User login
Preoperative preparation for gender-affirming vaginoplasty surgery
The field of gender-affirming surgery is one of the fastest growing surgical specialties in the country. Within the last few years, the number of procedures has increased markedly – with a total of 16,353 performed in 2020 compared with 8,304 in 2017.1,2 As the number of surgeries increases, so does the need for a standardized approach to preoperative evaluation and patient preparation.
Gender-affirming genital surgery for transfeminine individuals encompasses a spectrum of procedures that includes removal of the testicles (orchiectomy), creation of a neovaginal canal (full-depth vaginoplasty), and creation of external vulvar structures without a vaginal canal (zero-depth vaginoplasty). Each of these requires different levels of preoperative preparedness and medical optimization, and has unique postoperative challenges. Often, these postoperative complications can be mitigated with adequate patient education.
Many centers that offer genital gender-affirming surgery have a multidisciplinary team composed of a social worker, mental health providers, care coordinators, primary care providers, and surgeons. This team is essential to providing supportive services within their respective scope of practices.
The role of the mental health provider cannot be understated. While the updated standards of care from the World Professional Association for Transgender Health no longer require two letters from mental health providers prior to genital surgery, it is important to recognize that many insurance companies have not yet updated their policies and still require two letters. Even when insurance companies adjust their policies to reflect current standards, a mental health assessment is still necessary to determine if patients have any mental health issues that could negatively affect their surgical outcome.3 Furthermore, a continued relationship with a mental health provider is beneficial for patients as they go through a stressful and life-changing procedure.4
As with any surgery, understanding patient goals and expectations is a key element in achieving optimal patient satisfaction. Patients with high esthetic or functional expectations experience higher rates of disappointment after surgery and have more difficulty coping with complications.5
Decisions about proceeding with a particular type of genital surgery should consider a patient’s desire to have vaginal-receptive intercourse, their commitment to dilation, financial stability, a safe environment for recovery, a support network, and the ability to understand and cope with potential complications.4 Patients will present with a wide variety of educational backgrounds and medical literacy, and will have differing intellectual capabilities.4 Consultations should take into account potential challenges these factors may play in patients’ ability to understand this complex surgery.
An adequate amount of time should be allotted to addressing these challenges. In my practice, a consultation for a gender-affirming genital surgery takes approximately 60 minutes. A preoperative packet with information is mailed to the patient ahead of time that will be reviewed at the time of the visit. During the consultation, I utilize a visual presentation that details the preoperative requirements and different types of surgical procedures, shows preoperative and postoperative surgical results, and discusses potential complications. Before the consultation, I advise that patients bring a support person (ideally the person who will assist in postoperative care) and a list of questions that they may have.
Both full- and shallow-depth procedures are reviewed at the time of initial consultation. For patients who seek a full-depth vaginoplasty procedure, it is important to determine whether patients are committed to dilation and have a safe, supportive environment to do so. Patients may have physical limitations, such as obesity or mobility issues, that could make dilation difficult or even impossible. Patients may not have stable housing, may experience financial restrictions that would impede their ability to purchase necessary supplies, and lack a support person who can care for them in the immediate postoperative period. Many patients are unaware of the importance these social factors play in a successful outcome. Social workers and care coordinators are important resources when these challenges are encountered.
Medical optimization is not unlike other gynecologic procedures with a few exceptions. Obesity, diabetes, and smoking play larger roles in surgical complications than in other surgeries as vaginoplasty techniques use pedicled flaps that rely on adequate blood supply. Obesity, poorly controlled diabetes, and smoking are associated with increased rates of wound infection, poor wound healing, and graft loss. Smoking cessation for 8 weeks prior to surgery and for 4 weeks afterward is mandatory.
For patients with a history of smoking, a nicotine test is performed within 4 weeks of surgery. Many surgeons have body mass index requirements, typically ranging between 20 and 30 kg/m2, despite limited data. This paradigm is shifting to consider body fat distribution rather than BMI alone. Extensive body fat in the mons or groin area can increase the difficulty of pelvic floor dissection during surgery and impede visualization for dilation in the postoperative period. There are reports of patients dilating into their rectum or neourethra, which can have catastrophic consequences. For these patients, a zero-depth vaginoplasty or orchiectomy may initially be a safer option.
Many patients are justifiably excited to undergo the procedures as quality of life is typically improved after surgery. However, even with adequate counseling, many patients often underestimate the extensive recovery process. This surgical procedure requires extensive planning and adequate resources.4 Patients must be able to take off from work for prolonged periods of time (typically 6 weeks), which can serve as a source of financial stress. To maintain the integrity of suture lines in the genital region, prolonged or limited mobilization is recommended. This can create boredom and forces patients to rely on a caregiver for activities of daily living, such as household chores, cooking meals, and transportation.
Gender-affirming genital surgery is not only a complex surgical procedure but also requires extensive preoperative education and postoperative support. As this field continues to grow, patients, providers, and caregivers should work toward further developing a collaborative care model to optimize surgical outcomes and patient satisfaction.
Dr. Brandt is an ob.gyn. and fellowship-trained gender affirming surgeon in West Reading, Pa.
References
1. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2020.
2. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2017.
3. Coleman E et al. Standards of care for the health of transgender and gender diverse people. Version 8. Int J Transgender Health. 23(S1):S1-S258. doi :10.1080/26895269.2022.2100644.
4. Penkin A et al. In: Nikolavsky D and Blakely SA, eds. Urological care for the transgender patient: A comprehensive guide. Switzerland: Springer, 2021:37-44.
5. Waljee J et al. Surgery. 2014;155:799-808.
The field of gender-affirming surgery is one of the fastest growing surgical specialties in the country. Within the last few years, the number of procedures has increased markedly – with a total of 16,353 performed in 2020 compared with 8,304 in 2017.1,2 As the number of surgeries increases, so does the need for a standardized approach to preoperative evaluation and patient preparation.
Gender-affirming genital surgery for transfeminine individuals encompasses a spectrum of procedures that includes removal of the testicles (orchiectomy), creation of a neovaginal canal (full-depth vaginoplasty), and creation of external vulvar structures without a vaginal canal (zero-depth vaginoplasty). Each of these requires different levels of preoperative preparedness and medical optimization, and has unique postoperative challenges. Often, these postoperative complications can be mitigated with adequate patient education.
Many centers that offer genital gender-affirming surgery have a multidisciplinary team composed of a social worker, mental health providers, care coordinators, primary care providers, and surgeons. This team is essential to providing supportive services within their respective scope of practices.
The role of the mental health provider cannot be understated. While the updated standards of care from the World Professional Association for Transgender Health no longer require two letters from mental health providers prior to genital surgery, it is important to recognize that many insurance companies have not yet updated their policies and still require two letters. Even when insurance companies adjust their policies to reflect current standards, a mental health assessment is still necessary to determine if patients have any mental health issues that could negatively affect their surgical outcome.3 Furthermore, a continued relationship with a mental health provider is beneficial for patients as they go through a stressful and life-changing procedure.4
As with any surgery, understanding patient goals and expectations is a key element in achieving optimal patient satisfaction. Patients with high esthetic or functional expectations experience higher rates of disappointment after surgery and have more difficulty coping with complications.5
Decisions about proceeding with a particular type of genital surgery should consider a patient’s desire to have vaginal-receptive intercourse, their commitment to dilation, financial stability, a safe environment for recovery, a support network, and the ability to understand and cope with potential complications.4 Patients will present with a wide variety of educational backgrounds and medical literacy, and will have differing intellectual capabilities.4 Consultations should take into account potential challenges these factors may play in patients’ ability to understand this complex surgery.
An adequate amount of time should be allotted to addressing these challenges. In my practice, a consultation for a gender-affirming genital surgery takes approximately 60 minutes. A preoperative packet with information is mailed to the patient ahead of time that will be reviewed at the time of the visit. During the consultation, I utilize a visual presentation that details the preoperative requirements and different types of surgical procedures, shows preoperative and postoperative surgical results, and discusses potential complications. Before the consultation, I advise that patients bring a support person (ideally the person who will assist in postoperative care) and a list of questions that they may have.
Both full- and shallow-depth procedures are reviewed at the time of initial consultation. For patients who seek a full-depth vaginoplasty procedure, it is important to determine whether patients are committed to dilation and have a safe, supportive environment to do so. Patients may have physical limitations, such as obesity or mobility issues, that could make dilation difficult or even impossible. Patients may not have stable housing, may experience financial restrictions that would impede their ability to purchase necessary supplies, and lack a support person who can care for them in the immediate postoperative period. Many patients are unaware of the importance these social factors play in a successful outcome. Social workers and care coordinators are important resources when these challenges are encountered.
Medical optimization is not unlike other gynecologic procedures with a few exceptions. Obesity, diabetes, and smoking play larger roles in surgical complications than in other surgeries as vaginoplasty techniques use pedicled flaps that rely on adequate blood supply. Obesity, poorly controlled diabetes, and smoking are associated with increased rates of wound infection, poor wound healing, and graft loss. Smoking cessation for 8 weeks prior to surgery and for 4 weeks afterward is mandatory.
For patients with a history of smoking, a nicotine test is performed within 4 weeks of surgery. Many surgeons have body mass index requirements, typically ranging between 20 and 30 kg/m2, despite limited data. This paradigm is shifting to consider body fat distribution rather than BMI alone. Extensive body fat in the mons or groin area can increase the difficulty of pelvic floor dissection during surgery and impede visualization for dilation in the postoperative period. There are reports of patients dilating into their rectum or neourethra, which can have catastrophic consequences. For these patients, a zero-depth vaginoplasty or orchiectomy may initially be a safer option.
Many patients are justifiably excited to undergo the procedures as quality of life is typically improved after surgery. However, even with adequate counseling, many patients often underestimate the extensive recovery process. This surgical procedure requires extensive planning and adequate resources.4 Patients must be able to take off from work for prolonged periods of time (typically 6 weeks), which can serve as a source of financial stress. To maintain the integrity of suture lines in the genital region, prolonged or limited mobilization is recommended. This can create boredom and forces patients to rely on a caregiver for activities of daily living, such as household chores, cooking meals, and transportation.
Gender-affirming genital surgery is not only a complex surgical procedure but also requires extensive preoperative education and postoperative support. As this field continues to grow, patients, providers, and caregivers should work toward further developing a collaborative care model to optimize surgical outcomes and patient satisfaction.
Dr. Brandt is an ob.gyn. and fellowship-trained gender affirming surgeon in West Reading, Pa.
References
1. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2020.
2. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2017.
3. Coleman E et al. Standards of care for the health of transgender and gender diverse people. Version 8. Int J Transgender Health. 23(S1):S1-S258. doi :10.1080/26895269.2022.2100644.
4. Penkin A et al. In: Nikolavsky D and Blakely SA, eds. Urological care for the transgender patient: A comprehensive guide. Switzerland: Springer, 2021:37-44.
5. Waljee J et al. Surgery. 2014;155:799-808.
The field of gender-affirming surgery is one of the fastest growing surgical specialties in the country. Within the last few years, the number of procedures has increased markedly – with a total of 16,353 performed in 2020 compared with 8,304 in 2017.1,2 As the number of surgeries increases, so does the need for a standardized approach to preoperative evaluation and patient preparation.
Gender-affirming genital surgery for transfeminine individuals encompasses a spectrum of procedures that includes removal of the testicles (orchiectomy), creation of a neovaginal canal (full-depth vaginoplasty), and creation of external vulvar structures without a vaginal canal (zero-depth vaginoplasty). Each of these requires different levels of preoperative preparedness and medical optimization, and has unique postoperative challenges. Often, these postoperative complications can be mitigated with adequate patient education.
Many centers that offer genital gender-affirming surgery have a multidisciplinary team composed of a social worker, mental health providers, care coordinators, primary care providers, and surgeons. This team is essential to providing supportive services within their respective scope of practices.
The role of the mental health provider cannot be understated. While the updated standards of care from the World Professional Association for Transgender Health no longer require two letters from mental health providers prior to genital surgery, it is important to recognize that many insurance companies have not yet updated their policies and still require two letters. Even when insurance companies adjust their policies to reflect current standards, a mental health assessment is still necessary to determine if patients have any mental health issues that could negatively affect their surgical outcome.3 Furthermore, a continued relationship with a mental health provider is beneficial for patients as they go through a stressful and life-changing procedure.4
As with any surgery, understanding patient goals and expectations is a key element in achieving optimal patient satisfaction. Patients with high esthetic or functional expectations experience higher rates of disappointment after surgery and have more difficulty coping with complications.5
Decisions about proceeding with a particular type of genital surgery should consider a patient’s desire to have vaginal-receptive intercourse, their commitment to dilation, financial stability, a safe environment for recovery, a support network, and the ability to understand and cope with potential complications.4 Patients will present with a wide variety of educational backgrounds and medical literacy, and will have differing intellectual capabilities.4 Consultations should take into account potential challenges these factors may play in patients’ ability to understand this complex surgery.
An adequate amount of time should be allotted to addressing these challenges. In my practice, a consultation for a gender-affirming genital surgery takes approximately 60 minutes. A preoperative packet with information is mailed to the patient ahead of time that will be reviewed at the time of the visit. During the consultation, I utilize a visual presentation that details the preoperative requirements and different types of surgical procedures, shows preoperative and postoperative surgical results, and discusses potential complications. Before the consultation, I advise that patients bring a support person (ideally the person who will assist in postoperative care) and a list of questions that they may have.
Both full- and shallow-depth procedures are reviewed at the time of initial consultation. For patients who seek a full-depth vaginoplasty procedure, it is important to determine whether patients are committed to dilation and have a safe, supportive environment to do so. Patients may have physical limitations, such as obesity or mobility issues, that could make dilation difficult or even impossible. Patients may not have stable housing, may experience financial restrictions that would impede their ability to purchase necessary supplies, and lack a support person who can care for them in the immediate postoperative period. Many patients are unaware of the importance these social factors play in a successful outcome. Social workers and care coordinators are important resources when these challenges are encountered.
Medical optimization is not unlike other gynecologic procedures with a few exceptions. Obesity, diabetes, and smoking play larger roles in surgical complications than in other surgeries as vaginoplasty techniques use pedicled flaps that rely on adequate blood supply. Obesity, poorly controlled diabetes, and smoking are associated with increased rates of wound infection, poor wound healing, and graft loss. Smoking cessation for 8 weeks prior to surgery and for 4 weeks afterward is mandatory.
For patients with a history of smoking, a nicotine test is performed within 4 weeks of surgery. Many surgeons have body mass index requirements, typically ranging between 20 and 30 kg/m2, despite limited data. This paradigm is shifting to consider body fat distribution rather than BMI alone. Extensive body fat in the mons or groin area can increase the difficulty of pelvic floor dissection during surgery and impede visualization for dilation in the postoperative period. There are reports of patients dilating into their rectum or neourethra, which can have catastrophic consequences. For these patients, a zero-depth vaginoplasty or orchiectomy may initially be a safer option.
Many patients are justifiably excited to undergo the procedures as quality of life is typically improved after surgery. However, even with adequate counseling, many patients often underestimate the extensive recovery process. This surgical procedure requires extensive planning and adequate resources.4 Patients must be able to take off from work for prolonged periods of time (typically 6 weeks), which can serve as a source of financial stress. To maintain the integrity of suture lines in the genital region, prolonged or limited mobilization is recommended. This can create boredom and forces patients to rely on a caregiver for activities of daily living, such as household chores, cooking meals, and transportation.
Gender-affirming genital surgery is not only a complex surgical procedure but also requires extensive preoperative education and postoperative support. As this field continues to grow, patients, providers, and caregivers should work toward further developing a collaborative care model to optimize surgical outcomes and patient satisfaction.
Dr. Brandt is an ob.gyn. and fellowship-trained gender affirming surgeon in West Reading, Pa.
References
1. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2020.
2. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2017.
3. Coleman E et al. Standards of care for the health of transgender and gender diverse people. Version 8. Int J Transgender Health. 23(S1):S1-S258. doi :10.1080/26895269.2022.2100644.
4. Penkin A et al. In: Nikolavsky D and Blakely SA, eds. Urological care for the transgender patient: A comprehensive guide. Switzerland: Springer, 2021:37-44.
5. Waljee J et al. Surgery. 2014;155:799-808.
Surgeon gender not associated with maternal morbidity and hemorrhage after C-section
Surgeon gender was not associated with maternal morbidity or severe blood loss after cesarean delivery, a large prospective cohort study from France reports. The results have important implications for the promotion of gender equality among surgeons, obstetricians in particular, wrote a team led by Hanane Bouchghoul, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital. The report is in JAMA Surgery.
“Our findings are significant in that they add substantially to the string of studies contradicting the age-old dogma that men are better surgeons than women,” the authors wrote. Previous research has suggested slightly better outcomes with female surgeons or higher complication rates with male surgeons.
The results support those of a recent Canadian retrospective analysis suggesting that patients treated by male or female surgeons for various elective indications experience similar surgical outcomes but with a slight, statistically significant decrease in 30-day mortality when treated by female surgeons.
“Policy makers need to combat prejudice against women in surgical careers, particularly in obstetrics and gynecology, so that women no longer experience conscious or unconscious barriers or difficulties in their professional choices, training, and relationships with colleagues or patients,” study corresponding author Loïc Sentilhes, MD, PhD, of Bordeaux University Hospital, said in an interview.
Facing such barriers, women may doubt their ability to be surgeons, their legitimacy as surgeons, and may not consider this type of career, he continued. “Moreover a teacher may not be as involved in teaching young female surgeons as young male surgeons, or the doctor-patient relationship may be more complicated in the event of complications if the patient thinks that a female surgeon has less competence than a male surgeon.”
The analysis drew on data from the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery 2 trial, a multicenter, randomized, placebo-controlled study conducted from March 2018 through January 2020 in mothers from 27 French maternity hospitals.
Eligible participants had a cesarean delivery before or during labor at or after 34 weeks’ gestation. The primary endpoint was the incidence of a composite maternal morbidity variable, and the secondary endpoint was the incidence of postpartum hemorrhage, defined by a calculated estimated blood loss exceeding 1,000 mL or transfusion by day 2.
Among the 4,244 women included, male surgeons performed 943 cesarean deliveries (22.2%) and female surgeons performed 3,301 (77.8%). The percentage who were attending obstetricians was higher for men at 441 of 929 (47.5%) than women at 687 of 3,239 (21.2%).
The observed risk of maternal morbidity did not differ between male and female surgeons: 119 of 837 (14.2%) vs. 476 of 2,928 (16.3%), for an adjusted risk ratio (aRR) of 0.92 (95% confidence interval [CI], 0.77-1.13). Interaction between surgeon gender and level of experience with the risk of maternal morbidity was not statistically significant; nor did the groups differ specifically by risk for postpartum hemorrhage: aRR, 0.98 (95% CI, 0.85-1.13).
Despite the longstanding stereotype that men perform surgery better than women, and the traditional preponderance of male surgeons, the authors noted, postoperative morbidity and mortality may be lower after various surgeries performed by women.
The TRAAP2 trial
In an accompanying editorial, Amanda Fader, MD, of the department of obstetrics and gynecology at Johns Hopkins School of Medicine in Baltimore, and colleagues caution that the French study’s methodology may not fully account for the complex intersection of surgeon volume, experience, gender, clinical decision-making skills, and patient-level and clinical factors affecting outcomes.
That said, appraising surgical outcomes based on gender may be an essential step toward reducing implicit bias and dispelling engendered perceptions regarding gender and technical proficiency, the commentators stated. “To definitively dispel archaic, gender-based notions about performance in clinical or surgical settings, efforts must go beyond peer-reviewed research,” Dr. Fader said in an interview. “Medical institutions and leaders of clinical departments must make concerted efforts to recruit, mentor, support, and promote women and persons of all genders in medicine – as well as confront any discriminatory perceptions and experiences concerning sex, race and ethnicity, sexual orientation, or economic class.”
This study was supported by the French Ministry of Health under its Clinical Research Hospital Program. Dr. Sentilhes reported financial relationships with Dilafor, Bayer, GlaxoSmithKline, Sigvaris, and Ferring Pharmaceuticals. The editorial commentators disclosed no funding for their commentary or conflicts of interest.
Surgeon gender was not associated with maternal morbidity or severe blood loss after cesarean delivery, a large prospective cohort study from France reports. The results have important implications for the promotion of gender equality among surgeons, obstetricians in particular, wrote a team led by Hanane Bouchghoul, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital. The report is in JAMA Surgery.
“Our findings are significant in that they add substantially to the string of studies contradicting the age-old dogma that men are better surgeons than women,” the authors wrote. Previous research has suggested slightly better outcomes with female surgeons or higher complication rates with male surgeons.
The results support those of a recent Canadian retrospective analysis suggesting that patients treated by male or female surgeons for various elective indications experience similar surgical outcomes but with a slight, statistically significant decrease in 30-day mortality when treated by female surgeons.
“Policy makers need to combat prejudice against women in surgical careers, particularly in obstetrics and gynecology, so that women no longer experience conscious or unconscious barriers or difficulties in their professional choices, training, and relationships with colleagues or patients,” study corresponding author Loïc Sentilhes, MD, PhD, of Bordeaux University Hospital, said in an interview.
Facing such barriers, women may doubt their ability to be surgeons, their legitimacy as surgeons, and may not consider this type of career, he continued. “Moreover a teacher may not be as involved in teaching young female surgeons as young male surgeons, or the doctor-patient relationship may be more complicated in the event of complications if the patient thinks that a female surgeon has less competence than a male surgeon.”
The analysis drew on data from the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery 2 trial, a multicenter, randomized, placebo-controlled study conducted from March 2018 through January 2020 in mothers from 27 French maternity hospitals.
Eligible participants had a cesarean delivery before or during labor at or after 34 weeks’ gestation. The primary endpoint was the incidence of a composite maternal morbidity variable, and the secondary endpoint was the incidence of postpartum hemorrhage, defined by a calculated estimated blood loss exceeding 1,000 mL or transfusion by day 2.
Among the 4,244 women included, male surgeons performed 943 cesarean deliveries (22.2%) and female surgeons performed 3,301 (77.8%). The percentage who were attending obstetricians was higher for men at 441 of 929 (47.5%) than women at 687 of 3,239 (21.2%).
The observed risk of maternal morbidity did not differ between male and female surgeons: 119 of 837 (14.2%) vs. 476 of 2,928 (16.3%), for an adjusted risk ratio (aRR) of 0.92 (95% confidence interval [CI], 0.77-1.13). Interaction between surgeon gender and level of experience with the risk of maternal morbidity was not statistically significant; nor did the groups differ specifically by risk for postpartum hemorrhage: aRR, 0.98 (95% CI, 0.85-1.13).
Despite the longstanding stereotype that men perform surgery better than women, and the traditional preponderance of male surgeons, the authors noted, postoperative morbidity and mortality may be lower after various surgeries performed by women.
The TRAAP2 trial
In an accompanying editorial, Amanda Fader, MD, of the department of obstetrics and gynecology at Johns Hopkins School of Medicine in Baltimore, and colleagues caution that the French study’s methodology may not fully account for the complex intersection of surgeon volume, experience, gender, clinical decision-making skills, and patient-level and clinical factors affecting outcomes.
That said, appraising surgical outcomes based on gender may be an essential step toward reducing implicit bias and dispelling engendered perceptions regarding gender and technical proficiency, the commentators stated. “To definitively dispel archaic, gender-based notions about performance in clinical or surgical settings, efforts must go beyond peer-reviewed research,” Dr. Fader said in an interview. “Medical institutions and leaders of clinical departments must make concerted efforts to recruit, mentor, support, and promote women and persons of all genders in medicine – as well as confront any discriminatory perceptions and experiences concerning sex, race and ethnicity, sexual orientation, or economic class.”
This study was supported by the French Ministry of Health under its Clinical Research Hospital Program. Dr. Sentilhes reported financial relationships with Dilafor, Bayer, GlaxoSmithKline, Sigvaris, and Ferring Pharmaceuticals. The editorial commentators disclosed no funding for their commentary or conflicts of interest.
Surgeon gender was not associated with maternal morbidity or severe blood loss after cesarean delivery, a large prospective cohort study from France reports. The results have important implications for the promotion of gender equality among surgeons, obstetricians in particular, wrote a team led by Hanane Bouchghoul, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital. The report is in JAMA Surgery.
“Our findings are significant in that they add substantially to the string of studies contradicting the age-old dogma that men are better surgeons than women,” the authors wrote. Previous research has suggested slightly better outcomes with female surgeons or higher complication rates with male surgeons.
The results support those of a recent Canadian retrospective analysis suggesting that patients treated by male or female surgeons for various elective indications experience similar surgical outcomes but with a slight, statistically significant decrease in 30-day mortality when treated by female surgeons.
“Policy makers need to combat prejudice against women in surgical careers, particularly in obstetrics and gynecology, so that women no longer experience conscious or unconscious barriers or difficulties in their professional choices, training, and relationships with colleagues or patients,” study corresponding author Loïc Sentilhes, MD, PhD, of Bordeaux University Hospital, said in an interview.
Facing such barriers, women may doubt their ability to be surgeons, their legitimacy as surgeons, and may not consider this type of career, he continued. “Moreover a teacher may not be as involved in teaching young female surgeons as young male surgeons, or the doctor-patient relationship may be more complicated in the event of complications if the patient thinks that a female surgeon has less competence than a male surgeon.”
The analysis drew on data from the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery 2 trial, a multicenter, randomized, placebo-controlled study conducted from March 2018 through January 2020 in mothers from 27 French maternity hospitals.
Eligible participants had a cesarean delivery before or during labor at or after 34 weeks’ gestation. The primary endpoint was the incidence of a composite maternal morbidity variable, and the secondary endpoint was the incidence of postpartum hemorrhage, defined by a calculated estimated blood loss exceeding 1,000 mL or transfusion by day 2.
Among the 4,244 women included, male surgeons performed 943 cesarean deliveries (22.2%) and female surgeons performed 3,301 (77.8%). The percentage who were attending obstetricians was higher for men at 441 of 929 (47.5%) than women at 687 of 3,239 (21.2%).
The observed risk of maternal morbidity did not differ between male and female surgeons: 119 of 837 (14.2%) vs. 476 of 2,928 (16.3%), for an adjusted risk ratio (aRR) of 0.92 (95% confidence interval [CI], 0.77-1.13). Interaction between surgeon gender and level of experience with the risk of maternal morbidity was not statistically significant; nor did the groups differ specifically by risk for postpartum hemorrhage: aRR, 0.98 (95% CI, 0.85-1.13).
Despite the longstanding stereotype that men perform surgery better than women, and the traditional preponderance of male surgeons, the authors noted, postoperative morbidity and mortality may be lower after various surgeries performed by women.
The TRAAP2 trial
In an accompanying editorial, Amanda Fader, MD, of the department of obstetrics and gynecology at Johns Hopkins School of Medicine in Baltimore, and colleagues caution that the French study’s methodology may not fully account for the complex intersection of surgeon volume, experience, gender, clinical decision-making skills, and patient-level and clinical factors affecting outcomes.
That said, appraising surgical outcomes based on gender may be an essential step toward reducing implicit bias and dispelling engendered perceptions regarding gender and technical proficiency, the commentators stated. “To definitively dispel archaic, gender-based notions about performance in clinical or surgical settings, efforts must go beyond peer-reviewed research,” Dr. Fader said in an interview. “Medical institutions and leaders of clinical departments must make concerted efforts to recruit, mentor, support, and promote women and persons of all genders in medicine – as well as confront any discriminatory perceptions and experiences concerning sex, race and ethnicity, sexual orientation, or economic class.”
This study was supported by the French Ministry of Health under its Clinical Research Hospital Program. Dr. Sentilhes reported financial relationships with Dilafor, Bayer, GlaxoSmithKline, Sigvaris, and Ferring Pharmaceuticals. The editorial commentators disclosed no funding for their commentary or conflicts of interest.
FROM JAMA SURGERY
Update on secondary cytoreduction in recurrent ovarian cancer
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.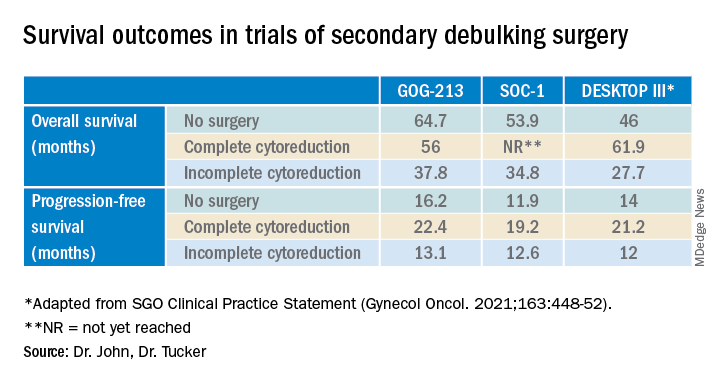
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Liability in robotic gyn surgery
The approach to hysterectomy has been debated, with the need for individualization case by case stressed, and the expertise of the operating surgeon considered.
CASE Was surgeon experience a factor in case complications?
VM is a 46-year-old woman (G5 P4014) reporting persistent uterine bleeding that is refractory to medical therapy. The patient has uterine fibroids, 6 weeks in size on examination, with “mild” prolapse noted. Additional medical diagnoses included vulvitis, ovarian cyst in the past, cystic mastopathy, and prior evidence of pelvic adhesion, noted at the time of ovarian cystectomy. Prior surgical records were not obtained by the operating surgeon, although her obstetric history includes 2 prior vaginal deliveries and 2 cesarean deliveries (CDs). The patient had an umbilical herniorraphy a number of years ago. Her medications include hormonal therapy, for presumed menopause, and medication for depression (she reported “doing well” on medication). She reported smoking 1 PPD and had a prior tubal ligation.
VM was previously evaluated for Lynch Syndrome and informed of the potential for increased risks of colon, endometrial, and several other cancers. She did not have cancer as of the time of planned surgery.
The patient underwent robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. The operating surgeon did not have a lot of experience with robotic hysterectomies but told the patient preoperatively “I have done a few.” Perioperatively, blood loss was minimal, urine output was recorded as 25 mL, and according to the operative report there were extensive pelvic adhesions and no complications. The “ureters were identified” when the broad ligament was opened at the time of skeletonization of the uterine vessels and documented accordingly. The intraoperative Foley was discontinued at the end of the procedure. The pathology report noted diffuse adenomyosis and uterine fibroids; the uterus weighed 250 g. In addition, a “large hemorrhagic corpus luteum cyst” was noted on the right ovary.
The patient presented for a postoperative visit reporting “leaking” serosanguinous fluid that began 2.5 weeks postoperatively and required her to wear 3 to 4 “Depends” every day. She also reported constipation since beginning her prescribed pain medication. She requested a copy of her medical records and said she was dissatisfied with the care she had received related to the hysterectomy; she was “seeking a second opinion from a urologist.” The urologist suggested evaluation of the “leaking,” and a Foley catheter was placed. When she stood up, however, there was leaking around the catheter, and she reported a “yellowish-green,” foul smelling discharge. She called the urologist’s office, stating, “I think I have a bowel obstruction.” The patient was instructed to proceed to the emergency department at her local hospital. She was released with a diagnosis of constipation. Upon follow-up urologic evaluation, a vulvovaginal fistula was noted. Management was a “simple fistula repair,” and the patient did well subsequently.
The patient brought suit against the hospital and operating gynecologist. In part the hospital records noted, “relatively inexperienced robotic surgeon.” The hospital was taken to task for granting privileges to an individual that had prior privilege “problems.”

Continue to: Medical opinion...
Medical opinion
This case demonstrates a number of issues. (We will discuss the credentials for the surgeon and hospital privileges in the legal considerations section.) From the medical perspective, the rate of urologic injury associated with all hysterectomies is 0.87%.1 Robotic hysterectomy has been reported at 0.92% in a series published from Henry Ford Hospital.1 The lowest rate of urologic injury is associated with vaginal hysterectomy, reported at 0.2%.2 Reported rates of urologic injury by approach to hysterectomy are1:
- robotic, 0.92%
- laparoscopic, 0.90%
- vaginal, 0.33%
- abdominal, 0.96%.
Complications by surgeon type also have been addressed, and the percent of total urologic complications are reported as1:
- ObGyn, 47%
- gyn oncologist, 47%
- urogynecologist, 6%.
Intraoperative conversion to laparotomy from initial robotic approach has been addressed in a retrospective study over a 2-year period, with operative times ranging from 1 hr, 50 min to 9 hrs of surgical time.1 The vast majority of intraoperative complications in a series reported from Finland were managed “within minutes,” and in the series of 83 patients, 5 (6%) required conversion to laparotomy.2 Intraoperative complications reported include failed entry, vascular injury, nerve injury, visceral injury, solid organ injury, tumor fragmentation, and anesthetic-related complications.3 Of note, the vascular injuries included inferior vena cava, common iliac, and external iliac.
Mortality rates in association with benign laparoscopic and robotic procedures have been addressed and noted to be 1:6,456 cases based upon a meta-analysis.4 The analysis included 124,216 patients. Laparoscopic versus robotic mortality rates were not statistically different. Mortality was more common among cases of undiagnosed rare colorectal injury. This mortality is on par with complications from Roux-en-Y gastric bypass procedures. Procedures such as sacrocolpopexy are equated with higher mortality (1:1,246) in comparison with benign hysterectomy.5
Infectious complications following either laparoscopic or robotic hysterectomy were reported at less than 1% and not statistically different for either approach.6 The series authored by Marra et al evaluated 176,016 patients.
Overall, robotic-assisted gynecologic complications are rare. One series was focused on gynecological oncologic cases.7 Specific categories of complications included7:
- patient positioning and pneumoperitoneum
- injury to surrounding organs
- bowel injury
- port site metastasis
- surgical emphysema
- vaginal cuff dehiscence
- anesthesia-related problems.
The authors concluded, “robotic assisted surgery in gynecological oncology is safe and the incidence of complications is low.”7 The major cause of death related to robotic surgery is vascular injury–related. The authors emphasized the importance of knowledge of anatomy, basic principles of “traction and counter-traction” and proper dissection along tissue planes as key to minimizing complications. Consider placement of stents for ureter identification, as appropriate. Barbed-suturing does not prevent dehiscence.
Continue to: Legal considerations...
Legal considerations
Robotic surgery presents many legal issues and promises to raise many more in the future. The law must control new technology while encouraging productive uses, and provide new remedies for harms while respecting traditional legal principles.8 There is no shortage of good ideas about controlling surgical robots,9 automated devices more generally,10 and artificial intelligence.11 Those issues will be important, and watching them unfold will be intriguing.
In the meantime, physicians and other health care professionals, health care facilities, technology companies, and patients must work within current legal structures in implementing and using robotic surgery. These are extraordinarily complex issues, so it is possible only to review the current landscape and speculate what the near future may hold.
Regulating surgical robots
The US Food and Drug Administration (FDA) is the primary regulator of robots used in medicine.12 It has the authority to regulate surgical devices, including surgical robots—which it refers to as “robotically-assisted surgical devices,” or RASD. In 2000, it approved Intuitive Surgical’s daVinci system for use in surgery. In 2017, the FDA expanded its clearance to include the Senhance System of TransEnterix Surgical Inc. for minimally invasive gynecologic surgery.13 In 2021, the FDA cleared the Hominis Surgical System for transvaginal hysterectomy “in certain patients.” However, the FDA emphasized that this clearance is for benign hysterectomy with salpingo-oophorectomy.14 (The FDA has cleared various robotic devices for several other areas of surgical practice, including neurosurgery, orthopedics, and urology.)
The use of robots in cancer surgery is limited. The FDA approved specific RASDs in some “surgical procedures commonly performed in patients with cancer, such as hysterectomy, prostatectomy, and colectomy.”15 However, it cautioned that this clearance was based only on a 30-day patient follow up. More specifically, the FDA “has not evaluated the safety or effectiveness of RASD devices for the prevention or treatment of cancer, based on cancer-related outcomes such as overall survival, recurrence, and disease-free survival.”15
The FDA has clearly warned physicians and patients that the agency has not granted the use of RASDs “for any cancer-related surgery marketing authorization, and therefore the survival benefits to patients compared to traditional surgery have not been established.”15 (This did not apply to the hysterectomy surgery as noted above. More specifically, that clearance did not apply to anything other than 30-day results, nor to the efficacy related to cancer survival.)
States also have some authority to regulate medical practice within their borders.9 When the FDA has approved a device as safe and effective, however, there are limits on what states can do to regulate or impose liability on the approved product. The Supreme Court held that the FDA approval “pre-empted” some state action regarding approved devices.16
Hospitals, of course, regulate what is allowed within the hospital. For example, it may require training before a physician is permitted to use equipment, limit the conditions for which the equipment may be used, or decline to obtain equipment for use in the hospitals.17 In the case of RASDs, however, the high cost of equipment may provide an incentive for hospitals to urge the wide use of the latest robotic acquisition.18
Regulation aims primarily to protect patients, usually from injury or inadequate treatment. Some robotic surgery is likely to be more expensive than the same surgery without robotic assistance. The cost to the patient is not usually part of the FDA’s consideration. Insurance companies (including Medicare and Medicaid), however, do care about costs and will set or negotiate how much the reimbursement will be for a procedure. Third-party payers may decline to cover the additional cost when there is no apparent benefit from using the robot.19 For some institutions, the public perception that it offers “the most modern technology” is an important public message and a strong incentive to have the equipment.20
There are inconsistent studies about the advantages and disadvantages of RADS in gynecologic procedures, although there are few randomized studies.21 The demonstrated advantages are generally identified as somewhat shorter recovery time.22 The ultimate goal will be to minimize risks while maximizing the many potential benefits of robotic surgery.23
Continue to: Liability...
Liability
A recent study by De Ravin and colleagues of robotic surgery liability found a 250% increase in the total number of robotic surgery–related malpractice claims reported in 7 recent years (2014-2021), compared with the prior 7 (2006-2013).24 However, the number of cases varied considerably from year to year. ObGyn had the most significant gain (from 19% to 49% of all claims). During the same time, urology claims declined from 56% to 16%. (The limitations of the study’s data are discussed later in this article.)
De Ravin et al reported the legal bases for the claims, but the specific legal claim was unclear in many cases.24 For example, the vast majority were classified as “negligent surgery.” Many cases made more than 1 legal claim for liability, so the total percentages were greater than 100%. Of the specific claims, many appear unrelated to robotic surgery (misdiagnosis, delayed treatment, or infection). However, there were a significant number of cases that raised issues that were related to robotic surgery. The following are those claims that probably relate to the “robotic” surgery, along with the percentage of cases making such a claim as reported24:
- “Patient not a candidate for surgery performed” appeared in about 13% of the cases.24 Such claims could include that the surgeon should have performed the surgery with traditional laparoscopy or open technique, but instead using a robot led to the injury. Physicians may feel pressure from patients or hospitals, because of the equipment’s cost, to use robotic surgery as it seems to be the modern approach (and therefore better). Neither reason is sufficient for using robotic assistance unless it will benefit the patient.
- “Failure to calibrate or operate robot” was in 11% of the claims.24 Physicians must properly calibrate and otherwise ensure that surgical equipment is operating correctly. In addition, the hospitals supplying the equipment must ensure that the equipment is maintained correctly. Finally, the equipment manufacturer may be liable through “products liability” if the equipment is defective.25 The expanding use of artificial intelligence in medical equipment (including surgical robots) is increasing the complexity of determining what “defective” means.11
- “Training deficiencies or credentialing” liability is a common problem with new technology. Physicians using new technology should be thoroughly trained and, where appropriate, certified in the use of the new technology.26 Early adopters of the technology should be especially cautious because good training may be challenging to obtain. In the study, the claims of inadequate training were particularly high during the early 7 years (35%), but dropped during the later time (4%).24
- “Improper positioning” of the patient or device or patient was raised in 7% of the cases.24
- “Manufacturing problems” were claimed in a small number of cases—13% in 2006-2013, but 2% in 2014-2021.24 These cases raise the complex question of products liability for robotic surgery and artificial intelligence (AI). Products liability has been part of surgical practice for many years. There usually will be liability if there are “defects” in a product, whether or not resulting from negligence. What a “defect” in a computer program means is a complicated issue that will be significant in future liability cases.27
Several other cases reported in the De Ravin study were probably related to robotic surgery. For example, Informed Consent and Failure to Monitor each appeared in more than 30%, of 2014-2021 cases, and Failure to Refer in 16% of the cases.24,27
The outcomes of the reported cases were mostly verdicts (or trial-related settlements) for defendants (doctors and hospitals). The defense prevailed 69% of the time in the early period and 78% of the time in 2014-2021. However, there were substantial damages in some cases. The range of damages in 2006-2013 was $95,000 to $6 million (mean, $2.5 million); in 2014-2021, it was $10,000 to $5 million (mean, $1.3 million).24
An earlier study looked at reported cases against Intuitive Surgical, maker of the daVinci system, from 2000-2017.28 Of the 108 claims in the study, 62% were gynecologic surgeries. Of these claims, 35% were dismissed, but “no other information regarding settlements or trial outcomes was available.” The study did not report the basis for the lawsuits involving gynecologic surgeries.
We should exercise caution in reviewing these studies. Although the studies were of considerable value, the authors note significant limitations of the databases available. The database was Westlaw in the first study discussed (“Robotic surgery: the impact”24) and Bloomberg in the second (“Robotic urologic”28). For example, the “impact” study was based on “jury verdict reports” excluding settlements, and the latter excluded class actions and cases settled. Thus the studies undoubtedly understated the number of claims made (those that resulted in settlement before a lawsuit was filed), cases filed but abandoned, and settlements made before trial.
Despite these limitations, the studies provide valuable insights into current malpractice risks and future directions. It is worth remembering that these cases nearly all involved a single robot, the daVinci, produced by Intuitive Surgical. It is not a “smart” robot and is commonly referred to as a “master-slave” machine. With much more intelligent and independent machines, the future will raise more complex problems in the FDA approval process and malpractice and product liability claims when things go wrong.
Continue to: What’s the verdict?...
What’s the verdict?
The case of VM and operating surgeon Dr. G illustrates several important legal aspects of using surgical robots. It also demonstrates that the presence of the robot assist still requires the surgeon’s careful attention to issues of informed consent, adequate specific training, and thorough follow up. In the following discussion, we divide the case review into the elements of negligence-malpractice (duty and breach, causation, and damages) and conclude with a thought about how to proceed when things have gone wrong.
Dr. G’s statement, “I’ve done a few,” is indefinite, but it may suggest that Dr. G. had not received full, supervised training in the robotic assist he was planning to use. That problem was underlined by the conclusion that Dr. G was a “relatively inexperienced robotic surgeon.” If so, that failure could constitute a breach of the duty of care to the patient. In addition, if it is inaccurate or did not provide information VM reasonably needed in consenting to Dr. G proceeding with the surgery, there could be an issue of whether there was a partial failure of fully informed consent.
The hospital also may have potential liability. It was “taken to task for granting privileges to an individual that had prior privilege ‘problems,’” suggesting that it had not performed adequate review before granting hospital privileges. Furthermore, if Dr. G was not sufficiently practiced or supervised in robotic surgery, the hospital, which allowed Dr. G to proceed, might also be negligent.
VM had a series of problems postsurgery that ultimately resulted in additional care and “simple fistula repair.” Assuming that there was negligence, the next question is whether that failure caused the injury. Causation may be the most difficult part of the case for VM to prove. It would require expert testimony that the inadequate surgery (inappropriate use of robotic surgery or other error during surgery) and follow up resulted in the formation or increase in the likelihood of the fistula.
VM would also have to prove damages. Damages are those costs (the economic value) of injuries that would not have occurred but for negligence. Damages would include most of the cost of the follow-up medical care and any related additional future care required, plus costs that were a consequence of the negligence (such as lost work). In addition, damages would include pain and suffering that resulted from the negligence, subject to caps in some states.
When the patient was dissatisfied and reported a postsurgical problem, the hospital and Dr. G may have had an opportunity to avoid further dissatisfaction, complaints, and ultimately a lawsuit. Effective approaches for dealing with such dissatisfaction may serve the institution’s and physician’s values and financial best interests.
The jury verdict was in favor of the plaintiff. Jurors felt the operating surgeon should have conveyed his experience with robotic surgery more clearly as part of the informed consent process.
“Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!”29
Medicine is still at the frontier of surgical robots. Over future decades, the number and sophistication of these machines will increase substantially. They likely will become much more like robots, guided by AI, and make independent judgments. These have the potential for significant medical progress that improves the treatment of patients. At the same time, the last 20 years suggest that robotic innovation will challenge medicine, the FDA and other regulators, lawmakers, and courts. In the future, regulators and patients should embrace genuine advances in robotic surgery but not be dazzled by these new machines’ luster (or potential for considerable profits).30
The public may be wildly optimistic about the benefits without balancing the risks. The AI that runs them will be essentially invisible and constantly changing. Physicians and regulators must develop new techniques for assessing and controlling the software. Real surgical robots require rigorous testing, cautious promotion, disciplined use, and perpetual review. ●
- Petersen S, Doe S, Rubinfield I, et al. Rate of urologic injury with robotic hysterectomy. J Min Invasc Gynecol. 2018;25:867-871.
- Makinen J, Johansson J, Toma C, et al. Morbidity of 10,110 hysterectomies by type approach. Hum Reprod. 2001;16:1473-1478.
- Karasu A, Kran G, Sanlikan F. Intraoperative complications and conversion to laparotomy in gynecologic robotic surgery. J Investig Surg. 2022;35:912-915.
- Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality rates in benign laparoscopic and robotic surgery: a systematic review and meta-analysis. J Min Invasc. 2020;27:603-612.
- Giurdano S, Victorzon M. Laparoscopic roux-en-Y gastric bypass in elderly patients (60 years or older): a meta-analysis of comparative studies. Scand J Surg. 2018;107:6-11.
- Marra A, Pulg-Asensio M, Edmond M, et al. Infectious complications of laparoscopic and robotic hysterectomy: a systematic literature review and meta-analysis. Int J Gynecol Cancer. 2019;29:518-530.
- Tse KY, Sheung H, Lim P. Robot-assisted gyneaecological cancer surgery-complications and prevention. Best Pract Res Clin Obstet Gynaecol. 2017;25:94-105.
- Hubbard FP. Sophisticated robots: balancing liability, regulation, and innovation. Fla Law Rev. 2014;66:1803-1872. https://scholarship.law.ufl.edu/cgi/viewcontent. cgi?article=1204&context=flr. Accessed December 20, 2022.
- Villanueva A. The legal battle with the future of autonomous surgical robotics. Ind Health Law Rev. 2020;17:367-392. https://journals.iupui.edu/index.php/ihlr/article /download/25051/23544. Accessed December 20, 2022.
- Lemley MA, Casey B. Remedies for robots. U Chi Law Rev. 2019;86:1311-1396. https://chicagounbound.uchicago.edu /cgi/viewcontent.cgi?article=6140&context=uclrev. Accessed December 20, 2022.
- Griffin F. Artificial intelligence and liability in health care. Health Matrix. 2021;31:65-106. https://scholarlycommons. law.case.edu/cgi/viewcontent.cgi?article=1659&context=hea lthmatrix. Accessed December 20, 2022.
- Britton D. Autonomous surgery: the law of autonomous surgical robots. J Law Tech Tex. 2017;1:152-189.
- US Food and Drug Administration. FDA clears new robotically-assisted surgical device for adult patients. October 13, 2017. https://www.fda.gov/news-events/press-announcements /fda-clears-new-robotically-assisted-surgical-device-adult -patients. Accessed December 20, 2022.
- US Food and Drug Administration. FDA authorizes first robotically-assisted surgical device for performing transvaginal hysterectomy. March 1, 2021. https://www.fda .gov/news-events/press-announcements/fda-authorizes -first-robotically-assisted-surgical-device-performing -transvaginal-hysterectomy. Accessed December 20, 2022.
- US Food and Drug Administration. Caution with robotically-assisted surgical devices in mastectomy: FDA Safety Communication, August 20, 2021. https://www.fda.gov/medical-devices/safety-communications/update-caution-robotically-assisted-surgical-devices-mastectomy-fda-safety-communication. Accessed December 22, 2022. Riegel v Medtronic, 552 US 312 (2008).
- Han ES, Advincula AP. Robotic surgery: advancements and inflection points in the field of gynecology. Obstet Gynecol Clin North Am. 2021;48:759-776.
- Witharm H. Robot-assisted surgery: an analysis of the legal and economic implications. Az J Interdisciplinary Studies. 2022;8:19-29. https://journals.librarypublishing.arizona.edu /azjis/article/id/5093/download/pdf/.
- Cameron S. Is daVinci robotic surgery a revolution or a rip-off? Healthline. August 10, 2016. https://www.healthline .com/health-news/is-da-vinci-robotic-surgery-revolution -or-ripoff-021215. Accessed December 20, 2022.
- Perez RE, Schwaitzberg SD. Robotic surgery: finding value in 2019 and beyond. Ann Laparosc Endosc Surg. 2019;4:1-7.
- Gitas G, Hanker L, Rody A, et al. Robotic surgery in gynecology: is the future already here? Minim Invasiv Therapy Allied Technol. 2022;4:1-0.
- Moon AS, Garofalo J, Koirala P, et al. Robotic surgery in gynecology. Surgical Clinics. 2020;100:445-460.
- Simshaw D, Terry N, Hauser K, et al. Regulating healthcare robots: maximizing opportunities while minimizing risks. Richmond J Law Tech. 2015;22:1-38. https://scholar works.iupui.edu/bitstream/handle/1805/11587/simshaw _2015_regulating.pdf?sequence=1&isAllowed=y. Accessed December 20, 2022.
- De Ravin E, Sell EA, Newman JG, et al. Medical malpractice in robotic surgery: a Westlaw database analysis. J Robotic Surg. 2022. https://doi.org/10.1007/s11701-022-01417-6. https:// link.springer.com/article/10.1007/s11701-022-014176#citeas. Accessed December 20, 2022.
- Beglinger C. A broken theory: the malfunction theory of strict products liability and the need for a new doctrine in the field of surgical robotics. Minnesotta Law Rev. 2019;104:1041-1093. . Accessed December 20, 2022.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasiv Gynecol. 2021;28:490-495.
- Koerner D. Doctor roboto: The no-man operation. U Tol L Rev. 2019;51:125-146.
- Nik-Ahd F, Souders CP, Zhao H, et al. Robotic urologic surgery: trends in litigation over the last decade. J Robotic Surg. 2019;13:729-734.
- Gültekin CalibriİB, Karabük E, Köse MF. “Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!” What is autonomous surgery and what are the latest developments? J Turk Ger Gynecol Assoc. 2021;22:58-70. https://www.ncbi .nlm.nih.gov/pmc/articles/PMC7944239/.
- Matsuzaki T. Ethical issues of artificial intelligence in medicine. California West Law Rev. 2018;55:255-273. https://scholarlycommons.law.cwsl.edu/cgi/viewcontent. cgi?article=1669&context=cwlr. Accessed December 20, 2022.
The approach to hysterectomy has been debated, with the need for individualization case by case stressed, and the expertise of the operating surgeon considered.
CASE Was surgeon experience a factor in case complications?
VM is a 46-year-old woman (G5 P4014) reporting persistent uterine bleeding that is refractory to medical therapy. The patient has uterine fibroids, 6 weeks in size on examination, with “mild” prolapse noted. Additional medical diagnoses included vulvitis, ovarian cyst in the past, cystic mastopathy, and prior evidence of pelvic adhesion, noted at the time of ovarian cystectomy. Prior surgical records were not obtained by the operating surgeon, although her obstetric history includes 2 prior vaginal deliveries and 2 cesarean deliveries (CDs). The patient had an umbilical herniorraphy a number of years ago. Her medications include hormonal therapy, for presumed menopause, and medication for depression (she reported “doing well” on medication). She reported smoking 1 PPD and had a prior tubal ligation.
VM was previously evaluated for Lynch Syndrome and informed of the potential for increased risks of colon, endometrial, and several other cancers. She did not have cancer as of the time of planned surgery.
The patient underwent robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. The operating surgeon did not have a lot of experience with robotic hysterectomies but told the patient preoperatively “I have done a few.” Perioperatively, blood loss was minimal, urine output was recorded as 25 mL, and according to the operative report there were extensive pelvic adhesions and no complications. The “ureters were identified” when the broad ligament was opened at the time of skeletonization of the uterine vessels and documented accordingly. The intraoperative Foley was discontinued at the end of the procedure. The pathology report noted diffuse adenomyosis and uterine fibroids; the uterus weighed 250 g. In addition, a “large hemorrhagic corpus luteum cyst” was noted on the right ovary.
The patient presented for a postoperative visit reporting “leaking” serosanguinous fluid that began 2.5 weeks postoperatively and required her to wear 3 to 4 “Depends” every day. She also reported constipation since beginning her prescribed pain medication. She requested a copy of her medical records and said she was dissatisfied with the care she had received related to the hysterectomy; she was “seeking a second opinion from a urologist.” The urologist suggested evaluation of the “leaking,” and a Foley catheter was placed. When she stood up, however, there was leaking around the catheter, and she reported a “yellowish-green,” foul smelling discharge. She called the urologist’s office, stating, “I think I have a bowel obstruction.” The patient was instructed to proceed to the emergency department at her local hospital. She was released with a diagnosis of constipation. Upon follow-up urologic evaluation, a vulvovaginal fistula was noted. Management was a “simple fistula repair,” and the patient did well subsequently.
The patient brought suit against the hospital and operating gynecologist. In part the hospital records noted, “relatively inexperienced robotic surgeon.” The hospital was taken to task for granting privileges to an individual that had prior privilege “problems.”

Continue to: Medical opinion...
Medical opinion
This case demonstrates a number of issues. (We will discuss the credentials for the surgeon and hospital privileges in the legal considerations section.) From the medical perspective, the rate of urologic injury associated with all hysterectomies is 0.87%.1 Robotic hysterectomy has been reported at 0.92% in a series published from Henry Ford Hospital.1 The lowest rate of urologic injury is associated with vaginal hysterectomy, reported at 0.2%.2 Reported rates of urologic injury by approach to hysterectomy are1:
- robotic, 0.92%
- laparoscopic, 0.90%
- vaginal, 0.33%
- abdominal, 0.96%.
Complications by surgeon type also have been addressed, and the percent of total urologic complications are reported as1:
- ObGyn, 47%
- gyn oncologist, 47%
- urogynecologist, 6%.
Intraoperative conversion to laparotomy from initial robotic approach has been addressed in a retrospective study over a 2-year period, with operative times ranging from 1 hr, 50 min to 9 hrs of surgical time.1 The vast majority of intraoperative complications in a series reported from Finland were managed “within minutes,” and in the series of 83 patients, 5 (6%) required conversion to laparotomy.2 Intraoperative complications reported include failed entry, vascular injury, nerve injury, visceral injury, solid organ injury, tumor fragmentation, and anesthetic-related complications.3 Of note, the vascular injuries included inferior vena cava, common iliac, and external iliac.
Mortality rates in association with benign laparoscopic and robotic procedures have been addressed and noted to be 1:6,456 cases based upon a meta-analysis.4 The analysis included 124,216 patients. Laparoscopic versus robotic mortality rates were not statistically different. Mortality was more common among cases of undiagnosed rare colorectal injury. This mortality is on par with complications from Roux-en-Y gastric bypass procedures. Procedures such as sacrocolpopexy are equated with higher mortality (1:1,246) in comparison with benign hysterectomy.5
Infectious complications following either laparoscopic or robotic hysterectomy were reported at less than 1% and not statistically different for either approach.6 The series authored by Marra et al evaluated 176,016 patients.
Overall, robotic-assisted gynecologic complications are rare. One series was focused on gynecological oncologic cases.7 Specific categories of complications included7:
- patient positioning and pneumoperitoneum
- injury to surrounding organs
- bowel injury
- port site metastasis
- surgical emphysema
- vaginal cuff dehiscence
- anesthesia-related problems.
The authors concluded, “robotic assisted surgery in gynecological oncology is safe and the incidence of complications is low.”7 The major cause of death related to robotic surgery is vascular injury–related. The authors emphasized the importance of knowledge of anatomy, basic principles of “traction and counter-traction” and proper dissection along tissue planes as key to minimizing complications. Consider placement of stents for ureter identification, as appropriate. Barbed-suturing does not prevent dehiscence.
Continue to: Legal considerations...
Legal considerations
Robotic surgery presents many legal issues and promises to raise many more in the future. The law must control new technology while encouraging productive uses, and provide new remedies for harms while respecting traditional legal principles.8 There is no shortage of good ideas about controlling surgical robots,9 automated devices more generally,10 and artificial intelligence.11 Those issues will be important, and watching them unfold will be intriguing.
In the meantime, physicians and other health care professionals, health care facilities, technology companies, and patients must work within current legal structures in implementing and using robotic surgery. These are extraordinarily complex issues, so it is possible only to review the current landscape and speculate what the near future may hold.
Regulating surgical robots
The US Food and Drug Administration (FDA) is the primary regulator of robots used in medicine.12 It has the authority to regulate surgical devices, including surgical robots—which it refers to as “robotically-assisted surgical devices,” or RASD. In 2000, it approved Intuitive Surgical’s daVinci system for use in surgery. In 2017, the FDA expanded its clearance to include the Senhance System of TransEnterix Surgical Inc. for minimally invasive gynecologic surgery.13 In 2021, the FDA cleared the Hominis Surgical System for transvaginal hysterectomy “in certain patients.” However, the FDA emphasized that this clearance is for benign hysterectomy with salpingo-oophorectomy.14 (The FDA has cleared various robotic devices for several other areas of surgical practice, including neurosurgery, orthopedics, and urology.)
The use of robots in cancer surgery is limited. The FDA approved specific RASDs in some “surgical procedures commonly performed in patients with cancer, such as hysterectomy, prostatectomy, and colectomy.”15 However, it cautioned that this clearance was based only on a 30-day patient follow up. More specifically, the FDA “has not evaluated the safety or effectiveness of RASD devices for the prevention or treatment of cancer, based on cancer-related outcomes such as overall survival, recurrence, and disease-free survival.”15
The FDA has clearly warned physicians and patients that the agency has not granted the use of RASDs “for any cancer-related surgery marketing authorization, and therefore the survival benefits to patients compared to traditional surgery have not been established.”15 (This did not apply to the hysterectomy surgery as noted above. More specifically, that clearance did not apply to anything other than 30-day results, nor to the efficacy related to cancer survival.)
States also have some authority to regulate medical practice within their borders.9 When the FDA has approved a device as safe and effective, however, there are limits on what states can do to regulate or impose liability on the approved product. The Supreme Court held that the FDA approval “pre-empted” some state action regarding approved devices.16
Hospitals, of course, regulate what is allowed within the hospital. For example, it may require training before a physician is permitted to use equipment, limit the conditions for which the equipment may be used, or decline to obtain equipment for use in the hospitals.17 In the case of RASDs, however, the high cost of equipment may provide an incentive for hospitals to urge the wide use of the latest robotic acquisition.18
Regulation aims primarily to protect patients, usually from injury or inadequate treatment. Some robotic surgery is likely to be more expensive than the same surgery without robotic assistance. The cost to the patient is not usually part of the FDA’s consideration. Insurance companies (including Medicare and Medicaid), however, do care about costs and will set or negotiate how much the reimbursement will be for a procedure. Third-party payers may decline to cover the additional cost when there is no apparent benefit from using the robot.19 For some institutions, the public perception that it offers “the most modern technology” is an important public message and a strong incentive to have the equipment.20
There are inconsistent studies about the advantages and disadvantages of RADS in gynecologic procedures, although there are few randomized studies.21 The demonstrated advantages are generally identified as somewhat shorter recovery time.22 The ultimate goal will be to minimize risks while maximizing the many potential benefits of robotic surgery.23
Continue to: Liability...
Liability
A recent study by De Ravin and colleagues of robotic surgery liability found a 250% increase in the total number of robotic surgery–related malpractice claims reported in 7 recent years (2014-2021), compared with the prior 7 (2006-2013).24 However, the number of cases varied considerably from year to year. ObGyn had the most significant gain (from 19% to 49% of all claims). During the same time, urology claims declined from 56% to 16%. (The limitations of the study’s data are discussed later in this article.)
De Ravin et al reported the legal bases for the claims, but the specific legal claim was unclear in many cases.24 For example, the vast majority were classified as “negligent surgery.” Many cases made more than 1 legal claim for liability, so the total percentages were greater than 100%. Of the specific claims, many appear unrelated to robotic surgery (misdiagnosis, delayed treatment, or infection). However, there were a significant number of cases that raised issues that were related to robotic surgery. The following are those claims that probably relate to the “robotic” surgery, along with the percentage of cases making such a claim as reported24:
- “Patient not a candidate for surgery performed” appeared in about 13% of the cases.24 Such claims could include that the surgeon should have performed the surgery with traditional laparoscopy or open technique, but instead using a robot led to the injury. Physicians may feel pressure from patients or hospitals, because of the equipment’s cost, to use robotic surgery as it seems to be the modern approach (and therefore better). Neither reason is sufficient for using robotic assistance unless it will benefit the patient.
- “Failure to calibrate or operate robot” was in 11% of the claims.24 Physicians must properly calibrate and otherwise ensure that surgical equipment is operating correctly. In addition, the hospitals supplying the equipment must ensure that the equipment is maintained correctly. Finally, the equipment manufacturer may be liable through “products liability” if the equipment is defective.25 The expanding use of artificial intelligence in medical equipment (including surgical robots) is increasing the complexity of determining what “defective” means.11
- “Training deficiencies or credentialing” liability is a common problem with new technology. Physicians using new technology should be thoroughly trained and, where appropriate, certified in the use of the new technology.26 Early adopters of the technology should be especially cautious because good training may be challenging to obtain. In the study, the claims of inadequate training were particularly high during the early 7 years (35%), but dropped during the later time (4%).24
- “Improper positioning” of the patient or device or patient was raised in 7% of the cases.24
- “Manufacturing problems” were claimed in a small number of cases—13% in 2006-2013, but 2% in 2014-2021.24 These cases raise the complex question of products liability for robotic surgery and artificial intelligence (AI). Products liability has been part of surgical practice for many years. There usually will be liability if there are “defects” in a product, whether or not resulting from negligence. What a “defect” in a computer program means is a complicated issue that will be significant in future liability cases.27
Several other cases reported in the De Ravin study were probably related to robotic surgery. For example, Informed Consent and Failure to Monitor each appeared in more than 30%, of 2014-2021 cases, and Failure to Refer in 16% of the cases.24,27
The outcomes of the reported cases were mostly verdicts (or trial-related settlements) for defendants (doctors and hospitals). The defense prevailed 69% of the time in the early period and 78% of the time in 2014-2021. However, there were substantial damages in some cases. The range of damages in 2006-2013 was $95,000 to $6 million (mean, $2.5 million); in 2014-2021, it was $10,000 to $5 million (mean, $1.3 million).24
An earlier study looked at reported cases against Intuitive Surgical, maker of the daVinci system, from 2000-2017.28 Of the 108 claims in the study, 62% were gynecologic surgeries. Of these claims, 35% were dismissed, but “no other information regarding settlements or trial outcomes was available.” The study did not report the basis for the lawsuits involving gynecologic surgeries.
We should exercise caution in reviewing these studies. Although the studies were of considerable value, the authors note significant limitations of the databases available. The database was Westlaw in the first study discussed (“Robotic surgery: the impact”24) and Bloomberg in the second (“Robotic urologic”28). For example, the “impact” study was based on “jury verdict reports” excluding settlements, and the latter excluded class actions and cases settled. Thus the studies undoubtedly understated the number of claims made (those that resulted in settlement before a lawsuit was filed), cases filed but abandoned, and settlements made before trial.
Despite these limitations, the studies provide valuable insights into current malpractice risks and future directions. It is worth remembering that these cases nearly all involved a single robot, the daVinci, produced by Intuitive Surgical. It is not a “smart” robot and is commonly referred to as a “master-slave” machine. With much more intelligent and independent machines, the future will raise more complex problems in the FDA approval process and malpractice and product liability claims when things go wrong.
Continue to: What’s the verdict?...
What’s the verdict?
The case of VM and operating surgeon Dr. G illustrates several important legal aspects of using surgical robots. It also demonstrates that the presence of the robot assist still requires the surgeon’s careful attention to issues of informed consent, adequate specific training, and thorough follow up. In the following discussion, we divide the case review into the elements of negligence-malpractice (duty and breach, causation, and damages) and conclude with a thought about how to proceed when things have gone wrong.
Dr. G’s statement, “I’ve done a few,” is indefinite, but it may suggest that Dr. G. had not received full, supervised training in the robotic assist he was planning to use. That problem was underlined by the conclusion that Dr. G was a “relatively inexperienced robotic surgeon.” If so, that failure could constitute a breach of the duty of care to the patient. In addition, if it is inaccurate or did not provide information VM reasonably needed in consenting to Dr. G proceeding with the surgery, there could be an issue of whether there was a partial failure of fully informed consent.
The hospital also may have potential liability. It was “taken to task for granting privileges to an individual that had prior privilege ‘problems,’” suggesting that it had not performed adequate review before granting hospital privileges. Furthermore, if Dr. G was not sufficiently practiced or supervised in robotic surgery, the hospital, which allowed Dr. G to proceed, might also be negligent.
VM had a series of problems postsurgery that ultimately resulted in additional care and “simple fistula repair.” Assuming that there was negligence, the next question is whether that failure caused the injury. Causation may be the most difficult part of the case for VM to prove. It would require expert testimony that the inadequate surgery (inappropriate use of robotic surgery or other error during surgery) and follow up resulted in the formation or increase in the likelihood of the fistula.
VM would also have to prove damages. Damages are those costs (the economic value) of injuries that would not have occurred but for negligence. Damages would include most of the cost of the follow-up medical care and any related additional future care required, plus costs that were a consequence of the negligence (such as lost work). In addition, damages would include pain and suffering that resulted from the negligence, subject to caps in some states.
When the patient was dissatisfied and reported a postsurgical problem, the hospital and Dr. G may have had an opportunity to avoid further dissatisfaction, complaints, and ultimately a lawsuit. Effective approaches for dealing with such dissatisfaction may serve the institution’s and physician’s values and financial best interests.
The jury verdict was in favor of the plaintiff. Jurors felt the operating surgeon should have conveyed his experience with robotic surgery more clearly as part of the informed consent process.
“Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!”29
Medicine is still at the frontier of surgical robots. Over future decades, the number and sophistication of these machines will increase substantially. They likely will become much more like robots, guided by AI, and make independent judgments. These have the potential for significant medical progress that improves the treatment of patients. At the same time, the last 20 years suggest that robotic innovation will challenge medicine, the FDA and other regulators, lawmakers, and courts. In the future, regulators and patients should embrace genuine advances in robotic surgery but not be dazzled by these new machines’ luster (or potential for considerable profits).30
The public may be wildly optimistic about the benefits without balancing the risks. The AI that runs them will be essentially invisible and constantly changing. Physicians and regulators must develop new techniques for assessing and controlling the software. Real surgical robots require rigorous testing, cautious promotion, disciplined use, and perpetual review. ●
The approach to hysterectomy has been debated, with the need for individualization case by case stressed, and the expertise of the operating surgeon considered.
CASE Was surgeon experience a factor in case complications?
VM is a 46-year-old woman (G5 P4014) reporting persistent uterine bleeding that is refractory to medical therapy. The patient has uterine fibroids, 6 weeks in size on examination, with “mild” prolapse noted. Additional medical diagnoses included vulvitis, ovarian cyst in the past, cystic mastopathy, and prior evidence of pelvic adhesion, noted at the time of ovarian cystectomy. Prior surgical records were not obtained by the operating surgeon, although her obstetric history includes 2 prior vaginal deliveries and 2 cesarean deliveries (CDs). The patient had an umbilical herniorraphy a number of years ago. Her medications include hormonal therapy, for presumed menopause, and medication for depression (she reported “doing well” on medication). She reported smoking 1 PPD and had a prior tubal ligation.
VM was previously evaluated for Lynch Syndrome and informed of the potential for increased risks of colon, endometrial, and several other cancers. She did not have cancer as of the time of planned surgery.
The patient underwent robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. The operating surgeon did not have a lot of experience with robotic hysterectomies but told the patient preoperatively “I have done a few.” Perioperatively, blood loss was minimal, urine output was recorded as 25 mL, and according to the operative report there were extensive pelvic adhesions and no complications. The “ureters were identified” when the broad ligament was opened at the time of skeletonization of the uterine vessels and documented accordingly. The intraoperative Foley was discontinued at the end of the procedure. The pathology report noted diffuse adenomyosis and uterine fibroids; the uterus weighed 250 g. In addition, a “large hemorrhagic corpus luteum cyst” was noted on the right ovary.
The patient presented for a postoperative visit reporting “leaking” serosanguinous fluid that began 2.5 weeks postoperatively and required her to wear 3 to 4 “Depends” every day. She also reported constipation since beginning her prescribed pain medication. She requested a copy of her medical records and said she was dissatisfied with the care she had received related to the hysterectomy; she was “seeking a second opinion from a urologist.” The urologist suggested evaluation of the “leaking,” and a Foley catheter was placed. When she stood up, however, there was leaking around the catheter, and she reported a “yellowish-green,” foul smelling discharge. She called the urologist’s office, stating, “I think I have a bowel obstruction.” The patient was instructed to proceed to the emergency department at her local hospital. She was released with a diagnosis of constipation. Upon follow-up urologic evaluation, a vulvovaginal fistula was noted. Management was a “simple fistula repair,” and the patient did well subsequently.
The patient brought suit against the hospital and operating gynecologist. In part the hospital records noted, “relatively inexperienced robotic surgeon.” The hospital was taken to task for granting privileges to an individual that had prior privilege “problems.”

Continue to: Medical opinion...
Medical opinion
This case demonstrates a number of issues. (We will discuss the credentials for the surgeon and hospital privileges in the legal considerations section.) From the medical perspective, the rate of urologic injury associated with all hysterectomies is 0.87%.1 Robotic hysterectomy has been reported at 0.92% in a series published from Henry Ford Hospital.1 The lowest rate of urologic injury is associated with vaginal hysterectomy, reported at 0.2%.2 Reported rates of urologic injury by approach to hysterectomy are1:
- robotic, 0.92%
- laparoscopic, 0.90%
- vaginal, 0.33%
- abdominal, 0.96%.
Complications by surgeon type also have been addressed, and the percent of total urologic complications are reported as1:
- ObGyn, 47%
- gyn oncologist, 47%
- urogynecologist, 6%.
Intraoperative conversion to laparotomy from initial robotic approach has been addressed in a retrospective study over a 2-year period, with operative times ranging from 1 hr, 50 min to 9 hrs of surgical time.1 The vast majority of intraoperative complications in a series reported from Finland were managed “within minutes,” and in the series of 83 patients, 5 (6%) required conversion to laparotomy.2 Intraoperative complications reported include failed entry, vascular injury, nerve injury, visceral injury, solid organ injury, tumor fragmentation, and anesthetic-related complications.3 Of note, the vascular injuries included inferior vena cava, common iliac, and external iliac.
Mortality rates in association with benign laparoscopic and robotic procedures have been addressed and noted to be 1:6,456 cases based upon a meta-analysis.4 The analysis included 124,216 patients. Laparoscopic versus robotic mortality rates were not statistically different. Mortality was more common among cases of undiagnosed rare colorectal injury. This mortality is on par with complications from Roux-en-Y gastric bypass procedures. Procedures such as sacrocolpopexy are equated with higher mortality (1:1,246) in comparison with benign hysterectomy.5
Infectious complications following either laparoscopic or robotic hysterectomy were reported at less than 1% and not statistically different for either approach.6 The series authored by Marra et al evaluated 176,016 patients.
Overall, robotic-assisted gynecologic complications are rare. One series was focused on gynecological oncologic cases.7 Specific categories of complications included7:
- patient positioning and pneumoperitoneum
- injury to surrounding organs
- bowel injury
- port site metastasis
- surgical emphysema
- vaginal cuff dehiscence
- anesthesia-related problems.
The authors concluded, “robotic assisted surgery in gynecological oncology is safe and the incidence of complications is low.”7 The major cause of death related to robotic surgery is vascular injury–related. The authors emphasized the importance of knowledge of anatomy, basic principles of “traction and counter-traction” and proper dissection along tissue planes as key to minimizing complications. Consider placement of stents for ureter identification, as appropriate. Barbed-suturing does not prevent dehiscence.
Continue to: Legal considerations...
Legal considerations
Robotic surgery presents many legal issues and promises to raise many more in the future. The law must control new technology while encouraging productive uses, and provide new remedies for harms while respecting traditional legal principles.8 There is no shortage of good ideas about controlling surgical robots,9 automated devices more generally,10 and artificial intelligence.11 Those issues will be important, and watching them unfold will be intriguing.
In the meantime, physicians and other health care professionals, health care facilities, technology companies, and patients must work within current legal structures in implementing and using robotic surgery. These are extraordinarily complex issues, so it is possible only to review the current landscape and speculate what the near future may hold.
Regulating surgical robots
The US Food and Drug Administration (FDA) is the primary regulator of robots used in medicine.12 It has the authority to regulate surgical devices, including surgical robots—which it refers to as “robotically-assisted surgical devices,” or RASD. In 2000, it approved Intuitive Surgical’s daVinci system for use in surgery. In 2017, the FDA expanded its clearance to include the Senhance System of TransEnterix Surgical Inc. for minimally invasive gynecologic surgery.13 In 2021, the FDA cleared the Hominis Surgical System for transvaginal hysterectomy “in certain patients.” However, the FDA emphasized that this clearance is for benign hysterectomy with salpingo-oophorectomy.14 (The FDA has cleared various robotic devices for several other areas of surgical practice, including neurosurgery, orthopedics, and urology.)
The use of robots in cancer surgery is limited. The FDA approved specific RASDs in some “surgical procedures commonly performed in patients with cancer, such as hysterectomy, prostatectomy, and colectomy.”15 However, it cautioned that this clearance was based only on a 30-day patient follow up. More specifically, the FDA “has not evaluated the safety or effectiveness of RASD devices for the prevention or treatment of cancer, based on cancer-related outcomes such as overall survival, recurrence, and disease-free survival.”15
The FDA has clearly warned physicians and patients that the agency has not granted the use of RASDs “for any cancer-related surgery marketing authorization, and therefore the survival benefits to patients compared to traditional surgery have not been established.”15 (This did not apply to the hysterectomy surgery as noted above. More specifically, that clearance did not apply to anything other than 30-day results, nor to the efficacy related to cancer survival.)
States also have some authority to regulate medical practice within their borders.9 When the FDA has approved a device as safe and effective, however, there are limits on what states can do to regulate or impose liability on the approved product. The Supreme Court held that the FDA approval “pre-empted” some state action regarding approved devices.16
Hospitals, of course, regulate what is allowed within the hospital. For example, it may require training before a physician is permitted to use equipment, limit the conditions for which the equipment may be used, or decline to obtain equipment for use in the hospitals.17 In the case of RASDs, however, the high cost of equipment may provide an incentive for hospitals to urge the wide use of the latest robotic acquisition.18
Regulation aims primarily to protect patients, usually from injury or inadequate treatment. Some robotic surgery is likely to be more expensive than the same surgery without robotic assistance. The cost to the patient is not usually part of the FDA’s consideration. Insurance companies (including Medicare and Medicaid), however, do care about costs and will set or negotiate how much the reimbursement will be for a procedure. Third-party payers may decline to cover the additional cost when there is no apparent benefit from using the robot.19 For some institutions, the public perception that it offers “the most modern technology” is an important public message and a strong incentive to have the equipment.20
There are inconsistent studies about the advantages and disadvantages of RADS in gynecologic procedures, although there are few randomized studies.21 The demonstrated advantages are generally identified as somewhat shorter recovery time.22 The ultimate goal will be to minimize risks while maximizing the many potential benefits of robotic surgery.23
Continue to: Liability...
Liability
A recent study by De Ravin and colleagues of robotic surgery liability found a 250% increase in the total number of robotic surgery–related malpractice claims reported in 7 recent years (2014-2021), compared with the prior 7 (2006-2013).24 However, the number of cases varied considerably from year to year. ObGyn had the most significant gain (from 19% to 49% of all claims). During the same time, urology claims declined from 56% to 16%. (The limitations of the study’s data are discussed later in this article.)
De Ravin et al reported the legal bases for the claims, but the specific legal claim was unclear in many cases.24 For example, the vast majority were classified as “negligent surgery.” Many cases made more than 1 legal claim for liability, so the total percentages were greater than 100%. Of the specific claims, many appear unrelated to robotic surgery (misdiagnosis, delayed treatment, or infection). However, there were a significant number of cases that raised issues that were related to robotic surgery. The following are those claims that probably relate to the “robotic” surgery, along with the percentage of cases making such a claim as reported24:
- “Patient not a candidate for surgery performed” appeared in about 13% of the cases.24 Such claims could include that the surgeon should have performed the surgery with traditional laparoscopy or open technique, but instead using a robot led to the injury. Physicians may feel pressure from patients or hospitals, because of the equipment’s cost, to use robotic surgery as it seems to be the modern approach (and therefore better). Neither reason is sufficient for using robotic assistance unless it will benefit the patient.
- “Failure to calibrate or operate robot” was in 11% of the claims.24 Physicians must properly calibrate and otherwise ensure that surgical equipment is operating correctly. In addition, the hospitals supplying the equipment must ensure that the equipment is maintained correctly. Finally, the equipment manufacturer may be liable through “products liability” if the equipment is defective.25 The expanding use of artificial intelligence in medical equipment (including surgical robots) is increasing the complexity of determining what “defective” means.11
- “Training deficiencies or credentialing” liability is a common problem with new technology. Physicians using new technology should be thoroughly trained and, where appropriate, certified in the use of the new technology.26 Early adopters of the technology should be especially cautious because good training may be challenging to obtain. In the study, the claims of inadequate training were particularly high during the early 7 years (35%), but dropped during the later time (4%).24
- “Improper positioning” of the patient or device or patient was raised in 7% of the cases.24
- “Manufacturing problems” were claimed in a small number of cases—13% in 2006-2013, but 2% in 2014-2021.24 These cases raise the complex question of products liability for robotic surgery and artificial intelligence (AI). Products liability has been part of surgical practice for many years. There usually will be liability if there are “defects” in a product, whether or not resulting from negligence. What a “defect” in a computer program means is a complicated issue that will be significant in future liability cases.27
Several other cases reported in the De Ravin study were probably related to robotic surgery. For example, Informed Consent and Failure to Monitor each appeared in more than 30%, of 2014-2021 cases, and Failure to Refer in 16% of the cases.24,27
The outcomes of the reported cases were mostly verdicts (or trial-related settlements) for defendants (doctors and hospitals). The defense prevailed 69% of the time in the early period and 78% of the time in 2014-2021. However, there were substantial damages in some cases. The range of damages in 2006-2013 was $95,000 to $6 million (mean, $2.5 million); in 2014-2021, it was $10,000 to $5 million (mean, $1.3 million).24
An earlier study looked at reported cases against Intuitive Surgical, maker of the daVinci system, from 2000-2017.28 Of the 108 claims in the study, 62% were gynecologic surgeries. Of these claims, 35% were dismissed, but “no other information regarding settlements or trial outcomes was available.” The study did not report the basis for the lawsuits involving gynecologic surgeries.
We should exercise caution in reviewing these studies. Although the studies were of considerable value, the authors note significant limitations of the databases available. The database was Westlaw in the first study discussed (“Robotic surgery: the impact”24) and Bloomberg in the second (“Robotic urologic”28). For example, the “impact” study was based on “jury verdict reports” excluding settlements, and the latter excluded class actions and cases settled. Thus the studies undoubtedly understated the number of claims made (those that resulted in settlement before a lawsuit was filed), cases filed but abandoned, and settlements made before trial.
Despite these limitations, the studies provide valuable insights into current malpractice risks and future directions. It is worth remembering that these cases nearly all involved a single robot, the daVinci, produced by Intuitive Surgical. It is not a “smart” robot and is commonly referred to as a “master-slave” machine. With much more intelligent and independent machines, the future will raise more complex problems in the FDA approval process and malpractice and product liability claims when things go wrong.
Continue to: What’s the verdict?...
What’s the verdict?
The case of VM and operating surgeon Dr. G illustrates several important legal aspects of using surgical robots. It also demonstrates that the presence of the robot assist still requires the surgeon’s careful attention to issues of informed consent, adequate specific training, and thorough follow up. In the following discussion, we divide the case review into the elements of negligence-malpractice (duty and breach, causation, and damages) and conclude with a thought about how to proceed when things have gone wrong.
Dr. G’s statement, “I’ve done a few,” is indefinite, but it may suggest that Dr. G. had not received full, supervised training in the robotic assist he was planning to use. That problem was underlined by the conclusion that Dr. G was a “relatively inexperienced robotic surgeon.” If so, that failure could constitute a breach of the duty of care to the patient. In addition, if it is inaccurate or did not provide information VM reasonably needed in consenting to Dr. G proceeding with the surgery, there could be an issue of whether there was a partial failure of fully informed consent.
The hospital also may have potential liability. It was “taken to task for granting privileges to an individual that had prior privilege ‘problems,’” suggesting that it had not performed adequate review before granting hospital privileges. Furthermore, if Dr. G was not sufficiently practiced or supervised in robotic surgery, the hospital, which allowed Dr. G to proceed, might also be negligent.
VM had a series of problems postsurgery that ultimately resulted in additional care and “simple fistula repair.” Assuming that there was negligence, the next question is whether that failure caused the injury. Causation may be the most difficult part of the case for VM to prove. It would require expert testimony that the inadequate surgery (inappropriate use of robotic surgery or other error during surgery) and follow up resulted in the formation or increase in the likelihood of the fistula.
VM would also have to prove damages. Damages are those costs (the economic value) of injuries that would not have occurred but for negligence. Damages would include most of the cost of the follow-up medical care and any related additional future care required, plus costs that were a consequence of the negligence (such as lost work). In addition, damages would include pain and suffering that resulted from the negligence, subject to caps in some states.
When the patient was dissatisfied and reported a postsurgical problem, the hospital and Dr. G may have had an opportunity to avoid further dissatisfaction, complaints, and ultimately a lawsuit. Effective approaches for dealing with such dissatisfaction may serve the institution’s and physician’s values and financial best interests.
The jury verdict was in favor of the plaintiff. Jurors felt the operating surgeon should have conveyed his experience with robotic surgery more clearly as part of the informed consent process.
“Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!”29
Medicine is still at the frontier of surgical robots. Over future decades, the number and sophistication of these machines will increase substantially. They likely will become much more like robots, guided by AI, and make independent judgments. These have the potential for significant medical progress that improves the treatment of patients. At the same time, the last 20 years suggest that robotic innovation will challenge medicine, the FDA and other regulators, lawmakers, and courts. In the future, regulators and patients should embrace genuine advances in robotic surgery but not be dazzled by these new machines’ luster (or potential for considerable profits).30
The public may be wildly optimistic about the benefits without balancing the risks. The AI that runs them will be essentially invisible and constantly changing. Physicians and regulators must develop new techniques for assessing and controlling the software. Real surgical robots require rigorous testing, cautious promotion, disciplined use, and perpetual review. ●
- Petersen S, Doe S, Rubinfield I, et al. Rate of urologic injury with robotic hysterectomy. J Min Invasc Gynecol. 2018;25:867-871.
- Makinen J, Johansson J, Toma C, et al. Morbidity of 10,110 hysterectomies by type approach. Hum Reprod. 2001;16:1473-1478.
- Karasu A, Kran G, Sanlikan F. Intraoperative complications and conversion to laparotomy in gynecologic robotic surgery. J Investig Surg. 2022;35:912-915.
- Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality rates in benign laparoscopic and robotic surgery: a systematic review and meta-analysis. J Min Invasc. 2020;27:603-612.
- Giurdano S, Victorzon M. Laparoscopic roux-en-Y gastric bypass in elderly patients (60 years or older): a meta-analysis of comparative studies. Scand J Surg. 2018;107:6-11.
- Marra A, Pulg-Asensio M, Edmond M, et al. Infectious complications of laparoscopic and robotic hysterectomy: a systematic literature review and meta-analysis. Int J Gynecol Cancer. 2019;29:518-530.
- Tse KY, Sheung H, Lim P. Robot-assisted gyneaecological cancer surgery-complications and prevention. Best Pract Res Clin Obstet Gynaecol. 2017;25:94-105.
- Hubbard FP. Sophisticated robots: balancing liability, regulation, and innovation. Fla Law Rev. 2014;66:1803-1872. https://scholarship.law.ufl.edu/cgi/viewcontent. cgi?article=1204&context=flr. Accessed December 20, 2022.
- Villanueva A. The legal battle with the future of autonomous surgical robotics. Ind Health Law Rev. 2020;17:367-392. https://journals.iupui.edu/index.php/ihlr/article /download/25051/23544. Accessed December 20, 2022.
- Lemley MA, Casey B. Remedies for robots. U Chi Law Rev. 2019;86:1311-1396. https://chicagounbound.uchicago.edu /cgi/viewcontent.cgi?article=6140&context=uclrev. Accessed December 20, 2022.
- Griffin F. Artificial intelligence and liability in health care. Health Matrix. 2021;31:65-106. https://scholarlycommons. law.case.edu/cgi/viewcontent.cgi?article=1659&context=hea lthmatrix. Accessed December 20, 2022.
- Britton D. Autonomous surgery: the law of autonomous surgical robots. J Law Tech Tex. 2017;1:152-189.
- US Food and Drug Administration. FDA clears new robotically-assisted surgical device for adult patients. October 13, 2017. https://www.fda.gov/news-events/press-announcements /fda-clears-new-robotically-assisted-surgical-device-adult -patients. Accessed December 20, 2022.
- US Food and Drug Administration. FDA authorizes first robotically-assisted surgical device for performing transvaginal hysterectomy. March 1, 2021. https://www.fda .gov/news-events/press-announcements/fda-authorizes -first-robotically-assisted-surgical-device-performing -transvaginal-hysterectomy. Accessed December 20, 2022.
- US Food and Drug Administration. Caution with robotically-assisted surgical devices in mastectomy: FDA Safety Communication, August 20, 2021. https://www.fda.gov/medical-devices/safety-communications/update-caution-robotically-assisted-surgical-devices-mastectomy-fda-safety-communication. Accessed December 22, 2022. Riegel v Medtronic, 552 US 312 (2008).
- Han ES, Advincula AP. Robotic surgery: advancements and inflection points in the field of gynecology. Obstet Gynecol Clin North Am. 2021;48:759-776.
- Witharm H. Robot-assisted surgery: an analysis of the legal and economic implications. Az J Interdisciplinary Studies. 2022;8:19-29. https://journals.librarypublishing.arizona.edu /azjis/article/id/5093/download/pdf/.
- Cameron S. Is daVinci robotic surgery a revolution or a rip-off? Healthline. August 10, 2016. https://www.healthline .com/health-news/is-da-vinci-robotic-surgery-revolution -or-ripoff-021215. Accessed December 20, 2022.
- Perez RE, Schwaitzberg SD. Robotic surgery: finding value in 2019 and beyond. Ann Laparosc Endosc Surg. 2019;4:1-7.
- Gitas G, Hanker L, Rody A, et al. Robotic surgery in gynecology: is the future already here? Minim Invasiv Therapy Allied Technol. 2022;4:1-0.
- Moon AS, Garofalo J, Koirala P, et al. Robotic surgery in gynecology. Surgical Clinics. 2020;100:445-460.
- Simshaw D, Terry N, Hauser K, et al. Regulating healthcare robots: maximizing opportunities while minimizing risks. Richmond J Law Tech. 2015;22:1-38. https://scholar works.iupui.edu/bitstream/handle/1805/11587/simshaw _2015_regulating.pdf?sequence=1&isAllowed=y. Accessed December 20, 2022.
- De Ravin E, Sell EA, Newman JG, et al. Medical malpractice in robotic surgery: a Westlaw database analysis. J Robotic Surg. 2022. https://doi.org/10.1007/s11701-022-01417-6. https:// link.springer.com/article/10.1007/s11701-022-014176#citeas. Accessed December 20, 2022.
- Beglinger C. A broken theory: the malfunction theory of strict products liability and the need for a new doctrine in the field of surgical robotics. Minnesotta Law Rev. 2019;104:1041-1093. . Accessed December 20, 2022.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasiv Gynecol. 2021;28:490-495.
- Koerner D. Doctor roboto: The no-man operation. U Tol L Rev. 2019;51:125-146.
- Nik-Ahd F, Souders CP, Zhao H, et al. Robotic urologic surgery: trends in litigation over the last decade. J Robotic Surg. 2019;13:729-734.
- Gültekin CalibriİB, Karabük E, Köse MF. “Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!” What is autonomous surgery and what are the latest developments? J Turk Ger Gynecol Assoc. 2021;22:58-70. https://www.ncbi .nlm.nih.gov/pmc/articles/PMC7944239/.
- Matsuzaki T. Ethical issues of artificial intelligence in medicine. California West Law Rev. 2018;55:255-273. https://scholarlycommons.law.cwsl.edu/cgi/viewcontent. cgi?article=1669&context=cwlr. Accessed December 20, 2022.
- Petersen S, Doe S, Rubinfield I, et al. Rate of urologic injury with robotic hysterectomy. J Min Invasc Gynecol. 2018;25:867-871.
- Makinen J, Johansson J, Toma C, et al. Morbidity of 10,110 hysterectomies by type approach. Hum Reprod. 2001;16:1473-1478.
- Karasu A, Kran G, Sanlikan F. Intraoperative complications and conversion to laparotomy in gynecologic robotic surgery. J Investig Surg. 2022;35:912-915.
- Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality rates in benign laparoscopic and robotic surgery: a systematic review and meta-analysis. J Min Invasc. 2020;27:603-612.
- Giurdano S, Victorzon M. Laparoscopic roux-en-Y gastric bypass in elderly patients (60 years or older): a meta-analysis of comparative studies. Scand J Surg. 2018;107:6-11.
- Marra A, Pulg-Asensio M, Edmond M, et al. Infectious complications of laparoscopic and robotic hysterectomy: a systematic literature review and meta-analysis. Int J Gynecol Cancer. 2019;29:518-530.
- Tse KY, Sheung H, Lim P. Robot-assisted gyneaecological cancer surgery-complications and prevention. Best Pract Res Clin Obstet Gynaecol. 2017;25:94-105.
- Hubbard FP. Sophisticated robots: balancing liability, regulation, and innovation. Fla Law Rev. 2014;66:1803-1872. https://scholarship.law.ufl.edu/cgi/viewcontent. cgi?article=1204&context=flr. Accessed December 20, 2022.
- Villanueva A. The legal battle with the future of autonomous surgical robotics. Ind Health Law Rev. 2020;17:367-392. https://journals.iupui.edu/index.php/ihlr/article /download/25051/23544. Accessed December 20, 2022.
- Lemley MA, Casey B. Remedies for robots. U Chi Law Rev. 2019;86:1311-1396. https://chicagounbound.uchicago.edu /cgi/viewcontent.cgi?article=6140&context=uclrev. Accessed December 20, 2022.
- Griffin F. Artificial intelligence and liability in health care. Health Matrix. 2021;31:65-106. https://scholarlycommons. law.case.edu/cgi/viewcontent.cgi?article=1659&context=hea lthmatrix. Accessed December 20, 2022.
- Britton D. Autonomous surgery: the law of autonomous surgical robots. J Law Tech Tex. 2017;1:152-189.
- US Food and Drug Administration. FDA clears new robotically-assisted surgical device for adult patients. October 13, 2017. https://www.fda.gov/news-events/press-announcements /fda-clears-new-robotically-assisted-surgical-device-adult -patients. Accessed December 20, 2022.
- US Food and Drug Administration. FDA authorizes first robotically-assisted surgical device for performing transvaginal hysterectomy. March 1, 2021. https://www.fda .gov/news-events/press-announcements/fda-authorizes -first-robotically-assisted-surgical-device-performing -transvaginal-hysterectomy. Accessed December 20, 2022.
- US Food and Drug Administration. Caution with robotically-assisted surgical devices in mastectomy: FDA Safety Communication, August 20, 2021. https://www.fda.gov/medical-devices/safety-communications/update-caution-robotically-assisted-surgical-devices-mastectomy-fda-safety-communication. Accessed December 22, 2022. Riegel v Medtronic, 552 US 312 (2008).
- Han ES, Advincula AP. Robotic surgery: advancements and inflection points in the field of gynecology. Obstet Gynecol Clin North Am. 2021;48:759-776.
- Witharm H. Robot-assisted surgery: an analysis of the legal and economic implications. Az J Interdisciplinary Studies. 2022;8:19-29. https://journals.librarypublishing.arizona.edu /azjis/article/id/5093/download/pdf/.
- Cameron S. Is daVinci robotic surgery a revolution or a rip-off? Healthline. August 10, 2016. https://www.healthline .com/health-news/is-da-vinci-robotic-surgery-revolution -or-ripoff-021215. Accessed December 20, 2022.
- Perez RE, Schwaitzberg SD. Robotic surgery: finding value in 2019 and beyond. Ann Laparosc Endosc Surg. 2019;4:1-7.
- Gitas G, Hanker L, Rody A, et al. Robotic surgery in gynecology: is the future already here? Minim Invasiv Therapy Allied Technol. 2022;4:1-0.
- Moon AS, Garofalo J, Koirala P, et al. Robotic surgery in gynecology. Surgical Clinics. 2020;100:445-460.
- Simshaw D, Terry N, Hauser K, et al. Regulating healthcare robots: maximizing opportunities while minimizing risks. Richmond J Law Tech. 2015;22:1-38. https://scholar works.iupui.edu/bitstream/handle/1805/11587/simshaw _2015_regulating.pdf?sequence=1&isAllowed=y. Accessed December 20, 2022.
- De Ravin E, Sell EA, Newman JG, et al. Medical malpractice in robotic surgery: a Westlaw database analysis. J Robotic Surg. 2022. https://doi.org/10.1007/s11701-022-01417-6. https:// link.springer.com/article/10.1007/s11701-022-014176#citeas. Accessed December 20, 2022.
- Beglinger C. A broken theory: the malfunction theory of strict products liability and the need for a new doctrine in the field of surgical robotics. Minnesotta Law Rev. 2019;104:1041-1093. . Accessed December 20, 2022.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasiv Gynecol. 2021;28:490-495.
- Koerner D. Doctor roboto: The no-man operation. U Tol L Rev. 2019;51:125-146.
- Nik-Ahd F, Souders CP, Zhao H, et al. Robotic urologic surgery: trends in litigation over the last decade. J Robotic Surg. 2019;13:729-734.
- Gültekin CalibriİB, Karabük E, Köse MF. “Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!” What is autonomous surgery and what are the latest developments? J Turk Ger Gynecol Assoc. 2021;22:58-70. https://www.ncbi .nlm.nih.gov/pmc/articles/PMC7944239/.
- Matsuzaki T. Ethical issues of artificial intelligence in medicine. California West Law Rev. 2018;55:255-273. https://scholarlycommons.law.cwsl.edu/cgi/viewcontent. cgi?article=1669&context=cwlr. Accessed December 20, 2022.
New guidelines on peds obesity call for aggressive treatment
and hope the problem solves itself. That’s the upshot of new guidelines from the American Academy of Pediatrics.
The authors of the guidelines also encourage primary care doctors to collaborate with other medical professionals to treat the comorbidities often linked to obesity, rather than take on the entire challenge themselves.
“It’s impossible to treat obesity within the four walls of the clinic. That’s one thing I have learned,” Ihuoma Eneli, MD, associate director of the AAP Institute for Healthy Childhood Weight, told this news organization. For example, a primary care doctor could partner with a gastroenterologist when treating a child who has nonalcoholic fatty liver disease, added Dr. Eneli, a professor of pediatrics at the Ohio State University, Columbus, who helped write the recommendations.
The new document updates 2007 recommendations from AAP about treating children and adolescents who are overweight or obese. The earlier statement focused on behavioral modification and healthy eating behaviors and paid less attention to weight-lowering medications or bariatric surgery for young people. That document did not offer specific advice to health care providers about how to address childhood overweight or obesity.
The 2023 guidelines recommend that pediatricians offer anyone aged 12 years and older with obesity – defined as a body mass index (BMI) at the 95th percentile or higher – the option of receiving weight-loss medications in addition to ongoing support for lifestyle modifications, such as exercising more and eating healthier foods.
The same approach holds for bariatric surgery once children reach age 13, and AAP stressed that no physician should ever stigmatize children or imply that they are to blame for their weight.
AAP did not receive any industry funding to develop the guidelines.
As children reach the threshold BMI levels, physicians should conduct complete physicals and order blood tests to get a fuller picture of the patients’ health.
These are the first guidelines from AAP aimed at giving pediatricians and other primary care providers concrete guidance for managing overweight and obesity in younger patients.
“Obesity is a complex, chronic disease, and that’s a frame shift here,” said Sandra S. Hassink, MD, leader of the guideline group and director of the AAP Institute for Healthy Childhood Weight.
Dr. Hassink compared obesity to asthma, another chronic disease that merits prompt attention and ongoing treatment. A physician would never let a child with asthma go untreated until their breathing problems are so severe that they turn blue, Dr. Hassink said; similarly, physicians should treat obesity in young people promptly and over time.
While some aspects of treating overweight and obesity are the same for children and adults, Dr. Hassink noted distinct differences. “Every child is embedded in a family and extended support structure,” Dr. Hassink said, which means that any obesity management technique needs the buy-in and support of the child’s family too.
AAP’s new advice reflects current understanding that excess weight or obesity in children is a result of biological and social factors, such as living in a food desert or experiencing the effects of structural racism.
The guidelines synthesize the results of hundreds of studies about the best way to treat excess weight in young people. If multiple studies were of high quality and all reached similar conclusions, they received an “A.” Less robust but still informative studies rated a “B.” In aggregate, the guideline about weight-lowering medication is based on “B” evidence that could shift with further research.
The authors recommend that clinicians calculate a child’s BMI beginning at age 2 years, with particular attention to those at the 85th percentile or higher for their age and sex (which would be defined as overweight), at the 95th percentile or higher (obesity), or at the 120th percentile and higher (severe obesity). Clinicians also should monitor blood pressure and cholesterol in their patients with overweight or obesity, particularly once they reach age 10.
Starting at age 6, providers should interview patients and their families about what would motivate them to lose weight, then tailor interventions to those factors rather than just make a blanket declaration that weight loss is necessary. This step should be coupled with intensive support – ideally, at least 26 hours of face-to-face support over the course of a year, although more is better – about effective exercise and dietary habits that result in weight loss.
The intensive support model should remain in place throughout childhood and adolescence and should be coupled with referrals for weight-loss medications or bariatric surgeries as needed once children reach age 12 or 13. Those age cutoffs are based on current evidence as to when weight-loss medications or surgery becomes effective, Dr. Hassink said, and could be shifted to lower ages if that’s what new evidence shows.
“Intensive health behavioral and lifestyle treatment is the base of all other treatment extensions,” Dr. Eneli said.
Young patients who needed weight-lowering medication used to have fewer options, according to Aaron S. Kelly, PhD, the Minnesota American Legion and Auxiliary Chair in Children’s Health at the University of Minnesota, Minneapolis.
.No longer.
Dr. Kelly was not involved in drafting the guidelines but was the lead investigator for trials of liraglutide (Saxenda), which in 2020 received U.S. Food and Drug Administration approval for treating obesity in adolescents. In 2022, the agency approved phentermine and topiramate extended-release capsules (Qsymia) for long-term weight management for patients aged 12 years and older, along with a once-weekly injection of semaglutide (Wegovy) patients in this age group. There are no weight-lowering medications for children younger than 12, Dr. Kelly said.
“Obesity is not a lifestyle problem. A lot of it is driven by the underlying biology,” Dr. Kelly said. “Really, what these medicines do is make it easier for people to make the right lifestyle choices by pushing back against the biology.”
For example, a drug can make people feel full for longer or disrupt chemical pathways that result in craving certain foods. Dr. Kelly emphasized that these drugs do not give license for people to eat as much as they want.
As for bariatric surgery, the new guidelines adhere closely to those in a 2019 AAP statement that bariatric surgery is safe and effective in pediatric settings. This is gratifying to Kirk W. Reichard, MD, MBA, a lead author of the 2019 article and director of the bariatric surgery program at Nemours Children’s Health.
Even if the information isn’t new as of 2023, Dr. Reichard said, AAP’s imprimatur could cause some eligible families to consider bariatric surgery when they may not have done so before.
Dr. Eneli, Dr. Hassink, and Dr. Reichard reported no relevant financial conflicts of interest. Dr. Kelly has relationships with Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus.
A version of this article first appeared on Medscape.com.
and hope the problem solves itself. That’s the upshot of new guidelines from the American Academy of Pediatrics.
The authors of the guidelines also encourage primary care doctors to collaborate with other medical professionals to treat the comorbidities often linked to obesity, rather than take on the entire challenge themselves.
“It’s impossible to treat obesity within the four walls of the clinic. That’s one thing I have learned,” Ihuoma Eneli, MD, associate director of the AAP Institute for Healthy Childhood Weight, told this news organization. For example, a primary care doctor could partner with a gastroenterologist when treating a child who has nonalcoholic fatty liver disease, added Dr. Eneli, a professor of pediatrics at the Ohio State University, Columbus, who helped write the recommendations.
The new document updates 2007 recommendations from AAP about treating children and adolescents who are overweight or obese. The earlier statement focused on behavioral modification and healthy eating behaviors and paid less attention to weight-lowering medications or bariatric surgery for young people. That document did not offer specific advice to health care providers about how to address childhood overweight or obesity.
The 2023 guidelines recommend that pediatricians offer anyone aged 12 years and older with obesity – defined as a body mass index (BMI) at the 95th percentile or higher – the option of receiving weight-loss medications in addition to ongoing support for lifestyle modifications, such as exercising more and eating healthier foods.
The same approach holds for bariatric surgery once children reach age 13, and AAP stressed that no physician should ever stigmatize children or imply that they are to blame for their weight.
AAP did not receive any industry funding to develop the guidelines.
As children reach the threshold BMI levels, physicians should conduct complete physicals and order blood tests to get a fuller picture of the patients’ health.
These are the first guidelines from AAP aimed at giving pediatricians and other primary care providers concrete guidance for managing overweight and obesity in younger patients.
“Obesity is a complex, chronic disease, and that’s a frame shift here,” said Sandra S. Hassink, MD, leader of the guideline group and director of the AAP Institute for Healthy Childhood Weight.
Dr. Hassink compared obesity to asthma, another chronic disease that merits prompt attention and ongoing treatment. A physician would never let a child with asthma go untreated until their breathing problems are so severe that they turn blue, Dr. Hassink said; similarly, physicians should treat obesity in young people promptly and over time.
While some aspects of treating overweight and obesity are the same for children and adults, Dr. Hassink noted distinct differences. “Every child is embedded in a family and extended support structure,” Dr. Hassink said, which means that any obesity management technique needs the buy-in and support of the child’s family too.
AAP’s new advice reflects current understanding that excess weight or obesity in children is a result of biological and social factors, such as living in a food desert or experiencing the effects of structural racism.
The guidelines synthesize the results of hundreds of studies about the best way to treat excess weight in young people. If multiple studies were of high quality and all reached similar conclusions, they received an “A.” Less robust but still informative studies rated a “B.” In aggregate, the guideline about weight-lowering medication is based on “B” evidence that could shift with further research.
The authors recommend that clinicians calculate a child’s BMI beginning at age 2 years, with particular attention to those at the 85th percentile or higher for their age and sex (which would be defined as overweight), at the 95th percentile or higher (obesity), or at the 120th percentile and higher (severe obesity). Clinicians also should monitor blood pressure and cholesterol in their patients with overweight or obesity, particularly once they reach age 10.
Starting at age 6, providers should interview patients and their families about what would motivate them to lose weight, then tailor interventions to those factors rather than just make a blanket declaration that weight loss is necessary. This step should be coupled with intensive support – ideally, at least 26 hours of face-to-face support over the course of a year, although more is better – about effective exercise and dietary habits that result in weight loss.
The intensive support model should remain in place throughout childhood and adolescence and should be coupled with referrals for weight-loss medications or bariatric surgeries as needed once children reach age 12 or 13. Those age cutoffs are based on current evidence as to when weight-loss medications or surgery becomes effective, Dr. Hassink said, and could be shifted to lower ages if that’s what new evidence shows.
“Intensive health behavioral and lifestyle treatment is the base of all other treatment extensions,” Dr. Eneli said.
Young patients who needed weight-lowering medication used to have fewer options, according to Aaron S. Kelly, PhD, the Minnesota American Legion and Auxiliary Chair in Children’s Health at the University of Minnesota, Minneapolis.
.No longer.
Dr. Kelly was not involved in drafting the guidelines but was the lead investigator for trials of liraglutide (Saxenda), which in 2020 received U.S. Food and Drug Administration approval for treating obesity in adolescents. In 2022, the agency approved phentermine and topiramate extended-release capsules (Qsymia) for long-term weight management for patients aged 12 years and older, along with a once-weekly injection of semaglutide (Wegovy) patients in this age group. There are no weight-lowering medications for children younger than 12, Dr. Kelly said.
“Obesity is not a lifestyle problem. A lot of it is driven by the underlying biology,” Dr. Kelly said. “Really, what these medicines do is make it easier for people to make the right lifestyle choices by pushing back against the biology.”
For example, a drug can make people feel full for longer or disrupt chemical pathways that result in craving certain foods. Dr. Kelly emphasized that these drugs do not give license for people to eat as much as they want.
As for bariatric surgery, the new guidelines adhere closely to those in a 2019 AAP statement that bariatric surgery is safe and effective in pediatric settings. This is gratifying to Kirk W. Reichard, MD, MBA, a lead author of the 2019 article and director of the bariatric surgery program at Nemours Children’s Health.
Even if the information isn’t new as of 2023, Dr. Reichard said, AAP’s imprimatur could cause some eligible families to consider bariatric surgery when they may not have done so before.
Dr. Eneli, Dr. Hassink, and Dr. Reichard reported no relevant financial conflicts of interest. Dr. Kelly has relationships with Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus.
A version of this article first appeared on Medscape.com.
and hope the problem solves itself. That’s the upshot of new guidelines from the American Academy of Pediatrics.
The authors of the guidelines also encourage primary care doctors to collaborate with other medical professionals to treat the comorbidities often linked to obesity, rather than take on the entire challenge themselves.
“It’s impossible to treat obesity within the four walls of the clinic. That’s one thing I have learned,” Ihuoma Eneli, MD, associate director of the AAP Institute for Healthy Childhood Weight, told this news organization. For example, a primary care doctor could partner with a gastroenterologist when treating a child who has nonalcoholic fatty liver disease, added Dr. Eneli, a professor of pediatrics at the Ohio State University, Columbus, who helped write the recommendations.
The new document updates 2007 recommendations from AAP about treating children and adolescents who are overweight or obese. The earlier statement focused on behavioral modification and healthy eating behaviors and paid less attention to weight-lowering medications or bariatric surgery for young people. That document did not offer specific advice to health care providers about how to address childhood overweight or obesity.
The 2023 guidelines recommend that pediatricians offer anyone aged 12 years and older with obesity – defined as a body mass index (BMI) at the 95th percentile or higher – the option of receiving weight-loss medications in addition to ongoing support for lifestyle modifications, such as exercising more and eating healthier foods.
The same approach holds for bariatric surgery once children reach age 13, and AAP stressed that no physician should ever stigmatize children or imply that they are to blame for their weight.
AAP did not receive any industry funding to develop the guidelines.
As children reach the threshold BMI levels, physicians should conduct complete physicals and order blood tests to get a fuller picture of the patients’ health.
These are the first guidelines from AAP aimed at giving pediatricians and other primary care providers concrete guidance for managing overweight and obesity in younger patients.
“Obesity is a complex, chronic disease, and that’s a frame shift here,” said Sandra S. Hassink, MD, leader of the guideline group and director of the AAP Institute for Healthy Childhood Weight.
Dr. Hassink compared obesity to asthma, another chronic disease that merits prompt attention and ongoing treatment. A physician would never let a child with asthma go untreated until their breathing problems are so severe that they turn blue, Dr. Hassink said; similarly, physicians should treat obesity in young people promptly and over time.
While some aspects of treating overweight and obesity are the same for children and adults, Dr. Hassink noted distinct differences. “Every child is embedded in a family and extended support structure,” Dr. Hassink said, which means that any obesity management technique needs the buy-in and support of the child’s family too.
AAP’s new advice reflects current understanding that excess weight or obesity in children is a result of biological and social factors, such as living in a food desert or experiencing the effects of structural racism.
The guidelines synthesize the results of hundreds of studies about the best way to treat excess weight in young people. If multiple studies were of high quality and all reached similar conclusions, they received an “A.” Less robust but still informative studies rated a “B.” In aggregate, the guideline about weight-lowering medication is based on “B” evidence that could shift with further research.
The authors recommend that clinicians calculate a child’s BMI beginning at age 2 years, with particular attention to those at the 85th percentile or higher for their age and sex (which would be defined as overweight), at the 95th percentile or higher (obesity), or at the 120th percentile and higher (severe obesity). Clinicians also should monitor blood pressure and cholesterol in their patients with overweight or obesity, particularly once they reach age 10.
Starting at age 6, providers should interview patients and their families about what would motivate them to lose weight, then tailor interventions to those factors rather than just make a blanket declaration that weight loss is necessary. This step should be coupled with intensive support – ideally, at least 26 hours of face-to-face support over the course of a year, although more is better – about effective exercise and dietary habits that result in weight loss.
The intensive support model should remain in place throughout childhood and adolescence and should be coupled with referrals for weight-loss medications or bariatric surgeries as needed once children reach age 12 or 13. Those age cutoffs are based on current evidence as to when weight-loss medications or surgery becomes effective, Dr. Hassink said, and could be shifted to lower ages if that’s what new evidence shows.
“Intensive health behavioral and lifestyle treatment is the base of all other treatment extensions,” Dr. Eneli said.
Young patients who needed weight-lowering medication used to have fewer options, according to Aaron S. Kelly, PhD, the Minnesota American Legion and Auxiliary Chair in Children’s Health at the University of Minnesota, Minneapolis.
.No longer.
Dr. Kelly was not involved in drafting the guidelines but was the lead investigator for trials of liraglutide (Saxenda), which in 2020 received U.S. Food and Drug Administration approval for treating obesity in adolescents. In 2022, the agency approved phentermine and topiramate extended-release capsules (Qsymia) for long-term weight management for patients aged 12 years and older, along with a once-weekly injection of semaglutide (Wegovy) patients in this age group. There are no weight-lowering medications for children younger than 12, Dr. Kelly said.
“Obesity is not a lifestyle problem. A lot of it is driven by the underlying biology,” Dr. Kelly said. “Really, what these medicines do is make it easier for people to make the right lifestyle choices by pushing back against the biology.”
For example, a drug can make people feel full for longer or disrupt chemical pathways that result in craving certain foods. Dr. Kelly emphasized that these drugs do not give license for people to eat as much as they want.
As for bariatric surgery, the new guidelines adhere closely to those in a 2019 AAP statement that bariatric surgery is safe and effective in pediatric settings. This is gratifying to Kirk W. Reichard, MD, MBA, a lead author of the 2019 article and director of the bariatric surgery program at Nemours Children’s Health.
Even if the information isn’t new as of 2023, Dr. Reichard said, AAP’s imprimatur could cause some eligible families to consider bariatric surgery when they may not have done so before.
Dr. Eneli, Dr. Hassink, and Dr. Reichard reported no relevant financial conflicts of interest. Dr. Kelly has relationships with Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
Findings question value of pessary for pelvic organ prolapse
The standard nonsurgical treatment for pelvic organ prolapse does not appear to work as well as surgery to correct the problem, Dutch researchers have found.
Pelvic organ prolapse is an uncomfortable condition, causing a troublesome vaginal bulge, often accompanied by urinary, bowel, or sexual dysfunction. Between 3% and 6% of women develop symptomatic prolapse, with the highest incidence in women aged 60-69 years – a fast-growing demographic.
Although many women choose surgical treatment, the American College of Obstetricians and Gynecologists recommends that women be offered a vaginal pessary as a noninvasive alternative, despite inconsistent data from observational studies on their effectiveness.
Lisa van der Vaart, MD, a doctoral student in ob.gyn. at the University of Amsterdam and the lead author of the new study, published in JAMA, said that differences in outcome measures, small sample size, and lack of long-term follow-up have bedeviled previous comparisons of the two techniques.
“We thought it was very important to perform a randomized control trial on this subject to improve counseling to women who suffer from symptomatic pelvic organ prolapse,” Dr. van der Vaart said.
She and her colleagues conducted a noninferiority randomized clinical trial that recruited 1,605 women with stage II or higher prolapse who were referred to specialty care at 21 hospitals in the Netherlands between 2015 and 2019. Of the 440 women who agreed to participate in the trial, 218 received a pessary, a device inserted into the vagina that provides support to tissues displaced by prolapse, and 222 underwent surgery.
The primary outcome was subjective improvement using a standardized questionnaire at 24 months; women were asked to rank their symptoms on a seven-point scale, and subjective improvement was defined as a response of much better or very much better.
“We saw a substantial amount of improvement in both groups,” Dr. van der Vaart said in an interview.
After 24 months of follow-up, outcome data were available for 173 women in the pessary group and 162 in the surgery group. For this intention-to treat population, 76.3% in the pessary group and 81.5% in the surgery group reported improvement.
Results were similar for the smaller group of participants who completed the study per protocol, without crossing over to a treatment to which they had not been allocated.
However, neither the intention-to-treat nor per-protocol analysis met the prespecified criteria for noninferiority, suggesting that use of a vaginal pessary is not equivalent to surgery.
The study also found differences in adverse events. Among women randomly assigned to surgery, 9% suffered a postoperative urinary tract infection, and 5.4% underwent additional therapy, such as pessary or repeat operation.
But use of a pessary also had downsides. The most common adverse event was discomfort (42.7%), and by 24 months, 60% of the participants in the pessary group had discontinued use.
Dr. van der Vaart said that she was surprised by the high number of women assigned to the pessary group who later elected to undergo surgery. “Women should be told that their chance of crossing over to a surgical intervention is quite high – more than 50% do eventually end up having surgery.”
Cheryl Iglesia, MD, director of the National Center for Advanced Pelvic Surgery at MedStar Health and professor of obstetrics and gynecology and urology at Georgetown University, both in Washington, was also struck by the high crossover rate. “We’ve had the same pessaries probably for the last 100 years,” she said. “We need to get better.”
Dr. Iglesia welcomed new approaches to making vaginal pessaries that are custom designed for each woman’s unique anatomy using 3D printing and pointed to promising initial clinical trials of disposable pessaries. With the aging of the population and demand for treatment of prolapse increasing, she cited a need for better nonsurgical alternatives: “We have a work-force issue and may not have enough adequately trained urogynecologists to meet the demand for prolapse repairs as our population ages.”
The study was funded by a grant from ZonMW, a Dutch governmental health care organization. Dr. van der Vaart reported grants from ZonMW during the conduct of the study.
A version of this article first appeared on Medscape.com.
The standard nonsurgical treatment for pelvic organ prolapse does not appear to work as well as surgery to correct the problem, Dutch researchers have found.
Pelvic organ prolapse is an uncomfortable condition, causing a troublesome vaginal bulge, often accompanied by urinary, bowel, or sexual dysfunction. Between 3% and 6% of women develop symptomatic prolapse, with the highest incidence in women aged 60-69 years – a fast-growing demographic.
Although many women choose surgical treatment, the American College of Obstetricians and Gynecologists recommends that women be offered a vaginal pessary as a noninvasive alternative, despite inconsistent data from observational studies on their effectiveness.
Lisa van der Vaart, MD, a doctoral student in ob.gyn. at the University of Amsterdam and the lead author of the new study, published in JAMA, said that differences in outcome measures, small sample size, and lack of long-term follow-up have bedeviled previous comparisons of the two techniques.
“We thought it was very important to perform a randomized control trial on this subject to improve counseling to women who suffer from symptomatic pelvic organ prolapse,” Dr. van der Vaart said.
She and her colleagues conducted a noninferiority randomized clinical trial that recruited 1,605 women with stage II or higher prolapse who were referred to specialty care at 21 hospitals in the Netherlands between 2015 and 2019. Of the 440 women who agreed to participate in the trial, 218 received a pessary, a device inserted into the vagina that provides support to tissues displaced by prolapse, and 222 underwent surgery.
The primary outcome was subjective improvement using a standardized questionnaire at 24 months; women were asked to rank their symptoms on a seven-point scale, and subjective improvement was defined as a response of much better or very much better.
“We saw a substantial amount of improvement in both groups,” Dr. van der Vaart said in an interview.
After 24 months of follow-up, outcome data were available for 173 women in the pessary group and 162 in the surgery group. For this intention-to treat population, 76.3% in the pessary group and 81.5% in the surgery group reported improvement.
Results were similar for the smaller group of participants who completed the study per protocol, without crossing over to a treatment to which they had not been allocated.
However, neither the intention-to-treat nor per-protocol analysis met the prespecified criteria for noninferiority, suggesting that use of a vaginal pessary is not equivalent to surgery.
The study also found differences in adverse events. Among women randomly assigned to surgery, 9% suffered a postoperative urinary tract infection, and 5.4% underwent additional therapy, such as pessary or repeat operation.
But use of a pessary also had downsides. The most common adverse event was discomfort (42.7%), and by 24 months, 60% of the participants in the pessary group had discontinued use.
Dr. van der Vaart said that she was surprised by the high number of women assigned to the pessary group who later elected to undergo surgery. “Women should be told that their chance of crossing over to a surgical intervention is quite high – more than 50% do eventually end up having surgery.”
Cheryl Iglesia, MD, director of the National Center for Advanced Pelvic Surgery at MedStar Health and professor of obstetrics and gynecology and urology at Georgetown University, both in Washington, was also struck by the high crossover rate. “We’ve had the same pessaries probably for the last 100 years,” she said. “We need to get better.”
Dr. Iglesia welcomed new approaches to making vaginal pessaries that are custom designed for each woman’s unique anatomy using 3D printing and pointed to promising initial clinical trials of disposable pessaries. With the aging of the population and demand for treatment of prolapse increasing, she cited a need for better nonsurgical alternatives: “We have a work-force issue and may not have enough adequately trained urogynecologists to meet the demand for prolapse repairs as our population ages.”
The study was funded by a grant from ZonMW, a Dutch governmental health care organization. Dr. van der Vaart reported grants from ZonMW during the conduct of the study.
A version of this article first appeared on Medscape.com.
The standard nonsurgical treatment for pelvic organ prolapse does not appear to work as well as surgery to correct the problem, Dutch researchers have found.
Pelvic organ prolapse is an uncomfortable condition, causing a troublesome vaginal bulge, often accompanied by urinary, bowel, or sexual dysfunction. Between 3% and 6% of women develop symptomatic prolapse, with the highest incidence in women aged 60-69 years – a fast-growing demographic.
Although many women choose surgical treatment, the American College of Obstetricians and Gynecologists recommends that women be offered a vaginal pessary as a noninvasive alternative, despite inconsistent data from observational studies on their effectiveness.
Lisa van der Vaart, MD, a doctoral student in ob.gyn. at the University of Amsterdam and the lead author of the new study, published in JAMA, said that differences in outcome measures, small sample size, and lack of long-term follow-up have bedeviled previous comparisons of the two techniques.
“We thought it was very important to perform a randomized control trial on this subject to improve counseling to women who suffer from symptomatic pelvic organ prolapse,” Dr. van der Vaart said.
She and her colleagues conducted a noninferiority randomized clinical trial that recruited 1,605 women with stage II or higher prolapse who were referred to specialty care at 21 hospitals in the Netherlands between 2015 and 2019. Of the 440 women who agreed to participate in the trial, 218 received a pessary, a device inserted into the vagina that provides support to tissues displaced by prolapse, and 222 underwent surgery.
The primary outcome was subjective improvement using a standardized questionnaire at 24 months; women were asked to rank their symptoms on a seven-point scale, and subjective improvement was defined as a response of much better or very much better.
“We saw a substantial amount of improvement in both groups,” Dr. van der Vaart said in an interview.
After 24 months of follow-up, outcome data were available for 173 women in the pessary group and 162 in the surgery group. For this intention-to treat population, 76.3% in the pessary group and 81.5% in the surgery group reported improvement.
Results were similar for the smaller group of participants who completed the study per protocol, without crossing over to a treatment to which they had not been allocated.
However, neither the intention-to-treat nor per-protocol analysis met the prespecified criteria for noninferiority, suggesting that use of a vaginal pessary is not equivalent to surgery.
The study also found differences in adverse events. Among women randomly assigned to surgery, 9% suffered a postoperative urinary tract infection, and 5.4% underwent additional therapy, such as pessary or repeat operation.
But use of a pessary also had downsides. The most common adverse event was discomfort (42.7%), and by 24 months, 60% of the participants in the pessary group had discontinued use.
Dr. van der Vaart said that she was surprised by the high number of women assigned to the pessary group who later elected to undergo surgery. “Women should be told that their chance of crossing over to a surgical intervention is quite high – more than 50% do eventually end up having surgery.”
Cheryl Iglesia, MD, director of the National Center for Advanced Pelvic Surgery at MedStar Health and professor of obstetrics and gynecology and urology at Georgetown University, both in Washington, was also struck by the high crossover rate. “We’ve had the same pessaries probably for the last 100 years,” she said. “We need to get better.”
Dr. Iglesia welcomed new approaches to making vaginal pessaries that are custom designed for each woman’s unique anatomy using 3D printing and pointed to promising initial clinical trials of disposable pessaries. With the aging of the population and demand for treatment of prolapse increasing, she cited a need for better nonsurgical alternatives: “We have a work-force issue and may not have enough adequately trained urogynecologists to meet the demand for prolapse repairs as our population ages.”
The study was funded by a grant from ZonMW, a Dutch governmental health care organization. Dr. van der Vaart reported grants from ZonMW during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA
Tips and tricks for a successful rollerball endometrial ablation
Rates of health care use after bariatric surgery in teens
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Meet the JCOM Author with Dr. Barkoudah: Neurosurgery Operating Room Efficiency During the COVID-19 Era
Birth method affects microbiome and vaccination response
Babies born vaginally have a different microbiome to those born by Caesarean section and have heightened responses to childhood vaccinations, according to a new study heralded as “interesting and important” by experts.
The microbiome is known to play a role in immune responses to vaccination. However, the relationship between early-life effects on intestinal microbiota composition and subsequent childhood vaccine responses had remained poorly understood. In the new study, “the findings suggest that vaginal birthing resulted in a microbiota composition associated with an increase in a specific type of antibody response to two routine childhood vaccines in healthy babies, compared with Caesarean section,” the authors said.
Researchers from the University of Edinburgh, with colleagues at Spaarne Hospital and University Medical Centre in Utrecht, and the National Institute for Public Health and the Environment in The Netherlands, tracked the development of the gut microbiome in a cohort of 120 healthy, full-term infants and assessed their antibody levels following two common childhood vaccinations.
The study, published in Nature Communications, found “a clear relationship between microbes in the gut of those babies and levels of antibodies.” Not only was vaginal birth associated with increased levels of Bifidobacterium and Escherichia coli in the gut microbiome in the first months of life but also with higher IgG antibody responses against both pneumococcal and meningococcal vaccines.
Antibody responses doubled after vaginal birth
The babies were given pneumococcal and meningococcal vaccinations at 8 and 12 weeks, and saliva was collected at follow-up visits at ages 12 and 18 months for antibody measurement. In the 101 babies tested for pneumococcal antibodies, the researchers found that antibody levels were twice as high among babies delivered naturally, compared with those delivered by C-section. High levels of two gut bacteria in particular – Bifidobacterium and E. coli – were associated with high antibody responses to the pneumococcal vaccine, showing that the microbiome mediated the link between mode of delivery and pneumococcal vaccine responses.
In 66 babies tested for anti-meningococcal antibodies, antibodies were 1.7 times higher for vaginally-born babies than those delivered via C-section, and high antibody levels were particularly associated with high levels of E. coli in the babies’ microbiome.
The results were also influenced by breast-feeding, which even among children born vaginally was linked with 3.5 times higher pneumococcal antibody levels, compared with those of formula-fed children. In contrast, levels of antibodies against meningococcus were unaffected by breast-feeding status.
Microbiome ‘sets level of infection protection’
The team said: “The baby acquires Bifidobacterium and E. coli bacteria through natural birth, and human milk is needed to provide the sugars for these bacteria to thrive on.” They explained: “The gut microbiome is seeded at birth, developing rapidly over the first few months of life, and is influenced mostly by delivery mode, breast-feeding, and antibiotic use.” The babies’ microbiome in early life contributes the immune system’s response to vaccines, they said, “and sets the level of protection against certain infections in childhood.”
Study lead Professor Debby Bogaert, chair of pediatric medicine at the University of Edinburgh, said: “I think it is especially interesting that we identified several beneficial microbes to be the link between mode of delivery and vaccine responses. In the future, we may be able to supplement those bacteria to children born by C-section shortly after birth through – for example, mother-to-baby ‘fecal transplants’ or the use of specifically designed probiotics.”
First author Dr. Emma de Koff, a microbiology trainee at the Amsterdam University Medical Center, said: “We expected to find a link between the gut microbiome and the babies’ vaccine responses, however we never thought to find the strongest effects in the first weeks of life.”
The findings “could help to inform conversations about C-sections between expectant mothers and their doctors,” commented the researchers, who said that they could also “shape the design of more tailored vaccination programs.” For example, in the future, vaccination schedules could be adjusted based on the method of delivery or analysis of the baby’s microbiome.
Potential to rectify immune system after Caesarean
Responding to the study, Professor Neil Mabbott, personal chair in immunopathology at the Roslin Institute of the University of Edinburgh, told the Science Media Centre: “This is a very interesting and important study. The authors show that infants delivered by a vaginal birth had higher responses to the two different types of vaccines against bacterial diseases, and this was associated with higher abundances of the potentially beneficial bacteria known as Bifidobacterium and E. coli in their intestines.”
He added: “This study raises the possibility that it may be possible to treat infants, especially Caesarean-delivered infants, with a bacterial supplement, or even a product produced by these beneficial bacteria, to help improve their immune systems, enhance their responses to certain vaccines and reduce their susceptibility to infections.”
The study raises important questions, he said, including whether the increased antibody levels from pneumococcal and meningococcal vaccinations following vaginal birth also leads to increased protection of the infants against infection or serious disease.
Sheena Cruickshank, immunologist and professor in biomedical sciences at the University of Manchester, England, commented: “It is now well established that the microbiome is important in immune development. In turn the mode of delivery and initial method of feeding is important in how the microbiome is first seeded in the baby.”
“However, other factors such as exposure to antibiotics and subsequent diet also play a role in how it then develops, making understanding the way the microbiome develops and changes quite complex. Microbes works as communities, and it can be difficult to determine whether changes in single species are important functionally. Breast milk also plays an important role in protecting the baby via transfer of maternal immunoglobulins, which will wane over a period of 6-12 months in the baby – thus ascertaining whether it’s the baby’s Ig is challenging.
“Given the complexity of the multitude of interactions, it is important that this is accounted for, and group sizes are large enough to ensure data is robust. Whilst this is an interesting study that adds to our knowledge of how the microbiome develops and the possible implications for immune development, it is still very preliminary, and the small group sizes warrant a need for further studies to verify this in larger groups.”
She added: “We will need to understand whether possible impacts of maternal delivery and feeding on immune development or vaccine responses can be restored by, for example, manipulating the microbiome.”
Professor Kim Barrett, vice dean for research at the University of California, Davis School of Medicine, said that, while further research was needed to uncover if and how manipulation of the human microbiome following C-section births might improve vaccine efficacy, “the work should at least lead to prompt additional consideration about an unintended consequence of the ever-increasing use of C-sections that may not be medically-necessary.”
Dr. Marie Lewis, researcher in gut microbiota at the University of Reading, England, said: “We have known for quite some time that the mode of delivery is incredibly important when it comes to the type of bacteria which colonize our guts. We also know that our gut bacteria in early life drive the development of our immune system, and natural births are linked with reduced risks of developing inflammatory conditions, such as asthma. It is therefore perhaps not really surprising that mode of delivery is also linked to responses to vaccinations.”
“The really interesting part here is the extent to which our gut microbiotas are accessible and changeable, and this important work could pave the way for administration of probiotics and prebiotics to improve vaccine responses in Caesarean-born children.”
‘Tantalizing data’
Dr. Chrissie Jones, associate professor of pediatric infectious diseases at the University of Southampton, and Southampton UK and education lead for the British Paediatric Allergy, Immunity, and Infection Group, said: “The link between method of delivery of the infant and the bacteria that live in the gut of the young infant has previously been shown. What is really interesting about this study is that, for the first time, the link between method of delivery (vaginal delivery vs. C-section), differences in bacterial communities of the gut, and differences in responses to vaccines is shown.”
“This study may give us fresh insights into the differences that we see in the amount of protective antibodies made after infant vaccination. It also gives us clues as to ways that we might be able to level the playing field for infants in the future – for instance, giving babies a safe cocktail of ‘friendly bacteria’ as a probiotic, or an additional dose of vaccine.”
“This study is the first step – it shows us a link or association but does not prove cause and effect that differences in the way babies are born alters how the immune system responds to vaccines. To prove this link we will need larger studies, but it is tantalizing data.”
The research was funded by Scotland’s Chief Scientist Office and the Netherlands Organisation for Scientific Research. DB received funding from OM pharma and Sanofi. All of the authors declared no other conflicts of interest.
A version of this article first appeared on Medscape.com.
Babies born vaginally have a different microbiome to those born by Caesarean section and have heightened responses to childhood vaccinations, according to a new study heralded as “interesting and important” by experts.
The microbiome is known to play a role in immune responses to vaccination. However, the relationship between early-life effects on intestinal microbiota composition and subsequent childhood vaccine responses had remained poorly understood. In the new study, “the findings suggest that vaginal birthing resulted in a microbiota composition associated with an increase in a specific type of antibody response to two routine childhood vaccines in healthy babies, compared with Caesarean section,” the authors said.
Researchers from the University of Edinburgh, with colleagues at Spaarne Hospital and University Medical Centre in Utrecht, and the National Institute for Public Health and the Environment in The Netherlands, tracked the development of the gut microbiome in a cohort of 120 healthy, full-term infants and assessed their antibody levels following two common childhood vaccinations.
The study, published in Nature Communications, found “a clear relationship between microbes in the gut of those babies and levels of antibodies.” Not only was vaginal birth associated with increased levels of Bifidobacterium and Escherichia coli in the gut microbiome in the first months of life but also with higher IgG antibody responses against both pneumococcal and meningococcal vaccines.
Antibody responses doubled after vaginal birth
The babies were given pneumococcal and meningococcal vaccinations at 8 and 12 weeks, and saliva was collected at follow-up visits at ages 12 and 18 months for antibody measurement. In the 101 babies tested for pneumococcal antibodies, the researchers found that antibody levels were twice as high among babies delivered naturally, compared with those delivered by C-section. High levels of two gut bacteria in particular – Bifidobacterium and E. coli – were associated with high antibody responses to the pneumococcal vaccine, showing that the microbiome mediated the link between mode of delivery and pneumococcal vaccine responses.
In 66 babies tested for anti-meningococcal antibodies, antibodies were 1.7 times higher for vaginally-born babies than those delivered via C-section, and high antibody levels were particularly associated with high levels of E. coli in the babies’ microbiome.
The results were also influenced by breast-feeding, which even among children born vaginally was linked with 3.5 times higher pneumococcal antibody levels, compared with those of formula-fed children. In contrast, levels of antibodies against meningococcus were unaffected by breast-feeding status.
Microbiome ‘sets level of infection protection’
The team said: “The baby acquires Bifidobacterium and E. coli bacteria through natural birth, and human milk is needed to provide the sugars for these bacteria to thrive on.” They explained: “The gut microbiome is seeded at birth, developing rapidly over the first few months of life, and is influenced mostly by delivery mode, breast-feeding, and antibiotic use.” The babies’ microbiome in early life contributes the immune system’s response to vaccines, they said, “and sets the level of protection against certain infections in childhood.”
Study lead Professor Debby Bogaert, chair of pediatric medicine at the University of Edinburgh, said: “I think it is especially interesting that we identified several beneficial microbes to be the link between mode of delivery and vaccine responses. In the future, we may be able to supplement those bacteria to children born by C-section shortly after birth through – for example, mother-to-baby ‘fecal transplants’ or the use of specifically designed probiotics.”
First author Dr. Emma de Koff, a microbiology trainee at the Amsterdam University Medical Center, said: “We expected to find a link between the gut microbiome and the babies’ vaccine responses, however we never thought to find the strongest effects in the first weeks of life.”
The findings “could help to inform conversations about C-sections between expectant mothers and their doctors,” commented the researchers, who said that they could also “shape the design of more tailored vaccination programs.” For example, in the future, vaccination schedules could be adjusted based on the method of delivery or analysis of the baby’s microbiome.
Potential to rectify immune system after Caesarean
Responding to the study, Professor Neil Mabbott, personal chair in immunopathology at the Roslin Institute of the University of Edinburgh, told the Science Media Centre: “This is a very interesting and important study. The authors show that infants delivered by a vaginal birth had higher responses to the two different types of vaccines against bacterial diseases, and this was associated with higher abundances of the potentially beneficial bacteria known as Bifidobacterium and E. coli in their intestines.”
He added: “This study raises the possibility that it may be possible to treat infants, especially Caesarean-delivered infants, with a bacterial supplement, or even a product produced by these beneficial bacteria, to help improve their immune systems, enhance their responses to certain vaccines and reduce their susceptibility to infections.”
The study raises important questions, he said, including whether the increased antibody levels from pneumococcal and meningococcal vaccinations following vaginal birth also leads to increased protection of the infants against infection or serious disease.
Sheena Cruickshank, immunologist and professor in biomedical sciences at the University of Manchester, England, commented: “It is now well established that the microbiome is important in immune development. In turn the mode of delivery and initial method of feeding is important in how the microbiome is first seeded in the baby.”
“However, other factors such as exposure to antibiotics and subsequent diet also play a role in how it then develops, making understanding the way the microbiome develops and changes quite complex. Microbes works as communities, and it can be difficult to determine whether changes in single species are important functionally. Breast milk also plays an important role in protecting the baby via transfer of maternal immunoglobulins, which will wane over a period of 6-12 months in the baby – thus ascertaining whether it’s the baby’s Ig is challenging.
“Given the complexity of the multitude of interactions, it is important that this is accounted for, and group sizes are large enough to ensure data is robust. Whilst this is an interesting study that adds to our knowledge of how the microbiome develops and the possible implications for immune development, it is still very preliminary, and the small group sizes warrant a need for further studies to verify this in larger groups.”
She added: “We will need to understand whether possible impacts of maternal delivery and feeding on immune development or vaccine responses can be restored by, for example, manipulating the microbiome.”
Professor Kim Barrett, vice dean for research at the University of California, Davis School of Medicine, said that, while further research was needed to uncover if and how manipulation of the human microbiome following C-section births might improve vaccine efficacy, “the work should at least lead to prompt additional consideration about an unintended consequence of the ever-increasing use of C-sections that may not be medically-necessary.”
Dr. Marie Lewis, researcher in gut microbiota at the University of Reading, England, said: “We have known for quite some time that the mode of delivery is incredibly important when it comes to the type of bacteria which colonize our guts. We also know that our gut bacteria in early life drive the development of our immune system, and natural births are linked with reduced risks of developing inflammatory conditions, such as asthma. It is therefore perhaps not really surprising that mode of delivery is also linked to responses to vaccinations.”
“The really interesting part here is the extent to which our gut microbiotas are accessible and changeable, and this important work could pave the way for administration of probiotics and prebiotics to improve vaccine responses in Caesarean-born children.”
‘Tantalizing data’
Dr. Chrissie Jones, associate professor of pediatric infectious diseases at the University of Southampton, and Southampton UK and education lead for the British Paediatric Allergy, Immunity, and Infection Group, said: “The link between method of delivery of the infant and the bacteria that live in the gut of the young infant has previously been shown. What is really interesting about this study is that, for the first time, the link between method of delivery (vaginal delivery vs. C-section), differences in bacterial communities of the gut, and differences in responses to vaccines is shown.”
“This study may give us fresh insights into the differences that we see in the amount of protective antibodies made after infant vaccination. It also gives us clues as to ways that we might be able to level the playing field for infants in the future – for instance, giving babies a safe cocktail of ‘friendly bacteria’ as a probiotic, or an additional dose of vaccine.”
“This study is the first step – it shows us a link or association but does not prove cause and effect that differences in the way babies are born alters how the immune system responds to vaccines. To prove this link we will need larger studies, but it is tantalizing data.”
The research was funded by Scotland’s Chief Scientist Office and the Netherlands Organisation for Scientific Research. DB received funding from OM pharma and Sanofi. All of the authors declared no other conflicts of interest.
A version of this article first appeared on Medscape.com.
Babies born vaginally have a different microbiome to those born by Caesarean section and have heightened responses to childhood vaccinations, according to a new study heralded as “interesting and important” by experts.
The microbiome is known to play a role in immune responses to vaccination. However, the relationship between early-life effects on intestinal microbiota composition and subsequent childhood vaccine responses had remained poorly understood. In the new study, “the findings suggest that vaginal birthing resulted in a microbiota composition associated with an increase in a specific type of antibody response to two routine childhood vaccines in healthy babies, compared with Caesarean section,” the authors said.
Researchers from the University of Edinburgh, with colleagues at Spaarne Hospital and University Medical Centre in Utrecht, and the National Institute for Public Health and the Environment in The Netherlands, tracked the development of the gut microbiome in a cohort of 120 healthy, full-term infants and assessed their antibody levels following two common childhood vaccinations.
The study, published in Nature Communications, found “a clear relationship between microbes in the gut of those babies and levels of antibodies.” Not only was vaginal birth associated with increased levels of Bifidobacterium and Escherichia coli in the gut microbiome in the first months of life but also with higher IgG antibody responses against both pneumococcal and meningococcal vaccines.
Antibody responses doubled after vaginal birth
The babies were given pneumococcal and meningococcal vaccinations at 8 and 12 weeks, and saliva was collected at follow-up visits at ages 12 and 18 months for antibody measurement. In the 101 babies tested for pneumococcal antibodies, the researchers found that antibody levels were twice as high among babies delivered naturally, compared with those delivered by C-section. High levels of two gut bacteria in particular – Bifidobacterium and E. coli – were associated with high antibody responses to the pneumococcal vaccine, showing that the microbiome mediated the link between mode of delivery and pneumococcal vaccine responses.
In 66 babies tested for anti-meningococcal antibodies, antibodies were 1.7 times higher for vaginally-born babies than those delivered via C-section, and high antibody levels were particularly associated with high levels of E. coli in the babies’ microbiome.
The results were also influenced by breast-feeding, which even among children born vaginally was linked with 3.5 times higher pneumococcal antibody levels, compared with those of formula-fed children. In contrast, levels of antibodies against meningococcus were unaffected by breast-feeding status.
Microbiome ‘sets level of infection protection’
The team said: “The baby acquires Bifidobacterium and E. coli bacteria through natural birth, and human milk is needed to provide the sugars for these bacteria to thrive on.” They explained: “The gut microbiome is seeded at birth, developing rapidly over the first few months of life, and is influenced mostly by delivery mode, breast-feeding, and antibiotic use.” The babies’ microbiome in early life contributes the immune system’s response to vaccines, they said, “and sets the level of protection against certain infections in childhood.”
Study lead Professor Debby Bogaert, chair of pediatric medicine at the University of Edinburgh, said: “I think it is especially interesting that we identified several beneficial microbes to be the link between mode of delivery and vaccine responses. In the future, we may be able to supplement those bacteria to children born by C-section shortly after birth through – for example, mother-to-baby ‘fecal transplants’ or the use of specifically designed probiotics.”
First author Dr. Emma de Koff, a microbiology trainee at the Amsterdam University Medical Center, said: “We expected to find a link between the gut microbiome and the babies’ vaccine responses, however we never thought to find the strongest effects in the first weeks of life.”
The findings “could help to inform conversations about C-sections between expectant mothers and their doctors,” commented the researchers, who said that they could also “shape the design of more tailored vaccination programs.” For example, in the future, vaccination schedules could be adjusted based on the method of delivery or analysis of the baby’s microbiome.
Potential to rectify immune system after Caesarean
Responding to the study, Professor Neil Mabbott, personal chair in immunopathology at the Roslin Institute of the University of Edinburgh, told the Science Media Centre: “This is a very interesting and important study. The authors show that infants delivered by a vaginal birth had higher responses to the two different types of vaccines against bacterial diseases, and this was associated with higher abundances of the potentially beneficial bacteria known as Bifidobacterium and E. coli in their intestines.”
He added: “This study raises the possibility that it may be possible to treat infants, especially Caesarean-delivered infants, with a bacterial supplement, or even a product produced by these beneficial bacteria, to help improve their immune systems, enhance their responses to certain vaccines and reduce their susceptibility to infections.”
The study raises important questions, he said, including whether the increased antibody levels from pneumococcal and meningococcal vaccinations following vaginal birth also leads to increased protection of the infants against infection or serious disease.
Sheena Cruickshank, immunologist and professor in biomedical sciences at the University of Manchester, England, commented: “It is now well established that the microbiome is important in immune development. In turn the mode of delivery and initial method of feeding is important in how the microbiome is first seeded in the baby.”
“However, other factors such as exposure to antibiotics and subsequent diet also play a role in how it then develops, making understanding the way the microbiome develops and changes quite complex. Microbes works as communities, and it can be difficult to determine whether changes in single species are important functionally. Breast milk also plays an important role in protecting the baby via transfer of maternal immunoglobulins, which will wane over a period of 6-12 months in the baby – thus ascertaining whether it’s the baby’s Ig is challenging.
“Given the complexity of the multitude of interactions, it is important that this is accounted for, and group sizes are large enough to ensure data is robust. Whilst this is an interesting study that adds to our knowledge of how the microbiome develops and the possible implications for immune development, it is still very preliminary, and the small group sizes warrant a need for further studies to verify this in larger groups.”
She added: “We will need to understand whether possible impacts of maternal delivery and feeding on immune development or vaccine responses can be restored by, for example, manipulating the microbiome.”
Professor Kim Barrett, vice dean for research at the University of California, Davis School of Medicine, said that, while further research was needed to uncover if and how manipulation of the human microbiome following C-section births might improve vaccine efficacy, “the work should at least lead to prompt additional consideration about an unintended consequence of the ever-increasing use of C-sections that may not be medically-necessary.”
Dr. Marie Lewis, researcher in gut microbiota at the University of Reading, England, said: “We have known for quite some time that the mode of delivery is incredibly important when it comes to the type of bacteria which colonize our guts. We also know that our gut bacteria in early life drive the development of our immune system, and natural births are linked with reduced risks of developing inflammatory conditions, such as asthma. It is therefore perhaps not really surprising that mode of delivery is also linked to responses to vaccinations.”
“The really interesting part here is the extent to which our gut microbiotas are accessible and changeable, and this important work could pave the way for administration of probiotics and prebiotics to improve vaccine responses in Caesarean-born children.”
‘Tantalizing data’
Dr. Chrissie Jones, associate professor of pediatric infectious diseases at the University of Southampton, and Southampton UK and education lead for the British Paediatric Allergy, Immunity, and Infection Group, said: “The link between method of delivery of the infant and the bacteria that live in the gut of the young infant has previously been shown. What is really interesting about this study is that, for the first time, the link between method of delivery (vaginal delivery vs. C-section), differences in bacterial communities of the gut, and differences in responses to vaccines is shown.”
“This study may give us fresh insights into the differences that we see in the amount of protective antibodies made after infant vaccination. It also gives us clues as to ways that we might be able to level the playing field for infants in the future – for instance, giving babies a safe cocktail of ‘friendly bacteria’ as a probiotic, or an additional dose of vaccine.”
“This study is the first step – it shows us a link or association but does not prove cause and effect that differences in the way babies are born alters how the immune system responds to vaccines. To prove this link we will need larger studies, but it is tantalizing data.”
The research was funded by Scotland’s Chief Scientist Office and the Netherlands Organisation for Scientific Research. DB received funding from OM pharma and Sanofi. All of the authors declared no other conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS










