User login
Update on high-grade vulvar interepithelial neoplasia
Vulvar squamous cell carcinomas (VSCC) comprise approximately 90% of all vulvar malignancies. Unlike cervical SCC, which are predominantly human papilloma virus (HPV) positive, only a minority of VSCC are HPV positive – on the order of 15%-25% of cases. Most cases occur in the setting of lichen sclerosus and are HPV negative.
Lichen sclerosus is a chronic inflammatory dermatitis typically involving the anogenital area, which in some cases can become seriously distorted (e.g. atrophy of the labia minora, clitoral phimosis, and introital stenosis). Although most cases are diagnosed in postmenopausal women, LS can affect women of any age. The true prevalence of lichen sclerosus is unknown. Recent studies have shown a prevalence of 1 in 60; among older women, it can even be as high as 1 in 30. While lichen sclerosus is a pruriginous condition, it is often asymptomatic. It is not considered a premalignant condition. The diagnosis is clinical; however, suspicious lesions (erosions/ulcerations, hyperkeratosis, pigmented areas, ecchymosis, warty or papular lesions), particularly when recalcitrant to adequate first-line therapy, should be biopsied.
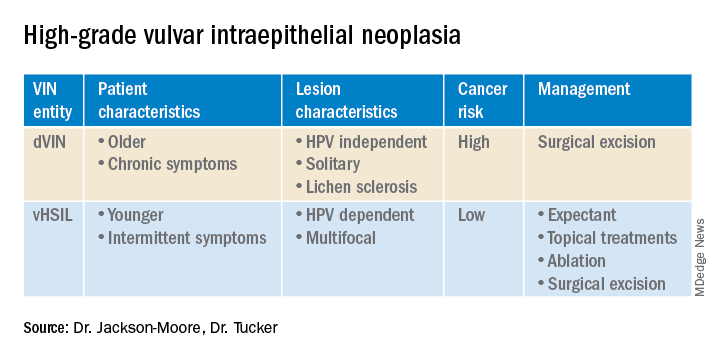
VSCC arises from precursor lesions or high-grade vulvar intraepithelial neoplasia (VIN). The 2015 International Society for the Study of Vulvovaginal Disease nomenclature classifies high-grade VIN into high-grade squamous intraepithelial lesion (HSIL) and differentiated VIN (dVIN). Most patients with high-grade VIN are diagnosed with HSIL or usual type VIN. A preponderance of these lesions (75%-85%) are HPV positive, predominantly HPV 16. Vulvar HSIL (vHSIL) lesions affect younger women. The lesions tend to be multifocal and extensive. On the other hand, dVIN typically affects older women and commonly develops as a solitary lesion. While dVIN accounts for only a small subset of patients with high-grade VIN, these lesions are HPV negative and associated with lichen sclerosus.
Both disease entities, vHSIL and dVIN, are increasing in incidence. There is a higher risk and shortened period of progression to cancer in patients with dVIN compared to HSIL. The cancer risk of vHSIL is relatively low. The 10-year cumulative VSCC risk reported in the literature is 10.3%; 9.7% for vHSIL and 50% for dVIN. Patients with vHSIL could benefit from less aggressive treatment modalities.
Patients present with a constellation of signs such as itching, pain, burning, bleeding, and discharge. Chronic symptoms portend HPV-independent lesions associated with lichen sclerosus while episodic signs are suggestive of HPV-positive lesions.
The recurrence risk of high-grade VIN is 46%-70%. Risk factors for recurrence include age greater than 50, immunosuppression, metasynchronous HSIL, and multifocal lesions. Recurrences occur in up to 50% of women who have undergone surgery. For those who undergo surgical treatment for high-grade VIN, recurrence is more common in the setting of positive margins, underlying lichen sclerosis, persistent HPV infection, and immunosuppression.
Management of high-grade VIN is determined by the lesion characteristics, patient characteristics, and medical expertise. Given the risk of progression of high-grade VIN to cancer and risk of underlying cancer, surgical therapy is typically recommended. The treatment of choice is surgical excision in cases of dVIN. Surgical treatments include CO2 laser ablation, wide local excision, and vulvectomy. Women who undergo surgical treatment for vHSIL have about a 50% chance of the condition recurring 1 year later, irrespective of whether treatment is by surgical excision or laser vaporization.
Since surgery can be associated with disfigurement and sexual dysfunction, alternatives to surgery should be considered in cases of vHSIL. The potential for effect on sexual function should be part of preoperative counseling and treatment. Women treated for VIN often experience increased inhibition of sexual excitement and increased inhibition of orgasm. One study found that in women undergoing vulvar excision for VIN, the impairment was found to be psychological in nature. Overall, the studies of sexual effect from treatment of VIN have found that women do not return to their pretreatment sexual function. However, the optimal management of vHSIL has not been determined. Nonsurgical options include topical therapies (imiquimod, 5-fluorouracil, cidofovir, and interferon) and nonpharmacologic treatments, such as photodynamic therapy.
Imiquimod, a topical immune modulator, is the most studied pharmacologic treatment of vHSIL. The drug induces secretion of cytokines, creating an immune response that clears the HPV infection. Imiquimod is safe and well tolerated. The clinical response rate varies between 35% and 81%. A recent study demonstrated the efficacy of imiquimod and the treatment was found to be noninferior to surgery. Adverse events differed, with local pain following surgical treatment and local pruritus and erythema associated with imiquimod use. Some patients did not respond to imiquimod; it was thought by the authors of the study that specific immunological factors affect the clinical response.
In conclusion, high-grade VIN is a heterogeneous disease made up of two distinct disease entities with rising incidence. In contrast to dVIN, the cancer risk is low for patients with vHSIL. Treatment should be driven by the clinical characteristics of the vulvar lesions, patients’ preferences, sexual activity, and compliance. Future directions include risk stratification of patients with vHSIL who are most likely to benefit from topical treatments, thus reducing overtreatment. Molecular biomarkers that could identify dVIN at an early stage are needed.
Dr. Jackson-Moore is associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
Cendejas BR et al. Am J Obstet Gynecol. 2015 Mar;212(3):291-7.
Lebreton M et al. J Gynecol Obstet Hum Reprod. 2020 Nov;49(9):101801.
Thuijs NB et al. Int J Cancer. 2021 Jan 1;148(1):90-8. doi: 10.1002/ijc.33198. .
Trutnovsky G et al. Lancet. 2022 May 7;399(10337):1790-8. Erratum in: Lancet. 2022 Oct 8;400(10359):1194.
Vulvar squamous cell carcinomas (VSCC) comprise approximately 90% of all vulvar malignancies. Unlike cervical SCC, which are predominantly human papilloma virus (HPV) positive, only a minority of VSCC are HPV positive – on the order of 15%-25% of cases. Most cases occur in the setting of lichen sclerosus and are HPV negative.
Lichen sclerosus is a chronic inflammatory dermatitis typically involving the anogenital area, which in some cases can become seriously distorted (e.g. atrophy of the labia minora, clitoral phimosis, and introital stenosis). Although most cases are diagnosed in postmenopausal women, LS can affect women of any age. The true prevalence of lichen sclerosus is unknown. Recent studies have shown a prevalence of 1 in 60; among older women, it can even be as high as 1 in 30. While lichen sclerosus is a pruriginous condition, it is often asymptomatic. It is not considered a premalignant condition. The diagnosis is clinical; however, suspicious lesions (erosions/ulcerations, hyperkeratosis, pigmented areas, ecchymosis, warty or papular lesions), particularly when recalcitrant to adequate first-line therapy, should be biopsied.
VSCC arises from precursor lesions or high-grade vulvar intraepithelial neoplasia (VIN). The 2015 International Society for the Study of Vulvovaginal Disease nomenclature classifies high-grade VIN into high-grade squamous intraepithelial lesion (HSIL) and differentiated VIN (dVIN). Most patients with high-grade VIN are diagnosed with HSIL or usual type VIN. A preponderance of these lesions (75%-85%) are HPV positive, predominantly HPV 16. Vulvar HSIL (vHSIL) lesions affect younger women. The lesions tend to be multifocal and extensive. On the other hand, dVIN typically affects older women and commonly develops as a solitary lesion. While dVIN accounts for only a small subset of patients with high-grade VIN, these lesions are HPV negative and associated with lichen sclerosus.
Both disease entities, vHSIL and dVIN, are increasing in incidence. There is a higher risk and shortened period of progression to cancer in patients with dVIN compared to HSIL. The cancer risk of vHSIL is relatively low. The 10-year cumulative VSCC risk reported in the literature is 10.3%; 9.7% for vHSIL and 50% for dVIN. Patients with vHSIL could benefit from less aggressive treatment modalities.
Patients present with a constellation of signs such as itching, pain, burning, bleeding, and discharge. Chronic symptoms portend HPV-independent lesions associated with lichen sclerosus while episodic signs are suggestive of HPV-positive lesions.
The recurrence risk of high-grade VIN is 46%-70%. Risk factors for recurrence include age greater than 50, immunosuppression, metasynchronous HSIL, and multifocal lesions. Recurrences occur in up to 50% of women who have undergone surgery. For those who undergo surgical treatment for high-grade VIN, recurrence is more common in the setting of positive margins, underlying lichen sclerosis, persistent HPV infection, and immunosuppression.
Management of high-grade VIN is determined by the lesion characteristics, patient characteristics, and medical expertise. Given the risk of progression of high-grade VIN to cancer and risk of underlying cancer, surgical therapy is typically recommended. The treatment of choice is surgical excision in cases of dVIN. Surgical treatments include CO2 laser ablation, wide local excision, and vulvectomy. Women who undergo surgical treatment for vHSIL have about a 50% chance of the condition recurring 1 year later, irrespective of whether treatment is by surgical excision or laser vaporization.
Since surgery can be associated with disfigurement and sexual dysfunction, alternatives to surgery should be considered in cases of vHSIL. The potential for effect on sexual function should be part of preoperative counseling and treatment. Women treated for VIN often experience increased inhibition of sexual excitement and increased inhibition of orgasm. One study found that in women undergoing vulvar excision for VIN, the impairment was found to be psychological in nature. Overall, the studies of sexual effect from treatment of VIN have found that women do not return to their pretreatment sexual function. However, the optimal management of vHSIL has not been determined. Nonsurgical options include topical therapies (imiquimod, 5-fluorouracil, cidofovir, and interferon) and nonpharmacologic treatments, such as photodynamic therapy.
Imiquimod, a topical immune modulator, is the most studied pharmacologic treatment of vHSIL. The drug induces secretion of cytokines, creating an immune response that clears the HPV infection. Imiquimod is safe and well tolerated. The clinical response rate varies between 35% and 81%. A recent study demonstrated the efficacy of imiquimod and the treatment was found to be noninferior to surgery. Adverse events differed, with local pain following surgical treatment and local pruritus and erythema associated with imiquimod use. Some patients did not respond to imiquimod; it was thought by the authors of the study that specific immunological factors affect the clinical response.

In conclusion, high-grade VIN is a heterogeneous disease made up of two distinct disease entities with rising incidence. In contrast to dVIN, the cancer risk is low for patients with vHSIL. Treatment should be driven by the clinical characteristics of the vulvar lesions, patients’ preferences, sexual activity, and compliance. Future directions include risk stratification of patients with vHSIL who are most likely to benefit from topical treatments, thus reducing overtreatment. Molecular biomarkers that could identify dVIN at an early stage are needed.
Dr. Jackson-Moore is associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
Cendejas BR et al. Am J Obstet Gynecol. 2015 Mar;212(3):291-7.
Lebreton M et al. J Gynecol Obstet Hum Reprod. 2020 Nov;49(9):101801.
Thuijs NB et al. Int J Cancer. 2021 Jan 1;148(1):90-8. doi: 10.1002/ijc.33198. .
Trutnovsky G et al. Lancet. 2022 May 7;399(10337):1790-8. Erratum in: Lancet. 2022 Oct 8;400(10359):1194.
Vulvar squamous cell carcinomas (VSCC) comprise approximately 90% of all vulvar malignancies. Unlike cervical SCC, which are predominantly human papilloma virus (HPV) positive, only a minority of VSCC are HPV positive – on the order of 15%-25% of cases. Most cases occur in the setting of lichen sclerosus and are HPV negative.
Lichen sclerosus is a chronic inflammatory dermatitis typically involving the anogenital area, which in some cases can become seriously distorted (e.g. atrophy of the labia minora, clitoral phimosis, and introital stenosis). Although most cases are diagnosed in postmenopausal women, LS can affect women of any age. The true prevalence of lichen sclerosus is unknown. Recent studies have shown a prevalence of 1 in 60; among older women, it can even be as high as 1 in 30. While lichen sclerosus is a pruriginous condition, it is often asymptomatic. It is not considered a premalignant condition. The diagnosis is clinical; however, suspicious lesions (erosions/ulcerations, hyperkeratosis, pigmented areas, ecchymosis, warty or papular lesions), particularly when recalcitrant to adequate first-line therapy, should be biopsied.
VSCC arises from precursor lesions or high-grade vulvar intraepithelial neoplasia (VIN). The 2015 International Society for the Study of Vulvovaginal Disease nomenclature classifies high-grade VIN into high-grade squamous intraepithelial lesion (HSIL) and differentiated VIN (dVIN). Most patients with high-grade VIN are diagnosed with HSIL or usual type VIN. A preponderance of these lesions (75%-85%) are HPV positive, predominantly HPV 16. Vulvar HSIL (vHSIL) lesions affect younger women. The lesions tend to be multifocal and extensive. On the other hand, dVIN typically affects older women and commonly develops as a solitary lesion. While dVIN accounts for only a small subset of patients with high-grade VIN, these lesions are HPV negative and associated with lichen sclerosus.
Both disease entities, vHSIL and dVIN, are increasing in incidence. There is a higher risk and shortened period of progression to cancer in patients with dVIN compared to HSIL. The cancer risk of vHSIL is relatively low. The 10-year cumulative VSCC risk reported in the literature is 10.3%; 9.7% for vHSIL and 50% for dVIN. Patients with vHSIL could benefit from less aggressive treatment modalities.
Patients present with a constellation of signs such as itching, pain, burning, bleeding, and discharge. Chronic symptoms portend HPV-independent lesions associated with lichen sclerosus while episodic signs are suggestive of HPV-positive lesions.
The recurrence risk of high-grade VIN is 46%-70%. Risk factors for recurrence include age greater than 50, immunosuppression, metasynchronous HSIL, and multifocal lesions. Recurrences occur in up to 50% of women who have undergone surgery. For those who undergo surgical treatment for high-grade VIN, recurrence is more common in the setting of positive margins, underlying lichen sclerosis, persistent HPV infection, and immunosuppression.
Management of high-grade VIN is determined by the lesion characteristics, patient characteristics, and medical expertise. Given the risk of progression of high-grade VIN to cancer and risk of underlying cancer, surgical therapy is typically recommended. The treatment of choice is surgical excision in cases of dVIN. Surgical treatments include CO2 laser ablation, wide local excision, and vulvectomy. Women who undergo surgical treatment for vHSIL have about a 50% chance of the condition recurring 1 year later, irrespective of whether treatment is by surgical excision or laser vaporization.
Since surgery can be associated with disfigurement and sexual dysfunction, alternatives to surgery should be considered in cases of vHSIL. The potential for effect on sexual function should be part of preoperative counseling and treatment. Women treated for VIN often experience increased inhibition of sexual excitement and increased inhibition of orgasm. One study found that in women undergoing vulvar excision for VIN, the impairment was found to be psychological in nature. Overall, the studies of sexual effect from treatment of VIN have found that women do not return to their pretreatment sexual function. However, the optimal management of vHSIL has not been determined. Nonsurgical options include topical therapies (imiquimod, 5-fluorouracil, cidofovir, and interferon) and nonpharmacologic treatments, such as photodynamic therapy.
Imiquimod, a topical immune modulator, is the most studied pharmacologic treatment of vHSIL. The drug induces secretion of cytokines, creating an immune response that clears the HPV infection. Imiquimod is safe and well tolerated. The clinical response rate varies between 35% and 81%. A recent study demonstrated the efficacy of imiquimod and the treatment was found to be noninferior to surgery. Adverse events differed, with local pain following surgical treatment and local pruritus and erythema associated with imiquimod use. Some patients did not respond to imiquimod; it was thought by the authors of the study that specific immunological factors affect the clinical response.

In conclusion, high-grade VIN is a heterogeneous disease made up of two distinct disease entities with rising incidence. In contrast to dVIN, the cancer risk is low for patients with vHSIL. Treatment should be driven by the clinical characteristics of the vulvar lesions, patients’ preferences, sexual activity, and compliance. Future directions include risk stratification of patients with vHSIL who are most likely to benefit from topical treatments, thus reducing overtreatment. Molecular biomarkers that could identify dVIN at an early stage are needed.
Dr. Jackson-Moore is associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
Cendejas BR et al. Am J Obstet Gynecol. 2015 Mar;212(3):291-7.
Lebreton M et al. J Gynecol Obstet Hum Reprod. 2020 Nov;49(9):101801.
Thuijs NB et al. Int J Cancer. 2021 Jan 1;148(1):90-8. doi: 10.1002/ijc.33198. .
Trutnovsky G et al. Lancet. 2022 May 7;399(10337):1790-8. Erratum in: Lancet. 2022 Oct 8;400(10359):1194.
Neurosurgery Operating Room Efficiency During the COVID-19 Era
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 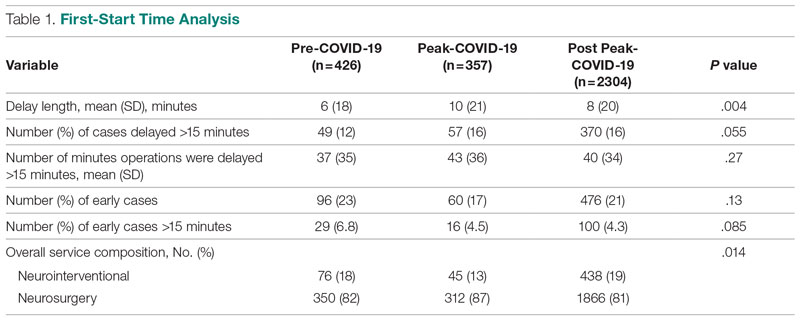
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.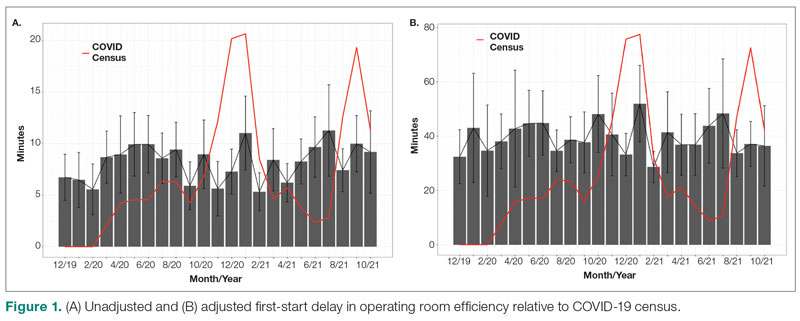
Turnover Time
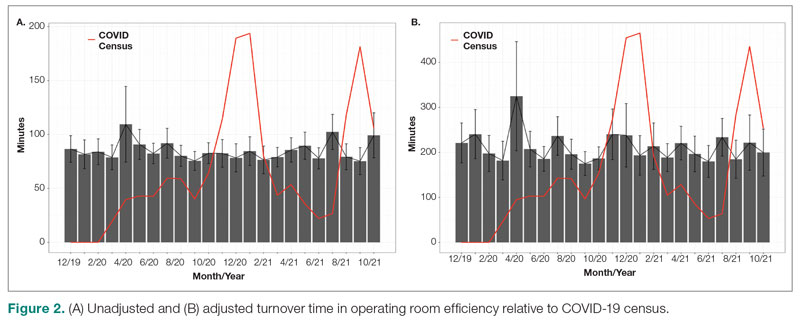
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.
Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.
Turnover Time
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.


Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.
Turnover Time
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.


Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
Anesthetic Choices and Postoperative Delirium Incidence: Propofol vs Sevoflurane
Study 1 Overview (Chang et al)
Objective: To assess the incidence of postoperative delirium (POD) following propofol- vs sevoflurane-based anesthesia in geriatric spine surgery patients.
Design: Retrospective, single-blinded observational study of propofol- and sevoflurane-based anesthesia cohorts.
Setting and participants: Patients eligible for this study were aged 65 years or older admitted to the SMG-SNU Boramae Medical Center (Seoul, South Korea). All patients underwent general anesthesia either via intravenous propofol or inhalational sevoflurane for spine surgery between January 2015 and December 2019. Patients were retrospectively identified via electronic medical records. Patient exclusion criteria included preoperative delirium, history of dementia, psychiatric disease, alcoholism, hepatic or renal dysfunction, postoperative mechanical ventilation dependence, other surgery within the recent 6 months, maintenance of intraoperative anesthesia with combined anesthetics, or incomplete medical record.
Main outcome measures: The primary outcome was the incidence of POD after administration of propofol- and sevoflurane-based anesthesia during hospitalization. Patients were screened for POD regularly by attending nurses using the Nursing Delirium Screening Scale (disorientation, inappropriate behavior, inappropriate communication, hallucination, and psychomotor retardation) during the entirety of the patient’s hospital stay; if 1 or more screening criteria were met, a psychiatrist was consulted for the proper diagnosis and management of delirium. A psychiatric diagnosis was required for a case to be counted toward the incidence of POD in this study. Secondary outcomes included postoperative 30-day complications (angina, myocardial infarction, transient ischemic attack/stroke, pneumonia, deep vein thrombosis, pulmonary embolism, acute kidney injury, or infection) and length of postoperative hospital stay.
Main results: POD occurred in 29 patients (10.3%) out of the total cohort of 281. POD was more common in the sevoflurane group than in the propofol group (15.7% vs 5.0%; P = .003). Using multivariable logistic regression, inhalational sevoflurane was associated with an increased risk of POD as compared to propofol-based anesthesia (odds ratio [OR], 4.120; 95% CI, 1.549-10.954; P = .005). There was no association between choice of anesthetic and postoperative 30-day complications or the length of postoperative hospital stay. Both older age (OR, 1.242; 95% CI, 1.130-1.366; P < .001) and higher pain score at postoperative day 1 (OR, 1.338; 95% CI, 1.056-1.696; P = .016) were associated with increased risk of POD.
Conclusion: Propofol-based anesthesia was associated with a lower incidence of and risk for POD than sevoflurane-based anesthesia in older patients undergoing spine surgery.
Study 2 Overview (Mei et al)
Objective: To determine the incidence and duration of POD in older patients after total knee/hip replacement (TKR/THR) under intravenous propofol or inhalational sevoflurane general anesthesia.
Design: Randomized clinical trial of propofol and sevoflurane groups.
Setting and participants: This study was conducted at the Shanghai Tenth People’s Hospital and involved 209 participants enrolled between June 2016 and November 2019. All participants were 60 years of age or older, scheduled for TKR/THR surgery under general anesthesia, American Society of Anesthesiologists (ASA) class I to III, and assessed to be of normal cognitive function preoperatively via a Mini-Mental State Examination. Participant exclusion criteria included preexisting delirium as assessed by the Confusion Assessment Method (CAM), prior diagnosed neurological diseases (eg, Parkinson’s disease), prior diagnosed mental disorders (eg, schizophrenia), or impaired vision or hearing that would influence cognitive assessments. All participants were randomly assigned to either sevoflurane or propofol anesthesia for their surgery via a computer-generated list. Of these, 103 received inhalational sevoflurane and 106 received intravenous propofol. All participants received standardized postoperative care.
Main outcome measures: All participants were interviewed by investigators, who were blinded to the anesthesia regimen, twice daily on postoperative days 1, 2, and 3 using CAM and a CAM-based scoring system (CAM-S) to assess delirium severity. The CAM encapsulated 4 criteria: acute onset and fluctuating course, agitation, disorganized thinking, and altered level of consciousness. To diagnose delirium, both the first and second criteria must be met, in addition to either the third or fourth criterion. The averages of the scores across the 3 postoperative days indicated delirium severity, while the incidence and duration of delirium was assessed by the presence of delirium as determined by CAM on any postoperative day.
Main results: All eligible participants (N = 209; mean [SD] age 71.2 [6.7] years; 29.2% male) were included in the final analysis. The incidence of POD was not statistically different between the propofol and sevoflurane groups (33.0% vs 23.3%; P = .119, Chi-square test). It was estimated that 316 participants in each arm of the study were needed to detect statistical differences. The number of days of POD per person were higher with propofol anesthesia as compared to sevoflurane (0.5 [0.8] vs 0.3 [0.5]; P = .049, Student’s t-test).
Conclusion: This underpowered study showed a 9.7% difference in the incidence of POD between older adults who received propofol (33.0%) and sevoflurane (23.3%) after THR/TKR. Further studies with a larger sample size are needed to compare general anesthetics and their role in POD.
Commentary
Delirium is characterized by an acute state of confusion with fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is often caused by medications and/or their related adverse effects, infections, electrolyte imbalances, and other clinical etiologies. Delirium often manifests in post-surgical settings, disproportionately affecting older patients and leading to increased risk of morbidity, mortality, hospital length of stay, and health care costs.1 Intraoperative risk factors for POD are determined by the degree of operative stress (eg, lower-risk surgeries put the patient at reduced risk for POD as compared to higher-risk surgeries) and are additive to preexisting patient-specific risk factors, such as older age and functional impairment.1 Because operative stress is associated with risk for POD, limiting operative stress in controlled ways, such as through the choice of anesthetic agent administered, may be a pragmatic way to manage operative risks and optimize outcomes, especially when serving a surgically vulnerable population.
In Study 1, Chang et al sought to assess whether 2 commonly utilized general anesthetics, propofol and sevoflurane, in older patients undergoing spine surgery differentially affected the incidence of POD. In this retrospective, single-blinded observational study of 281 geriatric patients, the researchers found that sevoflurane was associated with a higher risk of POD as compared to propofol. However, these anesthetics were not associated with surgical outcomes such as postoperative 30-day complications or the length of postoperative hospital stay. While these findings added new knowledge to this field of research, several limitations should be kept in mind when interpreting this study’s results. For instance, the sample size was relatively small, with all cases selected from a single center utilizing a retrospective analysis. In addition, although a standardized nursing screening tool was used as a method for delirium detection, hypoactive delirium or less symptomatic delirium may have been missed, which in turn would lead to an underestimation of POD incidence. The latter is a common limitation in delirium research.
In Study 2, Mei et al similarly explored the effects of general anesthetics on POD in older surgical patients. Specifically, using a randomized clinical trial design, the investigators compared propofol with sevoflurane in older patients who underwent TKR/THR, and their roles in POD severity and duration. Although the incidence of POD was higher in those who received propofol compared to sevoflurane, this trial was underpowered and the results did not reach statistical significance. In addition, while the duration of POD was slightly longer in the propofol group compared to the sevoflurane group (0.5 vs 0.3 days), it was unclear if this finding was clinically significant. Similar to many research studies in POD, limitations of Study 2 included a small sample size of 209 patients, with all participants enrolled from a single center. On the other hand, this study illustrated the feasibility of a method that allowed reproducible prospective assessment of POD time course using CAM and CAM-S.
Applications for Clinical Practice and System Implementation
The delineation of risk factors that contribute to delirium after surgery in older patients is key to mitigating risks for POD and improving clinical outcomes. An important step towards a better understanding of these modifiable risk factors is to clearly quantify intraoperative risk of POD attributable to specific anesthetics. While preclinical studies have shown differential neurotoxicity effects of propofol and sevoflurane, their impact on clinically important neurologic outcomes such as delirium and cognitive decline remains poorly understood. Although Studies 1 and 2 both provided head-to-head comparisons of propofol and sevoflurane as risk factors for POD in high-operative-stress surgeries in older patients, the results were inconsistent. That being said, this small incremental increase in knowledge was not unexpected in the course of discovery around a clinically complex research question. Importantly, these studies provided evidence regarding the methodological approaches that could be taken to further this line of research.
The mediating factors of the differences on neurologic outcomes between anesthetic agents are likely pharmacological, biological, and methodological. Pharmacologically, the differences between target receptors, such as GABAA (propofol, etomidate) or NMDA (ketamine), could be a defining feature in the difference in incidence of POD. Additionally, secondary actions of anesthetic agents on glycine, nicotinic, and acetylcholine receptors could play a role as well. Biologically, genes such as CYP2E1, CYP2B6, CYP2C9, GSTP1, UGT1A9, SULT1A1, and NQO1 have all been identified as genetic factors in the metabolism of anesthetics, and variations in such genes could result in different responses to anesthetics.2 Methodologically, routes of anesthetic administration (eg, inhalation vs intravenous), preexisting anatomical structures, or confounding medical conditions (eg, lower respiratory volume due to older age) may influence POD incidence, duration, or severity. Moreover, methodological differences between Studies 1 and 2, such as surgeries performed (spinal vs TKR/THR), patient populations (South Korean vs Chinese), and the diagnosis and monitoring of delirium (retrospective screening and diagnosis vs prospective CAM/CAM-S) may impact delirium outcomes. Thus, these factors should be considered in the design of future clinical trials undertaken to investigate the effects of anesthetics on POD.
Given the high prevalence of delirium and its associated adverse outcomes in the immediate postoperative period in older patients, further research is warranted to determine how anesthetics affect POD in order to optimize perioperative care and mitigate risks in this vulnerable population. Moreover, parallel investigations into how anesthetics differentially impact the development of transient or longer-term cognitive impairment after a surgical procedure (ie, postoperative cognitive dysfunction) in older adults are urgently needed in order to improve their cognitive health.
Practice Points
- Intravenous propofol and inhalational sevoflurane may be differentially associated with incidence, duration, and severity of POD in geriatric surgical patients.
- Further larger-scale studies are warranted to clarify the role of anesthetic choice in POD in order to optimize surgical outcomes in older patients.
–Jared Doan, BS, and Fred Ko, MD
Icahn School of Medicine at Mount Sinai
1. Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54(10):1578-1589. doi:10.1111/j.1532-5415.2006.00893.x
2. Mikstacki A, Skrzypczak-Zielinska M, Tamowicz B, et al. The impact of genetic factors on response to anaesthetics. Adv Med Sci. 2013;58(1):9-14. doi:10.2478/v10039-012-0065-z
Study 1 Overview (Chang et al)
Objective: To assess the incidence of postoperative delirium (POD) following propofol- vs sevoflurane-based anesthesia in geriatric spine surgery patients.
Design: Retrospective, single-blinded observational study of propofol- and sevoflurane-based anesthesia cohorts.
Setting and participants: Patients eligible for this study were aged 65 years or older admitted to the SMG-SNU Boramae Medical Center (Seoul, South Korea). All patients underwent general anesthesia either via intravenous propofol or inhalational sevoflurane for spine surgery between January 2015 and December 2019. Patients were retrospectively identified via electronic medical records. Patient exclusion criteria included preoperative delirium, history of dementia, psychiatric disease, alcoholism, hepatic or renal dysfunction, postoperative mechanical ventilation dependence, other surgery within the recent 6 months, maintenance of intraoperative anesthesia with combined anesthetics, or incomplete medical record.
Main outcome measures: The primary outcome was the incidence of POD after administration of propofol- and sevoflurane-based anesthesia during hospitalization. Patients were screened for POD regularly by attending nurses using the Nursing Delirium Screening Scale (disorientation, inappropriate behavior, inappropriate communication, hallucination, and psychomotor retardation) during the entirety of the patient’s hospital stay; if 1 or more screening criteria were met, a psychiatrist was consulted for the proper diagnosis and management of delirium. A psychiatric diagnosis was required for a case to be counted toward the incidence of POD in this study. Secondary outcomes included postoperative 30-day complications (angina, myocardial infarction, transient ischemic attack/stroke, pneumonia, deep vein thrombosis, pulmonary embolism, acute kidney injury, or infection) and length of postoperative hospital stay.
Main results: POD occurred in 29 patients (10.3%) out of the total cohort of 281. POD was more common in the sevoflurane group than in the propofol group (15.7% vs 5.0%; P = .003). Using multivariable logistic regression, inhalational sevoflurane was associated with an increased risk of POD as compared to propofol-based anesthesia (odds ratio [OR], 4.120; 95% CI, 1.549-10.954; P = .005). There was no association between choice of anesthetic and postoperative 30-day complications or the length of postoperative hospital stay. Both older age (OR, 1.242; 95% CI, 1.130-1.366; P < .001) and higher pain score at postoperative day 1 (OR, 1.338; 95% CI, 1.056-1.696; P = .016) were associated with increased risk of POD.
Conclusion: Propofol-based anesthesia was associated with a lower incidence of and risk for POD than sevoflurane-based anesthesia in older patients undergoing spine surgery.
Study 2 Overview (Mei et al)
Objective: To determine the incidence and duration of POD in older patients after total knee/hip replacement (TKR/THR) under intravenous propofol or inhalational sevoflurane general anesthesia.
Design: Randomized clinical trial of propofol and sevoflurane groups.
Setting and participants: This study was conducted at the Shanghai Tenth People’s Hospital and involved 209 participants enrolled between June 2016 and November 2019. All participants were 60 years of age or older, scheduled for TKR/THR surgery under general anesthesia, American Society of Anesthesiologists (ASA) class I to III, and assessed to be of normal cognitive function preoperatively via a Mini-Mental State Examination. Participant exclusion criteria included preexisting delirium as assessed by the Confusion Assessment Method (CAM), prior diagnosed neurological diseases (eg, Parkinson’s disease), prior diagnosed mental disorders (eg, schizophrenia), or impaired vision or hearing that would influence cognitive assessments. All participants were randomly assigned to either sevoflurane or propofol anesthesia for their surgery via a computer-generated list. Of these, 103 received inhalational sevoflurane and 106 received intravenous propofol. All participants received standardized postoperative care.
Main outcome measures: All participants were interviewed by investigators, who were blinded to the anesthesia regimen, twice daily on postoperative days 1, 2, and 3 using CAM and a CAM-based scoring system (CAM-S) to assess delirium severity. The CAM encapsulated 4 criteria: acute onset and fluctuating course, agitation, disorganized thinking, and altered level of consciousness. To diagnose delirium, both the first and second criteria must be met, in addition to either the third or fourth criterion. The averages of the scores across the 3 postoperative days indicated delirium severity, while the incidence and duration of delirium was assessed by the presence of delirium as determined by CAM on any postoperative day.
Main results: All eligible participants (N = 209; mean [SD] age 71.2 [6.7] years; 29.2% male) were included in the final analysis. The incidence of POD was not statistically different between the propofol and sevoflurane groups (33.0% vs 23.3%; P = .119, Chi-square test). It was estimated that 316 participants in each arm of the study were needed to detect statistical differences. The number of days of POD per person were higher with propofol anesthesia as compared to sevoflurane (0.5 [0.8] vs 0.3 [0.5]; P = .049, Student’s t-test).
Conclusion: This underpowered study showed a 9.7% difference in the incidence of POD between older adults who received propofol (33.0%) and sevoflurane (23.3%) after THR/TKR. Further studies with a larger sample size are needed to compare general anesthetics and their role in POD.
Commentary
Delirium is characterized by an acute state of confusion with fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is often caused by medications and/or their related adverse effects, infections, electrolyte imbalances, and other clinical etiologies. Delirium often manifests in post-surgical settings, disproportionately affecting older patients and leading to increased risk of morbidity, mortality, hospital length of stay, and health care costs.1 Intraoperative risk factors for POD are determined by the degree of operative stress (eg, lower-risk surgeries put the patient at reduced risk for POD as compared to higher-risk surgeries) and are additive to preexisting patient-specific risk factors, such as older age and functional impairment.1 Because operative stress is associated with risk for POD, limiting operative stress in controlled ways, such as through the choice of anesthetic agent administered, may be a pragmatic way to manage operative risks and optimize outcomes, especially when serving a surgically vulnerable population.
In Study 1, Chang et al sought to assess whether 2 commonly utilized general anesthetics, propofol and sevoflurane, in older patients undergoing spine surgery differentially affected the incidence of POD. In this retrospective, single-blinded observational study of 281 geriatric patients, the researchers found that sevoflurane was associated with a higher risk of POD as compared to propofol. However, these anesthetics were not associated with surgical outcomes such as postoperative 30-day complications or the length of postoperative hospital stay. While these findings added new knowledge to this field of research, several limitations should be kept in mind when interpreting this study’s results. For instance, the sample size was relatively small, with all cases selected from a single center utilizing a retrospective analysis. In addition, although a standardized nursing screening tool was used as a method for delirium detection, hypoactive delirium or less symptomatic delirium may have been missed, which in turn would lead to an underestimation of POD incidence. The latter is a common limitation in delirium research.
In Study 2, Mei et al similarly explored the effects of general anesthetics on POD in older surgical patients. Specifically, using a randomized clinical trial design, the investigators compared propofol with sevoflurane in older patients who underwent TKR/THR, and their roles in POD severity and duration. Although the incidence of POD was higher in those who received propofol compared to sevoflurane, this trial was underpowered and the results did not reach statistical significance. In addition, while the duration of POD was slightly longer in the propofol group compared to the sevoflurane group (0.5 vs 0.3 days), it was unclear if this finding was clinically significant. Similar to many research studies in POD, limitations of Study 2 included a small sample size of 209 patients, with all participants enrolled from a single center. On the other hand, this study illustrated the feasibility of a method that allowed reproducible prospective assessment of POD time course using CAM and CAM-S.
Applications for Clinical Practice and System Implementation
The delineation of risk factors that contribute to delirium after surgery in older patients is key to mitigating risks for POD and improving clinical outcomes. An important step towards a better understanding of these modifiable risk factors is to clearly quantify intraoperative risk of POD attributable to specific anesthetics. While preclinical studies have shown differential neurotoxicity effects of propofol and sevoflurane, their impact on clinically important neurologic outcomes such as delirium and cognitive decline remains poorly understood. Although Studies 1 and 2 both provided head-to-head comparisons of propofol and sevoflurane as risk factors for POD in high-operative-stress surgeries in older patients, the results were inconsistent. That being said, this small incremental increase in knowledge was not unexpected in the course of discovery around a clinically complex research question. Importantly, these studies provided evidence regarding the methodological approaches that could be taken to further this line of research.
The mediating factors of the differences on neurologic outcomes between anesthetic agents are likely pharmacological, biological, and methodological. Pharmacologically, the differences between target receptors, such as GABAA (propofol, etomidate) or NMDA (ketamine), could be a defining feature in the difference in incidence of POD. Additionally, secondary actions of anesthetic agents on glycine, nicotinic, and acetylcholine receptors could play a role as well. Biologically, genes such as CYP2E1, CYP2B6, CYP2C9, GSTP1, UGT1A9, SULT1A1, and NQO1 have all been identified as genetic factors in the metabolism of anesthetics, and variations in such genes could result in different responses to anesthetics.2 Methodologically, routes of anesthetic administration (eg, inhalation vs intravenous), preexisting anatomical structures, or confounding medical conditions (eg, lower respiratory volume due to older age) may influence POD incidence, duration, or severity. Moreover, methodological differences between Studies 1 and 2, such as surgeries performed (spinal vs TKR/THR), patient populations (South Korean vs Chinese), and the diagnosis and monitoring of delirium (retrospective screening and diagnosis vs prospective CAM/CAM-S) may impact delirium outcomes. Thus, these factors should be considered in the design of future clinical trials undertaken to investigate the effects of anesthetics on POD.
Given the high prevalence of delirium and its associated adverse outcomes in the immediate postoperative period in older patients, further research is warranted to determine how anesthetics affect POD in order to optimize perioperative care and mitigate risks in this vulnerable population. Moreover, parallel investigations into how anesthetics differentially impact the development of transient or longer-term cognitive impairment after a surgical procedure (ie, postoperative cognitive dysfunction) in older adults are urgently needed in order to improve their cognitive health.
Practice Points
- Intravenous propofol and inhalational sevoflurane may be differentially associated with incidence, duration, and severity of POD in geriatric surgical patients.
- Further larger-scale studies are warranted to clarify the role of anesthetic choice in POD in order to optimize surgical outcomes in older patients.
–Jared Doan, BS, and Fred Ko, MD
Icahn School of Medicine at Mount Sinai
Study 1 Overview (Chang et al)
Objective: To assess the incidence of postoperative delirium (POD) following propofol- vs sevoflurane-based anesthesia in geriatric spine surgery patients.
Design: Retrospective, single-blinded observational study of propofol- and sevoflurane-based anesthesia cohorts.
Setting and participants: Patients eligible for this study were aged 65 years or older admitted to the SMG-SNU Boramae Medical Center (Seoul, South Korea). All patients underwent general anesthesia either via intravenous propofol or inhalational sevoflurane for spine surgery between January 2015 and December 2019. Patients were retrospectively identified via electronic medical records. Patient exclusion criteria included preoperative delirium, history of dementia, psychiatric disease, alcoholism, hepatic or renal dysfunction, postoperative mechanical ventilation dependence, other surgery within the recent 6 months, maintenance of intraoperative anesthesia with combined anesthetics, or incomplete medical record.
Main outcome measures: The primary outcome was the incidence of POD after administration of propofol- and sevoflurane-based anesthesia during hospitalization. Patients were screened for POD regularly by attending nurses using the Nursing Delirium Screening Scale (disorientation, inappropriate behavior, inappropriate communication, hallucination, and psychomotor retardation) during the entirety of the patient’s hospital stay; if 1 or more screening criteria were met, a psychiatrist was consulted for the proper diagnosis and management of delirium. A psychiatric diagnosis was required for a case to be counted toward the incidence of POD in this study. Secondary outcomes included postoperative 30-day complications (angina, myocardial infarction, transient ischemic attack/stroke, pneumonia, deep vein thrombosis, pulmonary embolism, acute kidney injury, or infection) and length of postoperative hospital stay.
Main results: POD occurred in 29 patients (10.3%) out of the total cohort of 281. POD was more common in the sevoflurane group than in the propofol group (15.7% vs 5.0%; P = .003). Using multivariable logistic regression, inhalational sevoflurane was associated with an increased risk of POD as compared to propofol-based anesthesia (odds ratio [OR], 4.120; 95% CI, 1.549-10.954; P = .005). There was no association between choice of anesthetic and postoperative 30-day complications or the length of postoperative hospital stay. Both older age (OR, 1.242; 95% CI, 1.130-1.366; P < .001) and higher pain score at postoperative day 1 (OR, 1.338; 95% CI, 1.056-1.696; P = .016) were associated with increased risk of POD.
Conclusion: Propofol-based anesthesia was associated with a lower incidence of and risk for POD than sevoflurane-based anesthesia in older patients undergoing spine surgery.
Study 2 Overview (Mei et al)
Objective: To determine the incidence and duration of POD in older patients after total knee/hip replacement (TKR/THR) under intravenous propofol or inhalational sevoflurane general anesthesia.
Design: Randomized clinical trial of propofol and sevoflurane groups.
Setting and participants: This study was conducted at the Shanghai Tenth People’s Hospital and involved 209 participants enrolled between June 2016 and November 2019. All participants were 60 years of age or older, scheduled for TKR/THR surgery under general anesthesia, American Society of Anesthesiologists (ASA) class I to III, and assessed to be of normal cognitive function preoperatively via a Mini-Mental State Examination. Participant exclusion criteria included preexisting delirium as assessed by the Confusion Assessment Method (CAM), prior diagnosed neurological diseases (eg, Parkinson’s disease), prior diagnosed mental disorders (eg, schizophrenia), or impaired vision or hearing that would influence cognitive assessments. All participants were randomly assigned to either sevoflurane or propofol anesthesia for their surgery via a computer-generated list. Of these, 103 received inhalational sevoflurane and 106 received intravenous propofol. All participants received standardized postoperative care.
Main outcome measures: All participants were interviewed by investigators, who were blinded to the anesthesia regimen, twice daily on postoperative days 1, 2, and 3 using CAM and a CAM-based scoring system (CAM-S) to assess delirium severity. The CAM encapsulated 4 criteria: acute onset and fluctuating course, agitation, disorganized thinking, and altered level of consciousness. To diagnose delirium, both the first and second criteria must be met, in addition to either the third or fourth criterion. The averages of the scores across the 3 postoperative days indicated delirium severity, while the incidence and duration of delirium was assessed by the presence of delirium as determined by CAM on any postoperative day.
Main results: All eligible participants (N = 209; mean [SD] age 71.2 [6.7] years; 29.2% male) were included in the final analysis. The incidence of POD was not statistically different between the propofol and sevoflurane groups (33.0% vs 23.3%; P = .119, Chi-square test). It was estimated that 316 participants in each arm of the study were needed to detect statistical differences. The number of days of POD per person were higher with propofol anesthesia as compared to sevoflurane (0.5 [0.8] vs 0.3 [0.5]; P = .049, Student’s t-test).
Conclusion: This underpowered study showed a 9.7% difference in the incidence of POD between older adults who received propofol (33.0%) and sevoflurane (23.3%) after THR/TKR. Further studies with a larger sample size are needed to compare general anesthetics and their role in POD.
Commentary
Delirium is characterized by an acute state of confusion with fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is often caused by medications and/or their related adverse effects, infections, electrolyte imbalances, and other clinical etiologies. Delirium often manifests in post-surgical settings, disproportionately affecting older patients and leading to increased risk of morbidity, mortality, hospital length of stay, and health care costs.1 Intraoperative risk factors for POD are determined by the degree of operative stress (eg, lower-risk surgeries put the patient at reduced risk for POD as compared to higher-risk surgeries) and are additive to preexisting patient-specific risk factors, such as older age and functional impairment.1 Because operative stress is associated with risk for POD, limiting operative stress in controlled ways, such as through the choice of anesthetic agent administered, may be a pragmatic way to manage operative risks and optimize outcomes, especially when serving a surgically vulnerable population.
In Study 1, Chang et al sought to assess whether 2 commonly utilized general anesthetics, propofol and sevoflurane, in older patients undergoing spine surgery differentially affected the incidence of POD. In this retrospective, single-blinded observational study of 281 geriatric patients, the researchers found that sevoflurane was associated with a higher risk of POD as compared to propofol. However, these anesthetics were not associated with surgical outcomes such as postoperative 30-day complications or the length of postoperative hospital stay. While these findings added new knowledge to this field of research, several limitations should be kept in mind when interpreting this study’s results. For instance, the sample size was relatively small, with all cases selected from a single center utilizing a retrospective analysis. In addition, although a standardized nursing screening tool was used as a method for delirium detection, hypoactive delirium or less symptomatic delirium may have been missed, which in turn would lead to an underestimation of POD incidence. The latter is a common limitation in delirium research.
In Study 2, Mei et al similarly explored the effects of general anesthetics on POD in older surgical patients. Specifically, using a randomized clinical trial design, the investigators compared propofol with sevoflurane in older patients who underwent TKR/THR, and their roles in POD severity and duration. Although the incidence of POD was higher in those who received propofol compared to sevoflurane, this trial was underpowered and the results did not reach statistical significance. In addition, while the duration of POD was slightly longer in the propofol group compared to the sevoflurane group (0.5 vs 0.3 days), it was unclear if this finding was clinically significant. Similar to many research studies in POD, limitations of Study 2 included a small sample size of 209 patients, with all participants enrolled from a single center. On the other hand, this study illustrated the feasibility of a method that allowed reproducible prospective assessment of POD time course using CAM and CAM-S.
Applications for Clinical Practice and System Implementation
The delineation of risk factors that contribute to delirium after surgery in older patients is key to mitigating risks for POD and improving clinical outcomes. An important step towards a better understanding of these modifiable risk factors is to clearly quantify intraoperative risk of POD attributable to specific anesthetics. While preclinical studies have shown differential neurotoxicity effects of propofol and sevoflurane, their impact on clinically important neurologic outcomes such as delirium and cognitive decline remains poorly understood. Although Studies 1 and 2 both provided head-to-head comparisons of propofol and sevoflurane as risk factors for POD in high-operative-stress surgeries in older patients, the results were inconsistent. That being said, this small incremental increase in knowledge was not unexpected in the course of discovery around a clinically complex research question. Importantly, these studies provided evidence regarding the methodological approaches that could be taken to further this line of research.
The mediating factors of the differences on neurologic outcomes between anesthetic agents are likely pharmacological, biological, and methodological. Pharmacologically, the differences between target receptors, such as GABAA (propofol, etomidate) or NMDA (ketamine), could be a defining feature in the difference in incidence of POD. Additionally, secondary actions of anesthetic agents on glycine, nicotinic, and acetylcholine receptors could play a role as well. Biologically, genes such as CYP2E1, CYP2B6, CYP2C9, GSTP1, UGT1A9, SULT1A1, and NQO1 have all been identified as genetic factors in the metabolism of anesthetics, and variations in such genes could result in different responses to anesthetics.2 Methodologically, routes of anesthetic administration (eg, inhalation vs intravenous), preexisting anatomical structures, or confounding medical conditions (eg, lower respiratory volume due to older age) may influence POD incidence, duration, or severity. Moreover, methodological differences between Studies 1 and 2, such as surgeries performed (spinal vs TKR/THR), patient populations (South Korean vs Chinese), and the diagnosis and monitoring of delirium (retrospective screening and diagnosis vs prospective CAM/CAM-S) may impact delirium outcomes. Thus, these factors should be considered in the design of future clinical trials undertaken to investigate the effects of anesthetics on POD.
Given the high prevalence of delirium and its associated adverse outcomes in the immediate postoperative period in older patients, further research is warranted to determine how anesthetics affect POD in order to optimize perioperative care and mitigate risks in this vulnerable population. Moreover, parallel investigations into how anesthetics differentially impact the development of transient or longer-term cognitive impairment after a surgical procedure (ie, postoperative cognitive dysfunction) in older adults are urgently needed in order to improve their cognitive health.
Practice Points
- Intravenous propofol and inhalational sevoflurane may be differentially associated with incidence, duration, and severity of POD in geriatric surgical patients.
- Further larger-scale studies are warranted to clarify the role of anesthetic choice in POD in order to optimize surgical outcomes in older patients.
–Jared Doan, BS, and Fred Ko, MD
Icahn School of Medicine at Mount Sinai
1. Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54(10):1578-1589. doi:10.1111/j.1532-5415.2006.00893.x
2. Mikstacki A, Skrzypczak-Zielinska M, Tamowicz B, et al. The impact of genetic factors on response to anaesthetics. Adv Med Sci. 2013;58(1):9-14. doi:10.2478/v10039-012-0065-z
1. Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54(10):1578-1589. doi:10.1111/j.1532-5415.2006.00893.x
2. Mikstacki A, Skrzypczak-Zielinska M, Tamowicz B, et al. The impact of genetic factors on response to anaesthetics. Adv Med Sci. 2013;58(1):9-14. doi:10.2478/v10039-012-0065-z
A plane crash interrupts a doctor’s vacation
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Should every scheduled cesarean birth use an Enhanced Recovery after Surgery (ERAS) pathway?

Cesarean birth is one of the most common major surgical procedures performed in developed countries1 with over 1,170,000 cesarean births in the United States in 2021.2 Many surgeons and anesthesiologists believe that Enhanced Recovery after Surgery (ERAS) pathways improve surgical outcomes.3,4 Important goals of ERAS include setting patient expectations for the surgical procedure, accelerating patient recovery to full function, and minimizing perioperative complications such as severe nausea, aspiration, surgical site infection, wound complications, and perioperative anemia. The ERAS Society in 20185-7 and the Society for Obstetric Anesthesia and Perinatology (SOAP) in 20218 proposed ERAS pathways for cesarean birth. Both societies recommended that obstetric units consider adopting an ERAS pathway compatible with local clinical resources. In addition, the American College of Obstetricians and Gynecologists (ACOG) has provided guidance for implementing ERAS pathways for gynecologic surgery.9 The consistent use of standardized protocols to improve surgical care in obstetrics should lead to a reduction in care variation and improve health equity outcomes.
The clinical interventions recommended for ERAS cesarean birth occur sequentially in the preoperative, intraoperative, and postoperative phases of care. The recommendations associated with each of these phases are reviewed below. It is important to note that each obstetric unit should use a multidisciplinary process to develop an ERAS pathway that best supports local practice given clinician preferences, patient characteristics, and resource availability.
Preoperative components of ERAS
Standardized patient education (SPE). SPE is an important component of ERAS, although evidence to support the recommendation is limited. At a minimum a written handout describing steps in the cesarean birth process, or a patient-education video should be part of patient education. The University of Michigan Medical Center has produced a 3-minute video for patients explaining ERAS cesarean birth.10 The University of Maryland Medical Center has produced a 2.5-minute video in English and Spanish, explaining ERAS cesarean birth for patients.11 Some surgeons place a telephone call to patients the evening before surgery to help orient the patient to ERAS cesarean birth.
Breastfeeding education. An important goal of obstetric care is to optimize the rate of exclusive breastfeeding at birth. Breastfeeding education, including a commitment to support the initiation of breastfeeding within 1 hour of birth, may enhance the rate of exclusive breastfeeding. There are numerous videos available for patients about breastfeeding after cesarean birth (as an example, see: https://www.youtube.com/watch?v=9iOGn85NdTg).
Limit fasting. In the past, surgical guidelines recommended fasting after midnight prior to surgery. The ERAS Society recommends that patients should be encouraged to drink clear fluids up to 2 hours before surgery and may have a light meal up to 6 hours before surgery (Part 1).
Carbohydrate loading. Surgery causes a metabolic stress that is increased by fasting. Carbohydrate loading prior to surgery reduces the magnitude of the catabolic state caused by the combination of surgery and fasting.12 SOAP and the ERAS Society recommend oral carbohydrate fluid supplementation 2 hours before surgery for nondiabetic patients. SOAP suggests 32 oz of Gatorade or 16 oz of clear apple juice as options for carbohydrate loading. For diabetic patients, the carbohydrate load can be omitted. In fasting pregnant patients at term, gastric emptying was near complete 2 hours after consumption of 400 mL of a carbohydrate drink.13 In one study, consumption of 400 mL of a carbohydrate drink 2 hours before cesarean resulted in a 7% increase in the newborn blood glucose level at 20 min after delivery.14
Minimize preoperative anemia. Approximately 50% of pregnant women are iron deficient and approximately 10% are anemic in the third trimester.15,16 Cesarean birth is associated with significant blood loss necessitating the need to optimize red blood cell mass before surgery. Measuring ferritin to identify patients with iron deficiency and aggressive iron replacement, including intravenous iron if necessary, will reduce the prevalence of anemia prior to cesarean birth.17 Another cause of anemia in pregnancy is vitamin B12 (cobalamin) deficiency. Low vitamin B12 is especially common in pregnant patients who have previously had bariatric surgery. One study reported that, of 113 pregnant patients who were, on average, 3 years from a bariatric surgery procedure, 12% had vitamin B12 circulating levels < 130 pg/mL.18 Among pregnant patients who are anemic, and do not have a hemoglobinopathy, measuring ferritin, folic acid, and vitamin B12 will help identify the cause of anemia and guide treatment.19
Optimize preoperative physical condition. Improving healthy behaviors and reducing unhealthy behaviors preoperatively may enhance patient recovery to full function. In the weeks before scheduled cesarean birth, cessation of the use of tobacco products, optimizing activity and improving diet quality, including increasing protein intake, may best prepare patients for the metabolic stress of surgery.
Continue to: Intraoperative components of ERAS...
Intraoperative components of ERAS
Reduce the risk of surgical site infection (SSI) and wound complications. Bundles that include antibiotics, chlorhexidine (or an alternative antibacterial soap) and clippers have been shown to reduce SSI.20 Routine administration of preoperative antibiotics is a consensus recommendation and there is high adherence with this recommendation in the United States. Chlorhexidine-alcohol is the preferred solution for skin preparation. Vaginal preparation with povidine-iodine or chlorhexidine may be considered.6
Surgical technique. Blunt extension of a transverse hysterotomy may reduce blood loss. Closure of the hysterotomy incision in 2 layers is recommended to reduce uterine scar dehiscence in a subsequent pregnancy. If the patient has ≥2 cm of subcutaneous tissue, this layer should be approximated with sutures. Skin closure should be with subcuticular suture.6
Optimize uterotonic administration. Routine use of uterotonics reduces the risk of blood loss, transfusion, and postoperative anemia. There is high adherence with the use of uterotonic administration after birth in the United States.6,8
Ensure normothermia. Many patients become hypothermic during a cesarean birth. Active warming of the patient with an in-line IV fluid warmer and forced air warming over the patient’s body can reduce the risk of hypothermia.8
Initiate multimodal anesthesia. Anesthesiologists often use intrathecal or epidural morphine to enhance analgesia. Ketorolac administration prior to completion of the cesarean procedure and perioperative administration of acetaminophen may reduce postoperative pain.8 The use of preoperative antiemetics will reduce intraoperative and postoperative nausea and vomiting.
Initiate VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.6
Postoperative components of ERAS
Patient education to prepare for discharge home when ready. Patient education focused on home when ready is important in preparing the patient for discharge home.7 Completion of required newborn testing, lactation education, and contraception planning plus coordination of newborn pediatric follow-up is necessary before discharge.
Support early return of bowel function. Early return of bowel function is best supported by a multimodal approach including initiation of clear fluid intake immediately following surgery, encouraging consumption of a regular diet within 27 to 4 hours8 following surgery. Gum chewing for at least 5 minutes 3 times daily accelerates return of bowel function.8 In a meta-analysis of 10 randomized studies examining the effect of gum chewing after cesarean, the investigators reported that gum chewing shortened the time to passage of flatus and defecation.21
Early ambulation.
Sequentially advanced activity, starting with sitting on the edge of the bed, sitting in a chair, and ambulation within 8 hours of surgery, is recommended to facilitate faster recovery, reduce rates of complications, and enable transition to home.8
Early removal of the urinary catheter. It is recommended that the urinary catheter be removed within 12 hours after cesarean birth.8 Early removal of the urinary catheter increases patient mobility and reduces the length of hospitalization. Early removal of the urinary catheter may be associated with postoperative urinary retention and recatheterization in a small number of patients.
Prescribe routinely scheduled acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs) and ketorolac. A key component of ERAS cesarean birth is the standardized administration of nonopioid pain medicines, alternating doses of acetaminophen and an NSAID. ERAS cesarean birth is likely to result in a reduction in inpatient and postdischarge opioid use.22-24
VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.8
Auditing and reporting adherence with components of ERAS
In clinical practice there may be a gap between a clinician’s subjective perception of their performance and an independent audit of their clinical performance. ERAS pathways should be implemented with a commitment to performing audits and providing quantitative feedback to clinicians. Consistent use of measurement, feedback, and coaching can improve performance and reduce variation among individual clinicians. As an example, in one study of the use of a surgical safety checklist, 99% of the surgeons reported that they routinely used a surgical safety checklist, but the audit showed that the checklist was used in only 60% of cases.25 Gaps between self-reported performance and audited performance are common in clinical practice. Audits with feedback are critical to improving adherence with the components of an ERAS pathway.
Three independent systematic reviews and meta-analyses report that ERAS pathways reduce hospital length of stay without increasing the readmission rate.26-28 One meta-analysis reported that ERAS may also reduce time to first mobilization and result in earlier removal of the urinary catheter.26 ERAS pathways also may reduce postoperative complications, lower pain scores, and decrease opioid use.27 The general consensus among quality and safety experts is that reducing variation through standardization of pathways is generally associated with improved quality and enhanced safety. ERAS pathways have been widely accepted in multiple surgical fields. ERAS pathways should become the standard for performing cesarean procedures.●
1. Molina G, Weiser RG, Lipsitz SR, et al. Relationship between cesarean delivery rate and maternal and neonatal mortality. JAMA. 2015;314:2263-2270.
2. Hamilton BE, Martin JA, Osterman MJK. Births: provisional data for 2021. Vital Statistics Release; No. 20. Hyattsville, MD: National Center for Health Statistics. May 2022. https://www.cdc.gov/nchs/data/vsrr/vsrr020.pdf.
3. Berian JR, Ban KA, Liu JB, et al. Adherence to enhanced recovery protocols in NSQIP and association with colectomy outcomes. Ann Surg. 2019;486-493.
4. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292-298.
5. Wilson RD, Caughey AB, Wood SL, et al. Guidelines for antenatal and preoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 1). Am J Obstet Gynecol. 2018;219:523.e1-523.e15.
6. Caughey AB, Wood SL, Macones GA, et al Guidelines for intraoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 2). Am J Obstet Gynecol. 2018;219:533-544.
7. Macones GA, Caughey AB, Wood SL, et al. Guidelines for postoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 3). Am J Obstet Gynecol. 2019;221:247.e1-247.e9.
8. Bollag L, Lim G, Sultan P, et al. Society for Obstetric Anesthesia and Perinatology: Consensus statement and recommendations for enhanced recovery after cesarean. Anesth Analg. 2021;132:1362-1377.
9. Perioperative pathways: enhanced recovery after surgery. ACOG Committee Opinion No 750. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e120-130.
10. University of Michigan. ERAS: A patient education video. https://www.youtube.com/watch?v=CoFtgdluBc0. Accessed October 24, 2022.
11. University of Maryland. ERAS. https://www.umms.org/ummc/health-services/womens-health/ostetrics-gynecology/pregnancy-childbirth/labor-delivery/enhanced-recovery-after-cesarean. Accessed October 24, 2022.
12. Bilku DK, Dennison AR, Hall TC, et al. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
13. Popivanov P, Irwin R, Walsh M, et al. Gastric emptying of carbohydrate drinks in term parturients before elective caesarean surgery: an observational study. Int J Obstet Anesth. 2020;41:29-34.
14. He Y, Liu C, Han Y, et al. The impact of carbohydrate-rich supplement taken two hours before caesarean delivery on maternal and neonatal perioperative outcomes- a randomized clinical trial. BMC Pregnancy Childbirth. 2021;21:682.
15. Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med. 2021;34:1002-1005.
16. Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1996-2006. Am J Clin Nutr. 2011;93:1312-1320.
17. Nour N, Barbieri RL. Optimize detection and treatment of iron deficiency in pregnancy. OBG Manag. 2022;34:9-11.
18. Mead NC, Sakkatos P, Sakellaropoulos GC, et al. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg Obes Relat Dis. 2014;10:1166-1173.
19. Achebe MM, Gafter-Gvili A. How I treat anemia in pregnancy: iron, cobalamin and folate. Blood. 2017;129:940-949.
20. Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:735-746.
21. Wen Z, Shen M, Wu C, et al. Chewing gum for intestinal function recovery after caesarean section: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2017;17:105.
22. McCoy JA, Gutman S, Hamm RF, et al. The association between implementation of an enhanced recovery after cesarean pathway with standardized discharge prescriptions and opioid use and pain experience after cesarean delivery. Am J Perinatol. 2021;38:1341-1347.
23. Mullman L, Hilden P, Goral J, et al. Improved outcomes with an enhanced recovery approach to cesarean delivery. Obstet Gynecol. 2020;136:685-691.
24. Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
25. Sendlhofer G, Lumenta DB, Leitgeb K, et al. The gap between individual perception and compliance: a quantitative follow-up study of the surgical safety checklist application. PLoS One. 2016;11:e0149212.
26. Sultan P, Sharawi N, Blake L, et al. Impact of enhanced recovery after cesarean delivery on maternal outcomes: a systematic review and meta-analysis. Anaesth Crit Care Pain Med. 2021;40:100935.
27. Meng X, Chen K, Yang C, et al. The clinical efficacy and safety of enhanced recovery after surgery for cesarean section: a systematic review and meta-analysis of randomized controlled trials and observational studies. Front Med. 2021;8:694385.
28. Corson E, Hind D, Beever D, et al. Enhanced recovery after elective caesarean: a rapid review of clinical protocols and an umbrella review of systematic reviews. BMC Pregnancy Childbirth. 2017;17:91.

Cesarean birth is one of the most common major surgical procedures performed in developed countries1 with over 1,170,000 cesarean births in the United States in 2021.2 Many surgeons and anesthesiologists believe that Enhanced Recovery after Surgery (ERAS) pathways improve surgical outcomes.3,4 Important goals of ERAS include setting patient expectations for the surgical procedure, accelerating patient recovery to full function, and minimizing perioperative complications such as severe nausea, aspiration, surgical site infection, wound complications, and perioperative anemia. The ERAS Society in 20185-7 and the Society for Obstetric Anesthesia and Perinatology (SOAP) in 20218 proposed ERAS pathways for cesarean birth. Both societies recommended that obstetric units consider adopting an ERAS pathway compatible with local clinical resources. In addition, the American College of Obstetricians and Gynecologists (ACOG) has provided guidance for implementing ERAS pathways for gynecologic surgery.9 The consistent use of standardized protocols to improve surgical care in obstetrics should lead to a reduction in care variation and improve health equity outcomes.
The clinical interventions recommended for ERAS cesarean birth occur sequentially in the preoperative, intraoperative, and postoperative phases of care. The recommendations associated with each of these phases are reviewed below. It is important to note that each obstetric unit should use a multidisciplinary process to develop an ERAS pathway that best supports local practice given clinician preferences, patient characteristics, and resource availability.
Preoperative components of ERAS
Standardized patient education (SPE). SPE is an important component of ERAS, although evidence to support the recommendation is limited. At a minimum a written handout describing steps in the cesarean birth process, or a patient-education video should be part of patient education. The University of Michigan Medical Center has produced a 3-minute video for patients explaining ERAS cesarean birth.10 The University of Maryland Medical Center has produced a 2.5-minute video in English and Spanish, explaining ERAS cesarean birth for patients.11 Some surgeons place a telephone call to patients the evening before surgery to help orient the patient to ERAS cesarean birth.
Breastfeeding education. An important goal of obstetric care is to optimize the rate of exclusive breastfeeding at birth. Breastfeeding education, including a commitment to support the initiation of breastfeeding within 1 hour of birth, may enhance the rate of exclusive breastfeeding. There are numerous videos available for patients about breastfeeding after cesarean birth (as an example, see: https://www.youtube.com/watch?v=9iOGn85NdTg).
Limit fasting. In the past, surgical guidelines recommended fasting after midnight prior to surgery. The ERAS Society recommends that patients should be encouraged to drink clear fluids up to 2 hours before surgery and may have a light meal up to 6 hours before surgery (Part 1).
Carbohydrate loading. Surgery causes a metabolic stress that is increased by fasting. Carbohydrate loading prior to surgery reduces the magnitude of the catabolic state caused by the combination of surgery and fasting.12 SOAP and the ERAS Society recommend oral carbohydrate fluid supplementation 2 hours before surgery for nondiabetic patients. SOAP suggests 32 oz of Gatorade or 16 oz of clear apple juice as options for carbohydrate loading. For diabetic patients, the carbohydrate load can be omitted. In fasting pregnant patients at term, gastric emptying was near complete 2 hours after consumption of 400 mL of a carbohydrate drink.13 In one study, consumption of 400 mL of a carbohydrate drink 2 hours before cesarean resulted in a 7% increase in the newborn blood glucose level at 20 min after delivery.14
Minimize preoperative anemia. Approximately 50% of pregnant women are iron deficient and approximately 10% are anemic in the third trimester.15,16 Cesarean birth is associated with significant blood loss necessitating the need to optimize red blood cell mass before surgery. Measuring ferritin to identify patients with iron deficiency and aggressive iron replacement, including intravenous iron if necessary, will reduce the prevalence of anemia prior to cesarean birth.17 Another cause of anemia in pregnancy is vitamin B12 (cobalamin) deficiency. Low vitamin B12 is especially common in pregnant patients who have previously had bariatric surgery. One study reported that, of 113 pregnant patients who were, on average, 3 years from a bariatric surgery procedure, 12% had vitamin B12 circulating levels < 130 pg/mL.18 Among pregnant patients who are anemic, and do not have a hemoglobinopathy, measuring ferritin, folic acid, and vitamin B12 will help identify the cause of anemia and guide treatment.19
Optimize preoperative physical condition. Improving healthy behaviors and reducing unhealthy behaviors preoperatively may enhance patient recovery to full function. In the weeks before scheduled cesarean birth, cessation of the use of tobacco products, optimizing activity and improving diet quality, including increasing protein intake, may best prepare patients for the metabolic stress of surgery.
Continue to: Intraoperative components of ERAS...
Intraoperative components of ERAS
Reduce the risk of surgical site infection (SSI) and wound complications. Bundles that include antibiotics, chlorhexidine (or an alternative antibacterial soap) and clippers have been shown to reduce SSI.20 Routine administration of preoperative antibiotics is a consensus recommendation and there is high adherence with this recommendation in the United States. Chlorhexidine-alcohol is the preferred solution for skin preparation. Vaginal preparation with povidine-iodine or chlorhexidine may be considered.6
Surgical technique. Blunt extension of a transverse hysterotomy may reduce blood loss. Closure of the hysterotomy incision in 2 layers is recommended to reduce uterine scar dehiscence in a subsequent pregnancy. If the patient has ≥2 cm of subcutaneous tissue, this layer should be approximated with sutures. Skin closure should be with subcuticular suture.6
Optimize uterotonic administration. Routine use of uterotonics reduces the risk of blood loss, transfusion, and postoperative anemia. There is high adherence with the use of uterotonic administration after birth in the United States.6,8
Ensure normothermia. Many patients become hypothermic during a cesarean birth. Active warming of the patient with an in-line IV fluid warmer and forced air warming over the patient’s body can reduce the risk of hypothermia.8
Initiate multimodal anesthesia. Anesthesiologists often use intrathecal or epidural morphine to enhance analgesia. Ketorolac administration prior to completion of the cesarean procedure and perioperative administration of acetaminophen may reduce postoperative pain.8 The use of preoperative antiemetics will reduce intraoperative and postoperative nausea and vomiting.
Initiate VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.6
Postoperative components of ERAS
Patient education to prepare for discharge home when ready. Patient education focused on home when ready is important in preparing the patient for discharge home.7 Completion of required newborn testing, lactation education, and contraception planning plus coordination of newborn pediatric follow-up is necessary before discharge.
Support early return of bowel function. Early return of bowel function is best supported by a multimodal approach including initiation of clear fluid intake immediately following surgery, encouraging consumption of a regular diet within 27 to 4 hours8 following surgery. Gum chewing for at least 5 minutes 3 times daily accelerates return of bowel function.8 In a meta-analysis of 10 randomized studies examining the effect of gum chewing after cesarean, the investigators reported that gum chewing shortened the time to passage of flatus and defecation.21
Early ambulation.
Sequentially advanced activity, starting with sitting on the edge of the bed, sitting in a chair, and ambulation within 8 hours of surgery, is recommended to facilitate faster recovery, reduce rates of complications, and enable transition to home.8
Early removal of the urinary catheter. It is recommended that the urinary catheter be removed within 12 hours after cesarean birth.8 Early removal of the urinary catheter increases patient mobility and reduces the length of hospitalization. Early removal of the urinary catheter may be associated with postoperative urinary retention and recatheterization in a small number of patients.
Prescribe routinely scheduled acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs) and ketorolac. A key component of ERAS cesarean birth is the standardized administration of nonopioid pain medicines, alternating doses of acetaminophen and an NSAID. ERAS cesarean birth is likely to result in a reduction in inpatient and postdischarge opioid use.22-24
VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.8
Auditing and reporting adherence with components of ERAS
In clinical practice there may be a gap between a clinician’s subjective perception of their performance and an independent audit of their clinical performance. ERAS pathways should be implemented with a commitment to performing audits and providing quantitative feedback to clinicians. Consistent use of measurement, feedback, and coaching can improve performance and reduce variation among individual clinicians. As an example, in one study of the use of a surgical safety checklist, 99% of the surgeons reported that they routinely used a surgical safety checklist, but the audit showed that the checklist was used in only 60% of cases.25 Gaps between self-reported performance and audited performance are common in clinical practice. Audits with feedback are critical to improving adherence with the components of an ERAS pathway.
Three independent systematic reviews and meta-analyses report that ERAS pathways reduce hospital length of stay without increasing the readmission rate.26-28 One meta-analysis reported that ERAS may also reduce time to first mobilization and result in earlier removal of the urinary catheter.26 ERAS pathways also may reduce postoperative complications, lower pain scores, and decrease opioid use.27 The general consensus among quality and safety experts is that reducing variation through standardization of pathways is generally associated with improved quality and enhanced safety. ERAS pathways have been widely accepted in multiple surgical fields. ERAS pathways should become the standard for performing cesarean procedures.●

Cesarean birth is one of the most common major surgical procedures performed in developed countries1 with over 1,170,000 cesarean births in the United States in 2021.2 Many surgeons and anesthesiologists believe that Enhanced Recovery after Surgery (ERAS) pathways improve surgical outcomes.3,4 Important goals of ERAS include setting patient expectations for the surgical procedure, accelerating patient recovery to full function, and minimizing perioperative complications such as severe nausea, aspiration, surgical site infection, wound complications, and perioperative anemia. The ERAS Society in 20185-7 and the Society for Obstetric Anesthesia and Perinatology (SOAP) in 20218 proposed ERAS pathways for cesarean birth. Both societies recommended that obstetric units consider adopting an ERAS pathway compatible with local clinical resources. In addition, the American College of Obstetricians and Gynecologists (ACOG) has provided guidance for implementing ERAS pathways for gynecologic surgery.9 The consistent use of standardized protocols to improve surgical care in obstetrics should lead to a reduction in care variation and improve health equity outcomes.
The clinical interventions recommended for ERAS cesarean birth occur sequentially in the preoperative, intraoperative, and postoperative phases of care. The recommendations associated with each of these phases are reviewed below. It is important to note that each obstetric unit should use a multidisciplinary process to develop an ERAS pathway that best supports local practice given clinician preferences, patient characteristics, and resource availability.
Preoperative components of ERAS
Standardized patient education (SPE). SPE is an important component of ERAS, although evidence to support the recommendation is limited. At a minimum a written handout describing steps in the cesarean birth process, or a patient-education video should be part of patient education. The University of Michigan Medical Center has produced a 3-minute video for patients explaining ERAS cesarean birth.10 The University of Maryland Medical Center has produced a 2.5-minute video in English and Spanish, explaining ERAS cesarean birth for patients.11 Some surgeons place a telephone call to patients the evening before surgery to help orient the patient to ERAS cesarean birth.
Breastfeeding education. An important goal of obstetric care is to optimize the rate of exclusive breastfeeding at birth. Breastfeeding education, including a commitment to support the initiation of breastfeeding within 1 hour of birth, may enhance the rate of exclusive breastfeeding. There are numerous videos available for patients about breastfeeding after cesarean birth (as an example, see: https://www.youtube.com/watch?v=9iOGn85NdTg).
Limit fasting. In the past, surgical guidelines recommended fasting after midnight prior to surgery. The ERAS Society recommends that patients should be encouraged to drink clear fluids up to 2 hours before surgery and may have a light meal up to 6 hours before surgery (Part 1).
Carbohydrate loading. Surgery causes a metabolic stress that is increased by fasting. Carbohydrate loading prior to surgery reduces the magnitude of the catabolic state caused by the combination of surgery and fasting.12 SOAP and the ERAS Society recommend oral carbohydrate fluid supplementation 2 hours before surgery for nondiabetic patients. SOAP suggests 32 oz of Gatorade or 16 oz of clear apple juice as options for carbohydrate loading. For diabetic patients, the carbohydrate load can be omitted. In fasting pregnant patients at term, gastric emptying was near complete 2 hours after consumption of 400 mL of a carbohydrate drink.13 In one study, consumption of 400 mL of a carbohydrate drink 2 hours before cesarean resulted in a 7% increase in the newborn blood glucose level at 20 min after delivery.14
Minimize preoperative anemia. Approximately 50% of pregnant women are iron deficient and approximately 10% are anemic in the third trimester.15,16 Cesarean birth is associated with significant blood loss necessitating the need to optimize red blood cell mass before surgery. Measuring ferritin to identify patients with iron deficiency and aggressive iron replacement, including intravenous iron if necessary, will reduce the prevalence of anemia prior to cesarean birth.17 Another cause of anemia in pregnancy is vitamin B12 (cobalamin) deficiency. Low vitamin B12 is especially common in pregnant patients who have previously had bariatric surgery. One study reported that, of 113 pregnant patients who were, on average, 3 years from a bariatric surgery procedure, 12% had vitamin B12 circulating levels < 130 pg/mL.18 Among pregnant patients who are anemic, and do not have a hemoglobinopathy, measuring ferritin, folic acid, and vitamin B12 will help identify the cause of anemia and guide treatment.19
Optimize preoperative physical condition. Improving healthy behaviors and reducing unhealthy behaviors preoperatively may enhance patient recovery to full function. In the weeks before scheduled cesarean birth, cessation of the use of tobacco products, optimizing activity and improving diet quality, including increasing protein intake, may best prepare patients for the metabolic stress of surgery.
Continue to: Intraoperative components of ERAS...
Intraoperative components of ERAS
Reduce the risk of surgical site infection (SSI) and wound complications. Bundles that include antibiotics, chlorhexidine (or an alternative antibacterial soap) and clippers have been shown to reduce SSI.20 Routine administration of preoperative antibiotics is a consensus recommendation and there is high adherence with this recommendation in the United States. Chlorhexidine-alcohol is the preferred solution for skin preparation. Vaginal preparation with povidine-iodine or chlorhexidine may be considered.6
Surgical technique. Blunt extension of a transverse hysterotomy may reduce blood loss. Closure of the hysterotomy incision in 2 layers is recommended to reduce uterine scar dehiscence in a subsequent pregnancy. If the patient has ≥2 cm of subcutaneous tissue, this layer should be approximated with sutures. Skin closure should be with subcuticular suture.6
Optimize uterotonic administration. Routine use of uterotonics reduces the risk of blood loss, transfusion, and postoperative anemia. There is high adherence with the use of uterotonic administration after birth in the United States.6,8
Ensure normothermia. Many patients become hypothermic during a cesarean birth. Active warming of the patient with an in-line IV fluid warmer and forced air warming over the patient’s body can reduce the risk of hypothermia.8
Initiate multimodal anesthesia. Anesthesiologists often use intrathecal or epidural morphine to enhance analgesia. Ketorolac administration prior to completion of the cesarean procedure and perioperative administration of acetaminophen may reduce postoperative pain.8 The use of preoperative antiemetics will reduce intraoperative and postoperative nausea and vomiting.
Initiate VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.6
Postoperative components of ERAS
Patient education to prepare for discharge home when ready. Patient education focused on home when ready is important in preparing the patient for discharge home.7 Completion of required newborn testing, lactation education, and contraception planning plus coordination of newborn pediatric follow-up is necessary before discharge.
Support early return of bowel function. Early return of bowel function is best supported by a multimodal approach including initiation of clear fluid intake immediately following surgery, encouraging consumption of a regular diet within 27 to 4 hours8 following surgery. Gum chewing for at least 5 minutes 3 times daily accelerates return of bowel function.8 In a meta-analysis of 10 randomized studies examining the effect of gum chewing after cesarean, the investigators reported that gum chewing shortened the time to passage of flatus and defecation.21
Early ambulation.
Sequentially advanced activity, starting with sitting on the edge of the bed, sitting in a chair, and ambulation within 8 hours of surgery, is recommended to facilitate faster recovery, reduce rates of complications, and enable transition to home.8
Early removal of the urinary catheter. It is recommended that the urinary catheter be removed within 12 hours after cesarean birth.8 Early removal of the urinary catheter increases patient mobility and reduces the length of hospitalization. Early removal of the urinary catheter may be associated with postoperative urinary retention and recatheterization in a small number of patients.
Prescribe routinely scheduled acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs) and ketorolac. A key component of ERAS cesarean birth is the standardized administration of nonopioid pain medicines, alternating doses of acetaminophen and an NSAID. ERAS cesarean birth is likely to result in a reduction in inpatient and postdischarge opioid use.22-24
VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.8
Auditing and reporting adherence with components of ERAS
In clinical practice there may be a gap between a clinician’s subjective perception of their performance and an independent audit of their clinical performance. ERAS pathways should be implemented with a commitment to performing audits and providing quantitative feedback to clinicians. Consistent use of measurement, feedback, and coaching can improve performance and reduce variation among individual clinicians. As an example, in one study of the use of a surgical safety checklist, 99% of the surgeons reported that they routinely used a surgical safety checklist, but the audit showed that the checklist was used in only 60% of cases.25 Gaps between self-reported performance and audited performance are common in clinical practice. Audits with feedback are critical to improving adherence with the components of an ERAS pathway.
Three independent systematic reviews and meta-analyses report that ERAS pathways reduce hospital length of stay without increasing the readmission rate.26-28 One meta-analysis reported that ERAS may also reduce time to first mobilization and result in earlier removal of the urinary catheter.26 ERAS pathways also may reduce postoperative complications, lower pain scores, and decrease opioid use.27 The general consensus among quality and safety experts is that reducing variation through standardization of pathways is generally associated with improved quality and enhanced safety. ERAS pathways have been widely accepted in multiple surgical fields. ERAS pathways should become the standard for performing cesarean procedures.●
1. Molina G, Weiser RG, Lipsitz SR, et al. Relationship between cesarean delivery rate and maternal and neonatal mortality. JAMA. 2015;314:2263-2270.
2. Hamilton BE, Martin JA, Osterman MJK. Births: provisional data for 2021. Vital Statistics Release; No. 20. Hyattsville, MD: National Center for Health Statistics. May 2022. https://www.cdc.gov/nchs/data/vsrr/vsrr020.pdf.
3. Berian JR, Ban KA, Liu JB, et al. Adherence to enhanced recovery protocols in NSQIP and association with colectomy outcomes. Ann Surg. 2019;486-493.
4. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292-298.
5. Wilson RD, Caughey AB, Wood SL, et al. Guidelines for antenatal and preoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 1). Am J Obstet Gynecol. 2018;219:523.e1-523.e15.
6. Caughey AB, Wood SL, Macones GA, et al Guidelines for intraoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 2). Am J Obstet Gynecol. 2018;219:533-544.
7. Macones GA, Caughey AB, Wood SL, et al. Guidelines for postoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 3). Am J Obstet Gynecol. 2019;221:247.e1-247.e9.
8. Bollag L, Lim G, Sultan P, et al. Society for Obstetric Anesthesia and Perinatology: Consensus statement and recommendations for enhanced recovery after cesarean. Anesth Analg. 2021;132:1362-1377.
9. Perioperative pathways: enhanced recovery after surgery. ACOG Committee Opinion No 750. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e120-130.
10. University of Michigan. ERAS: A patient education video. https://www.youtube.com/watch?v=CoFtgdluBc0. Accessed October 24, 2022.
11. University of Maryland. ERAS. https://www.umms.org/ummc/health-services/womens-health/ostetrics-gynecology/pregnancy-childbirth/labor-delivery/enhanced-recovery-after-cesarean. Accessed October 24, 2022.
12. Bilku DK, Dennison AR, Hall TC, et al. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
13. Popivanov P, Irwin R, Walsh M, et al. Gastric emptying of carbohydrate drinks in term parturients before elective caesarean surgery: an observational study. Int J Obstet Anesth. 2020;41:29-34.
14. He Y, Liu C, Han Y, et al. The impact of carbohydrate-rich supplement taken two hours before caesarean delivery on maternal and neonatal perioperative outcomes- a randomized clinical trial. BMC Pregnancy Childbirth. 2021;21:682.
15. Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med. 2021;34:1002-1005.
16. Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1996-2006. Am J Clin Nutr. 2011;93:1312-1320.
17. Nour N, Barbieri RL. Optimize detection and treatment of iron deficiency in pregnancy. OBG Manag. 2022;34:9-11.
18. Mead NC, Sakkatos P, Sakellaropoulos GC, et al. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg Obes Relat Dis. 2014;10:1166-1173.
19. Achebe MM, Gafter-Gvili A. How I treat anemia in pregnancy: iron, cobalamin and folate. Blood. 2017;129:940-949.
20. Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:735-746.
21. Wen Z, Shen M, Wu C, et al. Chewing gum for intestinal function recovery after caesarean section: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2017;17:105.
22. McCoy JA, Gutman S, Hamm RF, et al. The association between implementation of an enhanced recovery after cesarean pathway with standardized discharge prescriptions and opioid use and pain experience after cesarean delivery. Am J Perinatol. 2021;38:1341-1347.
23. Mullman L, Hilden P, Goral J, et al. Improved outcomes with an enhanced recovery approach to cesarean delivery. Obstet Gynecol. 2020;136:685-691.
24. Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
25. Sendlhofer G, Lumenta DB, Leitgeb K, et al. The gap between individual perception and compliance: a quantitative follow-up study of the surgical safety checklist application. PLoS One. 2016;11:e0149212.
26. Sultan P, Sharawi N, Blake L, et al. Impact of enhanced recovery after cesarean delivery on maternal outcomes: a systematic review and meta-analysis. Anaesth Crit Care Pain Med. 2021;40:100935.
27. Meng X, Chen K, Yang C, et al. The clinical efficacy and safety of enhanced recovery after surgery for cesarean section: a systematic review and meta-analysis of randomized controlled trials and observational studies. Front Med. 2021;8:694385.
28. Corson E, Hind D, Beever D, et al. Enhanced recovery after elective caesarean: a rapid review of clinical protocols and an umbrella review of systematic reviews. BMC Pregnancy Childbirth. 2017;17:91.
1. Molina G, Weiser RG, Lipsitz SR, et al. Relationship between cesarean delivery rate and maternal and neonatal mortality. JAMA. 2015;314:2263-2270.
2. Hamilton BE, Martin JA, Osterman MJK. Births: provisional data for 2021. Vital Statistics Release; No. 20. Hyattsville, MD: National Center for Health Statistics. May 2022. https://www.cdc.gov/nchs/data/vsrr/vsrr020.pdf.
3. Berian JR, Ban KA, Liu JB, et al. Adherence to enhanced recovery protocols in NSQIP and association with colectomy outcomes. Ann Surg. 2019;486-493.
4. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292-298.
5. Wilson RD, Caughey AB, Wood SL, et al. Guidelines for antenatal and preoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 1). Am J Obstet Gynecol. 2018;219:523.e1-523.e15.
6. Caughey AB, Wood SL, Macones GA, et al Guidelines for intraoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 2). Am J Obstet Gynecol. 2018;219:533-544.
7. Macones GA, Caughey AB, Wood SL, et al. Guidelines for postoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 3). Am J Obstet Gynecol. 2019;221:247.e1-247.e9.
8. Bollag L, Lim G, Sultan P, et al. Society for Obstetric Anesthesia and Perinatology: Consensus statement and recommendations for enhanced recovery after cesarean. Anesth Analg. 2021;132:1362-1377.
9. Perioperative pathways: enhanced recovery after surgery. ACOG Committee Opinion No 750. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e120-130.
10. University of Michigan. ERAS: A patient education video. https://www.youtube.com/watch?v=CoFtgdluBc0. Accessed October 24, 2022.
11. University of Maryland. ERAS. https://www.umms.org/ummc/health-services/womens-health/ostetrics-gynecology/pregnancy-childbirth/labor-delivery/enhanced-recovery-after-cesarean. Accessed October 24, 2022.
12. Bilku DK, Dennison AR, Hall TC, et al. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
13. Popivanov P, Irwin R, Walsh M, et al. Gastric emptying of carbohydrate drinks in term parturients before elective caesarean surgery: an observational study. Int J Obstet Anesth. 2020;41:29-34.
14. He Y, Liu C, Han Y, et al. The impact of carbohydrate-rich supplement taken two hours before caesarean delivery on maternal and neonatal perioperative outcomes- a randomized clinical trial. BMC Pregnancy Childbirth. 2021;21:682.
15. Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med. 2021;34:1002-1005.
16. Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1996-2006. Am J Clin Nutr. 2011;93:1312-1320.
17. Nour N, Barbieri RL. Optimize detection and treatment of iron deficiency in pregnancy. OBG Manag. 2022;34:9-11.
18. Mead NC, Sakkatos P, Sakellaropoulos GC, et al. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg Obes Relat Dis. 2014;10:1166-1173.
19. Achebe MM, Gafter-Gvili A. How I treat anemia in pregnancy: iron, cobalamin and folate. Blood. 2017;129:940-949.
20. Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:735-746.
21. Wen Z, Shen M, Wu C, et al. Chewing gum for intestinal function recovery after caesarean section: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2017;17:105.
22. McCoy JA, Gutman S, Hamm RF, et al. The association between implementation of an enhanced recovery after cesarean pathway with standardized discharge prescriptions and opioid use and pain experience after cesarean delivery. Am J Perinatol. 2021;38:1341-1347.
23. Mullman L, Hilden P, Goral J, et al. Improved outcomes with an enhanced recovery approach to cesarean delivery. Obstet Gynecol. 2020;136:685-691.
24. Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
25. Sendlhofer G, Lumenta DB, Leitgeb K, et al. The gap between individual perception and compliance: a quantitative follow-up study of the surgical safety checklist application. PLoS One. 2016;11:e0149212.
26. Sultan P, Sharawi N, Blake L, et al. Impact of enhanced recovery after cesarean delivery on maternal outcomes: a systematic review and meta-analysis. Anaesth Crit Care Pain Med. 2021;40:100935.
27. Meng X, Chen K, Yang C, et al. The clinical efficacy and safety of enhanced recovery after surgery for cesarean section: a systematic review and meta-analysis of randomized controlled trials and observational studies. Front Med. 2021;8:694385.
28. Corson E, Hind D, Beever D, et al. Enhanced recovery after elective caesarean: a rapid review of clinical protocols and an umbrella review of systematic reviews. BMC Pregnancy Childbirth. 2017;17:91.
“Blind” endometrial sampling: A call to end the practice
Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.
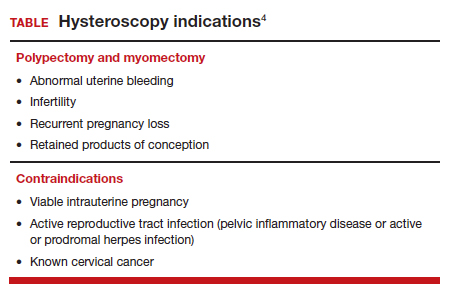
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.

Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.

OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.

Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.

OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.
Younger doctors call for more attention to patients with disabilities
As an undergraduate student at Northeastern University in Boston, Meghan Chin spent her summers working for a day program in Rhode Island. Her charges were adults with various forms of intellectual and developmental disabilities (IDD).
“I was very much a caretaker,” Ms. Chin, now 29, said. “It was everything from helping them get dressed in the morning to getting them to medical appointments.”
During one such visit Ms. Chin got a lesson about how health care looks from the viewpoint of someone with an IDD.
The patient was a woman in her 60s and she was having gastrointestinal issues; symptoms she could have articulated, if asked. “She was perfectly capable of telling a clinician where it hurt, how long she had experienced the problem, and what she had done or not done to alleviate it,” Ms. Chin said.
And of comprehending a response. But she was not given the opportunity.
“She would explain what was going on to the clinician,” Ms. Chin recalled. “And the clinician would turn to me and answer. It was this weird three-way conversation – as if she wasn’t even there in the room with us.”
Ms. Chin was incensed at the rude and disrespectful way the patient had been treated. But her charge didn’t seem upset or surprised. Just resigned. “Sadly, she had become used to this,” Ms. Chin said.
For the young aide, however, the experience was searing. “It didn’t seem right to me,” Ms. Chin said. “That’s why, when I went to medical school, I knew I wanted to do better for this population.”
Serendipity led her to Georgetown University, Washington, where she met Kim Bullock, MD, one of the country’s leading advocates for improved health care delivery to those with IDDs.
Dr. Bullock, an associate professor of family medicine, seeks to create better training and educational opportunities for medical students who will likely encounter patients with these disabilities in their practices.
When Dr. Bullock heard Ms. Chin’s story about the patient being ignored, she was not surprised.
“This is not an unusual or unique situation,” said Dr. Bullock, who is also director of Georgetown’s community health division and a faculty member of the university’s Center for Excellence for Developmental Disabilities. “In fact, it’s quite common and is part of what spurred my own interest in educating pre-med and medical students about effective communication techniques, particularly when addressing neurodiverse patients.”
More than 13% of Americans, or roughly 44 million people, have some form of disability, according to the National Institute on Disability at the University of New Hampshire, a figure that does not include those who are institutionalized. The Centers for Disease Control and Prevention estimates that 17% of children aged 3-17 years have a developmental disability.
Even so, many physicians feel ill-prepared to care for disabled patients. A survey of physicians, published in the journal Health Affairs, found that some lacked the resources and training to properly care for patients with disabilities, or that they struggled to coordinate care for such individuals. Some said they did not know which types of accessible equipment, like adjustable tables and chair scales, were needed or how to use them. And some said they actively try to avoid treating patients with disabilities.
Don’t assume
The first step at correcting the problem, Dr. Bullock said, is to not assume that all IDD patients are incapable of communicating. By talking not to the patient but to their caregiver or spouse or child, as the clinician did with Ms. Chin years ago, “we are taking away their agency, their autonomy to speak for and about themselves.”
Change involves altering physicians’ attitudes and assumptions toward this population, through education. But how?
“The medical school curriculum is tight as it is,” Dr. Bullock acknowledged. “There’s a lot of things students have to learn. People wonder: where we will add this?”
Her suggestion: Incorporate IDD all along the way, through programs or experiences that will enable medical students to see such patients “not as something separate, but as people that have special needs just as other populations have.”
Case in point: Operation House Call, a program in Massachusetts designed to support young health care professionals, by building “confidence, interest, and sensitivity” toward individuals with IDD.
Eight medical and allied health schools, including those at Harvard Medical School and Yale School of Nursing, participate in the program, the centerpiece of which is time spent by teams of medical students in the homes of families with neurodiverse members. “It’s transformational,” said Susan Feeney, DNP, NP-C, director of adult gerontology and family nurse practitioner programs at the graduate school of nursing at the University of Massachusetts, Worcester. “They spend a few hours at the homes of these families, have this interaction with them, and journal about their experiences.”
Dr. Feeney described as “transformational” the experience of the students after getting to know these families. “They all come back profoundly changed,” she told this news organization. “As a medical or health care professional, you meet people in an artificial environment of the clinic and hospital. Here, they become human, like you. It takes the stigma away.”
One area of medicine in which this is an exception is pediatrics, where interaction with children with IDD and their families is common – and close. “They’re going to be much more attuned to this,” Dr. Feeney said. “The problem is primary care or internal medicine. Once these children get into their mid and later 20s, and they need a practitioner to talk to about adult concerns.”
And with adulthood come other medical needs, as the physical demands of age fall no less heavily on individuals with IDDs than those without. For example: “Neurodiverse people get pregnant,” Dr. Bullock said. They also can get heart disease as they age; or require the care of a rheumatologist, a neurologist, an orthopedic surgeon, or any other medical specialty.
Generation gap
Fortunately, the next generation of physicians may be more open to this more inclusionary approach toward a widely misunderstood population.
Like Ms. Chin, Sarah Bdeir had experience with this population prior to beginning her training in medicine. She had volunteered at a school for people with IDD.
“It was one of the best experiences I’ve ever had,” Ms. Bdeir, now 23 and a first-year medical student at Wayne State University, Detroit, said. She found that the neurodiverse individuals she worked with had as many abilities as disabilities. “They are capable of learning, but they do it differently,” she said. “You have to adjust to the way they learn. And you have to step out of your own box.”
Ms. Bdeir also heard about Dr. Bullock’s work and is assisting her in a research project on how to better improve nutritional education for people with IDDs. And although she said it may take time for curriculum boards at medical schools to integrate this kind of training into their programs, she believes they will, in part because the rising cohort of medical students today have an eagerness to engage with and learn more about IDD patients.
As does Ms. Chin.
“When I talk to my peers about this, they’re very receptive,” Ms. Chin said. “They want to learn how to better support the IDD population. And they will learn. I believe in my generation of future doctors.”
A version of this article first appeared on Medscape.com.
As an undergraduate student at Northeastern University in Boston, Meghan Chin spent her summers working for a day program in Rhode Island. Her charges were adults with various forms of intellectual and developmental disabilities (IDD).
“I was very much a caretaker,” Ms. Chin, now 29, said. “It was everything from helping them get dressed in the morning to getting them to medical appointments.”
During one such visit Ms. Chin got a lesson about how health care looks from the viewpoint of someone with an IDD.
The patient was a woman in her 60s and she was having gastrointestinal issues; symptoms she could have articulated, if asked. “She was perfectly capable of telling a clinician where it hurt, how long she had experienced the problem, and what she had done or not done to alleviate it,” Ms. Chin said.
And of comprehending a response. But she was not given the opportunity.
“She would explain what was going on to the clinician,” Ms. Chin recalled. “And the clinician would turn to me and answer. It was this weird three-way conversation – as if she wasn’t even there in the room with us.”
Ms. Chin was incensed at the rude and disrespectful way the patient had been treated. But her charge didn’t seem upset or surprised. Just resigned. “Sadly, she had become used to this,” Ms. Chin said.
For the young aide, however, the experience was searing. “It didn’t seem right to me,” Ms. Chin said. “That’s why, when I went to medical school, I knew I wanted to do better for this population.”
Serendipity led her to Georgetown University, Washington, where she met Kim Bullock, MD, one of the country’s leading advocates for improved health care delivery to those with IDDs.
Dr. Bullock, an associate professor of family medicine, seeks to create better training and educational opportunities for medical students who will likely encounter patients with these disabilities in their practices.
When Dr. Bullock heard Ms. Chin’s story about the patient being ignored, she was not surprised.
“This is not an unusual or unique situation,” said Dr. Bullock, who is also director of Georgetown’s community health division and a faculty member of the university’s Center for Excellence for Developmental Disabilities. “In fact, it’s quite common and is part of what spurred my own interest in educating pre-med and medical students about effective communication techniques, particularly when addressing neurodiverse patients.”
More than 13% of Americans, or roughly 44 million people, have some form of disability, according to the National Institute on Disability at the University of New Hampshire, a figure that does not include those who are institutionalized. The Centers for Disease Control and Prevention estimates that 17% of children aged 3-17 years have a developmental disability.
Even so, many physicians feel ill-prepared to care for disabled patients. A survey of physicians, published in the journal Health Affairs, found that some lacked the resources and training to properly care for patients with disabilities, or that they struggled to coordinate care for such individuals. Some said they did not know which types of accessible equipment, like adjustable tables and chair scales, were needed or how to use them. And some said they actively try to avoid treating patients with disabilities.
Don’t assume
The first step at correcting the problem, Dr. Bullock said, is to not assume that all IDD patients are incapable of communicating. By talking not to the patient but to their caregiver or spouse or child, as the clinician did with Ms. Chin years ago, “we are taking away their agency, their autonomy to speak for and about themselves.”
Change involves altering physicians’ attitudes and assumptions toward this population, through education. But how?
“The medical school curriculum is tight as it is,” Dr. Bullock acknowledged. “There’s a lot of things students have to learn. People wonder: where we will add this?”
Her suggestion: Incorporate IDD all along the way, through programs or experiences that will enable medical students to see such patients “not as something separate, but as people that have special needs just as other populations have.”
Case in point: Operation House Call, a program in Massachusetts designed to support young health care professionals, by building “confidence, interest, and sensitivity” toward individuals with IDD.
Eight medical and allied health schools, including those at Harvard Medical School and Yale School of Nursing, participate in the program, the centerpiece of which is time spent by teams of medical students in the homes of families with neurodiverse members. “It’s transformational,” said Susan Feeney, DNP, NP-C, director of adult gerontology and family nurse practitioner programs at the graduate school of nursing at the University of Massachusetts, Worcester. “They spend a few hours at the homes of these families, have this interaction with them, and journal about their experiences.”
Dr. Feeney described as “transformational” the experience of the students after getting to know these families. “They all come back profoundly changed,” she told this news organization. “As a medical or health care professional, you meet people in an artificial environment of the clinic and hospital. Here, they become human, like you. It takes the stigma away.”
One area of medicine in which this is an exception is pediatrics, where interaction with children with IDD and their families is common – and close. “They’re going to be much more attuned to this,” Dr. Feeney said. “The problem is primary care or internal medicine. Once these children get into their mid and later 20s, and they need a practitioner to talk to about adult concerns.”
And with adulthood come other medical needs, as the physical demands of age fall no less heavily on individuals with IDDs than those without. For example: “Neurodiverse people get pregnant,” Dr. Bullock said. They also can get heart disease as they age; or require the care of a rheumatologist, a neurologist, an orthopedic surgeon, or any other medical specialty.
Generation gap
Fortunately, the next generation of physicians may be more open to this more inclusionary approach toward a widely misunderstood population.
Like Ms. Chin, Sarah Bdeir had experience with this population prior to beginning her training in medicine. She had volunteered at a school for people with IDD.
“It was one of the best experiences I’ve ever had,” Ms. Bdeir, now 23 and a first-year medical student at Wayne State University, Detroit, said. She found that the neurodiverse individuals she worked with had as many abilities as disabilities. “They are capable of learning, but they do it differently,” she said. “You have to adjust to the way they learn. And you have to step out of your own box.”
Ms. Bdeir also heard about Dr. Bullock’s work and is assisting her in a research project on how to better improve nutritional education for people with IDDs. And although she said it may take time for curriculum boards at medical schools to integrate this kind of training into their programs, she believes they will, in part because the rising cohort of medical students today have an eagerness to engage with and learn more about IDD patients.
As does Ms. Chin.
“When I talk to my peers about this, they’re very receptive,” Ms. Chin said. “They want to learn how to better support the IDD population. And they will learn. I believe in my generation of future doctors.”
A version of this article first appeared on Medscape.com.
As an undergraduate student at Northeastern University in Boston, Meghan Chin spent her summers working for a day program in Rhode Island. Her charges were adults with various forms of intellectual and developmental disabilities (IDD).
“I was very much a caretaker,” Ms. Chin, now 29, said. “It was everything from helping them get dressed in the morning to getting them to medical appointments.”
During one such visit Ms. Chin got a lesson about how health care looks from the viewpoint of someone with an IDD.
The patient was a woman in her 60s and she was having gastrointestinal issues; symptoms she could have articulated, if asked. “She was perfectly capable of telling a clinician where it hurt, how long she had experienced the problem, and what she had done or not done to alleviate it,” Ms. Chin said.
And of comprehending a response. But she was not given the opportunity.
“She would explain what was going on to the clinician,” Ms. Chin recalled. “And the clinician would turn to me and answer. It was this weird three-way conversation – as if she wasn’t even there in the room with us.”
Ms. Chin was incensed at the rude and disrespectful way the patient had been treated. But her charge didn’t seem upset or surprised. Just resigned. “Sadly, she had become used to this,” Ms. Chin said.
For the young aide, however, the experience was searing. “It didn’t seem right to me,” Ms. Chin said. “That’s why, when I went to medical school, I knew I wanted to do better for this population.”
Serendipity led her to Georgetown University, Washington, where she met Kim Bullock, MD, one of the country’s leading advocates for improved health care delivery to those with IDDs.
Dr. Bullock, an associate professor of family medicine, seeks to create better training and educational opportunities for medical students who will likely encounter patients with these disabilities in their practices.
When Dr. Bullock heard Ms. Chin’s story about the patient being ignored, she was not surprised.
“This is not an unusual or unique situation,” said Dr. Bullock, who is also director of Georgetown’s community health division and a faculty member of the university’s Center for Excellence for Developmental Disabilities. “In fact, it’s quite common and is part of what spurred my own interest in educating pre-med and medical students about effective communication techniques, particularly when addressing neurodiverse patients.”
More than 13% of Americans, or roughly 44 million people, have some form of disability, according to the National Institute on Disability at the University of New Hampshire, a figure that does not include those who are institutionalized. The Centers for Disease Control and Prevention estimates that 17% of children aged 3-17 years have a developmental disability.
Even so, many physicians feel ill-prepared to care for disabled patients. A survey of physicians, published in the journal Health Affairs, found that some lacked the resources and training to properly care for patients with disabilities, or that they struggled to coordinate care for such individuals. Some said they did not know which types of accessible equipment, like adjustable tables and chair scales, were needed or how to use them. And some said they actively try to avoid treating patients with disabilities.
Don’t assume
The first step at correcting the problem, Dr. Bullock said, is to not assume that all IDD patients are incapable of communicating. By talking not to the patient but to their caregiver or spouse or child, as the clinician did with Ms. Chin years ago, “we are taking away their agency, their autonomy to speak for and about themselves.”
Change involves altering physicians’ attitudes and assumptions toward this population, through education. But how?
“The medical school curriculum is tight as it is,” Dr. Bullock acknowledged. “There’s a lot of things students have to learn. People wonder: where we will add this?”
Her suggestion: Incorporate IDD all along the way, through programs or experiences that will enable medical students to see such patients “not as something separate, but as people that have special needs just as other populations have.”
Case in point: Operation House Call, a program in Massachusetts designed to support young health care professionals, by building “confidence, interest, and sensitivity” toward individuals with IDD.
Eight medical and allied health schools, including those at Harvard Medical School and Yale School of Nursing, participate in the program, the centerpiece of which is time spent by teams of medical students in the homes of families with neurodiverse members. “It’s transformational,” said Susan Feeney, DNP, NP-C, director of adult gerontology and family nurse practitioner programs at the graduate school of nursing at the University of Massachusetts, Worcester. “They spend a few hours at the homes of these families, have this interaction with them, and journal about their experiences.”
Dr. Feeney described as “transformational” the experience of the students after getting to know these families. “They all come back profoundly changed,” she told this news organization. “As a medical or health care professional, you meet people in an artificial environment of the clinic and hospital. Here, they become human, like you. It takes the stigma away.”
One area of medicine in which this is an exception is pediatrics, where interaction with children with IDD and their families is common – and close. “They’re going to be much more attuned to this,” Dr. Feeney said. “The problem is primary care or internal medicine. Once these children get into their mid and later 20s, and they need a practitioner to talk to about adult concerns.”
And with adulthood come other medical needs, as the physical demands of age fall no less heavily on individuals with IDDs than those without. For example: “Neurodiverse people get pregnant,” Dr. Bullock said. They also can get heart disease as they age; or require the care of a rheumatologist, a neurologist, an orthopedic surgeon, or any other medical specialty.
Generation gap
Fortunately, the next generation of physicians may be more open to this more inclusionary approach toward a widely misunderstood population.
Like Ms. Chin, Sarah Bdeir had experience with this population prior to beginning her training in medicine. She had volunteered at a school for people with IDD.
“It was one of the best experiences I’ve ever had,” Ms. Bdeir, now 23 and a first-year medical student at Wayne State University, Detroit, said. She found that the neurodiverse individuals she worked with had as many abilities as disabilities. “They are capable of learning, but they do it differently,” she said. “You have to adjust to the way they learn. And you have to step out of your own box.”
Ms. Bdeir also heard about Dr. Bullock’s work and is assisting her in a research project on how to better improve nutritional education for people with IDDs. And although she said it may take time for curriculum boards at medical schools to integrate this kind of training into their programs, she believes they will, in part because the rising cohort of medical students today have an eagerness to engage with and learn more about IDD patients.
As does Ms. Chin.
“When I talk to my peers about this, they’re very receptive,” Ms. Chin said. “They want to learn how to better support the IDD population. And they will learn. I believe in my generation of future doctors.”
A version of this article first appeared on Medscape.com.
Fertility physicians say they lack access to miscarriage drugs
In a survey taken before the Supreme Court’s Dobbs ruling regarding abortion rights, two-thirds of assisted reproduction technology (ART) physicians who don’t offer mifepristone/misoprostol to patients with early pregnancy loss (EPL) reported that they lack access to the drugs.
The numbers are likely higher now. In the wake of the court ruling, some physicians in states with new abortion restrictions fear they won’t be able to properly treat women with miscarriages. Access to mifepristone, a component of medication abortions along with misoprostol, is at the center of their concerns.
“These restrictions that were put in place to restrict abortion care have far-reaching implications regarding miscarriages and early pregnancy loss and the assisted reproduction community is not immune,” obstetrics and gynecology specialist Zachary Anderson, MD, a resident physician at the University of Southern California, Los Angeles, said in an interview. He presented the findings at the American Society for Reproductive Medicine’s 2022 meeting.
Early pregnancy loss – defined as a miscarriage within 12 weeks and 6 days of conception – is common in all pregnancies and affects an estimated 15% of those who rely on in vitro fertilization (IVF). In women who conceive through intrauterine insemination or IVF, “an abnormal karyotype embryo/fetus is the cause of miscarriage in more than two-thirds of cases,” Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview. “The options of management are observation – with no ability to determine when passage of the products of conception will occur – vs. mifepristone/misoprostol or suction D&C.”
Dr. Trolice added that “most woman select the medical treatment protocol, which is 200 mg mifepristone orally followed by 800 mcg misoprostol vaginally 24 hours later. If no signs of heavy bleeding occur after 3 hours following misoprostol, the patient should repeat the dose of 800 micrograms vaginally.”
According to the Reuters news service, some abortion bans target mifepristone. In October 2022, the American College of Obstetricians and Gynecologists asked the Food and Drug Administration to approve mifepristone for use in miscarriage management; such use is now off label, although it is approved to end early pregnancies in conjunction with misoprostol.
For the new study, researchers sent anonymous surveys to 826 members of the Society of Reproductive Endocrinology and Infertility and received 101 responses (12% response rate, 51% women, 86% non-Hispanic White, average age 52, 52% urban, and 51% in private practice).
More than two-thirds (70%) said they diagnosed early pregnancy loss at least once a week; 47% prefer treatment with misoprostol alone, 18% surgery in an operating room, 15% expectant management (monitoring a miscarriage as it occurs without medical intervention), 10% surgery in the office, and 3% mifepristone-misoprostol.
Of those who don’t offer mifepristone-misoprostol, 68% said they lack access, and 26% said they lack familiarity with the treatment.
Study coauthor Brian T. Nguyen, MD, MSc, assistant professor of obstetrics and gynecology at USC, said in an interview that mifepristone, a highly effective drug, is treated differently from other medications “for no good reason.”
Dr. Anderson, who led the study, urged colleagues to get the appropriate certification to prescribe mifepristone. “Providers overestimate how difficult it is to become certified to prescribe it,” he said.
Dr. Trolice, who is familiar with the study findings, said the response rate is low, and the results might be biased because those with preconceived opinions may be more likely to respond.
However, he said, “The results are not surprising in that medication is more commonly preferred (nearly 50%) given the devastation of a miscarriage and the desire to expedite resolution. Approximately one-third prefer surgical management, which would allow for genetic testing of the embryo/fetus to potentially determine a cause of the pregnancy loss.”
As for the medications used to treat early pregnancy loss, many ART physicians “treat pregnancy loss with misoprostol both pre- and post Dobbs,” he said. “The difficulty in obtaining mifepristone remains.”
The study authors and Dr. Trolice report no disclosures.
In a survey taken before the Supreme Court’s Dobbs ruling regarding abortion rights, two-thirds of assisted reproduction technology (ART) physicians who don’t offer mifepristone/misoprostol to patients with early pregnancy loss (EPL) reported that they lack access to the drugs.
The numbers are likely higher now. In the wake of the court ruling, some physicians in states with new abortion restrictions fear they won’t be able to properly treat women with miscarriages. Access to mifepristone, a component of medication abortions along with misoprostol, is at the center of their concerns.
“These restrictions that were put in place to restrict abortion care have far-reaching implications regarding miscarriages and early pregnancy loss and the assisted reproduction community is not immune,” obstetrics and gynecology specialist Zachary Anderson, MD, a resident physician at the University of Southern California, Los Angeles, said in an interview. He presented the findings at the American Society for Reproductive Medicine’s 2022 meeting.
Early pregnancy loss – defined as a miscarriage within 12 weeks and 6 days of conception – is common in all pregnancies and affects an estimated 15% of those who rely on in vitro fertilization (IVF). In women who conceive through intrauterine insemination or IVF, “an abnormal karyotype embryo/fetus is the cause of miscarriage in more than two-thirds of cases,” Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview. “The options of management are observation – with no ability to determine when passage of the products of conception will occur – vs. mifepristone/misoprostol or suction D&C.”
Dr. Trolice added that “most woman select the medical treatment protocol, which is 200 mg mifepristone orally followed by 800 mcg misoprostol vaginally 24 hours later. If no signs of heavy bleeding occur after 3 hours following misoprostol, the patient should repeat the dose of 800 micrograms vaginally.”
According to the Reuters news service, some abortion bans target mifepristone. In October 2022, the American College of Obstetricians and Gynecologists asked the Food and Drug Administration to approve mifepristone for use in miscarriage management; such use is now off label, although it is approved to end early pregnancies in conjunction with misoprostol.
For the new study, researchers sent anonymous surveys to 826 members of the Society of Reproductive Endocrinology and Infertility and received 101 responses (12% response rate, 51% women, 86% non-Hispanic White, average age 52, 52% urban, and 51% in private practice).
More than two-thirds (70%) said they diagnosed early pregnancy loss at least once a week; 47% prefer treatment with misoprostol alone, 18% surgery in an operating room, 15% expectant management (monitoring a miscarriage as it occurs without medical intervention), 10% surgery in the office, and 3% mifepristone-misoprostol.
Of those who don’t offer mifepristone-misoprostol, 68% said they lack access, and 26% said they lack familiarity with the treatment.
Study coauthor Brian T. Nguyen, MD, MSc, assistant professor of obstetrics and gynecology at USC, said in an interview that mifepristone, a highly effective drug, is treated differently from other medications “for no good reason.”
Dr. Anderson, who led the study, urged colleagues to get the appropriate certification to prescribe mifepristone. “Providers overestimate how difficult it is to become certified to prescribe it,” he said.
Dr. Trolice, who is familiar with the study findings, said the response rate is low, and the results might be biased because those with preconceived opinions may be more likely to respond.
However, he said, “The results are not surprising in that medication is more commonly preferred (nearly 50%) given the devastation of a miscarriage and the desire to expedite resolution. Approximately one-third prefer surgical management, which would allow for genetic testing of the embryo/fetus to potentially determine a cause of the pregnancy loss.”
As for the medications used to treat early pregnancy loss, many ART physicians “treat pregnancy loss with misoprostol both pre- and post Dobbs,” he said. “The difficulty in obtaining mifepristone remains.”
The study authors and Dr. Trolice report no disclosures.
In a survey taken before the Supreme Court’s Dobbs ruling regarding abortion rights, two-thirds of assisted reproduction technology (ART) physicians who don’t offer mifepristone/misoprostol to patients with early pregnancy loss (EPL) reported that they lack access to the drugs.
The numbers are likely higher now. In the wake of the court ruling, some physicians in states with new abortion restrictions fear they won’t be able to properly treat women with miscarriages. Access to mifepristone, a component of medication abortions along with misoprostol, is at the center of their concerns.
“These restrictions that were put in place to restrict abortion care have far-reaching implications regarding miscarriages and early pregnancy loss and the assisted reproduction community is not immune,” obstetrics and gynecology specialist Zachary Anderson, MD, a resident physician at the University of Southern California, Los Angeles, said in an interview. He presented the findings at the American Society for Reproductive Medicine’s 2022 meeting.
Early pregnancy loss – defined as a miscarriage within 12 weeks and 6 days of conception – is common in all pregnancies and affects an estimated 15% of those who rely on in vitro fertilization (IVF). In women who conceive through intrauterine insemination or IVF, “an abnormal karyotype embryo/fetus is the cause of miscarriage in more than two-thirds of cases,” Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview. “The options of management are observation – with no ability to determine when passage of the products of conception will occur – vs. mifepristone/misoprostol or suction D&C.”
Dr. Trolice added that “most woman select the medical treatment protocol, which is 200 mg mifepristone orally followed by 800 mcg misoprostol vaginally 24 hours later. If no signs of heavy bleeding occur after 3 hours following misoprostol, the patient should repeat the dose of 800 micrograms vaginally.”
According to the Reuters news service, some abortion bans target mifepristone. In October 2022, the American College of Obstetricians and Gynecologists asked the Food and Drug Administration to approve mifepristone for use in miscarriage management; such use is now off label, although it is approved to end early pregnancies in conjunction with misoprostol.
For the new study, researchers sent anonymous surveys to 826 members of the Society of Reproductive Endocrinology and Infertility and received 101 responses (12% response rate, 51% women, 86% non-Hispanic White, average age 52, 52% urban, and 51% in private practice).
More than two-thirds (70%) said they diagnosed early pregnancy loss at least once a week; 47% prefer treatment with misoprostol alone, 18% surgery in an operating room, 15% expectant management (monitoring a miscarriage as it occurs without medical intervention), 10% surgery in the office, and 3% mifepristone-misoprostol.
Of those who don’t offer mifepristone-misoprostol, 68% said they lack access, and 26% said they lack familiarity with the treatment.
Study coauthor Brian T. Nguyen, MD, MSc, assistant professor of obstetrics and gynecology at USC, said in an interview that mifepristone, a highly effective drug, is treated differently from other medications “for no good reason.”
Dr. Anderson, who led the study, urged colleagues to get the appropriate certification to prescribe mifepristone. “Providers overestimate how difficult it is to become certified to prescribe it,” he said.
Dr. Trolice, who is familiar with the study findings, said the response rate is low, and the results might be biased because those with preconceived opinions may be more likely to respond.
However, he said, “The results are not surprising in that medication is more commonly preferred (nearly 50%) given the devastation of a miscarriage and the desire to expedite resolution. Approximately one-third prefer surgical management, which would allow for genetic testing of the embryo/fetus to potentially determine a cause of the pregnancy loss.”
As for the medications used to treat early pregnancy loss, many ART physicians “treat pregnancy loss with misoprostol both pre- and post Dobbs,” he said. “The difficulty in obtaining mifepristone remains.”
The study authors and Dr. Trolice report no disclosures.
FROM ASRM 2022
Immediate skin-to-skin contact after cesarean section improves outcomes for parent, newborn
Birth parents are typically separated from their newborns following a cesarean section. However, a recent study published in the journal Nursing Open suggests immediate skin-to-skin contact may accelerate uterine contractions, reduce maternal blood loss, reduce newborn crying, improve patient satisfaction and comfort, and increase the rate of breastfeeding.
“[O]ur study contributes to scientific knowledge with key information to reduce maternal morbidity and mortality rates in mothers who have undergone scheduled cesarean sections,” José Miguel Pérez-Jiménez, MD, of the faculty of nursing, physiotherapy, and podiatry at Hospital Universitario Virgen Macarena, University of Sevilla, Spain, and colleagues wrote in their study. It promotes greater stability in the mothers by reducing the risk of postpartum hemorrhage, making it better to not separate mother and child in the first hours after this surgery, he said.
Dr. Pérez-Jiménez and colleagues evaluated 83 women who underwent a scheduled cesarean section in an unblinded, randomized controlled trial. The women were randomized to receive skin-to-skin contact in the operating room that continued in the postpartum unit, or the normal protocol after cesarean section that consisted of having the mother transferred to the postanesthesia recovery room while the newborn was sent to a maternity room with a parent or companion. Researchers assessed variables such as plasma hemoglobin, uterine contractions, breastfeeding, and postoperative pain, as well as subjective measures such as maternal satisfaction, comfort, previous cesarean section experience, and newborn crying.
Women who received usual care following cesarean section were more likely to have uterine contractions at the umbilical level compared with the skin-to-skin contact group (70% vs. 3%; P ≤ .0001), while the skin-to-skin group was more likely to have uterine contractions at the infraumbilical level (92.5% vs. 22.5%; P ≤ .0001). There was a statistically significant decrease in predischarge hemoglobin in the control group compared with the skin-to-skin group (10.522 vs. 11.075 g/dL; P ≤ .017); the level of hemoglobin reduction favored the skin-to-skin group (1.01 vs. 2.265 g/dL; P ≤ .0001). Women in the skin-to-skin group were more likely to report mild pain on a 10-point visual analog scale (VAS) after being transferred to the recovery room (1.48 vs. 6.23 points; P ≤ .0001) and being transferred to a maternity room or room in the postpartum unit (0.60 vs. 5.23 points; P ≤ .0001). Breastfeeding at birth was significantly higher among patients with immediate skin-to-skin contact compared with the control group (92.5% vs. 32.5%; P ≤ .0001), and continued at 1 month after birth (92.5% vs. 12.5%; P ≤ .0001). Newborns of mothers in the skin-to-skin group were significantly less likely to cry compared with newborns in the control group (90% vs. 55%; P ≤ .001).
When asked to rate their satisfaction on a 10-point Likert scale, women in the skin-to-skin contact group rated their experience significantly higher than did the control group (9.98 vs. 6.5; P ≤ .0001), and all women who had previously had a cesarean section in the skin-to-skin group (30%) rated their experience at 10 points compared with their previous cesarean section without skin-to-skin contact.
Implementing skin-to-skin contact after cesarean section
Betsy M. Collins, MD, MPH, assistant professor of obstetrics and gynecology at Emory University, Atlanta, said in an interview that while some of the findings were largely unsurprising and “confirmed a lot of the things that we already know about skin-to-skin [contact],” one major finding was the “stark difference” in the percentage of new birth parents who started breastfeeding after skin-to-skin contact and were still breastfeeding at 1 month postpartum compared with birth parents in the control group. She was not involved with the study and noted that the results complement recommendations from the World Health Organization on starting breastfeeding within the first hour after birth and continuing breastfeeding through the first 6 months of life.
“That was likely one of the greatest take-home points from the study ... that early skin-to-skin really promoted initiation of breastfeeding,” Dr. Collins said.
Two reasons why skin-to-skin contact after cesarean section isn’t regularly provided is that it can be difficult for personnel and safety reasons to have an extra nurse to continue monitoring the health of the newborn in the operating room, and there is a lack of culture supporting of skin-to-skin contact in the OR, Dr. Collins explained.
“Just like anything else, if it’s built into your standard operating procedure, then you have everything set up in place to do that initial assessment of the infant and then get the baby skin-to-skin as quickly as possible,” she said. If it’s your standard operating procedure to not provide skin-to-skin contact, she said, then there is a little bit more inertia to overcome to start providing it as a standard procedure.
At her center, Dr. Collins said skin-to-skin contact is initiated as soon as possible after birth, even in the operating room. The steps to implementing that policy involved getting the anesthesiology department on board with supporting the policy in the OR and training the circulating nursing staff to ensure a that nurse is available to monitor the newborn.
“I think the most important thing to know is that it’s absolutely doable and that you just have to have a champion just like any other quality initiative,” she said. One of the best ways to do that is to have the patients themselves request it, she noted, compared with its being requested by a physician or nurse.
“I think some patients are disappointed when they have to undergo cesarean delivery or feel like they’re missing out if they can’t have a vaginal delivery,” Dr. Collins said. Immediate skin-to-skin contact is “very good for not only physiology, as we read about in this paper – all the things they said about the benefits of skin-to-skin [contact] are true – but it’s really good for mental health. That bonding begins right away.”
As a birth parent, being separated from your newborn for several hours after a cesarean section, on the other hand, can be “pretty devastating,” Dr. Collins said.
“I think this is something that, once it becomes a standard of care, it will be expected that most hospitals should be doing this,” she said.
The authors and Dr. Collins report no relevant conflicts of interest.
Birth parents are typically separated from their newborns following a cesarean section. However, a recent study published in the journal Nursing Open suggests immediate skin-to-skin contact may accelerate uterine contractions, reduce maternal blood loss, reduce newborn crying, improve patient satisfaction and comfort, and increase the rate of breastfeeding.
“[O]ur study contributes to scientific knowledge with key information to reduce maternal morbidity and mortality rates in mothers who have undergone scheduled cesarean sections,” José Miguel Pérez-Jiménez, MD, of the faculty of nursing, physiotherapy, and podiatry at Hospital Universitario Virgen Macarena, University of Sevilla, Spain, and colleagues wrote in their study. It promotes greater stability in the mothers by reducing the risk of postpartum hemorrhage, making it better to not separate mother and child in the first hours after this surgery, he said.
Dr. Pérez-Jiménez and colleagues evaluated 83 women who underwent a scheduled cesarean section in an unblinded, randomized controlled trial. The women were randomized to receive skin-to-skin contact in the operating room that continued in the postpartum unit, or the normal protocol after cesarean section that consisted of having the mother transferred to the postanesthesia recovery room while the newborn was sent to a maternity room with a parent or companion. Researchers assessed variables such as plasma hemoglobin, uterine contractions, breastfeeding, and postoperative pain, as well as subjective measures such as maternal satisfaction, comfort, previous cesarean section experience, and newborn crying.
Women who received usual care following cesarean section were more likely to have uterine contractions at the umbilical level compared with the skin-to-skin contact group (70% vs. 3%; P ≤ .0001), while the skin-to-skin group was more likely to have uterine contractions at the infraumbilical level (92.5% vs. 22.5%; P ≤ .0001). There was a statistically significant decrease in predischarge hemoglobin in the control group compared with the skin-to-skin group (10.522 vs. 11.075 g/dL; P ≤ .017); the level of hemoglobin reduction favored the skin-to-skin group (1.01 vs. 2.265 g/dL; P ≤ .0001). Women in the skin-to-skin group were more likely to report mild pain on a 10-point visual analog scale (VAS) after being transferred to the recovery room (1.48 vs. 6.23 points; P ≤ .0001) and being transferred to a maternity room or room in the postpartum unit (0.60 vs. 5.23 points; P ≤ .0001). Breastfeeding at birth was significantly higher among patients with immediate skin-to-skin contact compared with the control group (92.5% vs. 32.5%; P ≤ .0001), and continued at 1 month after birth (92.5% vs. 12.5%; P ≤ .0001). Newborns of mothers in the skin-to-skin group were significantly less likely to cry compared with newborns in the control group (90% vs. 55%; P ≤ .001).
When asked to rate their satisfaction on a 10-point Likert scale, women in the skin-to-skin contact group rated their experience significantly higher than did the control group (9.98 vs. 6.5; P ≤ .0001), and all women who had previously had a cesarean section in the skin-to-skin group (30%) rated their experience at 10 points compared with their previous cesarean section without skin-to-skin contact.
Implementing skin-to-skin contact after cesarean section
Betsy M. Collins, MD, MPH, assistant professor of obstetrics and gynecology at Emory University, Atlanta, said in an interview that while some of the findings were largely unsurprising and “confirmed a lot of the things that we already know about skin-to-skin [contact],” one major finding was the “stark difference” in the percentage of new birth parents who started breastfeeding after skin-to-skin contact and were still breastfeeding at 1 month postpartum compared with birth parents in the control group. She was not involved with the study and noted that the results complement recommendations from the World Health Organization on starting breastfeeding within the first hour after birth and continuing breastfeeding through the first 6 months of life.
“That was likely one of the greatest take-home points from the study ... that early skin-to-skin really promoted initiation of breastfeeding,” Dr. Collins said.
Two reasons why skin-to-skin contact after cesarean section isn’t regularly provided is that it can be difficult for personnel and safety reasons to have an extra nurse to continue monitoring the health of the newborn in the operating room, and there is a lack of culture supporting of skin-to-skin contact in the OR, Dr. Collins explained.
“Just like anything else, if it’s built into your standard operating procedure, then you have everything set up in place to do that initial assessment of the infant and then get the baby skin-to-skin as quickly as possible,” she said. If it’s your standard operating procedure to not provide skin-to-skin contact, she said, then there is a little bit more inertia to overcome to start providing it as a standard procedure.
At her center, Dr. Collins said skin-to-skin contact is initiated as soon as possible after birth, even in the operating room. The steps to implementing that policy involved getting the anesthesiology department on board with supporting the policy in the OR and training the circulating nursing staff to ensure a that nurse is available to monitor the newborn.
“I think the most important thing to know is that it’s absolutely doable and that you just have to have a champion just like any other quality initiative,” she said. One of the best ways to do that is to have the patients themselves request it, she noted, compared with its being requested by a physician or nurse.
“I think some patients are disappointed when they have to undergo cesarean delivery or feel like they’re missing out if they can’t have a vaginal delivery,” Dr. Collins said. Immediate skin-to-skin contact is “very good for not only physiology, as we read about in this paper – all the things they said about the benefits of skin-to-skin [contact] are true – but it’s really good for mental health. That bonding begins right away.”
As a birth parent, being separated from your newborn for several hours after a cesarean section, on the other hand, can be “pretty devastating,” Dr. Collins said.
“I think this is something that, once it becomes a standard of care, it will be expected that most hospitals should be doing this,” she said.
The authors and Dr. Collins report no relevant conflicts of interest.
Birth parents are typically separated from their newborns following a cesarean section. However, a recent study published in the journal Nursing Open suggests immediate skin-to-skin contact may accelerate uterine contractions, reduce maternal blood loss, reduce newborn crying, improve patient satisfaction and comfort, and increase the rate of breastfeeding.
“[O]ur study contributes to scientific knowledge with key information to reduce maternal morbidity and mortality rates in mothers who have undergone scheduled cesarean sections,” José Miguel Pérez-Jiménez, MD, of the faculty of nursing, physiotherapy, and podiatry at Hospital Universitario Virgen Macarena, University of Sevilla, Spain, and colleagues wrote in their study. It promotes greater stability in the mothers by reducing the risk of postpartum hemorrhage, making it better to not separate mother and child in the first hours after this surgery, he said.
Dr. Pérez-Jiménez and colleagues evaluated 83 women who underwent a scheduled cesarean section in an unblinded, randomized controlled trial. The women were randomized to receive skin-to-skin contact in the operating room that continued in the postpartum unit, or the normal protocol after cesarean section that consisted of having the mother transferred to the postanesthesia recovery room while the newborn was sent to a maternity room with a parent or companion. Researchers assessed variables such as plasma hemoglobin, uterine contractions, breastfeeding, and postoperative pain, as well as subjective measures such as maternal satisfaction, comfort, previous cesarean section experience, and newborn crying.
Women who received usual care following cesarean section were more likely to have uterine contractions at the umbilical level compared with the skin-to-skin contact group (70% vs. 3%; P ≤ .0001), while the skin-to-skin group was more likely to have uterine contractions at the infraumbilical level (92.5% vs. 22.5%; P ≤ .0001). There was a statistically significant decrease in predischarge hemoglobin in the control group compared with the skin-to-skin group (10.522 vs. 11.075 g/dL; P ≤ .017); the level of hemoglobin reduction favored the skin-to-skin group (1.01 vs. 2.265 g/dL; P ≤ .0001). Women in the skin-to-skin group were more likely to report mild pain on a 10-point visual analog scale (VAS) after being transferred to the recovery room (1.48 vs. 6.23 points; P ≤ .0001) and being transferred to a maternity room or room in the postpartum unit (0.60 vs. 5.23 points; P ≤ .0001). Breastfeeding at birth was significantly higher among patients with immediate skin-to-skin contact compared with the control group (92.5% vs. 32.5%; P ≤ .0001), and continued at 1 month after birth (92.5% vs. 12.5%; P ≤ .0001). Newborns of mothers in the skin-to-skin group were significantly less likely to cry compared with newborns in the control group (90% vs. 55%; P ≤ .001).
When asked to rate their satisfaction on a 10-point Likert scale, women in the skin-to-skin contact group rated their experience significantly higher than did the control group (9.98 vs. 6.5; P ≤ .0001), and all women who had previously had a cesarean section in the skin-to-skin group (30%) rated their experience at 10 points compared with their previous cesarean section without skin-to-skin contact.
Implementing skin-to-skin contact after cesarean section
Betsy M. Collins, MD, MPH, assistant professor of obstetrics and gynecology at Emory University, Atlanta, said in an interview that while some of the findings were largely unsurprising and “confirmed a lot of the things that we already know about skin-to-skin [contact],” one major finding was the “stark difference” in the percentage of new birth parents who started breastfeeding after skin-to-skin contact and were still breastfeeding at 1 month postpartum compared with birth parents in the control group. She was not involved with the study and noted that the results complement recommendations from the World Health Organization on starting breastfeeding within the first hour after birth and continuing breastfeeding through the first 6 months of life.
“That was likely one of the greatest take-home points from the study ... that early skin-to-skin really promoted initiation of breastfeeding,” Dr. Collins said.
Two reasons why skin-to-skin contact after cesarean section isn’t regularly provided is that it can be difficult for personnel and safety reasons to have an extra nurse to continue monitoring the health of the newborn in the operating room, and there is a lack of culture supporting of skin-to-skin contact in the OR, Dr. Collins explained.
“Just like anything else, if it’s built into your standard operating procedure, then you have everything set up in place to do that initial assessment of the infant and then get the baby skin-to-skin as quickly as possible,” she said. If it’s your standard operating procedure to not provide skin-to-skin contact, she said, then there is a little bit more inertia to overcome to start providing it as a standard procedure.
At her center, Dr. Collins said skin-to-skin contact is initiated as soon as possible after birth, even in the operating room. The steps to implementing that policy involved getting the anesthesiology department on board with supporting the policy in the OR and training the circulating nursing staff to ensure a that nurse is available to monitor the newborn.
“I think the most important thing to know is that it’s absolutely doable and that you just have to have a champion just like any other quality initiative,” she said. One of the best ways to do that is to have the patients themselves request it, she noted, compared with its being requested by a physician or nurse.
“I think some patients are disappointed when they have to undergo cesarean delivery or feel like they’re missing out if they can’t have a vaginal delivery,” Dr. Collins said. Immediate skin-to-skin contact is “very good for not only physiology, as we read about in this paper – all the things they said about the benefits of skin-to-skin [contact] are true – but it’s really good for mental health. That bonding begins right away.”
As a birth parent, being separated from your newborn for several hours after a cesarean section, on the other hand, can be “pretty devastating,” Dr. Collins said.
“I think this is something that, once it becomes a standard of care, it will be expected that most hospitals should be doing this,” she said.
The authors and Dr. Collins report no relevant conflicts of interest.
FROM NURSING OPEN
Docs used permanent, not temporary stitches; lawsuits result
The first in what have come to be known as the “wrong stitches” cases has been settled, a story in The Ledger reports.
The former plaintiff in the now-settled suit is Carrie Monk, a Lakeland, Fla., resident who underwent total laparoscopic hysterectomy at Lakeland Regional Health Medical Center several years ago. (The medical center is managed by Lakeland Regional Health Systems.) D As a result, over the next 19 months, she experienced abdominal pain and constant bleeding, which in turn affected her personal life as well as her work as a nurse in the intensive care unit. She underwent follow-up surgery to have the permanent sutures removed, but two could not be identified and excised.
In July 2020, Ms. Monk filed a medical malpractice claim against Lakeland Regional Health, its medical center, and the ob-gyns who had performed her surgery. She was among the first of the women who had received the permanent sutures to do so.
On February 28, 2021, The Ledger ran a story on Ms. Monk’s suit. Less than 2 weeks later, Lakeland Regional Health sent letters to patients who had undergone “wrong stitch” surgeries, cautioning of possible postsurgical complications. The company reportedly kept secret how many letters it had sent out.
Since then, at least nine similar suits have been filed against Lakeland Regional Health, bringing the total number of such suits to 12. Four of these suits have been settled, including Ms. Monk’s. Of the remaining eight cases, several are in various pretrial stages.
Under the terms of her settlement, neither Ms. Monk nor her attorney may disclose what financial compensation or other awards she’s received. The attorney, however, referred to the settlement as “amicable.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
The first in what have come to be known as the “wrong stitches” cases has been settled, a story in The Ledger reports.
The former plaintiff in the now-settled suit is Carrie Monk, a Lakeland, Fla., resident who underwent total laparoscopic hysterectomy at Lakeland Regional Health Medical Center several years ago. (The medical center is managed by Lakeland Regional Health Systems.) D As a result, over the next 19 months, she experienced abdominal pain and constant bleeding, which in turn affected her personal life as well as her work as a nurse in the intensive care unit. She underwent follow-up surgery to have the permanent sutures removed, but two could not be identified and excised.
In July 2020, Ms. Monk filed a medical malpractice claim against Lakeland Regional Health, its medical center, and the ob-gyns who had performed her surgery. She was among the first of the women who had received the permanent sutures to do so.
On February 28, 2021, The Ledger ran a story on Ms. Monk’s suit. Less than 2 weeks later, Lakeland Regional Health sent letters to patients who had undergone “wrong stitch” surgeries, cautioning of possible postsurgical complications. The company reportedly kept secret how many letters it had sent out.
Since then, at least nine similar suits have been filed against Lakeland Regional Health, bringing the total number of such suits to 12. Four of these suits have been settled, including Ms. Monk’s. Of the remaining eight cases, several are in various pretrial stages.
Under the terms of her settlement, neither Ms. Monk nor her attorney may disclose what financial compensation or other awards she’s received. The attorney, however, referred to the settlement as “amicable.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
The first in what have come to be known as the “wrong stitches” cases has been settled, a story in The Ledger reports.
The former plaintiff in the now-settled suit is Carrie Monk, a Lakeland, Fla., resident who underwent total laparoscopic hysterectomy at Lakeland Regional Health Medical Center several years ago. (The medical center is managed by Lakeland Regional Health Systems.) D As a result, over the next 19 months, she experienced abdominal pain and constant bleeding, which in turn affected her personal life as well as her work as a nurse in the intensive care unit. She underwent follow-up surgery to have the permanent sutures removed, but two could not be identified and excised.
In July 2020, Ms. Monk filed a medical malpractice claim against Lakeland Regional Health, its medical center, and the ob-gyns who had performed her surgery. She was among the first of the women who had received the permanent sutures to do so.
On February 28, 2021, The Ledger ran a story on Ms. Monk’s suit. Less than 2 weeks later, Lakeland Regional Health sent letters to patients who had undergone “wrong stitch” surgeries, cautioning of possible postsurgical complications. The company reportedly kept secret how many letters it had sent out.
Since then, at least nine similar suits have been filed against Lakeland Regional Health, bringing the total number of such suits to 12. Four of these suits have been settled, including Ms. Monk’s. Of the remaining eight cases, several are in various pretrial stages.
Under the terms of her settlement, neither Ms. Monk nor her attorney may disclose what financial compensation or other awards she’s received. The attorney, however, referred to the settlement as “amicable.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.