User login
Moderate THST was most effective at treating thyroid cancer
Moderate thyroid hormone suppression therapy (THST) is associated with the best outcomes for patients with all stages of thyroid cancer, according to a prospective analysis of a multi-institutional registry published in the Journal of Clinical Endocrinology & Metabolism.
The researchers examined the outcomes of initial treatment for 4,941 patients with differentiated thyroid cancer (DTC), according to registry data from the National Thyroid Cancer Treatment Cooperative Study Group. The treatments included total/near total thyroidectomy (T/NTT), postoperative radioactive iodine-131 (131I), and THST. The median duration between treatment and follow-up for a patient was 6 years, with follow-up information available for all but 94 (1.9%) of the patients in the cohort.
Overall improvement was noted in stage III patients who received 131I (risk ratio, 0.66; P = .04) and stage IV patients who received both T/NTT and 131I (RR, 0.66; P = .049). In all stages, moderate THST was associated with significantly improved overall survival (RR stages I-IV: 0.13, 0.09, 0.13, and 0.33, respectively) and disease-free survival (DFS) (RR stages I-III: 0.52, 0.40, and 0.18, respectively); no additional survival benefit was achieved with more aggressive THST, even when distant metastatic disease was diagnosed during follow-up.
Lower initial stage and moderate THST were independent predictors of improved overall survival during follow-up years 1-3.
Consistent with previous research, this study also showed that T/NTT followed by 131I is associated with benefit in high-risk, but not low-risk patients.
“We report for the first time, in multivariate analysis of primary treatments for DTC, across all stages, only THST was associated with both improved stage-adjusted OS and DFS,” noted Dr. Aubrey A. Carhill and his colleagues.
“This analysis of the larger, more mature registry database extends and refines earlier observations regarding the impact of initial therapies on patient outcomes and further justifies the need for prospective, long-term, controlled studies,” the researchers noted.
Read the full study in the Journal of Clinical Endocrinology & Metabolism (doi:10.1210/JC.2015-1346).
Moderate thyroid hormone suppression therapy (THST) is associated with the best outcomes for patients with all stages of thyroid cancer, according to a prospective analysis of a multi-institutional registry published in the Journal of Clinical Endocrinology & Metabolism.
The researchers examined the outcomes of initial treatment for 4,941 patients with differentiated thyroid cancer (DTC), according to registry data from the National Thyroid Cancer Treatment Cooperative Study Group. The treatments included total/near total thyroidectomy (T/NTT), postoperative radioactive iodine-131 (131I), and THST. The median duration between treatment and follow-up for a patient was 6 years, with follow-up information available for all but 94 (1.9%) of the patients in the cohort.
Overall improvement was noted in stage III patients who received 131I (risk ratio, 0.66; P = .04) and stage IV patients who received both T/NTT and 131I (RR, 0.66; P = .049). In all stages, moderate THST was associated with significantly improved overall survival (RR stages I-IV: 0.13, 0.09, 0.13, and 0.33, respectively) and disease-free survival (DFS) (RR stages I-III: 0.52, 0.40, and 0.18, respectively); no additional survival benefit was achieved with more aggressive THST, even when distant metastatic disease was diagnosed during follow-up.
Lower initial stage and moderate THST were independent predictors of improved overall survival during follow-up years 1-3.
Consistent with previous research, this study also showed that T/NTT followed by 131I is associated with benefit in high-risk, but not low-risk patients.
“We report for the first time, in multivariate analysis of primary treatments for DTC, across all stages, only THST was associated with both improved stage-adjusted OS and DFS,” noted Dr. Aubrey A. Carhill and his colleagues.
“This analysis of the larger, more mature registry database extends and refines earlier observations regarding the impact of initial therapies on patient outcomes and further justifies the need for prospective, long-term, controlled studies,” the researchers noted.
Read the full study in the Journal of Clinical Endocrinology & Metabolism (doi:10.1210/JC.2015-1346).
Moderate thyroid hormone suppression therapy (THST) is associated with the best outcomes for patients with all stages of thyroid cancer, according to a prospective analysis of a multi-institutional registry published in the Journal of Clinical Endocrinology & Metabolism.
The researchers examined the outcomes of initial treatment for 4,941 patients with differentiated thyroid cancer (DTC), according to registry data from the National Thyroid Cancer Treatment Cooperative Study Group. The treatments included total/near total thyroidectomy (T/NTT), postoperative radioactive iodine-131 (131I), and THST. The median duration between treatment and follow-up for a patient was 6 years, with follow-up information available for all but 94 (1.9%) of the patients in the cohort.
Overall improvement was noted in stage III patients who received 131I (risk ratio, 0.66; P = .04) and stage IV patients who received both T/NTT and 131I (RR, 0.66; P = .049). In all stages, moderate THST was associated with significantly improved overall survival (RR stages I-IV: 0.13, 0.09, 0.13, and 0.33, respectively) and disease-free survival (DFS) (RR stages I-III: 0.52, 0.40, and 0.18, respectively); no additional survival benefit was achieved with more aggressive THST, even when distant metastatic disease was diagnosed during follow-up.
Lower initial stage and moderate THST were independent predictors of improved overall survival during follow-up years 1-3.
Consistent with previous research, this study also showed that T/NTT followed by 131I is associated with benefit in high-risk, but not low-risk patients.
“We report for the first time, in multivariate analysis of primary treatments for DTC, across all stages, only THST was associated with both improved stage-adjusted OS and DFS,” noted Dr. Aubrey A. Carhill and his colleagues.
“This analysis of the larger, more mature registry database extends and refines earlier observations regarding the impact of initial therapies on patient outcomes and further justifies the need for prospective, long-term, controlled studies,” the researchers noted.
Read the full study in the Journal of Clinical Endocrinology & Metabolism (doi:10.1210/JC.2015-1346).
Elevated IL-6 linked to complications after major abdominal surgery
On postoperative day 1, elevated interleukin-6 level was associated with postoperative complications, according to a single-center cohort study of patients who had major abdominal surgery.
“Up to 28% of patients undergoing major abdominal surgery experience postoperative complications, including wound infection, sepsis, anastomotic dehiscence, pneumonia,cardiovascular or respiratory events, and mortality” but an accurate means of identifying those in the risk category would contribute the development of prevention stratetgies, the investigators wrote.
Previous studies of cardiothoracic surgery have supported an association of systemic inflammation to poor outcomes. Dr. Thijs Rettig and colleagues at St. Antonius Hospital, Nieuwegein, the Netherlands, sought to clarify if markers of inflammation and major abdominal surgery correlate with outcomes. Their results were published in the July issue of Annals of Surgery.
Researchers conducted a prospective cohort study at a single center using data obtained from the Myocardial Injury and Complications after major abdominal surgery (MICOLON) study. Participants in the MICOLON study were individuals aged 45 years or older who underwent elective major abdominal surgery. Other inclusion criteria included major cardiovascular (CV) risk factors, coronary artery disease, cerebrovascular accident, diabetes, renal insufficiency, atrial fibrillation, left ventricular dysfunction, aortic valve stenosis, or two minor CV risk factors.
Interleukin-6 (IL-6), tumor necrosis factor (TNF)-alpha, and C-reactive protein (CRP) levels were obtained at baseline and postoperative days 1, 3, and 7 in 137 patients. Systemic inflammatory response syndrome (SIRS) scores were calculated within 48 hours of surgery.
Primary endpoints were 30-day mortality, sepsis, pneumonia, wound infection, anastomotic dehiscence, reoperation, new-onset atrial fibrillation, respiratory insufficiency, congestive heart failure, and myocardial infarction. Data were also collected on length of stay and patients were followed up at 30 days postoperatively for further complications.
With a mean age of 68 years, 59% of patients were male and 30% (n = 40) had an ASA score of 3 or higher. Colorectal (50%), gastroesophageal (22%), and pancreatic (10%) surgery were the most common procedures performed. After excluding 2 patients from analysis for elevated baseline IL-6, 135 patients were analyzed.
At least one postoperative complication was observed in 29% (n = 39) of study subjects with a mean onset of 5 days after surgery. Use of preoperative steroids, aspirin, and statins were not associated with complications; however, blood loss and longer surgery duration where associated with worse outcomes.
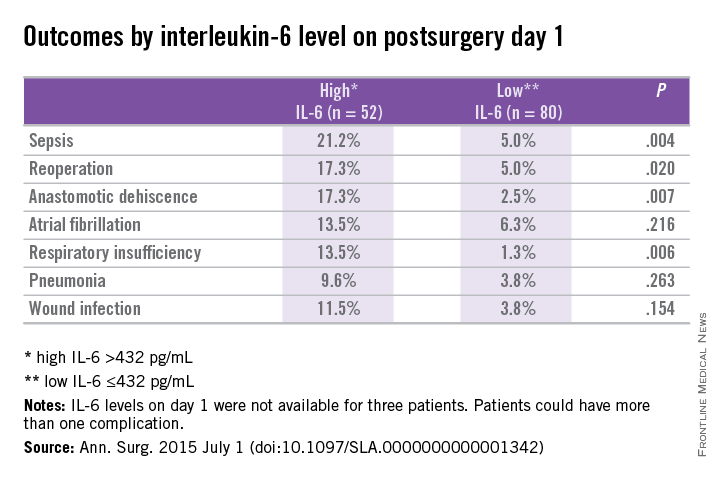
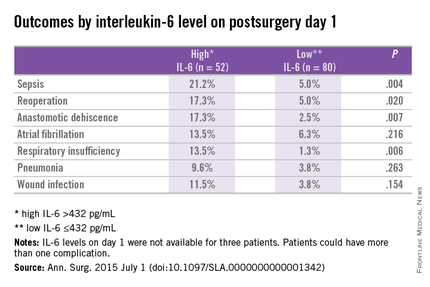
In patients with and without complications, differences in IL-6 levels were observed at day 1 at 596 pg/mL vs. 303 pg/mL (P < .01), day 3 at 128 pg/mL vs. 69 pg/mL (P < .01), and day 7 at 76 pg/mL vs. 27 pg/mL (P = .02).
On day 1, CRP was similar in both groups (90 mg/L vs. 78 mg/L; P = .131), but on days 3 (223 mg/L vs. 131 mg/L; P < .001) and 7 (131 mg/L vs. 63 mg/L; P < .001) differences were observed.
Differences in TNF-alpha were observed between groups on day 7 (0.5 pg/mL vs. 0, P < .01). The two groups demonstrated similar leukocyte counts postoperatively.
Prediction for postoperative complications was associated with an IL-6 of 432 pg/mL at day 1, which was 70% specific and 64% sensitive, and had a positive predictive value (PPV) of 44% and negative predictive value (NPV) of 84%. A longer hospital stay of 12 days vs. 7 days (P < .001) was associated with high IL-6 (> 432 pg/mL) vs. a low IL-6 (< 432 pg/mL) at day 1.
Elevated IL-6 level on postoperative day 1 was independently associated with postoperative complications by multivariant regression analysis (AOR: 3.3; 95% confidence interval, 1.3-8.5; P < .02).
The researchers concluded that an increased IL-6 level on postoperative day 1 was associated with increased length of stay and threefold increased risk of complications after major abdominal surgery. They further continued, “It is plausible that early recognition of postoperative complications optimizes the chance of better outcome. One way to enhance early detection of complications is using inflammatory markers as predictors of outcome.”
The authors reported no conflicts of interest.
On postoperative day 1, elevated interleukin-6 level was associated with postoperative complications, according to a single-center cohort study of patients who had major abdominal surgery.
“Up to 28% of patients undergoing major abdominal surgery experience postoperative complications, including wound infection, sepsis, anastomotic dehiscence, pneumonia,cardiovascular or respiratory events, and mortality” but an accurate means of identifying those in the risk category would contribute the development of prevention stratetgies, the investigators wrote.

Previous studies of cardiothoracic surgery have supported an association of systemic inflammation to poor outcomes. Dr. Thijs Rettig and colleagues at St. Antonius Hospital, Nieuwegein, the Netherlands, sought to clarify if markers of inflammation and major abdominal surgery correlate with outcomes. Their results were published in the July issue of Annals of Surgery.
Researchers conducted a prospective cohort study at a single center using data obtained from the Myocardial Injury and Complications after major abdominal surgery (MICOLON) study. Participants in the MICOLON study were individuals aged 45 years or older who underwent elective major abdominal surgery. Other inclusion criteria included major cardiovascular (CV) risk factors, coronary artery disease, cerebrovascular accident, diabetes, renal insufficiency, atrial fibrillation, left ventricular dysfunction, aortic valve stenosis, or two minor CV risk factors.
Interleukin-6 (IL-6), tumor necrosis factor (TNF)-alpha, and C-reactive protein (CRP) levels were obtained at baseline and postoperative days 1, 3, and 7 in 137 patients. Systemic inflammatory response syndrome (SIRS) scores were calculated within 48 hours of surgery.
Primary endpoints were 30-day mortality, sepsis, pneumonia, wound infection, anastomotic dehiscence, reoperation, new-onset atrial fibrillation, respiratory insufficiency, congestive heart failure, and myocardial infarction. Data were also collected on length of stay and patients were followed up at 30 days postoperatively for further complications.
With a mean age of 68 years, 59% of patients were male and 30% (n = 40) had an ASA score of 3 or higher. Colorectal (50%), gastroesophageal (22%), and pancreatic (10%) surgery were the most common procedures performed. After excluding 2 patients from analysis for elevated baseline IL-6, 135 patients were analyzed.
At least one postoperative complication was observed in 29% (n = 39) of study subjects with a mean onset of 5 days after surgery. Use of preoperative steroids, aspirin, and statins were not associated with complications; however, blood loss and longer surgery duration where associated with worse outcomes.
In patients with and without complications, differences in IL-6 levels were observed at day 1 at 596 pg/mL vs. 303 pg/mL (P < .01), day 3 at 128 pg/mL vs. 69 pg/mL (P < .01), and day 7 at 76 pg/mL vs. 27 pg/mL (P = .02).
On day 1, CRP was similar in both groups (90 mg/L vs. 78 mg/L; P = .131), but on days 3 (223 mg/L vs. 131 mg/L; P < .001) and 7 (131 mg/L vs. 63 mg/L; P < .001) differences were observed.
Differences in TNF-alpha were observed between groups on day 7 (0.5 pg/mL vs. 0, P < .01). The two groups demonstrated similar leukocyte counts postoperatively.
Prediction for postoperative complications was associated with an IL-6 of 432 pg/mL at day 1, which was 70% specific and 64% sensitive, and had a positive predictive value (PPV) of 44% and negative predictive value (NPV) of 84%. A longer hospital stay of 12 days vs. 7 days (P < .001) was associated with high IL-6 (> 432 pg/mL) vs. a low IL-6 (< 432 pg/mL) at day 1.
Elevated IL-6 level on postoperative day 1 was independently associated with postoperative complications by multivariant regression analysis (AOR: 3.3; 95% confidence interval, 1.3-8.5; P < .02).
The researchers concluded that an increased IL-6 level on postoperative day 1 was associated with increased length of stay and threefold increased risk of complications after major abdominal surgery. They further continued, “It is plausible that early recognition of postoperative complications optimizes the chance of better outcome. One way to enhance early detection of complications is using inflammatory markers as predictors of outcome.”
The authors reported no conflicts of interest.
On postoperative day 1, elevated interleukin-6 level was associated with postoperative complications, according to a single-center cohort study of patients who had major abdominal surgery.
“Up to 28% of patients undergoing major abdominal surgery experience postoperative complications, including wound infection, sepsis, anastomotic dehiscence, pneumonia,cardiovascular or respiratory events, and mortality” but an accurate means of identifying those in the risk category would contribute the development of prevention stratetgies, the investigators wrote.

Previous studies of cardiothoracic surgery have supported an association of systemic inflammation to poor outcomes. Dr. Thijs Rettig and colleagues at St. Antonius Hospital, Nieuwegein, the Netherlands, sought to clarify if markers of inflammation and major abdominal surgery correlate with outcomes. Their results were published in the July issue of Annals of Surgery.
Researchers conducted a prospective cohort study at a single center using data obtained from the Myocardial Injury and Complications after major abdominal surgery (MICOLON) study. Participants in the MICOLON study were individuals aged 45 years or older who underwent elective major abdominal surgery. Other inclusion criteria included major cardiovascular (CV) risk factors, coronary artery disease, cerebrovascular accident, diabetes, renal insufficiency, atrial fibrillation, left ventricular dysfunction, aortic valve stenosis, or two minor CV risk factors.
Interleukin-6 (IL-6), tumor necrosis factor (TNF)-alpha, and C-reactive protein (CRP) levels were obtained at baseline and postoperative days 1, 3, and 7 in 137 patients. Systemic inflammatory response syndrome (SIRS) scores were calculated within 48 hours of surgery.
Primary endpoints were 30-day mortality, sepsis, pneumonia, wound infection, anastomotic dehiscence, reoperation, new-onset atrial fibrillation, respiratory insufficiency, congestive heart failure, and myocardial infarction. Data were also collected on length of stay and patients were followed up at 30 days postoperatively for further complications.
With a mean age of 68 years, 59% of patients were male and 30% (n = 40) had an ASA score of 3 or higher. Colorectal (50%), gastroesophageal (22%), and pancreatic (10%) surgery were the most common procedures performed. After excluding 2 patients from analysis for elevated baseline IL-6, 135 patients were analyzed.
At least one postoperative complication was observed in 29% (n = 39) of study subjects with a mean onset of 5 days after surgery. Use of preoperative steroids, aspirin, and statins were not associated with complications; however, blood loss and longer surgery duration where associated with worse outcomes.
In patients with and without complications, differences in IL-6 levels were observed at day 1 at 596 pg/mL vs. 303 pg/mL (P < .01), day 3 at 128 pg/mL vs. 69 pg/mL (P < .01), and day 7 at 76 pg/mL vs. 27 pg/mL (P = .02).
On day 1, CRP was similar in both groups (90 mg/L vs. 78 mg/L; P = .131), but on days 3 (223 mg/L vs. 131 mg/L; P < .001) and 7 (131 mg/L vs. 63 mg/L; P < .001) differences were observed.
Differences in TNF-alpha were observed between groups on day 7 (0.5 pg/mL vs. 0, P < .01). The two groups demonstrated similar leukocyte counts postoperatively.
Prediction for postoperative complications was associated with an IL-6 of 432 pg/mL at day 1, which was 70% specific and 64% sensitive, and had a positive predictive value (PPV) of 44% and negative predictive value (NPV) of 84%. A longer hospital stay of 12 days vs. 7 days (P < .001) was associated with high IL-6 (> 432 pg/mL) vs. a low IL-6 (< 432 pg/mL) at day 1.
Elevated IL-6 level on postoperative day 1 was independently associated with postoperative complications by multivariant regression analysis (AOR: 3.3; 95% confidence interval, 1.3-8.5; P < .02).
The researchers concluded that an increased IL-6 level on postoperative day 1 was associated with increased length of stay and threefold increased risk of complications after major abdominal surgery. They further continued, “It is plausible that early recognition of postoperative complications optimizes the chance of better outcome. One way to enhance early detection of complications is using inflammatory markers as predictors of outcome.”
The authors reported no conflicts of interest.
FROM ANNALS OF SURGERY
Key clinical point: Postoperative complications after major abdominal surgery are associated with elevated IL-6.
Major finding: Elevated IL-6 level on postoperative day 1 was independently associated with postoperative complications by multivariant regression analysis (P < .02).
Data source: Prospective cohort study at a single center using data from the Myocardial Injury and Postoperative Complications after major abdominal surgery (MICOLON) study.
Disclosures: The authors reported no conflicts of interest.
New guidelines focus on pediatric thyroid nodules and cancer
The evaluation and treatment of thyroid nodules in children should differ from evaluation and treatment in adults in that ultrasound characteristics and clinical context should be used rather than size alone to identify nodules that warrant fine-needle aspiration, according to new pediatric-specific guidelines from the American Thyroid Association.
The Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer – the first-ever guidelines for the evaluation and management of thyroid nodules and cancer in children – also note that fine-needle aspiration (FNA) in children should be performed under ultrasound guidance, that preoperative FNA of a hyperfunctioning nodule in a child is not warranted as long as the lesion is removed, that a diffusely infiltrative form of papillary thyroid cancer may occur in children and should be considered in a clinically suspicious gland, and that surgery (lobectomy plus isthmusectomy) is favored over repeat FNA for most nodules with indeterminate cytology, Dr. Gary L. Francis of Virginia Commonwealth University and Children’s Hospital of Richmond, Va., and his colleagues from the American Thyroid Association Guidelines Task Force on Pediatric Thyroid Cancer determined based on an extensive literature search.
Together this guidance with respect to thyroid nodules represents just one of 34 recommendations contained in the guidelines, which, according to the authors, represent “the current optimal care for children and adolescents with these conditions.”
The guidelines were published in the July issue of Thyroid (2015;25:716-59).
Previous guidelines were geared toward adults, but thyroid neoplasms in children differ from those in adults with respect to pathophysiology, clinical presentation, and long-term outcomes. Further, therapy that may be appropriate in adults may not be appropriate for children at low risk for death but higher risk of long-term harm from certain treatments, they said.
For example, recent studies with long-term follow-up revealed an increase in all-cause mortality for survivors of childhood differentiated thyroid cancer (DTC), primarily caused by second malignancies in children treated with radiation.
“These observations, coupled with a better understanding of the excellent prognosis associated with pediatric DTC, have now prompted the American Thyroid Association to specifically address treatment of children with benign and malignant thyroid tumors,” they wrote.
While the task force acknowledged a paucity of randomized, double-blind, controlled clinical trials involving children with DTC, they note that “retrospective analysis of therapeutic options has led to a reconsideration of the former concept that all children with DTC should be similarly treated and has provided the opportunity ... to broaden the scope of acceptable therapy in an attempt to provide aggressive therapy when warranted and to limit overtreatment to those children who are unlikely to benefit.”
In addition to addressing the evaluation and management of thyroid nodules, the guidelines also address DTC, including preoperative staging, surgical management, postoperative staging, the role of radioactive iodine therapy, and goals for thyrotropin suppression. Management algorithms are proposed, and separate recommendations for papillary and follicular thyroid cancers are provided.
The authors note that since DTC recurrence has been reported as long as 40 years after initial therapy, children with DTC should be “followed for life, albeit with decreasing intensity for those with no evidence for disease.”
The guidelines are timely, as Surveillance, Epidemiology and End Results (SEER) program data indicate that new cases of thyroid cancer in persons under the age of 20 years represent 1.8% of all thyroid malignancies diagnosed in the United States, and that the incidence appears to be increasing.
Among 15- to 19-year old adolescents, thyroid cancer is the eighth most frequently diagnosed cancer, and it is the second most common cancer among girls, the authors said, noting that adolescents have a 10-fold greater incidence than do younger children, and that there is a female to male preponderance.
The development of pediatric-specific guidelines was critical, according to guidelines coauthor, Dr. Peter Angelos, professor of surgery and surgical ethics and chief of endocrine surgery at the University of Chicago Medicine and Biological Sciences.
“As they say, ‘children are not just small adults,’ ” he said in an interview.
In addition to the guidance provided on which types of nodules should be evaluated in children (since size alone should not be used to dictate who undergoes biopsy), a highlight of the guidelines is a recommendation that children with thyroid cancer be treated by multidisciplinary teams of physician in high-volume centers, he said.
“Thyroid cancer in children is different than in adults in that children have much higher rates of involved lymph nodes, but their overall prognosis is excellent despite the frequency of involved nodes. This confluence of findings pushes surgeons to do more aggressive operations to clear lymph nodes. This is a good thing, but unfortunately, can lead to higher complication rates (things such as permanently low calcium levels in the blood),” he said, adding that “the implications of finding a high-volume thyroid cancer surgeon with experience in thyroid cancer surgery on children are very significant and the guidelines make some recommendations about how many operations are necessary to constitute high volume.”
The push to limit the use of radioactive iodine in children further underscores the need for an experienced surgeon, he said.
“In an effort to avoid exposing children to radiation, surgeons are further pushed to be more aggressive in the operating room. Thus, it becomes even more important to see an experienced surgeon so that complications can be minimized. Even a seemingly ‘mild’ complication can be devastating for a child who will likely have to live with that complication for decades to come since the prognosis for thyroid cancer is so good,” he said.
An important potential benefit of treatment at centers with multidisciplinary interest and expertise is facilitation of additional research, particularly in areas of uncertainty, including the proper use of 131I, the interpretation of thyroglobulin (Tg) and TgAb (antibody) levels, the role of prospective ultrasound monitoring in presymptomatic children at risk for thyroid neoplasia, the use of novel targeted therapies for advanced disease that fails to respond to 131I, and the long-term psychosocial impacts of the disease on children and their families, the guideline authors said.
“These areas require well-designed long-term, multicenter studies that will be difficult to perform because of the rarity of pediatric DTC and the prolonged follow-up required to reach meaningful endpoints. Further research should be facilitated by ensuring that children with DTC are treated when possible at centers with multidisciplinary interest and expertise in this disease,” they concluded.
The guidelines were funded by the American Thyroid Association and ThyCa: Thyroid Cancer Survivors’ Association. Dr. Francis reported serving as an adviser to ThyCa and receiving research support from Grifols, Novo Nordisk, and the Juvenile Diabetes Research Foundation. Other authors reported relationships (consulting, receiving research support, and/or serving as a speaker) with Akrimax, IBSA Institut Biochimique, Pfizer, Novo Nordisk, Eli Lilly, AstraZeneca, Bayer Healthcare, Genzyme, Sobi, Henning, and Merck, and ThyCa.
The evaluation and treatment of thyroid nodules in children should differ from evaluation and treatment in adults in that ultrasound characteristics and clinical context should be used rather than size alone to identify nodules that warrant fine-needle aspiration, according to new pediatric-specific guidelines from the American Thyroid Association.
The Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer – the first-ever guidelines for the evaluation and management of thyroid nodules and cancer in children – also note that fine-needle aspiration (FNA) in children should be performed under ultrasound guidance, that preoperative FNA of a hyperfunctioning nodule in a child is not warranted as long as the lesion is removed, that a diffusely infiltrative form of papillary thyroid cancer may occur in children and should be considered in a clinically suspicious gland, and that surgery (lobectomy plus isthmusectomy) is favored over repeat FNA for most nodules with indeterminate cytology, Dr. Gary L. Francis of Virginia Commonwealth University and Children’s Hospital of Richmond, Va., and his colleagues from the American Thyroid Association Guidelines Task Force on Pediatric Thyroid Cancer determined based on an extensive literature search.
Together this guidance with respect to thyroid nodules represents just one of 34 recommendations contained in the guidelines, which, according to the authors, represent “the current optimal care for children and adolescents with these conditions.”
The guidelines were published in the July issue of Thyroid (2015;25:716-59).
Previous guidelines were geared toward adults, but thyroid neoplasms in children differ from those in adults with respect to pathophysiology, clinical presentation, and long-term outcomes. Further, therapy that may be appropriate in adults may not be appropriate for children at low risk for death but higher risk of long-term harm from certain treatments, they said.
For example, recent studies with long-term follow-up revealed an increase in all-cause mortality for survivors of childhood differentiated thyroid cancer (DTC), primarily caused by second malignancies in children treated with radiation.
“These observations, coupled with a better understanding of the excellent prognosis associated with pediatric DTC, have now prompted the American Thyroid Association to specifically address treatment of children with benign and malignant thyroid tumors,” they wrote.
While the task force acknowledged a paucity of randomized, double-blind, controlled clinical trials involving children with DTC, they note that “retrospective analysis of therapeutic options has led to a reconsideration of the former concept that all children with DTC should be similarly treated and has provided the opportunity ... to broaden the scope of acceptable therapy in an attempt to provide aggressive therapy when warranted and to limit overtreatment to those children who are unlikely to benefit.”
In addition to addressing the evaluation and management of thyroid nodules, the guidelines also address DTC, including preoperative staging, surgical management, postoperative staging, the role of radioactive iodine therapy, and goals for thyrotropin suppression. Management algorithms are proposed, and separate recommendations for papillary and follicular thyroid cancers are provided.
The authors note that since DTC recurrence has been reported as long as 40 years after initial therapy, children with DTC should be “followed for life, albeit with decreasing intensity for those with no evidence for disease.”
The guidelines are timely, as Surveillance, Epidemiology and End Results (SEER) program data indicate that new cases of thyroid cancer in persons under the age of 20 years represent 1.8% of all thyroid malignancies diagnosed in the United States, and that the incidence appears to be increasing.
Among 15- to 19-year old adolescents, thyroid cancer is the eighth most frequently diagnosed cancer, and it is the second most common cancer among girls, the authors said, noting that adolescents have a 10-fold greater incidence than do younger children, and that there is a female to male preponderance.
The development of pediatric-specific guidelines was critical, according to guidelines coauthor, Dr. Peter Angelos, professor of surgery and surgical ethics and chief of endocrine surgery at the University of Chicago Medicine and Biological Sciences.
“As they say, ‘children are not just small adults,’ ” he said in an interview.
In addition to the guidance provided on which types of nodules should be evaluated in children (since size alone should not be used to dictate who undergoes biopsy), a highlight of the guidelines is a recommendation that children with thyroid cancer be treated by multidisciplinary teams of physician in high-volume centers, he said.
“Thyroid cancer in children is different than in adults in that children have much higher rates of involved lymph nodes, but their overall prognosis is excellent despite the frequency of involved nodes. This confluence of findings pushes surgeons to do more aggressive operations to clear lymph nodes. This is a good thing, but unfortunately, can lead to higher complication rates (things such as permanently low calcium levels in the blood),” he said, adding that “the implications of finding a high-volume thyroid cancer surgeon with experience in thyroid cancer surgery on children are very significant and the guidelines make some recommendations about how many operations are necessary to constitute high volume.”
The push to limit the use of radioactive iodine in children further underscores the need for an experienced surgeon, he said.
“In an effort to avoid exposing children to radiation, surgeons are further pushed to be more aggressive in the operating room. Thus, it becomes even more important to see an experienced surgeon so that complications can be minimized. Even a seemingly ‘mild’ complication can be devastating for a child who will likely have to live with that complication for decades to come since the prognosis for thyroid cancer is so good,” he said.
An important potential benefit of treatment at centers with multidisciplinary interest and expertise is facilitation of additional research, particularly in areas of uncertainty, including the proper use of 131I, the interpretation of thyroglobulin (Tg) and TgAb (antibody) levels, the role of prospective ultrasound monitoring in presymptomatic children at risk for thyroid neoplasia, the use of novel targeted therapies for advanced disease that fails to respond to 131I, and the long-term psychosocial impacts of the disease on children and their families, the guideline authors said.
“These areas require well-designed long-term, multicenter studies that will be difficult to perform because of the rarity of pediatric DTC and the prolonged follow-up required to reach meaningful endpoints. Further research should be facilitated by ensuring that children with DTC are treated when possible at centers with multidisciplinary interest and expertise in this disease,” they concluded.
The guidelines were funded by the American Thyroid Association and ThyCa: Thyroid Cancer Survivors’ Association. Dr. Francis reported serving as an adviser to ThyCa and receiving research support from Grifols, Novo Nordisk, and the Juvenile Diabetes Research Foundation. Other authors reported relationships (consulting, receiving research support, and/or serving as a speaker) with Akrimax, IBSA Institut Biochimique, Pfizer, Novo Nordisk, Eli Lilly, AstraZeneca, Bayer Healthcare, Genzyme, Sobi, Henning, and Merck, and ThyCa.
The evaluation and treatment of thyroid nodules in children should differ from evaluation and treatment in adults in that ultrasound characteristics and clinical context should be used rather than size alone to identify nodules that warrant fine-needle aspiration, according to new pediatric-specific guidelines from the American Thyroid Association.
The Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer – the first-ever guidelines for the evaluation and management of thyroid nodules and cancer in children – also note that fine-needle aspiration (FNA) in children should be performed under ultrasound guidance, that preoperative FNA of a hyperfunctioning nodule in a child is not warranted as long as the lesion is removed, that a diffusely infiltrative form of papillary thyroid cancer may occur in children and should be considered in a clinically suspicious gland, and that surgery (lobectomy plus isthmusectomy) is favored over repeat FNA for most nodules with indeterminate cytology, Dr. Gary L. Francis of Virginia Commonwealth University and Children’s Hospital of Richmond, Va., and his colleagues from the American Thyroid Association Guidelines Task Force on Pediatric Thyroid Cancer determined based on an extensive literature search.
Together this guidance with respect to thyroid nodules represents just one of 34 recommendations contained in the guidelines, which, according to the authors, represent “the current optimal care for children and adolescents with these conditions.”
The guidelines were published in the July issue of Thyroid (2015;25:716-59).
Previous guidelines were geared toward adults, but thyroid neoplasms in children differ from those in adults with respect to pathophysiology, clinical presentation, and long-term outcomes. Further, therapy that may be appropriate in adults may not be appropriate for children at low risk for death but higher risk of long-term harm from certain treatments, they said.
For example, recent studies with long-term follow-up revealed an increase in all-cause mortality for survivors of childhood differentiated thyroid cancer (DTC), primarily caused by second malignancies in children treated with radiation.
“These observations, coupled with a better understanding of the excellent prognosis associated with pediatric DTC, have now prompted the American Thyroid Association to specifically address treatment of children with benign and malignant thyroid tumors,” they wrote.
While the task force acknowledged a paucity of randomized, double-blind, controlled clinical trials involving children with DTC, they note that “retrospective analysis of therapeutic options has led to a reconsideration of the former concept that all children with DTC should be similarly treated and has provided the opportunity ... to broaden the scope of acceptable therapy in an attempt to provide aggressive therapy when warranted and to limit overtreatment to those children who are unlikely to benefit.”
In addition to addressing the evaluation and management of thyroid nodules, the guidelines also address DTC, including preoperative staging, surgical management, postoperative staging, the role of radioactive iodine therapy, and goals for thyrotropin suppression. Management algorithms are proposed, and separate recommendations for papillary and follicular thyroid cancers are provided.
The authors note that since DTC recurrence has been reported as long as 40 years after initial therapy, children with DTC should be “followed for life, albeit with decreasing intensity for those with no evidence for disease.”
The guidelines are timely, as Surveillance, Epidemiology and End Results (SEER) program data indicate that new cases of thyroid cancer in persons under the age of 20 years represent 1.8% of all thyroid malignancies diagnosed in the United States, and that the incidence appears to be increasing.
Among 15- to 19-year old adolescents, thyroid cancer is the eighth most frequently diagnosed cancer, and it is the second most common cancer among girls, the authors said, noting that adolescents have a 10-fold greater incidence than do younger children, and that there is a female to male preponderance.
The development of pediatric-specific guidelines was critical, according to guidelines coauthor, Dr. Peter Angelos, professor of surgery and surgical ethics and chief of endocrine surgery at the University of Chicago Medicine and Biological Sciences.
“As they say, ‘children are not just small adults,’ ” he said in an interview.
In addition to the guidance provided on which types of nodules should be evaluated in children (since size alone should not be used to dictate who undergoes biopsy), a highlight of the guidelines is a recommendation that children with thyroid cancer be treated by multidisciplinary teams of physician in high-volume centers, he said.
“Thyroid cancer in children is different than in adults in that children have much higher rates of involved lymph nodes, but their overall prognosis is excellent despite the frequency of involved nodes. This confluence of findings pushes surgeons to do more aggressive operations to clear lymph nodes. This is a good thing, but unfortunately, can lead to higher complication rates (things such as permanently low calcium levels in the blood),” he said, adding that “the implications of finding a high-volume thyroid cancer surgeon with experience in thyroid cancer surgery on children are very significant and the guidelines make some recommendations about how many operations are necessary to constitute high volume.”
The push to limit the use of radioactive iodine in children further underscores the need for an experienced surgeon, he said.
“In an effort to avoid exposing children to radiation, surgeons are further pushed to be more aggressive in the operating room. Thus, it becomes even more important to see an experienced surgeon so that complications can be minimized. Even a seemingly ‘mild’ complication can be devastating for a child who will likely have to live with that complication for decades to come since the prognosis for thyroid cancer is so good,” he said.
An important potential benefit of treatment at centers with multidisciplinary interest and expertise is facilitation of additional research, particularly in areas of uncertainty, including the proper use of 131I, the interpretation of thyroglobulin (Tg) and TgAb (antibody) levels, the role of prospective ultrasound monitoring in presymptomatic children at risk for thyroid neoplasia, the use of novel targeted therapies for advanced disease that fails to respond to 131I, and the long-term psychosocial impacts of the disease on children and their families, the guideline authors said.
“These areas require well-designed long-term, multicenter studies that will be difficult to perform because of the rarity of pediatric DTC and the prolonged follow-up required to reach meaningful endpoints. Further research should be facilitated by ensuring that children with DTC are treated when possible at centers with multidisciplinary interest and expertise in this disease,” they concluded.
The guidelines were funded by the American Thyroid Association and ThyCa: Thyroid Cancer Survivors’ Association. Dr. Francis reported serving as an adviser to ThyCa and receiving research support from Grifols, Novo Nordisk, and the Juvenile Diabetes Research Foundation. Other authors reported relationships (consulting, receiving research support, and/or serving as a speaker) with Akrimax, IBSA Institut Biochimique, Pfizer, Novo Nordisk, Eli Lilly, AstraZeneca, Bayer Healthcare, Genzyme, Sobi, Henning, and Merck, and ThyCa.
FROM THYROID
Gene-testing predictive value can depend on institutional cancer prevalence
Molecular profiling may be useful to thyroid surgeons in a variety of scenarios, but results should be interpreted with proper knowledge of cancer prevalence at the clinician’s institution, report Dr. Robert L. Ferris and coauthors of the University of Pittsburgh Cancer Institute.
A large, prospective single-center study examined seven-gene mutational panel performance, and found that for the AUS/FLUS cytologic category, mutation identification had a positive predictive value of 88% for histologic cancers, with a false-positive rate of 12%, the authors said.
Results from two analyses of the gene expression classifier (GEC) test emphasized the importance of cancer prevalence at the institution in interpretation of negative predictive value (NPV) and positive predictive value (PPV). In the first study, though the overall calculated sensitivity for GEC was 94%, the high malignancy rate at the institution resulted in a lower estimated NPV of 90%. The second study found an estimated sensitivity and specificity to be 83% and 10%, respectively, and decreases in estimated NPV (94%) and PPV (16%), Dr. Ferris and his colleagues reported.
“Given the well established and frequently dramatic variations in cancer prevalence in thyroid cytology specimens, clinicians are urged to be aware of the prevalence of disease by cytologic category in their tested patients and carefully consider how local disease prevalence may change PPV and NPV of molecular diagnostic tests when applied to their unique clinical practice,” the authors said in the report.
Additionally, “the use of molecular profiling in cytologic indeterminate categories should be interpreted judiciously and with discretion by the clinician, who must be aware of institutional cytopathologic performance results, as well as the individual clinical and sonographic factors for each patient,” they concluded.
Read the full article in Thyroid (doi/pdf/10.1089/thy.2014.0502).
Molecular profiling may be useful to thyroid surgeons in a variety of scenarios, but results should be interpreted with proper knowledge of cancer prevalence at the clinician’s institution, report Dr. Robert L. Ferris and coauthors of the University of Pittsburgh Cancer Institute.
A large, prospective single-center study examined seven-gene mutational panel performance, and found that for the AUS/FLUS cytologic category, mutation identification had a positive predictive value of 88% for histologic cancers, with a false-positive rate of 12%, the authors said.
Results from two analyses of the gene expression classifier (GEC) test emphasized the importance of cancer prevalence at the institution in interpretation of negative predictive value (NPV) and positive predictive value (PPV). In the first study, though the overall calculated sensitivity for GEC was 94%, the high malignancy rate at the institution resulted in a lower estimated NPV of 90%. The second study found an estimated sensitivity and specificity to be 83% and 10%, respectively, and decreases in estimated NPV (94%) and PPV (16%), Dr. Ferris and his colleagues reported.
“Given the well established and frequently dramatic variations in cancer prevalence in thyroid cytology specimens, clinicians are urged to be aware of the prevalence of disease by cytologic category in their tested patients and carefully consider how local disease prevalence may change PPV and NPV of molecular diagnostic tests when applied to their unique clinical practice,” the authors said in the report.
Additionally, “the use of molecular profiling in cytologic indeterminate categories should be interpreted judiciously and with discretion by the clinician, who must be aware of institutional cytopathologic performance results, as well as the individual clinical and sonographic factors for each patient,” they concluded.
Read the full article in Thyroid (doi/pdf/10.1089/thy.2014.0502).
Molecular profiling may be useful to thyroid surgeons in a variety of scenarios, but results should be interpreted with proper knowledge of cancer prevalence at the clinician’s institution, report Dr. Robert L. Ferris and coauthors of the University of Pittsburgh Cancer Institute.
A large, prospective single-center study examined seven-gene mutational panel performance, and found that for the AUS/FLUS cytologic category, mutation identification had a positive predictive value of 88% for histologic cancers, with a false-positive rate of 12%, the authors said.
Results from two analyses of the gene expression classifier (GEC) test emphasized the importance of cancer prevalence at the institution in interpretation of negative predictive value (NPV) and positive predictive value (PPV). In the first study, though the overall calculated sensitivity for GEC was 94%, the high malignancy rate at the institution resulted in a lower estimated NPV of 90%. The second study found an estimated sensitivity and specificity to be 83% and 10%, respectively, and decreases in estimated NPV (94%) and PPV (16%), Dr. Ferris and his colleagues reported.
“Given the well established and frequently dramatic variations in cancer prevalence in thyroid cytology specimens, clinicians are urged to be aware of the prevalence of disease by cytologic category in their tested patients and carefully consider how local disease prevalence may change PPV and NPV of molecular diagnostic tests when applied to their unique clinical practice,” the authors said in the report.
Additionally, “the use of molecular profiling in cytologic indeterminate categories should be interpreted judiciously and with discretion by the clinician, who must be aware of institutional cytopathologic performance results, as well as the individual clinical and sonographic factors for each patient,” they concluded.
Read the full article in Thyroid (doi/pdf/10.1089/thy.2014.0502).
Routine screening sufficient for detecting occult cancer in patients with VTE
TORONTO – The prevalence of occult cancer is low in patients with a first unprovoked venous thromboembolism, according to results from a multicenter, randomized study presented at the International Society on Thrombosis and Haemostasis congress.
In addition, routine screening with the addition of a comprehensive CT scan of the abdomen and pelvis was no better than routine screening alone in detecting occult cancer in this population.
Those are key findings that Dr. Marc Carrier of the University of Ottawa presented from the Screening for Occult Malignancy in Patients with Idiopathic Venous Thromboembolism (SOME) trial, a multicenter, open-label, randomized controlled trial that compared the efficacy of conventional screening with or without comprehensive CT of the abdomen/pelvis for detecting occult cancers in patients with unprovoked venous thromboembolism (VTE). The results of this study were published the same day as his presentation in the New England Journal of Medicine.
“It has been described that up to 10% of patients with unprovoked VTE are diagnosed with cancer in the year following their VTE diagnosis,” Dr. Carrier said. “Therefore, it’s appealing for clinicians to screen these patients for occult cancer but it has led to a lot of great diversity in practices. Some clinicians prefer to use a limited screening strategy that would include a history, physical examination, routine blood tests, and a chest X-ray. Other clinicians prefer to use the limited screening strategy in combination with additional tests. That could be CT of the abdomen and pelvis, ultrasound, or tumor marker, or [computed axial tomography] scan. It’s hard for a physician to know what to use.”
For the SOME trial, a total of 854 patients with unprovoked VTE were randomized to two groups: 431 to limited occult cancer screening (basic blood work, chest X-ray, and breast/cervical/prostate cancer screening) and 423 to limited screening in combination with a comprehensive CT of the abdomen/pelvis. The comprehensive CT included a virtual colonoscopy and gastroscopy, a biphasic enhanced CT, a parenchymal pancreatogram, and a uniphasic enhanced CT of distended bladder. The primary outcome was confirmed cancer that was missed by the screening strategy and detected by the end of the 1-year follow-up period.
Dr. Carrier reported that 33 patients (3.9%) had a new diagnosis of cancer in the interval between randomization and 1-year follow-up: 14 in the limited-screening group and 19 in the limited-screening-plus-CT group, a difference that was not statistically significant (P = .28). In addition, the number of occult cancers missed by the end of the 1-year follow-up period was similar between the two groups: four in the limited-screening group and five in the limited-screening-plus-CT group.
He and his associates also found no significant differences between the limited-screening group and the limited-screening-plus-CT group in the rate of detection of early cancers (0.23% vs. 0.71%, respectively; P = .37), in overall mortality (1.4% vs. 1.2%; P > 0.99), or in cancer-related mortality (1.4% vs. 0.95%; P = .75).
“Occult cancers are not nearly as common as we thought they were, which is reassuring for clinicians and patients because then we don’t have to do a lot of investigations to try and find them, and often scare patients and expose them to radiation and additional procedures,” Dr. Carrier said in an interview. “Limited screening alone, which is what is recommended in Canada and in the United States for age- and gender-specific screening, is more than reasonable for these patients.”
The SOME trial was funded by the Heart and Stroke Foundation of Canada. Dr. Carrier had no relevant financial conflicts to disclose.
Therese Borden contributed to this article.
TORONTO – The prevalence of occult cancer is low in patients with a first unprovoked venous thromboembolism, according to results from a multicenter, randomized study presented at the International Society on Thrombosis and Haemostasis congress.
In addition, routine screening with the addition of a comprehensive CT scan of the abdomen and pelvis was no better than routine screening alone in detecting occult cancer in this population.
Those are key findings that Dr. Marc Carrier of the University of Ottawa presented from the Screening for Occult Malignancy in Patients with Idiopathic Venous Thromboembolism (SOME) trial, a multicenter, open-label, randomized controlled trial that compared the efficacy of conventional screening with or without comprehensive CT of the abdomen/pelvis for detecting occult cancers in patients with unprovoked venous thromboembolism (VTE). The results of this study were published the same day as his presentation in the New England Journal of Medicine.
“It has been described that up to 10% of patients with unprovoked VTE are diagnosed with cancer in the year following their VTE diagnosis,” Dr. Carrier said. “Therefore, it’s appealing for clinicians to screen these patients for occult cancer but it has led to a lot of great diversity in practices. Some clinicians prefer to use a limited screening strategy that would include a history, physical examination, routine blood tests, and a chest X-ray. Other clinicians prefer to use the limited screening strategy in combination with additional tests. That could be CT of the abdomen and pelvis, ultrasound, or tumor marker, or [computed axial tomography] scan. It’s hard for a physician to know what to use.”
For the SOME trial, a total of 854 patients with unprovoked VTE were randomized to two groups: 431 to limited occult cancer screening (basic blood work, chest X-ray, and breast/cervical/prostate cancer screening) and 423 to limited screening in combination with a comprehensive CT of the abdomen/pelvis. The comprehensive CT included a virtual colonoscopy and gastroscopy, a biphasic enhanced CT, a parenchymal pancreatogram, and a uniphasic enhanced CT of distended bladder. The primary outcome was confirmed cancer that was missed by the screening strategy and detected by the end of the 1-year follow-up period.
Dr. Carrier reported that 33 patients (3.9%) had a new diagnosis of cancer in the interval between randomization and 1-year follow-up: 14 in the limited-screening group and 19 in the limited-screening-plus-CT group, a difference that was not statistically significant (P = .28). In addition, the number of occult cancers missed by the end of the 1-year follow-up period was similar between the two groups: four in the limited-screening group and five in the limited-screening-plus-CT group.
He and his associates also found no significant differences between the limited-screening group and the limited-screening-plus-CT group in the rate of detection of early cancers (0.23% vs. 0.71%, respectively; P = .37), in overall mortality (1.4% vs. 1.2%; P > 0.99), or in cancer-related mortality (1.4% vs. 0.95%; P = .75).
“Occult cancers are not nearly as common as we thought they were, which is reassuring for clinicians and patients because then we don’t have to do a lot of investigations to try and find them, and often scare patients and expose them to radiation and additional procedures,” Dr. Carrier said in an interview. “Limited screening alone, which is what is recommended in Canada and in the United States for age- and gender-specific screening, is more than reasonable for these patients.”
The SOME trial was funded by the Heart and Stroke Foundation of Canada. Dr. Carrier had no relevant financial conflicts to disclose.
Therese Borden contributed to this article.
TORONTO – The prevalence of occult cancer is low in patients with a first unprovoked venous thromboembolism, according to results from a multicenter, randomized study presented at the International Society on Thrombosis and Haemostasis congress.
In addition, routine screening with the addition of a comprehensive CT scan of the abdomen and pelvis was no better than routine screening alone in detecting occult cancer in this population.
Those are key findings that Dr. Marc Carrier of the University of Ottawa presented from the Screening for Occult Malignancy in Patients with Idiopathic Venous Thromboembolism (SOME) trial, a multicenter, open-label, randomized controlled trial that compared the efficacy of conventional screening with or without comprehensive CT of the abdomen/pelvis for detecting occult cancers in patients with unprovoked venous thromboembolism (VTE). The results of this study were published the same day as his presentation in the New England Journal of Medicine.
“It has been described that up to 10% of patients with unprovoked VTE are diagnosed with cancer in the year following their VTE diagnosis,” Dr. Carrier said. “Therefore, it’s appealing for clinicians to screen these patients for occult cancer but it has led to a lot of great diversity in practices. Some clinicians prefer to use a limited screening strategy that would include a history, physical examination, routine blood tests, and a chest X-ray. Other clinicians prefer to use the limited screening strategy in combination with additional tests. That could be CT of the abdomen and pelvis, ultrasound, or tumor marker, or [computed axial tomography] scan. It’s hard for a physician to know what to use.”
For the SOME trial, a total of 854 patients with unprovoked VTE were randomized to two groups: 431 to limited occult cancer screening (basic blood work, chest X-ray, and breast/cervical/prostate cancer screening) and 423 to limited screening in combination with a comprehensive CT of the abdomen/pelvis. The comprehensive CT included a virtual colonoscopy and gastroscopy, a biphasic enhanced CT, a parenchymal pancreatogram, and a uniphasic enhanced CT of distended bladder. The primary outcome was confirmed cancer that was missed by the screening strategy and detected by the end of the 1-year follow-up period.
Dr. Carrier reported that 33 patients (3.9%) had a new diagnosis of cancer in the interval between randomization and 1-year follow-up: 14 in the limited-screening group and 19 in the limited-screening-plus-CT group, a difference that was not statistically significant (P = .28). In addition, the number of occult cancers missed by the end of the 1-year follow-up period was similar between the two groups: four in the limited-screening group and five in the limited-screening-plus-CT group.
He and his associates also found no significant differences between the limited-screening group and the limited-screening-plus-CT group in the rate of detection of early cancers (0.23% vs. 0.71%, respectively; P = .37), in overall mortality (1.4% vs. 1.2%; P > 0.99), or in cancer-related mortality (1.4% vs. 0.95%; P = .75).
“Occult cancers are not nearly as common as we thought they were, which is reassuring for clinicians and patients because then we don’t have to do a lot of investigations to try and find them, and often scare patients and expose them to radiation and additional procedures,” Dr. Carrier said in an interview. “Limited screening alone, which is what is recommended in Canada and in the United States for age- and gender-specific screening, is more than reasonable for these patients.”
The SOME trial was funded by the Heart and Stroke Foundation of Canada. Dr. Carrier had no relevant financial conflicts to disclose.
Therese Borden contributed to this article.
AT THE 2015 ISTH CONGRESS
Key clinical point: Occult cancers in patients with a first unprovoked VTE are not nearly as common as previously thought, and limited screening for such cancers is appropriate.
Major finding: There were no significant differences between the limited-screening group and the limited-screening-plus-CT group in the rate of detection of early cancers (0.23% vs. 0.71%); in overall mortality (1.4% vs. 1.2%), or in cancer-related mortality (1.4% vs. 0.95%).
Data source: A multicenter, open-label, randomized controlled trial of 854 patients with unprovoked VTE.
Disclosures: The trial was funded by the Heart and Stroke Foundation of Canada. Dr. Carrier reported having no financial disclosures.
ASCO supports endometrial cancer RT guidelines with some qualifications
The American Society of Clinical Oncology is supporting a guideline on postoperative radiation therapy for endometrial cancer, with some qualifications.
According to the guideline from the American Society for Radiation Oncology, surveillance only is a reasonable course of action in women without residual disease and for women with grade 1 or 2 cancer and less than 50% myometrial invasion (MI). For women with grade 1 or 2 cancer with more than 50% MI and women with grade 3 cancer with less than 50% MI, vaginal brachytherapy is as effective as pelvic radiation is at preventing recurrence and is preferable. Patients with grade 3 cancer above 50% MI will benefit from pelvic radiation therapy.
The ASCO Endorsement Panel gave several qualifications to highlight important points. Qualifications listed include the lack of survival benefit with external beam radiation therapy in early-stage disease, choosing vaginal brachytherapy over external beam radiation therapy in high-intermediate risk disease for locoregional control, chemotherapy in women with high-risk early-stage and advanced disease, the importance of clinical trials, and fertility and quality of life concerns, the panel reported.
Find the full study in the Journal of Clinical Oncology (doi:10.1200/JCO.2015.62.5459)
The American Society of Clinical Oncology is supporting a guideline on postoperative radiation therapy for endometrial cancer, with some qualifications.
According to the guideline from the American Society for Radiation Oncology, surveillance only is a reasonable course of action in women without residual disease and for women with grade 1 or 2 cancer and less than 50% myometrial invasion (MI). For women with grade 1 or 2 cancer with more than 50% MI and women with grade 3 cancer with less than 50% MI, vaginal brachytherapy is as effective as pelvic radiation is at preventing recurrence and is preferable. Patients with grade 3 cancer above 50% MI will benefit from pelvic radiation therapy.
The ASCO Endorsement Panel gave several qualifications to highlight important points. Qualifications listed include the lack of survival benefit with external beam radiation therapy in early-stage disease, choosing vaginal brachytherapy over external beam radiation therapy in high-intermediate risk disease for locoregional control, chemotherapy in women with high-risk early-stage and advanced disease, the importance of clinical trials, and fertility and quality of life concerns, the panel reported.
Find the full study in the Journal of Clinical Oncology (doi:10.1200/JCO.2015.62.5459)
The American Society of Clinical Oncology is supporting a guideline on postoperative radiation therapy for endometrial cancer, with some qualifications.
According to the guideline from the American Society for Radiation Oncology, surveillance only is a reasonable course of action in women without residual disease and for women with grade 1 or 2 cancer and less than 50% myometrial invasion (MI). For women with grade 1 or 2 cancer with more than 50% MI and women with grade 3 cancer with less than 50% MI, vaginal brachytherapy is as effective as pelvic radiation is at preventing recurrence and is preferable. Patients with grade 3 cancer above 50% MI will benefit from pelvic radiation therapy.
The ASCO Endorsement Panel gave several qualifications to highlight important points. Qualifications listed include the lack of survival benefit with external beam radiation therapy in early-stage disease, choosing vaginal brachytherapy over external beam radiation therapy in high-intermediate risk disease for locoregional control, chemotherapy in women with high-risk early-stage and advanced disease, the importance of clinical trials, and fertility and quality of life concerns, the panel reported.
Find the full study in the Journal of Clinical Oncology (doi:10.1200/JCO.2015.62.5459)
Contrast imaging detects some lung tumors in surgery
Injecting patients with a molecular contrast agent before lung cancer surgery and then using fluorescent imaging during the operation proved safe and somewhat effective at finding lung adenocarcinomas and discovering tumor metastases in a pilot clinical trial of 50 patients.
Investigators from the University of Pennsylvania, Philadelphia, and Emory (Atlanta) and Purdue (West Lafayette, Ind.) universities reported their findings online in the Journal of Thoracic and Cardiovascular Surgery (2015 [doi:10.1016/j.jtcvs.2015.05.014]).
“We believe the most important finding in this study was the ability to systemically inject our contrast agent into patients prior to surgery and have 92% of the lung adenocarcinomas fluoresce in the operating room,” Dr. Olugbenga Okusanya of the University of Pennsylvania, Philadelphia, and his colleagues said. Their approach overcomes some of the limitations of other contrast agents for imaging of lung cancer.
The approach involves intravenous administration of 0.1 mg/kg of a fluorescent folate receptor-alpha (FR-alpha)–targeted molecule contrast agent 4 hours before surgery. The specific agent they used is a synthetic conjugate between folate and fluorescein isothiocyanate that binds to serum proteins.
They set out to determine if an optical-targeted molecular contrast agent to FR-alpha could bind lung adenocarcinoma and then if real-time optical imaging, either in situ or ex vivo during surgery, would show the lesions. The 50 study subjects all had biopsy-confirmed lung adenocarcinoma. In the OR, the researchers used a gantry-mounted fluorescent imaging system to capture images of the tumors.
With the operating room lights switched off, imaging of the cancer was captured by fluorescence and white light in situ. The lung was deflated during imaging to expose as much of the lung surface to the imaging as possible. The in situ optical imaging required on average eight minutes to perform. Only seven tumors (14%) appeared fluorescent in situ before they were excised. Computed tomography (CT) before surgery showed that all seven of these tumors were below the pleural surface and within 1.2 cm of the lung surface.
Among the other 43 tumors, 39 exhibited uniform fluorescence once the surgical team exposed their surfaces while 4 – all invasive adenocarcinomas – did not. Final pathology confirmed that all 50 tumors were cancerous.
“With further refinements, this tool may prove useful in locating adenocarcinomas deeper in the lung parenchyma, lymph nodes and at pleural and resection margins,” Dr. Okusanya and his coauthors said.
The investigators noted that one advantage of this imaging strategy is that it can locate tumors of varying sizes, subtypes and metabolic activity with tumor-to-background ratios “not markedly different” among them. “This finding demonstrates that intraoperative molecular imaging may be potentially broadly applicable to all FR-alpha lung tumors,” they said.
Other advantages is that wavelength fluorophore poses no radiation exposure and that surgeons readily understand optical imaging “with no need for special training to interpret or process the data.” They did acknowledge that a significant drawback of the molecular contrast agent was its lack of penetration into the lung parenchyma.
“New molecular tools are emerging to identify lung adenocarcinomas during pulmonary resection,” Dr. Okusanya and colleagues said. “This technology will permit precise visualization of tumor margins, localization of small malignant ground-glass opacities, and accurate selection of lymph nodes with metastatic cancer cells. With miniaturization of imaging devices, this method will be particularly useful in minimally invasive surgery.”
The pilot clinical trial was partially supported by grant from the Biomedical Laboratory Research & Development Service of the Veterans Affairs Office of Research and Development and by the CHEST Foundation One Breath Clinical Research Award (SS).
Dr. Shuming Nie is a coauthor and a consultant for Spectropath, a start-up company that is developing advanced instrumentation and nanoparticle contrast agents. Dr. Philip S. Low is a coauthor and a consultant and stakeholder in OnTarget Laboratories. None of the other coauthors had any relationships to disclose.
“Not unexpectedly, the Achilles’ heel of the technique is tumor depth beneath the pleural surface,” Dr. Michael I. Ebright of Columbia University, New York, said in his commentary of the results of the pilot study. He noted that only 7 of the 50 tumors were visible before resection – and those were all large and close to the pleural surface.
“As we move toward the detection of smaller lesions through lung cancer screening, as well as toward the use of minimally invasive surgical techniques challenging direct palpation, more practical techniques for intraoperative localization are sorely needed,” Dr. Ebright said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.04.029]). But the techniques that have emerged are “time consuming, unreliable, or resource intensive.”
He likened the study results to a half-full glass of water: Those who see it half-empty will identify the weaknesses of the study because, among other findings, the technique does not image tumors more than 1 cm below the pleural surface; the half-full cohort will take the position that “new technology has to start somewhere, usually at proof of principle.”
But the in situ optical imaging approach is faster than the current localization options surgeons have, and the possibilities of the approach “are certainly exciting,” Dr. Ebright said.
“Imagination is an essential component of any technological progress,” Dr. Ebright said. “This study should be viewed as a launching pad rather than be judged solely on practicality in its current form.
Dr. Ebright is with the section of thoracic surgery, Columbia University Medical Center, New York.
“Not unexpectedly, the Achilles’ heel of the technique is tumor depth beneath the pleural surface,” Dr. Michael I. Ebright of Columbia University, New York, said in his commentary of the results of the pilot study. He noted that only 7 of the 50 tumors were visible before resection – and those were all large and close to the pleural surface.
“As we move toward the detection of smaller lesions through lung cancer screening, as well as toward the use of minimally invasive surgical techniques challenging direct palpation, more practical techniques for intraoperative localization are sorely needed,” Dr. Ebright said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.04.029]). But the techniques that have emerged are “time consuming, unreliable, or resource intensive.”
He likened the study results to a half-full glass of water: Those who see it half-empty will identify the weaknesses of the study because, among other findings, the technique does not image tumors more than 1 cm below the pleural surface; the half-full cohort will take the position that “new technology has to start somewhere, usually at proof of principle.”
But the in situ optical imaging approach is faster than the current localization options surgeons have, and the possibilities of the approach “are certainly exciting,” Dr. Ebright said.
“Imagination is an essential component of any technological progress,” Dr. Ebright said. “This study should be viewed as a launching pad rather than be judged solely on practicality in its current form.
Dr. Ebright is with the section of thoracic surgery, Columbia University Medical Center, New York.
“Not unexpectedly, the Achilles’ heel of the technique is tumor depth beneath the pleural surface,” Dr. Michael I. Ebright of Columbia University, New York, said in his commentary of the results of the pilot study. He noted that only 7 of the 50 tumors were visible before resection – and those were all large and close to the pleural surface.
“As we move toward the detection of smaller lesions through lung cancer screening, as well as toward the use of minimally invasive surgical techniques challenging direct palpation, more practical techniques for intraoperative localization are sorely needed,” Dr. Ebright said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.04.029]). But the techniques that have emerged are “time consuming, unreliable, or resource intensive.”
He likened the study results to a half-full glass of water: Those who see it half-empty will identify the weaknesses of the study because, among other findings, the technique does not image tumors more than 1 cm below the pleural surface; the half-full cohort will take the position that “new technology has to start somewhere, usually at proof of principle.”
But the in situ optical imaging approach is faster than the current localization options surgeons have, and the possibilities of the approach “are certainly exciting,” Dr. Ebright said.
“Imagination is an essential component of any technological progress,” Dr. Ebright said. “This study should be viewed as a launching pad rather than be judged solely on practicality in its current form.
Dr. Ebright is with the section of thoracic surgery, Columbia University Medical Center, New York.
Injecting patients with a molecular contrast agent before lung cancer surgery and then using fluorescent imaging during the operation proved safe and somewhat effective at finding lung adenocarcinomas and discovering tumor metastases in a pilot clinical trial of 50 patients.
Investigators from the University of Pennsylvania, Philadelphia, and Emory (Atlanta) and Purdue (West Lafayette, Ind.) universities reported their findings online in the Journal of Thoracic and Cardiovascular Surgery (2015 [doi:10.1016/j.jtcvs.2015.05.014]).
“We believe the most important finding in this study was the ability to systemically inject our contrast agent into patients prior to surgery and have 92% of the lung adenocarcinomas fluoresce in the operating room,” Dr. Olugbenga Okusanya of the University of Pennsylvania, Philadelphia, and his colleagues said. Their approach overcomes some of the limitations of other contrast agents for imaging of lung cancer.
The approach involves intravenous administration of 0.1 mg/kg of a fluorescent folate receptor-alpha (FR-alpha)–targeted molecule contrast agent 4 hours before surgery. The specific agent they used is a synthetic conjugate between folate and fluorescein isothiocyanate that binds to serum proteins.
They set out to determine if an optical-targeted molecular contrast agent to FR-alpha could bind lung adenocarcinoma and then if real-time optical imaging, either in situ or ex vivo during surgery, would show the lesions. The 50 study subjects all had biopsy-confirmed lung adenocarcinoma. In the OR, the researchers used a gantry-mounted fluorescent imaging system to capture images of the tumors.
With the operating room lights switched off, imaging of the cancer was captured by fluorescence and white light in situ. The lung was deflated during imaging to expose as much of the lung surface to the imaging as possible. The in situ optical imaging required on average eight minutes to perform. Only seven tumors (14%) appeared fluorescent in situ before they were excised. Computed tomography (CT) before surgery showed that all seven of these tumors were below the pleural surface and within 1.2 cm of the lung surface.
Among the other 43 tumors, 39 exhibited uniform fluorescence once the surgical team exposed their surfaces while 4 – all invasive adenocarcinomas – did not. Final pathology confirmed that all 50 tumors were cancerous.
“With further refinements, this tool may prove useful in locating adenocarcinomas deeper in the lung parenchyma, lymph nodes and at pleural and resection margins,” Dr. Okusanya and his coauthors said.
The investigators noted that one advantage of this imaging strategy is that it can locate tumors of varying sizes, subtypes and metabolic activity with tumor-to-background ratios “not markedly different” among them. “This finding demonstrates that intraoperative molecular imaging may be potentially broadly applicable to all FR-alpha lung tumors,” they said.
Other advantages is that wavelength fluorophore poses no radiation exposure and that surgeons readily understand optical imaging “with no need for special training to interpret or process the data.” They did acknowledge that a significant drawback of the molecular contrast agent was its lack of penetration into the lung parenchyma.
“New molecular tools are emerging to identify lung adenocarcinomas during pulmonary resection,” Dr. Okusanya and colleagues said. “This technology will permit precise visualization of tumor margins, localization of small malignant ground-glass opacities, and accurate selection of lymph nodes with metastatic cancer cells. With miniaturization of imaging devices, this method will be particularly useful in minimally invasive surgery.”
The pilot clinical trial was partially supported by grant from the Biomedical Laboratory Research & Development Service of the Veterans Affairs Office of Research and Development and by the CHEST Foundation One Breath Clinical Research Award (SS).
Dr. Shuming Nie is a coauthor and a consultant for Spectropath, a start-up company that is developing advanced instrumentation and nanoparticle contrast agents. Dr. Philip S. Low is a coauthor and a consultant and stakeholder in OnTarget Laboratories. None of the other coauthors had any relationships to disclose.
Injecting patients with a molecular contrast agent before lung cancer surgery and then using fluorescent imaging during the operation proved safe and somewhat effective at finding lung adenocarcinomas and discovering tumor metastases in a pilot clinical trial of 50 patients.
Investigators from the University of Pennsylvania, Philadelphia, and Emory (Atlanta) and Purdue (West Lafayette, Ind.) universities reported their findings online in the Journal of Thoracic and Cardiovascular Surgery (2015 [doi:10.1016/j.jtcvs.2015.05.014]).
“We believe the most important finding in this study was the ability to systemically inject our contrast agent into patients prior to surgery and have 92% of the lung adenocarcinomas fluoresce in the operating room,” Dr. Olugbenga Okusanya of the University of Pennsylvania, Philadelphia, and his colleagues said. Their approach overcomes some of the limitations of other contrast agents for imaging of lung cancer.
The approach involves intravenous administration of 0.1 mg/kg of a fluorescent folate receptor-alpha (FR-alpha)–targeted molecule contrast agent 4 hours before surgery. The specific agent they used is a synthetic conjugate between folate and fluorescein isothiocyanate that binds to serum proteins.
They set out to determine if an optical-targeted molecular contrast agent to FR-alpha could bind lung adenocarcinoma and then if real-time optical imaging, either in situ or ex vivo during surgery, would show the lesions. The 50 study subjects all had biopsy-confirmed lung adenocarcinoma. In the OR, the researchers used a gantry-mounted fluorescent imaging system to capture images of the tumors.
With the operating room lights switched off, imaging of the cancer was captured by fluorescence and white light in situ. The lung was deflated during imaging to expose as much of the lung surface to the imaging as possible. The in situ optical imaging required on average eight minutes to perform. Only seven tumors (14%) appeared fluorescent in situ before they were excised. Computed tomography (CT) before surgery showed that all seven of these tumors were below the pleural surface and within 1.2 cm of the lung surface.
Among the other 43 tumors, 39 exhibited uniform fluorescence once the surgical team exposed their surfaces while 4 – all invasive adenocarcinomas – did not. Final pathology confirmed that all 50 tumors were cancerous.
“With further refinements, this tool may prove useful in locating adenocarcinomas deeper in the lung parenchyma, lymph nodes and at pleural and resection margins,” Dr. Okusanya and his coauthors said.
The investigators noted that one advantage of this imaging strategy is that it can locate tumors of varying sizes, subtypes and metabolic activity with tumor-to-background ratios “not markedly different” among them. “This finding demonstrates that intraoperative molecular imaging may be potentially broadly applicable to all FR-alpha lung tumors,” they said.
Other advantages is that wavelength fluorophore poses no radiation exposure and that surgeons readily understand optical imaging “with no need for special training to interpret or process the data.” They did acknowledge that a significant drawback of the molecular contrast agent was its lack of penetration into the lung parenchyma.
“New molecular tools are emerging to identify lung adenocarcinomas during pulmonary resection,” Dr. Okusanya and colleagues said. “This technology will permit precise visualization of tumor margins, localization of small malignant ground-glass opacities, and accurate selection of lymph nodes with metastatic cancer cells. With miniaturization of imaging devices, this method will be particularly useful in minimally invasive surgery.”
The pilot clinical trial was partially supported by grant from the Biomedical Laboratory Research & Development Service of the Veterans Affairs Office of Research and Development and by the CHEST Foundation One Breath Clinical Research Award (SS).
Dr. Shuming Nie is a coauthor and a consultant for Spectropath, a start-up company that is developing advanced instrumentation and nanoparticle contrast agents. Dr. Philip S. Low is a coauthor and a consultant and stakeholder in OnTarget Laboratories. None of the other coauthors had any relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: A pilot clinical trial reported that molecular contrast imaging during lung cancer surgery is safe and somewhat effective at isolating malignancies.
Major finding: Among 50 patients, the imaging technique detected seven tumors close to the lung surface while 39 others showed fluorescence in the operating room once they were removed.
Data source: Single-center population of 50 patients with biopsy-proven lung adenocarcinoma.
Disclosures: The pilot clinical trial was partially supported by grant from the Biomedical Laboratory Research & Development Service of the Veterans Affairs Office of Research and Development and by the CHEST Foundation One Breath Clinical Research Award (SS). Dr. Nie is a coauthor and a consultant for Spectropath, a start-up company that is developing advanced instrumentation and nanoparticle contrast agents. Dr. Low is a coauthor and a consultant and stakeholder in OnTarget Laboratories. None of the other coauthors had any relationships to disclose.
Optical imaging has bright future for cancer surgery
For cancer patients and the surgeons who treat them, tumor margins matter. The amount of malignant tissue left behind after cancer surgery can make a profound difference in overall survival, but sparing healthy tissue while removing as much tumor as possible is limited by the surgeon’s ability to differentiate one from the other.
Now, new optical imaging techniques that deliver fluorescing molecules specifically to tumor targets show promise to allow both surgeons and pathologists to optimize cancer surgery. These intraoperative techniques use fluorescent molecules tagged to a ligand that binds to a target site on malignant cells. Thus, uptake occurs only in the malignant cells, highlighting them for the surgeon and for pathologists assessing margin adequacy.
Dr. Sunil Singhal of the University of Pennsylvania, Philadelphia, has coined the term “optical biopsy” to describe what’s happening during optical imaging-guided surgery. “For so long, we as surgeons have just had our eyes and our hands – and our intuition – to guide us. This technology allows us to really focus our attention where it needs to be in surgery.”
Optical imaging in surgery uses a variety of fluorescing molecules, some of which were originally derived from sources as diverse as scorpions and fireflies. Some of these glow in the visible spectrum, but increasingly, optical imaging techniques are employing molecules that fluoresce when excited by light in the near-infrared spectrum.
Using the near-infrared spectrum, said Dr. Eben Rosenthal, the Anne and John Doerr Medical Director of the Stanford (Calif.) Cancer Center, does two things: it allows for visualization deeper into tissues – up to 1 or 2 cm – and it improves the signal-to-noise ratio that can be a problem in the visual spectrum, where tissue autofluorescence occurs.
Cameras, endoscopes, and surgical microscopes can all be equipped to display the fluorescence and coregister the image with the white-light view the surgeon or pathologist normally sees. Surgeons using optical imaging techniques can usually continue to use the same tools and the same interface they are accustomed to.
Ligands can be small molecules, such as folate, or complex glycoproteins, such as monoclonal antibodies: all will bind selectively to sites on tumor cells. Patients are infused with the fluorescence-tagged ligand complexes before surgery, with timing appropriate to the half-lives of the agents, so infusions may happen the day before or the day of surgery.
For Dr. Rosenthal, using monoclonal antibodies to target tumors for optical imaging techniques affords several advantages. The antibodies are approved by the Food and Drug Administration, with known safety and side effect profiles. The antibodies can be tagged with an FDA-approved fluorescing molecule, allowing an easier approval process for the compound agent. Finally, the epidermal growth factor receptor (EGFR) target of these monoclonal antibodies is well expressed in many target tumor cells, including melanoma, breast, glioma, and colorectal cancers.
Dr. Singhal’s group has been working with fluorescein-tagged folate to identify pulmonary adenocarcinomas and is exploring use of other agents as well. “We like small molecules,” he said. “We can avoid lots of problems with biotoxicity.”
Annexin A2 (ANXA2) is a molecule expressed on the surface of cancer cells but found intracellularly in healthy cells, and it is the target of the chlorotoxin conjugate used by Dr. Jim Olson of Seattle’s Fred Hutchinson Cancer Research Center. Target malignancies may include glioblastoma, medullablastoma, sarcoma, and prostate and intestinal cancers.
Regardless of the target, however, optical imaging techniques may be particularly well suited for tumors occurring in areas where a generous dissection can have potentially devastating consequences, as in the head and neck, and for brain tumors. By giving the surgeon a clear delineation of malignant tissue, optical imaging can help the surgeon strike a balance to achieve the most conservative dissection that still achieves a complete gross dissection of the malignancy.
Achieving gross total dissection during cancer surgery is an important goal, said Dr. Olson. Citing devastating childhood brain tumors such as medulloblastomas and gliomablastomas, he noted that optical imaging can help surgeons avoid leaving behind any bulk areas of tumor, where hypoxic regions and therapy-resistant changes in tumor physiology may reduce the chance for cure. “Surgery is one of the most important elements of curing cancer,” he said. He pointed out that, in children with medulloblastomas, if nearly all tumor tissue is removed, less radiation is needed and survival is improved, even with less radiation – even if very small amounts of cancer remain.
Limitations of these technologies depend, to some extent, on the tissue targets, on substrates used to deliver the fluorescing molecules, and on the fluorescent agents themselves. Even with the enhanced tissue penetration of near-infrared light, the surgeon can really only see a centimeter or two beneath the surface of a solid organ. Said Dr. Singhal, “The basic limitation is that we’re talking about light.” However, if presurgery imaging identifies an area of concern, the surgeon can direct his or her dissection more carefully when the malignant tissue glows during surgery.
Dr. Rosenthal noted that pathologists also are making use of optical imaging to guide their processes. Since only about 5% of most tumor margins are sampled, there is an inevitable chance of sampling error with the resulting potential for a false-negative pathology result. “This technique augments the pathologist’s role and allows for improved biopsy results,” he said. Using image-guided pathology offers a way to test the margins immediately, helping the pathologist ascertain where the surgeon’s attention should be focused. Optical imaging, he said, “will in many ways change and enhance the experience for everybody.”
Dr. Olson, looking forward, asked, “If we can deliver the ability to light up, why can’t we deliver chemotherapy?” He sees an optical imaging future that will make flexible use of ligands to delineate tumors and test drug delivery.
There are many ways optical imaging can improve the practice of cancer surgery, Dr. Rosenthal said. “Historically, surgery has been a hard area to study because it’s so variable.” Following results from optical imaging–guided precision surgery, he said, will allow surgeons to identify and achieve “the right margin for the right cancer.”
Looking to the future, Dr. Singhal said, “it won’t be an either/or scenario. I think there will be multiple targets, multiple agents for this technology. We, as surgeons, have an obligation to our profession to move ahead with this.”
On Twitter @karioakes
For cancer patients and the surgeons who treat them, tumor margins matter. The amount of malignant tissue left behind after cancer surgery can make a profound difference in overall survival, but sparing healthy tissue while removing as much tumor as possible is limited by the surgeon’s ability to differentiate one from the other.
Now, new optical imaging techniques that deliver fluorescing molecules specifically to tumor targets show promise to allow both surgeons and pathologists to optimize cancer surgery. These intraoperative techniques use fluorescent molecules tagged to a ligand that binds to a target site on malignant cells. Thus, uptake occurs only in the malignant cells, highlighting them for the surgeon and for pathologists assessing margin adequacy.
Dr. Sunil Singhal of the University of Pennsylvania, Philadelphia, has coined the term “optical biopsy” to describe what’s happening during optical imaging-guided surgery. “For so long, we as surgeons have just had our eyes and our hands – and our intuition – to guide us. This technology allows us to really focus our attention where it needs to be in surgery.”
Optical imaging in surgery uses a variety of fluorescing molecules, some of which were originally derived from sources as diverse as scorpions and fireflies. Some of these glow in the visible spectrum, but increasingly, optical imaging techniques are employing molecules that fluoresce when excited by light in the near-infrared spectrum.
Using the near-infrared spectrum, said Dr. Eben Rosenthal, the Anne and John Doerr Medical Director of the Stanford (Calif.) Cancer Center, does two things: it allows for visualization deeper into tissues – up to 1 or 2 cm – and it improves the signal-to-noise ratio that can be a problem in the visual spectrum, where tissue autofluorescence occurs.
Cameras, endoscopes, and surgical microscopes can all be equipped to display the fluorescence and coregister the image with the white-light view the surgeon or pathologist normally sees. Surgeons using optical imaging techniques can usually continue to use the same tools and the same interface they are accustomed to.
Ligands can be small molecules, such as folate, or complex glycoproteins, such as monoclonal antibodies: all will bind selectively to sites on tumor cells. Patients are infused with the fluorescence-tagged ligand complexes before surgery, with timing appropriate to the half-lives of the agents, so infusions may happen the day before or the day of surgery.
For Dr. Rosenthal, using monoclonal antibodies to target tumors for optical imaging techniques affords several advantages. The antibodies are approved by the Food and Drug Administration, with known safety and side effect profiles. The antibodies can be tagged with an FDA-approved fluorescing molecule, allowing an easier approval process for the compound agent. Finally, the epidermal growth factor receptor (EGFR) target of these monoclonal antibodies is well expressed in many target tumor cells, including melanoma, breast, glioma, and colorectal cancers.
Dr. Singhal’s group has been working with fluorescein-tagged folate to identify pulmonary adenocarcinomas and is exploring use of other agents as well. “We like small molecules,” he said. “We can avoid lots of problems with biotoxicity.”
Annexin A2 (ANXA2) is a molecule expressed on the surface of cancer cells but found intracellularly in healthy cells, and it is the target of the chlorotoxin conjugate used by Dr. Jim Olson of Seattle’s Fred Hutchinson Cancer Research Center. Target malignancies may include glioblastoma, medullablastoma, sarcoma, and prostate and intestinal cancers.
Regardless of the target, however, optical imaging techniques may be particularly well suited for tumors occurring in areas where a generous dissection can have potentially devastating consequences, as in the head and neck, and for brain tumors. By giving the surgeon a clear delineation of malignant tissue, optical imaging can help the surgeon strike a balance to achieve the most conservative dissection that still achieves a complete gross dissection of the malignancy.
Achieving gross total dissection during cancer surgery is an important goal, said Dr. Olson. Citing devastating childhood brain tumors such as medulloblastomas and gliomablastomas, he noted that optical imaging can help surgeons avoid leaving behind any bulk areas of tumor, where hypoxic regions and therapy-resistant changes in tumor physiology may reduce the chance for cure. “Surgery is one of the most important elements of curing cancer,” he said. He pointed out that, in children with medulloblastomas, if nearly all tumor tissue is removed, less radiation is needed and survival is improved, even with less radiation – even if very small amounts of cancer remain.
Limitations of these technologies depend, to some extent, on the tissue targets, on substrates used to deliver the fluorescing molecules, and on the fluorescent agents themselves. Even with the enhanced tissue penetration of near-infrared light, the surgeon can really only see a centimeter or two beneath the surface of a solid organ. Said Dr. Singhal, “The basic limitation is that we’re talking about light.” However, if presurgery imaging identifies an area of concern, the surgeon can direct his or her dissection more carefully when the malignant tissue glows during surgery.
Dr. Rosenthal noted that pathologists also are making use of optical imaging to guide their processes. Since only about 5% of most tumor margins are sampled, there is an inevitable chance of sampling error with the resulting potential for a false-negative pathology result. “This technique augments the pathologist’s role and allows for improved biopsy results,” he said. Using image-guided pathology offers a way to test the margins immediately, helping the pathologist ascertain where the surgeon’s attention should be focused. Optical imaging, he said, “will in many ways change and enhance the experience for everybody.”
Dr. Olson, looking forward, asked, “If we can deliver the ability to light up, why can’t we deliver chemotherapy?” He sees an optical imaging future that will make flexible use of ligands to delineate tumors and test drug delivery.
There are many ways optical imaging can improve the practice of cancer surgery, Dr. Rosenthal said. “Historically, surgery has been a hard area to study because it’s so variable.” Following results from optical imaging–guided precision surgery, he said, will allow surgeons to identify and achieve “the right margin for the right cancer.”
Looking to the future, Dr. Singhal said, “it won’t be an either/or scenario. I think there will be multiple targets, multiple agents for this technology. We, as surgeons, have an obligation to our profession to move ahead with this.”
On Twitter @karioakes
For cancer patients and the surgeons who treat them, tumor margins matter. The amount of malignant tissue left behind after cancer surgery can make a profound difference in overall survival, but sparing healthy tissue while removing as much tumor as possible is limited by the surgeon’s ability to differentiate one from the other.
Now, new optical imaging techniques that deliver fluorescing molecules specifically to tumor targets show promise to allow both surgeons and pathologists to optimize cancer surgery. These intraoperative techniques use fluorescent molecules tagged to a ligand that binds to a target site on malignant cells. Thus, uptake occurs only in the malignant cells, highlighting them for the surgeon and for pathologists assessing margin adequacy.
Dr. Sunil Singhal of the University of Pennsylvania, Philadelphia, has coined the term “optical biopsy” to describe what’s happening during optical imaging-guided surgery. “For so long, we as surgeons have just had our eyes and our hands – and our intuition – to guide us. This technology allows us to really focus our attention where it needs to be in surgery.”
Optical imaging in surgery uses a variety of fluorescing molecules, some of which were originally derived from sources as diverse as scorpions and fireflies. Some of these glow in the visible spectrum, but increasingly, optical imaging techniques are employing molecules that fluoresce when excited by light in the near-infrared spectrum.
Using the near-infrared spectrum, said Dr. Eben Rosenthal, the Anne and John Doerr Medical Director of the Stanford (Calif.) Cancer Center, does two things: it allows for visualization deeper into tissues – up to 1 or 2 cm – and it improves the signal-to-noise ratio that can be a problem in the visual spectrum, where tissue autofluorescence occurs.
Cameras, endoscopes, and surgical microscopes can all be equipped to display the fluorescence and coregister the image with the white-light view the surgeon or pathologist normally sees. Surgeons using optical imaging techniques can usually continue to use the same tools and the same interface they are accustomed to.
Ligands can be small molecules, such as folate, or complex glycoproteins, such as monoclonal antibodies: all will bind selectively to sites on tumor cells. Patients are infused with the fluorescence-tagged ligand complexes before surgery, with timing appropriate to the half-lives of the agents, so infusions may happen the day before or the day of surgery.
For Dr. Rosenthal, using monoclonal antibodies to target tumors for optical imaging techniques affords several advantages. The antibodies are approved by the Food and Drug Administration, with known safety and side effect profiles. The antibodies can be tagged with an FDA-approved fluorescing molecule, allowing an easier approval process for the compound agent. Finally, the epidermal growth factor receptor (EGFR) target of these monoclonal antibodies is well expressed in many target tumor cells, including melanoma, breast, glioma, and colorectal cancers.
Dr. Singhal’s group has been working with fluorescein-tagged folate to identify pulmonary adenocarcinomas and is exploring use of other agents as well. “We like small molecules,” he said. “We can avoid lots of problems with biotoxicity.”
Annexin A2 (ANXA2) is a molecule expressed on the surface of cancer cells but found intracellularly in healthy cells, and it is the target of the chlorotoxin conjugate used by Dr. Jim Olson of Seattle’s Fred Hutchinson Cancer Research Center. Target malignancies may include glioblastoma, medullablastoma, sarcoma, and prostate and intestinal cancers.
Regardless of the target, however, optical imaging techniques may be particularly well suited for tumors occurring in areas where a generous dissection can have potentially devastating consequences, as in the head and neck, and for brain tumors. By giving the surgeon a clear delineation of malignant tissue, optical imaging can help the surgeon strike a balance to achieve the most conservative dissection that still achieves a complete gross dissection of the malignancy.
Achieving gross total dissection during cancer surgery is an important goal, said Dr. Olson. Citing devastating childhood brain tumors such as medulloblastomas and gliomablastomas, he noted that optical imaging can help surgeons avoid leaving behind any bulk areas of tumor, where hypoxic regions and therapy-resistant changes in tumor physiology may reduce the chance for cure. “Surgery is one of the most important elements of curing cancer,” he said. He pointed out that, in children with medulloblastomas, if nearly all tumor tissue is removed, less radiation is needed and survival is improved, even with less radiation – even if very small amounts of cancer remain.
Limitations of these technologies depend, to some extent, on the tissue targets, on substrates used to deliver the fluorescing molecules, and on the fluorescent agents themselves. Even with the enhanced tissue penetration of near-infrared light, the surgeon can really only see a centimeter or two beneath the surface of a solid organ. Said Dr. Singhal, “The basic limitation is that we’re talking about light.” However, if presurgery imaging identifies an area of concern, the surgeon can direct his or her dissection more carefully when the malignant tissue glows during surgery.
Dr. Rosenthal noted that pathologists also are making use of optical imaging to guide their processes. Since only about 5% of most tumor margins are sampled, there is an inevitable chance of sampling error with the resulting potential for a false-negative pathology result. “This technique augments the pathologist’s role and allows for improved biopsy results,” he said. Using image-guided pathology offers a way to test the margins immediately, helping the pathologist ascertain where the surgeon’s attention should be focused. Optical imaging, he said, “will in many ways change and enhance the experience for everybody.”
Dr. Olson, looking forward, asked, “If we can deliver the ability to light up, why can’t we deliver chemotherapy?” He sees an optical imaging future that will make flexible use of ligands to delineate tumors and test drug delivery.
There are many ways optical imaging can improve the practice of cancer surgery, Dr. Rosenthal said. “Historically, surgery has been a hard area to study because it’s so variable.” Following results from optical imaging–guided precision surgery, he said, will allow surgeons to identify and achieve “the right margin for the right cancer.”
Looking to the future, Dr. Singhal said, “it won’t be an either/or scenario. I think there will be multiple targets, multiple agents for this technology. We, as surgeons, have an obligation to our profession to move ahead with this.”
On Twitter @karioakes
AATS: Metformin linked to better progression-free survival in early-stage NSCLC
SEATTLE – Metformin use before surgery for stage I and II non–small cell lung cancer (NSCLC) was associated with improved progression-free survival at 5 years in a retrospective database study of 138 patients who also had type 2 diabetes.
The patients were treated for stage I and II NSCLC at Rush University Medical Center, Chicago. They also had type 2 diabetes; 81 (59%) were on metformin in the 6 months before pulmonary resection, and 57 (41%) were not, reported Rush medical student Robert Medairos, who is one of the study researchers.
At 5 years follow-up, progression-free survival was 60% in the metformin group, but about 35% in the no-metformin group (P = .01). Overall survival was about 90% at 5 years in both study arms.
A larger study or longer follow-up may show overall survival benefits for metformin users, Mr. Medairos said at the annual meeting of the American Association for Thoracic Surgery.
Patients were about 70 years old on average in both groups, with 35 pack-year smoking histories, and an average body mass index of about 30 kg/m2. Both study arms had slightly more men than women, and were otherwise balanced for ethnicity and comorbidities.
About 12% of patients in the metformin group and 40% in the no-metformin group, were on insulin prior to surgery. Mean preoperative creatinine was 1 mg/dL in the metformin group, and 1.7 mg/dL in the no-metformin group. There were trends towards higher-stage disease and more lymph node involvement in the no-metformin group. About half the patients in both arms had adenocarcinomas, but a greater proportion of patients in the no-metformin group had squamous cell carcinomas.
The Rush study might be the first to look into metformin for early NSCLC, but there have been several studies in advanced disease. A recent retrospective analysis of 750 diabetes patients with stage IV NSCLC found a median survival of 5 months for the 61% on metformin at diagnosis, but 3 months for those who were not (Am. J. Respir. Crit. Care. Med. 2015;191:448-54).
Currently, there are about a dozen ongoing trials of the drug for lung cancer, and scores more for prostate, breast, brain, uterine, colorectal, thyroid, and other cancers.
Metformin’s metabolic effects might reduce the ability of cancers to grow and metabolize, or the drug might somehow boost the antineoplastic effects of chemotherapeutics. “Our next step is to look at histological and tissues samples to see if metformin changes gene transcription in lung cancer cells,” Mr. Medairos said.
Mr. Medairos has no disclosures.
SEATTLE – Metformin use before surgery for stage I and II non–small cell lung cancer (NSCLC) was associated with improved progression-free survival at 5 years in a retrospective database study of 138 patients who also had type 2 diabetes.
The patients were treated for stage I and II NSCLC at Rush University Medical Center, Chicago. They also had type 2 diabetes; 81 (59%) were on metformin in the 6 months before pulmonary resection, and 57 (41%) were not, reported Rush medical student Robert Medairos, who is one of the study researchers.
At 5 years follow-up, progression-free survival was 60% in the metformin group, but about 35% in the no-metformin group (P = .01). Overall survival was about 90% at 5 years in both study arms.
A larger study or longer follow-up may show overall survival benefits for metformin users, Mr. Medairos said at the annual meeting of the American Association for Thoracic Surgery.
Patients were about 70 years old on average in both groups, with 35 pack-year smoking histories, and an average body mass index of about 30 kg/m2. Both study arms had slightly more men than women, and were otherwise balanced for ethnicity and comorbidities.
About 12% of patients in the metformin group and 40% in the no-metformin group, were on insulin prior to surgery. Mean preoperative creatinine was 1 mg/dL in the metformin group, and 1.7 mg/dL in the no-metformin group. There were trends towards higher-stage disease and more lymph node involvement in the no-metformin group. About half the patients in both arms had adenocarcinomas, but a greater proportion of patients in the no-metformin group had squamous cell carcinomas.
The Rush study might be the first to look into metformin for early NSCLC, but there have been several studies in advanced disease. A recent retrospective analysis of 750 diabetes patients with stage IV NSCLC found a median survival of 5 months for the 61% on metformin at diagnosis, but 3 months for those who were not (Am. J. Respir. Crit. Care. Med. 2015;191:448-54).
Currently, there are about a dozen ongoing trials of the drug for lung cancer, and scores more for prostate, breast, brain, uterine, colorectal, thyroid, and other cancers.
Metformin’s metabolic effects might reduce the ability of cancers to grow and metabolize, or the drug might somehow boost the antineoplastic effects of chemotherapeutics. “Our next step is to look at histological and tissues samples to see if metformin changes gene transcription in lung cancer cells,” Mr. Medairos said.
Mr. Medairos has no disclosures.
SEATTLE – Metformin use before surgery for stage I and II non–small cell lung cancer (NSCLC) was associated with improved progression-free survival at 5 years in a retrospective database study of 138 patients who also had type 2 diabetes.
The patients were treated for stage I and II NSCLC at Rush University Medical Center, Chicago. They also had type 2 diabetes; 81 (59%) were on metformin in the 6 months before pulmonary resection, and 57 (41%) were not, reported Rush medical student Robert Medairos, who is one of the study researchers.
At 5 years follow-up, progression-free survival was 60% in the metformin group, but about 35% in the no-metformin group (P = .01). Overall survival was about 90% at 5 years in both study arms.
A larger study or longer follow-up may show overall survival benefits for metformin users, Mr. Medairos said at the annual meeting of the American Association for Thoracic Surgery.
Patients were about 70 years old on average in both groups, with 35 pack-year smoking histories, and an average body mass index of about 30 kg/m2. Both study arms had slightly more men than women, and were otherwise balanced for ethnicity and comorbidities.
About 12% of patients in the metformin group and 40% in the no-metformin group, were on insulin prior to surgery. Mean preoperative creatinine was 1 mg/dL in the metformin group, and 1.7 mg/dL in the no-metformin group. There were trends towards higher-stage disease and more lymph node involvement in the no-metformin group. About half the patients in both arms had adenocarcinomas, but a greater proportion of patients in the no-metformin group had squamous cell carcinomas.
The Rush study might be the first to look into metformin for early NSCLC, but there have been several studies in advanced disease. A recent retrospective analysis of 750 diabetes patients with stage IV NSCLC found a median survival of 5 months for the 61% on metformin at diagnosis, but 3 months for those who were not (Am. J. Respir. Crit. Care. Med. 2015;191:448-54).
Currently, there are about a dozen ongoing trials of the drug for lung cancer, and scores more for prostate, breast, brain, uterine, colorectal, thyroid, and other cancers.
Metformin’s metabolic effects might reduce the ability of cancers to grow and metabolize, or the drug might somehow boost the antineoplastic effects of chemotherapeutics. “Our next step is to look at histological and tissues samples to see if metformin changes gene transcription in lung cancer cells,” Mr. Medairos said.
Mr. Medairos has no disclosures.
AT THE AATS ANNUAL MEETING
Key clinical point: Metformin might one day be part of routine lung cancer care.
Major finding: Five years after pulmonary resection for non–small cell lung cancer, progression-free survival was 60% in patients who were on metformin before surgery, but 35% in patients who were not (P = .01).
Data source: Retrospective study of 138 patients with type 2 diabetes and stage I and II non–small cell lung cancer.
Disclosures: The lead investigator has no relevant disclosures.
Invasive approaches ‘overused’ for evaluating pulmonary nodules
One in four intermediate-sized (8-20 mm) pulmonary nodules were found to be malignant, based on results from a retrospective study of 377 patients seen in 18 community pulmonology practices.
Malignant nodules were more likely to be found in current or former smokers and to be larger than the benign nodules.
Further, despite guideline recommendations for surveillance of pulmonary nodules with less than a 5% pretest probability of malignancy, 44% of 36 patients with low-risk pulmonary nodules underwent at least one invasive procedure.
“To our knowledge, this is the first study that documents the prevalence of cancer (25%) in patients with intermediate-sized nodules referred to community-based pulmonologists,” wrote Dr. Nichole T. Tanner of the Medical University of South Carolina, Charleston, and her coauthors in the study published online (Chest 2015 June 18 [doi:10.1378/chest.15-0630]). “This finding has implications for managing pulmonary nodules in the community; because the risk of cancer is substantial, patients should be managed based on their individualized risk for malignancy.”
Biopsy – either by transthoracic needle aspiration or bronchoscopy – was performed in 33% (125) of patients, of whom 35% were found to have a malignancy and 57% had a specific benign diagnosis; biopsies in 8% were nondiagnostic and those patients were followed for 2 years.
Referrals for surgery were given to 20% (77) of patients; 19 had previously undergone a biopsy, with 11 having a nondiagnostic biopsy and eight having a received a benign result. Of patients who underwent surgery, 65% were diagnosed with a malignancy, and 35% had benign disease. However, the rate of surgical resection was similar regardless of patients’ pretest probability of malignancy.
PET scanning, performed in 37% of patients, was more likely in those who had a biopsy or surgery. The overall accuracy of PET was 74%, and the false-negative rate decreased slightly as the size of the nodule increased.
“Guidelines recommend surgery if the pretest probability of cancer is high (pCA > 65%) because a negative biopsy would not dissuade the clinician from pursuing a definitive diagnosis and a positive biopsy would lead to surgery anyway.”
The researchers pointed out that there are a range of noninvasive options now available to determine the likelihood that a nodule is malignant, such as CT scanning with the use of volumetric software to measure diameter and volume-doubling time of a nodule in between scans.
The study was supported by Integrated Diagnostics. All but one study author declared consulting fees, grant funding, stock options, or employment with Integrated Diagnostics.
One in four intermediate-sized (8-20 mm) pulmonary nodules were found to be malignant, based on results from a retrospective study of 377 patients seen in 18 community pulmonology practices.
Malignant nodules were more likely to be found in current or former smokers and to be larger than the benign nodules.
Further, despite guideline recommendations for surveillance of pulmonary nodules with less than a 5% pretest probability of malignancy, 44% of 36 patients with low-risk pulmonary nodules underwent at least one invasive procedure.
“To our knowledge, this is the first study that documents the prevalence of cancer (25%) in patients with intermediate-sized nodules referred to community-based pulmonologists,” wrote Dr. Nichole T. Tanner of the Medical University of South Carolina, Charleston, and her coauthors in the study published online (Chest 2015 June 18 [doi:10.1378/chest.15-0630]). “This finding has implications for managing pulmonary nodules in the community; because the risk of cancer is substantial, patients should be managed based on their individualized risk for malignancy.”
Biopsy – either by transthoracic needle aspiration or bronchoscopy – was performed in 33% (125) of patients, of whom 35% were found to have a malignancy and 57% had a specific benign diagnosis; biopsies in 8% were nondiagnostic and those patients were followed for 2 years.
Referrals for surgery were given to 20% (77) of patients; 19 had previously undergone a biopsy, with 11 having a nondiagnostic biopsy and eight having a received a benign result. Of patients who underwent surgery, 65% were diagnosed with a malignancy, and 35% had benign disease. However, the rate of surgical resection was similar regardless of patients’ pretest probability of malignancy.
PET scanning, performed in 37% of patients, was more likely in those who had a biopsy or surgery. The overall accuracy of PET was 74%, and the false-negative rate decreased slightly as the size of the nodule increased.
“Guidelines recommend surgery if the pretest probability of cancer is high (pCA > 65%) because a negative biopsy would not dissuade the clinician from pursuing a definitive diagnosis and a positive biopsy would lead to surgery anyway.”
The researchers pointed out that there are a range of noninvasive options now available to determine the likelihood that a nodule is malignant, such as CT scanning with the use of volumetric software to measure diameter and volume-doubling time of a nodule in between scans.
The study was supported by Integrated Diagnostics. All but one study author declared consulting fees, grant funding, stock options, or employment with Integrated Diagnostics.
One in four intermediate-sized (8-20 mm) pulmonary nodules were found to be malignant, based on results from a retrospective study of 377 patients seen in 18 community pulmonology practices.
Malignant nodules were more likely to be found in current or former smokers and to be larger than the benign nodules.
Further, despite guideline recommendations for surveillance of pulmonary nodules with less than a 5% pretest probability of malignancy, 44% of 36 patients with low-risk pulmonary nodules underwent at least one invasive procedure.
“To our knowledge, this is the first study that documents the prevalence of cancer (25%) in patients with intermediate-sized nodules referred to community-based pulmonologists,” wrote Dr. Nichole T. Tanner of the Medical University of South Carolina, Charleston, and her coauthors in the study published online (Chest 2015 June 18 [doi:10.1378/chest.15-0630]). “This finding has implications for managing pulmonary nodules in the community; because the risk of cancer is substantial, patients should be managed based on their individualized risk for malignancy.”
Biopsy – either by transthoracic needle aspiration or bronchoscopy – was performed in 33% (125) of patients, of whom 35% were found to have a malignancy and 57% had a specific benign diagnosis; biopsies in 8% were nondiagnostic and those patients were followed for 2 years.
Referrals for surgery were given to 20% (77) of patients; 19 had previously undergone a biopsy, with 11 having a nondiagnostic biopsy and eight having a received a benign result. Of patients who underwent surgery, 65% were diagnosed with a malignancy, and 35% had benign disease. However, the rate of surgical resection was similar regardless of patients’ pretest probability of malignancy.
PET scanning, performed in 37% of patients, was more likely in those who had a biopsy or surgery. The overall accuracy of PET was 74%, and the false-negative rate decreased slightly as the size of the nodule increased.
“Guidelines recommend surgery if the pretest probability of cancer is high (pCA > 65%) because a negative biopsy would not dissuade the clinician from pursuing a definitive diagnosis and a positive biopsy would lead to surgery anyway.”
The researchers pointed out that there are a range of noninvasive options now available to determine the likelihood that a nodule is malignant, such as CT scanning with the use of volumetric software to measure diameter and volume-doubling time of a nodule in between scans.
The study was supported by Integrated Diagnostics. All but one study author declared consulting fees, grant funding, stock options, or employment with Integrated Diagnostics.
FROM CHEST
Key clinical point: One in four pulmonary nodules 8-20 mm in size are malignant, and invasive tests are being overused in low-risk patients in community settings.
Major finding: Despite guidelines recommending pulmonary nodes with a less than 5% pretest probability of malignancy be managed with surveillance only, 44% of low-risk patients in the study underwent at least one invasive procedure for what proved to be a benign nodule.
Data source: A multicenter, retrospective study in 377 patients.
Disclosures: The study was supported by Integrated Diagnostics. All but one author declared consulting fees, grant funding, stock options, or employment with Integrated Diagnostics.