibial tubercle, explained Dr. Harris, who founded the AAP section on sports medicine. “It’s a secondary center of ossification that appears at ages 10-12 years and fuses at ages 14-18.” Telltale signs are tenderness with or without swelling at the tibial tubercle that worsens with running, jumping, or impact activities. “If you ask [patients] to extend their knee against the resistance of your hand, you’ll probably reproduce the pain,” she said.
X-rays usually are not required. “You might do it to confirm the presence of an open apophysitis or to rule out other pathology, but other pathology is almost always unheard of at that area.”
Recommended treatment involves decreasing running and jumping activity as needed to keep symptoms manageable, and decreasing inflammation with ice and possibly nonsteroidal anti-inflammatory drugs. Wearing protective knee pads also can help. “Anytime the bump gets hit, kicked, or kneeled on, it will be more irritated,” she said. “Stretching quadriceps and especially hamstrings can help offload this problem.”.
Dr. Harris described the condition as self-limited although it can last 2-3 years. Potential complications considered minor include enlargement of the tibial tubercle, ununited ossicles in the patellar tendon, and avulsion of the tibial tubercle (rare). “Generally, Osgood-Schlatter is a harmless condition that you just want to manage,” she said.
Another common injury is Sinding-Larsen-Johansson syndrome, which is an apophysitis of the inferior pole of the patella that occurs in prepubescent boys and girls. “This is going to be knee pain, but there won’t be anything obvious for you to see or for them to point at,” Dr. Harris said. “You will focus on the inferior pole of the patella and palpate where the patellar tendon attaches to the knee cap. It’s analogous to jumper’s knee in adults.”
Lateral x-rays will reveal a small ossific fragment at the distal portion of the patella. Sinding-Larsen-Johansson disease typically resolves within 1 year. “It rarely interferes with activity; they just need an explanation of what’s going on,” she said.
Severs disease, which occurs in early puberty, is an apophysitis injury that affects the heels of children who participate in soccer and gymnastics. It’s marked by a traction/impact apophysitis at the site of insertion of the Achilles’ tendon at the posterior calcaneus. “At times, it can last for 2-3 years, but it is a self-limited condition,” Dr. Harris said. “Nothing ever bad comes from this other than the ups and downs of the pain. It’s pain at the base of the heel, not the Achilles’ tendon area, but patients will come in and some of them have been told they have Achilles’ tendinitis.”
Telltale signs include pain with heel walking and positive lateral squeeze test of the posterior calcaneus. “That reproduces the symptoms,” she said. “If they’re not symptomatic when you see them in the office, you can ask them to try this test after their next [sports] practice, and this will confirm the diagnosis. X-rays are not needed.”
Treatment involves modifying physical activity to keep symptoms manageable. Insertion of silicone heel cups can help, as can wearing shoes with good padding.
If a child of pubertal age presents with pain localized to the inner side of the arching foot, think tarsal navicular bone apophysitis, which is due to the presence of accessory navicular or open apophysitis. “It doesn’t really matter what the anatomy is; it’s all treated the same way, which is to support pronation with arch support,” Dr. Harris said. “If this doesn’t alleviate symptoms well enough, they need custom orthotics made.”
The final injury Dr. Harris discussed was Iselin’s disease, which is a secondary center of ossification at the site of insertion of the peroneal tendon at the base of the 5th metatarsal. Pain in the region is exacerbated by excessive lateral ankle movement. “On an x-ray, this looks like a small crescent of bone growing that hasn’t completely fused yet,” she said. “It’s often misread as a fracture, but it’s normal development.” Treatment consists of activity modification, icing, and NSAIDs as needed, and an ankle brace to provide lateral ankle support. She described Iselin’s as “harmless and short lived, from 6-10 months at the most.”
Dr. Harris reported having no financial disclosures.
On Twitter @dougbrunk
LAS VEGAS – Beware the diagnosis of tendinitis in children and adolescents who present with varying degrees of knee or foot pain.
“If you make the diagnosis of tendinitis, you’re probably wrong,” Dr. Sally S. Harris said at a pediatric update sponsored by the American Academy of Pediatrics California District 9. “Tendinitis rarely occurs in the pediatric and adolescent age group because tendinitis is a degenerative condition. So think of other things, especially apophysitis.”
An apophysis is a secondary center of ossification that contributes to the peripheral prominences around bones, such as around the ankles, elbows, pelvis, and knees: “The bumps, so to speak,” said Dr. Harris, who practices in the departments of sports medicine and pediatrics at Palo Alto (Calif.) Medical Foundation. “It’s a bone-related pain, an inflammation of that softer cartilage turning to bone that isn’t completely formed.”
Osgood-Schlatter disease ranks as the most-common apophysitis injury. This occurs during midpuberty and is marked by a prominent swollen bump on the front of the knee, just below the knee cap, where the patellar tendon attaches to the tibial tubercle, explained Dr. Harris, who founded the AAP section on sports medicine. “It’s a secondary center of ossification that appears at ages 10-12 years and fuses at ages 14-18.” Telltale signs are tenderness with or without swelling at the tibial tubercle that worsens with running, jumping, or impact activities. “If you ask [patients] to extend their knee against the resistance of your hand, you’ll probably reproduce the pain,” she said.
X-rays usually are not required. “You might do it to confirm the presence of an open apophysitis or to rule out other pathology, but other pathology is almost always unheard of at that area.”
Recommended treatment involves decreasing running and jumping activity as needed to keep symptoms manageable, and decreasing inflammation with ice and possibly nonsteroidal anti-inflammatory drugs. Wearing protective knee pads also can help. “Anytime the bump gets hit, kicked, or kneeled on, it will be more irritated,” she said. “Stretching quadriceps and especially hamstrings can help offload this problem.”.
Dr. Harris described the condition as self-limited although it can last 2-3 years. Potential complications considered minor include enlargement of the tibial tubercle, ununited ossicles in the patellar tendon, and avulsion of the tibial tubercle (rare). “Generally, Osgood-Schlatter is a harmless condition that you just want to manage,” she said.
Another common injury is Sinding-Larsen-Johansson syndrome, which is an apophysitis of the inferior pole of the patella that occurs in prepubescent boys and girls. “This is going to be knee pain, but there won’t be anything obvious for you to see or for them to point at,” Dr. Harris said. “You will focus on the inferior pole of the patella and palpate where the patellar tendon attaches to the knee cap. It’s analogous to jumper’s knee in adults.”
Lateral x-rays will reveal a small ossific fragment at the distal portion of the patella. Sinding-Larsen-Johansson disease typically resolves within 1 year. “It rarely interferes with activity; they just need an explanation of what’s going on,” she said.
Severs disease, which occurs in early puberty, is an apophysitis injury that affects the heels of children who participate in soccer and gymnastics. It’s marked by a traction/impact apophysitis at the site of insertion of the Achilles’ tendon at the posterior calcaneus. “At times, it can last for 2-3 years, but it is a self-limited condition,” Dr. Harris said. “Nothing ever bad comes from this other than the ups and downs of the pain. It’s pain at the base of the heel, not the Achilles’ tendon area, but patients will come in and some of them have been told they have Achilles’ tendinitis.”
Telltale signs include pain with heel walking and positive lateral squeeze test of the posterior calcaneus. “That reproduces the symptoms,” she said. “If they’re not symptomatic when you see them in the office, you can ask them to try this test after their next [sports] practice, and this will confirm the diagnosis. X-rays are not needed.”
Treatment involves modifying physical activity to keep symptoms manageable. Insertion of silicone heel cups can help, as can wearing shoes with good padding.
If a child of pubertal age presents with pain localized to the inner side of the arching foot, think tarsal navicular bone apophysitis, which is due to the presence of accessory navicular or open apophysitis. “It doesn’t really matter what the anatomy is; it’s all treated the same way, which is to support pronation with arch support,” Dr. Harris said. “If this doesn’t alleviate symptoms well enough, they need custom orthotics made.”
The final injury Dr. Harris discussed was Iselin’s disease, which is a secondary center of ossification at the site of insertion of the peroneal tendon at the base of the 5th metatarsal. Pain in the region is exacerbated by excessive lateral ankle movement. “On an x-ray, this looks like a small crescent of bone growing that hasn’t completely fused yet,” she said. “It’s often misread as a fracture, but it’s normal development.” Treatment consists of activity modification, icing, and NSAIDs as needed, and an ankle brace to provide lateral ankle support. She described Iselin’s as “harmless and short lived, from 6-10 months at the most.”
Dr. Harris reported having no financial disclosures.
On Twitter @dougbrunk
EXPERT ANALYSIS AT PEDIATRIC UPDATE
Midfoot Sprains in the National Football League
Midfoot (Lisfranc) joint injuries are uncommon in the general population, with a reported incidence ranging from 1 per 50,000 to 1 per 60,000 per year.1,2 The majority of these midfoot injuries result from high-velocity direct trauma involving severe disruption of the tarsometatarsal joint.1-6 Most of the literature on Lisfranc injuries are based on cohorts that include trauma patients. On the other hand, low-velocity indirect injuries of the tarsometatarsal joint have also been associated with midfoot or Lisfranc sprains.7 These injuries are even less extensively studied in athletes, who may sustain them from torsion or the shoe–surface interface.8
Foot and ankle injuries are among the most common injuries in athletes and represent 16% to 22% of all sports injuries.9 Although midfoot sprains are not common in the general population, sporting activities appear to result in a higher rate of midfoot injury, especially in elite athletes. In fact, midfoot sprains comprise the second most common athlete injury to the foot, after metatarsophalangeal joint injuries.10 Football players are especially prone to midfoot sprains; incidence is 4% per year, with offensive linemen sustaining 29.2% of midfoot sprains.10 The most common mechanism of injury is an axial longitudinal force while the foot is plantarflexed and slightly rotated.11,12
There is a paucity of literature detailing the impact of midfoot injuries on football players.8,10,13 A study of 23 collegiate football players found that they may have initially underwent a long period of acute disability but had very minor long-term complaints resulting in residual functional disability.10 However, there are no case series detailing the impact of midfoot sprains on professional football players for whom delayed return to sport can potentially have a devastating impact on a career in terms of both acute- and long-term disability.
We conducted a study to further define the mechanism of injury, diagnosis, treatment, and outcomes among National Football League (NFL) players with midfoot sprains. In addition, we aimed to provide a qualitative analysis of diagnostic and treatment algorithms being used by NFL team physicians in their management of midfoot sprains in these high-level contact athletes.
Materials and Methods
We evaluated midfoot sprains in NFL players in 2 specific phases. In phase 1, we retrospectively reviewed prospectively collected data involving midfoot sprains in professional players from a single NFL team over a 15-year period. In phase 2, we collated diagnostic and treatment algorithms for midfoot sprains among all 32 NFL team physicians by means of a structured questionnaire. Institutional review board approval was obtained for this study at the investigators’ institution.
In phase 1, a NFL team injury database was reviewed for midfoot sprains that had been prospectively entered by a team-certified athletic trainer after consultation with the head orthopedic team physician. All injury and diagnostic modalities and treatments were then analyzed. These included player position, foot and ankle protective gear (none, tape, brace, or unknown), playing surface (grass, AstroTurf, FieldTurf, or unknown), field condition (normal, wet, hard, or unknown), onset of injury (acute, chronic, or unknown), place of injury (game or practice), time of injury in game or practice (first quarter, second quarter, third quarter, fourth quarter, or unknown), type of play (collision, tackled, tackling, blocked, blocking, running/cutting, kicking, or unknown), and mechanism of injury (direct, torsion, shearing, or unknown).
Once the diagnosis was confirmed by physical examination and radiographic findings, midfoot sprain treatment was initiated based on the following algorithm protocols. Nondisplaced sprains were treated with a period of immobilization in a cam walker with progression to weight-bearing as tolerated (grade 1). Once asymptomatic, rehabilitation was initiated, including range of motion, strengthening, and proprioception, and gradual return to play as tolerated. Injuries with subtle diastasis (2-5 mm) were typically treated with nonoperative management in the same manner as the nondisplaced sprain protocol (grade 2); however, signs of gross instability indicated the potential requirement for surgical management. Some of these injuries underwent stress-testing to determine if there was gross instability. If the injury had subtle diastasis with instability or frank (>5 mm) displacement (grade 3), then surgical management was performed with closed versus open reduction and internal fixation (ORIF). The postoperative course included no weight-bearing for 4 to 6 weeks followed by partial weight-bearing for an additional 4 to 6 weeks. After approximately 8 to 12 postoperative weeks, screw removal was performed followed by progression to full weight-bearing and a comprehensive rehabilitation program, including range of motion, strengthening, proprioception, and gradual return to play. Return to play was allowed when the athlete was asymptomatic and had normal range of motion and strength. Time lost from participation was then recorded based on the dates of injury and return to play.
To further elucidate long-term postinjury playing status, we then gathered information from the www.NFL.com historical and current player databases as previously described by Shah and colleagues.14 From this website, we documented the number of regular-season and postseason games as well as the number of seasons before and after the injury. To be included in the series, the athlete had to have been on the active roster for an NFL franchise at the time of injury. Successful return to play was defined as actual return to play in regular season or postseason NFL games after the midfoot sprain.
In phase 2, a structured electronic questionnaire was sent to all 32 NFL team physicians. The questionnaire was compiled to gather information relating to current diagnostic, treatment, and outcome algorithms in the management of midfoot sprains involving professional football players. Each questionnaire was sent by e-mail to all survey participants and included an embedded link to a secure online survey resource (REDCap Survey Software Version 1.3.9; Vanderbilt University, Nashville, Tennessee). Once the electronic questionnaire was completed by each NFL team physician, results were exported in spreadsheet format for descriptive data analysis.
The retrospective case series and NFL team physician survey data were then analyzed. A descriptive analysis was performed for all variables, including means and minimum–maximum range for quantitative variables as well as frequencies and percentages for qualitative variables. Depending on injury severity, an independent-sample t test with corresponding P values was also calculated for time lost from participation.
Results
The retrospective review of the prospectively collected NFL injury database revealed there were 15 midfoot sprains during the study period. A statistical and descriptive analysis was performed for all study parameters, including player, field, injury, and outcome-specific data. For player, field, and injury-specific data, the results are summarized in the Table.
All grade 1 midfoot sprains (7 nondisplaced) and grade 2 midfoot sprains (5 with subtle diastasis and no instability) were treated with nonoperative management. The 12 players were allowed to return to play without the need for subsequent surgery within the same season. In the evaluation of return to play, based on the severity of the midfoot sprain, there was a statistically significant (P = .047) difference in mean (SD) time lost from participation between the grade 1 sprain group, 3.1 (1.9) days, and the grade 2 sprain group, 36 (26.1) days. Overall, nonoperative treatment of either grade 1 or grade 2 midfoot sprains resulted in a mean of 11.7 days of time lost from participation. In 1 patient with a grade 2 midfoot sprain, the injury occurred toward the end of the season, and the patient was not able to return to play during the remaining 42 days of the season. However, this patient returned to play the next season and had no residual problems.
Three grade 3 injuries (midfoot sprains with frank displacement) required surgical management with ORIF. One patient returned to play the same season, in 73 days; however, the other 2 patients had injuries toward the end of the season (29 and 77 days remaining) and were not able to return to play the same season. However, both these patients returned to play the next season and had no persistent problems. In terms of complications within the same season, there were no recurrent injuries reported after successful return to play.
When evaluating long-term postinjury playing status, we found that 11 (92%) of the 12 NFL players who had nonoperative treatment successfully returned to play. The only player who did not return to an NFL regular season or postseason game was an active-roster NFL player who never actually played in an NFL game before or after his midfoot sprain injury. Our series of NFL players played on average 1.9 years (range, 0-7 years) before the midfoot injury and 5.5 years (range, 0-14 years) after the midfoot injury. In terms of NFL regular-season and postseason games played, our cohort of NFL players played on average 24.0 games (range, 0-80 games) before the midfoot injury and 77.7 games (range, 0-226 games) after the midfoot injury. In fact, 10 of the 12 NFL players (83%) who had nonoperative treatment played more games and seasons after their midfoot injury.
The surveys from phase 2 were completed by all 32 NFL team physicians. When evaluating the severity of midfoot sprains, 63% of the NFL team physicians perform stress-view radiographs. To ascertain NFL team physicians’ management decisions, we evaluated midfoot sprain results according to injury severity, including amount of diastasis.
When managing midfoot sprains with no diastasis, 94% of the team physicians use immobilization, including 27 with a cam walker and 2 with a cast; however, 2 physicians (6%) use only ankle taping or an Ace bandage. Initial weight-bearing status varies among the NFL team physicians, but most (78%) choose to protect the player, including 17 non-weight-bearing, 8 partial weight-bearing, and 7 weight-bearing as tolerated. Most physicians ideally progress players to full weight-bearing by 3 weeks (12% immediately, 12% by week 1, 41% by week 2, 16% by week 3, and 19% from 4-6 weeks).
In the management of midfoot sprains with subtle diastasis, there is variation in treatment modes among the NFL team physicians, with 53% using nonoperative management (34% cam walker, 19% cast) and 47% suggesting operative management. Regardless of treatment, most physicians (97%) maintain initial non-weight-bearing restrictions. In fact, only 1 physician first recommended partial weight-bearing, which corresponded to initial treatment in a cam walker.
In terms of midfoot sprains with frank diastasis, 94% of the NFL team physicians indicated surgical management is warranted, with only 2 physicians (6%) recommending initial nonoperative management with a cam walker. Regardless of treatment, all the physicians (100%) implemented initial non-weight-bearing restrictions. Once surgical treatment was recommended, the preferred fixation method was ORIF using screws (94%) as opposed to closed reduction and internal fixation with percutaneous Kirschner wires (6%). Most of the physicians (59%) do not allow return to play until midfoot hardware is removed; however, 38% allow full participation with contact, and 3% allow partial participation with no contact. Removal of midfoot fixation is an important factor for most of the physicians before considering return to play, and 69% recommend hardware removal after 11 weeks. However, the specific timeline for hardware removal varied among these physicians, with 28% opting for removal at 11 to 12 weeks, 16% at 13 to 14 weeks, 12.5% at 7 to 8 weeks, 12.5% at 15 to 16 weeks, 12.5% at more than 16 weeks, 12.5% never, and 6% at 9 to 10 weeks.
The midfoot sprain treatment protocol (nonoperative vs operative management) based on injury severity was an important factor in considering return-to-play guidelines. When evaluating time lost from participation because of midfoot sprains, most of the NFL team physicians anticipated a period of 5 to 8 weeks when considering nonoperative management (56%) and more than 17 weeks after operative management (53%). In evaluating nonoperative management protocols, return-to-play guidelines were relatively expeditious, with 56% of the physicians estimating from 5 to 8 weeks, 22% from 1 to 4 weeks, 13% from 9 to 12 weeks, 6% from 13 to 16 weeks, and 3% longer than 20 weeks. In comparison to nonoperative management, return-to-play guidelines for operative management were prolonged, with 53% of the physicians estimating more than 20 weeks, 25% from 17 to 20 weeks, 13% from 13 to 16 weeks, and 9% from 9 to 12 weeks.
Discussion
Lisfranc and midfoot injuries remain a controversial topic in sports medicine. Several authors have argued that anatomical reduction of the tarsometatarsal joint in the setting of a Lisfranc injury yields optimal outcomes.15,16 Some studies have also suggested that purely ligamentous Lisfranc injuries may be more of a problem than bony injuries, which may have the benefit of osseous healing.15,17 Anatomical reduction can minimize the potential for arch collapse by maintaining the normal tarsometatarsal relationship. However, there are no long-term data to determine how midfoot arthrosis is affected by ORIF, which typically involves hardware traversing joints. Some have even argued that primary tarsometatarsal arthrodesis should be the treatment of choice, as the midfoot has limited native motion, and successful arthrodesis eliminates the potential for midfoot arthrosis.17,18 However, we are unaware of any studies that have routinely performed arthrodesis in an athletic population.
The majority of studies on midfoot injuries have evaluated individuals involved in traumatic accidents, most commonly motor vehicle collisions. The present study suggests there may be a subset of injuries in athletes that have yet to be adequately studied. Anecdotally, the NFL team physicians surveyed in our study suggested that midfoot sprains with no or subtle displacement may be treated with nonoperative measures while yielding satisfactory clinical outcomes. These results have been quantified in return-to-play status. Our subset of athletes from an NFL team corroborates these findings, even though the series was small (15 patients). Our survey results also suggest there is considerable variation in the “optimal” management plan among the physicians treating these elite athletes. Most would agree that the nondisplaced injuries can be managed conservatively and that the severely displaced injuries should be managed operatively, but the natural history of those injuries with subtle diastasis remains unclear. When operative intervention is implemented, hardware removal versus retention must also be considered when allowing for return to play. Although one would assume that motion-related hardware failure would be possible at the tarsometatarsal joints, this concept has yet to be clearly defined in the literature.
The present study also demonstrates that most athletes with these midfoot injuries can return to play at the elite NFL level, as evidenced by their short- and long-term return to play. However, it was not possible to differentiate the specific return-to-play level related to preinjury performance level. Furthermore, this relatively short-term NFL career follow-up study was not able to elucidate the long-term consequences of these injuries. In fact, arch collapse and acquired flatfoot deformity could eventually result from this injury, and long-term outcomes would be of particular interest in patients who have subtle diastasis and who are treated nonoperatively.
Although previous studies have supported operative management for Lisfranc injuries involving subtle diastasis, more than half of the NFL team physicians surveyed in this study use nonoperative treatment for these injuries.19 Future studies should evaluate stress-imaging to define the effect of stability or latent diastasis on long-term outcomes. Nonetheless, the present study demonstrates that a large cohort of NFL team physicians supports nonoperative management for these Lisfranc injuries with subtle diastasis, even in elite athletes. Additional prospective studies are needed to provide a more rigorous injury evaluation and closer follow-up, including subjective and objective outcomes, to further define the indications for management options for midfoot sprains in this population of contact athletes.
1. Aitken AP, Poulson D. Dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1963;45:246-260.
2. Hardcastle PH, Reschauer R, Kutscha-Lissberg E, Schoffmann W. Injuries to the tarsometatarsal joint. Incidence, classification and treatment. J Bone Joint Surg Br. 1982;64(3):349-356.
3. Arntz CT, Veith RG, Hansen ST Jr. Fractures and fracture-dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1988(2);70:173-181.
4. Goossens M, De Stoop N. Lisfranc’s fracture-dislocations: etiology, radiology, and results of treatment. A review of 20 cases. Clin Orthop. 1983;(176):154-162.
5. Myerson M. The diagnosis and treatment of injuries to the Lisfranc joint complex. Orthop Clin North Am. 1989;20(4):655-664.
6. Wiley JJ. The mechanism of tarso-metatarsal joint injuries. J Bone Joint Surg Br. 1971;53(3):474-482.
7. Faciszewski T, Burks RT, Manaster BJ. Subtle injuries of the Lisfranc joint. J Bone Joint Surg Am. 1990;72(10):1519-1522.
8. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
9. Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7(1):29-36.
10. Meyer SA, Callaghan JJ, Albright JP, Crowley ET, Powell JW. Midfoot sprains in collegiate football players. Am J Sports Med. 1994;22(3):392-401.
11. Shapiro MS, Wascher DC, Finerman GA. Rupture of Lisfranc’s ligament in athletes. Am J Sports Med. 1994;22(5):687-691.
12. Curtis MJ, Myerson M, Szura B. Tarsometatarsal joint injuries in the athlete. Am J Sports Med. 1993;21(4):497-502.
13. Harwood MI, Raikin SM. A Lisfranc fracture-dislocation in a football player. J Am Board Fam Pract. 2003;16(1):69-72.
14. Shah VM, Andrews JR, Fleisig GS, et al. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
15. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82(11):1609-1618.
16. Myerson MS, Cerrato RA. Current management of tarsometatarsal injuries in the athlete. J Bone Joint Surg Am. 2008;90(11):2522-2533.
17. Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88(3):514-520.
18. Coetzee JC, Ly TV. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 pt1):122-127.
19. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle. 2010;9:100-106.
Midfoot (Lisfranc) joint injuries are uncommon in the general population, with a reported incidence ranging from 1 per 50,000 to 1 per 60,000 per year.1,2 The majority of these midfoot injuries result from high-velocity direct trauma involving severe disruption of the tarsometatarsal joint.1-6 Most of the literature on Lisfranc injuries are based on cohorts that include trauma patients. On the other hand, low-velocity indirect injuries of the tarsometatarsal joint have also been associated with midfoot or Lisfranc sprains.7 These injuries are even less extensively studied in athletes, who may sustain them from torsion or the shoe–surface interface.8
Foot and ankle injuries are among the most common injuries in athletes and represent 16% to 22% of all sports injuries.9 Although midfoot sprains are not common in the general population, sporting activities appear to result in a higher rate of midfoot injury, especially in elite athletes. In fact, midfoot sprains comprise the second most common athlete injury to the foot, after metatarsophalangeal joint injuries.10 Football players are especially prone to midfoot sprains; incidence is 4% per year, with offensive linemen sustaining 29.2% of midfoot sprains.10 The most common mechanism of injury is an axial longitudinal force while the foot is plantarflexed and slightly rotated.11,12
There is a paucity of literature detailing the impact of midfoot injuries on football players.8,10,13 A study of 23 collegiate football players found that they may have initially underwent a long period of acute disability but had very minor long-term complaints resulting in residual functional disability.10 However, there are no case series detailing the impact of midfoot sprains on professional football players for whom delayed return to sport can potentially have a devastating impact on a career in terms of both acute- and long-term disability.
We conducted a study to further define the mechanism of injury, diagnosis, treatment, and outcomes among National Football League (NFL) players with midfoot sprains. In addition, we aimed to provide a qualitative analysis of diagnostic and treatment algorithms being used by NFL team physicians in their management of midfoot sprains in these high-level contact athletes.
Materials and Methods
We evaluated midfoot sprains in NFL players in 2 specific phases. In phase 1, we retrospectively reviewed prospectively collected data involving midfoot sprains in professional players from a single NFL team over a 15-year period. In phase 2, we collated diagnostic and treatment algorithms for midfoot sprains among all 32 NFL team physicians by means of a structured questionnaire. Institutional review board approval was obtained for this study at the investigators’ institution.
In phase 1, a NFL team injury database was reviewed for midfoot sprains that had been prospectively entered by a team-certified athletic trainer after consultation with the head orthopedic team physician. All injury and diagnostic modalities and treatments were then analyzed. These included player position, foot and ankle protective gear (none, tape, brace, or unknown), playing surface (grass, AstroTurf, FieldTurf, or unknown), field condition (normal, wet, hard, or unknown), onset of injury (acute, chronic, or unknown), place of injury (game or practice), time of injury in game or practice (first quarter, second quarter, third quarter, fourth quarter, or unknown), type of play (collision, tackled, tackling, blocked, blocking, running/cutting, kicking, or unknown), and mechanism of injury (direct, torsion, shearing, or unknown).
Once the diagnosis was confirmed by physical examination and radiographic findings, midfoot sprain treatment was initiated based on the following algorithm protocols. Nondisplaced sprains were treated with a period of immobilization in a cam walker with progression to weight-bearing as tolerated (grade 1). Once asymptomatic, rehabilitation was initiated, including range of motion, strengthening, and proprioception, and gradual return to play as tolerated. Injuries with subtle diastasis (2-5 mm) were typically treated with nonoperative management in the same manner as the nondisplaced sprain protocol (grade 2); however, signs of gross instability indicated the potential requirement for surgical management. Some of these injuries underwent stress-testing to determine if there was gross instability. If the injury had subtle diastasis with instability or frank (>5 mm) displacement (grade 3), then surgical management was performed with closed versus open reduction and internal fixation (ORIF). The postoperative course included no weight-bearing for 4 to 6 weeks followed by partial weight-bearing for an additional 4 to 6 weeks. After approximately 8 to 12 postoperative weeks, screw removal was performed followed by progression to full weight-bearing and a comprehensive rehabilitation program, including range of motion, strengthening, proprioception, and gradual return to play. Return to play was allowed when the athlete was asymptomatic and had normal range of motion and strength. Time lost from participation was then recorded based on the dates of injury and return to play.
To further elucidate long-term postinjury playing status, we then gathered information from the www.NFL.com historical and current player databases as previously described by Shah and colleagues.14 From this website, we documented the number of regular-season and postseason games as well as the number of seasons before and after the injury. To be included in the series, the athlete had to have been on the active roster for an NFL franchise at the time of injury. Successful return to play was defined as actual return to play in regular season or postseason NFL games after the midfoot sprain.
In phase 2, a structured electronic questionnaire was sent to all 32 NFL team physicians. The questionnaire was compiled to gather information relating to current diagnostic, treatment, and outcome algorithms in the management of midfoot sprains involving professional football players. Each questionnaire was sent by e-mail to all survey participants and included an embedded link to a secure online survey resource (REDCap Survey Software Version 1.3.9; Vanderbilt University, Nashville, Tennessee). Once the electronic questionnaire was completed by each NFL team physician, results were exported in spreadsheet format for descriptive data analysis.
The retrospective case series and NFL team physician survey data were then analyzed. A descriptive analysis was performed for all variables, including means and minimum–maximum range for quantitative variables as well as frequencies and percentages for qualitative variables. Depending on injury severity, an independent-sample t test with corresponding P values was also calculated for time lost from participation.
Results
The retrospective review of the prospectively collected NFL injury database revealed there were 15 midfoot sprains during the study period. A statistical and descriptive analysis was performed for all study parameters, including player, field, injury, and outcome-specific data. For player, field, and injury-specific data, the results are summarized in the Table.
All grade 1 midfoot sprains (7 nondisplaced) and grade 2 midfoot sprains (5 with subtle diastasis and no instability) were treated with nonoperative management. The 12 players were allowed to return to play without the need for subsequent surgery within the same season. In the evaluation of return to play, based on the severity of the midfoot sprain, there was a statistically significant (P = .047) difference in mean (SD) time lost from participation between the grade 1 sprain group, 3.1 (1.9) days, and the grade 2 sprain group, 36 (26.1) days. Overall, nonoperative treatment of either grade 1 or grade 2 midfoot sprains resulted in a mean of 11.7 days of time lost from participation. In 1 patient with a grade 2 midfoot sprain, the injury occurred toward the end of the season, and the patient was not able to return to play during the remaining 42 days of the season. However, this patient returned to play the next season and had no residual problems.
Three grade 3 injuries (midfoot sprains with frank displacement) required surgical management with ORIF. One patient returned to play the same season, in 73 days; however, the other 2 patients had injuries toward the end of the season (29 and 77 days remaining) and were not able to return to play the same season. However, both these patients returned to play the next season and had no persistent problems. In terms of complications within the same season, there were no recurrent injuries reported after successful return to play.
When evaluating long-term postinjury playing status, we found that 11 (92%) of the 12 NFL players who had nonoperative treatment successfully returned to play. The only player who did not return to an NFL regular season or postseason game was an active-roster NFL player who never actually played in an NFL game before or after his midfoot sprain injury. Our series of NFL players played on average 1.9 years (range, 0-7 years) before the midfoot injury and 5.5 years (range, 0-14 years) after the midfoot injury. In terms of NFL regular-season and postseason games played, our cohort of NFL players played on average 24.0 games (range, 0-80 games) before the midfoot injury and 77.7 games (range, 0-226 games) after the midfoot injury. In fact, 10 of the 12 NFL players (83%) who had nonoperative treatment played more games and seasons after their midfoot injury.
The surveys from phase 2 were completed by all 32 NFL team physicians. When evaluating the severity of midfoot sprains, 63% of the NFL team physicians perform stress-view radiographs. To ascertain NFL team physicians’ management decisions, we evaluated midfoot sprain results according to injury severity, including amount of diastasis.
When managing midfoot sprains with no diastasis, 94% of the team physicians use immobilization, including 27 with a cam walker and 2 with a cast; however, 2 physicians (6%) use only ankle taping or an Ace bandage. Initial weight-bearing status varies among the NFL team physicians, but most (78%) choose to protect the player, including 17 non-weight-bearing, 8 partial weight-bearing, and 7 weight-bearing as tolerated. Most physicians ideally progress players to full weight-bearing by 3 weeks (12% immediately, 12% by week 1, 41% by week 2, 16% by week 3, and 19% from 4-6 weeks).
In the management of midfoot sprains with subtle diastasis, there is variation in treatment modes among the NFL team physicians, with 53% using nonoperative management (34% cam walker, 19% cast) and 47% suggesting operative management. Regardless of treatment, most physicians (97%) maintain initial non-weight-bearing restrictions. In fact, only 1 physician first recommended partial weight-bearing, which corresponded to initial treatment in a cam walker.
In terms of midfoot sprains with frank diastasis, 94% of the NFL team physicians indicated surgical management is warranted, with only 2 physicians (6%) recommending initial nonoperative management with a cam walker. Regardless of treatment, all the physicians (100%) implemented initial non-weight-bearing restrictions. Once surgical treatment was recommended, the preferred fixation method was ORIF using screws (94%) as opposed to closed reduction and internal fixation with percutaneous Kirschner wires (6%). Most of the physicians (59%) do not allow return to play until midfoot hardware is removed; however, 38% allow full participation with contact, and 3% allow partial participation with no contact. Removal of midfoot fixation is an important factor for most of the physicians before considering return to play, and 69% recommend hardware removal after 11 weeks. However, the specific timeline for hardware removal varied among these physicians, with 28% opting for removal at 11 to 12 weeks, 16% at 13 to 14 weeks, 12.5% at 7 to 8 weeks, 12.5% at 15 to 16 weeks, 12.5% at more than 16 weeks, 12.5% never, and 6% at 9 to 10 weeks.
The midfoot sprain treatment protocol (nonoperative vs operative management) based on injury severity was an important factor in considering return-to-play guidelines. When evaluating time lost from participation because of midfoot sprains, most of the NFL team physicians anticipated a period of 5 to 8 weeks when considering nonoperative management (56%) and more than 17 weeks after operative management (53%). In evaluating nonoperative management protocols, return-to-play guidelines were relatively expeditious, with 56% of the physicians estimating from 5 to 8 weeks, 22% from 1 to 4 weeks, 13% from 9 to 12 weeks, 6% from 13 to 16 weeks, and 3% longer than 20 weeks. In comparison to nonoperative management, return-to-play guidelines for operative management were prolonged, with 53% of the physicians estimating more than 20 weeks, 25% from 17 to 20 weeks, 13% from 13 to 16 weeks, and 9% from 9 to 12 weeks.
Discussion
Lisfranc and midfoot injuries remain a controversial topic in sports medicine. Several authors have argued that anatomical reduction of the tarsometatarsal joint in the setting of a Lisfranc injury yields optimal outcomes.15,16 Some studies have also suggested that purely ligamentous Lisfranc injuries may be more of a problem than bony injuries, which may have the benefit of osseous healing.15,17 Anatomical reduction can minimize the potential for arch collapse by maintaining the normal tarsometatarsal relationship. However, there are no long-term data to determine how midfoot arthrosis is affected by ORIF, which typically involves hardware traversing joints. Some have even argued that primary tarsometatarsal arthrodesis should be the treatment of choice, as the midfoot has limited native motion, and successful arthrodesis eliminates the potential for midfoot arthrosis.17,18 However, we are unaware of any studies that have routinely performed arthrodesis in an athletic population.
The majority of studies on midfoot injuries have evaluated individuals involved in traumatic accidents, most commonly motor vehicle collisions. The present study suggests there may be a subset of injuries in athletes that have yet to be adequately studied. Anecdotally, the NFL team physicians surveyed in our study suggested that midfoot sprains with no or subtle displacement may be treated with nonoperative measures while yielding satisfactory clinical outcomes. These results have been quantified in return-to-play status. Our subset of athletes from an NFL team corroborates these findings, even though the series was small (15 patients). Our survey results also suggest there is considerable variation in the “optimal” management plan among the physicians treating these elite athletes. Most would agree that the nondisplaced injuries can be managed conservatively and that the severely displaced injuries should be managed operatively, but the natural history of those injuries with subtle diastasis remains unclear. When operative intervention is implemented, hardware removal versus retention must also be considered when allowing for return to play. Although one would assume that motion-related hardware failure would be possible at the tarsometatarsal joints, this concept has yet to be clearly defined in the literature.
The present study also demonstrates that most athletes with these midfoot injuries can return to play at the elite NFL level, as evidenced by their short- and long-term return to play. However, it was not possible to differentiate the specific return-to-play level related to preinjury performance level. Furthermore, this relatively short-term NFL career follow-up study was not able to elucidate the long-term consequences of these injuries. In fact, arch collapse and acquired flatfoot deformity could eventually result from this injury, and long-term outcomes would be of particular interest in patients who have subtle diastasis and who are treated nonoperatively.
Although previous studies have supported operative management for Lisfranc injuries involving subtle diastasis, more than half of the NFL team physicians surveyed in this study use nonoperative treatment for these injuries.19 Future studies should evaluate stress-imaging to define the effect of stability or latent diastasis on long-term outcomes. Nonetheless, the present study demonstrates that a large cohort of NFL team physicians supports nonoperative management for these Lisfranc injuries with subtle diastasis, even in elite athletes. Additional prospective studies are needed to provide a more rigorous injury evaluation and closer follow-up, including subjective and objective outcomes, to further define the indications for management options for midfoot sprains in this population of contact athletes.
Midfoot (Lisfranc) joint injuries are uncommon in the general population, with a reported incidence ranging from 1 per 50,000 to 1 per 60,000 per year.1,2 The majority of these midfoot injuries result from high-velocity direct trauma involving severe disruption of the tarsometatarsal joint.1-6 Most of the literature on Lisfranc injuries are based on cohorts that include trauma patients. On the other hand, low-velocity indirect injuries of the tarsometatarsal joint have also been associated with midfoot or Lisfranc sprains.7 These injuries are even less extensively studied in athletes, who may sustain them from torsion or the shoe–surface interface.8
Foot and ankle injuries are among the most common injuries in athletes and represent 16% to 22% of all sports injuries.9 Although midfoot sprains are not common in the general population, sporting activities appear to result in a higher rate of midfoot injury, especially in elite athletes. In fact, midfoot sprains comprise the second most common athlete injury to the foot, after metatarsophalangeal joint injuries.10 Football players are especially prone to midfoot sprains; incidence is 4% per year, with offensive linemen sustaining 29.2% of midfoot sprains.10 The most common mechanism of injury is an axial longitudinal force while the foot is plantarflexed and slightly rotated.11,12
There is a paucity of literature detailing the impact of midfoot injuries on football players.8,10,13 A study of 23 collegiate football players found that they may have initially underwent a long period of acute disability but had very minor long-term complaints resulting in residual functional disability.10 However, there are no case series detailing the impact of midfoot sprains on professional football players for whom delayed return to sport can potentially have a devastating impact on a career in terms of both acute- and long-term disability.
We conducted a study to further define the mechanism of injury, diagnosis, treatment, and outcomes among National Football League (NFL) players with midfoot sprains. In addition, we aimed to provide a qualitative analysis of diagnostic and treatment algorithms being used by NFL team physicians in their management of midfoot sprains in these high-level contact athletes.
Materials and Methods
We evaluated midfoot sprains in NFL players in 2 specific phases. In phase 1, we retrospectively reviewed prospectively collected data involving midfoot sprains in professional players from a single NFL team over a 15-year period. In phase 2, we collated diagnostic and treatment algorithms for midfoot sprains among all 32 NFL team physicians by means of a structured questionnaire. Institutional review board approval was obtained for this study at the investigators’ institution.
In phase 1, a NFL team injury database was reviewed for midfoot sprains that had been prospectively entered by a team-certified athletic trainer after consultation with the head orthopedic team physician. All injury and diagnostic modalities and treatments were then analyzed. These included player position, foot and ankle protective gear (none, tape, brace, or unknown), playing surface (grass, AstroTurf, FieldTurf, or unknown), field condition (normal, wet, hard, or unknown), onset of injury (acute, chronic, or unknown), place of injury (game or practice), time of injury in game or practice (first quarter, second quarter, third quarter, fourth quarter, or unknown), type of play (collision, tackled, tackling, blocked, blocking, running/cutting, kicking, or unknown), and mechanism of injury (direct, torsion, shearing, or unknown).
Once the diagnosis was confirmed by physical examination and radiographic findings, midfoot sprain treatment was initiated based on the following algorithm protocols. Nondisplaced sprains were treated with a period of immobilization in a cam walker with progression to weight-bearing as tolerated (grade 1). Once asymptomatic, rehabilitation was initiated, including range of motion, strengthening, and proprioception, and gradual return to play as tolerated. Injuries with subtle diastasis (2-5 mm) were typically treated with nonoperative management in the same manner as the nondisplaced sprain protocol (grade 2); however, signs of gross instability indicated the potential requirement for surgical management. Some of these injuries underwent stress-testing to determine if there was gross instability. If the injury had subtle diastasis with instability or frank (>5 mm) displacement (grade 3), then surgical management was performed with closed versus open reduction and internal fixation (ORIF). The postoperative course included no weight-bearing for 4 to 6 weeks followed by partial weight-bearing for an additional 4 to 6 weeks. After approximately 8 to 12 postoperative weeks, screw removal was performed followed by progression to full weight-bearing and a comprehensive rehabilitation program, including range of motion, strengthening, proprioception, and gradual return to play. Return to play was allowed when the athlete was asymptomatic and had normal range of motion and strength. Time lost from participation was then recorded based on the dates of injury and return to play.
To further elucidate long-term postinjury playing status, we then gathered information from the www.NFL.com historical and current player databases as previously described by Shah and colleagues.14 From this website, we documented the number of regular-season and postseason games as well as the number of seasons before and after the injury. To be included in the series, the athlete had to have been on the active roster for an NFL franchise at the time of injury. Successful return to play was defined as actual return to play in regular season or postseason NFL games after the midfoot sprain.
In phase 2, a structured electronic questionnaire was sent to all 32 NFL team physicians. The questionnaire was compiled to gather information relating to current diagnostic, treatment, and outcome algorithms in the management of midfoot sprains involving professional football players. Each questionnaire was sent by e-mail to all survey participants and included an embedded link to a secure online survey resource (REDCap Survey Software Version 1.3.9; Vanderbilt University, Nashville, Tennessee). Once the electronic questionnaire was completed by each NFL team physician, results were exported in spreadsheet format for descriptive data analysis.
The retrospective case series and NFL team physician survey data were then analyzed. A descriptive analysis was performed for all variables, including means and minimum–maximum range for quantitative variables as well as frequencies and percentages for qualitative variables. Depending on injury severity, an independent-sample t test with corresponding P values was also calculated for time lost from participation.
Results
The retrospective review of the prospectively collected NFL injury database revealed there were 15 midfoot sprains during the study period. A statistical and descriptive analysis was performed for all study parameters, including player, field, injury, and outcome-specific data. For player, field, and injury-specific data, the results are summarized in the Table.
All grade 1 midfoot sprains (7 nondisplaced) and grade 2 midfoot sprains (5 with subtle diastasis and no instability) were treated with nonoperative management. The 12 players were allowed to return to play without the need for subsequent surgery within the same season. In the evaluation of return to play, based on the severity of the midfoot sprain, there was a statistically significant (P = .047) difference in mean (SD) time lost from participation between the grade 1 sprain group, 3.1 (1.9) days, and the grade 2 sprain group, 36 (26.1) days. Overall, nonoperative treatment of either grade 1 or grade 2 midfoot sprains resulted in a mean of 11.7 days of time lost from participation. In 1 patient with a grade 2 midfoot sprain, the injury occurred toward the end of the season, and the patient was not able to return to play during the remaining 42 days of the season. However, this patient returned to play the next season and had no residual problems.
Three grade 3 injuries (midfoot sprains with frank displacement) required surgical management with ORIF. One patient returned to play the same season, in 73 days; however, the other 2 patients had injuries toward the end of the season (29 and 77 days remaining) and were not able to return to play the same season. However, both these patients returned to play the next season and had no persistent problems. In terms of complications within the same season, there were no recurrent injuries reported after successful return to play.
When evaluating long-term postinjury playing status, we found that 11 (92%) of the 12 NFL players who had nonoperative treatment successfully returned to play. The only player who did not return to an NFL regular season or postseason game was an active-roster NFL player who never actually played in an NFL game before or after his midfoot sprain injury. Our series of NFL players played on average 1.9 years (range, 0-7 years) before the midfoot injury and 5.5 years (range, 0-14 years) after the midfoot injury. In terms of NFL regular-season and postseason games played, our cohort of NFL players played on average 24.0 games (range, 0-80 games) before the midfoot injury and 77.7 games (range, 0-226 games) after the midfoot injury. In fact, 10 of the 12 NFL players (83%) who had nonoperative treatment played more games and seasons after their midfoot injury.
The surveys from phase 2 were completed by all 32 NFL team physicians. When evaluating the severity of midfoot sprains, 63% of the NFL team physicians perform stress-view radiographs. To ascertain NFL team physicians’ management decisions, we evaluated midfoot sprain results according to injury severity, including amount of diastasis.
When managing midfoot sprains with no diastasis, 94% of the team physicians use immobilization, including 27 with a cam walker and 2 with a cast; however, 2 physicians (6%) use only ankle taping or an Ace bandage. Initial weight-bearing status varies among the NFL team physicians, but most (78%) choose to protect the player, including 17 non-weight-bearing, 8 partial weight-bearing, and 7 weight-bearing as tolerated. Most physicians ideally progress players to full weight-bearing by 3 weeks (12% immediately, 12% by week 1, 41% by week 2, 16% by week 3, and 19% from 4-6 weeks).
In the management of midfoot sprains with subtle diastasis, there is variation in treatment modes among the NFL team physicians, with 53% using nonoperative management (34% cam walker, 19% cast) and 47% suggesting operative management. Regardless of treatment, most physicians (97%) maintain initial non-weight-bearing restrictions. In fact, only 1 physician first recommended partial weight-bearing, which corresponded to initial treatment in a cam walker.
In terms of midfoot sprains with frank diastasis, 94% of the NFL team physicians indicated surgical management is warranted, with only 2 physicians (6%) recommending initial nonoperative management with a cam walker. Regardless of treatment, all the physicians (100%) implemented initial non-weight-bearing restrictions. Once surgical treatment was recommended, the preferred fixation method was ORIF using screws (94%) as opposed to closed reduction and internal fixation with percutaneous Kirschner wires (6%). Most of the physicians (59%) do not allow return to play until midfoot hardware is removed; however, 38% allow full participation with contact, and 3% allow partial participation with no contact. Removal of midfoot fixation is an important factor for most of the physicians before considering return to play, and 69% recommend hardware removal after 11 weeks. However, the specific timeline for hardware removal varied among these physicians, with 28% opting for removal at 11 to 12 weeks, 16% at 13 to 14 weeks, 12.5% at 7 to 8 weeks, 12.5% at 15 to 16 weeks, 12.5% at more than 16 weeks, 12.5% never, and 6% at 9 to 10 weeks.
The midfoot sprain treatment protocol (nonoperative vs operative management) based on injury severity was an important factor in considering return-to-play guidelines. When evaluating time lost from participation because of midfoot sprains, most of the NFL team physicians anticipated a period of 5 to 8 weeks when considering nonoperative management (56%) and more than 17 weeks after operative management (53%). In evaluating nonoperative management protocols, return-to-play guidelines were relatively expeditious, with 56% of the physicians estimating from 5 to 8 weeks, 22% from 1 to 4 weeks, 13% from 9 to 12 weeks, 6% from 13 to 16 weeks, and 3% longer than 20 weeks. In comparison to nonoperative management, return-to-play guidelines for operative management were prolonged, with 53% of the physicians estimating more than 20 weeks, 25% from 17 to 20 weeks, 13% from 13 to 16 weeks, and 9% from 9 to 12 weeks.
Discussion
Lisfranc and midfoot injuries remain a controversial topic in sports medicine. Several authors have argued that anatomical reduction of the tarsometatarsal joint in the setting of a Lisfranc injury yields optimal outcomes.15,16 Some studies have also suggested that purely ligamentous Lisfranc injuries may be more of a problem than bony injuries, which may have the benefit of osseous healing.15,17 Anatomical reduction can minimize the potential for arch collapse by maintaining the normal tarsometatarsal relationship. However, there are no long-term data to determine how midfoot arthrosis is affected by ORIF, which typically involves hardware traversing joints. Some have even argued that primary tarsometatarsal arthrodesis should be the treatment of choice, as the midfoot has limited native motion, and successful arthrodesis eliminates the potential for midfoot arthrosis.17,18 However, we are unaware of any studies that have routinely performed arthrodesis in an athletic population.
The majority of studies on midfoot injuries have evaluated individuals involved in traumatic accidents, most commonly motor vehicle collisions. The present study suggests there may be a subset of injuries in athletes that have yet to be adequately studied. Anecdotally, the NFL team physicians surveyed in our study suggested that midfoot sprains with no or subtle displacement may be treated with nonoperative measures while yielding satisfactory clinical outcomes. These results have been quantified in return-to-play status. Our subset of athletes from an NFL team corroborates these findings, even though the series was small (15 patients). Our survey results also suggest there is considerable variation in the “optimal” management plan among the physicians treating these elite athletes. Most would agree that the nondisplaced injuries can be managed conservatively and that the severely displaced injuries should be managed operatively, but the natural history of those injuries with subtle diastasis remains unclear. When operative intervention is implemented, hardware removal versus retention must also be considered when allowing for return to play. Although one would assume that motion-related hardware failure would be possible at the tarsometatarsal joints, this concept has yet to be clearly defined in the literature.
The present study also demonstrates that most athletes with these midfoot injuries can return to play at the elite NFL level, as evidenced by their short- and long-term return to play. However, it was not possible to differentiate the specific return-to-play level related to preinjury performance level. Furthermore, this relatively short-term NFL career follow-up study was not able to elucidate the long-term consequences of these injuries. In fact, arch collapse and acquired flatfoot deformity could eventually result from this injury, and long-term outcomes would be of particular interest in patients who have subtle diastasis and who are treated nonoperatively.
Although previous studies have supported operative management for Lisfranc injuries involving subtle diastasis, more than half of the NFL team physicians surveyed in this study use nonoperative treatment for these injuries.19 Future studies should evaluate stress-imaging to define the effect of stability or latent diastasis on long-term outcomes. Nonetheless, the present study demonstrates that a large cohort of NFL team physicians supports nonoperative management for these Lisfranc injuries with subtle diastasis, even in elite athletes. Additional prospective studies are needed to provide a more rigorous injury evaluation and closer follow-up, including subjective and objective outcomes, to further define the indications for management options for midfoot sprains in this population of contact athletes.
1. Aitken AP, Poulson D. Dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1963;45:246-260.
2. Hardcastle PH, Reschauer R, Kutscha-Lissberg E, Schoffmann W. Injuries to the tarsometatarsal joint. Incidence, classification and treatment. J Bone Joint Surg Br. 1982;64(3):349-356.
3. Arntz CT, Veith RG, Hansen ST Jr. Fractures and fracture-dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1988(2);70:173-181.
4. Goossens M, De Stoop N. Lisfranc’s fracture-dislocations: etiology, radiology, and results of treatment. A review of 20 cases. Clin Orthop. 1983;(176):154-162.
5. Myerson M. The diagnosis and treatment of injuries to the Lisfranc joint complex. Orthop Clin North Am. 1989;20(4):655-664.
6. Wiley JJ. The mechanism of tarso-metatarsal joint injuries. J Bone Joint Surg Br. 1971;53(3):474-482.
7. Faciszewski T, Burks RT, Manaster BJ. Subtle injuries of the Lisfranc joint. J Bone Joint Surg Am. 1990;72(10):1519-1522.
8. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
9. Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7(1):29-36.
10. Meyer SA, Callaghan JJ, Albright JP, Crowley ET, Powell JW. Midfoot sprains in collegiate football players. Am J Sports Med. 1994;22(3):392-401.
11. Shapiro MS, Wascher DC, Finerman GA. Rupture of Lisfranc’s ligament in athletes. Am J Sports Med. 1994;22(5):687-691.
12. Curtis MJ, Myerson M, Szura B. Tarsometatarsal joint injuries in the athlete. Am J Sports Med. 1993;21(4):497-502.
13. Harwood MI, Raikin SM. A Lisfranc fracture-dislocation in a football player. J Am Board Fam Pract. 2003;16(1):69-72.
14. Shah VM, Andrews JR, Fleisig GS, et al. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
15. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82(11):1609-1618.
16. Myerson MS, Cerrato RA. Current management of tarsometatarsal injuries in the athlete. J Bone Joint Surg Am. 2008;90(11):2522-2533.
17. Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88(3):514-520.
18. Coetzee JC, Ly TV. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 pt1):122-127.
19. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle. 2010;9:100-106.
1. Aitken AP, Poulson D. Dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1963;45:246-260.
2. Hardcastle PH, Reschauer R, Kutscha-Lissberg E, Schoffmann W. Injuries to the tarsometatarsal joint. Incidence, classification and treatment. J Bone Joint Surg Br. 1982;64(3):349-356.
3. Arntz CT, Veith RG, Hansen ST Jr. Fractures and fracture-dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1988(2);70:173-181.
4. Goossens M, De Stoop N. Lisfranc’s fracture-dislocations: etiology, radiology, and results of treatment. A review of 20 cases. Clin Orthop. 1983;(176):154-162.
5. Myerson M. The diagnosis and treatment of injuries to the Lisfranc joint complex. Orthop Clin North Am. 1989;20(4):655-664.
6. Wiley JJ. The mechanism of tarso-metatarsal joint injuries. J Bone Joint Surg Br. 1971;53(3):474-482.
7. Faciszewski T, Burks RT, Manaster BJ. Subtle injuries of the Lisfranc joint. J Bone Joint Surg Am. 1990;72(10):1519-1522.
8. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
9. Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7(1):29-36.
10. Meyer SA, Callaghan JJ, Albright JP, Crowley ET, Powell JW. Midfoot sprains in collegiate football players. Am J Sports Med. 1994;22(3):392-401.
11. Shapiro MS, Wascher DC, Finerman GA. Rupture of Lisfranc’s ligament in athletes. Am J Sports Med. 1994;22(5):687-691.
12. Curtis MJ, Myerson M, Szura B. Tarsometatarsal joint injuries in the athlete. Am J Sports Med. 1993;21(4):497-502.
13. Harwood MI, Raikin SM. A Lisfranc fracture-dislocation in a football player. J Am Board Fam Pract. 2003;16(1):69-72.
14. Shah VM, Andrews JR, Fleisig GS, et al. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
15. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82(11):1609-1618.
16. Myerson MS, Cerrato RA. Current management of tarsometatarsal injuries in the athlete. J Bone Joint Surg Am. 2008;90(11):2522-2533.
17. Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88(3):514-520.
18. Coetzee JC, Ly TV. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 pt1):122-127.
19. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle. 2010;9:100-106.
Lower Extremity Injuries in Snowboarders
Epidemiology
The several studies of lower extremity injuries sustained while skiing and snowboarding have differed markedly with respect to patient demographics. Kim and colleagues1 compared snowboarding and skiing injuries over 18 seasons at a Vermont ski resort and found that the injury rate, assessed as mean number of days between injuries, was 400 for snowboarders and 345 for skiers. However, most snowboarding injuries were wrist injuries and generally of the upper extremity, whereas skiing injuries were mainly lower extremity injuries. Overall, young and inexperienced snowboarders had the highest injury rate. In a study on skiing and snowboarding injuries through 4 Utah seasons, Wasden and colleagues2 found that mean age at injury was 41 years for skiers and 23 years for snowboarders. This corroborates the finding from several studies1-3 that snowboarders tend to be younger. Snowboarding is a newer sport with many beginners. However, Ishimaru and colleagues4 found that lower extremity injuries may be associated with experienced snowboarders, who may be prone to take more risks and tackle more challenging slopes. Experienced snowboarders are also likely to sustain lower extremity injuries from falling, because of their risk-taking behavior.5
Although upper extremity injuries account for most snowboarding injuries, lower extremity injuries are a significant issue.6 Modern equipment and more challenging slopes have allowed snowboarders to attain great speeds going down slopes—leading to a surge in lower extremity injuries.7 Lower extremity injuries sustained during snowboarding are more likely to be on the leading side4; the ankle is the most frequent fracture site. Unlike snowboard equipment, modern ski equipment, including new boots and binding systems, is designed to reduce ankle injuries and lower leg fractures.6 The decline in foot, ankle, and tibia fractures can be attributed to taller and stiffer boots, which offer the lower extremities more protection.8
Mechanism of Injury
Talus Fractures
An increasingly common injury among snowboarders is a fracture of the lateral process of the talus; this injury accounts for 32% of snowboarders’ ankle fractures.6 The lateral process of the talus—wedge-shaped and covered in articular cartilage—is involved in the subtalar and ankle joints.9 A fracture here is often misdiagnosed as an ankle sprain (Figures 1–3).6,9,10 The exact mechanism of injury remains controversial, and several biomechanical factors seem to be involved. Funk and colleagues11 conducted a cadaveric study and concluded that eversion of an axially loaded, dorsiflexed ankle may be the primary injury mechanism for fracture. Furthermore, snowboarders have their feet in a position perpendicular to the board, and a fall parallel to the board could increase the eversion force on the ankle of the leading leg. Valderrabano and colleagues9 conducted a clinical study of 26 patients who sustained this injury from snowboarding. All the patients reported they had felt an axial impact from falling, jumping, or unexpectedly hitting a ground object, and 80% reported a rotational movement in the lower leg during the impact. The authors concluded that axial loading and dorsiflexion were not the only factors involved in lateral process talus fractures, and an external moment is necessary to cause this injury from a forward fall.9
Anterior Cruciate Ligament Injuries
Although snowboarders’ lower extremity injuries are primarily ankle injuries, snowboarders are also at risk for serious knee issues when landing from jumps. In skiers, anterior cruciate ligament (ACL) injuries have 5 well-established mechanisms, all involving separation of the feet and a twisting force in the knee (Figures 4, 5): boot-induced anterior drawer mechanism, phantom-foot mechanism, valgus-external rotation, forceful quadriceps muscle contraction, and a combination of internal rotation and extension.8,12 A valgus–external rotation mechanism of knee injury occurs when external rotation of the tibia results from the skier catching the inside edge of the front of the ski. A valgus force acts on the knee as the lower leg is abducted during forward momentum. The torque created on the knee joint is amplified by the length of the knee and commonly results in an ACL injury or medial collateral ligament injury.6 Reports indicate that the phantom-foot mechanism is the most common mechanism of ACL injury among skiers.6,13,14 In this situation, internal rotation of the knee results when an off-balance skier falls backward, which causes the knee to hyperflex. The skier catches an inside edge on the snow, which creates a torque that rotates the tibia relative to the femur and results in injury to the ACL.6,14 A boot-induced anterior drawer mechanism occurs during a landing, when the tail of the ski lands first and in an off-balance position, resulting in a load transmitted through the skis to the skier; this load causes an anterior drawer of the ski boot and tibia relative to the femur, straining the ACL and causing ACL rupture.6,13,14 In the forceful quadriceps muscle contraction mechanism of ACL injury, a forceful quadriceps contraction occurs after a jump to prevent a backward fall. With the knee in flexion, this quadriceps contraction causes an anterior translation of the tibia, resulting in ACL rupture.13,14
The mechanism of injury differs in snowboarding, in which both feet remain attached to the board. Davies and colleagues15 examined 35 snowboarders who sustained ACL injuries after a flat landing from a jump and concluded that snowboarders preparing for a landing exhibit more quadriceps contraction, which increases the loading force on the ACL during landing. Furthermore, the snowboarder’s stance on the board, with the front foot slightly rotated relative to the board, results in a slight internal tibial rotation of the knee and establishes a posture that makes the snowboarder susceptible to injury. However, the lower incidence of knee injuries among snowboarders compared with skiers may be attributable to the fact that there is a limited amount of torque that can be generated on either knee as both feet are fixed to the board.16
The increased quadriceps force in anticipation of a landing, combined with the internal tibial rotation of the knee caused by the snowboarder’s stance, may be the primary mechanism of ACL rupture in snowboarders.15
Injury Prevention Strategies
Prevention strategies require an identification of injury risk factors for snowboarders. Hasler and colleagues7 conducted a study with 306 patients to identify variables that presented a risk for snowboarders. Low readiness for speed, bad weather, and bad visibility, as well as snow conditions, were found to be significant risk factors.
Skiers’ overall injury rate has decreased over the past 60 years, and this decrease has been attributed in part to improved ski technique and instruction.17,18 Improperly adjusted ski bindings are the culprit in many equipment-related lower extremity injuries, and beginners are at much higher risk for such injuries. Lessons and comprehensive safety training could reduce this injury rate.17,19 Several awareness video and training programs focusing on injury prevention have reduced knee sprains in ski patrollers compared with controls by 62% in 1 study; a similar program reduced injury by 30% in nonprofessional skiers.17 A study of injured snowboarders during a winter in Scotland found that 37% of the patients had no formal instruction or training in correct snowboarding and falling technique.20 Training programs for snowboarders could yield meaningful results in injury prevention and avoidance of risk-taking behavior among snowboarders.
Advances in equipment have also had an impact on the incidence of skiing injuries. Ski bindings protect skiers in 2 ways. First, the binding keeps the boot attached to the ski and prevents unintended release on difficult terrain. Second, the binding releases the boot from the ski during extreme conditions to prevent the skier from experiencing extreme forces or moments that could result in injury. Functional failure in ski bindings has been implicated in increased incidence of knee injuries and ligament rupture. In a study of injuries sustained by recreational alpine skiers in Japan, Urabe and colleagues21 found that 96% of those injured stated that the ski bindings had not released at time of incident. The effects of binding adjustment and maintenance among snowboarders have not been fully investigated, and there are no set guidelines for individual snowboarders on appropriate binding level. However, as there is a range of binding adjustment options available, snowboarders may have an optimum level that maximizes both mobility and protection from injury.22
Soft-shelled boots may also increase injury risk for snowboarders. Such boots allow for a wider range of ankle motion and offer little protection from extreme joint movements. Soft boots are generally preferred among snowboarders because they allow for increased mobility for sharp turns and maneuvers. However, modification of the stiffness of boots that limit ankle and foot joint mobility could reduce the incidence of ankle fractures and sprains among snowboarders.22
Summary
Snowboarding has become increasingly popular worldwide. It attracts a loyal group of amateur athletes and has developed into a billion-dollar industry with a growing rank of professionals. Although most snowboarding injuries are upper extremity injuries, the foot, ankle, and knee represent commonly injured areas among recreational and experienced snowboarders. Advances in ski equipment have significantly reduced the incidence of ankle injuries, but rising knee ligament injuries continue to pose a challenge. Foot and ankle injuries remain an issue in snowboarders despite advances in equipment and safety. New snowboard designs and boot and binding modifications may hold promise in decreasing the risk for injury in these athletes.
1. Kim S, Endres NK, Johnson RJ, Ettlinger CF, Shealy JE. Snowboarding injuries: trends over time and comparisons with alpine skiing injuries. Am J Sports Med. 2012;40(4):770-776.
2. Wasden CC, McIntosh SE, Keith DS, McCowan C. An analysis of skiing and snowboarding injuries on Utah slopes. J Trauma. 2009;67(5):1022-1026.
3. Rust DA, Gilmore CJ, Treme G. Injury patterns at a large western United States ski resort with and without snowboarders: the Taos experience. Am J Sports Med. 2013;41(3):652-656.
4. Ishimaru D, Ogawa H, Sumi H, Sumi Y, Shimizu K. Lower extremity injuries in snowboarding. J Trauma. 2011;70(3):E48-E52.
5. Torjussen J, Bahr R. Injuries among competitive snowboarders at the national elite level. Am J Sports Med. 2005;33(3):370-377.
6. Deady LH, Salonen D. Skiing and snowboarding injuries: a review with a focus on mechanism of injury. Radiol Clin North Am. 2010;48(6):1113-1124.
7. Hasler RM, Berov S, Banneker L, et al. Are there risk factors for snowboard injuries? A case–control multicentre study of 559 snowboarders. Br J Sports Med. 2010;44(11):816-821.
8. St-Onge N, Chevalier Y, Hagemeister N, Van De Putte M, De Guise J. Effect of ski binding parameters on knee biomechanics: a three-dimensional computational study. Med Sci Sports Exerc. 2004;36(7):1218-1225.
9. Valderrabano V, Perren T, Ryf C, Rillmann P, Hintermann B. Snowboarder’s talus fracture: treatment outcome of 20 cases after 3.5 years. Am J Sports Med. 2005;33(6):871-880.
10. von Knoch F, Reckord U, von Knoch M, Sommer C. Fracture of the lateral process of the talus in snowboarders. J Bone Joint Surg Br. 2007;89(6):772-777.
11. Funk JR, Srinivasan SC, Crandall JR. Snowboarder’s talus fractures experimentally produced by eversion and dorsiflexion. Am J Sports Med. 2003;31(6):921-928.
12. Pujol N, Blanchi MP, Chambat P. The incidence of anterior cruciate ligament injuries among competitive alpine skiers: a 25-year investigation. Am J Sports Med. 2007;35(7):1070-1074.
13. Hame SL, Oakes DA, Markolf KL. Injury to the anterior cruciate ligament during alpine skiing: a biomechanical analysis of tibial torque and knee flexion angle. Am J Sports Med. 2002;30(4):537-540.
14. Bere T, Flørenes TW, Krosshaug T, Nordsletten L, Bahr R. Events leading to anterior cruciate ligament injury in World Cup alpine skiing: a systematic video analysis of 20 cases. Br J Sports Med. 2011;45(16):1294-1302.
15. Davies H, Tietjens B, Van Sterkenburg M, Mehgan A. Anterior cruciate ligament injuries in snowboarders: a quadriceps-induced injury. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1048-1051.
16. Bladin C, McCrory P, Pogorzelski A. Snowboarding injuries: current trends and future directions. Sports Med. 2004;34(2):133-139.
17. Rossi MJ, Lubowitz JH, Guttmann D. The skier’s knee. Arthroscopy. 2003;19(1):75-84.
18. Pressman A, Johnson DH. A review of ski injuries resulting in combined injury to the anterior cruciate ligament and medial collateral ligaments. Arthroscopy. 2003;19(2):194-202.
19. Hildebrandt C, Mildner E, Hotter B, Kirschner W, Höbenreich C, Raschner C. Accident prevention on ski slopes—perceptions of safety and knowledge of existing rules. Accid Anal Prev. 2011;43(4):1421-1426.
20. Langran M, Selvaraj S. Increased injury risk among first-day skiers, snowboarders, and skiboarders. Am J Sports Med. 2004;32(1):96-103.
21. Urabe Y, Ochi M, Onari K, Ikuta Y. Anterior cruciate ligament injury in recreational alpine skiers: analysis of mechanisms and strategy for prevention. J Orthop Sci. 2002;7(1):1-5.
22. McAlpine PR. Biomechanical Analysis of Snowboard Jump Landings: A Focus on the Ankle Joint Complex [doctoral thesis]. Auckland, New Zealand: University of Auckland; 2010.
Epidemiology
The several studies of lower extremity injuries sustained while skiing and snowboarding have differed markedly with respect to patient demographics. Kim and colleagues1 compared snowboarding and skiing injuries over 18 seasons at a Vermont ski resort and found that the injury rate, assessed as mean number of days between injuries, was 400 for snowboarders and 345 for skiers. However, most snowboarding injuries were wrist injuries and generally of the upper extremity, whereas skiing injuries were mainly lower extremity injuries. Overall, young and inexperienced snowboarders had the highest injury rate. In a study on skiing and snowboarding injuries through 4 Utah seasons, Wasden and colleagues2 found that mean age at injury was 41 years for skiers and 23 years for snowboarders. This corroborates the finding from several studies1-3 that snowboarders tend to be younger. Snowboarding is a newer sport with many beginners. However, Ishimaru and colleagues4 found that lower extremity injuries may be associated with experienced snowboarders, who may be prone to take more risks and tackle more challenging slopes. Experienced snowboarders are also likely to sustain lower extremity injuries from falling, because of their risk-taking behavior.5
Although upper extremity injuries account for most snowboarding injuries, lower extremity injuries are a significant issue.6 Modern equipment and more challenging slopes have allowed snowboarders to attain great speeds going down slopes—leading to a surge in lower extremity injuries.7 Lower extremity injuries sustained during snowboarding are more likely to be on the leading side4; the ankle is the most frequent fracture site. Unlike snowboard equipment, modern ski equipment, including new boots and binding systems, is designed to reduce ankle injuries and lower leg fractures.6 The decline in foot, ankle, and tibia fractures can be attributed to taller and stiffer boots, which offer the lower extremities more protection.8
Mechanism of Injury
Talus Fractures
An increasingly common injury among snowboarders is a fracture of the lateral process of the talus; this injury accounts for 32% of snowboarders’ ankle fractures.6 The lateral process of the talus—wedge-shaped and covered in articular cartilage—is involved in the subtalar and ankle joints.9 A fracture here is often misdiagnosed as an ankle sprain (Figures 1–3).6,9,10 The exact mechanism of injury remains controversial, and several biomechanical factors seem to be involved. Funk and colleagues11 conducted a cadaveric study and concluded that eversion of an axially loaded, dorsiflexed ankle may be the primary injury mechanism for fracture. Furthermore, snowboarders have their feet in a position perpendicular to the board, and a fall parallel to the board could increase the eversion force on the ankle of the leading leg. Valderrabano and colleagues9 conducted a clinical study of 26 patients who sustained this injury from snowboarding. All the patients reported they had felt an axial impact from falling, jumping, or unexpectedly hitting a ground object, and 80% reported a rotational movement in the lower leg during the impact. The authors concluded that axial loading and dorsiflexion were not the only factors involved in lateral process talus fractures, and an external moment is necessary to cause this injury from a forward fall.9
Anterior Cruciate Ligament Injuries
Although snowboarders’ lower extremity injuries are primarily ankle injuries, snowboarders are also at risk for serious knee issues when landing from jumps. In skiers, anterior cruciate ligament (ACL) injuries have 5 well-established mechanisms, all involving separation of the feet and a twisting force in the knee (Figures 4, 5): boot-induced anterior drawer mechanism, phantom-foot mechanism, valgus-external rotation, forceful quadriceps muscle contraction, and a combination of internal rotation and extension.8,12 A valgus–external rotation mechanism of knee injury occurs when external rotation of the tibia results from the skier catching the inside edge of the front of the ski. A valgus force acts on the knee as the lower leg is abducted during forward momentum. The torque created on the knee joint is amplified by the length of the knee and commonly results in an ACL injury or medial collateral ligament injury.6 Reports indicate that the phantom-foot mechanism is the most common mechanism of ACL injury among skiers.6,13,14 In this situation, internal rotation of the knee results when an off-balance skier falls backward, which causes the knee to hyperflex. The skier catches an inside edge on the snow, which creates a torque that rotates the tibia relative to the femur and results in injury to the ACL.6,14 A boot-induced anterior drawer mechanism occurs during a landing, when the tail of the ski lands first and in an off-balance position, resulting in a load transmitted through the skis to the skier; this load causes an anterior drawer of the ski boot and tibia relative to the femur, straining the ACL and causing ACL rupture.6,13,14 In the forceful quadriceps muscle contraction mechanism of ACL injury, a forceful quadriceps contraction occurs after a jump to prevent a backward fall. With the knee in flexion, this quadriceps contraction causes an anterior translation of the tibia, resulting in ACL rupture.13,14
The mechanism of injury differs in snowboarding, in which both feet remain attached to the board. Davies and colleagues15 examined 35 snowboarders who sustained ACL injuries after a flat landing from a jump and concluded that snowboarders preparing for a landing exhibit more quadriceps contraction, which increases the loading force on the ACL during landing. Furthermore, the snowboarder’s stance on the board, with the front foot slightly rotated relative to the board, results in a slight internal tibial rotation of the knee and establishes a posture that makes the snowboarder susceptible to injury. However, the lower incidence of knee injuries among snowboarders compared with skiers may be attributable to the fact that there is a limited amount of torque that can be generated on either knee as both feet are fixed to the board.16
The increased quadriceps force in anticipation of a landing, combined with the internal tibial rotation of the knee caused by the snowboarder’s stance, may be the primary mechanism of ACL rupture in snowboarders.15
Injury Prevention Strategies
Prevention strategies require an identification of injury risk factors for snowboarders. Hasler and colleagues7 conducted a study with 306 patients to identify variables that presented a risk for snowboarders. Low readiness for speed, bad weather, and bad visibility, as well as snow conditions, were found to be significant risk factors.
Skiers’ overall injury rate has decreased over the past 60 years, and this decrease has been attributed in part to improved ski technique and instruction.17,18 Improperly adjusted ski bindings are the culprit in many equipment-related lower extremity injuries, and beginners are at much higher risk for such injuries. Lessons and comprehensive safety training could reduce this injury rate.17,19 Several awareness video and training programs focusing on injury prevention have reduced knee sprains in ski patrollers compared with controls by 62% in 1 study; a similar program reduced injury by 30% in nonprofessional skiers.17 A study of injured snowboarders during a winter in Scotland found that 37% of the patients had no formal instruction or training in correct snowboarding and falling technique.20 Training programs for snowboarders could yield meaningful results in injury prevention and avoidance of risk-taking behavior among snowboarders.
Advances in equipment have also had an impact on the incidence of skiing injuries. Ski bindings protect skiers in 2 ways. First, the binding keeps the boot attached to the ski and prevents unintended release on difficult terrain. Second, the binding releases the boot from the ski during extreme conditions to prevent the skier from experiencing extreme forces or moments that could result in injury. Functional failure in ski bindings has been implicated in increased incidence of knee injuries and ligament rupture. In a study of injuries sustained by recreational alpine skiers in Japan, Urabe and colleagues21 found that 96% of those injured stated that the ski bindings had not released at time of incident. The effects of binding adjustment and maintenance among snowboarders have not been fully investigated, and there are no set guidelines for individual snowboarders on appropriate binding level. However, as there is a range of binding adjustment options available, snowboarders may have an optimum level that maximizes both mobility and protection from injury.22
Soft-shelled boots may also increase injury risk for snowboarders. Such boots allow for a wider range of ankle motion and offer little protection from extreme joint movements. Soft boots are generally preferred among snowboarders because they allow for increased mobility for sharp turns and maneuvers. However, modification of the stiffness of boots that limit ankle and foot joint mobility could reduce the incidence of ankle fractures and sprains among snowboarders.22
Summary
Snowboarding has become increasingly popular worldwide. It attracts a loyal group of amateur athletes and has developed into a billion-dollar industry with a growing rank of professionals. Although most snowboarding injuries are upper extremity injuries, the foot, ankle, and knee represent commonly injured areas among recreational and experienced snowboarders. Advances in ski equipment have significantly reduced the incidence of ankle injuries, but rising knee ligament injuries continue to pose a challenge. Foot and ankle injuries remain an issue in snowboarders despite advances in equipment and safety. New snowboard designs and boot and binding modifications may hold promise in decreasing the risk for injury in these athletes.
Epidemiology
The several studies of lower extremity injuries sustained while skiing and snowboarding have differed markedly with respect to patient demographics. Kim and colleagues1 compared snowboarding and skiing injuries over 18 seasons at a Vermont ski resort and found that the injury rate, assessed as mean number of days between injuries, was 400 for snowboarders and 345 for skiers. However, most snowboarding injuries were wrist injuries and generally of the upper extremity, whereas skiing injuries were mainly lower extremity injuries. Overall, young and inexperienced snowboarders had the highest injury rate. In a study on skiing and snowboarding injuries through 4 Utah seasons, Wasden and colleagues2 found that mean age at injury was 41 years for skiers and 23 years for snowboarders. This corroborates the finding from several studies1-3 that snowboarders tend to be younger. Snowboarding is a newer sport with many beginners. However, Ishimaru and colleagues4 found that lower extremity injuries may be associated with experienced snowboarders, who may be prone to take more risks and tackle more challenging slopes. Experienced snowboarders are also likely to sustain lower extremity injuries from falling, because of their risk-taking behavior.5
Although upper extremity injuries account for most snowboarding injuries, lower extremity injuries are a significant issue.6 Modern equipment and more challenging slopes have allowed snowboarders to attain great speeds going down slopes—leading to a surge in lower extremity injuries.7 Lower extremity injuries sustained during snowboarding are more likely to be on the leading side4; the ankle is the most frequent fracture site. Unlike snowboard equipment, modern ski equipment, including new boots and binding systems, is designed to reduce ankle injuries and lower leg fractures.6 The decline in foot, ankle, and tibia fractures can be attributed to taller and stiffer boots, which offer the lower extremities more protection.8
Mechanism of Injury
Talus Fractures
An increasingly common injury among snowboarders is a fracture of the lateral process of the talus; this injury accounts for 32% of snowboarders’ ankle fractures.6 The lateral process of the talus—wedge-shaped and covered in articular cartilage—is involved in the subtalar and ankle joints.9 A fracture here is often misdiagnosed as an ankle sprain (Figures 1–3).6,9,10 The exact mechanism of injury remains controversial, and several biomechanical factors seem to be involved. Funk and colleagues11 conducted a cadaveric study and concluded that eversion of an axially loaded, dorsiflexed ankle may be the primary injury mechanism for fracture. Furthermore, snowboarders have their feet in a position perpendicular to the board, and a fall parallel to the board could increase the eversion force on the ankle of the leading leg. Valderrabano and colleagues9 conducted a clinical study of 26 patients who sustained this injury from snowboarding. All the patients reported they had felt an axial impact from falling, jumping, or unexpectedly hitting a ground object, and 80% reported a rotational movement in the lower leg during the impact. The authors concluded that axial loading and dorsiflexion were not the only factors involved in lateral process talus fractures, and an external moment is necessary to cause this injury from a forward fall.9
Anterior Cruciate Ligament Injuries
Although snowboarders’ lower extremity injuries are primarily ankle injuries, snowboarders are also at risk for serious knee issues when landing from jumps. In skiers, anterior cruciate ligament (ACL) injuries have 5 well-established mechanisms, all involving separation of the feet and a twisting force in the knee (Figures 4, 5): boot-induced anterior drawer mechanism, phantom-foot mechanism, valgus-external rotation, forceful quadriceps muscle contraction, and a combination of internal rotation and extension.8,12 A valgus–external rotation mechanism of knee injury occurs when external rotation of the tibia results from the skier catching the inside edge of the front of the ski. A valgus force acts on the knee as the lower leg is abducted during forward momentum. The torque created on the knee joint is amplified by the length of the knee and commonly results in an ACL injury or medial collateral ligament injury.6 Reports indicate that the phantom-foot mechanism is the most common mechanism of ACL injury among skiers.6,13,14 In this situation, internal rotation of the knee results when an off-balance skier falls backward, which causes the knee to hyperflex. The skier catches an inside edge on the snow, which creates a torque that rotates the tibia relative to the femur and results in injury to the ACL.6,14 A boot-induced anterior drawer mechanism occurs during a landing, when the tail of the ski lands first and in an off-balance position, resulting in a load transmitted through the skis to the skier; this load causes an anterior drawer of the ski boot and tibia relative to the femur, straining the ACL and causing ACL rupture.6,13,14 In the forceful quadriceps muscle contraction mechanism of ACL injury, a forceful quadriceps contraction occurs after a jump to prevent a backward fall. With the knee in flexion, this quadriceps contraction causes an anterior translation of the tibia, resulting in ACL rupture.13,14
The mechanism of injury differs in snowboarding, in which both feet remain attached to the board. Davies and colleagues15 examined 35 snowboarders who sustained ACL injuries after a flat landing from a jump and concluded that snowboarders preparing for a landing exhibit more quadriceps contraction, which increases the loading force on the ACL during landing. Furthermore, the snowboarder’s stance on the board, with the front foot slightly rotated relative to the board, results in a slight internal tibial rotation of the knee and establishes a posture that makes the snowboarder susceptible to injury. However, the lower incidence of knee injuries among snowboarders compared with skiers may be attributable to the fact that there is a limited amount of torque that can be generated on either knee as both feet are fixed to the board.16
The increased quadriceps force in anticipation of a landing, combined with the internal tibial rotation of the knee caused by the snowboarder’s stance, may be the primary mechanism of ACL rupture in snowboarders.15
Injury Prevention Strategies
Prevention strategies require an identification of injury risk factors for snowboarders. Hasler and colleagues7 conducted a study with 306 patients to identify variables that presented a risk for snowboarders. Low readiness for speed, bad weather, and bad visibility, as well as snow conditions, were found to be significant risk factors.
Skiers’ overall injury rate has decreased over the past 60 years, and this decrease has been attributed in part to improved ski technique and instruction.17,18 Improperly adjusted ski bindings are the culprit in many equipment-related lower extremity injuries, and beginners are at much higher risk for such injuries. Lessons and comprehensive safety training could reduce this injury rate.17,19 Several awareness video and training programs focusing on injury prevention have reduced knee sprains in ski patrollers compared with controls by 62% in 1 study; a similar program reduced injury by 30% in nonprofessional skiers.17 A study of injured snowboarders during a winter in Scotland found that 37% of the patients had no formal instruction or training in correct snowboarding and falling technique.20 Training programs for snowboarders could yield meaningful results in injury prevention and avoidance of risk-taking behavior among snowboarders.
Advances in equipment have also had an impact on the incidence of skiing injuries. Ski bindings protect skiers in 2 ways. First, the binding keeps the boot attached to the ski and prevents unintended release on difficult terrain. Second, the binding releases the boot from the ski during extreme conditions to prevent the skier from experiencing extreme forces or moments that could result in injury. Functional failure in ski bindings has been implicated in increased incidence of knee injuries and ligament rupture. In a study of injuries sustained by recreational alpine skiers in Japan, Urabe and colleagues21 found that 96% of those injured stated that the ski bindings had not released at time of incident. The effects of binding adjustment and maintenance among snowboarders have not been fully investigated, and there are no set guidelines for individual snowboarders on appropriate binding level. However, as there is a range of binding adjustment options available, snowboarders may have an optimum level that maximizes both mobility and protection from injury.22
Soft-shelled boots may also increase injury risk for snowboarders. Such boots allow for a wider range of ankle motion and offer little protection from extreme joint movements. Soft boots are generally preferred among snowboarders because they allow for increased mobility for sharp turns and maneuvers. However, modification of the stiffness of boots that limit ankle and foot joint mobility could reduce the incidence of ankle fractures and sprains among snowboarders.22
Summary
Snowboarding has become increasingly popular worldwide. It attracts a loyal group of amateur athletes and has developed into a billion-dollar industry with a growing rank of professionals. Although most snowboarding injuries are upper extremity injuries, the foot, ankle, and knee represent commonly injured areas among recreational and experienced snowboarders. Advances in ski equipment have significantly reduced the incidence of ankle injuries, but rising knee ligament injuries continue to pose a challenge. Foot and ankle injuries remain an issue in snowboarders despite advances in equipment and safety. New snowboard designs and boot and binding modifications may hold promise in decreasing the risk for injury in these athletes.
1. Kim S, Endres NK, Johnson RJ, Ettlinger CF, Shealy JE. Snowboarding injuries: trends over time and comparisons with alpine skiing injuries. Am J Sports Med. 2012;40(4):770-776.
2. Wasden CC, McIntosh SE, Keith DS, McCowan C. An analysis of skiing and snowboarding injuries on Utah slopes. J Trauma. 2009;67(5):1022-1026.
3. Rust DA, Gilmore CJ, Treme G. Injury patterns at a large western United States ski resort with and without snowboarders: the Taos experience. Am J Sports Med. 2013;41(3):652-656.
4. Ishimaru D, Ogawa H, Sumi H, Sumi Y, Shimizu K. Lower extremity injuries in snowboarding. J Trauma. 2011;70(3):E48-E52.
5. Torjussen J, Bahr R. Injuries among competitive snowboarders at the national elite level. Am J Sports Med. 2005;33(3):370-377.
6. Deady LH, Salonen D. Skiing and snowboarding injuries: a review with a focus on mechanism of injury. Radiol Clin North Am. 2010;48(6):1113-1124.
7. Hasler RM, Berov S, Banneker L, et al. Are there risk factors for snowboard injuries? A case–control multicentre study of 559 snowboarders. Br J Sports Med. 2010;44(11):816-821.
8. St-Onge N, Chevalier Y, Hagemeister N, Van De Putte M, De Guise J. Effect of ski binding parameters on knee biomechanics: a three-dimensional computational study. Med Sci Sports Exerc. 2004;36(7):1218-1225.
9. Valderrabano V, Perren T, Ryf C, Rillmann P, Hintermann B. Snowboarder’s talus fracture: treatment outcome of 20 cases after 3.5 years. Am J Sports Med. 2005;33(6):871-880.
10. von Knoch F, Reckord U, von Knoch M, Sommer C. Fracture of the lateral process of the talus in snowboarders. J Bone Joint Surg Br. 2007;89(6):772-777.
11. Funk JR, Srinivasan SC, Crandall JR. Snowboarder’s talus fractures experimentally produced by eversion and dorsiflexion. Am J Sports Med. 2003;31(6):921-928.
12. Pujol N, Blanchi MP, Chambat P. The incidence of anterior cruciate ligament injuries among competitive alpine skiers: a 25-year investigation. Am J Sports Med. 2007;35(7):1070-1074.
13. Hame SL, Oakes DA, Markolf KL. Injury to the anterior cruciate ligament during alpine skiing: a biomechanical analysis of tibial torque and knee flexion angle. Am J Sports Med. 2002;30(4):537-540.
14. Bere T, Flørenes TW, Krosshaug T, Nordsletten L, Bahr R. Events leading to anterior cruciate ligament injury in World Cup alpine skiing: a systematic video analysis of 20 cases. Br J Sports Med. 2011;45(16):1294-1302.
15. Davies H, Tietjens B, Van Sterkenburg M, Mehgan A. Anterior cruciate ligament injuries in snowboarders: a quadriceps-induced injury. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1048-1051.
16. Bladin C, McCrory P, Pogorzelski A. Snowboarding injuries: current trends and future directions. Sports Med. 2004;34(2):133-139.
17. Rossi MJ, Lubowitz JH, Guttmann D. The skier’s knee. Arthroscopy. 2003;19(1):75-84.
18. Pressman A, Johnson DH. A review of ski injuries resulting in combined injury to the anterior cruciate ligament and medial collateral ligaments. Arthroscopy. 2003;19(2):194-202.
19. Hildebrandt C, Mildner E, Hotter B, Kirschner W, Höbenreich C, Raschner C. Accident prevention on ski slopes—perceptions of safety and knowledge of existing rules. Accid Anal Prev. 2011;43(4):1421-1426.
20. Langran M, Selvaraj S. Increased injury risk among first-day skiers, snowboarders, and skiboarders. Am J Sports Med. 2004;32(1):96-103.
21. Urabe Y, Ochi M, Onari K, Ikuta Y. Anterior cruciate ligament injury in recreational alpine skiers: analysis of mechanisms and strategy for prevention. J Orthop Sci. 2002;7(1):1-5.
22. McAlpine PR. Biomechanical Analysis of Snowboard Jump Landings: A Focus on the Ankle Joint Complex [doctoral thesis]. Auckland, New Zealand: University of Auckland; 2010.
1. Kim S, Endres NK, Johnson RJ, Ettlinger CF, Shealy JE. Snowboarding injuries: trends over time and comparisons with alpine skiing injuries. Am J Sports Med. 2012;40(4):770-776.
2. Wasden CC, McIntosh SE, Keith DS, McCowan C. An analysis of skiing and snowboarding injuries on Utah slopes. J Trauma. 2009;67(5):1022-1026.
3. Rust DA, Gilmore CJ, Treme G. Injury patterns at a large western United States ski resort with and without snowboarders: the Taos experience. Am J Sports Med. 2013;41(3):652-656.
4. Ishimaru D, Ogawa H, Sumi H, Sumi Y, Shimizu K. Lower extremity injuries in snowboarding. J Trauma. 2011;70(3):E48-E52.
5. Torjussen J, Bahr R. Injuries among competitive snowboarders at the national elite level. Am J Sports Med. 2005;33(3):370-377.
6. Deady LH, Salonen D. Skiing and snowboarding injuries: a review with a focus on mechanism of injury. Radiol Clin North Am. 2010;48(6):1113-1124.
7. Hasler RM, Berov S, Banneker L, et al. Are there risk factors for snowboard injuries? A case–control multicentre study of 559 snowboarders. Br J Sports Med. 2010;44(11):816-821.
8. St-Onge N, Chevalier Y, Hagemeister N, Van De Putte M, De Guise J. Effect of ski binding parameters on knee biomechanics: a three-dimensional computational study. Med Sci Sports Exerc. 2004;36(7):1218-1225.
9. Valderrabano V, Perren T, Ryf C, Rillmann P, Hintermann B. Snowboarder’s talus fracture: treatment outcome of 20 cases after 3.5 years. Am J Sports Med. 2005;33(6):871-880.
10. von Knoch F, Reckord U, von Knoch M, Sommer C. Fracture of the lateral process of the talus in snowboarders. J Bone Joint Surg Br. 2007;89(6):772-777.
11. Funk JR, Srinivasan SC, Crandall JR. Snowboarder’s talus fractures experimentally produced by eversion and dorsiflexion. Am J Sports Med. 2003;31(6):921-928.
12. Pujol N, Blanchi MP, Chambat P. The incidence of anterior cruciate ligament injuries among competitive alpine skiers: a 25-year investigation. Am J Sports Med. 2007;35(7):1070-1074.
13. Hame SL, Oakes DA, Markolf KL. Injury to the anterior cruciate ligament during alpine skiing: a biomechanical analysis of tibial torque and knee flexion angle. Am J Sports Med. 2002;30(4):537-540.
14. Bere T, Flørenes TW, Krosshaug T, Nordsletten L, Bahr R. Events leading to anterior cruciate ligament injury in World Cup alpine skiing: a systematic video analysis of 20 cases. Br J Sports Med. 2011;45(16):1294-1302.
15. Davies H, Tietjens B, Van Sterkenburg M, Mehgan A. Anterior cruciate ligament injuries in snowboarders: a quadriceps-induced injury. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1048-1051.
16. Bladin C, McCrory P, Pogorzelski A. Snowboarding injuries: current trends and future directions. Sports Med. 2004;34(2):133-139.
17. Rossi MJ, Lubowitz JH, Guttmann D. The skier’s knee. Arthroscopy. 2003;19(1):75-84.
18. Pressman A, Johnson DH. A review of ski injuries resulting in combined injury to the anterior cruciate ligament and medial collateral ligaments. Arthroscopy. 2003;19(2):194-202.
19. Hildebrandt C, Mildner E, Hotter B, Kirschner W, Höbenreich C, Raschner C. Accident prevention on ski slopes—perceptions of safety and knowledge of existing rules. Accid Anal Prev. 2011;43(4):1421-1426.
20. Langran M, Selvaraj S. Increased injury risk among first-day skiers, snowboarders, and skiboarders. Am J Sports Med. 2004;32(1):96-103.
21. Urabe Y, Ochi M, Onari K, Ikuta Y. Anterior cruciate ligament injury in recreational alpine skiers: analysis of mechanisms and strategy for prevention. J Orthop Sci. 2002;7(1):1-5.
22. McAlpine PR. Biomechanical Analysis of Snowboard Jump Landings: A Focus on the Ankle Joint Complex [doctoral thesis]. Auckland, New Zealand: University of Auckland; 2010.